
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 5
Nitrates, Nitrites and N-Nitroso Compounds
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of either the World Health Organization or the United Nations
Environment Programme
Published under the joint sponsorship of the United Nations
Environment Programme and the World Health Organization
World Health Organization
Geneva, 1978
ISBN No. 92 4 154065 6
(c) World Health Organization 1978
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. For rights of reproduction or
translation of WHO publications, in part or in toto, application
should be made to the Office of Publications, World Health
Organization, Geneva, Switzerland. The World Health Organization
welcomes such applications.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city or area or
of its authorities, or concerning the delimitation of its frontiers or
boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR NITRATES, NITRITES AND N-NITROSO
COMPOUNDS
1. SUMMARY AND RECOMMENDATIONS FOR FURTHER STUDIES
1.1. Summary
1.1.1. Analytical methods
1.1.1.1 Nitrates and nitrites
1.1.1.2 N-nitroso compounds
1.1.2. Sources and occurrence in the environment
1.1.2.1 Nitrates and nitrites
1.1.2.2 N-nitroso compounds
1.1.3. Metabolism
1.1.3.1 Nitrates and nitrites
1.1.3.2 N-nitroso compounds
1.1.4. Experimental studies in animals
1.1.4.1 Nitrates and nitrites
1.1.4.2 N-nitroso compounds
1.1.5. Epidemiological studies
1.1.5.1 Nitrates and nitrites
1.1.5.2 N-nitroso compounds
1.1.6. Evaluation of health risks
1.1.6.1 Nitrates and nitrites
1.1.6.2 N-nitroso compounds
1.2. Recommendations for further studies
1.2.1. Analytical problems
1.2.1.1 Nitrates and nitrites
1.2.1.2 N-nitroso compounds
1.2.2. Sources and levels in the environment
1.2.2.1 Nitrates and nitrites
1.2.2.2 N-nitroso compounds
1.2.3. Metabolism
1.2.3.1 Nitrates and nitrites
1.2.3.2 N-nitroso compounds
1.2.4. Experimental studies
1.2.5. Epidemiological and clinical studies
1.2.5.1 Nitrates and nitrites
1.2.5.2 N-nitroso compounds
2. CHEMISTRY AND ANALYTICAL METHODS
2.1. Chemical properties and reactions
2.1.1. Nitrates and nitrites
2.1.2. N-nitroso compounds
2.1.3. Formation of N-nitroso compounds in vitro
2.1.4. The effects of other substances on the formation of
N-nitroso compounds
2.2. Analytical methods
2.2.1. Nitrates and nitrites
2.2.2. N-nitroso compounds
3. SOURCES OF NITRATES, NITRITES AND N-NITROSO COMPOUNDS IN AIR,
WATER, SOIL, AND FOOD
3.1. Natural occurrence
3.1.1. Nitrates and nitrites
3.1.2. N-nitroso compounds
3.2. Sources related to man's activities
3.2.1. Nitrates and nitrites
3.2.1.1 Fertilizers
3.2.1.2 Animal wastes
3.2.1.3 Municipal, industrial, and transport
wastes
3.2.1.4 Deliberate addition of nitrates and
nitrites to food
3.2.2. N-nitroso compounds
3.2.2.1 Food
3.2.2.2 Tobacco
3.2.2.3 Industrial uses
4. TRANSPORT AND TRANSFORMATION IN ENVIRONMENTAL AND BIOLOGICAL
MEDIA
4.1. Nitrogen Cycle
4.2. Transformation in food
4.2.1. Reduction of nitrates to nitrites
4.2.2. Formation and degradation of N-nitroso compounds
4.3. Formation of N-nitroso compounds from drugs and pesticides
4.4. Formation of N-nitroso compounds in animal organisms
4.4.1. Formation of N-nitroso compounds in simulated
gastric juice
4.4.2. Formation of N-nitroso compounds in vivo
4.5. Formation of N-nitroso compounds by microorganisms
4.6. The effects of other chemicals on the formation of
N-nitroso compounds
5. ENVIRONMENTAL LEVELS AND EXPOSURES
5.1. Nitrates and nitrites
5.1.1. Ambient air
5.1.2. Water
5.1.3. Selected foods
5.1.4. Estimate of general population exposure
5.2. N-nitroso compounds
5.2.1. Ambient air
5.2.2. Water
5.2.3. Selected foods
5.2.4. Tobacco and tobacco smoke
5.2.5. Estimate of general population exposure
5.2.6. Occupational exposure to N-nitroso compounds
6. METABOLISM OF NITRATES, NITRITES, AND N-NITROSO COMPOUNDS
6.1. Gastrointestinal absorption
6.1.1. Nitrates and nitrites
6.1.2. N-nitroso compounds
6.2. Biotransformation and elimination
6.2.1. Nitrates and nitrites
6.2.2. N-nitroso compounds
7. EXPERIMENTAL ANIMAL STUDIES ON THE EFFECTS OF NITRATES, NITRITES,
AND N-NITROSO COMPOUNDS
7.1. Nitrates and nitrites
7.1.1. Acute and subacute toxicity studies
7.1.2. Chronic toxicity and carcinogenicity studies
7.1.3. Embryotoxicity
7.1.4. Mutagenicity
7.1.5. Interaction with nutritional factors
7.2. N-nitroso compounds
7.2.1. Acute and subacute toxicity studies
7.2.2. Carcinogenicity
7.2.2.1 Interspecies variation in response
7.2.2.2 Intraspecies variation in response
7.2.2.3 Dose-response relationships of N-nitroso
compounds
7.2.2.4 Tumour induction by combined
administration of nitrites, and amines or
amides
7.2.2.5 Dose-response relationship for
combinations of nitrites and amines
7.2.2.6 Transplacental carcinogenesis
7.2.2.7 Morphological studies
7.2.2.8 Biochemical mechanisms
7.2.2.9 Interaction with various chemical factors
7.2.2.10 Miscellaneous modifying factors
7.2.3. Embryotoxicity and teratogenicity
7.2.4. Mutagenicity
8. EFFECTS OF NITRATES, NITRITES, AND N-NITROSO COMPOUNDS ON MAN
8.1. Nitrates and nitrites
8.1.1. Epidemiological studies
8.1.1.1 Exposure through water
8.1.1.2 Exposure through vegetables
8.1.1.3 High accidental exposures
8.1.1.4 Ambient air exposures
8.1.2. Factors involved in susceptibility to nitrates
8.1.3. Dose-response relationships for nitrates and
nitrites
8.2. N-nitroso compounds
9. EVALUATION OF HEALTH RISKS TO MAN FROM EXPOSURE TO NITRATES,
NITRITES, AND N-NITROSO COMPOUNDS
9.1. Nitrates and nitrites
9.1.1. General considerations
9.1.2. Assessment of health risks
9.2. N-nitroso compounds
9.2.1. General considerations
9.2.2. Assessment of health risks
9.3. Reduction of exposure th
REFERENCES
NOTE TO READERS OF THE CRITERIA DOCUMENTS
While every effort has been made to present information in the
criteria documents as accurately as possible without unduly delaying
their publication, mistakes might have occurred and are likely to
occur in the future. In the interest of all users of the environmental
health criteria documents, readers are kindly requested to communicate
any errors found to the Division of Environmental Health, World Health
Organization, Geneva, Switzerland, in order that they may be included
in corrigenda which will appear in subsequent volumes.
In addition, experts in any particular field dealt with in the
criteria documents are kindly requested to make available to the WHO
Secretariat any important published information that may have
inadvertently been omitted and which may change the evaluation of
health risks from exposure to the environmental agent under
examination, so that the information may be considered in the event of
updating and re-evaluating the conclusions contained in the criteria
documents.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR NITRATES,
NITRITES, AND N-NITROSO COMPOUNDS
Lyons, France, 16-21 February 1976
Participants
Members
Dr. K. R. Bulusu, National Environmental Engineering Research
Institute, Nagpur, India
Mr T. J. Coomes, Food Chemistry, Composition and Safety, Ministry of
Agriculture, Fisheries and Food, London, England (Rapporteur)
Dr R. Kroes, Department of Oncology, National Institute of Public
Health, Bilthoven, Netherlands
Dr W. Lijinsky, Frederick Cancer Research Center, Frederick, MD, USA
Dr S.S. Mirvish, Eppley Institute for Research in Cancer, The
University of Nebraska College of Medicine, Omaha, NE, USA
Professor M. Nikonorow, Department of Food Research, State Institute
of Hygiene, Warsaw, Poland
Dr T. Petrova-Vergieva, Centre of Hygiene, Medical Academy, Sofia,
Bulgaria
Dr R. Preussmann, Institute for Toxicology and Chemotherapy, National
Cancer Research Centre, Heidelberg, Federal Republic of Germany
Dr P. Schmidt, Ministry of Health, Prague, Czechoslovakia
Professor K. Symon, Centre of General and Community Hygiene, Institute
of Hygiene and Epidemiology, Prague, Czechoslovakia (Vice
Chairman)
Professor R. Truhaut, Toxicological Research Centre, Faculty of
Pharmacy and Biology, Paris, France (Chairman)
Representatives of other organizations
Mme M.-Th. van der Venne, Commission of the European Communities,
Health Protection Directorate, Luxembourg
Mr G. Dorin, Natural Resources and Pollution Control Division,
Organization for Economic Cooperation and Development, Paris,
France
Secretariat
Dr L. Griciute, Chief, Unit of Environmental Carcinogenesis, IARC,
Lyons, France
Dr A. Kolbye, Bureau of Foods, US Food and Drug Administration,
Washington, DC, USA
Dr F. C. Lu, Chief, Food Additives, World Health Organization, Geneva,
Switzerland (Secretary)
Dr R. Montesano, Unit of Chemical Carcinogenesis, IARC, Lyons, France
Dr I. C. Munro, Toxicology Research Division, Health Protection
Branch, Department of National Health and Welfare, Ottawa, Canada
Mr E. A. Walker, Unit of Chemical Carcinogenesis, IARC, Lyons, France
a Unable to attend:
Dr E. Arrhenius, Director for Experimental Biology, Wallenberg
Laboratory, Lilla Frescati, Stockholm, Sweden
Dr V. Okulov, Petrov Research Institute of Oncology, Leningrad,
USSR
List of Abbreviations
DMN N-methyl- N-nitrosomethanamine ( N-nitrosodimethylamine,
dimethylnitrosamine)
DEN N-ethyl- N-nitrosoethanamine ( N-nitrosodiethylamine,
diethylnitrosamine)
DMA N-methylmethanamine (dimethylamine)
DEA N-ethylethanamine (diethylamine)
TMA N,N-dimethylmethanamine (trimethylamine)
TMAO trimethyloxamine (trimethylamine oxide)
ENVIRONMENTAL HEALTH CRITERIA FOR NITRATES, NITRITES, AND N-NITROSO
COMPOUNDS
A WHO Task Group on Environmental Health Criteria for Nitrates,
Nitrites and N-nitroso compounds met in Lyons from 16 to 20 February
1976. Dr Higginson, Director of the International Agency for Research
on Cancer opened the meeting on behalf of the Director-General. The
Task Group reviewed and amended the second draft of the criteria
document and made an evaluation of the health risks from exposure to
these compounds.
The preparation of the first draft of the criteria document was
based on national reviews of health effects research on nitrates,
nitrites, and N-nitroso compounds received from the national focal
points for collaboration in the WHO Environmental Health Criteria
Programme in Bulgaria, Canada, Czechoslovakia, the Federal Republic of
Germany, Netherlands, New Zealand, Poland, the United Kingdom, the
USA, and the USSR. Dr I. C. Munro, Toxicological Research Division,
Health Protection Branch of the Department of National Health and
Welfare, Ottawa, Ontario, Canada, prepared both the first draft and
the second draft which took into account the comments received from
the national focal points in Bulgaria, Canada, Czechoslovakia,
Finland, Japan, New Zealand, Poland, Sweden, USA, and the USSR; from
the United Nations Industrial Development Organization (UNIDO), the
Food and Agriculture Organization of the United Nations (FAO), the
United Nations Educational, Scientific and Cultural Organization
(UNESCO), the International Atomic Energy Agency (IAEA), the Health
Protection Directorate of the Commission of the European Communities
(CEC), and from the International Federation of Pharmaceutical
Manufacturers' Associations (IFPMA).
At the request of the Secretariat, comments were also received
from Dr S. Oden, Agricultural College, Department of Soil Science,
Division of Ecochemistry, Uppsala, Sweden.
The collaboration of these national institutions, international
organizations and individual experts is gratefully acknowledged.
Without their assistance this document could not have been completed.
The collaboration of the International Agency for Research on Cancer
in the preparation of the document and in acting as host to the Task
Group is also greatly appreciated.
The Secretariat wishes to thank Mr A. W. Kenny, Department of the
Environment, London, England and Mr D. A. H. Price, Chorley Wood,
Herts, England for their advice in the preparation of some sections of
the document and Dr Munro for his help in the final phases of editing.
This document is based primarily on national contributions and on
original publications listed in the reference section. In addition,
some recent publications reviewing the environmental health aspects of
nitrates, nitrites and N-nitroso compounds have been used. These
include reviews and symposia by the US National Academy of Sciences,
Washington, DC (Committee on Nitrate Accumulation, 1972), the US
Department of Health, Education and Welfare (1970), the US
Environmental Protection Agency,a the International Agency for
Research on Cancer (Bogovski 8: Walker, 1974; Bogovski et al., 1972a;
Walker et al., 1970), Druckrey et al. (1967), Lee (1970a), Magee &
Barnes (1967), Magee et al. (1976), Montesano & Bartch (1976), and Sen
(1974).
Details concerning the WHO Environmental Health Criteria
Programme including the definition of some terms frequently used in
the documents may be found in the general introduction to the
Environmental Health Criteria Programme published together with the
environmental health criteria document on mercury (Environmental
Health Criteria 1 -- Mercury, World Health Organization, Geneva,
1976).
a "US Environmental Protection Agency (1976) Scientific and
technical assessment report on nitrosamines. Preprint of document
submitted for publication in the STAR series, Washington DC,
Office of Reserch and Development, 210 pp.
1. SUMMARY AND RECOMMENDATIONS FOR FURTHER STUDIES
1.1 Summary
1.1.1 Analytical methods
1.1.1.1 Nitrates and nitrites
The Task Group recognized that interpretation of the results of
analyses of nitrates and nitrites in environmental and biological
media would vary according to the analytical methods employed (e.g.
spectrophotometry, spectrofluorimetry, nitrate specific electrode) and
that this would make meaningful comparisons of much data in the
literature difficult.
It was considered, however, that reported figures for water and
for meat products could be compared as far as the assessment of health
hazards was concerned. The Group also noted that, although the
principle underlying a particular method of analysis may well be the
same for a variety of substrates, difficulties usually arise during
the sampling, extraction, and clean-up procedures which vary in
complexity according to the nature of the substrate being analysed.
1.1.1.2 N-nitroso compounds
The detection and estimation of volatile N-nitroso compounds is
complicated by the following basic issues: they are likely to be
present in environmental media in concentrations of only 1 part in
109 parts; they occur in a complex matrix in food and biological
samples many components of which will contain nitrogen and will react
in a similar manner chemically; they must be isolated from this matrix
in a form that permits their estimation and unequivocal
identification. Whilst removal from the matrix is easy in the case of
N-nitroso compounds of low molecular weight because they are steam-
volatile, this approach cannot be used for non-volatile N-nitroso
compounds. Analysis of these compounds has received scant attention so
far, although some work on clean-up procedures exists, and the use of
the liquid chromatography technique is now under investigation
following its successful application to the separation of model
mixtures of N-nitroso- N-alkyl ureas and the analogous urethanes.
Irrespective of the isolation techniques used, the quantitative
determination of N-nitroso compounds requires a concomitant positive
identification of the molecular species determined. For this reason,
the preferred method of analysis, gas-liquid chromatography allied to
a nitrogen-sensitive detector, must be linked to high-resolution mass
spectrometry to confirm the presence of N-nitroso compounds. Results
should be considered positive only when or if mass-spectroscopic
techniques have confirmed their unequivocal presence.
1.1.2 Source and occurrence in the environment
1.1.2.1 Nitrates and nitrites
Nitrates are present naturally in soils, waters, all plant
materials, and in meats. They are also found in small concentrations
(1-40 µg/m3) in air as a result of air pollution. Levels in
cultivated soils and thus, levels in water, (which normally do not
exceed 10 mg/litre) may be increased by the use of commercial
nitrogenous fertilizers and by the return of wastes, derived from
animal husbandry or other sources, to the soil. Nitrate contents of
crops are influenced by the plant species, by genetic and
environmental factors, and by agricultural management practices. In
certain crops the levels may be very high (1000 mg/kg or more).
Nitrites are formed in nature by the action of nitrifying
bacteria as an intermediate stage in the formation of nitrates, but
concentrations in plants and water are usually very low. However,
microbiological conversion of nitrate to nitrite may occur during the
storage of fresh vegetables, particularly at room temperature, when
nitrite concentrations may rise to exceptionally high levels (about
3600 mg/kg dry weight). Both nitrates and nitrites are widely used in
the production and preservation of cured meat products and of some
fish. Such uses, which are controlled by law in many countries, are
considered vital for the prevention of botulism caused by the growth
of the toxin-producing strains of Clostridium botulinum that are
sometimes present in raw meat and that may persist in cooked meats.
The weekly intake of nitrates by a member of the general population in
England or in the USA has been roughly estimated to average about
400-500 mg but these figures cannot be applied generally because of
variations in feeding habits and in the nitrate concentrations in food
and water.
1.1.2.2 N-nitroso compounds
Low concentrations of N-nitroso compounds have been detected in
air, water, and food, notably in nitrite-treated meat products and
certain fish products. In most cases, the concentrations found in food
have been in the µg/kg range. No effective estimate of general
population exposure to N-nitroso compounds can be made on the basis
of these limited data. N-methyl- N-nitrosomethanamine
( N-nitrosodimethylamine, DMN) has been detected in urban air
samples and the presence of N-nitroso compounds, tentatively
identified as N-nitroso derivatives of some pesticides, has been
reported in samples from water treatment plants and river water in
the USA.
The in vivo formation of N-nitroso compounds from nitrates or
nitrites and amines or amides has been demonstrated in experimental
animals and in one case in man.
1.1.3 Metabolism
1.1.3.1 Nitrates and nitrites
In normal healthy individuals, nitrates and nitrites are rapidly
absorbed from the gastro-intestinal tract. Absorbed nitrite reacts
with haemoglobin to form methaemoglobin which, in adults, is rapidly
converted to oxyhaemoglobin by reducing systems such as NADH-
methaemoglobin reductase. In infants up to three months old and in
very young animals this enzyme system is not completely developed.
Under these conditions, the methaemoglobin formed may increase in the
body resulting in a characteristic clinical condition
(methaemoglobinaemia). Microorganisms present in the food and
gastrointestinal tract of very young infants may convert nitrates to
nitrites and thus exacerbate the problem in this age group. In healthy
individuals, absorbed nitrates are rapidly excreted by the kidneys.
1.1.3.2 N-nitroso compounds
Published information on the absorption, metabolism, and
elimination of N-nitroso compounds is limited. In cases where
experimental animal data are available, they demonstrate that
N-nitroso compounds are rapidly absorbed from the gastrointestinal
tract and that their biological half-time appears to be less than
24 h. A part of some compounds may be excreted unchanged via the
kidney, or even exhaled, but the greater part is metabollically
transformed (hydroxylation, chain shortening, ring-opening etc.) and
several metabolites of N-nitroso compounds have been identified
in urine. Significant amounts of some compounds such as DMN may be
degraded completely and the resulting carbon dioxide exhaled. The
extent of such degradation varies depending on the structure of the
compound and the animal species involved.
1.1.4 Experimental studies in animals
1.1.4.1 Nitrates and nitrites
The major effect of nitrates and nitrites is the induction of
methaemoglobinaemia, mostly readily observed in very young animals.
Most experimental work has been connected with this problem although
embryotoxic effects resulting in prenatal mortality, resorptions, and
decreased birthweights have been noted in rat pups whose mothers
received drinking water containing sodium nitrite. In adult rats given
drinking water containing nitrite for 24 months, methaemoglobin levels
were elevated but not to the point of producing overt toxic effects.
Animal species studied appeared to be fairly resistant to the
induction of methaemoglobinaemia by nitrites, since high doses were
required to induce even minimal changes. However, very young animals
have not been studied extensively or sufficiently. Nitrates and
nitrites do not appear to be carcinogenic but nitrite mutagenicity has
been demonstrated in several non-mammalian test systems.
1.1.4.2 N-nitroso compounds
In experimental animals, the most important biological actions of
N-nitroso compounds are their carcinogenicity and teratogenicity.
The carcinogenic action of N-nitroso compounds in animals is
known to occur in many different organs. In general, the routes of
administration do not influence the localization of the tumours.
However, both dose level and dose rate may affect the organ involved
and the type of tumour produced. Specific structural changes in both
dialkyl nitrosamines and cyclic nitrosamines affect their
carcinogenicity. N-nitroso compounds have been shown to be
transplacentally carcinogenic, when given to animals in the second
part of gestation, irrespective of the route of administration.
Carcinogenicity following the combined administration of amines or
amides and nitrites to animals has also been reported indicating the
in vivo formation of N-nitroso compounds.
The mutagenic action of nitrosamides, noted in test systems,
differs from that of nitrosamines in that the first group of compounds
has been found to be mutagenic in almost all test systems, whereas
nitrosamines seem only to be active in systems where metabolic
activation occurs.
Nitrosamines are known to have toxic and sometimes lethal effects
on animal embryos, whereas nitrosamides cause malformations in several
organs and systems.
1.1.5 Epidemiological studies
1.1.5.1 Nitrates and nitrites
Adults do not appear to be harmed directly by exposure to the
prevailing concentrations of nitrates and nitrites in the environment,
although some recent studies have indicated that nitrate aerosols in
the ambient air may act as respiratory irritants. However infants and
very young children are particularly susceptible to the induction of
methaemoglobinaemia by nitrates and nitrites, ingested in water and
food, and several cases of illness and death have been reported. In
most cases of methaemoglobinaemia, well-water containing high
concentrations of nitrates had been used in the reconstitution of
infant dried milk preparations. Most instances have been associated
with water containing more than 90 mg per litre but a few cases of
methaemoglobinaemia in infants have been associated with the
consumption of water containing less than 50 mg per litre. Cases of
methaemoglobinaemia in babies fed with spinach purée or carrot juice
(both of which may contain very high levels of nitrates) have been
reported, but there are too few data to establish dose-response
relationships.
1.1.5.2 N-nitroso compounds
So far, correlations have not been established that link cancer
in man with exposure to N-nitroso compounds or their precursors, but
the possible role of N-nitroso compounds and in particular their
in vivo formation in the development of nasopharyngeal, oesophageal,
and stomach cancer has been suggested.
1.1.6 Evaluation of health risks
1.1.6.1 Nitrates and nitrites
Epidemiological and clinical studies on man have shown that the
main toxic manifestation resulting from the ingestion of nitrates and
nitrites is methaemoglobinaemia. This has been confirmed by
experimental animal studies. On the basis of available data, the Task
Group concluded that the prevailing concentrations of nitrates and
nitrites in food and water did not constitute a health risk for adult
members of the general population and older children, but that the
risk may be higher for infants under 6 months of age and particularly
under 3 months. For this reason, the Group recommended that infant
dried milk preparations should be reconstituted with low-nitrate water
(at least below 45 mg/litre) and that low-nitrate vegetables should be
used in baby foods.
Also, the use of nitrates and nitrites as food additives should
be reduced to the minimum, and avoided in fresh meat and fish. Nitrate
levels in public water supplies should comply with the tentative limit
of 45 mg/litre recommended by the 1971 International Standards for
Drinking Water.
1.1.6.2 N-nitroso compounds
Although the precursors of N-nitroso compounds (nitrites,
amines, and amides) are known to be widely distributed in various
environmental media, information concerning N-nitroso compounds is
limited. However, they are known to be present in certain foods and
experimental animal studies have shown that they are formed in the
body from a variety of precursors. This may also occur in man.
N-nitroso compounds are carcinogenic in a wide range of animal
species, most are mutagenic in test systems, and some have been shown
to be teratogenic in animals.
Although there is no epidemiological or clinical evidence at
present, it is highly probable that these compounds may also be
carcinogenic in man. A quantitative estimation of the carcinogenic
risk to man associated with different levels of exposure is not
possible, at this time, because of inadequate data. For these reasons,
exposure to N-nitroso compounds and their precursors, (nitrites,
amines, and amides) should be kept as low as practically achievable.
1.2 Recommendations for Further Studies
1.2.1 Analytical problems
1.2.1.1 Nitrates and nitrites
The major need is for standardization of analytical methods. At
present, it is difficult to compare the studies reported by one
laboratory with those reported by another. While in many instances the
principle underlying the determination is the same for many of the
studies reported, the large variety of substrates containing nitrates
and nitrites gives rise to difficulties with respect to sampling,
extraction, and cleanup procedures. Further efforts are needed to
standardize these analytical procedures on an international basis. To
this end, the efforts of international and regional groups should be
supported.
1.2.1.2 N-nitroso compounds
The principal problem associated with the determination of
N-nitroso compounds in food and other environmental media results
from interference by other components of the substrates. At present,
positive identification of N-nitroso compounds can be made only by
mass spectroscopie techniques. Since such techniques are expensive and
not generally available, alternative methods are required. In
addition, methods for the detection and determination of nonvolatile
N-nitroso compounds should be developed further.
1.2.2 Sources and levels in the environment
1.2.2.1 Nitrates and nitrites
Research should be undertaken to find acceptable substitutes for
nitrates and nitrites in the preservation of certain foods such as
canned meats.
National surveys of nitrate levels in soils, water, plant
materials, foods, especially meat and milk products, and air are
required together with quantitative data concerning other factors
considered to have an effect on these levels. Similar information on
nitrite levels is required with particular reference to foods and to
areas where significant microbiological reduction of nitrates is
likely.
It is important that levels determined in survey work of this
nature should be reported on the basis of standardized analytical
methods to facilitate the eventual comparison of data from all
sources. National authorities should be encouraged to publish survey
data or to communicate them to the World Health Organization.
1.2.2.2 N-nitroso compounds
National surveys of food, air, and water for the presence of
volatile and, where possible, nonvolatile N-nitroso compounds are
required and any results reported should be confirmed by mass
spectroscopy. More studies are needed on the chemical conditions under
which N-nitroso compounds are formed (e.g. in mixtures of nitrites
and amines or amides). The use of ascorbic acid for the prevention of
nitrosamine formation and the inhibitory or catalytic effect of food
constituents on the formation of N-nitroso compounds also require
studies. The role of oxides of nitrogen as possible nitrosating agents
should be investigated in relation to the occurrence of N-nitroso
compounds in the environment (e.g. in the ambient and workroom air).
1.2.3 Metabolism
1.2.3.1 Nitrates and nitrites
Further work on the influence of ascorbic acid and other
ingredients of the stomach contents on the metabolism of nitrates and
nitrites is required. The treatment of infant dried milk formulae with
ascorbic acid or by the introduction of Lactobacilli to prevent
nitrate reduction should also be studied.
Other areas requiring investigation include: the influence of
gastro-enteric disease on the development of methaemoglobinaemia; the
influence of the total gut flora on nitrate metabolism in vivo; the
relationship between ingested nitrate and salivary nitrate and nitrite
levels.
1.2.3.2 N-nitroso compounds
More knowledge should be gained on the in vivo formation of
N-nitroso compounds in man and the factors involved. Studies
comparing the metabolism of N-nitroso compounds in experimental
animals and in man are considered to be of the greatest importance.
1.2.4 Experimental studies
Further research on the biological action of N-nitroso
compounds should concentrate on dose-response relationships especially
at low levels, and on their combined effects with other carcinogens,
and environmental pollutants. The influence of nutritional factors on
the carcinogenicity of N-nitroso compounds should be studied in more
detail.
More inhalation studies are necessary to assess the importance of
the recently reported occurrence of N-nitroso compounds in air and
further research is needed on the quantitative aspects of the
mutagenic activity of N-nitroso compounds and its possible
significance for man.
1.2.5 Epidemiological and clinical studies
1.2.5.1 Nitrates and nitrites
With respect to the adverse effects of nitrates and nitrites on
infants, there is a need to investigate the relationship between
methaemoglobinaemia and sudden infant death and to make further
studies on the role of gastroenteritis in increasing infant
susceptibility to nitrate poisoning. The role of acidified milk
preparations and Lactobacilli in protecting infants against
methaemoglobinaemia, and the possible protective role of ascorbic acid
fortification of infant milk preparations should also be elaborated.
1.2.5.2 N-nitroso compounds
Prospective and retrospective epidemiological studies in man,
exposed to N-nitroso compounds, are needed. Efforts should be made
to determine whether cancers, that are peculiar to special areas of
the world, might be due to exposure to N-nitroso compounds. Chemical
analyses of the environment for N-nitroso compounds and their
precursors should be carried out in conjunction with these
epidemiological studies.
2. CHEMISTRY AND ANALYTICAL METHODS
2.1 Chemical Properties and Reactions
2.1.1 Nitrates and nitrites
The nitrate ion (NO3-) is the conjugate base of nitric acid
(HNO3). Nitric acid is a strong acid (pKa = -1.37) which
dissociates in water yielding nitrate ions and hydroxonium ions
(H3O+). Salts of nitric acid (nitrates) are readily soluble in
water with the exception of the basic nitrates of mercury and bismuth.
The nitrite ion is the conjugate base of nitrous acid (HNO2)
which is a weak acid (pka = 3.37) and exists only in cold dilute
aqueous solution because it decomposes readily to give water and
dinitrogen trioxide (N2O3) or nitric acid, nitric oxide (NO), and
water. Salts of nitrous acid (nitrites) are much more stable than the
acid itself and are readily soluble in water with the exception of
silver nitrite.
In the environment (e.g. surface waters, soil) both nitrite and
nitrate ions can be formed from the ammonium ion (NH4+) in a two
step biological oxidation (nitrification) process:
2 NH4+ + 2OH- + 3O2 <=> 2 NO2- + 2H+ + 4H2O (1)
2 NO2- + O2 <=> 2 NO3- (2)
These two reactions are mediated by different microorganisms:
reaction (1) by an aerobic chemolithotroph Nitrosomonas; reaction
(2) by Nitrobacter which obtains almost all its energy from the
oxidation of nitrites.
Higher plants assimilate nitrite from the soil by (a) reduction
of nitrate to nitrite which is catalysed by nitrate reductase (NADPH)
(1.6.6.3), and (b) reduction of nitrite to ammonia catalysed by
nitrite reductase (1.7.99.3). Bacteria of many kinds can also reduce
nitrate to nitrite. However, because nitrite is easily oxidised to
nitrate the concentration of nitrites in environmental media such as
surface waters is usually very low (about 1 mg/litre) even when the
nitrate concentration is high (50-100 mg/litre).
These biochemical reactions are a part of the nitrogen cycle
which is further discussed in section 3.1.
2.1.2 N-nitroso compounds
N-nitroso compounds have a general structure
R1
\ N-N=O
R2/
They can be divided into two classes with different chemical
properties (Druckrey et al., 1967; Fridman et al., 1971):
(1) nitrosamines where R1 and R2 are alkyl or aryl groups;
and
(2) nitrosamides where R1 is an alkyl or aryl group, and R2
is an acyl group.
Nitrosamines are generally stable compounds that only slowly
decompose in the light or in aqueous acid solutions.
In contrast, nitrosamides are much less stable in aqueous acids
and unstable in basic solutions. Examples of nitrosamides are N-
alkyl- N-nitrosoureas (3) and N-alkyl- N-nitrosourethanes (4).
R - N - C - NH2 (3)
' "
NO O
R - N - C - OC2 H5 (4)
' "
NO O
The physical properties of N-nitroso compounds vary widely depending
on the substituent groups. Some like N-methyl- N-nitrosomethanamine
(dimethylnitrosamine, DMN) are oily liquids miscible with polar
solvents. Some are solids e.g. N-nitroso- N-phenylbenzenamine
(diphenylnitrosamine) and are only slightly soluble in ethanol and
practically insoluble in water. The lipid/water partition coefficients
vary widely. Nitrosamines show ultraviolet absorption peaks in water
at 230-240 nm and 330-350 nm. For nitrosamides, the long-wavelength
absorption peak in water is at 390-420 nm. Some N-nitroso compounds
are volatile (Mirvish, 1975, 1976; Sen, 1974). Physical properties of
N-nitroso compounds have been listed by Druckrey et al. (1967),
Fieser & Fieser (1967), and Weast (1976).
Nitrosamines may react by "transnitrosation" i.e. as nitrosating
agents to nucleophilica species (Buglass et al., 1974). This
reaction may have important biological implications.
a i.e. electron-rich.
2.1.3 Formation of N-nitroso compounds in vitro
The formation of N-nitroso compounds from amines and nitrites
has been reviewed by Mirvish (1975), Sander (1971a, 1971b), and Sander
& Schweinesberg (1972).
For example, for N-methylmethanamine (dimethylamine) (DMA) and
sodium nitrite in dilute hydrochloric acid solutions, nitrositation is
considered to proceed as follows (Mirvish, 1970):
NaNO2 + HCl <=> HNO2 + NaCl (5)
2HNO2 <=> N2O3 + H2O (6)
(CH3)2NH + N2O3 <=> (CH3)2HN - NO + NO2- (7)
The reaction rate depends on the concentration of nonionized
amine and nitrous acid. At pH > 1, the main nitrosating agent is
dinitrogen trioxide which is formed reversibly from 2 molecules of
nitrous acid. The rate of reaction (7) is proportional to the
concentration of dinitrogen trioxide, [N2O3], and hence to the
square of nitrous acid concentration, [HNO2]2, i.e.
rate (7) = k1[N2O3] [HNO2]2 (8)
The concentrations of nonionized amine and of free nitrous acid vary
with pH but k1, is independent of pH. For practical purposes it is
more convenient to rewrite equation (8) in terms of the total
concentrations of nitrite and DMA i.e.
rate (3) = k2 [total amine] [total nitrite]2 (9)
where k2 depends on pH; k2 and the reaction rate show maximum
values at pH = 3.4 corresponding to the strength of nitrous acid
(pka = 3.37). The reaction rate decreases tenfold for each 1-unit
increase in pH above pH = 3.4. Below this pH level, the nitrite is
almost completely converted to nitrous acid. The main effect of a
further reduction in pH is a continuous drop in nonionized amine
concentration, causing a decrease in the reaction rate. There is no
sharp pH limit for nitrosation. It can occur slowly at a pH of 5 or
even 6, as observed for DMA (Mirvish, 1970).
The nitrosation of amides, such as N-alkylureas and
N-alkylurethanes proceeds rapidly (Challis & Challis, 1970;
Mirvish, 1971; Sander & Burkle, 1969). In this case the nitrosating
agent is probably the nitracidium ion (H2NO2)+:
2HNO2 <=> (H2NO2)+ + NO2- (10)
or
HNO2 + H+ <=> (H2NO2)+ (10a)
and nitrosation is accomplished by the following reaction:
RNH.COR' + (H2NO2)+ -> RN(NO).COR' + H2O + H+ (12)
The reaction rate is again proportional to the concentrations of
nonionized alkylurea and nitracidium ions the formation of which can
be considered to proceed by equation (10a). Hence
rate (12) = k3 [RNH.COR'] [HNO2] [H+] (13)
or
rate (12) = k4 [total amide] [total nitrite] [H+] (14)
The reaction rate, which increases about tenfold for each 1-unit drop
in pH from 3 to 1, does not show a pH maximum; k4 depends on the
ionization of nitrite and, hence, on pH but it does not depend on the
ionization of amides, which are only slightly ionized above pH = 2.
Tables giving the rate constants for 15 amines and 21 amides
according to the above equations (Mirvish, 1975), indicate that the
most rapidly nitrosated classes of compounds are the N-alkylureas,
N-arylureas, N-alkylcarbamates, secondary aromatic amines,
secondary amine piperazine, morpholine derivatives, and tertiary
enamines.
It has been suggested that under mildly acidic conditions
tertiary amines also react with nitrous acid to produce nitrosamines
(Hein, 1963; Lijinsky, 1974; Lijinsky & Greenblatt, 1972; Lijinsky &
Singer, 1974; Lijinsky et al., 1972b; Roberts & Caserio, 1964; Smith &
Loeppky, 1967). Ender et al. (1967) studied the reaction between
nitrites and various methylamines including: methanamine
(monomethylamine); DMA; N,N-dimethylmethanamine (trimethylamine,
TMA); and trimethyloxamine (trimethylamine oxide, TMAO); they found
that DMN was produced in all cases. However, the rate of production
was proportional to the amount of nitrite present and increased with
decreasing pH values and increasing temperature. DMA was the most
reactive followed by TMA. Small amounts of DMN were formed from DMA
and sodium nitrite under very mild conditions (e.g. at 4°C). At
pH = 6.0, 2 to 2.5 times more DMN was formed than at pH = 6.5.
However, Malins et al. (1970), who failed to detect DMN formation at
pH levels of 5.8-6.4 after heating an aqueous mixture of sodium
nitrite and TMAO or DMA, found that trace amounts of DMN were
detectable in reaction mixtures consisting of TMA at concentrations of
400-2000 mg/litre and sodium nitrite at 400 mg/litre.
Fiddler et al. (1972) demonstrated the formation of DMN from
quaternary ammonium compounds and nitrite. The compounds studied
included N,N,N-trimethylethaminium chloride (neurine chloride),
2-(acetyloxy)- N,N,N-trimethylethanaminium chloride (acetylcholine
chloride), choline chloride, 1-carboxy- N,N,N-trimethylmethanaminium
hydroxide (betaine), and 3-carboxy-2-hydroxy- N,N,N-trimethyl-1-
propan-aminium chloride (carnitine chloride).
Nitrites are present in various foods and in saliva (Tannenbaum
et al., 1974) and can be formed in the infected bladder by bacterial
reduction. They may also be present in the stomach of infants and of
achlorhydric subjects where they are formed from nitrates, lower
acidity allowing the growth of nitrate-reducing bacteria.
Secondary amines are widely distributed in foods and have been
found in fish, eggs, rolls, biscuits, chocolate, soup cubes, meats,
and potatoes (Heyns, 1973, Lijinsky & Epstein, 1970). Tobacco and
tobacco smoke contain several secondary amines including pyrrolidine,
DMA, and piperidine (Neurath, 1972). Some aliphatic and heterocyclic
amines were identified in human blood and urine (Asatoor & Simenhoff,
1965; Perry et al., 1962). Other sources of secondary amines have been
given by Sander et al. (1971).
Methylguanidine, a natural constituent of beef (Kapeller-Adler &
Krael, 1930a) and shark, rayfish, and cod (Kapeller-Adler & Krael,
1930b), reacted with nitrite to produce N-methyl- N-nitrosourea and
N-methyl- N-nitrosocyanamide (Mirvish, 1971). The amino acids
1-proline, 1-hydroxyproline, and N-methylglycine (sarcosine) were
nitrosated 140-230 times more quickly than DMA at pH = 2.2-2.5
(Mirvish et al., 1973a).
N-nitroso compounds formed from 22 natural compounds were
listed by Mirvish (1975). In addition, nitrosation of N,N-bis (3
aminopropyl)-1, 4-butanediamine (spermine) and spermidine, two
polyamines, was reported by Ferguson et al. (1974) and Hildrum et al.
(1975).
2.1.4 The effects of other substances on the formation of N-nitroso
compounds
Several substances have been shown to catalyse the formation of
nitroso compounds from secondary amines and nitrite. Boyland & Walker
(1974) and Fan & Tannenbaum (1973) noted that chloride, bromide,
iodide, and thiocyanate catalysed the reaction while sulfate and
perchlorate ions did not have any effect. The effects of thiocyanate
have been studied more extensively; in its absence, the nitrosation of
N-methylbenzenamine ( N-methylaniline) and other secondary amines
is at a maximum at pH = 3, but in its presence, the reactions proceed
much more rapidly between pH levels of 1 and 2. Thiocyanate is present
in amounts of 110-330 mg/litre in human saliva. It has been estimated
that the thiocyanate concentration in the stomach is 3 times higher in
smokers than in nonsmokers.
Roller & Keefer (1974) reported a pronounced increase in the rate
of formation of DMN from DMA and nitrite in the presence of certain
carbonyl compounds and at a pH level higher than 3. Formaldehyde was
the most effective catalyst and the effect was appreciable even at
pH = 9. Challis & Bartlett (1975) reported that 3-[[3-(3,4-
dihydroxyphenyl)-1-oxo-2-propenyl]oxy]-1,4,5-trihydroxycyclohexane-
carboxilic acid (chlorogenic acid), a constituent of coffee was a
potent catalyst and in studies by Walker et al. (1975) 3,4,5-
trihydroxybenzoic acid (gallic acid) catalysed the nitrosation of
amines but only within a restricted pH range (around pH = 4).
On the other hand, Bogovski et al. (1972b) noted that tannins,
which are present in many foods, competed with secondary amines for
nitrite and thus led to a reduction in the amount of nitrosamine
formed. Similarly Challis (1973) demonstrated the preferential
nitrosation of phenols in the presence of amine to form
p-nitrosophenols suggesting a scavenging effect of phenols at
low pH.
Ascorbic acid inhibited the formation of DMN from oxytetracycline
and nitrite and also from aminophenazone (aminopyrine) and nitrite
(Mirvish et al., 1972, 1974). The same authors reported that gallic
acid, the active ingredient in tannins, completely inhibited
nitrosomorpholine formation from the parent amine and nitrite and that
sodium sulfite had a similar blocking activity.
The inhibitory effects of ascorbic acid and other inhibitory
agents on chemical nitrosation have recently been compared by Mirvish
et al., 1975 and it would seem, at present, that ascorbic acid is the
most effective and useful inhibitor of amine nitrosation.
2.2. Analytical Methods
2.2.1 Nitrates and nitrites
Methods for the determination of nitrates and nitrites in surface
and waste waters have been reviewed by Marculescu (1971). The most
suitable methods are colorimetric procedures using sodium salicylate
for nitrates and 4-aminobenzenesulfonic acid (sulfanilic acid) and
1-naphthalenamine (l-naphthylamine) for nitrites.
A standard procedure for determining nitrates in plants (HMSO,
1973) is based upon the reduction of nitrates to ammonia which is
removed by steam distillation and determined titrimetrically. The
nitrate electrode has been used in the determination of nitrates in
extracts of soils and herbage, and drainage water in the United
Kingdom (HMSO, 1974) and in the Federal Republic of Germany (Weil &
Quentin, 1973). Results indicated that several extraction procedures
applied to herbage gave higher values with the nitrate electrode than
with the standard distillation procedure. For drainage waters, better
agreement was obtained between the electrode and a spectrophotometric
procedure involving, 3,4-xylenol.
A variety of methods is available for the determination of
nitrates and nitrites in foods. A nitrate specific electrode for the
electrochemical determination of nitrate in spinach suspensions was
tested by Voogt (1969). Other anions Present in the spinach did not
have any direct influence on the precision of the results. Variations
in nitrate activity due to variations in the ionic strength of the
spinach extracts could be minimized by measuring the potential of the
extract in a 1% sodium sulfate solution. The precision of the method
was ± 2%. Kamm et al. (1965) developed a new method for the
determination of nitrates and nitrites in foods that would accurately
determine concentrations as low as 1 mg/kg. 1-Naphthylamine was
diazotized by nitrite and coupled with excess amine to give 4-(1-
naphthylazo)-1-naphthylamine which was measured spectrophoto-
metrically. Nitrate was quantitatively reduced by passage through a
cadmium column and determined as nitrite. Nitrite passed through the
column unaltered; thus nitrate was determined by difference.
Spectrophotometric and spectrofluorimetric methods for the
determination of low levels of nitrite in cheese were developed by
Rammel & Joerin (1972). The limits of detection for the two methods
were 50 µg and 3.0 µg of nitrite-N respectively, per kg of cheese or
milk products. A method to determine free and bound nitrite in meats
was published by Mirna (1974). Free nitrite was determined with the
Griess reagent whereas bound nitrite was liberated with Hg2+ in
aqueous acetone solution prior to diazotization. Methods of analysis
for nitrates and nitrites in several food products including meats,
cured meats, dry cure mix of curing pickle, flours, and baby foods
have been described (Horwitz, 1975) and adequate methods for the
determination of nitrates and nitrites in urine and blood are also
available (Shechter et al., 1972; Schneider & Yeary, 1973; Wegner,
1972).
2.2.2 N-nitroso compounds
The problems of estimating N-nitroso compounds in food and
other environmental media were recently reviewed by Bogovski & Walker
(1974), Bogovski et al. (1971a), Eisenbrand (1973), Fiddler (1975),
Scanlan (1975), Sen (1974), and Walker et al., (1976). The analytical
process can be divided into three major steps: extraction and
distillation from the specimen; purification; and qualitative and
quantitative determination.
The main difficulties in such analyses arise from the fact that
nitrosamines occur at very low concentrations and that they lack
suitable characteristics for trace analysis. They also suffer from
interference from other chemicals in the substrate which gives rise to
a considerable number of false-positive reports of the presence of
nitrosamines.
Most of the nitrosamines so far detected in foods are steam
volatile. Many analytical methods take advantage of this fact and, in
most of them, nitrosamines are isolated by distillation from an
aqueous, acidic, or basic solution. Distillation from an acidic
solution has the additional advantage of removing interfering amines.
Howard et al. (1970) digested fish samples with methanolic potassium
hydroxide before subjecting them to distillation. Telling et al.
(1971) reported improved recoveries of nitrosamines by vacuum
distillation. Other workers (Kröller, 1967; Sen et al., 1969a, 1972)
preferred initial extraction of the nitrosamines with ether or
methylene chloride prior to aqueous distillation. However, these
techniques cannot be used for the analysis of nonvolatile nitrosamines
such as nitrosoproline and nitrososarcosine.
Various column chromatographic procedures have been reported for
the clean-up of nitrosamines isolated from foods and biological
materials. These include ion-exchange (Alam et al., 1971a, 1971b; Sen
et al., 1969a, 1969b) or basic alumina columns (Sen, 1970; Sen et al.,
1970; Telling et al., 1972). These preliminary clean-ups have proved
to be extremely useful in cases where nitrosamines were estimated by
the conventional thin layer chromatography (TLC) and gas-liquid
chromatography (GLC) methods but such clean-ups were thought to be
unnecessary when a highly specific method such as high resolution gas-
liquid chromatography-mass spectroscopy (GLC-MS), was used for the
analysis.
Detection techniques can be divided into screening methods
suitable for routine surveys, and confirmation techniques to be used
if the results of the preliminary screening technique are positive.
Combined high resolution GLC-MS is believed to be the only reliable
confirmation technique available at the moment.
Eisenbrand & Preussman (1970) have described a colorimetric
technique in which nitrosamines are cleaved to nitrosyl bromide and
secondary amines, and the liberated NO+ ion is measured
colorimetrically after reacting with N-1-naphthalenyl-1,2-
ethanediamine (N(naphthyl-(1)-) ethylenediamine). The technique
appears to be reliable and applicable to a wide variety of
nitrosamines. The aminies formed after splitting may also be used to
estimate nitrosamines through the formation of fluorescent
5-(dimethylamino)-1-naphthalenesulphonyl (dansyl) derivatives.
Various TCL methods have been used for the detection and semi-
quantitative estimation of nitrosamines (Eisenbrand & Preussman, 1970;
Kröller, 1967; Möhler & Mayrhofer, 1968; Sen & Dalpé, 1972; Sen et
al., 1969a, 1973a; Yang & Brown, 1972). Most of these methods are
based on the principle of splitting the nitrosamines by UV radiation
into the parent secondary amines and nitrous acid, and subsequently
detecting these breakdown products with 2,2-dihydroxy-IH-indene-
1,3(2H)-dione (ninhydrin) N-phenylbenzeneamine (diphenylamine), and
Griess reagent, respectively. In some methods, nitrosamines are
reduced to hydrazines which are detected on TLC plates after the
formation of suitable derivatives.
GLC offers a rapid and sensitive technique for the analysis of
nitrosamines. In earlier work, the flame ionization detector was used
but it was later abandoned because of the lack of sensitivity, and
specifity. In more recent studies, various nitrogen-specific detectors
have been used such as the alkaline-flame ionization detector (Fiddler
et al., 1971; Howard et al., 1970; Kawabata, 1974), the Coulson
electrolytic conductivity detector (Crosby et al., 1972; Issenberg &
Tannenbaum, 1972; Panalaks et al., 1972; Rhoades & Johnson, 1970; Sen
et al., 1972, 1973a), and the microcoulometric detector (Newall &
Sisken, 1972). Each has some advantages and disadvantages, and the
reader is advised to consult the original papers for further details.
In one technique, nitrosamines were oxidized to the corresponding
nitramines which were then detected by an electron capture detector
(Althorpe et al., 1970; Castegnaro et al., 1972; Sen, 1970; Telling,
1972). Alliston et al. (1972) and Eisenbrand (1972) converted the
nitrosamines to the parent amines from which the heptafluoro-butyryl
derivatives were prepared and determined by electron capture detector.
Recently, Fine & Rufeh (1974) and Fine et al. (1974) have
reported a new instrument which is specific to the N-nitroso
functional group and is capable of detecting N-nitroso compounds in
foodstuffs at the µg/kg level with little or no concentration or
purification. In this technique, the N-nitroso compounds are cleaved
at the N-NO bond in the presence of a specific catalyst, and the
liberated NO is converted to excited nitrogen dioxide (NO2*) by
reaction with ozone. As the excited nitrogen dioxide rapidly decays,
it emits light in the near infrared region of the spectrum which can
be detected and measured. The instrument can be coupled either to a
GLC or a high pressure liquid chromatograph thus making it suitable
for the analysis of both volatile and nonvolatile nitrosamines.
