
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 6
PRINCIPLES AND METHODS FOR EVALUATING THE
TOXICITY OF CHEMICALS
PART I
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization
World Health Organization
Geneva, 1978
ISBN 92 4 154066 4
(c) World Health Organization 1978
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved. The designations
employed and the presentation of the material in this publication do
not imply the expression of any opinion whatsoever on the part of the
Secretariat of the World Health Organization concerning the legal
status of any country, territory, city or area or of its authorities,
or concerning the delimitation of its frontiers or boundaries. The
mention of specific companies or of certain manufacturers' products
does not imply that they are endorsed or recommended by the World
Health Organization in preference to others of a similar nature that
are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
PREFACE
REFERENCES
1. SOME GENERAL ASPECTS OF TOXICITY EVALUATION
1.1. Introduction
1.1.1. Defining toxicity, hazard, risk, and related terms
1.1.2. Laboratory testing
1.1.3. Toxicological field studies
1.1.4. Ecotoxicology
1.1.5. Priorities in the selection of chemicals for
testing
1.1.6. The extent of toxicity testing required
1.2. Dose-effect and dose-response relationship
1.2.1. Dose
1.2.2. Effect and response
1.2.3. Dose-effect and dose-response curves
1.2.4. Toxic effects due to a combination of chemicals
1.3. Interpretation of laboratory data
1.3.1. Distinction between adverse and nonadverse effects
1.3.2. Threshold: practical and theoretical considerations
1.3.3. Extrapolation of animal data to man
1.3.3.1 Species differences and related factors
1.3.3.2 Safety factors
1.3.3.3 Low-dose extrapolation
1.3.3.4 Other methods of extrapolation
1.4. Human data
1.4.1. Ethical considerations
1.4.2. Need for human investigations
1.5. The use of toxicological data in establishing environmental
health standards
1.5.1. Environmental health standards
1.5.2. Assessment of health risk and evaluation of
benefits
1.5.3. An example of toxicological information used in
standard setting
1.6. Limitations of safety evaluation
ANNEX
REFERENCES
2. FACTORS INFLUENCING THE DESIGN OF TOXICITY STUDIES
2.1. Introduction
2.2. Chemical and physical properties
2.2.1. General considerations
2.2.2. Physicochemical properties and the design of
toxicity studies
2.2.3. Impurities
2.3. Probable routes of exposure
2.3.1. General considerations
2.3.2. Specific variables related to route of exposure
2.3.2.1 Rate of absorption
2.3.2.2 Site of action
2.3.2.3 Biotransformation
2.3.2.4 Species
2.3.2.5 Unintended route
2.3.3. Special tests related to route
2.4. Selection and care of animals
2.4.1. General considerations
2.4.2. Animal variables
2.4.2.1 Selection of species
2.4.2.2 Animal models representing special
populations at risk
2.4.3. Cyclic variations in function or response
2.4.4. Environmental variables
2.4.4.1 Temperature
2.4.4.2 Caging
2.4.4.3 Diet and nutritional status
2.5. Statistical considerations
2.6. Nature of effects
2.6.1. Reversible and irreversible effects
2.6.2. Functional versus morphological changes
2.7. Dynamic aspects of predictive toxicology
2.7.1. Traditional versus new techniques
2.7.2. Toxicity of chemical analogues
2.7.3. Relation between site of metabolism and site of
injury
2.7.4. In vitro test systems
REFERENCES
3. ACUTE, SUBACUTE, AND CHRONIC TOXICITY TESTS
3.1. Introduction
3.2. General nature of test procedures
3.2.1. Housing, diet, and clinical examination of test
animals
3.3. Acute toxicity tests
3.3.1. Underlying principles
3.3.2. Experimental design
3.3.2.1 Selection of species
3.3.2.2 Selection of doses
3.3.2.3 Method of administration
3.3.2.4 Postmortem examination
3.3.3. Repeated high-dose studies
3.4. Subacute and chronic toxicity tests
3.4.1. Underlying principles
3.4.2. Experimental design
3.4.2.1 Selection of species and duration of
studies
3.4.2.2 Selection of doses
3.4.2.3 Method of administration
3.4.2.4 Biochemical organ function tests
3.4.2.5 Physiological measurements
3.4.2.6 Metabolic studies
3.4.2.7 Haematological information
3.4.2.8 Postmortem examination
3.4.2.9 Controls
3.4.3. Alternative approaches in chronic toxicity
3.4.3.1 Perinatal exposure
3.4.3.2 Use of nonrodent species
3.5. Evaluation and interpretation of the results of toxicity
tests
REFERENCES
4. CHEMOBIOKINETICS AND METABOLISM
4.1. Introduction
4.2. Absorption
4.2.1. General principles
4.2.2. Absorption from the lungs
4.2.3. Absorption from the skin
4.2.4. Gastrointestinal absorption
4.3. Distribution
4.4. Binding
4.4.1. Plasma-protein binding
4.4.2. Tissue binding
4.5. Excretion
4.5.1. Renal excretion
4.5.2. Biliary excretion
4.5.3. Enterohepatic circulation
4.5.4. Other routes of excretion
4.6. Metabolic transformation
4.6.1. Mechanism of metabolic transformation
4.6.1.1 Microsomal, mixed-function oxidations
4.6.1.2 Conjugation reactions
4.6.1.3 Extramicrosomal metabolic transformations
4.6.1.4 Nonenzymatic reactions
4.6.2. Species variability
4.6.3. Enzyme induction and inhibition
4.6.4. Metabolic saturation
4.7. Experimental design
4.8. Chemobiokinetics
4.8.1. One-compartment open model
4.8.2. Two compartment/multicompartment open systems
4.8.3. Repeated administration or repeated exposure
4.8.4. Kinetics of nonlinear or saturable systems
4.9. Linear and nonlinear one compartment open-model kinetics of
2,4,5-trichloro-phenoxyacetic acid (2,4,5-T)
4.10. Linear chemobiokinetics used to assess potential for
bioaccumulation of 2,3,6,7-tetrachlorodibenzo-p-dioxin
(TCDD)
ANNEX
REFERENCES
5. MORPHOLOGICAL STUDIES
5.1. Introduction
5.2. General recommendations
5.3. Gross observations
5.3.1. Autopsy techniques
5.3.2. Rat, mouse, guineapig, rabbit, monkey
5.3.3. Carnivores, swine
5.4. Selection, preservation, preparation, and storage of
tissues
5.4.1. Selection of tissues
5.4.2. Oral toxicity tests
5.4.3. Inhalation toxicity studies
5.4.4. Dermal toxicity studies
5.4.5. Special studies
5.5. Preservation of tissues
5.5.1. Immersion
5.5.2. Inflation
5.5.3. Perfusion
5.6. Trimming
5.7. Storage
5.8. Histological techniques
5.9. Special techniques
5.9.1. Enzyme histochemistry
5.9.2. Autoradiography
5.9.3. Immunofluorescence and immunoenzyme techniques
5.9.4. Electron microscopy
5.10. Microscopic examination
5.10.1. Number of animals and number of organs and tissues
studied microscopically
5.10.2. Description of the lesions
5.11. Presentation, evaluation, and interpretation of
pathological data
REFERENCES
6. INHALATION EXPOSURE
6.1. Introduction
6.2. Need for inhalation studies
6.3. Fate of inhaled materials
6.3.1. Nature of aerosols
6.3.2. Deposition
6.3.3. Clearance
6.4. Dose in inhalation studies
6.5. Choice of species
6.5.1. Anatomical differences
6.5.2. Physiological considerations
6.5.3. Disease and susceptibility states
6.6. Duration of exposure
6.6.1. Intermittent versus continuous exposure
6.7. Inhalation systems
6.7.1. Facilities required
6.7.2. Static systems
6.7.3. Dynamic systems
6.7.4. Typical whole-body systems
6.7.5. Construction materials
6.7.6. Engineering requirements
6.7.7. Special systems
6.7.7.1 Isolation units
6.7.7.2 Head and nose exposures
6.7.7.3 Instantaneous exposure systems
6.7.8. Variables to monitor
6.7.9. Human exposure facilities
6.8. Contaminant generation and characterization
6.8.1. Generation of vapours
6.8.2. Particle generators
6.8.2.1 Heterogeneous aerosols
6.8.3. Monitoring contaminant concentrations
6.8.3.1 Vapour sampling
6.8.3.2 Particulate sampling
6.9. Other methods of respiratory tract exposure
6.9.1. In vivo exposures
6.9.2. In vitro exposures
6.10. Biological end-points and interpretation of changes in
these end-points
6.10.1. Morphological changes
6.10.2. Functional changes
6.10.2.1 Measurement of respiratory frequency
6.10.2.2 Measurement of mechanics of respiration
6.10.3. Biochemical end-points
6.10.4. Other end-points in inhalation studies
REFERENCES
7. CARCINOGENICITY AND MUTAGENICITY
7.1. Introduction
7.2. Carcinogenicity
7.2.1. Long-term bioassays
7.2.1.1 Species, strain, and sex selection, and
size of groups
7.2.1.2 Route of administration
7.2.1.3 Inception and duration of tests
7.2.1.4 Dose-level and frequency of exposure
7.2.1.5 Combined treatment and cocarcinogenesis
7.2.1.6 Positive and untreated controls
7.2.1.7 Test material
7.2.1.8 Survey of animals, necropsy, and
histological examination
7.2.2. Short-term tests (rapid screening tests)
7.2.2.1 Metabolic activation, reaction with DNA,
and DNA repair
7.2.2.2 In vitro neoplastic transformation of
mammalian cells
7.2.2.3 Mutagenicity tests
7.2.2.4 Submammalian assay systems
7.2.2.5 Mammalian somatic cells
7.2.2.6 Host and tissue-(microsome) mediated
assays
7.2.3. Correlation between short- and long-term bioassays
for carcinogenicity
7.2.4. Significance of experimental testing for assessing
the possible carcinogenic risk of chemicals to man
7.3. Heritable mutations
7.3.1. Whole-animal tests
7.3.2. Monitoring of human populations
7.3.3. Significance of tests for heritable mutations
REFERENCES
NOTE TO READERS OF THE CRITERIA DOCUMENTS
While every effort has been made to present information in the
criteria documents as accurately as possible without unduly delaying
their publication, mistakes might have occurred and are likely to
occur in the future. In the interest of all users of the environmental
health criteria documents, readers are kindly requested to communicate
any errors found to the Division of Environmental Health, World Health
Organization, Geneva, Switzerland, in order that they may be included
in corrigenda which will appear in subsequent volumes.
In addition, experts in any particular field dealt with in the
criteria documents are kindly requested to make available to the WHO
Secretariat any important published information that may have
inadvertently been omitted and which may change the evaluation of
health risks from exposure to the environmental agent under
examination, so that the information may be considered in the event of
updating and re-evaluation of the conclusions contained in the
criteria documents.
PREFACE
The use of chemicals in practically every aspect of life has
grown very rapidly over the last few decades and international trade
in bulk chemicals, specialty chemicals, and consumer products has
increased proportionately, making imperative the need for continuous
review and reappraisal of procedures for evaluating their safety.
Concern about the possible health hazards that may arise from exposure
to chemicals has increased throughout the world, especially in the
industrialized countries. In many WHO Member States, this has resulted
in new laws and regulations which, in turn, have created a need to
assemble, analyse, and evaluate all available toxicological
information with a view to assessing hazard. Toxicologists have
responded by developing techniques for safety evaluation but these
often differ from one country to another. The differences are
sometimes slight, sometimes considerable; on occasion they have led to
unfortunate misunderstandings, and often to needless duplication of
work.
Ever since the World Health Organization started programmes on
food safety and drug evaluation, the need for some degree of
uniformity and for generally accepted principles and requirements for
toxicological testing and evaluation has been recognized. This has
resulted, in the last 20 years, in a number of technical reports and
guidelines on such topics as the general principles and methods for
the testing and evaluation of intentional and unintentional food
additives (WHO, 1957, 1958, 1967a, 1974a) and drugs (WHO, 1966, 1968,
1975a), on the evaluation of teratogenicity (WHO, 1967b),
mutagenicity, and carcinogenicity (WHO, 1961, 1969, 1971, 1974b,
1976a) and, more recently, on environmental and health monitoring and
the early detection of health impairment in occupational health (WHO,
1973, 1975b), on chemical and biochemical methodology for assessing
the hazards of pesticides to man (WHO, 1975c), and on the methods used
in establishing permissible levels of occupational exposure to harmful
agents (WHO, 1977). Several symposia have also been organized, to
discuss, for example, the methods used in the USSR for establishing
biologically safe levels of toxic substances (WHO, 1975d, 1975e), and
screening tests in chemical carcinogenesis (IARC, 1976). All these
publications remain a most useful source of information on selected
aspects of toxicological evaluation.
The need for more uniformity in methods of environmental health
risk evaluation was again raised at the 1973 World Health Assembly, in
resolution WHA26.58 on human health and the environment which inter
alia requested the Director-General to develop protocols for
experimental and epidemiological studies, uniform terminology, and
agreed definitions. Harmonization of toxicological and epidemiological
methods is also one of the objectives of the WHO Environmental Health
Criteria Programme (WHO, 1976b), initiated in 1973 in collaboration
with Member States and the United Nations Environment Programme
(UNEP), while a very recent (1977) World Health Assembly resolution
WHA30.47 requested the Director-General "to examine the possible
options for international cooperation with a view to accelerating and
making more effective the evaluation of health risks from exposure to
chemicals, and promoting the use of experimental and epidemiological
methods that will produce internationally comparable results".
Current concern about the health effects of chemicals is more
intense in some countries than in others, with the consequent
unevenness in political response reflected in variations in national
safety regulations. This situation is likely to continue for some
time. It is unrealistic and perhaps not really desirable at present,
to seek international standardization in safety testing and evaluation
as this might hinder the input of new ideas and the development of
improved methods and might lead either to the application of needless
tests or to failure to ask the essential questions. However, it is not
too early for scientists and decision-makers to try to understand the
similarities and differences in the safety evaluations made in
different countries. The underlying objectives are the same
everywhere, namely, to minimize harm and maximize safety and yet not
impede the beneficial use of chemicals. Similarly, the basic
scientific principles are globally accepted, so there is no reason why
there should not be a gradual harmonization of methods and procedures
for toxicological testing and evaluation.
With these views in mind and taking into account past work of
WHO, an attempt was made to set forth, comprehensively and on an
international basis, the principles and procedures for the safety
evaluation of all types of chemicals. More than 50 distinguished
experts from some 11 countries collaborated with the Organization and,
in a series of meetings and individual consultations, planned,
drafted, and revised this compilation of toxicological procedures,
providing at the same time an excellent example of international
cooperation. In addition, there was valuable support for the project
from the WHO collaborating centres at: the Institute of Hygiene and
Occupational Health, Sofia, Bulgaria; the National Institute of Public
Health, Bilthoven, Netherlands; the Department of Environmental
Hygiene, The Karolinska Institute, Stockholm, Sweden; the National
Institute of Environmental Health Sciences, Research Triangle Park,
North Carolina, USA; and the Sysin Institute of General and Communal
Hygiene, Moscow, USSR.
The general approach in preparing this publication has been to
present the underlying scientific principles, to evaluate the utility,
strengths and weaknesses of various methods and procedures, to help
the reader select the most suitable technique for a specific purpose
(bearing in mind that circumstances will often dictate the most
appropriate procedure) but not, as already mentioned, to prescribe
standard tests. While aiming at agreement on purely scientific issues,
it has not been sought on details of procedure, on the interpretation
of results, or on methods for setting environmental health standards.
Indeed, because there were often differences of opinion on these
matters, the solution adopted has been to present the different
viewpoints and interpretations. This explains a certain unevenness in
the text, particularly in those chapters prepared jointly by many
scientists from different countries.
Although an effort has been made to avoid inconsistency in
terminology, uniformity has not been possible; indeed, this is
something beyond the scope of the present monograph. However, WHO and
UNEP recently initiated another project that aims at internationally
agreed definitions for those terms most frequently used in
toxicological evaluation. Until this project is completed, it is
important to understand that some terms may have various meanings and
implications in different countries or in different scientific circles
and that it may be highly misleading to employ them outside the
national pattern of use or outside the context of a specialized field,
without precise definition. The reader is therefore warned to be wary
of the uncritical transfer of technical terms from one set of
circumstances to another.
Toxicology is a rapidly developing field, especially at this
time; it is hoped, nevertheless, that this monograph provides a valid
account of the present state of knowledge on the toxicity testing and
evaluation of chemicals as practiced by some of the leading experts in
the field. If it should also stimulate the exchange of knowledge and
experience and so contribute to greater efficiency and reliability in
toxicity testing and evaluation, it will have more than fulfilled its
purpose.
The work has been divided into two separate publications. The
first part contains the broad principles and more general aspects of
toxicity testing, the planning and evaluation of acute, subacute, and
chronic toxicity tests, chemobiokinetics and metabolism, morphological
tests, inhalation studies, and tests for carcinogenicity and
mutagenicity. Part 2 systematically covers some more specialized
procedures for safety evaluation, i.e. functional studies of organs
and systems, effects on reproduction, neurological and behavioural
studies, effects on the skin and the eye, cumulation and adaptation,
and finally discusses factors that could modify the outcome of
toxicity testing and evaluation.
The main authors mutually reviewed the chapters of the treatise,
which can therefore be considered to be a synthesis of various views
and opinions, but this does not detract from the merit of their own
contributions which are gratefully acknowledged. The WHO Secretariat
at the Meeting of the Main Authors in Geneva (28 July to 1 August
1975)a and at the Scientific Group in Lyons (1 to 5 December 1975)b
comprised: Dr M. El Batawi, Chief, Occupational Healtha;
Dr H. Bartsch, Unit of Chemical Carcinogenesis, IARC, Lyonsb:
Dr J. F. Copplestone, Vector Biology and Controlb; Dr F. C. Lu,
Chief, Food Additivesb; Dr R. Montesano, Unit of Chemical
Carcinogenesis, IARC, Lyonsa,b; Dr H. Nakajima, Drug Evaluation and
Monitoringa; Dr M. Vandekar, Vector Biology and Controla; and
Dr G. Vettorazzi, Food Additivesa. Dr V. B. Vouk, Chief, Control of
Environmental Pollution and Hazards was the Secretary of the Geneva
meeting, while Dr L. Tomatis, Chief, Unit of Chemical Carcinogenesis,
IARC, Lyons, and Dr Vouk were the Joint Secretaries of the Scientific
Group at Lyons. Representatives of other organizations who were
present at the meetings include: Dr M. Marcus (US Environmental
Protection Agency)a; Dr W. J. Hunter (Commission of the European
Communities)b; Mr C. Prior (Organization for Economic Cooperation
and Development)b; Dr V. Smirnyagin (International Council of
Scientific Unions)b. Miss S. Braman, Technical Assistant, Control of
Environmental Pollution and Hazards, serviced the two meetings and
helped throughout with the preparation of the manuscript.
The final editing was carried out by a group headed by Professor
N. Nelson who, indeed, presided over the whole project and to whom
special thanks are due for, without his ideas, enthusiasm and, above
all, profound knowledge of the subject, there would have been no
treatise.
a Participated in the Meeting of Main Authors, Geneva, 28 July to
1 August 1975.
b Participated in the Scientific Group on Methods of Toxicity
Evaluation of Chemicals, Lyons, 1-5 December 1975.
REFERENCES
IARC (1976) Screening tests in chemical carcinogenesis -- Proceedings
of a Workshop organized by IARC and the CEC, Brussels 1975.
IARC Sci. Publ. No. 12.
WHO (1957) WHO Technical Report Series No. 129 (General principles
governing the use of food additives: First report of the Joint
FAO/WHO Expert Committee on Food Additives.) 22 pp.
WHO (1958) WHO Technical Report Series No. 144 (Procedures for the
testing of intentional food additives to establish their safety
for use: Second report of the Joint FAO/WHO Expert Committee on
Food Additives.) 19 pp.
WHO (1961) WHO Technical Report Series No. 220 (Evaluation of the
carcinogenic hazards of food additives: Fifth report of the Joint
FAO/WHO Expert Committee on Food Additives.) 33 pp.
WHO (1966) WHO Technical Report Series No. 341 (Principles for
pre-clinical testing of drug safety: Report of a WHO Scientific
Group.) 22 pp.
WHO (1967a) WHO Technical Report Series No. 348 (Procedures for
investigating intentional and unintentional food additives:
Report of a WHO Scientific Group.) 25 pp.
WHO (1967b) WHO Technical Report Series No. 364 (Principles for the
testing of drugs for teratogenicity: Report of a WHO Scientific
Group.) 18 pp.
WHO (1968) WHO Technical Report Series No. 403 (Principles for the
clinical evaluation of drugs: Report of a WHO Scientific Group.)
32 pp.
WHO (1969) WHO Technical Report Series No. 426 (Principles for the
testing and evaluation of drugs for carcinogenicity: Report of a
WHO Scientific Group.) 26 pp.
WHO (1971) WHO Technical Report Series No. 482 (Evaluation and testing
of drugs for mutagenicity: principles and problems -- Report of a
WHO Scientific Group.) 18 pp.
WHO (1973) WHO Technical Report Series No. 535 (Environmental and
health monitoring in occupational health: Report of a WHO Expert
Committee.) 48 pp.
WHO (1974a) WHO Technical Report Series No. 539 (Toxicological
evaluation of certain food additives with a review of general
principles and of specifications: Seventeenth report of the Joint
FAO/WHO Expert Committee on Food Additives.) 40 pp.
WHO (1974b) WHO Technical Report Series No. 546 (Assessment of the
carcinogenicity and mutagenicity of chemicals: Report of a WHO
Scientific Group.) 19 pp.
WHO (1975a) WHO Technical Report Series No. 563 (Guidelines for
evaluation of drugs for use in man: Report of a WHO Scientific
Group.) 59 pp.
WHO (1975b) WHO Technical Report Series No. 571 (Early detection of
health impairment in occupational exposure to health hazards:
Report of a WHO Study Group.) 80 pp.
WHO (1975c) WHO Technical Report Series No. 560 (Chemical and
biochemical methodology for the assessment of hazards of
pesticides for man.) 26 pp.
WHO (1975d) Methods used in the USSR for establishing biologically
safe levels of toxic substances. Geneva, WHO, 171 pp.
WHO (1975e) Methods for studying biological effects of pollutants
(A review of methods used in the USSR). Copenhagen, WHO
Regional Office for Europe, 80 pp. (EURO publication 3109(4).)
WHO (1976a) WHO Technical Report Series No. 586 (Health hazards from
new environmental pollutants: Report of a WHO Study Group.)
96 pp.
WHO (1976b) Background and purpose of the WHO Environmental Health
Criteria Programme. (Reprint from Environmental Health
Criteria 1 Mercury.) Geneva, WHO, 9 pp.
WHO (1977) WHO Technical Report Series No. 601 (Methods used in
establishing permissible levels in occupational exposure to
harmful agents: Report of a WHO Expert Committee with the
participation of ILO.) 68 pp.
PRINCIPLES AND METHODS FOR THE TOXICITY EVALUATION OF CHEMICALS
Editorial Group
Dr F. A. Fairweather, Department of Health & Social Security, London,
England
Professor F. Kaloyanova, Institute of Hygiene & Occupational Health,
Sofia, Bulgaria
Dr G. N. Krasovskij, Laboratory of Water Toxicology, A. N. Sysin
Institute of General & Communal Hygiene, Moscow, USSR
Dr R. Kroes, Central Institute for Nutrition & Food Research, Zeist,
Netherlands
Dr R. Montesano, Unit of Chemical Carcinogenesis, International Agency
for Research on Cancer, Lyons, France
Professor S. D. Murphy, Division of Toxicology, Department of
Pharmacology, The University of Texas Health Sciences Center,
Houston, TX, USA
Professor N. Nelson, Institute of Environmental Medicine, New York
University, NY, USA (Chairman)
Professor D. V. Parke, Department of Biochemistry, University of
Surrey, Guildford, England
Professor I. V. Sanockij, Department of Toxicology, Institute of
Industrial Hygiene & Occupational Diseases, Moscow, USSR
Dr I. P. Ulanova, Department of Toxicology, Institute of Industrial
Hygiene & Occupational Diseases, Moscow, USSR
Dr V. B. Vouk, Control of Environmental Pollution and Hazards,
Division of Environmental Health, World Health Organization,
Geneva, Switzerland (Secretary)
Professor J. G. Wilson, Department of Pediatrics, Children's Hospital
Medical Center, University of Cincinnati, Cincinnati, OH, USA
PRINCIPLES AND METHODS FOR THE TOXICITY EVALUATION OF CHEMICALS
Contributors to Part 1
b Dr H. Bartsch, Unit of Chemical Carcinogenesis, International
Agency for Research on Cancer, Lyons, France (Chapter 7)
Dr S. M. Charbonneau, Toxicology Research Division, Health Protection
Branch, National Department of Health & Welfare, Ottawa, Canada
(Chapter 3)
a Dr R. T. Drew, Medical Department, Brookhaven National Laboratory,
Upton, NY, USA (Chapter 6)
a Dr H. L. Falk, National Institute of Environmental Health
Sciences, Research Triangle Park, NC, USA (Chapter 2)
Dr V. J. Feron, Central Institute for Food Research, Zeist,
Netherlands (Chapter 5)
a Dr P. Gehring, Toxicology Research Laboratory, Dow Chemical USA,
Midland, MI, USA (Chapter 4)
b Dr H. C. Grice, Toxicology Research Division, Health Protection
Branch, Department of National Health & Welfare, Ottawa, Canada
a,b Professor F. Kaloyanova, Institute of Hygiene & Occupational
Health, Sofia, Bulgaria
a Dr G. N. Krasovskij, Laboratory of Water Toxicology, A. N. Sysin
Institute of General & Communal Hygiene, Moscow, USSR (Chapter 1)
a,b Dr R. Kroes, Central Institute for Nutrition & Food Research,
Zeist, Netherlands (Chapters 1 & 5)
Dr J. E. LeBeau, Toxicology Research Laboratory, Dow Chemical USA,
Midland, MI, USA (Chapter 4)
a,b Dr S. Manyai, Biochemical Department, Institute of Occupational
Health, Budapest, Hungary (Chapter 4)
a,b Dr R. Montesano, Unit of Chemical Carcinogenesis, International
Agency for Research on Cancer, Lyons, France (Chapters 1 & 7)
a Dr I. C. Munro, Toxicology Research Division, Health Protection
Branch, Department of National Health & Welfare, Ottawa, Canada
(Chapters 1, 2 & 3)
a Professor S. D. Murphy, Division of Toxicology, Department of
Pharmacology, The University of Texas Health Sciences Center,
Houston, TX, USA (Chapters 1 & 2)
a,b Professor N. Nelson, Institute of Environmental Medicine, New
York University, NY, USA (Chapters 1 & 7)
b Professor G. Nordberg, Institute of Hygiene & Social Medicine,
Odense University, Odense, Denmark
a,b Professor D. V. Parke, Department of Biochemistry, University of
Surrey, Guildford, England (Chapters 1 & 2)
a,b Dr E. A. Pfitzer, Department of Toxicology, Research Division,
Hoffman-La Roche Inc., Nutley, NJ, USA (Chapters 1 & 2)
a Dr M. A. Pinigin, A. N. Sysin Institute of General & Communal
Hygiene, Moscow, USSR (Chapter 1)
Dr J. C. Ramsey, Toxicology Research Laboratory, Dow Chemical USA,
Midland, MI, USA (Chapter 4)
b Professor I. V. Sanockij, Department of Toxicology, Institute of
Industrial Hygiene & Occupational Diseases, Moscow, USSR
(Chapters 1 & 2)
Dr K. K. Sidorov, Department of Toxicology, Institute of Industrial
Hygiene & Occupational Diseases, Moscow, USSR (Chapter 6)
b Dr L. Tomatis, Unit of Chemical Carcinogenesis, International
Agency for Research on Cancer, Lyons, France (Chapter 7)
b Professeur R. Truhaut, Laboratoire de Toxicologie et d'Hygiène
industrielles, Faculté des Sciences pharmaceutiques et
biologiques. Université René Descartes, Paris, France
a Dr I. P. Ulanova, Department of Toxicology, Institute of
Industrial Hygiene & Occupational Diseases, Moscow, USSR
(Chapter 6)
a,b Dr V. B. Vouk, Control of Environmental Pollution and Hazards,
Division of Environmental Health, WHO, Geneva, Switzerland
(Chapter 1)
Dr Z. Zawidski, Toxicology Research Division, Health Protection
Branch, Department of National Health & Welfare, Ottawa, Canada
(Chapter 3)
a Participated in the Meeting of Main Authors, Geneva, 28 July to
1 August 1975.
b Participated in the Scientific Group on Methods of Toxicity
Evaluation of Chemicals, Lyons, 1-5 December 1975.
1. SOME GENERAL ASPECTS OF TOXICITY EVALUATION
1.1 Introduction
Toxicology is concerned both with the nature and mechanisms of
toxic lesions and the quantitative evaluation of the spectrum of
biological changes produced by exposure to chemicals. Every chemical
is toxic under certain conditions of exposure. An important corollary
is that for every chemical there should be some exposure condition
that is safe as regards man's health (Lazarev, 1938; Pravdin, 1934;
Smyth, 1963; Weil, 1972a) with the possible exception of chemical
carcinogens and mutagens (WHO, 1974a).
The quantitative evaluation of the biological changes caused by
chemicals aims at the establishment of dose-effect and dose-response
relationships that are of fundamental importance for health risk
evaluation.
1.1.1 Defining toxicity, hazard, risk, and related terms
In a general sense, the toxicity of a substance could be defined
as the capacity to cause injury to a living organism (NAS/NRC, 1970;
Sanockij, 1970). A highly toxic substance will damage an organism if
administered in very small amounts; a substance of low toxicity will
not produce an effect unless the amount is very large. Thus, toxicity
cannot be defined without reference to the quantity of a substance
administered or absorbed (dose), the way in which this quantity is
administered (e.g. inhalation, ingestion, injection) and distributed
in time (e.g. single dose, repeated doses), the type and severity of
injury, and the time needed to produce that injury.
There is no generally agreed definition of "hazard" associated
with a chemical, but the term is used to indicate the likelihood that
a chemical will cause an adverse health effect (injury) under the
conditions in which it is produced or used (Goldwater, 1968; NAS/NRC,
1970, Pravdin, 1934).
Risk is a statistical concept and has been defined by the
Preparatory Committee of the United Nations Conference on the Human
Environmenta, as the expected frequency of undesirable effects
arising from exposure to a pollutant. Estimates of risk may be
expressed in absolute terms or in relative terms. The absolute risk is
the excess risk due to exposure. The relative risk is the ratio
between the risk in the exposed population and the risk in the
unexposed population (BEIR, 1972; ICRP, 1966).
a Preparatory Committee of the United Nations Conference on the
Human Environment, Third Session, 13-24 September 1971
(A/Conf. 4818, pp. 45 & 46).
Safety is a term that has been used extensively but is difficult
to define. One definition is that "safety" is the practical certainty
that injury will not result from the substance when used in the
quantity and in the manner proposed for its use (NAS/NRC, 1970). This
definition is of little use unless "practical certainty" is defined in
some way, for example, in terms of a numerically specified low risk.
Another view is that "safety" should be judged in terms of socially
"acceptable" risks. Such judgments are largely outside the scope of
scientific evaluation but nevertheless require assessment both of the
probabilitiesa of various adverse effects and of their severity in
terms of human health or other concerns (NAS, 1975).
1.1.2 Laboratory testing
Human data on the toxicity of chemicals are obviously more
relevant to safety evaluation than those obtained from the exposure of
experimental animals (see section 1.4). However, controlled exposures
of man to hazardous or potentially hazardous substances are limited by
ethical considerations and information obtained by clinical or
epidemiological methods must be relied on. Where such information is
not available, as in the case of all new synthetic chemicals, data
must be obtained from tests on experimental animals and other
laboratory procedures. The degree of confidence with which human
health risks can be estimated from laboratory data depends on the
quality of the data, and the selection of appropriate laboratory
testing procedures is the main subject of this monograph.
1.1.3 Toxicological field studies
In the laboratory, only a small number of animal species are
available for testing. The testing of wild species, living in cages
under field conditions, may be useful but sometimes presents a variety
of problems. Successful trials require a large enough site (about
8 ha; 20 acres) with adequate and varied populations of birds,
mammals, fish, insects, and other species, and the area studied must
be considerably greater than that treated (Brown & Papworth, 1974).
Data obtained from field trials of chemicals are of considerable value
in supplementing data obtained with laboratory animal species and in
validating the projection of experimental results to the ecosystem,
including man. Studies of random events in natural ecosystems can also
provide useful data.
a i.e. the expected frequencies.
Sensitive analytical techniques now make it relatively simple to
conduct field studies in man by monitoring levels of a chemical or its
metabolites in blood, urine, hair, or saliva; this biological
monitoring together with environmental monitoring provides important
information on the exposure of mana. Regular periodic determination
of the profile of certain plasma enzymes and other biochemical
variables in the subject provides another valuable method for
monitoring health effects particularly under occupational exposure
conditions (WHO, 1973, 1975a, 1975b); changes in these profiles may
provide early warning of damage by toxic chemicals (Cuthbert, 1974).
1.1.4 Ecotoxicology
A new subdivision of toxicology, "ecotoxicology", has emerged
following observations that some persistent chemicals can exert toxic
effects at several points in an ecosystem. The appearance of a
chemical or the manifestation of a toxic effect may occur far away
from its initial point of introduction into the environment. Methods
for assessing the extent and significance of the movement of
pollutants and their degradation products through the environment to
target systems are discussed in a recent publication (NAS, 1975).
1.1.5 Priorities in the selection of chemicals for testing
In principle, all new chemicals require safety evaluation before
manufacture and sale, but, because of the large number of chemicals
that represent a possible hazard to human health and limited
resources, it is necessary to give priority to those that are directly
consumed by man, such as drugs and food additives, and those that are
widely used such as pesticides or household consumer products.
Industrial chemicals that can escape into the working or general
environment or can contaminate other products are another category of
concern.
Compounds of suspected high acute, chronic, or delayed toxicity
(such as carcinogenicity) or of high persistence in the environment,
or compounds which contain chemical groups known to be associated with
these properties, deserve the highest priority. This also applies to
compounds known to inhibit metabolic deactivation of chemicals as they
may represent a more insidious form of toxicity.
a Report of the Meeting of a Government Expert Group on Health
Related Monitoring. Unpublished WHO document CEP/77.6.
Chemicals resistant to metabolism, especially metabolism by
microflora, will have a high environmental persistence. Many
halogenated compounds come into this category, and should, therefore,
have some degree of priority. Compounds that accumulate in food chains
or are stored in the body, e.g. methylmercury and DDT, will be a
matter of concern. Such compounds are often highly lipid-soluble or
strongly bound to tissue proteins, or may undergo enterohepatic
recirculation with consequent slow excretion resulting in accumulation
in the organism.
Physicochemical properties can be an important consideration in
setting priorities for testing potential environmental pollutants. For
example, biomagnification of stable, fat-soluble substances may lead
to contamination of human food supplies as well as to adverse effects
in wildlife at the higher levels of food chains, even though the
intended use and sites of application of the substance would suggest
that primary exposure of these species is unlikely (Edwards, 1970).
Physicochemical properties such as vapour pressure, and particle size
and density are important in predicting the atmospheric transport of
chemicals (Fuchs, 1964; OECD, 1977). Adsorption of a chemical on soil
particles may increase the likelihood that the material will become
airborne or be transported by watercourses and subsequently deposited
in areas remote from its site of application (Cohen & Pinkerton,
1966), or it may retard the movement of a chemical through ground
water and thus reduce the likelihood of contamination of ground water
supplies near the site of application (Edwards, 1970; Hamaker et al.,
1966).
Even though certain predictions and comparisons of environmental
distribution and biomagnification of chemicals in the environment may
be made theoretically on the basis of the physicochemical properties
of the substances in question, more definitive information of this
nature can be obtained experimentally by the use of model ecosystems
such as those described by Metcalf et al. (1971) and Lu & Metcalf
(1975). These model ecosystems may be oversimplified, and they should
not replace experimental field studies or programmes for monitoring
environmental contaminants. However, their use in an early phase of
the overall evaluation of the toxicity of environmental chemicals may:
( a) help to determine order of priority of chemicals for study,
( b) identify the components of the environment (food, water, air)
most likely to be a source of human exposure and ( c) suggest whether
the chemicals are likely to accumulate in human tissues.
Furthermore, the systematic application of such model systems to
structure-distribution studies may help in determining with greater
certainty those physicochemical properties of substances that are most
useful in predicting the distribution and effects of chemicals in
ecosystems (Lu & Metcalf, 1975).
Information on production, use, and disposal are of great
importance in determining the sources and quantities of a chemical
released into the environment, in assessing the possible extent of
human exposure, and in identifying human populations that are likely
to be exposed.
In conclusion, essential criteria for priority in the selection
of chemicals for testing are: (a) indication or suspicion of hazard to
human health and type and severity of potential health effects; (b)
probable extent of production and use; (c) potential for persistence
in the environment; (d) potential for accumulation in biota and in the
environment, and (e) type and size of populations likely to be
exposed. A chemical of first priority for testing would rate highly
with respect to all or most of these criteria.
1.1.6 The extent of toxicity testing required
The extent of the toxicity testing required will depend on a
variety of considerations, and generally valid procedures cannot be
proposed. One scheme, proposed by Sanockij (1975a), for chemicals that
are being developed, is shown in Table 1.1. As a first step, it may be
useful to make an approximate estimation of toxicity based on the
chemical structure and the physical and chemical properties of the
substance, and on known correlations of these variables with
biological activity (Andreyeshcheva, 1976; WHO, 1976a). These
considerations may be of value for decisions on safety measures to be
taken during initial laboratory work. Extrapolation and interpolation
in homologous series may also be of value for decisions on safety
measures to be taken during initial laboratory work (Ljublina &
Miheev, 1974), but for some series of chemicals this is not
applicable.
A preliminary evaluation of toxicity should start when chemicals
are synthesized in the laboratory stage of the development of an
industrial process. The full evaluation of the chemicals involved,
both in respect to occupational and general population exposure, and
assessment of possible air, water, and food contamination, should be
initiated later, when it has been decided to proceed with full-scale
production of the chemical. Toxicity data obtained during the
development stages of a technological process could provide
information concerning the health hazards not only of the raw
materials and products, but also of the various other substances used
or produced as intermediates in the technological process, and of
gaseous and other wastes. Toxicological evaluation may also help in
the selection of an alternative technological process, less hazardous
to health.
Waste disposal by dispersion in air and water, the ease of
environmental degradation of the chemical, and the toxicity of the
degradation products, are other problems that need attention at an
early stage in the toxicological evaluation of new chemicals. For
example, resistance to degradation has to be taken into account when
formulating health criteria regulating the application and disposal of
pesticides (Medved & Spynu, 1970).
This phasing of toxicological studies may be useful in
coordinating testing at national and international levels.
Environmental and health standards will need to be defined
preferentially for those chemicals that show a significant degree of
toxicity and represent a health hazard, and are likely to be used
widely in industry, agriculture, or in consumer products.
Changes and developments in industrial processes, the development
of new chemicals, and changes in the use of existing chemicals, may
lead to new or increased hazards. This calls for a continuous
re-evaluation of priorities.
1.2 Dose-Effect and Dose-Response Relationships
1.2.1 Dose
Most commonly, the term "dose" is used to specify the amount of
chemical administered, usually expressed per unit body weight. If the
dose is administered into the stomach, on the skin, or into the
respiratory tract, transport across the membranes may be incomplete
and the absorbed dose will not be identical with the dose
administered. In environmental exposures, an estimate of the dose can
be made from the measurement of environmental and food concentrations
as a function of time, and involves the assessment of food intake,
inhalation rate, and the appropriate deposition and retention factors.
The doses in the organs and tissues of interest may be estimated
from:
(a) administered dose or intake;
(b) measurement of the concentrations in tissues and organ
samples;
(c) measurement of concentrations in excreta or exhaled air.
Table 1.1 The extent of toxicological evaluation required in relation to technological process development
Stages of technological Stages of toxicological Toxicological studies
development evaluation
1. Theoretical concept Preliminary toxicological Analysis of literature data on toxicity and
and process flow assessment hazards of raw materials, reagents,
diagram catalysers, semiproducts and additives
Assessment of toxicological parameters on
the basis of metabolic analogies, persistence,
the relationship between chemical
structure, chemical and physical properties.
and biological activity. Interpolation and
extrapolation in homologous series
2. Laboratory development Acute toxicity Acute and subacute experiments on
of the technological animals. Toxicological evaluation of
process technological unit processes
3. Pilot plant stage Subacute toxicity Subacute toxicity experiments on animals.
Studies of delayed effects. Medical
examination of workers.
Detailed toxicological Chronic toxicity studies and, when indicated,
evaluation effects on reproduction, carcinogenicity,
mutagenicity. Formulation of medical and
industrial hygiene requirements for
full-scale production
Table 1.1 (cont'd).
Stages of technological Stages of toxicological Toxicological studies
development evaluation
4. Design of industrial Additional studies Studies of the mechanism of action, early
scale process and differential diagnosis, experimental
therapy
5. Production and use Field studies Assessment of working and environmental
of chemicals conditions and of health status of workers
and general population
Epidemiological studies
Clinical evaluation of experimental
prophylactic, diagnostic and therapeutic
methods
Adjustment and correction of requirements
for health and environmental protection
The use of these three types of information for the purposes of tissue
and organ dose estimation requires the postulation of models to
describe the absorption, distribution, retention, biotransformation,
and excretion of the original chemical or its metabolites, as a
function of time (see Chapter 4).
When the site of toxic action is located at, or very near, the
site of application, for example, the skin, then the tissue dose
estimate may be very reliable. However, when the site of toxic action
is remote, for example, a liver cell, then the estimates of
toxicologically significant doses are much less reliable.
The presence of a chemical in the blood indicates absorption;
however, the blood concentration of a chemical is in a dynamic state,
reaching higher levels with increasing absorption but decreasing as
the distribution, tissue storage, metabolic transformation, and
excretion increase. The blood concentration of a chemical is useful as
an indicator of the dose only when it is related in a defined manner
to the concentration at the site or sites of action (organs and
tissues) (Task Group on Metal Toxicity, 1976).
1.2.2 Effect and response
"Effect" and "response" are often used interchangeably to denote
a biological change, either in an individual or in a population,
associated with an exposure or dose. Some toxicologists have, however,
found it useful to differentiate between an effect and a response by
applying the term "effect" to a biological change, and the term
"response" to the proportion of a population that demonstrates a
defined effect (Pfitzer, 1976; Task Group on Metal Toxicity, 1976).
In this terminology, response means the incidence rate of an
effect. For example, the LD50 value may be described as the dose
expected to cause a 50% response in a population tested for the lethal
effect of a chemical. This distinction will be made in the present
monograph, although it should be recognized that this terminology is
not generally accepted.
An effect can usually be measured on a graded scale of intensity
or severity and its magnitude related directly to the dose. Certain
effects, however, permit no gradation and can be expressed only as
"occurring" or "not occurring". Such effects are usually called
"quantal" (see for example, Finney, 1971). Typical examples of quantal
effects are death or occurrence of a tumoura.
The toxic action of chemicals usually affects the whole organism
but the primary damage may be localized in a specific target organ or
organs in which the toxic injury may manifest itself in terms of
dysfunction or overt disease (NIEHS, 1977). According to Sanockij
(1975a), the specificity of acute toxic action can be expressed in
terms of a "zone of specific action" (Zsp) which is the ratio
between the thresholdb dose of an acute effect at the level of the
total organism and the threshold dose for an acute effect at a
specific organ or system. If Zsp > 1, the toxic action is specific;
if Zsp < 1, it is non-specific.
Acute effects are those that occur or develop rapidly after a
single administration (Casarett, 1975) but acute effects may appear
after repeated or prolonged exposure as well. Chronic effects may also
result from a single exposure but more often they are a consequence of
repeated or prolonged exposures. Chronic effects are characterized not
only by their duration but also by certain pathological features. They
may arise from the accumulation of a toxic substance or its
metabolites in the body, or from a summation of acute effects. The
latent period (or the "time-to-occurrence" of an observable effect)
may sometimes be very long, particularly if the dose or exposure is
low. Other aspects of the nature of toxic effects are discussed in
section 2.6.
a A similar classification of effects is used in radiological
protection where a distinction is made between "nonstochastic" and
"stochastic" effects (ICRP, 1977). Nonstochastic effects are those
for which the severity of effect varies with the dose. Stochastic
effects are those for which the probability of occurrence, rather
than their severity, is regarded as a function of dose. Hereditary
effects and carcinogenesis induced by radiation are considered to
be stochastic.
b The threshold concept is discussed in section 1.3.2.
Not every effect is necessarily adverse or harmful. In some
cases, a graded effect may be either within the so-called "normal"
range of physiological variation, or an "adverse" effect, depending on
its intensity. The distinction between a physiological change and a
pathological effect (adverse effect) is sometimes very difficult to
make and there is much disagreement on this subject which will be
discussed in detail in section 1.3.1. The concept of biochemical
lesion introduced by Peters and his collaborators (Gavrilescu &
Peters, 1931; Peters, 1963, 1967), and based on the ideas of Claude
Bernard (Bernard, 1898), is of fundamental importance in this respect.
A biochemical lesion can be defined as the biochemical change or
defect which directly precedes pathological change or dysfunction
(Peters, 1967).
The Task Group on Metal Accumulation (1973) and the Task Group on
Metal Toxicity (1976) have defined the critical concentration for a
cell as the concentration (of a metal) at which undesirable (adverse)
functional changes, reversible or irreversible, occur in the cell.
Critical organ concentration has been defined as the mean
concentration in the organ at the time any of its cells reaches
critical concentration and critical organ as that particular organ
which first attains the critical concentration of a metal under
specified circumstances of exposure and for a given population. This
definition of "critical organ" differs from the generally accepted use
of the term, i.e. that the critical organ is the organ whose damage
(by radiation) results in the greatest injury to the individual (or
his descendants) (ICRP, 1965). However, some toxicologists question
the usefulness of the concept of a critical organ or tissue because it
diverts attention from the role that the various regulatory systems of
the body may have in relation to a toxic injury.
1.2.3 Dose-effect and dose-response curves
Dose-effect curves demonstrate the relation between dose and the
magnitude of a graded effect, either in an individual or in a
population. Such curves may have a variety of forms. Within a given
dose range they may be linear but more often they are not. Finney
(1952a) has discussed various transformations that can be used to make
dose-effect curves linear.
Dose-response curves demonstrate the relation between dose and
the proportion of individuals responding with a quantal effect. In
general, dose-response curves are S-shaped (increasing), and they have
upper and lower asymptotes, usually but not always 100 and 0% (see for
example Cornfield, 1954). One way of explaining the shape of
dose-response curves is that each individual in a population has a
unique "tolerance" and requires a certain dose before responding with
an effect. There exists, in principle, a low dose to which none will
respond and a high dose to which all will respond.
For each effect there will usually be a different dose-response
curve. Loewe (1959) and Hatch (1968) have discussed the relationship
between dose, effect, and response and its graphical representation in
a three-dimensional model.
If the experiment or observation is well designed (Chapters 2 and
3), the dose-response relationship will be based on data from many
individuals over a range of doses from minimum to maximum response.
Mathematical and statistical procedures are then used to establish the
curvilinear relationship that provides the best fit to all of the
data, expressed as mean values with their standard deviations at
different doses. Mathematical expressions for dose-effect and
dose-response relationships and the merits of applying normal,
log-normal, and other types of distributions are discussed in the
Appendix.
It should be pointed out that the shape of the dose-response
curve for the same substance and the same animal species may vary with
changes in experimental conditions, such as changes in the way in
which the dose is distributed in time (Weil, 1972a).
In evaluating human exposure to environmental chemicals, the dose
will usually be estimated as a function of concentration and time. In
some cases the concentration will be fairly constant and then the
time-effect and time-response relationships will be similar to the
dose-effect and dose-response relationships. However, in many cases
the concentration will vary, as will the time of exposure to specific
concentrations, and integrated relationships of dose-concentration-
time must be considered as well as dose-effect and time-effect
relationships (Druckrey, 1967; Golubev et al., 1973; Lazarev, 1963;
Weil, 1972a).
Haber's rule (ct=k) states that the product of concentration
(c) and time (t) results in a constant intensity of effect (k)
for some gases. This formula was later changed to ctb = k (where
b is constant) which fitted other biological data better (Lazarev &
Brusilovskaja, 1934), although it also has its limitations. The
extrapolation of concentration-time relationships has been used
successfully to obtain predictions of response following long-term
inhalation exposure to low concentrations (Pinigin, 1974).
Concentration-time relationships, such as the variation of the
fraction of the dose with time as in combinations of short-term peak
concentrations and prolonged low-level concentrations in air
pollution, and variable cycles of exposure, may influence the toxic
effect. Few systematic attempts to evaluate these factors have been
made, although Sidorenko & Pinigin (1975, 1976) have described some
principles for setting air quality standards from this viewpoint, and
Pinigin (1974) has dealt with the problems of intermittent inhalation
exposure. This problem has also been discussed by Ulanova et al.
(1973, 1976).
1.2.4 Toxic effects due to a combination of chemicals
When an organism is exposed to two or more chemicals, their joint
action may be:
(a) independent -- when the chemicals produce different effects
or have different modes of action;
(b) additive -- when the magnitude of an effect or response
produced by two or more chemicals is numerically equal to
the sum of the effects or responses that the chemicals would
produce individually;
(c) more than additive -- often called potentiation or
synergism;
(d) less than additive (antagonism, inhibition).
More specific terminology may be used when the mechanisms of
joint action are known or when definite assumptions are made about
them (Finney, 1971; Hewlett & Pluckett, 1961). The time intervals and
sequences between exposures to different chemicals are extremely
important, and the quality as well as the degree of joint action may
depend on these variables (Kagan, 1973; Kustov et al., 1974; Williams,
1969). Furthermore, the joint action at lethal dose levels may be
quite different from that at low dose levels, when the effects or
responses are often only additive or independent (Smyth et al., 1969;
Ulanova, 1969).
Most statistical models for joint action have been developed for
situations in which two or more chemicals are administered
simultaneously or within a short (few minutes) time interval. A model
proposed by Finney (1952b, 1971) is often used for predicting the
acute joint toxicity of chemicals. The model is strictly applicable to
mixtures of chemicals that act at the same site, producing the same
type of acute toxic effect and having parallel regression lines of
probits against log doses (see Appendix). For a mixture of, for
example, three chemicals, the equation for the median effective dose
(ED50) is
1 contour integralA contour integralB contour integralC
= + +
ED50 (A,B,C) ED50 (A) ED50 (B) ED50 (C)
where contour integralA, contour integralB and contour integralC are
the fractions of substances A, B, and C in the mixture. When all
the values on the right hand side of equation (1) are known, a predicted
ED50 (assuming additive joint action) can be calculated and compared
with the actual ED50 of the mixture determined experimentally. A
smaller than predicted ED50 demonstrates a more than additive
response (synergism), a greater than predicted ED50 indicates a less
than additive response (antagonism). Smyth et al. (1969) demonstrated
that this equation can give satisfactory results under conditions that
are less restrictive than stated above, for example in identifying the
type of acute joint action among randomly selected industrial
chemicals. Ball (1959) applied the equation to the estimation of
maximum allowable concentrations for occupational exposure to mixtures
of substances that exercise a "similar joint action", e.g. benzene and
toluene. Another model for estimating the results of joint action has
been developed using the isoeffective concentrations instead of ED50
in equation (1) (Pinigin, 1974).
The possibility of predicting the type of joint action is
enhanced if there is information on the metabolism and disposition of
the chemicals (Murphy, 1969; Williams, 1969). Basic principles
concerning the kinetics of reactions of chemicals with primary sites
(tissue receptor sites) and with secondary sites are important in
considering the joint action of chemicals (Gaddum, 1957; Schild et
al., 1961; Veldstra, 1956; Williams, 1969). The relevant factors seem
to be the relative affinities at the sites of action (e.g. target
enzymes, neuroeffector sites, and other vital target sites), and at
the sites of loss or sinks (e.g. detoxifying enzymes, nonvital tissue
binding sites, pathways of excretion, and storage sites), and the
intrinsic activity at the sites of actiona. Since there is a limited
number of sites of action and sinks within any organism, there will be
a limited dose range within which synergism or antagonism can be
demonstrated. This, of course, is only one area where more information
could help in predicting the effects of the joint action of chemicals.
Other areas where knowledge is insufficient are the possible effects
of low-level, prolonged exposures to mixtures of chemicals and the
effects of multiple stresses including chemicals, physical factors
such as heat and noise, and pre-existing disease (NIEHS, 1970).
a Relative affinity -- reciprocal of the dissociation constant for
the chemical-receptor complex. Intrinsic activity -- the capacity
of the chemical to produce an effect when it combines with a
reactive tissue site. For precise definitions see for example
Ariëns et al. (1957).
Simultaneous exposures to the same chemical in different media
(e.g. air, water, food) which is called "complex action" by some
toxicologists (Korbakova et al., 1971; Kustov et al., 1974; Pinigin,
1974; Spynu et al., 1972) is another aspect of multiple stresses which
has considerable practical importance.
1.3 Interpretation of Laboratory Data
It is essential that all experiments to evaluate toxicity should
be designed to be scientifically meaningful, and should not be
conducted merely to comply with statutory regulations. Thus, the
evaluation of each new chemical will not be an identical task and
procedures will differ, to some extent, from one compound to another.
The protocol for an experiment will evolve gradually during the
experiment, in accordance with earlier findings. It is useful to have
laboratory data validated by a study of the mechanisms involved in the
development of the toxic lesion. Furthermore, numerous endogenous and
environmental variables can modify the toxicity of chemicals, as
discussed in subsequent chapters. In some instances, the influence of
these variables is known, and can be controlled, but often this is not
the case and this may cause serious difficulties in the interpretation
of laboratory toxicity data.
In the present context, we are mainly interested in the
interpretation of laboratory data with a view to their application in
the evaluation of the health risk to man. The discussion will
therefore be limited to a few topics that are particularly relevant in
this respect.
1.3.1 Distinction between adverse and nonadverse effects
An adverse, or "abnormal" effect has often been defined in terms
of a measurement that is outside the "normal" range. The "normal"
range, in turn, is usually defined on the basis of measured values
observed in a group of presumably healthy individuals, and expressed
in statistical terms of a range representing 95% confidence limits of
the mean or, for individuals, in terms of 95% "tolerance" limitsa
established with a derived degree of confidence (95% or 99%). An
individual with a measured value outside this range may be either
a Tolerance limits are defined as m ± ks where m is the sample
mean, s is the sample standard deviation and k is a coefficient
that depends both on the size of the sample (N) and the required
degree of confidence. If the "normal" mean has been determined on
the basis of a very large sample, the 95% limits will be equal to
µ ± 1.96sigma where µ and sigma are the "true" or population values
of the mean and the standard deviation, respectively (see for
example Owen, 1955).
"abnormal" in fact, or one of that small group of "normal" individuals
who have extreme values. According to Sanockij (1970), the distinction
between "normal" and "abnormal" values based on statistical
considerations may be used as a criterion for adverse effects, if the
exposed population consists of adult, generally healthy individuals,
subject to periodical medical examination, such as workers. Departures
from "normal" values associated with a given exposure will then be
considered as adverse effects, if the observed changes are:
(a) statistically significant ( P < 0.05) in comparison with a
control group, and outside the limits (m ± 2 s) of
generally accepted "normal" values;
(b) statistically significant ( P < 0.05) in comparison with a
control group, but within the range of generally accepted
normal values, provided such changes persist for a
considerable time after the cessation of exposure; and
(c) statistically significant ( P < 0.05) in comparison with a
control group, but within the "normal" range, provided
statistically significant departures from the generally
accepted "normal" values become manifest under functional or
biochemical stress.
This statistical definition of adverse effects is less suitable
for the general population which includes some groups that may be
specially sensitive to environmental factors, particularly the very
young, the very old, those affected with disease, and those exposed to
other toxic materials or stresses. In this case, it is practically
impossible to define "normal" values, and any observable biological
change may be considered as an adverse effect under some
circumstances. For this reason, attempts have been made to set
criteria for adverse effects based on biological considerations and
not only on statistically significant differences with respect to an
unexposed population (control group). Although there is no general
agreement on such criteria and the ultimate decision on what is an
adverse effect will have to depend, in each case, on experience and
expert judgment, it may nevertheless be useful to give examples of
such criteria, which illustrate at the same time how different such
criteria may be.
A Committee for the Working Conference on Principles of Protocols
for Evaluating Chemicals in the Environment (NAS, 1975) defined
nonadverse effects as the absence of changes in morphology, growth,
development, and life span. Furthermore, nonadverse effects do not
result in impairment of the capacity to compensate for additional
stress. They are reversible following cessation of exposure without
detectable impairment of the ability of the organism to maintain
homeostasis, and do not enhance susceptibility to the deleterious
effects of other environmental influences.
On the other hand, adverse effects may be deduced as changes
that:
"1. occur with intermittent or continued exposure and that result in
impairment of functional capacity (as determined by anatomical,
physiological, and biochemical or behavioural parameters) or in a
decrement of the ability to compensate additional stress;
2. are irreversible during exposure or following cessation of
exposure if such changes cause detectable decrements in the
ability of the organism to maintain homeostasis; and
3. enhance the susceptibility of the organism to the deleterious
effects of other environmental influences."
Soviet toxicologists emphasize that criteria for differentiating
between adverse and nonadverse effects should not be based on overt
pathology (e.g. inflammation, necrosis, hyperplasia), and have
proposed, inter alia, a number of criteria based on metabolic and
biochemical changes. Such changes are considered to be adverse if:
(a) the metabolism of a substance becomes less efficient or the
elimination of a substance (expressed in terms of biological
half-time, T´) slows down with increasing doses of the
substance (Sanockij, 1956);
(b) enzymes that have a key significance in metabolism are
inhibited (Kustov & Tiunov, 1970);
(c) the inhibition of a certain enzyme results in an increase in
the concentration of the corresponding natural substrate in
the body and/or in a decreased capacity to metabolize the
specific substrates in a loading test (Kustov & Tiunov,
1970);
(d) the relative activities of different enzyme systems are
changed (e.g. the ratio of the activities of asparagine and
alanine transaminases (Kustov & Tiunov, 1970)).
Pokrovskij (1973) also attaches great importance to the changes
in the pattern of isoenzymes in the blood, and to the changes in the
subcellular membranes (e.g. lysosomal membranes) resulting from the
action of toxic substances.
Differentiation between "nonadverse" and "adverse" effects
requires considerable knowledge of the importance of reversible
changes and subtle departures from "normal" physiology and morphology
in terms of the organism's overall economy of life, ability to adapt
to other stresses, and their possible effects on life span. Newer and
improved methods of research have increasingly provided more sensitive
tests for subtle biological deviations such as induction of enzymes of
the smooth endoplasmic reticulum of the liver, or reversible
hypertrophy of the liver. These types of changes are produced by
relatively low doses of many chemicals and they are considered by some
authors to be adaptive and generally useful to health, and by others
to be indicative of injury (Hermann, 1974; Kustov & Tiunov, 1970;
Parke, 1975). One of the most challenging areas for basic research in
toxicology today is the acquisition of data that can be used to
estimate whether, or under what conditions, subtle changes in enzyme
activities, nerve action potentials, altered behavioural reaction etc.
indicate impairment of physiological function or predict impending
development of more serious irreversible injury, should exposure to
the chemical continue.
In addition to all these considerations, the possibility must be
kept in mind that an effect may not be seen because the number of
animals studied was inadequate, the observation time was too short, or
for other reasons.
1.3.2 Threshold: practical and theoretical considerations
The concept of "threshold" is complex and the term has to be
carefully defined, so that statements concerning this concept in
relation to the protection of human health are not confused by
semantic differences. A distinction should be made between the
threshold for individuals and thresholds for limited groups of
individuals or general populations.
The dependence of effect or response on the dose of a chemical
has already been discussed (section 1.2). As a rule, the intensity of
the effect or response decreases with reduction in dose, and a
biological reaction often reaches zero before the dose becomes equal
to zero. Below a certain limiting exposure level, or dose, i.e. below
the threshold, a chemical substance may not elicit a toxic effect. The
threshold for an adverse effect of a chemical is defined by some
toxicologists as the minimum exposure level or dose that gives rise to
biological changes beyond the limits of homeostatic adaptation. True
homeostatic adaptation should be carefully distinguished from
pathological processes (Sanockij, 1975a).
The existence of a threshold for all adverse effects is, however,
still a matter for discussion. Sanockij (1975b) has provided data
which show that small quantities of environmental chemicals may not
reach their receptor because the rate of elimination or metabolic
degradation is relatively more effective with smaller doses. It has
also been suggested that where effective repair processes are present,
even if a substance interacts with the receptor, it need not
necessarily produce an adverse effect.
For some toxic effects, such as neoplastic disease or mutations
of genetic material, it has been assumed by some authors that a single
molecule of a chemical is sufficient to initiate a process that may
progressively lead to an observed, harmful effect. For this reason, it
may not be possible to demonstrate that a threshold dose for a
carcinogen or a mutagen exists (Saffiotti, 1973).
Other scientists view carcinogenic or mutagenic chemicals as
toxic entities that may have special properties with regard to the
nature and characteristics of their adverse effects, but are subject
to the same physicochemical and biological interactions that are
considered to result in a threshold dose for other chemicals (Dinman,
1972; Sanockij, 1970; Stokinger, 1972; Weil, 1972a).
The question of the existence of a threshold for carcinogens and
mutagens was recently discussed by a WHO Scientific Group (WHO,
1974a), which concluded that "the existence of a threshold may be
envisaged. Nevertheless, the difficulties of determining a threshold
for a population are great. Therefore, mathematically derived
conclusions that it is impossible to demonstrate no-effect levels
experimentally cannot be ignored". A "no-effect" level for a group of
animals may occur because the dose is really below the theoretical
no-effect level (i.e. below the threshold) or because the number of
animals is too small. For example, in an experiment with 20 animals,
it is possible that none of the animals will show an effect whereas in
an experiment with 100 animals some response might be seen. However,
an upper limit for the probable response can be estimated
statistically. For instance, if in an experiment with 100 animals, no
response has been observed, it can be shown that there is a 95%
probability that, under the conditions of the experiment, the upper
limit of response is 3%, and that there is a 99% probability that the
response will not exceed 4.5%. Even in an experiment with 1000 animals
showing no response, the upper 95% confidence limit of response is 3
animals showing an effect per 1000 treated animals (Food & Drug
Administration, 1970).
Another reason for not having seen a response in an experiment
may be that the time of observation was too short. This may be the
case, for example, when the quantal effect considered is a cancer,
with a long latent period between exposure and appearance of tumours.
For these reasons the "no-effect level" has no real meaning and a
better term is "no-observed-effect level" (NAS, 1975).
1.3.3 Extrapolation of animal data to man
In many cases, studies with laboratory animals make it possible
to predict the toxic effects of chemicals in man. However, it is
important to realize that experimental animal models have their
limitations, and that the accuracy and reliability of a quantitative
prediction of toxicity in man depend on a number of conditions, such
as choice of animal species, design of the experiments, and methods of
extrapolation of animal data to man.
Hoel et al. (1975) considered the criteria for the adequacy of an
experiment to be used for the extrapolation of animal data to man.
They include: test animal species and strain (the animal should be
susceptible to induction of the effects under consideration); the
number of animals; the route of administration (which should include
the routes of human exposure); and the physical state and chemical
form of the agent. The side effects of the chemical and its organ
specificity should also be taken into account in the design of the
experiment. In interpreting the results, attention should be paid to
adequate survival of the animals, to possible intercurrent disease,
the quality and extent of pathological data, the quality and extent of
relevant data collection during the experiment, and the availability
of data at the time of interpretation.
1.3.3.1 Species differences and related factors
The most difficult problem in the extrapolation of animal data to
man is the conversion from one species to another. For most
substances, the pathogenesis of poisoning is the same in man and other
mammals, and for this reason the signs of intoxication are also
analogous. Thus, quantitative rather than qualitative differences in
toxic response are most common. Man may be more sensitive than certain
laboratory animals but there are also many cases where some animal
species are more sensitive than man. For example, the mouse is most
sensitive to atropine, the cat is less sensitive, while the dog and
the rabbit tolerate atropine in doses 100 times higher than the lethal
dose for man. However, the dog is more sensitive to hydrocyanic acid
than man (Elizarova, 1962).
Species differences in sensitivity can often be explained by
differences in metabolism, in particular by quantitative and
qualitative differences in the ability of an enzyme to detoxify
chemicals, and also by differences in the rates of absorption,
transport, distribution, and elimination of chemicals (Curry, 1970;
Ecobichon & Cormeau, 1973; Flynn et al., 1972; Hucker, 1970; Portman
et al., 1970; Sato & Moroi, 1971). Rall (1970) discussed various
factors to be considered in the selection of animal models for
pharmacotherapeutic studies in relation to the steps that intervene
between administration of the drug (or chemical) and the arrival of
the compound at the ultimate sites of action. After oral
administration, absorption in standard laboratory animals is generally
considered to be very similar to man, although there are quantitative
differences for some compounds. For example, species differences in
the absorption and action of some compounds are related to differences
in the bacterial flora of the gastrointestinal tract (Williams, 1972).
Rall further concluded that the distribution and storage of drugs are
reasonably consistent in mammalian species, including man, although
plasma binding tends to be more extensive in man than in small
mammalian species. Urinary excretion in different animal species
depends to some extent, on their different diets, since diet
influences urinary pH and thus the extent of ionization of compounds.
Biliary excretion is quite variable from species to species and
apparently is more extensive in mice and rabbits than in rats or man.
Species differences in response to chemicals appear to be mainly
related to rates of biotransformation which are generally more rapid
in small laboratory animals than in man.
One of the most potent bladder carcinogens, 2-naphthalenamine
(2-naphthylamine), produces bladder cancer in the dog, hamster, and
man, but not in the rat, rabbit, or guineapig. Species differences in
the carcinogenicity of 2-fluorenylacetamide (2-acetaminofluorene) have
been attributed to the different extents of metabolism to the
proximate carcinogen, the N-hydroxy derivative (Miller et al.,
1964). Similarly, strain differences in metabolism may also affect
toxicity (Mazze et al., 1973).
If metabolic information is available, differences in absorption,
distribution, biotransformation, and elimination of toxic substances
in man and animals should be taken into account when selecting
experimental animals.
Species differences in toxicity may also be due to differences in
cellular transport. Aflatoxin, which is more toxic to rats than to
mice, both as an acute poison and as a carcinogen, is transported more
slowly into the liver cells and is metabolized more rapidly in the
mouse than in the rat (Portman et al., 1970).
In determining the required duration of an animal experiment, it
is often useful to compare the life span of the animal with that of
man. Using the "body weight rule", the average life span for 70
species of mammals showed a linear correlation with body weight, but
the average life span of man was found to be an exception (Krasovskij,
1975). The regression equation obtained from a study of many mammals
showed that the average life span for a mammalian representative,
having the same body weight as man (70 kg) was equal to 15 years.
Thus, if this assumption is accepted the average life span of a rat
(about 2.5 years) corresponds to only 15-17 years of a man's life.
This inconsistency in the life spans of man and experimental animals
should be taken into account in the design and interpretation of
animal experiments for the evaluation of toxicity to man.
There are other problems in the evaluation of toxicity to man
from experiments on animals, such as where an effect is difficult to
measure or where similar conditions are difficult to obtain in animal
models, for example, intelligence and the more esoteric behavioural
changes. Furthermore, in animal experiments, the effects of social
factors, so important to man, cannot be evaluated.
For these reasons, when extrapolating from animals to man it is
prudent to apply a species conversion factor which should be
determined on the basis of biological considerations and the available
information on the test species (Hoel et al., 1975). There is no
definite rule for the species conversion factor. If the extrapolation
of data is based on the most sensitive species tested, some
toxicologists use a factor of 1 (Sabad et al., 1973), but others
recommend a factor as large as 10 (Weil, 1972a).
The unit of dose to be used has also to be considered in the
extrapolation of data to man and it has recently been recommended that
the dose per unit surface area approximately equivalent to the weight
raised to the power 2/3 should be used. If the dose is given in terms
of dietary concentration, there seems to be no need to make the
surface area adjustment (Hoel et al., 1975; Mantel & Schneiderman,
1975).
A separate problem, to which there appears to be no satisfactory
answer at present, is the conversion from an inbred animal strain to a
genetically highly heterogeneous human population (Hoel et al., 1975).
1.3.3.2 Safety factors
In almost all instances, laboratory data on the toxicity of
chemicals are drawn from experiments in which the adverse effect
occurs at a considerably higher incidence rate than would be
acceptable in man. For this reason alone, and apart from the
biological differences between laboratory species and man, an
extrapolation from a known dose-response range to an unknown range is
necessary. Indeed, essentially the same problem arises when a human
accident or epidemiological data are used as the starting point.
Traditionally, a safety factor has been introduced to provide for
uncertainties in extrapolation from animals to man, and from a small
group of individuals to a large population. Such safety factors have
ranged from 1 to as much as 5000. Because of the current uncertainty
regarding the mathematical and biological reliability of methods for
extrapolating from high doses to low doses, primary dependence on
somewhat arbitrary safety factors continues. However, means of
extrapolating from high to low doses are being intensively studied at
the present time, especially with respect to carcinogenicity.
Most regulatory authorities rely on the use of safety factors but
there are no precise guidelines for deciding the appropriate size of
such a factor. Sanockij (1962) and Sanockij & Sidorov (1975) have
discussed the rationale for different safety factors. In general, the
size of the safety factor will depend on (a) the nature of the toxic
effects, (b) the size and type of population to be protected, and (c)
the quality of toxicological information available. A factor of 2 to 5
or less may be considered as sufficient if the effect against which
individuals or a population are to be protected is not regarded as
very severe, if only a small number of workers are likely to be
exposed, and if the toxicological information is derived from human
data. On the other hand, a safety factor as large as 1000 or more may
be required if the possible effect is very serious, if the general
population is to be protected, and if the toxicological data are
derived from limited experiments on laboratory animals. In some cases,
the safety factor may be a value that has been used with reasonable
success and is, therefore, perpetuated.
For most food additives that are not considered to be
carcinogenic, it has been the accepted practice to divide the
no-adverse-effect dose (i.e., the maximum ineffective dose) in animals
by 100, to arrive at an acceptable daily intake (ADI) for man
(Vettorazzi, 1977; WHO, 1958). For pesticides and certain
environmental chemicals, safety factors ranging from less than 100 to
several thousand have been used (Vettorazzi, 1975). For some
occupational exposures, and for certain air pollutants (WHO, 1977)
much smaller safety factor have been proposed in the range of 2-5.
Safety factors have also been proposed for carcinogenic chemicals
ranging from 100 (Druckrey, 1967; Janyseva, 1972) to about 5000 (Weil,
1972a) but they have not been generally accepted.
1.3.3.3 Low-dose extrapolation
Low-dose extrapolation is based on mathematical models that are
used to predict the response at a given low dose or to predict that
dose which gives a predetermined low response. Such models may relate
the incidence of a quantal effect to dose, or they may consider the
distribution of the "time to occurrence" of a condition and its
relation to dose. In both cases, the results of extrapolation are
strongly dependent on the choice of the model. For example, the
Advisory Committee on Food Additives (FDA, 1970) noted that
dose-response data may fit several models equally well in the 2% to 5%
range, but the doses extrapolated to very low responses would differ
very strikingly: the ratio of ED1 to ED0.000001 would be either
100, 100 000, or 1 000 000 for the probit, logistic, or one-hit
curves, respectively.
Several extrapolation procedures have been proposed which will
give an upper limit to the dose corresponding to a low response. In
other words, the result of extrapolation will not be the best estimate
of the unknown dose required to give the desired response but a dose
that is most likely to be below the dose required to give this
response. Two procedures based on this approach have received
particular attention: one is based on the one-hit model, the other on
the probit model.
The one-hit model assumes that an effect can be induced after a
single susceptible target has been reached by a single biologically
effective unit of dose (see for example Cornfield, 1954). At low
doses, this model is numerically equivalent to the linear
dose-response model which is compatible with animal data for some
carcinogens (Druckrey, 1967) and with some human data such as the
incidence of lung cancer in relation to the number of cigarettes
smoked per day (Doll, 1967). In their simplest form (i.e. when the
true response at zero dose is assumed to be zero), the currently used
extrapolation procedures based on this model (Gross et al., 1970; Hoel
et al., 1975; Schneiderman, 1971) operate as follows: (1) the upper
99% confidence limit (UCL) is estimated for the observed response at a
dose d; (2) a desired limit is set for a low response ( R) e.g. 1
in 1 000 000; and (3) the dose ( de) that would produce a response
which is, with a 99% probability, lower than R is calculated from
the equation de = d*R/(UCL). Such procedures are more
conservative than the procedures based on any other currently used
dose-response model (probit, logit, or extreme-value models). In
addition, the one-hit model seems to have a reasonable biological
basis for carcinogenesis at low doses (Hoel et al., 1975).
Mantel & Bryan (1961) proposed the use of probits (see Annex to
this Chapter) and a log-normal distribution to describe the
variability of the sensitivities (tolerances) of individuals in a
population. The probit model gives a dose-response curve that is
concave at low-dose levels, and is less conservative than the linear
model based on the one-hit hypothesis. The Mantel-Bryan procedure (see
for example Schneiderman & Mantel, 1973) involves (1) the choice of a
desired limit of response (R) (e.g. 1 in 1 000 000); (2) the
estimation of the upper 99% confidence limit (UCL) for the observed
response at dose d, and (3) imposing a probit-log dose straight line
through UCL, with a slope (ß) equal to 1 (i.e. one probit per 10-fold
dose-range). The choice of the slope (ß) is critical in this
procedure. ß = 1 has been proposed because a slope greater than 1 is
usually (but not always) observed in carcinogenesis experiments. The
Mantel & Bryan procedure has been modified to take into account
response levels in control groups (Mantel et al., 1975).
A second category of models is based on the observation that the
median "time to occurrence" (latent period) of an effect such as
cancer may increase as the dose decreases but not proportionally. A
thousand-fold change in the dose usually causes an approximately
ten-fold change in the median time to tumour appearance and, with
decreasing dose levels, a dose may be reached which would predict
tumour occurrence beyond the life expectancy of the exposed
individuals. This would still be consistent with the hypothesis that
molecular changes in the cells, occurring in proportion to the
concentration of carcinogens, are the initiating event (for a recent
review see Jones & Grendon, 1975). One model (Altschuler, 1973; Blum,
1959; Druckrey, 1967) considers a log-normal distribution of the
time-to-occurrence with median time depending on dose but with
standard deviation independent of the dose. The dose d is related to
the median time (t) by d= c/tn, where n is assumed to be greater than
one, and c is a constant. Peto et al. (1972) compared this model
with another model in which the time to occurrence is considered to
have a Weibull distribution (Day, 1967; Peto & Lee, 1973). They found
that the Weibull distribution agreed with experimental data better
than the log-normal, but this no doubt depends on the type of cancer
involved. The low-dose extrapolation using these models would also be
strongly dependent on the choice of the model (Chand & Hoel, 1974).
Dose-"time-to-occurrence"-response relationships in cancer risk
assessment have also been considered by Janyseva & Antomonov (1976).
The application of all these procedures presents practical
difficulties (Hoel et al., 1975). Low-dose extrapolation is thus a
very difficult problem that cannot be solved by statistical methods
alone. Great caution should be exercised in using the existing methods
and their inherent limitations should always be kept in mind. Good
experimental data, combined with human data if available, and an
understanding of the mechanisms of toxic action are essential if the
task of low-dose extrapolation is to be accomplished satisfactorily.
1.3.3.4 Other methods of extrapolation
A method for extrapolation from one species to another based on
an established relationship between the indices of toxicity and body
weight for different animal species has also been suggested
(Krasovskij, 1976a). In mammals, the weights of internal organs, and
many physiological variables (pulse and respiration rates, consumption
of oxygen, food, and water, liver microsomal enzyme activity) show a
log-log linear relationship with body size of the animals (allometric
ratios). This regularity appears to be valid for more than 100
different variables including the period of gestation, litter size,
erythrocyte life span, and latent period of tumour development but
there are also other variables to which it does not apply. This "body
weight rule" (Krasovskij, 1975) may be expressed as follows: the
logarithms of biological variables of mammals show a linear regression
to the logarithms of the body weight.
Krasovskij (1976b) showed that values for the lethal dose for
dogs of several chemicals obtained from regression analysis of
toxicity in four other species of small mammals compared well with
predictions made from direct extrapolation from albino rats or from
the most sensitive animal species, or from the relationship of body
surface area, and also with predictions obtained by the method of
Ulanova (1969) and Van Noordwijk (1964). For the calculation of
extrapolation coefficients from regression equations, see Krasovskij
(1976b).
1.4 Human Data
1.4.1 Ethical considerations
In research involving human subjects, a number of elements, such
as the assessment of risk, potential benefit, and quality of consent,
have to be evaluated to ascertain whether ethical considerations are
satisfied. The essential provisions for protecting human subjects in
experimentation and research have been expounded by many international
and national organizations. Key factors include the right to informed
consent and freedom from coercion. The international instruments in
dealing with this matter are the Declaration of Helsinki (as revised
in Tokyo in 1975) and Article 7 of the International Covenant on Civil
and Political Rights, adopted by the United Nations General Assembly,
December 1966. Article 7 provides that "no-one shall be subjected
without his free consent to medical or scientific experimentation"
(Cranston, 1973; WHO, 1976b). Some countries possess specific codes of
ethics relating to human experimentation, and special problems of
experimentation that involve the use of fetuses, children, the
mentally ill, and prisoners require special consideration.
It is essential that human experimentation should only be
undertaken when there is adequate evidence from animal and other
studies that both the chemical and the circumstances of administration
are safe. Every experiment with human volunteers should be subject to
prior review and approval by a local ethical committee in order to
ensure that the intended study complies with the ethical principles
embodied in the Declaration of Helsinki and with other requirements of
national and local bodies.
Ideal conditions of truly informed consent may not always be
achieved in practice, consequently the burden of responsibility rests
mainly with the investigator and, to a lesser extent, with the peer
review body. Because of these difficulties, the guidelines and
procedures for the protection of human subjects should be constantly
reviewed and updated (WHO, 1976b).
In any case, collection of data from human subjects must be
accomplished with due respect for human rights and dignity. The use of
ethics committees with broad representation to review and approve all
such experimentation is recommended to protect the rights of human
subjects and to ensure responsible investigation.
1.4.2 Need for human investigations
Although there is general repugnance at the idea of using human
subjects to assess the safety of environmental chemicals, the question
is not whether or not human subjects should be used in toxicity
experiments but rather whether such chemicals, deemed from animal
toxicity studies to be relatively safe, should be released first to
controlled, carefully monitored groups of human subjects, instead of
being released indiscriminately to large populations with no
monitoring and with little or no opportunity to observe adverse
effects (Paget, 1970).
The prediction and prevention of possible toxic hazards that may
arise from the introduction of chemicals into the environment can be
made more valid if data from studies of the chemical in human subjects
are available. Three particular aspects of human toxicology have need
of such information, namely: (a) the selection, through comparative
consideration of metabolism, of the most appropriate animal species
for studies to predict the human response; (b) investigation of a
specific, reversible effect of the compound in the most sensitive
animal species, to determine whether there is a correlation with a
similar effect in man; and (c) study of effects specific to man.
Certain types of information about the effects of chemicals can
only be obtained by direct observations on man. Often, carefully
controlled experiments can provide significant information at doses
well below those anticipated to be "safe"; measurement of subtle
changes of reaction time, behavioural functions, and sensory responses
may be examples. In other cases, useful information may be obtained by
careful studies on human cells or tissue maintained by culture
techniques.
Human toxicological data include both the data obtained from
epidemiological surveys of populations exposed to a toxic chemical
under normal conditions of use, in cases of acute accidental poisoning
and in occupational exposure, and the data from experiments in
volunteers. Although an experiment is defined as observations under
controlled conditions of exposure, there is, at times, only a grey
area that distinguishes an experiment with human subjects from
observations on human subjects under natural conditions. For example,
some segments of human populations are at higher risk and should be
particularly closely monitored, e.g., those exposed to chemicals at
work or those receiving continuous treatment with medicines. The
periodic clinical evaluation of workers is normally the responsibility
of the employer and careful records of these examinations coupled with
measurement of exposure conditions often exist. If accidental
excessive exposure of an individual or a population should occur, it
is both ethical and pertinent to learn as much as possible,
recognizing always the right of the patient. Because of the wide
individual variation in the toxicity of chemicals to man, the final
evaluation should be based on information obtained from as widely
varied a human population as is compatible with the various ethical
principles involved.
1.5 The Use of Toxicological Data in Establishing Environmental
Health Standards
1.5.1 Environmental health standards
The aim of environmental health standards is to protect
individuals, human populations, and their progeny from the adverse
effects of hazardous environmental factors, including chemicals. A
sound principle of health protection is to keep all exposures as low
as reasonably achievable, subject to the condition that the
appropriate exposure limits, defined by the standard, are not
exceeded.
Environmental health standards for chemicals may be formulated
either in terms of concentrations in environmental components (e.g.,
air, water, food, consumer products) or in terms of amounts of
substances that may be taken into the body. These concentrations and
amounts should be sufficiently low that the threshold dose (if it
exists and can be determined) will not be reached, or that the
population of concern will not be subject to "unacceptable" risk, even
following life-time or working life-time exposure. In some cases, as
for irritant air pollutants, the distribution of exposure
concentrations in time should also be considered. Standards may also
prescribe the quantity of a substance to be used at any time and the
manner of its use.
Social, cultural, and economic considerations should be taken
into account in setting standards, but never to the detriment of
health protection which should be of primary concern.
It is obvious that a standard setting process will necessarily
involve many considerations besides toxicology. This process is often
very different in different countries and different types of society.
In general, however, it involves appraisal of toxicological data,
particularly of dose-response relationships, including the effects on
non-human targets (plants, animals, materials); social and economic
analysis, policy analysis and review of experience elsewhere, leading
eventually to an administrative or policy decision concerning the
standard. Other relevant questions include the technological
feasibility of achieving a standard, the cost and benefit of
implementing it, means of enforcement, other public health priorities
etc. Many of these topics are outside the scope of the present
monograph.
1.5.2 Assessment of health risk and evaluation of benefits
Assessment of health risk from a given exposure to an
environmental factor is an essential step in any procedure for setting
environmental health standards. Assessment of health risk involves
more than routine application of "safety factors", or low dose
extrapolation which provides estimates of response that are, strictly
speaking, applicable only to the conditions of the experiment. The
application of a "species conversion" factor has been discussed and
the difficulties pointed out (section 1.3.3.2). Questions such as the
incidence of effects in various age groups and the degree of life
shortening in affected individuals are all relevant to standard
setting. For this reason the study of "time-to-occurrence" models in
the extrapolation of data should be encouraged (Albert & Altschuler,
1976; Hoel et al., 1975). In addition, the seriousness of adverse
effects will have to be evaluated from the public health and social
viewpoint. Attention should also be paid to the heterogeneity of human
populations, and, at present, it is not clear how the existence of
susceptible groups, and the influence of nutrition and pre-existing
disease in human populations should be taken into account. The
existing methods of extrapolation from animal data to man deal with
exposures to single substances whereas the actual human environment
contains a large number of hazardous chemicals and other factors that
can interact and considerably modify the effects, for example, in
cancer induction (Bingham & Falk, 1959; Montesano et al., 1974). From
this viewpoint, the importance of epidemiology and systematic
surveillance of high risk groups cannot be overemphasized.
The acceptability of a given risk should also be considered in
standard setting. This, as well as the judgment on safety (which
involves decision on the acceptability of risk) exceeds the expertise
of toxicologists. This is a domain where society at large has a role
to play. Political decisions are also required on various social,
economic, and ecological concerns. The same applies to the evaluation
of benefits. As pointed out by a WHO Expert Committee (WHO, 1974a)
"the expertise needed for the evaluation of risk is different from
that needed for benefit evaluation. On the risk side, concern is
focused on adverse health effects on man, damage to the environment,
and misuse of natural resources. On the benefit side, the emphasis is
on value to the consumer and the country". The interaction of all
these factors is often described by the term "risk and benefit
analysis" (see for example Falk, 1975), which is only partly within
the area of toxicological expertise. The final judgment as to whether
the benefit does or does not justify a risk is for society to make.
1.5.3 An example of toxicological information used in standard
setting
Although standard setting procedures will differ from country to
country, and the requirements for toxicological information will vary
to a considerable extent, it may be useful, nevertheless, to describe,
as an example, the procedure used in the USSR for setting standards
for chemical pollutants in surface waters (Sysin, 1941; WHO, 1975a).
Information is first obtained on the likely concentrations of the
chemical in industrial waste waters and the physical and chemical
properties of the chemical. The stability of the substance under
environmental conditions is then evaluated by standard analytical
methods and the influence of the chemical on the self-purification
processes of natural waters is studied.
The toxicological investigations required include LD50 studies
for mice, rats, guineapigs and rabbits (Krasovskij, 1965) and subacute
experiments lasting 1-2 months, to provide data on functional
disturbances of organs and systems and on any cumulative properties of
the chemical (Krasovskij, 1970). These tests are followed by chronic
toxicity experiments lasting 6-8 months. Study of specific effects of
chemical water pollutants (e.g., mutagenicity, teratogenicity, and
effects on reproductive function) is also a necessary component of
toxicological investigations.
The subthreshold (maximum ineffective) concentration determined
by chronic experiments is then compared with the threshold
concentrations established for the other two indices of water quality
(i.e. effects on the self-purification of water and its organoleptic
properties), and the smallest concentration is assumed to be the
"hygienic" standard.
The total number of hygienic standards for hazardous substances
in water, developed in the USSR, has reached 500; of these, about 60%
have been established according to organoleptic criteria and 30%
according to the toxicological hazard index. These standards have been
incorporated in the water legislation of the country and serve as the
basis for practical control measures in protecting water bodies from
chemical pollution.
1.6 Limitations of Safety Evaluation
Experimental toxicology is a highly complex, multidisciplinary
science. The extrapolation of animal data to man requires
well-informed contributions from several scientific disciplines.
Absolute proof of safety for man of a chemical substance cannot be
obtained from the results of toxicological tests (Coon, 1973).
However, toxicological tests do provide guidance on the relative
toxicity of a compound and help in identifying likely modes of action
in man.
Acute toxicity studies in animals are of value in predicting
potential toxic effects of a chemical in human beings exposed to near
fatal doses. From the results of such studies, the nature of acute
responses in man may be anticipated with a view to initiating
life-supporting measures or first-aid or therapeutic procedures.
Short-term and subacute studies are particularly valuable in
determining the more subtle toxic effects of a chemical, whether or
not it has potential for cumulative toxicity and whether or not the
toxic effects are reversible upon cessation of exposure. These tests
are of value in estimating the potential hazard to man following
exposure of intermediate duration, usually 2-7 years.
Of greatest concern to toxicologists, regulatory officials, and
the general public are the possible chronic toxic effects of
chemicals. Chronic toxicity tests assist in establishing the degree of
risk to man that may be expected from low-level long-term exposure to
a chemical substance. Chemicals that tend to persist or concentrate in
the biosphere and as a result have the potential to affect large
segments of the population are of particular concern.
In extrapolating animal data to man, several factors must be
taken into consideration. These include the "no-effect level" derived
from animal experiments, the nature of the dose-response curve and the
nature of the toxic effects produced (Friedman, 1969). The known or
anticipated level of exposure in man and the potential number of
exposed individuals must also be considered (NAS, 1975). It is worth
pointing out that the so-called "no-effect level" is a statistically
derived value usually estimated within a 95% confidence interval and
that a 5% probability exists that the value is in error. It has been
noted, for example, that if a toxic effect occurs in only 1% of the
test animals, the effect will be entirely missed 37% of the time if
only 100 animals are used in each test (Friedman, 1969). In addition,
if the same effect occurs spontaneously in control animals, the
chances of detecting that response in treated animals becomes even
more remote.
Predictions of toxicity from laboratory animal studies are
dependent on the relevance of these studies to man, to wild-life, and
to environmental ecosystems. They are also dependent on the genetics,
nutrition, general health, and environmental circumstances of the
individuals exposed.
There may be a hereditary disposition in man to an increased
susceptibility to toxic chemicals, such as an increased tendency to
malignant tumours (Kellerman et al., 1973). Similarly, persons under
stress or treatment with immunosuppressive drugs may also be at
greater risk to chemical toxicity and chemical carcinogenesis. These
individuals will constitute abnormal populations for which the degree
of risk may not be predictable from animal studies or from human
studies carried out on healthy subjects, but these abnormal
populations may be of sufficient magnitude to merit special
consideration. Furthermore, genetic variations in laboratory animals
are paralleled by variations in the toxic response to chemicals, and
this puts additional limitations on predictions of possible human
toxicity from such animal data.
Similarly, the nutritional status of individuals may also result
in wide variation in susceptibility to toxic chemicals because
malnutrition may lead to reduction of natural protection afforded by
detoxication mechanisms.
Safety evaluation of chemicals is too frequently empirical and
there is often a tendency to mistake quantity of data for quality.
Regulatory agencies and toxicologists must be flexible and keep
abreast of new experimental techniques and methods and of fundamental
developments in the understanding of the mechanisms of toxicity. The
application of new methods could be more relevant and informative than
the routine use of old traditional ones, but these new methods should
not necessarily be expected to replace traditional procedures, nor
should they be applied routinely until adequately evaluated for
significance and reliability.
Whether new or old procedures are employed, it is very important
that the specific conditions under which the experiments are conducted
should be accessible to other scientists, so that the results from
different laboratories may be compared. Where it is not possible to
set forth such details in publications of toxicological
investigations, a central listing of detailed experimental procedures
and conditions would be desirable.
Annex
MATHEMATICAL EXPRESSIONS OF DOSE-EFFECT AND DOSE-RESPONSE
RELATIONSHIPS
Dose-effect and dose-response relationships may be plotted and an
empirical "best fit" of a curvilinear correlation may be expressed as
a mathematical equation. Alternatively, a visual inspection of the
graph may suggest a mathematical equation, such as linear,
exponential, or power function, and then the best-fit of the data
points to the equation may be calculated. A single set of data could
fit several mathematical equations equally well when the range of data
is limited. Therefore, care must be taken not to assume that
biological events follow a specific mathematical model unless the data
have been collected over a wide range of values.
Whenever possible, it is useful to develop a hypothesis for a
mechanism of toxic action on biological grounds, to derive the general
mathematical expression for the mechanism, and then to fit the data to
the equation to obtain the values for the constants in the equation
that will be specific for the conditions of the experiment. For
example, one mechanism of action may indicate that the law of mass
action (or chemical equilibrium) applies to the dose-effect
relationship. If one assumes 1) that one molecule of the chemical
binds reversibly with one receptor site; 2) that effect (E) is
directly proportional to the fraction of the total receptors bound by
the chemical; and 3) that the amount of bound chemical is very small
compared to the total concentration or dose (D), then the
application of the law of mass action leads to a relationship:
E = K1 D/( K2 + K1 D) where K1 and K2 are constants specific to the
experiment. Clark (1933) noted that this mathematical equation, which
gives an equilibrium curve asymptotically approaching the maximum
effect, has a very similar shape, over certain dose ranges, to a
logarithmic curve, such as E = K1 log( K2 D + 1), or a power function
curve, such as E = K1 DK2.
The sigmoid or S-shaped curve is a commonly observed curvilinear
expression for some dose-effect and most dose-response relationships.
The biological basis for this relationship may be partially understood
by the nature of the frequency distribution of individual
susceptibilities or resistances in a population. Most of the
individuals in a population will respond close to a central dose
level, and a few will respond only at very low or at very high dose
levels. This leads to a frequency distribution for the individual
responders as a function of dose. A frequency distribution, however,
does not describe a biological mechanism for susceptibility or
resistance, but the random occurrence of individuals with different
susceptibilities.
Fig. 1.1 shows a normal frequency distribution; the curve is
symmetrical around a central point. Its cumulative frequency
distribution provides the often observed sigmoid curve. A
dose-response relationship will be observed as a cumulative frequency
distribution because an individual who responds at a low dose will, of
course, also respond at higher doses. Thus the frequency of responders
at a given high dose includes all those that respond at that and all
lower doses.
Fig. 1.2 presents a distribution which is skewed towards the
high-dose levels. This distribution is often described as log-normal
because a logarithmic transformation of dose values results in a
normal frequency distribution (Fig. 1.3). In nature, many frequency
distributions are log normal in shape. This shape is also observed in
distributions where the central point is near zero; since the dose
level cannot be less than zero, there is only a narrow range in which
the more susceptible responders will cluster. The logarithmic scale
expands the zero point towards negative infinity, thus producing a
more symmetrical distribution around the central point. In addition to
normal and log-normal distributions, there are various other types of
skewed distributions.
The mathematical equation for the S-shaped curve is difficult to
handle and it is therefore often transformed into a straight line for
the presentation and evaluation of data. It is a mathematical
characteristic of the normal distribution that the points of
inflection of the curve on either side of the peak (or mean value) are
at values equal to plus and minus one standard deviation (S.D.) from
the mean (m). The integration of the normal distribution function
shows that the area under the curve from m - 1 S.D. to m + 1 S.D.
includes 68.3% of all members of the population. Thus 15.9% of the
population will be responders at doses equal to or less than the mean
minus 1 S.D., and 84.1% will be responders at doses equal to or less
than the mean plus 1 S.D. It may also be calculated that approximately
95.4% of the population will respond within a dose range given by the
mean ± 2 S.D., and approximately 99.9% will respond between the mean ±
3 S.D.
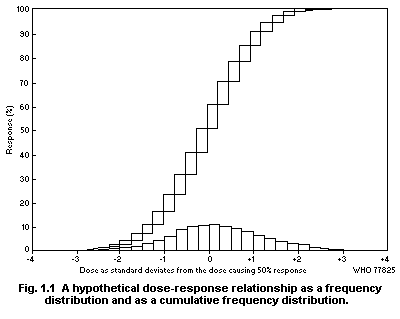
 Since m - 3 S.D., m - 2 S.D., m - 1 S.D., m, m + 1 S.D.,
m + 2 S.D., and m + 3 S.D. indicate equal dose intervals, the
corresponding percentage of responders, i.e. 0.1, 2.3, 15.9, 50, 84.1,
97.7, and 99.9 respectively, will give a straight line when these
percentages are plotted at equidistant intervals. Fig. 1.4 illustrates
this transformation; both the percent scale and the commonly used
"probit" scale are presented. Finney (1971) has presented the history
of the development and the utility of probit transformationa. Many
toxicologists use log-probability graph paper to express dose-response
relationships as a linear function for log-normal distributions of an
effect.
Fig. 1.5 shows two dose-response curves on the log-probability
graph paper. The ED50 (50% effective dose) value for chemical A is
10 dose units, while that for chemical B is 0.01 dose units. ED50
data are sometimes presented in the literature as a single dose value,
without providing confidence limits or the slope of the dose response
curve. It is clear in Fig. 1.5 that for chemicals A and B, not only
are the ED50 values three orders of magnitude apart but the test
systems respond in a very different manner.
As an example of the necessity for taking into account the
slopes, the practice of some toxicologists to study the effects of
repeated doses at 1/10 of the single dose ED50 can be considered.
For chemical A an effect would already be seen in 16% of the
population (ED16) after the first of the repeated doses, while for
chemical B it is quite probable that an effect will never be seen even
after many repeated doses (Fig. 1.5). This is predictable, if the
slopes of the dose-response curves are known.
Flat slopes, as for chemical A, are often indicative of such
factors as poor absorption, rapid excretion or detoxication, or of
toxic effects that become manifest some time after administration.
Steep slopes, as for chemical B, most frequently indicate rapid
absorption and rapid onset of toxic effects, as for example with
hydrogen cyanide or irritant gases. While slope is not an absolutely
reliable indicator of physiological or toxicological mechanisms, it is
useful to the experienced toxicologist, and should always be reported
along with its confidence limits.
a "Probit" = probability unit. Probit is the "standard deviate" or
the "normal equivalent deviate" (NED) increased by 5. The NED is
defined as the abscissa corresponding to a probability P in a
normal distribution with mean 0 and standard deviation 1.
Since m - 3 S.D., m - 2 S.D., m - 1 S.D., m, m + 1 S.D.,
m + 2 S.D., and m + 3 S.D. indicate equal dose intervals, the
corresponding percentage of responders, i.e. 0.1, 2.3, 15.9, 50, 84.1,
97.7, and 99.9 respectively, will give a straight line when these
percentages are plotted at equidistant intervals. Fig. 1.4 illustrates
this transformation; both the percent scale and the commonly used
"probit" scale are presented. Finney (1971) has presented the history
of the development and the utility of probit transformationa. Many
toxicologists use log-probability graph paper to express dose-response
relationships as a linear function for log-normal distributions of an
effect.
Fig. 1.5 shows two dose-response curves on the log-probability
graph paper. The ED50 (50% effective dose) value for chemical A is
10 dose units, while that for chemical B is 0.01 dose units. ED50
data are sometimes presented in the literature as a single dose value,
without providing confidence limits or the slope of the dose response
curve. It is clear in Fig. 1.5 that for chemicals A and B, not only
are the ED50 values three orders of magnitude apart but the test
systems respond in a very different manner.
As an example of the necessity for taking into account the
slopes, the practice of some toxicologists to study the effects of
repeated doses at 1/10 of the single dose ED50 can be considered.
For chemical A an effect would already be seen in 16% of the
population (ED16) after the first of the repeated doses, while for
chemical B it is quite probable that an effect will never be seen even
after many repeated doses (Fig. 1.5). This is predictable, if the
slopes of the dose-response curves are known.
Flat slopes, as for chemical A, are often indicative of such
factors as poor absorption, rapid excretion or detoxication, or of
toxic effects that become manifest some time after administration.
Steep slopes, as for chemical B, most frequently indicate rapid
absorption and rapid onset of toxic effects, as for example with
hydrogen cyanide or irritant gases. While slope is not an absolutely
reliable indicator of physiological or toxicological mechanisms, it is
useful to the experienced toxicologist, and should always be reported
along with its confidence limits.
a "Probit" = probability unit. Probit is the "standard deviate" or
the "normal equivalent deviate" (NED) increased by 5. The NED is
defined as the abscissa corresponding to a probability P in a
normal distribution with mean 0 and standard deviation 1.
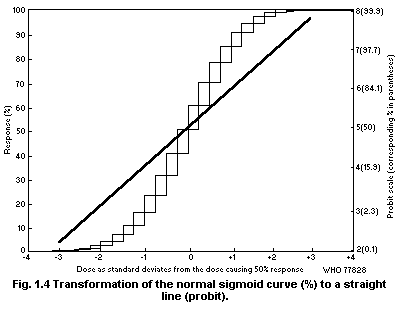
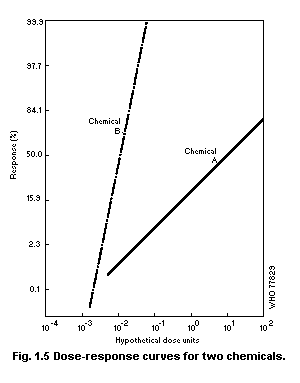 Fig. 1.6 illustrates the importance of parallelism of
dose-response curves for making any general statements about relative
effects. Chemicals C and D have identical ED50 values. However, any
statement about the relative equality of effect would only be true at
that particular dose. In fact, at higher doses, chemical C would be
more effective than D, and at lower doses, chemical D would be more
effective. Chemicals E and F, on the other hand, show relative
equi-effects in the ratio of 1 to 10 dose units over the entire dose
range. This parallelism of dose-response curves is essential for the
validity of general statements about relative toxicities. Special note
should be made, however, that the curves for chemicals E and F apply
only to one specific effect and one set of experimental conditions.
Observations of response for a different toxic effect, or
administration by a different route or to a different species may not
produce parallel dose-response curves for the same chemicals.
Fig. 1.6 illustrates the importance of parallelism of
dose-response curves for making any general statements about relative
effects. Chemicals C and D have identical ED50 values. However, any
statement about the relative equality of effect would only be true at
that particular dose. In fact, at higher doses, chemical C would be
more effective than D, and at lower doses, chemical D would be more
effective. Chemicals E and F, on the other hand, show relative
equi-effects in the ratio of 1 to 10 dose units over the entire dose
range. This parallelism of dose-response curves is essential for the
validity of general statements about relative toxicities. Special note
should be made, however, that the curves for chemicals E and F apply
only to one specific effect and one set of experimental conditions.
Observations of response for a different toxic effect, or
administration by a different route or to a different species may not
produce parallel dose-response curves for the same chemicals.
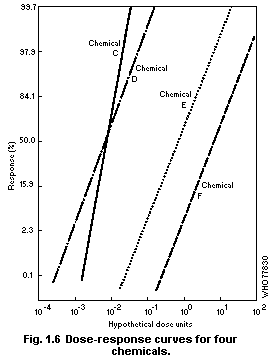 REFERENCES
ALBERT, R. E. & ALTSCHULER, B. (1973) Considerations relating to the
formulation of limits for unavoidable population exposures to
environmental carcinogens. In: Ballon, J. E., ed. Radionuclide
carcinogenesis, Springfield, Va, NIIS, pp. 233-253
(AEC Symposium Series CONF-72050).
ALBERT, R. E. & ALTSCHULER, B. (1976) Assessment of risks in terms of
life shortening, Environ. Health Perspect., 13: 91-94.
ANDREYESHCHEVA, N. G. (1976) Predicting biological effect as a
function of the chemical structure and the primary physical and
chemical properties of organic compounds. Environ. Health
Perspect., 13: 27-30.
ARIENS, E. J., VAN ROSSUM, J. M., & SIMONIS, A. M. (1957) Affinity,
intrinsic activity and drug interactions. Pharmacol. Rev.,
9: 218-236.
BALL, W. L. (1959) The investigation of present-time definitions and
conceptions of maximum allowable concentrations in different
countries. Pr. Lék., 11 (3): 127-128.
BEIR (1972) The effects of population exposure to low levels of
ionizing radiations -- Report of the Advisory Committee on the
Biological Effects of Ionizing Radiation. Washington, DC,
National Academy of Sciences -- National Research Council,
217 pp. (Govt. Printing Office Publication 0-489-797).
BERNARD, C. (1898) [Introduction to the study of experimental
medicine.] Paris, Librairie Delagrave (in French).
BINGHAM, E. & FALK, H. L. (1959) Environmental carcinogens --
modifying effect of cocarcinogens on the threshold response.
Arch. environ. Health, 19: 779-783.
BLUM, H. F. (1959) Carcinogenesis by ultraviolet light. Princeton,
NJ, Princeton University Press.
BROWN, B. M. & PAPWORTH, D. S. (1974) Environmental chemical hazards
to wildlife. In: Boyland, E. & Goulding, R., ed. Modern trends
in toxicology, London, Butterworths, Vol. 2, ch. 3, pp. 70-85.
CASARETT, L. J. (1975) Toxicologic evaluation. In: Casarett, L. J. &
Doull, J., ed. Toxicology, the basic science of poisons. New
York, Macmillan Publishing Co. Inc.
CHAND, N. & HOEL, D. G. (1974) A comparison of models for determining
safe levels of environmental agents. In: Proschan & Sefling, ed.
Reliability and biometry, Philadelphia, SIAM, pp. 382-401.
CLARK, A. J. (1933) Mode of action of drugs on cells. Baltimore,
Williams & Wilkins.
COHEN, J. M. & PINKERTON, C. (1966) Widespread translation of
pesticides by air transport and rain-out. In: Gould, R. F., ed.
Organic pesticides in the environment, American Chemical
Society, pp. 163-176 (Advances in Chemistry Series, vol. 60).
COON, J. (1973) Toxicology of naturally-occurring chemicals: a
perspective. In: Toxicants occurring naturally in foods
(2nd ed.). Washington, DC, National Academy of Sciences.
CORNFIELD, J. (1954) Measurement and comparison of toxicities: the
quantal response. In: Kempthorne, O., Bancroft, T. A., Gowen, J.
W., & Lush, J. L., ed. Statistics and mathematics in biology,
Ames, The Iowa State College Press, pp. 327-344.
CRANSTON, M. (1973) What are human rights? New York, Taplinger Pub.
Co. Inc., p. 111.
CURRY, S. H. (1970) Theoretical changes in drug distribution resulting
from changes in binding to plasma proteins and to tissues.
Pharm. Pharmacol., 22: 753-758.
CUTHBERT, J. W. (1974) Industrial toxicology. In: Boyland, E. &
Goulding, R., ed. Modern trends in toxicology. London,
Butterworths, pp. 86-115.
DAY, T. D. (1967) Carcinogenic action of cigarette smoke condensate on
mouse skin. Br. J. Cancer, 21: 56-81.
DINMAN, B. D. (1972) "Non-concept" of "no-threshold" chemicals in the
environment. Environ. Sci., 175 (4021): 495-497.
DOLL, R. (1967) Prevention of cancer: pointers from epidemiology.
London, Nuffield Provincial Hospital Trust.
DRUCKREY, H. (1967) Qualitative aspects of chemical carcinogenesis.
In: Truhaut, R., ed. Potential carcinogenic hazards from drugs,
evaluation of risks, New York, Springer-Verlag, pp. 60-78.
(UICC Monograph Series, Vol. 7.)
ECOBICHON, D. J. & CORMEAU, A.M. (1973) Pseudocholinesterases of
mammalian plasma: physiochemical properties and organophosphate
inhibition in 11 species. Toxicol. appl. Pharmacol.,
24: 92-100.
EDWARDS, C. A. (1970) Persistent pesticides in the environment.
Cleveland, Ohio, CRC Press.
ELIZAROVA, O. N. (1962) In: Opredelenie porogovyh doz promyslennyh
jadov pri peroral'nom vvedeni, Moscow, Medicina, pp. 107-108
(in Russian).
EPSTEIN, S.S. (1973) The Delaney amendment. J. prev. Med.,
2: 140-149.
FALK, H. L. (1975) Consideration of risks versus benefits. Environ.
Health Perspect., 11: 1-5.
FOOD & DRUG ADMINISTRATION (1970) Food and Drug Administration
Advisory Committee on Protocols for Safety Evaluation: Panel on
Carcinogenesis Report on Cancer Testing in the Safety Evaluation
of Food Additives and Pesticides. Toxicol. appl. Pharmacol.,
20: 419-438.
FINNEY, D. G. (1952a) Statistical method in biological assay,
London, Charles Griffin Ltd, 661 pp.
FINNEY, D. G. (1952b) Probit analysis (2nd ed.). London, Cambridge
University Press.
FINNEY, D. G. (1971) Probit analysis (3rd ed.). London, Cambridge
University Press.
FLYNN, D. I., LYNCH, M., & ZANNONI, V. G. (1972) Species differences
and drug metabolism. Biochem. Pharmacol., 21: 2577-2590.
FRIEDMAN, L. (1969) The role of the laboratory animal study of
intermediate duration for the evaluation of safety. In: Symposium
on the evaluation of the safety of food additives and chemical
residues. Toxicol. appl. Pharmacol., 16: 498-506.
FUCHS, N. A. (1964) The mechanics of aerosols, Oxford, Pergamon
Press, pp. 270-287.
GADDUM, J. H. (1957) Theories of drug antagonism. Pharmacol. Rev.,
9: 211-217.
GAVRILESCU, N. & PETERS, R. A. (1931) Biochemical lesions in vitamin B
deficiency. Biochem. J., 25: 1397-1409.
GOLDWATER, L. J. (1968) Toxicology. In: Sax, N. I., ed. Dangerous
properties of industrial materials, New York, Amsterdam, London,
Reinhold Book Corporation, pp. 1-23.
GOLUBEV, A. F., LJUBLINA, A. E., TOLOKOICEY, N. A., & FILOV, V. A.
(1973) In: Kolicestvennaja toksikologija. Leningrad, Medicina,
p. 217 (in Russian).
GROSS, M. A., FITZHUGH, O. G., & MANTEL, N. (1970) Evaluation of
safety of food additives: an illustration involving the influence
of methyl salicylate on rat reproduction. Biometrics,
26: 181-194.
HAMAKER, J. W., GORING, C. A. L., & YOUNGSON, C. R. (1966) Sorption
and leaching of 4-amino-3,5,6-trichloropicolinic acid in soils.
In: Gould, R. F., ed. Organic pesticides in the environment,
American Chemical Society, pp. 23-37 (Advances in Chemistry
Series, Vol. 60).
HATCH, T. F. (1968) Significant dimensions of the dose-response
relationship. Arch. environ. Health, 16: 571-578.
HATCH, T. F. & GROSS, P. (1964) Pulmonary deposition of inhaled
aerosols. New York, Academic Press.
HERMAN, R. S. (1974) Induction of liver growth by xenobiotic compounds
and other stimuli. CRC crit. Rev. Toxicol., 3(1): 97-158.
HEWLETT, P. S. & PLACKETT, R. L. (1961) Models for quantal responses
to mixtures of two drugs. In: Jonge, H. de, ed. Symposium on
quantitative methods in pharmacology. Amsterdam, North Holland
Publishing Co., pp. 328-336.
HOEL, D. G., GAYLOR, D. W., KIRSCHSTEIN, R. L., SAFFIOTTI, U., &
SCHNEIDERMAN, M. S. (1975) Estimation of risks of irreversible
delayed toxicity. J. Toxicol. environ. Health, 1(1): 133-151.
HUCKER, H. B. (1970) Species differences in drug metabolism.
Ann. Rev. Pharmacol., 10: 99-118.
ICRP (1965) Principles of environmental monitoring related to the
handling of radioactive materials. Report of Committee IV of the
International Commission on Radiological Protection. London,
Pergamon Press.
ICRP (1966) The evaluation of risk from radiation, a report prepared
for Committee I of the International Commission on Radiological
Protection. Oxford, London, Edinburgh, New York, Toronto, Paris,
Braunschweig, Pergamon Press (ICRP Publication 8).
ICRP (1977) Recommendations of the International Commission on
Radiological Protection (ICRP Publication 26 (in press)).
JANYSEVA, H. JA (1972) [On the establishment of MPCs of benz(a)pyrene
in the ambient air of built-up areas.] Gig. i Sanit.,
No. 7, pp. 87-91 (in Russian).
JANYSEVA, H. JA & ANTOMONOV, JU G. (1976) Predicting risk of tumour
occurrence in terms of life-shortening. Environ. Health
Perspect., 13: 95-100.
JONES, H. B. & GRENDON, A. (1975) Environmental factors in the origin
of cancer and estimation of possible hazards to man. Food
cosmet. Toxicol., 13: 251-267.
KAGAN, Y. S. (1973) [Methods of quantitative estimation of combined
and complex action on the organism of physical and chemical
factors in the environment.] Gig. i Sanit., No. 2, pp. 89-92
(in Russian).
KELLERMANN, G., SHAW, C. R., & LUYTEN-KELLERMANN, M. (1973) Aryl
hydrocarbon hydroxylase inducibility and bronchogenic carcinoma.
New Engl. J. Med., 289: 934-937.
KORBAKOVA, A. K., SUMSKAJA, N. I., ZAEVA, G. N., & NIKITENKO, T. K.
(1971) In: Naucnye osnovy sovremmenyh metodov gigieniceskogo
normirovanija himiceskih vescest'v v okruzajuscej srede.
Moscow, Institut gigieny truda i profzabolevanija AMN SSSR,
pp. 35-39.
KRASOVSKIJ, G. N. (1965) [Methodology for conducting an acute
experiment and for evaluating the results, and its basis.] In:
Sanitarnaja ohrana vodoemov ot zagrjaznenija promyslennymi
stocnymi vodami. No. 7, 247-268 (in Russian).
KRASOVSKIJ, G. N. (1970) [On methods for studying the cumulative
properties of toxic compounds.] Gig. i Sanit., No. 3, pp. 83-88
(in Russian).
KRASOVSKIJ, G. N. (1975) Species and sex differences in sensitivity to
toxic substances. In: Methods used in the USSR for establishing
biologically safe levels of toxic, Geneva, WHO, pp. 109-125.
KRASOVSKIJ, G. N. (1976a) [General standardization of biological
parameters for mammals according to body weight, and some
practical aspects of investigations.] In: Trudy MMI im Secenova
T.80 -- Novoe v metodah issledovannija, diagnostiki, lecenii i
profilaktiki vazneisih zabolevanii. Pp. 64-88 (in Russian).
KRASOVSKIJ, G. N. (1976b) Extrapolation of experimental data from
animals to man. Health Perspect., 13:51-58.
KUSTOV, V. V. & TIUNOV, L. A. (1970) [Enzyme system activity as a
means of estimating thresholds of the effects of toxins.] In:
Metody opredelenija toksicinosti i opasnosti himiceskih
vescestv. Moscow, Medicina, pp. 231-234 (in Russian).
KUSTOV, V. V., ZDANOV, A. M., & JUNOVSKIJ, G. D. (1974) [On the
evaluation of the combined effect of factors by the method of
partial regression.] Gig. Tr. Prof. Zabol., 16:33-36
(in Russian).
LAZAREV, N. V. (1938). Obscie osnovypromyslennoj toksikologii.
Moscow & Leningrad, Medgiz.
LAZAREV, N. V. (1963) Rukovodstvo po gigiena truda, Moscow, Medgiz.
LAZAREV, N. V. & BRUSILOVSKAJA, A. I. (1934) Fiziol. Z. SSSR, XVII,
pp. 611-619 (in Russian).
LJUBLINA, E. I. & MIHEEV, M. I. (1974) [Scientific bases for
establishing tentative safe levels of substances affecting man.]
In: Zurnal Vsesjuznogo Obcestva im. D. I. Mendeleeva,
XIX (2): 142-145 (in Russian).
LOEWE, S. (1959) Relationships between stimulus and response.
Science, 130: 692-695.
LU, P. Y. & METCALF, R. (1975) Environmental fate and biodegradability
of benzene derivatives as studied in a model aquatic ecosystem.
Environ. Health Perspect., 10: 269-284.
MANTEL, N. & BRYAN, W. R. (1961) Safety testing of carcinogenic
agents. J. Natl Cancer Inst., 27: 455-470.
MANTEL, N. & SCHNEIDERMAN, M. (1975) Estimating "safe" levels, a
hazardous undertaking. Cancer Res., 35: 1379-1386.
MANTEL, N., BOHIDAR, N., BROWN, C., CIMINERA, J., & TUKEY, J. (1975)
An improved "Mantel-Bryan" procedure for "safety" testing of
carcinogens. Cancer Res., 35: 865-872.
MAZZE, R. I., COUSINS, M. J., & KOSEK, J. C. (1973) Strain differences
in metabolism and susceptibility to the nephrotoxic effects of
methoxyfluorane in rats. J. Pharmacol. exp. Therapy,
184: 481-488.
MEDVED, L. I. & SPYNU, E. I. (1970) [Principles and methods of
hygienic standardization of pesticides. In: Principles and
methods of establishing maximum permissible concentrations of
harmful substances in the air of production premises.] Moscow,
Medicina (in Russian).
METCALF, R. L., KAPOOR, I. P., LU, P. Y., SCHUTH, C. K., & SHERMAN, P.
(1973) A model ecosystem for the evaluation of pesticide
biodegradability and ecological magnification. Environ. Sci.
Technol., 5: 709-744.
MILLER, E. C., MILLER, J. A., & ENOMOTOR, M. (1964) The comparative
carcinogenetics of 2-acetylaminofluorene and its N-hydroxy
metabolite in mice, hamsters and guinea pigs. Cancer Res.,
24: 2018-2032.
MONTESANO, R., MOHR, U., & MAGEE, P. N. (1974) Additive effect in the
induction of kidney tumours in rats treated with
dimethylnitrosamine and ethylmethanesulfonate. Br. J. Cancer,
29: 50-58.
MURPHY, S. D. (1969) Some relationships between effects of
insecticides and other stress conditions. Ann. NY Acad. Sci.,
160: 366-377.
NAS/NRC (1970) Evaluating the safety of food chemicals. Food
Protection Committee, Food and Nutrition Branch, Division of
Biology and Agriculture, National Research Council, Washington
DC, National Academy of Sciences. 55 pp.
NAS (1975) Principles for evaluating chemicals in the environment --
A report of the Committee for the Working Conference on
Principles of Protocols for Evaluating Chemicals in the
Environment. Washington, DC, National Academy of Sciences.
NIEHS (1970) Man's health and the environment, some research needs --
Report of the Task Force on Research Planning in Environmental
Health Science US Department of Health, Education and Welfare,
Public Health Service, National Institutes of Health, National
Institute of Environmental Health Sciences. Washington, DC, US
Govt Printing Office.
NIEHS (1977) Human health and the environment, some research needs --
Report of the Second Task Force for Research Planning in
Environmental Health US Department of Health, Education and
Welfare, Public Health Service, National Institutes of Health,
National Institute of Environmental Health Sciences. Washington,
DC, US Govt Printing Office (DHEW Publication No. NIH77-1277).
OECD (1977) The OECD programme on long range transport of air
pollutants. Measurements and findings. Paris, Organisation for
Economic Co-operation and Development, 268 pp.
OWEN, D. B. (1955) Handbook of statistical tables, London, Paris,
Pergamon Press, pp. 127-137.
PAGET, G. E. (1970) The design and interpretation of toxicity tests.
In: Paget, G. E., ed. Methods in toxicology, Philadelphia, F.
A. Davies, pp. 1-10.
PARKE, D. V. (1975) Induction of the drug-metabolizing enzymes. In:
Parke, D. V., ed. Enzyme induction, London, Plenum Press,
pp. 207-272.
PETERS, R. A. (1963) Biochemical lesions and lethal synthesis.
Oxford, London, New York, Paris, Pergamon Press.
PETERS, R. A. (1967) The biochemical lesion in thiamine deficiency.
In: Wolstenholme, G. E. W. & O'Connor, M., ed. Thiamine
deficiency, biochemical lesions and their significance, London,
J. & A. Churchill Ltd, pp. 1-8.
PETO, R. & LEE, P. N. (1973) Weibull distribution for continuous
carcinogenesis experiments. Biometrics, 29: 457-470.
PETO, R., LEE, P. N., & PAIGE, W. S. (1972) Statistical analysis of
the bioassay of continuous carcinogens. Br. J. Cancer,
26: 258-261.
PFITZER, E. A. (1976) General concepts and definitions for
dose-response and dose-effect relationships of toxic metals. In:
Nordberg, G., ed. Effects and dose-response relationships of
toxic metals. Amsterdam, Oxford, New York, Elsevier Scientific
Publishing Company.
PINIGIN, M. A. (1974) [Current problems of community toxicology in
relation to chemical air pollution.] In: Itogi nauki i tehniki.
Serija farmakologija. Himioterapevticeskie sredstva.
Toksikologija. Problemy toksikologii. Vol. 6, Moscow
(in Russian).
POKROVSKIJ, A. A. (1973) [Biochemical approaches to the evaluation of
toxic factors in the environment. In: Documentation of the
Conference on Methodological Approaches to the Study and
Evaluation of Toxic Factors in the Environment.] Moscow
(in Russian).
PORTMAN, R. S., PLOWMAN, K. M., & CAMPBELL, T. C. (1970) On mechanisms
affecting species susceptibility to aflatoxin. Biochim. Biophys.
Acta, 208: 487-495.
PRAVDIN, N. S. (1934) Rukovodstovo promyslennoi toksikologii.
Moscow, Biologija i Medicina.
RALL, D. F. (1970) Animal models for pharmacological studies. In:
Proceedings of the Symposium on Animal Models for Biomedical
Research III, Washington, DC, National Academy of Sciences,
pp. 125-146 (ISBN 0-309-01854-4).
SABAD, L. M., SANOCKIJ, I. V., ZAEVA, G. N., BUREVIC, T. C.,
KACNELSON, B. A., JANYSEVA, H. JA, & SUGAEVA, B. V. (1973) [On
the possibility of establishing MPCs for benz(a)pyrene in the air
of industrial premises.] Gig. i Sanit., No. 4, pp. 78-81
(in Russian).
SAFFIOTTI, U. (1973) Comments on the scientific basis for the "Delaney
Clause". J. Prev. Med., 2: 125-132.
SANOCKIJ, I. V. (1962) [The calculation of a safety factor for
experimentally based maximum permissible concentrations of
industrial poisons. In: Industrial toxicology and clinical
aspects of occupational diseases of chemical etiology.] Moscow,
Medgiz (in Russian).
SANOCKIJ, I. V. (1970) In: Sanockij, I. V., ed. Metody opredelenija
toksicnosti i opasnosti himiceskih vescestv. Moscow, Medicina,
pp. 11-12 (in Russian).
SANOCKIJ, I. V. (1975a) Investigation of new substances: permissible
limits and threshold of harmful action. In: Methods used in the
USSR for establishing biologically safe levels of toxic
substances, Geneva, WHO, pp. 9-18.
SANOCKIJ, I. V. (1975b) [The threshold concept for the reactions of
living systems to environmental influences and its consequences.
Soviet-American Symposium, Tbilisi.] Gidrometeoizdat,
pp. 112-120 (in Russian).
SANOCKIJ, I. V. & SIDOROV, K. K. (1975) [The current status of the
problem of safety factors in establishing the maximum permissible
concentrations of substances in the components of the general
environment.] Gig. i Sanit., No. 7, pp. 93-96 (in Russian).
SATO, T. & MOROI, K. (1971) Species and age differences in the
activity of isocarboxazid hydrolysing enzyme. Arch. int.
Pharmacodyn. Ther., 192: 128-134.
SCHILD, H. O., ARIENS, E. J., SIMONIS, A. M., DIKSTEIN, S., DE JONGH,
S. E., HEWLETT, P. S., & PLACKETT, R. L. (1961) Section on
mixtures of drugs. In: De Jonge, H. ed. Quantitative methods in
pharmacology, Proceedings of a Symposium held in Leyden on
May 10-13, 1960, Amsterdam, North Holland Publishing Company,
pp. 282-334.
SCHNEIDERMAN, M. A. (1971) A method for determining the dose
compatible with some "acceptable" level of risk. In: Chemicals
and the future of man -- Hearings before the Sub-Committee on
Executive Reorganization and Government Research of the Committee
on Government Operations, US Senate, Ninety-Second Congress,
First Session, 6 and 7 April 1971. Washington, DC, US Govt
Printing Office.
SCHNEIDERMAN, M. A. & MANTEL, N. (1973) The Delaney Clause and a
scheme for rewarding good experimentation. Prev. Med.,
2: 165-170.
SIDORENKO, G. I. & PINIGIN, M. A. (1975) Establishment of safe levels
of chemicals in communal hygiene: methodological approaches. In:
Methods used in the USSR for establishing biologically safe
levels of toxic substances, Geneva, WHO, pp. 126-138.
SIDORENKO, G. I. & PINIGIN, M. A. (1976) Concentration-time
relationship for various regimens of inhalation of organic
compounds. Environ. Health Perspect, 13: 17-21.
SMYTH, H. F., JR (1963) Industrial hygiene in retrospect and
prospect-toxicological aspects. Am. Ind. Hyg. Assoc. J.,
24: 222-226.
SMYTH, H. F. JR, WEIL, C. S., WEST, J. S., & CARPENTER, C. P. (1969)
An exploration of joint toxic action: twenty-seven industrial
chemicals intubated in rats in all possible pairs. Toxicol.
appl. Pharmacol, 14: 340-347.
SPYNU, E. I., VROCINSKIJ, K. K., ZOR'EVA, T. D., & MAL'KO, N. N.
(1972) [Complex hygienic standardization of new organophosphorus
pesticides in environmental components.] Gig. i. Sanit.,
No. 11, pp. 96-99 (in Russian).
STOKINGER, H. E. (1972) Concepts of thresholds in standards setting.
Arch. environ. Health, 25 (3): 153-157.
SYSIN, A. M., ed. (1941) [Maximum allowable concentrations of toxic
substances in water bodies.] Moscow and Leningrad, Stroizdat
(in Russian).
TASK GROUP ON METAL ACCUMULATION (1973) Accumulation of toxic metals
with special reference to their absorption, excretion and
biological half-time. Environ. Physiol. Biochem., 3: 65-107.
TASK GROUP ON METAL TOXICITY (1976) Consensus report for an
international meeting organized by the Sub-committee on the
Toxicology of Metals of the Permanent Commission and
International Association on Occupational Health, Tokyo, 18-23
November 1974. In: Nordberg, G., ed. Effects and dose-responses
relationships of toxic metals, Amsterdam, Oxford, New York,
Elsevier Scientific Publishing Company, pp. 7-111.
ULANOVA, I. P. (1969) [Problems of hygienic standardization of
mixtures of gases and vapours of chemical substances. In:
The toxicology of new industrial chemicals.] Moscow, Medicina,
pp. 33-39 (in Russian).
ULANOVA, I. P., AVILOVA, G. G., BAZAROVA, L. A., MAL'CEVA, N. M.,
MIGUKINA, N. V., HALEPO, A. I., & EITINGTON, A. I. (1973)
[Experimental data on adaptation to poisons under different
conditions of their action. Pharmacology, chemotherapeutic
substances, toxicology.] Itogi nauk i teh., No. 5, pp. 64-75
(in Russian).
ULANOVA, I. P., AVILOVA, G. G., MAL'CEVA, N. M., & HALEPO, A. I.
(1976) [Comparisons of reactions of the organism to continuous
and intermittent action of some chlorinated hydrocarbons.]
Gig. Tr. Profzabol., No. 7, pp. 22-25 (in Russian).
VAN NOORDWIJK, J. (1964) Communication between the experimental animal
and the pharmacologist. Stat. Neerl., 18 (4): 403-416.
VELDSTRA, H. (1956) Synergism and potentiation. Pharmacol. Rev.,
8: 339-387.
VETTORAZZI, G. (1975) Toxicological decisions and recommendations
resulting from the safety assessment of pesticide residues in
food. Crit. Rev. Toxicol., 4 (2): 125-183.
VETTORAZZI, G. (1977) Safety factors and their application in
toxicological evaluation. In: Hunter, W. J. & Smeets, J. G. P.
M., ed. The evaluation of toxicological data for the protection
of public health. Oxford, Pergamon Press (for the Commission of
the European Communities) pp. 207-223.
WEIL, C. S. (1972a) Statistics versus safety factors and scientific
judgment in the evaluation of safety for man. Toxicol. appl.
Pharmacol., 21: 454-463.
WEIL, C. S. (1972b) Guidelines for experiments to predict the degree
of safety of a material for man. Toxicol. appl. Pharmacol.,
21: 194-199.
WHO (1958) WHO Technical Report Series, No. 144 (Procedures for the
testing of intentional food additives to establish their safety
for use -- Second Report of the Joint FAO/WHO Expert Committee on
Food Additives.) 19 pp.
WHO (1973) WHO Technical Report Series, No. 535 (Environmental and
health monitoring in occupational health -- Report of a WHO
Expert Committee.) 48 pp.
WHO (1974a) WHO Technical Report Series, No. 546 (Assessment of
carcinogenicity and mutagenicity of chemicals -- Report of a WHO
Scientific Group.) 19 pp.
WHO (1974b) WHO Technical Report Series, No. 554 (Health aspects of
environmental pollution: planning and implementation of national
programmes -- Report of a WHO Expert Committee.) 57 pp.
WHO (1975a) WHO Technical Report Series, No. 571 (Early detection of
health impairment in occupational exposure to health hazards --
Report of a WHO Study Group.) 80 pp.
WHO (1975b) WHO Technical Report Series, No. 560 (Chemical and
biochemical methodology for the assessment of hazards of
pesticides for man -- Report of a WHO Scientific Group.) 26 pp.
WHO (1975c) Methods for studying biological effects of pollutants (a
review of methods used in the USSR). Report of a Working Group,
Moscow, November 1974. Copenhagen, WHO Regional Office for
Europe.
WHO (1976a) WHO Technical Report Series No. 586 (Health hazards of new
environmental pollutants -- Report of a WHO Study Group.) 96 pp.
WHO (1976b) Health aspects of human rights with special reference to
developments in biology and medicine. Geneva, World Health
Organization, pp. 25-27.
WHO (1977) Environmental Health Criteria 4: Oxides of nitrogen.
Geneva, WHO, 79 pp.
WILLIAMS, R. T. (1969) The fate of foreign compounds in man and
animals. Pure appl. Chem., 18: 129-141.
WILLIAMS, R. T. (1972) Toxicological implications of biotransformation
by intestinal microflora. Toxicol. appl. Pharmacol.,
23: 769-781.
2. FACTORS INFLUENCING THE DESIGN OF TOXICITY STUDIES
2.1 Introduction
The choice and sequence of toxicity tests will depend on the
questions or hypotheses that are developed. The nature and sequence of
tests used to satisfy requirements of regulatory agencies may differ
markedly from those used in an investigation of the basic mechanisms
of toxic action. Differences in approach will also depend on whether
the investigation is initiated to evaluate the toxicity of a chemical
prior to its introduction into use, i.e. prospective toxicology, or to
confirm in laboratory animals an epidemiological association that
suggests chemical-induced disease in man, i.e. retrospective
toxicology. Under ideal conditions, prospective toxicology will
eliminate the need for retrospective toxicity evaluation.
National or international regulatory or advisory bodies have
developed fairly specific guidelines or test protocols which are
expected to be applied to the toxicity evaluation of certain groups of
chemicals, introduced deliberately into our environment, e.g. food
additives and pesticides (Council of Europe, 1973; FDA, 1959; WHO,
1967). The development of guidelines for the systematic evaluation of
the toxicity of chemicals to which man is exposed, through his
occupation or through incidental contamination of the ambient
environment, is less common. Where specific guidelines have been
formulated, they usually require: test information on acute toxicity
in several species of experimental animals; some knowledge of the
biochemical disposition of the compounds; various short-term toxicity
tests; tests of the effects of the chemical on reproductive function;
chronic toxicity tests in one or more species; special tests on organ
function, clinical biochemistry and haematology, and other specific
tests as determined by the particular type and intended uses of the
chemical under consideration. Some commercial firms have developed
their own guidelines for toxicity tests and their sequence in the
premarket toxicological evaluation of products. Often the sequence of
tests will have certain checkpoints at which decisions will be made as
to whether continued development of the product (and more extensive
toxicity testing) is warranted.
In this chapter, various topics will be discussed from the
standpoint of the usefulness of certain types of information and the
influence that various factors may have in designing protocols for the
interpretation of data obtained in toxicity evaluation programmes.
2.2 Chemical and Physical Properties
2.2.1 General considerations
The late Horace Gerarde stated in an address that "toxicity is
the capacity of a substance to cause injury. It is an inherent,
unalterable molecular property which is dependent upon chemical
structure. There is nothing we can do about the toxicity of a chemical
except to know it"a.
The nature or quality of the toxic action inherent in a chemical
will depend to a large extent upon the functional group or groups
present in the molecule. Knowledge of the reactions that these
functional groups may undergo with reactive groups in critical
endogenous biochemical constituents provides a means of predicting the
nature of the toxic effects that may be expected. Smyth (1959) used
the permanence of threshold limit values over a period of years as a
criterion for evaluating various types of information used in setting
safety limits for industrial chemicals. For a limited number of
compounds, occupational threshold limit values (TLVs) had been
established on the basis of analogy with better known substances and
the permanence of these TLVs appeared to equal that of TLVs based upon
data from experimental toxicity studies and from human experience.
However, evaluation of toxicity by analogy with chemically-related
substances contains considerable potential for error, and requires a
great deal of toxicological information on very closely related
chemical substances. Very minor changes in structure may be
accompanied by profound changes in toxicity. The relationships between
physicochemical characteristics and toxicity, including the biological
activities of homologous series, have been reviewed by Ljublina &
Filov (1975).
2.2.2 Physicochemical properties and the design of toxicity studies
Zbinden (1973) included fourteen chemical and physical variables
in a check list of types of information useful in the toxicity
evaluation of new drugs. Although some of these variables may be
determined in the course of a toxicological evaluation, all of them
apply equally as well to environmental chemicals as to therapeutic
chemicals.
a Presented at the Flavor Manufacturers' Association of the United
States. Fall Symposium, 16 November 1972, Washington, DC.
Knowledge of the chemical structure is essential for the
preliminary prediction of the nature and site of toxic action,
assuming, of course, that some prior knowledge of the toxicity of
chemically-related compounds is available. It is also essential for
developing extraction and assay procedures for the determination of
tissue concentrations and allows for logical estimates of the nature
of metabolites that may be found. Indeed, without such knowledge,
logical design of an experiment is impossible.
The stability of the chemical at various pH values and the
photochemical properties are variables that must be considered as soon
as a substance arrives for testing in the toxicology laboratory, as
they may determine the manner in which the chemical should be stored
prior to administration to test animals or indicate the stability of
residues in tissue extracts. Many organic chemicals undergo
photochemical reactions that lead to either more or less toxic
products (Crosby, 1972) and organic esters are often readily
hydrolysed under conditions encountered during their laboratory
investigation (Eto, 1974). It may be necessary to exercise special
precautions to avoid chemical reactions during the preparation and
storage of test solutions or diets and during the analysis of tissues
and metabolic reaction mixtures. Furthermore, if a chemical is likely
to become activated by photochemical reactions, special tests for
phototoxicity may be required.
The organic solvent/water partition coefficient and pK are
physical properties of particular importance in the determination of
the absorption and distribution of a compound in living organisms as
well as in the development of appropriate extraction and assay
procedures for the chemical. Hansch & Dunn (1972) reviewed numerous
studies which suggest that characterization of the lipophilic nature
of compounds may allow systematic predictions of their relative
biological activities. Dillingham et al. (1973) applied these
principles when they compared the toxicity of substituted alcohols in
a tissue culture with their acute toxicity in mice and concluded that
tissue culture test systems may be useful in determining predictive
correlations between in vivo toxicity and the physicochemical
properties of compounds.
The extent of ionization of an organic compound will influence
its passage through lipoidal membranes (La Du et al., 1971). In
general, the unionized lipid-soluble form of an organic compound will
most readily pass through biological membranes. Although most of the
physicochemical principles of absorption and distribution have been
developed through systematic studies on medicinal chemicals, these
principles also apply to organic chemicals in the environment and to
the design of toxicity experiments (Loomis, 1974; also Chapter 4).
Patty (1958) discussed the influence of oil and water solubility and
of the coefficient of distribution of a vapour between blood and
alveolar air on the rates of equilibrium saturation and desaturation
of the body during inhalation experiments.
Particle size, shape, and density are of obvious importance in
studying the inhalation toxicities of aerosols, as they are important
factors in the determination of the site of deposition and the rates
and mechanisms of clearance from the respiratory tract (Hatch & Gross,
1964; also Chapter 6). Furthermore, the particle size of substances
given orally as suspensions can also markedly influence their toxicity
(Boyd, 1971). If critical judgements based on the relative toxicities
of the same or different substances, administered orally in
suspension, are to be made, it is necessary to ensure uniformity of
particle size.
Vapour pressure of a chemical substance is important in the
practical consideration of the likelihood of exposure of man through
inhalation, and the design of experimental inhalation toxicity studies
will be influenced by the ease with which a solid or liquid vaporizes
under controlled conditions. However, a high vapour pressure may
produce technical problems if the objective of the test is to
determine toxicity by the oral route of administration. Studies by
Jones et al. (1971) showed that for a large series of food flavouring
agents mixed in laboratory animal diets, the amount of loss from the
diet was inversely related to the boiling points of the flavouring
agents. Frequent chemical analyses of the diet, as well as frequent
preparation of fresh diets and/or restricted feeding periods to limit
the time for loss by vaporization are necessary to provide accurate
estimates of intake in feeding studies on substances with low boiling
points.
Knowledge of reactivity with, or binding to, macromolecules may
allow specific design of mechanism experiments, when these
macromolecules are essential tissue and cell constituents. Knowledge
of the chemical reactivity of a substance may also be of considerable
importance in the early planning of feeding studies, if the chemical
under test is likely to react with the macromolecules present in the
laboratory diet. On the one hand, chemical binding or adsorption on
macromolecules in the diet may markedly alter the rate and extent of
absorption of the test compound from the gastrointestinal tract; in
some cases, biologically reactive groups on the test chemical may be
neutralized by dietary constituents. On the other hand, reaction of
the test chemical with essential dietary constituents may contribute
to nutritional deficiency states, or new and more toxic compounds may
be formed. Several examples of these types of reactions have been
summarized by Golberg (1967).
2.2.3 Impurities
In the design of toxicity experiments, it is extremely important
to consider the chemical purity of the sample to be tested. In certain
cases, e.g. food additives and pesticides, the regulations will often
provide specifications of purity for the compound in actual use and
recommended test protocols may specify that toxicity evaluations are
to be conducted with samples that meet these specifications (WHO,
1967). However, there is always the possibility that, when testing the
technical product, the biological effects observed may be due to, or
modified by, trace contaminants. If the contaminants are unknown or
their biological activity unsuspected, toxicity tests may lead to
erroneous conclusions concerning the primary chemical in question. In
contrast, tests on highly purified samples may not detect the toxic
action of contaminants present in samples used commercially.
Furthermore, for many chemical substances used in manufacturing or
incidentally released into the environment, specifications of purity
may not be standardized. Therefore, one of the earliest and most
difficult decisions that must be made in the design of a toxicity
evaluation programme is the selection of the sample to be studied
(technical grade, highly purified, etc.)
Requirements for the purity of the compounds selected for
toxicological testing depend on the purpose of the testing as
discussed in section 2.1. During the development of a new
technological process, it may even be useful to test mixtures of
unknown composition. The information so obtained may alert organic
chemists in the research laboratory or pilot plant operators to the
possible hazards of these unknown mixtures which may vary as
procedures develop and improve. However, data obtained from such
studies will have a limited value. The determination of health
standards requires a compound of a high degree of purity or of highly
standardized composition combined with a precise knowledge of various
impurities. Only in this way will health standards have a universal
value. However, for practical purposes, and for extrapolation to human
exposure conditions it may be prudent, when a technical grade product
standardized by specifications is used in commerce, to select this
grade for toxicity testing and carefully characterize it with respect
to the nature and amounts of any impurities present. Scheduled
analytical spot checks during the course of the experiment to provide
assurance of chemical constancy is also desirable.
When a chemical substance used in commerce is not standardized
with respect to specifications for purity, experimental toxicity
evaluation on a test sample of high purity would, indeed, seem to be
the most rational selection. Data derived from this compound could
then be used to characterize, toxicologically, the action of the
primary chemical under consideration. When certain quantifiable
indices of toxicity have been identified, selected tests with typical
samples of commercial products could be conducted and the results
compared with the purified product to detect possible differences. Of
course, if the impurities represent a significant portion of the
product, or if their chemical properties or their chemical analogy to
other known substances suggest they may have serious toxic properties,
the impurities must be evaluated separately.
An alternative approach is to select a sample of a chemical that
most nearly represents the impure product used commercially, subject
this to comprehensive toxicity evaluation and then make selected,
critical toxicity comparison with purified samples of the primary
chemical as well as with the impurities.
Either approach contains uncertainties, and little would be
gained by short-term spot checks if the toxic action of the impurity
were only detectable after long-term exposure or a latent period. It
is in problem situations such as these that some of the
chemicophysical principles discussed earlier must be applied at
several levels of decision making: the sample to choose for testing;
the design of the evaluation protocol; or the decision (or regulation)
to produce a purer substance for routine use in commerce.
A recent, most controversial problem arising from the
contamination of a primary commercial product involves the apparent
teratogenic action of the herbicide (2,4,5-trichlorophenoxy)acetic
acid (2,4,5-T) (Panel on Herbicides 1971). It is now known that the
first studies to reveal this action were conducted with a sample of
the herbicide that contained a rather high concentration (about
30 mg/kg) of the contaminant 2,3,7,8-tetrachloro-dibenzo-4-dioxin
which is formed during the synthesis of the trichlorophenol precursor.
Tetrachlorodioxin is extremely toxic. For guineapigs, the ratio of the
LD50 for 2,4,5-T to the LD50 of the dioxin is about 630 000. In
female rats, the acute oral LD50 for dioxin is about 1/10 000 of the
oral LD50 for 2,4,5-T. The daily dose of dioxin in pregnant rats
that produced fetal toxicity was only about one four-hundredth of the
maternal LD50 of dioxin or about one four-millionth of the
single-close oral LD50 of 2,4,5-T for female rats. Thus, even if the
concentration of dioxin as a contaminant of 2,4,5-T is kept below
0.5 mg/kg, the major concern for the toxicity of 2,4,5-T should
apparently still be directed towards the contaminant rather than
towards the herbicide itself (at least insofar as effects on
reproduction are concerned).
This kind of problem is not new. Twenty-five years ago, marked
differences in the toxicity of different samples of the insecticide
parathion were traced to contamination with small quantities of the
oxygen analogue and the phosphorothiolate isomer, which are much more
potent anticholinesterases and more acutely toxic than the parent
insecticide (Diggle & Gage, 1951).
2.3 Probable Routes of Exposure
2.3.1 General considerations
Many chemicals will become distributed in various environmental
media or will be used for different purposes, and substantially
different populations may be at risk. Thus, it may be necessary to
obtain extensive test data by different routes of exposure. The choice
of route for practical purposes is generally dictated by: (a) the
likely route by which man will be exposed; and (b) whether the
chemical will produce local injury at the site of exposure. The second
question will often be resolved by acute or short-term studies on
animals dosed by oral, inhalation, dermal, and possibly ocular routes.
Details of test procedures are included in subsequent chapters.
Although it is usually wise to conduct experiments using the route
through which man will be exposed, other more convenient routes may be
chosen for many of the tests if it is determined that the major toxic
effects of a chemical are systemic, occurring only after absorption
and distribution in the body. Data on blood and tissue levels should
be obtained by several routes of exposure, including those that are
considered primarily experimental; with this information, it may be
possible to relate toxic effects to blood or tissue concentrations of
the test chemical and its metabolites. Such information greatly
facilitates comparison of experiments using different routes and may
either confirm or deny the validity of extrapolating data, obtained
experimentally by one route, to the evaluation of potential toxicity
by another perhaps more realistic route of exposure.
2.3.2 Specific variables related to route of exposure
2.3.2.1 Rate of absorption
As a general rule, one can predict that for the usual routes by
which man may be exposed, absorption of chemicals will be most rapid
when given by inhalation, less rapid when given by gavage, and slowest
with dermal application. This order may, however, be modified
depending upon various physicochemical properties of the substance
under test in relation to the microenvironment of the absorbing
surface (Klassen, 1975; Loomis, 1974). The rate of absorption will be
one determinant of the rate of onset of signs of acute poisoning. If
the rates of detoxification and excretion or of injury repair exceed
the limiting rate at which a chemical is absorbed, it is possible that
toxic signs observed by one route of administration will not be
detectable by another route for which absorption is slower (Casarett,
1975; Murphy, 1975). Comparative absorption-distribution kinetics by
different routes would determine such a possibility.
2.3.2.2 Site of action
Specific tests should be conducted to evaluate effects related to
local reactions with specific receptors present in the organ of
absorption. These may be morphological tests to detect evidence of
irritation, inflammation, or oedema, or they may be functional to
detect biochemical or reflex action or bronchoconstriction. In
addition, the route of exposure may determine the organ or
physiological system in which effects will be first observed or
detected at lowest doses. For example, pesticides, which are direct
inhibitors of acetylcholinesterase, when given in sufficient doses by
any route, will produce a characteristic toxic syndrome involving
essentially all organs or structures innervated by cholinergic nerves.
However, at low doses, only specific organs may be involved. If such
compounds are applied to the skin, local sweating and fasciculations
may occur in the absence of signs of systemic poisoning. Exposure by
inhalation may result in bronchoconstriction, exposure by ingestion
may cause gastrointestinal upset before, or at lower doses than,
generalized systemic effects (Henderson & Haggard, 1943; Holmstedt,
1959).
2.3.2.3 Biotransformation
The route of exposure may determine the likelihood and type of
biotransformation before the chemical contacts the specific sites of
action. Thus, when chemicals are administered by the oral (or
intraperitoneal) routes, they will be absorbed and transported first
through the portal circulation to the liver (Lukas et al., 1971). For
example, if, with low oral or dietary doses, the capacity of the liver
to detoxify the compounds exceeds the rate of absorption, an effective
injurious concentration may never reach critical sites of action in
other tissues. Absorption of the same quantity through the lung or
skin, which generally have less detoxifying capacities, may result in
toxic action. It is now known that the lung, skin, and intestinal
mucosa, although generally less active than the liver, also have the
capacity for biotransformation of foreign organic chemicals (Alvares
et al., 1973; Fouts, 1972; Lake et al., 1973; Wattenberg, 1972).
Although knowledge is incomplete with regard to the tissue
distribution of both activating and detoxifying enzyme systems, it is
likely that their relative distribution will determine, to some
extent, the specific tissues that will be most affected by low doses
of some compounds, when given by different routes of exposure.
2.3.2.4 Species
The relative susceptibility of different species to the action of
chemicals may differ depending upon the route of exposure. When
administering compounds by the oral route, such factors as vomiting
reflex (absent in rats) and/or differences in type and distribution of
microflora that may detoxify (or activate) the test compound can
influence the interpretation of the results (Williams, 1972). The
rates of penetration of compounds through the skin and the acute
dermal toxicities of various compounds differ markedly among species
and not always in a predictable manner (McCreesh, 1965). Many of the
problems encountered in dermal toxicity testing and suggestions for
further research have been discussed by Barnes (1968). Roe (1968)
discussed various problems encountered in the design and
interpretation of inhalation toxicity studies related to species
differences in the anatomy of the respiratory tree. Enzootic lung
infections are an additional problem in the use of some species of
animals for long-term inhalation studies.
2.3.2.5 Unintended route
Interpretation of results and measurement of actual dose-response
relationships can be made difficult, because appreciable oral
ingestion may occur with inhalation or dermal exposures. Animals
exposed by either of these routes may ingest the material as a result
of preening, unless dermal applications are covered or made
inaccessible to licking or unless special exposure chambers (e.g. head
only) are used for inhalation exposures (see Chapter 6). In addition,
in particle inhalation experiments, the physiological protective
mechanisms of clearance by mucous transport of the particles out of
the respiratory tree (Hatch & Gross, 1964) with subsequent swallowing
may result in gastrointestinal exposure. Some degree of lung exposure
to volatile compounds administered in the diet or by dermal
application is also likely.
2.3.3 Special tests related to route
When exposure to a compound is most likely to occur by
inhalation, it is useful to know the effect of variations in
ventilation rates, since this will be a common variable among an
exposed human population under different conditions of activity. This
may be accomplished by the use of exercise wheels or treadmills.
When dermal exposure is the likely route, it will be useful to
conduct some tests to determine the effect of different solvents on
penetration of the test compound through the skin. Studies of the
influence of factors such as sweating, abrasions, or the presence of
detergents on dermal absorption and toxicity will also aid in
estimating toxicities under conditions likely to be experienced by man
(see Chapter 11, Part 2).
Interpretation and implications of toxicity data obtained with
oral exposures can be enhanced by examination of the influence of
fasting, dietary variations, and, particularly, administration by
gavage versus inclusion in the diet or drinking water. These factors
are discussed in more detail in subsequent chapters, but it should be
stressed that quite different results and interpretations may ensue if
the same daily dose is given rapidly by gavage or gradually in the
diet. Interpretation of such experiments is greatly aided, if the
design includes comparative absorption and distribution kinetics.
2.4 Selection and Care of Animals
2.4.1 General considerations
The selection and care of laboratory animals to be used in
toxicity tests is especially important in determining the success of
the experiment itself, the extrapolation of the data to man, and the
cost of the evaluation programme. In order to provide data on a
sufficient number of animals for valid statistical analyses, it has
become common practice to use small laboratory rodents for most
large-scale toxicity test programmes. Dogs or nonhuman primates are
frequently included in some of the studies, and studies on at least
one nonrodent species are often required by the test protocols
recommended by regulatory and advisory agencies. Recommendations (with
appropriate references for detailed information) concerning the
selection and care of laboratory animals to be used in the usual broad
scale toxicity evaluation studies are included in Chapter 3. The
selection of animals to be used in various special test procedures is
discussed in subsequent chapters.
Animals and animal care practices should be selected to provide a
scientifically sound and reproducible experiment; however, some of the
variables that contribute to nonuniformity may actually be exploited,
in special studies, to obtain data that may be useful in extrapolation
to nonuniform human populations. For example, if the inherent toxicity
of an air pollutant is to be characterized, the occurrence of chronic
lung infections should be avoided. On the other hand, specially
designed experiments to test the influence of air pollutants on the
susceptibility of animals to lung infections have provided a sensitive
procedure for measuring the adverse effects of air pollutants on the
physiological protective mechanisms that confer resistance to
respiratory infections (Ehrlich, 1966). Epidemiologists could
certainly use such information in the design of studies on human
populations exposed to air pollutants and to infectious microorganisms
present in the environment.
The choice of animals and the environment in which they are used
in toxicity studies will ultimately be determined (as for any other
decisions relating to experimental design) by the nature of the
questions asked or the hypotheses formulated. Controlled introduction
of additional variables may be desired for special studies. The
important principle is that appropriate control conditions should be
included in such studies to allow comparisons with results obtained
under more conventional procedures.
2.4.2 Animal variables
The objective of most experimental toxicity studies is to predict
the adverse effects of chemicals in man. Therefore, in addition to
uniformity of response, the guiding principle for the selection of
appropriate test species is that the test animals should resemble man
as closely as possible with respect to absorption, distribution,
metabolic transformation, excretion, and effect at site(s) of action
of chemicals. Both male and female animals should be tested and the
test protocol should encompass exposures of animals at both ends of
the age spectrum (see Chapter 3).
It is generally recommended that random-bred rather than highly
inbred strains of animals be used in broad-scale toxicity testing, at
least until the action of the chemical is well characterized (Food &
Drug Administration, 1970). In more specialized toxicity tests, it may
be desirable to use inbred strains, for example, when animal models
are needed that represent a genetic variation in human population, or
when hypotheses on the mechanism of action are tested.
2.4.2.1 Selection of species
The Food Protection Committee (1970) indicated that sensitivity,
convenience, and similarity in metabolism to man are the prime factors
to be considered in the selection of animal species for toxicity
testing. In the absence of information to the contrary, it is
generally recommended that data obtained from the most sensitive
species should be used as the basis for the extrapolation of test
information to man.
There is now ample evidence of wide quantitative variations among
species in their rates of biotransformation of foreign compounds
(Committee on Problems of Drug Safety, 1969; Parke & Williams, 1969;
Williams, 1967). Since many organic chemicals are subject to
biotransformation at several reactive groups in the molecule, it is
important to identify and quantify the biotransformation and
distribution pathways of a chemical in man and in several laboratory
animal species as early as possible in toxicity evaluation studies. It
seems axiomatic that for costly chronic studies on experimental
animals, the species that is most representative of man with respect
to the metabolism of the test chemical should be chosen. Often, the
only information concerning the metabolism and distribution of the
test compound in man may be derived from limited studies on
individuals accidentally or occupationally exposed to uncontrolled or
unknown doses.
In vitro studies of metabolism using animal tissues and human
tissues obtained at autopsy or biopsy could help in comparisons of
similarities or differences in metabolism between man and laboratory
animals. Although this approach cannot, in itself, provide information
that may be obtained in studies on intact animals, it can, coupled
with knowledge of the physicochemical properties of the compound and
the kinetics of enzymatic biotransformation reactions in tissues of
various species, provide a logical basis for selection of species for
long-term toxicity tests. Decisions based on comparative human and
experimental animal metabolism data should take into account
information concerning several pathways of metabolism. This will help
to ensure the inclusion of data on quantitatively minor pathways of
metabolism that may result in products of major toxicological
importance. These considerations of variation in biotransformation can
also be applied to intraspecies variations related to age, sex, and
strain (Benke & Murphy, 1973; Jori et al., 1971a; MacLeod et al.,
1972; Parke & Williams, 1969).
Anatomical and morphological variations can also determine the
selection of species. This source of variation is likely to be of
particular importance in inhalation toxicity studies (Roe, 1968).
Tyler & Gillespie (1969) compared anatomical characteristics of the
lungs of human beings with several laboratory and domestic animal
species when considering appropriate animal models for human
emphysema. They grouped anatomically similar species into four
classes: (a) cattle, sheep, and swine; (b) dogs, cats, and rhesus
monkeys; (c) rabbits, rats, and guineapigs; and (d) horses and man.
From their studies on horses, they concluded that the pathophysiology
and the morphological characteristics of emphysema in horses closely
resembled the disease in man and that the horse could be a
particularly suitable laboratory animal for studies of this disease.
Obviously, in the usual toxicity studies on air pollutants, the costs
of using horses would be prohibitive. The reactivity of a chemical at
primary target sites must also be considered as a potential variable
contributing to species differences in toxicity. The acute toxicity of
certain organophosphorous insecticides in representative mammalian,
avian, and fish species appeared to be more readily related to species
differences in the reactivity of the target enzyme
(acetylcholinesterase (3.1.1.7)) than to differences in hepatic
biotransformation rates (Murphy et al., 1968).
In the absence of specific knowledge of comparative metabolism
and sites of action, it is appropriate to apply the principle that
quantitative and qualitative similarity of response in several
mammalian laboratory species enhances the confidence that man will
respond similarly. Tests on several species seem equally as useful for
predicting effects in a heterogenous human population as the selection
of test species based on the results of limited studies of metabolism
in a very few individual human subjects, who may or may not be
representative of a broad cross-section of the human population at
risk. Of course, any quantitative information on the disposition and
action of chemicals in man is useful, as it adds to the accumulation
of knowledge from which more specific guidelines for species selection
may be derived in the future.
2.4.2.2 Animal models representing special populations at risk
Because many chemicals in the environment are widely dispersed,
all segments of the human population may sustain some exposure. For
this reason, it may be useful to design special experiments to
evaluate toxicity in animal models that represent potentially
hypersusceptible segments of the human population. The very young and
the aged represent such segments generally, because in the very young,
natural protective mechanisms such as metabolic detoxification systems
may be incompletely developed and in the aged, cell repair processes
may be less active than in younger individuals. Evaluation of the
toxicity of chemicals in animal models of commonly occurring human
diseases may be of value. Thus, for example, epidemiological studies
suggest that individuals suffering from coronary artery disease may be
particularly susceptible to carbon monoxide, the severity of signs and
symptoms in patients suffering from cardiorespiratory disease appears
to be aggravated by air pollution, and asthmatic patients appear to
have a higher frequency of attacks during periods of high oxidant air
pollution (Heimann, 1967). Few attempts have been made to evaluate
experimentally the interactions between exposure to toxic chemicals
and model disease conditions. Taylor & Drew (1975) reported that an
inbred strain of cardiomyopathic hamsters was more susceptible to
acute toxicity and cardiac arrhythmias produced by inhaled
trichlorofluoromethane than were random-bred hamsters that were not
cardiomyopathic. Easton & Murphy (1967) suggested that their
observation of greater mortality and respiratory distress in
ozone-preexposed than in air-exposed guineapigs given histamine
injections or inhalation exposures might be analogous to the apparent
increase in frequency and severity of asthmatic attacks reported for
peak periods of photochemical air pollution.
Problems of standardization of disease conditions add another
dimension to toxicity studies. However, it seems that animal disease
models should be given more consideration in toxicity evaluations that
are intended to provide the basis for the safe use of chemicals to
which large human populations are exposed. Jones (1969) summarized
reference sources for animal models of a large number of specific
human diseases. Several papers in a series of symposia proceedings
published by the US National Academy of Sciences provide discussion
and references to (among other topics) animal models for commonly
occurring disease states of the lungs (Tyler & Gillespie, 1969), the
cerebrovascular system (Luginbuhl & Detweiler, 1968), the heart (Jobe,
1968), the kidney (Lerner & Dixon, 1968), atherosclerosis (Clarkson et
al., 1970), diabetes (Hackel et al., 1968), and chronic degenerative
diseases (Abinanti, 1971). Since gut microflora are changed in certain
gastrointestinal diseases in man, modifications of the quality and
distribution of microflora in experimental animals might be a useful
model for special tests (Williams, 1972). There are at least three
possible applications of these disease models to toxicity studies: (a)
evaluation of the susceptibility of diseased tissues to chemicals
known to exert their action on that tissue; (b) influence of the
disease state on the metabolism and distribution of chemicals that may
act on the diseased tissue or at other sites; and (c) research on the
mechanism of action of toxic chemicals using specific modification of
receptor function or biochemistry.
2.4.3 Cyclic variations in function or response
Many physiological variables undergo cyclic peaks and troughs of
activity (Altman & Dittmer, 1966) some of which are diurnal (24-h) and
others of longer duration. These rhythms may be completely under
intrinsic control or they may be partly or largely regulated by
environmental variables such as light and temperature. Boyd (1972)
considers most diurnal variations in susceptibility to drug toxicity
to be mainly related to eating and sleeping habits. Since rats are
nocturnal feeders, the greater quantity of food in the stomach early
in the morning compared with the afternoon may alter the acute
toxicity of chemicals given intragastrically. Attempts to standardize
this variable have led to recommendations that acute toxicity tests by
intragastric administration should be conducted on animals that have
fasted overnight (Food & Drug Administration, 1959). Intragastric
LD50 values are generally lower in rats fasted overnight compared
with those fed ad libitum; however, the differences are usually only
of the order of two- to three-fold (Boyd, 1972; Loomis, 1974). The
influence of fasting may, in some cases, be related to rates of
absorption from the gut in the presence or absence of food but this
cannot account for all such variations. A striking example has been
reported by Jaeger et al. (1975) in which acute toxicity and liver
injury in rats exposed through inhalation to several halogenated
olefins were enhanced 10- to 20-fold by overnight fasting. A diurnal
cycle of susceptibility of rats to the toxicity of inhaled vinylidene
chloride appeared to be related to the diurnal cycle of liver
glutathione concentrations (Jaeger et al., 1973) which may be
secondary to a diurnal cycle in feeding activities. The duration of
pentobarbital anaesthesia in mice under usual laboratory housing
conditions exhibited a diurnal cycle with the longest duration at
14h00 and the shortest (40-60% of that at 14h00) duration at 02h00
(Davis, 1962). The amplitude of the cycle was considerably reduced,
when animals were caged individually as opposed to group caging, and
constant light abolished the cycle. That circadian variation in the
action of certain organic chemicals may be related to circadian
variation in their biotransformation is suggested by the work of Jori
et al. (1971b).
Beuthin & Bousquet (1970) reported seasonal or circannual rhythms
for drug action and biotransformation rates in rats. The induction of
increased drug metabolism by phenobarbital also exhibited a seasonal
variation. Basal levels of hexobarbital metabolism were highest during
the winter months and lowest in summer, whereas the opposite cycle for
induction of hexobarbital oxidase by phenobarbital was observed. It
should be noted that studies of seasonal variations in the metabolism
or toxic action of chemicals must be carefully controlled with respect
to environmental variables that might produce similar variations in
response (see 2.4.4). Boyd (1972) suggests that seasonal variations in
susceptibility may be related to the hibernation reaction or to
weather conditions in the geographical area concerned.
Circadian variation in adrenocortical activity in rats was
investigated by Szot & Murphy (1971) in animals exposed acutely or
subacutely to the pesticides parathion and DDT. Although the degree of
stimulation of corticosterone secretion after single doses of
parathion varied depending upon the phase of the cycle at the time of
administration, feeding parathion or DDT in the diet at rather high
concentrations did not change the phase or the amplitude of the
natural adrenocortical rhythm or alter the stimulation of
corticosterone secretion produced by irritant stress.
In rodents, locomotor activity is greatest at night and Boyd
(1972) suggests that depression of activity is best demonstrated at
night or in rats starved for 3 days when their daytime activity is as
great as at night time. However, a more convenient approach may be to
reverse the lighting schedule, a procedure used successfully for
measuring the effects of various inhaled air pollutants on locomotor
activity in mice (Murphy, 1964).
The time of day at which biochemical or other tests are conducted
in control and experimental animals may influence the reproducibility
of the test data, if the biological variables under test display
rhythmic variation. An investigator may exploit these rhythmic
variations to advantage in special studies of factors that influence
susceptibility to chemical injury. However, if the aim is a broad
scale characterization of the toxicity of a chemical, the choice may
be to carefully standardize times of administration of chemicals and
of animal sampling to minimize both known and unrecognized circadian
variations as much as possible.
2.4.4 Environmental variables
There are numerous possible variations in the environment in
which experimental animals are housed or tested that can influence
their response to toxic chemicals. General considerations of these
variables are discussed by several authors (Boyd, 1972; Doull, 1972,
1975; Hurni, 1970; Morrison, 1968). Unless the purpose of the
experiment is to use these variables to predict possible alterations
in effects in man exposed to chemicals under similar environmental
variations, it is generally possible to minimize their influence on
the toxicity of chemicals by adopting good principles of animal care.
Reference sources are available to provide guidelines for proper
housing, diets, cage size requirements, etc. (DHEW, 1972; Universities
Federation for Animal Welfare, 1972).
Only brief comments will be made on some of the major
environmental variables that affect toxicity experiments or that can
be used for predicting mechanisms or possible implications to man.
2.4.4.1 Temperature
Major variations from the recommended environmental temperatures
and relative humidities can contribute not only to the impairment of
general health and increased susceptibility to infection of the
animals, but also to variation in their response to toxic chemicals.
The mechanisms of interactions between environmental and body
temperatures and drugs or toxic agents have been reviewed by Doull
(1972) and by Cremer & Bligh (1969). Since absorption, distribution,
metabolic transformation, excretion, and reactivity with receptor
sites depend on various temperature-dependent chemical reactions, it
might be expected that the toxicity of chemicals would be readily
influenced by temperature. However, since toxicity studies are usually
conducted with homotherms, only minor changes in core body temperature
occur with moderate changes in environmental temperatures. By the same
token, changes in environmental temperature will elicit homeostatic
changes in various physiological or biochemical systems. These may
then alter some of the physiological variables (e.g. ventilation,
circulation, body water, intermediary metabolism) that are
rate-limiting determinants of the absorption, deposition, and action
of toxic chemicals. Furthermore, toxic chemicals may exert their
action by disruption of the thermoregulatory mechanism as suggested
for cholinesterase inhibitors (Meeter & Wolthuis, 1968). Exposure to
toxic chemicals can also mimic the actions of extremes in
environmental temperature or other physical stressors (Murphy, 1969;
Szot & Murphy, 1970). Thus, fluctuations in environmental temperatures
can lead to functional changes that might be mistakenly attributed to
the action of the chemical or they may actually alter the toxicity. If
interference with physiological thermoregulatory mechanisms is a
likely action of the chemical, careful control of environmental
temperatures is necessary to ensure reproducibility of measurements of
this action.
2.4.4.2 Caging
The type of cage, grouping, bedding, and other factors related to
caging can markedly influence the toxicity of some chemicals and drugs
(Boyd, 1972; Doull, 1972; Hurni, 1970). The acute toxicity of
4-[1-hydroxy-2-[(1-methylethyl)amino]ethyl]-1,2-benzenediol
(isoproterenol) was markedly greater in rats caged singly for more
than three weeks than in rats caged in groups (Hatch et al., 1965).
Winter & Flataker (1962) reported that grouped rats held in "closed"
(sheet metal on four sides and bottom) cages were more resistant to
the acute toxicity of morphine and 1-[2-(4-amino-phenyl)ethyl]-
4-phenyl-4-piperidine carboxylic acid ethyl ester (anileridine) than
rats held in "open" (wire mesh) cages. These differences were
attributed to mechanical factors that prevented depressed rats from
maintaining an open airway in the wire mesh cages. Altered toxicity of
chemicals related to caging effects are generally purely experimental
variables and can be controlled by good practices of laboratory animal
housing.
2.4.4.3 Diet and nutritional status
Dietary variables can influence the toxicity of chemicals in
several ways. The toxicities of several pesticides were enhanced to
different degrees in rats given low protein diets (Boyd, 1969).
Protein-deficient diets protected rats against the acute
hepatotoxicity of carbon tetrachloride and N-methyl-
N-nitrosomethanamine (dimethylnitrosamine) (although the number of
kidney tumours after a single dose of the latter increased), while the
acute toxicity of chloroform was unchanged and the acute toxicity of
aflatoxins was markedly enhanced (McLean & McLean, 1969). These
effects could be explained, at least in part, by the reduction of
activity of hepatic mixed-function oxidases that generally results
from feeding low-protein diets. Whether or not a compound's toxicity
is increased or decreased in such circumstances will depend upon
whether microsomal biotransformation leads to the formation of more or
less toxic metabolites. Numerous other examples of macro and
micronutrient deficiencies, which alter the activity of the
drug-metabolizing enzyme systems and the toxicity of chemicals, have
been reviewed by Campbell & Hayes (1974). Intestinal and pulmonary
aryl hydrocarbon hydroxylase activity is modified by diet. Of
particular interest is the observation that changing rats from a
commercial, natural diet to a balanced, purified diet resulted in an
almost total loss of activity of this enzyme system in these tissues
(Wattenberg, 1972). Flavonoid compounds, present as natural
constituents of alfalfa meal (and other plants), may account for the
apparent induction of aryl hydrocarbon hydroxylase by natural diets.
The trace mineral content of diets can also influence the metabolism,
distribution, and action of toxic chemicals (Moffitt & Murphy, 1974).
The results of toxicity experiments can be markedly influenced if
care is not taken to ensure constancy of diets free from residues of
contaminating chemicals. However, since nutritional imbalances are
widespread in the human populations, controlled variation of
experimental diets to simulate major human deficiency states (e.g.
kwashiorkor resulting from protein deficiency) is an important area
for research in toxicology and should, perhaps, be included in
standard toxicity evaluations of select groups of chemicals.
2.5 Statistical Considerations
Although various protocols for toxicity testing recommend
specific numbers of animals to be used for various acute and chronic
tests (see Chapter 3), a useful guiding principle is that sufficient
animals should be used to allow statistically valid conclusions
concerning differences in the response of test animals compared to
controls and to provide a base for statistical extrapolations to
larger population samples. Statistical procedures allow the
experimenter to make ( a) descriptions of sets of data or population
characteristics, and ( b) statements of probability of events.
Various procedures provide for both enumerative data, or yes-no type
characteristics, and measurement data, or graded effects or
characteristics. Some procedures (t-test, F-test) are restricted to
observations that have specific frequency distributions, while others
(signed rank test, rank run test) are free of any assumptions about
distribution (i.e. nonparametric). Standard texts should be consulted
for the application of biostatistics to the design and analyses of
experiments. In practice, it is highly advisable to involve a
statistician in the experimental design as well as in the analysis.
The number of animals required to make statistically valid
conclusions regarding the differences between experimental and control
animals will depend upon the degree of confidence desired and the
magnitude of the possible sources of variation in the experiment. The
second consideration will depend upon the uniformity of the test
animals with respect to the biological system or systems under test.
This, in turn, will depend upon both genetic and environmental
factors. The reproducibility of the bioassay and chemical procedures
used in the tests will be another source of variation. A further
source of variation in toxicity testing is related to the constancy
and stability of the test chemical. Finally, there are the variables
introduced by the investigators (often the most difficult to control),
beginning with the care and attention given to accurate dosing
throughout the various steps of the experiment. Attempts must be made
to minimize all these sources of variation as far as possible, without
sacrificing any important aspect of the experiment.
When testing whether or not two sets of data may both be valid
samples from the same population with normal frequency distribution
(i.e. null hypothesis) or whether the control group is not
significantly different from the treatment group, statisticians
describe the appropriate sample size in terms of the desired "power"
of the test. Two types of decision errors exist. One is that a
significant difference between groups is stated to exist when, in
fact, there is no difference. This is called the type I error and the
experimenter must state what probabilities (alpha) for this error he
will accept; most commonly a probability of 0.05 is used, but an
experimenter may sometimes require a probability as low as 0.01, or
any other he chooses. The second type of decision error is that no
significant difference between groups is stated to exist when, in
fact, the groups are different. This is called the type II error, and
1-ß (the probability that one will not make this error) is the power
of the test. The power is directly related to the sample size and the
ratio of "differences between true means of the samples" to
"differences between experimental error of the means of the samples".
Once this ratio is fixed, the power increases solely as a function of
sample size. The experimental error (pooled variance) can be estimated
from previous experiments or a pilot study. The acceptable magnitude
of the differences between true means of the samples is at the
discretion of the experimenter; he must use expert judgment and should
have a reasonable rationale; it will usually be the smallest value
that is considered to be of practical importance.
Another important statistical consideration is related to the
selection of the valid number of sampling units. This may be
particularly true in considering quantal (all-none, yes-no) effects
that may be multiple occurrences within a single test animal, as, for
example, in reproduction and carcinogenesis studies where,
respectively, there may be a number of affected offspring or a number
of tumours resulting from the treatment of a single animal. The
selection of the appropriate unit, either the number of animals
exposed or the number of occurrences of an effect, can determine the
statistical significance of an observed effect. Weil (1970) suggests
that in reproduction studies the number of maternal animals (or
litters) and not the number of affected fetuses or offspring is the
valid sampling unit, and that in carcinogenesis studies the number of
tumour-bearing animals should be the sampling unit and not the total
number of tumours. Furthermore, in carcinogenesis studies, animals
risk death from factors other than the tumours; in some cases, animals
may have died before they had time to develop a tumour; in other
cases, information from some animals may be lost from the study
because of unexpected death and autolysis of tissues preventing tumour
identification. An adjusted tumour incidence may be estimated by the
life-table techniques in such experiments (McKinney et al., 1968).
Another problem may be associated with gross or histopathological
examination where, because of cost and time considerations, only
tissues of a fraction of the total number of animals exposed are
subjected to complete examination. This will reduce the likelihood
that statistically valid conclusions can be drawn from the data on
occurrence of lesions. In practice, reasonable compromises are usually
necessary. Irrespective of the statistical methods of analysis used in
both the design and interpretation of results of toxicity tests on
chemicals, they cannot replace careful experimentation and
comprehensive knowledge of the underlying biological mechanisms of the
various steps between exposure to a chemical and injury.
2.6 Nature of Effects
2.6.1 Reversible and irreversible effects
Reversible effects are characterized by the fact that the
deviation from normal structure or function induced by a chemical will
return to within normal limits (controls) following cessation of
exposure. With irreversible effects, the deviation persists or may
progress, even after exposure ceases. This might be further qualified
by time limits, that is, the time required for return to normality
after exposure should be a reasonable fraction of the remaining
lifetime of a young animal for it to be considered reversible.
Reversibility may also be qualified by the normal lifetime of a
specific cell or macromolecule that serves as the end-point for the
effect. For example, cholinesterase-inhibiting insecticides are
generally considered irreversible inhibitors if the rate of reversal
of inhibition corresponds approximately to the time required for
synthesis and replacement of the enzyme, a process with different
rates in different tissues. Certain effects of toxic chemicals are
unmistakably irreversible, including the production of terata, or
malignant tumours, production of mutations in offspring of exposed
animals, certain chronic neurological diseases, production of true
cirrhosis, or emphysema. These are rather gross manifestations of
certain specific chemical-cell interactions, and, either at the level
of the first affected molecule or at intervening points leading to
these manifestations, there are probably reversible effects.
Understanding these effects and determining the critical dose that
produces them will make it possible to predict truly adverse effects
more rapidly.
The rate of reversibility of an effect will depend upon the rate
of cellular injury and the rate at which this injury is repaired
(Casarett, 1975). The rate of injury will depend upon the
concentration and duration or frequency with which a test chemical
contacts responsive tissue constituents. It is, thus, dose and
dose-rate dependent. The rate of repair is determined intrinsically
and may involve several cell processes. It may vary between different
tissues and probably between different species and strains. From a
practical standpoint, it is generally impossible to measure the
specific processes involved in injury and repair in a standard
toxicity evaluation study. However, it is important to make
measurements of the reversibility of effects in early, acute and
subacute studies. Thus, the time required for a process to return to
normal after single doses (which produce various degrees of injury)
will provide a guideline for the selection of doses to be used in
subsequent acute or chronic studies. The predictive value of such
information will depend upon the persistence of the chemical in the
test organism. If the chemical produces an effect and then is rapidly
detoxified or excreted, it may be possible to predict, with reasonable
accuracy, doses or exposure schedules that would not produce
cumulative effects. The manner of exposure and possible actions other
than the one being measured would, of course, be important in drawing
such conclusions. For example, rapid reversibility after a single dose
might not be indicative of the rate of reversal with a repeated dosing
if the first dose, in addition to the measured effect, also altered
either the repair processes or the processes responsible for
detoxification of the chemical. An example of an apparent
self-inhibition of detoxification is the insecticide malathion which
is rapidly hydrolysed by carboxylesterases. These are, in turn,
inhibited by metabolites or contaminants of malathion (Murphy, 1967).
Repeated exposure studies are necessary to evaluate such
possibilities; thus, the design of short-term feeding or inhalation
studies should include extra groups of animals that can be removed
from exposure either at the end of the experiment or, preferably, at
selected intervals for measurements of rate of reversal of any
observed effect.
If the chemical persists or accumulates in the organism,
measurements and interpretation of rates of reversal of effects are
more complicated. For this reason, it is useful to have kinetic data
on absorption and disposition to correspond with data on rates of
production and reversal of effects. Further discussion of these and
related principles is provided by Hayes (1972) in relation to his
proposal that determination of "chronicity factors" (1-dose LD50
(mg/kg) ‰ 90-dose LD50 (mg/kg/day) in diet i.e. the ratio of single
dose LD50 to the daily dose given in diet for 90 days which results
in 50% mortality at that time) is useful in predicting candidate
chemicals requiring long-term studies. The use of such predictive
methods must also take into consideration potential for other effects
that could never be detected in a subacute study (e.g. tumorigenesis).
2.6.2 Functional versus morphological changes
Toxic effects are often classed as functional or morphological in
nature. There has been a traditional attitude that changes in gross or
microscopic structure are more serious than functional changes.
Indeed, altered structure often seems to have taken on the implication
of irreversibility while altered function is often considered a
reversible effect. This conclusion, of course, depends on the level of
understanding of mechanisms of injury, rates and mechanisms of repair,
and causal associations between related functional and morphological
changes. Furthermore, whether the change is regarded as functional or
morphological may depend on the manner of detection. For example,
accumulation of fat in a cell observed through a microscope will most
often be considered a structural change, but, if the same cells or
tissues were assayed for triglyceride content, the increased
triglyceride would probably be classified as a functional or
biochemical change. The introduction of enzyme histochemistry and
electron microscopy into toxicity evaluation studies makes the
distinction between morphological and functional effects even less
clear. It may therefore be inappropriate to attempt to make these
distinctions.
Rowe et al. (1959) reviewed data from studies on a large number
of chemicals repeatedly administered to animals over periods ranging
from one month to two years, and summarized the frequency with which a
certain effect was found and the frequency with which it was the only
effect found. The effects were considered on: mortality; food intake;
body weight; organ weights; the histopathology of virtually every
major organ; haematology; blood urea nitrogen; clinical urinalyses;
central nervous system (most probably gross behaviour); gross
pathology; and cholinesterase activity. The authors found that if
growth, liver weight, kidney weight, liver pathology, and kidney
pathology had been studied, the lowest dose level that caused any
effect would have been detected in 96% of the studies. Changes in food
intake, central nervous system depression, excessive mortality,
increased lung weight, testicular injury, haematological changes, and
cholinesterase depression were the most sensitive effects in one or
more of the remaining 4% of cases. The reader should consult the
original reference for details but it is important to note that of the
commonly used criteria of effects, liver and kidney micropathology
were quite sensitive indices.
There are, of course, well-known examples where functional
changes are the only manifestations of toxicity. Many of the
organophosphate and carbamate insecticides inhibit cholinesterase
(3.1.1.8) activity and produce signs and symptoms (even death) that
can be characterized as purely functional without the production of
morphological lesions, detectable by conventional techniques.
Similarly, irritant air pollutants can often cause bronchoconstriction
and respiratory distress, without any accompanying morphological
changes. Both the functional cholinergic signs and symptoms produced
by the anticholinesterase insecticides and the bronchoconstriction
produced by irritants provide means of early detection at low levels
of exposure. Although these effects are reversible, they are no less
important during the period of exposure than certain kinds of
morphological effects. On the other hand, certain kinds of
"functional" changes, e.g. increased level of plasma transaminase
activity, usually reflect some type of structural change in cells that
allow these enzymes to "leak" into plasma (Cornish, 1971).
It is not possible, with present knowledge, to conclude that
either functional or morphological changes represent the most
sensitive, the earliest, or the most serious effects of toxic
chemicals. Since maintenance of both integrated function and
integrated structure ultimately depends on chemical reactions among
cell constituents, it is logical to conclude that specific biochemical
changes are the first and most sensitive effects. Unfortunately, with
relatively few exceptions, the specific biochemical receptors for
toxic chemicals are unknown. The more information that can be obtained
with respect to time- and dose-relationships for functional and
morphological effects, the more predictive the tests will become. This
requires an approach to toxicity studies in which proof of a mechanism
will require an integrated biochemical, physiological, and
morphological approach. Dawkins & Rees (1959) provide a useful short
treatise on an integrated biochemical-pathological approach to studies
of several toxic chemicals. Although advances in both biochemistry and
pathology now allow even more precise studies than those outlined by
these authors, the general principles which they develop are still
applicable.
2.7 Dynamic Aspects of Predictive Toxicology
2.7.1 Traditional versus new techniques
The objective of any toxicity test programme is prediction:
prediction of biological disposition from physicochemical constants,
prediction of altered cell or organ system function from reaction with
macromolecules, prediction of irreversible consequences of reversible
changes, prediction of implications of selected measurable variables
to overall health and survival of the test organisms, prediction of
effects in individuals of one species from tests conducted in another,
and finally predictions of incidence in large populations from tests
on small samples. All of these predictions must be related
quantitatively to a dose and dose-rate or schedule that can ultimately
be related to probable amounts used, the manner of use or the
occurrence of the chemicals in the environment.
Generally, traditional approaches to toxicity evaluation have not
attempted to make predictions far removed from the final application
or interpretation of the data. Thus, as outlined in Chapter 3, test
organisms are exposed to a range of doses and their health status is
examined by biochemical, physiological, or pathological procedures
analogous to those used in clinical medicine. When this approach has
been comprehensive, judicious application of the data usually appears
to have been successful in preventing chemically-induced disease.
Abandoning this approach in favour of new, different, or short-cut
methods cannot be advocated without thorough verification of their
validity. On the other hand, serious consideration must be given to
the application of some short-term ways of predicting toxicity in
order to provide a practical means of evaluating the many chemicals
already in the environment and those new compounds that are
continuously being added to the environment and have not been
subjected to traditional tests. Preceding sections have discussed some
possibilities, the following sections contain brief comments and
examples for consideration in selecting tests.
2.7.2 Toxicity of chemical analogues
Although it may be possible to predict the toxicity of individual
compounds in a homologous series from detailed knowledge of some
members of the series, some special exceptions should be noted. A
classical example involves the series of fluorine-substituted
aliphatic alcohols and acids, in which high acute toxicity alternates
with odd and even numbers of carbon atoms, the latter being the most
toxic (Pattison, 1959). The odd number of total carbon atoms confers
high toxicity in a homologous series of fluoronitriles, however. This
demonstrates the possibility that detailed information concerning only
a few members of a homologous series might fail to predict the
toxicity of another member of the series.
Recently, Johnstone et al. (1974) examined a number of
biochemical effects in a series of isomerically pure compounds for
their potency as liver enzyme inducers. Potency for this effect
increased with increasing chlorination that was related to differences
in biotransformation and excretion rates; however, there were also
striking differences in the potency of positional isomers in the lower
chlorinated biphenyls.
The mechanism of the toxic action of organophosphorus
insecticides was known to be the inhibition of acetylcholinesterase
even before they were introduced into use 30 years ago. However,
quantitative prediction of their acute toxicity from in vitro tests
of their relative potency as anticholinesterases is still inadequate
because of incomplete knowledge of the dynamic relationships between
several pathways of metabolism which yield both more and less potent
metabolites (Murphy, 1975). Nevertheless, these compounds have been
subjected to a great deal of research on both their physicochemical
and biological properties which should be applicable to predictions of
their relative environmental persistence, interaction with other
compounds, and, ultimately, to the design of safe molecules (Eto,
1974).
Using model ecosystems, Lu & Metcalf (1975) studied
bioaccumulation, biodegradability, and comparative detoxification
mechanisms in several benzene derivatives with widely-varying
physicochemical constants and biological activities. They concluded
that biological disposition and action could be predicted by the basic
molecular properties of water solubility, the partition coefficient
for lipid/water, and reactivity as determined by electron density.
Johnson (1975) recently reviewed the problems encountered in the
pursuit of the mechanism of delayed peripheral neuropathy produced by
some organophosphorus esters. The structure-activity relationships
identified in this research may be considered as a model of thorough
investigation that began as a problem in retrospective toxicology and
led to promising developments applicable to prospective toxicology.
Some interesting aspects of this problem are: the particular
usefulness of a non-mammalian species, the hen, as a predictor of a
toxic action that occurs in man; the concept of primary metabolic
effects on central neurons as a precursor to pathological change
detected in peripheral nerve fibres; and the difficulties of detecting
a specific critical esterase inhibition that represented only a small
percentage of the total esterase activity. Production of peripheral
neuropathy appears to be characteristic of organophosphorus esters
which may not only phosphorylate the specific "neurotoxic esterase"
but are also capable of dealkylation (or aging) following
phosphorylation. Although the steps between this primary
phosphorylating-aging process and the eventual manifestation of
peripheral neuropathy are still unclear, it appears that it may be
possible to predict probable occurrence of a delayed, chronic disease
from studies of the primary chemical-macromolecular interaction of
neurotoxic esterase inhibition that occurs immediately following
exposure.
2.7.3 Relation between site of metabolism and site of injury
Although for many years it was thought that the biotransformation
of organic chemicals represented detoxification mechanisms, it is now
apparent that numerous compounds are enzymatically converted to
intrinsically more active compounds in vivo (Fouts, 1972; Murphy,
1975; Parke, 1968). The liver is generally the most active tissue in
catalysing these "activation" reactions, but it is not always the most
susceptible target tissue as, for example, in the case of activation
of phosphorothioate insecticides to phosphate insecticides. This may
be explained, in part, by the presence of detoxifying enzymes or
reactive but noncritical binding sites in the liver that may prevent
the phosphates from escaping to inhibit cholinesterase in nerve target
tissues. The brain tissue has only a small fraction of the liver's
capacity to activate phosphorothioates, but because the activation
occurs in the same tissue as the critical target site, activation in
the brain may be the most important in determining toxicity.
Recently, the characteristic hepatotoxicity of several chemicals
has been related to their enzymatic conversion to highly reactive
derivatives that covalently bind to essential liver cell constituents
at, or near, the site of activation (Brodie et al., 1971). A similar
possibility may explain the bronchiolar neurosis produced in rats and
mice by bromobenzene and other aromatic hydrocarbons (Reid et al.,
1973).
The detailed study of the metabolism, storage, or binding and
distribution of foreign chemicals in the lung is a relatively recent
activity and has focused largely on therapeutic chemicals (Bend et
al., 1973; Brown, 1974; Orton et al., 1973). Because inhalation is a
common route of exposure to a wide variety of air contaminants in
industrial and community environments, future toxicity studies would
benefit by the inclusion of metabolic studies concerning rates of
absorption from, and local actions in the lung. Witschi (1975) has
reviewed biochemical approaches that may be used in the evaluation of
toxic injury to the lung.
Since the intensity and duration of the toxic action of a
chemical depends on the concentration of the active form at critical
receptor sites of action, kinetic aspects of absorption, distribution,
and excretion (as well as biotransformation) will influence the
specific sites of action. This topic is discussed in detail in Chapter
4 but it is worthy of note that Dedrick (1973) developed several
useful kinetic models that might be applied in predicting species
differences or similarities in response.
2.7.4 In vitro test systems
Where appropriate, studies in experimental animals should be
supplemented by isolated perfused organ, tissue slice or extract, and
tissue culture techniques. Where possible, attempts should be made to
compare the tissues of human subjects available from autopsy or
therapeutic biopsies, with those of other species in their response to
toxic chemicals (Worden, 1974). When the mechanism(s) of toxicity have
been elucidated and the target organ(s) identified, specific species
comparisons and dose-response relationships can be studied by these
in vitro techniques.
Knowledge of a specific enzyme or biological macromolecule that
serves as a target for reaction with toxic chemicals may provide a
means for screening and predicting relative potencies or specific
actions of chemicals in intact organisms. However, as pointed out
previously for the organophosphorus insecticides, biotransformations
and membrane barriers to the distribution of chemicals in intact
animals will often invalidate conclusions drawn from in vitro
assays. This problem may be partly overcome by incorporating enzymic
biotransformation systems with the target macromolecule (or organism)
in the in vitro test system. Such an approach is used for screening
for potential mutagens in microorganism test systems (Malling &
Frantz, 1973) and has been applied to studies of the biochemical
actions of pesticides (Chow & Murphy, 1975; Cohen & Murphy, 1974). A
major problem in the use of in vitro test systems for predicting
toxicity is the difficulty of quantitatively relating concentrations
in the simplified in vitro systems to action in complex intact
organisms. With adequate correlative data in both in vitro and
in vivo systems this may become possible, but such information is
generally lacking at present. For the most part, therefore, in vitro
model test systems are qualitative predictors rather than
quantitative. This need not decrease their usefulness, however, as
long as this limitation is recognized in the interpretation of
results.
In general, in vitro test systems have been useful in
qualitatively predicting acute actions. However, as discussed earlier,
neurotoxic esterase inhibition provides promise for predicting delayed
chronic neuropathy produced by certain organophosphate compounds.
Major research efforts are now being devoted to in vitro test
systems for the prediction of mutagenesis and carcinogenesis. These
are discussed in detail in Chapter 7. There is general recognition of
the value of these test systems (Council of Environmental Mutagen
Society, 1975; Food & Drug Administration, 1970; Food Protection
Committee, 1970) as screening procedures, but much less agreement as
to the priority that they should have in the conduct and
interpretation of toxicity evaluations.
When it is possible to obtain comparisons between exposures of
organs or tissues of experimental animals and humans to toxic
chemicals, such comparisons will provide useful baseline data for the
future extrapolation of data from intact animal studies to man.
Culture systems of human cells may also be useful as comparative
systems. The difficulties of maintaining some human cell lines are
well documented, but primary cultures of differentiated mammary and
liver epithelia have been established and maintained (Buehring, 1972;
Lasfargues & Moore, 1971; Potter, 1972). Human lymphocytes have also
been used in vitro (Kellermann et al., 1973).
It may be possible to use these isolated systems to determine the
susceptibility to toxic chemicals of different cell types in different
organs and to determine the reversibility of adverse effects in these
cell lines and organs. Of particular usefulness would be the
determination of dose-response curves for many tissues and their
interspecies comparison. Such information could be used to predict
target cells and organs with a high degree of susceptibility or
resistance. However, as mentioned earlier, the usefulness of the data
may be limited if the cells, tissues, or organs are incapable of
metabolizing the chemical to a toxic form in the intact animal. Such a
biotransformation may even occur in a different tissue or organ from
the one under test in vitro. To overcome this problem,
biotransformation systems from animal or human tissues (e.g.
microsomal activating systems) are often added with the chemical to
the isolated culture systems.
One problem with these methods is the uncertainty that all the
steps of metabolism are equally duplicated, that is, in addition to
activation of a chemical there should also be an opportunity for the
chemical to be detoxified or conjugated and eliminated. Some of these
detoxification steps require different coenzymes or metabolites, and
the enzyme systems may not be limited to microsome fractions or liver
tissue. However, as long as it is realized that these in vitro test
systems may exaggerate the situation that occurs in vivo they can
prove useful, especially for tests where only very small quantities of
material are available, as might be the case with some impurities or
metabolites.
In summary, short-term, in vitro tests both for carcinogenicity
and other forms of toxicity show great promise (Golberg, 1974), and
although no single test is likely to be reliable, appropriate
combinations may provide valuable information concerning the
fundamental toxicity of environmental chemicals. This would currently
provide a useful adjunct to long-term studies in animal populations,
and may develop further in the future to provide the more reliable
method of assessment. Such tests will require much development and
will take years to validate and perhaps even longer to win public
confidence with regard to their reliability.
REFERENCES
ABINANTI, R. R. (1971) Chronic and degenerative diseases of man: the
value of natural and experimentally induced diseases of animals.
In: Animal models for biomedical research IV, Proceedings of a
Symposium, Washington DC, National Academy of Sciences,
pp. 31-46.
ALTMAN, P. L. & DITTMER, D. S., ed. (1966) Environmental biology,
Bethesda, MD, Federation of American Societies for Experimental
Biology, pp. 565-608.
ALVARES, A. P., LEIGH, S., KAPPAS, A, LEVIN, W., & CONNEY, A. H.
Induction of aryl hydrocarbon hydroxylase in human skin. Drug
Metabol. Disposition, 1: 386-390.
BARNES, J. M. (1968) Percutaneous toxicity. In: Boyland, E. &
Goulding, R., ed. Modern trends in toxicology, London,
Butterworths, pp. 18-38.
BEND, J. R., HOOK, G. E., & GRAM, T. E. (1973) Characterization of
lung microsomes as related to drug metabolism. Drug Metabol.
Disposition, 1: 358-367.
BENKE, G. M. & MURPHY, S. D. (1975) The influence of age on the
toxicity and metabolism of methyl parathion and parathion in male
and female rats. Toxicol. appl. Pharmacol., 31: 254-269.
BEUTHIN, P. K. & BOUSQUET, W. F. (1970) Long-term variation in basal
and phenobarbital-stimulated oxidative drug metabolism in the
rat. Biochem. Pharmacol., 19: 620-625.
BOYD, E. M. (1969) Dietary protein and pesticide toxicity in male
weanling rats. Bull. World Health Org., 40: 801-805.
BOYD, E. M. (1972) Predictive toxicometrics, Baltimore, Williams &
Wilkins, 408 pp.
BRODIE, B. B., CHO, A. K., KRISHNA, G., & REID, W. D. (1971) Drug
metabolism in man: past, present and future. Ann. NY Acad. Sci.,
179: 5-18.
BROWN, E. A. B. (1974) The localization, metabolism and effects of
drugs and toxicants in lung. Drug Metabol. Rev., 3: 33-87.
BUEHRING, G. C. (1972) Culture of human mammary epithelial cells:
keeping abreast with a new method. J. Natl Cancer Inst.,
49: 1433-1434.
CAMPBELL, T. & HAYES, J. R. (1974) Role of nutrition in the
drug-metabolizing enzyme system. Pharmacol. Rev., 26: 171-197.
CASARETT, L. J. (1975) Toxicologic evaluation. In: Casarett, L. J. &
Doull, J., ed. Toxicology -- the basic science of poisons, New
York, Macmillan, pp. 11-25.
CLARKSON, T. B., PRICHARD, R. W., BULLOCK, B.C., LEHNER, N. D. M.,
LOFLAND, H. B. & CLAIR, R. W. St. (1970) Animal models for
atherosclerosis. In: Animal models for biomedical research III.
Proceedings of a Symposium, Washington DC, National Academy of
Sciences, pp. 22-41.
CHOW, A. Y. K. & MURPHY, S. D. (1975) Production of a
methemoglobin-forming metabolite of 3,4-dichloroaniline by liver
in vitro. Bull. environ. Contam. Toxicol., 13: 9-13.
COHEN, S. D. & MURPHY, S. D. (1974) A simplified bioassay for
organophosphate detoxification and interactions. Toxicol. appl.
Pharmacol., 27: 537-550.
COMMITTEE ON PROBLEMS OF DRUG SAFETY (1969) Application of metabolic
data to the evaluation of drugs: report prepared by the Committee
on Problems of Drug Safety of the NAS/NRC Drug Research Board.
Clin. Pharmacol. Therap., 10: 607-634.
CORNISH, H. H. (1971) Problems posed by observations of serum enzyme
changes in toxicology. CRC Crit. Rev. Toxicol., 1: 1-132.
COUNCIL OF EUROPE (1973) Guide to the testing and toxicological
evaluation of flavouring substances. In: Natural flavouring
substances, their sources and added artificial flavouring
substances, Strasbourg, Council of Europe, pp. 403-410.
COUNCIL OF ENVIRONMENTAL MUTAGEN SOCIETY (1975) Environmental
mutagenic hazards. Science, 187: 503-514.
CREMER, J. E. & BLIGH, J. (1969) Body-temperature and response to
drugs. Brit. med. Bull., 25(3): 299-306.
CROSBY, D. G. (1972) Environmental photooxidation of pesticides. In:
Proceedings of a conference on degradation of synthetic organic
molecules in the biosphere, San Francisco, June 1971,
Washington DC, National Academy of Sciences, pp. 260-278.
DAVIS, W. M. (1962) Day-night periodicity in pentobarbital response of
mice and the influence of socio-physiological conditions.
Experientia (Basel), 18: 235-237.
DAWKINS, M. J. R. & REES, K. R. (1959) A biochemical approach to
pathology, London, Edward Arnold, 128 pp.
DEDRICK, R. L. (1973) Animal scale-up. J. Pharmacokin. & Biopharm.,
1: 435-461.
DHEW (1972) Guide for the care and use of laboratory animals
(prepared by Committee on Revision of the Guide for Laboratory
Animals and Care, ILAR, NRC). Washington DC, US Gov. Print.
Office (DHEW Publ. No. (NIH) 72.23).
DIGGLE, W. M. & GAGE, J. C. (1951) Cholinesterase inhibition in vivo
by O,O-diethyl O-p-nitrophenyl thiophosphate (Parathion
E 605). Biochem. J., 49: 491-494.
DILLINGHAM, E. O., MAST, R. W., BASS, G. E., & AUTIAN, J. (1973)
Toxicity of methyl- and halogen substituted alcohols in tissue
culture relative to structure-activity models and acute toxicity
in mice. J. Pharm. Sci., 62: 22-30.
DOULL, J. (1972) The effect of physical environmental factors on drug
response. In: Hayes, W. J., ed. Essays in toxicology, New York,
Academic Press, Vol. 3, pp. 37-63.
DOULL, J. (1973) Factors influencing toxicity. In: Casarett, L. J. &
Doull, J., ed. Toxicology -- the basic science of poisons, New
York, Macmillan, pp. 133-147.
EASTON, R. E. & MURPHY, S. D. (1967) Experimental ozone pre-exposure
and histamine: effect on the acute toxicity and respiratory
function effects of histamine in guinea pigs. Arch. environ.
Health, 15: 160-166.
EHRLICH, R. (1966) Effect of nitrogen dioxide on resistance to
respiratory infection. Bact. Rev., 30: 604-614.
ETO, M. (1974) Organophosphorus pesticides: organic and biological
chemistry. Cleveland, OH, CRC Press, pp. 387 (57-78).
FOOD & DRUG ADMINISTRATION (1959) Appraisal of the safety of
chemicals in foods, drugs and cosmetics. Austin, Texas,
Association of Food & Drug Officials of the USA, pp. 107.
FOOD & DRUG ADMINISTRATION (1970) Report on reproduction studies in
the safety evaluation of food additives and pesticide residues
(Report of panel on reproduction of the FDA Advisory Committee on
Protocols for Safety Evaluation). Toxicol. appl. Pharmacol.,
16: 264-269.
FOOD PROTECTION COMMITTEE (1970) Evaluating the safety of food
chemicals, Washington, DC, National Academy of Sciences, 55 pp.
FOUTS, J. R. (1972) Some studies and comments on hepatic and
extrahepatic microsomal toxication-detoxification system.
Environ. Health Perspect., Experimental Issue No. 2, Oct.
pp. 55-66.
GOLBERG, L. (1967) The amelioration of food (The Milroy Lectures).
J. Roy. Coll. Phys., 1 (4): 385-426.
GOLBERG, L. (1974) Short-term predictive tests. Proc. Eur. Soc. Drug
Toxicity, 15: 178-191.
HACKEL, D. B., MIKAT, E., LEBOVITZ, H., & SCHMIDT-NIELSEN, K. (1968)
Animal models for diabetes mellitus with special reference to the
sand rat (Prommomys obesus). In: Animal models for biomedical
research: Proceedings of a Symposium, Washington, DC, National
Academy of Sciences, pp, 14-20.
HANSCH, C. & DUNN, W. J. (1972) Linear relationships between
lipophilic character and biological activity of drugs.
J. pharm. Sc., 61: 1-18.
HATCH, A., BALEZO, T., WIBERG, G. S., & GRICE, H. C. (1965) The
importance of avoiding mental suffering in laboratory animals.
Anim. Welfare Inst. Rep., Vol. 14, No. 3.
HATCH, T. F. & GROSS, P. (1964) Pulmonary deposition and retention of
inhaled aerosols, New York, Academic Press, 184 pp.
HAYES, W. J. (1972) Tests for detecting and measuring long-term
toxicity. In: Hayes, W. J., ed. Essays in toxicology, New York,
Academic Press, Vol. 3, pp. 65-77.
HEIMANN, H. (1967) Status of air pollution health research. Arch.
environ. Health. 14: 488-503.
HENDERSON, Y. & HAGGARD, H. W. (1943) Noxious gases and the
principles of respiration influencing their action, New York,
Reinhold, 287 pp.
HOLMSTEDT, B. (1959) Pharmacology of organophosphorus cholinesterase
inhibitors. Pharmacol. Rev., 11: 567-688.
HURNI, H. (1970) The provision of laboratory animals. In: Paget, G.
E., ed. Methods in toxicology, Philadelphia, F. A. Davis,
pp. 11-48.
JAEGER, R. J., CONOLLY, R. B., & MURPHY, S. D. (1973) Diurnal
variation of hepatic glutathione concentration and its
correlation with 1,1-dichloroethylene inhalation toxicity in
rats. Res. Commun. Chem. Pathol. Pharmacol., 6: 465-471.
JAEGER, R. J., CONOLLY, R. B., & MURPHY, S. D. (1975) Short-term
inhalation toxicity of halogenated hydrocarbons. Arch. environ.
Health, 30: 26-31.
JOBE, C. L. (1968) Selection and development of animal models of
myocardial infarction. In: Proceedings of a Symposium on Animal
Models for Biomedial Research, Washington, DC, National Academy
of Sciences, pp. 101-108.
JOHNSON, M. K. (1975) The delayed neuropathy caused by some
organophosphorus esters: mechanism and challenge. CRC Crit. Rev.
Toxicol., 3: 289-316.
JOHNSTONE, G. J., ECOBICHON, D. J., & HUTZINGER, O. (1974) The
influence of pure polychlorinated biphenyl compounds on hepatic
function in the rat. Toxicol. appl. Pharmacol., 28: 66-81.
JONES, T. C. (1969) Mammalian and avian models of disease in man.
Fed. Proc., 28 (1): 162-169.
JONES, W. I., ROBACK, L. A., & TAYLOR, J. M. (1971) The loss of food
flavours from laboratory animal diets. II. Effect of laboratory
environment. J. Assoc. Offic. Anal. Chem., 54: 42-46.
JORI, A., PUGLIATTI, C., & PESCADOR, R. (1971a) Rat strain differences
in the activity of hepatic microsomal enzymes. Biochem.
Pharmacol., 20: 2695-2701.
JORI, A., SALLE, E., & SANTINI, V. (1971b) Daily rhythmic variation
and liver drug metabolism in rats. Biochem. Pharmacol.,
20: 2965-2969.
KLASSEN, C. D. (1975) Absorption, distribution and excretion of
toxicants. In: Casarett, J. L. & Doull, J., ed. The basic
science of poisons, New York, Macmillan, pp. 26-44.
KELLERMAN, G., LUYTEN-KELLERMAN, M. L., & SHAW, C. R. (1973) Genetic
variation of aryl hydrocarbon hydroxylase in human lymphocytes.
Am. J. Hum. Genet., 25: 327-331.
LA DU, B. N., MANDEL, G., & WAY, E. L., ed. (1971) Fundamentals of
drug metabolism and drug disposition, Baltimore, Williams &
Wilkins, 615 pp.
LAKE, B. G., HOPKINS, R., CHAKRABORTY, J., BRIDGES, J. W., & PARKE, V.
W. (1973) The influence of some hepatic enzyme inducers on
extrahepatic drug metabolism. Drug Metabol. Disposition,
1: 342-349.
LASFARGUES, E. Y. & MOORE, D. H. (1971) A method for the continuous
cultivation of mammary epithelium. In Vitro, 7: 21-25.
LERNER, R. A. & DIXON, F. J. (1968) Experimental and human
glomerulonephritis associated with antiglomerular basement
membrane antibodies. In: Proceedings of a Symposium on Animal
Models for Biomedical Research, Washington, DC, National Academy
of Sciences, pp. 109-130.
LJUBLINA, E. I. & FILOV, V. A. (1975) Chemical structure, physical and
chemical properties and biological activity. In: Methods used in
the USSR for establishing biologically safe levels of toxic
substances, Geneva, WHO, pp. 19-44.
LOOMIS, T. A. (1974) Essentials of toxicology, 2nd ed.,
Philadelphia, Lea & Febiger, 233 pp.
LU, P. Y. & METCALF, R. (1975) Environmental fate and biodegradability
of benzene derivatives as studied in a model aquatic ecosystem.
Environ. Health Perspect, 10: 269-284.
LUGINBUHL, H. & DETWEILER, D. K. (1968) Animal models for the study of
cerebrovascular disease. In: Proceedings of a Symposium on
Animal Models for Biomedial Research, Washington DC, National
Academy of Sciences, pp. 35-41.
LUKAS, G., BRINDLE, S., & GREENGARD, P. (1971) The route of absorption
of intraperitoneally administered compounds. J. Pharmacol. exp.
Therap., 178: 562-566.
MCLEOD, S. M., RENTON, K. W., & EADE, N. R. (1972) Development of
hepatic microsomal drug-oxidizing enzymes in immature male and
female rats. J. Pharmacol. exp. Therap., 183: 489-498.
MALLING, H. V. & FRANTZ, C. N. (1973) In vitro vs in vivo
metabolic activation of mutagens. Environ. Health Perspect.,
6: 71-82.
MCCREESH, A. H. (1965) Percutaneous toxicity. Toxicol. appl.
Pharmacol., 7: 20-26.
MCKINNEY, G. R., WEIKEL, J. H., WEBB, W. K., & DICK, R. J. (1968) Use
of life-table technique to estimate effects of certain steroids
on probability of tumour formation in a long-term study in rats.
Toxicol. appl. Pharmacol., 12: 68-79.
MCLEAN, A. E. M. & MCLEAN, E. K. (1969) Diet and toxicity. Brit. med.
Bull., 25: 278-281.
MEETER, E. & WOLTHUIS, O. L. (1968) The effects of cholinesterase
inhibitors on the body temperature of the rat. Environ. J.
Pharmacol., 4: 18-24.
MOFFITT, A. E. & MURPHY, S. D. (1974) Effect of excess and deficient
copper intake on hepatic microsomal metabolism and toxicity of
foreign chemicals. In: Hemphill, D. D., ed. Trace substances in
environmental health, Columbia, MO, University of Missouri
Press, Vol. VII, pp. 205-210.
MORRISON, J. K. (1968) The purpose and value of LD50 determinations.
In: Boyland, E. & Goulding, R., ed. Modern trends in toxicology,
Appleton-Century-Crofts, pp. 1-17.
MURPHY, S. D. (1964) A review of effects on animals of exposure to
auto exhaust and some of its components. Air Pollut. Control J.,
14: 303-308.
MURPHY, S. D. (1967) Malathion inhibition of esterases as a
determinant of malathion toxicity. J. Pharmacol. exp. Therap.,
156: 352-365.
MURPHY, S. D. (1969) Some relationships between effects of
insecticides and other stress conditions. Ann. NY Acad. Sci.,
160: 366-377.
MURPHY, S. D. (1975) Pesticides. In: Casarett, L. J. & Doull, J., ed.
Toxicology: the basic science of poisons, New York, Macmillan,
pp. 408-453.
MURPHY, S. D., LAUWERYS, R. R., & CHEEVEN, K. L. (1968) Comparative
anticholinesterase action of organophosphorus insecticides in
vertebrates. Toxicol. appl. Pharmacol., 12: 22-35.
ORTON, J. C., ANDERSON, M. W., PICKETT, R. D., ELING, T. E., & FOUTS,
J. R. (1973) Xenobiotic accumulation and metabolism by isolated
perfused rabbit lungs. J. Pharmacol. exp. therap.,
186: 482-497.
PANEL ON HERBICIDES (1971) Report on 2,4,5-T, Washington, DC, US
Govt Print. Office, 68 pp.
PARKE, D. V. (1968) The biochemistry of foreign compounds. Oxford,
Pergamon Press, 269 pp.
PARKE, D. V. & WILLIAMS, R. T. (1969) Metabolism of toxic substances.
Brit. med. Bull., 25: 256-262.
PATTISON, F. L. M. (1959) Toxic aliphatic fluorine compounds,
Amsterdam, Elsevier, 227 pp. (Elsevier Monographs).
PATTY, F. A. (1958) Industrial hygiene and toxicology, 2nd ed., New
York, Interscience, Vol. 1, 153 pp.
POTTER, V. R. (1972) Workshop on liver cell culture. Cancer Res.,
32: 1998-2000.
REID, W. D., HETT, K. F., HIK, J. M., & KRISHNA, G. (1973) Metabolism
and binding of aromatic hydrocarbons in the lung. Am. Rev. Resp.
Dis., 107: 539-551.
ROE, F. J. C. (1968) Inhalation tests. In: Boyland, E. & Goulding, R.,
ed. Modern trends in toxicology, London, Butterworths,
pp. 39-74.
ROWE, V. K., WOLF, M. A., NEIL, C. S., & SMITH, H. F. (1959) The
toxicological basis of threshold limit values: 2. Pathological
and biochemical criteria. Am. Ind. Hyg. Assoc. J., 20: 346-349.
SMYTH, H. F. (1959) The toxicological basis of threshold limit values:
1. Experience with threshold limit values based on animal data.
Am. Ind. Hyg. Assoc. J., 20: 341-345.
SZOT, R. J. & MURPHY, S. D. (1970) Phenobarbital and dexamethasone
inhibition of the adrenocortical response of rats to toxic
chemicals and other stresses. Toxicol. appl. Pharmacol.,
17: 761-773.
SZOT, J. R. & MURPHY, S. D. (1971) Relationships between cyclic
variations in adrenocortical secretary activity in rats and the
adrenocortical response to toxic chemical stress. Environ. Res.,
4: 530-538.
TAYLOR, G. J. & DREW, R. T. (1975) Cardiomyopathy predisposes hamsters
to trichlorofluoromethane toxicity. Toxicol. appl. Pharmacol.,
32: 177-183.
TYLER, W. S. & GILLESPIE, J. R. (1969) Structural and functional
alterations in horses with emphysema. In: Proceedings of a
Symposium on Animal Models for Biomedical Research, Washington,
DC, National Academy of Sciences, Vol. II, pp. 38-51.
UNIVERSITIES FEDERATION FOR ANIMAL WELFARE (1972) Handbook on the
care and management of laboratory animals, 4th ed., Baltimore,
Williams & Wilkins, 624 pp.
WATTENBERG, L. (1972) Dietary modification of intestinal and pulmonary
aryl hydrocarbon hydroxylase activity. Toxicol. appl.
Pharmacol., 23: 741-748.
WEIL, C. S. (1970) Selection of the valid numbers of sampling units
and a consideration of their combination in toxicological studies
in involving reproduction, teratogenesis and carcinogenesis.
Food Cosmet. Toxicol., 8: 177-182.
WHO (1967) WHO Technical Report Series, No. 348. (Procedures for
investigating intentional and unintentional food additives:
Report of a WHO Scientific Group) 25 pp.
WILLIAMS, R. T. (1967) Comparative patterns of drug metabolism. In:
Proceedings of an International Symposium on Comparative
Pharmacology. Fed. Proc., 26 (4): 1029-1046.
WILLIAMS, R. T. (1972) Toxicological implications of biotransformation
by intestinal microflora. Toxicol. appl. Pharmacol.,
23: 769-781.
WINTER, C. A. & FLATAKER, L. (1962) Cage design as a factor
influencing acute toxicity of respiratory depressant drugs in
rats. Toxicol. appl. Pharmacol., 4: 650-655.
WITSCHI, H. (1975) Exploitable biochemical approaches for the
evaluation of toxic lung damage. Essays in Toxicol.,
6: 125-191.
WORDEN, A. N. (1974) Toxicology and the environment. Toxicol.,
1: 3-27.
ZBINDEN, G. (1973) Progress in toxicology: special topics, New York,
Springer-Verlag, 88 pp.
3. ACUTE, SUBACUTE, AND CHRONIC TOXICITY TESTS
3.1 Introduction
The primary objective of toxicological testing is to determine
the effects of chemicals on biological systems and to obtain data on
the dose-response characteristics of the chemical. These data may
provide information on the degree of hazard to man and the environment
associated with a potential exposure related to a specific use of this
chemical. Elucidation of the metabolic behaviour of the chemical in
test animals increases confidence in defining the hazard (see Chapter
4). The degree of confidence with which hazard may be estimated
depends on the quality of the toxicological data. Selection of the
most appropriate test procedures coupled with strict adherence to
accepted experimental practices and astute observation are of
paramount importance in experimental toxicology.
3.2 General Nature of Test Procedures
Several types of toxicity testing procedures have been developed.
These include acute, subacute, and chronic studies. The major
difference between these tests is the dose employed and the length of
exposure to the chemical agent, but other differences in intent and
nature do exist and will be discussed. All of the tests share some
common characteristics. Each requires that groups of healthy animals,
housed under suitable conditions, be exposed to graded doses of the
test chemical. Rats, mice, guineapigs, rabbits, and hamsters are
commonly used for this purpose, but in some cases it may be necessary
to use dogs, swine, nonhuman primates, or other species. As a rule, a
control group is given the dosing vehicle or is sham treated.
Following treatment, the animals are closely observed for signs of
toxicity. Laboratory procedures designed to measure biological effects
are carried out on the treated and control animals. Detailed records
are maintained on each animal. Following completion of the test, all
animals, including controls, are subjected to a pathological
examination. Data should be analysed by appropriate statistical
procedures.
3.2.1 Housing, diet, and clinical examination of test animals
Animals should be healthy, genetically stable, and adequately
identified as to colony source. The controls and treated animals
should be of the same strain and species, age, and weight range, and
be supplied from the same source. Before starting the experiment, the
health status of all animals should be determined and monitored for
some time. During this time, a small randomly selected number of
animals from each shipment should be sacrificed and examined for
disease, parasites, and other specific pathogens. During the
quarantine period, animals may be caged together according to the
weight-space specifications. Acceptable standards for the housing and
care of experimental animals have been published (DHEW, 1972; Canadian
Council on Animal Care, 1973; Sontag et al., 1975).
During toxicity studies, rodents should be housed singly or in
pairs in stainless steel or plastic shoe-box cages while nonrodents
should be housed in suitable runs. The animals should be randomly
allotted to the cages and treatment regimes should be randomly applied
(Cox, 1958). Rodents should be allowed free access to food and water.
Nonrodents should be fed meal and given water ad libitum. The diet
fed to the animals should meet all of their nutritional requirements
(National Academy of Sciences, 1975) and should be free of toxic
chemical impurities that might influence the outcome of the test.
Periodic analysis of the diet to ensure its nutrient composition
should be undertaken since nutritional status may affect the nature of
toxic responses (Arcos, 1968). Although commercially available diets
of recognized quality are suitable for most subacute studies,
semipurified diets may be preferred because the nutrient and
nonnutrient components of the diet may be altered readily, where
necessary (Munro et al., 1974; Newberne, 1968).
Careful clinical observation of test animals is the most
neglected area in experimental toxicology. Few investigators are aware
or recognize that the skills required of a good medical diagnostician
are also required in assessing or diagnosing the toxic state or
condition of an animal. In toxicity studies, many animals may be lost
for evaluation because of death from intercurrent disease and
subsequent autolysis. With concerned, reliable staff, these losses can
be greatly reduced if a conscientious effort is made to recognize
early clinical signs of disease in the test animal. Ideally, each
animal on test should be looked upon as an individual patient. In this
way, there is an awareness of the idiosyncrasies of the animal and
departures from the normal will be more easily recognized. Once a
routine of careful clinical assessment has been established, it is
possible either to treat diseased test animals or, if necessary,
sacrifice them. In the latter case, the tissues are available for
histological examination. Otherwise, chronic disease effects might
render the tissues useless for the assessment of effects due to test
substances.
Detailed clinical examinations should be conducted weekly on the
test animals by competent, laboratory animal technicians under the
supervision of a veterinarian skilled in laboratory animal medicine
(Health and Welfare, Canada, 1973). These should include general
observation of the animals for overt signs of toxicity, quality of
hair, coat, general condition of the eyes, mouth, teeth, nose, and
ears (Leclair & Willard, 1970; Loomis, 1968). Assessment of cardiac
and respiratory function should be conducted by auscultation. If
neurological effects are anticipated, a detailed neurological
examination should be conducted. In larger species, this can be done
by skilled personnel using the methods of Charbonneau (1974), McGrath
(1960), and Mowbray & Cadell (1962). Examination of the eyes using
opthalmoscopic and slit-lamp techniques (Marzulli, 1968) may assist in
detecting ocular toxicity. The external and internal structures should
be carefully palpated and any tissue masses should be noted. Detailed
records of clinical evaluation should be maintained and should be
accessible to the attendant pathologist.
3.3 Acute Toxicity Tests
3.3.1 Underlying principles
Acute toxicity has been defined as the adverse effects occurring
within a short time of administration of single dose or multiple doses
given within 24 h (Hagan, 1959). When data are unavailable concerning
the toxicity of the test agent, acute toxicity studies are indicated
to identify the relative toxicity of the compound, to investigate its
mode of action and its specific toxic effect, and to determine the
existence of species differences.
The most frequently used acute toxicity test involves
determination of the median lethal dose (LD50) of the compound. The
LD50 has been defined as "a statistically derived expression of a
single dose of a material that can be expected to kill 50% of the
animals" (Gehring, 1973). The basic protocol for the determination of
the LD50 is well established and consists of treating groups of
animals with a mathematically-related series of doses in order to
determine the dose that kills 50% of the group and the dose-response
function. The LD50, being a calculated value, is always accompanied
by some estimation of the error of the value, such as the confidence
limits. The most commonly used methods for calculation of the LD50
are the graphic method of Litchfield & Wilcoxon (1947), the
logarithmic probit graph paper method of Miller & Tainter (1944), and
the method of moving averages of Thompson (1947) and Weil (1952). A
comparative review of these and other methods was published by
Armitage & Allen (1950). Death which occurs after the first 24 h is
more likely to be due to delayed toxic effects, which may be direct or
indirect. Signs occurring after the first 24-h period may give some
indication of the effect that the chemical may have at lower levels,
when administered for longer time periods.
3.3.2 Experimental design
3.3.2.1 Selection of species
The extent of species variation in toxicity testing has been well
documented in the reviews of Brodie (1964) and Rumke (1964). The
usefulness of determining species variability in order to assess the
applicability of toxicity data to man has been discussed by Hagan
(1959). Litchfield (1962) has postulated that if the toxicity of a
compound is the same in several species, there would appear to be an
increased likelihood that man would react in a similar manner.
The mouse, rat and dog are the most commonly used species for
acute toxicity testing. Both the rat and mouse should be used, as
marked differences in the LD50 between these two species are not
uncommon (Morrison et al., 1968).
The LD50 determination should be conducted in both male and
female animals, as differences in the LD50 between sexes have been
well documented (Hurst, 1958; Rumke, 1964) and are probably related,
in part, to differences in hepatic metabolism (Conney et al., 1965).
Acute toxicity may vary substantially with the age of the test
animal (Dieke & Richter, 1945; Lu et al., 1965; Scott et al., 1965;
Yeary & Benish, 1965), and animals of various ages should be used in
LD50 determinations. The effect of the age of the animal on the
LD50 is well documented and may be related to different levels of
drug metabolizing enzymes, absence of sex hormonal influences, or an
altered sensitivity of the central nervous system (Fouts & Hart, 1965;
Jondorf et al., 1959; Setnika & Magistretti, 1964).
The animals should be derived from previously untreated healthy
females. Weinberg et al. (1966) have demonstrated an effect of
treatment of dams during gestation with various compounds on the acute
oral toxicity in the newborn.
Furthermore, the animals should not have been previously used for
other studies, nor should there be a history of recent exposure to
anthelminthics or any other drug treatment.
The number of animals used should be sufficient for statistical
analysis and will depend on the method used for the calculation of the
LD50. Usually 8-10 rodents (4-6 animals of each sex) are used per
dose group (Leclair & Willard, 1970). Diechmann & LeBlanc (1943)
described a method using a total of 6 animals, while other methods
involved the use of 4-5 animals per dose group (Horn, 1956; Litchfield
& Wilcoxon, 1947; Thompson, 1947).
3.3.2.2 Selection of doses
The doses are selected to provide data for estimating the LD50
and to obtain information on the slope of the dose-response curve. At
least four doses, selected in logarithmic progression, should be used
(Weil, 1952).
In general, however, the doses can be arrived at only by
experimentation. The initial dose may be such that no effect is
manifested in the animals. In subsequent groups of animals, the dose
should be increased by a constant multiple until the dose of the
compound administered is sufficiently high that all of the animals in
the group die. Under these conditions, data can be obtained that can
be plotted to give a dose-response curve and from which the LD50
value may be calculated.
3.3.2.3 Method of administration
Generally, the chemical should be administered by the route by
which man would be exposed. If the route is oral, the compound should
be administered by gavage rather than mixed in the diet. In some
cases, the administration of the chemical along with the diet has been
shown to increase its toxicity compared with gavage dosing (Bein,
1963; Worden & Harper, 1963), but, in general, the oral toxicity of a
compound is greatest when it is administered by gavage to animals that
have fasted (Griffith, 1964). Griffith (1964) has demonstrated the
effect of the type and concentration of the vehicle on the LD50
value. The amount of the liquid or carrier administered should be
appropriate and the carrier should not, in itself, be toxic to the
animal.
In certain cases, even though the route of human exposure would
be oral, acute dermal, eye, and inhalation studies may be indicated to
assess the hazard to personnel handling the compound in the
laboratory.
3.3.2.4 Postmortem examination
In general, all animals dying during the observation period and
all surviving animals should be autopsied by a qualified pathologist
(Leclair & Willard, 1970). The autopsy should include gross and
histopathological examination of all organs.
If death is almost instantaneous and due to a pharmacological or
physical effect, i.e. massive gastrointestinal haemorrhage or acute
respiratory collapse, detailed histopathological examination of all
organs may not be indicated.
3.3.3 Repeated high-dose studies
Because of the inherent limitations of the LD50 in predicting
long-term toxicity, a short but intensive study or a series of such
studies may be indicated before commencing subacute tests. The purpose
of such studies is to define more precisely the doses to be used in
subacute tests and to elucidate more fully the organ systems affected.
The design of these repeated high-dose studies may vary but consists,
essentially, of repeated daily administration of a mathematically-
related series of doses to groups of animals for 5-21 days.
One type of repeated-dose study (Sontag et al., 1975) consists of
treating groups of five young adult animals of each sex at each of
five dose levels, the upper level being the one that is estimated to
produce no more than 10% lethality following a single dose, the
remaining doses being fractions of this dose.
A seven-day feeding study described by Weil et al. (1969)
consisted of treating five rodents of each sex at each of three or
four dose levels for seven days. Criteria of effects were mortality,
body weight gain, relative liver and kidney weights, and feed
consumption. This study showed that the results of the seven-day
feeding test were of significantly greater value in predicting dose
levels for the 90-day toxicity test than the LD50 values.
Daily observations, as described in section 3.1.3, should be
conducted and weekly body weight and food consumption (if the animals
are caged individually) monitored. For some test agents, especially
those with delayed toxicity or cumulative effects, other measurements,
such as organ function, body burden, absorption, and excretion of the
compound may be indicated. Animals should be necropsied and the
tissues should be examined for gross pathological changes and studied
histopathologically, if indicated.
3.4 Subacute and Chronic Toxicity Tests
3.4.1 Underlying principles
The subacute toxicity test generally involves daily or frequent
exposure to the compound over a period up to about 90 days. It
provides information on the major toxic effects of the test compound
and the target organs affected (Barnes, 1960). The latency of
development of the effect as related to dose, the relationship of the
blood and tissue levels of the compound to the development of lesions,
and the reversibility of the effects may also be studied. Data derived
from these studies are used for designing chronic toxicity tests in
which animals are exposed to the chemical for longer periods of time.
Man may be exposed for the greater part of his life-time to low
levels of a wide variety of environmental chemicals. Usually, the
degree of exposure is insufficient to produce overt signs of toxicity;
thus, cause-effect relationships cannot be easily established.
Epidemiological studies may assist in this respect, but, because man
is exposed simultaneously to several chemicals, it is difficult to
establish unequivocally the degree of hazard associated with any one
chemical. Acute and subacute toxicity tests are of limited value in
predicting chronic toxic effects because: (a) chemicals may produce
different toxic responses when administered repeatedly over a period;
and (b) during the aging process, factors such as altered tissue
sensitivity, changing metabolic and physiological capability, and
spontaneous disease may influence the degree and nature of toxic
responses. In addition, several important diseases such as heart
disease, chronic renal failure, and neoplasia are associated with
advancing age. These are multicausal in nature and thought to be due,
in part, to the presence of chemical substances, both natural and
synthetic, in the environment (WHO, 1972). Chronic toxicity tests, in
which animals are exposed for their entire lifetime to environmental
chemicals, have provided useful means of identifying those substances
of greatest public health concern. The tests are usually conducted
with the aim of establishing "no-observed-adverse-effect levels" that
may be used in setting acceptable daily intakes (ADIs), tolerance
limits for chemicals in food or water, or threshold limit values in
the case of occupational exposure. Since chronic toxicity testing is
expensive and requires specialized facilities and personnel, great
care must be taken in the design, execution, and interpretation of the
results of such studies.
3.4.2 Experimental design
3.4.2.1 Selection of species and duration of studies
In the subacute studies, if the compound has produced evidence of
toxicity in man and if sufficient toxicological and metabolic
information is available, it is often possible to select an
appropriate species on the basis of these data. For compounds about to
be put on the market about which little is known toxicologically, the
recommendations of the World Health Organization (WHO, 1958) and
competent national agencies (Friedman, 1969; Leclair & Willard 1970;
National Academy of Sciences, 1975) should be followed in selecting
appropriate test species. As a minimum recommendation, subacute
studies should be undertaken in two species, one rodent and one
nonrodent. Traditionally, the rat and dog are selected for subacute
toxicity testing because of their availability and the large amount of
background information available on them. When rats are used, the test
should be initiated just after weaning so that observations may be
made during the period of most rapid growth. A conventional strain
should be selected, so that the results in control and treated animals
can be compared with known literature values, and both sexes should be
tested to ascertain the influence of the sex hormones on the toxic
response. At least 10 animals of each sex should be included in each
dose group and the experiment should continue for 10% of the animals'
lifetime or about 3 months. If it is desired to study the pathogenesis
and reversibility of induced lesions or biochemokinetics, it is
recommended that observations be made at 3-week intervals during
exposure and last up to 3 months following termination of exposure.
In chronic toxicity testing, it is usual to expose the animals to
the chemical for the greater part of the life span. A wide variety of
animal species have been used in this type of work, although in most
cases rodents are the animals of choice, since large numbers can be
used to aid in the statistical interpretation of the results. Larger
animals should also be used (e.g. dog and monkey) for such species
have the advantage that larger samples of blood can be obtained on a
routine basis.
If the objective of the test is to study the carcinogenic
potential of a compound, the rat, mouse, or hamster is usually chosen
because of its shorter lifetime and the fact that large numbers may be
used to increase the sensitivity of the test.
When data on the metabolic fate of the test chemical in man is
not available, the species showing the greatest sensitivity in
subacute studies should be selected as the test species, provided the
species does not react atypically to the compound due to metabolic
peculiarities.
Sufficient numbers of animals should be included in the test to
ensure that a statistically valid design is achieved. Based on the
incidence of effects observed in subacute studies and the anticipated
incidence of chronic effects, the number of animals that should be
used can be calculated (Snedecor & Cochran, 1967).
Since it is usually the intention in chronic toxicity studies to
expose animals over the major portion of their life span, it is
essential to commence exposure early in life.
3.4.2.2 Selection of doses
Guidance on the selection of doses for subacute studies may be
obtained from the results of acute and repeated high-dose studies. For
compounds having a tendency to bioaccumulation, selection of doses is
particularly difficult. Kinetic studies may assist in establishing
acceptable dose levels since the half-time ( t´) for elimination
(Chapter 4) may provide guidance on the degree of bioaccumulation that
could be anticipated. To establish the nature of the toxic reaction,
the highest dose should provide a distinct toxic effect while the
lowest dose should not produce any detectable toxic reaction (Leclair
& Willard, 1970). To obtain maximum information on the dose-response
characteristics of the compound, at least two intermediate doses
should be included.
Information from subacute toxicity tests is of value in the
selection of appropriate dose levels, when commencing chronic toxicity
studies. In general, however, it is highly desirable to establish the
chemobiokinetic behaviour (Chapter 4) of the test compound and if
possible its major metabolites in the test species prior to
undertaking a chronic toxicity test. Particular attention should be
given to evidence for dose-dependent detoxification. Studies of this
nature will provide information on the degree to which the chemical
may be expected to accumulate in various body compartments and
unexpectedly produce evidence of toxicity. Since it is the object of
chronic toxicity tests to establish dose-response patterns and
"no-observed-adverse-effect levels", a minimum of three dose levels
should be used. The upper dose level should produce some slight
evidence of toxicity, but should be compatible with normal
physiological function (Leclair & Willard, 1970). The lowest dose
level would not be expected to produce evidence of toxicity (Health &
Welfare, Canada, 1973).
3.4.2.3 Method of administration
The route of administration in subacute and chronic studies
should be that through which man is likely to be exposed. For gases
and volatile industrial solvents, inhalation studies are recommended
(Magill et al., 1956) (Chapter 6), while for food additives,
pesticides, and other chemicals likely to come into contact with food
or water, the oral route is recommended (Leclair & Willard, 1970;
National Academy of Sciences, 1975). Incorporation of the test
chemical into the diet or drinking water is an appropriate means of
administration; however, care must be taken to ensure the stability of
the chemical in the dosing medium. The concentration of the test
chemical in the diet should be determined periodically to ensure
uniform dispersion and to aid in the quantification of achieved doses.
In some cases, the chemical may be unpalatable and administration by
gavage, or, in the case of dogs, by capsules may be necessary.
The diet is the preferred vehicle of administration, but it is
absolutely essential that the chemical be present in the diet in an
unaltered form; toxicity may be altered by interaction with dietary
constituents (Kello & Kostial, 1973). In rodent studies, the compound
may be administered in the diet as a fraction of the total diet, or a
sufficient quantity of the chemical may be added to the diet to
achieve predetermined dose levels (in mg per kg body weight per day).
In the latter case, it is necessary to adjust the dietary
concentration weekly or biweekly to maintain a constant dose level,
since food consumption per unit of body weight decreases as the animal
gets older. If, in rodent tests, the concentration of the test
compound in the diet is kept constant from weaning to maturity, the
actual dose received will be reduced by approximately 2.5 times over
the dosing period. This may have profound effects on the severity of
the toxic response and may be mistaken for tolerance. In chronic
toxicity tests, the chemical should be administered daily over the
entire treatment period. As an aid to interpretation of the test, only
one lot chemical should be used for the entire test unless the purity
of the chemical is definitely assured.
3.4.2.4 Biochemical organ function tests
In subacute studies, the use of a species such as the dog instead
of a rodent species permits the application of a wider range of
biochemical organ function tests because larger samples of blood can
be collected on a routine basis. Organ function studies should be
undertaken prior to initiation of the test, 3 and 10 days after the
start of dosing, at 30-day intervals thereafter throughout the test,
and terminally. The tests described for the chronic studies are also
applicable in subacute studies.
In the course of chronic toxicity tests, studies should be
undertaken to evaluate the functional integrity of various organ
systems. Assessment of the urinary system should commence with an
examination of the urine. Freshly-voided urine samples should be
obtained every one to three months from the test animals and examined
for the presence of occult blood, glucose, protein, and bilirubin
using simple diagnostic procedures. If positive effects are noted,
quantitative methods should be applied as outlined by Bergmeyer
(1965). Samples of freshly-voided urine should also be filtered
through Millipore filters and the filters stained according to the
Papanicolaou method (Frost, 1969) to detect the presence of renal
tubular cells or other cell types derived from the urinary system.
Urinary calculi and parasite eggs (Chapman, 1969) may be detected by
this method. Blood urea-nitrogen levels and other standard tests of
kidney function may be applied but they usually lack sufficient
sensitivity to detect subtle changes in kidney function.
Several test procedures are available for the assessment of liver
function. Most of these methods involve an examination of the serum
levels of hepatic enzymes that may be released in the serum following
liver injury (Czok, 1965; Henley et al., 1966; Zimmerman, 1974).
Korsrud et al. (1972) compared the sensitivity of various liver
function tests in the rat and noted that serum sorbitol dehydrogenase
(1.1.1.14) activity (an enzyme specific to the liver) correlated well
with the degree of histological alteration produced by hepatotoxic
agents such as carbon tetrachloride, 2,2'-iminobisethanol
(diethanolamine), and ethanethioamide (thioacetamide). However, Grice
et al. (1971) noted that pathological changes induced by these
compounds must be reasonably advanced before elevations are noted in
serum glutamic-oxaloacetic transaminase (2.6.1.1), lactate
dehydrogenase (1.1.1.27), or lactate dehydrogenase isoenzymes,
suggesting that changes in serum enzyme activity may not be as
sensitive an indicator of toxicity as pathomorphological examination.
Tests of liver function such as serum enzyme activities and various
clearance tests were reviewed recently by Balazs (1975). A complete
review of the principles and applications of these tests is given by
Cornish (1971) and further discussion of these methods is found in
Part II, Chapter 8. Suffice it to say, that a transient increase in
the activity of serum enzymes or other organ-derived constituents may
result from a transient change in organ homeostasis that produces no
lasting toxic effect.
For routine screening of organ function in large animals,
Charbonneau et al. (1974) used clinical procedures that can measure
the concentration of several serum enzymes and inorganic and other
constituents by automated methods. In general, these methods are not
sufficiently standardized or reproducible to detect minor alterations
in organ function but they do serve as a useful guide to general
clinical status.
3.4.2.5 Physiological measurements
In subacute studies, it is often possible to detect ensuing
pathological events through application of physiological function
tests.
In all studies, food consumption and body weight should be
recorded weekly in all animals. Weight gain per unit of food consumed
should be calculated (Munro et al., 1969). This gives a measure of the
efficiency of food use. The daily dose of chemical should be
calculated from data on food intake and body weight. Similar
measurements of food intake and body weight must be carried out in
chronic toxicity tests. If the test chemical is incorporated into the
drinking water, water intake must be measured. These measurements
should be conducted on a weekly basis during the entire test. The data
can be used to estimate the dose of chemical received and are
necessary in the establishment of dose-response relationships. Body
weight changes serve as a sensitive indication of the general health
status of test animals. Any rapid loss in body weight may signal the
onset of intoxication or disease. Computerized methods for recording
and analysing this type of data are available (Munro et al., 1972).
Under special circumstances, when the target organs of toxicity
have been identified during subacute studies, it is appropriate to
conduct measurements of the physiological function of organ systems.
Procedures such as electrocardiography (Grice et al., 1971),
electroencephalography (Flodmark & Steinwall, 1963; Harada et al.,
1967; Mann, 1970), electromyography (Chaffin, 1969), nerve conduction
studies and measurement of evoked potentials (Barnet et al., 1971;
Hrbek et al., 1972) may greatly assist in defining the functional
effects of chemicals (Chapter 8). Such tests are expensive to perform
and require highly specialized equipment and personnel. They have
limited application in routine testing but may be used to define
mechanisms of action. It is imperative that the results of such
studies be correlated with clinical and pathological findings (Grice,
1972; Osborne & Dent, 1973).
3.4.2.6 Metabolic studies
Subacute studies provide an excellent opportunity to undertake
metabolic investigations under conditions of repeated exposure that
may alter the nature of the metabolites and the rate of metabolic
transformation of the test compound. Urine and faeces can be collected
and examined for the presence of metabolites and, by undertaking
serial sacrifices at three-week intervals, the kinetics of
accumulation of the compound in various body compartments can be
evaluated.
To gain an understanding of the metabolic fate of a chemical that
may have a long biological half-time, such as hexachlorobenzene (Grant
et al., 1975), three extra groups of animals need to be studied for
tissue distribution to provide information that is necessary for
estimating the potential hazard to man. The principles of these
methods are reviewed in Chapter 4. Often it is desirable, in subacute
studies, to study the kinetics of the test compound and its
metabolites following completion of the dosing period. If extra groups
of animals are initially included for this purpose, much valuable
additional information on the compound may be obtained.
3.4.2.7 Haematological information
In subacute studies involving rodents, haematological studies
should be undertaken on randomly selected subgroups of animals prior
to initiation of the test, at 30-day intervals, and on all animals
terminally. Bone marrow should be examined terminally. Nonrodent test
animals should be examined at similar intervals.
In chronic toxicity studies involving rodents, haematological
studies of circulating blood cells should be undertaken on randomly
selected subgroups of animals prior to initiation of the test, at 3 to
6-month intervals and, on selected animals, terminally. Bone marrow
should be examined terminally and, if indicated, at interim times by
biopsy.
To assess the clinical state of nonrodents, haematological
variables should be examined frequently.
A set of test procedures is necessary for routine haematological
screening and the tests must be of sufficient sensitivity and accuracy
to be of practical value for use in large numbers of laboratory
animals (Cartwright, 1969; Schalm, 1967; Sirridge, 1967).
Quantification of blood cells and thorough study of cellular
morphology by a haematologist, experienced in small animal medicine,
is necessary in the study of haematological disorders. Haematological
evaluation of experimental animals is facilitated by the fact that
repeated sampling is relatively easy and small amounts of blood are
required, and that single-cell systems can be studied to obtain
information on cell production, destruction, defects, and dysfunction.
For erythroid evaluation, the numbers of circulating erythrocytes must
be counted and the haematocrit and haemoglobin concentration measured.
As an index of erythropoietic activity in the bone marrow, a
reticulocyte count should be carried out. Morphological assessment of
erythrocytes is mandatory. The number of circulating leucocytes should
be quantified and a differential count and morphological assessment
should be made. To evaluate the functional capacity and malignant
changes in the blood-forming organs, bone marrow should be examined
terminally. From bone marrow smears a differential count and
morphological assessment can be carried out. Imprints of lymph nodes
or spleen permit a detailed cytological study of normal and abnormal
cells present that may be of diagnostic significance.
To assess haemostatic function, it will be necessary to evaluate
platelets, coagulation systems, and fibrinolysis. Screening tests
include platelet count, clot retraction, one stage prothrombin time,
and activated partial thromboplastin time. More specific evaluation
may require factor assays, thrombin time, fibrinogen determination,
euglobulin clot lysis time, prothrombin consumption time, platelet
aggregation, and adhesiveness.
3.4.2.8 Postmortem examination
In every toxicity evaluation, all animals should be given a
thorough gross autopsy and detailed records kept on each animal.
Samples of all organs and supporting structures should be saved for
histopathological examination. Detailed autopsy methods are outlined
in Chapter 5.
In chronic toxicity testing it is often useful to incorporate
interim autopsy dates so that the progression of lesions may be
studied. At interim sacrifices and terminally (if sufficient animals
are in a healthy state), the major organs should be weighed. Organ
weights may serve as a useful index of toxicity; however, care must be
taken in the interpretation of the data. Decreased absolute organ
weights in treated animals may be merely a reflection of lower body
weight and calculation of organ to body weight ratios may increase the
usefulness of the data (Feron, 1973).
3.4.2.9 Controls
In the evaluation of both subacute and chronic toxicity, special
attention must be given to the control animals. The quality of data
obtained from the control animals has an important bearing on the
interpretation of results from the treated animals. Suitable numbers
of control animals of the same age and body weight as the treated
animals must be included in the experimental design in a statistically
randomized fashion.
Except for treatment with the test chemical, these animals should
be handled identically to the test subjects and all measurements
conducted on the treated animals must be carried out on the controls
with the same precision and frequency. In studies in which the
chemical is administered by gavage, the control animals should receive
the suspending vehicle in an amount equivalent to the treated animals.
The incidence of spontaneous lesions or of other changes in control
animals must be carefully noted and the interpretation of data
obtained from treated animals must include an appreciation of the role
that spontaneous disease processes may play in the manifestation of
chemical toxicity. It is particularly important, in studies with
rodents, to have detailed information on the incidence of neoplastic
diseases, since some species and strains (Sher, 1972) may have a high
background incidence of certain tumours which tends to reduce
longevity and decrease the chance of observing chronic toxic effects.
In addition, the chemical under test may alter the incidence of
spontaneous tumours and other diseases or may induce new tumours, and
this possibility must be taken into consideration in the evaluation of
the chronic toxicity of chemicals. In all cases, responses
attributable to the test compound must be compared with background
observations in controls. For this reason, the quality of the
toxicological data rests heavily on the adequacy of the control values
(Weil & Carpenter, 1969).
3.4.3 Alternative approaches in chronic toxicity
3.4.3.1 Perinatal exposure
The majority of chemicals to which man may be exposed are present
in air, food, or water for his entire lifetime. Recently, there has
been an attempt to duplicate the human situation in the chronic
toxicity test by exposing the test animals during the neonatal period
as well as throughout life (Friedman, 1969). In this approach, groups
of weaning animals (usually rodents) are exposed to the test chemical
until they reach sexual maturity. They are then mated, within dose
groups, and the treatment is continued during pregnancy and lactation.
Following weaning, the offspring are transferred to their parents'
diet and exposed for the balance of their lifetime to the test
chemical. The details of this test procedure have been outlined in a
recent Canadian Government publication (Health & Welfare, Canada,
1973) and by Epstein (1969). It is not known yet whether this
technique increases the sensitivity of the chronic toxicity test, but
it is known that exposure to carcinogens in the perinatal period will
often increase the incidence and decrease the latent period of
carcinogenesis (Tomatis & Mohr, ed., 1973).
Further study of this method is required to evaluate its
usefulness fully. It should be pointed out, however, that this
procedure adds considerably to the cost and length of the chronic
toxicity test.
3.4.3.2 Use of nonrodent species
In chronic toxicity studies with nonrodent species such as
nonhuman primates, dogs, or cats, it is often not feasible to expose
the animals to the test compound for their entire lifespan, even
though they may be the species of choice. Under such conditions,
careful examination of the kinetic and metabolic behaviour of the test
compound in these species may substitute, to some extent, for the
decreased treatment period (provided the anticipated endpoint is not
carcinogenesis). Carefully conducted kinetic studies will assist in
establishing when steady-state tissue concentrations of the test
chemical and its metabolites have been achieved. If treatment is
continued for a substantial period after the establishment of
steady-state kinetics without any increase in the degree of toxic
effects observed clinically, or during interim sacrifice, this may
partially substitute for a lifetime study and provide increased
assurance for those having to make regulatory decisions. If this
approach is not feasible, it may be possible to test human metabolites
in rodent species (Health & Welfare, Canada, 1973).
3.5 Evaluation and Interpretation of the Results of Toxicity Tests
The evaluation and interpretation of toxicity studies starts with
a clear definition of experimental objectives. The design of the
experiment should be such that the objectives can be reasonably
achieved. Well designed and carefully executed experiments add greatly
to the ease with which results can be evaluated and interpreted and
also to confidence in the experimental data.
The primary usefulness of the LD50 determination is to obtain
some idea of the magnitude of the acute toxic dose (Frazer & Sharratt,
1969) and information concerning the type of toxic effects of the
chemical. Such information includes whether death is immediate or
delayed, whether recovery from a near lethal dose is rapid or complete
or both, or whether the cause of death is narcosis with respiratory
failure, lung oedema, or liver necrosis.
However, the LD50 provides little information for the
assessment of the hazard from compounds to which the human population
is exposed for extended periods of time. Although it has been
suggested that compounds that do not show adverse effects when given
in doses of 3-5 g per kg body weight are essentially non-toxic
(National Academy of Sciences, 1975), there are numerous examples in
the literature of compounds with LD50 values greater than 5 g per kg
which produce toxic effects, when given in low doses for extended
periods of time (Frazer & Sharratt, 1968). If the main object of an
acute toxicity test is not to establish a value for the LD50 with
precision, but to learn something about the way in which the chemical
acts as a poison, as suggested by Paget & Barnes (1964), this can best
be accomplished by tests involving repeated daily administration to a
few animals for a period of 5-21 days. The information provided by the
LD50 regarding the effects of acute exposure to toxic compounds may
be useful as a guide for selecting doses for such studies.
The primary objective of subacute and chronic toxicity studies is
to determine the nature and severity of toxic effects and the
"no-observed-adverse-effect" dose level. These data may then be used
in the establishment of acceptable levels of exposure for man.
Data on group weight gain or body weight change should be plotted
against time, and differences between groups should be evaluated
statistically. Changes in body weight with time are best evaluated
statistically using trend analysis procedures (Armitage, 1955). Food
(and water) consumption data should be handled in a similar fashion.
Reduced body weight or weight gain in otherwise healthy treated
animals may be due to reduced food intake owing to its unpalatability
or to a specific toxic effect of the chemical resulting in reduced
efficiency of food use. Using data on the dietary concentration of the
test chemical, food consumption, and body weight, the mean daily dose
of chemical received (in mg/kg body weight/day or similar units)
should be calculated. Automated data processing procedures to
accomplish this are available (Munro et al., 1972).
Data on organ weights should be evaluated and interpreted with
great care. Increased relative (to body or brain weight) organ weights
may also result from adaptation to stress phenomena or from metabolic
overloading of biochemical pathways or physiological processes.
Increased liver weight, for example, may result from a stimulation of
de novo protein synthesis in the smooth endoplasmic reticulum (SER).
This results in a morphologically detectable increase in SER. The
biochemical counterpart of this increase is an increased ability of
the liver to metabolize certain foreign substances, sometimes
including the test compound and endogenous substrates, due to a
stimulation in the activity of hepatic mixed function oxidases
(Staubli et al., 1969). These adaptative changes may manifest
themselves clinically as tolerance. Often these changes are reversible
upon cessation of dosing and do not produce lasting toxicological
effects but the implications of chronically elevated levels of these
enzymes is not known. Certain enzyme inducers may cause impairment of
liver function and produce pathological and biochemical changes (Feuer
et al., 1965).
Data on biochemical and haematological effects should be
tabulated and compared with control values using statistical
procedures (Johnson, 1950). Any observed effects should be correlated
with clinical and pathological findings. A biochemical or
haematological change such as reduction in liver glycogen or an
alteration in white cell count may not be indicative of a toxic
effect, but an adaptation to a stress situation (National Academy of
Sciences, 1975). In general, changes in homeostasis must be carefully
evaluated since reversible shifts do not necessarily imply a toxic
effect in the absence of other toxic manifestations.
Changes in the functional state of physiological or neurological
processes, such as an alteration in the electrocardiogram or abnormal
behaviour, may result from pharmacological or pathological effects of
the test compound. Changes in functional state must be closely
correlated with their morphological counterpart in order to evaluate
their toxicological importance properly (Grice, 1972).
The cornerstone of experimental toxicology is the pathological
examination. Usually, decisions regarding the safety of a compound are
based on this evidence. All pathological findings in test animals
should be graded carefully and their incidence tabulated (see Chapter
5). Spontaneous lesions in control animals should also be noted and
compared to the observations in control animals in previous
experiments or in the literature (Peck, 1974) to ensure that the
incidence and nature of the lesions is representative of the strain.
Pathological data should be analysed rigorously using appropriate
statistical methods (Fleiss, 1973) and spurious observations
apparently unrelated to treatment should be identified. Lesions that
are dose-related should be studied in detail and correlated with gross
pathological findings, clinical observations, and other variables
(Grice, 1972).
It is not uncommon in chronic toxicity testing to find
pathological or other changes that occur in low incidence and that are
not dose-related but occur only in treated animals. Such reactions may
be idiosyncratic in nature or may be due to the hypersensitivity of
certain animals. Nevertheless, they deserve special attention since
they may be indicative of a hitherto unsuspected toxic effect. The
clinical history and other data from such animals should be reviewed
with great care and an attempt should be made to determine the reason
for the observed effects. Toxic effects that occur in extremely low
incidence present special problems in interpretation. There is no
substitute for experience in this respect and the prudent investigator
will consult the knowledgeable experts in this field (Zbinden, 1973).
REFERENCES
ARCOS, J. C. (1968) Chemical induction of cancer. Vol. 1, New York,
Academic Press.
ARMITAGE, P. & ALLEN, I. (1950) Methods of estimating the LD50 in
quantal response data. J. Hyg. (Lond.), 48: 298-322.
ARMITAGE, P. (1955) Tests for linear trends in proportions and
frequencies. Biometrics, 11: 375-385.
BALAZS, T. (1975) Toxic effects of chemicals in the liver.
FDA By-lines, 5: 291-303.
BARNES, J. M. (1960) Toxicity testing, In: Schilling, R. S. F., ed.
Modern trends in occupational health, London, Butterworths &
Co., pp. 20-32.
BARNET, A. B., OHRICH, E. S., & SHANKS, B. L. (1971) EEG evoked
responses to repetitive auditory stimulation in normal
Down's-syndrome infants. Dev. Med. Child. Neurol., 13: 321-329.
BEIN, H. J. (1963) Rational and irrational numbers in toxicology.
Proc. Euro. Soc. Study Drug Toxicity, 2: 15-26.
BERGMEYER, H. U. (1965) Methods of enzymatic analysis. New York,
Academic Press.
BRODIE, B. B. (1964) [The difficulties of transposing experimental
results obtained in animals to man.]. Actual. pharmacol.,
17: 1-23 (in French).
CANADIAN COUNCIL ON ANIMAL CARE (1973) Care of experimental animals
-- guide for Canada.
CARTWRIGHT, G. E. (1969) Diagnostic laboratory hematology, 4th ed.
New York, Grune & Stratton.
CHAFFIN, D. B. (1969) Surface electromyography frequency analysis as a
diagnostic tool. J. occup. Med., 11: 109-115.
CHAPMAN, W. H. (1969) Infection with Trichosomomoides crassicauda as
a factor in the induction of bladder tumours in rats fed
2-acetylaminofluorene. Invest. Urol., 7: 154-159.
CHARBONNEAU, S. M., MUNRO, I. C., NERA, E. A., WILLES, R. F.,
KUIPER-GOODMAN, T., IVERSON, F., MOODIE, C. A., STOLZ, D. R.,
ARMSTRONG, F. A. J., UTHE, J. F., & GRICE, H. C. (1974) Subacute
toxicity of methylmercury in the adult cat. Toxicol. appl.
Pharmacol., 27: 569-581.
CONNEY, A. H., SCHNEIDERMAN, K., JACOBSON, M., & KUNTZMAN, R. (1965)
Drug-induced changes in steroid metabolism. Ann. NY Acad. Sci.,
123: 98-109.
CORNISH, H. H. (1971) Problems posed by observations of serum enzyme
changes in toxicology. CRC Crit. Rev. Toxicol., 1: 1-32.
COX, D. R. (1958) Planning of experiments, New York, John Wiley,
Chapter 5.
CZOK, R. (1965) The behaviour of plasma enzymes in toxicological
experiments. Proc. Eur. Soc. Study Drug Toxicity, 5: 68-83.
DHEW (1972) Guide for the care and use of laboratory animals
(prepared by Committee for the Revision of the Guide for
Laboratory Animal Facilities and Care, Institute of Laboratory
Animal Resources). Washington DC, Govt Printing Office (DHEW
Publ. No. (NIH) 73-23).
DIECHMANN, W. B. & LEBLANC, T. J. (1943) Determination of the
approximate lethal dose with about six animals. J. ind. Hyg.
Toxicol., 25: 415-417.
DIEKE, S. H. & RICHTER, C. P. (1945) Acute toxicity of thiourea to
rats in relation to age, diet, strain and species variation.
J. Pharmacol. exp. Ther., 83: 195-202.
EPSTEIN, S. (1969) A catch-all toxicological screen. Experientia
(Basle), 25: 617.
FERON, V. J. (1973) An evaluation of the criterion "organ weight"
under conditions of growth retardation. Food Cosmet. Toxicol.
11: 85-94.
FEUER, G., GOLBERG, L., & LE PELLEY, J. R. (1965) Liver response
tests. I. Exploratory studies on glucose 6-phosphatase and other
liver enzymes. Food Cosmet. Toxicol., 3: 235-249.
FLEISS, J. L. (1973) Statistical methods for rates and proportions.
New York, John Wiley (Wiley Series in Probability and
Mathematical Statistics).
FLODMARK, S. & STEINWALL, O. (1963) Differentiated effects on certain
blood brain barrier phenomena and on the EEG produced by means of
intracarotidly applied mercuric dichloride. Acta. Physiol.
Scand., 57: 446-453.
FOUTS, J. R. & HART, L. G. (1965) Hepatic drug metabolism during the
perinatal period. Ann. NY Acad. Sci., 123: 245-251.
FRAZER, A. C. & SHARRATT, M. (1969) The value and limitations of
animal studies in the prediction of effects in man. In: The use
of animals in toxicological studies -- UFAW Symposium, England,
1968, Potters Bar, England, 41 pp.
FRIEDMAN, L. (1969) Symposium on the evaluation of the safety of food
additives and chemical residues. II. The role of the laboratory
animal study of intermediate duration for evaluation of safety.
Toxicol. appl. Pharmacol., 16: 498-506.
FROST, J. K. (1969) Manual for the tenth postgraduate institute for
pathologists in clinical cytopathology. Baltimore, MD, Johns
Hopkins Hospital.
GEHRING, P. J., ROWE, V. K., & MCCOLLISTER, S. B. (1973) Toxicology:
cost-time. Food Cosmet. Toxicol., 11: 1097-1110.
GRANT, D. L., HATINA, G. V., & MUNRO, I. C. (1975) Hexachlorobenzene
accumulation and decline of tissue residues and relationship to
some toxicity criteria in rats. Environ. Qual. Saf., Supplement
Vol. III, pp. 562-568.
GRICE, H. C. (1972) The changing role of pathology in modern safety
evaluation. CRC Crit. Rev. Toxicol., 1: 119-152.
GRICE, H. C., BARTH, M. L., CORNISH, H. H., FOSTER, G. V., & GRAY, R.
H. (1971) Experimental cobalt cardiomyopathy: correlation between
electrocardiography and pathology. Cardiol. Res., 4: 452-456.
GRIFFITH, J. F. (1964) Inter-laboratory variations in the
determination of acute oral LD50. Toxicol. appl. Pharmacol.,
6: 726-730.
HAGAN, J. M. (1959) Acute toxicity. In: Appraisal of the safety of
chemicals in food, drugs and cosmetics, Assoc. Food & Drug
Officials of USA, pp. 17-25.
HARADA, Y., MIYAMOTO, Y., NONAKA, I., OHTA, S., & NINOMIYA, T. (1967)
Electroencephalographic studies on Minamata Disease in children.
Dev. Med. Child Neurol., 10: 257-258.
HEALTH & WELFARE, CANADA (1970) Guide for the preparation of
submissions for food additives. Ottawa, Health & Welfare.
HEALTH & WELFARE, CANADA (1973) The testing of chemicals for
carcinogenicity, mutagenicity and teratogenicity. Ottawa,
Health & Welfare.
HENLEY, K. S., SCHMIDT, E., & SCHMIDT, W. (1966) Enzymes in serum,
their use in diagnosis. Springfield, IL, Thomas.
HORN, H. J. (1956) Simplified LD50 (or ED50) calculations.
Biometrics, 12: 311-321.
HRBEK, A., KARLBERG, P., KJELLMER, J., & OLSSON, T. (1972) Evoked EEG
responses in newborns with asphyxia and IRDs. Pediatr. Res.,
6: 61.
HURST, E. W. (1958) Sexual differences in the toxicity and therapeutic
action of chemical substances. In: Walpole, A. L. & Spinks, A.,
ed. The evaluation of drug toxicity, London, Churchill,
pp. 12-25.
JOHNSON, L. P. V. (1950) An introduction to applied biometrics.
Minneapolis, Burgers Publ., Co.
JONDORF, W. R., MAICKEL, R. P., & BRODIE, B. B. (1959) Instability of
newborn mice and guinea pigs to metabolize drugs. Biochem.
Pharmacol., 1: 352-354.
KELLO, D. & KOSTIAL, K. (1973) The effect of milk diets on lead
metabolism in rats. Environ. Res., 6: 355-360.
KORSRUD, G. O., GRICE, H. C., & MCLAUGHLAN, J. U. (1972) Sensitivity
of several serum enzymes in detecting carbon tetrachloride --
including liver damage in rats. Toxicol. appl. Pharmacol.,
22: 474-483.
LECLAIR, J. M. & WILLARD, J. W. (1970) Guide for the preparation of
submissions on tolerances for incidental contaminants and
agricultural chemicals in food. Ottawa, Canada, Food & Drug
Directorate, Dept of National Health & Welfare.
LITCHFIELD, J. T. (1962) Evaluation of the safety of new drugs by
means of tests in animals. Clin. Pharmacol. & Therap.,
3: 665-672.
LITCHFIELD, J. T. & WILCOXON, F. A. (1947) A simplified method of
evaluating dose-effect experiments. J. Pharm. exp. Ther.,
95: 99-113.
LOOMIS, T. A. (1968) Essentials of toxicology. Philadelphia, Lea &
Febiger.
LU, F. J., JESSUP, D. C., & LAVALLEE, A. (1965) Toxicity of pesticides
in young versus adult rats. Food Cosmet. Toxicol., 3: 591-596.
MAGILL, P. I., HOLDEN, F. R., & ACKLEY, C. (1956) Air Pollution
Handbook. New York, McGraw-Hill.
MANN, L. I. (1970) Developmental aspects and the effect of carbon
dioxide tension on fetal cephalic metabolism and
electroencephalogram. Exp. Neurol., 26: 148-159.
MARZULLI, F. N. (1968) Ocular side effects of drugs. Food Cosmet.
Toxicol., 6: 221-223.
MCGRATH, J. T. (1960) Neurological examination of the dog with
clinicopathological observation. 2nd ed. Philadelphia, Lea &
Febiger.
MILLER, L. C. & TAINTER, M. L. (1944) Estimation of the ED50 and its
error by means of log-probit graph paper. Proc. Soc. Exp. Biol.
Med. NY, 57: 261-264.
MORRISON, J. K., QUINTON, R. M., & REINERT, H. (1968) The purpose and
value of LD50 determinations. In: Boyland, E. & Goulding, R.,
ed. Modern trends in toxicology. London, Butterworths,
pp. 1-17.
MOWBRAY, J. B. & CADELL, T. E. (1962) Early behaviour patterns in
rhesus monkeys. J. Comp. Physiol. Psychol., 55: 350-357.
MUNRO, I. C., MIDDLETON, E. J., & GRICE, H. C. (1969) Biochemical and
pathological changes in rats fed brominated cottonseed oil for 80
days. Food Cosmet. Toxicol., 7: 25-33.
MUNRO, I. C., CHARBONNEAU, S. M., & WILLES, R. F. (1972) An automated
data acquisition and computer-based computation system for
application to toxicological studies in laboratory animals.
Lab. Anim. Sci., 22: 753-756.
MUNRO, I. C., MOODIE, C. A., KREWSKI, D., & GRICE, H. C. (1974)
Carcinogenicity study of commercial saccharin in the rat.
Toxicol. appl. Pharmacol., 32: 513-526.
NATIONAL ACADEMY OF SCIENCES (1975) Principles for evaluating
chemicals in the environment. Washington, DC, National Academy
of Sciences.
NEWBERNE, P., ROGERS, A. E., & WOGAN, G. N. (1968) Hepatorenal lesions
in rats fed a low lipotrope diet and exposed to aflatoxin.
J. Nutr., 94: 331-343.
OSBORNE, B. E. & DENT, N. J. (1973) Electrocardiography and blood
chemistry in the detection of myocardial lesions in dogs.
Food Cosmet. Toxicol., 11: 265-276.
PAGET, G. E. & BARNES, J. M. (1964) Toxicity tests. In: Laurence, D.
R. & Bacharach, A. L., ed. Evaluation of drug activities:
Pharmacometrics, London, New York, Academic Press,
Vol. 1, pp. 135-166.
PECK, H. M. (1974) Design of experiments to detect carcinogenic
effects of drugs. In: CRC Carcinogenesis testing of chemicals,
Cleveland, OH, CRC Press, pp. 1-13.
RUMKE, CHR. L. (1964) Some limitations of animal tests. In: Laurence,
D. R. & Bacharach, A. L., ed. Evaluation of drug activities:
Pharmacometrics, London, New York, Academic Press,
Vol. 1, pp. 125-133.
SCHALM, O. W. (1967) Veterinary haematology, 2nd ed. Philadelphia,
Lea & Febiger.
SCOTT, W. J., JOHNSTON, J. W., JOHNSTON, C. D., & BELILES, R. P.
(1966) Comparative acute toxicities of isoproterenol and
metraproterenol. Toxicol. appl. Pharmacol., 8: 353.
SETNIKAR, I. & MAGISTRETTI, M. J. (1964) The toxicity of central
nervous system stimulants in rats of different ages.
Proc. Eur. Soc. Drug Toxicity, 4: 132-139.
SHER, S. P. (1972) Mammary tumours in control rats: literature
tabulation. Toxicol. appl. Pharmacol., 22: 562-588.
SIRRIDGE, M. S. (1967) Laboratory evaluation of haemostasis.
Philadelphia, Lea & Febiger.
SNEDECOR, J. W. & COCHRAN, W. G. (1967) Statistical methods 6th ed.,
Ames, IA, Iowa State University Press, pp. 111-114, 221-223.
SONTAG, J. M., PAGE, N. P., & SAFFIOTTI, U. (1975) Guidelines for
carcinogen bioassay in small rodents. Bethesda, MD, National
Cancer Institute.
STAUBLI, W., HESS, R., & WEIBEL, E. R. (1969) Correlated morphometric
and biochemical studies on the liver cell. II. Effects of
phenobarbital on rat hepatocytes. J. cell. Biol., 42: 92-112.
THOMPSON, W. (1947) Use of moving averages and interpolation to
estimate median effective dose. Bac. Rev., 11: 115-141.
TOMATIS, L. & MOHR, U., ed. (1973) Transplacental carcinogenesis,
IARC Sci. Publ. No. 4, 181 pp.
WEIL, C. S. (1952) Relationship between short- and long-term feeding
studies in designing an effective toxicity test. Agric. Food
Chem., 11: 486-491.
WEIL, C. S., WOODSIDE, M.D., BERNARD, J. R., & CARPENTER, C. P. (1969)
Relationship between single peroral, one-week and ninety-day
feeding studies. Toxicol. appl. Pharmacol., 14: 426-431.
WEIL, C. S., & CARPENTER, C. P. (1969) Abnormal values in control
groups during repeated-dose toxicological studies. Toxicol.
appl. Pharmacol., 14: 335-339.
WEINBERG, M. S., GOLDHAMEN, R. E., & CARSON, S. (1966) Acute oral
toxicity of various drugs in newborn rats after treatment of the
dam during gestation. Toxicol. appl. Pharmacol., 9: 234-239.
WORDEN, A. N. & HARPER, K. H. (1963) Oral toxicity as influenced by
method of administration. Proc. Eur. Soc. Study Drug Toxicity,
2: 15-26.
WHO (1958) WHO Technical Report Series No. 144. (Procedures for the
testing of intentional food additives to establish their safety
for use -- 2nd report of the joint FAO/WHO Expert Committee on
Food Additives), 19 pp.
WHO (1972) Health hazards of the human environment, Geneva, WHO,
387 pp.
YEARLY, R. A. & BENISH, R. A. (1965) A comparison of the acute
toxicities of drugs in newborn and adult rats. Toxicol. appl.
Pharmacol., 7: 504.
ZBINDEN, G. (1973) Progress in toxicology: special topics. Vol. 1.
New York, Springer-Verlag.
ZIMMERMAN, H. J. (1974) Serum enzyme measurement in experimental
hepatotoxicity. Israel J. med. Sci., 10: 328-332.
4. CHEMOBIOKINETICS AND METABOLISM
4.1 Introduction
The objective of chemobiokinetic studies is to obtain data that
allow reliable assessment of the hazard of environmental chemicals to
man. Since effects are related to the amounts or concentrations of a
chemical in tissues and cells, it is imperative to elucidate the
dynamics of the toxicant at the target site. It should be emphasized
that the toxicant may be either the parent chemical or a metabolite or
degradation product formed from it. Thus, the qualitative
identification of the degradation products of a chemical together with
a quantitative characterization of their fate, as well as the fate of
the parent chemical, as a function of time, are inextricably
associated in a proper chemobiokinetic evaluation. In the context of
this chapter, the word "chemobiokinetics" has been used in place of
"pharmacokinetics" because too often the latter implies restriction of
this scientific discipline to drugs. The term chemobiokinetics is
proposed to emphasize its importance in evaluating the biological
effects of all chemicals.
4.2 Absorption
4.2.1 General principles
Absorption of a chemical into the body can take place,
potentially, by all routes of exposure. In assessing the toxicity and,
ultimately, the hazard of a chemical, the oral, dermal, and inhalation
routes of exposure are of primary importance. Following absorption,
the chemical is distributed by the blood to the various tissues.
Therefore, the rate of absorption is frequently estimated by
determining the concentration of the chemical in the plasma as a
function of time following exposure.
The route of administration can greatly influence the rate at
which a foreign chemical enters the body. Upon ingestion, the gastric
contents and pH of the stomach can influence the rate of absorption of
the chemical. In the small intestine, food may either enhance or delay
absorption. Indeed, the environment of the gastrointestinal tract (pH,
food, bacteria) may change the parent chemical into another chemical.
The inhalation route allows a chemical to pass rapidly into the blood
without encountering drastic changes in pH, food, or microflora. The
skin effectively retards the absorption of many chemicals; however, it
should not be considered as an absolute barrier. Some chemicals
readily penetrate intact skin and a minor abrasion of the skin may
greatly enhance the absorption of many chemicals.
In order that a chemical may be absorbed into the bloodstream, it
must cross one or more semipermeable membranes, such as the
gastrointestinal epithelium, the lining of the respiratory tract, or
the epidermis of the skin. Membranes are essentially lipoproteins with
aqueous pores through which water-soluble molecules can pass. The pore
size varies from 4 Å (intestinal epithelium and mast cells) to 30 Å
(capillaries), allowing the passage of molecules with molecular
weights less than 100-200 to approximately 60 000, respectively. Most
membranes have an electrical potential that may effectively preclude
the ready penetration of charged chemical species. Thus it is obvious
that the absorption of a chemical depends on its physicochemical
properties, molecular size, shape, degree of ionization, and lipid
solubility. For a more thorough discussion, the reader is referred to
Davson & Danielli (1952) and Schanker (1962a).
Three mechanisms have been proposed to explain how a chemical
passes across a cell membrane: (a) passive diffusion through the
membrane, (b) filtration through membranous pores, and (c) specialized
transport systems that carry water-soluble and large molecules across
the membrane by means of a "carrier".
Passive diffusion is considered to be the principal mechanism by
which chemicals can cross cell membranes. The rate of passive
diffusion of a molecule is proportional to the concentration gradient
across the membrane, the membrane thickness, the area available for
diffusion, and the diffusion constant, in accordance with Fick's Law
(La Du et al., 1972). The rate of passage is related directly to the
lipid solubility (Brodie, 1964). However, since absorption requires
passage through an aqueous- as well as a lipo-phase, the absorption of
a chemical with an extremely low solubility in water may be impeded in
spite of a high lipid-to-water partition coefficient. The passive
diffusion also depends on the extent of the ionization and the lipid
solubility of the ionized and nonionized species (Brodie, 1964).
Filtration is a process by which a chemical passes through the
aqueous pores in the membrane, and is governed by the size and shape
of the molecule. The bulk flow of water across the membrane produced
by an osmotic gradient or hydrostatic pressure can act as a carrier
for chemicals.
Specialized transport processes are needed to explain the
transport and kinetic behaviour of large, lipid insoluble molecules
and ions. Two types of carrier-mediated transport systems have been
recognized: active transport and facilitated diffusion. The carrier in
both systems is some component of the membrane that combines with the
chemical and assists its passage across the membrane. It has a limited
capacity and when it is saturated, the rate of transfer is no longer
dependent on the concentration of the chemical and assumes zero order
kinetics. Structure, conformation, size, and charge are important in
determining the affinity of a molecule for a carrier site, and
competition for carrier site will occur.
Active transport is a carrier-mediated transport system which
moves a molecule across a membrane against a concentration gradient,
or, if the molecule is an ion, against an electrochemical gradient. It
requires the expenditure of metabolic energy and can be inhibited by
poisons that interfere with cell metabolism. Active transport plays an
important role in the renal and biliary excretion of chemicals.
Facilitated diffusion is a carrier transport mechanism by which a
water-soluble molecule (i.e. glucose) is transported through a
membrane down a concentration gradient. No apparent energy is required
and metabolic poisons will not inhibit this process. The difference
between facilitated diffusion and active transport is that the latter
moves molecules against a concentration gradient, whereas the former
does not. For more complete discussion of membrane transport, refer to
La Du et al. (1972) and Goldstein et al. (1974).
Another active process, pinocytosis, has been implicated as a
mechanism for transferring large molecules and particles into cells.
In this process, the membrane engulfs the material and pinches off an
envelope containing the material within the cell.
4.2.2 Absorption from the lungs
The pulmonary epithelial lining is very thin, possesses a large
surface area, and is highly vascular. Thus, absorption of foreign
chemicals can take place at a very rapid rate. Most rapidly absorbed
are gases and aerosols with small particle size and a high
lipid-to-water partition coefficient. In most inhalation studies,
absorption may occur by routes other than the lungs and the
investigator should be aware of this in the interpretation of data.
A more complete discussion of the inhalation of chemicals is
presented in Chapter 6.
4.2.3 Absorption from the skin
The structure of the skin enables rapid penetration of
lipid-soluble compounds through the epidermis, a lipoprotein barrier,
whereas the highly porous dermis is permeable to both lipid- and
water-soluble substances (Katz & Poulsen, 1971). Factors which govern
penetration through the skin are hydration, pH, temperature, blood
supply, and metabolism as well as vehicle-skin interactions. Abrasion
of the skin may enhance absorption greatly. For a more complete
discussion of the principles for absorption through the skin and
experimental methods, refer to Part II, Chapter 11.
4.2.4 Gastrointestinal absorption
The gastrointestinal tract is one of the most important routes of
absorption of foreign compounds (Schanker, 1971). Chemicals can be
absorbed along any section of the gastrointestinal tract, but because
of the large surface area and rich blood supply, absorption is
favoured from the small intestines. In most parts, the movement of a
chemical across the epithelial lining of the gastrointestinal tract is
by diffusion and carrier transport mechanisms are involved to a lesser
degree.