The nonvolatile nitrosamines constitute a more varied group of
compounds than the volatile nitrosamines and, as such, cause
additional analytical problems. Nitrosoamino acids, such as
nitrososarcosine, nitrosoproline, and nitroso-2-hydroxyproline may be
analysed by conversion to volatile derivatives such as silyl ethers
(Eisenbrand et al., 1975).
Methods have been proposed for the determination of total
N-nitroso compounds, the general approach being to cleave the nitroso
group and measure the nitric oxide formed. Fan & Tannenbaum (1971)
eliminated the problem of nitrate interference by using long-wave
ultraviolet irradiation (360 nm) to split the nitroso group. The
released nitrite was diazotized and coupled to form a dyestuff before
colorimetric estimation. The method was designed for automation.
Eisenbrand & Preussmann (1970) used hydrobromic acid in a nonaqueous
medium to split the nitroso group. A method for splitting the nitroso
group which does not require any anhydrous medium has been proposed by
Fine et al. (1976a).
3. SOURCES OF NITRATES, NITRITES AND N-NITROSO COMPOUNDS IN AIR,
WATER, SOIL AND FOOD
3.1 Natural Occurrence
3.1.1 Nitrates and nitrites
Nitrates in soil and in surface and groundwater result from the
natural decomposition by microorganisms of organic nitrogenous
material such as the protein in plants, animals, and animal excreta.
The ammonium ion formed is oxidized to nitrites and nitrates (section
2.1.1). Natural occurrence of nitrates and nitrites in the environment
is a consequence of the nitrogen cycle (section 4.1) but normally
nitrites are only found in very low concentrations.
3.1.2 N-nitroso compounds
Systematic studies on the natural occurrence of N-nitroso
compounds have not been reported but a few studies show that these
compounds may occur in certain microorganisms (Murthy et al., 1966;
Vavra et al., 1960) and in one variety of mushroom (Hermann, 1960). At
least one of these compounds, strephozotrin, is a potent carcinogen
(Arison & Feudale, 1967; Sibay & Hayes, 1969). Other reports on the
natural occurrence of diethylnitrosamine (DEN) in certain plants have
still to be confirmed by modern analytical methods.
3.2 Sources Related to Man's Activities
3.2.1 Nitrates and nitrites
3.2.1.1 Fertilizers
Artificial fertilizers, a major source of environmental nitrates,
may be composed of a variety of chemicals including ammonium, calcium,
potassium, and sodium nitrates, and urea. The production of
nitrogenous fertilizers in the world has increased in terms of N from
15.8 million tonnes in 1961/62-1965/66 to 42.3 million tonnes in
1974/75 (United Nations, 1976).
The fact that plants cannot use soil nitrogen completely is of
great significance; nitrogen utilization may vary from 25 to 85%
depending on the crop and on agricultural techniques. Thus, to obtain
maximum production, a great excess of nitrogen fertilizer must be
applied to the soil and the resulting nitrogen runoff will be
substantially increased. For example, Kohl et al. (1971) showed that
as much as 55-60% of the nitrogen input in the Sangamon River feeding
Lake Decatur, IL, USA, was of fertilizer origin. Lee (1970b), Sawyer
(1947), and Sylvester (1961), have all published data showing that
nitrogen runoff is 3-10 times higher from fertilized areas than from
unfertilized areas in the same region. However, analysis of stream
waters did not show a clear relationship between the nitrate
concentrations in British rivers and the amounts of fertilizers used
on adjacent land (Tomlinson, 1970).
Brown & Smith (1967) observed that nitrogen fertilization tended
to increase the nitrate content of vegetables and attempts have been
made to correlate nitrogen application rates with the nitrate contents
of lettuce, radish, and spinach. In studies in Bulgaria, Biocev &
Pocinkova (1972) noted that the nitrate levels in spinach increased
when as little as 20 kg of nitrogen per ha was added to the soil.
Schuphan (1969) observed that application of four times the normal
amount of fertilizer resulted in considerably higher nitrate levels in
spinach but that the nitrite levels remained low.
3.2.1.2 Animal wastes
Another major source of nitrates is farm animal wastes which
contain large amounts of nitrogenous materials that may be converted
into nitrates. The problem is more acute where farming is carried out
intensively, a common practice in North America for both livestock and
poultry. Since a 450 kg steer excretes about 43 kg of nitrogen per
year, a 3200 head feedlot would produce 1400 tonnes annually on a
relatively small area--an amount equivalent to about 260 000 people.
Thus, such feedlots become "small area" sources of nitrogen runoff.
Only 10% of these wastes is returned to cultivated land (Standford et
al., 1969) and runoff studies demonstrate a considerable problem of
environmental pollution. Nye (1973) reported Gilbertson et al. (1970),
who found that the total nitrogen concentration in runoffs ranged from
about 50 to over 5500 mg/litre. Animal husbandry, even when carried
out on pastures or with the return of the animal wastes to cultivated
land, may still impose problems. Adriano et al. (1971) concluded that
wastes from a maximum of 7-8 cows could efficiently be used per
hectare of farmland or pasture and that higher application rates might
raise nitrate levels above 10 mg/litre in the subsoil waters.
3.2.1.3 Municipal, industrial, and transport wastes
Discharges of municipal and industrial wastes are concentrated
sources of nitrogen compounds that are, to a large extent, released
directly into surface waters. The amount of nitrogen in human wastes
is estimated to be about 5 kg per person per year (Committee on
Nitrate Accumulation, 1972). Even if treated, this waste will
represent a heavy water pollution load since secondary treatment
removes less than half of the nitrogen. Ammonium ions in the effluent
of septic tanks may be rapidly converted to nitrate which may
penetrate some distance from the tank. Sludge from treatment plants
and septic tanks has also to be disposed of and represents another
significant source of nitrogen pollution. Solid waste disposal
practices, particularly sanitary landfills and dumps, may represent a
source of water pollution by nitrogen compounds.
The nitrogen content of industrial wastes is highly variable;
fuel and food processing industries and petroleum refineries may
constitute important sources of nitrogen pollution. The
nitrogen/BODa ratio of food processing plant wastes is about 0.05
while for animal processing wastes this ratio amounts to 0.5.
(Committee on Nitrate Accumulation 1972). Oxides of nitrogen released
into the atmosphere from man-made sources such as motor vehicles,
fossil fuel combustion, and industrial processes amount to about 50
million tonnes per year on a global scale (Robinson & Robbins, 1972).
A considerable proportion of this fixed nitrogen is eventually
returned to the earth's surface as nitrate.
3.2.1.4 Deliberate addition of nitrates and nitrites to food
Nitrates and nitrites are widely used in the production of
certain meat products and in the preservation of fish in some
countries. Reasons for using these salts in food production have been
reviewed by Ingram (1974). Nitrite is used in meat curing to obtain
the characteristic pink colour and flavour of cured meat. While a
nitrite content of less than 5 mg/kg of meat is sufficient to give a
satisfactory colour for a limited period of time, up to 20 mg/kg may
be necessary to give commercially adequate colour stability and about
50 mg/kg to produce the characteristic flavour. However, detailed
experimental confirmation of these figures is lacking.
Curing meat gives an important degree of protection against
botulism and may provide similar protection against other bacteria
such as Clostridium welchii and staphylococci, although the
importance of this has not yet been assessed. The question of how much
nitrite is necessary to protect against botulism is very complex
because of several associated factors.
The addition of nitrates and nitrites to meats, meat products,
and cheese is governed by legislation in most countries, some of which
also allow the addition of these salts to fish products.
3.2.2 N-nitroso compounds
3.2.2.1 Food
The formation of N-nitroso compounds from nitrites and amines
during the storage and processing of food is discussed in section
4.2.2.
a BOD = biological oxygen demand.
3.2.2.2 Tobacco
Nitrosonornicotine has been found in unburned smoking tobacco,
chewing tobacco, and in snuff. The same compound has been identified
in the mainstream smoke of a popular nonfilter cigarette in the USA
(Hoffman et al., 1974).
3.2.2.3 Industrial uses
Although N-nitroso compounds do not appear to be extensively
used at present, Magee (1972) reports that patent applications have
been made in the UK for their use in the manufacture of dyestuffs,
lubricating oils, explosives, insecticides, and fungicides. Some
nitrosamines (nitrosodiphenylamine, N-N-dinitrosopentamethylenete-
tramine, polymerized N-nitroso 2,2,4-trimethyl-1,2-dihydroquinoline
and N-methyl- N, 4-dinitrosoaniline) are used as organic
accelerators and antioxidants in the production of rubber (Boyland et
al., 1968). DMN has been used as an industrial solvent, as a
nematocide, and in the synthesis of the rocket fuel 1,1-
dimenthylhydrazine. There is some evidence that DMN might also be
formed during the combustion of this rocket fuel (Simoneit &
Burlingame, 1971). There are patents for the use of DMN as a solvent
in the plastics and fibre industry, as an additive for lubricants, and
to increase the dielectric constant in condensers (Daiber, 1966).
Industrial uses may result in the occurrence of N-nitroso
compounds in the work environment and in industrial effluents. Fine et
al. (1976b) reported point sources of air pollution by DMN in
Baltimore, MD, and Belle, WV, in the USA. A factory using DMN as an
intermediate was shown to be the source in Baltimore and was shut
down; in Belle the source of DMN was an amine-manufacturing facility.
4. TRANSPORT AND TRANSFORMATION IN ENVIRONMENTAL
AND BIOLOGICAL MEDIA
4.1. Nitrogen Cycle
The continuous interchange between atmospheric and terrestrial
nitrogen takes place along a number of different pathways including
air, water, soil, microorganisms, plants, animals, and man. This
transfer and transformation of nitrogen is referred to as the nitrogen
cycle (Fig. 1).
The main factors affecting it are the climatic conditions, the
type and density of animal and plant populations, agricultural
practices, and animal husbandry. The nitrogen cycle has undergone
profound modifications through the agricultural and industrial
activities of man (Bolin & Arrhenius, 1977; Committee on Nitrate
Accumulation, 1972; Commoner, 1970; FAO/IAEA Panel of Experts, 1974).
Atmospheric nitrogen is in the form of dinitrogen (N2); the
great strength of the N = N bond is mainly responsible for its
chemical inertness. A part of atmospheric nitrogen is transformed by
microbial action and incorporated into living organisms. This process
is called nitrogen "fixation" and is estimated to amount globally to
150 million tonnes of fixed nitrogen per year. In industrial nitrogen
fixation, atmospheric nitrogen is combined with hydrogen at high
temperatures and pressures in the presence of suitable metal catalysts
(Haber-Bosch process) to produce ammonia. Industrial nitrogen fixation
accounts for about one quarter of the total world production of fixed
nitrogen (Bolin & Arrhenius, 1977). Various atmospheric processes
which have been discussed elsewhere (WHO, 1977) are minor sources of
fixed nitrogen.
Biological nitrogen fixation, i.e. its reduction to ammonia, can
be accomplished by only a limited number of organisms. Symbiotic
nitrogen fixation takes place in the root nodules of legumes such as
soya bean, clover, and alfalfa, which contain bacteria of the
Rhizobium species. There are also symbiotic processes with plants
other than legumes involving, for example, some cyanobacteria. A
number of free living bacteria and algae can also fix nitrogen (Burns
& Hardy, 1975; Quispel, 1974). The fixation is catalysed by a complex
enzyme nitrogenase (1.7.99.2). Ammonia produced by biological fixation
is then converted to nitrite and nitrate by the process of
nitrification (see section 2.1.1). Plants can assimilate only a part
of the nitrates present in soils; some leaches into ground water and
rivers and may reach estuaries and oceans, the rest is subjected to
denitrification, another natural biochemical process that degrades
nitrates to nitrogen or nitrous oxide (dinitrogen oxide) which are
released into the atmosphere. Denitrification takes place in the soil
and also at the interface between water and sediment in oceans,
rivers, lagoons, and lakes. Nitrates from natural fixation and
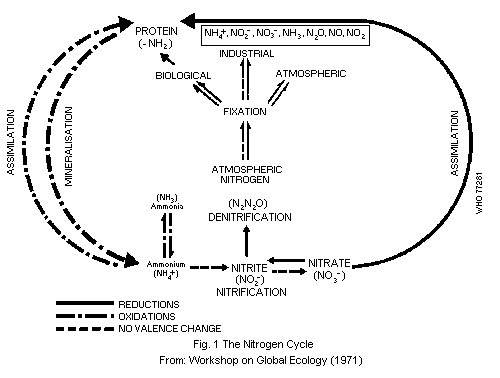 artificial fertilizers are ultimately used for the synthesis of
biological molecules, particularly proteins. Plants and animal waste
and dead tissues return fixed nitrogen to the soils, where part of it
is recycled and part returned to the atmosphere, thus completing the
nitrogen cycle. According to Delwiche (1970), nitrogen fixation on a
world basis may exceed denitrification by about 10%. The increased use
of industrial fertilizers has resulted in some areas in increased
concentrations of nitrates in bodies of water, resulting, in some
cases, in eutrophication.
4.2 Transformation in Foods
4.2.1 Reduction of nitrates to nitrites
Because of the ability of spinach to accumulate large quantities
of nitrates and reported cases of intoxication associated with the
consumption of this vegetable, several studies have been undertaken on
the conversion of nitrates to nitrites in spinach.
Data presented by Phillips (1968a) indicated that initial
nitrite-N contents of fresh, frozen, canned, and baby-food spinach
were generally less than 1 mg/kg fresh weight. However, several
authors have reported a rapid fall in nitrate levels and increase in
nitrite levels in fresh spinach during the first 4 days of storage at
room temperature (Achtzehn & Hawat, 1970; Phillips, 1968a; Schuphan,
1965). Higher nitrite levels occurred in spinach from fertilized
ground (Brown & Smith, 1967) and these could reach exceptionally high
values (3600 mg/kg dry weight) with excessive fertilization (Schuphan,
1965).
Under refrigeration, the nitrite-N contents of fresh spinach
increased very gradually throughout a storage period of 28 days
(Phillips, 1968a). Significant increases in nitrite-N levels did not
occur during the storage of frozen, canned, or baby-food spinach, but
increased concentrations were found in frozen spinach, that had been
left to thaw at room temperature for an excessively long period (39 h)
(Phillips, 1968a).
There was also a slight rise in nitrite levels when partially
consumed jars of commercial baby foods containing nitrates were stored
for 7 days at room temperature instead of under refrigeration
(Phillips, 1969). Selenka (1970) noted that nitrite formation in baby
foods was rapid in the presence of Escherichia coli and Pseudomonas
fluorescens, less rapid with Bacillus subtilis, and very slow with
Staphylococcus albus.
When foods consisted of a solid immersed in a liquid (e.g. canned
foods or frozen foods after thawing) nitrates were partially
transferred to the liquid portion or into the water in which the food
was cooked (Bodiphala & Ormrod, 1971). When large volumes of water and
long cooking times were employed (Kilgore et al., 1963), and when
canned vegetables were blanched in hot water instead of steam
(Johnson, 1966), significant amounts of nitrates were leached out of
the foods. Nitrate reductase (NADPH) (1.6.6.3) activity was rapidly
destroyed during cooking, thereby greatly diminishing further
conversion of nitrates to nitrites (Bodiphala & Ormrod, 1971). It is
also well known that the sterilization treatments necessary for
canning destroy microorganisms that could convert nitrates to
nitrites.
Conversion of nitrates to nitrites occurred more slowly in
vacuum-packed bacon than in unpackaged bacon, presumably due to the
low reducing ability of anaerobes (Cavett, 1962). Spencer (1967) found
that the nitrite content of vacuum-packed bacon decreased slowly on
storage. It has also been reported by Sebranek et al. (1974) that
nitrite levels in meat, determined 2 days after processing, were less
than half those originally added to frozen samples and samples
processed at 71°C, and that they decreased further during storage.
Frying, grilling, or boiling bacon or ham reduced the nitrites content
by 20-90% (Food Standards Committee, 1959).
When direct gas firing of spray dryers was employed, the nitrate-
N contents of dried milk products increased by 1-3 mg/kg compared with
those obtained using indirectly heated sprayers but nitrite-N levels
were unaffected (Manning et al., 1968). Air drying of potato and corn
starch led to the formation of only trace amounts of nitrite
(Gerritsen & De Willingen, 1969).
Nitrates may be reduced to nitrites when cooking is carried out
in aluminium utensils (Osteryoung, unpublished data)a. This
observation appears to be significant since some countries use
aluminium utensils for boiling milk and water, a practice which could
lead to the formation of sizeable quantities of nitrites. This effect
of aluminium should be investigated further.
4.2.2 Formation and degradation of N-nitroso compounds
The conditions under which various amines, amino acids, and
proteins in food could react with nitrite to form nitrosamines were
studied by Ender & Ceh (1971) and by Sen et al. (1970) who showed that
when cod, herring, hake, halibut, mackerel, or salmon were treated
with sodium nitrite at 200 mg/kg and cooked at 110°C for 60-70 min
there were only trace amounts (2.5-25 µg/kg) of DMN in the cooked
product. The highest levels were found in mackerel and hake, both of
which contain large amounts of DMA and TMA. Samples without added
nitrite did not contain any detectable nitrosamines.
a Nitrates as human and animal health hazards, Paper presented at
the Second Conference on Environmental Chemicals, Colorado State
University, 1973.
The formation of DMN was studied in aqueous model systems
containing methyl amines and sodium nitrite under conditions which
were more severe than those employed in the commercial processing of
nitrite-treated smoked chub (a fresh water fish containing small
amounts of TMA, TMAO, and DMA). The results of the studies showed that
not more than 10 µg of DMN per kg of the final product would be formed
during the smoking process (Malins et al., 1970).
Recently, Sen et al. (1973b) suggested that a major source of
nitrosamines in cured meat might arise from an interaction between the
nitrites and spices, such as black pepper and paprika, that are
present in curing mixtures. Nitrosopyrrolidine, nitrosopiperidine, and
DMN were found in a curing mixture used by a meat manufacturer in
Canada. The same authors (Sen et al. 1974a) have also studied the
effect of sodium nitrite concentration on the formation of
nitrosopyrrolidine and DMN in fried bacon. Bacon samples prepared with
sodium nitrite at 0, 50, 100, 150, or 200 mg/kg were analyzed for
nitrosopyrrolidine and DMN. No nitrosamine was detected in samples
prepared without nitrite but all treated samples contained 2-20 µg/kg
of nitrosamines. The level of nitrosopyrrolidine was related to the
initial concentration of nitrite in the bacon. It has also been shown
that nitrosamine formation in bacon increases with increasing
temperature and time of frying and that whereas baking, broiling, or
frying produce variable amounts of nitrosamines none is produced with
cooking in a microwave oven (Pensabene et al., 1974).
After deliberate nitrosation of eggs and meat with unusually
large amounts (1%) of sodium nitrite, some N-nitroso compounds
appeared to have been formed but the chemical nature of the compounds
detected was not clear (Walters, 1971).
No systematic studies on the formation of N-nitroso compounds
in cheese have been performed, although some types of cheese are known
to be processed with nitrate and nitrite.
There are few data on the fate of N-nitroso compounds during
the cooking, processing, or storage of food but some studies have
demonstrated that the volatile nitrosamine DMN and nitrosopyrrolidine
may be lost during the frying of bacon (Sen et al., 1973a).
4.3 Formation of N-nitroso Compounds from Drugs and Pesticides
Reaction with nitrite to form nitrosamines is not restricted to
food components. Lijinsky & Greenblatt (1972) and Lijinsky (1974)
reported that some antibiotics and other drugs that are widely used
can react with nitrites to form nitrosamines in alarmingly high
quantities. The drugs examined included oxytetracycline,
aminophenazone (aminopyrine), disulfiram, N,N-diethyl-3-
pyridinecarboxamide (nikethamide), tolazamide, and (E,E)-1-[5-(1,3-
benzodioxol-5-yl)-1-oxo-2,4-pentadienyl] piperidine (piperine).
Optimum conditions of temperature, pH, and concentration for these
reactions have been reported by Lijinsky et al. (1972a, 1972b, 1972c)
who more recently (Lijinsky, 1974) studied the reactions of
aminopyrine and other commonly used drugs with nitrous acid at rather
low concentrations to assess the magnitude of the hazard to man from
such interactions. The topic has been reviewed by Mirvish (1975) who
has listed 41 drugs and pesticides that have been nitrosated.
Pesticides listed include atrazine, simazine, ziram, and thiram.
4.4 Formation of N-nitroso Compounds in Animal Organisms
4.4.1 Formation of N-nitroso compounds in simulated gastric juice
Formation of DEN was demonstrated when DEA and nitrite were
incubated in the gastric juice of the rat, rabbit, cat, dog, and man.
More DEN was formed in human and rabbit gastric juices (pH 1-2 in both
cases) than in rat gastric juice (pH 4-5). (Sen et al., 1969a, 1969b).
The formation of nitrosamines by the interaction of some drugs
with nitrite in the presence of human gastric juice have been studied
by Scheunig & Ziebarth (1976). At 37°C and a pH = 2, for 1 h,
aminopyrine, sodium [(2,3 dihydro-1,5-dimethyl-3-oxo-2-phenyl-IH-
pyrazol-4-yl)methylamino] methanesulfonate (analgin), and piperazine
gave nitrosamine yields (calculated on the basis of nitrite used) of
69%, 11%, and 74.8% respectively.
In vitro studies have been carried out (Wells et al., 1974) in
which several foods (pork, egg, bread, milk, and cheese) were
incubated under simulated gastric conditions with concentrations of
nitrite similar to those used as food preservatives. The effect of the
thiocyanate ion as a catalyst for nitrosation was also studied since
it is secreted in saliva. Of the foods studied, only cheese produced
detectable amounts of volatile nitrosamines. The identity of the
nitrosamines was not indicated.
4.4.2 Formation of N-nitroso compounds in vivo
When DEA and nitrite were fed to cats and rabbits, considerable
amounts of DEN were detected in the stomach of the experimental
animals (Sen et al, 1969a, 1969b). Similar results were reported by
Sander & Sief (1969). Epstein (1972) reported the formation of
nitrosopiperidine in the gastrointestinal tract of rats treated with
nitrite and piperidine hydrochloride. When the nitrite concentration
was constant, nitrosopiperidine formation in the small intestine
increased with increasing concentrations of piperidine.
Nitrosopiperidine was also found in the stomach. Recently, Sander et
al. (1974a) demonstrated the formation of N-N'-dinitrosopiperazine,
DMN, and N-nitroso- N-methylbenzylamine in the stomach contents of
rats given the parent amines combined with nitrite. Considerable
individual variation in the degree of synthesis of N-N'-
dinitrosopiperazine was noted in the animals. In another recent
report, N-nitrosopyrrolidine was formed very rapidly in the stomach
of dogs from sodium nitrite and pyrrolidine (within 2-6 min) but after
30 min nearly all of it had disappeared, presumably due to its rapid
absorption (Mysliwy et al., 1974).
Indirect evidence of in vivo formation of N-nitroso compounds
has also been provided by some toxicity studies. Thus, hepatic
lesions, formed following administration of nitrite and some amines,
were similar to those produced by DMN or N-nitroso- N-
methylbenzylamine (Asahina et al., 1971). Similar effects were noted
where nitrite was administered up to 3 h after DMA, but the effect was
markedly reduced if the nitrite was given prior to the amine.
4.5 Formation of N-nitroso Compounds by Microorganisms
Studies conducted by Hawksworth & Hill (1971a), Klubes & Jondorf
(1971), and Sander & Sief (1969) suggested that nitrosamines could be
synthesized from secondary amines and nitrates or nitrites by
Escherichia coli and some species of streptococci. Fong & Chan
(1973b) demonstrated that homogenized Chinese salt fish inoculated
with Staphylococcus aureus (a nitrate-reducing bacterium) produced
considerable amounts of DMN.
Formation of nitrosamines in the presence of bacteria is unlikely
to occur in the large intestine, but the infected bladder and
achlorhydric stomach are likely sites (Hawksworth et al., 1974).
The ultimate mechanism of bacterial production of nitrosamines
remains to be ascertained. According to Hawksworth et al. (1974),
certain bacteria do reduce nitrate to nitrite but the formation of the
nitrosamine may be nonenzymatic and involve some heat-resistant
metabolite.
4.6 The Effects of Other Chemicals on the Formation of N-nitroso
Compounds
Fiddler et al. (1973) and Greenberg (1974) showed that high
levels of ascorbic acid reduced nitrosamine formation in frankfurter
sausages and in fried bacon. On the other hand, Nagata & Mirna (1974)
reported an increase in nitrosamine formation in meat products in the
presence of ascorbic acid. Other studies conducted on the inhibition
of nitrosamine formation by various compounds include a report by Sen
& Donaldson (1974) in which nitrosamine formation in human saliva was
inhibited by ascorbic acid. Ziebarth & Scheunig (1976) tested a number
of substances and beverages for the inhibition of the nitrosation of
several drugs under simulated gastric conditions. Of all the
substances investigated, ascorbic acid was regarded as the best
inhibitor because of its pronounced activity at pH values occurring in
the stomach and because it was not toxic in the amounts used.
5. ENVIRONMENTAL LEVELS AND EXPOSURES
5.1 Nitrates and Nitrites
5.1.1 Ambient air
Nitrate aerosols are the final stage in the atmospheric oxidation
of gaseous oxides of nitrogen, and substantial amounts of particulate
nitrates may be formed in urban areas affected by photochemical
pollution (Pitts & Lloyd, 1973). The concentration of nitrates in air
may range from about 1 to 40 µg/m3, depending on the sampling and
averaging periods. For example, the estimated annual mean values
(1968-1972) in Chattanooga, TN, USA, were between 1 and 6 µg/m3
(French et al., unpublished)a. The daily mean concentrations of
airborne nitrates in the central part of Tokyo ranged, in 1973, from
0.9 to 41.8 µg/m3 with an annual mean of 8.2 µg/m3. On the other
hand, in a small city with few industries (Matsue City) the daily
means were in the range of 1.1 -- 9.2 µg/m3, with an annual mean of
2.6 µg/m3 (Japan Environmental Sanitation Center, 1974).
5.1.2 Water
The concentrations of nitrates and nitrites in surface and ground
waters vary within wide limits, depending on geochemical conditions,
human and animal waste management practices, the extent to which
nitrogen-containing agriculture fertilizers are used locally, and on
industrial discharges of nitrogen compounds (section 3.2.1.).
In general, surface waters do not usually contain nitrate in
concentrations higher than 10 mg/litre, and nitrite concentrations
rarely exceed 1 mg/litre. However, a steady upward trend of nitrate
levels has been reported in recent years in some countries, both in
surface and ground waters. Thus, for example, in the River Thames,
England, nitrate concentrations increased from an average of
4 mg/litre in 1968 to an average of 9 mg/litre for the last quarter of
1973 (Water Research Centre, 1974). Similar increases have been
observed in several other English rivers (Casey, 1975; Owens, 1970;
Tomlinson, 1970). The nitrate concentrations are increasing in some
rivers that drain the great agricultural section of the centre of the
USA, and in selected small rivers the 45 mg/litre limit is sometimes
exceeded (Viets & Aldrich, 1973). A small increase in the nitrate
concentration of the Tamagawa River, Tokyo, Japan has also been
reported. From 1951-1965, the nitrate ion concentration rose from
7.9 mg/litre to 9.1 mg/litre. During the same period, the nitrite
concentration increased from 0.049 mg/litre to 0.53 mg/litre, i.e by a
factor of about 10 (Goto, 1973).
a French, J. G., Hasselblad, V., & Johnson, R. Aggravation of
asthma by air pollutants. 1971 -72 Southeastern CHESS studies.
Studies of 991 settlements in Bulgaria indicated that only 64
towns and villages had drinking water levels of nitrates between
30 mg/litre (Bulgarian standard) and 50 mg/litre. In 20 settlements,
situated in areas with intensive agriculture and stock breeding, the
nitrate concentrations exceeded 50 mg/litre. The reportb points out
that such problems did not exist some 10 years ago when smaller
quantities of nitrogen fertilizers were used in agriculture.
Much higher concentrations of nitrates are sometimes found in
ground water, particularly in water derived from dug wells. A survey
of over 2000 rural wells in Saskatchewan, Canada, revealed that 18.8%
contained nitrate concentrations of more than 50 mg/litre and 5.3% had
nitrate levels exceeding 300 mg/litre (Robertson & Draycott, 1948).
Hedlin (1971) also reported levels above 45 mg/litre in some wells in
a rural area of Manitoba, Canada. In many farm wells in central USA,
nitrate concentrations may range from 45-450 mg/litre. This problem is
neither new nor local, since such conditions have been recorded from
1895 to 1970 in Illinois, in 1939 in Iowa, and in 1970 in Minnesota
(Viets & Aldrich, 1973). The mean nitrate concentration in ground
water consumed by children affected by methaemoglobinaemia in
Czechoslovakia ranged from 18-257 mg/litre (Schmidt & Knotek, 1970).
According to Gruenar & Shuval (1970), about 180 wells for community
water supplies in the densely populated central and southern coastal
plain in Israel had nitrate concentrations exceeding 45 mg/litre. In
England, nitrate concentrations in some ground waters have been
reported to range from 12 mg/litre (Foster & Crease, 1974) to over
22 mg/litre (Reeves et al., 1974). Nitrate concentrations exceeding
45 mg/litre have not been reported in centralized water supplies in
the USSRc. However, high concentrations have been found from time
to time in dug wells, for example, 310-400 mg/litre in Leningrad
Oblast (Motylev, 1969), 110-200 mg/litre in the Tatar SSR (Petukhov
et al., 1972) and up to 430 mg/litre in the Moldvian SSR (Diskalenko,
1969).
5.1.3 Selected foods
According to the data compiled by the National Institute of
Environmental Health Sciences (NIEHS, 1970), the levels of nitrates in
vegetables vary considerably. The highest levels were found in beets,
egg plant, kale, and spinach and the lowest in tomatoes and peas;
similar findings were obtained in the German Democratic Republic by
Achtzehn & Hawat (1969). It is of interest to note that the nitrate
levels in vegetables reported by Jackson in 1967 were similar to those
reported by Richardson in 1907, when manure was used instead of
chemical fertilizers.
b Contribution to the WHO environmental health criteria document on
nitrates, nitrites and nitrosamines, Sofia, 1974.
c Contribution to the WHO environmental health criteria document on
nitrates, nitrites and nitrosamines, Moscow, 1974.
Nitrate contents vary not only between vegetable species but also
widely within a given species. This variation within a species may be
accounted for by such factors as temperature, sunlight, soil moisture,
and the level of available nitrogen in the soil (US Department of
Agriculture, 1965). A relationship between nitrate accumulation in
spinach and levels of fertilizer applied to the soil has been
demonstrated by a number of authors (Brown & Smith, 1967; Phillips,
1971). Furthermore, Schuphan (1965) reported exceptionally high levels
of nitrites (3600 mg/kg dry weight) in excessively fertilized fresh
spinach stored at room temperature.
A survey of the nitrate contents of fruits in the German
Democratic Republic, revealed that they were high in bananas and
strawberries but could not be detected in the other fruits examined
(Achtzehn & Hawat, 1969).
Cow's milk contained nitrate levels of 0-0.5 mg/litre (Simon et
al., 1964).
The levels of nitrates and nitrites in baby foods are of special
concern since infants are considerably more sensitive to the toxic
effects of nitrates than adults. Kamm et al. (1965) studied 194
prepared infant foods and found that, on average, fruits, dairy
products, puddings, egg products, meats, dry and concentrated food
supplements, and precooked cereal products contained nitrate levels of
less than 90 mg/kg. Vegetables, however, had a wide range of nitrate
contents varying from 0.9 to 2165 mg/kg, but nitrite levels never
exceeded 7 mg/kg. In studies on a number of canned foods, baby foods,
frozen foods, and vegetables, several varieties of fruit, spinach, and
beets generally had the highest nitrate contents (Bodiphala & Ormrod,
1971). Additional information may be found in articles by NIEHS (1970)
and Ashton (1970).
In a survey of various cured meats (Table 1, Ashton, 1970), the
highest nitrate content of 370-511 mg/kg was found in ham. In
analysing 197 samples of cured meat products, Panalaks et al. (1972)
found that the levels of nitrates and nitrites ranged from
0-3467 mg/kg and 0-252 mg/kg, respectively.
Dubrow & Kakisch (1960) analysed 338 samples of cheese and
reported that all were free of nitrites (less than 1 mg/kg). However,
40% of the samples contained nitrate levels of more than 1 mg/kg.
Rammell & Joerin (1972) also found low nitrite levels in cheese.
Table 1. Nitrate and nitrite contents of cured meats
Meat type Nitrate (mg/kg) Nitrite (mg/kg)
silverside 133-303 9-26
ham 370-511 7-150
luncheon meat 59-214 3.1-47
chopped ham & pork 53-101 22-62
corned beef 118-135 18-208
frankfurter sausage 119-141 8.5-10.3
From: Ashton (1970)
5.1.4 Estimate of general population exposure
One of the important sources of exposure to nitrates for man is
water. The level of nitrates in water may vary from practically nil to
over 200 mg/litre. In water from municipal supplies, however, it is
likely to be under 10 mg/litre. Thus, assuming an intake of 2 litres
of water per day, the daily intake of nitrates from this source would
normally be less than 20 mg, but with extremes of 0 and over 400 mg.
The other main sources of nitrates and nitrites are certain
vegetables and meat products. The intake from these sources is even
more variable because of marked differences not only in levels in
these foods but also in dietary patterns. However, Ashton (1970)
estimated the weekly intake of nitrates for a member of the general
population in the USA to be about 400 mg including 210 mg from
vegetables, 110 mg from meat products, and 85 mg from water (7 litres
per week). The estimate of Hill et al. (1973) for a member of the
general population in England included 225 mg from vegetables, 110 mg
from meat, and 105 mg from water in "control towns" and 645 mg from
water in Worksop, England. However these figures cannot be applied
generally because of variations in feeding habits and nitrate levels
in environmental media. Since the intake of nitrites is even more
variable, no estimates have been reported.
5.2 N-nitroso Compounds
5.2.1 Ambient air
The occurrence of N-nitroso compounds in urban air was reported
first by Bretschneider & Matz (1973) and confirmed by Fine et al.
(1976b) who found DMN at concentrations of about 1.2-3.5 µg/m3
(0.33-0.96 ppb) in an industrial district in Baltimore, MD, USA, and
about 0.06-0.17 µg/m3 (0.014 ppb - 0.051 ppb) in Belle, WV, USA.
N-nitroso compounds may be present in air, either due to their
formation in the air from secondary amines and oxides of nitrogen
(Neurath, 1972) or due to industrial omissions as in the instances
referred to.
5.2.2 Water
There are few reports on the occurrence of N-nitroso compounds
in water. Fine et al. (1976b) analysed samples from the Mississippi
river (New Orleans, LA) and from 3 water treatment plants in
Louisiana. Using the new N-nitroso compound-specific thermal energy
analyser (TEA) interfaced to both a gas chromatograph (GC) and a high
performance liquid chromatograph (LC), several peaks were tentatively
identified as belonging to N-nitroso derivatives of some pesticides.
The estimated concentrations were of the order of 0.1 µg/kg.
5.2.3 Selected foods
A summary of the reported occurrences of nitrosamines in meat and
fish products, adapted from Sen (1974) and updated, is presented in
Table 2. Only the results that were confirmed by mass spectroscopy are
quoted in this table. It was noted that the majority of some 50
publications dealt with the determination of nitrosamines,
particularly DMN, in processed pork meat. The methods employed for
analysis mainly involved gas chromatography. A few results were
confirmed by mass spectroscopic techniques. However, mass spectroscopy
confirmation is currently being employed more frequently than in the
past.
Table 2. Levels of nitrosamines in various meat and fish productsa
N-nitroso
Meat Country compounds Levels (c) Reference
or area foundb
dry sausage Canada DMN 10-20 µg/kg Sen (1972)
uncooked salami sausage Canada DMN 20-80 µg/kg Sen (1972)
salami sausage Netherlands DMN 0.3 µg/kg(e) Stephany et al. (1976)
DMN 0.1(e) Stephany et al. (1976)
NDBA 1.1(e) Stephany et al. (1976)
Netherlands NPY 0.4(e) Stephany et al. (1976)
NPIP 0.3(e) Stephany et al. (1976)
bacon Canada NPY 4-25 µg/kg Sen et al. (1973a)
Canada NPY 25-40 µg/kg Sen et al. (1974a)
Netherlands DMN 0.8 µg/kg(e) Stephany et al. (1976)
bacon DEN 0.2(g)
Netherlands NDBA 0.6(g) Stephany et al. (1976)
NPY 0.4(g)
Netherlands NPIP 0.6(g) Stephany et al. (1976)
USA NPY 7-35 µg/kg Pensabene et al. (1974)
bacon USA NPY 2, 28, 13 Fiddler et al. 1974
uncooked bacon Canada DMN 30 µg/kg Sen et al. (1973a)
fried bacon Netherlands DMN 2.4 µg/kg Groenen et al. (1976)
DEN 4.43 µg/kg
Netherlands DMN 1.1 µg/kg(e) Stephany et al. (1976)
DEN 0.2(g)
Netherlands NDBA 0.7(g) Stephany et al. (1976)
NPY 16.4(g)
Table 2 (Cont'd)
N-nitroso
Meat Country compounds Levels (c) Reference
or area foundb
Netherlands NPIP 3.9(g) Stephany et al. (1976)
UK NPY 1-40 µg/kg Crosby et al. (1972)
USA NPY 20-207 µg/kg Fazio et al. (1973)
smoked meat Netherlands DEN 7.91 µg/kg Groenen et al. (1976)
Netherlands DMN 3 Groenen et al. (1976)
smoked horse and Netherlands DMN 7.3 µg/kg(d) Stephany et al. (1976)
beef meat DEN 0.6(d) Stephany et al. (1976)
Netherlands NDBA 0.4(d) Stephany et al. (1976)
NPY 0.1(d) Stephany et al. (1976)
Netherlands NPIP 0.1(d) Stephany et al. (1976)
ham with layer Germany DMN 3 µg/kg Eisenbrand et al. (1975)
of pepper grains (Federal Republic of)
on the outside.
Only fat portion.
ham with layer Germany NPIP 6 µg/kg Eisenbrand et al. (1975)
of pepper grains (Federal Republic of)
on the outside.
Whole product Germany NPY 6
homogenized. (Federal Republic of)
ham, fried Germany DMN 1 µg/kg Eisenbrand et al. (1975)
(Federal Republic of) NPIP 8
NPY 19
Table 2 (Cont'd)
N-nitroso
Meat Country compounds Levels (c) Reference
or area foundb
ham with layer of Germany NPIP 4 µg/kg Eisenbrand et al. (1975)
pepper grains (Federal Republic of)
on the outside. Germany NPY 9
Whole product (Federal Republic of)
homogenized.
German type of Germany DMN 1 µg/kg Eisenbrand et al. (1975)
bacon, raw (Federal Republic of)
German type of Germany DMN 1 µg/kg Eisenbrand et al. (1975)
bacon, fried (Federal Republic of) NPIP 5 Eisenbrand et al. (1975)
Germany NPY 19 Eisenbrand et al. (1975)
(Federal Republic of)
smoked raw meat Germany DMN 2 µg/kg Eisenbrand et al. (1975)
(Federal Republic of)
smoked ham Germany DMN 8 µg/kg Eisenbrand et al. (1975)
(Federal Republic of)
smoked ham USA DMN 5 µg/kg Fazio et al. (1971b)
smoked ham USA DMN 5 µg/kg Fiddler et al. (1974)
cooked and smoked Netherlands DMN 0.4 µg/kg(f) Stephany et al. (1976)
ham DEN 0.6(f) Stephany et al. (1976)
Table 2 (Cont'd)
N-nitroso
Meat Country compounds Levels (c) Reference
or area foundb
Netherlands NDBA 0.4(f) Stephany et al. (1976)
NPY 0.3(f) Stephany et al. (1976)
Netherlands NPIP 0.4(f) Stephany et al. (1976)
cooked ham Netherlands DMN 6 µg/kg Groenen et al. (1976)
Bologna sausage Canada DEN 25 µg/kg Panalaks et al. (1974)
Canada NPY 20,100,105 Panalaks et al. (1974)
frankfurter sausage USA NPIP 50, 50, 60 µg/kg Wasserman et al. (1972)
spiced meat Canada DMN 5-48 µg/kg Sen et al. (1976)
products DEN 6-16
Canada NPIP 14-50 Sen et al. (1976)
Canada NPY 7-33 Sen et al. (1976)
fish meal Canada DMN 0.35-0.5 mg/kg Sen et al. (1972)
smoked, nitrate/ USA DMN 4-26 µg/kg Fazio at el. (1971a)
or nitrite treated
sable, salmon shad
fresh, smoked or UK DMN 1-9 µg/kg Crosby et al. (1972)
salted fish
salted white Hong Kong DMN 40-100 µg/kg Fong & Chan (1973a, 1973b)
herring
Table 2 (Cont'd)
N-nitroso
Meat Country compounds Levels (c) Reference
or area foundb
salted yellow Hong Kong DMN 10-60 µg/kg Fong & Chan (1973a, 1973b)
croakers
crude salt salted Hong Kong DMN 400 µg/kg Fong & Chan (1973a, 1973b)
white herring
crude salt salted Hong Kong DMN 200 µg/kg Fong & Chan (1973a, 1973b)
yellow croakers
prime salt salted Hong Kong DMN 10 µg/kg Fong & Chan (1973a, 1973b)
white herring
prime salt salted Hong Kong DMN 5 µg/kg Fong & Chan (1973a, 1973b)
yellow croakers
salted anchovies Hong Kong DMN 20 µg/kg Fong & Chan (1973a, 1973b)
whole herring meal Hong Kong DMN 300 µg/kg Fong & Chan (1973a, 1973b)
a Adapted from Sen (1974)
b DMN -- N-methyl- N-nitrosomethanamine c All values confirmed by mass spectroscopy
DEN -- N-ethyl- N nitrosoethanamine d mean of 4 samples
NDBA -- Nitroso- N-butylamine e mean of 5 samples
NPY -- Nitrosopyrrolidine f mean of 6 samples
NPIP -- Nitrosopiperidine g mean of 10 samples
Several authors who detected nitrosamines in foods by screening
methods but did not confirm these results by mass spectrometry include
Ender et al. (1964, 1967), Ender & Ceh (1967), Fong & Walsh (1971),
Freimuth & Glaser (1970), Hedler & Marquardt (1968), Kröller (1967),
Lembke & Moebus (1970), Möhler & Mayrhofer (1968, 1969), and Sakshaug
et al. (1965). Nevertheless, results by screening methods should not
be entirely ignored.
Considerable variations have been found in the levels of volatile
N-nitroso compounds in fried and grilled bacon. Attempts to
correlate these levels with levels of nitrates and nitrites did not
reveal any definite pattern. Telling et al. (1974) studied the effect
of various cooking temperatures on the levels of N-nitroso compounds
in grilled bacon. The results indicated that the levels of DMN
remained fairly constant as the cooking temperature was raised but
those of nitrosopyrrolidine increased.
Lipid soluble nitrosamines have an affinity for the fatty
portions of food (Sen et al. 1973a).
5.2.4 Tobacco and tobacco smoke
Since precursors for the formation of nitrosamines occur in
tobacco, Druckrey & Preussman (1962) thought it likely that tobacco or
tobacco smoke might contain trace amounts of nitrosamines. Initially,
studies on the nitrosamine content of tobacco products were hampered
due to interference from other compounds. Later, evidence suggesting
the presence of DMN, nitrosopyrrolidine, methylbutylnitrosamine, and
nitrosopiperidine in tobacco smoke was obtained (Kröller, 1967;
Neurath et al., 1964; Neurath, 1972, Serfontein & Hurter, 1966).
Although anabasine and nornicotine are constituents of tobacco smoke,
the corresponding nitroso derivatives were not detected by Neurath
(1972). Recently, however, Hoffman et al. (1974) reported the presence
of N-nitrosonornicotine at levels of up to 88 mg/kg in unburned
tobacco.
5.2.5 Estimate of general population exposure
The N-nitroso compounds that have been identified and
determined in meat and fish are listed in Table 2; consumption of
these foods constitutes a definite exposure of the general population
to these chemicals. However, at present, no estimate can be made of
the human exposure from these and other sources because insufficient
samples have been analysed and because the relevant food consumption
surveys have not been made.
5.2.6 Occupational exposure to N-nitroso compounds
The potential occupational hazards associated with the use or
manufacture of N-nitroso compounds in industry have been pointed out
by Magee & Barnes (1967).
Only a few quantitative data are available, however, on the
concentration of N-nitroso compounds in the work environment. In a
study by Bretschneider & Matz, (1976) DMN levels in the air in a
factory manufacturing DMA were roughly estimated to range from 0.001
to 0.43 µg/m3.
6. METABOLISM OF NITRATES, NITRITES, AND N-NITROSO COMPOUNDS
6.1 Gastrointestinal Absorption
6.1.1 Nitrates and nitrites
A part of ingested nitrates is readily absorbed and a part may be
metabolized by the microflora in the gastrointestinal tract (Ridder &
Oehme, 1974). Nitrites (NO2-), oxides of nitrogen (N2O5, NO2,
NO), hydroxylamine (NH2 OH), and ammonia (NH3) can be formed
depending upon the organisms present, the pH, and the available
nutrients (trace elements and carbohydrates), and may be absorbed.
Friedman et al. (1972) gave mice a single oral dose of 150 µg of
sodium nitrite. Measurement of the rate of disappearance showed that
the compound was rapidly absorbed and that food in the stomach had
little effect on absorption. Results using animals with a ligated
gastro-duodenal junction suggested that the major absorption site was
the gastric mucosa. Studies by Mirvish et al. (1974) on rats fed a
diet containing nitrite supported the previous observations on mice.
Experiments with food containing phenol red showed that a decrease in
nitrite levels in the stomach contents, especially in the glandular
part, that occurred within 5 h of feeding, was significantly greater
than that due to direct faecal elimination. This was attributed to
decomposition and other acid-catalyzed reactions of nitrite and to
direct absorption from the stomach.
6.1.2 N-nitroso compounds
Although a considerable degree of absorption of nitrosamines can
be inferred from the nature of their toxic effects following oral
administration, few reports could be found giving quantitative
information on absorption. Alarif & Epstein (1974) gave 3H-labelled
nitrosomethylurea and nitrosomethylurethane by gavage to groups of
pregnant guineapigs at doses of 2 mg/kg, and 5 mg/kg body weight,
respectively. The animals were killed 1 h after dosing. When the
uptake by maternal and fetal tissues was measured by liquid
scintillation counting and DNA determination, maternal levels were
generally higher than fetal levels. In studies by Juszkiewicz &
Kowalski (1974) DMN, DEN, and nitro-propylamine, administered orally
to goats at 20-30 mg/kg, appeared in the blood within “ h, and later
in the milk. The concentration in the milk, 2 h after administration
of DEN at 30 mg/kg, was 14 mg/litre; after 24 h only traces could be
found. Phillips et al. (1975a) examined the disappearance of DMN from
the stomach and small intestine of rats as an index of absorption, and
found that, while very little was absorbed from the stomach, DMN was
rapidly absorbed from the small intestine.
6.2 Biotransformation and Elimination
6.2.1 Nitrates and nitrites
In a study on 4 rats, 42-90% of nitrates, administered by stomach
tube, was excreted in the urine within 8 h of administration. Nitrites
were not detected in the urine either before or after administration
(Hawksworth & Hill, 1971b). The same authors carried out a study on
122 samples of human urine, and found that urinary nitrate
concentration was related to the amount of nitrate ingested.
The excretion of nitrates and nitrites in the saliva was studied
by Spiegelhalder et al. (1976) in 11 volunteers given various
vegetable juices with nitrate concentrations ranging from 30 to
550 mg/litre. The levels of nitrates and nitrites excreted were
proportional to the amounts of nitrates ingested. After ingesting
100 mg of nitrates, nitrite concentrations in the saliva increased, on
average, by 20 mg/litre.
6.2.2 N-nitroso compounds
Magee & Barnes (1967) have reviewed available information on the
metabolism and elimination of N-nitroso compounds. Magee (1956)
measured recovery of DMN from the whole body of the mouse and noted
that 97% of the total dose (0.05 mg/kg) could be recovered immediately
after oral administration and that the amounts recovered decreased
with time until at 4 h no DMN was recovered. Similar results were
obtained with the rat given DMN orally at 50 mg/kg, the concentration
fell rapidly with increasing time after injection so that only 30% of
the dose was recovered at 8 h and none at 24 h.
Metabolic transformation of DMN was demonstrated by Dutton &
Heath (1956) using 14C-labelled DMN. In both the mouse and the rat,
the main radioactive product was expired carbon dioxide. In the mouse,
65% of the injected 14C was recovered as expired carbon dioxide, 6 h
after a subcutaneous injection of DMN at 50 mg per kg body weight. In
the rat, about 40% of the radioactive 14C was recovered as expired
carbon dioxide, 8 h after the injection. At the end of the experiment,
the remainder of the 14C was fairly evenly distributed in the
tissues, apart from about 7% that was excreted in the urine.
Heath (1962) found that, while part of each of a number of
nitrosamines studied was excreted unchanged in urine and in expired
air, the greater part was decomposed. From the rates of expiration of
labelled carbon dioxide it was shown that decomposition of dimethyl-,
diethyl-, and N-butylmethylni-trosamine obeyed Michaelis-Menten
kinetics. The rate of decomposition was dose-dependent.