Although therapeutic amounts of drugs may be absorbed from the
buccal mucosa (Beckett & Hossie, 1971), absorption of environmental
chemicals from the mouth is minimal compared with that from the
stomach and intestine. Chemicals absorbed from the mouth are not
exposed to the gastrointestinal digestive juices and drug-metabolizing
enzymes. Furthermore, since they are not transported by the hepatic
portal system directly to the liver, their normally rapid metabolism
may be precluded, thus prolonging their effect.
The stomach is a significant site of absorption by passive
diffusion of many acid, and neutral, foreign compounds (Schanker et
al., 1957). Due to the acidity of the stomach, weak acids will exist
in the diffusible, nonionized, lipid-soluble form, whereas weak bases
will be highly ionized and therefore not generally absorbable.
Absorption from the small intestine is similar in principle to
that from the stomach (passive diffusion), except that the pH of the
intestinal contents (pH 6.6) may alter the fraction of the chemical in
the nonionized form favouring the absorption of both weakly-acid and
weakly-alkaline chemicals. The aqueous pore size, 4 Å, limits
absorption by filtration to molecules having a molecular weight of
less than 100-200. Rarely, an environmental chemical may be absorbed
from the intestinal tract by an active transport system that is
normally involved in the absorption of nutrients, e.g. sugars and
amino acids (Schanker, 1963).
Many factors can affect the absorption of foreign compounds from
the gastrointestinal tract (Brodie, 1964; Levine, 1970; Place &
Benson, 1971; Prescott, 1975): ( a) increased gastric emptying can
decrease gastric absorption and increase intestinal absorption; ( b)
increased intestinal peristalsis generally inhibits intestinal
absorption; ( c) gastric acid, intestinal digestive juices, and gut
microflora can all degrade chemicals to other absorbable or
nonabsorbable chemical species; ( d) food in the gastrointestinal tract
can impair absorption by producing a nonabsorbable complex, by
decreasing gastric emptying (especially fats), and by reducing,
mixing, or altering pH; ( e) normal digestion produces increased
gastrointestinal blood flow which will enhance absorption; ( f)
absorption of a solid will be impaired if dissolution in the
gastrointestinal tract does not take place. The practice of
administering chemicals admixed with the diet must take these factors
into account, especially the possible reaction of the chemical with
dietary constituents.
4.3 Distribution
Once absorbed, the distribution of a chemical is determined by
the relative plasma concentration, the rate of blood flow through
various organs and tissues, the rate by which the chemical penetrates
cell membranes, and the binding sites that are immediately available
in the plasma and tissues. After the initial distribution phase, the
rate by which a chemical penetrates cell membranes and the available
sites for binding are the predominating factors influencing the final
distribution of a chemical in the body.
When the plasma concentration of a chemical is high and the cell
membranes do not provide significant barriers to diffusion,
distribution is mainly to organs with high blood flow, e.g. brain,
liver, and kidney. A classic example of distribution and
redistribution is thiopental, a highly lipid-soluble chemical that,
after administration, is first distributed to the brain and
subsequently to muscle and body fat which have poor blood flow (Price
et al., 1960). Lipid-soluble, foreign compounds tend to be distributed
and localized in adipose tissue (Mark, 1971), in accordance with their
lipid-to-water partition coefficients, e.g. the chlorinated
hydrocarbon pesticides, dieldrin, DDT, and DDE (Rodomski et al., 1968)
and polychlorinated biphenyls (PCBs) (Allen et al., 1974).
Distribution of chemicals into organs and tissues is influenced by
membraneous barriers in the same way as absorption (see 4.2). For a
more detailed treatment, see Quastel (1965). The capillary membrane,
unlike other body or cell membranes, is freely permeable to foreign
compounds of a molecular weight of 60 000 or less, whether
lipid-soluble or not (Pappenheimer, 1953; Renkin, 1964); generally
chemicals pass these membranes readily, except in the brain,
testicles, and the eye (Gehring & Buerge, 1969).
The movement of foreign chemicals to the brain represents a
unique example that cannot be explained by the physicochemical
properties of the chemical and the tissue distribution. Many chemicals
fail to penetrate into the brain tissue or cerebrospinal fluid as
readily as into other tissues (Brodie & Hogben, 1957). The boundary
between blood and brain consists of several membranes; those of the
blood capillary wall, the glial cells closely surrounding the
capillary, and the membrane of the neurons or nerve cells. The
so-called "blood-brain barrier" is located at the capillary wall-glial
cell region. The capillary walls in the brain tend to be more like
cell membranes than capillary membranes. Therefore, ionized substances
and large water-soluble molecules such as proteins are almost entirely
excluded from passage (Rall, 1971). The chief mode of exit of both
lipid-soluble and polar compounds is by filtration across the
arachnoid villi. The method for studying the movement of chemicals
into and from the brain has been discussed by Rall (1971).
The red blood cell has unusual permeability in that organic
unions penetrate much more readily than cations. This may be explained
by the presence of positively charged membrane pores that will accept
anions but repel cations (Schanker et al., 1957).
4.4 Binding
A major factor, that can affect the distribution of a chemical,
is its affinity to bind to proteins and other macromolecules of the
body. Foreign chemicals have been shown to bind reversibly to such
substrates as albumen, globulins, haemoglobin, mucopolysaccharides,
nucleoproteins, and phospholipids (Shore et al., 1957). For a survey
of the biological implications of the protein binding of chemicals,
the reader is referred to Gillette (1973a).
Once a chemical is bound to a body constituent, it is temporarily
localized. This localization modifies the initial pattern of
distribution and affects the rates of absorption, metabolism, and
elimination of the chemical from the body.
4.4.1 Plasma-protein binding
Most chemicals show some degree of binding to plasma proteins,
the most important fraction of which is albumen. Albumen at pH = 7.3
contains a net negative charge; however, cationic groups must be
accessible because albumen has been shown to bind anions as well as
cations. Although the plasma proteins show an appreciable capacity for
binding many chemicals, this is limited, making it important to
understand such binding as a function of the concentration of the
chemical.
Since plasma proteins possess a limited number of binding sites
and the sites are somewhat nonspecific, two chemicals with an affinity
for the same binding site will compete with one another for binding.
The plasma proteins of various laboratory animals and man show
differences in the degree and nature of binding. This is due to
differences in the total concentrations and relative proportions of
the various plasma proteins as well as the composition and
conformation of albumens (Gillette, 1973b).
4.4.2 Tissue binding
The binding of chemicals to tissue constituents also contributes
to the localization of a chemical. Certain chemicals show a much
greater affinity for tissue than for plasma proteins, and in some
instances the affinity for tissue is quite specific. For example,
polycyclic aromatic compounds have been shown to have particular
affinity for the melanin in the eye (Potts, 1964).
Some metals and several chemicals and organic anions are bound to
proteins (Y and Z proteins or ligandins) in the liver (Levi et al.,
1969). These proteins may play a key role in the transfer of organic
anions from plasma to liver (Levi et al., 1969; Reyes et al., 1971),
and they also bind corticosteroids and azo-dye carcinogens (Litwack et
al., 1971). For further details concerning the nature and effects of
binding of chemicals by proteins and for methods of study, see
Chignell (1971), Gillette (1975), Keen (1971) and Settle et al.
(1971).
Many inorganic ions, particularly metals, as well as
tetracycline, are concentrated in various tissues and organs,
particularly in bones and teeth (Foreman, 1971). A convenient method
for studying the accumulation of chemicals in organs and tissue is
autoradiography (Roth, 1971). Valuable measurements may also be
obtained with classical chemical and radio-chemical techniques which
have the added advantage of being quantitative.
4.5 Excretion
Chemicals are excreted as the parent chemical, as metabolites, or
as conjugates of the parent chemical or its metabolites. The principal
routes of excretion are the urine and bile, and to a lesser degree
expired air, sweat, saliva, milk, and secretions of the
gastrointestinal tract.
4.5.1 Renal excretion
The kidneys are the most important route of excretion of foreign
compounds (Weiner, 1971). The three mechanisms of renal excretion are:
glomerular filtration, active tubular transport, and passive tubular
transport. Only compounds of high molecular weight or those bound
tightly to plasma proteins escape glomerular filtration and the
resulting filtrate contains approximately the same concentration of
foreign compounds as that found in the plasma in an unbound state.
Water and endogenous substrates are reabsorbed from the
glomerular filtrate as it passes down the tubule. In the tubule,
lipid-soluble, unionized chemicals pass in either direction by passive
diffusion. Thus, lipid-soluble chemicals may be reabsorbed by the
tubule, prolonging their retention in the body. Ionic chemicals, such
as conjugates and other metabolites, are poorly reabsorbed and pass
directly out of the body in the urine.
Active transport takes place in the proximal tubule of the
kidney. There are two distinct active transport processes. One process
is specific for organic anions and the other specific for organic
cations. Chemicals transported by the same transport process compete
with each other, and the excretion rate of one compound can be reduced
by the administration of the other. The active transport process can
be saturated as the concentration of the chemical in the plasma is
increased. When the active tubular secretion is saturated, that is,
when an increase in the concentration of the chemical in the plasma is
no longer accompanied by a proportional increase in the concentration
of the chemical in the urine, the concentration in the plasma is
referred to as the renal-plasma threshold.
The anionic secretory process is responsible for the excretion of
metabolites formed through conjugation of the parent chemical or its
degradation products with various endogenous substrates such as
glycine, sulfate, or glucuronic acid. These relatively polar,
lipid-insoluble metabolites are poorly reabsorbed from the tubules and
more readily excreted.
4.5.2 Biliary excretion
Biliary excretion is a major route for the excretion of foreign
chemicals (Smith, 1971a, 1973). It is has been demonstrated (Brauer,
1959; Schanker, 1962b; Sperber, 1963; Williams, 1965) that compounds
with high polarity, anionic and cationic conjugates of compounds bound
to plasma proteins, and compounds with molecular weights greater than
300 are actively transported against a concentration gradient into the
bile. It has also been shown that, once these compounds are in the
bile, they are not reabsorbed into the blood and are excreted into the
gastrointestinal tract (Schanker, 1965). Factors that influence the
biliary excretion of foreign chemicals and metabolites are considered
to be of two types: (a) physicochemical, relating to molecular size,
structural features, and polarity; and (b) biological, relating to
protein binding, renal excretion, metabolism, species, and sex. For a
comprehensive and detailed discussion of these subjects, the reader is
referred to Smith (1971a, 1973), and Stowe & Plaa (1968).
4.5.3 Enterohepatic circulation
Enterohepatic circulation is the phenomenon that occurs when a
compound is excreted via the bile into the gastrointestinal tract,
reabsorbed from the gastrointestinal tract and carried via the portal
system back to the liver, where it is again excreted via the bile and
recycled. Physiologically, enterohepatic circulation is important
because it permits reuse of endogenous biliary excretion products.
However, when a foreign compound is involved in enterohepatic
circulation, it must make its way either to the faeces or to the
peripheral blood to be excreted from the body. Thus, enterohepatic
circulation of a foreign compound serves to enhance its retention in
the body. There are examples in the literature (Gibson & Becker, 1967;
Keberle et al., 1962) which demonstrate that the half-life of a
compound involved in enterohepatic circulation can be decreased after
surgically interrupting the enterohepatic cycle. Administration of a
sequestering agent that binds the compound in the gastrointestinal
tract would serve the same purpose.
Smith (1973) has described the following factors that can affect
the enterohepatic circulation of a compound: ( a) the extent and rate
of excretion of the compound in the bile; ( b) the activity of the gall
bladder; ( c) the fate of the substance in the small intestine; and ( d)
the fate of the compound after reabsorption from the gut. Since many
foreign chemicals are excreted in the bile as unabsorbable conjugates,
the hydrolysis of these conjugates in the intestine may play a key
role in enterohepatic circulation. For a thorough discussion of
enterohepatic circulation, the reader is referred to Plaa (1975).
4.5.4 Other routes of excretion
In addition to excretion in bile and urine, other routes for the
excretion of foreign chemicals and their metabolites should not be
overlooked. In accordance with the pH partition theory, organic bases
highly ionized at the pH value of gastric juice may be secreted into
the stomach (Shore et al., 1957). Similarly, weak acids ionized at
neutral pH may be transferred from the plasma to the lumen of the
intestine. These chemicals are sequestered by the intestinal contents,
augmenting their excretion in the faeces.
Many volatile organic chemicals are excreted readily via exhaled
air (see Chapter 6). This route of excretion is common for carbon
dioxide, an ultimate end-product of an extensively metabolized organic
chemical. For this reason, the quantification of expired radiolabelled
carbon dioxide (14CO2) is very important in chemobiokinetic
studies using carbon-14-labelled compounds.
Many foreign compounds are excreted, to different degrees, in
milk, in either the aqueous or lipid phase (Rasmussen, 1971). Although
this route may be of minor importance for the elimination of a
chemical from the body, it should be given particular attention in
evaluating the hazard of chemicals to man. First, consumption of cow's
milk may constitute an important vehicle of exposure. Secondly, the
consumption of mother's milk by the newborn may provide very high
doses of a chemical that is concentrated in the milk. It should also
be noted that the volume of milk consumed by the newborn per unit body
weight may, in itself, magnify the dose received by this segment of
the population.
Chemicals are also excreted in sweat and saliva. The presence of
a chemical in sweat may lead to dermatitis. Although saliva is usually
swallowed and thus does not lead to elimination of the agent from the
body, recent work has shown that analysis of saliva for the presence
of a chemical may preclude the necessity for venepuncture to obtain
plasma for analysis.
4.6 Metabolic Transformation
Metabolic transformation or biotransformation are terms that have
been used to describe the process which converts a foreign chemical to
another derivative (metabolite) in the body. Metabolic transformation
has been the subject of several excellent reviews (Conney, 1967;
Conney & Burns, 1962; Dahm, 1971; Daly, 1971; Dutton, 1971; Garattini
et al., 1975; Gillette, 1971a,b & 1974a,b; Gillette et al., 1974;
Kuntzman, 1969; McClean, 1971; Smuckler, 1971; Weisburger &
Weisburger, 1971). It usually results in the formation of more polar
and water-soluble derivatives of a foreign chemical which can be more
readily excreted from the body. Generally, such metabolic
transformation of a foreign chemical also results in the formation of
a less toxic chemical. However, there are many cases where the
metabolites are more toxic than the parent chemicals (McLean, 1971;
Miller & Miller, 1971a).
A few compounds resist metabolic transformation. Most strong
acids and bases are excreted unchanged. Also the resistance of
long-acting nonpolar compounds (barbital, halogenated benzene, etc.),
to metabolic transformation might explain their slow elimination from
the body.
A metabolic activation is suggested, if a compound is more toxic
when given orally than intravenously, if there is a long delay between
the administration of a chemical and the onset of its biological
effect, or, if there is an increased effect following pretreatment
with compounds that induce metabolic transformation (Garattini et al.,
1975).
4.6.1 Mechanisms of metabolic transformation
Usually, the metabolic transformation of chemicals takes place to
the greatest extent in the liver and is catalysed by enzymes found in
the soluble, mitochondrial, and microsomal fractions of the cell.
Enzymes metabolizing foreign chemicals are also found, to a lesser
degree, in the cells of the gastrointestinal tract, kidney, lung,
placenta, and blood (Aitio, 1973; Gillette, 1963; Gram, 1973;
Hietanen, 1974; Hietanen & Valinio, 1973; Wattenberg & Leong, 1971;
Wattenberg et al., 1962; Witschi, 1975). It must be emphasized that
for a particular chemical, or a particular route of administration,
other organs may play a more important role in the metabolic
transformation of the chemical than the liver. The role of enzymatic
reactions carried out by the intestinal flora may be very important
and should not be overlooked (Scheline, 1968; Smith, 1971a).
Enzyme-catalysed, biochemical transformations can be classified into
four main types: (a) oxidations, (b) reductions, (c) hydrolyses and
(d) synthetic reactions (see Table 4.1 in Annex to this Chapter).
The metabolic transformation of a chemical can occur via various
pathways which can consist of a single reaction or multiple reactions.
If the metabolic pathway consists of one reaction it is usually
oxidation, reduction, or hydrolysis which tends to increase the
polarity of the compound. Multiple-reaction metabolic pathways can
consist of a series or any combination of oxidation, reduction, or
hydrolysis. The final reaction in a multiple-reaction pathway is
usually a conjugation reaction involving the addition of polar
endogenous functional groups (D-glucuronic acid, glycine etc.) which
usually render the molecule more polar, less lipid-soluble, and
therefore more readily excretable. The predominant sequence of
reactions or metabolic pathways is determined by many factors such as
the dose of the chemical, species, strain, age, sex, and certain
environmental variables.
4.6.1.1 Microsomal, mixed-function oxidations
The metabolism of a large variety of foreign compounds involves
oxidative processes. Microsomal oxidation refers to reactions
catalysed by the enzymes found in the microsomes of the endoplasmic
reticulum. These enzymes are sometimes referred to as microsomal,
mixed-function oxygenases (mono-oxygenases) (Mason, 1957). The
reactions require molecular oxygen and nicotinamide adenine
dinucleotide phosphate, reduced form (NADPH). The reduction
equivalents from NADPH are used to reduce molecular oxygen so that it
can be carried by a cytochrome called P-450 to the compound to be
oxygenated. The oxygen is then fixed into the compounds, usually as a
hydroxyl group (Estabrook, 1971; Estabrook et al., 1971).
The apparent sequence of events in the course of a mixed function
oxidation has been described (Boyd & Smellie, 1972; Estabrook et al.,
1972; Gillette, 1971c). The compound (substrate) forms a complex with
the oxidized cytochrome P-450; this is reduced either directly by
NADPH-cytochrome- c-reductase (1.6.2.4) or indirectly via an
unidentified electron carrier. The reduced cytochrome P-450-substrate
complex then reacts with oxygen to form an "active oxygen" complex,
which decomposes with the formation of the oxidized substrate and
oxidized cytochrome P-450. Substantial progress has been made in
elucidating this mechanism by the development of a method involving
the resolution and reconstitution of the components of the liver
microsomal hydroxylating system (Lu & Levin, 1974).
Measurement of mixed-function oxidase activities of liver
microsomes in vitro has become an important aspect in evaluating the
toxicity of chemicals. The mixed-function oxidase system may be either
a biotransformation system or a site of action of chemicals.
Measurements of the activity of this system can be performed using
either a 9000 g supernatant fraction (Henderson & Kersten, 1970;
Klinger, 1974) of a liver homogenate prepared in buffered KCl
solution, or a microsome fraction sedimented by centrifugation at
about 105 000 g (Flynn et al., 1972; Hewick & Fouts, 1970a,b; Liu et
al., 1975).
The reaction mixtures consisting of the particle-bound enzymes
have to be supplemented with an NADPH-generating system. This may be
fulfilled by the addition of NADPH and glucose-6-phosphate, if the
9000 g fraction is used, but if washed microsomes are used,
glucose-6-phosphate dehydrogenase (1.1.1.49) must also be added.
Isolated tissue cells, tissue cultures, or slices of organs, as
well as perfused organs can also be used for metabolic studies.
Because cytochrome P-450 is intimately associated with the
metabolism of many foreign chemicals, the following methods and
variables have been developed for ascertaining its activity in the
tissues of animals used in toxicological investigations.
The method of Omura & Sato (1964a,b) has been used to measure the
change in the microsomal content of cytochrome P-450 and cytochrome
b5. This method relies on a spectral shift of the pigment upon
exposure to carbon monoxide. An increase in the cytochrome P-450
content can be explained as a consequence of enzyme induction, whereas
the decrease of the haem pigment content may be the result of enhanced
permeability of microsomal membranes due to the damaging effects of
the chemical (Bond & De Matteis, 1969). The concentration of
cytochrome P-450 in the liver, however, is not always directly
proportional to the activity of the mixed-function oxidases.
Spectral changes of cytochrome P-450, determined in the presence
of various substrates, provide information about the binding between
the pigment and substrate (Hewick & Fouts, 1970a; Remmer et al.,
1966). Compounds may be classified into type I or type II according to
their spectral reactions with cytochrome P-450. When type I compounds
bind to cytochrome P-450, the characteristic spectral shift, spectral
difference, gives a peak at 385-390 nm and a trough at 418-427 nm,
whereas with type II compounds, the peak occurs at 425-435 nm and the
trough at 390-405 nm. Originally, it was thought that the magnitudes
of these spectral shifts, especially type I spectra, could be
correlated with microsomal biotransformations (Schenkman et al.,
1967). This correlation, however, is not universally applicable
(Davies et al., 1969; Gigon et al., 1969; Holtzman et al., 1968).
Thus, differences in the magnitude of these spectral changes are
difficult to interpret when they are detected in animals treated with
a chemical (Gillette et al., 1972). The same is true for the
ethylisocyanide difference spectra of cytochrome P-450 which are
characterized by two peaks at about 455 nm and 430 nm (Omura & Sato,
1964a).
Determination of the NADPH-cytochrome P-450 reductase activity,
the assumed rate limiting step in microsomal oxidations, has proved
useful in evaluating the effectiveness of the cytochrome P-450 system
prior to the oxygenation step (Fouts & Pohl, 1971; Gigon et al., 1969;
Hewick & Fouts, 1970b; Holtzman et al., 1968; Zannoni et al., 1972).
Measurement of the NADPH-cytochrome-c-reductase activity may give
information about the rate of flow of reducing equivalents from NADPH
to cytochrome P-450.
Determination of the rate of enzymatic conversion of a substrate
is a most valuable tool in elucidating the metabolic process. For this
purpose, however, it is essential to know the pathway for the
transformation of the chemical, and analytical methods are essential
to quantify the parent chemical and its reaction products. Selected
methods for monitoring some compounds and enzymatic reactions are
listed in Table 4.2 (see Annex to this Chapter).
There are large variations in the metabolism of foreign chemicals
as well as in susceptibility to metabolic inducers depending on the
species (Hucker, 1970), strain, age, and sex of animals.
Many variables must be considered as important factors in species
differences in the metabolism of foreign chemicals. Among these are
differences in binding, either to tissues or to plasma components,
such as albumen. Considerable variations in binding have been reported
for the same chemical in different species (Borgå et al., 1968; Kurz &
Friemel, 1967; Scholtan, 1963; Sturman & Smith, 1967; Witiak &
Whitehouse, 1969). More obvious are the concentrations and types of
foreign chemical-metabolizing enzymes in each species (Flynn et al.,
1972).
4.6.1.2 Conjugation reactions
The major conjugation mechanisms are: glucuronide synthesis,
"ethereal" sulfate synthesis, glutathione conjugation, glycine
conjugation, methylation, acetylation, and thiocyanate synthesis.
Glutamine conjugation has also been shown to occur in man and monkey.
The conjugates formed by these mechanisms are usually nontoxic,
therefore conjugation has also been referred to as a detoxification
mechanism.
These conjugations are biosynthetic reactions in which foreign
compounds or their metabolites containing suitable groups (hydroxyl,
amino, carbonyl, or epoxide) combine with some endogenous substrates
to form conjugates (Parke, 1968; Williams, 1967a, 1971). These
reactions require ATP as source of energy, coenzymes, and transferases
which are usually specific for the formation of conjugates of foreign
compounds. The conjugations usually proceed in at least two steps:
first, the extramicrosomal synthesis of acylcoenzyme and next the
transfer of the acyl moiety to the aglycone, which, in some but not
all cases, is localized in the microsomes. Thus, these reactions
cannot be considered as transformations, characteristic of microsomes.
In accordance with the coenzymes participating in these
reactions, they include:
formation of glucuronides (via uridine diphosphate glucuronic
acid, UDPGA);
formation of sulfate esters (via 3-phosphadenosine-5-
phosphosulfate, PAPS);
O-, N-, and S-methylation via 5'-[(3-amino-3-carboxypropyl)
methylsulfonio]-5'-dioxyadenosine( S-adenosylmethionine);
acetylations (via acetyl coenzyme A);
formation of peptide conjugates (via different acylcoenzyme A
derivatives);
formation of glutathione conjugates and mercapturic acids
(conjugations with glutathione).
Formation of glucuronides is probably the most important
microsomal conjugation mechanism (Dutton, 1971). It occurs in the
liver and to a lesser extent in the kidney, gastrointestinal tract,
and the skin. Biosynthesis of glucuronides can be measured in intact
animals by determining D-glucaric acid (Marsh, 1963) and
D-glucuronolactone dehydrogenase (1.1.1.70) (Marselos & Hanninen,
1974), by enhancement of D-glucuronolactone and aldehyde dehydrogenase
(1.2.1.3) by inducers of microsomal metabolism (Marselos & Hanninen,
1974), glucuronides (Gregory, 1960; Yuki & Fishman, 1963) and
L-ascorbic acid in urine. Elevation in urinary excretion of these
compounds may be an indicator of an adaptive acceleration of hepatic
glucuronide formation (Notten & Henderson, 1975). It should be
emphasized that increased excretion of D-glucaric acid can result from
enzyme induction; therefore it cannot be assumed that this occurrence
is indicative only of an increased glucuronide formation. Methods for
the measurement of glucuronide synthesis in whole organs and tissue
cultures, as well as in tissue slices, have been summarized by Dutton
(1966). In assays with homogenates and cell fractions the reaction
mixtures have to be supplemented with added UDPGA.
UDP-glucuronosyl transferase (2.4.1.17) activity can be
determined using 2-aminophenol (Burchell et al., 1972; Dutton &
Storey, 1962), 4-nitrophenol (Isselbacher 1956; Zakim & Vessey, 1973),
bilirubin (Heirwegh et al., 1972), 7-hydroxy-4-methyl-2H-I-
benzopyran-2-one (4-methylumbelliferone) (Aitio, 1973; Arias, 1962) or
morphine (Strickland et al., 1974).
In contrast to glucuronide synthesis, the formation of sulfate
esters is most probably an extramicrosomal process and is catalysed
generally by sulfate-conjugating enzymes in the presence of
3-phosphoadenosine-5-phosphosulfate as a co-enzyme (Roy, 1971). Among
the compounds of toxicological interest, phenols are converted by
sulfation to esters and excreted in the urine. Aminophenols yield
sulfamates. There are specific assays for the determination of
sulfotransferase (2.8.2) activity using 4-nitrophenol (Gregory &
Lipmann, 1957), or 3-(2-aminoethyl)-1H-indol-5-ol (serotonin) (Hidaka
et al., 1967) as acceptors.
The methyltransferases (2.1.1) catalyse O-, N- and
S-methylation of several physiologically active compounds and drugs
(Axelrod, 1971). They are widely distributed in different organs, but
only a small amount of catechol- O-methyltransferase (2.1.1.6) and
almost all of the phenol- O-methyltransferase (2.1.1.25) (Axelrod &
Daly, 1968) activity is localized in the microsomes of the liver. Only
microsomal transferases are induced by benzo(a)pyrene and inhibited by
SKF 525Aa. The methods used for the determination of
catechol- O-methyltransferase activity are based on the principle
that the enzyme catalyses the transfer of methyl groups to catechols
in the presence of S-adenosylmethionine as a methyl donor. The
substrates employed include adrenaline (Axelrod & Tomchick, 1958),
3,4-dihydrobenzoic acid (MacCaman, 1965), 3,4-dihydroxybenzeneacetic
acid (3,4-dihydrophenylacetic acid) (Assicot & Bohuon, 1969; Broch &
Guldberg, 1971) as well as I-(3,4-dihydroxyphenyl)ethanone
(3,4-dihydroxyacetophenone) (Borchardt, 1974). The end products of the
enzymatic reaction are measured either spectrofluorimetrically
(Axelrod & Tomchick, 1958; Borchardt, 1974; Broch & Guldberg, 1971),
or radiometrically using labelled methyl groups in the coenzyme
(MacCaman, 1965).
a Diethyl aminoethanol ester of diphenyl-propyl acetic acid.
Acetylation reactions of the amino group of foreign compounds are
catalysed by acetyltransferases (Weber, 1971). Substrates of these
enzyme reactions, localized in the soluble part of the cells, are
arylamines, hydrazines, and certain aliphatic amines. Coenzyme A is an
essential factor in these acetylations. Acetylation of arylamines has
been studied quantitatively, in vivo, in human beings and animals
(Williams, 1967b).
Methods for the determination of N-acetyltransferase (2.3.1.35)
activities in vitro summarized by Weber (1971) include colorimetric
(Brodie & Axelrod, 1948; Maher et al., 1957; Marshall, 1948; Shulert,
1961; Weber, 1970), spectrophotometric (Jenne & Boyer, 1962; Tabor et
al., 1953; Weber & Cohen, 1968; Weber et al., 1968) as well as
radiometric procedures (Stotz et al., 1969).
Conjugation of aromatic carboxylic acids (benzoic acid,
substituted benzoic acids, and heterocyclic carboxylic acids) with
amino acids by means of acetyl coenzyme A and ATP is called peptide
conjugation. Glycine is the most generally involved amino acid in this
reaction resulting in the formation of N-benzoylglycine (hippuric
acid). Indole-3-acetic acid, benzeneacetic acid, as well as
4-aminosalicylic acid, can conjugate with glutamine in man, and
several mammals. Determination of hippuric acids (Ogata et al., 1969)
enables the quantitative investigation of this conjugation reaction.
Conjugation of glutathione with foreign compounds, catalysed by
at least ten different glutathione S-transferases, is an important
pathway for the elimination of these compounds (Boyland, 1971).
Following the conjugation of foreign compounds with glutathione, the
conjugate is most frequently hydrolysed to the cysteine conjugate
which is excreted in the urine. Furthermore, the cysteine conjugate
may be acetylated and the resulting mercapturic acid excreted. The
significance of the mercapturic acid biosynthesis in man, however, is
difficult to assess.
Determination of glutathione S-transferase activities are based
on spectral change of the substrate (1,2-dichloro-4-nitrobenzene) due
to conjugation (Booth et al., 1961), or loss of glutathione content
(Boyland & Chasseaud, 1967; Boyland & Williams, 1965; Johnson, 1966)
or release of labile groups (Al-Kassab et al., 1963; Boyland &
Williams, 1965; Johnson, 1966) as well as on chromatographic
separation of the products (Suga et al., 1967). The determination of
the activity of gamma-glutamyltransferase (2.3.2.2), catalysing one
intermediary step of the overall mercapturic acid synthesis may also
be informative.
4.6.1.3 Extramicrosomal metabolic transformations
Foreign compounds, either transformed by oxidation or initially
having characteristic groups (hydroxyl, amino) may resemble normal
constituents of physiological metabolism. Thus, they may undergo
metabolic transformations similar to those of normal body
constituents: oxidation, reduction, deamination, hydrolysis. The
enzymes catalysing these reactions are localized in the cytosol or are
intrinsic compounds of the mitochondria.
In contrast to the extensive data in the literature on
enzyme-chemical interactions (MacMahon, 1971; Zeller, 1971) only a few
enzyme activities are commonly used to monitor toxicological events.
The alcohol dehydrogenase (1.1.1.1) of the liver is one of the
most important enzymes which catalyses the NAD-mediated oxidation of
various aliphatic and aromatic primary and secondary alcohols.
Determination of the activity of alcohol dehydrogenase is based on the
spectrophotometric measurement of the amount of NAD being reduced in
the presence of excess alcohol (Bonnichsen & Brink, 1955).
Among the amine oxidases, monoamine oxidase (1.4.3.4), localized
in the mitochondria, regulates the balance of the biogenic amines and
probably does not participate in the metabolism of foreign amines to a
great degree (Zeller, 1971). However, the fact that a large number of
substances (substrates and substrate analogues, alkyl and arylamines,
hydrazine derivatives, sulfhydryl reagents, etc.) inhibit this enzyme,
enables monoamine oxidase to be used as a tool in studies of the
toxicity of these inhibitors.
Monoamine oxidase activity can be measured manometrically
(Creasey, 1956) based on oxygen-consumption, by determination of
ammonia production (Cotzias & Dole, 1951), spectrophotometrically
(Dietrich & Erwin, 1969; Obata et al., 1971; Weissbach et al., 1960),
fluorimetrically (Takahashi & Takahara, 1968; Tufvesson, 1970) as well
as radiometrically (Otsuka & Kobayashi, 1964).
Hydrolysis by carboxylesterases (ali-esterases or arylesterases)
of foreign compounds containing ester groups may be important in
assessing their toxicity (La Du & Snady, 1971). Determination of
esterase activities using different substrates in the presence of the
chemical to be tested can disclose its possible inhibitory potency.
4.6.1.4 Nonenzymatic reactions
Although the foregoing sections have discussed enzymatic
modifications of chemicals, the investigator should not overlook
nonenzymatic, spontaneous reactions between chemicals and natural
constituents in the body that lead to the formation of metabolites,
e.g. the reaction of an alkylating agent with glutathione.
4.6.2 Species variability
A serious problem facing every research worker using an animal
species to study the metabolism of a foreign compound is whether or
not the metabolic pathway in the animal is similar to the metabolic
pathway in man. The problem is not only important in metabolic
studies, but is of utmost importance in using animal toxicity studies
to predict toxicological phenomena in man. Conney et al. (1974)
illustrated that the use of an animal species that metabolizes a
foreign compound in a similar manner to man will give a more precise
prediction of the type of toxicological phenomena to be expected in
man.
Different animal species have been shown to metabolize foreign
compounds at different rates. Quinn et al. (1958) has shown that
benzeneamine (aniline) has a metabolic half-time in the mouse of 35
minutes and in the dog of 167 minutes. In the same study it was
demonstrated that the metabolic half-time of an antipyrine in the rat
was 140 minutes, whereas in man it was 600 minutes.
Considerable species differences in metabolic pathways have also
been demonstrated. In the rat, mouse, and dog the carcinogen,
N-2-fluoranylacetamide (FAA), is N-hydroxylated to N-hydroxy-FAA
which is a more potent carcinogen than FAA. In the guineapig little or
no hydroxylation of FAA occurs. In toxicity studies, Miller & Miller
(1971b) and Weisburger et al. (1964) demonstrated that the rat, mouse,
and dog are susceptible to the carcinogenic activity of FAA, whereas
the guineapig is not. Thus, a difference in the metabolic pathways of
a foreign compound may greatly influence its toxicity.
Species variability in metabolism has been related to other
factors such as species differences in protein binding, and enzyme
concentration and type. Hucker (1970) described, in detail, species
differences in chemical metabolism and some of the factors responsible
for these differences.
4.6.3 Enzyme induction and inhibition
For some time it has been known that chemicals can increase the
activity of metabolizing enzyme systems. These chemicals have been
termed enzyme "inducers". Inducers exert their action by
quantitatively increasing the enzymes and components responsible for
the metabolism of foreign compounds. The importance of induction to
the toxicologist is two-fold. If metabolism leads to the formation of
excretable or nontoxic metabolites, induction will enhance
detoxification and excretion of the compound. However, if metabolism
leads to the production of a more toxic metabolite, induction will
increase the toxicity of a compound.
Many chemicals are known to increase metabolizing enzyme systems.
The reviews by Conney (1967), Kuntzman (1969), and Mannering (1968),
depict the large number of chemicals which induce metabolizing enzymes
and comprehensively review the factors involved in enzyme inductions.
Most inducers give maximum effects rather quickly -- within 2-3
days (Fouts, 1970). However, some require 2 weeks or longer (Gillette
et al., 1966; Hart & Fouts, 1965; Hoffman et al., 1968, 1970;
Kinoshita et al., 1966). Frequently, the degree of induction after
obtaining a maximum level may decline despite continuing treatment of
the animal with a chemical (Gillette et al., 1966; Hoffman et al.,
1968; Kinoshita et al., 1966).
Drug-metabolizing enzymes can also be depressed by foreign
chemicals, and these compounds are termed inhibitors. 2-(Diethylamino)
ethyl-alpha-phenyl-alpha-propyl benzeneacetate hydrochloride
(SKF-525A) is the best known of the inhibitors and is used routinely
in determining the effect of enzyme inhibition on the metabolism of
chemicals.
4.6.4 Metabolic saturation
In vivo saturation of metabolic pathways can play an important
role in determining the toxic profile of a chemical. A recent article
by Jollow et al. (1974) demonstrated the effect of enzyme saturation
on the metabolism and toxicity of bromobenzene. Bromobenzene was first
metabolically transformed to an epoxide which is hepatotoxic. After a
small nontoxic dose, approximately 75% was converted to the
glutathione conjugate and excreted as bromophenylmercapturic acid.
After a large toxic dose, only 45% was excreted as the mercapturic
acid. It was established that, at the toxic dose, the metabolic
conjugation pathway was overwhelmed due to lack of glutathione, which
resulted in an increased reaction of the epoxide hepatotoxin with DNA,
RNA, and protein.
It is very important to elucidate dose-dependent metabolism to
assess the hazard of a chemical. Frequently, the doses of a chemical
used to characterize toxicity are many times those encountered in the
environment. Toxicity incurred at these large doses may be influenced
by relative changes in metabolism and therefore must be interpreted
with caution and judgment in assessing the hazard of low doses.
4.7 Experimental Design
Since, for the most part, toxicity is a function of the
concentration of the toxicant in the tissues and cells, this
information together with its dynamics provides for inter- as well as
intra-species extrapolation of the results of toxicological effects.
The overall objectives of a chemobiokinetic study are to
determine the amount, rate, and nature of absorption, distribution,
metabolism, and excretion of a chemical. The approach to meeting those
objectives must be flexible and designed to meet the specific needs of
each chemical.
It is difficult to predict, without prior data, an animal species
that will metabolize a chemical similarly to man. Usually, initial
studies are performed in the rat and one other nonrodent species, such
as the dog or monkey, in an attempt to determine species variability.
If there are significant differences among species, it is important to
determine whether differences in the chemobiokinetic parameters
correlate with differences in toxicity or pharmacological activity.
Animals should be acclimatized to the environment of the metabolism
cage prior to the experiment. Light cycle, temperature, humidity, and
time of feeding should be standardized. The physical condition,
weight, and food and water consumption of each animal should be
monitored and recorded throughout the study.
There are advantages in using radioactively-labelled chemicals in
initial studies because of the ease with which radiochemical methods
(Chase & Rabinowitz, 1968) can be applied to chemobiokinetic studies.
An important advantage of using a radioactively-labelled chemical is
that it allows the establishment of the total recovery of the parent
chemical and its metabolites, i.e. the mass balance. To obtain this
the total radioactivity eliminated via the urine, faeces, and exhaled
air as well as that remaining in the carcass following termination of
the experiment should be determined. Until a reasonably good recovery
is obtained, 90% or greater, one can never be sure whether other
chemobiokinetic parameters obtained from the study are accurate.
Furthermore, the isolation and ultimate identification of unknown
metabolites is greatly enhanced by using radioactively-labelled
chemicals.
When using a radiolabelled chemical, the measurement of
radioactivity confirms the presence of the radioisotope, not the
chemical or its metabolites. In order to determine the identity of the
radioactively-labelled compound, the parent chemical and its
metabolites, analytical methods such as gas, high-pressure liquid, and
thin-layer chromatography and a combination of gas chromatography and
mass spectroscopy are frequently employed.
Until it is established that the radioactivity being monitored is
from the chemical in question, kinetic parameters apply to the
radioactivity only, not to the chemical studied. Difficulties can
arise if the radioactive atom does not remain an integral part of the
molecule under study. Tritium and carbon-14 are often incorporated
into the body pools of normal tissue components (Griffiths, 1968;
Rosenblum, 1965). Once the radioactivity is incorporated into these
compartments, its clearance depends on their rates of turnover.
Therefore, by monitoring radioactivity only, it can be falsely assumed
that a compound is being retained in the body.
Another very important reason for differentiating the parent
chemical from its metabolite is to assure that toxic effects that may
be present are associated with the parent chemical and not a
metabolite. Also persistence of a metabolite in the body rather than
the parent chemical may constitute the ultimate hazard.
In initial studies, consideration should be given to the
administration of the compound by intravenous injection as well as via
the route by which man is exposed to the chemical. The intravenous
route is used to provide a more definite assessment of the earlier
phases of distribution and/or elimination. Also, large variation in
rates of absorption will in some cases make the differentiation of the
early phases of distribution and elimination difficult. At least two
doses should be used. One dose should be equivalent to the dose
required to cause signs of toxicity. The second dose should be well
below the toxic dose and, if possible, equivalent to anticipated human
exposure levels.
Most frequently, kinetic parameters for elimination of a chemical
are established by sequential sampling of blood plasma and excreta,
following its administration. A preliminary probe study using one or
two animals is often needed to establish the time at which samples
should be collected, because this will vary with the species and the
chemical in question. After collection and until prepared for analysis
of the chemical or its metabolites, samples should be stored in a
manner that will preclude the breakdown of the chemical or its
metabolites. The data required from the initial chemobiokinetic
studies can be used to design further studies which may include the
following: distribution studies using autoradiography; the isolation
and identification of metabolites; studies to determine the
chemobiokinetic profile of metabolites; biliary excretion studies;
bioconcentration; and in vitro metabolism studies. The methods and
techniques needed to perform these studies are documented by La Du et
al. (1972).
4.8 Chemobiokinetics
Chemobiokinetics aims at quantification of the processes
discussed previously in this chapter. Thus, chemobiokinetics provides
quantitative information on the absorption, distribution,
biotransformation, and excretion of chemicals (including drugs and
endogenous substances) as a function of time. Since the classical
introduction of this discipline by Teorell (1937a,b), the concepts and
methods have been developed extensively, principally for application
to the clinical evaluation and/or use of drugs (Levy & Gibaldi, 1972,
1975; Wagner, 1968, 1971). The reader is also referred to Gehring et
al. (1976) who discuss the subject in greater detail.
One difficulty of many toxicologists and biologists on first
exposure to chemobiokinetics is the concept of compartments. The body
is composed of a large number of organs, tissues, cells, and fluids,
any one of which could be referred to morphologically and functionally
as a compartment. However, in chemobiokinetics, a compartment refers
collectively to those organs, tissues, cells, and fluids for which the
rates of uptake and subsequent clearance of a chemical are
sufficiently similar to preclude kinetic resolution. The rapidly
equilibrating compartment, referred to as the central compartment, may
be comprised of all those tissues with a profuse blood supply whereas
the slow or peripheral compartment may include tissues with a more
limited blood supply, i.e. fat and bone.
4.8.1 One-compartment open model
The simplest chemobiokinetic model is a one-compartment, open
model as shown in Fig. 4.1. In using this model, it is assumed that
the chemical equilibrates with all tissues to which it is distributed
sufficiently rapidly to preclude kinetic differentiation by the
techniques being used to characterize its movement in the body. For
example, if it requires 30 min for a chemical to attain equilibration
in the body after entering the blood stream, and if samples of blood,
tissues, and excreta are taken at 30 min intervals, it will appear
that the body consists of only one compartment.
Assuming that the rate of elimination of the chemical is
proportional to its concentration in the plasma, the concentration in
the plasma will be described by apparent first-order kinetics. The
rate of change of concentration in the plasma may be expressed in the
form of the linear differential equation
dC(t)
= - ke C(t) (1)
dt
where C(t) is the concentration at time t, and ke is the rate constant
for elimination. Solution of this differential equation with initial
condition C(t) = C(0) at time zero gives
C(t) = C(0) exp(- ke t) (exponential form) (2)
or,
(3)
REFERENCES
ALBERT, R. E. & ALTSCHULER, B. (1973) Considerations relating to the
formulation of limits for unavoidable population exposures to
environmental carcinogens. In: Ballon, J. E., ed. Radionuclide
carcinogenesis, Springfield, Va, NIIS, pp. 233-253
(AEC Symposium Series CONF-72050).
ALBERT, R. E. & ALTSCHULER, B. (1976) Assessment of risks in terms of
life shortening, Environ. Health Perspect., 13: 91-94.
ANDREYESHCHEVA, N. G. (1976) Predicting biological effect as a
function of the chemical structure and the primary physical and
chemical properties of organic compounds. Environ. Health
Perspect., 13: 27-30.
ARIENS, E. J., VAN ROSSUM, J. M., & SIMONIS, A. M. (1957) Affinity,
intrinsic activity and drug interactions. Pharmacol. Rev.,
9: 218-236.
BALL, W. L. (1959) The investigation of present-time definitions and
conceptions of maximum allowable concentrations in different
countries. Pr. Lék., 11 (3): 127-128.
BEIR (1972) The effects of population exposure to low levels of
ionizing radiations -- Report of the Advisory Committee on the
Biological Effects of Ionizing Radiation. Washington, DC,
National Academy of Sciences -- National Research Council,
217 pp. (Govt. Printing Office Publication 0-489-797).
BERNARD, C. (1898) [Introduction to the study of experimental
medicine.] Paris, Librairie Delagrave (in French).
BINGHAM, E. & FALK, H. L. (1959) Environmental carcinogens --
modifying effect of cocarcinogens on the threshold response.
Arch. environ. Health, 19: 779-783.
BLUM, H. F. (1959) Carcinogenesis by ultraviolet light. Princeton,
NJ, Princeton University Press.
BROWN, B. M. & PAPWORTH, D. S. (1974) Environmental chemical hazards
to wildlife. In: Boyland, E. & Goulding, R., ed. Modern trends
in toxicology, London, Butterworths, Vol. 2, ch. 3, pp. 70-85.
CASARETT, L. J. (1975) Toxicologic evaluation. In: Casarett, L. J. &
Doull, J., ed. Toxicology, the basic science of poisons. New
York, Macmillan Publishing Co. Inc.
CHAND, N. & HOEL, D. G. (1974) A comparison of models for determining
safe levels of environmental agents. In: Proschan & Sefling, ed.
Reliability and biometry, Philadelphia, SIAM, pp. 382-401.
CLARK, A. J. (1933) Mode of action of drugs on cells. Baltimore,
Williams & Wilkins.
COHEN, J. M. & PINKERTON, C. (1966) Widespread translation of
pesticides by air transport and rain-out. In: Gould, R. F., ed.
Organic pesticides in the environment, American Chemical
Society, pp. 163-176 (Advances in Chemistry Series, vol. 60).
COON, J. (1973) Toxicology of naturally-occurring chemicals: a
perspective. In: Toxicants occurring naturally in foods
(2nd ed.). Washington, DC, National Academy of Sciences.
CORNFIELD, J. (1954) Measurement and comparison of toxicities: the
quantal response. In: Kempthorne, O., Bancroft, T. A., Gowen, J.
W., & Lush, J. L., ed. Statistics and mathematics in biology,
Ames, The Iowa State College Press, pp. 327-344.
CRANSTON, M. (1973) What are human rights? New York, Taplinger Pub.
Co. Inc., p. 111.
CURRY, S. H. (1970) Theoretical changes in drug distribution resulting
from changes in binding to plasma proteins and to tissues.
Pharm. Pharmacol., 22: 753-758.
CUTHBERT, J. W. (1974) Industrial toxicology. In: Boyland, E. &
Goulding, R., ed. Modern trends in toxicology. London,
Butterworths, pp. 86-115.
DAY, T. D. (1967) Carcinogenic action of cigarette smoke condensate on
mouse skin. Br. J. Cancer, 21: 56-81.
DINMAN, B. D. (1972) "Non-concept" of "no-threshold" chemicals in the
environment. Environ. Sci., 175 (4021): 495-497.
DOLL, R. (1967) Prevention of cancer: pointers from epidemiology.
London, Nuffield Provincial Hospital Trust.
DRUCKREY, H. (1967) Qualitative aspects of chemical carcinogenesis.
In: Truhaut, R., ed. Potential carcinogenic hazards from drugs,
evaluation of risks, New York, Springer-Verlag, pp. 60-78.
(UICC Monograph Series, Vol. 7.)
ECOBICHON, D. J. & CORMEAU, A.M. (1973) Pseudocholinesterases of
mammalian plasma: physiochemical properties and organophosphate
inhibition in 11 species. Toxicol. appl. Pharmacol.,
24: 92-100.
EDWARDS, C. A. (1970) Persistent pesticides in the environment.
Cleveland, Ohio, CRC Press.
ELIZAROVA, O. N. (1962) In: Opredelenie porogovyh doz promyslennyh
jadov pri peroral'nom vvedeni, Moscow, Medicina, pp. 107-108
(in Russian).
EPSTEIN, S.S. (1973) The Delaney amendment. J. prev. Med.,
2: 140-149.
FALK, H. L. (1975) Consideration of risks versus benefits. Environ.
Health Perspect., 11: 1-5.
FOOD & DRUG ADMINISTRATION (1970) Food and Drug Administration
Advisory Committee on Protocols for Safety Evaluation: Panel on
Carcinogenesis Report on Cancer Testing in the Safety Evaluation
of Food Additives and Pesticides. Toxicol. appl. Pharmacol.,
20: 419-438.
FINNEY, D. G. (1952a) Statistical method in biological assay,
London, Charles Griffin Ltd, 661 pp.
FINNEY, D. G. (1952b) Probit analysis (2nd ed.). London, Cambridge
University Press.
FINNEY, D. G. (1971) Probit analysis (3rd ed.). London, Cambridge
University Press.
FLYNN, D. I., LYNCH, M., & ZANNONI, V. G. (1972) Species differences
and drug metabolism. Biochem. Pharmacol., 21: 2577-2590.
FRIEDMAN, L. (1969) The role of the laboratory animal study of
intermediate duration for the evaluation of safety. In: Symposium
on the evaluation of the safety of food additives and chemical
residues. Toxicol. appl. Pharmacol., 16: 498-506.
FUCHS, N. A. (1964) The mechanics of aerosols, Oxford, Pergamon
Press, pp. 270-287.
GADDUM, J. H. (1957) Theories of drug antagonism. Pharmacol. Rev.,
9: 211-217.
GAVRILESCU, N. & PETERS, R. A. (1931) Biochemical lesions in vitamin B
deficiency. Biochem. J., 25: 1397-1409.
GOLDWATER, L. J. (1968) Toxicology. In: Sax, N. I., ed. Dangerous
properties of industrial materials, New York, Amsterdam, London,
Reinhold Book Corporation, pp. 1-23.
GOLUBEV, A. F., LJUBLINA, A. E., TOLOKOICEY, N. A., & FILOV, V. A.
(1973) In: Kolicestvennaja toksikologija. Leningrad, Medicina,
p. 217 (in Russian).
GROSS, M. A., FITZHUGH, O. G., & MANTEL, N. (1970) Evaluation of
safety of food additives: an illustration involving the influence
of methyl salicylate on rat reproduction. Biometrics,
26: 181-194.
HAMAKER, J. W., GORING, C. A. L., & YOUNGSON, C. R. (1966) Sorption
and leaching of 4-amino-3,5,6-trichloropicolinic acid in soils.
In: Gould, R. F., ed. Organic pesticides in the environment,
American Chemical Society, pp. 23-37 (Advances in Chemistry
Series, Vol. 60).
HATCH, T. F. (1968) Significant dimensions of the dose-response
relationship. Arch. environ. Health, 16: 571-578.
HATCH, T. F. & GROSS, P. (1964) Pulmonary deposition of inhaled
aerosols. New York, Academic Press.
HERMAN, R. S. (1974) Induction of liver growth by xenobiotic compounds
and other stimuli. CRC crit. Rev. Toxicol., 3(1): 97-158.
HEWLETT, P. S. & PLACKETT, R. L. (1961) Models for quantal responses
to mixtures of two drugs. In: Jonge, H. de, ed. Symposium on
quantitative methods in pharmacology. Amsterdam, North Holland
Publishing Co., pp. 328-336.
HOEL, D. G., GAYLOR, D. W., KIRSCHSTEIN, R. L., SAFFIOTTI, U., &
SCHNEIDERMAN, M. S. (1975) Estimation of risks of irreversible
delayed toxicity. J. Toxicol. environ. Health, 1(1): 133-151.
HUCKER, H. B. (1970) Species differences in drug metabolism.
Ann. Rev. Pharmacol., 10: 99-118.
ICRP (1965) Principles of environmental monitoring related to the
handling of radioactive materials. Report of Committee IV of the
International Commission on Radiological Protection. London,
Pergamon Press.
ICRP (1966) The evaluation of risk from radiation, a report prepared
for Committee I of the International Commission on Radiological
Protection. Oxford, London, Edinburgh, New York, Toronto, Paris,
Braunschweig, Pergamon Press (ICRP Publication 8).
ICRP (1977) Recommendations of the International Commission on
Radiological Protection (ICRP Publication 26 (in press)).
JANYSEVA, H. JA (1972) [On the establishment of MPCs of benz(a)pyrene
in the ambient air of built-up areas.] Gig. i Sanit.,
No. 7, pp. 87-91 (in Russian).
JANYSEVA, H. JA & ANTOMONOV, JU G. (1976) Predicting risk of tumour
occurrence in terms of life-shortening. Environ. Health
Perspect., 13: 95-100.
JONES, H. B. & GRENDON, A. (1975) Environmental factors in the origin
of cancer and estimation of possible hazards to man. Food
cosmet. Toxicol., 13: 251-267.
KAGAN, Y. S. (1973) [Methods of quantitative estimation of combined
and complex action on the organism of physical and chemical
factors in the environment.] Gig. i Sanit., No. 2, pp. 89-92
(in Russian).
KELLERMANN, G., SHAW, C. R., & LUYTEN-KELLERMANN, M. (1973) Aryl
hydrocarbon hydroxylase inducibility and bronchogenic carcinoma.
New Engl. J. Med., 289: 934-937.
KORBAKOVA, A. K., SUMSKAJA, N. I., ZAEVA, G. N., & NIKITENKO, T. K.
(1971) In: Naucnye osnovy sovremmenyh metodov gigieniceskogo
normirovanija himiceskih vescest'v v okruzajuscej srede.
Moscow, Institut gigieny truda i profzabolevanija AMN SSSR,
pp. 35-39.
KRASOVSKIJ, G. N. (1965) [Methodology for conducting an acute
experiment and for evaluating the results, and its basis.] In:
Sanitarnaja ohrana vodoemov ot zagrjaznenija promyslennymi
stocnymi vodami. No. 7, 247-268 (in Russian).
KRASOVSKIJ, G. N. (1970) [On methods for studying the cumulative
properties of toxic compounds.] Gig. i Sanit., No. 3, pp. 83-88
(in Russian).
KRASOVSKIJ, G. N. (1975) Species and sex differences in sensitivity to
toxic substances. In: Methods used in the USSR for establishing
biologically safe levels of toxic, Geneva, WHO, pp. 109-125.
KRASOVSKIJ, G. N. (1976a) [General standardization of biological
parameters for mammals according to body weight, and some
practical aspects of investigations.] In: Trudy MMI im Secenova
T.80 -- Novoe v metodah issledovannija, diagnostiki, lecenii i
profilaktiki vazneisih zabolevanii. Pp. 64-88 (in Russian).
KRASOVSKIJ, G. N. (1976b) Extrapolation of experimental data from
animals to man. Health Perspect., 13:51-58.
KUSTOV, V. V. & TIUNOV, L. A. (1970) [Enzyme system activity as a
means of estimating thresholds of the effects of toxins.] In:
Metody opredelenija toksicinosti i opasnosti himiceskih
vescestv. Moscow, Medicina, pp. 231-234 (in Russian).
KUSTOV, V. V., ZDANOV, A. M., & JUNOVSKIJ, G. D. (1974) [On the
evaluation of the combined effect of factors by the method of
partial regression.] Gig. Tr. Prof. Zabol., 16:33-36
(in Russian).
LAZAREV, N. V. (1938). Obscie osnovypromyslennoj toksikologii.
Moscow & Leningrad, Medgiz.
LAZAREV, N. V. (1963) Rukovodstvo po gigiena truda, Moscow, Medgiz.
LAZAREV, N. V. & BRUSILOVSKAJA, A. I. (1934) Fiziol. Z. SSSR, XVII,
pp. 611-619 (in Russian).
LJUBLINA, E. I. & MIHEEV, M. I. (1974) [Scientific bases for
establishing tentative safe levels of substances affecting man.]
In: Zurnal Vsesjuznogo Obcestva im. D. I. Mendeleeva,
XIX (2): 142-145 (in Russian).
LOEWE, S. (1959) Relationships between stimulus and response.
Science, 130: 692-695.
LU, P. Y. & METCALF, R. (1975) Environmental fate and biodegradability
of benzene derivatives as studied in a model aquatic ecosystem.
Environ. Health Perspect., 10: 269-284.
MANTEL, N. & BRYAN, W. R. (1961) Safety testing of carcinogenic
agents. J. Natl Cancer Inst., 27: 455-470.
MANTEL, N. & SCHNEIDERMAN, M. (1975) Estimating "safe" levels, a
hazardous undertaking. Cancer Res., 35: 1379-1386.
MANTEL, N., BOHIDAR, N., BROWN, C., CIMINERA, J., & TUKEY, J. (1975)
An improved "Mantel-Bryan" procedure for "safety" testing of
carcinogens. Cancer Res., 35: 865-872.
MAZZE, R. I., COUSINS, M. J., & KOSEK, J. C. (1973) Strain differences
in metabolism and susceptibility to the nephrotoxic effects of
methoxyfluorane in rats. J. Pharmacol. exp. Therapy,
184: 481-488.
MEDVED, L. I. & SPYNU, E. I. (1970) [Principles and methods of
hygienic standardization of pesticides. In: Principles and
methods of establishing maximum permissible concentrations of
harmful substances in the air of production premises.] Moscow,
Medicina (in Russian).
METCALF, R. L., KAPOOR, I. P., LU, P. Y., SCHUTH, C. K., & SHERMAN, P.
(1973) A model ecosystem for the evaluation of pesticide
biodegradability and ecological magnification. Environ. Sci.
Technol., 5: 709-744.
MILLER, E. C., MILLER, J. A., & ENOMOTOR, M. (1964) The comparative
carcinogenetics of 2-acetylaminofluorene and its N-hydroxy
metabolite in mice, hamsters and guinea pigs. Cancer Res.,
24: 2018-2032.
MONTESANO, R., MOHR, U., & MAGEE, P. N. (1974) Additive effect in the
induction of kidney tumours in rats treated with
dimethylnitrosamine and ethylmethanesulfonate. Br. J. Cancer,
29: 50-58.
MURPHY, S. D. (1969) Some relationships between effects of
insecticides and other stress conditions. Ann. NY Acad. Sci.,
160: 366-377.
NAS/NRC (1970) Evaluating the safety of food chemicals. Food
Protection Committee, Food and Nutrition Branch, Division of
Biology and Agriculture, National Research Council, Washington
DC, National Academy of Sciences. 55 pp.
NAS (1975) Principles for evaluating chemicals in the environment --
A report of the Committee for the Working Conference on
Principles of Protocols for Evaluating Chemicals in the
Environment. Washington, DC, National Academy of Sciences.
NIEHS (1970) Man's health and the environment, some research needs --
Report of the Task Force on Research Planning in Environmental
Health Science US Department of Health, Education and Welfare,
Public Health Service, National Institutes of Health, National
Institute of Environmental Health Sciences. Washington, DC, US
Govt Printing Office.
NIEHS (1977) Human health and the environment, some research needs --
Report of the Second Task Force for Research Planning in
Environmental Health US Department of Health, Education and
Welfare, Public Health Service, National Institutes of Health,
National Institute of Environmental Health Sciences. Washington,
DC, US Govt Printing Office (DHEW Publication No. NIH77-1277).
OECD (1977) The OECD programme on long range transport of air
pollutants. Measurements and findings. Paris, Organisation for
Economic Co-operation and Development, 268 pp.
OWEN, D. B. (1955) Handbook of statistical tables, London, Paris,
Pergamon Press, pp. 127-137.
PAGET, G. E. (1970) The design and interpretation of toxicity tests.
In: Paget, G. E., ed. Methods in toxicology, Philadelphia, F.
A. Davies, pp. 1-10.
PARKE, D. V. (1975) Induction of the drug-metabolizing enzymes. In:
Parke, D. V., ed. Enzyme induction, London, Plenum Press,
pp. 207-272.
PETERS, R. A. (1963) Biochemical lesions and lethal synthesis.
Oxford, London, New York, Paris, Pergamon Press.
PETERS, R. A. (1967) The biochemical lesion in thiamine deficiency.
In: Wolstenholme, G. E. W. & O'Connor, M., ed. Thiamine
deficiency, biochemical lesions and their significance, London,
J. & A. Churchill Ltd, pp. 1-8.
PETO, R. & LEE, P. N. (1973) Weibull distribution for continuous
carcinogenesis experiments. Biometrics, 29: 457-470.
PETO, R., LEE, P. N., & PAIGE, W. S. (1972) Statistical analysis of
the bioassay of continuous carcinogens. Br. J. Cancer,
26: 258-261.
PFITZER, E. A. (1976) General concepts and definitions for
dose-response and dose-effect relationships of toxic metals. In:
Nordberg, G., ed. Effects and dose-response relationships of
toxic metals. Amsterdam, Oxford, New York, Elsevier Scientific
Publishing Company.
PINIGIN, M. A. (1974) [Current problems of community toxicology in
relation to chemical air pollution.] In: Itogi nauki i tehniki.
Serija farmakologija. Himioterapevticeskie sredstva.
Toksikologija. Problemy toksikologii. Vol. 6, Moscow
(in Russian).
POKROVSKIJ, A. A. (1973) [Biochemical approaches to the evaluation of
toxic factors in the environment. In: Documentation of the
Conference on Methodological Approaches to the Study and
Evaluation of Toxic Factors in the Environment.] Moscow
(in Russian).
PORTMAN, R. S., PLOWMAN, K. M., & CAMPBELL, T. C. (1970) On mechanisms
affecting species susceptibility to aflatoxin. Biochim. Biophys.
Acta, 208: 487-495.
PRAVDIN, N. S. (1934) Rukovodstovo promyslennoi toksikologii.
Moscow, Biologija i Medicina.
RALL, D. F. (1970) Animal models for pharmacological studies. In:
Proceedings of the Symposium on Animal Models for Biomedical
Research III, Washington, DC, National Academy of Sciences,
pp. 125-146 (ISBN 0-309-01854-4).
SABAD, L. M., SANOCKIJ, I. V., ZAEVA, G. N., BUREVIC, T. C.,
KACNELSON, B. A., JANYSEVA, H. JA, & SUGAEVA, B. V. (1973) [On
the possibility of establishing MPCs for benz(a)pyrene in the air
of industrial premises.] Gig. i Sanit., No. 4, pp. 78-81
(in Russian).
SAFFIOTTI, U. (1973) Comments on the scientific basis for the "Delaney
Clause". J. Prev. Med., 2: 125-132.
SANOCKIJ, I. V. (1962) [The calculation of a safety factor for
experimentally based maximum permissible concentrations of
industrial poisons. In: Industrial toxicology and clinical
aspects of occupational diseases of chemical etiology.] Moscow,
Medgiz (in Russian).
SANOCKIJ, I. V. (1970) In: Sanockij, I. V., ed. Metody opredelenija
toksicnosti i opasnosti himiceskih vescestv. Moscow, Medicina,
pp. 11-12 (in Russian).
SANOCKIJ, I. V. (1975a) Investigation of new substances: permissible
limits and threshold of harmful action. In: Methods used in the
USSR for establishing biologically safe levels of toxic
substances, Geneva, WHO, pp. 9-18.
SANOCKIJ, I. V. (1975b) [The threshold concept for the reactions of
living systems to environmental influences and its consequences.
Soviet-American Symposium, Tbilisi.] Gidrometeoizdat,
pp. 112-120 (in Russian).
SANOCKIJ, I. V. & SIDOROV, K. K. (1975) [The current status of the
problem of safety factors in establishing the maximum permissible
concentrations of substances in the components of the general
environment.] Gig. i Sanit., No. 7, pp. 93-96 (in Russian).
SATO, T. & MOROI, K. (1971) Species and age differences in the
activity of isocarboxazid hydrolysing enzyme. Arch. int.
Pharmacodyn. Ther., 192: 128-134.
SCHILD, H. O., ARIENS, E. J., SIMONIS, A. M., DIKSTEIN, S., DE JONGH,
S. E., HEWLETT, P. S., & PLACKETT, R. L. (1961) Section on
mixtures of drugs. In: De Jonge, H. ed. Quantitative methods in
pharmacology, Proceedings of a Symposium held in Leyden on
May 10-13, 1960, Amsterdam, North Holland Publishing Company,
pp. 282-334.
SCHNEIDERMAN, M. A. (1971) A method for determining the dose
compatible with some "acceptable" level of risk. In: Chemicals
and the future of man -- Hearings before the Sub-Committee on
Executive Reorganization and Government Research of the Committee
on Government Operations, US Senate, Ninety-Second Congress,
First Session, 6 and 7 April 1971. Washington, DC, US Govt
Printing Office.
SCHNEIDERMAN, M. A. & MANTEL, N. (1973) The Delaney Clause and a
scheme for rewarding good experimentation. Prev. Med.,
2: 165-170.
SIDORENKO, G. I. & PINIGIN, M. A. (1975) Establishment of safe levels
of chemicals in communal hygiene: methodological approaches. In:
Methods used in the USSR for establishing biologically safe
levels of toxic substances, Geneva, WHO, pp. 126-138.
SIDORENKO, G. I. & PINIGIN, M. A. (1976) Concentration-time
relationship for various regimens of inhalation of organic
compounds. Environ. Health Perspect, 13: 17-21.
SMYTH, H. F., JR (1963) Industrial hygiene in retrospect and
prospect-toxicological aspects. Am. Ind. Hyg. Assoc. J.,
24: 222-226.
SMYTH, H. F. JR, WEIL, C. S., WEST, J. S., & CARPENTER, C. P. (1969)
An exploration of joint toxic action: twenty-seven industrial
chemicals intubated in rats in all possible pairs. Toxicol.
appl. Pharmacol, 14: 340-347.
SPYNU, E. I., VROCINSKIJ, K. K., ZOR'EVA, T. D., & MAL'KO, N. N.
(1972) [Complex hygienic standardization of new organophosphorus
pesticides in environmental components.] Gig. i. Sanit.,
No. 11, pp. 96-99 (in Russian).
STOKINGER, H. E. (1972) Concepts of thresholds in standards setting.
Arch. environ. Health, 25 (3): 153-157.
SYSIN, A. M., ed. (1941) [Maximum allowable concentrations of toxic
substances in water bodies.] Moscow and Leningrad, Stroizdat
(in Russian).
TASK GROUP ON METAL ACCUMULATION (1973) Accumulation of toxic metals
with special reference to their absorption, excretion and
biological half-time. Environ. Physiol. Biochem., 3: 65-107.
TASK GROUP ON METAL TOXICITY (1976) Consensus report for an
international meeting organized by the Sub-committee on the
Toxicology of Metals of the Permanent Commission and
International Association on Occupational Health, Tokyo, 18-23
November 1974. In: Nordberg, G., ed. Effects and dose-responses
relationships of toxic metals, Amsterdam, Oxford, New York,
Elsevier Scientific Publishing Company, pp. 7-111.
ULANOVA, I. P. (1969) [Problems of hygienic standardization of
mixtures of gases and vapours of chemical substances. In:
The toxicology of new industrial chemicals.] Moscow, Medicina,
pp. 33-39 (in Russian).
ULANOVA, I. P., AVILOVA, G. G., BAZAROVA, L. A., MAL'CEVA, N. M.,
MIGUKINA, N. V., HALEPO, A. I., & EITINGTON, A. I. (1973)
[Experimental data on adaptation to poisons under different
conditions of their action. Pharmacology, chemotherapeutic
substances, toxicology.] Itogi nauk i teh., No. 5, pp. 64-75
(in Russian).
ULANOVA, I. P., AVILOVA, G. G., MAL'CEVA, N. M., & HALEPO, A. I.
(1976) [Comparisons of reactions of the organism to continuous
and intermittent action of some chlorinated hydrocarbons.]
Gig. Tr. Profzabol., No. 7, pp. 22-25 (in Russian).
VAN NOORDWIJK, J. (1964) Communication between the experimental animal
and the pharmacologist. Stat. Neerl., 18 (4): 403-416.
VELDSTRA, H. (1956) Synergism and potentiation. Pharmacol. Rev.,
8: 339-387.
VETTORAZZI, G. (1975) Toxicological decisions and recommendations
resulting from the safety assessment of pesticide residues in
food. Crit. Rev. Toxicol., 4 (2): 125-183.
VETTORAZZI, G. (1977) Safety factors and their application in
toxicological evaluation. In: Hunter, W. J. & Smeets, J. G. P.
M., ed. The evaluation of toxicological data for the protection
of public health. Oxford, Pergamon Press (for the Commission of
the European Communities) pp. 207-223.
WEIL, C. S. (1972a) Statistics versus safety factors and scientific
judgment in the evaluation of safety for man. Toxicol. appl.
Pharmacol., 21: 454-463.
WEIL, C. S. (1972b) Guidelines for experiments to predict the degree
of safety of a material for man. Toxicol. appl. Pharmacol.,
21: 194-199.
WHO (1958) WHO Technical Report Series, No. 144 (Procedures for the
testing of intentional food additives to establish their safety
for use -- Second Report of the Joint FAO/WHO Expert Committee on
Food Additives.) 19 pp.
WHO (1973) WHO Technical Report Series, No. 535 (Environmental and
health monitoring in occupational health -- Report of a WHO
Expert Committee.) 48 pp.
WHO (1974a) WHO Technical Report Series, No. 546 (Assessment of
carcinogenicity and mutagenicity of chemicals -- Report of a WHO
Scientific Group.) 19 pp.
WHO (1974b) WHO Technical Report Series, No. 554 (Health aspects of
environmental pollution: planning and implementation of national
programmes -- Report of a WHO Expert Committee.) 57 pp.
WHO (1975a) WHO Technical Report Series, No. 571 (Early detection of
health impairment in occupational exposure to health hazards --
Report of a WHO Study Group.) 80 pp.
WHO (1975b) WHO Technical Report Series, No. 560 (Chemical and
biochemical methodology for the assessment of hazards of
pesticides for man -- Report of a WHO Scientific Group.) 26 pp.
WHO (1975c) Methods for studying biological effects of pollutants (a
review of methods used in the USSR). Report of a Working Group,
Moscow, November 1974. Copenhagen, WHO Regional Office for
Europe.
WHO (1976a) WHO Technical Report Series No. 586 (Health hazards of new
environmental pollutants -- Report of a WHO Study Group.) 96 pp.
WHO (1976b) Health aspects of human rights with special reference to
developments in biology and medicine. Geneva, World Health
Organization, pp. 25-27.
WHO (1977) Environmental Health Criteria 4: Oxides of nitrogen.
Geneva, WHO, 79 pp.
WILLIAMS, R. T. (1969) The fate of foreign compounds in man and
animals. Pure appl. Chem., 18: 129-141.
WILLIAMS, R. T. (1972) Toxicological implications of biotransformation
by intestinal microflora. Toxicol. appl. Pharmacol.,
23: 769-781.
2. FACTORS INFLUENCING THE DESIGN OF TOXICITY STUDIES
2.1 Introduction
The choice and sequence of toxicity tests will depend on the
questions or hypotheses that are developed. The nature and sequence of
tests used to satisfy requirements of regulatory agencies may differ
markedly from those used in an investigation of the basic mechanisms
of toxic action. Differences in approach will also depend on whether
the investigation is initiated to evaluate the toxicity of a chemical
prior to its introduction into use, i.e. prospective toxicology, or to
confirm in laboratory animals an epidemiological association that
suggests chemical-induced disease in man, i.e. retrospective
toxicology. Under ideal conditions, prospective toxicology will
eliminate the need for retrospective toxicity evaluation.
National or international regulatory or advisory bodies have
developed fairly specific guidelines or test protocols which are
expected to be applied to the toxicity evaluation of certain groups of
chemicals, introduced deliberately into our environment, e.g. food
additives and pesticides (Council of Europe, 1973; FDA, 1959; WHO,
1967). The development of guidelines for the systematic evaluation of
the toxicity of chemicals to which man is exposed, through his
occupation or through incidental contamination of the ambient
environment, is less common. Where specific guidelines have been
formulated, they usually require: test information on acute toxicity
in several species of experimental animals; some knowledge of the
biochemical disposition of the compounds; various short-term toxicity
tests; tests of the effects of the chemical on reproductive function;
chronic toxicity tests in one or more species; special tests on organ
function, clinical biochemistry and haematology, and other specific
tests as determined by the particular type and intended uses of the
chemical under consideration. Some commercial firms have developed
their own guidelines for toxicity tests and their sequence in the
premarket toxicological evaluation of products. Often the sequence of
tests will have certain checkpoints at which decisions will be made as
to whether continued development of the product (and more extensive
toxicity testing) is warranted.
In this chapter, various topics will be discussed from the
standpoint of the usefulness of certain types of information and the
influence that various factors may have in designing protocols for the
interpretation of data obtained in toxicity evaluation programmes.
2.2 Chemical and Physical Properties
2.2.1 General considerations
The late Horace Gerarde stated in an address that "toxicity is
the capacity of a substance to cause injury. It is an inherent,
unalterable molecular property which is dependent upon chemical
structure. There is nothing we can do about the toxicity of a chemical
except to know it"a.
The nature or quality of the toxic action inherent in a chemical
will depend to a large extent upon the functional group or groups
present in the molecule. Knowledge of the reactions that these
functional groups may undergo with reactive groups in critical
endogenous biochemical constituents provides a means of predicting the
nature of the toxic effects that may be expected. Smyth (1959) used
the permanence of threshold limit values over a period of years as a
criterion for evaluating various types of information used in setting
safety limits for industrial chemicals. For a limited number of
compounds, occupational threshold limit values (TLVs) had been
established on the basis of analogy with better known substances and
the permanence of these TLVs appeared to equal that of TLVs based upon
data from experimental toxicity studies and from human experience.
However, evaluation of toxicity by analogy with chemically-related
substances contains considerable potential for error, and requires a
great deal of toxicological information on very closely related
chemical substances. Very minor changes in structure may be
accompanied by profound changes in toxicity. The relationships between
physicochemical characteristics and toxicity, including the biological
activities of homologous series, have been reviewed by Ljublina &
Filov (1975).
2.2.2 Physicochemical properties and the design of toxicity studies
Zbinden (1973) included fourteen chemical and physical variables
in a check list of types of information useful in the toxicity
evaluation of new drugs. Although some of these variables may be
determined in the course of a toxicological evaluation, all of them
apply equally as well to environmental chemicals as to therapeutic
chemicals.
a Presented at the Flavor Manufacturers' Association of the United
States. Fall Symposium, 16 November 1972, Washington, DC.
Knowledge of the chemical structure is essential for the
preliminary prediction of the nature and site of toxic action,
assuming, of course, that some prior knowledge of the toxicity of
chemically-related compounds is available. It is also essential for
developing extraction and assay procedures for the determination of
tissue concentrations and allows for logical estimates of the nature
of metabolites that may be found. Indeed, without such knowledge,
logical design of an experiment is impossible.
The stability of the chemical at various pH values and the
photochemical properties are variables that must be considered as soon
as a substance arrives for testing in the toxicology laboratory, as
they may determine the manner in which the chemical should be stored
prior to administration to test animals or indicate the stability of
residues in tissue extracts. Many organic chemicals undergo
photochemical reactions that lead to either more or less toxic
products (Crosby, 1972) and organic esters are often readily
hydrolysed under conditions encountered during their laboratory
investigation (Eto, 1974). It may be necessary to exercise special
precautions to avoid chemical reactions during the preparation and
storage of test solutions or diets and during the analysis of tissues
and metabolic reaction mixtures. Furthermore, if a chemical is likely
to become activated by photochemical reactions, special tests for
phototoxicity may be required.
The organic solvent/water partition coefficient and pK are
physical properties of particular importance in the determination of
the absorption and distribution of a compound in living organisms as
well as in the development of appropriate extraction and assay
procedures for the chemical. Hansch & Dunn (1972) reviewed numerous
studies which suggest that characterization of the lipophilic nature
of compounds may allow systematic predictions of their relative
biological activities. Dillingham et al. (1973) applied these
principles when they compared the toxicity of substituted alcohols in
a tissue culture with their acute toxicity in mice and concluded that
tissue culture test systems may be useful in determining predictive
correlations between in vivo toxicity and the physicochemical
properties of compounds.
The extent of ionization of an organic compound will influence
its passage through lipoidal membranes (La Du et al., 1971). In
general, the unionized lipid-soluble form of an organic compound will
most readily pass through biological membranes. Although most of the
physicochemical principles of absorption and distribution have been
developed through systematic studies on medicinal chemicals, these
principles also apply to organic chemicals in the environment and to
the design of toxicity experiments (Loomis, 1974; also Chapter 4).
Patty (1958) discussed the influence of oil and water solubility and
of the coefficient of distribution of a vapour between blood and
alveolar air on the rates of equilibrium saturation and desaturation
of the body during inhalation experiments.
Particle size, shape, and density are of obvious importance in
studying the inhalation toxicities of aerosols, as they are important
factors in the determination of the site of deposition and the rates
and mechanisms of clearance from the respiratory tract (Hatch & Gross,
1964; also Chapter 6). Furthermore, the particle size of substances
given orally as suspensions can also markedly influence their toxicity
(Boyd, 1971). If critical judgements based on the relative toxicities
of the same or different substances, administered orally in
suspension, are to be made, it is necessary to ensure uniformity of
particle size.
Vapour pressure of a chemical substance is important in the
practical consideration of the likelihood of exposure of man through
inhalation, and the design of experimental inhalation toxicity studies
will be influenced by the ease with which a solid or liquid vaporizes
under controlled conditions. However, a high vapour pressure may
produce technical problems if the objective of the test is to
determine toxicity by the oral route of administration. Studies by
Jones et al. (1971) showed that for a large series of food flavouring
agents mixed in laboratory animal diets, the amount of loss from the
diet was inversely related to the boiling points of the flavouring
agents. Frequent chemical analyses of the diet, as well as frequent
preparation of fresh diets and/or restricted feeding periods to limit
the time for loss by vaporization are necessary to provide accurate
estimates of intake in feeding studies on substances with low boiling
points.
Knowledge of reactivity with, or binding to, macromolecules may
allow specific design of mechanism experiments, when these
macromolecules are essential tissue and cell constituents. Knowledge
of the chemical reactivity of a substance may also be of considerable
importance in the early planning of feeding studies, if the chemical
under test is likely to react with the macromolecules present in the
laboratory diet. On the one hand, chemical binding or adsorption on
macromolecules in the diet may markedly alter the rate and extent of
absorption of the test compound from the gastrointestinal tract; in
some cases, biologically reactive groups on the test chemical may be
neutralized by dietary constituents. On the other hand, reaction of
the test chemical with essential dietary constituents may contribute
to nutritional deficiency states, or new and more toxic compounds may
be formed. Several examples of these types of reactions have been
summarized by Golberg (1967).
2.2.3 Impurities
In the design of toxicity experiments, it is extremely important
to consider the chemical purity of the sample to be tested. In certain
cases, e.g. food additives and pesticides, the regulations will often
provide specifications of purity for the compound in actual use and
recommended test protocols may specify that toxicity evaluations are
to be conducted with samples that meet these specifications (WHO,
1967). However, there is always the possibility that, when testing the
technical product, the biological effects observed may be due to, or
modified by, trace contaminants. If the contaminants are unknown or
their biological activity unsuspected, toxicity tests may lead to
erroneous conclusions concerning the primary chemical in question. In
contrast, tests on highly purified samples may not detect the toxic
action of contaminants present in samples used commercially.
Furthermore, for many chemical substances used in manufacturing or
incidentally released into the environment, specifications of purity
may not be standardized. Therefore, one of the earliest and most
difficult decisions that must be made in the design of a toxicity
evaluation programme is the selection of the sample to be studied
(technical grade, highly purified, etc.)
Requirements for the purity of the compounds selected for
toxicological testing depend on the purpose of the testing as
discussed in section 2.1. During the development of a new
technological process, it may even be useful to test mixtures of
unknown composition. The information so obtained may alert organic
chemists in the research laboratory or pilot plant operators to the
possible hazards of these unknown mixtures which may vary as
procedures develop and improve. However, data obtained from such
studies will have a limited value. The determination of health
standards requires a compound of a high degree of purity or of highly
standardized composition combined with a precise knowledge of various
impurities. Only in this way will health standards have a universal
value. However, for practical purposes, and for extrapolation to human
exposure conditions it may be prudent, when a technical grade product
standardized by specifications is used in commerce, to select this
grade for toxicity testing and carefully characterize it with respect
to the nature and amounts of any impurities present. Scheduled
analytical spot checks during the course of the experiment to provide
assurance of chemical constancy is also desirable.
When a chemical substance used in commerce is not standardized
with respect to specifications for purity, experimental toxicity
evaluation on a test sample of high purity would, indeed, seem to be
the most rational selection. Data derived from this compound could
then be used to characterize, toxicologically, the action of the
primary chemical under consideration. When certain quantifiable
indices of toxicity have been identified, selected tests with typical
samples of commercial products could be conducted and the results
compared with the purified product to detect possible differences. Of
course, if the impurities represent a significant portion of the
product, or if their chemical properties or their chemical analogy to
other known substances suggest they may have serious toxic properties,
the impurities must be evaluated separately.
An alternative approach is to select a sample of a chemical that
most nearly represents the impure product used commercially, subject
this to comprehensive toxicity evaluation and then make selected,
critical toxicity comparison with purified samples of the primary
chemical as well as with the impurities.
Either approach contains uncertainties, and little would be
gained by short-term spot checks if the toxic action of the impurity
were only detectable after long-term exposure or a latent period. It
is in problem situations such as these that some of the
chemicophysical principles discussed earlier must be applied at
several levels of decision making: the sample to choose for testing;
the design of the evaluation protocol; or the decision (or regulation)
to produce a purer substance for routine use in commerce.
A recent, most controversial problem arising from the
contamination of a primary commercial product involves the apparent
teratogenic action of the herbicide (2,4,5-trichlorophenoxy)acetic
acid (2,4,5-T) (Panel on Herbicides 1971). It is now known that the
first studies to reveal this action were conducted with a sample of
the herbicide that contained a rather high concentration (about
30 mg/kg) of the contaminant 2,3,7,8-tetrachloro-dibenzo-4-dioxin
which is formed during the synthesis of the trichlorophenol precursor.
Tetrachlorodioxin is extremely toxic. For guineapigs, the ratio of the
LD50 for 2,4,5-T to the LD50 of the dioxin is about 630 000. In
female rats, the acute oral LD50 for dioxin is about 1/10 000 of the
oral LD50 for 2,4,5-T. The daily dose of dioxin in pregnant rats
that produced fetal toxicity was only about one four-hundredth of the
maternal LD50 of dioxin or about one four-millionth of the
single-close oral LD50 of 2,4,5-T for female rats. Thus, even if the
concentration of dioxin as a contaminant of 2,4,5-T is kept below
0.5 mg/kg, the major concern for the toxicity of 2,4,5-T should
apparently still be directed towards the contaminant rather than
towards the herbicide itself (at least insofar as effects on
reproduction are concerned).
This kind of problem is not new. Twenty-five years ago, marked
differences in the toxicity of different samples of the insecticide
parathion were traced to contamination with small quantities of the
oxygen analogue and the phosphorothiolate isomer, which are much more
potent anticholinesterases and more acutely toxic than the parent
insecticide (Diggle & Gage, 1951).
2.3 Probable Routes of Exposure
2.3.1 General considerations
Many chemicals will become distributed in various environmental
media or will be used for different purposes, and substantially
different populations may be at risk. Thus, it may be necessary to
obtain extensive test data by different routes of exposure. The choice
of route for practical purposes is generally dictated by: (a) the
likely route by which man will be exposed; and (b) whether the
chemical will produce local injury at the site of exposure. The second
question will often be resolved by acute or short-term studies on
animals dosed by oral, inhalation, dermal, and possibly ocular routes.
Details of test procedures are included in subsequent chapters.
Although it is usually wise to conduct experiments using the route
through which man will be exposed, other more convenient routes may be
chosen for many of the tests if it is determined that the major toxic
effects of a chemical are systemic, occurring only after absorption
and distribution in the body. Data on blood and tissue levels should
be obtained by several routes of exposure, including those that are
considered primarily experimental; with this information, it may be
possible to relate toxic effects to blood or tissue concentrations of
the test chemical and its metabolites. Such information greatly
facilitates comparison of experiments using different routes and may
either confirm or deny the validity of extrapolating data, obtained
experimentally by one route, to the evaluation of potential toxicity
by another perhaps more realistic route of exposure.
2.3.2 Specific variables related to route of exposure
2.3.2.1 Rate of absorption
As a general rule, one can predict that for the usual routes by
which man may be exposed, absorption of chemicals will be most rapid
when given by inhalation, less rapid when given by gavage, and slowest
with dermal application. This order may, however, be modified
depending upon various physicochemical properties of the substance
under test in relation to the microenvironment of the absorbing
surface (Klassen, 1975; Loomis, 1974). The rate of absorption will be
one determinant of the rate of onset of signs of acute poisoning. If
the rates of detoxification and excretion or of injury repair exceed
the limiting rate at which a chemical is absorbed, it is possible that
toxic signs observed by one route of administration will not be
detectable by another route for which absorption is slower (Casarett,
1975; Murphy, 1975). Comparative absorption-distribution kinetics by
different routes would determine such a possibility.
2.3.2.2 Site of action
Specific tests should be conducted to evaluate effects related to
local reactions with specific receptors present in the organ of
absorption. These may be morphological tests to detect evidence of
irritation, inflammation, or oedema, or they may be functional to
detect biochemical or reflex action or bronchoconstriction. In
addition, the route of exposure may determine the organ or
physiological system in which effects will be first observed or
detected at lowest doses. For example, pesticides, which are direct
inhibitors of acetylcholinesterase, when given in sufficient doses by
any route, will produce a characteristic toxic syndrome involving
essentially all organs or structures innervated by cholinergic nerves.
However, at low doses, only specific organs may be involved. If such
compounds are applied to the skin, local sweating and fasciculations
may occur in the absence of signs of systemic poisoning. Exposure by
inhalation may result in bronchoconstriction, exposure by ingestion
may cause gastrointestinal upset before, or at lower doses than,
generalized systemic effects (Henderson & Haggard, 1943; Holmstedt,
1959).
2.3.2.3 Biotransformation
The route of exposure may determine the likelihood and type of
biotransformation before the chemical contacts the specific sites of
action. Thus, when chemicals are administered by the oral (or
intraperitoneal) routes, they will be absorbed and transported first
through the portal circulation to the liver (Lukas et al., 1971). For
example, if, with low oral or dietary doses, the capacity of the liver
to detoxify the compounds exceeds the rate of absorption, an effective
injurious concentration may never reach critical sites of action in
other tissues. Absorption of the same quantity through the lung or
skin, which generally have less detoxifying capacities, may result in
toxic action. It is now known that the lung, skin, and intestinal
mucosa, although generally less active than the liver, also have the
capacity for biotransformation of foreign organic chemicals (Alvares
et al., 1973; Fouts, 1972; Lake et al., 1973; Wattenberg, 1972).
Although knowledge is incomplete with regard to the tissue
distribution of both activating and detoxifying enzyme systems, it is
likely that their relative distribution will determine, to some
extent, the specific tissues that will be most affected by low doses
of some compounds, when given by different routes of exposure.
2.3.2.4 Species
The relative susceptibility of different species to the action of
chemicals may differ depending upon the route of exposure. When
administering compounds by the oral route, such factors as vomiting
reflex (absent in rats) and/or differences in type and distribution of
microflora that may detoxify (or activate) the test compound can
influence the interpretation of the results (Williams, 1972). The
rates of penetration of compounds through the skin and the acute
dermal toxicities of various compounds differ markedly among species
and not always in a predictable manner (McCreesh, 1965). Many of the
problems encountered in dermal toxicity testing and suggestions for
further research have been discussed by Barnes (1968). Roe (1968)
discussed various problems encountered in the design and
interpretation of inhalation toxicity studies related to species
differences in the anatomy of the respiratory tree. Enzootic lung
infections are an additional problem in the use of some species of
animals for long-term inhalation studies.
2.3.2.5 Unintended route
Interpretation of results and measurement of actual dose-response
relationships can be made difficult, because appreciable oral
ingestion may occur with inhalation or dermal exposures. Animals
exposed by either of these routes may ingest the material as a result
of preening, unless dermal applications are covered or made
inaccessible to licking or unless special exposure chambers (e.g. head
only) are used for inhalation exposures (see Chapter 6). In addition,
in particle inhalation experiments, the physiological protective
mechanisms of clearance by mucous transport of the particles out of
the respiratory tree (Hatch & Gross, 1964) with subsequent swallowing
may result in gastrointestinal exposure. Some degree of lung exposure
to volatile compounds administered in the diet or by dermal
application is also likely.
2.3.3 Special tests related to route
When exposure to a compound is most likely to occur by
inhalation, it is useful to know the effect of variations in
ventilation rates, since this will be a common variable among an
exposed human population under different conditions of activity. This
may be accomplished by the use of exercise wheels or treadmills.
When dermal exposure is the likely route, it will be useful to
conduct some tests to determine the effect of different solvents on
penetration of the test compound through the skin. Studies of the
influence of factors such as sweating, abrasions, or the presence of
detergents on dermal absorption and toxicity will also aid in
estimating toxicities under conditions likely to be experienced by man
(see Chapter 11, Part 2).
Interpretation and implications of toxicity data obtained with
oral exposures can be enhanced by examination of the influence of
fasting, dietary variations, and, particularly, administration by
gavage versus inclusion in the diet or drinking water. These factors
are discussed in more detail in subsequent chapters, but it should be
stressed that quite different results and interpretations may ensue if
the same daily dose is given rapidly by gavage or gradually in the
diet. Interpretation of such experiments is greatly aided, if the
design includes comparative absorption and distribution kinetics.
2.4 Selection and Care of Animals
2.4.1 General considerations
The selection and care of laboratory animals to be used in
toxicity tests is especially important in determining the success of
the experiment itself, the extrapolation of the data to man, and the
cost of the evaluation programme. In order to provide data on a
sufficient number of animals for valid statistical analyses, it has
become common practice to use small laboratory rodents for most
large-scale toxicity test programmes. Dogs or nonhuman primates are
frequently included in some of the studies, and studies on at least
one nonrodent species are often required by the test protocols
recommended by regulatory and advisory agencies. Recommendations (with
appropriate references for detailed information) concerning the
selection and care of laboratory animals to be used in the usual broad
scale toxicity evaluation studies are included in Chapter 3. The
selection of animals to be used in various special test procedures is
discussed in subsequent chapters.
Animals and animal care practices should be selected to provide a
scientifically sound and reproducible experiment; however, some of the
variables that contribute to nonuniformity may actually be exploited,
in special studies, to obtain data that may be useful in extrapolation
to nonuniform human populations. For example, if the inherent toxicity
of an air pollutant is to be characterized, the occurrence of chronic
lung infections should be avoided. On the other hand, specially
designed experiments to test the influence of air pollutants on the
susceptibility of animals to lung infections have provided a sensitive
procedure for measuring the adverse effects of air pollutants on the
physiological protective mechanisms that confer resistance to
respiratory infections (Ehrlich, 1966). Epidemiologists could
certainly use such information in the design of studies on human
populations exposed to air pollutants and to infectious microorganisms
present in the environment.
The choice of animals and the environment in which they are used
in toxicity studies will ultimately be determined (as for any other
decisions relating to experimental design) by the nature of the
questions asked or the hypotheses formulated. Controlled introduction
of additional variables may be desired for special studies. The
important principle is that appropriate control conditions should be
included in such studies to allow comparisons with results obtained
under more conventional procedures.
2.4.2 Animal variables
The objective of most experimental toxicity studies is to predict
the adverse effects of chemicals in man. Therefore, in addition to
uniformity of response, the guiding principle for the selection of
appropriate test species is that the test animals should resemble man
as closely as possible with respect to absorption, distribution,
metabolic transformation, excretion, and effect at site(s) of action
of chemicals. Both male and female animals should be tested and the
test protocol should encompass exposures of animals at both ends of
the age spectrum (see Chapter 3).
It is generally recommended that random-bred rather than highly
inbred strains of animals be used in broad-scale toxicity testing, at
least until the action of the chemical is well characterized (Food &
Drug Administration, 1970). In more specialized toxicity tests, it may
be desirable to use inbred strains, for example, when animal models
are needed that represent a genetic variation in human population, or
when hypotheses on the mechanism of action are tested.
2.4.2.1 Selection of species
The Food Protection Committee (1970) indicated that sensitivity,
convenience, and similarity in metabolism to man are the prime factors
to be considered in the selection of animal species for toxicity
testing. In the absence of information to the contrary, it is
generally recommended that data obtained from the most sensitive
species should be used as the basis for the extrapolation of test
information to man.
There is now ample evidence of wide quantitative variations among
species in their rates of biotransformation of foreign compounds
(Committee on Problems of Drug Safety, 1969; Parke & Williams, 1969;
Williams, 1967). Since many organic chemicals are subject to
biotransformation at several reactive groups in the molecule, it is
important to identify and quantify the biotransformation and
distribution pathways of a chemical in man and in several laboratory
animal species as early as possible in toxicity evaluation studies. It
seems axiomatic that for costly chronic studies on experimental
animals, the species that is most representative of man with respect
to the metabolism of the test chemical should be chosen. Often, the
only information concerning the metabolism and distribution of the
test compound in man may be derived from limited studies on
individuals accidentally or occupationally exposed to uncontrolled or
unknown doses.
In vitro studies of metabolism using animal tissues and human
tissues obtained at autopsy or biopsy could help in comparisons of
similarities or differences in metabolism between man and laboratory
animals. Although this approach cannot, in itself, provide information
that may be obtained in studies on intact animals, it can, coupled
with knowledge of the physicochemical properties of the compound and
the kinetics of enzymatic biotransformation reactions in tissues of
various species, provide a logical basis for selection of species for
long-term toxicity tests. Decisions based on comparative human and
experimental animal metabolism data should take into account
information concerning several pathways of metabolism. This will help
to ensure the inclusion of data on quantitatively minor pathways of
metabolism that may result in products of major toxicological
importance. These considerations of variation in biotransformation can
also be applied to intraspecies variations related to age, sex, and
strain (Benke & Murphy, 1973; Jori et al., 1971a; MacLeod et al.,
1972; Parke & Williams, 1969).
Anatomical and morphological variations can also determine the
selection of species. This source of variation is likely to be of
particular importance in inhalation toxicity studies (Roe, 1968).
Tyler & Gillespie (1969) compared anatomical characteristics of the
lungs of human beings with several laboratory and domestic animal
species when considering appropriate animal models for human
emphysema. They grouped anatomically similar species into four
classes: (a) cattle, sheep, and swine; (b) dogs, cats, and rhesus
monkeys; (c) rabbits, rats, and guineapigs; and (d) horses and man.
From their studies on horses, they concluded that the pathophysiology
and the morphological characteristics of emphysema in horses closely
resembled the disease in man and that the horse could be a
particularly suitable laboratory animal for studies of this disease.
Obviously, in the usual toxicity studies on air pollutants, the costs
of using horses would be prohibitive. The reactivity of a chemical at
primary target sites must also be considered as a potential variable
contributing to species differences in toxicity. The acute toxicity of
certain organophosphorous insecticides in representative mammalian,
avian, and fish species appeared to be more readily related to species
differences in the reactivity of the target enzyme
(acetylcholinesterase (3.1.1.7)) than to differences in hepatic
biotransformation rates (Murphy et al., 1968).
In the absence of specific knowledge of comparative metabolism
and sites of action, it is appropriate to apply the principle that
quantitative and qualitative similarity of response in several
mammalian laboratory species enhances the confidence that man will
respond similarly. Tests on several species seem equally as useful for
predicting effects in a heterogenous human population as the selection
of test species based on the results of limited studies of metabolism
in a very few individual human subjects, who may or may not be
representative of a broad cross-section of the human population at
risk. Of course, any quantitative information on the disposition and
action of chemicals in man is useful, as it adds to the accumulation
of knowledge from which more specific guidelines for species selection
may be derived in the future.
2.4.2.2 Animal models representing special populations at risk
Because many chemicals in the environment are widely dispersed,
all segments of the human population may sustain some exposure. For
this reason, it may be useful to design special experiments to
evaluate toxicity in animal models that represent potentially
hypersusceptible segments of the human population. The very young and
the aged represent such segments generally, because in the very young,
natural protective mechanisms such as metabolic detoxification systems
may be incompletely developed and in the aged, cell repair processes
may be less active than in younger individuals. Evaluation of the
toxicity of chemicals in animal models of commonly occurring human
diseases may be of value. Thus, for example, epidemiological studies
suggest that individuals suffering from coronary artery disease may be
particularly susceptible to carbon monoxide, the severity of signs and
symptoms in patients suffering from cardiorespiratory disease appears
to be aggravated by air pollution, and asthmatic patients appear to
have a higher frequency of attacks during periods of high oxidant air
pollution (Heimann, 1967). Few attempts have been made to evaluate
experimentally the interactions between exposure to toxic chemicals
and model disease conditions. Taylor & Drew (1975) reported that an
inbred strain of cardiomyopathic hamsters was more susceptible to
acute toxicity and cardiac arrhythmias produced by inhaled
trichlorofluoromethane than were random-bred hamsters that were not
cardiomyopathic. Easton & Murphy (1967) suggested that their
observation of greater mortality and respiratory distress in
ozone-preexposed than in air-exposed guineapigs given histamine
injections or inhalation exposures might be analogous to the apparent
increase in frequency and severity of asthmatic attacks reported for
peak periods of photochemical air pollution.
Problems of standardization of disease conditions add another
dimension to toxicity studies. However, it seems that animal disease
models should be given more consideration in toxicity evaluations that
are intended to provide the basis for the safe use of chemicals to
which large human populations are exposed. Jones (1969) summarized
reference sources for animal models of a large number of specific
human diseases. Several papers in a series of symposia proceedings
published by the US National Academy of Sciences provide discussion
and references to (among other topics) animal models for commonly
occurring disease states of the lungs (Tyler & Gillespie, 1969), the
cerebrovascular system (Luginbuhl & Detweiler, 1968), the heart (Jobe,
1968), the kidney (Lerner & Dixon, 1968), atherosclerosis (Clarkson et
al., 1970), diabetes (Hackel et al., 1968), and chronic degenerative
diseases (Abinanti, 1971). Since gut microflora are changed in certain
gastrointestinal diseases in man, modifications of the quality and
distribution of microflora in experimental animals might be a useful
model for special tests (Williams, 1972). There are at least three
possible applications of these disease models to toxicity studies: (a)
evaluation of the susceptibility of diseased tissues to chemicals
known to exert their action on that tissue; (b) influence of the
disease state on the metabolism and distribution of chemicals that may
act on the diseased tissue or at other sites; and (c) research on the
mechanism of action of toxic chemicals using specific modification of
receptor function or biochemistry.
2.4.3 Cyclic variations in function or response
Many physiological variables undergo cyclic peaks and troughs of
activity (Altman & Dittmer, 1966) some of which are diurnal (24-h) and
others of longer duration. These rhythms may be completely under
intrinsic control or they may be partly or largely regulated by
environmental variables such as light and temperature. Boyd (1972)
considers most diurnal variations in susceptibility to drug toxicity
to be mainly related to eating and sleeping habits. Since rats are
nocturnal feeders, the greater quantity of food in the stomach early
in the morning compared with the afternoon may alter the acute
toxicity of chemicals given intragastrically. Attempts to standardize
this variable have led to recommendations that acute toxicity tests by
intragastric administration should be conducted on animals that have
fasted overnight (Food & Drug Administration, 1959). Intragastric
LD50 values are generally lower in rats fasted overnight compared
with those fed ad libitum; however, the differences are usually only
of the order of two- to three-fold (Boyd, 1972; Loomis, 1974). The
influence of fasting may, in some cases, be related to rates of
absorption from the gut in the presence or absence of food but this
cannot account for all such variations. A striking example has been
reported by Jaeger et al. (1975) in which acute toxicity and liver
injury in rats exposed through inhalation to several halogenated
olefins were enhanced 10- to 20-fold by overnight fasting. A diurnal
cycle of susceptibility of rats to the toxicity of inhaled vinylidene
chloride appeared to be related to the diurnal cycle of liver
glutathione concentrations (Jaeger et al., 1973) which may be
secondary to a diurnal cycle in feeding activities. The duration of
pentobarbital anaesthesia in mice under usual laboratory housing
conditions exhibited a diurnal cycle with the longest duration at
14h00 and the shortest (40-60% of that at 14h00) duration at 02h00
(Davis, 1962). The amplitude of the cycle was considerably reduced,
when animals were caged individually as opposed to group caging, and
constant light abolished the cycle. That circadian variation in the
action of certain organic chemicals may be related to circadian
variation in their biotransformation is suggested by the work of Jori
et al. (1971b).
Beuthin & Bousquet (1970) reported seasonal or circannual rhythms
for drug action and biotransformation rates in rats. The induction of
increased drug metabolism by phenobarbital also exhibited a seasonal
variation. Basal levels of hexobarbital metabolism were highest during
the winter months and lowest in summer, whereas the opposite cycle for
induction of hexobarbital oxidase by phenobarbital was observed. It
should be noted that studies of seasonal variations in the metabolism
or toxic action of chemicals must be carefully controlled with respect
to environmental variables that might produce similar variations in
response (see 2.4.4). Boyd (1972) suggests that seasonal variations in
susceptibility may be related to the hibernation reaction or to
weather conditions in the geographical area concerned.
Circadian variation in adrenocortical activity in rats was
investigated by Szot & Murphy (1971) in animals exposed acutely or
subacutely to the pesticides parathion and DDT. Although the degree of
stimulation of corticosterone secretion after single doses of
parathion varied depending upon the phase of the cycle at the time of
administration, feeding parathion or DDT in the diet at rather high
concentrations did not change the phase or the amplitude of the
natural adrenocortical rhythm or alter the stimulation of
corticosterone secretion produced by irritant stress.
In rodents, locomotor activity is greatest at night and Boyd
(1972) suggests that depression of activity is best demonstrated at
night or in rats starved for 3 days when their daytime activity is as
great as at night time. However, a more convenient approach may be to
reverse the lighting schedule, a procedure used successfully for
measuring the effects of various inhaled air pollutants on locomotor
activity in mice (Murphy, 1964).
The time of day at which biochemical or other tests are conducted
in control and experimental animals may influence the reproducibility
of the test data, if the biological variables under test display
rhythmic variation. An investigator may exploit these rhythmic
variations to advantage in special studies of factors that influence
susceptibility to chemical injury. However, if the aim is a broad
scale characterization of the toxicity of a chemical, the choice may
be to carefully standardize times of administration of chemicals and
of animal sampling to minimize both known and unrecognized circadian
variations as much as possible.
2.4.4 Environmental variables
There are numerous possible variations in the environment in
which experimental animals are housed or tested that can influence
their response to toxic chemicals. General considerations of these
variables are discussed by several authors (Boyd, 1972; Doull, 1972,
1975; Hurni, 1970; Morrison, 1968). Unless the purpose of the
experiment is to use these variables to predict possible alterations
in effects in man exposed to chemicals under similar environmental
variations, it is generally possible to minimize their influence on
the toxicity of chemicals by adopting good principles of animal care.
Reference sources are available to provide guidelines for proper
housing, diets, cage size requirements, etc. (DHEW, 1972; Universities
Federation for Animal Welfare, 1972).
Only brief comments will be made on some of the major
environmental variables that affect toxicity experiments or that can
be used for predicting mechanisms or possible implications to man.
2.4.4.1 Temperature
Major variations from the recommended environmental temperatures
and relative humidities can contribute not only to the impairment of
general health and increased susceptibility to infection of the
animals, but also to variation in their response to toxic chemicals.
The mechanisms of interactions between environmental and body
temperatures and drugs or toxic agents have been reviewed by Doull
(1972) and by Cremer & Bligh (1969). Since absorption, distribution,
metabolic transformation, excretion, and reactivity with receptor
sites depend on various temperature-dependent chemical reactions, it
might be expected that the toxicity of chemicals would be readily
influenced by temperature. However, since toxicity studies are usually
conducted with homotherms, only minor changes in core body temperature
occur with moderate changes in environmental temperatures. By the same
token, changes in environmental temperature will elicit homeostatic
changes in various physiological or biochemical systems. These may
then alter some of the physiological variables (e.g. ventilation,
circulation, body water, intermediary metabolism) that are
rate-limiting determinants of the absorption, deposition, and action
of toxic chemicals. Furthermore, toxic chemicals may exert their
action by disruption of the thermoregulatory mechanism as suggested
for cholinesterase inhibitors (Meeter & Wolthuis, 1968). Exposure to
toxic chemicals can also mimic the actions of extremes in
environmental temperature or other physical stressors (Murphy, 1969;
Szot & Murphy, 1970). Thus, fluctuations in environmental temperatures
can lead to functional changes that might be mistakenly attributed to
the action of the chemical or they may actually alter the toxicity. If
interference with physiological thermoregulatory mechanisms is a
likely action of the chemical, careful control of environmental
temperatures is necessary to ensure reproducibility of measurements of
this action.
2.4.4.2 Caging
The type of cage, grouping, bedding, and other factors related to
caging can markedly influence the toxicity of some chemicals and drugs
(Boyd, 1972; Doull, 1972; Hurni, 1970). The acute toxicity of
4-[1-hydroxy-2-[(1-methylethyl)amino]ethyl]-1,2-benzenediol
(isoproterenol) was markedly greater in rats caged singly for more
than three weeks than in rats caged in groups (Hatch et al., 1965).
Winter & Flataker (1962) reported that grouped rats held in "closed"
(sheet metal on four sides and bottom) cages were more resistant to
the acute toxicity of morphine and 1-[2-(4-amino-phenyl)ethyl]-
4-phenyl-4-piperidine carboxylic acid ethyl ester (anileridine) than
rats held in "open" (wire mesh) cages. These differences were
attributed to mechanical factors that prevented depressed rats from
maintaining an open airway in the wire mesh cages. Altered toxicity of
chemicals related to caging effects are generally purely experimental
variables and can be controlled by good practices of laboratory animal
housing.
2.4.4.3 Diet and nutritional status
Dietary variables can influence the toxicity of chemicals in
several ways. The toxicities of several pesticides were enhanced to
different degrees in rats given low protein diets (Boyd, 1969).
Protein-deficient diets protected rats against the acute
hepatotoxicity of carbon tetrachloride and N-methyl-
N-nitrosomethanamine (dimethylnitrosamine) (although the number of
kidney tumours after a single dose of the latter increased), while the
acute toxicity of chloroform was unchanged and the acute toxicity of
aflatoxins was markedly enhanced (McLean & McLean, 1969). These
effects could be explained, at least in part, by the reduction of
activity of hepatic mixed-function oxidases that generally results
from feeding low-protein diets. Whether or not a compound's toxicity
is increased or decreased in such circumstances will depend upon
whether microsomal biotransformation leads to the formation of more or
less toxic metabolites. Numerous other examples of macro and
micronutrient deficiencies, which alter the activity of the
drug-metabolizing enzyme systems and the toxicity of chemicals, have
been reviewed by Campbell & Hayes (1974). Intestinal and pulmonary
aryl hydrocarbon hydroxylase activity is modified by diet. Of
particular interest is the observation that changing rats from a
commercial, natural diet to a balanced, purified diet resulted in an
almost total loss of activity of this enzyme system in these tissues
(Wattenberg, 1972). Flavonoid compounds, present as natural
constituents of alfalfa meal (and other plants), may account for the
apparent induction of aryl hydrocarbon hydroxylase by natural diets.
The trace mineral content of diets can also influence the metabolism,
distribution, and action of toxic chemicals (Moffitt & Murphy, 1974).
The results of toxicity experiments can be markedly influenced if
care is not taken to ensure constancy of diets free from residues of
contaminating chemicals. However, since nutritional imbalances are
widespread in the human populations, controlled variation of
experimental diets to simulate major human deficiency states (e.g.
kwashiorkor resulting from protein deficiency) is an important area
for research in toxicology and should, perhaps, be included in
standard toxicity evaluations of select groups of chemicals.
2.5 Statistical Considerations
Although various protocols for toxicity testing recommend
specific numbers of animals to be used for various acute and chronic
tests (see Chapter 3), a useful guiding principle is that sufficient
animals should be used to allow statistically valid conclusions
concerning differences in the response of test animals compared to
controls and to provide a base for statistical extrapolations to
larger population samples. Statistical procedures allow the
experimenter to make ( a) descriptions of sets of data or population
characteristics, and ( b) statements of probability of events.
Various procedures provide for both enumerative data, or yes-no type
characteristics, and measurement data, or graded effects or
characteristics. Some procedures (t-test, F-test) are restricted to
observations that have specific frequency distributions, while others
(signed rank test, rank run test) are free of any assumptions about
distribution (i.e. nonparametric). Standard texts should be consulted
for the application of biostatistics to the design and analyses of
experiments. In practice, it is highly advisable to involve a
statistician in the experimental design as well as in the analysis.
The number of animals required to make statistically valid
conclusions regarding the differences between experimental and control
animals will depend upon the degree of confidence desired and the
magnitude of the possible sources of variation in the experiment. The
second consideration will depend upon the uniformity of the test
animals with respect to the biological system or systems under test.
This, in turn, will depend upon both genetic and environmental
factors. The reproducibility of the bioassay and chemical procedures
used in the tests will be another source of variation. A further
source of variation in toxicity testing is related to the constancy
and stability of the test chemical. Finally, there are the variables
introduced by the investigators (often the most difficult to control),
beginning with the care and attention given to accurate dosing
throughout the various steps of the experiment. Attempts must be made
to minimize all these sources of variation as far as possible, without
sacrificing any important aspect of the experiment.
When testing whether or not two sets of data may both be valid
samples from the same population with normal frequency distribution
(i.e. null hypothesis) or whether the control group is not
significantly different from the treatment group, statisticians
describe the appropriate sample size in terms of the desired "power"
of the test. Two types of decision errors exist. One is that a
significant difference between groups is stated to exist when, in
fact, there is no difference. This is called the type I error and the
experimenter must state what probabilities (alpha) for this error he
will accept; most commonly a probability of 0.05 is used, but an
experimenter may sometimes require a probability as low as 0.01, or
any other he chooses. The second type of decision error is that no
significant difference between groups is stated to exist when, in
fact, the groups are different. This is called the type II error, and
1-ß (the probability that one will not make this error) is the power
of the test. The power is directly related to the sample size and the
ratio of "differences between true means of the samples" to
"differences between experimental error of the means of the samples".
Once this ratio is fixed, the power increases solely as a function of
sample size. The experimental error (pooled variance) can be estimated
from previous experiments or a pilot study. The acceptable magnitude
of the differences between true means of the samples is at the
discretion of the experimenter; he must use expert judgment and should
have a reasonable rationale; it will usually be the smallest value
that is considered to be of practical importance.
Another important statistical consideration is related to the
selection of the valid number of sampling units. This may be
particularly true in considering quantal (all-none, yes-no) effects
that may be multiple occurrences within a single test animal, as, for
example, in reproduction and carcinogenesis studies where,
respectively, there may be a number of affected offspring or a number
of tumours resulting from the treatment of a single animal. The
selection of the appropriate unit, either the number of animals
exposed or the number of occurrences of an effect, can determine the
statistical significance of an observed effect. Weil (1970) suggests
that in reproduction studies the number of maternal animals (or
litters) and not the number of affected fetuses or offspring is the
valid sampling unit, and that in carcinogenesis studies the number of
tumour-bearing animals should be the sampling unit and not the total
number of tumours. Furthermore, in carcinogenesis studies, animals
risk death from factors other than the tumours; in some cases, animals
may have died before they had time to develop a tumour; in other
cases, information from some animals may be lost from the study
because of unexpected death and autolysis of tissues preventing tumour
identification. An adjusted tumour incidence may be estimated by the
life-table techniques in such experiments (McKinney et al., 1968).
Another problem may be associated with gross or histopathological
examination where, because of cost and time considerations, only
tissues of a fraction of the total number of animals exposed are
subjected to complete examination. This will reduce the likelihood
that statistically valid conclusions can be drawn from the data on
occurrence of lesions. In practice, reasonable compromises are usually
necessary. Irrespective of the statistical methods of analysis used in
both the design and interpretation of results of toxicity tests on
chemicals, they cannot replace careful experimentation and
comprehensive knowledge of the underlying biological mechanisms of the
various steps between exposure to a chemical and injury.
2.6 Nature of Effects
2.6.1 Reversible and irreversible effects
Reversible effects are characterized by the fact that the
deviation from normal structure or function induced by a chemical will
return to within normal limits (controls) following cessation of
exposure. With irreversible effects, the deviation persists or may
progress, even after exposure ceases. This might be further qualified
by time limits, that is, the time required for return to normality
after exposure should be a reasonable fraction of the remaining
lifetime of a young animal for it to be considered reversible.
Reversibility may also be qualified by the normal lifetime of a
specific cell or macromolecule that serves as the end-point for the
effect. For example, cholinesterase-inhibiting insecticides are
generally considered irreversible inhibitors if the rate of reversal
of inhibition corresponds approximately to the time required for
synthesis and replacement of the enzyme, a process with different
rates in different tissues. Certain effects of toxic chemicals are
unmistakably irreversible, including the production of terata, or
malignant tumours, production of mutations in offspring of exposed
animals, certain chronic neurological diseases, production of true
cirrhosis, or emphysema. These are rather gross manifestations of
certain specific chemical-cell interactions, and, either at the level
of the first affected molecule or at intervening points leading to
these manifestations, there are probably reversible effects.
Understanding these effects and determining the critical dose that
produces them will make it possible to predict truly adverse effects
more rapidly.
The rate of reversibility of an effect will depend upon the rate
of cellular injury and the rate at which this injury is repaired
(Casarett, 1975). The rate of injury will depend upon the
concentration and duration or frequency with which a test chemical
contacts responsive tissue constituents. It is, thus, dose and
dose-rate dependent. The rate of repair is determined intrinsically
and may involve several cell processes. It may vary between different
tissues and probably between different species and strains. From a
practical standpoint, it is generally impossible to measure the
specific processes involved in injury and repair in a standard
toxicity evaluation study. However, it is important to make
measurements of the reversibility of effects in early, acute and
subacute studies. Thus, the time required for a process to return to
normal after single doses (which produce various degrees of injury)
will provide a guideline for the selection of doses to be used in
subsequent acute or chronic studies. The predictive value of such
information will depend upon the persistence of the chemical in the
test organism. If the chemical produces an effect and then is rapidly
detoxified or excreted, it may be possible to predict, with reasonable
accuracy, doses or exposure schedules that would not produce
cumulative effects. The manner of exposure and possible actions other
than the one being measured would, of course, be important in drawing
such conclusions. For example, rapid reversibility after a single dose
might not be indicative of the rate of reversal with a repeated dosing
if the first dose, in addition to the measured effect, also altered
either the repair processes or the processes responsible for
detoxification of the chemical. An example of an apparent
self-inhibition of detoxification is the insecticide malathion which
is rapidly hydrolysed by carboxylesterases. These are, in turn,
inhibited by metabolites or contaminants of malathion (Murphy, 1967).
Repeated exposure studies are necessary to evaluate such
possibilities; thus, the design of short-term feeding or inhalation
studies should include extra groups of animals that can be removed
from exposure either at the end of the experiment or, preferably, at
selected intervals for measurements of rate of reversal of any
observed effect.
If the chemical persists or accumulates in the organism,
measurements and interpretation of rates of reversal of effects are
more complicated. For this reason, it is useful to have kinetic data
on absorption and disposition to correspond with data on rates of
production and reversal of effects. Further discussion of these and
related principles is provided by Hayes (1972) in relation to his
proposal that determination of "chronicity factors" (1-dose LD50
(mg/kg) ‰ 90-dose LD50 (mg/kg/day) in diet i.e. the ratio of single
dose LD50 to the daily dose given in diet for 90 days which results
in 50% mortality at that time) is useful in predicting candidate
chemicals requiring long-term studies. The use of such predictive
methods must also take into consideration potential for other effects
that could never be detected in a subacute study (e.g. tumorigenesis).
2.6.2 Functional versus morphological changes
Toxic effects are often classed as functional or morphological in
nature. There has been a traditional attitude that changes in gross or
microscopic structure are more serious than functional changes.
Indeed, altered structure often seems to have taken on the implication
of irreversibility while altered function is often considered a
reversible effect. This conclusion, of course, depends on the level of
understanding of mechanisms of injury, rates and mechanisms of repair,
and causal associations between related functional and morphological
changes. Furthermore, whether the change is regarded as functional or
morphological may depend on the manner of detection. For example,
accumulation of fat in a cell observed through a microscope will most
often be considered a structural change, but, if the same cells or
tissues were assayed for triglyceride content, the increased
triglyceride would probably be classified as a functional or
biochemical change. The introduction of enzyme histochemistry and
electron microscopy into toxicity evaluation studies makes the
distinction between morphological and functional effects even less
clear. It may therefore be inappropriate to attempt to make these
distinctions.
Rowe et al. (1959) reviewed data from studies on a large number
of chemicals repeatedly administered to animals over periods ranging
from one month to two years, and summarized the frequency with which a
certain effect was found and the frequency with which it was the only
effect found. The effects were considered on: mortality; food intake;
body weight; organ weights; the histopathology of virtually every
major organ; haematology; blood urea nitrogen; clinical urinalyses;
central nervous system (most probably gross behaviour); gross
pathology; and cholinesterase activity. The authors found that if
growth, liver weight, kidney weight, liver pathology, and kidney
pathology had been studied, the lowest dose level that caused any
effect would have been detected in 96% of the studies. Changes in food
intake, central nervous system depression, excessive mortality,
increased lung weight, testicular injury, haematological changes, and
cholinesterase depression were the most sensitive effects in one or
more of the remaining 4% of cases. The reader should consult the
original reference for details but it is important to note that of the
commonly used criteria of effects, liver and kidney micropathology
were quite sensitive indices.
There are, of course, well-known examples where functional
changes are the only manifestations of toxicity. Many of the
organophosphate and carbamate insecticides inhibit cholinesterase
(3.1.1.8) activity and produce signs and symptoms (even death) that
can be characterized as purely functional without the production of
morphological lesions, detectable by conventional techniques.
Similarly, irritant air pollutants can often cause bronchoconstriction
and respiratory distress, without any accompanying morphological
changes. Both the functional cholinergic signs and symptoms produced
by the anticholinesterase insecticides and the bronchoconstriction
produced by irritants provide means of early detection at low levels
of exposure. Although these effects are reversible, they are no less
important during the period of exposure than certain kinds of
morphological effects. On the other hand, certain kinds of
"functional" changes, e.g. increased level of plasma transaminase
activity, usually reflect some type of structural change in cells that
allow these enzymes to "leak" into plasma (Cornish, 1971).
It is not possible, with present knowledge, to conclude that
either functional or morphological changes represent the most
sensitive, the earliest, or the most serious effects of toxic
chemicals. Since maintenance of both integrated function and
integrated structure ultimately depends on chemical reactions among
cell constituents, it is logical to conclude that specific biochemical
changes are the first and most sensitive effects. Unfortunately, with
relatively few exceptions, the specific biochemical receptors for
toxic chemicals are unknown. The more information that can be obtained
with respect to time- and dose-relationships for functional and
morphological effects, the more predictive the tests will become. This
requires an approach to toxicity studies in which proof of a mechanism
will require an integrated biochemical, physiological, and
morphological approach. Dawkins & Rees (1959) provide a useful short
treatise on an integrated biochemical-pathological approach to studies
of several toxic chemicals. Although advances in both biochemistry and
pathology now allow even more precise studies than those outlined by
these authors, the general principles which they develop are still
applicable.
2.7 Dynamic Aspects of Predictive Toxicology
2.7.1 Traditional versus new techniques
The objective of any toxicity test programme is prediction:
prediction of biological disposition from physicochemical constants,
prediction of altered cell or organ system function from reaction with
macromolecules, prediction of irreversible consequences of reversible
changes, prediction of implications of selected measurable variables
to overall health and survival of the test organisms, prediction of
effects in individuals of one species from tests conducted in another,
and finally predictions of incidence in large populations from tests
on small samples. All of these predictions must be related
quantitatively to a dose and dose-rate or schedule that can ultimately
be related to probable amounts used, the manner of use or the
occurrence of the chemicals in the environment.
Generally, traditional approaches to toxicity evaluation have not
attempted to make predictions far removed from the final application
or interpretation of the data. Thus, as outlined in Chapter 3, test
organisms are exposed to a range of doses and their health status is
examined by biochemical, physiological, or pathological procedures
analogous to those used in clinical medicine. When this approach has
been comprehensive, judicious application of the data usually appears
to have been successful in preventing chemically-induced disease.
Abandoning this approach in favour of new, different, or short-cut
methods cannot be advocated without thorough verification of their
validity. On the other hand, serious consideration must be given to
the application of some short-term ways of predicting toxicity in
order to provide a practical means of evaluating the many chemicals
already in the environment and those new compounds that are
continuously being added to the environment and have not been
subjected to traditional tests. Preceding sections have discussed some
possibilities, the following sections contain brief comments and
examples for consideration in selecting tests.
2.7.2 Toxicity of chemical analogues
Although it may be possible to predict the toxicity of individual
compounds in a homologous series from detailed knowledge of some
members of the series, some special exceptions should be noted. A
classical example involves the series of fluorine-substituted
aliphatic alcohols and acids, in which high acute toxicity alternates
with odd and even numbers of carbon atoms, the latter being the most
toxic (Pattison, 1959). The odd number of total carbon atoms confers
high toxicity in a homologous series of fluoronitriles, however. This
demonstrates the possibility that detailed information concerning only
a few members of a homologous series might fail to predict the
toxicity of another member of the series.
Recently, Johnstone et al. (1974) examined a number of
biochemical effects in a series of isomerically pure compounds for
their potency as liver enzyme inducers. Potency for this effect
increased with increasing chlorination that was related to differences
in biotransformation and excretion rates; however, there were also
striking differences in the potency of positional isomers in the lower
chlorinated biphenyls.
The mechanism of the toxic action of organophosphorus
insecticides was known to be the inhibition of acetylcholinesterase
even before they were introduced into use 30 years ago. However,
quantitative prediction of their acute toxicity from in vitro tests
of their relative potency as anticholinesterases is still inadequate
because of incomplete knowledge of the dynamic relationships between
several pathways of metabolism which yield both more and less potent
metabolites (Murphy, 1975). Nevertheless, these compounds have been
subjected to a great deal of research on both their physicochemical
and biological properties which should be applicable to predictions of
their relative environmental persistence, interaction with other
compounds, and, ultimately, to the design of safe molecules (Eto,
1974).
Using model ecosystems, Lu & Metcalf (1975) studied
bioaccumulation, biodegradability, and comparative detoxification
mechanisms in several benzene derivatives with widely-varying
physicochemical constants and biological activities. They concluded
that biological disposition and action could be predicted by the basic
molecular properties of water solubility, the partition coefficient
for lipid/water, and reactivity as determined by electron density.
Johnson (1975) recently reviewed the problems encountered in the
pursuit of the mechanism of delayed peripheral neuropathy produced by
some organophosphorus esters. The structure-activity relationships
identified in this research may be considered as a model of thorough
investigation that began as a problem in retrospective toxicology and
led to promising developments applicable to prospective toxicology.
Some interesting aspects of this problem are: the particular
usefulness of a non-mammalian species, the hen, as a predictor of a
toxic action that occurs in man; the concept of primary metabolic
effects on central neurons as a precursor to pathological change
detected in peripheral nerve fibres; and the difficulties of detecting
a specific critical esterase inhibition that represented only a small
percentage of the total esterase activity. Production of peripheral
neuropathy appears to be characteristic of organophosphorus esters
which may not only phosphorylate the specific "neurotoxic esterase"
but are also capable of dealkylation (or aging) following
phosphorylation. Although the steps between this primary
phosphorylating-aging process and the eventual manifestation of
peripheral neuropathy are still unclear, it appears that it may be
possible to predict probable occurrence of a delayed, chronic disease
from studies of the primary chemical-macromolecular interaction of
neurotoxic esterase inhibition that occurs immediately following
exposure.
2.7.3 Relation between site of metabolism and site of injury
Although for many years it was thought that the biotransformation
of organic chemicals represented detoxification mechanisms, it is now
apparent that numerous compounds are enzymatically converted to
intrinsically more active compounds in vivo (Fouts, 1972; Murphy,
1975; Parke, 1968). The liver is generally the most active tissue in
catalysing these "activation" reactions, but it is not always the most
susceptible target tissue as, for example, in the case of activation
of phosphorothioate insecticides to phosphate insecticides. This may
be explained, in part, by the presence of detoxifying enzymes or
reactive but noncritical binding sites in the liver that may prevent
the phosphates from escaping to inhibit cholinesterase in nerve target
tissues. The brain tissue has only a small fraction of the liver's
capacity to activate phosphorothioates, but because the activation
occurs in the same tissue as the critical target site, activation in
the brain may be the most important in determining toxicity.
Recently, the characteristic hepatotoxicity of several chemicals
has been related to their enzymatic conversion to highly reactive
derivatives that covalently bind to essential liver cell constituents
at, or near, the site of activation (Brodie et al., 1971). A similar
possibility may explain the bronchiolar neurosis produced in rats and
mice by bromobenzene and other aromatic hydrocarbons (Reid et al.,
1973).
The detailed study of the metabolism, storage, or binding and
distribution of foreign chemicals in the lung is a relatively recent
activity and has focused largely on therapeutic chemicals (Bend et
al., 1973; Brown, 1974; Orton et al., 1973). Because inhalation is a
common route of exposure to a wide variety of air contaminants in
industrial and community environments, future toxicity studies would
benefit by the inclusion of metabolic studies concerning rates of
absorption from, and local actions in the lung. Witschi (1975) has
reviewed biochemical approaches that may be used in the evaluation of
toxic injury to the lung.
Since the intensity and duration of the toxic action of a
chemical depends on the concentration of the active form at critical
receptor sites of action, kinetic aspects of absorption, distribution,
and excretion (as well as biotransformation) will influence the
specific sites of action. This topic is discussed in detail in Chapter
4 but it is worthy of note that Dedrick (1973) developed several
useful kinetic models that might be applied in predicting species
differences or similarities in response.
2.7.4 In vitro test systems
Where appropriate, studies in experimental animals should be
supplemented by isolated perfused organ, tissue slice or extract, and
tissue culture techniques. Where possible, attempts should be made to
compare the tissues of human subjects available from autopsy or
therapeutic biopsies, with those of other species in their response to
toxic chemicals (Worden, 1974). When the mechanism(s) of toxicity have
been elucidated and the target organ(s) identified, specific species
comparisons and dose-response relationships can be studied by these
in vitro techniques.
Knowledge of a specific enzyme or biological macromolecule that
serves as a target for reaction with toxic chemicals may provide a
means for screening and predicting relative potencies or specific
actions of chemicals in intact organisms. However, as pointed out
previously for the organophosphorus insecticides, biotransformations
and membrane barriers to the distribution of chemicals in intact
animals will often invalidate conclusions drawn from in vitro
assays. This problem may be partly overcome by incorporating enzymic
biotransformation systems with the target macromolecule (or organism)
in the in vitro test system. Such an approach is used for screening
for potential mutagens in microorganism test systems (Malling &
Frantz, 1973) and has been applied to studies of the biochemical
actions of pesticides (Chow & Murphy, 1975; Cohen & Murphy, 1974). A
major problem in the use of in vitro test systems for predicting
toxicity is the difficulty of quantitatively relating concentrations
in the simplified in vitro systems to action in complex intact
organisms. With adequate correlative data in both in vitro and
in vivo systems this may become possible, but such information is
generally lacking at present. For the most part, therefore, in vitro
model test systems are qualitative predictors rather than
quantitative. This need not decrease their usefulness, however, as
long as this limitation is recognized in the interpretation of
results.
In general, in vitro test systems have been useful in
qualitatively predicting acute actions. However, as discussed earlier,
neurotoxic esterase inhibition provides promise for predicting delayed
chronic neuropathy produced by certain organophosphate compounds.
Major research efforts are now being devoted to in vitro test
systems for the prediction of mutagenesis and carcinogenesis. These
are discussed in detail in Chapter 7. There is general recognition of
the value of these test systems (Council of Environmental Mutagen
Society, 1975; Food & Drug Administration, 1970; Food Protection
Committee, 1970) as screening procedures, but much less agreement as
to the priority that they should have in the conduct and
interpretation of toxicity evaluations.
When it is possible to obtain comparisons between exposures of
organs or tissues of experimental animals and humans to toxic
chemicals, such comparisons will provide useful baseline data for the
future extrapolation of data from intact animal studies to man.
Culture systems of human cells may also be useful as comparative
systems. The difficulties of maintaining some human cell lines are
well documented, but primary cultures of differentiated mammary and
liver epithelia have been established and maintained (Buehring, 1972;
Lasfargues & Moore, 1971; Potter, 1972). Human lymphocytes have also
been used in vitro (Kellermann et al., 1973).
It may be possible to use these isolated systems to determine the
susceptibility to toxic chemicals of different cell types in different
organs and to determine the reversibility of adverse effects in these
cell lines and organs. Of particular usefulness would be the
determination of dose-response curves for many tissues and their
interspecies comparison. Such information could be used to predict
target cells and organs with a high degree of susceptibility or
resistance. However, as mentioned earlier, the usefulness of the data
may be limited if the cells, tissues, or organs are incapable of
metabolizing the chemical to a toxic form in the intact animal. Such a
biotransformation may even occur in a different tissue or organ from
the one under test in vitro. To overcome this problem,
biotransformation systems from animal or human tissues (e.g.
microsomal activating systems) are often added with the chemical to
the isolated culture systems.
One problem with these methods is the uncertainty that all the
steps of metabolism are equally duplicated, that is, in addition to
activation of a chemical there should also be an opportunity for the
chemical to be detoxified or conjugated and eliminated. Some of these
detoxification steps require different coenzymes or metabolites, and
the enzyme systems may not be limited to microsome fractions or liver
tissue. However, as long as it is realized that these in vitro test
systems may exaggerate the situation that occurs in vivo they can
prove useful, especially for tests where only very small quantities of
material are available, as might be the case with some impurities or
metabolites.
In summary, short-term, in vitro tests both for carcinogenicity
and other forms of toxicity show great promise (Golberg, 1974), and
although no single test is likely to be reliable, appropriate
combinations may provide valuable information concerning the
fundamental toxicity of environmental chemicals. This would currently
provide a useful adjunct to long-term studies in animal populations,
and may develop further in the future to provide the more reliable
method of assessment. Such tests will require much development and
will take years to validate and perhaps even longer to win public
confidence with regard to their reliability.
REFERENCES
ABINANTI, R. R. (1971) Chronic and degenerative diseases of man: the
value of natural and experimentally induced diseases of animals.
In: Animal models for biomedical research IV, Proceedings of a
Symposium, Washington DC, National Academy of Sciences,
pp. 31-46.
ALTMAN, P. L. & DITTMER, D. S., ed. (1966) Environmental biology,
Bethesda, MD, Federation of American Societies for Experimental
Biology, pp. 565-608.
ALVARES, A. P., LEIGH, S., KAPPAS, A, LEVIN, W., & CONNEY, A. H.
Induction of aryl hydrocarbon hydroxylase in human skin. Drug
Metabol. Disposition, 1: 386-390.
BARNES, J. M. (1968) Percutaneous toxicity. In: Boyland, E. &
Goulding, R., ed. Modern trends in toxicology, London,
Butterworths, pp. 18-38.
BEND, J. R., HOOK, G. E., & GRAM, T. E. (1973) Characterization of
lung microsomes as related to drug metabolism. Drug Metabol.
Disposition, 1: 358-367.
BENKE, G. M. & MURPHY, S. D. (1975) The influence of age on the
toxicity and metabolism of methyl parathion and parathion in male
and female rats. Toxicol. appl. Pharmacol., 31: 254-269.
BEUTHIN, P. K. & BOUSQUET, W. F. (1970) Long-term variation in basal
and phenobarbital-stimulated oxidative drug metabolism in the
rat. Biochem. Pharmacol., 19: 620-625.
BOYD, E. M. (1969) Dietary protein and pesticide toxicity in male
weanling rats. Bull. World Health Org., 40: 801-805.
BOYD, E. M. (1972) Predictive toxicometrics, Baltimore, Williams &
Wilkins, 408 pp.
BRODIE, B. B., CHO, A. K., KRISHNA, G., & REID, W. D. (1971) Drug
metabolism in man: past, present and future. Ann. NY Acad. Sci.,
179: 5-18.
BROWN, E. A. B. (1974) The localization, metabolism and effects of
drugs and toxicants in lung. Drug Metabol. Rev., 3: 33-87.
BUEHRING, G. C. (1972) Culture of human mammary epithelial cells:
keeping abreast with a new method. J. Natl Cancer Inst.,
49: 1433-1434.
CAMPBELL, T. & HAYES, J. R. (1974) Role of nutrition in the
drug-metabolizing enzyme system. Pharmacol. Rev., 26: 171-197.
CASARETT, L. J. (1975) Toxicologic evaluation. In: Casarett, L. J. &
Doull, J., ed. Toxicology -- the basic science of poisons, New
York, Macmillan, pp. 11-25.
CLARKSON, T. B., PRICHARD, R. W., BULLOCK, B.C., LEHNER, N. D. M.,
LOFLAND, H. B. & CLAIR, R. W. St. (1970) Animal models for
atherosclerosis. In: Animal models for biomedical research III.
Proceedings of a Symposium, Washington DC, National Academy of
Sciences, pp. 22-41.
CHOW, A. Y. K. & MURPHY, S. D. (1975) Production of a
methemoglobin-forming metabolite of 3,4-dichloroaniline by liver
in vitro. Bull. environ. Contam. Toxicol., 13: 9-13.
COHEN, S. D. & MURPHY, S. D. (1974) A simplified bioassay for
organophosphate detoxification and interactions. Toxicol. appl.
Pharmacol., 27: 537-550.
COMMITTEE ON PROBLEMS OF DRUG SAFETY (1969) Application of metabolic
data to the evaluation of drugs: report prepared by the Committee
on Problems of Drug Safety of the NAS/NRC Drug Research Board.
Clin. Pharmacol. Therap., 10: 607-634.
CORNISH, H. H. (1971) Problems posed by observations of serum enzyme
changes in toxicology. CRC Crit. Rev. Toxicol., 1: 1-132.
COUNCIL OF EUROPE (1973) Guide to the testing and toxicological
evaluation of flavouring substances. In: Natural flavouring
substances, their sources and added artificial flavouring
substances, Strasbourg, Council of Europe, pp. 403-410.
COUNCIL OF ENVIRONMENTAL MUTAGEN SOCIETY (1975) Environmental
mutagenic hazards. Science, 187: 503-514.
CREMER, J. E. & BLIGH, J. (1969) Body-temperature and response to
drugs. Brit. med. Bull., 25(3): 299-306.
CROSBY, D. G. (1972) Environmental photooxidation of pesticides. In:
Proceedings of a conference on degradation of synthetic organic
molecules in the biosphere, San Francisco, June 1971,
Washington DC, National Academy of Sciences, pp. 260-278.
DAVIS, W. M. (1962) Day-night periodicity in pentobarbital response of
mice and the influence of socio-physiological conditions.
Experientia (Basel), 18: 235-237.
DAWKINS, M. J. R. & REES, K. R. (1959) A biochemical approach to
pathology, London, Edward Arnold, 128 pp.
DEDRICK, R. L. (1973) Animal scale-up. J. Pharmacokin. & Biopharm.,
1: 435-461.
DHEW (1972) Guide for the care and use of laboratory animals
(prepared by Committee on Revision of the Guide for Laboratory
Animals and Care, ILAR, NRC). Washington DC, US Gov. Print.
Office (DHEW Publ. No. (NIH) 72.23).
DIGGLE, W. M. & GAGE, J. C. (1951) Cholinesterase inhibition in vivo
by O,O-diethyl O-p-nitrophenyl thiophosphate (Parathion
E 605). Biochem. J., 49: 491-494.
DILLINGHAM, E. O., MAST, R. W., BASS, G. E., & AUTIAN, J. (1973)
Toxicity of methyl- and halogen substituted alcohols in tissue
culture relative to structure-activity models and acute toxicity
in mice. J. Pharm. Sci., 62: 22-30.
DOULL, J. (1972) The effect of physical environmental factors on drug
response. In: Hayes, W. J., ed. Essays in toxicology, New York,
Academic Press, Vol. 3, pp. 37-63.
DOULL, J. (1973) Factors influencing toxicity. In: Casarett, L. J. &
Doull, J., ed. Toxicology -- the basic science of poisons, New
York, Macmillan, pp. 133-147.
EASTON, R. E. & MURPHY, S. D. (1967) Experimental ozone pre-exposure
and histamine: effect on the acute toxicity and respiratory
function effects of histamine in guinea pigs. Arch. environ.
Health, 15: 160-166.
EHRLICH, R. (1966) Effect of nitrogen dioxide on resistance to
respiratory infection. Bact. Rev., 30: 604-614.
ETO, M. (1974) Organophosphorus pesticides: organic and biological
chemistry. Cleveland, OH, CRC Press, pp. 387 (57-78).
FOOD & DRUG ADMINISTRATION (1959) Appraisal of the safety of
chemicals in foods, drugs and cosmetics. Austin, Texas,
Association of Food & Drug Officials of the USA, pp. 107.
FOOD & DRUG ADMINISTRATION (1970) Report on reproduction studies in
the safety evaluation of food additives and pesticide residues
(Report of panel on reproduction of the FDA Advisory Committee on
Protocols for Safety Evaluation). Toxicol. appl. Pharmacol.,
16: 264-269.
FOOD PROTECTION COMMITTEE (1970) Evaluating the safety of food
chemicals, Washington, DC, National Academy of Sciences, 55 pp.
FOUTS, J. R. (1972) Some studies and comments on hepatic and
extrahepatic microsomal toxication-detoxification system.
Environ. Health Perspect., Experimental Issue No. 2, Oct.
pp. 55-66.
GOLBERG, L. (1967) The amelioration of food (The Milroy Lectures).
J. Roy. Coll. Phys., 1 (4): 385-426.
GOLBERG, L. (1974) Short-term predictive tests. Proc. Eur. Soc. Drug
Toxicity, 15: 178-191.
HACKEL, D. B., MIKAT, E., LEBOVITZ, H., & SCHMIDT-NIELSEN, K. (1968)
Animal models for diabetes mellitus with special reference to the
sand rat (Prommomys obesus). In: Animal models for biomedical
research: Proceedings of a Symposium, Washington, DC, National
Academy of Sciences, pp, 14-20.
HANSCH, C. & DUNN, W. J. (1972) Linear relationships between
lipophilic character and biological activity of drugs.
J. pharm. Sc., 61: 1-18.
HATCH, A., BALEZO, T., WIBERG, G. S., & GRICE, H. C. (1965) The
importance of avoiding mental suffering in laboratory animals.
Anim. Welfare Inst. Rep., Vol. 14, No. 3.
HATCH, T. F. & GROSS, P. (1964) Pulmonary deposition and retention of
inhaled aerosols, New York, Academic Press, 184 pp.
HAYES, W. J. (1972) Tests for detecting and measuring long-term
toxicity. In: Hayes, W. J., ed. Essays in toxicology, New York,
Academic Press, Vol. 3, pp. 65-77.
HEIMANN, H. (1967) Status of air pollution health research. Arch.
environ. Health. 14: 488-503.
HENDERSON, Y. & HAGGARD, H. W. (1943) Noxious gases and the
principles of respiration influencing their action, New York,
Reinhold, 287 pp.
HOLMSTEDT, B. (1959) Pharmacology of organophosphorus cholinesterase
inhibitors. Pharmacol. Rev., 11: 567-688.
HURNI, H. (1970) The provision of laboratory animals. In: Paget, G.
E., ed. Methods in toxicology, Philadelphia, F. A. Davis,
pp. 11-48.
JAEGER, R. J., CONOLLY, R. B., & MURPHY, S. D. (1973) Diurnal
variation of hepatic glutathione concentration and its
correlation with 1,1-dichloroethylene inhalation toxicity in
rats. Res. Commun. Chem. Pathol. Pharmacol., 6: 465-471.
JAEGER, R. J., CONOLLY, R. B., & MURPHY, S. D. (1975) Short-term
inhalation toxicity of halogenated hydrocarbons. Arch. environ.
Health, 30: 26-31.
JOBE, C. L. (1968) Selection and development of animal models of
myocardial infarction. In: Proceedings of a Symposium on Animal
Models for Biomedial Research, Washington, DC, National Academy
of Sciences, pp. 101-108.
JOHNSON, M. K. (1975) The delayed neuropathy caused by some
organophosphorus esters: mechanism and challenge. CRC Crit. Rev.
Toxicol., 3: 289-316.
JOHNSTONE, G. J., ECOBICHON, D. J., & HUTZINGER, O. (1974) The
influence of pure polychlorinated biphenyl compounds on hepatic
function in the rat. Toxicol. appl. Pharmacol., 28: 66-81.
JONES, T. C. (1969) Mammalian and avian models of disease in man.
Fed. Proc., 28 (1): 162-169.
JONES, W. I., ROBACK, L. A., & TAYLOR, J. M. (1971) The loss of food
flavours from laboratory animal diets. II. Effect of laboratory
environment. J. Assoc. Offic. Anal. Chem., 54: 42-46.
JORI, A., PUGLIATTI, C., & PESCADOR, R. (1971a) Rat strain differences
in the activity of hepatic microsomal enzymes. Biochem.
Pharmacol., 20: 2695-2701.
JORI, A., SALLE, E., & SANTINI, V. (1971b) Daily rhythmic variation
and liver drug metabolism in rats. Biochem. Pharmacol.,
20: 2965-2969.
KLASSEN, C. D. (1975) Absorption, distribution and excretion of
toxicants. In: Casarett, J. L. & Doull, J., ed. The basic
science of poisons, New York, Macmillan, pp. 26-44.
KELLERMAN, G., LUYTEN-KELLERMAN, M. L., & SHAW, C. R. (1973) Genetic
variation of aryl hydrocarbon hydroxylase in human lymphocytes.
Am. J. Hum. Genet., 25: 327-331.
LA DU, B. N., MANDEL, G., & WAY, E. L., ed. (1971) Fundamentals of
drug metabolism and drug disposition, Baltimore, Williams &
Wilkins, 615 pp.
LAKE, B. G., HOPKINS, R., CHAKRABORTY, J., BRIDGES, J. W., & PARKE, V.
W. (1973) The influence of some hepatic enzyme inducers on
extrahepatic drug metabolism. Drug Metabol. Disposition,
1: 342-349.
LASFARGUES, E. Y. & MOORE, D. H. (1971) A method for the continuous
cultivation of mammary epithelium. In Vitro, 7: 21-25.
LERNER, R. A. & DIXON, F. J. (1968) Experimental and human
glomerulonephritis associated with antiglomerular basement
membrane antibodies. In: Proceedings of a Symposium on Animal
Models for Biomedical Research, Washington, DC, National Academy
of Sciences, pp. 109-130.
LJUBLINA, E. I. & FILOV, V. A. (1975) Chemical structure, physical and
chemical properties and biological activity. In: Methods used in
the USSR for establishing biologically safe levels of toxic
substances, Geneva, WHO, pp. 19-44.
LOOMIS, T. A. (1974) Essentials of toxicology, 2nd ed.,
Philadelphia, Lea & Febiger, 233 pp.
LU, P. Y. & METCALF, R. (1975) Environmental fate and biodegradability
of benzene derivatives as studied in a model aquatic ecosystem.
Environ. Health Perspect, 10: 269-284.
LUGINBUHL, H. & DETWEILER, D. K. (1968) Animal models for the study of
cerebrovascular disease. In: Proceedings of a Symposium on
Animal Models for Biomedial Research, Washington DC, National
Academy of Sciences, pp. 35-41.
LUKAS, G., BRINDLE, S., & GREENGARD, P. (1971) The route of absorption
of intraperitoneally administered compounds. J. Pharmacol. exp.
Therap., 178: 562-566.
MCLEOD, S. M., RENTON, K. W., & EADE, N. R. (1972) Development of
hepatic microsomal drug-oxidizing enzymes in immature male and
female rats. J. Pharmacol. exp. Therap., 183: 489-498.
MALLING, H. V. & FRANTZ, C. N. (1973) In vitro vs in vivo
metabolic activation of mutagens. Environ. Health Perspect.,
6: 71-82.
MCCREESH, A. H. (1965) Percutaneous toxicity. Toxicol. appl.
Pharmacol., 7: 20-26.
MCKINNEY, G. R., WEIKEL, J. H., WEBB, W. K., & DICK, R. J. (1968) Use
of life-table technique to estimate effects of certain steroids
on probability of tumour formation in a long-term study in rats.
Toxicol. appl. Pharmacol., 12: 68-79.
MCLEAN, A. E. M. & MCLEAN, E. K. (1969) Diet and toxicity. Brit. med.
Bull., 25: 278-281.
MEETER, E. & WOLTHUIS, O. L. (1968) The effects of cholinesterase
inhibitors on the body temperature of the rat. Environ. J.
Pharmacol., 4: 18-24.
MOFFITT, A. E. & MURPHY, S. D. (1974) Effect of excess and deficient
copper intake on hepatic microsomal metabolism and toxicity of
foreign chemicals. In: Hemphill, D. D., ed. Trace substances in
environmental health, Columbia, MO, University of Missouri
Press, Vol. VII, pp. 205-210.
MORRISON, J. K. (1968) The purpose and value of LD50 determinations.
In: Boyland, E. & Goulding, R., ed. Modern trends in toxicology,
Appleton-Century-Crofts, pp. 1-17.
MURPHY, S. D. (1964) A review of effects on animals of exposure to
auto exhaust and some of its components. Air Pollut. Control J.,
14: 303-308.
MURPHY, S. D. (1967) Malathion inhibition of esterases as a
determinant of malathion toxicity. J. Pharmacol. exp. Therap.,
156: 352-365.
MURPHY, S. D. (1969) Some relationships between effects of
insecticides and other stress conditions. Ann. NY Acad. Sci.,
160: 366-377.
MURPHY, S. D. (1975) Pesticides. In: Casarett, L. J. & Doull, J., ed.
Toxicology: the basic science of poisons, New York, Macmillan,
pp. 408-453.
MURPHY, S. D., LAUWERYS, R. R., & CHEEVEN, K. L. (1968) Comparative
anticholinesterase action of organophosphorus insecticides in
vertebrates. Toxicol. appl. Pharmacol., 12: 22-35.
ORTON, J. C., ANDERSON, M. W., PICKETT, R. D., ELING, T. E., & FOUTS,
J. R. (1973) Xenobiotic accumulation and metabolism by isolated
perfused rabbit lungs. J. Pharmacol. exp. therap.,
186: 482-497.
PANEL ON HERBICIDES (1971) Report on 2,4,5-T, Washington, DC, US
Govt Print. Office, 68 pp.
PARKE, D. V. (1968) The biochemistry of foreign compounds. Oxford,
Pergamon Press, 269 pp.
PARKE, D. V. & WILLIAMS, R. T. (1969) Metabolism of toxic substances.
Brit. med. Bull., 25: 256-262.
PATTISON, F. L. M. (1959) Toxic aliphatic fluorine compounds,
Amsterdam, Elsevier, 227 pp. (Elsevier Monographs).
PATTY, F. A. (1958) Industrial hygiene and toxicology, 2nd ed., New
York, Interscience, Vol. 1, 153 pp.
POTTER, V. R. (1972) Workshop on liver cell culture. Cancer Res.,
32: 1998-2000.
REID, W. D., HETT, K. F., HIK, J. M., & KRISHNA, G. (1973) Metabolism
and binding of aromatic hydrocarbons in the lung. Am. Rev. Resp.
Dis., 107: 539-551.
ROE, F. J. C. (1968) Inhalation tests. In: Boyland, E. & Goulding, R.,
ed. Modern trends in toxicology, London, Butterworths,
pp. 39-74.
ROWE, V. K., WOLF, M. A., NEIL, C. S., & SMITH, H. F. (1959) The
toxicological basis of threshold limit values: 2. Pathological
and biochemical criteria. Am. Ind. Hyg. Assoc. J., 20: 346-349.
SMYTH, H. F. (1959) The toxicological basis of threshold limit values:
1. Experience with threshold limit values based on animal data.
Am. Ind. Hyg. Assoc. J., 20: 341-345.
SZOT, R. J. & MURPHY, S. D. (1970) Phenobarbital and dexamethasone
inhibition of the adrenocortical response of rats to toxic
chemicals and other stresses. Toxicol. appl. Pharmacol.,
17: 761-773.
SZOT, J. R. & MURPHY, S. D. (1971) Relationships between cyclic
variations in adrenocortical secretary activity in rats and the
adrenocortical response to toxic chemical stress. Environ. Res.,
4: 530-538.
TAYLOR, G. J. & DREW, R. T. (1975) Cardiomyopathy predisposes hamsters
to trichlorofluoromethane toxicity. Toxicol. appl. Pharmacol.,
32: 177-183.
TYLER, W. S. & GILLESPIE, J. R. (1969) Structural and functional
alterations in horses with emphysema. In: Proceedings of a
Symposium on Animal Models for Biomedical Research, Washington,
DC, National Academy of Sciences, Vol. II, pp. 38-51.
UNIVERSITIES FEDERATION FOR ANIMAL WELFARE (1972) Handbook on the
care and management of laboratory animals, 4th ed., Baltimore,
Williams & Wilkins, 624 pp.
WATTENBERG, L. (1972) Dietary modification of intestinal and pulmonary
aryl hydrocarbon hydroxylase activity. Toxicol. appl.
Pharmacol., 23: 741-748.
WEIL, C. S. (1970) Selection of the valid numbers of sampling units
and a consideration of their combination in toxicological studies
in involving reproduction, teratogenesis and carcinogenesis.
Food Cosmet. Toxicol., 8: 177-182.
WHO (1967) WHO Technical Report Series, No. 348. (Procedures for
investigating intentional and unintentional food additives:
Report of a WHO Scientific Group) 25 pp.
WILLIAMS, R. T. (1967) Comparative patterns of drug metabolism. In:
Proceedings of an International Symposium on Comparative
Pharmacology. Fed. Proc., 26 (4): 1029-1046.
WILLIAMS, R. T. (1972) Toxicological implications of biotransformation
by intestinal microflora. Toxicol. appl. Pharmacol.,
23: 769-781.
WINTER, C. A. & FLATAKER, L. (1962) Cage design as a factor
influencing acute toxicity of respiratory depressant drugs in
rats. Toxicol. appl. Pharmacol., 4: 650-655.
WITSCHI, H. (1975) Exploitable biochemical approaches for the
evaluation of toxic lung damage. Essays in Toxicol.,
6: 125-191.
WORDEN, A. N. (1974) Toxicology and the environment. Toxicol.,
1: 3-27.
ZBINDEN, G. (1973) Progress in toxicology: special topics, New York,
Springer-Verlag, 88 pp.
3. ACUTE, SUBACUTE, AND CHRONIC TOXICITY TESTS
3.1 Introduction
The primary objective of toxicological testing is to determine
the effects of chemicals on biological systems and to obtain data on
the dose-response characteristics of the chemical. These data may
provide information on the degree of hazard to man and the environment
associated with a potential exposure related to a specific use of this
chemical. Elucidation of the metabolic behaviour of the chemical in
test animals increases confidence in defining the hazard (see Chapter
4). The degree of confidence with which hazard may be estimated
depends on the quality of the toxicological data. Selection of the
most appropriate test procedures coupled with strict adherence to
accepted experimental practices and astute observation are of
paramount importance in experimental toxicology.
3.2 General Nature of Test Procedures
Several types of toxicity testing procedures have been developed.
These include acute, subacute, and chronic studies. The major
difference between these tests is the dose employed and the length of
exposure to the chemical agent, but other differences in intent and
nature do exist and will be discussed. All of the tests share some
common characteristics. Each requires that groups of healthy animals,
housed under suitable conditions, be exposed to graded doses of the
test chemical. Rats, mice, guineapigs, rabbits, and hamsters are
commonly used for this purpose, but in some cases it may be necessary
to use dogs, swine, nonhuman primates, or other species. As a rule, a
control group is given the dosing vehicle or is sham treated.
Following treatment, the animals are closely observed for signs of
toxicity. Laboratory procedures designed to measure biological effects
are carried out on the treated and control animals. Detailed records
are maintained on each animal. Following completion of the test, all
animals, including controls, are subjected to a pathological
examination. Data should be analysed by appropriate statistical
procedures.
3.2.1 Housing, diet, and clinical examination of test animals
Animals should be healthy, genetically stable, and adequately
identified as to colony source. The controls and treated animals
should be of the same strain and species, age, and weight range, and
be supplied from the same source. Before starting the experiment, the
health status of all animals should be determined and monitored for
some time. During this time, a small randomly selected number of
animals from each shipment should be sacrificed and examined for
disease, parasites, and other specific pathogens. During the
quarantine period, animals may be caged together according to the
weight-space specifications. Acceptable standards for the housing and
care of experimental animals have been published (DHEW, 1972; Canadian
Council on Animal Care, 1973; Sontag et al., 1975).
During toxicity studies, rodents should be housed singly or in
pairs in stainless steel or plastic shoe-box cages while nonrodents
should be housed in suitable runs. The animals should be randomly
allotted to the cages and treatment regimes should be randomly applied
(Cox, 1958). Rodents should be allowed free access to food and water.
Nonrodents should be fed meal and given water ad libitum. The diet
fed to the animals should meet all of their nutritional requirements
(National Academy of Sciences, 1975) and should be free of toxic
chemical impurities that might influence the outcome of the test.
Periodic analysis of the diet to ensure its nutrient composition
should be undertaken since nutritional status may affect the nature of
toxic responses (Arcos, 1968). Although commercially available diets
of recognized quality are suitable for most subacute studies,
semipurified diets may be preferred because the nutrient and
nonnutrient components of the diet may be altered readily, where
necessary (Munro et al., 1974; Newberne, 1968).
Careful clinical observation of test animals is the most
neglected area in experimental toxicology. Few investigators are aware
or recognize that the skills required of a good medical diagnostician
are also required in assessing or diagnosing the toxic state or
condition of an animal. In toxicity studies, many animals may be lost
for evaluation because of death from intercurrent disease and
subsequent autolysis. With concerned, reliable staff, these losses can
be greatly reduced if a conscientious effort is made to recognize
early clinical signs of disease in the test animal. Ideally, each
animal on test should be looked upon as an individual patient. In this
way, there is an awareness of the idiosyncrasies of the animal and
departures from the normal will be more easily recognized. Once a
routine of careful clinical assessment has been established, it is
possible either to treat diseased test animals or, if necessary,
sacrifice them. In the latter case, the tissues are available for
histological examination. Otherwise, chronic disease effects might
render the tissues useless for the assessment of effects due to test
substances.
Detailed clinical examinations should be conducted weekly on the
test animals by competent, laboratory animal technicians under the
supervision of a veterinarian skilled in laboratory animal medicine
(Health and Welfare, Canada, 1973). These should include general
observation of the animals for overt signs of toxicity, quality of
hair, coat, general condition of the eyes, mouth, teeth, nose, and
ears (Leclair & Willard, 1970; Loomis, 1968). Assessment of cardiac
and respiratory function should be conducted by auscultation. If
neurological effects are anticipated, a detailed neurological
examination should be conducted. In larger species, this can be done
by skilled personnel using the methods of Charbonneau (1974), McGrath
(1960), and Mowbray & Cadell (1962). Examination of the eyes using
opthalmoscopic and slit-lamp techniques (Marzulli, 1968) may assist in
detecting ocular toxicity. The external and internal structures should
be carefully palpated and any tissue masses should be noted. Detailed
records of clinical evaluation should be maintained and should be
accessible to the attendant pathologist.
3.3 Acute Toxicity Tests
3.3.1 Underlying principles
Acute toxicity has been defined as the adverse effects occurring
within a short time of administration of single dose or multiple doses
given within 24 h (Hagan, 1959). When data are unavailable concerning
the toxicity of the test agent, acute toxicity studies are indicated
to identify the relative toxicity of the compound, to investigate its
mode of action and its specific toxic effect, and to determine the
existence of species differences.
The most frequently used acute toxicity test involves
determination of the median lethal dose (LD50) of the compound. The
LD50 has been defined as "a statistically derived expression of a
single dose of a material that can be expected to kill 50% of the
animals" (Gehring, 1973). The basic protocol for the determination of
the LD50 is well established and consists of treating groups of
animals with a mathematically-related series of doses in order to
determine the dose that kills 50% of the group and the dose-response
function. The LD50, being a calculated value, is always accompanied
by some estimation of the error of the value, such as the confidence
limits. The most commonly used methods for calculation of the LD50
are the graphic method of Litchfield & Wilcoxon (1947), the
logarithmic probit graph paper method of Miller & Tainter (1944), and
the method of moving averages of Thompson (1947) and Weil (1952). A
comparative review of these and other methods was published by
Armitage & Allen (1950). Death which occurs after the first 24 h is
more likely to be due to delayed toxic effects, which may be direct or
indirect. Signs occurring after the first 24-h period may give some
indication of the effect that the chemical may have at lower levels,
when administered for longer time periods.
3.3.2 Experimental design
3.3.2.1 Selection of species
The extent of species variation in toxicity testing has been well
documented in the reviews of Brodie (1964) and Rumke (1964). The
usefulness of determining species variability in order to assess the
applicability of toxicity data to man has been discussed by Hagan
(1959). Litchfield (1962) has postulated that if the toxicity of a
compound is the same in several species, there would appear to be an
increased likelihood that man would react in a similar manner.
The mouse, rat and dog are the most commonly used species for
acute toxicity testing. Both the rat and mouse should be used, as
marked differences in the LD50 between these two species are not
uncommon (Morrison et al., 1968).
The LD50 determination should be conducted in both male and
female animals, as differences in the LD50 between sexes have been
well documented (Hurst, 1958; Rumke, 1964) and are probably related,
in part, to differences in hepatic metabolism (Conney et al., 1965).
Acute toxicity may vary substantially with the age of the test
animal (Dieke & Richter, 1945; Lu et al., 1965; Scott et al., 1965;
Yeary & Benish, 1965), and animals of various ages should be used in
LD50 determinations. The effect of the age of the animal on the
LD50 is well documented and may be related to different levels of
drug metabolizing enzymes, absence of sex hormonal influences, or an
altered sensitivity of the central nervous system (Fouts & Hart, 1965;
Jondorf et al., 1959; Setnika & Magistretti, 1964).
The animals should be derived from previously untreated healthy
females. Weinberg et al. (1966) have demonstrated an effect of
treatment of dams during gestation with various compounds on the acute
oral toxicity in the newborn.
Furthermore, the animals should not have been previously used for
other studies, nor should there be a history of recent exposure to
anthelminthics or any other drug treatment.
The number of animals used should be sufficient for statistical
analysis and will depend on the method used for the calculation of the
LD50. Usually 8-10 rodents (4-6 animals of each sex) are used per
dose group (Leclair & Willard, 1970). Diechmann & LeBlanc (1943)
described a method using a total of 6 animals, while other methods
involved the use of 4-5 animals per dose group (Horn, 1956; Litchfield
& Wilcoxon, 1947; Thompson, 1947).
3.3.2.2 Selection of doses
The doses are selected to provide data for estimating the LD50
and to obtain information on the slope of the dose-response curve. At
least four doses, selected in logarithmic progression, should be used
(Weil, 1952).
In general, however, the doses can be arrived at only by
experimentation. The initial dose may be such that no effect is
manifested in the animals. In subsequent groups of animals, the dose
should be increased by a constant multiple until the dose of the
compound administered is sufficiently high that all of the animals in
the group die. Under these conditions, data can be obtained that can
be plotted to give a dose-response curve and from which the LD50
value may be calculated.
3.3.2.3 Method of administration
Generally, the chemical should be administered by the route by
which man would be exposed. If the route is oral, the compound should
be administered by gavage rather than mixed in the diet. In some
cases, the administration of the chemical along with the diet has been
shown to increase its toxicity compared with gavage dosing (Bein,
1963; Worden & Harper, 1963), but, in general, the oral toxicity of a
compound is greatest when it is administered by gavage to animals that
have fasted (Griffith, 1964). Griffith (1964) has demonstrated the
effect of the type and concentration of the vehicle on the LD50
value. The amount of the liquid or carrier administered should be
appropriate and the carrier should not, in itself, be toxic to the
animal.
In certain cases, even though the route of human exposure would
be oral, acute dermal, eye, and inhalation studies may be indicated to
assess the hazard to personnel handling the compound in the
laboratory.
3.3.2.4 Postmortem examination
In general, all animals dying during the observation period and
all surviving animals should be autopsied by a qualified pathologist
(Leclair & Willard, 1970). The autopsy should include gross and
histopathological examination of all organs.
If death is almost instantaneous and due to a pharmacological or
physical effect, i.e. massive gastrointestinal haemorrhage or acute
respiratory collapse, detailed histopathological examination of all
organs may not be indicated.
3.3.3 Repeated high-dose studies
Because of the inherent limitations of the LD50 in predicting
long-term toxicity, a short but intensive study or a series of such
studies may be indicated before commencing subacute tests. The purpose
of such studies is to define more precisely the doses to be used in
subacute tests and to elucidate more fully the organ systems affected.
The design of these repeated high-dose studies may vary but consists,
essentially, of repeated daily administration of a mathematically-
related series of doses to groups of animals for 5-21 days.
One type of repeated-dose study (Sontag et al., 1975) consists of
treating groups of five young adult animals of each sex at each of
five dose levels, the upper level being the one that is estimated to
produce no more than 10% lethality following a single dose, the
remaining doses being fractions of this dose.
A seven-day feeding study described by Weil et al. (1969)
consisted of treating five rodents of each sex at each of three or
four dose levels for seven days. Criteria of effects were mortality,
body weight gain, relative liver and kidney weights, and feed
consumption. This study showed that the results of the seven-day
feeding test were of significantly greater value in predicting dose
levels for the 90-day toxicity test than the LD50 values.
Daily observations, as described in section 3.1.3, should be
conducted and weekly body weight and food consumption (if the animals
are caged individually) monitored. For some test agents, especially
those with delayed toxicity or cumulative effects, other measurements,
such as organ function, body burden, absorption, and excretion of the
compound may be indicated. Animals should be necropsied and the
tissues should be examined for gross pathological changes and studied
histopathologically, if indicated.
3.4 Subacute and Chronic Toxicity Tests
3.4.1 Underlying principles
The subacute toxicity test generally involves daily or frequent
exposure to the compound over a period up to about 90 days. It
provides information on the major toxic effects of the test compound
and the target organs affected (Barnes, 1960). The latency of
development of the effect as related to dose, the relationship of the
blood and tissue levels of the compound to the development of lesions,
and the reversibility of the effects may also be studied. Data derived
from these studies are used for designing chronic toxicity tests in
which animals are exposed to the chemical for longer periods of time.
Man may be exposed for the greater part of his life-time to low
levels of a wide variety of environmental chemicals. Usually, the
degree of exposure is insufficient to produce overt signs of toxicity;
thus, cause-effect relationships cannot be easily established.
Epidemiological studies may assist in this respect, but, because man
is exposed simultaneously to several chemicals, it is difficult to
establish unequivocally the degree of hazard associated with any one
chemical. Acute and subacute toxicity tests are of limited value in
predicting chronic toxic effects because: (a) chemicals may produce
different toxic responses when administered repeatedly over a period;
and (b) during the aging process, factors such as altered tissue
sensitivity, changing metabolic and physiological capability, and
spontaneous disease may influence the degree and nature of toxic
responses. In addition, several important diseases such as heart
disease, chronic renal failure, and neoplasia are associated with
advancing age. These are multicausal in nature and thought to be due,
in part, to the presence of chemical substances, both natural and
synthetic, in the environment (WHO, 1972). Chronic toxicity tests, in
which animals are exposed for their entire lifetime to environmental
chemicals, have provided useful means of identifying those substances
of greatest public health concern. The tests are usually conducted
with the aim of establishing "no-observed-adverse-effect levels" that
may be used in setting acceptable daily intakes (ADIs), tolerance
limits for chemicals in food or water, or threshold limit values in
the case of occupational exposure. Since chronic toxicity testing is
expensive and requires specialized facilities and personnel, great
care must be taken in the design, execution, and interpretation of the
results of such studies.
3.4.2 Experimental design
3.4.2.1 Selection of species and duration of studies
In the subacute studies, if the compound has produced evidence of
toxicity in man and if sufficient toxicological and metabolic
information is available, it is often possible to select an
appropriate species on the basis of these data. For compounds about to
be put on the market about which little is known toxicologically, the
recommendations of the World Health Organization (WHO, 1958) and
competent national agencies (Friedman, 1969; Leclair & Willard 1970;
National Academy of Sciences, 1975) should be followed in selecting
appropriate test species. As a minimum recommendation, subacute
studies should be undertaken in two species, one rodent and one
nonrodent. Traditionally, the rat and dog are selected for subacute
toxicity testing because of their availability and the large amount of
background information available on them. When rats are used, the test
should be initiated just after weaning so that observations may be
made during the period of most rapid growth. A conventional strain
should be selected, so that the results in control and treated animals
can be compared with known literature values, and both sexes should be
tested to ascertain the influence of the sex hormones on the toxic
response. At least 10 animals of each sex should be included in each
dose group and the experiment should continue for 10% of the animals'
lifetime or about 3 months. If it is desired to study the pathogenesis
and reversibility of induced lesions or biochemokinetics, it is
recommended that observations be made at 3-week intervals during
exposure and last up to 3 months following termination of exposure.
In chronic toxicity testing, it is usual to expose the animals to
the chemical for the greater part of the life span. A wide variety of
animal species have been used in this type of work, although in most
cases rodents are the animals of choice, since large numbers can be
used to aid in the statistical interpretation of the results. Larger
animals should also be used (e.g. dog and monkey) for such species
have the advantage that larger samples of blood can be obtained on a
routine basis.
If the objective of the test is to study the carcinogenic
potential of a compound, the rat, mouse, or hamster is usually chosen
because of its shorter lifetime and the fact that large numbers may be
used to increase the sensitivity of the test.
When data on the metabolic fate of the test chemical in man is
not available, the species showing the greatest sensitivity in
subacute studies should be selected as the test species, provided the
species does not react atypically to the compound due to metabolic
peculiarities.
Sufficient numbers of animals should be included in the test to
ensure that a statistically valid design is achieved. Based on the
incidence of effects observed in subacute studies and the anticipated
incidence of chronic effects, the number of animals that should be
used can be calculated (Snedecor & Cochran, 1967).
Since it is usually the intention in chronic toxicity studies to
expose animals over the major portion of their life span, it is
essential to commence exposure early in life.
3.4.2.2 Selection of doses
Guidance on the selection of doses for subacute studies may be
obtained from the results of acute and repeated high-dose studies. For
compounds having a tendency to bioaccumulation, selection of doses is
particularly difficult. Kinetic studies may assist in establishing
acceptable dose levels since the half-time ( t´) for elimination
(Chapter 4) may provide guidance on the degree of bioaccumulation that
could be anticipated. To establish the nature of the toxic reaction,
the highest dose should provide a distinct toxic effect while the
lowest dose should not produce any detectable toxic reaction (Leclair
& Willard, 1970). To obtain maximum information on the dose-response
characteristics of the compound, at least two intermediate doses
should be included.
Information from subacute toxicity tests is of value in the
selection of appropriate dose levels, when commencing chronic toxicity
studies. In general, however, it is highly desirable to establish the
chemobiokinetic behaviour (Chapter 4) of the test compound and if
possible its major metabolites in the test species prior to
undertaking a chronic toxicity test. Particular attention should be
given to evidence for dose-dependent detoxification. Studies of this
nature will provide information on the degree to which the chemical
may be expected to accumulate in various body compartments and
unexpectedly produce evidence of toxicity. Since it is the object of
chronic toxicity tests to establish dose-response patterns and
"no-observed-adverse-effect levels", a minimum of three dose levels
should be used. The upper dose level should produce some slight
evidence of toxicity, but should be compatible with normal
physiological function (Leclair & Willard, 1970). The lowest dose
level would not be expected to produce evidence of toxicity (Health &
Welfare, Canada, 1973).
3.4.2.3 Method of administration
The route of administration in subacute and chronic studies
should be that through which man is likely to be exposed. For gases
and volatile industrial solvents, inhalation studies are recommended
(Magill et al., 1956) (Chapter 6), while for food additives,
pesticides, and other chemicals likely to come into contact with food
or water, the oral route is recommended (Leclair & Willard, 1970;
National Academy of Sciences, 1975). Incorporation of the test
chemical into the diet or drinking water is an appropriate means of
administration; however, care must be taken to ensure the stability of
the chemical in the dosing medium. The concentration of the test
chemical in the diet should be determined periodically to ensure
uniform dispersion and to aid in the quantification of achieved doses.
In some cases, the chemical may be unpalatable and administration by
gavage, or, in the case of dogs, by capsules may be necessary.
The diet is the preferred vehicle of administration, but it is
absolutely essential that the chemical be present in the diet in an
unaltered form; toxicity may be altered by interaction with dietary
constituents (Kello & Kostial, 1973). In rodent studies, the compound
may be administered in the diet as a fraction of the total diet, or a
sufficient quantity of the chemical may be added to the diet to
achieve predetermined dose levels (in mg per kg body weight per day).
In the latter case, it is necessary to adjust the dietary
concentration weekly or biweekly to maintain a constant dose level,
since food consumption per unit of body weight decreases as the animal
gets older. If, in rodent tests, the concentration of the test
compound in the diet is kept constant from weaning to maturity, the
actual dose received will be reduced by approximately 2.5 times over
the dosing period. This may have profound effects on the severity of
the toxic response and may be mistaken for tolerance. In chronic
toxicity tests, the chemical should be administered daily over the
entire treatment period. As an aid to interpretation of the test, only
one lot chemical should be used for the entire test unless the purity
of the chemical is definitely assured.
3.4.2.4 Biochemical organ function tests
In subacute studies, the use of a species such as the dog instead
of a rodent species permits the application of a wider range of
biochemical organ function tests because larger samples of blood can
be collected on a routine basis. Organ function studies should be
undertaken prior to initiation of the test, 3 and 10 days after the
start of dosing, at 30-day intervals thereafter throughout the test,
and terminally. The tests described for the chronic studies are also
applicable in subacute studies.
In the course of chronic toxicity tests, studies should be
undertaken to evaluate the functional integrity of various organ
systems. Assessment of the urinary system should commence with an
examination of the urine. Freshly-voided urine samples should be
obtained every one to three months from the test animals and examined
for the presence of occult blood, glucose, protein, and bilirubin
using simple diagnostic procedures. If positive effects are noted,
quantitative methods should be applied as outlined by Bergmeyer
(1965). Samples of freshly-voided urine should also be filtered
through Millipore filters and the filters stained according to the
Papanicolaou method (Frost, 1969) to detect the presence of renal
tubular cells or other cell types derived from the urinary system.
Urinary calculi and parasite eggs (Chapman, 1969) may be detected by
this method. Blood urea-nitrogen levels and other standard tests of
kidney function may be applied but they usually lack sufficient
sensitivity to detect subtle changes in kidney function.
Several test procedures are available for the assessment of liver
function. Most of these methods involve an examination of the serum
levels of hepatic enzymes that may be released in the serum following
liver injury (Czok, 1965; Henley et al., 1966; Zimmerman, 1974).
Korsrud et al. (1972) compared the sensitivity of various liver
function tests in the rat and noted that serum sorbitol dehydrogenase
(1.1.1.14) activity (an enzyme specific to the liver) correlated well
with the degree of histological alteration produced by hepatotoxic
agents such as carbon tetrachloride, 2,2'-iminobisethanol
(diethanolamine), and ethanethioamide (thioacetamide). However, Grice
et al. (1971) noted that pathological changes induced by these
compounds must be reasonably advanced before elevations are noted in
serum glutamic-oxaloacetic transaminase (2.6.1.1), lactate
dehydrogenase (1.1.1.27), or lactate dehydrogenase isoenzymes,
suggesting that changes in serum enzyme activity may not be as
sensitive an indicator of toxicity as pathomorphological examination.
Tests of liver function such as serum enzyme activities and various
clearance tests were reviewed recently by Balazs (1975). A complete
review of the principles and applications of these tests is given by
Cornish (1971) and further discussion of these methods is found in
Part II, Chapter 8. Suffice it to say, that a transient increase in
the activity of serum enzymes or other organ-derived constituents may
result from a transient change in organ homeostasis that produces no
lasting toxic effect.
For routine screening of organ function in large animals,
Charbonneau et al. (1974) used clinical procedures that can measure
the concentration of several serum enzymes and inorganic and other
constituents by automated methods. In general, these methods are not
sufficiently standardized or reproducible to detect minor alterations
in organ function but they do serve as a useful guide to general
clinical status.
3.4.2.5 Physiological measurements
In subacute studies, it is often possible to detect ensuing
pathological events through application of physiological function
tests.
In all studies, food consumption and body weight should be
recorded weekly in all animals. Weight gain per unit of food consumed
should be calculated (Munro et al., 1969). This gives a measure of the
efficiency of food use. The daily dose of chemical should be
calculated from data on food intake and body weight. Similar
measurements of food intake and body weight must be carried out in
chronic toxicity tests. If the test chemical is incorporated into the
drinking water, water intake must be measured. These measurements
should be conducted on a weekly basis during the entire test. The data
can be used to estimate the dose of chemical received and are
necessary in the establishment of dose-response relationships. Body
weight changes serve as a sensitive indication of the general health
status of test animals. Any rapid loss in body weight may signal the
onset of intoxication or disease. Computerized methods for recording
and analysing this type of data are available (Munro et al., 1972).
Under special circumstances, when the target organs of toxicity
have been identified during subacute studies, it is appropriate to
conduct measurements of the physiological function of organ systems.
Procedures such as electrocardiography (Grice et al., 1971),
electroencephalography (Flodmark & Steinwall, 1963; Harada et al.,
1967; Mann, 1970), electromyography (Chaffin, 1969), nerve conduction
studies and measurement of evoked potentials (Barnet et al., 1971;
Hrbek et al., 1972) may greatly assist in defining the functional
effects of chemicals (Chapter 8). Such tests are expensive to perform
and require highly specialized equipment and personnel. They have
limited application in routine testing but may be used to define
mechanisms of action. It is imperative that the results of such
studies be correlated with clinical and pathological findings (Grice,
1972; Osborne & Dent, 1973).
3.4.2.6 Metabolic studies
Subacute studies provide an excellent opportunity to undertake
metabolic investigations under conditions of repeated exposure that
may alter the nature of the metabolites and the rate of metabolic
transformation of the test compound. Urine and faeces can be collected
and examined for the presence of metabolites and, by undertaking
serial sacrifices at three-week intervals, the kinetics of
accumulation of the compound in various body compartments can be
evaluated.
To gain an understanding of the metabolic fate of a chemical that
may have a long biological half-time, such as hexachlorobenzene (Grant
et al., 1975), three extra groups of animals need to be studied for
tissue distribution to provide information that is necessary for
estimating the potential hazard to man. The principles of these
methods are reviewed in Chapter 4. Often it is desirable, in subacute
studies, to study the kinetics of the test compound and its
metabolites following completion of the dosing period. If extra groups
of animals are initially included for this purpose, much valuable
additional information on the compound may be obtained.
3.4.2.7 Haematological information
In subacute studies involving rodents, haematological studies
should be undertaken on randomly selected subgroups of animals prior
to initiation of the test, at 30-day intervals, and on all animals
terminally. Bone marrow should be examined terminally. Nonrodent test
animals should be examined at similar intervals.
In chronic toxicity studies involving rodents, haematological
studies of circulating blood cells should be undertaken on randomly
selected subgroups of animals prior to initiation of the test, at 3 to
6-month intervals and, on selected animals, terminally. Bone marrow
should be examined terminally and, if indicated, at interim times by
biopsy.
To assess the clinical state of nonrodents, haematological
variables should be examined frequently.
A set of test procedures is necessary for routine haematological
screening and the tests must be of sufficient sensitivity and accuracy
to be of practical value for use in large numbers of laboratory
animals (Cartwright, 1969; Schalm, 1967; Sirridge, 1967).
Quantification of blood cells and thorough study of cellular
morphology by a haematologist, experienced in small animal medicine,
is necessary in the study of haematological disorders. Haematological
evaluation of experimental animals is facilitated by the fact that
repeated sampling is relatively easy and small amounts of blood are
required, and that single-cell systems can be studied to obtain
information on cell production, destruction, defects, and dysfunction.
For erythroid evaluation, the numbers of circulating erythrocytes must
be counted and the haematocrit and haemoglobin concentration measured.
As an index of erythropoietic activity in the bone marrow, a
reticulocyte count should be carried out. Morphological assessment of
erythrocytes is mandatory. The number of circulating leucocytes should
be quantified and a differential count and morphological assessment
should be made. To evaluate the functional capacity and malignant
changes in the blood-forming organs, bone marrow should be examined
terminally. From bone marrow smears a differential count and
morphological assessment can be carried out. Imprints of lymph nodes
or spleen permit a detailed cytological study of normal and abnormal
cells present that may be of diagnostic significance.
To assess haemostatic function, it will be necessary to evaluate
platelets, coagulation systems, and fibrinolysis. Screening tests
include platelet count, clot retraction, one stage prothrombin time,
and activated partial thromboplastin time. More specific evaluation
may require factor assays, thrombin time, fibrinogen determination,
euglobulin clot lysis time, prothrombin consumption time, platelet
aggregation, and adhesiveness.
3.4.2.8 Postmortem examination
In every toxicity evaluation, all animals should be given a
thorough gross autopsy and detailed records kept on each animal.
Samples of all organs and supporting structures should be saved for
histopathological examination. Detailed autopsy methods are outlined
in Chapter 5.
In chronic toxicity testing it is often useful to incorporate
interim autopsy dates so that the progression of lesions may be
studied. At interim sacrifices and terminally (if sufficient animals
are in a healthy state), the major organs should be weighed. Organ
weights may serve as a useful index of toxicity; however, care must be
taken in the interpretation of the data. Decreased absolute organ
weights in treated animals may be merely a reflection of lower body
weight and calculation of organ to body weight ratios may increase the
usefulness of the data (Feron, 1973).
3.4.2.9 Controls
In the evaluation of both subacute and chronic toxicity, special
attention must be given to the control animals. The quality of data
obtained from the control animals has an important bearing on the
interpretation of results from the treated animals. Suitable numbers
of control animals of the same age and body weight as the treated
animals must be included in the experimental design in a statistically
randomized fashion.
Except for treatment with the test chemical, these animals should
be handled identically to the test subjects and all measurements
conducted on the treated animals must be carried out on the controls
with the same precision and frequency. In studies in which the
chemical is administered by gavage, the control animals should receive
the suspending vehicle in an amount equivalent to the treated animals.
The incidence of spontaneous lesions or of other changes in control
animals must be carefully noted and the interpretation of data
obtained from treated animals must include an appreciation of the role
that spontaneous disease processes may play in the manifestation of
chemical toxicity. It is particularly important, in studies with
rodents, to have detailed information on the incidence of neoplastic
diseases, since some species and strains (Sher, 1972) may have a high
background incidence of certain tumours which tends to reduce
longevity and decrease the chance of observing chronic toxic effects.
In addition, the chemical under test may alter the incidence of
spontaneous tumours and other diseases or may induce new tumours, and
this possibility must be taken into consideration in the evaluation of
the chronic toxicity of chemicals. In all cases, responses
attributable to the test compound must be compared with background
observations in controls. For this reason, the quality of the
toxicological data rests heavily on the adequacy of the control values
(Weil & Carpenter, 1969).
3.4.3 Alternative approaches in chronic toxicity
3.4.3.1 Perinatal exposure
The majority of chemicals to which man may be exposed are present
in air, food, or water for his entire lifetime. Recently, there has
been an attempt to duplicate the human situation in the chronic
toxicity test by exposing the test animals during the neonatal period
as well as throughout life (Friedman, 1969). In this approach, groups
of weaning animals (usually rodents) are exposed to the test chemical
until they reach sexual maturity. They are then mated, within dose
groups, and the treatment is continued during pregnancy and lactation.
Following weaning, the offspring are transferred to their parents'
diet and exposed for the balance of their lifetime to the test
chemical. The details of this test procedure have been outlined in a
recent Canadian Government publication (Health & Welfare, Canada,
1973) and by Epstein (1969). It is not known yet whether this
technique increases the sensitivity of the chronic toxicity test, but
it is known that exposure to carcinogens in the perinatal period will
often increase the incidence and decrease the latent period of
carcinogenesis (Tomatis & Mohr, ed., 1973).
Further study of this method is required to evaluate its
usefulness fully. It should be pointed out, however, that this
procedure adds considerably to the cost and length of the chronic
toxicity test.
3.4.3.2 Use of nonrodent species
In chronic toxicity studies with nonrodent species such as
nonhuman primates, dogs, or cats, it is often not feasible to expose
the animals to the test compound for their entire lifespan, even
though they may be the species of choice. Under such conditions,
careful examination of the kinetic and metabolic behaviour of the test
compound in these species may substitute, to some extent, for the
decreased treatment period (provided the anticipated endpoint is not
carcinogenesis). Carefully conducted kinetic studies will assist in
establishing when steady-state tissue concentrations of the test
chemical and its metabolites have been achieved. If treatment is
continued for a substantial period after the establishment of
steady-state kinetics without any increase in the degree of toxic
effects observed clinically, or during interim sacrifice, this may
partially substitute for a lifetime study and provide increased
assurance for those having to make regulatory decisions. If this
approach is not feasible, it may be possible to test human metabolites
in rodent species (Health & Welfare, Canada, 1973).
3.5 Evaluation and Interpretation of the Results of Toxicity Tests
The evaluation and interpretation of toxicity studies starts with
a clear definition of experimental objectives. The design of the
experiment should be such that the objectives can be reasonably
achieved. Well designed and carefully executed experiments add greatly
to the ease with which results can be evaluated and interpreted and
also to confidence in the experimental data.
The primary usefulness of the LD50 determination is to obtain
some idea of the magnitude of the acute toxic dose (Frazer & Sharratt,
1969) and information concerning the type of toxic effects of the
chemical. Such information includes whether death is immediate or
delayed, whether recovery from a near lethal dose is rapid or complete
or both, or whether the cause of death is narcosis with respiratory
failure, lung oedema, or liver necrosis.
However, the LD50 provides little information for the
assessment of the hazard from compounds to which the human population
is exposed for extended periods of time. Although it has been
suggested that compounds that do not show adverse effects when given
in doses of 3-5 g per kg body weight are essentially non-toxic
(National Academy of Sciences, 1975), there are numerous examples in
the literature of compounds with LD50 values greater than 5 g per kg
which produce toxic effects, when given in low doses for extended
periods of time (Frazer & Sharratt, 1968). If the main object of an
acute toxicity test is not to establish a value for the LD50 with
precision, but to learn something about the way in which the chemical
acts as a poison, as suggested by Paget & Barnes (1964), this can best
be accomplished by tests involving repeated daily administration to a
few animals for a period of 5-21 days. The information provided by the
LD50 regarding the effects of acute exposure to toxic compounds may
be useful as a guide for selecting doses for such studies.
The primary objective of subacute and chronic toxicity studies is
to determine the nature and severity of toxic effects and the
"no-observed-adverse-effect" dose level. These data may then be used
in the establishment of acceptable levels of exposure for man.
Data on group weight gain or body weight change should be plotted
against time, and differences between groups should be evaluated
statistically. Changes in body weight with time are best evaluated
statistically using trend analysis procedures (Armitage, 1955). Food
(and water) consumption data should be handled in a similar fashion.
Reduced body weight or weight gain in otherwise healthy treated
animals may be due to reduced food intake owing to its unpalatability
or to a specific toxic effect of the chemical resulting in reduced
efficiency of food use. Using data on the dietary concentration of the
test chemical, food consumption, and body weight, the mean daily dose
of chemical received (in mg/kg body weight/day or similar units)
should be calculated. Automated data processing procedures to
accomplish this are available (Munro et al., 1972).
Data on organ weights should be evaluated and interpreted with
great care. Increased relative (to body or brain weight) organ weights
may also result from adaptation to stress phenomena or from metabolic
overloading of biochemical pathways or physiological processes.
Increased liver weight, for example, may result from a stimulation of
de novo protein synthesis in the smooth endoplasmic reticulum (SER).
This results in a morphologically detectable increase in SER. The
biochemical counterpart of this increase is an increased ability of
the liver to metabolize certain foreign substances, sometimes
including the test compound and endogenous substrates, due to a
stimulation in the activity of hepatic mixed function oxidases
(Staubli et al., 1969). These adaptative changes may manifest
themselves clinically as tolerance. Often these changes are reversible
upon cessation of dosing and do not produce lasting toxicological
effects but the implications of chronically elevated levels of these
enzymes is not known. Certain enzyme inducers may cause impairment of
liver function and produce pathological and biochemical changes (Feuer
et al., 1965).
Data on biochemical and haematological effects should be
tabulated and compared with control values using statistical
procedures (Johnson, 1950). Any observed effects should be correlated
with clinical and pathological findings. A biochemical or
haematological change such as reduction in liver glycogen or an
alteration in white cell count may not be indicative of a toxic
effect, but an adaptation to a stress situation (National Academy of
Sciences, 1975). In general, changes in homeostasis must be carefully
evaluated since reversible shifts do not necessarily imply a toxic
effect in the absence of other toxic manifestations.
Changes in the functional state of physiological or neurological
processes, such as an alteration in the electrocardiogram or abnormal
behaviour, may result from pharmacological or pathological effects of
the test compound. Changes in functional state must be closely
correlated with their morphological counterpart in order to evaluate
their toxicological importance properly (Grice, 1972).
The cornerstone of experimental toxicology is the pathological
examination. Usually, decisions regarding the safety of a compound are
based on this evidence. All pathological findings in test animals
should be graded carefully and their incidence tabulated (see Chapter
5). Spontaneous lesions in control animals should also be noted and
compared to the observations in control animals in previous
experiments or in the literature (Peck, 1974) to ensure that the
incidence and nature of the lesions is representative of the strain.
Pathological data should be analysed rigorously using appropriate
statistical methods (Fleiss, 1973) and spurious observations
apparently unrelated to treatment should be identified. Lesions that
are dose-related should be studied in detail and correlated with gross
pathological findings, clinical observations, and other variables
(Grice, 1972).
It is not uncommon in chronic toxicity testing to find
pathological or other changes that occur in low incidence and that are
not dose-related but occur only in treated animals. Such reactions may
be idiosyncratic in nature or may be due to the hypersensitivity of
certain animals. Nevertheless, they deserve special attention since
they may be indicative of a hitherto unsuspected toxic effect. The
clinical history and other data from such animals should be reviewed
with great care and an attempt should be made to determine the reason
for the observed effects. Toxic effects that occur in extremely low
incidence present special problems in interpretation. There is no
substitute for experience in this respect and the prudent investigator
will consult the knowledgeable experts in this field (Zbinden, 1973).
REFERENCES
ARCOS, J. C. (1968) Chemical induction of cancer. Vol. 1, New York,
Academic Press.
ARMITAGE, P. & ALLEN, I. (1950) Methods of estimating the LD50 in
quantal response data. J. Hyg. (Lond.), 48: 298-322.
ARMITAGE, P. (1955) Tests for linear trends in proportions and
frequencies. Biometrics, 11: 375-385.
BALAZS, T. (1975) Toxic effects of chemicals in the liver.
FDA By-lines, 5: 291-303.
BARNES, J. M. (1960) Toxicity testing, In: Schilling, R. S. F., ed.
Modern trends in occupational health, London, Butterworths &
Co., pp. 20-32.
BARNET, A. B., OHRICH, E. S., & SHANKS, B. L. (1971) EEG evoked
responses to repetitive auditory stimulation in normal
Down's-syndrome infants. Dev. Med. Child. Neurol., 13: 321-329.
BEIN, H. J. (1963) Rational and irrational numbers in toxicology.
Proc. Euro. Soc. Study Drug Toxicity, 2: 15-26.
BERGMEYER, H. U. (1965) Methods of enzymatic analysis. New York,
Academic Press.
BRODIE, B. B. (1964) [The difficulties of transposing experimental
results obtained in animals to man.]. Actual. pharmacol.,
17: 1-23 (in French).
CANADIAN COUNCIL ON ANIMAL CARE (1973) Care of experimental animals
-- guide for Canada.
CARTWRIGHT, G. E. (1969) Diagnostic laboratory hematology, 4th ed.
New York, Grune & Stratton.
CHAFFIN, D. B. (1969) Surface electromyography frequency analysis as a
diagnostic tool. J. occup. Med., 11: 109-115.
CHAPMAN, W. H. (1969) Infection with Trichosomomoides crassicauda as
a factor in the induction of bladder tumours in rats fed
2-acetylaminofluorene. Invest. Urol., 7: 154-159.
CHARBONNEAU, S. M., MUNRO, I. C., NERA, E. A., WILLES, R. F.,
KUIPER-GOODMAN, T., IVERSON, F., MOODIE, C. A., STOLZ, D. R.,
ARMSTRONG, F. A. J., UTHE, J. F., & GRICE, H. C. (1974) Subacute
toxicity of methylmercury in the adult cat. Toxicol. appl.
Pharmacol., 27: 569-581.
CONNEY, A. H., SCHNEIDERMAN, K., JACOBSON, M., & KUNTZMAN, R. (1965)
Drug-induced changes in steroid metabolism. Ann. NY Acad. Sci.,
123: 98-109.
CORNISH, H. H. (1971) Problems posed by observations of serum enzyme
changes in toxicology. CRC Crit. Rev. Toxicol., 1: 1-32.
COX, D. R. (1958) Planning of experiments, New York, John Wiley,
Chapter 5.
CZOK, R. (1965) The behaviour of plasma enzymes in toxicological
experiments. Proc. Eur. Soc. Study Drug Toxicity, 5: 68-83.
DHEW (1972) Guide for the care and use of laboratory animals
(prepared by Committee for the Revision of the Guide for
Laboratory Animal Facilities and Care, Institute of Laboratory
Animal Resources). Washington DC, Govt Printing Office (DHEW
Publ. No. (NIH) 73-23).
DIECHMANN, W. B. & LEBLANC, T. J. (1943) Determination of the
approximate lethal dose with about six animals. J. ind. Hyg.
Toxicol., 25: 415-417.
DIEKE, S. H. & RICHTER, C. P. (1945) Acute toxicity of thiourea to
rats in relation to age, diet, strain and species variation.
J. Pharmacol. exp. Ther., 83: 195-202.
EPSTEIN, S. (1969) A catch-all toxicological screen. Experientia
(Basle), 25: 617.
FERON, V. J. (1973) An evaluation of the criterion "organ weight"
under conditions of growth retardation. Food Cosmet. Toxicol.
11: 85-94.
FEUER, G., GOLBERG, L., & LE PELLEY, J. R. (1965) Liver response
tests. I. Exploratory studies on glucose 6-phosphatase and other
liver enzymes. Food Cosmet. Toxicol., 3: 235-249.
FLEISS, J. L. (1973) Statistical methods for rates and proportions.
New York, John Wiley (Wiley Series in Probability and
Mathematical Statistics).
FLODMARK, S. & STEINWALL, O. (1963) Differentiated effects on certain
blood brain barrier phenomena and on the EEG produced by means of
intracarotidly applied mercuric dichloride. Acta. Physiol.
Scand., 57: 446-453.
FOUTS, J. R. & HART, L. G. (1965) Hepatic drug metabolism during the
perinatal period. Ann. NY Acad. Sci., 123: 245-251.
FRAZER, A. C. & SHARRATT, M. (1969) The value and limitations of
animal studies in the prediction of effects in man. In: The use
of animals in toxicological studies -- UFAW Symposium, England,
1968, Potters Bar, England, 41 pp.
FRIEDMAN, L. (1969) Symposium on the evaluation of the safety of food
additives and chemical residues. II. The role of the laboratory
animal study of intermediate duration for evaluation of safety.
Toxicol. appl. Pharmacol., 16: 498-506.
FROST, J. K. (1969) Manual for the tenth postgraduate institute for
pathologists in clinical cytopathology. Baltimore, MD, Johns
Hopkins Hospital.
GEHRING, P. J., ROWE, V. K., & MCCOLLISTER, S. B. (1973) Toxicology:
cost-time. Food Cosmet. Toxicol., 11: 1097-1110.
GRANT, D. L., HATINA, G. V., & MUNRO, I. C. (1975) Hexachlorobenzene
accumulation and decline of tissue residues and relationship to
some toxicity criteria in rats. Environ. Qual. Saf., Supplement
Vol. III, pp. 562-568.
GRICE, H. C. (1972) The changing role of pathology in modern safety
evaluation. CRC Crit. Rev. Toxicol., 1: 119-152.
GRICE, H. C., BARTH, M. L., CORNISH, H. H., FOSTER, G. V., & GRAY, R.
H. (1971) Experimental cobalt cardiomyopathy: correlation between
electrocardiography and pathology. Cardiol. Res., 4: 452-456.
GRIFFITH, J. F. (1964) Inter-laboratory variations in the
determination of acute oral LD50. Toxicol. appl. Pharmacol.,
6: 726-730.
HAGAN, J. M. (1959) Acute toxicity. In: Appraisal of the safety of
chemicals in food, drugs and cosmetics, Assoc. Food & Drug
Officials of USA, pp. 17-25.
HARADA, Y., MIYAMOTO, Y., NONAKA, I., OHTA, S., & NINOMIYA, T. (1967)
Electroencephalographic studies on Minamata Disease in children.
Dev. Med. Child Neurol., 10: 257-258.
HEALTH & WELFARE, CANADA (1970) Guide for the preparation of
submissions for food additives. Ottawa, Health & Welfare.
HEALTH & WELFARE, CANADA (1973) The testing of chemicals for
carcinogenicity, mutagenicity and teratogenicity. Ottawa,
Health & Welfare.
HENLEY, K. S., SCHMIDT, E., & SCHMIDT, W. (1966) Enzymes in serum,
their use in diagnosis. Springfield, IL, Thomas.
HORN, H. J. (1956) Simplified LD50 (or ED50) calculations.
Biometrics, 12: 311-321.
HRBEK, A., KARLBERG, P., KJELLMER, J., & OLSSON, T. (1972) Evoked EEG
responses in newborns with asphyxia and IRDs. Pediatr. Res.,
6: 61.
HURST, E. W. (1958) Sexual differences in the toxicity and therapeutic
action of chemical substances. In: Walpole, A. L. & Spinks, A.,
ed. The evaluation of drug toxicity, London, Churchill,
pp. 12-25.
JOHNSON, L. P. V. (1950) An introduction to applied biometrics.
Minneapolis, Burgers Publ., Co.
JONDORF, W. R., MAICKEL, R. P., & BRODIE, B. B. (1959) Instability of
newborn mice and guinea pigs to metabolize drugs. Biochem.
Pharmacol., 1: 352-354.
KELLO, D. & KOSTIAL, K. (1973) The effect of milk diets on lead
metabolism in rats. Environ. Res., 6: 355-360.
KORSRUD, G. O., GRICE, H. C., & MCLAUGHLAN, J. U. (1972) Sensitivity
of several serum enzymes in detecting carbon tetrachloride --
including liver damage in rats. Toxicol. appl. Pharmacol.,
22: 474-483.
LECLAIR, J. M. & WILLARD, J. W. (1970) Guide for the preparation of
submissions on tolerances for incidental contaminants and
agricultural chemicals in food. Ottawa, Canada, Food & Drug
Directorate, Dept of National Health & Welfare.
LITCHFIELD, J. T. (1962) Evaluation of the safety of new drugs by
means of tests in animals. Clin. Pharmacol. & Therap.,
3: 665-672.
LITCHFIELD, J. T. & WILCOXON, F. A. (1947) A simplified method of
evaluating dose-effect experiments. J. Pharm. exp. Ther.,
95: 99-113.
LOOMIS, T. A. (1968) Essentials of toxicology. Philadelphia, Lea &
Febiger.
LU, F. J., JESSUP, D. C., & LAVALLEE, A. (1965) Toxicity of pesticides
in young versus adult rats. Food Cosmet. Toxicol., 3: 591-596.
MAGILL, P. I., HOLDEN, F. R., & ACKLEY, C. (1956) Air Pollution
Handbook. New York, McGraw-Hill.
MANN, L. I. (1970) Developmental aspects and the effect of carbon
dioxide tension on fetal cephalic metabolism and
electroencephalogram. Exp. Neurol., 26: 148-159.
MARZULLI, F. N. (1968) Ocular side effects of drugs. Food Cosmet.
Toxicol., 6: 221-223.
MCGRATH, J. T. (1960) Neurological examination of the dog with
clinicopathological observation. 2nd ed. Philadelphia, Lea &
Febiger.
MILLER, L. C. & TAINTER, M. L. (1944) Estimation of the ED50 and its
error by means of log-probit graph paper. Proc. Soc. Exp. Biol.
Med. NY, 57: 261-264.
MORRISON, J. K., QUINTON, R. M., & REINERT, H. (1968) The purpose and
value of LD50 determinations. In: Boyland, E. & Goulding, R.,
ed. Modern trends in toxicology. London, Butterworths,
pp. 1-17.
MOWBRAY, J. B. & CADELL, T. E. (1962) Early behaviour patterns in
rhesus monkeys. J. Comp. Physiol. Psychol., 55: 350-357.
MUNRO, I. C., MIDDLETON, E. J., & GRICE, H. C. (1969) Biochemical and
pathological changes in rats fed brominated cottonseed oil for 80
days. Food Cosmet. Toxicol., 7: 25-33.
MUNRO, I. C., CHARBONNEAU, S. M., & WILLES, R. F. (1972) An automated
data acquisition and computer-based computation system for
application to toxicological studies in laboratory animals.
Lab. Anim. Sci., 22: 753-756.
MUNRO, I. C., MOODIE, C. A., KREWSKI, D., & GRICE, H. C. (1974)
Carcinogenicity study of commercial saccharin in the rat.
Toxicol. appl. Pharmacol., 32: 513-526.
NATIONAL ACADEMY OF SCIENCES (1975) Principles for evaluating
chemicals in the environment. Washington, DC, National Academy
of Sciences.
NEWBERNE, P., ROGERS, A. E., & WOGAN, G. N. (1968) Hepatorenal lesions
in rats fed a low lipotrope diet and exposed to aflatoxin.
J. Nutr., 94: 331-343.
OSBORNE, B. E. & DENT, N. J. (1973) Electrocardiography and blood
chemistry in the detection of myocardial lesions in dogs.
Food Cosmet. Toxicol., 11: 265-276.
PAGET, G. E. & BARNES, J. M. (1964) Toxicity tests. In: Laurence, D.
R. & Bacharach, A. L., ed. Evaluation of drug activities:
Pharmacometrics, London, New York, Academic Press,
Vol. 1, pp. 135-166.
PECK, H. M. (1974) Design of experiments to detect carcinogenic
effects of drugs. In: CRC Carcinogenesis testing of chemicals,
Cleveland, OH, CRC Press, pp. 1-13.
RUMKE, CHR. L. (1964) Some limitations of animal tests. In: Laurence,
D. R. & Bacharach, A. L., ed. Evaluation of drug activities:
Pharmacometrics, London, New York, Academic Press,
Vol. 1, pp. 125-133.
SCHALM, O. W. (1967) Veterinary haematology, 2nd ed. Philadelphia,
Lea & Febiger.
SCOTT, W. J., JOHNSTON, J. W., JOHNSTON, C. D., & BELILES, R. P.
(1966) Comparative acute toxicities of isoproterenol and
metraproterenol. Toxicol. appl. Pharmacol., 8: 353.
SETNIKAR, I. & MAGISTRETTI, M. J. (1964) The toxicity of central
nervous system stimulants in rats of different ages.
Proc. Eur. Soc. Drug Toxicity, 4: 132-139.
SHER, S. P. (1972) Mammary tumours in control rats: literature
tabulation. Toxicol. appl. Pharmacol., 22: 562-588.
SIRRIDGE, M. S. (1967) Laboratory evaluation of haemostasis.
Philadelphia, Lea & Febiger.
SNEDECOR, J. W. & COCHRAN, W. G. (1967) Statistical methods 6th ed.,
Ames, IA, Iowa State University Press, pp. 111-114, 221-223.
SONTAG, J. M., PAGE, N. P., & SAFFIOTTI, U. (1975) Guidelines for
carcinogen bioassay in small rodents. Bethesda, MD, National
Cancer Institute.
STAUBLI, W., HESS, R., & WEIBEL, E. R. (1969) Correlated morphometric
and biochemical studies on the liver cell. II. Effects of
phenobarbital on rat hepatocytes. J. cell. Biol., 42: 92-112.
THOMPSON, W. (1947) Use of moving averages and interpolation to
estimate median effective dose. Bac. Rev., 11: 115-141.
TOMATIS, L. & MOHR, U., ed. (1973) Transplacental carcinogenesis,
IARC Sci. Publ. No. 4, 181 pp.
WEIL, C. S. (1952) Relationship between short- and long-term feeding
studies in designing an effective toxicity test. Agric. Food
Chem., 11: 486-491.
WEIL, C. S., WOODSIDE, M.D., BERNARD, J. R., & CARPENTER, C. P. (1969)
Relationship between single peroral, one-week and ninety-day
feeding studies. Toxicol. appl. Pharmacol., 14: 426-431.
WEIL, C. S., & CARPENTER, C. P. (1969) Abnormal values in control
groups during repeated-dose toxicological studies. Toxicol.
appl. Pharmacol., 14: 335-339.
WEINBERG, M. S., GOLDHAMEN, R. E., & CARSON, S. (1966) Acute oral
toxicity of various drugs in newborn rats after treatment of the
dam during gestation. Toxicol. appl. Pharmacol., 9: 234-239.
WORDEN, A. N. & HARPER, K. H. (1963) Oral toxicity as influenced by
method of administration. Proc. Eur. Soc. Study Drug Toxicity,
2: 15-26.
WHO (1958) WHO Technical Report Series No. 144. (Procedures for the
testing of intentional food additives to establish their safety
for use -- 2nd report of the joint FAO/WHO Expert Committee on
Food Additives), 19 pp.
WHO (1972) Health hazards of the human environment, Geneva, WHO,
387 pp.
YEARLY, R. A. & BENISH, R. A. (1965) A comparison of the acute
toxicities of drugs in newborn and adult rats. Toxicol. appl.
Pharmacol., 7: 504.
ZBINDEN, G. (1973) Progress in toxicology: special topics. Vol. 1.
New York, Springer-Verlag.
ZIMMERMAN, H. J. (1974) Serum enzyme measurement in experimental
hepatotoxicity. Israel J. med. Sci., 10: 328-332.
4. CHEMOBIOKINETICS AND METABOLISM
4.1 Introduction
The objective of chemobiokinetic studies is to obtain data that
allow reliable assessment of the hazard of environmental chemicals to
man. Since effects are related to the amounts or concentrations of a
chemical in tissues and cells, it is imperative to elucidate the
dynamics of the toxicant at the target site. It should be emphasized
that the toxicant may be either the parent chemical or a metabolite or
degradation product formed from it. Thus, the qualitative
identification of the degradation products of a chemical together with
a quantitative characterization of their fate, as well as the fate of
the parent chemical, as a function of time, are inextricably
associated in a proper chemobiokinetic evaluation. In the context of
this chapter, the word "chemobiokinetics" has been used in place of
"pharmacokinetics" because too often the latter implies restriction of
this scientific discipline to drugs. The term chemobiokinetics is
proposed to emphasize its importance in evaluating the biological
effects of all chemicals.
4.2 Absorption
4.2.1 General principles
Absorption of a chemical into the body can take place,
potentially, by all routes of exposure. In assessing the toxicity and,
ultimately, the hazard of a chemical, the oral, dermal, and inhalation
routes of exposure are of primary importance. Following absorption,
the chemical is distributed by the blood to the various tissues.
Therefore, the rate of absorption is frequently estimated by
determining the concentration of the chemical in the plasma as a
function of time following exposure.
The route of administration can greatly influence the rate at
which a foreign chemical enters the body. Upon ingestion, the gastric
contents and pH of the stomach can influence the rate of absorption of
the chemical. In the small intestine, food may either enhance or delay
absorption. Indeed, the environment of the gastrointestinal tract (pH,
food, bacteria) may change the parent chemical into another chemical.
The inhalation route allows a chemical to pass rapidly into the blood
without encountering drastic changes in pH, food, or microflora. The
skin effectively retards the absorption of many chemicals; however, it
should not be considered as an absolute barrier. Some chemicals
readily penetrate intact skin and a minor abrasion of the skin may
greatly enhance the absorption of many chemicals.
In order that a chemical may be absorbed into the bloodstream, it
must cross one or more semipermeable membranes, such as the
gastrointestinal epithelium, the lining of the respiratory tract, or
the epidermis of the skin. Membranes are essentially lipoproteins with
aqueous pores through which water-soluble molecules can pass. The pore
size varies from 4 Å (intestinal epithelium and mast cells) to 30 Å
(capillaries), allowing the passage of molecules with molecular
weights less than 100-200 to approximately 60 000, respectively. Most
membranes have an electrical potential that may effectively preclude
the ready penetration of charged chemical species. Thus it is obvious
that the absorption of a chemical depends on its physicochemical
properties, molecular size, shape, degree of ionization, and lipid
solubility. For a more thorough discussion, the reader is referred to
Davson & Danielli (1952) and Schanker (1962a).
Three mechanisms have been proposed to explain how a chemical
passes across a cell membrane: (a) passive diffusion through the
membrane, (b) filtration through membranous pores, and (c) specialized
transport systems that carry water-soluble and large molecules across
the membrane by means of a "carrier".
Passive diffusion is considered to be the principal mechanism by
which chemicals can cross cell membranes. The rate of passive
diffusion of a molecule is proportional to the concentration gradient
across the membrane, the membrane thickness, the area available for
diffusion, and the diffusion constant, in accordance with Fick's Law
(La Du et al., 1972). The rate of passage is related directly to the
lipid solubility (Brodie, 1964). However, since absorption requires
passage through an aqueous- as well as a lipo-phase, the absorption of
a chemical with an extremely low solubility in water may be impeded in
spite of a high lipid-to-water partition coefficient. The passive
diffusion also depends on the extent of the ionization and the lipid
solubility of the ionized and nonionized species (Brodie, 1964).
Filtration is a process by which a chemical passes through the
aqueous pores in the membrane, and is governed by the size and shape
of the molecule. The bulk flow of water across the membrane produced
by an osmotic gradient or hydrostatic pressure can act as a carrier
for chemicals.
Specialized transport processes are needed to explain the
transport and kinetic behaviour of large, lipid insoluble molecules
and ions. Two types of carrier-mediated transport systems have been
recognized: active transport and facilitated diffusion. The carrier in
both systems is some component of the membrane that combines with the
chemical and assists its passage across the membrane. It has a limited
capacity and when it is saturated, the rate of transfer is no longer
dependent on the concentration of the chemical and assumes zero order
kinetics. Structure, conformation, size, and charge are important in
determining the affinity of a molecule for a carrier site, and
competition for carrier site will occur.
Active transport is a carrier-mediated transport system which
moves a molecule across a membrane against a concentration gradient,
or, if the molecule is an ion, against an electrochemical gradient. It
requires the expenditure of metabolic energy and can be inhibited by
poisons that interfere with cell metabolism. Active transport plays an
important role in the renal and biliary excretion of chemicals.
Facilitated diffusion is a carrier transport mechanism by which a
water-soluble molecule (i.e. glucose) is transported through a
membrane down a concentration gradient. No apparent energy is required
and metabolic poisons will not inhibit this process. The difference
between facilitated diffusion and active transport is that the latter
moves molecules against a concentration gradient, whereas the former
does not. For more complete discussion of membrane transport, refer to
La Du et al. (1972) and Goldstein et al. (1974).
Another active process, pinocytosis, has been implicated as a
mechanism for transferring large molecules and particles into cells.
In this process, the membrane engulfs the material and pinches off an
envelope containing the material within the cell.
4.2.2 Absorption from the lungs
The pulmonary epithelial lining is very thin, possesses a large
surface area, and is highly vascular. Thus, absorption of foreign
chemicals can take place at a very rapid rate. Most rapidly absorbed
are gases and aerosols with small particle size and a high
lipid-to-water partition coefficient. In most inhalation studies,
absorption may occur by routes other than the lungs and the
investigator should be aware of this in the interpretation of data.
A more complete discussion of the inhalation of chemicals is
presented in Chapter 6.
4.2.3 Absorption from the skin
The structure of the skin enables rapid penetration of
lipid-soluble compounds through the epidermis, a lipoprotein barrier,
whereas the highly porous dermis is permeable to both lipid- and
water-soluble substances (Katz & Poulsen, 1971). Factors which govern
penetration through the skin are hydration, pH, temperature, blood
supply, and metabolism as well as vehicle-skin interactions. Abrasion
of the skin may enhance absorption greatly. For a more complete
discussion of the principles for absorption through the skin and
experimental methods, refer to Part II, Chapter 11.
4.2.4 Gastrointestinal absorption
The gastrointestinal tract is one of the most important routes of
absorption of foreign compounds (Schanker, 1971). Chemicals can be
absorbed along any section of the gastrointestinal tract, but because
of the large surface area and rich blood supply, absorption is
favoured from the small intestines. In most parts, the movement of a
chemical across the epithelial lining of the gastrointestinal tract is
by diffusion and carrier transport mechanisms are involved to a lesser
degree.
Although therapeutic amounts of drugs may be absorbed from the
buccal mucosa (Beckett & Hossie, 1971), absorption of environmental
chemicals from the mouth is minimal compared with that from the
stomach and intestine. Chemicals absorbed from the mouth are not
exposed to the gastrointestinal digestive juices and drug-metabolizing
enzymes. Furthermore, since they are not transported by the hepatic
portal system directly to the liver, their normally rapid metabolism
may be precluded, thus prolonging their effect.
The stomach is a significant site of absorption by passive
diffusion of many acid, and neutral, foreign compounds (Schanker et
al., 1957). Due to the acidity of the stomach, weak acids will exist
in the diffusible, nonionized, lipid-soluble form, whereas weak bases
will be highly ionized and therefore not generally absorbable.
Absorption from the small intestine is similar in principle to
that from the stomach (passive diffusion), except that the pH of the
intestinal contents (pH 6.6) may alter the fraction of the chemical in
the nonionized form favouring the absorption of both weakly-acid and
weakly-alkaline chemicals. The aqueous pore size, 4 Å, limits
absorption by filtration to molecules having a molecular weight of
less than 100-200. Rarely, an environmental chemical may be absorbed
from the intestinal tract by an active transport system that is
normally involved in the absorption of nutrients, e.g. sugars and
amino acids (Schanker, 1963).
Many factors can affect the absorption of foreign compounds from
the gastrointestinal tract (Brodie, 1964; Levine, 1970; Place &
Benson, 1971; Prescott, 1975): ( a) increased gastric emptying can
decrease gastric absorption and increase intestinal absorption; ( b)
increased intestinal peristalsis generally inhibits intestinal
absorption; ( c) gastric acid, intestinal digestive juices, and gut
microflora can all degrade chemicals to other absorbable or
nonabsorbable chemical species; ( d) food in the gastrointestinal tract
can impair absorption by producing a nonabsorbable complex, by
decreasing gastric emptying (especially fats), and by reducing,
mixing, or altering pH; ( e) normal digestion produces increased
gastrointestinal blood flow which will enhance absorption; ( f)
absorption of a solid will be impaired if dissolution in the
gastrointestinal tract does not take place. The practice of
administering chemicals admixed with the diet must take these factors
into account, especially the possible reaction of the chemical with
dietary constituents.
4.3 Distribution
Once absorbed, the distribution of a chemical is determined by
the relative plasma concentration, the rate of blood flow through
various organs and tissues, the rate by which the chemical penetrates
cell membranes, and the binding sites that are immediately available
in the plasma and tissues. After the initial distribution phase, the
rate by which a chemical penetrates cell membranes and the available
sites for binding are the predominating factors influencing the final
distribution of a chemical in the body.
When the plasma concentration of a chemical is high and the cell
membranes do not provide significant barriers to diffusion,
distribution is mainly to organs with high blood flow, e.g. brain,
liver, and kidney. A classic example of distribution and
redistribution is thiopental, a highly lipid-soluble chemical that,
after administration, is first distributed to the brain and
subsequently to muscle and body fat which have poor blood flow (Price
et al., 1960). Lipid-soluble, foreign compounds tend to be distributed
and localized in adipose tissue (Mark, 1971), in accordance with their
lipid-to-water partition coefficients, e.g. the chlorinated
hydrocarbon pesticides, dieldrin, DDT, and DDE (Rodomski et al., 1968)
and polychlorinated biphenyls (PCBs) (Allen et al., 1974).
Distribution of chemicals into organs and tissues is influenced by
membraneous barriers in the same way as absorption (see 4.2). For a
more detailed treatment, see Quastel (1965). The capillary membrane,
unlike other body or cell membranes, is freely permeable to foreign
compounds of a molecular weight of 60 000 or less, whether
lipid-soluble or not (Pappenheimer, 1953; Renkin, 1964); generally
chemicals pass these membranes readily, except in the brain,
testicles, and the eye (Gehring & Buerge, 1969).
The movement of foreign chemicals to the brain represents a
unique example that cannot be explained by the physicochemical
properties of the chemical and the tissue distribution. Many chemicals
fail to penetrate into the brain tissue or cerebrospinal fluid as
readily as into other tissues (Brodie & Hogben, 1957). The boundary
between blood and brain consists of several membranes; those of the
blood capillary wall, the glial cells closely surrounding the
capillary, and the membrane of the neurons or nerve cells. The
so-called "blood-brain barrier" is located at the capillary wall-glial
cell region. The capillary walls in the brain tend to be more like
cell membranes than capillary membranes. Therefore, ionized substances
and large water-soluble molecules such as proteins are almost entirely
excluded from passage (Rall, 1971). The chief mode of exit of both
lipid-soluble and polar compounds is by filtration across the
arachnoid villi. The method for studying the movement of chemicals
into and from the brain has been discussed by Rall (1971).
The red blood cell has unusual permeability in that organic
unions penetrate much more readily than cations. This may be explained
by the presence of positively charged membrane pores that will accept
anions but repel cations (Schanker et al., 1957).
4.4 Binding
A major factor, that can affect the distribution of a chemical,
is its affinity to bind to proteins and other macromolecules of the
body. Foreign chemicals have been shown to bind reversibly to such
substrates as albumen, globulins, haemoglobin, mucopolysaccharides,
nucleoproteins, and phospholipids (Shore et al., 1957). For a survey
of the biological implications of the protein binding of chemicals,
the reader is referred to Gillette (1973a).
Once a chemical is bound to a body constituent, it is temporarily
localized. This localization modifies the initial pattern of
distribution and affects the rates of absorption, metabolism, and
elimination of the chemical from the body.
4.4.1 Plasma-protein binding
Most chemicals show some degree of binding to plasma proteins,
the most important fraction of which is albumen. Albumen at pH = 7.3
contains a net negative charge; however, cationic groups must be
accessible because albumen has been shown to bind anions as well as
cations. Although the plasma proteins show an appreciable capacity for
binding many chemicals, this is limited, making it important to
understand such binding as a function of the concentration of the
chemical.
Since plasma proteins possess a limited number of binding sites
and the sites are somewhat nonspecific, two chemicals with an affinity
for the same binding site will compete with one another for binding.
The plasma proteins of various laboratory animals and man show
differences in the degree and nature of binding. This is due to
differences in the total concentrations and relative proportions of
the various plasma proteins as well as the composition and
conformation of albumens (Gillette, 1973b).
4.4.2 Tissue binding
The binding of chemicals to tissue constituents also contributes
to the localization of a chemical. Certain chemicals show a much
greater affinity for tissue than for plasma proteins, and in some
instances the affinity for tissue is quite specific. For example,
polycyclic aromatic compounds have been shown to have particular
affinity for the melanin in the eye (Potts, 1964).
Some metals and several chemicals and organic anions are bound to
proteins (Y and Z proteins or ligandins) in the liver (Levi et al.,
1969). These proteins may play a key role in the transfer of organic
anions from plasma to liver (Levi et al., 1969; Reyes et al., 1971),
and they also bind corticosteroids and azo-dye carcinogens (Litwack et
al., 1971). For further details concerning the nature and effects of
binding of chemicals by proteins and for methods of study, see
Chignell (1971), Gillette (1975), Keen (1971) and Settle et al.
(1971).
Many inorganic ions, particularly metals, as well as
tetracycline, are concentrated in various tissues and organs,
particularly in bones and teeth (Foreman, 1971). A convenient method
for studying the accumulation of chemicals in organs and tissue is
autoradiography (Roth, 1971). Valuable measurements may also be
obtained with classical chemical and radio-chemical techniques which
have the added advantage of being quantitative.
4.5 Excretion
Chemicals are excreted as the parent chemical, as metabolites, or
as conjugates of the parent chemical or its metabolites. The principal
routes of excretion are the urine and bile, and to a lesser degree
expired air, sweat, saliva, milk, and secretions of the
gastrointestinal tract.
4.5.1 Renal excretion
The kidneys are the most important route of excretion of foreign
compounds (Weiner, 1971). The three mechanisms of renal excretion are:
glomerular filtration, active tubular transport, and passive tubular
transport. Only compounds of high molecular weight or those bound
tightly to plasma proteins escape glomerular filtration and the
resulting filtrate contains approximately the same concentration of
foreign compounds as that found in the plasma in an unbound state.
Water and endogenous substrates are reabsorbed from the
glomerular filtrate as it passes down the tubule. In the tubule,
lipid-soluble, unionized chemicals pass in either direction by passive
diffusion. Thus, lipid-soluble chemicals may be reabsorbed by the
tubule, prolonging their retention in the body. Ionic chemicals, such
as conjugates and other metabolites, are poorly reabsorbed and pass
directly out of the body in the urine.
Active transport takes place in the proximal tubule of the
kidney. There are two distinct active transport processes. One process
is specific for organic anions and the other specific for organic
cations. Chemicals transported by the same transport process compete
with each other, and the excretion rate of one compound can be reduced
by the administration of the other. The active transport process can
be saturated as the concentration of the chemical in the plasma is
increased. When the active tubular secretion is saturated, that is,
when an increase in the concentration of the chemical in the plasma is
no longer accompanied by a proportional increase in the concentration
of the chemical in the urine, the concentration in the plasma is
referred to as the renal-plasma threshold.
The anionic secretory process is responsible for the excretion of
metabolites formed through conjugation of the parent chemical or its
degradation products with various endogenous substrates such as
glycine, sulfate, or glucuronic acid. These relatively polar,
lipid-insoluble metabolites are poorly reabsorbed from the tubules and
more readily excreted.
4.5.2 Biliary excretion
Biliary excretion is a major route for the excretion of foreign
chemicals (Smith, 1971a, 1973). It is has been demonstrated (Brauer,
1959; Schanker, 1962b; Sperber, 1963; Williams, 1965) that compounds
with high polarity, anionic and cationic conjugates of compounds bound
to plasma proteins, and compounds with molecular weights greater than
300 are actively transported against a concentration gradient into the
bile. It has also been shown that, once these compounds are in the
bile, they are not reabsorbed into the blood and are excreted into the
gastrointestinal tract (Schanker, 1965). Factors that influence the
biliary excretion of foreign chemicals and metabolites are considered
to be of two types: (a) physicochemical, relating to molecular size,
structural features, and polarity; and (b) biological, relating to
protein binding, renal excretion, metabolism, species, and sex. For a
comprehensive and detailed discussion of these subjects, the reader is
referred to Smith (1971a, 1973), and Stowe & Plaa (1968).
4.5.3 Enterohepatic circulation
Enterohepatic circulation is the phenomenon that occurs when a
compound is excreted via the bile into the gastrointestinal tract,
reabsorbed from the gastrointestinal tract and carried via the portal
system back to the liver, where it is again excreted via the bile and
recycled. Physiologically, enterohepatic circulation is important
because it permits reuse of endogenous biliary excretion products.
However, when a foreign compound is involved in enterohepatic
circulation, it must make its way either to the faeces or to the
peripheral blood to be excreted from the body. Thus, enterohepatic
circulation of a foreign compound serves to enhance its retention in
the body. There are examples in the literature (Gibson & Becker, 1967;
Keberle et al., 1962) which demonstrate that the half-life of a
compound involved in enterohepatic circulation can be decreased after
surgically interrupting the enterohepatic cycle. Administration of a
sequestering agent that binds the compound in the gastrointestinal
tract would serve the same purpose.
Smith (1973) has described the following factors that can affect
the enterohepatic circulation of a compound: ( a) the extent and rate
of excretion of the compound in the bile; ( b) the activity of the gall
bladder; ( c) the fate of the substance in the small intestine; and ( d)
the fate of the compound after reabsorption from the gut. Since many
foreign chemicals are excreted in the bile as unabsorbable conjugates,
the hydrolysis of these conjugates in the intestine may play a key
role in enterohepatic circulation. For a thorough discussion of
enterohepatic circulation, the reader is referred to Plaa (1975).
4.5.4 Other routes of excretion
In addition to excretion in bile and urine, other routes for the
excretion of foreign chemicals and their metabolites should not be
overlooked. In accordance with the pH partition theory, organic bases
highly ionized at the pH value of gastric juice may be secreted into
the stomach (Shore et al., 1957). Similarly, weak acids ionized at
neutral pH may be transferred from the plasma to the lumen of the
intestine. These chemicals are sequestered by the intestinal contents,
augmenting their excretion in the faeces.
Many volatile organic chemicals are excreted readily via exhaled
air (see Chapter 6). This route of excretion is common for carbon
dioxide, an ultimate end-product of an extensively metabolized organic
chemical. For this reason, the quantification of expired radiolabelled
carbon dioxide (14CO2) is very important in chemobiokinetic
studies using carbon-14-labelled compounds.
Many foreign compounds are excreted, to different degrees, in
milk, in either the aqueous or lipid phase (Rasmussen, 1971). Although
this route may be of minor importance for the elimination of a
chemical from the body, it should be given particular attention in
evaluating the hazard of chemicals to man. First, consumption of cow's
milk may constitute an important vehicle of exposure. Secondly, the
consumption of mother's milk by the newborn may provide very high
doses of a chemical that is concentrated in the milk. It should also
be noted that the volume of milk consumed by the newborn per unit body
weight may, in itself, magnify the dose received by this segment of
the population.
Chemicals are also excreted in sweat and saliva. The presence of
a chemical in sweat may lead to dermatitis. Although saliva is usually
swallowed and thus does not lead to elimination of the agent from the
body, recent work has shown that analysis of saliva for the presence
of a chemical may preclude the necessity for venepuncture to obtain
plasma for analysis.
4.6 Metabolic Transformation
Metabolic transformation or biotransformation are terms that have
been used to describe the process which converts a foreign chemical to
another derivative (metabolite) in the body. Metabolic transformation
has been the subject of several excellent reviews (Conney, 1967;
Conney & Burns, 1962; Dahm, 1971; Daly, 1971; Dutton, 1971; Garattini
et al., 1975; Gillette, 1971a,b & 1974a,b; Gillette et al., 1974;
Kuntzman, 1969; McClean, 1971; Smuckler, 1971; Weisburger &
Weisburger, 1971). It usually results in the formation of more polar
and water-soluble derivatives of a foreign chemical which can be more
readily excreted from the body. Generally, such metabolic
transformation of a foreign chemical also results in the formation of
a less toxic chemical. However, there are many cases where the
metabolites are more toxic than the parent chemicals (McLean, 1971;
Miller & Miller, 1971a).
A few compounds resist metabolic transformation. Most strong
acids and bases are excreted unchanged. Also the resistance of
long-acting nonpolar compounds (barbital, halogenated benzene, etc.),
to metabolic transformation might explain their slow elimination from
the body.
A metabolic activation is suggested, if a compound is more toxic
when given orally than intravenously, if there is a long delay between
the administration of a chemical and the onset of its biological
effect, or, if there is an increased effect following pretreatment
with compounds that induce metabolic transformation (Garattini et al.,
1975).
4.6.1 Mechanisms of metabolic transformation
Usually, the metabolic transformation of chemicals takes place to
the greatest extent in the liver and is catalysed by enzymes found in
the soluble, mitochondrial, and microsomal fractions of the cell.
Enzymes metabolizing foreign chemicals are also found, to a lesser
degree, in the cells of the gastrointestinal tract, kidney, lung,
placenta, and blood (Aitio, 1973; Gillette, 1963; Gram, 1973;
Hietanen, 1974; Hietanen & Valinio, 1973; Wattenberg & Leong, 1971;
Wattenberg et al., 1962; Witschi, 1975). It must be emphasized that
for a particular chemical, or a particular route of administration,
other organs may play a more important role in the metabolic
transformation of the chemical than the liver. The role of enzymatic
reactions carried out by the intestinal flora may be very important
and should not be overlooked (Scheline, 1968; Smith, 1971a).
Enzyme-catalysed, biochemical transformations can be classified into
four main types: (a) oxidations, (b) reductions, (c) hydrolyses and
(d) synthetic reactions (see Table 4.1 in Annex to this Chapter).
The metabolic transformation of a chemical can occur via various
pathways which can consist of a single reaction or multiple reactions.
If the metabolic pathway consists of one reaction it is usually
oxidation, reduction, or hydrolysis which tends to increase the
polarity of the compound. Multiple-reaction metabolic pathways can
consist of a series or any combination of oxidation, reduction, or
hydrolysis. The final reaction in a multiple-reaction pathway is
usually a conjugation reaction involving the addition of polar
endogenous functional groups (D-glucuronic acid, glycine etc.) which
usually render the molecule more polar, less lipid-soluble, and
therefore more readily excretable. The predominant sequence of
reactions or metabolic pathways is determined by many factors such as
the dose of the chemical, species, strain, age, sex, and certain
environmental variables.
4.6.1.1 Microsomal, mixed-function oxidations
The metabolism of a large variety of foreign compounds involves
oxidative processes. Microsomal oxidation refers to reactions
catalysed by the enzymes found in the microsomes of the endoplasmic
reticulum. These enzymes are sometimes referred to as microsomal,
mixed-function oxygenases (mono-oxygenases) (Mason, 1957). The
reactions require molecular oxygen and nicotinamide adenine
dinucleotide phosphate, reduced form (NADPH). The reduction
equivalents from NADPH are used to reduce molecular oxygen so that it
can be carried by a cytochrome called P-450 to the compound to be
oxygenated. The oxygen is then fixed into the compounds, usually as a
hydroxyl group (Estabrook, 1971; Estabrook et al., 1971).
The apparent sequence of events in the course of a mixed function
oxidation has been described (Boyd & Smellie, 1972; Estabrook et al.,
1972; Gillette, 1971c). The compound (substrate) forms a complex with
the oxidized cytochrome P-450; this is reduced either directly by
NADPH-cytochrome- c-reductase (1.6.2.4) or indirectly via an
unidentified electron carrier. The reduced cytochrome P-450-substrate
complex then reacts with oxygen to form an "active oxygen" complex,
which decomposes with the formation of the oxidized substrate and
oxidized cytochrome P-450. Substantial progress has been made in
elucidating this mechanism by the development of a method involving
the resolution and reconstitution of the components of the liver
microsomal hydroxylating system (Lu & Levin, 1974).
Measurement of mixed-function oxidase activities of liver
microsomes in vitro has become an important aspect in evaluating the
toxicity of chemicals. The mixed-function oxidase system may be either
a biotransformation system or a site of action of chemicals.
Measurements of the activity of this system can be performed using
either a 9000 g supernatant fraction (Henderson & Kersten, 1970;
Klinger, 1974) of a liver homogenate prepared in buffered KCl
solution, or a microsome fraction sedimented by centrifugation at
about 105 000 g (Flynn et al., 1972; Hewick & Fouts, 1970a,b; Liu et
al., 1975).
The reaction mixtures consisting of the particle-bound enzymes
have to be supplemented with an NADPH-generating system. This may be
fulfilled by the addition of NADPH and glucose-6-phosphate, if the
9000 g fraction is used, but if washed microsomes are used,
glucose-6-phosphate dehydrogenase (1.1.1.49) must also be added.
Isolated tissue cells, tissue cultures, or slices of organs, as
well as perfused organs can also be used for metabolic studies.
Because cytochrome P-450 is intimately associated with the
metabolism of many foreign chemicals, the following methods and
variables have been developed for ascertaining its activity in the
tissues of animals used in toxicological investigations.
The method of Omura & Sato (1964a,b) has been used to measure the
change in the microsomal content of cytochrome P-450 and cytochrome
b5. This method relies on a spectral shift of the pigment upon
exposure to carbon monoxide. An increase in the cytochrome P-450
content can be explained as a consequence of enzyme induction, whereas
the decrease of the haem pigment content may be the result of enhanced
permeability of microsomal membranes due to the damaging effects of
the chemical (Bond & De Matteis, 1969). The concentration of
cytochrome P-450 in the liver, however, is not always directly
proportional to the activity of the mixed-function oxidases.
Spectral changes of cytochrome P-450, determined in the presence
of various substrates, provide information about the binding between
the pigment and substrate (Hewick & Fouts, 1970a; Remmer et al.,
1966). Compounds may be classified into type I or type II according to
their spectral reactions with cytochrome P-450. When type I compounds
bind to cytochrome P-450, the characteristic spectral shift, spectral
difference, gives a peak at 385-390 nm and a trough at 418-427 nm,
whereas with type II compounds, the peak occurs at 425-435 nm and the
trough at 390-405 nm. Originally, it was thought that the magnitudes
of these spectral shifts, especially type I spectra, could be
correlated with microsomal biotransformations (Schenkman et al.,
1967). This correlation, however, is not universally applicable
(Davies et al., 1969; Gigon et al., 1969; Holtzman et al., 1968).
Thus, differences in the magnitude of these spectral changes are
difficult to interpret when they are detected in animals treated with
a chemical (Gillette et al., 1972). The same is true for the
ethylisocyanide difference spectra of cytochrome P-450 which are
characterized by two peaks at about 455 nm and 430 nm (Omura & Sato,
1964a).
Determination of the NADPH-cytochrome P-450 reductase activity,
the assumed rate limiting step in microsomal oxidations, has proved
useful in evaluating the effectiveness of the cytochrome P-450 system
prior to the oxygenation step (Fouts & Pohl, 1971; Gigon et al., 1969;
Hewick & Fouts, 1970b; Holtzman et al., 1968; Zannoni et al., 1972).
Measurement of the NADPH-cytochrome-c-reductase activity may give
information about the rate of flow of reducing equivalents from NADPH
to cytochrome P-450.
Determination of the rate of enzymatic conversion of a substrate
is a most valuable tool in elucidating the metabolic process. For this
purpose, however, it is essential to know the pathway for the
transformation of the chemical, and analytical methods are essential
to quantify the parent chemical and its reaction products. Selected
methods for monitoring some compounds and enzymatic reactions are
listed in Table 4.2 (see Annex to this Chapter).
There are large variations in the metabolism of foreign chemicals
as well as in susceptibility to metabolic inducers depending on the
species (Hucker, 1970), strain, age, and sex of animals.
Many variables must be considered as important factors in species
differences in the metabolism of foreign chemicals. Among these are
differences in binding, either to tissues or to plasma components,
such as albumen. Considerable variations in binding have been reported
for the same chemical in different species (Borgå et al., 1968; Kurz &
Friemel, 1967; Scholtan, 1963; Sturman & Smith, 1967; Witiak &
Whitehouse, 1969). More obvious are the concentrations and types of
foreign chemical-metabolizing enzymes in each species (Flynn et al.,
1972).
4.6.1.2 Conjugation reactions
The major conjugation mechanisms are: glucuronide synthesis,
"ethereal" sulfate synthesis, glutathione conjugation, glycine
conjugation, methylation, acetylation, and thiocyanate synthesis.
Glutamine conjugation has also been shown to occur in man and monkey.
The conjugates formed by these mechanisms are usually nontoxic,
therefore conjugation has also been referred to as a detoxification
mechanism.
These conjugations are biosynthetic reactions in which foreign
compounds or their metabolites containing suitable groups (hydroxyl,
amino, carbonyl, or epoxide) combine with some endogenous substrates
to form conjugates (Parke, 1968; Williams, 1967a, 1971). These
reactions require ATP as source of energy, coenzymes, and transferases
which are usually specific for the formation of conjugates of foreign
compounds. The conjugations usually proceed in at least two steps:
first, the extramicrosomal synthesis of acylcoenzyme and next the
transfer of the acyl moiety to the aglycone, which, in some but not
all cases, is localized in the microsomes. Thus, these reactions
cannot be considered as transformations, characteristic of microsomes.
In accordance with the coenzymes participating in these
reactions, they include:
formation of glucuronides (via uridine diphosphate glucuronic
acid, UDPGA);
formation of sulfate esters (via 3-phosphadenosine-5-
phosphosulfate, PAPS);
O-, N-, and S-methylation via 5'-[(3-amino-3-carboxypropyl)
methylsulfonio]-5'-dioxyadenosine( S-adenosylmethionine);
acetylations (via acetyl coenzyme A);
formation of peptide conjugates (via different acylcoenzyme A
derivatives);
formation of glutathione conjugates and mercapturic acids
(conjugations with glutathione).
Formation of glucuronides is probably the most important
microsomal conjugation mechanism (Dutton, 1971). It occurs in the
liver and to a lesser extent in the kidney, gastrointestinal tract,
and the skin. Biosynthesis of glucuronides can be measured in intact
animals by determining D-glucaric acid (Marsh, 1963) and
D-glucuronolactone dehydrogenase (1.1.1.70) (Marselos & Hanninen,
1974), by enhancement of D-glucuronolactone and aldehyde dehydrogenase
(1.2.1.3) by inducers of microsomal metabolism (Marselos & Hanninen,
1974), glucuronides (Gregory, 1960; Yuki & Fishman, 1963) and
L-ascorbic acid in urine. Elevation in urinary excretion of these
compounds may be an indicator of an adaptive acceleration of hepatic
glucuronide formation (Notten & Henderson, 1975). It should be
emphasized that increased excretion of D-glucaric acid can result from
enzyme induction; therefore it cannot be assumed that this occurrence
is indicative only of an increased glucuronide formation. Methods for
the measurement of glucuronide synthesis in whole organs and tissue
cultures, as well as in tissue slices, have been summarized by Dutton
(1966). In assays with homogenates and cell fractions the reaction
mixtures have to be supplemented with added UDPGA.
UDP-glucuronosyl transferase (2.4.1.17) activity can be
determined using 2-aminophenol (Burchell et al., 1972; Dutton &
Storey, 1962), 4-nitrophenol (Isselbacher 1956; Zakim & Vessey, 1973),
bilirubin (Heirwegh et al., 1972), 7-hydroxy-4-methyl-2H-I-
benzopyran-2-one (4-methylumbelliferone) (Aitio, 1973; Arias, 1962) or
morphine (Strickland et al., 1974).
In contrast to glucuronide synthesis, the formation of sulfate
esters is most probably an extramicrosomal process and is catalysed
generally by sulfate-conjugating enzymes in the presence of
3-phosphoadenosine-5-phosphosulfate as a co-enzyme (Roy, 1971). Among
the compounds of toxicological interest, phenols are converted by
sulfation to esters and excreted in the urine. Aminophenols yield
sulfamates. There are specific assays for the determination of
sulfotransferase (2.8.2) activity using 4-nitrophenol (Gregory &
Lipmann, 1957), or 3-(2-aminoethyl)-1H-indol-5-ol (serotonin) (Hidaka
et al., 1967) as acceptors.
The methyltransferases (2.1.1) catalyse O-, N- and
S-methylation of several physiologically active compounds and drugs
(Axelrod, 1971). They are widely distributed in different organs, but
only a small amount of catechol- O-methyltransferase (2.1.1.6) and
almost all of the phenol- O-methyltransferase (2.1.1.25) (Axelrod &
Daly, 1968) activity is localized in the microsomes of the liver. Only
microsomal transferases are induced by benzo(a)pyrene and inhibited by
SKF 525Aa. The methods used for the determination of
catechol- O-methyltransferase activity are based on the principle
that the enzyme catalyses the transfer of methyl groups to catechols
in the presence of S-adenosylmethionine as a methyl donor. The
substrates employed include adrenaline (Axelrod & Tomchick, 1958),
3,4-dihydrobenzoic acid (MacCaman, 1965), 3,4-dihydroxybenzeneacetic
acid (3,4-dihydrophenylacetic acid) (Assicot & Bohuon, 1969; Broch &
Guldberg, 1971) as well as I-(3,4-dihydroxyphenyl)ethanone
(3,4-dihydroxyacetophenone) (Borchardt, 1974). The end products of the
enzymatic reaction are measured either spectrofluorimetrically
(Axelrod & Tomchick, 1958; Borchardt, 1974; Broch & Guldberg, 1971),
or radiometrically using labelled methyl groups in the coenzyme
(MacCaman, 1965).
a Diethyl aminoethanol ester of diphenyl-propyl acetic acid.
Acetylation reactions of the amino group of foreign compounds are
catalysed by acetyltransferases (Weber, 1971). Substrates of these
enzyme reactions, localized in the soluble part of the cells, are
arylamines, hydrazines, and certain aliphatic amines. Coenzyme A is an
essential factor in these acetylations. Acetylation of arylamines has
been studied quantitatively, in vivo, in human beings and animals
(Williams, 1967b).
Methods for the determination of N-acetyltransferase (2.3.1.35)
activities in vitro summarized by Weber (1971) include colorimetric
(Brodie & Axelrod, 1948; Maher et al., 1957; Marshall, 1948; Shulert,
1961; Weber, 1970), spectrophotometric (Jenne & Boyer, 1962; Tabor et
al., 1953; Weber & Cohen, 1968; Weber et al., 1968) as well as
radiometric procedures (Stotz et al., 1969).
Conjugation of aromatic carboxylic acids (benzoic acid,
substituted benzoic acids, and heterocyclic carboxylic acids) with
amino acids by means of acetyl coenzyme A and ATP is called peptide
conjugation. Glycine is the most generally involved amino acid in this
reaction resulting in the formation of N-benzoylglycine (hippuric
acid). Indole-3-acetic acid, benzeneacetic acid, as well as
4-aminosalicylic acid, can conjugate with glutamine in man, and
several mammals. Determination of hippuric acids (Ogata et al., 1969)
enables the quantitative investigation of this conjugation reaction.
Conjugation of glutathione with foreign compounds, catalysed by
at least ten different glutathione S-transferases, is an important
pathway for the elimination of these compounds (Boyland, 1971).
Following the conjugation of foreign compounds with glutathione, the
conjugate is most frequently hydrolysed to the cysteine conjugate
which is excreted in the urine. Furthermore, the cysteine conjugate
may be acetylated and the resulting mercapturic acid excreted. The
significance of the mercapturic acid biosynthesis in man, however, is
difficult to assess.
Determination of glutathione S-transferase activities are based
on spectral change of the substrate (1,2-dichloro-4-nitrobenzene) due
to conjugation (Booth et al., 1961), or loss of glutathione content
(Boyland & Chasseaud, 1967; Boyland & Williams, 1965; Johnson, 1966)
or release of labile groups (Al-Kassab et al., 1963; Boyland &
Williams, 1965; Johnson, 1966) as well as on chromatographic
separation of the products (Suga et al., 1967). The determination of
the activity of gamma-glutamyltransferase (2.3.2.2), catalysing one
intermediary step of the overall mercapturic acid synthesis may also
be informative.
4.6.1.3 Extramicrosomal metabolic transformations
Foreign compounds, either transformed by oxidation or initially
having characteristic groups (hydroxyl, amino) may resemble normal
constituents of physiological metabolism. Thus, they may undergo
metabolic transformations similar to those of normal body
constituents: oxidation, reduction, deamination, hydrolysis. The
enzymes catalysing these reactions are localized in the cytosol or are
intrinsic compounds of the mitochondria.
In contrast to the extensive data in the literature on
enzyme-chemical interactions (MacMahon, 1971; Zeller, 1971) only a few
enzyme activities are commonly used to monitor toxicological events.
The alcohol dehydrogenase (1.1.1.1) of the liver is one of the
most important enzymes which catalyses the NAD-mediated oxidation of
various aliphatic and aromatic primary and secondary alcohols.
Determination of the activity of alcohol dehydrogenase is based on the
spectrophotometric measurement of the amount of NAD being reduced in
the presence of excess alcohol (Bonnichsen & Brink, 1955).
Among the amine oxidases, monoamine oxidase (1.4.3.4), localized
in the mitochondria, regulates the balance of the biogenic amines and
probably does not participate in the metabolism of foreign amines to a
great degree (Zeller, 1971). However, the fact that a large number of
substances (substrates and substrate analogues, alkyl and arylamines,
hydrazine derivatives, sulfhydryl reagents, etc.) inhibit this enzyme,
enables monoamine oxidase to be used as a tool in studies of the
toxicity of these inhibitors.
Monoamine oxidase activity can be measured manometrically
(Creasey, 1956) based on oxygen-consumption, by determination of
ammonia production (Cotzias & Dole, 1951), spectrophotometrically
(Dietrich & Erwin, 1969; Obata et al., 1971; Weissbach et al., 1960),
fluorimetrically (Takahashi & Takahara, 1968; Tufvesson, 1970) as well
as radiometrically (Otsuka & Kobayashi, 1964).
Hydrolysis by carboxylesterases (ali-esterases or arylesterases)
of foreign compounds containing ester groups may be important in
assessing their toxicity (La Du & Snady, 1971). Determination of
esterase activities using different substrates in the presence of the
chemical to be tested can disclose its possible inhibitory potency.
4.6.1.4 Nonenzymatic reactions
Although the foregoing sections have discussed enzymatic
modifications of chemicals, the investigator should not overlook
nonenzymatic, spontaneous reactions between chemicals and natural
constituents in the body that lead to the formation of metabolites,
e.g. the reaction of an alkylating agent with glutathione.
4.6.2 Species variability
A serious problem facing every research worker using an animal
species to study the metabolism of a foreign compound is whether or
not the metabolic pathway in the animal is similar to the metabolic
pathway in man. The problem is not only important in metabolic
studies, but is of utmost importance in using animal toxicity studies
to predict toxicological phenomena in man. Conney et al. (1974)
illustrated that the use of an animal species that metabolizes a
foreign compound in a similar manner to man will give a more precise
prediction of the type of toxicological phenomena to be expected in
man.
Different animal species have been shown to metabolize foreign
compounds at different rates. Quinn et al. (1958) has shown that
benzeneamine (aniline) has a metabolic half-time in the mouse of 35
minutes and in the dog of 167 minutes. In the same study it was
demonstrated that the metabolic half-time of an antipyrine in the rat
was 140 minutes, whereas in man it was 600 minutes.
Considerable species differences in metabolic pathways have also
been demonstrated. In the rat, mouse, and dog the carcinogen,
N-2-fluoranylacetamide (FAA), is N-hydroxylated to N-hydroxy-FAA
which is a more potent carcinogen than FAA. In the guineapig little or
no hydroxylation of FAA occurs. In toxicity studies, Miller & Miller
(1971b) and Weisburger et al. (1964) demonstrated that the rat, mouse,
and dog are susceptible to the carcinogenic activity of FAA, whereas
the guineapig is not. Thus, a difference in the metabolic pathways of
a foreign compound may greatly influence its toxicity.
Species variability in metabolism has been related to other
factors such as species differences in protein binding, and enzyme
concentration and type. Hucker (1970) described, in detail, species
differences in chemical metabolism and some of the factors responsible
for these differences.
4.6.3 Enzyme induction and inhibition
For some time it has been known that chemicals can increase the
activity of metabolizing enzyme systems. These chemicals have been
termed enzyme "inducers". Inducers exert their action by
quantitatively increasing the enzymes and components responsible for
the metabolism of foreign compounds. The importance of induction to
the toxicologist is two-fold. If metabolism leads to the formation of
excretable or nontoxic metabolites, induction will enhance
detoxification and excretion of the compound. However, if metabolism
leads to the production of a more toxic metabolite, induction will
increase the toxicity of a compound.
Many chemicals are known to increase metabolizing enzyme systems.
The reviews by Conney (1967), Kuntzman (1969), and Mannering (1968),
depict the large number of chemicals which induce metabolizing enzymes
and comprehensively review the factors involved in enzyme inductions.
Most inducers give maximum effects rather quickly -- within 2-3
days (Fouts, 1970). However, some require 2 weeks or longer (Gillette
et al., 1966; Hart & Fouts, 1965; Hoffman et al., 1968, 1970;
Kinoshita et al., 1966). Frequently, the degree of induction after
obtaining a maximum level may decline despite continuing treatment of
the animal with a chemical (Gillette et al., 1966; Hoffman et al.,
1968; Kinoshita et al., 1966).
Drug-metabolizing enzymes can also be depressed by foreign
chemicals, and these compounds are termed inhibitors. 2-(Diethylamino)
ethyl-alpha-phenyl-alpha-propyl benzeneacetate hydrochloride
(SKF-525A) is the best known of the inhibitors and is used routinely
in determining the effect of enzyme inhibition on the metabolism of
chemicals.
4.6.4 Metabolic saturation
In vivo saturation of metabolic pathways can play an important
role in determining the toxic profile of a chemical. A recent article
by Jollow et al. (1974) demonstrated the effect of enzyme saturation
on the metabolism and toxicity of bromobenzene. Bromobenzene was first
metabolically transformed to an epoxide which is hepatotoxic. After a
small nontoxic dose, approximately 75% was converted to the
glutathione conjugate and excreted as bromophenylmercapturic acid.
After a large toxic dose, only 45% was excreted as the mercapturic
acid. It was established that, at the toxic dose, the metabolic
conjugation pathway was overwhelmed due to lack of glutathione, which
resulted in an increased reaction of the epoxide hepatotoxin with DNA,
RNA, and protein.
It is very important to elucidate dose-dependent metabolism to
assess the hazard of a chemical. Frequently, the doses of a chemical
used to characterize toxicity are many times those encountered in the
environment. Toxicity incurred at these large doses may be influenced
by relative changes in metabolism and therefore must be interpreted
with caution and judgment in assessing the hazard of low doses.
4.7 Experimental Design
Since, for the most part, toxicity is a function of the
concentration of the toxicant in the tissues and cells, this
information together with its dynamics provides for inter- as well as
intra-species extrapolation of the results of toxicological effects.
The overall objectives of a chemobiokinetic study are to
determine the amount, rate, and nature of absorption, distribution,
metabolism, and excretion of a chemical. The approach to meeting those
objectives must be flexible and designed to meet the specific needs of
each chemical.
It is difficult to predict, without prior data, an animal species
that will metabolize a chemical similarly to man. Usually, initial
studies are performed in the rat and one other nonrodent species, such
as the dog or monkey, in an attempt to determine species variability.
If there are significant differences among species, it is important to
determine whether differences in the chemobiokinetic parameters
correlate with differences in toxicity or pharmacological activity.
Animals should be acclimatized to the environment of the metabolism
cage prior to the experiment. Light cycle, temperature, humidity, and
time of feeding should be standardized. The physical condition,
weight, and food and water consumption of each animal should be
monitored and recorded throughout the study.
There are advantages in using radioactively-labelled chemicals in
initial studies because of the ease with which radiochemical methods
(Chase & Rabinowitz, 1968) can be applied to chemobiokinetic studies.
An important advantage of using a radioactively-labelled chemical is
that it allows the establishment of the total recovery of the parent
chemical and its metabolites, i.e. the mass balance. To obtain this
the total radioactivity eliminated via the urine, faeces, and exhaled
air as well as that remaining in the carcass following termination of
the experiment should be determined. Until a reasonably good recovery
is obtained, 90% or greater, one can never be sure whether other
chemobiokinetic parameters obtained from the study are accurate.
Furthermore, the isolation and ultimate identification of unknown
metabolites is greatly enhanced by using radioactively-labelled
chemicals.
When using a radiolabelled chemical, the measurement of
radioactivity confirms the presence of the radioisotope, not the
chemical or its metabolites. In order to determine the identity of the
radioactively-labelled compound, the parent chemical and its
metabolites, analytical methods such as gas, high-pressure liquid, and
thin-layer chromatography and a combination of gas chromatography and
mass spectroscopy are frequently employed.
Until it is established that the radioactivity being monitored is
from the chemical in question, kinetic parameters apply to the
radioactivity only, not to the chemical studied. Difficulties can
arise if the radioactive atom does not remain an integral part of the
molecule under study. Tritium and carbon-14 are often incorporated
into the body pools of normal tissue components (Griffiths, 1968;
Rosenblum, 1965). Once the radioactivity is incorporated into these
compartments, its clearance depends on their rates of turnover.
Therefore, by monitoring radioactivity only, it can be falsely assumed
that a compound is being retained in the body.
Another very important reason for differentiating the parent
chemical from its metabolite is to assure that toxic effects that may
be present are associated with the parent chemical and not a
metabolite. Also persistence of a metabolite in the body rather than
the parent chemical may constitute the ultimate hazard.
In initial studies, consideration should be given to the
administration of the compound by intravenous injection as well as via
the route by which man is exposed to the chemical. The intravenous
route is used to provide a more definite assessment of the earlier
phases of distribution and/or elimination. Also, large variation in
rates of absorption will in some cases make the differentiation of the
early phases of distribution and elimination difficult. At least two
doses should be used. One dose should be equivalent to the dose
required to cause signs of toxicity. The second dose should be well
below the toxic dose and, if possible, equivalent to anticipated human
exposure levels.
Most frequently, kinetic parameters for elimination of a chemical
are established by sequential sampling of blood plasma and excreta,
following its administration. A preliminary probe study using one or
two animals is often needed to establish the time at which samples
should be collected, because this will vary with the species and the
chemical in question. After collection and until prepared for analysis
of the chemical or its metabolites, samples should be stored in a
manner that will preclude the breakdown of the chemical or its
metabolites. The data required from the initial chemobiokinetic
studies can be used to design further studies which may include the
following: distribution studies using autoradiography; the isolation
and identification of metabolites; studies to determine the
chemobiokinetic profile of metabolites; biliary excretion studies;
bioconcentration; and in vitro metabolism studies. The methods and
techniques needed to perform these studies are documented by La Du et
al. (1972).
4.8 Chemobiokinetics
Chemobiokinetics aims at quantification of the processes
discussed previously in this chapter. Thus, chemobiokinetics provides
quantitative information on the absorption, distribution,
biotransformation, and excretion of chemicals (including drugs and
endogenous substances) as a function of time. Since the classical
introduction of this discipline by Teorell (1937a,b), the concepts and
methods have been developed extensively, principally for application
to the clinical evaluation and/or use of drugs (Levy & Gibaldi, 1972,
1975; Wagner, 1968, 1971). The reader is also referred to Gehring et
al. (1976) who discuss the subject in greater detail.
One difficulty of many toxicologists and biologists on first
exposure to chemobiokinetics is the concept of compartments. The body
is composed of a large number of organs, tissues, cells, and fluids,
any one of which could be referred to morphologically and functionally
as a compartment. However, in chemobiokinetics, a compartment refers
collectively to those organs, tissues, cells, and fluids for which the
rates of uptake and subsequent clearance of a chemical are
sufficiently similar to preclude kinetic resolution. The rapidly
equilibrating compartment, referred to as the central compartment, may
be comprised of all those tissues with a profuse blood supply whereas
the slow or peripheral compartment may include tissues with a more
limited blood supply, i.e. fat and bone.
4.8.1 One-compartment open model
The simplest chemobiokinetic model is a one-compartment, open
model as shown in Fig. 4.1. In using this model, it is assumed that
the chemical equilibrates with all tissues to which it is distributed
sufficiently rapidly to preclude kinetic differentiation by the
techniques being used to characterize its movement in the body. For
example, if it requires 30 min for a chemical to attain equilibration
in the body after entering the blood stream, and if samples of blood,
tissues, and excreta are taken at 30 min intervals, it will appear
that the body consists of only one compartment.
Assuming that the rate of elimination of the chemical is
proportional to its concentration in the plasma, the concentration in
the plasma will be described by apparent first-order kinetics. The
rate of change of concentration in the plasma may be expressed in the
form of the linear differential equation
dC(t)
= - ke C(t) (1)
dt
where C(t) is the concentration at time t, and ke is the rate constant
for elimination. Solution of this differential equation with initial
condition C(t) = C(0) at time zero gives
C(t) = C(0) exp(- ke t) (exponential form) (2)
or,
(3)
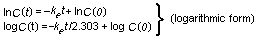 (4)
(4)
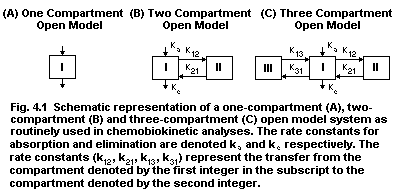 In these equations, C(0) is the concentration of the chemical in the
plasma at time zero. A plot of C(t) versus time on semilogarithmic
paper will yield a straight line (Fig. 4.2) with slope - ke and
intercept C(0).
In these equations, C(0) is the concentration of the chemical in the
plasma at time zero. A plot of C(t) versus time on semilogarithmic
paper will yield a straight line (Fig. 4.2) with slope - ke and
intercept C(0).
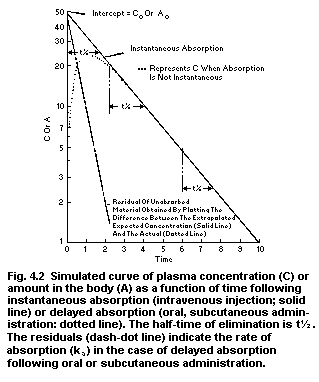 Having determined ke, which is measured in units of reciprocal
time, the time required to reduce the plasma concentration by one-half
is estimated; this time is referred to as the t´ or half-time. It
can be determined from the equation
ln 2 0.693
t´ = = (5)
ke ke
When the chemical is not absorbed instantaneously, the mathematics
needed to describe the concentration in plasma as a function of time
become somewhat more complicated. Assuming apparent first-order
absorption as well as elimination, the concentration C(t) in plasma
is given by the expression
contour integral* D0* ka
C(t) = {exp (- ke t) - exp (- ka t)} (6)
Vd( ka - ke)
In this expression, the terms not previously mentioned are D0, the
dose; contour integral, the fraction of dose absorbed; Vd, the
apparent volume of distribution; and ka, the apparent first-order
absorption rate constant.
The elimination rate constant, ke, is determined as described
previously using that portion of the solid line representing the
plasma concentration after absorption is essentially complete. In Fig.
4.2, this occurs when the dotted line blends into the solid line. The
rate constant for absorption, ka, may be estimated by projecting
the solid line backward to the origin. The difference between the
experimentally-determined values used to characterize the dotted line
are subtracted from those predicted by the backward projection at
corresponding times. Subsequently, the values obtained by this "curve
stripping" procedure are plotted producing a curve like the dash-dash
line in Fig. 4.2. Using this procedure, the t´ for absorption and
ka are determined.
The volume of distribution, Vd, is a term used to describe the
apparent volume to which a chemical is distributed when it is assumed
that the affinity of the plasma and all tissues is equivalent. An
analogy is placing a known amount of a dye in a liquid contained in a
system of unknown volume. After the concentration of the dye has
attained a constant value, the volume of the system can be determined
by dividing the dose, D0, by the concentration to give the volume
of distribution, Vd.
In the plasma, the concentration of the chemical declines because
of elimination as well as distribution to tissues. Therefore, to
estimate Vd, it is necessary to project the elimination phase of
the curve back to the origin. The value obtained at the time zero
intercept by this projection is divided into D0 to obtain the
volume of distribution, Vd, in ml/kg.
The value of Vd provides some important information about the
distribution of the chemical in the body. As the distribution to the
tissues increases, for whatever reason, physicochemical affinity,
active transport into cells, Vd increases. If the distribution of a
chemical in the human body is limited to plasma, extracellular fluid,
or total body water, the respective values of Vd will be
approximately 40, 170, and 580 ml/kg. If a chemical has a high
affinity for a particular tissue, for example, the affinity of a
lipophilic chemical for fat, Vd may exceed significantly 1000 mg/kg.
When the volume of distribution is known, the amount of chemical in
the body at any time t, A(t), can be calculated from the equation
A(t) = C(t)Vd (7)
Until now, concepts relating only to the concentration of the chemical
in the plasma have been discussed.
However, these concepts are equally applicable to other tissues
or, for that matter, to excreta, expired air, or urine. In the case of
urine, the concentrating power of the kidney must be accounted for to
normalize the data. If the affinity of the chemical for the various
tissues and excreta is equivalent and if rapid equilibration is
assumed, the concentration curves will be superimposable. However,
this would be an unusual occurrence. Because of the differences in
affinity, it is more likely that a family of parallel concentration
curves will be obtained. It is emphasized that these curves will be
parallel only after an apparent steady state has been achieved between
the tissues.
In addition to concentration, the same concepts apply if one
desires to characterize the total amount of chemical in the body,
A(t), as a function of time following exposure. For example, if a
dose D0 is ingested and apparent first-order kinetics is assumed,
the amount of the chemical in the body is given by the expression
A(t) = D0 exp (- ke t) (8)
Using equation (7), equation (8) can be shown to be equivalent to
C(t) = C(0) exp (- ke t) (9)
Logarithmic transformation of equations (8) or (9) may be used to
obtain curves like those in Fig. 4.2. The dotted curve would apply if
the chemical were applied to the skin and subsequently absorbed.
One caution must be emphasized in resolving the kinetics of the
amount of an agent in the body. Usually, it is not adequate to
determine the amount of the agent excreted and calculate the amount
remaining in the body by subtracting the cumulative amount excreted
from the original dose. This can be done if, and only if, the agent is
metabolically transformed to a very limited degree and, essentially,
all of the original dose is recovered. This seldom happens.
To circumvent the problem just described, the amount of the
chemical excreted over designated time intervals is determined until a
significant amount can no longer be detected. Assume that the rate of
excretion is proportional to the amount of chemical in the body,
A(t). Let B(t) be the cumulative amount excreted to time t after
administration. Then
dA(t)
= ke A(t) (10)
dt
or,
A(t) = D0 exp(- ke t) (11)
And
dB(t)
= kex A(t) (12)
dt
dB(t) kex
= D0 exp(- ke t) (13)
dt ke
or,
kex
B(t) = D0 {1 - exp (- ke t)} (14)
ke
In these equations, ke represents the apparent first-order overall
elimination rate constant and kex is the rate constant for
excretion via the route being analysed. If Ei is the amount
excreted in the ith time interval of duration deltat then
Ei = B( ti) - B( ti - delta t) (15)
where B( ti) is the amount excreted between administration and ti,
the time at the end of the ith time interval.
In terms of the dose administered D0, and the rate constant
ke,
kex
Ei = D0 exp(- ke ti) {exp( kedelta t) - 1} (16)
ke
the logarithmic form of which is
Having determined ke, which is measured in units of reciprocal
time, the time required to reduce the plasma concentration by one-half
is estimated; this time is referred to as the t´ or half-time. It
can be determined from the equation
ln 2 0.693
t´ = = (5)
ke ke
When the chemical is not absorbed instantaneously, the mathematics
needed to describe the concentration in plasma as a function of time
become somewhat more complicated. Assuming apparent first-order
absorption as well as elimination, the concentration C(t) in plasma
is given by the expression
contour integral* D0* ka
C(t) = {exp (- ke t) - exp (- ka t)} (6)
Vd( ka - ke)
In this expression, the terms not previously mentioned are D0, the
dose; contour integral, the fraction of dose absorbed; Vd, the
apparent volume of distribution; and ka, the apparent first-order
absorption rate constant.
The elimination rate constant, ke, is determined as described
previously using that portion of the solid line representing the
plasma concentration after absorption is essentially complete. In Fig.
4.2, this occurs when the dotted line blends into the solid line. The
rate constant for absorption, ka, may be estimated by projecting
the solid line backward to the origin. The difference between the
experimentally-determined values used to characterize the dotted line
are subtracted from those predicted by the backward projection at
corresponding times. Subsequently, the values obtained by this "curve
stripping" procedure are plotted producing a curve like the dash-dash
line in Fig. 4.2. Using this procedure, the t´ for absorption and
ka are determined.
The volume of distribution, Vd, is a term used to describe the
apparent volume to which a chemical is distributed when it is assumed
that the affinity of the plasma and all tissues is equivalent. An
analogy is placing a known amount of a dye in a liquid contained in a
system of unknown volume. After the concentration of the dye has
attained a constant value, the volume of the system can be determined
by dividing the dose, D0, by the concentration to give the volume
of distribution, Vd.
In the plasma, the concentration of the chemical declines because
of elimination as well as distribution to tissues. Therefore, to
estimate Vd, it is necessary to project the elimination phase of
the curve back to the origin. The value obtained at the time zero
intercept by this projection is divided into D0 to obtain the
volume of distribution, Vd, in ml/kg.
The value of Vd provides some important information about the
distribution of the chemical in the body. As the distribution to the
tissues increases, for whatever reason, physicochemical affinity,
active transport into cells, Vd increases. If the distribution of a
chemical in the human body is limited to plasma, extracellular fluid,
or total body water, the respective values of Vd will be
approximately 40, 170, and 580 ml/kg. If a chemical has a high
affinity for a particular tissue, for example, the affinity of a
lipophilic chemical for fat, Vd may exceed significantly 1000 mg/kg.
When the volume of distribution is known, the amount of chemical in
the body at any time t, A(t), can be calculated from the equation
A(t) = C(t)Vd (7)
Until now, concepts relating only to the concentration of the chemical
in the plasma have been discussed.
However, these concepts are equally applicable to other tissues
or, for that matter, to excreta, expired air, or urine. In the case of
urine, the concentrating power of the kidney must be accounted for to
normalize the data. If the affinity of the chemical for the various
tissues and excreta is equivalent and if rapid equilibration is
assumed, the concentration curves will be superimposable. However,
this would be an unusual occurrence. Because of the differences in
affinity, it is more likely that a family of parallel concentration
curves will be obtained. It is emphasized that these curves will be
parallel only after an apparent steady state has been achieved between
the tissues.
In addition to concentration, the same concepts apply if one
desires to characterize the total amount of chemical in the body,
A(t), as a function of time following exposure. For example, if a
dose D0 is ingested and apparent first-order kinetics is assumed,
the amount of the chemical in the body is given by the expression
A(t) = D0 exp (- ke t) (8)
Using equation (7), equation (8) can be shown to be equivalent to
C(t) = C(0) exp (- ke t) (9)
Logarithmic transformation of equations (8) or (9) may be used to
obtain curves like those in Fig. 4.2. The dotted curve would apply if
the chemical were applied to the skin and subsequently absorbed.
One caution must be emphasized in resolving the kinetics of the
amount of an agent in the body. Usually, it is not adequate to
determine the amount of the agent excreted and calculate the amount
remaining in the body by subtracting the cumulative amount excreted
from the original dose. This can be done if, and only if, the agent is
metabolically transformed to a very limited degree and, essentially,
all of the original dose is recovered. This seldom happens.
To circumvent the problem just described, the amount of the
chemical excreted over designated time intervals is determined until a
significant amount can no longer be detected. Assume that the rate of
excretion is proportional to the amount of chemical in the body,
A(t). Let B(t) be the cumulative amount excreted to time t after
administration. Then
dA(t)
= ke A(t) (10)
dt
or,
A(t) = D0 exp(- ke t) (11)
And
dB(t)
= kex A(t) (12)
dt
dB(t) kex
= D0 exp(- ke t) (13)
dt ke
or,
kex
B(t) = D0 {1 - exp (- ke t)} (14)
ke
In these equations, ke represents the apparent first-order overall
elimination rate constant and kex is the rate constant for
excretion via the route being analysed. If Ei is the amount
excreted in the ith time interval of duration deltat then
Ei = B( ti) - B( ti - delta t) (15)
where B( ti) is the amount excreted between administration and ti,
the time at the end of the ith time interval.
In terms of the dose administered D0, and the rate constant
ke,
kex
Ei = D0 exp(- ke ti) {exp( kedelta t) - 1} (16)
ke
the logarithmic form of which is
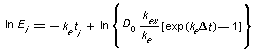 (17)
A semilogarithmic plot of Ei versus ti will give a straight line
with slope - ke. The above expression can be modified to accommodate
unequal time intervals, but in doing so graphic insights are lost.
In using excretion data to resolve kinetic parameters, it is
desirable to keep the collection intervals as short as practical.
Ideally, the collection intervals should be shorter than the t´ for
elimination of the chemical; otherwise resolution of a biphasic
excretion pattern may be precluded. Biphasic refers to two kinetically
distinct excretion phases. For a volatile chemical excreted to some
degree by exhalation, determination of the chemical exhaled as a
function of time may be particularly useful for resolving its
biochemokinetics.
As already stated, the excretion rate of a chemical by one route
of excretion may be different from its overall rate of elimination.
This is true because the agent may be eliminated by other routes
and/or metabolically transformed. The following scheme may be used to
depict a chemical that is eliminated by a metabolic pathway as well as
by excretion in the urine and exhalation:
/ ku excretion in urine
C - kr excretion via exhalation
\ kmx metabolic transformation to compound y
In this case, the overall elimination constant will be ke = ku +
kr + kmx.
The various metabolic transformation and excretion rates may be
estimated using the following equations:
ku= U infinity( ke/ D0) (18)
kr = R infinity( ke/ D0) (19)
kmx = X infinity( ke/ D0) (20)
Uinfinity and Rinfinity are the total amounts of the parent chemical
excreted in urine and expired air. Xinfinity is the total amount of
metabolite, X, recovered from excreta. For excretion of the chemical
in the urine, the differential equation is:
dU
= ku D0 exp(- ke t) (21)
dt
The solution of the equation (21) yields
U inifnity = ku D0/ ke (22)
and
ku = U infinity ke/ D0 (23)
When the urinary excretion of a chemical is determined, it is
frequently desirable to determine its renal clearance in order to
ascertain whether the chemical is actively secreted, reabsorbed, or
only passively filtered by the kidney in the excretion process. Renal
clearance is defined as the urinary excretion rate, delta U/delta
t, divided by the plasma concentration, C:
Rc = (delta U/delta t)/ C (24)
If the plasma concentration is changing during the urinary collection
interval, the concentration at the midpoint of the interval is used
frequently. It may also be shown using equations (9), (21), and (24)
that
Rc = ku Vd (25)
which precludes the necessity of knowing the plasma concentration.
Renal clearance values for inulin measure excretion via glomerular
filtration. For man, the normal value is 125 ± 15 ml/min (Pitts,
1963). If the renal clearance of a chemical exceeds this value in man,
it constitutes evidence that the chemical is actively secreted. If it
is less, it indicates the chemical is actively reabsorbed. If the
compound is bound to a significant degree to protein, it may be
necessary to determine and use the concentration of unbound chemical
in plasma in order to obtain a realistic value for renal clearance.
4.8.2 Two-compartment/multicompartment open systems
Rapidly equilibrating compartments in which the chemical has
reached equilibrium with plasma before the first blood samples are
taken will appear kinetically as one compartment, but a "deep" or more
slowly equilibrating compartment will give rise to a plasma
concentration curve that appears biphasic. The model used to describe
this system is a two-compartment open model (Fig. 4.1). The central
and the "shallow" or rapidly equilibrating compartments are considered
as one. The major sites of metabolic transformation and excretion are
the liver and the kidneys. Since these organs are perfused with blood,
it can be assumed, generally, that they are part of the central
compartment and that elimination occurs from the central compartment.
Fig. 4.3 is a simulated plasma concentration curve representing a
two-compartment system following rapid intravenous administration of a
chemical. The chemical has first been rapidly distributed to
well-perfused tissues, then more slowly to other tissues comprising
the deep compartment.