When the metabolic transformation of dialkylnitrosamines,
especially of the bladder carcinogen di- N-butylnitrosamine was
investigated, major urinary metabolites with retained N-nitroso
structure were identified (Blattmann & Preussman, 1973, 1974;
Blattmann et al., 1974; Okada & Suzuki, 1972; Okada et al., 1975).
Hydroxylation, particularly at the terminal CH3 group, has been
observed as well as chain shortening. Butyl (3-carboxypropyl)
nitrosamine (BCPN), a major metabolite of butyl (4-hydroxy butyl)-
nitrosamine (BBN) was an equally potent and selective bladder
carcinogen (Okada & Suzuki, 1972). Ring-opening was observed during
metabolism of nitrosomorpholine (Stewart et al., 1974).
7. EXPERIMENTAL STUDIES ON THE EFFECTS OF NITRATES, NITRITES, AND
N-NITROSO COMPOUNDS
7.1 Nitrates and Nitrites
7.1.1 Acute and subacute toxicity studies
Acute nitrate poisoning was first recognized in cattlea by Mayo
as early as 1895 (Wright & Davidson, 1964), while Comly (1945) was the
first to report nitrate poisoning from well water in infants in the
USA. As a result of these and other reports, several studies have been
conducted on the toxicity of nitrates in a wide variety of animal
species. They are mainly centred on the formation of methaemoglobin
that accompanies excessive exposure to nitrates and nitrites. The
acute toxicity of nitrates and nitrites was recently reviewed by the
Committee on Nitrate Accumulation (1972).
Although the outstanding feature of nitrate toxicity is the
development of methaemoglobinaemia, nitrates may also cause
vasodilation which aggravates the effects of the methaemoglobinaemia.
The nitrite ion formed by reduction of nitrates, oxidizes the iron in
the haemoglobin molecule from the ferrous to the ferric state. The
resultant methaemoglobin is incapable of reversibly binding oxygen
(Bosch et al., 1950). Clinical signs of nitrate toxicity, attributable
to hypoxia appear when methaemoglobin values exceed about 20% (section
8.1). Oxidation of haemoglobin to methaemoglobin by the nitrite ion
occurs at different rates for each animal species, but there is little
difference between individuals of the same species (Smith & Beutler,
1966). Similarly, the reduction of methaemoglobin in erythrocytes
mainly by the enzyme system, NADH--methaemoglobin reductase, is
characteristically different for each animal species. The chemical
induction of methaemoglobinaemia has recently been reviewed by Smith
(1969, 1975). These physiological processes appear to be related, even
though there is a large variation in the rate of formation of
methaemoglobin and its subsequent reduction. This may help to explain
the difference in species susceptibility and the variation in signs
seen in nitrate poisoning.
In an effort to develop sensitive tests for the detection of the
possible effects of subclinical methaemoglobinaemia, behavioural
studies with mice were undertaken by Behroozi et al., 1971. Groups of
57 black, 6J, male mice were given nitrites in their drinking water at
doses aimed at producing methaemoglobin levels varying from slightly
above normal to 15%, which can be considered to be in the subclinical
a For further discussion of nitrate intoxication in livestock,
that may involve significant economic loss, see Oehme (1975).
range. Sodium nitrite doses in water were 100, 1000, 1500, or
2000 mg/litre. The results showed a significant reduction of overall
motor activity in the groups receiving the highest levels of
nitrites. There was a significant inverse relationship between the
methaemoglobin level and motor activity, with a coefficient of
correlation of 0.65. An effort to counteract the methaemoglobinaemia
was made by giving ascorbic acid to the group receiving the highest
level of nitrites (2000 mg/litre). (See section 7.1.5). The effect
was to reduce the methaemoglobin levels to almost normal but the
motor activity level of the group so treated remained low and about
equal to the equivalent group that had not received an antidote.
These experiments seemed to indicate that the nitrites had some form
of sedative effect on the treated mice, that was not necessarily
associated with the development of methaemoglobinaemia.
Experiments on rabbits have shown that methaemoglobinaemia caused
by nitrates in water also affects cardiac activity by increasing the
number of cardiac contractions to an extent directly proportional to
the increase in methaemoglobin levels. At the 10-15% methaemoglobin
level, the electrocardiogram shows a shortening of the Q-T interval
and a reduction in the T wave, which may even become negative (Garbuz,
1968, 1971).
7.1.2 Chronic toxicity and carcinogenicity studies
In a study conducted by Shuval & Gruener (1972), groups of 8 male
rats (3 months old) were given tap water (control) or 100, 1000, 2000,
or 3000 mg of sodium nitrite per litre of drinking water. After 24
months, there were no significant differences in growth and
development, mortality, and total haemoglobin levels between the
control and treated groups. However, the methaemoglobin levels in the
groups receiving sodium nitrite at 1000, 2000, or 3000 mg/litre were
raised significantly throughout the study and averaged 5%, 12%, and
22% of total haemoglobin respectively. The methaemoglobin levels in
the group receiving 100 mg/litre were slightly above those of the
control group for the first 60 days only. There were some changes in
the liver and spleen of treated animals but the main pathological
changes occurred in the heart and lungs. In the heart, small foci of
cells and fibrosis were seen in some animals with pronounced
degenerative foci in animals receiving the highest concentrations of
nitrites. The coronary arteries were thin and dilated. The bronchi
were frequently dilated with the walls infiltrated by lymphocytes and
the mucosa and muscle were often atrophied. Emphysema was the rule.
These changes, which were present in 1 or 2 control rats and in a
small number of those receiving nitrite levels of 100 mg/litre, were
found with increasing frequency and severity in the 3 highest dose
groups.
Druckery et al. (1963a) did not observe extensive methaemoglobin
formation in rats given 100 mg sodium nitrite per kg body weight in
the drinking water, but there was a slight reduction in the life span.
Studies by Van Logten et al. (1972) in which groups of 30 male and 30
female rats received sodium nitrite at concentrations of 0, 0.02, or
0.05% with or without glucono--lactone (GDL) in the diet for 29
months did not demonstrate any significant haematological or
biochemical effects. Carcinogenic action, which could be related to
the administration of sodium nitrite with or without DEA or GDL, was
not observed in either of these studies.
It has been reported that prolonged administration to rats of
1/20 of the LD50 of calcium and sodium nitrates disturbed the energy
conversion processes such as glycolysis and the pentose phosphate
cycle, changed the activity of the glutathione-ascorbic acid system in
the blood and in the hepatic and cerebral tissues, raised the levels
of methaemoglobin and of NADH-methaemoglobin reductase activity, and
reduced the haemoglobin levels (Diskalenko & Dobrjanskaja, 1972;
Diskalenko & Trofimenko, 1972).
Greenblatt & Mirvish (1972) gave 3 groups of about 40, 7-9 week
old, male, strain A mice, 1 or 2 g of sodium nitrite or 12.3 g of
sodium nitrate/litre of water respectively, for 20 weeks and did not
observe any increase in lung tumour incidence in comparison with
untreated controls. Lijinsky et al. (1973a) reported similar negative
results when groups of 30 rats were given sodium nitrate at 5 g/litre
or sodium nitrite at 2 g/litre in their drinking water at a daily rate
of 20 ml/rat throughout most of their lifetime. Other studies by
Lijinsky et al. (1973c) on groups of 30 rats given 20 ml of sodium
nitrite at 2 g/litre daily, for 5 days a week, also produced negative
results. Taylor & Lijinsky (1975) reported that tumour formation in 57
Sprague-Dawley rats, exposed for 2 years to a drinking solution
containing sodium nitrite at 2 g/litre, was no greater than in the
controls.
7.1.3 Embryotoxicity
A study has been reported by Shuval & Gruener (1972) in which 2
groups of 12 pregnant rats were given 2000 or 3000 mg of sodium
nitrite per litre of drinking water, respectively. A control group did
not receive any treatment. Pregnant rats developed anaemia and had
higher methaemoglobin levels than nonpregnant rats receiving similar
doses. There was a pronounced increase in mortality among the newborn
rats of treated dams compared with those of untreated controls,
particularly in the 3-week period before weaning. Mortality in the
offspring was 6% in controls, 30% in those given 2000 mg/litre and
53% in those given 3000 mg/litre. Birthweights were similar in all
groups but growth was markedly reduced in pups of treated dams. Such
pups had thin hair coats. Treatment of 2 groups of 10 and 15 pregnant
rats with 1% and 0.3%, respectively, of sodium nitrate in the diet did
not result in any embryotoxic or teratogenic effects on the 9th and
10th days of gestation (Alexandrov & Jänisch, 1971).
Sleight & Atallah (1968) conducted a study in which 46 female
guineapigs, divided into 12 groups each containing at least 1 male,
were given potassium nitrate in doses ranging from 300 to 10 000 mg/kg
body weight in the water and potassium nitrite in amounts ranging from
300 to 10 000 mg/kg for periods ranging from 100-240 days.
Reproduction in the female was grossly impaired in the high nitrate
group. Fetal losses were 100% in females given 5000 or 10 000 mg/kg of
the nitrite and one female died. Reproduction was maintained at lower
levels of treatment. Apparently male fertility was not impaired, since
conception took place at all levels of treatment. Food and water
consumption and weight gains of treated animals were normal except for
a diminished rate of gain at a nitrite level of 10 000 mg/kg body
weight. Uterine and cervical inflammatory lesions and degenerative
placental lesions were present in females in which the fetuses had
been aborted, mummified, or absorbed. Sinha & Sleight (1971) reported
studies in which 4 pregnant guineapigs, given sodium nitrite at 50 mg
per kg of body weight, subcutaneously, underwent normal parturition.
However, fetal deaths followed by abortion, occurred in 3 guineapigs
given sodium nitrite at 60 mg/kg. The fetal deaths occurred
approximately 1 h after nitrite administration, when the maternal and
fetal methaemoglobin levels were highest. At the time of death, there
were no noticeable changes in the placenta; pathological changes
developed after the death of the fetuses. There were lower blood pO2
values in the fetuses of the guineapigs treated with nitrite at
60 mg/kg than in those of the controls. Fetal death did not occur in
pregnant animals given sodium nitrite at 60 mg/kg combined with
simultaneous intraperitoneal treatment with 10 mg of methylene blue
per kg body weight. The data suggest that fetal death resulted from
hypoxia, mainly induced by maternal methaemoglobinaemia.
Studies by Shuval & Gruener (1972) indicated that
methaemoglobinaemia might be induced transplacentally and that the
observed limit for transplacentally induced methaemoglobinemia was a
sodium nitrite dose of 2.5 mg/kg body weight. A steep increase in
effect occurred with increasing doses of sodium nitrite.
7.1.4 Mutagenicity
The mutagenic potential of nitrates and nitrites has not been
studied extensively; in fact, no data are available on their mutagenic
action in the mammalian systems. The mutagenicity of nitrates and
nitrites with respect to the transformation of DNA has been reported
(Bressler et al., 1968; Horn & Herriot, 1962; Strack et al., 1964) and
nitrous acid has been shown to be mutagenic in bacterial systems such
as Escherichia coli (Kaudewitz, 1959; Verly et al., 1967) and
Salmonella typhimurium (de Serres et al., 1967). Positive results in
mutagenic studies have been reported with the yeast Saccharamyces
cerevisiae (Nashed & Jabbur, 1966; Zimmerman & Schwaier, 1967;
Zimmerman et al., 1966), as well as with Aspergillus nidulans
(Siddiqi, 1962), A. niger, and A. amstelodami (Steinberg & Thom,
1940), and tobacco mosaic Virus (Sehgal & Krause, 1968).
7.1.5 Interaction with nutritional factors
Studies by Kociba & Sleight (1970) showed that maternal blood
levels of methaemoglobin were significantly higher in 12 ascorbic
acid-deficient, pregnant guineapigs, following the subcutaneous
administration of sodium nitrite at 40 mg/kg body weight, than in
those on a normal diet. Following subcutaneous administration of
sodium nitrite at 50 mg/kg, there was a higher percentage of fetal
death in the ascorbic acid-deficient guineapigs.
Experiments were conducted by Stoewsand (1973) with young, male,
guineapigs (number of animals not stated) to investigate the influence
of feeding beets with naturally occurring low and high amounts of
nitrates and nitrites, and the influence of dietary supplementation
with ascorbic acid and methionine on methaemoglobinaemia, induced by
orally administered sodium nitrite at doses of 25 or 50 mg/kg body
weight. Low-nitrate beet diets seemed to "protect" guineapigs from
nitrite intoxication. In addition, a 1% diet containing L-ascorbic
acid and 1% methione reduced nitrite-induced methaemoglobin blood
levels.
Sell & Roberts (1963) showed that ad libitum feeding of diets
containing 0.4% potassium nitrite to 6 groups of 50 chicks depressed
growth, irrespective of the amount of vitamin A administered. Also,
all the test animals, except those given massive injections of vitamin
A, exhibited reduced liver stores of the vitamin and enlarged thyroid
glands.
Studies by Phillips (1966) demonstrated that the liver vitamin A
contents of rats fed 1% potassium nitrite in diets containing carotene
or vitamin A, were less than those of control rats. It was suggested
that the dietary nitrite degraded the carotene and vitamin A in the
digestive tract before their absorption.
7.2. N-nitroso Compounds
7.2.1 Acute and subacute toxicity studies
The acute toxicity of N-nitroso compounds is not of great
toxicological significance because there is no relationship between
acute toxicity and the carcinogenic potential of this class of
compounds (Druckrey et al., 1967, Magee & Barnes, 1967). Because DMN
was reported to cause cirrhosis and other toxic effects in industrial
workers, Barnes & Magee (1954) examined the toxicity of this compound.
Doses of 20-40 mg/kg body weight given to rats, dogs, rabbits, and
guineapigs produced severe hepatic damage. A single dose of DMN given
to rats orally or by intravenous, intraperitoneal, or subcutaneous
injection, produced centrilobular necrosis accompanied by haemmorhages
in the liver. In rats given 20 mg DMN/kg body weight the liver cells
in the centrilobular and mid-zonal regions became pale and, after
18 h, the cytoplasm was amorphous and vacuolated; the nuclei were pale
and irregular in outline. By 24 h, the cells were necrotic and
confluent areas became haemorrhagic. Haemorrhage was usually more
pronounced after 48 h but after 72 h the recovery process had begun
and was almost complete in 3 weeks (Barnes & Magee, 1954; Magee &
Barnes, 1962). A detailed study by light and electronmicroscopy of
changes in the liver cytoplasm of rats treated with
N-nitrosomorpholine has been reported by Bannasch (1968).
The acute toxicity of acyl-alkyl-nitrosamides and diazoalkanes
was reported by Druckery et al. (1967), Magee & Barnes (1967), and
Shank (1975). The acute toxicity (LD50) of the nitroso compounds
varied widely; some were only mildly toxic while others produced
highly destructive lesions. N-nitroso- N-methylurethane, for
example, when given orally, produced severe necrotic lesions in the
stomach, congestion of the lungs, and periportal necrosis of the liver
(Schmähl & Thomas, 1962; Schoental, 1960). N-nitroso- N-methylurea
also produced inflammatory haemorrhagic lesions of the stomach,
intestine, and pancreas and a reduction in the bone marrow when given
orally to rats, (Druckrey et al., 1961, 1967).
The acute toxicity of some N-nitroso compounds, e.g. nitroso-
piperidine and nitroso-morpholine, was not manifested as liver damage
but as neurotoxic effects, e.g. convulsions (Lee & Lijinsky, 1966).
The acute toxicity of nitrosomines formed in vivo was studied
by Asahina et al. (1971). Sodium nitrite was administered by gavage to
mice at 100 or 150 mg/kg bodyweight alone or in combination with DMA
and methylbenzylamine at doses of 500-2500 mg/kg and 800-1600 mg/kg,
respectively. Combinations of the amines and nitrite produced hepatic
lesions similar to those produced by DMN or nitrosome-thylbenzylamine.
Similar effects were noted when nitrite was administered up to 3 h
after DMA but these effects were markedly reduced if the nitrite was
given prior to the amine. Lijinsky & Greenblatt (1972) reported
hepatic necrosis following coadministration of aminopyrine and sodium
nitrite.
In a number of studies, ascorbic acid has been shown to have an
inhibitory effect on: a) nitrosamine formation in the stomach and
small intestine of rats treated with 7-chloro-2-methylamino-5-phenyl-
3H-1, 4-benzodiazepine-4-oxide, (chlordiazepoxide, Librium) and sodium
nitrite (Preda et al., 1976); b) hepatotoxicity induced by the
combined administration of sodium nitrite and aminopyrine in rats
(Kamm et al., 1973) and mice (Greenblatt, 1973); c) liver necrosis
produced by DMA and nitrite administered to rats by gavage (Cardesa et
al., 1974); d) the teratogenic and transplacental carcinogenic effects
produced in rats by treatment with alkylurea and nitrite (Ivankovic et
al., 1973); and e) the induction of lung adenomas in mice by prolonged
treatment with morpholine, piperazine, and methylurea plus nitrite
(Mirvish et al., 1975).
Species differences exist with respect to the toxic effects of
N-nitroso compounds. Mink appear to be especially sensitive to DMN,
and as in other species, the effects are seen primarily in the liver.
Carter et al. (1969) noted widespread liver degeneration and necrosis
of hepatocytes in mink given DMN at 2.5 or 5.0 mg/kg in the diet for
7-11 days. The liver lesions were accompanied by bile-duct
proliferation, ascites, and haemorrhage of the gastrointestinal tract.
Sheep and cattle are also more sensitive to nitrosamines than
laboratory animals (Sakshaug et al, 1965; Koppang, 1964). Sheep, given
a single dose of DMN at 5 mg/kg body weight or 12 doses of 0.5 mg/kg,
died or were severely affected displaying anoxia, lack of rumination,
ataxia, and respiratory difficulties, while cattle given DMN at
0.1 mg/kg body weight showed pronounced hepatotoxic effects in 1-6 months.
Pathological and biochemical effects, observed in the liver of a
number of animal species including the rat following continuous
administration of nitrosamides, have been reviewed by Magee & Barnes
(1967) and Magee et al. (1976); the main effect is inhibition of
protein synthesis which might be a result of an accelerated breakdown
of messenger ribonucleic acid (RNA).
7.2.2 Carcinogenicity
The carcinogenic activity of N-nitroso compounds has been
summarized in several reviews (Druckrey, et al., 1967; Magee & Barnes,
1967; Magee et al., 1976). Various animal species including mammals,
birds, fish, and amphibia have been shown to be susceptible to the
carcinogenic action of nitrosamines. At present, some 80 nitrosamines
and 23 nitrosamides have been tested in rats and about 80% of the
nitrosamines and practically all the nitrosamides have proved to be
carcinogenic (Montesano & Bartch, 1976). These carcinogens show a
marked organ specificity as shown in Table 3.
Nitrosamines produce a carcinogenic effect in the liver,
oesophagus, respiratory system, and kidney, whereas nitrosamides
affect the peripheral and central nervous systems, and the gastro-
intestinal tract organs.
The dose schedule seems to play an important role in this organ
specificity. For example, in rats, long-term exposure to relatively
low doses of DMN induced mainly liver tumours, whereas a single or a
few high doses over a short period induced mainly kidney tumours
(Magee & Barnes, 1962). Similar responses in relation to the liver and
oesophagus were observed with DEN in rats (Druckey et al., 1967). The
route of administration does not seem to play an important role in the
carcinogenicity of this chemical. It is worthwhile pointing out that
many of these chemicals were carcinogenic following a single
administration and that, furthermore, exposure of rats to a single
dose during pregnancy resulted in carcinogenesis in the immediate
descendants and also in the two succeeding generations (Tomatis et
al., 1977).
Nitrosamines exert their adverse biological effects after being
metabolically activated by microsomal mixed function oxidases to form
reactive intermediates. On the other hand, nitrosamides decompose
enzymatically to reactive, and in most cases, alkylating derivatives.
The importance of hydroxilation of the alpha-hydrogen atoms of the
nitrosamines is demonstrated by the lack of carcinogenicity of
compounds, such as diphenylnitrosamine, which do not have this alpha-
hydrogen (Magee et al., 1976).
Table 3. Localization of tumours induced by N-nitroso
compounds in ratsa
Number of N-nitroso compounds
affecting target organ
Target organ Nitrosamines Nitrosamides
liver 35 2
oesophagus-pharynx 32 3
nasal cavities 18 -
respiratory tract 10 1
kidney 8 9
tongue 8 -
forestomach 7 11
bladder 4 1
central and peripheral 2 9
nervous system
ear duct 2 1
testis 1 -
ovary 1 2
mammary glands 1 1
sites of injection 3 4
intestine - 7
glandular stomach - 6
skin - 3
jaw - 1
uterus - 2
vagina - 1
haemopoietic system - 2
a From: Montesano & Bartch (1976)
7.2.2.1 Interspecies variation in response
Several N-nitroso compounds have been tested in different
animal species. DEN, tested in more than 20 species including
primates, induced tumours of the liver in all of them, together with
various tumours of other organs. In some cases, there have been marked
differences between species in response to N-nitroso compounds. For
example, nitrosoheptamethyleneimine produced squamous carcinoma of the
lung in rats (Lijinsky et al., 1969), the same effects in European
hamsters, but tumours of the forestomach and the lung in Syrian
hamsters (Lijinsky et al., 1970). In all three species, this compound
also induced oesophageal tumours. Nitrosomethylurethane induced
tumours of the pancreas in guineapigs (Druckrey et al., 1968) and
forestomach carcinomas in rats. Bis-2-hydroxypropyl-nitrosamine
produced pancreatic tumours in Syrian hamsters. These results show
the difficulty of attributing, with certainty, any particular tumour
response of man to a particular N-nitroso compound.
7.2.2.2 Intraspecies variation in response
Kuwahra et al. (1972) administered DMN orally or by subcutaneous
or intraperitoneal injections, to 8 to 12-week-old, male and female
mice of the DDD, BALB/C, and SJL/J strains. Tumours were found mainly
in the retroperitoneum and abdominal cavity and the incidence and
distribution were little affected by the strain of mouse but markedly
by the route of administration. Clapp et al. (1971) noted that DEN
induced forestomach and oesophageal squamous cell carcinomas in both
BALB/C and RF/Un strains of mice but induced liver haemangiosarcomas
in the former and hepatomas in the latter. In addition, DEN induced a
high incidence of lung tumours in RF mice but a very low incidence in
BALB/C mice. In both strains, DMN induced lung adenomas and liver
haemangiosarcomas. Tissue sensitivity did not appear to be related to
the spontaneous tumour incidence of the strain.
7.2.2.3 Dose-response relationships of N-nitroso compounds
Druckrey et al. (1963b) were the first to study the dose-response
relationships of N-nitroso compounds in relation to minimum effect
doses. They concluded, from studies in rats given DEN, that the
carcinogenic effect was related to dose and induction time in such a
way that d.t2.3 = constant, where d represents the daily dose and
t the induction time. Even a low dose of 0.15 mg/kg body weight
resulted in liver carcinomas in 27 out of 30 surviving animals. The
average induction time was 609 ± 38 days. The lowest dose studied
(0.075 mg/kg body weight) produced liver and oesophageal tumours in
all four surviving animals with an induction time of 830 days.
Mohr & Hilfrich (1972) gave a single intravenous injection of DEN
to rats at 8 dose levels between 1.25 and 160 mg/kg body weight. A
dose-response relationship was noted; at the lowest dose of 1.25
mg/kg, only one kidney tumour was observed in 20 treated rats, but, at
higher doses, the incidence of these tumours increased. Single dose
experiments with ethylnitrosourea in a transplacental carcinogenesis
experiment also showed that the incidence of induced tumours (mainly
of the brain and nervous system in the progeny) was directly
proportional to the dose of the carcinogen. Four doses between 1 and
50 mg/kg were used and even in the lowest dose group 36/41 animals in
the progeny died with tumours (Swenberg et al., 1972). Terracini et
al. (1967) fed rats concentrations of DMN ranging from 2 to 50 mg/kg
diet. At 2 and 5 mg/kg the incidence of liver tumours in the survivors
was 1/26 and 8/74, respectively, at 60 weeks. At 20 and 50 mg/kg,
liver tumours were observed in more than 60% of the test animals.
From this study, it can be concluded that a dietary level of DMN of
5 mg/kg is still carcinogenic in the rat.
A current dose-response study on rats receiving oral doses of
N-nitrosopyrrolidine has been reported by Preussmann (unpublished
data).a Liver tumours were induced in groups receiving 10, 3, and
1 mg/kg body weight per day, respectively, but not in a group
receiving 0.3 mg/kg per day.
Clapp & Toya (1970) gave male, RF mice DMN in the drinking water
for various lengths of time, with cumulative doses ranging from 87 to
243 mg/kg body weight. The incidence of lung adenomas in all treated
groups was higher than that in the untreated control groups. Whereas,
apparently, the incidence of hepatocellular tumours was not affected,
the incidence of liver haemangiosarcomas increased in the higher dose
groups and reached a maximum of 96%. Bertram & Craig (1973) using 2
groups of 100, C57BL/6 mice found that the incidence of bladder
tumours fell from 80% in animals given 30 mg of nitrosodi- N-
butylamine per kg/body weight per day to 36% at 7.5 mg/kg body weight
per day. Sander & Schweinsberg (1973) noted that an increasing
incidence of tumours of the oesophagus and forestomach was induced in
NMRI mice by adding a given dose of methylbenzylnitrosamine to the
drinking water at various times to provide total doses ranging from
1.4 to 44 mg/kg body weight.
a Paper presented at the Proceedings of the Second International
Symposium on Nitrite in Meat Products, Zeist, September 1976.
In studies conducted by Vesselinovitch (1969), DMN was
administered repeatedly by intraperitoneal injection to 73 male and 54
female C57BL x C3H mice starting at 7 days of age. The injections were
given at 3 day intervals for a total of six doses of 1, 2, or 4 mg/kg
body weight. Mice killed at 66 weeks of age showed an increase in the
incidence of hepatomas, hepatocarcinomas, lung adenomas, and
haemangiomas as the dose increased. The incidence of liver tumours was
higher in males (75%) than in females (28%).
Tomatis & cefis (1967) gave a dose of 3.6 mg of DMN by stomach
tube to one group of 30 Syrian golden hamsters in 3 administrations of
1.6 mg, 1.0 mg, and 1.0 mg over a 5-week period. A second group
received a single dose of 1.6 mg. Both dosing regimes produced liver
cell carcinomas and a few cholangiomatous lesions but no kidney
tumours. Montesano & Saffiotti (1968) administered 12, weekly,
subcutaneous injections of DEN at 0.5, 1.0, 2.0, or 4.0 mg to 4 groups
of 36 Syrian golden hamsters. The results demonstrated a positive
dose-response relationship for tumour induction in the upper
respiratory tract (nasal cavities, larynx, and trachea). The incidence
of nasal cavity tumours, which developed early, varied from 6/35 to
27/36. The incidence of tumours of the larynx varied from 6/35 to
26/36 and that of tumours of the trachea from 29/35 to 35/36.
7.2.2.4 Tumour induction by combined administration of nitrites
and amines or amides
Carcinogenic effects following the combined administration of
secondary and tertiary amines or amides with nitrite have been
reported. Tumours were not observed when sodium nitrite or the amine
or amide were given singly. Tumours of the same type occurred at the
same site when the N-nitroso compound, believed to be formed from
the nitrite and amine or amide, was given as a positive control. In
some of the experiments, the nitrite was given in the drinking water
and the amine or amide in the food. In others, the compounds were
combined in the same medium (drinking water or food).
Preussman (1975) reviewed studies in which tumours that had been
produced at certain sites by the oral administration of combinations
of amines and nitrite, were compared with the tumours induced by the
corresponding N-nitroso compounds. Table 4 has been adapted from
Preussman (1975) and updated.
Table 4. In vivo formation of N-nitroso compounds following oral administration of sodium nitrite and amino
compounds as demonstrated by specific carcinogenesisa
sodium nitrite + amine Expected tumour Reference
oral administration Observed tumour site for
amino compounds site corresponding
N-nitroso compound
Rat
diethylamine -- liver Druckrey et al.
(1963b)
Sander et al.
(1968)
Sander (1971a)
triethylamine -- liver (for Schweinsberg &
diethylnitrosamine Sander (1972)
morpholine liver, kidney, lung liver Sander & Burkle
N-methylbenzylamine oesophagus oesophagus (1969), Sander
(1971c)
piperidine -- oesophagus
liver
N-methylanaline oesophagus, nasal oesophagus
cavity
N-methylcyclohexylamine oesophagus oesophagus Sander (1971a)
Table 4 (Cont'd)
sodium nitrite + amine Expected tumour Reference
oral administration Observed tumour site for
amino compounds site corresponding
N-nitroso compound
N-methylbenzylamine oesophagus oesophagus
phenylbenzylamine negative untested
N,N'-dibenzylethylenediamine negative untested
indole negative untested
morpholine liver (kidney) liver (kidney) Shank & Newberne
(1972)
aminopyrine liver liver (for Lijinsky et al.
(Pyramidon) dimethylnitrosamine) (1973c)
heptamethyleneamine lung lung
oesophagus oesophagus
aminopyrine liver liver Taylor &
(for DMN) Lijinsky
(1975)
oxytetracycline liver (for DMN) liver Greenblatt et al. (1973)
proline negative untested
Table 4 (Cont'd)
sodium nitrite + amine Expected tumour Reference
oral administration Observed tumour site for
amino compounds site corresponding
N-nitroso compound
hydroxyproline negative untested
arginine negative untested
N-methylacetamide negative forestomach
N-methylurethane negative forestomach
N-ethylurethane negative (carcinogenic Sander (1971b)
following
i.v. administration)
acetanilide negative untested
glycylglycine negative untested
N-methyluracil negative untested
N-methylguanidine negative untested
N-phenylurea negative (carcinogenic Sander (1971b)
following
s.c. administration)
N-methylthiourea negative untested
N-methylurea brain, nervous brain, nervous
system, kidney system, kidney
Table 4 (Cont'd)
sodium nitrite + amine Expected tumour Reference
oral administration Observed tumour site for
amino compounds site corresponding
N-nitroso compound
N-ethylurea brain, nervous brain, nervous
system, kidney system, kidney
N'N'-dimethylurea brain, nervous brain, nervous Sander (1971b)
system, kidney system, kidney
imidazolidinone kidney (carcinogenic Sander & Burkle
following (1971)
s.c. administration)
ethylurea (during brain, nervous brain, nervous Ivankovic &
pregnancy, system system (in Preussmann
transplacental) (in descendants) descendants) (1970); Osske
et al. (1972)
Mouse
dimethylamine lung (adenoma)
piperazine lung (adenoma) lung (adenoma) Greenblatt et al. (1971)
morpholine lung (adenoma) lung (adenoma)
N-methylaniline lung (adenoma) lung (adenoma)
morpholine lung (adenoma) lung (adenoma)
Table 4 (Cont'd)
sodium nitrite + amine Expected tumour Reference
oral administration Observed tumour site for
amino compounds site corresponding
N-nitroso compound
N-methylbenzylamine oesophagus oesophagus Sander (1971a)
forestomach forestomach
piperazine lung (adenoma) lung (adenoma) Greenblatt &
Mirvish (1972)
N-methylurea lung (adenoma) lung (adenoma) Mirvish et al. (1973b)
N-ethylurea lung (adenoma) lung (adenoma)
a Adapted from Preussmann (1975)
7.2.2.5 Dose-response relationship for combinations of nitrite
and amines
Greenblatt & Mirvish (1972) conducted a study in which groups of
40, male, strain A mice, given 0.69-18.75 g of piperazine per kg of
food and 0.05-2.0 g of sodium nitrite per litre of drinking water for
20-25 weeks, were killed 10-13 weeks later. The yield of lung adenomas
was statistically significantly greater than in untreated controls,
when doses as low as 0.69 g piperazine/kg plus 1.0 g sodium nitrite/
litre, or 6.25 g piperazine/kg plus 0.25 g sodium nitrite/litre were
given. In the animals given 6.25 g piperazine per kg of food, plus
2.0 g sodium nitrite per litre of water, 39 out of 40 had adenomas,
in contrast with 5 out of 39 controls. A progressive decrease in the
incidence of adenomas was seen with reduction in the nitrite level.
On the other hand, when the nitrite level was maintained at
1.0 g/litre, the tumour incidence increased marginally when the dose
of piperazine was increased from 0.69 g to 18.75 g/kg of food.
Nitrite and piperazine administered alone yielded negative results.
When various concentrations of nitrite and morpholine (up to
1000 mg/kg) were fed to groups of Sprague-Dawley rats, the
development of hepatocellular carcinomas and angiosarcomas identical
to those produced by N-nitrosomorpholine was noted (Newberne &
Shank, 1973).
A 2-3% incidence of hepatomas was induced with only 5 mg of
morpholine plus 1000 mg of sodium nitrite per kg of food, or 1000 mg
of morpholine plus 50 mg sodium nitrite/kg. The tumour incidence was
98% when the concentration of both components was 1000 mg/kg, and 0%
when diet alone was given.
7.2.2.6 Transplacental carcinogenesis
Induction of neoplasms in offspring as a result of prenatal
exposure to various N-nitroso compounds and related substances has
been reported in different animal species including the rat, mouse,
golden hamster, guineapig, rabbit, dog, and monkey (Magee et al.,
1976). The various routes of administration (subcutaneous,
intraperitoneal, intravenous, oral, and inhalation) were equally
effective. However, a critical factor was the time of treatment during
gestation.
Since many of these substances appeared to be metabolically
activated to exert their carcinogenic action, the lack of an adequate
metabolic system during the first period of pregnancy may explain the
failure to observe tumours, when exposure was limited to this period.
DMN induced tumours in the offspring only when administered during the
last days of pregnancy (Alexandrov, 1968a). The data of Magee (1972)
are in keeping with these findings; the formation of 7-methylguanine
in the nucleic acids of fetal rat tissues was detected following
treatment with DMN on the 21st day of pregnancy but not on the
15th day.
When considering the effect of different acyl alkyl nitrosamides,
N-ethyl- N-nitrosourea was found to be more active than its methyl
analogue (Alexandrov, 1969b; Ivankovic & Druckrey, 1968).
The sensitivity of the nervous system at various stages of
prenatal development was examined in rats and golden hamsters by
Ivankovic & Druckrey (1968) by means of single intravenous injections
of N-ethyl- N-nitrosourea on different days during gestation. A
high incidence of tumours was observed after treatment on the 18th day
or shortly before delivery (21st day) but none developed when the
mother animals were treated before the 12th day. A positive dose-
response relationship was obtained with a single dose in the range of
5 to 80 mg/kg bodyweight on the 15th day. A single dose as low as
2 mg/kg was sufficient to produce malignant, neurogenic tumours in
2/25 newborn whereas in adult animals an effect was produced in 50%
of the animals by a single dose at 160 mg/kg. Thus a 50-times higher
sensitivity was demonstrated for the fetal nervous system. Similar
results were obtained by Swenberg et al. (1972) in Sprague-Dawley and
Fisher rats using a dose of N-ethyl- N-nitrosourea as low as
1 mg/kg. In a recent study on rats, Maekawa & Odashima (1975) explored
the effects of subcutaneous injections of N-butyl-1-nitrosourea on
embryonal, fetal, and newborn nervous systems. Treatment during early
gestation led only to the death of the embryo. When the compound was
given in the middle of the pregnancy (8-14 days), a high incidence of
nervous system tumours and pituitary tumours was found in the
offspring. Treatment late in pregnancy (15-21 days) led to the
development of nervous system tumours in 33/36 offspring.
When lactating animals were given the compounds, tumours were
induced in 19/39 of the offspring, the target organs being mainly the
testes and uterus.
Tomatis (1977) reported an increased incidence of cancer in the
descendants (second and third generation) of transplacentally treated
rats. In certain cases, the tumour-producing doses were lower than
those for adult animals. The target organs were usually similar in
fetal, newborn, and adult animals but the nervous system has been
shown to be highly sensitive to certain compounds, notably the
nitrosoureas. The induction time for transplacental tumours may be
shorter than that in adults.
7.2.2.7 Morphological studies
Morphological events associated with the development of hepatic
tumours, following exposure to N-nitroso compounds, were studied by
Rabes et al. (1970), Scherer et al. (1972), and Schmitz-Moorman et al.
(1972). Schmitz-Moorman et al. (1972) followed the carcinogenesis
induced by DMN in rat liver, using histological and histochemical
procedures. In the initial phase, there were vacuolar changes
accompanied by a decrease in the RNA and glycogen contents. In the
second phase, glycogen storage was noted while in the third phase,
basophilic cells with atypical nuclei were observed and could not be
distinguished from microcarcinomas. The RNA content of these cells was
substantially higher. The activities of acid phosphatase (3.1.3.2),
succinic dehydrogenase (1.3.99.1) and glucose-6-phosphatase (3.1.3.9)
substantially decreased. In the last carcinogenic stage, dramatic
changes were noted in several enzymes (Jennissen et al., 1971).
Early changes in the lung tissue of mice exposed to carcinogenic
doses of DMN were reported by Calafat et al. (1970) and DEN-induced
alterations in the respiratory tract of hamsters were reported by
Althoff et al. (1971). Greenblatt & Rijhsinghani (1969) compared the
cytopathological changes induced by DEN and DMN in the nasal
epithelium of hamsters; other light microscopic studies have been
published by Bertram & Craig (1972), Boyland et al. (1968), Hicks et
al. (1973), and Terracini et al., (1967). The histological appearance
of tumours under the electron microscope has been described by
Kirkland & Pick (1973). Veno-occlusive disease in the liver of rats
given DMN was reported by Butler & Hard (1971) who had also studied
the effects of this compound on rat testes (Hard & Butler, 1970c).
Other studies, by light microscopy, to observe early changes
related to the formation of neoplasms induced in the kidneys of rats
treated with DMN, were reported by Benemanski & Litvinov (1969), Hard
& Butler (1970a, 1970b), and Hard et al. (1971).
Electron microscopic studies of DMN-induced liver and kidney
tumours have been reported in rats (Bhathal & Hurley, 1973; Geil et
al., 1968; Hard & Butler, 1971a, 1971b, 1971c; Ireton et al., 1972;
Jasmin & Cha, 1969; Svoboda & Higginson, 1968) and in mice (Takayama,
1968). Treatment with DEN resulted in ultrastructural changes in the
lungs of hamsters (Stracks & Feron, 1973) and in the liver of monkeys
(Williams, 1970) and rats (Bader et al., 1971; Bruni, 1973). Early
morphological changes in the bladder of rats exposed to N-butyl- N-
butanol-4-nitrosamine were reported by Riedel & Piper (1973).
7.2.2.8 Biochemical mechanisms
Extensive work on the biochemical mechanisms of carcinogenesis
produced by N-nitroso compounds has been reviewed by Magee & Barnes
(1967) and more recently by Magee et al. (1976). Alkylation of nucleic
acids by N-nitroso compounds or their metabolites has been
investigated extensively and has been suggested as the mechanism of
carcinogenicity (Krüger, 1972, 1973; Lijinsky et al., 1973b; Swann &
Magee, 1971; Takayama & Muramatsu, 1969). In early studies, it was
thought that the 7 position of guanine was the significant site of the
reaction.
Following the important discovery by Loveless and Hampton (1969)
of the O,6-alkylation of deoxyguanosine by nitrosomethylurea (NMU)
and the implications of this reaction in terms of carcinogenicity,
O'Connor et al. (1973) estimated the amount of methylation at the
O,6-position of guanine DNA isolated from animals treated with DMN.
This base accounted for 4-6% of the methylation after DMN treatment.
O,6-methylguanine was lost (by "excision") from DNA with a half life
of approximately 13 h. The excision of the abnormal components of DNA,
O,6-methylguanine, and the unstable acid-labile products, may be
important processes in liver carcinogenesis. O'Connor et al. (1973)
suggested that events leading to the development of tumours may be
related to the efficiency of the cellular excision system for certain
products of alkylation rather than to the level of alkylation obtained
at a particular site.
7.2.2.9 Interaction with various chemical factors
Several studies are available on the combined effects of
N-nitroso compounds and other carcinogens. Schmähl et al. (1963)
showed that combined oral administration of the hepatocarcinogens DEN
and N,N-dimethyl-4-(phenylazo)benzenamine (4-dimethyl-
aminoazobenzene) significantly reduced the time for tumour induction
and that only 66% of the total dose administered when single compounds
were used, was necessary for tumour induction. Takayama & Imaizumi
(1969) also demonstrated synergism with the combined administration of
DMN and 4-dimethylaminoazo-benzene. Liver tumours were induced in rats
by sequential administration of the two carcinogens in doses that did
not induce tumours when each carcinogen was given alone and the tumour
induction time was reduced. The syncarcinogenic effects of a single
dose of a combination of DEN and carbon tetrachloride were observed by
both Pound et al. (1973) and Schmähl et al. (1965). The incidence of
liver cancer increased and the induction time decreased in combined
treatments compared with treatment with the individual compounds.
Schmähl (1970) administered hepatocarcinogens (DMN, DEN,
nitrosomorpholine, and 4-dimethylaminoazobenzene) to rats in such low
daily doses that the administration of any one of the compounds alone
did not lead to tumours during the lifetime of the animals. However,
combined administration induced tumours in 43% of the treated animals.
Combined administration to rats of DMN plus 1,2-dihydro-3-methyl-
benz[j]aceanthrylene (3-methylcholanthrene) did not increase the
number of liver tumours compared with that induced by DMN alone but
resulted in tumours in the lungs, that were not seen in the treatment
with DMN alone (Hoch-Ligeti et al., 1968).
1-Isothiocyanatonaphthalene (1-napthyl-isothiocyanate) and
3-methyl-cholanthrene failed to inhibit the hepatocarcinogenic
effects of DEN (Makiura et al., 1973). Similarly, local treatment of
the glandular mucosa of the stomach of rats with 4-nitroquinoline-
1-oxide or p-dimethylaminoazobenzene did not have any effect on
the production of liver or oesophagal tumours due to oral
administration of DEN (Odashima, 1969). It has been reported that
ionizing radiation did not increase tumour incidence in animals
exposed to nitrosamines (Flaks et al., 1973; Schmähl et al., 1966).
The effect of many other substances on nitrosamine carcinogenesis
has been studied. For example, various dusts including aluminium (III)
oxide, magnesium (II) oxide, and carbon had little effect on the
respiratory carcinogenesis of DEN in hamsters (Stenback et al., 1973)
but other studies on hamsters demonstrated that iron (III) oxide
markedly increased the incidence of respiratory tract tumours induced
by DEN (Feron et al., 1972). The synergistic effects of various
substances on respiratory carcinogenesis in hamsters given N-nitroso
compounds were reviewed by Montesano (1970).
The effect of noncarcinogenic chemicals on the incidence of other
tumours induced by N-nitroso compounds has also been studied. In
rats given DEN, liver tumour incidence was decreased by the
administration of calcium heparin (Platt & Hering, 1973),
aminoacetonitrile (Hadjiolov, 1971), and reserpine (Lacassagne et al.,
1968). Lactoflavin, nicotinamide, or 2,6-bis-(diethanolamino)-4,8-
dipiperidino-pyrimido [5,4-d] pyrimidine (dipyridamole) (Schmähl &
Stackelberg, 1968) and hydrocortisone (Schmähl et al., 1971) did not
influence the incidence of liver tumours induced in rats by DEN.
Tryptophan was shown to inhibit the production of liver tumours, but
not bladder tumours in rats exposed to N-nitrosodibutylamine
(Okajima et al., 1971). N-(2-chloroethyl)- N (phenylmethyl)
benzenemethanamine (dibenamine) did not have any effect on the
incidence of oral, pharyngeal, or oesophageal tumours in DEN-treated
rats, but did significantly reduce the number and severity of hepatic
neoplasms (Weisburger et al., 1974). In mice, treatment with
phenobarbital decreased the toxicity and carcinogenicity of DEN (Kunz
et al., 1969).
7.2.2.10 Miscellaneous modifying factors
Nasal infection of mice with the influenza viruses PR8/FIK and
A2/Bethesda 10/63 followed by treatment with DEN significantly
increased lung tumour incidence in comparison with that in mice
treated only with DEN (Schmidt-Ruppin & Papadoupulu, 1972). The Motol
virus has been shown to increase hepatic carcinoma in mice given DEN
(Kordac et al., 1969). Rats with chronic respiratory disease showed an
increased lung tumour incidence when given nitrosoheptamethylenemine
compared with germ-free or specific pathogen-free animals (Schreiber
et al., 1972).
Feeding with a protein-deficient diet protected rats against the
lethal and hepatotoxic effects of DMN (McLean & Verschuuren, 1969) but
increased the incidence of renal carcinomas (McLean & Magee, 1970).
Low protein diets and low zinc intake failed to influence the
incidence of oesophageal tumours in rats treated with N-methyl- N-
nitroso-'pentyl' amine (Van Rensburg, 1972). A single intraperitoneal
injection of DMA induced nasal tumours in rats, previously starved for
48 h (Noronha & Goodall, 1972).
The carcinogenic activity of nitroso- N-(hydroxybutyl)
butanamine (butyl-( N-hydroxybutyl) nitrosamine) appears to be
influenced by sex hormones (Bertram & Craig, 1972). Tumours developed
much earlier in male, than in female mice. This difference was
abolished, however, if the males were castrated or, conversely, if the
females were treated with testosterone.
Surgical manipulation of experimental animals may influence the
induction of tumours by N-nitroso compounds. This has been shown for
partial hepatectomy (Craddock, 1971; Grunthal et al., 1970; Rabes et
al., 1971) unilateral nephrectomy (Ito et al., 1969) and ureter
ligation (Ito et al., 1971).
7.2.3 Embryotoxicity and teratogenicity
Whereas nitrosamines are reported to have toxic and lethal
effects on the embryo, usually at dose levels that are toxic to the
mother animals, nitrosamides ( N-ethyl-and N'methyl urea) bring about
malformations of several of the organs and systems of the developing
organisms at levels not toxic to the pregnant animal. Animal
experiments have shown that immature tissues are especially sensitive
to these compounds. Dose-response relationships have been established
and the existence of a noneffect level has been indicated.
DMN, administered orally at 30 mg/kg to pregnant rats, produced
increased prenatal mortality (Alexandrov, 1967). Toxic effects on the
embryo were noted following intravenous or intraperitoneal
administration of DMN at various times during gestation but no
teratological abnormalities were observed. Similar results were
observed with DEN and with nitroso- N,N-bis butanamine (nitrosodi-
N-butylamine) in rats (Alexandrov 1967), and with DEN in hamsters
(Pielsticker, 1967). The aromatic nitroso compounds such as
nitrosomethylbenzenamine (nitrosomethylaniline) were toxic to the
embryo and had a mild teratogenic action when given to rats at the
maximum tolerated doses (Alexandrov, 1968a, 1968b).
Treatment of pregnant rats with a single dose of 10-30mg of
nitrosomethylurea per kg body weight on day 9 of gestation produced
anophthalmia, hydrocephaly, exencephaly, and occasionally spina bifida
while treatment on days 12-15 produced microcephaly (Alexandrov,
1969a; Koyama et al., 1970; Napaikov, 1971; Von Kreybig, 1965a, 1965b;
Von Kreybig & Schmidt, 1966, 1967).
The teratogenic action of nitrosoethylurea in rats was described
by Druckrey et al. (1966) and Ivankovic & Druckrey (1968) who noted
that oligodactylia and syndactylia of the fore and hind limbs were
dose-related. Von Kreybig & Schmidt (1967) and von Kreybig (1968)
confirmed these effects and also found brain anomalies. Napalkov
(1971) and Wechsler (1970, 1971) reported similar results. The
teratogenic and embryotoxic effects of N-nitroso compounds including
the pathogenesis of brain lesions were reviewed by Wechsler (1973).
7.2.4 Mutagenicity
The mutagenicity of N-nitroso compounds was reviewed by Magee &
Barnes (1967) and more recently by Montesano & Bartch (1976). Until
recently, the data available were mainly concerned with the
nitrosamides, the data on nitrosamines being limited to test systems
which do not take into consideration the metabolic activation of these
compounds by mammalian enzymes. As with other biological effects,
there is a clear distinction between the mutagenic action of
nitrosamides and nitrosamines. Nitrosamides were found to be mutagenic
to almost all genetic indicators; this was attributed to the
nonenzymatic formation of alkylating reactants.
On the other hand, nitrosamines have been reported, prior to
1970, to have a much more limited range of mutagenic activity; they
were found to be mutagenic in tests with Drosophila melanogaster
(Pasternak, 1964) but no such activity was observed in assays in
which bacteria, yeast, or fungi were used. Malling (1966) showed that
DMN and DEN were mutagenic for Neurospora crassa when the conidia
were suspended in Udenfriend's hydroxylating model system and the
treatment carried out under conditions believed to give rise to the
same metabolic products as those formed by the action of liver enzymes
in rat and mouse. Garbidge & Legator (1969), using the host-mediated
assay, were the first to show that DMN, administered to mice, induced
mutations in Salmonella O,phimurium, which had been injected
beforehand and was then reisolated from the peritoneal cavity. Most of
the mutagenicity assays were carried out using bacteria or fungi as
genetic indicators in the presence or absence of a microsomal
activation system. Only a few data are available on the mutagenicity
of DMN in mammalian systems such as the dominant lethal test or
cytogenetic studies. However, in general, the last two systems have a
very low sensitivity and false negatives can easily result with
N-nitroso compounds. The early observation of Pasternak (1962)
that DMN induced lethal mutations in Drosophila melanogaster
demonstrated the possible value of this system for testing the
mutagenicity of compounds that require metabolic activation.