Assuming all the transfer processes are first order, the system
of linear differential equations describing the two-compartment model
shown in Fig. 4.1 is as follows:
dC(t) k21 VD CD (t)
= - k12 C(t) - ke C(t) + (26)
dt Vd
dCD( t) k12 Vd C( t)
= - k21 CD( t) (27)
dt VD
(17)
A semilogarithmic plot of Ei versus ti will give a straight line
with slope - ke. The above expression can be modified to accommodate
unequal time intervals, but in doing so graphic insights are lost.
In using excretion data to resolve kinetic parameters, it is
desirable to keep the collection intervals as short as practical.
Ideally, the collection intervals should be shorter than the t´ for
elimination of the chemical; otherwise resolution of a biphasic
excretion pattern may be precluded. Biphasic refers to two kinetically
distinct excretion phases. For a volatile chemical excreted to some
degree by exhalation, determination of the chemical exhaled as a
function of time may be particularly useful for resolving its
biochemokinetics.
As already stated, the excretion rate of a chemical by one route
of excretion may be different from its overall rate of elimination.
This is true because the agent may be eliminated by other routes
and/or metabolically transformed. The following scheme may be used to
depict a chemical that is eliminated by a metabolic pathway as well as
by excretion in the urine and exhalation:
/ ku excretion in urine
C - kr excretion via exhalation
\ kmx metabolic transformation to compound y
In this case, the overall elimination constant will be ke = ku +
kr + kmx.
The various metabolic transformation and excretion rates may be
estimated using the following equations:
ku= U infinity( ke/ D0) (18)
kr = R infinity( ke/ D0) (19)
kmx = X infinity( ke/ D0) (20)
Uinfinity and Rinfinity are the total amounts of the parent chemical
excreted in urine and expired air. Xinfinity is the total amount of
metabolite, X, recovered from excreta. For excretion of the chemical
in the urine, the differential equation is:
dU
= ku D0 exp(- ke t) (21)
dt
The solution of the equation (21) yields
U inifnity = ku D0/ ke (22)
and
ku = U infinity ke/ D0 (23)
When the urinary excretion of a chemical is determined, it is
frequently desirable to determine its renal clearance in order to
ascertain whether the chemical is actively secreted, reabsorbed, or
only passively filtered by the kidney in the excretion process. Renal
clearance is defined as the urinary excretion rate, delta U/delta
t, divided by the plasma concentration, C:
Rc = (delta U/delta t)/ C (24)
If the plasma concentration is changing during the urinary collection
interval, the concentration at the midpoint of the interval is used
frequently. It may also be shown using equations (9), (21), and (24)
that
Rc = ku Vd (25)
which precludes the necessity of knowing the plasma concentration.
Renal clearance values for inulin measure excretion via glomerular
filtration. For man, the normal value is 125 ± 15 ml/min (Pitts,
1963). If the renal clearance of a chemical exceeds this value in man,
it constitutes evidence that the chemical is actively secreted. If it
is less, it indicates the chemical is actively reabsorbed. If the
compound is bound to a significant degree to protein, it may be
necessary to determine and use the concentration of unbound chemical
in plasma in order to obtain a realistic value for renal clearance.
4.8.2 Two-compartment/multicompartment open systems
Rapidly equilibrating compartments in which the chemical has
reached equilibrium with plasma before the first blood samples are
taken will appear kinetically as one compartment, but a "deep" or more
slowly equilibrating compartment will give rise to a plasma
concentration curve that appears biphasic. The model used to describe
this system is a two-compartment open model (Fig. 4.1). The central
and the "shallow" or rapidly equilibrating compartments are considered
as one. The major sites of metabolic transformation and excretion are
the liver and the kidneys. Since these organs are perfused with blood,
it can be assumed, generally, that they are part of the central
compartment and that elimination occurs from the central compartment.
Fig. 4.3 is a simulated plasma concentration curve representing a
two-compartment system following rapid intravenous administration of a
chemical. The chemical has first been rapidly distributed to
well-perfused tissues, then more slowly to other tissues comprising
the deep compartment.
Assuming all the transfer processes are first order, the system
of linear differential equations describing the two-compartment model
shown in Fig. 4.1 is as follows:
dC(t) k21 VD CD (t)
= - k12 C(t) - ke C(t) + (26)
dt Vd
dCD( t) k12 Vd C( t)
= - k21 CD( t) (27)
dt VD
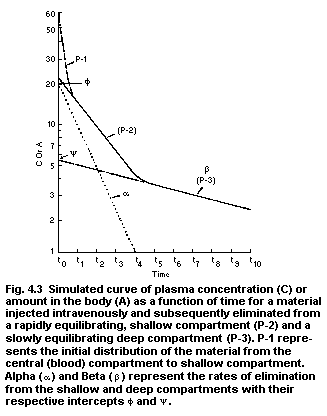 where C(t) and CD (t) are concentrations of the chemical in the
central and deep compartments respectively. The apparent volumes of
distribution for these compartments are Vd for the central
compartment and VD for the slow exchange compartment. If the
apparent volumetric flow rates between the two compartments are the
same, i.e. k12 Vd = k21 VD, the differential equation system can be
solved with initial conditions C(0) = D0/ Vd and CD(0) = 0 at time
zero to give the following mathematical representation for the solid
curve in Fig. 4.3:
C(t) = phiexp(-alpha t) + psi exp(-ß t) (28)
ß is the slope of the line for the slow phase of elimination and alpha
is the slope for the rapid phase of elimination. The value of ß is
determined as previously described and a technique called feathering
is used to obtain alpha. This technique constitutes projecting the
solid line for the slow phase backward to the origin (dash-dash line)
and subtracting the respective projected values from the experimental
values used to delineate the rapid phase of clearance. These values
are replotted (dotted line). The slope of this line is alpha. The
values for phi and psi are the intercepts at the ordinate for the
rapid and slow elimination phases, respectively.
The rate constants k12, k21, and ke (Fig. 4.1) may be
determined as follows:
phiß + psi alpha
k21 = (29)
phi + psi
alphaß
ke = (30)
k21
k12 = alpha + ß - ( k21 + ke) (31)
k12 is of particular importance because from it the amount of
chemical in the deep compartment (AD (t)) is readily calculated
from the equation
k12 D0
AD( t) = {exp(-alpha t) - exp(-ß t)} (32)
ß - alpha
Using this information, toxicologists can ascertain whether there may
be correlations between the effect of a chemical and its presence in a
deep compartment. Indeed, for the toxicologist, a prominent slow phase
for the elimination of a chemical is a red flag suggesting that with
repeated administration cumulative toxicity may constitute a problem.
These concepts developed for the plasma concentration of a
chemical conforming to a two-compartment open-model system can be
extended to describe the amount of the agent in the body or the amount
excreted. Also, an absorption component may be added which would give
a function involving the sum of three exponential terms:
C( t) = phiexp(-alpha t) + psi exp(-ß t) + (phi + psi)exp (- ka t) (33)
4.8.3 Repeated administration or repeated exposure
The concentration of a chemical in the plasma or tissues or the
amount of chemical in the body following repeated administration or
exposure is illustrated in Fig. 4.4 for a one-compartment open system.
Mathematical representation of these concentrations is obtained by
addition of the exponential terms for each dose so that the
concentration of the chemical at time t following the nth dose is
given by
where C(t) and CD (t) are concentrations of the chemical in the
central and deep compartments respectively. The apparent volumes of
distribution for these compartments are Vd for the central
compartment and VD for the slow exchange compartment. If the
apparent volumetric flow rates between the two compartments are the
same, i.e. k12 Vd = k21 VD, the differential equation system can be
solved with initial conditions C(0) = D0/ Vd and CD(0) = 0 at time
zero to give the following mathematical representation for the solid
curve in Fig. 4.3:
C(t) = phiexp(-alpha t) + psi exp(-ß t) (28)
ß is the slope of the line for the slow phase of elimination and alpha
is the slope for the rapid phase of elimination. The value of ß is
determined as previously described and a technique called feathering
is used to obtain alpha. This technique constitutes projecting the
solid line for the slow phase backward to the origin (dash-dash line)
and subtracting the respective projected values from the experimental
values used to delineate the rapid phase of clearance. These values
are replotted (dotted line). The slope of this line is alpha. The
values for phi and psi are the intercepts at the ordinate for the
rapid and slow elimination phases, respectively.
The rate constants k12, k21, and ke (Fig. 4.1) may be
determined as follows:
phiß + psi alpha
k21 = (29)
phi + psi
alphaß
ke = (30)
k21
k12 = alpha + ß - ( k21 + ke) (31)
k12 is of particular importance because from it the amount of
chemical in the deep compartment (AD (t)) is readily calculated
from the equation
k12 D0
AD( t) = {exp(-alpha t) - exp(-ß t)} (32)
ß - alpha
Using this information, toxicologists can ascertain whether there may
be correlations between the effect of a chemical and its presence in a
deep compartment. Indeed, for the toxicologist, a prominent slow phase
for the elimination of a chemical is a red flag suggesting that with
repeated administration cumulative toxicity may constitute a problem.
These concepts developed for the plasma concentration of a
chemical conforming to a two-compartment open-model system can be
extended to describe the amount of the agent in the body or the amount
excreted. Also, an absorption component may be added which would give
a function involving the sum of three exponential terms:
C( t) = phiexp(-alpha t) + psi exp(-ß t) + (phi + psi)exp (- ka t) (33)
4.8.3 Repeated administration or repeated exposure
The concentration of a chemical in the plasma or tissues or the
amount of chemical in the body following repeated administration or
exposure is illustrated in Fig. 4.4 for a one-compartment open system.
Mathematical representation of these concentrations is obtained by
addition of the exponential terms for each dose so that the
concentration of the chemical at time t following the nth dose is
given by
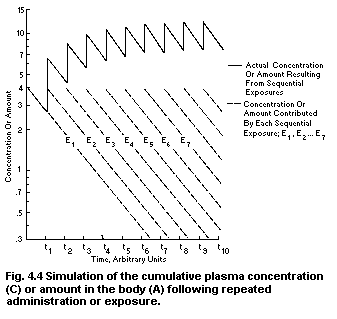
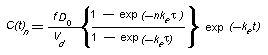 (34)
where tau is the interval between doses. After a large number of doses,
the term exp (-nketau ) approaches zero, and the value for the
concentration of chemical becomes
(34)
where tau is the interval between doses. After a large number of doses,
the term exp (-nketau ) approaches zero, and the value for the
concentration of chemical becomes
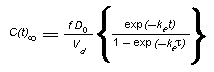 (35)
Once the plateau concentration is reached, further exposure to the
same dose at the same frequency will not result in any further
increase in concentration. At the plateau, the maximum concentration
which will occur immediately following the last exposure is given by:
(35)
Once the plateau concentration is reached, further exposure to the
same dose at the same frequency will not result in any further
increase in concentration. At the plateau, the maximum concentration
which will occur immediately following the last exposure is given by:
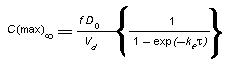 (36)
The minimum concentration will occur immediately before the next
exposure and is given by:
(36)
The minimum concentration will occur immediately before the next
exposure and is given by:
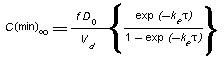 The expression defining the average concentration after the plateau
has been attained is:
contour integral D0
C(av)infinity = (38)
Vd ketau
If the exposure or the route of administration is such that the
first-order rate of absorption, ka, must be considered, the plasma
concentration following n repetitive doses at a dose interval tau is
given by:
The expression defining the average concentration after the plateau
has been attained is:
contour integral D0
C(av)infinity = (38)
Vd ketau
If the exposure or the route of administration is such that the
first-order rate of absorption, ka, must be considered, the plasma
concentration following n repetitive doses at a dose interval tau is
given by:
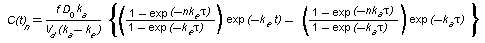 The rate constant for absorption, ka, may be replaced by the rate
constant for delivery of a substance being inhaled.
4.8.4 Kinetics of nonlinear or saturable systems
Dose-response curves for an effect arising from the
administration of a range of dose levels of a toxic agent usually
follow a log-normal distribution. Extrapolation of the logarithmic
probability transformation of these curves predicts that some
individuals will respond at an infinitesimally small dose, while
others will never respond, no matter how large the dose. The
assumption inherent in such extrapolation beyond the range of observed
data is that the chemobiokinetic profile of the compound in question
is independent of the dose level administered.
Assuming dose-independence, a 10-fold increase in the plasma
concentration of a chemical will result from a 10-fold increase in the
administered dose. However, many metabolic and excretory processes are
saturable and, as the dose of chemical begins to overwhelm these
processes, it may be expected that there will be a disproportionate
increase in toxicity. Therefore, nonlinear chemobiokinetics is of the
utmost importance in toxicology.
Many metabolic and active transfer processes as well as some
passive protein-binding processes have a finite capacity for reactions
with a chemical. The rate of these nonlinear processes can be defined
by the Michaelis-Menten equation
- dC(t) Vm C(t)
= (40)
dt Km + C(t)
where C(t) represents concentration of the chemical at time t,
Vm is the maximum rate of the process, and Km is the
concentration of chemical at which the rate of the process is equal to
one-half of Vm. Although this equation has been found useful in
delineating in vivo nonlinear kinetics, the constants should be
referred to as apparent in vivo constants, since they are
undoubtedly influenced by many other biological processes. Two
important limiting cases for this equation are as follows. When the
concentration of chemical is much smaller than Km( C( t) « Km) then
equation (40) reduces to
- dC( t) Vm
= C( t) (41)
dt Km
and the ratio of Vm/ Km will approximate an apparent first-order
rate constant. However, when the concentration is much greater than
Km (C(t) » Km) then the rate is described by
- dC( t)
= Vm (42)
dt
In this case, the rate is no longer dependent on the prevailing
concentration, but has become zero order and thus independent of
concentration.
Fig. 4.5 displays a typical concentration versus time curve for a
chemical the elimination of which follows nonlinear or
Michaelis-Menten kinetics. As long as the concentration remains
significantly less than Km, the log-linear portion of the plot is
applicable and all the principles of apparent first-order kinetics
apply. But, as the concentration approaches and then exceeds Km, the
semi-logarithm plot becomes nonlinear. In this region of zero-order
kinetics, the plot will be linear if rectangular coordinates are used.
The rate constant for absorption, ka, may be replaced by the rate
constant for delivery of a substance being inhaled.
4.8.4 Kinetics of nonlinear or saturable systems
Dose-response curves for an effect arising from the
administration of a range of dose levels of a toxic agent usually
follow a log-normal distribution. Extrapolation of the logarithmic
probability transformation of these curves predicts that some
individuals will respond at an infinitesimally small dose, while
others will never respond, no matter how large the dose. The
assumption inherent in such extrapolation beyond the range of observed
data is that the chemobiokinetic profile of the compound in question
is independent of the dose level administered.
Assuming dose-independence, a 10-fold increase in the plasma
concentration of a chemical will result from a 10-fold increase in the
administered dose. However, many metabolic and excretory processes are
saturable and, as the dose of chemical begins to overwhelm these
processes, it may be expected that there will be a disproportionate
increase in toxicity. Therefore, nonlinear chemobiokinetics is of the
utmost importance in toxicology.
Many metabolic and active transfer processes as well as some
passive protein-binding processes have a finite capacity for reactions
with a chemical. The rate of these nonlinear processes can be defined
by the Michaelis-Menten equation
- dC(t) Vm C(t)
= (40)
dt Km + C(t)
where C(t) represents concentration of the chemical at time t,
Vm is the maximum rate of the process, and Km is the
concentration of chemical at which the rate of the process is equal to
one-half of Vm. Although this equation has been found useful in
delineating in vivo nonlinear kinetics, the constants should be
referred to as apparent in vivo constants, since they are
undoubtedly influenced by many other biological processes. Two
important limiting cases for this equation are as follows. When the
concentration of chemical is much smaller than Km( C( t) « Km) then
equation (40) reduces to
- dC( t) Vm
= C( t) (41)
dt Km
and the ratio of Vm/ Km will approximate an apparent first-order
rate constant. However, when the concentration is much greater than
Km (C(t) » Km) then the rate is described by
- dC( t)
= Vm (42)
dt
In this case, the rate is no longer dependent on the prevailing
concentration, but has become zero order and thus independent of
concentration.
Fig. 4.5 displays a typical concentration versus time curve for a
chemical the elimination of which follows nonlinear or
Michaelis-Menten kinetics. As long as the concentration remains
significantly less than Km, the log-linear portion of the plot is
applicable and all the principles of apparent first-order kinetics
apply. But, as the concentration approaches and then exceeds Km, the
semi-logarithm plot becomes nonlinear. In this region of zero-order
kinetics, the plot will be linear if rectangular coordinates are used.
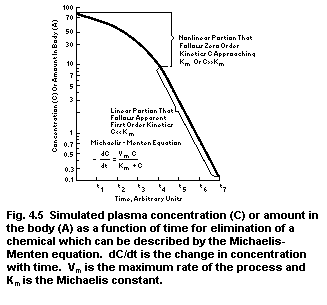 4.9 Linear and Nonlinear One Compartment Open-model Kinetics of
2,4,5-Trichlorophenoxyacetic acid (2,4,5-T)
To illustrate the use of chemobiokinetics in toxicology, some
results obtained from studies with 2,4,5-T are presented below.
2,4,5-T, a herbicide, has been reported to be teratogenic, fetotoxic,
and embryotoxic at doses of 100 mg/kg/day during the period of
organogenesis (Collins & Williams, 1971; Courtney & Moore, 1971;
Courtney et al., 1970; Roll, 1971; Sparschu et al., 1971).
4.9 Linear and Nonlinear One Compartment Open-model Kinetics of
2,4,5-Trichlorophenoxyacetic acid (2,4,5-T)
To illustrate the use of chemobiokinetics in toxicology, some
results obtained from studies with 2,4,5-T are presented below.
2,4,5-T, a herbicide, has been reported to be teratogenic, fetotoxic,
and embryotoxic at doses of 100 mg/kg/day during the period of
organogenesis (Collins & Williams, 1971; Courtney & Moore, 1971;
Courtney et al., 1970; Roll, 1971; Sparschu et al., 1971).
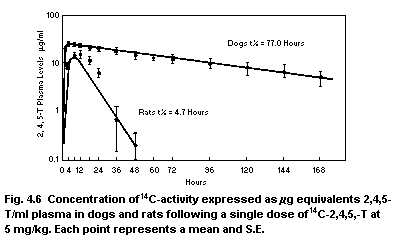 To elucidate the potential hazard of this compound, 5 mg/kg of
14C ring-labelled 2,4,5-T was administered as a single oral dose to
rats and dogs (Piper et al., 1973). The plasma concentration versus
time curves (Fig. 4.6) indicated compliance with a one-compartment
open model system having apparent first-order rates of absorption and
clearance; the t´ values for the clearance of 2,4,5-T from the
plasma of rats and dogs were 4.7 and 77 h, respectively. For
elimination from the body via the urine (Fig. 4.7), the t´ values
were 13.6 and 86.6 h. Since clearance of 2,4,5-T from the plasma of
rats was more rapid than its elimination in the urine, the compound
may have been actively concentrated in the kidneys prior to excretion
in the urine. Also, the much slower elimination by dogs than rats
correlates with the higher toxicity in dogs; the single oral LD50 is
100 mg/kg and 300 mg/kg for dogs and rats, respectively (Drill &
Heratyka, 1953; Rowe & Hymas, 1954).
Another species difference was demonstrated by the fact that
virtually all the 14C excreted by the rats was through the urine
while approximately 20% of that excreted by dogs was through the
faeces. Also, no breakdown products of 2,4,5-T could be detected in
the urine of rats given 5 mg/kg, but about 10% of the 14C activity
in the urine of dogs was attributable to breakdown products.
If an active secretory process in the kidney was the primary
elimination process in rats, then this nonlinear process should be
saturable by the administration of higher doses. Figs. 4.8 and 4.9
show that this is the case, since the t´ for both the clearance
of 2,4,5-T from plasma and its urinary elimination increase with
increasing dose. At doses of 100 or 200 mg/kg, the process was
saturated and the rates of elimination from the plasma and from the
body were the same. Further evidence of nonlinear kinetics was the
fact that a larger percentage of the 14C administered as
14C-2,4,5-T was excreted through the faeces as the dose was
increased. Also, degradation products of 2,4,5-T were found in the
urine of rats given 100 or 200 mg/kg, but not 5 or 50 mg/kg.
The nonlinear chemobiokinetics of 2,4,5-T were further
characterized following intravenous doses in rats of 5 or 100 mg/kg
(Sauerhoff et al., 1975). Clearance from the plasma of rats given
100 mg/kg followed classical Michaelis-Menten kinetics (Fig. 4.10).
The values for Vm and Km were calculated to be 16.6 ± 1.82 µg/h/g of
plasma and 127.6 ± 25.9 µg/g of plasma, respectively. During the
log-linear phase of excretion the t´ was 5.3 ± 1.2 h.
To elucidate the potential hazard of this compound, 5 mg/kg of
14C ring-labelled 2,4,5-T was administered as a single oral dose to
rats and dogs (Piper et al., 1973). The plasma concentration versus
time curves (Fig. 4.6) indicated compliance with a one-compartment
open model system having apparent first-order rates of absorption and
clearance; the t´ values for the clearance of 2,4,5-T from the
plasma of rats and dogs were 4.7 and 77 h, respectively. For
elimination from the body via the urine (Fig. 4.7), the t´ values
were 13.6 and 86.6 h. Since clearance of 2,4,5-T from the plasma of
rats was more rapid than its elimination in the urine, the compound
may have been actively concentrated in the kidneys prior to excretion
in the urine. Also, the much slower elimination by dogs than rats
correlates with the higher toxicity in dogs; the single oral LD50 is
100 mg/kg and 300 mg/kg for dogs and rats, respectively (Drill &
Heratyka, 1953; Rowe & Hymas, 1954).
Another species difference was demonstrated by the fact that
virtually all the 14C excreted by the rats was through the urine
while approximately 20% of that excreted by dogs was through the
faeces. Also, no breakdown products of 2,4,5-T could be detected in
the urine of rats given 5 mg/kg, but about 10% of the 14C activity
in the urine of dogs was attributable to breakdown products.
If an active secretory process in the kidney was the primary
elimination process in rats, then this nonlinear process should be
saturable by the administration of higher doses. Figs. 4.8 and 4.9
show that this is the case, since the t´ for both the clearance
of 2,4,5-T from plasma and its urinary elimination increase with
increasing dose. At doses of 100 or 200 mg/kg, the process was
saturated and the rates of elimination from the plasma and from the
body were the same. Further evidence of nonlinear kinetics was the
fact that a larger percentage of the 14C administered as
14C-2,4,5-T was excreted through the faeces as the dose was
increased. Also, degradation products of 2,4,5-T were found in the
urine of rats given 100 or 200 mg/kg, but not 5 or 50 mg/kg.
The nonlinear chemobiokinetics of 2,4,5-T were further
characterized following intravenous doses in rats of 5 or 100 mg/kg
(Sauerhoff et al., 1975). Clearance from the plasma of rats given
100 mg/kg followed classical Michaelis-Menten kinetics (Fig. 4.10).
The values for Vm and Km were calculated to be 16.6 ± 1.82 µg/h/g of
plasma and 127.6 ± 25.9 µg/g of plasma, respectively. During the
log-linear phase of excretion the t´ was 5.3 ± 1.2 h.
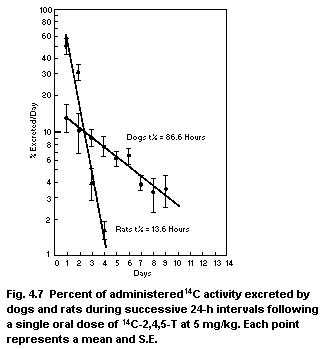
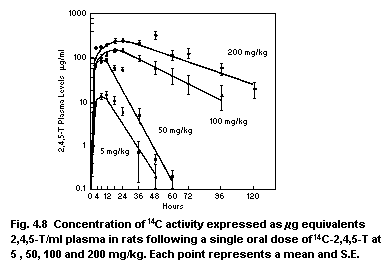 In the experiments of Sauerhoff et al. (1975), the volume of
distribution increased from 190 to 235 ml/kg in rats given 5 and
100 mg/kg, respectively. This increase in the volume of distribution
indicates that with increasing dose a larger fraction of the dose is
distributed into various tissues and cells. Thus, a disproportionate
increase in toxicity may be expected. The fate of 2,4,5-T following
oral doses of 5 mg/kg has also been investigated in man (Gehring et
al., 1973). The elimination of 2,4,5-T from the plasma and in the
urine followed apparent first order kinetics with t1/2 of 23.1 h
(Figs. 4.11, 4.12, 4.13). A comparison of the elimination rates in man
with those in rats and dogs indicates that the toxicity of 2,4,5-T to
man would lie somewhere between that to rats and dogs. The peak plasma
levels attained with a dose of 5 mg/kg, which are higher in man than
in either rats or dogs, are associated with a greater degree of plasma
protein binding in man. Also, the volume of distribution in man of
80 ml/kg is attested to the retention of 2,4,5-T in the vascular
compartment.
Fig. 4.14 illustrates simulated levels of 2,4,5-T that would be
attained in the plasma of man with repeated ingestion. If 0.25 mg/kg
were ingested daily, a level equalling that attained by ingesting a
single dose of 5 mg/kg, as in this study, would never be reached.
In the experiments of Sauerhoff et al. (1975), the volume of
distribution increased from 190 to 235 ml/kg in rats given 5 and
100 mg/kg, respectively. This increase in the volume of distribution
indicates that with increasing dose a larger fraction of the dose is
distributed into various tissues and cells. Thus, a disproportionate
increase in toxicity may be expected. The fate of 2,4,5-T following
oral doses of 5 mg/kg has also been investigated in man (Gehring et
al., 1973). The elimination of 2,4,5-T from the plasma and in the
urine followed apparent first order kinetics with t1/2 of 23.1 h
(Figs. 4.11, 4.12, 4.13). A comparison of the elimination rates in man
with those in rats and dogs indicates that the toxicity of 2,4,5-T to
man would lie somewhere between that to rats and dogs. The peak plasma
levels attained with a dose of 5 mg/kg, which are higher in man than
in either rats or dogs, are associated with a greater degree of plasma
protein binding in man. Also, the volume of distribution in man of
80 ml/kg is attested to the retention of 2,4,5-T in the vascular
compartment.
Fig. 4.14 illustrates simulated levels of 2,4,5-T that would be
attained in the plasma of man with repeated ingestion. If 0.25 mg/kg
were ingested daily, a level equalling that attained by ingesting a
single dose of 5 mg/kg, as in this study, would never be reached.
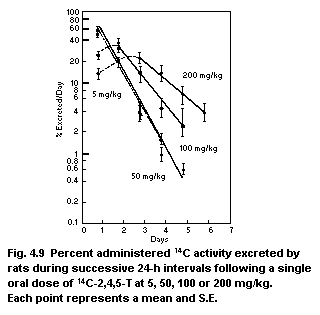 Additional studies on 2,4,5-T have demonstrated that it is
actively secreted by the kidney (Hook et al., 1974). This process of
elimination is saturable at high doses and the capacity for excretion
in dogs is more limited than in rats. As indicated previously, when
doses of 2,4,5-T are given that exceed the capacity for renal
excretion, the compound finds its way into more tissues and cells, is
eliminated more slowly, and undergoes a greater degree of metabolic
transformation. Thus, to use the toxicity incurred by high doses of
2,4,5-T to make statistical estimates of the toxicity that may be
incurred at low doses violates a basic a priori assumption.
Additional studies on 2,4,5-T have demonstrated that it is
actively secreted by the kidney (Hook et al., 1974). This process of
elimination is saturable at high doses and the capacity for excretion
in dogs is more limited than in rats. As indicated previously, when
doses of 2,4,5-T are given that exceed the capacity for renal
excretion, the compound finds its way into more tissues and cells, is
eliminated more slowly, and undergoes a greater degree of metabolic
transformation. Thus, to use the toxicity incurred by high doses of
2,4,5-T to make statistical estimates of the toxicity that may be
incurred at low doses violates a basic a priori assumption.
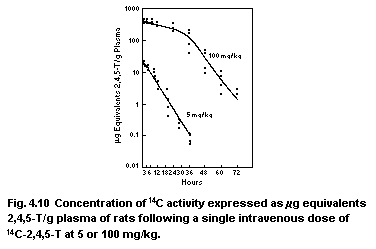
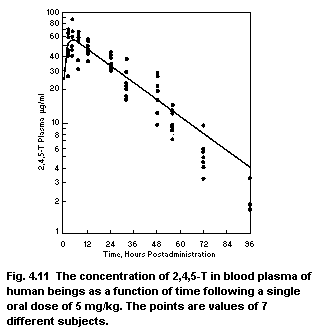
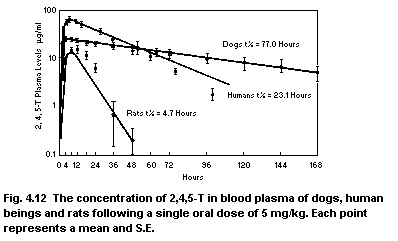
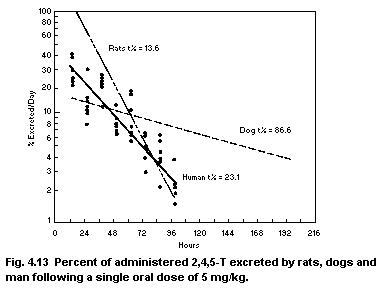
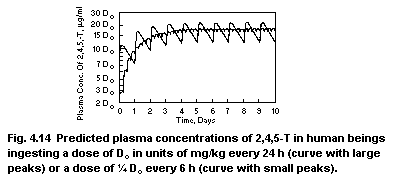 The nonlinear chemobiokinetics of toxic doses of 2,4,5-T is an
example for many other compounds (Gehring et al., 1976). Indeed, it is
likely that for most compounds, toxicity may coincide with the
saturation of the detoxification process, operative at low doses.
Recently, Gillette (1974a,b) has given special consideration to the
chemobiokinetics of reactive metabolites of chemicals that react with
macromolecules (DNA, RNA, and protein) causing toxic effects. The
concepts presented in these papers are very important to the
toxicologist because they indicate possible threshold mechanisms for
toxicity, in particular chronic toxicity.
4.10 Linear Chemobiokinetics Used to Assess Potential for
Bioaccumulation of 2,3,6,7-tetrachlorodibenzo-p-dioxin (TCDD)
TCDD is a highly toxic compound formed as an unwanted contaminant
in the manufacture of 2,4,5-trichlorophenol (Schwetz et al., 1973).
Use of trichlorophenol to manufacture 2,4,5-trichlorophenoxyacetic
acid may result in contamination of 2,4,5-T with TCDD. The
physicochemical properties of TCDD suggest that exposure to small
amounts may result in the persistent accumulation of the highly toxic
material and, eventually, in toxic effects. To elucidate the
propensity of TCDD to accumulate in the body, a series of
pharmacokinetic studies was conducted (Rose et al., 1975). In these
studies, one group of rats was given a single oral dose of 14C-TCDD
at 1 µg/kg and the excretion of 14C activity in urine, expired air,
and faeces was determined. Other groups of rats were given orally
0.01, 0.1 or 1.0 µg of 14C-TCDD/kg/day, from Monday to Friday, for
up to 7 weeks. In addition to determining the amounts of 14C
activity excreted in the urine and faeces of these rats, the amounts
remaining in the body were calculated as a function of time and the
levels of 14C-activity residing in various tissues after 1, 3, and 7
weeks of administration were determined.
Since the overall recovery of 14C in rats given a single oral
dose of 14C-TCDD was 97 ± 8%, the amounts of 14C activity
remaining in the bodies of the rats as a function of time was
calculated by subtracting the cumulative amount excreted from the
original dose. The resulting body burdens of 14C are depicted in
Fig. 4.15. The halftime for elimination of 14C from the body ranged
from 21 to 39 days. All of the 14C activity was eliminated via the
faeces.
The concentration of 14C activity in the bodies of rats given
0.1 or 1.0 µg/kg/day, from Monday to Friday, for 7 weeks as a function
of time are shown in Fig. 4.16. The data show clearly that, with
repeated exposure, the concentration of 14C activity in the body
increases but the rate of increase decreases with time and the amount
in the body begins to plateau, even though exposure continues.
The nonlinear chemobiokinetics of toxic doses of 2,4,5-T is an
example for many other compounds (Gehring et al., 1976). Indeed, it is
likely that for most compounds, toxicity may coincide with the
saturation of the detoxification process, operative at low doses.
Recently, Gillette (1974a,b) has given special consideration to the
chemobiokinetics of reactive metabolites of chemicals that react with
macromolecules (DNA, RNA, and protein) causing toxic effects. The
concepts presented in these papers are very important to the
toxicologist because they indicate possible threshold mechanisms for
toxicity, in particular chronic toxicity.
4.10 Linear Chemobiokinetics Used to Assess Potential for
Bioaccumulation of 2,3,6,7-tetrachlorodibenzo-p-dioxin (TCDD)
TCDD is a highly toxic compound formed as an unwanted contaminant
in the manufacture of 2,4,5-trichlorophenol (Schwetz et al., 1973).
Use of trichlorophenol to manufacture 2,4,5-trichlorophenoxyacetic
acid may result in contamination of 2,4,5-T with TCDD. The
physicochemical properties of TCDD suggest that exposure to small
amounts may result in the persistent accumulation of the highly toxic
material and, eventually, in toxic effects. To elucidate the
propensity of TCDD to accumulate in the body, a series of
pharmacokinetic studies was conducted (Rose et al., 1975). In these
studies, one group of rats was given a single oral dose of 14C-TCDD
at 1 µg/kg and the excretion of 14C activity in urine, expired air,
and faeces was determined. Other groups of rats were given orally
0.01, 0.1 or 1.0 µg of 14C-TCDD/kg/day, from Monday to Friday, for
up to 7 weeks. In addition to determining the amounts of 14C
activity excreted in the urine and faeces of these rats, the amounts
remaining in the body were calculated as a function of time and the
levels of 14C-activity residing in various tissues after 1, 3, and 7
weeks of administration were determined.
Since the overall recovery of 14C in rats given a single oral
dose of 14C-TCDD was 97 ± 8%, the amounts of 14C activity
remaining in the bodies of the rats as a function of time was
calculated by subtracting the cumulative amount excreted from the
original dose. The resulting body burdens of 14C are depicted in
Fig. 4.15. The halftime for elimination of 14C from the body ranged
from 21 to 39 days. All of the 14C activity was eliminated via the
faeces.
The concentration of 14C activity in the bodies of rats given
0.1 or 1.0 µg/kg/day, from Monday to Friday, for 7 weeks as a function
of time are shown in Fig. 4.16. The data show clearly that, with
repeated exposure, the concentration of 14C activity in the body
increases but the rate of increase decreases with time and the amount
in the body begins to plateau, even though exposure continues.
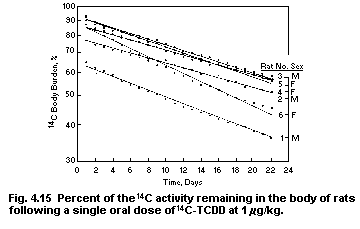
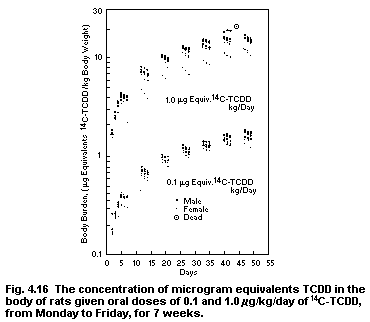 The average overall recovery of administered 14C was 97.7 ± 9%
of the cumulative dose of 14C. Mathematical analyses of the data
presented in Fig. 4.16 revealed a rate constant for excretion of TCDD
of 0.0293 ± 0.0050 days-1 which corresponds to a half-time of 23.7
days. The fraction of each dose absorbed was 0.861 ± 0.078. Using
these values, it may be calculated that the ultimate steady state body
burden would be 21.3 D0 for rats given a daily dose of D0, 5
consecutive days weekly for an infinite number of weeks. If D0 were
administered every day for an infinite time, the ultimate steady state
body burden would be 29.0 D0. Within the 7 weeks of this study, the
rats had attained 79.1% of the ultimate steady state body burden. The
time required to reach 90% of the ultimate steady state body burden
would be 78.5 days.
The concentrations of 14C-activity in the liver and fat of rats
given 14C-TCDD at concentrations of 0.01, 0.1, or 1.0 µg/kg/day,
from Monday to Friday, for 1, 3, or 7 weeks are illustrated
graphically in Figs. 4.17 and 4.18, respectively. Just like the body
burden levels, the levels in these tissues increase at a decreasing
rate and begin to plateau. It should also be noted that at each time
of measurement, there is a direct relationship between the dose being
administered and the level in the tissue. This latter observation is
illustrated more clearly in Figs. 4.19 and 4.20, where the
concentrations of 14C-activity in the liver and the fat have been
divided by the dose. This shows that over the range of doses given,
0.01 to 1.0 µg/kg/day, the relative degree of accumulation of
14C-TCDD by these tissues is not influenced by dose.
Mathematical evaluation of the data presented in Fig. 4.17-4.20
revealed that the rates for the clearance of TCDD from liver and fat
were 0.026 ± 0.000 and 0.029 ± 0.001 days-1 respectively. These
rates are essentially the same as the rate of elimination from the
body in toto, which is not unexpected because these tissues
contained the bulk of the 14C-TCDD in the body. The ultimate steady
state concentrations in liver and fat that would be attained with an
infinite duration of exposure are 0.250 ± 0.000 and 0.058 ± 0.003
D0 µg TCDD/g where D0 equals the dose being administered in µg/kg.
The times required to reach specified fractions of the ultimate steady
state concentrations would be identical no matter what dose, D0, is
being administered.
The 14C-activity in liver tissue from rats given 0.1 or
1.0 µg/kg/day, from Monday to Friday, weekly for 7 weeks, was
demonstrated by gas chromatography and by a combination of gas
chromatography and mass spectrometry to be due to 14C-TCDD. Also
important was the finding that the 14C-TCDD present in the liver was
readily extractable, indicating that TCDD does not bind irreversibly
with tissue. With regard to the assessment of the hazard of repeated
exposure to very small amounts of TCDD, the results show that TCDD
would not continue to accumulate in the body with prolonged repeated
exposure. In rats, 93% of the ultimate steady state level of TCDD in
the body would be attained within 90 days. Recently, a toxicological
evaluation of TCDD was conducted in rats given doses of 0.001, 0.01,
0.1 and 1.0 µg TCDD/kg/day, from Monday to Friday, for 13 weeks
(Kociba et al., 1975). Perceptible adverse effects did not develop in
rats given 0.001 or 0.01 µg TCDD/kg/day. Adverse effects including
hepatic pathology and functional changes, atrophy of the thymus, and
haematological alterations were observed in rats receiving 0.1 or
1.0 µg TCDD/kg/day. Indeed, some rats receiving 1.0 µg TCDD/kg/day
died. The results of the studies on the fate and accumulation of TCDD
in rats given repeated daily doses showed clearly that even with more
prolonged exposure those rats which received 0.01 µg TCDD/kg/day would
not continue to accumulate TCDD in the body and its tissues to the
extent leading to the toxic manifestations as seen in those rats
receiving 0.1 or 1.0 µg/kg/day. Since the levels of TCDD in the
tissues had essentially plateaued within 90 days, more prolonged
exposure would not be expected to lead to the attainment of toxic
amounts of TCDD in the body or its tissues.
The average overall recovery of administered 14C was 97.7 ± 9%
of the cumulative dose of 14C. Mathematical analyses of the data
presented in Fig. 4.16 revealed a rate constant for excretion of TCDD
of 0.0293 ± 0.0050 days-1 which corresponds to a half-time of 23.7
days. The fraction of each dose absorbed was 0.861 ± 0.078. Using
these values, it may be calculated that the ultimate steady state body
burden would be 21.3 D0 for rats given a daily dose of D0, 5
consecutive days weekly for an infinite number of weeks. If D0 were
administered every day for an infinite time, the ultimate steady state
body burden would be 29.0 D0. Within the 7 weeks of this study, the
rats had attained 79.1% of the ultimate steady state body burden. The
time required to reach 90% of the ultimate steady state body burden
would be 78.5 days.
The concentrations of 14C-activity in the liver and fat of rats
given 14C-TCDD at concentrations of 0.01, 0.1, or 1.0 µg/kg/day,
from Monday to Friday, for 1, 3, or 7 weeks are illustrated
graphically in Figs. 4.17 and 4.18, respectively. Just like the body
burden levels, the levels in these tissues increase at a decreasing
rate and begin to plateau. It should also be noted that at each time
of measurement, there is a direct relationship between the dose being
administered and the level in the tissue. This latter observation is
illustrated more clearly in Figs. 4.19 and 4.20, where the
concentrations of 14C-activity in the liver and the fat have been
divided by the dose. This shows that over the range of doses given,
0.01 to 1.0 µg/kg/day, the relative degree of accumulation of
14C-TCDD by these tissues is not influenced by dose.
Mathematical evaluation of the data presented in Fig. 4.17-4.20
revealed that the rates for the clearance of TCDD from liver and fat
were 0.026 ± 0.000 and 0.029 ± 0.001 days-1 respectively. These
rates are essentially the same as the rate of elimination from the
body in toto, which is not unexpected because these tissues
contained the bulk of the 14C-TCDD in the body. The ultimate steady
state concentrations in liver and fat that would be attained with an
infinite duration of exposure are 0.250 ± 0.000 and 0.058 ± 0.003
D0 µg TCDD/g where D0 equals the dose being administered in µg/kg.
The times required to reach specified fractions of the ultimate steady
state concentrations would be identical no matter what dose, D0, is
being administered.
The 14C-activity in liver tissue from rats given 0.1 or
1.0 µg/kg/day, from Monday to Friday, weekly for 7 weeks, was
demonstrated by gas chromatography and by a combination of gas
chromatography and mass spectrometry to be due to 14C-TCDD. Also
important was the finding that the 14C-TCDD present in the liver was
readily extractable, indicating that TCDD does not bind irreversibly
with tissue. With regard to the assessment of the hazard of repeated
exposure to very small amounts of TCDD, the results show that TCDD
would not continue to accumulate in the body with prolonged repeated
exposure. In rats, 93% of the ultimate steady state level of TCDD in
the body would be attained within 90 days. Recently, a toxicological
evaluation of TCDD was conducted in rats given doses of 0.001, 0.01,
0.1 and 1.0 µg TCDD/kg/day, from Monday to Friday, for 13 weeks
(Kociba et al., 1975). Perceptible adverse effects did not develop in
rats given 0.001 or 0.01 µg TCDD/kg/day. Adverse effects including
hepatic pathology and functional changes, atrophy of the thymus, and
haematological alterations were observed in rats receiving 0.1 or
1.0 µg TCDD/kg/day. Indeed, some rats receiving 1.0 µg TCDD/kg/day
died. The results of the studies on the fate and accumulation of TCDD
in rats given repeated daily doses showed clearly that even with more
prolonged exposure those rats which received 0.01 µg TCDD/kg/day would
not continue to accumulate TCDD in the body and its tissues to the
extent leading to the toxic manifestations as seen in those rats
receiving 0.1 or 1.0 µg/kg/day. Since the levels of TCDD in the
tissues had essentially plateaued within 90 days, more prolonged
exposure would not be expected to lead to the attainment of toxic
amounts of TCDD in the body or its tissues.
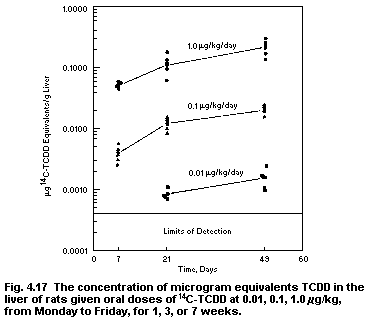
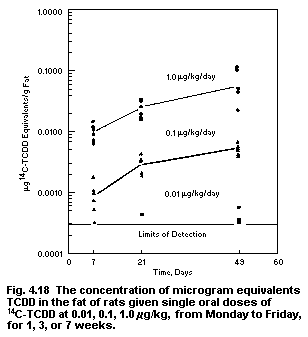
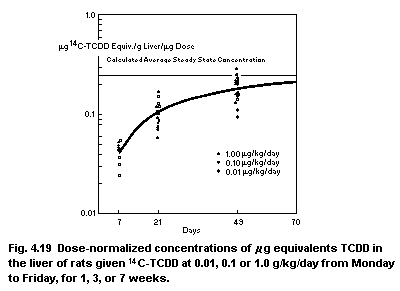
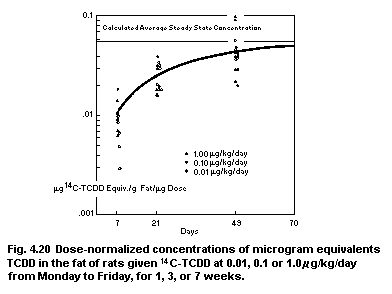 Annex
Table 4.1 Different types of drug-metabolizing reactions
I. OXIDATIONS
(a) Microsomal oxidations
(Ciaccio, 1971; Dahm, 1971; Daly, 1971; Gillette, 1971b, Gram, 1971;
Smuckler, 1971; Weisburger & Weisburger, 1971.)
Aliphatic oxidation RCH3 ----> RCH2OH
Annex
Table 4.1 Different types of drug-metabolizing reactions
I. OXIDATIONS
(a) Microsomal oxidations
(Ciaccio, 1971; Dahm, 1971; Daly, 1971; Gillette, 1971b, Gram, 1971;
Smuckler, 1971; Weisburger & Weisburger, 1971.)
Aliphatic oxidation RCH3 ----> RCH2OH
 O
/ \
Epoxidation R - CH2 - CH2 - R ----> R - CH - CH - R
O
/ \
Epoxidation R - CH2 - CH2 - R ----> R - CH - CH - R
 CH3 H
/ /
N-dealkylation R - N ----> R - N + CH2O
\ \
CH3 CH3
O-dealkylation R - O - CH3 ----> R - OH + CH2O
S-dealkylation R - S - CH3 ----> R - SH + CH2O
Table 4.1 (contd.)
Metalloalkane dealkylation Pb(C2H5)4 ----> PbH(C2H5)3
R R
\ \
N-oxidation R - N ----> R - N = O + H+
/ /
R R
CH3 H
/ /
N-dealkylation R - N ----> R - N + CH2O
\ \
CH3 CH3
O-dealkylation R - O - CH3 ----> R - OH + CH2O
S-dealkylation R - S - CH3 ----> R - SH + CH2O
Table 4.1 (contd.)
Metalloalkane dealkylation Pb(C2H5)4 ----> PbH(C2H5)3
R R
\ \
N-oxidation R - N ----> R - N = O + H+
/ /
R R
 N-hydroxylation
Sulfoxidation
N-hydroxylation
Sulfoxidation
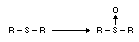 Desulfuration R R
\ \
C=S ----> C=O
/ /
R R
Desulfuration R R
\ \
C=S ----> C=O
/ /
R R
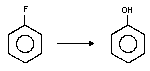 Dehalogenation
(b) Nonmicrosomal oxidations
Monoamine and diamine oxidation
O2 H2O
RCH2NH2 ---> RCH = NH ---> RCHO + NH3
Table 4.1 (contd.)
Alcohol dehydrogenation RCH2OH + NAD+ ----> R - CHO + NADH + H+
Aldehyde dehydrogenation R - CHO + NAD+ ----> R - COOH + NADH + H+
II. REDUCTIONS
(a) Microsomal reductions
Nitro reduction RNO2 ----> RNO ----> RNHOH ----> RNH2
Azo reduction RN = NR ----> RNHNHR ----> RNH2 + RNH2
Reductive dehalogenation R - CCl3 ----> R - CHCl2
(b) Nonmicrosomal reductions
R R
\ \
Aldehyde reduction C = O ----> CHOH
/ /
R R
III. HYDROLYSIS
Ester hydrolysis R - CO - O - R1 ----> R - COOH + R1 - OH
Amide hydrolysis R - CO - NH2 ----> R - COOH + NH3
Table 4.1 (contd.)
IV. CONJUGATION
(a) UDPGA-medicated conjugations
O-glucuronide formation ether type:
Dehalogenation
(b) Nonmicrosomal oxidations
Monoamine and diamine oxidation
O2 H2O
RCH2NH2 ---> RCH = NH ---> RCHO + NH3
Table 4.1 (contd.)
Alcohol dehydrogenation RCH2OH + NAD+ ----> R - CHO + NADH + H+
Aldehyde dehydrogenation R - CHO + NAD+ ----> R - COOH + NADH + H+
II. REDUCTIONS
(a) Microsomal reductions
Nitro reduction RNO2 ----> RNO ----> RNHOH ----> RNH2
Azo reduction RN = NR ----> RNHNHR ----> RNH2 + RNH2
Reductive dehalogenation R - CCl3 ----> R - CHCl2
(b) Nonmicrosomal reductions
R R
\ \
Aldehyde reduction C = O ----> CHOH
/ /
R R
III. HYDROLYSIS
Ester hydrolysis R - CO - O - R1 ----> R - COOH + R1 - OH
Amide hydrolysis R - CO - NH2 ----> R - COOH + NH3
Table 4.1 (contd.)
IV. CONJUGATION
(a) UDPGA-medicated conjugations
O-glucuronide formation ether type:
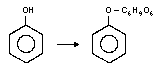 ester type:
ester type:
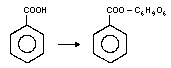 N-glucuronide formation
N-glucuronide formation
 Table 4.1 (contd.)
IV. CONJUGATION (cont'd)
S-glucuronide formation
Table 4.1 (contd.)
IV. CONJUGATION (cont'd)
S-glucuronide formation
 (b) PAPS-medicated conjugation
Sulfate ester formation
(b) PAPS-medicated conjugation
Sulfate ester formation
 Table 4.1 (contd.)
(c) Methylations
N-methylation
Table 4.1 (contd.)
(c) Methylations
N-methylation
 O-methylation
O-methylation
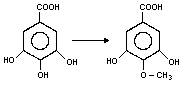 S-methylation C2H5SH -----> C2H5S-CH3
Table 4.1 (contd.)
(d) Acetylations
S-methylation C2H5SH -----> C2H5S-CH3
Table 4.1 (contd.)
(d) Acetylations
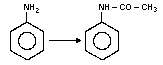 (e) Peptide conjugations
(e) Peptide conjugations
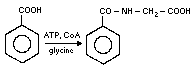 (f) Glutathione conjugations
(f) Glutathione conjugations
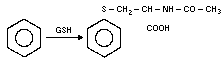 Table 4.2 Methods for the determination of several mixed-function
oxidase activities
(a) Aryl hydrocarbon hydroxylation (using 3,4-benzpyrene as
substrate)
(Nebert & Gelboin, 1968a,b; Wattenberg et al., 1962)
(b) Aliphatic side-chain hydroxylation (of pentobarbital)
(Cooper & Brodie, 1955)
(c) 4-hydroxylation (of aniline)
(Brodie & Axelrod, 1948; Chabra et al., 1972; Gilbert &
Golberg, 1965; Henderson & Kersten, 1970; Hilton & Santorelli,
1970; Imai et al., 1966; Kato & Gillette, 1965; Schenkman
et al., 1967; Sternsen & Hes, 1975)
(d) N-hydroxylation (of aniline)
(Herr & Kiese, 1959)
(e) N-oxidation (determination of amine oxides)
(Fok & Ziegler, 1970; Ziegler et al., 1973)
(f) Nitro reduction
(Fouts & Brodie, 1957; Hietbrink & DuBois, 1965)
(g) N-demethylation (of aminopyrine)
(Brodie & Axelrod, 1950; Chrastil & Wilson, 1975; Cochin &
Axelrod, 1959; Dewaide & Henderson, 1968; Feuer et al., 1971;
Kinoshita et al., 1966; Klinger, 1974; La Du et al., 1955;
MacMahon, 1962; Nash, 1953; Pederson & Aust, 1970; Poland &
Nebert, 1973; Schoene et al., 1972)
(h) N-demethylation (of benzphetamine)
(Hewick & Fouts, 1970a,b; Liu et al., 1975; Lu et al., 1969;
Nash, 1953)
(i) N-demethylation (of ethylmorphine)
(Anders & Mannering, 1966)
(j) O-demethylation (of O-nitroanisole)
(Christensen & Wissing, 1972; Kinoshita et al., 1966; Netter,
1960; Netter & Seidel, 1964; Schoene et al., 1972;
Zannoni, 1971)
(k) O-dealkylation (of ethylumbelliferone)
(Ullrich & Weber, 1972)
REFERENCES
AITIO, A. (1973) Glucuronide synthesis in the rat and guinea pig lung.
Xenobiotica, 3: 13-22.
AL-KASSAB, S., BOYLAND, E., & WILLIAMS, K. (1963) An enzyme from rat
liver catalysing conjugations with glutathione; replacement of
nitro groups. Biochem. J., 87: 4-9.
ALLEN, J. R., NORBOCK, D. H., & HSU, I. C. (1974) Tissue modifications
in monkeys as related to absorption, distribution and excretion
of polychlorinated biphenyls. Arch. environ. Contam. Toxicol.,
2: 86-95.
ANDERS, M. W. & MANNERING, G. J. (1966) Inhibition of drug metabolism.
I. Kinetics of the inhibition of the N-demethylation of
ethylmorphine by 2-diethylaminoethyl 2, 2-diphenylvalerate HCl
(SKF 525A) and related compounds. Mol. Pharmacol., 2: 319-327.
ARIAS, I. M. (1962) Chronic unconjugated hyperbilirubinaemia without
overt signs of haemolysis in adolescents and adults. J. clin.
Invest., 41: 2233-2245.
ASSICOT, M. & BOHUON, C. (1969) A simple and rapid fluorimetric
determination of catechol- O-methyl transferase activity.
Life Sci., 8: 93-100.
AXELROD, J. (1971) Methyltransferase enzymes in the metabolism of
physiologically active compounds and drugs. In: Brodie, B. B. &
Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 2, pp. 609-619.
AXELROD, J. & DALY, J. W. (1968) Phenol- O-methyl transferase.
Biochim. Biophys. Acta, 159: 472-478.
AXELROD, J. & TOMCHICK, R. (1958) Enzymatic O-methylation of
epinephrine and other catechols. J. biol. Chem., 233: 702-705.
BAKER, R. C., COONS, L. B., & HODGSON, E. (1973) Low speed preparation
of microsomes: a comparative study. Chem. biol. Interactions,
6: 307-316.
BECKETT, A. H. & HOSSIE, R. D. (1971) Buccal absorption of drugs. In:
Brodie, B. B. & Gillette, J. R., ed. Concepts in biochemical
pharmacology, Berlin, Springer-Verlag, Vol. 1, pp. 25-46.
BOND, E. J. & DE MATTEIS, F. (1969) Biochemical changes in rat liver
after administration of carbon disulfide with reference to
microsomal changes. Biochem. Pharmacol., 18: 2531-2549.
BONNICHSEN, R. K. & BRINK, N. G. (1955) Liver alcohol dehydrogenase.
In: Colowick, S. P. & Kaplan, N. O., ed. Methods in enzymology,
New York, Academic Press, Vol. 1, pp. 495-500.
BOOTH, J., BOYLAND, E., & SIMS, P. (1961) An enzyme from rat liver
catalysing conjugations with glutathione. Biochem. J.,
79: 516-524.
BORCHARDT, R. T. (1974) A rapid spectrophotometric assay for
catechol- O-methyl transferase. Anal. Biochem., 58: 382-389.
BORGÅ, O., AZARNOFF, D. L., & SJÖQVIST, F. (1968) Species differences
in the plasmaprotein binding of desipramine. J. Pharm.
Pharmacol., 20: 571.
BOYD, G. S. & SMELLIE, R. M. S. (1972) Biological hydroxylation
mechanism. New York, Academic Press.
BOYLAND, E. (1971) Mercapturic acid conjugation. In: Brodie, B. B. &
Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 2, pp. 584-608.
BOYLAND, E. & CHASSEAUD, L. F. (1967) Enzyme-catalysed conjugations of
glutathione with unsaturated compounds. Biochem. J.,
104: 95-102.
BOYLAND, E. & WILLIAMS, K. (1965) A new enzyme catalysing the
conjugations of epoxides. Biochem. J., 94: 190-197.
BRAUER, R. W. (1959) Mechanisms of bile secretion. J. Am. Med.
Assoc., 169: 1462-1466.
BROCH, O. J., JR & GULDBERG, H. C. (1971) On the determination of
catechol- O-methyl transferase activity in tissue homogenates.
Acta Pharmacol. Toxicol., 30: 266-277.
BRODIE, B. B. (1964) Physicochemical factors in drug absorption. In:
Binns, T. B., ed. Absorption and distribution of drugs,
Baltimore, Williams & Wilkins, pp. 16-48.
BRODIE, B. B. & AXELROD, J. (1948) The estimation of acetanilide and
its metabolic products, aniline, N-acetyl- p-aminophenol and
p-aminophenol (free and total conjugates) in biological fluids
and tissues. J. Pharmac. exp. Ther., 94: 22-28.
BRODIE, B. B. & AXELROD, J. (1950) The fate of aminopyrine (Pyramidon)
in man and methods for the estimation of aminopyrine and its
metabolites in biological material. J. Pharmac. exp. Ther.,
99: 171-184.
BRODIE, B. B. & HOGBEN, C. A. M. (1957) Some physicochemical factors
in drug action. J. Pharm. Pharmacol., 9: 345-347.
BURCHELL, B., DUTTON, G. J., & NEMETH, A. M. (1972) Development of
phenobarbital-sensitive control mechanisms for uridine
diphosphate glucuronyl-transferase activity in chick liver.
J. cell. Biol., 55: 448-456.
CHASE, G. D. & RABINOWITZ, J. L. (1968) Principles of radioisotope
methodology, 3rd ed. Minneapolis, Burgess Publishing Company.
CHABHRA, R. S., GRAM, T. E., & FOUTS, J. R. (1972) A comparative study
of two procedures used in the determination of hepatic microsomal
aniline hydroxylation. Toxicol. appl. Pharmacol., 22: 50-58.
CHIGNELL, C. F. (1971) Physical methods for studying drug-protein
binding. In: Brodie, B. B. & Gillette, J. R., ed. Concepts in
biochemical pharmacology, Berlin, Springer-Verlag, Vol. 1,
pp. 187-212.
CHRASTIL, J. & WILSON, J. T. (1975) A sensitive colorimetric method
for formaldehyde. Anal. Biochem., 63: 202-207.
CHRISTENSEN, F. & WISSING, F. (1972) Inhibition of microsomal
drug-metabolizing enzymes from rat liver by various
4-hydroxy-coumarin derivatives. Biochem. Pharmacol.,
21: 975-984.
CIACCIO, E. I. (1971) Intimate study of drug action. II. Fate of drugs
in the body. In: Dipalma, J. R, ed. Drills pharmacology in
medicine, New York, McGraw-Hill, pp. 36-66.
COCHIN, J. & AXELROD, J. (1959) Biochemical and pharmacological
changes in the rat following chronic administration of morphine,
nalorphine and normorphine. J. Pharmac. exp. Ther.,
125: 105-110.
COLLINS, T. F. X. & WILLIAMS, C. H. (1971) Teratogenic studies with
2,4,5-T and 2,4-D in the hamster. Bull. environ. Contam.
Toxicol., 6: 559-567.
CONNEY, A. H. (1967) Pharmacological implications of microsomal enzyme
induction. Pharmacol. Rev., 19: 317-366.
CONNEY, A. H. & BURNS, J. J. (1962) Factors influencing drug
metabolism. Adv. Pharmacol., 1: 31-54.
CONNEY, A. H., COUTINHO, C., KOECHLIN, B., SWARM, R., CHERIPHO, J. A.,
IMPELLIZERI, C., & BARUTH, H. (1974) From animals to man:
metabolic considerations. Clin. Pharmacol. Ther., 16: 176-182.
COOPER, J. R. & BRODIE, B. B. (1965) Enzymatic oxidation of
pentobarbital and thiopental. J. Pharmacol. exp. Ther.,
120: 75-87.
COTZIAS, G. C. & DOLE, V. P. (1951) Metabolism of amines. I.
Microdetermination of aminoamine oxidase in tissues.
J. biol. Chem., 190: 665-672.
COURTNEY, D. K., GAYLOR, D. W., HOGAN, M. D., FLAK, H. L., BATES, R.
R., & MITCHELL, I. (1970) Teratogenic evaluation of 2,4,5-T.
Science, 168: 864-866.
COURTNEY, D. K. & MOORE, J. A. (1971) Teratology studies with
2,4,5-trichlorophenoxyacetic acid and
2,3,7,8-tetrachlorodibenzo- p-dioxin. Toxicol. appl.
Pharmacol., 20: 396-403.
CREASEY, N. H. (1956) Factors which interfere with the manometric
assay of monoamine oxidase. Biochem. J., 64: 178-183.
DAHM, P. A. (1971) Oxidative desulfuration and dealkylation of
selected organophosphate insecticides. In: Brodie, B. B. &
Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 2, pp. 362-366.
DALY, J. (1971) Enzymatic oxidation of carbon. In: Brodie, B. B. &
Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 2, pp. 285-311.
DAVIES, D. S., GIGON, P. L., & GILLETTE, J. R. (1969) Species and sex
differences in electron transport systems in liver microsomes and
their relationship to ethylmorphine demethylation. Life Sci.,
8: 85-91.
DAVSON, H. & DANIELLI, J. F. (1952) The permeability of natural
membranes, 2nd ed. Cambridge, Cambridge Univ. Press.
DEITRICH, R. A. & ERWIN, V. G. (1969) Spectrophotometric assay for
monoamine oxidase. Anal. Biochem., 30: 395-402.
DEWAIDE, J. H. & HENDERSON, P. T. (1968) Hepatic N-demethylation of
aminopyrine in rat and trout. Biochem. Pharmacol.,
17: 1901-1907.
DRILL, V. A. & HERATJKA, T. (1953) Toxicity of
2,4-dichlorophenoxyacetic acid and 2,4,5-trichlorophenoxyacetic
acid. Arch. ind. Hyg. occup. Med, 7: 61-67.
DUTTON, G. J. (1966) The biosynthesis of glucuronides. In: Dutton, G.
J. ed. Glucuronic acid, free and combined. New York, Academic
Press.
DUTTON, G. J. (1971) Glucuronide-forming enzymes. In: Brodie, B. B. &
Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 2, pp. 378-400.
DUTTON, G. J. & STOREY, I. D. E. (1962) Glucuronide-forming enzymes.
In: Colowick, S. P. & Kaplan, N. O., ed. Methods in enzymology,
New York, Academic Press, Vol. 5, pp. 159-164.
ESTABROOK, R. W. (1971) Cytochrome P-450. Its function in the
oxidative metabolism of drugs. In: Brodie, B. B. & Gillette, J.
R., ed. Concepts in biochemical pharmacology, Berlin,
Springer-Verlag, Vol. 2, pp. 264-284.
ESTABROOK, R. W., FRANKLIN, M. R., COHEN, B., SHIGAMATSU, A., &
HILDEBRANDT, A. G. (1971) Influence of hepatic microsomal mixed
function oxidation reactions on cellular metabolic control.
Metabolism, 20: 186-199.
ESTABROOK, R. W., GILLETTE, J. R., & LEIBMAN, K. C. (1972)
Microsomes and drug oxidations. Baltimore, Williams & Wilkins.
FEUER, G., SOSA-LUCERO, J. C., LUMB, F., & MODDEL, G. (1971) Failure
of various drugs to induce drug metabolizing enzymes in
extrahepatic tissues of the rat. Toxicol. appl. Pharmacol.,
19: 579-589.
FLYNN, E. J., LYNCH, M., & ZANNONI, V. G. (1969) Species differences
in hepatic microsomal electron transport. Fed. Proc., 28: 483.
FLYNN, E. J., LYNCH, M., & ZANNONI, V. G. (1972) Species differences
and drug metabolism. Biochem. Pharmacol., 21: 2577-2590.
FOK, A. K. & ZIEGLER, D. M. (1970) Estimation of amine oxides in the
presence of hepatic microsomes. Biochem. Biophys. Res. Commun.,
41: 534-540.
FOREMAN, H. (1971) Translocation of drugs into bone. In: Brodie, B. B.
& Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 1, pp. 249-257.
FOUTS, J. R. (1970) The stimulation and inhibition of hepatic
microsomal drug-metabolising enzymes with special reference to
the effects of environmental contaminants. Toxicol. appl.
Pharmacol., 17: 804-809.
FOUTS, J. R. & BRODIE, B. B. (1957) The enzymatic reduction of
chloramphenicol, p-nitrobenzoic acid and other aromatic nitro
compounds in mammals. J. Pharmacol. exp. Ther. 119: 197-207.
FOUTS, J. R. & POHL, H. J. (1971) Further studies on the effects of
metal ions on rat liver microsomal reduced nicotinamide adenine
dinucleotide phosphate-cytochrome P-450 reductase. J. Pharmacol.
exp. Ther., 179: 91-100.
GARATTINI, S., MARCUCCI, F., & MUSSINI, E. (1975) Biotransformation of
drugs to pharmacologically active metabolites. In: Gillette, J.
R. & Mitchell, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 3, pp. 113-119.
GEHRING, P. J. & BUERGE, J. (1969) The distribution of
2,4-dinitrophenol relative to its cataractogenic activity in
ducklings and rabbits. Toxicol. appl. Pharmacol., 15: 574-592.
GEHRING, P. J., KRAMER, C. D., SCHWETZ, B. A., ROSE, J. Q., & ROWE, V.
K. (1973) The fate of 2,4,5-trichlorophenoxyacetic acid (2,4,5-T)
following oral administration to man. Toxicol. appl. Pharmacol.,
26: 352-361.
GEHRING, P. J., BLAU, G. E., & WATANABE, P. G. (1976) Pharmacokinetic
studies in evaluation of the toxicological and environmental
hazard of chemicals. In: Advances in modern toxicology -- Newer
concepts in safety evaluation. Washington, Hemisphere Publ.
Corp.
GIBSON, J. E. & BECKER, B. A. (1967) Demonstration of enhanced
lethality of drugs in hypoexcretory animals. J. pharm. Sci.,
56: 1503-1505.
GIGON, P. L., GRAM, T. E., & GILLETTE, J. R. (1969) Studies on the
rate of reduction of hepatic microsomal cytochrome P-450 by
reduced nicotinamide adenine dinucleotide phosphate. Effect of
drug substrates. Mol. Pharmacol., 5: 109-122.
GILBERT, D. & GOLBERG, L. (1965) Liver response tests. III. Liver
enlargement and stimulation of microsomal processing enzyme
activity. Food Cosmet. Toxicol., 3: 417-432.
GILLETTE, J. R. (1963) Metabolism of drugs and other foreign compounds
by enzymatic mechanisms. Arzneimittel Forsch., 6: 11-73.
GILLETTE, J. R. (1971a) Factors affecting drug metabolism. Ann. NY
Acad. Sci., 179: 43-66.
GILLETTE, J. R. (1971b) Reductive enzymes. In: Brodie, B. B. &
Gillette, J. R. ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 2, pp. 349-361.
GILLETTE, J. R. (1971c) Effects of various inducers on electron
transport systems associated with drug metabolism by liver
microsomes. Metabolism, 20: 215-227.
GILLETTE, J. R. (1973a) Overview of drug-protein binding. Ann. NY
Acad. Sci., 226: 6-17.
GILLETTE, J. R. (1973b) The importance of tissue distribution in
pharmacokinetics. J. Pharmacol. Biopharm., 1: 497-519.
GILLETTE, J. R. (1974a) A perspective on the role of chemically
reactive metabolites of foreign chemicals in toxicity. I.
Correlation of changes in covalent binding of reactivity
metabolites with changes in the incidence and severity of
toxicity. Biochem. Pharmacol., 23: 2785-2794.
GILLETTE, J. R. (1974b) A perspective on the role of chemically
reactive metabolites of foreign compounds in toxicity: II.
Alterations in the kinetics of covalent binding. Biochem.
Pharmacol., 23: 2927-2938.
GILLETTE, J. R. (1975) Other aspects of pharmacokinetics. In:
Gillette, J. R. & Mitchell, J. R., ed. Concepts in biochemical
pharmacology, Berlin, Springer-Verlag, Vol. 3, pp. 35-85.
GILLETTE, J. R., CHAN, T. W., & TERRIERE, L. C. (1966) Interactions
between DDT analogues and microsomal epoxidase systems.
J. agric. Food Chem., 14: 540-545.
GILLETTE, J. R. DAVIS, D. C., & SASAME, (1972) Cytochrome P-450 and
its role in drug metabolism. Annu. Rev. Pharmacol., 12: 57-84.
GILLETTE, J. R., MITCHELL, J. R., & BRODIE, B. B. (1974) Biochemical
mechanisms of drug toxicity. Annu. Rev. Pharmacol.,
14: 271-288.
GOLDSTEIN, A., ARONOW, L., & KOLMAN, S. M. (1974) Principles of drug
action. In: The basis of pharmacology, 2nd ed., New York,
Wiley, pp. 106-205.
GRAM, T. E. (1971) Enzymatic N-, O- and S-dealkylation of foreign
compounds by hepatic microsomes. In: Brodie, B. B. & Gillette, J.
R., ed. Concepts in biochemical pharmacology, Berlin,
Springer-Verlag, Vol. 2, pp. 334-348.
GRAM, T. E. (1973) Comparative aspects of mixed function oxidation by
lung and liver of rabbits. Drug. Metabol. Rev., 2: 1-32.
GREGORY, J. D. (1960) The effect of borate on the carbazole reaction.
Arch. Biochem. Biophys., 89: 157-159.
GREGORY, J. D. & LIPMANN, F. (1957) The transfer of sulfate among
phenolic compounds with 3,5-diphosphoadenosine as co-enzyme.
J. Biol. Chem., 229: 1081-1090.
GRIFFITHS, M. H. (1968) The metabolism of N-triphenylmorphine in the
dog and rat. Biochem. J., 108: 731-740.
HART, L. G. & FOUTS, J. R. (1965) Further studies on the stimulation
of hepatic microsomal drug metabolizing enzymes by DDT and its
analogues. Arch. exp. Pathol., 249: 486-500.
HEIRWEGH, K. P. M., VAN DER VIJER, M., & FEVERY, J. (1972) Assay and
properties of digitonin-activated bilirubin uridine diphosphate
glucuronyl-transferase from rat liver. Biochem. J.,
129: 605-618.
HENDERSON, P. TH. & KERSTEN, K. J. (1970) Metabolism of drugs during
liver regeneration. Biochem. Pharmacol., 19: 2343-2351.
HERR, F. & KIESE, M. (1959) [Determination of nitrosobenzol in the
blood.] Arch. exp. Path. Pharmakol., 235: 351-353 (in German).
HEWICK, D. S. & FOUTS, J. R. (1970a) Effects of storage on hepatic
microsomal cytochromes and substrate-induced difference spectra.
Biochem. Pharmacol., 19: 457-472.
HEWICK, D. S. & FOUTS, J. R. (1970b) The metabolism in vitro and
hepatic microsomal interactions of some enantiomeric drug
substrates. Biochem. J., 117: 833-841.
HIDAKA, H., NAGATSU, T., & YAGI, K. (1967) A rapid and simple assay of
serotonin sulfokinase activity. Anal. Biochem., 17: 388-392.
HIETANEN, E. (1974) Effect of sex and castration on hepatic and
intestinal activity of drug-metabolizing enzymes. Pharmacology,
12: 84-89.
HIETANEN, E. & VALINIO, H. (1973) Interspecies variations in small
intestinal and hepatic drug hydroxylation and glucuronidation.
Acta Pharmacol. Toxicol., 33: 57-64.
HIETBRINK, B. E. & DUBOIS, K. P. (1965) Influence of X-radiation on
development of enzymes responsible for desulfuration of an
organic phosphorothioate and reduction of p-nitrobenzoic acid
in the livers of male rats. Radiat. Res., 22: 598-603.
HILTON, J. & SARTORELLI, A. C. (1970) Induction by phenobarbital of
microsomal mixed oxidase enzymes in regenerating liver. J. biol.
Chem., 245: 4187-4192.
HOFFMAN, D. G., WORTH, H. M., & ANDERSON, R. C. (1968) Stimulation of
hepatic microsomal drug-metabolizing enzymes by alpha alpha-bis
p-chlorophenyl-3-pyridine methanol and a method for determining
no-effect levels in rats. Toxicol. appl. Pharmacol.,
12: 464-472.
HOFFMAN, D. F., WORTH, H. M., & ANDERSON, R. C. (1970) Stimulation of
hepatic drug-metabolizing enzymes by chlorophenothane (DDT); the
relationship to liver enlargement and hepatotoxicity in the rat.
Toxicol. appl. Pharmacol., 16: 171-178.
HOLTZMAN, J. L., GRAM, T. E., GIGON, P. L., & GILLETTE, J. R. (1968)
The distribution of the components of mixed function oxidase
between the rough and the smooth endoplasmic reticulum of liver
cells. Biochem. J., 110: 407-412.
HOOK, J. B., BAIBIE, M. D., JOHNSON, J. T., & GEHRING, P. J. (1974)
In vitro analysis of transport of 2,4,5-trichlorophenoxyacetic
acid (2,4,5-T) by rat and dog kidney. Food Cosmet. Toxicol.,
12: 209-218.
HUCKER, H. B. (1970) Species differences in drug metabolism.
Annu. Rev. Pharmacol., 10: 99-118.
IMAI, Y., ITO, A., & SATOR, R. R. (1966) Evidence for biochemically
different types of vesicles in the hepatic microsomal fraction.
J. Biochem., 60: 417-428.
ISSELBACHER, K. J. (1956) Enzymatic mechanisms of hormone metabolism.
II. Mechanism of hormonal glucuronide formation. In: Pincus, G.,
ed. Recent progress in hormone research, New York, Academic
Press, pp. 134-151.
JENNE, J. W. & BOYER, P. D. (1962) Kinetic characteristics of the
acetylation of isoniazid and p-aminosalicylic acid by a liver
enzyme preparation. Biochim. Biophys. Acta, 65: 121-127.
JOHNSON, M. K. (1966) Metabolism of iodomethane in the rat.
Biochem. J., 98: 38-43.
JOLLOW, P. J., MITCHELL, J. R., ZAMPOGLIONE, N., & GILLETTE, J. R.
(1974) Bromobenzene-induced liver necrosis: protective role of
glutathione and evidence for 3,4-bromobenzene oxide as the
hepatotoxic metabolite. Pharmacology, 11: 151-169.
KATO, R. & GILLETTE, J. R. (1965) Effect of starvation on
NADPH-dependent enzymes in liver microsomes of male and female
rats. J. Pharmacol. exp. Ther., 150: 279-284.
KATZ, M. & POULSEN, B. J. (1971) Absorption of drugs through the skin.
In: Brodie, B. B. & Gillette, J. R., ed. Concepts in biochemical
pharmacology, Berlin, Springer-Verlag, Vol. 1, pp. 103-174.
KEBERLE, H., HOFFMAN, K., & BERNHARD, K. (1962) The metabolism of
glutethimide (Doridnene). Experientia (Basel), 18: 105-111.
KEEN, P. (1971) Effect of binding to plasma proteins on the
distribution, activity and elimination of drugs. In: Brodie, B.
B. & Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 1, pp. 213-233.
KINOSHITA, F. K., FRAWLEY, J. P., & DUBOIS, K. P. (1966) Quantitative
measurement of induction of hepatic microsomal enzymes by various
dietary levels of DDT and toxaphene in rat. Toxicol. appl.
Pharmacol., 9: 505-513.
KLINGER, W. (1974) Optimal conditions for the first step of
amidopyrine- N-demethylation by 900 × g liver supernatant of
newborn and adult rats. Acta Biol. Med. Germ., 33: 181-186.
KUNTZMAN, R. (1969) Drugs and enzyme induction. Annu. Rev.
Pharmacol., 9: 21-36.
KURZ, H. & FRIEMEL, G. (1967) [Species-specific differences in the
binding of plasma proteins.] Arch. Pharmakol. exp. Path.,
257: 35-36 (in German).
LA DU, B. N. & SNADY, H. (1971) Esterases of human tissues. In:
Brodie, B. B. & Gillette, J. R., ed. Concepts in biochemical
pharmacology, Berlin, Springer-Verlag, Vol. 2, pp. 477-499.
LA DU, B. N., GAUDETTE, L., TROUSOF, N., & BRODIE, B. B. (1955)
Enzymatic dealkylation of aminopyrine (Pyramidon) and other
alkylamines. J. biol. Chem., 214: 741-752.
LA DU, B. N., MANDEL, G. H., & WAY, E. L. (1972) Fundamentals of drug
metabolism and drug disposition. Baltimore, Williams & Wilkins.
LEVI, A. J., GATMAITAN, Z., & ARIAS, I. M. (1969) Two hepatic
cytoplasmic protein fractions, Y and Z, and their possible role
in hepatic uptake of bilirubin, sulfobromophthalein, and other
anions, J. clin. Invest., 48: 2156-2167.
LEVINE, R. R. (1970) Factors affecting gastrointestinal absorption of
drugs. Digest Dis. 15: 171-188.
LEVY, G. & GIBALDI, M. (1972) Pharmacokinetics of drug action.
Annu. Rev. Pharmacol., 12: 85-98.
LEVY, G. & GIBALDI, M. (1975) Pharmacokinetics. In: Gillette, J. R. &
Mitchell, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 3, pp. 1-34.
LITWACK, G., KETTERER, B., & ARIAS, I. M. (1971) Ligondin: a hepatic
protein which binds steroids, bilirubin, carcinogens and a number
of exogenous organic anions. Nature (Lond.), 234: 466-467.
LIU, S. J., RAMSEY, R. K., & FALLON, H. J. (1975) Effects of ethanol
on hepatic microsomal drug-metabolizing enzymes in the rat.
Biochem. Pharmacol., 24: 369-378.
LU, A. Y. H. & LEVIN, W. (1974) The resolution and reconstitution of
the liver microsomal hydroxylation system. Biochim. Biophys.
Acta, 344: 205-240.
LU, A. Y. H., STROBEL, H. W., & COON, M. J. (1969) Hydroxylation of
benzphetamine and other drugs by a solubilized form of cytochrome
P-450 from liver microsomes: lipid requirement for drug
demethylation. Biochem. Biophys. Res. Commun., 36: 545-551.
LUCIS, O. J., SCHACKH, Z. A., & EMBIL, J. A., JR (1970) Cadmium as a
trace element and cadmium-binding components in human cells.
Experientia (Basel), 26: 1109-1112.
MACCAMAN, R. E. (1965) Microdetermination of catechol- O-methyl
transferase. Life Sci., 4: 2353-2359.
MCLEAN, A. E. M. (1971) Conversion by the liver of inactive molecules
into toxic molecules. In: Aldridge, W. N., ed. Mechanism of
toxicity, London, Macmillan, pp. 219-228.
MACMAHON, R. E. (1962) The competitive inhibition of the
N-demethylation of butynamine by 2,4-dichloro-6-
phenolphenoxy-ethylamine (DPEA). J. Pharmacol. exp. Ther.,
138: 382-386.
MACMAHON, R. E. (1971) Enzymatic oxidation and reduction of alcohols,
aldehydes and ketones. In: Brodie, B. B. & Gillette, J. R., ed.
Concepts in biochemical pharmacology, Berlin, Springer-Verlag,
Vol. 2, pp. 500-517.
MAHER, J. R., WHITNEY, J. M., CHAMBERS, J. S., & STANONIS, D. J.
(1957) The quantitative determination of isoniazid and
para-aminosalicylic acid in body fluids. Am. Rev. Tuberc.,
76: 852-861.
MANNERING, G. J. (1968) Significance of stimulation and inhibition of
drug metabolism. In: Burger, A., ed. Selected pharmaceutical
testing methods, New York, Marcel Dekker, pp. 51-119.
MANNERING, G. J. (1971a) Inhibition of drug metabolism. In: Brodie, B.
B. & Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 2, pp. 452-476.
MANNERING, G. J. (1971b) Properties of cytochrome P-450 as affected by
environmental factors: qualitative changes due to administration
of polycyclic hydrocarbons. Metabolism, 20: 228-245.
MARK, L. C. (1971) Translocation of drugs and other exogenous
chemicals into adipose tissue. In: Brodie, B. B. & Gillette, J.
R., ed. Concepts in biochemical pharmacology, Berlin,
Springer-Verlag, Vol. 1, pp. 258-275.
MARSELOS, M. & HÄNNINEN, O. (1974) Enhancement of D-glucuronolactone
and acetaldehyde dehydrogenase activities in the rat liver by
inducers of drug metabolism. Biochem. Pharmacol.,
23: 1457-1466.
MARSH, C. A. (1963) Metabolism of D-glucuronolactone in mammalian
systems; conversion of D-glucuronolactone into D-glucaric acid by
tissue preparations. Biochem. J., 87: 82-90.
MARSHALL, E. K., JR (1948) Determination of par-amino-salicylic acid
in blood. Proc. Soc. Exp. Biol. Med., 68: 471-472.
MASON, H. S. (1957) Mechanism of oxygen metabolism. Adv. Enzymol.,
19: 79-233.
MILLER, E. C. & MILLER, J. A. (1971a) The mutagenicity of chemical
carcinogens: correlations, problems and interpretations. In:
Hollaender, A., ed. Chemical mutagens, New York, Plenum,
Vol. 1, pp. 83-119.
MILLER, J. A. & MILLER, E. C. (1971b) Chemical carcinogenesis:
mechanisms and approaches to its control. J. Natl Cancer Inst.,
47: v-xiv.
NASH, T. (1953) The colorimetric estimation of formaldehyde by means
of the Hantsch reaction. Biochem. J., 55: 416-421.
NEBERT, D. W. & GELBOIN, H. V. (1968a) Substrate inducible microsomal
aryl hydroxylase. I. Assay and properties of induced enzymes.
J. Biol. Chem., 243: 6242-6249.
NEBERT, D. W. & GELBOIN, H. V. (1968b) Substrate inducible microsomal
aryl hydroxylase. II. Cellular responses during enzymic
induction. J. Biol. Chem., 243: 6250-6261.
NETTER, K. J. (1960) [A method for the direct measurement of
O-demethylization in liver microsomes and its application to
the inhibitory effects of SKF 525A on microsomes.]
Arch. Pharmacol., 238: 292-300 (in German).
NETTER, K. J. & SEIDEL, G. (1964) An adaptively stimulated
O-demethylating system in rat liver microsomes and its kinetic
properties. J. Pharmacol. exp. Ther., 146: 61-65.
NOTTON, W. R. & HENDERSON, P. TH. (1975) The influence of N-hexane
treatment on the glucuronic acid pathway and activity of some
drug-metabolizing enzymes in the guinea pig. Biochem.
Pharmacol., 24: 127- 131.
OBATA, F., USHIWATA, A., & NAKAMURA, Y. (1971) Spectrophotometric
assay of monoamine oxidase using 2,4,6-trinitrobenzene-1-sulfonic
acid. J. Biochem., 69: 349-354.
OGATA, M., TOMOKUNI, K., & TAKATSUKA, Y. (1969) Quantitative
determination in urine of hippuric acid and m- or p-methyl
hippuric acid, metabolites of toluene and m- or p-xylene.
Brit. J. ind. Med., 26: 330-334.
OMURA, T. & SATO, R. (1964a) The carbon monoxide-binding pigment of
liver microsomes: I. Evidence for its hemoprotein nature.
J. biol. Chem., 239: 2370-2378.
OMURA, T. & SATO, R. (1964b) The carbon monoxide-binding pigment of
liver microsomes: II. Solubilization, purification and
properties. J. biol. Chem., 239: 2379-2385.
OTSUKA, S. & KOBAYASHI, Y. (1964) A radioisotopic assay for monamine
oxidase determinations in human plasma. Biochem. Pharmacol.,
13: 995-1006.
PAPPENHEIMER, J. R. (1953) Passage of molecules through capillary
walls. Physiol. Rev., 33: 387-423.
PARKE, D. V. (1968) The biochemistry of foreign compounds. New York,
Pergamon Press.
PEDERSON, T. C. & AUST, S. D. (1970) Aminopyrine demethylase: kinetic
evidence for multiple microsomal activities. Biochem.
Pharmacol., 19: 2221-2230.
PIPER, W. N., ROSE, J. Q., LENG, M. L. & GEHRING, P. J. (1973) The
fate of 2,4,5-trichlorophenoxyacetic acid (2,4,5-T) following
oral administration to rats and dogs. Toxicol. appl. Pharmacol.,
26: 339-351.
PITTS, R. F. (1963) Physiology of the kidney and body fluids.
Yearbook, Chicago, Medical Publishers Inc.
PLAA, G. L. (1975) The enterohepatic circulation. In: Gillette, J. R.
& Mitchell, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 3, pp. 130-149.
PLACE, V. A. & BENSON, H. (1971) Dietary influences on therapy with
drugs. J. Mond. Pharm., 14: 261-278.
POLAND, A. P. & NEBERT, D. W. (1973) A sensitive radiometric assay of
aminopyrine N-demethylation. J. Pharmacol. exp. Ther.,
184: 269-277.
POTTS, A. M. (1964) The reaction of uveal pigments in vitro with
polycyclic compounds. Invest. Ophthalmol., 3: 405-416.
PRESCOTT, L. F. (1975) Pathological and physiological factors
affecting drug absorption, distribution, elimination and response
in man. In: Gillette, J. R. & Mitchell, J. R., ed. Concepts in
biochemical pharmacology, Berlin, Springer-Verlag,
Vol. 3, pp. 234-257.
PRICE, H. L., KOVNAT, P. J., SOFER, J. N., CONNER, E. H., & PRICE, M.
L. (1960) The uptake of thiopental by body tissues and its
relation to the duration of narcosis. Clin. Pharmacol. Ther.,
1: 16-22.
QUASTEL, J. H. (1965) Molecular transport at cell membranes.
Proc. Roy. Soc. Br., 163: 169-186.
QUINN, G. P., AXELROD, J., & BRODIE, B. B. (1958) Species, strain and
sex differences in metabolism of hexobarbitone, aminopyrine,
antipyrine and aniline. Biochem. Pharmacol., 1: 152-159.
RALL, D. P. (1971) Drug entry into brain and cerebrospinal fluid. In:
Brodie, B. B. & Gillette, J. R., ed. Concepts in biochemical
pharmacology, Berlin, Springer-Verlag, Vol. 1, pp. 240-248.
RASMUSSEN, F. (1971) Excretion of drugs by milk. In: Brodie, B. B. &
Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 1, pp. 390-402.
REMMER, H., SCHENKMAN, J., ESTABROOK, R. W., SASAME, H., GILLETTE, J.
R., NARASHIMHULU, S., COOPER, D. Y., & ROSENTHAL, O. (1966) Drug
interaction with hepatic microsomal cytochrome. Mol. Pharmacol.,
2: 187-190.
RENKIN, E. M. (1964) Transport of large molecules across capillary
walls. Physiologist, 7: 13.
REYES, H., LEVI, A. J., GATMAITAN, Z., & ARIAS, I. M. (1971) Studies
on Y and Z, two hepatic cytoplasmic organic anion-binding
proteins: Effect of drugs, chemicals, hormones and cholestosis.
J. Clin. Invest., 50: 2242-2251.
RODOMSKI, J. L., DEICHMANN, W. B., & CLIZER, E. E. (1968) Pesticide
concentrations in the liver, brain and adipose tissue of terminal
hospital patients. Food Cosmet. Toxicol., 6: 209-220.
ROLL, R. (1971) Investigations concerning the teratogenic effect of
2,4,5-T in mice. Food Cosmet. Toxicol., 9: 671-676.
ROSE, J. Q., RAMSEY, J. C., WENTZLER, T. G., HUMMEL, R. A., & GEHRING,
P. J. (1975) The fate of 2,3,7,8-tetrachlorodibenzo- p-dioxin
(TCDD) following single and repeated oral doses to the rat.
Toxicol. appl. Pharmacol., 36: 209-226.
ROSENBLUM, C. (1965) Non-metabolite residues in radioactive tracer
studies. In: Roth, L. J., ed. Isotopes in experimental
pharmacology, Chicago, University of Chicago Press,
pp. 353-360.
ROTH, L. J. (1971) The use of autoradiography in experimental
pharmacology. In: Brodie, B. B. & Gillette, J. R., ed. Concepts
in biochemical pharmacology, Berlin, Springer-Verlag,
Vol. 1, pp. 286-316.
ROWE, V. K. & HYMAS, T. A. (1954) Summary of toxicological information
of 2,4-D and 2,4,5-T type herbicides and an evaluation of the
hazards to livestock associated with their use. Am. J. Vet.
Res., 15: 622-629.
ROY, A. B. (1971) Sulfate conjugation enzymes. In: Brodie, B. B. &
Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 2, pp. 536-563.
SAUERHOFF, M. W., BRAUN, W. H., BLAU, G. E., & GEHRING, P. J. (1975)
The dose-dependent pharmacokinetic profile of
2,4,5-trichlorophenoxyacetic acid following intravenous
administration to rats. Toxicol. appl. Pharmacol., 36: 491-501.
SCHANKER, L. S. (1962a) Passage of drugs across body membranes.
Pharmacol. Rev., 14: 501-530.
SCHANKER, L. S. (1962b) Concentrative transfer of an organic cation
from blood into bile. Biochem. Pharmacol., 11: 253-254.
SCHANKER, L. S. (1963) Passage of drugs across the gastrointestinal
epithelium. In: Hogben, C. A. M., ed. Proceedings of the 1st
International Pharmacological Meeting, Oxford, Pergamon Press,
Vol. 4, pp. 120-130.
SCHANKER, L. S. (1965) Hepatic transport of organic cations. In:
Taylor, W., ed. The biliary system. Oxford, Blackwell.
SCHANKER, L. S. (1971) Absorption of drugs from the gastrointestinal
tract. In: Brodie, B. B. & Gillette, J. R., ed. Concepts in
biochemical pharmacology, Berlin, Springer-Verlag,
Vol. 1, pp. 9-24.
SCHANKER, L. S., SHORE, P. A., BRODIE, B. B., & HOGBEN, C. A. M.
(1957) Absorption of drugs from the stomach. I. The rat.
J. Pharmacol. exp. Ther., 120: 528-539.
SCHELINE, R. R. (1968) Drug metabolism by intestinal microorganisms.
J. pharm. Sci., 57: 2021-2037.
SCHENKMAN, J. B., REMMER, H., & ESTABROOK, R. W. (1967) Spectral
studies of drug interaction with hepatic microsomal cytochrome.
Mol. Pharmacol., 3: 113-123.
SCHOENE, B., FLEISCHMANN, R. A., REMMER, H., & OLDERSHAUSEN, H. F.
(1972) Determination of drug-metabolizing enzymes in needle
biopsies of human liver. Eur. J. clin. Pharmacol., 4: 65-73.
SCHOLTAN, W. (1963) On the protein binding of long-acting
sulfonamides. Chemotherapia, 6: 180-195.
SCHWETZ, B. A., NORRIS, J. M., SPARSCHU, G. L., ROWE, V. K., GEHRING,
P. J., EMERSON, J. K., & GERBIG, C. G. (1973) Toxicology of
chlorinated dibenzo- p-dioxins. Environ. Health Perspect.
(Experimental issue), 5: 87-100.
SETTLE, W., HEGEMAN, S., & FEATHERSTONE, R. M. (1971) The nature of
drug-protein interaction. In: Brodie, B. B. & Gillette, J. R.,
ed. Concepts in biochemical pharmacology, Berlin,
Springer-Verlag, Vol. 1, pp. 175-186.
SHORE, P. A., BRODIE, B. B., & HOGBEN, C. A. M. (1957) The gastric
secretion of drugs -- a pH partition hypothesis. J. Pharmacol.
exp. Ther., 119: 361-369.
SHULERT, A. R. (1961) Physiological disposition of hydralazine
(1-hydrazinophthalazine) and a method for its determination in
biological fluids. Arch. int. Pharmacodyn., 132: 1-15.
SMITH, R. L. (1971a) Excretion of drugs in bile. In: Brodie, B. B. &
Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 1, pp. 354-389.
SMITH, R. L. (1971b) The role of the gut flora in the conversion of
inactive compounds to active metabolites. In: Aldridge, W. N.,
ed. Mechanism of toxicity, London, Macmillan, pp. 229-247.
SMITH, R. L. (1973) The excretory function of bile: the elimination
of drugs and toxic substances in bile. London, England, Chapman
& Hall.
SMUCKLER, E. A. (1971) Metabolism of halogenated compounds. In:
Brodie, B. B. & Gillette, J. R., ed. Concepts in biochemical
pharmacology, Berlin, Springer-Verlag, Vol. 2, pp. 367-377.
SPARSCHU, G. L., DUNN, F. L., LISOWE, R. W. & ROWE, V. K. (1971) Study
of the effects of high levels of 2,4,5-trichlorophenoxyacetic
acid on fetal development in the rat. Food Cosmet. Toxicol.,
9: 527-530.
SPERBER, I. (1963) Drugs and membranes. In: Hogben, C. A. M., ed.
Proceedings of the 1st International Pharmacological Meeting,
Oxford, Pergamon Press, Vol. 4.
STERRSON, L. A. & HES, J. (1975) Electrochemical method for the
determination of aniline hydroxylation. Anal. Biochem.,
67: 74-80.
STOTZ, E., STEINBERG, M. S., COHEN, S. N., & WEBER, W. W. (1969)
Acetylation of 5-hydroxytryptamine by isoniazide
N-acetyltransferase. Biochim. Biophys. Acta, 184: 210-212.
STOWE, C. M. & PLAA, G. L. (1968) Extrarenal excretion of drugs and
chemicals. Annu. Rev. Pharmacol., 8: 337-356.
STRICKLAND, R. D., GREGORY, D. H., & LYNCH, J. L. (1974) A method for
assaying hepatic morphine UDP-glucuronyl transferase. Biochem.
Med., 11: 180-188.
STURMAN, J. A. & SMITH, M. J. H. (1967) The binding of salicylate to
plasma proteins in different species. J. Pharm. Pharmacol.,
19: 621-623.
SUGA, T., OHATA, I., KUMAOKA, H., & AKAGI, M. (1967) Studies on
mercapturic acids: investigation of glutathione-conjugating
enzyme by the method of thin-layer chromatography.
Chem. Pharmacol. Bull., 15: 1059-1064.
TABOR, H., MEHLER, A. H., & STADTMAN, E. R. (1953) The enzymatic
acetylation of amines. J. biol. Chem., 204: 127-138.
TAKAHASHI, H. & TAKAHARA, S. (1968) A sensitive fluorimetric assay for
monoamine oxidase based on the formation of 4,6-quinolinediol
from 5-hydroxykynurenamine. J. Biochem., 64: 7-11.
TEORELL, T. (1937a) Kinetics of distribution of substances
administered to the body: I. The extra-vascular modes of
administration. Arch. int. Pharmacodyn., 57: 205-226.
TEORELL, T. (1937b) Kinetics of distribution of substances
administered to the body: II. The intra-vascular modes of
administration. Arch. int. Pharmacodyn., 57: 227-240.
TUFVESSON, G. (1970) Fluorimetric determination of amine oxidase
activity in human blood serum with kynuramine as substrate.
Scand. J. clin. Lab. Invest., 26: 151-154.
ULLRICH, V. & WEBER, P. (1972) The O-dealkylation of
7-ethoxycoumarin by liver microsomes: a direct fluorimetric test.
Z. Physiol. Chem., 353: 1171-1177.
WAGNER, J. P. (1968) Pharmacokinetics. Ann. Rev. Pharmacol.,
8: 67-94.
WAGNER, J. P. (1971) Biopharmaceutics and relevant pharmacokinetics.
Hamilton, Drug Intelligence Publications.
WATTERNBERG, L. W. & LEONG, J. L. (1971) Tissue distribution studies
of polycyclic hydrocarbon hydroxylase activity. In: Brodie, B. B.
& Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 2, pp. 422-430.
WATTENBERG, L. W., LEONG, J. L., & STRAND, P. J. (1962) Benzpyrene
hydroxylase activity in the gastrointestinal tract. Cancer Res.,
22: 1120-1125.
WEBER, W. W. (1970) N-acetyltransferase (mammalian liver). In:
Tabor, H. & Tabor, C. W., ed. Methods in enzymology, 17B. New
York, Academic Press.
WEBER, W. W. (1971) Acetylating, deacetylating and amino
acid-conjugating enzymes. In: Brodie, B. B. & Gillette, J. R.,
ed. Concepts in biochemical pharmacology, Berlin,
Springer-Verlag, Vol. 2, pp. 564-583.
WEBER, W. W. & COHEN, S. N. (1968) The mechanism of isoniazid
acetylation by human N-acetyltransferase. Biochim. Biophys.
Acta, 151: 276-278.
WEBER, W. W., COHEN, S. N., & STEINBERG, M. S. (1968) Purification and
properties of N-acetyltransferase from mammalian liver.
Ann. NY Acad. Sci., 151: 734-741.
WEINER, I. M. (1971) Excretion of drugs by the kidney. In: Brodie, B.
B. & Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 1, pp. 328-353.
WEISBURGER, H. H. & WEISBURGER, E. R. (1971) N-oxidation enzymes.
In: Brodie, B. B. & Gillette, J. R., ed. Concepts in biochemical
pharmacology, Berlin, Springer-Verlag, Vol. 2, pp. 312-333.
WEISBURGER, H. H., GRANTHAM, P. H., & WEISBURGER, E. R. (1964)
Metabolism of N-2-fluorenylacetamide in the hamster. Toxicol.
appl. Pharmacol., 6: 427-433.
WEISSBACH, H., SMITH, T. E., FALY, J. W., WITKOP, B., & UDENFRIEND, S.
(1960) A rapid spectrophotometric assay of monoamine oxidase
based on the rate of disappearance of kynuramine. J. Biol.
Chem., 235: 1160-1163.
WILLIAMS, C. H., JR & KAMIN, H. (1962) Microsomal triphosphopyridine
nucleotide-cytochrome c reductase of liver. J. Biol. Chem.,
237: 587-595.
WILLIAMS, R. T. (1965) The influence of enterohepatic circulation on
toxicity of drugs. Ann. NY Acad. Sci, 123: 110-124.
WILLIAMS, R. T. (1967a) The biogenesis of conjugation and detoxication
products. In: Bernfield, P, ed. Biogenesis of natural compounds,
2nd ed., Oxford, Pergamon Press, pp. 589-639.
WILLIAMS, R. T. (1967b) Comparative patterns of drug metabolism.
Fed. Proc., 26: 1029-1039.
WILLIAMS, R. T. (1971) Introduction: Pathways of drug metabolism. In:
Brodie, B. B. & Gillette, J. R., ed. Concepts in biochemical
pharmacology, Berlin, Springer-Verlag, Vol. 2, pp. 226-242.
WITIAK, D. T. & WHITEHOUSE, M. W. (1969) Species differences in the
albumin binding of 2,4,6-trinitrobenzaldehyde, chlorphenoxyacetic
acids, 2-(4'-hydroxybenzeneazo) benzoic acid and some other
drugs; the unique behaviour of rat albumin. Biochem. Pharmacol.,
18: 971-977.
WITSCHI, H. (1975) Exploitable biochemical approaches for evaluation
of toxic lung damage. In: Hayes, W. J. Jr., ed. Essays in
toxicology, New York, Academic Press, Vol. 6, pp. 125-191.
YUKI, H. & FISHMAN, W. H (1963) A carbazole method for the
differential analysis of glucuronate, glucosiduronate and
hyaluronate. Biochim. Biophys. Acta, 69: 576-578.
ZAKIM, D. & VESSEY, D. A. (1973) Techniques for the characterization
of UDP-glucuronyl-transferase, glucose-6-phosphatase and other
tightly bound microsomal enzymes. In: Glick, D., ed. Methods of
biochemical analysis, New York, Wiley, Vol. 21, pp. 2-37.
ZANNONI, V. G. (1971) Experiments illustrating drug distribution and
excretion. In: La Du, B. N., Mandel, H. G, & Way, E. L, ed.
Fundamentals of drug metabolism and drug disposition,
Baltimore, Williams & Wilkins, pp. 583-590.
ZANNONI, V. G., FLYNN, E. J., & LYNCH, M. (1972) Ascorbic acid and
drug metabolism. Biochem. Pharmacol., 21: 1377-1392.
ZELLER, E. A. (1971) Amine oxidases. In: Brodie, B. B. & Gillette, J.
R., ed. Concepts in biochemical pharmacology, Berlin,
Springer-Verlag, Vol. 2, pp. 518-535.
ZIEGLER, D. M., MCKEE, E. M., & POULSEN, L. L. (1973) Microsomal
flavo-protein-catalyzed N-oxidation of arylamines. Drug Metab.
Disposition, 1: 314-320.
5. MORPHOLOGICAL STUDIES
5.1 Introduction
Morphological studies are often the corner stones of toxicity
experiments. The variety of such studies leads to many different
approaches from the viewpoint of pathology, and skill and flexibility
in working procedures seem to be far more important than a strict
schedule.
On the other hand, it is advisable to have some general
guidelines on pathological procedures for routine quality testing for
toxicity, even though specific questions may often be posed, special
experiments may have to be carried out, and special animals,
techniques, and examinations used in order to elucidate certain
problems.
This chapter deals with the various phases of morphological
studies with a view to providing some general recommendations
concerning the procedures to be followed. It must be emphasized,
however, that these recommendations are only a guide, and that it will
be the pathologist's special responsibility to see that studies are
carried out in the way most likely to ensure optimum results.
5.2 General Recommendations
Gross necropsy facilities should be in close proximity to those
of the pathologist. The autopsy room must be equipped with adequate
dissection tables, dissection materials, running water, drains,
lighting, ventilation, and facilities for disinfection. In addition,
gross photography facilities are necessary. Cooling facilities must be
available for the storage of dead animals until necropsy, but the
animals should not be frozen (Sontag et al., 1975). To carry out
proper experiments and to prevent the loss of a considerable number of
animals by cannibalism or autolysis, it is essential that animals be
observed at least once a day, including Saturdays and Sundays. Animals
in moribund condition should be killed.
For the trimming of fixed tissues, a well-ventilated area,
preferably with an exhaust hood, and running water and drains, is
required.
The histology laboratory should be separated from the autopsy
room and should be equipped with tissue-processing equipment,
microtomes, cryostat, embedding and staining facilities, and supplies
(Sontag et al., 1975). Storage facilities are necessary for the fixed
tissues, as well as for the tissue block and histological slide files.
The facilities should be vermin-proof and temperature controlled or at
least cool.
Veterinary or medical pathologists with experience in laboratory
animal pathology or others with appropriate training and experience
should be responsible for all pathology procedures. In addition, the
pathologist should participate in the design and conduct of
experiments.
Histology technicians with appropriate training and experience in
the histological field will guarantee good histotechnical work,
whereas technicians trained and experienced in laboratory animal
dissection will be of great help in performing necropsies. Technicians
must be able to recognize and adequately describe gross abnormalities.
Personnel should be available at the weekends to perform
necropsies on animals found dead or killed in extremis.
5.3 Gross Observations
Gross pathology can be performed in most cases by experienced
technicians under the guidance of the pathologist. They should follow
a certain scheme of necropsy technique and must be able to recognize
abnormalities. A checklist should be used to ensure that all organs
and tissues are inspected and dissected. Information on clinical signs
must be available. Abnormalities found must be recorded on autopsy
cards. The descriptions must be clear and provide details such as
location, size, colour, texture, number, etc.
A great number of lesions will be within the scale of known
abnormalities for the animal species and breed used. Lesions found
beyond this normal scale should always be examined by the pathologist
himself. It is advisable for all new lesions, especially those
attributable to the treatment, to be photographed.
Dead animals should be necropsied unless cannibalism or autolysis
precludes this. Autolysis should not readily be accepted as an excuse
for not performing an autopsy. An inadequate gross necropsy cannot be
replaced by microscopic examination, no matter how well performed. On
the other hand, a well-performed gross necropsy may provide optimum
information for microscopic examination and may, in certain cases,
facilitate more selective microscopic examination.
Several ways of killing animals are available and the methods
depend on the facilities, the purpose of the experiment, and the
species used. Sacrifice of animals by anaesthesia (by ether or
barbiturates) is widely used as well as asphyxiation by carbon
dioxide. In rodents, the blood of the body can be removed by cardiac
puncture or puncturing the abdominal aorta, and in dogs by puncture of
the common carotid artery. Alternatively in small rodents decapitation
can be performed, though this may result in blood aspiration and
damage to certain tissues or organs. On the other hand decapitation
will probably give more constant results with respect to the total
blood loss, and consequently lead to fairly consistent organ weights.
5.3.1 Autopsy techniques
It is difficult, if not impossible, to prescribe working
procedures or techniques that will be adequate in all conditions.
Often, observations made when the animals were alive or experience
with other structurally-related compounds may focus attention on
certain organs or tissues. Sometimes fixation by perfusion is
necessary for proper examination of specific tissues (for example, the
central nervous system).
Large numbers of animals should not be sacrificed at any one
time, if too few technicians are available to perform the necropsies.
Animals should be killed in sequence, especially where it is necessary
to sample tissues for biochemical, enzyme-histochemical, or analytical
studies. However, organs and tissues should be weighed as soon as
possible after death to ensure that they have not dried out and that
they can be fixed quickly. Special procedures may shorten the time
needed for full autopsy especially in the case of small rodents. The
exact organization of working procedures depends largely on the
facilities and labour available, but good preparation and teamwork are
crucial factors.
Sacrifice should be carried out so that animals of the
experimental and control groups are killed at approximately the same
time of day, thus preventing the introduction of variations in
physiological status dependent on circadian rhythm. In certain cases,
however, this may not be feasible.
Special care is necessary when test elements or compounds are
analysed after sacrifice. At times it may be necessary to kill the
animals in a given order to prevent contamination (e.g. sacrifice of
the control first, followed by the lowest dose level, then the
intermediate dose level, and finally the high-dose level).
Autopsy techniques have already been described (Roe, 1965) and
only a general outline of the procedures is given.
5.3.2 Rat, mouse, guineapig, rabbit, monkey
The necropsy starts with external examination including the body
orifices. Thereafter, the animals are fixed on their backs. After a
median incision, the skin is partly removed and the subcutis,
superficial lymph nodes, salivary glands, and mammary glands can be
inspected and dissected. The abdomen can be opened and the negative
pressure of the thorax may be checked and the thorax opened.
Thyroid and thymus can then be inspected and dissected.
Trachea, lungs, and heart are removed en bloc. The heart is
detached, but the trachea and lungs remain intact to provide easy
handling for inflation of the lungs with fixative (see under fixation
methods).
Abdominal organs are removed. The examination of liver and kidney
should include making a routine number of parallel slices of the
organs to examine their cut surfaces. The gastrointestinal tract
should be removed from the abdominal cavity and opened. In chronic
toxicity experiments, the entire gastrointestinal tract must be
opened, the mucosal surface examined, and representative parts taken
for histological examination. The oesophagus and parts of the
intestine can be rolled according to the Swiss roll technique in order
to obtain a considerable length of mucosa musculature and serosa in
the microscopic section. Brain, peripheral nerves, skeletal
musculature, and bones (including a joint) are removed. The spinal
cord may best be fixed in situ.
5.3.3 Carnivores, swine
After external examination, necropsy is performed when the animal
is lying on its right side. Left front and hind legs are removed
without opening the apertura thoracis superior. The skin is partly
removed after a median ventral incision from mouth to os pubis.
Subcutis, fat, mammary glands, salivary glands, and superficial lymph
nodes may be inspected and dissected. In addition, the different
joints of the legs are opened and inspected. After opening the
abdomen, the thorax is checked for negative pressure and opened by
removing the left side of the thoracic wall. Then the right side of
the pleural cavity is inspected. The oesophagus is removed by cutting
it cranially. The thoracic and abdominal organs are removed and
individually inspected and opened. The nasopharynx is then opened and
inspected, and the tongue, larynx, pharynx, tonsils, brain, spinal
cord, peripheral nerves, and musculature collected. Most organs should
be sliced in parallel slices to examine the cut surfaces.
5.4 Selection, Preservation, Preparation, and Storage of Tissues
5.4.1 Selection of tissues
Which organs and tissues should be collected for fixation is
determined by the type of toxicity experiment and the compound being
tested. Sufficient material should be collected to prevent the
necessity of having to repeat a certain experiment. Since it is
impossible to provide strict rules for selection, only a general
outline will be given.
5.4.2 Oral toxicity tests
In acute toxicity experiments, restrictive microscopic
examination may be necessary in certain cases. Some laboratories fix
liver and kidneys, others do not. The additional information obtained
from histological examination of these organs in acute experiments is,
usually, limited.
In range-finding tests, restrictive pathology is common practice.
Normally, the heart, liver, spleen, and kidneys and all grossly
abnormal tissues are collected for fixation. When the compound tested
is given by stomach tube, it is advisable to include lungs,
oesophagus, and stomach in the histological study. In subacute
(90-day) and chronic studies, it is advisable to select all tissues
and organs for fixation in buffered formal saline. This normally
includes the following organs and tissues:
brain gall bladder (if present)
pituitary oesophagus
thyroid (parathyroid) stomach
thymus duodenum
(trachea) jejunum
lungs ileum
heart caecum
sternum (bone marrow) colon
salivary glands rectum
liver urinary bladder
spleen lymph nodes -- mandibular or mesenteric
kidneys lymph nodes -- popliteal or axillary
adrenals mammary glands
pancreas (thigh) musculature
gonads peripheral nerve
accessory genital organs (eyes)
aorta (femur -- incl. joint)
(skin) spinal cord (at three levels)
(exorbital lachrymal glands)
The tissues mentioned between brackets are sometimes considered to be
optional. In addition, all tissues containing grossly observed lesions
should be fixed.
The same selection is usually made in carcinogenicity tests. In
both chronic toxicity and carcinogenicity experiments, it is also very
important to collect all organs and tissues of animals that have died
or have been killed in extremis. Prompt examination of the tissues
of these animals may provide valuable information leading to a more
meaningful pathological examination of animals, that die or are
sacrificed later on, or at the end of the experiment. In cases where
only part of an organ or tissue is taken, it is important that the
same part of that organ or tissue be selected at approximately the
same site in all animals.
5.4.3 Inhalation toxicity studies
In acute inhalation studies, it may be worthwhile, in some cases,
to select lungs for fixation and subsequent microscopic examination,
as well as liver and kidneys. In most cases, however, this seems
unnecessary since the pulmonary lesions are usually of the same type
(hyperaemia and oedema) and can be detected by gross inspection.
Examination of the entire respiratory tract is necessary in
subacute inhalation studies. This includes nasal cavity, pharynx,
larynx, trachea, main bronchi, and lungs. The exact orientation of
these organs for trimming, embedding, and sectioning is of prime
importance and needs special attention. More organs and tissues may be
selected in the inhalation studies, selection usually being effected
in the same way as for oral studies.
5.4.4 Dermal toxicity studies
In acute dermal toxicity studies, it is advisable to perform
microscopic pathology on liver and kidneys. The presence of
degenerative changes in these organs indicates that the compound is
active transdermally. Examination of the skin is also advisable.
In subacute dermal toxicity studies, the organs and tissues are
selected in the same way as that described for oral tests. Of course,
the skin deserves special attention in that both treated areas and
normal skin in comparable areas are selected.
5.4.5 Special studies
In the case of sensitization or irritation studies, the selection
of tissues depends completely on the type of test. In eye irritation
studies, the examination of eye and conjunctivae seems adequate
whereas in a Landstainer-Draize test, and especially in a maximization
test, the skin may be examined. In all these cases, it may, or may
not, be necessary to select more tissues.
5.5 Preservation of Tissues
Preservation of tissues can be performed by immersion, inflation
or distension, and perfusion. Immersion is most commonly used, but in
some circumstances may not result in satisfactory preservation.
Microscopic examination of the lungs, for example, cannot be done
adequately if they have not been inflated or have not been fixed by
perfusion. When the central nervous system is a target organ, it is an
absolute necessity to use perfusion to ensure proper fixation, as
fixation by immersion leads to many artifacts that are
indistinguishable from certain degenerative changes. When animals in
long-term tests are killed in extremis, it is advisable to use
fixation by perfusion. Perfusion is usually essential for electron
microscope studies of tissues.
With perfusion, the best conditions for microscopic examination
are obtained while effective gross examination remains possible. All
tissues should be fixed in 10% neutral buffered formalin or another
appropriate fixative.
5.5.1 Immersion
Immersion is the most used and usually the most appropriate
method of preservation in toxicity experiments. The tissues are placed
in the preservative and fixed for 24-48 h. Fixation at higher
temperatures (40-50°C), in a vacuum, or by use of microwaves (Gordon &
Daniel, 1974) may shorten the fixation time considerably. Tissues
thicker than 0.5 cm should not be fixed. The preservative/tissue ratio
is very important and must be greater than 10:1 (volume fixation
fluid/volume tissue) to obtain acceptable fixation. The fixation of
intact animals with opened abdomen should not be carried out. All
tissues and organs must be fixed separately, and some may need special
attention. Skin and peripheral nerves must be fixed in a straight (but
not stretched) and flat position. To achieve this, these tissues can
first be attached to a piece of thick filter paper. The spinal cord
can best be fixed in situ before it is taken out. Special fixing
solutions may be used in certain cases such as Bouin's fixative for
the fixation of ovaries, testes, thyroid, and adrenals or Zenker's
solution for the fixation of the eyes.
As certain organs (pituitary, thyroid, ovaries, adrenals, and
lymph nodes) of some of the smaller rodents are rather small, they
should be fixed separately in smaller jars to prevent loss; various
fixatives may be used.
5.5.2 Inflation
Inflation is used to preserve lungs effectively. To prevent the
formation of artifacts that may be misjudged as emphysema, it is
necessary for inflation to be carried out at constant pressure
(Chevalier, 1971; Fawell & Lewis, 1971). In certain cases, however,
even fixation of the lungs by inflation may not be adequate and
perfusion will be preferred (for example, in inhalation studies).
Inflation with fixative is also necessary for correct fixation of
the urinary bladder. If the bladder is distended, urine must be
replaced by fixative via the urethra using a syringe with a blunt
needle. Contracted empty bladders should be partly distended with
fixative. The reflux of fixative in the bladder is prevented by
ligation of the urethra. Inflation may also be used for fixation of
the digestive tract. Here again, ligation is necessary. In these
cases, the organs have to be bisected after fixation and the interior
surface inspected.
5.5.3 Perfusion
The best way to preserve tissues is by perfusion, which is
usually effected by infusion in the left ventricle of the heart and by
opening the sinus venosus to provide for proper circulation. Perfusion
of isolated organs (liver, kidneys) is another possibility. Before
perfusion, the animals are anaesthetized with a barbiturate,
administered in combination with nitrate and heparin to ensure
vasodilation and prevent clotting. A solution consisting of 77.5 ml
sodium nitrite (1.25%) in water, 10 ml heparin (5000 IU/ml) and 12 ml
pentobarbital (60 mg/ml), of which 10 ml is administered per kg body
weight, is satisfactory. Then the blood is removed with an isotonic
saline solution using slight overpressure. When all the blood has left
the body via the sinus venosus or right atrium, the body may be
perfused with the fixative.
Perfusion is sometimes not possible especially in experiments
where the recording of organ weights is important (i.e. in subchronic
toxicity studies). This difficulty can be solved by increasing the
number of experimental animals, though this may lead to a considerable
increase in costs. Perfusion of large numbers of animals is possible
using simple facilities at low cost (Fig. 5.1).
5.6 Trimming
It is important that the trimming be carried out by well-trained
people. It must be emphasized that knowledge of pathological phenomena
is necessary, as it frequently happens that the person responsible for
trimming cuts out the "good looking" areas and discards the tumours
present in the organs. The tissues must be sliced in such a way that
the cut surfaces present the largest possible area for examination.
The use of a special trimming scheme during the procedure may be
helpful.
The kidneys should be sectioned through the cortex and medulla,
one kidney mid-longitudinally, the other mid-transversely.
The brain must be cross-sectioned at, at least, three sites: the
frontal cortex with basal ganglia, parietal cortex with thalamus, and
cerebellum with pons.
The lungs should be sectioned transversely, parallel to the long
axis of the body. These sections must include the main bronchi and
carina.
The hollow organs should be trimmed in such a way that a
cross-section from mucosa to serosa is obtained.
Table 4.2 Methods for the determination of several mixed-function
oxidase activities
(a) Aryl hydrocarbon hydroxylation (using 3,4-benzpyrene as
substrate)
(Nebert & Gelboin, 1968a,b; Wattenberg et al., 1962)
(b) Aliphatic side-chain hydroxylation (of pentobarbital)
(Cooper & Brodie, 1955)
(c) 4-hydroxylation (of aniline)
(Brodie & Axelrod, 1948; Chabra et al., 1972; Gilbert &
Golberg, 1965; Henderson & Kersten, 1970; Hilton & Santorelli,
1970; Imai et al., 1966; Kato & Gillette, 1965; Schenkman
et al., 1967; Sternsen & Hes, 1975)
(d) N-hydroxylation (of aniline)
(Herr & Kiese, 1959)
(e) N-oxidation (determination of amine oxides)
(Fok & Ziegler, 1970; Ziegler et al., 1973)
(f) Nitro reduction
(Fouts & Brodie, 1957; Hietbrink & DuBois, 1965)
(g) N-demethylation (of aminopyrine)
(Brodie & Axelrod, 1950; Chrastil & Wilson, 1975; Cochin &
Axelrod, 1959; Dewaide & Henderson, 1968; Feuer et al., 1971;
Kinoshita et al., 1966; Klinger, 1974; La Du et al., 1955;
MacMahon, 1962; Nash, 1953; Pederson & Aust, 1970; Poland &
Nebert, 1973; Schoene et al., 1972)
(h) N-demethylation (of benzphetamine)
(Hewick & Fouts, 1970a,b; Liu et al., 1975; Lu et al., 1969;
Nash, 1953)
(i) N-demethylation (of ethylmorphine)
(Anders & Mannering, 1966)
(j) O-demethylation (of O-nitroanisole)
(Christensen & Wissing, 1972; Kinoshita et al., 1966; Netter,
1960; Netter & Seidel, 1964; Schoene et al., 1972;
Zannoni, 1971)
(k) O-dealkylation (of ethylumbelliferone)
(Ullrich & Weber, 1972)
REFERENCES
AITIO, A. (1973) Glucuronide synthesis in the rat and guinea pig lung.
Xenobiotica, 3: 13-22.
AL-KASSAB, S., BOYLAND, E., & WILLIAMS, K. (1963) An enzyme from rat
liver catalysing conjugations with glutathione; replacement of
nitro groups. Biochem. J., 87: 4-9.
ALLEN, J. R., NORBOCK, D. H., & HSU, I. C. (1974) Tissue modifications
in monkeys as related to absorption, distribution and excretion
of polychlorinated biphenyls. Arch. environ. Contam. Toxicol.,
2: 86-95.
ANDERS, M. W. & MANNERING, G. J. (1966) Inhibition of drug metabolism.
I. Kinetics of the inhibition of the N-demethylation of
ethylmorphine by 2-diethylaminoethyl 2, 2-diphenylvalerate HCl
(SKF 525A) and related compounds. Mol. Pharmacol., 2: 319-327.
ARIAS, I. M. (1962) Chronic unconjugated hyperbilirubinaemia without
overt signs of haemolysis in adolescents and adults. J. clin.
Invest., 41: 2233-2245.
ASSICOT, M. & BOHUON, C. (1969) A simple and rapid fluorimetric
determination of catechol- O-methyl transferase activity.
Life Sci., 8: 93-100.
AXELROD, J. (1971) Methyltransferase enzymes in the metabolism of
physiologically active compounds and drugs. In: Brodie, B. B. &
Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 2, pp. 609-619.
AXELROD, J. & DALY, J. W. (1968) Phenol- O-methyl transferase.
Biochim. Biophys. Acta, 159: 472-478.
AXELROD, J. & TOMCHICK, R. (1958) Enzymatic O-methylation of
epinephrine and other catechols. J. biol. Chem., 233: 702-705.
BAKER, R. C., COONS, L. B., & HODGSON, E. (1973) Low speed preparation
of microsomes: a comparative study. Chem. biol. Interactions,
6: 307-316.
BECKETT, A. H. & HOSSIE, R. D. (1971) Buccal absorption of drugs. In:
Brodie, B. B. & Gillette, J. R., ed. Concepts in biochemical
pharmacology, Berlin, Springer-Verlag, Vol. 1, pp. 25-46.
BOND, E. J. & DE MATTEIS, F. (1969) Biochemical changes in rat liver
after administration of carbon disulfide with reference to
microsomal changes. Biochem. Pharmacol., 18: 2531-2549.
BONNICHSEN, R. K. & BRINK, N. G. (1955) Liver alcohol dehydrogenase.
In: Colowick, S. P. & Kaplan, N. O., ed. Methods in enzymology,
New York, Academic Press, Vol. 1, pp. 495-500.
BOOTH, J., BOYLAND, E., & SIMS, P. (1961) An enzyme from rat liver
catalysing conjugations with glutathione. Biochem. J.,
79: 516-524.
BORCHARDT, R. T. (1974) A rapid spectrophotometric assay for
catechol- O-methyl transferase. Anal. Biochem., 58: 382-389.
BORGÅ, O., AZARNOFF, D. L., & SJÖQVIST, F. (1968) Species differences
in the plasmaprotein binding of desipramine. J. Pharm.
Pharmacol., 20: 571.
BOYD, G. S. & SMELLIE, R. M. S. (1972) Biological hydroxylation
mechanism. New York, Academic Press.
BOYLAND, E. (1971) Mercapturic acid conjugation. In: Brodie, B. B. &
Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 2, pp. 584-608.
BOYLAND, E. & CHASSEAUD, L. F. (1967) Enzyme-catalysed conjugations of
glutathione with unsaturated compounds. Biochem. J.,
104: 95-102.
BOYLAND, E. & WILLIAMS, K. (1965) A new enzyme catalysing the
conjugations of epoxides. Biochem. J., 94: 190-197.
BRAUER, R. W. (1959) Mechanisms of bile secretion. J. Am. Med.
Assoc., 169: 1462-1466.
BROCH, O. J., JR & GULDBERG, H. C. (1971) On the determination of
catechol- O-methyl transferase activity in tissue homogenates.
Acta Pharmacol. Toxicol., 30: 266-277.
BRODIE, B. B. (1964) Physicochemical factors in drug absorption. In:
Binns, T. B., ed. Absorption and distribution of drugs,
Baltimore, Williams & Wilkins, pp. 16-48.
BRODIE, B. B. & AXELROD, J. (1948) The estimation of acetanilide and
its metabolic products, aniline, N-acetyl- p-aminophenol and
p-aminophenol (free and total conjugates) in biological fluids
and tissues. J. Pharmac. exp. Ther., 94: 22-28.
BRODIE, B. B. & AXELROD, J. (1950) The fate of aminopyrine (Pyramidon)
in man and methods for the estimation of aminopyrine and its
metabolites in biological material. J. Pharmac. exp. Ther.,
99: 171-184.
BRODIE, B. B. & HOGBEN, C. A. M. (1957) Some physicochemical factors
in drug action. J. Pharm. Pharmacol., 9: 345-347.
BURCHELL, B., DUTTON, G. J., & NEMETH, A. M. (1972) Development of
phenobarbital-sensitive control mechanisms for uridine
diphosphate glucuronyl-transferase activity in chick liver.
J. cell. Biol., 55: 448-456.
CHASE, G. D. & RABINOWITZ, J. L. (1968) Principles of radioisotope
methodology, 3rd ed. Minneapolis, Burgess Publishing Company.
CHABHRA, R. S., GRAM, T. E., & FOUTS, J. R. (1972) A comparative study
of two procedures used in the determination of hepatic microsomal
aniline hydroxylation. Toxicol. appl. Pharmacol., 22: 50-58.
CHIGNELL, C. F. (1971) Physical methods for studying drug-protein
binding. In: Brodie, B. B. & Gillette, J. R., ed. Concepts in
biochemical pharmacology, Berlin, Springer-Verlag, Vol. 1,
pp. 187-212.
CHRASTIL, J. & WILSON, J. T. (1975) A sensitive colorimetric method
for formaldehyde. Anal. Biochem., 63: 202-207.
CHRISTENSEN, F. & WISSING, F. (1972) Inhibition of microsomal
drug-metabolizing enzymes from rat liver by various
4-hydroxy-coumarin derivatives. Biochem. Pharmacol.,
21: 975-984.
CIACCIO, E. I. (1971) Intimate study of drug action. II. Fate of drugs
in the body. In: Dipalma, J. R, ed. Drills pharmacology in
medicine, New York, McGraw-Hill, pp. 36-66.
COCHIN, J. & AXELROD, J. (1959) Biochemical and pharmacological
changes in the rat following chronic administration of morphine,
nalorphine and normorphine. J. Pharmac. exp. Ther.,
125: 105-110.
COLLINS, T. F. X. & WILLIAMS, C. H. (1971) Teratogenic studies with
2,4,5-T and 2,4-D in the hamster. Bull. environ. Contam.
Toxicol., 6: 559-567.
CONNEY, A. H. (1967) Pharmacological implications of microsomal enzyme
induction. Pharmacol. Rev., 19: 317-366.
CONNEY, A. H. & BURNS, J. J. (1962) Factors influencing drug
metabolism. Adv. Pharmacol., 1: 31-54.
CONNEY, A. H., COUTINHO, C., KOECHLIN, B., SWARM, R., CHERIPHO, J. A.,
IMPELLIZERI, C., & BARUTH, H. (1974) From animals to man:
metabolic considerations. Clin. Pharmacol. Ther., 16: 176-182.
COOPER, J. R. & BRODIE, B. B. (1965) Enzymatic oxidation of
pentobarbital and thiopental. J. Pharmacol. exp. Ther.,
120: 75-87.
COTZIAS, G. C. & DOLE, V. P. (1951) Metabolism of amines. I.
Microdetermination of aminoamine oxidase in tissues.
J. biol. Chem., 190: 665-672.
COURTNEY, D. K., GAYLOR, D. W., HOGAN, M. D., FLAK, H. L., BATES, R.
R., & MITCHELL, I. (1970) Teratogenic evaluation of 2,4,5-T.
Science, 168: 864-866.
COURTNEY, D. K. & MOORE, J. A. (1971) Teratology studies with
2,4,5-trichlorophenoxyacetic acid and
2,3,7,8-tetrachlorodibenzo- p-dioxin. Toxicol. appl.
Pharmacol., 20: 396-403.
CREASEY, N. H. (1956) Factors which interfere with the manometric
assay of monoamine oxidase. Biochem. J., 64: 178-183.
DAHM, P. A. (1971) Oxidative desulfuration and dealkylation of
selected organophosphate insecticides. In: Brodie, B. B. &
Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 2, pp. 362-366.
DALY, J. (1971) Enzymatic oxidation of carbon. In: Brodie, B. B. &
Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 2, pp. 285-311.
DAVIES, D. S., GIGON, P. L., & GILLETTE, J. R. (1969) Species and sex
differences in electron transport systems in liver microsomes and
their relationship to ethylmorphine demethylation. Life Sci.,
8: 85-91.
DAVSON, H. & DANIELLI, J. F. (1952) The permeability of natural
membranes, 2nd ed. Cambridge, Cambridge Univ. Press.
DEITRICH, R. A. & ERWIN, V. G. (1969) Spectrophotometric assay for
monoamine oxidase. Anal. Biochem., 30: 395-402.
DEWAIDE, J. H. & HENDERSON, P. T. (1968) Hepatic N-demethylation of
aminopyrine in rat and trout. Biochem. Pharmacol.,
17: 1901-1907.
DRILL, V. A. & HERATJKA, T. (1953) Toxicity of
2,4-dichlorophenoxyacetic acid and 2,4,5-trichlorophenoxyacetic
acid. Arch. ind. Hyg. occup. Med, 7: 61-67.
DUTTON, G. J. (1966) The biosynthesis of glucuronides. In: Dutton, G.
J. ed. Glucuronic acid, free and combined. New York, Academic
Press.
DUTTON, G. J. (1971) Glucuronide-forming enzymes. In: Brodie, B. B. &
Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 2, pp. 378-400.
DUTTON, G. J. & STOREY, I. D. E. (1962) Glucuronide-forming enzymes.
In: Colowick, S. P. & Kaplan, N. O., ed. Methods in enzymology,
New York, Academic Press, Vol. 5, pp. 159-164.
ESTABROOK, R. W. (1971) Cytochrome P-450. Its function in the
oxidative metabolism of drugs. In: Brodie, B. B. & Gillette, J.
R., ed. Concepts in biochemical pharmacology, Berlin,
Springer-Verlag, Vol. 2, pp. 264-284.
ESTABROOK, R. W., FRANKLIN, M. R., COHEN, B., SHIGAMATSU, A., &
HILDEBRANDT, A. G. (1971) Influence of hepatic microsomal mixed
function oxidation reactions on cellular metabolic control.
Metabolism, 20: 186-199.
ESTABROOK, R. W., GILLETTE, J. R., & LEIBMAN, K. C. (1972)
Microsomes and drug oxidations. Baltimore, Williams & Wilkins.
FEUER, G., SOSA-LUCERO, J. C., LUMB, F., & MODDEL, G. (1971) Failure
of various drugs to induce drug metabolizing enzymes in
extrahepatic tissues of the rat. Toxicol. appl. Pharmacol.,
19: 579-589.
FLYNN, E. J., LYNCH, M., & ZANNONI, V. G. (1969) Species differences
in hepatic microsomal electron transport. Fed. Proc., 28: 483.
FLYNN, E. J., LYNCH, M., & ZANNONI, V. G. (1972) Species differences
and drug metabolism. Biochem. Pharmacol., 21: 2577-2590.
FOK, A. K. & ZIEGLER, D. M. (1970) Estimation of amine oxides in the
presence of hepatic microsomes. Biochem. Biophys. Res. Commun.,
41: 534-540.
FOREMAN, H. (1971) Translocation of drugs into bone. In: Brodie, B. B.
& Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 1, pp. 249-257.
FOUTS, J. R. (1970) The stimulation and inhibition of hepatic
microsomal drug-metabolising enzymes with special reference to
the effects of environmental contaminants. Toxicol. appl.
Pharmacol., 17: 804-809.
FOUTS, J. R. & BRODIE, B. B. (1957) The enzymatic reduction of
chloramphenicol, p-nitrobenzoic acid and other aromatic nitro
compounds in mammals. J. Pharmacol. exp. Ther. 119: 197-207.
FOUTS, J. R. & POHL, H. J. (1971) Further studies on the effects of
metal ions on rat liver microsomal reduced nicotinamide adenine
dinucleotide phosphate-cytochrome P-450 reductase. J. Pharmacol.
exp. Ther., 179: 91-100.
GARATTINI, S., MARCUCCI, F., & MUSSINI, E. (1975) Biotransformation of
drugs to pharmacologically active metabolites. In: Gillette, J.
R. & Mitchell, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 3, pp. 113-119.
GEHRING, P. J. & BUERGE, J. (1969) The distribution of
2,4-dinitrophenol relative to its cataractogenic activity in
ducklings and rabbits. Toxicol. appl. Pharmacol., 15: 574-592.
GEHRING, P. J., KRAMER, C. D., SCHWETZ, B. A., ROSE, J. Q., & ROWE, V.
K. (1973) The fate of 2,4,5-trichlorophenoxyacetic acid (2,4,5-T)
following oral administration to man. Toxicol. appl. Pharmacol.,
26: 352-361.
GEHRING, P. J., BLAU, G. E., & WATANABE, P. G. (1976) Pharmacokinetic
studies in evaluation of the toxicological and environmental
hazard of chemicals. In: Advances in modern toxicology -- Newer
concepts in safety evaluation. Washington, Hemisphere Publ.
Corp.
GIBSON, J. E. & BECKER, B. A. (1967) Demonstration of enhanced
lethality of drugs in hypoexcretory animals. J. pharm. Sci.,
56: 1503-1505.
GIGON, P. L., GRAM, T. E., & GILLETTE, J. R. (1969) Studies on the
rate of reduction of hepatic microsomal cytochrome P-450 by
reduced nicotinamide adenine dinucleotide phosphate. Effect of
drug substrates. Mol. Pharmacol., 5: 109-122.
GILBERT, D. & GOLBERG, L. (1965) Liver response tests. III. Liver
enlargement and stimulation of microsomal processing enzyme
activity. Food Cosmet. Toxicol., 3: 417-432.
GILLETTE, J. R. (1963) Metabolism of drugs and other foreign compounds
by enzymatic mechanisms. Arzneimittel Forsch., 6: 11-73.
GILLETTE, J. R. (1971a) Factors affecting drug metabolism. Ann. NY
Acad. Sci., 179: 43-66.
GILLETTE, J. R. (1971b) Reductive enzymes. In: Brodie, B. B. &
Gillette, J. R. ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 2, pp. 349-361.
GILLETTE, J. R. (1971c) Effects of various inducers on electron
transport systems associated with drug metabolism by liver
microsomes. Metabolism, 20: 215-227.
GILLETTE, J. R. (1973a) Overview of drug-protein binding. Ann. NY
Acad. Sci., 226: 6-17.
GILLETTE, J. R. (1973b) The importance of tissue distribution in
pharmacokinetics. J. Pharmacol. Biopharm., 1: 497-519.
GILLETTE, J. R. (1974a) A perspective on the role of chemically
reactive metabolites of foreign chemicals in toxicity. I.
Correlation of changes in covalent binding of reactivity
metabolites with changes in the incidence and severity of
toxicity. Biochem. Pharmacol., 23: 2785-2794.
GILLETTE, J. R. (1974b) A perspective on the role of chemically
reactive metabolites of foreign compounds in toxicity: II.
Alterations in the kinetics of covalent binding. Biochem.
Pharmacol., 23: 2927-2938.
GILLETTE, J. R. (1975) Other aspects of pharmacokinetics. In:
Gillette, J. R. & Mitchell, J. R., ed. Concepts in biochemical
pharmacology, Berlin, Springer-Verlag, Vol. 3, pp. 35-85.
GILLETTE, J. R., CHAN, T. W., & TERRIERE, L. C. (1966) Interactions
between DDT analogues and microsomal epoxidase systems.
J. agric. Food Chem., 14: 540-545.
GILLETTE, J. R. DAVIS, D. C., & SASAME, (1972) Cytochrome P-450 and
its role in drug metabolism. Annu. Rev. Pharmacol., 12: 57-84.
GILLETTE, J. R., MITCHELL, J. R., & BRODIE, B. B. (1974) Biochemical
mechanisms of drug toxicity. Annu. Rev. Pharmacol.,
14: 271-288.
GOLDSTEIN, A., ARONOW, L., & KOLMAN, S. M. (1974) Principles of drug
action. In: The basis of pharmacology, 2nd ed., New York,
Wiley, pp. 106-205.
GRAM, T. E. (1971) Enzymatic N-, O- and S-dealkylation of foreign
compounds by hepatic microsomes. In: Brodie, B. B. & Gillette, J.
R., ed. Concepts in biochemical pharmacology, Berlin,
Springer-Verlag, Vol. 2, pp. 334-348.
GRAM, T. E. (1973) Comparative aspects of mixed function oxidation by
lung and liver of rabbits. Drug. Metabol. Rev., 2: 1-32.
GREGORY, J. D. (1960) The effect of borate on the carbazole reaction.
Arch. Biochem. Biophys., 89: 157-159.
GREGORY, J. D. & LIPMANN, F. (1957) The transfer of sulfate among
phenolic compounds with 3,5-diphosphoadenosine as co-enzyme.
J. Biol. Chem., 229: 1081-1090.
GRIFFITHS, M. H. (1968) The metabolism of N-triphenylmorphine in the
dog and rat. Biochem. J., 108: 731-740.
HART, L. G. & FOUTS, J. R. (1965) Further studies on the stimulation
of hepatic microsomal drug metabolizing enzymes by DDT and its
analogues. Arch. exp. Pathol., 249: 486-500.
HEIRWEGH, K. P. M., VAN DER VIJER, M., & FEVERY, J. (1972) Assay and
properties of digitonin-activated bilirubin uridine diphosphate
glucuronyl-transferase from rat liver. Biochem. J.,
129: 605-618.
HENDERSON, P. TH. & KERSTEN, K. J. (1970) Metabolism of drugs during
liver regeneration. Biochem. Pharmacol., 19: 2343-2351.
HERR, F. & KIESE, M. (1959) [Determination of nitrosobenzol in the
blood.] Arch. exp. Path. Pharmakol., 235: 351-353 (in German).
HEWICK, D. S. & FOUTS, J. R. (1970a) Effects of storage on hepatic
microsomal cytochromes and substrate-induced difference spectra.
Biochem. Pharmacol., 19: 457-472.
HEWICK, D. S. & FOUTS, J. R. (1970b) The metabolism in vitro and
hepatic microsomal interactions of some enantiomeric drug
substrates. Biochem. J., 117: 833-841.
HIDAKA, H., NAGATSU, T., & YAGI, K. (1967) A rapid and simple assay of
serotonin sulfokinase activity. Anal. Biochem., 17: 388-392.
HIETANEN, E. (1974) Effect of sex and castration on hepatic and
intestinal activity of drug-metabolizing enzymes. Pharmacology,
12: 84-89.
HIETANEN, E. & VALINIO, H. (1973) Interspecies variations in small
intestinal and hepatic drug hydroxylation and glucuronidation.
Acta Pharmacol. Toxicol., 33: 57-64.
HIETBRINK, B. E. & DUBOIS, K. P. (1965) Influence of X-radiation on
development of enzymes responsible for desulfuration of an
organic phosphorothioate and reduction of p-nitrobenzoic acid
in the livers of male rats. Radiat. Res., 22: 598-603.
HILTON, J. & SARTORELLI, A. C. (1970) Induction by phenobarbital of
microsomal mixed oxidase enzymes in regenerating liver. J. biol.
Chem., 245: 4187-4192.
HOFFMAN, D. G., WORTH, H. M., & ANDERSON, R. C. (1968) Stimulation of
hepatic microsomal drug-metabolizing enzymes by alpha alpha-bis
p-chlorophenyl-3-pyridine methanol and a method for determining
no-effect levels in rats. Toxicol. appl. Pharmacol.,
12: 464-472.
HOFFMAN, D. F., WORTH, H. M., & ANDERSON, R. C. (1970) Stimulation of
hepatic drug-metabolizing enzymes by chlorophenothane (DDT); the
relationship to liver enlargement and hepatotoxicity in the rat.
Toxicol. appl. Pharmacol., 16: 171-178.
HOLTZMAN, J. L., GRAM, T. E., GIGON, P. L., & GILLETTE, J. R. (1968)
The distribution of the components of mixed function oxidase
between the rough and the smooth endoplasmic reticulum of liver
cells. Biochem. J., 110: 407-412.
HOOK, J. B., BAIBIE, M. D., JOHNSON, J. T., & GEHRING, P. J. (1974)
In vitro analysis of transport of 2,4,5-trichlorophenoxyacetic
acid (2,4,5-T) by rat and dog kidney. Food Cosmet. Toxicol.,
12: 209-218.
HUCKER, H. B. (1970) Species differences in drug metabolism.
Annu. Rev. Pharmacol., 10: 99-118.
IMAI, Y., ITO, A., & SATOR, R. R. (1966) Evidence for biochemically
different types of vesicles in the hepatic microsomal fraction.
J. Biochem., 60: 417-428.
ISSELBACHER, K. J. (1956) Enzymatic mechanisms of hormone metabolism.
II. Mechanism of hormonal glucuronide formation. In: Pincus, G.,
ed. Recent progress in hormone research, New York, Academic
Press, pp. 134-151.
JENNE, J. W. & BOYER, P. D. (1962) Kinetic characteristics of the
acetylation of isoniazid and p-aminosalicylic acid by a liver
enzyme preparation. Biochim. Biophys. Acta, 65: 121-127.
JOHNSON, M. K. (1966) Metabolism of iodomethane in the rat.
Biochem. J., 98: 38-43.
JOLLOW, P. J., MITCHELL, J. R., ZAMPOGLIONE, N., & GILLETTE, J. R.
(1974) Bromobenzene-induced liver necrosis: protective role of
glutathione and evidence for 3,4-bromobenzene oxide as the
hepatotoxic metabolite. Pharmacology, 11: 151-169.
KATO, R. & GILLETTE, J. R. (1965) Effect of starvation on
NADPH-dependent enzymes in liver microsomes of male and female
rats. J. Pharmacol. exp. Ther., 150: 279-284.
KATZ, M. & POULSEN, B. J. (1971) Absorption of drugs through the skin.
In: Brodie, B. B. & Gillette, J. R., ed. Concepts in biochemical
pharmacology, Berlin, Springer-Verlag, Vol. 1, pp. 103-174.
KEBERLE, H., HOFFMAN, K., & BERNHARD, K. (1962) The metabolism of
glutethimide (Doridnene). Experientia (Basel), 18: 105-111.
KEEN, P. (1971) Effect of binding to plasma proteins on the
distribution, activity and elimination of drugs. In: Brodie, B.
B. & Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 1, pp. 213-233.
KINOSHITA, F. K., FRAWLEY, J. P., & DUBOIS, K. P. (1966) Quantitative
measurement of induction of hepatic microsomal enzymes by various
dietary levels of DDT and toxaphene in rat. Toxicol. appl.
Pharmacol., 9: 505-513.
KLINGER, W. (1974) Optimal conditions for the first step of
amidopyrine- N-demethylation by 900 × g liver supernatant of
newborn and adult rats. Acta Biol. Med. Germ., 33: 181-186.
KUNTZMAN, R. (1969) Drugs and enzyme induction. Annu. Rev.
Pharmacol., 9: 21-36.
KURZ, H. & FRIEMEL, G. (1967) [Species-specific differences in the
binding of plasma proteins.] Arch. Pharmakol. exp. Path.,
257: 35-36 (in German).
LA DU, B. N. & SNADY, H. (1971) Esterases of human tissues. In:
Brodie, B. B. & Gillette, J. R., ed. Concepts in biochemical
pharmacology, Berlin, Springer-Verlag, Vol. 2, pp. 477-499.
LA DU, B. N., GAUDETTE, L., TROUSOF, N., & BRODIE, B. B. (1955)
Enzymatic dealkylation of aminopyrine (Pyramidon) and other
alkylamines. J. biol. Chem., 214: 741-752.
LA DU, B. N., MANDEL, G. H., & WAY, E. L. (1972) Fundamentals of drug
metabolism and drug disposition. Baltimore, Williams & Wilkins.
LEVI, A. J., GATMAITAN, Z., & ARIAS, I. M. (1969) Two hepatic
cytoplasmic protein fractions, Y and Z, and their possible role
in hepatic uptake of bilirubin, sulfobromophthalein, and other
anions, J. clin. Invest., 48: 2156-2167.
LEVINE, R. R. (1970) Factors affecting gastrointestinal absorption of
drugs. Digest Dis. 15: 171-188.
LEVY, G. & GIBALDI, M. (1972) Pharmacokinetics of drug action.
Annu. Rev. Pharmacol., 12: 85-98.
LEVY, G. & GIBALDI, M. (1975) Pharmacokinetics. In: Gillette, J. R. &
Mitchell, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 3, pp. 1-34.
LITWACK, G., KETTERER, B., & ARIAS, I. M. (1971) Ligondin: a hepatic
protein which binds steroids, bilirubin, carcinogens and a number
of exogenous organic anions. Nature (Lond.), 234: 466-467.
LIU, S. J., RAMSEY, R. K., & FALLON, H. J. (1975) Effects of ethanol
on hepatic microsomal drug-metabolizing enzymes in the rat.
Biochem. Pharmacol., 24: 369-378.
LU, A. Y. H. & LEVIN, W. (1974) The resolution and reconstitution of
the liver microsomal hydroxylation system. Biochim. Biophys.
Acta, 344: 205-240.
LU, A. Y. H., STROBEL, H. W., & COON, M. J. (1969) Hydroxylation of
benzphetamine and other drugs by a solubilized form of cytochrome
P-450 from liver microsomes: lipid requirement for drug
demethylation. Biochem. Biophys. Res. Commun., 36: 545-551.
LUCIS, O. J., SCHACKH, Z. A., & EMBIL, J. A., JR (1970) Cadmium as a
trace element and cadmium-binding components in human cells.
Experientia (Basel), 26: 1109-1112.
MACCAMAN, R. E. (1965) Microdetermination of catechol- O-methyl
transferase. Life Sci., 4: 2353-2359.
MCLEAN, A. E. M. (1971) Conversion by the liver of inactive molecules
into toxic molecules. In: Aldridge, W. N., ed. Mechanism of
toxicity, London, Macmillan, pp. 219-228.
MACMAHON, R. E. (1962) The competitive inhibition of the
N-demethylation of butynamine by 2,4-dichloro-6-
phenolphenoxy-ethylamine (DPEA). J. Pharmacol. exp. Ther.,
138: 382-386.
MACMAHON, R. E. (1971) Enzymatic oxidation and reduction of alcohols,
aldehydes and ketones. In: Brodie, B. B. & Gillette, J. R., ed.
Concepts in biochemical pharmacology, Berlin, Springer-Verlag,
Vol. 2, pp. 500-517.
MAHER, J. R., WHITNEY, J. M., CHAMBERS, J. S., & STANONIS, D. J.
(1957) The quantitative determination of isoniazid and
para-aminosalicylic acid in body fluids. Am. Rev. Tuberc.,
76: 852-861.
MANNERING, G. J. (1968) Significance of stimulation and inhibition of
drug metabolism. In: Burger, A., ed. Selected pharmaceutical
testing methods, New York, Marcel Dekker, pp. 51-119.
MANNERING, G. J. (1971a) Inhibition of drug metabolism. In: Brodie, B.
B. & Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 2, pp. 452-476.
MANNERING, G. J. (1971b) Properties of cytochrome P-450 as affected by
environmental factors: qualitative changes due to administration
of polycyclic hydrocarbons. Metabolism, 20: 228-245.
MARK, L. C. (1971) Translocation of drugs and other exogenous
chemicals into adipose tissue. In: Brodie, B. B. & Gillette, J.
R., ed. Concepts in biochemical pharmacology, Berlin,
Springer-Verlag, Vol. 1, pp. 258-275.
MARSELOS, M. & HÄNNINEN, O. (1974) Enhancement of D-glucuronolactone
and acetaldehyde dehydrogenase activities in the rat liver by
inducers of drug metabolism. Biochem. Pharmacol.,
23: 1457-1466.
MARSH, C. A. (1963) Metabolism of D-glucuronolactone in mammalian
systems; conversion of D-glucuronolactone into D-glucaric acid by
tissue preparations. Biochem. J., 87: 82-90.
MARSHALL, E. K., JR (1948) Determination of par-amino-salicylic acid
in blood. Proc. Soc. Exp. Biol. Med., 68: 471-472.
MASON, H. S. (1957) Mechanism of oxygen metabolism. Adv. Enzymol.,
19: 79-233.
MILLER, E. C. & MILLER, J. A. (1971a) The mutagenicity of chemical
carcinogens: correlations, problems and interpretations. In:
Hollaender, A., ed. Chemical mutagens, New York, Plenum,
Vol. 1, pp. 83-119.
MILLER, J. A. & MILLER, E. C. (1971b) Chemical carcinogenesis:
mechanisms and approaches to its control. J. Natl Cancer Inst.,
47: v-xiv.
NASH, T. (1953) The colorimetric estimation of formaldehyde by means
of the Hantsch reaction. Biochem. J., 55: 416-421.
NEBERT, D. W. & GELBOIN, H. V. (1968a) Substrate inducible microsomal
aryl hydroxylase. I. Assay and properties of induced enzymes.
J. Biol. Chem., 243: 6242-6249.
NEBERT, D. W. & GELBOIN, H. V. (1968b) Substrate inducible microsomal
aryl hydroxylase. II. Cellular responses during enzymic
induction. J. Biol. Chem., 243: 6250-6261.
NETTER, K. J. (1960) [A method for the direct measurement of
O-demethylization in liver microsomes and its application to
the inhibitory effects of SKF 525A on microsomes.]
Arch. Pharmacol., 238: 292-300 (in German).
NETTER, K. J. & SEIDEL, G. (1964) An adaptively stimulated
O-demethylating system in rat liver microsomes and its kinetic
properties. J. Pharmacol. exp. Ther., 146: 61-65.
NOTTON, W. R. & HENDERSON, P. TH. (1975) The influence of N-hexane
treatment on the glucuronic acid pathway and activity of some
drug-metabolizing enzymes in the guinea pig. Biochem.
Pharmacol., 24: 127- 131.
OBATA, F., USHIWATA, A., & NAKAMURA, Y. (1971) Spectrophotometric
assay of monoamine oxidase using 2,4,6-trinitrobenzene-1-sulfonic
acid. J. Biochem., 69: 349-354.
OGATA, M., TOMOKUNI, K., & TAKATSUKA, Y. (1969) Quantitative
determination in urine of hippuric acid and m- or p-methyl
hippuric acid, metabolites of toluene and m- or p-xylene.
Brit. J. ind. Med., 26: 330-334.
OMURA, T. & SATO, R. (1964a) The carbon monoxide-binding pigment of
liver microsomes: I. Evidence for its hemoprotein nature.
J. biol. Chem., 239: 2370-2378.
OMURA, T. & SATO, R. (1964b) The carbon monoxide-binding pigment of
liver microsomes: II. Solubilization, purification and
properties. J. biol. Chem., 239: 2379-2385.
OTSUKA, S. & KOBAYASHI, Y. (1964) A radioisotopic assay for monamine
oxidase determinations in human plasma. Biochem. Pharmacol.,
13: 995-1006.
PAPPENHEIMER, J. R. (1953) Passage of molecules through capillary
walls. Physiol. Rev., 33: 387-423.
PARKE, D. V. (1968) The biochemistry of foreign compounds. New York,
Pergamon Press.
PEDERSON, T. C. & AUST, S. D. (1970) Aminopyrine demethylase: kinetic
evidence for multiple microsomal activities. Biochem.
Pharmacol., 19: 2221-2230.
PIPER, W. N., ROSE, J. Q., LENG, M. L. & GEHRING, P. J. (1973) The
fate of 2,4,5-trichlorophenoxyacetic acid (2,4,5-T) following
oral administration to rats and dogs. Toxicol. appl. Pharmacol.,
26: 339-351.
PITTS, R. F. (1963) Physiology of the kidney and body fluids.
Yearbook, Chicago, Medical Publishers Inc.
PLAA, G. L. (1975) The enterohepatic circulation. In: Gillette, J. R.
& Mitchell, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 3, pp. 130-149.
PLACE, V. A. & BENSON, H. (1971) Dietary influences on therapy with
drugs. J. Mond. Pharm., 14: 261-278.
POLAND, A. P. & NEBERT, D. W. (1973) A sensitive radiometric assay of
aminopyrine N-demethylation. J. Pharmacol. exp. Ther.,
184: 269-277.
POTTS, A. M. (1964) The reaction of uveal pigments in vitro with
polycyclic compounds. Invest. Ophthalmol., 3: 405-416.
PRESCOTT, L. F. (1975) Pathological and physiological factors
affecting drug absorption, distribution, elimination and response
in man. In: Gillette, J. R. & Mitchell, J. R., ed. Concepts in
biochemical pharmacology, Berlin, Springer-Verlag,
Vol. 3, pp. 234-257.
PRICE, H. L., KOVNAT, P. J., SOFER, J. N., CONNER, E. H., & PRICE, M.
L. (1960) The uptake of thiopental by body tissues and its
relation to the duration of narcosis. Clin. Pharmacol. Ther.,
1: 16-22.
QUASTEL, J. H. (1965) Molecular transport at cell membranes.
Proc. Roy. Soc. Br., 163: 169-186.
QUINN, G. P., AXELROD, J., & BRODIE, B. B. (1958) Species, strain and
sex differences in metabolism of hexobarbitone, aminopyrine,
antipyrine and aniline. Biochem. Pharmacol., 1: 152-159.
RALL, D. P. (1971) Drug entry into brain and cerebrospinal fluid. In:
Brodie, B. B. & Gillette, J. R., ed. Concepts in biochemical
pharmacology, Berlin, Springer-Verlag, Vol. 1, pp. 240-248.
RASMUSSEN, F. (1971) Excretion of drugs by milk. In: Brodie, B. B. &
Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 1, pp. 390-402.
REMMER, H., SCHENKMAN, J., ESTABROOK, R. W., SASAME, H., GILLETTE, J.
R., NARASHIMHULU, S., COOPER, D. Y., & ROSENTHAL, O. (1966) Drug
interaction with hepatic microsomal cytochrome. Mol. Pharmacol.,
2: 187-190.
RENKIN, E. M. (1964) Transport of large molecules across capillary
walls. Physiologist, 7: 13.
REYES, H., LEVI, A. J., GATMAITAN, Z., & ARIAS, I. M. (1971) Studies
on Y and Z, two hepatic cytoplasmic organic anion-binding
proteins: Effect of drugs, chemicals, hormones and cholestosis.
J. Clin. Invest., 50: 2242-2251.
RODOMSKI, J. L., DEICHMANN, W. B., & CLIZER, E. E. (1968) Pesticide
concentrations in the liver, brain and adipose tissue of terminal
hospital patients. Food Cosmet. Toxicol., 6: 209-220.
ROLL, R. (1971) Investigations concerning the teratogenic effect of
2,4,5-T in mice. Food Cosmet. Toxicol., 9: 671-676.
ROSE, J. Q., RAMSEY, J. C., WENTZLER, T. G., HUMMEL, R. A., & GEHRING,
P. J. (1975) The fate of 2,3,7,8-tetrachlorodibenzo- p-dioxin
(TCDD) following single and repeated oral doses to the rat.
Toxicol. appl. Pharmacol., 36: 209-226.
ROSENBLUM, C. (1965) Non-metabolite residues in radioactive tracer
studies. In: Roth, L. J., ed. Isotopes in experimental
pharmacology, Chicago, University of Chicago Press,
pp. 353-360.
ROTH, L. J. (1971) The use of autoradiography in experimental
pharmacology. In: Brodie, B. B. & Gillette, J. R., ed. Concepts
in biochemical pharmacology, Berlin, Springer-Verlag,
Vol. 1, pp. 286-316.
ROWE, V. K. & HYMAS, T. A. (1954) Summary of toxicological information
of 2,4-D and 2,4,5-T type herbicides and an evaluation of the
hazards to livestock associated with their use. Am. J. Vet.
Res., 15: 622-629.
ROY, A. B. (1971) Sulfate conjugation enzymes. In: Brodie, B. B. &
Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 2, pp. 536-563.
SAUERHOFF, M. W., BRAUN, W. H., BLAU, G. E., & GEHRING, P. J. (1975)
The dose-dependent pharmacokinetic profile of
2,4,5-trichlorophenoxyacetic acid following intravenous
administration to rats. Toxicol. appl. Pharmacol., 36: 491-501.
SCHANKER, L. S. (1962a) Passage of drugs across body membranes.
Pharmacol. Rev., 14: 501-530.
SCHANKER, L. S. (1962b) Concentrative transfer of an organic cation
from blood into bile. Biochem. Pharmacol., 11: 253-254.
SCHANKER, L. S. (1963) Passage of drugs across the gastrointestinal
epithelium. In: Hogben, C. A. M., ed. Proceedings of the 1st
International Pharmacological Meeting, Oxford, Pergamon Press,
Vol. 4, pp. 120-130.
SCHANKER, L. S. (1965) Hepatic transport of organic cations. In:
Taylor, W., ed. The biliary system. Oxford, Blackwell.
SCHANKER, L. S. (1971) Absorption of drugs from the gastrointestinal
tract. In: Brodie, B. B. & Gillette, J. R., ed. Concepts in
biochemical pharmacology, Berlin, Springer-Verlag,
Vol. 1, pp. 9-24.
SCHANKER, L. S., SHORE, P. A., BRODIE, B. B., & HOGBEN, C. A. M.
(1957) Absorption of drugs from the stomach. I. The rat.
J. Pharmacol. exp. Ther., 120: 528-539.
SCHELINE, R. R. (1968) Drug metabolism by intestinal microorganisms.
J. pharm. Sci., 57: 2021-2037.
SCHENKMAN, J. B., REMMER, H., & ESTABROOK, R. W. (1967) Spectral
studies of drug interaction with hepatic microsomal cytochrome.
Mol. Pharmacol., 3: 113-123.
SCHOENE, B., FLEISCHMANN, R. A., REMMER, H., & OLDERSHAUSEN, H. F.
(1972) Determination of drug-metabolizing enzymes in needle
biopsies of human liver. Eur. J. clin. Pharmacol., 4: 65-73.
SCHOLTAN, W. (1963) On the protein binding of long-acting
sulfonamides. Chemotherapia, 6: 180-195.
SCHWETZ, B. A., NORRIS, J. M., SPARSCHU, G. L., ROWE, V. K., GEHRING,
P. J., EMERSON, J. K., & GERBIG, C. G. (1973) Toxicology of
chlorinated dibenzo- p-dioxins. Environ. Health Perspect.
(Experimental issue), 5: 87-100.
SETTLE, W., HEGEMAN, S., & FEATHERSTONE, R. M. (1971) The nature of
drug-protein interaction. In: Brodie, B. B. & Gillette, J. R.,
ed. Concepts in biochemical pharmacology, Berlin,
Springer-Verlag, Vol. 1, pp. 175-186.
SHORE, P. A., BRODIE, B. B., & HOGBEN, C. A. M. (1957) The gastric
secretion of drugs -- a pH partition hypothesis. J. Pharmacol.
exp. Ther., 119: 361-369.
SHULERT, A. R. (1961) Physiological disposition of hydralazine
(1-hydrazinophthalazine) and a method for its determination in
biological fluids. Arch. int. Pharmacodyn., 132: 1-15.
SMITH, R. L. (1971a) Excretion of drugs in bile. In: Brodie, B. B. &
Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 1, pp. 354-389.
SMITH, R. L. (1971b) The role of the gut flora in the conversion of
inactive compounds to active metabolites. In: Aldridge, W. N.,
ed. Mechanism of toxicity, London, Macmillan, pp. 229-247.
SMITH, R. L. (1973) The excretory function of bile: the elimination
of drugs and toxic substances in bile. London, England, Chapman
& Hall.
SMUCKLER, E. A. (1971) Metabolism of halogenated compounds. In:
Brodie, B. B. & Gillette, J. R., ed. Concepts in biochemical
pharmacology, Berlin, Springer-Verlag, Vol. 2, pp. 367-377.
SPARSCHU, G. L., DUNN, F. L., LISOWE, R. W. & ROWE, V. K. (1971) Study
of the effects of high levels of 2,4,5-trichlorophenoxyacetic
acid on fetal development in the rat. Food Cosmet. Toxicol.,
9: 527-530.
SPERBER, I. (1963) Drugs and membranes. In: Hogben, C. A. M., ed.
Proceedings of the 1st International Pharmacological Meeting,
Oxford, Pergamon Press, Vol. 4.
STERRSON, L. A. & HES, J. (1975) Electrochemical method for the
determination of aniline hydroxylation. Anal. Biochem.,
67: 74-80.
STOTZ, E., STEINBERG, M. S., COHEN, S. N., & WEBER, W. W. (1969)
Acetylation of 5-hydroxytryptamine by isoniazide
N-acetyltransferase. Biochim. Biophys. Acta, 184: 210-212.
STOWE, C. M. & PLAA, G. L. (1968) Extrarenal excretion of drugs and
chemicals. Annu. Rev. Pharmacol., 8: 337-356.
STRICKLAND, R. D., GREGORY, D. H., & LYNCH, J. L. (1974) A method for
assaying hepatic morphine UDP-glucuronyl transferase. Biochem.
Med., 11: 180-188.
STURMAN, J. A. & SMITH, M. J. H. (1967) The binding of salicylate to
plasma proteins in different species. J. Pharm. Pharmacol.,
19: 621-623.
SUGA, T., OHATA, I., KUMAOKA, H., & AKAGI, M. (1967) Studies on
mercapturic acids: investigation of glutathione-conjugating
enzyme by the method of thin-layer chromatography.
Chem. Pharmacol. Bull., 15: 1059-1064.
TABOR, H., MEHLER, A. H., & STADTMAN, E. R. (1953) The enzymatic
acetylation of amines. J. biol. Chem., 204: 127-138.
TAKAHASHI, H. & TAKAHARA, S. (1968) A sensitive fluorimetric assay for
monoamine oxidase based on the formation of 4,6-quinolinediol
from 5-hydroxykynurenamine. J. Biochem., 64: 7-11.
TEORELL, T. (1937a) Kinetics of distribution of substances
administered to the body: I. The extra-vascular modes of
administration. Arch. int. Pharmacodyn., 57: 205-226.
TEORELL, T. (1937b) Kinetics of distribution of substances
administered to the body: II. The intra-vascular modes of
administration. Arch. int. Pharmacodyn., 57: 227-240.
TUFVESSON, G. (1970) Fluorimetric determination of amine oxidase
activity in human blood serum with kynuramine as substrate.
Scand. J. clin. Lab. Invest., 26: 151-154.
ULLRICH, V. & WEBER, P. (1972) The O-dealkylation of
7-ethoxycoumarin by liver microsomes: a direct fluorimetric test.
Z. Physiol. Chem., 353: 1171-1177.
WAGNER, J. P. (1968) Pharmacokinetics. Ann. Rev. Pharmacol.,
8: 67-94.
WAGNER, J. P. (1971) Biopharmaceutics and relevant pharmacokinetics.
Hamilton, Drug Intelligence Publications.
WATTERNBERG, L. W. & LEONG, J. L. (1971) Tissue distribution studies
of polycyclic hydrocarbon hydroxylase activity. In: Brodie, B. B.
& Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 2, pp. 422-430.
WATTENBERG, L. W., LEONG, J. L., & STRAND, P. J. (1962) Benzpyrene
hydroxylase activity in the gastrointestinal tract. Cancer Res.,
22: 1120-1125.
WEBER, W. W. (1970) N-acetyltransferase (mammalian liver). In:
Tabor, H. & Tabor, C. W., ed. Methods in enzymology, 17B. New
York, Academic Press.
WEBER, W. W. (1971) Acetylating, deacetylating and amino
acid-conjugating enzymes. In: Brodie, B. B. & Gillette, J. R.,
ed. Concepts in biochemical pharmacology, Berlin,
Springer-Verlag, Vol. 2, pp. 564-583.
WEBER, W. W. & COHEN, S. N. (1968) The mechanism of isoniazid
acetylation by human N-acetyltransferase. Biochim. Biophys.
Acta, 151: 276-278.
WEBER, W. W., COHEN, S. N., & STEINBERG, M. S. (1968) Purification and
properties of N-acetyltransferase from mammalian liver.
Ann. NY Acad. Sci., 151: 734-741.
WEINER, I. M. (1971) Excretion of drugs by the kidney. In: Brodie, B.
B. & Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 1, pp. 328-353.
WEISBURGER, H. H. & WEISBURGER, E. R. (1971) N-oxidation enzymes.
In: Brodie, B. B. & Gillette, J. R., ed. Concepts in biochemical
pharmacology, Berlin, Springer-Verlag, Vol. 2, pp. 312-333.
WEISBURGER, H. H., GRANTHAM, P. H., & WEISBURGER, E. R. (1964)
Metabolism of N-2-fluorenylacetamide in the hamster. Toxicol.
appl. Pharmacol., 6: 427-433.
WEISSBACH, H., SMITH, T. E., FALY, J. W., WITKOP, B., & UDENFRIEND, S.
(1960) A rapid spectrophotometric assay of monoamine oxidase
based on the rate of disappearance of kynuramine. J. Biol.
Chem., 235: 1160-1163.
WILLIAMS, C. H., JR & KAMIN, H. (1962) Microsomal triphosphopyridine
nucleotide-cytochrome c reductase of liver. J. Biol. Chem.,
237: 587-595.
WILLIAMS, R. T. (1965) The influence of enterohepatic circulation on
toxicity of drugs. Ann. NY Acad. Sci, 123: 110-124.
WILLIAMS, R. T. (1967a) The biogenesis of conjugation and detoxication
products. In: Bernfield, P, ed. Biogenesis of natural compounds,
2nd ed., Oxford, Pergamon Press, pp. 589-639.
WILLIAMS, R. T. (1967b) Comparative patterns of drug metabolism.
Fed. Proc., 26: 1029-1039.
WILLIAMS, R. T. (1971) Introduction: Pathways of drug metabolism. In:
Brodie, B. B. & Gillette, J. R., ed. Concepts in biochemical
pharmacology, Berlin, Springer-Verlag, Vol. 2, pp. 226-242.
WITIAK, D. T. & WHITEHOUSE, M. W. (1969) Species differences in the
albumin binding of 2,4,6-trinitrobenzaldehyde, chlorphenoxyacetic
acids, 2-(4'-hydroxybenzeneazo) benzoic acid and some other
drugs; the unique behaviour of rat albumin. Biochem. Pharmacol.,
18: 971-977.
WITSCHI, H. (1975) Exploitable biochemical approaches for evaluation
of toxic lung damage. In: Hayes, W. J. Jr., ed. Essays in
toxicology, New York, Academic Press, Vol. 6, pp. 125-191.
YUKI, H. & FISHMAN, W. H (1963) A carbazole method for the
differential analysis of glucuronate, glucosiduronate and
hyaluronate. Biochim. Biophys. Acta, 69: 576-578.
ZAKIM, D. & VESSEY, D. A. (1973) Techniques for the characterization
of UDP-glucuronyl-transferase, glucose-6-phosphatase and other
tightly bound microsomal enzymes. In: Glick, D., ed. Methods of
biochemical analysis, New York, Wiley, Vol. 21, pp. 2-37.
ZANNONI, V. G. (1971) Experiments illustrating drug distribution and
excretion. In: La Du, B. N., Mandel, H. G, & Way, E. L, ed.
Fundamentals of drug metabolism and drug disposition,
Baltimore, Williams & Wilkins, pp. 583-590.
ZANNONI, V. G., FLYNN, E. J., & LYNCH, M. (1972) Ascorbic acid and
drug metabolism. Biochem. Pharmacol., 21: 1377-1392.
ZELLER, E. A. (1971) Amine oxidases. In: Brodie, B. B. & Gillette, J.
R., ed. Concepts in biochemical pharmacology, Berlin,
Springer-Verlag, Vol. 2, pp. 518-535.
ZIEGLER, D. M., MCKEE, E. M., & POULSEN, L. L. (1973) Microsomal
flavo-protein-catalyzed N-oxidation of arylamines. Drug Metab.
Disposition, 1: 314-320.
5. MORPHOLOGICAL STUDIES
5.1 Introduction
Morphological studies are often the corner stones of toxicity
experiments. The variety of such studies leads to many different
approaches from the viewpoint of pathology, and skill and flexibility
in working procedures seem to be far more important than a strict
schedule.
On the other hand, it is advisable to have some general
guidelines on pathological procedures for routine quality testing for
toxicity, even though specific questions may often be posed, special
experiments may have to be carried out, and special animals,
techniques, and examinations used in order to elucidate certain
problems.
This chapter deals with the various phases of morphological
studies with a view to providing some general recommendations
concerning the procedures to be followed. It must be emphasized,
however, that these recommendations are only a guide, and that it will
be the pathologist's special responsibility to see that studies are
carried out in the way most likely to ensure optimum results.
5.2 General Recommendations
Gross necropsy facilities should be in close proximity to those
of the pathologist. The autopsy room must be equipped with adequate
dissection tables, dissection materials, running water, drains,
lighting, ventilation, and facilities for disinfection. In addition,
gross photography facilities are necessary. Cooling facilities must be
available for the storage of dead animals until necropsy, but the
animals should not be frozen (Sontag et al., 1975). To carry out
proper experiments and to prevent the loss of a considerable number of
animals by cannibalism or autolysis, it is essential that animals be
observed at least once a day, including Saturdays and Sundays. Animals
in moribund condition should be killed.
For the trimming of fixed tissues, a well-ventilated area,
preferably with an exhaust hood, and running water and drains, is
required.
The histology laboratory should be separated from the autopsy
room and should be equipped with tissue-processing equipment,
microtomes, cryostat, embedding and staining facilities, and supplies
(Sontag et al., 1975). Storage facilities are necessary for the fixed
tissues, as well as for the tissue block and histological slide files.
The facilities should be vermin-proof and temperature controlled or at
least cool.
Veterinary or medical pathologists with experience in laboratory
animal pathology or others with appropriate training and experience
should be responsible for all pathology procedures. In addition, the
pathologist should participate in the design and conduct of
experiments.
Histology technicians with appropriate training and experience in
the histological field will guarantee good histotechnical work,
whereas technicians trained and experienced in laboratory animal
dissection will be of great help in performing necropsies. Technicians
must be able to recognize and adequately describe gross abnormalities.
Personnel should be available at the weekends to perform
necropsies on animals found dead or killed in extremis.
5.3 Gross Observations
Gross pathology can be performed in most cases by experienced
technicians under the guidance of the pathologist. They should follow
a certain scheme of necropsy technique and must be able to recognize
abnormalities. A checklist should be used to ensure that all organs
and tissues are inspected and dissected. Information on clinical signs
must be available. Abnormalities found must be recorded on autopsy
cards. The descriptions must be clear and provide details such as
location, size, colour, texture, number, etc.
A great number of lesions will be within the scale of known
abnormalities for the animal species and breed used. Lesions found
beyond this normal scale should always be examined by the pathologist
himself. It is advisable for all new lesions, especially those
attributable to the treatment, to be photographed.
Dead animals should be necropsied unless cannibalism or autolysis
precludes this. Autolysis should not readily be accepted as an excuse
for not performing an autopsy. An inadequate gross necropsy cannot be
replaced by microscopic examination, no matter how well performed. On
the other hand, a well-performed gross necropsy may provide optimum
information for microscopic examination and may, in certain cases,
facilitate more selective microscopic examination.
Several ways of killing animals are available and the methods
depend on the facilities, the purpose of the experiment, and the
species used. Sacrifice of animals by anaesthesia (by ether or
barbiturates) is widely used as well as asphyxiation by carbon
dioxide. In rodents, the blood of the body can be removed by cardiac
puncture or puncturing the abdominal aorta, and in dogs by puncture of
the common carotid artery. Alternatively in small rodents decapitation
can be performed, though this may result in blood aspiration and
damage to certain tissues or organs. On the other hand decapitation
will probably give more constant results with respect to the total
blood loss, and consequently lead to fairly consistent organ weights.
5.3.1 Autopsy techniques
It is difficult, if not impossible, to prescribe working
procedures or techniques that will be adequate in all conditions.
Often, observations made when the animals were alive or experience
with other structurally-related compounds may focus attention on
certain organs or tissues. Sometimes fixation by perfusion is
necessary for proper examination of specific tissues (for example, the
central nervous system).
Large numbers of animals should not be sacrificed at any one
time, if too few technicians are available to perform the necropsies.
Animals should be killed in sequence, especially where it is necessary
to sample tissues for biochemical, enzyme-histochemical, or analytical
studies. However, organs and tissues should be weighed as soon as
possible after death to ensure that they have not dried out and that
they can be fixed quickly. Special procedures may shorten the time
needed for full autopsy especially in the case of small rodents. The
exact organization of working procedures depends largely on the
facilities and labour available, but good preparation and teamwork are
crucial factors.
Sacrifice should be carried out so that animals of the
experimental and control groups are killed at approximately the same
time of day, thus preventing the introduction of variations in
physiological status dependent on circadian rhythm. In certain cases,
however, this may not be feasible.
Special care is necessary when test elements or compounds are
analysed after sacrifice. At times it may be necessary to kill the
animals in a given order to prevent contamination (e.g. sacrifice of
the control first, followed by the lowest dose level, then the
intermediate dose level, and finally the high-dose level).
Autopsy techniques have already been described (Roe, 1965) and
only a general outline of the procedures is given.
5.3.2 Rat, mouse, guineapig, rabbit, monkey
The necropsy starts with external examination including the body
orifices. Thereafter, the animals are fixed on their backs. After a
median incision, the skin is partly removed and the subcutis,
superficial lymph nodes, salivary glands, and mammary glands can be
inspected and dissected. The abdomen can be opened and the negative
pressure of the thorax may be checked and the thorax opened.
Thyroid and thymus can then be inspected and dissected.
Trachea, lungs, and heart are removed en bloc. The heart is
detached, but the trachea and lungs remain intact to provide easy
handling for inflation of the lungs with fixative (see under fixation
methods).
Abdominal organs are removed. The examination of liver and kidney
should include making a routine number of parallel slices of the
organs to examine their cut surfaces. The gastrointestinal tract
should be removed from the abdominal cavity and opened. In chronic
toxicity experiments, the entire gastrointestinal tract must be
opened, the mucosal surface examined, and representative parts taken
for histological examination. The oesophagus and parts of the
intestine can be rolled according to the Swiss roll technique in order
to obtain a considerable length of mucosa musculature and serosa in
the microscopic section. Brain, peripheral nerves, skeletal
musculature, and bones (including a joint) are removed. The spinal
cord may best be fixed in situ.
5.3.3 Carnivores, swine
After external examination, necropsy is performed when the animal
is lying on its right side. Left front and hind legs are removed
without opening the apertura thoracis superior. The skin is partly
removed after a median ventral incision from mouth to os pubis.
Subcutis, fat, mammary glands, salivary glands, and superficial lymph
nodes may be inspected and dissected. In addition, the different
joints of the legs are opened and inspected. After opening the
abdomen, the thorax is checked for negative pressure and opened by
removing the left side of the thoracic wall. Then the right side of
the pleural cavity is inspected. The oesophagus is removed by cutting
it cranially. The thoracic and abdominal organs are removed and
individually inspected and opened. The nasopharynx is then opened and
inspected, and the tongue, larynx, pharynx, tonsils, brain, spinal
cord, peripheral nerves, and musculature collected. Most organs should
be sliced in parallel slices to examine the cut surfaces.
5.4 Selection, Preservation, Preparation, and Storage of Tissues
5.4.1 Selection of tissues
Which organs and tissues should be collected for fixation is
determined by the type of toxicity experiment and the compound being
tested. Sufficient material should be collected to prevent the
necessity of having to repeat a certain experiment. Since it is
impossible to provide strict rules for selection, only a general
outline will be given.
5.4.2 Oral toxicity tests
In acute toxicity experiments, restrictive microscopic
examination may be necessary in certain cases. Some laboratories fix
liver and kidneys, others do not. The additional information obtained
from histological examination of these organs in acute experiments is,
usually, limited.
In range-finding tests, restrictive pathology is common practice.
Normally, the heart, liver, spleen, and kidneys and all grossly
abnormal tissues are collected for fixation. When the compound tested
is given by stomach tube, it is advisable to include lungs,
oesophagus, and stomach in the histological study. In subacute
(90-day) and chronic studies, it is advisable to select all tissues
and organs for fixation in buffered formal saline. This normally
includes the following organs and tissues:
brain gall bladder (if present)
pituitary oesophagus
thyroid (parathyroid) stomach
thymus duodenum
(trachea) jejunum
lungs ileum
heart caecum
sternum (bone marrow) colon
salivary glands rectum
liver urinary bladder
spleen lymph nodes -- mandibular or mesenteric
kidneys lymph nodes -- popliteal or axillary
adrenals mammary glands
pancreas (thigh) musculature
gonads peripheral nerve
accessory genital organs (eyes)
aorta (femur -- incl. joint)
(skin) spinal cord (at three levels)
(exorbital lachrymal glands)
The tissues mentioned between brackets are sometimes considered to be
optional. In addition, all tissues containing grossly observed lesions
should be fixed.
The same selection is usually made in carcinogenicity tests. In
both chronic toxicity and carcinogenicity experiments, it is also very
important to collect all organs and tissues of animals that have died
or have been killed in extremis. Prompt examination of the tissues
of these animals may provide valuable information leading to a more
meaningful pathological examination of animals, that die or are
sacrificed later on, or at the end of the experiment. In cases where
only part of an organ or tissue is taken, it is important that the
same part of that organ or tissue be selected at approximately the
same site in all animals.
5.4.3 Inhalation toxicity studies
In acute inhalation studies, it may be worthwhile, in some cases,
to select lungs for fixation and subsequent microscopic examination,
as well as liver and kidneys. In most cases, however, this seems
unnecessary since the pulmonary lesions are usually of the same type
(hyperaemia and oedema) and can be detected by gross inspection.
Examination of the entire respiratory tract is necessary in
subacute inhalation studies. This includes nasal cavity, pharynx,
larynx, trachea, main bronchi, and lungs. The exact orientation of
these organs for trimming, embedding, and sectioning is of prime
importance and needs special attention. More organs and tissues may be
selected in the inhalation studies, selection usually being effected
in the same way as for oral studies.
5.4.4 Dermal toxicity studies
In acute dermal toxicity studies, it is advisable to perform
microscopic pathology on liver and kidneys. The presence of
degenerative changes in these organs indicates that the compound is
active transdermally. Examination of the skin is also advisable.
In subacute dermal toxicity studies, the organs and tissues are
selected in the same way as that described for oral tests. Of course,
the skin deserves special attention in that both treated areas and
normal skin in comparable areas are selected.
5.4.5 Special studies
In the case of sensitization or irritation studies, the selection
of tissues depends completely on the type of test. In eye irritation
studies, the examination of eye and conjunctivae seems adequate
whereas in a Landstainer-Draize test, and especially in a maximization
test, the skin may be examined. In all these cases, it may, or may
not, be necessary to select more tissues.
5.5 Preservation of Tissues
Preservation of tissues can be performed by immersion, inflation
or distension, and perfusion. Immersion is most commonly used, but in
some circumstances may not result in satisfactory preservation.
Microscopic examination of the lungs, for example, cannot be done
adequately if they have not been inflated or have not been fixed by
perfusion. When the central nervous system is a target organ, it is an
absolute necessity to use perfusion to ensure proper fixation, as
fixation by immersion leads to many artifacts that are
indistinguishable from certain degenerative changes. When animals in
long-term tests are killed in extremis, it is advisable to use
fixation by perfusion. Perfusion is usually essential for electron
microscope studies of tissues.
With perfusion, the best conditions for microscopic examination
are obtained while effective gross examination remains possible. All
tissues should be fixed in 10% neutral buffered formalin or another
appropriate fixative.
5.5.1 Immersion
Immersion is the most used and usually the most appropriate
method of preservation in toxicity experiments. The tissues are placed
in the preservative and fixed for 24-48 h. Fixation at higher
temperatures (40-50°C), in a vacuum, or by use of microwaves (Gordon &
Daniel, 1974) may shorten the fixation time considerably. Tissues
thicker than 0.5 cm should not be fixed. The preservative/tissue ratio
is very important and must be greater than 10:1 (volume fixation
fluid/volume tissue) to obtain acceptable fixation. The fixation of
intact animals with opened abdomen should not be carried out. All
tissues and organs must be fixed separately, and some may need special
attention. Skin and peripheral nerves must be fixed in a straight (but
not stretched) and flat position. To achieve this, these tissues can
first be attached to a piece of thick filter paper. The spinal cord
can best be fixed in situ before it is taken out. Special fixing
solutions may be used in certain cases such as Bouin's fixative for
the fixation of ovaries, testes, thyroid, and adrenals or Zenker's
solution for the fixation of the eyes.
As certain organs (pituitary, thyroid, ovaries, adrenals, and
lymph nodes) of some of the smaller rodents are rather small, they
should be fixed separately in smaller jars to prevent loss; various
fixatives may be used.
5.5.2 Inflation
Inflation is used to preserve lungs effectively. To prevent the
formation of artifacts that may be misjudged as emphysema, it is
necessary for inflation to be carried out at constant pressure
(Chevalier, 1971; Fawell & Lewis, 1971). In certain cases, however,
even fixation of the lungs by inflation may not be adequate and
perfusion will be preferred (for example, in inhalation studies).
Inflation with fixative is also necessary for correct fixation of
the urinary bladder. If the bladder is distended, urine must be
replaced by fixative via the urethra using a syringe with a blunt
needle. Contracted empty bladders should be partly distended with
fixative. The reflux of fixative in the bladder is prevented by
ligation of the urethra. Inflation may also be used for fixation of
the digestive tract. Here again, ligation is necessary. In these
cases, the organs have to be bisected after fixation and the interior
surface inspected.
5.5.3 Perfusion
The best way to preserve tissues is by perfusion, which is
usually effected by infusion in the left ventricle of the heart and by
opening the sinus venosus to provide for proper circulation. Perfusion
of isolated organs (liver, kidneys) is another possibility. Before
perfusion, the animals are anaesthetized with a barbiturate,
administered in combination with nitrate and heparin to ensure
vasodilation and prevent clotting. A solution consisting of 77.5 ml
sodium nitrite (1.25%) in water, 10 ml heparin (5000 IU/ml) and 12 ml
pentobarbital (60 mg/ml), of which 10 ml is administered per kg body
weight, is satisfactory. Then the blood is removed with an isotonic
saline solution using slight overpressure. When all the blood has left
the body via the sinus venosus or right atrium, the body may be
perfused with the fixative.
Perfusion is sometimes not possible especially in experiments
where the recording of organ weights is important (i.e. in subchronic
toxicity studies). This difficulty can be solved by increasing the
number of experimental animals, though this may lead to a considerable
increase in costs. Perfusion of large numbers of animals is possible
using simple facilities at low cost (Fig. 5.1).
5.6 Trimming
It is important that the trimming be carried out by well-trained
people. It must be emphasized that knowledge of pathological phenomena
is necessary, as it frequently happens that the person responsible for
trimming cuts out the "good looking" areas and discards the tumours
present in the organs. The tissues must be sliced in such a way that
the cut surfaces present the largest possible area for examination.
The use of a special trimming scheme during the procedure may be
helpful.
The kidneys should be sectioned through the cortex and medulla,
one kidney mid-longitudinally, the other mid-transversely.
The brain must be cross-sectioned at, at least, three sites: the
frontal cortex with basal ganglia, parietal cortex with thalamus, and
cerebellum with pons.
The lungs should be sectioned transversely, parallel to the long
axis of the body. These sections must include the main bronchi and
carina.
The hollow organs should be trimmed in such a way that a
cross-section from mucosa to serosa is obtained.
 Tumours or tumorous masses usually need to be trimmed in several
portions. Preferably, tissues surrounding the tumour should be
included.
When intestines are fixed as a roll, the roll can best be
embedded as such, since, trimming of these rolls is practically
impossible, even after fixation.
For certain organs, special trimming procedures are needed. For
example, the nasal cavity should be sectioned transversely at three
sites. The trimming of the larynx/pharynx is crucial in order to
obtain a section that can be properly interpreted.
Trimmed tissues should have a maximum thickness of 2-3 mm for
satisfactory processing.
5.7 Storage
Material not used for processing should not be discarded, but
should be stored in airtight jars or plastic bags to ensure that the
tissues do not dry out. Plastic bags are an excellent way of storing
tissues in minimum space. The bags should be clearly and permanently
labelled. The tissues should be stored, at least, until the
microscopic examination has been completed and the findings adequately
evaluated. If at all possible, the tissues should be stored for a long
period (e.g. material from 90-day studies should be stored for 2 years
and that from chronic experiments for 5-10 years), but storage
facilities may prevent this. Tissue blocks and sections can be stored
in a cool area for a considerable time (10 years or more).
5.8 Histological Techniques
Embedding in paraffin or polymer-containing paraffins or waxes is
advisable. The embedding procedures may be shortened considerably by
using automatic tissue-processing equipment and special frames in
which the paraffin blocks can be made in large numbers (Fig. 5.2).
Proper and exact labelling of the blocks is a necessity. The blocks
may be prepared in such a way that they can be placed as they are in
the microtomes.
Tissue sectioning can be performed at a thickness of 4-6 mm and
sections can be stained routinely with haematoxylin and eosin or a
comparable routine stain. Serial sectioning can best be done with the
help of an engine-powdered microtome.
The use of semithin sections (1 µm) is of considerable importance
for specific organs such as bone-marrow, kidneys, lymph nodes, spleen,
and endocrine glands. These sections can now be made on special
Tumours or tumorous masses usually need to be trimmed in several
portions. Preferably, tissues surrounding the tumour should be
included.
When intestines are fixed as a roll, the roll can best be
embedded as such, since, trimming of these rolls is practically
impossible, even after fixation.
For certain organs, special trimming procedures are needed. For
example, the nasal cavity should be sectioned transversely at three
sites. The trimming of the larynx/pharynx is crucial in order to
obtain a section that can be properly interpreted.
Trimmed tissues should have a maximum thickness of 2-3 mm for
satisfactory processing.
5.7 Storage
Material not used for processing should not be discarded, but
should be stored in airtight jars or plastic bags to ensure that the
tissues do not dry out. Plastic bags are an excellent way of storing
tissues in minimum space. The bags should be clearly and permanently
labelled. The tissues should be stored, at least, until the
microscopic examination has been completed and the findings adequately
evaluated. If at all possible, the tissues should be stored for a long
period (e.g. material from 90-day studies should be stored for 2 years
and that from chronic experiments for 5-10 years), but storage
facilities may prevent this. Tissue blocks and sections can be stored
in a cool area for a considerable time (10 years or more).
5.8 Histological Techniques
Embedding in paraffin or polymer-containing paraffins or waxes is
advisable. The embedding procedures may be shortened considerably by
using automatic tissue-processing equipment and special frames in
which the paraffin blocks can be made in large numbers (Fig. 5.2).
Proper and exact labelling of the blocks is a necessity. The blocks
may be prepared in such a way that they can be placed as they are in
the microtomes.
Tissue sectioning can be performed at a thickness of 4-6 mm and
sections can be stained routinely with haematoxylin and eosin or a
comparable routine stain. Serial sectioning can best be done with the
help of an engine-powdered microtome.
The use of semithin sections (1 µm) is of considerable importance
for specific organs such as bone-marrow, kidneys, lymph nodes, spleen,
and endocrine glands. These sections can now be made on special
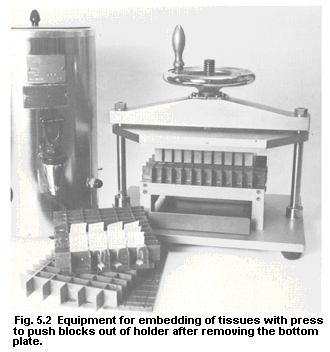 microtomes, that cut the normal paraffin blocks with glass knives. Of
course, this procedure should only be followed when specific details
have to be followed during the microscopic examination. The use of
semithin Epon sections yields even better results.
It will often be necessary to use special staining techniques on
tissues in order to provide more information on the presence of
carbohydrates, proteins, fats, elements, or certain structural
organizations. Special fixation is sometimes necessary for appropriate
staining. Bones and calcified tissues have to be decalcified. Eyes
usually need to be fixed and embedded differently. Blood vessels may
be stained with Sudan black in order to study vascular lesions.
5.9 Special Techniques
5.9.1 Enzyme histochemistry
Enzyme histochemistry is a technique used to detect the activity
or presence of an enzyme in a tissue by incubating the fresh tissue in
an appropriate medium, so that a fine coloured granular precipitation
forms wherever the enzyme is present.
The use of the enzyme-histochemical technique is not common in
toxicity testing. Nevertheless, the technique is a valuable one, since
it provides information about the metabolic activity and function of
the tissues. Furthermore, it introduces the possibility of correlating
biochemical with histological findings that may lead to a more correct
interpretation. Enzyme-histochemical investigations also make it
possible to visualize certain differences in enzyme activity within
the structural organization of tissues.
In some cases, a decrease in enzyme activity in certain cells is
compensated for by an increase in other cells within the same organ.
Such biological differences can only be detected by
enzyme-histological investigation, when the results of biochemical
determinations are within normal limits and no alterations are seen
using conventional histological techniques. Enzyme-histological
investigations can easily be incorporated in routine toxicity testing,
if necessary.
Relatively small pieces of tissue are quickly frozen in an inert
liquid (i.e. isopentane), or cooled in liquid nitrogen or a mixture of
solid carbon dioxide and methanol. Storage of the tissues is effected
at -70°C. Cryostat sections are prepared and incubated in specially
prepared media. Good reference works for the methods have been
published (Barka & Anderson, 1965; Pearse, 1968, 1972). To obtain
optimum information, the prepared section should be examined by
semiquantitative methods.
An enzyme histochemical investigation can also be performed at
the electron microscope stage (Geyer, 1973). In this case, the
activity or presence of an enzyme is determined by the deposition of
electron-dense material at the sites where the enzyme is located. It
is evident that these delicate techniques are not easy to apply in
routine toxicity testing. They may, however, be of great importance in
specific studies on the biological effects of a compound on cellular
components, or to detect early damage.
5.9.2 Autoradiography
Autoradiography is based on the principle that radioactive
substances present in tissues are able to produce an image on a
photographic film or plate. The radiations emitted by the radioactive
substances must be of relatively weak energy, so that they will have a
short range in tissues and emulsions. When the range is too long,
developed silver grains can be found in the emulsion far away from the
radioactive source.
In this respect only alpha particles, beta particles, and Auger
electrons are useful since electromagnetic radiations such as gamma
and X-rays give poor results due to their penetrating power.
Tritium (3H) has been most frequently used in autoradiographic
studies, although 14C and 32P are also being used as tracers.
Autoradiography, at both light microscopic and ultrastructural
levels, can be used in kinetic studies on tissues by incorporating
radioactive-labelled bases in DNA or RNA resulting in the production
of silver grains on a photographic film covering the section. The
different methods have been extensively described for nondiffusible
substances (Baserga & Malamud, 1969; Rogers, 1967) as well as for
diffusible substances (Roth & Stumpf, 1969).
Apart from studying the kinetics of tissues, autoradiographic
techniques can also be used to study the distribution of radiolabelled
compounds and their metabolites. This, again, can be done at light
microscopic and ultrastructural levels.
The technique of whole-body autoradiography as developed by
Ullberg et at. (1972) is a valuable tool, in this respect, since the
distribution of a compound can qualitatively (or even
semiquantitatively) be studied in the whole body. In addition, pieces
can be cut out to use for microautoradiographic research.
Substances may also be labelled with fluorescent chemicals
permitting their detection by illumination of the sections with
ultra-violet radiation. The technique of fluorescence microscopy can
also be used for the detection of certain dyes that possess
autofluorescing properties. Some substances, such as the
catecholamines, can be made visible by reaction with formaldehyde
which converts mines into fluorescent substances (Eränko & Räisänen,
1966). Fluorescence in tissues can be measured quantitatively, thus
facilitating controlled conditions (Ploem et al., 1974).
Autoradiographic studies cannot be incorporated easily into
routine toxicity experiments. However, the technique may be a very
important tool in special studies.
5.9.3 Immunofluorescence and immunoenzyme techniques
Immunofluorescence techniques are primarily used in determining
the presence or absence of certain antibodies or antigens in tissues.
Antigens are detected by binding them to specific antibodies. If a
specific antiserum is conjugated with a fluorescent dye, the specific
antigen-antibody complex formed can be seen by studying the tissue
section using ultraviolet radiation microscopy. This direct
immunofluorescent technique can be replaced by a more sensitive
indirect method in which the substance actually rendered fluorescent
is not the antigen under consideration but an intermediate material
the distribution of which corresponds precisely to that of the antigen
being studied. Information on the principle of these techniques is
available (Goldman, 1968; Nairn & Marrack, 1964).
The use of enzyme conjugates has recently been developed
(Sternberger, 1974). This system is used when antigen-antibody
complexes have to be localized at ultrastructural levels.
Immunofluorescence or immunoenzyme techniques will not be used in
most routine toxicity experiments, but they may be of importance in
special studies such as those described in Chapter 7.
5.9.4 Electron microscopy
Transmission electron microscopy is the most commonly used
technique for studying the ultrastructure of tissues. Different
fixation, embedding, and cutting techniques have to be used to obtain
ultrathin sections of tissues (Flauert, 1973, 1974, 1975; Hayat, 1970,
1972, 1973). At the ultra-structural level, very minute changes can be
detected and they have to be distinguished from the many artifacts
that can be introduced during the different processing procedures.
Under optimum conditions, tissues can be examined at high
magnifications.
As tissue examination by electron microscopy is very time
consuming and costly, it is important only to select tissues from
those experiments that justify it. Ultrastructural studies have
contributed enormously to our knowledge of molecular biology. In the
case of toxicology, ultrastructural studies are always carried out as
a secondary investigation, and are usually applied to
ultrastructurally, specific, pathological changes already studied in
detail at the light microscopic level, in order to secure a better
judgment of the importance of the lesion. Ultrastructural studies are
also used to confirm that target organs that appear normal at a
certain close level using light microscopy, do not in fact show
pathological changes.
Scanning electron microscopy (SEM) techniques, that have come
into use in recent years, provide 3-dimensional images of complete
biological units and also chemical information (Hayes, 1973). Here
again, special processing procedures may be needed, although, in
certain cases, formalin-fixed and paraffin-embedded material can be
used. Natural surfaces, dissected material, sectioned tissue, and
living specimens may be studied. SEM is a valuable tool in biology
but, for toxicological investigations, its use is still rather
restricted, apart from the possibility of obtaining quantitative
chemical information. The development of analytical electron
microscopy, using the principles of X-ray microanalysis, also makes it
possible to correlate tissue ultrastructure and chemistry (Hayes,
1973; Weavers, 1973).
5.10 Microscopic Examination
Routine histopathological examination is very important and
should be carried out correctly. First of all, the sections to be
studied should be of good quality. Additional sections and special
stains must be prepared if necessary. Special stains are used to study
and describe individual lesions; they may also be used to examine
certain organs or lesions to permit better judgment of
semiquantitative comparison. For example, when haemosiderosis is found
to be an important effect, special stains, based on the reaction of
the dye with iron, facilitate semiquantitative analysis of the degree
of the lesion.
Good microscopic equipment is necessary to perform optimum
microscopic examination. Sources of ultraviolet radiation and
polarized light should be available.
The microscopic examination must be carried out by well-trained
pathologists or other persons trained and experienced in the field of
laboratory animal pathology. The use of parapathologists for routine
microscopy in toxicity and carcinogenicity experiments is extremely
valuable and is important for overcoming a shortage of personnel,
trained in laboratory animal pathology (Toxicol. appl. Pharmacol.,
1975). Experience has shown that well-trained parapathologists can
become very skilful in microscopy and in screening sections for
abnormalities. With further experience, they are also able to describe
certain lesions. It is the pathologist's duty to see that all lesions
observed are described and interpreted correctly, and to check the
sections for any lesions that may have been overlooked.
It is a serious mistake, in an experiment, to undertake
microscopic examination before the results of other examinations such
as biochemical determinations, haematology, and organ weights, are
available, for the information obtained from these procedures may give
important directions for the microscopic study. For example, an
increased thyroid weight may lead to more careful examination of the
thyroid for hyperplasia and may prompt histometric determinations.
Lower lymph node weight may point to semiquantitative examination of
these organs with regard to reduced immunocapacity (Cottier et al.,
1972). Differences or changes in the blood picture call for more
detailed study of the bone marrow, for example, by preparing and
studying 1 µm sections, to detect suspected haemopoietic disturbances.
Gross observations should always be correlated with microscopic
findings. It is a false assumption to think that a microscopic
examination will be more objective when performed blindly; knowledge
of clinical signs and macroscopy are indispensable for a meaningful
examination. Of course, the pathologist must be careful not to let
knowledge of clinical effects influence his objective evaluation of
the tissues; thus, sections of the tissues considered to be involved
may have to be re-examined blindly. If such a re-examination is
carried out, semiquantitative scoring of the extent of the lesion will
also help to detect a possible dose-effect relationship. Should there
be any doubt in interpreting the significance of a lesion, it is
advisable to consult other pathologists, all of whom should re-examine
the slides blindly.
5.10.1 Number of animals and number of organs and tissues studied
microscopically
In acute and rangefinding tests, the organs and tissues are
usually fixed and examined microscopically after processing. In
subacute and long-term studies, and, sometimes, in rangefinding tests,
it is customary initially to examine only the tissues of the highest
dose group, the control group and the target organs, if known. In
addition, all grossly observed lesions are processed in the
intermediate and low-level groups. If the results of the microscopic
examination of the highest dose group indicate a need to examine
certain organs at lower levels, this can be done at a second stage.
In chronic toxicity experiments, all males and females of the
highest dose group should be examined. It is also advisable to examine
all control animals completely, since this will be the only way to
ascertain the incidence of tumours and other "lesions" normally
occurring in the strain of animals used. Such information is
indispensable for correctly evaluating the significance of changes
observed in exposed animals.
In carcinogenicity experiments, where larger numbers of animals
per group are usually used, some laboratories restrict microscopic
examination mainly to the grossly observed abnormalities and tumours,
and perform complete histological examinations on only 15-20 male and
15-20 female survivors of the highest dose group and of the control
group, on the assumption that a well-performed gross examination will
detect most of the tumours present. Histological examination of all
tissues of 15-20 animals of each sex will give some information on the
possible presence of certain pre-malignant hyperplastic or neoplastic
changes not grossly observed. If such lesions are found, the
histological examination should cover all animals, while small organs
which may bear tumours that cannot be grossly observed (thyroid,
pituitary, adrenals) must be examined histologically.
5.10.2 Description of the lesions
For the microscopic examination, it is essential to use a
check-list to detect losses, especially of small organs lost in the
processing procedure. All organs and tissues examined should be
listed, even though no abnormalities are seen, while all lesions found
should be described clearly and accurately. It is essential to
describe lesions in a semiquantitative way using such words as slight,
moderate, strong, and very strong to indicate the extent of the
lesion; it is also essential to indicate the criteria used to
investigate and classify abnormalities. It will often be necessary to
re-examine certain lesions in order to obtain a well-balanced
semiquantitative judgment. Several reference works may be of help in
the classification of tumours and other lesions (Beveridge & Sobin,
1974; Cotchin & Roe, 1967; Ribelin & McCoy, 1965; and Turusov, 1973).
Certain lesions found by microscopic examination may, in fact,
consist of several entities. In such cases, it may be important to
classify these entities independently. For example, perilobular liver
degeneration may be combined with bile duct proliferation, but one of
the two phenomena may be more extensive than the other with respect to
dose level and time. For a better interpretation, it is preferable to
describe and classify such lesions independently.
5.11 Presentation, Evaluation, and Interpretation of Pathological
Data
A well-performed and well-described microscopic examination is
not complete if the results cannot be studied and compared easily and
adequately. The lesions found in the different groups should be
tabulated according to organ, group, and sex in order to facilitate
comparison of the incidence of such lesions in the different groups.
If, in addition, a semiquantitative estimate of the extent of the
lesions is made, it will be easy to see whether they are
compound-induced and increase with time and dose.
It is important that these tables include details such as the
number of animals necropsied and the number examined microscopically,
per group and sex. In addition, it is advisable to state, the exact
number of organs or tissues examined microscopically, since it is well
known that, in every toxicity experiment, organs and tissues get lost
during the processing procedure. In this way, the loss can be
adequately estimated and the lesions found can be expressed against
the real number of organs or tissues examined.
For correct interpretation of the tumour tables, it is advisable
to include hyperplastic and preneoplastic lesions as individual
groups. The table should also include the number of days that elapsed
from the start of the experiment until the observation of each rumour.
This first observation may be clinical, for example, when mammary, ear
duct, or skin tumours are involved and the time that has elapsed can
then be called the "induction time". However, usually, tumours are
found after death or killing in extremis, or at sacrifice at the end
of the experiment. Thus, in these cases, it seems most appropriate to
use the term "time of observation". The inclusion of the "time of
observation" of tumours in the table is of help to the investigator
since it provides information on shorter or prolonged latency periods
(e.g. time that has elapsed between initiation and the appearance of
tumours). In addition, it reminds the reader to take into account the
survival differences when expressing the results.
In long-term experiments, the number of lesions found may be
quite large. Furthermore, some lesions may have been noticed during a
certain period in life, when the animals died or were sacrificed
in extremis, while others will have been noticed at the end of an
experiment. When such lesions are tabulated in the same table, this
information may get lost. It seems, therefore, most appropriate in
such long-term experiments to pool the results of the pathological
examination for certain periods, for example the first 12 months of
life and then for 3- to 6-monthly intervals, and to give the results
of the examination undertaken at the end of the experiment separately.
It is obvious that in preparing such tables, the use of an
automated data acquisition and computer-based system will be of great
help. These systems have been described for application to
toxicological studies, especially for animal weights, food and drug
consumption, organ weights, and biochemical data (Munro et al., 1972).
In pathology, such systems offer considerable potential. The necessity
for the correct coding of pathological changes, and the use of a code
system set up so that detailed information about lesions can still be
obtained, is obvious. Several systems have been described (Becker,
1973; Enlander, 1975; Smith et al., 1972), and may be adapted for this
purpose.
Pathological examinations must always be evaluated and
interpreted in connexion with other phenomena found. It must be
decided whether lesions noticed are in fact pathological, and whether
they are found in connexion with changes in other variables such as
organ weights, biochemical tests, etc. If compound-induced lesions are
found, they may not only be present at the highest dose level, but
also at an intermediate dose level. In the latter case, it is
important to determine whether there is a dose-dependent increase in
the severity and extent of the lesion, a finding which would
strengthen the assumption that the lesion is compound-induced.
Moreover, a clear dose-response relationship permits better evaluation
of the potential risk of a compound. Whenever lesions are found only
at the highest dose level, the sections at all dose levels should be
re-examined blindly to remove any doubt. Often the two sexes will show
a different sensitivity to the action of a compound.
Problems may arise when certain lesions are found more frequently
and to a greater extent in the treated groups compared with the
control group and compared with the common incidence of the lesion in
the strain used. The usual attitude towards this phenomenon is to
consider it as an effect or response.
In the evaluation of tumour incidence, criteria such as increased
tumour incidence, decrease in latency period, and the appearance of
tumours in organs where they do not occur spontaneously, have to be
considered. For this reason, detailed information on the spontaneous
occurrence of tumours in the species and strain used is essential,
particularly when tests indicate that the compound possesses a weak
carcinogenic action. Simple comparison of tumour incidence in control
animals and experimental animals at one point in time may lead to
erroneous conclusions, especially when only one of the above three
criteria is met. Many factors other than treatment may influence the
incidence of spontaneous tumours.
Statistical evaluation of the results is often necessary, but, as
yet, there are no agreed procedures for comparing statistically the
incidence of malignant tumours in controls and experimental groups.
REFERENCES
BARKA, T. & ANDERSON, P. J. (1965) Histochemistry: theory, practice
and bibliography. New York, Harper & Row; London, Evanston.
BASERGA, R. & MALAMUD, D. (1969) Autoradiography: techniques and
application. New York, Harper & Row; London, Evanston.
BECKER, H. (1973) Experiences with a test-stage free-text processing
system in pathology, using display terminals. Path. Europ.,
8: 307-313.
BEVERIDGE, W. I. B. & SOBIN, L. H., ed. (1974) International
histological classification of tumours of domestic animals.
Bull. World Health Org., 50: 1-142.
CHEVALIER, H. J. (1971) [A method for the fixation of the lungs of
small animals.] Z. Versuchstierk., 13: 101-104 (in German).
COHRS, P., JAFFE, R, & MEESEN, H. (1958) [Pathology of laboratory
animals] Berlin, Springer-Verlag, Vol. 2 (in German).
COTCHIN, E. & ROE, F. J. C. (1967) Pathology of laboratory rats and
mice. Oxford, Edinburgh, Blackwell.
COTTIER, H., TURK, J., & SOBIN, L. (1972) A proposal for a
standardized system of reporting human lymph node morphology in
relation to immunological function. Bull. World Health Organ.,
47: 375-408.
ENLANDER, D. (1975) Computer data-processing of medical diagnosis in
pathology. Am. J. clin. Pathol., 63: 538-544.
ERÄNKO, O. & RAISANEN, L. (1966) In vitro release and uptake of
noradrenaline in the rat iris. In: Ebler, U.S. V., Rosell, S. &
Uunäs, B., ed. Mechanism of release of biogenic amines. New
York, Pergamon Press.
FAWELL, J. K. & LEWIS, D. J. (1971) A simple apparatus for the
inflation fixation of lungs at constant pressure. Lab. Anim.,
5: 267-270.
FLAUERT, A. M. (1973, 1974, 1975) Practical methods in electron
microscopy. Amsterdam, Oxford, North Holland Publ.; New York,
Elsevier.
GEYER, G. (1973) [Ultrahistochemistry, histochemical working
guidelines for electron-microscopy.] Stuttgart, Gustav Fischer
Verlag (in German).
GOLDMAN, M. (1968) Fluorescent antibody methods. New York, London,
Academic Press.
GORDON, H. W. & DANIEL, E. J. (1974) Microwave fixation of human
tissues. Am. J. med. Technol., 40(10): 441-442.
HAYAT, M. A. (1970, 1972, 1973) Principles and techniques of electron
microscopy. In: Biological Applications Vol. I, II, III, IV.
New York, Van Nostrand Reinhold Company.
HAYES, T. I. (1973) Scanning electron microscopy in biology. In
Koehler, J. H., ed. Advanced techniques in biological electron
microscopy, Berlin, Heidelberg, New York, Springer-Verlag,
pp. 153-214.
MUNRO, I. C., CHARBONNEAU, S. M., & WILLES, R. F. (1972) An automated
data acquisition and computer-based computation system for
application of toxicological studies in laboratory animals.
Lab. Anim. Sci., 22: 753-756.
NAIRN, R. E. & MARRACK, J. R. (1964) Fluorescent protein tracing.
Edinburgh, London, Livingstone.
PEARSE, A. G. E. (1968, 1972) Histochemistry, theoretical and
applied. 3rd ed., Edinburgh, Churchill Livingstone, Vol. I &
II.
PLOEM, J. S., DE STERKE, J. A., BONNET, J., & WASMUND, H. (1974) A
micro-spectrofluorometer with epi-illumination operated under
computer control. J. Histochem. Cytochem., 22: 668-677.
RIBELIN, W. E. & MCCOY, J. R. (1965) The pathology of laboratory
animals. Springfield, Charles Thomas.
ROE, F. J. C. (1965) Spontaneous tumours in rats and mice. Food
Cosmet. Toxicol., 3: 707-720.
ROGERS, A. W. (1967) Techniques of autoradiography. Amsterdam,
London, New York, Elsevier.
ROTH, L. J. & STUMPF, W. E. (1969) Autoradiography of diffusible
substances. New York, London, Academic Press.
SMITH, J. E., MCGAVIN, M. D, & GRONWALL, R. (1972) English-language
computer-based system for storage and retrieval of pathological
diagnosis. Vet. Pathol. 9: 152-158.
SONTAG, J. M., PAGE, N. P., & SAFFIOTTA, U. (1975) Guidelines for
carcinogen bioassay in small rodents. Bethesda MD, National
Cancer Institute.
STERNBERGER, L. A. (1974) Immunocytochemistry. Englewood Cliffs, NJ,
Prentice-Hall. Toxicol. appl. Pharmacol. (1975) 31: 1-3, A
training programme for parapathologists.
TURUSOV, V. S. ed. (1973) Pathology of tumours in laboratory animals,
Vol. 1, Lyon, IARC (Sci. Publ. No. 5).
ULLBERG, S., HAMMARSTROEM, L., & APPELGREN, L-E. (1972)
Autoradiography in pharmacology. In: Cohen, Y., ed. I.E.P.T.
Section 78, Oxford, New York, Pergamon Press, Vol. 1,
pp. 221-239.
WEAVERS, B. A. (1973) The potentiality of EMMA-4, the analytical
electron microscopy in histochemistry: a review. Histochem. J.,
5: 173-193.
6. INHALATION EXPOSUREa
6.1 Introduction
Advances in technology, throughout the world, have increased the
number and amount of chemicals in the atmosphere. The health effects
of inhalation of these chemicals can be predicted to some extent by
experimental investigation. In order to simulate environmental
conditions, a special technology has evolved relating to the design
and operation of inhalation chambers and the generation and
characterization of aerosols and vapours. This chapter will introduce
this technology and review the significance of certain biological
end-points commonly measured in inhalation studies.
This chapter is not intended to be all-inclusive, nor to provide
complete instructions on inhalation technology. No toxicological
protocol is sufficient to cover all situations with all materials.
Hence, the expertise of the individual investigator and the objectives
of the investigation will govern the selection of the final protocol
and the biological end-points relevant to the particular compound
concerned.
The costs associated with evaluation of toxicity by the
inhalation route are considerably higher than for toxicity studies
using other routes of exposure because of the cost of the specialized
equipment used in inhalation studies and the time required for its
calibration. In general, it can be estimated that the total cost of
any type of inhalation study, short- or long-term, will be two to
three times that of a comparable oral study. Because of this, it is
important to determine when inhalation studies should be performed and
when, and if, studies by other routes would suffice. Finally, in
addition to costs, other resources should be considered. In most
countries, the number of laboratories capable of performing inhalation
studies is limited. Thus, it is essential to develop priorities for
the selection of compounds for evaluation of inhalation toxicity.
6.2 Need for Inhalation Studies
The effect of a compound depends on its concentration at the
receptors of the affected organ or system. The concentration at
different sites and times is a function of the route of entry. Thus
the route of entry is an important factor with regard to toxicity. No
good substitute model for inhalation exposure as a means of direct
exposure of the lungs has been developed, although intratracheal
exposure has been frequently used, particularly in pulmonary
a The assistance of Dr J. O'Neil, Dr M. Amdur, and Dr W. Busey in
the preparation of this chapter is gratefully acknowledged.
carcinogenesis studies. The distribution kinetics are different with
pulsed exposures compared with constant inhalation exposures. With
pulsed exposures, such as oral, intravenous, intraperitoneal, etc.,
the concentration of the test material will reach a peak and then
usually fall off, depending on the distribution coefficients for each
compound and organ in question. With continuous exposure,
concentrations in many body compartments will attain an equilibrium,
depending again on the concentration of the test material and the
distribution coefficient. Thus, quantitative toxicity is often quite
different, depending on the route of entry.
With the exception of certain drugs, man's exposure to
environmental agents comes either from skin contact, ingestion, or
inhalation. Inhalation is of particular importance in occupational
exposure and in exposure to air pollutants. For airborne substances,
the lung is the first organ that foreign chemicals encounter and it is
one of the body's first lines of defence. Chemicals that enter the
lung can either exert a direct effect on the cells of the lung, or be
absorbed into the systemic circulation. Blood passing from the lung to
the heart and then into the peripheral circulation can carry agents
directly to other organs without passing through the detoxication
processes of the liver. This contrasts with oral exposure where
chemicals may be absorbed into blood that immediately passes through
the liver and can be metabolically transformed into either more or
less toxic compounds.
Direct contact with irritants causes local inflammation in the
respiratory system, the degree of which may depend on the local
concentration and not on the total dose. For example, Kljackina (1973)
demonstrated that inhaled bromine acts as a specific irritant of the
respiratory system whereas bromine administered orally results in
changes in the nervous system.
Thus, specific reasons for performing inhalation studies include:
(a) determination of specific responses of the respiratory tract;
(b) assessment of the toxic hazard of agents whose principal route
of exposure is via inhalation; (c) investigation into the mechanism
of toxicity of inhaled materials; and (d) study of the comparative
toxicity of agents administered by different routes.
6.3 Fate of Inhaled Materials
6.3.1 Nature of aerosols
The nature and characteristics of aerosols have been treated in
detail elsewhere (Cadle, 1965; Mercer, 1973a,b; Raabe, 1970), but it
may be useful to review a few general concepts. Aerosols consist of
finely divided particles ranging in size from about 0.01 to 100 µm in
diameter. The particles in a cloud are usually of many sizes and the
size can be expressed as a size-frequency distribution curve which
usually best fits a log-normal distribution. There are several ways to
express the diameter of a particle, the common ones being count median
diameter, mass median diameter, and aerodynamic mass median diameter.
The last term is important as it considers each distribution as if it
were made up of unit density spheres and measures its diameter as if
it were acting aerodynamically like a unit density sphere. Thus,
aerosol behaviour can be compared, regardless of the individual shape
and density of the particles.
The division of a solid into fine particles that become airborne
results in a large increase in the surface area of the material. The
consequence is a generally increased chemical reactivity of the
material, thus accelerating all physical and chemical processes, such
as oxidation, dissolution, evaporation, absorption, and electrical
activity. The physiological activity of these particles also
increases. The sizes of interest from a biological standpoint range
from about 0.1 to 10 µm. Larger particles do not usually enter the
respiratory tract or, if they do, they are deposited in the nose.
6.3.2 Deposition
Material that enters the respiratory tract with the inspired air
can either be deposited or exhaled. Many factors affect the deposition
of particles or the absorption of vapours in the respiratory system.
Retention of vapours is governed by the diffusion rates of the vapour,
the solubility of the vapour in the various body compartments, and the
degree to which these compartments have attained the equilibrium. This
in turn, depends on the duration of the exposure, the concentration,
and the rate of removal of the vapours. This subject has recently been
reviewed by Stupfel & Mordelet-Dambrine (1974).
Many more factors govern the deposition of particulates in the
respiratory tract. Roe (1968) has divided these into physical and
chemical characteristics of the particle, anatomical, physiological,
and pathological factors. The size, density, and shape of the
particles are the physical variables that determine their aerodynamic
behaviour. The Task Group on Lung Dynamics (Morrow et al., 1966) has
discussed deposition as a function of particle size.
Three distinct physical processes act on particles suspended in
the atmosphere to cause them to be deposited in the respiratory tract.
Inertial impaction results from the tendency of particles to move in
straight lines. The repeated branching and subdivision of the
respiratory tract causes particles to be impacted on surfaces,
particularly near the bifurcations. Inertial forces are greater with
larger particles. Sedimentation due to gravity also causes particles
to strike the surface of the respiratory tract. Finally, Brownian
movement, particularly of smaller particles, causes them to be
deposited in the lung. The effectiveness of these mechanisms depends
on the anatomy of the respiratory tract, the size of the particle, and
the breathing pattern. During artificially induced hyperventilation in
rabbits, more material was deposited in comparison with controls
(Velickovskij & Kacnelson, 1964). Normal deposition and deposition in
diseased states are discussed in detail by Albert et al. (1973), Brain
& Valberg (1974), Goldberg & Laurenco (1973), Macklem et al. (1973)
and Stuart (1974).
Aerosols are deposited all along the respiratory tract. Large
particles (5-10 µm) are mainly deposited in the upper respiratory
tract, including the nasal cavity. The depth of penetration increases
as particle size decreases and particles in the 1-2 µm range are, for
the most part, deposited in the alveoli. As a first approximation, 25%
of inhaled particles are exhaled, 50% are deposited in the upper
respiratory tract, and 25% are deposited in the lower respiratory
tract (Morrow et al., 1966).
6.3.3 Clearance
Soluble particles readily dissolve at the site of deposition.
Generally, they enter the bloodstream and then behave as if they had
been intravenously injected. Insoluble particles can be removed from
the respiratory tract by several mechanisms depending on the site of
deposition. Particles that are deposited on the mucous blanket are
carried towards the pharynx by the cilia and are usually swallowed or
expectorated. Hence, it is impossible to completely separate
respiratory exposure from gastrointestinal exposure. The rate of
clearance by the mucociliary escalator has been measured in man by a
number of investigators (Albert et al., 1969, 1973; Camner et al.,
1972, 1973; Morrow, 1970; Sanchis et al., 1972) and animals (Albert et
al., 1973; Thomas, 1969; Velickovskij & Kacnelson, 1964; Watson et
al., 1969).
Particles that are deposited in the deep non-ciliated portion of
the lung clear more slowly. One mechanism responsible for alveolar
clearance is phagocytosis. Although alveolar macrophages engulf
particles within a few hours, there is evidence that macrophages do
not carry them actively to the mucociliary escalator in the first few
days following inhalation (Camner et al., 1977). Recent studies
indicate that there are other mechanisms of alveolar clearance. Tucker
et al. (1973) have shown alveolar clearance by normal interstitial
drainage pathways. Casarett (1972) and Morrow (1973) described a
possible mechanism whereby particles could move from the alveolus up
to the terminal bronchiole, and then to the ciliated epithelium for
removal on the mucus. Very fine particles can also enter the blood
directly through the lymphatic system. Finally, particles can dissolve
and be absorbed into the bloodstream. Some particles remain in the
alveolar region of the lung for considerable periods of time, during
which dissolution forces can operate.
6.4 Dose in Inhalation Studies
The term dose has many meanings depending on the background and
expertise of the investigator. In toxicology, dose is usually defined
as the mass of material introduced into the animal, and it is often
divided by the body weight. Hence, toxicologists often speak of dose
in mg/kg. Even this is misleading, to some extent, because the
material may not interact in any way, and because of other reasons
that become apparent in species comparisons. The actual dose or amount
of a substance entering the internal milieu of the body depends on the
concentration and the particle size of the inhaled material, the
duration of the exposure, and the breathing variables of the test
species. Thus, the actual absorbed dose is difficult to determine and
inhalation toxicologists often refer to exposure conditions instead of
dose. The exposure must be defined both in terms of concentration (C)
and time (t), and is sometimes expressed as the product of these two
(Ct) (MacFarland, 1968) (see also Chapter 1). While the Ct product
is not a true dose, it can be used in a similar fashion. Haber (1924)
recognized this and Haber's rule states that, for a fixed Ct
product, the response will be the same. This rule holds reasonably
well over limited ranges of C and t, but deviations occur when
extreme values of the variables are examined.
More frequently, inhalation toxicologists keep the time constant
and vary the concentration of the test material. Hence, they measure
an LC50, i.e. the median concentration to which animals are exposed
for a specified time that will kill 50% of the animals within a fixed
period of time after exposure. It is implicit in LC50 data that the
durations of both the exposure and post-exposure period are
standardized. Comparative LC50 data are often obtained for 4-h
exposures and a 14-day post-exposure observation period (Carpenter et
al., 1949; Pozzani et al., 1959).
6.5 Choice of Species
The ideal subject for studies relevant to man is man himself.
However, human volunteers can only be used where the toxicological
hazard is already reasonably well defined and accepted. Human studies
have been conducted recently with chlorinated hydrocarbon solvents and
common air pollutants (Andersen et al., 1974; Hazucha et al., 1973;
Stewart, 1972; Stewart et al., 1970a,b, 1973). These experiments were
well controlled and monitored and the exposure levels were low.
Rats, dogs, and monkeys have been the species most used for
inhalation toxicity studies, although investigators have also used
mice, hamsters, guineapigs, rabbits, cats, miniature swine, and
donkeys. The choice of species should be made, primarily, with a view
to extrapolating the experimental results to man. However, choice on
this basis alone is difficult since the validity of such an
extrapolation is often uncertain. When selecting the particular
species for study, the following factors must be considered: the
comparative morphology of the respiratory tract; the presence or
absence of lung disease or susceptible states; and the similarity of
biochemical and physiological responses to those in man.
6.5.1 Anatomical differences
The respiratory systems of various laboratory animals and man
differ widely. As man is a primate, it is sometimes erroneously
assumed that the monkey is a good model for inhalation studies, but
marked differences are noted when comparing human and sub-human
primate respiratory systems. In the monkey, the end airway is always a
respiratory bronchiole, whereas this is rarely the case in man.
Furthermore, the monkey's lung is not lobulated.
The most commonly used laboratory animals are quadrupeds, and
their respiratory systems are horizontal and not vertical as in the
primate. This is important because the aerodynamics of a horizontally
arranged lung are different, and particle deposition will also be
different. In human subjects, maximum dust deposition is in the upper
portion of the lung; in experimental animals, maximum deposition in
the more ventral portions of the lung is usual.
The gross anatomy of the respiratory systems of the various
laboratory animals and man is quite different. In animals such as the
rat and guineapig, the nose contains highly developed tortuous
turbinates. In monkeys and man with relatively smaller noses, the
turbinates are less complex. These differences in nasal anatomy are
especially important in studies involving exposure to particulates.
More particles impinge in more complex turbinate systems and will not,
therefore, reach the deeper portions of the respiratory system.
The subgross anatomy of the lungs of various laboratory animals
and man has been detailed and compared by McLaughlin et el. (1961a,b,
1966). The authors have grouped the common laboratory animals and man
in three basic categories based on subgross pulmonary anatomy
(Table 6.1). They grouped the various animals on the basis of lung
lobulation, the presence or absence of respiratory bronchioles, the
presence or absence of terminal bronchioles, pulmonary artery/
bronchial artery shunts, and the termination of the bronchial
arteries. Their studies indicate that the pulmonary anatomy of the
horse most closely resembles that of man.
6.5.2 Physiological considerations
Pertinent differences in the lung physiology of various species
must be considered in inhalation toxicology. Normal values or ranges
for several species, including man, are summarized in Table 6.2
(Sanockij, 1970a).
Table 6.1 Comparative subgross anatomy of the lunga
Species Lung No. of lobes Lobulation Respiratory Terminal Pulmonary/artery Termination Pleura
type bronchioles bronchioles Bronchial/artery of bronchial
left right shunts arteries
Cow I 3 5(4) well developed extremely poor present present distal airway thick
development
Sheep I 3 4 well developed extremely poor present present distal airway thick
development
Pig I 3 4 well developed extremely poor present not present distal airway thick
development
Monkey II 3 4 not present very well absent not present distal airway thin
developed
Dog II 3 4 not present very well absent not present distal airway thin
developed
Cat II 3 4 not present very well absent not present distal airway thin
developed
Guineapig IIa 3 4 not present fairly well absent present distal airway thin
developed
Rat IIa 1 4 not present fairly well absent few present distal airway thin
developed
Rabbit IIa 3 4 not present fairly well absent present tertiary thin
developed bronchus
Horse III (3) (4) imperfectly poorly present present distal airway thick
developed developed and alveoli
Man III 2 3 imperfectly poorly present present distal airway thick
developed developed and alveoli
a From: McLaughlin et al. (1961a,b, 1966).
Table 6.2 Some physiological indices of man and animalsa
Man Dog Cat Rabbit Guineapig Rat Mouse
Body surface (m2) 1.8 0.528 0.2 0.18 0.040 0.030 0.006
Relation body surface to body weight 0.0257 0.044 0.066 0.072 0.12 0.15 0.3
(m2/kg)
Basal metabolism (kJ/kg) 105 222 -- 188 360 615 711
Frequency of respiration (min) 14-18 10-30 20--30 50-100 80-135 110-135 140-210
Size of alveoli (µm) 150 100 100 -- -- 50 30
Surface of lungs (m2) 50 100 7.2 5.21 1.47 0.56 0.12
Relation of lung surface to body weight 0.7 8.3 2.8 2.5 3.2 3.3 5.4
(m2/kg)
Inhaled air (ml) 616 40-60 -- -- 1.75 0.865 0.154
Lung ventilation (ml/min) 8732 -- 1000 600 155 73 25
Relation of lung ventilation to body 0.13 -- 0.30 0.29 0.33 0.05 1.24
weight (ml/min/g)
Consumption of oxygen (ml/kg/hr) 203.1 3600 9420 522.7 2180 2199 3910
Elimination of CO2 (ml/kg/h) 168.8 -- -- -- -- 2650 4240
Coefficient of respiration 0.82 -- -- 0.83 -- 0.82 0.85-1.33
Pulse frequency for 1 min 70-72 90-130 120-180 150-240 206-280 300-500 520-780
a From: Sanockij (1970a).
6.5.3 Disease and susceptibility states
Most toxicological investigations are performed on healthy
animals. However, epidemiological studies have indicated that during
air pollution episodes the populations at greatest risk are the young,
the aged, and those people with pre-existing cardiopulmonary disease.
One task of the inhalation toxicologist is to identify those segments
of the population that are particularly susceptible to the presence of
airborne contaminants. Certain animal models of diseased or stressed
states have been described (Boyd et al., 1974; Drew & Taylor, 1974;
Silver et al., 1973; Taylor & Drew, 1975; see also Chapter 2) which
could be used or could be adapted for use in inhalation studies. The
most commonly used model of this nature is the papain-induced
emphysematous animal (Gross et al., 1965; Martorana et al., 1973;
Niewoehner & Kleinermann, 1973; Snider et al., 1974). Both aged and
neonatal animals could be used as models of high susceptibility
groups. Disease and susceptibility states are important considerations
in the selection of the species and, in some cases, even the strain of
animal to be investigated since it is well known that the incidence of
cancer differs considerably among certain species and strains.
For example, Kuschner et al. (1975) recently reported a high
incidence of respiratory tract tumours in rats after exposure to
oxybis[chloro-methane] (bis(chloromethyl)ether) but only a few tumours
in hamsters. These tumours were about equally divided between
esthesioneuro-epitheliomas and bronchogenic squamous cell carcinomas.
Leong et al. (1975) repeated these experiments; however, all the
tumours in Leong's study were nasal tumours with no bronchogenic
carcinomas. The only difference noted in the protocol was that Leong
et al. used specific-pathogen-free, caesarean-derived rats, whereas
Kuschner et al. used Sprague-Dawley rats, that were not
specific-pathogen-free.
6.6 Duration of Exposure
Acute inhalation toxicity studies usually consist of a single
exposure (or occasionally a few exposures) of not more than 8 h.
Repeated exposure studies consist of a number of daily exposures for
fixed periods of time. Occasionally, investigators terminate exposure
after several days or weeks, and then maintain the animals in the
colony to observe delayed development of long-term effects (Kuschner
et al., 1975).
The duration of chronic studies varies considerably. One logical
proposal is based on the life span of the test species (Sanockij,
1970b). If, for example, one considers that toxic signs will appear in
man after an exposure over 10% of his life span (7 years), animals
should also be exposed for 10% of their life span. Thus, rats should
be exposed for 3-4 months, and larger animals for a somewhat longer
period. Some authors (Sidorenko & Pinigin, 1970) consider that even
continuous exposure for 3-4 months is insufficient to simulate
lifetime exposure in man, and many studies last longer, some up to 5
years (Lewis et al., 1974). Powell & Hosey (1965) consider the minimum
duration of a chronic study to be one year, the animals being exposed
6 h a day for 5 days a week. This corresponds to a significant portion
of a rodent's lifetime.
Chronic studies are conducted to determine the effects of
long-term exposure to compounds and particularly (in the USSR) to
establish minimum effect levels (Limch). In order to evaluate the
effects of long-term exposures, such as elevated incidences of
infection, emphysema, or the induction of cancer, inhalation controls
should be run concurrently. Two species are usually used. Occasionally
the toxic effects and the mechanism of chronic toxicity are entirely
different from those manifested in acute exposures. Benzene, for
example, is a central nervous system depressant at high
concentrations, while, at low concentrations over long periods of
exposure, it affects the hematopoietic system.
The selection of concentration for chronic studies is difficult.
For example, in the USA, concentrations are chosen that do not produce
mortality and produce only minimal changes in other biological indices
of toxicity during limited studies. In the USSR, the concentration
selected is below the Limac. In order to investigate dose-response
relationships, it is advisable to use at least three different dose
levels, hoping that the highest level chosen will produce quantifiable
effects and that the lowest level selected will produce minimal or
even no effects. In the USSR, research workers often use as the
highest level the concentration which, in single short-term exposures
of human subjects, does not produce any effect during the study of
reflex reactions.
When investigating the various biological indices of toxicity, it
is necessary to carry out measurements more frequently during the
early stages of the study (Camner et al., 1972, 1973). Studies carried
out at the Institute of Labour Hygiene and Occupational Medicine of
the USSR show that the time to the display of the first signs (period
for initial decompensation) varies considerably. Thus, the variables
are recorded at 1, 4, 8, 15, and 30 days and monthly thereafter. After
terminating the exposure, the frequency of measurements may be
increased again to record any early changes.
6.6.1 Intermittent versus continuous exposures
Long-term exposures are usually patterned on projected industrial
experience, giving the animals a daily exposure of 6-7 h, 5 days a
week (intermittent exposure), or on a possible environmental exposure,
with 22-24 h of exposure per day, 7 days a week (continuous exposure),
with about an hour for feeding the animals and maintaining the
chambers. In both cases, the animals are usually exposed to a fixed
concentration of test materials. Thus, neither situation approaches
actual human experience, where concentrations of atmospheric
pollutants are continuously fluctuating by one or two orders of
magnitude. A major difference to consider between intermittent and
continuous exposure is that with the former there is a 17-18 h period
in which animals may recover from the effects of each daily exposure,
and an even longer recovery period at weekends. The recovery period in
some cases is extremely important as in the case of continuous versus
intermittent exposure to dichlormethane (Haun, 1972).
The choice of intermittent or continuous exposure depends on the
objectives of the study and on the human experience that is to be
simulated. However, certain technical difficulties must be considered.
For example, the advantages of continuous exposure for simulating
environmental conditions may be offset by the necessity of watering
and feeding during exposure, and by the need for more complicated (and
reliable) aerosol and vapour generation and monitoring techniques.
Intermittent systems require simpler chambers, since provision for
food and water is not necessary. The contaminant dispersal systems are
also simpler as they need to operate for only 6-7 h per day.
6.7 Inhalation Systems
6.7.1 Facilities required
It is more advantageous to build facilities designed specifically
for inhalation studies than to modify existing buildings. High
cellings are necessary for housing exposure chambers and related
equipment, such as aerosol and vapour generators, filters, flowmeters,
etc. A constant supply of clean filtered air with temperature and
humidity controls should be available for both the chamber rooms and
the chambers themselves. Adequate floor space should provide access to
at least two sides of the chambers, and the chambers themselves should
be separated by a small space to avoid heat transfer between chambers.
6.7.2 Static systems
Inhalation systems can be characterized as static, when the agent
is introduced into a chamber as a batch and then mixed, or dynamic,
when airflow and introduction (and removal) of agent are continuous.
The duration of static exposure is limited by: (a) the gradual
depletion of oxygen; (b) the accumulation of carbon dioxide;
(c) the accumulation of water vapour; and (d) the gradual increase
in temperature inside the chamber. In spite of these limitations,
static systems are of great practical usefulness in assessing acute
toxicity, particularly when the supply of material is limited.
Procedures similar to those described by Draize et al. (1959) are in
use today for screening commercial products. Another use of static
systems is for the exposure of animals to biological aerosols. It is
difficult to generate a viable biological aerosol particle
continuously because of the limited amount of material available;
thus, material is dispersed in a large chamber, mixed, and the animals
exposed through nose tubes connected to the test atmosphere (Jemski &
Phillips, 1965).
6.7.3 Dynamic systems
Today, most inhalation facilities use dynamic systems where the
airflow and introduction of agents are continuous. The theoretical or
nominal concentration of chemicals in a chamber can be calculated as
follows:
flow of chemical
concentration =
flow of air
Many factors, including wall loss, losses on the skin and fur of
animals, and uptake by the test animals, cause the actual
concentration to be somewhat less than the nominal concentration.
Thus, the concentration should always be measured by an appropriate
instrument or technique rather than reporting the nominal
concentration.
When material is introduced into a chamber, the concentration
builds up exponentially according to equations originally described
and verified by Silver (1946). If perfect mixing occurs, the
concentration can be calculated according to the following equation:
C = (w/b) (1 - exp (-bt/a)) (1)
where C = the concentration of material at time t; w = amount of
material introduced per minute; a = volume of the chamber;
b = flow of air through the chamber.
The fraction of equilibrium concentration (w/b) attained in
time t is:
C
= 1 - exp (1 bt/a) (2)
w/b
Thus, the time required to reach 99% (t99) of the equilibrium
concentration is:
0.99 = 1 - exp (-bt99/a) (3)
or
t99 = 4.6052 a/b
This equation may be given the general form:
tx = Ka/b (4)
where x equals % nominal concentration attained in time t and
microtomes, that cut the normal paraffin blocks with glass knives. Of
course, this procedure should only be followed when specific details
have to be followed during the microscopic examination. The use of
semithin Epon sections yields even better results.
It will often be necessary to use special staining techniques on
tissues in order to provide more information on the presence of
carbohydrates, proteins, fats, elements, or certain structural
organizations. Special fixation is sometimes necessary for appropriate
staining. Bones and calcified tissues have to be decalcified. Eyes
usually need to be fixed and embedded differently. Blood vessels may
be stained with Sudan black in order to study vascular lesions.
5.9 Special Techniques
5.9.1 Enzyme histochemistry
Enzyme histochemistry is a technique used to detect the activity
or presence of an enzyme in a tissue by incubating the fresh tissue in
an appropriate medium, so that a fine coloured granular precipitation
forms wherever the enzyme is present.
The use of the enzyme-histochemical technique is not common in
toxicity testing. Nevertheless, the technique is a valuable one, since
it provides information about the metabolic activity and function of
the tissues. Furthermore, it introduces the possibility of correlating
biochemical with histological findings that may lead to a more correct
interpretation. Enzyme-histochemical investigations also make it
possible to visualize certain differences in enzyme activity within
the structural organization of tissues.
In some cases, a decrease in enzyme activity in certain cells is
compensated for by an increase in other cells within the same organ.
Such biological differences can only be detected by
enzyme-histological investigation, when the results of biochemical
determinations are within normal limits and no alterations are seen
using conventional histological techniques. Enzyme-histological
investigations can easily be incorporated in routine toxicity testing,
if necessary.
Relatively small pieces of tissue are quickly frozen in an inert
liquid (i.e. isopentane), or cooled in liquid nitrogen or a mixture of
solid carbon dioxide and methanol. Storage of the tissues is effected
at -70°C. Cryostat sections are prepared and incubated in specially
prepared media. Good reference works for the methods have been
published (Barka & Anderson, 1965; Pearse, 1968, 1972). To obtain
optimum information, the prepared section should be examined by
semiquantitative methods.
An enzyme histochemical investigation can also be performed at
the electron microscope stage (Geyer, 1973). In this case, the
activity or presence of an enzyme is determined by the deposition of
electron-dense material at the sites where the enzyme is located. It
is evident that these delicate techniques are not easy to apply in
routine toxicity testing. They may, however, be of great importance in
specific studies on the biological effects of a compound on cellular
components, or to detect early damage.
5.9.2 Autoradiography
Autoradiography is based on the principle that radioactive
substances present in tissues are able to produce an image on a
photographic film or plate. The radiations emitted by the radioactive
substances must be of relatively weak energy, so that they will have a
short range in tissues and emulsions. When the range is too long,
developed silver grains can be found in the emulsion far away from the
radioactive source.
In this respect only alpha particles, beta particles, and Auger
electrons are useful since electromagnetic radiations such as gamma
and X-rays give poor results due to their penetrating power.
Tritium (3H) has been most frequently used in autoradiographic
studies, although 14C and 32P are also being used as tracers.
Autoradiography, at both light microscopic and ultrastructural
levels, can be used in kinetic studies on tissues by incorporating
radioactive-labelled bases in DNA or RNA resulting in the production
of silver grains on a photographic film covering the section. The
different methods have been extensively described for nondiffusible
substances (Baserga & Malamud, 1969; Rogers, 1967) as well as for
diffusible substances (Roth & Stumpf, 1969).
Apart from studying the kinetics of tissues, autoradiographic
techniques can also be used to study the distribution of radiolabelled
compounds and their metabolites. This, again, can be done at light
microscopic and ultrastructural levels.
The technique of whole-body autoradiography as developed by
Ullberg et at. (1972) is a valuable tool, in this respect, since the
distribution of a compound can qualitatively (or even
semiquantitatively) be studied in the whole body. In addition, pieces
can be cut out to use for microautoradiographic research.
Substances may also be labelled with fluorescent chemicals
permitting their detection by illumination of the sections with
ultra-violet radiation. The technique of fluorescence microscopy can
also be used for the detection of certain dyes that possess
autofluorescing properties. Some substances, such as the
catecholamines, can be made visible by reaction with formaldehyde
which converts mines into fluorescent substances (Eränko & Räisänen,
1966). Fluorescence in tissues can be measured quantitatively, thus
facilitating controlled conditions (Ploem et al., 1974).
Autoradiographic studies cannot be incorporated easily into
routine toxicity experiments. However, the technique may be a very
important tool in special studies.
5.9.3 Immunofluorescence and immunoenzyme techniques
Immunofluorescence techniques are primarily used in determining
the presence or absence of certain antibodies or antigens in tissues.
Antigens are detected by binding them to specific antibodies. If a
specific antiserum is conjugated with a fluorescent dye, the specific
antigen-antibody complex formed can be seen by studying the tissue
section using ultraviolet radiation microscopy. This direct
immunofluorescent technique can be replaced by a more sensitive
indirect method in which the substance actually rendered fluorescent
is not the antigen under consideration but an intermediate material
the distribution of which corresponds precisely to that of the antigen
being studied. Information on the principle of these techniques is
available (Goldman, 1968; Nairn & Marrack, 1964).
The use of enzyme conjugates has recently been developed
(Sternberger, 1974). This system is used when antigen-antibody
complexes have to be localized at ultrastructural levels.
Immunofluorescence or immunoenzyme techniques will not be used in
most routine toxicity experiments, but they may be of importance in
special studies such as those described in Chapter 7.
5.9.4 Electron microscopy
Transmission electron microscopy is the most commonly used
technique for studying the ultrastructure of tissues. Different
fixation, embedding, and cutting techniques have to be used to obtain
ultrathin sections of tissues (Flauert, 1973, 1974, 1975; Hayat, 1970,
1972, 1973). At the ultra-structural level, very minute changes can be
detected and they have to be distinguished from the many artifacts
that can be introduced during the different processing procedures.
Under optimum conditions, tissues can be examined at high
magnifications.
As tissue examination by electron microscopy is very time
consuming and costly, it is important only to select tissues from
those experiments that justify it. Ultrastructural studies have
contributed enormously to our knowledge of molecular biology. In the
case of toxicology, ultrastructural studies are always carried out as
a secondary investigation, and are usually applied to
ultrastructurally, specific, pathological changes already studied in
detail at the light microscopic level, in order to secure a better
judgment of the importance of the lesion. Ultrastructural studies are
also used to confirm that target organs that appear normal at a
certain close level using light microscopy, do not in fact show
pathological changes.
Scanning electron microscopy (SEM) techniques, that have come
into use in recent years, provide 3-dimensional images of complete
biological units and also chemical information (Hayes, 1973). Here
again, special processing procedures may be needed, although, in
certain cases, formalin-fixed and paraffin-embedded material can be
used. Natural surfaces, dissected material, sectioned tissue, and
living specimens may be studied. SEM is a valuable tool in biology
but, for toxicological investigations, its use is still rather
restricted, apart from the possibility of obtaining quantitative
chemical information. The development of analytical electron
microscopy, using the principles of X-ray microanalysis, also makes it
possible to correlate tissue ultrastructure and chemistry (Hayes,
1973; Weavers, 1973).
5.10 Microscopic Examination
Routine histopathological examination is very important and
should be carried out correctly. First of all, the sections to be
studied should be of good quality. Additional sections and special
stains must be prepared if necessary. Special stains are used to study
and describe individual lesions; they may also be used to examine
certain organs or lesions to permit better judgment of
semiquantitative comparison. For example, when haemosiderosis is found
to be an important effect, special stains, based on the reaction of
the dye with iron, facilitate semiquantitative analysis of the degree
of the lesion.
Good microscopic equipment is necessary to perform optimum
microscopic examination. Sources of ultraviolet radiation and
polarized light should be available.
The microscopic examination must be carried out by well-trained
pathologists or other persons trained and experienced in the field of
laboratory animal pathology. The use of parapathologists for routine
microscopy in toxicity and carcinogenicity experiments is extremely
valuable and is important for overcoming a shortage of personnel,
trained in laboratory animal pathology (Toxicol. appl. Pharmacol.,
1975). Experience has shown that well-trained parapathologists can
become very skilful in microscopy and in screening sections for
abnormalities. With further experience, they are also able to describe
certain lesions. It is the pathologist's duty to see that all lesions
observed are described and interpreted correctly, and to check the
sections for any lesions that may have been overlooked.
It is a serious mistake, in an experiment, to undertake
microscopic examination before the results of other examinations such
as biochemical determinations, haematology, and organ weights, are
available, for the information obtained from these procedures may give
important directions for the microscopic study. For example, an
increased thyroid weight may lead to more careful examination of the
thyroid for hyperplasia and may prompt histometric determinations.
Lower lymph node weight may point to semiquantitative examination of
these organs with regard to reduced immunocapacity (Cottier et al.,
1972). Differences or changes in the blood picture call for more
detailed study of the bone marrow, for example, by preparing and
studying 1 µm sections, to detect suspected haemopoietic disturbances.
Gross observations should always be correlated with microscopic
findings. It is a false assumption to think that a microscopic
examination will be more objective when performed blindly; knowledge
of clinical signs and macroscopy are indispensable for a meaningful
examination. Of course, the pathologist must be careful not to let
knowledge of clinical effects influence his objective evaluation of
the tissues; thus, sections of the tissues considered to be involved
may have to be re-examined blindly. If such a re-examination is
carried out, semiquantitative scoring of the extent of the lesion will
also help to detect a possible dose-effect relationship. Should there
be any doubt in interpreting the significance of a lesion, it is
advisable to consult other pathologists, all of whom should re-examine
the slides blindly.
5.10.1 Number of animals and number of organs and tissues studied
microscopically
In acute and rangefinding tests, the organs and tissues are
usually fixed and examined microscopically after processing. In
subacute and long-term studies, and, sometimes, in rangefinding tests,
it is customary initially to examine only the tissues of the highest
dose group, the control group and the target organs, if known. In
addition, all grossly observed lesions are processed in the
intermediate and low-level groups. If the results of the microscopic
examination of the highest dose group indicate a need to examine
certain organs at lower levels, this can be done at a second stage.
In chronic toxicity experiments, all males and females of the
highest dose group should be examined. It is also advisable to examine
all control animals completely, since this will be the only way to
ascertain the incidence of tumours and other "lesions" normally
occurring in the strain of animals used. Such information is
indispensable for correctly evaluating the significance of changes
observed in exposed animals.
In carcinogenicity experiments, where larger numbers of animals
per group are usually used, some laboratories restrict microscopic
examination mainly to the grossly observed abnormalities and tumours,
and perform complete histological examinations on only 15-20 male and
15-20 female survivors of the highest dose group and of the control
group, on the assumption that a well-performed gross examination will
detect most of the tumours present. Histological examination of all
tissues of 15-20 animals of each sex will give some information on the
possible presence of certain pre-malignant hyperplastic or neoplastic
changes not grossly observed. If such lesions are found, the
histological examination should cover all animals, while small organs
which may bear tumours that cannot be grossly observed (thyroid,
pituitary, adrenals) must be examined histologically.
5.10.2 Description of the lesions
For the microscopic examination, it is essential to use a
check-list to detect losses, especially of small organs lost in the
processing procedure. All organs and tissues examined should be
listed, even though no abnormalities are seen, while all lesions found
should be described clearly and accurately. It is essential to
describe lesions in a semiquantitative way using such words as slight,
moderate, strong, and very strong to indicate the extent of the
lesion; it is also essential to indicate the criteria used to
investigate and classify abnormalities. It will often be necessary to
re-examine certain lesions in order to obtain a well-balanced
semiquantitative judgment. Several reference works may be of help in
the classification of tumours and other lesions (Beveridge & Sobin,
1974; Cotchin & Roe, 1967; Ribelin & McCoy, 1965; and Turusov, 1973).
Certain lesions found by microscopic examination may, in fact,
consist of several entities. In such cases, it may be important to
classify these entities independently. For example, perilobular liver
degeneration may be combined with bile duct proliferation, but one of
the two phenomena may be more extensive than the other with respect to
dose level and time. For a better interpretation, it is preferable to
describe and classify such lesions independently.
5.11 Presentation, Evaluation, and Interpretation of Pathological
Data
A well-performed and well-described microscopic examination is
not complete if the results cannot be studied and compared easily and
adequately. The lesions found in the different groups should be
tabulated according to organ, group, and sex in order to facilitate
comparison of the incidence of such lesions in the different groups.
If, in addition, a semiquantitative estimate of the extent of the
lesions is made, it will be easy to see whether they are
compound-induced and increase with time and dose.
It is important that these tables include details such as the
number of animals necropsied and the number examined microscopically,
per group and sex. In addition, it is advisable to state, the exact
number of organs or tissues examined microscopically, since it is well
known that, in every toxicity experiment, organs and tissues get lost
during the processing procedure. In this way, the loss can be
adequately estimated and the lesions found can be expressed against
the real number of organs or tissues examined.
For correct interpretation of the tumour tables, it is advisable
to include hyperplastic and preneoplastic lesions as individual
groups. The table should also include the number of days that elapsed
from the start of the experiment until the observation of each rumour.
This first observation may be clinical, for example, when mammary, ear
duct, or skin tumours are involved and the time that has elapsed can
then be called the "induction time". However, usually, tumours are
found after death or killing in extremis, or at sacrifice at the end
of the experiment. Thus, in these cases, it seems most appropriate to
use the term "time of observation". The inclusion of the "time of
observation" of tumours in the table is of help to the investigator
since it provides information on shorter or prolonged latency periods
(e.g. time that has elapsed between initiation and the appearance of
tumours). In addition, it reminds the reader to take into account the
survival differences when expressing the results.
In long-term experiments, the number of lesions found may be
quite large. Furthermore, some lesions may have been noticed during a
certain period in life, when the animals died or were sacrificed
in extremis, while others will have been noticed at the end of an
experiment. When such lesions are tabulated in the same table, this
information may get lost. It seems, therefore, most appropriate in
such long-term experiments to pool the results of the pathological
examination for certain periods, for example the first 12 months of
life and then for 3- to 6-monthly intervals, and to give the results
of the examination undertaken at the end of the experiment separately.
It is obvious that in preparing such tables, the use of an
automated data acquisition and computer-based system will be of great
help. These systems have been described for application to
toxicological studies, especially for animal weights, food and drug
consumption, organ weights, and biochemical data (Munro et al., 1972).
In pathology, such systems offer considerable potential. The necessity
for the correct coding of pathological changes, and the use of a code
system set up so that detailed information about lesions can still be
obtained, is obvious. Several systems have been described (Becker,
1973; Enlander, 1975; Smith et al., 1972), and may be adapted for this
purpose.
Pathological examinations must always be evaluated and
interpreted in connexion with other phenomena found. It must be
decided whether lesions noticed are in fact pathological, and whether
they are found in connexion with changes in other variables such as
organ weights, biochemical tests, etc. If compound-induced lesions are
found, they may not only be present at the highest dose level, but
also at an intermediate dose level. In the latter case, it is
important to determine whether there is a dose-dependent increase in
the severity and extent of the lesion, a finding which would
strengthen the assumption that the lesion is compound-induced.
Moreover, a clear dose-response relationship permits better evaluation
of the potential risk of a compound. Whenever lesions are found only
at the highest dose level, the sections at all dose levels should be
re-examined blindly to remove any doubt. Often the two sexes will show
a different sensitivity to the action of a compound.
Problems may arise when certain lesions are found more frequently
and to a greater extent in the treated groups compared with the
control group and compared with the common incidence of the lesion in
the strain used. The usual attitude towards this phenomenon is to
consider it as an effect or response.
In the evaluation of tumour incidence, criteria such as increased
tumour incidence, decrease in latency period, and the appearance of
tumours in organs where they do not occur spontaneously, have to be
considered. For this reason, detailed information on the spontaneous
occurrence of tumours in the species and strain used is essential,
particularly when tests indicate that the compound possesses a weak
carcinogenic action. Simple comparison of tumour incidence in control
animals and experimental animals at one point in time may lead to
erroneous conclusions, especially when only one of the above three
criteria is met. Many factors other than treatment may influence the
incidence of spontaneous tumours.
Statistical evaluation of the results is often necessary, but, as
yet, there are no agreed procedures for comparing statistically the
incidence of malignant tumours in controls and experimental groups.
REFERENCES
BARKA, T. & ANDERSON, P. J. (1965) Histochemistry: theory, practice
and bibliography. New York, Harper & Row; London, Evanston.
BASERGA, R. & MALAMUD, D. (1969) Autoradiography: techniques and
application. New York, Harper & Row; London, Evanston.
BECKER, H. (1973) Experiences with a test-stage free-text processing
system in pathology, using display terminals. Path. Europ.,
8: 307-313.
BEVERIDGE, W. I. B. & SOBIN, L. H., ed. (1974) International
histological classification of tumours of domestic animals.
Bull. World Health Org., 50: 1-142.
CHEVALIER, H. J. (1971) [A method for the fixation of the lungs of
small animals.] Z. Versuchstierk., 13: 101-104 (in German).
COHRS, P., JAFFE, R, & MEESEN, H. (1958) [Pathology of laboratory
animals] Berlin, Springer-Verlag, Vol. 2 (in German).
COTCHIN, E. & ROE, F. J. C. (1967) Pathology of laboratory rats and
mice. Oxford, Edinburgh, Blackwell.
COTTIER, H., TURK, J., & SOBIN, L. (1972) A proposal for a
standardized system of reporting human lymph node morphology in
relation to immunological function. Bull. World Health Organ.,
47: 375-408.
ENLANDER, D. (1975) Computer data-processing of medical diagnosis in
pathology. Am. J. clin. Pathol., 63: 538-544.
ERÄNKO, O. & RAISANEN, L. (1966) In vitro release and uptake of
noradrenaline in the rat iris. In: Ebler, U.S. V., Rosell, S. &
Uunäs, B., ed. Mechanism of release of biogenic amines. New
York, Pergamon Press.
FAWELL, J. K. & LEWIS, D. J. (1971) A simple apparatus for the
inflation fixation of lungs at constant pressure. Lab. Anim.,
5: 267-270.
FLAUERT, A. M. (1973, 1974, 1975) Practical methods in electron
microscopy. Amsterdam, Oxford, North Holland Publ.; New York,
Elsevier.
GEYER, G. (1973) [Ultrahistochemistry, histochemical working
guidelines for electron-microscopy.] Stuttgart, Gustav Fischer
Verlag (in German).
GOLDMAN, M. (1968) Fluorescent antibody methods. New York, London,
Academic Press.
GORDON, H. W. & DANIEL, E. J. (1974) Microwave fixation of human
tissues. Am. J. med. Technol., 40(10): 441-442.
HAYAT, M. A. (1970, 1972, 1973) Principles and techniques of electron
microscopy. In: Biological Applications Vol. I, II, III, IV.
New York, Van Nostrand Reinhold Company.
HAYES, T. I. (1973) Scanning electron microscopy in biology. In
Koehler, J. H., ed. Advanced techniques in biological electron
microscopy, Berlin, Heidelberg, New York, Springer-Verlag,
pp. 153-214.
MUNRO, I. C., CHARBONNEAU, S. M., & WILLES, R. F. (1972) An automated
data acquisition and computer-based computation system for
application of toxicological studies in laboratory animals.
Lab. Anim. Sci., 22: 753-756.
NAIRN, R. E. & MARRACK, J. R. (1964) Fluorescent protein tracing.
Edinburgh, London, Livingstone.
PEARSE, A. G. E. (1968, 1972) Histochemistry, theoretical and
applied. 3rd ed., Edinburgh, Churchill Livingstone, Vol. I &
II.
PLOEM, J. S., DE STERKE, J. A., BONNET, J., & WASMUND, H. (1974) A
micro-spectrofluorometer with epi-illumination operated under
computer control. J. Histochem. Cytochem., 22: 668-677.
RIBELIN, W. E. & MCCOY, J. R. (1965) The pathology of laboratory
animals. Springfield, Charles Thomas.
ROE, F. J. C. (1965) Spontaneous tumours in rats and mice. Food
Cosmet. Toxicol., 3: 707-720.
ROGERS, A. W. (1967) Techniques of autoradiography. Amsterdam,
London, New York, Elsevier.
ROTH, L. J. & STUMPF, W. E. (1969) Autoradiography of diffusible
substances. New York, London, Academic Press.
SMITH, J. E., MCGAVIN, M. D, & GRONWALL, R. (1972) English-language
computer-based system for storage and retrieval of pathological
diagnosis. Vet. Pathol. 9: 152-158.
SONTAG, J. M., PAGE, N. P., & SAFFIOTTA, U. (1975) Guidelines for
carcinogen bioassay in small rodents. Bethesda MD, National
Cancer Institute.
STERNBERGER, L. A. (1974) Immunocytochemistry. Englewood Cliffs, NJ,
Prentice-Hall. Toxicol. appl. Pharmacol. (1975) 31: 1-3, A
training programme for parapathologists.
TURUSOV, V. S. ed. (1973) Pathology of tumours in laboratory animals,
Vol. 1, Lyon, IARC (Sci. Publ. No. 5).
ULLBERG, S., HAMMARSTROEM, L., & APPELGREN, L-E. (1972)
Autoradiography in pharmacology. In: Cohen, Y., ed. I.E.P.T.
Section 78, Oxford, New York, Pergamon Press, Vol. 1,
pp. 221-239.
WEAVERS, B. A. (1973) The potentiality of EMMA-4, the analytical
electron microscopy in histochemistry: a review. Histochem. J.,
5: 173-193.
6. INHALATION EXPOSUREa
6.1 Introduction
Advances in technology, throughout the world, have increased the
number and amount of chemicals in the atmosphere. The health effects
of inhalation of these chemicals can be predicted to some extent by
experimental investigation. In order to simulate environmental
conditions, a special technology has evolved relating to the design
and operation of inhalation chambers and the generation and
characterization of aerosols and vapours. This chapter will introduce
this technology and review the significance of certain biological
end-points commonly measured in inhalation studies.
This chapter is not intended to be all-inclusive, nor to provide
complete instructions on inhalation technology. No toxicological
protocol is sufficient to cover all situations with all materials.
Hence, the expertise of the individual investigator and the objectives
of the investigation will govern the selection of the final protocol
and the biological end-points relevant to the particular compound
concerned.
The costs associated with evaluation of toxicity by the
inhalation route are considerably higher than for toxicity studies
using other routes of exposure because of the cost of the specialized
equipment used in inhalation studies and the time required for its
calibration. In general, it can be estimated that the total cost of
any type of inhalation study, short- or long-term, will be two to
three times that of a comparable oral study. Because of this, it is
important to determine when inhalation studies should be performed and
when, and if, studies by other routes would suffice. Finally, in
addition to costs, other resources should be considered. In most
countries, the number of laboratories capable of performing inhalation
studies is limited. Thus, it is essential to develop priorities for
the selection of compounds for evaluation of inhalation toxicity.
6.2 Need for Inhalation Studies
The effect of a compound depends on its concentration at the
receptors of the affected organ or system. The concentration at
different sites and times is a function of the route of entry. Thus
the route of entry is an important factor with regard to toxicity. No
good substitute model for inhalation exposure as a means of direct
exposure of the lungs has been developed, although intratracheal
exposure has been frequently used, particularly in pulmonary
a The assistance of Dr J. O'Neil, Dr M. Amdur, and Dr W. Busey in
the preparation of this chapter is gratefully acknowledged.
carcinogenesis studies. The distribution kinetics are different with
pulsed exposures compared with constant inhalation exposures. With
pulsed exposures, such as oral, intravenous, intraperitoneal, etc.,
the concentration of the test material will reach a peak and then
usually fall off, depending on the distribution coefficients for each
compound and organ in question. With continuous exposure,
concentrations in many body compartments will attain an equilibrium,
depending again on the concentration of the test material and the
distribution coefficient. Thus, quantitative toxicity is often quite
different, depending on the route of entry.
With the exception of certain drugs, man's exposure to
environmental agents comes either from skin contact, ingestion, or
inhalation. Inhalation is of particular importance in occupational
exposure and in exposure to air pollutants. For airborne substances,
the lung is the first organ that foreign chemicals encounter and it is
one of the body's first lines of defence. Chemicals that enter the
lung can either exert a direct effect on the cells of the lung, or be
absorbed into the systemic circulation. Blood passing from the lung to
the heart and then into the peripheral circulation can carry agents
directly to other organs without passing through the detoxication
processes of the liver. This contrasts with oral exposure where
chemicals may be absorbed into blood that immediately passes through
the liver and can be metabolically transformed into either more or
less toxic compounds.
Direct contact with irritants causes local inflammation in the
respiratory system, the degree of which may depend on the local
concentration and not on the total dose. For example, Kljackina (1973)
demonstrated that inhaled bromine acts as a specific irritant of the
respiratory system whereas bromine administered orally results in
changes in the nervous system.
Thus, specific reasons for performing inhalation studies include:
(a) determination of specific responses of the respiratory tract;
(b) assessment of the toxic hazard of agents whose principal route
of exposure is via inhalation; (c) investigation into the mechanism
of toxicity of inhaled materials; and (d) study of the comparative
toxicity of agents administered by different routes.
6.3 Fate of Inhaled Materials
6.3.1 Nature of aerosols
The nature and characteristics of aerosols have been treated in
detail elsewhere (Cadle, 1965; Mercer, 1973a,b; Raabe, 1970), but it
may be useful to review a few general concepts. Aerosols consist of
finely divided particles ranging in size from about 0.01 to 100 µm in
diameter. The particles in a cloud are usually of many sizes and the
size can be expressed as a size-frequency distribution curve which
usually best fits a log-normal distribution. There are several ways to
express the diameter of a particle, the common ones being count median
diameter, mass median diameter, and aerodynamic mass median diameter.
The last term is important as it considers each distribution as if it
were made up of unit density spheres and measures its diameter as if
it were acting aerodynamically like a unit density sphere. Thus,
aerosol behaviour can be compared, regardless of the individual shape
and density of the particles.
The division of a solid into fine particles that become airborne
results in a large increase in the surface area of the material. The
consequence is a generally increased chemical reactivity of the
material, thus accelerating all physical and chemical processes, such
as oxidation, dissolution, evaporation, absorption, and electrical
activity. The physiological activity of these particles also
increases. The sizes of interest from a biological standpoint range
from about 0.1 to 10 µm. Larger particles do not usually enter the
respiratory tract or, if they do, they are deposited in the nose.
6.3.2 Deposition
Material that enters the respiratory tract with the inspired air
can either be deposited or exhaled. Many factors affect the deposition
of particles or the absorption of vapours in the respiratory system.
Retention of vapours is governed by the diffusion rates of the vapour,
the solubility of the vapour in the various body compartments, and the
degree to which these compartments have attained the equilibrium. This
in turn, depends on the duration of the exposure, the concentration,
and the rate of removal of the vapours. This subject has recently been
reviewed by Stupfel & Mordelet-Dambrine (1974).
Many more factors govern the deposition of particulates in the
respiratory tract. Roe (1968) has divided these into physical and
chemical characteristics of the particle, anatomical, physiological,
and pathological factors. The size, density, and shape of the
particles are the physical variables that determine their aerodynamic
behaviour. The Task Group on Lung Dynamics (Morrow et al., 1966) has
discussed deposition as a function of particle size.
Three distinct physical processes act on particles suspended in
the atmosphere to cause them to be deposited in the respiratory tract.
Inertial impaction results from the tendency of particles to move in
straight lines. The repeated branching and subdivision of the
respiratory tract causes particles to be impacted on surfaces,
particularly near the bifurcations. Inertial forces are greater with
larger particles. Sedimentation due to gravity also causes particles
to strike the surface of the respiratory tract. Finally, Brownian
movement, particularly of smaller particles, causes them to be
deposited in the lung. The effectiveness of these mechanisms depends
on the anatomy of the respiratory tract, the size of the particle, and
the breathing pattern. During artificially induced hyperventilation in
rabbits, more material was deposited in comparison with controls
(Velickovskij & Kacnelson, 1964). Normal deposition and deposition in
diseased states are discussed in detail by Albert et al. (1973), Brain
& Valberg (1974), Goldberg & Laurenco (1973), Macklem et al. (1973)
and Stuart (1974).
Aerosols are deposited all along the respiratory tract. Large
particles (5-10 µm) are mainly deposited in the upper respiratory
tract, including the nasal cavity. The depth of penetration increases
as particle size decreases and particles in the 1-2 µm range are, for
the most part, deposited in the alveoli. As a first approximation, 25%
of inhaled particles are exhaled, 50% are deposited in the upper
respiratory tract, and 25% are deposited in the lower respiratory
tract (Morrow et al., 1966).
6.3.3 Clearance
Soluble particles readily dissolve at the site of deposition.
Generally, they enter the bloodstream and then behave as if they had
been intravenously injected. Insoluble particles can be removed from
the respiratory tract by several mechanisms depending on the site of
deposition. Particles that are deposited on the mucous blanket are
carried towards the pharynx by the cilia and are usually swallowed or
expectorated. Hence, it is impossible to completely separate
respiratory exposure from gastrointestinal exposure. The rate of
clearance by the mucociliary escalator has been measured in man by a
number of investigators (Albert et al., 1969, 1973; Camner et al.,
1972, 1973; Morrow, 1970; Sanchis et al., 1972) and animals (Albert et
al., 1973; Thomas, 1969; Velickovskij & Kacnelson, 1964; Watson et
al., 1969).
Particles that are deposited in the deep non-ciliated portion of
the lung clear more slowly. One mechanism responsible for alveolar
clearance is phagocytosis. Although alveolar macrophages engulf
particles within a few hours, there is evidence that macrophages do
not carry them actively to the mucociliary escalator in the first few
days following inhalation (Camner et al., 1977). Recent studies
indicate that there are other mechanisms of alveolar clearance. Tucker
et al. (1973) have shown alveolar clearance by normal interstitial
drainage pathways. Casarett (1972) and Morrow (1973) described a
possible mechanism whereby particles could move from the alveolus up
to the terminal bronchiole, and then to the ciliated epithelium for
removal on the mucus. Very fine particles can also enter the blood
directly through the lymphatic system. Finally, particles can dissolve
and be absorbed into the bloodstream. Some particles remain in the
alveolar region of the lung for considerable periods of time, during
which dissolution forces can operate.
6.4 Dose in Inhalation Studies
The term dose has many meanings depending on the background and
expertise of the investigator. In toxicology, dose is usually defined
as the mass of material introduced into the animal, and it is often
divided by the body weight. Hence, toxicologists often speak of dose
in mg/kg. Even this is misleading, to some extent, because the
material may not interact in any way, and because of other reasons
that become apparent in species comparisons. The actual dose or amount
of a substance entering the internal milieu of the body depends on the
concentration and the particle size of the inhaled material, the
duration of the exposure, and the breathing variables of the test
species. Thus, the actual absorbed dose is difficult to determine and
inhalation toxicologists often refer to exposure conditions instead of
dose. The exposure must be defined both in terms of concentration (C)
and time (t), and is sometimes expressed as the product of these two
(Ct) (MacFarland, 1968) (see also Chapter 1). While the Ct product
is not a true dose, it can be used in a similar fashion. Haber (1924)
recognized this and Haber's rule states that, for a fixed Ct
product, the response will be the same. This rule holds reasonably
well over limited ranges of C and t, but deviations occur when
extreme values of the variables are examined.
More frequently, inhalation toxicologists keep the time constant
and vary the concentration of the test material. Hence, they measure
an LC50, i.e. the median concentration to which animals are exposed
for a specified time that will kill 50% of the animals within a fixed
period of time after exposure. It is implicit in LC50 data that the
durations of both the exposure and post-exposure period are
standardized. Comparative LC50 data are often obtained for 4-h
exposures and a 14-day post-exposure observation period (Carpenter et
al., 1949; Pozzani et al., 1959).
6.5 Choice of Species
The ideal subject for studies relevant to man is man himself.
However, human volunteers can only be used where the toxicological
hazard is already reasonably well defined and accepted. Human studies
have been conducted recently with chlorinated hydrocarbon solvents and
common air pollutants (Andersen et al., 1974; Hazucha et al., 1973;
Stewart, 1972; Stewart et al., 1970a,b, 1973). These experiments were
well controlled and monitored and the exposure levels were low.
Rats, dogs, and monkeys have been the species most used for
inhalation toxicity studies, although investigators have also used
mice, hamsters, guineapigs, rabbits, cats, miniature swine, and
donkeys. The choice of species should be made, primarily, with a view
to extrapolating the experimental results to man. However, choice on
this basis alone is difficult since the validity of such an
extrapolation is often uncertain. When selecting the particular
species for study, the following factors must be considered: the
comparative morphology of the respiratory tract; the presence or
absence of lung disease or susceptible states; and the similarity of
biochemical and physiological responses to those in man.
6.5.1 Anatomical differences
The respiratory systems of various laboratory animals and man
differ widely. As man is a primate, it is sometimes erroneously
assumed that the monkey is a good model for inhalation studies, but
marked differences are noted when comparing human and sub-human
primate respiratory systems. In the monkey, the end airway is always a
respiratory bronchiole, whereas this is rarely the case in man.
Furthermore, the monkey's lung is not lobulated.
The most commonly used laboratory animals are quadrupeds, and
their respiratory systems are horizontal and not vertical as in the
primate. This is important because the aerodynamics of a horizontally
arranged lung are different, and particle deposition will also be
different. In human subjects, maximum dust deposition is in the upper
portion of the lung; in experimental animals, maximum deposition in
the more ventral portions of the lung is usual.
The gross anatomy of the respiratory systems of the various
laboratory animals and man is quite different. In animals such as the
rat and guineapig, the nose contains highly developed tortuous
turbinates. In monkeys and man with relatively smaller noses, the
turbinates are less complex. These differences in nasal anatomy are
especially important in studies involving exposure to particulates.
More particles impinge in more complex turbinate systems and will not,
therefore, reach the deeper portions of the respiratory system.
The subgross anatomy of the lungs of various laboratory animals
and man has been detailed and compared by McLaughlin et el. (1961a,b,
1966). The authors have grouped the common laboratory animals and man
in three basic categories based on subgross pulmonary anatomy
(Table 6.1). They grouped the various animals on the basis of lung
lobulation, the presence or absence of respiratory bronchioles, the
presence or absence of terminal bronchioles, pulmonary artery/
bronchial artery shunts, and the termination of the bronchial
arteries. Their studies indicate that the pulmonary anatomy of the
horse most closely resembles that of man.
6.5.2 Physiological considerations
Pertinent differences in the lung physiology of various species
must be considered in inhalation toxicology. Normal values or ranges
for several species, including man, are summarized in Table 6.2
(Sanockij, 1970a).
Table 6.1 Comparative subgross anatomy of the lunga
Species Lung No. of lobes Lobulation Respiratory Terminal Pulmonary/artery Termination Pleura
type bronchioles bronchioles Bronchial/artery of bronchial
left right shunts arteries
Cow I 3 5(4) well developed extremely poor present present distal airway thick
development
Sheep I 3 4 well developed extremely poor present present distal airway thick
development
Pig I 3 4 well developed extremely poor present not present distal airway thick
development
Monkey II 3 4 not present very well absent not present distal airway thin
developed
Dog II 3 4 not present very well absent not present distal airway thin
developed
Cat II 3 4 not present very well absent not present distal airway thin
developed
Guineapig IIa 3 4 not present fairly well absent present distal airway thin
developed
Rat IIa 1 4 not present fairly well absent few present distal airway thin
developed
Rabbit IIa 3 4 not present fairly well absent present tertiary thin
developed bronchus
Horse III (3) (4) imperfectly poorly present present distal airway thick
developed developed and alveoli
Man III 2 3 imperfectly poorly present present distal airway thick
developed developed and alveoli
a From: McLaughlin et al. (1961a,b, 1966).