The mutagenicity of certain nitrosated pesticides, herbicides,
and primary amines has been examined. The N-nitrosated derivatives
of the pesticides propoxur and carbaryl, which are aryl- N-methyl
carbamates, and of the herbicide benzothiazuron, a methylurea
derivative, induced mitotic gene conversion in Saccharomyces
cerevisiae (Sibert & Eisenbrand, 1974). N-nitrosocarbaryl was
also found to be mutagenic in Haemophilus infiuenzae (Elespuru
et al., 1974). Endo et al. (1973) screened a number of
N-nitrosated guanidine derivatives for their ability to induce
base pair substitutions in Salmonella typhimurium and a mutagenic
effect was observed for many of these compounds; nitrosated
methylguanidine was the most potent.
The mutagenicity of sodium nitrite, DMA, methylurea, and
ethylurea given orally to mice, has been demonstrated in a host-
mediated assay using a strain of Salmonella typhimurium as an
indicator (Couch & Friedman, 1975). When combined with sodium nitrite,
both ethylurea and methylurea had a greater effect than DMA.
Other properties of N-nitroso compounds such as cell
transformation in vitro and their influence on DNA repair mechanisms
are being investigated, but as yet, there are not sufficient data for
their evaluation.
8. EFFECTS OF NITRATES, NITRITES AND N-NITROSO COMPOUNDS ON MAN
8.1 Nitrates and Nitrites
In the erythrocytes of healthy individuals, the process of
methaemoglobin formation and reduction is continuous. The mean content
of methaemoglobin in healthy populations is usually reported to be
below 2% of the total haemoglobin concentration (Committee on Nitrate
Accumulation, 1972; Gobbi et al., 1974; Smith, 1972). However,
Goldsmith et al. (1975) recently found mean levels in Californian
populations ranging up to 2.11% with 1% of the adults and 8% of
infants having methaemoglobin levels exceeding 4%. Higher values are
found in premature than in full-term infants and levels in infants are
higher than those in older children and adults (Kravitz et al., 1956).
At a level of about 10%, methaemoglobinaemia may produce symptomless
cyanosis, whereas levels of 20-50% are associated with conspicuous
cyanosis accompanied by hypoxic signs and symptoms, such as weakness,
exertional dyspnoea, headaches, tachycardia, and loss of consciousness
(Arena, 1970; Committee of Nitrate Accumulation, 1972; Jaffé & Heller,
1964). The lethal concentration of methaemoglobin is not known, but
death may occur at levels exceeding 50% (Committee of Nitrate
Accumulation, 1972).
8.1.1 Epidemiologicai studies
The National Academy of Sciences, USA (Committee on Nitrate
Accumulation, 1972) recently reviewed about 350 cases of
methaemoglobinaemia in the USA and about 1000 cases in Europe that
were reported to be associated with the intake of nitrates in well
water or in food. There were 41 fatalities in the USA and about 80 in
Europe. Only one case of infant methaemoglobinaemia resulting from the
consumption of water from a municipal water supply was reported in the
USA (Vigil et al., 1965).
8.1.1.1 Exposure through water
The toxicity to man of nitrates in water was first reported by
Comly (1945). He noted high levels of methaemoglobin and the
associated signs of nitrate toxicity in 2 infants who had consumed
water containing high concentrations of nitrates (619 and
388 mg/litre). Since then, several epidemiological and case studies
have been carried out in various parts of the world, particularly in
areas with naturally high nitrate levels in water.
Robertson & Riddell (1949) reported 10 cases in which infants
receiving powdered milk preparations made with well waters with
nitrate concentrations exceeding 75 mg/litre had blood levels of
methaemoglobin ranging from 5 to 50% of total haemoglobin. Two of
the infants, whose dried milk preparations had been reconstituted
with well waters containing nitrate levels of 1200-1300mg/litre, had
methaemoglobin levels of 25% and 44% of total haemoglobin,
respectively; both cyanosed rapidly and died before therapy could be
applied.
In the state of Minnesota (USA), Bosch et al. (1950) reported 139
cases of infant methaemoglobinaemia due to the ingestion of well water
with a high nitrate content (over 89 mg/litre); the mortality rate was
10%. In Kansas, 13 cases of infant methaemoglobinaemia including 3
deaths caused by drinking well water, were reported between the early
1940s and 1950 (Walton, 1951). Many other cases in the USA have been
reported by this author. On the other hand, infant methaemoglobinaemia
was absent in urban areas of New York and the province of Ontario,
Canada where nitrate levels in water were low (Ciavaglia & Thompson,
1969). Knotek & Schmidt (1964) reported 115 cases of methaemo-
globinaemia from a total of 5800 children born in central
Czechoslovakia between 1953 and 1960. Of these, 8% were fatal, 52%
were severe, and 40% were mild. Most deaths were associated with the
consumption of water containing nitrate concentrations ranging from
70 to 250 mg/litre. In these cases, methaemoglobinaemia was
invariably associated with the consumption of infant milk
preparations, which contained microorganisms such as B. subtills,
capable of reducing nitrates to nitrites. These workers reported the
inhibitory effect of buttermilk on this conversion. They attributed
this effect to the presence of Streptococcus lactis which
produces the antibiotic nisin and is capable of preventing the growth
of the spores of B. subtilis.
Shuval & Gruener (1972) studied communities with various
concentrations of nitrates in the drinking water, in Israel. Infants
from rural communities with reported nitrate levels of 50 --
90 mg/litre in the water supplies were compared with controls where
the average level was 5 mg/litre. They did not find any definite cases
of methaemoglobinaemia nor were there any significant differences in
the methaemoglobin levels. However, there was widespread consumption
of citrus juices and the water consumption in infant milk preparations
was low (94% of the infants studied were breast fed or received whole
cow's milk). Soviet literature contains information concerning a
comparatively small number of cases of symptomless methaemoglobinaemia
caused by nitrates in water (Diskalenko, 1969; Motylev, 1969). In
children whose drinking water contained a high level of nitrates, the
methaemoglobin level did not usually exceed 10% although higher levels
were found occasionally. Sattelmacher (1962) and Simon et al. (1964)
compiled 1060 and 745 cases, respectively, of infant
methaemoglobinaemia due to nitrate-contaminated water in the Federal
Republic of Germany. Most of the cases were associated with water
from private wells and in 84-90% of the cases the water contained
nitrate concentrations exceeding 100 mg/litre (although a few cases
of methaemoglobinaemia were reported with water containing less than
50 mg/litre).
Commoner et al. (1972) found small, but statistically
significant, subclinical elevations in methaemoglobin levels in adults
exposed to high intakes of nitrates in a rural area when compared with
an urban control population. There is also a small amount of data
showing that under similar conditions, pregnant women in rural areas
have higher methaemoglobin levels than those in urban areas. This is
of particular interest since previous workers have reported an
increased susceptibility to nitrates in pregnant women. (Skrivan,
1971). Thus, there is concern over the effects on the fetus of the
general lowering of oxygen tension. Gelperin et al. (1971) recently
reported the presence of methaemoglobinaemia in a newborn infant,
presumably exposed to nitrates transplacentally. In this study, 72
mothers and infants tested were exposed to water with nitrate
concentrations ranging from 28 to 45 mg/litre over a 2-month period.
During the 2 weeks of maximum concentration (45 mg/litre), the average
methaemoglobin levels were 1.18% for mothers and 1.91% for newborns
with those of one mother and one infant rising to 6.39% and 5.87%,
respectively.
Children aged 12-14 years who drank water with a nitrate level of
105 mg/litre were noted to have slightly delayed reactions to light
and sound stimuli combined with a mean methaemoglobin level of 5.3% in
comparison with control children drinking water with a nitrate level
of 8 mg/litre whose methaemoglobin levels average 0.75% (Petukhov &
Ivanov, 1970).
8.1.1.2 Exposure through vegetables
Cases of methaemoglobinaemia, some resulting in death, have been
observed following the consumption of spinach. Hölscher & Natzschka
(1964) reported 2 cases in young infants (aged 2 and 3.5 months) who
had eaten spinach purée. Fresh spinach from the same source contained
only traces of nitrates but had nitrite ion levels of 2180 mg/kg.
Fourteen further cases of methaemoglobinaemia in infants (aged 2-10
months) were reported from the Federal Republic of Germany (Sinios &
Wodsak, 1965). Unprocessed spinach was used almost exclusively in the
preparation of the infants' meals and preparation took place at least
24 h before the mealtime. Since in most cases some of the same spinach
had been eaten 24 and 48 h before without causing illness, the authors
assumed that the nitrites were formed within the final 24 h of
storage. Information on 7 cases showed that the mother tasted the
spinach before feeding the child and no change in taste was apparent.
None of the children refused the meal which caused the poisoning.
Conversion of nitrates to nitrites in fresh spinach was demonstrated
by Schuphan (1965) (section 4.2.1). Keating et al. (1973) reported a
case of methaemoglobinaemia in an infant given carrot juice. Other
cases of food-induced methaemoglobinaemia have recently been reviewed
by Luhrs (1973).
8.1.1.3 High accidental exposures through food
Certain meat products contain nitrates and nitrites. Normally, no
adverse effects result from consumption of such products. However, in
1955, an outbreak of 10 cases of methaemoglobinaemia in children
occurred in New Orleans, USA, attributed to the consumption of large
amounts of nitrites in sausage meats (Orgeron et al., 1957). Further
studies revealed that the meats had nitrite concentrations of more
than 200 mg/kg and that some had levels as high as 6570 mg/kg. Singley
(1962) reported 3 cases of methaemoglobinaemia resulting from the
consumption of fish, that had been adulterated with sodium nitrite.
One patient died and it was assessed that he had consumed
approximately 33 mg sodium nitrite/kg body weight. Other cases of
poisoning involving the consumption of nitrite-treated sausage and
frankfurters were reported by Bakshi et al. (1967) and Henderson &
Raskin (1972).
8.1.1.4 Ambient air exposures
Effects on man of nitrate aerosols in ambient air were not
considered by the Task Group. However, because of the recent concern
with the role of nitrates in urban air pollution, it may be of
interest to briefly summarize the current information on this problem.
Airborne nitrates may act as respiratory irritants (Knelson & Lee,
1977) and a recent study conducted by the US Environmental Protection
Agency in the New York-New Jersey metropolitan area showed that
increased asthmatic attacks were significantly associated with
elevated levels of suspended nitrates in six of the seven communities
studied. No such effect was observed with nitrogen dioxide. Another
study in two south-eastern communities in the USA showed some evidence
that a combination of suspended nitrates and suspended sulfates
increased the risk of asthma attacks more than either pollutant did
alone. Because of the present difficulties in measuring suspended
nitrates, these results should be considered as qualitative instead of
quantitative (French et al., unpublished data)a.
8.1.2 Factors involved in susceptibility to nitrates
The work of Marriott et al. (1933), who investigated the acidity
and bacterial flora of 200 infants suffering from diarrhoea, supports
the hypothesis that lack of acidity in the gastric juices of newborn
infants might permit the growth of nitrate-reducing organisms in the
upper gastrointestinal tract and, thus, the reduction of nitrates to
nitrites before the former could be completely absorbed. The pH of the
a French, J. G., Hasselblad, V., & Johnson, R. Aggravation of
asthma by air pollutants. 1971-72 Southeastern CHESS studies.
stomach contents of healthy infants varied from 2.0 to 5.0 (average
3.7) and that for infants suffering from bacillary dysentery ranged
from 2.0 to 5.0 (average 3.0). However, for infants with nonspecified
diarrhoea the pH varied from 4.6 to 6.5 (average 5.6).
According to estimates made by Burden (1961), the water intake of
young infants is nearly 10 times higher than that of adults on a per
unit body weight basis.
The susceptibility of infants to methaemoglobinaemia during the
first six months of life, and especially during the first trimester
(Bailey, 1966; Kübler, 1965) can be explained by various mechanisms,
none of which is fully understood at present. It has been reported
that fetal haemoglobin, which in newborn infants makes up to 60-80% of
the total haemoglobin decreasing to about 20-30% in 3 months (British
Medical Journal, 1966; Kübler, 1965), is more readily oxidized to
methaemoglobin than adult haemoglobin (Betke, 1953; Künzer &
Schneider, 1953). This might explain why premature infants, who
frequently have a higher percentage of fetal haemoglobin than full-
term infants are more susceptible to methaemoglobinaemia. Keohane &
Metcalfe (1960) reported that the sensitivity of erythrocytes to
oxidation to methaemoglobin on exposure to nitrites gradually declined
during childhood until the age of puberty, after which it decreased
rapidly. This decline in sensitivity was not related to the
disappearance of fetal haemoglobin and the authors suggested that some
other factor might be responsible. The susceptibility of newborn and
young infants to develop methaemoglobinaemia could also be attributed
to the incomplete development of the NADH methaemoglobin reductase
system. Several studies have shown that the erythrocytes of newborn
and young infants have a lower capacity to reduce methaemoglobin than
those of older children and adults and that the erythrocytes of
premature newborns have a lower reduction capacity than those of full-
term infants (Bartos et al., 1966; Ross, 1963; Ross & Desforges,
1959). Except in rare cases of hereditary enzyme deficiency (Balsamo
et al., 1964), this deficiency in the NADH methaemoglobin reductase
system seems to disappear after the first 3-4 months of life (Bartos
et al., 1966: Künzer & Schneider, 1953).
The haemoglobin of pregnant women and that of patients suffering
from carcinomata has also been reported to be sensitive to oxidation
to methaemoglobin. However, this sensitivity disappeared in the first
instance after delivery and in the second, after radical extirpation
of the neoplasms (Metcalf, 1961).
8.1.3 Dose-response relationships for nitrates and nitrites
Estimates of the effective exposure of the general population to
nitrates and nitrites have been discussed briefly in section 5.1.4.
However, this information is not sufficient to relate environmental
concentrations to actual intake of nitrates or nitrites in cases of
observed methaemoglobinaemia. Retrospective epidemiological studies
discussed in section 8.1.1 provide little information on intake
because in most cases either the data concerning concentrations in
water or food were not reliable enough, or the amounts of water or
food consumed had not been measured. However, a study reported by
Winton et al. (1971) considered the nitrate intake from water in some
detail. The study was conducted in southern California and central
Illinois, USA, and involved 111 infants whose ages ranged from less
than two weeks to six months. The mother of each infant was asked
about the fluid intake of the infant in the previous 24 h, the method
of formula preparation, and the possible inclusion of any other source
of nitrate in the diet (e.g. vegetables) or administration of
methaemoglobin-forming medicines. The variables that determined the
nitrate dose were the daily fluid intake per body weight (which might
increase in hot and arid climates, or with fever), the fraction of the
total daily fluid intake taken in the form of water, and the
concentration of nitrates in the water. Using this method, it was
possible to estimate that a daily dose of 10 mg/kg body weight could
be obtained from water containing a nitrate concentration of
50 mg/litre. The daily water intake varied from 10% of the total daily
fluid intake for breast-fed infants or for those receiving ready-to-
feed preparations to 90% for infants who were fed preparations made
with powder. This shows that no generalization is possible, at
present, about the relationship between the nitrate concentration in
drinking water and the dose of nitrate, and that estimates of nitrate
dose from nitrate concentrations in water or food would have to be
made for individual cases, taking into account local conditions and
dietary habits.
The relationship between the doses of nitrate and methaemoglobin
levels is even more difficult to establish because of large individual
differences in response, depending on age and many other host
variables. For example, using the method described in the previous
paragraph, Winton et al. (1971) found that in a group of 111 infants,
63 received a nitrate dose of less than 1 mg/kg body weight, 23 were
exposed to 1-4.9 mg/kg, 20 to 5.0-9.9 mg/kg, and 5 infants to
10-15.5 mg/kg. However, only 3 infants appeared to have methaemoglobin
levels above normal (0-2.9%) and they were the youngest of the five
who had received more than 10 mg/kg. The highest methaemoglobin level
(5.3%) was found in 30-day-old baby who had received 15.5 mg/kg.
Another possible approach is to analyse the available retrospective
epidemiological data. This has been done by the Committee on Nitrate
Accumulation (1972) and by Diskalenko (1968) who concluded that only
very crude estimates of correlations between nitrate concentration in
water and methaemoglobin levels could be obtained, probably because of
the delay between the analyses of water and blood, difficulties in
identifying the sources of water consumed, and the heterogeneity of
the population samples studied, particularly with respect to age. The
analyses performed by the Committee on Nitrate Accumulation (1972)
showed, for example, that an increase in the nitrate concentration in
water from 0-49 mg/litre to 49-98 mg/litre, increased the
methaemoglobin level, on average, from 1.0% to 1.3% in infants aged
0-3 months, but did not affect the methaemoglobin level (0.8%) in
infants aged 3-6 months (Simon et al., 1964). Methaemoglobin and
haemoglobin levels, measured in 96 infants from 22 localities in
Rheinhessen, Federal Republic of Germany, during official maternal
counselling, were correlated with the nitrate concentrations of the
drinking water in the localities concerned by Würkert (1974,
unpublished data)a. Nitrate concentrations of 0-5 mg/litre,
31-50 mg/litre, and over 100 mg/litre corresponded to methaemoglobin
levels of 1.65%, 2.44%, and 6.59%, respectively. Higher methaemoglobin
levels were found in the presence of infections and in infants given
tea to drink or vegetable nutrients.
The last question pertains to the relation between the level of
methaemoglobin and clinical signs and symptoms of methaemoglobinaemia.
Normally, methaemoglobin is present in the blood at a concentration of
less than 2% of total haemoglobin.
Subclinical methaemoglobinaemia (less than 10% methaemoglobin)
has not been considered to be of direct health significance, but
Petukhov & Ivanov (1970) reported behavioural effects at these levels.
Clinical signs of methaemoglobinaemia such as cyanosis become
apparent at a methaemoglobin level of about 10%. Hypoxic signs and
symptoms may develop at levels exceeding 20% and death may occur at
levels of 50% or more.
In conclusion, the available information does not permit the
establishment of a quantitative dose-response relationship for human
exposure to nitrates in water or food.
8.2 N-nitroso Compounds
Freund (1937) first described acute intoxication by DMN. Barnes &
Magee (1954) described 2 cases of industrial intoxication due to DMN
in which one individual had hepatic cirrhosis at death; the other
survived but, 6 months later, was shown to have a hard liver suspected
of being cirrhotic. Watrous (1947) and Wrigley (1948) reported cases
of accidental exposure to N-nitroso-methylurethane. Reddening of the
conjunctiva and erythema of the face and feet developed quickly, and a
respiratory disorder developed later.
No reports are available concerning carcinogenesis in industrial
or other workers exposed to N-nitroso compounds, nor have
relationships been established, from epidemiological and analytical
data, that link cancer in man with exposure to N-nitroso compounds or
their possible precursors such as nitrates, nitrites, and compounds
a Thesis reported in the contributed of the Federal Republic of
Germany to the WHO environmental health critera document on
Nitrates, nitrites and N-nitroso compounds.
that can be nitrosated, occurring as food components, drugs, and
pesticides. A recent review of these data is available (Mirvish,
1976).
Some reports have been concerned with the possible etiological
role of N-nitroso compounds in nasopharyngeal cancer in south-east
Asia (Clifford, 1970; Fong & Chan, 1973a) and oesophageal cancer in
South Africa, Iran, and China (Burrell et al., 1966; Coordinating
Group for Research on the Etiology of Esophageal Cancer of North
China, 1974; Day, 1975; Harmozdian et al., 1975). However, no
relationship has been established; nitrosamines were detected in food
from these areas but this was not confirmed by mass spectroscopy
(Eisenbrand et al., 1976; Purchase et al., 1975).
The epidemiology of stomach cancer has been discussed from a
similar point of view by Correa et al. (1975), Endo et al. (1973),
Haenzel & Correa (1975), Hill et al. (1973), Mirvish (1971), and
Weisburger & Raineri (1975). It was suggested that nitrosamides might
be formed in the stomach from amides occurring in the diet, and might
then act locally on this organ. Relationships have been sought between
the occurrence of stomach cancer and the nitrate contents of the soil
or water in Chile, Colombia, and the United Kingdom (Hawksworth et
al., 1974; Hill et al., 1973; Zaldivar & Wetterstrand, 1975) but none
was established.
9. EVALUATION OF HEALTH RISKS TO MAN FROM EXPOSURE TO NITRATES,
NITRITES, AND N-NITROSO COMPOUNDS
9.1 Nitrates and Nitrites
9.1.1 General considerations
Man is exposed to nitrates and nitrites mainly through water and
food. Nitrate concentrations may be particularly high in drinking
water derived from dug wells. Nitrates in food may occur naturally or
may be added for various technological or even public health reasons
(e.g. addition of nitrates and nitrites to certain meat products to
protect against botulism). Although intake of very large doses of
nitrates can be fatal to man, such intake is not likely to occur
through environmental exposure, except in the case of infants and very
young children who are high risk groups because of their
susceptibility to nitrates and nitrites. Weekly intakes of nitrates by
members of the general population are difficult to evaluate but rough
estimates are available for England and the USA giving values of about
400-450 mg/week (85-105 mg from water; 210-225 mg from vegetables, and
about 110 mg from meat products).
These figures cannot be applied generally and a separate
estimation of the nitrate intake from food and water should be made
for each case, especially when the subjects are infants or young
children (section 8.1.3). Exposure to nitrates can also occur through
the inhalation of polluted air.
The assessment of health risks to man (section 9.1.2) has been
based on epidemiological studies and clinical evidence. The animal
data discussion in section 7.1, confirm the findings in man that
methaemoglobinaemia is the main toxic effect of nitrate and nitrite
ingestion. Methaemoglobinaemia is caused by nitrites, the reduction
products of nitrates. The reduction usually occurs through microbial
action either in the environment or in the body. The health risks from
exposure to nitrates is therefore related not only to their
concentration in drinking water and food, but also to the presence or
absence of conditions conducive to their reduction to nitrites. Young
infants constitute the most vulnerable group for the following
reasons:
(1) Lower acidity in their stomach allows the growth of certain
microbes that contain enzymes capable of reducing nitrates
to nitrites;
(2) Fetal haemoglobin, which constitutes a considerable
proportion of the haemoglobin of the young infant, and the
erythrocytes during childhood may be more susceptible to
conversion to methaemoglobin by the action of nitrites;
(3) The enzyme system capable of reducing methaemoglobin to
haemoglobin is deficient in the young infant; and
(4) The fluid intake of the young infant is higher than that of
the adult in relation to the body weight.
9.1.2 Assessment of health risks
Precise dose-response relationships could not be established by
the Task Group because of the existence of various strongly modifying
factors and the lack of accurate quantitative data. However, on the
basis of the available information, the Task Group reached the
following conclusions:
(a) General population -- The prevailing levels of nitrates and
nitrites in water and food do not seem to have any harmful
effects in adults and older children, although there are reports
of susceptible individuals who have been affected by meat treated
with nitrites, and of cases of poisoning resulting from the
ingestion of certain foods accidently containing excessive
amounts of nitrites (8.1.1.3). Subclinical methaemoglobinaemia
may also be found in individuals consuming water containing high
levels of nitrates (8.1.3).
(b) Susceptible group -- Infants less than 6 months old and
especially those under 3 months of age are particularly
susceptible to methaemoglobinaemia caused by intake of water
containing elevated levels of nitrates, especially when they are
fed with preparations made from dried milk of low acidity. While
a few cases of methaemoglobinaemia have been reported associated
with water nitrate levels of less than 50 mg/litre, most cases
occur with nitrate levels of 90 mg/litre or more. Nitrates in
water may cause death of the infant, but the lowest level that
may be fatal cannot be estimated at present.
The ingestion of vegetables (e.g. spinach, carrots) containing
elevated nitrate and/or nitrite levels may also cause
methaemoglobinaemia in infants, especially in those aged between 6
months and 1 year. Storage of vegetables, other than in the frozen or
canned state, is likely to increase the nitrite level and hence the
risk.
(c) Effects of airborne nitrates -- A few recent studies have
indicated that airborne nitrates may act as respiratory irritants
but, at present, adequate quantitative data are not available and
the Task Group did not consider this exposure in their health
risk evaluation.
9.2 N-nitroso Compounds
9.2.1 General considerations
Nitrites (and indirectly nitrates) can react with amines and
amides to form nitrosamines and nitrosamides. The precursors of these
N-nitroso compounds are widely distributed in various environmental
media. Information concerning the presence of N-nitroso compounds
per se is limited although they have been identified in certain
foods such as luncheon meats and in air and water samples. The
conditions under which N-nitroso compounds can be formed are
outlined in sections 4.2-4.6.
More than 80% of over one hundred N-nitroso compounds tested
proved to be carcinogenic in animal experiments giving rise to tumours
in many organs and also producing tumours transplacentally.
N-nitroso compounds are carcinogenic in a wide range of animal
species; most are mutagenic in test systems and some have been shown
to be teratogenic to animals.
The possible health hazard from N-nitroso compounds is not
confined to those present in the environment. Their formation, from a
variety of precursors in the body of animals, has been demonstrated,
and this may also occur in man.
9.2.2 Assessment of health risks
A dose-response relationship has been shown to exist in different
species of rodents for some carcinogenic N-nitroso compounds. As the
dose is reduced, the tumour incidence decreases and the time for
tumour induction increases and may exceed the life span of the animals.
Although there is no clinical or epidemiological evidence, it is
highly probable that these compounds are also carcinogenic to man.
However, present limitations concerning available dose-response data
in animals and their interpretation, and inadequate knowledge of the
biomechanism of cancer induction preclude a quantitative estimation of
the carcinogenic risk to man that may be associated with different
exposures to N-nitroso compounds.
9.3 Reduction of Exposure
The assessments of health risk given in sections 9.1.2
[(a) and (b)] and 9.2.2 lead to a number of practical conclusions
concerning the need to reduce the exposure to nitrates, nitrites, and
N-nitroso compounds.
As regards the exposure to nitrates and nitrites, the Task Group
made the following specific recommendations:
(a) Infant dried milk preparations should be reconstituted only
with water containing low levels of nitrates. If such water is
not available, breast feeding or the use of cow's milk should be
encouraged.
(b) Only vegetables with a low nitrate content should be used in
the preparation of baby foods. If vegetables known to contain
high levels of nitrates are used, appropriate food processing
precautions should be instituted. Nitrates and nitrites should
not be added to baby foods.
(c) The use of nitrates and nitrites in foods as preservatives
should be reduced to the minimum level that provides protection
against botulism. This applies particularly to cured and canned
meats and to fish. The use of nitrates and nitrites on fresh
meats or fish should be avoided.
(d) Nitrate levels in public drinking water should comply with,
or preferably be lower than, the tentative limit of
45 mg/litre recommended in the International Standards for
Drinking Water (WHO, 1971).
With respect to the carcinogenic risk from exposure to
N-nitroso compounds, it is prudent to assume that any exposure may
involve some degree of risk, and that exposure should therefore be
kept as low as practically achievable. This may not be an easy task in
many instances, since these compounds may occur in the environment in
concentrations of the order of parts per billion, as a result of a
variety of natural and technological processes, and, moreover, they
may be formed in vivo from nitrates, amines, and amides, which
are ubiquitous. Obviously the recommendations (a) to (d) will also
contribute to the reduction of carcinogenic risk related to
N-nitroso compounds.
REFERENCES
ACHTZEHN, M. K. & HAWAT, H. (1969) [Nitrate accumulation in vegetables
a possibility of nitrate intoxication in infants.] Die Nahrung,
13: 667-676 (in German).
ACHTZEHN, M. K. & HAWAT, H. (1970) [On nitrate formation in vegetables
and vegetable products. Part I. Raw spinach.] Die Nahrung,
14: 383-394 (in German).
ADRIANO, C. C., PRATT, D. F., & BISHOP, S. E. (1971) Fate of inorganic
forms of nitrogen and salt from land disposed manures from
dairies. In: Livestock waste management and pollution
abatement. St. Joseph, MI (Am. Soc. Agric. Eng. Proc. No. 271).
ALAM, B. S., SAPORESCHETZ, I. B., & EPSTEIN, S.S. (1971a) Formation of
N-nitroso-piperidine from piperidine and sodium nitrite in the
stomach and the isolated intestinal loop of the rat. Nature
(Lond.), 232: 116-118.
ALAM, B. S., SAPORESCHETZ, I. B., & EPSTEIN, S.S. (1971b) Synthesis of
nitrosopiperidine from nitrate and piperidine in the gastro-
intestinai tract of the rat. Nature (Lond.), 232: 199-200.
ALARIF, A. & EPSTEIN, S.S. (1974) The uptake and metabolism of
14 C-labelled nitrosomethylurethane and nitrosomethylurea in
guinea pigs and their in vitro metabolism in the guinea pig
and human pancreas. IARC Sci. Publ. No. 9, pp. 215-219.
ALEXANDROV, V. A. (1967) [On the mechanism of carcinogenicity of
N-nitrosodimethylamine in connection with peculiarities of
its effect on embryogenesis] Vop. Onkol., 13: 87-92
(in Russian).
ALEXANDROV, V. A. (1968a) Blastomogenic effect of dimethylnitrosamine
on pregnant rats and their offspring. Nature (Lond.),
218: 280-281.
ALEXANDROV, V. A. (1968b) [Effect of N-nitroso- N-methylaniline and
N-nitroso- N-ethylaniline on the rat embryo] Vop. Onkol.,
14: 37-38 (in Russian).
ALEXANDROV, V. A. (1969a) [Transplacental blastomogenic action of
N-nitrosomethylurea in rat offspring] Vop. Onkol.;
15: 55-61 (in Russian).
ALEXANDROV, V. A. (1969b) Uterine, vaginal and mammary tumors induced
by nitrosoureas in pregnant rats. Nature (Lond.),
222: 1064-1065.
ALEXANDROV, V. A. & JANISCH, W. (1971) Teratogenic effect of ethylurea
and nitrite in rats. Experientia (Basel), 27: 538-539.
ALLISTON, T. G., COX, G. B., & KIRK, R. S. (1972)The determination of
steam-volatile N-nitrosamines in foodstuffs by formation of
electron-capturing derivatives from electrochemically derived
amines. Analyst, 97: 915-920.
ALTHOFF, J., WILSON, R., & MOHR, U. (1971) Diethylnitrosamine-induced
alterations in the tracheobronchial system of Syrian golden
hamsters. J. Natl Cancer Inst., 46: 1067-1071.
ALTHORPE, J., GODDARD, D. A., & SISSONS, D. J., TELLING, G. M. (1970)
The gas chromatographic determination of nitrosamines at the
picogram level by conversion to their corresponding nitramines.
J. Chromatogr., 53: 371-373.
ARENA, J. M. (1970) Poisoning, toxicology, symptoms and treatment.
2nd ed., Springfield, I, Bannerstone House, p. 9.
ARISON, R. N. & FEUDALE, E. L. (1967) Induction of renal tumour by
streptozotoc n in rats. Nature (Lond.), 214: 1254-1255.
ASAHINA, S., FRIEDMAN, M. A., ARNOLD, E., MILLAR, G. N., MISHKIN, M.,
BISHOP, Y., & EPSTEIN, S.S. (1971) Acute synergistic toxicity and
hepatic necrosis following oral administration of sodium nitrite
and secondary amines to mice. Cancer Res., 31: 1201-1205.
ASATOOR, A.M. & SIMENHOFF, M. L. (1965)The origin of urinary
dimethylamine. Biochim. Biophys. Acta, 111: 384-392.
ASHTON, M. R. (1970) The occurrence of nitrates and nitrites in foods.
BFMIRA lit. surv. No 7, pp. 32.
BADER, G., STILLER, D., & ULLRICH, K. (1971) [Ultrastructural changes
in toxically injured and proliferating liver cells due to long-
term application of diethylnitrosamine] Arch.
Geschwulstforsch., 37: 327-343 (in German).
BAILEY, W. P. (1966) Methemoglobinemia-acute nitrate poisoning in
infants: Second report. J. Am. Osteopath. Assoc.,
66: 431-434.
BAKSHI, S. P., FAHEY, J. L., & PIERCE, L. E. (1967) Sausage cyanosis--
Acquired methemoglobinemia nitrite poisoning.
N. Eng. J. Med. 277: 1072.
BALSAMO, P., HARDY, W. R., & SCOTT, E. M. (1964) Hereditary
methaemoglobinaemia due to diaphorase deficiency in Navajo
Indians. J. Pediat., 65: 928-931.
BANNASCH, P. (1968) In: The cytoplasm of hepatocytes during
carcinogenesis. New York, Springer Verlag.
BARNES, J. M. & MAGEE, P.M. (1954) Some toxic properties of
dimethylnitrosamine. Br. J. ind. Med., 11: 167-174.
BARTOS, H. R., DESFORGES, J. F., & CLARK, N. (1966) Erythrocyte DPNH
dependent diaphorase levels in infants. Pediatrics, 37: 991-993.
BEHROOZI, K., GUTTMAN, R., GRUENER, N., & SHUVAL, H. I. (1971) Changes
in the motor activity of mice given sodium nitrite drinking
solution. In: Proceedings of a Symposium on Environmental
Physiology, Beersheba, December, 1971.
BNEMANSKIJ, V. V. & LITVINOV, N. N. (1969) [Some data on the histo-
and morphogenesis of experimental tumours of the kidneys in
animals.] Arkh. Pat., 31: 79-84 (in Russian).
BERTRAM, J. S. & CRAIG, A. W. (1972) Specific induction of bladder
cancer in mice by butyl- (4-hydroxybutyl) -nitrosamine and the
effects of hormonal modifications on the sex difference in
response. Eur. J. Cancer, 8(6): 587-594.
BERTRAM, J. S. & CRAIG, A. W. (1973) Induction of bladder tumours in
mice with dibutylnitrosamine. Br. J. Cancer, 24
(2): 352-359.
BETKE, K. (1953) [Comparative examination of the oxidation of fetal
and adult oxyhaemoglobin by sodium nitrite.] Naturwiss., 40: 60
(in German).
BHATHAL, P.S. & HURLEY, J. V. (1973) An electron microscope study of
the production of ascites in acute dimethylnitrosamine (NDMA)-
induced liver injury. J. Pathol., 111: 103-116.
BIOCEV, IV. & POCINKOVA, Z. (1972) [Effect of applied artificial
fertilizers on the soil, drinking water and foodstuffs of
vegetable origin. Report on the International Congress of
Country Hygiene, Varna] (in Russian).
BLATTMANN, L. & PREUSSMAN, R. (1973) [The structure of metabolites of
carcinogenic dialkylnitrosamine in the urine of rats]
Z. Krebsforsch., 79: 3-5 (in German).
BLATTMANN, L. & PREUSSMAN, R. (1974a). [The biotransformation of
carcinogenic dialkylnitrosamines. Further urine metabolites of
di-n-butyl- and di-n-pentylnitrosamines.] Z. Krebsforsch.,
81: 75-78 (in German).
BLATTMANN, L., JOSWIG, N., & PREUSSMAN, T. (1974b). [The structure of
the metabolites of the carcinogenic methyl-n-butyl-nitrosamine in
the urine of rats.] Z. Krebsforsch., 81: 71-73 (in German).
BODIPHALA, T. & ORMROD, D. P. (1971) Factors affecting the nitrate
content of vegetable and fruit foods. Can. Inst. Food Technol.,
J., 4(1): 6-8.
BOGOVSKI, P. & WALKER, E. A., ed. (1974) N-nitroso compounds in the
environment. Proceedings of a Working Conference, the
International Agency for Research on Cancer, Lyons, France,
17-20 October, 1973, Lyons, International Agency for Research on
Cancer, pp. 243.
BOGGYSKI, P., PREUSSMANN, R., & WALKER, E. A., ed. (1972a) N-nitroso
compounds analysis and formation. Proceedings of a Working
Conference, Deutsches Krebsforschungszentrum, Heidelberg,
Federal Republic of Germany, 13-15 October, 1971, Lyons,
International Agency for Research on Cancer, pp. 140.
BOGGYSKI, P., CASTEGNARO, M., PIGNETTELI, B., & WALKER, E. A. (1972b)
The inhibiting effects of tannins on the formation of
nitrosamines. IARC Sci. Publ. No. 3, pp. 127-129.
BOLIN, B. & ARRHENIUS, E., ed. (1977) An essential life factor and a
growing environmental hazard. Report from Nobel Symposium No. 38,
Ambio, 6 (2-3): 96-105.
BOSCH, H. M., ROSENFIELD, A. B., HUSTON, R., SHIPMAN, H. R., &
WOODWARD, F. L. (1950) Methemogiobinemia and Minnesota well
supplies. J. Am. Water Assoc., 42: 161-170.
BOYLAND, E. & WALKER, S. A. (1974) Effect of thiocyanate on
nitrosation of amines. Nature (Lond.), 248: 601-602.
BOYLAND, E., CARTER, R. L., GORROD, J. W., & ROE, F. J. C. (1968)
Carcinogenic properties of certain rubber additives. Eur. J.
Cancer, 4: 233-239.
BRESLER, S. E., KALININ, V. L., & PERUMOV, D. A. (1968) Inactivation
and mutagenesis on isolated DNA. IV Possibility of integration of
lethal damage into the chromosome of Bacillus subtills during
transformation. Mutat. Res., 5: 329-341.
BRETSCHNEIDER, K. & MATZ, J. (1973) [Nitrosamines (NA) in the
atmospheric air and in the air at the workplace.] Arch.
Geschwulstforsch., 42: 36-41 (in German).
BRETSCHNEIDER, K. & MATZ, J. (1976) Occurrence and analysis of
nitrosamines in air. IARC Sci. Publ. No. 14, pp. 395-399.
British Medical Journal (1966), 1: 250-251. Spinach--a risk to
babies.
BROWN, J. R. & SMITH, G. E. (1967) Nitrate accumulation in vegetable
crops as influenced by soil fertility practises. Columbia,
University of Missouri, pp. 43 (Res. Bull. No. 920).
BRUNI, C. (1973) Distinctive cells similar to fetal hepatocytes
associated with liver carcinogenesis by diethylnitrosamine.
Electron microscopic study. J. Natl Cancer Inst.,
50: 1513-1528.
BUGLASS, A. J., CHALLIS, B.C., & OSBORNE, M. R. (1974)
Transnitrosation and decomposition of N- nitrosamines.
N- nitroso compounds in the environment. IARC Sci. Publ., No. 9,
pp. 94-100.
BURDEN, E. H. W. J. (1961) The toxicology of nitrates and nitrites
with particular reference to the potability of water supplies.
Analyst, 86 (1024): 429-433.
BURNS, R. C. & HARDY, R. W. F. (1975) Nitrogen fixation in bacteria
and higher plants. Berlin, Springer-Verlag.
BURRELL, R. J. W., ROACH, W. A., & SHADWELL, A. (1966) Esophageal
cancer in the Bantu of the Transkei associated with mineral
deficiency in garden plants. J. Natl Cancer Inst.,
36: 201-214.
BUTLER, W. H. & HARD, G. C. (1971) Hepatoxicity of dimethylnitrosamine
in the rat with special reference to veno-occlusive disease.
Exp. mol. Pathol., 15: 209-219.
CALAFAT, J., DEN ENGELSE, L, & EMMELOT, P. (1970) Studies on lung
tumours. II. Morphological alterations induced by
dimethylnitrosamine in mouse lung and liver and their relevance
to tumourigenesis. Chem. biol. Interact.,
2: 309-320.
CARDESA, A., MIRVlSH, S.S., HAVEN, G. T., & SHUBIK, P. (1974)
Inhibitory effect of ascorbic acid on the acute toxicity of
dimethylamine plus nitrite in the rat. Proc. Soc. Exp. Biol.
Med., 145: 124-128.
CARTER, R. L., PERCIVAL, W. H., & ROE, F. J. C. (1969) Exceptional
sensitivity of mink to the hepatotoxic effects of
dimethylnitrosamine. J. Pathol., 97 (1): 79-88.
CASEY, H. (1975) Variation in chemical composition of the River Frome,
England from 1965 to 1972. Freshwater Biol.,
5: 507-514.
CASTEGNARO, M., PIGNATELLI, B., & WALKER, E. A. (1972) N-Nitroso
compounds: Analysis and formation. IARC Sci. Publ, No. 3,
pp. 87-89.
CAVETT, J. J. (1962) The microbiology of vacuum-packed sliced bacon.
J. appl. Bact., 25: 282-289.
CHALLIS, B.C. (1973) Rapid nitrosation of phenols anti its
implications for health hazards from dietary nitrites. Nature
(Load.), 244: 466.
CHALLIS, B.C. & BARTLETT, C. D. (1975) Possible cocarcinogenic effects
of coffee constituents. Nature (Lond.),
254: 532-533.
CHALLIS, B.C. & CHALLIS, J. A. (1970) Reactions of the carboxamide
group. In: Zabicky, J., ed. The chemistry of amides, London,
Interscience, pp. 733-848.
CIAVAGLIA, F. & THOMPSON, R. P. (1969) Infant methemoglobinemia.
Absence in areas with low concentrations of nitrate-nitrogen in
water supply. N.Y. State J. Med., 69: 3128-3129.
CLAPP, N. K. & TOYA, R. E. (1970) Effect of cumulative dose and dose
rate on dimethylnitrosamine oncogenesis in RF mice.
J. Natl Cancer Inst., 45: 495-498.
CLAPP, N. K., TYNDALL, R. L., & OTTEN, J. A. (1971) Differences in
tumor types and organ susceptibility in BALB-C and RF mice
following dimethylnitrosamine and diethyinitrosamine. Cancer
Res., 31: 196-198.
CLIFFORD, P. (1970) On the epidemiology of nasopharyngeal carcinoma.
Int. J. Cancer, 5: 287-309.
COLLINS-THOMPSON, D. L., SEN, N. P., ARIS, B., & SCHWENGHAMER, L.
(1972) Non-enzymic in vitro formation of nitrosamines by
bacteria isolated from meat products. Can. J. Microbiol,
18 (12): 1968-1971.
COMLY, H. H. (1945) Cyanosis in infants caused by nitrates in well
water. J. Am. Med. Assoc., 129: 112-116.
COMMITTEE ON NITRATE ACCUMULATION (1972) Accumulation of nitrate.
Washington, DC, National Academy of Sciences. p. 48.
COMMONER, B. (1970) Threats to the integrity of the nitrogen cycle.
In: Singer, F. S., ed. Global effects of environmental
pollution, NY, Springer Verlag, pp. 70-95.
COMMONER, B., SHEARER, G., & KOHL, D. (1972) A study of certain
ecological, public and economic consequences of the use of
inorganic nitrogen fertilizer, St. Louis, Washington
University. (1st year Progress Report NSF Grant No. GI29926x).
COOKE, G. E. & WILTIAMS, R. J. B. (1970) Losses of nitrogen and
phosphorus from agricultural land. Water Treat. Exam.,
19 (3): 253-276.
COORDINATING GROUP FOR RESEARCH ON THE ETIOLOGY OF ESOPHAGEAL CANCER
OF NORTH CHINA (1974) The epidemiology of esophageal cancer in
North China and preliminary results in the investigation of its
etiological factors. Abstract, 11th International Cancer
Congress, Florence, Italy.
CORREA, P., HAENSZEL, W., CUELLO, C., TANNENBAUM, S., & ARCHER, M.
(1975) A model for gastric cancer epidemiology, Lancet, July 12,
pp. 58-59.
COUCH, D. B. & FRIEDMAN, M. A. (1975) Interactive mutagenicity of
sodium nitrite, dimethylamine, methylurea and ethylurea. Mutat.
res., 31: 109-114.
CRADDOCK, V. M. (1971) Liver carcinomas induced in rats by single
administration of dimethylnitrosamine after partial hepatectomy.
J. Natl Cancer IMNST, 47: 899-907.
CROSBY, N. T., FOREMAN, K. K., PALFRAMEN, J. F., & SAWYER, R. (1972)
Determination of volatile nitrosamines in food products at the
µg/kg level. IARC Sci. Publ. No. 3, pp. 38-42.
DAIBER, D. (1966) [Methods for the quantitative and qualitative
estimation of organic N- nitroso compounds.] Freiburg (Thesis)
(in German).
DAY, N. E. (1975) Some aspects of the epidemiology of esophageal
cancer. Cancer Res., 35 (2): 3304-3307.
DELWICHE, C. C. (1970) The nitrogen cycle. Sci. Am., 223: 137-146.
DISKALENKO, A. P. (1968) Methemoglobinemia of water-nitrate origin in
the Moldavian SSR. Gig. i Sanit., 33: 32-37.
DISKALENKO, A. P. (1969) [Methaemoglobinaemia caused by nitrates in
water and its prophylaxis.] Kisinev (in Russian).
DISKALENKO, A. P. & DOBRJANSKAJA, E. V. (1972) [Changes in redox
processes in hepatic and cerebral tissues as a result of the
ingestion of nitrates with drinking water. In: Topical
questions of hygiene and epidemiology] Kisinev, pp. 22-23 (in
Russian).
DISKALENKO, A. P. & TROFIMENKO, JU. N. (1972) [Changes in the activity
of the glutathione-ascorbic acid system and respiration in
erythrocytes as a result of the ingestion of nitrites with
drinking water. In: Topical questions of hygiene and
epidemiology Kisinev, pp. 23-25 (in Russian).
DOWNS, E. F. (1950) Cyanosis of infants caused by nitrate
concentrations in rural water-supplies. Bull. World Health
Organ., 3: 165-169.
DRUCKREY, H. & PREUSSMANN, R. (1962) [The production of lung cancer in
rats by subcutaneous injection of diamylnitrosamine.]
Naturwiss., 49: 111-112 (in German).
DRUCKREY, H., PREUSSMAN, R., SCHMÄHL, D., & MÜLLER, M. (1961) [The
chemical constitution and carcinogenic effects of the
nitrosamines.] Naturwiss., 48: 134-135 (in German).
DRUCKREY, H., STEINHOFF, D., BEUTHNER, H., SCHNEIDER, H., & KLÄRNER,
P. (1963a) [Screening of nitrite for chronic toxicity in rats.]
Arzneimittel-Forsch., 13: 320-323 (in German).
DRUCKREY, H., SCHILDBACH, A., SCHMAHL, D., PREUSSMANN, R., &
IVANKOVIC, S. (1963b) [Quantitative analysis of the carcinogenic
action of diethylnitrosamine.] Arzneimittel-Forsch,
13: 841-851 (in German).
DRUCKREY, H., IVANKOVlC, S., & PREUSSMANN, R. (1966) Teratogenic and
carcinogenic effects in the offspring after single injection of
ethylnitrosourea in pregnant rats. Nature (Lond.),
210: 1378-1379.
DRUCKREY, H., PREUSSMANN, R., IVANKOVIC, S., & SCHMÄHL, D. (1967)
[Organotropic carcinogenic effects of 65 different N-nitroso-
compounds in BD-rats.] Z. Krebsforsch, 69: 103-201 (in German).
DRUCKREY, H., LANDSCHÜTZ, CH., & PREUSSMANN, R. (1968) [Oesophageal
carcinoma due to inhalation of methylbutylnitrosamine (MBNA) in
rats.] Z. Krebsforsch., 71: 135-139 (in German).
DRUCKREY, H., LANDSCHOTZ, CH., & IVANKOVlC, S. (1970a) [Transplacental
production of malignant tumours in the nervous system II.
Ethylnitroso urea in 10 genetically defined rat strains.] Z.
Krebsforsch., 73: 371-386 (in German).
DUBROW, H. & KABISCH, W. (1960) [Investigations of the evidence of
nitrates and nitrites in cheese.] Milchwiss., 15 (11): 543-549
(in German).
DURFOR, C. N. & BECKER, E. (1965) Public water supplies of the 100
largest cities in the United States. Washington DC, US Dept
Interior (Geological Survey Water Supply Paper 1812).
DUTTON, A. H. & HEATH, D. F. (1956) Demethylation of
dimethylnitrosamine in rats and mice. Nature (Lond.),
178: 644.
EISENBRAND, G. (1972) Determination of volatile nitrosamines at low
levels in food by acid-catalysed denitrosation and formation of
derivatives from the resulting amines. IARC Sci. Publ. No. 3,
pp. 64-71.
EISENBRAND, G. (1973) Determination of volatile nitrosamines: a
review. In: Proceedings of the International Symposium on
Nitrite in Meat Products. Zeist, pp. 45-52.
EISENBRAND, G. & PREUSSMANN, R. (1970) [A new method for the
colorimetric determination of nitrosamines following cleavage of
the N-nitroso group with hydrogen bromide in glacial acetic
acid.] Arzneimittel-Forsch., 20: 1513-1517 (in German).
EISENBRAND, G., JANZOWSKI, C., & PREUSSMANN, R. (1975) Gas
chromatographic determination of N-nitrosoamino acids by
trimethylsilylation and single-ion mass fragmentography.
J. Chromatogr., 115: 602-606.
EISENBRAND, G., VON RAPPARD, E., ZAPPE, R., & PREUSSMANN, R. (1976)
Trace analysis of volatile nitrosamines by a modified nitrogen
specific detector in pyrolytic mode and by ion-specific
determination of heptafiuorobutyrantides in a GC/MS system.
IARC Sci. Publ. No. 14, pp. 65-75.
EISENSTARK, A. & ROSNER, J. L. (1964) Chemically induced reversions in
the cysC region of Salmonella typhimurium. Genetics,
49: 343-355.
ELESPURU, R. K. & LIJINSKY, W. (1973) The formation of carcinogenic
nitroso compounds from nitrite and some types of agricultural
chemicals. Food Cosmet. Toxicol., 11 (3): 807-811.
ELESPURU, R., LIJINSKY, W., & SETLOW, J. K. (1974) Nitrosocarbaryl as
a potent mutagen of environmental significance. Nature (Lond.),
247: 386-387.
ENDER, F. & CEH, L. (1967) Occurrence and determination of
nitrosamines in foodstuffs for human and animal nutrition.
Alkylierend Wirkend Verbindungen, Second Conference on Tobacco
Research., Freiburg, 1960, pp. 83-91.
ENDER, F. & CEH, L. Z. (1971) Conditions and chemical reaction
mechanisms by which nitrosamines may be formed in biological
products with reference to their possible occurrence in food
products. Z. Lebensm. Unters. Forsch., 145 (3): 133-142.
ENDER, F., HAVRE, G., HELGEBOSTAD, A., KOPPANG, N., MADSEN, R., & CEH,
L. (1964) Isolation and identification of a hepatoxic factor in
herring meal produced from sodium nitrite preserved herring.
Naturwiss., 51: 637-638.
ENDER, F., HAVRE, G., MADSEN, R., CEH, L., & HELGEBOSTAD, A. (1967)
[Studies on conditions under which N-nitrosodime-thylamine is
formed in herring meal produced from nitrite-preserved herring.]
Z. Tierphysiol. Tierernähr. Futtermittelk., 22: 181-189 (in
German).
ENDO, H., TAKAHESHI, K., & AOYAGI, H. (1973) Screening of compounds
structurally and functionally related to N-methyl- N-nitroso
guanidine, a gastric carcinogen. Gann, 65: 45-54.
EPPSON, H. F., GLENN, M. W., ELLIS, W. W., & GILBERT, C. S. (1960)
Nitrate in the diet of pregnant ewes. JAVMA, 137 (10): 611-614.
EPSTEIN, S. (1972) In vivo studies on interactions between secondary
amines and nitrites or nitrates. IARC Sci. Publ. No. 3,
pp. 109-115.
FAN, T-Y. & TANNENBAUM, S. R. (1972) Automatic colorimetric
determination of N-nitroso compounds. J. agric. Food Chem.,
19: 1267-1269.
FAN, T-Y. & TANNENBAUM, S. R. (1973) Factors influencing the rate of
formation of nitrosomorpholine from morpholine and nitrite:
acceleration by thiocyanate and other anions
J. agric. Food Chem, 21: 237-240.
FAO/IAEA Panel of Experts (1974) Effects of agricultural production
on nitrates in food and water with particular reference to
isotope studies. Proceedings and Report of a Panel of Experts,
Vienna, 4-8 June 1973, Vienna, International Atomic Energy
Agency, pp. 158.
FASSETT, D. W. (1966) Nitrates and nitrites. In: Toxicants occurring
naturally in foods. Washington, DC, National Academy of
Sciences, pp. 250-449 (Publ. No. 1354).
FAZIO, T., DAMICO, J. N., HOWARD, J. W., WHITE, R. H., & WATTS, J. O.
(1971a) Gas chromatographic determination and mass spectrometric
confirmation of N-nitro-sodimethylamine in smoke-processed
marine fish. J. agric. Food Chem,
19(2): 250-253.
FAZlO, T., WHITE, R. H., & HOWARD, J. W. (1971b) Analysis of nitrite-
and/or nitrate-processed meats for
N-nitrosodimethylamine. J. Assoc. Off Anal. Chem.,
54(5): 1157-1159.
FAZIO, T., HOWARD, J. W., & WHITE, R. (1972) Multidetection method
for analysis of volatile nitrosamines in foods. IARC Sci. Publ.
No. 3, pp. 16-24.
FAZIO, T., WHITE, R. H., DUSOLD, L. R., & HOWARD, J. W. (1973)
Nitrosopyrrolidine in cooked bacon. J. Assoc. Off. Anal. Chem.,
56 (4): 919-921.
FERGUSON, J. H., MYSLIWY, T. J., & ARCHER, M. C. (1974) The
nitrosation of spermidine and spermine. IARC Sci. Publ. No. 9,
pp. 90-93.
FERON, V. J., EMMELOT, P., & VOSSENAAR, T. (1972) Lower respiratory
tract tumors in Syrian golden hamsters after intratracheal
instillations of diethylnitrosamine alone and with ferric oxide.
Eur. J. Cancer, 8: 445-449.
FIDDLER, W. (1975) The occurrence and determination of N-nitroso
compounds. Toxicoi. appl. Pharmacol., 31: 352-360.
FIDDLER, W., DOERR, R. C., ERTEL, J. R., & WASSERMAN, A. E. (1971)
Gas-liquid chromatographic determination of
N-nitrosodimethylamine in ham. J. Assoc. Off Anal. Chem, 54
(5): 1160-1163.
FIDDLER, W., PENSABENE, J. W., DOERR, R. C., & WASSERMAN, A. E. (1972)
Formation of N-nitrosodimethylamine from naturally occurring
quaternary ammonium compounds and tertiary amines. Nature
(Lond.), 236: 307.
FIDDLER, W., PENSABENE, J. W., PIOTROWSKI, E.G., DOERR, R. C., &
WASSERMAN, A. E. (1973) Use of sodium ascorbate or erythorbate to
inhibit formation of N-Nitroso-dimethylamine in frankfurters.
J. Food Sci., 38: 1084.
FIDDLER, W., PENSABENE, J. W., FAGAN, J. C., THORN, E. J., PIOTROWSKA,
E.G., & WASSERMAN, A. (1974) The role of lean and adipose tissue
on the formation of nitrosopyrrolidine in fried bacon. J. Food
Sci., 39: 1070-1071.
FIESER, L. F. & FIESER, M. (1967) Reagents for organic synthesis.
New York, Wiley, pp. 747-749.
FINE, D. H. & RUFFEH, F. (1974) Description of the thermal energy
analyser for N- nitroso compounds. IARC Sci. Publ. No. 9,
pp. 40-44.
FINE, D. H., RUFEH, F., & LIEB, D. (1974) Group analysis of volatile
and non-volatile N-nitroso compounds. Nature (Lond.),
247: 309-310.
FINE, D. H., HUFFMAN, F., ROUNBEHLER, D. P., & BETCHER, N.M. (1976a)
Analysis of N-nitroso compounds by combined high- performance
liquid chromatography and thermal energy analysis. IARC Sci.
Publ. No. 14, pp. 43-50.
FINE, D. H., ROUNBEHLER, D. P., BELCHER, N.M., & EPSTEIN, S.S. (1976b)
N-nitroso compounds in air and water. IARC Sci. Publ. No. 14,
pp. 401-408.
FLAKS, A., HAM LTON, J. M., CLAYSON, D. B., & BURCH, P. R. J. (1973)
The combined effect of radiation and chemical carcinogens in
female A x 1F mice. Br. J. cancer, 28: 227-231.
FONG, Y. Y. & CHAN, W. C. (1973a) Dimethylnitrosamine in Chinese
marine salt fish. Food Cosmet. Toxicol., 11: 841-845.
FONG, Y. Y. & CHAN, W. C. (1973b) Bacterial production of
dimethylnitrosamine in salted fish. Nature (Lond.),
243: 421-422.
FONG, Y. Y. & WALSH, E. O'F. (1971) Carcinogenic nitrosamines in
Cantonese salt-dried fish. Lancet, 2: 1032.
FOOD STANDARDS COMMITTEE (1959) Preservatives in food. London, Her
Majesty's Stationery Office.
FOSTER, S.S. D. & CREASE, R. I. (1974) Nitrate pollution of chalk
groundwater in east Yorkshire -- a hydrological appraisal. J.
Inst. Water Eng., 28: 178-194.
FREIMURTH, U. & GLASER, E. (1970) [The appearance of nitrosamines in
food.] Die Nahrung., 14 (5): 357-361 (in German).
FREUND, H. A. (1937) Clinical manifestations and studies in
parenchymatous hepatitis. Ann. int. Med., 10: 1144-1155.
FRIDMAN, A. L., MUKHAMETSIN, F. M., & NOMIKOV, S.S. (1971) [Advances
in the chemistry of aliphatic nitrosamines.] Russ. chem. Rev.,
40 (1): 34-50 (in Russian).
FRIEDMAN, M. A., GREENE, E. J., & EPSTEIN, S.S. (1972) Rapid gastric
absorption of sodium nitrite in mice. J. pharm. Sci.,
61 (9): 1492-1494.
GARBIDGE, M. G. & LEGATOR, M. S. (1969) A host-mediated microbic assay
for the detection of mutagenic contents. Proc. Soc. Exp. Biol.
Med., 130: 831-834.
GARBUZ, A.M. (1968) [The effect of symptomless methaemoglo-binaemia
on some bodily functions.] Author's summary of a thesis for the
degree of Candidate of Medical Sciences, Leningrad (in Russian).
GARBUZ, A.M. (1971) [Changes in the body's functional condition in
symptomless methaemoglobinaemia. In: Methaemoglobinaemia caused
by various factors and methods of preventing it.] Summaries of
papers read at the First Scientific Conference on
Methaemoglobinaemia, Leningrad, pp. 18-21 (in Russian).
GEIL, J. H., STENGER, R. J., BEHKI, R. M., & MORGAN, W. S. (1968)
Hepatotoxic and carcinogenic effects of dimethylnitrosamine in
low dosage. Light and electron microscopic study.
J. Natl Cancer Inst., 40: 713-730.
GELPERIN, A., JACOBS, E. E., & KLETKE, L. S. (1971) The development of
methemoglobin in mothers and newborn infants from nitrate in
water supplies. Ill. med. J., 140: 42-44.
GERRITSEN, G. A. & DE WILLIGEN, A. H. A. (1969) [The nitrite content
of potato and corn starch.] Die Starke, 21: 101-105 (in French).
GILBERTSON, C. B., MCCALLA, T. M., ELLIS, J. R., CROSS, O. E., &
WOODS, W. R. (1970) The effect of animal density and surface
slope on characteristics of runoff solid waste, and nitrate
movement on unpaved feedlots. Lincoln, Univ. Nebraska Coll.
Agric. and Home Econ.
GOBBI, A., SOVERINI, R., & GRISLER, R. (1974) [Normal
methemoglobinemia values observed in 500 inhabitants of the City
of Milan.] Med. Lavoro, 65: 306-310 (in Italian).
GOLDSMITH, J. R., ROKAW, S. N., & SHEARER, L. N. (1975) Distributions
of percentage methemoglobin in several population groups in
California. Int. J. Epidem., 4: 207-212.
GOTO, M. (1973) Inorganic chemicals in the environment -- with special
reference to the pollution problems in Japan. Environ. Qual.
Saf., 2: 72-77.
GROENEN, P. J., JONK, C., VAN INGEN, C., & TEN NOEVER DE BRAUW, M. C.
(1976) Determination of eight volatile nitrosamines in thirty
cured meat products with capillary gas chromatography-high
resolution mass spectrometry: the presence of
nitrosodiethylamine and the absence of nitrosopyrrolidine. IARC
Sci. Publ., No. 14, pp. 321-331.
GREENBERG, R. A. (1974) Ascorbate and nitrosamine formation in cured
meats. In: Proceedings of the International Symposium on
Nitrite in Meat Products, Zeist, 10-14 September, 1973,
Wageningen, Centre for Agricultural Publishing and Documentation,
pp. 179-188.
GREENBLATT, M. (1973) Ascorbic acid blocking of aminopyrine
nitrosation in NZO-Bl mice. J. Natl Cancer Inst.,
50: 1055-1056.
GREENBLATT, M. & MIRVISH, S.S. (1973) Dose-response studies with
concurrent administration of piperazine and sodium nitrite to
strain A mice. J. Natl Cancer Inst., 50 (1): 119-124.
GREENBLATT, M. & RIJHSINGHANI, K. (1969) Comparative cytopathologic
alterations induced by alkylnitrosamines in nasal epithelium of
the Syrian hamster. J. Natl Cancer Inst., 42: 421-433.
GREENBLATT, M., MIRVISH, S., & SO, B. T. (1971) Nitrosamine studies:
Induction of lung adenomas by concurrent administration of sodium
nitrite and secondary amines in Swiss mice. J. Natl Cancer
Inst., 46: 1029-1034.
GREENBLATT, M., KOMMINENI, V., CONRAD, E., WALLCAVE, L., & LIHNSKY, W.
(1972) In vivo conversion of phenmetrazine into its N-nitroso
derivative. Nature (Lond.), 236
(62): 25-26.
GREENBLATT, M., KOMMINENI, V. R. C., & LIJINSKY, W. (1973) Null effect
of concurrent feeding of sodium nitrite and amino acids to MRC
rats. J. Natl Cancer Inst., 50: 799-802.
GRUENER, N. & SHUVAL, H. I. (1970) Health aspects of nitrates in
drinking water. In: Shuval, H. I., ed. Developments in water
quality research. Ann Arbor, Humphrey Science Publ., pp. 89-106.
GRUNTHAL, D., HELLENBROICH, D. O., SANGER, P., & MAAS, H. (1970)[The
effect of partial hepatectomies on the hepatoma rate after
administration of diethyl-nitrosamine.]
Z. Naturforsch., 25: 1277-1281 (in German).
HADJIOLOV, D. (1971) [The inhibition of dimethylnitrosamine
carcinogenesis in rat liver by aminoacetonitrile.]
Z. Krebsforsch., 76: 91-92 (in German).
HAENSZEL, W. & CORREA, P. (1975) - Developments in the epidemiology of
stomach cancer over the past decade. Cancer Res.,
35: 3452-3459.
HANSEN, H. H. & MUGGIA, F. M. (1971) Treatment of malignant brain
tumours with nitrosoureas. Cancer Chemother. Rep. Part 1, 55
(2): 99-100.
HARD, G. C. & BUTLER, W. H. (1970a) Cellular analysis of renal
neoplasia. Induction of renal tumors in dietary-conditioned rats
by dimethylnitrosamine, with a reappraisal of morphological
characteristics. Cancer Res., 30: 2796-2805.
HARD, G. C. & BUTLER, W. H. (1970b) Cellular analysis of renal
neoplasia. Light microscope study of the development of
interstitial lesions induced in the rat kidney by a single
carcinogenic dose of dimethylnitrosamine. Cancer Res.,
30: 2806-2815.
HARD, G. C. & BUTLER, W. H. (1970c) Toxicity of dimethylni-trosamine
for the rat testis. J. Pathol., 102: 201-207.
HARD, G. C. & BUTLER, W. H. (1971a) Ultrastructural aspects of renal
adenocarcinoma induced in the rat by dimethylnitrosamine. Cancer
Res., 31: 366-372.
HARD, G. C. & BUTLER, W. H. (1971b) Ultrastructural study of the
development of interstitial lesions leading to mesenchymal
neoplasia induced in the rat renal cortex by dimethylnitrosamine.
Cancer Res., 31: 337-347.
HARD, G. C. & BUTLER, W. H. (1971c) Morphogenesis of epithelial
neoplasms induced in the rat kidney by dimethylnitrosamine.
Cancer Res., 31: 1496-1505.
HARD, G. C., BORLAND, R., & BUTLER, W. H. (1971) Altered morphology
and behavior of kidney fibroblasts in-vitro following in-vivo
treatment of rats with a carcinogenic dose of
dimethylnitrosamine. Experientia (Basel), 27: 1208-1209.
HARMOZDIAN, H., DAY, N. E., ARAMESH, B., & MAHBOUBI, E. (1975) Dietary
factors and esophageal cancer in the Caspian littoral of Iran.
Cancer Res., 35: 3493-3498.
HASHIMOTO, Y., SUZUKI, E., & OKADA, M. (1972) [Induction of urinary
bladder tumors in ACI/N rats by butyl (3-carboxypropyl)
nitrosamine, a major urinary metabolite of butyl-(4-hydroxbutyl)
nitrosamine.] Gann, 63: 637-638 (in Japanese).
HAWKSWORTH, G. & HILL, M. J. (1971a) The formation of nitrosamines by
human intestinal bacteria. Biochem. J., 122: 28p-29p.
HAWKSWORTH, G. & HILL, M. J. (1971b) Bacteria and the
N-nitrosation of secondary amines. Br. J. Cancer,
25: 520-526.
HAWKSWORTH, G., HILL, M. J., GORDILL, G., & CUELLO, C. (1974)
Possible relationship between nitrates, nitrosamines and
gastric cancer in S.U. Columbia. IARC Sci. Publ. No. 9,
pp. 229-234.
HEATH, D. F. (1962) The decomposition and toxicity of
dialkylnitrosamines in rats. Blochem. J., 85: 72-91.
HEDLER, L. & MARQUARDT, P. (1968) Occurrence of diethynitrosamine in
some samples of food. Food Cosmet. Toxicol, 6: 341-348.
HEDLIN, R. A. (1971) Nitrate contamination of ground water in the
Neepawa-Langruth area of Manitoba. Can. J. Soil Sci,
51: 75-84.
HEIN, G. E. (1963) Reaction of tertiary amines with nitrous acid,
J. chem. Educ, 40 (4): 181-184.
HENDERSON, W. R. & RASKIN, N.H. (1972) "Hot-Dog" headache: Individual
susceptibility to nitrite. Lancet, 2: 1162-1163.
HEPPEL, L. A. & PORTERFIELD, V. T. (1949) Metabolism of inorganic
nitrite and nitrate esters I. The coupled oxidation of nitrite by
peroxide-forming systems and catalase. J. biol. Chem, No. 178,
pp. 549-556.
HERMANN, H. (1960) [P. Methylnitrosaminebenzaldehyde, a metabolic
product of Clytocybe suaveolens.] Naturwiss., 47: 162
(in German).
HEYNS, K. (1973) [On nitrosamines in nutrients.] Getreide Mehl Brot,
27: 249-253 (in German).
HICKS, R. M., WAKEFIELD, J. ST. J., & CHOWANIEC, J. (1973)
Co-carcinogenic action of saccharin in the chemical induction of
bladder cancer. Nature (Lond.), 243: 347-349.
HILDRUM, K. I., SCANLAN, R. A., & LIBBEY, L. M. (1975) Identification
of gamma-butenyl-(beta-propenyl) nitrosamine, the principal
volatile nitrosamine formed in the nitrosation of spermidine or
sperminc. J. agric. Food Chem., 23
(1): 34-37.
HILL, M. J., HAWKSWORTH, G., & TATTERSALL, G. (1973) Bacteria,
nitrosamines and cancer of the stomach. Br. J. Cancer,
28 (6): 562-567.
HMSO (1973) The determination of nitrate-nitrogen in herbage. London,
Her Majesty's Stationery Office, pp. 3 (Tech. Bull. 27).
HMSO (1974) Annual Report 1972, London, Her Majesty's Stationery
Office, pp. 206.
HOCH-LIGETI, C., ARGUS, M. F., & ARCOS, J. C. (1968) Combined
carcinogenic effects of dimethylnitrosamine and 3-methyl-
cholanthrene in the rat. J. Natl Cancer Inst., 40: 535-549.
HOFFMAN, D., HECHT, S.S., ORNAF, R. M., & WYDNER, E. L. (1974)
N-nitrosonornicotine in tobacco. Science, 186: 265-267.
HÖLSCHER, P.M. & NATZSCHKA, J. (1964) [Methaemoglobinaemia in young
children due to the nitrite contents of spinach.] Dtsch Med.
Wschr, 89: 1751-1754 (in German).
HORN, E. E. & HERRIOT, R. M. (1962) The mutagenic action of nitrous
acid on "single-stranded" (denatured) haemophilus transforming
DNA. Proc. Natl Acad. Sci. US, 48: 1409-1416.
HORWlTZ, W. (1975) Association of Official Analytical Chemists,
Official methods of analysis. 12th ed. Washington, DC,
pp. 1094.
HOWARD, J. W., FAZlO, T., & WATTS, J. O. (1970) Extraction and gas
chromatographic determination of N-nitrosodimethy-lamine in
smoked fish application to smoked nitrite-treated chub. J.
Assoc. Off. Anal. Chem., 53(2): 269-274.
INGRAM, M. (1974) The microbiological effects of nitrite. In:
Proceedings of the International Symposium on Nitrite in Meat
Products, Zeist, 10-14 September, 1973, Wageningen, Centre for
Agricultural Publishing and Documentation, pp. 63-75.
IRETON, H. J., McGIVEN, A. R., & DAVIES, D. J. (1972) Renal
mesenchymal tumours induced in rats by dimethylnitrosamine. Light
and electron-microscope studies. J. Pathol., 108: 181-185.
ISSENBERG, P. & TANNENBAUM, S. (1972) Approaches to the
determination of volatile and non volatile N- nitroso
compounds in foods and beverages. IARC Sci. Publ., No. 3,
pp. 31-37.
ITO, N., HIASA, Y., TAMAI, A., OKAJIMA, E., & KITAMORA, H. (1969)
Histogenesis of urinary bladder tumors induced by N-butyl- N-
(4-hydroxybutyl) nitrosamine in rats. Gann, 60: 401-410.
ITO, N., MAKIURA, S., YOKOTA, Y., KAMAMOTO, Y., HIASA, Y., & SUGIHARA,
S. (1971) Effect of unilateral ureter ligation on development of
tumors in the urinary system of rats treated with N-butyl- N-
(4-hydroxybutyl) nitrosamine. Gann, 62: 359-365.
IVANKOVIC, S. & DRUCKREY, H. (1968) [Transplacental induction of
malignant tumours of the nervous system. I Ethylnitrosourea (ENU)
in BD-IX-rats.] Z. Krebsforsch., 71: 320-360 (in German).
IVANKOVIC, S. & PREUSSMANN, R. (1970) [Transplacental induction of
malignant tumours by the oral administration of ethylurea and
nitrite in rats.] Naturwiss., 57: 460-461 (in German).
IVANKOVIC, S., PREUSSMANN, R., SCHMÄHL, D., & gELLER, J. (1973)
[Prevention of nitrosamide induced hydrocephali by ascorbic acid
after prenatal administration of ethylurea and nitrite to rats.]
Z. Krebsforsch, 79: 145-147 (in German).
JACKSON, W. A., STEEL, J. S., & BOSWELL, V. R. (1967) Nitrates in
edible vegetables and vegetable products. Proc. Am. Soc. Hort.
Sci., 90: 349-352.
JAFFÉ, E. R. & HELLER, P. (1964) Methemoglobinemia in man. In: Moore,
C. V. & Brown, E. B., ed. Progress in hematology, vol. IV, New
York, Grune & Stratton, pp. 48-71.
JAPAN ENVIRONMENTAL SANITATION CENTER (1974) [Report of 1973 results
of the analysis for suspended particulate matter in the National
Air Sampling Network.] Kawasaki, Japan, pp. 14, 24 (in Japanese).
JASMIN, G. & CHA, J. W. (1969) Renal adenomas induced in rats by
dimethylnitrosamine. An electron microscopic study. Arch.
Pathol., 87: 267-278.
JENISSEN, H., HOSHINO, J., & KROGER, H. (1971) [The influence of
carcinogenic nitrosamines on the induction of serine dehydratase
in the rat liver.] Z. Krebsforsch., 75: 246-252 (in German).
JOHNSON, J. H. (1966) Internal can corrosion due to high nitrate
content of canned vegetables. Proc. Fla. State Hort. Sci.,
79: 239-242.
JUSKIEWICZ, T. & KOWALSKI, B. (1974) Passage of nitrosamines from
rumen into milk in goats. IARC Sci. Publ. No. 9, pp. 173-176.
KAMM, L., MCKEOWN, G. G., & SMITH, D. M. (1965) New colorimetric
method for the determination of the nitrate and nitrite content
of baby foods. J. Assoc. Off. Agric. Chem., 48 (5): 892-897.
KAMM, J. J., DASHMAN, T., CONNEY, A. H., & BURNS, J. J. (1973)
Protective effect of ascorbic acid on hepatoxicity caused by
sodium nitrite and aminopyrin. Proc. Natl Acad. Sci.,
70: 747-749.
KAPELLER-ADLER, R. & KRAEL, J. (1930a) [Research on nitrogen
distribution in the muscle of different animal species I,]
Biochem. Z., 221: 437-460 (in German).
KAPELLER-ADLER, R. & KRAEL, J. (1930b) [Research on nitrogen
distribution in the muscle of different animal species II.]
Biochem. Z., 224: 364-377 (in German).
KAUDEWITZ, F. (1959) Production of bacterial mutants with nitrous
acid. Nature (Lond.), 183: 1829-1830.
KAWABATA, T. (1974) Addendum, N- nitroso compounds: Recent studies
in Japan. IARC Sci. Publ. No. 9, pp. 154-158.
KEATING, J.P., LELL, M. E., STRAUSS, A. W., ZARKOWSKY, H., & SMITH, G.
E. (1973) Infantile methemoglobinemia caused by carrot juice. N.
Eng. J. Med., 288: 824-826.
KEOHANE, K. W. & METCALF, W. K. (1960) An investigation of the
differing sensitivity of juvenile and adult erythrocytes to
methemoglobinization. Phys. med. Biol., 5: 27-35.
KILGORE, L., STASCH, A. R. & BARRENTINE, B. F. (1963) Nitrate content
of beets, collards, turnip greens. J. Am. Diet. Assoc.,
43: 39-42.
KING, E. J. (1959) Qualitative analysis and electrolytic solutions.
New York, Harcourt, Brace & Co., pp. 519.
KIRKLAND, D. J. & PICK, C. R. (1973) The histological appearance of
tumours derived from rat embryo cells transformed in vitro
spontaneously and after treatment with nitrosomethylurea. Br.
J. Cancer, 28: 440-452.
KLUBES, P. & JONDORF, W. R. (1971) Dimethyl nitrosamine formation from
sodium nitrite and dimethylamine by bacterial flora of rat
intestine. Res. Commun. chem. Pathol. Pharmacol,
2 (1): 24-34.
KNELSON, J. H. & LEE, R. E. (1977) Oxides of nitrogen in the
atmosphere: Origin, fate and public health implications. Ambio,
6 (2-3): 126-130.
KNOTEK, Z. & SCHMIDT, P. (1964) Pathogenesis, incidence and
possibilities of preventing alimentary nitrate methemoglobinemia
in infants. Pediatrics, 34: 78-83.
KOCIBA, R. J. & SLEIGHT, S. D. (1970) Nitrite toxicosis in the
ascorbic acid-deficient guinea pig. Toxicol. appl. Pharmacol.
16: 424-429.
KOHL, D. H., SHEARER, G. B., & COMMONER, B. (1971) Fertilizer
nitrogen: Contribution to nitrate in surface water in a corn belt
watershed. Science, 174: 1331-1334.
KOPPANG, N. (1964) An outbreak of toxic liver injury in ruminants.
Nord. vet. Med., 16: 305-322.
KORDAC, V., SCHON, E., & BRAUN, A. (1969) Hepatocancerogenesis in mice
with simultaneous administration of diethylnitrosamine and the
virus Motol. Neoplasma, 16: 485-490.
KOYAMA, T., HANDA, J., HANDA, H., & MATSUMOTO, S. (1970)
Methylnitrosourea-induced malformations of brain in SD-JCL rat.
Arch. Neurol., 22: 342-347.
KRAVITZ, H., ELEGANT, L. D., KAISER, E., & KAGAN, B. M. (1956)
Methemoglobin values in premature and mature infants and
children. A.M.A.J. Dis. Child., 91: 1-5.
KRÖLLER, E. (1967) [Investigations of the evidence of nitrosamines in
tobacco smoke and food.] Dtsch. Lebensm. Rundsch., 63(10):
303-305 (in German).
KRÜGER, F. W. (1972) [On the biochemistry of carcinogenic
N-nitroso compounds.] Ärztliche Forsch., 26 (6): 197-202 (in
German).
KRÜGER, F. W. (1973) Metabolism of nitrosamines in vivo. II. On the
methylation of nucleic acids by aliphatic di-n-alkylnitrosamines
in vivo, caused by beta-oxidation: The increased formation of
7-methyl-guanine after application of beta-hydroxypropyl-
nitrosamine compared to that after application of di- N-propyl-
nitrosamine. Z. Krebsforsch., 79: 90-97.
KÜBLER, W. (1958) [The importance of the nitrate content of vegetables
in child nutrition.] Z. Kinderheilk, 81: 405-416 (in German).
KÜBLER, W. (1965) [Methaemoglobinaemia in infants after feeding with
spinach.] Dtsch. med. Wschr., 90: 1881-1882 (in German).
KUNZ, W., SCHAUDE, G., & THOMAS, C. (1969) [The effect of
phenobarbital and halogenated hydrocarbons on nitrosamine
carcinogenesis.] Z. Krebsforsch., 72: 291-304 (in German).
KÜNZER, W. & SCHNEIDER, D. (1953) [The activity of the reducing enzyme
system in erythrocytes of young infants.] Acta haemat., 9: 346
(in German).
KUWAHARA, A., OTSUKA, H., & NAGMATSU, A. (1972) Induction of
hemangiomatous lesions with dimethylnitrosamine. Influence of
route of administration and strain of mice. Gann,
63: 499-502.
LACASSAGNE, A., BUU-HOI, N., GIAO, N. B., & FERRANDO, R. (1968)
Retarding effect of resirpine on the cancerization of rat liver
induced by diethylnitrosamine. Bull. Cancer,
55 (1): 87-89.
LEE, D. H. K. (1970a) Nitrates, nitrites and methemoglobinemia.
Environ. Res., 3: 484-511.
LEE, G. F. (1970b) Eutrophication. Occasional Paper No. 2,
University of Wisconsin, Water Resources Center, Madison,
pp. 12-13.
LEE, K. Y. & LIJINSKY, W. (1966) Alkylation of rat liver RNA by cyclic
N-nitrosamines in vivo, J. Natl Cancer Inst., 37
(A): 401-407.
LEHMAN, A. J. (1958) Quarterly report to the editor on topics of
current interest. Nitrates and nitrites in meat products. Assoc.
Food Drug Off. US Q. Bull., 22: 136-138.
LEMBKE, A. & MOEBUS, O. (1970) Is there a connection between the
occurrence of nitrite-producing microorganisms in cheese and the
formation of nitrosamine? Proceedings of the 18th
International Dairy Congress A.2.3, p. 139.
LIJINSKY, W. (1974) Reaction of drugs with nitrous acid as a source of
carcinogenic nitrosamines. Cancer Res., 34: 255-258.
LIJINSKY, W. & EPSTEIN, S.S. (1970) Nitrosamines as environmental
carcinogens. Nature (Lond.), 225: 21-23.
LIJINSKY, W. & GREENBLATT, M. (1972) Carcinogen dimethylnitrosamine
produced in vivo from nitrite and aminopyrine. Nature (New
Biology), 236: 177-178.
LIJINSKY, W. & SINGER, G. (1974) Formation of nitrosamines from
tertiary amines and nitrous acid. IARC Sci. Publ. No. 9,
pp. 111-114.
LIJINSKY, W., TOMATIS, L, & WENGEN, C. E. M. (1969) Lung tumors in
rats treated with N-nitrosoheptamethyleneimine and
N-nitrosooctamethyleneimine. Proc. Soc. Exp. Biol. Med.,
130: 945-949.
LIJINSKY, W., FERRERO, A., MONTESANO, R., & WENYON, C. E. M. (1970)
Tumorigenecity of cyclic nitrosamines in Syrian golden hamsters.
Z. Krebsforsch., 74: 185-189.
LIJINSKY, W., CONRAD, E., & VAN DE BOGART, R. (1972a) Carcinogenic
nitrosamines formed by drug/nitrite interactions. Nature
(Lond.), 239: 165-167.
LIJINSKY, W., CONRAD, E., & VAN DE BOGART, R. (1972b) Formation of
carcinogenic nitrosamines by interaction of drugs with nitrite.
IARC Sci. Publ. No. 3, pp. 130-133.
LIJINSKY, W., KEEFER, L., CONRAD, E., & VAN DE BOGART, R. (1972c)
Nitrosation of tertiary amines and some biologic implications.
J. Natl Cancer Inst., 49 (5): 1239-1249.
LIJINSKY, W., GREENBLATT, M., & KOMMINENI, C. (1973a) Brief
communication: Feeding studies of nitrilotriacetic acid and
derivatives in rats. J. Natl Cancer Inst., 50: 1061-1063.
LIJINSKY, W., KEEFER, L., LOO, J., & ROSS, A. E. (1973b) Studies of
alkylation of nucleic acids in rats by cyclic nitrosamines.
Cancer Res., 33: 1634-1641.
LIJINSKY, W., TAYLOR, H. W., SNYDER, C., & NETTESHEIM, P. (1973c)
Malignant tumors of liver and lung in rats fed aminopyrine or
heptamethyleneimine together with nitrite. Nature (Lond.),
244: 176-178.
LOVELESS, A. & HAMPTON, C. (1969) Inactivation and mutation of
coliphage T2 by N-methyl- and N-ethyl- N-nitrosourea.
Murat. Res., 7: 1-12.
LUHRS, C. E. (1973) Nitrogenous materials in the environment. Draft
report. US Environmental Protection Agency, Hazardous Materials
Advisory Committee.
MAEKAWA, H. & ODASHIMA, S. (1975) Induction of tumours of the nervous
system in the HCI/N rat with N-buyl-I-nitrosourea administered
transplacentally, neonatally or via maternal milk. Gann,
66: 175-183.
MAGEE, P. N. (1956) Toxic liver injury. The metabolism of
dimethylnitrosamine. Biochem. J., 64: 676-682.
MAGEE, P. N. (1972) Possibilities of hazard from nitrosamines in
industry. Ann. occup. Hyg., 15: 19-22.
MAGEE, P. N. & BARNES, J. M. (1962) Induction of kidney tumours in the
rat with dimethyl ( N-nitrosodimethylamine) nitrosamine.
J. Pathol. Bacteriol., 84: 19-31.
MAGEE, P. N. & BARNES, J. M. (1967) Carcinogenic nitroso compounds.
Adv. Cancer Res., 10: 163-246.
MAGEE, P. N., MONTESANO, R., & PREUSSMANN, R. (1976) N-nitroso
compounds and related carcinogens. In: Searle, E. ed., Chemical
carcinogens. ACS monograph No. 173, pp 491-625.
MAKIURA, S., KAMMAMOTO, Y., SUGIHARA, S., HIRAO, K., HIASA, Y., ARAI,
M., & ITO, N. (1973) Effect of 1-naphthyl isothiocyanate and
3-methylcholanthrene on hepato-carcinogenesis in rats treated with
diethylnitrosamine. Gann, 64: 101-104.
MALINS, D.C., ROUBAL, W. T., & ROBISCH, P. A. (1970) The possible
nitrosation of amines in smoked chub. J. agric. Food. Chem.,
18(4): 740-741.
MALLING, H. V. (1966) Mutagenicity of two potent carcinogens
dimethylnitrosamine and diethylnitrosamine in Neurospora
crassa. Mutat. Res., 3: 537-540.
MANNING, P. B., COULTRER, S. T., & JENNESS, R. (1968) Determination of
nitrate and nitrite in milk and dry milk products. J. Dairy
Sci., 51: (11): 1725-1730.
MARCULESCU, V. (1917) Determination of nitrates in water. Stud.
Prot. Epurarea Apelor., 15: 127-161
MARRIOTT, W. M., HARTMANN, A. F., & SENN, M. J. E. (1933) Observations
on the nature and treatment of diarrhea and the associated
systemic disturbances. J. Pediatr., 3: 181-191.
MCLEAN, A. E. M. & MAGEE, P. N. (1970) Increased renal carcinogenesis
by dimethylnitrosamine in protein deficient rats. Br. J. exp.
Pathol., 51: 587-590.
MCLEAN, A. E. & VERSCHUUREN, H. G. (1969) Effects of diet and
microsomal enzyme induction on the toxicity of
dimethylnitrosamine. Br. J. exp. Pathol., 50: 22-25.
METCALF, W. K. (1961) The sensitivity of haemoglobin to oxidation in
various conditions. Nature (Lond.), 190: 543-544.
MIRNA, A. (1974) Determination of free and bound nitrite. In:
Proceedings of the International Symposium on Nitrite in Meat
Products, Ziest, 10-14 September 1973, Wageningen, Centre for
Agricultural Publishing and Documentation, pp. 21-28.
MIRVISH, S.S. (1970) Kinetics of dimethylamine nitrosation in relation
to nitrosamine carcinogenesis. J. Natl Cancer Inst., 44
(3): 633-639.
MIRVISH, S.S. (1971) Kinetics of nitrosamide formation from
alkylureas, N-alkylurethanes and alkylguanidines: possible
implications for the etiology of human gastric cancer. J. Natl
Cancer Inst., 46: 1183-1193.
MIRVISH, S.S. (1975) Formation of N-nitroso compounds: chemistry,
kinetics, and in vivo occurrence. Toxicol. appl. Pharmacol,
31: 325-351.
MIRVISH, S.S. (in press) N-nitroso compounds, nitrite and nitrate:
possible implications for the causation of human cancer. J.
Water Pollut.
MIRVISH, S., WALLCAVE, L., EAGEN, M., & SHUBIK, P. (1972) Ascorbate-
nitrite reaction; possible means of blocking the formation of
carcinogenic N-nitroso compounds. Science, 177: 65-68.
MIRVISH, S.S., SAMS, J., FAN, T. Y., & TANNENBAUM, S. R. (1973a)
Kinetics of nitrosation of the amino acids proline hydroxyproline
and sarcosine. J. Natl Cancer Inst., 51: 1833-1840.
MIRVISH, S.S., CARDESA, A., WALLCAVE, L., & SHUBIK, P. (1973b) Effect
on sodium ascorabte on lung adenoma induction by amines plus
nitrite. Proc. Am. Assoc. Cancer Res., 14: 102.
MIRVISH, S.S., ARNOLD, S., CONRAD, E., GHADIRIAN, P., KOMMINENI, V. R.
C., & SAMS, J. (1974) The formation of N- nitroso compounds,
preparation of heptafiuorobutyryl derivatives of ureas, and the
fate of nitrite in the rat stomach. IARC Sci. Publ. No. 9,
pp. 67-70.
MIRVISH, S.S., CARDESA, A., WALLCAVE, L., & SHUBIK, P. (1975)
Induction of mouse lung adenomas by amines or ureas plus nitrite
and by N-nitroso compounds: effects of ascorbates, gallic acid,
thyocyanate, and caffeine. J. Natl Cancer Inst., 55: 633-636.
MIRVISH, S.S., ISSENBERG, P., & SORNSON, H. C. (1976) Air-water and
ether-water distribution of N-nitroso compounds: implications
for laboratory safety, analytical methodology, and
carcinogenicity for the rat esophagus, nose, and liver. J. Natl
Cancer Inst., 56 (6): 1125-1129.
MÖHLER, K. & MAYRHOFER, O. L. (1968) [Evidence and determination of
nitrosamines in food.] Z. Lebensm. Unter-Forsch., 135 (6):
313-318 (in German).
MÖHLER, K. & MAYRHOFER, O. L. (1969) [Effect of various factors on the
formation of nitrosamines in meat products.] 15th European
Meeting of the Meat Research Workers, Helsinki, 17-24 August
1969, pp. 302-304 (in German).
MOHR, U. & HILFRICH, J. (1972) Brief communication: Effect of a single
dose of N-diethylnitrosamine on the rat kidney. J. Natl
Cancer Inst., 49: 1729-1731.
MONTESANG, R. (1970) Systemic carcinogens ( N-nitroso compounds) and
synergistic or additive effects of respiratory carcinogenesis.
Tumori, 56: 335-344.
MONTESANO, R. & BARTCH, H. (1976) Mutagenic and carcinogenic
N-nitroso compounds: possible environmental hazards. Mutat.
Res., 32: 179-228.
MONTESANO, R. & SAFFIOTTI, U. (1968) Carcinogenic response of the
respiratory tract of Syrian golden hamsters to different doses of
diethylnitrosamine. Cancer Res., 28: 2197-2210.
MOTYLEV, V. D. (1969) [The question of age-related methaemoglobinaemia
caused by nitrates and nitrites and its prophylaxis.] Author's
summary of a thesis for the degree of Candidate of Medical
Sciences, Leningrad, (in Russian).
MURTHY, Y. K. S., THIEMANN, J. E., CORONELLI, C. I., & SENSl, P.
(1966) Alanosine, a new antiviral and antitumour agent isolated
from a streptomyces. Nature (Lond.), 211: 1198-1199.
MYSLIWY, T. S., WICK, E. L., ARCHER, M. C., SHANK, R. C., & NEWBERNE,
P.M. (1974) Formation of N-nitrosopyrrolidine in a dog's
stomach. Br. J. Cancer, 30: 279-283.
NAGAWA, Y. & MIRNA, A. (1974) [Influence exerted by processing upon
the formation of nitrosamines in meat products.]
Fleischwirtschaft., 1781-1788 (in German).
NAPALKOV, N. P. (1971) [Experimentation on transplacental
blastomogenesis as a tool for study of etiopathogenesis of
tumours in children.] Vop. Onkol., 17 (8): 3-15 (in Russian).
NASHED, N. & JUBBUR, G. (1966) A genetic and functional
characterization of adenine mutants induced in yeast by
1-nitroso-imidazolidone-2 and nitrous acid. Z. Verer-bungsl,
98: 106-110.
NEURATH, H. (1972) Nitrosamine formation from precursors in tobacco
smoke. IARC Sci. Publ. No. 3, pp. 134-136.
NEURATH, G. & SCHREIBER, O. (1974) Investigations on amines in the
human environment. Sci. Publ. No. 9, pp. 211-214.
NEURATH, G., PIRMANN, B., & WICHERNOTT, (1964) [On the question of
nitroso compounds in tobacco smoke.] Beitr. Tabakforsch.,
2(7): 311-319 (in German).
NEWBERNE, P.M. & SHANK, R. C. (1973) Induction of liver and lung
tumors in rats by the simultaneous administration of sodium
nitrite and morpholine. Food Cosmet. Toxicol., 11: 819-825.
NEWELL, J. E. & SISKEN, H. R. (1972) Determination of nitrosodi-
methylamine in the low parts per billion. J. agric. Food Chem.,
20 (3): 711-713.
NIEHS (1970) Nitrates, nitrites and methemoglobinemia. North
Carolina, National Institute of Environmental Health Sciences,
pp. 43 (Rep. No. 2).
NOROHNA, R. F. & GOODALL, C. M. (1972) Nasal tumors in starved rats
injected once with dimethylnitrosamine. N. Z. med. J.,
75: 374-375.
NYE, J. C. (1973) Nitrogenous compounds in the environment. Draft
Report, Washington, DC, US Environmental Protection Agency,
Hazardous Materials Advisory Committee, pp. 95-109.
O'CONNOR, P. J., CAPPS, M. J., & CRAIG, A. W. (1973) Comparative
studies of the hepatocarcinogen N,N-dimethylnitrosamine in
vivo: reaction sites in rat liver DNA and the significance of
their relative stabilities. Br. J. Cancer, 27: 153-166.
ODASHIMA, S. (1969) Experimental carcinoma of the glandular stomach in
rats. I. Effect of 7, 12-dimethylbenz-[a]anthracene or
4-nitroquinolene 1-oxide placed on glandular stomach combined with
oral administration of N,N1-(2,7-fluorenylene) bisacetamide
or N-nitrosodiethylamine. Gann, 60: 211-222.
OEHME, F. W. (1975) Vetinary toxicology. In: Caserett, L. J. & Doulle,
J., ed. Toxicology, the basic science of poisons. New York,
Macmillan Publishing Co., Inc., pp. 701-727.
OKADA, M. & SUZUKI, E. (1972) Metabolism of butyl (4-hydroxybutyl)
nitrosamine in rats. Gann, 63 (3): 391-392.
OKADA, M., SUZUKI, E., ANJYO, T., & MOCHIZUKI, M. (1975) Mutagenicity
of gamma-acetoxy-dialkylnitrosamines: model compounds for an
ultimate carcinogen. Gann, 66 (4): 457-458.
OKAJIMA, E., HIRAMATSU, T., MOTOMIYA, Y., IRWA, K., IJUIN, M., & ITO,
N. (1971) Effect of DL-tryptophan on tumorigenesis in the urinary
bladder and liver of rats treated with N-nitrosodibutylamine.
Gann, 62: 163-169.
OLSON. O. E., NELSON, D. L., & EMERICK, R. J. (1963) Effect of nitrate
and some of its reduction products on carotine stability.
J. agric. Food Chem, 11: 140-143.
ORGERON, J. D., MARTIN, J. D., CARAWAY, C. T., MARTINE, R. M., &
HAUSER, G. H. (1957) Methemoglobinemia from eating meat with high
nitrite content. Public Health Rep., 72 (3): 189-193.
OSSKE, G., WARZOK, R., & SCHNEIDER, J. (1972) [Transplacental tumour
induction by endogenous N-ethyl- N-nitrosourea in the rat.]
J. Arch. Geschwulstforsch., 40: 244-247 (in German).
OWENS, M. (1970) Nutrient balances in rivers. Proc. Soc. Water
Treat. Exam., 19: 239-247.
PANALAKS, T., IYENGAR, J. R., & SEN, N. P. (1972) Nitrate, nitrite and
dimethylnitrosamine in cured meat products. J. Assoc. Off.
Anal. Chem, 56 (3): 621-625.
PANALAKS, T., IYENGAR, J. R., DONALDSON, B. A., MILES, W. F., & SEN.
N. P. (1974) Further survey of cured meat products for volatile
N-nitrosamines. J. Assoc. Off. Anal. Chem., 57 (4): 806-812.
PASTERNAK, L. (1962) [Mutagenic effects of dimethylnitrosamine with
Drosophila melanogaster.] Naturwiss., 16: 381 (in German).
PASTERNAK, L. (1964) Investigation into the mutagenic effect of
several nitrosamine and nitrosamide compounds. Arzneintittel-
Forsch., 14: 802-804.
PENSABENE, J. W., FIDDLER, W., GATES, R. A., FAGAN, J. C., &
WASSERMAN, A. E. (1974) Effect of frying and other cooking
conditions on nitrosopyrrolidine formation in bacon.
J. Food Sci., 39: 314-136.
PERRY, T. L., SHAW, K. N. F., WALKER, D., & REDLICH, D. (1962) Urinary
excretion of amines in normal children. Pediatrics, 30: 576-584.
PETUKHOV, N. I., RYVKIN, A. I., GAINULLEN, G. G., & LANDYSHEVA, V. I.
(1972) [Methaemoglobinaemia in children and adults caused by
nitrates in water] Gig. i Sanit., 3: 14-17 (in Russian).
PETUKHOV, N. I. & IVANOV, A. V. (1970) Investigation of certain
psychophysiological reactions in children suffering from
methemoglobinemia due to nitrates in water. Gig. i Sanit.,
35 (1): 26-28.
PHILLIPS, J. C., LAKE, B. G., HEADING, C. E., GANGOLLI, S. D., &
LLOYD, A. G. (1975a) Studies on the metabolism of dimethyl-
nitrosamine in the rat. I. Effect of dose, route of
administration and sex. Food Cosmet. Toxicol., 13: 203-209.
PHILLIPS, J. C., HEADING, C. E., LAKE, B. O., GANGOLLI, S. D., &
LLOYD, A. G. (1975b) Studies on the metabolism of
dimethylnitrosamine in the rat. II. The effects of phenobarbitone
and 20-methylcholanthrene on the in vitro and in vivo
metabolism and acute toxicity of dimethylnitrosamine in young
and mature rats. Food Cosmet. Toxicol., 13: 611-617.
PHILLIPS, W. E. J. (1966) Effect of dietary nitrite on the liver
storage of vitamin A in the rat. Can. J. Blochem.,
44 (1): 1-7.
PHILLIPS, W. E. J. (1968a) Changes in the nitrate and nitrite contents
of fresh and processed spinach during storage. J. agric. Food
Chem., 16 (1): 88-91.
PHILLIPS, W. E. J. (1968b) Nitrate content of foods -- public health
implications. Can. Inst. Food Technol. J., 1 (3): 98-103.
PHILLIPS, W. E. J. (1969) Lack of nitrite accumulation in partially
consumed jars of baby food. Can. Inst. Food Technol. J.,
2 (4): 160-161.
PHILLIPS, W. E. J. (1971) Naturally occurring nitrate and nitrite in
foods in relation to infant methemoglobinemia. Food Cosmet.
Toxicol., 9: 219-228.
PIELSTICKER, K. (1967) Absence of malformations following
diethylnitrosamine in the golden hamster. Naturwiss.,
54: 340-341.
PISCIOTTA, A. V., EBBE, S. N., & HINZ, J. E. (1959) Clinical and
laboratory features of two variants of methemoglobin M disease.
J. Lab. clin. Med, 54: 73-87.
PITTS, J. N. Jr. & LLOYD, A. C. (1973) Discharges into the atmosphere.
In: Nitrogenous compounds in the environment. Washington, US
Environmental Protection Agency, pp. 43-65 (Rep No. EPA-SAB-73-
001).
PLATT, D. & HERING, F. J. (1973) The effect of Calciparin on
diethylnitrosamine-induced liver tumors in the rat.
Arzneimitteljbrsch., 23 (7): 956-961.
POUND, A, W. LAWSON, T. A., & HORN, L. (1973) Increased carcinogenic
action of dimethylnitrosamine after prior administration of
carbon tetrachloride. Br. J. Cancer, 27: 451-459.
PREDA, N., POPA, L., GALEA, V., & SIMU, G. (1976) N-nitroso compound
formation by chiordiazepoxide and nitrite interaction in vitro
and in vivo: protective action of ascorbic acid. IARC Sci.
Publ. No. 14, pp. 301-304.