Table 6.2 Some physiological indices of man and animalsa
Man Dog Cat Rabbit Guineapig Rat Mouse
Body surface (m2) 1.8 0.528 0.2 0.18 0.040 0.030 0.006
Relation body surface to body weight 0.0257 0.044 0.066 0.072 0.12 0.15 0.3
(m2/kg)
Basal metabolism (kJ/kg) 105 222 -- 188 360 615 711
Frequency of respiration (min) 14-18 10-30 20--30 50-100 80-135 110-135 140-210
Size of alveoli (µm) 150 100 100 -- -- 50 30
Surface of lungs (m2) 50 100 7.2 5.21 1.47 0.56 0.12
Relation of lung surface to body weight 0.7 8.3 2.8 2.5 3.2 3.3 5.4
(m2/kg)
Inhaled air (ml) 616 40-60 -- -- 1.75 0.865 0.154
Lung ventilation (ml/min) 8732 -- 1000 600 155 73 25
Relation of lung ventilation to body 0.13 -- 0.30 0.29 0.33 0.05 1.24
weight (ml/min/g)
Consumption of oxygen (ml/kg/hr) 203.1 3600 9420 522.7 2180 2199 3910
Elimination of CO2 (ml/kg/h) 168.8 -- -- -- -- 2650 4240
Coefficient of respiration 0.82 -- -- 0.83 -- 0.82 0.85-1.33
Pulse frequency for 1 min 70-72 90-130 120-180 150-240 206-280 300-500 520-780
a From: Sanockij (1970a).
6.5.3 Disease and susceptibility states
Most toxicological investigations are performed on healthy
animals. However, epidemiological studies have indicated that during
air pollution episodes the populations at greatest risk are the young,
the aged, and those people with pre-existing cardiopulmonary disease.
One task of the inhalation toxicologist is to identify those segments
of the population that are particularly susceptible to the presence of
airborne contaminants. Certain animal models of diseased or stressed
states have been described (Boyd et al., 1974; Drew & Taylor, 1974;
Silver et al., 1973; Taylor & Drew, 1975; see also Chapter 2) which
could be used or could be adapted for use in inhalation studies. The
most commonly used model of this nature is the papain-induced
emphysematous animal (Gross et al., 1965; Martorana et al., 1973;
Niewoehner & Kleinermann, 1973; Snider et al., 1974). Both aged and
neonatal animals could be used as models of high susceptibility
groups. Disease and susceptibility states are important considerations
in the selection of the species and, in some cases, even the strain of
animal to be investigated since it is well known that the incidence of
cancer differs considerably among certain species and strains.
For example, Kuschner et al. (1975) recently reported a high
incidence of respiratory tract tumours in rats after exposure to
oxybis[chloro-methane] (bis(chloromethyl)ether) but only a few tumours
in hamsters. These tumours were about equally divided between
esthesioneuro-epitheliomas and bronchogenic squamous cell carcinomas.
Leong et al. (1975) repeated these experiments; however, all the
tumours in Leong's study were nasal tumours with no bronchogenic
carcinomas. The only difference noted in the protocol was that Leong
et al. used specific-pathogen-free, caesarean-derived rats, whereas
Kuschner et al. used Sprague-Dawley rats, that were not
specific-pathogen-free.
6.6 Duration of Exposure
Acute inhalation toxicity studies usually consist of a single
exposure (or occasionally a few exposures) of not more than 8 h.
Repeated exposure studies consist of a number of daily exposures for
fixed periods of time. Occasionally, investigators terminate exposure
after several days or weeks, and then maintain the animals in the
colony to observe delayed development of long-term effects (Kuschner
et al., 1975).
The duration of chronic studies varies considerably. One logical
proposal is based on the life span of the test species (Sanockij,
1970b). If, for example, one considers that toxic signs will appear in
man after an exposure over 10% of his life span (7 years), animals
should also be exposed for 10% of their life span. Thus, rats should
be exposed for 3-4 months, and larger animals for a somewhat longer
period. Some authors (Sidorenko & Pinigin, 1970) consider that even
continuous exposure for 3-4 months is insufficient to simulate
lifetime exposure in man, and many studies last longer, some up to 5
years (Lewis et al., 1974). Powell & Hosey (1965) consider the minimum
duration of a chronic study to be one year, the animals being exposed
6 h a day for 5 days a week. This corresponds to a significant portion
of a rodent's lifetime.
Chronic studies are conducted to determine the effects of
long-term exposure to compounds and particularly (in the USSR) to
establish minimum effect levels (Limch). In order to evaluate the
effects of long-term exposures, such as elevated incidences of
infection, emphysema, or the induction of cancer, inhalation controls
should be run concurrently. Two species are usually used. Occasionally
the toxic effects and the mechanism of chronic toxicity are entirely
different from those manifested in acute exposures. Benzene, for
example, is a central nervous system depressant at high
concentrations, while, at low concentrations over long periods of
exposure, it affects the hematopoietic system.
The selection of concentration for chronic studies is difficult.
For example, in the USA, concentrations are chosen that do not produce
mortality and produce only minimal changes in other biological indices
of toxicity during limited studies. In the USSR, the concentration
selected is below the Limac. In order to investigate dose-response
relationships, it is advisable to use at least three different dose
levels, hoping that the highest level chosen will produce quantifiable
effects and that the lowest level selected will produce minimal or
even no effects. In the USSR, research workers often use as the
highest level the concentration which, in single short-term exposures
of human subjects, does not produce any effect during the study of
reflex reactions.
When investigating the various biological indices of toxicity, it
is necessary to carry out measurements more frequently during the
early stages of the study (Camner et al., 1972, 1973). Studies carried
out at the Institute of Labour Hygiene and Occupational Medicine of
the USSR show that the time to the display of the first signs (period
for initial decompensation) varies considerably. Thus, the variables
are recorded at 1, 4, 8, 15, and 30 days and monthly thereafter. After
terminating the exposure, the frequency of measurements may be
increased again to record any early changes.
6.6.1 Intermittent versus continuous exposures
Long-term exposures are usually patterned on projected industrial
experience, giving the animals a daily exposure of 6-7 h, 5 days a
week (intermittent exposure), or on a possible environmental exposure,
with 22-24 h of exposure per day, 7 days a week (continuous exposure),
with about an hour for feeding the animals and maintaining the
chambers. In both cases, the animals are usually exposed to a fixed
concentration of test materials. Thus, neither situation approaches
actual human experience, where concentrations of atmospheric
pollutants are continuously fluctuating by one or two orders of
magnitude. A major difference to consider between intermittent and
continuous exposure is that with the former there is a 17-18 h period
in which animals may recover from the effects of each daily exposure,
and an even longer recovery period at weekends. The recovery period in
some cases is extremely important as in the case of continuous versus
intermittent exposure to dichlormethane (Haun, 1972).
The choice of intermittent or continuous exposure depends on the
objectives of the study and on the human experience that is to be
simulated. However, certain technical difficulties must be considered.
For example, the advantages of continuous exposure for simulating
environmental conditions may be offset by the necessity of watering
and feeding during exposure, and by the need for more complicated (and
reliable) aerosol and vapour generation and monitoring techniques.
Intermittent systems require simpler chambers, since provision for
food and water is not necessary. The contaminant dispersal systems are
also simpler as they need to operate for only 6-7 h per day.
6.7 Inhalation Systems
6.7.1 Facilities required
It is more advantageous to build facilities designed specifically
for inhalation studies than to modify existing buildings. High
cellings are necessary for housing exposure chambers and related
equipment, such as aerosol and vapour generators, filters, flowmeters,
etc. A constant supply of clean filtered air with temperature and
humidity controls should be available for both the chamber rooms and
the chambers themselves. Adequate floor space should provide access to
at least two sides of the chambers, and the chambers themselves should
be separated by a small space to avoid heat transfer between chambers.
6.7.2 Static systems
Inhalation systems can be characterized as static, when the agent
is introduced into a chamber as a batch and then mixed, or dynamic,
when airflow and introduction (and removal) of agent are continuous.
The duration of static exposure is limited by: (a) the gradual
depletion of oxygen; (b) the accumulation of carbon dioxide;
(c) the accumulation of water vapour; and (d) the gradual increase
in temperature inside the chamber. In spite of these limitations,
static systems are of great practical usefulness in assessing acute
toxicity, particularly when the supply of material is limited.
Procedures similar to those described by Draize et al. (1959) are in
use today for screening commercial products. Another use of static
systems is for the exposure of animals to biological aerosols. It is
difficult to generate a viable biological aerosol particle
continuously because of the limited amount of material available;
thus, material is dispersed in a large chamber, mixed, and the animals
exposed through nose tubes connected to the test atmosphere (Jemski &
Phillips, 1965).
6.7.3 Dynamic systems
Today, most inhalation facilities use dynamic systems where the
airflow and introduction of agents are continuous. The theoretical or
nominal concentration of chemicals in a chamber can be calculated as
follows:
flow of chemical
concentration =
flow of air
Many factors, including wall loss, losses on the skin and fur of
animals, and uptake by the test animals, cause the actual
concentration to be somewhat less than the nominal concentration.
Thus, the concentration should always be measured by an appropriate
instrument or technique rather than reporting the nominal
concentration.
When material is introduced into a chamber, the concentration
builds up exponentially according to equations originally described
and verified by Silver (1946). If perfect mixing occurs, the
concentration can be calculated according to the following equation:
C = (w/b) (1 - exp (-bt/a)) (1)
where C = the concentration of material at time t; w = amount of
material introduced per minute; a = volume of the chamber;
b = flow of air through the chamber.
The fraction of equilibrium concentration (w/b) attained in
time t is:
C
= 1 - exp (1 bt/a) (2)
w/b
Thus, the time required to reach 99% (t99) of the equilibrium
concentration is:
0.99 = 1 - exp (-bt99/a) (3)
or
t99 = 4.6052 a/b
This equation may be given the general form:
tx = Ka/b (4)
where x equals % nominal concentration attained in time t and
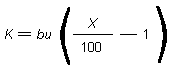 Values of K for various values of x are tablulated below:
x K
99 4.6
95 3.0
90 2.3
85 1.9
80 1.6
There are several features of interest in the above equations.
Since the concentration build-up is exponential, the concentration
will theoretically never reach a constant value. However, in practice,
the concentration is not detectably different once the equilibration
time is equal to t99 or longer. The clearance curve of removal of
chemical from the chamber after the flow of chemical is discontinued
also follows an exponential curve (Fig. 6.1). Thus, to ensure that
little or no material remains in the chamber, it should be operated
for t99 after discontinuing the flow of chemical. This procedure
also compensates for the time required for build-up of material in the
chamber. Finally, it should be noted that in the general equation,
tx is only a function of the volume of the chamber and the flow of
air through the chamber. Thus, if the ratio of a/b = 1,
t99 = 4.6 min, t95 = 3 min, etc. A 150-litre chamber operated at
30 litre/min would have a/b = 5 and t99 = 5 (4.6 min) = 23 min.
MacFarland (1976) has recently reviewed these principles and has also
discussed ways of decreasing t99 by manipulating flow rate.
6.7.4 Typical whole-body systems
Inhalation exposure technology has been recently reviewed by Drew
& Laskin (1973) and earlier descriptions and requirements have been
given by Frazer et al. (1959), Hinners et al. (1966, 1968), and Roe
(1968). The simplest inhalation system would be a box with facilities
for air intake and exhaust. This concept has been used for many years
in chambers similar to that described by Drew & Laskin (1973)
(Fig. 6.2). In this system, a cylindrical glass battery jar is mounted
in a horizontal position; a frame holds the jar and provides support
Values of K for various values of x are tablulated below:
x K
99 4.6
95 3.0
90 2.3
85 1.9
80 1.6
There are several features of interest in the above equations.
Since the concentration build-up is exponential, the concentration
will theoretically never reach a constant value. However, in practice,
the concentration is not detectably different once the equilibration
time is equal to t99 or longer. The clearance curve of removal of
chemical from the chamber after the flow of chemical is discontinued
also follows an exponential curve (Fig. 6.1). Thus, to ensure that
little or no material remains in the chamber, it should be operated
for t99 after discontinuing the flow of chemical. This procedure
also compensates for the time required for build-up of material in the
chamber. Finally, it should be noted that in the general equation,
tx is only a function of the volume of the chamber and the flow of
air through the chamber. Thus, if the ratio of a/b = 1,
t99 = 4.6 min, t95 = 3 min, etc. A 150-litre chamber operated at
30 litre/min would have a/b = 5 and t99 = 5 (4.6 min) = 23 min.
MacFarland (1976) has recently reviewed these principles and has also
discussed ways of decreasing t99 by manipulating flow rate.
6.7.4 Typical whole-body systems
Inhalation exposure technology has been recently reviewed by Drew
& Laskin (1973) and earlier descriptions and requirements have been
given by Frazer et al. (1959), Hinners et al. (1966, 1968), and Roe
(1968). The simplest inhalation system would be a box with facilities
for air intake and exhaust. This concept has been used for many years
in chambers similar to that described by Drew & Laskin (1973)
(Fig. 6.2). In this system, a cylindrical glass battery jar is mounted
in a horizontal position; a frame holds the jar and provides support
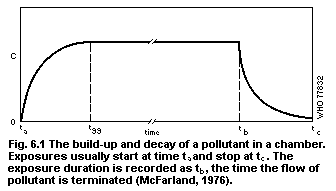 for a panel which is mounted against the open end and serves as a
closure. Various openings can be cut in the panel for the introduction
of the pollutant and for monitoring the concentration.
Studies at the University of Rochester on the toxicity of
radioactive materials contributed significantly to the development of
the technology of inhalation exposure. The original test chamber was
cylindrical with cones on the top and bottom. A modified chamber in
the shape of a hexagon with pyramidal ends (Wilson & Laskin, 1950) is
known as the "Rochester Chamber". A final modification with a square
cross-section as shown schematically in Fig. 6.3 is known as the "NYU
Chamber" (New York University Chamber) (Laskin et al., 1970). These
two shapes are currently in use in several laboratories, although
cubes, cylinders, spheres, modified hemispheres, and even chambers
with an elliptical cross-section have all been used (Drew & Laskin,
1973). The two major considerations that influence the shape of the
chamber are uniformity of distribution and wall loss of the test
substance (MacFarland, 1976) with secondary importance placed on
caging supports, accessibility, and costs.
A typical chamber is shown schematically in Fig. 6.3. The unit
has a volume of 1.3 m3 and is about 3 m in height. The body is made
of stainless steel and the windows can be made of either glass or
lucite. Clean air is supplied at the top with the pollutant being
injected in a perpendicular direction to the incoming airstream.
Chambers with tangential pollutant introduction are also common.
Airflow is usually down through the chamber and the air is removed
through the side arm of a Y fitting at the bottom. Animal wastes are
removed at the bottom via the building drains, usually through a trap
or a valve. The trap also maintains the integrity of the system and
allows operation at a pressure slightly (1-2 cm H20) below ambient.
Chambers of this general shape have been built with volumes ranging
from 128 litres up to 5 m3, although MacFarland (1976) suggests that
the pollutant concentration in chambers of less than 1 m3 may not be
uniform.
The size of the chamber depends on the number and size of the
animals to be exposed. The total animal volume should not exceed
approximately 5% of the total chamber volume. Experience has shown
that above 5% surface losses begin to cause excessive concentration
losses and thermal considerations also begin to play a limiting role.
6.7.5 Construction materials
Inhalation chambers should be constructed of materials that do
not react with the test material and are easily cleaned. The most
versatile materials are stainless steel and glass. However, many other
materials have been used, including aluminium, wood, wood coated with
epoxy paints, lucite, and various fibre panels. The chamber should
have at least two sides made almost completely of transparent material
for a panel which is mounted against the open end and serves as a
closure. Various openings can be cut in the panel for the introduction
of the pollutant and for monitoring the concentration.
Studies at the University of Rochester on the toxicity of
radioactive materials contributed significantly to the development of
the technology of inhalation exposure. The original test chamber was
cylindrical with cones on the top and bottom. A modified chamber in
the shape of a hexagon with pyramidal ends (Wilson & Laskin, 1950) is
known as the "Rochester Chamber". A final modification with a square
cross-section as shown schematically in Fig. 6.3 is known as the "NYU
Chamber" (New York University Chamber) (Laskin et al., 1970). These
two shapes are currently in use in several laboratories, although
cubes, cylinders, spheres, modified hemispheres, and even chambers
with an elliptical cross-section have all been used (Drew & Laskin,
1973). The two major considerations that influence the shape of the
chamber are uniformity of distribution and wall loss of the test
substance (MacFarland, 1976) with secondary importance placed on
caging supports, accessibility, and costs.
A typical chamber is shown schematically in Fig. 6.3. The unit
has a volume of 1.3 m3 and is about 3 m in height. The body is made
of stainless steel and the windows can be made of either glass or
lucite. Clean air is supplied at the top with the pollutant being
injected in a perpendicular direction to the incoming airstream.
Chambers with tangential pollutant introduction are also common.
Airflow is usually down through the chamber and the air is removed
through the side arm of a Y fitting at the bottom. Animal wastes are
removed at the bottom via the building drains, usually through a trap
or a valve. The trap also maintains the integrity of the system and
allows operation at a pressure slightly (1-2 cm H20) below ambient.
Chambers of this general shape have been built with volumes ranging
from 128 litres up to 5 m3, although MacFarland (1976) suggests that
the pollutant concentration in chambers of less than 1 m3 may not be
uniform.
The size of the chamber depends on the number and size of the
animals to be exposed. The total animal volume should not exceed
approximately 5% of the total chamber volume. Experience has shown
that above 5% surface losses begin to cause excessive concentration
losses and thermal considerations also begin to play a limiting role.
6.7.5 Construction materials
Inhalation chambers should be constructed of materials that do
not react with the test material and are easily cleaned. The most
versatile materials are stainless steel and glass. However, many other
materials have been used, including aluminium, wood, wood coated with
epoxy paints, lucite, and various fibre panels. The chamber should
have at least two sides made almost completely of transparent material
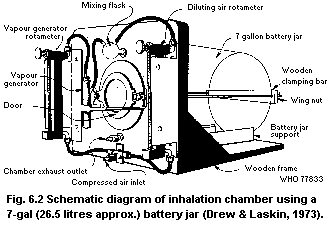
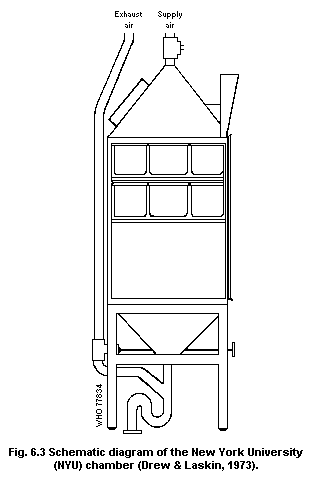 in order to view the animals during exposure. The inside surfaces
should be as free as possible from perturbations and rough surfaces
and edges, in order to facilitate cleaning. Openings should be
included to monitor the variables needed to characterize the exposure
(section 6.7.8).
6.7.6 Engineering requirements
Accurate control of airflow in the chambers is essential. The
usual procedure is to supply filtered, conditioned air in excess of
that required and then tap off the common supply for each chamber. In
most experiments, the ratio of chamber volume to airflow ranges from 1
to about 6.
When handling hazardous materials, special safety precautions
must be taken to protect operating personnel and the surrounding
environment. The chambers are operated at slightly negative pressure
to ensure that any leaks draw air into the system. Chamber effluents
must be cleaned, usually by filters or scrubbers, or at least diluted,
before being released into the environment. Stack effluents should be
monitored.
Food and water must be provided in the chambers, when animals are
being exposed continuously. Facilities for cleaning the chambers,
while the animals are in them, are also necessary, though in many
laboratories the animals are removed from the chamber for 45 min-1 h
to permit it being serviced. Racks for supporting animal cages must be
included and exposure cages should have all six sides made of wire
mesh (stainless steel) to ensure good mixing.
6.7.7 Special systems
6.7.7.1 Isolation units
When handling particularly hazardous materials, additional
precautions are necessary. These can be fairly simple, such as
operating a battery jar in a fume hood, or complex, such as the
chamber-within-a-chamber concept described by Laskin et al. (1970). In
this system (Fig. 6.4) the aerosol is separated by two barriers from
the operator with separate glove boxes for generation and removal of
the aerosol. In addition, living quarters are provided behind a
barrier with pass boxes for food and animal wastes to be moved into
and out of the chamber.
6.7.7.2 Head and nose exposures
Early in the development of inhalation exposure systems,
investigators realized the value of head or nose only exposures
(Saito, 1912). Stokinger (1949) described chambers with openings for
head-only exposures for several species, and Henderson (1952)
in order to view the animals during exposure. The inside surfaces
should be as free as possible from perturbations and rough surfaces
and edges, in order to facilitate cleaning. Openings should be
included to monitor the variables needed to characterize the exposure
(section 6.7.8).
6.7.6 Engineering requirements
Accurate control of airflow in the chambers is essential. The
usual procedure is to supply filtered, conditioned air in excess of
that required and then tap off the common supply for each chamber. In
most experiments, the ratio of chamber volume to airflow ranges from 1
to about 6.
When handling hazardous materials, special safety precautions
must be taken to protect operating personnel and the surrounding
environment. The chambers are operated at slightly negative pressure
to ensure that any leaks draw air into the system. Chamber effluents
must be cleaned, usually by filters or scrubbers, or at least diluted,
before being released into the environment. Stack effluents should be
monitored.
Food and water must be provided in the chambers, when animals are
being exposed continuously. Facilities for cleaning the chambers,
while the animals are in them, are also necessary, though in many
laboratories the animals are removed from the chamber for 45 min-1 h
to permit it being serviced. Racks for supporting animal cages must be
included and exposure cages should have all six sides made of wire
mesh (stainless steel) to ensure good mixing.
6.7.7 Special systems
6.7.7.1 Isolation units
When handling particularly hazardous materials, additional
precautions are necessary. These can be fairly simple, such as
operating a battery jar in a fume hood, or complex, such as the
chamber-within-a-chamber concept described by Laskin et al. (1970). In
this system (Fig. 6.4) the aerosol is separated by two barriers from
the operator with separate glove boxes for generation and removal of
the aerosol. In addition, living quarters are provided behind a
barrier with pass boxes for food and animal wastes to be moved into
and out of the chamber.
6.7.7.2 Head and nose exposures
Early in the development of inhalation exposure systems,
investigators realized the value of head or nose only exposures
(Saito, 1912). Stokinger (1949) described chambers with openings for
head-only exposures for several species, and Henderson (1952)
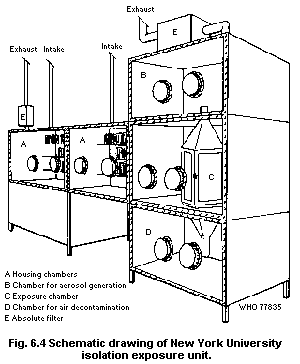 described an apparatus consisting of a cylindrical chamber with
openings arranged along two sides to enable nose exposure of mice to
biological aerosols. Nose exposure systems are used in situations
where: (a) skin absorption is not desirable; (b) the amount of test
material is limited; (c) the material is hazardous; and (d) there is
no need for a large chamber. They are most commonly used for acute
exposures, since it is difficult to restrain the animals for long
periods of time.
Nose exposure systems have been used extensively for two
particular situations -- exposure to radioactive aerosols and exposure
to cigarette smoke. Investigators at the Lovelace Foundation for
Medical Education and Research in Albuquerque have developed a series
of nose exposure units (Boecker et al., 1964; Raabe et al., 1973;
Thomas & Lie, 1963). The technical difficulties of exposing animals to
cigarette smoke has prompted the development of several systems
designed specifically for this purpose (Hoffman & Wynder, 1970; Stuart
et al., 1970). One device has been developed by Homburger et al.
(1967) and two have been described by Dontenwill (1970). Albert et al.
(1974) have developed a device to expose donkeys to cigarette smoke
via nose tubes. Devices for exposing dogs to cigarette smoke have also
been described (Cahan & Kirman, 1968).
A nose exposure system for exposure to dusts has been developed
at the Institute of Hygiene and Occupational Medicine in Moscow
(Valeznev et al., 1970) (Fig. 6.5). It consists of a completely
enclosed system in which up to 40 rodents can be exposed
simultaneously. A very complex system adaptable to both head-only and
whole-body exposures is routinely used at the Medical Institute in
Kiev (Balasov et al., 1968). A schematic diagram of this system is
shown in Fig. 6.6.
6.7.7.3 Instantaneous exposure systems
Occasionally, it is necessary to expose animals to a
concentration of material, while avoiding the time required to attain
uniform concentrations. In this case, the air in the chamber is
equilibrated with the test material and the animals rapidly inserted
into the chamber. Sometimes double chambers are used for this purpose.
The animals are placed in the upper half and the pollutant is
introduced into the lower half. When the desired concentration is
reached in the lower half, a trap door opens and the animals fall into
the lower half, while the mechanism immediately closes. Other
investigators have described various drawer arrangements for rapid
insertion of animals into test atmospheres.
described an apparatus consisting of a cylindrical chamber with
openings arranged along two sides to enable nose exposure of mice to
biological aerosols. Nose exposure systems are used in situations
where: (a) skin absorption is not desirable; (b) the amount of test
material is limited; (c) the material is hazardous; and (d) there is
no need for a large chamber. They are most commonly used for acute
exposures, since it is difficult to restrain the animals for long
periods of time.
Nose exposure systems have been used extensively for two
particular situations -- exposure to radioactive aerosols and exposure
to cigarette smoke. Investigators at the Lovelace Foundation for
Medical Education and Research in Albuquerque have developed a series
of nose exposure units (Boecker et al., 1964; Raabe et al., 1973;
Thomas & Lie, 1963). The technical difficulties of exposing animals to
cigarette smoke has prompted the development of several systems
designed specifically for this purpose (Hoffman & Wynder, 1970; Stuart
et al., 1970). One device has been developed by Homburger et al.
(1967) and two have been described by Dontenwill (1970). Albert et al.
(1974) have developed a device to expose donkeys to cigarette smoke
via nose tubes. Devices for exposing dogs to cigarette smoke have also
been described (Cahan & Kirman, 1968).
A nose exposure system for exposure to dusts has been developed
at the Institute of Hygiene and Occupational Medicine in Moscow
(Valeznev et al., 1970) (Fig. 6.5). It consists of a completely
enclosed system in which up to 40 rodents can be exposed
simultaneously. A very complex system adaptable to both head-only and
whole-body exposures is routinely used at the Medical Institute in
Kiev (Balasov et al., 1968). A schematic diagram of this system is
shown in Fig. 6.6.
6.7.7.3 Instantaneous exposure systems
Occasionally, it is necessary to expose animals to a
concentration of material, while avoiding the time required to attain
uniform concentrations. In this case, the air in the chamber is
equilibrated with the test material and the animals rapidly inserted
into the chamber. Sometimes double chambers are used for this purpose.
The animals are placed in the upper half and the pollutant is
introduced into the lower half. When the desired concentration is
reached in the lower half, a trap door opens and the animals fall into
the lower half, while the mechanism immediately closes. Other
investigators have described various drawer arrangements for rapid
insertion of animals into test atmospheres.
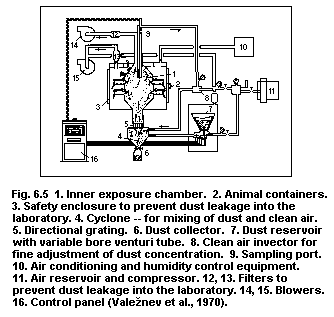
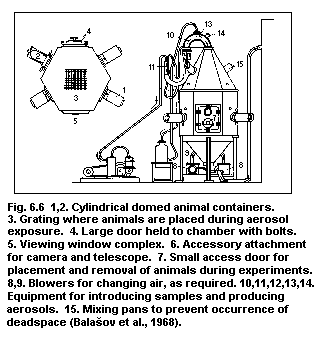 6.7.8 Variables to monitor
It is necessary to monitor several variables during the operation
of an inhalation chamber. The most obvious is the concentration in the
chamber of the pollutant in question. This can be done continuously by
automated samplers and recorded on a strip chart recorder or manually
at periodic intervals using a variety of sampling techniques. When
aerosols are involved, particle size should be determined. The flow of
pollutant, the flow of air, and the chamber pressure should all be
recorded frequently. Chamber temperature and humidity are other
variables that should be monitored. It is also useful to measure the
pressure drop across intake and exhaust filters in order to know when
to replace them.
6.7.9 Human exposure facilities
Ethical principles will always be of first concern, when
considering the exposure of human subjects to materials for toxicity
evaluation. However, there are situations where controlled exposure of
human beings can provide useful information with minimum risk. One of
the more modern facilities has been described by Stewart et al.
(1970a,b). It consists of a room approximately 6 m × 6 m × 2.7 m,
completely air conditioned and operated at slightly negative pressure.
Activity within this chamber is strictly sedentary and comfortable
chairs and study desks are provided. Meals are served during
exposures, with coffee and soft drinks available continuously. All
subjects are under continuous surveillance by medical personnel and
all activities are visually monitored by closed circuit television.
Such facilities are particularly useful for studying psychomotor
effects and other sensitive indicators of exposure to low
concentrations of organic vapours.
The odour threshold concentrations used in the USSR for
establishing maximum permissible concentrations for single exposures
are determined in human subjects over short periods (5-10 min).
Obviously such studies can only be carried out at very low
concentrations considered to be safe. The minimum concentration sensed
by the most sensitive individual is accepted as the odour threshold
(Rjazanov, 1964).
Reflex reactions produced in man by irritating the receptive
zones of the respiratory organs with subsensory concentrations of
atmospheric pollutants, were established by measuring the light
sensitivity of the eye, the bioelectric activity of the cerebrum, etc.
(Bustueva et al., 1960; Rjazanov, 1964). Changes in encephalographic
responses resulted from exposure to small concentrations of these
substances. The maximum concentration that did not produce an effect
on the bioelectric activity of the cerebrum was, in most cases, 3-4
times lower than the odour threshold concentration (Krotov, 1971;
Sidorenko & Pinigin, 1972).
6.8 Contaminant Generation and Characterization
Extensive reviews have been published by several investigators on
methods of generating and characterizing vapours and particles (Bryan,
1970; Cotabish et al., 1961; Drew & Lippmann, 1971; Lodge, 1968;
Mercer, 1973a,b; Nelson, 1971; Raabe, 1970).
6.8.1 Generation of vapours
Vapours can be generated by using one of several flow-dilution
devices (Cotabish et al., 1961; Drew & Lippmann, 1971; Nelson, 1971;
Saltzman, 1971; Saltzman & Warburg, 1965). If the contaminant is a
liquid at room temperature, a vaporization step must be included. One
procedure is to use a motor-driven syringe and to apply the liquid to
a wick or heated plate in a calibrated stream of air (Nelson & Griggs,
1968). Another method is to saturate the airstream with vapour and
then dilute it with air to the desired concentration (Cotabish et al.,
1961). A third technique, originally described by O'Keefe & Ortman
(1966), consists of using permeation tubes. These are especially
useful when using low concentrations of test materials for
standardization procedures. In theory, there is no reason why they
cannot be scaled up for use in inhalation studies.
6.8.2 Particle generators
The generation of particulate contaminants is usually more
difficult than vapour generation. The contaminant may be generated
from a dry powder or from a liquid and the particles generated may be
of uniform size (monodisperse) or may vary greatly in size
(heterogeneous).
6.8.2.1 Heterogeneous aerosols
The Wright dust feed (1950) is one of the better known
instruments for generating aerosols from a dry powder. A gear drives
the surface of a packed cylinder of finely ground powder against a
scraping mechanism. A high velocity airstream disperses the powder.
Proper use of the Wright dust feed is dependent upon the control of
the relative humidity of the airstream and the packing density of the
powder. Other devices for producing aerosols from dry powders have
been described (Crider et al., 1968; Deichman, 1944; Dimmick, 1959;
Stead et al., 1944). Since the particle size of the resultant aerosols
depends upon the size of the original powder, elutriators and cyclones
are sometimes included to limit the maximum size. Laskin et al. (1971)
described generators that include such devices for producing freshly
ground polyurethane aerosols.
Agglomeration, usually caused by electrical charge, is a serious
problem with dry dust aerosols. The electrical behaviour of aerosols
has been reviewed by Whitby & Liu (1966). A mechanical solution to
agglomeration has been proposed by Drew & Laskin (1971) who described
a fluidizing dust generator.
Wet dispersion generators break liquid into droplets. The liquid
may be a solution or a suspension of the test material. In most cases,
the liquid is drawn into filaments or films that are broken into
droplets. A number of compressed air-driven generators (nebulizers),
that produce droplets of many sizes, have been described (Dautrebande,
1962; Laskin, 1948; Lauterbach et al., 1956; Raabe, 1970). The
resulting aerosols are polydisperse although relatively narrow size
distribution can be attained with some nebulizers.
Monodisperse aerosols are occasionally needed by investigators,
especially when studying regional deposition. The most popular device
for dispensing uniform droplets is the spinning disc generator first
described by Walton & Prewett (1949). Primary droplets thrown off the
perimeter of a spinning disc are uniform in size. The liquid is fed on
to the centre of the disc and accumulates at the edge until broken off
by centrifugal force. Some secondary, smaller droplets are also
produced but these can be separated dynamically. Several investigators
have successfully used the spinning disc for inhalation studies
(Albert et al., 1964; Kajland et al., 1964; Lippmann & Albert, 1967;
Philipson, 1973). Another device employing a controlled condensation
process originally described by LaMer & Sinclair (1943) has been found
to be suitable for inhalation studies. A third principle, consisting
of size specific collection, resuspension and subsequent dispersion,
has also been used (Kotrappa et al., 1972).
6.8.3 Monitoring contaminant concentrations
The techniques and equipment for monitoring contaminant
concentrations have been reviewed by a number of authors (Lippman,
1971; Powell & Hosey, 1965). In many cases, the characteristics of the
contaminant determine the sampling technique. Sometimes a number of
techniques are available and the method of choice may depend upon the
availability of equipment, cost of reagents, time for analysis, or
other factors. Automated instrumentation is currently available for a
number of contaminants. However, when using automatic devices,
a second method, usually chemical, should be used to verify the
instrument performance.
6.8.3.1 Vapour sampling
Two basic methods for the collection of gaseous samples are
employed. The first involves the use of a gas collector, such as an
evacuated flask or bottle, to obtain a definite volume of air at a
known temperature and pressure. The second method involves the passage
of a known volume of air through a collecting medium to remove the
desired contaminants from the sampled atmosphere. The samples are then
analysed by appropriate analytical techniques.
Since the assays are related to the volume of air sampled, the
instrumentation for monitoring airflow or volume should be accurately
calibrated. It is also important that there are no leaks in the
sampling train, thus assuring that all the air measured has passed
through the collecting medium.
A number of devices, currently available, measure the
concentration of vapours continuously; many record the result
graphically. Many detection principles are used including
conductivity, colorimetry, and spectrophotometry. Recent commercial
instrumentation using the principle of infrared spectrometry are
especially useful. Most of these instruments have been described by
Nader (1971).
6.8.3.2 Particulate sampling
When monitoring particulate atmospheres, mass concentration and
particle size must be determined. The mass concentration can be
measured by techniques similar to those used for monitoring vapours.
The material can be collected, then assayed by appropriate chemical
methods. Gravimetric analysis can also be performed by weighing the
filter paper before and after collecting a sample. The resulting mass
can be related to the volume of air that was sampled.
Particle collection techniques include filtration, impingement,
thermal and electrostatic precipitation, and sedimentation. The basic
principles for these techniques and specific examples of each method
have been reviewed (Lippmann, 1971). These principles should be
thoroughly understood prior to selection of a method for a specific
contaminant.
The techniques for measuring particle size and numbers have been
discussed in detail by Mercer (1973a,b). Direct methods consisting of
both conventional and electron microscopy can be used. In both cases,
proper sampling methods to ensure collection of a representative
sample should be followed. Electrostatic and thermal precipitators or
an impinger can be used. After using an impinger, the dust can be
counted in a standard haematology counting cell or counted directly
with a Coulter Counter.
There are two indirect methods of assessing particle size; the
use of cascade impactors and the use of light scattering devices. The
theory of cascade impaction has been described by Mercer (1963, 1964,
1965). The theory of light scattering devices has been reviewed by
Hodkinson (1966) and commercially available instruments have been
listed by Swift (1971). Many factors, including shape, opacity, and
others, some unknown, affect the amount of light scattered and the
measured number and size of the particles. These devices should,
therefore, be used cautiously.
6.9 Other Methods of Respiratory Tract Exposure
6.9.1 In vivo exposures
In addition to direct inhalation, several other methods of
exposing the respiratory tract have been described (Roe, 1968).
Andervont (1937) originally described a thread implantation technique
whereby a thread impregnated with a test compound was literally passed
through a lobe of the lung. This technique was modified by Kuschner et
al. (1957) who devised a pellet that could be impregnated with the
test material or coated with radioactive materials. This pellet had
hooks which held it in the lumen of the bronchus or bronchiole. This
technique has been used to demonstrate the carcinogenicity of a number
of compounds including benzo(a)pyrene, 3-methylcholanthrene, and
calcium chromate (Laskin et al., 1970). A technique whereby hamsters
are anaesthetized and then intratracheally intubated with various
materials is in use in a number of laboratories (Laskin et al., 1970;
Little & O'Toole, 1974; Saffiotti, 1970; Schreiber et al., 1972). In
two laboratories, this technique has been coupled with inhalation
exposures to study the potential cocarcinogenicity of various agents
(Laskin & Nettesheim, 1974, personal communication).
6.9.2 In vitro exposures
A few in vitro techniques that show promise as regards
elucidation of the mechanisms of toxicity of inhaled materials should
be mentioned. A number of investigators (Niemeier & Bingham, 1972;
O'Neill & Tierney, 1974; Orton et al., 1973) are using various,
isolated, perfused lung preparations to study toxicity. Such
preparations could be particularly useful in studying the transfer of
materials from the lung to the blood. These preparations are also used
to study pulmonary metabolism as are lung tissue slices (O'Neil &
Tierney, 1974). Finally, several laboratories are developing methods
of culturing tracheal rings and sections (Griesemer et al., 1974; Lane
& Miller, 1975). These preparations show great promise for
toxicological evaluation (section 2.7.4).
6.10 Biological End-points and Interpretation of Changes in these
End-points
Throughout the course of an inhalation study, there are a number
of biological indicators to observe. The classical indices of toxicity
include weight change and mortality, organ-to-body weight ratios, and
both gross and microscopic changes in the morphology of the various
tissues and organs. While the study is in progress, respiratory,
physiological indices can be monitored and the excretion of certain
chemicals in breath, urine, and faeces can also be followed. Some
procedures particularly useful in inhalation toxicity studies are
discussed below.
6.10.1 Morphological changes
The primary function of the lung is the exchange of oxygen and
carbon dioxide between the blood and alveolar air. The walls of the
alveoli are lined by a single, flattened layer of epithelial cells
that are in close proximity to endothelial cells lining capillaries.
The mean distance from the lumen of the capillary to the lumen of the
alveolus is less than one micron. Because this distance is so small,
any thickening or inflammation of the alveolar wall will severely
disrupt the diffusion of gases.
The air in the alveolus arrives through a series of branching
bronchi and bronchioles the specialized epithelial lining of which is
instrumental in removing particulate matter from the lung. Any lesions
that result in narrowing of the lumen of these bronchi and bronchioles
cause a disruption in the ventilation of that portion of the lung.
Chemicals affecting the specialized ciliated epithelial lining, the
bronchioles, and the bronchi may interrupt the clearance mechanism,
resulting in a build-up of inhaled particulates in the lung.
The type of morphological change observed in the lung as a result
of the inhalation of materials depends upon the concentration of the
inhaled material and the length of time for which animals are exposed.
Short-term inhalation of high concentrations of certain materials may
result in acute changes in the lung such as oedema, necrosis, and
purulent inflammation. However, the inhalation of the same material at
lower concentrations for longer periods of time may result in chronic
changes such as fibrosis and even neoplasia. Table 6.3 lists a number
of chemicals and the response elicited in the lung following their
inhalation.
Techniques for quantifying morphological changes are, at best,
limited. The usual practice is to assign some number to the degree of
damage and then to apply statistical procedures to these numbers.
These procedures are, however, subject to individual interpretation.
Assessment of the degree of fibrosis is possible (Roe, 1968), although
such measurements should be checked by special staining procedures.
Interpretation of any morphological changes in the lung must take
into consideration the concentration and duration of exposure.
Exposure to certain materials will elicit immediate acute
morphological changes which, with time, tend to resolve and disappear.
This phenomenon suggests that the animal is able to adapt to the
effects of some chemicals, if the concentration is not too high. An
Table 6.3 Responses of the lung to various chemicals
Chemical Bronchiolar Loss of Oedema Epithelial Fibrosis Emphysema Granuloma
epithelial goblet metaplasia
hyperplasia cells
nitrogen dioxide x x x x x
ozone x x x x x
allergens x
sulfuric acid x x
bis-chloromethyl ether
silica x x
coal dust x x
beryllium x x x
cotton dust
nickel compounds
Table 6.3 (cont'd)
Necrosis Neoplasia Bronchial Alveolar Nonsuppurative
constriction epithelial alveolitis
hyperplasia
nitrogen dioxide x
ozone
allergens x
sulfuric acid x x
bis-chloromethyl ether x
silica
coal dust
beryllium x
cotton dust x
nickel compounds x
example of this adaptive phenomenon is seen with ozone. Nonlethal
exposures to ozone have a protective effect against subsequent lethal
exposures which would result in marked pulmonary oedema (Alpert &
Lewis, 1971).
6.10.2 Functional changes
There are certain advantages in using alterations in respiratory
functions as indices of toxicity: (a) quantitative measurements can
usually be made at concentrations far below those needed to produce
morphological effects; (b) many effects may be measured at the level
of reversible rather than irreversible changes; (c) such measurements
may elucidate mechanisms of action of materials, and (d) in many
instances similar data can be obtained from experimental animals and
man.
6.10.2.1 Measurement of respiratory frequency
Alteration of respiratory frequency in mice may be used as a
simple screening test for assessing irritant potency (Alarie, 1973).
The changes produced are dose-related, which permits construction of
dose-effect curves and calculation of the concentration required to
produce, for example, a 50% decrease in respiratory frequency. Some
irritants, mainly those affecting the upper respiratory tract,
decrease frequency. Other irritants, mainly those which penetrate to
the deeper areas of the lung, increase frequency. Measurement of
respiratory frequency alone is a very sensitive measure of effect for
those irritants that increase frequency (e.g. ozone and nitrogen
dioxide), but it is a relatively insensitive measure of effect for
those irritants that decrease frequency (e.g. sulfur dioxide and
formaldehyde).
6.10.2.2 Measurement of mechanics of respiration
Alterations in pulmonary flow resistance or pulmonary compliance
may be used to assess irritant potency. The method of Amdur & Mead
(1958) required three basic measurements: intrapleural pressure, tidal
volume, and the rate of flow of gas in and out of the respiratory
tract. Intrapleural pressure is estimated by placing a fluid-filled
catheter in the pleural space, while the animal is under anaesthesia.
Once the catheter is positioned, no further anaesthesia is necessary.
Tidal volume is obtained by placing the animal in a body
plethysmograph and measuring the pressure changes as the animal
breathes quietly. Rate of gas flow is obtained by electrical
differentiation of the volume signal. This method provides data on
both resistance and compliance, but is generally limited to single
exposure studies of a few hours duration. The guineapig has been the
species most commonly used for this method; however, plethysmographic
studies have been successfully carried out on several other rodents
including mice (Alarie, 1973), rats (Palacek, 1969) and rabbits
(Davidson et al., 1966).
The method of Murphy & Ulrich (1964) does not involve surgical
intervention and, thus, makes it possible to perform measurements
repeatedly on the same animals. The animal is placed in a body
plethysmograph to which an oscillating sine wave pressure is applied.
The animal's face is fitted with a mask containing a pneumotachograph
screen for measuring flow. Changes in resistance are calculated for
the alterations in flow produced by the superimposed pressure
oscillations. This technique has been used in toxicological studies
without making compliance measurements. However, by measuring
oesophageal pressure reflecting intrapleural pressure, it should be
possible to estimate compliance changes. A compliance measurement is
described by Mead (1960) that involves no surgical intervention.
The observed changes in respiratory mechanics are dose-related
and permit the construction of dose-effect curves. These may be used
to compare irritant potency or to study such things as the effect of
inert particles on the reaction to irritant gases. The deep lung
irritants that increase respiratory frequency tend to show a decrease
in compliance as the primary alteration in the mechanical behaviour of
the lung. Resistance changes with such irritants are minimal. The
irritants that slow respiratory frequency tend to show an increase in
resistance accompanied by a smaller decrease in compliance, as the
primary alteration in pulmonary mechanics.
An increase in pulmonary flow resistance can result from a
narrowing of the lumen of the bronchi mediated by constriction of the
smooth muscle. The effect, in this situation, usually occurs rapidly
on exposure to the irritant. In the case of a gaseous irritant, the
effect is fairly rapidly reversed when irritant exposure ceases. In
the case of a particulate irritant which remains in the lung, the
effect is less readily reversible. Swelling of the respiratory mucosa
or an increase in mucus secretion can also cause an increase in flow
resistance. The effect, in this situation, usually takes some time to
develop.
Much of the damage to the lung is to the small airways which
change either in calibre or stability or both. It would be very useful
if this damage could be detected early during an exposure before more
serious disease develops. There are three tests which are promising
and have been useful in human subjects. They are the maximal
expiratory flow volume curve, reviewed by Hyatt & Black (1973), the
alveolar closing volume (Dollfuss et al., 1967) and the frequency
dependency of compliance (Woolcock et al., 1969). Animal models that
provide the same data have not yet been fully developed.
6.10.3 Biochemical end-points
In the last decade, much research has centred around the
elucidation of some of the biochemical aspects of the lung. It has
only recently been demonstrated that the lung is capable of
biotransforming many foreign chemicals and several authors have
investigated the role of cytochrome P-450-dependent enzyme systems in
pulmonary microsomes (Bend et al., 1972; Chhabra & Fouts, 1974;
Chhabra et al., 1974; Fouts & Devereux, 1972; Harper et al., 1975;
Hook et al., 1972). The importance of intermediary metabolism is now
being considered (Tierney, 1974). The biochemistry of pulmonary
surfactants and their role in pulmonary defence mechanisms has been
reviewed by King (1974). Investigations of the lung collagen have
recently been reviewed by Crystal (1974). Witschi (1975) has provided
an excellent summary of biochemical approaches for the evaluation of
pulmonary toxicity.
6.10.4 Other end-points in inhalation studies
Exposure by inhalation is only a means by which the substance
enters the animal. This chapter has concentrated on the lung as the
critical organ. However, there are end-points that are not necessarily
related to the lung. Inhalation studies have recently been reported to
assess the teratological effects of inhaled materials (Schwetz et al.,
1975). They have also been used in behavioural studies (Weiss &
Laties, 1975; Xintaras et al., 1974).
REFERENCES
ALARIE, Y. (1973) Sensory irritation of the upper airways by airborne
chemicals. Toxicol. appl. Pharmacol., 24: 279-297.
ALBERT, R. E., PETROW, H. G., SALAM, A. S., & SPIEGELMAN, J. R. (1964)
Fabrication of monodisperse lucite and iron oxide particles with
a spinning disc generator. Health Phys., 10: 933-940.
ALBERT, R. E., LIPPMANN, M., & BRISCOE, W. (1969) The characteristics
of bronchial clearance and the effects of cigarette smoking.
Arch. environ. Health, 18: 738-755.
ALBERT, R. E., LIPPMANN, M., PETERSON, H. T., JR, BERGER, J., SANBORN,
K., & BORWAG, D. (1973) Bronchial deposition and clearance of
aerosols. Arch. intern. Med., 131: 115-131.
ALBERT, R. E., BERGER, J., SANSORN, K., & LIPPMANN, M. (1974) Effects
of cigarette smoke components on bronchial clearance in the
donkey. Arch. environ. Health, 29: 96-101.
ALPERT, S. M. & LEWIS, T. R. (1971) Ozone tolerance studies utilizing
unilateral lung exposure. J. appl. Physiol., 192: 364-368.
AMDUR, M. O. & MEAD, J. (1958) Mechanics of respiration in
unanaesthetized guinea pigs. Am. J. Physiol., 192: 364-368.
ANDERSEN, I., LUNDQVIST, G. R., JENSON, P. L., & PROCTOR, D. F. (1974)
Human response to controlled levels of sulfur dioxide. Arch.
environ. Health, 28: 21-39.
ANDERVONT, H. B. (1937) Pulmonary tumours in mice: IV. Lung tumours
induced by subcutaneous injection of 1,2,5,6-dibenzanthracene in
different media and by its direct contact with lung tissue.
Public Health Rep., 52: 1584.
BALASOV, V. E., BARTENEV, V. D., SAVICKIJ, I. V., & TRAHTENSERG, I. M.
(1968) [Toxicological assessment of volatile substances leaking
from synthetic materials.] Zdorov'e, Kiev (in Russian).
BEND, J. R., HOOK, G. E. R., EASTERLING, R. E., GRAM, T. E., & FOUTS,
J. R. (1972) A comparative study of the hepatic and pulmonary
microsomal mixed function oxidase systems in the rabbit.
J. Pharmacol. exp. Ther., 183: 206-217.
BOECKER, B. B., AQUILAR, F. L., & MERCER, T. T. (1964) The design of a
canine inhalation exposure apparatus incorporating a whole-body
plethysmograph. Health Phys., 10: 1077-1089.
BOYD, M. R., BURKA, L. T., HARRIS, T. M., & WILSON, B. J. (1974)
Lung-toxic furanoterpenoids produced by sweet potatoes (Ipomoea
batatas) following microbial infection. Biochim. Biophys.
Acta, 337: 184-195.
BRIAN, J. D. & VALBERG, P. A. (1974) Models of lung retention based on
International Congress of Radiation Protection Task Group Report.
Arch. environ. Health, 28: 1-11.
BRYAN, R. J. (1970) Generation and monitoring of gases for inhalation
studies. In: Hanna, M. G. Jr, Nettesheim, P., & Gilbert, J. R.,
ed. Inhalation carcinogenesis. Springfield VA, Clearinghouse
for Federal Scientific and Technical Information, NBS, US Dept of
Commerce (CONF-691001).
BUSTUEVA, K. A., POLEZAEV, E. F., & SOMENENKO, A. D. (1960) [A study
of threshold values for reflex action of atmospheric pollutants.]
Gig. i Sanit., No. 1, pp. 57-61 (in Russian).
CADLE, R. D. (1965) Particle size. Princeton, NJ, Van
Nostrand-Reinhold.
CAHAN, W. G. & KIRMAN, D. (1968) An effective system and procedure for
cigarette smoking by dogs. J. surg. Res., 8: 567-575.
CAMNER, P., PHILIPSON, K., & FRIBERG, L. (1972) Tracheobronchial
clearance in twins. Arch. environ. Health, 24: 82-87.
CAMNER, P., MOSSBERG, B., & PHILIPSON, K. (1973) Tracheobronchial
clearance and chronic obstructuve lung disease. Scand. J.
respir. Dis., 54: 272-281.
CAMNER, P., HELLSTRÖM, P. A., LUNDBERG, M., & PHILIPSON, K. (1977)
Lung clearance of 4 µm particles coated with silver, carbon and
beryllium. Arch, environ. Health, 32: 58-62.
CARPENTER, C. P., SMYTH, H. F., JR, & POZZANI, U. C. (1949) The assay
of acute vapour toxicity and the grading and interpretation of
results on 96 chemical compounds. J. ind. Hyg. Toxicol.,
31: 343-346.
CASARETT, L. J. (1972) The vital sacs: alveolar clearance mechanisms
in inhalation toxicology. In: Hayes, W. J., ed. Essays in
toxicology. New York, London, Academic Press.
CHHABRA, R. S. & FOUTS, J. R. (1974) Sex differences in the metabolism
of xenobiotics by extrahepatic tissue in rats. Drug Metab.
Disposition, 2: 375-379.
CHHABRA, R. S., POHL, R. J., & FOUTS, J. R. (1974) A comparative study
of xenobiotic-metabolizing enzymes in liver and intestine of
various animal species. Drug Metab. Disposition, 2: 443-447.
COTABISH, H. N., MCCONNAUGHEY, P. W., & MESSER, H. C. (1961) Making
known concentrations for instrument calibration. Am. Ind. Hyg.
Assoc. J., 22: 392-402.
CRIDER, W. L., BARKELEY, N. P., & STRONG, A. A. (1968) Dry-powder
aerosol dispensing device with long-time output stability.
Rev. Sci. Instrum., 39: 152-155.
CRYSTAL, R. G. (1974) Lung collagen: definition, diversity and
development. Fed. Proc., 33: 2248-2255.
DAUTREBANDE, L. (1952) Microaerosols, New York, Academic Press,
pp. 7-22.
DAVIDSON, J. T., WASSERMAN, K., LILLINGTON, G. A., & SCHMIDT, R. W.
(1966) Pulmonary function testing in the rabbit. J. appl.
Physiol., 21: 1094-1098.
DEICHMANN, W. B. (1944) An apparatus designed for the production of
controlled dust concentrations in the air breathed by
experimental animals. J. ind. Hyg. Toxicol., 26: 334-335.
DIMMICK, R. L. (1959) Jet disperser for compacted powders in the
one-to-ten micron range. Am. Med. Assoc. Arch. ind. Health,
20: 8-14.
DOLLFUSS, R. E., MILIC-EMILI, J., & BATES, D. V. (1967) Regional
ventilation of the lung studied with boluses of 133 xenon.
Respir. Phys., 2: 234-246.
DONTENWILL, W. (1970) Experimental investigations on the effect of
cigarette smoke inhalation on small laboratory animals. In:
Hanna, M. G. Jr, Nettesheim, P., & Gilbert, J. R., ed.
Inhalation carcinogenesis. Springfield VA, Clearinghouse for
Federal Scientific and Technical Information, NBS, US Dept of
Commerce.
DRAIZE, J. H., NELSON, A. A., NEWBURGER, S. N., & KELLEY, E. A. (1959)
Inhalation toxicity studies of six types of aerosol hair sprays.
Proc. Sci. Sec. Toilet Goods Assoc., 31: 28-32.
DREW, J. H. & LASKIN, S. (1971) A new dust-generating system for
inhalation studies. Am. Ind. Hyg. Assoc. J., 32: 327-330.
DREW, R. T. & LASKIN, S. (1973) Environmental inhalation chambers.
In: Day, W. I., ed. Methods of animal experimentation. New
York, Academic Press.
DREW, R. T. & LIPPMANN, M. (1971) Calibration of air sampling
instruments: II. Production of test atmospheres for instrument
calibraton. In: Lippmann, M., ed. Air sampling instruments, 4th
ed., Cincinnati, OH, ACGIH, pp. I-1.
DREW, R. T. & TAYLOR, G. J. (1975) Cardiophysical studies with
stressed animals. In: Proceedings of the 5th Annual Conference
on Environmental Toxicology, Dayton, Ohio, 1974, US Govt
Printing Office, pp. 121-129.
FOUTS, J. R. & DEVEREUX, T. R. (1972) Developmental aspects of hepatic
and extrahepatic drug-metabolizing enzyme systems: microsomal
enzymes and components in rabbit liver and lung during the first
month of life. J. Pharmacol. exp. Ther., 183: 458-468.
FRASER, D. A., BALES, R. E., LIPPMANN, M., & STOKINGER, H. E. (1959)
Exposure chambers for research in animal inhalation. USPHS
(Public Health Monograph 57).
GOLDBERG, I. S. & LAURENCO, R. V. (1973) Deposition of aerosols in
pulmonary disease. Arch. intern. Med., 131: 88-92.
GRIESEMER, R. A., KENDRICK, J., & NETTESHEIM, P. (1974) Tracheal
grafts. In: Proceedings on Experimental Respiratory
Carcinogenesis, Seattle 1974, Springer-Verlag, pp. 537-547.
GROSS, P., PFITZER, E. A., TOLKER, E., BABYAK, M. A., & KASCHAK, M.
(1965) Experimental emphysema: its production with papain in
normal and silicotic rats. Arch. environ. Health, 11: 50-58.
HABER, F. (1924) [Five lectures for the years 1920-1923.] Berlin,
Springer-Verlag (in German).
HARPER, C., DREW, R. T., & FOUTS, J. R. (1975) Species differences in
benzene hydroxylation to phenol by pulmonary and hepatic
microsomes. Drug Metab. Disposition, 3: 381-388.
HAUN, C. C. (1972) Continuous animal exposure to methylene chloride.
In: Proceedings of the 2nd Annual Conference on Environmental
Toxicology, Dayton, Ohio, 1971, US Govt Printing Office,
pp. 309-326.
HAZUCHA, M., SILVERMAN, F., PARENT, C., FIELD, S., & BATES, D. V.
(1973) Pulmonary function in man after short-term exposure to
ozone. Arch. environ. Health, 27: 183-188.
HENDERSON, D. W. (1952) Apparatus for study of airborne infection.
J. Hyg. (Lond.), 50: 53-68.
HINNERS, R. G., BURKART, J. K., & CONTNER, G. L. (1966) Animal
exposure chambers in air pollution studies. Arch. environ.
Health, 13: 609-615.
HINNERS, R. G., BORKART, J. K., & PUNTE, C. L. (1968) Animal
inhalation exposure chambers. Arch. environ. Health,
16: 194-206.
HODKINSON, J. R. (1966) The optical measurements of aerosols. In:
Davies, C. N., ed. Aerosol science, New York, Academic Press,
pp. 287-357.
HOFFMANN, D. & WYNDER, E. L. (1970) Chamber development and aerosol
dispersion. In: Hanna, M. G. Jr, Nettesheim, P., & Gilbert, J.
R., ed. Inhalation carcinogenesis. Springfield, VA.
Clearinghouse for Federal Scientific and Technical Information,
NBS, US Dept of Commerce (CONF-691001).
HOMBURGER, F., BERNFELD, P., BOGDONOFF, P., KELLEY, T., & WALTON, R.
(1967) Inhalation by small animals of fresh cigarette smoke
generated by new smoking machine. Toxicol. appl. Pharmacol.,
10: 382.
HOOK, G. E. R., BEND, J. R., HOEL, D., FOUTS, J. R., & GRAM, T. E.
(1972) Preparation of lung microsomes and a comparison of the
distribution of enzymes between subcellular fractions of rabbit
lung and liver. J. Pharmacol. exp. Ther., 182: 474-490.
HYATT, R. E. & BLACK, L. F. (1973) The flow-volume curve: a current
perspective. Am. Rev. Respir. Dis., 107: 191-199.
JEMSKI, J. V. & PHILLIPS, G. B. (1965) Aerosol challenge of animals.
In: Gay, W. I., ed. Methods of animal experimentation, New
York, Academic Press, Vol. 1, pp. 273-341.
KAJLAND, A., EDFORS, M., FRIBERG, L., & HOLMA, B. (1964) Radioactive
monodisperse test aerosols and lung clearance studies. Health
Phys., 10: 941-945.
KING, R. J. (1974) The surfactant system of the lung. Fed. Proc.,
33: 2238-2247.
KLJACKINA, A. M. (1973) In: Toxicology of new industrial chemical,
Moscow, Medicina, Vol. 13, p. 23.
KOTRAPPA, P., BOYD, H. A., & WILKINSON, C. J. (1972) Technology for
the production of monodisperse aerosols of oxides of transuranic
elements for inhalation experiments. Health Phys., 22: 837-843.
KROTOY, YU. A. (1971) [Setting of approximate maximum permissible
concentrations of atmospheric pollutants by calculation.]
Gig. i Sanit., 12: 8-12 (in Russian).
KUSCHNER, M., LASKIN, S., CHRISTOFANO, E., & NELSON, N. (1957)
Experimental carcinoma of the lung. In: Proceedings of the 3rd
National Cancer Conference, Philadelphia, Lippincott,
pp. 485-495.
KUSCHNER, M., LASKIN, S., DREW, R. T., CAPIELLO, V., & NELSON, N.
(1975) Inhalation carcinogenicity of alpha haloethers: III.
Lifetime and limited period inhalation studies with
bis(chloromethyl) ether at 0.1 ppm. Arch. environ. Health,
30: 73-77.
LAMER, V. K. & SINCLAIR, D. (1943) An improved homogeneous aerosol
generator. Washington DC, US Dept of Commerce (OSRD Report
No. 1668).
LANE, B. P. & MILLER, S. L. (1975) Dose dependence of
carcinogen-induced changes in tracheal epithelium in organ
culture. In: Proceedings of the Symposium on Experimental
Respiratory Carcinogenesis, Seattle, 1974, Springer-Verlag,
pp. 507-513.
LASKIN, S. (1948) AEC Project Quarterly Report UR-38. Rochester, NY,
University of Rochester.
LASKIN, S., KUSCHNER, M., & DREW, R. T. (1970) Studies in pulmonary
carcinogenesis. In: Hanna, M. G. Jr, Nettesheim, P., & Gilbert,
J. R., ed. Inhalation carcinogenesis. Springfield VA,
Clearinghouse for Federal Scientific and Technical Information,
NBS, US Dept of Commerce (CONF-691001).
LASKIN, S., DREW, R. T., CAPIELLO, V. P., & KUSCHNER, M. (1971)
Inhalation studies with freshly generated polyurethane foam dust.
In: Mercer, T. T., Morrow, P. E., & Storer, W., ed. Assessment
of airborne particles, Springfield, IL, Thomas, pp. 382-404.
LAUTERBACH, K. E., HAYES, D., & COELHO, M. A. (1956) An improved
aerosol generator. Am. Med. Assoc. Arch. ind. Health,
13: 156-160.
LEONG, B. J. K., KOCIBA, R. J., JERSEY, G. C., & GEHRING, P. J. (1975)
Effects from repeated inhalation of parts per billion of
bis(chloromethyl) ether in rats. Toxicol. appl. Pharmacol.,
35: 175.
LEWIS, T. R., MOORMAN, W. J., YANG, Y., & STARA, J. F. (1974)
Long-term exposure to auto exhaust and other pollutant mixtures.
Arch. environ. Health, 29: 102-106.
LIPPMANN, M. & ALBERT, R. E. (1967) A compact electric motor-driven
spinning disc aerosol generator. Am. Ind. Hyg. Assoc. J.,
28: 501-506.
LIPPMANN, M., ed. (1971) Air sampling instruments, 4th ed.
Cincinnati, OH, ACGIH.
LITTLE, J. B. & O'TOOLE, W. F. (1974) Respiratory trace tumours in
hamsters induced by benzo(a)pyrene and polonium210 alpha
radiation. Cancer Res., 34: 3026-3039.
LODGE, J. P. (1968) Production of controlled test atmospheres.
In: Stern, A. C., ed. Air pollution, 2nd ed., New York,
Academic Press, Vol. II, pp. 465.
MACFARLAND, H. N. (1968) Exposure chambers -- design and operation.
In: Proceedings of the 7th Annual Technical Meeting of the
American Association of Contamination Control, Chicago, 1968,
pp. 19-25.
MACFARLAND, H. N. (1976) Respiratory toxicology. In: Hayes, W. J. Jr,
ed. Essays in toxicology, New York, San Francisco, London,
Academic Press, Vol. 7, pp. 121-154.
MACKLEM, P. T., HOGG, W. E., & BRUTON, J. (1973) Peripheral airway
obstruction and particulate deposition in the lung. Arch.
intern. Med., 131: 93-100.
MARTORANA, P. A., MCKEEL, N. W., RICHARD, J. W., & SHARE, N. N. (1973)
Function-structure correlation studies on excised hamster lungs
in papain-induced emphysema. Can. J. Physiol. Pharmacol.,
51: 635-641.
MCLAUGHLIN, R. F., TYLER, W. S., & CANADA, R. O. (1961a) Subgross
pulmonary anatomy in various mammals and man. J. Am. Med. Assoc.
175: 694-697.
MCLAUGHLIN, R. F., TYLER, W. S., & CANADA, R. O. (1961b) Study of the
subgross pulmonary anatomy of various mammals. Am. J. Anat.,
108: 149-164.
MCLAUGHLIN, F. R., TYLER, W. S., & CANADA, R. O. (1966) Subgross
pulmonary anatomy of the rabbit, rat and guinea pig with
additional notes on the human lung. Am. Rev. Respir. Dis.,
94: 380-387.
MEAD, J. (1960) Control of respiratory frequency. J. appl. Physiol.,
15: 325-360.
MERCER, T. T. (1963) On the calibration of cascade impactors. Ann.
occup. Hyg., 6: 1-14.
MERCER, T. T. (1964) The stage constants of cascade impactors. Ann.
occup. Hyg., 7: 115-125.
MERCER, T. T. (1965) The interpretation of cascade impactor data. Am.
Ind. Hyg. Assoc. J., 26: 236-241.
MERCER, T. T. (1973a) Production and characterization of aerosols.
Arch. intern. Med., 131: 39-50.
MERCER, T. T. (1973b) Aerosol technology in hazard evaluation. New
York, London, Academic Press.
MORROW, P. E. (1970) Models for the study of particulate retention and
elimination in the lung. In: Hanna, M. G., Nettesheim, P. &
Gilbert, J. R., ed. Inhalation carcinogenesis. Springfield VA,
Clearinghouse for Federal Scientific and Technical Information,
NBS, US Dept of Commerce (CONF-691001).
MORROW, P. E. (1973) Alveolar clearance of aerosols. Arch. intern.
Med., 131: 101-108.
MORROW, P. E., HODGE, H. C., NEWMAN, W. F., MAYNARD, E. A., BLANCHET,
H. J. JR, FASSET, D. W., BIRK, R. E., & MAVRODT, S. (1966)
Deposition and retention models for internal dosimetry of the
human respiratory tract. Health Phys., 12: 173-207.
MURPHY, S. D. & ULRICH, G. E. (1964) Multi-animal test system for
measuring effects of irritant gases and vapours on respiratory
function of guinea pig. Am. Ind. Hyg. Assoc. J., 25: 28-36.
NADER, J. S. (1971) Direct-reading instruments for analysing airborne
gases and vapours. In: Lippmann, M., ed. Air sampling
instruments, 4th ed., Cincinnati, ACGIH, p. J-1.
NELSON, G. O. (1971) Controlled test atmospheres: principles and
techniques. Ann Arbor, MI, Ann Arbor Sci. Publ.
NELSON, G. O. & GRIGGS, K. E. (1968) Precision dynamic method for
producing known concentrations of gas and solvent vapour in air.
Rev. Sci. Instrum., 39: 927-928.
NIEMEIER, R. W. & BINGHAM, E. (1972) An isolated perfused lung
preparation for metabolic studies. Life Sci., 11(II): 807-820.
NIEWOEHNER, D. R. & KLEINERMAN, J. (1973) Effects of experimental
emphysema and bronchiolitis on lung mechanics and morphometry.
J. appl. Physiol., 35: 25-31.
O'KEEEE, A. E. & ORTMAN, G. O. (1966) Primary standards for trace gas
analysis. Anal. Chem., 38: 760-763.
O'NEIL, J. J. & TIERNEY, D. G. (1974) Rat lung metabolism: glucose
utilization by isolated perfused lungs and tissue slices. Am. J.
Physiol., 226: 867-873.
ORTON, T. C., ANDERSON, M. W., PICKETT, R. D., ELING, T. E., & FOUTS,
J. R. (1973) Xenobiotic accumulation and metabolism by isolated
perfused rabbit lungs. J. Pharmacol. exp. Ther., 186: 482-497.
PALACEK, F. (1969) Measurement of ventilatory mechanics in the rat.
J. appl. Physiol., 27: 149-156.
PHILIPSON, K. (1973) On the production of monodisperse particles with
a spinning disc. Aerosol Sci., 4: 51-57.
POWELL, C. H. & HOSEY, A. D. (1965) The industrial environment, its
evaluation and control (US PHS Publ. 614).
POZZANI, U. C., WEIL, C. S., & CARPENTER, C. P. (1959) The
toxicological basis of threshold limit values: 5. The
experimental inhalation of vapour mixtures by rats with notes
upon the relationship between single-dose oral data. Am. Ind.
Hyg. Assoc. J., 20: 364-369.
RAABE, O. G. (1970) Generation and characterization of aerosols. In:
Hanna, M. G. Jr, Nettesheim, P., & Gilbert, J. R., ed.
Inhalation carcinogenesis. Clearinghouse for Federal Scientific
and Technical Information, NBS, Springfield VA, US Dept of
Commerce (CONF-691001).
RAABE, O. G., BENNICK, J. E., LIGHT, M. E., HOBBS, C. N., THOMAS, R.
L., & TILLERY, M. (1973) An improved apparatus for acute
inhalation exposure of rodents to radioactive aerosols. Toxicol.
appl. Pharmacol., 26: 264-273.
RJAZANOV, V. A. (1964) Criteria and methods for establishing maximum
permissible concentrations of atmospheric pollutants in the USSR.
In: Maximum permissible concentrations of atmospheric
pollutants, Moscow, Medicina, Vol. 8, pp. 5-21.
ROE, F. J. C. (1968) Inhalation tests. In: Boyland, E. & Goulding, R.,
ed. Modern trends in toxicology, London, Butterworth, Vol. 1.
SAFFIOTTI, U. (1970) Experimental respiratory tract carcinogenesis and
its relation to inhalation exposures. In: Hanna, M. G. Jr,
Nettesheim, P., & Gilbert, J. R., ed. Inhalation carcinogenesis.
Springfield, VA, Clearinghouse for Federal Scientific and
Technical Information, NBS, US Sept of Commerce (CONF-691001).
SAITO, Y. (1912) [Experimental investigations on the quantitative
absorption of dust by animals at accurately known concentrations
of dust in air.] Arch. Hyg., 75: 134-151 (in German).
SALTZMAN, B. E. (1971) Permeation tubes as calibrated sources of gas.
In: Proceedings of 1st Annual Conference on Environmental
Toxicology, Dayton, Ohio, 1970, US Govt Printing Office,
pp. 309-326.
SALTZMAN, B. E. & WARBURG, A. F. (1965) Precision flow dilution system
for standard low concentrations of nitrogen dioxide. Anal.
Chem., 37: 1261-1264.
SANCHIS, J., DOLOVICH, M., CHALMERS, R., & NEWHOUSE, M. (1972)
Quantification of regional aerosol clearance in the normal human
lung. J. appl. Physiol., 33: 757-762.
SANOCKIJ, I. V., ed. (1970a) [Methods for determining toxicity and
hazards of chemicals.] Moscow, Medicina, pp. 62-63 (in
Russian).
SANOCKIJ, I. V. (1970b) [Complete toxicometry.] In: [Methods for
determining the toxicity and hazards of chemicals.] Moscow,
Medicina, pp. 50-54 (in Russian).
SCHREIBER, H., NETTESHEIM, P., & MARTIN, D. H. (1972) Rapid
development of bronchiolo-alveolar squamous cell tumours in rats
after intratracheal injection of 3-methylcholanthrene. J. Natl
Cancer Inst., 49: 541-554.
SCHWETZ, B. A., LEONG, B. J. K., & GEHRING, P. J. (1975) The effect of
maternally inhaled trichloroethylene, perchloroethylene, methyl
chloroform and methylene chloride on embryonal and fetal
development in mice and rats. Toxicol. appl. Pharmacol.,
32: 84-96.
SIDORENKO, G. I. & PINIGIN, M. A. (1970) Rapid determination of
maximum permissible concentrations of atmospheric pollutants.
Gig. i Sanit., No. 3, pp. 93-96.
SILVER, M. J., HOCH, W., KOCSIS, J. J., INGERMAN, C. M., & SMITH, J.
B. (1973) Arachadonic acid causes sudden death in rabbits.
Science, 183: 1085-1086.
SILVER, S. D. (1946) Constant flow gassing chambers: principles
influencing design and operations. J. lab. clin. Med.,
31: 1153-1161.
SNIDER, G. L., HAYES, J. A., FRANZBLAU, C., KAGEN, H. M., STONE, P.S.,
& KORTHY, A. L. (1974) Relationship between elastolytic activity
and experimental emphysema-inducing properties of papain
preparations. Am. Rev. Respir. Dis., 10: 254-262.
STEAD, F. M., DERNEHL, C. U. & NAU, C. A. (1944) A dust feed apparatus
useful for exposure of small animals to small and fixed
concentrations of dust. J. ind. Hyg. Toxicol., 26: 90-93.
STEWART, R. D. (1972) Use of human volunteers for the toxicological
evaluation of materials. In: Symposium on an Appraisal of
Halogenated Fire Extinguishing Agents. Washington DC, National
Academy of Sciences.
STEWART, R. D., DODD, H. C., GAY, H. H., & ERLEY, D. S. (1970a)
Experimental human exposure to trichloroethylene. Arch. environ.
Health, 20: 64-71.
STEWART, R. D., PETERSON, J. E., BARETTA, E. D., BACHARD, R. T.,
HOSCO, M. T., & HERRMANN, A. A. (1970b) Experimental human
exposure to carbon monoxide. Arch. environ. Health,
21: 154-164.
STEWART, R. D., PETERSON, J. E., FISHER, T. N., HOSKO, M. J., BARETTA,
E. D., DODD, H. C., & HERRMANN, A. A. (1973) Experimental human
exposure to high concentrations of carbon monoxide. Arch.
environ. Health, 26: 1-7.
STOKINGER, H. E. (1949) Toxicity following inhalation. In Voegtlin, C.
& Hodge, C., ed. Pharmacology and toxicology of uranium
compounds, 1st ed., New York, McGraw-Hill, Vol. III.
STUART, B. O. (1974) Deposition of inhaled aerosols. Arch. intern.
Med., 131: 60-75.
STUART, B. O., WILLARD, D. H., & HOWARD, E. B. (1970) Uranium mine air
contaminants in dogs and hamsters. In: Hanna, M. G. Jr,
Nettesheim, P., & Gilbert, J. R., ed. Inhalation carcinogenesis.
Springfield, VA, Clearinghouse for Federal Scientific and
Technical Information, NBS, US Dept of Commerce (CONF-691001).
STUPFEL, M. & MORDELET-DAMBRINE, M. (1974) Penetration of pollutants
in the airways. Bull. Physio-Path. Respir., 10: 481-509.
SWIFT, D. L. (1971) Direct-reading instruments for analysing airborne
particles. In: Lippmann, M., ed. Air sampling instruments, 4th
ed., Cincinnati, OH, ACGIH, p. T-1.
TAYLOR, G. J. & DREW, R. T. (1975) Cardiomyopathy predisposes hamsters
to trichlorofluoromethane toxicity. Toxicol. appl. Pharmacol.,
32: 177-183.
THOMAS, R. G. & LIE, R. (1963) Procedures and equipment used in
inhalation studies on small animals. LF-11, Lovelace Foundation
for Medical Education and Research.
THOMAS, R. L. (1969) Deposition and initial translocation of inhaled
particles in small laboratory animals. Health Phys.,
16: 417-428.
TIERNEY, D. G. (1974) Intermediary metabolism of the lung. Fed.
Proc., 33: 2232-2237.
TUCKER, A. D., WYATT, J. H., & UNDRY, D. (1973) Clearance of inhaled
particles from alveoli by normal interstitial drainage pathways.
J. appl. Physiol., 35, 719-732.
VALEZNEV, I. P., ELOVSKAJA, L. T., & MOISEJCEV, P. I. (1970)
[Equipment for research on biological effects of aerosols.]
Ofic. bjull. Gos. kom. Min. SSSR po delam izobretenij i
otkrytij, No. 10, avtonskoe svidetel'stvo No. 265307 (in
Russian).
VELICKOVSKIJ, B. T. & KACNELSON, B. A. (1964) [Etiology and
pathogenesis of silicosis.] Moscow, Medicina (in Russian).
WALTON, W. H. & PREWETT, W. C. (1949) The production of sprays and
mists of uniform drop size by means of spinning disc type
sprayers. Proc. Phys. Soc. (London), 62: 341-350.
WATSON, J. A., SPRITZER, A. A., AULD, J. A. & GUETTHOFF, M. A. (1969)
Deposition and clearance following inhalation and intra-tracheal
injection of particles. Arch. environ. Health, 19: 51-58.
WEISS, B. & LATIES, V. (1975) Behavioural toxicology. New York,
Plenum Press.
WHITBY, K. T. & LIU, B. Y. H. (1966) The electrical behaviour of
aerosols. In: Davies, C. N., ed. Aerosol Science, New York,
Academic Press.
WILSON, R. H. & LASKIN, S. (1950) Improved design for an animal
inhalation exposure unit. Rochester, NY, University of
Rochester (AEC Proj. Rep. U-116), p. 80.
WITSCHI, H. P. (1975) Exploitable biochemical approaches for the
evaluation of toxic lung damage. In: Hayes, W. Jr, ed. Essays in
toxicology, New York, Academic Press, Vol. 6.
WOOLCOCK, A. J., VINCENT, N. J., & MACKLEM, P. T. (1969) Frequency
dependence of compliance as a test for obstruction in the small
airways. J. clin. Invest., 48: 1097-1106.
WRIGHT, B. M. (1950) A new dust-feed mechanism. J. Sci. Instrum.,
27: 12-15.
XINTARAS, C., JOHNSON, B. L., & DE GROOT, I., ed. (1974) Behavioural
toxicology. Washington DC, US Govt Printing Office.
7. CARCINOGENICITY AND MUTAGENICITY
7.1 Introduction
This chapter deals with the two related but separate processes,
heritable mutations and cancer. Cancer involves the conversion of
normal cells to malignant cells and the development of what is usually
an irreversible malignant disease process in the present generation
frequently leading to a fatal outcome for the bearer of the
malignancy. Heritable mutations are mutations that are transmissible
to later generations and the target cells are the germ cells of either
sex.
Developments in the last two decades make it possible to discuss
certain aspects of mutagenesis and carcinogenesis in parallel. In
other words, there is now increasing evidence that, in most cases,
somatic mutations are probably involved in the conversion of normal to
malignant cells. Thus, the ability of a chemical or physical agent to
produce mutations is relevant both to the question of heritable
mutations (mutations of the germ cell) and carcinogenicity.
There is a well-established body of knowledge that shows a close
correspondence between cancer of the whole animal as studied in the
laboratory and the occurrence of cancer in man; this is especially
true of occupational cancer. More recently, a fairly close correlation
has been shown between tests of isolated, simple, biological systems,
e.g. reverse bacterial mutations and the response to carcinogens of
whole animals, and human beings.
A similar situation does not exist with respect to patterns in
human heritable mutations. Thus, although there is good evidence of
correlation between in vitro tests for mutagenicity and heritable
mutations in insects and experimental mammals, it can only be
inferred, at present, that this also applies to man. The inference,
however, is extremely strong and not subject to serious doubt. Thus,
there is every reason to expect, that, in general, man will respond
biologically in a manner quite similar to that of other species when
exposed to mutagens that reach the germ cells. The lack of full
correlation stems more from the lack of appropriate studies in man
than from any uncertainty concerning the underlying biological
considerations. At present, methods for the detection of mutations in
man are difficult, cumbersome, and insensitive, and it is imperative
to operate on the assumption that agents capable of producing germ
cell mutations in laboratory studies would also be capable of
producing similar mutations in man.
Thus, two quite different disease processes can have one common
level of consideration, namely, their ability to produce alterations
in the genetic material of cells. The following discussion is
concerned with methods for carcinogenicity testing. First, the
traditional lifetime exposure of the entire organism to the test
compound is considered. This is followed by a discussion of various
in vitro systems for the examination of mutagenicity including DNA
damage, mutagenicity in bacteria and eukaryotic organisms, and
transformation of cell cultures. (The first three of these are
particularly relevant to mutagenicity of the germ cell or of the
somatic cell.) Following this, the general problem of examination for
heritable mutations is considered. This includes inquiries at three
levels: injury to DNA, point mutations, and chromosome alterations.
Whole animal and isolated test systems are available to make relevant
determinations. However, the whole field of mutagenicity testing is
now in a period of rapid evolution with advances being made in a
number of areas. It can, therefore, be anticipated that present
procedures will undergo alteration and, it is hoped, will improve
within the near future.
7.2 Carcinogenicity
7.2.1 Long-term bioassays
The fact that environmental factors have a direct effect in
producing cancer in man is shown by: (a) the unequivocal evidence of
the chemical origin of occupational cancer, for example, urinary
bladder tumours in workers exposed to aromatic amines, lung cancers in
workers exposed to bis(chloromethyl)ether, etc.; (b) the
well-documented cases of iatrogenic cancer; (c) the positive
correlation between cigarette smoking and lung cancer; (d) the
differences in cancer incidence in urban and rural populations and
(e) the results of studies on migrants showing that for some types
of cancer they acquire incidences similar to those found in the host
countries. Results obtained in experimental studies on carcinogenesis
confirm the direct carcinogenic effect of chemicals. However, there is
not necessarily a correlation between acute toxicity and
carcinogenicity; a chemical with a high acute toxicity may not have
any, or only a very low carcinogenic potential. Conversely, a chemical
that produces a very minor, or no evident toxic effect in acute or
subacute experiments may be a very powerful carcinogen. Knowledge of
the acute and subacute toxicity of the test chemical however, is
important in order to permit better planning of the experiment. For
instance, the premature death of the test animals, due to unforeseen
side-effects, may thus be avoided.
7.2.1.1 Species, strain, and sex selection, and size of groups
The description of methods and recommended procedures in this
chapter applies to long-term testing in rodents and specifically to
the mouse, rat, and Syrian golden hamster (FDA, 1971; NCI, 1976; Weil,
1972; Weisburger, 1975; Weisburger & Weisburger, 1967). Rodents have
been preferred to other species because of their susceptibility to
tumour induction, their relatively short life span, the limited cost
of their maintenance, their widespread use in pharmacological and
toxicological studies, the availability of inbred strains and, as a
consequence of these characteristics, the large amount of information
available on their physiology and pathology. Nonrodents, in particular
dogs and primates, have rarely been used in the past; though used more
at the present time, their much higher cost of maintenance, the long
period of observation, and the impracticability of using sufficiently
large numbers put nonrodents at a disadvantage for long-term
bioassays. Also, comparative studies on the metabolism of various
chemicals have not indicated necessarily that primates and dogs are
closer to man in this respect than rodents. Nevertheless, many of the
procedures for long-term bioassays in rodents are applicable to assays
in nonrodents.
Long-term bioassays of the carcinogenicity of environmental
chemicals are carried out to assess a possible risk to man and to
estimate the need for primary preventive measures. Because of the
urgent nature of these problems, it is recommended that a compound of
unknown activity should be tested on two animal species. Although a
positive (that is, carcinogenic) effect in one species is considered
as adequate warning, only negative findings in two species can be
regarded as adequate negative evidence.
Among rodents, the species of choice are undoubtedly the mouse,
the rat, and the Syrian golden hamslet. The European hamster has
recently been introduced, particularly for studies in lung pathology,
but its use cannot be recommended for routine testing. Guineapigs and
rabbits have been used occasionally but they have few of the
advantages of smaller rodents combined with the disadvantages implied
by a much longer life span, higher cost of maintenance, and the
scarcity or absence of inbred strains.
Of the three rodent species of choice, the mouse and the rat have
been more widely used than the hamster. However, the last species has
proved to be an excellent tool for revealing carcinogenic effects in
the respiratory tract and urinary bladder. Furthermore, hamster cells
are widely used for in vitro transformation assays, and, since in
most cases these assays require the back transplantation of the cells
in syngenic hosts, the use of the hamster has recently been extended.
The hamster is generally randomly bred but a pure strain is becoming
available.
The use of inbred strains undoubtedly has the advantage of making
available animals with known characteristics such as an average life
span, and a spontaneous tumour rate with little variability.
Random-bred animals or, even better, animals bred with maximum
avoidance of inbreeding, are considered more resistant to infections
and perhaps more liable than many inbred strains to reveal any
carcinogenic effect on an unsuspected target organ. It is common
experience that carcinogenic activity is more easily recognized by
the increased frequency and earlier appearance of tumours at sites
where tumours occur spontaneously. Inbred strains are often known to
have one or two particularly susceptible organs and the induction of
tumours in these organs could, in part, be regarded as an
intensification of whatever underlies the spontaneous occurrence of
tumours in that organ. A carefully planned experiment must take this
possibility into account, and preference should be given to strains
with a low incidence of tumours. Noninbred strains are inconvenient in
that, in many cases, background tumour incidence in untreated controls
is unpredictable. Moreover, experimental groups initiated at different
times can rarely be compared with each other since inter- as well as
intragroup variation may be considerable.
Hybrid mice of two known inbred strains are excellent as they are
particularly robust and long-lived, but they are, of course, more
difficult to obtain and are likely to be more expensive than inbred
strains.
In selecting the species, it is important to be aware that there
are, within each species, particular susceptibilites; for instance, it
is easier to induce liver tumours in the mouse than in the rat, and
conversely it is much easier to induce subcutaneous tumours in the rat
than in the mouse. The skin of the mouse and the rabbit is possibly
more sensitive to tumour induction by skin painting than that of the
rat and the hamster, particularly when polycyclic hydrocarbons are
used. The hamster is more susceptible to urinary bladder
carcinogenesis than the mouse and possibly the rat.
It is essential that experimental groups should be composed of an
equal number of animals of each sex, since differences in response to
the carcinogenic activity of chemicals are well documented. Thus, the
use of one sex only would not show the full range of activity of the
test chemical.
The group should be sufficiently large to permit statistical
evaluation of the results. The UICC Committee on Carcinogenicity
Testing (UICC, 1969) recommended that the number of animals should be
such that it "would be likely to yield reasonable (e.g. 90%)
statistical significance as between the main induced tumour incidence
and its spontaneous incidence". A sufficient number of animals must be
alive at the time that the first tumour appears and it is suggested
that this number should never be less than 25 per sex.
7.2.1.2 Route of administration
It has been agreed that the chemical under test should be
administered by the route of human exposure. This applies to food
additives and food contaminants in particular, but it is equally
desirable for drugs. This rule cannot always be applied to
environmental or industrial chemicals. It is well known that the three
main routes of exposure in man are inhalation, ingestion, and skin
absorption. Inhalation is often predominant in man but in experimental
carcinogenesis the inhalation route, while highly desirable in
principle, has rarely been used in the past because of lack of
adequately equipped laboratories. However, it is now being widely used
in programmes concerning smoking and health.
When the route of exposure is per os, the chemical is
administered either mixed in the diet or drinking water, or by gavage,
the choice depending on the specific characteristics of the test
chemical; a volatile compound, for example, should never be mixed in
the diet while, for a drug, the route by which it will be used in man
should be selected, i.e. per os, or by intravenous, subcutaneous or
topical application.
7.2.1.3 Inception and duration of tests
Most commonly, tests are initiated in young adult animals from 7
to 9 weeks of age. The start of treatment soon after weaning is
recommended as a routine procedure. One objective of carcinogenicity
testing is to obtain the maximum possible carcinogenic effect of the
test chemical in order to compensate for the limited number of
individuals at risk. This is accomplished by employing high levels of
exposure (see 7.1.4) and by starting the treatment when the animals
are at their most sensitive age. For some years following the pioneer
work of Pietra et al. (1961), treatment immediately after birth and
within the first 24 h of life was thought to be the most sensitive
model. Careful reviews of the data available have indicated that
neonatal treatment alone can be recommended in some instances, but not
as a general routine procedure (Della Porta & Terracini, 1969). For a
single neonatal administration, only a very limited quantity of the
chemical to be tested is needed and this could be advantageous, when
only a small amount of the chemical is available.
The main limitation of the use of a single neonatal treatment is
that the newborn animal may not have developed sufficient metabolic
competence to metabolize the test chemical. Another limitation is that
not all chemical carcinogens are active after one, or even a few
exposures.
More recently, prenatal exposure has received considerable
attention, since it has been demonstrated that some tissues, notably
nervous tissue, are more susceptible to certain carcinogens during
fetal life than later (Druckrey et al., 1967; Napalkov, 1973; Napalkov
& Alexandrov, 1968; Tomatis, 1973). At present, however, it can only
be speculated that prenatal exposure will reveal the carcinogenic
effect of a chemical that would not have been revealed had treatment
started at a later age. A sensitive procedure might be to integrate
prenatal exposure with long-term, postnatal exposure, in order to
obtain maximum sensitivity of the bioassay system. To achieve this,
animals mated at the age of 8-9 weeks, should be exposed to the test
chemical during the second half of pregnancy. The parents so treated
can constitute a group under conventional long-term testing, if the
treatment is continued after delivery. Their offspring, already
exposed in utero, and possibly after birth through their mother's
milk and excreta, should be exposed directly to the test chemical from
the age of weaning onwards. In this way, two different experimental
groups can be obtained: one for which exposure was started at the
young adult stage (the parents) and the other for which exposure was
started prenatally (the offspring) (Tomatis, 1974b).
The duration of a positive test, i.e. where exposure to the test
chemical is followed by an increased incidence of tumours, depends on
the time of appearance and the rapidity of growth of the induced
tumours. To agree in advance on a given duration of exposure is of
prime importance for negative tests. Some research workers have
adopted a duration for carcinogenicity tests of 2 years in rats and 18
months in mice, while others have preferred an observation period
extending over the entire life span of the animals. A long finite
period -- that is, not less than 2 years -- is recommended in
preference to the entire life span of the animals, for the following
reasons: (a) induced tumours usually occur within this observation
period; (b) "spontaneous" tumours appear with highest frequency late
in life and their appearance may make it more difficult to evaluate
the carcinogenicity of a compound, particularly if of low potency;
(c) a few animals may far exceed the normal life span of the species
and extend unnecessarily the duration of the experiment; (d) tests
are very expensive and any justifiable abbreviation means good economy
(Health & Welfare, Canada, 1973; Tomatis, 1974a; UICC, 1969; WHO,
1961).
7.2.1.4 Dose-level and frequency of exposure
If only one level is to be used, the highest dose allowing long
survival of the majority of the animals should be chosen. The
selection of this dose should be made from results obtained in
subacute toxicity tests. Information on the LD50 of certain
compounds may be available and this can be useful in deciding the
level of the dose for the subacute studies.
While it would be very difficult to give a definition of the
maximum tolerated dose, it seems quite reasonable to insist that it
allows adequate survival of the animals and does not produce a
reduction in weight of more than 10% compared with the controls
(Friedman, 1974). If two dose levels are chosen, the second should be
one quarter or one third of the first dose.
If the goal of the test is merely to ascertain the possible
carcinogenicity of a compound, then the assay protocol should achieve
the maximum sensitivity of the test; in this case, the highest
tolerated dose is the most appropriate. If, however, additional
information has to be collected, in particular regarding the
establishment of a possible minimum effect or no-observed-effect
level, then initiation of a dose-response experiment should be
envisaged. In this case, a minimum of three doses, and preferably
four, should be included. An advantage of including several dose
levels, the lowest of which does not decrease the life span of the
animals, is the possibility of collecting other data on chronic
toxicity not otherwise available through a carcinogenicity test at
high dose levels.
Frequency of exposure may vary according to the route chosen. If
the chemical is administered in the drinking water or mixed in the
diet, exposure is continuous. If the chemical is given by gavage, the
frequency can be two to three times per week. Topical applications may
be made daily, while subcutaneous or intravenous injections must be
more widely spaced, e.g. once or twice weekly. The duration of
treatment should cover almost the entire observation period.
7.2.1.5 Combined treatment and cocarcinogenesis
An investigation on the effect of more than one agent, given
simultaneously, may approximate the actual environmental situation,
where there is never exposure to a single chemical. In particular, it
may be useful in revealing the carcinogenic effect of a chemical of
very low carcinogenic potency and thereby help to identify situations
or populations that may be exposed to an otherwise unsuspected high
risk.
Response to an environmental chemical may be modified by the
action of other chemicals that may either alter the rate and/or
pathway of metabolism of the test chemical, or have a cocarcinogenic
effect. A cocarcinogenic effect may be additive, when the chemical has
a carcinogenic effect on its own which adds to the effect produced by
the test chemical; it may be synergistic, when its effect, combined
with that of the test chemical when given alone, exceeds the summation
of the separate effects; or it may act as an incomplete carcinogen,
that is, only as initiator or promoter in the two-stage carcinogenic
process (UICC, 1969) (see Chapter 13).
It is also essential to keep in mind that dietary components may
influence the incidence of tumours in test animals. This may occur
either because of the unsuspected presence of carcinogens (such as
aflatoxins or nitrosamines) or because of substances that may modify
the response to the test chemical by altering, for instance, the
hepatic microsomal enzyme activity.
7.2.1.6 Positive and untreated controls
An adequate group of untreated animals, serving as controls,
should always be included in the planning of a carcinogenicity test.
The size of the control group should never be smaller than the size of
the treated group and, preferably, should be larger. When more than
one chemical is tested simultaneously, the same control group can be
used, provided that its size is appropriately increased.
Besides these controls, i.e. animals not receiving treatment, and
which could be called negative controls, the inclusion of positive
controls in planning an experiment has recently been recommended, i.e.
inclusion of a group of animals receiving a known carcinogen at a dose
level that has already produced carcinogenic effects in several
laboratory studies. Such controls ensure: (a) more confidence in the
outcome of tests carried out on compounds of unknown activity
(Weisburger, 1974); (b) assessment of the relative carcinogenic
potency of the test chemical, and (c) an indirect check on the
reliability of the test laboratory. While it does not seem essential
to include a positive control in every test, it is highly advisable
for every laboratory to check the sensitivity of its bioassay system,
periodically, with selected known carcinogenic chemicals. It is also
advisable that a group of animals should receive only the vehicle
(i.e. acetone, dimethylsulfoxide, etc.) in which the chemical under
test is eventually administered.
7.2.1.7 Test material
Long-term studies should not be started until sufficient
information on the identity and purity of the test chemical has been
assembled. The importance of knowing the toxicity of impurities is
well demonstrated by the case of TCDD present as an impurity in the
herbicide 2,4,5-T. Drugs or food additives should be tested in the
form and degree of purity intended for human consumption (Health &
Welfare, Canada, 1973; National Academy of Sciences, 1960; WHO, 1961,
1969). In this case, as well as for mixtures of chemicals to which man
may be exposed, it may be highly advisable to carry out additional
studies in order to identify positively the carcinogenic component of
the mixture.
7.2.1.8 Survey of animals, necropsy, and histological examination
In order to present results correctly, detailed records of all
experimental procedures and surveillance of the animals during the
entire observation period should be maintained. While all pertinent
details of the experiment may not be published, it is a good rule for
the investigator to keep them for discussion with other interested
scientists (UICC, 1969). If the results are to be published, it is
essential that adequate information be given on the test chemical and
test animal, as well as on all observations made on the experimental
and control groups (WHO, 1961). A detailed list of items to be
considered is given in the UICC (1969) report.
In the case of neoplasms (Turusov, 1973, 1976), it is extremely
important to give an exact description of the criteria used to
classify lesions as hyperplastic, preneoplastic or neoplastic, benign
or malignant. Many times, pathologists do not agree on certain
diagnoses and it is far easier to interpret the results when the
criteria used for classification are known. Terms such as hepatoma,
which do not indicate the specific tissue of origin, should not be
used (Reuber, 1974), while terms such as cholangioma or adenomatosis
require additional qualification. The term should clearly indicate
whether the lesion is considered malignant. Thus, specific
descriptions should be given, e.g. in the case of "hepatoma" it may be
either a liver cell adenoma or a hepatocellular carcinoma, while
"cholangioma" may be cholangiocellular adenoma or cholangiocellular
carcinoma. In addition, carcinomas may be subdivided into well,
moderately, and poorly differentiated carcinemas.
A lesion with atypical cells or with focal malignant change
should be classified separately rather than under malignant tumours.
7.2.2 Short-term tests (rapid screening tests)
To date, there are no reliable alternatives to long-term
bioassays for testing the carcinogenicity of chemicals; this means a
delay of two years or more before a carcinogenicity bioassay yields
results. However, recent advances in mutagenicity testing hold out
hopes of a short-term test, at least for selecting, from among the
many chemicals to be tested, those most in need of early attention. So
far, about 80% of carcinogens have been shown to be mutagenic and with
the continuously increasing sensitivity of the models now employed an
even higher correlation is possible. At present, mutagenicity testing
represents a valuable system for screening chemicals to be submitted
to long-term carcinogenicity testing; it cannot replace long-term
testing, until a satisfactory positive correlation is established and
the possibility of false negatives eliminated.
A mutation is any heritable change in genetic material including
a chemical transformation of an individual gene (point mutation) or a
change involving rearrangement of parts of a chromosome (chromosome
mutation). The question of whether or not a mutagenic event is a
prerequisite for carcinogenesis has long been debated. Many mutagens
have been shown to be carcinogenic but other known carcinogens are
known not to be mutagenic. Other mechanisms not involving DNA have
also been implied in the process of carcinogenesis (Pitot, 1974).
Recent short-term mutagenicity testing procedures (McCann & Ames,
1976: McCann et al., 1975; Sugimura et al., 1976) have shown greater
correlation between mutagenicity and carcinogenicity; consequently,
some thought is now being given to using these assays as preliminary
screens for potential carcinogens. However, at the moment, the fact
that a particular compound has mutagenic activity can only be
considered, in terms of carcinogenicity, as indicative of potential
reactivity with DNA.
7.2.2.1 Metabolic activation, reaction with DNA, and DNA repair
The synthetic and naturally occurring chemical carcinogens
include a variety of chemicals having no common structural feature.
However, it is becoming clear that the ultimate reactive forms of many
chemical carcinogens are electrophilic (electron-deficient) reactants
(Miller, 1970). Although some chemical carcinogens, such as direct
alkylating agents and metal ions, are electrophiles per se, the
majority require metabolic activation to reactive forms (ultimate
carcinogens) (Fig. 7.1).
The formation of these electrophilic reactants from exogenous
chemicals, in an animal species or in an organ, is the result of the
balance between in vivo activation and deactivation reactions
carried out predominantly by enzymes localized in the endoplasmic
reticulum of the cell. The ultimately available concentration of
reactive metabolites seems to account for some of the organ and
species specificities shown by several chemical carcinogens.
During the last decade, much progress has been made in
understanding the metabolism of various classes of chemical
carcinogens (polynuclear hydrocarbons, Sims & Grover, 1974;
N-nitroso compounds, Magee et al., 1976; aromatic amines, Kriek,
1974; Weisburger & Weisburger, 1973; miscellaneous compounds, Miller,
1973). The activity of microsomal mixed function oxidases is
influenced by various drugs or environmental chemicals, that may
stimulate or inhibit the formation of the ultimate carcinogens (Conney
& Burns, 1972).
These ultimate carcinogens bind covalently with cellular
macromolecules such as DNA, RNA, or proteins, which, directly or
indirectly, leads to heritable changes in the affected cells. Although
the critical targets of chemical carcinogens are not known, there is a
great deal of evidence supporting the theory that DNA is one major
target in carcinogenesis (Farber, 1973).
Some specific binding sites for the metabolites of carcinogens
have been identified in the bases of DNA. The carcinogenic N-nitroso
compounds are examples (Magee et al., 1976). The main site of
alkylation of DNA by alkylnitroso compounds is the N,7 position of
guanine, as with other alkylating agents. Other sites are also
attacked and the significance of these DNA interactions is being
investigated. The formation of 7-alkylguanine, however, seems to be of
no obvious importance in the process of tumour induction, since no
quantitative or qualitative correlation between the occurrence of this
alkylated base in the DNA of treated animals and the carcinogenicity
of nitrosamines or alkylating agents has been observed (Magee et al.,
1976). Alkylation of guanine bases in DNA at the 7-position does not
seem to produce mutations in bacteriophages (Loveless & Hampton,
1969), nor does it alter the coding properties of synthetic
polynucleotides in vivo (Ludlum, 1970). Various laboratories have
examined the biological importance of the alkylated O,6 position of
guanine residues in DNA which, in consequence, is able to induce
mutations in phage (Loveless, 1969). It has been shown that there is
an aberrant base pairing with a polymer containing alkylated
6.7.8 Variables to monitor
It is necessary to monitor several variables during the operation
of an inhalation chamber. The most obvious is the concentration in the
chamber of the pollutant in question. This can be done continuously by
automated samplers and recorded on a strip chart recorder or manually
at periodic intervals using a variety of sampling techniques. When
aerosols are involved, particle size should be determined. The flow of
pollutant, the flow of air, and the chamber pressure should all be
recorded frequently. Chamber temperature and humidity are other
variables that should be monitored. It is also useful to measure the
pressure drop across intake and exhaust filters in order to know when
to replace them.
6.7.9 Human exposure facilities
Ethical principles will always be of first concern, when
considering the exposure of human subjects to materials for toxicity
evaluation. However, there are situations where controlled exposure of
human beings can provide useful information with minimum risk. One of
the more modern facilities has been described by Stewart et al.
(1970a,b). It consists of a room approximately 6 m × 6 m × 2.7 m,
completely air conditioned and operated at slightly negative pressure.
Activity within this chamber is strictly sedentary and comfortable
chairs and study desks are provided. Meals are served during
exposures, with coffee and soft drinks available continuously. All
subjects are under continuous surveillance by medical personnel and
all activities are visually monitored by closed circuit television.
Such facilities are particularly useful for studying psychomotor
effects and other sensitive indicators of exposure to low
concentrations of organic vapours.
The odour threshold concentrations used in the USSR for
establishing maximum permissible concentrations for single exposures
are determined in human subjects over short periods (5-10 min).
Obviously such studies can only be carried out at very low
concentrations considered to be safe. The minimum concentration sensed
by the most sensitive individual is accepted as the odour threshold
(Rjazanov, 1964).
Reflex reactions produced in man by irritating the receptive
zones of the respiratory organs with subsensory concentrations of
atmospheric pollutants, were established by measuring the light
sensitivity of the eye, the bioelectric activity of the cerebrum, etc.
(Bustueva et al., 1960; Rjazanov, 1964). Changes in encephalographic
responses resulted from exposure to small concentrations of these
substances. The maximum concentration that did not produce an effect
on the bioelectric activity of the cerebrum was, in most cases, 3-4
times lower than the odour threshold concentration (Krotov, 1971;
Sidorenko & Pinigin, 1972).
6.8 Contaminant Generation and Characterization
Extensive reviews have been published by several investigators on
methods of generating and characterizing vapours and particles (Bryan,
1970; Cotabish et al., 1961; Drew & Lippmann, 1971; Lodge, 1968;
Mercer, 1973a,b; Nelson, 1971; Raabe, 1970).
6.8.1 Generation of vapours
Vapours can be generated by using one of several flow-dilution
devices (Cotabish et al., 1961; Drew & Lippmann, 1971; Nelson, 1971;
Saltzman, 1971; Saltzman & Warburg, 1965). If the contaminant is a
liquid at room temperature, a vaporization step must be included. One
procedure is to use a motor-driven syringe and to apply the liquid to
a wick or heated plate in a calibrated stream of air (Nelson & Griggs,
1968). Another method is to saturate the airstream with vapour and
then dilute it with air to the desired concentration (Cotabish et al.,
1961). A third technique, originally described by O'Keefe & Ortman
(1966), consists of using permeation tubes. These are especially
useful when using low concentrations of test materials for
standardization procedures. In theory, there is no reason why they
cannot be scaled up for use in inhalation studies.
6.8.2 Particle generators
The generation of particulate contaminants is usually more
difficult than vapour generation. The contaminant may be generated
from a dry powder or from a liquid and the particles generated may be
of uniform size (monodisperse) or may vary greatly in size
(heterogeneous).
6.8.2.1 Heterogeneous aerosols
The Wright dust feed (1950) is one of the better known
instruments for generating aerosols from a dry powder. A gear drives
the surface of a packed cylinder of finely ground powder against a
scraping mechanism. A high velocity airstream disperses the powder.
Proper use of the Wright dust feed is dependent upon the control of
the relative humidity of the airstream and the packing density of the
powder. Other devices for producing aerosols from dry powders have
been described (Crider et al., 1968; Deichman, 1944; Dimmick, 1959;
Stead et al., 1944). Since the particle size of the resultant aerosols
depends upon the size of the original powder, elutriators and cyclones
are sometimes included to limit the maximum size. Laskin et al. (1971)
described generators that include such devices for producing freshly
ground polyurethane aerosols.
Agglomeration, usually caused by electrical charge, is a serious
problem with dry dust aerosols. The electrical behaviour of aerosols
has been reviewed by Whitby & Liu (1966). A mechanical solution to
agglomeration has been proposed by Drew & Laskin (1971) who described
a fluidizing dust generator.
Wet dispersion generators break liquid into droplets. The liquid
may be a solution or a suspension of the test material. In most cases,
the liquid is drawn into filaments or films that are broken into
droplets. A number of compressed air-driven generators (nebulizers),
that produce droplets of many sizes, have been described (Dautrebande,
1962; Laskin, 1948; Lauterbach et al., 1956; Raabe, 1970). The
resulting aerosols are polydisperse although relatively narrow size
distribution can be attained with some nebulizers.
Monodisperse aerosols are occasionally needed by investigators,
especially when studying regional deposition. The most popular device
for dispensing uniform droplets is the spinning disc generator first
described by Walton & Prewett (1949). Primary droplets thrown off the
perimeter of a spinning disc are uniform in size. The liquid is fed on
to the centre of the disc and accumulates at the edge until broken off
by centrifugal force. Some secondary, smaller droplets are also
produced but these can be separated dynamically. Several investigators
have successfully used the spinning disc for inhalation studies
(Albert et al., 1964; Kajland et al., 1964; Lippmann & Albert, 1967;
Philipson, 1973). Another device employing a controlled condensation
process originally described by LaMer & Sinclair (1943) has been found
to be suitable for inhalation studies. A third principle, consisting
of size specific collection, resuspension and subsequent dispersion,
has also been used (Kotrappa et al., 1972).
6.8.3 Monitoring contaminant concentrations
The techniques and equipment for monitoring contaminant
concentrations have been reviewed by a number of authors (Lippman,
1971; Powell & Hosey, 1965). In many cases, the characteristics of the
contaminant determine the sampling technique. Sometimes a number of
techniques are available and the method of choice may depend upon the
availability of equipment, cost of reagents, time for analysis, or
other factors. Automated instrumentation is currently available for a
number of contaminants. However, when using automatic devices,
a second method, usually chemical, should be used to verify the
instrument performance.
6.8.3.1 Vapour sampling
Two basic methods for the collection of gaseous samples are
employed. The first involves the use of a gas collector, such as an
evacuated flask or bottle, to obtain a definite volume of air at a
known temperature and pressure. The second method involves the passage
of a known volume of air through a collecting medium to remove the
desired contaminants from the sampled atmosphere. The samples are then
analysed by appropriate analytical techniques.
Since the assays are related to the volume of air sampled, the
instrumentation for monitoring airflow or volume should be accurately
calibrated. It is also important that there are no leaks in the
sampling train, thus assuring that all the air measured has passed
through the collecting medium.
A number of devices, currently available, measure the
concentration of vapours continuously; many record the result
graphically. Many detection principles are used including
conductivity, colorimetry, and spectrophotometry. Recent commercial
instrumentation using the principle of infrared spectrometry are
especially useful. Most of these instruments have been described by
Nader (1971).
6.8.3.2 Particulate sampling
When monitoring particulate atmospheres, mass concentration and
particle size must be determined. The mass concentration can be
measured by techniques similar to those used for monitoring vapours.
The material can be collected, then assayed by appropriate chemical
methods. Gravimetric analysis can also be performed by weighing the
filter paper before and after collecting a sample. The resulting mass
can be related to the volume of air that was sampled.
Particle collection techniques include filtration, impingement,
thermal and electrostatic precipitation, and sedimentation. The basic
principles for these techniques and specific examples of each method
have been reviewed (Lippmann, 1971). These principles should be
thoroughly understood prior to selection of a method for a specific
contaminant.
The techniques for measuring particle size and numbers have been
discussed in detail by Mercer (1973a,b). Direct methods consisting of
both conventional and electron microscopy can be used. In both cases,
proper sampling methods to ensure collection of a representative
sample should be followed. Electrostatic and thermal precipitators or
an impinger can be used. After using an impinger, the dust can be
counted in a standard haematology counting cell or counted directly
with a Coulter Counter.
There are two indirect methods of assessing particle size; the
use of cascade impactors and the use of light scattering devices. The
theory of cascade impaction has been described by Mercer (1963, 1964,
1965). The theory of light scattering devices has been reviewed by
Hodkinson (1966) and commercially available instruments have been
listed by Swift (1971). Many factors, including shape, opacity, and
others, some unknown, affect the amount of light scattered and the
measured number and size of the particles. These devices should,
therefore, be used cautiously.
6.9 Other Methods of Respiratory Tract Exposure
6.9.1 In vivo exposures
In addition to direct inhalation, several other methods of
exposing the respiratory tract have been described (Roe, 1968).
Andervont (1937) originally described a thread implantation technique
whereby a thread impregnated with a test compound was literally passed
through a lobe of the lung. This technique was modified by Kuschner et
al. (1957) who devised a pellet that could be impregnated with the
test material or coated with radioactive materials. This pellet had
hooks which held it in the lumen of the bronchus or bronchiole. This
technique has been used to demonstrate the carcinogenicity of a number
of compounds including benzo(a)pyrene, 3-methylcholanthrene, and
calcium chromate (Laskin et al., 1970). A technique whereby hamsters
are anaesthetized and then intratracheally intubated with various
materials is in use in a number of laboratories (Laskin et al., 1970;
Little & O'Toole, 1974; Saffiotti, 1970; Schreiber et al., 1972). In
two laboratories, this technique has been coupled with inhalation
exposures to study the potential cocarcinogenicity of various agents
(Laskin & Nettesheim, 1974, personal communication).
6.9.2 In vitro exposures
A few in vitro techniques that show promise as regards
elucidation of the mechanisms of toxicity of inhaled materials should
be mentioned. A number of investigators (Niemeier & Bingham, 1972;
O'Neill & Tierney, 1974; Orton et al., 1973) are using various,
isolated, perfused lung preparations to study toxicity. Such
preparations could be particularly useful in studying the transfer of
materials from the lung to the blood. These preparations are also used
to study pulmonary metabolism as are lung tissue slices (O'Neil &
Tierney, 1974). Finally, several laboratories are developing methods
of culturing tracheal rings and sections (Griesemer et al., 1974; Lane
& Miller, 1975). These preparations show great promise for
toxicological evaluation (section 2.7.4).
6.10 Biological End-points and Interpretation of Changes in these
End-points
Throughout the course of an inhalation study, there are a number
of biological indicators to observe. The classical indices of toxicity
include weight change and mortality, organ-to-body weight ratios, and
both gross and microscopic changes in the morphology of the various
tissues and organs. While the study is in progress, respiratory,
physiological indices can be monitored and the excretion of certain
chemicals in breath, urine, and faeces can also be followed. Some
procedures particularly useful in inhalation toxicity studies are
discussed below.
6.10.1 Morphological changes
The primary function of the lung is the exchange of oxygen and
carbon dioxide between the blood and alveolar air. The walls of the
alveoli are lined by a single, flattened layer of epithelial cells
that are in close proximity to endothelial cells lining capillaries.
The mean distance from the lumen of the capillary to the lumen of the
alveolus is less than one micron. Because this distance is so small,
any thickening or inflammation of the alveolar wall will severely
disrupt the diffusion of gases.
The air in the alveolus arrives through a series of branching
bronchi and bronchioles the specialized epithelial lining of which is
instrumental in removing particulate matter from the lung. Any lesions
that result in narrowing of the lumen of these bronchi and bronchioles
cause a disruption in the ventilation of that portion of the lung.
Chemicals affecting the specialized ciliated epithelial lining, the
bronchioles, and the bronchi may interrupt the clearance mechanism,
resulting in a build-up of inhaled particulates in the lung.
The type of morphological change observed in the lung as a result
of the inhalation of materials depends upon the concentration of the
inhaled material and the length of time for which animals are exposed.
Short-term inhalation of high concentrations of certain materials may
result in acute changes in the lung such as oedema, necrosis, and
purulent inflammation. However, the inhalation of the same material at
lower concentrations for longer periods of time may result in chronic
changes such as fibrosis and even neoplasia. Table 6.3 lists a number
of chemicals and the response elicited in the lung following their
inhalation.
Techniques for quantifying morphological changes are, at best,
limited. The usual practice is to assign some number to the degree of
damage and then to apply statistical procedures to these numbers.
These procedures are, however, subject to individual interpretation.
Assessment of the degree of fibrosis is possible (Roe, 1968), although
such measurements should be checked by special staining procedures.
Interpretation of any morphological changes in the lung must take
into consideration the concentration and duration of exposure.
Exposure to certain materials will elicit immediate acute
morphological changes which, with time, tend to resolve and disappear.
This phenomenon suggests that the animal is able to adapt to the
effects of some chemicals, if the concentration is not too high. An
Table 6.3 Responses of the lung to various chemicals
Chemical Bronchiolar Loss of Oedema Epithelial Fibrosis Emphysema Granuloma
epithelial goblet metaplasia
hyperplasia cells
nitrogen dioxide x x x x x
ozone x x x x x
allergens x
sulfuric acid x x
bis-chloromethyl ether
silica x x
coal dust x x
beryllium x x x
cotton dust
nickel compounds
Table 6.3 (cont'd)
Necrosis Neoplasia Bronchial Alveolar Nonsuppurative
constriction epithelial alveolitis
hyperplasia
nitrogen dioxide x
ozone
allergens x
sulfuric acid x x
bis-chloromethyl ether x
silica
coal dust
beryllium x
cotton dust x
nickel compounds x
example of this adaptive phenomenon is seen with ozone. Nonlethal
exposures to ozone have a protective effect against subsequent lethal
exposures which would result in marked pulmonary oedema (Alpert &
Lewis, 1971).
6.10.2 Functional changes
There are certain advantages in using alterations in respiratory
functions as indices of toxicity: (a) quantitative measurements can
usually be made at concentrations far below those needed to produce
morphological effects; (b) many effects may be measured at the level
of reversible rather than irreversible changes; (c) such measurements
may elucidate mechanisms of action of materials, and (d) in many
instances similar data can be obtained from experimental animals and
man.
6.10.2.1 Measurement of respiratory frequency
Alteration of respiratory frequency in mice may be used as a
simple screening test for assessing irritant potency (Alarie, 1973).
The changes produced are dose-related, which permits construction of
dose-effect curves and calculation of the concentration required to
produce, for example, a 50% decrease in respiratory frequency. Some
irritants, mainly those affecting the upper respiratory tract,
decrease frequency. Other irritants, mainly those which penetrate to
the deeper areas of the lung, increase frequency. Measurement of
respiratory frequency alone is a very sensitive measure of effect for
those irritants that increase frequency (e.g. ozone and nitrogen
dioxide), but it is a relatively insensitive measure of effect for
those irritants that decrease frequency (e.g. sulfur dioxide and
formaldehyde).
6.10.2.2 Measurement of mechanics of respiration
Alterations in pulmonary flow resistance or pulmonary compliance
may be used to assess irritant potency. The method of Amdur & Mead
(1958) required three basic measurements: intrapleural pressure, tidal
volume, and the rate of flow of gas in and out of the respiratory
tract. Intrapleural pressure is estimated by placing a fluid-filled
catheter in the pleural space, while the animal is under anaesthesia.
Once the catheter is positioned, no further anaesthesia is necessary.
Tidal volume is obtained by placing the animal in a body
plethysmograph and measuring the pressure changes as the animal
breathes quietly. Rate of gas flow is obtained by electrical
differentiation of the volume signal. This method provides data on
both resistance and compliance, but is generally limited to single
exposure studies of a few hours duration. The guineapig has been the
species most commonly used for this method; however, plethysmographic
studies have been successfully carried out on several other rodents
including mice (Alarie, 1973), rats (Palacek, 1969) and rabbits
(Davidson et al., 1966).
The method of Murphy & Ulrich (1964) does not involve surgical
intervention and, thus, makes it possible to perform measurements
repeatedly on the same animals. The animal is placed in a body
plethysmograph to which an oscillating sine wave pressure is applied.
The animal's face is fitted with a mask containing a pneumotachograph
screen for measuring flow. Changes in resistance are calculated for
the alterations in flow produced by the superimposed pressure
oscillations. This technique has been used in toxicological studies
without making compliance measurements. However, by measuring
oesophageal pressure reflecting intrapleural pressure, it should be
possible to estimate compliance changes. A compliance measurement is
described by Mead (1960) that involves no surgical intervention.
The observed changes in respiratory mechanics are dose-related
and permit the construction of dose-effect curves. These may be used
to compare irritant potency or to study such things as the effect of
inert particles on the reaction to irritant gases. The deep lung
irritants that increase respiratory frequency tend to show a decrease
in compliance as the primary alteration in the mechanical behaviour of
the lung. Resistance changes with such irritants are minimal. The
irritants that slow respiratory frequency tend to show an increase in
resistance accompanied by a smaller decrease in compliance, as the
primary alteration in pulmonary mechanics.
An increase in pulmonary flow resistance can result from a
narrowing of the lumen of the bronchi mediated by constriction of the
smooth muscle. The effect, in this situation, usually occurs rapidly
on exposure to the irritant. In the case of a gaseous irritant, the
effect is fairly rapidly reversed when irritant exposure ceases. In
the case of a particulate irritant which remains in the lung, the
effect is less readily reversible. Swelling of the respiratory mucosa
or an increase in mucus secretion can also cause an increase in flow
resistance. The effect, in this situation, usually takes some time to
develop.
Much of the damage to the lung is to the small airways which
change either in calibre or stability or both. It would be very useful
if this damage could be detected early during an exposure before more
serious disease develops. There are three tests which are promising
and have been useful in human subjects. They are the maximal
expiratory flow volume curve, reviewed by Hyatt & Black (1973), the
alveolar closing volume (Dollfuss et al., 1967) and the frequency
dependency of compliance (Woolcock et al., 1969). Animal models that
provide the same data have not yet been fully developed.
6.10.3 Biochemical end-points
In the last decade, much research has centred around the
elucidation of some of the biochemical aspects of the lung. It has
only recently been demonstrated that the lung is capable of
biotransforming many foreign chemicals and several authors have
investigated the role of cytochrome P-450-dependent enzyme systems in
pulmonary microsomes (Bend et al., 1972; Chhabra & Fouts, 1974;
Chhabra et al., 1974; Fouts & Devereux, 1972; Harper et al., 1975;
Hook et al., 1972). The importance of intermediary metabolism is now
being considered (Tierney, 1974). The biochemistry of pulmonary
surfactants and their role in pulmonary defence mechanisms has been
reviewed by King (1974). Investigations of the lung collagen have
recently been reviewed by Crystal (1974). Witschi (1975) has provided
an excellent summary of biochemical approaches for the evaluation of
pulmonary toxicity.
6.10.4 Other end-points in inhalation studies
Exposure by inhalation is only a means by which the substance
enters the animal. This chapter has concentrated on the lung as the
critical organ. However, there are end-points that are not necessarily
related to the lung. Inhalation studies have recently been reported to
assess the teratological effects of inhaled materials (Schwetz et al.,
1975). They have also been used in behavioural studies (Weiss &
Laties, 1975; Xintaras et al., 1974).
REFERENCES
ALARIE, Y. (1973) Sensory irritation of the upper airways by airborne
chemicals. Toxicol. appl. Pharmacol., 24: 279-297.
ALBERT, R. E., PETROW, H. G., SALAM, A. S., & SPIEGELMAN, J. R. (1964)
Fabrication of monodisperse lucite and iron oxide particles with
a spinning disc generator. Health Phys., 10: 933-940.
ALBERT, R. E., LIPPMANN, M., & BRISCOE, W. (1969) The characteristics
of bronchial clearance and the effects of cigarette smoking.
Arch. environ. Health, 18: 738-755.
ALBERT, R. E., LIPPMANN, M., PETERSON, H. T., JR, BERGER, J., SANBORN,
K., & BORWAG, D. (1973) Bronchial deposition and clearance of
aerosols. Arch. intern. Med., 131: 115-131.
ALBERT, R. E., BERGER, J., SANSORN, K., & LIPPMANN, M. (1974) Effects
of cigarette smoke components on bronchial clearance in the
donkey. Arch. environ. Health, 29: 96-101.
ALPERT, S. M. & LEWIS, T. R. (1971) Ozone tolerance studies utilizing
unilateral lung exposure. J. appl. Physiol., 192: 364-368.
AMDUR, M. O. & MEAD, J. (1958) Mechanics of respiration in
unanaesthetized guinea pigs. Am. J. Physiol., 192: 364-368.
ANDERSEN, I., LUNDQVIST, G. R., JENSON, P. L., & PROCTOR, D. F. (1974)
Human response to controlled levels of sulfur dioxide. Arch.
environ. Health, 28: 21-39.
ANDERVONT, H. B. (1937) Pulmonary tumours in mice: IV. Lung tumours
induced by subcutaneous injection of 1,2,5,6-dibenzanthracene in
different media and by its direct contact with lung tissue.
Public Health Rep., 52: 1584.
BALASOV, V. E., BARTENEV, V. D., SAVICKIJ, I. V., & TRAHTENSERG, I. M.
(1968) [Toxicological assessment of volatile substances leaking
from synthetic materials.] Zdorov'e, Kiev (in Russian).
BEND, J. R., HOOK, G. E. R., EASTERLING, R. E., GRAM, T. E., & FOUTS,
J. R. (1972) A comparative study of the hepatic and pulmonary
microsomal mixed function oxidase systems in the rabbit.
J. Pharmacol. exp. Ther., 183: 206-217.
BOECKER, B. B., AQUILAR, F. L., & MERCER, T. T. (1964) The design of a
canine inhalation exposure apparatus incorporating a whole-body
plethysmograph. Health Phys., 10: 1077-1089.
BOYD, M. R., BURKA, L. T., HARRIS, T. M., & WILSON, B. J. (1974)
Lung-toxic furanoterpenoids produced by sweet potatoes (Ipomoea
batatas) following microbial infection. Biochim. Biophys.
Acta, 337: 184-195.
BRIAN, J. D. & VALBERG, P. A. (1974) Models of lung retention based on
International Congress of Radiation Protection Task Group Report.
Arch. environ. Health, 28: 1-11.
BRYAN, R. J. (1970) Generation and monitoring of gases for inhalation
studies. In: Hanna, M. G. Jr, Nettesheim, P., & Gilbert, J. R.,
ed. Inhalation carcinogenesis. Springfield VA, Clearinghouse
for Federal Scientific and Technical Information, NBS, US Dept of
Commerce (CONF-691001).
BUSTUEVA, K. A., POLEZAEV, E. F., & SOMENENKO, A. D. (1960) [A study
of threshold values for reflex action of atmospheric pollutants.]
Gig. i Sanit., No. 1, pp. 57-61 (in Russian).
CADLE, R. D. (1965) Particle size. Princeton, NJ, Van
Nostrand-Reinhold.
CAHAN, W. G. & KIRMAN, D. (1968) An effective system and procedure for
cigarette smoking by dogs. J. surg. Res., 8: 567-575.
CAMNER, P., PHILIPSON, K., & FRIBERG, L. (1972) Tracheobronchial
clearance in twins. Arch. environ. Health, 24: 82-87.
CAMNER, P., MOSSBERG, B., & PHILIPSON, K. (1973) Tracheobronchial
clearance and chronic obstructuve lung disease. Scand. J.
respir. Dis., 54: 272-281.
CAMNER, P., HELLSTRÖM, P. A., LUNDBERG, M., & PHILIPSON, K. (1977)
Lung clearance of 4 µm particles coated with silver, carbon and
beryllium. Arch, environ. Health, 32: 58-62.
CARPENTER, C. P., SMYTH, H. F., JR, & POZZANI, U. C. (1949) The assay
of acute vapour toxicity and the grading and interpretation of
results on 96 chemical compounds. J. ind. Hyg. Toxicol.,
31: 343-346.
CASARETT, L. J. (1972) The vital sacs: alveolar clearance mechanisms
in inhalation toxicology. In: Hayes, W. J., ed. Essays in
toxicology. New York, London, Academic Press.
CHHABRA, R. S. & FOUTS, J. R. (1974) Sex differences in the metabolism
of xenobiotics by extrahepatic tissue in rats. Drug Metab.
Disposition, 2: 375-379.
CHHABRA, R. S., POHL, R. J., & FOUTS, J. R. (1974) A comparative study
of xenobiotic-metabolizing enzymes in liver and intestine of
various animal species. Drug Metab. Disposition, 2: 443-447.
COTABISH, H. N., MCCONNAUGHEY, P. W., & MESSER, H. C. (1961) Making
known concentrations for instrument calibration. Am. Ind. Hyg.
Assoc. J., 22: 392-402.
CRIDER, W. L., BARKELEY, N. P., & STRONG, A. A. (1968) Dry-powder
aerosol dispensing device with long-time output stability.
Rev. Sci. Instrum., 39: 152-155.
CRYSTAL, R. G. (1974) Lung collagen: definition, diversity and
development. Fed. Proc., 33: 2248-2255.
DAUTREBANDE, L. (1952) Microaerosols, New York, Academic Press,
pp. 7-22.
DAVIDSON, J. T., WASSERMAN, K., LILLINGTON, G. A., & SCHMIDT, R. W.
(1966) Pulmonary function testing in the rabbit. J. appl.
Physiol., 21: 1094-1098.
DEICHMANN, W. B. (1944) An apparatus designed for the production of
controlled dust concentrations in the air breathed by
experimental animals. J. ind. Hyg. Toxicol., 26: 334-335.
DIMMICK, R. L. (1959) Jet disperser for compacted powders in the
one-to-ten micron range. Am. Med. Assoc. Arch. ind. Health,
20: 8-14.
DOLLFUSS, R. E., MILIC-EMILI, J., & BATES, D. V. (1967) Regional
ventilation of the lung studied with boluses of 133 xenon.
Respir. Phys., 2: 234-246.
DONTENWILL, W. (1970) Experimental investigations on the effect of
cigarette smoke inhalation on small laboratory animals. In:
Hanna, M. G. Jr, Nettesheim, P., & Gilbert, J. R., ed.
Inhalation carcinogenesis. Springfield VA, Clearinghouse for
Federal Scientific and Technical Information, NBS, US Dept of
Commerce.
DRAIZE, J. H., NELSON, A. A., NEWBURGER, S. N., & KELLEY, E. A. (1959)
Inhalation toxicity studies of six types of aerosol hair sprays.
Proc. Sci. Sec. Toilet Goods Assoc., 31: 28-32.
DREW, J. H. & LASKIN, S. (1971) A new dust-generating system for
inhalation studies. Am. Ind. Hyg. Assoc. J., 32: 327-330.
DREW, R. T. & LASKIN, S. (1973) Environmental inhalation chambers.
In: Day, W. I., ed. Methods of animal experimentation. New
York, Academic Press.
DREW, R. T. & LIPPMANN, M. (1971) Calibration of air sampling
instruments: II. Production of test atmospheres for instrument
calibraton. In: Lippmann, M., ed. Air sampling instruments, 4th
ed., Cincinnati, OH, ACGIH, pp. I-1.
DREW, R. T. & TAYLOR, G. J. (1975) Cardiophysical studies with
stressed animals. In: Proceedings of the 5th Annual Conference
on Environmental Toxicology, Dayton, Ohio, 1974, US Govt
Printing Office, pp. 121-129.
FOUTS, J. R. & DEVEREUX, T. R. (1972) Developmental aspects of hepatic
and extrahepatic drug-metabolizing enzyme systems: microsomal
enzymes and components in rabbit liver and lung during the first
month of life. J. Pharmacol. exp. Ther., 183: 458-468.
FRASER, D. A., BALES, R. E., LIPPMANN, M., & STOKINGER, H. E. (1959)
Exposure chambers for research in animal inhalation. USPHS
(Public Health Monograph 57).
GOLDBERG, I. S. & LAURENCO, R. V. (1973) Deposition of aerosols in
pulmonary disease. Arch. intern. Med., 131: 88-92.
GRIESEMER, R. A., KENDRICK, J., & NETTESHEIM, P. (1974) Tracheal
grafts. In: Proceedings on Experimental Respiratory
Carcinogenesis, Seattle 1974, Springer-Verlag, pp. 537-547.
GROSS, P., PFITZER, E. A., TOLKER, E., BABYAK, M. A., & KASCHAK, M.
(1965) Experimental emphysema: its production with papain in
normal and silicotic rats. Arch. environ. Health, 11: 50-58.
HABER, F. (1924) [Five lectures for the years 1920-1923.] Berlin,
Springer-Verlag (in German).
HARPER, C., DREW, R. T., & FOUTS, J. R. (1975) Species differences in
benzene hydroxylation to phenol by pulmonary and hepatic
microsomes. Drug Metab. Disposition, 3: 381-388.
HAUN, C. C. (1972) Continuous animal exposure to methylene chloride.
In: Proceedings of the 2nd Annual Conference on Environmental
Toxicology, Dayton, Ohio, 1971, US Govt Printing Office,
pp. 309-326.
HAZUCHA, M., SILVERMAN, F., PARENT, C., FIELD, S., & BATES, D. V.
(1973) Pulmonary function in man after short-term exposure to
ozone. Arch. environ. Health, 27: 183-188.
HENDERSON, D. W. (1952) Apparatus for study of airborne infection.
J. Hyg. (Lond.), 50: 53-68.
HINNERS, R. G., BURKART, J. K., & CONTNER, G. L. (1966) Animal
exposure chambers in air pollution studies. Arch. environ.
Health, 13: 609-615.
HINNERS, R. G., BORKART, J. K., & PUNTE, C. L. (1968) Animal
inhalation exposure chambers. Arch. environ. Health,
16: 194-206.
HODKINSON, J. R. (1966) The optical measurements of aerosols. In:
Davies, C. N., ed. Aerosol science, New York, Academic Press,
pp. 287-357.
HOFFMANN, D. & WYNDER, E. L. (1970) Chamber development and aerosol
dispersion. In: Hanna, M. G. Jr, Nettesheim, P., & Gilbert, J.
R., ed. Inhalation carcinogenesis. Springfield, VA.
Clearinghouse for Federal Scientific and Technical Information,
NBS, US Dept of Commerce (CONF-691001).
HOMBURGER, F., BERNFELD, P., BOGDONOFF, P., KELLEY, T., & WALTON, R.
(1967) Inhalation by small animals of fresh cigarette smoke
generated by new smoking machine. Toxicol. appl. Pharmacol.,
10: 382.
HOOK, G. E. R., BEND, J. R., HOEL, D., FOUTS, J. R., & GRAM, T. E.
(1972) Preparation of lung microsomes and a comparison of the
distribution of enzymes between subcellular fractions of rabbit
lung and liver. J. Pharmacol. exp. Ther., 182: 474-490.
HYATT, R. E. & BLACK, L. F. (1973) The flow-volume curve: a current
perspective. Am. Rev. Respir. Dis., 107: 191-199.
JEMSKI, J. V. & PHILLIPS, G. B. (1965) Aerosol challenge of animals.
In: Gay, W. I., ed. Methods of animal experimentation, New
York, Academic Press, Vol. 1, pp. 273-341.
KAJLAND, A., EDFORS, M., FRIBERG, L., & HOLMA, B. (1964) Radioactive
monodisperse test aerosols and lung clearance studies. Health
Phys., 10: 941-945.
KING, R. J. (1974) The surfactant system of the lung. Fed. Proc.,
33: 2238-2247.
KLJACKINA, A. M. (1973) In: Toxicology of new industrial chemical,
Moscow, Medicina, Vol. 13, p. 23.
KOTRAPPA, P., BOYD, H. A., & WILKINSON, C. J. (1972) Technology for
the production of monodisperse aerosols of oxides of transuranic
elements for inhalation experiments. Health Phys., 22: 837-843.
KROTOY, YU. A. (1971) [Setting of approximate maximum permissible
concentrations of atmospheric pollutants by calculation.]
Gig. i Sanit., 12: 8-12 (in Russian).
KUSCHNER, M., LASKIN, S., CHRISTOFANO, E., & NELSON, N. (1957)
Experimental carcinoma of the lung. In: Proceedings of the 3rd
National Cancer Conference, Philadelphia, Lippincott,
pp. 485-495.
KUSCHNER, M., LASKIN, S., DREW, R. T., CAPIELLO, V., & NELSON, N.
(1975) Inhalation carcinogenicity of alpha haloethers: III.
Lifetime and limited period inhalation studies with
bis(chloromethyl) ether at 0.1 ppm. Arch. environ. Health,
30: 73-77.
LAMER, V. K. & SINCLAIR, D. (1943) An improved homogeneous aerosol
generator. Washington DC, US Dept of Commerce (OSRD Report
No. 1668).
LANE, B. P. & MILLER, S. L. (1975) Dose dependence of
carcinogen-induced changes in tracheal epithelium in organ
culture. In: Proceedings of the Symposium on Experimental
Respiratory Carcinogenesis, Seattle, 1974, Springer-Verlag,
pp. 507-513.
LASKIN, S. (1948) AEC Project Quarterly Report UR-38. Rochester, NY,
University of Rochester.
LASKIN, S., KUSCHNER, M., & DREW, R. T. (1970) Studies in pulmonary
carcinogenesis. In: Hanna, M. G. Jr, Nettesheim, P., & Gilbert,
J. R., ed. Inhalation carcinogenesis. Springfield VA,
Clearinghouse for Federal Scientific and Technical Information,
NBS, US Dept of Commerce (CONF-691001).
LASKIN, S., DREW, R. T., CAPIELLO, V. P., & KUSCHNER, M. (1971)
Inhalation studies with freshly generated polyurethane foam dust.
In: Mercer, T. T., Morrow, P. E., & Storer, W., ed. Assessment
of airborne particles, Springfield, IL, Thomas, pp. 382-404.
LAUTERBACH, K. E., HAYES, D., & COELHO, M. A. (1956) An improved
aerosol generator. Am. Med. Assoc. Arch. ind. Health,
13: 156-160.
LEONG, B. J. K., KOCIBA, R. J., JERSEY, G. C., & GEHRING, P. J. (1975)
Effects from repeated inhalation of parts per billion of
bis(chloromethyl) ether in rats. Toxicol. appl. Pharmacol.,
35: 175.
LEWIS, T. R., MOORMAN, W. J., YANG, Y., & STARA, J. F. (1974)
Long-term exposure to auto exhaust and other pollutant mixtures.
Arch. environ. Health, 29: 102-106.
LIPPMANN, M. & ALBERT, R. E. (1967) A compact electric motor-driven
spinning disc aerosol generator. Am. Ind. Hyg. Assoc. J.,
28: 501-506.
LIPPMANN, M., ed. (1971) Air sampling instruments, 4th ed.
Cincinnati, OH, ACGIH.
LITTLE, J. B. & O'TOOLE, W. F. (1974) Respiratory trace tumours in
hamsters induced by benzo(a)pyrene and polonium210 alpha
radiation. Cancer Res., 34: 3026-3039.
LODGE, J. P. (1968) Production of controlled test atmospheres.
In: Stern, A. C., ed. Air pollution, 2nd ed., New York,
Academic Press, Vol. II, pp. 465.
MACFARLAND, H. N. (1968) Exposure chambers -- design and operation.
In: Proceedings of the 7th Annual Technical Meeting of the
American Association of Contamination Control, Chicago, 1968,
pp. 19-25.
MACFARLAND, H. N. (1976) Respiratory toxicology. In: Hayes, W. J. Jr,
ed. Essays in toxicology, New York, San Francisco, London,
Academic Press, Vol. 7, pp. 121-154.
MACKLEM, P. T., HOGG, W. E., & BRUTON, J. (1973) Peripheral airway
obstruction and particulate deposition in the lung. Arch.
intern. Med., 131: 93-100.
MARTORANA, P. A., MCKEEL, N. W., RICHARD, J. W., & SHARE, N. N. (1973)
Function-structure correlation studies on excised hamster lungs
in papain-induced emphysema. Can. J. Physiol. Pharmacol.,
51: 635-641.
MCLAUGHLIN, R. F., TYLER, W. S., & CANADA, R. O. (1961a) Subgross
pulmonary anatomy in various mammals and man. J. Am. Med. Assoc.
175: 694-697.
MCLAUGHLIN, R. F., TYLER, W. S., & CANADA, R. O. (1961b) Study of the
subgross pulmonary anatomy of various mammals. Am. J. Anat.,
108: 149-164.
MCLAUGHLIN, F. R., TYLER, W. S., & CANADA, R. O. (1966) Subgross
pulmonary anatomy of the rabbit, rat and guinea pig with
additional notes on the human lung. Am. Rev. Respir. Dis.,
94: 380-387.
MEAD, J. (1960) Control of respiratory frequency. J. appl. Physiol.,
15: 325-360.
MERCER, T. T. (1963) On the calibration of cascade impactors. Ann.
occup. Hyg., 6: 1-14.
MERCER, T. T. (1964) The stage constants of cascade impactors. Ann.
occup. Hyg., 7: 115-125.
MERCER, T. T. (1965) The interpretation of cascade impactor data. Am.
Ind. Hyg. Assoc. J., 26: 236-241.
MERCER, T. T. (1973a) Production and characterization of aerosols.
Arch. intern. Med., 131: 39-50.
MERCER, T. T. (1973b) Aerosol technology in hazard evaluation. New
York, London, Academic Press.
MORROW, P. E. (1970) Models for the study of particulate retention and
elimination in the lung. In: Hanna, M. G., Nettesheim, P. &
Gilbert, J. R., ed. Inhalation carcinogenesis. Springfield VA,
Clearinghouse for Federal Scientific and Technical Information,
NBS, US Dept of Commerce (CONF-691001).
MORROW, P. E. (1973) Alveolar clearance of aerosols. Arch. intern.
Med., 131: 101-108.
MORROW, P. E., HODGE, H. C., NEWMAN, W. F., MAYNARD, E. A., BLANCHET,
H. J. JR, FASSET, D. W., BIRK, R. E., & MAVRODT, S. (1966)
Deposition and retention models for internal dosimetry of the
human respiratory tract. Health Phys., 12: 173-207.
MURPHY, S. D. & ULRICH, G. E. (1964) Multi-animal test system for
measuring effects of irritant gases and vapours on respiratory
function of guinea pig. Am. Ind. Hyg. Assoc. J., 25: 28-36.
NADER, J. S. (1971) Direct-reading instruments for analysing airborne
gases and vapours. In: Lippmann, M., ed. Air sampling
instruments, 4th ed., Cincinnati, ACGIH, p. J-1.
NELSON, G. O. (1971) Controlled test atmospheres: principles and
techniques. Ann Arbor, MI, Ann Arbor Sci. Publ.
NELSON, G. O. & GRIGGS, K. E. (1968) Precision dynamic method for
producing known concentrations of gas and solvent vapour in air.
Rev. Sci. Instrum., 39: 927-928.
NIEMEIER, R. W. & BINGHAM, E. (1972) An isolated perfused lung
preparation for metabolic studies. Life Sci., 11(II): 807-820.
NIEWOEHNER, D. R. & KLEINERMAN, J. (1973) Effects of experimental
emphysema and bronchiolitis on lung mechanics and morphometry.
J. appl. Physiol., 35: 25-31.
O'KEEEE, A. E. & ORTMAN, G. O. (1966) Primary standards for trace gas
analysis. Anal. Chem., 38: 760-763.
O'NEIL, J. J. & TIERNEY, D. G. (1974) Rat lung metabolism: glucose
utilization by isolated perfused lungs and tissue slices. Am. J.
Physiol., 226: 867-873.
ORTON, T. C., ANDERSON, M. W., PICKETT, R. D., ELING, T. E., & FOUTS,
J. R. (1973) Xenobiotic accumulation and metabolism by isolated
perfused rabbit lungs. J. Pharmacol. exp. Ther., 186: 482-497.
PALACEK, F. (1969) Measurement of ventilatory mechanics in the rat.
J. appl. Physiol., 27: 149-156.
PHILIPSON, K. (1973) On the production of monodisperse particles with
a spinning disc. Aerosol Sci., 4: 51-57.
POWELL, C. H. & HOSEY, A. D. (1965) The industrial environment, its
evaluation and control (US PHS Publ. 614).
POZZANI, U. C., WEIL, C. S., & CARPENTER, C. P. (1959) The
toxicological basis of threshold limit values: 5. The
experimental inhalation of vapour mixtures by rats with notes
upon the relationship between single-dose oral data. Am. Ind.
Hyg. Assoc. J., 20: 364-369.
RAABE, O. G. (1970) Generation and characterization of aerosols. In:
Hanna, M. G. Jr, Nettesheim, P., & Gilbert, J. R., ed.
Inhalation carcinogenesis. Clearinghouse for Federal Scientific
and Technical Information, NBS, Springfield VA, US Dept of
Commerce (CONF-691001).
RAABE, O. G., BENNICK, J. E., LIGHT, M. E., HOBBS, C. N., THOMAS, R.
L., & TILLERY, M. (1973) An improved apparatus for acute
inhalation exposure of rodents to radioactive aerosols. Toxicol.
appl. Pharmacol., 26: 264-273.
RJAZANOV, V. A. (1964) Criteria and methods for establishing maximum
permissible concentrations of atmospheric pollutants in the USSR.
In: Maximum permissible concentrations of atmospheric
pollutants, Moscow, Medicina, Vol. 8, pp. 5-21.
ROE, F. J. C. (1968) Inhalation tests. In: Boyland, E. & Goulding, R.,
ed. Modern trends in toxicology, London, Butterworth, Vol. 1.
SAFFIOTTI, U. (1970) Experimental respiratory tract carcinogenesis and
its relation to inhalation exposures. In: Hanna, M. G. Jr,
Nettesheim, P., & Gilbert, J. R., ed. Inhalation carcinogenesis.
Springfield, VA, Clearinghouse for Federal Scientific and
Technical Information, NBS, US Sept of Commerce (CONF-691001).
SAITO, Y. (1912) [Experimental investigations on the quantitative
absorption of dust by animals at accurately known concentrations
of dust in air.] Arch. Hyg., 75: 134-151 (in German).
SALTZMAN, B. E. (1971) Permeation tubes as calibrated sources of gas.
In: Proceedings of 1st Annual Conference on Environmental
Toxicology, Dayton, Ohio, 1970, US Govt Printing Office,
pp. 309-326.
SALTZMAN, B. E. & WARBURG, A. F. (1965) Precision flow dilution system
for standard low concentrations of nitrogen dioxide. Anal.
Chem., 37: 1261-1264.
SANCHIS, J., DOLOVICH, M., CHALMERS, R., & NEWHOUSE, M. (1972)
Quantification of regional aerosol clearance in the normal human
lung. J. appl. Physiol., 33: 757-762.
SANOCKIJ, I. V., ed. (1970a) [Methods for determining toxicity and
hazards of chemicals.] Moscow, Medicina, pp. 62-63 (in
Russian).
SANOCKIJ, I. V. (1970b) [Complete toxicometry.] In: [Methods for
determining the toxicity and hazards of chemicals.] Moscow,
Medicina, pp. 50-54 (in Russian).
SCHREIBER, H., NETTESHEIM, P., & MARTIN, D. H. (1972) Rapid
development of bronchiolo-alveolar squamous cell tumours in rats
after intratracheal injection of 3-methylcholanthrene. J. Natl
Cancer Inst., 49: 541-554.
SCHWETZ, B. A., LEONG, B. J. K., & GEHRING, P. J. (1975) The effect of
maternally inhaled trichloroethylene, perchloroethylene, methyl
chloroform and methylene chloride on embryonal and fetal
development in mice and rats. Toxicol. appl. Pharmacol.,
32: 84-96.
SIDORENKO, G. I. & PINIGIN, M. A. (1970) Rapid determination of
maximum permissible concentrations of atmospheric pollutants.
Gig. i Sanit., No. 3, pp. 93-96.
SILVER, M. J., HOCH, W., KOCSIS, J. J., INGERMAN, C. M., & SMITH, J.
B. (1973) Arachadonic acid causes sudden death in rabbits.
Science, 183: 1085-1086.
SILVER, S. D. (1946) Constant flow gassing chambers: principles
influencing design and operations. J. lab. clin. Med.,
31: 1153-1161.
SNIDER, G. L., HAYES, J. A., FRANZBLAU, C., KAGEN, H. M., STONE, P.S.,
& KORTHY, A. L. (1974) Relationship between elastolytic activity
and experimental emphysema-inducing properties of papain
preparations. Am. Rev. Respir. Dis., 10: 254-262.
STEAD, F. M., DERNEHL, C. U. & NAU, C. A. (1944) A dust feed apparatus
useful for exposure of small animals to small and fixed
concentrations of dust. J. ind. Hyg. Toxicol., 26: 90-93.
STEWART, R. D. (1972) Use of human volunteers for the toxicological
evaluation of materials. In: Symposium on an Appraisal of
Halogenated Fire Extinguishing Agents. Washington DC, National
Academy of Sciences.
STEWART, R. D., DODD, H. C., GAY, H. H., & ERLEY, D. S. (1970a)
Experimental human exposure to trichloroethylene. Arch. environ.
Health, 20: 64-71.
STEWART, R. D., PETERSON, J. E., BARETTA, E. D., BACHARD, R. T.,
HOSCO, M. T., & HERRMANN, A. A. (1970b) Experimental human
exposure to carbon monoxide. Arch. environ. Health,
21: 154-164.
STEWART, R. D., PETERSON, J. E., FISHER, T. N., HOSKO, M. J., BARETTA,
E. D., DODD, H. C., & HERRMANN, A. A. (1973) Experimental human
exposure to high concentrations of carbon monoxide. Arch.
environ. Health, 26: 1-7.
STOKINGER, H. E. (1949) Toxicity following inhalation. In Voegtlin, C.
& Hodge, C., ed. Pharmacology and toxicology of uranium
compounds, 1st ed., New York, McGraw-Hill, Vol. III.
STUART, B. O. (1974) Deposition of inhaled aerosols. Arch. intern.
Med., 131: 60-75.
STUART, B. O., WILLARD, D. H., & HOWARD, E. B. (1970) Uranium mine air
contaminants in dogs and hamsters. In: Hanna, M. G. Jr,
Nettesheim, P., & Gilbert, J. R., ed. Inhalation carcinogenesis.
Springfield, VA, Clearinghouse for Federal Scientific and
Technical Information, NBS, US Dept of Commerce (CONF-691001).
STUPFEL, M. & MORDELET-DAMBRINE, M. (1974) Penetration of pollutants
in the airways. Bull. Physio-Path. Respir., 10: 481-509.
SWIFT, D. L. (1971) Direct-reading instruments for analysing airborne
particles. In: Lippmann, M., ed. Air sampling instruments, 4th
ed., Cincinnati, OH, ACGIH, p. T-1.
TAYLOR, G. J. & DREW, R. T. (1975) Cardiomyopathy predisposes hamsters
to trichlorofluoromethane toxicity. Toxicol. appl. Pharmacol.,
32: 177-183.
THOMAS, R. G. & LIE, R. (1963) Procedures and equipment used in
inhalation studies on small animals. LF-11, Lovelace Foundation
for Medical Education and Research.
THOMAS, R. L. (1969) Deposition and initial translocation of inhaled
particles in small laboratory animals. Health Phys.,
16: 417-428.
TIERNEY, D. G. (1974) Intermediary metabolism of the lung. Fed.
Proc., 33: 2232-2237.
TUCKER, A. D., WYATT, J. H., & UNDRY, D. (1973) Clearance of inhaled
particles from alveoli by normal interstitial drainage pathways.
J. appl. Physiol., 35, 719-732.
VALEZNEV, I. P., ELOVSKAJA, L. T., & MOISEJCEV, P. I. (1970)
[Equipment for research on biological effects of aerosols.]
Ofic. bjull. Gos. kom. Min. SSSR po delam izobretenij i
otkrytij, No. 10, avtonskoe svidetel'stvo No. 265307 (in
Russian).
VELICKOVSKIJ, B. T. & KACNELSON, B. A. (1964) [Etiology and
pathogenesis of silicosis.] Moscow, Medicina (in Russian).
WALTON, W. H. & PREWETT, W. C. (1949) The production of sprays and
mists of uniform drop size by means of spinning disc type
sprayers. Proc. Phys. Soc. (London), 62: 341-350.
WATSON, J. A., SPRITZER, A. A., AULD, J. A. & GUETTHOFF, M. A. (1969)
Deposition and clearance following inhalation and intra-tracheal
injection of particles. Arch. environ. Health, 19: 51-58.
WEISS, B. & LATIES, V. (1975) Behavioural toxicology. New York,
Plenum Press.
WHITBY, K. T. & LIU, B. Y. H. (1966) The electrical behaviour of
aerosols. In: Davies, C. N., ed. Aerosol Science, New York,
Academic Press.
WILSON, R. H. & LASKIN, S. (1950) Improved design for an animal
inhalation exposure unit. Rochester, NY, University of
Rochester (AEC Proj. Rep. U-116), p. 80.
WITSCHI, H. P. (1975) Exploitable biochemical approaches for the
evaluation of toxic lung damage. In: Hayes, W. Jr, ed. Essays in
toxicology, New York, Academic Press, Vol. 6.
WOOLCOCK, A. J., VINCENT, N. J., & MACKLEM, P. T. (1969) Frequency
dependence of compliance as a test for obstruction in the small
airways. J. clin. Invest., 48: 1097-1106.
WRIGHT, B. M. (1950) A new dust-feed mechanism. J. Sci. Instrum.,
27: 12-15.
XINTARAS, C., JOHNSON, B. L., & DE GROOT, I., ed. (1974) Behavioural
toxicology. Washington DC, US Govt Printing Office.
7. CARCINOGENICITY AND MUTAGENICITY
7.1 Introduction
This chapter deals with the two related but separate processes,
heritable mutations and cancer. Cancer involves the conversion of
normal cells to malignant cells and the development of what is usually
an irreversible malignant disease process in the present generation
frequently leading to a fatal outcome for the bearer of the
malignancy. Heritable mutations are mutations that are transmissible
to later generations and the target cells are the germ cells of either
sex.
Developments in the last two decades make it possible to discuss
certain aspects of mutagenesis and carcinogenesis in parallel. In
other words, there is now increasing evidence that, in most cases,
somatic mutations are probably involved in the conversion of normal to
malignant cells. Thus, the ability of a chemical or physical agent to
produce mutations is relevant both to the question of heritable
mutations (mutations of the germ cell) and carcinogenicity.
There is a well-established body of knowledge that shows a close
correspondence between cancer of the whole animal as studied in the
laboratory and the occurrence of cancer in man; this is especially
true of occupational cancer. More recently, a fairly close correlation
has been shown between tests of isolated, simple, biological systems,
e.g. reverse bacterial mutations and the response to carcinogens of
whole animals, and human beings.
A similar situation does not exist with respect to patterns in
human heritable mutations. Thus, although there is good evidence of
correlation between in vitro tests for mutagenicity and heritable
mutations in insects and experimental mammals, it can only be
inferred, at present, that this also applies to man. The inference,
however, is extremely strong and not subject to serious doubt. Thus,
there is every reason to expect, that, in general, man will respond
biologically in a manner quite similar to that of other species when
exposed to mutagens that reach the germ cells. The lack of full
correlation stems more from the lack of appropriate studies in man
than from any uncertainty concerning the underlying biological
considerations. At present, methods for the detection of mutations in
man are difficult, cumbersome, and insensitive, and it is imperative
to operate on the assumption that agents capable of producing germ
cell mutations in laboratory studies would also be capable of
producing similar mutations in man.
Thus, two quite different disease processes can have one common
level of consideration, namely, their ability to produce alterations
in the genetic material of cells. The following discussion is
concerned with methods for carcinogenicity testing. First, the
traditional lifetime exposure of the entire organism to the test
compound is considered. This is followed by a discussion of various
in vitro systems for the examination of mutagenicity including DNA
damage, mutagenicity in bacteria and eukaryotic organisms, and
transformation of cell cultures. (The first three of these are
particularly relevant to mutagenicity of the germ cell or of the
somatic cell.) Following this, the general problem of examination for
heritable mutations is considered. This includes inquiries at three
levels: injury to DNA, point mutations, and chromosome alterations.
Whole animal and isolated test systems are available to make relevant
determinations. However, the whole field of mutagenicity testing is
now in a period of rapid evolution with advances being made in a
number of areas. It can, therefore, be anticipated that present
procedures will undergo alteration and, it is hoped, will improve
within the near future.
7.2 Carcinogenicity
7.2.1 Long-term bioassays
The fact that environmental factors have a direct effect in
producing cancer in man is shown by: (a) the unequivocal evidence of
the chemical origin of occupational cancer, for example, urinary
bladder tumours in workers exposed to aromatic amines, lung cancers in
workers exposed to bis(chloromethyl)ether, etc.; (b) the
well-documented cases of iatrogenic cancer; (c) the positive
correlation between cigarette smoking and lung cancer; (d) the
differences in cancer incidence in urban and rural populations and
(e) the results of studies on migrants showing that for some types
of cancer they acquire incidences similar to those found in the host
countries. Results obtained in experimental studies on carcinogenesis
confirm the direct carcinogenic effect of chemicals. However, there is
not necessarily a correlation between acute toxicity and
carcinogenicity; a chemical with a high acute toxicity may not have
any, or only a very low carcinogenic potential. Conversely, a chemical
that produces a very minor, or no evident toxic effect in acute or
subacute experiments may be a very powerful carcinogen. Knowledge of
the acute and subacute toxicity of the test chemical however, is
important in order to permit better planning of the experiment. For
instance, the premature death of the test animals, due to unforeseen
side-effects, may thus be avoided.
7.2.1.1 Species, strain, and sex selection, and size of groups
The description of methods and recommended procedures in this
chapter applies to long-term testing in rodents and specifically to
the mouse, rat, and Syrian golden hamster (FDA, 1971; NCI, 1976; Weil,
1972; Weisburger, 1975; Weisburger & Weisburger, 1967). Rodents have
been preferred to other species because of their susceptibility to
tumour induction, their relatively short life span, the limited cost
of their maintenance, their widespread use in pharmacological and
toxicological studies, the availability of inbred strains and, as a
consequence of these characteristics, the large amount of information
available on their physiology and pathology. Nonrodents, in particular
dogs and primates, have rarely been used in the past; though used more
at the present time, their much higher cost of maintenance, the long
period of observation, and the impracticability of using sufficiently
large numbers put nonrodents at a disadvantage for long-term
bioassays. Also, comparative studies on the metabolism of various
chemicals have not indicated necessarily that primates and dogs are
closer to man in this respect than rodents. Nevertheless, many of the
procedures for long-term bioassays in rodents are applicable to assays
in nonrodents.
Long-term bioassays of the carcinogenicity of environmental
chemicals are carried out to assess a possible risk to man and to
estimate the need for primary preventive measures. Because of the
urgent nature of these problems, it is recommended that a compound of
unknown activity should be tested on two animal species. Although a
positive (that is, carcinogenic) effect in one species is considered
as adequate warning, only negative findings in two species can be
regarded as adequate negative evidence.
Among rodents, the species of choice are undoubtedly the mouse,
the rat, and the Syrian golden hamslet. The European hamster has
recently been introduced, particularly for studies in lung pathology,
but its use cannot be recommended for routine testing. Guineapigs and
rabbits have been used occasionally but they have few of the
advantages of smaller rodents combined with the disadvantages implied
by a much longer life span, higher cost of maintenance, and the
scarcity or absence of inbred strains.
Of the three rodent species of choice, the mouse and the rat have
been more widely used than the hamster. However, the last species has
proved to be an excellent tool for revealing carcinogenic effects in
the respiratory tract and urinary bladder. Furthermore, hamster cells
are widely used for in vitro transformation assays, and, since in
most cases these assays require the back transplantation of the cells
in syngenic hosts, the use of the hamster has recently been extended.
The hamster is generally randomly bred but a pure strain is becoming
available.
The use of inbred strains undoubtedly has the advantage of making
available animals with known characteristics such as an average life
span, and a spontaneous tumour rate with little variability.
Random-bred animals or, even better, animals bred with maximum
avoidance of inbreeding, are considered more resistant to infections
and perhaps more liable than many inbred strains to reveal any
carcinogenic effect on an unsuspected target organ. It is common
experience that carcinogenic activity is more easily recognized by
the increased frequency and earlier appearance of tumours at sites
where tumours occur spontaneously. Inbred strains are often known to
have one or two particularly susceptible organs and the induction of
tumours in these organs could, in part, be regarded as an
intensification of whatever underlies the spontaneous occurrence of
tumours in that organ. A carefully planned experiment must take this
possibility into account, and preference should be given to strains
with a low incidence of tumours. Noninbred strains are inconvenient in
that, in many cases, background tumour incidence in untreated controls
is unpredictable. Moreover, experimental groups initiated at different
times can rarely be compared with each other since inter- as well as
intragroup variation may be considerable.
Hybrid mice of two known inbred strains are excellent as they are
particularly robust and long-lived, but they are, of course, more
difficult to obtain and are likely to be more expensive than inbred
strains.
In selecting the species, it is important to be aware that there
are, within each species, particular susceptibilites; for instance, it
is easier to induce liver tumours in the mouse than in the rat, and
conversely it is much easier to induce subcutaneous tumours in the rat
than in the mouse. The skin of the mouse and the rabbit is possibly
more sensitive to tumour induction by skin painting than that of the
rat and the hamster, particularly when polycyclic hydrocarbons are
used. The hamster is more susceptible to urinary bladder
carcinogenesis than the mouse and possibly the rat.
It is essential that experimental groups should be composed of an
equal number of animals of each sex, since differences in response to
the carcinogenic activity of chemicals are well documented. Thus, the
use of one sex only would not show the full range of activity of the
test chemical.
The group should be sufficiently large to permit statistical
evaluation of the results. The UICC Committee on Carcinogenicity
Testing (UICC, 1969) recommended that the number of animals should be
such that it "would be likely to yield reasonable (e.g. 90%)
statistical significance as between the main induced tumour incidence
and its spontaneous incidence". A sufficient number of animals must be
alive at the time that the first tumour appears and it is suggested
that this number should never be less than 25 per sex.
7.2.1.2 Route of administration
It has been agreed that the chemical under test should be
administered by the route of human exposure. This applies to food
additives and food contaminants in particular, but it is equally
desirable for drugs. This rule cannot always be applied to
environmental or industrial chemicals. It is well known that the three
main routes of exposure in man are inhalation, ingestion, and skin
absorption. Inhalation is often predominant in man but in experimental
carcinogenesis the inhalation route, while highly desirable in
principle, has rarely been used in the past because of lack of
adequately equipped laboratories. However, it is now being widely used
in programmes concerning smoking and health.
When the route of exposure is per os, the chemical is
administered either mixed in the diet or drinking water, or by gavage,
the choice depending on the specific characteristics of the test
chemical; a volatile compound, for example, should never be mixed in
the diet while, for a drug, the route by which it will be used in man
should be selected, i.e. per os, or by intravenous, subcutaneous or
topical application.
7.2.1.3 Inception and duration of tests
Most commonly, tests are initiated in young adult animals from 7
to 9 weeks of age. The start of treatment soon after weaning is
recommended as a routine procedure. One objective of carcinogenicity
testing is to obtain the maximum possible carcinogenic effect of the
test chemical in order to compensate for the limited number of
individuals at risk. This is accomplished by employing high levels of
exposure (see 7.1.4) and by starting the treatment when the animals
are at their most sensitive age. For some years following the pioneer
work of Pietra et al. (1961), treatment immediately after birth and
within the first 24 h of life was thought to be the most sensitive
model. Careful reviews of the data available have indicated that
neonatal treatment alone can be recommended in some instances, but not
as a general routine procedure (Della Porta & Terracini, 1969). For a
single neonatal administration, only a very limited quantity of the
chemical to be tested is needed and this could be advantageous, when
only a small amount of the chemical is available.
The main limitation of the use of a single neonatal treatment is
that the newborn animal may not have developed sufficient metabolic
competence to metabolize the test chemical. Another limitation is that
not all chemical carcinogens are active after one, or even a few
exposures.
More recently, prenatal exposure has received considerable
attention, since it has been demonstrated that some tissues, notably
nervous tissue, are more susceptible to certain carcinogens during
fetal life than later (Druckrey et al., 1967; Napalkov, 1973; Napalkov
& Alexandrov, 1968; Tomatis, 1973). At present, however, it can only
be speculated that prenatal exposure will reveal the carcinogenic
effect of a chemical that would not have been revealed had treatment
started at a later age. A sensitive procedure might be to integrate
prenatal exposure with long-term, postnatal exposure, in order to
obtain maximum sensitivity of the bioassay system. To achieve this,
animals mated at the age of 8-9 weeks, should be exposed to the test
chemical during the second half of pregnancy. The parents so treated
can constitute a group under conventional long-term testing, if the
treatment is continued after delivery. Their offspring, already
exposed in utero, and possibly after birth through their mother's
milk and excreta, should be exposed directly to the test chemical from
the age of weaning onwards. In this way, two different experimental
groups can be obtained: one for which exposure was started at the
young adult stage (the parents) and the other for which exposure was
started prenatally (the offspring) (Tomatis, 1974b).
The duration of a positive test, i.e. where exposure to the test
chemical is followed by an increased incidence of tumours, depends on
the time of appearance and the rapidity of growth of the induced
tumours. To agree in advance on a given duration of exposure is of
prime importance for negative tests. Some research workers have
adopted a duration for carcinogenicity tests of 2 years in rats and 18
months in mice, while others have preferred an observation period
extending over the entire life span of the animals. A long finite
period -- that is, not less than 2 years -- is recommended in
preference to the entire life span of the animals, for the following
reasons: (a) induced tumours usually occur within this observation
period; (b) "spontaneous" tumours appear with highest frequency late
in life and their appearance may make it more difficult to evaluate
the carcinogenicity of a compound, particularly if of low potency;
(c) a few animals may far exceed the normal life span of the species
and extend unnecessarily the duration of the experiment; (d) tests
are very expensive and any justifiable abbreviation means good economy
(Health & Welfare, Canada, 1973; Tomatis, 1974a; UICC, 1969; WHO,
1961).
7.2.1.4 Dose-level and frequency of exposure
If only one level is to be used, the highest dose allowing long
survival of the majority of the animals should be chosen. The
selection of this dose should be made from results obtained in
subacute toxicity tests. Information on the LD50 of certain
compounds may be available and this can be useful in deciding the
level of the dose for the subacute studies.
While it would be very difficult to give a definition of the
maximum tolerated dose, it seems quite reasonable to insist that it
allows adequate survival of the animals and does not produce a
reduction in weight of more than 10% compared with the controls
(Friedman, 1974). If two dose levels are chosen, the second should be
one quarter or one third of the first dose.
If the goal of the test is merely to ascertain the possible
carcinogenicity of a compound, then the assay protocol should achieve
the maximum sensitivity of the test; in this case, the highest
tolerated dose is the most appropriate. If, however, additional
information has to be collected, in particular regarding the
establishment of a possible minimum effect or no-observed-effect
level, then initiation of a dose-response experiment should be
envisaged. In this case, a minimum of three doses, and preferably
four, should be included. An advantage of including several dose
levels, the lowest of which does not decrease the life span of the
animals, is the possibility of collecting other data on chronic
toxicity not otherwise available through a carcinogenicity test at
high dose levels.
Frequency of exposure may vary according to the route chosen. If
the chemical is administered in the drinking water or mixed in the
diet, exposure is continuous. If the chemical is given by gavage, the
frequency can be two to three times per week. Topical applications may
be made daily, while subcutaneous or intravenous injections must be
more widely spaced, e.g. once or twice weekly. The duration of
treatment should cover almost the entire observation period.
7.2.1.5 Combined treatment and cocarcinogenesis
An investigation on the effect of more than one agent, given
simultaneously, may approximate the actual environmental situation,
where there is never exposure to a single chemical. In particular, it
may be useful in revealing the carcinogenic effect of a chemical of
very low carcinogenic potency and thereby help to identify situations
or populations that may be exposed to an otherwise unsuspected high
risk.
Response to an environmental chemical may be modified by the
action of other chemicals that may either alter the rate and/or
pathway of metabolism of the test chemical, or have a cocarcinogenic
effect. A cocarcinogenic effect may be additive, when the chemical has
a carcinogenic effect on its own which adds to the effect produced by
the test chemical; it may be synergistic, when its effect, combined
with that of the test chemical when given alone, exceeds the summation
of the separate effects; or it may act as an incomplete carcinogen,
that is, only as initiator or promoter in the two-stage carcinogenic
process (UICC, 1969) (see Chapter 13).
It is also essential to keep in mind that dietary components may
influence the incidence of tumours in test animals. This may occur
either because of the unsuspected presence of carcinogens (such as
aflatoxins or nitrosamines) or because of substances that may modify
the response to the test chemical by altering, for instance, the
hepatic microsomal enzyme activity.
7.2.1.6 Positive and untreated controls
An adequate group of untreated animals, serving as controls,
should always be included in the planning of a carcinogenicity test.
The size of the control group should never be smaller than the size of
the treated group and, preferably, should be larger. When more than
one chemical is tested simultaneously, the same control group can be
used, provided that its size is appropriately increased.
Besides these controls, i.e. animals not receiving treatment, and
which could be called negative controls, the inclusion of positive
controls in planning an experiment has recently been recommended, i.e.
inclusion of a group of animals receiving a known carcinogen at a dose
level that has already produced carcinogenic effects in several
laboratory studies. Such controls ensure: (a) more confidence in the
outcome of tests carried out on compounds of unknown activity
(Weisburger, 1974); (b) assessment of the relative carcinogenic
potency of the test chemical, and (c) an indirect check on the
reliability of the test laboratory. While it does not seem essential
to include a positive control in every test, it is highly advisable
for every laboratory to check the sensitivity of its bioassay system,
periodically, with selected known carcinogenic chemicals. It is also
advisable that a group of animals should receive only the vehicle
(i.e. acetone, dimethylsulfoxide, etc.) in which the chemical under
test is eventually administered.
7.2.1.7 Test material
Long-term studies should not be started until sufficient
information on the identity and purity of the test chemical has been
assembled. The importance of knowing the toxicity of impurities is
well demonstrated by the case of TCDD present as an impurity in the
herbicide 2,4,5-T. Drugs or food additives should be tested in the
form and degree of purity intended for human consumption (Health &
Welfare, Canada, 1973; National Academy of Sciences, 1960; WHO, 1961,
1969). In this case, as well as for mixtures of chemicals to which man
may be exposed, it may be highly advisable to carry out additional
studies in order to identify positively the carcinogenic component of
the mixture.
7.2.1.8 Survey of animals, necropsy, and histological examination
In order to present results correctly, detailed records of all
experimental procedures and surveillance of the animals during the
entire observation period should be maintained. While all pertinent
details of the experiment may not be published, it is a good rule for
the investigator to keep them for discussion with other interested
scientists (UICC, 1969). If the results are to be published, it is
essential that adequate information be given on the test chemical and
test animal, as well as on all observations made on the experimental
and control groups (WHO, 1961). A detailed list of items to be
considered is given in the UICC (1969) report.
In the case of neoplasms (Turusov, 1973, 1976), it is extremely
important to give an exact description of the criteria used to
classify lesions as hyperplastic, preneoplastic or neoplastic, benign
or malignant. Many times, pathologists do not agree on certain
diagnoses and it is far easier to interpret the results when the
criteria used for classification are known. Terms such as hepatoma,
which do not indicate the specific tissue of origin, should not be
used (Reuber, 1974), while terms such as cholangioma or adenomatosis
require additional qualification. The term should clearly indicate
whether the lesion is considered malignant. Thus, specific
descriptions should be given, e.g. in the case of "hepatoma" it may be
either a liver cell adenoma or a hepatocellular carcinoma, while
"cholangioma" may be cholangiocellular adenoma or cholangiocellular
carcinoma. In addition, carcinomas may be subdivided into well,
moderately, and poorly differentiated carcinemas.
A lesion with atypical cells or with focal malignant change
should be classified separately rather than under malignant tumours.
7.2.2 Short-term tests (rapid screening tests)
To date, there are no reliable alternatives to long-term
bioassays for testing the carcinogenicity of chemicals; this means a
delay of two years or more before a carcinogenicity bioassay yields
results. However, recent advances in mutagenicity testing hold out
hopes of a short-term test, at least for selecting, from among the
many chemicals to be tested, those most in need of early attention. So
far, about 80% of carcinogens have been shown to be mutagenic and with
the continuously increasing sensitivity of the models now employed an
even higher correlation is possible. At present, mutagenicity testing
represents a valuable system for screening chemicals to be submitted
to long-term carcinogenicity testing; it cannot replace long-term
testing, until a satisfactory positive correlation is established and
the possibility of false negatives eliminated.
A mutation is any heritable change in genetic material including
a chemical transformation of an individual gene (point mutation) or a
change involving rearrangement of parts of a chromosome (chromosome
mutation). The question of whether or not a mutagenic event is a
prerequisite for carcinogenesis has long been debated. Many mutagens
have been shown to be carcinogenic but other known carcinogens are
known not to be mutagenic. Other mechanisms not involving DNA have
also been implied in the process of carcinogenesis (Pitot, 1974).
Recent short-term mutagenicity testing procedures (McCann & Ames,
1976: McCann et al., 1975; Sugimura et al., 1976) have shown greater
correlation between mutagenicity and carcinogenicity; consequently,
some thought is now being given to using these assays as preliminary
screens for potential carcinogens. However, at the moment, the fact
that a particular compound has mutagenic activity can only be
considered, in terms of carcinogenicity, as indicative of potential
reactivity with DNA.
7.2.2.1 Metabolic activation, reaction with DNA, and DNA repair
The synthetic and naturally occurring chemical carcinogens
include a variety of chemicals having no common structural feature.
However, it is becoming clear that the ultimate reactive forms of many
chemical carcinogens are electrophilic (electron-deficient) reactants
(Miller, 1970). Although some chemical carcinogens, such as direct
alkylating agents and metal ions, are electrophiles per se, the
majority require metabolic activation to reactive forms (ultimate
carcinogens) (Fig. 7.1).
The formation of these electrophilic reactants from exogenous
chemicals, in an animal species or in an organ, is the result of the
balance between in vivo activation and deactivation reactions
carried out predominantly by enzymes localized in the endoplasmic
reticulum of the cell. The ultimately available concentration of
reactive metabolites seems to account for some of the organ and
species specificities shown by several chemical carcinogens.
During the last decade, much progress has been made in
understanding the metabolism of various classes of chemical
carcinogens (polynuclear hydrocarbons, Sims & Grover, 1974;
N-nitroso compounds, Magee et al., 1976; aromatic amines, Kriek,
1974; Weisburger & Weisburger, 1973; miscellaneous compounds, Miller,
1973). The activity of microsomal mixed function oxidases is
influenced by various drugs or environmental chemicals, that may
stimulate or inhibit the formation of the ultimate carcinogens (Conney
& Burns, 1972).
These ultimate carcinogens bind covalently with cellular
macromolecules such as DNA, RNA, or proteins, which, directly or
indirectly, leads to heritable changes in the affected cells. Although
the critical targets of chemical carcinogens are not known, there is a
great deal of evidence supporting the theory that DNA is one major
target in carcinogenesis (Farber, 1973).
Some specific binding sites for the metabolites of carcinogens
have been identified in the bases of DNA. The carcinogenic N-nitroso
compounds are examples (Magee et al., 1976). The main site of
alkylation of DNA by alkylnitroso compounds is the N,7 position of
guanine, as with other alkylating agents. Other sites are also
attacked and the significance of these DNA interactions is being
investigated. The formation of 7-alkylguanine, however, seems to be of
no obvious importance in the process of tumour induction, since no
quantitative or qualitative correlation between the occurrence of this
alkylated base in the DNA of treated animals and the carcinogenicity
of nitrosamines or alkylating agents has been observed (Magee et al.,
1976). Alkylation of guanine bases in DNA at the 7-position does not
seem to produce mutations in bacteriophages (Loveless & Hampton,
1969), nor does it alter the coding properties of synthetic
polynucleotides in vivo (Ludlum, 1970). Various laboratories have
examined the biological importance of the alkylated O,6 position of
guanine residues in DNA which, in consequence, is able to induce
mutations in phage (Loveless, 1969). It has been shown that there is
an aberrant base pairing with a polymer containing alkylated
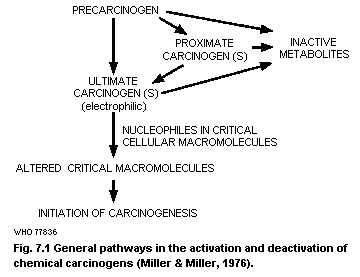 O,6-guanine residues (Gerchman & Ludlum, 1973). Goth & Rajewsky
(1974) showed, in vivo, that the initial degree of alkylation at the
O,6 position in the DNA was, apparently, not correlated with the
tissue-specific carcinogenicity of ethylnitrosourea which induces
brain, but not liver, tumours. However, O,6-ethylguanine persists
much longer in brain DNA than N,7-ethylguanine. The O,6-alkyl
elimination is also much slower from brain than from the liver DNA.
From these findings it becomes apparent that variations in the
sensitivity of different organs to the carcinogenic action of
N-nitroso compounds might be attributed to different enzyme
activities capable of repairing lesions in cellular DNA and/or to
different rates of cell division in the target and non-target organs
(Craddock, 1976; Margison et al., 1976; Pegg & Nicoll, 1976; Pegg,
1977).
Possibly these repair systems, which recognize damaged DNA, are
also impaired in the case of some types of cancer in man. The skin of
patients suffering from the hereditary disease xeroderma pigmentosum
is extremely sensitive to sunlight and such persons have a very high
incidence of skin cancer. It has been shown that the cells of the skin
of these patients are deficient in the capacity to repair UV-damaged
DNA and that this deficiency is caused by lack of enzymes required for
the excision of damaged regions from DNA (Cleaver, 1969).
Some chemicals cause changes in the biological properties of DNA
without covalent binding, e.g. by intercalation which could cause
frameshift mutations. Since radioactive labelled chemicals are needed
for most of these studies, technical problems associated with these
methods render the tests impractical for routine screening. However,
they will continue to contribute considerably to the understanding of
the mechanism of interaction of a chemical or its activating
metabolite with specific sites in DNA.
Initial lesions in DNA can either lead to a permanent change,
such as a mutation, or can be removed by cellular repair processes. In
bacteria and mammalian cells, three major repair processes can be
considered. First, the photoreactivation repair process that repairs
only UV damage to DNA and involves a direct enzymatic cleavage of
pyrimidine dimers to monomers upon exposure of cells to visible light.
This repair process is present in most prokaryotes and eukaryotes and
it was recently identified in human cells (Sutherland et al., 1975).
The second process, post-replication repair, which is also called
recombination repair, is limited to the DNA synthesis period. Although
its mechanism is not well known, it has been proposed that it is
caused by the presence of damaged bases on parental DNA strands too
close to the replication fork to be recognized by the third process,
excision repair. Unlike post-replication repair, excision repair
occurs throughout the cell cycle and is present in a large variety of
organisms. During this process, damaged bases are excised, producing a
single-strand break. This gap is repaired by replacing the original
bases with bases complementary to those of the opposite intact strand.
Various enzymes are involved in this process, namely endonuclease, a
DNA exonuclease, a DNA polymerase and a DNA ligase (Cleaver, 1974).
From studies on prokaryotes, the concept arose that excision
repair is error-free (Kondo, 1973). It is thought that mutations
originate during semi-conservative replication as a consequence of the
fact that DNA templates containing damage have not yet been or cannot
be repaired by excision repair. Since DNA replication on damaged
templates involves post-replication repair, this repair process is
considered error-prone and responsible for mutagenesis. Whether
miscoding and mutagenesis of DNA templates is the result of lack of
excision repair enzymes or due to error-prone post-replicative repair
is not clear at present.
The isolation of xeroderma pigmentosum-variant fibroblasts, that
have normal excision repair but are deficient in post-replication
repair (Maher et al., 1976) may lead to a better understanding of the
role of these repair processes in mutagenesis and carcinogenesis.
Recent efforts have been devoted to the development in mammalian
systems of a screening test for the detection of possible mutagens or
carcinogens based on excision repair. The focus on this type of repair
is mainly based on the greater availability of procedures thought to
be appropriate to the problem. There are four different ways of
examining this form of repair in mammalian systems and all are based
on the assumption that chemical mutagens or carcinogens interact with
DNA, inducing molecular alterations which result in DNA repair that
can be measured as unscheduled incorporation of various DNA precursors
(Cleaver, 1975; Legator & Flamm, 1973). The common basis of all these
tests is the differentiation between repair and normal
semiconservative synthesis of DNA.
One procedure, "unscheduled DNA synthesis", involves
autoradiography of cultured cells, and consists of the incorporation
of precursors during resynthesis of short nucleotide sequences that
have been eliminated from DNA strands following their damage by
chemicals (Cleaver, 1973; Stich & San, 1970). This procedure permits a
quantitative evaluation of DNA repair synthesis in various types of
cell in a tissue and can be applied to an in vivo system (Stich &
Kieser, 1974), thus allowing the detection of indirect mutagens and/or
carcinogens.
Another process uses 5-bromodeoxyuridine (BrdU), which is an
analogue of thymidine. During normal replication, the incorporation of
BrdU produces a DNA with a high buoyant density; this does not occur
when BrdU is incorporated in DNA during the repair synthesis. Thus, by
using radiolabelled BrdU, or radiolabelled thymidine and non-labelled
BrdU, it is possible to differentiate, on cesium chloride density
gradients, semi-conservative replication of DNA from the repair
synthesis according to the different buoyant densities.
A third procedure also uses BrdU but the differentiation between
semi-conservative and repair DNA synthesis is done by different means.
Cells exposed to the chemicals are incubated with BrdU and the DNA is
subjected to long wavelength ultra-violet radiation. Breaks appear in
the DNA at the sites of incorporation of BrdU and cause slower
sedimentation of the DNA in alkaline sucrose gradients (Smith &
Hanawalt, 1969). This technique appears to be a very sensitive one for
measuring single-strand breaks but it still seems to be prone to
artefacts and cannot be considered suitable for large-scale studies
(Cleaver, 1975).
Another method involves the total suppression of normal DNA
synthesis, for example, by hydroxyurea, thus the incorporation of
precursors into DNA reflects only repair synthesis and not normal
replication.
Some of the above techniques are time-consuming, costly and not
yet standardized, so that it is difficult to adapt them for
large-scale studies. The most promising approach appears to be the
measurement of unscheduled DNA synthesis, but criticisms have also
been made of the use of this system as an indicator of mutagenic or
carcinogenic potential (Cleaver et al., 1975). One limitation is that
unscheduled synthesis is an average measure of repairable damage in
all cells of a population or in all sites of the DNA, whereas
mutagenesis and carcinogenesis seem to depend on the amount of
unrepaired damage in a small percentage of cells and in a few specific
DNA sites. Due to these limitations and to the current limited
understanding of these processes, none of these variables could, by
themselves, be a reliable indicator of a potential mutagenic or
carcinogenic chemical. However, these studies associated with studies
of DNA damage as well as of the expression of this damage are
essential in the understanding and development of reliable screening
systems.
Metabolic activation of chemicals to electrophiles, DNA damage,
and subsequent repair processes are important factors in the
initiation of cancer. The phenotypic expression (tumour development)
of these initial molecular changes is modulated by a number of factors
(Farber, 1973) that influence the macroscopic appearance of cancer at
a later stage (Pitot, 1977). However, the initial molecular changes,
induced by chemical carcinogens, appear prerequisite for initiation of
the cancer process. Further studies on the alteration of DNA induced
by chemical carcinogens and on the repair of such lesions before cell
division by tissues, whether target or not, are required to evaluate
the role of DNA repair processes in chemical carcinogenesis.
7.2.2.2 In vitro neoplastic transformation of mammalian cells
The term transformation in such studies refers to the in vitro
observations of various changes present in the cells treated in vitro
with the chemicals, when compared with untreated control cells.
Transformed cells may differ from control cells in various ways such
as: (a) alteration of cellular and colony morphology; (b) increased
plating efficiency; (c) agglutinability by plant lectins; (d) altered
glycolytic patterns; (e) resistance to toxicity of some chemicals; (f)
altered surface properties (contact inhibition of movement and growth,
population density, growth in suspension, ability to grow in agar or
other semisolid media); (g) sensitivity to activated macrophages and
lymphocytes; (h) establishment of cell lines with the potential to be
sub-cultured indefinitely in vitro; and (i) appearance of new
antigens (Fedoroff, 1967). However, the unequivocal criterion of
malignant transformation is the capacity of transformed cells to
develop a malignant neoplasm when injected into a syngenic,
immunosuppressed or thymus-free host, or into privileged sites
(Giovannella et al., 1972; Sanford, 1965).
Recently, Sanford (1974) critically reviewed the significance of
these various in vitro changes in relation to the acquisition of
neoplastic potential. It was stressed that these criteria of
neoplastic transformation cannot completely replace the in vivo
assay for tumour production. With reference to the problems of the
application of tissue culture to the rapid detection and
characterization of neoplastic transformation in vitro, the reader
is referred to the proceedings of the symposium "New Horizons for
Tissue Culture in Cancer Research" ( J. Natl Cancer Inst., 1974).
In most in vitro transformation experiments the cells used have
been fibroblasts. Earle & Nettleship (1943) reported the
transformation by 1,2-dihydro-3-methyl-benz[j]aceanthrylene
(3-methylcholanthrene) of a long-term culture of fibroblasts as
demonstrated by the development of tumours after inoculation of the
cells into mice. However, this observation was attributed to
spontaneous transformation, since Sanford et at. (1950) observed that
the cells were tumorigenic also without treatment with chemicals. The
first clear demonstration of transformation of cells in culture by
chemicals was that of Berwald & Sachs (1963) who observed the
transformation of Syrian hamster embryo secondary cells with
3-methylcholanthrene and benzo(a)pyrene, but not with urethane or
solvent. Since this report, various cells types originating from
different animal species have been used for in vitro transformation.
This topic has recently been reviewed by Heidelberger (1973) and
Kuroki (1975), who critically examined the advantages and
disadvantages of the various strains and lines of fibroblastic cells,
namely of the Syrian hamster embryo, fibroblastic cells derived from
mouse ventral prostate, cell lines derived from embryo cells of Swiss
mouse BALB/c, C3H, AKR, and C57/B1 strains, Chinese hamster lung
cells, as well as various tissues from organ cultures. Transformation
of the above cell lines was obtained with polynuclear hydrocarbons and
with chemicals that do not require metabolic activation.
Some chemical carcinogens that are active in vivo have failed
to induce transformation, when applied directly to hamster embryo
cells; this is presumably because such compounds require metabolic
conversion to active intermediates that are lacking or are present in
insufficient concentration in these cells. However, neoplastic
transformation was detected in fibroblasts obtained from embryos whose
mothers had been exposed to indirect carcinogens during pregnancy
(Di Paolo et al., 1972, 1973). A similar approach was used by Borland
& Hard (1974), who cultured kidney cells at various times following
in vivo treatment of rats with N-methyl- N-nitrosomethanamine
(dimethylnitrosamine). The cells isolated from treated rats showed
various morphological and behavioural changes associated with
transformation. Laerum & Rajewsky (1975) reported the development of
glioblastomas following injection of glial cells originating from the
brain of rat embryos, the mother having been treated with
ethylnitrosourea during pregnancy.
Epithelial cultures from rat liver were recently established in
various laboratories and used for transformation studies (Iype, 1974;
Katsuta & Takaoka, 1972; Montesano et al, 1975; Weinstein et al.,
1975; Williams et al., 1973). The transformation of these cells by
various carcinogens that need metabolic activation was determined by
the development of carcinomas after their back-transplantation into
suitable hosts. Carcinomas were observed following inoculation of
epithelial cells from mouse skin, or rat urinary bladder or salivary
glands, treated with chemical carcinogens in vitro (Brown, 1973;
Fusenig et al., 1973; Hashimoto & Kitagawa, 1974).
One disadvantage of these epithelial cells is that the
morphological criteria for transformation of fibroblast cultures
(piling up of cells, crisscross arrangement of cells etc.) do not
apply possibly because normal epithelial cells have little or no
locomotion and they continue to divide even when in close contact with
other cells (Weinstein et al., 1975). However, the capacity for growth
in soft agar appears to provide a reliable and reproducible
correlation with the tumorigenicity of these cells (Weinstein et al.,
1975; Montesano et al., 1977). Although, at present, this system
provides only a qualitative and not a quantitative assay of in vitro
transformation, it is the only instance of unequivocal production of
carcinoma. The establishment of reliable criteria, measurable in
vitro, for distinguishing normal from transformed epithelial cells
in culture is essential for the development of quantitative systems
for the transformation of these epithelial cells. Recently the
neoplastic transformation of human diploid cells by chemical
carcinogens has been described (Kakunaga, 1977).
Cell culture has been extremely useful in elucidating the
cellular and molecular mechanism of chemical carcinogens and it holds
great promise as a test for screening for the potential
carcinogenicity of environmental chemicals. However, some time is
needed before this test may be used for routine testing in a
reproducible way.
7.2.2.3 Mutagenicity tests
The growing experimental evidence linking the carcinogenic
activity of numerous chemicals with their capacity to be converted
into electrophilic derivatives, that may also exert a mutagenic
effect, has led to the suggestion that a relationship between chemical
carcinogenesis and mutagenesis may exist (Miller & Miller, 1971a,b).
Such a correlation has so far been limited to those changes of the
genotype that appear as a consequence of structural or functional
alterations of nucleic acids. Not all chemical mutagens have been
shown to be carcinogenic. However, most chemical carcinogens, several
of which cause cancer in man, have now been found to be mutagens, when
tested by one of the mutagenicity test procedures that combine
microbial, mammalian, or other animal cell systems as genetic targets
with an in vitro or in vivo metabolic activation system. The
growing empirical relationship between chemical mutagens and
carcinogens does not imply that the two processes are identical, but
it offers a promising method for the use of mutagenesis as a rapid
prescreening assay for carcinogenesis (Bartsch & Grover, 1976;
Bridges, 1976; Council of the Environmental Mutagenic Society, 1975;
IARC, 1976; McCann & Ames, 1976; Purchase et al., 1976; Stoltz et al.,
1974; Sugimura et al., 1976; WHO, 1974).
The choice of mutagen-detecting assay depends on various
considerations, i.e. the chemical structure and pharmacological
activity of the chemical, the type of human exposure, and the nature
of the population at risk. The same considerations should be taken
into account in assessing the strength of the evidence of mutagenicity
and its relevance to man.
7.2.2.4 Submammalian assay systems
Bacterial phages have been used to test reactive forms of
chemical carcinogens. Chemicals inducing point mutations can be
detected by reacting the test compound with the free phage or with
phage during its duplication inside bacteria (Corbett et al., 1970;
Drake, 1971).
In the bacterial transformation of DNA (Freese & Strack, 1962),
genetic information contained in the DNA isolated from one strain of
bacteria can be transformed to that of a recipient strain. Purified
bacterial DNA is readily accessible to reactive forms of mutagens.
Thus, this system has been used to quantify the mutagenic potential of
a number of chemicals. Extensive studies have been made on the
inactivation of Bacillus subtilis DNA and transformation of the
tryptophane-requiring strain T-3 (Herriott, 1971; Maher et al., 1968).
The reverse mutationa system of Salmonella typhimurium uses
the genetically well-defined histidine-requiring mutants developed by
Ames and his colleagues (Ames, 1971; Ames et al., 1972a,b, 1973;
McCann et al., 1975).
These revert to prototrophy by single-base pair substitutions,
e.g. strain TA1535 or by base pair insertion (frameshift), e.g.
strains TA1536, TA1537 and TA1538. Most of the theoretically possible
types of mutation may be detected with a set of these test strains,
where a mutation of one of the genes responsible for excision repair
(UVrB) has produced a 100-fold increase in sensitivity. Penetration of
larger molecules through the bacterial cell walls has also been
facilitated by the use of deep-rough mutants deficient in the exterior
polysaccharide coat. Two newly developed test strains TA100 and TA98
were obtained by transferring an ampicillin resistance factor
(R factor) to the standard test strains TA1535 and TA1538,
respectively (McCann et al., 1975). These strains are effective in
detecting classes of mutagens that were not previously detected with
the original strains. The tests are usually performed by adding a few
crystals or a drop of solution of the test chemical in
sulfinylbis[methane] (dimethylsulfoxide) or water to a uniform lawn of
one of the histidine-requiring mutants on the surface of a Petri plate
containing histidine-poor medium, or by incorporating the test
compound, a postmitochondrial tissue fraction, cofactors (NADPH or
NADPH+ and glucose 6-phosphate) and the bacterial test strain in
histidine-poor soft agar. For general mutagenicity screening, a liver
homogenate (9000 g supernatant) from rats induced with a mixture of
polychlorinated biphenyls (Aroclor 1254) is recommended as a metabolic
activation system. For routine testing, the strains TA1535, TA1537 and
TA1538 can be used in combination with the strains carrying the R
factor (TA100 and TA98). This method of testing using various groups
of chemicals and various experimental conditions is described in more
detail by Ames et al. (1975).
Using the genetically well characterized Escherichia coli
strains, reverse and forward mutation can be scored using nutritional
resistance or fermentative markers (Bridges et al., 1972; Mohn, 1973).
Prophage lambda induction in E. coli strains by chemical mutagens
activated by liver microsomal enzymes has been described as a
sensitive test for the detection of potential mutagens and carcinogens
(Moreau et al., 1976).
a Mutations in which the function of a given gene is lost are
called forward mutations. Mutations that bring about the
restoration of gene function are called reverse mutations or back
mutations.
Mutagenesis has been extensively studied in Neurospora crassa.
Heterokaryon has been developed, which is heterozygous for two
closely-linked loci in the ad-3 region. Mutants at the ad-3A and
ad-3B loci have a requirement for adenine and can be selected
directly on the basis of accumulation of a reddish-purple pigment in
the mycelium. The ad-3 mutations can be characterized by a series of
simple genetic tests to distinguish point mutations from deletions and
to obtain a presumptive identification of the genetic alterations in
the point mutations at the ad-3B locus at a molecular level (de
Serres & Malling, 1971).
Saccharomyces cerevisiae and S. pombe have been used to
investigate the effects of carcinogenic and mutagenic compounds in
these organisms. Mitotic gene conversion, in which a sequence of a few
hundred nucleotides of one chromosome is replaced by one corresponding
sequence from a homologous chromosome, can be studied in diploid cells
of the yeast S. cerevisiae strain D-4 heteroallelic at the loci
trp-5 and ade-2. This strain carries two different inactive
alleles of two genes ( trp-5 and ade-2) which are located on
different chromosomes and the functional defects of which lead to a
nutritional requirement. Mitotic gene conversion, which is increased
by many types of mutagenic treatment, can transfer the intact region
of one of these alleles to the defective region of the other, thus
producing a heterozygotic diploid cell with full functional activity.
The mechanism is presumably based on the formation of single-strand
breaks in DNA and probably involves repair processes. Positive results
are only obtained with agents or metabolites which either bind with
DNA covalently or interfere with DNA metabolism. In contrast with most
microbial mutation systems, mitotic gene conversion does not show a
response specific to any type of mutagen. In addition to gene
conversion, forward and reverse mutation can be measured with
S. cerevisiae (Loprieno et al., 1976; Marquardt, 1974; Mortimer &
Manney, 1971; Zimmerman, 1973).
7.2.2.5 Mammalian somatic cells
The application of cultured cell lines is somewhat restricted at
present by requirements for karyotypic stability and high plating
efficiency. A widely used system developed by Chu (1971) employs cell
lines derived from the lung, ovary, and other tissues of Chinese
hamster, which usually maintain a near-diploid chromosome number and
exhibit active growth and high cloning efficiency. Two reviews discuss
this topic in detail (De Mars, 1974; Thompson & Baker, 1973).
Selective media have been developed to detect both forward and reverse
mutations at three genetic loci involving enzymes in the salvage
pathways of purines and pyrimidines. In Chinese hamster, as in man,
the use of preformed hypoxanthine and guanine is controlled by an
X-linked gene. Mutant cells at these loci are deficient in the enzyme
hypoxanthine-guanine phosphoribosyl transferase and are resistant to
certain purine analogues. Similarly, mutant cells deficient in
adenosine phosphoribosyl transferase cannot metabolize preformed
adenosine or its analogues for incorporation into nucleic acid. The
third type of drug-resistant mutants currently studied in these and
other mammalian cells are those deficient in thymidine kinase. Such
cells exhibit resistance to the thymidine analogue,
5-bromodeoxyuridine. Cells carrying mutation at these loci can be
selected from the wild type in an environment containing an
appropriate purine or pyrimidine analogue. Revertants to the wild type
can be recovered in selective media in which the cells are supplied
with a natural purine or pyrimidine while the normal de novo pathway
of purine or pyrimidine synthesis is inhibited by an antimetabolite.
It has been demonstrated that Chinese hamster cells treated with
physical or chemical mutagens undergo a significant increase in the
frequency of forward and reverse mutations compared with the
spontaneous frequency (Huberman & Sachs, 1974; Huberman et al., 1971,
1972). A liver microsomal activation system can be added for the
metabolic activation of chemicals (Krahn & Heidelberger, 1975; Kuroki
et al., 1977).
Forward mutations from thymidine kinase +/- cells to thymidine
kinase -/- have been induced by treatment of cells with X-rays or
chemical mutagens (mouse lymphoma L5178 Y cells). These cells can also
be grown in the presence of in vitro or in vivo metabolic
activation systems, which makes them suitable for host-mediated
mutagenicity tests (Nahas & Capizzi, 1974).
Forward mutations to hypoxanthine-guanine phosphoribosyl
transferase deficiency (resistance to 8-azaguanine) in diploid human
fibroblasts in culture have been shown to occur either spontaneously
or after X-radiation (Albertini & De Mars, 1973). Chemical induction
of forward mutation from hypoxanthine-guanine phosphoribosyl
transferase - to + in human lymphoblastoid cell line has also been
reported (Sato et al., 1972).
7.2.2.6 Host and tissue-(microsome) mediated assays
These take into account the conversion of chemicals into
mutagenic metabolites, and are particularly suitable for the detection
of chemicals that are not mutagenic per se but require metabolic
activation. They can be performed in vivo (host mediated) or in
vitro (tissue mediated) using various indicator organisms, such as
bacteria, fungi, yeast, or mammalian cells.
In the host-mediated assay, the indicator organisms are injected
into the interperitoneal cavity of an animal (Legator & Malling,
1971), which is then treated with the test compound by another route.
After a given length of time, the animal is killed and the indicator
organism is recovered and scored for mutants. Comparison between the
mutagenic action of the compound on a test strain directly and the
host-mediated assay indicates whether the host can activate or
inactivate the test compound. The limitations of the host-mediated
assay are the high spontaneous mutation rates of the indicator
organism in the host, the host's effect on cell survival and the
selection of heterogenic cell populations. In order to measure a
significant increase over the spontaneous mutation rate, doses well
above LD50 have to be given to rats or mice. This type of assay is
also limited since it does not identify the site of conversion to a
mutagenic metabolite. Modifications have been reported (Mohn, 1973).
In the tissue-mediated assay, the indicator organisms are
incubated in vitro in the presence of a tissue fraction plus
appropriate cofactors and the test compound. The mutants are
subsequently isolated and scored.
Ames et al. (1973a,b) have developed a mutagenicity assay that
combines a Salmonella strain, a liver microsomal preparation, and
cofactors in a soft agar layer on a Petri dish. Bartsch et al.
(1975b), Czygan et al. (1973) and Malling (1974) have described a
liquid incubation system where bacteria, a tissue preparation, and the
test compound are incubated in liquid suspension. Test compounds that
are gases at room temperature or are volatile can be assayed by
exposing Petri dishes containing bacteria, tissue fraction, and
cofactors to a mixture of gas and oxygen at 37°C (Bartsch et al.,
1975a). The mutagenicity of urinary metabolites, excreted as
conjugates in experimental animals treated with an indirect mutagen,
may be detected by treating the urine with hydrolytic enzymes in the
presence of a bacterial test strain or yeast, and an in vitro
metabolic activation system (Commoner et al., 1974; Durston & Ames,
1974; Marquardt, 1974). In another modification, Huberman & Sachs
(1974) used lethally irradiated rat fibroblasts that retain a
drug-metabolizing capacity and cocultivated them with Chinese hamster
V79 cells which are used as genetic indicators.
The mutagenicity tests previously described (7.2.2.4, 7.2.2.5,
7.2.2.6) all have individual advantages and limitations determined
either by the genetic indicators or by the metabolic activation
system. The use of submammalian organisms for the detection and
classification of mutants, induced by chemicals, is greatly
facilitated by the relatively small genome to which fine genetic
mapping and biochemical analysis can be applied and by the short
generation time. Huge populations of some of these organisms can be
raised and they can easily be handled when analysing multiple
mutations.
The relevance of such data from submammalian systems to mammalian
cells is based on the assumption that the basic principles of
heredity, and the structure and functions of DNA in terms of
reactivity, the triplet code, transcriptional, and translational
mechanisms, are essentially the same for all living cells irrespective
of their evolutionary level. However, this extrapolation is hampered
by lack of knowledge of repair processes that play a role in mutation
fixation and expression and are insufficiently understood in mammals.
It is obvious that normal human diploid cells are most desirable for
this test, but current studies suggest that reliable conclusions can
be drawn from results with non-human mammalian cells.
The other factor, related to the fact that many chemicals are not
active per se, is the metabolic activation of the compounds. In many
cases, reactive metabolites with a limited life span may fail to reach
or react with the genetic indicator either because they are further
metabolized to inactive compounds, or because they react with other
cellular constituents. For this reason, mutagenicity assays in intact
animals (host-mediated assays) may give negative results, as in the
case of N-methyl- N'-nitro- N-nitrosoguanidine (MNNG) and acridine
mustard (ICR-170), proven to be extremely potent mutagens. Metabolism
in animals is affected by exogenous and endogenous factors such as
chemicals causing enzyme induction and inhibition. Other modifying
factors are age, sex, and strain of animals, diurnal and seasonal
rhythms, differences between the fetal and adult state, mode of
administration, cellular uptake, and distribution and excretion of the
chemical.
The tissue-mediated mutagenicity test cannot, with certainty,
reproduce the in vivo situation, but obvious advantages are the high
sensitivity, good reproducibility, low cost, and the possibility of
testing a large number of chemicals. In addition, in vitro testing
with well-characterized genetic indicators allows the use of human
tissue such as human liver to determine their ability to generate a
mutagen (Bartsch, 1976).
7.2.3 Correlation between short and long-term bioassays for
carcinogenicity
Short-term tests (rapid screening tests) are procedures that do
not have the in vivo production of a visible tumour in animals as an
end point. The variables used in short-term tests to detect chemical
carcinogens are based on an interaction of carcinogens and/or their
metabolites with macromolecules, the induction of chromosomal
aberrations, mutagenesis, DNA repair, and DNA binding.
In the evaluation of these methods, the major question is which
screening tests, singly or collectively, serve as reliable indicators
or predictors of the potential carcinogenic hazard of the chemical.
The answer can only be obtained by testing a representative number of
compounds. A valid test should demonstrate that compounds with known
carcinogenic properties are positive, within the limit of the test,
and negative compounds are negative. If a test is required for
preliminary screening, a small proportion of false negatives or
positive results may be acceptable, but for a final test, no false
negative results are acceptable.
Rapid screening tests should be relatively simple and
inexpensive. They can be used in three ways: to trace carcinogens
and/or mutagens in the complex environment of man; as a tool for
prescreening chemicals to be submitted to a more lengthy set of
bioassays; or for better extrapolation from experimental animal data
to man and for improving the relevance of long-term bioassays in
experimental animals.
Considering reproducibility, cost, the number of chemicals that
can be examined in a short time, and the scientific basis of the test,
tissue-mediated mutagenicity procedures using well-characterized
genetic indicators and a metabolically defined in vitro activation
system, appear at present to be the most promising short-term tests.
With current methods there is still the chance of false negative
results, depending on the systems used, either for lack of appropriate
cofactors for activation or because of the extreme reactivity and/or
toxicity of the compound or its metabolites. However, the number of
false negative results in in vitro tissue-mediated assays is small
compared with other mutagenicity test procedures (Montesano & Bartsch,
1976).
The increasing evidence of a possible correlation between
mutagenicity and carcinogenicity certainly does not mean that one
biological effect may be equated with another. Thus, the mutagenic
activity of a chemical cannot, at present, automatically be assumed to
imply a definite carcinogenic effect in man, nor can these results
replace long-term carcinogenicity testing in animals.
Furthermore, it is still unknown whether all carcinogens will be
found to be mutagenic, and all mutagens, carcinogenic. Examples are
the strong mutagens, nitrous acid, hydroxylamine, and base analogues,
for which no carcinogenic effect in animals has so far been reported;
they do not act via electrophilic intermediates, a mechanism that has
now been recognized for must ultimate carcinogenic forms. Nor have
steroidal sex hormones, carcinogenic in animals, been reported to be
mutagenic, as yet. On the other hand, various chromium salts have
recently been shown to be mutagenic in bacteria (Venitt & Levy, 1974).
Development of cancer in vivo is determined by a variety of
factors that cannot be duplicated in an in vitro short-term testing
system because:
(a) The concentration of ultimate reactive metabolites available
to react in organs in animal species with cellular
macromolecules, which is a consequence of a balance between
metabolic activation and detoxication processes, is only
partly reflected by in vitro testing procedures.
(b) Species and organ specificity of a chemical carcinogen might
be determined, in part, by organ-specific DNA repair.
(c) Since chemical carcinogenesis is thought to be a multi-step
process in which the early, apparently irreversible,
initiation of a cell is followed by several subsequent
stimuli provoking cellular replications, leading to an overt
tumour, a short-term test capable of detecting complete
carcinogens may be useful to detect initiating agents but
cannot at the present time detect the action of promoting
agents.
Currently used short-term tests, in particular tissue-mediated
mutagenicity assays are effective in predicting, with a certain
accuracy, the carcinogenic potential of chemicals (McCann & Ames,
1976; Purchase et al., 1976; Sugimura et al., 1976), but give no
indication of the target organs or species specificity of their
carcinogenic activity. Furthermore, the relative potency of a chemical
to induce biological effects, defined as the endpoints, for the most
frequently used rapid screening tests cannot at the present time be
reliably correlated with its carcinogenic potency (Bartsch et al.,
1977; Meselson & Russell, 1977). More data are needed to compare
dose-response curves obtained in rapid screening tests on a given
chemical with those of other biological effects obtained in vivo
(long-term bioassays).
7.2.4 Significance of experimental testing for assessing the possible
carcinogenic risk of chemicals to man
Experience acquired, so far, in long-term carcinogenicity testing
has shown that nearly all compounds that are carcinogenic in man are
also carcinogenic in one or several animal species, even though the
tumour type may not be the same as in man. The concept that animal
carcinogenicity data are predictive of a human carcinogenic risk and
useful in preventing human cancer was accepted in 1941 in the case of
2-actylaminofluorene (AAF). This chemical was not used on a worldwide
basis as an insecticide, because some experiments had already shown
its carcinogenicity before it was marketed (Wilson et al., 1941); its
restriction was facilitated by the existence of a number of
substitutes at the time when the results of the first experiments on
AAF were reported. This cautious attitude has not been consistently
applied in other situations, on the grounds that the experimental data
were insufficient or inadequate to evaluate the possible hazard to
man. This is shown by the various relationships between experimental
carcinogenicity data and possible human hazard that are considered in
the adoption of preventive measures.
The first observation that some aromatic amines were involved in
the causation of urinary bladder tumours in man dates from 1896; it
was confirmed in 1907 and reconfirmed many times thereafter (IARC,
1974). In 1938, Hueper et al. reported the induction of bladder cancer
in dogs exposed to 2-naphthalenamine (2-naphthylamine). Preventive
measures were taken only in the late 1950s, when conspicuous
epidemiological evidence had accumulated. In this case, the human
epidemiological evidence was judged insufficient, and the experimental
evidence, when it became available, was also deemed insufficient until
further epidemiological findings came to hand.
The delay in taking preventive measures applies also to
carcinogens on which, contrary to the case of aromatic amines,
experimental evidence preceded observations of their effect in man,
e.g. diethylstilbestrol, bis(chloromethyl)ether, and chloroethylene
(vinyl chloride). (Tomatis et al. 1978; Montesano & Tomatis, 1977).
One of the main objections to carcinogenicity tests on animals is
that the experimental system used tries to produce the maximum
possible carcinogenic effect of the test chemical, does not reflect
the human situation, and is therefore misleading. It is difficult,
however, to accept this argument because, in most instances where a
chemical was found by epidemiological investigations to be associated
with cancer in man, the incidence was so high that the association was
clear without animal studies. This has been the case for high risk
groups such as occupational cancer groups. However, the risk is not
confined to these groups but also applies to other populations where
cancer incidence may be too low for detection by normal
epidemiological methods, hence the need to carry out animal
experiments under conditions that permit confident judgment of the
carcinogenicity or inactivity of a chemical.
Cancer testing in animals has reached a relatively sophisticated
stage and an exhaustive study of a chemical in animals is sufficient
evidence of a potential cancer risk for man. An assessment of the
validity of experimental results is essential for the successful
prevention of cancer in man. This does not preclude further research
for the development of short-term tests, but, at the present time,
these cannot replace long-term carcinogenicity testing.
7.3 Heritable Mutations
As noted in the introduction, direct methods for assessing
whether a chemical has the potential to cause heritable mutations in
man do not exist at present. Nontheless, information relative to the
possible production in man of germ cell mutations can be derived from
a variety of sources. These fall into three major categories: (a)
primary DNA damage involving DNA alteration, stimulation of DNA
repair, gene mutations tests including mutagenicity assessment using
bacterial or other microorganisms, with and without metabolic
activation; (b) whole animal tests for point or gene mutations
including insects, e.g. drosophila recessive, and the specific locus
test in mice; (c) chromosomal mutations including cytogenetic tests in
mammals, the dominant lethal test in mammals, and the heritable
translocation test in rodents. Examples of the first category have
already been presented. These tests provide information relevant to
heritable mutations and section 7.2.2 should be consulted for fuller
details. Obviously, tests employing isolated systems only provide
information on mutagenic action on the specific systems tested. This
information cannot be fully interpreted without evidence concerning
the access of this ultimate mutagen to the germ cells.
An extensive examination of relevant test procedures for
heritable mutations has recently been completed (DHEW, 1977).
7.3.1 Whole-animal tests
Insects. Drosophila melanogaster is one of the best genetically
characterized species and is widely used. Drosophila of either sex can
be treated and mutation frequencies from successive germ cell stages
may be obtained after observation of three generations. The X-lined
recessive lethal test is one of the most sensitive tests with
drosophila, since the X-chromosome represents about 1/5th of the whole
genome. In contrast to most microorganisms, insects possess an enzyme
system that appears to metabolize foreign compounds in a fashion
similar to that of vertebrates (Abrahamson & Lewis, 1971; Fahmy &
Fahmy, 1972, 1973; Sobels & Vogel, 1976).
Mouse specific locus test. The specific locus test is a method
of inducing, detecting, and measuring the rate of mutation at several
recessive loci. It consists essentially of mating treated or untreated
wild type mice, either male or female, to a strain homozygous for a
number of known recessive genes. The recessive genes are such that
they are readily expressed as visible phenotypes in homozygous state.
If a mutation occurs in any of the test loci in the germ cells of
treated animals, it may be detected in the offspring. If no mutation
has occurred following treatment, the progeny from the cross will all
be of the wild type (Cattanach, 1971; Russell, 1951).
Chromosomal mutations. Although the molecular basis is not
understood, a change in the whole chromosome, i.e. a structural
chromosome aberration, occurring as a consequence of a misrepair of
chromosomal breaks, may lead to deletions, duplications, and
translocations. Changes in the whole chromosome complement, i.e.
numerical chromosome aberrations, arise through nondisjunction, as in
failure of a pair of chromosomes to separate during gametogenesis,
meiotic nondisjunction, or during mitotic division. The resulting
daughter cells are either trisomic with an extra chromosome or
monosomic and lacking a chromosome. Anaphase lag occurs during nuclear
division in the progeny cells, and a chromosome may be either lost or
gained. In somatic cells, nondisjunction and anaphase lag can lead to
mosaicism.
Chromosomal damage may be studied in a number of test systems
that are best divided into in vivo and in vitro systems, on the
basis of their capability to metabolize the test compound (Frohberg,
1973). The short-term human lymphocyte culture in vitro is commonly
used to assess the effects of chemicals upon chromosomes. Metabolic
activation of the test compound can be achieved by addition of a liver
microsomal system (Bimboes & Greim, 1976).
The micronucleus test. As an in vivo cytogenetic method, the
micronucleus test is a procedure for the detection of aberrations
involving anaphase chromosome behaviour using bone-marrow
erythroblasts. The test is based on the formation of micronuclei from
particles of chromatin material which, due to chromosome breakage or
spindle disjunction, do not migrate to the poles during anaphase and
are not incorporated into the telophase nuclei of the dividing cells.
The procedure is to treat animals with clastogenic agents and, at an
appropriate time after treatment, to aspirate bone-marrow samples into
calf serum. This is then centrifuged and smears are made from the
resuspended pellets of cells. The smears are air-dried and stained.
The evaluation of the bone-marrow preparations involves examination of
2000-5000 erythrocytes per specimen; the total number of polychromatic
and normochromatic erythrocytes with and without micronuclei are
recorded (Schmid, 1973). Sister chromatid exchanges, induced by
mutagens in vivo and in vitro can be scored in peripheral
lymphocytes or Chinese hamster cells, using the differential straining
of chromatids substituted with 5-bromodeoxyuridine in place of
thymidine (Natarajan et al., 1976; Smythe & Evans, 1976; Stetka &
Wolf, 1976).
Dominant lethal tests and other in vivo systems. The dominant
lethal test is based on the preimplantation loss of eggs or on the
formation of dead embryonic implants following the injection of
mutagens into a male or female mouse at a specific time before mating
(Bateman & Epstein, 1971). Dominant lethal tests assume that a single
mutation has occurred in the eggs or sperm, which is lethal to the
embryo and heterozygous at the affected locus. However, a disturbingly
high number of mutagens have given a negative response with this test.
The detection of mutations arising from compounds that are metabolized
to transient reactive intermediates not produced in germ cells or not
reaching them from other organs may limit this test. Furthermore,
chemical agents causing spermatogenic arrest or cytocidal effect on
the sperms may give false positive results.
Heritable translocation in male mammals. The heritable
translocation test has the important feature of measuring sexually
transmissible germ cell mutations in rodent spermatogonia. Generoso et
al. (1974) have described details of the technique and discussed its
usefulness for the routine screening of substances that cause
chromosomal mutations.
Young adult male mice are treated and females mated with the
exposed males on a schedule that can be used for the comparison of the
sensitivity of the different male germ cell stages. Male progeny from
these matings are collected and mated for the determination of
semisterility and sterility. Progeny with reduced fertility are
subjected to cytogenetic analysis. The cytological examination of
dividing spermatocytes, from animals treated with test chemicals that
cause breaks on two nonhomologous chromosomes, yields aberrant
chromosomal figures. These are recognized as rings or chains of four
chromosomes as opposed to the normal bivalent chromosomal
configuration.
A proper evaluation of this test cannot be made at this time
because of insufficient data in terms of the variety of chemicals
tested to date.
7.3.2 Monitoring of human populations
In spite of the variety of test systems available for detecting
the mutagenic effects of chemicals, it is difficult, from the results
obtained at present, to evaluate with confidence the transmissible
genetic effects caused by the chemical exposure of man. However, the
potential hazards of mutations are such that every effort should be
made to reduce the risk.
Judgment on the potential mutagenic hazard to man should be based
on various considerations such as strength of the experimental
evidence of mutagenicity, the exposure pattern to the chemical, and
the pharmacological properties of the compounds. This is difficult and
complex. Although human studies are difficult, expensive, and often
subject to misinterpretation, only studies which directly estimate the
extent to which environmental factors change human genetic material
give definitive answers.
Proper monitoring of the human population (Crow, 1971; Sutton,
1972) can be carried out by three main approaches:
(a) biochemical: detection of inherited protein variants;
(b) cytogenetic: screening of blood of newborn infants or of
fetuses delivered by spontaneous abortion for chromosomal
changes;
(c) phenotype: surveillance of genetically determined disease or
anomalies.
The validity of these monitoring systems can only be properly
evaluated if, and when, a mutagen is actually discovered.
7.3.3 Significance of tests for heritable mutations
As mentioned earlier, there is, at this time, no direct
correlation between laboratory tests for heritable mutations and human
experience. However, the available body of information from nonhuman
mammals and lower life forms clearly points to the ability of
chemicals to produce alterations in germ cells which are inherited in
succeeding generations. Accordingly, the implications of such data for
man are so persuasive that they must be taken into account in
establishing safety measures for the introduction of chemicals into
use. This is especially true, at this time, since techniques for
detecting mutations in human populations are so insensitive that a
significantly mutagenic chemical could easily escape attention.
These considerations will almost certainly lead to increasing
concern in establishing regulatory and control procedures. In fact,
the Environmental Protection Agency in the USA has recently proposed
preliminary guidelines for conducting tests for heritable mutations as
part of the routine registration procedure for pesticides.
REFERENCES
ABRAHAMSON, S. & LEWIS, E. B. (1971) The detection of mutations in
Drosophila melanogaster. In: Hollaender, A., ed. Chemical
mutagens, principles and methods for their detection, Vol. 2,
461-488.
ALBERTINI, R. J. & DEMARS, R. (1973) Somatic cell mutation detection
and quantification of X-ray induced mutation in cultured human
diploid fibroblasts. Mutat. Res., 18, 199-244.
AMES, B. N. (1971) The detection of chemical mutagens with enteric
bacteria. In: Hollaender, A., ed. Chemical mutagens, principles
and methods for their detection, Vol. 1, pp. 267-282.
AMES, B. N., SIMS, P., & GROVER, P. L. (1972a) Epoxides of
carcinogenic polycyclic hydrocarbons are frameshift mutagens.
Science, 176, 47-49.
AMES, B. N., GURNEY, E.G., MILLER, J. A., & BARTSCH, H. (1972b)
Carcinogens as frameshift mutagens: Metabolites and derivatives
of 2-acetylaminofluorene and other aromatic amine carcinogens.
Proc. Natl Acad. Sci., 69: 3128-3132.
AMES, B. N., LEE, F. D., & DURSTON, W. E. (1973a) An improved
bacterial test system for the detection and classification of
mutagens and carcinogens. Proc. Natl Acad. Sci., 70: 782-786.
AMES, B. N., DURSTON, W. E., YAMASAKI, E., & LEE, F. D. (1973b)
Carcinogens are mutagens: a simple test system combining liver
homogenates for activation and bacteria for detection. Proc.
Natl Acad. Sci., 70; 2281-2285.
AMES, B. N., MCCANN, J., & YAMASAKI, E. (1975) Methods for detecting
carcinogens and mutagens with the Salmonella/mammalian-
microsome mutagenicity test. Mutat. Res., 31: 347-364.
BARTSCH, H. (1976) Predictive value of mutagenicity tests in chemical
carcinogenesis. Mutat. Res., 38: 177-190.
BARTSCH, H. & GROVER, P. L. (1976) Chemical carcinogenesis and
mutagenesis. In: Symington, T. & Carter, R. L., ed. Scientific
foundations of oncology, London, William Heinemann Medical
Books Ltd, pp. 334-342.
BARTSCH, H., MALAVEILLE, C., & MONTESANO, R. (1975a) Human, rat and
mouse liver-mediated mutagenicity of vinyl chloride in
S. typhimurium strains. Int. J. Cancer, 15: 429-437.
BARTSCH, H., MALAVEILLE, C., & MONTESANO, R. (1975b) In vitro
metabolism and microsome-mediated mutagenicity of
dialkylnitrosamines in rat, hamster and mouse tissues. Cancer
Res., 35: 644-651.
BARTSCH, H., MALAVEILLE, C., STICH, H. F., MILLER, E. C., & MILLER, J.
A. (1977) Comparative electrophilicity, mutagenicity, DNA repair
induction activity, and carcinogenicity of some N- and O-Acyl
derivatives of N-Hydroxy-2-aminofluorene. Cancer Res.,
37: 1461-1467.
BATEMAN, A. J. & EPSTEIN, S.S. (1971) Dominant lethal mutations in
mammals. In: Hollaender, A., ed. Chemical mutagens, principles
and methods for their detection, Vol. 2, pp. 541-568.
BERWALD, Y. & SACHS, L. (1963) In vitro transformation with chemical
carcinogens. Nature (Lond.), 200: 1182-1184.
BIMBOES, D. & GREIM, H. (1976) Human lymphocytes as target cells in a
metabolizing test system in vitro for detecting potential
mutagens. Mutat. Res., 35: 155-160.
BORLAND, R. & HARD, G. C. (1974) Early appearance of "transformed"
cells from the kidneys of rats treated with a "single"
carcinogenic dose of dimethylnitrosamine (DMN) detected by
culture in vitro. Europ. J. Cancer, 10: 177-184.
BRIDGES, B. A. (1976) Short-term screening tests for carcinogens.
Nature (Lond.), 261: 195-200.
BRIDGES, B. A., MOTTERSHEAD, R. P., ROTHWELL, M. A., & GREEN, M. H. L.
(1972) Repair deficient bacterial strains suitable for
mutagenicity screening tests with fungicide captan. Chem.-Biol.
Interact., 5: 77-84.
BROWN, A.M. (1973) In vitro transformation of submandibular gland
epithelial cells and fibroblasts of adult rats by
methylcholanthrene. Cancer Res., 33: 2779-2789.
CATTANACH, B. M. (1971) Specific locus mutation in mice. In:
Hollaender, A., ed. Chemical mutagens, principles and methods
for their detection, Vol. 2, pp. 535-540.
CHU, E. H. Y. (1971) Induction and analysis of gene mutations in
mammalian cells in culture. In: Hollaender, A., ed. Chemical
mutagens, principles and methods for their detection, Vol. 2,
pp. 411-444.
CLEAVER, J. E. (1969) Xeroderma pigmentosum: a human disease in
which an initial state of DNA repair is defective. Proc. Natl
Acad. Sci., 63: 428-435.
CLEAVER, J. E. (1973) DNA repair with purines and pyrimidines in
radiation- and carcinogen-damaged normal and Xeroderma
pigmentosum human cells. Cancer Res., 33: 362-369.
CLEAVER, J. E. (1974) Repair processes for photochemical damage in
mammalian cells. In: Lett, J. T., Adler, H., & Zelle, M., ed.
Advances in radiation biology, New York, Academic Press, Vol.
4, pp. 1-75.
CLEAVER, J. E. (1975) Methods for studying repair of DNA damaged by
physical and chemical carcinogens. In: Bush, E., ed. Methods in
cancer research, New York, Academic Press, Vol. 11, pp.
123-165.
CLEAVER, J. E., GOTH, R., & FRIEDBERG, E. C. (1975) Value of
measurements of DNA repair levels in predicting carcinogenic
potential of chemicals. In: Montesano, R., Bartsch, H., &
Tomatis, L., ed. Screening tests in chemical carcinogenesis,
IARC Sci. Publ. No. 12, pp. 639-661.
COMMONER, B., VITHAYATHIL, A. J., & HENRY, J. I. (1974) Detection of
metabolic carcinogen intermediates in urine of carcinogen-fed
rats by means of bacterial mutagenesis. Nature (Lond.),
249: 850-852.
CONNEY, A. H. & BURNS, J. J. (1972) Metabolic interactions among
environmental chemicals and drugs. Science, 178: 576-586.
CORBETT, T. H., HEIDELBERGER, C., & DOVE, W. F. (1970) Determination
of the mutagenic activity to bacteriophage T-4 of carcinogenic
and non-carcinogenic compounds. Mol. Pharmacol., 6(6): 667-679.
COUNCIL OF THE ENVIRONMENTAL MUTAGEN SOCIETY (1975) Environmental
mutagenic hazards, mutagenicity screening is now both feasible
and necessary for chemicals entering the environment. Science,
187: 503-514.
CRADDOCK, V. M. (1976) Replication and repair of DNA in liver of rats
treated with dimethylnitrosamine and with methyl
methanesulphonate. In: Magee, P. N. et al., ed. Fundamentals in
cancer prevention, Tokyo, Univ. Tokyo Press; Baltimore, Univ.
Park Press, pp. 293-311.
CROW, J. F. (1971) Human population monitoring. In: Hollaender, A, ed.
Chemical mutagens, principles and methods for their detection,
Vol. 2, pp. 591-606.
CZYGAN, P. J., GREIM, H., GARRO, A. J., HUTTERER, F., SCHAFFAER, F.,
POPPER, H., ROSENTHAL, O., & COOPER, D. Y. (1973) Microsomal
metabolism of DMN and the cytochrome activation to a mutagen.
Cancer Res,, 33: 2983-2986.
DELLA PORTA, G. & TERRACINI, B. (1969) Chemical carcinogenesis in
infant animals. Progr. Exp. Tumour Res., 11: 334-363.
DE MARS, R. (1974) Resistance of cultured human fibroblasts and other
cells to purine and pyrimidine analogues in relation to
mutagenesis detection. Mutat. Res., 24: 335-364.
DHEW (1977) Approaches to determining the mutagenic properties of
chemicals: Risks to future generations. Report of a working
group of the Subcommittee on Environmental Mutagenesis.
DI PAOLO, J. A., NELSON, R. L., & DONOVAN, P. J. (1972) In vitro
transformation of Syrian hamster embryo cells by diverse chemical
carcinogens. Nature (Lond.), 235: 278-280.
DI PAOLO, J. A., NELSON, R. L., DONOVAN, P. J., & EVANS, C. H. (1973)
Host-mediated in vivo-in vitro assay for chemical
carcinogenesis. Arch. Pathol., 95: 380-385.
DE SERRES, F. J. & MALLING, H. V. (1971) Measurement of recessive
lethal damage over the entire genome and at two specific loci in
the ad-3 region of a two-component heterokaryon of Neurospora
Crassa. In: Hollaender, A., ed. Chemical mutagens, principles
and methods for their detection, Vol. 2, pp. 311-342.
DRAKE, J. W. (1971) Mutagen screening with virulent bacteriophages.
In: Hollaender, A., ed. Chemical mutagens, principles and
methods for their detection, Vol. 1, pp. 219-234.
DRUCKREY, H., PREUSSMAN, R., IVANKOVIC, S., & SCHMÄL D. (1967)
[Organotropic carcinogenic effects of 65 different N-nitroso
compounds on BD-rats.] Z. Krebsforsch., 69 103-201.
DURSTON, W. E. & AMES, B. N. (1974) A simple method for the detection
of mutagens in urine: Studies with the carcinogen
2-acetylaminofluorene. Proc. Natl Acad. Sci., 71: 737-741.
EARLE, W. R. & NETTLESHIP, A. (1943) Production of malignancy in
vitro. V. Results of injections of cultures into mice. J. Natl
Cancer Inst., 4 213-227.
FAHMY O. G. & FAHMY, M.D. (1972) Mutagenic properties of AAF and its
metabolites. Int. J. Cancer, 9 284-298.
FAHMY O. G. & FAHMY, M.D. (1973) Mutagenic properties of
benzo(a)pyrene. Cancer Res., 33: 302-309.
FARBER, E. (1973) Carcinogenesis -- cellular evolution as a unifying
thread: presidential address. Cancer Res., 33: 2537-2550.
FDA (1971) Panel on carcinogenesis report on cancer testing in the
safety evaluation of food additives and pesticides. Toxic. appl.
Pharmacol., 20: 419-438.
FEDOROFF, S. (1967) Proposed usage of animal tissue culture terms,
J. Natl Cancer Inst., 38: 607-611.
FREESE, E. & STRACK, H. B. (1962) Induction of mutations in
transforming DNA by hydroxylamine. Proc. Natl Acad. Sci.,
48: 1796-1803.
FRIEDMAN, L. (1974) Dose selection and administration. In: Goldberg,
L., ed. Carcinogenesis testing of chemicals, Cleveland, CRC
Press, pp. 21-22.
FROHBERG, H. (1973) Critique of in vivo cytogenic test systems.
Agents Actions, 3: 119-123.
FUSENIG, N. R., SAMSEL, W., THON, W., & WORST, P. K. M. (1973)
Malignant transformation of epidermal cells in culture by DMBA.
In: Prunieras, M., Robert, L., & Rosenfeld, C., ed.
Differentiation of eukaryotic cells in culture. Colloque of
INSERM, Vol. 19, pp. 219-228.
GERCHMAN, L. L. & LUDLUM, D. B. (1973) The properties of
O6-methylguanine in template for RNA polymerase. Biochim.
biophys. Acta, 308: 310-316.
GIOVANELLA B, C., YIM, S. O., STEHLIN, J. S., & WILLIAMS, L. J. Jr
(1972) Brief communications: Development of invasive tumors in
the "nude" mouse after injection of cultured human melanoma
cells. J. Natl Cancer Inst., 48: 1531-1533.
GOTH, R. & RAJEWSKY, M. F. (1974) Molecular and cellular mechanisms
associated with pulse-carcinogenesis in the rat nervous system by
ethylnitrosourea: ethylation of nucleic acids and elimination
rates of ethylated bases from the DNA of different tissues.
Z. Krebsforsch., 82: 37-64.
HASHIMOTO, Y. & KITAGAWA, H. (1974) In vitro neoplastic
transformation of epithelial cell of rat urinary bladder by
nitrosamines. Nature (Lond.), 252: 497-499.
HEALTH AND WELFARE, CANADA (1973) The testing of chemicals for
carcinogenicity, mutagenicity, teratogenicity.
HEIDELBERGER, C. (1973) Chemical oncogenesis in culture. Adv. Cancer
Res., 18: 317-366.
HERRIOTT, R. M. (1971) Effects on DNA: Transforming principle. In:
Hollaender, A., ed. Chemical mutagens, principles and methods
for their detection, Vol. 1, pp. 175-218.
HUBERMAN, E. & SACHS, L. (1974) Cell-mediated mutagenesis of mammalian
cells with chemical carcinogens. Int. J. Cancer, 13: 326-333.
HUBERMAN, E., ASPIRAS, L., HEIDELBERGER, C., GROVER, P. L., & SIMS, P.
(1971) Mutagenicity to mammalian cells of epoxides and other
derivatives of polycyclic hydrocarbons. Proc. Natl Acad. Sci.,
68: 3195-3199.
HUBERMAN, E., DONOVAN, P. J., & DIPAOLO, J. A. (1972) Mutation and
transformation of cultured mammalian cells by
N-acetoxy- N-2-fluorenylacetamide. J. Natl Cancer Inst.
48: 837-840.
HUEPER, W. C., WILEY, F. H., & WOLFE, H. D. (1938) Experimental
production of bladder tumours in dogs by administration of
ß-naphthylamine. J. industr. Hyg. Toxicol., 20: 46-84.
IARC (1976) Monographs on the evaluation of carcinogenic risk of
chemicals to man: some aromatic amines, hydrazine and related
substances, N- nitroso compounds and miscellaneous alkylating
agents. Lyons, International Agency for Research on Cancer,
Vol. 4.
IYPE P. T. (1974) Studies on chemical carcinogenesis in vitro using
adult rat fiver cells. In: Montesano, R. & Tomatis, L., ed.
Chemical carcinogenesis essays, IARC Sci. Publ. No. 10,
pp. 119-133.
J. Natl Cancer Inst., 53: 1427-1519 (1974) Proceedings of the
Symposium "New Horizons for Tissue Culture in Cancer Research".
KAKUNAGA, T. The transformation of human diploid cells by chemical
carcinogens. In: Hiatt, H. H. et al., ed. Origins of human
cancer (1977) Cold Spring Harbor Laboratory. pp. 1537-1548.
KATSUTA, H. & TAKAOKA, T. (1972) Carcinogenesis in tissue culture.
XIV. Malignant transformation of rat liver parenchymal cells
treated with 4-nitroquinoline 1-oxide in tissue culture. J. Natl
Cancer Inst., 49: 1563-1536.
KONDO, S. (1973) Evidence that mutations are induced by errors in
repair and replication. Genet. suppl., 73: 109-122.
KRAHN, D. F. & HEIDELBERGER, C. (1975) Microsome-mediated mutagenesis
in Chinese hamster cells by chemical oncogens. Proc. Am. Assoc.
Cancer Res., 16: 74.
KRIEK, E. (1974) Carcinogenesis by aromatic amines. Biochem.
Biophys. Acta, 355: 177-203.
KUROKI, T. (1975) Contributions of tissue culture to the study of
chemical carcinogenesis: A review. In: Recent topics in chemical
carcinogenesis, Gann, Monograph on Cancer Res., 17: 69-85.
KUROKI, T., DREVON, C., & MONTESANO, R. (1977) Microsome-mediated
mutagenesis in V-79 Chinese hamster cells by various
nitrosamines. Cancer Res., 1044-1050.
LAERUM, O. D. & RAJEWSKY, M. F. (1975) Neoplastic transformation of
foetal rat brain cells in culture following exposure to
ethylnitrosourea in vivo. J. Natl Cancer Inst., 55: 1177-1187.
LEGATOR, M. S. & FLAMM, W. G. (1973) Environmental mutagenesis and
repair. Ann. Rev. Biochem., 683-708.
LEGATOR, M. S. & MALLING, H. V. (1971) The host-mediated assay, a
practical procedure for evaluating potential mutagenic agents in
mammals. In: Hollaender A., ed. Chemical mutagens, principles
and methods for their detection, Vol. 2, pp. 569-590.
LOPRIENO, N., BARALE, R., BARONCELLI, S., BARTSCH, H., BRONZETTI, G.,
CAMMELLINI, A., CORSI, C., FREZZA, D., NIERI, R., LEPORINI, C.,
ROSELLINI, D., & ROSSI, A.M. (1976) Induction of gene mutations
and gene conversions by vinyl chloride metabolites in yeast.
Cancer Res., 37: 253-257.
LOVELESS, A. (1969) Possible relevance of O6-alkylation of
deoxyguanosine to the mutagenicity and carcinogenicity of
nitrosamines and nitrosamides. Nature (Lond.), 233: 206-207.
LOVELESS, A. & HAMPTON, C. L. (1969) Inactivation and mutation of
coliphage T2 by N-methyl- and N-ethyl- N-nitrosourea.
Mutation Res., 7: 1-12.
LUDLUM, D. B. (1970) Alkylated polycytidic acid templates for RNA
polymerase. Biochim. biophys. Acta, 213: 142-148.
MAGEE, P. N., MONTESANO, R., & PREUSSMAN, R. (1976) N-nitroso
compounds and related carcinogens. In: Searle, C., ed. Chemical
carcinogens, ACS monograph No. 173, pp. 491-625.
MAHER, V., MILLER, E. C., MILLER, J. A., & SZYBALSKI, W. (1968)
Mutations and decreases in density of transforming DNA produced
by derivatives of the carcinogens 2-acetylaminofluorene and
N-methyl-4-aminoazobenzene. Mol. Pharmacol., 4: 411-426.
MAHER, V. M., CURREN, R. D., OUELLETTE, L. M., & MCCORMICK, J. J.
(1976) Effect of DNA repair on the frequency of mutations induced
in human cells by ultraviolet irradiation and by chemical
carcinogens. In: Magee, P. N., et al, ed. Fundamentals in cancer
prevention, Tokyo, Univ. of Tokyo Press; Baltimore, Univ Park
Press, pp. 363-382.
MALLING, H. V. (1974) Mutagenic activation of dimethylnitrosamine and
diethylnitrosamine in the host-mediated assay and the microsomal
system. Mutat. Res., 26: 465-472.
MARGISON, G. P., MARGISON, J. M., & MONTESANO, R. (1976) Methylated
purines in the deoxyribonucleic acid of various Syrian golden
hamster tissues after administration of a hepatocarcinogenic dose
of dimethylnitrosamine. Biochem. J., 157: 627-634.
MARQUARDT, H. (1974) Mutation and recombination experiments with yeast
as pre-screening tests for carcinogenic effects.
Z. Krebsforsch., 81: 333-346.
MCCANN, J. & AMES, B. N. (1976) Detection of carcinogens as mutagens
in the Salmonella/microsome test: Assay of 300 chemicals:
Discussion. Proc. Natl Acad. Sci., 73: 950-954.
MCCANN, J., SPINGARN, N. E., KOBORI, J., & AMES, B. N. (1975)
Detection of carcinogens as mutagens: bacterial tester strains
with R factor plasmids. Proc. Natl Acad. Sci., 72: 979-983.
MESELSON, M. & RUSSELL, K. (1977) Comparisons of carcinogenic and
mutagenic potency. In: Hiatt, H. H. et at. ed. Origins of human
cancer. Cold Spring Harbor Laboratory. pp. 1473-1481.
MILLER, J. A. (1970) Carcinogenesis by chemicals: an overview -- G. H.
A. Clowes Memorial Lecture. Cancer Res., 30: 559-576.
MILLER, J. A. (1973) Naturally occurring substances that can induce
tumors. Proc. Natl Acad. Sci., 23: 508-549.
MILLER, E. C. & MILLER, J. A. (1971a) The mutagenicity of chemical
carcinogens: correlations, problems, and interpretations. In:
Hollaender, A., ed. Chemical mutagens, principles and methods
for their detection, Vol. 1, pp. 83-120.
MILLER, J. A. & MILLER, E. C. (1971b) Chemical carcinogenesis,
mechanisms and approaches to its control. J. Natl Cancer Inst.,
47: V-XIV.
MILLER, E. C. & MILLER, J. A. (1976) The metabolism of chemical
carcinogens to reactive electrophiles and their possible
mechanisms of action in carcinogenesis. In: Searle, C. E., ed.
Chemical carcinogens, ACS Monograph 173. Washington, DC,
American Chemical Society, pp. 737-762.
MOHN, G. (1973) Revertants of an Escherichia coli K-12 strain with
high sensitivity to radiations and chemicals. Murat. Res.,
19: 349-355.
MOHN, G. & ELLENBERGER, J. (1973) Mammalian blood mediated
mutagenicity test using a multi-purpose strain of E. coli,
K-12. Mutat. Res., 19: 257-260.
MONTESANO, R. & BARTSCH, H. (1976) Mutagenic and carcinogenic
N-nitroso compounds: Possible environmental hazards. Mutat.
Res., 32: 179-228.
MONTESANO, R., SAINT VINCENT, L., & TOMATIS, L. (1973)
Malignant transformation in vitro of rat liver cells by
dimethylnitrosamine and N-methyl- N'-nitro-
N-nitrosoguanidine. Brit. J. Cancer, 38: 215-220.
MONTESANO, R., SAINT VINCENT, L., DREVON, C,, & TOMATIS, L. (1975)
Production of epithelial and mesenchymal tumours with rat liver
cells transformed in vitro. Int. J. Cancer. 55: 1177-1187.
MONTESANO, R., DREVON, C., KUROKI, T., SAINT VINCENT, L., HANDLEMAN,
S., SANFORD, K. K., DEFEO, D. & WEINSTEIN, I. B. (1977) Test for
malignant transformation of rat liver cells in culture: Cytology,
growth in soft agar and production of plasminogen activator.
J. Natl Cancer Inst., 59: 1651-1658.
MONTESANO, R., BARTSCH, H., & TOMATIS, L., ed. (1976) Screening tests
in chemical carcinogenesis. IARC Sci. Publ. No. 12.
MONTESANO, R. & TOMATIS, L. (1977) Legislation concerning chemical
carcinogens in several industrialized countries. Cancer Res.,
37: 310-316.
MOREAU, P., BAILONE, A., & DEVORET, R. (1976) Prophage lambda
induction in Escherichia coli K12 envA uvrB: A highly
sensitive test for potential carcinogens. Proc. Natl Acad. Sci.,
73: 3700-3704.
MORTIMER, R. K. & MANNEY, T. R. (1971) Mutation induction in yeast.
In: Hollaender, A., ed. Chemical mutagens, principles and
methods for their detection, Vol. 1, pp. 289-310.
NAHAS, A. & CAPIZZI, R. L. (1974) Effect of in vivo treatment with
L asparaginal on the in vitro uptake and phosphorylation of
some anti-leukemic agents. Cancer Res., 34: 2687-2693.
NAPALKOV, N. P. (1973) Some general considerations on the problem of
transplacental carcinogenesis. In: Tomatis, L. & Mohr, U., ed.
Transplacental carcinogenesis, IARC Sci. Publ. No. 4, pp. 1-13.
NAPALKOV, N. P. & ALEXANDROV, V. A. (1968) On the effects of
blastomogenic substances on the organism during embryogenesis.
Z. Krebsforsch., 71: 32-50.
NATARAJAN, A. T., TATES, A.D., VAN BUUL, P. P. W., MEIJERS, M., & DE
VOGEL, N. (1976) Cytogenetic effects of mutagens/carcinogens
after activation in a microsomal system in vitro. I. Induction
of chromosome aberrations and sister chromatid exchanges by
diethylnitrosamine (DEN) and dimethylnitrosamine (DMN) in CHO
cells in the presence of rat-liver microsomes. Murat. Res.,
37: 83-90.
MATIONAL ACADEMY OF SCIENCES (1960) Problems in the evaluation of
carcinogenic hazard from use of food additives. Washington, Natl
Res. Council (Publ. No. 479).
NCI (1976) NCI Technical Report Series, No. 1 (Guidelines for
carcinogen bioassay in small rodents), pp. 1-65 (DHEW Publication
No. (NIH) 76-701).
PEGG, A. E. (1977) Formation and metabolism of alkylated nucleosides:
Possible role in carcinogenesis by nitroso compounds and
alkylating ageNTs. Adv. Cancer Res., 25: 195-269.
PEGG, T. & NICOLL, J. W. (1976) Nitrosamine carcinogenesis: The
importance of the persistence in DNA of alkylated bases in the
organotropism of tumour induction. In: Montesano, R., Bartsch,
H., & Tomatis, L., ed. Screening tests in chemical
carcinogenesis, IARC Sci. Publ. No. 12, pp. 571-592.
PIETRA, G., RAPPAPORT, H., & SHUBIK, P. (1961) The effects of
carcinogenic chemicals in newborn mice. Cancer, 14: 308-317.
PITOT, H. C. (1974) Neoplasia: A somatic mutation or a heritable
change in cytoplasmic membranes? J. Natl Cancer Inst.,
53: 905-911.
PITOT, H. C. (1977) The natural history of neoplasia. Am. J. Path.,
89: 402-411.
PURCHASE, I. F. H., LONGSTAFF, E., ASHBY, J., STYLES, J. A., ANDERSON,
D., LEFEVRE, P. A., & WESTWOOD, F. R. (1976) Evaluation of six
short-term tests for detecting organic chemical carcinogens and
recommendations for their use. Nature (Lond.), 264: 624-627.
REUBER, M. (1974) Criteria for tumor diagnosis and classification of
malignancy. In: Golberg, L., ed. Carcinogenesis testing of
chemicals, Cleveland, CRC Press, pp. 71-73.
ROSENKRANZ, H. S. (1973) Aspects of microbiology in cancer research.
Rev. Microbiol., 1622: 383-400.
RUSSELL, W. L. (1951) X-ray induced mutations in mice. Cold Spring
Harbour Symp. Quant. Biol., 16: 327-336.
SANFORD, K. K. (1965) Malignant transformation of cells in vitro.
Inter. Rev. Cytol., 18: 249-311.
SANFORD, K. K. (1974) Biological manifestations of oncogenesis in
vitro: a critique. J. Natl Cancer Inst., 53: 1481-1485.
SANFORD, K. K., EARLE, W. R., SHELTON, E., SCHILLING, E. L., DUCHESNE,
E. M., LIKELY, G. D., & BECKER, E. M. (1950) Production of
malignancy in vitro. XII. Further transformations of mouse
fibroblasts to sarcomatous cells. J. Natl Cancer Inst.,
11: 351-367.
SATO, K., SLESINSKI, R. S, & LITTLEFIELD, J. W. (1972) Chemical
mutagenesis at the phosphoribosyl transferase locus in cultured
human lymphoblasts. Proc. Natl Acad. Sci., 69: 1244-1248.
SCHMID, W. (1973) Chemical mutagen testing on in vivo somatic
mammalian cells. Agents Actions, 3: 77-85.
SIMS, P. & GROVER, P. L. (1974) Epoxides in polycyclic aromatic
hydrocarbon metabolism and carcinogenesis. Advances in Cancer
Res., 20: 165-274.
SMITH, W. E. & HANAWALT, P. C. (1969) Molecular photobiology:
Inactivation and recovery. New York, Academic Press.
SMYTHE, D. R. & EVANS, H. J. (1976) Mapping of sister-chromatid
exchanges in human chromosomes using G-Banding and
autoradiography. Mutat. Res., 35: 139-154.
SOBELS, F. H. & VOGEL, E. (1976) The capacity of drosophila for
detecting relevant genetic damage. Mutat. Res., 41: 95-106.
STETKA, D. G. & WOLFF, S. (1976) Sister chromatid exchange as an assay
for genetic damage induced by mutagen-carcinogens. I. In vitro
test for compounds requiring metabolic activation. Mutat. Res.,
41: 333-342.
STICH, H. F. & KIESER, D. (1974) Use of DNA repair synthesis in
detecting organo-tropic actions of chemical carcinogens.
Proc. Soc. Exp. Biol Med., 145: 1339-1342.
STICH, H. F. & SAN, R. H. C. (1970) DNA repair and chromatid anomalies
in mammalian cells exposed to 4 nitroquinoline 1-oxide. Mutat.
Res., 10: 389-404.
STOLTZ, D. R., POIRIER, L. A., IRVING, C. C., STICH, H. F.,
WEISBURGER, J. H., & GRICE, H. C. (1974) Evaluation of short-term
tests for carcinogenicity. Toxicol. appl. Pharmacol.,
29: 157-180.
SUGIMURA, T., SATO, S., NAGAO, M., YAHAGI, T., MATSUSHIMA, T., SEINO,
Y., TAKEUCHI, M., & KAWACHI, T. (1976) Overlapping of carcinogens
and mutagens. In: Magee, P. N., Takayama, S., Sugimura, T., &
Matsushima, T., ed. Fundamentals in cancer prevention, Tokyo,
University of Tokyo Press; Baltimore, Univ. Park Press,
pp. 191-215.
SUTHERLAND, B. M., RICE, M., & WAGNER, E. K. (1975) Xeroderma
pigmentosum cells contain low levels of photoreactivating
enzyme. Proc. Natl Acad. Sci., 72: 103-107.
SUTTON, H. E. (1972) Monitoring somatic mutations in human
populations. In: Sutton, H. E. & Harris, M. I., ed. Mutagenic
effects of environmental contaminants, pp. 122-128.
THOMPSON, L. H. & BAKER, R.M. (1973) Isolation of mutants of cultured
mammalian cells. In: Prescott, D. M., ed. Methods of cell
biology, New York, Academic Press, pp. 209-280.
TOMATIS, L. (1973) Transplacental carcinogenesis. In: Raven, R. W.,
ed. Modern trends in oncology -- 1, London, Butterworths,
pp. 99-126.
TOMATIS, L. (1974a) The validity of long-term bioassays in
carcinogenicity testing. Chemical and vital oncogenesis. Excerpts
Medica Int. Congr. Ser. No. 350, Vol. 2, pp. 82-93.
TOMATIS, L. (1974b) Inception and duration of tests. In: Golberg, L.,
ed. Carcinogenesis testing of chemicals, Cleveland, CRC Press,
pp. 23-27.
TOMATIS, L., AGTHE, C., BARTSCH, H., HUFF, J., MONTESANO, R., SARACCI,
R., WALKER, E., & WILBOURN, J. (1978) Evaluation of the
carcinogenicity of chemicals: A review of the monograph program
of the International Agency for Research on Cancer. Cancer Res.,
38: 877-885.
TURUSOV, V. S., ed. (1973, 1976) Pathology of tumours in laboratory
animals, Vol. I, Part 1 and 2, IARC Sci. Publ. No. 5 and No. 6.
Lyon.
UICC (1969) UICC Technical Report Series Vol. 2 (Carcinogenicity
testing). Geneva, International Union Against Cancer, 56 pp.
VENITT, S. & LEVY, L. S. (1974) Mutagenicity of chromates in bacteria
and its relevance to chromate carcinogenesis. Nature (Lond.),
250: 493-495.
WEIL, C. S. (1972) Statistics vs safety factors and scientific
judgements in the evaluation of safety for man. Toxicol. appl.
Pharmacol., 21: 454-463.
WEINSTEIN, I. B., ORENSTEIN, J. M., GEBERT, T., KAIGHN, M. E., &
STADLER, V. C. (1975) Growth and structural properties of
epithelial cell cultures established from normal rat liver and
chemically induced hepatomas. Cancer Res., 35: 253-263.
WEISBURGER, J. H. (1974) Inclusion of positive control. In: Golberg,
L., ed. Carcinogenesis testing of chemicals, Cleveland, CRC
Press, pp. 29-34.
WEISBURGER, E. K. (1975) A critical evaluation of the methods used for
determining carcinogenicity. J. clin. Pharmacol., 15: 5-15.
WEISBURGER, J. H. & WEISBURGER E. K. (1967) Tests for chemical
carcinogens. In: Busch, H., ed. Methods in cancer research,
Academic Press, Vol. 1, pp. 307-398.
WEISBURGER, J. H. & WEISBURGER, E. K. (1973) Biochemical formation and
pharmacological, toxicological and pathological properties of
hydroxylamines and hydroxamic acids. Pharmacol. Rev. 25: 1-66.
WHO (1961) WHO Technical Report Series No. 220 (Evaluation of
carcinogenic hazards of food additives), 33 pp.
WHO (1964) WHO Technical Report Series No. 276 (Prevention of cancer).
WHO (1969) WHO Technical Report Series No. 426 (Principles for the
testing and evaluation of drugs for carcinogenicity).
WHO (1974) WHO Technical Report Series, No. 546 (Assessment of the
carcinogenicity and mutagenicity of chemicals), 19 pp.
WILLIAMS, G. M., ELLIOT, J. M., & WEISBURGER, J. H. (1973) Carcinoma
after malignant conversion in vitro of epithelial-like cells
from rat liver following exposure to chemical carcinogens.
Cancer Res., 33: 606-612.
WILSON, R. H., DEEDS, F., & COX, A. J. (1941) The toxicity and
carcinogenicity of 2-acetaminofluorene. Cancer Res.,
1: 595-608.
ZIMMERMANN, F. K. (1973) Detection of genetically active chemicals
using various yeast systems. In: Hollaender, A., ed. Chemical
mutagens, principles and methods for their detection, Vol. 3,
pp. 209-240.
O,6-guanine residues (Gerchman & Ludlum, 1973). Goth & Rajewsky
(1974) showed, in vivo, that the initial degree of alkylation at the
O,6 position in the DNA was, apparently, not correlated with the
tissue-specific carcinogenicity of ethylnitrosourea which induces
brain, but not liver, tumours. However, O,6-ethylguanine persists
much longer in brain DNA than N,7-ethylguanine. The O,6-alkyl
elimination is also much slower from brain than from the liver DNA.
From these findings it becomes apparent that variations in the
sensitivity of different organs to the carcinogenic action of
N-nitroso compounds might be attributed to different enzyme
activities capable of repairing lesions in cellular DNA and/or to
different rates of cell division in the target and non-target organs
(Craddock, 1976; Margison et al., 1976; Pegg & Nicoll, 1976; Pegg,
1977).
Possibly these repair systems, which recognize damaged DNA, are
also impaired in the case of some types of cancer in man. The skin of
patients suffering from the hereditary disease xeroderma pigmentosum
is extremely sensitive to sunlight and such persons have a very high
incidence of skin cancer. It has been shown that the cells of the skin
of these patients are deficient in the capacity to repair UV-damaged
DNA and that this deficiency is caused by lack of enzymes required for
the excision of damaged regions from DNA (Cleaver, 1969).
Some chemicals cause changes in the biological properties of DNA
without covalent binding, e.g. by intercalation which could cause
frameshift mutations. Since radioactive labelled chemicals are needed
for most of these studies, technical problems associated with these
methods render the tests impractical for routine screening. However,
they will continue to contribute considerably to the understanding of
the mechanism of interaction of a chemical or its activating
metabolite with specific sites in DNA.
Initial lesions in DNA can either lead to a permanent change,
such as a mutation, or can be removed by cellular repair processes. In
bacteria and mammalian cells, three major repair processes can be
considered. First, the photoreactivation repair process that repairs
only UV damage to DNA and involves a direct enzymatic cleavage of
pyrimidine dimers to monomers upon exposure of cells to visible light.
This repair process is present in most prokaryotes and eukaryotes and
it was recently identified in human cells (Sutherland et al., 1975).
The second process, post-replication repair, which is also called
recombination repair, is limited to the DNA synthesis period. Although
its mechanism is not well known, it has been proposed that it is
caused by the presence of damaged bases on parental DNA strands too
close to the replication fork to be recognized by the third process,
excision repair. Unlike post-replication repair, excision repair
occurs throughout the cell cycle and is present in a large variety of
organisms. During this process, damaged bases are excised, producing a
single-strand break. This gap is repaired by replacing the original
bases with bases complementary to those of the opposite intact strand.
Various enzymes are involved in this process, namely endonuclease, a
DNA exonuclease, a DNA polymerase and a DNA ligase (Cleaver, 1974).
From studies on prokaryotes, the concept arose that excision
repair is error-free (Kondo, 1973). It is thought that mutations
originate during semi-conservative replication as a consequence of the
fact that DNA templates containing damage have not yet been or cannot
be repaired by excision repair. Since DNA replication on damaged
templates involves post-replication repair, this repair process is
considered error-prone and responsible for mutagenesis. Whether
miscoding and mutagenesis of DNA templates is the result of lack of
excision repair enzymes or due to error-prone post-replicative repair
is not clear at present.
The isolation of xeroderma pigmentosum-variant fibroblasts, that
have normal excision repair but are deficient in post-replication
repair (Maher et al., 1976) may lead to a better understanding of the
role of these repair processes in mutagenesis and carcinogenesis.
Recent efforts have been devoted to the development in mammalian
systems of a screening test for the detection of possible mutagens or
carcinogens based on excision repair. The focus on this type of repair
is mainly based on the greater availability of procedures thought to
be appropriate to the problem. There are four different ways of
examining this form of repair in mammalian systems and all are based
on the assumption that chemical mutagens or carcinogens interact with
DNA, inducing molecular alterations which result in DNA repair that
can be measured as unscheduled incorporation of various DNA precursors
(Cleaver, 1975; Legator & Flamm, 1973). The common basis of all these
tests is the differentiation between repair and normal
semiconservative synthesis of DNA.
One procedure, "unscheduled DNA synthesis", involves
autoradiography of cultured cells, and consists of the incorporation
of precursors during resynthesis of short nucleotide sequences that
have been eliminated from DNA strands following their damage by
chemicals (Cleaver, 1973; Stich & San, 1970). This procedure permits a
quantitative evaluation of DNA repair synthesis in various types of
cell in a tissue and can be applied to an in vivo system (Stich &
Kieser, 1974), thus allowing the detection of indirect mutagens and/or
carcinogens.
Another process uses 5-bromodeoxyuridine (BrdU), which is an
analogue of thymidine. During normal replication, the incorporation of
BrdU produces a DNA with a high buoyant density; this does not occur
when BrdU is incorporated in DNA during the repair synthesis. Thus, by
using radiolabelled BrdU, or radiolabelled thymidine and non-labelled
BrdU, it is possible to differentiate, on cesium chloride density
gradients, semi-conservative replication of DNA from the repair
synthesis according to the different buoyant densities.
A third procedure also uses BrdU but the differentiation between
semi-conservative and repair DNA synthesis is done by different means.
Cells exposed to the chemicals are incubated with BrdU and the DNA is
subjected to long wavelength ultra-violet radiation. Breaks appear in
the DNA at the sites of incorporation of BrdU and cause slower
sedimentation of the DNA in alkaline sucrose gradients (Smith &
Hanawalt, 1969). This technique appears to be a very sensitive one for
measuring single-strand breaks but it still seems to be prone to
artefacts and cannot be considered suitable for large-scale studies
(Cleaver, 1975).
Another method involves the total suppression of normal DNA
synthesis, for example, by hydroxyurea, thus the incorporation of
precursors into DNA reflects only repair synthesis and not normal
replication.
Some of the above techniques are time-consuming, costly and not
yet standardized, so that it is difficult to adapt them for
large-scale studies. The most promising approach appears to be the
measurement of unscheduled DNA synthesis, but criticisms have also
been made of the use of this system as an indicator of mutagenic or
carcinogenic potential (Cleaver et al., 1975). One limitation is that
unscheduled synthesis is an average measure of repairable damage in
all cells of a population or in all sites of the DNA, whereas
mutagenesis and carcinogenesis seem to depend on the amount of
unrepaired damage in a small percentage of cells and in a few specific
DNA sites. Due to these limitations and to the current limited
understanding of these processes, none of these variables could, by
themselves, be a reliable indicator of a potential mutagenic or
carcinogenic chemical. However, these studies associated with studies
of DNA damage as well as of the expression of this damage are
essential in the understanding and development of reliable screening
systems.
Metabolic activation of chemicals to electrophiles, DNA damage,
and subsequent repair processes are important factors in the
initiation of cancer. The phenotypic expression (tumour development)
of these initial molecular changes is modulated by a number of factors
(Farber, 1973) that influence the macroscopic appearance of cancer at
a later stage (Pitot, 1977). However, the initial molecular changes,
induced by chemical carcinogens, appear prerequisite for initiation of
the cancer process. Further studies on the alteration of DNA induced
by chemical carcinogens and on the repair of such lesions before cell
division by tissues, whether target or not, are required to evaluate
the role of DNA repair processes in chemical carcinogenesis.
7.2.2.2 In vitro neoplastic transformation of mammalian cells
The term transformation in such studies refers to the in vitro
observations of various changes present in the cells treated in vitro
with the chemicals, when compared with untreated control cells.
Transformed cells may differ from control cells in various ways such
as: (a) alteration of cellular and colony morphology; (b) increased
plating efficiency; (c) agglutinability by plant lectins; (d) altered
glycolytic patterns; (e) resistance to toxicity of some chemicals; (f)
altered surface properties (contact inhibition of movement and growth,
population density, growth in suspension, ability to grow in agar or
other semisolid media); (g) sensitivity to activated macrophages and
lymphocytes; (h) establishment of cell lines with the potential to be
sub-cultured indefinitely in vitro; and (i) appearance of new
antigens (Fedoroff, 1967). However, the unequivocal criterion of
malignant transformation is the capacity of transformed cells to
develop a malignant neoplasm when injected into a syngenic,
immunosuppressed or thymus-free host, or into privileged sites
(Giovannella et al., 1972; Sanford, 1965).
Recently, Sanford (1974) critically reviewed the significance of
these various in vitro changes in relation to the acquisition of
neoplastic potential. It was stressed that these criteria of
neoplastic transformation cannot completely replace the in vivo
assay for tumour production. With reference to the problems of the
application of tissue culture to the rapid detection and
characterization of neoplastic transformation in vitro, the reader
is referred to the proceedings of the symposium "New Horizons for
Tissue Culture in Cancer Research" ( J. Natl Cancer Inst., 1974).
In most in vitro transformation experiments the cells used have
been fibroblasts. Earle & Nettleship (1943) reported the
transformation by 1,2-dihydro-3-methyl-benz[j]aceanthrylene
(3-methylcholanthrene) of a long-term culture of fibroblasts as
demonstrated by the development of tumours after inoculation of the
cells into mice. However, this observation was attributed to
spontaneous transformation, since Sanford et at. (1950) observed that
the cells were tumorigenic also without treatment with chemicals. The
first clear demonstration of transformation of cells in culture by
chemicals was that of Berwald & Sachs (1963) who observed the
transformation of Syrian hamster embryo secondary cells with
3-methylcholanthrene and benzo(a)pyrene, but not with urethane or
solvent. Since this report, various cells types originating from
different animal species have been used for in vitro transformation.
This topic has recently been reviewed by Heidelberger (1973) and
Kuroki (1975), who critically examined the advantages and
disadvantages of the various strains and lines of fibroblastic cells,
namely of the Syrian hamster embryo, fibroblastic cells derived from
mouse ventral prostate, cell lines derived from embryo cells of Swiss
mouse BALB/c, C3H, AKR, and C57/B1 strains, Chinese hamster lung
cells, as well as various tissues from organ cultures. Transformation
of the above cell lines was obtained with polynuclear hydrocarbons and
with chemicals that do not require metabolic activation.
Some chemical carcinogens that are active in vivo have failed
to induce transformation, when applied directly to hamster embryo
cells; this is presumably because such compounds require metabolic
conversion to active intermediates that are lacking or are present in
insufficient concentration in these cells. However, neoplastic
transformation was detected in fibroblasts obtained from embryos whose
mothers had been exposed to indirect carcinogens during pregnancy
(Di Paolo et al., 1972, 1973). A similar approach was used by Borland
& Hard (1974), who cultured kidney cells at various times following
in vivo treatment of rats with N-methyl- N-nitrosomethanamine
(dimethylnitrosamine). The cells isolated from treated rats showed
various morphological and behavioural changes associated with
transformation. Laerum & Rajewsky (1975) reported the development of
glioblastomas following injection of glial cells originating from the
brain of rat embryos, the mother having been treated with
ethylnitrosourea during pregnancy.
Epithelial cultures from rat liver were recently established in
various laboratories and used for transformation studies (Iype, 1974;
Katsuta & Takaoka, 1972; Montesano et al, 1975; Weinstein et al.,
1975; Williams et al., 1973). The transformation of these cells by
various carcinogens that need metabolic activation was determined by
the development of carcinomas after their back-transplantation into
suitable hosts. Carcinomas were observed following inoculation of
epithelial cells from mouse skin, or rat urinary bladder or salivary
glands, treated with chemical carcinogens in vitro (Brown, 1973;
Fusenig et al., 1973; Hashimoto & Kitagawa, 1974).
One disadvantage of these epithelial cells is that the
morphological criteria for transformation of fibroblast cultures
(piling up of cells, crisscross arrangement of cells etc.) do not
apply possibly because normal epithelial cells have little or no
locomotion and they continue to divide even when in close contact with
other cells (Weinstein et al., 1975). However, the capacity for growth
in soft agar appears to provide a reliable and reproducible
correlation with the tumorigenicity of these cells (Weinstein et al.,
1975; Montesano et al., 1977). Although, at present, this system
provides only a qualitative and not a quantitative assay of in vitro
transformation, it is the only instance of unequivocal production of
carcinoma. The establishment of reliable criteria, measurable in
vitro, for distinguishing normal from transformed epithelial cells
in culture is essential for the development of quantitative systems
for the transformation of these epithelial cells. Recently the
neoplastic transformation of human diploid cells by chemical
carcinogens has been described (Kakunaga, 1977).
Cell culture has been extremely useful in elucidating the
cellular and molecular mechanism of chemical carcinogens and it holds
great promise as a test for screening for the potential
carcinogenicity of environmental chemicals. However, some time is
needed before this test may be used for routine testing in a
reproducible way.
7.2.2.3 Mutagenicity tests
The growing experimental evidence linking the carcinogenic
activity of numerous chemicals with their capacity to be converted
into electrophilic derivatives, that may also exert a mutagenic
effect, has led to the suggestion that a relationship between chemical
carcinogenesis and mutagenesis may exist (Miller & Miller, 1971a,b).
Such a correlation has so far been limited to those changes of the
genotype that appear as a consequence of structural or functional
alterations of nucleic acids. Not all chemical mutagens have been
shown to be carcinogenic. However, most chemical carcinogens, several
of which cause cancer in man, have now been found to be mutagens, when
tested by one of the mutagenicity test procedures that combine
microbial, mammalian, or other animal cell systems as genetic targets
with an in vitro or in vivo metabolic activation system. The
growing empirical relationship between chemical mutagens and
carcinogens does not imply that the two processes are identical, but
it offers a promising method for the use of mutagenesis as a rapid
prescreening assay for carcinogenesis (Bartsch & Grover, 1976;
Bridges, 1976; Council of the Environmental Mutagenic Society, 1975;
IARC, 1976; McCann & Ames, 1976; Purchase et al., 1976; Stoltz et al.,
1974; Sugimura et al., 1976; WHO, 1974).
The choice of mutagen-detecting assay depends on various
considerations, i.e. the chemical structure and pharmacological
activity of the chemical, the type of human exposure, and the nature
of the population at risk. The same considerations should be taken
into account in assessing the strength of the evidence of mutagenicity
and its relevance to man.
7.2.2.4 Submammalian assay systems
Bacterial phages have been used to test reactive forms of
chemical carcinogens. Chemicals inducing point mutations can be
detected by reacting the test compound with the free phage or with
phage during its duplication inside bacteria (Corbett et al., 1970;
Drake, 1971).
In the bacterial transformation of DNA (Freese & Strack, 1962),
genetic information contained in the DNA isolated from one strain of
bacteria can be transformed to that of a recipient strain. Purified
bacterial DNA is readily accessible to reactive forms of mutagens.
Thus, this system has been used to quantify the mutagenic potential of
a number of chemicals. Extensive studies have been made on the
inactivation of Bacillus subtilis DNA and transformation of the
tryptophane-requiring strain T-3 (Herriott, 1971; Maher et al., 1968).
The reverse mutationa system of Salmonella typhimurium uses
the genetically well-defined histidine-requiring mutants developed by
Ames and his colleagues (Ames, 1971; Ames et al., 1972a,b, 1973;
McCann et al., 1975).
These revert to prototrophy by single-base pair substitutions,
e.g. strain TA1535 or by base pair insertion (frameshift), e.g.
strains TA1536, TA1537 and TA1538. Most of the theoretically possible
types of mutation may be detected with a set of these test strains,
where a mutation of one of the genes responsible for excision repair
(UVrB) has produced a 100-fold increase in sensitivity. Penetration of
larger molecules through the bacterial cell walls has also been
facilitated by the use of deep-rough mutants deficient in the exterior
polysaccharide coat. Two newly developed test strains TA100 and TA98
were obtained by transferring an ampicillin resistance factor
(R factor) to the standard test strains TA1535 and TA1538,
respectively (McCann et al., 1975). These strains are effective in
detecting classes of mutagens that were not previously detected with
the original strains. The tests are usually performed by adding a few
crystals or a drop of solution of the test chemical in
sulfinylbis[methane] (dimethylsulfoxide) or water to a uniform lawn of
one of the histidine-requiring mutants on the surface of a Petri plate
containing histidine-poor medium, or by incorporating the test
compound, a postmitochondrial tissue fraction, cofactors (NADPH or
NADPH+ and glucose 6-phosphate) and the bacterial test strain in
histidine-poor soft agar. For general mutagenicity screening, a liver
homogenate (9000 g supernatant) from rats induced with a mixture of
polychlorinated biphenyls (Aroclor 1254) is recommended as a metabolic
activation system. For routine testing, the strains TA1535, TA1537 and
TA1538 can be used in combination with the strains carrying the R
factor (TA100 and TA98). This method of testing using various groups
of chemicals and various experimental conditions is described in more
detail by Ames et al. (1975).
Using the genetically well characterized Escherichia coli
strains, reverse and forward mutation can be scored using nutritional
resistance or fermentative markers (Bridges et al., 1972; Mohn, 1973).
Prophage lambda induction in E. coli strains by chemical mutagens
activated by liver microsomal enzymes has been described as a
sensitive test for the detection of potential mutagens and carcinogens
(Moreau et al., 1976).
a Mutations in which the function of a given gene is lost are
called forward mutations. Mutations that bring about the
restoration of gene function are called reverse mutations or back
mutations.
Mutagenesis has been extensively studied in Neurospora crassa.
Heterokaryon has been developed, which is heterozygous for two
closely-linked loci in the ad-3 region. Mutants at the ad-3A and
ad-3B loci have a requirement for adenine and can be selected
directly on the basis of accumulation of a reddish-purple pigment in
the mycelium. The ad-3 mutations can be characterized by a series of
simple genetic tests to distinguish point mutations from deletions and
to obtain a presumptive identification of the genetic alterations in
the point mutations at the ad-3B locus at a molecular level (de
Serres & Malling, 1971).
Saccharomyces cerevisiae and S. pombe have been used to
investigate the effects of carcinogenic and mutagenic compounds in
these organisms. Mitotic gene conversion, in which a sequence of a few
hundred nucleotides of one chromosome is replaced by one corresponding
sequence from a homologous chromosome, can be studied in diploid cells
of the yeast S. cerevisiae strain D-4 heteroallelic at the loci
trp-5 and ade-2. This strain carries two different inactive
alleles of two genes ( trp-5 and ade-2) which are located on
different chromosomes and the functional defects of which lead to a
nutritional requirement. Mitotic gene conversion, which is increased
by many types of mutagenic treatment, can transfer the intact region
of one of these alleles to the defective region of the other, thus
producing a heterozygotic diploid cell with full functional activity.
The mechanism is presumably based on the formation of single-strand
breaks in DNA and probably involves repair processes. Positive results
are only obtained with agents or metabolites which either bind with
DNA covalently or interfere with DNA metabolism. In contrast with most
microbial mutation systems, mitotic gene conversion does not show a
response specific to any type of mutagen. In addition to gene
conversion, forward and reverse mutation can be measured with
S. cerevisiae (Loprieno et al., 1976; Marquardt, 1974; Mortimer &
Manney, 1971; Zimmerman, 1973).
7.2.2.5 Mammalian somatic cells
The application of cultured cell lines is somewhat restricted at
present by requirements for karyotypic stability and high plating
efficiency. A widely used system developed by Chu (1971) employs cell
lines derived from the lung, ovary, and other tissues of Chinese
hamster, which usually maintain a near-diploid chromosome number and
exhibit active growth and high cloning efficiency. Two reviews discuss
this topic in detail (De Mars, 1974; Thompson & Baker, 1973).
Selective media have been developed to detect both forward and reverse
mutations at three genetic loci involving enzymes in the salvage
pathways of purines and pyrimidines. In Chinese hamster, as in man,
the use of preformed hypoxanthine and guanine is controlled by an
X-linked gene. Mutant cells at these loci are deficient in the enzyme
hypoxanthine-guanine phosphoribosyl transferase and are resistant to
certain purine analogues. Similarly, mutant cells deficient in
adenosine phosphoribosyl transferase cannot metabolize preformed
adenosine or its analogues for incorporation into nucleic acid. The
third type of drug-resistant mutants currently studied in these and
other mammalian cells are those deficient in thymidine kinase. Such
cells exhibit resistance to the thymidine analogue,
5-bromodeoxyuridine. Cells carrying mutation at these loci can be
selected from the wild type in an environment containing an
appropriate purine or pyrimidine analogue. Revertants to the wild type
can be recovered in selective media in which the cells are supplied
with a natural purine or pyrimidine while the normal de novo pathway
of purine or pyrimidine synthesis is inhibited by an antimetabolite.
It has been demonstrated that Chinese hamster cells treated with
physical or chemical mutagens undergo a significant increase in the
frequency of forward and reverse mutations compared with the
spontaneous frequency (Huberman & Sachs, 1974; Huberman et al., 1971,
1972). A liver microsomal activation system can be added for the
metabolic activation of chemicals (Krahn & Heidelberger, 1975; Kuroki
et al., 1977).
Forward mutations from thymidine kinase +/- cells to thymidine
kinase -/- have been induced by treatment of cells with X-rays or
chemical mutagens (mouse lymphoma L5178 Y cells). These cells can also
be grown in the presence of in vitro or in vivo metabolic
activation systems, which makes them suitable for host-mediated
mutagenicity tests (Nahas & Capizzi, 1974).
Forward mutations to hypoxanthine-guanine phosphoribosyl
transferase deficiency (resistance to 8-azaguanine) in diploid human
fibroblasts in culture have been shown to occur either spontaneously
or after X-radiation (Albertini & De Mars, 1973). Chemical induction
of forward mutation from hypoxanthine-guanine phosphoribosyl
transferase - to + in human lymphoblastoid cell line has also been
reported (Sato et al., 1972).