PREUSSMAN, R. (1975) [Carcinogenic chemicals in the human
environment.] Handbuch der Allg. Pathol., VI/6/11. 448-482 (in
German).
PURCHASE, I. F. H., TUSTIN, R. C., & van RENSBERG, S. J. (1975)
Biological testing of food grown in the Transkei. Food Cosmet.
Toxicol, 13: 639-647.
QUISPEL, A., ed. (1974) -- The biology of nitrogen fixation.
Amsterdam, North Holland Publishing Company,
RABES, H., HARTENSTEIN, R., & SCHOLZE, P. (1970) Specific stages of
cellular response to homeostatic control during
diethylnitrosamine-induced liver carcinogenesis. Experientia
(Basel), 26: 1356-1359.
RABES, H., HARTENSTEZN, R., & GMINDER, J. (1971) Kidney neoplasms
induced by diethylnitrosamine in partially hepatectomized rats.
Naturwiss., 58: 102-103.
RAMMELL, C. G. & JOERIN, M. N. (1972) Determination of nitrite in
cheese and other dairy products. J. Dairy Res., 39: 89-94.
REDDY, B. S. & THOMAS, J. W. (1962) Interrelationship between thyroid
status and nitrate on carotine conversion. J. anim. Sci.,
21: 1010.
REEVES, M. J., BIRTLES, A. B., COURCHE, R. C., & ALDRICK, R. J. (1974)
Groundwater resources of the Vale of York. London, Water
Resources Board Report, pp. 90.
RHOADES, J. W. & JOHNSON, D. E. (1970) Gas-chromatography and
selective detection of N-nitrosamines. J. Chromatogr. Sci.,
8: 616-618.
RICHARDSON, W. D. (1907) Nitrates in vegetable foods in cured meats
and elsewhere. J. Am. Chem. Soc, 29: 1757-1767.
RIDDER, W. E. & OEHME, F. W. (1974) Nitrates as an environmental,
animal, and human hazard. Clin. Toxicol 7 (2): 145-159.
RIEDEL, B. & PIPER, R. (1973) Early changes of the rat bladder mucosa
after experimental cancerogenesis with N-butyl- N-butanol
(4)-nitrosamine.] (author's transl.). Urol. Int, 28: 322-327.
ROBERTS, J. D. & CASERIO, M. C. (1964) Basic Principles of Organic
Chemistry, New York, Benjamin, pp. 656-666.
ROBERTS, W. K. & SELL, J. L. (1963) Vitamin A destruction by nitrite
in vitro and in vivo. J. anim. Sci., 22: 1081-1085.
ROBERTSON, H. E. & DRAYCOTT, M. E. (1948) Nitrate poisoning of
infants by contaminated drinking water. Abstracts of papers
presented at the fifteenth Annual Christmas Meeting of the
Laboratory Section, Canadian Public Health Association, London,
Ontario, 13-14 December, p. 30.
ROBERTSON, H. E. & RIDDELL, W. A. (1949) Cyanosis of infants produced
by high nitrate concentration in rural waters of Saskatchewan.
Can. J. Public Health, 40: 72-77.
ROBINSON, E. & ROBBINS, R. C. (1972) Emissions, concentrations and
fate of gaseous atmospheric pollutants. In: Strauss, W., ed. Air
pollution control Part II, New York, Wiley-Interscience,
pp. 1-93,
ROLLER, P. P. & KEEFER, L. K. (1974) Catalysis of nitrosation
reactions by electrophilic species. IARC Sci. Publ. No. 9,
pp. 86-89.
ROSS, J. D. (1963) Deficient activity of DPNH-dependent methemoglobin
diaphorase in cord blood erythrocytes. Blood, 21 (1): 51-62.
ROSS, J. D. & DESFORGES, J. F. (1959) Reduction of methemoglobin by
erythrocytes from cord blood. Pediatrics, 23: 718-726.
SAKSHAUG, J., SÖGNEN, E., HANSEN, M. A., & KOPPANG, N. (1965)
Dimethylnitrosamine; its hepatotoxic effect in sheep and its
occurrence in toxic batches of herring meal. Nature (Lond.),
206: 1261-1262.
SANDER, J. (1971a) [Investigations on the formation of carcinogenic
nitroso compounds in the stomach of experimental animals and its
significance for man. I] Arzneimittel-Forsch., 21: 1572-1580
(in German).
SANDER, J. (1971b) [Investigations on the formation of carcinogenic
nitroso compounds in the stomach of experimental animals and its
significance for man.] Arzneimittel-Forsch., 21: 1703-1707 (in
German).
SANDER, J. (1971c)[Further experiments on tumour induction by oral
administration of small doses of N-methylbenzylamine and sodium
nitrite.] Z. Krebsforsch., 76: 93-96 (in German).
SANDER, J. & BÜRKLE, G. (1969) [Induction of malignant tumors in rats
by simultaneous feeding of nitrite and secondary amines.]
Z. Krebsforsch., 73: 54-66 (in German).
SANDER, J. & SEIF, F. (1969) [Bacterial reduction of nitrate in the
stomach of man as a cause of nitrosamine formation.]
Arzneimittel-Forsch, 19: 1091-1093 (in German).
SANDER, J. & SCHWEINSBERG, F. (1972) In vivo and in vitro
experiments on the formation of N- nitroso compounds from
amines or amides and nitrate or nitrite. IARC Sci. Publ. No. 3,
pp. 97-103.
SANDER, J. & SCHWEZNSBERG, F. (1973) [Tumor induction in mice with
methylbenzylnitrosamine in low doses.] Z. Krebsforsch,
79: 157-161 (in German).
SANDER, J., SCHWEINSBERG, F., & MENZ, H. P. (1968) [Investigations on
the formation of carcinogenic nitrosamine in the stomach.]
Hoppe-Seyler's Z. physiol. Chem, 349: 1691-1697 (in German).
SANDER, J., BURKLE, G., FLOHE, L., & AEIKENS, B. (1971) [ In vitro
investigations on the possibility of the formation of
carcinogenic nitrosamide in the stomach.] Arzneim-Forsch.,
(Drug Res.), 21 (3): 411-414 (in German).
SANDER, J., LABAR, J., LADENSTEIN, M., & SCHWEINSBERG, F. (1974a)
Quantitative measurement of in vivo nitrosamine formation.
IARC Sci. Publ. No. 9, pp. 123-131.
SANDER, J., LABAR, J., LADENSTEIN, M., & SCHWEINSBERG, F. (1974b)
Experiments on the degradation of nitrosamines in plants. IARC
Sci. Publ. No. 9, pp. 205-210.
SATTELMACHER, P. G. (1962) [Methemoglobinemia from nitrates in
drinking water.] Schriflenr. Ver. Wasser Boden. Lufthyg., No. 21
(in German).
SAWYER, C. N. (1947) Fertilization of lakes by agricultural and urban
drainage. J. New England Water Works Assoc., 109-127.
SCANLAN, R. A. (1975) Nitrosamines in food. CRC Critical Reviews in
Food Technology, April 1975, pp. 357-402.
SCHERER, E., HOFFMAN, M., EMMELOT, P., & FRIEDRICH-FRESKA, M. (1972)
Quantitative study on foci of altered liver cells induced in the
rat by a single dose of diethylnitrosamine and partial
hepatectomy. J. Natl Cancer Inst., 49: 93-106.
SCHEUNIG, O. & ZIEBARTH, D. (1976) Formation of nitrosamines by
interaction of some drugs with nitrite in human gastric juice.
IARC Sci. Publ. No. 14, pp. 269-277.
SCHMÄHL, D. (1963) [The origin, growth and chemotherapy of malignant
tumours.] Arzneimittei-Forsch., 13 (Supplement): 612 (in
German).
SCHMÄHL, D. (1970) [Experimental studies on syncarcinogenesis VI: The
addition of minimal doses of four different hepatotropic
carcinogens in liver cancer induction in the rat.]
Z. Krebsforsch., 74: 457-466 (in German).
SCHMÄHL, D. & STACKELBERG, S. YON (1968)[The influence of lactoflavin,
nicotinamide or dipyridamole on the carcinogenic activity of
diethylnitrosamine in rats.] Arzneimittel-Forsch., 18: 318-320
(in German).
SCHMÄHL, D. & THOMAS, C. (1962) [Acute nitrosomethylurethane poisoning
in rats following oral application.] Arzneimittel-Forsch.,
12: 585-587 (in German).
SCHMÄHL, D., THOMAS, C., & KÖNIG, K. (1963) [Studies of
syncarcinogenesis: I. Experiments on the induction of cancer in
rats by the simultaneous administration of diethyinitrosamine and
4-dimethylamino-azobenzene.] Z. Krebsforsch., 65: 351-377
(in German).
SCHMÄHL, D., THOMAS, C., SATTLER, W., & SCHIELD, G. F. (1965)
[Experimental studies of syncarcinogenesis: III. Attempts to
induce cancer in rats by administering diethylnitrosamine and
CC14 (or ethyl alcohol) simultaneously. In addition, an
experimental contribution regarding "alcoholic cirrhosis".]
Z. Krebsforsch., 66: 526-532 (in German).
SCHMÄHL, D., STUTZ, E., & THOMAS, C. (1966) [Experimental studies on
syncarcinogenesis. V. Experiments on cancer generation in rats
with the simultaneous application of X-ray radiation and
diethylnitrosamine or 4-dimethyl-aminobiphenyl.]
Z. Krebsforsch., 68: 68-72 (in German).
SCHMÄHL, D., WAGNER, R., & SCHERF, H. R. (1971) [Influence of
immunosuppressive drugs on cancer formation in rat liver by
diethyinitrosamine and on the induction of fibrosarcoma by
3,4-benzopyrene.] Arzneimittel-Forsch, 21 (3): 403-404
(in German).
SCHMIDT, P. & KNOTEK, Z. (1970) Epidemiological evaluation of
nitrates as ground water contaminants in Czechoslovakia (Paper
presented to the Sixth International Water Pollution Research
Conference, San Francisco).
SCHMID-RUPPIN, K. H. & PAPADOUPOULU, G. (1972) [Effect of
diethylnitrosamine (DENA) and influenza viruses on the induction
of lung carcinomas in mice.] Z. Krebsforsch., 77: 150-154
(in German).
SCHMITZ-MOORMAN, P., GEDIGK, P., & DHARAMADHACH, A. (1972) [Early
histological and histochemical changes during experimental
production of liver carcinoma by diethylnitrosamine.]
Z. Krebsforsch, 77: 9-16 (in German).
SCHNEIDER, N. R. & YEARY, R. A. (1973) Measurement of nitrite and
nitrate in blood. Am. J. vet. Res., 34 (1): 133-136.
SCHOENTAL, R. (1960) Carcinogenic action of diagomethane and of
nitroso- N-methylurethane. Nature (Lond.), 188: 420-421.
SCHREIBER, H., NETTESHEIM, P., LIJINSKY, W., RICHTER, C. B., &
WALBURO, H. E. (1972) Induction of lung cancer in germfree,
specific-pathogen-free and infected rats by
N-nitrosoheptamethyleneimine. Enhancement by respiratory
infection. J. Natl Cancer Inst., 49: 1107-1114.
SCHUPHAN, W. (1965) [The nitrate content of spinach (Spinacia
oleracea, L) in relation to methaemoglobinaemia in children.]
Z. Ernähr. Wiss., 5 (3-4): 207-209 (in German).
SCHUPHAN, W. (1969) [The formation of nitrate and nitrite in plant
metabolism.] Nutritio Dieta, 11: 120-132 (in German).
SCHWEINSBERG, F. & SANDER, J. (1972) [Carcinogenic nitrosamine from
simple aliphatic tertiary amines and nitrite.] Hoppe-Seyler's
Z. physiol. Chem., 353: 1671-1676 (in German).
SEBRANEK, J. G., CASSENS, R. G., & HOEKSTRA, W. G. (1974) Fate of
added nitrite. In: Proceedings of the International Symposium
on Nitrite in Meat Products, Ziest, 10-14 September, 1973,
Wageningen, Centre for Agricultural Publishing and
Documentation, pp. 139-147.
SEHGAL, O. P. & KRAUSE, G. F. (1968) Efficiency of nitrous acid as an
inactivating and mutagenic agent of intact tobacco mosaic virus
and its isolated nucleic acid. J. Virol., 2 (10): 966-971.
SELENKA, F. (1970) Formation and reduction of nitrite in infant
nutrients containing nitrate. Archiv. Hyg., 154 (4): 336-345.
SELL, J. L. & ROBERTS, W. K. (1963) Effects of dietary nitrite on the
chick: Growth, liver vitamin A stores and thyroid weight. J.
Nutr, 79: 171-178.
SEN, N. P. (1970) Gas-liquid chromatographic determination of
dimethylnitrosamine as dimethylnitramine at picogram levels.
J. Chromatogr, 51: 301-304.
SEN, N. P. (1972) The evidence for the presence of dimethylnitrosamine
in meat products. Food Cosmet. Toxicol., 10: 219-223.
SEN, N. P. (1974) Nitrosamines in toxic constituents in animal
foodstuffs. New York, Academic Press Inc., pp. 131.
SEN, N. P. & DALPE, C. (1972) A simple thin-layer chromatographic
technique for the semi-quantitative determination of volatile
nitrosamines in alcoholic beverages. Analyst,
97: 216-220.
SEN, N. P. & DONALDSON, B. (1974) The effect of ascorbic acid and
glutathiane on the formation of nitrosopiperazines from
piperazine adipate and nitrite. IARC Sci. Publ.
No. 9, pp. 103-106.
SEN, N. P., SMITH, D.C., SCHWINGHAMER, L., & MARLEAU, J. J. (1969a)
Diethylnitrosamine and other N-nitrosamines in foods. J.
Assoc. Off. Anal. Chem., 52 (1): 47-52.
SEN, N. P., SMITH, D.C., & SCHWINGHAMER, L. (1969b) Formation of
N-nitrosamines from secondary amines and nitrite in human and
animal gastric juice. Food Cosmet. Toxicol., 7: 301-307.
SEN, N. P., SMITH, D.C., SCHWINGHAMER, L., & HOWSAM, B. (1970)
Formation of nitrosamines in nitrite-treated fish. Can. Inst.
Food Technol. J., 3 (2): 66-69.
SEN, N. P., SCHWINGHAMER, L. A., DONALDSON, B. A., & MILES, W. F.
(1972) N-nitrosodimethylamine in fish meal. J. agric. Food
Chem., 20 (6): 1280-1281.
SEN, N. P., DONALDSON, B., IYENGAR, J. R., & PANALAKS, T. (1973a)
Nitrosopyrrolidine and dimethylnitrosamine in bacon. Nature
(Lond.), 241: 473-474.
SEN, N. P., MILES, W. F., DONALDSON, B., PANALAKS, T., & IYENGAR, J.
R. (1973b) Formation of nitrosamines in a meat curing mixture.
Nature (Lond.), 245: 104-105.
SEN, N. P., IYENGAR, J. R., DONALDSON, B. A., & PANALAKS, T. (1974a)
Effect of sodium nitrite concentration on the formation of
nitrosopyrrolidine and dimethylnitrosamine in fried bacon. J.
agric. Food Chem, 22 (3): 540-541.
SEN, N. P., DONALDSON, B., CHARBONNEAU, C., & MILES, W. F. (1974b)
Effect of additives on the formation of nitrosamines in meat
curing mixtures containing spices and nitrite. J. agric. Food
Chem., 22 (6): 1125-1130.
SEN, N. P., IYENGAR, J. R., MILES, W. F., & PANALAKS, T. (1976)
Nitrosamines in cured meat products. IARC Sci. Publ.
No. 14, pp. 333-342.
SERFONTEIN, W. J. & HURTER, P. (1966) Nitrosamines as environmental
carcinogens. II. Evidence for the presence of nitrosamines in
tobacco smoke condensate. Cancer Res., 26: 575-579.
DE SERRES, F. J., BROCKMAN, H. E., BARNETT, W. E., & KOLMARK, H. G.
(1967) Allelic complementation among nitrous acid-induced ad-3B
mutants of Neurospora crassa. Mutat. Res., 4: 415-424.
SHANK, R. C. (1975) Toxicology of N-nitroso compounds. Toxicol.
appl. Pharmacol., 31: 361-368.
SHANK, R. C. & NEWnERNE, P.M. (1972) Nitrite-morpholine induced
hepatomas. Food Cosmet. Toxicol., 10: 887-888.
SHEARER, L. A., GOLDSMITH, J. R., YOUNG, C., KEARNS, O. A., & TAMPLIN,
B. R. (1972) Methaemoglobin levels in infants in an area with
high nitrate water supply. Am. J. Public Health, 62
(9): 1174-1180.
SCHECHTER, H., GARDNER, N., & SHUVAL, H. I. (1972) A micromethod for
the determination of nitrite in blood. Anal. Chem. Acta.,
60: 93-99.
SHUVAL, H. I. & GRUENER, N. (1972) Epidemiological and toxicological
aspects of nitrates and nitrites in the environment. Am. J.
Public Health, 62: 1045-1052.
SIBAY, T. M. & HAYES, J. A. (1969) Potential carcinogenic effect of
streptozotocin. Lancet, II: 912.
SIBERT, D. & EISENBRAND, G. (1974) Induction of mitotic gene
conversion in Saccharomyces cerevisae by N-nitrosated
pesticides. Mutat. Res., 22: 121-126.
SIDDIQI, O. H. (1962) Mutagenic action of nitrous acid on
Asperigillus nidulans. Genet. Res., 3 (2): 303-314.
SIMON, C. (1966) Nitrite poisoning from spinach. Lancet, I
(7442): 872.
SIMON, C., MANZKE, H., KAY, H., & MROWETZ, G. (1964) On the
occurrence, pathogenesis, and possibilities for prophylaxis of
the methemoglobinemia caused by nitrite. Z. Kinderheilkunde,
91: 124-138.
SIMONEIT, B. R. & BURLINGAME, A. L. (1971) Organic analyses of
selected areas of Surveyor III recovered on the Apollo 12
mission. Nature (Lond.), 234: 210-211.
SINGLEY, T. L. (1962) Secondary methemoglobineamia due to the
adulteration of fish with sodium nitrite. Ann. internal Med.,
57 (5): 800-803.
SINHA, D. P. & SLEIGHT, S. D. (1971) Pathogenesis of abortion in acute
nitrite toxicosis in guinea pigs. Toxicol. appl. Pharmacol.,
18 (2): 340-347.
SINIOS VON, A. & WODSAK, W. (1965) [Spinach toxicity in children.]
Dtsch Med. Wochenschr, 90: 1856-1863 (in German).
SKRIVAN, J. (1971) Methemoglobinemia in pregnancy. Acta
Universitatis Carolinae Medica, 17: 123-160.
SLEIGHT, S. D. & ATALLAH, O. A. (1968) Reproduction in the guinea pig
as affected by chronic administration of potassium nitrate and
potassium nitrite. Toxicol. appl. Pharmacol., 12: 179-185.
SMITH, C. H. (1972) Polycythemia, methemogiobinemia,
sulfhemoglobinemia, and miscellaneous anemias In: Blood
diseases of infancy and childhood, 3rd ed., Saint Louis, The C.
V. Mosby Company, pp. 450-454.
SMITH, G. E. (1964) Nitrate problems in plants and water supplies in
Missouri. In: 2nd Annual Symposium on Relation of Geology and
Trace Elements to Nutrition.
SMITH, G. E. (1966) Causes of nitrate accumulation in plants and water
supplies. In: 18th Annual Midwest Fertilizer Conference,
Chicago, Illinois.
SMITH, J. E. & BEUTLER, E. (1966) Methemoglobin formation and
reduction in man and various animals species. Am. J. Physiol.,
210: 347-350.
SMITH, P. A. S. & LOEPPKY, R. N. (1967) Nitrosative cleavage of
tertiary amines. J. Am. Chem. Soc., 89 (5): 1147-1157.
SMITH, R. P. (1969) The significance of methaemoglobinaemia in
toxicology. In: Blood, F. R., ed. Essays in toxicology Volume
I, New York and London, Academic Press, pp. 84-113.
SMITH, R. P. (1975) Toxicology of the formed elements of the blood.
In: Casseret, L. J. & Doull, J., ed. Toxicology the basic
science of poisons. New York, Macmillan Publishing Co., Inc.,
pp. 225-243.
SPENCER, R. (1967) A study of factors affecting the quality and
shelf life of vacuum packed bacon, and of the behaviour of
Wiltshire cooked bacon packed and stored under controlled
conditions. (BFMIRA Res. Rep. No. 136).
SPIEGELHALDER, B., EISENBRAND, G., & PREUSSMAN, R. (1976) The
influence of dietary intake of nitrate on the nitrite content in
human saliva: a factor of possible relevance for in vivo
formation of N-nitroso compounds. Food Cosmet. Toxicol.,
14 (6): 545-548.
STANDFORD, G., ENGLAND, C. B., & TAYLOR, A. W. (1969) Fertilizer use
and Water quality, US Department of Agriculture, ARS,
pp. 41-168.
STEINBERG, R. A. & THOM, C. (1940) Mutations and reversions in
reproductivity of Aspergilli with nitrite, colchicine and
d-lysine. Proc. Natl Acad. Sci., 26 (6): 363-366.
STENBACK, F. G., FERRERO, A., & SHUBIK, P. (1973) Synergistic effects
of diethylnitrosamine and different dusts on respiratory
carcinogenesis in hamsters. Cancer Res., 33: 2209-2214.
STEPHANY, R. W., FREUDENTHAL, J., & SCHULLER, P. L. (1976)
Quantitative and qualitative determination of some volatile
nitrosamines in various meat products, IARC Sci. Publ.
No. 14, pp. 343-354.
STEWART, B. W., SWANN, P. F., HOLSMAN, J. W., & MAGEE, P. N. (1974)
Cellular injury and carcinogenesis. Evidence for the alkylation
of rat liver nucleic acids in vivo by N-nitrosomorpholine. Z.
Krebsforsch., 82 (1): 1-12.
STOEWSAND, G. S. (1973) Nitrite-induced methemoglobinemia in guinea
pigs: Influence of diets containing beets with varying amounts of
nitrate, and the effect of ascorbic acid, and methionine. J.
Nutr., 103 (3): 419-424.
STONE, I. M., LASCHIVER, C., & SALETAN, L. T. (1968) Nitrates in
worts, beers and brewing. Waller Stein Lab. Communications, 31
(106): 193-201.
STRACK, H. B., FREESE, E. B., & FREESE, E. (1964) Comparison of
mutation and inactivation rates induced in bacteriophage and
transforming DNA by various mutagens. Mutat. Res.,
1: 10-21.
STRACKS W. & FERON, V. J. (1973) Ultrastructure of pulmonary adenomas
induced by intratracheal instillation of diethylnitrosamine in
Syrian golden hamsters. Eur. J. Cancer, 9: 359-362.
SVOBODA, D. & HIGGINSON, J. (1968) A comparison of ultrastructural
changes in rat liver due to chemical carcinogens. Cancer Res.,
28: 1703-1733.
SWANN, P. F. & KAUFMAN, D. G. (1973) The dose response for the
induction of kidney tumors in the rat by a single dose of
dimethylnitrosamine. Br. J. Cancer, 28: 83-84.
SWANN, P. F. & MAGEE, P. W. (1971) Nitrosamine-induced carcinogenesis:
The alkylation of N-7 of guanine of nucleic acids of the rat by
diethylnitrosamine, N-ethyl- N-nitrosourea and
ethylmethanesulphonate. Biochem. J., 125: 841-847.
SWENBERG, J. A., KOESTNER, A., WESCHLER, W., & DENLINGER, R. H. (1972)
Quantitative aspects of transplacentai tumor induction with
ethylnitrosourea in rats. Cancer Res., 32: 2656-2660.
SYLVESTER, R. O. (1961) Nutrient content of drainage water forested,
urban and agricultural areas. In: Algae and Metropolitan
Wastes. Transactions of the 1960 Seminar, Robert A. Taft Sanitary
Engineering Center, pp. 80-87, (Technical Report W61-3).
TAKAYAMA, S. (1968) The histological and autoradiographical studies of
mouse liver during the course of carcinogenesis by
dimethylnitrosamine. Z. Krebsforsch., 71: 246-254.
TAKAYAMA, S. & IMAIZUMI, T. (1969) Sequential effects of chemically
different carcinogens, dimethylnitrosamine and 4-dimethylaminoazo-
benzene, on hepatocarcinogenesis in rats. Int. J. Cancer,
4: 373-383.
TAKAYAMA, S. & MURAMATSU, M. (1969) Incorporation of tritiated
dimethylnitrosamine into subcellular fractions of mouse liver
after long-term administration of dimethylnitrosamine. Z.
Krebsforsch, 73: 172-179.
TANNENBAUM, S. R., SINSKEY, A. J., WEISMAN, M., & BISHOP, W. (1974)
Nitrite in human saliva. Its possible relationship to nitrosamine
formation. J. Natl Cancer Inst., 53 (1): 79-84.
TANNENBAUM, S. R., WEISMAN, M., & FELT, D. (1976) The effect of
nitrate intake on nitrite formation in human saliva. Food
Cosmet. Toxicol., 14 (6): 549-552.
TAYLOR, H. W. & LIJINSKY, W. (1975) Tumour induction in rats by
feeding heptamethyleneimine and nitrite in water. Cancer Res.,
35: 812-815.
TELLING G. M. (1972) A gas-liquid chromatographic procedure for the
detection of volatile N-nitrosamines at the 10 parts per
billion level in foodstuffs after conversion to their
corresponding nitramines. J. Chromatogr., 73: 79-87.
TELLING, G. M., BRYCE, T. A., & ALTHORPE, J. (1971) Use of vacuum
distillation and gas chromatography mass spectrometry for
determination of low levels of volatile nitrosamines in meat
products. J. agric. Food Chem., 19(5): 937-940.
TELLING G. M., BRICE, T. A., HOAR, D., OSBORNE, D., & WELTI, D. (1974)
Progress in the analysis of volatile N-nitroso compounds.
IARC Sci. Publ. No. 9, pp. 12-17.
TERRACINI, B., MAGEE, P. N., & BARNES, J. M. (1967) Hepatic pathology
in rats on low dietary levels of dimethylnitrosamine. Br. J.
Cancer, 21: 559-565.
THOMAS, J. F. J. (1948) Water Survey Report No. 2, Ottawa river
drainage basin. Canada Department of Mines and Technical
Surveys, pp. 11-23 (Publ. No. 834).
TOMATIS, L. & CEFIS, F. (1967) The effects of multiple and single
administration of dimethylnitrosamine to hamsters. Tumori,
53: 447-451.
TOMATIS, L. (1977) Prenatal exposure to chemical carcinogens and its
effect on subsequent generations. In: Conference on Perinatal
Carcinogenesis. Washington, DC, National Cancer Institute
Monograph.
TOMLINSON, T. E. (1970) Trends in nitrate concentrations in English
rivers in relation to fertilizer. Water Treat. Exam.,
pp. 277-293.
TOUSSAINT, W. & SELENKA, F. (1970) Methemoglobin formation in young
infants. A contribution to drinking water hygiene on Rheinhessen.
Mschr. Kinderheilk., 118(6): 282-284.
UNGERER, O., EISENBRAND, G., & PREUSSMANN, R. (1974) The reaction of
nitrite with pesticides. Formation, chemical properties and
carcinogenic activity of the N-nitroso compounds of the
herbicide N-methyl-N1-(2-benzothiazolyl)-urea. Z.
Krebsforsch., 81 (3-4): 217-242.
UNITED NATIONS (1976) Statistical Yearbook, 1975, New York, p. 298.
US DEPARTMENT OF AGRICULTURE (1965) Agricultural Information
Bulletin, No. 299, Washington, DC.
US DEPARTMENT OF HEALTH, EDUCATION AND WELFARE (1966) Air Quality
Data from the National Air Sampling Networks and Contributing
State and Local Networks, 1964-1965, Cincinnati, OH, USDHEW
Public Health Service Division of Air Pollution, pp. 125.
US DEPARTMENT OF HEALTH, EDUCATION AND WELFARE (1970) Nitrates,
nitrites and methaemoglobinaemia, Research Triangle Park, N.C.,
National Institute of Environmental Health Sciences, pp. 43
(Environ. Rev. No. 2).
VAN LOGTEN, M. J., DEN TONKELAAR, E. M., KROES, R. BERKVENS, J. M., &
VAN ESCH, G. J. (1972) Long-term experiment with canned meat
treated with sodium nitrate and glucono-delta-lactone in rats.
Food Cosmet. Toxicol., 10: 475-488.
VAN RENSBURG, S. J. (1972) Failure of low protein and zinc intakes to
influence nitrosamine-induced oesophageal carcinogenesis. S.
Afr. reed. J, 46: 1137-1138.
VAVRA, J. J., DE BOER, C., DIETZ, A., HANKA, L. J., & SOKOLSKI, W. T.
(1960) Antibiotics Annual 1959-1960, NY, Antiobiotica Inc,
pp. 230.
VERLY, W. G., BARBASON, H., DUSART, J, & PETITPAS-DEWANDRE, A. (1967)
A comparative study of the action of ethyl methane sulphonate and
HNO2 on the mutation to streptomycin resistance of Escherichia
coil K 12. Biochim. Biophys. Acta, 145: 752-762.
VESSELINOVITCH, S. D. (1969) The sex-dependent difference in the
development of liver tumors in mice administered
dimethylnitrosamine. Cancer Res., 29: 1024-1027.
VIETS, F. B. & ALDRICH, S. R. (1973) Nitrogenous materials in the
environment, Draft Report, US Environmental Protection Agency,
Hazardous Materials Advisory Committee.
VIGIL, J., WARBURTON, S., HAYNES, W. A., & KAISER, L. R. (1965)
Nitrates in municipal water supply cause methemoglobinemia in
infant. Public Health Rep., 80 (12): 1119-1121.
VON KREYBIG, T. (1965a) [The effect of teratogenic compounds on the
early stages of prenatal development in the rat.] Naunyn-
Schmiederbergs Arch: exp. Path. Pharmacol., 252: 196-204
(in German).
VON KREYBIG, T. (1965b) [The effect of carcinogen methylnitrosourea
doses on the embryonal development in the rat.] Z. Krebsforsch.,
67: 46-50 (in German).
VON KREYBIG, T. (1968) [Experimental prenatal toxicology.]
Arzneimittel-Forsch., Suppl. 17, pp. 1-211 (in German).
VON KREYBIG, T. & SCHMIDT, W. (1966) [On chemical teratogenesis in the
rat.] ArzneimitteI-Forsch., 8: 989-1001 (in German).
VON KREYBIG, T. & SCHMIDT, W. (1967) [Chemically induced fetopathies
in the rat.] Arzneimittel-Forsch., 9: 1093-1100 (in German)
VOOGT, P. (1906) The determination of nitrate with a nitrate selective
electrode. Dtsch Lebensm. Rundschau., 65: 196-198.
WALKER, E. A., PIGNATELLI, B., & CASTEGNARO, M. (1975) Effects of
gallic acid on nitrosamine formation. Nature (Lond.),
258: 176.
WALKER, E. A., BOGOVSKI, P., & GRICUITE, L., ed. (1976) Environmental
N-nitroso compounds analysis and formation. Proceedings of a
Working Conference, the Polytechnical Institute, Tallinin,
Estonian SSR, 1-3 October, 1975, Lyons, International Agency for
Research on Cancer, pp. 512.
WALTERS, C. L. (1971) The detection and estimation of trace amounts of
N-nitrosamines in a food matrix. Lab. Pract., 20: 574-578.
WALTON, G. (1951) Survey of literature relating to infant
methemoglobinemia due to nitrate-contaminated water. Am. J.
Public Health, 41: 986-996.
WASSERMAN, A. E., FIDDLER, W., DOERR, R. C., OSMAN, S. F., & DOOLEY,
C. J. (1972) Dimethylnitrosamine in frankfurters. Food Cosmet.
Toxicol., 10: 681-684.
WATER RESEARCH CENTRE (1974) Notes on water pollution, No. 66,
pp. 4.
WATROUS, R. M. (1947) Health hazards of the pharmaceutical industry.
Br. J. ind. Med., 4: 111-125.
WEAST, R. C. ed. (1976) Handbook of chemistry and physics, 57th
edition, Cleveland, OH, CRC Press, pp. C-81-C-685.
WESCHLER, W. (1970) Oncogenic, teratogenic and mutagenic effects of
methyl- and ethylnitrosourea. In: 6th International Congress of
Neuropathology, Paris. Masson, pp. 128-129.
WESCHLER, W. (1971) Teratogenic effects of the neurotropic resorptive
carcinogens methyl-and ethylnitrosourea in rats. J. Neuropath.
exp. Neurol., 30: 120-121.
WESCHLER, W. (1973) Carcinogenic and teratogenic effects of
ethylnitrosourea and methylnitrosourea during pregnancy in the
rat. IARC Sci. Publ. No. 4, pp. 127-142.
WEGNER, T. N. (1972) Simple and sensitive procedure for determining
nitrate and nitrite in mixtures in biological fluids. J. Dairy
Sci., 55 (5): 642-644.
WEIL, L. & QUENTIN, L.-E. (1973) Nitrogen compounds in water and
nitrate determination with ion-sensitive electrode. Vom Wasser,
40: 125-133.
WEISBERGER, J. H. & RAINERI, R. (1975) Dietary factors and the
etiology of gastric cancer. Cancer Res., 35: 3469-3474.
WEISBERGER, E. K., WARD, J. M., & BROWN, C. (1974) Dibenamine:
Selective protection against diethylnitrosamine-induced hepatic
carcinogenesis but not oral, pharyngeal and esophageal
carcinogenesis. Toxicol. appl. Pharmacol., 28: 477-484.
WELLS, B. E., WALKER, R., & WALTERS, C. L. (1974) Interactions between
sodium nitrite and foodstuffs under gastric conditions. J. Sci.
Food Agric., 25: 1048.
WHO (1971) International standards for drinking water. Third ed,
Geneva, World Health Organization, pp. 70.
WHO (1977) Environmental health criteria 4--Oxides of nitrogen,
Geneva, World Health Organization, pp. 81.
WILLIAMS, A. O. (1970) Ultrastructure of liver cell carcinoma in
Macaca mulata monkey. Exp. mol. Pathol., 13: 359-369.
WINTON, E. F., TARBIFF, R. G., & MCCABE, L. J. (1971) Nitrate in
drinking water. J. Am. Water Works Assoc., 63: 95-98.
WORKSHOP ON GLOBAL ECOLOGY (1971) Man in the living environment, The
Institute of Ecology Report of the workshop on global ecological
problems, Chicago, IL, Field Museum of Natural History, pp. 267.
WRIGHT, A. J. & DAVIDSON, K. L. (1964) Nitrate accumulation in crops
and nitrate poisoning in animals. Advan. Agron., 16: 197-247.
WRIGLEY, F. (1948) Toxic effects of nitroso-methyl urethane.
Br. J. ind. Med., 5: 26-27.
YANG, K. W. & BROWN, E. V. (1972) A sensitive analysis for
nitrosamines. Anal. Lett., 5 (5): 293-304.
ZALDIVAR & WETTERSTRAND (1975) Further evidence of a positive
correlation between exposure to nitrate fertilizers (NaNO3 and
KNO3) and gastric cancer death rates: Nitrites and
nitrosamines. Experientia (Basel), 31: 1354-1355.
ZIEBARTH, D. & SCHEUNIG, G. (1976) Effects of some inhibitors on the
nitrosation of drugs in human gastric juice. IARC Sci. Publ. No.
14, pp. 279-290.
ZIMMERMAN, F. K. & SCHWAIER, R. (1967) Induction of a mitotic gene
conversion with nitrous acid, 1-methyl-3-nitro-1-nitrosoguanidine
and other alkylating agents in Saccharomyces cerevisiae. Mol.
gen. Genet., 100: 63-76.
ZIMMERMAN, F. K., SCHWAIER, R., & LAER, U. V. (1966) Mitotic
recombination induced in Saccharomyces cerevisiae with nitrous
acid, diethylsulphate and carcinogenic, alkylating nitrosamides.
Z. Vererbungsl., 98: 230-246.
artificial fertilizers are ultimately used for the synthesis of
biological molecules, particularly proteins. Plants and animal waste
and dead tissues return fixed nitrogen to the soils, where part of it
is recycled and part returned to the atmosphere, thus completing the
nitrogen cycle. According to Delwiche (1970), nitrogen fixation on a
world basis may exceed denitrification by about 10%. The increased use
of industrial fertilizers has resulted in some areas in increased
concentrations of nitrates in bodies of water, resulting, in some
cases, in eutrophication.
4.2 Transformation in Foods
4.2.1 Reduction of nitrates to nitrites
Because of the ability of spinach to accumulate large quantities
of nitrates and reported cases of intoxication associated with the
consumption of this vegetable, several studies have been undertaken on
the conversion of nitrates to nitrites in spinach.
Data presented by Phillips (1968a) indicated that initial
nitrite-N contents of fresh, frozen, canned, and baby-food spinach
were generally less than 1 mg/kg fresh weight. However, several
authors have reported a rapid fall in nitrate levels and increase in
nitrite levels in fresh spinach during the first 4 days of storage at
room temperature (Achtzehn & Hawat, 1970; Phillips, 1968a; Schuphan,
1965). Higher nitrite levels occurred in spinach from fertilized
ground (Brown & Smith, 1967) and these could reach exceptionally high
values (3600 mg/kg dry weight) with excessive fertilization (Schuphan,
1965).
Under refrigeration, the nitrite-N contents of fresh spinach
increased very gradually throughout a storage period of 28 days
(Phillips, 1968a). Significant increases in nitrite-N levels did not
occur during the storage of frozen, canned, or baby-food spinach, but
increased concentrations were found in frozen spinach, that had been
left to thaw at room temperature for an excessively long period (39 h)
(Phillips, 1968a).
There was also a slight rise in nitrite levels when partially
consumed jars of commercial baby foods containing nitrates were stored
for 7 days at room temperature instead of under refrigeration
(Phillips, 1969). Selenka (1970) noted that nitrite formation in baby
foods was rapid in the presence of Escherichia coli and Pseudomonas
fluorescens, less rapid with Bacillus subtilis, and very slow with
Staphylococcus albus.
When foods consisted of a solid immersed in a liquid (e.g. canned
foods or frozen foods after thawing) nitrates were partially
transferred to the liquid portion or into the water in which the food
was cooked (Bodiphala & Ormrod, 1971). When large volumes of water and
long cooking times were employed (Kilgore et al., 1963), and when
canned vegetables were blanched in hot water instead of steam
(Johnson, 1966), significant amounts of nitrates were leached out of
the foods. Nitrate reductase (NADPH) (1.6.6.3) activity was rapidly
destroyed during cooking, thereby greatly diminishing further
conversion of nitrates to nitrites (Bodiphala & Ormrod, 1971). It is
also well known that the sterilization treatments necessary for
canning destroy microorganisms that could convert nitrates to
nitrites.
Conversion of nitrates to nitrites occurred more slowly in
vacuum-packed bacon than in unpackaged bacon, presumably due to the
low reducing ability of anaerobes (Cavett, 1962). Spencer (1967) found
that the nitrite content of vacuum-packed bacon decreased slowly on
storage. It has also been reported by Sebranek et al. (1974) that
nitrite levels in meat, determined 2 days after processing, were less
than half those originally added to frozen samples and samples
processed at 71°C, and that they decreased further during storage.
Frying, grilling, or boiling bacon or ham reduced the nitrites content
by 20-90% (Food Standards Committee, 1959).
When direct gas firing of spray dryers was employed, the nitrate-
N contents of dried milk products increased by 1-3 mg/kg compared with
those obtained using indirectly heated sprayers but nitrite-N levels
were unaffected (Manning et al., 1968). Air drying of potato and corn
starch led to the formation of only trace amounts of nitrite
(Gerritsen & De Willingen, 1969).
Nitrates may be reduced to nitrites when cooking is carried out
in aluminium utensils (Osteryoung, unpublished data)a. This
observation appears to be significant since some countries use
aluminium utensils for boiling milk and water, a practice which could
lead to the formation of sizeable quantities of nitrites. This effect
of aluminium should be investigated further.
4.2.2 Formation and degradation of N-nitroso compounds
The conditions under which various amines, amino acids, and
proteins in food could react with nitrite to form nitrosamines were
studied by Ender & Ceh (1971) and by Sen et al. (1970) who showed that
when cod, herring, hake, halibut, mackerel, or salmon were treated
with sodium nitrite at 200 mg/kg and cooked at 110°C for 60-70 min
there were only trace amounts (2.5-25 µg/kg) of DMN in the cooked
product. The highest levels were found in mackerel and hake, both of
which contain large amounts of DMA and TMA. Samples without added
nitrite did not contain any detectable nitrosamines.
a Nitrates as human and animal health hazards, Paper presented at
the Second Conference on Environmental Chemicals, Colorado State
University, 1973.
The formation of DMN was studied in aqueous model systems
containing methyl amines and sodium nitrite under conditions which
were more severe than those employed in the commercial processing of
nitrite-treated smoked chub (a fresh water fish containing small
amounts of TMA, TMAO, and DMA). The results of the studies showed that
not more than 10 µg of DMN per kg of the final product would be formed
during the smoking process (Malins et al., 1970).
Recently, Sen et al. (1973b) suggested that a major source of
nitrosamines in cured meat might arise from an interaction between the
nitrites and spices, such as black pepper and paprika, that are
present in curing mixtures. Nitrosopyrrolidine, nitrosopiperidine, and
DMN were found in a curing mixture used by a meat manufacturer in
Canada. The same authors (Sen et al. 1974a) have also studied the
effect of sodium nitrite concentration on the formation of
nitrosopyrrolidine and DMN in fried bacon. Bacon samples prepared with
sodium nitrite at 0, 50, 100, 150, or 200 mg/kg were analyzed for
nitrosopyrrolidine and DMN. No nitrosamine was detected in samples
prepared without nitrite but all treated samples contained 2-20 µg/kg
of nitrosamines. The level of nitrosopyrrolidine was related to the
initial concentration of nitrite in the bacon. It has also been shown
that nitrosamine formation in bacon increases with increasing
temperature and time of frying and that whereas baking, broiling, or
frying produce variable amounts of nitrosamines none is produced with
cooking in a microwave oven (Pensabene et al., 1974).
After deliberate nitrosation of eggs and meat with unusually
large amounts (1%) of sodium nitrite, some N-nitroso compounds
appeared to have been formed but the chemical nature of the compounds
detected was not clear (Walters, 1971).
No systematic studies on the formation of N-nitroso compounds
in cheese have been performed, although some types of cheese are known
to be processed with nitrate and nitrite.
There are few data on the fate of N-nitroso compounds during
the cooking, processing, or storage of food but some studies have
demonstrated that the volatile nitrosamine DMN and nitrosopyrrolidine
may be lost during the frying of bacon (Sen et al., 1973a).
4.3 Formation of N-nitroso Compounds from Drugs and Pesticides
Reaction with nitrite to form nitrosamines is not restricted to
food components. Lijinsky & Greenblatt (1972) and Lijinsky (1974)
reported that some antibiotics and other drugs that are widely used
can react with nitrites to form nitrosamines in alarmingly high
quantities. The drugs examined included oxytetracycline,
aminophenazone (aminopyrine), disulfiram, N,N-diethyl-3-
pyridinecarboxamide (nikethamide), tolazamide, and (E,E)-1-[5-(1,3-
benzodioxol-5-yl)-1-oxo-2,4-pentadienyl] piperidine (piperine).
Optimum conditions of temperature, pH, and concentration for these
reactions have been reported by Lijinsky et al. (1972a, 1972b, 1972c)
who more recently (Lijinsky, 1974) studied the reactions of
aminopyrine and other commonly used drugs with nitrous acid at rather
low concentrations to assess the magnitude of the hazard to man from
such interactions. The topic has been reviewed by Mirvish (1975) who
has listed 41 drugs and pesticides that have been nitrosated.
Pesticides listed include atrazine, simazine, ziram, and thiram.
4.4 Formation of N-nitroso Compounds in Animal Organisms
4.4.1 Formation of N-nitroso compounds in simulated gastric juice
Formation of DEN was demonstrated when DEA and nitrite were
incubated in the gastric juice of the rat, rabbit, cat, dog, and man.
More DEN was formed in human and rabbit gastric juices (pH 1-2 in both
cases) than in rat gastric juice (pH 4-5). (Sen et al., 1969a, 1969b).
The formation of nitrosamines by the interaction of some drugs
with nitrite in the presence of human gastric juice have been studied
by Scheunig & Ziebarth (1976). At 37°C and a pH = 2, for 1 h,
aminopyrine, sodium [(2,3 dihydro-1,5-dimethyl-3-oxo-2-phenyl-IH-
pyrazol-4-yl)methylamino] methanesulfonate (analgin), and piperazine
gave nitrosamine yields (calculated on the basis of nitrite used) of
69%, 11%, and 74.8% respectively.
In vitro studies have been carried out (Wells et al., 1974) in
which several foods (pork, egg, bread, milk, and cheese) were
incubated under simulated gastric conditions with concentrations of
nitrite similar to those used as food preservatives. The effect of the
thiocyanate ion as a catalyst for nitrosation was also studied since
it is secreted in saliva. Of the foods studied, only cheese produced
detectable amounts of volatile nitrosamines. The identity of the
nitrosamines was not indicated.
4.4.2 Formation of N-nitroso compounds in vivo
When DEA and nitrite were fed to cats and rabbits, considerable
amounts of DEN were detected in the stomach of the experimental
animals (Sen et al, 1969a, 1969b). Similar results were reported by
Sander & Sief (1969). Epstein (1972) reported the formation of
nitrosopiperidine in the gastrointestinal tract of rats treated with
nitrite and piperidine hydrochloride. When the nitrite concentration
was constant, nitrosopiperidine formation in the small intestine
increased with increasing concentrations of piperidine.
Nitrosopiperidine was also found in the stomach. Recently, Sander et
al. (1974a) demonstrated the formation of N-N'-dinitrosopiperazine,
DMN, and N-nitroso- N-methylbenzylamine in the stomach contents of
rats given the parent amines combined with nitrite. Considerable
individual variation in the degree of synthesis of N-N'-
dinitrosopiperazine was noted in the animals. In another recent
report, N-nitrosopyrrolidine was formed very rapidly in the stomach
of dogs from sodium nitrite and pyrrolidine (within 2-6 min) but after
30 min nearly all of it had disappeared, presumably due to its rapid
absorption (Mysliwy et al., 1974).
Indirect evidence of in vivo formation of N-nitroso compounds
has also been provided by some toxicity studies. Thus, hepatic
lesions, formed following administration of nitrite and some amines,
were similar to those produced by DMN or N-nitroso- N-
methylbenzylamine (Asahina et al., 1971). Similar effects were noted
where nitrite was administered up to 3 h after DMA, but the effect was
markedly reduced if the nitrite was given prior to the amine.
4.5 Formation of N-nitroso Compounds by Microorganisms
Studies conducted by Hawksworth & Hill (1971a), Klubes & Jondorf
(1971), and Sander & Sief (1969) suggested that nitrosamines could be
synthesized from secondary amines and nitrates or nitrites by
Escherichia coli and some species of streptococci. Fong & Chan
(1973b) demonstrated that homogenized Chinese salt fish inoculated
with Staphylococcus aureus (a nitrate-reducing bacterium) produced
considerable amounts of DMN.
Formation of nitrosamines in the presence of bacteria is unlikely
to occur in the large intestine, but the infected bladder and
achlorhydric stomach are likely sites (Hawksworth et al., 1974).
The ultimate mechanism of bacterial production of nitrosamines
remains to be ascertained. According to Hawksworth et al. (1974),
certain bacteria do reduce nitrate to nitrite but the formation of the
nitrosamine may be nonenzymatic and involve some heat-resistant
metabolite.
4.6 The Effects of Other Chemicals on the Formation of N-nitroso
Compounds
Fiddler et al. (1973) and Greenberg (1974) showed that high
levels of ascorbic acid reduced nitrosamine formation in frankfurter
sausages and in fried bacon. On the other hand, Nagata & Mirna (1974)
reported an increase in nitrosamine formation in meat products in the
presence of ascorbic acid. Other studies conducted on the inhibition
of nitrosamine formation by various compounds include a report by Sen
& Donaldson (1974) in which nitrosamine formation in human saliva was
inhibited by ascorbic acid. Ziebarth & Scheunig (1976) tested a number
of substances and beverages for the inhibition of the nitrosation of
several drugs under simulated gastric conditions. Of all the
substances investigated, ascorbic acid was regarded as the best
inhibitor because of its pronounced activity at pH values occurring in
the stomach and because it was not toxic in the amounts used.
5. ENVIRONMENTAL LEVELS AND EXPOSURES
5.1 Nitrates and Nitrites
5.1.1 Ambient air
Nitrate aerosols are the final stage in the atmospheric oxidation
of gaseous oxides of nitrogen, and substantial amounts of particulate
nitrates may be formed in urban areas affected by photochemical
pollution (Pitts & Lloyd, 1973). The concentration of nitrates in air
may range from about 1 to 40 µg/m3, depending on the sampling and
averaging periods. For example, the estimated annual mean values
(1968-1972) in Chattanooga, TN, USA, were between 1 and 6 µg/m3
(French et al., unpublished)a. The daily mean concentrations of
airborne nitrates in the central part of Tokyo ranged, in 1973, from
0.9 to 41.8 µg/m3 with an annual mean of 8.2 µg/m3. On the other
hand, in a small city with few industries (Matsue City) the daily
means were in the range of 1.1 -- 9.2 µg/m3, with an annual mean of
2.6 µg/m3 (Japan Environmental Sanitation Center, 1974).
5.1.2 Water
The concentrations of nitrates and nitrites in surface and ground
waters vary within wide limits, depending on geochemical conditions,
human and animal waste management practices, the extent to which
nitrogen-containing agriculture fertilizers are used locally, and on
industrial discharges of nitrogen compounds (section 3.2.1.).
In general, surface waters do not usually contain nitrate in
concentrations higher than 10 mg/litre, and nitrite concentrations
rarely exceed 1 mg/litre. However, a steady upward trend of nitrate
levels has been reported in recent years in some countries, both in
surface and ground waters. Thus, for example, in the River Thames,
England, nitrate concentrations increased from an average of
4 mg/litre in 1968 to an average of 9 mg/litre for the last quarter of
1973 (Water Research Centre, 1974). Similar increases have been
observed in several other English rivers (Casey, 1975; Owens, 1970;
Tomlinson, 1970). The nitrate concentrations are increasing in some
rivers that drain the great agricultural section of the centre of the
USA, and in selected small rivers the 45 mg/litre limit is sometimes
exceeded (Viets & Aldrich, 1973). A small increase in the nitrate
concentration of the Tamagawa River, Tokyo, Japan has also been
reported. From 1951-1965, the nitrate ion concentration rose from
7.9 mg/litre to 9.1 mg/litre. During the same period, the nitrite
concentration increased from 0.049 mg/litre to 0.53 mg/litre, i.e by a
factor of about 10 (Goto, 1973).
a French, J. G., Hasselblad, V., & Johnson, R. Aggravation of
asthma by air pollutants. 1971 -72 Southeastern CHESS studies.
Studies of 991 settlements in Bulgaria indicated that only 64
towns and villages had drinking water levels of nitrates between
30 mg/litre (Bulgarian standard) and 50 mg/litre. In 20 settlements,
situated in areas with intensive agriculture and stock breeding, the
nitrate concentrations exceeded 50 mg/litre. The reportb points out
that such problems did not exist some 10 years ago when smaller
quantities of nitrogen fertilizers were used in agriculture.
Much higher concentrations of nitrates are sometimes found in
ground water, particularly in water derived from dug wells. A survey
of over 2000 rural wells in Saskatchewan, Canada, revealed that 18.8%
contained nitrate concentrations of more than 50 mg/litre and 5.3% had
nitrate levels exceeding 300 mg/litre (Robertson & Draycott, 1948).
Hedlin (1971) also reported levels above 45 mg/litre in some wells in
a rural area of Manitoba, Canada. In many farm wells in central USA,
nitrate concentrations may range from 45-450 mg/litre. This problem is
neither new nor local, since such conditions have been recorded from
1895 to 1970 in Illinois, in 1939 in Iowa, and in 1970 in Minnesota
(Viets & Aldrich, 1973). The mean nitrate concentration in ground
water consumed by children affected by methaemoglobinaemia in
Czechoslovakia ranged from 18-257 mg/litre (Schmidt & Knotek, 1970).
According to Gruenar & Shuval (1970), about 180 wells for community
water supplies in the densely populated central and southern coastal
plain in Israel had nitrate concentrations exceeding 45 mg/litre. In
England, nitrate concentrations in some ground waters have been
reported to range from 12 mg/litre (Foster & Crease, 1974) to over
22 mg/litre (Reeves et al., 1974). Nitrate concentrations exceeding
45 mg/litre have not been reported in centralized water supplies in
the USSRc. However, high concentrations have been found from time
to time in dug wells, for example, 310-400 mg/litre in Leningrad
Oblast (Motylev, 1969), 110-200 mg/litre in the Tatar SSR (Petukhov
et al., 1972) and up to 430 mg/litre in the Moldvian SSR (Diskalenko,
1969).
5.1.3 Selected foods
According to the data compiled by the National Institute of
Environmental Health Sciences (NIEHS, 1970), the levels of nitrates in
vegetables vary considerably. The highest levels were found in beets,
egg plant, kale, and spinach and the lowest in tomatoes and peas;
similar findings were obtained in the German Democratic Republic by
Achtzehn & Hawat (1969). It is of interest to note that the nitrate
levels in vegetables reported by Jackson in 1967 were similar to those
reported by Richardson in 1907, when manure was used instead of
chemical fertilizers.
b Contribution to the WHO environmental health criteria document on
nitrates, nitrites and nitrosamines, Sofia, 1974.
c Contribution to the WHO environmental health criteria document on
nitrates, nitrites and nitrosamines, Moscow, 1974.
Nitrate contents vary not only between vegetable species but also
widely within a given species. This variation within a species may be
accounted for by such factors as temperature, sunlight, soil moisture,
and the level of available nitrogen in the soil (US Department of
Agriculture, 1965). A relationship between nitrate accumulation in
spinach and levels of fertilizer applied to the soil has been
demonstrated by a number of authors (Brown & Smith, 1967; Phillips,
1971). Furthermore, Schuphan (1965) reported exceptionally high levels
of nitrites (3600 mg/kg dry weight) in excessively fertilized fresh
spinach stored at room temperature.
A survey of the nitrate contents of fruits in the German
Democratic Republic, revealed that they were high in bananas and
strawberries but could not be detected in the other fruits examined
(Achtzehn & Hawat, 1969).
Cow's milk contained nitrate levels of 0-0.5 mg/litre (Simon et
al., 1964).
The levels of nitrates and nitrites in baby foods are of special
concern since infants are considerably more sensitive to the toxic
effects of nitrates than adults. Kamm et al. (1965) studied 194
prepared infant foods and found that, on average, fruits, dairy
products, puddings, egg products, meats, dry and concentrated food
supplements, and precooked cereal products contained nitrate levels of
less than 90 mg/kg. Vegetables, however, had a wide range of nitrate
contents varying from 0.9 to 2165 mg/kg, but nitrite levels never
exceeded 7 mg/kg. In studies on a number of canned foods, baby foods,
frozen foods, and vegetables, several varieties of fruit, spinach, and
beets generally had the highest nitrate contents (Bodiphala & Ormrod,
1971). Additional information may be found in articles by NIEHS (1970)
and Ashton (1970).
In a survey of various cured meats (Table 1, Ashton, 1970), the
highest nitrate content of 370-511 mg/kg was found in ham. In
analysing 197 samples of cured meat products, Panalaks et al. (1972)
found that the levels of nitrates and nitrites ranged from
0-3467 mg/kg and 0-252 mg/kg, respectively.
Dubrow & Kakisch (1960) analysed 338 samples of cheese and
reported that all were free of nitrites (less than 1 mg/kg). However,
40% of the samples contained nitrate levels of more than 1 mg/kg.
Rammell & Joerin (1972) also found low nitrite levels in cheese.
Table 1. Nitrate and nitrite contents of cured meats
Meat type Nitrate (mg/kg) Nitrite (mg/kg)
silverside 133-303 9-26
ham 370-511 7-150
luncheon meat 59-214 3.1-47
chopped ham & pork 53-101 22-62
corned beef 118-135 18-208
frankfurter sausage 119-141 8.5-10.3
From: Ashton (1970)
5.1.4 Estimate of general population exposure
One of the important sources of exposure to nitrates for man is
water. The level of nitrates in water may vary from practically nil to
over 200 mg/litre. In water from municipal supplies, however, it is
likely to be under 10 mg/litre. Thus, assuming an intake of 2 litres
of water per day, the daily intake of nitrates from this source would
normally be less than 20 mg, but with extremes of 0 and over 400 mg.
The other main sources of nitrates and nitrites are certain
vegetables and meat products. The intake from these sources is even
more variable because of marked differences not only in levels in
these foods but also in dietary patterns. However, Ashton (1970)
estimated the weekly intake of nitrates for a member of the general
population in the USA to be about 400 mg including 210 mg from
vegetables, 110 mg from meat products, and 85 mg from water (7 litres
per week). The estimate of Hill et al. (1973) for a member of the
general population in England included 225 mg from vegetables, 110 mg
from meat, and 105 mg from water in "control towns" and 645 mg from
water in Worksop, England. However these figures cannot be applied
generally because of variations in feeding habits and nitrate levels
in environmental media. Since the intake of nitrites is even more
variable, no estimates have been reported.
5.2 N-nitroso Compounds
5.2.1 Ambient air
The occurrence of N-nitroso compounds in urban air was reported
first by Bretschneider & Matz (1973) and confirmed by Fine et al.
(1976b) who found DMN at concentrations of about 1.2-3.5 µg/m3
(0.33-0.96 ppb) in an industrial district in Baltimore, MD, USA, and
about 0.06-0.17 µg/m3 (0.014 ppb - 0.051 ppb) in Belle, WV, USA.
N-nitroso compounds may be present in air, either due to their
formation in the air from secondary amines and oxides of nitrogen
(Neurath, 1972) or due to industrial omissions as in the instances
referred to.
5.2.2 Water
There are few reports on the occurrence of N-nitroso compounds
in water. Fine et al. (1976b) analysed samples from the Mississippi
river (New Orleans, LA) and from 3 water treatment plants in
Louisiana. Using the new N-nitroso compound-specific thermal energy
analyser (TEA) interfaced to both a gas chromatograph (GC) and a high
performance liquid chromatograph (LC), several peaks were tentatively
identified as belonging to N-nitroso derivatives of some pesticides.
The estimated concentrations were of the order of 0.1 µg/kg.
5.2.3 Selected foods
A summary of the reported occurrences of nitrosamines in meat and
fish products, adapted from Sen (1974) and updated, is presented in
Table 2. Only the results that were confirmed by mass spectroscopy are
quoted in this table. It was noted that the majority of some 50
publications dealt with the determination of nitrosamines,
particularly DMN, in processed pork meat. The methods employed for
analysis mainly involved gas chromatography. A few results were
confirmed by mass spectroscopic techniques. However, mass spectroscopy
confirmation is currently being employed more frequently than in the
past.
Table 2. Levels of nitrosamines in various meat and fish productsa
N-nitroso
Meat Country compounds Levels (c) Reference
or area foundb
dry sausage Canada DMN 10-20 µg/kg Sen (1972)
uncooked salami sausage Canada DMN 20-80 µg/kg Sen (1972)
salami sausage Netherlands DMN 0.3 µg/kg(e) Stephany et al. (1976)
DMN 0.1(e) Stephany et al. (1976)
NDBA 1.1(e) Stephany et al. (1976)
Netherlands NPY 0.4(e) Stephany et al. (1976)
NPIP 0.3(e) Stephany et al. (1976)
bacon Canada NPY 4-25 µg/kg Sen et al. (1973a)
Canada NPY 25-40 µg/kg Sen et al. (1974a)
Netherlands DMN 0.8 µg/kg(e) Stephany et al. (1976)
bacon DEN 0.2(g)
Netherlands NDBA 0.6(g) Stephany et al. (1976)
NPY 0.4(g)
Netherlands NPIP 0.6(g) Stephany et al. (1976)
USA NPY 7-35 µg/kg Pensabene et al. (1974)
bacon USA NPY 2, 28, 13 Fiddler et al. 1974
uncooked bacon Canada DMN 30 µg/kg Sen et al. (1973a)
fried bacon Netherlands DMN 2.4 µg/kg Groenen et al. (1976)
DEN 4.43 µg/kg
Netherlands DMN 1.1 µg/kg(e) Stephany et al. (1976)
DEN 0.2(g)
Netherlands NDBA 0.7(g) Stephany et al. (1976)
NPY 16.4(g)
Table 2 (Cont'd)
N-nitroso
Meat Country compounds Levels (c) Reference
or area foundb
Netherlands NPIP 3.9(g) Stephany et al. (1976)
UK NPY 1-40 µg/kg Crosby et al. (1972)
USA NPY 20-207 µg/kg Fazio et al. (1973)
smoked meat Netherlands DEN 7.91 µg/kg Groenen et al. (1976)
Netherlands DMN 3 Groenen et al. (1976)
smoked horse and Netherlands DMN 7.3 µg/kg(d) Stephany et al. (1976)
beef meat DEN 0.6(d) Stephany et al. (1976)
Netherlands NDBA 0.4(d) Stephany et al. (1976)
NPY 0.1(d) Stephany et al. (1976)
Netherlands NPIP 0.1(d) Stephany et al. (1976)
ham with layer Germany DMN 3 µg/kg Eisenbrand et al. (1975)
of pepper grains (Federal Republic of)
on the outside.
Only fat portion.
ham with layer Germany NPIP 6 µg/kg Eisenbrand et al. (1975)
of pepper grains (Federal Republic of)
on the outside.
Whole product Germany NPY 6
homogenized. (Federal Republic of)
ham, fried Germany DMN 1 µg/kg Eisenbrand et al. (1975)
(Federal Republic of) NPIP 8
NPY 19
Table 2 (Cont'd)
N-nitroso
Meat Country compounds Levels (c) Reference
or area foundb
ham with layer of Germany NPIP 4 µg/kg Eisenbrand et al. (1975)
pepper grains (Federal Republic of)
on the outside. Germany NPY 9
Whole product (Federal Republic of)
homogenized.
German type of Germany DMN 1 µg/kg Eisenbrand et al. (1975)
bacon, raw (Federal Republic of)
German type of Germany DMN 1 µg/kg Eisenbrand et al. (1975)
bacon, fried (Federal Republic of) NPIP 5 Eisenbrand et al. (1975)
Germany NPY 19 Eisenbrand et al. (1975)
(Federal Republic of)
smoked raw meat Germany DMN 2 µg/kg Eisenbrand et al. (1975)
(Federal Republic of)
smoked ham Germany DMN 8 µg/kg Eisenbrand et al. (1975)
(Federal Republic of)
smoked ham USA DMN 5 µg/kg Fazio et al. (1971b)
smoked ham USA DMN 5 µg/kg Fiddler et al. (1974)
cooked and smoked Netherlands DMN 0.4 µg/kg(f) Stephany et al. (1976)
ham DEN 0.6(f) Stephany et al. (1976)
Table 2 (Cont'd)
N-nitroso
Meat Country compounds Levels (c) Reference
or area foundb
Netherlands NDBA 0.4(f) Stephany et al. (1976)
NPY 0.3(f) Stephany et al. (1976)
Netherlands NPIP 0.4(f) Stephany et al. (1976)
cooked ham Netherlands DMN 6 µg/kg Groenen et al. (1976)
Bologna sausage Canada DEN 25 µg/kg Panalaks et al. (1974)
Canada NPY 20,100,105 Panalaks et al. (1974)
frankfurter sausage USA NPIP 50, 50, 60 µg/kg Wasserman et al. (1972)
spiced meat Canada DMN 5-48 µg/kg Sen et al. (1976)
products DEN 6-16
Canada NPIP 14-50 Sen et al. (1976)
Canada NPY 7-33 Sen et al. (1976)
fish meal Canada DMN 0.35-0.5 mg/kg Sen et al. (1972)
smoked, nitrate/ USA DMN 4-26 µg/kg Fazio at el. (1971a)
or nitrite treated
sable, salmon shad
fresh, smoked or UK DMN 1-9 µg/kg Crosby et al. (1972)
salted fish
salted white Hong Kong DMN 40-100 µg/kg Fong & Chan (1973a, 1973b)
herring
Table 2 (Cont'd)
N-nitroso
Meat Country compounds Levels (c) Reference
or area foundb
salted yellow Hong Kong DMN 10-60 µg/kg Fong & Chan (1973a, 1973b)
croakers
crude salt salted Hong Kong DMN 400 µg/kg Fong & Chan (1973a, 1973b)
white herring
crude salt salted Hong Kong DMN 200 µg/kg Fong & Chan (1973a, 1973b)
yellow croakers
prime salt salted Hong Kong DMN 10 µg/kg Fong & Chan (1973a, 1973b)
white herring
prime salt salted Hong Kong DMN 5 µg/kg Fong & Chan (1973a, 1973b)
yellow croakers
salted anchovies Hong Kong DMN 20 µg/kg Fong & Chan (1973a, 1973b)
whole herring meal Hong Kong DMN 300 µg/kg Fong & Chan (1973a, 1973b)
a Adapted from Sen (1974)
b DMN -- N-methyl- N-nitrosomethanamine c All values confirmed by mass spectroscopy
DEN -- N-ethyl- N nitrosoethanamine d mean of 4 samples
NDBA -- Nitroso- N-butylamine e mean of 5 samples
NPY -- Nitrosopyrrolidine f mean of 6 samples
NPIP -- Nitrosopiperidine g mean of 10 samples
Several authors who detected nitrosamines in foods by screening
methods but did not confirm these results by mass spectrometry include
Ender et al. (1964, 1967), Ender & Ceh (1967), Fong & Walsh (1971),
Freimuth & Glaser (1970), Hedler & Marquardt (1968), Kröller (1967),
Lembke & Moebus (1970), Möhler & Mayrhofer (1968, 1969), and Sakshaug
et al. (1965). Nevertheless, results by screening methods should not
be entirely ignored.
Considerable variations have been found in the levels of volatile
N-nitroso compounds in fried and grilled bacon. Attempts to
correlate these levels with levels of nitrates and nitrites did not
reveal any definite pattern. Telling et al. (1974) studied the effect
of various cooking temperatures on the levels of N-nitroso compounds
in grilled bacon. The results indicated that the levels of DMN
remained fairly constant as the cooking temperature was raised but
those of nitrosopyrrolidine increased.
Lipid soluble nitrosamines have an affinity for the fatty
portions of food (Sen et al. 1973a).
5.2.4 Tobacco and tobacco smoke
Since precursors for the formation of nitrosamines occur in
tobacco, Druckrey & Preussman (1962) thought it likely that tobacco or
tobacco smoke might contain trace amounts of nitrosamines. Initially,
studies on the nitrosamine content of tobacco products were hampered
due to interference from other compounds. Later, evidence suggesting
the presence of DMN, nitrosopyrrolidine, methylbutylnitrosamine, and
nitrosopiperidine in tobacco smoke was obtained (Kröller, 1967;
Neurath et al., 1964; Neurath, 1972, Serfontein & Hurter, 1966).
Although anabasine and nornicotine are constituents of tobacco smoke,
the corresponding nitroso derivatives were not detected by Neurath
(1972). Recently, however, Hoffman et al. (1974) reported the presence
of N-nitrosonornicotine at levels of up to 88 mg/kg in unburned
tobacco.
5.2.5 Estimate of general population exposure
The N-nitroso compounds that have been identified and
determined in meat and fish are listed in Table 2; consumption of
these foods constitutes a definite exposure of the general population
to these chemicals. However, at present, no estimate can be made of
the human exposure from these and other sources because insufficient
samples have been analysed and because the relevant food consumption
surveys have not been made.
5.2.6 Occupational exposure to N-nitroso compounds
The potential occupational hazards associated with the use or
manufacture of N-nitroso compounds in industry have been pointed out
by Magee & Barnes (1967).
Only a few quantitative data are available, however, on the
concentration of N-nitroso compounds in the work environment. In a
study by Bretschneider & Matz, (1976) DMN levels in the air in a
factory manufacturing DMA were roughly estimated to range from 0.001
to 0.43 µg/m3.
6. METABOLISM OF NITRATES, NITRITES, AND N-NITROSO COMPOUNDS
6.1 Gastrointestinal Absorption
6.1.1 Nitrates and nitrites
A part of ingested nitrates is readily absorbed and a part may be
metabolized by the microflora in the gastrointestinal tract (Ridder &
Oehme, 1974). Nitrites (NO2-), oxides of nitrogen (N2O5, NO2,
NO), hydroxylamine (NH2 OH), and ammonia (NH3) can be formed
depending upon the organisms present, the pH, and the available
nutrients (trace elements and carbohydrates), and may be absorbed.
Friedman et al. (1972) gave mice a single oral dose of 150 µg of
sodium nitrite. Measurement of the rate of disappearance showed that
the compound was rapidly absorbed and that food in the stomach had
little effect on absorption. Results using animals with a ligated
gastro-duodenal junction suggested that the major absorption site was
the gastric mucosa. Studies by Mirvish et al. (1974) on rats fed a
diet containing nitrite supported the previous observations on mice.
Experiments with food containing phenol red showed that a decrease in
nitrite levels in the stomach contents, especially in the glandular
part, that occurred within 5 h of feeding, was significantly greater
than that due to direct faecal elimination. This was attributed to
decomposition and other acid-catalyzed reactions of nitrite and to
direct absorption from the stomach.
6.1.2 N-nitroso compounds
Although a considerable degree of absorption of nitrosamines can
be inferred from the nature of their toxic effects following oral
administration, few reports could be found giving quantitative
information on absorption. Alarif & Epstein (1974) gave 3H-labelled
nitrosomethylurea and nitrosomethylurethane by gavage to groups of
pregnant guineapigs at doses of 2 mg/kg, and 5 mg/kg body weight,
respectively. The animals were killed 1 h after dosing. When the
uptake by maternal and fetal tissues was measured by liquid
scintillation counting and DNA determination, maternal levels were
generally higher than fetal levels. In studies by Juszkiewicz &
Kowalski (1974) DMN, DEN, and nitro-propylamine, administered orally
to goats at 20-30 mg/kg, appeared in the blood within “ h, and later
in the milk. The concentration in the milk, 2 h after administration
of DEN at 30 mg/kg, was 14 mg/litre; after 24 h only traces could be
found. Phillips et al. (1975a) examined the disappearance of DMN from
the stomach and small intestine of rats as an index of absorption, and
found that, while very little was absorbed from the stomach, DMN was
rapidly absorbed from the small intestine.
6.2 Biotransformation and Elimination
6.2.1 Nitrates and nitrites
In a study on 4 rats, 42-90% of nitrates, administered by stomach
tube, was excreted in the urine within 8 h of administration. Nitrites
were not detected in the urine either before or after administration
(Hawksworth & Hill, 1971b). The same authors carried out a study on
122 samples of human urine, and found that urinary nitrate
concentration was related to the amount of nitrate ingested.
The excretion of nitrates and nitrites in the saliva was studied
by Spiegelhalder et al. (1976) in 11 volunteers given various
vegetable juices with nitrate concentrations ranging from 30 to
550 mg/litre. The levels of nitrates and nitrites excreted were
proportional to the amounts of nitrates ingested. After ingesting
100 mg of nitrates, nitrite concentrations in the saliva increased, on
average, by 20 mg/litre.
6.2.2 N-nitroso compounds
Magee & Barnes (1967) have reviewed available information on the
metabolism and elimination of N-nitroso compounds. Magee (1956)
measured recovery of DMN from the whole body of the mouse and noted
that 97% of the total dose (0.05 mg/kg) could be recovered immediately
after oral administration and that the amounts recovered decreased
with time until at 4 h no DMN was recovered. Similar results were
obtained with the rat given DMN orally at 50 mg/kg, the concentration
fell rapidly with increasing time after injection so that only 30% of
the dose was recovered at 8 h and none at 24 h.
Metabolic transformation of DMN was demonstrated by Dutton &
Heath (1956) using 14C-labelled DMN. In both the mouse and the rat,
the main radioactive product was expired carbon dioxide. In the mouse,
65% of the injected 14C was recovered as expired carbon dioxide, 6 h
after a subcutaneous injection of DMN at 50 mg per kg body weight. In
the rat, about 40% of the radioactive 14C was recovered as expired
carbon dioxide, 8 h after the injection. At the end of the experiment,
the remainder of the 14C was fairly evenly distributed in the
tissues, apart from about 7% that was excreted in the urine.
Heath (1962) found that, while part of each of a number of
nitrosamines studied was excreted unchanged in urine and in expired
air, the greater part was decomposed. From the rates of expiration of
labelled carbon dioxide it was shown that decomposition of dimethyl-,
diethyl-, and N-butylmethylni-trosamine obeyed Michaelis-Menten
kinetics. The rate of decomposition was dose-dependent.
When the metabolic transformation of dialkylnitrosamines,
especially of the bladder carcinogen di- N-butylnitrosamine was
investigated, major urinary metabolites with retained N-nitroso
structure were identified (Blattmann & Preussman, 1973, 1974;
Blattmann et al., 1974; Okada & Suzuki, 1972; Okada et al., 1975).
Hydroxylation, particularly at the terminal CH3 group, has been
observed as well as chain shortening. Butyl (3-carboxypropyl)
nitrosamine (BCPN), a major metabolite of butyl (4-hydroxy butyl)-
nitrosamine (BBN) was an equally potent and selective bladder
carcinogen (Okada & Suzuki, 1972). Ring-opening was observed during
metabolism of nitrosomorpholine (Stewart et al., 1974).
7. EXPERIMENTAL STUDIES ON THE EFFECTS OF NITRATES, NITRITES, AND
N-NITROSO COMPOUNDS
7.1 Nitrates and Nitrites
7.1.1 Acute and subacute toxicity studies
Acute nitrate poisoning was first recognized in cattlea by Mayo
as early as 1895 (Wright & Davidson, 1964), while Comly (1945) was the
first to report nitrate poisoning from well water in infants in the
USA. As a result of these and other reports, several studies have been
conducted on the toxicity of nitrates in a wide variety of animal
species. They are mainly centred on the formation of methaemoglobin
that accompanies excessive exposure to nitrates and nitrites. The
acute toxicity of nitrates and nitrites was recently reviewed by the
Committee on Nitrate Accumulation (1972).
Although the outstanding feature of nitrate toxicity is the
development of methaemoglobinaemia, nitrates may also cause
vasodilation which aggravates the effects of the methaemoglobinaemia.
The nitrite ion formed by reduction of nitrates, oxidizes the iron in
the haemoglobin molecule from the ferrous to the ferric state. The
resultant methaemoglobin is incapable of reversibly binding oxygen
(Bosch et al., 1950). Clinical signs of nitrate toxicity, attributable
to hypoxia appear when methaemoglobin values exceed about 20% (section
8.1). Oxidation of haemoglobin to methaemoglobin by the nitrite ion
occurs at different rates for each animal species, but there is little
difference between individuals of the same species (Smith & Beutler,
1966). Similarly, the reduction of methaemoglobin in erythrocytes
mainly by the enzyme system, NADH--methaemoglobin reductase, is
characteristically different for each animal species. The chemical
induction of methaemoglobinaemia has recently been reviewed by Smith
(1969, 1975). These physiological processes appear to be related, even
though there is a large variation in the rate of formation of
methaemoglobin and its subsequent reduction. This may help to explain
the difference in species susceptibility and the variation in signs
seen in nitrate poisoning.
In an effort to develop sensitive tests for the detection of the
possible effects of subclinical methaemoglobinaemia, behavioural
studies with mice were undertaken by Behroozi et al., 1971. Groups of
57 black, 6J, male mice were given nitrites in their drinking water at
doses aimed at producing methaemoglobin levels varying from slightly
above normal to 15%, which can be considered to be in the subclinical
a For further discussion of nitrate intoxication in livestock,
that may involve significant economic loss, see Oehme (1975).
range. Sodium nitrite doses in water were 100, 1000, 1500, or
2000 mg/litre. The results showed a significant reduction of overall
motor activity in the groups receiving the highest levels of
nitrites. There was a significant inverse relationship between the
methaemoglobin level and motor activity, with a coefficient of
correlation of 0.65. An effort to counteract the methaemoglobinaemia
was made by giving ascorbic acid to the group receiving the highest
level of nitrites (2000 mg/litre). (See section 7.1.5). The effect
was to reduce the methaemoglobin levels to almost normal but the
motor activity level of the group so treated remained low and about
equal to the equivalent group that had not received an antidote.
These experiments seemed to indicate that the nitrites had some form
of sedative effect on the treated mice, that was not necessarily
associated with the development of methaemoglobinaemia.
Experiments on rabbits have shown that methaemoglobinaemia caused
by nitrates in water also affects cardiac activity by increasing the
number of cardiac contractions to an extent directly proportional to
the increase in methaemoglobin levels. At the 10-15% methaemoglobin
level, the electrocardiogram shows a shortening of the Q-T interval
and a reduction in the T wave, which may even become negative (Garbuz,
1968, 1971).
7.1.2 Chronic toxicity and carcinogenicity studies
In a study conducted by Shuval & Gruener (1972), groups of 8 male
rats (3 months old) were given tap water (control) or 100, 1000, 2000,
or 3000 mg of sodium nitrite per litre of drinking water. After 24
months, there were no significant differences in growth and
development, mortality, and total haemoglobin levels between the
control and treated groups. However, the methaemoglobin levels in the
groups receiving sodium nitrite at 1000, 2000, or 3000 mg/litre were
raised significantly throughout the study and averaged 5%, 12%, and
22% of total haemoglobin respectively. The methaemoglobin levels in
the group receiving 100 mg/litre were slightly above those of the
control group for the first 60 days only. There were some changes in
the liver and spleen of treated animals but the main pathological
changes occurred in the heart and lungs. In the heart, small foci of
cells and fibrosis were seen in some animals with pronounced
degenerative foci in animals receiving the highest concentrations of
nitrites. The coronary arteries were thin and dilated. The bronchi
were frequently dilated with the walls infiltrated by lymphocytes and
the mucosa and muscle were often atrophied. Emphysema was the rule.
These changes, which were present in 1 or 2 control rats and in a
small number of those receiving nitrite levels of 100 mg/litre, were
found with increasing frequency and severity in the 3 highest dose
groups.
Druckery et al. (1963a) did not observe extensive methaemoglobin
formation in rats given 100 mg sodium nitrite per kg body weight in
the drinking water, but there was a slight reduction in the life span.
Studies by Van Logten et al. (1972) in which groups of 30 male and 30
female rats received sodium nitrite at concentrations of 0, 0.02, or
0.05% with or without glucono--lactone (GDL) in the diet for 29
months did not demonstrate any significant haematological or
biochemical effects. Carcinogenic action, which could be related to
the administration of sodium nitrite with or without DEA or GDL, was
not observed in either of these studies.
It has been reported that prolonged administration to rats of
1/20 of the LD50 of calcium and sodium nitrates disturbed the energy
conversion processes such as glycolysis and the pentose phosphate
cycle, changed the activity of the glutathione-ascorbic acid system in
the blood and in the hepatic and cerebral tissues, raised the levels
of methaemoglobin and of NADH-methaemoglobin reductase activity, and
reduced the haemoglobin levels (Diskalenko & Dobrjanskaja, 1972;
Diskalenko & Trofimenko, 1972).
Greenblatt & Mirvish (1972) gave 3 groups of about 40, 7-9 week
old, male, strain A mice, 1 or 2 g of sodium nitrite or 12.3 g of
sodium nitrate/litre of water respectively, for 20 weeks and did not
observe any increase in lung tumour incidence in comparison with
untreated controls. Lijinsky et al. (1973a) reported similar negative
results when groups of 30 rats were given sodium nitrate at 5 g/litre
or sodium nitrite at 2 g/litre in their drinking water at a daily rate
of 20 ml/rat throughout most of their lifetime. Other studies by
Lijinsky et al. (1973c) on groups of 30 rats given 20 ml of sodium
nitrite at 2 g/litre daily, for 5 days a week, also produced negative
results. Taylor & Lijinsky (1975) reported that tumour formation in 57
Sprague-Dawley rats, exposed for 2 years to a drinking solution
containing sodium nitrite at 2 g/litre, was no greater than in the
controls.
7.1.3 Embryotoxicity
A study has been reported by Shuval & Gruener (1972) in which 2
groups of 12 pregnant rats were given 2000 or 3000 mg of sodium
nitrite per litre of drinking water, respectively. A control group did
not receive any treatment. Pregnant rats developed anaemia and had
higher methaemoglobin levels than nonpregnant rats receiving similar
doses. There was a pronounced increase in mortality among the newborn
rats of treated dams compared with those of untreated controls,
particularly in the 3-week period before weaning. Mortality in the
offspring was 6% in controls, 30% in those given 2000 mg/litre and
53% in those given 3000 mg/litre. Birthweights were similar in all
groups but growth was markedly reduced in pups of treated dams. Such
pups had thin hair coats. Treatment of 2 groups of 10 and 15 pregnant
rats with 1% and 0.3%, respectively, of sodium nitrate in the diet did
not result in any embryotoxic or teratogenic effects on the 9th and
10th days of gestation (Alexandrov & Jänisch, 1971).
Sleight & Atallah (1968) conducted a study in which 46 female
guineapigs, divided into 12 groups each containing at least 1 male,
were given potassium nitrate in doses ranging from 300 to 10 000 mg/kg
body weight in the water and potassium nitrite in amounts ranging from
300 to 10 000 mg/kg for periods ranging from 100-240 days.
Reproduction in the female was grossly impaired in the high nitrate
group. Fetal losses were 100% in females given 5000 or 10 000 mg/kg of
the nitrite and one female died. Reproduction was maintained at lower
levels of treatment. Apparently male fertility was not impaired, since
conception took place at all levels of treatment. Food and water
consumption and weight gains of treated animals were normal except for
a diminished rate of gain at a nitrite level of 10 000 mg/kg body
weight. Uterine and cervical inflammatory lesions and degenerative
placental lesions were present in females in which the fetuses had
been aborted, mummified, or absorbed. Sinha & Sleight (1971) reported
studies in which 4 pregnant guineapigs, given sodium nitrite at 50 mg
per kg of body weight, subcutaneously, underwent normal parturition.
However, fetal deaths followed by abortion, occurred in 3 guineapigs
given sodium nitrite at 60 mg/kg. The fetal deaths occurred
approximately 1 h after nitrite administration, when the maternal and
fetal methaemoglobin levels were highest. At the time of death, there
were no noticeable changes in the placenta; pathological changes
developed after the death of the fetuses. There were lower blood pO2
values in the fetuses of the guineapigs treated with nitrite at
60 mg/kg than in those of the controls. Fetal death did not occur in
pregnant animals given sodium nitrite at 60 mg/kg combined with
simultaneous intraperitoneal treatment with 10 mg of methylene blue
per kg body weight. The data suggest that fetal death resulted from
hypoxia, mainly induced by maternal methaemoglobinaemia.
Studies by Shuval & Gruener (1972) indicated that
methaemoglobinaemia might be induced transplacentally and that the
observed limit for transplacentally induced methaemoglobinemia was a
sodium nitrite dose of 2.5 mg/kg body weight. A steep increase in
effect occurred with increasing doses of sodium nitrite.
7.1.4 Mutagenicity
The mutagenic potential of nitrates and nitrites has not been
studied extensively; in fact, no data are available on their mutagenic
action in the mammalian systems. The mutagenicity of nitrates and
nitrites with respect to the transformation of DNA has been reported
(Bressler et al., 1968; Horn & Herriot, 1962; Strack et al., 1964) and
nitrous acid has been shown to be mutagenic in bacterial systems such
as Escherichia coli (Kaudewitz, 1959; Verly et al., 1967) and
Salmonella typhimurium (de Serres et al., 1967). Positive results in
mutagenic studies have been reported with the yeast Saccharamyces
cerevisiae (Nashed & Jabbur, 1966; Zimmerman & Schwaier, 1967;
Zimmerman et al., 1966), as well as with Aspergillus nidulans
(Siddiqi, 1962), A. niger, and A. amstelodami (Steinberg & Thom,
1940), and tobacco mosaic Virus (Sehgal & Krause, 1968).
7.1.5 Interaction with nutritional factors
Studies by Kociba & Sleight (1970) showed that maternal blood
levels of methaemoglobin were significantly higher in 12 ascorbic
acid-deficient, pregnant guineapigs, following the subcutaneous
administration of sodium nitrite at 40 mg/kg body weight, than in
those on a normal diet. Following subcutaneous administration of
sodium nitrite at 50 mg/kg, there was a higher percentage of fetal
death in the ascorbic acid-deficient guineapigs.
Experiments were conducted by Stoewsand (1973) with young, male,
guineapigs (number of animals not stated) to investigate the influence
of feeding beets with naturally occurring low and high amounts of
nitrates and nitrites, and the influence of dietary supplementation
with ascorbic acid and methionine on methaemoglobinaemia, induced by
orally administered sodium nitrite at doses of 25 or 50 mg/kg body
weight. Low-nitrate beet diets seemed to "protect" guineapigs from
nitrite intoxication. In addition, a 1% diet containing L-ascorbic
acid and 1% methione reduced nitrite-induced methaemoglobin blood
levels.
Sell & Roberts (1963) showed that ad libitum feeding of diets
containing 0.4% potassium nitrite to 6 groups of 50 chicks depressed
growth, irrespective of the amount of vitamin A administered. Also,
all the test animals, except those given massive injections of vitamin
A, exhibited reduced liver stores of the vitamin and enlarged thyroid
glands.
Studies by Phillips (1966) demonstrated that the liver vitamin A
contents of rats fed 1% potassium nitrite in diets containing carotene
or vitamin A, were less than those of control rats. It was suggested
that the dietary nitrite degraded the carotene and vitamin A in the
digestive tract before their absorption.
7.2. N-nitroso Compounds
7.2.1 Acute and subacute toxicity studies
The acute toxicity of N-nitroso compounds is not of great
toxicological significance because there is no relationship between
acute toxicity and the carcinogenic potential of this class of
compounds (Druckrey et al., 1967, Magee & Barnes, 1967). Because DMN
was reported to cause cirrhosis and other toxic effects in industrial
workers, Barnes & Magee (1954) examined the toxicity of this compound.
Doses of 20-40 mg/kg body weight given to rats, dogs, rabbits, and
guineapigs produced severe hepatic damage. A single dose of DMN given
to rats orally or by intravenous, intraperitoneal, or subcutaneous
injection, produced centrilobular necrosis accompanied by haemmorhages
in the liver. In rats given 20 mg DMN/kg body weight the liver cells
in the centrilobular and mid-zonal regions became pale and, after
18 h, the cytoplasm was amorphous and vacuolated; the nuclei were pale
and irregular in outline. By 24 h, the cells were necrotic and
confluent areas became haemorrhagic. Haemorrhage was usually more
pronounced after 48 h but after 72 h the recovery process had begun
and was almost complete in 3 weeks (Barnes & Magee, 1954; Magee &
Barnes, 1962). A detailed study by light and electronmicroscopy of
changes in the liver cytoplasm of rats treated with
N-nitrosomorpholine has been reported by Bannasch (1968).
The acute toxicity of acyl-alkyl-nitrosamides and diazoalkanes
was reported by Druckery et al. (1967), Magee & Barnes (1967), and
Shank (1975). The acute toxicity (LD50) of the nitroso compounds
varied widely; some were only mildly toxic while others produced
highly destructive lesions. N-nitroso- N-methylurethane, for
example, when given orally, produced severe necrotic lesions in the
stomach, congestion of the lungs, and periportal necrosis of the liver
(Schmähl & Thomas, 1962; Schoental, 1960). N-nitroso- N-methylurea
also produced inflammatory haemorrhagic lesions of the stomach,
intestine, and pancreas and a reduction in the bone marrow when given
orally to rats, (Druckrey et al., 1961, 1967).
The acute toxicity of some N-nitroso compounds, e.g. nitroso-
piperidine and nitroso-morpholine, was not manifested as liver damage
but as neurotoxic effects, e.g. convulsions (Lee & Lijinsky, 1966).
The acute toxicity of nitrosomines formed in vivo was studied
by Asahina et al. (1971). Sodium nitrite was administered by gavage to
mice at 100 or 150 mg/kg bodyweight alone or in combination with DMA
and methylbenzylamine at doses of 500-2500 mg/kg and 800-1600 mg/kg,
respectively. Combinations of the amines and nitrite produced hepatic
lesions similar to those produced by DMN or nitrosome-thylbenzylamine.
Similar effects were noted when nitrite was administered up to 3 h
after DMA but these effects were markedly reduced if the nitrite was
given prior to the amine. Lijinsky & Greenblatt (1972) reported
hepatic necrosis following coadministration of aminopyrine and sodium
nitrite.
In a number of studies, ascorbic acid has been shown to have an
inhibitory effect on: a) nitrosamine formation in the stomach and
small intestine of rats treated with 7-chloro-2-methylamino-5-phenyl-
3H-1, 4-benzodiazepine-4-oxide, (chlordiazepoxide, Librium) and sodium
nitrite (Preda et al., 1976); b) hepatotoxicity induced by the
combined administration of sodium nitrite and aminopyrine in rats
(Kamm et al., 1973) and mice (Greenblatt, 1973); c) liver necrosis
produced by DMA and nitrite administered to rats by gavage (Cardesa et
al., 1974); d) the teratogenic and transplacental carcinogenic effects
produced in rats by treatment with alkylurea and nitrite (Ivankovic et
al., 1973); and e) the induction of lung adenomas in mice by prolonged
treatment with morpholine, piperazine, and methylurea plus nitrite
(Mirvish et al., 1975).
Species differences exist with respect to the toxic effects of
N-nitroso compounds. Mink appear to be especially sensitive to DMN,
and as in other species, the effects are seen primarily in the liver.
Carter et al. (1969) noted widespread liver degeneration and necrosis
of hepatocytes in mink given DMN at 2.5 or 5.0 mg/kg in the diet for
7-11 days. The liver lesions were accompanied by bile-duct
proliferation, ascites, and haemorrhage of the gastrointestinal tract.
Sheep and cattle are also more sensitive to nitrosamines than
laboratory animals (Sakshaug et al, 1965; Koppang, 1964). Sheep, given
a single dose of DMN at 5 mg/kg body weight or 12 doses of 0.5 mg/kg,
died or were severely affected displaying anoxia, lack of rumination,
ataxia, and respiratory difficulties, while cattle given DMN at
0.1 mg/kg body weight showed pronounced hepatotoxic effects in 1-6 months.
Pathological and biochemical effects, observed in the liver of a
number of animal species including the rat following continuous
administration of nitrosamides, have been reviewed by Magee & Barnes
(1967) and Magee et al. (1976); the main effect is inhibition of
protein synthesis which might be a result of an accelerated breakdown
of messenger ribonucleic acid (RNA).
7.2.2 Carcinogenicity
The carcinogenic activity of N-nitroso compounds has been
summarized in several reviews (Druckrey, et al., 1967; Magee & Barnes,
1967; Magee et al., 1976). Various animal species including mammals,
birds, fish, and amphibia have been shown to be susceptible to the
carcinogenic action of nitrosamines. At present, some 80 nitrosamines
and 23 nitrosamides have been tested in rats and about 80% of the
nitrosamines and practically all the nitrosamides have proved to be
carcinogenic (Montesano & Bartch, 1976). These carcinogens show a
marked organ specificity as shown in Table 3.
Nitrosamines produce a carcinogenic effect in the liver,
oesophagus, respiratory system, and kidney, whereas nitrosamides
affect the peripheral and central nervous systems, and the gastro-
intestinal tract organs.
The dose schedule seems to play an important role in this organ
specificity. For example, in rats, long-term exposure to relatively
low doses of DMN induced mainly liver tumours, whereas a single or a
few high doses over a short period induced mainly kidney tumours
(Magee & Barnes, 1962). Similar responses in relation to the liver and
oesophagus were observed with DEN in rats (Druckey et al., 1967). The
route of administration does not seem to play an important role in the
carcinogenicity of this chemical. It is worthwhile pointing out that
many of these chemicals were carcinogenic following a single
administration and that, furthermore, exposure of rats to a single
dose during pregnancy resulted in carcinogenesis in the immediate
descendants and also in the two succeeding generations (Tomatis et
al., 1977).
Nitrosamines exert their adverse biological effects after being
metabolically activated by microsomal mixed function oxidases to form
reactive intermediates. On the other hand, nitrosamides decompose
enzymatically to reactive, and in most cases, alkylating derivatives.
The importance of hydroxilation of the alpha-hydrogen atoms of the
nitrosamines is demonstrated by the lack of carcinogenicity of
compounds, such as diphenylnitrosamine, which do not have this alpha-
hydrogen (Magee et al., 1976).
Table 3. Localization of tumours induced by N-nitroso
compounds in ratsa
Number of N-nitroso compounds
affecting target organ
Target organ Nitrosamines Nitrosamides
liver 35 2
oesophagus-pharynx 32 3
nasal cavities 18 -
respiratory tract 10 1
kidney 8 9
tongue 8 -
forestomach 7 11
bladder 4 1
central and peripheral 2 9
nervous system
ear duct 2 1
testis 1 -
ovary 1 2
mammary glands 1 1
sites of injection 3 4
intestine - 7
glandular stomach - 6
skin - 3
jaw - 1
uterus - 2
vagina - 1
haemopoietic system - 2
a From: Montesano & Bartch (1976)
7.2.2.1 Interspecies variation in response
Several N-nitroso compounds have been tested in different
animal species. DEN, tested in more than 20 species including
primates, induced tumours of the liver in all of them, together with
various tumours of other organs. In some cases, there have been marked
differences between species in response to N-nitroso compounds. For
example, nitrosoheptamethyleneimine produced squamous carcinoma of the
lung in rats (Lijinsky et al., 1969), the same effects in European
hamsters, but tumours of the forestomach and the lung in Syrian
hamsters (Lijinsky et al., 1970). In all three species, this compound
also induced oesophageal tumours. Nitrosomethylurethane induced
tumours of the pancreas in guineapigs (Druckrey et al., 1968) and
forestomach carcinomas in rats. Bis-2-hydroxypropyl-nitrosamine
produced pancreatic tumours in Syrian hamsters. These results show
the difficulty of attributing, with certainty, any particular tumour
response of man to a particular N-nitroso compound.
7.2.2.2 Intraspecies variation in response
Kuwahra et al. (1972) administered DMN orally or by subcutaneous
or intraperitoneal injections, to 8 to 12-week-old, male and female
mice of the DDD, BALB/C, and SJL/J strains. Tumours were found mainly
in the retroperitoneum and abdominal cavity and the incidence and
distribution were little affected by the strain of mouse but markedly
by the route of administration. Clapp et al. (1971) noted that DEN
induced forestomach and oesophageal squamous cell carcinomas in both
BALB/C and RF/Un strains of mice but induced liver haemangiosarcomas
in the former and hepatomas in the latter. In addition, DEN induced a
high incidence of lung tumours in RF mice but a very low incidence in
BALB/C mice. In both strains, DMN induced lung adenomas and liver
haemangiosarcomas. Tissue sensitivity did not appear to be related to
the spontaneous tumour incidence of the strain.
7.2.2.3 Dose-response relationships of N-nitroso compounds
Druckrey et al. (1963b) were the first to study the dose-response
relationships of N-nitroso compounds in relation to minimum effect
doses. They concluded, from studies in rats given DEN, that the
carcinogenic effect was related to dose and induction time in such a
way that d.t2.3 = constant, where d represents the daily dose and
t the induction time. Even a low dose of 0.15 mg/kg body weight
resulted in liver carcinomas in 27 out of 30 surviving animals. The
average induction time was 609 ± 38 days. The lowest dose studied
(0.075 mg/kg body weight) produced liver and oesophageal tumours in
all four surviving animals with an induction time of 830 days.
Mohr & Hilfrich (1972) gave a single intravenous injection of DEN
to rats at 8 dose levels between 1.25 and 160 mg/kg body weight. A
dose-response relationship was noted; at the lowest dose of 1.25
mg/kg, only one kidney tumour was observed in 20 treated rats, but, at
higher doses, the incidence of these tumours increased. Single dose
experiments with ethylnitrosourea in a transplacental carcinogenesis
experiment also showed that the incidence of induced tumours (mainly
of the brain and nervous system in the progeny) was directly
proportional to the dose of the carcinogen. Four doses between 1 and
50 mg/kg were used and even in the lowest dose group 36/41 animals in
the progeny died with tumours (Swenberg et al., 1972). Terracini et
al. (1967) fed rats concentrations of DMN ranging from 2 to 50 mg/kg
diet. At 2 and 5 mg/kg the incidence of liver tumours in the survivors
was 1/26 and 8/74, respectively, at 60 weeks. At 20 and 50 mg/kg,
liver tumours were observed in more than 60% of the test animals.
From this study, it can be concluded that a dietary level of DMN of
5 mg/kg is still carcinogenic in the rat.
A current dose-response study on rats receiving oral doses of
N-nitrosopyrrolidine has been reported by Preussmann (unpublished
data).a Liver tumours were induced in groups receiving 10, 3, and
1 mg/kg body weight per day, respectively, but not in a group
receiving 0.3 mg/kg per day.
Clapp & Toya (1970) gave male, RF mice DMN in the drinking water
for various lengths of time, with cumulative doses ranging from 87 to
243 mg/kg body weight. The incidence of lung adenomas in all treated
groups was higher than that in the untreated control groups. Whereas,
apparently, the incidence of hepatocellular tumours was not affected,
the incidence of liver haemangiosarcomas increased in the higher dose
groups and reached a maximum of 96%. Bertram & Craig (1973) using 2
groups of 100, C57BL/6 mice found that the incidence of bladder
tumours fell from 80% in animals given 30 mg of nitrosodi- N-
butylamine per kg/body weight per day to 36% at 7.5 mg/kg body weight
per day. Sander & Schweinsberg (1973) noted that an increasing
incidence of tumours of the oesophagus and forestomach was induced in
NMRI mice by adding a given dose of methylbenzylnitrosamine to the
drinking water at various times to provide total doses ranging from
1.4 to 44 mg/kg body weight.
a Paper presented at the Proceedings of the Second International
Symposium on Nitrite in Meat Products, Zeist, September 1976.
In studies conducted by Vesselinovitch (1969), DMN was
administered repeatedly by intraperitoneal injection to 73 male and 54
female C57BL x C3H mice starting at 7 days of age. The injections were
given at 3 day intervals for a total of six doses of 1, 2, or 4 mg/kg
body weight. Mice killed at 66 weeks of age showed an increase in the
incidence of hepatomas, hepatocarcinomas, lung adenomas, and
haemangiomas as the dose increased. The incidence of liver tumours was
higher in males (75%) than in females (28%).