7.2.2.6 Host and tissue-(microsome) mediated assays
These take into account the conversion of chemicals into
mutagenic metabolites, and are particularly suitable for the detection
of chemicals that are not mutagenic per se but require metabolic
activation. They can be performed in vivo (host mediated) or in
vitro (tissue mediated) using various indicator organisms, such as
bacteria, fungi, yeast, or mammalian cells.
In the host-mediated assay, the indicator organisms are injected
into the interperitoneal cavity of an animal (Legator & Malling,
1971), which is then treated with the test compound by another route.
After a given length of time, the animal is killed and the indicator
organism is recovered and scored for mutants. Comparison between the
mutagenic action of the compound on a test strain directly and the
host-mediated assay indicates whether the host can activate or
inactivate the test compound. The limitations of the host-mediated
assay are the high spontaneous mutation rates of the indicator
organism in the host, the host's effect on cell survival and the
selection of heterogenic cell populations. In order to measure a
significant increase over the spontaneous mutation rate, doses well
above LD50 have to be given to rats or mice. This type of assay is
also limited since it does not identify the site of conversion to a
mutagenic metabolite. Modifications have been reported (Mohn, 1973).
In the tissue-mediated assay, the indicator organisms are
incubated in vitro in the presence of a tissue fraction plus
appropriate cofactors and the test compound. The mutants are
subsequently isolated and scored.
Ames et al. (1973a,b) have developed a mutagenicity assay that
combines a Salmonella strain, a liver microsomal preparation, and
cofactors in a soft agar layer on a Petri dish. Bartsch et al.
(1975b), Czygan et al. (1973) and Malling (1974) have described a
liquid incubation system where bacteria, a tissue preparation, and the
test compound are incubated in liquid suspension. Test compounds that
are gases at room temperature or are volatile can be assayed by
exposing Petri dishes containing bacteria, tissue fraction, and
cofactors to a mixture of gas and oxygen at 37°C (Bartsch et al.,
1975a). The mutagenicity of urinary metabolites, excreted as
conjugates in experimental animals treated with an indirect mutagen,
may be detected by treating the urine with hydrolytic enzymes in the
presence of a bacterial test strain or yeast, and an in vitro
metabolic activation system (Commoner et al., 1974; Durston & Ames,
1974; Marquardt, 1974). In another modification, Huberman & Sachs
(1974) used lethally irradiated rat fibroblasts that retain a
drug-metabolizing capacity and cocultivated them with Chinese hamster
V79 cells which are used as genetic indicators.
The mutagenicity tests previously described (7.2.2.4, 7.2.2.5,
7.2.2.6) all have individual advantages and limitations determined
either by the genetic indicators or by the metabolic activation
system. The use of submammalian organisms for the detection and
classification of mutants, induced by chemicals, is greatly
facilitated by the relatively small genome to which fine genetic
mapping and biochemical analysis can be applied and by the short
generation time. Huge populations of some of these organisms can be
raised and they can easily be handled when analysing multiple
mutations.
The relevance of such data from submammalian systems to mammalian
cells is based on the assumption that the basic principles of
heredity, and the structure and functions of DNA in terms of
reactivity, the triplet code, transcriptional, and translational
mechanisms, are essentially the same for all living cells irrespective
of their evolutionary level. However, this extrapolation is hampered
by lack of knowledge of repair processes that play a role in mutation
fixation and expression and are insufficiently understood in mammals.
It is obvious that normal human diploid cells are most desirable for
this test, but current studies suggest that reliable conclusions can
be drawn from results with non-human mammalian cells.
The other factor, related to the fact that many chemicals are not
active per se, is the metabolic activation of the compounds. In many
cases, reactive metabolites with a limited life span may fail to reach
or react with the genetic indicator either because they are further
metabolized to inactive compounds, or because they react with other
cellular constituents. For this reason, mutagenicity assays in intact
animals (host-mediated assays) may give negative results, as in the
case of N-methyl- N'-nitro- N-nitrosoguanidine (MNNG) and acridine
mustard (ICR-170), proven to be extremely potent mutagens. Metabolism
in animals is affected by exogenous and endogenous factors such as
chemicals causing enzyme induction and inhibition. Other modifying
factors are age, sex, and strain of animals, diurnal and seasonal
rhythms, differences between the fetal and adult state, mode of
administration, cellular uptake, and distribution and excretion of the
chemical.
The tissue-mediated mutagenicity test cannot, with certainty,
reproduce the in vivo situation, but obvious advantages are the high
sensitivity, good reproducibility, low cost, and the possibility of
testing a large number of chemicals. In addition, in vitro testing
with well-characterized genetic indicators allows the use of human
tissue such as human liver to determine their ability to generate a
mutagen (Bartsch, 1976).
7.2.3 Correlation between short and long-term bioassays for
carcinogenicity
Short-term tests (rapid screening tests) are procedures that do
not have the in vivo production of a visible tumour in animals as an
end point. The variables used in short-term tests to detect chemical
carcinogens are based on an interaction of carcinogens and/or their
metabolites with macromolecules, the induction of chromosomal
aberrations, mutagenesis, DNA repair, and DNA binding.
In the evaluation of these methods, the major question is which
screening tests, singly or collectively, serve as reliable indicators
or predictors of the potential carcinogenic hazard of the chemical.
The answer can only be obtained by testing a representative number of
compounds. A valid test should demonstrate that compounds with known
carcinogenic properties are positive, within the limit of the test,
and negative compounds are negative. If a test is required for
preliminary screening, a small proportion of false negatives or
positive results may be acceptable, but for a final test, no false
negative results are acceptable.
Rapid screening tests should be relatively simple and
inexpensive. They can be used in three ways: to trace carcinogens
and/or mutagens in the complex environment of man; as a tool for
prescreening chemicals to be submitted to a more lengthy set of
bioassays; or for better extrapolation from experimental animal data
to man and for improving the relevance of long-term bioassays in
experimental animals.
Considering reproducibility, cost, the number of chemicals that
can be examined in a short time, and the scientific basis of the test,
tissue-mediated mutagenicity procedures using well-characterized
genetic indicators and a metabolically defined in vitro activation
system, appear at present to be the most promising short-term tests.
With current methods there is still the chance of false negative
results, depending on the systems used, either for lack of appropriate
cofactors for activation or because of the extreme reactivity and/or
toxicity of the compound or its metabolites. However, the number of
false negative results in in vitro tissue-mediated assays is small
compared with other mutagenicity test procedures (Montesano & Bartsch,
1976).
The increasing evidence of a possible correlation between
mutagenicity and carcinogenicity certainly does not mean that one
biological effect may be equated with another. Thus, the mutagenic
activity of a chemical cannot, at present, automatically be assumed to
imply a definite carcinogenic effect in man, nor can these results
replace long-term carcinogenicity testing in animals.
Furthermore, it is still unknown whether all carcinogens will be
found to be mutagenic, and all mutagens, carcinogenic. Examples are
the strong mutagens, nitrous acid, hydroxylamine, and base analogues,
for which no carcinogenic effect in animals has so far been reported;
they do not act via electrophilic intermediates, a mechanism that has
now been recognized for must ultimate carcinogenic forms. Nor have
steroidal sex hormones, carcinogenic in animals, been reported to be
mutagenic, as yet. On the other hand, various chromium salts have
recently been shown to be mutagenic in bacteria (Venitt & Levy, 1974).
Development of cancer in vivo is determined by a variety of
factors that cannot be duplicated in an in vitro short-term testing
system because:
(a) The concentration of ultimate reactive metabolites available
to react in organs in animal species with cellular
macromolecules, which is a consequence of a balance between
metabolic activation and detoxication processes, is only
partly reflected by in vitro testing procedures.
(b) Species and organ specificity of a chemical carcinogen might
be determined, in part, by organ-specific DNA repair.
(c) Since chemical carcinogenesis is thought to be a multi-step
process in which the early, apparently irreversible,
initiation of a cell is followed by several subsequent
stimuli provoking cellular replications, leading to an overt
tumour, a short-term test capable of detecting complete
carcinogens may be useful to detect initiating agents but
cannot at the present time detect the action of promoting
agents.
Currently used short-term tests, in particular tissue-mediated
mutagenicity assays are effective in predicting, with a certain
accuracy, the carcinogenic potential of chemicals (McCann & Ames,
1976; Purchase et al., 1976; Sugimura et al., 1976), but give no
indication of the target organs or species specificity of their
carcinogenic activity. Furthermore, the relative potency of a chemical
to induce biological effects, defined as the endpoints, for the most
frequently used rapid screening tests cannot at the present time be
reliably correlated with its carcinogenic potency (Bartsch et al.,
1977; Meselson & Russell, 1977). More data are needed to compare
dose-response curves obtained in rapid screening tests on a given
chemical with those of other biological effects obtained in vivo
(long-term bioassays).
7.2.4 Significance of experimental testing for assessing the possible
carcinogenic risk of chemicals to man
Experience acquired, so far, in long-term carcinogenicity testing
has shown that nearly all compounds that are carcinogenic in man are
also carcinogenic in one or several animal species, even though the
tumour type may not be the same as in man. The concept that animal
carcinogenicity data are predictive of a human carcinogenic risk and
useful in preventing human cancer was accepted in 1941 in the case of
2-actylaminofluorene (AAF). This chemical was not used on a worldwide
basis as an insecticide, because some experiments had already shown
its carcinogenicity before it was marketed (Wilson et al., 1941); its
restriction was facilitated by the existence of a number of
substitutes at the time when the results of the first experiments on
AAF were reported. This cautious attitude has not been consistently
applied in other situations, on the grounds that the experimental data
were insufficient or inadequate to evaluate the possible hazard to
man. This is shown by the various relationships between experimental
carcinogenicity data and possible human hazard that are considered in
the adoption of preventive measures.
The first observation that some aromatic amines were involved in
the causation of urinary bladder tumours in man dates from 1896; it
was confirmed in 1907 and reconfirmed many times thereafter (IARC,
1974). In 1938, Hueper et al. reported the induction of bladder cancer
in dogs exposed to 2-naphthalenamine (2-naphthylamine). Preventive
measures were taken only in the late 1950s, when conspicuous
epidemiological evidence had accumulated. In this case, the human
epidemiological evidence was judged insufficient, and the experimental
evidence, when it became available, was also deemed insufficient until
further epidemiological findings came to hand.
The delay in taking preventive measures applies also to
carcinogens on which, contrary to the case of aromatic amines,
experimental evidence preceded observations of their effect in man,
e.g. diethylstilbestrol, bis(chloromethyl)ether, and chloroethylene
(vinyl chloride). (Tomatis et al. 1978; Montesano & Tomatis, 1977).
One of the main objections to carcinogenicity tests on animals is
that the experimental system used tries to produce the maximum
possible carcinogenic effect of the test chemical, does not reflect
the human situation, and is therefore misleading. It is difficult,
however, to accept this argument because, in most instances where a
chemical was found by epidemiological investigations to be associated
with cancer in man, the incidence was so high that the association was
clear without animal studies. This has been the case for high risk
groups such as occupational cancer groups. However, the risk is not
confined to these groups but also applies to other populations where
cancer incidence may be too low for detection by normal
epidemiological methods, hence the need to carry out animal
experiments under conditions that permit confident judgment of the
carcinogenicity or inactivity of a chemical.
Cancer testing in animals has reached a relatively sophisticated
stage and an exhaustive study of a chemical in animals is sufficient
evidence of a potential cancer risk for man. An assessment of the
validity of experimental results is essential for the successful
prevention of cancer in man. This does not preclude further research
for the development of short-term tests, but, at the present time,
these cannot replace long-term carcinogenicity testing.
7.3 Heritable Mutations
As noted in the introduction, direct methods for assessing
whether a chemical has the potential to cause heritable mutations in
man do not exist at present. Nontheless, information relative to the
possible production in man of germ cell mutations can be derived from
a variety of sources. These fall into three major categories: (a)
primary DNA damage involving DNA alteration, stimulation of DNA
repair, gene mutations tests including mutagenicity assessment using
bacterial or other microorganisms, with and without metabolic
activation; (b) whole animal tests for point or gene mutations
including insects, e.g. drosophila recessive, and the specific locus
test in mice; (c) chromosomal mutations including cytogenetic tests in
mammals, the dominant lethal test in mammals, and the heritable
translocation test in rodents. Examples of the first category have
already been presented. These tests provide information relevant to
heritable mutations and section 7.2.2 should be consulted for fuller
details. Obviously, tests employing isolated systems only provide
information on mutagenic action on the specific systems tested. This
information cannot be fully interpreted without evidence concerning
the access of this ultimate mutagen to the germ cells.
An extensive examination of relevant test procedures for
heritable mutations has recently been completed (DHEW, 1977).
7.3.1 Whole-animal tests
Insects. Drosophila melanogaster is one of the best genetically
characterized species and is widely used. Drosophila of either sex can
be treated and mutation frequencies from successive germ cell stages
may be obtained after observation of three generations. The X-lined
recessive lethal test is one of the most sensitive tests with
drosophila, since the X-chromosome represents about 1/5th of the whole
genome. In contrast to most microorganisms, insects possess an enzyme
system that appears to metabolize foreign compounds in a fashion
similar to that of vertebrates (Abrahamson & Lewis, 1971; Fahmy &
Fahmy, 1972, 1973; Sobels & Vogel, 1976).
Mouse specific locus test. The specific locus test is a method
of inducing, detecting, and measuring the rate of mutation at several
recessive loci. It consists essentially of mating treated or untreated
wild type mice, either male or female, to a strain homozygous for a
number of known recessive genes. The recessive genes are such that
they are readily expressed as visible phenotypes in homozygous state.
If a mutation occurs in any of the test loci in the germ cells of
treated animals, it may be detected in the offspring. If no mutation
has occurred following treatment, the progeny from the cross will all
be of the wild type (Cattanach, 1971; Russell, 1951).
Chromosomal mutations. Although the molecular basis is not
understood, a change in the whole chromosome, i.e. a structural
chromosome aberration, occurring as a consequence of a misrepair of
chromosomal breaks, may lead to deletions, duplications, and
translocations. Changes in the whole chromosome complement, i.e.
numerical chromosome aberrations, arise through nondisjunction, as in
failure of a pair of chromosomes to separate during gametogenesis,
meiotic nondisjunction, or during mitotic division. The resulting
daughter cells are either trisomic with an extra chromosome or
monosomic and lacking a chromosome. Anaphase lag occurs during nuclear
division in the progeny cells, and a chromosome may be either lost or
gained. In somatic cells, nondisjunction and anaphase lag can lead to
mosaicism.
Chromosomal damage may be studied in a number of test systems
that are best divided into in vivo and in vitro systems, on the
basis of their capability to metabolize the test compound (Frohberg,
1973). The short-term human lymphocyte culture in vitro is commonly
used to assess the effects of chemicals upon chromosomes. Metabolic
activation of the test compound can be achieved by addition of a liver
microsomal system (Bimboes & Greim, 1976).
The micronucleus test. As an in vivo cytogenetic method, the
micronucleus test is a procedure for the detection of aberrations
involving anaphase chromosome behaviour using bone-marrow
erythroblasts. The test is based on the formation of micronuclei from
particles of chromatin material which, due to chromosome breakage or
spindle disjunction, do not migrate to the poles during anaphase and
are not incorporated into the telophase nuclei of the dividing cells.
The procedure is to treat animals with clastogenic agents and, at an
appropriate time after treatment, to aspirate bone-marrow samples into
calf serum. This is then centrifuged and smears are made from the
resuspended pellets of cells. The smears are air-dried and stained.
The evaluation of the bone-marrow preparations involves examination of
2000-5000 erythrocytes per specimen; the total number of polychromatic
and normochromatic erythrocytes with and without micronuclei are
recorded (Schmid, 1973). Sister chromatid exchanges, induced by
mutagens in vivo and in vitro can be scored in peripheral
lymphocytes or Chinese hamster cells, using the differential straining
of chromatids substituted with 5-bromodeoxyuridine in place of
thymidine (Natarajan et al., 1976; Smythe & Evans, 1976; Stetka &
Wolf, 1976).
Dominant lethal tests and other in vivo systems. The dominant
lethal test is based on the preimplantation loss of eggs or on the
formation of dead embryonic implants following the injection of
mutagens into a male or female mouse at a specific time before mating
(Bateman & Epstein, 1971). Dominant lethal tests assume that a single
mutation has occurred in the eggs or sperm, which is lethal to the
embryo and heterozygous at the affected locus. However, a disturbingly
high number of mutagens have given a negative response with this test.
The detection of mutations arising from compounds that are metabolized
to transient reactive intermediates not produced in germ cells or not
reaching them from other organs may limit this test. Furthermore,
chemical agents causing spermatogenic arrest or cytocidal effect on
the sperms may give false positive results.
Heritable translocation in male mammals. The heritable
translocation test has the important feature of measuring sexually
transmissible germ cell mutations in rodent spermatogonia. Generoso et
al. (1974) have described details of the technique and discussed its
usefulness for the routine screening of substances that cause
chromosomal mutations.
Young adult male mice are treated and females mated with the
exposed males on a schedule that can be used for the comparison of the
sensitivity of the different male germ cell stages. Male progeny from
these matings are collected and mated for the determination of
semisterility and sterility. Progeny with reduced fertility are
subjected to cytogenetic analysis. The cytological examination of
dividing spermatocytes, from animals treated with test chemicals that
cause breaks on two nonhomologous chromosomes, yields aberrant
chromosomal figures. These are recognized as rings or chains of four
chromosomes as opposed to the normal bivalent chromosomal
configuration.
A proper evaluation of this test cannot be made at this time
because of insufficient data in terms of the variety of chemicals
tested to date.
7.3.2 Monitoring of human populations
In spite of the variety of test systems available for detecting
the mutagenic effects of chemicals, it is difficult, from the results
obtained at present, to evaluate with confidence the transmissible
genetic effects caused by the chemical exposure of man. However, the
potential hazards of mutations are such that every effort should be
made to reduce the risk.
Judgment on the potential mutagenic hazard to man should be based
on various considerations such as strength of the experimental
evidence of mutagenicity, the exposure pattern to the chemical, and
the pharmacological properties of the compounds. This is difficult and
complex. Although human studies are difficult, expensive, and often
subject to misinterpretation, only studies which directly estimate the
extent to which environmental factors change human genetic material
give definitive answers.
Proper monitoring of the human population (Crow, 1971; Sutton,
1972) can be carried out by three main approaches:
(a) biochemical: detection of inherited protein variants;
(b) cytogenetic: screening of blood of newborn infants or of
fetuses delivered by spontaneous abortion for chromosomal
changes;
(c) phenotype: surveillance of genetically determined disease or
anomalies.
The validity of these monitoring systems can only be properly
evaluated if, and when, a mutagen is actually discovered.
7.3.3 Significance of tests for heritable mutations
As mentioned earlier, there is, at this time, no direct
correlation between laboratory tests for heritable mutations and human
experience. However, the available body of information from nonhuman
mammals and lower life forms clearly points to the ability of
chemicals to produce alterations in germ cells which are inherited in
succeeding generations. Accordingly, the implications of such data for
man are so persuasive that they must be taken into account in
establishing safety measures for the introduction of chemicals into
use. This is especially true, at this time, since techniques for
detecting mutations in human populations are so insensitive that a
significantly mutagenic chemical could easily escape attention.
These considerations will almost certainly lead to increasing
concern in establishing regulatory and control procedures. In fact,
the Environmental Protection Agency in the USA has recently proposed
preliminary guidelines for conducting tests for heritable mutations as
part of the routine registration procedure for pesticides.
REFERENCES
ABRAHAMSON, S. & LEWIS, E. B. (1971) The detection of mutations in
Drosophila melanogaster. In: Hollaender, A., ed. Chemical
mutagens, principles and methods for their detection, Vol. 2,
461-488.
ALBERTINI, R. J. & DEMARS, R. (1973) Somatic cell mutation detection
and quantification of X-ray induced mutation in cultured human
diploid fibroblasts. Mutat. Res., 18, 199-244.
AMES, B. N. (1971) The detection of chemical mutagens with enteric
bacteria. In: Hollaender, A., ed. Chemical mutagens, principles
and methods for their detection, Vol. 1, pp. 267-282.
AMES, B. N., SIMS, P., & GROVER, P. L. (1972a) Epoxides of
carcinogenic polycyclic hydrocarbons are frameshift mutagens.
Science, 176, 47-49.
AMES, B. N., GURNEY, E.G., MILLER, J. A., & BARTSCH, H. (1972b)
Carcinogens as frameshift mutagens: Metabolites and derivatives
of 2-acetylaminofluorene and other aromatic amine carcinogens.
Proc. Natl Acad. Sci., 69: 3128-3132.
AMES, B. N., LEE, F. D., & DURSTON, W. E. (1973a) An improved
bacterial test system for the detection and classification of
mutagens and carcinogens. Proc. Natl Acad. Sci., 70: 782-786.
AMES, B. N., DURSTON, W. E., YAMASAKI, E., & LEE, F. D. (1973b)
Carcinogens are mutagens: a simple test system combining liver
homogenates for activation and bacteria for detection. Proc.
Natl Acad. Sci., 70; 2281-2285.
AMES, B. N., MCCANN, J., & YAMASAKI, E. (1975) Methods for detecting
carcinogens and mutagens with the Salmonella/mammalian-
microsome mutagenicity test. Mutat. Res., 31: 347-364.
BARTSCH, H. (1976) Predictive value of mutagenicity tests in chemical
carcinogenesis. Mutat. Res., 38: 177-190.
BARTSCH, H. & GROVER, P. L. (1976) Chemical carcinogenesis and
mutagenesis. In: Symington, T. & Carter, R. L., ed. Scientific
foundations of oncology, London, William Heinemann Medical
Books Ltd, pp. 334-342.
BARTSCH, H., MALAVEILLE, C., & MONTESANO, R. (1975a) Human, rat and
mouse liver-mediated mutagenicity of vinyl chloride in
S. typhimurium strains. Int. J. Cancer, 15: 429-437.
BARTSCH, H., MALAVEILLE, C., & MONTESANO, R. (1975b) In vitro
metabolism and microsome-mediated mutagenicity of
dialkylnitrosamines in rat, hamster and mouse tissues. Cancer
Res., 35: 644-651.
BARTSCH, H., MALAVEILLE, C., STICH, H. F., MILLER, E. C., & MILLER, J.
A. (1977) Comparative electrophilicity, mutagenicity, DNA repair
induction activity, and carcinogenicity of some N- and O-Acyl
derivatives of N-Hydroxy-2-aminofluorene. Cancer Res.,
37: 1461-1467.
BATEMAN, A. J. & EPSTEIN, S.S. (1971) Dominant lethal mutations in
mammals. In: Hollaender, A., ed. Chemical mutagens, principles
and methods for their detection, Vol. 2, pp. 541-568.
BERWALD, Y. & SACHS, L. (1963) In vitro transformation with chemical
carcinogens. Nature (Lond.), 200: 1182-1184.
BIMBOES, D. & GREIM, H. (1976) Human lymphocytes as target cells in a
metabolizing test system in vitro for detecting potential
mutagens. Mutat. Res., 35: 155-160.
BORLAND, R. & HARD, G. C. (1974) Early appearance of "transformed"
cells from the kidneys of rats treated with a "single"
carcinogenic dose of dimethylnitrosamine (DMN) detected by
culture in vitro. Europ. J. Cancer, 10: 177-184.
BRIDGES, B. A. (1976) Short-term screening tests for carcinogens.
Nature (Lond.), 261: 195-200.
BRIDGES, B. A., MOTTERSHEAD, R. P., ROTHWELL, M. A., & GREEN, M. H. L.
(1972) Repair deficient bacterial strains suitable for
mutagenicity screening tests with fungicide captan. Chem.-Biol.
Interact., 5: 77-84.
BROWN, A.M. (1973) In vitro transformation of submandibular gland
epithelial cells and fibroblasts of adult rats by
methylcholanthrene. Cancer Res., 33: 2779-2789.
CATTANACH, B. M. (1971) Specific locus mutation in mice. In:
Hollaender, A., ed. Chemical mutagens, principles and methods
for their detection, Vol. 2, pp. 535-540.
CHU, E. H. Y. (1971) Induction and analysis of gene mutations in
mammalian cells in culture. In: Hollaender, A., ed. Chemical
mutagens, principles and methods for their detection, Vol. 2,
pp. 411-444.
CLEAVER, J. E. (1969) Xeroderma pigmentosum: a human disease in
which an initial state of DNA repair is defective. Proc. Natl
Acad. Sci., 63: 428-435.
CLEAVER, J. E. (1973) DNA repair with purines and pyrimidines in
radiation- and carcinogen-damaged normal and Xeroderma
pigmentosum human cells. Cancer Res., 33: 362-369.
CLEAVER, J. E. (1974) Repair processes for photochemical damage in
mammalian cells. In: Lett, J. T., Adler, H., & Zelle, M., ed.
Advances in radiation biology, New York, Academic Press, Vol.
4, pp. 1-75.
CLEAVER, J. E. (1975) Methods for studying repair of DNA damaged by
physical and chemical carcinogens. In: Bush, E., ed. Methods in
cancer research, New York, Academic Press, Vol. 11, pp.
123-165.
CLEAVER, J. E., GOTH, R., & FRIEDBERG, E. C. (1975) Value of
measurements of DNA repair levels in predicting carcinogenic
potential of chemicals. In: Montesano, R., Bartsch, H., &
Tomatis, L., ed. Screening tests in chemical carcinogenesis,
IARC Sci. Publ. No. 12, pp. 639-661.
COMMONER, B., VITHAYATHIL, A. J., & HENRY, J. I. (1974) Detection of
metabolic carcinogen intermediates in urine of carcinogen-fed
rats by means of bacterial mutagenesis. Nature (Lond.),
249: 850-852.
CONNEY, A. H. & BURNS, J. J. (1972) Metabolic interactions among
environmental chemicals and drugs. Science, 178: 576-586.
CORBETT, T. H., HEIDELBERGER, C., & DOVE, W. F. (1970) Determination
of the mutagenic activity to bacteriophage T-4 of carcinogenic
and non-carcinogenic compounds. Mol. Pharmacol., 6(6): 667-679.
COUNCIL OF THE ENVIRONMENTAL MUTAGEN SOCIETY (1975) Environmental
mutagenic hazards, mutagenicity screening is now both feasible
and necessary for chemicals entering the environment. Science,
187: 503-514.
CRADDOCK, V. M. (1976) Replication and repair of DNA in liver of rats
treated with dimethylnitrosamine and with methyl
methanesulphonate. In: Magee, P. N. et al., ed. Fundamentals in
cancer prevention, Tokyo, Univ. Tokyo Press; Baltimore, Univ.
Park Press, pp. 293-311.
CROW, J. F. (1971) Human population monitoring. In: Hollaender, A, ed.
Chemical mutagens, principles and methods for their detection,
Vol. 2, pp. 591-606.
CZYGAN, P. J., GREIM, H., GARRO, A. J., HUTTERER, F., SCHAFFAER, F.,
POPPER, H., ROSENTHAL, O., & COOPER, D. Y. (1973) Microsomal
metabolism of DMN and the cytochrome activation to a mutagen.
Cancer Res,, 33: 2983-2986.
DELLA PORTA, G. & TERRACINI, B. (1969) Chemical carcinogenesis in
infant animals. Progr. Exp. Tumour Res., 11: 334-363.
DE MARS, R. (1974) Resistance of cultured human fibroblasts and other
cells to purine and pyrimidine analogues in relation to
mutagenesis detection. Mutat. Res., 24: 335-364.
DHEW (1977) Approaches to determining the mutagenic properties of
chemicals: Risks to future generations. Report of a working
group of the Subcommittee on Environmental Mutagenesis.
DI PAOLO, J. A., NELSON, R. L., & DONOVAN, P. J. (1972) In vitro
transformation of Syrian hamster embryo cells by diverse chemical
carcinogens. Nature (Lond.), 235: 278-280.
DI PAOLO, J. A., NELSON, R. L., DONOVAN, P. J., & EVANS, C. H. (1973)
Host-mediated in vivo-in vitro assay for chemical
carcinogenesis. Arch. Pathol., 95: 380-385.
DE SERRES, F. J. & MALLING, H. V. (1971) Measurement of recessive
lethal damage over the entire genome and at two specific loci in
the ad-3 region of a two-component heterokaryon of Neurospora
Crassa. In: Hollaender, A., ed. Chemical mutagens, principles
and methods for their detection, Vol. 2, pp. 311-342.
DRAKE, J. W. (1971) Mutagen screening with virulent bacteriophages.
In: Hollaender, A., ed. Chemical mutagens, principles and
methods for their detection, Vol. 1, pp. 219-234.
DRUCKREY, H., PREUSSMAN, R., IVANKOVIC, S., & SCHMÄL D. (1967)
[Organotropic carcinogenic effects of 65 different N-nitroso
compounds on BD-rats.] Z. Krebsforsch., 69 103-201.
DURSTON, W. E. & AMES, B. N. (1974) A simple method for the detection
of mutagens in urine: Studies with the carcinogen
2-acetylaminofluorene. Proc. Natl Acad. Sci., 71: 737-741.
EARLE, W. R. & NETTLESHIP, A. (1943) Production of malignancy in
vitro. V. Results of injections of cultures into mice. J. Natl
Cancer Inst., 4 213-227.
FAHMY O. G. & FAHMY, M.D. (1972) Mutagenic properties of AAF and its
metabolites. Int. J. Cancer, 9 284-298.
FAHMY O. G. & FAHMY, M.D. (1973) Mutagenic properties of
benzo(a)pyrene. Cancer Res., 33: 302-309.
FARBER, E. (1973) Carcinogenesis -- cellular evolution as a unifying
thread: presidential address. Cancer Res., 33: 2537-2550.
FDA (1971) Panel on carcinogenesis report on cancer testing in the
safety evaluation of food additives and pesticides. Toxic. appl.
Pharmacol., 20: 419-438.
FEDOROFF, S. (1967) Proposed usage of animal tissue culture terms,
J. Natl Cancer Inst., 38: 607-611.
FREESE, E. & STRACK, H. B. (1962) Induction of mutations in
transforming DNA by hydroxylamine. Proc. Natl Acad. Sci.,
48: 1796-1803.
FRIEDMAN, L. (1974) Dose selection and administration. In: Goldberg,
L., ed. Carcinogenesis testing of chemicals, Cleveland, CRC
Press, pp. 21-22.
FROHBERG, H. (1973) Critique of in vivo cytogenic test systems.
Agents Actions, 3: 119-123.
FUSENIG, N. R., SAMSEL, W., THON, W., & WORST, P. K. M. (1973)
Malignant transformation of epidermal cells in culture by DMBA.
In: Prunieras, M., Robert, L., & Rosenfeld, C., ed.
Differentiation of eukaryotic cells in culture. Colloque of
INSERM, Vol. 19, pp. 219-228.
GERCHMAN, L. L. & LUDLUM, D. B. (1973) The properties of
O6-methylguanine in template for RNA polymerase. Biochim.
biophys. Acta, 308: 310-316.
GIOVANELLA B, C., YIM, S. O., STEHLIN, J. S., & WILLIAMS, L. J. Jr
(1972) Brief communications: Development of invasive tumors in
the "nude" mouse after injection of cultured human melanoma
cells. J. Natl Cancer Inst., 48: 1531-1533.
GOTH, R. & RAJEWSKY, M. F. (1974) Molecular and cellular mechanisms
associated with pulse-carcinogenesis in the rat nervous system by
ethylnitrosourea: ethylation of nucleic acids and elimination
rates of ethylated bases from the DNA of different tissues.
Z. Krebsforsch., 82: 37-64.
HASHIMOTO, Y. & KITAGAWA, H. (1974) In vitro neoplastic
transformation of epithelial cell of rat urinary bladder by
nitrosamines. Nature (Lond.), 252: 497-499.
HEALTH AND WELFARE, CANADA (1973) The testing of chemicals for
carcinogenicity, mutagenicity, teratogenicity.
HEIDELBERGER, C. (1973) Chemical oncogenesis in culture. Adv. Cancer
Res., 18: 317-366.
HERRIOTT, R. M. (1971) Effects on DNA: Transforming principle. In:
Hollaender, A., ed. Chemical mutagens, principles and methods
for their detection, Vol. 1, pp. 175-218.
HUBERMAN, E. & SACHS, L. (1974) Cell-mediated mutagenesis of mammalian
cells with chemical carcinogens. Int. J. Cancer, 13: 326-333.
HUBERMAN, E., ASPIRAS, L., HEIDELBERGER, C., GROVER, P. L., & SIMS, P.
(1971) Mutagenicity to mammalian cells of epoxides and other
derivatives of polycyclic hydrocarbons. Proc. Natl Acad. Sci.,
68: 3195-3199.
HUBERMAN, E., DONOVAN, P. J., & DIPAOLO, J. A. (1972) Mutation and
transformation of cultured mammalian cells by
N-acetoxy- N-2-fluorenylacetamide. J. Natl Cancer Inst.
48: 837-840.
HUEPER, W. C., WILEY, F. H., & WOLFE, H. D. (1938) Experimental
production of bladder tumours in dogs by administration of
ß-naphthylamine. J. industr. Hyg. Toxicol., 20: 46-84.
IARC (1976) Monographs on the evaluation of carcinogenic risk of
chemicals to man: some aromatic amines, hydrazine and related
substances, N- nitroso compounds and miscellaneous alkylating
agents. Lyons, International Agency for Research on Cancer,
Vol. 4.
IYPE P. T. (1974) Studies on chemical carcinogenesis in vitro using
adult rat fiver cells. In: Montesano, R. & Tomatis, L., ed.
Chemical carcinogenesis essays, IARC Sci. Publ. No. 10,
pp. 119-133.
J. Natl Cancer Inst., 53: 1427-1519 (1974) Proceedings of the
Symposium "New Horizons for Tissue Culture in Cancer Research".
KAKUNAGA, T. The transformation of human diploid cells by chemical
carcinogens. In: Hiatt, H. H. et al., ed. Origins of human
cancer (1977) Cold Spring Harbor Laboratory. pp. 1537-1548.
KATSUTA, H. & TAKAOKA, T. (1972) Carcinogenesis in tissue culture.
XIV. Malignant transformation of rat liver parenchymal cells
treated with 4-nitroquinoline 1-oxide in tissue culture. J. Natl
Cancer Inst., 49: 1563-1536.
KONDO, S. (1973) Evidence that mutations are induced by errors in
repair and replication. Genet. suppl., 73: 109-122.
KRAHN, D. F. & HEIDELBERGER, C. (1975) Microsome-mediated mutagenesis
in Chinese hamster cells by chemical oncogens. Proc. Am. Assoc.
Cancer Res., 16: 74.
KRIEK, E. (1974) Carcinogenesis by aromatic amines. Biochem.
Biophys. Acta, 355: 177-203.
KUROKI, T. (1975) Contributions of tissue culture to the study of
chemical carcinogenesis: A review. In: Recent topics in chemical
carcinogenesis, Gann, Monograph on Cancer Res., 17: 69-85.
KUROKI, T., DREVON, C., & MONTESANO, R. (1977) Microsome-mediated
mutagenesis in V-79 Chinese hamster cells by various
nitrosamines. Cancer Res., 1044-1050.
LAERUM, O. D. & RAJEWSKY, M. F. (1975) Neoplastic transformation of
foetal rat brain cells in culture following exposure to
ethylnitrosourea in vivo. J. Natl Cancer Inst., 55: 1177-1187.
LEGATOR, M. S. & FLAMM, W. G. (1973) Environmental mutagenesis and
repair. Ann. Rev. Biochem., 683-708.
LEGATOR, M. S. & MALLING, H. V. (1971) The host-mediated assay, a
practical procedure for evaluating potential mutagenic agents in
mammals. In: Hollaender A., ed. Chemical mutagens, principles
and methods for their detection, Vol. 2, pp. 569-590.
LOPRIENO, N., BARALE, R., BARONCELLI, S., BARTSCH, H., BRONZETTI, G.,
CAMMELLINI, A., CORSI, C., FREZZA, D., NIERI, R., LEPORINI, C.,
ROSELLINI, D., & ROSSI, A.M. (1976) Induction of gene mutations
and gene conversions by vinyl chloride metabolites in yeast.
Cancer Res., 37: 253-257.
LOVELESS, A. (1969) Possible relevance of O6-alkylation of
deoxyguanosine to the mutagenicity and carcinogenicity of
nitrosamines and nitrosamides. Nature (Lond.), 233: 206-207.
LOVELESS, A. & HAMPTON, C. L. (1969) Inactivation and mutation of
coliphage T2 by N-methyl- and N-ethyl- N-nitrosourea.
Mutation Res., 7: 1-12.
LUDLUM, D. B. (1970) Alkylated polycytidic acid templates for RNA
polymerase. Biochim. biophys. Acta, 213: 142-148.
MAGEE, P. N., MONTESANO, R., & PREUSSMAN, R. (1976) N-nitroso
compounds and related carcinogens. In: Searle, C., ed. Chemical
carcinogens, ACS monograph No. 173, pp. 491-625.
MAHER, V., MILLER, E. C., MILLER, J. A., & SZYBALSKI, W. (1968)
Mutations and decreases in density of transforming DNA produced
by derivatives of the carcinogens 2-acetylaminofluorene and
N-methyl-4-aminoazobenzene. Mol. Pharmacol., 4: 411-426.
MAHER, V. M., CURREN, R. D., OUELLETTE, L. M., & MCCORMICK, J. J.
(1976) Effect of DNA repair on the frequency of mutations induced
in human cells by ultraviolet irradiation and by chemical
carcinogens. In: Magee, P. N., et al, ed. Fundamentals in cancer
prevention, Tokyo, Univ. of Tokyo Press; Baltimore, Univ Park
Press, pp. 363-382.
MALLING, H. V. (1974) Mutagenic activation of dimethylnitrosamine and
diethylnitrosamine in the host-mediated assay and the microsomal
system. Mutat. Res., 26: 465-472.
MARGISON, G. P., MARGISON, J. M., & MONTESANO, R. (1976) Methylated
purines in the deoxyribonucleic acid of various Syrian golden
hamster tissues after administration of a hepatocarcinogenic dose
of dimethylnitrosamine. Biochem. J., 157: 627-634.
MARQUARDT, H. (1974) Mutation and recombination experiments with yeast
as pre-screening tests for carcinogenic effects.
Z. Krebsforsch., 81: 333-346.
MCCANN, J. & AMES, B. N. (1976) Detection of carcinogens as mutagens
in the Salmonella/microsome test: Assay of 300 chemicals:
Discussion. Proc. Natl Acad. Sci., 73: 950-954.
MCCANN, J., SPINGARN, N. E., KOBORI, J., & AMES, B. N. (1975)
Detection of carcinogens as mutagens: bacterial tester strains
with R factor plasmids. Proc. Natl Acad. Sci., 72: 979-983.
MESELSON, M. & RUSSELL, K. (1977) Comparisons of carcinogenic and
mutagenic potency. In: Hiatt, H. H. et at. ed. Origins of human
cancer. Cold Spring Harbor Laboratory. pp. 1473-1481.
MILLER, J. A. (1970) Carcinogenesis by chemicals: an overview -- G. H.
A. Clowes Memorial Lecture. Cancer Res., 30: 559-576.
MILLER, J. A. (1973) Naturally occurring substances that can induce
tumors. Proc. Natl Acad. Sci., 23: 508-549.
MILLER, E. C. & MILLER, J. A. (1971a) The mutagenicity of chemical
carcinogens: correlations, problems, and interpretations. In:
Hollaender, A., ed. Chemical mutagens, principles and methods
for their detection, Vol. 1, pp. 83-120.
MILLER, J. A. & MILLER, E. C. (1971b) Chemical carcinogenesis,
mechanisms and approaches to its control. J. Natl Cancer Inst.,
47: V-XIV.
MILLER, E. C. & MILLER, J. A. (1976) The metabolism of chemical
carcinogens to reactive electrophiles and their possible
mechanisms of action in carcinogenesis. In: Searle, C. E., ed.
Chemical carcinogens, ACS Monograph 173. Washington, DC,
American Chemical Society, pp. 737-762.
MOHN, G. (1973) Revertants of an Escherichia coli K-12 strain with
high sensitivity to radiations and chemicals. Murat. Res.,
19: 349-355.
MOHN, G. & ELLENBERGER, J. (1973) Mammalian blood mediated
mutagenicity test using a multi-purpose strain of E. coli,
K-12. Mutat. Res., 19: 257-260.
MONTESANO, R. & BARTSCH, H. (1976) Mutagenic and carcinogenic
N-nitroso compounds: Possible environmental hazards. Mutat.
Res., 32: 179-228.
MONTESANO, R., SAINT VINCENT, L., & TOMATIS, L. (1973)
Malignant transformation in vitro of rat liver cells by
dimethylnitrosamine and N-methyl- N'-nitro-
N-nitrosoguanidine. Brit. J. Cancer, 38: 215-220.
MONTESANO, R., SAINT VINCENT, L., DREVON, C,, & TOMATIS, L. (1975)
Production of epithelial and mesenchymal tumours with rat liver
cells transformed in vitro. Int. J. Cancer. 55: 1177-1187.
MONTESANO, R., DREVON, C., KUROKI, T., SAINT VINCENT, L., HANDLEMAN,
S., SANFORD, K. K., DEFEO, D. & WEINSTEIN, I. B. (1977) Test for
malignant transformation of rat liver cells in culture: Cytology,
growth in soft agar and production of plasminogen activator.
J. Natl Cancer Inst., 59: 1651-1658.
MONTESANO, R., BARTSCH, H., & TOMATIS, L., ed. (1976) Screening tests
in chemical carcinogenesis. IARC Sci. Publ. No. 12.
MONTESANO, R. & TOMATIS, L. (1977) Legislation concerning chemical
carcinogens in several industrialized countries. Cancer Res.,
37: 310-316.
MOREAU, P., BAILONE, A., & DEVORET, R. (1976) Prophage lambda
induction in Escherichia coli K12 envA uvrB: A highly
sensitive test for potential carcinogens. Proc. Natl Acad. Sci.,
73: 3700-3704.
MORTIMER, R. K. & MANNEY, T. R. (1971) Mutation induction in yeast.
In: Hollaender, A., ed. Chemical mutagens, principles and
methods for their detection, Vol. 1, pp. 289-310.
NAHAS, A. & CAPIZZI, R. L. (1974) Effect of in vivo treatment with
L asparaginal on the in vitro uptake and phosphorylation of
some anti-leukemic agents. Cancer Res., 34: 2687-2693.
NAPALKOV, N. P. (1973) Some general considerations on the problem of
transplacental carcinogenesis. In: Tomatis, L. & Mohr, U., ed.
Transplacental carcinogenesis, IARC Sci. Publ. No. 4, pp. 1-13.
NAPALKOV, N. P. & ALEXANDROV, V. A. (1968) On the effects of
blastomogenic substances on the organism during embryogenesis.
Z. Krebsforsch., 71: 32-50.
NATARAJAN, A. T., TATES, A.D., VAN BUUL, P. P. W., MEIJERS, M., & DE
VOGEL, N. (1976) Cytogenetic effects of mutagens/carcinogens
after activation in a microsomal system in vitro. I. Induction
of chromosome aberrations and sister chromatid exchanges by
diethylnitrosamine (DEN) and dimethylnitrosamine (DMN) in CHO
cells in the presence of rat-liver microsomes. Murat. Res.,
37: 83-90.
MATIONAL ACADEMY OF SCIENCES (1960) Problems in the evaluation of
carcinogenic hazard from use of food additives. Washington, Natl
Res. Council (Publ. No. 479).
NCI (1976) NCI Technical Report Series, No. 1 (Guidelines for
carcinogen bioassay in small rodents), pp. 1-65 (DHEW Publication
No. (NIH) 76-701).
PEGG, A. E. (1977) Formation and metabolism of alkylated nucleosides:
Possible role in carcinogenesis by nitroso compounds and
alkylating ageNTs. Adv. Cancer Res., 25: 195-269.
PEGG, T. & NICOLL, J. W. (1976) Nitrosamine carcinogenesis: The
importance of the persistence in DNA of alkylated bases in the
organotropism of tumour induction. In: Montesano, R., Bartsch,
H., & Tomatis, L., ed. Screening tests in chemical
carcinogenesis, IARC Sci. Publ. No. 12, pp. 571-592.
PIETRA, G., RAPPAPORT, H., & SHUBIK, P. (1961) The effects of
carcinogenic chemicals in newborn mice. Cancer, 14: 308-317.
PITOT, H. C. (1974) Neoplasia: A somatic mutation or a heritable
change in cytoplasmic membranes? J. Natl Cancer Inst.,
53: 905-911.
PITOT, H. C. (1977) The natural history of neoplasia. Am. J. Path.,
89: 402-411.
PURCHASE, I. F. H., LONGSTAFF, E., ASHBY, J., STYLES, J. A., ANDERSON,
D., LEFEVRE, P. A., & WESTWOOD, F. R. (1976) Evaluation of six
short-term tests for detecting organic chemical carcinogens and
recommendations for their use. Nature (Lond.), 264: 624-627.
REUBER, M. (1974) Criteria for tumor diagnosis and classification of
malignancy. In: Golberg, L., ed. Carcinogenesis testing of
chemicals, Cleveland, CRC Press, pp. 71-73.
ROSENKRANZ, H. S. (1973) Aspects of microbiology in cancer research.
Rev. Microbiol., 1622: 383-400.
RUSSELL, W. L. (1951) X-ray induced mutations in mice. Cold Spring
Harbour Symp. Quant. Biol., 16: 327-336.
SANFORD, K. K. (1965) Malignant transformation of cells in vitro.
Inter. Rev. Cytol., 18: 249-311.
SANFORD, K. K. (1974) Biological manifestations of oncogenesis in
vitro: a critique. J. Natl Cancer Inst., 53: 1481-1485.
SANFORD, K. K., EARLE, W. R., SHELTON, E., SCHILLING, E. L., DUCHESNE,
E. M., LIKELY, G. D., & BECKER, E. M. (1950) Production of
malignancy in vitro. XII. Further transformations of mouse
fibroblasts to sarcomatous cells. J. Natl Cancer Inst.,
11: 351-367.
SATO, K., SLESINSKI, R. S, & LITTLEFIELD, J. W. (1972) Chemical
mutagenesis at the phosphoribosyl transferase locus in cultured
human lymphoblasts. Proc. Natl Acad. Sci., 69: 1244-1248.
SCHMID, W. (1973) Chemical mutagen testing on in vivo somatic
mammalian cells. Agents Actions, 3: 77-85.
SIMS, P. & GROVER, P. L. (1974) Epoxides in polycyclic aromatic
hydrocarbon metabolism and carcinogenesis. Advances in Cancer
Res., 20: 165-274.
SMITH, W. E. & HANAWALT, P. C. (1969) Molecular photobiology:
Inactivation and recovery. New York, Academic Press.
SMYTHE, D. R. & EVANS, H. J. (1976) Mapping of sister-chromatid
exchanges in human chromosomes using G-Banding and
autoradiography. Mutat. Res., 35: 139-154.
SOBELS, F. H. & VOGEL, E. (1976) The capacity of drosophila for
detecting relevant genetic damage. Mutat. Res., 41: 95-106.
STETKA, D. G. & WOLFF, S. (1976) Sister chromatid exchange as an assay
for genetic damage induced by mutagen-carcinogens. I. In vitro
test for compounds requiring metabolic activation. Mutat. Res.,
41: 333-342.
STICH, H. F. & KIESER, D. (1974) Use of DNA repair synthesis in
detecting organo-tropic actions of chemical carcinogens.
Proc. Soc. Exp. Biol Med., 145: 1339-1342.
STICH, H. F. & SAN, R. H. C. (1970) DNA repair and chromatid anomalies
in mammalian cells exposed to 4 nitroquinoline 1-oxide. Mutat.
Res., 10: 389-404.
STOLTZ, D. R., POIRIER, L. A., IRVING, C. C., STICH, H. F.,
WEISBURGER, J. H., & GRICE, H. C. (1974) Evaluation of short-term
tests for carcinogenicity. Toxicol. appl. Pharmacol.,
29: 157-180.
SUGIMURA, T., SATO, S., NAGAO, M., YAHAGI, T., MATSUSHIMA, T., SEINO,
Y., TAKEUCHI, M., & KAWACHI, T. (1976) Overlapping of carcinogens
and mutagens. In: Magee, P. N., Takayama, S., Sugimura, T., &
Matsushima, T., ed. Fundamentals in cancer prevention, Tokyo,
University of Tokyo Press; Baltimore, Univ. Park Press,
pp. 191-215.
SUTHERLAND, B. M., RICE, M., & WAGNER, E. K. (1975) Xeroderma
pigmentosum cells contain low levels of photoreactivating
enzyme. Proc. Natl Acad. Sci., 72: 103-107.
SUTTON, H. E. (1972) Monitoring somatic mutations in human
populations. In: Sutton, H. E. & Harris, M. I., ed. Mutagenic
effects of environmental contaminants, pp. 122-128.
THOMPSON, L. H. & BAKER, R.M. (1973) Isolation of mutants of cultured
mammalian cells. In: Prescott, D. M., ed. Methods of cell
biology, New York, Academic Press, pp. 209-280.
TOMATIS, L. (1973) Transplacental carcinogenesis. In: Raven, R. W.,
ed. Modern trends in oncology -- 1, London, Butterworths,
pp. 99-126.
TOMATIS, L. (1974a) The validity of long-term bioassays in
carcinogenicity testing. Chemical and vital oncogenesis. Excerpts
Medica Int. Congr. Ser. No. 350, Vol. 2, pp. 82-93.
TOMATIS, L. (1974b) Inception and duration of tests. In: Golberg, L.,
ed. Carcinogenesis testing of chemicals, Cleveland, CRC Press,
pp. 23-27.
TOMATIS, L., AGTHE, C., BARTSCH, H., HUFF, J., MONTESANO, R., SARACCI,
R., WALKER, E., & WILBOURN, J. (1978) Evaluation of the
carcinogenicity of chemicals: A review of the monograph program
of the International Agency for Research on Cancer. Cancer Res.,
38: 877-885.
TURUSOV, V. S., ed. (1973, 1976) Pathology of tumours in laboratory
animals, Vol. I, Part 1 and 2, IARC Sci. Publ. No. 5 and No. 6.
Lyon.
UICC (1969) UICC Technical Report Series Vol. 2 (Carcinogenicity
testing). Geneva, International Union Against Cancer, 56 pp.
VENITT, S. & LEVY, L. S. (1974) Mutagenicity of chromates in bacteria
and its relevance to chromate carcinogenesis. Nature (Lond.),
250: 493-495.
WEIL, C. S. (1972) Statistics vs safety factors and scientific
judgements in the evaluation of safety for man. Toxicol. appl.
Pharmacol., 21: 454-463.
WEINSTEIN, I. B., ORENSTEIN, J. M., GEBERT, T., KAIGHN, M. E., &
STADLER, V. C. (1975) Growth and structural properties of
epithelial cell cultures established from normal rat liver and
chemically induced hepatomas. Cancer Res., 35: 253-263.
WEISBURGER, J. H. (1974) Inclusion of positive control. In: Golberg,
L., ed. Carcinogenesis testing of chemicals, Cleveland, CRC
Press, pp. 29-34.
WEISBURGER, E. K. (1975) A critical evaluation of the methods used for
determining carcinogenicity. J. clin. Pharmacol., 15: 5-15.
WEISBURGER, J. H. & WEISBURGER E. K. (1967) Tests for chemical
carcinogens. In: Busch, H., ed. Methods in cancer research,
Academic Press, Vol. 1, pp. 307-398.
WEISBURGER, J. H. & WEISBURGER, E. K. (1973) Biochemical formation and
pharmacological, toxicological and pathological properties of
hydroxylamines and hydroxamic acids. Pharmacol. Rev. 25: 1-66.
WHO (1961) WHO Technical Report Series No. 220 (Evaluation of
carcinogenic hazards of food additives), 33 pp.
WHO (1964) WHO Technical Report Series No. 276 (Prevention of cancer).
WHO (1969) WHO Technical Report Series No. 426 (Principles for the
testing and evaluation of drugs for carcinogenicity).
WHO (1974) WHO Technical Report Series, No. 546 (Assessment of the
carcinogenicity and mutagenicity of chemicals), 19 pp.
WILLIAMS, G. M., ELLIOT, J. M., & WEISBURGER, J. H. (1973) Carcinoma
after malignant conversion in vitro of epithelial-like cells
from rat liver following exposure to chemical carcinogens.
Cancer Res., 33: 606-612.
WILSON, R. H., DEEDS, F., & COX, A. J. (1941) The toxicity and
carcinogenicity of 2-acetaminofluorene. Cancer Res.,
1: 595-608.
ZIMMERMANN, F. K. (1973) Detection of genetically active chemicals
using various yeast systems. In: Hollaender, A., ed. Chemical
mutagens, principles and methods for their detection, Vol. 3,
pp. 209-240.


 Since m - 3 S.D., m - 2 S.D., m - 1 S.D., m, m + 1 S.D.,
m + 2 S.D., and m + 3 S.D. indicate equal dose intervals, the
corresponding percentage of responders, i.e. 0.1, 2.3, 15.9, 50, 84.1,
97.7, and 99.9 respectively, will give a straight line when these
percentages are plotted at equidistant intervals. Fig. 1.4 illustrates
this transformation; both the percent scale and the commonly used
"probit" scale are presented. Finney (1971) has presented the history
of the development and the utility of probit transformationa. Many
toxicologists use log-probability graph paper to express dose-response
relationships as a linear function for log-normal distributions of an
effect.
Fig. 1.5 shows two dose-response curves on the log-probability
graph paper. The ED50 (50% effective dose) value for chemical A is
10 dose units, while that for chemical B is 0.01 dose units. ED50
data are sometimes presented in the literature as a single dose value,
without providing confidence limits or the slope of the dose response
curve. It is clear in Fig. 1.5 that for chemicals A and B, not only
are the ED50 values three orders of magnitude apart but the test
systems respond in a very different manner.
As an example of the necessity for taking into account the
slopes, the practice of some toxicologists to study the effects of
repeated doses at 1/10 of the single dose ED50 can be considered.
For chemical A an effect would already be seen in 16% of the
population (ED16) after the first of the repeated doses, while for
chemical B it is quite probable that an effect will never be seen even
after many repeated doses (Fig. 1.5). This is predictable, if the
slopes of the dose-response curves are known.
Flat slopes, as for chemical A, are often indicative of such
factors as poor absorption, rapid excretion or detoxication, or of
toxic effects that become manifest some time after administration.
Steep slopes, as for chemical B, most frequently indicate rapid
absorption and rapid onset of toxic effects, as for example with
hydrogen cyanide or irritant gases. While slope is not an absolutely
reliable indicator of physiological or toxicological mechanisms, it is
useful to the experienced toxicologist, and should always be reported
along with its confidence limits.
a "Probit" = probability unit. Probit is the "standard deviate" or
the "normal equivalent deviate" (NED) increased by 5. The NED is
defined as the abscissa corresponding to a probability P in a
normal distribution with mean 0 and standard deviation 1.
Since m - 3 S.D., m - 2 S.D., m - 1 S.D., m, m + 1 S.D.,
m + 2 S.D., and m + 3 S.D. indicate equal dose intervals, the
corresponding percentage of responders, i.e. 0.1, 2.3, 15.9, 50, 84.1,
97.7, and 99.9 respectively, will give a straight line when these
percentages are plotted at equidistant intervals. Fig. 1.4 illustrates
this transformation; both the percent scale and the commonly used
"probit" scale are presented. Finney (1971) has presented the history
of the development and the utility of probit transformationa. Many
toxicologists use log-probability graph paper to express dose-response
relationships as a linear function for log-normal distributions of an
effect.
Fig. 1.5 shows two dose-response curves on the log-probability
graph paper. The ED50 (50% effective dose) value for chemical A is
10 dose units, while that for chemical B is 0.01 dose units. ED50
data are sometimes presented in the literature as a single dose value,
without providing confidence limits or the slope of the dose response
curve. It is clear in Fig. 1.5 that for chemicals A and B, not only
are the ED50 values three orders of magnitude apart but the test
systems respond in a very different manner.
As an example of the necessity for taking into account the
slopes, the practice of some toxicologists to study the effects of
repeated doses at 1/10 of the single dose ED50 can be considered.
For chemical A an effect would already be seen in 16% of the
population (ED16) after the first of the repeated doses, while for
chemical B it is quite probable that an effect will never be seen even
after many repeated doses (Fig. 1.5). This is predictable, if the
slopes of the dose-response curves are known.
Flat slopes, as for chemical A, are often indicative of such
factors as poor absorption, rapid excretion or detoxication, or of
toxic effects that become manifest some time after administration.
Steep slopes, as for chemical B, most frequently indicate rapid
absorption and rapid onset of toxic effects, as for example with
hydrogen cyanide or irritant gases. While slope is not an absolutely
reliable indicator of physiological or toxicological mechanisms, it is
useful to the experienced toxicologist, and should always be reported
along with its confidence limits.
a "Probit" = probability unit. Probit is the "standard deviate" or
the "normal equivalent deviate" (NED) increased by 5. The NED is
defined as the abscissa corresponding to a probability P in a
normal distribution with mean 0 and standard deviation 1.

 Fig. 1.6 illustrates the importance of parallelism of
dose-response curves for making any general statements about relative
effects. Chemicals C and D have identical ED50 values. However, any
statement about the relative equality of effect would only be true at
that particular dose. In fact, at higher doses, chemical C would be
more effective than D, and at lower doses, chemical D would be more
effective. Chemicals E and F, on the other hand, show relative
equi-effects in the ratio of 1 to 10 dose units over the entire dose
range. This parallelism of dose-response curves is essential for the
validity of general statements about relative toxicities. Special note
should be made, however, that the curves for chemicals E and F apply
only to one specific effect and one set of experimental conditions.
Observations of response for a different toxic effect, or
administration by a different route or to a different species may not
produce parallel dose-response curves for the same chemicals.
Fig. 1.6 illustrates the importance of parallelism of
dose-response curves for making any general statements about relative
effects. Chemicals C and D have identical ED50 values. However, any
statement about the relative equality of effect would only be true at
that particular dose. In fact, at higher doses, chemical C would be
more effective than D, and at lower doses, chemical D would be more
effective. Chemicals E and F, on the other hand, show relative
equi-effects in the ratio of 1 to 10 dose units over the entire dose
range. This parallelism of dose-response curves is essential for the
validity of general statements about relative toxicities. Special note
should be made, however, that the curves for chemicals E and F apply
only to one specific effect and one set of experimental conditions.
Observations of response for a different toxic effect, or
administration by a different route or to a different species may not
produce parallel dose-response curves for the same chemicals.
 REFERENCES
ALBERT, R. E. & ALTSCHULER, B. (1973) Considerations relating to the
formulation of limits for unavoidable population exposures to
environmental carcinogens. In: Ballon, J. E., ed. Radionuclide
carcinogenesis, Springfield, Va, NIIS, pp. 233-253
(AEC Symposium Series CONF-72050).
ALBERT, R. E. & ALTSCHULER, B. (1976) Assessment of risks in terms of
life shortening, Environ. Health Perspect., 13: 91-94.
ANDREYESHCHEVA, N. G. (1976) Predicting biological effect as a
function of the chemical structure and the primary physical and
chemical properties of organic compounds. Environ. Health
Perspect., 13: 27-30.
ARIENS, E. J., VAN ROSSUM, J. M., & SIMONIS, A. M. (1957) Affinity,
intrinsic activity and drug interactions. Pharmacol. Rev.,
9: 218-236.
BALL, W. L. (1959) The investigation of present-time definitions and
conceptions of maximum allowable concentrations in different
countries. Pr. Lék., 11 (3): 127-128.
BEIR (1972) The effects of population exposure to low levels of
ionizing radiations -- Report of the Advisory Committee on the
Biological Effects of Ionizing Radiation. Washington, DC,
National Academy of Sciences -- National Research Council,
217 pp. (Govt. Printing Office Publication 0-489-797).
BERNARD, C. (1898) [Introduction to the study of experimental
medicine.] Paris, Librairie Delagrave (in French).
BINGHAM, E. & FALK, H. L. (1959) Environmental carcinogens --
modifying effect of cocarcinogens on the threshold response.
Arch. environ. Health, 19: 779-783.
BLUM, H. F. (1959) Carcinogenesis by ultraviolet light. Princeton,
NJ, Princeton University Press.
BROWN, B. M. & PAPWORTH, D. S. (1974) Environmental chemical hazards
to wildlife. In: Boyland, E. & Goulding, R., ed. Modern trends
in toxicology, London, Butterworths, Vol. 2, ch. 3, pp. 70-85.
CASARETT, L. J. (1975) Toxicologic evaluation. In: Casarett, L. J. &
Doull, J., ed. Toxicology, the basic science of poisons. New
York, Macmillan Publishing Co. Inc.
CHAND, N. & HOEL, D. G. (1974) A comparison of models for determining
safe levels of environmental agents. In: Proschan & Sefling, ed.
Reliability and biometry, Philadelphia, SIAM, pp. 382-401.
CLARK, A. J. (1933) Mode of action of drugs on cells. Baltimore,
Williams & Wilkins.
COHEN, J. M. & PINKERTON, C. (1966) Widespread translation of
pesticides by air transport and rain-out. In: Gould, R. F., ed.
Organic pesticides in the environment, American Chemical
Society, pp. 163-176 (Advances in Chemistry Series, vol. 60).
COON, J. (1973) Toxicology of naturally-occurring chemicals: a
perspective. In: Toxicants occurring naturally in foods
(2nd ed.). Washington, DC, National Academy of Sciences.
CORNFIELD, J. (1954) Measurement and comparison of toxicities: the
quantal response. In: Kempthorne, O., Bancroft, T. A., Gowen, J.
W., & Lush, J. L., ed. Statistics and mathematics in biology,
Ames, The Iowa State College Press, pp. 327-344.
CRANSTON, M. (1973) What are human rights? New York, Taplinger Pub.
Co. Inc., p. 111.
CURRY, S. H. (1970) Theoretical changes in drug distribution resulting
from changes in binding to plasma proteins and to tissues.
Pharm. Pharmacol., 22: 753-758.
CUTHBERT, J. W. (1974) Industrial toxicology. In: Boyland, E. &
Goulding, R., ed. Modern trends in toxicology. London,
Butterworths, pp. 86-115.
DAY, T. D. (1967) Carcinogenic action of cigarette smoke condensate on
mouse skin. Br. J. Cancer, 21: 56-81.
DINMAN, B. D. (1972) "Non-concept" of "no-threshold" chemicals in the
environment. Environ. Sci., 175 (4021): 495-497.
DOLL, R. (1967) Prevention of cancer: pointers from epidemiology.
London, Nuffield Provincial Hospital Trust.
DRUCKREY, H. (1967) Qualitative aspects of chemical carcinogenesis.
In: Truhaut, R., ed. Potential carcinogenic hazards from drugs,
evaluation of risks, New York, Springer-Verlag, pp. 60-78.
(UICC Monograph Series, Vol. 7.)
ECOBICHON, D. J. & CORMEAU, A.M. (1973) Pseudocholinesterases of
mammalian plasma: physiochemical properties and organophosphate
inhibition in 11 species. Toxicol. appl. Pharmacol.,
24: 92-100.
EDWARDS, C. A. (1970) Persistent pesticides in the environment.
Cleveland, Ohio, CRC Press.
ELIZAROVA, O. N. (1962) In: Opredelenie porogovyh doz promyslennyh
jadov pri peroral'nom vvedeni, Moscow, Medicina, pp. 107-108
(in Russian).
EPSTEIN, S.S. (1973) The Delaney amendment. J. prev. Med.,
2: 140-149.
FALK, H. L. (1975) Consideration of risks versus benefits. Environ.
Health Perspect., 11: 1-5.
FOOD & DRUG ADMINISTRATION (1970) Food and Drug Administration
Advisory Committee on Protocols for Safety Evaluation: Panel on
Carcinogenesis Report on Cancer Testing in the Safety Evaluation
of Food Additives and Pesticides. Toxicol. appl. Pharmacol.,
20: 419-438.
FINNEY, D. G. (1952a) Statistical method in biological assay,
London, Charles Griffin Ltd, 661 pp.
FINNEY, D. G. (1952b) Probit analysis (2nd ed.). London, Cambridge
University Press.
FINNEY, D. G. (1971) Probit analysis (3rd ed.). London, Cambridge
University Press.
FLYNN, D. I., LYNCH, M., & ZANNONI, V. G. (1972) Species differences
and drug metabolism. Biochem. Pharmacol., 21: 2577-2590.
FRIEDMAN, L. (1969) The role of the laboratory animal study of
intermediate duration for the evaluation of safety. In: Symposium
on the evaluation of the safety of food additives and chemical
residues. Toxicol. appl. Pharmacol., 16: 498-506.
FUCHS, N. A. (1964) The mechanics of aerosols, Oxford, Pergamon
Press, pp. 270-287.
GADDUM, J. H. (1957) Theories of drug antagonism. Pharmacol. Rev.,
9: 211-217.
GAVRILESCU, N. & PETERS, R. A. (1931) Biochemical lesions in vitamin B
deficiency. Biochem. J., 25: 1397-1409.
GOLDWATER, L. J. (1968) Toxicology. In: Sax, N. I., ed. Dangerous
properties of industrial materials, New York, Amsterdam, London,
Reinhold Book Corporation, pp. 1-23.
GOLUBEV, A. F., LJUBLINA, A. E., TOLOKOICEY, N. A., & FILOV, V. A.
(1973) In: Kolicestvennaja toksikologija. Leningrad, Medicina,
p. 217 (in Russian).
GROSS, M. A., FITZHUGH, O. G., & MANTEL, N. (1970) Evaluation of
safety of food additives: an illustration involving the influence
of methyl salicylate on rat reproduction. Biometrics,
26: 181-194.
HAMAKER, J. W., GORING, C. A. L., & YOUNGSON, C. R. (1966) Sorption
and leaching of 4-amino-3,5,6-trichloropicolinic acid in soils.
In: Gould, R. F., ed. Organic pesticides in the environment,
American Chemical Society, pp. 23-37 (Advances in Chemistry
Series, Vol. 60).
HATCH, T. F. (1968) Significant dimensions of the dose-response
relationship. Arch. environ. Health, 16: 571-578.
HATCH, T. F. & GROSS, P. (1964) Pulmonary deposition of inhaled
aerosols. New York, Academic Press.
HERMAN, R. S. (1974) Induction of liver growth by xenobiotic compounds
and other stimuli. CRC crit. Rev. Toxicol., 3(1): 97-158.
HEWLETT, P. S. & PLACKETT, R. L. (1961) Models for quantal responses
to mixtures of two drugs. In: Jonge, H. de, ed. Symposium on
quantitative methods in pharmacology. Amsterdam, North Holland
Publishing Co., pp. 328-336.
HOEL, D. G., GAYLOR, D. W., KIRSCHSTEIN, R. L., SAFFIOTTI, U., &
SCHNEIDERMAN, M. S. (1975) Estimation of risks of irreversible
delayed toxicity. J. Toxicol. environ. Health, 1(1): 133-151.
HUCKER, H. B. (1970) Species differences in drug metabolism.
Ann. Rev. Pharmacol., 10: 99-118.
ICRP (1965) Principles of environmental monitoring related to the
handling of radioactive materials. Report of Committee IV of the
International Commission on Radiological Protection. London,
Pergamon Press.
ICRP (1966) The evaluation of risk from radiation, a report prepared
for Committee I of the International Commission on Radiological
Protection. Oxford, London, Edinburgh, New York, Toronto, Paris,
Braunschweig, Pergamon Press (ICRP Publication 8).
ICRP (1977) Recommendations of the International Commission on
Radiological Protection (ICRP Publication 26 (in press)).
JANYSEVA, H. JA (1972) [On the establishment of MPCs of benz(a)pyrene
in the ambient air of built-up areas.] Gig. i Sanit.,
No. 7, pp. 87-91 (in Russian).
JANYSEVA, H. JA & ANTOMONOV, JU G. (1976) Predicting risk of tumour
occurrence in terms of life-shortening. Environ. Health
Perspect., 13: 95-100.
JONES, H. B. & GRENDON, A. (1975) Environmental factors in the origin
of cancer and estimation of possible hazards to man. Food
cosmet. Toxicol., 13: 251-267.
KAGAN, Y. S. (1973) [Methods of quantitative estimation of combined
and complex action on the organism of physical and chemical
factors in the environment.] Gig. i Sanit., No. 2, pp. 89-92
(in Russian).
KELLERMANN, G., SHAW, C. R., & LUYTEN-KELLERMANN, M. (1973) Aryl
hydrocarbon hydroxylase inducibility and bronchogenic carcinoma.
New Engl. J. Med., 289: 934-937.
KORBAKOVA, A. K., SUMSKAJA, N. I., ZAEVA, G. N., & NIKITENKO, T. K.
(1971) In: Naucnye osnovy sovremmenyh metodov gigieniceskogo
normirovanija himiceskih vescest'v v okruzajuscej srede.
Moscow, Institut gigieny truda i profzabolevanija AMN SSSR,
pp. 35-39.
KRASOVSKIJ, G. N. (1965) [Methodology for conducting an acute
experiment and for evaluating the results, and its basis.] In:
Sanitarnaja ohrana vodoemov ot zagrjaznenija promyslennymi
stocnymi vodami. No. 7, 247-268 (in Russian).
KRASOVSKIJ, G. N. (1970) [On methods for studying the cumulative
properties of toxic compounds.] Gig. i Sanit., No. 3, pp. 83-88
(in Russian).
KRASOVSKIJ, G. N. (1975) Species and sex differences in sensitivity to
toxic substances. In: Methods used in the USSR for establishing
biologically safe levels of toxic, Geneva, WHO, pp. 109-125.
KRASOVSKIJ, G. N. (1976a) [General standardization of biological
parameters for mammals according to body weight, and some
practical aspects of investigations.] In: Trudy MMI im Secenova
T.80 -- Novoe v metodah issledovannija, diagnostiki, lecenii i
profilaktiki vazneisih zabolevanii. Pp. 64-88 (in Russian).
KRASOVSKIJ, G. N. (1976b) Extrapolation of experimental data from
animals to man. Health Perspect., 13:51-58.
KUSTOV, V. V. & TIUNOV, L. A. (1970) [Enzyme system activity as a
means of estimating thresholds of the effects of toxins.] In:
Metody opredelenija toksicinosti i opasnosti himiceskih
vescestv. Moscow, Medicina, pp. 231-234 (in Russian).
KUSTOV, V. V., ZDANOV, A. M., & JUNOVSKIJ, G. D. (1974) [On the
evaluation of the combined effect of factors by the method of
partial regression.] Gig. Tr. Prof. Zabol., 16:33-36
(in Russian).
LAZAREV, N. V. (1938). Obscie osnovypromyslennoj toksikologii.
Moscow & Leningrad, Medgiz.
LAZAREV, N. V. (1963) Rukovodstvo po gigiena truda, Moscow, Medgiz.
LAZAREV, N. V. & BRUSILOVSKAJA, A. I. (1934) Fiziol. Z. SSSR, XVII,
pp. 611-619 (in Russian).
LJUBLINA, E. I. & MIHEEV, M. I. (1974) [Scientific bases for
establishing tentative safe levels of substances affecting man.]
In: Zurnal Vsesjuznogo Obcestva im. D. I. Mendeleeva,
XIX (2): 142-145 (in Russian).
LOEWE, S. (1959) Relationships between stimulus and response.
Science, 130: 692-695.
LU, P. Y. & METCALF, R. (1975) Environmental fate and biodegradability
of benzene derivatives as studied in a model aquatic ecosystem.
Environ. Health Perspect., 10: 269-284.
MANTEL, N. & BRYAN, W. R. (1961) Safety testing of carcinogenic
agents. J. Natl Cancer Inst., 27: 455-470.
MANTEL, N. & SCHNEIDERMAN, M. (1975) Estimating "safe" levels, a
hazardous undertaking. Cancer Res., 35: 1379-1386.
MANTEL, N., BOHIDAR, N., BROWN, C., CIMINERA, J., & TUKEY, J. (1975)
An improved "Mantel-Bryan" procedure for "safety" testing of
carcinogens. Cancer Res., 35: 865-872.
MAZZE, R. I., COUSINS, M. J., & KOSEK, J. C. (1973) Strain differences
in metabolism and susceptibility to the nephrotoxic effects of
methoxyfluorane in rats. J. Pharmacol. exp. Therapy,
184: 481-488.
MEDVED, L. I. & SPYNU, E. I. (1970) [Principles and methods of
hygienic standardization of pesticides. In: Principles and
methods of establishing maximum permissible concentrations of
harmful substances in the air of production premises.] Moscow,
Medicina (in Russian).
METCALF, R. L., KAPOOR, I. P., LU, P. Y., SCHUTH, C. K., & SHERMAN, P.
(1973) A model ecosystem for the evaluation of pesticide
biodegradability and ecological magnification. Environ. Sci.
Technol., 5: 709-744.
MILLER, E. C., MILLER, J. A., & ENOMOTOR, M. (1964) The comparative
carcinogenetics of 2-acetylaminofluorene and its N-hydroxy
metabolite in mice, hamsters and guinea pigs. Cancer Res.,
24: 2018-2032.
MONTESANO, R., MOHR, U., & MAGEE, P. N. (1974) Additive effect in the
induction of kidney tumours in rats treated with
dimethylnitrosamine and ethylmethanesulfonate. Br. J. Cancer,
29: 50-58.
MURPHY, S. D. (1969) Some relationships between effects of
insecticides and other stress conditions. Ann. NY Acad. Sci.,
160: 366-377.
NAS/NRC (1970) Evaluating the safety of food chemicals. Food
Protection Committee, Food and Nutrition Branch, Division of
Biology and Agriculture, National Research Council, Washington
DC, National Academy of Sciences. 55 pp.
NAS (1975) Principles for evaluating chemicals in the environment --
A report of the Committee for the Working Conference on
Principles of Protocols for Evaluating Chemicals in the
Environment. Washington, DC, National Academy of Sciences.
NIEHS (1970) Man's health and the environment, some research needs --
Report of the Task Force on Research Planning in Environmental
Health Science US Department of Health, Education and Welfare,
Public Health Service, National Institutes of Health, National
Institute of Environmental Health Sciences. Washington, DC, US
Govt Printing Office.
NIEHS (1977) Human health and the environment, some research needs --
Report of the Second Task Force for Research Planning in
Environmental Health US Department of Health, Education and
Welfare, Public Health Service, National Institutes of Health,
National Institute of Environmental Health Sciences. Washington,
DC, US Govt Printing Office (DHEW Publication No. NIH77-1277).
OECD (1977) The OECD programme on long range transport of air
pollutants. Measurements and findings. Paris, Organisation for
Economic Co-operation and Development, 268 pp.
OWEN, D. B. (1955) Handbook of statistical tables, London, Paris,
Pergamon Press, pp. 127-137.
PAGET, G. E. (1970) The design and interpretation of toxicity tests.
In: Paget, G. E., ed. Methods in toxicology, Philadelphia, F.
A. Davies, pp. 1-10.
PARKE, D. V. (1975) Induction of the drug-metabolizing enzymes. In:
Parke, D. V., ed. Enzyme induction, London, Plenum Press,
pp. 207-272.
PETERS, R. A. (1963) Biochemical lesions and lethal synthesis.
Oxford, London, New York, Paris, Pergamon Press.
PETERS, R. A. (1967) The biochemical lesion in thiamine deficiency.
In: Wolstenholme, G. E. W. & O'Connor, M., ed. Thiamine
deficiency, biochemical lesions and their significance, London,
J. & A. Churchill Ltd, pp. 1-8.
PETO, R. & LEE, P. N. (1973) Weibull distribution for continuous
carcinogenesis experiments. Biometrics, 29: 457-470.
PETO, R., LEE, P. N., & PAIGE, W. S. (1972) Statistical analysis of
the bioassay of continuous carcinogens. Br. J. Cancer,
26: 258-261.
PFITZER, E. A. (1976) General concepts and definitions for
dose-response and dose-effect relationships of toxic metals. In:
Nordberg, G., ed. Effects and dose-response relationships of
toxic metals. Amsterdam, Oxford, New York, Elsevier Scientific
Publishing Company.
PINIGIN, M. A. (1974) [Current problems of community toxicology in
relation to chemical air pollution.] In: Itogi nauki i tehniki.
Serija farmakologija. Himioterapevticeskie sredstva.
Toksikologija. Problemy toksikologii. Vol. 6, Moscow
(in Russian).
POKROVSKIJ, A. A. (1973) [Biochemical approaches to the evaluation of
toxic factors in the environment. In: Documentation of the
Conference on Methodological Approaches to the Study and
Evaluation of Toxic Factors in the Environment.] Moscow
(in Russian).
PORTMAN, R. S., PLOWMAN, K. M., & CAMPBELL, T. C. (1970) On mechanisms
affecting species susceptibility to aflatoxin. Biochim. Biophys.
Acta, 208: 487-495.
PRAVDIN, N. S. (1934) Rukovodstovo promyslennoi toksikologii.
Moscow, Biologija i Medicina.
RALL, D. F. (1970) Animal models for pharmacological studies. In:
Proceedings of the Symposium on Animal Models for Biomedical
Research III, Washington, DC, National Academy of Sciences,
pp. 125-146 (ISBN 0-309-01854-4).
SABAD, L. M., SANOCKIJ, I. V., ZAEVA, G. N., BUREVIC, T. C.,
KACNELSON, B. A., JANYSEVA, H. JA, & SUGAEVA, B. V. (1973) [On
the possibility of establishing MPCs for benz(a)pyrene in the air
of industrial premises.] Gig. i Sanit., No. 4, pp. 78-81
(in Russian).
SAFFIOTTI, U. (1973) Comments on the scientific basis for the "Delaney
Clause". J. Prev. Med., 2: 125-132.
SANOCKIJ, I. V. (1962) [The calculation of a safety factor for
experimentally based maximum permissible concentrations of
industrial poisons. In: Industrial toxicology and clinical
aspects of occupational diseases of chemical etiology.] Moscow,
Medgiz (in Russian).
SANOCKIJ, I. V. (1970) In: Sanockij, I. V., ed. Metody opredelenija
toksicnosti i opasnosti himiceskih vescestv. Moscow, Medicina,
pp. 11-12 (in Russian).
SANOCKIJ, I. V. (1975a) Investigation of new substances: permissible
limits and threshold of harmful action. In: Methods used in the
USSR for establishing biologically safe levels of toxic
substances, Geneva, WHO, pp. 9-18.
SANOCKIJ, I. V. (1975b) [The threshold concept for the reactions of
living systems to environmental influences and its consequences.
Soviet-American Symposium, Tbilisi.] Gidrometeoizdat,
pp. 112-120 (in Russian).
SANOCKIJ, I. V. & SIDOROV, K. K. (1975) [The current status of the
problem of safety factors in establishing the maximum permissible
concentrations of substances in the components of the general
environment.] Gig. i Sanit., No. 7, pp. 93-96 (in Russian).
SATO, T. & MOROI, K. (1971) Species and age differences in the
activity of isocarboxazid hydrolysing enzyme. Arch. int.
Pharmacodyn. Ther., 192: 128-134.
SCHILD, H. O., ARIENS, E. J., SIMONIS, A. M., DIKSTEIN, S., DE JONGH,
S. E., HEWLETT, P. S., & PLACKETT, R. L. (1961) Section on
mixtures of drugs. In: De Jonge, H. ed. Quantitative methods in
pharmacology, Proceedings of a Symposium held in Leyden on
May 10-13, 1960, Amsterdam, North Holland Publishing Company,
pp. 282-334.
SCHNEIDERMAN, M. A. (1971) A method for determining the dose
compatible with some "acceptable" level of risk. In: Chemicals
and the future of man -- Hearings before the Sub-Committee on
Executive Reorganization and Government Research of the Committee
on Government Operations, US Senate, Ninety-Second Congress,
First Session, 6 and 7 April 1971. Washington, DC, US Govt
Printing Office.
SCHNEIDERMAN, M. A. & MANTEL, N. (1973) The Delaney Clause and a
scheme for rewarding good experimentation. Prev. Med.,
2: 165-170.
SIDORENKO, G. I. & PINIGIN, M. A. (1975) Establishment of safe levels
of chemicals in communal hygiene: methodological approaches. In:
Methods used in the USSR for establishing biologically safe
levels of toxic substances, Geneva, WHO, pp. 126-138.
SIDORENKO, G. I. & PINIGIN, M. A. (1976) Concentration-time
relationship for various regimens of inhalation of organic
compounds. Environ. Health Perspect, 13: 17-21.
SMYTH, H. F., JR (1963) Industrial hygiene in retrospect and
prospect-toxicological aspects. Am. Ind. Hyg. Assoc. J.,
24: 222-226.
SMYTH, H. F. JR, WEIL, C. S., WEST, J. S., & CARPENTER, C. P. (1969)
An exploration of joint toxic action: twenty-seven industrial
chemicals intubated in rats in all possible pairs. Toxicol.
appl. Pharmacol, 14: 340-347.
SPYNU, E. I., VROCINSKIJ, K. K., ZOR'EVA, T. D., & MAL'KO, N. N.
(1972) [Complex hygienic standardization of new organophosphorus
pesticides in environmental components.] Gig. i. Sanit.,
No. 11, pp. 96-99 (in Russian).
STOKINGER, H. E. (1972) Concepts of thresholds in standards setting.
Arch. environ. Health, 25 (3): 153-157.
SYSIN, A. M., ed. (1941) [Maximum allowable concentrations of toxic
substances in water bodies.] Moscow and Leningrad, Stroizdat
(in Russian).
TASK GROUP ON METAL ACCUMULATION (1973) Accumulation of toxic metals
with special reference to their absorption, excretion and
biological half-time. Environ. Physiol. Biochem., 3: 65-107.
TASK GROUP ON METAL TOXICITY (1976) Consensus report for an
international meeting organized by the Sub-committee on the
Toxicology of Metals of the Permanent Commission and
International Association on Occupational Health, Tokyo, 18-23
November 1974. In: Nordberg, G., ed. Effects and dose-responses
relationships of toxic metals, Amsterdam, Oxford, New York,
Elsevier Scientific Publishing Company, pp. 7-111.
ULANOVA, I. P. (1969) [Problems of hygienic standardization of
mixtures of gases and vapours of chemical substances. In:
The toxicology of new industrial chemicals.] Moscow, Medicina,
pp. 33-39 (in Russian).
ULANOVA, I. P., AVILOVA, G. G., BAZAROVA, L. A., MAL'CEVA, N. M.,
MIGUKINA, N. V., HALEPO, A. I., & EITINGTON, A. I. (1973)
[Experimental data on adaptation to poisons under different
conditions of their action. Pharmacology, chemotherapeutic
substances, toxicology.] Itogi nauk i teh., No. 5, pp. 64-75
(in Russian).
ULANOVA, I. P., AVILOVA, G. G., MAL'CEVA, N. M., & HALEPO, A. I.
(1976) [Comparisons of reactions of the organism to continuous
and intermittent action of some chlorinated hydrocarbons.]
Gig. Tr. Profzabol., No. 7, pp. 22-25 (in Russian).
VAN NOORDWIJK, J. (1964) Communication between the experimental animal
and the pharmacologist. Stat. Neerl., 18 (4): 403-416.
VELDSTRA, H. (1956) Synergism and potentiation. Pharmacol. Rev.,
8: 339-387.
VETTORAZZI, G. (1975) Toxicological decisions and recommendations
resulting from the safety assessment of pesticide residues in
food. Crit. Rev. Toxicol., 4 (2): 125-183.
VETTORAZZI, G. (1977) Safety factors and their application in
toxicological evaluation. In: Hunter, W. J. & Smeets, J. G. P.
M., ed. The evaluation of toxicological data for the protection
of public health. Oxford, Pergamon Press (for the Commission of
the European Communities) pp. 207-223.
WEIL, C. S. (1972a) Statistics versus safety factors and scientific
judgment in the evaluation of safety for man. Toxicol. appl.
Pharmacol., 21: 454-463.
WEIL, C. S. (1972b) Guidelines for experiments to predict the degree
of safety of a material for man. Toxicol. appl. Pharmacol.,
21: 194-199.
WHO (1958) WHO Technical Report Series, No. 144 (Procedures for the
testing of intentional food additives to establish their safety
for use -- Second Report of the Joint FAO/WHO Expert Committee on
Food Additives.) 19 pp.
WHO (1973) WHO Technical Report Series, No. 535 (Environmental and
health monitoring in occupational health -- Report of a WHO
Expert Committee.) 48 pp.
WHO (1974a) WHO Technical Report Series, No. 546 (Assessment of
carcinogenicity and mutagenicity of chemicals -- Report of a WHO
Scientific Group.) 19 pp.
WHO (1974b) WHO Technical Report Series, No. 554 (Health aspects of
environmental pollution: planning and implementation of national
programmes -- Report of a WHO Expert Committee.) 57 pp.
WHO (1975a) WHO Technical Report Series, No. 571 (Early detection of
health impairment in occupational exposure to health hazards --
Report of a WHO Study Group.) 80 pp.
WHO (1975b) WHO Technical Report Series, No. 560 (Chemical and
biochemical methodology for the assessment of hazards of
pesticides for man -- Report of a WHO Scientific Group.) 26 pp.
WHO (1975c) Methods for studying biological effects of pollutants (a
review of methods used in the USSR). Report of a Working Group,
Moscow, November 1974. Copenhagen, WHO Regional Office for
Europe.
WHO (1976a) WHO Technical Report Series No. 586 (Health hazards of new
environmental pollutants -- Report of a WHO Study Group.) 96 pp.
WHO (1976b) Health aspects of human rights with special reference to
developments in biology and medicine. Geneva, World Health
Organization, pp. 25-27.
WHO (1977) Environmental Health Criteria 4: Oxides of nitrogen.
Geneva, WHO, 79 pp.
WILLIAMS, R. T. (1969) The fate of foreign compounds in man and
animals. Pure appl. Chem., 18: 129-141.
WILLIAMS, R. T. (1972) Toxicological implications of biotransformation
by intestinal microflora. Toxicol. appl. Pharmacol.,
23: 769-781.
2. FACTORS INFLUENCING THE DESIGN OF TOXICITY STUDIES
2.1 Introduction
The choice and sequence of toxicity tests will depend on the
questions or hypotheses that are developed. The nature and sequence of
tests used to satisfy requirements of regulatory agencies may differ
markedly from those used in an investigation of the basic mechanisms
of toxic action. Differences in approach will also depend on whether
the investigation is initiated to evaluate the toxicity of a chemical
prior to its introduction into use, i.e. prospective toxicology, or to
confirm in laboratory animals an epidemiological association that
suggests chemical-induced disease in man, i.e. retrospective
toxicology. Under ideal conditions, prospective toxicology will
eliminate the need for retrospective toxicity evaluation.
National or international regulatory or advisory bodies have
developed fairly specific guidelines or test protocols which are
expected to be applied to the toxicity evaluation of certain groups of
chemicals, introduced deliberately into our environment, e.g. food
additives and pesticides (Council of Europe, 1973; FDA, 1959; WHO,
1967). The development of guidelines for the systematic evaluation of
the toxicity of chemicals to which man is exposed, through his
occupation or through incidental contamination of the ambient
environment, is less common. Where specific guidelines have been
formulated, they usually require: test information on acute toxicity
in several species of experimental animals; some knowledge of the
biochemical disposition of the compounds; various short-term toxicity
tests; tests of the effects of the chemical on reproductive function;
chronic toxicity tests in one or more species; special tests on organ
function, clinical biochemistry and haematology, and other specific
tests as determined by the particular type and intended uses of the
chemical under consideration. Some commercial firms have developed
their own guidelines for toxicity tests and their sequence in the
premarket toxicological evaluation of products. Often the sequence of
tests will have certain checkpoints at which decisions will be made as
to whether continued development of the product (and more extensive
toxicity testing) is warranted.
In this chapter, various topics will be discussed from the
standpoint of the usefulness of certain types of information and the
influence that various factors may have in designing protocols for the
interpretation of data obtained in toxicity evaluation programmes.
2.2 Chemical and Physical Properties
2.2.1 General considerations
The late Horace Gerarde stated in an address that "toxicity is
the capacity of a substance to cause injury. It is an inherent,
unalterable molecular property which is dependent upon chemical
structure. There is nothing we can do about the toxicity of a chemical
except to know it"a.
The nature or quality of the toxic action inherent in a chemical
will depend to a large extent upon the functional group or groups
present in the molecule. Knowledge of the reactions that these
functional groups may undergo with reactive groups in critical
endogenous biochemical constituents provides a means of predicting the
nature of the toxic effects that may be expected. Smyth (1959) used
the permanence of threshold limit values over a period of years as a
criterion for evaluating various types of information used in setting
safety limits for industrial chemicals. For a limited number of
compounds, occupational threshold limit values (TLVs) had been
established on the basis of analogy with better known substances and
the permanence of these TLVs appeared to equal that of TLVs based upon
data from experimental toxicity studies and from human experience.
However, evaluation of toxicity by analogy with chemically-related
substances contains considerable potential for error, and requires a
great deal of toxicological information on very closely related
chemical substances. Very minor changes in structure may be
accompanied by profound changes in toxicity. The relationships between
physicochemical characteristics and toxicity, including the biological
activities of homologous series, have been reviewed by Ljublina &
Filov (1975).
2.2.2 Physicochemical properties and the design of toxicity studies
Zbinden (1973) included fourteen chemical and physical variables
in a check list of types of information useful in the toxicity
evaluation of new drugs. Although some of these variables may be
determined in the course of a toxicological evaluation, all of them
apply equally as well to environmental chemicals as to therapeutic
chemicals.
a Presented at the Flavor Manufacturers' Association of the United
States. Fall Symposium, 16 November 1972, Washington, DC.
Knowledge of the chemical structure is essential for the
preliminary prediction of the nature and site of toxic action,
assuming, of course, that some prior knowledge of the toxicity of
chemically-related compounds is available. It is also essential for
developing extraction and assay procedures for the determination of
tissue concentrations and allows for logical estimates of the nature
of metabolites that may be found. Indeed, without such knowledge,
logical design of an experiment is impossible.
The stability of the chemical at various pH values and the
photochemical properties are variables that must be considered as soon
as a substance arrives for testing in the toxicology laboratory, as
they may determine the manner in which the chemical should be stored
prior to administration to test animals or indicate the stability of
residues in tissue extracts. Many organic chemicals undergo
photochemical reactions that lead to either more or less toxic
products (Crosby, 1972) and organic esters are often readily
hydrolysed under conditions encountered during their laboratory
investigation (Eto, 1974). It may be necessary to exercise special
precautions to avoid chemical reactions during the preparation and
storage of test solutions or diets and during the analysis of tissues
and metabolic reaction mixtures. Furthermore, if a chemical is likely
to become activated by photochemical reactions, special tests for
phototoxicity may be required.
The organic solvent/water partition coefficient and pK are
physical properties of particular importance in the determination of
the absorption and distribution of a compound in living organisms as
well as in the development of appropriate extraction and assay
procedures for the chemical. Hansch & Dunn (1972) reviewed numerous
studies which suggest that characterization of the lipophilic nature
of compounds may allow systematic predictions of their relative
biological activities. Dillingham et al. (1973) applied these
principles when they compared the toxicity of substituted alcohols in
a tissue culture with their acute toxicity in mice and concluded that
tissue culture test systems may be useful in determining predictive
correlations between in vivo toxicity and the physicochemical
properties of compounds.
The extent of ionization of an organic compound will influence
its passage through lipoidal membranes (La Du et al., 1971). In
general, the unionized lipid-soluble form of an organic compound will
most readily pass through biological membranes. Although most of the
physicochemical principles of absorption and distribution have been
developed through systematic studies on medicinal chemicals, these
principles also apply to organic chemicals in the environment and to
the design of toxicity experiments (Loomis, 1974; also Chapter 4).
Patty (1958) discussed the influence of oil and water solubility and
of the coefficient of distribution of a vapour between blood and
alveolar air on the rates of equilibrium saturation and desaturation
of the body during inhalation experiments.
Particle size, shape, and density are of obvious importance in
studying the inhalation toxicities of aerosols, as they are important
factors in the determination of the site of deposition and the rates
and mechanisms of clearance from the respiratory tract (Hatch & Gross,
1964; also Chapter 6). Furthermore, the particle size of substances
given orally as suspensions can also markedly influence their toxicity
(Boyd, 1971). If critical judgements based on the relative toxicities
of the same or different substances, administered orally in
suspension, are to be made, it is necessary to ensure uniformity of
particle size.
Vapour pressure of a chemical substance is important in the
practical consideration of the likelihood of exposure of man through
inhalation, and the design of experimental inhalation toxicity studies
will be influenced by the ease with which a solid or liquid vaporizes
under controlled conditions. However, a high vapour pressure may
produce technical problems if the objective of the test is to
determine toxicity by the oral route of administration. Studies by
Jones et al. (1971) showed that for a large series of food flavouring
agents mixed in laboratory animal diets, the amount of loss from the
diet was inversely related to the boiling points of the flavouring
agents. Frequent chemical analyses of the diet, as well as frequent
preparation of fresh diets and/or restricted feeding periods to limit
the time for loss by vaporization are necessary to provide accurate
estimates of intake in feeding studies on substances with low boiling
points.
Knowledge of reactivity with, or binding to, macromolecules may
allow specific design of mechanism experiments, when these
macromolecules are essential tissue and cell constituents. Knowledge
of the chemical reactivity of a substance may also be of considerable
importance in the early planning of feeding studies, if the chemical
under test is likely to react with the macromolecules present in the
laboratory diet. On the one hand, chemical binding or adsorption on
macromolecules in the diet may markedly alter the rate and extent of
absorption of the test compound from the gastrointestinal tract; in
some cases, biologically reactive groups on the test chemical may be
neutralized by dietary constituents. On the other hand, reaction of
the test chemical with essential dietary constituents may contribute
to nutritional deficiency states, or new and more toxic compounds may
be formed. Several examples of these types of reactions have been
summarized by Golberg (1967).
2.2.3 Impurities
In the design of toxicity experiments, it is extremely important
to consider the chemical purity of the sample to be tested. In certain
cases, e.g. food additives and pesticides, the regulations will often
provide specifications of purity for the compound in actual use and
recommended test protocols may specify that toxicity evaluations are
to be conducted with samples that meet these specifications (WHO,
1967). However, there is always the possibility that, when testing the
technical product, the biological effects observed may be due to, or
modified by, trace contaminants. If the contaminants are unknown or
their biological activity unsuspected, toxicity tests may lead to
erroneous conclusions concerning the primary chemical in question. In
contrast, tests on highly purified samples may not detect the toxic
action of contaminants present in samples used commercially.
Furthermore, for many chemical substances used in manufacturing or
incidentally released into the environment, specifications of purity
may not be standardized. Therefore, one of the earliest and most
difficult decisions that must be made in the design of a toxicity
evaluation programme is the selection of the sample to be studied
(technical grade, highly purified, etc.)
Requirements for the purity of the compounds selected for
toxicological testing depend on the purpose of the testing as
discussed in section 2.1. During the development of a new
technological process, it may even be useful to test mixtures of
unknown composition. The information so obtained may alert organic
chemists in the research laboratory or pilot plant operators to the
possible hazards of these unknown mixtures which may vary as
procedures develop and improve. However, data obtained from such
studies will have a limited value. The determination of health
standards requires a compound of a high degree of purity or of highly
standardized composition combined with a precise knowledge of various
impurities. Only in this way will health standards have a universal
value. However, for practical purposes, and for extrapolation to human
exposure conditions it may be prudent, when a technical grade product
standardized by specifications is used in commerce, to select this
grade for toxicity testing and carefully characterize it with respect
to the nature and amounts of any impurities present. Scheduled
analytical spot checks during the course of the experiment to provide
assurance of chemical constancy is also desirable.
When a chemical substance used in commerce is not standardized
with respect to specifications for purity, experimental toxicity
evaluation on a test sample of high purity would, indeed, seem to be
the most rational selection. Data derived from this compound could
then be used to characterize, toxicologically, the action of the
primary chemical under consideration. When certain quantifiable
indices of toxicity have been identified, selected tests with typical
samples of commercial products could be conducted and the results
compared with the purified product to detect possible differences. Of
course, if the impurities represent a significant portion of the
product, or if their chemical properties or their chemical analogy to
other known substances suggest they may have serious toxic properties,
the impurities must be evaluated separately.
An alternative approach is to select a sample of a chemical that
most nearly represents the impure product used commercially, subject
this to comprehensive toxicity evaluation and then make selected,
critical toxicity comparison with purified samples of the primary
chemical as well as with the impurities.
Either approach contains uncertainties, and little would be
gained by short-term spot checks if the toxic action of the impurity
were only detectable after long-term exposure or a latent period. It
is in problem situations such as these that some of the
chemicophysical principles discussed earlier must be applied at
several levels of decision making: the sample to choose for testing;
the design of the evaluation protocol; or the decision (or regulation)
to produce a purer substance for routine use in commerce.
A recent, most controversial problem arising from the
contamination of a primary commercial product involves the apparent
teratogenic action of the herbicide (2,4,5-trichlorophenoxy)acetic
acid (2,4,5-T) (Panel on Herbicides 1971). It is now known that the
first studies to reveal this action were conducted with a sample of
the herbicide that contained a rather high concentration (about
30 mg/kg) of the contaminant 2,3,7,8-tetrachloro-dibenzo-4-dioxin
which is formed during the synthesis of the trichlorophenol precursor.
Tetrachlorodioxin is extremely toxic. For guineapigs, the ratio of the
LD50 for 2,4,5-T to the LD50 of the dioxin is about 630 000. In
female rats, the acute oral LD50 for dioxin is about 1/10 000 of the
oral LD50 for 2,4,5-T. The daily dose of dioxin in pregnant rats
that produced fetal toxicity was only about one four-hundredth of the
maternal LD50 of dioxin or about one four-millionth of the
single-close oral LD50 of 2,4,5-T for female rats. Thus, even if the
concentration of dioxin as a contaminant of 2,4,5-T is kept below
0.5 mg/kg, the major concern for the toxicity of 2,4,5-T should
apparently still be directed towards the contaminant rather than
towards the herbicide itself (at least insofar as effects on
reproduction are concerned).
This kind of problem is not new. Twenty-five years ago, marked
differences in the toxicity of different samples of the insecticide
parathion were traced to contamination with small quantities of the
oxygen analogue and the phosphorothiolate isomer, which are much more
potent anticholinesterases and more acutely toxic than the parent
insecticide (Diggle & Gage, 1951).
2.3 Probable Routes of Exposure
2.3.1 General considerations
Many chemicals will become distributed in various environmental
media or will be used for different purposes, and substantially
different populations may be at risk. Thus, it may be necessary to
obtain extensive test data by different routes of exposure. The choice
of route for practical purposes is generally dictated by: (a) the
likely route by which man will be exposed; and (b) whether the
chemical will produce local injury at the site of exposure. The second
question will often be resolved by acute or short-term studies on
animals dosed by oral, inhalation, dermal, and possibly ocular routes.
Details of test procedures are included in subsequent chapters.
Although it is usually wise to conduct experiments using the route
through which man will be exposed, other more convenient routes may be
chosen for many of the tests if it is determined that the major toxic
effects of a chemical are systemic, occurring only after absorption
and distribution in the body. Data on blood and tissue levels should
be obtained by several routes of exposure, including those that are
considered primarily experimental; with this information, it may be
possible to relate toxic effects to blood or tissue concentrations of
the test chemical and its metabolites. Such information greatly
facilitates comparison of experiments using different routes and may
either confirm or deny the validity of extrapolating data, obtained
experimentally by one route, to the evaluation of potential toxicity
by another perhaps more realistic route of exposure.
2.3.2 Specific variables related to route of exposure
2.3.2.1 Rate of absorption
As a general rule, one can predict that for the usual routes by
which man may be exposed, absorption of chemicals will be most rapid
when given by inhalation, less rapid when given by gavage, and slowest
with dermal application. This order may, however, be modified
depending upon various physicochemical properties of the substance
under test in relation to the microenvironment of the absorbing
surface (Klassen, 1975; Loomis, 1974). The rate of absorption will be
one determinant of the rate of onset of signs of acute poisoning. If
the rates of detoxification and excretion or of injury repair exceed
the limiting rate at which a chemical is absorbed, it is possible that
toxic signs observed by one route of administration will not be
detectable by another route for which absorption is slower (Casarett,
1975; Murphy, 1975). Comparative absorption-distribution kinetics by
different routes would determine such a possibility.
2.3.2.2 Site of action
Specific tests should be conducted to evaluate effects related to
local reactions with specific receptors present in the organ of
absorption. These may be morphological tests to detect evidence of
irritation, inflammation, or oedema, or they may be functional to
detect biochemical or reflex action or bronchoconstriction. In
addition, the route of exposure may determine the organ or
physiological system in which effects will be first observed or
detected at lowest doses. For example, pesticides, which are direct
inhibitors of acetylcholinesterase, when given in sufficient doses by
any route, will produce a characteristic toxic syndrome involving
essentially all organs or structures innervated by cholinergic nerves.
However, at low doses, only specific organs may be involved. If such
compounds are applied to the skin, local sweating and fasciculations
may occur in the absence of signs of systemic poisoning. Exposure by
inhalation may result in bronchoconstriction, exposure by ingestion
may cause gastrointestinal upset before, or at lower doses than,
generalized systemic effects (Henderson & Haggard, 1943; Holmstedt,
1959).
2.3.2.3 Biotransformation
The route of exposure may determine the likelihood and type of
biotransformation before the chemical contacts the specific sites of
action. Thus, when chemicals are administered by the oral (or
intraperitoneal) routes, they will be absorbed and transported first
through the portal circulation to the liver (Lukas et al., 1971). For
example, if, with low oral or dietary doses, the capacity of the liver
to detoxify the compounds exceeds the rate of absorption, an effective
injurious concentration may never reach critical sites of action in
other tissues. Absorption of the same quantity through the lung or
skin, which generally have less detoxifying capacities, may result in
toxic action. It is now known that the lung, skin, and intestinal
mucosa, although generally less active than the liver, also have the
capacity for biotransformation of foreign organic chemicals (Alvares
et al., 1973; Fouts, 1972; Lake et al., 1973; Wattenberg, 1972).
Although knowledge is incomplete with regard to the tissue
distribution of both activating and detoxifying enzyme systems, it is
likely that their relative distribution will determine, to some
extent, the specific tissues that will be most affected by low doses
of some compounds, when given by different routes of exposure.
2.3.2.4 Species
The relative susceptibility of different species to the action of
chemicals may differ depending upon the route of exposure. When
administering compounds by the oral route, such factors as vomiting
reflex (absent in rats) and/or differences in type and distribution of
microflora that may detoxify (or activate) the test compound can
influence the interpretation of the results (Williams, 1972). The
rates of penetration of compounds through the skin and the acute
dermal toxicities of various compounds differ markedly among species
and not always in a predictable manner (McCreesh, 1965). Many of the
problems encountered in dermal toxicity testing and suggestions for
further research have been discussed by Barnes (1968). Roe (1968)
discussed various problems encountered in the design and
interpretation of inhalation toxicity studies related to species
differences in the anatomy of the respiratory tree. Enzootic lung
infections are an additional problem in the use of some species of
animals for long-term inhalation studies.
2.3.2.5 Unintended route
Interpretation of results and measurement of actual dose-response
relationships can be made difficult, because appreciable oral
ingestion may occur with inhalation or dermal exposures. Animals
exposed by either of these routes may ingest the material as a result
of preening, unless dermal applications are covered or made
inaccessible to licking or unless special exposure chambers (e.g. head
only) are used for inhalation exposures (see Chapter 6). In addition,
in particle inhalation experiments, the physiological protective
mechanisms of clearance by mucous transport of the particles out of
the respiratory tree (Hatch & Gross, 1964) with subsequent swallowing
may result in gastrointestinal exposure. Some degree of lung exposure
to volatile compounds administered in the diet or by dermal
application is also likely.
2.3.3 Special tests related to route
When exposure to a compound is most likely to occur by
inhalation, it is useful to know the effect of variations in
ventilation rates, since this will be a common variable among an
exposed human population under different conditions of activity. This
may be accomplished by the use of exercise wheels or treadmills.
When dermal exposure is the likely route, it will be useful to
conduct some tests to determine the effect of different solvents on
penetration of the test compound through the skin. Studies of the
influence of factors such as sweating, abrasions, or the presence of
detergents on dermal absorption and toxicity will also aid in
estimating toxicities under conditions likely to be experienced by man
(see Chapter 11, Part 2).
Interpretation and implications of toxicity data obtained with
oral exposures can be enhanced by examination of the influence of
fasting, dietary variations, and, particularly, administration by
gavage versus inclusion in the diet or drinking water. These factors
are discussed in more detail in subsequent chapters, but it should be
stressed that quite different results and interpretations may ensue if
the same daily dose is given rapidly by gavage or gradually in the
diet. Interpretation of such experiments is greatly aided, if the
design includes comparative absorption and distribution kinetics.
2.4 Selection and Care of Animals
2.4.1 General considerations
The selection and care of laboratory animals to be used in
toxicity tests is especially important in determining the success of
the experiment itself, the extrapolation of the data to man, and the
cost of the evaluation programme. In order to provide data on a
sufficient number of animals for valid statistical analyses, it has
become common practice to use small laboratory rodents for most
large-scale toxicity test programmes. Dogs or nonhuman primates are
frequently included in some of the studies, and studies on at least
one nonrodent species are often required by the test protocols
recommended by regulatory and advisory agencies. Recommendations (with
appropriate references for detailed information) concerning the
selection and care of laboratory animals to be used in the usual broad
scale toxicity evaluation studies are included in Chapter 3. The
selection of animals to be used in various special test procedures is
discussed in subsequent chapters.
Animals and animal care practices should be selected to provide a
scientifically sound and reproducible experiment; however, some of the
variables that contribute to nonuniformity may actually be exploited,
in special studies, to obtain data that may be useful in extrapolation
to nonuniform human populations. For example, if the inherent toxicity
of an air pollutant is to be characterized, the occurrence of chronic
lung infections should be avoided. On the other hand, specially
designed experiments to test the influence of air pollutants on the
susceptibility of animals to lung infections have provided a sensitive
procedure for measuring the adverse effects of air pollutants on the
physiological protective mechanisms that confer resistance to
respiratory infections (Ehrlich, 1966). Epidemiologists could
certainly use such information in the design of studies on human
populations exposed to air pollutants and to infectious microorganisms
present in the environment.
The choice of animals and the environment in which they are used
in toxicity studies will ultimately be determined (as for any other
decisions relating to experimental design) by the nature of the
questions asked or the hypotheses formulated. Controlled introduction
of additional variables may be desired for special studies. The
important principle is that appropriate control conditions should be
included in such studies to allow comparisons with results obtained
under more conventional procedures.
2.4.2 Animal variables
The objective of most experimental toxicity studies is to predict
the adverse effects of chemicals in man. Therefore, in addition to
uniformity of response, the guiding principle for the selection of
appropriate test species is that the test animals should resemble man
as closely as possible with respect to absorption, distribution,
metabolic transformation, excretion, and effect at site(s) of action
of chemicals. Both male and female animals should be tested and the
test protocol should encompass exposures of animals at both ends of
the age spectrum (see Chapter 3).
It is generally recommended that random-bred rather than highly
inbred strains of animals be used in broad-scale toxicity testing, at
least until the action of the chemical is well characterized (Food &
Drug Administration, 1970). In more specialized toxicity tests, it may
be desirable to use inbred strains, for example, when animal models
are needed that represent a genetic variation in human population, or
when hypotheses on the mechanism of action are tested.
2.4.2.1 Selection of species
The Food Protection Committee (1970) indicated that sensitivity,
convenience, and similarity in metabolism to man are the prime factors
to be considered in the selection of animal species for toxicity
testing. In the absence of information to the contrary, it is
generally recommended that data obtained from the most sensitive
species should be used as the basis for the extrapolation of test
information to man.
There is now ample evidence of wide quantitative variations among
species in their rates of biotransformation of foreign compounds
(Committee on Problems of Drug Safety, 1969; Parke & Williams, 1969;
Williams, 1967). Since many organic chemicals are subject to
biotransformation at several reactive groups in the molecule, it is
important to identify and quantify the biotransformation and
distribution pathways of a chemical in man and in several laboratory
animal species as early as possible in toxicity evaluation studies. It
seems axiomatic that for costly chronic studies on experimental
animals, the species that is most representative of man with respect
to the metabolism of the test chemical should be chosen. Often, the
only information concerning the metabolism and distribution of the
test compound in man may be derived from limited studies on
individuals accidentally or occupationally exposed to uncontrolled or
unknown doses.
In vitro studies of metabolism using animal tissues and human
tissues obtained at autopsy or biopsy could help in comparisons of
similarities or differences in metabolism between man and laboratory
animals. Although this approach cannot, in itself, provide information
that may be obtained in studies on intact animals, it can, coupled
with knowledge of the physicochemical properties of the compound and
the kinetics of enzymatic biotransformation reactions in tissues of
various species, provide a logical basis for selection of species for
long-term toxicity tests. Decisions based on comparative human and
experimental animal metabolism data should take into account
information concerning several pathways of metabolism. This will help
to ensure the inclusion of data on quantitatively minor pathways of
metabolism that may result in products of major toxicological
importance. These considerations of variation in biotransformation can
also be applied to intraspecies variations related to age, sex, and
strain (Benke & Murphy, 1973; Jori et al., 1971a; MacLeod et al.,
1972; Parke & Williams, 1969).
Anatomical and morphological variations can also determine the
selection of species. This source of variation is likely to be of
particular importance in inhalation toxicity studies (Roe, 1968).
Tyler & Gillespie (1969) compared anatomical characteristics of the
lungs of human beings with several laboratory and domestic animal
species when considering appropriate animal models for human
emphysema. They grouped anatomically similar species into four
classes: (a) cattle, sheep, and swine; (b) dogs, cats, and rhesus
monkeys; (c) rabbits, rats, and guineapigs; and (d) horses and man.
From their studies on horses, they concluded that the pathophysiology
and the morphological characteristics of emphysema in horses closely
resembled the disease in man and that the horse could be a
particularly suitable laboratory animal for studies of this disease.
Obviously, in the usual toxicity studies on air pollutants, the costs
of using horses would be prohibitive. The reactivity of a chemical at
primary target sites must also be considered as a potential variable
contributing to species differences in toxicity. The acute toxicity of
certain organophosphorous insecticides in representative mammalian,
avian, and fish species appeared to be more readily related to species
differences in the reactivity of the target enzyme
(acetylcholinesterase (3.1.1.7)) than to differences in hepatic
biotransformation rates (Murphy et al., 1968).
In the absence of specific knowledge of comparative metabolism
and sites of action, it is appropriate to apply the principle that
quantitative and qualitative similarity of response in several
mammalian laboratory species enhances the confidence that man will
respond similarly. Tests on several species seem equally as useful for
predicting effects in a heterogenous human population as the selection
of test species based on the results of limited studies of metabolism
in a very few individual human subjects, who may or may not be
representative of a broad cross-section of the human population at
risk. Of course, any quantitative information on the disposition and
action of chemicals in man is useful, as it adds to the accumulation
of knowledge from which more specific guidelines for species selection
may be derived in the future.
2.4.2.2 Animal models representing special populations at risk
Because many chemicals in the environment are widely dispersed,
all segments of the human population may sustain some exposure. For
this reason, it may be useful to design special experiments to
evaluate toxicity in animal models that represent potentially
hypersusceptible segments of the human population. The very young and
the aged represent such segments generally, because in the very young,
natural protective mechanisms such as metabolic detoxification systems
may be incompletely developed and in the aged, cell repair processes
may be less active than in younger individuals. Evaluation of the
toxicity of chemicals in animal models of commonly occurring human
diseases may be of value. Thus, for example, epidemiological studies
suggest that individuals suffering from coronary artery disease may be
particularly susceptible to carbon monoxide, the severity of signs and
symptoms in patients suffering from cardiorespiratory disease appears
to be aggravated by air pollution, and asthmatic patients appear to
have a higher frequency of attacks during periods of high oxidant air
pollution (Heimann, 1967). Few attempts have been made to evaluate
experimentally the interactions between exposure to toxic chemicals
and model disease conditions. Taylor & Drew (1975) reported that an
inbred strain of cardiomyopathic hamsters was more susceptible to
acute toxicity and cardiac arrhythmias produced by inhaled
trichlorofluoromethane than were random-bred hamsters that were not
cardiomyopathic. Easton & Murphy (1967) suggested that their
observation of greater mortality and respiratory distress in
ozone-preexposed than in air-exposed guineapigs given histamine
injections or inhalation exposures might be analogous to the apparent
increase in frequency and severity of asthmatic attacks reported for
peak periods of photochemical air pollution.
Problems of standardization of disease conditions add another
dimension to toxicity studies. However, it seems that animal disease
models should be given more consideration in toxicity evaluations that
are intended to provide the basis for the safe use of chemicals to
which large human populations are exposed. Jones (1969) summarized
reference sources for animal models of a large number of specific
human diseases. Several papers in a series of symposia proceedings
published by the US National Academy of Sciences provide discussion
and references to (among other topics) animal models for commonly
occurring disease states of the lungs (Tyler & Gillespie, 1969), the
cerebrovascular system (Luginbuhl & Detweiler, 1968), the heart (Jobe,
1968), the kidney (Lerner & Dixon, 1968), atherosclerosis (Clarkson et
al., 1970), diabetes (Hackel et al., 1968), and chronic degenerative
diseases (Abinanti, 1971). Since gut microflora are changed in certain
gastrointestinal diseases in man, modifications of the quality and
distribution of microflora in experimental animals might be a useful
model for special tests (Williams, 1972). There are at least three
possible applications of these disease models to toxicity studies: (a)
evaluation of the susceptibility of diseased tissues to chemicals
known to exert their action on that tissue; (b) influence of the
disease state on the metabolism and distribution of chemicals that may
act on the diseased tissue or at other sites; and (c) research on the
mechanism of action of toxic chemicals using specific modification of
receptor function or biochemistry.
2.4.3 Cyclic variations in function or response
Many physiological variables undergo cyclic peaks and troughs of
activity (Altman & Dittmer, 1966) some of which are diurnal (24-h) and
others of longer duration. These rhythms may be completely under
intrinsic control or they may be partly or largely regulated by
environmental variables such as light and temperature. Boyd (1972)
considers most diurnal variations in susceptibility to drug toxicity
to be mainly related to eating and sleeping habits. Since rats are
nocturnal feeders, the greater quantity of food in the stomach early
in the morning compared with the afternoon may alter the acute
toxicity of chemicals given intragastrically. Attempts to standardize
this variable have led to recommendations that acute toxicity tests by
intragastric administration should be conducted on animals that have
fasted overnight (Food & Drug Administration, 1959). Intragastric
LD50 values are generally lower in rats fasted overnight compared
with those fed ad libitum; however, the differences are usually only
of the order of two- to three-fold (Boyd, 1972; Loomis, 1974). The
influence of fasting may, in some cases, be related to rates of
absorption from the gut in the presence or absence of food but this
cannot account for all such variations. A striking example has been
reported by Jaeger et al. (1975) in which acute toxicity and liver
injury in rats exposed through inhalation to several halogenated
olefins were enhanced 10- to 20-fold by overnight fasting. A diurnal
cycle of susceptibility of rats to the toxicity of inhaled vinylidene
chloride appeared to be related to the diurnal cycle of liver
glutathione concentrations (Jaeger et al., 1973) which may be
secondary to a diurnal cycle in feeding activities. The duration of
pentobarbital anaesthesia in mice under usual laboratory housing
conditions exhibited a diurnal cycle with the longest duration at
14h00 and the shortest (40-60% of that at 14h00) duration at 02h00
(Davis, 1962). The amplitude of the cycle was considerably reduced,
when animals were caged individually as opposed to group caging, and
constant light abolished the cycle. That circadian variation in the
action of certain organic chemicals may be related to circadian
variation in their biotransformation is suggested by the work of Jori
et al. (1971b).
Beuthin & Bousquet (1970) reported seasonal or circannual rhythms
for drug action and biotransformation rates in rats. The induction of
increased drug metabolism by phenobarbital also exhibited a seasonal
variation. Basal levels of hexobarbital metabolism were highest during
the winter months and lowest in summer, whereas the opposite cycle for
induction of hexobarbital oxidase by phenobarbital was observed. It
should be noted that studies of seasonal variations in the metabolism
or toxic action of chemicals must be carefully controlled with respect
to environmental variables that might produce similar variations in
response (see 2.4.4). Boyd (1972) suggests that seasonal variations in
susceptibility may be related to the hibernation reaction or to
weather conditions in the geographical area concerned.
Circadian variation in adrenocortical activity in rats was
investigated by Szot & Murphy (1971) in animals exposed acutely or
subacutely to the pesticides parathion and DDT. Although the degree of
stimulation of corticosterone secretion after single doses of
parathion varied depending upon the phase of the cycle at the time of
administration, feeding parathion or DDT in the diet at rather high
concentrations did not change the phase or the amplitude of the
natural adrenocortical rhythm or alter the stimulation of
corticosterone secretion produced by irritant stress.
In rodents, locomotor activity is greatest at night and Boyd
(1972) suggests that depression of activity is best demonstrated at
night or in rats starved for 3 days when their daytime activity is as
great as at night time. However, a more convenient approach may be to
reverse the lighting schedule, a procedure used successfully for
measuring the effects of various inhaled air pollutants on locomotor
activity in mice (Murphy, 1964).
The time of day at which biochemical or other tests are conducted
in control and experimental animals may influence the reproducibility
of the test data, if the biological variables under test display
rhythmic variation. An investigator may exploit these rhythmic
variations to advantage in special studies of factors that influence
susceptibility to chemical injury. However, if the aim is a broad
scale characterization of the toxicity of a chemical, the choice may
be to carefully standardize times of administration of chemicals and
of animal sampling to minimize both known and unrecognized circadian
variations as much as possible.
2.4.4 Environmental variables
There are numerous possible variations in the environment in
which experimental animals are housed or tested that can influence
their response to toxic chemicals. General considerations of these
variables are discussed by several authors (Boyd, 1972; Doull, 1972,
1975; Hurni, 1970; Morrison, 1968). Unless the purpose of the
experiment is to use these variables to predict possible alterations
in effects in man exposed to chemicals under similar environmental
variations, it is generally possible to minimize their influence on
the toxicity of chemicals by adopting good principles of animal care.
Reference sources are available to provide guidelines for proper
housing, diets, cage size requirements, etc. (DHEW, 1972; Universities
Federation for Animal Welfare, 1972).
Only brief comments will be made on some of the major
environmental variables that affect toxicity experiments or that can
be used for predicting mechanisms or possible implications to man.
2.4.4.1 Temperature
Major variations from the recommended environmental temperatures
and relative humidities can contribute not only to the impairment of
general health and increased susceptibility to infection of the
animals, but also to variation in their response to toxic chemicals.
The mechanisms of interactions between environmental and body
temperatures and drugs or toxic agents have been reviewed by Doull
(1972) and by Cremer & Bligh (1969). Since absorption, distribution,
metabolic transformation, excretion, and reactivity with receptor
sites depend on various temperature-dependent chemical reactions, it
might be expected that the toxicity of chemicals would be readily
influenced by temperature. However, since toxicity studies are usually
conducted with homotherms, only minor changes in core body temperature
occur with moderate changes in environmental temperatures. By the same
token, changes in environmental temperature will elicit homeostatic
changes in various physiological or biochemical systems. These may
then alter some of the physiological variables (e.g. ventilation,
circulation, body water, intermediary metabolism) that are
rate-limiting determinants of the absorption, deposition, and action
of toxic chemicals. Furthermore, toxic chemicals may exert their
action by disruption of the thermoregulatory mechanism as suggested
for cholinesterase inhibitors (Meeter & Wolthuis, 1968). Exposure to
toxic chemicals can also mimic the actions of extremes in
environmental temperature or other physical stressors (Murphy, 1969;
Szot & Murphy, 1970). Thus, fluctuations in environmental temperatures
can lead to functional changes that might be mistakenly attributed to
the action of the chemical or they may actually alter the toxicity. If
interference with physiological thermoregulatory mechanisms is a
likely action of the chemical, careful control of environmental
temperatures is necessary to ensure reproducibility of measurements of
this action.
2.4.4.2 Caging
The type of cage, grouping, bedding, and other factors related to
caging can markedly influence the toxicity of some chemicals and drugs
(Boyd, 1972; Doull, 1972; Hurni, 1970). The acute toxicity of
4-[1-hydroxy-2-[(1-methylethyl)amino]ethyl]-1,2-benzenediol
(isoproterenol) was markedly greater in rats caged singly for more
than three weeks than in rats caged in groups (Hatch et al., 1965).
Winter & Flataker (1962) reported that grouped rats held in "closed"
(sheet metal on four sides and bottom) cages were more resistant to
the acute toxicity of morphine and 1-[2-(4-amino-phenyl)ethyl]-
4-phenyl-4-piperidine carboxylic acid ethyl ester (anileridine) than
rats held in "open" (wire mesh) cages. These differences were
attributed to mechanical factors that prevented depressed rats from
maintaining an open airway in the wire mesh cages. Altered toxicity of
chemicals related to caging effects are generally purely experimental
variables and can be controlled by good practices of laboratory animal
housing.
2.4.4.3 Diet and nutritional status
Dietary variables can influence the toxicity of chemicals in
several ways. The toxicities of several pesticides were enhanced to
different degrees in rats given low protein diets (Boyd, 1969).
Protein-deficient diets protected rats against the acute
hepatotoxicity of carbon tetrachloride and N-methyl-
N-nitrosomethanamine (dimethylnitrosamine) (although the number of
kidney tumours after a single dose of the latter increased), while the
acute toxicity of chloroform was unchanged and the acute toxicity of
aflatoxins was markedly enhanced (McLean & McLean, 1969). These
effects could be explained, at least in part, by the reduction of
activity of hepatic mixed-function oxidases that generally results
from feeding low-protein diets. Whether or not a compound's toxicity
is increased or decreased in such circumstances will depend upon
whether microsomal biotransformation leads to the formation of more or
less toxic metabolites. Numerous other examples of macro and
micronutrient deficiencies, which alter the activity of the
drug-metabolizing enzyme systems and the toxicity of chemicals, have
been reviewed by Campbell & Hayes (1974). Intestinal and pulmonary
aryl hydrocarbon hydroxylase activity is modified by diet. Of
particular interest is the observation that changing rats from a
commercial, natural diet to a balanced, purified diet resulted in an
almost total loss of activity of this enzyme system in these tissues
(Wattenberg, 1972). Flavonoid compounds, present as natural
constituents of alfalfa meal (and other plants), may account for the
apparent induction of aryl hydrocarbon hydroxylase by natural diets.
The trace mineral content of diets can also influence the metabolism,
distribution, and action of toxic chemicals (Moffitt & Murphy, 1974).
The results of toxicity experiments can be markedly influenced if
care is not taken to ensure constancy of diets free from residues of
contaminating chemicals. However, since nutritional imbalances are
widespread in the human populations, controlled variation of
experimental diets to simulate major human deficiency states (e.g.
kwashiorkor resulting from protein deficiency) is an important area
for research in toxicology and should, perhaps, be included in
standard toxicity evaluations of select groups of chemicals.
2.5 Statistical Considerations
Although various protocols for toxicity testing recommend
specific numbers of animals to be used for various acute and chronic
tests (see Chapter 3), a useful guiding principle is that sufficient
animals should be used to allow statistically valid conclusions
concerning differences in the response of test animals compared to
controls and to provide a base for statistical extrapolations to
larger population samples. Statistical procedures allow the
experimenter to make ( a) descriptions of sets of data or population
characteristics, and ( b) statements of probability of events.
Various procedures provide for both enumerative data, or yes-no type
characteristics, and measurement data, or graded effects or
characteristics. Some procedures (t-test, F-test) are restricted to
observations that have specific frequency distributions, while others
(signed rank test, rank run test) are free of any assumptions about
distribution (i.e. nonparametric). Standard texts should be consulted
for the application of biostatistics to the design and analyses of
experiments. In practice, it is highly advisable to involve a
statistician in the experimental design as well as in the analysis.
The number of animals required to make statistically valid
conclusions regarding the differences between experimental and control
animals will depend upon the degree of confidence desired and the
magnitude of the possible sources of variation in the experiment. The
second consideration will depend upon the uniformity of the test
animals with respect to the biological system or systems under test.
This, in turn, will depend upon both genetic and environmental
factors. The reproducibility of the bioassay and chemical procedures
used in the tests will be another source of variation. A further
source of variation in toxicity testing is related to the constancy
and stability of the test chemical. Finally, there are the variables
introduced by the investigators (often the most difficult to control),
beginning with the care and attention given to accurate dosing
throughout the various steps of the experiment. Attempts must be made
to minimize all these sources of variation as far as possible, without
sacrificing any important aspect of the experiment.
When testing whether or not two sets of data may both be valid
samples from the same population with normal frequency distribution
(i.e. null hypothesis) or whether the control group is not
significantly different from the treatment group, statisticians
describe the appropriate sample size in terms of the desired "power"
of the test. Two types of decision errors exist. One is that a
significant difference between groups is stated to exist when, in
fact, there is no difference. This is called the type I error and the
experimenter must state what probabilities (alpha) for this error he
will accept; most commonly a probability of 0.05 is used, but an
experimenter may sometimes require a probability as low as 0.01, or
any other he chooses. The second type of decision error is that no
significant difference between groups is stated to exist when, in
fact, the groups are different. This is called the type II error, and
1-ß (the probability that one will not make this error) is the power
of the test. The power is directly related to the sample size and the
ratio of "differences between true means of the samples" to
"differences between experimental error of the means of the samples".
Once this ratio is fixed, the power increases solely as a function of
sample size. The experimental error (pooled variance) can be estimated
from previous experiments or a pilot study. The acceptable magnitude
of the differences between true means of the samples is at the
discretion of the experimenter; he must use expert judgment and should
have a reasonable rationale; it will usually be the smallest value
that is considered to be of practical importance.
Another important statistical consideration is related to the
selection of the valid number of sampling units. This may be
particularly true in considering quantal (all-none, yes-no) effects
that may be multiple occurrences within a single test animal, as, for
example, in reproduction and carcinogenesis studies where,
respectively, there may be a number of affected offspring or a number
of tumours resulting from the treatment of a single animal. The
selection of the appropriate unit, either the number of animals
exposed or the number of occurrences of an effect, can determine the
statistical significance of an observed effect. Weil (1970) suggests
that in reproduction studies the number of maternal animals (or
litters) and not the number of affected fetuses or offspring is the
valid sampling unit, and that in carcinogenesis studies the number of
tumour-bearing animals should be the sampling unit and not the total
number of tumours. Furthermore, in carcinogenesis studies, animals
risk death from factors other than the tumours; in some cases, animals
may have died before they had time to develop a tumour; in other
cases, information from some animals may be lost from the study
because of unexpected death and autolysis of tissues preventing tumour
identification. An adjusted tumour incidence may be estimated by the
life-table techniques in such experiments (McKinney et al., 1968).
Another problem may be associated with gross or histopathological
examination where, because of cost and time considerations, only
tissues of a fraction of the total number of animals exposed are
subjected to complete examination. This will reduce the likelihood
that statistically valid conclusions can be drawn from the data on
occurrence of lesions. In practice, reasonable compromises are usually
necessary. Irrespective of the statistical methods of analysis used in
both the design and interpretation of results of toxicity tests on
chemicals, they cannot replace careful experimentation and
comprehensive knowledge of the underlying biological mechanisms of the
various steps between exposure to a chemical and injury.
2.6 Nature of Effects
2.6.1 Reversible and irreversible effects
Reversible effects are characterized by the fact that the
deviation from normal structure or function induced by a chemical will
return to within normal limits (controls) following cessation of
exposure. With irreversible effects, the deviation persists or may
progress, even after exposure ceases. This might be further qualified
by time limits, that is, the time required for return to normality
after exposure should be a reasonable fraction of the remaining
lifetime of a young animal for it to be considered reversible.
Reversibility may also be qualified by the normal lifetime of a
specific cell or macromolecule that serves as the end-point for the
effect. For example, cholinesterase-inhibiting insecticides are
generally considered irreversible inhibitors if the rate of reversal
of inhibition corresponds approximately to the time required for
synthesis and replacement of the enzyme, a process with different
rates in different tissues. Certain effects of toxic chemicals are
unmistakably irreversible, including the production of terata, or
malignant tumours, production of mutations in offspring of exposed
animals, certain chronic neurological diseases, production of true
cirrhosis, or emphysema. These are rather gross manifestations of
certain specific chemical-cell interactions, and, either at the level
of the first affected molecule or at intervening points leading to
these manifestations, there are probably reversible effects.
Understanding these effects and determining the critical dose that
produces them will make it possible to predict truly adverse effects
more rapidly.
The rate of reversibility of an effect will depend upon the rate
of cellular injury and the rate at which this injury is repaired
(Casarett, 1975). The rate of injury will depend upon the
concentration and duration or frequency with which a test chemical
contacts responsive tissue constituents. It is, thus, dose and
dose-rate dependent. The rate of repair is determined intrinsically
and may involve several cell processes. It may vary between different
tissues and probably between different species and strains. From a
practical standpoint, it is generally impossible to measure the
specific processes involved in injury and repair in a standard
toxicity evaluation study. However, it is important to make
measurements of the reversibility of effects in early, acute and
subacute studies. Thus, the time required for a process to return to
normal after single doses (which produce various degrees of injury)
will provide a guideline for the selection of doses to be used in
subsequent acute or chronic studies. The predictive value of such
information will depend upon the persistence of the chemical in the
test organism. If the chemical produces an effect and then is rapidly
detoxified or excreted, it may be possible to predict, with reasonable
accuracy, doses or exposure schedules that would not produce
cumulative effects. The manner of exposure and possible actions other
than the one being measured would, of course, be important in drawing
such conclusions. For example, rapid reversibility after a single dose
might not be indicative of the rate of reversal with a repeated dosing
if the first dose, in addition to the measured effect, also altered
either the repair processes or the processes responsible for
detoxification of the chemical. An example of an apparent
self-inhibition of detoxification is the insecticide malathion which
is rapidly hydrolysed by carboxylesterases. These are, in turn,
inhibited by metabolites or contaminants of malathion (Murphy, 1967).
Repeated exposure studies are necessary to evaluate such
possibilities; thus, the design of short-term feeding or inhalation
studies should include extra groups of animals that can be removed
from exposure either at the end of the experiment or, preferably, at
selected intervals for measurements of rate of reversal of any
observed effect.
If the chemical persists or accumulates in the organism,
measurements and interpretation of rates of reversal of effects are
more complicated. For this reason, it is useful to have kinetic data
on absorption and disposition to correspond with data on rates of
production and reversal of effects. Further discussion of these and
related principles is provided by Hayes (1972) in relation to his
proposal that determination of "chronicity factors" (1-dose LD50
(mg/kg) ‰ 90-dose LD50 (mg/kg/day) in diet i.e. the ratio of single
dose LD50 to the daily dose given in diet for 90 days which results
in 50% mortality at that time) is useful in predicting candidate
chemicals requiring long-term studies. The use of such predictive
methods must also take into consideration potential for other effects
that could never be detected in a subacute study (e.g. tumorigenesis).
2.6.2 Functional versus morphological changes
Toxic effects are often classed as functional or morphological in
nature. There has been a traditional attitude that changes in gross or
microscopic structure are more serious than functional changes.
Indeed, altered structure often seems to have taken on the implication
of irreversibility while altered function is often considered a
reversible effect. This conclusion, of course, depends on the level of
understanding of mechanisms of injury, rates and mechanisms of repair,
and causal associations between related functional and morphological
changes. Furthermore, whether the change is regarded as functional or
morphological may depend on the manner of detection. For example,
accumulation of fat in a cell observed through a microscope will most
often be considered a structural change, but, if the same cells or
tissues were assayed for triglyceride content, the increased
triglyceride would probably be classified as a functional or
biochemical change. The introduction of enzyme histochemistry and
electron microscopy into toxicity evaluation studies makes the
distinction between morphological and functional effects even less
clear. It may therefore be inappropriate to attempt to make these
distinctions.
Rowe et al. (1959) reviewed data from studies on a large number
of chemicals repeatedly administered to animals over periods ranging
from one month to two years, and summarized the frequency with which a
certain effect was found and the frequency with which it was the only
effect found. The effects were considered on: mortality; food intake;
body weight; organ weights; the histopathology of virtually every
major organ; haematology; blood urea nitrogen; clinical urinalyses;
central nervous system (most probably gross behaviour); gross
pathology; and cholinesterase activity. The authors found that if
growth, liver weight, kidney weight, liver pathology, and kidney
pathology had been studied, the lowest dose level that caused any
effect would have been detected in 96% of the studies. Changes in food
intake, central nervous system depression, excessive mortality,
increased lung weight, testicular injury, haematological changes, and
cholinesterase depression were the most sensitive effects in one or
more of the remaining 4% of cases. The reader should consult the
original reference for details but it is important to note that of the
commonly used criteria of effects, liver and kidney micropathology
were quite sensitive indices.
There are, of course, well-known examples where functional
changes are the only manifestations of toxicity. Many of the
organophosphate and carbamate insecticides inhibit cholinesterase
(3.1.1.8) activity and produce signs and symptoms (even death) that
can be characterized as purely functional without the production of
morphological lesions, detectable by conventional techniques.
Similarly, irritant air pollutants can often cause bronchoconstriction
and respiratory distress, without any accompanying morphological
changes. Both the functional cholinergic signs and symptoms produced
by the anticholinesterase insecticides and the bronchoconstriction
produced by irritants provide means of early detection at low levels
of exposure. Although these effects are reversible, they are no less
important during the period of exposure than certain kinds of
morphological effects. On the other hand, certain kinds of
"functional" changes, e.g. increased level of plasma transaminase
activity, usually reflect some type of structural change in cells that
allow these enzymes to "leak" into plasma (Cornish, 1971).
It is not possible, with present knowledge, to conclude that
either functional or morphological changes represent the most
sensitive, the earliest, or the most serious effects of toxic
chemicals. Since maintenance of both integrated function and
integrated structure ultimately depends on chemical reactions among
cell constituents, it is logical to conclude that specific biochemical
changes are the first and most sensitive effects. Unfortunately, with
relatively few exceptions, the specific biochemical receptors for
toxic chemicals are unknown. The more information that can be obtained
with respect to time- and dose-relationships for functional and
morphological effects, the more predictive the tests will become. This
requires an approach to toxicity studies in which proof of a mechanism
will require an integrated biochemical, physiological, and
morphological approach. Dawkins & Rees (1959) provide a useful short
treatise on an integrated biochemical-pathological approach to studies
of several toxic chemicals. Although advances in both biochemistry and
pathology now allow even more precise studies than those outlined by
these authors, the general principles which they develop are still
applicable.
2.7 Dynamic Aspects of Predictive Toxicology
2.7.1 Traditional versus new techniques
The objective of any toxicity test programme is prediction:
prediction of biological disposition from physicochemical constants,
prediction of altered cell or organ system function from reaction with
macromolecules, prediction of irreversible consequences of reversible
changes, prediction of implications of selected measurable variables
to overall health and survival of the test organisms, prediction of
effects in individuals of one species from tests conducted in another,
and finally predictions of incidence in large populations from tests
on small samples. All of these predictions must be related
quantitatively to a dose and dose-rate or schedule that can ultimately
be related to probable amounts used, the manner of use or the
occurrence of the chemicals in the environment.
Generally, traditional approaches to toxicity evaluation have not
attempted to make predictions far removed from the final application
or interpretation of the data. Thus, as outlined in Chapter 3, test
organisms are exposed to a range of doses and their health status is
examined by biochemical, physiological, or pathological procedures
analogous to those used in clinical medicine. When this approach has
been comprehensive, judicious application of the data usually appears
to have been successful in preventing chemically-induced disease.
Abandoning this approach in favour of new, different, or short-cut
methods cannot be advocated without thorough verification of their
validity. On the other hand, serious consideration must be given to
the application of some short-term ways of predicting toxicity in
order to provide a practical means of evaluating the many chemicals
already in the environment and those new compounds that are
continuously being added to the environment and have not been
subjected to traditional tests. Preceding sections have discussed some
possibilities, the following sections contain brief comments and
examples for consideration in selecting tests.
2.7.2 Toxicity of chemical analogues
Although it may be possible to predict the toxicity of individual
compounds in a homologous series from detailed knowledge of some
members of the series, some special exceptions should be noted. A
classical example involves the series of fluorine-substituted
aliphatic alcohols and acids, in which high acute toxicity alternates
with odd and even numbers of carbon atoms, the latter being the most
toxic (Pattison, 1959). The odd number of total carbon atoms confers
high toxicity in a homologous series of fluoronitriles, however. This
demonstrates the possibility that detailed information concerning only
a few members of a homologous series might fail to predict the
toxicity of another member of the series.
Recently, Johnstone et al. (1974) examined a number of
biochemical effects in a series of isomerically pure compounds for
their potency as liver enzyme inducers. Potency for this effect
increased with increasing chlorination that was related to differences
in biotransformation and excretion rates; however, there were also
striking differences in the potency of positional isomers in the lower
chlorinated biphenyls.
The mechanism of the toxic action of organophosphorus
insecticides was known to be the inhibition of acetylcholinesterase
even before they were introduced into use 30 years ago. However,
quantitative prediction of their acute toxicity from in vitro tests
of their relative potency as anticholinesterases is still inadequate
because of incomplete knowledge of the dynamic relationships between
several pathways of metabolism which yield both more and less potent
metabolites (Murphy, 1975). Nevertheless, these compounds have been
subjected to a great deal of research on both their physicochemical
and biological properties which should be applicable to predictions of
their relative environmental persistence, interaction with other
compounds, and, ultimately, to the design of safe molecules (Eto,
1974).
Using model ecosystems, Lu & Metcalf (1975) studied
bioaccumulation, biodegradability, and comparative detoxification
mechanisms in several benzene derivatives with widely-varying
physicochemical constants and biological activities. They concluded
that biological disposition and action could be predicted by the basic
molecular properties of water solubility, the partition coefficient
for lipid/water, and reactivity as determined by electron density.
Johnson (1975) recently reviewed the problems encountered in the
pursuit of the mechanism of delayed peripheral neuropathy produced by
some organophosphorus esters. The structure-activity relationships
identified in this research may be considered as a model of thorough
investigation that began as a problem in retrospective toxicology and
led to promising developments applicable to prospective toxicology.
Some interesting aspects of this problem are: the particular
usefulness of a non-mammalian species, the hen, as a predictor of a
toxic action that occurs in man; the concept of primary metabolic
effects on central neurons as a precursor to pathological change
detected in peripheral nerve fibres; and the difficulties of detecting
a specific critical esterase inhibition that represented only a small
percentage of the total esterase activity. Production of peripheral
neuropathy appears to be characteristic of organophosphorus esters
which may not only phosphorylate the specific "neurotoxic esterase"
but are also capable of dealkylation (or aging) following
phosphorylation. Although the steps between this primary
phosphorylating-aging process and the eventual manifestation of
peripheral neuropathy are still unclear, it appears that it may be
possible to predict probable occurrence of a delayed, chronic disease
from studies of the primary chemical-macromolecular interaction of
neurotoxic esterase inhibition that occurs immediately following
exposure.
2.7.3 Relation between site of metabolism and site of injury
Although for many years it was thought that the biotransformation
of organic chemicals represented detoxification mechanisms, it is now
apparent that numerous compounds are enzymatically converted to
intrinsically more active compounds in vivo (Fouts, 1972; Murphy,
1975; Parke, 1968). The liver is generally the most active tissue in
catalysing these "activation" reactions, but it is not always the most
susceptible target tissue as, for example, in the case of activation
of phosphorothioate insecticides to phosphate insecticides. This may
be explained, in part, by the presence of detoxifying enzymes or
reactive but noncritical binding sites in the liver that may prevent
the phosphates from escaping to inhibit cholinesterase in nerve target
tissues. The brain tissue has only a small fraction of the liver's
capacity to activate phosphorothioates, but because the activation
occurs in the same tissue as the critical target site, activation in
the brain may be the most important in determining toxicity.
Recently, the characteristic hepatotoxicity of several chemicals
has been related to their enzymatic conversion to highly reactive
derivatives that covalently bind to essential liver cell constituents
at, or near, the site of activation (Brodie et al., 1971). A similar
possibility may explain the bronchiolar neurosis produced in rats and
mice by bromobenzene and other aromatic hydrocarbons (Reid et al.,
1973).
The detailed study of the metabolism, storage, or binding and
distribution of foreign chemicals in the lung is a relatively recent
activity and has focused largely on therapeutic chemicals (Bend et
al., 1973; Brown, 1974; Orton et al., 1973). Because inhalation is a
common route of exposure to a wide variety of air contaminants in
industrial and community environments, future toxicity studies would
benefit by the inclusion of metabolic studies concerning rates of
absorption from, and local actions in the lung. Witschi (1975) has
reviewed biochemical approaches that may be used in the evaluation of
toxic injury to the lung.
Since the intensity and duration of the toxic action of a
chemical depends on the concentration of the active form at critical
receptor sites of action, kinetic aspects of absorption, distribution,
and excretion (as well as biotransformation) will influence the
specific sites of action. This topic is discussed in detail in Chapter
4 but it is worthy of note that Dedrick (1973) developed several
useful kinetic models that might be applied in predicting species
differences or similarities in response.
2.7.4 In vitro test systems
Where appropriate, studies in experimental animals should be
supplemented by isolated perfused organ, tissue slice or extract, and
tissue culture techniques. Where possible, attempts should be made to
compare the tissues of human subjects available from autopsy or
therapeutic biopsies, with those of other species in their response to
toxic chemicals (Worden, 1974). When the mechanism(s) of toxicity have
been elucidated and the target organ(s) identified, specific species
comparisons and dose-response relationships can be studied by these
in vitro techniques.
Knowledge of a specific enzyme or biological macromolecule that
serves as a target for reaction with toxic chemicals may provide a
means for screening and predicting relative potencies or specific
actions of chemicals in intact organisms. However, as pointed out
previously for the organophosphorus insecticides, biotransformations
and membrane barriers to the distribution of chemicals in intact
animals will often invalidate conclusions drawn from in vitro
assays. This problem may be partly overcome by incorporating enzymic
biotransformation systems with the target macromolecule (or organism)
in the in vitro test system. Such an approach is used for screening
for potential mutagens in microorganism test systems (Malling &
Frantz, 1973) and has been applied to studies of the biochemical
actions of pesticides (Chow & Murphy, 1975; Cohen & Murphy, 1974). A
major problem in the use of in vitro test systems for predicting
toxicity is the difficulty of quantitatively relating concentrations
in the simplified in vitro systems to action in complex intact
organisms. With adequate correlative data in both in vitro and
in vivo systems this may become possible, but such information is
generally lacking at present. For the most part, therefore, in vitro
model test systems are qualitative predictors rather than
quantitative. This need not decrease their usefulness, however, as
long as this limitation is recognized in the interpretation of
results.
In general, in vitro test systems have been useful in
qualitatively predicting acute actions. However, as discussed earlier,
neurotoxic esterase inhibition provides promise for predicting delayed
chronic neuropathy produced by certain organophosphate compounds.
Major research efforts are now being devoted to in vitro test
systems for the prediction of mutagenesis and carcinogenesis. These
are discussed in detail in Chapter 7. There is general recognition of
the value of these test systems (Council of Environmental Mutagen
Society, 1975; Food & Drug Administration, 1970; Food Protection
Committee, 1970) as screening procedures, but much less agreement as
to the priority that they should have in the conduct and
interpretation of toxicity evaluations.
When it is possible to obtain comparisons between exposures of
organs or tissues of experimental animals and humans to toxic
chemicals, such comparisons will provide useful baseline data for the
future extrapolation of data from intact animal studies to man.
Culture systems of human cells may also be useful as comparative
systems. The difficulties of maintaining some human cell lines are
well documented, but primary cultures of differentiated mammary and
liver epithelia have been established and maintained (Buehring, 1972;
Lasfargues & Moore, 1971; Potter, 1972). Human lymphocytes have also
been used in vitro (Kellermann et al., 1973).
It may be possible to use these isolated systems to determine the
susceptibility to toxic chemicals of different cell types in different
organs and to determine the reversibility of adverse effects in these
cell lines and organs. Of particular usefulness would be the
determination of dose-response curves for many tissues and their
interspecies comparison. Such information could be used to predict
target cells and organs with a high degree of susceptibility or
resistance. However, as mentioned earlier, the usefulness of the data
may be limited if the cells, tissues, or organs are incapable of
metabolizing the chemical to a toxic form in the intact animal. Such a
biotransformation may even occur in a different tissue or organ from
the one under test in vitro. To overcome this problem,
biotransformation systems from animal or human tissues (e.g.
microsomal activating systems) are often added with the chemical to
the isolated culture systems.
One problem with these methods is the uncertainty that all the
steps of metabolism are equally duplicated, that is, in addition to
activation of a chemical there should also be an opportunity for the
chemical to be detoxified or conjugated and eliminated. Some of these
detoxification steps require different coenzymes or metabolites, and
the enzyme systems may not be limited to microsome fractions or liver
tissue. However, as long as it is realized that these in vitro test
systems may exaggerate the situation that occurs in vivo they can
prove useful, especially for tests where only very small quantities of
material are available, as might be the case with some impurities or
metabolites.
In summary, short-term, in vitro tests both for carcinogenicity
and other forms of toxicity show great promise (Golberg, 1974), and
although no single test is likely to be reliable, appropriate
combinations may provide valuable information concerning the
fundamental toxicity of environmental chemicals. This would currently
provide a useful adjunct to long-term studies in animal populations,
and may develop further in the future to provide the more reliable
method of assessment. Such tests will require much development and
will take years to validate and perhaps even longer to win public
confidence with regard to their reliability.
REFERENCES
ABINANTI, R. R. (1971) Chronic and degenerative diseases of man: the
value of natural and experimentally induced diseases of animals.
In: Animal models for biomedical research IV, Proceedings of a
Symposium, Washington DC, National Academy of Sciences,
pp. 31-46.
ALTMAN, P. L. & DITTMER, D. S., ed. (1966) Environmental biology,
Bethesda, MD, Federation of American Societies for Experimental
Biology, pp. 565-608.
ALVARES, A. P., LEIGH, S., KAPPAS, A, LEVIN, W., & CONNEY, A. H.
Induction of aryl hydrocarbon hydroxylase in human skin. Drug
Metabol. Disposition, 1: 386-390.
BARNES, J. M. (1968) Percutaneous toxicity. In: Boyland, E. &
Goulding, R., ed. Modern trends in toxicology, London,
Butterworths, pp. 18-38.
BEND, J. R., HOOK, G. E., & GRAM, T. E. (1973) Characterization of
lung microsomes as related to drug metabolism. Drug Metabol.
Disposition, 1: 358-367.
BENKE, G. M. & MURPHY, S. D. (1975) The influence of age on the
toxicity and metabolism of methyl parathion and parathion in male
and female rats. Toxicol. appl. Pharmacol., 31: 254-269.
BEUTHIN, P. K. & BOUSQUET, W. F. (1970) Long-term variation in basal
and phenobarbital-stimulated oxidative drug metabolism in the
rat. Biochem. Pharmacol., 19: 620-625.
BOYD, E. M. (1969) Dietary protein and pesticide toxicity in male
weanling rats. Bull. World Health Org., 40: 801-805.
BOYD, E. M. (1972) Predictive toxicometrics, Baltimore, Williams &
Wilkins, 408 pp.
BRODIE, B. B., CHO, A. K., KRISHNA, G., & REID, W. D. (1971) Drug
metabolism in man: past, present and future. Ann. NY Acad. Sci.,
179: 5-18.
BROWN, E. A. B. (1974) The localization, metabolism and effects of
drugs and toxicants in lung. Drug Metabol. Rev., 3: 33-87.
BUEHRING, G. C. (1972) Culture of human mammary epithelial cells:
keeping abreast with a new method. J. Natl Cancer Inst.,
49: 1433-1434.
CAMPBELL, T. & HAYES, J. R. (1974) Role of nutrition in the
drug-metabolizing enzyme system. Pharmacol. Rev., 26: 171-197.
CASARETT, L. J. (1975) Toxicologic evaluation. In: Casarett, L. J. &
Doull, J., ed. Toxicology -- the basic science of poisons, New
York, Macmillan, pp. 11-25.
CLARKSON, T. B., PRICHARD, R. W., BULLOCK, B.C., LEHNER, N. D. M.,
LOFLAND, H. B. & CLAIR, R. W. St. (1970) Animal models for
atherosclerosis. In: Animal models for biomedical research III.
Proceedings of a Symposium, Washington DC, National Academy of
Sciences, pp. 22-41.
CHOW, A. Y. K. & MURPHY, S. D. (1975) Production of a
methemoglobin-forming metabolite of 3,4-dichloroaniline by liver
in vitro. Bull. environ. Contam. Toxicol., 13: 9-13.
COHEN, S. D. & MURPHY, S. D. (1974) A simplified bioassay for
organophosphate detoxification and interactions. Toxicol. appl.
Pharmacol., 27: 537-550.
COMMITTEE ON PROBLEMS OF DRUG SAFETY (1969) Application of metabolic
data to the evaluation of drugs: report prepared by the Committee
on Problems of Drug Safety of the NAS/NRC Drug Research Board.
Clin. Pharmacol. Therap., 10: 607-634.
CORNISH, H. H. (1971) Problems posed by observations of serum enzyme
changes in toxicology. CRC Crit. Rev. Toxicol., 1: 1-132.
COUNCIL OF EUROPE (1973) Guide to the testing and toxicological
evaluation of flavouring substances. In: Natural flavouring
substances, their sources and added artificial flavouring
substances, Strasbourg, Council of Europe, pp. 403-410.
COUNCIL OF ENVIRONMENTAL MUTAGEN SOCIETY (1975) Environmental
mutagenic hazards. Science, 187: 503-514.
CREMER, J. E. & BLIGH, J. (1969) Body-temperature and response to
drugs. Brit. med. Bull., 25(3): 299-306.
CROSBY, D. G. (1972) Environmental photooxidation of pesticides. In:
Proceedings of a conference on degradation of synthetic organic
molecules in the biosphere, San Francisco, June 1971,
Washington DC, National Academy of Sciences, pp. 260-278.
DAVIS, W. M. (1962) Day-night periodicity in pentobarbital response of
mice and the influence of socio-physiological conditions.
Experientia (Basel), 18: 235-237.
DAWKINS, M. J. R. & REES, K. R. (1959) A biochemical approach to
pathology, London, Edward Arnold, 128 pp.
DEDRICK, R. L. (1973) Animal scale-up. J. Pharmacokin. & Biopharm.,
1: 435-461.
DHEW (1972) Guide for the care and use of laboratory animals
(prepared by Committee on Revision of the Guide for Laboratory
Animals and Care, ILAR, NRC). Washington DC, US Gov. Print.
Office (DHEW Publ. No. (NIH) 72.23).
DIGGLE, W. M. & GAGE, J. C. (1951) Cholinesterase inhibition in vivo
by O,O-diethyl O-p-nitrophenyl thiophosphate (Parathion
E 605). Biochem. J., 49: 491-494.
DILLINGHAM, E. O., MAST, R. W., BASS, G. E., & AUTIAN, J. (1973)
Toxicity of methyl- and halogen substituted alcohols in tissue
culture relative to structure-activity models and acute toxicity
in mice. J. Pharm. Sci., 62: 22-30.
DOULL, J. (1972) The effect of physical environmental factors on drug
response. In: Hayes, W. J., ed. Essays in toxicology, New York,
Academic Press, Vol. 3, pp. 37-63.
DOULL, J. (1973) Factors influencing toxicity. In: Casarett, L. J. &
Doull, J., ed. Toxicology -- the basic science of poisons, New
York, Macmillan, pp. 133-147.
EASTON, R. E. & MURPHY, S. D. (1967) Experimental ozone pre-exposure
and histamine: effect on the acute toxicity and respiratory
function effects of histamine in guinea pigs. Arch. environ.
Health, 15: 160-166.
EHRLICH, R. (1966) Effect of nitrogen dioxide on resistance to
respiratory infection. Bact. Rev., 30: 604-614.
ETO, M. (1974) Organophosphorus pesticides: organic and biological
chemistry. Cleveland, OH, CRC Press, pp. 387 (57-78).
FOOD & DRUG ADMINISTRATION (1959) Appraisal of the safety of
chemicals in foods, drugs and cosmetics. Austin, Texas,
Association of Food & Drug Officials of the USA, pp. 107.
FOOD & DRUG ADMINISTRATION (1970) Report on reproduction studies in
the safety evaluation of food additives and pesticide residues
(Report of panel on reproduction of the FDA Advisory Committee on
Protocols for Safety Evaluation). Toxicol. appl. Pharmacol.,
16: 264-269.
FOOD PROTECTION COMMITTEE (1970) Evaluating the safety of food
chemicals, Washington, DC, National Academy of Sciences, 55 pp.
FOUTS, J. R. (1972) Some studies and comments on hepatic and
extrahepatic microsomal toxication-detoxification system.
Environ. Health Perspect., Experimental Issue No. 2, Oct.
pp. 55-66.
GOLBERG, L. (1967) The amelioration of food (The Milroy Lectures).
J. Roy. Coll. Phys., 1 (4): 385-426.
GOLBERG, L. (1974) Short-term predictive tests. Proc. Eur. Soc. Drug
Toxicity, 15: 178-191.
HACKEL, D. B., MIKAT, E., LEBOVITZ, H., & SCHMIDT-NIELSEN, K. (1968)
Animal models for diabetes mellitus with special reference to the
sand rat (Prommomys obesus). In: Animal models for biomedical
research: Proceedings of a Symposium, Washington, DC, National
Academy of Sciences, pp, 14-20.
HANSCH, C. & DUNN, W. J. (1972) Linear relationships between
lipophilic character and biological activity of drugs.
J. pharm. Sc., 61: 1-18.
HATCH, A., BALEZO, T., WIBERG, G. S., & GRICE, H. C. (1965) The
importance of avoiding mental suffering in laboratory animals.
Anim. Welfare Inst. Rep., Vol. 14, No. 3.
HATCH, T. F. & GROSS, P. (1964) Pulmonary deposition and retention of
inhaled aerosols, New York, Academic Press, 184 pp.
HAYES, W. J. (1972) Tests for detecting and measuring long-term
toxicity. In: Hayes, W. J., ed. Essays in toxicology, New York,
Academic Press, Vol. 3, pp. 65-77.
HEIMANN, H. (1967) Status of air pollution health research. Arch.
environ. Health. 14: 488-503.
HENDERSON, Y. & HAGGARD, H. W. (1943) Noxious gases and the
principles of respiration influencing their action, New York,
Reinhold, 287 pp.
HOLMSTEDT, B. (1959) Pharmacology of organophosphorus cholinesterase
inhibitors. Pharmacol. Rev., 11: 567-688.
HURNI, H. (1970) The provision of laboratory animals. In: Paget, G.
E., ed. Methods in toxicology, Philadelphia, F. A. Davis,
pp. 11-48.
JAEGER, R. J., CONOLLY, R. B., & MURPHY, S. D. (1973) Diurnal
variation of hepatic glutathione concentration and its
correlation with 1,1-dichloroethylene inhalation toxicity in
rats. Res. Commun. Chem. Pathol. Pharmacol., 6: 465-471.
JAEGER, R. J., CONOLLY, R. B., & MURPHY, S. D. (1975) Short-term
inhalation toxicity of halogenated hydrocarbons. Arch. environ.
Health, 30: 26-31.
JOBE, C. L. (1968) Selection and development of animal models of
myocardial infarction. In: Proceedings of a Symposium on Animal
Models for Biomedial Research, Washington, DC, National Academy
of Sciences, pp. 101-108.
JOHNSON, M. K. (1975) The delayed neuropathy caused by some
organophosphorus esters: mechanism and challenge. CRC Crit. Rev.
Toxicol., 3: 289-316.
JOHNSTONE, G. J., ECOBICHON, D. J., & HUTZINGER, O. (1974) The
influence of pure polychlorinated biphenyl compounds on hepatic
function in the rat. Toxicol. appl. Pharmacol., 28: 66-81.
JONES, T. C. (1969) Mammalian and avian models of disease in man.
Fed. Proc., 28 (1): 162-169.
JONES, W. I., ROBACK, L. A., & TAYLOR, J. M. (1971) The loss of food
flavours from laboratory animal diets. II. Effect of laboratory
environment. J. Assoc. Offic. Anal. Chem., 54: 42-46.
JORI, A., PUGLIATTI, C., & PESCADOR, R. (1971a) Rat strain differences
in the activity of hepatic microsomal enzymes. Biochem.
Pharmacol., 20: 2695-2701.
JORI, A., SALLE, E., & SANTINI, V. (1971b) Daily rhythmic variation
and liver drug metabolism in rats. Biochem. Pharmacol.,
20: 2965-2969.
KLASSEN, C. D. (1975) Absorption, distribution and excretion of
toxicants. In: Casarett, J. L. & Doull, J., ed. The basic
science of poisons, New York, Macmillan, pp. 26-44.
KELLERMAN, G., LUYTEN-KELLERMAN, M. L., & SHAW, C. R. (1973) Genetic
variation of aryl hydrocarbon hydroxylase in human lymphocytes.
Am. J. Hum. Genet., 25: 327-331.
LA DU, B. N., MANDEL, G., & WAY, E. L., ed. (1971) Fundamentals of
drug metabolism and drug disposition, Baltimore, Williams &
Wilkins, 615 pp.
LAKE, B. G., HOPKINS, R., CHAKRABORTY, J., BRIDGES, J. W., & PARKE, V.
W. (1973) The influence of some hepatic enzyme inducers on
extrahepatic drug metabolism. Drug Metabol. Disposition,
1: 342-349.
LASFARGUES, E. Y. & MOORE, D. H. (1971) A method for the continuous
cultivation of mammary epithelium. In Vitro, 7: 21-25.
LERNER, R. A. & DIXON, F. J. (1968) Experimental and human
glomerulonephritis associated with antiglomerular basement
membrane antibodies. In: Proceedings of a Symposium on Animal
Models for Biomedical Research, Washington, DC, National Academy
of Sciences, pp. 109-130.
LJUBLINA, E. I. & FILOV, V. A. (1975) Chemical structure, physical and
chemical properties and biological activity. In: Methods used in
the USSR for establishing biologically safe levels of toxic
substances, Geneva, WHO, pp. 19-44.
LOOMIS, T. A. (1974) Essentials of toxicology, 2nd ed.,
Philadelphia, Lea & Febiger, 233 pp.
LU, P. Y. & METCALF, R. (1975) Environmental fate and biodegradability
of benzene derivatives as studied in a model aquatic ecosystem.
Environ. Health Perspect, 10: 269-284.
LUGINBUHL, H. & DETWEILER, D. K. (1968) Animal models for the study of
cerebrovascular disease. In: Proceedings of a Symposium on
Animal Models for Biomedial Research, Washington DC, National
Academy of Sciences, pp. 35-41.
LUKAS, G., BRINDLE, S., & GREENGARD, P. (1971) The route of absorption
of intraperitoneally administered compounds. J. Pharmacol. exp.
Therap., 178: 562-566.
MCLEOD, S. M., RENTON, K. W., & EADE, N. R. (1972) Development of
hepatic microsomal drug-oxidizing enzymes in immature male and
female rats. J. Pharmacol. exp. Therap., 183: 489-498.
MALLING, H. V. & FRANTZ, C. N. (1973) In vitro vs in vivo
metabolic activation of mutagens. Environ. Health Perspect.,
6: 71-82.
MCCREESH, A. H. (1965) Percutaneous toxicity. Toxicol. appl.
Pharmacol., 7: 20-26.
MCKINNEY, G. R., WEIKEL, J. H., WEBB, W. K., & DICK, R. J. (1968) Use
of life-table technique to estimate effects of certain steroids
on probability of tumour formation in a long-term study in rats.
Toxicol. appl. Pharmacol., 12: 68-79.
MCLEAN, A. E. M. & MCLEAN, E. K. (1969) Diet and toxicity. Brit. med.
Bull., 25: 278-281.
MEETER, E. & WOLTHUIS, O. L. (1968) The effects of cholinesterase
inhibitors on the body temperature of the rat. Environ. J.
Pharmacol., 4: 18-24.
MOFFITT, A. E. & MURPHY, S. D. (1974) Effect of excess and deficient
copper intake on hepatic microsomal metabolism and toxicity of
foreign chemicals. In: Hemphill, D. D., ed. Trace substances in
environmental health, Columbia, MO, University of Missouri
Press, Vol. VII, pp. 205-210.
MORRISON, J. K. (1968) The purpose and value of LD50 determinations.
In: Boyland, E. & Goulding, R., ed. Modern trends in toxicology,
Appleton-Century-Crofts, pp. 1-17.
MURPHY, S. D. (1964) A review of effects on animals of exposure to
auto exhaust and some of its components. Air Pollut. Control J.,
14: 303-308.
MURPHY, S. D. (1967) Malathion inhibition of esterases as a
determinant of malathion toxicity. J. Pharmacol. exp. Therap.,
156: 352-365.
MURPHY, S. D. (1969) Some relationships between effects of
insecticides and other stress conditions. Ann. NY Acad. Sci.,
160: 366-377.
MURPHY, S. D. (1975) Pesticides. In: Casarett, L. J. & Doull, J., ed.
Toxicology: the basic science of poisons, New York, Macmillan,
pp. 408-453.
MURPHY, S. D., LAUWERYS, R. R., & CHEEVEN, K. L. (1968) Comparative
anticholinesterase action of organophosphorus insecticides in
vertebrates. Toxicol. appl. Pharmacol., 12: 22-35.
ORTON, J. C., ANDERSON, M. W., PICKETT, R. D., ELING, T. E., & FOUTS,
J. R. (1973) Xenobiotic accumulation and metabolism by isolated
perfused rabbit lungs. J. Pharmacol. exp. therap.,
186: 482-497.
PANEL ON HERBICIDES (1971) Report on 2,4,5-T, Washington, DC, US
Govt Print. Office, 68 pp.
PARKE, D. V. (1968) The biochemistry of foreign compounds. Oxford,
Pergamon Press, 269 pp.
PARKE, D. V. & WILLIAMS, R. T. (1969) Metabolism of toxic substances.
Brit. med. Bull., 25: 256-262.
PATTISON, F. L. M. (1959) Toxic aliphatic fluorine compounds,
Amsterdam, Elsevier, 227 pp. (Elsevier Monographs).
PATTY, F. A. (1958) Industrial hygiene and toxicology, 2nd ed., New
York, Interscience, Vol. 1, 153 pp.
POTTER, V. R. (1972) Workshop on liver cell culture. Cancer Res.,
32: 1998-2000.
REID, W. D., HETT, K. F., HIK, J. M., & KRISHNA, G. (1973) Metabolism
and binding of aromatic hydrocarbons in the lung. Am. Rev. Resp.
Dis., 107: 539-551.
ROE, F. J. C. (1968) Inhalation tests. In: Boyland, E. & Goulding, R.,
ed. Modern trends in toxicology, London, Butterworths,
pp. 39-74.
ROWE, V. K., WOLF, M. A., NEIL, C. S., & SMITH, H. F. (1959) The
toxicological basis of threshold limit values: 2. Pathological
and biochemical criteria. Am. Ind. Hyg. Assoc. J., 20: 346-349.
SMYTH, H. F. (1959) The toxicological basis of threshold limit values:
1. Experience with threshold limit values based on animal data.
Am. Ind. Hyg. Assoc. J., 20: 341-345.
SZOT, R. J. & MURPHY, S. D. (1970) Phenobarbital and dexamethasone
inhibition of the adrenocortical response of rats to toxic
chemicals and other stresses. Toxicol. appl. Pharmacol.,
17: 761-773.
SZOT, J. R. & MURPHY, S. D. (1971) Relationships between cyclic
variations in adrenocortical secretary activity in rats and the
adrenocortical response to toxic chemical stress. Environ. Res.,
4: 530-538.
TAYLOR, G. J. & DREW, R. T. (1975) Cardiomyopathy predisposes hamsters
to trichlorofluoromethane toxicity. Toxicol. appl. Pharmacol.,
32: 177-183.
TYLER, W. S. & GILLESPIE, J. R. (1969) Structural and functional
alterations in horses with emphysema. In: Proceedings of a
Symposium on Animal Models for Biomedical Research, Washington,
DC, National Academy of Sciences, Vol. II, pp. 38-51.
UNIVERSITIES FEDERATION FOR ANIMAL WELFARE (1972) Handbook on the
care and management of laboratory animals, 4th ed., Baltimore,
Williams & Wilkins, 624 pp.
WATTENBERG, L. (1972) Dietary modification of intestinal and pulmonary
aryl hydrocarbon hydroxylase activity. Toxicol. appl.
Pharmacol., 23: 741-748.
WEIL, C. S. (1970) Selection of the valid numbers of sampling units
and a consideration of their combination in toxicological studies
in involving reproduction, teratogenesis and carcinogenesis.
Food Cosmet. Toxicol., 8: 177-182.
WHO (1967) WHO Technical Report Series, No. 348. (Procedures for
investigating intentional and unintentional food additives:
Report of a WHO Scientific Group) 25 pp.
WILLIAMS, R. T. (1967) Comparative patterns of drug metabolism. In:
Proceedings of an International Symposium on Comparative
Pharmacology. Fed. Proc., 26 (4): 1029-1046.
WILLIAMS, R. T. (1972) Toxicological implications of biotransformation
by intestinal microflora. Toxicol. appl. Pharmacol.,
23: 769-781.
WINTER, C. A. & FLATAKER, L. (1962) Cage design as a factor
influencing acute toxicity of respiratory depressant drugs in
rats. Toxicol. appl. Pharmacol., 4: 650-655.
WITSCHI, H. (1975) Exploitable biochemical approaches for the
evaluation of toxic lung damage. Essays in Toxicol.,
6: 125-191.
WORDEN, A. N. (1974) Toxicology and the environment. Toxicol.,
1: 3-27.
ZBINDEN, G. (1973) Progress in toxicology: special topics, New York,
Springer-Verlag, 88 pp.
3. ACUTE, SUBACUTE, AND CHRONIC TOXICITY TESTS
3.1 Introduction
The primary objective of toxicological testing is to determine
the effects of chemicals on biological systems and to obtain data on
the dose-response characteristics of the chemical. These data may
provide information on the degree of hazard to man and the environment
associated with a potential exposure related to a specific use of this
chemical. Elucidation of the metabolic behaviour of the chemical in
test animals increases confidence in defining the hazard (see Chapter
4). The degree of confidence with which hazard may be estimated
depends on the quality of the toxicological data. Selection of the
most appropriate test procedures coupled with strict adherence to
accepted experimental practices and astute observation are of
paramount importance in experimental toxicology.
3.2 General Nature of Test Procedures
Several types of toxicity testing procedures have been developed.
These include acute, subacute, and chronic studies. The major
difference between these tests is the dose employed and the length of
exposure to the chemical agent, but other differences in intent and
nature do exist and will be discussed. All of the tests share some
common characteristics. Each requires that groups of healthy animals,
housed under suitable conditions, be exposed to graded doses of the
test chemical. Rats, mice, guineapigs, rabbits, and hamsters are
commonly used for this purpose, but in some cases it may be necessary
to use dogs, swine, nonhuman primates, or other species. As a rule, a
control group is given the dosing vehicle or is sham treated.
Following treatment, the animals are closely observed for signs of
toxicity. Laboratory procedures designed to measure biological effects
are carried out on the treated and control animals. Detailed records
are maintained on each animal. Following completion of the test, all
animals, including controls, are subjected to a pathological
examination. Data should be analysed by appropriate statistical
procedures.
3.2.1 Housing, diet, and clinical examination of test animals
Animals should be healthy, genetically stable, and adequately
identified as to colony source. The controls and treated animals
should be of the same strain and species, age, and weight range, and
be supplied from the same source. Before starting the experiment, the
health status of all animals should be determined and monitored for
some time. During this time, a small randomly selected number of
animals from each shipment should be sacrificed and examined for
disease, parasites, and other specific pathogens. During the
quarantine period, animals may be caged together according to the
weight-space specifications. Acceptable standards for the housing and
care of experimental animals have been published (DHEW, 1972; Canadian
Council on Animal Care, 1973; Sontag et al., 1975).
During toxicity studies, rodents should be housed singly or in
pairs in stainless steel or plastic shoe-box cages while nonrodents
should be housed in suitable runs. The animals should be randomly
allotted to the cages and treatment regimes should be randomly applied
(Cox, 1958). Rodents should be allowed free access to food and water.
Nonrodents should be fed meal and given water ad libitum. The diet
fed to the animals should meet all of their nutritional requirements
(National Academy of Sciences, 1975) and should be free of toxic
chemical impurities that might influence the outcome of the test.
Periodic analysis of the diet to ensure its nutrient composition
should be undertaken since nutritional status may affect the nature of
toxic responses (Arcos, 1968). Although commercially available diets
of recognized quality are suitable for most subacute studies,
semipurified diets may be preferred because the nutrient and
nonnutrient components of the diet may be altered readily, where
necessary (Munro et al., 1974; Newberne, 1968).
Careful clinical observation of test animals is the most
neglected area in experimental toxicology. Few investigators are aware
or recognize that the skills required of a good medical diagnostician
are also required in assessing or diagnosing the toxic state or
condition of an animal. In toxicity studies, many animals may be lost
for evaluation because of death from intercurrent disease and
subsequent autolysis. With concerned, reliable staff, these losses can
be greatly reduced if a conscientious effort is made to recognize
early clinical signs of disease in the test animal. Ideally, each
animal on test should be looked upon as an individual patient. In this
way, there is an awareness of the idiosyncrasies of the animal and
departures from the normal will be more easily recognized. Once a
routine of careful clinical assessment has been established, it is
possible either to treat diseased test animals or, if necessary,
sacrifice them. In the latter case, the tissues are available for
histological examination. Otherwise, chronic disease effects might
render the tissues useless for the assessment of effects due to test
substances.
Detailed clinical examinations should be conducted weekly on the
test animals by competent, laboratory animal technicians under the
supervision of a veterinarian skilled in laboratory animal medicine
(Health and Welfare, Canada, 1973). These should include general
observation of the animals for overt signs of toxicity, quality of
hair, coat, general condition of the eyes, mouth, teeth, nose, and
ears (Leclair & Willard, 1970; Loomis, 1968). Assessment of cardiac
and respiratory function should be conducted by auscultation. If
neurological effects are anticipated, a detailed neurological
examination should be conducted. In larger species, this can be done
by skilled personnel using the methods of Charbonneau (1974), McGrath
(1960), and Mowbray & Cadell (1962). Examination of the eyes using
opthalmoscopic and slit-lamp techniques (Marzulli, 1968) may assist in
detecting ocular toxicity. The external and internal structures should
be carefully palpated and any tissue masses should be noted. Detailed
records of clinical evaluation should be maintained and should be
accessible to the attendant pathologist.
3.3 Acute Toxicity Tests
3.3.1 Underlying principles
Acute toxicity has been defined as the adverse effects occurring
within a short time of administration of single dose or multiple doses
given within 24 h (Hagan, 1959). When data are unavailable concerning
the toxicity of the test agent, acute toxicity studies are indicated
to identify the relative toxicity of the compound, to investigate its
mode of action and its specific toxic effect, and to determine the
existence of species differences.
The most frequently used acute toxicity test involves
determination of the median lethal dose (LD50) of the compound. The
LD50 has been defined as "a statistically derived expression of a
single dose of a material that can be expected to kill 50% of the
animals" (Gehring, 1973). The basic protocol for the determination of
the LD50 is well established and consists of treating groups of
animals with a mathematically-related series of doses in order to
determine the dose that kills 50% of the group and the dose-response
function. The LD50, being a calculated value, is always accompanied
by some estimation of the error of the value, such as the confidence
limits. The most commonly used methods for calculation of the LD50
are the graphic method of Litchfield & Wilcoxon (1947), the
logarithmic probit graph paper method of Miller & Tainter (1944), and
the method of moving averages of Thompson (1947) and Weil (1952). A
comparative review of these and other methods was published by
Armitage & Allen (1950). Death which occurs after the first 24 h is
more likely to be due to delayed toxic effects, which may be direct or
indirect. Signs occurring after the first 24-h period may give some
indication of the effect that the chemical may have at lower levels,
when administered for longer time periods.
3.3.2 Experimental design
3.3.2.1 Selection of species
The extent of species variation in toxicity testing has been well
documented in the reviews of Brodie (1964) and Rumke (1964). The
usefulness of determining species variability in order to assess the
applicability of toxicity data to man has been discussed by Hagan
(1959). Litchfield (1962) has postulated that if the toxicity of a
compound is the same in several species, there would appear to be an
increased likelihood that man would react in a similar manner.
The mouse, rat and dog are the most commonly used species for
acute toxicity testing. Both the rat and mouse should be used, as
marked differences in the LD50 between these two species are not
uncommon (Morrison et al., 1968).
The LD50 determination should be conducted in both male and
female animals, as differences in the LD50 between sexes have been
well documented (Hurst, 1958; Rumke, 1964) and are probably related,
in part, to differences in hepatic metabolism (Conney et al., 1965).
Acute toxicity may vary substantially with the age of the test
animal (Dieke & Richter, 1945; Lu et al., 1965; Scott et al., 1965;
Yeary & Benish, 1965), and animals of various ages should be used in
LD50 determinations. The effect of the age of the animal on the
LD50 is well documented and may be related to different levels of
drug metabolizing enzymes, absence of sex hormonal influences, or an
altered sensitivity of the central nervous system (Fouts & Hart, 1965;
Jondorf et al., 1959; Setnika & Magistretti, 1964).
The animals should be derived from previously untreated healthy
females. Weinberg et al. (1966) have demonstrated an effect of
treatment of dams during gestation with various compounds on the acute
oral toxicity in the newborn.
Furthermore, the animals should not have been previously used for
other studies, nor should there be a history of recent exposure to
anthelminthics or any other drug treatment.
The number of animals used should be sufficient for statistical
analysis and will depend on the method used for the calculation of the
LD50. Usually 8-10 rodents (4-6 animals of each sex) are used per
dose group (Leclair & Willard, 1970). Diechmann & LeBlanc (1943)
described a method using a total of 6 animals, while other methods
involved the use of 4-5 animals per dose group (Horn, 1956; Litchfield
& Wilcoxon, 1947; Thompson, 1947).
3.3.2.2 Selection of doses
The doses are selected to provide data for estimating the LD50
and to obtain information on the slope of the dose-response curve. At
least four doses, selected in logarithmic progression, should be used
(Weil, 1952).
In general, however, the doses can be arrived at only by
experimentation. The initial dose may be such that no effect is
manifested in the animals. In subsequent groups of animals, the dose
should be increased by a constant multiple until the dose of the
compound administered is sufficiently high that all of the animals in
the group die. Under these conditions, data can be obtained that can
be plotted to give a dose-response curve and from which the LD50
value may be calculated.
3.3.2.3 Method of administration
Generally, the chemical should be administered by the route by
which man would be exposed. If the route is oral, the compound should
be administered by gavage rather than mixed in the diet. In some
cases, the administration of the chemical along with the diet has been
shown to increase its toxicity compared with gavage dosing (Bein,
1963; Worden & Harper, 1963), but, in general, the oral toxicity of a
compound is greatest when it is administered by gavage to animals that
have fasted (Griffith, 1964). Griffith (1964) has demonstrated the
effect of the type and concentration of the vehicle on the LD50
value. The amount of the liquid or carrier administered should be
appropriate and the carrier should not, in itself, be toxic to the
animal.
In certain cases, even though the route of human exposure would
be oral, acute dermal, eye, and inhalation studies may be indicated to
assess the hazard to personnel handling the compound in the
laboratory.
3.3.2.4 Postmortem examination
In general, all animals dying during the observation period and
all surviving animals should be autopsied by a qualified pathologist
(Leclair & Willard, 1970). The autopsy should include gross and
histopathological examination of all organs.
If death is almost instantaneous and due to a pharmacological or
physical effect, i.e. massive gastrointestinal haemorrhage or acute
respiratory collapse, detailed histopathological examination of all
organs may not be indicated.
3.3.3 Repeated high-dose studies
Because of the inherent limitations of the LD50 in predicting
long-term toxicity, a short but intensive study or a series of such
studies may be indicated before commencing subacute tests. The purpose
of such studies is to define more precisely the doses to be used in
subacute tests and to elucidate more fully the organ systems affected.
The design of these repeated high-dose studies may vary but consists,
essentially, of repeated daily administration of a mathematically-
related series of doses to groups of animals for 5-21 days.
One type of repeated-dose study (Sontag et al., 1975) consists of
treating groups of five young adult animals of each sex at each of
five dose levels, the upper level being the one that is estimated to
produce no more than 10% lethality following a single dose, the
remaining doses being fractions of this dose.
A seven-day feeding study described by Weil et al. (1969)
consisted of treating five rodents of each sex at each of three or
four dose levels for seven days. Criteria of effects were mortality,
body weight gain, relative liver and kidney weights, and feed
consumption. This study showed that the results of the seven-day
feeding test were of significantly greater value in predicting dose
levels for the 90-day toxicity test than the LD50 values.
Daily observations, as described in section 3.1.3, should be
conducted and weekly body weight and food consumption (if the animals
are caged individually) monitored. For some test agents, especially
those with delayed toxicity or cumulative effects, other measurements,
such as organ function, body burden, absorption, and excretion of the
compound may be indicated. Animals should be necropsied and the
tissues should be examined for gross pathological changes and studied
histopathologically, if indicated.
3.4 Subacute and Chronic Toxicity Tests
3.4.1 Underlying principles
The subacute toxicity test generally involves daily or frequent
exposure to the compound over a period up to about 90 days. It
provides information on the major toxic effects of the test compound
and the target organs affected (Barnes, 1960). The latency of
development of the effect as related to dose, the relationship of the
blood and tissue levels of the compound to the development of lesions,
and the reversibility of the effects may also be studied. Data derived
from these studies are used for designing chronic toxicity tests in
which animals are exposed to the chemical for longer periods of time.
Man may be exposed for the greater part of his life-time to low
levels of a wide variety of environmental chemicals. Usually, the
degree of exposure is insufficient to produce overt signs of toxicity;
thus, cause-effect relationships cannot be easily established.
Epidemiological studies may assist in this respect, but, because man
is exposed simultaneously to several chemicals, it is difficult to
establish unequivocally the degree of hazard associated with any one
chemical. Acute and subacute toxicity tests are of limited value in
predicting chronic toxic effects because: (a) chemicals may produce
different toxic responses when administered repeatedly over a period;
and (b) during the aging process, factors such as altered tissue
sensitivity, changing metabolic and physiological capability, and
spontaneous disease may influence the degree and nature of toxic
responses. In addition, several important diseases such as heart
disease, chronic renal failure, and neoplasia are associated with
advancing age. These are multicausal in nature and thought to be due,
in part, to the presence of chemical substances, both natural and
synthetic, in the environment (WHO, 1972). Chronic toxicity tests, in
which animals are exposed for their entire lifetime to environmental
chemicals, have provided useful means of identifying those substances
of greatest public health concern. The tests are usually conducted
with the aim of establishing "no-observed-adverse-effect levels" that
may be used in setting acceptable daily intakes (ADIs), tolerance
limits for chemicals in food or water, or threshold limit values in
the case of occupational exposure. Since chronic toxicity testing is
expensive and requires specialized facilities and personnel, great
care must be taken in the design, execution, and interpretation of the
results of such studies.
3.4.2 Experimental design
3.4.2.1 Selection of species and duration of studies
In the subacute studies, if the compound has produced evidence of
toxicity in man and if sufficient toxicological and metabolic
information is available, it is often possible to select an
appropriate species on the basis of these data. For compounds about to
be put on the market about which little is known toxicologically, the
recommendations of the World Health Organization (WHO, 1958) and
competent national agencies (Friedman, 1969; Leclair & Willard 1970;
National Academy of Sciences, 1975) should be followed in selecting
appropriate test species. As a minimum recommendation, subacute
studies should be undertaken in two species, one rodent and one
nonrodent. Traditionally, the rat and dog are selected for subacute
toxicity testing because of their availability and the large amount of
background information available on them. When rats are used, the test
should be initiated just after weaning so that observations may be
made during the period of most rapid growth. A conventional strain
should be selected, so that the results in control and treated animals
can be compared with known literature values, and both sexes should be
tested to ascertain the influence of the sex hormones on the toxic
response. At least 10 animals of each sex should be included in each
dose group and the experiment should continue for 10% of the animals'
lifetime or about 3 months. If it is desired to study the pathogenesis
and reversibility of induced lesions or biochemokinetics, it is
recommended that observations be made at 3-week intervals during
exposure and last up to 3 months following termination of exposure.
In chronic toxicity testing, it is usual to expose the animals to
the chemical for the greater part of the life span. A wide variety of
animal species have been used in this type of work, although in most
cases rodents are the animals of choice, since large numbers can be
used to aid in the statistical interpretation of the results. Larger
animals should also be used (e.g. dog and monkey) for such species
have the advantage that larger samples of blood can be obtained on a
routine basis.
If the objective of the test is to study the carcinogenic
potential of a compound, the rat, mouse, or hamster is usually chosen
because of its shorter lifetime and the fact that large numbers may be
used to increase the sensitivity of the test.
When data on the metabolic fate of the test chemical in man is
not available, the species showing the greatest sensitivity in
subacute studies should be selected as the test species, provided the
species does not react atypically to the compound due to metabolic
peculiarities.
Sufficient numbers of animals should be included in the test to
ensure that a statistically valid design is achieved. Based on the
incidence of effects observed in subacute studies and the anticipated
incidence of chronic effects, the number of animals that should be
used can be calculated (Snedecor & Cochran, 1967).
Since it is usually the intention in chronic toxicity studies to
expose animals over the major portion of their life span, it is
essential to commence exposure early in life.
3.4.2.2 Selection of doses
Guidance on the selection of doses for subacute studies may be
obtained from the results of acute and repeated high-dose studies. For
compounds having a tendency to bioaccumulation, selection of doses is
particularly difficult. Kinetic studies may assist in establishing
acceptable dose levels since the half-time ( t´) for elimination
(Chapter 4) may provide guidance on the degree of bioaccumulation that
could be anticipated. To establish the nature of the toxic reaction,
the highest dose should provide a distinct toxic effect while the
lowest dose should not produce any detectable toxic reaction (Leclair
& Willard, 1970). To obtain maximum information on the dose-response
characteristics of the compound, at least two intermediate doses
should be included.
Information from subacute toxicity tests is of value in the
selection of appropriate dose levels, when commencing chronic toxicity
studies. In general, however, it is highly desirable to establish the
chemobiokinetic behaviour (Chapter 4) of the test compound and if
possible its major metabolites in the test species prior to
undertaking a chronic toxicity test. Particular attention should be
given to evidence for dose-dependent detoxification. Studies of this
nature will provide information on the degree to which the chemical
may be expected to accumulate in various body compartments and
unexpectedly produce evidence of toxicity. Since it is the object of
chronic toxicity tests to establish dose-response patterns and
"no-observed-adverse-effect levels", a minimum of three dose levels
should be used. The upper dose level should produce some slight
evidence of toxicity, but should be compatible with normal
physiological function (Leclair & Willard, 1970). The lowest dose
level would not be expected to produce evidence of toxicity (Health &
Welfare, Canada, 1973).
3.4.2.3 Method of administration
The route of administration in subacute and chronic studies
should be that through which man is likely to be exposed. For gases
and volatile industrial solvents, inhalation studies are recommended
(Magill et al., 1956) (Chapter 6), while for food additives,
pesticides, and other chemicals likely to come into contact with food
or water, the oral route is recommended (Leclair & Willard, 1970;
National Academy of Sciences, 1975). Incorporation of the test
chemical into the diet or drinking water is an appropriate means of
administration; however, care must be taken to ensure the stability of
the chemical in the dosing medium. The concentration of the test
chemical in the diet should be determined periodically to ensure
uniform dispersion and to aid in the quantification of achieved doses.
In some cases, the chemical may be unpalatable and administration by
gavage, or, in the case of dogs, by capsules may be necessary.
The diet is the preferred vehicle of administration, but it is
absolutely essential that the chemical be present in the diet in an
unaltered form; toxicity may be altered by interaction with dietary
constituents (Kello & Kostial, 1973). In rodent studies, the compound
may be administered in the diet as a fraction of the total diet, or a
sufficient quantity of the chemical may be added to the diet to
achieve predetermined dose levels (in mg per kg body weight per day).
In the latter case, it is necessary to adjust the dietary
concentration weekly or biweekly to maintain a constant dose level,
since food consumption per unit of body weight decreases as the animal
gets older. If, in rodent tests, the concentration of the test
compound in the diet is kept constant from weaning to maturity, the
actual dose received will be reduced by approximately 2.5 times over
the dosing period. This may have profound effects on the severity of
the toxic response and may be mistaken for tolerance. In chronic
toxicity tests, the chemical should be administered daily over the
entire treatment period. As an aid to interpretation of the test, only
one lot chemical should be used for the entire test unless the purity
of the chemical is definitely assured.
3.4.2.4 Biochemical organ function tests
In subacute studies, the use of a species such as the dog instead
of a rodent species permits the application of a wider range of
biochemical organ function tests because larger samples of blood can
be collected on a routine basis. Organ function studies should be
undertaken prior to initiation of the test, 3 and 10 days after the
start of dosing, at 30-day intervals thereafter throughout the test,
and terminally. The tests described for the chronic studies are also
applicable in subacute studies.
In the course of chronic toxicity tests, studies should be
undertaken to evaluate the functional integrity of various organ
systems. Assessment of the urinary system should commence with an
examination of the urine. Freshly-voided urine samples should be
obtained every one to three months from the test animals and examined
for the presence of occult blood, glucose, protein, and bilirubin
using simple diagnostic procedures. If positive effects are noted,
quantitative methods should be applied as outlined by Bergmeyer
(1965). Samples of freshly-voided urine should also be filtered
through Millipore filters and the filters stained according to the
Papanicolaou method (Frost, 1969) to detect the presence of renal
tubular cells or other cell types derived from the urinary system.
Urinary calculi and parasite eggs (Chapman, 1969) may be detected by
this method. Blood urea-nitrogen levels and other standard tests of
kidney function may be applied but they usually lack sufficient
sensitivity to detect subtle changes in kidney function.
Several test procedures are available for the assessment of liver
function. Most of these methods involve an examination of the serum
levels of hepatic enzymes that may be released in the serum following
liver injury (Czok, 1965; Henley et al., 1966; Zimmerman, 1974).
Korsrud et al. (1972) compared the sensitivity of various liver
function tests in the rat and noted that serum sorbitol dehydrogenase
(1.1.1.14) activity (an enzyme specific to the liver) correlated well
with the degree of histological alteration produced by hepatotoxic
agents such as carbon tetrachloride, 2,2'-iminobisethanol
(diethanolamine), and ethanethioamide (thioacetamide). However, Grice
et al. (1971) noted that pathological changes induced by these
compounds must be reasonably advanced before elevations are noted in
serum glutamic-oxaloacetic transaminase (2.6.1.1), lactate
dehydrogenase (1.1.1.27), or lactate dehydrogenase isoenzymes,
suggesting that changes in serum enzyme activity may not be as
sensitive an indicator of toxicity as pathomorphological examination.
Tests of liver function such as serum enzyme activities and various
clearance tests were reviewed recently by Balazs (1975). A complete
review of the principles and applications of these tests is given by
Cornish (1971) and further discussion of these methods is found in
Part II, Chapter 8. Suffice it to say, that a transient increase in
the activity of serum enzymes or other organ-derived constituents may
result from a transient change in organ homeostasis that produces no
lasting toxic effect.
For routine screening of organ function in large animals,
Charbonneau et al. (1974) used clinical procedures that can measure
the concentration of several serum enzymes and inorganic and other
constituents by automated methods. In general, these methods are not
sufficiently standardized or reproducible to detect minor alterations
in organ function but they do serve as a useful guide to general
clinical status.
3.4.2.5 Physiological measurements
In subacute studies, it is often possible to detect ensuing
pathological events through application of physiological function
tests.
In all studies, food consumption and body weight should be
recorded weekly in all animals. Weight gain per unit of food consumed
should be calculated (Munro et al., 1969). This gives a measure of the
efficiency of food use. The daily dose of chemical should be
calculated from data on food intake and body weight. Similar
measurements of food intake and body weight must be carried out in
chronic toxicity tests. If the test chemical is incorporated into the
drinking water, water intake must be measured. These measurements
should be conducted on a weekly basis during the entire test. The data
can be used to estimate the dose of chemical received and are
necessary in the establishment of dose-response relationships. Body
weight changes serve as a sensitive indication of the general health
status of test animals. Any rapid loss in body weight may signal the
onset of intoxication or disease. Computerized methods for recording
and analysing this type of data are available (Munro et al., 1972).
Under special circumstances, when the target organs of toxicity
have been identified during subacute studies, it is appropriate to
conduct measurements of the physiological function of organ systems.
Procedures such as electrocardiography (Grice et al., 1971),
electroencephalography (Flodmark & Steinwall, 1963; Harada et al.,
1967; Mann, 1970), electromyography (Chaffin, 1969), nerve conduction
studies and measurement of evoked potentials (Barnet et al., 1971;
Hrbek et al., 1972) may greatly assist in defining the functional
effects of chemicals (Chapter 8). Such tests are expensive to perform
and require highly specialized equipment and personnel. They have
limited application in routine testing but may be used to define
mechanisms of action. It is imperative that the results of such
studies be correlated with clinical and pathological findings (Grice,
1972; Osborne & Dent, 1973).
3.4.2.6 Metabolic studies
Subacute studies provide an excellent opportunity to undertake
metabolic investigations under conditions of repeated exposure that
may alter the nature of the metabolites and the rate of metabolic
transformation of the test compound. Urine and faeces can be collected
and examined for the presence of metabolites and, by undertaking
serial sacrifices at three-week intervals, the kinetics of
accumulation of the compound in various body compartments can be
evaluated.
To gain an understanding of the metabolic fate of a chemical that
may have a long biological half-time, such as hexachlorobenzene (Grant
et al., 1975), three extra groups of animals need to be studied for
tissue distribution to provide information that is necessary for
estimating the potential hazard to man. The principles of these
methods are reviewed in Chapter 4. Often it is desirable, in subacute
studies, to study the kinetics of the test compound and its
metabolites following completion of the dosing period. If extra groups
of animals are initially included for this purpose, much valuable
additional information on the compound may be obtained.
3.4.2.7 Haematological information
In subacute studies involving rodents, haematological studies
should be undertaken on randomly selected subgroups of animals prior
to initiation of the test, at 30-day intervals, and on all animals
terminally. Bone marrow should be examined terminally. Nonrodent test
animals should be examined at similar intervals.
In chronic toxicity studies involving rodents, haematological
studies of circulating blood cells should be undertaken on randomly
selected subgroups of animals prior to initiation of the test, at 3 to
6-month intervals and, on selected animals, terminally. Bone marrow
should be examined terminally and, if indicated, at interim times by
biopsy.
To assess the clinical state of nonrodents, haematological
variables should be examined frequently.
A set of test procedures is necessary for routine haematological
screening and the tests must be of sufficient sensitivity and accuracy
to be of practical value for use in large numbers of laboratory
animals (Cartwright, 1969; Schalm, 1967; Sirridge, 1967).
Quantification of blood cells and thorough study of cellular
morphology by a haematologist, experienced in small animal medicine,
is necessary in the study of haematological disorders. Haematological
evaluation of experimental animals is facilitated by the fact that
repeated sampling is relatively easy and small amounts of blood are
required, and that single-cell systems can be studied to obtain
information on cell production, destruction, defects, and dysfunction.
For erythroid evaluation, the numbers of circulating erythrocytes must
be counted and the haematocrit and haemoglobin concentration measured.
As an index of erythropoietic activity in the bone marrow, a
reticulocyte count should be carried out. Morphological assessment of
erythrocytes is mandatory. The number of circulating leucocytes should
be quantified and a differential count and morphological assessment
should be made. To evaluate the functional capacity and malignant
changes in the blood-forming organs, bone marrow should be examined
terminally. From bone marrow smears a differential count and
morphological assessment can be carried out. Imprints of lymph nodes
or spleen permit a detailed cytological study of normal and abnormal
cells present that may be of diagnostic significance.
To assess haemostatic function, it will be necessary to evaluate
platelets, coagulation systems, and fibrinolysis. Screening tests
include platelet count, clot retraction, one stage prothrombin time,
and activated partial thromboplastin time. More specific evaluation
may require factor assays, thrombin time, fibrinogen determination,
euglobulin clot lysis time, prothrombin consumption time, platelet
aggregation, and adhesiveness.
3.4.2.8 Postmortem examination
In every toxicity evaluation, all animals should be given a
thorough gross autopsy and detailed records kept on each animal.
Samples of all organs and supporting structures should be saved for
histopathological examination. Detailed autopsy methods are outlined
in Chapter 5.
In chronic toxicity testing it is often useful to incorporate
interim autopsy dates so that the progression of lesions may be
studied. At interim sacrifices and terminally (if sufficient animals
are in a healthy state), the major organs should be weighed. Organ
weights may serve as a useful index of toxicity; however, care must be
taken in the interpretation of the data. Decreased absolute organ
weights in treated animals may be merely a reflection of lower body
weight and calculation of organ to body weight ratios may increase the
usefulness of the data (Feron, 1973).
3.4.2.9 Controls
In the evaluation of both subacute and chronic toxicity, special
attention must be given to the control animals. The quality of data
obtained from the control animals has an important bearing on the
interpretation of results from the treated animals. Suitable numbers
of control animals of the same age and body weight as the treated
animals must be included in the experimental design in a statistically
randomized fashion.
Except for treatment with the test chemical, these animals should
be handled identically to the test subjects and all measurements
conducted on the treated animals must be carried out on the controls
with the same precision and frequency. In studies in which the
chemical is administered by gavage, the control animals should receive
the suspending vehicle in an amount equivalent to the treated animals.
The incidence of spontaneous lesions or of other changes in control
animals must be carefully noted and the interpretation of data
obtained from treated animals must include an appreciation of the role
that spontaneous disease processes may play in the manifestation of
chemical toxicity. It is particularly important, in studies with
rodents, to have detailed information on the incidence of neoplastic
diseases, since some species and strains (Sher, 1972) may have a high
background incidence of certain tumours which tends to reduce
longevity and decrease the chance of observing chronic toxic effects.
In addition, the chemical under test may alter the incidence of
spontaneous tumours and other diseases or may induce new tumours, and
this possibility must be taken into consideration in the evaluation of
the chronic toxicity of chemicals. In all cases, responses
attributable to the test compound must be compared with background
observations in controls. For this reason, the quality of the
toxicological data rests heavily on the adequacy of the control values
(Weil & Carpenter, 1969).
3.4.3 Alternative approaches in chronic toxicity
3.4.3.1 Perinatal exposure
The majority of chemicals to which man may be exposed are present
in air, food, or water for his entire lifetime. Recently, there has
been an attempt to duplicate the human situation in the chronic
toxicity test by exposing the test animals during the neonatal period
as well as throughout life (Friedman, 1969). In this approach, groups
of weaning animals (usually rodents) are exposed to the test chemical
until they reach sexual maturity. They are then mated, within dose
groups, and the treatment is continued during pregnancy and lactation.
Following weaning, the offspring are transferred to their parents'
diet and exposed for the balance of their lifetime to the test
chemical. The details of this test procedure have been outlined in a
recent Canadian Government publication (Health & Welfare, Canada,
1973) and by Epstein (1969). It is not known yet whether this
technique increases the sensitivity of the chronic toxicity test, but
it is known that exposure to carcinogens in the perinatal period will
often increase the incidence and decrease the latent period of
carcinogenesis (Tomatis & Mohr, ed., 1973).
Further study of this method is required to evaluate its
usefulness fully. It should be pointed out, however, that this
procedure adds considerably to the cost and length of the chronic
toxicity test.
3.4.3.2 Use of nonrodent species
In chronic toxicity studies with nonrodent species such as
nonhuman primates, dogs, or cats, it is often not feasible to expose
the animals to the test compound for their entire lifespan, even
though they may be the species of choice. Under such conditions,
careful examination of the kinetic and metabolic behaviour of the test
compound in these species may substitute, to some extent, for the
decreased treatment period (provided the anticipated endpoint is not
carcinogenesis). Carefully conducted kinetic studies will assist in
establishing when steady-state tissue concentrations of the test
chemical and its metabolites have been achieved. If treatment is
continued for a substantial period after the establishment of
steady-state kinetics without any increase in the degree of toxic
effects observed clinically, or during interim sacrifice, this may
partially substitute for a lifetime study and provide increased
assurance for those having to make regulatory decisions. If this
approach is not feasible, it may be possible to test human metabolites
in rodent species (Health & Welfare, Canada, 1973).
3.5 Evaluation and Interpretation of the Results of Toxicity Tests
The evaluation and interpretation of toxicity studies starts with
a clear definition of experimental objectives. The design of the
experiment should be such that the objectives can be reasonably
achieved. Well designed and carefully executed experiments add greatly
to the ease with which results can be evaluated and interpreted and
also to confidence in the experimental data.
The primary usefulness of the LD50 determination is to obtain
some idea of the magnitude of the acute toxic dose (Frazer & Sharratt,
1969) and information concerning the type of toxic effects of the
chemical. Such information includes whether death is immediate or
delayed, whether recovery from a near lethal dose is rapid or complete
or both, or whether the cause of death is narcosis with respiratory
failure, lung oedema, or liver necrosis.
However, the LD50 provides little information for the
assessment of the hazard from compounds to which the human population
is exposed for extended periods of time. Although it has been
suggested that compounds that do not show adverse effects when given
in doses of 3-5 g per kg body weight are essentially non-toxic
(National Academy of Sciences, 1975), there are numerous examples in
the literature of compounds with LD50 values greater than 5 g per kg
which produce toxic effects, when given in low doses for extended
periods of time (Frazer & Sharratt, 1968). If the main object of an
acute toxicity test is not to establish a value for the LD50 with
precision, but to learn something about the way in which the chemical
acts as a poison, as suggested by Paget & Barnes (1964), this can best
be accomplished by tests involving repeated daily administration to a
few animals for a period of 5-21 days. The information provided by the
LD50 regarding the effects of acute exposure to toxic compounds may
be useful as a guide for selecting doses for such studies.
The primary objective of subacute and chronic toxicity studies is
to determine the nature and severity of toxic effects and the
"no-observed-adverse-effect" dose level. These data may then be used
in the establishment of acceptable levels of exposure for man.
Data on group weight gain or body weight change should be plotted
against time, and differences between groups should be evaluated
statistically. Changes in body weight with time are best evaluated
statistically using trend analysis procedures (Armitage, 1955). Food
(and water) consumption data should be handled in a similar fashion.
Reduced body weight or weight gain in otherwise healthy treated
animals may be due to reduced food intake owing to its unpalatability
or to a specific toxic effect of the chemical resulting in reduced
efficiency of food use. Using data on the dietary concentration of the
test chemical, food consumption, and body weight, the mean daily dose
of chemical received (in mg/kg body weight/day or similar units)
should be calculated. Automated data processing procedures to
accomplish this are available (Munro et al., 1972).
Data on organ weights should be evaluated and interpreted with
great care. Increased relative (to body or brain weight) organ weights
may also result from adaptation to stress phenomena or from metabolic
overloading of biochemical pathways or physiological processes.
Increased liver weight, for example, may result from a stimulation of
de novo protein synthesis in the smooth endoplasmic reticulum (SER).
This results in a morphologically detectable increase in SER. The
biochemical counterpart of this increase is an increased ability of
the liver to metabolize certain foreign substances, sometimes
including the test compound and endogenous substrates, due to a
stimulation in the activity of hepatic mixed function oxidases
(Staubli et al., 1969). These adaptative changes may manifest
themselves clinically as tolerance. Often these changes are reversible
upon cessation of dosing and do not produce lasting toxicological
effects but the implications of chronically elevated levels of these
enzymes is not known. Certain enzyme inducers may cause impairment of
liver function and produce pathological and biochemical changes (Feuer
et al., 1965).
Data on biochemical and haematological effects should be
tabulated and compared with control values using statistical
procedures (Johnson, 1950). Any observed effects should be correlated
with clinical and pathological findings. A biochemical or
haematological change such as reduction in liver glycogen or an
alteration in white cell count may not be indicative of a toxic
effect, but an adaptation to a stress situation (National Academy of
Sciences, 1975). In general, changes in homeostasis must be carefully
evaluated since reversible shifts do not necessarily imply a toxic
effect in the absence of other toxic manifestations.
Changes in the functional state of physiological or neurological
processes, such as an alteration in the electrocardiogram or abnormal
behaviour, may result from pharmacological or pathological effects of
the test compound. Changes in functional state must be closely
correlated with their morphological counterpart in order to evaluate
their toxicological importance properly (Grice, 1972).
The cornerstone of experimental toxicology is the pathological
examination. Usually, decisions regarding the safety of a compound are
based on this evidence. All pathological findings in test animals
should be graded carefully and their incidence tabulated (see Chapter
5). Spontaneous lesions in control animals should also be noted and
compared to the observations in control animals in previous
experiments or in the literature (Peck, 1974) to ensure that the
incidence and nature of the lesions is representative of the strain.
Pathological data should be analysed rigorously using appropriate
statistical methods (Fleiss, 1973) and spurious observations
apparently unrelated to treatment should be identified. Lesions that
are dose-related should be studied in detail and correlated with gross
pathological findings, clinical observations, and other variables
(Grice, 1972).
It is not uncommon in chronic toxicity testing to find
pathological or other changes that occur in low incidence and that are
not dose-related but occur only in treated animals. Such reactions may
be idiosyncratic in nature or may be due to the hypersensitivity of
certain animals. Nevertheless, they deserve special attention since
they may be indicative of a hitherto unsuspected toxic effect. The
clinical history and other data from such animals should be reviewed
with great care and an attempt should be made to determine the reason
for the observed effects. Toxic effects that occur in extremely low
incidence present special problems in interpretation. There is no
substitute for experience in this respect and the prudent investigator
will consult the knowledgeable experts in this field (Zbinden, 1973).
REFERENCES
ARCOS, J. C. (1968) Chemical induction of cancer. Vol. 1, New York,
Academic Press.
ARMITAGE, P. & ALLEN, I. (1950) Methods of estimating the LD50 in
quantal response data. J. Hyg. (Lond.), 48: 298-322.
ARMITAGE, P. (1955) Tests for linear trends in proportions and
frequencies. Biometrics, 11: 375-385.
BALAZS, T. (1975) Toxic effects of chemicals in the liver.
FDA By-lines, 5: 291-303.
BARNES, J. M. (1960) Toxicity testing, In: Schilling, R. S. F., ed.
Modern trends in occupational health, London, Butterworths &
Co., pp. 20-32.
BARNET, A. B., OHRICH, E. S., & SHANKS, B. L. (1971) EEG evoked
responses to repetitive auditory stimulation in normal
Down's-syndrome infants. Dev. Med. Child. Neurol., 13: 321-329.
BEIN, H. J. (1963) Rational and irrational numbers in toxicology.
Proc. Euro. Soc. Study Drug Toxicity, 2: 15-26.
BERGMEYER, H. U. (1965) Methods of enzymatic analysis. New York,
Academic Press.
BRODIE, B. B. (1964) [The difficulties of transposing experimental
results obtained in animals to man.]. Actual. pharmacol.,
17: 1-23 (in French).
CANADIAN COUNCIL ON ANIMAL CARE (1973) Care of experimental animals
-- guide for Canada.
CARTWRIGHT, G. E. (1969) Diagnostic laboratory hematology, 4th ed.
New York, Grune & Stratton.
CHAFFIN, D. B. (1969) Surface electromyography frequency analysis as a
diagnostic tool. J. occup. Med., 11: 109-115.
CHAPMAN, W. H. (1969) Infection with Trichosomomoides crassicauda as
a factor in the induction of bladder tumours in rats fed
2-acetylaminofluorene. Invest. Urol., 7: 154-159.
CHARBONNEAU, S. M., MUNRO, I. C., NERA, E. A., WILLES, R. F.,
KUIPER-GOODMAN, T., IVERSON, F., MOODIE, C. A., STOLZ, D. R.,
ARMSTRONG, F. A. J., UTHE, J. F., & GRICE, H. C. (1974) Subacute
toxicity of methylmercury in the adult cat. Toxicol. appl.
Pharmacol., 27: 569-581.
CONNEY, A. H., SCHNEIDERMAN, K., JACOBSON, M., & KUNTZMAN, R. (1965)
Drug-induced changes in steroid metabolism. Ann. NY Acad. Sci.,
123: 98-109.
CORNISH, H. H. (1971) Problems posed by observations of serum enzyme
changes in toxicology. CRC Crit. Rev. Toxicol., 1: 1-32.
COX, D. R. (1958) Planning of experiments, New York, John Wiley,
Chapter 5.
CZOK, R. (1965) The behaviour of plasma enzymes in toxicological
experiments. Proc. Eur. Soc. Study Drug Toxicity, 5: 68-83.
DHEW (1972) Guide for the care and use of laboratory animals
(prepared by Committee for the Revision of the Guide for
Laboratory Animal Facilities and Care, Institute of Laboratory
Animal Resources). Washington DC, Govt Printing Office (DHEW
Publ. No. (NIH) 73-23).
DIECHMANN, W. B. & LEBLANC, T. J. (1943) Determination of the
approximate lethal dose with about six animals. J. ind. Hyg.
Toxicol., 25: 415-417.
DIEKE, S. H. & RICHTER, C. P. (1945) Acute toxicity of thiourea to
rats in relation to age, diet, strain and species variation.
J. Pharmacol. exp. Ther., 83: 195-202.
EPSTEIN, S. (1969) A catch-all toxicological screen. Experientia
(Basle), 25: 617.
FERON, V. J. (1973) An evaluation of the criterion "organ weight"
under conditions of growth retardation. Food Cosmet. Toxicol.
11: 85-94.
FEUER, G., GOLBERG, L., & LE PELLEY, J. R. (1965) Liver response
tests. I. Exploratory studies on glucose 6-phosphatase and other
liver enzymes. Food Cosmet. Toxicol., 3: 235-249.
FLEISS, J. L. (1973) Statistical methods for rates and proportions.
New York, John Wiley (Wiley Series in Probability and
Mathematical Statistics).
FLODMARK, S. & STEINWALL, O. (1963) Differentiated effects on certain
blood brain barrier phenomena and on the EEG produced by means of
intracarotidly applied mercuric dichloride. Acta. Physiol.
Scand., 57: 446-453.
FOUTS, J. R. & HART, L. G. (1965) Hepatic drug metabolism during the
perinatal period. Ann. NY Acad. Sci., 123: 245-251.
FRAZER, A. C. & SHARRATT, M. (1969) The value and limitations of
animal studies in the prediction of effects in man. In: The use
of animals in toxicological studies -- UFAW Symposium, England,
1968, Potters Bar, England, 41 pp.
FRIEDMAN, L. (1969) Symposium on the evaluation of the safety of food
additives and chemical residues. II. The role of the laboratory
animal study of intermediate duration for evaluation of safety.
Toxicol. appl. Pharmacol., 16: 498-506.
FROST, J. K. (1969) Manual for the tenth postgraduate institute for
pathologists in clinical cytopathology. Baltimore, MD, Johns
Hopkins Hospital.
GEHRING, P. J., ROWE, V. K., & MCCOLLISTER, S. B. (1973) Toxicology:
cost-time. Food Cosmet. Toxicol., 11: 1097-1110.
GRANT, D. L., HATINA, G. V., & MUNRO, I. C. (1975) Hexachlorobenzene
accumulation and decline of tissue residues and relationship to
some toxicity criteria in rats. Environ. Qual. Saf., Supplement
Vol. III, pp. 562-568.
GRICE, H. C. (1972) The changing role of pathology in modern safety
evaluation. CRC Crit. Rev. Toxicol., 1: 119-152.
GRICE, H. C., BARTH, M. L., CORNISH, H. H., FOSTER, G. V., & GRAY, R.
H. (1971) Experimental cobalt cardiomyopathy: correlation between
electrocardiography and pathology. Cardiol. Res., 4: 452-456.
GRIFFITH, J. F. (1964) Inter-laboratory variations in the
determination of acute oral LD50. Toxicol. appl. Pharmacol.,
6: 726-730.
HAGAN, J. M. (1959) Acute toxicity. In: Appraisal of the safety of
chemicals in food, drugs and cosmetics, Assoc. Food & Drug
Officials of USA, pp. 17-25.
HARADA, Y., MIYAMOTO, Y., NONAKA, I., OHTA, S., & NINOMIYA, T. (1967)
Electroencephalographic studies on Minamata Disease in children.
Dev. Med. Child Neurol., 10: 257-258.
HEALTH & WELFARE, CANADA (1970) Guide for the preparation of
submissions for food additives. Ottawa, Health & Welfare.
HEALTH & WELFARE, CANADA (1973) The testing of chemicals for
carcinogenicity, mutagenicity and teratogenicity. Ottawa,
Health & Welfare.
HENLEY, K. S., SCHMIDT, E., & SCHMIDT, W. (1966) Enzymes in serum,
their use in diagnosis. Springfield, IL, Thomas.
HORN, H. J. (1956) Simplified LD50 (or ED50) calculations.
Biometrics, 12: 311-321.
HRBEK, A., KARLBERG, P., KJELLMER, J., & OLSSON, T. (1972) Evoked EEG
responses in newborns with asphyxia and IRDs. Pediatr. Res.,
6: 61.
HURST, E. W. (1958) Sexual differences in the toxicity and therapeutic
action of chemical substances. In: Walpole, A. L. & Spinks, A.,
ed. The evaluation of drug toxicity, London, Churchill,
pp. 12-25.
JOHNSON, L. P. V. (1950) An introduction to applied biometrics.
Minneapolis, Burgers Publ., Co.
JONDORF, W. R., MAICKEL, R. P., & BRODIE, B. B. (1959) Instability of
newborn mice and guinea pigs to metabolize drugs. Biochem.
Pharmacol., 1: 352-354.
KELLO, D. & KOSTIAL, K. (1973) The effect of milk diets on lead
metabolism in rats. Environ. Res., 6: 355-360.
KORSRUD, G. O., GRICE, H. C., & MCLAUGHLAN, J. U. (1972) Sensitivity
of several serum enzymes in detecting carbon tetrachloride --
including liver damage in rats. Toxicol. appl. Pharmacol.,
22: 474-483.
LECLAIR, J. M. & WILLARD, J. W. (1970) Guide for the preparation of
submissions on tolerances for incidental contaminants and
agricultural chemicals in food. Ottawa, Canada, Food & Drug
Directorate, Dept of National Health & Welfare.
LITCHFIELD, J. T. (1962) Evaluation of the safety of new drugs by
means of tests in animals. Clin. Pharmacol. & Therap.,
3: 665-672.
LITCHFIELD, J. T. & WILCOXON, F. A. (1947) A simplified method of
evaluating dose-effect experiments. J. Pharm. exp. Ther.,
95: 99-113.
LOOMIS, T. A. (1968) Essentials of toxicology. Philadelphia, Lea &
Febiger.
LU, F. J., JESSUP, D. C., & LAVALLEE, A. (1965) Toxicity of pesticides
in young versus adult rats. Food Cosmet. Toxicol., 3: 591-596.
MAGILL, P. I., HOLDEN, F. R., & ACKLEY, C. (1956) Air Pollution
Handbook. New York, McGraw-Hill.
MANN, L. I. (1970) Developmental aspects and the effect of carbon
dioxide tension on fetal cephalic metabolism and
electroencephalogram. Exp. Neurol., 26: 148-159.
MARZULLI, F. N. (1968) Ocular side effects of drugs. Food Cosmet.
Toxicol., 6: 221-223.
MCGRATH, J. T. (1960) Neurological examination of the dog with
clinicopathological observation. 2nd ed. Philadelphia, Lea &
Febiger.
MILLER, L. C. & TAINTER, M. L. (1944) Estimation of the ED50 and its
error by means of log-probit graph paper. Proc. Soc. Exp. Biol.
Med. NY, 57: 261-264.
MORRISON, J. K., QUINTON, R. M., & REINERT, H. (1968) The purpose and
value of LD50 determinations. In: Boyland, E. & Goulding, R.,
ed. Modern trends in toxicology. London, Butterworths,
pp. 1-17.
MOWBRAY, J. B. & CADELL, T. E. (1962) Early behaviour patterns in
rhesus monkeys. J. Comp. Physiol. Psychol., 55: 350-357.
MUNRO, I. C., MIDDLETON, E. J., & GRICE, H. C. (1969) Biochemical and
pathological changes in rats fed brominated cottonseed oil for 80
days. Food Cosmet. Toxicol., 7: 25-33.
MUNRO, I. C., CHARBONNEAU, S. M., & WILLES, R. F. (1972) An automated
data acquisition and computer-based computation system for
application to toxicological studies in laboratory animals.
Lab. Anim. Sci., 22: 753-756.
MUNRO, I. C., MOODIE, C. A., KREWSKI, D., & GRICE, H. C. (1974)
Carcinogenicity study of commercial saccharin in the rat.
Toxicol. appl. Pharmacol., 32: 513-526.
NATIONAL ACADEMY OF SCIENCES (1975) Principles for evaluating
chemicals in the environment. Washington, DC, National Academy
of Sciences.
NEWBERNE, P., ROGERS, A. E., & WOGAN, G. N. (1968) Hepatorenal lesions
in rats fed a low lipotrope diet and exposed to aflatoxin.
J. Nutr., 94: 331-343.
OSBORNE, B. E. & DENT, N. J. (1973) Electrocardiography and blood
chemistry in the detection of myocardial lesions in dogs.
Food Cosmet. Toxicol., 11: 265-276.
PAGET, G. E. & BARNES, J. M. (1964) Toxicity tests. In: Laurence, D.
R. & Bacharach, A. L., ed. Evaluation of drug activities:
Pharmacometrics, London, New York, Academic Press,
Vol. 1, pp. 135-166.
PECK, H. M. (1974) Design of experiments to detect carcinogenic
effects of drugs. In: CRC Carcinogenesis testing of chemicals,
Cleveland, OH, CRC Press, pp. 1-13.
RUMKE, CHR. L. (1964) Some limitations of animal tests. In: Laurence,
D. R. & Bacharach, A. L., ed. Evaluation of drug activities:
Pharmacometrics, London, New York, Academic Press,
Vol. 1, pp. 125-133.
SCHALM, O. W. (1967) Veterinary haematology, 2nd ed. Philadelphia,
Lea & Febiger.
SCOTT, W. J., JOHNSTON, J. W., JOHNSTON, C. D., & BELILES, R. P.
(1966) Comparative acute toxicities of isoproterenol and
metraproterenol. Toxicol. appl. Pharmacol., 8: 353.
SETNIKAR, I. & MAGISTRETTI, M. J. (1964) The toxicity of central
nervous system stimulants in rats of different ages.
Proc. Eur. Soc. Drug Toxicity, 4: 132-139.
SHER, S. P. (1972) Mammary tumours in control rats: literature
tabulation. Toxicol. appl. Pharmacol., 22: 562-588.
SIRRIDGE, M. S. (1967) Laboratory evaluation of haemostasis.
Philadelphia, Lea & Febiger.
SNEDECOR, J. W. & COCHRAN, W. G. (1967) Statistical methods 6th ed.,
Ames, IA, Iowa State University Press, pp. 111-114, 221-223.
SONTAG, J. M., PAGE, N. P., & SAFFIOTTI, U. (1975) Guidelines for
carcinogen bioassay in small rodents. Bethesda, MD, National
Cancer Institute.
STAUBLI, W., HESS, R., & WEIBEL, E. R. (1969) Correlated morphometric
and biochemical studies on the liver cell. II. Effects of
phenobarbital on rat hepatocytes. J. cell. Biol., 42: 92-112.
THOMPSON, W. (1947) Use of moving averages and interpolation to
estimate median effective dose. Bac. Rev., 11: 115-141.
TOMATIS, L. & MOHR, U., ed. (1973) Transplacental carcinogenesis,
IARC Sci. Publ. No. 4, 181 pp.
WEIL, C. S. (1952) Relationship between short- and long-term feeding
studies in designing an effective toxicity test. Agric. Food
Chem., 11: 486-491.
WEIL, C. S., WOODSIDE, M.D., BERNARD, J. R., & CARPENTER, C. P. (1969)
Relationship between single peroral, one-week and ninety-day
feeding studies. Toxicol. appl. Pharmacol., 14: 426-431.
WEIL, C. S., & CARPENTER, C. P. (1969) Abnormal values in control
groups during repeated-dose toxicological studies. Toxicol.
appl. Pharmacol., 14: 335-339.
WEINBERG, M. S., GOLDHAMEN, R. E., & CARSON, S. (1966) Acute oral
toxicity of various drugs in newborn rats after treatment of the
dam during gestation. Toxicol. appl. Pharmacol., 9: 234-239.
WORDEN, A. N. & HARPER, K. H. (1963) Oral toxicity as influenced by
method of administration. Proc. Eur. Soc. Study Drug Toxicity,
2: 15-26.
WHO (1958) WHO Technical Report Series No. 144. (Procedures for the
testing of intentional food additives to establish their safety
for use -- 2nd report of the joint FAO/WHO Expert Committee on
Food Additives), 19 pp.
WHO (1972) Health hazards of the human environment, Geneva, WHO,
387 pp.
YEARLY, R. A. & BENISH, R. A. (1965) A comparison of the acute
toxicities of drugs in newborn and adult rats. Toxicol. appl.
Pharmacol., 7: 504.
ZBINDEN, G. (1973) Progress in toxicology: special topics. Vol. 1.
New York, Springer-Verlag.
ZIMMERMAN, H. J. (1974) Serum enzyme measurement in experimental
hepatotoxicity. Israel J. med. Sci., 10: 328-332.
4. CHEMOBIOKINETICS AND METABOLISM
4.1 Introduction
The objective of chemobiokinetic studies is to obtain data that
allow reliable assessment of the hazard of environmental chemicals to
man. Since effects are related to the amounts or concentrations of a
chemical in tissues and cells, it is imperative to elucidate the
dynamics of the toxicant at the target site. It should be emphasized
that the toxicant may be either the parent chemical or a metabolite or
degradation product formed from it. Thus, the qualitative
identification of the degradation products of a chemical together with
a quantitative characterization of their fate, as well as the fate of
the parent chemical, as a function of time, are inextricably
associated in a proper chemobiokinetic evaluation. In the context of
this chapter, the word "chemobiokinetics" has been used in place of
"pharmacokinetics" because too often the latter implies restriction of
this scientific discipline to drugs. The term chemobiokinetics is
proposed to emphasize its importance in evaluating the biological
effects of all chemicals.
4.2 Absorption
4.2.1 General principles
Absorption of a chemical into the body can take place,
potentially, by all routes of exposure. In assessing the toxicity and,
ultimately, the hazard of a chemical, the oral, dermal, and inhalation
routes of exposure are of primary importance. Following absorption,
the chemical is distributed by the blood to the various tissues.
Therefore, the rate of absorption is frequently estimated by
determining the concentration of the chemical in the plasma as a
function of time following exposure.
The route of administration can greatly influence the rate at
which a foreign chemical enters the body. Upon ingestion, the gastric
contents and pH of the stomach can influence the rate of absorption of
the chemical. In the small intestine, food may either enhance or delay
absorption. Indeed, the environment of the gastrointestinal tract (pH,
food, bacteria) may change the parent chemical into another chemical.
The inhalation route allows a chemical to pass rapidly into the blood
without encountering drastic changes in pH, food, or microflora. The
skin effectively retards the absorption of many chemicals; however, it
should not be considered as an absolute barrier. Some chemicals
readily penetrate intact skin and a minor abrasion of the skin may
greatly enhance the absorption of many chemicals.
In order that a chemical may be absorbed into the bloodstream, it
must cross one or more semipermeable membranes, such as the
gastrointestinal epithelium, the lining of the respiratory tract, or
the epidermis of the skin. Membranes are essentially lipoproteins with
aqueous pores through which water-soluble molecules can pass. The pore
size varies from 4 Å (intestinal epithelium and mast cells) to 30 Å
(capillaries), allowing the passage of molecules with molecular
weights less than 100-200 to approximately 60 000, respectively. Most
membranes have an electrical potential that may effectively preclude
the ready penetration of charged chemical species. Thus it is obvious
that the absorption of a chemical depends on its physicochemical
properties, molecular size, shape, degree of ionization, and lipid
solubility. For a more thorough discussion, the reader is referred to
Davson & Danielli (1952) and Schanker (1962a).
Three mechanisms have been proposed to explain how a chemical
passes across a cell membrane: (a) passive diffusion through the
membrane, (b) filtration through membranous pores, and (c) specialized
transport systems that carry water-soluble and large molecules across
the membrane by means of a "carrier".
Passive diffusion is considered to be the principal mechanism by
which chemicals can cross cell membranes. The rate of passive
diffusion of a molecule is proportional to the concentration gradient
across the membrane, the membrane thickness, the area available for
diffusion, and the diffusion constant, in accordance with Fick's Law
(La Du et al., 1972). The rate of passage is related directly to the
lipid solubility (Brodie, 1964). However, since absorption requires
passage through an aqueous- as well as a lipo-phase, the absorption of
a chemical with an extremely low solubility in water may be impeded in
spite of a high lipid-to-water partition coefficient. The passive
diffusion also depends on the extent of the ionization and the lipid
solubility of the ionized and nonionized species (Brodie, 1964).
Filtration is a process by which a chemical passes through the
aqueous pores in the membrane, and is governed by the size and shape
of the molecule. The bulk flow of water across the membrane produced
by an osmotic gradient or hydrostatic pressure can act as a carrier
for chemicals.
Specialized transport processes are needed to explain the
transport and kinetic behaviour of large, lipid insoluble molecules
and ions. Two types of carrier-mediated transport systems have been
recognized: active transport and facilitated diffusion. The carrier in
both systems is some component of the membrane that combines with the
chemical and assists its passage across the membrane. It has a limited
capacity and when it is saturated, the rate of transfer is no longer
dependent on the concentration of the chemical and assumes zero order
kinetics. Structure, conformation, size, and charge are important in
determining the affinity of a molecule for a carrier site, and
competition for carrier site will occur.
Active transport is a carrier-mediated transport system which
moves a molecule across a membrane against a concentration gradient,
or, if the molecule is an ion, against an electrochemical gradient. It
requires the expenditure of metabolic energy and can be inhibited by
poisons that interfere with cell metabolism. Active transport plays an
important role in the renal and biliary excretion of chemicals.
Facilitated diffusion is a carrier transport mechanism by which a
water-soluble molecule (i.e. glucose) is transported through a
membrane down a concentration gradient. No apparent energy is required
and metabolic poisons will not inhibit this process. The difference
between facilitated diffusion and active transport is that the latter
moves molecules against a concentration gradient, whereas the former
does not. For more complete discussion of membrane transport, refer to
La Du et al. (1972) and Goldstein et al. (1974).
Another active process, pinocytosis, has been implicated as a
mechanism for transferring large molecules and particles into cells.
In this process, the membrane engulfs the material and pinches off an
envelope containing the material within the cell.
4.2.2 Absorption from the lungs
The pulmonary epithelial lining is very thin, possesses a large
surface area, and is highly vascular. Thus, absorption of foreign
chemicals can take place at a very rapid rate. Most rapidly absorbed
are gases and aerosols with small particle size and a high
lipid-to-water partition coefficient. In most inhalation studies,
absorption may occur by routes other than the lungs and the
investigator should be aware of this in the interpretation of data.
A more complete discussion of the inhalation of chemicals is
presented in Chapter 6.
4.2.3 Absorption from the skin
The structure of the skin enables rapid penetration of
lipid-soluble compounds through the epidermis, a lipoprotein barrier,
whereas the highly porous dermis is permeable to both lipid- and
water-soluble substances (Katz & Poulsen, 1971). Factors which govern
penetration through the skin are hydration, pH, temperature, blood
supply, and metabolism as well as vehicle-skin interactions. Abrasion
of the skin may enhance absorption greatly. For a more complete
discussion of the principles for absorption through the skin and
experimental methods, refer to Part II, Chapter 11.
4.2.4 Gastrointestinal absorption
The gastrointestinal tract is one of the most important routes of
absorption of foreign compounds (Schanker, 1971). Chemicals can be
absorbed along any section of the gastrointestinal tract, but because
of the large surface area and rich blood supply, absorption is
favoured from the small intestines. In most parts, the movement of a
chemical across the epithelial lining of the gastrointestinal tract is
by diffusion and carrier transport mechanisms are involved to a lesser
degree.
Although therapeutic amounts of drugs may be absorbed from the
buccal mucosa (Beckett & Hossie, 1971), absorption of environmental
chemicals from the mouth is minimal compared with that from the
stomach and intestine. Chemicals absorbed from the mouth are not
exposed to the gastrointestinal digestive juices and drug-metabolizing
enzymes. Furthermore, since they are not transported by the hepatic
portal system directly to the liver, their normally rapid metabolism
may be precluded, thus prolonging their effect.
The stomach is a significant site of absorption by passive
diffusion of many acid, and neutral, foreign compounds (Schanker et
al., 1957). Due to the acidity of the stomach, weak acids will exist
in the diffusible, nonionized, lipid-soluble form, whereas weak bases
will be highly ionized and therefore not generally absorbable.
Absorption from the small intestine is similar in principle to
that from the stomach (passive diffusion), except that the pH of the
intestinal contents (pH 6.6) may alter the fraction of the chemical in
the nonionized form favouring the absorption of both weakly-acid and
weakly-alkaline chemicals. The aqueous pore size, 4 Å, limits
absorption by filtration to molecules having a molecular weight of
less than 100-200. Rarely, an environmental chemical may be absorbed
from the intestinal tract by an active transport system that is
normally involved in the absorption of nutrients, e.g. sugars and
amino acids (Schanker, 1963).
Many factors can affect the absorption of foreign compounds from
the gastrointestinal tract (Brodie, 1964; Levine, 1970; Place &
Benson, 1971; Prescott, 1975): ( a) increased gastric emptying can
decrease gastric absorption and increase intestinal absorption; ( b)
increased intestinal peristalsis generally inhibits intestinal
absorption; ( c) gastric acid, intestinal digestive juices, and gut
microflora can all degrade chemicals to other absorbable or
nonabsorbable chemical species; ( d) food in the gastrointestinal tract
can impair absorption by producing a nonabsorbable complex, by
decreasing gastric emptying (especially fats), and by reducing,
mixing, or altering pH; ( e) normal digestion produces increased
gastrointestinal blood flow which will enhance absorption; ( f)
absorption of a solid will be impaired if dissolution in the
gastrointestinal tract does not take place. The practice of
administering chemicals admixed with the diet must take these factors
into account, especially the possible reaction of the chemical with
dietary constituents.
4.3 Distribution
Once absorbed, the distribution of a chemical is determined by
the relative plasma concentration, the rate of blood flow through
various organs and tissues, the rate by which the chemical penetrates
cell membranes, and the binding sites that are immediately available
in the plasma and tissues. After the initial distribution phase, the
rate by which a chemical penetrates cell membranes and the available
sites for binding are the predominating factors influencing the final
distribution of a chemical in the body.
When the plasma concentration of a chemical is high and the cell
membranes do not provide significant barriers to diffusion,
distribution is mainly to organs with high blood flow, e.g. brain,
liver, and kidney. A classic example of distribution and
redistribution is thiopental, a highly lipid-soluble chemical that,
after administration, is first distributed to the brain and
subsequently to muscle and body fat which have poor blood flow (Price
et al., 1960). Lipid-soluble, foreign compounds tend to be distributed
and localized in adipose tissue (Mark, 1971), in accordance with their
lipid-to-water partition coefficients, e.g. the chlorinated
hydrocarbon pesticides, dieldrin, DDT, and DDE (Rodomski et al., 1968)
and polychlorinated biphenyls (PCBs) (Allen et al., 1974).
Distribution of chemicals into organs and tissues is influenced by
membraneous barriers in the same way as absorption (see 4.2). For a
more detailed treatment, see Quastel (1965). The capillary membrane,
unlike other body or cell membranes, is freely permeable to foreign
compounds of a molecular weight of 60 000 or less, whether
lipid-soluble or not (Pappenheimer, 1953; Renkin, 1964); generally
chemicals pass these membranes readily, except in the brain,
testicles, and the eye (Gehring & Buerge, 1969).
The movement of foreign chemicals to the brain represents a
unique example that cannot be explained by the physicochemical
properties of the chemical and the tissue distribution. Many chemicals
fail to penetrate into the brain tissue or cerebrospinal fluid as
readily as into other tissues (Brodie & Hogben, 1957). The boundary
between blood and brain consists of several membranes; those of the
blood capillary wall, the glial cells closely surrounding the
capillary, and the membrane of the neurons or nerve cells. The
so-called "blood-brain barrier" is located at the capillary wall-glial
cell region. The capillary walls in the brain tend to be more like
cell membranes than capillary membranes. Therefore, ionized substances
and large water-soluble molecules such as proteins are almost entirely
excluded from passage (Rall, 1971). The chief mode of exit of both
lipid-soluble and polar compounds is by filtration across the
arachnoid villi. The method for studying the movement of chemicals
into and from the brain has been discussed by Rall (1971).
The red blood cell has unusual permeability in that organic
unions penetrate much more readily than cations. This may be explained
by the presence of positively charged membrane pores that will accept
anions but repel cations (Schanker et al., 1957).
4.4 Binding
A major factor, that can affect the distribution of a chemical,
is its affinity to bind to proteins and other macromolecules of the
body. Foreign chemicals have been shown to bind reversibly to such
substrates as albumen, globulins, haemoglobin, mucopolysaccharides,
nucleoproteins, and phospholipids (Shore et al., 1957). For a survey
of the biological implications of the protein binding of chemicals,
the reader is referred to Gillette (1973a).
Once a chemical is bound to a body constituent, it is temporarily
localized. This localization modifies the initial pattern of
distribution and affects the rates of absorption, metabolism, and
elimination of the chemical from the body.
4.4.1 Plasma-protein binding
Most chemicals show some degree of binding to plasma proteins,
the most important fraction of which is albumen. Albumen at pH = 7.3
contains a net negative charge; however, cationic groups must be
accessible because albumen has been shown to bind anions as well as
cations. Although the plasma proteins show an appreciable capacity for
binding many chemicals, this is limited, making it important to
understand such binding as a function of the concentration of the
chemical.
Since plasma proteins possess a limited number of binding sites
and the sites are somewhat nonspecific, two chemicals with an affinity
for the same binding site will compete with one another for binding.
The plasma proteins of various laboratory animals and man show
differences in the degree and nature of binding. This is due to
differences in the total concentrations and relative proportions of
the various plasma proteins as well as the composition and
conformation of albumens (Gillette, 1973b).
4.4.2 Tissue binding
The binding of chemicals to tissue constituents also contributes
to the localization of a chemical. Certain chemicals show a much
greater affinity for tissue than for plasma proteins, and in some
instances the affinity for tissue is quite specific. For example,
polycyclic aromatic compounds have been shown to have particular
affinity for the melanin in the eye (Potts, 1964).
Some metals and several chemicals and organic anions are bound to
proteins (Y and Z proteins or ligandins) in the liver (Levi et al.,
1969). These proteins may play a key role in the transfer of organic
anions from plasma to liver (Levi et al., 1969; Reyes et al., 1971),
and they also bind corticosteroids and azo-dye carcinogens (Litwack et
al., 1971). For further details concerning the nature and effects of
binding of chemicals by proteins and for methods of study, see
Chignell (1971), Gillette (1975), Keen (1971) and Settle et al.
(1971).
Many inorganic ions, particularly metals, as well as
tetracycline, are concentrated in various tissues and organs,
particularly in bones and teeth (Foreman, 1971). A convenient method
for studying the accumulation of chemicals in organs and tissue is
autoradiography (Roth, 1971). Valuable measurements may also be
obtained with classical chemical and radio-chemical techniques which
have the added advantage of being quantitative.
4.5 Excretion
Chemicals are excreted as the parent chemical, as metabolites, or
as conjugates of the parent chemical or its metabolites. The principal
routes of excretion are the urine and bile, and to a lesser degree
expired air, sweat, saliva, milk, and secretions of the
gastrointestinal tract.
4.5.1 Renal excretion
The kidneys are the most important route of excretion of foreign
compounds (Weiner, 1971). The three mechanisms of renal excretion are:
glomerular filtration, active tubular transport, and passive tubular
transport. Only compounds of high molecular weight or those bound
tightly to plasma proteins escape glomerular filtration and the
resulting filtrate contains approximately the same concentration of
foreign compounds as that found in the plasma in an unbound state.
Water and endogenous substrates are reabsorbed from the
glomerular filtrate as it passes down the tubule. In the tubule,
lipid-soluble, unionized chemicals pass in either direction by passive
diffusion. Thus, lipid-soluble chemicals may be reabsorbed by the
tubule, prolonging their retention in the body. Ionic chemicals, such
as conjugates and other metabolites, are poorly reabsorbed and pass
directly out of the body in the urine.
Active transport takes place in the proximal tubule of the
kidney. There are two distinct active transport processes. One process
is specific for organic anions and the other specific for organic
cations. Chemicals transported by the same transport process compete
with each other, and the excretion rate of one compound can be reduced
by the administration of the other. The active transport process can
be saturated as the concentration of the chemical in the plasma is
increased. When the active tubular secretion is saturated, that is,
when an increase in the concentration of the chemical in the plasma is
no longer accompanied by a proportional increase in the concentration
of the chemical in the urine, the concentration in the plasma is
referred to as the renal-plasma threshold.
The anionic secretory process is responsible for the excretion of
metabolites formed through conjugation of the parent chemical or its
degradation products with various endogenous substrates such as
glycine, sulfate, or glucuronic acid. These relatively polar,
lipid-insoluble metabolites are poorly reabsorbed from the tubules and
more readily excreted.
4.5.2 Biliary excretion
Biliary excretion is a major route for the excretion of foreign
chemicals (Smith, 1971a, 1973). It is has been demonstrated (Brauer,
1959; Schanker, 1962b; Sperber, 1963; Williams, 1965) that compounds
with high polarity, anionic and cationic conjugates of compounds bound
to plasma proteins, and compounds with molecular weights greater than
300 are actively transported against a concentration gradient into the
bile. It has also been shown that, once these compounds are in the
bile, they are not reabsorbed into the blood and are excreted into the
gastrointestinal tract (Schanker, 1965). Factors that influence the
biliary excretion of foreign chemicals and metabolites are considered
to be of two types: (a) physicochemical, relating to molecular size,
structural features, and polarity; and (b) biological, relating to
protein binding, renal excretion, metabolism, species, and sex. For a
comprehensive and detailed discussion of these subjects, the reader is
referred to Smith (1971a, 1973), and Stowe & Plaa (1968).
4.5.3 Enterohepatic circulation
Enterohepatic circulation is the phenomenon that occurs when a
compound is excreted via the bile into the gastrointestinal tract,
reabsorbed from the gastrointestinal tract and carried via the portal
system back to the liver, where it is again excreted via the bile and
recycled. Physiologically, enterohepatic circulation is important
because it permits reuse of endogenous biliary excretion products.
However, when a foreign compound is involved in enterohepatic
circulation, it must make its way either to the faeces or to the
peripheral blood to be excreted from the body. Thus, enterohepatic
circulation of a foreign compound serves to enhance its retention in
the body. There are examples in the literature (Gibson & Becker, 1967;
Keberle et al., 1962) which demonstrate that the half-life of a
compound involved in enterohepatic circulation can be decreased after
surgically interrupting the enterohepatic cycle. Administration of a
sequestering agent that binds the compound in the gastrointestinal
tract would serve the same purpose.
Smith (1973) has described the following factors that can affect
the enterohepatic circulation of a compound: ( a) the extent and rate
of excretion of the compound in the bile; ( b) the activity of the gall
bladder; ( c) the fate of the substance in the small intestine; and ( d)
the fate of the compound after reabsorption from the gut. Since many
foreign chemicals are excreted in the bile as unabsorbable conjugates,
the hydrolysis of these conjugates in the intestine may play a key
role in enterohepatic circulation. For a thorough discussion of
enterohepatic circulation, the reader is referred to Plaa (1975).
4.5.4 Other routes of excretion
In addition to excretion in bile and urine, other routes for the
excretion of foreign chemicals and their metabolites should not be
overlooked. In accordance with the pH partition theory, organic bases
highly ionized at the pH value of gastric juice may be secreted into
the stomach (Shore et al., 1957). Similarly, weak acids ionized at
neutral pH may be transferred from the plasma to the lumen of the
intestine. These chemicals are sequestered by the intestinal contents,
augmenting their excretion in the faeces.
Many volatile organic chemicals are excreted readily via exhaled
air (see Chapter 6). This route of excretion is common for carbon
dioxide, an ultimate end-product of an extensively metabolized organic
chemical. For this reason, the quantification of expired radiolabelled
carbon dioxide (14CO2) is very important in chemobiokinetic
studies using carbon-14-labelled compounds.
Many foreign compounds are excreted, to different degrees, in
milk, in either the aqueous or lipid phase (Rasmussen, 1971). Although
this route may be of minor importance for the elimination of a
chemical from the body, it should be given particular attention in
evaluating the hazard of chemicals to man. First, consumption of cow's
milk may constitute an important vehicle of exposure. Secondly, the
consumption of mother's milk by the newborn may provide very high
doses of a chemical that is concentrated in the milk. It should also
be noted that the volume of milk consumed by the newborn per unit body
weight may, in itself, magnify the dose received by this segment of
the population.
Chemicals are also excreted in sweat and saliva. The presence of
a chemical in sweat may lead to dermatitis. Although saliva is usually
swallowed and thus does not lead to elimination of the agent from the
body, recent work has shown that analysis of saliva for the presence
of a chemical may preclude the necessity for venepuncture to obtain
plasma for analysis.
4.6 Metabolic Transformation
Metabolic transformation or biotransformation are terms that have
been used to describe the process which converts a foreign chemical to
another derivative (metabolite) in the body. Metabolic transformation
has been the subject of several excellent reviews (Conney, 1967;
Conney & Burns, 1962; Dahm, 1971; Daly, 1971; Dutton, 1971; Garattini
et al., 1975; Gillette, 1971a,b & 1974a,b; Gillette et al., 1974;
Kuntzman, 1969; McClean, 1971; Smuckler, 1971; Weisburger &
Weisburger, 1971). It usually results in the formation of more polar
and water-soluble derivatives of a foreign chemical which can be more
readily excreted from the body. Generally, such metabolic
transformation of a foreign chemical also results in the formation of
a less toxic chemical. However, there are many cases where the
metabolites are more toxic than the parent chemicals (McLean, 1971;
Miller & Miller, 1971a).
A few compounds resist metabolic transformation. Most strong
acids and bases are excreted unchanged. Also the resistance of
long-acting nonpolar compounds (barbital, halogenated benzene, etc.),
to metabolic transformation might explain their slow elimination from
the body.
A metabolic activation is suggested, if a compound is more toxic
when given orally than intravenously, if there is a long delay between
the administration of a chemical and the onset of its biological
effect, or, if there is an increased effect following pretreatment
with compounds that induce metabolic transformation (Garattini et al.,
1975).
4.6.1 Mechanisms of metabolic transformation
Usually, the metabolic transformation of chemicals takes place to
the greatest extent in the liver and is catalysed by enzymes found in
the soluble, mitochondrial, and microsomal fractions of the cell.
Enzymes metabolizing foreign chemicals are also found, to a lesser
degree, in the cells of the gastrointestinal tract, kidney, lung,
placenta, and blood (Aitio, 1973; Gillette, 1963; Gram, 1973;
Hietanen, 1974; Hietanen & Valinio, 1973; Wattenberg & Leong, 1971;
Wattenberg et al., 1962; Witschi, 1975). It must be emphasized that
for a particular chemical, or a particular route of administration,
other organs may play a more important role in the metabolic
transformation of the chemical than the liver. The role of enzymatic
reactions carried out by the intestinal flora may be very important
and should not be overlooked (Scheline, 1968; Smith, 1971a).
Enzyme-catalysed, biochemical transformations can be classified into
four main types: (a) oxidations, (b) reductions, (c) hydrolyses and
(d) synthetic reactions (see Table 4.1 in Annex to this Chapter).
The metabolic transformation of a chemical can occur via various
pathways which can consist of a single reaction or multiple reactions.
If the metabolic pathway consists of one reaction it is usually
oxidation, reduction, or hydrolysis which tends to increase the
polarity of the compound. Multiple-reaction metabolic pathways can
consist of a series or any combination of oxidation, reduction, or
hydrolysis. The final reaction in a multiple-reaction pathway is
usually a conjugation reaction involving the addition of polar
endogenous functional groups (D-glucuronic acid, glycine etc.) which
usually render the molecule more polar, less lipid-soluble, and
therefore more readily excretable. The predominant sequence of
reactions or metabolic pathways is determined by many factors such as
the dose of the chemical, species, strain, age, sex, and certain
environmental variables.
4.6.1.1 Microsomal, mixed-function oxidations
The metabolism of a large variety of foreign compounds involves
oxidative processes. Microsomal oxidation refers to reactions
catalysed by the enzymes found in the microsomes of the endoplasmic
reticulum. These enzymes are sometimes referred to as microsomal,
mixed-function oxygenases (mono-oxygenases) (Mason, 1957). The
reactions require molecular oxygen and nicotinamide adenine
dinucleotide phosphate, reduced form (NADPH). The reduction
equivalents from NADPH are used to reduce molecular oxygen so that it
can be carried by a cytochrome called P-450 to the compound to be
oxygenated. The oxygen is then fixed into the compounds, usually as a
hydroxyl group (Estabrook, 1971; Estabrook et al., 1971).
The apparent sequence of events in the course of a mixed function
oxidation has been described (Boyd & Smellie, 1972; Estabrook et al.,
1972; Gillette, 1971c). The compound (substrate) forms a complex with
the oxidized cytochrome P-450; this is reduced either directly by
NADPH-cytochrome- c-reductase (1.6.2.4) or indirectly via an
unidentified electron carrier. The reduced cytochrome P-450-substrate
complex then reacts with oxygen to form an "active oxygen" complex,
which decomposes with the formation of the oxidized substrate and
oxidized cytochrome P-450. Substantial progress has been made in
elucidating this mechanism by the development of a method involving
the resolution and reconstitution of the components of the liver
microsomal hydroxylating system (Lu & Levin, 1974).
Measurement of mixed-function oxidase activities of liver
microsomes in vitro has become an important aspect in evaluating the
toxicity of chemicals. The mixed-function oxidase system may be either
a biotransformation system or a site of action of chemicals.
Measurements of the activity of this system can be performed using
either a 9000 g supernatant fraction (Henderson & Kersten, 1970;
Klinger, 1974) of a liver homogenate prepared in buffered KCl
solution, or a microsome fraction sedimented by centrifugation at
about 105 000 g (Flynn et al., 1972; Hewick & Fouts, 1970a,b; Liu et
al., 1975).
The reaction mixtures consisting of the particle-bound enzymes
have to be supplemented with an NADPH-generating system. This may be
fulfilled by the addition of NADPH and glucose-6-phosphate, if the
9000 g fraction is used, but if washed microsomes are used,
glucose-6-phosphate dehydrogenase (1.1.1.49) must also be added.
Isolated tissue cells, tissue cultures, or slices of organs, as
well as perfused organs can also be used for metabolic studies.
Because cytochrome P-450 is intimately associated with the
metabolism of many foreign chemicals, the following methods and
variables have been developed for ascertaining its activity in the
tissues of animals used in toxicological investigations.
The method of Omura & Sato (1964a,b) has been used to measure the
change in the microsomal content of cytochrome P-450 and cytochrome
b5. This method relies on a spectral shift of the pigment upon
exposure to carbon monoxide. An increase in the cytochrome P-450
content can be explained as a consequence of enzyme induction, whereas
the decrease of the haem pigment content may be the result of enhanced
permeability of microsomal membranes due to the damaging effects of
the chemical (Bond & De Matteis, 1969). The concentration of
cytochrome P-450 in the liver, however, is not always directly
proportional to the activity of the mixed-function oxidases.
Spectral changes of cytochrome P-450, determined in the presence
of various substrates, provide information about the binding between
the pigment and substrate (Hewick & Fouts, 1970a; Remmer et al.,
1966). Compounds may be classified into type I or type II according to
their spectral reactions with cytochrome P-450. When type I compounds
bind to cytochrome P-450, the characteristic spectral shift, spectral
difference, gives a peak at 385-390 nm and a trough at 418-427 nm,
whereas with type II compounds, the peak occurs at 425-435 nm and the
trough at 390-405 nm. Originally, it was thought that the magnitudes
of these spectral shifts, especially type I spectra, could be
correlated with microsomal biotransformations (Schenkman et al.,
1967). This correlation, however, is not universally applicable
(Davies et al., 1969; Gigon et al., 1969; Holtzman et al., 1968).
Thus, differences in the magnitude of these spectral changes are
difficult to interpret when they are detected in animals treated with
a chemical (Gillette et al., 1972). The same is true for the
ethylisocyanide difference spectra of cytochrome P-450 which are
characterized by two peaks at about 455 nm and 430 nm (Omura & Sato,
1964a).
Determination of the NADPH-cytochrome P-450 reductase activity,
the assumed rate limiting step in microsomal oxidations, has proved
useful in evaluating the effectiveness of the cytochrome P-450 system
prior to the oxygenation step (Fouts & Pohl, 1971; Gigon et al., 1969;
Hewick & Fouts, 1970b; Holtzman et al., 1968; Zannoni et al., 1972).
Measurement of the NADPH-cytochrome-c-reductase activity may give
information about the rate of flow of reducing equivalents from NADPH
to cytochrome P-450.
Determination of the rate of enzymatic conversion of a substrate
is a most valuable tool in elucidating the metabolic process. For this
purpose, however, it is essential to know the pathway for the
transformation of the chemical, and analytical methods are essential
to quantify the parent chemical and its reaction products. Selected
methods for monitoring some compounds and enzymatic reactions are
listed in Table 4.2 (see Annex to this Chapter).
There are large variations in the metabolism of foreign chemicals
as well as in susceptibility to metabolic inducers depending on the
species (Hucker, 1970), strain, age, and sex of animals.
Many variables must be considered as important factors in species
differences in the metabolism of foreign chemicals. Among these are
differences in binding, either to tissues or to plasma components,
such as albumen. Considerable variations in binding have been reported
for the same chemical in different species (Borgå et al., 1968; Kurz &
Friemel, 1967; Scholtan, 1963; Sturman & Smith, 1967; Witiak &
Whitehouse, 1969). More obvious are the concentrations and types of
foreign chemical-metabolizing enzymes in each species (Flynn et al.,
1972).
4.6.1.2 Conjugation reactions
The major conjugation mechanisms are: glucuronide synthesis,
"ethereal" sulfate synthesis, glutathione conjugation, glycine
conjugation, methylation, acetylation, and thiocyanate synthesis.
Glutamine conjugation has also been shown to occur in man and monkey.
The conjugates formed by these mechanisms are usually nontoxic,
therefore conjugation has also been referred to as a detoxification
mechanism.
These conjugations are biosynthetic reactions in which foreign
compounds or their metabolites containing suitable groups (hydroxyl,
amino, carbonyl, or epoxide) combine with some endogenous substrates
to form conjugates (Parke, 1968; Williams, 1967a, 1971). These
reactions require ATP as source of energy, coenzymes, and transferases
which are usually specific for the formation of conjugates of foreign
compounds. The conjugations usually proceed in at least two steps:
first, the extramicrosomal synthesis of acylcoenzyme and next the
transfer of the acyl moiety to the aglycone, which, in some but not
all cases, is localized in the microsomes. Thus, these reactions
cannot be considered as transformations, characteristic of microsomes.
In accordance with the coenzymes participating in these
reactions, they include:
formation of glucuronides (via uridine diphosphate glucuronic
acid, UDPGA);
formation of sulfate esters (via 3-phosphadenosine-5-
phosphosulfate, PAPS);
O-, N-, and S-methylation via 5'-[(3-amino-3-carboxypropyl)
methylsulfonio]-5'-dioxyadenosine( S-adenosylmethionine);
acetylations (via acetyl coenzyme A);
formation of peptide conjugates (via different acylcoenzyme A
derivatives);
formation of glutathione conjugates and mercapturic acids
(conjugations with glutathione).
Formation of glucuronides is probably the most important
microsomal conjugation mechanism (Dutton, 1971). It occurs in the
liver and to a lesser extent in the kidney, gastrointestinal tract,
and the skin. Biosynthesis of glucuronides can be measured in intact
animals by determining D-glucaric acid (Marsh, 1963) and
D-glucuronolactone dehydrogenase (1.1.1.70) (Marselos & Hanninen,
1974), by enhancement of D-glucuronolactone and aldehyde dehydrogenase
(1.2.1.3) by inducers of microsomal metabolism (Marselos & Hanninen,
1974), glucuronides (Gregory, 1960; Yuki & Fishman, 1963) and
L-ascorbic acid in urine. Elevation in urinary excretion of these
compounds may be an indicator of an adaptive acceleration of hepatic
glucuronide formation (Notten & Henderson, 1975). It should be
emphasized that increased excretion of D-glucaric acid can result from
enzyme induction; therefore it cannot be assumed that this occurrence
is indicative only of an increased glucuronide formation. Methods for
the measurement of glucuronide synthesis in whole organs and tissue
cultures, as well as in tissue slices, have been summarized by Dutton
(1966). In assays with homogenates and cell fractions the reaction
mixtures have to be supplemented with added UDPGA.
UDP-glucuronosyl transferase (2.4.1.17) activity can be
determined using 2-aminophenol (Burchell et al., 1972; Dutton &
Storey, 1962), 4-nitrophenol (Isselbacher 1956; Zakim & Vessey, 1973),
bilirubin (Heirwegh et al., 1972), 7-hydroxy-4-methyl-2H-I-
benzopyran-2-one (4-methylumbelliferone) (Aitio, 1973; Arias, 1962) or
morphine (Strickland et al., 1974).
In contrast to glucuronide synthesis, the formation of sulfate
esters is most probably an extramicrosomal process and is catalysed
generally by sulfate-conjugating enzymes in the presence of
3-phosphoadenosine-5-phosphosulfate as a co-enzyme (Roy, 1971). Among
the compounds of toxicological interest, phenols are converted by
sulfation to esters and excreted in the urine. Aminophenols yield
sulfamates. There are specific assays for the determination of
sulfotransferase (2.8.2) activity using 4-nitrophenol (Gregory &
Lipmann, 1957), or 3-(2-aminoethyl)-1H-indol-5-ol (serotonin) (Hidaka
et al., 1967) as acceptors.
The methyltransferases (2.1.1) catalyse O-, N- and
S-methylation of several physiologically active compounds and drugs
(Axelrod, 1971). They are widely distributed in different organs, but
only a small amount of catechol- O-methyltransferase (2.1.1.6) and
almost all of the phenol- O-methyltransferase (2.1.1.25) (Axelrod &
Daly, 1968) activity is localized in the microsomes of the liver. Only
microsomal transferases are induced by benzo(a)pyrene and inhibited by
SKF 525Aa. The methods used for the determination of
catechol- O-methyltransferase activity are based on the principle
that the enzyme catalyses the transfer of methyl groups to catechols
in the presence of S-adenosylmethionine as a methyl donor. The
substrates employed include adrenaline (Axelrod & Tomchick, 1958),
3,4-dihydrobenzoic acid (MacCaman, 1965), 3,4-dihydroxybenzeneacetic
acid (3,4-dihydrophenylacetic acid) (Assicot & Bohuon, 1969; Broch &
Guldberg, 1971) as well as I-(3,4-dihydroxyphenyl)ethanone
(3,4-dihydroxyacetophenone) (Borchardt, 1974). The end products of the
enzymatic reaction are measured either spectrofluorimetrically
(Axelrod & Tomchick, 1958; Borchardt, 1974; Broch & Guldberg, 1971),
or radiometrically using labelled methyl groups in the coenzyme
(MacCaman, 1965).
a Diethyl aminoethanol ester of diphenyl-propyl acetic acid.
Acetylation reactions of the amino group of foreign compounds are
catalysed by acetyltransferases (Weber, 1971). Substrates of these
enzyme reactions, localized in the soluble part of the cells, are
arylamines, hydrazines, and certain aliphatic amines. Coenzyme A is an
essential factor in these acetylations. Acetylation of arylamines has
been studied quantitatively, in vivo, in human beings and animals
(Williams, 1967b).
Methods for the determination of N-acetyltransferase (2.3.1.35)
activities in vitro summarized by Weber (1971) include colorimetric
(Brodie & Axelrod, 1948; Maher et al., 1957; Marshall, 1948; Shulert,
1961; Weber, 1970), spectrophotometric (Jenne & Boyer, 1962; Tabor et
al., 1953; Weber & Cohen, 1968; Weber et al., 1968) as well as
radiometric procedures (Stotz et al., 1969).
Conjugation of aromatic carboxylic acids (benzoic acid,
substituted benzoic acids, and heterocyclic carboxylic acids) with
amino acids by means of acetyl coenzyme A and ATP is called peptide
conjugation. Glycine is the most generally involved amino acid in this
reaction resulting in the formation of N-benzoylglycine (hippuric
acid). Indole-3-acetic acid, benzeneacetic acid, as well as
4-aminosalicylic acid, can conjugate with glutamine in man, and
several mammals. Determination of hippuric acids (Ogata et al., 1969)
enables the quantitative investigation of this conjugation reaction.
Conjugation of glutathione with foreign compounds, catalysed by
at least ten different glutathione S-transferases, is an important
pathway for the elimination of these compounds (Boyland, 1971).
Following the conjugation of foreign compounds with glutathione, the
conjugate is most frequently hydrolysed to the cysteine conjugate
which is excreted in the urine. Furthermore, the cysteine conjugate
may be acetylated and the resulting mercapturic acid excreted. The
significance of the mercapturic acid biosynthesis in man, however, is
difficult to assess.
Determination of glutathione S-transferase activities are based
on spectral change of the substrate (1,2-dichloro-4-nitrobenzene) due
to conjugation (Booth et al., 1961), or loss of glutathione content
(Boyland & Chasseaud, 1967; Boyland & Williams, 1965; Johnson, 1966)
or release of labile groups (Al-Kassab et al., 1963; Boyland &
Williams, 1965; Johnson, 1966) as well as on chromatographic
separation of the products (Suga et al., 1967). The determination of
the activity of gamma-glutamyltransferase (2.3.2.2), catalysing one
intermediary step of the overall mercapturic acid synthesis may also
be informative.
4.6.1.3 Extramicrosomal metabolic transformations
Foreign compounds, either transformed by oxidation or initially
having characteristic groups (hydroxyl, amino) may resemble normal
constituents of physiological metabolism. Thus, they may undergo
metabolic transformations similar to those of normal body
constituents: oxidation, reduction, deamination, hydrolysis. The
enzymes catalysing these reactions are localized in the cytosol or are
intrinsic compounds of the mitochondria.
In contrast to the extensive data in the literature on
enzyme-chemical interactions (MacMahon, 1971; Zeller, 1971) only a few
enzyme activities are commonly used to monitor toxicological events.
The alcohol dehydrogenase (1.1.1.1) of the liver is one of the
most important enzymes which catalyses the NAD-mediated oxidation of
various aliphatic and aromatic primary and secondary alcohols.
Determination of the activity of alcohol dehydrogenase is based on the
spectrophotometric measurement of the amount of NAD being reduced in
the presence of excess alcohol (Bonnichsen & Brink, 1955).
Among the amine oxidases, monoamine oxidase (1.4.3.4), localized
in the mitochondria, regulates the balance of the biogenic amines and
probably does not participate in the metabolism of foreign amines to a
great degree (Zeller, 1971). However, the fact that a large number of
substances (substrates and substrate analogues, alkyl and arylamines,
hydrazine derivatives, sulfhydryl reagents, etc.) inhibit this enzyme,
enables monoamine oxidase to be used as a tool in studies of the
toxicity of these inhibitors.
Monoamine oxidase activity can be measured manometrically
(Creasey, 1956) based on oxygen-consumption, by determination of
ammonia production (Cotzias & Dole, 1951), spectrophotometrically
(Dietrich & Erwin, 1969; Obata et al., 1971; Weissbach et al., 1960),
fluorimetrically (Takahashi & Takahara, 1968; Tufvesson, 1970) as well
as radiometrically (Otsuka & Kobayashi, 1964).
Hydrolysis by carboxylesterases (ali-esterases or arylesterases)
of foreign compounds containing ester groups may be important in
assessing their toxicity (La Du & Snady, 1971). Determination of
esterase activities using different substrates in the presence of the
chemical to be tested can disclose its possible inhibitory potency.
4.6.1.4 Nonenzymatic reactions
Although the foregoing sections have discussed enzymatic
modifications of chemicals, the investigator should not overlook
nonenzymatic, spontaneous reactions between chemicals and natural
constituents in the body that lead to the formation of metabolites,
e.g. the reaction of an alkylating agent with glutathione.
4.6.2 Species variability
A serious problem facing every research worker using an animal
species to study the metabolism of a foreign compound is whether or
not the metabolic pathway in the animal is similar to the metabolic
pathway in man. The problem is not only important in metabolic
studies, but is of utmost importance in using animal toxicity studies
to predict toxicological phenomena in man. Conney et al. (1974)
illustrated that the use of an animal species that metabolizes a
foreign compound in a similar manner to man will give a more precise
prediction of the type of toxicological phenomena to be expected in
man.
Different animal species have been shown to metabolize foreign
compounds at different rates. Quinn et al. (1958) has shown that
benzeneamine (aniline) has a metabolic half-time in the mouse of 35
minutes and in the dog of 167 minutes. In the same study it was
demonstrated that the metabolic half-time of an antipyrine in the rat
was 140 minutes, whereas in man it was 600 minutes.
Considerable species differences in metabolic pathways have also
been demonstrated. In the rat, mouse, and dog the carcinogen,
N-2-fluoranylacetamide (FAA), is N-hydroxylated to N-hydroxy-FAA
which is a more potent carcinogen than FAA. In the guineapig little or
no hydroxylation of FAA occurs. In toxicity studies, Miller & Miller
(1971b) and Weisburger et al. (1964) demonstrated that the rat, mouse,
and dog are susceptible to the carcinogenic activity of FAA, whereas
the guineapig is not. Thus, a difference in the metabolic pathways of
a foreign compound may greatly influence its toxicity.
Species variability in metabolism has been related to other
factors such as species differences in protein binding, and enzyme
concentration and type. Hucker (1970) described, in detail, species
differences in chemical metabolism and some of the factors responsible
for these differences.
4.6.3 Enzyme induction and inhibition
For some time it has been known that chemicals can increase the
activity of metabolizing enzyme systems. These chemicals have been
termed enzyme "inducers". Inducers exert their action by
quantitatively increasing the enzymes and components responsible for
the metabolism of foreign compounds. The importance of induction to
the toxicologist is two-fold. If metabolism leads to the formation of
excretable or nontoxic metabolites, induction will enhance
detoxification and excretion of the compound. However, if metabolism
leads to the production of a more toxic metabolite, induction will
increase the toxicity of a compound.
Many chemicals are known to increase metabolizing enzyme systems.
The reviews by Conney (1967), Kuntzman (1969), and Mannering (1968),
depict the large number of chemicals which induce metabolizing enzymes
and comprehensively review the factors involved in enzyme inductions.
Most inducers give maximum effects rather quickly -- within 2-3
days (Fouts, 1970). However, some require 2 weeks or longer (Gillette
et al., 1966; Hart & Fouts, 1965; Hoffman et al., 1968, 1970;
Kinoshita et al., 1966). Frequently, the degree of induction after
obtaining a maximum level may decline despite continuing treatment of
the animal with a chemical (Gillette et al., 1966; Hoffman et al.,
1968; Kinoshita et al., 1966).
Drug-metabolizing enzymes can also be depressed by foreign
chemicals, and these compounds are termed inhibitors. 2-(Diethylamino)
ethyl-alpha-phenyl-alpha-propyl benzeneacetate hydrochloride
(SKF-525A) is the best known of the inhibitors and is used routinely
in determining the effect of enzyme inhibition on the metabolism of
chemicals.
4.6.4 Metabolic saturation
In vivo saturation of metabolic pathways can play an important
role in determining the toxic profile of a chemical. A recent article
by Jollow et al. (1974) demonstrated the effect of enzyme saturation
on the metabolism and toxicity of bromobenzene. Bromobenzene was first
metabolically transformed to an epoxide which is hepatotoxic. After a
small nontoxic dose, approximately 75% was converted to the
glutathione conjugate and excreted as bromophenylmercapturic acid.
After a large toxic dose, only 45% was excreted as the mercapturic
acid. It was established that, at the toxic dose, the metabolic
conjugation pathway was overwhelmed due to lack of glutathione, which
resulted in an increased reaction of the epoxide hepatotoxin with DNA,
RNA, and protein.
It is very important to elucidate dose-dependent metabolism to
assess the hazard of a chemical. Frequently, the doses of a chemical
used to characterize toxicity are many times those encountered in the
environment. Toxicity incurred at these large doses may be influenced
by relative changes in metabolism and therefore must be interpreted
with caution and judgment in assessing the hazard of low doses.
4.7 Experimental Design
Since, for the most part, toxicity is a function of the
concentration of the toxicant in the tissues and cells, this
information together with its dynamics provides for inter- as well as
intra-species extrapolation of the results of toxicological effects.
The overall objectives of a chemobiokinetic study are to
determine the amount, rate, and nature of absorption, distribution,
metabolism, and excretion of a chemical. The approach to meeting those
objectives must be flexible and designed to meet the specific needs of
each chemical.
It is difficult to predict, without prior data, an animal species
that will metabolize a chemical similarly to man. Usually, initial
studies are performed in the rat and one other nonrodent species, such
as the dog or monkey, in an attempt to determine species variability.
If there are significant differences among species, it is important to
determine whether differences in the chemobiokinetic parameters
correlate with differences in toxicity or pharmacological activity.
Animals should be acclimatized to the environment of the metabolism
cage prior to the experiment. Light cycle, temperature, humidity, and
time of feeding should be standardized. The physical condition,
weight, and food and water consumption of each animal should be
monitored and recorded throughout the study.
There are advantages in using radioactively-labelled chemicals in
initial studies because of the ease with which radiochemical methods
(Chase & Rabinowitz, 1968) can be applied to chemobiokinetic studies.
An important advantage of using a radioactively-labelled chemical is
that it allows the establishment of the total recovery of the parent
chemical and its metabolites, i.e. the mass balance. To obtain this
the total radioactivity eliminated via the urine, faeces, and exhaled
air as well as that remaining in the carcass following termination of
the experiment should be determined. Until a reasonably good recovery
is obtained, 90% or greater, one can never be sure whether other
chemobiokinetic parameters obtained from the study are accurate.
Furthermore, the isolation and ultimate identification of unknown
metabolites is greatly enhanced by using radioactively-labelled
chemicals.
When using a radiolabelled chemical, the measurement of
radioactivity confirms the presence of the radioisotope, not the
chemical or its metabolites. In order to determine the identity of the
radioactively-labelled compound, the parent chemical and its
metabolites, analytical methods such as gas, high-pressure liquid, and
thin-layer chromatography and a combination of gas chromatography and
mass spectroscopy are frequently employed.
Until it is established that the radioactivity being monitored is
from the chemical in question, kinetic parameters apply to the
radioactivity only, not to the chemical studied. Difficulties can
arise if the radioactive atom does not remain an integral part of the
molecule under study. Tritium and carbon-14 are often incorporated
into the body pools of normal tissue components (Griffiths, 1968;
Rosenblum, 1965). Once the radioactivity is incorporated into these
compartments, its clearance depends on their rates of turnover.
Therefore, by monitoring radioactivity only, it can be falsely assumed
that a compound is being retained in the body.
Another very important reason for differentiating the parent
chemical from its metabolite is to assure that toxic effects that may
be present are associated with the parent chemical and not a
metabolite. Also persistence of a metabolite in the body rather than
the parent chemical may constitute the ultimate hazard.
In initial studies, consideration should be given to the
administration of the compound by intravenous injection as well as via
the route by which man is exposed to the chemical. The intravenous
route is used to provide a more definite assessment of the earlier
phases of distribution and/or elimination. Also, large variation in
rates of absorption will in some cases make the differentiation of the
early phases of distribution and elimination difficult. At least two
doses should be used. One dose should be equivalent to the dose
required to cause signs of toxicity. The second dose should be well
below the toxic dose and, if possible, equivalent to anticipated human
exposure levels.
Most frequently, kinetic parameters for elimination of a chemical
are established by sequential sampling of blood plasma and excreta,
following its administration. A preliminary probe study using one or
two animals is often needed to establish the time at which samples
should be collected, because this will vary with the species and the
chemical in question. After collection and until prepared for analysis
of the chemical or its metabolites, samples should be stored in a
manner that will preclude the breakdown of the chemical or its
metabolites. The data required from the initial chemobiokinetic
studies can be used to design further studies which may include the
following: distribution studies using autoradiography; the isolation
and identification of metabolites; studies to determine the
chemobiokinetic profile of metabolites; biliary excretion studies;
bioconcentration; and in vitro metabolism studies. The methods and
techniques needed to perform these studies are documented by La Du et
al. (1972).
4.8 Chemobiokinetics
Chemobiokinetics aims at quantification of the processes
discussed previously in this chapter. Thus, chemobiokinetics provides
quantitative information on the absorption, distribution,
biotransformation, and excretion of chemicals (including drugs and
endogenous substances) as a function of time. Since the classical
introduction of this discipline by Teorell (1937a,b), the concepts and
methods have been developed extensively, principally for application
to the clinical evaluation and/or use of drugs (Levy & Gibaldi, 1972,
1975; Wagner, 1968, 1971). The reader is also referred to Gehring et
al. (1976) who discuss the subject in greater detail.
One difficulty of many toxicologists and biologists on first
exposure to chemobiokinetics is the concept of compartments. The body
is composed of a large number of organs, tissues, cells, and fluids,
any one of which could be referred to morphologically and functionally
as a compartment. However, in chemobiokinetics, a compartment refers
collectively to those organs, tissues, cells, and fluids for which the
rates of uptake and subsequent clearance of a chemical are
sufficiently similar to preclude kinetic resolution. The rapidly
equilibrating compartment, referred to as the central compartment, may
be comprised of all those tissues with a profuse blood supply whereas
the slow or peripheral compartment may include tissues with a more
limited blood supply, i.e. fat and bone.
4.8.1 One-compartment open model
The simplest chemobiokinetic model is a one-compartment, open
model as shown in Fig. 4.1. In using this model, it is assumed that
the chemical equilibrates with all tissues to which it is distributed
sufficiently rapidly to preclude kinetic differentiation by the
techniques being used to characterize its movement in the body. For
example, if it requires 30 min for a chemical to attain equilibration
in the body after entering the blood stream, and if samples of blood,
tissues, and excreta are taken at 30 min intervals, it will appear
that the body consists of only one compartment.
Assuming that the rate of elimination of the chemical is
proportional to its concentration in the plasma, the concentration in
the plasma will be described by apparent first-order kinetics. The
rate of change of concentration in the plasma may be expressed in the
form of the linear differential equation
dC(t)
= - ke C(t) (1)
dt
where C(t) is the concentration at time t, and ke is the rate constant
for elimination. Solution of this differential equation with initial
condition C(t) = C(0) at time zero gives
C(t) = C(0) exp(- ke t) (exponential form) (2)
or,
(3)
REFERENCES
ALBERT, R. E. & ALTSCHULER, B. (1973) Considerations relating to the
formulation of limits for unavoidable population exposures to
environmental carcinogens. In: Ballon, J. E., ed. Radionuclide
carcinogenesis, Springfield, Va, NIIS, pp. 233-253
(AEC Symposium Series CONF-72050).
ALBERT, R. E. & ALTSCHULER, B. (1976) Assessment of risks in terms of
life shortening, Environ. Health Perspect., 13: 91-94.
ANDREYESHCHEVA, N. G. (1976) Predicting biological effect as a
function of the chemical structure and the primary physical and
chemical properties of organic compounds. Environ. Health
Perspect., 13: 27-30.
ARIENS, E. J., VAN ROSSUM, J. M., & SIMONIS, A. M. (1957) Affinity,
intrinsic activity and drug interactions. Pharmacol. Rev.,
9: 218-236.
BALL, W. L. (1959) The investigation of present-time definitions and
conceptions of maximum allowable concentrations in different
countries. Pr. Lék., 11 (3): 127-128.
BEIR (1972) The effects of population exposure to low levels of
ionizing radiations -- Report of the Advisory Committee on the
Biological Effects of Ionizing Radiation. Washington, DC,
National Academy of Sciences -- National Research Council,
217 pp. (Govt. Printing Office Publication 0-489-797).
BERNARD, C. (1898) [Introduction to the study of experimental
medicine.] Paris, Librairie Delagrave (in French).
BINGHAM, E. & FALK, H. L. (1959) Environmental carcinogens --
modifying effect of cocarcinogens on the threshold response.
Arch. environ. Health, 19: 779-783.
BLUM, H. F. (1959) Carcinogenesis by ultraviolet light. Princeton,
NJ, Princeton University Press.
BROWN, B. M. & PAPWORTH, D. S. (1974) Environmental chemical hazards
to wildlife. In: Boyland, E. & Goulding, R., ed. Modern trends
in toxicology, London, Butterworths, Vol. 2, ch. 3, pp. 70-85.
CASARETT, L. J. (1975) Toxicologic evaluation. In: Casarett, L. J. &
Doull, J., ed. Toxicology, the basic science of poisons. New
York, Macmillan Publishing Co. Inc.
CHAND, N. & HOEL, D. G. (1974) A comparison of models for determining
safe levels of environmental agents. In: Proschan & Sefling, ed.
Reliability and biometry, Philadelphia, SIAM, pp. 382-401.
CLARK, A. J. (1933) Mode of action of drugs on cells. Baltimore,
Williams & Wilkins.
COHEN, J. M. & PINKERTON, C. (1966) Widespread translation of
pesticides by air transport and rain-out. In: Gould, R. F., ed.
Organic pesticides in the environment, American Chemical
Society, pp. 163-176 (Advances in Chemistry Series, vol. 60).
COON, J. (1973) Toxicology of naturally-occurring chemicals: a
perspective. In: Toxicants occurring naturally in foods
(2nd ed.). Washington, DC, National Academy of Sciences.
CORNFIELD, J. (1954) Measurement and comparison of toxicities: the
quantal response. In: Kempthorne, O., Bancroft, T. A., Gowen, J.
W., & Lush, J. L., ed. Statistics and mathematics in biology,
Ames, The Iowa State College Press, pp. 327-344.
CRANSTON, M. (1973) What are human rights? New York, Taplinger Pub.
Co. Inc., p. 111.
CURRY, S. H. (1970) Theoretical changes in drug distribution resulting
from changes in binding to plasma proteins and to tissues.
Pharm. Pharmacol., 22: 753-758.
CUTHBERT, J. W. (1974) Industrial toxicology. In: Boyland, E. &
Goulding, R., ed. Modern trends in toxicology. London,
Butterworths, pp. 86-115.
DAY, T. D. (1967) Carcinogenic action of cigarette smoke condensate on
mouse skin. Br. J. Cancer, 21: 56-81.
DINMAN, B. D. (1972) "Non-concept" of "no-threshold" chemicals in the
environment. Environ. Sci., 175 (4021): 495-497.
DOLL, R. (1967) Prevention of cancer: pointers from epidemiology.
London, Nuffield Provincial Hospital Trust.
DRUCKREY, H. (1967) Qualitative aspects of chemical carcinogenesis.
In: Truhaut, R., ed. Potential carcinogenic hazards from drugs,
evaluation of risks, New York, Springer-Verlag, pp. 60-78.
(UICC Monograph Series, Vol. 7.)
ECOBICHON, D. J. & CORMEAU, A.M. (1973) Pseudocholinesterases of
mammalian plasma: physiochemical properties and organophosphate
inhibition in 11 species. Toxicol. appl. Pharmacol.,
24: 92-100.
EDWARDS, C. A. (1970) Persistent pesticides in the environment.
Cleveland, Ohio, CRC Press.
ELIZAROVA, O. N. (1962) In: Opredelenie porogovyh doz promyslennyh
jadov pri peroral'nom vvedeni, Moscow, Medicina, pp. 107-108
(in Russian).
EPSTEIN, S.S. (1973) The Delaney amendment. J. prev. Med.,
2: 140-149.
FALK, H. L. (1975) Consideration of risks versus benefits. Environ.
Health Perspect., 11: 1-5.
FOOD & DRUG ADMINISTRATION (1970) Food and Drug Administration
Advisory Committee on Protocols for Safety Evaluation: Panel on
Carcinogenesis Report on Cancer Testing in the Safety Evaluation
of Food Additives and Pesticides. Toxicol. appl. Pharmacol.,
20: 419-438.
FINNEY, D. G. (1952a) Statistical method in biological assay,
London, Charles Griffin Ltd, 661 pp.
FINNEY, D. G. (1952b) Probit analysis (2nd ed.). London, Cambridge
University Press.
FINNEY, D. G. (1971) Probit analysis (3rd ed.). London, Cambridge
University Press.
FLYNN, D. I., LYNCH, M., & ZANNONI, V. G. (1972) Species differences
and drug metabolism. Biochem. Pharmacol., 21: 2577-2590.
FRIEDMAN, L. (1969) The role of the laboratory animal study of
intermediate duration for the evaluation of safety. In: Symposium
on the evaluation of the safety of food additives and chemical
residues. Toxicol. appl. Pharmacol., 16: 498-506.
FUCHS, N. A. (1964) The mechanics of aerosols, Oxford, Pergamon
Press, pp. 270-287.
GADDUM, J. H. (1957) Theories of drug antagonism. Pharmacol. Rev.,
9: 211-217.
GAVRILESCU, N. & PETERS, R. A. (1931) Biochemical lesions in vitamin B
deficiency. Biochem. J., 25: 1397-1409.
GOLDWATER, L. J. (1968) Toxicology. In: Sax, N. I., ed. Dangerous
properties of industrial materials, New York, Amsterdam, London,
Reinhold Book Corporation, pp. 1-23.
GOLUBEV, A. F., LJUBLINA, A. E., TOLOKOICEY, N. A., & FILOV, V. A.
(1973) In: Kolicestvennaja toksikologija. Leningrad, Medicina,
p. 217 (in Russian).
GROSS, M. A., FITZHUGH, O. G., & MANTEL, N. (1970) Evaluation of
safety of food additives: an illustration involving the influence
of methyl salicylate on rat reproduction. Biometrics,
26: 181-194.
HAMAKER, J. W., GORING, C. A. L., & YOUNGSON, C. R. (1966) Sorption
and leaching of 4-amino-3,5,6-trichloropicolinic acid in soils.
In: Gould, R. F., ed. Organic pesticides in the environment,
American Chemical Society, pp. 23-37 (Advances in Chemistry
Series, Vol. 60).
HATCH, T. F. (1968) Significant dimensions of the dose-response
relationship. Arch. environ. Health, 16: 571-578.
HATCH, T. F. & GROSS, P. (1964) Pulmonary deposition of inhaled
aerosols. New York, Academic Press.
HERMAN, R. S. (1974) Induction of liver growth by xenobiotic compounds
and other stimuli. CRC crit. Rev. Toxicol., 3(1): 97-158.
HEWLETT, P. S. & PLACKETT, R. L. (1961) Models for quantal responses
to mixtures of two drugs. In: Jonge, H. de, ed. Symposium on
quantitative methods in pharmacology. Amsterdam, North Holland
Publishing Co., pp. 328-336.
HOEL, D. G., GAYLOR, D. W., KIRSCHSTEIN, R. L., SAFFIOTTI, U., &
SCHNEIDERMAN, M. S. (1975) Estimation of risks of irreversible
delayed toxicity. J. Toxicol. environ. Health, 1(1): 133-151.
HUCKER, H. B. (1970) Species differences in drug metabolism.
Ann. Rev. Pharmacol., 10: 99-118.
ICRP (1965) Principles of environmental monitoring related to the
handling of radioactive materials. Report of Committee IV of the
International Commission on Radiological Protection. London,
Pergamon Press.
ICRP (1966) The evaluation of risk from radiation, a report prepared
for Committee I of the International Commission on Radiological
Protection. Oxford, London, Edinburgh, New York, Toronto, Paris,
Braunschweig, Pergamon Press (ICRP Publication 8).
ICRP (1977) Recommendations of the International Commission on
Radiological Protection (ICRP Publication 26 (in press)).
JANYSEVA, H. JA (1972) [On the establishment of MPCs of benz(a)pyrene
in the ambient air of built-up areas.] Gig. i Sanit.,
No. 7, pp. 87-91 (in Russian).
JANYSEVA, H. JA & ANTOMONOV, JU G. (1976) Predicting risk of tumour
occurrence in terms of life-shortening. Environ. Health
Perspect., 13: 95-100.
JONES, H. B. & GRENDON, A. (1975) Environmental factors in the origin
of cancer and estimation of possible hazards to man. Food
cosmet. Toxicol., 13: 251-267.
KAGAN, Y. S. (1973) [Methods of quantitative estimation of combined
and complex action on the organism of physical and chemical
factors in the environment.] Gig. i Sanit., No. 2, pp. 89-92
(in Russian).
KELLERMANN, G., SHAW, C. R., & LUYTEN-KELLERMANN, M. (1973) Aryl
hydrocarbon hydroxylase inducibility and bronchogenic carcinoma.
New Engl. J. Med., 289: 934-937.
KORBAKOVA, A. K., SUMSKAJA, N. I., ZAEVA, G. N., & NIKITENKO, T. K.
(1971) In: Naucnye osnovy sovremmenyh metodov gigieniceskogo
normirovanija himiceskih vescest'v v okruzajuscej srede.
Moscow, Institut gigieny truda i profzabolevanija AMN SSSR,
pp. 35-39.
KRASOVSKIJ, G. N. (1965) [Methodology for conducting an acute
experiment and for evaluating the results, and its basis.] In:
Sanitarnaja ohrana vodoemov ot zagrjaznenija promyslennymi
stocnymi vodami. No. 7, 247-268 (in Russian).
KRASOVSKIJ, G. N. (1970) [On methods for studying the cumulative
properties of toxic compounds.] Gig. i Sanit., No. 3, pp. 83-88
(in Russian).
KRASOVSKIJ, G. N. (1975) Species and sex differences in sensitivity to
toxic substances. In: Methods used in the USSR for establishing
biologically safe levels of toxic, Geneva, WHO, pp. 109-125.
KRASOVSKIJ, G. N. (1976a) [General standardization of biological
parameters for mammals according to body weight, and some
practical aspects of investigations.] In: Trudy MMI im Secenova
T.80 -- Novoe v metodah issledovannija, diagnostiki, lecenii i
profilaktiki vazneisih zabolevanii. Pp. 64-88 (in Russian).
KRASOVSKIJ, G. N. (1976b) Extrapolation of experimental data from
animals to man. Health Perspect., 13:51-58.
KUSTOV, V. V. & TIUNOV, L. A. (1970) [Enzyme system activity as a
means of estimating thresholds of the effects of toxins.] In:
Metody opredelenija toksicinosti i opasnosti himiceskih
vescestv. Moscow, Medicina, pp. 231-234 (in Russian).
KUSTOV, V. V., ZDANOV, A. M., & JUNOVSKIJ, G. D. (1974) [On the
evaluation of the combined effect of factors by the method of
partial regression.] Gig. Tr. Prof. Zabol., 16:33-36
(in Russian).
LAZAREV, N. V. (1938). Obscie osnovypromyslennoj toksikologii.
Moscow & Leningrad, Medgiz.
LAZAREV, N. V. (1963) Rukovodstvo po gigiena truda, Moscow, Medgiz.
LAZAREV, N. V. & BRUSILOVSKAJA, A. I. (1934) Fiziol. Z. SSSR, XVII,
pp. 611-619 (in Russian).
LJUBLINA, E. I. & MIHEEV, M. I. (1974) [Scientific bases for
establishing tentative safe levels of substances affecting man.]
In: Zurnal Vsesjuznogo Obcestva im. D. I. Mendeleeva,
XIX (2): 142-145 (in Russian).
LOEWE, S. (1959) Relationships between stimulus and response.
Science, 130: 692-695.
LU, P. Y. & METCALF, R. (1975) Environmental fate and biodegradability
of benzene derivatives as studied in a model aquatic ecosystem.
Environ. Health Perspect., 10: 269-284.
MANTEL, N. & BRYAN, W. R. (1961) Safety testing of carcinogenic
agents. J. Natl Cancer Inst., 27: 455-470.
MANTEL, N. & SCHNEIDERMAN, M. (1975) Estimating "safe" levels, a
hazardous undertaking. Cancer Res., 35: 1379-1386.
MANTEL, N., BOHIDAR, N., BROWN, C., CIMINERA, J., & TUKEY, J. (1975)
An improved "Mantel-Bryan" procedure for "safety" testing of
carcinogens. Cancer Res., 35: 865-872.
MAZZE, R. I., COUSINS, M. J., & KOSEK, J. C. (1973) Strain differences
in metabolism and susceptibility to the nephrotoxic effects of
methoxyfluorane in rats. J. Pharmacol. exp. Therapy,
184: 481-488.
MEDVED, L. I. & SPYNU, E. I. (1970) [Principles and methods of
hygienic standardization of pesticides. In: Principles and
methods of establishing maximum permissible concentrations of
harmful substances in the air of production premises.] Moscow,
Medicina (in Russian).
METCALF, R. L., KAPOOR, I. P., LU, P. Y., SCHUTH, C. K., & SHERMAN, P.
(1973) A model ecosystem for the evaluation of pesticide
biodegradability and ecological magnification. Environ. Sci.
Technol., 5: 709-744.
MILLER, E. C., MILLER, J. A., & ENOMOTOR, M. (1964) The comparative
carcinogenetics of 2-acetylaminofluorene and its N-hydroxy
metabolite in mice, hamsters and guinea pigs. Cancer Res.,
24: 2018-2032.
MONTESANO, R., MOHR, U., & MAGEE, P. N. (1974) Additive effect in the
induction of kidney tumours in rats treated with
dimethylnitrosamine and ethylmethanesulfonate. Br. J. Cancer,
29: 50-58.
MURPHY, S. D. (1969) Some relationships between effects of
insecticides and other stress conditions. Ann. NY Acad. Sci.,
160: 366-377.
NAS/NRC (1970) Evaluating the safety of food chemicals. Food
Protection Committee, Food and Nutrition Branch, Division of
Biology and Agriculture, National Research Council, Washington
DC, National Academy of Sciences. 55 pp.
NAS (1975) Principles for evaluating chemicals in the environment --
A report of the Committee for the Working Conference on
Principles of Protocols for Evaluating Chemicals in the
Environment. Washington, DC, National Academy of Sciences.
NIEHS (1970) Man's health and the environment, some research needs --
Report of the Task Force on Research Planning in Environmental
Health Science US Department of Health, Education and Welfare,
Public Health Service, National Institutes of Health, National
Institute of Environmental Health Sciences. Washington, DC, US
Govt Printing Office.
NIEHS (1977) Human health and the environment, some research needs --
Report of the Second Task Force for Research Planning in
Environmental Health US Department of Health, Education and
Welfare, Public Health Service, National Institutes of Health,
National Institute of Environmental Health Sciences. Washington,
DC, US Govt Printing Office (DHEW Publication No. NIH77-1277).
OECD (1977) The OECD programme on long range transport of air
pollutants. Measurements and findings. Paris, Organisation for
Economic Co-operation and Development, 268 pp.
OWEN, D. B. (1955) Handbook of statistical tables, London, Paris,
Pergamon Press, pp. 127-137.
PAGET, G. E. (1970) The design and interpretation of toxicity tests.
In: Paget, G. E., ed. Methods in toxicology, Philadelphia, F.
A. Davies, pp. 1-10.
PARKE, D. V. (1975) Induction of the drug-metabolizing enzymes. In:
Parke, D. V., ed. Enzyme induction, London, Plenum Press,
pp. 207-272.
PETERS, R. A. (1963) Biochemical lesions and lethal synthesis.
Oxford, London, New York, Paris, Pergamon Press.
PETERS, R. A. (1967) The biochemical lesion in thiamine deficiency.
In: Wolstenholme, G. E. W. & O'Connor, M., ed. Thiamine
deficiency, biochemical lesions and their significance, London,
J. & A. Churchill Ltd, pp. 1-8.
PETO, R. & LEE, P. N. (1973) Weibull distribution for continuous
carcinogenesis experiments. Biometrics, 29: 457-470.
PETO, R., LEE, P. N., & PAIGE, W. S. (1972) Statistical analysis of
the bioassay of continuous carcinogens. Br. J. Cancer,
26: 258-261.
PFITZER, E. A. (1976) General concepts and definitions for
dose-response and dose-effect relationships of toxic metals. In:
Nordberg, G., ed. Effects and dose-response relationships of
toxic metals. Amsterdam, Oxford, New York, Elsevier Scientific
Publishing Company.
PINIGIN, M. A. (1974) [Current problems of community toxicology in
relation to chemical air pollution.] In: Itogi nauki i tehniki.
Serija farmakologija. Himioterapevticeskie sredstva.
Toksikologija. Problemy toksikologii. Vol. 6, Moscow
(in Russian).
POKROVSKIJ, A. A. (1973) [Biochemical approaches to the evaluation of
toxic factors in the environment. In: Documentation of the
Conference on Methodological Approaches to the Study and
Evaluation of Toxic Factors in the Environment.] Moscow
(in Russian).
PORTMAN, R. S., PLOWMAN, K. M., & CAMPBELL, T. C. (1970) On mechanisms
affecting species susceptibility to aflatoxin. Biochim. Biophys.
Acta, 208: 487-495.
PRAVDIN, N. S. (1934) Rukovodstovo promyslennoi toksikologii.
Moscow, Biologija i Medicina.
RALL, D. F. (1970) Animal models for pharmacological studies. In:
Proceedings of the Symposium on Animal Models for Biomedical
Research III, Washington, DC, National Academy of Sciences,
pp. 125-146 (ISBN 0-309-01854-4).
SABAD, L. M., SANOCKIJ, I. V., ZAEVA, G. N., BUREVIC, T. C.,
KACNELSON, B. A., JANYSEVA, H. JA, & SUGAEVA, B. V. (1973) [On
the possibility of establishing MPCs for benz(a)pyrene in the air
of industrial premises.] Gig. i Sanit., No. 4, pp. 78-81
(in Russian).
SAFFIOTTI, U. (1973) Comments on the scientific basis for the "Delaney
Clause". J. Prev. Med., 2: 125-132.
SANOCKIJ, I. V. (1962) [The calculation of a safety factor for
experimentally based maximum permissible concentrations of
industrial poisons. In: Industrial toxicology and clinical
aspects of occupational diseases of chemical etiology.] Moscow,
Medgiz (in Russian).
SANOCKIJ, I. V. (1970) In: Sanockij, I. V., ed. Metody opredelenija
toksicnosti i opasnosti himiceskih vescestv. Moscow, Medicina,
pp. 11-12 (in Russian).
SANOCKIJ, I. V. (1975a) Investigation of new substances: permissible
limits and threshold of harmful action. In: Methods used in the
USSR for establishing biologically safe levels of toxic
substances, Geneva, WHO, pp. 9-18.
SANOCKIJ, I. V. (1975b) [The threshold concept for the reactions of
living systems to environmental influences and its consequences.
Soviet-American Symposium, Tbilisi.] Gidrometeoizdat,
pp. 112-120 (in Russian).
SANOCKIJ, I. V. & SIDOROV, K. K. (1975) [The current status of the
problem of safety factors in establishing the maximum permissible
concentrations of substances in the components of the general
environment.] Gig. i Sanit., No. 7, pp. 93-96 (in Russian).
SATO, T. & MOROI, K. (1971) Species and age differences in the
activity of isocarboxazid hydrolysing enzyme. Arch. int.
Pharmacodyn. Ther., 192: 128-134.
SCHILD, H. O., ARIENS, E. J., SIMONIS, A. M., DIKSTEIN, S., DE JONGH,
S. E., HEWLETT, P. S., & PLACKETT, R. L. (1961) Section on
mixtures of drugs. In: De Jonge, H. ed. Quantitative methods in
pharmacology, Proceedings of a Symposium held in Leyden on
May 10-13, 1960, Amsterdam, North Holland Publishing Company,
pp. 282-334.
SCHNEIDERMAN, M. A. (1971) A method for determining the dose
compatible with some "acceptable" level of risk. In: Chemicals
and the future of man -- Hearings before the Sub-Committee on
Executive Reorganization and Government Research of the Committee
on Government Operations, US Senate, Ninety-Second Congress,
First Session, 6 and 7 April 1971. Washington, DC, US Govt
Printing Office.
SCHNEIDERMAN, M. A. & MANTEL, N. (1973) The Delaney Clause and a
scheme for rewarding good experimentation. Prev. Med.,
2: 165-170.
SIDORENKO, G. I. & PINIGIN, M. A. (1975) Establishment of safe levels
of chemicals in communal hygiene: methodological approaches. In:
Methods used in the USSR for establishing biologically safe
levels of toxic substances, Geneva, WHO, pp. 126-138.
SIDORENKO, G. I. & PINIGIN, M. A. (1976) Concentration-time
relationship for various regimens of inhalation of organic
compounds. Environ. Health Perspect, 13: 17-21.
SMYTH, H. F., JR (1963) Industrial hygiene in retrospect and
prospect-toxicological aspects. Am. Ind. Hyg. Assoc. J.,
24: 222-226.
SMYTH, H. F. JR, WEIL, C. S., WEST, J. S., & CARPENTER, C. P. (1969)
An exploration of joint toxic action: twenty-seven industrial
chemicals intubated in rats in all possible pairs. Toxicol.
appl. Pharmacol, 14: 340-347.
SPYNU, E. I., VROCINSKIJ, K. K., ZOR'EVA, T. D., & MAL'KO, N. N.
(1972) [Complex hygienic standardization of new organophosphorus
pesticides in environmental components.] Gig. i. Sanit.,
No. 11, pp. 96-99 (in Russian).
STOKINGER, H. E. (1972) Concepts of thresholds in standards setting.
Arch. environ. Health, 25 (3): 153-157.
SYSIN, A. M., ed. (1941) [Maximum allowable concentrations of toxic
substances in water bodies.] Moscow and Leningrad, Stroizdat
(in Russian).
TASK GROUP ON METAL ACCUMULATION (1973) Accumulation of toxic metals
with special reference to their absorption, excretion and
biological half-time. Environ. Physiol. Biochem., 3: 65-107.
TASK GROUP ON METAL TOXICITY (1976) Consensus report for an
international meeting organized by the Sub-committee on the
Toxicology of Metals of the Permanent Commission and
International Association on Occupational Health, Tokyo, 18-23
November 1974. In: Nordberg, G., ed. Effects and dose-responses
relationships of toxic metals, Amsterdam, Oxford, New York,
Elsevier Scientific Publishing Company, pp. 7-111.
ULANOVA, I. P. (1969) [Problems of hygienic standardization of
mixtures of gases and vapours of chemical substances. In:
The toxicology of new industrial chemicals.] Moscow, Medicina,
pp. 33-39 (in Russian).
ULANOVA, I. P., AVILOVA, G. G., BAZAROVA, L. A., MAL'CEVA, N. M.,
MIGUKINA, N. V., HALEPO, A. I., & EITINGTON, A. I. (1973)
[Experimental data on adaptation to poisons under different
conditions of their action. Pharmacology, chemotherapeutic
substances, toxicology.] Itogi nauk i teh., No. 5, pp. 64-75
(in Russian).
ULANOVA, I. P., AVILOVA, G. G., MAL'CEVA, N. M., & HALEPO, A. I.
(1976) [Comparisons of reactions of the organism to continuous
and intermittent action of some chlorinated hydrocarbons.]
Gig. Tr. Profzabol., No. 7, pp. 22-25 (in Russian).
VAN NOORDWIJK, J. (1964) Communication between the experimental animal
and the pharmacologist. Stat. Neerl., 18 (4): 403-416.
VELDSTRA, H. (1956) Synergism and potentiation. Pharmacol. Rev.,
8: 339-387.
VETTORAZZI, G. (1975) Toxicological decisions and recommendations
resulting from the safety assessment of pesticide residues in
food. Crit. Rev. Toxicol., 4 (2): 125-183.
VETTORAZZI, G. (1977) Safety factors and their application in
toxicological evaluation. In: Hunter, W. J. & Smeets, J. G. P.
M., ed. The evaluation of toxicological data for the protection
of public health. Oxford, Pergamon Press (for the Commission of
the European Communities) pp. 207-223.
WEIL, C. S. (1972a) Statistics versus safety factors and scientific
judgment in the evaluation of safety for man. Toxicol. appl.
Pharmacol., 21: 454-463.
WEIL, C. S. (1972b) Guidelines for experiments to predict the degree
of safety of a material for man. Toxicol. appl. Pharmacol.,
21: 194-199.
WHO (1958) WHO Technical Report Series, No. 144 (Procedures for the
testing of intentional food additives to establish their safety
for use -- Second Report of the Joint FAO/WHO Expert Committee on
Food Additives.) 19 pp.
WHO (1973) WHO Technical Report Series, No. 535 (Environmental and
health monitoring in occupational health -- Report of a WHO
Expert Committee.) 48 pp.
WHO (1974a) WHO Technical Report Series, No. 546 (Assessment of
carcinogenicity and mutagenicity of chemicals -- Report of a WHO
Scientific Group.) 19 pp.
WHO (1974b) WHO Technical Report Series, No. 554 (Health aspects of
environmental pollution: planning and implementation of national
programmes -- Report of a WHO Expert Committee.) 57 pp.
WHO (1975a) WHO Technical Report Series, No. 571 (Early detection of
health impairment in occupational exposure to health hazards --
Report of a WHO Study Group.) 80 pp.
WHO (1975b) WHO Technical Report Series, No. 560 (Chemical and
biochemical methodology for the assessment of hazards of
pesticides for man -- Report of a WHO Scientific Group.) 26 pp.
WHO (1975c) Methods for studying biological effects of pollutants (a
review of methods used in the USSR). Report of a Working Group,
Moscow, November 1974. Copenhagen, WHO Regional Office for
Europe.
WHO (1976a) WHO Technical Report Series No. 586 (Health hazards of new
environmental pollutants -- Report of a WHO Study Group.) 96 pp.
WHO (1976b) Health aspects of human rights with special reference to
developments in biology and medicine. Geneva, World Health
Organization, pp. 25-27.
WHO (1977) Environmental Health Criteria 4: Oxides of nitrogen.
Geneva, WHO, 79 pp.
WILLIAMS, R. T. (1969) The fate of foreign compounds in man and
animals. Pure appl. Chem., 18: 129-141.
WILLIAMS, R. T. (1972) Toxicological implications of biotransformation
by intestinal microflora. Toxicol. appl. Pharmacol.,
23: 769-781.
2. FACTORS INFLUENCING THE DESIGN OF TOXICITY STUDIES
2.1 Introduction
The choice and sequence of toxicity tests will depend on the
questions or hypotheses that are developed. The nature and sequence of
tests used to satisfy requirements of regulatory agencies may differ
markedly from those used in an investigation of the basic mechanisms
of toxic action. Differences in approach will also depend on whether
the investigation is initiated to evaluate the toxicity of a chemical
prior to its introduction into use, i.e. prospective toxicology, or to
confirm in laboratory animals an epidemiological association that
suggests chemical-induced disease in man, i.e. retrospective
toxicology. Under ideal conditions, prospective toxicology will
eliminate the need for retrospective toxicity evaluation.
National or international regulatory or advisory bodies have
developed fairly specific guidelines or test protocols which are
expected to be applied to the toxicity evaluation of certain groups of
chemicals, introduced deliberately into our environment, e.g. food
additives and pesticides (Council of Europe, 1973; FDA, 1959; WHO,
1967). The development of guidelines for the systematic evaluation of
the toxicity of chemicals to which man is exposed, through his
occupation or through incidental contamination of the ambient
environment, is less common. Where specific guidelines have been
formulated, they usually require: test information on acute toxicity
in several species of experimental animals; some knowledge of the
biochemical disposition of the compounds; various short-term toxicity
tests; tests of the effects of the chemical on reproductive function;
chronic toxicity tests in one or more species; special tests on organ
function, clinical biochemistry and haematology, and other specific
tests as determined by the particular type and intended uses of the
chemical under consideration. Some commercial firms have developed
their own guidelines for toxicity tests and their sequence in the
premarket toxicological evaluation of products. Often the sequence of
tests will have certain checkpoints at which decisions will be made as
to whether continued development of the product (and more extensive
toxicity testing) is warranted.
In this chapter, various topics will be discussed from the
standpoint of the usefulness of certain types of information and the
influence that various factors may have in designing protocols for the
interpretation of data obtained in toxicity evaluation programmes.
2.2 Chemical and Physical Properties
2.2.1 General considerations
The late Horace Gerarde stated in an address that "toxicity is
the capacity of a substance to cause injury. It is an inherent,
unalterable molecular property which is dependent upon chemical
structure. There is nothing we can do about the toxicity of a chemical
except to know it"a.
The nature or quality of the toxic action inherent in a chemical
will depend to a large extent upon the functional group or groups
present in the molecule. Knowledge of the reactions that these
functional groups may undergo with reactive groups in critical
endogenous biochemical constituents provides a means of predicting the
nature of the toxic effects that may be expected. Smyth (1959) used
the permanence of threshold limit values over a period of years as a
criterion for evaluating various types of information used in setting
safety limits for industrial chemicals. For a limited number of
compounds, occupational threshold limit values (TLVs) had been
established on the basis of analogy with better known substances and
the permanence of these TLVs appeared to equal that of TLVs based upon
data from experimental toxicity studies and from human experience.
However, evaluation of toxicity by analogy with chemically-related
substances contains considerable potential for error, and requires a
great deal of toxicological information on very closely related
chemical substances. Very minor changes in structure may be
accompanied by profound changes in toxicity. The relationships between
physicochemical characteristics and toxicity, including the biological
activities of homologous series, have been reviewed by Ljublina &
Filov (1975).
2.2.2 Physicochemical properties and the design of toxicity studies
Zbinden (1973) included fourteen chemical and physical variables
in a check list of types of information useful in the toxicity
evaluation of new drugs. Although some of these variables may be
determined in the course of a toxicological evaluation, all of them
apply equally as well to environmental chemicals as to therapeutic
chemicals.
a Presented at the Flavor Manufacturers' Association of the United
States. Fall Symposium, 16 November 1972, Washington, DC.
Knowledge of the chemical structure is essential for the
preliminary prediction of the nature and site of toxic action,
assuming, of course, that some prior knowledge of the toxicity of
chemically-related compounds is available. It is also essential for
developing extraction and assay procedures for the determination of
tissue concentrations and allows for logical estimates of the nature
of metabolites that may be found. Indeed, without such knowledge,
logical design of an experiment is impossible.
The stability of the chemical at various pH values and the
photochemical properties are variables that must be considered as soon
as a substance arrives for testing in the toxicology laboratory, as
they may determine the manner in which the chemical should be stored
prior to administration to test animals or indicate the stability of
residues in tissue extracts. Many organic chemicals undergo
photochemical reactions that lead to either more or less toxic
products (Crosby, 1972) and organic esters are often readily
hydrolysed under conditions encountered during their laboratory
investigation (Eto, 1974). It may be necessary to exercise special
precautions to avoid chemical reactions during the preparation and
storage of test solutions or diets and during the analysis of tissues
and metabolic reaction mixtures. Furthermore, if a chemical is likely
to become activated by photochemical reactions, special tests for
phototoxicity may be required.
The organic solvent/water partition coefficient and pK are
physical properties of particular importance in the determination of
the absorption and distribution of a compound in living organisms as
well as in the development of appropriate extraction and assay
procedures for the chemical. Hansch & Dunn (1972) reviewed numerous
studies which suggest that characterization of the lipophilic nature
of compounds may allow systematic predictions of their relative
biological activities. Dillingham et al. (1973) applied these
principles when they compared the toxicity of substituted alcohols in
a tissue culture with their acute toxicity in mice and concluded that
tissue culture test systems may be useful in determining predictive
correlations between in vivo toxicity and the physicochemical
properties of compounds.
The extent of ionization of an organic compound will influence
its passage through lipoidal membranes (La Du et al., 1971). In
general, the unionized lipid-soluble form of an organic compound will
most readily pass through biological membranes. Although most of the
physicochemical principles of absorption and distribution have been
developed through systematic studies on medicinal chemicals, these
principles also apply to organic chemicals in the environment and to
the design of toxicity experiments (Loomis, 1974; also Chapter 4).
Patty (1958) discussed the influence of oil and water solubility and
of the coefficient of distribution of a vapour between blood and
alveolar air on the rates of equilibrium saturation and desaturation
of the body during inhalation experiments.
Particle size, shape, and density are of obvious importance in
studying the inhalation toxicities of aerosols, as they are important
factors in the determination of the site of deposition and the rates
and mechanisms of clearance from the respiratory tract (Hatch & Gross,
1964; also Chapter 6). Furthermore, the particle size of substances
given orally as suspensions can also markedly influence their toxicity
(Boyd, 1971). If critical judgements based on the relative toxicities
of the same or different substances, administered orally in
suspension, are to be made, it is necessary to ensure uniformity of
particle size.
Vapour pressure of a chemical substance is important in the
practical consideration of the likelihood of exposure of man through
inhalation, and the design of experimental inhalation toxicity studies
will be influenced by the ease with which a solid or liquid vaporizes
under controlled conditions. However, a high vapour pressure may
produce technical problems if the objective of the test is to
determine toxicity by the oral route of administration. Studies by
Jones et al. (1971) showed that for a large series of food flavouring
agents mixed in laboratory animal diets, the amount of loss from the
diet was inversely related to the boiling points of the flavouring
agents. Frequent chemical analyses of the diet, as well as frequent
preparation of fresh diets and/or restricted feeding periods to limit
the time for loss by vaporization are necessary to provide accurate
estimates of intake in feeding studies on substances with low boiling
points.
Knowledge of reactivity with, or binding to, macromolecules may
allow specific design of mechanism experiments, when these
macromolecules are essential tissue and cell constituents. Knowledge
of the chemical reactivity of a substance may also be of considerable
importance in the early planning of feeding studies, if the chemical
under test is likely to react with the macromolecules present in the
laboratory diet. On the one hand, chemical binding or adsorption on
macromolecules in the diet may markedly alter the rate and extent of
absorption of the test compound from the gastrointestinal tract; in
some cases, biologically reactive groups on the test chemical may be
neutralized by dietary constituents. On the other hand, reaction of
the test chemical with essential dietary constituents may contribute
to nutritional deficiency states, or new and more toxic compounds may
be formed. Several examples of these types of reactions have been
summarized by Golberg (1967).
2.2.3 Impurities
In the design of toxicity experiments, it is extremely important
to consider the chemical purity of the sample to be tested. In certain
cases, e.g. food additives and pesticides, the regulations will often
provide specifications of purity for the compound in actual use and
recommended test protocols may specify that toxicity evaluations are
to be conducted with samples that meet these specifications (WHO,
1967). However, there is always the possibility that, when testing the
technical product, the biological effects observed may be due to, or
modified by, trace contaminants. If the contaminants are unknown or
their biological activity unsuspected, toxicity tests may lead to
erroneous conclusions concerning the primary chemical in question. In
contrast, tests on highly purified samples may not detect the toxic
action of contaminants present in samples used commercially.
Furthermore, for many chemical substances used in manufacturing or
incidentally released into the environment, specifications of purity
may not be standardized. Therefore, one of the earliest and most
difficult decisions that must be made in the design of a toxicity
evaluation programme is the selection of the sample to be studied
(technical grade, highly purified, etc.)
Requirements for the purity of the compounds selected for
toxicological testing depend on the purpose of the testing as
discussed in section 2.1. During the development of a new
technological process, it may even be useful to test mixtures of
unknown composition. The information so obtained may alert organic
chemists in the research laboratory or pilot plant operators to the
possible hazards of these unknown mixtures which may vary as
procedures develop and improve. However, data obtained from such
studies will have a limited value. The determination of health
standards requires a compound of a high degree of purity or of highly
standardized composition combined with a precise knowledge of various
impurities. Only in this way will health standards have a universal
value. However, for practical purposes, and for extrapolation to human
exposure conditions it may be prudent, when a technical grade product
standardized by specifications is used in commerce, to select this
grade for toxicity testing and carefully characterize it with respect
to the nature and amounts of any impurities present. Scheduled
analytical spot checks during the course of the experiment to provide
assurance of chemical constancy is also desirable.
When a chemical substance used in commerce is not standardized
with respect to specifications for purity, experimental toxicity
evaluation on a test sample of high purity would, indeed, seem to be
the most rational selection. Data derived from this compound could
then be used to characterize, toxicologically, the action of the
primary chemical under consideration. When certain quantifiable
indices of toxicity have been identified, selected tests with typical
samples of commercial products could be conducted and the results
compared with the purified product to detect possible differences. Of
course, if the impurities represent a significant portion of the
product, or if their chemical properties or their chemical analogy to
other known substances suggest they may have serious toxic properties,
the impurities must be evaluated separately.
An alternative approach is to select a sample of a chemical that
most nearly represents the impure product used commercially, subject
this to comprehensive toxicity evaluation and then make selected,
critical toxicity comparison with purified samples of the primary
chemical as well as with the impurities.
Either approach contains uncertainties, and little would be
gained by short-term spot checks if the toxic action of the impurity
were only detectable after long-term exposure or a latent period. It
is in problem situations such as these that some of the
chemicophysical principles discussed earlier must be applied at
several levels of decision making: the sample to choose for testing;
the design of the evaluation protocol; or the decision (or regulation)
to produce a purer substance for routine use in commerce.
A recent, most controversial problem arising from the
contamination of a primary commercial product involves the apparent
teratogenic action of the herbicide (2,4,5-trichlorophenoxy)acetic
acid (2,4,5-T) (Panel on Herbicides 1971). It is now known that the
first studies to reveal this action were conducted with a sample of
the herbicide that contained a rather high concentration (about
30 mg/kg) of the contaminant 2,3,7,8-tetrachloro-dibenzo-4-dioxin
which is formed during the synthesis of the trichlorophenol precursor.
Tetrachlorodioxin is extremely toxic. For guineapigs, the ratio of the
LD50 for 2,4,5-T to the LD50 of the dioxin is about 630 000. In
female rats, the acute oral LD50 for dioxin is about 1/10 000 of the
oral LD50 for 2,4,5-T. The daily dose of dioxin in pregnant rats
that produced fetal toxicity was only about one four-hundredth of the
maternal LD50 of dioxin or about one four-millionth of the
single-close oral LD50 of 2,4,5-T for female rats. Thus, even if the
concentration of dioxin as a contaminant of 2,4,5-T is kept below
0.5 mg/kg, the major concern for the toxicity of 2,4,5-T should
apparently still be directed towards the contaminant rather than
towards the herbicide itself (at least insofar as effects on
reproduction are concerned).
This kind of problem is not new. Twenty-five years ago, marked
differences in the toxicity of different samples of the insecticide
parathion were traced to contamination with small quantities of the
oxygen analogue and the phosphorothiolate isomer, which are much more
potent anticholinesterases and more acutely toxic than the parent
insecticide (Diggle & Gage, 1951).
2.3 Probable Routes of Exposure
2.3.1 General considerations
Many chemicals will become distributed in various environmental
media or will be used for different purposes, and substantially
different populations may be at risk. Thus, it may be necessary to
obtain extensive test data by different routes of exposure. The choice
of route for practical purposes is generally dictated by: (a) the
likely route by which man will be exposed; and (b) whether the
chemical will produce local injury at the site of exposure. The second
question will often be resolved by acute or short-term studies on
animals dosed by oral, inhalation, dermal, and possibly ocular routes.
Details of test procedures are included in subsequent chapters.
Although it is usually wise to conduct experiments using the route
through which man will be exposed, other more convenient routes may be
chosen for many of the tests if it is determined that the major toxic
effects of a chemical are systemic, occurring only after absorption
and distribution in the body. Data on blood and tissue levels should
be obtained by several routes of exposure, including those that are
considered primarily experimental; with this information, it may be
possible to relate toxic effects to blood or tissue concentrations of
the test chemical and its metabolites. Such information greatly
facilitates comparison of experiments using different routes and may
either confirm or deny the validity of extrapolating data, obtained
experimentally by one route, to the evaluation of potential toxicity
by another perhaps more realistic route of exposure.
2.3.2 Specific variables related to route of exposure
2.3.2.1 Rate of absorption
As a general rule, one can predict that for the usual routes by
which man may be exposed, absorption of chemicals will be most rapid
when given by inhalation, less rapid when given by gavage, and slowest
with dermal application. This order may, however, be modified
depending upon various physicochemical properties of the substance
under test in relation to the microenvironment of the absorbing
surface (Klassen, 1975; Loomis, 1974). The rate of absorption will be
one determinant of the rate of onset of signs of acute poisoning. If
the rates of detoxification and excretion or of injury repair exceed
the limiting rate at which a chemical is absorbed, it is possible that
toxic signs observed by one route of administration will not be
detectable by another route for which absorption is slower (Casarett,
1975; Murphy, 1975). Comparative absorption-distribution kinetics by
different routes would determine such a possibility.
2.3.2.2 Site of action
Specific tests should be conducted to evaluate effects related to
local reactions with specific receptors present in the organ of
absorption. These may be morphological tests to detect evidence of
irritation, inflammation, or oedema, or they may be functional to
detect biochemical or reflex action or bronchoconstriction. In
addition, the route of exposure may determine the organ or
physiological system in which effects will be first observed or
detected at lowest doses. For example, pesticides, which are direct
inhibitors of acetylcholinesterase, when given in sufficient doses by
any route, will produce a characteristic toxic syndrome involving
essentially all organs or structures innervated by cholinergic nerves.
However, at low doses, only specific organs may be involved. If such
compounds are applied to the skin, local sweating and fasciculations
may occur in the absence of signs of systemic poisoning. Exposure by
inhalation may result in bronchoconstriction, exposure by ingestion
may cause gastrointestinal upset before, or at lower doses than,
generalized systemic effects (Henderson & Haggard, 1943; Holmstedt,
1959).
2.3.2.3 Biotransformation
The route of exposure may determine the likelihood and type of
biotransformation before the chemical contacts the specific sites of
action. Thus, when chemicals are administered by the oral (or
intraperitoneal) routes, they will be absorbed and transported first
through the portal circulation to the liver (Lukas et al., 1971). For
example, if, with low oral or dietary doses, the capacity of the liver
to detoxify the compounds exceeds the rate of absorption, an effective
injurious concentration may never reach critical sites of action in
other tissues. Absorption of the same quantity through the lung or
skin, which generally have less detoxifying capacities, may result in
toxic action. It is now known that the lung, skin, and intestinal
mucosa, although generally less active than the liver, also have the
capacity for biotransformation of foreign organic chemicals (Alvares
et al., 1973; Fouts, 1972; Lake et al., 1973; Wattenberg, 1972).
Although knowledge is incomplete with regard to the tissue
distribution of both activating and detoxifying enzyme systems, it is
likely that their relative distribution will determine, to some
extent, the specific tissues that will be most affected by low doses
of some compounds, when given by different routes of exposure.
2.3.2.4 Species
The relative susceptibility of different species to the action of
chemicals may differ depending upon the route of exposure. When
administering compounds by the oral route, such factors as vomiting
reflex (absent in rats) and/or differences in type and distribution of
microflora that may detoxify (or activate) the test compound can
influence the interpretation of the results (Williams, 1972). The
rates of penetration of compounds through the skin and the acute
dermal toxicities of various compounds differ markedly among species
and not always in a predictable manner (McCreesh, 1965). Many of the
problems encountered in dermal toxicity testing and suggestions for
further research have been discussed by Barnes (1968). Roe (1968)
discussed various problems encountered in the design and
interpretation of inhalation toxicity studies related to species
differences in the anatomy of the respiratory tree. Enzootic lung
infections are an additional problem in the use of some species of
animals for long-term inhalation studies.
2.3.2.5 Unintended route
Interpretation of results and measurement of actual dose-response
relationships can be made difficult, because appreciable oral
ingestion may occur with inhalation or dermal exposures. Animals
exposed by either of these routes may ingest the material as a result
of preening, unless dermal applications are covered or made
inaccessible to licking or unless special exposure chambers (e.g. head
only) are used for inhalation exposures (see Chapter 6). In addition,
in particle inhalation experiments, the physiological protective
mechanisms of clearance by mucous transport of the particles out of
the respiratory tree (Hatch & Gross, 1964) with subsequent swallowing
may result in gastrointestinal exposure. Some degree of lung exposure
to volatile compounds administered in the diet or by dermal
application is also likely.
2.3.3 Special tests related to route
When exposure to a compound is most likely to occur by
inhalation, it is useful to know the effect of variations in
ventilation rates, since this will be a common variable among an
exposed human population under different conditions of activity. This
may be accomplished by the use of exercise wheels or treadmills.
When dermal exposure is the likely route, it will be useful to
conduct some tests to determine the effect of different solvents on
penetration of the test compound through the skin. Studies of the
influence of factors such as sweating, abrasions, or the presence of
detergents on dermal absorption and toxicity will also aid in
estimating toxicities under conditions likely to be experienced by man
(see Chapter 11, Part 2).
Interpretation and implications of toxicity data obtained with
oral exposures can be enhanced by examination of the influence of
fasting, dietary variations, and, particularly, administration by
gavage versus inclusion in the diet or drinking water. These factors
are discussed in more detail in subsequent chapters, but it should be
stressed that quite different results and interpretations may ensue if
the same daily dose is given rapidly by gavage or gradually in the
diet. Interpretation of such experiments is greatly aided, if the
design includes comparative absorption and distribution kinetics.
2.4 Selection and Care of Animals
2.4.1 General considerations
The selection and care of laboratory animals to be used in
toxicity tests is especially important in determining the success of
the experiment itself, the extrapolation of the data to man, and the
cost of the evaluation programme. In order to provide data on a
sufficient number of animals for valid statistical analyses, it has
become common practice to use small laboratory rodents for most
large-scale toxicity test programmes. Dogs or nonhuman primates are
frequently included in some of the studies, and studies on at least
one nonrodent species are often required by the test protocols
recommended by regulatory and advisory agencies. Recommendations (with
appropriate references for detailed information) concerning the
selection and care of laboratory animals to be used in the usual broad
scale toxicity evaluation studies are included in Chapter 3. The
selection of animals to be used in various special test procedures is
discussed in subsequent chapters.
Animals and animal care practices should be selected to provide a
scientifically sound and reproducible experiment; however, some of the
variables that contribute to nonuniformity may actually be exploited,
in special studies, to obtain data that may be useful in extrapolation
to nonuniform human populations. For example, if the inherent toxicity
of an air pollutant is to be characterized, the occurrence of chronic
lung infections should be avoided. On the other hand, specially
designed experiments to test the influence of air pollutants on the
susceptibility of animals to lung infections have provided a sensitive
procedure for measuring the adverse effects of air pollutants on the
physiological protective mechanisms that confer resistance to
respiratory infections (Ehrlich, 1966). Epidemiologists could
certainly use such information in the design of studies on human
populations exposed to air pollutants and to infectious microorganisms
present in the environment.
The choice of animals and the environment in which they are used
in toxicity studies will ultimately be determined (as for any other
decisions relating to experimental design) by the nature of the
questions asked or the hypotheses formulated. Controlled introduction
of additional variables may be desired for special studies. The
important principle is that appropriate control conditions should be
included in such studies to allow comparisons with results obtained
under more conventional procedures.
2.4.2 Animal variables
The objective of most experimental toxicity studies is to predict
the adverse effects of chemicals in man. Therefore, in addition to
uniformity of response, the guiding principle for the selection of
appropriate test species is that the test animals should resemble man
as closely as possible with respect to absorption, distribution,
metabolic transformation, excretion, and effect at site(s) of action
of chemicals. Both male and female animals should be tested and the
test protocol should encompass exposures of animals at both ends of
the age spectrum (see Chapter 3).
It is generally recommended that random-bred rather than highly
inbred strains of animals be used in broad-scale toxicity testing, at
least until the action of the chemical is well characterized (Food &
Drug Administration, 1970). In more specialized toxicity tests, it may
be desirable to use inbred strains, for example, when animal models
are needed that represent a genetic variation in human population, or
when hypotheses on the mechanism of action are tested.
2.4.2.1 Selection of species
The Food Protection Committee (1970) indicated that sensitivity,
convenience, and similarity in metabolism to man are the prime factors
to be considered in the selection of animal species for toxicity
testing. In the absence of information to the contrary, it is
generally recommended that data obtained from the most sensitive
species should be used as the basis for the extrapolation of test
information to man.
There is now ample evidence of wide quantitative variations among
species in their rates of biotransformation of foreign compounds
(Committee on Problems of Drug Safety, 1969; Parke & Williams, 1969;
Williams, 1967). Since many organic chemicals are subject to
biotransformation at several reactive groups in the molecule, it is
important to identify and quantify the biotransformation and
distribution pathways of a chemical in man and in several laboratory
animal species as early as possible in toxicity evaluation studies. It
seems axiomatic that for costly chronic studies on experimental
animals, the species that is most representative of man with respect
to the metabolism of the test chemical should be chosen. Often, the
only information concerning the metabolism and distribution of the
test compound in man may be derived from limited studies on
individuals accidentally or occupationally exposed to uncontrolled or
unknown doses.
In vitro studies of metabolism using animal tissues and human
tissues obtained at autopsy or biopsy could help in comparisons of
similarities or differences in metabolism between man and laboratory
animals. Although this approach cannot, in itself, provide information
that may be obtained in studies on intact animals, it can, coupled
with knowledge of the physicochemical properties of the compound and
the kinetics of enzymatic biotransformation reactions in tissues of
various species, provide a logical basis for selection of species for
long-term toxicity tests. Decisions based on comparative human and
experimental animal metabolism data should take into account
information concerning several pathways of metabolism. This will help
to ensure the inclusion of data on quantitatively minor pathways of
metabolism that may result in products of major toxicological
importance. These considerations of variation in biotransformation can
also be applied to intraspecies variations related to age, sex, and
strain (Benke & Murphy, 1973; Jori et al., 1971a; MacLeod et al.,
1972; Parke & Williams, 1969).
Anatomical and morphological variations can also determine the
selection of species. This source of variation is likely to be of
particular importance in inhalation toxicity studies (Roe, 1968).
Tyler & Gillespie (1969) compared anatomical characteristics of the
lungs of human beings with several laboratory and domestic animal
species when considering appropriate animal models for human
emphysema. They grouped anatomically similar species into four
classes: (a) cattle, sheep, and swine; (b) dogs, cats, and rhesus
monkeys; (c) rabbits, rats, and guineapigs; and (d) horses and man.
From their studies on horses, they concluded that the pathophysiology
and the morphological characteristics of emphysema in horses closely
resembled the disease in man and that the horse could be a
particularly suitable laboratory animal for studies of this disease.
Obviously, in the usual toxicity studies on air pollutants, the costs
of using horses would be prohibitive. The reactivity of a chemical at
primary target sites must also be considered as a potential variable
contributing to species differences in toxicity. The acute toxicity of
certain organophosphorous insecticides in representative mammalian,
avian, and fish species appeared to be more readily related to species
differences in the reactivity of the target enzyme
(acetylcholinesterase (3.1.1.7)) than to differences in hepatic
biotransformation rates (Murphy et al., 1968).
In the absence of specific knowledge of comparative metabolism
and sites of action, it is appropriate to apply the principle that
quantitative and qualitative similarity of response in several
mammalian laboratory species enhances the confidence that man will
respond similarly. Tests on several species seem equally as useful for
predicting effects in a heterogenous human population as the selection
of test species based on the results of limited studies of metabolism
in a very few individual human subjects, who may or may not be
representative of a broad cross-section of the human population at
risk. Of course, any quantitative information on the disposition and
action of chemicals in man is useful, as it adds to the accumulation
of knowledge from which more specific guidelines for species selection
may be derived in the future.
2.4.2.2 Animal models representing special populations at risk
Because many chemicals in the environment are widely dispersed,
all segments of the human population may sustain some exposure. For
this reason, it may be useful to design special experiments to
evaluate toxicity in animal models that represent potentially
hypersusceptible segments of the human population. The very young and
the aged represent such segments generally, because in the very young,
natural protective mechanisms such as metabolic detoxification systems
may be incompletely developed and in the aged, cell repair processes
may be less active than in younger individuals. Evaluation of the
toxicity of chemicals in animal models of commonly occurring human
diseases may be of value. Thus, for example, epidemiological studies
suggest that individuals suffering from coronary artery disease may be
particularly susceptible to carbon monoxide, the severity of signs and
symptoms in patients suffering from cardiorespiratory disease appears
to be aggravated by air pollution, and asthmatic patients appear to
have a higher frequency of attacks during periods of high oxidant air
pollution (Heimann, 1967). Few attempts have been made to evaluate
experimentally the interactions between exposure to toxic chemicals
and model disease conditions. Taylor & Drew (1975) reported that an
inbred strain of cardiomyopathic hamsters was more susceptible to
acute toxicity and cardiac arrhythmias produced by inhaled
trichlorofluoromethane than were random-bred hamsters that were not
cardiomyopathic. Easton & Murphy (1967) suggested that their
observation of greater mortality and respiratory distress in
ozone-preexposed than in air-exposed guineapigs given histamine
injections or inhalation exposures might be analogous to the apparent
increase in frequency and severity of asthmatic attacks reported for
peak periods of photochemical air pollution.
Problems of standardization of disease conditions add another
dimension to toxicity studies. However, it seems that animal disease
models should be given more consideration in toxicity evaluations that
are intended to provide the basis for the safe use of chemicals to
which large human populations are exposed. Jones (1969) summarized
reference sources for animal models of a large number of specific
human diseases. Several papers in a series of symposia proceedings
published by the US National Academy of Sciences provide discussion
and references to (among other topics) animal models for commonly
occurring disease states of the lungs (Tyler & Gillespie, 1969), the
cerebrovascular system (Luginbuhl & Detweiler, 1968), the heart (Jobe,
1968), the kidney (Lerner & Dixon, 1968), atherosclerosis (Clarkson et
al., 1970), diabetes (Hackel et al., 1968), and chronic degenerative
diseases (Abinanti, 1971). Since gut microflora are changed in certain
gastrointestinal diseases in man, modifications of the quality and
distribution of microflora in experimental animals might be a useful
model for special tests (Williams, 1972). There are at least three
possible applications of these disease models to toxicity studies: (a)
evaluation of the susceptibility of diseased tissues to chemicals
known to exert their action on that tissue; (b) influence of the
disease state on the metabolism and distribution of chemicals that may
act on the diseased tissue or at other sites; and (c) research on the
mechanism of action of toxic chemicals using specific modification of
receptor function or biochemistry.
2.4.3 Cyclic variations in function or response
Many physiological variables undergo cyclic peaks and troughs of
activity (Altman & Dittmer, 1966) some of which are diurnal (24-h) and
others of longer duration. These rhythms may be completely under
intrinsic control or they may be partly or largely regulated by
environmental variables such as light and temperature. Boyd (1972)
considers most diurnal variations in susceptibility to drug toxicity
to be mainly related to eating and sleeping habits. Since rats are
nocturnal feeders, the greater quantity of food in the stomach early
in the morning compared with the afternoon may alter the acute
toxicity of chemicals given intragastrically. Attempts to standardize
this variable have led to recommendations that acute toxicity tests by
intragastric administration should be conducted on animals that have
fasted overnight (Food & Drug Administration, 1959). Intragastric
LD50 values are generally lower in rats fasted overnight compared
with those fed ad libitum; however, the differences are usually only
of the order of two- to three-fold (Boyd, 1972; Loomis, 1974). The
influence of fasting may, in some cases, be related to rates of
absorption from the gut in the presence or absence of food but this
cannot account for all such variations. A striking example has been
reported by Jaeger et al. (1975) in which acute toxicity and liver
injury in rats exposed through inhalation to several halogenated
olefins were enhanced 10- to 20-fold by overnight fasting. A diurnal
cycle of susceptibility of rats to the toxicity of inhaled vinylidene
chloride appeared to be related to the diurnal cycle of liver
glutathione concentrations (Jaeger et al., 1973) which may be
secondary to a diurnal cycle in feeding activities. The duration of
pentobarbital anaesthesia in mice under usual laboratory housing
conditions exhibited a diurnal cycle with the longest duration at
14h00 and the shortest (40-60% of that at 14h00) duration at 02h00
(Davis, 1962). The amplitude of the cycle was considerably reduced,
when animals were caged individually as opposed to group caging, and
constant light abolished the cycle. That circadian variation in the
action of certain organic chemicals may be related to circadian
variation in their biotransformation is suggested by the work of Jori
et al. (1971b).
Beuthin & Bousquet (1970) reported seasonal or circannual rhythms
for drug action and biotransformation rates in rats. The induction of
increased drug metabolism by phenobarbital also exhibited a seasonal
variation. Basal levels of hexobarbital metabolism were highest during
the winter months and lowest in summer, whereas the opposite cycle for
induction of hexobarbital oxidase by phenobarbital was observed. It
should be noted that studies of seasonal variations in the metabolism
or toxic action of chemicals must be carefully controlled with respect
to environmental variables that might produce similar variations in
response (see 2.4.4). Boyd (1972) suggests that seasonal variations in
susceptibility may be related to the hibernation reaction or to
weather conditions in the geographical area concerned.
Circadian variation in adrenocortical activity in rats was
investigated by Szot & Murphy (1971) in animals exposed acutely or
subacutely to the pesticides parathion and DDT. Although the degree of
stimulation of corticosterone secretion after single doses of
parathion varied depending upon the phase of the cycle at the time of
administration, feeding parathion or DDT in the diet at rather high
concentrations did not change the phase or the amplitude of the
natural adrenocortical rhythm or alter the stimulation of
corticosterone secretion produced by irritant stress.
In rodents, locomotor activity is greatest at night and Boyd
(1972) suggests that depression of activity is best demonstrated at
night or in rats starved for 3 days when their daytime activity is as
great as at night time. However, a more convenient approach may be to
reverse the lighting schedule, a procedure used successfully for
measuring the effects of various inhaled air pollutants on locomotor
activity in mice (Murphy, 1964).
The time of day at which biochemical or other tests are conducted
in control and experimental animals may influence the reproducibility
of the test data, if the biological variables under test display
rhythmic variation. An investigator may exploit these rhythmic
variations to advantage in special studies of factors that influence
susceptibility to chemical injury. However, if the aim is a broad
scale characterization of the toxicity of a chemical, the choice may
be to carefully standardize times of administration of chemicals and
of animal sampling to minimize both known and unrecognized circadian
variations as much as possible.
2.4.4 Environmental variables
There are numerous possible variations in the environment in
which experimental animals are housed or tested that can influence
their response to toxic chemicals. General considerations of these
variables are discussed by several authors (Boyd, 1972; Doull, 1972,
1975; Hurni, 1970; Morrison, 1968). Unless the purpose of the
experiment is to use these variables to predict possible alterations
in effects in man exposed to chemicals under similar environmental
variations, it is generally possible to minimize their influence on
the toxicity of chemicals by adopting good principles of animal care.
Reference sources are available to provide guidelines for proper
housing, diets, cage size requirements, etc. (DHEW, 1972; Universities
Federation for Animal Welfare, 1972).
Only brief comments will be made on some of the major
environmental variables that affect toxicity experiments or that can
be used for predicting mechanisms or possible implications to man.
2.4.4.1 Temperature
Major variations from the recommended environmental temperatures
and relative humidities can contribute not only to the impairment of
general health and increased susceptibility to infection of the
animals, but also to variation in their response to toxic chemicals.
The mechanisms of interactions between environmental and body
temperatures and drugs or toxic agents have been reviewed by Doull
(1972) and by Cremer & Bligh (1969). Since absorption, distribution,
metabolic transformation, excretion, and reactivity with receptor
sites depend on various temperature-dependent chemical reactions, it
might be expected that the toxicity of chemicals would be readily
influenced by temperature. However, since toxicity studies are usually
conducted with homotherms, only minor changes in core body temperature
occur with moderate changes in environmental temperatures. By the same
token, changes in environmental temperature will elicit homeostatic
changes in various physiological or biochemical systems. These may
then alter some of the physiological variables (e.g. ventilation,
circulation, body water, intermediary metabolism) that are
rate-limiting determinants of the absorption, deposition, and action
of toxic chemicals. Furthermore, toxic chemicals may exert their
action by disruption of the thermoregulatory mechanism as suggested
for cholinesterase inhibitors (Meeter & Wolthuis, 1968). Exposure to
toxic chemicals can also mimic the actions of extremes in
environmental temperature or other physical stressors (Murphy, 1969;
Szot & Murphy, 1970). Thus, fluctuations in environmental temperatures
can lead to functional changes that might be mistakenly attributed to
the action of the chemical or they may actually alter the toxicity. If
interference with physiological thermoregulatory mechanisms is a
likely action of the chemical, careful control of environmental
temperatures is necessary to ensure reproducibility of measurements of
this action.
2.4.4.2 Caging
The type of cage, grouping, bedding, and other factors related to
caging can markedly influence the toxicity of some chemicals and drugs
(Boyd, 1972; Doull, 1972; Hurni, 1970). The acute toxicity of
4-[1-hydroxy-2-[(1-methylethyl)amino]ethyl]-1,2-benzenediol
(isoproterenol) was markedly greater in rats caged singly for more
than three weeks than in rats caged in groups (Hatch et al., 1965).
Winter & Flataker (1962) reported that grouped rats held in "closed"
(sheet metal on four sides and bottom) cages were more resistant to
the acute toxicity of morphine and 1-[2-(4-amino-phenyl)ethyl]-
4-phenyl-4-piperidine carboxylic acid ethyl ester (anileridine) than
rats held in "open" (wire mesh) cages. These differences were
attributed to mechanical factors that prevented depressed rats from
maintaining an open airway in the wire mesh cages. Altered toxicity of
chemicals related to caging effects are generally purely experimental
variables and can be controlled by good practices of laboratory animal
housing.
2.4.4.3 Diet and nutritional status
Dietary variables can influence the toxicity of chemicals in
several ways. The toxicities of several pesticides were enhanced to
different degrees in rats given low protein diets (Boyd, 1969).
Protein-deficient diets protected rats against the acute
hepatotoxicity of carbon tetrachloride and N-methyl-
N-nitrosomethanamine (dimethylnitrosamine) (although the number of
kidney tumours after a single dose of the latter increased), while the
acute toxicity of chloroform was unchanged and the acute toxicity of
aflatoxins was markedly enhanced (McLean & McLean, 1969). These
effects could be explained, at least in part, by the reduction of
activity of hepatic mixed-function oxidases that generally results
from feeding low-protein diets. Whether or not a compound's toxicity
is increased or decreased in such circumstances will depend upon
whether microsomal biotransformation leads to the formation of more or
less toxic metabolites. Numerous other examples of macro and
micronutrient deficiencies, which alter the activity of the
drug-metabolizing enzyme systems and the toxicity of chemicals, have
been reviewed by Campbell & Hayes (1974). Intestinal and pulmonary
aryl hydrocarbon hydroxylase activity is modified by diet. Of
particular interest is the observation that changing rats from a
commercial, natural diet to a balanced, purified diet resulted in an
almost total loss of activity of this enzyme system in these tissues
(Wattenberg, 1972). Flavonoid compounds, present as natural
constituents of alfalfa meal (and other plants), may account for the
apparent induction of aryl hydrocarbon hydroxylase by natural diets.
The trace mineral content of diets can also influence the metabolism,
distribution, and action of toxic chemicals (Moffitt & Murphy, 1974).
The results of toxicity experiments can be markedly influenced if
care is not taken to ensure constancy of diets free from residues of
contaminating chemicals. However, since nutritional imbalances are
widespread in the human populations, controlled variation of
experimental diets to simulate major human deficiency states (e.g.
kwashiorkor resulting from protein deficiency) is an important area
for research in toxicology and should, perhaps, be included in
standard toxicity evaluations of select groups of chemicals.
2.5 Statistical Considerations
Although various protocols for toxicity testing recommend
specific numbers of animals to be used for various acute and chronic
tests (see Chapter 3), a useful guiding principle is that sufficient
animals should be used to allow statistically valid conclusions
concerning differences in the response of test animals compared to
controls and to provide a base for statistical extrapolations to
larger population samples. Statistical procedures allow the
experimenter to make ( a) descriptions of sets of data or population
characteristics, and ( b) statements of probability of events.
Various procedures provide for both enumerative data, or yes-no type
characteristics, and measurement data, or graded effects or
characteristics. Some procedures (t-test, F-test) are restricted to
observations that have specific frequency distributions, while others
(signed rank test, rank run test) are free of any assumptions about
distribution (i.e. nonparametric). Standard texts should be consulted
for the application of biostatistics to the design and analyses of
experiments. In practice, it is highly advisable to involve a
statistician in the experimental design as well as in the analysis.
The number of animals required to make statistically valid
conclusions regarding the differences between experimental and control
animals will depend upon the degree of confidence desired and the
magnitude of the possible sources of variation in the experiment. The
second consideration will depend upon the uniformity of the test
animals with respect to the biological system or systems under test.
This, in turn, will depend upon both genetic and environmental
factors. The reproducibility of the bioassay and chemical procedures
used in the tests will be another source of variation. A further
source of variation in toxicity testing is related to the constancy
and stability of the test chemical. Finally, there are the variables
introduced by the investigators (often the most difficult to control),
beginning with the care and attention given to accurate dosing
throughout the various steps of the experiment. Attempts must be made
to minimize all these sources of variation as far as possible, without
sacrificing any important aspect of the experiment.
When testing whether or not two sets of data may both be valid
samples from the same population with normal frequency distribution
(i.e. null hypothesis) or whether the control group is not
significantly different from the treatment group, statisticians
describe the appropriate sample size in terms of the desired "power"
of the test. Two types of decision errors exist. One is that a
significant difference between groups is stated to exist when, in
fact, there is no difference. This is called the type I error and the
experimenter must state what probabilities (alpha) for this error he
will accept; most commonly a probability of 0.05 is used, but an
experimenter may sometimes require a probability as low as 0.01, or
any other he chooses. The second type of decision error is that no
significant difference between groups is stated to exist when, in
fact, the groups are different. This is called the type II error, and
1-ß (the probability that one will not make this error) is the power
of the test. The power is directly related to the sample size and the
ratio of "differences between true means of the samples" to
"differences between experimental error of the means of the samples".
Once this ratio is fixed, the power increases solely as a function of
sample size. The experimental error (pooled variance) can be estimated
from previous experiments or a pilot study. The acceptable magnitude
of the differences between true means of the samples is at the
discretion of the experimenter; he must use expert judgment and should
have a reasonable rationale; it will usually be the smallest value
that is considered to be of practical importance.
Another important statistical consideration is related to the
selection of the valid number of sampling units. This may be
particularly true in considering quantal (all-none, yes-no) effects
that may be multiple occurrences within a single test animal, as, for
example, in reproduction and carcinogenesis studies where,
respectively, there may be a number of affected offspring or a number
of tumours resulting from the treatment of a single animal. The
selection of the appropriate unit, either the number of animals
exposed or the number of occurrences of an effect, can determine the
statistical significance of an observed effect. Weil (1970) suggests
that in reproduction studies the number of maternal animals (or
litters) and not the number of affected fetuses or offspring is the
valid sampling unit, and that in carcinogenesis studies the number of
tumour-bearing animals should be the sampling unit and not the total
number of tumours. Furthermore, in carcinogenesis studies, animals
risk death from factors other than the tumours; in some cases, animals
may have died before they had time to develop a tumour; in other
cases, information from some animals may be lost from the study
because of unexpected death and autolysis of tissues preventing tumour
identification. An adjusted tumour incidence may be estimated by the
life-table techniques in such experiments (McKinney et al., 1968).
Another problem may be associated with gross or histopathological
examination where, because of cost and time considerations, only
tissues of a fraction of the total number of animals exposed are
subjected to complete examination. This will reduce the likelihood
that statistically valid conclusions can be drawn from the data on
occurrence of lesions. In practice, reasonable compromises are usually
necessary. Irrespective of the statistical methods of analysis used in
both the design and interpretation of results of toxicity tests on
chemicals, they cannot replace careful experimentation and
comprehensive knowledge of the underlying biological mechanisms of the
various steps between exposure to a chemical and injury.
2.6 Nature of Effects
2.6.1 Reversible and irreversible effects
Reversible effects are characterized by the fact that the
deviation from normal structure or function induced by a chemical will
return to within normal limits (controls) following cessation of
exposure. With irreversible effects, the deviation persists or may
progress, even after exposure ceases. This might be further qualified
by time limits, that is, the time required for return to normality
after exposure should be a reasonable fraction of the remaining
lifetime of a young animal for it to be considered reversible.
Reversibility may also be qualified by the normal lifetime of a
specific cell or macromolecule that serves as the end-point for the
effect. For example, cholinesterase-inhibiting insecticides are
generally considered irreversible inhibitors if the rate of reversal
of inhibition corresponds approximately to the time required for
synthesis and replacement of the enzyme, a process with different
rates in different tissues. Certain effects of toxic chemicals are
unmistakably irreversible, including the production of terata, or
malignant tumours, production of mutations in offspring of exposed
animals, certain chronic neurological diseases, production of true
cirrhosis, or emphysema. These are rather gross manifestations of
certain specific chemical-cell interactions, and, either at the level
of the first affected molecule or at intervening points leading to
these manifestations, there are probably reversible effects.
Understanding these effects and determining the critical dose that
produces them will make it possible to predict truly adverse effects
more rapidly.
The rate of reversibility of an effect will depend upon the rate
of cellular injury and the rate at which this injury is repaired
(Casarett, 1975). The rate of injury will depend upon the
concentration and duration or frequency with which a test chemical
contacts responsive tissue constituents. It is, thus, dose and
dose-rate dependent. The rate of repair is determined intrinsically
and may involve several cell processes. It may vary between different
tissues and probably between different species and strains. From a
practical standpoint, it is generally impossible to measure the
specific processes involved in injury and repair in a standard
toxicity evaluation study. However, it is important to make
measurements of the reversibility of effects in early, acute and
subacute studies. Thus, the time required for a process to return to
normal after single doses (which produce various degrees of injury)
will provide a guideline for the selection of doses to be used in
subsequent acute or chronic studies. The predictive value of such
information will depend upon the persistence of the chemical in the
test organism. If the chemical produces an effect and then is rapidly
detoxified or excreted, it may be possible to predict, with reasonable
accuracy, doses or exposure schedules that would not produce
cumulative effects. The manner of exposure and possible actions other
than the one being measured would, of course, be important in drawing
such conclusions. For example, rapid reversibility after a single dose
might not be indicative of the rate of reversal with a repeated dosing
if the first dose, in addition to the measured effect, also altered
either the repair processes or the processes responsible for
detoxification of the chemical. An example of an apparent
self-inhibition of detoxification is the insecticide malathion which
is rapidly hydrolysed by carboxylesterases. These are, in turn,
inhibited by metabolites or contaminants of malathion (Murphy, 1967).
Repeated exposure studies are necessary to evaluate such
possibilities; thus, the design of short-term feeding or inhalation
studies should include extra groups of animals that can be removed
from exposure either at the end of the experiment or, preferably, at
selected intervals for measurements of rate of reversal of any
observed effect.
If the chemical persists or accumulates in the organism,
measurements and interpretation of rates of reversal of effects are
more complicated. For this reason, it is useful to have kinetic data
on absorption and disposition to correspond with data on rates of
production and reversal of effects. Further discussion of these and
related principles is provided by Hayes (1972) in relation to his
proposal that determination of "chronicity factors" (1-dose LD50
(mg/kg) ‰ 90-dose LD50 (mg/kg/day) in diet i.e. the ratio of single
dose LD50 to the daily dose given in diet for 90 days which results
in 50% mortality at that time) is useful in predicting candidate
chemicals requiring long-term studies. The use of such predictive
methods must also take into consideration potential for other effects
that could never be detected in a subacute study (e.g. tumorigenesis).
2.6.2 Functional versus morphological changes
Toxic effects are often classed as functional or morphological in
nature. There has been a traditional attitude that changes in gross or
microscopic structure are more serious than functional changes.
Indeed, altered structure often seems to have taken on the implication
of irreversibility while altered function is often considered a
reversible effect. This conclusion, of course, depends on the level of
understanding of mechanisms of injury, rates and mechanisms of repair,
and causal associations between related functional and morphological
changes. Furthermore, whether the change is regarded as functional or
morphological may depend on the manner of detection. For example,
accumulation of fat in a cell observed through a microscope will most
often be considered a structural change, but, if the same cells or
tissues were assayed for triglyceride content, the increased
triglyceride would probably be classified as a functional or
biochemical change. The introduction of enzyme histochemistry and
electron microscopy into toxicity evaluation studies makes the
distinction between morphological and functional effects even less
clear. It may therefore be inappropriate to attempt to make these
distinctions.
Rowe et al. (1959) reviewed data from studies on a large number
of chemicals repeatedly administered to animals over periods ranging
from one month to two years, and summarized the frequency with which a
certain effect was found and the frequency with which it was the only
effect found. The effects were considered on: mortality; food intake;
body weight; organ weights; the histopathology of virtually every
major organ; haematology; blood urea nitrogen; clinical urinalyses;
central nervous system (most probably gross behaviour); gross
pathology; and cholinesterase activity. The authors found that if
growth, liver weight, kidney weight, liver pathology, and kidney
pathology had been studied, the lowest dose level that caused any
effect would have been detected in 96% of the studies. Changes in food
intake, central nervous system depression, excessive mortality,
increased lung weight, testicular injury, haematological changes, and
cholinesterase depression were the most sensitive effects in one or
more of the remaining 4% of cases. The reader should consult the
original reference for details but it is important to note that of the
commonly used criteria of effects, liver and kidney micropathology
were quite sensitive indices.
There are, of course, well-known examples where functional
changes are the only manifestations of toxicity. Many of the
organophosphate and carbamate insecticides inhibit cholinesterase
(3.1.1.8) activity and produce signs and symptoms (even death) that
can be characterized as purely functional without the production of
morphological lesions, detectable by conventional techniques.
Similarly, irritant air pollutants can often cause bronchoconstriction
and respiratory distress, without any accompanying morphological
changes. Both the functional cholinergic signs and symptoms produced
by the anticholinesterase insecticides and the bronchoconstriction
produced by irritants provide means of early detection at low levels
of exposure. Although these effects are reversible, they are no less
important during the period of exposure than certain kinds of
morphological effects. On the other hand, certain kinds of
"functional" changes, e.g. increased level of plasma transaminase
activity, usually reflect some type of structural change in cells that
allow these enzymes to "leak" into plasma (Cornish, 1971).
It is not possible, with present knowledge, to conclude that
either functional or morphological changes represent the most
sensitive, the earliest, or the most serious effects of toxic
chemicals. Since maintenance of both integrated function and
integrated structure ultimately depends on chemical reactions among
cell constituents, it is logical to conclude that specific biochemical
changes are the first and most sensitive effects. Unfortunately, with
relatively few exceptions, the specific biochemical receptors for
toxic chemicals are unknown. The more information that can be obtained
with respect to time- and dose-relationships for functional and
morphological effects, the more predictive the tests will become. This
requires an approach to toxicity studies in which proof of a mechanism
will require an integrated biochemical, physiological, and
morphological approach. Dawkins & Rees (1959) provide a useful short
treatise on an integrated biochemical-pathological approach to studies
of several toxic chemicals. Although advances in both biochemistry and
pathology now allow even more precise studies than those outlined by
these authors, the general principles which they develop are still
applicable.
2.7 Dynamic Aspects of Predictive Toxicology
2.7.1 Traditional versus new techniques
The objective of any toxicity test programme is prediction:
prediction of biological disposition from physicochemical constants,
prediction of altered cell or organ system function from reaction with
macromolecules, prediction of irreversible consequences of reversible
changes, prediction of implications of selected measurable variables
to overall health and survival of the test organisms, prediction of
effects in individuals of one species from tests conducted in another,
and finally predictions of incidence in large populations from tests
on small samples. All of these predictions must be related
quantitatively to a dose and dose-rate or schedule that can ultimately
be related to probable amounts used, the manner of use or the
occurrence of the chemicals in the environment.
Generally, traditional approaches to toxicity evaluation have not
attempted to make predictions far removed from the final application
or interpretation of the data. Thus, as outlined in Chapter 3, test
organisms are exposed to a range of doses and their health status is
examined by biochemical, physiological, or pathological procedures
analogous to those used in clinical medicine. When this approach has
been comprehensive, judicious application of the data usually appears
to have been successful in preventing chemically-induced disease.
Abandoning this approach in favour of new, different, or short-cut
methods cannot be advocated without thorough verification of their
validity. On the other hand, serious consideration must be given to
the application of some short-term ways of predicting toxicity in
order to provide a practical means of evaluating the many chemicals
already in the environment and those new compounds that are
continuously being added to the environment and have not been
subjected to traditional tests. Preceding sections have discussed some
possibilities, the following sections contain brief comments and
examples for consideration in selecting tests.
2.7.2 Toxicity of chemical analogues
Although it may be possible to predict the toxicity of individual
compounds in a homologous series from detailed knowledge of some
members of the series, some special exceptions should be noted. A
classical example involves the series of fluorine-substituted
aliphatic alcohols and acids, in which high acute toxicity alternates
with odd and even numbers of carbon atoms, the latter being the most
toxic (Pattison, 1959). The odd number of total carbon atoms confers
high toxicity in a homologous series of fluoronitriles, however. This
demonstrates the possibility that detailed information concerning only
a few members of a homologous series might fail to predict the
toxicity of another member of the series.
Recently, Johnstone et al. (1974) examined a number of
biochemical effects in a series of isomerically pure compounds for
their potency as liver enzyme inducers. Potency for this effect
increased with increasing chlorination that was related to differences
in biotransformation and excretion rates; however, there were also
striking differences in the potency of positional isomers in the lower
chlorinated biphenyls.
The mechanism of the toxic action of organophosphorus
insecticides was known to be the inhibition of acetylcholinesterase
even before they were introduced into use 30 years ago. However,
quantitative prediction of their acute toxicity from in vitro tests
of their relative potency as anticholinesterases is still inadequate
because of incomplete knowledge of the dynamic relationships between
several pathways of metabolism which yield both more and less potent
metabolites (Murphy, 1975). Nevertheless, these compounds have been
subjected to a great deal of research on both their physicochemical
and biological properties which should be applicable to predictions of
their relative environmental persistence, interaction with other
compounds, and, ultimately, to the design of safe molecules (Eto,
1974).
Using model ecosystems, Lu & Metcalf (1975) studied
bioaccumulation, biodegradability, and comparative detoxification
mechanisms in several benzene derivatives with widely-varying
physicochemical constants and biological activities. They concluded
that biological disposition and action could be predicted by the basic
molecular properties of water solubility, the partition coefficient
for lipid/water, and reactivity as determined by electron density.
Johnson (1975) recently reviewed the problems encountered in the
pursuit of the mechanism of delayed peripheral neuropathy produced by
some organophosphorus esters. The structure-activity relationships
identified in this research may be considered as a model of thorough
investigation that began as a problem in retrospective toxicology and
led to promising developments applicable to prospective toxicology.
Some interesting aspects of this problem are: the particular
usefulness of a non-mammalian species, the hen, as a predictor of a
toxic action that occurs in man; the concept of primary metabolic
effects on central neurons as a precursor to pathological change
detected in peripheral nerve fibres; and the difficulties of detecting
a specific critical esterase inhibition that represented only a small
percentage of the total esterase activity. Production of peripheral
neuropathy appears to be characteristic of organophosphorus esters
which may not only phosphorylate the specific "neurotoxic esterase"
but are also capable of dealkylation (or aging) following
phosphorylation. Although the steps between this primary
phosphorylating-aging process and the eventual manifestation of
peripheral neuropathy are still unclear, it appears that it may be
possible to predict probable occurrence of a delayed, chronic disease
from studies of the primary chemical-macromolecular interaction of
neurotoxic esterase inhibition that occurs immediately following
exposure.
2.7.3 Relation between site of metabolism and site of injury
Although for many years it was thought that the biotransformation
of organic chemicals represented detoxification mechanisms, it is now
apparent that numerous compounds are enzymatically converted to
intrinsically more active compounds in vivo (Fouts, 1972; Murphy,
1975; Parke, 1968). The liver is generally the most active tissue in
catalysing these "activation" reactions, but it is not always the most
susceptible target tissue as, for example, in the case of activation
of phosphorothioate insecticides to phosphate insecticides. This may
be explained, in part, by the presence of detoxifying enzymes or
reactive but noncritical binding sites in the liver that may prevent
the phosphates from escaping to inhibit cholinesterase in nerve target
tissues. The brain tissue has only a small fraction of the liver's
capacity to activate phosphorothioates, but because the activation
occurs in the same tissue as the critical target site, activation in
the brain may be the most important in determining toxicity.
Recently, the characteristic hepatotoxicity of several chemicals
has been related to their enzymatic conversion to highly reactive
derivatives that covalently bind to essential liver cell constituents
at, or near, the site of activation (Brodie et al., 1971). A similar
possibility may explain the bronchiolar neurosis produced in rats and
mice by bromobenzene and other aromatic hydrocarbons (Reid et al.,
1973).
The detailed study of the metabolism, storage, or binding and
distribution of foreign chemicals in the lung is a relatively recent
activity and has focused largely on therapeutic chemicals (Bend et
al., 1973; Brown, 1974; Orton et al., 1973). Because inhalation is a
common route of exposure to a wide variety of air contaminants in
industrial and community environments, future toxicity studies would
benefit by the inclusion of metabolic studies concerning rates of
absorption from, and local actions in the lung. Witschi (1975) has
reviewed biochemical approaches that may be used in the evaluation of
toxic injury to the lung.
Since the intensity and duration of the toxic action of a
chemical depends on the concentration of the active form at critical
receptor sites of action, kinetic aspects of absorption, distribution,
and excretion (as well as biotransformation) will influence the
specific sites of action. This topic is discussed in detail in Chapter
4 but it is worthy of note that Dedrick (1973) developed several
useful kinetic models that might be applied in predicting species
differences or similarities in response.
2.7.4 In vitro test systems
Where appropriate, studies in experimental animals should be
supplemented by isolated perfused organ, tissue slice or extract, and
tissue culture techniques. Where possible, attempts should be made to
compare the tissues of human subjects available from autopsy or
therapeutic biopsies, with those of other species in their response to
toxic chemicals (Worden, 1974). When the mechanism(s) of toxicity have
been elucidated and the target organ(s) identified, specific species
comparisons and dose-response relationships can be studied by these
in vitro techniques.
Knowledge of a specific enzyme or biological macromolecule that
serves as a target for reaction with toxic chemicals may provide a
means for screening and predicting relative potencies or specific
actions of chemicals in intact organisms. However, as pointed out
previously for the organophosphorus insecticides, biotransformations
and membrane barriers to the distribution of chemicals in intact
animals will often invalidate conclusions drawn from in vitro
assays. This problem may be partly overcome by incorporating enzymic
biotransformation systems with the target macromolecule (or organism)
in the in vitro test system. Such an approach is used for screening
for potential mutagens in microorganism test systems (Malling &
Frantz, 1973) and has been applied to studies of the biochemical
actions of pesticides (Chow & Murphy, 1975; Cohen & Murphy, 1974). A
major problem in the use of in vitro test systems for predicting
toxicity is the difficulty of quantitatively relating concentrations
in the simplified in vitro systems to action in complex intact
organisms. With adequate correlative data in both in vitro and
in vivo systems this may become possible, but such information is
generally lacking at present. For the most part, therefore, in vitro
model test systems are qualitative predictors rather than
quantitative. This need not decrease their usefulness, however, as
long as this limitation is recognized in the interpretation of
results.
In general, in vitro test systems have been useful in
qualitatively predicting acute actions. However, as discussed earlier,
neurotoxic esterase inhibition provides promise for predicting delayed
chronic neuropathy produced by certain organophosphate compounds.
Major research efforts are now being devoted to in vitro test
systems for the prediction of mutagenesis and carcinogenesis. These
are discussed in detail in Chapter 7. There is general recognition of
the value of these test systems (Council of Environmental Mutagen
Society, 1975; Food & Drug Administration, 1970; Food Protection
Committee, 1970) as screening procedures, but much less agreement as
to the priority that they should have in the conduct and
interpretation of toxicity evaluations.
When it is possible to obtain comparisons between exposures of
organs or tissues of experimental animals and humans to toxic
chemicals, such comparisons will provide useful baseline data for the
future extrapolation of data from intact animal studies to man.
Culture systems of human cells may also be useful as comparative
systems. The difficulties of maintaining some human cell lines are
well documented, but primary cultures of differentiated mammary and
liver epithelia have been established and maintained (Buehring, 1972;
Lasfargues & Moore, 1971; Potter, 1972). Human lymphocytes have also
been used in vitro (Kellermann et al., 1973).
It may be possible to use these isolated systems to determine the
susceptibility to toxic chemicals of different cell types in different
organs and to determine the reversibility of adverse effects in these
cell lines and organs. Of particular usefulness would be the
determination of dose-response curves for many tissues and their
interspecies comparison. Such information could be used to predict
target cells and organs with a high degree of susceptibility or
resistance. However, as mentioned earlier, the usefulness of the data
may be limited if the cells, tissues, or organs are incapable of
metabolizing the chemical to a toxic form in the intact animal. Such a
biotransformation may even occur in a different tissue or organ from
the one under test in vitro. To overcome this problem,
biotransformation systems from animal or human tissues (e.g.
microsomal activating systems) are often added with the chemical to
the isolated culture systems.
One problem with these methods is the uncertainty that all the
steps of metabolism are equally duplicated, that is, in addition to
activation of a chemical there should also be an opportunity for the
chemical to be detoxified or conjugated and eliminated. Some of these
detoxification steps require different coenzymes or metabolites, and
the enzyme systems may not be limited to microsome fractions or liver
tissue. However, as long as it is realized that these in vitro test
systems may exaggerate the situation that occurs in vivo they can
prove useful, especially for tests where only very small quantities of
material are available, as might be the case with some impurities or
metabolites.
In summary, short-term, in vitro tests both for carcinogenicity
and other forms of toxicity show great promise (Golberg, 1974), and
although no single test is likely to be reliable, appropriate
combinations may provide valuable information concerning the
fundamental toxicity of environmental chemicals. This would currently
provide a useful adjunct to long-term studies in animal populations,
and may develop further in the future to provide the more reliable
method of assessment. Such tests will require much development and
will take years to validate and perhaps even longer to win public
confidence with regard to their reliability.
REFERENCES
ABINANTI, R. R. (1971) Chronic and degenerative diseases of man: the
value of natural and experimentally induced diseases of animals.
In: Animal models for biomedical research IV, Proceedings of a
Symposium, Washington DC, National Academy of Sciences,
pp. 31-46.
ALTMAN, P. L. & DITTMER, D. S., ed. (1966) Environmental biology,
Bethesda, MD, Federation of American Societies for Experimental
Biology, pp. 565-608.
ALVARES, A. P., LEIGH, S., KAPPAS, A, LEVIN, W., & CONNEY, A. H.
Induction of aryl hydrocarbon hydroxylase in human skin. Drug
Metabol. Disposition, 1: 386-390.
BARNES, J. M. (1968) Percutaneous toxicity. In: Boyland, E. &
Goulding, R., ed. Modern trends in toxicology, London,
Butterworths, pp. 18-38.
BEND, J. R., HOOK, G. E., & GRAM, T. E. (1973) Characterization of
lung microsomes as related to drug metabolism. Drug Metabol.
Disposition, 1: 358-367.
BENKE, G. M. & MURPHY, S. D. (1975) The influence of age on the
toxicity and metabolism of methyl parathion and parathion in male
and female rats. Toxicol. appl. Pharmacol., 31: 254-269.
BEUTHIN, P. K. & BOUSQUET, W. F. (1970) Long-term variation in basal
and phenobarbital-stimulated oxidative drug metabolism in the
rat. Biochem. Pharmacol., 19: 620-625.
BOYD, E. M. (1969) Dietary protein and pesticide toxicity in male
weanling rats. Bull. World Health Org., 40: 801-805.
BOYD, E. M. (1972) Predictive toxicometrics, Baltimore, Williams &
Wilkins, 408 pp.
BRODIE, B. B., CHO, A. K., KRISHNA, G., & REID, W. D. (1971) Drug
metabolism in man: past, present and future. Ann. NY Acad. Sci.,
179: 5-18.
BROWN, E. A. B. (1974) The localization, metabolism and effects of
drugs and toxicants in lung. Drug Metabol. Rev., 3: 33-87.
BUEHRING, G. C. (1972) Culture of human mammary epithelial cells:
keeping abreast with a new method. J. Natl Cancer Inst.,
49: 1433-1434.
CAMPBELL, T. & HAYES, J. R. (1974) Role of nutrition in the
drug-metabolizing enzyme system. Pharmacol. Rev., 26: 171-197.
CASARETT, L. J. (1975) Toxicologic evaluation. In: Casarett, L. J. &
Doull, J., ed. Toxicology -- the basic science of poisons, New
York, Macmillan, pp. 11-25.
CLARKSON, T. B., PRICHARD, R. W., BULLOCK, B.C., LEHNER, N. D. M.,
LOFLAND, H. B. & CLAIR, R. W. St. (1970) Animal models for
atherosclerosis. In: Animal models for biomedical research III.
Proceedings of a Symposium, Washington DC, National Academy of
Sciences, pp. 22-41.
CHOW, A. Y. K. & MURPHY, S. D. (1975) Production of a
methemoglobin-forming metabolite of 3,4-dichloroaniline by liver
in vitro. Bull. environ. Contam. Toxicol., 13: 9-13.
COHEN, S. D. & MURPHY, S. D. (1974) A simplified bioassay for
organophosphate detoxification and interactions. Toxicol. appl.
Pharmacol., 27: 537-550.
COMMITTEE ON PROBLEMS OF DRUG SAFETY (1969) Application of metabolic
data to the evaluation of drugs: report prepared by the Committee
on Problems of Drug Safety of the NAS/NRC Drug Research Board.
Clin. Pharmacol. Therap., 10: 607-634.
CORNISH, H. H. (1971) Problems posed by observations of serum enzyme
changes in toxicology. CRC Crit. Rev. Toxicol., 1: 1-132.
COUNCIL OF EUROPE (1973) Guide to the testing and toxicological
evaluation of flavouring substances. In: Natural flavouring
substances, their sources and added artificial flavouring
substances, Strasbourg, Council of Europe, pp. 403-410.
COUNCIL OF ENVIRONMENTAL MUTAGEN SOCIETY (1975) Environmental
mutagenic hazards. Science, 187: 503-514.
CREMER, J. E. & BLIGH, J. (1969) Body-temperature and response to
drugs. Brit. med. Bull., 25(3): 299-306.
CROSBY, D. G. (1972) Environmental photooxidation of pesticides. In:
Proceedings of a conference on degradation of synthetic organic
molecules in the biosphere, San Francisco, June 1971,
Washington DC, National Academy of Sciences, pp. 260-278.
DAVIS, W. M. (1962) Day-night periodicity in pentobarbital response of
mice and the influence of socio-physiological conditions.
Experientia (Basel), 18: 235-237.
DAWKINS, M. J. R. & REES, K. R. (1959) A biochemical approach to
pathology, London, Edward Arnold, 128 pp.
DEDRICK, R. L. (1973) Animal scale-up. J. Pharmacokin. & Biopharm.,
1: 435-461.
DHEW (1972) Guide for the care and use of laboratory animals
(prepared by Committee on Revision of the Guide for Laboratory
Animals and Care, ILAR, NRC). Washington DC, US Gov. Print.
Office (DHEW Publ. No. (NIH) 72.23).
DIGGLE, W. M. & GAGE, J. C. (1951) Cholinesterase inhibition in vivo
by O,O-diethyl O-p-nitrophenyl thiophosphate (Parathion
E 605). Biochem. J., 49: 491-494.
DILLINGHAM, E. O., MAST, R. W., BASS, G. E., & AUTIAN, J. (1973)
Toxicity of methyl- and halogen substituted alcohols in tissue
culture relative to structure-activity models and acute toxicity
in mice. J. Pharm. Sci., 62: 22-30.
DOULL, J. (1972) The effect of physical environmental factors on drug
response. In: Hayes, W. J., ed. Essays in toxicology, New York,
Academic Press, Vol. 3, pp. 37-63.
DOULL, J. (1973) Factors influencing toxicity. In: Casarett, L. J. &
Doull, J., ed. Toxicology -- the basic science of poisons, New
York, Macmillan, pp. 133-147.
EASTON, R. E. & MURPHY, S. D. (1967) Experimental ozone pre-exposure
and histamine: effect on the acute toxicity and respiratory
function effects of histamine in guinea pigs. Arch. environ.
Health, 15: 160-166.
EHRLICH, R. (1966) Effect of nitrogen dioxide on resistance to
respiratory infection. Bact. Rev., 30: 604-614.
ETO, M. (1974) Organophosphorus pesticides: organic and biological
chemistry. Cleveland, OH, CRC Press, pp. 387 (57-78).
FOOD & DRUG ADMINISTRATION (1959) Appraisal of the safety of
chemicals in foods, drugs and cosmetics. Austin, Texas,
Association of Food & Drug Officials of the USA, pp. 107.
FOOD & DRUG ADMINISTRATION (1970) Report on reproduction studies in
the safety evaluation of food additives and pesticide residues
(Report of panel on reproduction of the FDA Advisory Committee on
Protocols for Safety Evaluation). Toxicol. appl. Pharmacol.,
16: 264-269.
FOOD PROTECTION COMMITTEE (1970) Evaluating the safety of food
chemicals, Washington, DC, National Academy of Sciences, 55 pp.
FOUTS, J. R. (1972) Some studies and comments on hepatic and
extrahepatic microsomal toxication-detoxification system.
Environ. Health Perspect., Experimental Issue No. 2, Oct.
pp. 55-66.
GOLBERG, L. (1967) The amelioration of food (The Milroy Lectures).
J. Roy. Coll. Phys., 1 (4): 385-426.
GOLBERG, L. (1974) Short-term predictive tests. Proc. Eur. Soc. Drug
Toxicity, 15: 178-191.
HACKEL, D. B., MIKAT, E., LEBOVITZ, H., & SCHMIDT-NIELSEN, K. (1968)
Animal models for diabetes mellitus with special reference to the
sand rat (Prommomys obesus). In: Animal models for biomedical
research: Proceedings of a Symposium, Washington, DC, National
Academy of Sciences, pp, 14-20.
HANSCH, C. & DUNN, W. J. (1972) Linear relationships between
lipophilic character and biological activity of drugs.
J. pharm. Sc., 61: 1-18.
HATCH, A., BALEZO, T., WIBERG, G. S., & GRICE, H. C. (1965) The
importance of avoiding mental suffering in laboratory animals.
Anim. Welfare Inst. Rep., Vol. 14, No. 3.
HATCH, T. F. & GROSS, P. (1964) Pulmonary deposition and retention of
inhaled aerosols, New York, Academic Press, 184 pp.
HAYES, W. J. (1972) Tests for detecting and measuring long-term
toxicity. In: Hayes, W. J., ed. Essays in toxicology, New York,
Academic Press, Vol. 3, pp. 65-77.
HEIMANN, H. (1967) Status of air pollution health research. Arch.
environ. Health. 14: 488-503.
HENDERSON, Y. & HAGGARD, H. W. (1943) Noxious gases and the
principles of respiration influencing their action, New York,
Reinhold, 287 pp.
HOLMSTEDT, B. (1959) Pharmacology of organophosphorus cholinesterase
inhibitors. Pharmacol. Rev., 11: 567-688.
HURNI, H. (1970) The provision of laboratory animals. In: Paget, G.
E., ed. Methods in toxicology, Philadelphia, F. A. Davis,
pp. 11-48.
JAEGER, R. J., CONOLLY, R. B., & MURPHY, S. D. (1973) Diurnal
variation of hepatic glutathione concentration and its
correlation with 1,1-dichloroethylene inhalation toxicity in
rats. Res. Commun. Chem. Pathol. Pharmacol., 6: 465-471.
JAEGER, R. J., CONOLLY, R. B., & MURPHY, S. D. (1975) Short-term
inhalation toxicity of halogenated hydrocarbons. Arch. environ.
Health, 30: 26-31.
JOBE, C. L. (1968) Selection and development of animal models of
myocardial infarction. In: Proceedings of a Symposium on Animal
Models for Biomedial Research, Washington, DC, National Academy
of Sciences, pp. 101-108.
JOHNSON, M. K. (1975) The delayed neuropathy caused by some
organophosphorus esters: mechanism and challenge. CRC Crit. Rev.
Toxicol., 3: 289-316.
JOHNSTONE, G. J., ECOBICHON, D. J., & HUTZINGER, O. (1974) The
influence of pure polychlorinated biphenyl compounds on hepatic
function in the rat. Toxicol. appl. Pharmacol., 28: 66-81.
JONES, T. C. (1969) Mammalian and avian models of disease in man.
Fed. Proc., 28 (1): 162-169.
JONES, W. I., ROBACK, L. A., & TAYLOR, J. M. (1971) The loss of food
flavours from laboratory animal diets. II. Effect of laboratory
environment. J. Assoc. Offic. Anal. Chem., 54: 42-46.
JORI, A., PUGLIATTI, C., & PESCADOR, R. (1971a) Rat strain differences
in the activity of hepatic microsomal enzymes. Biochem.
Pharmacol., 20: 2695-2701.
JORI, A., SALLE, E., & SANTINI, V. (1971b) Daily rhythmic variation
and liver drug metabolism in rats. Biochem. Pharmacol.,
20: 2965-2969.
KLASSEN, C. D. (1975) Absorption, distribution and excretion of
toxicants. In: Casarett, J. L. & Doull, J., ed. The basic
science of poisons, New York, Macmillan, pp. 26-44.
KELLERMAN, G., LUYTEN-KELLERMAN, M. L., & SHAW, C. R. (1973) Genetic
variation of aryl hydrocarbon hydroxylase in human lymphocytes.
Am. J. Hum. Genet., 25: 327-331.
LA DU, B. N., MANDEL, G., & WAY, E. L., ed. (1971) Fundamentals of
drug metabolism and drug disposition, Baltimore, Williams &
Wilkins, 615 pp.
LAKE, B. G., HOPKINS, R., CHAKRABORTY, J., BRIDGES, J. W., & PARKE, V.
W. (1973) The influence of some hepatic enzyme inducers on
extrahepatic drug metabolism. Drug Metabol. Disposition,
1: 342-349.
LASFARGUES, E. Y. & MOORE, D. H. (1971) A method for the continuous
cultivation of mammary epithelium. In Vitro, 7: 21-25.
LERNER, R. A. & DIXON, F. J. (1968) Experimental and human
glomerulonephritis associated with antiglomerular basement
membrane antibodies. In: Proceedings of a Symposium on Animal
Models for Biomedical Research, Washington, DC, National Academy
of Sciences, pp. 109-130.
LJUBLINA, E. I. & FILOV, V. A. (1975) Chemical structure, physical and
chemical properties and biological activity. In: Methods used in
the USSR for establishing biologically safe levels of toxic
substances, Geneva, WHO, pp. 19-44.
LOOMIS, T. A. (1974) Essentials of toxicology, 2nd ed.,
Philadelphia, Lea & Febiger, 233 pp.
LU, P. Y. & METCALF, R. (1975) Environmental fate and biodegradability
of benzene derivatives as studied in a model aquatic ecosystem.
Environ. Health Perspect, 10: 269-284.
LUGINBUHL, H. & DETWEILER, D. K. (1968) Animal models for the study of
cerebrovascular disease. In: Proceedings of a Symposium on
Animal Models for Biomedial Research, Washington DC, National
Academy of Sciences, pp. 35-41.
LUKAS, G., BRINDLE, S., & GREENGARD, P. (1971) The route of absorption
of intraperitoneally administered compounds. J. Pharmacol. exp.
Therap., 178: 562-566.
MCLEOD, S. M., RENTON, K. W., & EADE, N. R. (1972) Development of
hepatic microsomal drug-oxidizing enzymes in immature male and
female rats. J. Pharmacol. exp. Therap., 183: 489-498.
MALLING, H. V. & FRANTZ, C. N. (1973) In vitro vs in vivo
metabolic activation of mutagens. Environ. Health Perspect.,
6: 71-82.
MCCREESH, A. H. (1965) Percutaneous toxicity. Toxicol. appl.
Pharmacol., 7: 20-26.
MCKINNEY, G. R., WEIKEL, J. H., WEBB, W. K., & DICK, R. J. (1968) Use
of life-table technique to estimate effects of certain steroids
on probability of tumour formation in a long-term study in rats.
Toxicol. appl. Pharmacol., 12: 68-79.
MCLEAN, A. E. M. & MCLEAN, E. K. (1969) Diet and toxicity. Brit. med.
Bull., 25: 278-281.
MEETER, E. & WOLTHUIS, O. L. (1968) The effects of cholinesterase
inhibitors on the body temperature of the rat. Environ. J.
Pharmacol., 4: 18-24.
MOFFITT, A. E. & MURPHY, S. D. (1974) Effect of excess and deficient
copper intake on hepatic microsomal metabolism and toxicity of
foreign chemicals. In: Hemphill, D. D., ed. Trace substances in
environmental health, Columbia, MO, University of Missouri
Press, Vol. VII, pp. 205-210.
MORRISON, J. K. (1968) The purpose and value of LD50 determinations.
In: Boyland, E. & Goulding, R., ed. Modern trends in toxicology,
Appleton-Century-Crofts, pp. 1-17.
MURPHY, S. D. (1964) A review of effects on animals of exposure to
auto exhaust and some of its components. Air Pollut. Control J.,
14: 303-308.
MURPHY, S. D. (1967) Malathion inhibition of esterases as a
determinant of malathion toxicity. J. Pharmacol. exp. Therap.,
156: 352-365.
MURPHY, S. D. (1969) Some relationships between effects of
insecticides and other stress conditions. Ann. NY Acad. Sci.,
160: 366-377.
MURPHY, S. D. (1975) Pesticides. In: Casarett, L. J. & Doull, J., ed.
Toxicology: the basic science of poisons, New York, Macmillan,
pp. 408-453.
MURPHY, S. D., LAUWERYS, R. R., & CHEEVEN, K. L. (1968) Comparative
anticholinesterase action of organophosphorus insecticides in
vertebrates. Toxicol. appl. Pharmacol., 12: 22-35.
ORTON, J. C., ANDERSON, M. W., PICKETT, R. D., ELING, T. E., & FOUTS,
J. R. (1973) Xenobiotic accumulation and metabolism by isolated
perfused rabbit lungs. J. Pharmacol. exp. therap.,
186: 482-497.
PANEL ON HERBICIDES (1971) Report on 2,4,5-T, Washington, DC, US
Govt Print. Office, 68 pp.
PARKE, D. V. (1968) The biochemistry of foreign compounds. Oxford,
Pergamon Press, 269 pp.
PARKE, D. V. & WILLIAMS, R. T. (1969) Metabolism of toxic substances.
Brit. med. Bull., 25: 256-262.
PATTISON, F. L. M. (1959) Toxic aliphatic fluorine compounds,
Amsterdam, Elsevier, 227 pp. (Elsevier Monographs).
PATTY, F. A. (1958) Industrial hygiene and toxicology, 2nd ed., New
York, Interscience, Vol. 1, 153 pp.
POTTER, V. R. (1972) Workshop on liver cell culture. Cancer Res.,
32: 1998-2000.
REID, W. D., HETT, K. F., HIK, J. M., & KRISHNA, G. (1973) Metabolism
and binding of aromatic hydrocarbons in the lung. Am. Rev. Resp.
Dis., 107: 539-551.
ROE, F. J. C. (1968) Inhalation tests. In: Boyland, E. & Goulding, R.,
ed. Modern trends in toxicology, London, Butterworths,
pp. 39-74.
ROWE, V. K., WOLF, M. A., NEIL, C. S., & SMITH, H. F. (1959) The
toxicological basis of threshold limit values: 2. Pathological
and biochemical criteria. Am. Ind. Hyg. Assoc. J., 20: 346-349.
SMYTH, H. F. (1959) The toxicological basis of threshold limit values:
1. Experience with threshold limit values based on animal data.
Am. Ind. Hyg. Assoc. J., 20: 341-345.
SZOT, R. J. & MURPHY, S. D. (1970) Phenobarbital and dexamethasone
inhibition of the adrenocortical response of rats to toxic
chemicals and other stresses. Toxicol. appl. Pharmacol.,
17: 761-773.
SZOT, J. R. & MURPHY, S. D. (1971) Relationships between cyclic
variations in adrenocortical secretary activity in rats and the
adrenocortical response to toxic chemical stress. Environ. Res.,
4: 530-538.
TAYLOR, G. J. & DREW, R. T. (1975) Cardiomyopathy predisposes hamsters
to trichlorofluoromethane toxicity. Toxicol. appl. Pharmacol.,
32: 177-183.
TYLER, W. S. & GILLESPIE, J. R. (1969) Structural and functional
alterations in horses with emphysema. In: Proceedings of a
Symposium on Animal Models for Biomedical Research, Washington,
DC, National Academy of Sciences, Vol. II, pp. 38-51.
UNIVERSITIES FEDERATION FOR ANIMAL WELFARE (1972) Handbook on the
care and management of laboratory animals, 4th ed., Baltimore,
Williams & Wilkins, 624 pp.
WATTENBERG, L. (1972) Dietary modification of intestinal and pulmonary
aryl hydrocarbon hydroxylase activity. Toxicol. appl.
Pharmacol., 23: 741-748.
WEIL, C. S. (1970) Selection of the valid numbers of sampling units
and a consideration of their combination in toxicological studies
in involving reproduction, teratogenesis and carcinogenesis.
Food Cosmet. Toxicol., 8: 177-182.
WHO (1967) WHO Technical Report Series, No. 348. (Procedures for
investigating intentional and unintentional food additives:
Report of a WHO Scientific Group) 25 pp.
WILLIAMS, R. T. (1967) Comparative patterns of drug metabolism. In:
Proceedings of an International Symposium on Comparative
Pharmacology. Fed. Proc., 26 (4): 1029-1046.
WILLIAMS, R. T. (1972) Toxicological implications of biotransformation
by intestinal microflora. Toxicol. appl. Pharmacol.,
23: 769-781.
WINTER, C. A. & FLATAKER, L. (1962) Cage design as a factor
influencing acute toxicity of respiratory depressant drugs in
rats. Toxicol. appl. Pharmacol., 4: 650-655.
WITSCHI, H. (1975) Exploitable biochemical approaches for the
evaluation of toxic lung damage. Essays in Toxicol.,
6: 125-191.
WORDEN, A. N. (1974) Toxicology and the environment. Toxicol.,
1: 3-27.
ZBINDEN, G. (1973) Progress in toxicology: special topics, New York,
Springer-Verlag, 88 pp.
3. ACUTE, SUBACUTE, AND CHRONIC TOXICITY TESTS
3.1 Introduction
The primary objective of toxicological testing is to determine
the effects of chemicals on biological systems and to obtain data on
the dose-response characteristics of the chemical. These data may
provide information on the degree of hazard to man and the environment
associated with a potential exposure related to a specific use of this
chemical. Elucidation of the metabolic behaviour of the chemical in
test animals increases confidence in defining the hazard (see Chapter
4). The degree of confidence with which hazard may be estimated
depends on the quality of the toxicological data. Selection of the
most appropriate test procedures coupled with strict adherence to
accepted experimental practices and astute observation are of
paramount importance in experimental toxicology.
3.2 General Nature of Test Procedures
Several types of toxicity testing procedures have been developed.
These include acute, subacute, and chronic studies. The major
difference between these tests is the dose employed and the length of
exposure to the chemical agent, but other differences in intent and
nature do exist and will be discussed. All of the tests share some
common characteristics. Each requires that groups of healthy animals,
housed under suitable conditions, be exposed to graded doses of the
test chemical. Rats, mice, guineapigs, rabbits, and hamsters are
commonly used for this purpose, but in some cases it may be necessary
to use dogs, swine, nonhuman primates, or other species. As a rule, a
control group is given the dosing vehicle or is sham treated.
Following treatment, the animals are closely observed for signs of
toxicity. Laboratory procedures designed to measure biological effects
are carried out on the treated and control animals. Detailed records
are maintained on each animal. Following completion of the test, all
animals, including controls, are subjected to a pathological
examination. Data should be analysed by appropriate statistical
procedures.
3.2.1 Housing, diet, and clinical examination of test animals
Animals should be healthy, genetically stable, and adequately
identified as to colony source. The controls and treated animals
should be of the same strain and species, age, and weight range, and
be supplied from the same source. Before starting the experiment, the
health status of all animals should be determined and monitored for
some time. During this time, a small randomly selected number of
animals from each shipment should be sacrificed and examined for
disease, parasites, and other specific pathogens. During the
quarantine period, animals may be caged together according to the
weight-space specifications. Acceptable standards for the housing and
care of experimental animals have been published (DHEW, 1972; Canadian
Council on Animal Care, 1973; Sontag et al., 1975).
During toxicity studies, rodents should be housed singly or in
pairs in stainless steel or plastic shoe-box cages while nonrodents
should be housed in suitable runs. The animals should be randomly
allotted to the cages and treatment regimes should be randomly applied
(Cox, 1958). Rodents should be allowed free access to food and water.
Nonrodents should be fed meal and given water ad libitum. The diet
fed to the animals should meet all of their nutritional requirements
(National Academy of Sciences, 1975) and should be free of toxic
chemical impurities that might influence the outcome of the test.
Periodic analysis of the diet to ensure its nutrient composition
should be undertaken since nutritional status may affect the nature of
toxic responses (Arcos, 1968). Although commercially available diets
of recognized quality are suitable for most subacute studies,
semipurified diets may be preferred because the nutrient and
nonnutrient components of the diet may be altered readily, where
necessary (Munro et al., 1974; Newberne, 1968).
Careful clinical observation of test animals is the most
neglected area in experimental toxicology. Few investigators are aware
or recognize that the skills required of a good medical diagnostician
are also required in assessing or diagnosing the toxic state or
condition of an animal. In toxicity studies, many animals may be lost
for evaluation because of death from intercurrent disease and
subsequent autolysis. With concerned, reliable staff, these losses can
be greatly reduced if a conscientious effort is made to recognize
early clinical signs of disease in the test animal. Ideally, each
animal on test should be looked upon as an individual patient. In this
way, there is an awareness of the idiosyncrasies of the animal and
departures from the normal will be more easily recognized. Once a
routine of careful clinical assessment has been established, it is
possible either to treat diseased test animals or, if necessary,
sacrifice them. In the latter case, the tissues are available for
histological examination. Otherwise, chronic disease effects might
render the tissues useless for the assessment of effects due to test
substances.
Detailed clinical examinations should be conducted weekly on the
test animals by competent, laboratory animal technicians under the
supervision of a veterinarian skilled in laboratory animal medicine
(Health and Welfare, Canada, 1973). These should include general
observation of the animals for overt signs of toxicity, quality of
hair, coat, general condition of the eyes, mouth, teeth, nose, and
ears (Leclair & Willard, 1970; Loomis, 1968). Assessment of cardiac
and respiratory function should be conducted by auscultation. If
neurological effects are anticipated, a detailed neurological
examination should be conducted. In larger species, this can be done
by skilled personnel using the methods of Charbonneau (1974), McGrath
(1960), and Mowbray & Cadell (1962). Examination of the eyes using
opthalmoscopic and slit-lamp techniques (Marzulli, 1968) may assist in
detecting ocular toxicity. The external and internal structures should
be carefully palpated and any tissue masses should be noted. Detailed
records of clinical evaluation should be maintained and should be
accessible to the attendant pathologist.
3.3 Acute Toxicity Tests
3.3.1 Underlying principles
Acute toxicity has been defined as the adverse effects occurring
within a short time of administration of single dose or multiple doses
given within 24 h (Hagan, 1959). When data are unavailable concerning
the toxicity of the test agent, acute toxicity studies are indicated
to identify the relative toxicity of the compound, to investigate its
mode of action and its specific toxic effect, and to determine the
existence of species differences.
The most frequently used acute toxicity test involves
determination of the median lethal dose (LD50) of the compound. The
LD50 has been defined as "a statistically derived expression of a
single dose of a material that can be expected to kill 50% of the
animals" (Gehring, 1973). The basic protocol for the determination of
the LD50 is well established and consists of treating groups of
animals with a mathematically-related series of doses in order to
determine the dose that kills 50% of the group and the dose-response
function. The LD50, being a calculated value, is always accompanied
by some estimation of the error of the value, such as the confidence
limits. The most commonly used methods for calculation of the LD50
are the graphic method of Litchfield & Wilcoxon (1947), the
logarithmic probit graph paper method of Miller & Tainter (1944), and
the method of moving averages of Thompson (1947) and Weil (1952). A
comparative review of these and other methods was published by
Armitage & Allen (1950). Death which occurs after the first 24 h is
more likely to be due to delayed toxic effects, which may be direct or
indirect. Signs occurring after the first 24-h period may give some
indication of the effect that the chemical may have at lower levels,
when administered for longer time periods.
3.3.2 Experimental design
3.3.2.1 Selection of species
The extent of species variation in toxicity testing has been well
documented in the reviews of Brodie (1964) and Rumke (1964). The
usefulness of determining species variability in order to assess the
applicability of toxicity data to man has been discussed by Hagan
(1959). Litchfield (1962) has postulated that if the toxicity of a
compound is the same in several species, there would appear to be an
increased likelihood that man would react in a similar manner.
The mouse, rat and dog are the most commonly used species for
acute toxicity testing. Both the rat and mouse should be used, as
marked differences in the LD50 between these two species are not
uncommon (Morrison et al., 1968).
The LD50 determination should be conducted in both male and
female animals, as differences in the LD50 between sexes have been
well documented (Hurst, 1958; Rumke, 1964) and are probably related,
in part, to differences in hepatic metabolism (Conney et al., 1965).
Acute toxicity may vary substantially with the age of the test
animal (Dieke & Richter, 1945; Lu et al., 1965; Scott et al., 1965;
Yeary & Benish, 1965), and animals of various ages should be used in
LD50 determinations. The effect of the age of the animal on the
LD50 is well documented and may be related to different levels of
drug metabolizing enzymes, absence of sex hormonal influences, or an
altered sensitivity of the central nervous system (Fouts & Hart, 1965;
Jondorf et al., 1959; Setnika & Magistretti, 1964).
The animals should be derived from previously untreated healthy
females. Weinberg et al. (1966) have demonstrated an effect of
treatment of dams during gestation with various compounds on the acute
oral toxicity in the newborn.
Furthermore, the animals should not have been previously used for
other studies, nor should there be a history of recent exposure to
anthelminthics or any other drug treatment.
The number of animals used should be sufficient for statistical
analysis and will depend on the method used for the calculation of the
LD50. Usually 8-10 rodents (4-6 animals of each sex) are used per
dose group (Leclair & Willard, 1970). Diechmann & LeBlanc (1943)
described a method using a total of 6 animals, while other methods
involved the use of 4-5 animals per dose group (Horn, 1956; Litchfield
& Wilcoxon, 1947; Thompson, 1947).
3.3.2.2 Selection of doses
The doses are selected to provide data for estimating the LD50
and to obtain information on the slope of the dose-response curve. At
least four doses, selected in logarithmic progression, should be used
(Weil, 1952).
In general, however, the doses can be arrived at only by
experimentation. The initial dose may be such that no effect is
manifested in the animals. In subsequent groups of animals, the dose
should be increased by a constant multiple until the dose of the
compound administered is sufficiently high that all of the animals in
the group die. Under these conditions, data can be obtained that can
be plotted to give a dose-response curve and from which the LD50
value may be calculated.
3.3.2.3 Method of administration
Generally, the chemical should be administered by the route by
which man would be exposed. If the route is oral, the compound should
be administered by gavage rather than mixed in the diet. In some
cases, the administration of the chemical along with the diet has been
shown to increase its toxicity compared with gavage dosing (Bein,
1963; Worden & Harper, 1963), but, in general, the oral toxicity of a
compound is greatest when it is administered by gavage to animals that
have fasted (Griffith, 1964). Griffith (1964) has demonstrated the
effect of the type and concentration of the vehicle on the LD50
value. The amount of the liquid or carrier administered should be
appropriate and the carrier should not, in itself, be toxic to the
animal.
In certain cases, even though the route of human exposure would
be oral, acute dermal, eye, and inhalation studies may be indicated to
assess the hazard to personnel handling the compound in the
laboratory.
3.3.2.4 Postmortem examination
In general, all animals dying during the observation period and
all surviving animals should be autopsied by a qualified pathologist
(Leclair & Willard, 1970). The autopsy should include gross and
histopathological examination of all organs.
If death is almost instantaneous and due to a pharmacological or
physical effect, i.e. massive gastrointestinal haemorrhage or acute
respiratory collapse, detailed histopathological examination of all
organs may not be indicated.
3.3.3 Repeated high-dose studies
Because of the inherent limitations of the LD50 in predicting
long-term toxicity, a short but intensive study or a series of such
studies may be indicated before commencing subacute tests. The purpose
of such studies is to define more precisely the doses to be used in
subacute tests and to elucidate more fully the organ systems affected.
The design of these repeated high-dose studies may vary but consists,
essentially, of repeated daily administration of a mathematically-
related series of doses to groups of animals for 5-21 days.
One type of repeated-dose study (Sontag et al., 1975) consists of
treating groups of five young adult animals of each sex at each of
five dose levels, the upper level being the one that is estimated to
produce no more than 10% lethality following a single dose, the
remaining doses being fractions of this dose.
A seven-day feeding study described by Weil et al. (1969)
consisted of treating five rodents of each sex at each of three or
four dose levels for seven days. Criteria of effects were mortality,
body weight gain, relative liver and kidney weights, and feed
consumption. This study showed that the results of the seven-day
feeding test were of significantly greater value in predicting dose
levels for the 90-day toxicity test than the LD50 values.
Daily observations, as described in section 3.1.3, should be
conducted and weekly body weight and food consumption (if the animals
are caged individually) monitored. For some test agents, especially
those with delayed toxicity or cumulative effects, other measurements,
such as organ function, body burden, absorption, and excretion of the
compound may be indicated. Animals should be necropsied and the
tissues should be examined for gross pathological changes and studied
histopathologically, if indicated.
3.4 Subacute and Chronic Toxicity Tests
3.4.1 Underlying principles
The subacute toxicity test generally involves daily or frequent
exposure to the compound over a period up to about 90 days. It
provides information on the major toxic effects of the test compound
and the target organs affected (Barnes, 1960). The latency of
development of the effect as related to dose, the relationship of the
blood and tissue levels of the compound to the development of lesions,
and the reversibility of the effects may also be studied. Data derived
from these studies are used for designing chronic toxicity tests in
which animals are exposed to the chemical for longer periods of time.
Man may be exposed for the greater part of his life-time to low
levels of a wide variety of environmental chemicals. Usually, the
degree of exposure is insufficient to produce overt signs of toxicity;
thus, cause-effect relationships cannot be easily established.
Epidemiological studies may assist in this respect, but, because man
is exposed simultaneously to several chemicals, it is difficult to
establish unequivocally the degree of hazard associated with any one
chemical. Acute and subacute toxicity tests are of limited value in
predicting chronic toxic effects because: (a) chemicals may produce
different toxic responses when administered repeatedly over a period;
and (b) during the aging process, factors such as altered tissue
sensitivity, changing metabolic and physiological capability, and
spontaneous disease may influence the degree and nature of toxic
responses. In addition, several important diseases such as heart
disease, chronic renal failure, and neoplasia are associated with
advancing age. These are multicausal in nature and thought to be due,
in part, to the presence of chemical substances, both natural and
synthetic, in the environment (WHO, 1972). Chronic toxicity tests, in
which animals are exposed for their entire lifetime to environmental
chemicals, have provided useful means of identifying those substances
of greatest public health concern. The tests are usually conducted
with the aim of establishing "no-observed-adverse-effect levels" that
may be used in setting acceptable daily intakes (ADIs), tolerance
limits for chemicals in food or water, or threshold limit values in
the case of occupational exposure. Since chronic toxicity testing is
expensive and requires specialized facilities and personnel, great
care must be taken in the design, execution, and interpretation of the
results of such studies.
3.4.2 Experimental design
3.4.2.1 Selection of species and duration of studies
In the subacute studies, if the compound has produced evidence of
toxicity in man and if sufficient toxicological and metabolic
information is available, it is often possible to select an
appropriate species on the basis of these data. For compounds about to
be put on the market about which little is known toxicologically, the
recommendations of the World Health Organization (WHO, 1958) and
competent national agencies (Friedman, 1969; Leclair & Willard 1970;
National Academy of Sciences, 1975) should be followed in selecting
appropriate test species. As a minimum recommendation, subacute
studies should be undertaken in two species, one rodent and one
nonrodent. Traditionally, the rat and dog are selected for subacute
toxicity testing because of their availability and the large amount of
background information available on them. When rats are used, the test
should be initiated just after weaning so that observations may be
made during the period of most rapid growth. A conventional strain
should be selected, so that the results in control and treated animals
can be compared with known literature values, and both sexes should be
tested to ascertain the influence of the sex hormones on the toxic
response. At least 10 animals of each sex should be included in each
dose group and the experiment should continue for 10% of the animals'
lifetime or about 3 months. If it is desired to study the pathogenesis
and reversibility of induced lesions or biochemokinetics, it is
recommended that observations be made at 3-week intervals during
exposure and last up to 3 months following termination of exposure.
In chronic toxicity testing, it is usual to expose the animals to
the chemical for the greater part of the life span. A wide variety of
animal species have been used in this type of work, although in most
cases rodents are the animals of choice, since large numbers can be
used to aid in the statistical interpretation of the results. Larger
animals should also be used (e.g. dog and monkey) for such species
have the advantage that larger samples of blood can be obtained on a
routine basis.
If the objective of the test is to study the carcinogenic
potential of a compound, the rat, mouse, or hamster is usually chosen
because of its shorter lifetime and the fact that large numbers may be
used to increase the sensitivity of the test.
When data on the metabolic fate of the test chemical in man is
not available, the species showing the greatest sensitivity in
subacute studies should be selected as the test species, provided the
species does not react atypically to the compound due to metabolic
peculiarities.
Sufficient numbers of animals should be included in the test to
ensure that a statistically valid design is achieved. Based on the
incidence of effects observed in subacute studies and the anticipated
incidence of chronic effects, the number of animals that should be
used can be calculated (Snedecor & Cochran, 1967).
Since it is usually the intention in chronic toxicity studies to
expose animals over the major portion of their life span, it is
essential to commence exposure early in life.
3.4.2.2 Selection of doses
Guidance on the selection of doses for subacute studies may be
obtained from the results of acute and repeated high-dose studies. For
compounds having a tendency to bioaccumulation, selection of doses is
particularly difficult. Kinetic studies may assist in establishing
acceptable dose levels since the half-time ( t´) for elimination
(Chapter 4) may provide guidance on the degree of bioaccumulation that
could be anticipated. To establish the nature of the toxic reaction,
the highest dose should provide a distinct toxic effect while the
lowest dose should not produce any detectable toxic reaction (Leclair
& Willard, 1970). To obtain maximum information on the dose-response
characteristics of the compound, at least two intermediate doses
should be included.
Information from subacute toxicity tests is of value in the
selection of appropriate dose levels, when commencing chronic toxicity
studies. In general, however, it is highly desirable to establish the
chemobiokinetic behaviour (Chapter 4) of the test compound and if
possible its major metabolites in the test species prior to
undertaking a chronic toxicity test. Particular attention should be
given to evidence for dose-dependent detoxification. Studies of this
nature will provide information on the degree to which the chemical
may be expected to accumulate in various body compartments and
unexpectedly produce evidence of toxicity. Since it is the object of
chronic toxicity tests to establish dose-response patterns and
"no-observed-adverse-effect levels", a minimum of three dose levels
should be used. The upper dose level should produce some slight
evidence of toxicity, but should be compatible with normal
physiological function (Leclair & Willard, 1970). The lowest dose
level would not be expected to produce evidence of toxicity (Health &
Welfare, Canada, 1973).
3.4.2.3 Method of administration
The route of administration in subacute and chronic studies
should be that through which man is likely to be exposed. For gases
and volatile industrial solvents, inhalation studies are recommended
(Magill et al., 1956) (Chapter 6), while for food additives,
pesticides, and other chemicals likely to come into contact with food
or water, the oral route is recommended (Leclair & Willard, 1970;
National Academy of Sciences, 1975). Incorporation of the test
chemical into the diet or drinking water is an appropriate means of
administration; however, care must be taken to ensure the stability of
the chemical in the dosing medium. The concentration of the test
chemical in the diet should be determined periodically to ensure
uniform dispersion and to aid in the quantification of achieved doses.
In some cases, the chemical may be unpalatable and administration by
gavage, or, in the case of dogs, by capsules may be necessary.
The diet is the preferred vehicle of administration, but it is
absolutely essential that the chemical be present in the diet in an
unaltered form; toxicity may be altered by interaction with dietary
constituents (Kello & Kostial, 1973). In rodent studies, the compound
may be administered in the diet as a fraction of the total diet, or a
sufficient quantity of the chemical may be added to the diet to
achieve predetermined dose levels (in mg per kg body weight per day).
In the latter case, it is necessary to adjust the dietary
concentration weekly or biweekly to maintain a constant dose level,
since food consumption per unit of body weight decreases as the animal
gets older. If, in rodent tests, the concentration of the test
compound in the diet is kept constant from weaning to maturity, the
actual dose received will be reduced by approximately 2.5 times over
the dosing period. This may have profound effects on the severity of
the toxic response and may be mistaken for tolerance. In chronic
toxicity tests, the chemical should be administered daily over the
entire treatment period. As an aid to interpretation of the test, only
one lot chemical should be used for the entire test unless the purity
of the chemical is definitely assured.
3.4.2.4 Biochemical organ function tests
In subacute studies, the use of a species such as the dog instead
of a rodent species permits the application of a wider range of
biochemical organ function tests because larger samples of blood can
be collected on a routine basis. Organ function studies should be
undertaken prior to initiation of the test, 3 and 10 days after the
start of dosing, at 30-day intervals thereafter throughout the test,
and terminally. The tests described for the chronic studies are also
applicable in subacute studies.
In the course of chronic toxicity tests, studies should be
undertaken to evaluate the functional integrity of various organ
systems. Assessment of the urinary system should commence with an
examination of the urine. Freshly-voided urine samples should be
obtained every one to three months from the test animals and examined
for the presence of occult blood, glucose, protein, and bilirubin
using simple diagnostic procedures. If positive effects are noted,
quantitative methods should be applied as outlined by Bergmeyer
(1965). Samples of freshly-voided urine should also be filtered
through Millipore filters and the filters stained according to the
Papanicolaou method (Frost, 1969) to detect the presence of renal
tubular cells or other cell types derived from the urinary system.
Urinary calculi and parasite eggs (Chapman, 1969) may be detected by
this method. Blood urea-nitrogen levels and other standard tests of
kidney function may be applied but they usually lack sufficient
sensitivity to detect subtle changes in kidney function.
Several test procedures are available for the assessment of liver
function. Most of these methods involve an examination of the serum
levels of hepatic enzymes that may be released in the serum following
liver injury (Czok, 1965; Henley et al., 1966; Zimmerman, 1974).
Korsrud et al. (1972) compared the sensitivity of various liver
function tests in the rat and noted that serum sorbitol dehydrogenase
(1.1.1.14) activity (an enzyme specific to the liver) correlated well
with the degree of histological alteration produced by hepatotoxic
agents such as carbon tetrachloride, 2,2'-iminobisethanol
(diethanolamine), and ethanethioamide (thioacetamide). However, Grice
et al. (1971) noted that pathological changes induced by these
compounds must be reasonably advanced before elevations are noted in
serum glutamic-oxaloacetic transaminase (2.6.1.1), lactate
dehydrogenase (1.1.1.27), or lactate dehydrogenase isoenzymes,
suggesting that changes in serum enzyme activity may not be as
sensitive an indicator of toxicity as pathomorphological examination.
Tests of liver function such as serum enzyme activities and various
clearance tests were reviewed recently by Balazs (1975). A complete
review of the principles and applications of these tests is given by
Cornish (1971) and further discussion of these methods is found in
Part II, Chapter 8. Suffice it to say, that a transient increase in
the activity of serum enzymes or other organ-derived constituents may
result from a transient change in organ homeostasis that produces no
lasting toxic effect.
For routine screening of organ function in large animals,
Charbonneau et al. (1974) used clinical procedures that can measure
the concentration of several serum enzymes and inorganic and other
constituents by automated methods. In general, these methods are not
sufficiently standardized or reproducible to detect minor alterations
in organ function but they do serve as a useful guide to general
clinical status.
3.4.2.5 Physiological measurements
In subacute studies, it is often possible to detect ensuing
pathological events through application of physiological function
tests.
In all studies, food consumption and body weight should be
recorded weekly in all animals. Weight gain per unit of food consumed
should be calculated (Munro et al., 1969). This gives a measure of the
efficiency of food use. The daily dose of chemical should be
calculated from data on food intake and body weight. Similar
measurements of food intake and body weight must be carried out in
chronic toxicity tests. If the test chemical is incorporated into the
drinking water, water intake must be measured. These measurements
should be conducted on a weekly basis during the entire test. The data
can be used to estimate the dose of chemical received and are
necessary in the establishment of dose-response relationships. Body
weight changes serve as a sensitive indication of the general health
status of test animals. Any rapid loss in body weight may signal the
onset of intoxication or disease. Computerized methods for recording
and analysing this type of data are available (Munro et al., 1972).
Under special circumstances, when the target organs of toxicity
have been identified during subacute studies, it is appropriate to
conduct measurements of the physiological function of organ systems.
Procedures such as electrocardiography (Grice et al., 1971),
electroencephalography (Flodmark & Steinwall, 1963; Harada et al.,
1967; Mann, 1970), electromyography (Chaffin, 1969), nerve conduction
studies and measurement of evoked potentials (Barnet et al., 1971;
Hrbek et al., 1972) may greatly assist in defining the functional
effects of chemicals (Chapter 8). Such tests are expensive to perform
and require highly specialized equipment and personnel. They have
limited application in routine testing but may be used to define
mechanisms of action. It is imperative that the results of such
studies be correlated with clinical and pathological findings (Grice,
1972; Osborne & Dent, 1973).
3.4.2.6 Metabolic studies
Subacute studies provide an excellent opportunity to undertake
metabolic investigations under conditions of repeated exposure that
may alter the nature of the metabolites and the rate of metabolic
transformation of the test compound. Urine and faeces can be collected
and examined for the presence of metabolites and, by undertaking
serial sacrifices at three-week intervals, the kinetics of
accumulation of the compound in various body compartments can be
evaluated.
To gain an understanding of the metabolic fate of a chemical that
may have a long biological half-time, such as hexachlorobenzene (Grant
et al., 1975), three extra groups of animals need to be studied for
tissue distribution to provide information that is necessary for
estimating the potential hazard to man. The principles of these
methods are reviewed in Chapter 4. Often it is desirable, in subacute
studies, to study the kinetics of the test compound and its
metabolites following completion of the dosing period. If extra groups
of animals are initially included for this purpose, much valuable
additional information on the compound may be obtained.
3.4.2.7 Haematological information
In subacute studies involving rodents, haematological studies
should be undertaken on randomly selected subgroups of animals prior
to initiation of the test, at 30-day intervals, and on all animals
terminally. Bone marrow should be examined terminally. Nonrodent test
animals should be examined at similar intervals.
In chronic toxicity studies involving rodents, haematological
studies of circulating blood cells should be undertaken on randomly
selected subgroups of animals prior to initiation of the test, at 3 to
6-month intervals and, on selected animals, terminally. Bone marrow
should be examined terminally and, if indicated, at interim times by
biopsy.
To assess the clinical state of nonrodents, haematological
variables should be examined frequently.
A set of test procedures is necessary for routine haematological
screening and the tests must be of sufficient sensitivity and accuracy
to be of practical value for use in large numbers of laboratory
animals (Cartwright, 1969; Schalm, 1967; Sirridge, 1967).
Quantification of blood cells and thorough study of cellular
morphology by a haematologist, experienced in small animal medicine,
is necessary in the study of haematological disorders. Haematological
evaluation of experimental animals is facilitated by the fact that
repeated sampling is relatively easy and small amounts of blood are
required, and that single-cell systems can be studied to obtain
information on cell production, destruction, defects, and dysfunction.
For erythroid evaluation, the numbers of circulating erythrocytes must
be counted and the haematocrit and haemoglobin concentration measured.
As an index of erythropoietic activity in the bone marrow, a
reticulocyte count should be carried out. Morphological assessment of
erythrocytes is mandatory. The number of circulating leucocytes should
be quantified and a differential count and morphological assessment
should be made. To evaluate the functional capacity and malignant
changes in the blood-forming organs, bone marrow should be examined
terminally. From bone marrow smears a differential count and
morphological assessment can be carried out. Imprints of lymph nodes
or spleen permit a detailed cytological study of normal and abnormal
cells present that may be of diagnostic significance.
To assess haemostatic function, it will be necessary to evaluate
platelets, coagulation systems, and fibrinolysis. Screening tests
include platelet count, clot retraction, one stage prothrombin time,
and activated partial thromboplastin time. More specific evaluation
may require factor assays, thrombin time, fibrinogen determination,
euglobulin clot lysis time, prothrombin consumption time, platelet
aggregation, and adhesiveness.
3.4.2.8 Postmortem examination
In every toxicity evaluation, all animals should be given a
thorough gross autopsy and detailed records kept on each animal.
Samples of all organs and supporting structures should be saved for
histopathological examination. Detailed autopsy methods are outlined
in Chapter 5.
In chronic toxicity testing it is often useful to incorporate
interim autopsy dates so that the progression of lesions may be
studied. At interim sacrifices and terminally (if sufficient animals
are in a healthy state), the major organs should be weighed. Organ
weights may serve as a useful index of toxicity; however, care must be
taken in the interpretation of the data. Decreased absolute organ
weights in treated animals may be merely a reflection of lower body
weight and calculation of organ to body weight ratios may increase the
usefulness of the data (Feron, 1973).
3.4.2.9 Controls
In the evaluation of both subacute and chronic toxicity, special
attention must be given to the control animals. The quality of data
obtained from the control animals has an important bearing on the
interpretation of results from the treated animals. Suitable numbers
of control animals of the same age and body weight as the treated
animals must be included in the experimental design in a statistically
randomized fashion.
Except for treatment with the test chemical, these animals should
be handled identically to the test subjects and all measurements
conducted on the treated animals must be carried out on the controls
with the same precision and frequency. In studies in which the
chemical is administered by gavage, the control animals should receive
the suspending vehicle in an amount equivalent to the treated animals.
The incidence of spontaneous lesions or of other changes in control
animals must be carefully noted and the interpretation of data
obtained from treated animals must include an appreciation of the role
that spontaneous disease processes may play in the manifestation of
chemical toxicity. It is particularly important, in studies with
rodents, to have detailed information on the incidence of neoplastic
diseases, since some species and strains (Sher, 1972) may have a high
background incidence of certain tumours which tends to reduce
longevity and decrease the chance of observing chronic toxic effects.
In addition, the chemical under test may alter the incidence of
spontaneous tumours and other diseases or may induce new tumours, and
this possibility must be taken into consideration in the evaluation of
the chronic toxicity of chemicals. In all cases, responses
attributable to the test compound must be compared with background
observations in controls. For this reason, the quality of the
toxicological data rests heavily on the adequacy of the control values
(Weil & Carpenter, 1969).
3.4.3 Alternative approaches in chronic toxicity
3.4.3.1 Perinatal exposure
The majority of chemicals to which man may be exposed are present
in air, food, or water for his entire lifetime. Recently, there has
been an attempt to duplicate the human situation in the chronic
toxicity test by exposing the test animals during the neonatal period
as well as throughout life (Friedman, 1969). In this approach, groups
of weaning animals (usually rodents) are exposed to the test chemical
until they reach sexual maturity. They are then mated, within dose
groups, and the treatment is continued during pregnancy and lactation.
Following weaning, the offspring are transferred to their parents'
diet and exposed for the balance of their lifetime to the test
chemical. The details of this test procedure have been outlined in a
recent Canadian Government publication (Health & Welfare, Canada,
1973) and by Epstein (1969). It is not known yet whether this
technique increases the sensitivity of the chronic toxicity test, but
it is known that exposure to carcinogens in the perinatal period will
often increase the incidence and decrease the latent period of
carcinogenesis (Tomatis & Mohr, ed., 1973).
Further study of this method is required to evaluate its
usefulness fully. It should be pointed out, however, that this
procedure adds considerably to the cost and length of the chronic
toxicity test.
3.4.3.2 Use of nonrodent species
In chronic toxicity studies with nonrodent species such as
nonhuman primates, dogs, or cats, it is often not feasible to expose
the animals to the test compound for their entire lifespan, even
though they may be the species of choice. Under such conditions,
careful examination of the kinetic and metabolic behaviour of the test
compound in these species may substitute, to some extent, for the
decreased treatment period (provided the anticipated endpoint is not
carcinogenesis). Carefully conducted kinetic studies will assist in
establishing when steady-state tissue concentrations of the test
chemical and its metabolites have been achieved. If treatment is
continued for a substantial period after the establishment of
steady-state kinetics without any increase in the degree of toxic
effects observed clinically, or during interim sacrifice, this may
partially substitute for a lifetime study and provide increased
assurance for those having to make regulatory decisions. If this
approach is not feasible, it may be possible to test human metabolites
in rodent species (Health & Welfare, Canada, 1973).
3.5 Evaluation and Interpretation of the Results of Toxicity Tests
The evaluation and interpretation of toxicity studies starts with
a clear definition of experimental objectives. The design of the
experiment should be such that the objectives can be reasonably
achieved. Well designed and carefully executed experiments add greatly
to the ease with which results can be evaluated and interpreted and
also to confidence in the experimental data.
The primary usefulness of the LD50 determination is to obtain
some idea of the magnitude of the acute toxic dose (Frazer & Sharratt,
1969) and information concerning the type of toxic effects of the
chemical. Such information includes whether death is immediate or
delayed, whether recovery from a near lethal dose is rapid or complete
or both, or whether the cause of death is narcosis with respiratory
failure, lung oedema, or liver necrosis.
However, the LD50 provides little information for the
assessment of the hazard from compounds to which the human population
is exposed for extended periods of time. Although it has been
suggested that compounds that do not show adverse effects when given
in doses of 3-5 g per kg body weight are essentially non-toxic
(National Academy of Sciences, 1975), there are numerous examples in
the literature of compounds with LD50 values greater than 5 g per kg
which produce toxic effects, when given in low doses for extended
periods of time (Frazer & Sharratt, 1968). If the main object of an
acute toxicity test is not to establish a value for the LD50 with
precision, but to learn something about the way in which the chemical
acts as a poison, as suggested by Paget & Barnes (1964), this can best
be accomplished by tests involving repeated daily administration to a
few animals for a period of 5-21 days. The information provided by the
LD50 regarding the effects of acute exposure to toxic compounds may
be useful as a guide for selecting doses for such studies.
The primary objective of subacute and chronic toxicity studies is
to determine the nature and severity of toxic effects and the
"no-observed-adverse-effect" dose level. These data may then be used
in the establishment of acceptable levels of exposure for man.
Data on group weight gain or body weight change should be plotted
against time, and differences between groups should be evaluated
statistically. Changes in body weight with time are best evaluated
statistically using trend analysis procedures (Armitage, 1955). Food
(and water) consumption data should be handled in a similar fashion.
Reduced body weight or weight gain in otherwise healthy treated
animals may be due to reduced food intake owing to its unpalatability
or to a specific toxic effect of the chemical resulting in reduced
efficiency of food use. Using data on the dietary concentration of the
test chemical, food consumption, and body weight, the mean daily dose
of chemical received (in mg/kg body weight/day or similar units)
should be calculated. Automated data processing procedures to
accomplish this are available (Munro et al., 1972).
Data on organ weights should be evaluated and interpreted with
great care. Increased relative (to body or brain weight) organ weights
may also result from adaptation to stress phenomena or from metabolic
overloading of biochemical pathways or physiological processes.
Increased liver weight, for example, may result from a stimulation of
de novo protein synthesis in the smooth endoplasmic reticulum (SER).
This results in a morphologically detectable increase in SER. The
biochemical counterpart of this increase is an increased ability of
the liver to metabolize certain foreign substances, sometimes
including the test compound and endogenous substrates, due to a
stimulation in the activity of hepatic mixed function oxidases
(Staubli et al., 1969). These adaptative changes may manifest
themselves clinically as tolerance. Often these changes are reversible
upon cessation of dosing and do not produce lasting toxicological
effects but the implications of chronically elevated levels of these
enzymes is not known. Certain enzyme inducers may cause impairment of
liver function and produce pathological and biochemical changes (Feuer
et al., 1965).
Data on biochemical and haematological effects should be
tabulated and compared with control values using statistical
procedures (Johnson, 1950). Any observed effects should be correlated
with clinical and pathological findings. A biochemical or
haematological change such as reduction in liver glycogen or an
alteration in white cell count may not be indicative of a toxic
effect, but an adaptation to a stress situation (National Academy of
Sciences, 1975). In general, changes in homeostasis must be carefully
evaluated since reversible shifts do not necessarily imply a toxic
effect in the absence of other toxic manifestations.
Changes in the functional state of physiological or neurological
processes, such as an alteration in the electrocardiogram or abnormal
behaviour, may result from pharmacological or pathological effects of
the test compound. Changes in functional state must be closely
correlated with their morphological counterpart in order to evaluate
their toxicological importance properly (Grice, 1972).
The cornerstone of experimental toxicology is the pathological
examination. Usually, decisions regarding the safety of a compound are
based on this evidence. All pathological findings in test animals
should be graded carefully and their incidence tabulated (see Chapter
5). Spontaneous lesions in control animals should also be noted and
compared to the observations in control animals in previous
experiments or in the literature (Peck, 1974) to ensure that the
incidence and nature of the lesions is representative of the strain.
Pathological data should be analysed rigorously using appropriate
statistical methods (Fleiss, 1973) and spurious observations
apparently unrelated to treatment should be identified. Lesions that
are dose-related should be studied in detail and correlated with gross
pathological findings, clinical observations, and other variables
(Grice, 1972).
It is not uncommon in chronic toxicity testing to find
pathological or other changes that occur in low incidence and that are
not dose-related but occur only in treated animals. Such reactions may
be idiosyncratic in nature or may be due to the hypersensitivity of
certain animals. Nevertheless, they deserve special attention since
they may be indicative of a hitherto unsuspected toxic effect. The
clinical history and other data from such animals should be reviewed
with great care and an attempt should be made to determine the reason
for the observed effects. Toxic effects that occur in extremely low
incidence present special problems in interpretation. There is no
substitute for experience in this respect and the prudent investigator
will consult the knowledgeable experts in this field (Zbinden, 1973).
REFERENCES
ARCOS, J. C. (1968) Chemical induction of cancer. Vol. 1, New York,
Academic Press.
ARMITAGE, P. & ALLEN, I. (1950) Methods of estimating the LD50 in
quantal response data. J. Hyg. (Lond.), 48: 298-322.
ARMITAGE, P. (1955) Tests for linear trends in proportions and
frequencies. Biometrics, 11: 375-385.
BALAZS, T. (1975) Toxic effects of chemicals in the liver.
FDA By-lines, 5: 291-303.
BARNES, J. M. (1960) Toxicity testing, In: Schilling, R. S. F., ed.
Modern trends in occupational health, London, Butterworths &
Co., pp. 20-32.
BARNET, A. B., OHRICH, E. S., & SHANKS, B. L. (1971) EEG evoked
responses to repetitive auditory stimulation in normal
Down's-syndrome infants. Dev. Med. Child. Neurol., 13: 321-329.
BEIN, H. J. (1963) Rational and irrational numbers in toxicology.
Proc. Euro. Soc. Study Drug Toxicity, 2: 15-26.
BERGMEYER, H. U. (1965) Methods of enzymatic analysis. New York,
Academic Press.
BRODIE, B. B. (1964) [The difficulties of transposing experimental
results obtained in animals to man.]. Actual. pharmacol.,
17: 1-23 (in French).
CANADIAN COUNCIL ON ANIMAL CARE (1973) Care of experimental animals
-- guide for Canada.
CARTWRIGHT, G. E. (1969) Diagnostic laboratory hematology, 4th ed.
New York, Grune & Stratton.
CHAFFIN, D. B. (1969) Surface electromyography frequency analysis as a
diagnostic tool. J. occup. Med., 11: 109-115.
CHAPMAN, W. H. (1969) Infection with Trichosomomoides crassicauda as
a factor in the induction of bladder tumours in rats fed
2-acetylaminofluorene. Invest. Urol., 7: 154-159.
CHARBONNEAU, S. M., MUNRO, I. C., NERA, E. A., WILLES, R. F.,
KUIPER-GOODMAN, T., IVERSON, F., MOODIE, C. A., STOLZ, D. R.,
ARMSTRONG, F. A. J., UTHE, J. F., & GRICE, H. C. (1974) Subacute
toxicity of methylmercury in the adult cat. Toxicol. appl.
Pharmacol., 27: 569-581.
CONNEY, A. H., SCHNEIDERMAN, K., JACOBSON, M., & KUNTZMAN, R. (1965)
Drug-induced changes in steroid metabolism. Ann. NY Acad. Sci.,
123: 98-109.
CORNISH, H. H. (1971) Problems posed by observations of serum enzyme
changes in toxicology. CRC Crit. Rev. Toxicol., 1: 1-32.
COX, D. R. (1958) Planning of experiments, New York, John Wiley,
Chapter 5.
CZOK, R. (1965) The behaviour of plasma enzymes in toxicological
experiments. Proc. Eur. Soc. Study Drug Toxicity, 5: 68-83.
DHEW (1972) Guide for the care and use of laboratory animals
(prepared by Committee for the Revision of the Guide for
Laboratory Animal Facilities and Care, Institute of Laboratory
Animal Resources). Washington DC, Govt Printing Office (DHEW
Publ. No. (NIH) 73-23).
DIECHMANN, W. B. & LEBLANC, T. J. (1943) Determination of the
approximate lethal dose with about six animals. J. ind. Hyg.
Toxicol., 25: 415-417.
DIEKE, S. H. & RICHTER, C. P. (1945) Acute toxicity of thiourea to
rats in relation to age, diet, strain and species variation.
J. Pharmacol. exp. Ther., 83: 195-202.
EPSTEIN, S. (1969) A catch-all toxicological screen. Experientia
(Basle), 25: 617.
FERON, V. J. (1973) An evaluation of the criterion "organ weight"
under conditions of growth retardation. Food Cosmet. Toxicol.
11: 85-94.
FEUER, G., GOLBERG, L., & LE PELLEY, J. R. (1965) Liver response
tests. I. Exploratory studies on glucose 6-phosphatase and other
liver enzymes. Food Cosmet. Toxicol., 3: 235-249.
FLEISS, J. L. (1973) Statistical methods for rates and proportions.
New York, John Wiley (Wiley Series in Probability and
Mathematical Statistics).
FLODMARK, S. & STEINWALL, O. (1963) Differentiated effects on certain
blood brain barrier phenomena and on the EEG produced by means of
intracarotidly applied mercuric dichloride. Acta. Physiol.
Scand., 57: 446-453.
FOUTS, J. R. & HART, L. G. (1965) Hepatic drug metabolism during the
perinatal period. Ann. NY Acad. Sci., 123: 245-251.
FRAZER, A. C. & SHARRATT, M. (1969) The value and limitations of
animal studies in the prediction of effects in man. In: The use
of animals in toxicological studies -- UFAW Symposium, England,
1968, Potters Bar, England, 41 pp.
FRIEDMAN, L. (1969) Symposium on the evaluation of the safety of food
additives and chemical residues. II. The role of the laboratory
animal study of intermediate duration for evaluation of safety.
Toxicol. appl. Pharmacol., 16: 498-506.
FROST, J. K. (1969) Manual for the tenth postgraduate institute for
pathologists in clinical cytopathology. Baltimore, MD, Johns
Hopkins Hospital.
GEHRING, P. J., ROWE, V. K., & MCCOLLISTER, S. B. (1973) Toxicology:
cost-time. Food Cosmet. Toxicol., 11: 1097-1110.
GRANT, D. L., HATINA, G. V., & MUNRO, I. C. (1975) Hexachlorobenzene
accumulation and decline of tissue residues and relationship to
some toxicity criteria in rats. Environ. Qual. Saf., Supplement
Vol. III, pp. 562-568.
GRICE, H. C. (1972) The changing role of pathology in modern safety
evaluation. CRC Crit. Rev. Toxicol., 1: 119-152.
GRICE, H. C., BARTH, M. L., CORNISH, H. H., FOSTER, G. V., & GRAY, R.
H. (1971) Experimental cobalt cardiomyopathy: correlation between
electrocardiography and pathology. Cardiol. Res., 4: 452-456.
GRIFFITH, J. F. (1964) Inter-laboratory variations in the
determination of acute oral LD50. Toxicol. appl. Pharmacol.,
6: 726-730.
HAGAN, J. M. (1959) Acute toxicity. In: Appraisal of the safety of
chemicals in food, drugs and cosmetics, Assoc. Food & Drug
Officials of USA, pp. 17-25.
HARADA, Y., MIYAMOTO, Y., NONAKA, I., OHTA, S., & NINOMIYA, T. (1967)
Electroencephalographic studies on Minamata Disease in children.
Dev. Med. Child Neurol., 10: 257-258.
HEALTH & WELFARE, CANADA (1970) Guide for the preparation of
submissions for food additives. Ottawa, Health & Welfare.
HEALTH & WELFARE, CANADA (1973) The testing of chemicals for
carcinogenicity, mutagenicity and teratogenicity. Ottawa,
Health & Welfare.
HENLEY, K. S., SCHMIDT, E., & SCHMIDT, W. (1966) Enzymes in serum,
their use in diagnosis. Springfield, IL, Thomas.
HORN, H. J. (1956) Simplified LD50 (or ED50) calculations.
Biometrics, 12: 311-321.
HRBEK, A., KARLBERG, P., KJELLMER, J., & OLSSON, T. (1972) Evoked EEG
responses in newborns with asphyxia and IRDs. Pediatr. Res.,
6: 61.
HURST, E. W. (1958) Sexual differences in the toxicity and therapeutic
action of chemical substances. In: Walpole, A. L. & Spinks, A.,
ed. The evaluation of drug toxicity, London, Churchill,
pp. 12-25.
JOHNSON, L. P. V. (1950) An introduction to applied biometrics.
Minneapolis, Burgers Publ., Co.
JONDORF, W. R., MAICKEL, R. P., & BRODIE, B. B. (1959) Instability of
newborn mice and guinea pigs to metabolize drugs. Biochem.
Pharmacol., 1: 352-354.
KELLO, D. & KOSTIAL, K. (1973) The effect of milk diets on lead
metabolism in rats. Environ. Res., 6: 355-360.
KORSRUD, G. O., GRICE, H. C., & MCLAUGHLAN, J. U. (1972) Sensitivity
of several serum enzymes in detecting carbon tetrachloride --
including liver damage in rats. Toxicol. appl. Pharmacol.,
22: 474-483.
LECLAIR, J. M. & WILLARD, J. W. (1970) Guide for the preparation of
submissions on tolerances for incidental contaminants and
agricultural chemicals in food. Ottawa, Canada, Food & Drug
Directorate, Dept of National Health & Welfare.
LITCHFIELD, J. T. (1962) Evaluation of the safety of new drugs by
means of tests in animals. Clin. Pharmacol. & Therap.,
3: 665-672.
LITCHFIELD, J. T. & WILCOXON, F. A. (1947) A simplified method of
evaluating dose-effect experiments. J. Pharm. exp. Ther.,
95: 99-113.
LOOMIS, T. A. (1968) Essentials of toxicology. Philadelphia, Lea &
Febiger.
LU, F. J., JESSUP, D. C., & LAVALLEE, A. (1965) Toxicity of pesticides
in young versus adult rats. Food Cosmet. Toxicol., 3: 591-596.
MAGILL, P. I., HOLDEN, F. R., & ACKLEY, C. (1956) Air Pollution
Handbook. New York, McGraw-Hill.
MANN, L. I. (1970) Developmental aspects and the effect of carbon
dioxide tension on fetal cephalic metabolism and
electroencephalogram. Exp. Neurol., 26: 148-159.
MARZULLI, F. N. (1968) Ocular side effects of drugs. Food Cosmet.
Toxicol., 6: 221-223.
MCGRATH, J. T. (1960) Neurological examination of the dog with
clinicopathological observation. 2nd ed. Philadelphia, Lea &
Febiger.
MILLER, L. C. & TAINTER, M. L. (1944) Estimation of the ED50 and its
error by means of log-probit graph paper. Proc. Soc. Exp. Biol.
Med. NY, 57: 261-264.
MORRISON, J. K., QUINTON, R. M., & REINERT, H. (1968) The purpose and
value of LD50 determinations. In: Boyland, E. & Goulding, R.,
ed. Modern trends in toxicology. London, Butterworths,
pp. 1-17.
MOWBRAY, J. B. & CADELL, T. E. (1962) Early behaviour patterns in
rhesus monkeys. J. Comp. Physiol. Psychol., 55: 350-357.
MUNRO, I. C., MIDDLETON, E. J., & GRICE, H. C. (1969) Biochemical and
pathological changes in rats fed brominated cottonseed oil for 80
days. Food Cosmet. Toxicol., 7: 25-33.
MUNRO, I. C., CHARBONNEAU, S. M., & WILLES, R. F. (1972) An automated
data acquisition and computer-based computation system for
application to toxicological studies in laboratory animals.
Lab. Anim. Sci., 22: 753-756.
MUNRO, I. C., MOODIE, C. A., KREWSKI, D., & GRICE, H. C. (1974)
Carcinogenicity study of commercial saccharin in the rat.
Toxicol. appl. Pharmacol., 32: 513-526.
NATIONAL ACADEMY OF SCIENCES (1975) Principles for evaluating
chemicals in the environment. Washington, DC, National Academy
of Sciences.
NEWBERNE, P., ROGERS, A. E., & WOGAN, G. N. (1968) Hepatorenal lesions
in rats fed a low lipotrope diet and exposed to aflatoxin.
J. Nutr., 94: 331-343.
OSBORNE, B. E. & DENT, N. J. (1973) Electrocardiography and blood
chemistry in the detection of myocardial lesions in dogs.
Food Cosmet. Toxicol., 11: 265-276.
PAGET, G. E. & BARNES, J. M. (1964) Toxicity tests. In: Laurence, D.
R. & Bacharach, A. L., ed. Evaluation of drug activities:
Pharmacometrics, London, New York, Academic Press,
Vol. 1, pp. 135-166.
PECK, H. M. (1974) Design of experiments to detect carcinogenic
effects of drugs. In: CRC Carcinogenesis testing of chemicals,
Cleveland, OH, CRC Press, pp. 1-13.
RUMKE, CHR. L. (1964) Some limitations of animal tests. In: Laurence,
D. R. & Bacharach, A. L., ed. Evaluation of drug activities:
Pharmacometrics, London, New York, Academic Press,
Vol. 1, pp. 125-133.
SCHALM, O. W. (1967) Veterinary haematology, 2nd ed. Philadelphia,
Lea & Febiger.
SCOTT, W. J., JOHNSTON, J. W., JOHNSTON, C. D., & BELILES, R. P.
(1966) Comparative acute toxicities of isoproterenol and
metraproterenol. Toxicol. appl. Pharmacol., 8: 353.
SETNIKAR, I. & MAGISTRETTI, M. J. (1964) The toxicity of central
nervous system stimulants in rats of different ages.
Proc. Eur. Soc. Drug Toxicity, 4: 132-139.
SHER, S. P. (1972) Mammary tumours in control rats: literature
tabulation. Toxicol. appl. Pharmacol., 22: 562-588.
SIRRIDGE, M. S. (1967) Laboratory evaluation of haemostasis.
Philadelphia, Lea & Febiger.
SNEDECOR, J. W. & COCHRAN, W. G. (1967) Statistical methods 6th ed.,
Ames, IA, Iowa State University Press, pp. 111-114, 221-223.
SONTAG, J. M., PAGE, N. P., & SAFFIOTTI, U. (1975) Guidelines for
carcinogen bioassay in small rodents. Bethesda, MD, National
Cancer Institute.
STAUBLI, W., HESS, R., & WEIBEL, E. R. (1969) Correlated morphometric
and biochemical studies on the liver cell. II. Effects of
phenobarbital on rat hepatocytes. J. cell. Biol., 42: 92-112.
THOMPSON, W. (1947) Use of moving averages and interpolation to
estimate median effective dose. Bac. Rev., 11: 115-141.
TOMATIS, L. & MOHR, U., ed. (1973) Transplacental carcinogenesis,
IARC Sci. Publ. No. 4, 181 pp.
WEIL, C. S. (1952) Relationship between short- and long-term feeding
studies in designing an effective toxicity test. Agric. Food
Chem., 11: 486-491.
WEIL, C. S., WOODSIDE, M.D., BERNARD, J. R., & CARPENTER, C. P. (1969)
Relationship between single peroral, one-week and ninety-day
feeding studies. Toxicol. appl. Pharmacol., 14: 426-431.
WEIL, C. S., & CARPENTER, C. P. (1969) Abnormal values in control
groups during repeated-dose toxicological studies. Toxicol.
appl. Pharmacol., 14: 335-339.
WEINBERG, M. S., GOLDHAMEN, R. E., & CARSON, S. (1966) Acute oral
toxicity of various drugs in newborn rats after treatment of the
dam during gestation. Toxicol. appl. Pharmacol., 9: 234-239.
WORDEN, A. N. & HARPER, K. H. (1963) Oral toxicity as influenced by
method of administration. Proc. Eur. Soc. Study Drug Toxicity,
2: 15-26.
WHO (1958) WHO Technical Report Series No. 144. (Procedures for the
testing of intentional food additives to establish their safety
for use -- 2nd report of the joint FAO/WHO Expert Committee on
Food Additives), 19 pp.
WHO (1972) Health hazards of the human environment, Geneva, WHO,
387 pp.
YEARLY, R. A. & BENISH, R. A. (1965) A comparison of the acute
toxicities of drugs in newborn and adult rats. Toxicol. appl.
Pharmacol., 7: 504.
ZBINDEN, G. (1973) Progress in toxicology: special topics. Vol. 1.
New York, Springer-Verlag.
ZIMMERMAN, H. J. (1974) Serum enzyme measurement in experimental
hepatotoxicity. Israel J. med. Sci., 10: 328-332.
4. CHEMOBIOKINETICS AND METABOLISM
4.1 Introduction
The objective of chemobiokinetic studies is to obtain data that
allow reliable assessment of the hazard of environmental chemicals to
man. Since effects are related to the amounts or concentrations of a
chemical in tissues and cells, it is imperative to elucidate the
dynamics of the toxicant at the target site. It should be emphasized
that the toxicant may be either the parent chemical or a metabolite or
degradation product formed from it. Thus, the qualitative
identification of the degradation products of a chemical together with
a quantitative characterization of their fate, as well as the fate of
the parent chemical, as a function of time, are inextricably
associated in a proper chemobiokinetic evaluation. In the context of
this chapter, the word "chemobiokinetics" has been used in place of
"pharmacokinetics" because too often the latter implies restriction of
this scientific discipline to drugs. The term chemobiokinetics is
proposed to emphasize its importance in evaluating the biological
effects of all chemicals.
4.2 Absorption
4.2.1 General principles
Absorption of a chemical into the body can take place,
potentially, by all routes of exposure. In assessing the toxicity and,
ultimately, the hazard of a chemical, the oral, dermal, and inhalation
routes of exposure are of primary importance. Following absorption,
the chemical is distributed by the blood to the various tissues.
Therefore, the rate of absorption is frequently estimated by
determining the concentration of the chemical in the plasma as a
function of time following exposure.
The route of administration can greatly influence the rate at
which a foreign chemical enters the body. Upon ingestion, the gastric
contents and pH of the stomach can influence the rate of absorption of
the chemical. In the small intestine, food may either enhance or delay
absorption. Indeed, the environment of the gastrointestinal tract (pH,
food, bacteria) may change the parent chemical into another chemical.
The inhalation route allows a chemical to pass rapidly into the blood
without encountering drastic changes in pH, food, or microflora. The
skin effectively retards the absorption of many chemicals; however, it
should not be considered as an absolute barrier. Some chemicals
readily penetrate intact skin and a minor abrasion of the skin may
greatly enhance the absorption of many chemicals.
In order that a chemical may be absorbed into the bloodstream, it
must cross one or more semipermeable membranes, such as the
gastrointestinal epithelium, the lining of the respiratory tract, or
the epidermis of the skin. Membranes are essentially lipoproteins with
aqueous pores through which water-soluble molecules can pass. The pore
size varies from 4 Å (intestinal epithelium and mast cells) to 30 Å
(capillaries), allowing the passage of molecules with molecular
weights less than 100-200 to approximately 60 000, respectively. Most
membranes have an electrical potential that may effectively preclude
the ready penetration of charged chemical species. Thus it is obvious
that the absorption of a chemical depends on its physicochemical
properties, molecular size, shape, degree of ionization, and lipid
solubility. For a more thorough discussion, the reader is referred to
Davson & Danielli (1952) and Schanker (1962a).
Three mechanisms have been proposed to explain how a chemical
passes across a cell membrane: (a) passive diffusion through the
membrane, (b) filtration through membranous pores, and (c) specialized
transport systems that carry water-soluble and large molecules across
the membrane by means of a "carrier".
Passive diffusion is considered to be the principal mechanism by
which chemicals can cross cell membranes. The rate of passive
diffusion of a molecule is proportional to the concentration gradient
across the membrane, the membrane thickness, the area available for
diffusion, and the diffusion constant, in accordance with Fick's Law
(La Du et al., 1972). The rate of passage is related directly to the
lipid solubility (Brodie, 1964). However, since absorption requires
passage through an aqueous- as well as a lipo-phase, the absorption of
a chemical with an extremely low solubility in water may be impeded in
spite of a high lipid-to-water partition coefficient. The passive
diffusion also depends on the extent of the ionization and the lipid
solubility of the ionized and nonionized species (Brodie, 1964).
Filtration is a process by which a chemical passes through the
aqueous pores in the membrane, and is governed by the size and shape
of the molecule. The bulk flow of water across the membrane produced
by an osmotic gradient or hydrostatic pressure can act as a carrier
for chemicals.
Specialized transport processes are needed to explain the
transport and kinetic behaviour of large, lipid insoluble molecules
and ions. Two types of carrier-mediated transport systems have been
recognized: active transport and facilitated diffusion. The carrier in
both systems is some component of the membrane that combines with the
chemical and assists its passage across the membrane. It has a limited
capacity and when it is saturated, the rate of transfer is no longer
dependent on the concentration of the chemical and assumes zero order
kinetics. Structure, conformation, size, and charge are important in
determining the affinity of a molecule for a carrier site, and
competition for carrier site will occur.
Active transport is a carrier-mediated transport system which
moves a molecule across a membrane against a concentration gradient,
or, if the molecule is an ion, against an electrochemical gradient. It
requires the expenditure of metabolic energy and can be inhibited by
poisons that interfere with cell metabolism. Active transport plays an
important role in the renal and biliary excretion of chemicals.
Facilitated diffusion is a carrier transport mechanism by which a
water-soluble molecule (i.e. glucose) is transported through a
membrane down a concentration gradient. No apparent energy is required
and metabolic poisons will not inhibit this process. The difference
between facilitated diffusion and active transport is that the latter
moves molecules against a concentration gradient, whereas the former
does not. For more complete discussion of membrane transport, refer to
La Du et al. (1972) and Goldstein et al. (1974).
Another active process, pinocytosis, has been implicated as a
mechanism for transferring large molecules and particles into cells.
In this process, the membrane engulfs the material and pinches off an
envelope containing the material within the cell.
4.2.2 Absorption from the lungs
The pulmonary epithelial lining is very thin, possesses a large
surface area, and is highly vascular. Thus, absorption of foreign
chemicals can take place at a very rapid rate. Most rapidly absorbed
are gases and aerosols with small particle size and a high
lipid-to-water partition coefficient. In most inhalation studies,
absorption may occur by routes other than the lungs and the
investigator should be aware of this in the interpretation of data.
A more complete discussion of the inhalation of chemicals is
presented in Chapter 6.
4.2.3 Absorption from the skin
The structure of the skin enables rapid penetration of
lipid-soluble compounds through the epidermis, a lipoprotein barrier,
whereas the highly porous dermis is permeable to both lipid- and
water-soluble substances (Katz & Poulsen, 1971). Factors which govern
penetration through the skin are hydration, pH, temperature, blood
supply, and metabolism as well as vehicle-skin interactions. Abrasion
of the skin may enhance absorption greatly. For a more complete
discussion of the principles for absorption through the skin and
experimental methods, refer to Part II, Chapter 11.
4.2.4 Gastrointestinal absorption
The gastrointestinal tract is one of the most important routes of
absorption of foreign compounds (Schanker, 1971). Chemicals can be
absorbed along any section of the gastrointestinal tract, but because
of the large surface area and rich blood supply, absorption is
favoured from the small intestines. In most parts, the movement of a
chemical across the epithelial lining of the gastrointestinal tract is
by diffusion and carrier transport mechanisms are involved to a lesser
degree.
Although therapeutic amounts of drugs may be absorbed from the
buccal mucosa (Beckett & Hossie, 1971), absorption of environmental
chemicals from the mouth is minimal compared with that from the
stomach and intestine. Chemicals absorbed from the mouth are not
exposed to the gastrointestinal digestive juices and drug-metabolizing
enzymes. Furthermore, since they are not transported by the hepatic
portal system directly to the liver, their normally rapid metabolism
may be precluded, thus prolonging their effect.
The stomach is a significant site of absorption by passive
diffusion of many acid, and neutral, foreign compounds (Schanker et
al., 1957). Due to the acidity of the stomach, weak acids will exist
in the diffusible, nonionized, lipid-soluble form, whereas weak bases
will be highly ionized and therefore not generally absorbable.
Absorption from the small intestine is similar in principle to
that from the stomach (passive diffusion), except that the pH of the
intestinal contents (pH 6.6) may alter the fraction of the chemical in
the nonionized form favouring the absorption of both weakly-acid and
weakly-alkaline chemicals. The aqueous pore size, 4 Å, limits
absorption by filtration to molecules having a molecular weight of
less than 100-200. Rarely, an environmental chemical may be absorbed
from the intestinal tract by an active transport system that is
normally involved in the absorption of nutrients, e.g. sugars and
amino acids (Schanker, 1963).
Many factors can affect the absorption of foreign compounds from
the gastrointestinal tract (Brodie, 1964; Levine, 1970; Place &
Benson, 1971; Prescott, 1975): ( a) increased gastric emptying can
decrease gastric absorption and increase intestinal absorption; ( b)
increased intestinal peristalsis generally inhibits intestinal
absorption; ( c) gastric acid, intestinal digestive juices, and gut
microflora can all degrade chemicals to other absorbable or
nonabsorbable chemical species; ( d) food in the gastrointestinal tract
can impair absorption by producing a nonabsorbable complex, by
decreasing gastric emptying (especially fats), and by reducing,
mixing, or altering pH; ( e) normal digestion produces increased
gastrointestinal blood flow which will enhance absorption; ( f)
absorption of a solid will be impaired if dissolution in the
gastrointestinal tract does not take place. The practice of
administering chemicals admixed with the diet must take these factors
into account, especially the possible reaction of the chemical with
dietary constituents.
4.3 Distribution
Once absorbed, the distribution of a chemical is determined by
the relative plasma concentration, the rate of blood flow through
various organs and tissues, the rate by which the chemical penetrates
cell membranes, and the binding sites that are immediately available
in the plasma and tissues. After the initial distribution phase, the
rate by which a chemical penetrates cell membranes and the available
sites for binding are the predominating factors influencing the final
distribution of a chemical in the body.
When the plasma concentration of a chemical is high and the cell
membranes do not provide significant barriers to diffusion,
distribution is mainly to organs with high blood flow, e.g. brain,
liver, and kidney. A classic example of distribution and
redistribution is thiopental, a highly lipid-soluble chemical that,
after administration, is first distributed to the brain and
subsequently to muscle and body fat which have poor blood flow (Price
et al., 1960). Lipid-soluble, foreign compounds tend to be distributed
and localized in adipose tissue (Mark, 1971), in accordance with their
lipid-to-water partition coefficients, e.g. the chlorinated
hydrocarbon pesticides, dieldrin, DDT, and DDE (Rodomski et al., 1968)
and polychlorinated biphenyls (PCBs) (Allen et al., 1974).
Distribution of chemicals into organs and tissues is influenced by
membraneous barriers in the same way as absorption (see 4.2). For a
more detailed treatment, see Quastel (1965). The capillary membrane,
unlike other body or cell membranes, is freely permeable to foreign
compounds of a molecular weight of 60 000 or less, whether
lipid-soluble or not (Pappenheimer, 1953; Renkin, 1964); generally
chemicals pass these membranes readily, except in the brain,
testicles, and the eye (Gehring & Buerge, 1969).
The movement of foreign chemicals to the brain represents a
unique example that cannot be explained by the physicochemical
properties of the chemical and the tissue distribution. Many chemicals
fail to penetrate into the brain tissue or cerebrospinal fluid as
readily as into other tissues (Brodie & Hogben, 1957). The boundary
between blood and brain consists of several membranes; those of the
blood capillary wall, the glial cells closely surrounding the
capillary, and the membrane of the neurons or nerve cells. The
so-called "blood-brain barrier" is located at the capillary wall-glial
cell region. The capillary walls in the brain tend to be more like
cell membranes than capillary membranes. Therefore, ionized substances
and large water-soluble molecules such as proteins are almost entirely
excluded from passage (Rall, 1971). The chief mode of exit of both
lipid-soluble and polar compounds is by filtration across the
arachnoid villi. The method for studying the movement of chemicals
into and from the brain has been discussed by Rall (1971).
The red blood cell has unusual permeability in that organic
unions penetrate much more readily than cations. This may be explained
by the presence of positively charged membrane pores that will accept
anions but repel cations (Schanker et al., 1957).
4.4 Binding
A major factor, that can affect the distribution of a chemical,
is its affinity to bind to proteins and other macromolecules of the
body. Foreign chemicals have been shown to bind reversibly to such
substrates as albumen, globulins, haemoglobin, mucopolysaccharides,
nucleoproteins, and phospholipids (Shore et al., 1957). For a survey
of the biological implications of the protein binding of chemicals,
the reader is referred to Gillette (1973a).
Once a chemical is bound to a body constituent, it is temporarily
localized. This localization modifies the initial pattern of
distribution and affects the rates of absorption, metabolism, and
elimination of the chemical from the body.
4.4.1 Plasma-protein binding
Most chemicals show some degree of binding to plasma proteins,
the most important fraction of which is albumen. Albumen at pH = 7.3
contains a net negative charge; however, cationic groups must be
accessible because albumen has been shown to bind anions as well as
cations. Although the plasma proteins show an appreciable capacity for
binding many chemicals, this is limited, making it important to
understand such binding as a function of the concentration of the
chemical.
Since plasma proteins possess a limited number of binding sites
and the sites are somewhat nonspecific, two chemicals with an affinity
for the same binding site will compete with one another for binding.
The plasma proteins of various laboratory animals and man show
differences in the degree and nature of binding. This is due to
differences in the total concentrations and relative proportions of
the various plasma proteins as well as the composition and
conformation of albumens (Gillette, 1973b).
4.4.2 Tissue binding
The binding of chemicals to tissue constituents also contributes
to the localization of a chemical. Certain chemicals show a much
greater affinity for tissue than for plasma proteins, and in some
instances the affinity for tissue is quite specific. For example,
polycyclic aromatic compounds have been shown to have particular
affinity for the melanin in the eye (Potts, 1964).
Some metals and several chemicals and organic anions are bound to
proteins (Y and Z proteins or ligandins) in the liver (Levi et al.,
1969). These proteins may play a key role in the transfer of organic
anions from plasma to liver (Levi et al., 1969; Reyes et al., 1971),
and they also bind corticosteroids and azo-dye carcinogens (Litwack et
al., 1971). For further details concerning the nature and effects of
binding of chemicals by proteins and for methods of study, see
Chignell (1971), Gillette (1975), Keen (1971) and Settle et al.
(1971).
Many inorganic ions, particularly metals, as well as
tetracycline, are concentrated in various tissues and organs,
particularly in bones and teeth (Foreman, 1971). A convenient method
for studying the accumulation of chemicals in organs and tissue is
autoradiography (Roth, 1971). Valuable measurements may also be
obtained with classical chemical and radio-chemical techniques which
have the added advantage of being quantitative.
4.5 Excretion
Chemicals are excreted as the parent chemical, as metabolites, or
as conjugates of the parent chemical or its metabolites. The principal
routes of excretion are the urine and bile, and to a lesser degree
expired air, sweat, saliva, milk, and secretions of the
gastrointestinal tract.
4.5.1 Renal excretion
The kidneys are the most important route of excretion of foreign
compounds (Weiner, 1971). The three mechanisms of renal excretion are:
glomerular filtration, active tubular transport, and passive tubular
transport. Only compounds of high molecular weight or those bound
tightly to plasma proteins escape glomerular filtration and the
resulting filtrate contains approximately the same concentration of
foreign compounds as that found in the plasma in an unbound state.
Water and endogenous substrates are reabsorbed from the
glomerular filtrate as it passes down the tubule. In the tubule,
lipid-soluble, unionized chemicals pass in either direction by passive
diffusion. Thus, lipid-soluble chemicals may be reabsorbed by the
tubule, prolonging their retention in the body. Ionic chemicals, such
as conjugates and other metabolites, are poorly reabsorbed and pass
directly out of the body in the urine.
Active transport takes place in the proximal tubule of the
kidney. There are two distinct active transport processes. One process
is specific for organic anions and the other specific for organic
cations. Chemicals transported by the same transport process compete
with each other, and the excretion rate of one compound can be reduced
by the administration of the other. The active transport process can
be saturated as the concentration of the chemical in the plasma is
increased. When the active tubular secretion is saturated, that is,
when an increase in the concentration of the chemical in the plasma is
no longer accompanied by a proportional increase in the concentration
of the chemical in the urine, the concentration in the plasma is
referred to as the renal-plasma threshold.
The anionic secretory process is responsible for the excretion of
metabolites formed through conjugation of the parent chemical or its
degradation products with various endogenous substrates such as
glycine, sulfate, or glucuronic acid. These relatively polar,
lipid-insoluble metabolites are poorly reabsorbed from the tubules and
more readily excreted.
4.5.2 Biliary excretion
Biliary excretion is a major route for the excretion of foreign
chemicals (Smith, 1971a, 1973). It is has been demonstrated (Brauer,
1959; Schanker, 1962b; Sperber, 1963; Williams, 1965) that compounds
with high polarity, anionic and cationic conjugates of compounds bound
to plasma proteins, and compounds with molecular weights greater than
300 are actively transported against a concentration gradient into the
bile. It has also been shown that, once these compounds are in the
bile, they are not reabsorbed into the blood and are excreted into the
gastrointestinal tract (Schanker, 1965). Factors that influence the
biliary excretion of foreign chemicals and metabolites are considered
to be of two types: (a) physicochemical, relating to molecular size,
structural features, and polarity; and (b) biological, relating to
protein binding, renal excretion, metabolism, species, and sex. For a
comprehensive and detailed discussion of these subjects, the reader is
referred to Smith (1971a, 1973), and Stowe & Plaa (1968).
4.5.3 Enterohepatic circulation
Enterohepatic circulation is the phenomenon that occurs when a
compound is excreted via the bile into the gastrointestinal tract,
reabsorbed from the gastrointestinal tract and carried via the portal
system back to the liver, where it is again excreted via the bile and
recycled. Physiologically, enterohepatic circulation is important
because it permits reuse of endogenous biliary excretion products.
However, when a foreign compound is involved in enterohepatic
circulation, it must make its way either to the faeces or to the
peripheral blood to be excreted from the body. Thus, enterohepatic
circulation of a foreign compound serves to enhance its retention in
the body. There are examples in the literature (Gibson & Becker, 1967;
Keberle et al., 1962) which demonstrate that the half-life of a
compound involved in enterohepatic circulation can be decreased after
surgically interrupting the enterohepatic cycle. Administration of a
sequestering agent that binds the compound in the gastrointestinal
tract would serve the same purpose.
Smith (1973) has described the following factors that can affect
the enterohepatic circulation of a compound: ( a) the extent and rate
of excretion of the compound in the bile; ( b) the activity of the gall
bladder; ( c) the fate of the substance in the small intestine; and ( d)
the fate of the compound after reabsorption from the gut. Since many
foreign chemicals are excreted in the bile as unabsorbable conjugates,
the hydrolysis of these conjugates in the intestine may play a key
role in enterohepatic circulation. For a thorough discussion of
enterohepatic circulation, the reader is referred to Plaa (1975).
4.5.4 Other routes of excretion
In addition to excretion in bile and urine, other routes for the
excretion of foreign chemicals and their metabolites should not be
overlooked. In accordance with the pH partition theory, organic bases
highly ionized at the pH value of gastric juice may be secreted into
the stomach (Shore et al., 1957). Similarly, weak acids ionized at
neutral pH may be transferred from the plasma to the lumen of the
intestine. These chemicals are sequestered by the intestinal contents,
augmenting their excretion in the faeces.
Many volatile organic chemicals are excreted readily via exhaled
air (see Chapter 6). This route of excretion is common for carbon
dioxide, an ultimate end-product of an extensively metabolized organic
chemical. For this reason, the quantification of expired radiolabelled
carbon dioxide (14CO2) is very important in chemobiokinetic
studies using carbon-14-labelled compounds.
Many foreign compounds are excreted, to different degrees, in
milk, in either the aqueous or lipid phase (Rasmussen, 1971). Although
this route may be of minor importance for the elimination of a
chemical from the body, it should be given particular attention in
evaluating the hazard of chemicals to man. First, consumption of cow's
milk may constitute an important vehicle of exposure. Secondly, the
consumption of mother's milk by the newborn may provide very high
doses of a chemical that is concentrated in the milk. It should also
be noted that the volume of milk consumed by the newborn per unit body
weight may, in itself, magnify the dose received by this segment of
the population.
Chemicals are also excreted in sweat and saliva. The presence of
a chemical in sweat may lead to dermatitis. Although saliva is usually
swallowed and thus does not lead to elimination of the agent from the
body, recent work has shown that analysis of saliva for the presence
of a chemical may preclude the necessity for venepuncture to obtain
plasma for analysis.
4.6 Metabolic Transformation
Metabolic transformation or biotransformation are terms that have
been used to describe the process which converts a foreign chemical to
another derivative (metabolite) in the body. Metabolic transformation
has been the subject of several excellent reviews (Conney, 1967;
Conney & Burns, 1962; Dahm, 1971; Daly, 1971; Dutton, 1971; Garattini
et al., 1975; Gillette, 1971a,b & 1974a,b; Gillette et al., 1974;
Kuntzman, 1969; McClean, 1971; Smuckler, 1971; Weisburger &
Weisburger, 1971). It usually results in the formation of more polar
and water-soluble derivatives of a foreign chemical which can be more
readily excreted from the body. Generally, such metabolic
transformation of a foreign chemical also results in the formation of
a less toxic chemical. However, there are many cases where the
metabolites are more toxic than the parent chemicals (McLean, 1971;
Miller & Miller, 1971a).
A few compounds resist metabolic transformation. Most strong
acids and bases are excreted unchanged. Also the resistance of
long-acting nonpolar compounds (barbital, halogenated benzene, etc.),
to metabolic transformation might explain their slow elimination from
the body.
A metabolic activation is suggested, if a compound is more toxic
when given orally than intravenously, if there is a long delay between
the administration of a chemical and the onset of its biological
effect, or, if there is an increased effect following pretreatment
with compounds that induce metabolic transformation (Garattini et al.,
1975).
4.6.1 Mechanisms of metabolic transformation
Usually, the metabolic transformation of chemicals takes place to
the greatest extent in the liver and is catalysed by enzymes found in
the soluble, mitochondrial, and microsomal fractions of the cell.
Enzymes metabolizing foreign chemicals are also found, to a lesser
degree, in the cells of the gastrointestinal tract, kidney, lung,
placenta, and blood (Aitio, 1973; Gillette, 1963; Gram, 1973;
Hietanen, 1974; Hietanen & Valinio, 1973; Wattenberg & Leong, 1971;
Wattenberg et al., 1962; Witschi, 1975). It must be emphasized that
for a particular chemical, or a particular route of administration,
other organs may play a more important role in the metabolic
transformation of the chemical than the liver. The role of enzymatic
reactions carried out by the intestinal flora may be very important
and should not be overlooked (Scheline, 1968; Smith, 1971a).
Enzyme-catalysed, biochemical transformations can be classified into
four main types: (a) oxidations, (b) reductions, (c) hydrolyses and
(d) synthetic reactions (see Table 4.1 in Annex to this Chapter).
The metabolic transformation of a chemical can occur via various
pathways which can consist of a single reaction or multiple reactions.
If the metabolic pathway consists of one reaction it is usually
oxidation, reduction, or hydrolysis which tends to increase the
polarity of the compound. Multiple-reaction metabolic pathways can
consist of a series or any combination of oxidation, reduction, or
hydrolysis. The final reaction in a multiple-reaction pathway is
usually a conjugation reaction involving the addition of polar
endogenous functional groups (D-glucuronic acid, glycine etc.) which
usually render the molecule more polar, less lipid-soluble, and
therefore more readily excretable. The predominant sequence of
reactions or metabolic pathways is determined by many factors such as
the dose of the chemical, species, strain, age, sex, and certain
environmental variables.
4.6.1.1 Microsomal, mixed-function oxidations
The metabolism of a large variety of foreign compounds involves
oxidative processes. Microsomal oxidation refers to reactions
catalysed by the enzymes found in the microsomes of the endoplasmic
reticulum. These enzymes are sometimes referred to as microsomal,
mixed-function oxygenases (mono-oxygenases) (Mason, 1957). The
reactions require molecular oxygen and nicotinamide adenine
dinucleotide phosphate, reduced form (NADPH). The reduction
equivalents from NADPH are used to reduce molecular oxygen so that it
can be carried by a cytochrome called P-450 to the compound to be
oxygenated. The oxygen is then fixed into the compounds, usually as a
hydroxyl group (Estabrook, 1971; Estabrook et al., 1971).
The apparent sequence of events in the course of a mixed function
oxidation has been described (Boyd & Smellie, 1972; Estabrook et al.,
1972; Gillette, 1971c). The compound (substrate) forms a complex with
the oxidized cytochrome P-450; this is reduced either directly by
NADPH-cytochrome- c-reductase (1.6.2.4) or indirectly via an
unidentified electron carrier. The reduced cytochrome P-450-substrate
complex then reacts with oxygen to form an "active oxygen" complex,
which decomposes with the formation of the oxidized substrate and
oxidized cytochrome P-450. Substantial progress has been made in
elucidating this mechanism by the development of a method involving
the resolution and reconstitution of the components of the liver
microsomal hydroxylating system (Lu & Levin, 1974).
Measurement of mixed-function oxidase activities of liver
microsomes in vitro has become an important aspect in evaluating the
toxicity of chemicals. The mixed-function oxidase system may be either
a biotransformation system or a site of action of chemicals.
Measurements of the activity of this system can be performed using
either a 9000 g supernatant fraction (Henderson & Kersten, 1970;
Klinger, 1974) of a liver homogenate prepared in buffered KCl
solution, or a microsome fraction sedimented by centrifugation at
about 105 000 g (Flynn et al., 1972; Hewick & Fouts, 1970a,b; Liu et
al., 1975).
The reaction mixtures consisting of the particle-bound enzymes
have to be supplemented with an NADPH-generating system. This may be
fulfilled by the addition of NADPH and glucose-6-phosphate, if the
9000 g fraction is used, but if washed microsomes are used,
glucose-6-phosphate dehydrogenase (1.1.1.49) must also be added.
Isolated tissue cells, tissue cultures, or slices of organs, as
well as perfused organs can also be used for metabolic studies.
Because cytochrome P-450 is intimately associated with the
metabolism of many foreign chemicals, the following methods and
variables have been developed for ascertaining its activity in the
tissues of animals used in toxicological investigations.
The method of Omura & Sato (1964a,b) has been used to measure the
change in the microsomal content of cytochrome P-450 and cytochrome
b5. This method relies on a spectral shift of the pigment upon
exposure to carbon monoxide. An increase in the cytochrome P-450
content can be explained as a consequence of enzyme induction, whereas
the decrease of the haem pigment content may be the result of enhanced
permeability of microsomal membranes due to the damaging effects of
the chemical (Bond & De Matteis, 1969). The concentration of
cytochrome P-450 in the liver, however, is not always directly
proportional to the activity of the mixed-function oxidases.
Spectral changes of cytochrome P-450, determined in the presence
of various substrates, provide information about the binding between
the pigment and substrate (Hewick & Fouts, 1970a; Remmer et al.,
1966). Compounds may be classified into type I or type II according to
their spectral reactions with cytochrome P-450. When type I compounds
bind to cytochrome P-450, the characteristic spectral shift, spectral
difference, gives a peak at 385-390 nm and a trough at 418-427 nm,
whereas with type II compounds, the peak occurs at 425-435 nm and the
trough at 390-405 nm. Originally, it was thought that the magnitudes
of these spectral shifts, especially type I spectra, could be
correlated with microsomal biotransformations (Schenkman et al.,
1967). This correlation, however, is not universally applicable
(Davies et al., 1969; Gigon et al., 1969; Holtzman et al., 1968).
Thus, differences in the magnitude of these spectral changes are
difficult to interpret when they are detected in animals treated with
a chemical (Gillette et al., 1972). The same is true for the
ethylisocyanide difference spectra of cytochrome P-450 which are
characterized by two peaks at about 455 nm and 430 nm (Omura & Sato,
1964a).
Determination of the NADPH-cytochrome P-450 reductase activity,
the assumed rate limiting step in microsomal oxidations, has proved
useful in evaluating the effectiveness of the cytochrome P-450 system
prior to the oxygenation step (Fouts & Pohl, 1971; Gigon et al., 1969;
Hewick & Fouts, 1970b; Holtzman et al., 1968; Zannoni et al., 1972).
Measurement of the NADPH-cytochrome-c-reductase activity may give
information about the rate of flow of reducing equivalents from NADPH
to cytochrome P-450.
Determination of the rate of enzymatic conversion of a substrate
is a most valuable tool in elucidating the metabolic process. For this
purpose, however, it is essential to know the pathway for the
transformation of the chemical, and analytical methods are essential
to quantify the parent chemical and its reaction products. Selected
methods for monitoring some compounds and enzymatic reactions are
listed in Table 4.2 (see Annex to this Chapter).
There are large variations in the metabolism of foreign chemicals
as well as in susceptibility to metabolic inducers depending on the
species (Hucker, 1970), strain, age, and sex of animals.
Many variables must be considered as important factors in species
differences in the metabolism of foreign chemicals. Among these are
differences in binding, either to tissues or to plasma components,
such as albumen. Considerable variations in binding have been reported
for the same chemical in different species (Borgå et al., 1968; Kurz &
Friemel, 1967; Scholtan, 1963; Sturman & Smith, 1967; Witiak &
Whitehouse, 1969). More obvious are the concentrations and types of
foreign chemical-metabolizing enzymes in each species (Flynn et al.,
1972).
4.6.1.2 Conjugation reactions
The major conjugation mechanisms are: glucuronide synthesis,
"ethereal" sulfate synthesis, glutathione conjugation, glycine
conjugation, methylation, acetylation, and thiocyanate synthesis.
Glutamine conjugation has also been shown to occur in man and monkey.
The conjugates formed by these mechanisms are usually nontoxic,
therefore conjugation has also been referred to as a detoxification
mechanism.
These conjugations are biosynthetic reactions in which foreign
compounds or their metabolites containing suitable groups (hydroxyl,
amino, carbonyl, or epoxide) combine with some endogenous substrates
to form conjugates (Parke, 1968; Williams, 1967a, 1971). These
reactions require ATP as source of energy, coenzymes, and transferases
which are usually specific for the formation of conjugates of foreign
compounds. The conjugations usually proceed in at least two steps:
first, the extramicrosomal synthesis of acylcoenzyme and next the
transfer of the acyl moiety to the aglycone, which, in some but not
all cases, is localized in the microsomes. Thus, these reactions
cannot be considered as transformations, characteristic of microsomes.
In accordance with the coenzymes participating in these
reactions, they include:
formation of glucuronides (via uridine diphosphate glucuronic
acid, UDPGA);
formation of sulfate esters (via 3-phosphadenosine-5-
phosphosulfate, PAPS);
O-, N-, and S-methylation via 5'-[(3-amino-3-carboxypropyl)
methylsulfonio]-5'-dioxyadenosine( S-adenosylmethionine);
acetylations (via acetyl coenzyme A);
formation of peptide conjugates (via different acylcoenzyme A
derivatives);
formation of glutathione conjugates and mercapturic acids
(conjugations with glutathione).
Formation of glucuronides is probably the most important
microsomal conjugation mechanism (Dutton, 1971). It occurs in the
liver and to a lesser extent in the kidney, gastrointestinal tract,
and the skin. Biosynthesis of glucuronides can be measured in intact
animals by determining D-glucaric acid (Marsh, 1963) and
D-glucuronolactone dehydrogenase (1.1.1.70) (Marselos & Hanninen,
1974), by enhancement of D-glucuronolactone and aldehyde dehydrogenase
(1.2.1.3) by inducers of microsomal metabolism (Marselos & Hanninen,
1974), glucuronides (Gregory, 1960; Yuki & Fishman, 1963) and
L-ascorbic acid in urine. Elevation in urinary excretion of these
compounds may be an indicator of an adaptive acceleration of hepatic
glucuronide formation (Notten & Henderson, 1975). It should be
emphasized that increased excretion of D-glucaric acid can result from
enzyme induction; therefore it cannot be assumed that this occurrence
is indicative only of an increased glucuronide formation. Methods for
the measurement of glucuronide synthesis in whole organs and tissue
cultures, as well as in tissue slices, have been summarized by Dutton
(1966). In assays with homogenates and cell fractions the reaction
mixtures have to be supplemented with added UDPGA.
UDP-glucuronosyl transferase (2.4.1.17) activity can be
determined using 2-aminophenol (Burchell et al., 1972; Dutton &
Storey, 1962), 4-nitrophenol (Isselbacher 1956; Zakim & Vessey, 1973),
bilirubin (Heirwegh et al., 1972), 7-hydroxy-4-methyl-2H-I-
benzopyran-2-one (4-methylumbelliferone) (Aitio, 1973; Arias, 1962) or
morphine (Strickland et al., 1974).
In contrast to glucuronide synthesis, the formation of sulfate
esters is most probably an extramicrosomal process and is catalysed
generally by sulfate-conjugating enzymes in the presence of
3-phosphoadenosine-5-phosphosulfate as a co-enzyme (Roy, 1971). Among
the compounds of toxicological interest, phenols are converted by
sulfation to esters and excreted in the urine. Aminophenols yield
sulfamates. There are specific assays for the determination of
sulfotransferase (2.8.2) activity using 4-nitrophenol (Gregory &
Lipmann, 1957), or 3-(2-aminoethyl)-1H-indol-5-ol (serotonin) (Hidaka
et al., 1967) as acceptors.
The methyltransferases (2.1.1) catalyse O-, N- and
S-methylation of several physiologically active compounds and drugs
(Axelrod, 1971). They are widely distributed in different organs, but
only a small amount of catechol- O-methyltransferase (2.1.1.6) and
almost all of the phenol- O-methyltransferase (2.1.1.25) (Axelrod &
Daly, 1968) activity is localized in the microsomes of the liver. Only
microsomal transferases are induced by benzo(a)pyrene and inhibited by
SKF 525Aa. The methods used for the determination of
catechol- O-methyltransferase activity are based on the principle
that the enzyme catalyses the transfer of methyl groups to catechols
in the presence of S-adenosylmethionine as a methyl donor. The
substrates employed include adrenaline (Axelrod & Tomchick, 1958),
3,4-dihydrobenzoic acid (MacCaman, 1965), 3,4-dihydroxybenzeneacetic
acid (3,4-dihydrophenylacetic acid) (Assicot & Bohuon, 1969; Broch &
Guldberg, 1971) as well as I-(3,4-dihydroxyphenyl)ethanone
(3,4-dihydroxyacetophenone) (Borchardt, 1974). The end products of the
enzymatic reaction are measured either spectrofluorimetrically
(Axelrod & Tomchick, 1958; Borchardt, 1974; Broch & Guldberg, 1971),
or radiometrically using labelled methyl groups in the coenzyme
(MacCaman, 1965).
a Diethyl aminoethanol ester of diphenyl-propyl acetic acid.
Acetylation reactions of the amino group of foreign compounds are
catalysed by acetyltransferases (Weber, 1971). Substrates of these
enzyme reactions, localized in the soluble part of the cells, are
arylamines, hydrazines, and certain aliphatic amines. Coenzyme A is an
essential factor in these acetylations. Acetylation of arylamines has
been studied quantitatively, in vivo, in human beings and animals
(Williams, 1967b).
Methods for the determination of N-acetyltransferase (2.3.1.35)
activities in vitro summarized by Weber (1971) include colorimetric
(Brodie & Axelrod, 1948; Maher et al., 1957; Marshall, 1948; Shulert,
1961; Weber, 1970), spectrophotometric (Jenne & Boyer, 1962; Tabor et
al., 1953; Weber & Cohen, 1968; Weber et al., 1968) as well as
radiometric procedures (Stotz et al., 1969).
Conjugation of aromatic carboxylic acids (benzoic acid,
substituted benzoic acids, and heterocyclic carboxylic acids) with
amino acids by means of acetyl coenzyme A and ATP is called peptide
conjugation. Glycine is the most generally involved amino acid in this
reaction resulting in the formation of N-benzoylglycine (hippuric
acid). Indole-3-acetic acid, benzeneacetic acid, as well as
4-aminosalicylic acid, can conjugate with glutamine in man, and
several mammals. Determination of hippuric acids (Ogata et al., 1969)
enables the quantitative investigation of this conjugation reaction.
Conjugation of glutathione with foreign compounds, catalysed by
at least ten different glutathione S-transferases, is an important
pathway for the elimination of these compounds (Boyland, 1971).
Following the conjugation of foreign compounds with glutathione, the
conjugate is most frequently hydrolysed to the cysteine conjugate
which is excreted in the urine. Furthermore, the cysteine conjugate
may be acetylated and the resulting mercapturic acid excreted. The
significance of the mercapturic acid biosynthesis in man, however, is
difficult to assess.
Determination of glutathione S-transferase activities are based
on spectral change of the substrate (1,2-dichloro-4-nitrobenzene) due
to conjugation (Booth et al., 1961), or loss of glutathione content
(Boyland & Chasseaud, 1967; Boyland & Williams, 1965; Johnson, 1966)
or release of labile groups (Al-Kassab et al., 1963; Boyland &
Williams, 1965; Johnson, 1966) as well as on chromatographic
separation of the products (Suga et al., 1967). The determination of
the activity of gamma-glutamyltransferase (2.3.2.2), catalysing one
intermediary step of the overall mercapturic acid synthesis may also
be informative.
4.6.1.3 Extramicrosomal metabolic transformations
Foreign compounds, either transformed by oxidation or initially
having characteristic groups (hydroxyl, amino) may resemble normal
constituents of physiological metabolism. Thus, they may undergo
metabolic transformations similar to those of normal body
constituents: oxidation, reduction, deamination, hydrolysis. The
enzymes catalysing these reactions are localized in the cytosol or are
intrinsic compounds of the mitochondria.
In contrast to the extensive data in the literature on
enzyme-chemical interactions (MacMahon, 1971; Zeller, 1971) only a few
enzyme activities are commonly used to monitor toxicological events.
The alcohol dehydrogenase (1.1.1.1) of the liver is one of the
most important enzymes which catalyses the NAD-mediated oxidation of
various aliphatic and aromatic primary and secondary alcohols.
Determination of the activity of alcohol dehydrogenase is based on the
spectrophotometric measurement of the amount of NAD being reduced in
the presence of excess alcohol (Bonnichsen & Brink, 1955).
Among the amine oxidases, monoamine oxidase (1.4.3.4), localized
in the mitochondria, regulates the balance of the biogenic amines and
probably does not participate in the metabolism of foreign amines to a
great degree (Zeller, 1971). However, the fact that a large number of
substances (substrates and substrate analogues, alkyl and arylamines,
hydrazine derivatives, sulfhydryl reagents, etc.) inhibit this enzyme,
enables monoamine oxidase to be used as a tool in studies of the
toxicity of these inhibitors.
Monoamine oxidase activity can be measured manometrically
(Creasey, 1956) based on oxygen-consumption, by determination of
ammonia production (Cotzias & Dole, 1951), spectrophotometrically
(Dietrich & Erwin, 1969; Obata et al., 1971; Weissbach et al., 1960),
fluorimetrically (Takahashi & Takahara, 1968; Tufvesson, 1970) as well
as radiometrically (Otsuka & Kobayashi, 1964).
Hydrolysis by carboxylesterases (ali-esterases or arylesterases)
of foreign compounds containing ester groups may be important in
assessing their toxicity (La Du & Snady, 1971). Determination of
esterase activities using different substrates in the presence of the
chemical to be tested can disclose its possible inhibitory potency.
4.6.1.4 Nonenzymatic reactions
Although the foregoing sections have discussed enzymatic
modifications of chemicals, the investigator should not overlook
nonenzymatic, spontaneous reactions between chemicals and natural
constituents in the body that lead to the formation of metabolites,
e.g. the reaction of an alkylating agent with glutathione.
4.6.2 Species variability
A serious problem facing every research worker using an animal
species to study the metabolism of a foreign compound is whether or
not the metabolic pathway in the animal is similar to the metabolic
pathway in man. The problem is not only important in metabolic
studies, but is of utmost importance in using animal toxicity studies
to predict toxicological phenomena in man. Conney et al. (1974)
illustrated that the use of an animal species that metabolizes a
foreign compound in a similar manner to man will give a more precise
prediction of the type of toxicological phenomena to be expected in
man.
Different animal species have been shown to metabolize foreign
compounds at different rates. Quinn et al. (1958) has shown that
benzeneamine (aniline) has a metabolic half-time in the mouse of 35
minutes and in the dog of 167 minutes. In the same study it was
demonstrated that the metabolic half-time of an antipyrine in the rat
was 140 minutes, whereas in man it was 600 minutes.
Considerable species differences in metabolic pathways have also
been demonstrated. In the rat, mouse, and dog the carcinogen,
N-2-fluoranylacetamide (FAA), is N-hydroxylated to N-hydroxy-FAA
which is a more potent carcinogen than FAA. In the guineapig little or
no hydroxylation of FAA occurs. In toxicity studies, Miller & Miller
(1971b) and Weisburger et al. (1964) demonstrated that the rat, mouse,
and dog are susceptible to the carcinogenic activity of FAA, whereas
the guineapig is not. Thus, a difference in the metabolic pathways of
a foreign compound may greatly influence its toxicity.
Species variability in metabolism has been related to other
factors such as species differences in protein binding, and enzyme
concentration and type. Hucker (1970) described, in detail, species
differences in chemical metabolism and some of the factors responsible
for these differences.
4.6.3 Enzyme induction and inhibition
For some time it has been known that chemicals can increase the
activity of metabolizing enzyme systems. These chemicals have been
termed enzyme "inducers". Inducers exert their action by
quantitatively increasing the enzymes and components responsible for
the metabolism of foreign compounds. The importance of induction to
the toxicologist is two-fold. If metabolism leads to the formation of
excretable or nontoxic metabolites, induction will enhance
detoxification and excretion of the compound. However, if metabolism
leads to the production of a more toxic metabolite, induction will
increase the toxicity of a compound.
Many chemicals are known to increase metabolizing enzyme systems.
The reviews by Conney (1967), Kuntzman (1969), and Mannering (1968),
depict the large number of chemicals which induce metabolizing enzymes
and comprehensively review the factors involved in enzyme inductions.
Most inducers give maximum effects rather quickly -- within 2-3
days (Fouts, 1970). However, some require 2 weeks or longer (Gillette
et al., 1966; Hart & Fouts, 1965; Hoffman et al., 1968, 1970;
Kinoshita et al., 1966). Frequently, the degree of induction after
obtaining a maximum level may decline despite continuing treatment of
the animal with a chemical (Gillette et al., 1966; Hoffman et al.,
1968; Kinoshita et al., 1966).
Drug-metabolizing enzymes can also be depressed by foreign
chemicals, and these compounds are termed inhibitors. 2-(Diethylamino)
ethyl-alpha-phenyl-alpha-propyl benzeneacetate hydrochloride
(SKF-525A) is the best known of the inhibitors and is used routinely
in determining the effect of enzyme inhibition on the metabolism of
chemicals.
4.6.4 Metabolic saturation
In vivo saturation of metabolic pathways can play an important
role in determining the toxic profile of a chemical. A recent article
by Jollow et al. (1974) demonstrated the effect of enzyme saturation
on the metabolism and toxicity of bromobenzene. Bromobenzene was first
metabolically transformed to an epoxide which is hepatotoxic. After a
small nontoxic dose, approximately 75% was converted to the
glutathione conjugate and excreted as bromophenylmercapturic acid.
After a large toxic dose, only 45% was excreted as the mercapturic
acid. It was established that, at the toxic dose, the metabolic
conjugation pathway was overwhelmed due to lack of glutathione, which
resulted in an increased reaction of the epoxide hepatotoxin with DNA,
RNA, and protein.
It is very important to elucidate dose-dependent metabolism to
assess the hazard of a chemical. Frequently, the doses of a chemical
used to characterize toxicity are many times those encountered in the
environment. Toxicity incurred at these large doses may be influenced
by relative changes in metabolism and therefore must be interpreted
with caution and judgment in assessing the hazard of low doses.
4.7 Experimental Design
Since, for the most part, toxicity is a function of the
concentration of the toxicant in the tissues and cells, this
information together with its dynamics provides for inter- as well as
intra-species extrapolation of the results of toxicological effects.
The overall objectives of a chemobiokinetic study are to
determine the amount, rate, and nature of absorption, distribution,
metabolism, and excretion of a chemical. The approach to meeting those
objectives must be flexible and designed to meet the specific needs of
each chemical.
It is difficult to predict, without prior data, an animal species
that will metabolize a chemical similarly to man. Usually, initial
studies are performed in the rat and one other nonrodent species, such
as the dog or monkey, in an attempt to determine species variability.
If there are significant differences among species, it is important to
determine whether differences in the chemobiokinetic parameters
correlate with differences in toxicity or pharmacological activity.
Animals should be acclimatized to the environment of the metabolism
cage prior to the experiment. Light cycle, temperature, humidity, and
time of feeding should be standardized. The physical condition,
weight, and food and water consumption of each animal should be
monitored and recorded throughout the study.
There are advantages in using radioactively-labelled chemicals in
initial studies because of the ease with which radiochemical methods
(Chase & Rabinowitz, 1968) can be applied to chemobiokinetic studies.
An important advantage of using a radioactively-labelled chemical is
that it allows the establishment of the total recovery of the parent
chemical and its metabolites, i.e. the mass balance. To obtain this
the total radioactivity eliminated via the urine, faeces, and exhaled
air as well as that remaining in the carcass following termination of
the experiment should be determined. Until a reasonably good recovery
is obtained, 90% or greater, one can never be sure whether other
chemobiokinetic parameters obtained from the study are accurate.
Furthermore, the isolation and ultimate identification of unknown
metabolites is greatly enhanced by using radioactively-labelled
chemicals.
When using a radiolabelled chemical, the measurement of
radioactivity confirms the presence of the radioisotope, not the
chemical or its metabolites. In order to determine the identity of the
radioactively-labelled compound, the parent chemical and its
metabolites, analytical methods such as gas, high-pressure liquid, and
thin-layer chromatography and a combination of gas chromatography and
mass spectroscopy are frequently employed.
Until it is established that the radioactivity being monitored is
from the chemical in question, kinetic parameters apply to the
radioactivity only, not to the chemical studied. Difficulties can
arise if the radioactive atom does not remain an integral part of the
molecule under study. Tritium and carbon-14 are often incorporated
into the body pools of normal tissue components (Griffiths, 1968;
Rosenblum, 1965). Once the radioactivity is incorporated into these
compartments, its clearance depends on their rates of turnover.
Therefore, by monitoring radioactivity only, it can be falsely assumed
that a compound is being retained in the body.
Another very important reason for differentiating the parent
chemical from its metabolite is to assure that toxic effects that may
be present are associated with the parent chemical and not a
metabolite. Also persistence of a metabolite in the body rather than
the parent chemical may constitute the ultimate hazard.
In initial studies, consideration should be given to the
administration of the compound by intravenous injection as well as via
the route by which man is exposed to the chemical. The intravenous
route is used to provide a more definite assessment of the earlier
phases of distribution and/or elimination. Also, large variation in
rates of absorption will in some cases make the differentiation of the
early phases of distribution and elimination difficult. At least two
doses should be used. One dose should be equivalent to the dose
required to cause signs of toxicity. The second dose should be well
below the toxic dose and, if possible, equivalent to anticipated human
exposure levels.
Most frequently, kinetic parameters for elimination of a chemical
are established by sequential sampling of blood plasma and excreta,
following its administration. A preliminary probe study using one or
two animals is often needed to establish the time at which samples
should be collected, because this will vary with the species and the
chemical in question. After collection and until prepared for analysis
of the chemical or its metabolites, samples should be stored in a
manner that will preclude the breakdown of the chemical or its
metabolites. The data required from the initial chemobiokinetic
studies can be used to design further studies which may include the
following: distribution studies using autoradiography; the isolation
and identification of metabolites; studies to determine the
chemobiokinetic profile of metabolites; biliary excretion studies;
bioconcentration; and in vitro metabolism studies. The methods and
techniques needed to perform these studies are documented by La Du et
al. (1972).
4.8 Chemobiokinetics
Chemobiokinetics aims at quantification of the processes
discussed previously in this chapter. Thus, chemobiokinetics provides
quantitative information on the absorption, distribution,
biotransformation, and excretion of chemicals (including drugs and
endogenous substances) as a function of time. Since the classical
introduction of this discipline by Teorell (1937a,b), the concepts and
methods have been developed extensively, principally for application
to the clinical evaluation and/or use of drugs (Levy & Gibaldi, 1972,
1975; Wagner, 1968, 1971). The reader is also referred to Gehring et
al. (1976) who discuss the subject in greater detail.
One difficulty of many toxicologists and biologists on first
exposure to chemobiokinetics is the concept of compartments. The body
is composed of a large number of organs, tissues, cells, and fluids,
any one of which could be referred to morphologically and functionally
as a compartment. However, in chemobiokinetics, a compartment refers
collectively to those organs, tissues, cells, and fluids for which the
rates of uptake and subsequent clearance of a chemical are
sufficiently similar to preclude kinetic resolution. The rapidly
equilibrating compartment, referred to as the central compartment, may
be comprised of all those tissues with a profuse blood supply whereas
the slow or peripheral compartment may include tissues with a more
limited blood supply, i.e. fat and bone.
4.8.1 One-compartment open model
The simplest chemobiokinetic model is a one-compartment, open
model as shown in Fig. 4.1. In using this model, it is assumed that
the chemical equilibrates with all tissues to which it is distributed
sufficiently rapidly to preclude kinetic differentiation by the
techniques being used to characterize its movement in the body. For
example, if it requires 30 min for a chemical to attain equilibration
in the body after entering the blood stream, and if samples of blood,
tissues, and excreta are taken at 30 min intervals, it will appear
that the body consists of only one compartment.
Assuming that the rate of elimination of the chemical is
proportional to its concentration in the plasma, the concentration in
the plasma will be described by apparent first-order kinetics. The
rate of change of concentration in the plasma may be expressed in the
form of the linear differential equation
dC(t)
= - ke C(t) (1)
dt
where C(t) is the concentration at time t, and ke is the rate constant
for elimination. Solution of this differential equation with initial
condition C(t) = C(0) at time zero gives
C(t) = C(0) exp(- ke t) (exponential form) (2)
or,
(3)
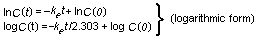 (4)
(4)
 In these equations, C(0) is the concentration of the chemical in the
plasma at time zero. A plot of C(t) versus time on semilogarithmic
paper will yield a straight line (Fig. 4.2) with slope - ke and
intercept C(0).
In these equations, C(0) is the concentration of the chemical in the
plasma at time zero. A plot of C(t) versus time on semilogarithmic
paper will yield a straight line (Fig. 4.2) with slope - ke and
intercept C(0).
 Having determined ke, which is measured in units of reciprocal
time, the time required to reduce the plasma concentration by one-half
is estimated; this time is referred to as the t´ or half-time. It
can be determined from the equation
ln 2 0.693
t´ = = (5)
ke ke
When the chemical is not absorbed instantaneously, the mathematics
needed to describe the concentration in plasma as a function of time
become somewhat more complicated. Assuming apparent first-order
absorption as well as elimination, the concentration C(t) in plasma
is given by the expression
contour integral* D0* ka
C(t) = {exp (- ke t) - exp (- ka t)} (6)
Vd( ka - ke)
In this expression, the terms not previously mentioned are D0, the
dose; contour integral, the fraction of dose absorbed; Vd, the
apparent volume of distribution; and ka, the apparent first-order
absorption rate constant.
The elimination rate constant, ke, is determined as described
previously using that portion of the solid line representing the
plasma concentration after absorption is essentially complete. In Fig.
4.2, this occurs when the dotted line blends into the solid line. The
rate constant for absorption, ka, may be estimated by projecting
the solid line backward to the origin. The difference between the
experimentally-determined values used to characterize the dotted line
are subtracted from those predicted by the backward projection at
corresponding times. Subsequently, the values obtained by this "curve
stripping" procedure are plotted producing a curve like the dash-dash
line in Fig. 4.2. Using this procedure, the t´ for absorption and
ka are determined.
The volume of distribution, Vd, is a term used to describe the
apparent volume to which a chemical is distributed when it is assumed
that the affinity of the plasma and all tissues is equivalent. An
analogy is placing a known amount of a dye in a liquid contained in a
system of unknown volume. After the concentration of the dye has
attained a constant value, the volume of the system can be determined
by dividing the dose, D0, by the concentration to give the volume
of distribution, Vd.
In the plasma, the concentration of the chemical declines because
of elimination as well as distribution to tissues. Therefore, to
estimate Vd, it is necessary to project the elimination phase of
the curve back to the origin. The value obtained at the time zero
intercept by this projection is divided into D0 to obtain the
volume of distribution, Vd, in ml/kg.
The value of Vd provides some important information about the
distribution of the chemical in the body. As the distribution to the
tissues increases, for whatever reason, physicochemical affinity,
active transport into cells, Vd increases. If the distribution of a
chemical in the human body is limited to plasma, extracellular fluid,
or total body water, the respective values of Vd will be
approximately 40, 170, and 580 ml/kg. If a chemical has a high
affinity for a particular tissue, for example, the affinity of a
lipophilic chemical for fat, Vd may exceed significantly 1000 mg/kg.
When the volume of distribution is known, the amount of chemical in
the body at any time t, A(t), can be calculated from the equation
A(t) = C(t)Vd (7)
Until now, concepts relating only to the concentration of the chemical
in the plasma have been discussed.
However, these concepts are equally applicable to other tissues
or, for that matter, to excreta, expired air, or urine. In the case of
urine, the concentrating power of the kidney must be accounted for to
normalize the data. If the affinity of the chemical for the various
tissues and excreta is equivalent and if rapid equilibration is
assumed, the concentration curves will be superimposable. However,
this would be an unusual occurrence. Because of the differences in
affinity, it is more likely that a family of parallel concentration
curves will be obtained. It is emphasized that these curves will be
parallel only after an apparent steady state has been achieved between
the tissues.
In addition to concentration, the same concepts apply if one
desires to characterize the total amount of chemical in the body,
A(t), as a function of time following exposure. For example, if a
dose D0 is ingested and apparent first-order kinetics is assumed,
the amount of the chemical in the body is given by the expression
A(t) = D0 exp (- ke t) (8)
Using equation (7), equation (8) can be shown to be equivalent to
C(t) = C(0) exp (- ke t) (9)
Logarithmic transformation of equations (8) or (9) may be used to
obtain curves like those in Fig. 4.2. The dotted curve would apply if
the chemical were applied to the skin and subsequently absorbed.
One caution must be emphasized in resolving the kinetics of the
amount of an agent in the body. Usually, it is not adequate to
determine the amount of the agent excreted and calculate the amount
remaining in the body by subtracting the cumulative amount excreted
from the original dose. This can be done if, and only if, the agent is
metabolically transformed to a very limited degree and, essentially,
all of the original dose is recovered. This seldom happens.
To circumvent the problem just described, the amount of the
chemical excreted over designated time intervals is determined until a
significant amount can no longer be detected. Assume that the rate of
excretion is proportional to the amount of chemical in the body,
A(t). Let B(t) be the cumulative amount excreted to time t after
administration. Then
dA(t)
= ke A(t) (10)
dt
or,
A(t) = D0 exp(- ke t) (11)
And
dB(t)
= kex A(t) (12)
dt
dB(t) kex
= D0 exp(- ke t) (13)
dt ke
or,
kex
B(t) = D0 {1 - exp (- ke t)} (14)
ke
In these equations, ke represents the apparent first-order overall
elimination rate constant and kex is the rate constant for
excretion via the route being analysed. If Ei is the amount
excreted in the ith time interval of duration deltat then
Ei = B( ti) - B( ti - delta t) (15)
where B( ti) is the amount excreted between administration and ti,
the time at the end of the ith time interval.
In terms of the dose administered D0, and the rate constant
ke,
kex
Ei = D0 exp(- ke ti) {exp( kedelta t) - 1} (16)
ke
the logarithmic form of which is
Having determined ke, which is measured in units of reciprocal
time, the time required to reduce the plasma concentration by one-half
is estimated; this time is referred to as the t´ or half-time. It
can be determined from the equation
ln 2 0.693
t´ = = (5)
ke ke
When the chemical is not absorbed instantaneously, the mathematics
needed to describe the concentration in plasma as a function of time
become somewhat more complicated. Assuming apparent first-order
absorption as well as elimination, the concentration C(t) in plasma
is given by the expression
contour integral* D0* ka
C(t) = {exp (- ke t) - exp (- ka t)} (6)
Vd( ka - ke)
In this expression, the terms not previously mentioned are D0, the
dose; contour integral, the fraction of dose absorbed; Vd, the
apparent volume of distribution; and ka, the apparent first-order
absorption rate constant.
The elimination rate constant, ke, is determined as described
previously using that portion of the solid line representing the
plasma concentration after absorption is essentially complete. In Fig.
4.2, this occurs when the dotted line blends into the solid line. The
rate constant for absorption, ka, may be estimated by projecting
the solid line backward to the origin. The difference between the
experimentally-determined values used to characterize the dotted line
are subtracted from those predicted by the backward projection at
corresponding times. Subsequently, the values obtained by this "curve
stripping" procedure are plotted producing a curve like the dash-dash
line in Fig. 4.2. Using this procedure, the t´ for absorption and
ka are determined.
The volume of distribution, Vd, is a term used to describe the
apparent volume to which a chemical is distributed when it is assumed
that the affinity of the plasma and all tissues is equivalent. An
analogy is placing a known amount of a dye in a liquid contained in a
system of unknown volume. After the concentration of the dye has
attained a constant value, the volume of the system can be determined
by dividing the dose, D0, by the concentration to give the volume
of distribution, Vd.
In the plasma, the concentration of the chemical declines because
of elimination as well as distribution to tissues. Therefore, to
estimate Vd, it is necessary to project the elimination phase of
the curve back to the origin. The value obtained at the time zero
intercept by this projection is divided into D0 to obtain the
volume of distribution, Vd, in ml/kg.
The value of Vd provides some important information about the
distribution of the chemical in the body. As the distribution to the
tissues increases, for whatever reason, physicochemical affinity,
active transport into cells, Vd increases. If the distribution of a
chemical in the human body is limited to plasma, extracellular fluid,
or total body water, the respective values of Vd will be
approximately 40, 170, and 580 ml/kg. If a chemical has a high
affinity for a particular tissue, for example, the affinity of a
lipophilic chemical for fat, Vd may exceed significantly 1000 mg/kg.
When the volume of distribution is known, the amount of chemical in
the body at any time t, A(t), can be calculated from the equation
A(t) = C(t)Vd (7)
Until now, concepts relating only to the concentration of the chemical
in the plasma have been discussed.
However, these concepts are equally applicable to other tissues
or, for that matter, to excreta, expired air, or urine. In the case of
urine, the concentrating power of the kidney must be accounted for to
normalize the data. If the affinity of the chemical for the various
tissues and excreta is equivalent and if rapid equilibration is
assumed, the concentration curves will be superimposable. However,
this would be an unusual occurrence. Because of the differences in
affinity, it is more likely that a family of parallel concentration
curves will be obtained. It is emphasized that these curves will be
parallel only after an apparent steady state has been achieved between
the tissues.
In addition to concentration, the same concepts apply if one
desires to characterize the total amount of chemical in the body,
A(t), as a function of time following exposure. For example, if a
dose D0 is ingested and apparent first-order kinetics is assumed,
the amount of the chemical in the body is given by the expression
A(t) = D0 exp (- ke t) (8)
Using equation (7), equation (8) can be shown to be equivalent to
C(t) = C(0) exp (- ke t) (9)
Logarithmic transformation of equations (8) or (9) may be used to
obtain curves like those in Fig. 4.2. The dotted curve would apply if
the chemical were applied to the skin and subsequently absorbed.
One caution must be emphasized in resolving the kinetics of the
amount of an agent in the body. Usually, it is not adequate to
determine the amount of the agent excreted and calculate the amount
remaining in the body by subtracting the cumulative amount excreted
from the original dose. This can be done if, and only if, the agent is
metabolically transformed to a very limited degree and, essentially,
all of the original dose is recovered. This seldom happens.
To circumvent the problem just described, the amount of the
chemical excreted over designated time intervals is determined until a
significant amount can no longer be detected. Assume that the rate of
excretion is proportional to the amount of chemical in the body,
A(t). Let B(t) be the cumulative amount excreted to time t after
administration. Then
dA(t)
= ke A(t) (10)
dt
or,
A(t) = D0 exp(- ke t) (11)
And
dB(t)
= kex A(t) (12)
dt
dB(t) kex
= D0 exp(- ke t) (13)
dt ke
or,
kex
B(t) = D0 {1 - exp (- ke t)} (14)
ke
In these equations, ke represents the apparent first-order overall
elimination rate constant and kex is the rate constant for
excretion via the route being analysed. If Ei is the amount
excreted in the ith time interval of duration deltat then
Ei = B( ti) - B( ti - delta t) (15)
where B( ti) is the amount excreted between administration and ti,
the time at the end of the ith time interval.
In terms of the dose administered D0, and the rate constant
ke,
kex
Ei = D0 exp(- ke ti) {exp( kedelta t) - 1} (16)
ke
the logarithmic form of which is
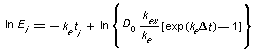 (17)
A semilogarithmic plot of Ei versus ti will give a straight line
with slope - ke. The above expression can be modified to accommodate
unequal time intervals, but in doing so graphic insights are lost.
In using excretion data to resolve kinetic parameters, it is
desirable to keep the collection intervals as short as practical.
Ideally, the collection intervals should be shorter than the t´ for
elimination of the chemical; otherwise resolution of a biphasic
excretion pattern may be precluded. Biphasic refers to two kinetically
distinct excretion phases. For a volatile chemical excreted to some
degree by exhalation, determination of the chemical exhaled as a
function of time may be particularly useful for resolving its
biochemokinetics.
As already stated, the excretion rate of a chemical by one route
of excretion may be different from its overall rate of elimination.
This is true because the agent may be eliminated by other routes
and/or metabolically transformed. The following scheme may be used to
depict a chemical that is eliminated by a metabolic pathway as well as
by excretion in the urine and exhalation:
/ ku excretion in urine
C - kr excretion via exhalation
\ kmx metabolic transformation to compound y
In this case, the overall elimination constant will be ke = ku +
kr + kmx.
The various metabolic transformation and excretion rates may be
estimated using the following equations:
ku= U infinity( ke/ D0) (18)
kr = R infinity( ke/ D0) (19)
kmx = X infinity( ke/ D0) (20)
Uinfinity and Rinfinity are the total amounts of the parent chemical
excreted in urine and expired air. Xinfinity is the total amount of
metabolite, X, recovered from excreta. For excretion of the chemical
in the urine, the differential equation is:
dU
= ku D0 exp(- ke t) (21)
dt
The solution of the equation (21) yields
U inifnity = ku D0/ ke (22)
and
ku = U infinity ke/ D0 (23)
When the urinary excretion of a chemical is determined, it is
frequently desirable to determine its renal clearance in order to
ascertain whether the chemical is actively secreted, reabsorbed, or
only passively filtered by the kidney in the excretion process. Renal
clearance is defined as the urinary excretion rate, delta U/delta
t, divided by the plasma concentration, C:
Rc = (delta U/delta t)/ C (24)
If the plasma concentration is changing during the urinary collection
interval, the concentration at the midpoint of the interval is used
frequently. It may also be shown using equations (9), (21), and (24)
that
Rc = ku Vd (25)
which precludes the necessity of knowing the plasma concentration.
Renal clearance values for inulin measure excretion via glomerular
filtration. For man, the normal value is 125 ± 15 ml/min (Pitts,
1963). If the renal clearance of a chemical exceeds this value in man,
it constitutes evidence that the chemical is actively secreted. If it
is less, it indicates the chemical is actively reabsorbed. If the
compound is bound to a significant degree to protein, it may be
necessary to determine and use the concentration of unbound chemical
in plasma in order to obtain a realistic value for renal clearance.
4.8.2 Two-compartment/multicompartment open systems
Rapidly equilibrating compartments in which the chemical has
reached equilibrium with plasma before the first blood samples are
taken will appear kinetically as one compartment, but a "deep" or more
slowly equilibrating compartment will give rise to a plasma
concentration curve that appears biphasic. The model used to describe
this system is a two-compartment open model (Fig. 4.1). The central
and the "shallow" or rapidly equilibrating compartments are considered
as one. The major sites of metabolic transformation and excretion are
the liver and the kidneys. Since these organs are perfused with blood,
it can be assumed, generally, that they are part of the central
compartment and that elimination occurs from the central compartment.
Fig. 4.3 is a simulated plasma concentration curve representing a
two-compartment system following rapid intravenous administration of a
chemical. The chemical has first been rapidly distributed to
well-perfused tissues, then more slowly to other tissues comprising
the deep compartment.
Assuming all the transfer processes are first order, the system
of linear differential equations describing the two-compartment model
shown in Fig. 4.1 is as follows:
dC(t) k21 VD CD (t)
= - k12 C(t) - ke C(t) + (26)
dt Vd
dCD( t) k12 Vd C( t)
= - k21 CD( t) (27)
dt VD
(17)
A semilogarithmic plot of Ei versus ti will give a straight line
with slope - ke. The above expression can be modified to accommodate
unequal time intervals, but in doing so graphic insights are lost.
In using excretion data to resolve kinetic parameters, it is
desirable to keep the collection intervals as short as practical.
Ideally, the collection intervals should be shorter than the t´ for
elimination of the chemical; otherwise resolution of a biphasic
excretion pattern may be precluded. Biphasic refers to two kinetically
distinct excretion phases. For a volatile chemical excreted to some
degree by exhalation, determination of the chemical exhaled as a
function of time may be particularly useful for resolving its
biochemokinetics.
As already stated, the excretion rate of a chemical by one route
of excretion may be different from its overall rate of elimination.
This is true because the agent may be eliminated by other routes
and/or metabolically transformed. The following scheme may be used to
depict a chemical that is eliminated by a metabolic pathway as well as
by excretion in the urine and exhalation:
/ ku excretion in urine
C - kr excretion via exhalation
\ kmx metabolic transformation to compound y
In this case, the overall elimination constant will be ke = ku +
kr + kmx.
The various metabolic transformation and excretion rates may be
estimated using the following equations:
ku= U infinity( ke/ D0) (18)
kr = R infinity( ke/ D0) (19)
kmx = X infinity( ke/ D0) (20)
Uinfinity and Rinfinity are the total amounts of the parent chemical
excreted in urine and expired air. Xinfinity is the total amount of
metabolite, X, recovered from excreta. For excretion of the chemical
in the urine, the differential equation is:
dU
= ku D0 exp(- ke t) (21)
dt
The solution of the equation (21) yields
U inifnity = ku D0/ ke (22)
and
ku = U infinity ke/ D0 (23)
When the urinary excretion of a chemical is determined, it is
frequently desirable to determine its renal clearance in order to
ascertain whether the chemical is actively secreted, reabsorbed, or
only passively filtered by the kidney in the excretion process. Renal
clearance is defined as the urinary excretion rate, delta U/delta
t, divided by the plasma concentration, C:
Rc = (delta U/delta t)/ C (24)
If the plasma concentration is changing during the urinary collection
interval, the concentration at the midpoint of the interval is used
frequently. It may also be shown using equations (9), (21), and (24)
that
Rc = ku Vd (25)
which precludes the necessity of knowing the plasma concentration.
Renal clearance values for inulin measure excretion via glomerular
filtration. For man, the normal value is 125 ± 15 ml/min (Pitts,
1963). If the renal clearance of a chemical exceeds this value in man,
it constitutes evidence that the chemical is actively secreted. If it
is less, it indicates the chemical is actively reabsorbed. If the
compound is bound to a significant degree to protein, it may be
necessary to determine and use the concentration of unbound chemical
in plasma in order to obtain a realistic value for renal clearance.
4.8.2 Two-compartment/multicompartment open systems
Rapidly equilibrating compartments in which the chemical has
reached equilibrium with plasma before the first blood samples are
taken will appear kinetically as one compartment, but a "deep" or more
slowly equilibrating compartment will give rise to a plasma
concentration curve that appears biphasic. The model used to describe
this system is a two-compartment open model (Fig. 4.1). The central
and the "shallow" or rapidly equilibrating compartments are considered
as one. The major sites of metabolic transformation and excretion are
the liver and the kidneys. Since these organs are perfused with blood,
it can be assumed, generally, that they are part of the central
compartment and that elimination occurs from the central compartment.
Fig. 4.3 is a simulated plasma concentration curve representing a
two-compartment system following rapid intravenous administration of a
chemical. The chemical has first been rapidly distributed to
well-perfused tissues, then more slowly to other tissues comprising
the deep compartment.
Assuming all the transfer processes are first order, the system
of linear differential equations describing the two-compartment model
shown in Fig. 4.1 is as follows:
dC(t) k21 VD CD (t)
= - k12 C(t) - ke C(t) + (26)
dt Vd
dCD( t) k12 Vd C( t)
= - k21 CD( t) (27)
dt VD
 where C(t) and CD (t) are concentrations of the chemical in the
central and deep compartments respectively. The apparent volumes of
distribution for these compartments are Vd for the central
compartment and VD for the slow exchange compartment. If the
apparent volumetric flow rates between the two compartments are the
same, i.e. k12 Vd = k21 VD, the differential equation system can be
solved with initial conditions C(0) = D0/ Vd and CD(0) = 0 at time
zero to give the following mathematical representation for the solid
curve in Fig. 4.3:
C(t) = phiexp(-alpha t) + psi exp(-ß t) (28)
ß is the slope of the line for the slow phase of elimination and alpha
is the slope for the rapid phase of elimination. The value of ß is
determined as previously described and a technique called feathering
is used to obtain alpha. This technique constitutes projecting the
solid line for the slow phase backward to the origin (dash-dash line)
and subtracting the respective projected values from the experimental
values used to delineate the rapid phase of clearance. These values
are replotted (dotted line). The slope of this line is alpha. The
values for phi and psi are the intercepts at the ordinate for the
rapid and slow elimination phases, respectively.
The rate constants k12, k21, and ke (Fig. 4.1) may be
determined as follows:
phiß + psi alpha
k21 = (29)
phi + psi
alphaß
ke = (30)
k21
k12 = alpha + ß - ( k21 + ke) (31)
k12 is of particular importance because from it the amount of
chemical in the deep compartment (AD (t)) is readily calculated
from the equation
k12 D0
AD( t) = {exp(-alpha t) - exp(-ß t)} (32)
ß - alpha
Using this information, toxicologists can ascertain whether there may
be correlations between the effect of a chemical and its presence in a
deep compartment. Indeed, for the toxicologist, a prominent slow phase
for the elimination of a chemical is a red flag suggesting that with
repeated administration cumulative toxicity may constitute a problem.
These concepts developed for the plasma concentration of a
chemical conforming to a two-compartment open-model system can be
extended to describe the amount of the agent in the body or the amount
excreted. Also, an absorption component may be added which would give
a function involving the sum of three exponential terms:
C( t) = phiexp(-alpha t) + psi exp(-ß t) + (phi + psi)exp (- ka t) (33)
4.8.3 Repeated administration or repeated exposure
The concentration of a chemical in the plasma or tissues or the
amount of chemical in the body following repeated administration or
exposure is illustrated in Fig. 4.4 for a one-compartment open system.
Mathematical representation of these concentrations is obtained by
addition of the exponential terms for each dose so that the
concentration of the chemical at time t following the nth dose is
given by
where C(t) and CD (t) are concentrations of the chemical in the
central and deep compartments respectively. The apparent volumes of
distribution for these compartments are Vd for the central
compartment and VD for the slow exchange compartment. If the
apparent volumetric flow rates between the two compartments are the
same, i.e. k12 Vd = k21 VD, the differential equation system can be
solved with initial conditions C(0) = D0/ Vd and CD(0) = 0 at time
zero to give the following mathematical representation for the solid
curve in Fig. 4.3:
C(t) = phiexp(-alpha t) + psi exp(-ß t) (28)
ß is the slope of the line for the slow phase of elimination and alpha
is the slope for the rapid phase of elimination. The value of ß is
determined as previously described and a technique called feathering
is used to obtain alpha. This technique constitutes projecting the
solid line for the slow phase backward to the origin (dash-dash line)
and subtracting the respective projected values from the experimental
values used to delineate the rapid phase of clearance. These values
are replotted (dotted line). The slope of this line is alpha. The
values for phi and psi are the intercepts at the ordinate for the
rapid and slow elimination phases, respectively.
The rate constants k12, k21, and ke (Fig. 4.1) may be
determined as follows:
phiß + psi alpha
k21 = (29)
phi + psi
alphaß
ke = (30)
k21
k12 = alpha + ß - ( k21 + ke) (31)
k12 is of particular importance because from it the amount of
chemical in the deep compartment (AD (t)) is readily calculated
from the equation
k12 D0
AD( t) = {exp(-alpha t) - exp(-ß t)} (32)
ß - alpha
Using this information, toxicologists can ascertain whether there may
be correlations between the effect of a chemical and its presence in a
deep compartment. Indeed, for the toxicologist, a prominent slow phase
for the elimination of a chemical is a red flag suggesting that with
repeated administration cumulative toxicity may constitute a problem.
These concepts developed for the plasma concentration of a
chemical conforming to a two-compartment open-model system can be
extended to describe the amount of the agent in the body or the amount
excreted. Also, an absorption component may be added which would give
a function involving the sum of three exponential terms:
C( t) = phiexp(-alpha t) + psi exp(-ß t) + (phi + psi)exp (- ka t) (33)
4.8.3 Repeated administration or repeated exposure
The concentration of a chemical in the plasma or tissues or the
amount of chemical in the body following repeated administration or
exposure is illustrated in Fig. 4.4 for a one-compartment open system.
Mathematical representation of these concentrations is obtained by
addition of the exponential terms for each dose so that the
concentration of the chemical at time t following the nth dose is
given by

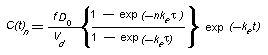 (34)
where tau is the interval between doses. After a large number of doses,
the term exp (-nketau ) approaches zero, and the value for the
concentration of chemical becomes
(34)
where tau is the interval between doses. After a large number of doses,
the term exp (-nketau ) approaches zero, and the value for the
concentration of chemical becomes
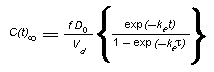 (35)
Once the plateau concentration is reached, further exposure to the
same dose at the same frequency will not result in any further
increase in concentration. At the plateau, the maximum concentration
which will occur immediately following the last exposure is given by:
(35)
Once the plateau concentration is reached, further exposure to the
same dose at the same frequency will not result in any further
increase in concentration. At the plateau, the maximum concentration
which will occur immediately following the last exposure is given by:
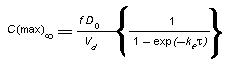 (36)
The minimum concentration will occur immediately before the next
exposure and is given by:
(36)
The minimum concentration will occur immediately before the next
exposure and is given by:
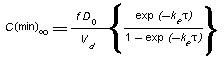 The expression defining the average concentration after the plateau
has been attained is:
contour integral D0
C(av)infinity = (38)
Vd ketau
If the exposure or the route of administration is such that the
first-order rate of absorption, ka, must be considered, the plasma
concentration following n repetitive doses at a dose interval tau is
given by:
The expression defining the average concentration after the plateau
has been attained is:
contour integral D0
C(av)infinity = (38)
Vd ketau
If the exposure or the route of administration is such that the
first-order rate of absorption, ka, must be considered, the plasma
concentration following n repetitive doses at a dose interval tau is
given by:
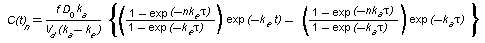 The rate constant for absorption, ka, may be replaced by the rate
constant for delivery of a substance being inhaled.
4.8.4 Kinetics of nonlinear or saturable systems
Dose-response curves for an effect arising from the
administration of a range of dose levels of a toxic agent usually
follow a log-normal distribution. Extrapolation of the logarithmic
probability transformation of these curves predicts that some
individuals will respond at an infinitesimally small dose, while
others will never respond, no matter how large the dose. The
assumption inherent in such extrapolation beyond the range of observed
data is that the chemobiokinetic profile of the compound in question
is independent of the dose level administered.
Assuming dose-independence, a 10-fold increase in the plasma
concentration of a chemical will result from a 10-fold increase in the
administered dose. However, many metabolic and excretory processes are
saturable and, as the dose of chemical begins to overwhelm these
processes, it may be expected that there will be a disproportionate
increase in toxicity. Therefore, nonlinear chemobiokinetics is of the
utmost importance in toxicology.
Many metabolic and active transfer processes as well as some
passive protein-binding processes have a finite capacity for reactions
with a chemical. The rate of these nonlinear processes can be defined
by the Michaelis-Menten equation
- dC(t) Vm C(t)
= (40)
dt Km + C(t)
where C(t) represents concentration of the chemical at time t,
Vm is the maximum rate of the process, and Km is the
concentration of chemical at which the rate of the process is equal to
one-half of Vm. Although this equation has been found useful in
delineating in vivo nonlinear kinetics, the constants should be
referred to as apparent in vivo constants, since they are
undoubtedly influenced by many other biological processes. Two
important limiting cases for this equation are as follows. When the
concentration of chemical is much smaller than Km( C( t) « Km) then
equation (40) reduces to
- dC( t) Vm
= C( t) (41)
dt Km
and the ratio of Vm/ Km will approximate an apparent first-order
rate constant. However, when the concentration is much greater than
Km (C(t) » Km) then the rate is described by
- dC( t)
= Vm (42)
dt
In this case, the rate is no longer dependent on the prevailing
concentration, but has become zero order and thus independent of
concentration.
Fig. 4.5 displays a typical concentration versus time curve for a
chemical the elimination of which follows nonlinear or
Michaelis-Menten kinetics. As long as the concentration remains
significantly less than Km, the log-linear portion of the plot is
applicable and all the principles of apparent first-order kinetics
apply. But, as the concentration approaches and then exceeds Km, the
semi-logarithm plot becomes nonlinear. In this region of zero-order
kinetics, the plot will be linear if rectangular coordinates are used.
The rate constant for absorption, ka, may be replaced by the rate
constant for delivery of a substance being inhaled.
4.8.4 Kinetics of nonlinear or saturable systems
Dose-response curves for an effect arising from the
administration of a range of dose levels of a toxic agent usually
follow a log-normal distribution. Extrapolation of the logarithmic
probability transformation of these curves predicts that some
individuals will respond at an infinitesimally small dose, while
others will never respond, no matter how large the dose. The
assumption inherent in such extrapolation beyond the range of observed
data is that the chemobiokinetic profile of the compound in question
is independent of the dose level administered.
Assuming dose-independence, a 10-fold increase in the plasma
concentration of a chemical will result from a 10-fold increase in the
administered dose. However, many metabolic and excretory processes are
saturable and, as the dose of chemical begins to overwhelm these
processes, it may be expected that there will be a disproportionate
increase in toxicity. Therefore, nonlinear chemobiokinetics is of the
utmost importance in toxicology.
Many metabolic and active transfer processes as well as some
passive protein-binding processes have a finite capacity for reactions
with a chemical. The rate of these nonlinear processes can be defined
by the Michaelis-Menten equation
- dC(t) Vm C(t)
= (40)
dt Km + C(t)
where C(t) represents concentration of the chemical at time t,
Vm is the maximum rate of the process, and Km is the
concentration of chemical at which the rate of the process is equal to
one-half of Vm. Although this equation has been found useful in
delineating in vivo nonlinear kinetics, the constants should be
referred to as apparent in vivo constants, since they are
undoubtedly influenced by many other biological processes. Two
important limiting cases for this equation are as follows. When the
concentration of chemical is much smaller than Km( C( t) « Km) then
equation (40) reduces to
- dC( t) Vm
= C( t) (41)
dt Km
and the ratio of Vm/ Km will approximate an apparent first-order
rate constant. However, when the concentration is much greater than
Km (C(t) » Km) then the rate is described by
- dC( t)
= Vm (42)
dt
In this case, the rate is no longer dependent on the prevailing
concentration, but has become zero order and thus independent of
concentration.
Fig. 4.5 displays a typical concentration versus time curve for a
chemical the elimination of which follows nonlinear or
Michaelis-Menten kinetics. As long as the concentration remains
significantly less than Km, the log-linear portion of the plot is
applicable and all the principles of apparent first-order kinetics
apply. But, as the concentration approaches and then exceeds Km, the
semi-logarithm plot becomes nonlinear. In this region of zero-order
kinetics, the plot will be linear if rectangular coordinates are used.
 4.9 Linear and Nonlinear One Compartment Open-model Kinetics of
2,4,5-Trichlorophenoxyacetic acid (2,4,5-T)
To illustrate the use of chemobiokinetics in toxicology, some
results obtained from studies with 2,4,5-T are presented below.
2,4,5-T, a herbicide, has been reported to be teratogenic, fetotoxic,
and embryotoxic at doses of 100 mg/kg/day during the period of
organogenesis (Collins & Williams, 1971; Courtney & Moore, 1971;
Courtney et al., 1970; Roll, 1971; Sparschu et al., 1971).
4.9 Linear and Nonlinear One Compartment Open-model Kinetics of
2,4,5-Trichlorophenoxyacetic acid (2,4,5-T)
To illustrate the use of chemobiokinetics in toxicology, some
results obtained from studies with 2,4,5-T are presented below.
2,4,5-T, a herbicide, has been reported to be teratogenic, fetotoxic,
and embryotoxic at doses of 100 mg/kg/day during the period of
organogenesis (Collins & Williams, 1971; Courtney & Moore, 1971;
Courtney et al., 1970; Roll, 1971; Sparschu et al., 1971).
 To elucidate the potential hazard of this compound, 5 mg/kg of
14C ring-labelled 2,4,5-T was administered as a single oral dose to
rats and dogs (Piper et al., 1973). The plasma concentration versus
time curves (Fig. 4.6) indicated compliance with a one-compartment
open model system having apparent first-order rates of absorption and
clearance; the t´ values for the clearance of 2,4,5-T from the
plasma of rats and dogs were 4.7 and 77 h, respectively. For
elimination from the body via the urine (Fig. 4.7), the t´ values
were 13.6 and 86.6 h. Since clearance of 2,4,5-T from the plasma of
rats was more rapid than its elimination in the urine, the compound
may have been actively concentrated in the kidneys prior to excretion
in the urine. Also, the much slower elimination by dogs than rats
correlates with the higher toxicity in dogs; the single oral LD50 is
100 mg/kg and 300 mg/kg for dogs and rats, respectively (Drill &
Heratyka, 1953; Rowe & Hymas, 1954).
Another species difference was demonstrated by the fact that
virtually all the 14C excreted by the rats was through the urine
while approximately 20% of that excreted by dogs was through the
faeces. Also, no breakdown products of 2,4,5-T could be detected in
the urine of rats given 5 mg/kg, but about 10% of the 14C activity
in the urine of dogs was attributable to breakdown products.
If an active secretory process in the kidney was the primary
elimination process in rats, then this nonlinear process should be
saturable by the administration of higher doses. Figs. 4.8 and 4.9
show that this is the case, since the t´ for both the clearance
of 2,4,5-T from plasma and its urinary elimination increase with
increasing dose. At doses of 100 or 200 mg/kg, the process was
saturated and the rates of elimination from the plasma and from the
body were the same. Further evidence of nonlinear kinetics was the
fact that a larger percentage of the 14C administered as
14C-2,4,5-T was excreted through the faeces as the dose was
increased. Also, degradation products of 2,4,5-T were found in the
urine of rats given 100 or 200 mg/kg, but not 5 or 50 mg/kg.
The nonlinear chemobiokinetics of 2,4,5-T were further
characterized following intravenous doses in rats of 5 or 100 mg/kg
(Sauerhoff et al., 1975). Clearance from the plasma of rats given
100 mg/kg followed classical Michaelis-Menten kinetics (Fig. 4.10).
The values for Vm and Km were calculated to be 16.6 ± 1.82 µg/h/g of
plasma and 127.6 ± 25.9 µg/g of plasma, respectively. During the
log-linear phase of excretion the t´ was 5.3 ± 1.2 h.
To elucidate the potential hazard of this compound, 5 mg/kg of
14C ring-labelled 2,4,5-T was administered as a single oral dose to
rats and dogs (Piper et al., 1973). The plasma concentration versus
time curves (Fig. 4.6) indicated compliance with a one-compartment
open model system having apparent first-order rates of absorption and
clearance; the t´ values for the clearance of 2,4,5-T from the
plasma of rats and dogs were 4.7 and 77 h, respectively. For
elimination from the body via the urine (Fig. 4.7), the t´ values
were 13.6 and 86.6 h. Since clearance of 2,4,5-T from the plasma of
rats was more rapid than its elimination in the urine, the compound
may have been actively concentrated in the kidneys prior to excretion
in the urine. Also, the much slower elimination by dogs than rats
correlates with the higher toxicity in dogs; the single oral LD50 is
100 mg/kg and 300 mg/kg for dogs and rats, respectively (Drill &
Heratyka, 1953; Rowe & Hymas, 1954).
Another species difference was demonstrated by the fact that
virtually all the 14C excreted by the rats was through the urine
while approximately 20% of that excreted by dogs was through the
faeces. Also, no breakdown products of 2,4,5-T could be detected in
the urine of rats given 5 mg/kg, but about 10% of the 14C activity
in the urine of dogs was attributable to breakdown products.
If an active secretory process in the kidney was the primary
elimination process in rats, then this nonlinear process should be
saturable by the administration of higher doses. Figs. 4.8 and 4.9
show that this is the case, since the t´ for both the clearance
of 2,4,5-T from plasma and its urinary elimination increase with
increasing dose. At doses of 100 or 200 mg/kg, the process was
saturated and the rates of elimination from the plasma and from the
body were the same. Further evidence of nonlinear kinetics was the
fact that a larger percentage of the 14C administered as
14C-2,4,5-T was excreted through the faeces as the dose was
increased. Also, degradation products of 2,4,5-T were found in the
urine of rats given 100 or 200 mg/kg, but not 5 or 50 mg/kg.
The nonlinear chemobiokinetics of 2,4,5-T were further
characterized following intravenous doses in rats of 5 or 100 mg/kg
(Sauerhoff et al., 1975). Clearance from the plasma of rats given
100 mg/kg followed classical Michaelis-Menten kinetics (Fig. 4.10).
The values for Vm and Km were calculated to be 16.6 ± 1.82 µg/h/g of
plasma and 127.6 ± 25.9 µg/g of plasma, respectively. During the
log-linear phase of excretion the t´ was 5.3 ± 1.2 h.

 In the experiments of Sauerhoff et al. (1975), the volume of
distribution increased from 190 to 235 ml/kg in rats given 5 and
100 mg/kg, respectively. This increase in the volume of distribution
indicates that with increasing dose a larger fraction of the dose is
distributed into various tissues and cells. Thus, a disproportionate
increase in toxicity may be expected. The fate of 2,4,5-T following
oral doses of 5 mg/kg has also been investigated in man (Gehring et
al., 1973). The elimination of 2,4,5-T from the plasma and in the
urine followed apparent first order kinetics with t1/2 of 23.1 h
(Figs. 4.11, 4.12, 4.13). A comparison of the elimination rates in man
with those in rats and dogs indicates that the toxicity of 2,4,5-T to
man would lie somewhere between that to rats and dogs. The peak plasma
levels attained with a dose of 5 mg/kg, which are higher in man than
in either rats or dogs, are associated with a greater degree of plasma
protein binding in man. Also, the volume of distribution in man of
80 ml/kg is attested to the retention of 2,4,5-T in the vascular
compartment.
Fig. 4.14 illustrates simulated levels of 2,4,5-T that would be
attained in the plasma of man with repeated ingestion. If 0.25 mg/kg
were ingested daily, a level equalling that attained by ingesting a
single dose of 5 mg/kg, as in this study, would never be reached.
In the experiments of Sauerhoff et al. (1975), the volume of
distribution increased from 190 to 235 ml/kg in rats given 5 and
100 mg/kg, respectively. This increase in the volume of distribution
indicates that with increasing dose a larger fraction of the dose is
distributed into various tissues and cells. Thus, a disproportionate
increase in toxicity may be expected. The fate of 2,4,5-T following
oral doses of 5 mg/kg has also been investigated in man (Gehring et
al., 1973). The elimination of 2,4,5-T from the plasma and in the
urine followed apparent first order kinetics with t1/2 of 23.1 h
(Figs. 4.11, 4.12, 4.13). A comparison of the elimination rates in man
with those in rats and dogs indicates that the toxicity of 2,4,5-T to
man would lie somewhere between that to rats and dogs. The peak plasma
levels attained with a dose of 5 mg/kg, which are higher in man than
in either rats or dogs, are associated with a greater degree of plasma
protein binding in man. Also, the volume of distribution in man of
80 ml/kg is attested to the retention of 2,4,5-T in the vascular
compartment.
Fig. 4.14 illustrates simulated levels of 2,4,5-T that would be
attained in the plasma of man with repeated ingestion. If 0.25 mg/kg
were ingested daily, a level equalling that attained by ingesting a
single dose of 5 mg/kg, as in this study, would never be reached.
 Additional studies on 2,4,5-T have demonstrated that it is
actively secreted by the kidney (Hook et al., 1974). This process of
elimination is saturable at high doses and the capacity for excretion
in dogs is more limited than in rats. As indicated previously, when
doses of 2,4,5-T are given that exceed the capacity for renal
excretion, the compound finds its way into more tissues and cells, is
eliminated more slowly, and undergoes a greater degree of metabolic
transformation. Thus, to use the toxicity incurred by high doses of
2,4,5-T to make statistical estimates of the toxicity that may be
incurred at low doses violates a basic a priori assumption.
Additional studies on 2,4,5-T have demonstrated that it is
actively secreted by the kidney (Hook et al., 1974). This process of
elimination is saturable at high doses and the capacity for excretion
in dogs is more limited than in rats. As indicated previously, when
doses of 2,4,5-T are given that exceed the capacity for renal
excretion, the compound finds its way into more tissues and cells, is
eliminated more slowly, and undergoes a greater degree of metabolic
transformation. Thus, to use the toxicity incurred by high doses of
2,4,5-T to make statistical estimates of the toxicity that may be
incurred at low doses violates a basic a priori assumption.




 The nonlinear chemobiokinetics of toxic doses of 2,4,5-T is an
example for many other compounds (Gehring et al., 1976). Indeed, it is
likely that for most compounds, toxicity may coincide with the
saturation of the detoxification process, operative at low doses.
Recently, Gillette (1974a,b) has given special consideration to the
chemobiokinetics of reactive metabolites of chemicals that react with
macromolecules (DNA, RNA, and protein) causing toxic effects. The
concepts presented in these papers are very important to the
toxicologist because they indicate possible threshold mechanisms for
toxicity, in particular chronic toxicity.
4.10 Linear Chemobiokinetics Used to Assess Potential for
Bioaccumulation of 2,3,6,7-tetrachlorodibenzo-p-dioxin (TCDD)
TCDD is a highly toxic compound formed as an unwanted contaminant
in the manufacture of 2,4,5-trichlorophenol (Schwetz et al., 1973).
Use of trichlorophenol to manufacture 2,4,5-trichlorophenoxyacetic
acid may result in contamination of 2,4,5-T with TCDD. The
physicochemical properties of TCDD suggest that exposure to small
amounts may result in the persistent accumulation of the highly toxic
material and, eventually, in toxic effects. To elucidate the
propensity of TCDD to accumulate in the body, a series of
pharmacokinetic studies was conducted (Rose et al., 1975). In these
studies, one group of rats was given a single oral dose of 14C-TCDD
at 1 µg/kg and the excretion of 14C activity in urine, expired air,
and faeces was determined. Other groups of rats were given orally
0.01, 0.1 or 1.0 µg of 14C-TCDD/kg/day, from Monday to Friday, for
up to 7 weeks. In addition to determining the amounts of 14C
activity excreted in the urine and faeces of these rats, the amounts
remaining in the body were calculated as a function of time and the
levels of 14C-activity residing in various tissues after 1, 3, and 7
weeks of administration were determined.
Since the overall recovery of 14C in rats given a single oral
dose of 14C-TCDD was 97 ± 8%, the amounts of 14C activity
remaining in the bodies of the rats as a function of time was
calculated by subtracting the cumulative amount excreted from the
original dose. The resulting body burdens of 14C are depicted in
Fig. 4.15. The halftime for elimination of 14C from the body ranged
from 21 to 39 days. All of the 14C activity was eliminated via the
faeces.
The concentration of 14C activity in the bodies of rats given
0.1 or 1.0 µg/kg/day, from Monday to Friday, for 7 weeks as a function
of time are shown in Fig. 4.16. The data show clearly that, with
repeated exposure, the concentration of 14C activity in the body
increases but the rate of increase decreases with time and the amount
in the body begins to plateau, even though exposure continues.
The nonlinear chemobiokinetics of toxic doses of 2,4,5-T is an
example for many other compounds (Gehring et al., 1976). Indeed, it is
likely that for most compounds, toxicity may coincide with the
saturation of the detoxification process, operative at low doses.
Recently, Gillette (1974a,b) has given special consideration to the
chemobiokinetics of reactive metabolites of chemicals that react with
macromolecules (DNA, RNA, and protein) causing toxic effects. The
concepts presented in these papers are very important to the
toxicologist because they indicate possible threshold mechanisms for
toxicity, in particular chronic toxicity.
4.10 Linear Chemobiokinetics Used to Assess Potential for
Bioaccumulation of 2,3,6,7-tetrachlorodibenzo-p-dioxin (TCDD)
TCDD is a highly toxic compound formed as an unwanted contaminant
in the manufacture of 2,4,5-trichlorophenol (Schwetz et al., 1973).
Use of trichlorophenol to manufacture 2,4,5-trichlorophenoxyacetic
acid may result in contamination of 2,4,5-T with TCDD. The
physicochemical properties of TCDD suggest that exposure to small
amounts may result in the persistent accumulation of the highly toxic
material and, eventually, in toxic effects. To elucidate the
propensity of TCDD to accumulate in the body, a series of
pharmacokinetic studies was conducted (Rose et al., 1975). In these
studies, one group of rats was given a single oral dose of 14C-TCDD
at 1 µg/kg and the excretion of 14C activity in urine, expired air,
and faeces was determined. Other groups of rats were given orally
0.01, 0.1 or 1.0 µg of 14C-TCDD/kg/day, from Monday to Friday, for
up to 7 weeks. In addition to determining the amounts of 14C
activity excreted in the urine and faeces of these rats, the amounts
remaining in the body were calculated as a function of time and the
levels of 14C-activity residing in various tissues after 1, 3, and 7
weeks of administration were determined.
Since the overall recovery of 14C in rats given a single oral
dose of 14C-TCDD was 97 ± 8%, the amounts of 14C activity
remaining in the bodies of the rats as a function of time was
calculated by subtracting the cumulative amount excreted from the
original dose. The resulting body burdens of 14C are depicted in
Fig. 4.15. The halftime for elimination of 14C from the body ranged
from 21 to 39 days. All of the 14C activity was eliminated via the
faeces.
The concentration of 14C activity in the bodies of rats given
0.1 or 1.0 µg/kg/day, from Monday to Friday, for 7 weeks as a function
of time are shown in Fig. 4.16. The data show clearly that, with
repeated exposure, the concentration of 14C activity in the body
increases but the rate of increase decreases with time and the amount
in the body begins to plateau, even though exposure continues.

 The average overall recovery of administered 14C was 97.7 ± 9%
of the cumulative dose of 14C. Mathematical analyses of the data
presented in Fig. 4.16 revealed a rate constant for excretion of TCDD
of 0.0293 ± 0.0050 days-1 which corresponds to a half-time of 23.7
days. The fraction of each dose absorbed was 0.861 ± 0.078. Using
these values, it may be calculated that the ultimate steady state body
burden would be 21.3 D0 for rats given a daily dose of D0, 5
consecutive days weekly for an infinite number of weeks. If D0 were
administered every day for an infinite time, the ultimate steady state
body burden would be 29.0 D0. Within the 7 weeks of this study, the
rats had attained 79.1% of the ultimate steady state body burden. The
time required to reach 90% of the ultimate steady state body burden
would be 78.5 days.
The concentrations of 14C-activity in the liver and fat of rats
given 14C-TCDD at concentrations of 0.01, 0.1, or 1.0 µg/kg/day,
from Monday to Friday, for 1, 3, or 7 weeks are illustrated
graphically in Figs. 4.17 and 4.18, respectively. Just like the body
burden levels, the levels in these tissues increase at a decreasing
rate and begin to plateau. It should also be noted that at each time
of measurement, there is a direct relationship between the dose being
administered and the level in the tissue. This latter observation is
illustrated more clearly in Figs. 4.19 and 4.20, where the
concentrations of 14C-activity in the liver and the fat have been
divided by the dose. This shows that over the range of doses given,
0.01 to 1.0 µg/kg/day, the relative degree of accumulation of
14C-TCDD by these tissues is not influenced by dose.
Mathematical evaluation of the data presented in Fig. 4.17-4.20
revealed that the rates for the clearance of TCDD from liver and fat
were 0.026 ± 0.000 and 0.029 ± 0.001 days-1 respectively. These
rates are essentially the same as the rate of elimination from the
body in toto, which is not unexpected because these tissues
contained the bulk of the 14C-TCDD in the body. The ultimate steady
state concentrations in liver and fat that would be attained with an
infinite duration of exposure are 0.250 ± 0.000 and 0.058 ± 0.003
D0 µg TCDD/g where D0 equals the dose being administered in µg/kg.
The times required to reach specified fractions of the ultimate steady
state concentrations would be identical no matter what dose, D0, is
being administered.
The 14C-activity in liver tissue from rats given 0.1 or
1.0 µg/kg/day, from Monday to Friday, weekly for 7 weeks, was
demonstrated by gas chromatography and by a combination of gas
chromatography and mass spectrometry to be due to 14C-TCDD. Also
important was the finding that the 14C-TCDD present in the liver was
readily extractable, indicating that TCDD does not bind irreversibly
with tissue. With regard to the assessment of the hazard of repeated
exposure to very small amounts of TCDD, the results show that TCDD
would not continue to accumulate in the body with prolonged repeated
exposure. In rats, 93% of the ultimate steady state level of TCDD in
the body would be attained within 90 days. Recently, a toxicological
evaluation of TCDD was conducted in rats given doses of 0.001, 0.01,
0.1 and 1.0 µg TCDD/kg/day, from Monday to Friday, for 13 weeks
(Kociba et al., 1975). Perceptible adverse effects did not develop in
rats given 0.001 or 0.01 µg TCDD/kg/day. Adverse effects including
hepatic pathology and functional changes, atrophy of the thymus, and
haematological alterations were observed in rats receiving 0.1 or
1.0 µg TCDD/kg/day. Indeed, some rats receiving 1.0 µg TCDD/kg/day
died. The results of the studies on the fate and accumulation of TCDD
in rats given repeated daily doses showed clearly that even with more
prolonged exposure those rats which received 0.01 µg TCDD/kg/day would
not continue to accumulate TCDD in the body and its tissues to the
extent leading to the toxic manifestations as seen in those rats
receiving 0.1 or 1.0 µg/kg/day. Since the levels of TCDD in the
tissues had essentially plateaued within 90 days, more prolonged
exposure would not be expected to lead to the attainment of toxic
amounts of TCDD in the body or its tissues.
The average overall recovery of administered 14C was 97.7 ± 9%
of the cumulative dose of 14C. Mathematical analyses of the data
presented in Fig. 4.16 revealed a rate constant for excretion of TCDD
of 0.0293 ± 0.0050 days-1 which corresponds to a half-time of 23.7
days. The fraction of each dose absorbed was 0.861 ± 0.078. Using
these values, it may be calculated that the ultimate steady state body
burden would be 21.3 D0 for rats given a daily dose of D0, 5
consecutive days weekly for an infinite number of weeks. If D0 were
administered every day for an infinite time, the ultimate steady state
body burden would be 29.0 D0. Within the 7 weeks of this study, the
rats had attained 79.1% of the ultimate steady state body burden. The
time required to reach 90% of the ultimate steady state body burden
would be 78.5 days.
The concentrations of 14C-activity in the liver and fat of rats
given 14C-TCDD at concentrations of 0.01, 0.1, or 1.0 µg/kg/day,
from Monday to Friday, for 1, 3, or 7 weeks are illustrated
graphically in Figs. 4.17 and 4.18, respectively. Just like the body
burden levels, the levels in these tissues increase at a decreasing
rate and begin to plateau. It should also be noted that at each time
of measurement, there is a direct relationship between the dose being
administered and the level in the tissue. This latter observation is
illustrated more clearly in Figs. 4.19 and 4.20, where the
concentrations of 14C-activity in the liver and the fat have been
divided by the dose. This shows that over the range of doses given,
0.01 to 1.0 µg/kg/day, the relative degree of accumulation of
14C-TCDD by these tissues is not influenced by dose.
Mathematical evaluation of the data presented in Fig. 4.17-4.20
revealed that the rates for the clearance of TCDD from liver and fat
were 0.026 ± 0.000 and 0.029 ± 0.001 days-1 respectively. These
rates are essentially the same as the rate of elimination from the
body in toto, which is not unexpected because these tissues
contained the bulk of the 14C-TCDD in the body. The ultimate steady
state concentrations in liver and fat that would be attained with an
infinite duration of exposure are 0.250 ± 0.000 and 0.058 ± 0.003
D0 µg TCDD/g where D0 equals the dose being administered in µg/kg.
The times required to reach specified fractions of the ultimate steady
state concentrations would be identical no matter what dose, D0, is
being administered.
The 14C-activity in liver tissue from rats given 0.1 or
1.0 µg/kg/day, from Monday to Friday, weekly for 7 weeks, was
demonstrated by gas chromatography and by a combination of gas
chromatography and mass spectrometry to be due to 14C-TCDD. Also
important was the finding that the 14C-TCDD present in the liver was
readily extractable, indicating that TCDD does not bind irreversibly
with tissue. With regard to the assessment of the hazard of repeated
exposure to very small amounts of TCDD, the results show that TCDD
would not continue to accumulate in the body with prolonged repeated
exposure. In rats, 93% of the ultimate steady state level of TCDD in
the body would be attained within 90 days. Recently, a toxicological
evaluation of TCDD was conducted in rats given doses of 0.001, 0.01,
0.1 and 1.0 µg TCDD/kg/day, from Monday to Friday, for 13 weeks
(Kociba et al., 1975). Perceptible adverse effects did not develop in
rats given 0.001 or 0.01 µg TCDD/kg/day. Adverse effects including
hepatic pathology and functional changes, atrophy of the thymus, and
haematological alterations were observed in rats receiving 0.1 or
1.0 µg TCDD/kg/day. Indeed, some rats receiving 1.0 µg TCDD/kg/day
died. The results of the studies on the fate and accumulation of TCDD
in rats given repeated daily doses showed clearly that even with more
prolonged exposure those rats which received 0.01 µg TCDD/kg/day would
not continue to accumulate TCDD in the body and its tissues to the
extent leading to the toxic manifestations as seen in those rats
receiving 0.1 or 1.0 µg/kg/day. Since the levels of TCDD in the
tissues had essentially plateaued within 90 days, more prolonged
exposure would not be expected to lead to the attainment of toxic
amounts of TCDD in the body or its tissues.



 Annex
Table 4.1 Different types of drug-metabolizing reactions
I. OXIDATIONS
(a) Microsomal oxidations
(Ciaccio, 1971; Dahm, 1971; Daly, 1971; Gillette, 1971b, Gram, 1971;
Smuckler, 1971; Weisburger & Weisburger, 1971.)
Aliphatic oxidation RCH3 ----> RCH2OH
Annex
Table 4.1 Different types of drug-metabolizing reactions
I. OXIDATIONS
(a) Microsomal oxidations
(Ciaccio, 1971; Dahm, 1971; Daly, 1971; Gillette, 1971b, Gram, 1971;
Smuckler, 1971; Weisburger & Weisburger, 1971.)
Aliphatic oxidation RCH3 ----> RCH2OH
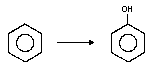 O
/ \
Epoxidation R - CH2 - CH2 - R ----> R - CH - CH - R
O
/ \
Epoxidation R - CH2 - CH2 - R ----> R - CH - CH - R
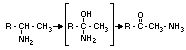 CH3 H
/ /
N-dealkylation R - N ----> R - N + CH2O
\ \
CH3 CH3
O-dealkylation R - O - CH3 ----> R - OH + CH2O
S-dealkylation R - S - CH3 ----> R - SH + CH2O
Table 4.1 (contd.)
Metalloalkane dealkylation Pb(C2H5)4 ----> PbH(C2H5)3
R R
\ \
N-oxidation R - N ----> R - N = O + H+
/ /
R R
CH3 H
/ /
N-dealkylation R - N ----> R - N + CH2O
\ \
CH3 CH3
O-dealkylation R - O - CH3 ----> R - OH + CH2O
S-dealkylation R - S - CH3 ----> R - SH + CH2O
Table 4.1 (contd.)
Metalloalkane dealkylation Pb(C2H5)4 ----> PbH(C2H5)3
R R
\ \
N-oxidation R - N ----> R - N = O + H+
/ /
R R
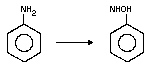 N-hydroxylation
Sulfoxidation
N-hydroxylation
Sulfoxidation
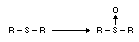 Desulfuration R R
\ \
C=S ----> C=O
/ /
R R
Desulfuration R R
\ \
C=S ----> C=O
/ /
R R
 Dehalogenation
(b) Nonmicrosomal oxidations
Monoamine and diamine oxidation
O2 H2O
RCH2NH2 ---> RCH = NH ---> RCHO + NH3
Table 4.1 (contd.)
Alcohol dehydrogenation RCH2OH + NAD+ ----> R - CHO + NADH + H+
Aldehyde dehydrogenation R - CHO + NAD+ ----> R - COOH + NADH + H+
II. REDUCTIONS
(a) Microsomal reductions
Nitro reduction RNO2 ----> RNO ----> RNHOH ----> RNH2
Azo reduction RN = NR ----> RNHNHR ----> RNH2 + RNH2
Reductive dehalogenation R - CCl3 ----> R - CHCl2
(b) Nonmicrosomal reductions
R R
\ \
Aldehyde reduction C = O ----> CHOH
/ /
R R
III. HYDROLYSIS
Ester hydrolysis R - CO - O - R1 ----> R - COOH + R1 - OH
Amide hydrolysis R - CO - NH2 ----> R - COOH + NH3
Table 4.1 (contd.)
IV. CONJUGATION
(a) UDPGA-medicated conjugations
O-glucuronide formation ether type:
Dehalogenation
(b) Nonmicrosomal oxidations
Monoamine and diamine oxidation
O2 H2O
RCH2NH2 ---> RCH = NH ---> RCHO + NH3
Table 4.1 (contd.)
Alcohol dehydrogenation RCH2OH + NAD+ ----> R - CHO + NADH + H+
Aldehyde dehydrogenation R - CHO + NAD+ ----> R - COOH + NADH + H+
II. REDUCTIONS
(a) Microsomal reductions
Nitro reduction RNO2 ----> RNO ----> RNHOH ----> RNH2
Azo reduction RN = NR ----> RNHNHR ----> RNH2 + RNH2
Reductive dehalogenation R - CCl3 ----> R - CHCl2
(b) Nonmicrosomal reductions
R R
\ \
Aldehyde reduction C = O ----> CHOH
/ /
R R
III. HYDROLYSIS
Ester hydrolysis R - CO - O - R1 ----> R - COOH + R1 - OH
Amide hydrolysis R - CO - NH2 ----> R - COOH + NH3
Table 4.1 (contd.)
IV. CONJUGATION
(a) UDPGA-medicated conjugations
O-glucuronide formation ether type:
 ester type:
ester type:
 N-glucuronide formation
N-glucuronide formation
 Table 4.1 (contd.)
IV. CONJUGATION (cont'd)
S-glucuronide formation
Table 4.1 (contd.)
IV. CONJUGATION (cont'd)
S-glucuronide formation
 (b) PAPS-medicated conjugation
Sulfate ester formation
(b) PAPS-medicated conjugation
Sulfate ester formation
 Table 4.1 (contd.)
(c) Methylations
N-methylation
Table 4.1 (contd.)
(c) Methylations
N-methylation
 O-methylation
O-methylation
 S-methylation C2H5SH -----> C2H5S-CH3
Table 4.1 (contd.)
(d) Acetylations
S-methylation C2H5SH -----> C2H5S-CH3
Table 4.1 (contd.)
(d) Acetylations
 (e) Peptide conjugations
(e) Peptide conjugations
 (f) Glutathione conjugations
(f) Glutathione conjugations
 Table 4.2 Methods for the determination of several mixed-function
oxidase activities
(a) Aryl hydrocarbon hydroxylation (using 3,4-benzpyrene as
substrate)
(Nebert & Gelboin, 1968a,b; Wattenberg et al., 1962)
(b) Aliphatic side-chain hydroxylation (of pentobarbital)
(Cooper & Brodie, 1955)
(c) 4-hydroxylation (of aniline)
(Brodie & Axelrod, 1948; Chabra et al., 1972; Gilbert &
Golberg, 1965; Henderson & Kersten, 1970; Hilton & Santorelli,
1970; Imai et al., 1966; Kato & Gillette, 1965; Schenkman
et al., 1967; Sternsen & Hes, 1975)
(d) N-hydroxylation (of aniline)
(Herr & Kiese, 1959)
(e) N-oxidation (determination of amine oxides)
(Fok & Ziegler, 1970; Ziegler et al., 1973)
(f) Nitro reduction
(Fouts & Brodie, 1957; Hietbrink & DuBois, 1965)
(g) N-demethylation (of aminopyrine)
(Brodie & Axelrod, 1950; Chrastil & Wilson, 1975; Cochin &
Axelrod, 1959; Dewaide & Henderson, 1968; Feuer et al., 1971;
Kinoshita et al., 1966; Klinger, 1974; La Du et al., 1955;
MacMahon, 1962; Nash, 1953; Pederson & Aust, 1970; Poland &
Nebert, 1973; Schoene et al., 1972)
(h) N-demethylation (of benzphetamine)
(Hewick & Fouts, 1970a,b; Liu et al., 1975; Lu et al., 1969;
Nash, 1953)
(i) N-demethylation (of ethylmorphine)
(Anders & Mannering, 1966)
(j) O-demethylation (of O-nitroanisole)
(Christensen & Wissing, 1972; Kinoshita et al., 1966; Netter,
1960; Netter & Seidel, 1964; Schoene et al., 1972;
Zannoni, 1971)
(k) O-dealkylation (of ethylumbelliferone)
(Ullrich & Weber, 1972)
REFERENCES
AITIO, A. (1973) Glucuronide synthesis in the rat and guinea pig lung.
Xenobiotica, 3: 13-22.
AL-KASSAB, S., BOYLAND, E., & WILLIAMS, K. (1963) An enzyme from rat
liver catalysing conjugations with glutathione; replacement of
nitro groups. Biochem. J., 87: 4-9.
ALLEN, J. R., NORBOCK, D. H., & HSU, I. C. (1974) Tissue modifications
in monkeys as related to absorption, distribution and excretion
of polychlorinated biphenyls. Arch. environ. Contam. Toxicol.,
2: 86-95.
ANDERS, M. W. & MANNERING, G. J. (1966) Inhibition of drug metabolism.
I. Kinetics of the inhibition of the N-demethylation of
ethylmorphine by 2-diethylaminoethyl 2, 2-diphenylvalerate HCl
(SKF 525A) and related compounds. Mol. Pharmacol., 2: 319-327.
ARIAS, I. M. (1962) Chronic unconjugated hyperbilirubinaemia without
overt signs of haemolysis in adolescents and adults. J. clin.
Invest., 41: 2233-2245.
ASSICOT, M. & BOHUON, C. (1969) A simple and rapid fluorimetric
determination of catechol- O-methyl transferase activity.
Life Sci., 8: 93-100.
AXELROD, J. (1971) Methyltransferase enzymes in the metabolism of
physiologically active compounds and drugs. In: Brodie, B. B. &
Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 2, pp. 609-619.
AXELROD, J. & DALY, J. W. (1968) Phenol- O-methyl transferase.
Biochim. Biophys. Acta, 159: 472-478.
AXELROD, J. & TOMCHICK, R. (1958) Enzymatic O-methylation of
epinephrine and other catechols. J. biol. Chem., 233: 702-705.
BAKER, R. C., COONS, L. B., & HODGSON, E. (1973) Low speed preparation
of microsomes: a comparative study. Chem. biol. Interactions,
6: 307-316.
BECKETT, A. H. & HOSSIE, R. D. (1971) Buccal absorption of drugs. In:
Brodie, B. B. & Gillette, J. R., ed. Concepts in biochemical
pharmacology, Berlin, Springer-Verlag, Vol. 1, pp. 25-46.
BOND, E. J. & DE MATTEIS, F. (1969) Biochemical changes in rat liver
after administration of carbon disulfide with reference to
microsomal changes. Biochem. Pharmacol., 18: 2531-2549.
BONNICHSEN, R. K. & BRINK, N. G. (1955) Liver alcohol dehydrogenase.
In: Colowick, S. P. & Kaplan, N. O., ed. Methods in enzymology,
New York, Academic Press, Vol. 1, pp. 495-500.
BOOTH, J., BOYLAND, E., & SIMS, P. (1961) An enzyme from rat liver
catalysing conjugations with glutathione. Biochem. J.,
79: 516-524.
BORCHARDT, R. T. (1974) A rapid spectrophotometric assay for
catechol- O-methyl transferase. Anal. Biochem., 58: 382-389.
BORGÅ, O., AZARNOFF, D. L., & SJÖQVIST, F. (1968) Species differences
in the plasmaprotein binding of desipramine. J. Pharm.
Pharmacol., 20: 571.
BOYD, G. S. & SMELLIE, R. M. S. (1972) Biological hydroxylation
mechanism. New York, Academic Press.
BOYLAND, E. (1971) Mercapturic acid conjugation. In: Brodie, B. B. &
Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 2, pp. 584-608.
BOYLAND, E. & CHASSEAUD, L. F. (1967) Enzyme-catalysed conjugations of
glutathione with unsaturated compounds. Biochem. J.,
104: 95-102.
BOYLAND, E. & WILLIAMS, K. (1965) A new enzyme catalysing the
conjugations of epoxides. Biochem. J., 94: 190-197.
BRAUER, R. W. (1959) Mechanisms of bile secretion. J. Am. Med.
Assoc., 169: 1462-1466.
BROCH, O. J., JR & GULDBERG, H. C. (1971) On the determination of
catechol- O-methyl transferase activity in tissue homogenates.
Acta Pharmacol. Toxicol., 30: 266-277.
BRODIE, B. B. (1964) Physicochemical factors in drug absorption. In:
Binns, T. B., ed. Absorption and distribution of drugs,
Baltimore, Williams & Wilkins, pp. 16-48.
BRODIE, B. B. & AXELROD, J. (1948) The estimation of acetanilide and
its metabolic products, aniline, N-acetyl- p-aminophenol and
p-aminophenol (free and total conjugates) in biological fluids
and tissues. J. Pharmac. exp. Ther., 94: 22-28.
BRODIE, B. B. & AXELROD, J. (1950) The fate of aminopyrine (Pyramidon)
in man and methods for the estimation of aminopyrine and its
metabolites in biological material. J. Pharmac. exp. Ther.,
99: 171-184.
BRODIE, B. B. & HOGBEN, C. A. M. (1957) Some physicochemical factors
in drug action. J. Pharm. Pharmacol., 9: 345-347.
BURCHELL, B., DUTTON, G. J., & NEMETH, A. M. (1972) Development of
phenobarbital-sensitive control mechanisms for uridine
diphosphate glucuronyl-transferase activity in chick liver.
J. cell. Biol., 55: 448-456.
CHASE, G. D. & RABINOWITZ, J. L. (1968) Principles of radioisotope
methodology, 3rd ed. Minneapolis, Burgess Publishing Company.
CHABHRA, R. S., GRAM, T. E., & FOUTS, J. R. (1972) A comparative study
of two procedures used in the determination of hepatic microsomal
aniline hydroxylation. Toxicol. appl. Pharmacol., 22: 50-58.
CHIGNELL, C. F. (1971) Physical methods for studying drug-protein
binding. In: Brodie, B. B. & Gillette, J. R., ed. Concepts in
biochemical pharmacology, Berlin, Springer-Verlag, Vol. 1,
pp. 187-212.
CHRASTIL, J. & WILSON, J. T. (1975) A sensitive colorimetric method
for formaldehyde. Anal. Biochem., 63: 202-207.
CHRISTENSEN, F. & WISSING, F. (1972) Inhibition of microsomal
drug-metabolizing enzymes from rat liver by various
4-hydroxy-coumarin derivatives. Biochem. Pharmacol.,
21: 975-984.
CIACCIO, E. I. (1971) Intimate study of drug action. II. Fate of drugs
in the body. In: Dipalma, J. R, ed. Drills pharmacology in
medicine, New York, McGraw-Hill, pp. 36-66.
COCHIN, J. & AXELROD, J. (1959) Biochemical and pharmacological
changes in the rat following chronic administration of morphine,
nalorphine and normorphine. J. Pharmac. exp. Ther.,
125: 105-110.
COLLINS, T. F. X. & WILLIAMS, C. H. (1971) Teratogenic studies with
2,4,5-T and 2,4-D in the hamster. Bull. environ. Contam.
Toxicol., 6: 559-567.
CONNEY, A. H. (1967) Pharmacological implications of microsomal enzyme
induction. Pharmacol. Rev., 19: 317-366.
CONNEY, A. H. & BURNS, J. J. (1962) Factors influencing drug
metabolism. Adv. Pharmacol., 1: 31-54.
CONNEY, A. H., COUTINHO, C., KOECHLIN, B., SWARM, R., CHERIPHO, J. A.,
IMPELLIZERI, C., & BARUTH, H. (1974) From animals to man:
metabolic considerations. Clin. Pharmacol. Ther., 16: 176-182.
COOPER, J. R. & BRODIE, B. B. (1965) Enzymatic oxidation of
pentobarbital and thiopental. J. Pharmacol. exp. Ther.,
120: 75-87.
COTZIAS, G. C. & DOLE, V. P. (1951) Metabolism of amines. I.
Microdetermination of aminoamine oxidase in tissues.
J. biol. Chem., 190: 665-672.
COURTNEY, D. K., GAYLOR, D. W., HOGAN, M. D., FLAK, H. L., BATES, R.
R., & MITCHELL, I. (1970) Teratogenic evaluation of 2,4,5-T.
Science, 168: 864-866.
COURTNEY, D. K. & MOORE, J. A. (1971) Teratology studies with
2,4,5-trichlorophenoxyacetic acid and
2,3,7,8-tetrachlorodibenzo- p-dioxin. Toxicol. appl.
Pharmacol., 20: 396-403.
CREASEY, N. H. (1956) Factors which interfere with the manometric
assay of monoamine oxidase. Biochem. J., 64: 178-183.
DAHM, P. A. (1971) Oxidative desulfuration and dealkylation of
selected organophosphate insecticides. In: Brodie, B. B. &
Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 2, pp. 362-366.
DALY, J. (1971) Enzymatic oxidation of carbon. In: Brodie, B. B. &
Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 2, pp. 285-311.
DAVIES, D. S., GIGON, P. L., & GILLETTE, J. R. (1969) Species and sex
differences in electron transport systems in liver microsomes and
their relationship to ethylmorphine demethylation. Life Sci.,
8: 85-91.
DAVSON, H. & DANIELLI, J. F. (1952) The permeability of natural
membranes, 2nd ed. Cambridge, Cambridge Univ. Press.
DEITRICH, R. A. & ERWIN, V. G. (1969) Spectrophotometric assay for
monoamine oxidase. Anal. Biochem., 30: 395-402.
DEWAIDE, J. H. & HENDERSON, P. T. (1968) Hepatic N-demethylation of
aminopyrine in rat and trout. Biochem. Pharmacol.,
17: 1901-1907.
DRILL, V. A. & HERATJKA, T. (1953) Toxicity of
2,4-dichlorophenoxyacetic acid and 2,4,5-trichlorophenoxyacetic
acid. Arch. ind. Hyg. occup. Med, 7: 61-67.
DUTTON, G. J. (1966) The biosynthesis of glucuronides. In: Dutton, G.
J. ed. Glucuronic acid, free and combined. New York, Academic
Press.
DUTTON, G. J. (1971) Glucuronide-forming enzymes. In: Brodie, B. B. &
Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 2, pp. 378-400.
DUTTON, G. J. & STOREY, I. D. E. (1962) Glucuronide-forming enzymes.
In: Colowick, S. P. & Kaplan, N. O., ed. Methods in enzymology,
New York, Academic Press, Vol. 5, pp. 159-164.
ESTABROOK, R. W. (1971) Cytochrome P-450. Its function in the
oxidative metabolism of drugs. In: Brodie, B. B. & Gillette, J.
R., ed. Concepts in biochemical pharmacology, Berlin,
Springer-Verlag, Vol. 2, pp. 264-284.
ESTABROOK, R. W., FRANKLIN, M. R., COHEN, B., SHIGAMATSU, A., &
HILDEBRANDT, A. G. (1971) Influence of hepatic microsomal mixed
function oxidation reactions on cellular metabolic control.
Metabolism, 20: 186-199.
ESTABROOK, R. W., GILLETTE, J. R., & LEIBMAN, K. C. (1972)
Microsomes and drug oxidations. Baltimore, Williams & Wilkins.
FEUER, G., SOSA-LUCERO, J. C., LUMB, F., & MODDEL, G. (1971) Failure
of various drugs to induce drug metabolizing enzymes in
extrahepatic tissues of the rat. Toxicol. appl. Pharmacol.,
19: 579-589.
FLYNN, E. J., LYNCH, M., & ZANNONI, V. G. (1969) Species differences
in hepatic microsomal electron transport. Fed. Proc., 28: 483.
FLYNN, E. J., LYNCH, M., & ZANNONI, V. G. (1972) Species differences
and drug metabolism. Biochem. Pharmacol., 21: 2577-2590.
FOK, A. K. & ZIEGLER, D. M. (1970) Estimation of amine oxides in the
presence of hepatic microsomes. Biochem. Biophys. Res. Commun.,
41: 534-540.
FOREMAN, H. (1971) Translocation of drugs into bone. In: Brodie, B. B.
& Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 1, pp. 249-257.
FOUTS, J. R. (1970) The stimulation and inhibition of hepatic
microsomal drug-metabolising enzymes with special reference to
the effects of environmental contaminants. Toxicol. appl.
Pharmacol., 17: 804-809.
FOUTS, J. R. & BRODIE, B. B. (1957) The enzymatic reduction of
chloramphenicol, p-nitrobenzoic acid and other aromatic nitro
compounds in mammals. J. Pharmacol. exp. Ther. 119: 197-207.
FOUTS, J. R. & POHL, H. J. (1971) Further studies on the effects of
metal ions on rat liver microsomal reduced nicotinamide adenine
dinucleotide phosphate-cytochrome P-450 reductase. J. Pharmacol.
exp. Ther., 179: 91-100.
GARATTINI, S., MARCUCCI, F., & MUSSINI, E. (1975) Biotransformation of
drugs to pharmacologically active metabolites. In: Gillette, J.
R. & Mitchell, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 3, pp. 113-119.
GEHRING, P. J. & BUERGE, J. (1969) The distribution of
2,4-dinitrophenol relative to its cataractogenic activity in
ducklings and rabbits. Toxicol. appl. Pharmacol., 15: 574-592.
GEHRING, P. J., KRAMER, C. D., SCHWETZ, B. A., ROSE, J. Q., & ROWE, V.
K. (1973) The fate of 2,4,5-trichlorophenoxyacetic acid (2,4,5-T)
following oral administration to man. Toxicol. appl. Pharmacol.,
26: 352-361.
GEHRING, P. J., BLAU, G. E., & WATANABE, P. G. (1976) Pharmacokinetic
studies in evaluation of the toxicological and environmental
hazard of chemicals. In: Advances in modern toxicology -- Newer
concepts in safety evaluation. Washington, Hemisphere Publ.
Corp.
GIBSON, J. E. & BECKER, B. A. (1967) Demonstration of enhanced
lethality of drugs in hypoexcretory animals. J. pharm. Sci.,
56: 1503-1505.
GIGON, P. L., GRAM, T. E., & GILLETTE, J. R. (1969) Studies on the
rate of reduction of hepatic microsomal cytochrome P-450 by
reduced nicotinamide adenine dinucleotide phosphate. Effect of
drug substrates. Mol. Pharmacol., 5: 109-122.
GILBERT, D. & GOLBERG, L. (1965) Liver response tests. III. Liver
enlargement and stimulation of microsomal processing enzyme
activity. Food Cosmet. Toxicol., 3: 417-432.
GILLETTE, J. R. (1963) Metabolism of drugs and other foreign compounds
by enzymatic mechanisms. Arzneimittel Forsch., 6: 11-73.
GILLETTE, J. R. (1971a) Factors affecting drug metabolism. Ann. NY
Acad. Sci., 179: 43-66.
GILLETTE, J. R. (1971b) Reductive enzymes. In: Brodie, B. B. &
Gillette, J. R. ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 2, pp. 349-361.
GILLETTE, J. R. (1971c) Effects of various inducers on electron
transport systems associated with drug metabolism by liver
microsomes. Metabolism, 20: 215-227.
GILLETTE, J. R. (1973a) Overview of drug-protein binding. Ann. NY
Acad. Sci., 226: 6-17.
GILLETTE, J. R. (1973b) The importance of tissue distribution in
pharmacokinetics. J. Pharmacol. Biopharm., 1: 497-519.
GILLETTE, J. R. (1974a) A perspective on the role of chemically
reactive metabolites of foreign chemicals in toxicity. I.
Correlation of changes in covalent binding of reactivity
metabolites with changes in the incidence and severity of
toxicity. Biochem. Pharmacol., 23: 2785-2794.
GILLETTE, J. R. (1974b) A perspective on the role of chemically
reactive metabolites of foreign compounds in toxicity: II.
Alterations in the kinetics of covalent binding. Biochem.
Pharmacol., 23: 2927-2938.
GILLETTE, J. R. (1975) Other aspects of pharmacokinetics. In:
Gillette, J. R. & Mitchell, J. R., ed. Concepts in biochemical
pharmacology, Berlin, Springer-Verlag, Vol. 3, pp. 35-85.
GILLETTE, J. R., CHAN, T. W., & TERRIERE, L. C. (1966) Interactions
between DDT analogues and microsomal epoxidase systems.
J. agric. Food Chem., 14: 540-545.
GILLETTE, J. R. DAVIS, D. C., & SASAME, (1972) Cytochrome P-450 and
its role in drug metabolism. Annu. Rev. Pharmacol., 12: 57-84.
GILLETTE, J. R., MITCHELL, J. R., & BRODIE, B. B. (1974) Biochemical
mechanisms of drug toxicity. Annu. Rev. Pharmacol.,
14: 271-288.
GOLDSTEIN, A., ARONOW, L., & KOLMAN, S. M. (1974) Principles of drug
action. In: The basis of pharmacology, 2nd ed., New York,
Wiley, pp. 106-205.
GRAM, T. E. (1971) Enzymatic N-, O- and S-dealkylation of foreign
compounds by hepatic microsomes. In: Brodie, B. B. & Gillette, J.
R., ed. Concepts in biochemical pharmacology, Berlin,
Springer-Verlag, Vol. 2, pp. 334-348.
GRAM, T. E. (1973) Comparative aspects of mixed function oxidation by
lung and liver of rabbits. Drug. Metabol. Rev., 2: 1-32.
GREGORY, J. D. (1960) The effect of borate on the carbazole reaction.
Arch. Biochem. Biophys., 89: 157-159.
GREGORY, J. D. & LIPMANN, F. (1957) The transfer of sulfate among
phenolic compounds with 3,5-diphosphoadenosine as co-enzyme.
J. Biol. Chem., 229: 1081-1090.
GRIFFITHS, M. H. (1968) The metabolism of N-triphenylmorphine in the
dog and rat. Biochem. J., 108: 731-740.
HART, L. G. & FOUTS, J. R. (1965) Further studies on the stimulation
of hepatic microsomal drug metabolizing enzymes by DDT and its
analogues. Arch. exp. Pathol., 249: 486-500.
HEIRWEGH, K. P. M., VAN DER VIJER, M., & FEVERY, J. (1972) Assay and
properties of digitonin-activated bilirubin uridine diphosphate
glucuronyl-transferase from rat liver. Biochem. J.,
129: 605-618.
HENDERSON, P. TH. & KERSTEN, K. J. (1970) Metabolism of drugs during
liver regeneration. Biochem. Pharmacol., 19: 2343-2351.
HERR, F. & KIESE, M. (1959) [Determination of nitrosobenzol in the
blood.] Arch. exp. Path. Pharmakol., 235: 351-353 (in German).
HEWICK, D. S. & FOUTS, J. R. (1970a) Effects of storage on hepatic
microsomal cytochromes and substrate-induced difference spectra.
Biochem. Pharmacol., 19: 457-472.
HEWICK, D. S. & FOUTS, J. R. (1970b) The metabolism in vitro and
hepatic microsomal interactions of some enantiomeric drug
substrates. Biochem. J., 117: 833-841.
HIDAKA, H., NAGATSU, T., & YAGI, K. (1967) A rapid and simple assay of
serotonin sulfokinase activity. Anal. Biochem., 17: 388-392.
HIETANEN, E. (1974) Effect of sex and castration on hepatic and
intestinal activity of drug-metabolizing enzymes. Pharmacology,
12: 84-89.
HIETANEN, E. & VALINIO, H. (1973) Interspecies variations in small
intestinal and hepatic drug hydroxylation and glucuronidation.
Acta Pharmacol. Toxicol., 33: 57-64.
HIETBRINK, B. E. & DUBOIS, K. P. (1965) Influence of X-radiation on
development of enzymes responsible for desulfuration of an
organic phosphorothioate and reduction of p-nitrobenzoic acid
in the livers of male rats. Radiat. Res., 22: 598-603.
HILTON, J. & SARTORELLI, A. C. (1970) Induction by phenobarbital of
microsomal mixed oxidase enzymes in regenerating liver. J. biol.
Chem., 245: 4187-4192.
HOFFMAN, D. G., WORTH, H. M., & ANDERSON, R. C. (1968) Stimulation of
hepatic microsomal drug-metabolizing enzymes by alpha alpha-bis
p-chlorophenyl-3-pyridine methanol and a method for determining
no-effect levels in rats. Toxicol. appl. Pharmacol.,
12: 464-472.
HOFFMAN, D. F., WORTH, H. M., & ANDERSON, R. C. (1970) Stimulation of
hepatic drug-metabolizing enzymes by chlorophenothane (DDT); the
relationship to liver enlargement and hepatotoxicity in the rat.
Toxicol. appl. Pharmacol., 16: 171-178.
HOLTZMAN, J. L., GRAM, T. E., GIGON, P. L., & GILLETTE, J. R. (1968)
The distribution of the components of mixed function oxidase
between the rough and the smooth endoplasmic reticulum of liver
cells. Biochem. J., 110: 407-412.
HOOK, J. B., BAIBIE, M. D., JOHNSON, J. T., & GEHRING, P. J. (1974)
In vitro analysis of transport of 2,4,5-trichlorophenoxyacetic
acid (2,4,5-T) by rat and dog kidney. Food Cosmet. Toxicol.,
12: 209-218.
HUCKER, H. B. (1970) Species differences in drug metabolism.
Annu. Rev. Pharmacol., 10: 99-118.
IMAI, Y., ITO, A., & SATOR, R. R. (1966) Evidence for biochemically
different types of vesicles in the hepatic microsomal fraction.
J. Biochem., 60: 417-428.
ISSELBACHER, K. J. (1956) Enzymatic mechanisms of hormone metabolism.
II. Mechanism of hormonal glucuronide formation. In: Pincus, G.,
ed. Recent progress in hormone research, New York, Academic
Press, pp. 134-151.
JENNE, J. W. & BOYER, P. D. (1962) Kinetic characteristics of the
acetylation of isoniazid and p-aminosalicylic acid by a liver
enzyme preparation. Biochim. Biophys. Acta, 65: 121-127.
JOHNSON, M. K. (1966) Metabolism of iodomethane in the rat.
Biochem. J., 98: 38-43.
JOLLOW, P. J., MITCHELL, J. R., ZAMPOGLIONE, N., & GILLETTE, J. R.
(1974) Bromobenzene-induced liver necrosis: protective role of
glutathione and evidence for 3,4-bromobenzene oxide as the
hepatotoxic metabolite. Pharmacology, 11: 151-169.
KATO, R. & GILLETTE, J. R. (1965) Effect of starvation on
NADPH-dependent enzymes in liver microsomes of male and female
rats. J. Pharmacol. exp. Ther., 150: 279-284.
KATZ, M. & POULSEN, B. J. (1971) Absorption of drugs through the skin.
In: Brodie, B. B. & Gillette, J. R., ed. Concepts in biochemical
pharmacology, Berlin, Springer-Verlag, Vol. 1, pp. 103-174.
KEBERLE, H., HOFFMAN, K., & BERNHARD, K. (1962) The metabolism of
glutethimide (Doridnene). Experientia (Basel), 18: 105-111.
KEEN, P. (1971) Effect of binding to plasma proteins on the
distribution, activity and elimination of drugs. In: Brodie, B.
B. & Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 1, pp. 213-233.
KINOSHITA, F. K., FRAWLEY, J. P., & DUBOIS, K. P. (1966) Quantitative
measurement of induction of hepatic microsomal enzymes by various
dietary levels of DDT and toxaphene in rat. Toxicol. appl.
Pharmacol., 9: 505-513.
KLINGER, W. (1974) Optimal conditions for the first step of
amidopyrine- N-demethylation by 900 × g liver supernatant of
newborn and adult rats. Acta Biol. Med. Germ., 33: 181-186.
KUNTZMAN, R. (1969) Drugs and enzyme induction. Annu. Rev.
Pharmacol., 9: 21-36.
KURZ, H. & FRIEMEL, G. (1967) [Species-specific differences in the
binding of plasma proteins.] Arch. Pharmakol. exp. Path.,
257: 35-36 (in German).
LA DU, B. N. & SNADY, H. (1971) Esterases of human tissues. In:
Brodie, B. B. & Gillette, J. R., ed. Concepts in biochemical
pharmacology, Berlin, Springer-Verlag, Vol. 2, pp. 477-499.
LA DU, B. N., GAUDETTE, L., TROUSOF, N., & BRODIE, B. B. (1955)
Enzymatic dealkylation of aminopyrine (Pyramidon) and other
alkylamines. J. biol. Chem., 214: 741-752.
LA DU, B. N., MANDEL, G. H., & WAY, E. L. (1972) Fundamentals of drug
metabolism and drug disposition. Baltimore, Williams & Wilkins.
LEVI, A. J., GATMAITAN, Z., & ARIAS, I. M. (1969) Two hepatic
cytoplasmic protein fractions, Y and Z, and their possible role
in hepatic uptake of bilirubin, sulfobromophthalein, and other
anions, J. clin. Invest., 48: 2156-2167.
LEVINE, R. R. (1970) Factors affecting gastrointestinal absorption of
drugs. Digest Dis. 15: 171-188.
LEVY, G. & GIBALDI, M. (1972) Pharmacokinetics of drug action.
Annu. Rev. Pharmacol., 12: 85-98.
LEVY, G. & GIBALDI, M. (1975) Pharmacokinetics. In: Gillette, J. R. &
Mitchell, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 3, pp. 1-34.
LITWACK, G., KETTERER, B., & ARIAS, I. M. (1971) Ligondin: a hepatic
protein which binds steroids, bilirubin, carcinogens and a number
of exogenous organic anions. Nature (Lond.), 234: 466-467.
LIU, S. J., RAMSEY, R. K., & FALLON, H. J. (1975) Effects of ethanol
on hepatic microsomal drug-metabolizing enzymes in the rat.
Biochem. Pharmacol., 24: 369-378.
LU, A. Y. H. & LEVIN, W. (1974) The resolution and reconstitution of
the liver microsomal hydroxylation system. Biochim. Biophys.
Acta, 344: 205-240.
LU, A. Y. H., STROBEL, H. W., & COON, M. J. (1969) Hydroxylation of
benzphetamine and other drugs by a solubilized form of cytochrome
P-450 from liver microsomes: lipid requirement for drug
demethylation. Biochem. Biophys. Res. Commun., 36: 545-551.
LUCIS, O. J., SCHACKH, Z. A., & EMBIL, J. A., JR (1970) Cadmium as a
trace element and cadmium-binding components in human cells.
Experientia (Basel), 26: 1109-1112.
MACCAMAN, R. E. (1965) Microdetermination of catechol- O-methyl
transferase. Life Sci., 4: 2353-2359.
MCLEAN, A. E. M. (1971) Conversion by the liver of inactive molecules
into toxic molecules. In: Aldridge, W. N., ed. Mechanism of
toxicity, London, Macmillan, pp. 219-228.
MACMAHON, R. E. (1962) The competitive inhibition of the
N-demethylation of butynamine by 2,4-dichloro-6-
phenolphenoxy-ethylamine (DPEA). J. Pharmacol. exp. Ther.,
138: 382-386.
MACMAHON, R. E. (1971) Enzymatic oxidation and reduction of alcohols,
aldehydes and ketones. In: Brodie, B. B. & Gillette, J. R., ed.
Concepts in biochemical pharmacology, Berlin, Springer-Verlag,
Vol. 2, pp. 500-517.
MAHER, J. R., WHITNEY, J. M., CHAMBERS, J. S., & STANONIS, D. J.
(1957) The quantitative determination of isoniazid and
para-aminosalicylic acid in body fluids. Am. Rev. Tuberc.,
76: 852-861.
MANNERING, G. J. (1968) Significance of stimulation and inhibition of
drug metabolism. In: Burger, A., ed. Selected pharmaceutical
testing methods, New York, Marcel Dekker, pp. 51-119.
MANNERING, G. J. (1971a) Inhibition of drug metabolism. In: Brodie, B.
B. & Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 2, pp. 452-476.
MANNERING, G. J. (1971b) Properties of cytochrome P-450 as affected by
environmental factors: qualitative changes due to administration
of polycyclic hydrocarbons. Metabolism, 20: 228-245.
MARK, L. C. (1971) Translocation of drugs and other exogenous
chemicals into adipose tissue. In: Brodie, B. B. & Gillette, J.
R., ed. Concepts in biochemical pharmacology, Berlin,
Springer-Verlag, Vol. 1, pp. 258-275.
MARSELOS, M. & HÄNNINEN, O. (1974) Enhancement of D-glucuronolactone
and acetaldehyde dehydrogenase activities in the rat liver by
inducers of drug metabolism. Biochem. Pharmacol.,
23: 1457-1466.
MARSH, C. A. (1963) Metabolism of D-glucuronolactone in mammalian
systems; conversion of D-glucuronolactone into D-glucaric acid by
tissue preparations. Biochem. J., 87: 82-90.
MARSHALL, E. K., JR (1948) Determination of par-amino-salicylic acid
in blood. Proc. Soc. Exp. Biol. Med., 68: 471-472.
MASON, H. S. (1957) Mechanism of oxygen metabolism. Adv. Enzymol.,
19: 79-233.
MILLER, E. C. & MILLER, J. A. (1971a) The mutagenicity of chemical
carcinogens: correlations, problems and interpretations. In:
Hollaender, A., ed. Chemical mutagens, New York, Plenum,
Vol. 1, pp. 83-119.
MILLER, J. A. & MILLER, E. C. (1971b) Chemical carcinogenesis:
mechanisms and approaches to its control. J. Natl Cancer Inst.,
47: v-xiv.
NASH, T. (1953) The colorimetric estimation of formaldehyde by means
of the Hantsch reaction. Biochem. J., 55: 416-421.
NEBERT, D. W. & GELBOIN, H. V. (1968a) Substrate inducible microsomal
aryl hydroxylase. I. Assay and properties of induced enzymes.
J. Biol. Chem., 243: 6242-6249.
NEBERT, D. W. & GELBOIN, H. V. (1968b) Substrate inducible microsomal
aryl hydroxylase. II. Cellular responses during enzymic
induction. J. Biol. Chem., 243: 6250-6261.
NETTER, K. J. (1960) [A method for the direct measurement of
O-demethylization in liver microsomes and its application to
the inhibitory effects of SKF 525A on microsomes.]
Arch. Pharmacol., 238: 292-300 (in German).
NETTER, K. J. & SEIDEL, G. (1964) An adaptively stimulated
O-demethylating system in rat liver microsomes and its kinetic
properties. J. Pharmacol. exp. Ther., 146: 61-65.
NOTTON, W. R. & HENDERSON, P. TH. (1975) The influence of N-hexane
treatment on the glucuronic acid pathway and activity of some
drug-metabolizing enzymes in the guinea pig. Biochem.
Pharmacol., 24: 127- 131.
OBATA, F., USHIWATA, A., & NAKAMURA, Y. (1971) Spectrophotometric
assay of monoamine oxidase using 2,4,6-trinitrobenzene-1-sulfonic
acid. J. Biochem., 69: 349-354.
OGATA, M., TOMOKUNI, K., & TAKATSUKA, Y. (1969) Quantitative
determination in urine of hippuric acid and m- or p-methyl
hippuric acid, metabolites of toluene and m- or p-xylene.
Brit. J. ind. Med., 26: 330-334.
OMURA, T. & SATO, R. (1964a) The carbon monoxide-binding pigment of
liver microsomes: I. Evidence for its hemoprotein nature.
J. biol. Chem., 239: 2370-2378.
OMURA, T. & SATO, R. (1964b) The carbon monoxide-binding pigment of
liver microsomes: II. Solubilization, purification and
properties. J. biol. Chem., 239: 2379-2385.
OTSUKA, S. & KOBAYASHI, Y. (1964) A radioisotopic assay for monamine
oxidase determinations in human plasma. Biochem. Pharmacol.,
13: 995-1006.
PAPPENHEIMER, J. R. (1953) Passage of molecules through capillary
walls. Physiol. Rev., 33: 387-423.
PARKE, D. V. (1968) The biochemistry of foreign compounds. New York,
Pergamon Press.
PEDERSON, T. C. & AUST, S. D. (1970) Aminopyrine demethylase: kinetic
evidence for multiple microsomal activities. Biochem.
Pharmacol., 19: 2221-2230.
PIPER, W. N., ROSE, J. Q., LENG, M. L. & GEHRING, P. J. (1973) The
fate of 2,4,5-trichlorophenoxyacetic acid (2,4,5-T) following
oral administration to rats and dogs. Toxicol. appl. Pharmacol.,
26: 339-351.
PITTS, R. F. (1963) Physiology of the kidney and body fluids.
Yearbook, Chicago, Medical Publishers Inc.
PLAA, G. L. (1975) The enterohepatic circulation. In: Gillette, J. R.
& Mitchell, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 3, pp. 130-149.
PLACE, V. A. & BENSON, H. (1971) Dietary influences on therapy with
drugs. J. Mond. Pharm., 14: 261-278.
POLAND, A. P. & NEBERT, D. W. (1973) A sensitive radiometric assay of
aminopyrine N-demethylation. J. Pharmacol. exp. Ther.,
184: 269-277.
POTTS, A. M. (1964) The reaction of uveal pigments in vitro with
polycyclic compounds. Invest. Ophthalmol., 3: 405-416.
PRESCOTT, L. F. (1975) Pathological and physiological factors
affecting drug absorption, distribution, elimination and response
in man. In: Gillette, J. R. & Mitchell, J. R., ed. Concepts in
biochemical pharmacology, Berlin, Springer-Verlag,
Vol. 3, pp. 234-257.
PRICE, H. L., KOVNAT, P. J., SOFER, J. N., CONNER, E. H., & PRICE, M.
L. (1960) The uptake of thiopental by body tissues and its
relation to the duration of narcosis. Clin. Pharmacol. Ther.,
1: 16-22.
QUASTEL, J. H. (1965) Molecular transport at cell membranes.
Proc. Roy. Soc. Br., 163: 169-186.
QUINN, G. P., AXELROD, J., & BRODIE, B. B. (1958) Species, strain and
sex differences in metabolism of hexobarbitone, aminopyrine,
antipyrine and aniline. Biochem. Pharmacol., 1: 152-159.
RALL, D. P. (1971) Drug entry into brain and cerebrospinal fluid. In:
Brodie, B. B. & Gillette, J. R., ed. Concepts in biochemical
pharmacology, Berlin, Springer-Verlag, Vol. 1, pp. 240-248.
RASMUSSEN, F. (1971) Excretion of drugs by milk. In: Brodie, B. B. &
Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 1, pp. 390-402.
REMMER, H., SCHENKMAN, J., ESTABROOK, R. W., SASAME, H., GILLETTE, J.
R., NARASHIMHULU, S., COOPER, D. Y., & ROSENTHAL, O. (1966) Drug
interaction with hepatic microsomal cytochrome. Mol. Pharmacol.,
2: 187-190.
RENKIN, E. M. (1964) Transport of large molecules across capillary
walls. Physiologist, 7: 13.
REYES, H., LEVI, A. J., GATMAITAN, Z., & ARIAS, I. M. (1971) Studies
on Y and Z, two hepatic cytoplasmic organic anion-binding
proteins: Effect of drugs, chemicals, hormones and cholestosis.
J. Clin. Invest., 50: 2242-2251.
RODOMSKI, J. L., DEICHMANN, W. B., & CLIZER, E. E. (1968) Pesticide
concentrations in the liver, brain and adipose tissue of terminal
hospital patients. Food Cosmet. Toxicol., 6: 209-220.
ROLL, R. (1971) Investigations concerning the teratogenic effect of
2,4,5-T in mice. Food Cosmet. Toxicol., 9: 671-676.
ROSE, J. Q., RAMSEY, J. C., WENTZLER, T. G., HUMMEL, R. A., & GEHRING,
P. J. (1975) The fate of 2,3,7,8-tetrachlorodibenzo- p-dioxin
(TCDD) following single and repeated oral doses to the rat.
Toxicol. appl. Pharmacol., 36: 209-226.
ROSENBLUM, C. (1965) Non-metabolite residues in radioactive tracer
studies. In: Roth, L. J., ed. Isotopes in experimental
pharmacology, Chicago, University of Chicago Press,
pp. 353-360.
ROTH, L. J. (1971) The use of autoradiography in experimental
pharmacology. In: Brodie, B. B. & Gillette, J. R., ed. Concepts
in biochemical pharmacology, Berlin, Springer-Verlag,
Vol. 1, pp. 286-316.
ROWE, V. K. & HYMAS, T. A. (1954) Summary of toxicological information
of 2,4-D and 2,4,5-T type herbicides and an evaluation of the
hazards to livestock associated with their use. Am. J. Vet.
Res., 15: 622-629.
ROY, A. B. (1971) Sulfate conjugation enzymes. In: Brodie, B. B. &
Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 2, pp. 536-563.
SAUERHOFF, M. W., BRAUN, W. H., BLAU, G. E., & GEHRING, P. J. (1975)
The dose-dependent pharmacokinetic profile of
2,4,5-trichlorophenoxyacetic acid following intravenous
administration to rats. Toxicol. appl. Pharmacol., 36: 491-501.
SCHANKER, L. S. (1962a) Passage of drugs across body membranes.
Pharmacol. Rev., 14: 501-530.
SCHANKER, L. S. (1962b) Concentrative transfer of an organic cation
from blood into bile. Biochem. Pharmacol., 11: 253-254.
SCHANKER, L. S. (1963) Passage of drugs across the gastrointestinal
epithelium. In: Hogben, C. A. M., ed. Proceedings of the 1st
International Pharmacological Meeting, Oxford, Pergamon Press,
Vol. 4, pp. 120-130.
SCHANKER, L. S. (1965) Hepatic transport of organic cations. In:
Taylor, W., ed. The biliary system. Oxford, Blackwell.
SCHANKER, L. S. (1971) Absorption of drugs from the gastrointestinal
tract. In: Brodie, B. B. & Gillette, J. R., ed. Concepts in
biochemical pharmacology, Berlin, Springer-Verlag,
Vol. 1, pp. 9-24.
SCHANKER, L. S., SHORE, P. A., BRODIE, B. B., & HOGBEN, C. A. M.
(1957) Absorption of drugs from the stomach. I. The rat.
J. Pharmacol. exp. Ther., 120: 528-539.
SCHELINE, R. R. (1968) Drug metabolism by intestinal microorganisms.
J. pharm. Sci., 57: 2021-2037.
SCHENKMAN, J. B., REMMER, H., & ESTABROOK, R. W. (1967) Spectral
studies of drug interaction with hepatic microsomal cytochrome.
Mol. Pharmacol., 3: 113-123.
SCHOENE, B., FLEISCHMANN, R. A., REMMER, H., & OLDERSHAUSEN, H. F.
(1972) Determination of drug-metabolizing enzymes in needle
biopsies of human liver. Eur. J. clin. Pharmacol., 4: 65-73.
SCHOLTAN, W. (1963) On the protein binding of long-acting
sulfonamides. Chemotherapia, 6: 180-195.
SCHWETZ, B. A., NORRIS, J. M., SPARSCHU, G. L., ROWE, V. K., GEHRING,
P. J., EMERSON, J. K., & GERBIG, C. G. (1973) Toxicology of
chlorinated dibenzo- p-dioxins. Environ. Health Perspect.
(Experimental issue), 5: 87-100.
SETTLE, W., HEGEMAN, S., & FEATHERSTONE, R. M. (1971) The nature of
drug-protein interaction. In: Brodie, B. B. & Gillette, J. R.,
ed. Concepts in biochemical pharmacology, Berlin,
Springer-Verlag, Vol. 1, pp. 175-186.
SHORE, P. A., BRODIE, B. B., & HOGBEN, C. A. M. (1957) The gastric
secretion of drugs -- a pH partition hypothesis. J. Pharmacol.
exp. Ther., 119: 361-369.
SHULERT, A. R. (1961) Physiological disposition of hydralazine
(1-hydrazinophthalazine) and a method for its determination in
biological fluids. Arch. int. Pharmacodyn., 132: 1-15.
SMITH, R. L. (1971a) Excretion of drugs in bile. In: Brodie, B. B. &
Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 1, pp. 354-389.
SMITH, R. L. (1971b) The role of the gut flora in the conversion of
inactive compounds to active metabolites. In: Aldridge, W. N.,
ed. Mechanism of toxicity, London, Macmillan, pp. 229-247.
SMITH, R. L. (1973) The excretory function of bile: the elimination
of drugs and toxic substances in bile. London, England, Chapman
& Hall.
SMUCKLER, E. A. (1971) Metabolism of halogenated compounds. In:
Brodie, B. B. & Gillette, J. R., ed. Concepts in biochemical
pharmacology, Berlin, Springer-Verlag, Vol. 2, pp. 367-377.
SPARSCHU, G. L., DUNN, F. L., LISOWE, R. W. & ROWE, V. K. (1971) Study
of the effects of high levels of 2,4,5-trichlorophenoxyacetic
acid on fetal development in the rat. Food Cosmet. Toxicol.,
9: 527-530.
SPERBER, I. (1963) Drugs and membranes. In: Hogben, C. A. M., ed.
Proceedings of the 1st International Pharmacological Meeting,
Oxford, Pergamon Press, Vol. 4.
STERRSON, L. A. & HES, J. (1975) Electrochemical method for the
determination of aniline hydroxylation. Anal. Biochem.,
67: 74-80.
STOTZ, E., STEINBERG, M. S., COHEN, S. N., & WEBER, W. W. (1969)
Acetylation of 5-hydroxytryptamine by isoniazide
N-acetyltransferase. Biochim. Biophys. Acta, 184: 210-212.
STOWE, C. M. & PLAA, G. L. (1968) Extrarenal excretion of drugs and
chemicals. Annu. Rev. Pharmacol., 8: 337-356.
STRICKLAND, R. D., GREGORY, D. H., & LYNCH, J. L. (1974) A method for
assaying hepatic morphine UDP-glucuronyl transferase. Biochem.
Med., 11: 180-188.
STURMAN, J. A. & SMITH, M. J. H. (1967) The binding of salicylate to
plasma proteins in different species. J. Pharm. Pharmacol.,
19: 621-623.
SUGA, T., OHATA, I., KUMAOKA, H., & AKAGI, M. (1967) Studies on
mercapturic acids: investigation of glutathione-conjugating
enzyme by the method of thin-layer chromatography.
Chem. Pharmacol. Bull., 15: 1059-1064.
TABOR, H., MEHLER, A. H., & STADTMAN, E. R. (1953) The enzymatic
acetylation of amines. J. biol. Chem., 204: 127-138.
TAKAHASHI, H. & TAKAHARA, S. (1968) A sensitive fluorimetric assay for
monoamine oxidase based on the formation of 4,6-quinolinediol
from 5-hydroxykynurenamine. J. Biochem., 64: 7-11.
TEORELL, T. (1937a) Kinetics of distribution of substances
administered to the body: I. The extra-vascular modes of
administration. Arch. int. Pharmacodyn., 57: 205-226.
TEORELL, T. (1937b) Kinetics of distribution of substances
administered to the body: II. The intra-vascular modes of
administration. Arch. int. Pharmacodyn., 57: 227-240.
TUFVESSON, G. (1970) Fluorimetric determination of amine oxidase
activity in human blood serum with kynuramine as substrate.
Scand. J. clin. Lab. Invest., 26: 151-154.
ULLRICH, V. & WEBER, P. (1972) The O-dealkylation of
7-ethoxycoumarin by liver microsomes: a direct fluorimetric test.
Z. Physiol. Chem., 353: 1171-1177.
WAGNER, J. P. (1968) Pharmacokinetics. Ann. Rev. Pharmacol.,
8: 67-94.
WAGNER, J. P. (1971) Biopharmaceutics and relevant pharmacokinetics.
Hamilton, Drug Intelligence Publications.
WATTERNBERG, L. W. & LEONG, J. L. (1971) Tissue distribution studies
of polycyclic hydrocarbon hydroxylase activity. In: Brodie, B. B.
& Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 2, pp. 422-430.
WATTENBERG, L. W., LEONG, J. L., & STRAND, P. J. (1962) Benzpyrene
hydroxylase activity in the gastrointestinal tract. Cancer Res.,
22: 1120-1125.
WEBER, W. W. (1970) N-acetyltransferase (mammalian liver). In:
Tabor, H. & Tabor, C. W., ed. Methods in enzymology, 17B. New
York, Academic Press.
WEBER, W. W. (1971) Acetylating, deacetylating and amino
acid-conjugating enzymes. In: Brodie, B. B. & Gillette, J. R.,
ed. Concepts in biochemical pharmacology, Berlin,
Springer-Verlag, Vol. 2, pp. 564-583.
WEBER, W. W. & COHEN, S. N. (1968) The mechanism of isoniazid
acetylation by human N-acetyltransferase. Biochim. Biophys.
Acta, 151: 276-278.
WEBER, W. W., COHEN, S. N., & STEINBERG, M. S. (1968) Purification and
properties of N-acetyltransferase from mammalian liver.
Ann. NY Acad. Sci., 151: 734-741.
WEINER, I. M. (1971) Excretion of drugs by the kidney. In: Brodie, B.
B. & Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 1, pp. 328-353.
WEISBURGER, H. H. & WEISBURGER, E. R. (1971) N-oxidation enzymes.
In: Brodie, B. B. & Gillette, J. R., ed. Concepts in biochemical
pharmacology, Berlin, Springer-Verlag, Vol. 2, pp. 312-333.
WEISBURGER, H. H., GRANTHAM, P. H., & WEISBURGER, E. R. (1964)
Metabolism of N-2-fluorenylacetamide in the hamster. Toxicol.
appl. Pharmacol., 6: 427-433.
WEISSBACH, H., SMITH, T. E., FALY, J. W., WITKOP, B., & UDENFRIEND, S.
(1960) A rapid spectrophotometric assay of monoamine oxidase
based on the rate of disappearance of kynuramine. J. Biol.
Chem., 235: 1160-1163.
WILLIAMS, C. H., JR & KAMIN, H. (1962) Microsomal triphosphopyridine
nucleotide-cytochrome c reductase of liver. J. Biol. Chem.,
237: 587-595.
WILLIAMS, R. T. (1965) The influence of enterohepatic circulation on
toxicity of drugs. Ann. NY Acad. Sci, 123: 110-124.
WILLIAMS, R. T. (1967a) The biogenesis of conjugation and detoxication
products. In: Bernfield, P, ed. Biogenesis of natural compounds,
2nd ed., Oxford, Pergamon Press, pp. 589-639.
WILLIAMS, R. T. (1967b) Comparative patterns of drug metabolism.
Fed. Proc., 26: 1029-1039.
WILLIAMS, R. T. (1971) Introduction: Pathways of drug metabolism. In:
Brodie, B. B. & Gillette, J. R., ed. Concepts in biochemical
pharmacology, Berlin, Springer-Verlag, Vol. 2, pp. 226-242.
WITIAK, D. T. & WHITEHOUSE, M. W. (1969) Species differences in the
albumin binding of 2,4,6-trinitrobenzaldehyde, chlorphenoxyacetic
acids, 2-(4'-hydroxybenzeneazo) benzoic acid and some other
drugs; the unique behaviour of rat albumin. Biochem. Pharmacol.,
18: 971-977.
WITSCHI, H. (1975) Exploitable biochemical approaches for evaluation
of toxic lung damage. In: Hayes, W. J. Jr., ed. Essays in
toxicology, New York, Academic Press, Vol. 6, pp. 125-191.
YUKI, H. & FISHMAN, W. H (1963) A carbazole method for the
differential analysis of glucuronate, glucosiduronate and
hyaluronate. Biochim. Biophys. Acta, 69: 576-578.
ZAKIM, D. & VESSEY, D. A. (1973) Techniques for the characterization
of UDP-glucuronyl-transferase, glucose-6-phosphatase and other
tightly bound microsomal enzymes. In: Glick, D., ed. Methods of
biochemical analysis, New York, Wiley, Vol. 21, pp. 2-37.
ZANNONI, V. G. (1971) Experiments illustrating drug distribution and
excretion. In: La Du, B. N., Mandel, H. G, & Way, E. L, ed.
Fundamentals of drug metabolism and drug disposition,
Baltimore, Williams & Wilkins, pp. 583-590.
ZANNONI, V. G., FLYNN, E. J., & LYNCH, M. (1972) Ascorbic acid and
drug metabolism. Biochem. Pharmacol., 21: 1377-1392.
ZELLER, E. A. (1971) Amine oxidases. In: Brodie, B. B. & Gillette, J.
R., ed. Concepts in biochemical pharmacology, Berlin,
Springer-Verlag, Vol. 2, pp. 518-535.
ZIEGLER, D. M., MCKEE, E. M., & POULSEN, L. L. (1973) Microsomal
flavo-protein-catalyzed N-oxidation of arylamines. Drug Metab.
Disposition, 1: 314-320.
5. MORPHOLOGICAL STUDIES
5.1 Introduction
Morphological studies are often the corner stones of toxicity
experiments. The variety of such studies leads to many different
approaches from the viewpoint of pathology, and skill and flexibility
in working procedures seem to be far more important than a strict
schedule.
On the other hand, it is advisable to have some general
guidelines on pathological procedures for routine quality testing for
toxicity, even though specific questions may often be posed, special
experiments may have to be carried out, and special animals,
techniques, and examinations used in order to elucidate certain
problems.
This chapter deals with the various phases of morphological
studies with a view to providing some general recommendations
concerning the procedures to be followed. It must be emphasized,
however, that these recommendations are only a guide, and that it will
be the pathologist's special responsibility to see that studies are
carried out in the way most likely to ensure optimum results.
5.2 General Recommendations
Gross necropsy facilities should be in close proximity to those
of the pathologist. The autopsy room must be equipped with adequate
dissection tables, dissection materials, running water, drains,
lighting, ventilation, and facilities for disinfection. In addition,
gross photography facilities are necessary. Cooling facilities must be
available for the storage of dead animals until necropsy, but the
animals should not be frozen (Sontag et al., 1975). To carry out
proper experiments and to prevent the loss of a considerable number of
animals by cannibalism or autolysis, it is essential that animals be
observed at least once a day, including Saturdays and Sundays. Animals
in moribund condition should be killed.
For the trimming of fixed tissues, a well-ventilated area,
preferably with an exhaust hood, and running water and drains, is
required.
The histology laboratory should be separated from the autopsy
room and should be equipped with tissue-processing equipment,
microtomes, cryostat, embedding and staining facilities, and supplies
(Sontag et al., 1975). Storage facilities are necessary for the fixed
tissues, as well as for the tissue block and histological slide files.
The facilities should be vermin-proof and temperature controlled or at
least cool.
Veterinary or medical pathologists with experience in laboratory
animal pathology or others with appropriate training and experience
should be responsible for all pathology procedures. In addition, the
pathologist should participate in the design and conduct of
experiments.
Histology technicians with appropriate training and experience in
the histological field will guarantee good histotechnical work,
whereas technicians trained and experienced in laboratory animal
dissection will be of great help in performing necropsies. Technicians
must be able to recognize and adequately describe gross abnormalities.
Personnel should be available at the weekends to perform
necropsies on animals found dead or killed in extremis.
5.3 Gross Observations
Gross pathology can be performed in most cases by experienced
technicians under the guidance of the pathologist. They should follow
a certain scheme of necropsy technique and must be able to recognize
abnormalities. A checklist should be used to ensure that all organs
and tissues are inspected and dissected. Information on clinical signs
must be available. Abnormalities found must be recorded on autopsy
cards. The descriptions must be clear and provide details such as
location, size, colour, texture, number, etc.
A great number of lesions will be within the scale of known
abnormalities for the animal species and breed used. Lesions found
beyond this normal scale should always be examined by the pathologist
himself. It is advisable for all new lesions, especially those
attributable to the treatment, to be photographed.
Dead animals should be necropsied unless cannibalism or autolysis
precludes this. Autolysis should not readily be accepted as an excuse
for not performing an autopsy. An inadequate gross necropsy cannot be
replaced by microscopic examination, no matter how well performed. On
the other hand, a well-performed gross necropsy may provide optimum
information for microscopic examination and may, in certain cases,
facilitate more selective microscopic examination.
Several ways of killing animals are available and the methods
depend on the facilities, the purpose of the experiment, and the
species used. Sacrifice of animals by anaesthesia (by ether or
barbiturates) is widely used as well as asphyxiation by carbon
dioxide. In rodents, the blood of the body can be removed by cardiac
puncture or puncturing the abdominal aorta, and in dogs by puncture of
the common carotid artery. Alternatively in small rodents decapitation
can be performed, though this may result in blood aspiration and
damage to certain tissues or organs. On the other hand decapitation
will probably give more constant results with respect to the total
blood loss, and consequently lead to fairly consistent organ weights.
5.3.1 Autopsy techniques
It is difficult, if not impossible, to prescribe working
procedures or techniques that will be adequate in all conditions.
Often, observations made when the animals were alive or experience
with other structurally-related compounds may focus attention on
certain organs or tissues. Sometimes fixation by perfusion is
necessary for proper examination of specific tissues (for example, the
central nervous system).
Large numbers of animals should not be sacrificed at any one
time, if too few technicians are available to perform the necropsies.
Animals should be killed in sequence, especially where it is necessary
to sample tissues for biochemical, enzyme-histochemical, or analytical
studies. However, organs and tissues should be weighed as soon as
possible after death to ensure that they have not dried out and that
they can be fixed quickly. Special procedures may shorten the time
needed for full autopsy especially in the case of small rodents. The
exact organization of working procedures depends largely on the
facilities and labour available, but good preparation and teamwork are
crucial factors.
Sacrifice should be carried out so that animals of the
experimental and control groups are killed at approximately the same
time of day, thus preventing the introduction of variations in
physiological status dependent on circadian rhythm. In certain cases,
however, this may not be feasible.
Special care is necessary when test elements or compounds are
analysed after sacrifice. At times it may be necessary to kill the
animals in a given order to prevent contamination (e.g. sacrifice of
the control first, followed by the lowest dose level, then the
intermediate dose level, and finally the high-dose level).
Autopsy techniques have already been described (Roe, 1965) and
only a general outline of the procedures is given.
5.3.2 Rat, mouse, guineapig, rabbit, monkey
The necropsy starts with external examination including the body
orifices. Thereafter, the animals are fixed on their backs. After a
median incision, the skin is partly removed and the subcutis,
superficial lymph nodes, salivary glands, and mammary glands can be
inspected and dissected. The abdomen can be opened and the negative
pressure of the thorax may be checked and the thorax opened.
Thyroid and thymus can then be inspected and dissected.
Trachea, lungs, and heart are removed en bloc. The heart is
detached, but the trachea and lungs remain intact to provide easy
handling for inflation of the lungs with fixative (see under fixation
methods).
Abdominal organs are removed. The examination of liver and kidney
should include making a routine number of parallel slices of the
organs to examine their cut surfaces. The gastrointestinal tract
should be removed from the abdominal cavity and opened. In chronic
toxicity experiments, the entire gastrointestinal tract must be
opened, the mucosal surface examined, and representative parts taken
for histological examination. The oesophagus and parts of the
intestine can be rolled according to the Swiss roll technique in order
to obtain a considerable length of mucosa musculature and serosa in
the microscopic section. Brain, peripheral nerves, skeletal
musculature, and bones (including a joint) are removed. The spinal
cord may best be fixed in situ.
5.3.3 Carnivores, swine
After external examination, necropsy is performed when the animal
is lying on its right side. Left front and hind legs are removed
without opening the apertura thoracis superior. The skin is partly
removed after a median ventral incision from mouth to os pubis.
Subcutis, fat, mammary glands, salivary glands, and superficial lymph
nodes may be inspected and dissected. In addition, the different
joints of the legs are opened and inspected. After opening the
abdomen, the thorax is checked for negative pressure and opened by
removing the left side of the thoracic wall. Then the right side of
the pleural cavity is inspected. The oesophagus is removed by cutting
it cranially. The thoracic and abdominal organs are removed and
individually inspected and opened. The nasopharynx is then opened and
inspected, and the tongue, larynx, pharynx, tonsils, brain, spinal
cord, peripheral nerves, and musculature collected. Most organs should
be sliced in parallel slices to examine the cut surfaces.
5.4 Selection, Preservation, Preparation, and Storage of Tissues
5.4.1 Selection of tissues
Which organs and tissues should be collected for fixation is
determined by the type of toxicity experiment and the compound being
tested. Sufficient material should be collected to prevent the
necessity of having to repeat a certain experiment. Since it is
impossible to provide strict rules for selection, only a general
outline will be given.
5.4.2 Oral toxicity tests
In acute toxicity experiments, restrictive microscopic
examination may be necessary in certain cases. Some laboratories fix
liver and kidneys, others do not. The additional information obtained
from histological examination of these organs in acute experiments is,
usually, limited.
In range-finding tests, restrictive pathology is common practice.
Normally, the heart, liver, spleen, and kidneys and all grossly
abnormal tissues are collected for fixation. When the compound tested
is given by stomach tube, it is advisable to include lungs,
oesophagus, and stomach in the histological study. In subacute
(90-day) and chronic studies, it is advisable to select all tissues
and organs for fixation in buffered formal saline. This normally
includes the following organs and tissues:
brain gall bladder (if present)
pituitary oesophagus
thyroid (parathyroid) stomach
thymus duodenum
(trachea) jejunum
lungs ileum
heart caecum
sternum (bone marrow) colon
salivary glands rectum
liver urinary bladder
spleen lymph nodes -- mandibular or mesenteric
kidneys lymph nodes -- popliteal or axillary
adrenals mammary glands
pancreas (thigh) musculature
gonads peripheral nerve
accessory genital organs (eyes)
aorta (femur -- incl. joint)
(skin) spinal cord (at three levels)
(exorbital lachrymal glands)
The tissues mentioned between brackets are sometimes considered to be
optional. In addition, all tissues containing grossly observed lesions
should be fixed.
The same selection is usually made in carcinogenicity tests. In
both chronic toxicity and carcinogenicity experiments, it is also very
important to collect all organs and tissues of animals that have died
or have been killed in extremis. Prompt examination of the tissues
of these animals may provide valuable information leading to a more
meaningful pathological examination of animals, that die or are
sacrificed later on, or at the end of the experiment. In cases where
only part of an organ or tissue is taken, it is important that the
same part of that organ or tissue be selected at approximately the
same site in all animals.
5.4.3 Inhalation toxicity studies
In acute inhalation studies, it may be worthwhile, in some cases,
to select lungs for fixation and subsequent microscopic examination,
as well as liver and kidneys. In most cases, however, this seems
unnecessary since the pulmonary lesions are usually of the same type
(hyperaemia and oedema) and can be detected by gross inspection.
Examination of the entire respiratory tract is necessary in
subacute inhalation studies. This includes nasal cavity, pharynx,
larynx, trachea, main bronchi, and lungs. The exact orientation of
these organs for trimming, embedding, and sectioning is of prime
importance and needs special attention. More organs and tissues may be
selected in the inhalation studies, selection usually being effected
in the same way as for oral studies.
5.4.4 Dermal toxicity studies
In acute dermal toxicity studies, it is advisable to perform
microscopic pathology on liver and kidneys. The presence of
degenerative changes in these organs indicates that the compound is
active transdermally. Examination of the skin is also advisable.
In subacute dermal toxicity studies, the organs and tissues are
selected in the same way as that described for oral tests. Of course,
the skin deserves special attention in that both treated areas and
normal skin in comparable areas are selected.
5.4.5 Special studies
In the case of sensitization or irritation studies, the selection
of tissues depends completely on the type of test. In eye irritation
studies, the examination of eye and conjunctivae seems adequate
whereas in a Landstainer-Draize test, and especially in a maximization
test, the skin may be examined. In all these cases, it may, or may
not, be necessary to select more tissues.
5.5 Preservation of Tissues
Preservation of tissues can be performed by immersion, inflation
or distension, and perfusion. Immersion is most commonly used, but in
some circumstances may not result in satisfactory preservation.
Microscopic examination of the lungs, for example, cannot be done
adequately if they have not been inflated or have not been fixed by
perfusion. When the central nervous system is a target organ, it is an
absolute necessity to use perfusion to ensure proper fixation, as
fixation by immersion leads to many artifacts that are
indistinguishable from certain degenerative changes. When animals in
long-term tests are killed in extremis, it is advisable to use
fixation by perfusion. Perfusion is usually essential for electron
microscope studies of tissues.
With perfusion, the best conditions for microscopic examination
are obtained while effective gross examination remains possible. All
tissues should be fixed in 10% neutral buffered formalin or another
appropriate fixative.
5.5.1 Immersion
Immersion is the most used and usually the most appropriate
method of preservation in toxicity experiments. The tissues are placed
in the preservative and fixed for 24-48 h. Fixation at higher
temperatures (40-50°C), in a vacuum, or by use of microwaves (Gordon &
Daniel, 1974) may shorten the fixation time considerably. Tissues
thicker than 0.5 cm should not be fixed. The preservative/tissue ratio
is very important and must be greater than 10:1 (volume fixation
fluid/volume tissue) to obtain acceptable fixation. The fixation of
intact animals with opened abdomen should not be carried out. All
tissues and organs must be fixed separately, and some may need special
attention. Skin and peripheral nerves must be fixed in a straight (but
not stretched) and flat position. To achieve this, these tissues can
first be attached to a piece of thick filter paper. The spinal cord
can best be fixed in situ before it is taken out. Special fixing
solutions may be used in certain cases such as Bouin's fixative for
the fixation of ovaries, testes, thyroid, and adrenals or Zenker's
solution for the fixation of the eyes.
As certain organs (pituitary, thyroid, ovaries, adrenals, and
lymph nodes) of some of the smaller rodents are rather small, they
should be fixed separately in smaller jars to prevent loss; various
fixatives may be used.
5.5.2 Inflation
Inflation is used to preserve lungs effectively. To prevent the
formation of artifacts that may be misjudged as emphysema, it is
necessary for inflation to be carried out at constant pressure
(Chevalier, 1971; Fawell & Lewis, 1971). In certain cases, however,
even fixation of the lungs by inflation may not be adequate and
perfusion will be preferred (for example, in inhalation studies).
Inflation with fixative is also necessary for correct fixation of
the urinary bladder. If the bladder is distended, urine must be
replaced by fixative via the urethra using a syringe with a blunt
needle. Contracted empty bladders should be partly distended with
fixative. The reflux of fixative in the bladder is prevented by
ligation of the urethra. Inflation may also be used for fixation of
the digestive tract. Here again, ligation is necessary. In these
cases, the organs have to be bisected after fixation and the interior
surface inspected.
5.5.3 Perfusion
The best way to preserve tissues is by perfusion, which is
usually effected by infusion in the left ventricle of the heart and by
opening the sinus venosus to provide for proper circulation. Perfusion
of isolated organs (liver, kidneys) is another possibility. Before
perfusion, the animals are anaesthetized with a barbiturate,
administered in combination with nitrate and heparin to ensure
vasodilation and prevent clotting. A solution consisting of 77.5 ml
sodium nitrite (1.25%) in water, 10 ml heparin (5000 IU/ml) and 12 ml
pentobarbital (60 mg/ml), of which 10 ml is administered per kg body
weight, is satisfactory. Then the blood is removed with an isotonic
saline solution using slight overpressure. When all the blood has left
the body via the sinus venosus or right atrium, the body may be
perfused with the fixative.
Perfusion is sometimes not possible especially in experiments
where the recording of organ weights is important (i.e. in subchronic
toxicity studies). This difficulty can be solved by increasing the
number of experimental animals, though this may lead to a considerable
increase in costs. Perfusion of large numbers of animals is possible
using simple facilities at low cost (Fig. 5.1).
5.6 Trimming
It is important that the trimming be carried out by well-trained
people. It must be emphasized that knowledge of pathological phenomena
is necessary, as it frequently happens that the person responsible for
trimming cuts out the "good looking" areas and discards the tumours
present in the organs. The tissues must be sliced in such a way that
the cut surfaces present the largest possible area for examination.
The use of a special trimming scheme during the procedure may be
helpful.
The kidneys should be sectioned through the cortex and medulla,
one kidney mid-longitudinally, the other mid-transversely.
The brain must be cross-sectioned at, at least, three sites: the
frontal cortex with basal ganglia, parietal cortex with thalamus, and
cerebellum with pons.
The lungs should be sectioned transversely, parallel to the long
axis of the body. These sections must include the main bronchi and
carina.
The hollow organs should be trimmed in such a way that a
cross-section from mucosa to serosa is obtained.
Table 4.2 Methods for the determination of several mixed-function
oxidase activities
(a) Aryl hydrocarbon hydroxylation (using 3,4-benzpyrene as
substrate)
(Nebert & Gelboin, 1968a,b; Wattenberg et al., 1962)
(b) Aliphatic side-chain hydroxylation (of pentobarbital)
(Cooper & Brodie, 1955)
(c) 4-hydroxylation (of aniline)
(Brodie & Axelrod, 1948; Chabra et al., 1972; Gilbert &
Golberg, 1965; Henderson & Kersten, 1970; Hilton & Santorelli,
1970; Imai et al., 1966; Kato & Gillette, 1965; Schenkman
et al., 1967; Sternsen & Hes, 1975)
(d) N-hydroxylation (of aniline)
(Herr & Kiese, 1959)
(e) N-oxidation (determination of amine oxides)
(Fok & Ziegler, 1970; Ziegler et al., 1973)
(f) Nitro reduction
(Fouts & Brodie, 1957; Hietbrink & DuBois, 1965)
(g) N-demethylation (of aminopyrine)
(Brodie & Axelrod, 1950; Chrastil & Wilson, 1975; Cochin &
Axelrod, 1959; Dewaide & Henderson, 1968; Feuer et al., 1971;
Kinoshita et al., 1966; Klinger, 1974; La Du et al., 1955;
MacMahon, 1962; Nash, 1953; Pederson & Aust, 1970; Poland &
Nebert, 1973; Schoene et al., 1972)
(h) N-demethylation (of benzphetamine)
(Hewick & Fouts, 1970a,b; Liu et al., 1975; Lu et al., 1969;
Nash, 1953)
(i) N-demethylation (of ethylmorphine)
(Anders & Mannering, 1966)
(j) O-demethylation (of O-nitroanisole)
(Christensen & Wissing, 1972; Kinoshita et al., 1966; Netter,
1960; Netter & Seidel, 1964; Schoene et al., 1972;
Zannoni, 1971)
(k) O-dealkylation (of ethylumbelliferone)
(Ullrich & Weber, 1972)
REFERENCES
AITIO, A. (1973) Glucuronide synthesis in the rat and guinea pig lung.
Xenobiotica, 3: 13-22.
AL-KASSAB, S., BOYLAND, E., & WILLIAMS, K. (1963) An enzyme from rat
liver catalysing conjugations with glutathione; replacement of
nitro groups. Biochem. J., 87: 4-9.
ALLEN, J. R., NORBOCK, D. H., & HSU, I. C. (1974) Tissue modifications
in monkeys as related to absorption, distribution and excretion
of polychlorinated biphenyls. Arch. environ. Contam. Toxicol.,
2: 86-95.
ANDERS, M. W. & MANNERING, G. J. (1966) Inhibition of drug metabolism.
I. Kinetics of the inhibition of the N-demethylation of
ethylmorphine by 2-diethylaminoethyl 2, 2-diphenylvalerate HCl
(SKF 525A) and related compounds. Mol. Pharmacol., 2: 319-327.
ARIAS, I. M. (1962) Chronic unconjugated hyperbilirubinaemia without
overt signs of haemolysis in adolescents and adults. J. clin.
Invest., 41: 2233-2245.
ASSICOT, M. & BOHUON, C. (1969) A simple and rapid fluorimetric
determination of catechol- O-methyl transferase activity.
Life Sci., 8: 93-100.
AXELROD, J. (1971) Methyltransferase enzymes in the metabolism of
physiologically active compounds and drugs. In: Brodie, B. B. &
Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 2, pp. 609-619.
AXELROD, J. & DALY, J. W. (1968) Phenol- O-methyl transferase.
Biochim. Biophys. Acta, 159: 472-478.
AXELROD, J. & TOMCHICK, R. (1958) Enzymatic O-methylation of
epinephrine and other catechols. J. biol. Chem., 233: 702-705.
BAKER, R. C., COONS, L. B., & HODGSON, E. (1973) Low speed preparation
of microsomes: a comparative study. Chem. biol. Interactions,
6: 307-316.
BECKETT, A. H. & HOSSIE, R. D. (1971) Buccal absorption of drugs. In:
Brodie, B. B. & Gillette, J. R., ed. Concepts in biochemical
pharmacology, Berlin, Springer-Verlag, Vol. 1, pp. 25-46.
BOND, E. J. & DE MATTEIS, F. (1969) Biochemical changes in rat liver
after administration of carbon disulfide with reference to
microsomal changes. Biochem. Pharmacol., 18: 2531-2549.
BONNICHSEN, R. K. & BRINK, N. G. (1955) Liver alcohol dehydrogenase.
In: Colowick, S. P. & Kaplan, N. O., ed. Methods in enzymology,
New York, Academic Press, Vol. 1, pp. 495-500.
BOOTH, J., BOYLAND, E., & SIMS, P. (1961) An enzyme from rat liver
catalysing conjugations with glutathione. Biochem. J.,
79: 516-524.
BORCHARDT, R. T. (1974) A rapid spectrophotometric assay for
catechol- O-methyl transferase. Anal. Biochem., 58: 382-389.
BORGÅ, O., AZARNOFF, D. L., & SJÖQVIST, F. (1968) Species differences
in the plasmaprotein binding of desipramine. J. Pharm.
Pharmacol., 20: 571.
BOYD, G. S. & SMELLIE, R. M. S. (1972) Biological hydroxylation
mechanism. New York, Academic Press.
BOYLAND, E. (1971) Mercapturic acid conjugation. In: Brodie, B. B. &
Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 2, pp. 584-608.
BOYLAND, E. & CHASSEAUD, L. F. (1967) Enzyme-catalysed conjugations of
glutathione with unsaturated compounds. Biochem. J.,
104: 95-102.
BOYLAND, E. & WILLIAMS, K. (1965) A new enzyme catalysing the
conjugations of epoxides. Biochem. J., 94: 190-197.
BRAUER, R. W. (1959) Mechanisms of bile secretion. J. Am. Med.
Assoc., 169: 1462-1466.
BROCH, O. J., JR & GULDBERG, H. C. (1971) On the determination of
catechol- O-methyl transferase activity in tissue homogenates.
Acta Pharmacol. Toxicol., 30: 266-277.
BRODIE, B. B. (1964) Physicochemical factors in drug absorption. In:
Binns, T. B., ed. Absorption and distribution of drugs,
Baltimore, Williams & Wilkins, pp. 16-48.
BRODIE, B. B. & AXELROD, J. (1948) The estimation of acetanilide and
its metabolic products, aniline, N-acetyl- p-aminophenol and
p-aminophenol (free and total conjugates) in biological fluids
and tissues. J. Pharmac. exp. Ther., 94: 22-28.
BRODIE, B. B. & AXELROD, J. (1950) The fate of aminopyrine (Pyramidon)
in man and methods for the estimation of aminopyrine and its
metabolites in biological material. J. Pharmac. exp. Ther.,
99: 171-184.
BRODIE, B. B. & HOGBEN, C. A. M. (1957) Some physicochemical factors
in drug action. J. Pharm. Pharmacol., 9: 345-347.
BURCHELL, B., DUTTON, G. J., & NEMETH, A. M. (1972) Development of
phenobarbital-sensitive control mechanisms for uridine
diphosphate glucuronyl-transferase activity in chick liver.
J. cell. Biol., 55: 448-456.
CHASE, G. D. & RABINOWITZ, J. L. (1968) Principles of radioisotope
methodology, 3rd ed. Minneapolis, Burgess Publishing Company.
CHABHRA, R. S., GRAM, T. E., & FOUTS, J. R. (1972) A comparative study
of two procedures used in the determination of hepatic microsomal
aniline hydroxylation. Toxicol. appl. Pharmacol., 22: 50-58.
CHIGNELL, C. F. (1971) Physical methods for studying drug-protein
binding. In: Brodie, B. B. & Gillette, J. R., ed. Concepts in
biochemical pharmacology, Berlin, Springer-Verlag, Vol. 1,
pp. 187-212.
CHRASTIL, J. & WILSON, J. T. (1975) A sensitive colorimetric method
for formaldehyde. Anal. Biochem., 63: 202-207.
CHRISTENSEN, F. & WISSING, F. (1972) Inhibition of microsomal
drug-metabolizing enzymes from rat liver by various
4-hydroxy-coumarin derivatives. Biochem. Pharmacol.,
21: 975-984.
CIACCIO, E. I. (1971) Intimate study of drug action. II. Fate of drugs
in the body. In: Dipalma, J. R, ed. Drills pharmacology in
medicine, New York, McGraw-Hill, pp. 36-66.
COCHIN, J. & AXELROD, J. (1959) Biochemical and pharmacological
changes in the rat following chronic administration of morphine,
nalorphine and normorphine. J. Pharmac. exp. Ther.,
125: 105-110.
COLLINS, T. F. X. & WILLIAMS, C. H. (1971) Teratogenic studies with
2,4,5-T and 2,4-D in the hamster. Bull. environ. Contam.
Toxicol., 6: 559-567.
CONNEY, A. H. (1967) Pharmacological implications of microsomal enzyme
induction. Pharmacol. Rev., 19: 317-366.
CONNEY, A. H. & BURNS, J. J. (1962) Factors influencing drug
metabolism. Adv. Pharmacol., 1: 31-54.
CONNEY, A. H., COUTINHO, C., KOECHLIN, B., SWARM, R., CHERIPHO, J. A.,
IMPELLIZERI, C., & BARUTH, H. (1974) From animals to man:
metabolic considerations. Clin. Pharmacol. Ther., 16: 176-182.
COOPER, J. R. & BRODIE, B. B. (1965) Enzymatic oxidation of
pentobarbital and thiopental. J. Pharmacol. exp. Ther.,
120: 75-87.
COTZIAS, G. C. & DOLE, V. P. (1951) Metabolism of amines. I.
Microdetermination of aminoamine oxidase in tissues.
J. biol. Chem., 190: 665-672.
COURTNEY, D. K., GAYLOR, D. W., HOGAN, M. D., FLAK, H. L., BATES, R.
R., & MITCHELL, I. (1970) Teratogenic evaluation of 2,4,5-T.
Science, 168: 864-866.
COURTNEY, D. K. & MOORE, J. A. (1971) Teratology studies with
2,4,5-trichlorophenoxyacetic acid and
2,3,7,8-tetrachlorodibenzo- p-dioxin. Toxicol. appl.
Pharmacol., 20: 396-403.
CREASEY, N. H. (1956) Factors which interfere with the manometric
assay of monoamine oxidase. Biochem. J., 64: 178-183.
DAHM, P. A. (1971) Oxidative desulfuration and dealkylation of
selected organophosphate insecticides. In: Brodie, B. B. &
Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 2, pp. 362-366.
DALY, J. (1971) Enzymatic oxidation of carbon. In: Brodie, B. B. &
Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 2, pp. 285-311.
DAVIES, D. S., GIGON, P. L., & GILLETTE, J. R. (1969) Species and sex
differences in electron transport systems in liver microsomes and
their relationship to ethylmorphine demethylation. Life Sci.,
8: 85-91.
DAVSON, H. & DANIELLI, J. F. (1952) The permeability of natural
membranes, 2nd ed. Cambridge, Cambridge Univ. Press.
DEITRICH, R. A. & ERWIN, V. G. (1969) Spectrophotometric assay for
monoamine oxidase. Anal. Biochem., 30: 395-402.
DEWAIDE, J. H. & HENDERSON, P. T. (1968) Hepatic N-demethylation of
aminopyrine in rat and trout. Biochem. Pharmacol.,
17: 1901-1907.
DRILL, V. A. & HERATJKA, T. (1953) Toxicity of
2,4-dichlorophenoxyacetic acid and 2,4,5-trichlorophenoxyacetic
acid. Arch. ind. Hyg. occup. Med, 7: 61-67.
DUTTON, G. J. (1966) The biosynthesis of glucuronides. In: Dutton, G.
J. ed. Glucuronic acid, free and combined. New York, Academic
Press.
DUTTON, G. J. (1971) Glucuronide-forming enzymes. In: Brodie, B. B. &
Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 2, pp. 378-400.
DUTTON, G. J. & STOREY, I. D. E. (1962) Glucuronide-forming enzymes.
In: Colowick, S. P. & Kaplan, N. O., ed. Methods in enzymology,
New York, Academic Press, Vol. 5, pp. 159-164.
ESTABROOK, R. W. (1971) Cytochrome P-450. Its function in the
oxidative metabolism of drugs. In: Brodie, B. B. & Gillette, J.
R., ed. Concepts in biochemical pharmacology, Berlin,
Springer-Verlag, Vol. 2, pp. 264-284.
ESTABROOK, R. W., FRANKLIN, M. R., COHEN, B., SHIGAMATSU, A., &
HILDEBRANDT, A. G. (1971) Influence of hepatic microsomal mixed
function oxidation reactions on cellular metabolic control.
Metabolism, 20: 186-199.
ESTABROOK, R. W., GILLETTE, J. R., & LEIBMAN, K. C. (1972)
Microsomes and drug oxidations. Baltimore, Williams & Wilkins.
FEUER, G., SOSA-LUCERO, J. C., LUMB, F., & MODDEL, G. (1971) Failure
of various drugs to induce drug metabolizing enzymes in
extrahepatic tissues of the rat. Toxicol. appl. Pharmacol.,
19: 579-589.
FLYNN, E. J., LYNCH, M., & ZANNONI, V. G. (1969) Species differences
in hepatic microsomal electron transport. Fed. Proc., 28: 483.
FLYNN, E. J., LYNCH, M., & ZANNONI, V. G. (1972) Species differences
and drug metabolism. Biochem. Pharmacol., 21: 2577-2590.
FOK, A. K. & ZIEGLER, D. M. (1970) Estimation of amine oxides in the
presence of hepatic microsomes. Biochem. Biophys. Res. Commun.,
41: 534-540.
FOREMAN, H. (1971) Translocation of drugs into bone. In: Brodie, B. B.
& Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 1, pp. 249-257.
FOUTS, J. R. (1970) The stimulation and inhibition of hepatic
microsomal drug-metabolising enzymes with special reference to
the effects of environmental contaminants. Toxicol. appl.
Pharmacol., 17: 804-809.
FOUTS, J. R. & BRODIE, B. B. (1957) The enzymatic reduction of
chloramphenicol, p-nitrobenzoic acid and other aromatic nitro
compounds in mammals. J. Pharmacol. exp. Ther. 119: 197-207.
FOUTS, J. R. & POHL, H. J. (1971) Further studies on the effects of
metal ions on rat liver microsomal reduced nicotinamide adenine
dinucleotide phosphate-cytochrome P-450 reductase. J. Pharmacol.
exp. Ther., 179: 91-100.
GARATTINI, S., MARCUCCI, F., & MUSSINI, E. (1975) Biotransformation of
drugs to pharmacologically active metabolites. In: Gillette, J.
R. & Mitchell, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 3, pp. 113-119.
GEHRING, P. J. & BUERGE, J. (1969) The distribution of
2,4-dinitrophenol relative to its cataractogenic activity in
ducklings and rabbits. Toxicol. appl. Pharmacol., 15: 574-592.
GEHRING, P. J., KRAMER, C. D., SCHWETZ, B. A., ROSE, J. Q., & ROWE, V.
K. (1973) The fate of 2,4,5-trichlorophenoxyacetic acid (2,4,5-T)
following oral administration to man. Toxicol. appl. Pharmacol.,
26: 352-361.
GEHRING, P. J., BLAU, G. E., & WATANABE, P. G. (1976) Pharmacokinetic
studies in evaluation of the toxicological and environmental
hazard of chemicals. In: Advances in modern toxicology -- Newer
concepts in safety evaluation. Washington, Hemisphere Publ.
Corp.
GIBSON, J. E. & BECKER, B. A. (1967) Demonstration of enhanced
lethality of drugs in hypoexcretory animals. J. pharm. Sci.,
56: 1503-1505.
GIGON, P. L., GRAM, T. E., & GILLETTE, J. R. (1969) Studies on the
rate of reduction of hepatic microsomal cytochrome P-450 by
reduced nicotinamide adenine dinucleotide phosphate. Effect of
drug substrates. Mol. Pharmacol., 5: 109-122.
GILBERT, D. & GOLBERG, L. (1965) Liver response tests. III. Liver
enlargement and stimulation of microsomal processing enzyme
activity. Food Cosmet. Toxicol., 3: 417-432.
GILLETTE, J. R. (1963) Metabolism of drugs and other foreign compounds
by enzymatic mechanisms. Arzneimittel Forsch., 6: 11-73.
GILLETTE, J. R. (1971a) Factors affecting drug metabolism. Ann. NY
Acad. Sci., 179: 43-66.
GILLETTE, J. R. (1971b) Reductive enzymes. In: Brodie, B. B. &
Gillette, J. R. ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 2, pp. 349-361.
GILLETTE, J. R. (1971c) Effects of various inducers on electron
transport systems associated with drug metabolism by liver
microsomes. Metabolism, 20: 215-227.
GILLETTE, J. R. (1973a) Overview of drug-protein binding. Ann. NY
Acad. Sci., 226: 6-17.
GILLETTE, J. R. (1973b) The importance of tissue distribution in
pharmacokinetics. J. Pharmacol. Biopharm., 1: 497-519.
GILLETTE, J. R. (1974a) A perspective on the role of chemically
reactive metabolites of foreign chemicals in toxicity. I.
Correlation of changes in covalent binding of reactivity
metabolites with changes in the incidence and severity of
toxicity. Biochem. Pharmacol., 23: 2785-2794.
GILLETTE, J. R. (1974b) A perspective on the role of chemically
reactive metabolites of foreign compounds in toxicity: II.
Alterations in the kinetics of covalent binding. Biochem.
Pharmacol., 23: 2927-2938.
GILLETTE, J. R. (1975) Other aspects of pharmacokinetics. In:
Gillette, J. R. & Mitchell, J. R., ed. Concepts in biochemical
pharmacology, Berlin, Springer-Verlag, Vol. 3, pp. 35-85.
GILLETTE, J. R., CHAN, T. W., & TERRIERE, L. C. (1966) Interactions
between DDT analogues and microsomal epoxidase systems.
J. agric. Food Chem., 14: 540-545.
GILLETTE, J. R. DAVIS, D. C., & SASAME, (1972) Cytochrome P-450 and
its role in drug metabolism. Annu. Rev. Pharmacol., 12: 57-84.
GILLETTE, J. R., MITCHELL, J. R., & BRODIE, B. B. (1974) Biochemical
mechanisms of drug toxicity. Annu. Rev. Pharmacol.,
14: 271-288.
GOLDSTEIN, A., ARONOW, L., & KOLMAN, S. M. (1974) Principles of drug
action. In: The basis of pharmacology, 2nd ed., New York,
Wiley, pp. 106-205.
GRAM, T. E. (1971) Enzymatic N-, O- and S-dealkylation of foreign
compounds by hepatic microsomes. In: Brodie, B. B. & Gillette, J.
R., ed. Concepts in biochemical pharmacology, Berlin,
Springer-Verlag, Vol. 2, pp. 334-348.
GRAM, T. E. (1973) Comparative aspects of mixed function oxidation by
lung and liver of rabbits. Drug. Metabol. Rev., 2: 1-32.
GREGORY, J. D. (1960) The effect of borate on the carbazole reaction.
Arch. Biochem. Biophys., 89: 157-159.
GREGORY, J. D. & LIPMANN, F. (1957) The transfer of sulfate among
phenolic compounds with 3,5-diphosphoadenosine as co-enzyme.
J. Biol. Chem., 229: 1081-1090.
GRIFFITHS, M. H. (1968) The metabolism of N-triphenylmorphine in the
dog and rat. Biochem. J., 108: 731-740.
HART, L. G. & FOUTS, J. R. (1965) Further studies on the stimulation
of hepatic microsomal drug metabolizing enzymes by DDT and its
analogues. Arch. exp. Pathol., 249: 486-500.
HEIRWEGH, K. P. M., VAN DER VIJER, M., & FEVERY, J. (1972) Assay and
properties of digitonin-activated bilirubin uridine diphosphate
glucuronyl-transferase from rat liver. Biochem. J.,
129: 605-618.
HENDERSON, P. TH. & KERSTEN, K. J. (1970) Metabolism of drugs during
liver regeneration. Biochem. Pharmacol., 19: 2343-2351.
HERR, F. & KIESE, M. (1959) [Determination of nitrosobenzol in the
blood.] Arch. exp. Path. Pharmakol., 235: 351-353 (in German).
HEWICK, D. S. & FOUTS, J. R. (1970a) Effects of storage on hepatic
microsomal cytochromes and substrate-induced difference spectra.
Biochem. Pharmacol., 19: 457-472.
HEWICK, D. S. & FOUTS, J. R. (1970b) The metabolism in vitro and
hepatic microsomal interactions of some enantiomeric drug
substrates. Biochem. J., 117: 833-841.
HIDAKA, H., NAGATSU, T., & YAGI, K. (1967) A rapid and simple assay of
serotonin sulfokinase activity. Anal. Biochem., 17: 388-392.
HIETANEN, E. (1974) Effect of sex and castration on hepatic and
intestinal activity of drug-metabolizing enzymes. Pharmacology,
12: 84-89.
HIETANEN, E. & VALINIO, H. (1973) Interspecies variations in small
intestinal and hepatic drug hydroxylation and glucuronidation.
Acta Pharmacol. Toxicol., 33: 57-64.
HIETBRINK, B. E. & DUBOIS, K. P. (1965) Influence of X-radiation on
development of enzymes responsible for desulfuration of an
organic phosphorothioate and reduction of p-nitrobenzoic acid
in the livers of male rats. Radiat. Res., 22: 598-603.
HILTON, J. & SARTORELLI, A. C. (1970) Induction by phenobarbital of
microsomal mixed oxidase enzymes in regenerating liver. J. biol.
Chem., 245: 4187-4192.
HOFFMAN, D. G., WORTH, H. M., & ANDERSON, R. C. (1968) Stimulation of
hepatic microsomal drug-metabolizing enzymes by alpha alpha-bis
p-chlorophenyl-3-pyridine methanol and a method for determining
no-effect levels in rats. Toxicol. appl. Pharmacol.,
12: 464-472.
HOFFMAN, D. F., WORTH, H. M., & ANDERSON, R. C. (1970) Stimulation of
hepatic drug-metabolizing enzymes by chlorophenothane (DDT); the
relationship to liver enlargement and hepatotoxicity in the rat.
Toxicol. appl. Pharmacol., 16: 171-178.
HOLTZMAN, J. L., GRAM, T. E., GIGON, P. L., & GILLETTE, J. R. (1968)
The distribution of the components of mixed function oxidase
between the rough and the smooth endoplasmic reticulum of liver
cells. Biochem. J., 110: 407-412.
HOOK, J. B., BAIBIE, M. D., JOHNSON, J. T., & GEHRING, P. J. (1974)
In vitro analysis of transport of 2,4,5-trichlorophenoxyacetic
acid (2,4,5-T) by rat and dog kidney. Food Cosmet. Toxicol.,
12: 209-218.
HUCKER, H. B. (1970) Species differences in drug metabolism.
Annu. Rev. Pharmacol., 10: 99-118.
IMAI, Y., ITO, A., & SATOR, R. R. (1966) Evidence for biochemically
different types of vesicles in the hepatic microsomal fraction.
J. Biochem., 60: 417-428.
ISSELBACHER, K. J. (1956) Enzymatic mechanisms of hormone metabolism.
II. Mechanism of hormonal glucuronide formation. In: Pincus, G.,
ed. Recent progress in hormone research, New York, Academic
Press, pp. 134-151.
JENNE, J. W. & BOYER, P. D. (1962) Kinetic characteristics of the
acetylation of isoniazid and p-aminosalicylic acid by a liver
enzyme preparation. Biochim. Biophys. Acta, 65: 121-127.
JOHNSON, M. K. (1966) Metabolism of iodomethane in the rat.
Biochem. J., 98: 38-43.
JOLLOW, P. J., MITCHELL, J. R., ZAMPOGLIONE, N., & GILLETTE, J. R.
(1974) Bromobenzene-induced liver necrosis: protective role of
glutathione and evidence for 3,4-bromobenzene oxide as the
hepatotoxic metabolite. Pharmacology, 11: 151-169.
KATO, R. & GILLETTE, J. R. (1965) Effect of starvation on
NADPH-dependent enzymes in liver microsomes of male and female
rats. J. Pharmacol. exp. Ther., 150: 279-284.
KATZ, M. & POULSEN, B. J. (1971) Absorption of drugs through the skin.
In: Brodie, B. B. & Gillette, J. R., ed. Concepts in biochemical
pharmacology, Berlin, Springer-Verlag, Vol. 1, pp. 103-174.
KEBERLE, H., HOFFMAN, K., & BERNHARD, K. (1962) The metabolism of
glutethimide (Doridnene). Experientia (Basel), 18: 105-111.
KEEN, P. (1971) Effect of binding to plasma proteins on the
distribution, activity and elimination of drugs. In: Brodie, B.
B. & Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 1, pp. 213-233.
KINOSHITA, F. K., FRAWLEY, J. P., & DUBOIS, K. P. (1966) Quantitative
measurement of induction of hepatic microsomal enzymes by various
dietary levels of DDT and toxaphene in rat. Toxicol. appl.
Pharmacol., 9: 505-513.
KLINGER, W. (1974) Optimal conditions for the first step of
amidopyrine- N-demethylation by 900 × g liver supernatant of
newborn and adult rats. Acta Biol. Med. Germ., 33: 181-186.
KUNTZMAN, R. (1969) Drugs and enzyme induction. Annu. Rev.
Pharmacol., 9: 21-36.
KURZ, H. & FRIEMEL, G. (1967) [Species-specific differences in the
binding of plasma proteins.] Arch. Pharmakol. exp. Path.,
257: 35-36 (in German).
LA DU, B. N. & SNADY, H. (1971) Esterases of human tissues. In:
Brodie, B. B. & Gillette, J. R., ed. Concepts in biochemical
pharmacology, Berlin, Springer-Verlag, Vol. 2, pp. 477-499.
LA DU, B. N., GAUDETTE, L., TROUSOF, N., & BRODIE, B. B. (1955)
Enzymatic dealkylation of aminopyrine (Pyramidon) and other
alkylamines. J. biol. Chem., 214: 741-752.
LA DU, B. N., MANDEL, G. H., & WAY, E. L. (1972) Fundamentals of drug
metabolism and drug disposition. Baltimore, Williams & Wilkins.
LEVI, A. J., GATMAITAN, Z., & ARIAS, I. M. (1969) Two hepatic
cytoplasmic protein fractions, Y and Z, and their possible role
in hepatic uptake of bilirubin, sulfobromophthalein, and other
anions, J. clin. Invest., 48: 2156-2167.
LEVINE, R. R. (1970) Factors affecting gastrointestinal absorption of
drugs. Digest Dis. 15: 171-188.
LEVY, G. & GIBALDI, M. (1972) Pharmacokinetics of drug action.
Annu. Rev. Pharmacol., 12: 85-98.
LEVY, G. & GIBALDI, M. (1975) Pharmacokinetics. In: Gillette, J. R. &
Mitchell, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 3, pp. 1-34.
LITWACK, G., KETTERER, B., & ARIAS, I. M. (1971) Ligondin: a hepatic
protein which binds steroids, bilirubin, carcinogens and a number
of exogenous organic anions. Nature (Lond.), 234: 466-467.
LIU, S. J., RAMSEY, R. K., & FALLON, H. J. (1975) Effects of ethanol
on hepatic microsomal drug-metabolizing enzymes in the rat.
Biochem. Pharmacol., 24: 369-378.
LU, A. Y. H. & LEVIN, W. (1974) The resolution and reconstitution of
the liver microsomal hydroxylation system. Biochim. Biophys.
Acta, 344: 205-240.
LU, A. Y. H., STROBEL, H. W., & COON, M. J. (1969) Hydroxylation of
benzphetamine and other drugs by a solubilized form of cytochrome
P-450 from liver microsomes: lipid requirement for drug
demethylation. Biochem. Biophys. Res. Commun., 36: 545-551.
LUCIS, O. J., SCHACKH, Z. A., & EMBIL, J. A., JR (1970) Cadmium as a
trace element and cadmium-binding components in human cells.
Experientia (Basel), 26: 1109-1112.
MACCAMAN, R. E. (1965) Microdetermination of catechol- O-methyl
transferase. Life Sci., 4: 2353-2359.
MCLEAN, A. E. M. (1971) Conversion by the liver of inactive molecules
into toxic molecules. In: Aldridge, W. N., ed. Mechanism of
toxicity, London, Macmillan, pp. 219-228.
MACMAHON, R. E. (1962) The competitive inhibition of the
N-demethylation of butynamine by 2,4-dichloro-6-
phenolphenoxy-ethylamine (DPEA). J. Pharmacol. exp. Ther.,
138: 382-386.
MACMAHON, R. E. (1971) Enzymatic oxidation and reduction of alcohols,
aldehydes and ketones. In: Brodie, B. B. & Gillette, J. R., ed.
Concepts in biochemical pharmacology, Berlin, Springer-Verlag,
Vol. 2, pp. 500-517.
MAHER, J. R., WHITNEY, J. M., CHAMBERS, J. S., & STANONIS, D. J.
(1957) The quantitative determination of isoniazid and
para-aminosalicylic acid in body fluids. Am. Rev. Tuberc.,
76: 852-861.
MANNERING, G. J. (1968) Significance of stimulation and inhibition of
drug metabolism. In: Burger, A., ed. Selected pharmaceutical
testing methods, New York, Marcel Dekker, pp. 51-119.
MANNERING, G. J. (1971a) Inhibition of drug metabolism. In: Brodie, B.
B. & Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 2, pp. 452-476.
MANNERING, G. J. (1971b) Properties of cytochrome P-450 as affected by
environmental factors: qualitative changes due to administration
of polycyclic hydrocarbons. Metabolism, 20: 228-245.
MARK, L. C. (1971) Translocation of drugs and other exogenous
chemicals into adipose tissue. In: Brodie, B. B. & Gillette, J.
R., ed. Concepts in biochemical pharmacology, Berlin,
Springer-Verlag, Vol. 1, pp. 258-275.
MARSELOS, M. & HÄNNINEN, O. (1974) Enhancement of D-glucuronolactone
and acetaldehyde dehydrogenase activities in the rat liver by
inducers of drug metabolism. Biochem. Pharmacol.,
23: 1457-1466.
MARSH, C. A. (1963) Metabolism of D-glucuronolactone in mammalian
systems; conversion of D-glucuronolactone into D-glucaric acid by
tissue preparations. Biochem. J., 87: 82-90.
MARSHALL, E. K., JR (1948) Determination of par-amino-salicylic acid
in blood. Proc. Soc. Exp. Biol. Med., 68: 471-472.
MASON, H. S. (1957) Mechanism of oxygen metabolism. Adv. Enzymol.,
19: 79-233.
MILLER, E. C. & MILLER, J. A. (1971a) The mutagenicity of chemical
carcinogens: correlations, problems and interpretations. In:
Hollaender, A., ed. Chemical mutagens, New York, Plenum,
Vol. 1, pp. 83-119.
MILLER, J. A. & MILLER, E. C. (1971b) Chemical carcinogenesis:
mechanisms and approaches to its control. J. Natl Cancer Inst.,
47: v-xiv.
NASH, T. (1953) The colorimetric estimation of formaldehyde by means
of the Hantsch reaction. Biochem. J., 55: 416-421.
NEBERT, D. W. & GELBOIN, H. V. (1968a) Substrate inducible microsomal
aryl hydroxylase. I. Assay and properties of induced enzymes.
J. Biol. Chem., 243: 6242-6249.
NEBERT, D. W. & GELBOIN, H. V. (1968b) Substrate inducible microsomal
aryl hydroxylase. II. Cellular responses during enzymic
induction. J. Biol. Chem., 243: 6250-6261.
NETTER, K. J. (1960) [A method for the direct measurement of
O-demethylization in liver microsomes and its application to
the inhibitory effects of SKF 525A on microsomes.]
Arch. Pharmacol., 238: 292-300 (in German).
NETTER, K. J. & SEIDEL, G. (1964) An adaptively stimulated
O-demethylating system in rat liver microsomes and its kinetic
properties. J. Pharmacol. exp. Ther., 146: 61-65.
NOTTON, W. R. & HENDERSON, P. TH. (1975) The influence of N-hexane
treatment on the glucuronic acid pathway and activity of some
drug-metabolizing enzymes in the guinea pig. Biochem.
Pharmacol., 24: 127- 131.
OBATA, F., USHIWATA, A., & NAKAMURA, Y. (1971) Spectrophotometric
assay of monoamine oxidase using 2,4,6-trinitrobenzene-1-sulfonic
acid. J. Biochem., 69: 349-354.
OGATA, M., TOMOKUNI, K., & TAKATSUKA, Y. (1969) Quantitative
determination in urine of hippuric acid and m- or p-methyl
hippuric acid, metabolites of toluene and m- or p-xylene.
Brit. J. ind. Med., 26: 330-334.
OMURA, T. & SATO, R. (1964a) The carbon monoxide-binding pigment of
liver microsomes: I. Evidence for its hemoprotein nature.
J. biol. Chem., 239: 2370-2378.
OMURA, T. & SATO, R. (1964b) The carbon monoxide-binding pigment of
liver microsomes: II. Solubilization, purification and
properties. J. biol. Chem., 239: 2379-2385.
OTSUKA, S. & KOBAYASHI, Y. (1964) A radioisotopic assay for monamine
oxidase determinations in human plasma. Biochem. Pharmacol.,
13: 995-1006.
PAPPENHEIMER, J. R. (1953) Passage of molecules through capillary
walls. Physiol. Rev., 33: 387-423.
PARKE, D. V. (1968) The biochemistry of foreign compounds. New York,
Pergamon Press.
PEDERSON, T. C. & AUST, S. D. (1970) Aminopyrine demethylase: kinetic
evidence for multiple microsomal activities. Biochem.
Pharmacol., 19: 2221-2230.
PIPER, W. N., ROSE, J. Q., LENG, M. L. & GEHRING, P. J. (1973) The
fate of 2,4,5-trichlorophenoxyacetic acid (2,4,5-T) following
oral administration to rats and dogs. Toxicol. appl. Pharmacol.,
26: 339-351.
PITTS, R. F. (1963) Physiology of the kidney and body fluids.
Yearbook, Chicago, Medical Publishers Inc.
PLAA, G. L. (1975) The enterohepatic circulation. In: Gillette, J. R.
& Mitchell, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 3, pp. 130-149.
PLACE, V. A. & BENSON, H. (1971) Dietary influences on therapy with
drugs. J. Mond. Pharm., 14: 261-278.
POLAND, A. P. & NEBERT, D. W. (1973) A sensitive radiometric assay of
aminopyrine N-demethylation. J. Pharmacol. exp. Ther.,
184: 269-277.
POTTS, A. M. (1964) The reaction of uveal pigments in vitro with
polycyclic compounds. Invest. Ophthalmol., 3: 405-416.
PRESCOTT, L. F. (1975) Pathological and physiological factors
affecting drug absorption, distribution, elimination and response
in man. In: Gillette, J. R. & Mitchell, J. R., ed. Concepts in
biochemical pharmacology, Berlin, Springer-Verlag,
Vol. 3, pp. 234-257.
PRICE, H. L., KOVNAT, P. J., SOFER, J. N., CONNER, E. H., & PRICE, M.
L. (1960) The uptake of thiopental by body tissues and its
relation to the duration of narcosis. Clin. Pharmacol. Ther.,
1: 16-22.
QUASTEL, J. H. (1965) Molecular transport at cell membranes.
Proc. Roy. Soc. Br., 163: 169-186.
QUINN, G. P., AXELROD, J., & BRODIE, B. B. (1958) Species, strain and
sex differences in metabolism of hexobarbitone, aminopyrine,
antipyrine and aniline. Biochem. Pharmacol., 1: 152-159.
RALL, D. P. (1971) Drug entry into brain and cerebrospinal fluid. In:
Brodie, B. B. & Gillette, J. R., ed. Concepts in biochemical
pharmacology, Berlin, Springer-Verlag, Vol. 1, pp. 240-248.
RASMUSSEN, F. (1971) Excretion of drugs by milk. In: Brodie, B. B. &
Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 1, pp. 390-402.
REMMER, H., SCHENKMAN, J., ESTABROOK, R. W., SASAME, H., GILLETTE, J.
R., NARASHIMHULU, S., COOPER, D. Y., & ROSENTHAL, O. (1966) Drug
interaction with hepatic microsomal cytochrome. Mol. Pharmacol.,
2: 187-190.
RENKIN, E. M. (1964) Transport of large molecules across capillary
walls. Physiologist, 7: 13.
REYES, H., LEVI, A. J., GATMAITAN, Z., & ARIAS, I. M. (1971) Studies
on Y and Z, two hepatic cytoplasmic organic anion-binding
proteins: Effect of drugs, chemicals, hormones and cholestosis.
J. Clin. Invest., 50: 2242-2251.
RODOMSKI, J. L., DEICHMANN, W. B., & CLIZER, E. E. (1968) Pesticide
concentrations in the liver, brain and adipose tissue of terminal
hospital patients. Food Cosmet. Toxicol., 6: 209-220.
ROLL, R. (1971) Investigations concerning the teratogenic effect of
2,4,5-T in mice. Food Cosmet. Toxicol., 9: 671-676.
ROSE, J. Q., RAMSEY, J. C., WENTZLER, T. G., HUMMEL, R. A., & GEHRING,
P. J. (1975) The fate of 2,3,7,8-tetrachlorodibenzo- p-dioxin
(TCDD) following single and repeated oral doses to the rat.
Toxicol. appl. Pharmacol., 36: 209-226.
ROSENBLUM, C. (1965) Non-metabolite residues in radioactive tracer
studies. In: Roth, L. J., ed. Isotopes in experimental
pharmacology, Chicago, University of Chicago Press,
pp. 353-360.
ROTH, L. J. (1971) The use of autoradiography in experimental
pharmacology. In: Brodie, B. B. & Gillette, J. R., ed. Concepts
in biochemical pharmacology, Berlin, Springer-Verlag,
Vol. 1, pp. 286-316.
ROWE, V. K. & HYMAS, T. A. (1954) Summary of toxicological information
of 2,4-D and 2,4,5-T type herbicides and an evaluation of the
hazards to livestock associated with their use. Am. J. Vet.
Res., 15: 622-629.
ROY, A. B. (1971) Sulfate conjugation enzymes. In: Brodie, B. B. &
Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 2, pp. 536-563.
SAUERHOFF, M. W., BRAUN, W. H., BLAU, G. E., & GEHRING, P. J. (1975)
The dose-dependent pharmacokinetic profile of
2,4,5-trichlorophenoxyacetic acid following intravenous
administration to rats. Toxicol. appl. Pharmacol., 36: 491-501.
SCHANKER, L. S. (1962a) Passage of drugs across body membranes.
Pharmacol. Rev., 14: 501-530.
SCHANKER, L. S. (1962b) Concentrative transfer of an organic cation
from blood into bile. Biochem. Pharmacol., 11: 253-254.
SCHANKER, L. S. (1963) Passage of drugs across the gastrointestinal
epithelium. In: Hogben, C. A. M., ed. Proceedings of the 1st
International Pharmacological Meeting, Oxford, Pergamon Press,
Vol. 4, pp. 120-130.
SCHANKER, L. S. (1965) Hepatic transport of organic cations. In:
Taylor, W., ed. The biliary system. Oxford, Blackwell.
SCHANKER, L. S. (1971) Absorption of drugs from the gastrointestinal
tract. In: Brodie, B. B. & Gillette, J. R., ed. Concepts in
biochemical pharmacology, Berlin, Springer-Verlag,
Vol. 1, pp. 9-24.
SCHANKER, L. S., SHORE, P. A., BRODIE, B. B., & HOGBEN, C. A. M.
(1957) Absorption of drugs from the stomach. I. The rat.
J. Pharmacol. exp. Ther., 120: 528-539.
SCHELINE, R. R. (1968) Drug metabolism by intestinal microorganisms.
J. pharm. Sci., 57: 2021-2037.
SCHENKMAN, J. B., REMMER, H., & ESTABROOK, R. W. (1967) Spectral
studies of drug interaction with hepatic microsomal cytochrome.
Mol. Pharmacol., 3: 113-123.
SCHOENE, B., FLEISCHMANN, R. A., REMMER, H., & OLDERSHAUSEN, H. F.
(1972) Determination of drug-metabolizing enzymes in needle
biopsies of human liver. Eur. J. clin. Pharmacol., 4: 65-73.
SCHOLTAN, W. (1963) On the protein binding of long-acting
sulfonamides. Chemotherapia, 6: 180-195.
SCHWETZ, B. A., NORRIS, J. M., SPARSCHU, G. L., ROWE, V. K., GEHRING,
P. J., EMERSON, J. K., & GERBIG, C. G. (1973) Toxicology of
chlorinated dibenzo- p-dioxins. Environ. Health Perspect.
(Experimental issue), 5: 87-100.
SETTLE, W., HEGEMAN, S., & FEATHERSTONE, R. M. (1971) The nature of
drug-protein interaction. In: Brodie, B. B. & Gillette, J. R.,
ed. Concepts in biochemical pharmacology, Berlin,
Springer-Verlag, Vol. 1, pp. 175-186.
SHORE, P. A., BRODIE, B. B., & HOGBEN, C. A. M. (1957) The gastric
secretion of drugs -- a pH partition hypothesis. J. Pharmacol.
exp. Ther., 119: 361-369.
SHULERT, A. R. (1961) Physiological disposition of hydralazine
(1-hydrazinophthalazine) and a method for its determination in
biological fluids. Arch. int. Pharmacodyn., 132: 1-15.
SMITH, R. L. (1971a) Excretion of drugs in bile. In: Brodie, B. B. &
Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 1, pp. 354-389.
SMITH, R. L. (1971b) The role of the gut flora in the conversion of
inactive compounds to active metabolites. In: Aldridge, W. N.,
ed. Mechanism of toxicity, London, Macmillan, pp. 229-247.
SMITH, R. L. (1973) The excretory function of bile: the elimination
of drugs and toxic substances in bile. London, England, Chapman
& Hall.
SMUCKLER, E. A. (1971) Metabolism of halogenated compounds. In:
Brodie, B. B. & Gillette, J. R., ed. Concepts in biochemical
pharmacology, Berlin, Springer-Verlag, Vol. 2, pp. 367-377.
SPARSCHU, G. L., DUNN, F. L., LISOWE, R. W. & ROWE, V. K. (1971) Study
of the effects of high levels of 2,4,5-trichlorophenoxyacetic
acid on fetal development in the rat. Food Cosmet. Toxicol.,
9: 527-530.
SPERBER, I. (1963) Drugs and membranes. In: Hogben, C. A. M., ed.
Proceedings of the 1st International Pharmacological Meeting,
Oxford, Pergamon Press, Vol. 4.
STERRSON, L. A. & HES, J. (1975) Electrochemical method for the
determination of aniline hydroxylation. Anal. Biochem.,
67: 74-80.
STOTZ, E., STEINBERG, M. S., COHEN, S. N., & WEBER, W. W. (1969)
Acetylation of 5-hydroxytryptamine by isoniazide
N-acetyltransferase. Biochim. Biophys. Acta, 184: 210-212.
STOWE, C. M. & PLAA, G. L. (1968) Extrarenal excretion of drugs and
chemicals. Annu. Rev. Pharmacol., 8: 337-356.
STRICKLAND, R. D., GREGORY, D. H., & LYNCH, J. L. (1974) A method for
assaying hepatic morphine UDP-glucuronyl transferase. Biochem.
Med., 11: 180-188.
STURMAN, J. A. & SMITH, M. J. H. (1967) The binding of salicylate to
plasma proteins in different species. J. Pharm. Pharmacol.,
19: 621-623.
SUGA, T., OHATA, I., KUMAOKA, H., & AKAGI, M. (1967) Studies on
mercapturic acids: investigation of glutathione-conjugating
enzyme by the method of thin-layer chromatography.
Chem. Pharmacol. Bull., 15: 1059-1064.
TABOR, H., MEHLER, A. H., & STADTMAN, E. R. (1953) The enzymatic
acetylation of amines. J. biol. Chem., 204: 127-138.
TAKAHASHI, H. & TAKAHARA, S. (1968) A sensitive fluorimetric assay for
monoamine oxidase based on the formation of 4,6-quinolinediol
from 5-hydroxykynurenamine. J. Biochem., 64: 7-11.
TEORELL, T. (1937a) Kinetics of distribution of substances
administered to the body: I. The extra-vascular modes of
administration. Arch. int. Pharmacodyn., 57: 205-226.
TEORELL, T. (1937b) Kinetics of distribution of substances
administered to the body: II. The intra-vascular modes of
administration. Arch. int. Pharmacodyn., 57: 227-240.
TUFVESSON, G. (1970) Fluorimetric determination of amine oxidase
activity in human blood serum with kynuramine as substrate.
Scand. J. clin. Lab. Invest., 26: 151-154.
ULLRICH, V. & WEBER, P. (1972) The O-dealkylation of
7-ethoxycoumarin by liver microsomes: a direct fluorimetric test.
Z. Physiol. Chem., 353: 1171-1177.
WAGNER, J. P. (1968) Pharmacokinetics. Ann. Rev. Pharmacol.,
8: 67-94.
WAGNER, J. P. (1971) Biopharmaceutics and relevant pharmacokinetics.
Hamilton, Drug Intelligence Publications.
WATTERNBERG, L. W. & LEONG, J. L. (1971) Tissue distribution studies
of polycyclic hydrocarbon hydroxylase activity. In: Brodie, B. B.
& Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 2, pp. 422-430.
WATTENBERG, L. W., LEONG, J. L., & STRAND, P. J. (1962) Benzpyrene
hydroxylase activity in the gastrointestinal tract. Cancer Res.,
22: 1120-1125.
WEBER, W. W. (1970) N-acetyltransferase (mammalian liver). In:
Tabor, H. & Tabor, C. W., ed. Methods in enzymology, 17B. New
York, Academic Press.
WEBER, W. W. (1971) Acetylating, deacetylating and amino
acid-conjugating enzymes. In: Brodie, B. B. & Gillette, J. R.,
ed. Concepts in biochemical pharmacology, Berlin,
Springer-Verlag, Vol. 2, pp. 564-583.
WEBER, W. W. & COHEN, S. N. (1968) The mechanism of isoniazid
acetylation by human N-acetyltransferase. Biochim. Biophys.
Acta, 151: 276-278.
WEBER, W. W., COHEN, S. N., & STEINBERG, M. S. (1968) Purification and
properties of N-acetyltransferase from mammalian liver.
Ann. NY Acad. Sci., 151: 734-741.
WEINER, I. M. (1971) Excretion of drugs by the kidney. In: Brodie, B.
B. & Gillette, J. R., ed. Concepts in biochemical pharmacology,
Berlin, Springer-Verlag, Vol. 1, pp. 328-353.
WEISBURGER, H. H. & WEISBURGER, E. R. (1971) N-oxidation enzymes.
In: Brodie, B. B. & Gillette, J. R., ed. Concepts in biochemical
pharmacology, Berlin, Springer-Verlag, Vol. 2, pp. 312-333.
WEISBURGER, H. H., GRANTHAM, P. H., & WEISBURGER, E. R. (1964)
Metabolism of N-2-fluorenylacetamide in the hamster. Toxicol.
appl. Pharmacol., 6: 427-433.
WEISSBACH, H., SMITH, T. E., FALY, J. W., WITKOP, B., & UDENFRIEND, S.
(1960) A rapid spectrophotometric assay of monoamine oxidase
based on the rate of disappearance of kynuramine. J. Biol.
Chem., 235: 1160-1163.
WILLIAMS, C. H., JR & KAMIN, H. (1962) Microsomal triphosphopyridine
nucleotide-cytochrome c reductase of liver. J. Biol. Chem.,
237: 587-595.
WILLIAMS, R. T. (1965) The influence of enterohepatic circulation on
toxicity of drugs. Ann. NY Acad. Sci, 123: 110-124.
WILLIAMS, R. T. (1967a) The biogenesis of conjugation and detoxication
products. In: Bernfield, P, ed. Biogenesis of natural compounds,
2nd ed., Oxford, Pergamon Press, pp. 589-639.
WILLIAMS, R. T. (1967b) Comparative patterns of drug metabolism.
Fed. Proc., 26: 1029-1039.
WILLIAMS, R. T. (1971) Introduction: Pathways of drug metabolism. In:
Brodie, B. B. & Gillette, J. R., ed. Concepts in biochemical
pharmacology, Berlin, Springer-Verlag, Vol. 2, pp. 226-242.
WITIAK, D. T. & WHITEHOUSE, M. W. (1969) Species differences in the
albumin binding of 2,4,6-trinitrobenzaldehyde, chlorphenoxyacetic
acids, 2-(4'-hydroxybenzeneazo) benzoic acid and some other
drugs; the unique behaviour of rat albumin. Biochem. Pharmacol.,
18: 971-977.
WITSCHI, H. (1975) Exploitable biochemical approaches for evaluation
of toxic lung damage. In: Hayes, W. J. Jr., ed. Essays in
toxicology, New York, Academic Press, Vol. 6, pp. 125-191.
YUKI, H. & FISHMAN, W. H (1963) A carbazole method for the
differential analysis of glucuronate, glucosiduronate and
hyaluronate. Biochim. Biophys. Acta, 69: 576-578.
ZAKIM, D. & VESSEY, D. A. (1973) Techniques for the characterization
of UDP-glucuronyl-transferase, glucose-6-phosphatase and other
tightly bound microsomal enzymes. In: Glick, D., ed. Methods of
biochemical analysis, New York, Wiley, Vol. 21, pp. 2-37.
ZANNONI, V. G. (1971) Experiments illustrating drug distribution and
excretion. In: La Du, B. N., Mandel, H. G, & Way, E. L, ed.
Fundamentals of drug metabolism and drug disposition,
Baltimore, Williams & Wilkins, pp. 583-590.
ZANNONI, V. G., FLYNN, E. J., & LYNCH, M. (1972) Ascorbic acid and
drug metabolism. Biochem. Pharmacol., 21: 1377-1392.
ZELLER, E. A. (1971) Amine oxidases. In: Brodie, B. B. & Gillette, J.
R., ed. Concepts in biochemical pharmacology, Berlin,
Springer-Verlag, Vol. 2, pp. 518-535.
ZIEGLER, D. M., MCKEE, E. M., & POULSEN, L. L. (1973) Microsomal
flavo-protein-catalyzed N-oxidation of arylamines. Drug Metab.
Disposition, 1: 314-320.
5. MORPHOLOGICAL STUDIES
5.1 Introduction
Morphological studies are often the corner stones of toxicity
experiments. The variety of such studies leads to many different
approaches from the viewpoint of pathology, and skill and flexibility
in working procedures seem to be far more important than a strict
schedule.
On the other hand, it is advisable to have some general
guidelines on pathological procedures for routine quality testing for
toxicity, even though specific questions may often be posed, special
experiments may have to be carried out, and special animals,
techniques, and examinations used in order to elucidate certain
problems.
This chapter deals with the various phases of morphological
studies with a view to providing some general recommendations
concerning the procedures to be followed. It must be emphasized,
however, that these recommendations are only a guide, and that it will
be the pathologist's special responsibility to see that studies are
carried out in the way most likely to ensure optimum results.
5.2 General Recommendations
Gross necropsy facilities should be in close proximity to those
of the pathologist. The autopsy room must be equipped with adequate
dissection tables, dissection materials, running water, drains,
lighting, ventilation, and facilities for disinfection. In addition,
gross photography facilities are necessary. Cooling facilities must be
available for the storage of dead animals until necropsy, but the
animals should not be frozen (Sontag et al., 1975). To carry out
proper experiments and to prevent the loss of a considerable number of
animals by cannibalism or autolysis, it is essential that animals be
observed at least once a day, including Saturdays and Sundays. Animals
in moribund condition should be killed.
For the trimming of fixed tissues, a well-ventilated area,
preferably with an exhaust hood, and running water and drains, is
required.
The histology laboratory should be separated from the autopsy
room and should be equipped with tissue-processing equipment,
microtomes, cryostat, embedding and staining facilities, and supplies
(Sontag et al., 1975). Storage facilities are necessary for the fixed
tissues, as well as for the tissue block and histological slide files.
The facilities should be vermin-proof and temperature controlled or at
least cool.
Veterinary or medical pathologists with experience in laboratory
animal pathology or others with appropriate training and experience
should be responsible for all pathology procedures. In addition, the
pathologist should participate in the design and conduct of
experiments.
Histology technicians with appropriate training and experience in
the histological field will guarantee good histotechnical work,
whereas technicians trained and experienced in laboratory animal
dissection will be of great help in performing necropsies. Technicians
must be able to recognize and adequately describe gross abnormalities.
Personnel should be available at the weekends to perform
necropsies on animals found dead or killed in extremis.
5.3 Gross Observations
Gross pathology can be performed in most cases by experienced
technicians under the guidance of the pathologist. They should follow
a certain scheme of necropsy technique and must be able to recognize
abnormalities. A checklist should be used to ensure that all organs
and tissues are inspected and dissected. Information on clinical signs
must be available. Abnormalities found must be recorded on autopsy
cards. The descriptions must be clear and provide details such as
location, size, colour, texture, number, etc.
A great number of lesions will be within the scale of known
abnormalities for the animal species and breed used. Lesions found
beyond this normal scale should always be examined by the pathologist
himself. It is advisable for all new lesions, especially those
attributable to the treatment, to be photographed.
Dead animals should be necropsied unless cannibalism or autolysis
precludes this. Autolysis should not readily be accepted as an excuse
for not performing an autopsy. An inadequate gross necropsy cannot be
replaced by microscopic examination, no matter how well performed. On
the other hand, a well-performed gross necropsy may provide optimum
information for microscopic examination and may, in certain cases,
facilitate more selective microscopic examination.
Several ways of killing animals are available and the methods
depend on the facilities, the purpose of the experiment, and the
species used. Sacrifice of animals by anaesthesia (by ether or
barbiturates) is widely used as well as asphyxiation by carbon
dioxide. In rodents, the blood of the body can be removed by cardiac
puncture or puncturing the abdominal aorta, and in dogs by puncture of
the common carotid artery. Alternatively in small rodents decapitation
can be performed, though this may result in blood aspiration and
damage to certain tissues or organs. On the other hand decapitation
will probably give more constant results with respect to the total
blood loss, and consequently lead to fairly consistent organ weights.
5.3.1 Autopsy techniques
It is difficult, if not impossible, to prescribe working
procedures or techniques that will be adequate in all conditions.
Often, observations made when the animals were alive or experience
with other structurally-related compounds may focus attention on
certain organs or tissues. Sometimes fixation by perfusion is
necessary for proper examination of specific tissues (for example, the
central nervous system).
Large numbers of animals should not be sacrificed at any one
time, if too few technicians are available to perform the necropsies.
Animals should be killed in sequence, especially where it is necessary
to sample tissues for biochemical, enzyme-histochemical, or analytical
studies. However, organs and tissues should be weighed as soon as
possible after death to ensure that they have not dried out and that
they can be fixed quickly. Special procedures may shorten the time
needed for full autopsy especially in the case of small rodents. The
exact organization of working procedures depends largely on the
facilities and labour available, but good preparation and teamwork are
crucial factors.
Sacrifice should be carried out so that animals of the
experimental and control groups are killed at approximately the same
time of day, thus preventing the introduction of variations in
physiological status dependent on circadian rhythm. In certain cases,
however, this may not be feasible.
Special care is necessary when test elements or compounds are
analysed after sacrifice. At times it may be necessary to kill the
animals in a given order to prevent contamination (e.g. sacrifice of
the control first, followed by the lowest dose level, then the
intermediate dose level, and finally the high-dose level).
Autopsy techniques have already been described (Roe, 1965) and
only a general outline of the procedures is given.
5.3.2 Rat, mouse, guineapig, rabbit, monkey
The necropsy starts with external examination including the body
orifices. Thereafter, the animals are fixed on their backs. After a
median incision, the skin is partly removed and the subcutis,
superficial lymph nodes, salivary glands, and mammary glands can be
inspected and dissected. The abdomen can be opened and the negative
pressure of the thorax may be checked and the thorax opened.
Thyroid and thymus can then be inspected and dissected.
Trachea, lungs, and heart are removed en bloc. The heart is
detached, but the trachea and lungs remain intact to provide easy
handling for inflation of the lungs with fixative (see under fixation
methods).
Abdominal organs are removed. The examination of liver and kidney
should include making a routine number of parallel slices of the
organs to examine their cut surfaces. The gastrointestinal tract
should be removed from the abdominal cavity and opened. In chronic
toxicity experiments, the entire gastrointestinal tract must be
opened, the mucosal surface examined, and representative parts taken
for histological examination. The oesophagus and parts of the
intestine can be rolled according to the Swiss roll technique in order
to obtain a considerable length of mucosa musculature and serosa in
the microscopic section. Brain, peripheral nerves, skeletal
musculature, and bones (including a joint) are removed. The spinal
cord may best be fixed in situ.
5.3.3 Carnivores, swine
After external examination, necropsy is performed when the animal
is lying on its right side. Left front and hind legs are removed
without opening the apertura thoracis superior. The skin is partly
removed after a median ventral incision from mouth to os pubis.
Subcutis, fat, mammary glands, salivary glands, and superficial lymph
nodes may be inspected and dissected. In addition, the different
joints of the legs are opened and inspected. After opening the
abdomen, the thorax is checked for negative pressure and opened by
removing the left side of the thoracic wall. Then the right side of
the pleural cavity is inspected. The oesophagus is removed by cutting
it cranially. The thoracic and abdominal organs are removed and
individually inspected and opened. The nasopharynx is then opened and
inspected, and the tongue, larynx, pharynx, tonsils, brain, spinal
cord, peripheral nerves, and musculature collected. Most organs should
be sliced in parallel slices to examine the cut surfaces.
5.4 Selection, Preservation, Preparation, and Storage of Tissues
5.4.1 Selection of tissues
Which organs and tissues should be collected for fixation is
determined by the type of toxicity experiment and the compound being
tested. Sufficient material should be collected to prevent the
necessity of having to repeat a certain experiment. Since it is
impossible to provide strict rules for selection, only a general
outline will be given.
5.4.2 Oral toxicity tests
In acute toxicity experiments, restrictive microscopic
examination may be necessary in certain cases. Some laboratories fix
liver and kidneys, others do not. The additional information obtained
from histological examination of these organs in acute experiments is,
usually, limited.
In range-finding tests, restrictive pathology is common practice.
Normally, the heart, liver, spleen, and kidneys and all grossly
abnormal tissues are collected for fixation. When the compound tested
is given by stomach tube, it is advisable to include lungs,
oesophagus, and stomach in the histological study. In subacute
(90-day) and chronic studies, it is advisable to select all tissues
and organs for fixation in buffered formal saline. This normally
includes the following organs and tissues:
brain gall bladder (if present)
pituitary oesophagus
thyroid (parathyroid) stomach
thymus duodenum
(trachea) jejunum
lungs ileum
heart caecum
sternum (bone marrow) colon
salivary glands rectum
liver urinary bladder
spleen lymph nodes -- mandibular or mesenteric
kidneys lymph nodes -- popliteal or axillary
adrenals mammary glands
pancreas (thigh) musculature
gonads peripheral nerve
accessory genital organs (eyes)
aorta (femur -- incl. joint)
(skin) spinal cord (at three levels)
(exorbital lachrymal glands)
The tissues mentioned between brackets are sometimes considered to be
optional. In addition, all tissues containing grossly observed lesions
should be fixed.
The same selection is usually made in carcinogenicity tests. In
both chronic toxicity and carcinogenicity experiments, it is also very
important to collect all organs and tissues of animals that have died
or have been killed in extremis. Prompt examination of the tissues
of these animals may provide valuable information leading to a more
meaningful pathological examination of animals, that die or are
sacrificed later on, or at the end of the experiment. In cases where
only part of an organ or tissue is taken, it is important that the
same part of that organ or tissue be selected at approximately the
same site in all animals.
5.4.3 Inhalation toxicity studies
In acute inhalation studies, it may be worthwhile, in some cases,
to select lungs for fixation and subsequent microscopic examination,
as well as liver and kidneys. In most cases, however, this seems
unnecessary since the pulmonary lesions are usually of the same type
(hyperaemia and oedema) and can be detected by gross inspection.
Examination of the entire respiratory tract is necessary in
subacute inhalation studies. This includes nasal cavity, pharynx,
larynx, trachea, main bronchi, and lungs. The exact orientation of
these organs for trimming, embedding, and sectioning is of prime
importance and needs special attention. More organs and tissues may be
selected in the inhalation studies, selection usually being effected
in the same way as for oral studies.
5.4.4 Dermal toxicity studies
In acute dermal toxicity studies, it is advisable to perform
microscopic pathology on liver and kidneys. The presence of
degenerative changes in these organs indicates that the compound is
active transdermally. Examination of the skin is also advisable.
In subacute dermal toxicity studies, the organs and tissues are
selected in the same way as that described for oral tests. Of course,
the skin deserves special attention in that both treated areas and
normal skin in comparable areas are selected.
5.4.5 Special studies
In the case of sensitization or irritation studies, the selection
of tissues depends completely on the type of test. In eye irritation
studies, the examination of eye and conjunctivae seems adequate
whereas in a Landstainer-Draize test, and especially in a maximization
test, the skin may be examined. In all these cases, it may, or may
not, be necessary to select more tissues.
5.5 Preservation of Tissues
Preservation of tissues can be performed by immersion, inflation
or distension, and perfusion. Immersion is most commonly used, but in
some circumstances may not result in satisfactory preservation.
Microscopic examination of the lungs, for example, cannot be done
adequately if they have not been inflated or have not been fixed by
perfusion. When the central nervous system is a target organ, it is an
absolute necessity to use perfusion to ensure proper fixation, as
fixation by immersion leads to many artifacts that are
indistinguishable from certain degenerative changes. When animals in
long-term tests are killed in extremis, it is advisable to use
fixation by perfusion. Perfusion is usually essential for electron
microscope studies of tissues.
With perfusion, the best conditions for microscopic examination
are obtained while effective gross examination remains possible. All
tissues should be fixed in 10% neutral buffered formalin or another
appropriate fixative.
5.5.1 Immersion
Immersion is the most used and usually the most appropriate
method of preservation in toxicity experiments. The tissues are placed
in the preservative and fixed for 24-48 h. Fixation at higher
temperatures (40-50°C), in a vacuum, or by use of microwaves (Gordon &
Daniel, 1974) may shorten the fixation time considerably. Tissues
thicker than 0.5 cm should not be fixed. The preservative/tissue ratio
is very important and must be greater than 10:1 (volume fixation
fluid/volume tissue) to obtain acceptable fixation. The fixation of
intact animals with opened abdomen should not be carried out. All
tissues and organs must be fixed separately, and some may need special
attention. Skin and peripheral nerves must be fixed in a straight (but
not stretched) and flat position. To achieve this, these tissues can
first be attached to a piece of thick filter paper. The spinal cord
can best be fixed in situ before it is taken out. Special fixing
solutions may be used in certain cases such as Bouin's fixative for
the fixation of ovaries, testes, thyroid, and adrenals or Zenker's
solution for the fixation of the eyes.
As certain organs (pituitary, thyroid, ovaries, adrenals, and
lymph nodes) of some of the smaller rodents are rather small, they
should be fixed separately in smaller jars to prevent loss; various
fixatives may be used.
5.5.2 Inflation
Inflation is used to preserve lungs effectively. To prevent the
formation of artifacts that may be misjudged as emphysema, it is
necessary for inflation to be carried out at constant pressure
(Chevalier, 1971; Fawell & Lewis, 1971). In certain cases, however,
even fixation of the lungs by inflation may not be adequate and
perfusion will be preferred (for example, in inhalation studies).
Inflation with fixative is also necessary for correct fixation of
the urinary bladder. If the bladder is distended, urine must be
replaced by fixative via the urethra using a syringe with a blunt
needle. Contracted empty bladders should be partly distended with
fixative. The reflux of fixative in the bladder is prevented by
ligation of the urethra. Inflation may also be used for fixation of
the digestive tract. Here again, ligation is necessary. In these
cases, the organs have to be bisected after fixation and the interior
surface inspected.
5.5.3 Perfusion
The best way to preserve tissues is by perfusion, which is
usually effected by infusion in the left ventricle of the heart and by
opening the sinus venosus to provide for proper circulation. Perfusion
of isolated organs (liver, kidneys) is another possibility. Before
perfusion, the animals are anaesthetized with a barbiturate,
administered in combination with nitrate and heparin to ensure
vasodilation and prevent clotting. A solution consisting of 77.5 ml
sodium nitrite (1.25%) in water, 10 ml heparin (5000 IU/ml) and 12 ml
pentobarbital (60 mg/ml), of which 10 ml is administered per kg body
weight, is satisfactory. Then the blood is removed with an isotonic
saline solution using slight overpressure. When all the blood has left
the body via the sinus venosus or right atrium, the body may be
perfused with the fixative.
Perfusion is sometimes not possible especially in experiments
where the recording of organ weights is important (i.e. in subchronic
toxicity studies). This difficulty can be solved by increasing the
number of experimental animals, though this may lead to a considerable
increase in costs. Perfusion of large numbers of animals is possible
using simple facilities at low cost (Fig. 5.1).
5.6 Trimming
It is important that the trimming be carried out by well-trained
people. It must be emphasized that knowledge of pathological phenomena
is necessary, as it frequently happens that the person responsible for
trimming cuts out the "good looking" areas and discards the tumours
present in the organs. The tissues must be sliced in such a way that
the cut surfaces present the largest possible area for examination.
The use of a special trimming scheme during the procedure may be
helpful.
The kidneys should be sectioned through the cortex and medulla,
one kidney mid-longitudinally, the other mid-transversely.
The brain must be cross-sectioned at, at least, three sites: the
frontal cortex with basal ganglia, parietal cortex with thalamus, and
cerebellum with pons.
The lungs should be sectioned transversely, parallel to the long
axis of the body. These sections must include the main bronchi and
carina.
The hollow organs should be trimmed in such a way that a
cross-section from mucosa to serosa is obtained.
 Tumours or tumorous masses usually need to be trimmed in several
portions. Preferably, tissues surrounding the tumour should be
included.
When intestines are fixed as a roll, the roll can best be
embedded as such, since, trimming of these rolls is practically
impossible, even after fixation.
For certain organs, special trimming procedures are needed. For
example, the nasal cavity should be sectioned transversely at three
sites. The trimming of the larynx/pharynx is crucial in order to
obtain a section that can be properly interpreted.
Trimmed tissues should have a maximum thickness of 2-3 mm for
satisfactory processing.
5.7 Storage
Material not used for processing should not be discarded, but
should be stored in airtight jars or plastic bags to ensure that the
tissues do not dry out. Plastic bags are an excellent way of storing
tissues in minimum space. The bags should be clearly and permanently
labelled. The tissues should be stored, at least, until the
microscopic examination has been completed and the findings adequately
evaluated. If at all possible, the tissues should be stored for a long
period (e.g. material from 90-day studies should be stored for 2 years
and that from chronic experiments for 5-10 years), but storage
facilities may prevent this. Tissue blocks and sections can be stored
in a cool area for a considerable time (10 years or more).
5.8 Histological Techniques
Embedding in paraffin or polymer-containing paraffins or waxes is
advisable. The embedding procedures may be shortened considerably by
using automatic tissue-processing equipment and special frames in
which the paraffin blocks can be made in large numbers (Fig. 5.2).
Proper and exact labelling of the blocks is a necessity. The blocks
may be prepared in such a way that they can be placed as they are in
the microtomes.
Tissue sectioning can be performed at a thickness of 4-6 mm and
sections can be stained routinely with haematoxylin and eosin or a
comparable routine stain. Serial sectioning can best be done with the
help of an engine-powdered microtome.
The use of semithin sections (1 µm) is of considerable importance
for specific organs such as bone-marrow, kidneys, lymph nodes, spleen,
and endocrine glands. These sections can now be made on special
Tumours or tumorous masses usually need to be trimmed in several
portions. Preferably, tissues surrounding the tumour should be
included.
When intestines are fixed as a roll, the roll can best be
embedded as such, since, trimming of these rolls is practically
impossible, even after fixation.
For certain organs, special trimming procedures are needed. For
example, the nasal cavity should be sectioned transversely at three
sites. The trimming of the larynx/pharynx is crucial in order to
obtain a section that can be properly interpreted.
Trimmed tissues should have a maximum thickness of 2-3 mm for
satisfactory processing.
5.7 Storage
Material not used for processing should not be discarded, but
should be stored in airtight jars or plastic bags to ensure that the
tissues do not dry out. Plastic bags are an excellent way of storing
tissues in minimum space. The bags should be clearly and permanently
labelled. The tissues should be stored, at least, until the
microscopic examination has been completed and the findings adequately
evaluated. If at all possible, the tissues should be stored for a long
period (e.g. material from 90-day studies should be stored for 2 years
and that from chronic experiments for 5-10 years), but storage
facilities may prevent this. Tissue blocks and sections can be stored
in a cool area for a considerable time (10 years or more).
5.8 Histological Techniques
Embedding in paraffin or polymer-containing paraffins or waxes is
advisable. The embedding procedures may be shortened considerably by
using automatic tissue-processing equipment and special frames in
which the paraffin blocks can be made in large numbers (Fig. 5.2).
Proper and exact labelling of the blocks is a necessity. The blocks
may be prepared in such a way that they can be placed as they are in
the microtomes.
Tissue sectioning can be performed at a thickness of 4-6 mm and
sections can be stained routinely with haematoxylin and eosin or a
comparable routine stain. Serial sectioning can best be done with the
help of an engine-powdered microtome.
The use of semithin sections (1 µm) is of considerable importance
for specific organs such as bone-marrow, kidneys, lymph nodes, spleen,
and endocrine glands. These sections can now be made on special
 microtomes, that cut the normal paraffin blocks with glass knives. Of
course, this procedure should only be followed when specific details
have to be followed during the microscopic examination. The use of
semithin Epon sections yields even better results.
It will often be necessary to use special staining techniques on
tissues in order to provide more information on the presence of
carbohydrates, proteins, fats, elements, or certain structural
organizations. Special fixation is sometimes necessary for appropriate
staining. Bones and calcified tissues have to be decalcified. Eyes
usually need to be fixed and embedded differently. Blood vessels may
be stained with Sudan black in order to study vascular lesions.
5.9 Special Techniques
5.9.1 Enzyme histochemistry
Enzyme histochemistry is a technique used to detect the activity
or presence of an enzyme in a tissue by incubating the fresh tissue in
an appropriate medium, so that a fine coloured granular precipitation
forms wherever the enzyme is present.
The use of the enzyme-histochemical technique is not common in
toxicity testing. Nevertheless, the technique is a valuable one, since
it provides information about the metabolic activity and function of
the tissues. Furthermore, it introduces the possibility of correlating
biochemical with histological findings that may lead to a more correct
interpretation. Enzyme-histochemical investigations also make it
possible to visualize certain differences in enzyme activity within
the structural organization of tissues.
In some cases, a decrease in enzyme activity in certain cells is
compensated for by an increase in other cells within the same organ.
Such biological differences can only be detected by
enzyme-histological investigation, when the results of biochemical
determinations are within normal limits and no alterations are seen
using conventional histological techniques. Enzyme-histological
investigations can easily be incorporated in routine toxicity testing,
if necessary.
Relatively small pieces of tissue are quickly frozen in an inert
liquid (i.e. isopentane), or cooled in liquid nitrogen or a mixture of
solid carbon dioxide and methanol. Storage of the tissues is effected
at -70°C. Cryostat sections are prepared and incubated in specially
prepared media. Good reference works for the methods have been
published (Barka & Anderson, 1965; Pearse, 1968, 1972). To obtain
optimum information, the prepared section should be examined by
semiquantitative methods.
An enzyme histochemical investigation can also be performed at
the electron microscope stage (Geyer, 1973). In this case, the
activity or presence of an enzyme is determined by the deposition of
electron-dense material at the sites where the enzyme is located. It
is evident that these delicate techniques are not easy to apply in
routine toxicity testing. They may, however, be of great importance in
specific studies on the biological effects of a compound on cellular
components, or to detect early damage.
5.9.2 Autoradiography
Autoradiography is based on the principle that radioactive
substances present in tissues are able to produce an image on a
photographic film or plate. The radiations emitted by the radioactive
substances must be of relatively weak energy, so that they will have a
short range in tissues and emulsions. When the range is too long,
developed silver grains can be found in the emulsion far away from the
radioactive source.
In this respect only alpha particles, beta particles, and Auger
electrons are useful since electromagnetic radiations such as gamma
and X-rays give poor results due to their penetrating power.
Tritium (3H) has been most frequently used in autoradiographic
studies, although 14C and 32P are also being used as tracers.
Autoradiography, at both light microscopic and ultrastructural
levels, can be used in kinetic studies on tissues by incorporating
radioactive-labelled bases in DNA or RNA resulting in the production
of silver grains on a photographic film covering the section. The
different methods have been extensively described for nondiffusible
substances (Baserga & Malamud, 1969; Rogers, 1967) as well as for
diffusible substances (Roth & Stumpf, 1969).
Apart from studying the kinetics of tissues, autoradiographic
techniques can also be used to study the distribution of radiolabelled
compounds and their metabolites. This, again, can be done at light
microscopic and ultrastructural levels.
The technique of whole-body autoradiography as developed by
Ullberg et at. (1972) is a valuable tool, in this respect, since the
distribution of a compound can qualitatively (or even
semiquantitatively) be studied in the whole body. In addition, pieces
can be cut out to use for microautoradiographic research.
Substances may also be labelled with fluorescent chemicals
permitting their detection by illumination of the sections with
ultra-violet radiation. The technique of fluorescence microscopy can
also be used for the detection of certain dyes that possess
autofluorescing properties. Some substances, such as the
catecholamines, can be made visible by reaction with formaldehyde
which converts mines into fluorescent substances (Eränko & Räisänen,
1966). Fluorescence in tissues can be measured quantitatively, thus
facilitating controlled conditions (Ploem et al., 1974).
Autoradiographic studies cannot be incorporated easily into
routine toxicity experiments. However, the technique may be a very
important tool in special studies.
5.9.3 Immunofluorescence and immunoenzyme techniques
Immunofluorescence techniques are primarily used in determining
the presence or absence of certain antibodies or antigens in tissues.
Antigens are detected by binding them to specific antibodies. If a
specific antiserum is conjugated with a fluorescent dye, the specific
antigen-antibody complex formed can be seen by studying the tissue
section using ultraviolet radiation microscopy. This direct
immunofluorescent technique can be replaced by a more sensitive
indirect method in which the substance actually rendered fluorescent
is not the antigen under consideration but an intermediate material
the distribution of which corresponds precisely to that of the antigen
being studied. Information on the principle of these techniques is
available (Goldman, 1968; Nairn & Marrack, 1964).
The use of enzyme conjugates has recently been developed
(Sternberger, 1974). This system is used when antigen-antibody
complexes have to be localized at ultrastructural levels.
Immunofluorescence or immunoenzyme techniques will not be used in
most routine toxicity experiments, but they may be of importance in
special studies such as those described in Chapter 7.
5.9.4 Electron microscopy
Transmission electron microscopy is the most commonly used
technique for studying the ultrastructure of tissues. Different
fixation, embedding, and cutting techniques have to be used to obtain
ultrathin sections of tissues (Flauert, 1973, 1974, 1975; Hayat, 1970,
1972, 1973). At the ultra-structural level, very minute changes can be
detected and they have to be distinguished from the many artifacts
that can be introduced during the different processing procedures.
Under optimum conditions, tissues can be examined at high
magnifications.
As tissue examination by electron microscopy is very time
consuming and costly, it is important only to select tissues from
those experiments that justify it. Ultrastructural studies have
contributed enormously to our knowledge of molecular biology. In the
case of toxicology, ultrastructural studies are always carried out as
a secondary investigation, and are usually applied to
ultrastructurally, specific, pathological changes already studied in
detail at the light microscopic level, in order to secure a better
judgment of the importance of the lesion. Ultrastructural studies are
also used to confirm that target organs that appear normal at a
certain close level using light microscopy, do not in fact show
pathological changes.
Scanning electron microscopy (SEM) techniques, that have come
into use in recent years, provide 3-dimensional images of complete
biological units and also chemical information (Hayes, 1973). Here
again, special processing procedures may be needed, although, in
certain cases, formalin-fixed and paraffin-embedded material can be
used. Natural surfaces, dissected material, sectioned tissue, and
living specimens may be studied. SEM is a valuable tool in biology
but, for toxicological investigations, its use is still rather
restricted, apart from the possibility of obtaining quantitative
chemical information. The development of analytical electron
microscopy, using the principles of X-ray microanalysis, also makes it
possible to correlate tissue ultrastructure and chemistry (Hayes,
1973; Weavers, 1973).
5.10 Microscopic Examination
Routine histopathological examination is very important and
should be carried out correctly. First of all, the sections to be
studied should be of good quality. Additional sections and special
stains must be prepared if necessary. Special stains are used to study
and describe individual lesions; they may also be used to examine
certain organs or lesions to permit better judgment of
semiquantitative comparison. For example, when haemosiderosis is found
to be an important effect, special stains, based on the reaction of
the dye with iron, facilitate semiquantitative analysis of the degree
of the lesion.
Good microscopic equipment is necessary to perform optimum
microscopic examination. Sources of ultraviolet radiation and
polarized light should be available.
The microscopic examination must be carried out by well-trained
pathologists or other persons trained and experienced in the field of
laboratory animal pathology. The use of parapathologists for routine
microscopy in toxicity and carcinogenicity experiments is extremely
valuable and is important for overcoming a shortage of personnel,
trained in laboratory animal pathology (Toxicol. appl. Pharmacol.,
1975). Experience has shown that well-trained parapathologists can
become very skilful in microscopy and in screening sections for
abnormalities. With further experience, they are also able to describe
certain lesions. It is the pathologist's duty to see that all lesions
observed are described and interpreted correctly, and to check the
sections for any lesions that may have been overlooked.
It is a serious mistake, in an experiment, to undertake
microscopic examination before the results of other examinations such
as biochemical determinations, haematology, and organ weights, are
available, for the information obtained from these procedures may give
important directions for the microscopic study. For example, an
increased thyroid weight may lead to more careful examination of the
thyroid for hyperplasia and may prompt histometric determinations.
Lower lymph node weight may point to semiquantitative examination of
these organs with regard to reduced immunocapacity (Cottier et al.,
1972). Differences or changes in the blood picture call for more
detailed study of the bone marrow, for example, by preparing and
studying 1 µm sections, to detect suspected haemopoietic disturbances.
Gross observations should always be correlated with microscopic
findings. It is a false assumption to think that a microscopic
examination will be more objective when performed blindly; knowledge
of clinical signs and macroscopy are indispensable for a meaningful
examination. Of course, the pathologist must be careful not to let
knowledge of clinical effects influence his objective evaluation of
the tissues; thus, sections of the tissues considered to be involved
may have to be re-examined blindly. If such a re-examination is
carried out, semiquantitative scoring of the extent of the lesion will
also help to detect a possible dose-effect relationship. Should there
be any doubt in interpreting the significance of a lesion, it is
advisable to consult other pathologists, all of whom should re-examine
the slides blindly.
5.10.1 Number of animals and number of organs and tissues studied
microscopically
In acute and rangefinding tests, the organs and tissues are
usually fixed and examined microscopically after processing. In
subacute and long-term studies, and, sometimes, in rangefinding tests,
it is customary initially to examine only the tissues of the highest
dose group, the control group and the target organs, if known. In
addition, all grossly observed lesions are processed in the
intermediate and low-level groups. If the results of the microscopic
examination of the highest dose group indicate a need to examine
certain organs at lower levels, this can be done at a second stage.
In chronic toxicity experiments, all males and females of the
highest dose group should be examined. It is also advisable to examine
all control animals completely, since this will be the only way to
ascertain the incidence of tumours and other "lesions" normally
occurring in the strain of animals used. Such information is
indispensable for correctly evaluating the significance of changes
observed in exposed animals.
In carcinogenicity experiments, where larger numbers of animals
per group are usually used, some laboratories restrict microscopic
examination mainly to the grossly observed abnormalities and tumours,
and perform complete histological examinations on only 15-20 male and
15-20 female survivors of the highest dose group and of the control
group, on the assumption that a well-performed gross examination will
detect most of the tumours present. Histological examination of all
tissues of 15-20 animals of each sex will give some information on the
possible presence of certain pre-malignant hyperplastic or neoplastic
changes not grossly observed. If such lesions are found, the
histological examination should cover all animals, while small organs
which may bear tumours that cannot be grossly observed (thyroid,
pituitary, adrenals) must be examined histologically.
5.10.2 Description of the lesions
For the microscopic examination, it is essential to use a
check-list to detect losses, especially of small organs lost in the
processing procedure. All organs and tissues examined should be
listed, even though no abnormalities are seen, while all lesions found
should be described clearly and accurately. It is essential to
describe lesions in a semiquantitative way using such words as slight,
moderate, strong, and very strong to indicate the extent of the
lesion; it is also essential to indicate the criteria used to
investigate and classify abnormalities. It will often be necessary to
re-examine certain lesions in order to obtain a well-balanced
semiquantitative judgment. Several reference works may be of help in
the classification of tumours and other lesions (Beveridge & Sobin,
1974; Cotchin & Roe, 1967; Ribelin & McCoy, 1965; and Turusov, 1973).
Certain lesions found by microscopic examination may, in fact,
consist of several entities. In such cases, it may be important to
classify these entities independently. For example, perilobular liver
degeneration may be combined with bile duct proliferation, but one of
the two phenomena may be more extensive than the other with respect to
dose level and time. For a better interpretation, it is preferable to
describe and classify such lesions independently.
5.11 Presentation, Evaluation, and Interpretation of Pathological
Data
A well-performed and well-described microscopic examination is
not complete if the results cannot be studied and compared easily and
adequately. The lesions found in the different groups should be
tabulated according to organ, group, and sex in order to facilitate
comparison of the incidence of such lesions in the different groups.
If, in addition, a semiquantitative estimate of the extent of the
lesions is made, it will be easy to see whether they are
compound-induced and increase with time and dose.
It is important that these tables include details such as the
number of animals necropsied and the number examined microscopically,
per group and sex. In addition, it is advisable to state, the exact
number of organs or tissues examined microscopically, since it is well
known that, in every toxicity experiment, organs and tissues get lost
during the processing procedure. In this way, the loss can be
adequately estimated and the lesions found can be expressed against
the real number of organs or tissues examined.
For correct interpretation of the tumour tables, it is advisable
to include hyperplastic and preneoplastic lesions as individual
groups. The table should also include the number of days that elapsed
from the start of the experiment until the observation of each rumour.
This first observation may be clinical, for example, when mammary, ear
duct, or skin tumours are involved and the time that has elapsed can
then be called the "induction time". However, usually, tumours are
found after death or killing in extremis, or at sacrifice at the end
of the experiment. Thus, in these cases, it seems most appropriate to
use the term "time of observation". The inclusion of the "time of
observation" of tumours in the table is of help to the investigator
since it provides information on shorter or prolonged latency periods
(e.g. time that has elapsed between initiation and the appearance of
tumours). In addition, it reminds the reader to take into account the
survival differences when expressing the results.
In long-term experiments, the number of lesions found may be
quite large. Furthermore, some lesions may have been noticed during a
certain period in life, when the animals died or were sacrificed
in extremis, while others will have been noticed at the end of an
experiment. When such lesions are tabulated in the same table, this
information may get lost. It seems, therefore, most appropriate in
such long-term experiments to pool the results of the pathological
examination for certain periods, for example the first 12 months of
life and then for 3- to 6-monthly intervals, and to give the results
of the examination undertaken at the end of the experiment separately.
It is obvious that in preparing such tables, the use of an
automated data acquisition and computer-based system will be of great
help. These systems have been described for application to
toxicological studies, especially for animal weights, food and drug
consumption, organ weights, and biochemical data (Munro et al., 1972).
In pathology, such systems offer considerable potential. The necessity
for the correct coding of pathological changes, and the use of a code
system set up so that detailed information about lesions can still be
obtained, is obvious. Several systems have been described (Becker,
1973; Enlander, 1975; Smith et al., 1972), and may be adapted for this
purpose.
Pathological examinations must always be evaluated and
interpreted in connexion with other phenomena found. It must be
decided whether lesions noticed are in fact pathological, and whether
they are found in connexion with changes in other variables such as
organ weights, biochemical tests, etc. If compound-induced lesions are
found, they may not only be present at the highest dose level, but
also at an intermediate dose level. In the latter case, it is
important to determine whether there is a dose-dependent increase in
the severity and extent of the lesion, a finding which would
strengthen the assumption that the lesion is compound-induced.
Moreover, a clear dose-response relationship permits better evaluation
of the potential risk of a compound. Whenever lesions are found only
at the highest dose level, the sections at all dose levels should be
re-examined blindly to remove any doubt. Often the two sexes will show
a different sensitivity to the action of a compound.
Problems may arise when certain lesions are found more frequently
and to a greater extent in the treated groups compared with the
control group and compared with the common incidence of the lesion in
the strain used. The usual attitude towards this phenomenon is to
consider it as an effect or response.
In the evaluation of tumour incidence, criteria such as increased
tumour incidence, decrease in latency period, and the appearance of
tumours in organs where they do not occur spontaneously, have to be
considered. For this reason, detailed information on the spontaneous
occurrence of tumours in the species and strain used is essential,
particularly when tests indicate that the compound possesses a weak
carcinogenic action. Simple comparison of tumour incidence in control
animals and experimental animals at one point in time may lead to
erroneous conclusions, especially when only one of the above three
criteria is met. Many factors other than treatment may influence the
incidence of spontaneous tumours.
Statistical evaluation of the results is often necessary, but, as
yet, there are no agreed procedures for comparing statistically the
incidence of malignant tumours in controls and experimental groups.
REFERENCES
BARKA, T. & ANDERSON, P. J. (1965) Histochemistry: theory, practice
and bibliography. New York, Harper & Row; London, Evanston.
BASERGA, R. & MALAMUD, D. (1969) Autoradiography: techniques and
application. New York, Harper & Row; London, Evanston.
BECKER, H. (1973) Experiences with a test-stage free-text processing
system in pathology, using display terminals. Path. Europ.,
8: 307-313.
BEVERIDGE, W. I. B. & SOBIN, L. H., ed. (1974) International
histological classification of tumours of domestic animals.
Bull. World Health Org., 50: 1-142.
CHEVALIER, H. J. (1971) [A method for the fixation of the lungs of
small animals.] Z. Versuchstierk., 13: 101-104 (in German).
COHRS, P., JAFFE, R, & MEESEN, H. (1958) [Pathology of laboratory
animals] Berlin, Springer-Verlag, Vol. 2 (in German).
COTCHIN, E. & ROE, F. J. C. (1967) Pathology of laboratory rats and
mice. Oxford, Edinburgh, Blackwell.
COTTIER, H., TURK, J., & SOBIN, L. (1972) A proposal for a
standardized system of reporting human lymph node morphology in
relation to immunological function. Bull. World Health Organ.,
47: 375-408.
ENLANDER, D. (1975) Computer data-processing of medical diagnosis in
pathology. Am. J. clin. Pathol., 63: 538-544.
ERÄNKO, O. & RAISANEN, L. (1966) In vitro release and uptake of
noradrenaline in the rat iris. In: Ebler, U.S. V., Rosell, S. &
Uunäs, B., ed. Mechanism of release of biogenic amines. New
York, Pergamon Press.
FAWELL, J. K. & LEWIS, D. J. (1971) A simple apparatus for the
inflation fixation of lungs at constant pressure. Lab. Anim.,
5: 267-270.
FLAUERT, A. M. (1973, 1974, 1975) Practical methods in electron
microscopy. Amsterdam, Oxford, North Holland Publ.; New York,
Elsevier.
GEYER, G. (1973) [Ultrahistochemistry, histochemical working
guidelines for electron-microscopy.] Stuttgart, Gustav Fischer
Verlag (in German).
GOLDMAN, M. (1968) Fluorescent antibody methods. New York, London,
Academic Press.
GORDON, H. W. & DANIEL, E. J. (1974) Microwave fixation of human
tissues. Am. J. med. Technol., 40(10): 441-442.
HAYAT, M. A. (1970, 1972, 1973) Principles and techniques of electron
microscopy. In: Biological Applications Vol. I, II, III, IV.
New York, Van Nostrand Reinhold Company.
HAYES, T. I. (1973) Scanning electron microscopy in biology. In
Koehler, J. H., ed. Advanced techniques in biological electron
microscopy, Berlin, Heidelberg, New York, Springer-Verlag,
pp. 153-214.
MUNRO, I. C., CHARBONNEAU, S. M., & WILLES, R. F. (1972) An automated
data acquisition and computer-based computation system for
application of toxicological studies in laboratory animals.
Lab. Anim. Sci., 22: 753-756.
NAIRN, R. E. & MARRACK, J. R. (1964) Fluorescent protein tracing.
Edinburgh, London, Livingstone.
PEARSE, A. G. E. (1968, 1972) Histochemistry, theoretical and
applied. 3rd ed., Edinburgh, Churchill Livingstone, Vol. I &
II.
PLOEM, J. S., DE STERKE, J. A., BONNET, J., & WASMUND, H. (1974) A
micro-spectrofluorometer with epi-illumination operated under
computer control. J. Histochem. Cytochem., 22: 668-677.
RIBELIN, W. E. & MCCOY, J. R. (1965) The pathology of laboratory
animals. Springfield, Charles Thomas.
ROE, F. J. C. (1965) Spontaneous tumours in rats and mice. Food
Cosmet. Toxicol., 3: 707-720.
ROGERS, A. W. (1967) Techniques of autoradiography. Amsterdam,
London, New York, Elsevier.
ROTH, L. J. & STUMPF, W. E. (1969) Autoradiography of diffusible
substances. New York, London, Academic Press.
SMITH, J. E., MCGAVIN, M. D, & GRONWALL, R. (1972) English-language
computer-based system for storage and retrieval of pathological
diagnosis. Vet. Pathol. 9: 152-158.
SONTAG, J. M., PAGE, N. P., & SAFFIOTTA, U. (1975) Guidelines for
carcinogen bioassay in small rodents. Bethesda MD, National
Cancer Institute.
STERNBERGER, L. A. (1974) Immunocytochemistry. Englewood Cliffs, NJ,
Prentice-Hall. Toxicol. appl. Pharmacol. (1975) 31: 1-3, A
training programme for parapathologists.
TURUSOV, V. S. ed. (1973) Pathology of tumours in laboratory animals,
Vol. 1, Lyon, IARC (Sci. Publ. No. 5).
ULLBERG, S., HAMMARSTROEM, L., & APPELGREN, L-E. (1972)
Autoradiography in pharmacology. In: Cohen, Y., ed. I.E.P.T.
Section 78, Oxford, New York, Pergamon Press, Vol. 1,
pp. 221-239.
WEAVERS, B. A. (1973) The potentiality of EMMA-4, the analytical
electron microscopy in histochemistry: a review. Histochem. J.,
5: 173-193.
6. INHALATION EXPOSUREa
6.1 Introduction
Advances in technology, throughout the world, have increased the
number and amount of chemicals in the atmosphere. The health effects
of inhalation of these chemicals can be predicted to some extent by
experimental investigation. In order to simulate environmental
conditions, a special technology has evolved relating to the design
and operation of inhalation chambers and the generation and
characterization of aerosols and vapours. This chapter will introduce
this technology and review the significance of certain biological
end-points commonly measured in inhalation studies.
This chapter is not intended to be all-inclusive, nor to provide
complete instructions on inhalation technology. No toxicological
protocol is sufficient to cover all situations with all materials.
Hence, the expertise of the individual investigator and the objectives
of the investigation will govern the selection of the final protocol
and the biological end-points relevant to the particular compound
concerned.
The costs associated with evaluation of toxicity by the
inhalation route are considerably higher than for toxicity studies
using other routes of exposure because of the cost of the specialized
equipment used in inhalation studies and the time required for its
calibration. In general, it can be estimated that the total cost of
any type of inhalation study, short- or long-term, will be two to
three times that of a comparable oral study. Because of this, it is
important to determine when inhalation studies should be performed and
when, and if, studies by other routes would suffice. Finally, in
addition to costs, other resources should be considered. In most
countries, the number of laboratories capable of performing inhalation
studies is limited. Thus, it is essential to develop priorities for
the selection of compounds for evaluation of inhalation toxicity.
6.2 Need for Inhalation Studies
The effect of a compound depends on its concentration at the
receptors of the affected organ or system. The concentration at
different sites and times is a function of the route of entry. Thus
the route of entry is an important factor with regard to toxicity. No
good substitute model for inhalation exposure as a means of direct
exposure of the lungs has been developed, although intratracheal
exposure has been frequently used, particularly in pulmonary
a The assistance of Dr J. O'Neil, Dr M. Amdur, and Dr W. Busey in
the preparation of this chapter is gratefully acknowledged.
carcinogenesis studies. The distribution kinetics are different with
pulsed exposures compared with constant inhalation exposures. With
pulsed exposures, such as oral, intravenous, intraperitoneal, etc.,
the concentration of the test material will reach a peak and then
usually fall off, depending on the distribution coefficients for each
compound and organ in question. With continuous exposure,
concentrations in many body compartments will attain an equilibrium,
depending again on the concentration of the test material and the
distribution coefficient. Thus, quantitative toxicity is often quite
different, depending on the route of entry.
With the exception of certain drugs, man's exposure to
environmental agents comes either from skin contact, ingestion, or
inhalation. Inhalation is of particular importance in occupational
exposure and in exposure to air pollutants. For airborne substances,
the lung is the first organ that foreign chemicals encounter and it is
one of the body's first lines of defence. Chemicals that enter the
lung can either exert a direct effect on the cells of the lung, or be
absorbed into the systemic circulation. Blood passing from the lung to
the heart and then into the peripheral circulation can carry agents
directly to other organs without passing through the detoxication
processes of the liver. This contrasts with oral exposure where
chemicals may be absorbed into blood that immediately passes through
the liver and can be metabolically transformed into either more or
less toxic compounds.
Direct contact with irritants causes local inflammation in the
respiratory system, the degree of which may depend on the local
concentration and not on the total dose. For example, Kljackina (1973)
demonstrated that inhaled bromine acts as a specific irritant of the
respiratory system whereas bromine administered orally results in
changes in the nervous system.
Thus, specific reasons for performing inhalation studies include:
(a) determination of specific responses of the respiratory tract;
(b) assessment of the toxic hazard of agents whose principal route
of exposure is via inhalation; (c) investigation into the mechanism
of toxicity of inhaled materials; and (d) study of the comparative
toxicity of agents administered by different routes.
6.3 Fate of Inhaled Materials
6.3.1 Nature of aerosols
The nature and characteristics of aerosols have been treated in
detail elsewhere (Cadle, 1965; Mercer, 1973a,b; Raabe, 1970), but it
may be useful to review a few general concepts. Aerosols consist of
finely divided particles ranging in size from about 0.01 to 100 µm in
diameter. The particles in a cloud are usually of many sizes and the
size can be expressed as a size-frequency distribution curve which
usually best fits a log-normal distribution. There are several ways to
express the diameter of a particle, the common ones being count median
diameter, mass median diameter, and aerodynamic mass median diameter.
The last term is important as it considers each distribution as if it
were made up of unit density spheres and measures its diameter as if
it were acting aerodynamically like a unit density sphere. Thus,
aerosol behaviour can be compared, regardless of the individual shape
and density of the particles.
The division of a solid into fine particles that become airborne
results in a large increase in the surface area of the material. The
consequence is a generally increased chemical reactivity of the
material, thus accelerating all physical and chemical processes, such
as oxidation, dissolution, evaporation, absorption, and electrical
activity. The physiological activity of these particles also
increases. The sizes of interest from a biological standpoint range
from about 0.1 to 10 µm. Larger particles do not usually enter the
respiratory tract or, if they do, they are deposited in the nose.
6.3.2 Deposition
Material that enters the respiratory tract with the inspired air
can either be deposited or exhaled. Many factors affect the deposition
of particles or the absorption of vapours in the respiratory system.
Retention of vapours is governed by the diffusion rates of the vapour,
the solubility of the vapour in the various body compartments, and the
degree to which these compartments have attained the equilibrium. This
in turn, depends on the duration of the exposure, the concentration,
and the rate of removal of the vapours. This subject has recently been
reviewed by Stupfel & Mordelet-Dambrine (1974).
Many more factors govern the deposition of particulates in the
respiratory tract. Roe (1968) has divided these into physical and
chemical characteristics of the particle, anatomical, physiological,
and pathological factors. The size, density, and shape of the
particles are the physical variables that determine their aerodynamic
behaviour. The Task Group on Lung Dynamics (Morrow et al., 1966) has
discussed deposition as a function of particle size.
Three distinct physical processes act on particles suspended in
the atmosphere to cause them to be deposited in the respiratory tract.
Inertial impaction results from the tendency of particles to move in
straight lines. The repeated branching and subdivision of the
respiratory tract causes particles to be impacted on surfaces,
particularly near the bifurcations. Inertial forces are greater with
larger particles. Sedimentation due to gravity also causes particles
to strike the surface of the respiratory tract. Finally, Brownian
movement, particularly of smaller particles, causes them to be
deposited in the lung. The effectiveness of these mechanisms depends
on the anatomy of the respiratory tract, the size of the particle, and
the breathing pattern. During artificially induced hyperventilation in
rabbits, more material was deposited in comparison with controls
(Velickovskij & Kacnelson, 1964). Normal deposition and deposition in
diseased states are discussed in detail by Albert et al. (1973), Brain
& Valberg (1974), Goldberg & Laurenco (1973), Macklem et al. (1973)
and Stuart (1974).
Aerosols are deposited all along the respiratory tract. Large
particles (5-10 µm) are mainly deposited in the upper respiratory
tract, including the nasal cavity. The depth of penetration increases
as particle size decreases and particles in the 1-2 µm range are, for
the most part, deposited in the alveoli. As a first approximation, 25%
of inhaled particles are exhaled, 50% are deposited in the upper
respiratory tract, and 25% are deposited in the lower respiratory
tract (Morrow et al., 1966).
6.3.3 Clearance
Soluble particles readily dissolve at the site of deposition.
Generally, they enter the bloodstream and then behave as if they had
been intravenously injected. Insoluble particles can be removed from
the respiratory tract by several mechanisms depending on the site of
deposition. Particles that are deposited on the mucous blanket are
carried towards the pharynx by the cilia and are usually swallowed or
expectorated. Hence, it is impossible to completely separate
respiratory exposure from gastrointestinal exposure. The rate of
clearance by the mucociliary escalator has been measured in man by a
number of investigators (Albert et al., 1969, 1973; Camner et al.,
1972, 1973; Morrow, 1970; Sanchis et al., 1972) and animals (Albert et
al., 1973; Thomas, 1969; Velickovskij & Kacnelson, 1964; Watson et
al., 1969).
Particles that are deposited in the deep non-ciliated portion of
the lung clear more slowly. One mechanism responsible for alveolar
clearance is phagocytosis. Although alveolar macrophages engulf
particles within a few hours, there is evidence that macrophages do
not carry them actively to the mucociliary escalator in the first few
days following inhalation (Camner et al., 1977). Recent studies
indicate that there are other mechanisms of alveolar clearance. Tucker
et al. (1973) have shown alveolar clearance by normal interstitial
drainage pathways. Casarett (1972) and Morrow (1973) described a
possible mechanism whereby particles could move from the alveolus up
to the terminal bronchiole, and then to the ciliated epithelium for
removal on the mucus. Very fine particles can also enter the blood
directly through the lymphatic system. Finally, particles can dissolve
and be absorbed into the bloodstream. Some particles remain in the
alveolar region of the lung for considerable periods of time, during
which dissolution forces can operate.
6.4 Dose in Inhalation Studies
The term dose has many meanings depending on the background and
expertise of the investigator. In toxicology, dose is usually defined
as the mass of material introduced into the animal, and it is often
divided by the body weight. Hence, toxicologists often speak of dose
in mg/kg. Even this is misleading, to some extent, because the
material may not interact in any way, and because of other reasons
that become apparent in species comparisons. The actual dose or amount
of a substance entering the internal milieu of the body depends on the
concentration and the particle size of the inhaled material, the
duration of the exposure, and the breathing variables of the test
species. Thus, the actual absorbed dose is difficult to determine and
inhalation toxicologists often refer to exposure conditions instead of
dose. The exposure must be defined both in terms of concentration (C)
and time (t), and is sometimes expressed as the product of these two
(Ct) (MacFarland, 1968) (see also Chapter 1). While the Ct product
is not a true dose, it can be used in a similar fashion. Haber (1924)
recognized this and Haber's rule states that, for a fixed Ct
product, the response will be the same. This rule holds reasonably
well over limited ranges of C and t, but deviations occur when
extreme values of the variables are examined.
More frequently, inhalation toxicologists keep the time constant
and vary the concentration of the test material. Hence, they measure
an LC50, i.e. the median concentration to which animals are exposed
for a specified time that will kill 50% of the animals within a fixed
period of time after exposure. It is implicit in LC50 data that the
durations of both the exposure and post-exposure period are
standardized. Comparative LC50 data are often obtained for 4-h
exposures and a 14-day post-exposure observation period (Carpenter et
al., 1949; Pozzani et al., 1959).
6.5 Choice of Species
The ideal subject for studies relevant to man is man himself.
However, human volunteers can only be used where the toxicological
hazard is already reasonably well defined and accepted. Human studies
have been conducted recently with chlorinated hydrocarbon solvents and
common air pollutants (Andersen et al., 1974; Hazucha et al., 1973;
Stewart, 1972; Stewart et al., 1970a,b, 1973). These experiments were
well controlled and monitored and the exposure levels were low.
Rats, dogs, and monkeys have been the species most used for
inhalation toxicity studies, although investigators have also used
mice, hamsters, guineapigs, rabbits, cats, miniature swine, and
donkeys. The choice of species should be made, primarily, with a view
to extrapolating the experimental results to man. However, choice on
this basis alone is difficult since the validity of such an
extrapolation is often uncertain. When selecting the particular
species for study, the following factors must be considered: the
comparative morphology of the respiratory tract; the presence or
absence of lung disease or susceptible states; and the similarity of
biochemical and physiological responses to those in man.
6.5.1 Anatomical differences
The respiratory systems of various laboratory animals and man
differ widely. As man is a primate, it is sometimes erroneously
assumed that the monkey is a good model for inhalation studies, but
marked differences are noted when comparing human and sub-human
primate respiratory systems. In the monkey, the end airway is always a
respiratory bronchiole, whereas this is rarely the case in man.
Furthermore, the monkey's lung is not lobulated.
The most commonly used laboratory animals are quadrupeds, and
their respiratory systems are horizontal and not vertical as in the
primate. This is important because the aerodynamics of a horizontally
arranged lung are different, and particle deposition will also be
different. In human subjects, maximum dust deposition is in the upper
portion of the lung; in experimental animals, maximum deposition in
the more ventral portions of the lung is usual.
The gross anatomy of the respiratory systems of the various
laboratory animals and man is quite different. In animals such as the
rat and guineapig, the nose contains highly developed tortuous
turbinates. In monkeys and man with relatively smaller noses, the
turbinates are less complex. These differences in nasal anatomy are
especially important in studies involving exposure to particulates.
More particles impinge in more complex turbinate systems and will not,
therefore, reach the deeper portions of the respiratory system.
The subgross anatomy of the lungs of various laboratory animals
and man has been detailed and compared by McLaughlin et el. (1961a,b,
1966). The authors have grouped the common laboratory animals and man
in three basic categories based on subgross pulmonary anatomy
(Table 6.1). They grouped the various animals on the basis of lung
lobulation, the presence or absence of respiratory bronchioles, the
presence or absence of terminal bronchioles, pulmonary artery/
bronchial artery shunts, and the termination of the bronchial
arteries. Their studies indicate that the pulmonary anatomy of the
horse most closely resembles that of man.
6.5.2 Physiological considerations
Pertinent differences in the lung physiology of various species
must be considered in inhalation toxicology. Normal values or ranges
for several species, including man, are summarized in Table 6.2
(Sanockij, 1970a).
Table 6.1 Comparative subgross anatomy of the lunga
Species Lung No. of lobes Lobulation Respiratory Terminal Pulmonary/artery Termination Pleura
type bronchioles bronchioles Bronchial/artery of bronchial
left right shunts arteries
Cow I 3 5(4) well developed extremely poor present present distal airway thick
development
Sheep I 3 4 well developed extremely poor present present distal airway thick
development
Pig I 3 4 well developed extremely poor present not present distal airway thick
development
Monkey II 3 4 not present very well absent not present distal airway thin
developed
Dog II 3 4 not present very well absent not present distal airway thin
developed
Cat II 3 4 not present very well absent not present distal airway thin
developed
Guineapig IIa 3 4 not present fairly well absent present distal airway thin
developed
Rat IIa 1 4 not present fairly well absent few present distal airway thin
developed
Rabbit IIa 3 4 not present fairly well absent present tertiary thin
developed bronchus
Horse III (3) (4) imperfectly poorly present present distal airway thick
developed developed and alveoli
Man III 2 3 imperfectly poorly present present distal airway thick
developed developed and alveoli
a From: McLaughlin et al. (1961a,b, 1966).
Table 6.2 Some physiological indices of man and animalsa
Man Dog Cat Rabbit Guineapig Rat Mouse
Body surface (m2) 1.8 0.528 0.2 0.18 0.040 0.030 0.006
Relation body surface to body weight 0.0257 0.044 0.066 0.072 0.12 0.15 0.3
(m2/kg)
Basal metabolism (kJ/kg) 105 222 -- 188 360 615 711
Frequency of respiration (min) 14-18 10-30 20--30 50-100 80-135 110-135 140-210
Size of alveoli (µm) 150 100 100 -- -- 50 30
Surface of lungs (m2) 50 100 7.2 5.21 1.47 0.56 0.12
Relation of lung surface to body weight 0.7 8.3 2.8 2.5 3.2 3.3 5.4
(m2/kg)
Inhaled air (ml) 616 40-60 -- -- 1.75 0.865 0.154
Lung ventilation (ml/min) 8732 -- 1000 600 155 73 25
Relation of lung ventilation to body 0.13 -- 0.30 0.29 0.33 0.05 1.24
weight (ml/min/g)
Consumption of oxygen (ml/kg/hr) 203.1 3600 9420 522.7 2180 2199 3910
Elimination of CO2 (ml/kg/h) 168.8 -- -- -- -- 2650 4240
Coefficient of respiration 0.82 -- -- 0.83 -- 0.82 0.85-1.33
Pulse frequency for 1 min 70-72 90-130 120-180 150-240 206-280 300-500 520-780
a From: Sanockij (1970a).
6.5.3 Disease and susceptibility states
Most toxicological investigations are performed on healthy
animals. However, epidemiological studies have indicated that during
air pollution episodes the populations at greatest risk are the young,
the aged, and those people with pre-existing cardiopulmonary disease.
One task of the inhalation toxicologist is to identify those segments
of the population that are particularly susceptible to the presence of
airborne contaminants. Certain animal models of diseased or stressed
states have been described (Boyd et al., 1974; Drew & Taylor, 1974;
Silver et al., 1973; Taylor & Drew, 1975; see also Chapter 2) which
could be used or could be adapted for use in inhalation studies. The
most commonly used model of this nature is the papain-induced
emphysematous animal (Gross et al., 1965; Martorana et al., 1973;
Niewoehner & Kleinermann, 1973; Snider et al., 1974). Both aged and
neonatal animals could be used as models of high susceptibility
groups. Disease and susceptibility states are important considerations
in the selection of the species and, in some cases, even the strain of
animal to be investigated since it is well known that the incidence of
cancer differs considerably among certain species and strains.
For example, Kuschner et al. (1975) recently reported a high
incidence of respiratory tract tumours in rats after exposure to
oxybis[chloro-methane] (bis(chloromethyl)ether) but only a few tumours
in hamsters. These tumours were about equally divided between
esthesioneuro-epitheliomas and bronchogenic squamous cell carcinomas.
Leong et al. (1975) repeated these experiments; however, all the
tumours in Leong's study were nasal tumours with no bronchogenic
carcinomas. The only difference noted in the protocol was that Leong
et al. used specific-pathogen-free, caesarean-derived rats, whereas
Kuschner et al. used Sprague-Dawley rats, that were not
specific-pathogen-free.
6.6 Duration of Exposure
Acute inhalation toxicity studies usually consist of a single
exposure (or occasionally a few exposures) of not more than 8 h.
Repeated exposure studies consist of a number of daily exposures for
fixed periods of time. Occasionally, investigators terminate exposure
after several days or weeks, and then maintain the animals in the
colony to observe delayed development of long-term effects (Kuschner
et al., 1975).
The duration of chronic studies varies considerably. One logical
proposal is based on the life span of the test species (Sanockij,
1970b). If, for example, one considers that toxic signs will appear in
man after an exposure over 10% of his life span (7 years), animals
should also be exposed for 10% of their life span. Thus, rats should
be exposed for 3-4 months, and larger animals for a somewhat longer
period. Some authors (Sidorenko & Pinigin, 1970) consider that even
continuous exposure for 3-4 months is insufficient to simulate
lifetime exposure in man, and many studies last longer, some up to 5
years (Lewis et al., 1974). Powell & Hosey (1965) consider the minimum
duration of a chronic study to be one year, the animals being exposed
6 h a day for 5 days a week. This corresponds to a significant portion
of a rodent's lifetime.
Chronic studies are conducted to determine the effects of
long-term exposure to compounds and particularly (in the USSR) to
establish minimum effect levels (Limch). In order to evaluate the
effects of long-term exposures, such as elevated incidences of
infection, emphysema, or the induction of cancer, inhalation controls
should be run concurrently. Two species are usually used. Occasionally
the toxic effects and the mechanism of chronic toxicity are entirely
different from those manifested in acute exposures. Benzene, for
example, is a central nervous system depressant at high
concentrations, while, at low concentrations over long periods of
exposure, it affects the hematopoietic system.
The selection of concentration for chronic studies is difficult.
For example, in the USA, concentrations are chosen that do not produce
mortality and produce only minimal changes in other biological indices
of toxicity during limited studies. In the USSR, the concentration
selected is below the Limac. In order to investigate dose-response
relationships, it is advisable to use at least three different dose
levels, hoping that the highest level chosen will produce quantifiable
effects and that the lowest level selected will produce minimal or
even no effects. In the USSR, research workers often use as the
highest level the concentration which, in single short-term exposures
of human subjects, does not produce any effect during the study of
reflex reactions.
When investigating the various biological indices of toxicity, it
is necessary to carry out measurements more frequently during the
early stages of the study (Camner et al., 1972, 1973). Studies carried
out at the Institute of Labour Hygiene and Occupational Medicine of
the USSR show that the time to the display of the first signs (period
for initial decompensation) varies considerably. Thus, the variables
are recorded at 1, 4, 8, 15, and 30 days and monthly thereafter. After
terminating the exposure, the frequency of measurements may be
increased again to record any early changes.
6.6.1 Intermittent versus continuous exposures
Long-term exposures are usually patterned on projected industrial
experience, giving the animals a daily exposure of 6-7 h, 5 days a
week (intermittent exposure), or on a possible environmental exposure,
with 22-24 h of exposure per day, 7 days a week (continuous exposure),
with about an hour for feeding the animals and maintaining the
chambers. In both cases, the animals are usually exposed to a fixed
concentration of test materials. Thus, neither situation approaches
actual human experience, where concentrations of atmospheric
pollutants are continuously fluctuating by one or two orders of
magnitude. A major difference to consider between intermittent and
continuous exposure is that with the former there is a 17-18 h period
in which animals may recover from the effects of each daily exposure,
and an even longer recovery period at weekends. The recovery period in
some cases is extremely important as in the case of continuous versus
intermittent exposure to dichlormethane (Haun, 1972).
The choice of intermittent or continuous exposure depends on the
objectives of the study and on the human experience that is to be
simulated. However, certain technical difficulties must be considered.
For example, the advantages of continuous exposure for simulating
environmental conditions may be offset by the necessity of watering
and feeding during exposure, and by the need for more complicated (and
reliable) aerosol and vapour generation and monitoring techniques.
Intermittent systems require simpler chambers, since provision for
food and water is not necessary. The contaminant dispersal systems are
also simpler as they need to operate for only 6-7 h per day.
6.7 Inhalation Systems
6.7.1 Facilities required
It is more advantageous to build facilities designed specifically
for inhalation studies than to modify existing buildings. High
cellings are necessary for housing exposure chambers and related
equipment, such as aerosol and vapour generators, filters, flowmeters,
etc. A constant supply of clean filtered air with temperature and
humidity controls should be available for both the chamber rooms and
the chambers themselves. Adequate floor space should provide access to
at least two sides of the chambers, and the chambers themselves should
be separated by a small space to avoid heat transfer between chambers.
6.7.2 Static systems
Inhalation systems can be characterized as static, when the agent
is introduced into a chamber as a batch and then mixed, or dynamic,
when airflow and introduction (and removal) of agent are continuous.
The duration of static exposure is limited by: (a) the gradual
depletion of oxygen; (b) the accumulation of carbon dioxide;
(c) the accumulation of water vapour; and (d) the gradual increase
in temperature inside the chamber. In spite of these limitations,
static systems are of great practical usefulness in assessing acute
toxicity, particularly when the supply of material is limited.
Procedures similar to those described by Draize et al. (1959) are in
use today for screening commercial products. Another use of static
systems is for the exposure of animals to biological aerosols. It is
difficult to generate a viable biological aerosol particle
continuously because of the limited amount of material available;
thus, material is dispersed in a large chamber, mixed, and the animals
exposed through nose tubes connected to the test atmosphere (Jemski &
Phillips, 1965).
6.7.3 Dynamic systems
Today, most inhalation facilities use dynamic systems where the
airflow and introduction of agents are continuous. The theoretical or
nominal concentration of chemicals in a chamber can be calculated as
follows:
flow of chemical
concentration =
flow of air
Many factors, including wall loss, losses on the skin and fur of
animals, and uptake by the test animals, cause the actual
concentration to be somewhat less than the nominal concentration.
Thus, the concentration should always be measured by an appropriate
instrument or technique rather than reporting the nominal
concentration.
When material is introduced into a chamber, the concentration
builds up exponentially according to equations originally described
and verified by Silver (1946). If perfect mixing occurs, the
concentration can be calculated according to the following equation:
C = (w/b) (1 - exp (-bt/a)) (1)
where C = the concentration of material at time t; w = amount of
material introduced per minute; a = volume of the chamber;
b = flow of air through the chamber.
The fraction of equilibrium concentration (w/b) attained in
time t is:
C
= 1 - exp (1 bt/a) (2)
w/b
Thus, the time required to reach 99% (t99) of the equilibrium
concentration is:
0.99 = 1 - exp (-bt99/a) (3)
or
t99 = 4.6052 a/b
This equation may be given the general form:
tx = Ka/b (4)
where x equals % nominal concentration attained in time t and
microtomes, that cut the normal paraffin blocks with glass knives. Of
course, this procedure should only be followed when specific details
have to be followed during the microscopic examination. The use of
semithin Epon sections yields even better results.
It will often be necessary to use special staining techniques on
tissues in order to provide more information on the presence of
carbohydrates, proteins, fats, elements, or certain structural
organizations. Special fixation is sometimes necessary for appropriate
staining. Bones and calcified tissues have to be decalcified. Eyes
usually need to be fixed and embedded differently. Blood vessels may
be stained with Sudan black in order to study vascular lesions.
5.9 Special Techniques
5.9.1 Enzyme histochemistry
Enzyme histochemistry is a technique used to detect the activity
or presence of an enzyme in a tissue by incubating the fresh tissue in
an appropriate medium, so that a fine coloured granular precipitation
forms wherever the enzyme is present.
The use of the enzyme-histochemical technique is not common in
toxicity testing. Nevertheless, the technique is a valuable one, since
it provides information about the metabolic activity and function of
the tissues. Furthermore, it introduces the possibility of correlating
biochemical with histological findings that may lead to a more correct
interpretation. Enzyme-histochemical investigations also make it
possible to visualize certain differences in enzyme activity within
the structural organization of tissues.
In some cases, a decrease in enzyme activity in certain cells is
compensated for by an increase in other cells within the same organ.
Such biological differences can only be detected by
enzyme-histological investigation, when the results of biochemical
determinations are within normal limits and no alterations are seen
using conventional histological techniques. Enzyme-histological
investigations can easily be incorporated in routine toxicity testing,
if necessary.
Relatively small pieces of tissue are quickly frozen in an inert
liquid (i.e. isopentane), or cooled in liquid nitrogen or a mixture of
solid carbon dioxide and methanol. Storage of the tissues is effected
at -70°C. Cryostat sections are prepared and incubated in specially
prepared media. Good reference works for the methods have been
published (Barka & Anderson, 1965; Pearse, 1968, 1972). To obtain
optimum information, the prepared section should be examined by
semiquantitative methods.
An enzyme histochemical investigation can also be performed at
the electron microscope stage (Geyer, 1973). In this case, the
activity or presence of an enzyme is determined by the deposition of
electron-dense material at the sites where the enzyme is located. It
is evident that these delicate techniques are not easy to apply in
routine toxicity testing. They may, however, be of great importance in
specific studies on the biological effects of a compound on cellular
components, or to detect early damage.
5.9.2 Autoradiography
Autoradiography is based on the principle that radioactive
substances present in tissues are able to produce an image on a
photographic film or plate. The radiations emitted by the radioactive
substances must be of relatively weak energy, so that they will have a
short range in tissues and emulsions. When the range is too long,
developed silver grains can be found in the emulsion far away from the
radioactive source.
In this respect only alpha particles, beta particles, and Auger
electrons are useful since electromagnetic radiations such as gamma
and X-rays give poor results due to their penetrating power.
Tritium (3H) has been most frequently used in autoradiographic
studies, although 14C and 32P are also being used as tracers.
Autoradiography, at both light microscopic and ultrastructural
levels, can be used in kinetic studies on tissues by incorporating
radioactive-labelled bases in DNA or RNA resulting in the production
of silver grains on a photographic film covering the section. The
different methods have been extensively described for nondiffusible
substances (Baserga & Malamud, 1969; Rogers, 1967) as well as for
diffusible substances (Roth & Stumpf, 1969).
Apart from studying the kinetics of tissues, autoradiographic
techniques can also be used to study the distribution of radiolabelled
compounds and their metabolites. This, again, can be done at light
microscopic and ultrastructural levels.
The technique of whole-body autoradiography as developed by
Ullberg et at. (1972) is a valuable tool, in this respect, since the
distribution of a compound can qualitatively (or even
semiquantitatively) be studied in the whole body. In addition, pieces
can be cut out to use for microautoradiographic research.
Substances may also be labelled with fluorescent chemicals
permitting their detection by illumination of the sections with
ultra-violet radiation. The technique of fluorescence microscopy can
also be used for the detection of certain dyes that possess
autofluorescing properties. Some substances, such as the
catecholamines, can be made visible by reaction with formaldehyde
which converts mines into fluorescent substances (Eränko & Räisänen,
1966). Fluorescence in tissues can be measured quantitatively, thus
facilitating controlled conditions (Ploem et al., 1974).
Autoradiographic studies cannot be incorporated easily into
routine toxicity experiments. However, the technique may be a very
important tool in special studies.
5.9.3 Immunofluorescence and immunoenzyme techniques
Immunofluorescence techniques are primarily used in determining
the presence or absence of certain antibodies or antigens in tissues.
Antigens are detected by binding them to specific antibodies. If a
specific antiserum is conjugated with a fluorescent dye, the specific
antigen-antibody complex formed can be seen by studying the tissue
section using ultraviolet radiation microscopy. This direct
immunofluorescent technique can be replaced by a more sensitive
indirect method in which the substance actually rendered fluorescent
is not the antigen under consideration but an intermediate material
the distribution of which corresponds precisely to that of the antigen
being studied. Information on the principle of these techniques is
available (Goldman, 1968; Nairn & Marrack, 1964).
The use of enzyme conjugates has recently been developed
(Sternberger, 1974). This system is used when antigen-antibody
complexes have to be localized at ultrastructural levels.
Immunofluorescence or immunoenzyme techniques will not be used in
most routine toxicity experiments, but they may be of importance in
special studies such as those described in Chapter 7.
5.9.4 Electron microscopy
Transmission electron microscopy is the most commonly used
technique for studying the ultrastructure of tissues. Different
fixation, embedding, and cutting techniques have to be used to obtain
ultrathin sections of tissues (Flauert, 1973, 1974, 1975; Hayat, 1970,
1972, 1973). At the ultra-structural level, very minute changes can be
detected and they have to be distinguished from the many artifacts
that can be introduced during the different processing procedures.
Under optimum conditions, tissues can be examined at high
magnifications.
As tissue examination by electron microscopy is very time
consuming and costly, it is important only to select tissues from
those experiments that justify it. Ultrastructural studies have
contributed enormously to our knowledge of molecular biology. In the
case of toxicology, ultrastructural studies are always carried out as
a secondary investigation, and are usually applied to
ultrastructurally, specific, pathological changes already studied in
detail at the light microscopic level, in order to secure a better
judgment of the importance of the lesion. Ultrastructural studies are
also used to confirm that target organs that appear normal at a
certain close level using light microscopy, do not in fact show
pathological changes.
Scanning electron microscopy (SEM) techniques, that have come
into use in recent years, provide 3-dimensional images of complete
biological units and also chemical information (Hayes, 1973). Here
again, special processing procedures may be needed, although, in
certain cases, formalin-fixed and paraffin-embedded material can be
used. Natural surfaces, dissected material, sectioned tissue, and
living specimens may be studied. SEM is a valuable tool in biology
but, for toxicological investigations, its use is still rather
restricted, apart from the possibility of obtaining quantitative
chemical information. The development of analytical electron
microscopy, using the principles of X-ray microanalysis, also makes it
possible to correlate tissue ultrastructure and chemistry (Hayes,
1973; Weavers, 1973).
5.10 Microscopic Examination
Routine histopathological examination is very important and
should be carried out correctly. First of all, the sections to be
studied should be of good quality. Additional sections and special
stains must be prepared if necessary. Special stains are used to study
and describe individual lesions; they may also be used to examine
certain organs or lesions to permit better judgment of
semiquantitative comparison. For example, when haemosiderosis is found
to be an important effect, special stains, based on the reaction of
the dye with iron, facilitate semiquantitative analysis of the degree
of the lesion.
Good microscopic equipment is necessary to perform optimum
microscopic examination. Sources of ultraviolet radiation and
polarized light should be available.
The microscopic examination must be carried out by well-trained
pathologists or other persons trained and experienced in the field of
laboratory animal pathology. The use of parapathologists for routine
microscopy in toxicity and carcinogenicity experiments is extremely
valuable and is important for overcoming a shortage of personnel,
trained in laboratory animal pathology (Toxicol. appl. Pharmacol.,
1975). Experience has shown that well-trained parapathologists can
become very skilful in microscopy and in screening sections for
abnormalities. With further experience, they are also able to describe
certain lesions. It is the pathologist's duty to see that all lesions
observed are described and interpreted correctly, and to check the
sections for any lesions that may have been overlooked.
It is a serious mistake, in an experiment, to undertake
microscopic examination before the results of other examinations such
as biochemical determinations, haematology, and organ weights, are
available, for the information obtained from these procedures may give
important directions for the microscopic study. For example, an
increased thyroid weight may lead to more careful examination of the
thyroid for hyperplasia and may prompt histometric determinations.
Lower lymph node weight may point to semiquantitative examination of
these organs with regard to reduced immunocapacity (Cottier et al.,
1972). Differences or changes in the blood picture call for more
detailed study of the bone marrow, for example, by preparing and
studying 1 µm sections, to detect suspected haemopoietic disturbances.
Gross observations should always be correlated with microscopic
findings. It is a false assumption to think that a microscopic
examination will be more objective when performed blindly; knowledge
of clinical signs and macroscopy are indispensable for a meaningful
examination. Of course, the pathologist must be careful not to let
knowledge of clinical effects influence his objective evaluation of
the tissues; thus, sections of the tissues considered to be involved
may have to be re-examined blindly. If such a re-examination is
carried out, semiquantitative scoring of the extent of the lesion will
also help to detect a possible dose-effect relationship. Should there
be any doubt in interpreting the significance of a lesion, it is
advisable to consult other pathologists, all of whom should re-examine
the slides blindly.
5.10.1 Number of animals and number of organs and tissues studied
microscopically
In acute and rangefinding tests, the organs and tissues are
usually fixed and examined microscopically after processing. In
subacute and long-term studies, and, sometimes, in rangefinding tests,
it is customary initially to examine only the tissues of the highest
dose group, the control group and the target organs, if known. In
addition, all grossly observed lesions are processed in the
intermediate and low-level groups. If the results of the microscopic
examination of the highest dose group indicate a need to examine
certain organs at lower levels, this can be done at a second stage.
In chronic toxicity experiments, all males and females of the
highest dose group should be examined. It is also advisable to examine
all control animals completely, since this will be the only way to
ascertain the incidence of tumours and other "lesions" normally
occurring in the strain of animals used. Such information is
indispensable for correctly evaluating the significance of changes
observed in exposed animals.
In carcinogenicity experiments, where larger numbers of animals
per group are usually used, some laboratories restrict microscopic
examination mainly to the grossly observed abnormalities and tumours,
and perform complete histological examinations on only 15-20 male and
15-20 female survivors of the highest dose group and of the control
group, on the assumption that a well-performed gross examination will
detect most of the tumours present. Histological examination of all
tissues of 15-20 animals of each sex will give some information on the
possible presence of certain pre-malignant hyperplastic or neoplastic
changes not grossly observed. If such lesions are found, the
histological examination should cover all animals, while small organs
which may bear tumours that cannot be grossly observed (thyroid,
pituitary, adrenals) must be examined histologically.
5.10.2 Description of the lesions
For the microscopic examination, it is essential to use a
check-list to detect losses, especially of small organs lost in the
processing procedure. All organs and tissues examined should be
listed, even though no abnormalities are seen, while all lesions found
should be described clearly and accurately. It is essential to
describe lesions in a semiquantitative way using such words as slight,
moderate, strong, and very strong to indicate the extent of the
lesion; it is also essential to indicate the criteria used to
investigate and classify abnormalities. It will often be necessary to
re-examine certain lesions in order to obtain a well-balanced
semiquantitative judgment. Several reference works may be of help in
the classification of tumours and other lesions (Beveridge & Sobin,
1974; Cotchin & Roe, 1967; Ribelin & McCoy, 1965; and Turusov, 1973).
Certain lesions found by microscopic examination may, in fact,
consist of several entities. In such cases, it may be important to
classify these entities independently. For example, perilobular liver
degeneration may be combined with bile duct proliferation, but one of
the two phenomena may be more extensive than the other with respect to
dose level and time. For a better interpretation, it is preferable to
describe and classify such lesions independently.
5.11 Presentation, Evaluation, and Interpretation of Pathological
Data
A well-performed and well-described microscopic examination is
not complete if the results cannot be studied and compared easily and
adequately. The lesions found in the different groups should be
tabulated according to organ, group, and sex in order to facilitate
comparison of the incidence of such lesions in the different groups.
If, in addition, a semiquantitative estimate of the extent of the
lesions is made, it will be easy to see whether they are
compound-induced and increase with time and dose.
It is important that these tables include details such as the
number of animals necropsied and the number examined microscopically,
per group and sex. In addition, it is advisable to state, the exact
number of organs or tissues examined microscopically, since it is well
known that, in every toxicity experiment, organs and tissues get lost
during the processing procedure. In this way, the loss can be
adequately estimated and the lesions found can be expressed against
the real number of organs or tissues examined.
For correct interpretation of the tumour tables, it is advisable
to include hyperplastic and preneoplastic lesions as individual
groups. The table should also include the number of days that elapsed
from the start of the experiment until the observation of each rumour.
This first observation may be clinical, for example, when mammary, ear
duct, or skin tumours are involved and the time that has elapsed can
then be called the "induction time". However, usually, tumours are
found after death or killing in extremis, or at sacrifice at the end
of the experiment. Thus, in these cases, it seems most appropriate to
use the term "time of observation". The inclusion of the "time of
observation" of tumours in the table is of help to the investigator
since it provides information on shorter or prolonged latency periods
(e.g. time that has elapsed between initiation and the appearance of
tumours). In addition, it reminds the reader to take into account the
survival differences when expressing the results.
In long-term experiments, the number of lesions found may be
quite large. Furthermore, some lesions may have been noticed during a
certain period in life, when the animals died or were sacrificed
in extremis, while others will have been noticed at the end of an
experiment. When such lesions are tabulated in the same table, this
information may get lost. It seems, therefore, most appropriate in
such long-term experiments to pool the results of the pathological
examination for certain periods, for example the first 12 months of
life and then for 3- to 6-monthly intervals, and to give the results
of the examination undertaken at the end of the experiment separately.
It is obvious that in preparing such tables, the use of an
automated data acquisition and computer-based system will be of great
help. These systems have been described for application to
toxicological studies, especially for animal weights, food and drug
consumption, organ weights, and biochemical data (Munro et al., 1972).
In pathology, such systems offer considerable potential. The necessity
for the correct coding of pathological changes, and the use of a code
system set up so that detailed information about lesions can still be
obtained, is obvious. Several systems have been described (Becker,
1973; Enlander, 1975; Smith et al., 1972), and may be adapted for this
purpose.
Pathological examinations must always be evaluated and
interpreted in connexion with other phenomena found. It must be
decided whether lesions noticed are in fact pathological, and whether
they are found in connexion with changes in other variables such as
organ weights, biochemical tests, etc. If compound-induced lesions are
found, they may not only be present at the highest dose level, but
also at an intermediate dose level. In the latter case, it is
important to determine whether there is a dose-dependent increase in
the severity and extent of the lesion, a finding which would
strengthen the assumption that the lesion is compound-induced.
Moreover, a clear dose-response relationship permits better evaluation
of the potential risk of a compound. Whenever lesions are found only
at the highest dose level, the sections at all dose levels should be
re-examined blindly to remove any doubt. Often the two sexes will show
a different sensitivity to the action of a compound.
Problems may arise when certain lesions are found more frequently
and to a greater extent in the treated groups compared with the
control group and compared with the common incidence of the lesion in
the strain used. The usual attitude towards this phenomenon is to
consider it as an effect or response.
In the evaluation of tumour incidence, criteria such as increased
tumour incidence, decrease in latency period, and the appearance of
tumours in organs where they do not occur spontaneously, have to be
considered. For this reason, detailed information on the spontaneous
occurrence of tumours in the species and strain used is essential,
particularly when tests indicate that the compound possesses a weak
carcinogenic action. Simple comparison of tumour incidence in control
animals and experimental animals at one point in time may lead to
erroneous conclusions, especially when only one of the above three
criteria is met. Many factors other than treatment may influence the
incidence of spontaneous tumours.
Statistical evaluation of the results is often necessary, but, as
yet, there are no agreed procedures for comparing statistically the
incidence of malignant tumours in controls and experimental groups.
REFERENCES
BARKA, T. & ANDERSON, P. J. (1965) Histochemistry: theory, practice
and bibliography. New York, Harper & Row; London, Evanston.
BASERGA, R. & MALAMUD, D. (1969) Autoradiography: techniques and
application. New York, Harper & Row; London, Evanston.
BECKER, H. (1973) Experiences with a test-stage free-text processing
system in pathology, using display terminals. Path. Europ.,
8: 307-313.
BEVERIDGE, W. I. B. & SOBIN, L. H., ed. (1974) International
histological classification of tumours of domestic animals.
Bull. World Health Org., 50: 1-142.
CHEVALIER, H. J. (1971) [A method for the fixation of the lungs of
small animals.] Z. Versuchstierk., 13: 101-104 (in German).
COHRS, P., JAFFE, R, & MEESEN, H. (1958) [Pathology of laboratory
animals] Berlin, Springer-Verlag, Vol. 2 (in German).
COTCHIN, E. & ROE, F. J. C. (1967) Pathology of laboratory rats and
mice. Oxford, Edinburgh, Blackwell.
COTTIER, H., TURK, J., & SOBIN, L. (1972) A proposal for a
standardized system of reporting human lymph node morphology in
relation to immunological function. Bull. World Health Organ.,
47: 375-408.
ENLANDER, D. (1975) Computer data-processing of medical diagnosis in
pathology. Am. J. clin. Pathol., 63: 538-544.
ERÄNKO, O. & RAISANEN, L. (1966) In vitro release and uptake of
noradrenaline in the rat iris. In: Ebler, U.S. V., Rosell, S. &
Uunäs, B., ed. Mechanism of release of biogenic amines. New
York, Pergamon Press.
FAWELL, J. K. & LEWIS, D. J. (1971) A simple apparatus for the
inflation fixation of lungs at constant pressure. Lab. Anim.,
5: 267-270.
FLAUERT, A. M. (1973, 1974, 1975) Practical methods in electron
microscopy. Amsterdam, Oxford, North Holland Publ.; New York,
Elsevier.
GEYER, G. (1973) [Ultrahistochemistry, histochemical working
guidelines for electron-microscopy.] Stuttgart, Gustav Fischer
Verlag (in German).
GOLDMAN, M. (1968) Fluorescent antibody methods. New York, London,
Academic Press.
GORDON, H. W. & DANIEL, E. J. (1974) Microwave fixation of human
tissues. Am. J. med. Technol., 40(10): 441-442.
HAYAT, M. A. (1970, 1972, 1973) Principles and techniques of electron
microscopy. In: Biological Applications Vol. I, II, III, IV.
New York, Van Nostrand Reinhold Company.
HAYES, T. I. (1973) Scanning electron microscopy in biology. In
Koehler, J. H., ed. Advanced techniques in biological electron
microscopy, Berlin, Heidelberg, New York, Springer-Verlag,
pp. 153-214.
MUNRO, I. C., CHARBONNEAU, S. M., & WILLES, R. F. (1972) An automated
data acquisition and computer-based computation system for
application of toxicological studies in laboratory animals.
Lab. Anim. Sci., 22: 753-756.
NAIRN, R. E. & MARRACK, J. R. (1964) Fluorescent protein tracing.
Edinburgh, London, Livingstone.
PEARSE, A. G. E. (1968, 1972) Histochemistry, theoretical and
applied. 3rd ed., Edinburgh, Churchill Livingstone, Vol. I &
II.
PLOEM, J. S., DE STERKE, J. A., BONNET, J., & WASMUND, H. (1974) A
micro-spectrofluorometer with epi-illumination operated under
computer control. J. Histochem. Cytochem., 22: 668-677.
RIBELIN, W. E. & MCCOY, J. R. (1965) The pathology of laboratory
animals. Springfield, Charles Thomas.
ROE, F. J. C. (1965) Spontaneous tumours in rats and mice. Food
Cosmet. Toxicol., 3: 707-720.
ROGERS, A. W. (1967) Techniques of autoradiography. Amsterdam,
London, New York, Elsevier.
ROTH, L. J. & STUMPF, W. E. (1969) Autoradiography of diffusible
substances. New York, London, Academic Press.
SMITH, J. E., MCGAVIN, M. D, & GRONWALL, R. (1972) English-language
computer-based system for storage and retrieval of pathological
diagnosis. Vet. Pathol. 9: 152-158.
SONTAG, J. M., PAGE, N. P., & SAFFIOTTA, U. (1975) Guidelines for
carcinogen bioassay in small rodents. Bethesda MD, National
Cancer Institute.
STERNBERGER, L. A. (1974) Immunocytochemistry. Englewood Cliffs, NJ,
Prentice-Hall. Toxicol. appl. Pharmacol. (1975) 31: 1-3, A
training programme for parapathologists.
TURUSOV, V. S. ed. (1973) Pathology of tumours in laboratory animals,
Vol. 1, Lyon, IARC (Sci. Publ. No. 5).
ULLBERG, S., HAMMARSTROEM, L., & APPELGREN, L-E. (1972)
Autoradiography in pharmacology. In: Cohen, Y., ed. I.E.P.T.
Section 78, Oxford, New York, Pergamon Press, Vol. 1,
pp. 221-239.
WEAVERS, B. A. (1973) The potentiality of EMMA-4, the analytical
electron microscopy in histochemistry: a review. Histochem. J.,
5: 173-193.
6. INHALATION EXPOSUREa
6.1 Introduction
Advances in technology, throughout the world, have increased the
number and amount of chemicals in the atmosphere. The health effects
of inhalation of these chemicals can be predicted to some extent by
experimental investigation. In order to simulate environmental
conditions, a special technology has evolved relating to the design
and operation of inhalation chambers and the generation and
characterization of aerosols and vapours. This chapter will introduce
this technology and review the significance of certain biological
end-points commonly measured in inhalation studies.
This chapter is not intended to be all-inclusive, nor to provide
complete instructions on inhalation technology. No toxicological
protocol is sufficient to cover all situations with all materials.
Hence, the expertise of the individual investigator and the objectives
of the investigation will govern the selection of the final protocol
and the biological end-points relevant to the particular compound
concerned.
The costs associated with evaluation of toxicity by the
inhalation route are considerably higher than for toxicity studies
using other routes of exposure because of the cost of the specialized
equipment used in inhalation studies and the time required for its
calibration. In general, it can be estimated that the total cost of
any type of inhalation study, short- or long-term, will be two to
three times that of a comparable oral study. Because of this, it is
important to determine when inhalation studies should be performed and
when, and if, studies by other routes would suffice. Finally, in
addition to costs, other resources should be considered. In most
countries, the number of laboratories capable of performing inhalation
studies is limited. Thus, it is essential to develop priorities for
the selection of compounds for evaluation of inhalation toxicity.
6.2 Need for Inhalation Studies
The effect of a compound depends on its concentration at the
receptors of the affected organ or system. The concentration at
different sites and times is a function of the route of entry. Thus
the route of entry is an important factor with regard to toxicity. No
good substitute model for inhalation exposure as a means of direct
exposure of the lungs has been developed, although intratracheal
exposure has been frequently used, particularly in pulmonary
a The assistance of Dr J. O'Neil, Dr M. Amdur, and Dr W. Busey in
the preparation of this chapter is gratefully acknowledged.
carcinogenesis studies. The distribution kinetics are different with
pulsed exposures compared with constant inhalation exposures. With
pulsed exposures, such as oral, intravenous, intraperitoneal, etc.,
the concentration of the test material will reach a peak and then
usually fall off, depending on the distribution coefficients for each
compound and organ in question. With continuous exposure,
concentrations in many body compartments will attain an equilibrium,
depending again on the concentration of the test material and the
distribution coefficient. Thus, quantitative toxicity is often quite
different, depending on the route of entry.
With the exception of certain drugs, man's exposure to
environmental agents comes either from skin contact, ingestion, or
inhalation. Inhalation is of particular importance in occupational
exposure and in exposure to air pollutants. For airborne substances,
the lung is the first organ that foreign chemicals encounter and it is
one of the body's first lines of defence. Chemicals that enter the
lung can either exert a direct effect on the cells of the lung, or be
absorbed into the systemic circulation. Blood passing from the lung to
the heart and then into the peripheral circulation can carry agents
directly to other organs without passing through the detoxication
processes of the liver. This contrasts with oral exposure where
chemicals may be absorbed into blood that immediately passes through
the liver and can be metabolically transformed into either more or
less toxic compounds.
Direct contact with irritants causes local inflammation in the
respiratory system, the degree of which may depend on the local
concentration and not on the total dose. For example, Kljackina (1973)
demonstrated that inhaled bromine acts as a specific irritant of the
respiratory system whereas bromine administered orally results in
changes in the nervous system.
Thus, specific reasons for performing inhalation studies include:
(a) determination of specific responses of the respiratory tract;
(b) assessment of the toxic hazard of agents whose principal route
of exposure is via inhalation; (c) investigation into the mechanism
of toxicity of inhaled materials; and (d) study of the comparative
toxicity of agents administered by different routes.
6.3 Fate of Inhaled Materials
6.3.1 Nature of aerosols
The nature and characteristics of aerosols have been treated in
detail elsewhere (Cadle, 1965; Mercer, 1973a,b; Raabe, 1970), but it
may be useful to review a few general concepts. Aerosols consist of
finely divided particles ranging in size from about 0.01 to 100 µm in
diameter. The particles in a cloud are usually of many sizes and the
size can be expressed as a size-frequency distribution curve which
usually best fits a log-normal distribution. There are several ways to
express the diameter of a particle, the common ones being count median
diameter, mass median diameter, and aerodynamic mass median diameter.
The last term is important as it considers each distribution as if it
were made up of unit density spheres and measures its diameter as if
it were acting aerodynamically like a unit density sphere. Thus,
aerosol behaviour can be compared, regardless of the individual shape
and density of the particles.
The division of a solid into fine particles that become airborne
results in a large increase in the surface area of the material. The
consequence is a generally increased chemical reactivity of the
material, thus accelerating all physical and chemical processes, such
as oxidation, dissolution, evaporation, absorption, and electrical
activity. The physiological activity of these particles also
increases. The sizes of interest from a biological standpoint range
from about 0.1 to 10 µm. Larger particles do not usually enter the
respiratory tract or, if they do, they are deposited in the nose.
6.3.2 Deposition
Material that enters the respiratory tract with the inspired air
can either be deposited or exhaled. Many factors affect the deposition
of particles or the absorption of vapours in the respiratory system.
Retention of vapours is governed by the diffusion rates of the vapour,
the solubility of the vapour in the various body compartments, and the
degree to which these compartments have attained the equilibrium. This
in turn, depends on the duration of the exposure, the concentration,
and the rate of removal of the vapours. This subject has recently been
reviewed by Stupfel & Mordelet-Dambrine (1974).
Many more factors govern the deposition of particulates in the
respiratory tract. Roe (1968) has divided these into physical and
chemical characteristics of the particle, anatomical, physiological,
and pathological factors. The size, density, and shape of the
particles are the physical variables that determine their aerodynamic
behaviour. The Task Group on Lung Dynamics (Morrow et al., 1966) has
discussed deposition as a function of particle size.
Three distinct physical processes act on particles suspended in
the atmosphere to cause them to be deposited in the respiratory tract.
Inertial impaction results from the tendency of particles to move in
straight lines. The repeated branching and subdivision of the
respiratory tract causes particles to be impacted on surfaces,
particularly near the bifurcations. Inertial forces are greater with
larger particles. Sedimentation due to gravity also causes particles
to strike the surface of the respiratory tract. Finally, Brownian
movement, particularly of smaller particles, causes them to be
deposited in the lung. The effectiveness of these mechanisms depends
on the anatomy of the respiratory tract, the size of the particle, and
the breathing pattern. During artificially induced hyperventilation in
rabbits, more material was deposited in comparison with controls
(Velickovskij & Kacnelson, 1964). Normal deposition and deposition in
diseased states are discussed in detail by Albert et al. (1973), Brain
& Valberg (1974), Goldberg & Laurenco (1973), Macklem et al. (1973)
and Stuart (1974).
Aerosols are deposited all along the respiratory tract. Large
particles (5-10 µm) are mainly deposited in the upper respiratory
tract, including the nasal cavity. The depth of penetration increases
as particle size decreases and particles in the 1-2 µm range are, for
the most part, deposited in the alveoli. As a first approximation, 25%
of inhaled particles are exhaled, 50% are deposited in the upper
respiratory tract, and 25% are deposited in the lower respiratory
tract (Morrow et al., 1966).
6.3.3 Clearance
Soluble particles readily dissolve at the site of deposition.
Generally, they enter the bloodstream and then behave as if they had
been intravenously injected. Insoluble particles can be removed from
the respiratory tract by several mechanisms depending on the site of
deposition. Particles that are deposited on the mucous blanket are
carried towards the pharynx by the cilia and are usually swallowed or
expectorated. Hence, it is impossible to completely separate
respiratory exposure from gastrointestinal exposure. The rate of
clearance by the mucociliary escalator has been measured in man by a
number of investigators (Albert et al., 1969, 1973; Camner et al.,
1972, 1973; Morrow, 1970; Sanchis et al., 1972) and animals (Albert et
al., 1973; Thomas, 1969; Velickovskij & Kacnelson, 1964; Watson et
al., 1969).
Particles that are deposited in the deep non-ciliated portion of
the lung clear more slowly. One mechanism responsible for alveolar
clearance is phagocytosis. Although alveolar macrophages engulf
particles within a few hours, there is evidence that macrophages do
not carry them actively to the mucociliary escalator in the first few
days following inhalation (Camner et al., 1977). Recent studies
indicate that there are other mechanisms of alveolar clearance. Tucker
et al. (1973) have shown alveolar clearance by normal interstitial
drainage pathways. Casarett (1972) and Morrow (1973) described a
possible mechanism whereby particles could move from the alveolus up
to the terminal bronchiole, and then to the ciliated epithelium for
removal on the mucus. Very fine particles can also enter the blood
directly through the lymphatic system. Finally, particles can dissolve
and be absorbed into the bloodstream. Some particles remain in the
alveolar region of the lung for considerable periods of time, during
which dissolution forces can operate.
6.4 Dose in Inhalation Studies
The term dose has many meanings depending on the background and
expertise of the investigator. In toxicology, dose is usually defined
as the mass of material introduced into the animal, and it is often
divided by the body weight. Hence, toxicologists often speak of dose
in mg/kg. Even this is misleading, to some extent, because the
material may not interact in any way, and because of other reasons
that become apparent in species comparisons. The actual dose or amount
of a substance entering the internal milieu of the body depends on the
concentration and the particle size of the inhaled material, the
duration of the exposure, and the breathing variables of the test
species. Thus, the actual absorbed dose is difficult to determine and
inhalation toxicologists often refer to exposure conditions instead of
dose. The exposure must be defined both in terms of concentration (C)
and time (t), and is sometimes expressed as the product of these two
(Ct) (MacFarland, 1968) (see also Chapter 1). While the Ct product
is not a true dose, it can be used in a similar fashion. Haber (1924)
recognized this and Haber's rule states that, for a fixed Ct
product, the response will be the same. This rule holds reasonably
well over limited ranges of C and t, but deviations occur when
extreme values of the variables are examined.
More frequently, inhalation toxicologists keep the time constant
and vary the concentration of the test material. Hence, they measure
an LC50, i.e. the median concentration to which animals are exposed
for a specified time that will kill 50% of the animals within a fixed
period of time after exposure. It is implicit in LC50 data that the
durations of both the exposure and post-exposure period are
standardized. Comparative LC50 data are often obtained for 4-h
exposures and a 14-day post-exposure observation period (Carpenter et
al., 1949; Pozzani et al., 1959).
6.5 Choice of Species
The ideal subject for studies relevant to man is man himself.
However, human volunteers can only be used where the toxicological
hazard is already reasonably well defined and accepted. Human studies
have been conducted recently with chlorinated hydrocarbon solvents and
common air pollutants (Andersen et al., 1974; Hazucha et al., 1973;
Stewart, 1972; Stewart et al., 1970a,b, 1973). These experiments were
well controlled and monitored and the exposure levels were low.
Rats, dogs, and monkeys have been the species most used for
inhalation toxicity studies, although investigators have also used
mice, hamsters, guineapigs, rabbits, cats, miniature swine, and
donkeys. The choice of species should be made, primarily, with a view
to extrapolating the experimental results to man. However, choice on
this basis alone is difficult since the validity of such an
extrapolation is often uncertain. When selecting the particular
species for study, the following factors must be considered: the
comparative morphology of the respiratory tract; the presence or
absence of lung disease or susceptible states; and the similarity of
biochemical and physiological responses to those in man.
6.5.1 Anatomical differences
The respiratory systems of various laboratory animals and man
differ widely. As man is a primate, it is sometimes erroneously
assumed that the monkey is a good model for inhalation studies, but
marked differences are noted when comparing human and sub-human
primate respiratory systems. In the monkey, the end airway is always a
respiratory bronchiole, whereas this is rarely the case in man.
Furthermore, the monkey's lung is not lobulated.
The most commonly used laboratory animals are quadrupeds, and
their respiratory systems are horizontal and not vertical as in the
primate. This is important because the aerodynamics of a horizontally
arranged lung are different, and particle deposition will also be
different. In human subjects, maximum dust deposition is in the upper
portion of the lung; in experimental animals, maximum deposition in
the more ventral portions of the lung is usual.
The gross anatomy of the respiratory systems of the various
laboratory animals and man is quite different. In animals such as the
rat and guineapig, the nose contains highly developed tortuous
turbinates. In monkeys and man with relatively smaller noses, the
turbinates are less complex. These differences in nasal anatomy are
especially important in studies involving exposure to particulates.
More particles impinge in more complex turbinate systems and will not,
therefore, reach the deeper portions of the respiratory system.
The subgross anatomy of the lungs of various laboratory animals
and man has been detailed and compared by McLaughlin et el. (1961a,b,
1966). The authors have grouped the common laboratory animals and man
in three basic categories based on subgross pulmonary anatomy
(Table 6.1). They grouped the various animals on the basis of lung
lobulation, the presence or absence of respiratory bronchioles, the
presence or absence of terminal bronchioles, pulmonary artery/
bronchial artery shunts, and the termination of the bronchial
arteries. Their studies indicate that the pulmonary anatomy of the
horse most closely resembles that of man.
6.5.2 Physiological considerations
Pertinent differences in the lung physiology of various species
must be considered in inhalation toxicology. Normal values or ranges
for several species, including man, are summarized in Table 6.2
(Sanockij, 1970a).
Table 6.1 Comparative subgross anatomy of the lunga
Species Lung No. of lobes Lobulation Respiratory Terminal Pulmonary/artery Termination Pleura
type bronchioles bronchioles Bronchial/artery of bronchial
left right shunts arteries
Cow I 3 5(4) well developed extremely poor present present distal airway thick
development
Sheep I 3 4 well developed extremely poor present present distal airway thick
development
Pig I 3 4 well developed extremely poor present not present distal airway thick
development
Monkey II 3 4 not present very well absent not present distal airway thin
developed
Dog II 3 4 not present very well absent not present distal airway thin
developed
Cat II 3 4 not present very well absent not present distal airway thin
developed
Guineapig IIa 3 4 not present fairly well absent present distal airway thin
developed
Rat IIa 1 4 not present fairly well absent few present distal airway thin
developed
Rabbit IIa 3 4 not present fairly well absent present tertiary thin
developed bronchus
Horse III (3) (4) imperfectly poorly present present distal airway thick
developed developed and alveoli
Man III 2 3 imperfectly poorly present present distal airway thick
developed developed and alveoli
a From: McLaughlin et al. (1961a,b, 1966).
Table 6.2 Some physiological indices of man and animalsa
Man Dog Cat Rabbit Guineapig Rat Mouse
Body surface (m2) 1.8 0.528 0.2 0.18 0.040 0.030 0.006
Relation body surface to body weight 0.0257 0.044 0.066 0.072 0.12 0.15 0.3
(m2/kg)
Basal metabolism (kJ/kg) 105 222 -- 188 360 615 711
Frequency of respiration (min) 14-18 10-30 20--30 50-100 80-135 110-135 140-210
Size of alveoli (µm) 150 100 100 -- -- 50 30
Surface of lungs (m2) 50 100 7.2 5.21 1.47 0.56 0.12
Relation of lung surface to body weight 0.7 8.3 2.8 2.5 3.2 3.3 5.4
(m2/kg)
Inhaled air (ml) 616 40-60 -- -- 1.75 0.865 0.154
Lung ventilation (ml/min) 8732 -- 1000 600 155 73 25
Relation of lung ventilation to body 0.13 -- 0.30 0.29 0.33 0.05 1.24
weight (ml/min/g)
Consumption of oxygen (ml/kg/hr) 203.1 3600 9420 522.7 2180 2199 3910
Elimination of CO2 (ml/kg/h) 168.8 -- -- -- -- 2650 4240
Coefficient of respiration 0.82 -- -- 0.83 -- 0.82 0.85-1.33
Pulse frequency for 1 min 70-72 90-130 120-180 150-240 206-280 300-500 520-780
a From: Sanockij (1970a).
6.5.3 Disease and susceptibility states
Most toxicological investigations are performed on healthy
animals. However, epidemiological studies have indicated that during
air pollution episodes the populations at greatest risk are the young,
the aged, and those people with pre-existing cardiopulmonary disease.
One task of the inhalation toxicologist is to identify those segments
of the population that are particularly susceptible to the presence of
airborne contaminants. Certain animal models of diseased or stressed
states have been described (Boyd et al., 1974; Drew & Taylor, 1974;
Silver et al., 1973; Taylor & Drew, 1975; see also Chapter 2) which
could be used or could be adapted for use in inhalation studies. The
most commonly used model of this nature is the papain-induced
emphysematous animal (Gross et al., 1965; Martorana et al., 1973;
Niewoehner & Kleinermann, 1973; Snider et al., 1974). Both aged and
neonatal animals could be used as models of high susceptibility
groups. Disease and susceptibility states are important considerations
in the selection of the species and, in some cases, even the strain of
animal to be investigated since it is well known that the incidence of
cancer differs considerably among certain species and strains.
For example, Kuschner et al. (1975) recently reported a high
incidence of respiratory tract tumours in rats after exposure to
oxybis[chloro-methane] (bis(chloromethyl)ether) but only a few tumours
in hamsters. These tumours were about equally divided between
esthesioneuro-epitheliomas and bronchogenic squamous cell carcinomas.
Leong et al. (1975) repeated these experiments; however, all the
tumours in Leong's study were nasal tumours with no bronchogenic
carcinomas. The only difference noted in the protocol was that Leong
et al. used specific-pathogen-free, caesarean-derived rats, whereas
Kuschner et al. used Sprague-Dawley rats, that were not
specific-pathogen-free.
6.6 Duration of Exposure
Acute inhalation toxicity studies usually consist of a single
exposure (or occasionally a few exposures) of not more than 8 h.
Repeated exposure studies consist of a number of daily exposures for
fixed periods of time. Occasionally, investigators terminate exposure
after several days or weeks, and then maintain the animals in the
colony to observe delayed development of long-term effects (Kuschner
et al., 1975).
The duration of chronic studies varies considerably. One logical
proposal is based on the life span of the test species (Sanockij,
1970b). If, for example, one considers that toxic signs will appear in
man after an exposure over 10% of his life span (7 years), animals
should also be exposed for 10% of their life span. Thus, rats should
be exposed for 3-4 months, and larger animals for a somewhat longer
period. Some authors (Sidorenko & Pinigin, 1970) consider that even
continuous exposure for 3-4 months is insufficient to simulate
lifetime exposure in man, and many studies last longer, some up to 5
years (Lewis et al., 1974). Powell & Hosey (1965) consider the minimum
duration of a chronic study to be one year, the animals being exposed
6 h a day for 5 days a week. This corresponds to a significant portion
of a rodent's lifetime.
Chronic studies are conducted to determine the effects of
long-term exposure to compounds and particularly (in the USSR) to
establish minimum effect levels (Limch). In order to evaluate the
effects of long-term exposures, such as elevated incidences of
infection, emphysema, or the induction of cancer, inhalation controls
should be run concurrently. Two species are usually used. Occasionally
the toxic effects and the mechanism of chronic toxicity are entirely
different from those manifested in acute exposures. Benzene, for
example, is a central nervous system depressant at high
concentrations, while, at low concentrations over long periods of
exposure, it affects the hematopoietic system.
The selection of concentration for chronic studies is difficult.
For example, in the USA, concentrations are chosen that do not produce
mortality and produce only minimal changes in other biological indices
of toxicity during limited studies. In the USSR, the concentration
selected is below the Limac. In order to investigate dose-response
relationships, it is advisable to use at least three different dose
levels, hoping that the highest level chosen will produce quantifiable
effects and that the lowest level selected will produce minimal or
even no effects. In the USSR, research workers often use as the
highest level the concentration which, in single short-term exposures
of human subjects, does not produce any effect during the study of
reflex reactions.
When investigating the various biological indices of toxicity, it
is necessary to carry out measurements more frequently during the
early stages of the study (Camner et al., 1972, 1973). Studies carried
out at the Institute of Labour Hygiene and Occupational Medicine of
the USSR show that the time to the display of the first signs (period
for initial decompensation) varies considerably. Thus, the variables
are recorded at 1, 4, 8, 15, and 30 days and monthly thereafter. After
terminating the exposure, the frequency of measurements may be
increased again to record any early changes.
6.6.1 Intermittent versus continuous exposures
Long-term exposures are usually patterned on projected industrial
experience, giving the animals a daily exposure of 6-7 h, 5 days a
week (intermittent exposure), or on a possible environmental exposure,
with 22-24 h of exposure per day, 7 days a week (continuous exposure),
with about an hour for feeding the animals and maintaining the
chambers. In both cases, the animals are usually exposed to a fixed
concentration of test materials. Thus, neither situation approaches
actual human experience, where concentrations of atmospheric
pollutants are continuously fluctuating by one or two orders of
magnitude. A major difference to consider between intermittent and
continuous exposure is that with the former there is a 17-18 h period
in which animals may recover from the effects of each daily exposure,
and an even longer recovery period at weekends. The recovery period in
some cases is extremely important as in the case of continuous versus
intermittent exposure to dichlormethane (Haun, 1972).
The choice of intermittent or continuous exposure depends on the
objectives of the study and on the human experience that is to be
simulated. However, certain technical difficulties must be considered.
For example, the advantages of continuous exposure for simulating
environmental conditions may be offset by the necessity of watering
and feeding during exposure, and by the need for more complicated (and
reliable) aerosol and vapour generation and monitoring techniques.
Intermittent systems require simpler chambers, since provision for
food and water is not necessary. The contaminant dispersal systems are
also simpler as they need to operate for only 6-7 h per day.
6.7 Inhalation Systems
6.7.1 Facilities required
It is more advantageous to build facilities designed specifically
for inhalation studies than to modify existing buildings. High
cellings are necessary for housing exposure chambers and related
equipment, such as aerosol and vapour generators, filters, flowmeters,
etc. A constant supply of clean filtered air with temperature and
humidity controls should be available for both the chamber rooms and
the chambers themselves. Adequate floor space should provide access to
at least two sides of the chambers, and the chambers themselves should
be separated by a small space to avoid heat transfer between chambers.
6.7.2 Static systems
Inhalation systems can be characterized as static, when the agent
is introduced into a chamber as a batch and then mixed, or dynamic,
when airflow and introduction (and removal) of agent are continuous.
The duration of static exposure is limited by: (a) the gradual
depletion of oxygen; (b) the accumulation of carbon dioxide;
(c) the accumulation of water vapour; and (d) the gradual increase
in temperature inside the chamber. In spite of these limitations,
static systems are of great practical usefulness in assessing acute
toxicity, particularly when the supply of material is limited.
Procedures similar to those described by Draize et al. (1959) are in
use today for screening commercial products. Another use of static
systems is for the exposure of animals to biological aerosols. It is
difficult to generate a viable biological aerosol particle
continuously because of the limited amount of material available;
thus, material is dispersed in a large chamber, mixed, and the animals
exposed through nose tubes connected to the test atmosphere (Jemski &
Phillips, 1965).
6.7.3 Dynamic systems
Today, most inhalation facilities use dynamic systems where the
airflow and introduction of agents are continuous. The theoretical or
nominal concentration of chemicals in a chamber can be calculated as
follows:
flow of chemical
concentration =
flow of air
Many factors, including wall loss, losses on the skin and fur of
animals, and uptake by the test animals, cause the actual
concentration to be somewhat less than the nominal concentration.
Thus, the concentration should always be measured by an appropriate
instrument or technique rather than reporting the nominal
concentration.
When material is introduced into a chamber, the concentration
builds up exponentially according to equations originally described
and verified by Silver (1946). If perfect mixing occurs, the
concentration can be calculated according to the following equation:
C = (w/b) (1 - exp (-bt/a)) (1)
where C = the concentration of material at time t; w = amount of
material introduced per minute; a = volume of the chamber;
b = flow of air through the chamber.
The fraction of equilibrium concentration (w/b) attained in
time t is:
C
= 1 - exp (1 bt/a) (2)
w/b
Thus, the time required to reach 99% (t99) of the equilibrium
concentration is:
0.99 = 1 - exp (-bt99/a) (3)
or
t99 = 4.6052 a/b
This equation may be given the general form:
tx = Ka/b (4)
where x equals % nominal concentration attained in time t and
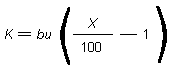 Values of K for various values of x are tablulated below:
x K
99 4.6
95 3.0
90 2.3
85 1.9
80 1.6
There are several features of interest in the above equations.
Since the concentration build-up is exponential, the concentration
will theoretically never reach a constant value. However, in practice,
the concentration is not detectably different once the equilibration
time is equal to t99 or longer. The clearance curve of removal of
chemical from the chamber after the flow of chemical is discontinued
also follows an exponential curve (Fig. 6.1). Thus, to ensure that
little or no material remains in the chamber, it should be operated
for t99 after discontinuing the flow of chemical. This procedure
also compensates for the time required for build-up of material in the
chamber. Finally, it should be noted that in the general equation,
tx is only a function of the volume of the chamber and the flow of
air through the chamber. Thus, if the ratio of a/b = 1,
t99 = 4.6 min, t95 = 3 min, etc. A 150-litre chamber operated at
30 litre/min would have a/b = 5 and t99 = 5 (4.6 min) = 23 min.
MacFarland (1976) has recently reviewed these principles and has also
discussed ways of decreasing t99 by manipulating flow rate.
6.7.4 Typical whole-body systems
Inhalation exposure technology has been recently reviewed by Drew
& Laskin (1973) and earlier descriptions and requirements have been
given by Frazer et al. (1959), Hinners et al. (1966, 1968), and Roe
(1968). The simplest inhalation system would be a box with facilities
for air intake and exhaust. This concept has been used for many years
in chambers similar to that described by Drew & Laskin (1973)
(Fig. 6.2). In this system, a cylindrical glass battery jar is mounted
in a horizontal position; a frame holds the jar and provides support
Values of K for various values of x are tablulated below:
x K
99 4.6
95 3.0
90 2.3
85 1.9
80 1.6
There are several features of interest in the above equations.
Since the concentration build-up is exponential, the concentration
will theoretically never reach a constant value. However, in practice,
the concentration is not detectably different once the equilibration
time is equal to t99 or longer. The clearance curve of removal of
chemical from the chamber after the flow of chemical is discontinued
also follows an exponential curve (Fig. 6.1). Thus, to ensure that
little or no material remains in the chamber, it should be operated
for t99 after discontinuing the flow of chemical. This procedure
also compensates for the time required for build-up of material in the
chamber. Finally, it should be noted that in the general equation,
tx is only a function of the volume of the chamber and the flow of
air through the chamber. Thus, if the ratio of a/b = 1,
t99 = 4.6 min, t95 = 3 min, etc. A 150-litre chamber operated at
30 litre/min would have a/b = 5 and t99 = 5 (4.6 min) = 23 min.
MacFarland (1976) has recently reviewed these principles and has also
discussed ways of decreasing t99 by manipulating flow rate.
6.7.4 Typical whole-body systems
Inhalation exposure technology has been recently reviewed by Drew
& Laskin (1973) and earlier descriptions and requirements have been
given by Frazer et al. (1959), Hinners et al. (1966, 1968), and Roe
(1968). The simplest inhalation system would be a box with facilities
for air intake and exhaust. This concept has been used for many years
in chambers similar to that described by Drew & Laskin (1973)
(Fig. 6.2). In this system, a cylindrical glass battery jar is mounted
in a horizontal position; a frame holds the jar and provides support
 for a panel which is mounted against the open end and serves as a
closure. Various openings can be cut in the panel for the introduction
of the pollutant and for monitoring the concentration.
Studies at the University of Rochester on the toxicity of
radioactive materials contributed significantly to the development of
the technology of inhalation exposure. The original test chamber was
cylindrical with cones on the top and bottom. A modified chamber in
the shape of a hexagon with pyramidal ends (Wilson & Laskin, 1950) is
known as the "Rochester Chamber". A final modification with a square
cross-section as shown schematically in Fig. 6.3 is known as the "NYU
Chamber" (New York University Chamber) (Laskin et al., 1970). These
two shapes are currently in use in several laboratories, although
cubes, cylinders, spheres, modified hemispheres, and even chambers
with an elliptical cross-section have all been used (Drew & Laskin,
1973). The two major considerations that influence the shape of the
chamber are uniformity of distribution and wall loss of the test
substance (MacFarland, 1976) with secondary importance placed on
caging supports, accessibility, and costs.
A typical chamber is shown schematically in Fig. 6.3. The unit
has a volume of 1.3 m3 and is about 3 m in height. The body is made
of stainless steel and the windows can be made of either glass or
lucite. Clean air is supplied at the top with the pollutant being
injected in a perpendicular direction to the incoming airstream.
Chambers with tangential pollutant introduction are also common.
Airflow is usually down through the chamber and the air is removed
through the side arm of a Y fitting at the bottom. Animal wastes are
removed at the bottom via the building drains, usually through a trap
or a valve. The trap also maintains the integrity of the system and
allows operation at a pressure slightly (1-2 cm H20) below ambient.
Chambers of this general shape have been built with volumes ranging
from 128 litres up to 5 m3, although MacFarland (1976) suggests that
the pollutant concentration in chambers of less than 1 m3 may not be
uniform.
The size of the chamber depends on the number and size of the
animals to be exposed. The total animal volume should not exceed
approximately 5% of the total chamber volume. Experience has shown
that above 5% surface losses begin to cause excessive concentration
losses and thermal considerations also begin to play a limiting role.
6.7.5 Construction materials
Inhalation chambers should be constructed of materials that do
not react with the test material and are easily cleaned. The most
versatile materials are stainless steel and glass. However, many other
materials have been used, including aluminium, wood, wood coated with
epoxy paints, lucite, and various fibre panels. The chamber should
have at least two sides made almost completely of transparent material
for a panel which is mounted against the open end and serves as a
closure. Various openings can be cut in the panel for the introduction
of the pollutant and for monitoring the concentration.
Studies at the University of Rochester on the toxicity of
radioactive materials contributed significantly to the development of
the technology of inhalation exposure. The original test chamber was
cylindrical with cones on the top and bottom. A modified chamber in
the shape of a hexagon with pyramidal ends (Wilson & Laskin, 1950) is
known as the "Rochester Chamber". A final modification with a square
cross-section as shown schematically in Fig. 6.3 is known as the "NYU
Chamber" (New York University Chamber) (Laskin et al., 1970). These
two shapes are currently in use in several laboratories, although
cubes, cylinders, spheres, modified hemispheres, and even chambers
with an elliptical cross-section have all been used (Drew & Laskin,
1973). The two major considerations that influence the shape of the
chamber are uniformity of distribution and wall loss of the test
substance (MacFarland, 1976) with secondary importance placed on
caging supports, accessibility, and costs.
A typical chamber is shown schematically in Fig. 6.3. The unit
has a volume of 1.3 m3 and is about 3 m in height. The body is made
of stainless steel and the windows can be made of either glass or
lucite. Clean air is supplied at the top with the pollutant being
injected in a perpendicular direction to the incoming airstream.
Chambers with tangential pollutant introduction are also common.
Airflow is usually down through the chamber and the air is removed
through the side arm of a Y fitting at the bottom. Animal wastes are
removed at the bottom via the building drains, usually through a trap
or a valve. The trap also maintains the integrity of the system and
allows operation at a pressure slightly (1-2 cm H20) below ambient.
Chambers of this general shape have been built with volumes ranging
from 128 litres up to 5 m3, although MacFarland (1976) suggests that
the pollutant concentration in chambers of less than 1 m3 may not be
uniform.
The size of the chamber depends on the number and size of the
animals to be exposed. The total animal volume should not exceed
approximately 5% of the total chamber volume. Experience has shown
that above 5% surface losses begin to cause excessive concentration
losses and thermal considerations also begin to play a limiting role.
6.7.5 Construction materials
Inhalation chambers should be constructed of materials that do
not react with the test material and are easily cleaned. The most
versatile materials are stainless steel and glass. However, many other
materials have been used, including aluminium, wood, wood coated with
epoxy paints, lucite, and various fibre panels. The chamber should
have at least two sides made almost completely of transparent material

 in order to view the animals during exposure. The inside surfaces
should be as free as possible from perturbations and rough surfaces
and edges, in order to facilitate cleaning. Openings should be
included to monitor the variables needed to characterize the exposure
(section 6.7.8).
6.7.6 Engineering requirements
Accurate control of airflow in the chambers is essential. The
usual procedure is to supply filtered, conditioned air in excess of
that required and then tap off the common supply for each chamber. In
most experiments, the ratio of chamber volume to airflow ranges from 1
to about 6.
When handling hazardous materials, special safety precautions
must be taken to protect operating personnel and the surrounding
environment. The chambers are operated at slightly negative pressure
to ensure that any leaks draw air into the system. Chamber effluents
must be cleaned, usually by filters or scrubbers, or at least diluted,
before being released into the environment. Stack effluents should be
monitored.
Food and water must be provided in the chambers, when animals are
being exposed continuously. Facilities for cleaning the chambers,
while the animals are in them, are also necessary, though in many
laboratories the animals are removed from the chamber for 45 min-1 h
to permit it being serviced. Racks for supporting animal cages must be
included and exposure cages should have all six sides made of wire
mesh (stainless steel) to ensure good mixing.
6.7.7 Special systems
6.7.7.1 Isolation units
When handling particularly hazardous materials, additional
precautions are necessary. These can be fairly simple, such as
operating a battery jar in a fume hood, or complex, such as the
chamber-within-a-chamber concept described by Laskin et al. (1970). In
this system (Fig. 6.4) the aerosol is separated by two barriers from
the operator with separate glove boxes for generation and removal of
the aerosol. In addition, living quarters are provided behind a
barrier with pass boxes for food and animal wastes to be moved into
and out of the chamber.
6.7.7.2 Head and nose exposures
Early in the development of inhalation exposure systems,
investigators realized the value of head or nose only exposures
(Saito, 1912). Stokinger (1949) described chambers with openings for
head-only exposures for several species, and Henderson (1952)
in order to view the animals during exposure. The inside surfaces
should be as free as possible from perturbations and rough surfaces
and edges, in order to facilitate cleaning. Openings should be
included to monitor the variables needed to characterize the exposure
(section 6.7.8).
6.7.6 Engineering requirements
Accurate control of airflow in the chambers is essential. The
usual procedure is to supply filtered, conditioned air in excess of
that required and then tap off the common supply for each chamber. In
most experiments, the ratio of chamber volume to airflow ranges from 1
to about 6.
When handling hazardous materials, special safety precautions
must be taken to protect operating personnel and the surrounding
environment. The chambers are operated at slightly negative pressure
to ensure that any leaks draw air into the system. Chamber effluents
must be cleaned, usually by filters or scrubbers, or at least diluted,
before being released into the environment. Stack effluents should be
monitored.
Food and water must be provided in the chambers, when animals are
being exposed continuously. Facilities for cleaning the chambers,
while the animals are in them, are also necessary, though in many
laboratories the animals are removed from the chamber for 45 min-1 h
to permit it being serviced. Racks for supporting animal cages must be
included and exposure cages should have all six sides made of wire
mesh (stainless steel) to ensure good mixing.
6.7.7 Special systems
6.7.7.1 Isolation units
When handling particularly hazardous materials, additional
precautions are necessary. These can be fairly simple, such as
operating a battery jar in a fume hood, or complex, such as the
chamber-within-a-chamber concept described by Laskin et al. (1970). In
this system (Fig. 6.4) the aerosol is separated by two barriers from
the operator with separate glove boxes for generation and removal of
the aerosol. In addition, living quarters are provided behind a
barrier with pass boxes for food and animal wastes to be moved into
and out of the chamber.
6.7.7.2 Head and nose exposures
Early in the development of inhalation exposure systems,
investigators realized the value of head or nose only exposures
(Saito, 1912). Stokinger (1949) described chambers with openings for
head-only exposures for several species, and Henderson (1952)
 described an apparatus consisting of a cylindrical chamber with
openings arranged along two sides to enable nose exposure of mice to
biological aerosols. Nose exposure systems are used in situations
where: (a) skin absorption is not desirable; (b) the amount of test
material is limited; (c) the material is hazardous; and (d) there is
no need for a large chamber. They are most commonly used for acute
exposures, since it is difficult to restrain the animals for long
periods of time.
Nose exposure systems have been used extensively for two
particular situations -- exposure to radioactive aerosols and exposure
to cigarette smoke. Investigators at the Lovelace Foundation for
Medical Education and Research in Albuquerque have developed a series
of nose exposure units (Boecker et al., 1964; Raabe et al., 1973;
Thomas & Lie, 1963). The technical difficulties of exposing animals to
cigarette smoke has prompted the development of several systems
designed specifically for this purpose (Hoffman & Wynder, 1970; Stuart
et al., 1970). One device has been developed by Homburger et al.
(1967) and two have been described by Dontenwill (1970). Albert et al.
(1974) have developed a device to expose donkeys to cigarette smoke
via nose tubes. Devices for exposing dogs to cigarette smoke have also
been described (Cahan & Kirman, 1968).
A nose exposure system for exposure to dusts has been developed
at the Institute of Hygiene and Occupational Medicine in Moscow
(Valeznev et al., 1970) (Fig. 6.5). It consists of a completely
enclosed system in which up to 40 rodents can be exposed
simultaneously. A very complex system adaptable to both head-only and
whole-body exposures is routinely used at the Medical Institute in
Kiev (Balasov et al., 1968). A schematic diagram of this system is
shown in Fig. 6.6.
6.7.7.3 Instantaneous exposure systems
Occasionally, it is necessary to expose animals to a
concentration of material, while avoiding the time required to attain
uniform concentrations. In this case, the air in the chamber is
equilibrated with the test material and the animals rapidly inserted
into the chamber. Sometimes double chambers are used for this purpose.
The animals are placed in the upper half and the pollutant is
introduced into the lower half. When the desired concentration is
reached in the lower half, a trap door opens and the animals fall into
the lower half, while the mechanism immediately closes. Other
investigators have described various drawer arrangements for rapid
insertion of animals into test atmospheres.
described an apparatus consisting of a cylindrical chamber with
openings arranged along two sides to enable nose exposure of mice to
biological aerosols. Nose exposure systems are used in situations
where: (a) skin absorption is not desirable; (b) the amount of test
material is limited; (c) the material is hazardous; and (d) there is
no need for a large chamber. They are most commonly used for acute
exposures, since it is difficult to restrain the animals for long
periods of time.
Nose exposure systems have been used extensively for two
particular situations -- exposure to radioactive aerosols and exposure
to cigarette smoke. Investigators at the Lovelace Foundation for
Medical Education and Research in Albuquerque have developed a series
of nose exposure units (Boecker et al., 1964; Raabe et al., 1973;
Thomas & Lie, 1963). The technical difficulties of exposing animals to
cigarette smoke has prompted the development of several systems
designed specifically for this purpose (Hoffman & Wynder, 1970; Stuart
et al., 1970). One device has been developed by Homburger et al.
(1967) and two have been described by Dontenwill (1970). Albert et al.
(1974) have developed a device to expose donkeys to cigarette smoke
via nose tubes. Devices for exposing dogs to cigarette smoke have also
been described (Cahan & Kirman, 1968).
A nose exposure system for exposure to dusts has been developed
at the Institute of Hygiene and Occupational Medicine in Moscow
(Valeznev et al., 1970) (Fig. 6.5). It consists of a completely
enclosed system in which up to 40 rodents can be exposed
simultaneously. A very complex system adaptable to both head-only and
whole-body exposures is routinely used at the Medical Institute in
Kiev (Balasov et al., 1968). A schematic diagram of this system is
shown in Fig. 6.6.
6.7.7.3 Instantaneous exposure systems
Occasionally, it is necessary to expose animals to a
concentration of material, while avoiding the time required to attain
uniform concentrations. In this case, the air in the chamber is
equilibrated with the test material and the animals rapidly inserted
into the chamber. Sometimes double chambers are used for this purpose.
The animals are placed in the upper half and the pollutant is
introduced into the lower half. When the desired concentration is
reached in the lower half, a trap door opens and the animals fall into
the lower half, while the mechanism immediately closes. Other
investigators have described various drawer arrangements for rapid
insertion of animals into test atmospheres.

 6.7.8 Variables to monitor
It is necessary to monitor several variables during the operation
of an inhalation chamber. The most obvious is the concentration in the
chamber of the pollutant in question. This can be done continuously by
automated samplers and recorded on a strip chart recorder or manually
at periodic intervals using a variety of sampling techniques. When
aerosols are involved, particle size should be determined. The flow of
pollutant, the flow of air, and the chamber pressure should all be
recorded frequently. Chamber temperature and humidity are other
variables that should be monitored. It is also useful to measure the
pressure drop across intake and exhaust filters in order to know when
to replace them.
6.7.9 Human exposure facilities
Ethical principles will always be of first concern, when
considering the exposure of human subjects to materials for toxicity
evaluation. However, there are situations where controlled exposure of
human beings can provide useful information with minimum risk. One of
the more modern facilities has been described by Stewart et al.
(1970a,b). It consists of a room approximately 6 m × 6 m × 2.7 m,
completely air conditioned and operated at slightly negative pressure.
Activity within this chamber is strictly sedentary and comfortable
chairs and study desks are provided. Meals are served during
exposures, with coffee and soft drinks available continuously. All
subjects are under continuous surveillance by medical personnel and
all activities are visually monitored by closed circuit television.
Such facilities are particularly useful for studying psychomotor
effects and other sensitive indicators of exposure to low
concentrations of organic vapours.
The odour threshold concentrations used in the USSR for
establishing maximum permissible concentrations for single exposures
are determined in human subjects over short periods (5-10 min).
Obviously such studies can only be carried out at very low
concentrations considered to be safe. The minimum concentration sensed
by the most sensitive individual is accepted as the odour threshold
(Rjazanov, 1964).
Reflex reactions produced in man by irritating the receptive
zones of the respiratory organs with subsensory concentrations of
atmospheric pollutants, were established by measuring the light
sensitivity of the eye, the bioelectric activity of the cerebrum, etc.
(Bustueva et al., 1960; Rjazanov, 1964). Changes in encephalographic
responses resulted from exposure to small concentrations of these
substances. The maximum concentration that did not produce an effect
on the bioelectric activity of the cerebrum was, in most cases, 3-4
times lower than the odour threshold concentration (Krotov, 1971;
Sidorenko & Pinigin, 1972).
6.8 Contaminant Generation and Characterization
Extensive reviews have been published by several investigators on
methods of generating and characterizing vapours and particles (Bryan,
1970; Cotabish et al., 1961; Drew & Lippmann, 1971; Lodge, 1968;
Mercer, 1973a,b; Nelson, 1971; Raabe, 1970).
6.8.1 Generation of vapours
Vapours can be generated by using one of several flow-dilution
devices (Cotabish et al., 1961; Drew & Lippmann, 1971; Nelson, 1971;
Saltzman, 1971; Saltzman & Warburg, 1965). If the contaminant is a
liquid at room temperature, a vaporization step must be included. One
procedure is to use a motor-driven syringe and to apply the liquid to
a wick or heated plate in a calibrated stream of air (Nelson & Griggs,
1968). Another method is to saturate the airstream with vapour and
then dilute it with air to the desired concentration (Cotabish et al.,
1961). A third technique, originally described by O'Keefe & Ortman
(1966), consists of using permeation tubes. These are especially
useful when using low concentrations of test materials for
standardization procedures. In theory, there is no reason why they
cannot be scaled up for use in inhalation studies.
6.8.2 Particle generators
The generation of particulate contaminants is usually more
difficult than vapour generation. The contaminant may be generated
from a dry powder or from a liquid and the particles generated may be
of uniform size (monodisperse) or may vary greatly in size
(heterogeneous).
6.8.2.1 Heterogeneous aerosols
The Wright dust feed (1950) is one of the better known
instruments for generating aerosols from a dry powder. A gear drives
the surface of a packed cylinder of finely ground powder against a
scraping mechanism. A high velocity airstream disperses the powder.
Proper use of the Wright dust feed is dependent upon the control of
the relative humidity of the airstream and the packing density of the
powder. Other devices for producing aerosols from dry powders have
been described (Crider et al., 1968; Deichman, 1944; Dimmick, 1959;
Stead et al., 1944). Since the particle size of the resultant aerosols
depends upon the size of the original powder, elutriators and cyclones
are sometimes included to limit the maximum size. Laskin et al. (1971)
described generators that include such devices for producing freshly
ground polyurethane aerosols.
Agglomeration, usually caused by electrical charge, is a serious
problem with dry dust aerosols. The electrical behaviour of aerosols
has been reviewed by Whitby & Liu (1966). A mechanical solution to
agglomeration has been proposed by Drew & Laskin (1971) who described
a fluidizing dust generator.
Wet dispersion generators break liquid into droplets. The liquid
may be a solution or a suspension of the test material. In most cases,
the liquid is drawn into filaments or films that are broken into
droplets. A number of compressed air-driven generators (nebulizers),
that produce droplets of many sizes, have been described (Dautrebande,
1962; Laskin, 1948; Lauterbach et al., 1956; Raabe, 1970). The
resulting aerosols are polydisperse although relatively narrow size
distribution can be attained with some nebulizers.
Monodisperse aerosols are occasionally needed by investigators,
especially when studying regional deposition. The most popular device
for dispensing uniform droplets is the spinning disc generator first
described by Walton & Prewett (1949). Primary droplets thrown off the
perimeter of a spinning disc are uniform in size. The liquid is fed on
to the centre of the disc and accumulates at the edge until broken off
by centrifugal force. Some secondary, smaller droplets are also
produced but these can be separated dynamically. Several investigators
have successfully used the spinning disc for inhalation studies
(Albert et al., 1964; Kajland et al., 1964; Lippmann & Albert, 1967;
Philipson, 1973). Another device employing a controlled condensation
process originally described by LaMer & Sinclair (1943) has been found
to be suitable for inhalation studies. A third principle, consisting
of size specific collection, resuspension and subsequent dispersion,
has also been used (Kotrappa et al., 1972).
6.8.3 Monitoring contaminant concentrations
The techniques and equipment for monitoring contaminant
concentrations have been reviewed by a number of authors (Lippman,
1971; Powell & Hosey, 1965). In many cases, the characteristics of the
contaminant determine the sampling technique. Sometimes a number of
techniques are available and the method of choice may depend upon the
availability of equipment, cost of reagents, time for analysis, or
other factors. Automated instrumentation is currently available for a
number of contaminants. However, when using automatic devices,
a second method, usually chemical, should be used to verify the
instrument performance.
6.8.3.1 Vapour sampling
Two basic methods for the collection of gaseous samples are
employed. The first involves the use of a gas collector, such as an
evacuated flask or bottle, to obtain a definite volume of air at a
known temperature and pressure. The second method involves the passage
of a known volume of air through a collecting medium to remove the
desired contaminants from the sampled atmosphere. The samples are then
analysed by appropriate analytical techniques.
Since the assays are related to the volume of air sampled, the
instrumentation for monitoring airflow or volume should be accurately
calibrated. It is also important that there are no leaks in the
sampling train, thus assuring that all the air measured has passed
through the collecting medium.
A number of devices, currently available, measure the
concentration of vapours continuously; many record the result
graphically. Many detection principles are used including
conductivity, colorimetry, and spectrophotometry. Recent commercial
instrumentation using the principle of infrared spectrometry are
especially useful. Most of these instruments have been described by
Nader (1971).
6.8.3.2 Particulate sampling
When monitoring particulate atmospheres, mass concentration and
particle size must be determined. The mass concentration can be
measured by techniques similar to those used for monitoring vapours.
The material can be collected, then assayed by appropriate chemical
methods. Gravimetric analysis can also be performed by weighing the
filter paper before and after collecting a sample. The resulting mass
can be related to the volume of air that was sampled.
Particle collection techniques include filtration, impingement,
thermal and electrostatic precipitation, and sedimentation. The basic
principles for these techniques and specific examples of each method
have been reviewed (Lippmann, 1971). These principles should be
thoroughly understood prior to selection of a method for a specific
contaminant.
The techniques for measuring particle size and numbers have been
discussed in detail by Mercer (1973a,b). Direct methods consisting of
both conventional and electron microscopy can be used. In both cases,
proper sampling methods to ensure collection of a representative
sample should be followed. Electrostatic and thermal precipitators or
an impinger can be used. After using an impinger, the dust can be
counted in a standard haematology counting cell or counted directly
with a Coulter Counter.
There are two indirect methods of assessing particle size; the
use of cascade impactors and the use of light scattering devices. The
theory of cascade impaction has been described by Mercer (1963, 1964,
1965). The theory of light scattering devices has been reviewed by
Hodkinson (1966) and commercially available instruments have been
listed by Swift (1971). Many factors, including shape, opacity, and
others, some unknown, affect the amount of light scattered and the
measured number and size of the particles. These devices should,
therefore, be used cautiously.
6.9 Other Methods of Respiratory Tract Exposure
6.9.1 In vivo exposures
In addition to direct inhalation, several other methods of
exposing the respiratory tract have been described (Roe, 1968).
Andervont (1937) originally described a thread implantation technique
whereby a thread impregnated with a test compound was literally passed
through a lobe of the lung. This technique was modified by Kuschner et
al. (1957) who devised a pellet that could be impregnated with the
test material or coated with radioactive materials. This pellet had
hooks which held it in the lumen of the bronchus or bronchiole. This
technique has been used to demonstrate the carcinogenicity of a number
of compounds including benzo(a)pyrene, 3-methylcholanthrene, and
calcium chromate (Laskin et al., 1970). A technique whereby hamsters
are anaesthetized and then intratracheally intubated with various
materials is in use in a number of laboratories (Laskin et al., 1970;
Little & O'Toole, 1974; Saffiotti, 1970; Schreiber et al., 1972). In
two laboratories, this technique has been coupled with inhalation
exposures to study the potential cocarcinogenicity of various agents
(Laskin & Nettesheim, 1974, personal communication).
6.9.2 In vitro exposures
A few in vitro techniques that show promise as regards
elucidation of the mechanisms of toxicity of inhaled materials should
be mentioned. A number of investigators (Niemeier & Bingham, 1972;
O'Neill & Tierney, 1974; Orton et al., 1973) are using various,
isolated, perfused lung preparations to study toxicity. Such
preparations could be particularly useful in studying the transfer of
materials from the lung to the blood. These preparations are also used
to study pulmonary metabolism as are lung tissue slices (O'Neil &
Tierney, 1974). Finally, several laboratories are developing methods
of culturing tracheal rings and sections (Griesemer et al., 1974; Lane
& Miller, 1975). These preparations show great promise for
toxicological evaluation (section 2.7.4).
6.10 Biological End-points and Interpretation of Changes in these
End-points
Throughout the course of an inhalation study, there are a number
of biological indicators to observe. The classical indices of toxicity
include weight change and mortality, organ-to-body weight ratios, and
both gross and microscopic changes in the morphology of the various
tissues and organs. While the study is in progress, respiratory,
physiological indices can be monitored and the excretion of certain
chemicals in breath, urine, and faeces can also be followed. Some
procedures particularly useful in inhalation toxicity studies are
discussed below.
6.10.1 Morphological changes
The primary function of the lung is the exchange of oxygen and
carbon dioxide between the blood and alveolar air. The walls of the
alveoli are lined by a single, flattened layer of epithelial cells
that are in close proximity to endothelial cells lining capillaries.
The mean distance from the lumen of the capillary to the lumen of the
alveolus is less than one micron. Because this distance is so small,
any thickening or inflammation of the alveolar wall will severely
disrupt the diffusion of gases.
The air in the alveolus arrives through a series of branching
bronchi and bronchioles the specialized epithelial lining of which is
instrumental in removing particulate matter from the lung. Any lesions
that result in narrowing of the lumen of these bronchi and bronchioles
cause a disruption in the ventilation of that portion of the lung.
Chemicals affecting the specialized ciliated epithelial lining, the
bronchioles, and the bronchi may interrupt the clearance mechanism,
resulting in a build-up of inhaled particulates in the lung.
The type of morphological change observed in the lung as a result
of the inhalation of materials depends upon the concentration of the
inhaled material and the length of time for which animals are exposed.
Short-term inhalation of high concentrations of certain materials may
result in acute changes in the lung such as oedema, necrosis, and
purulent inflammation. However, the inhalation of the same material at
lower concentrations for longer periods of time may result in chronic
changes such as fibrosis and even neoplasia. Table 6.3 lists a number
of chemicals and the response elicited in the lung following their
inhalation.
Techniques for quantifying morphological changes are, at best,
limited. The usual practice is to assign some number to the degree of
damage and then to apply statistical procedures to these numbers.
These procedures are, however, subject to individual interpretation.
Assessment of the degree of fibrosis is possible (Roe, 1968), although
such measurements should be checked by special staining procedures.
Interpretation of any morphological changes in the lung must take
into consideration the concentration and duration of exposure.
Exposure to certain materials will elicit immediate acute
morphological changes which, with time, tend to resolve and disappear.
This phenomenon suggests that the animal is able to adapt to the
effects of some chemicals, if the concentration is not too high. An
Table 6.3 Responses of the lung to various chemicals
Chemical Bronchiolar Loss of Oedema Epithelial Fibrosis Emphysema Granuloma
epithelial goblet metaplasia
hyperplasia cells
nitrogen dioxide x x x x x
ozone x x x x x
allergens x
sulfuric acid x x
bis-chloromethyl ether
silica x x
coal dust x x
beryllium x x x
cotton dust
nickel compounds
Table 6.3 (cont'd)
Necrosis Neoplasia Bronchial Alveolar Nonsuppurative
constriction epithelial alveolitis
hyperplasia
nitrogen dioxide x
ozone
allergens x
sulfuric acid x x
bis-chloromethyl ether x
silica
coal dust
beryllium x
cotton dust x
nickel compounds x
example of this adaptive phenomenon is seen with ozone. Nonlethal
exposures to ozone have a protective effect against subsequent lethal
exposures which would result in marked pulmonary oedema (Alpert &
Lewis, 1971).
6.10.2 Functional changes
There are certain advantages in using alterations in respiratory
functions as indices of toxicity: (a) quantitative measurements can
usually be made at concentrations far below those needed to produce
morphological effects; (b) many effects may be measured at the level
of reversible rather than irreversible changes; (c) such measurements
may elucidate mechanisms of action of materials, and (d) in many
instances similar data can be obtained from experimental animals and
man.
6.10.2.1 Measurement of respiratory frequency
Alteration of respiratory frequency in mice may be used as a
simple screening test for assessing irritant potency (Alarie, 1973).
The changes produced are dose-related, which permits construction of
dose-effect curves and calculation of the concentration required to
produce, for example, a 50% decrease in respiratory frequency. Some
irritants, mainly those affecting the upper respiratory tract,
decrease frequency. Other irritants, mainly those which penetrate to
the deeper areas of the lung, increase frequency. Measurement of
respiratory frequency alone is a very sensitive measure of effect for
those irritants that increase frequency (e.g. ozone and nitrogen
dioxide), but it is a relatively insensitive measure of effect for
those irritants that decrease frequency (e.g. sulfur dioxide and
formaldehyde).
6.10.2.2 Measurement of mechanics of respiration
Alterations in pulmonary flow resistance or pulmonary compliance
may be used to assess irritant potency. The method of Amdur & Mead
(1958) required three basic measurements: intrapleural pressure, tidal
volume, and the rate of flow of gas in and out of the respiratory
tract. Intrapleural pressure is estimated by placing a fluid-filled
catheter in the pleural space, while the animal is under anaesthesia.
Once the catheter is positioned, no further anaesthesia is necessary.
Tidal volume is obtained by placing the animal in a body
plethysmograph and measuring the pressure changes as the animal
breathes quietly. Rate of gas flow is obtained by electrical
differentiation of the volume signal. This method provides data on
both resistance and compliance, but is generally limited to single
exposure studies of a few hours duration. The guineapig has been the
species most commonly used for this method; however, plethysmographic
studies have been successfully carried out on several other rodents
including mice (Alarie, 1973), rats (Palacek, 1969) and rabbits
(Davidson et al., 1966).
The method of Murphy & Ulrich (1964) does not involve surgical
intervention and, thus, makes it possible to perform measurements
repeatedly on the same animals. The animal is placed in a body
plethysmograph to which an oscillating sine wave pressure is applied.
The animal's face is fitted with a mask containing a pneumotachograph
screen for measuring flow. Changes in resistance are calculated for
the alterations in flow produced by the superimposed pressure
oscillations. This technique has been used in toxicological studies
without making compliance measurements. However, by measuring
oesophageal pressure reflecting intrapleural pressure, it should be
possible to estimate compliance changes. A compliance measurement is
described by Mead (1960) that involves no surgical intervention.
The observed changes in respiratory mechanics are dose-related
and permit the construction of dose-effect curves. These may be used
to compare irritant potency or to study such things as the effect of
inert particles on the reaction to irritant gases. The deep lung
irritants that increase respiratory frequency tend to show a decrease
in compliance as the primary alteration in the mechanical behaviour of
the lung. Resistance changes with such irritants are minimal. The
irritants that slow respiratory frequency tend to show an increase in
resistance accompanied by a smaller decrease in compliance, as the
primary alteration in pulmonary mechanics.
An increase in pulmonary flow resistance can result from a
narrowing of the lumen of the bronchi mediated by constriction of the
smooth muscle. The effect, in this situation, usually occurs rapidly
on exposure to the irritant. In the case of a gaseous irritant, the
effect is fairly rapidly reversed when irritant exposure ceases. In
the case of a particulate irritant which remains in the lung, the
effect is less readily reversible. Swelling of the respiratory mucosa
or an increase in mucus secretion can also cause an increase in flow
resistance. The effect, in this situation, usually takes some time to
develop.
Much of the damage to the lung is to the small airways which
change either in calibre or stability or both. It would be very useful
if this damage could be detected early during an exposure before more
serious disease develops. There are three tests which are promising
and have been useful in human subjects. They are the maximal
expiratory flow volume curve, reviewed by Hyatt & Black (1973), the
alveolar closing volume (Dollfuss et al., 1967) and the frequency
dependency of compliance (Woolcock et al., 1969). Animal models that
provide the same data have not yet been fully developed.
6.10.3 Biochemical end-points
In the last decade, much research has centred around the
elucidation of some of the biochemical aspects of the lung. It has
only recently been demonstrated that the lung is capable of
biotransforming many foreign chemicals and several authors have
investigated the role of cytochrome P-450-dependent enzyme systems in
pulmonary microsomes (Bend et al., 1972; Chhabra & Fouts, 1974;
Chhabra et al., 1974; Fouts & Devereux, 1972; Harper et al., 1975;
Hook et al., 1972). The importance of intermediary metabolism is now
being considered (Tierney, 1974). The biochemistry of pulmonary
surfactants and their role in pulmonary defence mechanisms has been
reviewed by King (1974). Investigations of the lung collagen have
recently been reviewed by Crystal (1974). Witschi (1975) has provided
an excellent summary of biochemical approaches for the evaluation of
pulmonary toxicity.
6.10.4 Other end-points in inhalation studies
Exposure by inhalation is only a means by which the substance
enters the animal. This chapter has concentrated on the lung as the
critical organ. However, there are end-points that are not necessarily
related to the lung. Inhalation studies have recently been reported to
assess the teratological effects of inhaled materials (Schwetz et al.,
1975). They have also been used in behavioural studies (Weiss &
Laties, 1975; Xintaras et al., 1974).
REFERENCES
ALARIE, Y. (1973) Sensory irritation of the upper airways by airborne
chemicals. Toxicol. appl. Pharmacol., 24: 279-297.
ALBERT, R. E., PETROW, H. G., SALAM, A. S., & SPIEGELMAN, J. R. (1964)
Fabrication of monodisperse lucite and iron oxide particles with
a spinning disc generator. Health Phys., 10: 933-940.
ALBERT, R. E., LIPPMANN, M., & BRISCOE, W. (1969) The characteristics
of bronchial clearance and the effects of cigarette smoking.
Arch. environ. Health, 18: 738-755.
ALBERT, R. E., LIPPMANN, M., PETERSON, H. T., JR, BERGER, J., SANBORN,
K., & BORWAG, D. (1973) Bronchial deposition and clearance of
aerosols. Arch. intern. Med., 131: 115-131.
ALBERT, R. E., BERGER, J., SANSORN, K., & LIPPMANN, M. (1974) Effects
of cigarette smoke components on bronchial clearance in the
donkey. Arch. environ. Health, 29: 96-101.
ALPERT, S. M. & LEWIS, T. R. (1971) Ozone tolerance studies utilizing
unilateral lung exposure. J. appl. Physiol., 192: 364-368.
AMDUR, M. O. & MEAD, J. (1958) Mechanics of respiration in
unanaesthetized guinea pigs. Am. J. Physiol., 192: 364-368.
ANDERSEN, I., LUNDQVIST, G. R., JENSON, P. L., & PROCTOR, D. F. (1974)
Human response to controlled levels of sulfur dioxide. Arch.
environ. Health, 28: 21-39.
ANDERVONT, H. B. (1937) Pulmonary tumours in mice: IV. Lung tumours
induced by subcutaneous injection of 1,2,5,6-dibenzanthracene in
different media and by its direct contact with lung tissue.
Public Health Rep., 52: 1584.
BALASOV, V. E., BARTENEV, V. D., SAVICKIJ, I. V., & TRAHTENSERG, I. M.
(1968) [Toxicological assessment of volatile substances leaking
from synthetic materials.] Zdorov'e, Kiev (in Russian).
BEND, J. R., HOOK, G. E. R., EASTERLING, R. E., GRAM, T. E., & FOUTS,
J. R. (1972) A comparative study of the hepatic and pulmonary
microsomal mixed function oxidase systems in the rabbit.
J. Pharmacol. exp. Ther., 183: 206-217.
BOECKER, B. B., AQUILAR, F. L., & MERCER, T. T. (1964) The design of a
canine inhalation exposure apparatus incorporating a whole-body
plethysmograph. Health Phys., 10: 1077-1089.
BOYD, M. R., BURKA, L. T., HARRIS, T. M., & WILSON, B. J. (1974)
Lung-toxic furanoterpenoids produced by sweet potatoes (Ipomoea
batatas) following microbial infection. Biochim. Biophys.
Acta, 337: 184-195.
BRIAN, J. D. & VALBERG, P. A. (1974) Models of lung retention based on
International Congress of Radiation Protection Task Group Report.
Arch. environ. Health, 28: 1-11.
BRYAN, R. J. (1970) Generation and monitoring of gases for inhalation
studies. In: Hanna, M. G. Jr, Nettesheim, P., & Gilbert, J. R.,
ed. Inhalation carcinogenesis. Springfield VA, Clearinghouse
for Federal Scientific and Technical Information, NBS, US Dept of
Commerce (CONF-691001).
BUSTUEVA, K. A., POLEZAEV, E. F., & SOMENENKO, A. D. (1960) [A study
of threshold values for reflex action of atmospheric pollutants.]
Gig. i Sanit., No. 1, pp. 57-61 (in Russian).
CADLE, R. D. (1965) Particle size. Princeton, NJ, Van
Nostrand-Reinhold.
CAHAN, W. G. & KIRMAN, D. (1968) An effective system and procedure for
cigarette smoking by dogs. J. surg. Res., 8: 567-575.
CAMNER, P., PHILIPSON, K., & FRIBERG, L. (1972) Tracheobronchial
clearance in twins. Arch. environ. Health, 24: 82-87.
CAMNER, P., MOSSBERG, B., & PHILIPSON, K. (1973) Tracheobronchial
clearance and chronic obstructuve lung disease. Scand. J.
respir. Dis., 54: 272-281.
CAMNER, P., HELLSTRÖM, P. A., LUNDBERG, M., & PHILIPSON, K. (1977)
Lung clearance of 4 µm particles coated with silver, carbon and
beryllium. Arch, environ. Health, 32: 58-62.
CARPENTER, C. P., SMYTH, H. F., JR, & POZZANI, U. C. (1949) The assay
of acute vapour toxicity and the grading and interpretation of
results on 96 chemical compounds. J. ind. Hyg. Toxicol.,
31: 343-346.
CASARETT, L. J. (1972) The vital sacs: alveolar clearance mechanisms
in inhalation toxicology. In: Hayes, W. J., ed. Essays in
toxicology. New York, London, Academic Press.
CHHABRA, R. S. & FOUTS, J. R. (1974) Sex differences in the metabolism
of xenobiotics by extrahepatic tissue in rats. Drug Metab.
Disposition, 2: 375-379.
CHHABRA, R. S., POHL, R. J., & FOUTS, J. R. (1974) A comparative study
of xenobiotic-metabolizing enzymes in liver and intestine of
various animal species. Drug Metab. Disposition, 2: 443-447.
COTABISH, H. N., MCCONNAUGHEY, P. W., & MESSER, H. C. (1961) Making
known concentrations for instrument calibration. Am. Ind. Hyg.
Assoc. J., 22: 392-402.
CRIDER, W. L., BARKELEY, N. P., & STRONG, A. A. (1968) Dry-powder
aerosol dispensing device with long-time output stability.
Rev. Sci. Instrum., 39: 152-155.
CRYSTAL, R. G. (1974) Lung collagen: definition, diversity and
development. Fed. Proc., 33: 2248-2255.
DAUTREBANDE, L. (1952) Microaerosols, New York, Academic Press,
pp. 7-22.
DAVIDSON, J. T., WASSERMAN, K., LILLINGTON, G. A., & SCHMIDT, R. W.
(1966) Pulmonary function testing in the rabbit. J. appl.
Physiol., 21: 1094-1098.
DEICHMANN, W. B. (1944) An apparatus designed for the production of
controlled dust concentrations in the air breathed by
experimental animals. J. ind. Hyg. Toxicol., 26: 334-335.
DIMMICK, R. L. (1959) Jet disperser for compacted powders in the
one-to-ten micron range. Am. Med. Assoc. Arch. ind. Health,
20: 8-14.
DOLLFUSS, R. E., MILIC-EMILI, J., & BATES, D. V. (1967) Regional
ventilation of the lung studied with boluses of 133 xenon.
Respir. Phys., 2: 234-246.
DONTENWILL, W. (1970) Experimental investigations on the effect of
cigarette smoke inhalation on small laboratory animals. In:
Hanna, M. G. Jr, Nettesheim, P., & Gilbert, J. R., ed.
Inhalation carcinogenesis. Springfield VA, Clearinghouse for
Federal Scientific and Technical Information, NBS, US Dept of
Commerce.
DRAIZE, J. H., NELSON, A. A., NEWBURGER, S. N., & KELLEY, E. A. (1959)
Inhalation toxicity studies of six types of aerosol hair sprays.
Proc. Sci. Sec. Toilet Goods Assoc., 31: 28-32.
DREW, J. H. & LASKIN, S. (1971) A new dust-generating system for
inhalation studies. Am. Ind. Hyg. Assoc. J., 32: 327-330.
DREW, R. T. & LASKIN, S. (1973) Environmental inhalation chambers.
In: Day, W. I., ed. Methods of animal experimentation. New
York, Academic Press.
DREW, R. T. & LIPPMANN, M. (1971) Calibration of air sampling
instruments: II. Production of test atmospheres for instrument
calibraton. In: Lippmann, M., ed. Air sampling instruments, 4th
ed., Cincinnati, OH, ACGIH, pp. I-1.
DREW, R. T. & TAYLOR, G. J. (1975) Cardiophysical studies with
stressed animals. In: Proceedings of the 5th Annual Conference
on Environmental Toxicology, Dayton, Ohio, 1974, US Govt
Printing Office, pp. 121-129.
FOUTS, J. R. & DEVEREUX, T. R. (1972) Developmental aspects of hepatic
and extrahepatic drug-metabolizing enzyme systems: microsomal
enzymes and components in rabbit liver and lung during the first
month of life. J. Pharmacol. exp. Ther., 183: 458-468.
FRASER, D. A., BALES, R. E., LIPPMANN, M., & STOKINGER, H. E. (1959)
Exposure chambers for research in animal inhalation. USPHS
(Public Health Monograph 57).
GOLDBERG, I. S. & LAURENCO, R. V. (1973) Deposition of aerosols in
pulmonary disease. Arch. intern. Med., 131: 88-92.
GRIESEMER, R. A., KENDRICK, J., & NETTESHEIM, P. (1974) Tracheal
grafts. In: Proceedings on Experimental Respiratory
Carcinogenesis, Seattle 1974, Springer-Verlag, pp. 537-547.
GROSS, P., PFITZER, E. A., TOLKER, E., BABYAK, M. A., & KASCHAK, M.
(1965) Experimental emphysema: its production with papain in
normal and silicotic rats. Arch. environ. Health, 11: 50-58.
HABER, F. (1924) [Five lectures for the years 1920-1923.] Berlin,
Springer-Verlag (in German).
HARPER, C., DREW, R. T., & FOUTS, J. R. (1975) Species differences in
benzene hydroxylation to phenol by pulmonary and hepatic
microsomes. Drug Metab. Disposition, 3: 381-388.
HAUN, C. C. (1972) Continuous animal exposure to methylene chloride.
In: Proceedings of the 2nd Annual Conference on Environmental
Toxicology, Dayton, Ohio, 1971, US Govt Printing Office,
pp. 309-326.
HAZUCHA, M., SILVERMAN, F., PARENT, C., FIELD, S., & BATES, D. V.
(1973) Pulmonary function in man after short-term exposure to
ozone. Arch. environ. Health, 27: 183-188.
HENDERSON, D. W. (1952) Apparatus for study of airborne infection.
J. Hyg. (Lond.), 50: 53-68.
HINNERS, R. G., BURKART, J. K., & CONTNER, G. L. (1966) Animal
exposure chambers in air pollution studies. Arch. environ.
Health, 13: 609-615.
HINNERS, R. G., BORKART, J. K., & PUNTE, C. L. (1968) Animal
inhalation exposure chambers. Arch. environ. Health,
16: 194-206.
HODKINSON, J. R. (1966) The optical measurements of aerosols. In:
Davies, C. N., ed. Aerosol science, New York, Academic Press,
pp. 287-357.
HOFFMANN, D. & WYNDER, E. L. (1970) Chamber development and aerosol
dispersion. In: Hanna, M. G. Jr, Nettesheim, P., & Gilbert, J.
R., ed. Inhalation carcinogenesis. Springfield, VA.
Clearinghouse for Federal Scientific and Technical Information,
NBS, US Dept of Commerce (CONF-691001).
HOMBURGER, F., BERNFELD, P., BOGDONOFF, P., KELLEY, T., & WALTON, R.
(1967) Inhalation by small animals of fresh cigarette smoke
generated by new smoking machine. Toxicol. appl. Pharmacol.,
10: 382.
HOOK, G. E. R., BEND, J. R., HOEL, D., FOUTS, J. R., & GRAM, T. E.
(1972) Preparation of lung microsomes and a comparison of the
distribution of enzymes between subcellular fractions of rabbit
lung and liver. J. Pharmacol. exp. Ther., 182: 474-490.
HYATT, R. E. & BLACK, L. F. (1973) The flow-volume curve: a current
perspective. Am. Rev. Respir. Dis., 107: 191-199.
JEMSKI, J. V. & PHILLIPS, G. B. (1965) Aerosol challenge of animals.
In: Gay, W. I., ed. Methods of animal experimentation, New
York, Academic Press, Vol. 1, pp. 273-341.
KAJLAND, A., EDFORS, M., FRIBERG, L., & HOLMA, B. (1964) Radioactive
monodisperse test aerosols and lung clearance studies. Health
Phys., 10: 941-945.
KING, R. J. (1974) The surfactant system of the lung. Fed. Proc.,
33: 2238-2247.
KLJACKINA, A. M. (1973) In: Toxicology of new industrial chemical,
Moscow, Medicina, Vol. 13, p. 23.
KOTRAPPA, P., BOYD, H. A., & WILKINSON, C. J. (1972) Technology for
the production of monodisperse aerosols of oxides of transuranic
elements for inhalation experiments. Health Phys., 22: 837-843.
KROTOY, YU. A. (1971) [Setting of approximate maximum permissible
concentrations of atmospheric pollutants by calculation.]
Gig. i Sanit., 12: 8-12 (in Russian).
KUSCHNER, M., LASKIN, S., CHRISTOFANO, E., & NELSON, N. (1957)
Experimental carcinoma of the lung. In: Proceedings of the 3rd
National Cancer Conference, Philadelphia, Lippincott,
pp. 485-495.
KUSCHNER, M., LASKIN, S., DREW, R. T., CAPIELLO, V., & NELSON, N.
(1975) Inhalation carcinogenicity of alpha haloethers: III.
Lifetime and limited period inhalation studies with
bis(chloromethyl) ether at 0.1 ppm. Arch. environ. Health,
30: 73-77.
LAMER, V. K. & SINCLAIR, D. (1943) An improved homogeneous aerosol
generator. Washington DC, US Dept of Commerce (OSRD Report
No. 1668).
LANE, B. P. & MILLER, S. L. (1975) Dose dependence of
carcinogen-induced changes in tracheal epithelium in organ
culture. In: Proceedings of the Symposium on Experimental
Respiratory Carcinogenesis, Seattle, 1974, Springer-Verlag,
pp. 507-513.
LASKIN, S. (1948) AEC Project Quarterly Report UR-38. Rochester, NY,
University of Rochester.
LASKIN, S., KUSCHNER, M., & DREW, R. T. (1970) Studies in pulmonary
carcinogenesis. In: Hanna, M. G. Jr, Nettesheim, P., & Gilbert,
J. R., ed. Inhalation carcinogenesis. Springfield VA,
Clearinghouse for Federal Scientific and Technical Information,
NBS, US Dept of Commerce (CONF-691001).
LASKIN, S., DREW, R. T., CAPIELLO, V. P., & KUSCHNER, M. (1971)
Inhalation studies with freshly generated polyurethane foam dust.
In: Mercer, T. T., Morrow, P. E., & Storer, W., ed. Assessment
of airborne particles, Springfield, IL, Thomas, pp. 382-404.
LAUTERBACH, K. E., HAYES, D., & COELHO, M. A. (1956) An improved
aerosol generator. Am. Med. Assoc. Arch. ind. Health,
13: 156-160.
LEONG, B. J. K., KOCIBA, R. J., JERSEY, G. C., & GEHRING, P. J. (1975)
Effects from repeated inhalation of parts per billion of
bis(chloromethyl) ether in rats. Toxicol. appl. Pharmacol.,
35: 175.
LEWIS, T. R., MOORMAN, W. J., YANG, Y., & STARA, J. F. (1974)
Long-term exposure to auto exhaust and other pollutant mixtures.
Arch. environ. Health, 29: 102-106.
LIPPMANN, M. & ALBERT, R. E. (1967) A compact electric motor-driven
spinning disc aerosol generator. Am. Ind. Hyg. Assoc. J.,
28: 501-506.
LIPPMANN, M., ed. (1971) Air sampling instruments, 4th ed.
Cincinnati, OH, ACGIH.
LITTLE, J. B. & O'TOOLE, W. F. (1974) Respiratory trace tumours in
hamsters induced by benzo(a)pyrene and polonium210 alpha
radiation. Cancer Res., 34: 3026-3039.
LODGE, J. P. (1968) Production of controlled test atmospheres.
In: Stern, A. C., ed. Air pollution, 2nd ed., New York,
Academic Press, Vol. II, pp. 465.
MACFARLAND, H. N. (1968) Exposure chambers -- design and operation.
In: Proceedings of the 7th Annual Technical Meeting of the
American Association of Contamination Control, Chicago, 1968,
pp. 19-25.
MACFARLAND, H. N. (1976) Respiratory toxicology. In: Hayes, W. J. Jr,
ed. Essays in toxicology, New York, San Francisco, London,
Academic Press, Vol. 7, pp. 121-154.
MACKLEM, P. T., HOGG, W. E., & BRUTON, J. (1973) Peripheral airway
obstruction and particulate deposition in the lung. Arch.
intern. Med., 131: 93-100.
MARTORANA, P. A., MCKEEL, N. W., RICHARD, J. W., & SHARE, N. N. (1973)
Function-structure correlation studies on excised hamster lungs
in papain-induced emphysema. Can. J. Physiol. Pharmacol.,
51: 635-641.
MCLAUGHLIN, R. F., TYLER, W. S., & CANADA, R. O. (1961a) Subgross
pulmonary anatomy in various mammals and man. J. Am. Med. Assoc.
175: 694-697.
MCLAUGHLIN, R. F., TYLER, W. S., & CANADA, R. O. (1961b) Study of the
subgross pulmonary anatomy of various mammals. Am. J. Anat.,
108: 149-164.
MCLAUGHLIN, F. R., TYLER, W. S., & CANADA, R. O. (1966) Subgross
pulmonary anatomy of the rabbit, rat and guinea pig with
additional notes on the human lung. Am. Rev. Respir. Dis.,
94: 380-387.
MEAD, J. (1960) Control of respiratory frequency. J. appl. Physiol.,
15: 325-360.
MERCER, T. T. (1963) On the calibration of cascade impactors. Ann.
occup. Hyg., 6: 1-14.
MERCER, T. T. (1964) The stage constants of cascade impactors. Ann.
occup. Hyg., 7: 115-125.
MERCER, T. T. (1965) The interpretation of cascade impactor data. Am.
Ind. Hyg. Assoc. J., 26: 236-241.
MERCER, T. T. (1973a) Production and characterization of aerosols.
Arch. intern. Med., 131: 39-50.
MERCER, T. T. (1973b) Aerosol technology in hazard evaluation. New
York, London, Academic Press.
MORROW, P. E. (1970) Models for the study of particulate retention and
elimination in the lung. In: Hanna, M. G., Nettesheim, P. &
Gilbert, J. R., ed. Inhalation carcinogenesis. Springfield VA,
Clearinghouse for Federal Scientific and Technical Information,
NBS, US Dept of Commerce (CONF-691001).
MORROW, P. E. (1973) Alveolar clearance of aerosols. Arch. intern.
Med., 131: 101-108.
MORROW, P. E., HODGE, H. C., NEWMAN, W. F., MAYNARD, E. A., BLANCHET,
H. J. JR, FASSET, D. W., BIRK, R. E., & MAVRODT, S. (1966)
Deposition and retention models for internal dosimetry of the
human respiratory tract. Health Phys., 12: 173-207.
MURPHY, S. D. & ULRICH, G. E. (1964) Multi-animal test system for
measuring effects of irritant gases and vapours on respiratory
function of guinea pig. Am. Ind. Hyg. Assoc. J., 25: 28-36.
NADER, J. S. (1971) Direct-reading instruments for analysing airborne
gases and vapours. In: Lippmann, M., ed. Air sampling
instruments, 4th ed., Cincinnati, ACGIH, p. J-1.
NELSON, G. O. (1971) Controlled test atmospheres: principles and
techniques. Ann Arbor, MI, Ann Arbor Sci. Publ.
NELSON, G. O. & GRIGGS, K. E. (1968) Precision dynamic method for
producing known concentrations of gas and solvent vapour in air.
Rev. Sci. Instrum., 39: 927-928.
NIEMEIER, R. W. & BINGHAM, E. (1972) An isolated perfused lung
preparation for metabolic studies. Life Sci., 11(II): 807-820.
NIEWOEHNER, D. R. & KLEINERMAN, J. (1973) Effects of experimental
emphysema and bronchiolitis on lung mechanics and morphometry.
J. appl. Physiol., 35: 25-31.
O'KEEEE, A. E. & ORTMAN, G. O. (1966) Primary standards for trace gas
analysis. Anal. Chem., 38: 760-763.
O'NEIL, J. J. & TIERNEY, D. G. (1974) Rat lung metabolism: glucose
utilization by isolated perfused lungs and tissue slices. Am. J.
Physiol., 226: 867-873.
ORTON, T. C., ANDERSON, M. W., PICKETT, R. D., ELING, T. E., & FOUTS,
J. R. (1973) Xenobiotic accumulation and metabolism by isolated
perfused rabbit lungs. J. Pharmacol. exp. Ther., 186: 482-497.
PALACEK, F. (1969) Measurement of ventilatory mechanics in the rat.
J. appl. Physiol., 27: 149-156.
PHILIPSON, K. (1973) On the production of monodisperse particles with
a spinning disc. Aerosol Sci., 4: 51-57.
POWELL, C. H. & HOSEY, A. D. (1965) The industrial environment, its
evaluation and control (US PHS Publ. 614).
POZZANI, U. C., WEIL, C. S., & CARPENTER, C. P. (1959) The
toxicological basis of threshold limit values: 5. The
experimental inhalation of vapour mixtures by rats with notes
upon the relationship between single-dose oral data. Am. Ind.
Hyg. Assoc. J., 20: 364-369.
RAABE, O. G. (1970) Generation and characterization of aerosols. In:
Hanna, M. G. Jr, Nettesheim, P., & Gilbert, J. R., ed.
Inhalation carcinogenesis. Clearinghouse for Federal Scientific
and Technical Information, NBS, Springfield VA, US Dept of
Commerce (CONF-691001).
RAABE, O. G., BENNICK, J. E., LIGHT, M. E., HOBBS, C. N., THOMAS, R.
L., & TILLERY, M. (1973) An improved apparatus for acute
inhalation exposure of rodents to radioactive aerosols. Toxicol.
appl. Pharmacol., 26: 264-273.
RJAZANOV, V. A. (1964) Criteria and methods for establishing maximum
permissible concentrations of atmospheric pollutants in the USSR.
In: Maximum permissible concentrations of atmospheric
pollutants, Moscow, Medicina, Vol. 8, pp. 5-21.
ROE, F. J. C. (1968) Inhalation tests. In: Boyland, E. & Goulding, R.,
ed. Modern trends in toxicology, London, Butterworth, Vol. 1.
SAFFIOTTI, U. (1970) Experimental respiratory tract carcinogenesis and
its relation to inhalation exposures. In: Hanna, M. G. Jr,
Nettesheim, P., & Gilbert, J. R., ed. Inhalation carcinogenesis.
Springfield, VA, Clearinghouse for Federal Scientific and
Technical Information, NBS, US Sept of Commerce (CONF-691001).
SAITO, Y. (1912) [Experimental investigations on the quantitative
absorption of dust by animals at accurately known concentrations
of dust in air.] Arch. Hyg., 75: 134-151 (in German).
SALTZMAN, B. E. (1971) Permeation tubes as calibrated sources of gas.
In: Proceedings of 1st Annual Conference on Environmental
Toxicology, Dayton, Ohio, 1970, US Govt Printing Office,
pp. 309-326.
SALTZMAN, B. E. & WARBURG, A. F. (1965) Precision flow dilution system
for standard low concentrations of nitrogen dioxide. Anal.
Chem., 37: 1261-1264.
SANCHIS, J., DOLOVICH, M., CHALMERS, R., & NEWHOUSE, M. (1972)
Quantification of regional aerosol clearance in the normal human
lung. J. appl. Physiol., 33: 757-762.
SANOCKIJ, I. V., ed. (1970a) [Methods for determining toxicity and
hazards of chemicals.] Moscow, Medicina, pp. 62-63 (in
Russian).
SANOCKIJ, I. V. (1970b) [Complete toxicometry.] In: [Methods for
determining the toxicity and hazards of chemicals.] Moscow,
Medicina, pp. 50-54 (in Russian).
SCHREIBER, H., NETTESHEIM, P., & MARTIN, D. H. (1972) Rapid
development of bronchiolo-alveolar squamous cell tumours in rats
after intratracheal injection of 3-methylcholanthrene. J. Natl
Cancer Inst., 49: 541-554.
SCHWETZ, B. A., LEONG, B. J. K., & GEHRING, P. J. (1975) The effect of
maternally inhaled trichloroethylene, perchloroethylene, methyl
chloroform and methylene chloride on embryonal and fetal
development in mice and rats. Toxicol. appl. Pharmacol.,
32: 84-96.
SIDORENKO, G. I. & PINIGIN, M. A. (1970) Rapid determination of
maximum permissible concentrations of atmospheric pollutants.
Gig. i Sanit., No. 3, pp. 93-96.
SILVER, M. J., HOCH, W., KOCSIS, J. J., INGERMAN, C. M., & SMITH, J.
B. (1973) Arachadonic acid causes sudden death in rabbits.
Science, 183: 1085-1086.
SILVER, S. D. (1946) Constant flow gassing chambers: principles
influencing design and operations. J. lab. clin. Med.,
31: 1153-1161.
SNIDER, G. L., HAYES, J. A., FRANZBLAU, C., KAGEN, H. M., STONE, P.S.,
& KORTHY, A. L. (1974) Relationship between elastolytic activity
and experimental emphysema-inducing properties of papain
preparations. Am. Rev. Respir. Dis., 10: 254-262.
STEAD, F. M., DERNEHL, C. U. & NAU, C. A. (1944) A dust feed apparatus
useful for exposure of small animals to small and fixed
concentrations of dust. J. ind. Hyg. Toxicol., 26: 90-93.
STEWART, R. D. (1972) Use of human volunteers for the toxicological
evaluation of materials. In: Symposium on an Appraisal of
Halogenated Fire Extinguishing Agents. Washington DC, National
Academy of Sciences.
STEWART, R. D., DODD, H. C., GAY, H. H., & ERLEY, D. S. (1970a)
Experimental human exposure to trichloroethylene. Arch. environ.
Health, 20: 64-71.
STEWART, R. D., PETERSON, J. E., BARETTA, E. D., BACHARD, R. T.,
HOSCO, M. T., & HERRMANN, A. A. (1970b) Experimental human
exposure to carbon monoxide. Arch. environ. Health,
21: 154-164.
STEWART, R. D., PETERSON, J. E., FISHER, T. N., HOSKO, M. J., BARETTA,
E. D., DODD, H. C., & HERRMANN, A. A. (1973) Experimental human
exposure to high concentrations of carbon monoxide. Arch.
environ. Health, 26: 1-7.
STOKINGER, H. E. (1949) Toxicity following inhalation. In Voegtlin, C.
& Hodge, C., ed. Pharmacology and toxicology of uranium
compounds, 1st ed., New York, McGraw-Hill, Vol. III.
STUART, B. O. (1974) Deposition of inhaled aerosols. Arch. intern.
Med., 131: 60-75.
STUART, B. O., WILLARD, D. H., & HOWARD, E. B. (1970) Uranium mine air
contaminants in dogs and hamsters. In: Hanna, M. G. Jr,
Nettesheim, P., & Gilbert, J. R., ed. Inhalation carcinogenesis.
Springfield, VA, Clearinghouse for Federal Scientific and
Technical Information, NBS, US Dept of Commerce (CONF-691001).
STUPFEL, M. & MORDELET-DAMBRINE, M. (1974) Penetration of pollutants
in the airways. Bull. Physio-Path. Respir., 10: 481-509.
SWIFT, D. L. (1971) Direct-reading instruments for analysing airborne
particles. In: Lippmann, M., ed. Air sampling instruments, 4th
ed., Cincinnati, OH, ACGIH, p. T-1.
TAYLOR, G. J. & DREW, R. T. (1975) Cardiomyopathy predisposes hamsters
to trichlorofluoromethane toxicity. Toxicol. appl. Pharmacol.,
32: 177-183.
THOMAS, R. G. & LIE, R. (1963) Procedures and equipment used in
inhalation studies on small animals. LF-11, Lovelace Foundation
for Medical Education and Research.
THOMAS, R. L. (1969) Deposition and initial translocation of inhaled
particles in small laboratory animals. Health Phys.,
16: 417-428.
TIERNEY, D. G. (1974) Intermediary metabolism of the lung. Fed.
Proc., 33: 2232-2237.
TUCKER, A. D., WYATT, J. H., & UNDRY, D. (1973) Clearance of inhaled
particles from alveoli by normal interstitial drainage pathways.
J. appl. Physiol., 35, 719-732.
VALEZNEV, I. P., ELOVSKAJA, L. T., & MOISEJCEV, P. I. (1970)
[Equipment for research on biological effects of aerosols.]
Ofic. bjull. Gos. kom. Min. SSSR po delam izobretenij i
otkrytij, No. 10, avtonskoe svidetel'stvo No. 265307 (in
Russian).
VELICKOVSKIJ, B. T. & KACNELSON, B. A. (1964) [Etiology and
pathogenesis of silicosis.] Moscow, Medicina (in Russian).
WALTON, W. H. & PREWETT, W. C. (1949) The production of sprays and
mists of uniform drop size by means of spinning disc type
sprayers. Proc. Phys. Soc. (London), 62: 341-350.
WATSON, J. A., SPRITZER, A. A., AULD, J. A. & GUETTHOFF, M. A. (1969)
Deposition and clearance following inhalation and intra-tracheal
injection of particles. Arch. environ. Health, 19: 51-58.
WEISS, B. & LATIES, V. (1975) Behavioural toxicology. New York,
Plenum Press.
WHITBY, K. T. & LIU, B. Y. H. (1966) The electrical behaviour of
aerosols. In: Davies, C. N., ed. Aerosol Science, New York,
Academic Press.
WILSON, R. H. & LASKIN, S. (1950) Improved design for an animal
inhalation exposure unit. Rochester, NY, University of
Rochester (AEC Proj. Rep. U-116), p. 80.
WITSCHI, H. P. (1975) Exploitable biochemical approaches for the
evaluation of toxic lung damage. In: Hayes, W. Jr, ed. Essays in
toxicology, New York, Academic Press, Vol. 6.
WOOLCOCK, A. J., VINCENT, N. J., & MACKLEM, P. T. (1969) Frequency
dependence of compliance as a test for obstruction in the small
airways. J. clin. Invest., 48: 1097-1106.
WRIGHT, B. M. (1950) A new dust-feed mechanism. J. Sci. Instrum.,
27: 12-15.
XINTARAS, C., JOHNSON, B. L., & DE GROOT, I., ed. (1974) Behavioural
toxicology. Washington DC, US Govt Printing Office.
7. CARCINOGENICITY AND MUTAGENICITY
7.1 Introduction
This chapter deals with the two related but separate processes,
heritable mutations and cancer. Cancer involves the conversion of
normal cells to malignant cells and the development of what is usually
an irreversible malignant disease process in the present generation
frequently leading to a fatal outcome for the bearer of the
malignancy. Heritable mutations are mutations that are transmissible
to later generations and the target cells are the germ cells of either
sex.
Developments in the last two decades make it possible to discuss
certain aspects of mutagenesis and carcinogenesis in parallel. In
other words, there is now increasing evidence that, in most cases,
somatic mutations are probably involved in the conversion of normal to
malignant cells. Thus, the ability of a chemical or physical agent to
produce mutations is relevant both to the question of heritable
mutations (mutations of the germ cell) and carcinogenicity.
There is a well-established body of knowledge that shows a close
correspondence between cancer of the whole animal as studied in the
laboratory and the occurrence of cancer in man; this is especially
true of occupational cancer. More recently, a fairly close correlation
has been shown between tests of isolated, simple, biological systems,
e.g. reverse bacterial mutations and the response to carcinogens of
whole animals, and human beings.
A similar situation does not exist with respect to patterns in
human heritable mutations. Thus, although there is good evidence of
correlation between in vitro tests for mutagenicity and heritable
mutations in insects and experimental mammals, it can only be
inferred, at present, that this also applies to man. The inference,
however, is extremely strong and not subject to serious doubt. Thus,
there is every reason to expect, that, in general, man will respond
biologically in a manner quite similar to that of other species when
exposed to mutagens that reach the germ cells. The lack of full
correlation stems more from the lack of appropriate studies in man
than from any uncertainty concerning the underlying biological
considerations. At present, methods for the detection of mutations in
man are difficult, cumbersome, and insensitive, and it is imperative
to operate on the assumption that agents capable of producing germ
cell mutations in laboratory studies would also be capable of
producing similar mutations in man.
Thus, two quite different disease processes can have one common
level of consideration, namely, their ability to produce alterations
in the genetic material of cells. The following discussion is
concerned with methods for carcinogenicity testing. First, the
traditional lifetime exposure of the entire organism to the test
compound is considered. This is followed by a discussion of various
in vitro systems for the examination of mutagenicity including DNA
damage, mutagenicity in bacteria and eukaryotic organisms, and
transformation of cell cultures. (The first three of these are
particularly relevant to mutagenicity of the germ cell or of the
somatic cell.) Following this, the general problem of examination for
heritable mutations is considered. This includes inquiries at three
levels: injury to DNA, point mutations, and chromosome alterations.
Whole animal and isolated test systems are available to make relevant
determinations. However, the whole field of mutagenicity testing is
now in a period of rapid evolution with advances being made in a
number of areas. It can, therefore, be anticipated that present
procedures will undergo alteration and, it is hoped, will improve
within the near future.
7.2 Carcinogenicity
7.2.1 Long-term bioassays
The fact that environmental factors have a direct effect in
producing cancer in man is shown by: (a) the unequivocal evidence of
the chemical origin of occupational cancer, for example, urinary
bladder tumours in workers exposed to aromatic amines, lung cancers in
workers exposed to bis(chloromethyl)ether, etc.; (b) the
well-documented cases of iatrogenic cancer; (c) the positive
correlation between cigarette smoking and lung cancer; (d) the
differences in cancer incidence in urban and rural populations and
(e) the results of studies on migrants showing that for some types
of cancer they acquire incidences similar to those found in the host
countries. Results obtained in experimental studies on carcinogenesis
confirm the direct carcinogenic effect of chemicals. However, there is
not necessarily a correlation between acute toxicity and
carcinogenicity; a chemical with a high acute toxicity may not have
any, or only a very low carcinogenic potential. Conversely, a chemical
that produces a very minor, or no evident toxic effect in acute or
subacute experiments may be a very powerful carcinogen. Knowledge of
the acute and subacute toxicity of the test chemical however, is
important in order to permit better planning of the experiment. For
instance, the premature death of the test animals, due to unforeseen
side-effects, may thus be avoided.
7.2.1.1 Species, strain, and sex selection, and size of groups
The description of methods and recommended procedures in this
chapter applies to long-term testing in rodents and specifically to
the mouse, rat, and Syrian golden hamster (FDA, 1971; NCI, 1976; Weil,
1972; Weisburger, 1975; Weisburger & Weisburger, 1967). Rodents have
been preferred to other species because of their susceptibility to
tumour induction, their relatively short life span, the limited cost
of their maintenance, their widespread use in pharmacological and
toxicological studies, the availability of inbred strains and, as a
consequence of these characteristics, the large amount of information
available on their physiology and pathology. Nonrodents, in particular
dogs and primates, have rarely been used in the past; though used more
at the present time, their much higher cost of maintenance, the long
period of observation, and the impracticability of using sufficiently
large numbers put nonrodents at a disadvantage for long-term
bioassays. Also, comparative studies on the metabolism of various
chemicals have not indicated necessarily that primates and dogs are
closer to man in this respect than rodents. Nevertheless, many of the
procedures for long-term bioassays in rodents are applicable to assays
in nonrodents.
Long-term bioassays of the carcinogenicity of environmental
chemicals are carried out to assess a possible risk to man and to
estimate the need for primary preventive measures. Because of the
urgent nature of these problems, it is recommended that a compound of
unknown activity should be tested on two animal species. Although a
positive (that is, carcinogenic) effect in one species is considered
as adequate warning, only negative findings in two species can be
regarded as adequate negative evidence.
Among rodents, the species of choice are undoubtedly the mouse,
the rat, and the Syrian golden hamslet. The European hamster has
recently been introduced, particularly for studies in lung pathology,
but its use cannot be recommended for routine testing. Guineapigs and
rabbits have been used occasionally but they have few of the
advantages of smaller rodents combined with the disadvantages implied
by a much longer life span, higher cost of maintenance, and the
scarcity or absence of inbred strains.
Of the three rodent species of choice, the mouse and the rat have
been more widely used than the hamster. However, the last species has
proved to be an excellent tool for revealing carcinogenic effects in
the respiratory tract and urinary bladder. Furthermore, hamster cells
are widely used for in vitro transformation assays, and, since in
most cases these assays require the back transplantation of the cells
in syngenic hosts, the use of the hamster has recently been extended.
The hamster is generally randomly bred but a pure strain is becoming
available.
The use of inbred strains undoubtedly has the advantage of making
available animals with known characteristics such as an average life
span, and a spontaneous tumour rate with little variability.
Random-bred animals or, even better, animals bred with maximum
avoidance of inbreeding, are considered more resistant to infections
and perhaps more liable than many inbred strains to reveal any
carcinogenic effect on an unsuspected target organ. It is common
experience that carcinogenic activity is more easily recognized by
the increased frequency and earlier appearance of tumours at sites
where tumours occur spontaneously. Inbred strains are often known to
have one or two particularly susceptible organs and the induction of
tumours in these organs could, in part, be regarded as an
intensification of whatever underlies the spontaneous occurrence of
tumours in that organ. A carefully planned experiment must take this
possibility into account, and preference should be given to strains
with a low incidence of tumours. Noninbred strains are inconvenient in
that, in many cases, background tumour incidence in untreated controls
is unpredictable. Moreover, experimental groups initiated at different
times can rarely be compared with each other since inter- as well as
intragroup variation may be considerable.
Hybrid mice of two known inbred strains are excellent as they are
particularly robust and long-lived, but they are, of course, more
difficult to obtain and are likely to be more expensive than inbred
strains.
In selecting the species, it is important to be aware that there
are, within each species, particular susceptibilites; for instance, it
is easier to induce liver tumours in the mouse than in the rat, and
conversely it is much easier to induce subcutaneous tumours in the rat
than in the mouse. The skin of the mouse and the rabbit is possibly
more sensitive to tumour induction by skin painting than that of the
rat and the hamster, particularly when polycyclic hydrocarbons are
used. The hamster is more susceptible to urinary bladder
carcinogenesis than the mouse and possibly the rat.
It is essential that experimental groups should be composed of an
equal number of animals of each sex, since differences in response to
the carcinogenic activity of chemicals are well documented. Thus, the
use of one sex only would not show the full range of activity of the
test chemical.
The group should be sufficiently large to permit statistical
evaluation of the results. The UICC Committee on Carcinogenicity
Testing (UICC, 1969) recommended that the number of animals should be
such that it "would be likely to yield reasonable (e.g. 90%)
statistical significance as between the main induced tumour incidence
and its spontaneous incidence". A sufficient number of animals must be
alive at the time that the first tumour appears and it is suggested
that this number should never be less than 25 per sex.
7.2.1.2 Route of administration
It has been agreed that the chemical under test should be
administered by the route of human exposure. This applies to food
additives and food contaminants in particular, but it is equally
desirable for drugs. This rule cannot always be applied to
environmental or industrial chemicals. It is well known that the three
main routes of exposure in man are inhalation, ingestion, and skin
absorption. Inhalation is often predominant in man but in experimental
carcinogenesis the inhalation route, while highly desirable in
principle, has rarely been used in the past because of lack of
adequately equipped laboratories. However, it is now being widely used
in programmes concerning smoking and health.
When the route of exposure is per os, the chemical is
administered either mixed in the diet or drinking water, or by gavage,
the choice depending on the specific characteristics of the test
chemical; a volatile compound, for example, should never be mixed in
the diet while, for a drug, the route by which it will be used in man
should be selected, i.e. per os, or by intravenous, subcutaneous or
topical application.
7.2.1.3 Inception and duration of tests
Most commonly, tests are initiated in young adult animals from 7
to 9 weeks of age. The start of treatment soon after weaning is
recommended as a routine procedure. One objective of carcinogenicity
testing is to obtain the maximum possible carcinogenic effect of the
test chemical in order to compensate for the limited number of
individuals at risk. This is accomplished by employing high levels of
exposure (see 7.1.4) and by starting the treatment when the animals
are at their most sensitive age. For some years following the pioneer
work of Pietra et al. (1961), treatment immediately after birth and
within the first 24 h of life was thought to be the most sensitive
model. Careful reviews of the data available have indicated that
neonatal treatment alone can be recommended in some instances, but not
as a general routine procedure (Della Porta & Terracini, 1969). For a
single neonatal administration, only a very limited quantity of the
chemical to be tested is needed and this could be advantageous, when
only a small amount of the chemical is available.
The main limitation of the use of a single neonatal treatment is
that the newborn animal may not have developed sufficient metabolic
competence to metabolize the test chemical. Another limitation is that
not all chemical carcinogens are active after one, or even a few
exposures.
More recently, prenatal exposure has received considerable
attention, since it has been demonstrated that some tissues, notably
nervous tissue, are more susceptible to certain carcinogens during
fetal life than later (Druckrey et al., 1967; Napalkov, 1973; Napalkov
& Alexandrov, 1968; Tomatis, 1973). At present, however, it can only
be speculated that prenatal exposure will reveal the carcinogenic
effect of a chemical that would not have been revealed had treatment
started at a later age. A sensitive procedure might be to integrate
prenatal exposure with long-term, postnatal exposure, in order to
obtain maximum sensitivity of the bioassay system. To achieve this,
animals mated at the age of 8-9 weeks, should be exposed to the test
chemical during the second half of pregnancy. The parents so treated
can constitute a group under conventional long-term testing, if the
treatment is continued after delivery. Their offspring, already
exposed in utero, and possibly after birth through their mother's
milk and excreta, should be exposed directly to the test chemical from
the age of weaning onwards. In this way, two different experimental
groups can be obtained: one for which exposure was started at the
young adult stage (the parents) and the other for which exposure was
started prenatally (the offspring) (Tomatis, 1974b).
The duration of a positive test, i.e. where exposure to the test
chemical is followed by an increased incidence of tumours, depends on
the time of appearance and the rapidity of growth of the induced
tumours. To agree in advance on a given duration of exposure is of
prime importance for negative tests. Some research workers have
adopted a duration for carcinogenicity tests of 2 years in rats and 18
months in mice, while others have preferred an observation period
extending over the entire life span of the animals. A long finite
period -- that is, not less than 2 years -- is recommended in
preference to the entire life span of the animals, for the following
reasons: (a) induced tumours usually occur within this observation
period; (b) "spontaneous" tumours appear with highest frequency late
in life and their appearance may make it more difficult to evaluate
the carcinogenicity of a compound, particularly if of low potency;
(c) a few animals may far exceed the normal life span of the species
and extend unnecessarily the duration of the experiment; (d) tests
are very expensive and any justifiable abbreviation means good economy
(Health & Welfare, Canada, 1973; Tomatis, 1974a; UICC, 1969; WHO,
1961).
7.2.1.4 Dose-level and frequency of exposure
If only one level is to be used, the highest dose allowing long
survival of the majority of the animals should be chosen. The
selection of this dose should be made from results obtained in
subacute toxicity tests. Information on the LD50 of certain
compounds may be available and this can be useful in deciding the
level of the dose for the subacute studies.
While it would be very difficult to give a definition of the
maximum tolerated dose, it seems quite reasonable to insist that it
allows adequate survival of the animals and does not produce a
reduction in weight of more than 10% compared with the controls
(Friedman, 1974). If two dose levels are chosen, the second should be
one quarter or one third of the first dose.
If the goal of the test is merely to ascertain the possible
carcinogenicity of a compound, then the assay protocol should achieve
the maximum sensitivity of the test; in this case, the highest
tolerated dose is the most appropriate. If, however, additional
information has to be collected, in particular regarding the
establishment of a possible minimum effect or no-observed-effect
level, then initiation of a dose-response experiment should be
envisaged. In this case, a minimum of three doses, and preferably
four, should be included. An advantage of including several dose
levels, the lowest of which does not decrease the life span of the
animals, is the possibility of collecting other data on chronic
toxicity not otherwise available through a carcinogenicity test at
high dose levels.
Frequency of exposure may vary according to the route chosen. If
the chemical is administered in the drinking water or mixed in the
diet, exposure is continuous. If the chemical is given by gavage, the
frequency can be two to three times per week. Topical applications may
be made daily, while subcutaneous or intravenous injections must be
more widely spaced, e.g. once or twice weekly. The duration of
treatment should cover almost the entire observation period.
7.2.1.5 Combined treatment and cocarcinogenesis
An investigation on the effect of more than one agent, given
simultaneously, may approximate the actual environmental situation,
where there is never exposure to a single chemical. In particular, it
may be useful in revealing the carcinogenic effect of a chemical of
very low carcinogenic potency and thereby help to identify situations
or populations that may be exposed to an otherwise unsuspected high
risk.
Response to an environmental chemical may be modified by the
action of other chemicals that may either alter the rate and/or
pathway of metabolism of the test chemical, or have a cocarcinogenic
effect. A cocarcinogenic effect may be additive, when the chemical has
a carcinogenic effect on its own which adds to the effect produced by
the test chemical; it may be synergistic, when its effect, combined
with that of the test chemical when given alone, exceeds the summation
of the separate effects; or it may act as an incomplete carcinogen,
that is, only as initiator or promoter in the two-stage carcinogenic
process (UICC, 1969) (see Chapter 13).
It is also essential to keep in mind that dietary components may
influence the incidence of tumours in test animals. This may occur
either because of the unsuspected presence of carcinogens (such as
aflatoxins or nitrosamines) or because of substances that may modify
the response to the test chemical by altering, for instance, the
hepatic microsomal enzyme activity.
7.2.1.6 Positive and untreated controls
An adequate group of untreated animals, serving as controls,
should always be included in the planning of a carcinogenicity test.
The size of the control group should never be smaller than the size of
the treated group and, preferably, should be larger. When more than
one chemical is tested simultaneously, the same control group can be
used, provided that its size is appropriately increased.
Besides these controls, i.e. animals not receiving treatment, and
which could be called negative controls, the inclusion of positive
controls in planning an experiment has recently been recommended, i.e.
inclusion of a group of animals receiving a known carcinogen at a dose
level that has already produced carcinogenic effects in several
laboratory studies. Such controls ensure: (a) more confidence in the
outcome of tests carried out on compounds of unknown activity
(Weisburger, 1974); (b) assessment of the relative carcinogenic
potency of the test chemical, and (c) an indirect check on the
reliability of the test laboratory. While it does not seem essential
to include a positive control in every test, it is highly advisable
for every laboratory to check the sensitivity of its bioassay system,
periodically, with selected known carcinogenic chemicals. It is also
advisable that a group of animals should receive only the vehicle
(i.e. acetone, dimethylsulfoxide, etc.) in which the chemical under
test is eventually administered.
7.2.1.7 Test material
Long-term studies should not be started until sufficient
information on the identity and purity of the test chemical has been
assembled. The importance of knowing the toxicity of impurities is
well demonstrated by the case of TCDD present as an impurity in the
herbicide 2,4,5-T. Drugs or food additives should be tested in the
form and degree of purity intended for human consumption (Health &
Welfare, Canada, 1973; National Academy of Sciences, 1960; WHO, 1961,
1969). In this case, as well as for mixtures of chemicals to which man
may be exposed, it may be highly advisable to carry out additional
studies in order to identify positively the carcinogenic component of
the mixture.
7.2.1.8 Survey of animals, necropsy, and histological examination
In order to present results correctly, detailed records of all
experimental procedures and surveillance of the animals during the
entire observation period should be maintained. While all pertinent
details of the experiment may not be published, it is a good rule for
the investigator to keep them for discussion with other interested
scientists (UICC, 1969). If the results are to be published, it is
essential that adequate information be given on the test chemical and
test animal, as well as on all observations made on the experimental
and control groups (WHO, 1961). A detailed list of items to be
considered is given in the UICC (1969) report.
In the case of neoplasms (Turusov, 1973, 1976), it is extremely
important to give an exact description of the criteria used to
classify lesions as hyperplastic, preneoplastic or neoplastic, benign
or malignant. Many times, pathologists do not agree on certain
diagnoses and it is far easier to interpret the results when the
criteria used for classification are known. Terms such as hepatoma,
which do not indicate the specific tissue of origin, should not be
used (Reuber, 1974), while terms such as cholangioma or adenomatosis
require additional qualification. The term should clearly indicate
whether the lesion is considered malignant. Thus, specific
descriptions should be given, e.g. in the case of "hepatoma" it may be
either a liver cell adenoma or a hepatocellular carcinoma, while
"cholangioma" may be cholangiocellular adenoma or cholangiocellular
carcinoma. In addition, carcinomas may be subdivided into well,
moderately, and poorly differentiated carcinemas.
A lesion with atypical cells or with focal malignant change
should be classified separately rather than under malignant tumours.
7.2.2 Short-term tests (rapid screening tests)
To date, there are no reliable alternatives to long-term
bioassays for testing the carcinogenicity of chemicals; this means a
delay of two years or more before a carcinogenicity bioassay yields
results. However, recent advances in mutagenicity testing hold out
hopes of a short-term test, at least for selecting, from among the
many chemicals to be tested, those most in need of early attention. So
far, about 80% of carcinogens have been shown to be mutagenic and with
the continuously increasing sensitivity of the models now employed an
even higher correlation is possible. At present, mutagenicity testing
represents a valuable system for screening chemicals to be submitted
to long-term carcinogenicity testing; it cannot replace long-term
testing, until a satisfactory positive correlation is established and
the possibility of false negatives eliminated.
A mutation is any heritable change in genetic material including
a chemical transformation of an individual gene (point mutation) or a
change involving rearrangement of parts of a chromosome (chromosome
mutation). The question of whether or not a mutagenic event is a
prerequisite for carcinogenesis has long been debated. Many mutagens
have been shown to be carcinogenic but other known carcinogens are
known not to be mutagenic. Other mechanisms not involving DNA have
also been implied in the process of carcinogenesis (Pitot, 1974).
Recent short-term mutagenicity testing procedures (McCann & Ames,
1976: McCann et al., 1975; Sugimura et al., 1976) have shown greater
correlation between mutagenicity and carcinogenicity; consequently,
some thought is now being given to using these assays as preliminary
screens for potential carcinogens. However, at the moment, the fact
that a particular compound has mutagenic activity can only be
considered, in terms of carcinogenicity, as indicative of potential
reactivity with DNA.
7.2.2.1 Metabolic activation, reaction with DNA, and DNA repair
The synthetic and naturally occurring chemical carcinogens
include a variety of chemicals having no common structural feature.
However, it is becoming clear that the ultimate reactive forms of many
chemical carcinogens are electrophilic (electron-deficient) reactants
(Miller, 1970). Although some chemical carcinogens, such as direct
alkylating agents and metal ions, are electrophiles per se, the
majority require metabolic activation to reactive forms (ultimate
carcinogens) (Fig. 7.1).
The formation of these electrophilic reactants from exogenous
chemicals, in an animal species or in an organ, is the result of the
balance between in vivo activation and deactivation reactions
carried out predominantly by enzymes localized in the endoplasmic
reticulum of the cell. The ultimately available concentration of
reactive metabolites seems to account for some of the organ and
species specificities shown by several chemical carcinogens.
During the last decade, much progress has been made in
understanding the metabolism of various classes of chemical
carcinogens (polynuclear hydrocarbons, Sims & Grover, 1974;
N-nitroso compounds, Magee et al., 1976; aromatic amines, Kriek,
1974; Weisburger & Weisburger, 1973; miscellaneous compounds, Miller,
1973). The activity of microsomal mixed function oxidases is
influenced by various drugs or environmental chemicals, that may
stimulate or inhibit the formation of the ultimate carcinogens (Conney
& Burns, 1972).
These ultimate carcinogens bind covalently with cellular
macromolecules such as DNA, RNA, or proteins, which, directly or
indirectly, leads to heritable changes in the affected cells. Although
the critical targets of chemical carcinogens are not known, there is a
great deal of evidence supporting the theory that DNA is one major
target in carcinogenesis (Farber, 1973).
Some specific binding sites for the metabolites of carcinogens
have been identified in the bases of DNA. The carcinogenic N-nitroso
compounds are examples (Magee et al., 1976). The main site of
alkylation of DNA by alkylnitroso compounds is the N,7 position of
guanine, as with other alkylating agents. Other sites are also
attacked and the significance of these DNA interactions is being
investigated. The formation of 7-alkylguanine, however, seems to be of
no obvious importance in the process of tumour induction, since no
quantitative or qualitative correlation between the occurrence of this
alkylated base in the DNA of treated animals and the carcinogenicity
of nitrosamines or alkylating agents has been observed (Magee et al.,
1976). Alkylation of guanine bases in DNA at the 7-position does not
seem to produce mutations in bacteriophages (Loveless & Hampton,
1969), nor does it alter the coding properties of synthetic
polynucleotides in vivo (Ludlum, 1970). Various laboratories have
examined the biological importance of the alkylated O,6 position of
guanine residues in DNA which, in consequence, is able to induce
mutations in phage (Loveless, 1969). It has been shown that there is
an aberrant base pairing with a polymer containing alkylated
6.7.8 Variables to monitor
It is necessary to monitor several variables during the operation
of an inhalation chamber. The most obvious is the concentration in the
chamber of the pollutant in question. This can be done continuously by
automated samplers and recorded on a strip chart recorder or manually
at periodic intervals using a variety of sampling techniques. When
aerosols are involved, particle size should be determined. The flow of
pollutant, the flow of air, and the chamber pressure should all be
recorded frequently. Chamber temperature and humidity are other
variables that should be monitored. It is also useful to measure the
pressure drop across intake and exhaust filters in order to know when
to replace them.
6.7.9 Human exposure facilities
Ethical principles will always be of first concern, when
considering the exposure of human subjects to materials for toxicity
evaluation. However, there are situations where controlled exposure of
human beings can provide useful information with minimum risk. One of
the more modern facilities has been described by Stewart et al.
(1970a,b). It consists of a room approximately 6 m × 6 m × 2.7 m,
completely air conditioned and operated at slightly negative pressure.
Activity within this chamber is strictly sedentary and comfortable
chairs and study desks are provided. Meals are served during
exposures, with coffee and soft drinks available continuously. All
subjects are under continuous surveillance by medical personnel and
all activities are visually monitored by closed circuit television.
Such facilities are particularly useful for studying psychomotor
effects and other sensitive indicators of exposure to low
concentrations of organic vapours.
The odour threshold concentrations used in the USSR for
establishing maximum permissible concentrations for single exposures
are determined in human subjects over short periods (5-10 min).
Obviously such studies can only be carried out at very low
concentrations considered to be safe. The minimum concentration sensed
by the most sensitive individual is accepted as the odour threshold
(Rjazanov, 1964).
Reflex reactions produced in man by irritating the receptive
zones of the respiratory organs with subsensory concentrations of
atmospheric pollutants, were established by measuring the light
sensitivity of the eye, the bioelectric activity of the cerebrum, etc.
(Bustueva et al., 1960; Rjazanov, 1964). Changes in encephalographic
responses resulted from exposure to small concentrations of these
substances. The maximum concentration that did not produce an effect
on the bioelectric activity of the cerebrum was, in most cases, 3-4
times lower than the odour threshold concentration (Krotov, 1971;
Sidorenko & Pinigin, 1972).
6.8 Contaminant Generation and Characterization
Extensive reviews have been published by several investigators on
methods of generating and characterizing vapours and particles (Bryan,
1970; Cotabish et al., 1961; Drew & Lippmann, 1971; Lodge, 1968;
Mercer, 1973a,b; Nelson, 1971; Raabe, 1970).
6.8.1 Generation of vapours
Vapours can be generated by using one of several flow-dilution
devices (Cotabish et al., 1961; Drew & Lippmann, 1971; Nelson, 1971;
Saltzman, 1971; Saltzman & Warburg, 1965). If the contaminant is a
liquid at room temperature, a vaporization step must be included. One
procedure is to use a motor-driven syringe and to apply the liquid to
a wick or heated plate in a calibrated stream of air (Nelson & Griggs,
1968). Another method is to saturate the airstream with vapour and
then dilute it with air to the desired concentration (Cotabish et al.,
1961). A third technique, originally described by O'Keefe & Ortman
(1966), consists of using permeation tubes. These are especially
useful when using low concentrations of test materials for
standardization procedures. In theory, there is no reason why they
cannot be scaled up for use in inhalation studies.
6.8.2 Particle generators
The generation of particulate contaminants is usually more
difficult than vapour generation. The contaminant may be generated
from a dry powder or from a liquid and the particles generated may be
of uniform size (monodisperse) or may vary greatly in size
(heterogeneous).
6.8.2.1 Heterogeneous aerosols
The Wright dust feed (1950) is one of the better known
instruments for generating aerosols from a dry powder. A gear drives
the surface of a packed cylinder of finely ground powder against a
scraping mechanism. A high velocity airstream disperses the powder.
Proper use of the Wright dust feed is dependent upon the control of
the relative humidity of the airstream and the packing density of the
powder. Other devices for producing aerosols from dry powders have
been described (Crider et al., 1968; Deichman, 1944; Dimmick, 1959;
Stead et al., 1944). Since the particle size of the resultant aerosols
depends upon the size of the original powder, elutriators and cyclones
are sometimes included to limit the maximum size. Laskin et al. (1971)
described generators that include such devices for producing freshly
ground polyurethane aerosols.
Agglomeration, usually caused by electrical charge, is a serious
problem with dry dust aerosols. The electrical behaviour of aerosols
has been reviewed by Whitby & Liu (1966). A mechanical solution to
agglomeration has been proposed by Drew & Laskin (1971) who described
a fluidizing dust generator.
Wet dispersion generators break liquid into droplets. The liquid
may be a solution or a suspension of the test material. In most cases,
the liquid is drawn into filaments or films that are broken into
droplets. A number of compressed air-driven generators (nebulizers),
that produce droplets of many sizes, have been described (Dautrebande,
1962; Laskin, 1948; Lauterbach et al., 1956; Raabe, 1970). The
resulting aerosols are polydisperse although relatively narrow size
distribution can be attained with some nebulizers.
Monodisperse aerosols are occasionally needed by investigators,
especially when studying regional deposition. The most popular device
for dispensing uniform droplets is the spinning disc generator first
described by Walton & Prewett (1949). Primary droplets thrown off the
perimeter of a spinning disc are uniform in size. The liquid is fed on
to the centre of the disc and accumulates at the edge until broken off
by centrifugal force. Some secondary, smaller droplets are also
produced but these can be separated dynamically. Several investigators
have successfully used the spinning disc for inhalation studies
(Albert et al., 1964; Kajland et al., 1964; Lippmann & Albert, 1967;
Philipson, 1973). Another device employing a controlled condensation
process originally described by LaMer & Sinclair (1943) has been found
to be suitable for inhalation studies. A third principle, consisting
of size specific collection, resuspension and subsequent dispersion,
has also been used (Kotrappa et al., 1972).
6.8.3 Monitoring contaminant concentrations
The techniques and equipment for monitoring contaminant
concentrations have been reviewed by a number of authors (Lippman,
1971; Powell & Hosey, 1965). In many cases, the characteristics of the
contaminant determine the sampling technique. Sometimes a number of
techniques are available and the method of choice may depend upon the
availability of equipment, cost of reagents, time for analysis, or
other factors. Automated instrumentation is currently available for a
number of contaminants. However, when using automatic devices,
a second method, usually chemical, should be used to verify the
instrument performance.
6.8.3.1 Vapour sampling
Two basic methods for the collection of gaseous samples are
employed. The first involves the use of a gas collector, such as an
evacuated flask or bottle, to obtain a definite volume of air at a
known temperature and pressure. The second method involves the passage
of a known volume of air through a collecting medium to remove the
desired contaminants from the sampled atmosphere. The samples are then
analysed by appropriate analytical techniques.
Since the assays are related to the volume of air sampled, the
instrumentation for monitoring airflow or volume should be accurately
calibrated. It is also important that there are no leaks in the
sampling train, thus assuring that all the air measured has passed
through the collecting medium.
A number of devices, currently available, measure the
concentration of vapours continuously; many record the result
graphically. Many detection principles are used including
conductivity, colorimetry, and spectrophotometry. Recent commercial
instrumentation using the principle of infrared spectrometry are
especially useful. Most of these instruments have been described by
Nader (1971).
6.8.3.2 Particulate sampling
When monitoring particulate atmospheres, mass concentration and
particle size must be determined. The mass concentration can be
measured by techniques similar to those used for monitoring vapours.
The material can be collected, then assayed by appropriate chemical
methods. Gravimetric analysis can also be performed by weighing the
filter paper before and after collecting a sample. The resulting mass
can be related to the volume of air that was sampled.
Particle collection techniques include filtration, impingement,
thermal and electrostatic precipitation, and sedimentation. The basic
principles for these techniques and specific examples of each method
have been reviewed (Lippmann, 1971). These principles should be
thoroughly understood prior to selection of a method for a specific
contaminant.
The techniques for measuring particle size and numbers have been
discussed in detail by Mercer (1973a,b). Direct methods consisting of
both conventional and electron microscopy can be used. In both cases,
proper sampling methods to ensure collection of a representative
sample should be followed. Electrostatic and thermal precipitators or
an impinger can be used. After using an impinger, the dust can be
counted in a standard haematology counting cell or counted directly
with a Coulter Counter.
There are two indirect methods of assessing particle size; the
use of cascade impactors and the use of light scattering devices. The
theory of cascade impaction has been described by Mercer (1963, 1964,
1965). The theory of light scattering devices has been reviewed by
Hodkinson (1966) and commercially available instruments have been
listed by Swift (1971). Many factors, including shape, opacity, and
others, some unknown, affect the amount of light scattered and the
measured number and size of the particles. These devices should,
therefore, be used cautiously.
6.9 Other Methods of Respiratory Tract Exposure
6.9.1 In vivo exposures
In addition to direct inhalation, several other methods of
exposing the respiratory tract have been described (Roe, 1968).
Andervont (1937) originally described a thread implantation technique
whereby a thread impregnated with a test compound was literally passed
through a lobe of the lung. This technique was modified by Kuschner et
al. (1957) who devised a pellet that could be impregnated with the
test material or coated with radioactive materials. This pellet had
hooks which held it in the lumen of the bronchus or bronchiole. This
technique has been used to demonstrate the carcinogenicity of a number
of compounds including benzo(a)pyrene, 3-methylcholanthrene, and
calcium chromate (Laskin et al., 1970). A technique whereby hamsters
are anaesthetized and then intratracheally intubated with various
materials is in use in a number of laboratories (Laskin et al., 1970;
Little & O'Toole, 1974; Saffiotti, 1970; Schreiber et al., 1972). In
two laboratories, this technique has been coupled with inhalation
exposures to study the potential cocarcinogenicity of various agents
(Laskin & Nettesheim, 1974, personal communication).
6.9.2 In vitro exposures
A few in vitro techniques that show promise as regards
elucidation of the mechanisms of toxicity of inhaled materials should
be mentioned. A number of investigators (Niemeier & Bingham, 1972;
O'Neill & Tierney, 1974; Orton et al., 1973) are using various,
isolated, perfused lung preparations to study toxicity. Such
preparations could be particularly useful in studying the transfer of
materials from the lung to the blood. These preparations are also used
to study pulmonary metabolism as are lung tissue slices (O'Neil &
Tierney, 1974). Finally, several laboratories are developing methods
of culturing tracheal rings and sections (Griesemer et al., 1974; Lane
& Miller, 1975). These preparations show great promise for
toxicological evaluation (section 2.7.4).
6.10 Biological End-points and Interpretation of Changes in these
End-points
Throughout the course of an inhalation study, there are a number
of biological indicators to observe. The classical indices of toxicity
include weight change and mortality, organ-to-body weight ratios, and
both gross and microscopic changes in the morphology of the various
tissues and organs. While the study is in progress, respiratory,
physiological indices can be monitored and the excretion of certain
chemicals in breath, urine, and faeces can also be followed. Some
procedures particularly useful in inhalation toxicity studies are
discussed below.
6.10.1 Morphological changes
The primary function of the lung is the exchange of oxygen and
carbon dioxide between the blood and alveolar air. The walls of the
alveoli are lined by a single, flattened layer of epithelial cells
that are in close proximity to endothelial cells lining capillaries.
The mean distance from the lumen of the capillary to the lumen of the
alveolus is less than one micron. Because this distance is so small,
any thickening or inflammation of the alveolar wall will severely
disrupt the diffusion of gases.
The air in the alveolus arrives through a series of branching
bronchi and bronchioles the specialized epithelial lining of which is
instrumental in removing particulate matter from the lung. Any lesions
that result in narrowing of the lumen of these bronchi and bronchioles
cause a disruption in the ventilation of that portion of the lung.
Chemicals affecting the specialized ciliated epithelial lining, the
bronchioles, and the bronchi may interrupt the clearance mechanism,
resulting in a build-up of inhaled particulates in the lung.
The type of morphological change observed in the lung as a result
of the inhalation of materials depends upon the concentration of the
inhaled material and the length of time for which animals are exposed.
Short-term inhalation of high concentrations of certain materials may
result in acute changes in the lung such as oedema, necrosis, and
purulent inflammation. However, the inhalation of the same material at
lower concentrations for longer periods of time may result in chronic
changes such as fibrosis and even neoplasia. Table 6.3 lists a number
of chemicals and the response elicited in the lung following their
inhalation.
Techniques for quantifying morphological changes are, at best,
limited. The usual practice is to assign some number to the degree of
damage and then to apply statistical procedures to these numbers.
These procedures are, however, subject to individual interpretation.
Assessment of the degree of fibrosis is possible (Roe, 1968), although
such measurements should be checked by special staining procedures.
Interpretation of any morphological changes in the lung must take
into consideration the concentration and duration of exposure.
Exposure to certain materials will elicit immediate acute
morphological changes which, with time, tend to resolve and disappear.
This phenomenon suggests that the animal is able to adapt to the
effects of some chemicals, if the concentration is not too high. An
Table 6.3 Responses of the lung to various chemicals
Chemical Bronchiolar Loss of Oedema Epithelial Fibrosis Emphysema Granuloma
epithelial goblet metaplasia
hyperplasia cells
nitrogen dioxide x x x x x
ozone x x x x x
allergens x
sulfuric acid x x
bis-chloromethyl ether
silica x x
coal dust x x
beryllium x x x
cotton dust
nickel compounds
Table 6.3 (cont'd)
Necrosis Neoplasia Bronchial Alveolar Nonsuppurative
constriction epithelial alveolitis
hyperplasia
nitrogen dioxide x
ozone
allergens x
sulfuric acid x x
bis-chloromethyl ether x
silica
coal dust
beryllium x
cotton dust x
nickel compounds x
example of this adaptive phenomenon is seen with ozone. Nonlethal
exposures to ozone have a protective effect against subsequent lethal
exposures which would result in marked pulmonary oedema (Alpert &
Lewis, 1971).
6.10.2 Functional changes
There are certain advantages in using alterations in respiratory
functions as indices of toxicity: (a) quantitative measurements can
usually be made at concentrations far below those needed to produce
morphological effects; (b) many effects may be measured at the level
of reversible rather than irreversible changes; (c) such measurements
may elucidate mechanisms of action of materials, and (d) in many
instances similar data can be obtained from experimental animals and
man.
6.10.2.1 Measurement of respiratory frequency
Alteration of respiratory frequency in mice may be used as a
simple screening test for assessing irritant potency (Alarie, 1973).
The changes produced are dose-related, which permits construction of
dose-effect curves and calculation of the concentration required to
produce, for example, a 50% decrease in respiratory frequency. Some
irritants, mainly those affecting the upper respiratory tract,
decrease frequency. Other irritants, mainly those which penetrate to
the deeper areas of the lung, increase frequency. Measurement of
respiratory frequency alone is a very sensitive measure of effect for
those irritants that increase frequency (e.g. ozone and nitrogen
dioxide), but it is a relatively insensitive measure of effect for
those irritants that decrease frequency (e.g. sulfur dioxide and
formaldehyde).
6.10.2.2 Measurement of mechanics of respiration
Alterations in pulmonary flow resistance or pulmonary compliance
may be used to assess irritant potency. The method of Amdur & Mead
(1958) required three basic measurements: intrapleural pressure, tidal
volume, and the rate of flow of gas in and out of the respiratory
tract. Intrapleural pressure is estimated by placing a fluid-filled
catheter in the pleural space, while the animal is under anaesthesia.
Once the catheter is positioned, no further anaesthesia is necessary.
Tidal volume is obtained by placing the animal in a body
plethysmograph and measuring the pressure changes as the animal
breathes quietly. Rate of gas flow is obtained by electrical
differentiation of the volume signal. This method provides data on
both resistance and compliance, but is generally limited to single
exposure studies of a few hours duration. The guineapig has been the
species most commonly used for this method; however, plethysmographic
studies have been successfully carried out on several other rodents
including mice (Alarie, 1973), rats (Palacek, 1969) and rabbits
(Davidson et al., 1966).
The method of Murphy & Ulrich (1964) does not involve surgical
intervention and, thus, makes it possible to perform measurements
repeatedly on the same animals. The animal is placed in a body
plethysmograph to which an oscillating sine wave pressure is applied.
The animal's face is fitted with a mask containing a pneumotachograph
screen for measuring flow. Changes in resistance are calculated for
the alterations in flow produced by the superimposed pressure
oscillations. This technique has been used in toxicological studies
without making compliance measurements. However, by measuring
oesophageal pressure reflecting intrapleural pressure, it should be
possible to estimate compliance changes. A compliance measurement is
described by Mead (1960) that involves no surgical intervention.
The observed changes in respiratory mechanics are dose-related
and permit the construction of dose-effect curves. These may be used
to compare irritant potency or to study such things as the effect of
inert particles on the reaction to irritant gases. The deep lung
irritants that increase respiratory frequency tend to show a decrease
in compliance as the primary alteration in the mechanical behaviour of
the lung. Resistance changes with such irritants are minimal. The
irritants that slow respiratory frequency tend to show an increase in
resistance accompanied by a smaller decrease in compliance, as the
primary alteration in pulmonary mechanics.
An increase in pulmonary flow resistance can result from a
narrowing of the lumen of the bronchi mediated by constriction of the
smooth muscle. The effect, in this situation, usually occurs rapidly
on exposure to the irritant. In the case of a gaseous irritant, the
effect is fairly rapidly reversed when irritant exposure ceases. In
the case of a particulate irritant which remains in the lung, the
effect is less readily reversible. Swelling of the respiratory mucosa
or an increase in mucus secretion can also cause an increase in flow
resistance. The effect, in this situation, usually takes some time to
develop.
Much of the damage to the lung is to the small airways which
change either in calibre or stability or both. It would be very useful
if this damage could be detected early during an exposure before more
serious disease develops. There are three tests which are promising
and have been useful in human subjects. They are the maximal
expiratory flow volume curve, reviewed by Hyatt & Black (1973), the
alveolar closing volume (Dollfuss et al., 1967) and the frequency
dependency of compliance (Woolcock et al., 1969). Animal models that
provide the same data have not yet been fully developed.
6.10.3 Biochemical end-points
In the last decade, much research has centred around the
elucidation of some of the biochemical aspects of the lung. It has
only recently been demonstrated that the lung is capable of
biotransforming many foreign chemicals and several authors have
investigated the role of cytochrome P-450-dependent enzyme systems in
pulmonary microsomes (Bend et al., 1972; Chhabra & Fouts, 1974;
Chhabra et al., 1974; Fouts & Devereux, 1972; Harper et al., 1975;
Hook et al., 1972). The importance of intermediary metabolism is now
being considered (Tierney, 1974). The biochemistry of pulmonary
surfactants and their role in pulmonary defence mechanisms has been
reviewed by King (1974). Investigations of the lung collagen have
recently been reviewed by Crystal (1974). Witschi (1975) has provided
an excellent summary of biochemical approaches for the evaluation of
pulmonary toxicity.
6.10.4 Other end-points in inhalation studies
Exposure by inhalation is only a means by which the substance
enters the animal. This chapter has concentrated on the lung as the
critical organ. However, there are end-points that are not necessarily
related to the lung. Inhalation studies have recently been reported to
assess the teratological effects of inhaled materials (Schwetz et al.,
1975). They have also been used in behavioural studies (Weiss &
Laties, 1975; Xintaras et al., 1974).
REFERENCES
ALARIE, Y. (1973) Sensory irritation of the upper airways by airborne
chemicals. Toxicol. appl. Pharmacol., 24: 279-297.
ALBERT, R. E., PETROW, H. G., SALAM, A. S., & SPIEGELMAN, J. R. (1964)
Fabrication of monodisperse lucite and iron oxide particles with
a spinning disc generator. Health Phys., 10: 933-940.
ALBERT, R. E., LIPPMANN, M., & BRISCOE, W. (1969) The characteristics
of bronchial clearance and the effects of cigarette smoking.
Arch. environ. Health, 18: 738-755.
ALBERT, R. E., LIPPMANN, M., PETERSON, H. T., JR, BERGER, J., SANBORN,
K., & BORWAG, D. (1973) Bronchial deposition and clearance of
aerosols. Arch. intern. Med., 131: 115-131.
ALBERT, R. E., BERGER, J., SANSORN, K., & LIPPMANN, M. (1974) Effects
of cigarette smoke components on bronchial clearance in the
donkey. Arch. environ. Health, 29: 96-101.
ALPERT, S. M. & LEWIS, T. R. (1971) Ozone tolerance studies utilizing
unilateral lung exposure. J. appl. Physiol., 192: 364-368.
AMDUR, M. O. & MEAD, J. (1958) Mechanics of respiration in
unanaesthetized guinea pigs. Am. J. Physiol., 192: 364-368.
ANDERSEN, I., LUNDQVIST, G. R., JENSON, P. L., & PROCTOR, D. F. (1974)
Human response to controlled levels of sulfur dioxide. Arch.
environ. Health, 28: 21-39.
ANDERVONT, H. B. (1937) Pulmonary tumours in mice: IV. Lung tumours
induced by subcutaneous injection of 1,2,5,6-dibenzanthracene in
different media and by its direct contact with lung tissue.
Public Health Rep., 52: 1584.
BALASOV, V. E., BARTENEV, V. D., SAVICKIJ, I. V., & TRAHTENSERG, I. M.
(1968) [Toxicological assessment of volatile substances leaking
from synthetic materials.] Zdorov'e, Kiev (in Russian).
BEND, J. R., HOOK, G. E. R., EASTERLING, R. E., GRAM, T. E., & FOUTS,
J. R. (1972) A comparative study of the hepatic and pulmonary
microsomal mixed function oxidase systems in the rabbit.
J. Pharmacol. exp. Ther., 183: 206-217.
BOECKER, B. B., AQUILAR, F. L., & MERCER, T. T. (1964) The design of a
canine inhalation exposure apparatus incorporating a whole-body
plethysmograph. Health Phys., 10: 1077-1089.
BOYD, M. R., BURKA, L. T., HARRIS, T. M., & WILSON, B. J. (1974)
Lung-toxic furanoterpenoids produced by sweet potatoes (Ipomoea
batatas) following microbial infection. Biochim. Biophys.
Acta, 337: 184-195.
BRIAN, J. D. & VALBERG, P. A. (1974) Models of lung retention based on
International Congress of Radiation Protection Task Group Report.
Arch. environ. Health, 28: 1-11.
BRYAN, R. J. (1970) Generation and monitoring of gases for inhalation
studies. In: Hanna, M. G. Jr, Nettesheim, P., & Gilbert, J. R.,
ed. Inhalation carcinogenesis. Springfield VA, Clearinghouse
for Federal Scientific and Technical Information, NBS, US Dept of
Commerce (CONF-691001).
BUSTUEVA, K. A., POLEZAEV, E. F., & SOMENENKO, A. D. (1960) [A study
of threshold values for reflex action of atmospheric pollutants.]
Gig. i Sanit., No. 1, pp. 57-61 (in Russian).
CADLE, R. D. (1965) Particle size. Princeton, NJ, Van
Nostrand-Reinhold.
CAHAN, W. G. & KIRMAN, D. (1968) An effective system and procedure for
cigarette smoking by dogs. J. surg. Res., 8: 567-575.
CAMNER, P., PHILIPSON, K., & FRIBERG, L. (1972) Tracheobronchial
clearance in twins. Arch. environ. Health, 24: 82-87.
CAMNER, P., MOSSBERG, B., & PHILIPSON, K. (1973) Tracheobronchial
clearance and chronic obstructuve lung disease. Scand. J.
respir. Dis., 54: 272-281.
CAMNER, P., HELLSTRÖM, P. A., LUNDBERG, M., & PHILIPSON, K. (1977)
Lung clearance of 4 µm particles coated with silver, carbon and
beryllium. Arch, environ. Health, 32: 58-62.
CARPENTER, C. P., SMYTH, H. F., JR, & POZZANI, U. C. (1949) The assay
of acute vapour toxicity and the grading and interpretation of
results on 96 chemical compounds. J. ind. Hyg. Toxicol.,
31: 343-346.
CASARETT, L. J. (1972) The vital sacs: alveolar clearance mechanisms
in inhalation toxicology. In: Hayes, W. J., ed. Essays in
toxicology. New York, London, Academic Press.
CHHABRA, R. S. & FOUTS, J. R. (1974) Sex differences in the metabolism
of xenobiotics by extrahepatic tissue in rats. Drug Metab.
Disposition, 2: 375-379.
CHHABRA, R. S., POHL, R. J., & FOUTS, J. R. (1974) A comparative study
of xenobiotic-metabolizing enzymes in liver and intestine of
various animal species. Drug Metab. Disposition, 2: 443-447.
COTABISH, H. N., MCCONNAUGHEY, P. W., & MESSER, H. C. (1961) Making
known concentrations for instrument calibration. Am. Ind. Hyg.
Assoc. J., 22: 392-402.
CRIDER, W. L., BARKELEY, N. P., & STRONG, A. A. (1968) Dry-powder
aerosol dispensing device with long-time output stability.
Rev. Sci. Instrum., 39: 152-155.
CRYSTAL, R. G. (1974) Lung collagen: definition, diversity and
development. Fed. Proc., 33: 2248-2255.
DAUTREBANDE, L. (1952) Microaerosols, New York, Academic Press,
pp. 7-22.
DAVIDSON, J. T., WASSERMAN, K., LILLINGTON, G. A., & SCHMIDT, R. W.
(1966) Pulmonary function testing in the rabbit. J. appl.
Physiol., 21: 1094-1098.
DEICHMANN, W. B. (1944) An apparatus designed for the production of
controlled dust concentrations in the air breathed by
experimental animals. J. ind. Hyg. Toxicol., 26: 334-335.
DIMMICK, R. L. (1959) Jet disperser for compacted powders in the
one-to-ten micron range. Am. Med. Assoc. Arch. ind. Health,
20: 8-14.
DOLLFUSS, R. E., MILIC-EMILI, J., & BATES, D. V. (1967) Regional
ventilation of the lung studied with boluses of 133 xenon.
Respir. Phys., 2: 234-246.
DONTENWILL, W. (1970) Experimental investigations on the effect of
cigarette smoke inhalation on small laboratory animals. In:
Hanna, M. G. Jr, Nettesheim, P., & Gilbert, J. R., ed.
Inhalation carcinogenesis. Springfield VA, Clearinghouse for
Federal Scientific and Technical Information, NBS, US Dept of
Commerce.
DRAIZE, J. H., NELSON, A. A., NEWBURGER, S. N., & KELLEY, E. A. (1959)
Inhalation toxicity studies of six types of aerosol hair sprays.
Proc. Sci. Sec. Toilet Goods Assoc., 31: 28-32.
DREW, J. H. & LASKIN, S. (1971) A new dust-generating system for
inhalation studies. Am. Ind. Hyg. Assoc. J., 32: 327-330.
DREW, R. T. & LASKIN, S. (1973) Environmental inhalation chambers.
In: Day, W. I., ed. Methods of animal experimentation. New
York, Academic Press.
DREW, R. T. & LIPPMANN, M. (1971) Calibration of air sampling
instruments: II. Production of test atmospheres for instrument
calibraton. In: Lippmann, M., ed. Air sampling instruments, 4th
ed., Cincinnati, OH, ACGIH, pp. I-1.
DREW, R. T. & TAYLOR, G. J. (1975) Cardiophysical studies with
stressed animals. In: Proceedings of the 5th Annual Conference
on Environmental Toxicology, Dayton, Ohio, 1974, US Govt
Printing Office, pp. 121-129.
FOUTS, J. R. & DEVEREUX, T. R. (1972) Developmental aspects of hepatic
and extrahepatic drug-metabolizing enzyme systems: microsomal
enzymes and components in rabbit liver and lung during the first
month of life. J. Pharmacol. exp. Ther., 183: 458-468.
FRASER, D. A., BALES, R. E., LIPPMANN, M., & STOKINGER, H. E. (1959)
Exposure chambers for research in animal inhalation. USPHS
(Public Health Monograph 57).
GOLDBERG, I. S. & LAURENCO, R. V. (1973) Deposition of aerosols in
pulmonary disease. Arch. intern. Med., 131: 88-92.
GRIESEMER, R. A., KENDRICK, J., & NETTESHEIM, P. (1974) Tracheal
grafts. In: Proceedings on Experimental Respiratory
Carcinogenesis, Seattle 1974, Springer-Verlag, pp. 537-547.
GROSS, P., PFITZER, E. A., TOLKER, E., BABYAK, M. A., & KASCHAK, M.
(1965) Experimental emphysema: its production with papain in
normal and silicotic rats. Arch. environ. Health, 11: 50-58.
HABER, F. (1924) [Five lectures for the years 1920-1923.] Berlin,
Springer-Verlag (in German).
HARPER, C., DREW, R. T., & FOUTS, J. R. (1975) Species differences in
benzene hydroxylation to phenol by pulmonary and hepatic
microsomes. Drug Metab. Disposition, 3: 381-388.
HAUN, C. C. (1972) Continuous animal exposure to methylene chloride.
In: Proceedings of the 2nd Annual Conference on Environmental
Toxicology, Dayton, Ohio, 1971, US Govt Printing Office,
pp. 309-326.
HAZUCHA, M., SILVERMAN, F., PARENT, C., FIELD, S., & BATES, D. V.
(1973) Pulmonary function in man after short-term exposure to
ozone. Arch. environ. Health, 27: 183-188.
HENDERSON, D. W. (1952) Apparatus for study of airborne infection.
J. Hyg. (Lond.), 50: 53-68.
HINNERS, R. G., BURKART, J. K., & CONTNER, G. L. (1966) Animal
exposure chambers in air pollution studies. Arch. environ.
Health, 13: 609-615.
HINNERS, R. G., BORKART, J. K., & PUNTE, C. L. (1968) Animal
inhalation exposure chambers. Arch. environ. Health,
16: 194-206.
HODKINSON, J. R. (1966) The optical measurements of aerosols. In:
Davies, C. N., ed. Aerosol science, New York, Academic Press,
pp. 287-357.
HOFFMANN, D. & WYNDER, E. L. (1970) Chamber development and aerosol
dispersion. In: Hanna, M. G. Jr, Nettesheim, P., & Gilbert, J.
R., ed. Inhalation carcinogenesis. Springfield, VA.
Clearinghouse for Federal Scientific and Technical Information,
NBS, US Dept of Commerce (CONF-691001).
HOMBURGER, F., BERNFELD, P., BOGDONOFF, P., KELLEY, T., & WALTON, R.
(1967) Inhalation by small animals of fresh cigarette smoke
generated by new smoking machine. Toxicol. appl. Pharmacol.,
10: 382.
HOOK, G. E. R., BEND, J. R., HOEL, D., FOUTS, J. R., & GRAM, T. E.
(1972) Preparation of lung microsomes and a comparison of the
distribution of enzymes between subcellular fractions of rabbit
lung and liver. J. Pharmacol. exp. Ther., 182: 474-490.
HYATT, R. E. & BLACK, L. F. (1973) The flow-volume curve: a current
perspective. Am. Rev. Respir. Dis., 107: 191-199.
JEMSKI, J. V. & PHILLIPS, G. B. (1965) Aerosol challenge of animals.
In: Gay, W. I., ed. Methods of animal experimentation, New
York, Academic Press, Vol. 1, pp. 273-341.
KAJLAND, A., EDFORS, M., FRIBERG, L., & HOLMA, B. (1964) Radioactive
monodisperse test aerosols and lung clearance studies. Health
Phys., 10: 941-945.
KING, R. J. (1974) The surfactant system of the lung. Fed. Proc.,
33: 2238-2247.
KLJACKINA, A. M. (1973) In: Toxicology of new industrial chemical,
Moscow, Medicina, Vol. 13, p. 23.
KOTRAPPA, P., BOYD, H. A., & WILKINSON, C. J. (1972) Technology for
the production of monodisperse aerosols of oxides of transuranic
elements for inhalation experiments. Health Phys., 22: 837-843.
KROTOY, YU. A. (1971) [Setting of approximate maximum permissible
concentrations of atmospheric pollutants by calculation.]
Gig. i Sanit., 12: 8-12 (in Russian).
KUSCHNER, M., LASKIN, S., CHRISTOFANO, E., & NELSON, N. (1957)
Experimental carcinoma of the lung. In: Proceedings of the 3rd
National Cancer Conference, Philadelphia, Lippincott,
pp. 485-495.
KUSCHNER, M., LASKIN, S., DREW, R. T., CAPIELLO, V., & NELSON, N.
(1975) Inhalation carcinogenicity of alpha haloethers: III.
Lifetime and limited period inhalation studies with
bis(chloromethyl) ether at 0.1 ppm. Arch. environ. Health,
30: 73-77.
LAMER, V. K. & SINCLAIR, D. (1943) An improved homogeneous aerosol
generator. Washington DC, US Dept of Commerce (OSRD Report
No. 1668).
LANE, B. P. & MILLER, S. L. (1975) Dose dependence of
carcinogen-induced changes in tracheal epithelium in organ
culture. In: Proceedings of the Symposium on Experimental
Respiratory Carcinogenesis, Seattle, 1974, Springer-Verlag,
pp. 507-513.
LASKIN, S. (1948) AEC Project Quarterly Report UR-38. Rochester, NY,
University of Rochester.
LASKIN, S., KUSCHNER, M., & DREW, R. T. (1970) Studies in pulmonary
carcinogenesis. In: Hanna, M. G. Jr, Nettesheim, P., & Gilbert,
J. R., ed. Inhalation carcinogenesis. Springfield VA,
Clearinghouse for Federal Scientific and Technical Information,
NBS, US Dept of Commerce (CONF-691001).
LASKIN, S., DREW, R. T., CAPIELLO, V. P., & KUSCHNER, M. (1971)
Inhalation studies with freshly generated polyurethane foam dust.
In: Mercer, T. T., Morrow, P. E., & Storer, W., ed. Assessment
of airborne particles, Springfield, IL, Thomas, pp. 382-404.
LAUTERBACH, K. E., HAYES, D., & COELHO, M. A. (1956) An improved
aerosol generator. Am. Med. Assoc. Arch. ind. Health,
13: 156-160.
LEONG, B. J. K., KOCIBA, R. J., JERSEY, G. C., & GEHRING, P. J. (1975)
Effects from repeated inhalation of parts per billion of
bis(chloromethyl) ether in rats. Toxicol. appl. Pharmacol.,
35: 175.
LEWIS, T. R., MOORMAN, W. J., YANG, Y., & STARA, J. F. (1974)
Long-term exposure to auto exhaust and other pollutant mixtures.
Arch. environ. Health, 29: 102-106.
LIPPMANN, M. & ALBERT, R. E. (1967) A compact electric motor-driven
spinning disc aerosol generator. Am. Ind. Hyg. Assoc. J.,
28: 501-506.
LIPPMANN, M., ed. (1971) Air sampling instruments, 4th ed.
Cincinnati, OH, ACGIH.
LITTLE, J. B. & O'TOOLE, W. F. (1974) Respiratory trace tumours in
hamsters induced by benzo(a)pyrene and polonium210 alpha
radiation. Cancer Res., 34: 3026-3039.
LODGE, J. P. (1968) Production of controlled test atmospheres.
In: Stern, A. C., ed. Air pollution, 2nd ed., New York,
Academic Press, Vol. II, pp. 465.
MACFARLAND, H. N. (1968) Exposure chambers -- design and operation.
In: Proceedings of the 7th Annual Technical Meeting of the
American Association of Contamination Control, Chicago, 1968,
pp. 19-25.
MACFARLAND, H. N. (1976) Respiratory toxicology. In: Hayes, W. J. Jr,
ed. Essays in toxicology, New York, San Francisco, London,
Academic Press, Vol. 7, pp. 121-154.
MACKLEM, P. T., HOGG, W. E., & BRUTON, J. (1973) Peripheral airway
obstruction and particulate deposition in the lung. Arch.
intern. Med., 131: 93-100.
MARTORANA, P. A., MCKEEL, N. W., RICHARD, J. W., & SHARE, N. N. (1973)
Function-structure correlation studies on excised hamster lungs
in papain-induced emphysema. Can. J. Physiol. Pharmacol.,
51: 635-641.
MCLAUGHLIN, R. F., TYLER, W. S., & CANADA, R. O. (1961a) Subgross
pulmonary anatomy in various mammals and man. J. Am. Med. Assoc.
175: 694-697.
MCLAUGHLIN, R. F., TYLER, W. S., & CANADA, R. O. (1961b) Study of the
subgross pulmonary anatomy of various mammals. Am. J. Anat.,
108: 149-164.
MCLAUGHLIN, F. R., TYLER, W. S., & CANADA, R. O. (1966) Subgross
pulmonary anatomy of the rabbit, rat and guinea pig with
additional notes on the human lung. Am. Rev. Respir. Dis.,
94: 380-387.
MEAD, J. (1960) Control of respiratory frequency. J. appl. Physiol.,
15: 325-360.
MERCER, T. T. (1963) On the calibration of cascade impactors. Ann.
occup. Hyg., 6: 1-14.
MERCER, T. T. (1964) The stage constants of cascade impactors. Ann.
occup. Hyg., 7: 115-125.
MERCER, T. T. (1965) The interpretation of cascade impactor data. Am.
Ind. Hyg. Assoc. J., 26: 236-241.
MERCER, T. T. (1973a) Production and characterization of aerosols.
Arch. intern. Med., 131: 39-50.
MERCER, T. T. (1973b) Aerosol technology in hazard evaluation. New
York, London, Academic Press.
MORROW, P. E. (1970) Models for the study of particulate retention and
elimination in the lung. In: Hanna, M. G., Nettesheim, P. &
Gilbert, J. R., ed. Inhalation carcinogenesis. Springfield VA,
Clearinghouse for Federal Scientific and Technical Information,
NBS, US Dept of Commerce (CONF-691001).
MORROW, P. E. (1973) Alveolar clearance of aerosols. Arch. intern.
Med., 131: 101-108.
MORROW, P. E., HODGE, H. C., NEWMAN, W. F., MAYNARD, E. A., BLANCHET,
H. J. JR, FASSET, D. W., BIRK, R. E., & MAVRODT, S. (1966)
Deposition and retention models for internal dosimetry of the
human respiratory tract. Health Phys., 12: 173-207.
MURPHY, S. D. & ULRICH, G. E. (1964) Multi-animal test system for
measuring effects of irritant gases and vapours on respiratory
function of guinea pig. Am. Ind. Hyg. Assoc. J., 25: 28-36.
NADER, J. S. (1971) Direct-reading instruments for analysing airborne
gases and vapours. In: Lippmann, M., ed. Air sampling
instruments, 4th ed., Cincinnati, ACGIH, p. J-1.
NELSON, G. O. (1971) Controlled test atmospheres: principles and
techniques. Ann Arbor, MI, Ann Arbor Sci. Publ.
NELSON, G. O. & GRIGGS, K. E. (1968) Precision dynamic method for
producing known concentrations of gas and solvent vapour in air.
Rev. Sci. Instrum., 39: 927-928.
NIEMEIER, R. W. & BINGHAM, E. (1972) An isolated perfused lung
preparation for metabolic studies. Life Sci., 11(II): 807-820.
NIEWOEHNER, D. R. & KLEINERMAN, J. (1973) Effects of experimental
emphysema and bronchiolitis on lung mechanics and morphometry.
J. appl. Physiol., 35: 25-31.
O'KEEEE, A. E. & ORTMAN, G. O. (1966) Primary standards for trace gas
analysis. Anal. Chem., 38: 760-763.
O'NEIL, J. J. & TIERNEY, D. G. (1974) Rat lung metabolism: glucose
utilization by isolated perfused lungs and tissue slices. Am. J.
Physiol., 226: 867-873.
ORTON, T. C., ANDERSON, M. W., PICKETT, R. D., ELING, T. E., & FOUTS,
J. R. (1973) Xenobiotic accumulation and metabolism by isolated
perfused rabbit lungs. J. Pharmacol. exp. Ther., 186: 482-497.
PALACEK, F. (1969) Measurement of ventilatory mechanics in the rat.
J. appl. Physiol., 27: 149-156.
PHILIPSON, K. (1973) On the production of monodisperse particles with
a spinning disc. Aerosol Sci., 4: 51-57.
POWELL, C. H. & HOSEY, A. D. (1965) The industrial environment, its
evaluation and control (US PHS Publ. 614).
POZZANI, U. C., WEIL, C. S., & CARPENTER, C. P. (1959) The
toxicological basis of threshold limit values: 5. The
experimental inhalation of vapour mixtures by rats with notes
upon the relationship between single-dose oral data. Am. Ind.
Hyg. Assoc. J., 20: 364-369.
RAABE, O. G. (1970) Generation and characterization of aerosols. In:
Hanna, M. G. Jr, Nettesheim, P., & Gilbert, J. R., ed.
Inhalation carcinogenesis. Clearinghouse for Federal Scientific
and Technical Information, NBS, Springfield VA, US Dept of
Commerce (CONF-691001).
RAABE, O. G., BENNICK, J. E., LIGHT, M. E., HOBBS, C. N., THOMAS, R.
L., & TILLERY, M. (1973) An improved apparatus for acute
inhalation exposure of rodents to radioactive aerosols. Toxicol.
appl. Pharmacol., 26: 264-273.
RJAZANOV, V. A. (1964) Criteria and methods for establishing maximum
permissible concentrations of atmospheric pollutants in the USSR.
In: Maximum permissible concentrations of atmospheric
pollutants, Moscow, Medicina, Vol. 8, pp. 5-21.
ROE, F. J. C. (1968) Inhalation tests. In: Boyland, E. & Goulding, R.,
ed. Modern trends in toxicology, London, Butterworth, Vol. 1.
SAFFIOTTI, U. (1970) Experimental respiratory tract carcinogenesis and
its relation to inhalation exposures. In: Hanna, M. G. Jr,
Nettesheim, P., & Gilbert, J. R., ed. Inhalation carcinogenesis.
Springfield, VA, Clearinghouse for Federal Scientific and
Technical Information, NBS, US Sept of Commerce (CONF-691001).
SAITO, Y. (1912) [Experimental investigations on the quantitative
absorption of dust by animals at accurately known concentrations
of dust in air.] Arch. Hyg., 75: 134-151 (in German).
SALTZMAN, B. E. (1971) Permeation tubes as calibrated sources of gas.
In: Proceedings of 1st Annual Conference on Environmental
Toxicology, Dayton, Ohio, 1970, US Govt Printing Office,
pp. 309-326.
SALTZMAN, B. E. & WARBURG, A. F. (1965) Precision flow dilution system
for standard low concentrations of nitrogen dioxide. Anal.
Chem., 37: 1261-1264.
SANCHIS, J., DOLOVICH, M., CHALMERS, R., & NEWHOUSE, M. (1972)
Quantification of regional aerosol clearance in the normal human
lung. J. appl. Physiol., 33: 757-762.
SANOCKIJ, I. V., ed. (1970a) [Methods for determining toxicity and
hazards of chemicals.] Moscow, Medicina, pp. 62-63 (in
Russian).
SANOCKIJ, I. V. (1970b) [Complete toxicometry.] In: [Methods for
determining the toxicity and hazards of chemicals.] Moscow,
Medicina, pp. 50-54 (in Russian).
SCHREIBER, H., NETTESHEIM, P., & MARTIN, D. H. (1972) Rapid
development of bronchiolo-alveolar squamous cell tumours in rats
after intratracheal injection of 3-methylcholanthrene. J. Natl
Cancer Inst., 49: 541-554.
SCHWETZ, B. A., LEONG, B. J. K., & GEHRING, P. J. (1975) The effect of
maternally inhaled trichloroethylene, perchloroethylene, methyl
chloroform and methylene chloride on embryonal and fetal
development in mice and rats. Toxicol. appl. Pharmacol.,
32: 84-96.
SIDORENKO, G. I. & PINIGIN, M. A. (1970) Rapid determination of
maximum permissible concentrations of atmospheric pollutants.
Gig. i Sanit., No. 3, pp. 93-96.
SILVER, M. J., HOCH, W., KOCSIS, J. J., INGERMAN, C. M., & SMITH, J.
B. (1973) Arachadonic acid causes sudden death in rabbits.
Science, 183: 1085-1086.
SILVER, S. D. (1946) Constant flow gassing chambers: principles
influencing design and operations. J. lab. clin. Med.,
31: 1153-1161.
SNIDER, G. L., HAYES, J. A., FRANZBLAU, C., KAGEN, H. M., STONE, P.S.,
& KORTHY, A. L. (1974) Relationship between elastolytic activity
and experimental emphysema-inducing properties of papain
preparations. Am. Rev. Respir. Dis., 10: 254-262.
STEAD, F. M., DERNEHL, C. U. & NAU, C. A. (1944) A dust feed apparatus
useful for exposure of small animals to small and fixed
concentrations of dust. J. ind. Hyg. Toxicol., 26: 90-93.
STEWART, R. D. (1972) Use of human volunteers for the toxicological
evaluation of materials. In: Symposium on an Appraisal of
Halogenated Fire Extinguishing Agents. Washington DC, National
Academy of Sciences.
STEWART, R. D., DODD, H. C., GAY, H. H., & ERLEY, D. S. (1970a)
Experimental human exposure to trichloroethylene. Arch. environ.
Health, 20: 64-71.
STEWART, R. D., PETERSON, J. E., BARETTA, E. D., BACHARD, R. T.,
HOSCO, M. T., & HERRMANN, A. A. (1970b) Experimental human
exposure to carbon monoxide. Arch. environ. Health,
21: 154-164.
STEWART, R. D., PETERSON, J. E., FISHER, T. N., HOSKO, M. J., BARETTA,
E. D., DODD, H. C., & HERRMANN, A. A. (1973) Experimental human
exposure to high concentrations of carbon monoxide. Arch.
environ. Health, 26: 1-7.
STOKINGER, H. E. (1949) Toxicity following inhalation. In Voegtlin, C.
& Hodge, C., ed. Pharmacology and toxicology of uranium
compounds, 1st ed., New York, McGraw-Hill, Vol. III.
STUART, B. O. (1974) Deposition of inhaled aerosols. Arch. intern.
Med., 131: 60-75.
STUART, B. O., WILLARD, D. H., & HOWARD, E. B. (1970) Uranium mine air
contaminants in dogs and hamsters. In: Hanna, M. G. Jr,
Nettesheim, P., & Gilbert, J. R., ed. Inhalation carcinogenesis.
Springfield, VA, Clearinghouse for Federal Scientific and
Technical Information, NBS, US Dept of Commerce (CONF-691001).
STUPFEL, M. & MORDELET-DAMBRINE, M. (1974) Penetration of pollutants
in the airways. Bull. Physio-Path. Respir., 10: 481-509.
SWIFT, D. L. (1971) Direct-reading instruments for analysing airborne
particles. In: Lippmann, M., ed. Air sampling instruments, 4th
ed., Cincinnati, OH, ACGIH, p. T-1.
TAYLOR, G. J. & DREW, R. T. (1975) Cardiomyopathy predisposes hamsters
to trichlorofluoromethane toxicity. Toxicol. appl. Pharmacol.,
32: 177-183.
THOMAS, R. G. & LIE, R. (1963) Procedures and equipment used in
inhalation studies on small animals. LF-11, Lovelace Foundation
for Medical Education and Research.
THOMAS, R. L. (1969) Deposition and initial translocation of inhaled
particles in small laboratory animals. Health Phys.,
16: 417-428.
TIERNEY, D. G. (1974) Intermediary metabolism of the lung. Fed.
Proc., 33: 2232-2237.
TUCKER, A. D., WYATT, J. H., & UNDRY, D. (1973) Clearance of inhaled
particles from alveoli by normal interstitial drainage pathways.
J. appl. Physiol., 35, 719-732.
VALEZNEV, I. P., ELOVSKAJA, L. T., & MOISEJCEV, P. I. (1970)
[Equipment for research on biological effects of aerosols.]
Ofic. bjull. Gos. kom. Min. SSSR po delam izobretenij i
otkrytij, No. 10, avtonskoe svidetel'stvo No. 265307 (in
Russian).
VELICKOVSKIJ, B. T. & KACNELSON, B. A. (1964) [Etiology and
pathogenesis of silicosis.] Moscow, Medicina (in Russian).
WALTON, W. H. & PREWETT, W. C. (1949) The production of sprays and
mists of uniform drop size by means of spinning disc type
sprayers. Proc. Phys. Soc. (London), 62: 341-350.
WATSON, J. A., SPRITZER, A. A., AULD, J. A. & GUETTHOFF, M. A. (1969)
Deposition and clearance following inhalation and intra-tracheal
injection of particles. Arch. environ. Health, 19: 51-58.
WEISS, B. & LATIES, V. (1975) Behavioural toxicology. New York,
Plenum Press.
WHITBY, K. T. & LIU, B. Y. H. (1966) The electrical behaviour of
aerosols. In: Davies, C. N., ed. Aerosol Science, New York,
Academic Press.
WILSON, R. H. & LASKIN, S. (1950) Improved design for an animal
inhalation exposure unit. Rochester, NY, University of
Rochester (AEC Proj. Rep. U-116), p. 80.
WITSCHI, H. P. (1975) Exploitable biochemical approaches for the
evaluation of toxic lung damage. In: Hayes, W. Jr, ed. Essays in
toxicology, New York, Academic Press, Vol. 6.
WOOLCOCK, A. J., VINCENT, N. J., & MACKLEM, P. T. (1969) Frequency
dependence of compliance as a test for obstruction in the small
airways. J. clin. Invest., 48: 1097-1106.
WRIGHT, B. M. (1950) A new dust-feed mechanism. J. Sci. Instrum.,
27: 12-15.
XINTARAS, C., JOHNSON, B. L., & DE GROOT, I., ed. (1974) Behavioural
toxicology. Washington DC, US Govt Printing Office.
7. CARCINOGENICITY AND MUTAGENICITY
7.1 Introduction
This chapter deals with the two related but separate processes,
heritable mutations and cancer. Cancer involves the conversion of
normal cells to malignant cells and the development of what is usually
an irreversible malignant disease process in the present generation
frequently leading to a fatal outcome for the bearer of the
malignancy. Heritable mutations are mutations that are transmissible
to later generations and the target cells are the germ cells of either
sex.
Developments in the last two decades make it possible to discuss
certain aspects of mutagenesis and carcinogenesis in parallel. In
other words, there is now increasing evidence that, in most cases,
somatic mutations are probably involved in the conversion of normal to
malignant cells. Thus, the ability of a chemical or physical agent to
produce mutations is relevant both to the question of heritable
mutations (mutations of the germ cell) and carcinogenicity.
There is a well-established body of knowledge that shows a close
correspondence between cancer of the whole animal as studied in the
laboratory and the occurrence of cancer in man; this is especially
true of occupational cancer. More recently, a fairly close correlation
has been shown between tests of isolated, simple, biological systems,
e.g. reverse bacterial mutations and the response to carcinogens of
whole animals, and human beings.
A similar situation does not exist with respect to patterns in
human heritable mutations. Thus, although there is good evidence of
correlation between in vitro tests for mutagenicity and heritable
mutations in insects and experimental mammals, it can only be
inferred, at present, that this also applies to man. The inference,
however, is extremely strong and not subject to serious doubt. Thus,
there is every reason to expect, that, in general, man will respond
biologically in a manner quite similar to that of other species when
exposed to mutagens that reach the germ cells. The lack of full
correlation stems more from the lack of appropriate studies in man
than from any uncertainty concerning the underlying biological
considerations. At present, methods for the detection of mutations in
man are difficult, cumbersome, and insensitive, and it is imperative
to operate on the assumption that agents capable of producing germ
cell mutations in laboratory studies would also be capable of
producing similar mutations in man.
Thus, two quite different disease processes can have one common
level of consideration, namely, their ability to produce alterations
in the genetic material of cells. The following discussion is
concerned with methods for carcinogenicity testing. First, the
traditional lifetime exposure of the entire organism to the test
compound is considered. This is followed by a discussion of various
in vitro systems for the examination of mutagenicity including DNA
damage, mutagenicity in bacteria and eukaryotic organisms, and
transformation of cell cultures. (The first three of these are
particularly relevant to mutagenicity of the germ cell or of the
somatic cell.) Following this, the general problem of examination for
heritable mutations is considered. This includes inquiries at three
levels: injury to DNA, point mutations, and chromosome alterations.
Whole animal and isolated test systems are available to make relevant
determinations. However, the whole field of mutagenicity testing is
now in a period of rapid evolution with advances being made in a
number of areas. It can, therefore, be anticipated that present
procedures will undergo alteration and, it is hoped, will improve
within the near future.
7.2 Carcinogenicity
7.2.1 Long-term bioassays
The fact that environmental factors have a direct effect in
producing cancer in man is shown by: (a) the unequivocal evidence of
the chemical origin of occupational cancer, for example, urinary
bladder tumours in workers exposed to aromatic amines, lung cancers in
workers exposed to bis(chloromethyl)ether, etc.; (b) the
well-documented cases of iatrogenic cancer; (c) the positive
correlation between cigarette smoking and lung cancer; (d) the
differences in cancer incidence in urban and rural populations and
(e) the results of studies on migrants showing that for some types
of cancer they acquire incidences similar to those found in the host
countries. Results obtained in experimental studies on carcinogenesis
confirm the direct carcinogenic effect of chemicals. However, there is
not necessarily a correlation between acute toxicity and
carcinogenicity; a chemical with a high acute toxicity may not have
any, or only a very low carcinogenic potential. Conversely, a chemical
that produces a very minor, or no evident toxic effect in acute or
subacute experiments may be a very powerful carcinogen. Knowledge of
the acute and subacute toxicity of the test chemical however, is
important in order to permit better planning of the experiment. For
instance, the premature death of the test animals, due to unforeseen
side-effects, may thus be avoided.
7.2.1.1 Species, strain, and sex selection, and size of groups
The description of methods and recommended procedures in this
chapter applies to long-term testing in rodents and specifically to
the mouse, rat, and Syrian golden hamster (FDA, 1971; NCI, 1976; Weil,
1972; Weisburger, 1975; Weisburger & Weisburger, 1967). Rodents have
been preferred to other species because of their susceptibility to
tumour induction, their relatively short life span, the limited cost
of their maintenance, their widespread use in pharmacological and
toxicological studies, the availability of inbred strains and, as a
consequence of these characteristics, the large amount of information
available on their physiology and pathology. Nonrodents, in particular
dogs and primates, have rarely been used in the past; though used more
at the present time, their much higher cost of maintenance, the long
period of observation, and the impracticability of using sufficiently
large numbers put nonrodents at a disadvantage for long-term
bioassays. Also, comparative studies on the metabolism of various
chemicals have not indicated necessarily that primates and dogs are
closer to man in this respect than rodents. Nevertheless, many of the
procedures for long-term bioassays in rodents are applicable to assays
in nonrodents.
Long-term bioassays of the carcinogenicity of environmental
chemicals are carried out to assess a possible risk to man and to
estimate the need for primary preventive measures. Because of the
urgent nature of these problems, it is recommended that a compound of
unknown activity should be tested on two animal species. Although a
positive (that is, carcinogenic) effect in one species is considered
as adequate warning, only negative findings in two species can be
regarded as adequate negative evidence.
Among rodents, the species of choice are undoubtedly the mouse,
the rat, and the Syrian golden hamslet. The European hamster has
recently been introduced, particularly for studies in lung pathology,
but its use cannot be recommended for routine testing. Guineapigs and
rabbits have been used occasionally but they have few of the
advantages of smaller rodents combined with the disadvantages implied
by a much longer life span, higher cost of maintenance, and the
scarcity or absence of inbred strains.
Of the three rodent species of choice, the mouse and the rat have
been more widely used than the hamster. However, the last species has
proved to be an excellent tool for revealing carcinogenic effects in
the respiratory tract and urinary bladder. Furthermore, hamster cells
are widely used for in vitro transformation assays, and, since in
most cases these assays require the back transplantation of the cells
in syngenic hosts, the use of the hamster has recently been extended.
The hamster is generally randomly bred but a pure strain is becoming
available.
The use of inbred strains undoubtedly has the advantage of making
available animals with known characteristics such as an average life
span, and a spontaneous tumour rate with little variability.
Random-bred animals or, even better, animals bred with maximum
avoidance of inbreeding, are considered more resistant to infections
and perhaps more liable than many inbred strains to reveal any
carcinogenic effect on an unsuspected target organ. It is common
experience that carcinogenic activity is more easily recognized by
the increased frequency and earlier appearance of tumours at sites
where tumours occur spontaneously. Inbred strains are often known to
have one or two particularly susceptible organs and the induction of
tumours in these organs could, in part, be regarded as an
intensification of whatever underlies the spontaneous occurrence of
tumours in that organ. A carefully planned experiment must take this
possibility into account, and preference should be given to strains
with a low incidence of tumours. Noninbred strains are inconvenient in
that, in many cases, background tumour incidence in untreated controls
is unpredictable. Moreover, experimental groups initiated at different
times can rarely be compared with each other since inter- as well as
intragroup variation may be considerable.
Hybrid mice of two known inbred strains are excellent as they are
particularly robust and long-lived, but they are, of course, more
difficult to obtain and are likely to be more expensive than inbred
strains.
In selecting the species, it is important to be aware that there
are, within each species, particular susceptibilites; for instance, it
is easier to induce liver tumours in the mouse than in the rat, and
conversely it is much easier to induce subcutaneous tumours in the rat
than in the mouse. The skin of the mouse and the rabbit is possibly
more sensitive to tumour induction by skin painting than that of the
rat and the hamster, particularly when polycyclic hydrocarbons are
used. The hamster is more susceptible to urinary bladder
carcinogenesis than the mouse and possibly the rat.
It is essential that experimental groups should be composed of an
equal number of animals of each sex, since differences in response to
the carcinogenic activity of chemicals are well documented. Thus, the
use of one sex only would not show the full range of activity of the
test chemical.
The group should be sufficiently large to permit statistical
evaluation of the results. The UICC Committee on Carcinogenicity
Testing (UICC, 1969) recommended that the number of animals should be
such that it "would be likely to yield reasonable (e.g. 90%)
statistical significance as between the main induced tumour incidence
and its spontaneous incidence". A sufficient number of animals must be
alive at the time that the first tumour appears and it is suggested
that this number should never be less than 25 per sex.
7.2.1.2 Route of administration
It has been agreed that the chemical under test should be
administered by the route of human exposure. This applies to food
additives and food contaminants in particular, but it is equally
desirable for drugs. This rule cannot always be applied to
environmental or industrial chemicals. It is well known that the three
main routes of exposure in man are inhalation, ingestion, and skin
absorption. Inhalation is often predominant in man but in experimental
carcinogenesis the inhalation route, while highly desirable in
principle, has rarely been used in the past because of lack of
adequately equipped laboratories. However, it is now being widely used
in programmes concerning smoking and health.
When the route of exposure is per os, the chemical is
administered either mixed in the diet or drinking water, or by gavage,
the choice depending on the specific characteristics of the test
chemical; a volatile compound, for example, should never be mixed in
the diet while, for a drug, the route by which it will be used in man
should be selected, i.e. per os, or by intravenous, subcutaneous or
topical application.
7.2.1.3 Inception and duration of tests
Most commonly, tests are initiated in young adult animals from 7
to 9 weeks of age. The start of treatment soon after weaning is
recommended as a routine procedure. One objective of carcinogenicity
testing is to obtain the maximum possible carcinogenic effect of the
test chemical in order to compensate for the limited number of
individuals at risk. This is accomplished by employing high levels of
exposure (see 7.1.4) and by starting the treatment when the animals
are at their most sensitive age. For some years following the pioneer
work of Pietra et al. (1961), treatment immediately after birth and
within the first 24 h of life was thought to be the most sensitive
model. Careful reviews of the data available have indicated that
neonatal treatment alone can be recommended in some instances, but not
as a general routine procedure (Della Porta & Terracini, 1969). For a
single neonatal administration, only a very limited quantity of the
chemical to be tested is needed and this could be advantageous, when
only a small amount of the chemical is available.
The main limitation of the use of a single neonatal treatment is
that the newborn animal may not have developed sufficient metabolic
competence to metabolize the test chemical. Another limitation is that
not all chemical carcinogens are active after one, or even a few
exposures.
More recently, prenatal exposure has received considerable
attention, since it has been demonstrated that some tissues, notably
nervous tissue, are more susceptible to certain carcinogens during
fetal life than later (Druckrey et al., 1967; Napalkov, 1973; Napalkov
& Alexandrov, 1968; Tomatis, 1973). At present, however, it can only
be speculated that prenatal exposure will reveal the carcinogenic
effect of a chemical that would not have been revealed had treatment
started at a later age. A sensitive procedure might be to integrate
prenatal exposure with long-term, postnatal exposure, in order to
obtain maximum sensitivity of the bioassay system. To achieve this,
animals mated at the age of 8-9 weeks, should be exposed to the test
chemical during the second half of pregnancy. The parents so treated
can constitute a group under conventional long-term testing, if the
treatment is continued after delivery. Their offspring, already
exposed in utero, and possibly after birth through their mother's
milk and excreta, should be exposed directly to the test chemical from
the age of weaning onwards. In this way, two different experimental
groups can be obtained: one for which exposure was started at the
young adult stage (the parents) and the other for which exposure was
started prenatally (the offspring) (Tomatis, 1974b).
The duration of a positive test, i.e. where exposure to the test
chemical is followed by an increased incidence of tumours, depends on
the time of appearance and the rapidity of growth of the induced
tumours. To agree in advance on a given duration of exposure is of
prime importance for negative tests. Some research workers have
adopted a duration for carcinogenicity tests of 2 years in rats and 18
months in mice, while others have preferred an observation period
extending over the entire life span of the animals. A long finite
period -- that is, not less than 2 years -- is recommended in
preference to the entire life span of the animals, for the following
reasons: (a) induced tumours usually occur within this observation
period; (b) "spontaneous" tumours appear with highest frequency late
in life and their appearance may make it more difficult to evaluate
the carcinogenicity of a compound, particularly if of low potency;
(c) a few animals may far exceed the normal life span of the species
and extend unnecessarily the duration of the experiment; (d) tests
are very expensive and any justifiable abbreviation means good economy
(Health & Welfare, Canada, 1973; Tomatis, 1974a; UICC, 1969; WHO,
1961).
7.2.1.4 Dose-level and frequency of exposure
If only one level is to be used, the highest dose allowing long
survival of the majority of the animals should be chosen. The
selection of this dose should be made from results obtained in
subacute toxicity tests. Information on the LD50 of certain
compounds may be available and this can be useful in deciding the
level of the dose for the subacute studies.
While it would be very difficult to give a definition of the
maximum tolerated dose, it seems quite reasonable to insist that it
allows adequate survival of the animals and does not produce a
reduction in weight of more than 10% compared with the controls
(Friedman, 1974). If two dose levels are chosen, the second should be
one quarter or one third of the first dose.
If the goal of the test is merely to ascertain the possible
carcinogenicity of a compound, then the assay protocol should achieve
the maximum sensitivity of the test; in this case, the highest
tolerated dose is the most appropriate. If, however, additional
information has to be collected, in particular regarding the
establishment of a possible minimum effect or no-observed-effect
level, then initiation of a dose-response experiment should be
envisaged. In this case, a minimum of three doses, and preferably
four, should be included. An advantage of including several dose
levels, the lowest of which does not decrease the life span of the
animals, is the possibility of collecting other data on chronic
toxicity not otherwise available through a carcinogenicity test at
high dose levels.
Frequency of exposure may vary according to the route chosen. If
the chemical is administered in the drinking water or mixed in the
diet, exposure is continuous. If the chemical is given by gavage, the
frequency can be two to three times per week. Topical applications may
be made daily, while subcutaneous or intravenous injections must be
more widely spaced, e.g. once or twice weekly. The duration of
treatment should cover almost the entire observation period.
7.2.1.5 Combined treatment and cocarcinogenesis
An investigation on the effect of more than one agent, given
simultaneously, may approximate the actual environmental situation,
where there is never exposure to a single chemical. In particular, it
may be useful in revealing the carcinogenic effect of a chemical of
very low carcinogenic potency and thereby help to identify situations
or populations that may be exposed to an otherwise unsuspected high
risk.
Response to an environmental chemical may be modified by the
action of other chemicals that may either alter the rate and/or
pathway of metabolism of the test chemical, or have a cocarcinogenic
effect. A cocarcinogenic effect may be additive, when the chemical has
a carcinogenic effect on its own which adds to the effect produced by
the test chemical; it may be synergistic, when its effect, combined
with that of the test chemical when given alone, exceeds the summation
of the separate effects; or it may act as an incomplete carcinogen,
that is, only as initiator or promoter in the two-stage carcinogenic
process (UICC, 1969) (see Chapter 13).
It is also essential to keep in mind that dietary components may
influence the incidence of tumours in test animals. This may occur
either because of the unsuspected presence of carcinogens (such as
aflatoxins or nitrosamines) or because of substances that may modify
the response to the test chemical by altering, for instance, the
hepatic microsomal enzyme activity.
7.2.1.6 Positive and untreated controls
An adequate group of untreated animals, serving as controls,
should always be included in the planning of a carcinogenicity test.
The size of the control group should never be smaller than the size of
the treated group and, preferably, should be larger. When more than
one chemical is tested simultaneously, the same control group can be
used, provided that its size is appropriately increased.
Besides these controls, i.e. animals not receiving treatment, and
which could be called negative controls, the inclusion of positive
controls in planning an experiment has recently been recommended, i.e.
inclusion of a group of animals receiving a known carcinogen at a dose
level that has already produced carcinogenic effects in several
laboratory studies. Such controls ensure: (a) more confidence in the
outcome of tests carried out on compounds of unknown activity
(Weisburger, 1974); (b) assessment of the relative carcinogenic
potency of the test chemical, and (c) an indirect check on the
reliability of the test laboratory. While it does not seem essential
to include a positive control in every test, it is highly advisable
for every laboratory to check the sensitivity of its bioassay system,
periodically, with selected known carcinogenic chemicals. It is also
advisable that a group of animals should receive only the vehicle
(i.e. acetone, dimethylsulfoxide, etc.) in which the chemical under
test is eventually administered.
7.2.1.7 Test material
Long-term studies should not be started until sufficient
information on the identity and purity of the test chemical has been
assembled. The importance of knowing the toxicity of impurities is
well demonstrated by the case of TCDD present as an impurity in the
herbicide 2,4,5-T. Drugs or food additives should be tested in the
form and degree of purity intended for human consumption (Health &
Welfare, Canada, 1973; National Academy of Sciences, 1960; WHO, 1961,
1969). In this case, as well as for mixtures of chemicals to which man
may be exposed, it may be highly advisable to carry out additional
studies in order to identify positively the carcinogenic component of
the mixture.
7.2.1.8 Survey of animals, necropsy, and histological examination
In order to present results correctly, detailed records of all
experimental procedures and surveillance of the animals during the
entire observation period should be maintained. While all pertinent
details of the experiment may not be published, it is a good rule for
the investigator to keep them for discussion with other interested
scientists (UICC, 1969). If the results are to be published, it is
essential that adequate information be given on the test chemical and
test animal, as well as on all observations made on the experimental
and control groups (WHO, 1961). A detailed list of items to be
considered is given in the UICC (1969) report.
In the case of neoplasms (Turusov, 1973, 1976), it is extremely
important to give an exact description of the criteria used to
classify lesions as hyperplastic, preneoplastic or neoplastic, benign
or malignant. Many times, pathologists do not agree on certain
diagnoses and it is far easier to interpret the results when the
criteria used for classification are known. Terms such as hepatoma,
which do not indicate the specific tissue of origin, should not be
used (Reuber, 1974), while terms such as cholangioma or adenomatosis
require additional qualification. The term should clearly indicate
whether the lesion is considered malignant. Thus, specific
descriptions should be given, e.g. in the case of "hepatoma" it may be
either a liver cell adenoma or a hepatocellular carcinoma, while
"cholangioma" may be cholangiocellular adenoma or cholangiocellular
carcinoma. In addition, carcinomas may be subdivided into well,
moderately, and poorly differentiated carcinemas.
A lesion with atypical cells or with focal malignant change
should be classified separately rather than under malignant tumours.
7.2.2 Short-term tests (rapid screening tests)
To date, there are no reliable alternatives to long-term
bioassays for testing the carcinogenicity of chemicals; this means a
delay of two years or more before a carcinogenicity bioassay yields
results. However, recent advances in mutagenicity testing hold out
hopes of a short-term test, at least for selecting, from among the
many chemicals to be tested, those most in need of early attention. So
far, about 80% of carcinogens have been shown to be mutagenic and with
the continuously increasing sensitivity of the models now employed an
even higher correlation is possible. At present, mutagenicity testing
represents a valuable system for screening chemicals to be submitted
to long-term carcinogenicity testing; it cannot replace long-term
testing, until a satisfactory positive correlation is established and
the possibility of false negatives eliminated.
A mutation is any heritable change in genetic material including
a chemical transformation of an individual gene (point mutation) or a
change involving rearrangement of parts of a chromosome (chromosome
mutation). The question of whether or not a mutagenic event is a
prerequisite for carcinogenesis has long been debated. Many mutagens
have been shown to be carcinogenic but other known carcinogens are
known not to be mutagenic. Other mechanisms not involving DNA have
also been implied in the process of carcinogenesis (Pitot, 1974).
Recent short-term mutagenicity testing procedures (McCann & Ames,
1976: McCann et al., 1975; Sugimura et al., 1976) have shown greater
correlation between mutagenicity and carcinogenicity; consequently,
some thought is now being given to using these assays as preliminary
screens for potential carcinogens. However, at the moment, the fact
that a particular compound has mutagenic activity can only be
considered, in terms of carcinogenicity, as indicative of potential
reactivity with DNA.
7.2.2.1 Metabolic activation, reaction with DNA, and DNA repair
The synthetic and naturally occurring chemical carcinogens
include a variety of chemicals having no common structural feature.
However, it is becoming clear that the ultimate reactive forms of many
chemical carcinogens are electrophilic (electron-deficient) reactants
(Miller, 1970). Although some chemical carcinogens, such as direct
alkylating agents and metal ions, are electrophiles per se, the
majority require metabolic activation to reactive forms (ultimate
carcinogens) (Fig. 7.1).
The formation of these electrophilic reactants from exogenous
chemicals, in an animal species or in an organ, is the result of the
balance between in vivo activation and deactivation reactions
carried out predominantly by enzymes localized in the endoplasmic
reticulum of the cell. The ultimately available concentration of
reactive metabolites seems to account for some of the organ and
species specificities shown by several chemical carcinogens.
During the last decade, much progress has been made in
understanding the metabolism of various classes of chemical
carcinogens (polynuclear hydrocarbons, Sims & Grover, 1974;
N-nitroso compounds, Magee et al., 1976; aromatic amines, Kriek,
1974; Weisburger & Weisburger, 1973; miscellaneous compounds, Miller,
1973). The activity of microsomal mixed function oxidases is
influenced by various drugs or environmental chemicals, that may
stimulate or inhibit the formation of the ultimate carcinogens (Conney
& Burns, 1972).
These ultimate carcinogens bind covalently with cellular
macromolecules such as DNA, RNA, or proteins, which, directly or
indirectly, leads to heritable changes in the affected cells. Although
the critical targets of chemical carcinogens are not known, there is a
great deal of evidence supporting the theory that DNA is one major
target in carcinogenesis (Farber, 1973).
Some specific binding sites for the metabolites of carcinogens
have been identified in the bases of DNA. The carcinogenic N-nitroso
compounds are examples (Magee et al., 1976). The main site of
alkylation of DNA by alkylnitroso compounds is the N,7 position of
guanine, as with other alkylating agents. Other sites are also
attacked and the significance of these DNA interactions is being
investigated. The formation of 7-alkylguanine, however, seems to be of
no obvious importance in the process of tumour induction, since no
quantitative or qualitative correlation between the occurrence of this
alkylated base in the DNA of treated animals and the carcinogenicity
of nitrosamines or alkylating agents has been observed (Magee et al.,
1976). Alkylation of guanine bases in DNA at the 7-position does not
seem to produce mutations in bacteriophages (Loveless & Hampton,
1969), nor does it alter the coding properties of synthetic
polynucleotides in vivo (Ludlum, 1970). Various laboratories have
examined the biological importance of the alkylated O,6 position of
guanine residues in DNA which, in consequence, is able to induce
mutations in phage (Loveless, 1969). It has been shown that there is
an aberrant base pairing with a polymer containing alkylated
 O,6-guanine residues (Gerchman & Ludlum, 1973). Goth & Rajewsky
(1974) showed, in vivo, that the initial degree of alkylation at the
O,6 position in the DNA was, apparently, not correlated with the
tissue-specific carcinogenicity of ethylnitrosourea which induces
brain, but not liver, tumours. However, O,6-ethylguanine persists
much longer in brain DNA than N,7-ethylguanine. The O,6-alkyl
elimination is also much slower from brain than from the liver DNA.
From these findings it becomes apparent that variations in the
sensitivity of different organs to the carcinogenic action of
N-nitroso compounds might be attributed to different enzyme
activities capable of repairing lesions in cellular DNA and/or to
different rates of cell division in the target and non-target organs
(Craddock, 1976; Margison et al., 1976; Pegg & Nicoll, 1976; Pegg,
1977).
Possibly these repair systems, which recognize damaged DNA, are
also impaired in the case of some types of cancer in man. The skin of
patients suffering from the hereditary disease xeroderma pigmentosum
is extremely sensitive to sunlight and such persons have a very high
incidence of skin cancer. It has been shown that the cells of the skin
of these patients are deficient in the capacity to repair UV-damaged
DNA and that this deficiency is caused by lack of enzymes required for
the excision of damaged regions from DNA (Cleaver, 1969).
Some chemicals cause changes in the biological properties of DNA
without covalent binding, e.g. by intercalation which could cause
frameshift mutations. Since radioactive labelled chemicals are needed
for most of these studies, technical problems associated with these
methods render the tests impractical for routine screening. However,
they will continue to contribute considerably to the understanding of
the mechanism of interaction of a chemical or its activating
metabolite with specific sites in DNA.
Initial lesions in DNA can either lead to a permanent change,
such as a mutation, or can be removed by cellular repair processes. In
bacteria and mammalian cells, three major repair processes can be
considered. First, the photoreactivation repair process that repairs
only UV damage to DNA and involves a direct enzymatic cleavage of
pyrimidine dimers to monomers upon exposure of cells to visible light.
This repair process is present in most prokaryotes and eukaryotes and
it was recently identified in human cells (Sutherland et al., 1975).
The second process, post-replication repair, which is also called
recombination repair, is limited to the DNA synthesis period. Although
its mechanism is not well known, it has been proposed that it is
caused by the presence of damaged bases on parental DNA strands too
close to the replication fork to be recognized by the third process,
excision repair. Unlike post-replication repair, excision repair
occurs throughout the cell cycle and is present in a large variety of
organisms. During this process, damaged bases are excised, producing a
single-strand break. This gap is repaired by replacing the original
bases with bases complementary to those of the opposite intact strand.
Various enzymes are involved in this process, namely endonuclease, a
DNA exonuclease, a DNA polymerase and a DNA ligase (Cleaver, 1974).
From studies on prokaryotes, the concept arose that excision
repair is error-free (Kondo, 1973). It is thought that mutations
originate during semi-conservative replication as a consequence of the
fact that DNA templates containing damage have not yet been or cannot
be repaired by excision repair. Since DNA replication on damaged
templates involves post-replication repair, this repair process is
considered error-prone and responsible for mutagenesis. Whether
miscoding and mutagenesis of DNA templates is the result of lack of
excision repair enzymes or due to error-prone post-replicative repair
is not clear at present.
The isolation of xeroderma pigmentosum-variant fibroblasts, that
have normal excision repair but are deficient in post-replication
repair (Maher et al., 1976) may lead to a better understanding of the
role of these repair processes in mutagenesis and carcinogenesis.
Recent efforts have been devoted to the development in mammalian
systems of a screening test for the detection of possible mutagens or
carcinogens based on excision repair. The focus on this type of repair
is mainly based on the greater availability of procedures thought to
be appropriate to the problem. There are four different ways of
examining this form of repair in mammalian systems and all are based
on the assumption that chemical mutagens or carcinogens interact with
DNA, inducing molecular alterations which result in DNA repair that
can be measured as unscheduled incorporation of various DNA precursors
(Cleaver, 1975; Legator & Flamm, 1973). The common basis of all these
tests is the differentiation between repair and normal
semiconservative synthesis of DNA.
One procedure, "unscheduled DNA synthesis", involves
autoradiography of cultured cells, and consists of the incorporation
of precursors during resynthesis of short nucleotide sequences that
have been eliminated from DNA strands following their damage by
chemicals (Cleaver, 1973; Stich & San, 1970). This procedure permits a
quantitative evaluation of DNA repair synthesis in various types of
cell in a tissue and can be applied to an in vivo system (Stich &
Kieser, 1974), thus allowing the detection of indirect mutagens and/or
carcinogens.
Another process uses 5-bromodeoxyuridine (BrdU), which is an
analogue of thymidine. During normal replication, the incorporation of
BrdU produces a DNA with a high buoyant density; this does not occur
when BrdU is incorporated in DNA during the repair synthesis. Thus, by
using radiolabelled BrdU, or radiolabelled thymidine and non-labelled
BrdU, it is possible to differentiate, on cesium chloride density
gradients, semi-conservative replication of DNA from the repair
synthesis according to the different buoyant densities.
A third procedure also uses BrdU but the differentiation between
semi-conservative and repair DNA synthesis is done by different means.
Cells exposed to the chemicals are incubated with BrdU and the DNA is
subjected to long wavelength ultra-violet radiation. Breaks appear in
the DNA at the sites of incorporation of BrdU and cause slower
sedimentation of the DNA in alkaline sucrose gradients (Smith &
Hanawalt, 1969). This technique appears to be a very sensitive one for
measuring single-strand breaks but it still seems to be prone to
artefacts and cannot be considered suitable for large-scale studies
(Cleaver, 1975).
Another method involves the total suppression of normal DNA
synthesis, for example, by hydroxyurea, thus the incorporation of
precursors into DNA reflects only repair synthesis and not normal
replication.
Some of the above techniques are time-consuming, costly and not
yet standardized, so that it is difficult to adapt them for
large-scale studies. The most promising approach appears to be the
measurement of unscheduled DNA synthesis, but criticisms have also
been made of the use of this system as an indicator of mutagenic or
carcinogenic potential (Cleaver et al., 1975). One limitation is that
unscheduled synthesis is an average measure of repairable damage in
all cells of a population or in all sites of the DNA, whereas
mutagenesis and carcinogenesis seem to depend on the amount of
unrepaired damage in a small percentage of cells and in a few specific
DNA sites. Due to these limitations and to the current limited
understanding of these processes, none of these variables could, by
themselves, be a reliable indicator of a potential mutagenic or
carcinogenic chemical. However, these studies associated with studies
of DNA damage as well as of the expression of this damage are
essential in the understanding and development of reliable screening
systems.
Metabolic activation of chemicals to electrophiles, DNA damage,
and subsequent repair processes are important factors in the
initiation of cancer. The phenotypic expression (tumour development)
of these initial molecular changes is modulated by a number of factors
(Farber, 1973) that influence the macroscopic appearance of cancer at
a later stage (Pitot, 1977). However, the initial molecular changes,
induced by chemical carcinogens, appear prerequisite for initiation of
the cancer process. Further studies on the alteration of DNA induced
by chemical carcinogens and on the repair of such lesions before cell
division by tissues, whether target or not, are required to evaluate
the role of DNA repair processes in chemical carcinogenesis.
7.2.2.2 In vitro neoplastic transformation of mammalian cells
The term transformation in such studies refers to the in vitro
observations of various changes present in the cells treated in vitro
with the chemicals, when compared with untreated control cells.
Transformed cells may differ from control cells in various ways such
as: (a) alteration of cellular and colony morphology; (b) increased
plating efficiency; (c) agglutinability by plant lectins; (d) altered
glycolytic patterns; (e) resistance to toxicity of some chemicals; (f)
altered surface properties (contact inhibition of movement and growth,
population density, growth in suspension, ability to grow in agar or
other semisolid media); (g) sensitivity to activated macrophages and
lymphocytes; (h) establishment of cell lines with the potential to be
sub-cultured indefinitely in vitro; and (i) appearance of new
antigens (Fedoroff, 1967). However, the unequivocal criterion of
malignant transformation is the capacity of transformed cells to
develop a malignant neoplasm when injected into a syngenic,
immunosuppressed or thymus-free host, or into privileged sites
(Giovannella et al., 1972; Sanford, 1965).
Recently, Sanford (1974) critically reviewed the significance of
these various in vitro changes in relation to the acquisition of
neoplastic potential. It was stressed that these criteria of
neoplastic transformation cannot completely replace the in vivo
assay for tumour production. With reference to the problems of the
application of tissue culture to the rapid detection and
characterization of neoplastic transformation in vitro, the reader
is referred to the proceedings of the symposium "New Horizons for
Tissue Culture in Cancer Research" ( J. Natl Cancer Inst., 1974).
In most in vitro transformation experiments the cells used have
been fibroblasts. Earle & Nettleship (1943) reported the
transformation by 1,2-dihydro-3-methyl-benz[j]aceanthrylene
(3-methylcholanthrene) of a long-term culture of fibroblasts as
demonstrated by the development of tumours after inoculation of the
cells into mice. However, this observation was attributed to
spontaneous transformation, since Sanford et at. (1950) observed that
the cells were tumorigenic also without treatment with chemicals. The
first clear demonstration of transformation of cells in culture by
chemicals was that of Berwald & Sachs (1963) who observed the
transformation of Syrian hamster embryo secondary cells with
3-methylcholanthrene and benzo(a)pyrene, but not with urethane or
solvent. Since this report, various cells types originating from
different animal species have been used for in vitro transformation.
This topic has recently been reviewed by Heidelberger (1973) and
Kuroki (1975), who critically examined the advantages and
disadvantages of the various strains and lines of fibroblastic cells,
namely of the Syrian hamster embryo, fibroblastic cells derived from
mouse ventral prostate, cell lines derived from embryo cells of Swiss
mouse BALB/c, C3H, AKR, and C57/B1 strains, Chinese hamster lung
cells, as well as various tissues from organ cultures. Transformation
of the above cell lines was obtained with polynuclear hydrocarbons and
with chemicals that do not require metabolic activation.
Some chemical carcinogens that are active in vivo have failed
to induce transformation, when applied directly to hamster embryo
cells; this is presumably because such compounds require metabolic
conversion to active intermediates that are lacking or are present in
insufficient concentration in these cells. However, neoplastic
transformation was detected in fibroblasts obtained from embryos whose
mothers had been exposed to indirect carcinogens during pregnancy
(Di Paolo et al., 1972, 1973). A similar approach was used by Borland
& Hard (1974), who cultured kidney cells at various times following
in vivo treatment of rats with N-methyl- N-nitrosomethanamine
(dimethylnitrosamine). The cells isolated from treated rats showed
various morphological and behavioural changes associated with
transformation. Laerum & Rajewsky (1975) reported the development of
glioblastomas following injection of glial cells originating from the
brain of rat embryos, the mother having been treated with
ethylnitrosourea during pregnancy.
Epithelial cultures from rat liver were recently established in
various laboratories and used for transformation studies (Iype, 1974;
Katsuta & Takaoka, 1972; Montesano et al, 1975; Weinstein et al.,
1975; Williams et al., 1973). The transformation of these cells by
various carcinogens that need metabolic activation was determined by
the development of carcinomas after their back-transplantation into
suitable hosts. Carcinomas were observed following inoculation of
epithelial cells from mouse skin, or rat urinary bladder or salivary
glands, treated with chemical carcinogens in vitro (Brown, 1973;
Fusenig et al., 1973; Hashimoto & Kitagawa, 1974).
One disadvantage of these epithelial cells is that the
morphological criteria for transformation of fibroblast cultures
(piling up of cells, crisscross arrangement of cells etc.) do not
apply possibly because normal epithelial cells have little or no
locomotion and they continue to divide even when in close contact with
other cells (Weinstein et al., 1975). However, the capacity for growth
in soft agar appears to provide a reliable and reproducible
correlation with the tumorigenicity of these cells (Weinstein et al.,
1975; Montesano et al., 1977). Although, at present, this system
provides only a qualitative and not a quantitative assay of in vitro
transformation, it is the only instance of unequivocal production of
carcinoma. The establishment of reliable criteria, measurable in
vitro, for distinguishing normal from transformed epithelial cells
in culture is essential for the development of quantitative systems
for the transformation of these epithelial cells. Recently the
neoplastic transformation of human diploid cells by chemical
carcinogens has been described (Kakunaga, 1977).
Cell culture has been extremely useful in elucidating the
cellular and molecular mechanism of chemical carcinogens and it holds
great promise as a test for screening for the potential
carcinogenicity of environmental chemicals. However, some time is
needed before this test may be used for routine testing in a
reproducible way.
7.2.2.3 Mutagenicity tests
The growing experimental evidence linking the carcinogenic
activity of numerous chemicals with their capacity to be converted
into electrophilic derivatives, that may also exert a mutagenic
effect, has led to the suggestion that a relationship between chemical
carcinogenesis and mutagenesis may exist (Miller & Miller, 1971a,b).
Such a correlation has so far been limited to those changes of the
genotype that appear as a consequence of structural or functional
alterations of nucleic acids. Not all chemical mutagens have been
shown to be carcinogenic. However, most chemical carcinogens, several
of which cause cancer in man, have now been found to be mutagens, when
tested by one of the mutagenicity test procedures that combine
microbial, mammalian, or other animal cell systems as genetic targets
with an in vitro or in vivo metabolic activation system. The
growing empirical relationship between chemical mutagens and
carcinogens does not imply that the two processes are identical, but
it offers a promising method for the use of mutagenesis as a rapid
prescreening assay for carcinogenesis (Bartsch & Grover, 1976;
Bridges, 1976; Council of the Environmental Mutagenic Society, 1975;
IARC, 1976; McCann & Ames, 1976; Purchase et al., 1976; Stoltz et al.,
1974; Sugimura et al., 1976; WHO, 1974).
The choice of mutagen-detecting assay depends on various
considerations, i.e. the chemical structure and pharmacological
activity of the chemical, the type of human exposure, and the nature
of the population at risk. The same considerations should be taken
into account in assessing the strength of the evidence of mutagenicity
and its relevance to man.
7.2.2.4 Submammalian assay systems
Bacterial phages have been used to test reactive forms of
chemical carcinogens. Chemicals inducing point mutations can be
detected by reacting the test compound with the free phage or with
phage during its duplication inside bacteria (Corbett et al., 1970;
Drake, 1971).
In the bacterial transformation of DNA (Freese & Strack, 1962),
genetic information contained in the DNA isolated from one strain of
bacteria can be transformed to that of a recipient strain. Purified
bacterial DNA is readily accessible to reactive forms of mutagens.
Thus, this system has been used to quantify the mutagenic potential of
a number of chemicals. Extensive studies have been made on the
inactivation of Bacillus subtilis DNA and transformation of the
tryptophane-requiring strain T-3 (Herriott, 1971; Maher et al., 1968).
The reverse mutationa system of Salmonella typhimurium uses
the genetically well-defined histidine-requiring mutants developed by
Ames and his colleagues (Ames, 1971; Ames et al., 1972a,b, 1973;
McCann et al., 1975).
These revert to prototrophy by single-base pair substitutions,
e.g. strain TA1535 or by base pair insertion (frameshift), e.g.
strains TA1536, TA1537 and TA1538. Most of the theoretically possible
types of mutation may be detected with a set of these test strains,
where a mutation of one of the genes responsible for excision repair
(UVrB) has produced a 100-fold increase in sensitivity. Penetration of
larger molecules through the bacterial cell walls has also been
facilitated by the use of deep-rough mutants deficient in the exterior
polysaccharide coat. Two newly developed test strains TA100 and TA98
were obtained by transferring an ampicillin resistance factor
(R factor) to the standard test strains TA1535 and TA1538,
respectively (McCann et al., 1975). These strains are effective in
detecting classes of mutagens that were not previously detected with
the original strains. The tests are usually performed by adding a few
crystals or a drop of solution of the test chemical in
sulfinylbis[methane] (dimethylsulfoxide) or water to a uniform lawn of
one of the histidine-requiring mutants on the surface of a Petri plate
containing histidine-poor medium, or by incorporating the test
compound, a postmitochondrial tissue fraction, cofactors (NADPH or
NADPH+ and glucose 6-phosphate) and the bacterial test strain in
histidine-poor soft agar. For general mutagenicity screening, a liver
homogenate (9000 g supernatant) from rats induced with a mixture of
polychlorinated biphenyls (Aroclor 1254) is recommended as a metabolic
activation system. For routine testing, the strains TA1535, TA1537 and
TA1538 can be used in combination with the strains carrying the R
factor (TA100 and TA98). This method of testing using various groups
of chemicals and various experimental conditions is described in more
detail by Ames et al. (1975).
Using the genetically well characterized Escherichia coli
strains, reverse and forward mutation can be scored using nutritional
resistance or fermentative markers (Bridges et al., 1972; Mohn, 1973).
Prophage lambda induction in E. coli strains by chemical mutagens
activated by liver microsomal enzymes has been described as a
sensitive test for the detection of potential mutagens and carcinogens
(Moreau et al., 1976).
a Mutations in which the function of a given gene is lost are
called forward mutations. Mutations that bring about the
restoration of gene function are called reverse mutations or back
mutations.
Mutagenesis has been extensively studied in Neurospora crassa.
Heterokaryon has been developed, which is heterozygous for two
closely-linked loci in the ad-3 region. Mutants at the ad-3A and
ad-3B loci have a requirement for adenine and can be selected
directly on the basis of accumulation of a reddish-purple pigment in
the mycelium. The ad-3 mutations can be characterized by a series of
simple genetic tests to distinguish point mutations from deletions and
to obtain a presumptive identification of the genetic alterations in
the point mutations at the ad-3B locus at a molecular level (de
Serres & Malling, 1971).
Saccharomyces cerevisiae and S. pombe have been used to
investigate the effects of carcinogenic and mutagenic compounds in
these organisms. Mitotic gene conversion, in which a sequence of a few
hundred nucleotides of one chromosome is replaced by one corresponding
sequence from a homologous chromosome, can be studied in diploid cells
of the yeast S. cerevisiae strain D-4 heteroallelic at the loci
trp-5 and ade-2. This strain carries two different inactive
alleles of two genes ( trp-5 and ade-2) which are located on
different chromosomes and the functional defects of which lead to a
nutritional requirement. Mitotic gene conversion, which is increased
by many types of mutagenic treatment, can transfer the intact region
of one of these alleles to the defective region of the other, thus
producing a heterozygotic diploid cell with full functional activity.
The mechanism is presumably based on the formation of single-strand
breaks in DNA and probably involves repair processes. Positive results
are only obtained with agents or metabolites which either bind with
DNA covalently or interfere with DNA metabolism. In contrast with most
microbial mutation systems, mitotic gene conversion does not show a
response specific to any type of mutagen. In addition to gene
conversion, forward and reverse mutation can be measured with
S. cerevisiae (Loprieno et al., 1976; Marquardt, 1974; Mortimer &
Manney, 1971; Zimmerman, 1973).
7.2.2.5 Mammalian somatic cells
The application of cultured cell lines is somewhat restricted at
present by requirements for karyotypic stability and high plating
efficiency. A widely used system developed by Chu (1971) employs cell
lines derived from the lung, ovary, and other tissues of Chinese
hamster, which usually maintain a near-diploid chromosome number and
exhibit active growth and high cloning efficiency. Two reviews discuss
this topic in detail (De Mars, 1974; Thompson & Baker, 1973).
Selective media have been developed to detect both forward and reverse
mutations at three genetic loci involving enzymes in the salvage
pathways of purines and pyrimidines. In Chinese hamster, as in man,
the use of preformed hypoxanthine and guanine is controlled by an
X-linked gene. Mutant cells at these loci are deficient in the enzyme
hypoxanthine-guanine phosphoribosyl transferase and are resistant to
certain purine analogues. Similarly, mutant cells deficient in
adenosine phosphoribosyl transferase cannot metabolize preformed
adenosine or its analogues for incorporation into nucleic acid. The
third type of drug-resistant mutants currently studied in these and
other mammalian cells are those deficient in thymidine kinase. Such
cells exhibit resistance to the thymidine analogue,
5-bromodeoxyuridine. Cells carrying mutation at these loci can be
selected from the wild type in an environment containing an
appropriate purine or pyrimidine analogue. Revertants to the wild type
can be recovered in selective media in which the cells are supplied
with a natural purine or pyrimidine while the normal de novo pathway
of purine or pyrimidine synthesis is inhibited by an antimetabolite.
It has been demonstrated that Chinese hamster cells treated with
physical or chemical mutagens undergo a significant increase in the
frequency of forward and reverse mutations compared with the
spontaneous frequency (Huberman & Sachs, 1974; Huberman et al., 1971,
1972). A liver microsomal activation system can be added for the
metabolic activation of chemicals (Krahn & Heidelberger, 1975; Kuroki
et al., 1977).
Forward mutations from thymidine kinase +/- cells to thymidine
kinase -/- have been induced by treatment of cells with X-rays or
chemical mutagens (mouse lymphoma L5178 Y cells). These cells can also
be grown in the presence of in vitro or in vivo metabolic
activation systems, which makes them suitable for host-mediated
mutagenicity tests (Nahas & Capizzi, 1974).
Forward mutations to hypoxanthine-guanine phosphoribosyl
transferase deficiency (resistance to 8-azaguanine) in diploid human
fibroblasts in culture have been shown to occur either spontaneously
or after X-radiation (Albertini & De Mars, 1973). Chemical induction
of forward mutation from hypoxanthine-guanine phosphoribosyl
transferase - to + in human lymphoblastoid cell line has also been
reported (Sato et al., 1972).
7.2.2.6 Host and tissue-(microsome) mediated assays
These take into account the conversion of chemicals into
mutagenic metabolites, and are particularly suitable for the detection
of chemicals that are not mutagenic per se but require metabolic
activation. They can be performed in vivo (host mediated) or in
vitro (tissue mediated) using various indicator organisms, such as
bacteria, fungi, yeast, or mammalian cells.
In the host-mediated assay, the indicator organisms are injected
into the interperitoneal cavity of an animal (Legator & Malling,
1971), which is then treated with the test compound by another route.
After a given length of time, the animal is killed and the indicator
organism is recovered and scored for mutants. Comparison between the
mutagenic action of the compound on a test strain directly and the
host-mediated assay indicates whether the host can activate or
inactivate the test compound. The limitations of the host-mediated
assay are the high spontaneous mutation rates of the indicator
organism in the host, the host's effect on cell survival and the
selection of heterogenic cell populations. In order to measure a
significant increase over the spontaneous mutation rate, doses well
above LD50 have to be given to rats or mice. This type of assay is
also limited since it does not identify the site of conversion to a
mutagenic metabolite. Modifications have been reported (Mohn, 1973).
In the tissue-mediated assay, the indicator organisms are
incubated in vitro in the presence of a tissue fraction plus
appropriate cofactors and the test compound. The mutants are
subsequently isolated and scored.
Ames et al. (1973a,b) have developed a mutagenicity assay that
combines a Salmonella strain, a liver microsomal preparation, and
cofactors in a soft agar layer on a Petri dish. Bartsch et al.
(1975b), Czygan et al. (1973) and Malling (1974) have described a
liquid incubation system where bacteria, a tissue preparation, and the
test compound are incubated in liquid suspension. Test compounds that
are gases at room temperature or are volatile can be assayed by
exposing Petri dishes containing bacteria, tissue fraction, and
cofactors to a mixture of gas and oxygen at 37°C (Bartsch et al.,
1975a). The mutagenicity of urinary metabolites, excreted as
conjugates in experimental animals treated with an indirect mutagen,
may be detected by treating the urine with hydrolytic enzymes in the
presence of a bacterial test strain or yeast, and an in vitro
metabolic activation system (Commoner et al., 1974; Durston & Ames,
1974; Marquardt, 1974). In another modification, Huberman & Sachs
(1974) used lethally irradiated rat fibroblasts that retain a
drug-metabolizing capacity and cocultivated them with Chinese hamster
V79 cells which are used as genetic indicators.
The mutagenicity tests previously described (7.2.2.4, 7.2.2.5,
7.2.2.6) all have individual advantages and limitations determined
either by the genetic indicators or by the metabolic activation
system. The use of submammalian organisms for the detection and
classification of mutants, induced by chemicals, is greatly
facilitated by the relatively small genome to which fine genetic
mapping and biochemical analysis can be applied and by the short
generation time. Huge populations of some of these organisms can be
raised and they can easily be handled when analysing multiple
mutations.
The relevance of such data from submammalian systems to mammalian
cells is based on the assumption that the basic principles of
heredity, and the structure and functions of DNA in terms of
reactivity, the triplet code, transcriptional, and translational
mechanisms, are essentially the same for all living cells irrespective
of their evolutionary level. However, this extrapolation is hampered
by lack of knowledge of repair processes that play a role in mutation
fixation and expression and are insufficiently understood in mammals.
It is obvious that normal human diploid cells are most desirable for
this test, but current studies suggest that reliable conclusions can
be drawn from results with non-human mammalian cells.
The other factor, related to the fact that many chemicals are not
active per se, is the metabolic activation of the compounds. In many
cases, reactive metabolites with a limited life span may fail to reach
or react with the genetic indicator either because they are further
metabolized to inactive compounds, or because they react with other
cellular constituents. For this reason, mutagenicity assays in intact
animals (host-mediated assays) may give negative results, as in the
case of N-methyl- N'-nitro- N-nitrosoguanidine (MNNG) and acridine
mustard (ICR-170), proven to be extremely potent mutagens. Metabolism
in animals is affected by exogenous and endogenous factors such as
chemicals causing enzyme induction and inhibition. Other modifying
factors are age, sex, and strain of animals, diurnal and seasonal
rhythms, differences between the fetal and adult state, mode of
administration, cellular uptake, and distribution and excretion of the
chemical.
The tissue-mediated mutagenicity test cannot, with certainty,
reproduce the in vivo situation, but obvious advantages are the high
sensitivity, good reproducibility, low cost, and the possibility of
testing a large number of chemicals. In addition, in vitro testing
with well-characterized genetic indicators allows the use of human
tissue such as human liver to determine their ability to generate a
mutagen (Bartsch, 1976).
7.2.3 Correlation between short and long-term bioassays for
carcinogenicity
Short-term tests (rapid screening tests) are procedures that do
not have the in vivo production of a visible tumour in animals as an
end point. The variables used in short-term tests to detect chemical
carcinogens are based on an interaction of carcinogens and/or their
metabolites with macromolecules, the induction of chromosomal
aberrations, mutagenesis, DNA repair, and DNA binding.
In the evaluation of these methods, the major question is which
screening tests, singly or collectively, serve as reliable indicators
or predictors of the potential carcinogenic hazard of the chemical.
The answer can only be obtained by testing a representative number of
compounds. A valid test should demonstrate that compounds with known
carcinogenic properties are positive, within the limit of the test,
and negative compounds are negative. If a test is required for
preliminary screening, a small proportion of false negatives or
positive results may be acceptable, but for a final test, no false
negative results are acceptable.
Rapid screening tests should be relatively simple and
inexpensive. They can be used in three ways: to trace carcinogens
and/or mutagens in the complex environment of man; as a tool for
prescreening chemicals to be submitted to a more lengthy set of
bioassays; or for better extrapolation from experimental animal data
to man and for improving the relevance of long-term bioassays in
experimental animals.
Considering reproducibility, cost, the number of chemicals that
can be examined in a short time, and the scientific basis of the test,
tissue-mediated mutagenicity procedures using well-characterized
genetic indicators and a metabolically defined in vitro activation
system, appear at present to be the most promising short-term tests.
With current methods there is still the chance of false negative
results, depending on the systems used, either for lack of appropriate
cofactors for activation or because of the extreme reactivity and/or
toxicity of the compound or its metabolites. However, the number of
false negative results in in vitro tissue-mediated assays is small
compared with other mutagenicity test procedures (Montesano & Bartsch,
1976).
The increasing evidence of a possible correlation between
mutagenicity and carcinogenicity certainly does not mean that one
biological effect may be equated with another. Thus, the mutagenic
activity of a chemical cannot, at present, automatically be assumed to
imply a definite carcinogenic effect in man, nor can these results
replace long-term carcinogenicity testing in animals.
Furthermore, it is still unknown whether all carcinogens will be
found to be mutagenic, and all mutagens, carcinogenic. Examples are
the strong mutagens, nitrous acid, hydroxylamine, and base analogues,
for which no carcinogenic effect in animals has so far been reported;
they do not act via electrophilic intermediates, a mechanism that has
now been recognized for must ultimate carcinogenic forms. Nor have
steroidal sex hormones, carcinogenic in animals, been reported to be
mutagenic, as yet. On the other hand, various chromium salts have
recently been shown to be mutagenic in bacteria (Venitt & Levy, 1974).
Development of cancer in vivo is determined by a variety of
factors that cannot be duplicated in an in vitro short-term testing
system because:
(a) The concentration of ultimate reactive metabolites available
to react in organs in animal species with cellular
macromolecules, which is a consequence of a balance between
metabolic activation and detoxication processes, is only
partly reflected by in vitro testing procedures.
(b) Species and organ specificity of a chemical carcinogen might
be determined, in part, by organ-specific DNA repair.
(c) Since chemical carcinogenesis is thought to be a multi-step
process in which the early, apparently irreversible,
initiation of a cell is followed by several subsequent
stimuli provoking cellular replications, leading to an overt
tumour, a short-term test capable of detecting complete
carcinogens may be useful to detect initiating agents but
cannot at the present time detect the action of promoting
agents.
Currently used short-term tests, in particular tissue-mediated
mutagenicity assays are effective in predicting, with a certain
accuracy, the carcinogenic potential of chemicals (McCann & Ames,
1976; Purchase et al., 1976; Sugimura et al., 1976), but give no
indication of the target organs or species specificity of their
carcinogenic activity. Furthermore, the relative potency of a chemical
to induce biological effects, defined as the endpoints, for the most
frequently used rapid screening tests cannot at the present time be
reliably correlated with its carcinogenic potency (Bartsch et al.,
1977; Meselson & Russell, 1977). More data are needed to compare
dose-response curves obtained in rapid screening tests on a given
chemical with those of other biological effects obtained in vivo
(long-term bioassays).
7.2.4 Significance of experimental testing for assessing the possible
carcinogenic risk of chemicals to man
Experience acquired, so far, in long-term carcinogenicity testing
has shown that nearly all compounds that are carcinogenic in man are
also carcinogenic in one or several animal species, even though the
tumour type may not be the same as in man. The concept that animal
carcinogenicity data are predictive of a human carcinogenic risk and
useful in preventing human cancer was accepted in 1941 in the case of
2-actylaminofluorene (AAF). This chemical was not used on a worldwide
basis as an insecticide, because some experiments had already shown
its carcinogenicity before it was marketed (Wilson et al., 1941); its
restriction was facilitated by the existence of a number of
substitutes at the time when the results of the first experiments on
AAF were reported. This cautious attitude has not been consistently
applied in other situations, on the grounds that the experimental data
were insufficient or inadequate to evaluate the possible hazard to
man. This is shown by the various relationships between experimental
carcinogenicity data and possible human hazard that are considered in
the adoption of preventive measures.
The first observation that some aromatic amines were involved in
the causation of urinary bladder tumours in man dates from 1896; it
was confirmed in 1907 and reconfirmed many times thereafter (IARC,
1974). In 1938, Hueper et al. reported the induction of bladder cancer
in dogs exposed to 2-naphthalenamine (2-naphthylamine). Preventive
measures were taken only in the late 1950s, when conspicuous
epidemiological evidence had accumulated. In this case, the human
epidemiological evidence was judged insufficient, and the experimental
evidence, when it became available, was also deemed insufficient until
further epidemiological findings came to hand.
The delay in taking preventive measures applies also to
carcinogens on which, contrary to the case of aromatic amines,
experimental evidence preceded observations of their effect in man,
e.g. diethylstilbestrol, bis(chloromethyl)ether, and chloroethylene
(vinyl chloride). (Tomatis et al. 1978; Montesano & Tomatis, 1977).
One of the main objections to carcinogenicity tests on animals is
that the experimental system used tries to produce the maximum
possible carcinogenic effect of the test chemical, does not reflect
the human situation, and is therefore misleading. It is difficult,
however, to accept this argument because, in most instances where a
chemical was found by epidemiological investigations to be associated
with cancer in man, the incidence was so high that the association was
clear without animal studies. This has been the case for high risk
groups such as occupational cancer groups. However, the risk is not
confined to these groups but also applies to other populations where
cancer incidence may be too low for detection by normal
epidemiological methods, hence the need to carry out animal
experiments under conditions that permit confident judgment of the
carcinogenicity or inactivity of a chemical.
Cancer testing in animals has reached a relatively sophisticated
stage and an exhaustive study of a chemical in animals is sufficient
evidence of a potential cancer risk for man. An assessment of the
validity of experimental results is essential for the successful
prevention of cancer in man. This does not preclude further research
for the development of short-term tests, but, at the present time,
these cannot replace long-term carcinogenicity testing.
7.3 Heritable Mutations
As noted in the introduction, direct methods for assessing
whether a chemical has the potential to cause heritable mutations in
man do not exist at present. Nontheless, information relative to the
possible production in man of germ cell mutations can be derived from
a variety of sources. These fall into three major categories: (a)
primary DNA damage involving DNA alteration, stimulation of DNA
repair, gene mutations tests including mutagenicity assessment using
bacterial or other microorganisms, with and without metabolic
activation; (b) whole animal tests for point or gene mutations
including insects, e.g. drosophila recessive, and the specific locus
test in mice; (c) chromosomal mutations including cytogenetic tests in
mammals, the dominant lethal test in mammals, and the heritable
translocation test in rodents. Examples of the first category have
already been presented. These tests provide information relevant to
heritable mutations and section 7.2.2 should be consulted for fuller
details. Obviously, tests employing isolated systems only provide
information on mutagenic action on the specific systems tested. This
information cannot be fully interpreted without evidence concerning
the access of this ultimate mutagen to the germ cells.
An extensive examination of relevant test procedures for
heritable mutations has recently been completed (DHEW, 1977).
7.3.1 Whole-animal tests
Insects. Drosophila melanogaster is one of the best genetically
characterized species and is widely used. Drosophila of either sex can
be treated and mutation frequencies from successive germ cell stages
may be obtained after observation of three generations. The X-lined
recessive lethal test is one of the most sensitive tests with
drosophila, since the X-chromosome represents about 1/5th of the whole
genome. In contrast to most microorganisms, insects possess an enzyme
system that appears to metabolize foreign compounds in a fashion
similar to that of vertebrates (Abrahamson & Lewis, 1971; Fahmy &
Fahmy, 1972, 1973; Sobels & Vogel, 1976).
Mouse specific locus test. The specific locus test is a method
of inducing, detecting, and measuring the rate of mutation at several
recessive loci. It consists essentially of mating treated or untreated
wild type mice, either male or female, to a strain homozygous for a
number of known recessive genes. The recessive genes are such that
they are readily expressed as visible phenotypes in homozygous state.
If a mutation occurs in any of the test loci in the germ cells of
treated animals, it may be detected in the offspring. If no mutation
has occurred following treatment, the progeny from the cross will all
be of the wild type (Cattanach, 1971; Russell, 1951).
Chromosomal mutations. Although the molecular basis is not
understood, a change in the whole chromosome, i.e. a structural
chromosome aberration, occurring as a consequence of a misrepair of
chromosomal breaks, may lead to deletions, duplications, and
translocations. Changes in the whole chromosome complement, i.e.
numerical chromosome aberrations, arise through nondisjunction, as in
failure of a pair of chromosomes to separate during gametogenesis,
meiotic nondisjunction, or during mitotic division. The resulting
daughter cells are either trisomic with an extra chromosome or
monosomic and lacking a chromosome. Anaphase lag occurs during nuclear
division in the progeny cells, and a chromosome may be either lost or
gained. In somatic cells, nondisjunction and anaphase lag can lead to
mosaicism.
Chromosomal damage may be studied in a number of test systems
that are best divided into in vivo and in vitro systems, on the
basis of their capability to metabolize the test compound (Frohberg,
1973). The short-term human lymphocyte culture in vitro is commonly
used to assess the effects of chemicals upon chromosomes. Metabolic
activation of the test compound can be achieved by addition of a liver
microsomal system (Bimboes & Greim, 1976).
The micronucleus test. As an in vivo cytogenetic method, the
micronucleus test is a procedure for the detection of aberrations
involving anaphase chromosome behaviour using bone-marrow
erythroblasts. The test is based on the formation of micronuclei from
particles of chromatin material which, due to chromosome breakage or
spindle disjunction, do not migrate to the poles during anaphase and
are not incorporated into the telophase nuclei of the dividing cells.
The procedure is to treat animals with clastogenic agents and, at an
appropriate time after treatment, to aspirate bone-marrow samples into
calf serum. This is then centrifuged and smears are made from the
resuspended pellets of cells. The smears are air-dried and stained.
The evaluation of the bone-marrow preparations involves examination of
2000-5000 erythrocytes per specimen; the total number of polychromatic
and normochromatic erythrocytes with and without micronuclei are
recorded (Schmid, 1973). Sister chromatid exchanges, induced by
mutagens in vivo and in vitro can be scored in peripheral
lymphocytes or Chinese hamster cells, using the differential straining
of chromatids substituted with 5-bromodeoxyuridine in place of
thymidine (Natarajan et al., 1976; Smythe & Evans, 1976; Stetka &
Wolf, 1976).
Dominant lethal tests and other in vivo systems. The dominant
lethal test is based on the preimplantation loss of eggs or on the
formation of dead embryonic implants following the injection of
mutagens into a male or female mouse at a specific time before mating
(Bateman & Epstein, 1971). Dominant lethal tests assume that a single
mutation has occurred in the eggs or sperm, which is lethal to the
embryo and heterozygous at the affected locus. However, a disturbingly
high number of mutagens have given a negative response with this test.
The detection of mutations arising from compounds that are metabolized
to transient reactive intermediates not produced in germ cells or not
reaching them from other organs may limit this test. Furthermore,
chemical agents causing spermatogenic arrest or cytocidal effect on
the sperms may give false positive results.
Heritable translocation in male mammals. The heritable
translocation test has the important feature of measuring sexually
transmissible germ cell mutations in rodent spermatogonia. Generoso et
al. (1974) have described details of the technique and discussed its
usefulness for the routine screening of substances that cause
chromosomal mutations.
Young adult male mice are treated and females mated with the
exposed males on a schedule that can be used for the comparison of the
sensitivity of the different male germ cell stages. Male progeny from
these matings are collected and mated for the determination of
semisterility and sterility. Progeny with reduced fertility are
subjected to cytogenetic analysis. The cytological examination of
dividing spermatocytes, from animals treated with test chemicals that
cause breaks on two nonhomologous chromosomes, yields aberrant
chromosomal figures. These are recognized as rings or chains of four
chromosomes as opposed to the normal bivalent chromosomal
configuration.
A proper evaluation of this test cannot be made at this time
because of insufficient data in terms of the variety of chemicals
tested to date.
7.3.2 Monitoring of human populations
In spite of the variety of test systems available for detecting
the mutagenic effects of chemicals, it is difficult, from the results
obtained at present, to evaluate with confidence the transmissible
genetic effects caused by the chemical exposure of man. However, the
potential hazards of mutations are such that every effort should be
made to reduce the risk.
Judgment on the potential mutagenic hazard to man should be based
on various considerations such as strength of the experimental
evidence of mutagenicity, the exposure pattern to the chemical, and
the pharmacological properties of the compounds. This is difficult and
complex. Although human studies are difficult, expensive, and often
subject to misinterpretation, only studies which directly estimate the
extent to which environmental factors change human genetic material
give definitive answers.
Proper monitoring of the human population (Crow, 1971; Sutton,
1972) can be carried out by three main approaches:
(a) biochemical: detection of inherited protein variants;
(b) cytogenetic: screening of blood of newborn infants or of
fetuses delivered by spontaneous abortion for chromosomal
changes;
(c) phenotype: surveillance of genetically determined disease or
anomalies.
The validity of these monitoring systems can only be properly
evaluated if, and when, a mutagen is actually discovered.
7.3.3 Significance of tests for heritable mutations
As mentioned earlier, there is, at this time, no direct
correlation between laboratory tests for heritable mutations and human
experience. However, the available body of information from nonhuman
mammals and lower life forms clearly points to the ability of
chemicals to produce alterations in germ cells which are inherited in
succeeding generations. Accordingly, the implications of such data for
man are so persuasive that they must be taken into account in
establishing safety measures for the introduction of chemicals into
use. This is especially true, at this time, since techniques for
detecting mutations in human populations are so insensitive that a
significantly mutagenic chemical could easily escape attention.
These considerations will almost certainly lead to increasing
concern in establishing regulatory and control procedures. In fact,
the Environmental Protection Agency in the USA has recently proposed
preliminary guidelines for conducting tests for heritable mutations as
part of the routine registration procedure for pesticides.
REFERENCES
ABRAHAMSON, S. & LEWIS, E. B. (1971) The detection of mutations in
Drosophila melanogaster. In: Hollaender, A., ed. Chemical
mutagens, principles and methods for their detection, Vol. 2,
461-488.
ALBERTINI, R. J. & DEMARS, R. (1973) Somatic cell mutation detection
and quantification of X-ray induced mutation in cultured human
diploid fibroblasts. Mutat. Res., 18, 199-244.
AMES, B. N. (1971) The detection of chemical mutagens with enteric
bacteria. In: Hollaender, A., ed. Chemical mutagens, principles
and methods for their detection, Vol. 1, pp. 267-282.
AMES, B. N., SIMS, P., & GROVER, P. L. (1972a) Epoxides of
carcinogenic polycyclic hydrocarbons are frameshift mutagens.
Science, 176, 47-49.
AMES, B. N., GURNEY, E.G., MILLER, J. A., & BARTSCH, H. (1972b)
Carcinogens as frameshift mutagens: Metabolites and derivatives
of 2-acetylaminofluorene and other aromatic amine carcinogens.
Proc. Natl Acad. Sci., 69: 3128-3132.
AMES, B. N., LEE, F. D., & DURSTON, W. E. (1973a) An improved
bacterial test system for the detection and classification of
mutagens and carcinogens. Proc. Natl Acad. Sci., 70: 782-786.
AMES, B. N., DURSTON, W. E., YAMASAKI, E., & LEE, F. D. (1973b)
Carcinogens are mutagens: a simple test system combining liver
homogenates for activation and bacteria for detection. Proc.
Natl Acad. Sci., 70; 2281-2285.
AMES, B. N., MCCANN, J., & YAMASAKI, E. (1975) Methods for detecting
carcinogens and mutagens with the Salmonella/mammalian-
microsome mutagenicity test. Mutat. Res., 31: 347-364.
BARTSCH, H. (1976) Predictive value of mutagenicity tests in chemical
carcinogenesis. Mutat. Res., 38: 177-190.
BARTSCH, H. & GROVER, P. L. (1976) Chemical carcinogenesis and
mutagenesis. In: Symington, T. & Carter, R. L., ed. Scientific
foundations of oncology, London, William Heinemann Medical
Books Ltd, pp. 334-342.
BARTSCH, H., MALAVEILLE, C., & MONTESANO, R. (1975a) Human, rat and
mouse liver-mediated mutagenicity of vinyl chloride in
S. typhimurium strains. Int. J. Cancer, 15: 429-437.
BARTSCH, H., MALAVEILLE, C., & MONTESANO, R. (1975b) In vitro
metabolism and microsome-mediated mutagenicity of
dialkylnitrosamines in rat, hamster and mouse tissues. Cancer
Res., 35: 644-651.
BARTSCH, H., MALAVEILLE, C., STICH, H. F., MILLER, E. C., & MILLER, J.
A. (1977) Comparative electrophilicity, mutagenicity, DNA repair
induction activity, and carcinogenicity of some N- and O-Acyl
derivatives of N-Hydroxy-2-aminofluorene. Cancer Res.,
37: 1461-1467.
BATEMAN, A. J. & EPSTEIN, S.S. (1971) Dominant lethal mutations in
mammals. In: Hollaender, A., ed. Chemical mutagens, principles
and methods for their detection, Vol. 2, pp. 541-568.
BERWALD, Y. & SACHS, L. (1963) In vitro transformation with chemical
carcinogens. Nature (Lond.), 200: 1182-1184.
BIMBOES, D. & GREIM, H. (1976) Human lymphocytes as target cells in a
metabolizing test system in vitro for detecting potential
mutagens. Mutat. Res., 35: 155-160.
BORLAND, R. & HARD, G. C. (1974) Early appearance of "transformed"
cells from the kidneys of rats treated with a "single"
carcinogenic dose of dimethylnitrosamine (DMN) detected by
culture in vitro. Europ. J. Cancer, 10: 177-184.
BRIDGES, B. A. (1976) Short-term screening tests for carcinogens.
Nature (Lond.), 261: 195-200.
BRIDGES, B. A., MOTTERSHEAD, R. P., ROTHWELL, M. A., & GREEN, M. H. L.
(1972) Repair deficient bacterial strains suitable for
mutagenicity screening tests with fungicide captan. Chem.-Biol.
Interact., 5: 77-84.
BROWN, A.M. (1973) In vitro transformation of submandibular gland
epithelial cells and fibroblasts of adult rats by
methylcholanthrene. Cancer Res., 33: 2779-2789.
CATTANACH, B. M. (1971) Specific locus mutation in mice. In:
Hollaender, A., ed. Chemical mutagens, principles and methods
for their detection, Vol. 2, pp. 535-540.
CHU, E. H. Y. (1971) Induction and analysis of gene mutations in
mammalian cells in culture. In: Hollaender, A., ed. Chemical
mutagens, principles and methods for their detection, Vol. 2,
pp. 411-444.
CLEAVER, J. E. (1969) Xeroderma pigmentosum: a human disease in
which an initial state of DNA repair is defective. Proc. Natl
Acad. Sci., 63: 428-435.
CLEAVER, J. E. (1973) DNA repair with purines and pyrimidines in
radiation- and carcinogen-damaged normal and Xeroderma
pigmentosum human cells. Cancer Res., 33: 362-369.
CLEAVER, J. E. (1974) Repair processes for photochemical damage in
mammalian cells. In: Lett, J. T., Adler, H., & Zelle, M., ed.
Advances in radiation biology, New York, Academic Press, Vol.
4, pp. 1-75.
CLEAVER, J. E. (1975) Methods for studying repair of DNA damaged by
physical and chemical carcinogens. In: Bush, E., ed. Methods in
cancer research, New York, Academic Press, Vol. 11, pp.
123-165.
CLEAVER, J. E., GOTH, R., & FRIEDBERG, E. C. (1975) Value of
measurements of DNA repair levels in predicting carcinogenic
potential of chemicals. In: Montesano, R., Bartsch, H., &
Tomatis, L., ed. Screening tests in chemical carcinogenesis,
IARC Sci. Publ. No. 12, pp. 639-661.
COMMONER, B., VITHAYATHIL, A. J., & HENRY, J. I. (1974) Detection of
metabolic carcinogen intermediates in urine of carcinogen-fed
rats by means of bacterial mutagenesis. Nature (Lond.),
249: 850-852.
CONNEY, A. H. & BURNS, J. J. (1972) Metabolic interactions among
environmental chemicals and drugs. Science, 178: 576-586.
CORBETT, T. H., HEIDELBERGER, C., & DOVE, W. F. (1970) Determination
of the mutagenic activity to bacteriophage T-4 of carcinogenic
and non-carcinogenic compounds. Mol. Pharmacol., 6(6): 667-679.
COUNCIL OF THE ENVIRONMENTAL MUTAGEN SOCIETY (1975) Environmental
mutagenic hazards, mutagenicity screening is now both feasible
and necessary for chemicals entering the environment. Science,
187: 503-514.
CRADDOCK, V. M. (1976) Replication and repair of DNA in liver of rats
treated with dimethylnitrosamine and with methyl
methanesulphonate. In: Magee, P. N. et al., ed. Fundamentals in
cancer prevention, Tokyo, Univ. Tokyo Press; Baltimore, Univ.
Park Press, pp. 293-311.
CROW, J. F. (1971) Human population monitoring. In: Hollaender, A, ed.
Chemical mutagens, principles and methods for their detection,
Vol. 2, pp. 591-606.
CZYGAN, P. J., GREIM, H., GARRO, A. J., HUTTERER, F., SCHAFFAER, F.,
POPPER, H., ROSENTHAL, O., & COOPER, D. Y. (1973) Microsomal
metabolism of DMN and the cytochrome activation to a mutagen.
Cancer Res,, 33: 2983-2986.
DELLA PORTA, G. & TERRACINI, B. (1969) Chemical carcinogenesis in
infant animals. Progr. Exp. Tumour Res., 11: 334-363.
DE MARS, R. (1974) Resistance of cultured human fibroblasts and other
cells to purine and pyrimidine analogues in relation to
mutagenesis detection. Mutat. Res., 24: 335-364.
DHEW (1977) Approaches to determining the mutagenic properties of
chemicals: Risks to future generations. Report of a working
group of the Subcommittee on Environmental Mutagenesis.
DI PAOLO, J. A., NELSON, R. L., & DONOVAN, P. J. (1972) In vitro
transformation of Syrian hamster embryo cells by diverse chemical
carcinogens. Nature (Lond.), 235: 278-280.
DI PAOLO, J. A., NELSON, R. L., DONOVAN, P. J., & EVANS, C. H. (1973)
Host-mediated in vivo-in vitro assay for chemical
carcinogenesis. Arch. Pathol., 95: 380-385.
DE SERRES, F. J. & MALLING, H. V. (1971) Measurement of recessive
lethal damage over the entire genome and at two specific loci in
the ad-3 region of a two-component heterokaryon of Neurospora
Crassa. In: Hollaender, A., ed. Chemical mutagens, principles
and methods for their detection, Vol. 2, pp. 311-342.
DRAKE, J. W. (1971) Mutagen screening with virulent bacteriophages.
In: Hollaender, A., ed. Chemical mutagens, principles and
methods for their detection, Vol. 1, pp. 219-234.
DRUCKREY, H., PREUSSMAN, R., IVANKOVIC, S., & SCHMÄL D. (1967)
[Organotropic carcinogenic effects of 65 different N-nitroso
compounds on BD-rats.] Z. Krebsforsch., 69 103-201.
DURSTON, W. E. & AMES, B. N. (1974) A simple method for the detection
of mutagens in urine: Studies with the carcinogen
2-acetylaminofluorene. Proc. Natl Acad. Sci., 71: 737-741.
EARLE, W. R. & NETTLESHIP, A. (1943) Production of malignancy in
vitro. V. Results of injections of cultures into mice. J. Natl
Cancer Inst., 4 213-227.
FAHMY O. G. & FAHMY, M.D. (1972) Mutagenic properties of AAF and its
metabolites. Int. J. Cancer, 9 284-298.
FAHMY O. G. & FAHMY, M.D. (1973) Mutagenic properties of
benzo(a)pyrene. Cancer Res., 33: 302-309.
FARBER, E. (1973) Carcinogenesis -- cellular evolution as a unifying
thread: presidential address. Cancer Res., 33: 2537-2550.
FDA (1971) Panel on carcinogenesis report on cancer testing in the
safety evaluation of food additives and pesticides. Toxic. appl.
Pharmacol., 20: 419-438.
FEDOROFF, S. (1967) Proposed usage of animal tissue culture terms,
J. Natl Cancer Inst., 38: 607-611.
FREESE, E. & STRACK, H. B. (1962) Induction of mutations in
transforming DNA by hydroxylamine. Proc. Natl Acad. Sci.,
48: 1796-1803.
FRIEDMAN, L. (1974) Dose selection and administration. In: Goldberg,
L., ed. Carcinogenesis testing of chemicals, Cleveland, CRC
Press, pp. 21-22.
FROHBERG, H. (1973) Critique of in vivo cytogenic test systems.
Agents Actions, 3: 119-123.
FUSENIG, N. R., SAMSEL, W., THON, W., & WORST, P. K. M. (1973)
Malignant transformation of epidermal cells in culture by DMBA.
In: Prunieras, M., Robert, L., & Rosenfeld, C., ed.
Differentiation of eukaryotic cells in culture. Colloque of
INSERM, Vol. 19, pp. 219-228.
GERCHMAN, L. L. & LUDLUM, D. B. (1973) The properties of
O6-methylguanine in template for RNA polymerase. Biochim.
biophys. Acta, 308: 310-316.
GIOVANELLA B, C., YIM, S. O., STEHLIN, J. S., & WILLIAMS, L. J. Jr
(1972) Brief communications: Development of invasive tumors in
the "nude" mouse after injection of cultured human melanoma
cells. J. Natl Cancer Inst., 48: 1531-1533.
GOTH, R. & RAJEWSKY, M. F. (1974) Molecular and cellular mechanisms
associated with pulse-carcinogenesis in the rat nervous system by
ethylnitrosourea: ethylation of nucleic acids and elimination
rates of ethylated bases from the DNA of different tissues.
Z. Krebsforsch., 82: 37-64.
HASHIMOTO, Y. & KITAGAWA, H. (1974) In vitro neoplastic
transformation of epithelial cell of rat urinary bladder by
nitrosamines. Nature (Lond.), 252: 497-499.
HEALTH AND WELFARE, CANADA (1973) The testing of chemicals for
carcinogenicity, mutagenicity, teratogenicity.
HEIDELBERGER, C. (1973) Chemical oncogenesis in culture. Adv. Cancer
Res., 18: 317-366.
HERRIOTT, R. M. (1971) Effects on DNA: Transforming principle. In:
Hollaender, A., ed. Chemical mutagens, principles and methods
for their detection, Vol. 1, pp. 175-218.
HUBERMAN, E. & SACHS, L. (1974) Cell-mediated mutagenesis of mammalian
cells with chemical carcinogens. Int. J. Cancer, 13: 326-333.
HUBERMAN, E., ASPIRAS, L., HEIDELBERGER, C., GROVER, P. L., & SIMS, P.
(1971) Mutagenicity to mammalian cells of epoxides and other
derivatives of polycyclic hydrocarbons. Proc. Natl Acad. Sci.,
68: 3195-3199.
HUBERMAN, E., DONOVAN, P. J., & DIPAOLO, J. A. (1972) Mutation and
transformation of cultured mammalian cells by
N-acetoxy- N-2-fluorenylacetamide. J. Natl Cancer Inst.
48: 837-840.
HUEPER, W. C., WILEY, F. H., & WOLFE, H. D. (1938) Experimental
production of bladder tumours in dogs by administration of
ß-naphthylamine. J. industr. Hyg. Toxicol., 20: 46-84.
IARC (1976) Monographs on the evaluation of carcinogenic risk of
chemicals to man: some aromatic amines, hydrazine and related
substances, N- nitroso compounds and miscellaneous alkylating
agents. Lyons, International Agency for Research on Cancer,
Vol. 4.
IYPE P. T. (1974) Studies on chemical carcinogenesis in vitro using
adult rat fiver cells. In: Montesano, R. & Tomatis, L., ed.
Chemical carcinogenesis essays, IARC Sci. Publ. No. 10,
pp. 119-133.
J. Natl Cancer Inst., 53: 1427-1519 (1974) Proceedings of the
Symposium "New Horizons for Tissue Culture in Cancer Research".
KAKUNAGA, T. The transformation of human diploid cells by chemical
carcinogens. In: Hiatt, H. H. et al., ed. Origins of human
cancer (1977) Cold Spring Harbor Laboratory. pp. 1537-1548.
KATSUTA, H. & TAKAOKA, T. (1972) Carcinogenesis in tissue culture.
XIV. Malignant transformation of rat liver parenchymal cells
treated with 4-nitroquinoline 1-oxide in tissue culture. J. Natl
Cancer Inst., 49: 1563-1536.
KONDO, S. (1973) Evidence that mutations are induced by errors in
repair and replication. Genet. suppl., 73: 109-122.
KRAHN, D. F. & HEIDELBERGER, C. (1975) Microsome-mediated mutagenesis
in Chinese hamster cells by chemical oncogens. Proc. Am. Assoc.
Cancer Res., 16: 74.
KRIEK, E. (1974) Carcinogenesis by aromatic amines. Biochem.
Biophys. Acta, 355: 177-203.
KUROKI, T. (1975) Contributions of tissue culture to the study of
chemical carcinogenesis: A review. In: Recent topics in chemical
carcinogenesis, Gann, Monograph on Cancer Res., 17: 69-85.
KUROKI, T., DREVON, C., & MONTESANO, R. (1977) Microsome-mediated
mutagenesis in V-79 Chinese hamster cells by various
nitrosamines. Cancer Res., 1044-1050.
LAERUM, O. D. & RAJEWSKY, M. F. (1975) Neoplastic transformation of
foetal rat brain cells in culture following exposure to
ethylnitrosourea in vivo. J. Natl Cancer Inst., 55: 1177-1187.
LEGATOR, M. S. & FLAMM, W. G. (1973) Environmental mutagenesis and
repair. Ann. Rev. Biochem., 683-708.
LEGATOR, M. S. & MALLING, H. V. (1971) The host-mediated assay, a
practical procedure for evaluating potential mutagenic agents in
mammals. In: Hollaender A., ed. Chemical mutagens, principles
and methods for their detection, Vol. 2, pp. 569-590.
LOPRIENO, N., BARALE, R., BARONCELLI, S., BARTSCH, H., BRONZETTI, G.,
CAMMELLINI, A., CORSI, C., FREZZA, D., NIERI, R., LEPORINI, C.,
ROSELLINI, D., & ROSSI, A.M. (1976) Induction of gene mutations
and gene conversions by vinyl chloride metabolites in yeast.
Cancer Res., 37: 253-257.
LOVELESS, A. (1969) Possible relevance of O6-alkylation of
deoxyguanosine to the mutagenicity and carcinogenicity of
nitrosamines and nitrosamides. Nature (Lond.), 233: 206-207.
LOVELESS, A. & HAMPTON, C. L. (1969) Inactivation and mutation of
coliphage T2 by N-methyl- and N-ethyl- N-nitrosourea.
Mutation Res., 7: 1-12.
LUDLUM, D. B. (1970) Alkylated polycytidic acid templates for RNA
polymerase. Biochim. biophys. Acta, 213: 142-148.
MAGEE, P. N., MONTESANO, R., & PREUSSMAN, R. (1976) N-nitroso
compounds and related carcinogens. In: Searle, C., ed. Chemical
carcinogens, ACS monograph No. 173, pp. 491-625.
MAHER, V., MILLER, E. C., MILLER, J. A., & SZYBALSKI, W. (1968)
Mutations and decreases in density of transforming DNA produced
by derivatives of the carcinogens 2-acetylaminofluorene and
N-methyl-4-aminoazobenzene. Mol. Pharmacol., 4: 411-426.
MAHER, V. M., CURREN, R. D., OUELLETTE, L. M., & MCCORMICK, J. J.
(1976) Effect of DNA repair on the frequency of mutations induced
in human cells by ultraviolet irradiation and by chemical
carcinogens. In: Magee, P. N., et al, ed. Fundamentals in cancer
prevention, Tokyo, Univ. of Tokyo Press; Baltimore, Univ Park
Press, pp. 363-382.
MALLING, H. V. (1974) Mutagenic activation of dimethylnitrosamine and
diethylnitrosamine in the host-mediated assay and the microsomal
system. Mutat. Res., 26: 465-472.
MARGISON, G. P., MARGISON, J. M., & MONTESANO, R. (1976) Methylated
purines in the deoxyribonucleic acid of various Syrian golden
hamster tissues after administration of a hepatocarcinogenic dose
of dimethylnitrosamine. Biochem. J., 157: 627-634.
MARQUARDT, H. (1974) Mutation and recombination experiments with yeast
as pre-screening tests for carcinogenic effects.
Z. Krebsforsch., 81: 333-346.
MCCANN, J. & AMES, B. N. (1976) Detection of carcinogens as mutagens
in the Salmonella/microsome test: Assay of 300 chemicals:
Discussion. Proc. Natl Acad. Sci., 73: 950-954.
MCCANN, J., SPINGARN, N. E., KOBORI, J., & AMES, B. N. (1975)
Detection of carcinogens as mutagens: bacterial tester strains
with R factor plasmids. Proc. Natl Acad. Sci., 72: 979-983.
MESELSON, M. & RUSSELL, K. (1977) Comparisons of carcinogenic and
mutagenic potency. In: Hiatt, H. H. et at. ed. Origins of human
cancer. Cold Spring Harbor Laboratory. pp. 1473-1481.
MILLER, J. A. (1970) Carcinogenesis by chemicals: an overview -- G. H.
A. Clowes Memorial Lecture. Cancer Res., 30: 559-576.
MILLER, J. A. (1973) Naturally occurring substances that can induce
tumors. Proc. Natl Acad. Sci., 23: 508-549.
MILLER, E. C. & MILLER, J. A. (1971a) The mutagenicity of chemical
carcinogens: correlations, problems, and interpretations. In:
Hollaender, A., ed. Chemical mutagens, principles and methods
for their detection, Vol. 1, pp. 83-120.
MILLER, J. A. & MILLER, E. C. (1971b) Chemical carcinogenesis,
mechanisms and approaches to its control. J. Natl Cancer Inst.,
47: V-XIV.
MILLER, E. C. & MILLER, J. A. (1976) The metabolism of chemical
carcinogens to reactive electrophiles and their possible
mechanisms of action in carcinogenesis. In: Searle, C. E., ed.
Chemical carcinogens, ACS Monograph 173. Washington, DC,
American Chemical Society, pp. 737-762.
MOHN, G. (1973) Revertants of an Escherichia coli K-12 strain with
high sensitivity to radiations and chemicals. Murat. Res.,
19: 349-355.
MOHN, G. & ELLENBERGER, J. (1973) Mammalian blood mediated
mutagenicity test using a multi-purpose strain of E. coli,
K-12. Mutat. Res., 19: 257-260.
MONTESANO, R. & BARTSCH, H. (1976) Mutagenic and carcinogenic
N-nitroso compounds: Possible environmental hazards. Mutat.
Res., 32: 179-228.
MONTESANO, R., SAINT VINCENT, L., & TOMATIS, L. (1973)
Malignant transformation in vitro of rat liver cells by
dimethylnitrosamine and N-methyl- N'-nitro-
N-nitrosoguanidine. Brit. J. Cancer, 38: 215-220.
MONTESANO, R., SAINT VINCENT, L., DREVON, C,, & TOMATIS, L. (1975)
Production of epithelial and mesenchymal tumours with rat liver
cells transformed in vitro. Int. J. Cancer. 55: 1177-1187.
MONTESANO, R., DREVON, C., KUROKI, T., SAINT VINCENT, L., HANDLEMAN,
S., SANFORD, K. K., DEFEO, D. & WEINSTEIN, I. B. (1977) Test for
malignant transformation of rat liver cells in culture: Cytology,
growth in soft agar and production of plasminogen activator.
J. Natl Cancer Inst., 59: 1651-1658.
MONTESANO, R., BARTSCH, H., & TOMATIS, L., ed. (1976) Screening tests
in chemical carcinogenesis. IARC Sci. Publ. No. 12.
MONTESANO, R. & TOMATIS, L. (1977) Legislation concerning chemical
carcinogens in several industrialized countries. Cancer Res.,
37: 310-316.
MOREAU, P., BAILONE, A., & DEVORET, R. (1976) Prophage lambda
induction in Escherichia coli K12 envA uvrB: A highly
sensitive test for potential carcinogens. Proc. Natl Acad. Sci.,
73: 3700-3704.
MORTIMER, R. K. & MANNEY, T. R. (1971) Mutation induction in yeast.
In: Hollaender, A., ed. Chemical mutagens, principles and
methods for their detection, Vol. 1, pp. 289-310.
NAHAS, A. & CAPIZZI, R. L. (1974) Effect of in vivo treatment with
L asparaginal on the in vitro uptake and phosphorylation of
some anti-leukemic agents. Cancer Res., 34: 2687-2693.
NAPALKOV, N. P. (1973) Some general considerations on the problem of
transplacental carcinogenesis. In: Tomatis, L. & Mohr, U., ed.
Transplacental carcinogenesis, IARC Sci. Publ. No. 4, pp. 1-13.
NAPALKOV, N. P. & ALEXANDROV, V. A. (1968) On the effects of
blastomogenic substances on the organism during embryogenesis.
Z. Krebsforsch., 71: 32-50.
NATARAJAN, A. T., TATES, A.D., VAN BUUL, P. P. W., MEIJERS, M., & DE
VOGEL, N. (1976) Cytogenetic effects of mutagens/carcinogens
after activation in a microsomal system in vitro. I. Induction
of chromosome aberrations and sister chromatid exchanges by
diethylnitrosamine (DEN) and dimethylnitrosamine (DMN) in CHO
cells in the presence of rat-liver microsomes. Murat. Res.,
37: 83-90.
MATIONAL ACADEMY OF SCIENCES (1960) Problems in the evaluation of
carcinogenic hazard from use of food additives. Washington, Natl
Res. Council (Publ. No. 479).
NCI (1976) NCI Technical Report Series, No. 1 (Guidelines for
carcinogen bioassay in small rodents), pp. 1-65 (DHEW Publication
No. (NIH) 76-701).
PEGG, A. E. (1977) Formation and metabolism of alkylated nucleosides:
Possible role in carcinogenesis by nitroso compounds and
alkylating ageNTs. Adv. Cancer Res., 25: 195-269.
PEGG, T. & NICOLL, J. W. (1976) Nitrosamine carcinogenesis: The
importance of the persistence in DNA of alkylated bases in the
organotropism of tumour induction. In: Montesano, R., Bartsch,
H., & Tomatis, L., ed. Screening tests in chemical
carcinogenesis, IARC Sci. Publ. No. 12, pp. 571-592.
PIETRA, G., RAPPAPORT, H., & SHUBIK, P. (1961) The effects of
carcinogenic chemicals in newborn mice. Cancer, 14: 308-317.
PITOT, H. C. (1974) Neoplasia: A somatic mutation or a heritable
change in cytoplasmic membranes? J. Natl Cancer Inst.,
53: 905-911.
PITOT, H. C. (1977) The natural history of neoplasia. Am. J. Path.,
89: 402-411.
PURCHASE, I. F. H., LONGSTAFF, E., ASHBY, J., STYLES, J. A., ANDERSON,
D., LEFEVRE, P. A., & WESTWOOD, F. R. (1976) Evaluation of six
short-term tests for detecting organic chemical carcinogens and
recommendations for their use. Nature (Lond.), 264: 624-627.
REUBER, M. (1974) Criteria for tumor diagnosis and classification of
malignancy. In: Golberg, L., ed. Carcinogenesis testing of
chemicals, Cleveland, CRC Press, pp. 71-73.
ROSENKRANZ, H. S. (1973) Aspects of microbiology in cancer research.
Rev. Microbiol., 1622: 383-400.
RUSSELL, W. L. (1951) X-ray induced mutations in mice. Cold Spring
Harbour Symp. Quant. Biol., 16: 327-336.
SANFORD, K. K. (1965) Malignant transformation of cells in vitro.
Inter. Rev. Cytol., 18: 249-311.
SANFORD, K. K. (1974) Biological manifestations of oncogenesis in
vitro: a critique. J. Natl Cancer Inst., 53: 1481-1485.
SANFORD, K. K., EARLE, W. R., SHELTON, E., SCHILLING, E. L., DUCHESNE,
E. M., LIKELY, G. D., & BECKER, E. M. (1950) Production of
malignancy in vitro. XII. Further transformations of mouse
fibroblasts to sarcomatous cells. J. Natl Cancer Inst.,
11: 351-367.
SATO, K., SLESINSKI, R. S, & LITTLEFIELD, J. W. (1972) Chemical
mutagenesis at the phosphoribosyl transferase locus in cultured
human lymphoblasts. Proc. Natl Acad. Sci., 69: 1244-1248.
SCHMID, W. (1973) Chemical mutagen testing on in vivo somatic
mammalian cells. Agents Actions, 3: 77-85.
SIMS, P. & GROVER, P. L. (1974) Epoxides in polycyclic aromatic
hydrocarbon metabolism and carcinogenesis. Advances in Cancer
Res., 20: 165-274.
SMITH, W. E. & HANAWALT, P. C. (1969) Molecular photobiology:
Inactivation and recovery. New York, Academic Press.
SMYTHE, D. R. & EVANS, H. J. (1976) Mapping of sister-chromatid
exchanges in human chromosomes using G-Banding and
autoradiography. Mutat. Res., 35: 139-154.
SOBELS, F. H. & VOGEL, E. (1976) The capacity of drosophila for
detecting relevant genetic damage. Mutat. Res., 41: 95-106.
STETKA, D. G. & WOLFF, S. (1976) Sister chromatid exchange as an assay
for genetic damage induced by mutagen-carcinogens. I. In vitro
test for compounds requiring metabolic activation. Mutat. Res.,
41: 333-342.
STICH, H. F. & KIESER, D. (1974) Use of DNA repair synthesis in
detecting organo-tropic actions of chemical carcinogens.
Proc. Soc. Exp. Biol Med., 145: 1339-1342.
STICH, H. F. & SAN, R. H. C. (1970) DNA repair and chromatid anomalies
in mammalian cells exposed to 4 nitroquinoline 1-oxide. Mutat.
Res., 10: 389-404.
STOLTZ, D. R., POIRIER, L. A., IRVING, C. C., STICH, H. F.,
WEISBURGER, J. H., & GRICE, H. C. (1974) Evaluation of short-term
tests for carcinogenicity. Toxicol. appl. Pharmacol.,
29: 157-180.
SUGIMURA, T., SATO, S., NAGAO, M., YAHAGI, T., MATSUSHIMA, T., SEINO,
Y., TAKEUCHI, M., & KAWACHI, T. (1976) Overlapping of carcinogens
and mutagens. In: Magee, P. N., Takayama, S., Sugimura, T., &
Matsushima, T., ed. Fundamentals in cancer prevention, Tokyo,
University of Tokyo Press; Baltimore, Univ. Park Press,
pp. 191-215.
SUTHERLAND, B. M., RICE, M., & WAGNER, E. K. (1975) Xeroderma
pigmentosum cells contain low levels of photoreactivating
enzyme. Proc. Natl Acad. Sci., 72: 103-107.
SUTTON, H. E. (1972) Monitoring somatic mutations in human
populations. In: Sutton, H. E. & Harris, M. I., ed. Mutagenic
effects of environmental contaminants, pp. 122-128.
THOMPSON, L. H. & BAKER, R.M. (1973) Isolation of mutants of cultured
mammalian cells. In: Prescott, D. M., ed. Methods of cell
biology, New York, Academic Press, pp. 209-280.
TOMATIS, L. (1973) Transplacental carcinogenesis. In: Raven, R. W.,
ed. Modern trends in oncology -- 1, London, Butterworths,
pp. 99-126.
TOMATIS, L. (1974a) The validity of long-term bioassays in
carcinogenicity testing. Chemical and vital oncogenesis. Excerpts
Medica Int. Congr. Ser. No. 350, Vol. 2, pp. 82-93.
TOMATIS, L. (1974b) Inception and duration of tests. In: Golberg, L.,
ed. Carcinogenesis testing of chemicals, Cleveland, CRC Press,
pp. 23-27.
TOMATIS, L., AGTHE, C., BARTSCH, H., HUFF, J., MONTESANO, R., SARACCI,
R., WALKER, E., & WILBOURN, J. (1978) Evaluation of the
carcinogenicity of chemicals: A review of the monograph program
of the International Agency for Research on Cancer. Cancer Res.,
38: 877-885.
TURUSOV, V. S., ed. (1973, 1976) Pathology of tumours in laboratory
animals, Vol. I, Part 1 and 2, IARC Sci. Publ. No. 5 and No. 6.
Lyon.
UICC (1969) UICC Technical Report Series Vol. 2 (Carcinogenicity
testing). Geneva, International Union Against Cancer, 56 pp.
VENITT, S. & LEVY, L. S. (1974) Mutagenicity of chromates in bacteria
and its relevance to chromate carcinogenesis. Nature (Lond.),
250: 493-495.
WEIL, C. S. (1972) Statistics vs safety factors and scientific
judgements in the evaluation of safety for man. Toxicol. appl.
Pharmacol., 21: 454-463.
WEINSTEIN, I. B., ORENSTEIN, J. M., GEBERT, T., KAIGHN, M. E., &
STADLER, V. C. (1975) Growth and structural properties of
epithelial cell cultures established from normal rat liver and
chemically induced hepatomas. Cancer Res., 35: 253-263.
WEISBURGER, J. H. (1974) Inclusion of positive control. In: Golberg,
L., ed. Carcinogenesis testing of chemicals, Cleveland, CRC
Press, pp. 29-34.
WEISBURGER, E. K. (1975) A critical evaluation of the methods used for
determining carcinogenicity. J. clin. Pharmacol., 15: 5-15.
WEISBURGER, J. H. & WEISBURGER E. K. (1967) Tests for chemical
carcinogens. In: Busch, H., ed. Methods in cancer research,
Academic Press, Vol. 1, pp. 307-398.
WEISBURGER, J. H. & WEISBURGER, E. K. (1973) Biochemical formation and
pharmacological, toxicological and pathological properties of
hydroxylamines and hydroxamic acids. Pharmacol. Rev. 25: 1-66.
WHO (1961) WHO Technical Report Series No. 220 (Evaluation of
carcinogenic hazards of food additives), 33 pp.
WHO (1964) WHO Technical Report Series No. 276 (Prevention of cancer).
WHO (1969) WHO Technical Report Series No. 426 (Principles for the
testing and evaluation of drugs for carcinogenicity).
WHO (1974) WHO Technical Report Series, No. 546 (Assessment of the
carcinogenicity and mutagenicity of chemicals), 19 pp.
WILLIAMS, G. M., ELLIOT, J. M., & WEISBURGER, J. H. (1973) Carcinoma
after malignant conversion in vitro of epithelial-like cells
from rat liver following exposure to chemical carcinogens.
Cancer Res., 33: 606-612.
WILSON, R. H., DEEDS, F., & COX, A. J. (1941) The toxicity and
carcinogenicity of 2-acetaminofluorene. Cancer Res.,
1: 595-608.
ZIMMERMANN, F. K. (1973) Detection of genetically active chemicals
using various yeast systems. In: Hollaender, A., ed. Chemical
mutagens, principles and methods for their detection, Vol. 3,
pp. 209-240.
O,6-guanine residues (Gerchman & Ludlum, 1973). Goth & Rajewsky
(1974) showed, in vivo, that the initial degree of alkylation at the
O,6 position in the DNA was, apparently, not correlated with the
tissue-specific carcinogenicity of ethylnitrosourea which induces
brain, but not liver, tumours. However, O,6-ethylguanine persists
much longer in brain DNA than N,7-ethylguanine. The O,6-alkyl
elimination is also much slower from brain than from the liver DNA.
From these findings it becomes apparent that variations in the
sensitivity of different organs to the carcinogenic action of
N-nitroso compounds might be attributed to different enzyme
activities capable of repairing lesions in cellular DNA and/or to
different rates of cell division in the target and non-target organs
(Craddock, 1976; Margison et al., 1976; Pegg & Nicoll, 1976; Pegg,
1977).
Possibly these repair systems, which recognize damaged DNA, are
also impaired in the case of some types of cancer in man. The skin of
patients suffering from the hereditary disease xeroderma pigmentosum
is extremely sensitive to sunlight and such persons have a very high
incidence of skin cancer. It has been shown that the cells of the skin
of these patients are deficient in the capacity to repair UV-damaged
DNA and that this deficiency is caused by lack of enzymes required for
the excision of damaged regions from DNA (Cleaver, 1969).
Some chemicals cause changes in the biological properties of DNA
without covalent binding, e.g. by intercalation which could cause
frameshift mutations. Since radioactive labelled chemicals are needed
for most of these studies, technical problems associated with these
methods render the tests impractical for routine screening. However,
they will continue to contribute considerably to the understanding of
the mechanism of interaction of a chemical or its activating
metabolite with specific sites in DNA.
Initial lesions in DNA can either lead to a permanent change,
such as a mutation, or can be removed by cellular repair processes. In
bacteria and mammalian cells, three major repair processes can be
considered. First, the photoreactivation repair process that repairs
only UV damage to DNA and involves a direct enzymatic cleavage of
pyrimidine dimers to monomers upon exposure of cells to visible light.
This repair process is present in most prokaryotes and eukaryotes and
it was recently identified in human cells (Sutherland et al., 1975).
The second process, post-replication repair, which is also called
recombination repair, is limited to the DNA synthesis period. Although
its mechanism is not well known, it has been proposed that it is
caused by the presence of damaged bases on parental DNA strands too
close to the replication fork to be recognized by the third process,
excision repair. Unlike post-replication repair, excision repair
occurs throughout the cell cycle and is present in a large variety of
organisms. During this process, damaged bases are excised, producing a
single-strand break. This gap is repaired by replacing the original
bases with bases complementary to those of the opposite intact strand.
Various enzymes are involved in this process, namely endonuclease, a
DNA exonuclease, a DNA polymerase and a DNA ligase (Cleaver, 1974).
From studies on prokaryotes, the concept arose that excision
repair is error-free (Kondo, 1973). It is thought that mutations
originate during semi-conservative replication as a consequence of the
fact that DNA templates containing damage have not yet been or cannot
be repaired by excision repair. Since DNA replication on damaged
templates involves post-replication repair, this repair process is
considered error-prone and responsible for mutagenesis. Whether
miscoding and mutagenesis of DNA templates is the result of lack of
excision repair enzymes or due to error-prone post-replicative repair
is not clear at present.
The isolation of xeroderma pigmentosum-variant fibroblasts, that
have normal excision repair but are deficient in post-replication
repair (Maher et al., 1976) may lead to a better understanding of the
role of these repair processes in mutagenesis and carcinogenesis.
Recent efforts have been devoted to the development in mammalian
systems of a screening test for the detection of possible mutagens or
carcinogens based on excision repair. The focus on this type of repair
is mainly based on the greater availability of procedures thought to
be appropriate to the problem. There are four different ways of
examining this form of repair in mammalian systems and all are based
on the assumption that chemical mutagens or carcinogens interact with
DNA, inducing molecular alterations which result in DNA repair that
can be measured as unscheduled incorporation of various DNA precursors
(Cleaver, 1975; Legator & Flamm, 1973). The common basis of all these
tests is the differentiation between repair and normal
semiconservative synthesis of DNA.
One procedure, "unscheduled DNA synthesis", involves
autoradiography of cultured cells, and consists of the incorporation
of precursors during resynthesis of short nucleotide sequences that
have been eliminated from DNA strands following their damage by
chemicals (Cleaver, 1973; Stich & San, 1970). This procedure permits a
quantitative evaluation of DNA repair synthesis in various types of
cell in a tissue and can be applied to an in vivo system (Stich &
Kieser, 1974), thus allowing the detection of indirect mutagens and/or
carcinogens.
Another process uses 5-bromodeoxyuridine (BrdU), which is an
analogue of thymidine. During normal replication, the incorporation of
BrdU produces a DNA with a high buoyant density; this does not occur
when BrdU is incorporated in DNA during the repair synthesis. Thus, by
using radiolabelled BrdU, or radiolabelled thymidine and non-labelled
BrdU, it is possible to differentiate, on cesium chloride density
gradients, semi-conservative replication of DNA from the repair
synthesis according to the different buoyant densities.
A third procedure also uses BrdU but the differentiation between
semi-conservative and repair DNA synthesis is done by different means.
Cells exposed to the chemicals are incubated with BrdU and the DNA is
subjected to long wavelength ultra-violet radiation. Breaks appear in
the DNA at the sites of incorporation of BrdU and cause slower
sedimentation of the DNA in alkaline sucrose gradients (Smith &
Hanawalt, 1969). This technique appears to be a very sensitive one for
measuring single-strand breaks but it still seems to be prone to
artefacts and cannot be considered suitable for large-scale studies
(Cleaver, 1975).
Another method involves the total suppression of normal DNA
synthesis, for example, by hydroxyurea, thus the incorporation of
precursors into DNA reflects only repair synthesis and not normal
replication.
Some of the above techniques are time-consuming, costly and not
yet standardized, so that it is difficult to adapt them for
large-scale studies. The most promising approach appears to be the
measurement of unscheduled DNA synthesis, but criticisms have also
been made of the use of this system as an indicator of mutagenic or
carcinogenic potential (Cleaver et al., 1975). One limitation is that
unscheduled synthesis is an average measure of repairable damage in
all cells of a population or in all sites of the DNA, whereas
mutagenesis and carcinogenesis seem to depend on the amount of
unrepaired damage in a small percentage of cells and in a few specific
DNA sites. Due to these limitations and to the current limited
understanding of these processes, none of these variables could, by
themselves, be a reliable indicator of a potential mutagenic or
carcinogenic chemical. However, these studies associated with studies
of DNA damage as well as of the expression of this damage are
essential in the understanding and development of reliable screening
systems.
Metabolic activation of chemicals to electrophiles, DNA damage,
and subsequent repair processes are important factors in the
initiation of cancer. The phenotypic expression (tumour development)
of these initial molecular changes is modulated by a number of factors
(Farber, 1973) that influence the macroscopic appearance of cancer at
a later stage (Pitot, 1977). However, the initial molecular changes,
induced by chemical carcinogens, appear prerequisite for initiation of
the cancer process. Further studies on the alteration of DNA induced
by chemical carcinogens and on the repair of such lesions before cell
division by tissues, whether target or not, are required to evaluate
the role of DNA repair processes in chemical carcinogenesis.
7.2.2.2 In vitro neoplastic transformation of mammalian cells
The term transformation in such studies refers to the in vitro
observations of various changes present in the cells treated in vitro
with the chemicals, when compared with untreated control cells.
Transformed cells may differ from control cells in various ways such
as: (a) alteration of cellular and colony morphology; (b) increased
plating efficiency; (c) agglutinability by plant lectins; (d) altered
glycolytic patterns; (e) resistance to toxicity of some chemicals; (f)
altered surface properties (contact inhibition of movement and growth,
population density, growth in suspension, ability to grow in agar or
other semisolid media); (g) sensitivity to activated macrophages and
lymphocytes; (h) establishment of cell lines with the potential to be
sub-cultured indefinitely in vitro; and (i) appearance of new
antigens (Fedoroff, 1967). However, the unequivocal criterion of
malignant transformation is the capacity of transformed cells to
develop a malignant neoplasm when injected into a syngenic,
immunosuppressed or thymus-free host, or into privileged sites
(Giovannella et al., 1972; Sanford, 1965).
Recently, Sanford (1974) critically reviewed the significance of
these various in vitro changes in relation to the acquisition of
neoplastic potential. It was stressed that these criteria of
neoplastic transformation cannot completely replace the in vivo
assay for tumour production. With reference to the problems of the
application of tissue culture to the rapid detection and
characterization of neoplastic transformation in vitro, the reader
is referred to the proceedings of the symposium "New Horizons for
Tissue Culture in Cancer Research" ( J. Natl Cancer Inst., 1974).
In most in vitro transformation experiments the cells used have
been fibroblasts. Earle & Nettleship (1943) reported the
transformation by 1,2-dihydro-3-methyl-benz[j]aceanthrylene
(3-methylcholanthrene) of a long-term culture of fibroblasts as
demonstrated by the development of tumours after inoculation of the
cells into mice. However, this observation was attributed to
spontaneous transformation, since Sanford et at. (1950) observed that
the cells were tumorigenic also without treatment with chemicals. The
first clear demonstration of transformation of cells in culture by
chemicals was that of Berwald & Sachs (1963) who observed the
transformation of Syrian hamster embryo secondary cells with
3-methylcholanthrene and benzo(a)pyrene, but not with urethane or
solvent. Since this report, various cells types originating from
different animal species have been used for in vitro transformation.
This topic has recently been reviewed by Heidelberger (1973) and
Kuroki (1975), who critically examined the advantages and
disadvantages of the various strains and lines of fibroblastic cells,
namely of the Syrian hamster embryo, fibroblastic cells derived from
mouse ventral prostate, cell lines derived from embryo cells of Swiss
mouse BALB/c, C3H, AKR, and C57/B1 strains, Chinese hamster lung
cells, as well as various tissues from organ cultures. Transformation
of the above cell lines was obtained with polynuclear hydrocarbons and
with chemicals that do not require metabolic activation.
Some chemical carcinogens that are active in vivo have failed
to induce transformation, when applied directly to hamster embryo
cells; this is presumably because such compounds require metabolic
conversion to active intermediates that are lacking or are present in
insufficient concentration in these cells. However, neoplastic
transformation was detected in fibroblasts obtained from embryos whose
mothers had been exposed to indirect carcinogens during pregnancy
(Di Paolo et al., 1972, 1973). A similar approach was used by Borland
& Hard (1974), who cultured kidney cells at various times following
in vivo treatment of rats with N-methyl- N-nitrosomethanamine
(dimethylnitrosamine). The cells isolated from treated rats showed
various morphological and behavioural changes associated with
transformation. Laerum & Rajewsky (1975) reported the development of
glioblastomas following injection of glial cells originating from the
brain of rat embryos, the mother having been treated with
ethylnitrosourea during pregnancy.
Epithelial cultures from rat liver were recently established in
various laboratories and used for transformation studies (Iype, 1974;
Katsuta & Takaoka, 1972; Montesano et al, 1975; Weinstein et al.,
1975; Williams et al., 1973). The transformation of these cells by
various carcinogens that need metabolic activation was determined by
the development of carcinomas after their back-transplantation into
suitable hosts. Carcinomas were observed following inoculation of
epithelial cells from mouse skin, or rat urinary bladder or salivary
glands, treated with chemical carcinogens in vitro (Brown, 1973;
Fusenig et al., 1973; Hashimoto & Kitagawa, 1974).
One disadvantage of these epithelial cells is that the
morphological criteria for transformation of fibroblast cultures
(piling up of cells, crisscross arrangement of cells etc.) do not
apply possibly because normal epithelial cells have little or no
locomotion and they continue to divide even when in close contact with
other cells (Weinstein et al., 1975). However, the capacity for growth
in soft agar appears to provide a reliable and reproducible
correlation with the tumorigenicity of these cells (Weinstein et al.,
1975; Montesano et al., 1977). Although, at present, this system
provides only a qualitative and not a quantitative assay of in vitro
transformation, it is the only instance of unequivocal production of
carcinoma. The establishment of reliable criteria, measurable in
vitro, for distinguishing normal from transformed epithelial cells
in culture is essential for the development of quantitative systems
for the transformation of these epithelial cells. Recently the
neoplastic transformation of human diploid cells by chemical
carcinogens has been described (Kakunaga, 1977).
Cell culture has been extremely useful in elucidating the
cellular and molecular mechanism of chemical carcinogens and it holds
great promise as a test for screening for the potential
carcinogenicity of environmental chemicals. However, some time is
needed before this test may be used for routine testing in a
reproducible way.
7.2.2.3 Mutagenicity tests
The growing experimental evidence linking the carcinogenic
activity of numerous chemicals with their capacity to be converted
into electrophilic derivatives, that may also exert a mutagenic
effect, has led to the suggestion that a relationship between chemical
carcinogenesis and mutagenesis may exist (Miller & Miller, 1971a,b).
Such a correlation has so far been limited to those changes of the
genotype that appear as a consequence of structural or functional
alterations of nucleic acids. Not all chemical mutagens have been
shown to be carcinogenic. However, most chemical carcinogens, several
of which cause cancer in man, have now been found to be mutagens, when
tested by one of the mutagenicity test procedures that combine
microbial, mammalian, or other animal cell systems as genetic targets
with an in vitro or in vivo metabolic activation system. The
growing empirical relationship between chemical mutagens and
carcinogens does not imply that the two processes are identical, but
it offers a promising method for the use of mutagenesis as a rapid
prescreening assay for carcinogenesis (Bartsch & Grover, 1976;
Bridges, 1976; Council of the Environmental Mutagenic Society, 1975;
IARC, 1976; McCann & Ames, 1976; Purchase et al., 1976; Stoltz et al.,
1974; Sugimura et al., 1976; WHO, 1974).
The choice of mutagen-detecting assay depends on various
considerations, i.e. the chemical structure and pharmacological
activity of the chemical, the type of human exposure, and the nature
of the population at risk. The same considerations should be taken
into account in assessing the strength of the evidence of mutagenicity
and its relevance to man.
7.2.2.4 Submammalian assay systems
Bacterial phages have been used to test reactive forms of
chemical carcinogens. Chemicals inducing point mutations can be
detected by reacting the test compound with the free phage or with
phage during its duplication inside bacteria (Corbett et al., 1970;
Drake, 1971).
In the bacterial transformation of DNA (Freese & Strack, 1962),
genetic information contained in the DNA isolated from one strain of
bacteria can be transformed to that of a recipient strain. Purified
bacterial DNA is readily accessible to reactive forms of mutagens.
Thus, this system has been used to quantify the mutagenic potential of
a number of chemicals. Extensive studies have been made on the
inactivation of Bacillus subtilis DNA and transformation of the
tryptophane-requiring strain T-3 (Herriott, 1971; Maher et al., 1968).
The reverse mutationa system of Salmonella typhimurium uses
the genetically well-defined histidine-requiring mutants developed by
Ames and his colleagues (Ames, 1971; Ames et al., 1972a,b, 1973;
McCann et al., 1975).
These revert to prototrophy by single-base pair substitutions,
e.g. strain TA1535 or by base pair insertion (frameshift), e.g.
strains TA1536, TA1537 and TA1538. Most of the theoretically possible
types of mutation may be detected with a set of these test strains,
where a mutation of one of the genes responsible for excision repair
(UVrB) has produced a 100-fold increase in sensitivity. Penetration of
larger molecules through the bacterial cell walls has also been
facilitated by the use of deep-rough mutants deficient in the exterior
polysaccharide coat. Two newly developed test strains TA100 and TA98
were obtained by transferring an ampicillin resistance factor
(R factor) to the standard test strains TA1535 and TA1538,
respectively (McCann et al., 1975). These strains are effective in
detecting classes of mutagens that were not previously detected with
the original strains. The tests are usually performed by adding a few
crystals or a drop of solution of the test chemical in
sulfinylbis[methane] (dimethylsulfoxide) or water to a uniform lawn of
one of the histidine-requiring mutants on the surface of a Petri plate
containing histidine-poor medium, or by incorporating the test
compound, a postmitochondrial tissue fraction, cofactors (NADPH or
NADPH+ and glucose 6-phosphate) and the bacterial test strain in
histidine-poor soft agar. For general mutagenicity screening, a liver
homogenate (9000 g supernatant) from rats induced with a mixture of
polychlorinated biphenyls (Aroclor 1254) is recommended as a metabolic
activation system. For routine testing, the strains TA1535, TA1537 and
TA1538 can be used in combination with the strains carrying the R
factor (TA100 and TA98). This method of testing using various groups
of chemicals and various experimental conditions is described in more
detail by Ames et al. (1975).
Using the genetically well characterized Escherichia coli
strains, reverse and forward mutation can be scored using nutritional
resistance or fermentative markers (Bridges et al., 1972; Mohn, 1973).
Prophage lambda induction in E. coli strains by chemical mutagens
activated by liver microsomal enzymes has been described as a
sensitive test for the detection of potential mutagens and carcinogens
(Moreau et al., 1976).
a Mutations in which the function of a given gene is lost are
called forward mutations. Mutations that bring about the
restoration of gene function are called reverse mutations or back
mutations.
Mutagenesis has been extensively studied in Neurospora crassa.
Heterokaryon has been developed, which is heterozygous for two
closely-linked loci in the ad-3 region. Mutants at the ad-3A and
ad-3B loci have a requirement for adenine and can be selected
directly on the basis of accumulation of a reddish-purple pigment in
the mycelium. The ad-3 mutations can be characterized by a series of
simple genetic tests to distinguish point mutations from deletions and
to obtain a presumptive identification of the genetic alterations in
the point mutations at the ad-3B locus at a molecular level (de
Serres & Malling, 1971).
Saccharomyces cerevisiae and S. pombe have been used to
investigate the effects of carcinogenic and mutagenic compounds in
these organisms. Mitotic gene conversion, in which a sequence of a few
hundred nucleotides of one chromosome is replaced by one corresponding
sequence from a homologous chromosome, can be studied in diploid cells
of the yeast S. cerevisiae strain D-4 heteroallelic at the loci
trp-5 and ade-2. This strain carries two different inactive
alleles of two genes ( trp-5 and ade-2) which are located on
different chromosomes and the functional defects of which lead to a
nutritional requirement. Mitotic gene conversion, which is increased
by many types of mutagenic treatment, can transfer the intact region
of one of these alleles to the defective region of the other, thus
producing a heterozygotic diploid cell with full functional activity.
The mechanism is presumably based on the formation of single-strand
breaks in DNA and probably involves repair processes. Positive results
are only obtained with agents or metabolites which either bind with
DNA covalently or interfere with DNA metabolism. In contrast with most
microbial mutation systems, mitotic gene conversion does not show a
response specific to any type of mutagen. In addition to gene
conversion, forward and reverse mutation can be measured with
S. cerevisiae (Loprieno et al., 1976; Marquardt, 1974; Mortimer &
Manney, 1971; Zimmerman, 1973).
7.2.2.5 Mammalian somatic cells
The application of cultured cell lines is somewhat restricted at
present by requirements for karyotypic stability and high plating
efficiency. A widely used system developed by Chu (1971) employs cell
lines derived from the lung, ovary, and other tissues of Chinese
hamster, which usually maintain a near-diploid chromosome number and
exhibit active growth and high cloning efficiency. Two reviews discuss
this topic in detail (De Mars, 1974; Thompson & Baker, 1973).
Selective media have been developed to detect both forward and reverse
mutations at three genetic loci involving enzymes in the salvage
pathways of purines and pyrimidines. In Chinese hamster, as in man,
the use of preformed hypoxanthine and guanine is controlled by an
X-linked gene. Mutant cells at these loci are deficient in the enzyme
hypoxanthine-guanine phosphoribosyl transferase and are resistant to
certain purine analogues. Similarly, mutant cells deficient in
adenosine phosphoribosyl transferase cannot metabolize preformed
adenosine or its analogues for incorporation into nucleic acid. The
third type of drug-resistant mutants currently studied in these and
other mammalian cells are those deficient in thymidine kinase. Such
cells exhibit resistance to the thymidine analogue,
5-bromodeoxyuridine. Cells carrying mutation at these loci can be
selected from the wild type in an environment containing an
appropriate purine or pyrimidine analogue. Revertants to the wild type
can be recovered in selective media in which the cells are supplied
with a natural purine or pyrimidine while the normal de novo pathway
of purine or pyrimidine synthesis is inhibited by an antimetabolite.
It has been demonstrated that Chinese hamster cells treated with
physical or chemical mutagens undergo a significant increase in the
frequency of forward and reverse mutations compared with the
spontaneous frequency (Huberman & Sachs, 1974; Huberman et al., 1971,
1972). A liver microsomal activation system can be added for the
metabolic activation of chemicals (Krahn & Heidelberger, 1975; Kuroki
et al., 1977).
Forward mutations from thymidine kinase +/- cells to thymidine
kinase -/- have been induced by treatment of cells with X-rays or
chemical mutagens (mouse lymphoma L5178 Y cells). These cells can also
be grown in the presence of in vitro or in vivo metabolic
activation systems, which makes them suitable for host-mediated
mutagenicity tests (Nahas & Capizzi, 1974).
Forward mutations to hypoxanthine-guanine phosphoribosyl
transferase deficiency (resistance to 8-azaguanine) in diploid human
fibroblasts in culture have been shown to occur either spontaneously
or after X-radiation (Albertini & De Mars, 1973). Chemical induction
of forward mutation from hypoxanthine-guanine phosphoribosyl
transferase - to + in human lymphoblastoid cell line has also been
reported (Sato et al., 1972).
7.2.2.6 Host and tissue-(microsome) mediated assays
These take into account the conversion of chemicals into
mutagenic metabolites, and are particularly suitable for the detection
of chemicals that are not mutagenic per se but require metabolic
activation. They can be performed in vivo (host mediated) or in
vitro (tissue mediated) using various indicator organisms, such as
bacteria, fungi, yeast, or mammalian cells.
In the host-mediated assay, the indicator organisms are injected
into the interperitoneal cavity of an animal (Legator & Malling,
1971), which is then treated with the test compound by another route.
After a given length of time, the animal is killed and the indicator
organism is recovered and scored for mutants. Comparison between the
mutagenic action of the compound on a test strain directly and the
host-mediated assay indicates whether the host can activate or
inactivate the test compound. The limitations of the host-mediated
assay are the high spontaneous mutation rates of the indicator
organism in the host, the host's effect on cell survival and the
selection of heterogenic cell populations. In order to measure a
significant increase over the spontaneous mutation rate, doses well
above LD50 have to be given to rats or mice. This type of assay is
also limited since it does not identify the site of conversion to a
mutagenic metabolite. Modifications have been reported (Mohn, 1973).
In the tissue-mediated assay, the indicator organisms are
incubated in vitro in the presence of a tissue fraction plus
appropriate cofactors and the test compound. The mutants are
subsequently isolated and scored.
Ames et al. (1973a,b) have developed a mutagenicity assay that
combines a Salmonella strain, a liver microsomal preparation, and
cofactors in a soft agar layer on a Petri dish. Bartsch et al.
(1975b), Czygan et al. (1973) and Malling (1974) have described a
liquid incubation system where bacteria, a tissue preparation, and the
test compound are incubated in liquid suspension. Test compounds that
are gases at room temperature or are volatile can be assayed by
exposing Petri dishes containing bacteria, tissue fraction, and
cofactors to a mixture of gas and oxygen at 37°C (Bartsch et al.,
1975a). The mutagenicity of urinary metabolites, excreted as
conjugates in experimental animals treated with an indirect mutagen,
may be detected by treating the urine with hydrolytic enzymes in the
presence of a bacterial test strain or yeast, and an in vitro
metabolic activation system (Commoner et al., 1974; Durston & Ames,
1974; Marquardt, 1974). In another modification, Huberman & Sachs
(1974) used lethally irradiated rat fibroblasts that retain a
drug-metabolizing capacity and cocultivated them with Chinese hamster
V79 cells which are used as genetic indicators.
The mutagenicity tests previously described (7.2.2.4, 7.2.2.5,
7.2.2.6) all have individual advantages and limitations determined
either by the genetic indicators or by the metabolic activation
system. The use of submammalian organisms for the detection and
classification of mutants, induced by chemicals, is greatly
facilitated by the relatively small genome to which fine genetic
mapping and biochemical analysis can be applied and by the short
generation time. Huge populations of some of these organisms can be
raised and they can easily be handled when analysing multiple
mutations.
The relevance of such data from submammalian systems to mammalian
cells is based on the assumption that the basic principles of
heredity, and the structure and functions of DNA in terms of
reactivity, the triplet code, transcriptional, and translational
mechanisms, are essentially the same for all living cells irrespective
of their evolutionary level. However, this extrapolation is hampered
by lack of knowledge of repair processes that play a role in mutation
fixation and expression and are insufficiently understood in mammals.
It is obvious that normal human diploid cells are most desirable for
this test, but current studies suggest that reliable conclusions can
be drawn from results with non-human mammalian cells.
The other factor, related to the fact that many chemicals are not
active per se, is the metabolic activation of the compounds. In many
cases, reactive metabolites with a limited life span may fail to reach
or react with the genetic indicator either because they are further
metabolized to inactive compounds, or because they react with other
cellular constituents. For this reason, mutagenicity assays in intact
animals (host-mediated assays) may give negative results, as in the
case of N-methyl- N'-nitro- N-nitrosoguanidine (MNNG) and acridine
mustard (ICR-170), proven to be extremely potent mutagens. Metabolism
in animals is affected by exogenous and endogenous factors such as
chemicals causing enzyme induction and inhibition. Other modifying
factors are age, sex, and strain of animals, diurnal and seasonal
rhythms, differences between the fetal and adult state, mode of
administration, cellular uptake, and distribution and excretion of the
chemical.
The tissue-mediated mutagenicity test cannot, with certainty,
reproduce the in vivo situation, but obvious advantages are the high
sensitivity, good reproducibility, low cost, and the possibility of
testing a large number of chemicals. In addition, in vitro testing
with well-characterized genetic indicators allows the use of human
tissue such as human liver to determine their ability to generate a
mutagen (Bartsch, 1976).
7.2.3 Correlation between short and long-term bioassays for
carcinogenicity
Short-term tests (rapid screening tests) are procedures that do
not have the in vivo production of a visible tumour in animals as an
end point. The variables used in short-term tests to detect chemical
carcinogens are based on an interaction of carcinogens and/or their
metabolites with macromolecules, the induction of chromosomal
aberrations, mutagenesis, DNA repair, and DNA binding.
In the evaluation of these methods, the major question is which
screening tests, singly or collectively, serve as reliable indicators
or predictors of the potential carcinogenic hazard of the chemical.
The answer can only be obtained by testing a representative number of
compounds. A valid test should demonstrate that compounds with known
carcinogenic properties are positive, within the limit of the test,
and negative compounds are negative. If a test is required for
preliminary screening, a small proportion of false negatives or
positive results may be acceptable, but for a final test, no false
negative results are acceptable.
Rapid screening tests should be relatively simple and
inexpensive. They can be used in three ways: to trace carcinogens
and/or mutagens in the complex environment of man; as a tool for
prescreening chemicals to be submitted to a more lengthy set of
bioassays; or for better extrapolation from experimental animal data
to man and for improving the relevance of long-term bioassays in
experimental animals.
Considering reproducibility, cost, the number of chemicals that
can be examined in a short time, and the scientific basis of the test,
tissue-mediated mutagenicity procedures using well-characterized
genetic indicators and a metabolically defined in vitro activation
system, appear at present to be the most promising short-term tests.
With current methods there is still the chance of false negative
results, depending on the systems used, either for lack of appropriate
cofactors for activation or because of the extreme reactivity and/or
toxicity of the compound or its metabolites. However, the number of
false negative results in in vitro tissue-mediated assays is small
compared with other mutagenicity test procedures (Montesano & Bartsch,
1976).
The increasing evidence of a possible correlation between
mutagenicity and carcinogenicity certainly does not mean that one
biological effect may be equated with another. Thus, the mutagenic
activity of a chemical cannot, at present, automatically be assumed to
imply a definite carcinogenic effect in man, nor can these results
replace long-term carcinogenicity testing in animals.
Furthermore, it is still unknown whether all carcinogens will be
found to be mutagenic, and all mutagens, carcinogenic. Examples are
the strong mutagens, nitrous acid, hydroxylamine, and base analogues,
for which no carcinogenic effect in animals has so far been reported;
they do not act via electrophilic intermediates, a mechanism that has
now been recognized for must ultimate carcinogenic forms. Nor have
steroidal sex hormones, carcinogenic in animals, been reported to be
mutagenic, as yet. On the other hand, various chromium salts have
recently been shown to be mutagenic in bacteria (Venitt & Levy, 1974).
Development of cancer in vivo is determined by a variety of
factors that cannot be duplicated in an in vitro short-term testing
system because:
(a) The concentration of ultimate reactive metabolites available
to react in organs in animal species with cellular
macromolecules, which is a consequence of a balance between
metabolic activation and detoxication processes, is only
partly reflected by in vitro testing procedures.
(b) Species and organ specificity of a chemical carcinogen might
be determined, in part, by organ-specific DNA repair.
(c) Since chemical carcinogenesis is thought to be a multi-step
process in which the early, apparently irreversible,
initiation of a cell is followed by several subsequent
stimuli provoking cellular replications, leading to an overt
tumour, a short-term test capable of detecting complete
carcinogens may be useful to detect initiating agents but
cannot at the present time detect the action of promoting
agents.
Currently used short-term tests, in particular tissue-mediated
mutagenicity assays are effective in predicting, with a certain
accuracy, the carcinogenic potential of chemicals (McCann & Ames,
1976; Purchase et al., 1976; Sugimura et al., 1976), but give no
indication of the target organs or species specificity of their
carcinogenic activity. Furthermore, the relative potency of a chemical
to induce biological effects, defined as the endpoints, for the most
frequently used rapid screening tests cannot at the present time be
reliably correlated with its carcinogenic potency (Bartsch et al.,
1977; Meselson & Russell, 1977). More data are needed to compare
dose-response curves obtained in rapid screening tests on a given
chemical with those of other biological effects obtained in vivo
(long-term bioassays).
7.2.4 Significance of experimental testing for assessing the possible
carcinogenic risk of chemicals to man
Experience acquired, so far, in long-term carcinogenicity testing
has shown that nearly all compounds that are carcinogenic in man are
also carcinogenic in one or several animal species, even though the
tumour type may not be the same as in man. The concept that animal
carcinogenicity data are predictive of a human carcinogenic risk and
useful in preventing human cancer was accepted in 1941 in the case of
2-actylaminofluorene (AAF). This chemical was not used on a worldwide
basis as an insecticide, because some experiments had already shown
its carcinogenicity before it was marketed (Wilson et al., 1941); its
restriction was facilitated by the existence of a number of
substitutes at the time when the results of the first experiments on
AAF were reported. This cautious attitude has not been consistently
applied in other situations, on the grounds that the experimental data
were insufficient or inadequate to evaluate the possible hazard to
man. This is shown by the various relationships between experimental
carcinogenicity data and possible human hazard that are considered in
the adoption of preventive measures.
The first observation that some aromatic amines were involved in
the causation of urinary bladder tumours in man dates from 1896; it
was confirmed in 1907 and reconfirmed many times thereafter (IARC,
1974). In 1938, Hueper et al. reported the induction of bladder cancer
in dogs exposed to 2-naphthalenamine (2-naphthylamine). Preventive
measures were taken only in the late 1950s, when conspicuous
epidemiological evidence had accumulated. In this case, the human
epidemiological evidence was judged insufficient, and the experimental
evidence, when it became available, was also deemed insufficient until
further epidemiological findings came to hand.
The delay in taking preventive measures applies also to
carcinogens on which, contrary to the case of aromatic amines,
experimental evidence preceded observations of their effect in man,
e.g. diethylstilbestrol, bis(chloromethyl)ether, and chloroethylene
(vinyl chloride). (Tomatis et al. 1978; Montesano & Tomatis, 1977).
One of the main objections to carcinogenicity tests on animals is
that the experimental system used tries to produce the maximum
possible carcinogenic effect of the test chemical, does not reflect
the human situation, and is therefore misleading. It is difficult,
however, to accept this argument because, in most instances where a
chemical was found by epidemiological investigations to be associated
with cancer in man, the incidence was so high that the association was
clear without animal studies. This has been the case for high risk
groups such as occupational cancer groups. However, the risk is not
confined to these groups but also applies to other populations where
cancer incidence may be too low for detection by normal
epidemiological methods, hence the need to carry out animal
experiments under conditions that permit confident judgment of the
carcinogenicity or inactivity of a chemical.
Cancer testing in animals has reached a relatively sophisticated
stage and an exhaustive study of a chemical in animals is sufficient
evidence of a potential cancer risk for man. An assessment of the
validity of experimental results is essential for the successful
prevention of cancer in man. This does not preclude further research
for the development of short-term tests, but, at the present time,
these cannot replace long-term carcinogenicity testing.
7.3 Heritable Mutations
As noted in the introduction, direct methods for assessing
whether a chemical has the potential to cause heritable mutations in
man do not exist at present. Nontheless, information relative to the
possible production in man of germ cell mutations can be derived from
a variety of sources. These fall into three major categories: (a)
primary DNA damage involving DNA alteration, stimulation of DNA
repair, gene mutations tests including mutagenicity assessment using
bacterial or other microorganisms, with and without metabolic
activation; (b) whole animal tests for point or gene mutations
including insects, e.g. drosophila recessive, and the specific locus
test in mice; (c) chromosomal mutations including cytogenetic tests in
mammals, the dominant lethal test in mammals, and the heritable
translocation test in rodents. Examples of the first category have
already been presented. These tests provide information relevant to
heritable mutations and section 7.2.2 should be consulted for fuller
details. Obviously, tests employing isolated systems only provide
information on mutagenic action on the specific systems tested. This
information cannot be fully interpreted without evidence concerning
the access of this ultimate mutagen to the germ cells.
An extensive examination of relevant test procedures for
heritable mutations has recently been completed (DHEW, 1977).
7.3.1 Whole-animal tests
Insects. Drosophila melanogaster is one of the best genetically
characterized species and is widely used. Drosophila of either sex can
be treated and mutation frequencies from successive germ cell stages
may be obtained after observation of three generations. The X-lined
recessive lethal test is one of the most sensitive tests with
drosophila, since the X-chromosome represents about 1/5th of the whole
genome. In contrast to most microorganisms, insects possess an enzyme
system that appears to metabolize foreign compounds in a fashion
similar to that of vertebrates (Abrahamson & Lewis, 1971; Fahmy &
Fahmy, 1972, 1973; Sobels & Vogel, 1976).
Mouse specific locus test. The specific locus test is a method
of inducing, detecting, and measuring the rate of mutation at several
recessive loci. It consists essentially of mating treated or untreated
wild type mice, either male or female, to a strain homozygous for a
number of known recessive genes. The recessive genes are such that
they are readily expressed as visible phenotypes in homozygous state.
If a mutation occurs in any of the test loci in the germ cells of
treated animals, it may be detected in the offspring. If no mutation
has occurred following treatment, the progeny from the cross will all
be of the wild type (Cattanach, 1971; Russell, 1951).
Chromosomal mutations. Although the molecular basis is not
understood, a change in the whole chromosome, i.e. a structural
chromosome aberration, occurring as a consequence of a misrepair of
chromosomal breaks, may lead to deletions, duplications, and
translocations. Changes in the whole chromosome complement, i.e.
numerical chromosome aberrations, arise through nondisjunction, as in
failure of a pair of chromosomes to separate during gametogenesis,
meiotic nondisjunction, or during mitotic division. The resulting
daughter cells are either trisomic with an extra chromosome or
monosomic and lacking a chromosome. Anaphase lag occurs during nuclear
division in the progeny cells, and a chromosome may be either lost or
gained. In somatic cells, nondisjunction and anaphase lag can lead to
mosaicism.
Chromosomal damage may be studied in a number of test systems
that are best divided into in vivo and in vitro systems, on the
basis of their capability to metabolize the test compound (Frohberg,
1973). The short-term human lymphocyte culture in vitro is commonly
used to assess the effects of chemicals upon chromosomes. Metabolic
activation of the test compound can be achieved by addition of a liver
microsomal system (Bimboes & Greim, 1976).
The micronucleus test. As an in vivo cytogenetic method, the
micronucleus test is a procedure for the detection of aberrations
involving anaphase chromosome behaviour using bone-marrow
erythroblasts. The test is based on the formation of micronuclei from
particles of chromatin material which, due to chromosome breakage or
spindle disjunction, do not migrate to the poles during anaphase and
are not incorporated into the telophase nuclei of the dividing cells.
The procedure is to treat animals with clastogenic agents and, at an
appropriate time after treatment, to aspirate bone-marrow samples into
calf serum. This is then centrifuged and smears are made from the
resuspended pellets of cells. The smears are air-dried and stained.
The evaluation of the bone-marrow preparations involves examination of
2000-5000 erythrocytes per specimen; the total number of polychromatic
and normochromatic erythrocytes with and without micronuclei are
recorded (Schmid, 1973). Sister chromatid exchanges, induced by
mutagens in vivo and in vitro can be scored in peripheral
lymphocytes or Chinese hamster cells, using the differential straining
of chromatids substituted with 5-bromodeoxyuridine in place of
thymidine (Natarajan et al., 1976; Smythe & Evans, 1976; Stetka &
Wolf, 1976).
Dominant lethal tests and other in vivo systems. The dominant
lethal test is based on the preimplantation loss of eggs or on the
formation of dead embryonic implants following the injection of
mutagens into a male or female mouse at a specific time before mating
(Bateman & Epstein, 1971). Dominant lethal tests assume that a single
mutation has occurred in the eggs or sperm, which is lethal to the
embryo and heterozygous at the affected locus. However, a disturbingly
high number of mutagens have given a negative response with this test.
The detection of mutations arising from compounds that are metabolized
to transient reactive intermediates not produced in germ cells or not
reaching them from other organs may limit this test. Furthermore,
chemical agents causing spermatogenic arrest or cytocidal effect on
the sperms may give false positive results.
Heritable translocation in male mammals. The heritable
translocation test has the important feature of measuring sexually
transmissible germ cell mutations in rodent spermatogonia. Generoso et
al. (1974) have described details of the technique and discussed its
usefulness for the routine screening of substances that cause
chromosomal mutations.
Young adult male mice are treated and females mated with the
exposed males on a schedule that can be used for the comparison of the
sensitivity of the different male germ cell stages. Male progeny from
these matings are collected and mated for the determination of
semisterility and sterility. Progeny with reduced fertility are
subjected to cytogenetic analysis. The cytological examination of
dividing spermatocytes, from animals treated with test chemicals that
cause breaks on two nonhomologous chromosomes, yields aberrant
chromosomal figures. These are recognized as rings or chains of four
chromosomes as opposed to the normal bivalent chromosomal
configuration.
A proper evaluation of this test cannot be made at this time
because of insufficient data in terms of the variety of chemicals
tested to date.
7.3.2 Monitoring of human populations
In spite of the variety of test systems available for detecting
the mutagenic effects of chemicals, it is difficult, from the results
obtained at present, to evaluate with confidence the transmissible
genetic effects caused by the chemical exposure of man. However, the
potential hazards of mutations are such that every effort should be
made to reduce the risk.
Judgment on the potential mutagenic hazard to man should be based
on various considerations such as strength of the experimental
evidence of mutagenicity, the exposure pattern to the chemical, and
the pharmacological properties of the compounds. This is difficult and
complex. Although human studies are difficult, expensive, and often
subject to misinterpretation, only studies which directly estimate the
extent to which environmental factors change human genetic material
give definitive answers.
Proper monitoring of the human population (Crow, 1971; Sutton,
1972) can be carried out by three main approaches:
(a) biochemical: detection of inherited protein variants;
(b) cytogenetic: screening of blood of newborn infants or of
fetuses delivered by spontaneous abortion for chromosomal
changes;
(c) phenotype: surveillance of genetically determined disease or
anomalies.
The validity of these monitoring systems can only be properly
evaluated if, and when, a mutagen is actually discovered.
7.3.3 Significance of tests for heritable mutations
As mentioned earlier, there is, at this time, no direct
correlation between laboratory tests for heritable mutations and human
experience. However, the available body of information from nonhuman
mammals and lower life forms clearly points to the ability of
chemicals to produce alterations in germ cells which are inherited in
succeeding generations. Accordingly, the implications of such data for
man are so persuasive that they must be taken into account in
establishing safety measures for the introduction of chemicals into
use. This is especially true, at this time, since techniques for
detecting mutations in human populations are so insensitive that a
significantly mutagenic chemical could easily escape attention.
These considerations will almost certainly lead to increasing
concern in establishing regulatory and control procedures. In fact,
the Environmental Protection Agency in the USA has recently proposed
preliminary guidelines for conducting tests for heritable mutations as
part of the routine registration procedure for pesticides.
REFERENCES
ABRAHAMSON, S. & LEWIS, E. B. (1971) The detection of mutations in
Drosophila melanogaster. In: Hollaender, A., ed. Chemical
mutagens, principles and methods for their detection, Vol. 2,
461-488.
ALBERTINI, R. J. & DEMARS, R. (1973) Somatic cell mutation detection
and quantification of X-ray induced mutation in cultured human
diploid fibroblasts. Mutat. Res., 18, 199-244.
AMES, B. N. (1971) The detection of chemical mutagens with enteric
bacteria. In: Hollaender, A., ed. Chemical mutagens, principles
and methods for their detection, Vol. 1, pp. 267-282.
AMES, B. N., SIMS, P., & GROVER, P. L. (1972a) Epoxides of
carcinogenic polycyclic hydrocarbons are frameshift mutagens.
Science, 176, 47-49.
AMES, B. N., GURNEY, E.G., MILLER, J. A., & BARTSCH, H. (1972b)
Carcinogens as frameshift mutagens: Metabolites and derivatives
of 2-acetylaminofluorene and other aromatic amine carcinogens.
Proc. Natl Acad. Sci., 69: 3128-3132.
AMES, B. N., LEE, F. D., & DURSTON, W. E. (1973a) An improved
bacterial test system for the detection and classification of
mutagens and carcinogens. Proc. Natl Acad. Sci., 70: 782-786.
AMES, B. N., DURSTON, W. E., YAMASAKI, E., & LEE, F. D. (1973b)
Carcinogens are mutagens: a simple test system combining liver
homogenates for activation and bacteria for detection. Proc.
Natl Acad. Sci., 70; 2281-2285.
AMES, B. N., MCCANN, J., & YAMASAKI, E. (1975) Methods for detecting
carcinogens and mutagens with the Salmonella/mammalian-
microsome mutagenicity test. Mutat. Res., 31: 347-364.
BARTSCH, H. (1976) Predictive value of mutagenicity tests in chemical
carcinogenesis. Mutat. Res., 38: 177-190.
BARTSCH, H. & GROVER, P. L. (1976) Chemical carcinogenesis and
mutagenesis. In: Symington, T. & Carter, R. L., ed. Scientific
foundations of oncology, London, William Heinemann Medical
Books Ltd, pp. 334-342.
BARTSCH, H., MALAVEILLE, C., & MONTESANO, R. (1975a) Human, rat and
mouse liver-mediated mutagenicity of vinyl chloride in
S. typhimurium strains. Int. J. Cancer, 15: 429-437.
BARTSCH, H., MALAVEILLE, C., & MONTESANO, R. (1975b) In vitro
metabolism and microsome-mediated mutagenicity of
dialkylnitrosamines in rat, hamster and mouse tissues. Cancer
Res., 35: 644-651.
BARTSCH, H., MALAVEILLE, C., STICH, H. F., MILLER, E. C., & MILLER, J.
A. (1977) Comparative electrophilicity, mutagenicity, DNA repair
induction activity, and carcinogenicity of some N- and O-Acyl
derivatives of N-Hydroxy-2-aminofluorene. Cancer Res.,
37: 1461-1467.
BATEMAN, A. J. & EPSTEIN, S.S. (1971) Dominant lethal mutations in
mammals. In: Hollaender, A., ed. Chemical mutagens, principles
and methods for their detection, Vol. 2, pp. 541-568.
BERWALD, Y. & SACHS, L. (1963) In vitro transformation with chemical
carcinogens. Nature (Lond.), 200: 1182-1184.
BIMBOES, D. & GREIM, H. (1976) Human lymphocytes as target cells in a
metabolizing test system in vitro for detecting potential
mutagens. Mutat. Res., 35: 155-160.
BORLAND, R. & HARD, G. C. (1974) Early appearance of "transformed"
cells from the kidneys of rats treated with a "single"
carcinogenic dose of dimethylnitrosamine (DMN) detected by
culture in vitro. Europ. J. Cancer, 10: 177-184.
BRIDGES, B. A. (1976) Short-term screening tests for carcinogens.
Nature (Lond.), 261: 195-200.
BRIDGES, B. A., MOTTERSHEAD, R. P., ROTHWELL, M. A., & GREEN, M. H. L.
(1972) Repair deficient bacterial strains suitable for
mutagenicity screening tests with fungicide captan. Chem.-Biol.
Interact., 5: 77-84.
BROWN, A.M. (1973) In vitro transformation of submandibular gland
epithelial cells and fibroblasts of adult rats by
methylcholanthrene. Cancer Res., 33: 2779-2789.
CATTANACH, B. M. (1971) Specific locus mutation in mice. In:
Hollaender, A., ed. Chemical mutagens, principles and methods
for their detection, Vol. 2, pp. 535-540.
CHU, E. H. Y. (1971) Induction and analysis of gene mutations in
mammalian cells in culture. In: Hollaender, A., ed. Chemical
mutagens, principles and methods for their detection, Vol. 2,
pp. 411-444.
CLEAVER, J. E. (1969) Xeroderma pigmentosum: a human disease in
which an initial state of DNA repair is defective. Proc. Natl
Acad. Sci., 63: 428-435.
CLEAVER, J. E. (1973) DNA repair with purines and pyrimidines in
radiation- and carcinogen-damaged normal and Xeroderma
pigmentosum human cells. Cancer Res., 33: 362-369.
CLEAVER, J. E. (1974) Repair processes for photochemical damage in
mammalian cells. In: Lett, J. T., Adler, H., & Zelle, M., ed.
Advances in radiation biology, New York, Academic Press, Vol.
4, pp. 1-75.
CLEAVER, J. E. (1975) Methods for studying repair of DNA damaged by
physical and chemical carcinogens. In: Bush, E., ed. Methods in
cancer research, New York, Academic Press, Vol. 11, pp.
123-165.
CLEAVER, J. E., GOTH, R., & FRIEDBERG, E. C. (1975) Value of
measurements of DNA repair levels in predicting carcinogenic
potential of chemicals. In: Montesano, R., Bartsch, H., &
Tomatis, L., ed. Screening tests in chemical carcinogenesis,
IARC Sci. Publ. No. 12, pp. 639-661.
COMMONER, B., VITHAYATHIL, A. J., & HENRY, J. I. (1974) Detection of
metabolic carcinogen intermediates in urine of carcinogen-fed
rats by means of bacterial mutagenesis. Nature (Lond.),
249: 850-852.
CONNEY, A. H. & BURNS, J. J. (1972) Metabolic interactions among
environmental chemicals and drugs. Science, 178: 576-586.
CORBETT, T. H., HEIDELBERGER, C., & DOVE, W. F. (1970) Determination
of the mutagenic activity to bacteriophage T-4 of carcinogenic
and non-carcinogenic compounds. Mol. Pharmacol., 6(6): 667-679.
COUNCIL OF THE ENVIRONMENTAL MUTAGEN SOCIETY (1975) Environmental
mutagenic hazards, mutagenicity screening is now both feasible
and necessary for chemicals entering the environment. Science,
187: 503-514.
CRADDOCK, V. M. (1976) Replication and repair of DNA in liver of rats
treated with dimethylnitrosamine and with methyl
methanesulphonate. In: Magee, P. N. et al., ed. Fundamentals in
cancer prevention, Tokyo, Univ. Tokyo Press; Baltimore, Univ.
Park Press, pp. 293-311.
CROW, J. F. (1971) Human population monitoring. In: Hollaender, A, ed.
Chemical mutagens, principles and methods for their detection,
Vol. 2, pp. 591-606.
CZYGAN, P. J., GREIM, H., GARRO, A. J., HUTTERER, F., SCHAFFAER, F.,
POPPER, H., ROSENTHAL, O., & COOPER, D. Y. (1973) Microsomal
metabolism of DMN and the cytochrome activation to a mutagen.
Cancer Res,, 33: 2983-2986.
DELLA PORTA, G. & TERRACINI, B. (1969) Chemical carcinogenesis in
infant animals. Progr. Exp. Tumour Res., 11: 334-363.
DE MARS, R. (1974) Resistance of cultured human fibroblasts and other
cells to purine and pyrimidine analogues in relation to
mutagenesis detection. Mutat. Res., 24: 335-364.
DHEW (1977) Approaches to determining the mutagenic properties of
chemicals: Risks to future generations. Report of a working
group of the Subcommittee on Environmental Mutagenesis.
DI PAOLO, J. A., NELSON, R. L., & DONOVAN, P. J. (1972) In vitro
transformation of Syrian hamster embryo cells by diverse chemical
carcinogens. Nature (Lond.), 235: 278-280.
DI PAOLO, J. A., NELSON, R. L., DONOVAN, P. J., & EVANS, C. H. (1973)
Host-mediated in vivo-in vitro assay for chemical
carcinogenesis. Arch. Pathol., 95: 380-385.
DE SERRES, F. J. & MALLING, H. V. (1971) Measurement of recessive
lethal damage over the entire genome and at two specific loci in
the ad-3 region of a two-component heterokaryon of Neurospora
Crassa. In: Hollaender, A., ed. Chemical mutagens, principles
and methods for their detection, Vol. 2, pp. 311-342.
DRAKE, J. W. (1971) Mutagen screening with virulent bacteriophages.
In: Hollaender, A., ed. Chemical mutagens, principles and
methods for their detection, Vol. 1, pp. 219-234.
DRUCKREY, H., PREUSSMAN, R., IVANKOVIC, S., & SCHMÄL D. (1967)
[Organotropic carcinogenic effects of 65 different N-nitroso
compounds on BD-rats.] Z. Krebsforsch., 69 103-201.
DURSTON, W. E. & AMES, B. N. (1974) A simple method for the detection
of mutagens in urine: Studies with the carcinogen
2-acetylaminofluorene. Proc. Natl Acad. Sci., 71: 737-741.
EARLE, W. R. & NETTLESHIP, A. (1943) Production of malignancy in
vitro. V. Results of injections of cultures into mice. J. Natl
Cancer Inst., 4 213-227.
FAHMY O. G. & FAHMY, M.D. (1972) Mutagenic properties of AAF and its
metabolites. Int. J. Cancer, 9 284-298.
FAHMY O. G. & FAHMY, M.D. (1973) Mutagenic properties of
benzo(a)pyrene. Cancer Res., 33: 302-309.
FARBER, E. (1973) Carcinogenesis -- cellular evolution as a unifying
thread: presidential address. Cancer Res., 33: 2537-2550.
FDA (1971) Panel on carcinogenesis report on cancer testing in the
safety evaluation of food additives and pesticides. Toxic. appl.
Pharmacol., 20: 419-438.
FEDOROFF, S. (1967) Proposed usage of animal tissue culture terms,
J. Natl Cancer Inst., 38: 607-611.
FREESE, E. & STRACK, H. B. (1962) Induction of mutations in
transforming DNA by hydroxylamine. Proc. Natl Acad. Sci.,
48: 1796-1803.
FRIEDMAN, L. (1974) Dose selection and administration. In: Goldberg,
L., ed. Carcinogenesis testing of chemicals, Cleveland, CRC
Press, pp. 21-22.
FROHBERG, H. (1973) Critique of in vivo cytogenic test systems.
Agents Actions, 3: 119-123.
FUSENIG, N. R., SAMSEL, W., THON, W., & WORST, P. K. M. (1973)
Malignant transformation of epidermal cells in culture by DMBA.
In: Prunieras, M., Robert, L., & Rosenfeld, C., ed.
Differentiation of eukaryotic cells in culture. Colloque of
INSERM, Vol. 19, pp. 219-228.
GERCHMAN, L. L. & LUDLUM, D. B. (1973) The properties of
O6-methylguanine in template for RNA polymerase. Biochim.
biophys. Acta, 308: 310-316.
GIOVANELLA B, C., YIM, S. O., STEHLIN, J. S., & WILLIAMS, L. J. Jr
(1972) Brief communications: Development of invasive tumors in
the "nude" mouse after injection of cultured human melanoma
cells. J. Natl Cancer Inst., 48: 1531-1533.
GOTH, R. & RAJEWSKY, M. F. (1974) Molecular and cellular mechanisms
associated with pulse-carcinogenesis in the rat nervous system by
ethylnitrosourea: ethylation of nucleic acids and elimination
rates of ethylated bases from the DNA of different tissues.
Z. Krebsforsch., 82: 37-64.
HASHIMOTO, Y. & KITAGAWA, H. (1974) In vitro neoplastic
transformation of epithelial cell of rat urinary bladder by
nitrosamines. Nature (Lond.), 252: 497-499.
HEALTH AND WELFARE, CANADA (1973) The testing of chemicals for
carcinogenicity, mutagenicity, teratogenicity.
HEIDELBERGER, C. (1973) Chemical oncogenesis in culture. Adv. Cancer
Res., 18: 317-366.
HERRIOTT, R. M. (1971) Effects on DNA: Transforming principle. In:
Hollaender, A., ed. Chemical mutagens, principles and methods
for their detection, Vol. 1, pp. 175-218.
HUBERMAN, E. & SACHS, L. (1974) Cell-mediated mutagenesis of mammalian
cells with chemical carcinogens. Int. J. Cancer, 13: 326-333.
HUBERMAN, E., ASPIRAS, L., HEIDELBERGER, C., GROVER, P. L., & SIMS, P.
(1971) Mutagenicity to mammalian cells of epoxides and other
derivatives of polycyclic hydrocarbons. Proc. Natl Acad. Sci.,
68: 3195-3199.
HUBERMAN, E., DONOVAN, P. J., & DIPAOLO, J. A. (1972) Mutation and
transformation of cultured mammalian cells by
N-acetoxy- N-2-fluorenylacetamide. J. Natl Cancer Inst.
48: 837-840.
HUEPER, W. C., WILEY, F. H., & WOLFE, H. D. (1938) Experimental
production of bladder tumours in dogs by administration of
ß-naphthylamine. J. industr. Hyg. Toxicol., 20: 46-84.
IARC (1976) Monographs on the evaluation of carcinogenic risk of
chemicals to man: some aromatic amines, hydrazine and related
substances, N- nitroso compounds and miscellaneous alkylating
agents. Lyons, International Agency for Research on Cancer,
Vol. 4.
IYPE P. T. (1974) Studies on chemical carcinogenesis in vitro using
adult rat fiver cells. In: Montesano, R. & Tomatis, L., ed.
Chemical carcinogenesis essays, IARC Sci. Publ. No. 10,
pp. 119-133.
J. Natl Cancer Inst., 53: 1427-1519 (1974) Proceedings of the
Symposium "New Horizons for Tissue Culture in Cancer Research".
KAKUNAGA, T. The transformation of human diploid cells by chemical
carcinogens. In: Hiatt, H. H. et al., ed. Origins of human
cancer (1977) Cold Spring Harbor Laboratory. pp. 1537-1548.
KATSUTA, H. & TAKAOKA, T. (1972) Carcinogenesis in tissue culture.
XIV. Malignant transformation of rat liver parenchymal cells
treated with 4-nitroquinoline 1-oxide in tissue culture. J. Natl
Cancer Inst., 49: 1563-1536.
KONDO, S. (1973) Evidence that mutations are induced by errors in
repair and replication. Genet. suppl., 73: 109-122.
KRAHN, D. F. & HEIDELBERGER, C. (1975) Microsome-mediated mutagenesis
in Chinese hamster cells by chemical oncogens. Proc. Am. Assoc.
Cancer Res., 16: 74.
KRIEK, E. (1974) Carcinogenesis by aromatic amines. Biochem.
Biophys. Acta, 355: 177-203.
KUROKI, T. (1975) Contributions of tissue culture to the study of
chemical carcinogenesis: A review. In: Recent topics in chemical
carcinogenesis, Gann, Monograph on Cancer Res., 17: 69-85.
KUROKI, T., DREVON, C., & MONTESANO, R. (1977) Microsome-mediated
mutagenesis in V-79 Chinese hamster cells by various
nitrosamines. Cancer Res., 1044-1050.
LAERUM, O. D. & RAJEWSKY, M. F. (1975) Neoplastic transformation of
foetal rat brain cells in culture following exposure to
ethylnitrosourea in vivo. J. Natl Cancer Inst., 55: 1177-1187.
LEGATOR, M. S. & FLAMM, W. G. (1973) Environmental mutagenesis and
repair. Ann. Rev. Biochem., 683-708.
LEGATOR, M. S. & MALLING, H. V. (1971) The host-mediated assay, a
practical procedure for evaluating potential mutagenic agents in
mammals. In: Hollaender A., ed. Chemical mutagens, principles
and methods for their detection, Vol. 2, pp. 569-590.
LOPRIENO, N., BARALE, R., BARONCELLI, S., BARTSCH, H., BRONZETTI, G.,
CAMMELLINI, A., CORSI, C., FREZZA, D., NIERI, R., LEPORINI, C.,
ROSELLINI, D., & ROSSI, A.M. (1976) Induction of gene mutations
and gene conversions by vinyl chloride metabolites in yeast.
Cancer Res., 37: 253-257.
LOVELESS, A. (1969) Possible relevance of O6-alkylation of
deoxyguanosine to the mutagenicity and carcinogenicity of
nitrosamines and nitrosamides. Nature (Lond.), 233: 206-207.
LOVELESS, A. & HAMPTON, C. L. (1969) Inactivation and mutation of
coliphage T2 by N-methyl- and N-ethyl- N-nitrosourea.
Mutation Res., 7: 1-12.
LUDLUM, D. B. (1970) Alkylated polycytidic acid templates for RNA
polymerase. Biochim. biophys. Acta, 213: 142-148.
MAGEE, P. N., MONTESANO, R., & PREUSSMAN, R. (1976) N-nitroso
compounds and related carcinogens. In: Searle, C., ed. Chemical
carcinogens, ACS monograph No. 173, pp. 491-625.
MAHER, V., MILLER, E. C., MILLER, J. A., & SZYBALSKI, W. (1968)
Mutations and decreases in density of transforming DNA produced
by derivatives of the carcinogens 2-acetylaminofluorene and
N-methyl-4-aminoazobenzene. Mol. Pharmacol., 4: 411-426.
MAHER, V. M., CURREN, R. D., OUELLETTE, L. M., & MCCORMICK, J. J.
(1976) Effect of DNA repair on the frequency of mutations induced
in human cells by ultraviolet irradiation and by chemical
carcinogens. In: Magee, P. N., et al, ed. Fundamentals in cancer
prevention, Tokyo, Univ. of Tokyo Press; Baltimore, Univ Park
Press, pp. 363-382.
MALLING, H. V. (1974) Mutagenic activation of dimethylnitrosamine and
diethylnitrosamine in the host-mediated assay and the microsomal
system. Mutat. Res., 26: 465-472.
MARGISON, G. P., MARGISON, J. M., & MONTESANO, R. (1976) Methylated
purines in the deoxyribonucleic acid of various Syrian golden
hamster tissues after administration of a hepatocarcinogenic dose
of dimethylnitrosamine. Biochem. J., 157: 627-634.
MARQUARDT, H. (1974) Mutation and recombination experiments with yeast
as pre-screening tests for carcinogenic effects.
Z. Krebsforsch., 81: 333-346.
MCCANN, J. & AMES, B. N. (1976) Detection of carcinogens as mutagens
in the Salmonella/microsome test: Assay of 300 chemicals:
Discussion. Proc. Natl Acad. Sci., 73: 950-954.
MCCANN, J., SPINGARN, N. E., KOBORI, J., & AMES, B. N. (1975)
Detection of carcinogens as mutagens: bacterial tester strains
with R factor plasmids. Proc. Natl Acad. Sci., 72: 979-983.
MESELSON, M. & RUSSELL, K. (1977) Comparisons of carcinogenic and
mutagenic potency. In: Hiatt, H. H. et at. ed. Origins of human
cancer. Cold Spring Harbor Laboratory. pp. 1473-1481.
MILLER, J. A. (1970) Carcinogenesis by chemicals: an overview -- G. H.
A. Clowes Memorial Lecture. Cancer Res., 30: 559-576.
MILLER, J. A. (1973) Naturally occurring substances that can induce
tumors. Proc. Natl Acad. Sci., 23: 508-549.
MILLER, E. C. & MILLER, J. A. (1971a) The mutagenicity of chemical
carcinogens: correlations, problems, and interpretations. In:
Hollaender, A., ed. Chemical mutagens, principles and methods
for their detection, Vol. 1, pp. 83-120.
MILLER, J. A. & MILLER, E. C. (1971b) Chemical carcinogenesis,
mechanisms and approaches to its control. J. Natl Cancer Inst.,
47: V-XIV.
MILLER, E. C. & MILLER, J. A. (1976) The metabolism of chemical
carcinogens to reactive electrophiles and their possible
mechanisms of action in carcinogenesis. In: Searle, C. E., ed.
Chemical carcinogens, ACS Monograph 173. Washington, DC,
American Chemical Society, pp. 737-762.
MOHN, G. (1973) Revertants of an Escherichia coli K-12 strain with
high sensitivity to radiations and chemicals. Murat. Res.,
19: 349-355.
MOHN, G. & ELLENBERGER, J. (1973) Mammalian blood mediated
mutagenicity test using a multi-purpose strain of E. coli,
K-12. Mutat. Res., 19: 257-260.
MONTESANO, R. & BARTSCH, H. (1976) Mutagenic and carcinogenic
N-nitroso compounds: Possible environmental hazards. Mutat.
Res., 32: 179-228.
MONTESANO, R., SAINT VINCENT, L., & TOMATIS, L. (1973)
Malignant transformation in vitro of rat liver cells by
dimethylnitrosamine and N-methyl- N'-nitro-
N-nitrosoguanidine. Brit. J. Cancer, 38: 215-220.
MONTESANO, R., SAINT VINCENT, L., DREVON, C,, & TOMATIS, L. (1975)
Production of epithelial and mesenchymal tumours with rat liver
cells transformed in vitro. Int. J. Cancer. 55: 1177-1187.
MONTESANO, R., DREVON, C., KUROKI, T., SAINT VINCENT, L., HANDLEMAN,
S., SANFORD, K. K., DEFEO, D. & WEINSTEIN, I. B. (1977) Test for
malignant transformation of rat liver cells in culture: Cytology,
growth in soft agar and production of plasminogen activator.
J. Natl Cancer Inst., 59: 1651-1658.
MONTESANO, R., BARTSCH, H., & TOMATIS, L., ed. (1976) Screening tests
in chemical carcinogenesis. IARC Sci. Publ. No. 12.
MONTESANO, R. & TOMATIS, L. (1977) Legislation concerning chemical
carcinogens in several industrialized countries. Cancer Res.,
37: 310-316.
MOREAU, P., BAILONE, A., & DEVORET, R. (1976) Prophage lambda
induction in Escherichia coli K12 envA uvrB: A highly
sensitive test for potential carcinogens. Proc. Natl Acad. Sci.,
73: 3700-3704.
MORTIMER, R. K. & MANNEY, T. R. (1971) Mutation induction in yeast.
In: Hollaender, A., ed. Chemical mutagens, principles and
methods for their detection, Vol. 1, pp. 289-310.
NAHAS, A. & CAPIZZI, R. L. (1974) Effect of in vivo treatment with
L asparaginal on the in vitro uptake and phosphorylation of
some anti-leukemic agents. Cancer Res., 34: 2687-2693.
NAPALKOV, N. P. (1973) Some general considerations on the problem of
transplacental carcinogenesis. In: Tomatis, L. & Mohr, U., ed.
Transplacental carcinogenesis, IARC Sci. Publ. No. 4, pp. 1-13.
NAPALKOV, N. P. & ALEXANDROV, V. A. (1968) On the effects of
blastomogenic substances on the organism during embryogenesis.
Z. Krebsforsch., 71: 32-50.
NATARAJAN, A. T., TATES, A.D., VAN BUUL, P. P. W., MEIJERS, M., & DE
VOGEL, N. (1976) Cytogenetic effects of mutagens/carcinogens
after activation in a microsomal system in vitro. I. Induction
of chromosome aberrations and sister chromatid exchanges by
diethylnitrosamine (DEN) and dimethylnitrosamine (DMN) in CHO
cells in the presence of rat-liver microsomes. Murat. Res.,
37: 83-90.
MATIONAL ACADEMY OF SCIENCES (1960) Problems in the evaluation of
carcinogenic hazard from use of food additives. Washington, Natl
Res. Council (Publ. No. 479).
NCI (1976) NCI Technical Report Series, No. 1 (Guidelines for
carcinogen bioassay in small rodents), pp. 1-65 (DHEW Publication
No. (NIH) 76-701).
PEGG, A. E. (1977) Formation and metabolism of alkylated nucleosides:
Possible role in carcinogenesis by nitroso compounds and
alkylating ageNTs. Adv. Cancer Res., 25: 195-269.
PEGG, T. & NICOLL, J. W. (1976) Nitrosamine carcinogenesis: The
importance of the persistence in DNA of alkylated bases in the
organotropism of tumour induction. In: Montesano, R., Bartsch,
H., & Tomatis, L., ed. Screening tests in chemical
carcinogenesis, IARC Sci. Publ. No. 12, pp. 571-592.
PIETRA, G., RAPPAPORT, H., & SHUBIK, P. (1961) The effects of
carcinogenic chemicals in newborn mice. Cancer, 14: 308-317.
PITOT, H. C. (1974) Neoplasia: A somatic mutation or a heritable
change in cytoplasmic membranes? J. Natl Cancer Inst.,
53: 905-911.
PITOT, H. C. (1977) The natural history of neoplasia. Am. J. Path.,
89: 402-411.
PURCHASE, I. F. H., LONGSTAFF, E., ASHBY, J., STYLES, J. A., ANDERSON,
D., LEFEVRE, P. A., & WESTWOOD, F. R. (1976) Evaluation of six
short-term tests for detecting organic chemical carcinogens and
recommendations for their use. Nature (Lond.), 264: 624-627.
REUBER, M. (1974) Criteria for tumor diagnosis and classification of
malignancy. In: Golberg, L., ed. Carcinogenesis testing of
chemicals, Cleveland, CRC Press, pp. 71-73.
ROSENKRANZ, H. S. (1973) Aspects of microbiology in cancer research.
Rev. Microbiol., 1622: 383-400.
RUSSELL, W. L. (1951) X-ray induced mutations in mice. Cold Spring
Harbour Symp. Quant. Biol., 16: 327-336.
SANFORD, K. K. (1965) Malignant transformation of cells in vitro.
Inter. Rev. Cytol., 18: 249-311.
SANFORD, K. K. (1974) Biological manifestations of oncogenesis in
vitro: a critique. J. Natl Cancer Inst., 53: 1481-1485.
SANFORD, K. K., EARLE, W. R., SHELTON, E., SCHILLING, E. L., DUCHESNE,
E. M., LIKELY, G. D., & BECKER, E. M. (1950) Production of
malignancy in vitro. XII. Further transformations of mouse
fibroblasts to sarcomatous cells. J. Natl Cancer Inst.,
11: 351-367.
SATO, K., SLESINSKI, R. S, & LITTLEFIELD, J. W. (1972) Chemical
mutagenesis at the phosphoribosyl transferase locus in cultured
human lymphoblasts. Proc. Natl Acad. Sci., 69: 1244-1248.
SCHMID, W. (1973) Chemical mutagen testing on in vivo somatic
mammalian cells. Agents Actions, 3: 77-85.
SIMS, P. & GROVER, P. L. (1974) Epoxides in polycyclic aromatic
hydrocarbon metabolism and carcinogenesis. Advances in Cancer
Res., 20: 165-274.
SMITH, W. E. & HANAWALT, P. C. (1969) Molecular photobiology:
Inactivation and recovery. New York, Academic Press.
SMYTHE, D. R. & EVANS, H. J. (1976) Mapping of sister-chromatid
exchanges in human chromosomes using G-Banding and
autoradiography. Mutat. Res., 35: 139-154.
SOBELS, F. H. & VOGEL, E. (1976) The capacity of drosophila for
detecting relevant genetic damage. Mutat. Res., 41: 95-106.
STETKA, D. G. & WOLFF, S. (1976) Sister chromatid exchange as an assay
for genetic damage induced by mutagen-carcinogens. I. In vitro
test for compounds requiring metabolic activation. Mutat. Res.,
41: 333-342.
STICH, H. F. & KIESER, D. (1974) Use of DNA repair synthesis in
detecting organo-tropic actions of chemical carcinogens.
Proc. Soc. Exp. Biol Med., 145: 1339-1342.
STICH, H. F. & SAN, R. H. C. (1970) DNA repair and chromatid anomalies
in mammalian cells exposed to 4 nitroquinoline 1-oxide. Mutat.
Res., 10: 389-404.
STOLTZ, D. R., POIRIER, L. A., IRVING, C. C., STICH, H. F.,
WEISBURGER, J. H., & GRICE, H. C. (1974) Evaluation of short-term
tests for carcinogenicity. Toxicol. appl. Pharmacol.,
29: 157-180.
SUGIMURA, T., SATO, S., NAGAO, M., YAHAGI, T., MATSUSHIMA, T., SEINO,
Y., TAKEUCHI, M., & KAWACHI, T. (1976) Overlapping of carcinogens
and mutagens. In: Magee, P. N., Takayama, S., Sugimura, T., &
Matsushima, T., ed. Fundamentals in cancer prevention, Tokyo,
University of Tokyo Press; Baltimore, Univ. Park Press,
pp. 191-215.
SUTHERLAND, B. M., RICE, M., & WAGNER, E. K. (1975) Xeroderma
pigmentosum cells contain low levels of photoreactivating
enzyme. Proc. Natl Acad. Sci., 72: 103-107.
SUTTON, H. E. (1972) Monitoring somatic mutations in human
populations. In: Sutton, H. E. & Harris, M. I., ed. Mutagenic
effects of environmental contaminants, pp. 122-128.
THOMPSON, L. H. & BAKER, R.M. (1973) Isolation of mutants of cultured
mammalian cells. In: Prescott, D. M., ed. Methods of cell
biology, New York, Academic Press, pp. 209-280.
TOMATIS, L. (1973) Transplacental carcinogenesis. In: Raven, R. W.,
ed. Modern trends in oncology -- 1, London, Butterworths,
pp. 99-126.
TOMATIS, L. (1974a) The validity of long-term bioassays in
carcinogenicity testing. Chemical and vital oncogenesis. Excerpts
Medica Int. Congr. Ser. No. 350, Vol. 2, pp. 82-93.
TOMATIS, L. (1974b) Inception and duration of tests. In: Golberg, L.,
ed. Carcinogenesis testing of chemicals, Cleveland, CRC Press,
pp. 23-27.
TOMATIS, L., AGTHE, C., BARTSCH, H., HUFF, J., MONTESANO, R., SARACCI,
R., WALKER, E., & WILBOURN, J. (1978) Evaluation of the
carcinogenicity of chemicals: A review of the monograph program
of the International Agency for Research on Cancer. Cancer Res.,
38: 877-885.
TURUSOV, V. S., ed. (1973, 1976) Pathology of tumours in laboratory
animals, Vol. I, Part 1 and 2, IARC Sci. Publ. No. 5 and No. 6.
Lyon.
UICC (1969) UICC Technical Report Series Vol. 2 (Carcinogenicity
testing). Geneva, International Union Against Cancer, 56 pp.
VENITT, S. & LEVY, L. S. (1974) Mutagenicity of chromates in bacteria
and its relevance to chromate carcinogenesis. Nature (Lond.),
250: 493-495.
WEIL, C. S. (1972) Statistics vs safety factors and scientific
judgements in the evaluation of safety for man. Toxicol. appl.
Pharmacol., 21: 454-463.
WEINSTEIN, I. B., ORENSTEIN, J. M., GEBERT, T., KAIGHN, M. E., &
STADLER, V. C. (1975) Growth and structural properties of
epithelial cell cultures established from normal rat liver and
chemically induced hepatomas. Cancer Res., 35: 253-263.
WEISBURGER, J. H. (1974) Inclusion of positive control. In: Golberg,
L., ed. Carcinogenesis testing of chemicals, Cleveland, CRC
Press, pp. 29-34.
WEISBURGER, E. K. (1975) A critical evaluation of the methods used for
determining carcinogenicity. J. clin. Pharmacol., 15: 5-15.
WEISBURGER, J. H. & WEISBURGER E. K. (1967) Tests for chemical
carcinogens. In: Busch, H., ed. Methods in cancer research,
Academic Press, Vol. 1, pp. 307-398.
WEISBURGER, J. H. & WEISBURGER, E. K. (1973) Biochemical formation and
pharmacological, toxicological and pathological properties of
hydroxylamines and hydroxamic acids. Pharmacol. Rev. 25: 1-66.
WHO (1961) WHO Technical Report Series No. 220 (Evaluation of
carcinogenic hazards of food additives), 33 pp.
WHO (1964) WHO Technical Report Series No. 276 (Prevention of cancer).
WHO (1969) WHO Technical Report Series No. 426 (Principles for the
testing and evaluation of drugs for carcinogenicity).
WHO (1974) WHO Technical Report Series, No. 546 (Assessment of the
carcinogenicity and mutagenicity of chemicals), 19 pp.
WILLIAMS, G. M., ELLIOT, J. M., & WEISBURGER, J. H. (1973) Carcinoma
after malignant conversion in vitro of epithelial-like cells
from rat liver following exposure to chemical carcinogens.
Cancer Res., 33: 606-612.
WILSON, R. H., DEEDS, F., & COX, A. J. (1941) The toxicity and
carcinogenicity of 2-acetaminofluorene. Cancer Res.,
1: 595-608.
ZIMMERMANN, F. K. (1973) Detection of genetically active chemicals
using various yeast systems. In: Hollaender, A., ed. Chemical
mutagens, principles and methods for their detection, Vol. 3,
pp. 209-240.