Tomatis & cefis (1967) gave a dose of 3.6 mg of DMN by stomach
tube to one group of 30 Syrian golden hamsters in 3 administrations of
1.6 mg, 1.0 mg, and 1.0 mg over a 5-week period. A second group
received a single dose of 1.6 mg. Both dosing regimes produced liver
cell carcinomas and a few cholangiomatous lesions but no kidney
tumours. Montesano & Saffiotti (1968) administered 12, weekly,
subcutaneous injections of DEN at 0.5, 1.0, 2.0, or 4.0 mg to 4 groups
of 36 Syrian golden hamsters. The results demonstrated a positive
dose-response relationship for tumour induction in the upper
respiratory tract (nasal cavities, larynx, and trachea). The incidence
of nasal cavity tumours, which developed early, varied from 6/35 to
27/36. The incidence of tumours of the larynx varied from 6/35 to
26/36 and that of tumours of the trachea from 29/35 to 35/36.
7.2.2.4 Tumour induction by combined administration of nitrites
and amines or amides
Carcinogenic effects following the combined administration of
secondary and tertiary amines or amides with nitrite have been
reported. Tumours were not observed when sodium nitrite or the amine
or amide were given singly. Tumours of the same type occurred at the
same site when the N-nitroso compound, believed to be formed from
the nitrite and amine or amide, was given as a positive control. In
some of the experiments, the nitrite was given in the drinking water
and the amine or amide in the food. In others, the compounds were
combined in the same medium (drinking water or food).
Preussman (1975) reviewed studies in which tumours that had been
produced at certain sites by the oral administration of combinations
of amines and nitrite, were compared with the tumours induced by the
corresponding N-nitroso compounds. Table 4 has been adapted from
Preussman (1975) and updated.
Table 4. In vivo formation of N-nitroso compounds following oral administration of sodium nitrite and amino
compounds as demonstrated by specific carcinogenesisa
sodium nitrite + amine Expected tumour Reference
oral administration Observed tumour site for
amino compounds site corresponding
N-nitroso compound
Rat
diethylamine -- liver Druckrey et al.
(1963b)
Sander et al.
(1968)
Sander (1971a)
triethylamine -- liver (for Schweinsberg &
diethylnitrosamine Sander (1972)
morpholine liver, kidney, lung liver Sander & Burkle
N-methylbenzylamine oesophagus oesophagus (1969), Sander
(1971c)
piperidine -- oesophagus
liver
N-methylanaline oesophagus, nasal oesophagus
cavity
N-methylcyclohexylamine oesophagus oesophagus Sander (1971a)
Table 4 (Cont'd)
sodium nitrite + amine Expected tumour Reference
oral administration Observed tumour site for
amino compounds site corresponding
N-nitroso compound
N-methylbenzylamine oesophagus oesophagus
phenylbenzylamine negative untested
N,N'-dibenzylethylenediamine negative untested
indole negative untested
morpholine liver (kidney) liver (kidney) Shank & Newberne
(1972)
aminopyrine liver liver (for Lijinsky et al.
(Pyramidon) dimethylnitrosamine) (1973c)
heptamethyleneamine lung lung
oesophagus oesophagus
aminopyrine liver liver Taylor &
(for DMN) Lijinsky
(1975)
oxytetracycline liver (for DMN) liver Greenblatt et al. (1973)
proline negative untested
Table 4 (Cont'd)
sodium nitrite + amine Expected tumour Reference
oral administration Observed tumour site for
amino compounds site corresponding
N-nitroso compound
hydroxyproline negative untested
arginine negative untested
N-methylacetamide negative forestomach
N-methylurethane negative forestomach
N-ethylurethane negative (carcinogenic Sander (1971b)
following
i.v. administration)
acetanilide negative untested
glycylglycine negative untested
N-methyluracil negative untested
N-methylguanidine negative untested
N-phenylurea negative (carcinogenic Sander (1971b)
following
s.c. administration)
N-methylthiourea negative untested
N-methylurea brain, nervous brain, nervous
system, kidney system, kidney
Table 4 (Cont'd)
sodium nitrite + amine Expected tumour Reference
oral administration Observed tumour site for
amino compounds site corresponding
N-nitroso compound
N-ethylurea brain, nervous brain, nervous
system, kidney system, kidney
N'N'-dimethylurea brain, nervous brain, nervous Sander (1971b)
system, kidney system, kidney
imidazolidinone kidney (carcinogenic Sander & Burkle
following (1971)
s.c. administration)
ethylurea (during brain, nervous brain, nervous Ivankovic &
pregnancy, system system (in Preussmann
transplacental) (in descendants) descendants) (1970); Osske
et al. (1972)
Mouse
dimethylamine lung (adenoma)
piperazine lung (adenoma) lung (adenoma) Greenblatt et al. (1971)
morpholine lung (adenoma) lung (adenoma)
N-methylaniline lung (adenoma) lung (adenoma)
morpholine lung (adenoma) lung (adenoma)
Table 4 (Cont'd)
sodium nitrite + amine Expected tumour Reference
oral administration Observed tumour site for
amino compounds site corresponding
N-nitroso compound
N-methylbenzylamine oesophagus oesophagus Sander (1971a)
forestomach forestomach
piperazine lung (adenoma) lung (adenoma) Greenblatt &
Mirvish (1972)
N-methylurea lung (adenoma) lung (adenoma) Mirvish et al. (1973b)
N-ethylurea lung (adenoma) lung (adenoma)
a Adapted from Preussmann (1975)
7.2.2.5 Dose-response relationship for combinations of nitrite
and amines
Greenblatt & Mirvish (1972) conducted a study in which groups of
40, male, strain A mice, given 0.69-18.75 g of piperazine per kg of
food and 0.05-2.0 g of sodium nitrite per litre of drinking water for
20-25 weeks, were killed 10-13 weeks later. The yield of lung adenomas
was statistically significantly greater than in untreated controls,
when doses as low as 0.69 g piperazine/kg plus 1.0 g sodium nitrite/
litre, or 6.25 g piperazine/kg plus 0.25 g sodium nitrite/litre were
given. In the animals given 6.25 g piperazine per kg of food, plus
2.0 g sodium nitrite per litre of water, 39 out of 40 had adenomas,
in contrast with 5 out of 39 controls. A progressive decrease in the
incidence of adenomas was seen with reduction in the nitrite level.
On the other hand, when the nitrite level was maintained at
1.0 g/litre, the tumour incidence increased marginally when the dose
of piperazine was increased from 0.69 g to 18.75 g/kg of food.
Nitrite and piperazine administered alone yielded negative results.
When various concentrations of nitrite and morpholine (up to
1000 mg/kg) were fed to groups of Sprague-Dawley rats, the
development of hepatocellular carcinomas and angiosarcomas identical
to those produced by N-nitrosomorpholine was noted (Newberne &
Shank, 1973).
A 2-3% incidence of hepatomas was induced with only 5 mg of
morpholine plus 1000 mg of sodium nitrite per kg of food, or 1000 mg
of morpholine plus 50 mg sodium nitrite/kg. The tumour incidence was
98% when the concentration of both components was 1000 mg/kg, and 0%
when diet alone was given.
7.2.2.6 Transplacental carcinogenesis
Induction of neoplasms in offspring as a result of prenatal
exposure to various N-nitroso compounds and related substances has
been reported in different animal species including the rat, mouse,
golden hamster, guineapig, rabbit, dog, and monkey (Magee et al.,
1976). The various routes of administration (subcutaneous,
intraperitoneal, intravenous, oral, and inhalation) were equally
effective. However, a critical factor was the time of treatment during
gestation.
Since many of these substances appeared to be metabolically
activated to exert their carcinogenic action, the lack of an adequate
metabolic system during the first period of pregnancy may explain the
failure to observe tumours, when exposure was limited to this period.
DMN induced tumours in the offspring only when administered during the
last days of pregnancy (Alexandrov, 1968a). The data of Magee (1972)
are in keeping with these findings; the formation of 7-methylguanine
in the nucleic acids of fetal rat tissues was detected following
treatment with DMN on the 21st day of pregnancy but not on the
15th day.
When considering the effect of different acyl alkyl nitrosamides,
N-ethyl- N-nitrosourea was found to be more active than its methyl
analogue (Alexandrov, 1969b; Ivankovic & Druckrey, 1968).
The sensitivity of the nervous system at various stages of
prenatal development was examined in rats and golden hamsters by
Ivankovic & Druckrey (1968) by means of single intravenous injections
of N-ethyl- N-nitrosourea on different days during gestation. A
high incidence of tumours was observed after treatment on the 18th day
or shortly before delivery (21st day) but none developed when the
mother animals were treated before the 12th day. A positive dose-
response relationship was obtained with a single dose in the range of
5 to 80 mg/kg bodyweight on the 15th day. A single dose as low as
2 mg/kg was sufficient to produce malignant, neurogenic tumours in
2/25 newborn whereas in adult animals an effect was produced in 50%
of the animals by a single dose at 160 mg/kg. Thus a 50-times higher
sensitivity was demonstrated for the fetal nervous system. Similar
results were obtained by Swenberg et al. (1972) in Sprague-Dawley and
Fisher rats using a dose of N-ethyl- N-nitrosourea as low as
1 mg/kg. In a recent study on rats, Maekawa & Odashima (1975) explored
the effects of subcutaneous injections of N-butyl-1-nitrosourea on
embryonal, fetal, and newborn nervous systems. Treatment during early
gestation led only to the death of the embryo. When the compound was
given in the middle of the pregnancy (8-14 days), a high incidence of
nervous system tumours and pituitary tumours was found in the
offspring. Treatment late in pregnancy (15-21 days) led to the
development of nervous system tumours in 33/36 offspring.
When lactating animals were given the compounds, tumours were
induced in 19/39 of the offspring, the target organs being mainly the
testes and uterus.
Tomatis (1977) reported an increased incidence of cancer in the
descendants (second and third generation) of transplacentally treated
rats. In certain cases, the tumour-producing doses were lower than
those for adult animals. The target organs were usually similar in
fetal, newborn, and adult animals but the nervous system has been
shown to be highly sensitive to certain compounds, notably the
nitrosoureas. The induction time for transplacental tumours may be
shorter than that in adults.
7.2.2.7 Morphological studies
Morphological events associated with the development of hepatic
tumours, following exposure to N-nitroso compounds, were studied by
Rabes et al. (1970), Scherer et al. (1972), and Schmitz-Moorman et al.
(1972). Schmitz-Moorman et al. (1972) followed the carcinogenesis
induced by DMN in rat liver, using histological and histochemical
procedures. In the initial phase, there were vacuolar changes
accompanied by a decrease in the RNA and glycogen contents. In the
second phase, glycogen storage was noted while in the third phase,
basophilic cells with atypical nuclei were observed and could not be
distinguished from microcarcinomas. The RNA content of these cells was
substantially higher. The activities of acid phosphatase (3.1.3.2),
succinic dehydrogenase (1.3.99.1) and glucose-6-phosphatase (3.1.3.9)
substantially decreased. In the last carcinogenic stage, dramatic
changes were noted in several enzymes (Jennissen et al., 1971).
Early changes in the lung tissue of mice exposed to carcinogenic
doses of DMN were reported by Calafat et al. (1970) and DEN-induced
alterations in the respiratory tract of hamsters were reported by
Althoff et al. (1971). Greenblatt & Rijhsinghani (1969) compared the
cytopathological changes induced by DEN and DMN in the nasal
epithelium of hamsters; other light microscopic studies have been
published by Bertram & Craig (1972), Boyland et al. (1968), Hicks et
al. (1973), and Terracini et al., (1967). The histological appearance
of tumours under the electron microscope has been described by
Kirkland & Pick (1973). Veno-occlusive disease in the liver of rats
given DMN was reported by Butler & Hard (1971) who had also studied
the effects of this compound on rat testes (Hard & Butler, 1970c).
Other studies, by light microscopy, to observe early changes
related to the formation of neoplasms induced in the kidneys of rats
treated with DMN, were reported by Benemanski & Litvinov (1969), Hard
& Butler (1970a, 1970b), and Hard et al. (1971).
Electron microscopic studies of DMN-induced liver and kidney
tumours have been reported in rats (Bhathal & Hurley, 1973; Geil et
al., 1968; Hard & Butler, 1971a, 1971b, 1971c; Ireton et al., 1972;
Jasmin & Cha, 1969; Svoboda & Higginson, 1968) and in mice (Takayama,
1968). Treatment with DEN resulted in ultrastructural changes in the
lungs of hamsters (Stracks & Feron, 1973) and in the liver of monkeys
(Williams, 1970) and rats (Bader et al., 1971; Bruni, 1973). Early
morphological changes in the bladder of rats exposed to N-butyl- N-
butanol-4-nitrosamine were reported by Riedel & Piper (1973).
7.2.2.8 Biochemical mechanisms
Extensive work on the biochemical mechanisms of carcinogenesis
produced by N-nitroso compounds has been reviewed by Magee & Barnes
(1967) and more recently by Magee et al. (1976). Alkylation of nucleic
acids by N-nitroso compounds or their metabolites has been
investigated extensively and has been suggested as the mechanism of
carcinogenicity (Krüger, 1972, 1973; Lijinsky et al., 1973b; Swann &
Magee, 1971; Takayama & Muramatsu, 1969). In early studies, it was
thought that the 7 position of guanine was the significant site of the
reaction.
Following the important discovery by Loveless and Hampton (1969)
of the O,6-alkylation of deoxyguanosine by nitrosomethylurea (NMU)
and the implications of this reaction in terms of carcinogenicity,
O'Connor et al. (1973) estimated the amount of methylation at the
O,6-position of guanine DNA isolated from animals treated with DMN.
This base accounted for 4-6% of the methylation after DMN treatment.
O,6-methylguanine was lost (by "excision") from DNA with a half life
of approximately 13 h. The excision of the abnormal components of DNA,
O,6-methylguanine, and the unstable acid-labile products, may be
important processes in liver carcinogenesis. O'Connor et al. (1973)
suggested that events leading to the development of tumours may be
related to the efficiency of the cellular excision system for certain
products of alkylation rather than to the level of alkylation obtained
at a particular site.
7.2.2.9 Interaction with various chemical factors
Several studies are available on the combined effects of
N-nitroso compounds and other carcinogens. Schmähl et al. (1963)
showed that combined oral administration of the hepatocarcinogens DEN
and N,N-dimethyl-4-(phenylazo)benzenamine (4-dimethyl-
aminoazobenzene) significantly reduced the time for tumour induction
and that only 66% of the total dose administered when single compounds
were used, was necessary for tumour induction. Takayama & Imaizumi
(1969) also demonstrated synergism with the combined administration of
DMN and 4-dimethylaminoazo-benzene. Liver tumours were induced in rats
by sequential administration of the two carcinogens in doses that did
not induce tumours when each carcinogen was given alone and the tumour
induction time was reduced. The syncarcinogenic effects of a single
dose of a combination of DEN and carbon tetrachloride were observed by
both Pound et al. (1973) and Schmähl et al. (1965). The incidence of
liver cancer increased and the induction time decreased in combined
treatments compared with treatment with the individual compounds.
Schmähl (1970) administered hepatocarcinogens (DMN, DEN,
nitrosomorpholine, and 4-dimethylaminoazobenzene) to rats in such low
daily doses that the administration of any one of the compounds alone
did not lead to tumours during the lifetime of the animals. However,
combined administration induced tumours in 43% of the treated animals.
Combined administration to rats of DMN plus 1,2-dihydro-3-methyl-
benz[j]aceanthrylene (3-methylcholanthrene) did not increase the
number of liver tumours compared with that induced by DMN alone but
resulted in tumours in the lungs, that were not seen in the treatment
with DMN alone (Hoch-Ligeti et al., 1968).
1-Isothiocyanatonaphthalene (1-napthyl-isothiocyanate) and
3-methyl-cholanthrene failed to inhibit the hepatocarcinogenic
effects of DEN (Makiura et al., 1973). Similarly, local treatment of
the glandular mucosa of the stomach of rats with 4-nitroquinoline-
1-oxide or p-dimethylaminoazobenzene did not have any effect on
the production of liver or oesophagal tumours due to oral
administration of DEN (Odashima, 1969). It has been reported that
ionizing radiation did not increase tumour incidence in animals
exposed to nitrosamines (Flaks et al., 1973; Schmähl et al., 1966).
The effect of many other substances on nitrosamine carcinogenesis
has been studied. For example, various dusts including aluminium (III)
oxide, magnesium (II) oxide, and carbon had little effect on the
respiratory carcinogenesis of DEN in hamsters (Stenback et al., 1973)
but other studies on hamsters demonstrated that iron (III) oxide
markedly increased the incidence of respiratory tract tumours induced
by DEN (Feron et al., 1972). The synergistic effects of various
substances on respiratory carcinogenesis in hamsters given N-nitroso
compounds were reviewed by Montesano (1970).
The effect of noncarcinogenic chemicals on the incidence of other
tumours induced by N-nitroso compounds has also been studied. In
rats given DEN, liver tumour incidence was decreased by the
administration of calcium heparin (Platt & Hering, 1973),
aminoacetonitrile (Hadjiolov, 1971), and reserpine (Lacassagne et al.,
1968). Lactoflavin, nicotinamide, or 2,6-bis-(diethanolamino)-4,8-
dipiperidino-pyrimido [5,4-d] pyrimidine (dipyridamole) (Schmähl &
Stackelberg, 1968) and hydrocortisone (Schmähl et al., 1971) did not
influence the incidence of liver tumours induced in rats by DEN.
Tryptophan was shown to inhibit the production of liver tumours, but
not bladder tumours in rats exposed to N-nitrosodibutylamine
(Okajima et al., 1971). N-(2-chloroethyl)- N (phenylmethyl)
benzenemethanamine (dibenamine) did not have any effect on the
incidence of oral, pharyngeal, or oesophageal tumours in DEN-treated
rats, but did significantly reduce the number and severity of hepatic
neoplasms (Weisburger et al., 1974). In mice, treatment with
phenobarbital decreased the toxicity and carcinogenicity of DEN (Kunz
et al., 1969).
7.2.2.10 Miscellaneous modifying factors
Nasal infection of mice with the influenza viruses PR8/FIK and
A2/Bethesda 10/63 followed by treatment with DEN significantly
increased lung tumour incidence in comparison with that in mice
treated only with DEN (Schmidt-Ruppin & Papadoupulu, 1972). The Motol
virus has been shown to increase hepatic carcinoma in mice given DEN
(Kordac et al., 1969). Rats with chronic respiratory disease showed an
increased lung tumour incidence when given nitrosoheptamethylenemine
compared with germ-free or specific pathogen-free animals (Schreiber
et al., 1972).
Feeding with a protein-deficient diet protected rats against the
lethal and hepatotoxic effects of DMN (McLean & Verschuuren, 1969) but
increased the incidence of renal carcinomas (McLean & Magee, 1970).
Low protein diets and low zinc intake failed to influence the
incidence of oesophageal tumours in rats treated with N-methyl- N-
nitroso-'pentyl' amine (Van Rensburg, 1972). A single intraperitoneal
injection of DMA induced nasal tumours in rats, previously starved for
48 h (Noronha & Goodall, 1972).
The carcinogenic activity of nitroso- N-(hydroxybutyl)
butanamine (butyl-( N-hydroxybutyl) nitrosamine) appears to be
influenced by sex hormones (Bertram & Craig, 1972). Tumours developed
much earlier in male, than in female mice. This difference was
abolished, however, if the males were castrated or, conversely, if the
females were treated with testosterone.
Surgical manipulation of experimental animals may influence the
induction of tumours by N-nitroso compounds. This has been shown for
partial hepatectomy (Craddock, 1971; Grunthal et al., 1970; Rabes et
al., 1971) unilateral nephrectomy (Ito et al., 1969) and ureter
ligation (Ito et al., 1971).
7.2.3 Embryotoxicity and teratogenicity
Whereas nitrosamines are reported to have toxic and lethal
effects on the embryo, usually at dose levels that are toxic to the
mother animals, nitrosamides ( N-ethyl-and N'methyl urea) bring about
malformations of several of the organs and systems of the developing
organisms at levels not toxic to the pregnant animal. Animal
experiments have shown that immature tissues are especially sensitive
to these compounds. Dose-response relationships have been established
and the existence of a noneffect level has been indicated.
DMN, administered orally at 30 mg/kg to pregnant rats, produced
increased prenatal mortality (Alexandrov, 1967). Toxic effects on the
embryo were noted following intravenous or intraperitoneal
administration of DMN at various times during gestation but no
teratological abnormalities were observed. Similar results were
observed with DEN and with nitroso- N,N-bis butanamine (nitrosodi-
N-butylamine) in rats (Alexandrov 1967), and with DEN in hamsters
(Pielsticker, 1967). The aromatic nitroso compounds such as
nitrosomethylbenzenamine (nitrosomethylaniline) were toxic to the
embryo and had a mild teratogenic action when given to rats at the
maximum tolerated doses (Alexandrov, 1968a, 1968b).
Treatment of pregnant rats with a single dose of 10-30mg of
nitrosomethylurea per kg body weight on day 9 of gestation produced
anophthalmia, hydrocephaly, exencephaly, and occasionally spina bifida
while treatment on days 12-15 produced microcephaly (Alexandrov,
1969a; Koyama et al., 1970; Napaikov, 1971; Von Kreybig, 1965a, 1965b;
Von Kreybig & Schmidt, 1966, 1967).
The teratogenic action of nitrosoethylurea in rats was described
by Druckrey et al. (1966) and Ivankovic & Druckrey (1968) who noted
that oligodactylia and syndactylia of the fore and hind limbs were
dose-related. Von Kreybig & Schmidt (1967) and von Kreybig (1968)
confirmed these effects and also found brain anomalies. Napalkov
(1971) and Wechsler (1970, 1971) reported similar results. The
teratogenic and embryotoxic effects of N-nitroso compounds including
the pathogenesis of brain lesions were reviewed by Wechsler (1973).
7.2.4 Mutagenicity
The mutagenicity of N-nitroso compounds was reviewed by Magee &
Barnes (1967) and more recently by Montesano & Bartch (1976). Until
recently, the data available were mainly concerned with the
nitrosamides, the data on nitrosamines being limited to test systems
which do not take into consideration the metabolic activation of these
compounds by mammalian enzymes. As with other biological effects,
there is a clear distinction between the mutagenic action of
nitrosamides and nitrosamines. Nitrosamides were found to be mutagenic
to almost all genetic indicators; this was attributed to the
nonenzymatic formation of alkylating reactants.
On the other hand, nitrosamines have been reported, prior to
1970, to have a much more limited range of mutagenic activity; they
were found to be mutagenic in tests with Drosophila melanogaster
(Pasternak, 1964) but no such activity was observed in assays in
which bacteria, yeast, or fungi were used. Malling (1966) showed that
DMN and DEN were mutagenic for Neurospora crassa when the conidia
were suspended in Udenfriend's hydroxylating model system and the
treatment carried out under conditions believed to give rise to the
same metabolic products as those formed by the action of liver enzymes
in rat and mouse. Garbidge & Legator (1969), using the host-mediated
assay, were the first to show that DMN, administered to mice, induced
mutations in Salmonella O,phimurium, which had been injected
beforehand and was then reisolated from the peritoneal cavity. Most of
the mutagenicity assays were carried out using bacteria or fungi as
genetic indicators in the presence or absence of a microsomal
activation system. Only a few data are available on the mutagenicity
of DMN in mammalian systems such as the dominant lethal test or
cytogenetic studies. However, in general, the last two systems have a
very low sensitivity and false negatives can easily result with
N-nitroso compounds. The early observation of Pasternak (1962)
that DMN induced lethal mutations in Drosophila melanogaster
demonstrated the possible value of this system for testing the
mutagenicity of compounds that require metabolic activation.
The mutagenicity of certain nitrosated pesticides, herbicides,
and primary amines has been examined. The N-nitrosated derivatives
of the pesticides propoxur and carbaryl, which are aryl- N-methyl
carbamates, and of the herbicide benzothiazuron, a methylurea
derivative, induced mitotic gene conversion in Saccharomyces
cerevisiae (Sibert & Eisenbrand, 1974). N-nitrosocarbaryl was
also found to be mutagenic in Haemophilus infiuenzae (Elespuru
et al., 1974). Endo et al. (1973) screened a number of
N-nitrosated guanidine derivatives for their ability to induce
base pair substitutions in Salmonella typhimurium and a mutagenic
effect was observed for many of these compounds; nitrosated
methylguanidine was the most potent.
The mutagenicity of sodium nitrite, DMA, methylurea, and
ethylurea given orally to mice, has been demonstrated in a host-
mediated assay using a strain of Salmonella typhimurium as an
indicator (Couch & Friedman, 1975). When combined with sodium nitrite,
both ethylurea and methylurea had a greater effect than DMA.
Other properties of N-nitroso compounds such as cell
transformation in vitro and their influence on DNA repair mechanisms
are being investigated, but as yet, there are not sufficient data for
their evaluation.
8. EFFECTS OF NITRATES, NITRITES AND N-NITROSO COMPOUNDS ON MAN
8.1 Nitrates and Nitrites
In the erythrocytes of healthy individuals, the process of
methaemoglobin formation and reduction is continuous. The mean content
of methaemoglobin in healthy populations is usually reported to be
below 2% of the total haemoglobin concentration (Committee on Nitrate
Accumulation, 1972; Gobbi et al., 1974; Smith, 1972). However,
Goldsmith et al. (1975) recently found mean levels in Californian
populations ranging up to 2.11% with 1% of the adults and 8% of
infants having methaemoglobin levels exceeding 4%. Higher values are
found in premature than in full-term infants and levels in infants are
higher than those in older children and adults (Kravitz et al., 1956).
At a level of about 10%, methaemoglobinaemia may produce symptomless
cyanosis, whereas levels of 20-50% are associated with conspicuous
cyanosis accompanied by hypoxic signs and symptoms, such as weakness,
exertional dyspnoea, headaches, tachycardia, and loss of consciousness
(Arena, 1970; Committee of Nitrate Accumulation, 1972; Jaffé & Heller,
1964). The lethal concentration of methaemoglobin is not known, but
death may occur at levels exceeding 50% (Committee of Nitrate
Accumulation, 1972).
8.1.1 Epidemiologicai studies
The National Academy of Sciences, USA (Committee on Nitrate
Accumulation, 1972) recently reviewed about 350 cases of
methaemoglobinaemia in the USA and about 1000 cases in Europe that
were reported to be associated with the intake of nitrates in well
water or in food. There were 41 fatalities in the USA and about 80 in
Europe. Only one case of infant methaemoglobinaemia resulting from the
consumption of water from a municipal water supply was reported in the
USA (Vigil et al., 1965).
8.1.1.1 Exposure through water
The toxicity to man of nitrates in water was first reported by
Comly (1945). He noted high levels of methaemoglobin and the
associated signs of nitrate toxicity in 2 infants who had consumed
water containing high concentrations of nitrates (619 and
388 mg/litre). Since then, several epidemiological and case studies
have been carried out in various parts of the world, particularly in
areas with naturally high nitrate levels in water.
Robertson & Riddell (1949) reported 10 cases in which infants
receiving powdered milk preparations made with well waters with
nitrate concentrations exceeding 75 mg/litre had blood levels of
methaemoglobin ranging from 5 to 50% of total haemoglobin. Two of
the infants, whose dried milk preparations had been reconstituted
with well waters containing nitrate levels of 1200-1300mg/litre, had
methaemoglobin levels of 25% and 44% of total haemoglobin,
respectively; both cyanosed rapidly and died before therapy could be
applied.
In the state of Minnesota (USA), Bosch et al. (1950) reported 139
cases of infant methaemoglobinaemia due to the ingestion of well water
with a high nitrate content (over 89 mg/litre); the mortality rate was
10%. In Kansas, 13 cases of infant methaemoglobinaemia including 3
deaths caused by drinking well water, were reported between the early
1940s and 1950 (Walton, 1951). Many other cases in the USA have been
reported by this author. On the other hand, infant methaemoglobinaemia
was absent in urban areas of New York and the province of Ontario,
Canada where nitrate levels in water were low (Ciavaglia & Thompson,
1969). Knotek & Schmidt (1964) reported 115 cases of methaemo-
globinaemia from a total of 5800 children born in central
Czechoslovakia between 1953 and 1960. Of these, 8% were fatal, 52%
were severe, and 40% were mild. Most deaths were associated with the
consumption of water containing nitrate concentrations ranging from
70 to 250 mg/litre. In these cases, methaemoglobinaemia was
invariably associated with the consumption of infant milk
preparations, which contained microorganisms such as B. subtills,
capable of reducing nitrates to nitrites. These workers reported the
inhibitory effect of buttermilk on this conversion. They attributed
this effect to the presence of Streptococcus lactis which
produces the antibiotic nisin and is capable of preventing the growth
of the spores of B. subtilis.
Shuval & Gruener (1972) studied communities with various
concentrations of nitrates in the drinking water, in Israel. Infants
from rural communities with reported nitrate levels of 50 --
90 mg/litre in the water supplies were compared with controls where
the average level was 5 mg/litre. They did not find any definite cases
of methaemoglobinaemia nor were there any significant differences in
the methaemoglobin levels. However, there was widespread consumption
of citrus juices and the water consumption in infant milk preparations
was low (94% of the infants studied were breast fed or received whole
cow's milk). Soviet literature contains information concerning a
comparatively small number of cases of symptomless methaemoglobinaemia
caused by nitrates in water (Diskalenko, 1969; Motylev, 1969). In
children whose drinking water contained a high level of nitrates, the
methaemoglobin level did not usually exceed 10% although higher levels
were found occasionally. Sattelmacher (1962) and Simon et al. (1964)
compiled 1060 and 745 cases, respectively, of infant
methaemoglobinaemia due to nitrate-contaminated water in the Federal
Republic of Germany. Most of the cases were associated with water
from private wells and in 84-90% of the cases the water contained
nitrate concentrations exceeding 100 mg/litre (although a few cases
of methaemoglobinaemia were reported with water containing less than
50 mg/litre).
Commoner et al. (1972) found small, but statistically
significant, subclinical elevations in methaemoglobin levels in adults
exposed to high intakes of nitrates in a rural area when compared with
an urban control population. There is also a small amount of data
showing that under similar conditions, pregnant women in rural areas
have higher methaemoglobin levels than those in urban areas. This is
of particular interest since previous workers have reported an
increased susceptibility to nitrates in pregnant women. (Skrivan,
1971). Thus, there is concern over the effects on the fetus of the
general lowering of oxygen tension. Gelperin et al. (1971) recently
reported the presence of methaemoglobinaemia in a newborn infant,
presumably exposed to nitrates transplacentally. In this study, 72
mothers and infants tested were exposed to water with nitrate
concentrations ranging from 28 to 45 mg/litre over a 2-month period.
During the 2 weeks of maximum concentration (45 mg/litre), the average
methaemoglobin levels were 1.18% for mothers and 1.91% for newborns
with those of one mother and one infant rising to 6.39% and 5.87%,
respectively.
Children aged 12-14 years who drank water with a nitrate level of
105 mg/litre were noted to have slightly delayed reactions to light
and sound stimuli combined with a mean methaemoglobin level of 5.3% in
comparison with control children drinking water with a nitrate level
of 8 mg/litre whose methaemoglobin levels average 0.75% (Petukhov &
Ivanov, 1970).
8.1.1.2 Exposure through vegetables
Cases of methaemoglobinaemia, some resulting in death, have been
observed following the consumption of spinach. Hölscher & Natzschka
(1964) reported 2 cases in young infants (aged 2 and 3.5 months) who
had eaten spinach purée. Fresh spinach from the same source contained
only traces of nitrates but had nitrite ion levels of 2180 mg/kg.
Fourteen further cases of methaemoglobinaemia in infants (aged 2-10
months) were reported from the Federal Republic of Germany (Sinios &
Wodsak, 1965). Unprocessed spinach was used almost exclusively in the
preparation of the infants' meals and preparation took place at least
24 h before the mealtime. Since in most cases some of the same spinach
had been eaten 24 and 48 h before without causing illness, the authors
assumed that the nitrites were formed within the final 24 h of
storage. Information on 7 cases showed that the mother tasted the
spinach before feeding the child and no change in taste was apparent.
None of the children refused the meal which caused the poisoning.
Conversion of nitrates to nitrites in fresh spinach was demonstrated
by Schuphan (1965) (section 4.2.1). Keating et al. (1973) reported a
case of methaemoglobinaemia in an infant given carrot juice. Other
cases of food-induced methaemoglobinaemia have recently been reviewed
by Luhrs (1973).
8.1.1.3 High accidental exposures through food
Certain meat products contain nitrates and nitrites. Normally, no
adverse effects result from consumption of such products. However, in
1955, an outbreak of 10 cases of methaemoglobinaemia in children
occurred in New Orleans, USA, attributed to the consumption of large
amounts of nitrites in sausage meats (Orgeron et al., 1957). Further
studies revealed that the meats had nitrite concentrations of more
than 200 mg/kg and that some had levels as high as 6570 mg/kg. Singley
(1962) reported 3 cases of methaemoglobinaemia resulting from the
consumption of fish, that had been adulterated with sodium nitrite.
One patient died and it was assessed that he had consumed
approximately 33 mg sodium nitrite/kg body weight. Other cases of
poisoning involving the consumption of nitrite-treated sausage and
frankfurters were reported by Bakshi et al. (1967) and Henderson &
Raskin (1972).
8.1.1.4 Ambient air exposures
Effects on man of nitrate aerosols in ambient air were not
considered by the Task Group. However, because of the recent concern
with the role of nitrates in urban air pollution, it may be of
interest to briefly summarize the current information on this problem.
Airborne nitrates may act as respiratory irritants (Knelson & Lee,
1977) and a recent study conducted by the US Environmental Protection
Agency in the New York-New Jersey metropolitan area showed that
increased asthmatic attacks were significantly associated with
elevated levels of suspended nitrates in six of the seven communities
studied. No such effect was observed with nitrogen dioxide. Another
study in two south-eastern communities in the USA showed some evidence
that a combination of suspended nitrates and suspended sulfates
increased the risk of asthma attacks more than either pollutant did
alone. Because of the present difficulties in measuring suspended
nitrates, these results should be considered as qualitative instead of
quantitative (French et al., unpublished data)a.
8.1.2 Factors involved in susceptibility to nitrates
The work of Marriott et al. (1933), who investigated the acidity
and bacterial flora of 200 infants suffering from diarrhoea, supports
the hypothesis that lack of acidity in the gastric juices of newborn
infants might permit the growth of nitrate-reducing organisms in the
upper gastrointestinal tract and, thus, the reduction of nitrates to
nitrites before the former could be completely absorbed. The pH of the
a French, J. G., Hasselblad, V., & Johnson, R. Aggravation of
asthma by air pollutants. 1971-72 Southeastern CHESS studies.
stomach contents of healthy infants varied from 2.0 to 5.0 (average
3.7) and that for infants suffering from bacillary dysentery ranged
from 2.0 to 5.0 (average 3.0). However, for infants with nonspecified
diarrhoea the pH varied from 4.6 to 6.5 (average 5.6).
According to estimates made by Burden (1961), the water intake of
young infants is nearly 10 times higher than that of adults on a per
unit body weight basis.
The susceptibility of infants to methaemoglobinaemia during the
first six months of life, and especially during the first trimester
(Bailey, 1966; Kübler, 1965) can be explained by various mechanisms,
none of which is fully understood at present. It has been reported
that fetal haemoglobin, which in newborn infants makes up to 60-80% of
the total haemoglobin decreasing to about 20-30% in 3 months (British
Medical Journal, 1966; Kübler, 1965), is more readily oxidized to
methaemoglobin than adult haemoglobin (Betke, 1953; Künzer &
Schneider, 1953). This might explain why premature infants, who
frequently have a higher percentage of fetal haemoglobin than full-
term infants are more susceptible to methaemoglobinaemia. Keohane &
Metcalfe (1960) reported that the sensitivity of erythrocytes to
oxidation to methaemoglobin on exposure to nitrites gradually declined
during childhood until the age of puberty, after which it decreased
rapidly. This decline in sensitivity was not related to the
disappearance of fetal haemoglobin and the authors suggested that some
other factor might be responsible. The susceptibility of newborn and
young infants to develop methaemoglobinaemia could also be attributed
to the incomplete development of the NADH methaemoglobin reductase
system. Several studies have shown that the erythrocytes of newborn
and young infants have a lower capacity to reduce methaemoglobin than
those of older children and adults and that the erythrocytes of
premature newborns have a lower reduction capacity than those of full-
term infants (Bartos et al., 1966; Ross, 1963; Ross & Desforges,
1959). Except in rare cases of hereditary enzyme deficiency (Balsamo
et al., 1964), this deficiency in the NADH methaemoglobin reductase
system seems to disappear after the first 3-4 months of life (Bartos
et al., 1966: Künzer & Schneider, 1953).
The haemoglobin of pregnant women and that of patients suffering
from carcinomata has also been reported to be sensitive to oxidation
to methaemoglobin. However, this sensitivity disappeared in the first
instance after delivery and in the second, after radical extirpation
of the neoplasms (Metcalf, 1961).
8.1.3 Dose-response relationships for nitrates and nitrites
Estimates of the effective exposure of the general population to
nitrates and nitrites have been discussed briefly in section 5.1.4.
However, this information is not sufficient to relate environmental
concentrations to actual intake of nitrates or nitrites in cases of
observed methaemoglobinaemia. Retrospective epidemiological studies
discussed in section 8.1.1 provide little information on intake
because in most cases either the data concerning concentrations in
water or food were not reliable enough, or the amounts of water or
food consumed had not been measured. However, a study reported by
Winton et al. (1971) considered the nitrate intake from water in some
detail. The study was conducted in southern California and central
Illinois, USA, and involved 111 infants whose ages ranged from less
than two weeks to six months. The mother of each infant was asked
about the fluid intake of the infant in the previous 24 h, the method
of formula preparation, and the possible inclusion of any other source
of nitrate in the diet (e.g. vegetables) or administration of
methaemoglobin-forming medicines. The variables that determined the
nitrate dose were the daily fluid intake per body weight (which might
increase in hot and arid climates, or with fever), the fraction of the
total daily fluid intake taken in the form of water, and the
concentration of nitrates in the water. Using this method, it was
possible to estimate that a daily dose of 10 mg/kg body weight could
be obtained from water containing a nitrate concentration of
50 mg/litre. The daily water intake varied from 10% of the total daily
fluid intake for breast-fed infants or for those receiving ready-to-
feed preparations to 90% for infants who were fed preparations made
with powder. This shows that no generalization is possible, at
present, about the relationship between the nitrate concentration in
drinking water and the dose of nitrate, and that estimates of nitrate
dose from nitrate concentrations in water or food would have to be
made for individual cases, taking into account local conditions and
dietary habits.
The relationship between the doses of nitrate and methaemoglobin
levels is even more difficult to establish because of large individual
differences in response, depending on age and many other host
variables. For example, using the method described in the previous
paragraph, Winton et al. (1971) found that in a group of 111 infants,
63 received a nitrate dose of less than 1 mg/kg body weight, 23 were
exposed to 1-4.9 mg/kg, 20 to 5.0-9.9 mg/kg, and 5 infants to
10-15.5 mg/kg. However, only 3 infants appeared to have methaemoglobin
levels above normal (0-2.9%) and they were the youngest of the five
who had received more than 10 mg/kg. The highest methaemoglobin level
(5.3%) was found in 30-day-old baby who had received 15.5 mg/kg.
Another possible approach is to analyse the available retrospective
epidemiological data. This has been done by the Committee on Nitrate
Accumulation (1972) and by Diskalenko (1968) who concluded that only
very crude estimates of correlations between nitrate concentration in
water and methaemoglobin levels could be obtained, probably because of
the delay between the analyses of water and blood, difficulties in
identifying the sources of water consumed, and the heterogeneity of
the population samples studied, particularly with respect to age. The
analyses performed by the Committee on Nitrate Accumulation (1972)
showed, for example, that an increase in the nitrate concentration in
water from 0-49 mg/litre to 49-98 mg/litre, increased the
methaemoglobin level, on average, from 1.0% to 1.3% in infants aged
0-3 months, but did not affect the methaemoglobin level (0.8%) in
infants aged 3-6 months (Simon et al., 1964). Methaemoglobin and
haemoglobin levels, measured in 96 infants from 22 localities in
Rheinhessen, Federal Republic of Germany, during official maternal
counselling, were correlated with the nitrate concentrations of the
drinking water in the localities concerned by Würkert (1974,
unpublished data)a. Nitrate concentrations of 0-5 mg/litre,
31-50 mg/litre, and over 100 mg/litre corresponded to methaemoglobin
levels of 1.65%, 2.44%, and 6.59%, respectively. Higher methaemoglobin
levels were found in the presence of infections and in infants given
tea to drink or vegetable nutrients.
The last question pertains to the relation between the level of
methaemoglobin and clinical signs and symptoms of methaemoglobinaemia.
Normally, methaemoglobin is present in the blood at a concentration of
less than 2% of total haemoglobin.
Subclinical methaemoglobinaemia (less than 10% methaemoglobin)
has not been considered to be of direct health significance, but
Petukhov & Ivanov (1970) reported behavioural effects at these levels.
Clinical signs of methaemoglobinaemia such as cyanosis become
apparent at a methaemoglobin level of about 10%. Hypoxic signs and
symptoms may develop at levels exceeding 20% and death may occur at
levels of 50% or more.
In conclusion, the available information does not permit the
establishment of a quantitative dose-response relationship for human
exposure to nitrates in water or food.
8.2 N-nitroso Compounds
Freund (1937) first described acute intoxication by DMN. Barnes &
Magee (1954) described 2 cases of industrial intoxication due to DMN
in which one individual had hepatic cirrhosis at death; the other
survived but, 6 months later, was shown to have a hard liver suspected
of being cirrhotic. Watrous (1947) and Wrigley (1948) reported cases
of accidental exposure to N-nitroso-methylurethane. Reddening of the
conjunctiva and erythema of the face and feet developed quickly, and a
respiratory disorder developed later.
No reports are available concerning carcinogenesis in industrial
or other workers exposed to N-nitroso compounds, nor have
relationships been established, from epidemiological and analytical
data, that link cancer in man with exposure to N-nitroso compounds or
their possible precursors such as nitrates, nitrites, and compounds
a Thesis reported in the contributed of the Federal Republic of
Germany to the WHO environmental health critera document on
Nitrates, nitrites and N-nitroso compounds.
that can be nitrosated, occurring as food components, drugs, and
pesticides. A recent review of these data is available (Mirvish,
1976).
Some reports have been concerned with the possible etiological
role of N-nitroso compounds in nasopharyngeal cancer in south-east
Asia (Clifford, 1970; Fong & Chan, 1973a) and oesophageal cancer in
South Africa, Iran, and China (Burrell et al., 1966; Coordinating
Group for Research on the Etiology of Esophageal Cancer of North
China, 1974; Day, 1975; Harmozdian et al., 1975). However, no
relationship has been established; nitrosamines were detected in food
from these areas but this was not confirmed by mass spectroscopy
(Eisenbrand et al., 1976; Purchase et al., 1975).
The epidemiology of stomach cancer has been discussed from a
similar point of view by Correa et al. (1975), Endo et al. (1973),
Haenzel & Correa (1975), Hill et al. (1973), Mirvish (1971), and
Weisburger & Raineri (1975). It was suggested that nitrosamides might
be formed in the stomach from amides occurring in the diet, and might
then act locally on this organ. Relationships have been sought between
the occurrence of stomach cancer and the nitrate contents of the soil
or water in Chile, Colombia, and the United Kingdom (Hawksworth et
al., 1974; Hill et al., 1973; Zaldivar & Wetterstrand, 1975) but none
was established.
9. EVALUATION OF HEALTH RISKS TO MAN FROM EXPOSURE TO NITRATES,
NITRITES, AND N-NITROSO COMPOUNDS
9.1 Nitrates and Nitrites
9.1.1 General considerations
Man is exposed to nitrates and nitrites mainly through water and
food. Nitrate concentrations may be particularly high in drinking
water derived from dug wells. Nitrates in food may occur naturally or
may be added for various technological or even public health reasons
(e.g. addition of nitrates and nitrites to certain meat products to
protect against botulism). Although intake of very large doses of
nitrates can be fatal to man, such intake is not likely to occur
through environmental exposure, except in the case of infants and very
young children who are high risk groups because of their
susceptibility to nitrates and nitrites. Weekly intakes of nitrates by
members of the general population are difficult to evaluate but rough
estimates are available for England and the USA giving values of about
400-450 mg/week (85-105 mg from water; 210-225 mg from vegetables, and
about 110 mg from meat products).
These figures cannot be applied generally and a separate
estimation of the nitrate intake from food and water should be made
for each case, especially when the subjects are infants or young
children (section 8.1.3). Exposure to nitrates can also occur through
the inhalation of polluted air.
The assessment of health risks to man (section 9.1.2) has been
based on epidemiological studies and clinical evidence. The animal
data discussion in section 7.1, confirm the findings in man that
methaemoglobinaemia is the main toxic effect of nitrate and nitrite
ingestion. Methaemoglobinaemia is caused by nitrites, the reduction
products of nitrates. The reduction usually occurs through microbial
action either in the environment or in the body. The health risks from
exposure to nitrates is therefore related not only to their
concentration in drinking water and food, but also to the presence or
absence of conditions conducive to their reduction to nitrites. Young
infants constitute the most vulnerable group for the following
reasons:
(1) Lower acidity in their stomach allows the growth of certain
microbes that contain enzymes capable of reducing nitrates
to nitrites;
(2) Fetal haemoglobin, which constitutes a considerable
proportion of the haemoglobin of the young infant, and the
erythrocytes during childhood may be more susceptible to
conversion to methaemoglobin by the action of nitrites;
(3) The enzyme system capable of reducing methaemoglobin to
haemoglobin is deficient in the young infant; and
(4) The fluid intake of the young infant is higher than that of
the adult in relation to the body weight.
9.1.2 Assessment of health risks
Precise dose-response relationships could not be established by
the Task Group because of the existence of various strongly modifying
factors and the lack of accurate quantitative data. However, on the
basis of the available information, the Task Group reached the
following conclusions:
(a) General population -- The prevailing levels of nitrates and
nitrites in water and food do not seem to have any harmful
effects in adults and older children, although there are reports
of susceptible individuals who have been affected by meat treated
with nitrites, and of cases of poisoning resulting from the
ingestion of certain foods accidently containing excessive
amounts of nitrites (8.1.1.3). Subclinical methaemoglobinaemia
may also be found in individuals consuming water containing high
levels of nitrates (8.1.3).
(b) Susceptible group -- Infants less than 6 months old and
especially those under 3 months of age are particularly
susceptible to methaemoglobinaemia caused by intake of water
containing elevated levels of nitrates, especially when they are
fed with preparations made from dried milk of low acidity. While
a few cases of methaemoglobinaemia have been reported associated
with water nitrate levels of less than 50 mg/litre, most cases
occur with nitrate levels of 90 mg/litre or more. Nitrates in
water may cause death of the infant, but the lowest level that
may be fatal cannot be estimated at present.
The ingestion of vegetables (e.g. spinach, carrots) containing
elevated nitrate and/or nitrite levels may also cause
methaemoglobinaemia in infants, especially in those aged between 6
months and 1 year. Storage of vegetables, other than in the frozen or
canned state, is likely to increase the nitrite level and hence the
risk.
(c) Effects of airborne nitrates -- A few recent studies have
indicated that airborne nitrates may act as respiratory irritants
but, at present, adequate quantitative data are not available and
the Task Group did not consider this exposure in their health
risk evaluation.
9.2 N-nitroso Compounds
9.2.1 General considerations
Nitrites (and indirectly nitrates) can react with amines and
amides to form nitrosamines and nitrosamides. The precursors of these
N-nitroso compounds are widely distributed in various environmental
media. Information concerning the presence of N-nitroso compounds
per se is limited although they have been identified in certain
foods such as luncheon meats and in air and water samples. The
conditions under which N-nitroso compounds can be formed are
outlined in sections 4.2-4.6.
More than 80% of over one hundred N-nitroso compounds tested
proved to be carcinogenic in animal experiments giving rise to tumours
in many organs and also producing tumours transplacentally.
N-nitroso compounds are carcinogenic in a wide range of animal
species; most are mutagenic in test systems and some have been shown
to be teratogenic to animals.
The possible health hazard from N-nitroso compounds is not
confined to those present in the environment. Their formation, from a
variety of precursors in the body of animals, has been demonstrated,
and this may also occur in man.
9.2.2 Assessment of health risks
A dose-response relationship has been shown to exist in different
species of rodents for some carcinogenic N-nitroso compounds. As the
dose is reduced, the tumour incidence decreases and the time for
tumour induction increases and may exceed the life span of the animals.
Although there is no clinical or epidemiological evidence, it is
highly probable that these compounds are also carcinogenic to man.
However, present limitations concerning available dose-response data
in animals and their interpretation, and inadequate knowledge of the
biomechanism of cancer induction preclude a quantitative estimation of
the carcinogenic risk to man that may be associated with different
exposures to N-nitroso compounds.
9.3 Reduction of Exposure
The assessments of health risk given in sections 9.1.2
[(a) and (b)] and 9.2.2 lead to a number of practical conclusions
concerning the need to reduce the exposure to nitrates, nitrites, and
N-nitroso compounds.
As regards the exposure to nitrates and nitrites, the Task Group
made the following specific recommendations:
(a) Infant dried milk preparations should be reconstituted only
with water containing low levels of nitrates. If such water is
not available, breast feeding or the use of cow's milk should be
encouraged.
(b) Only vegetables with a low nitrate content should be used in
the preparation of baby foods. If vegetables known to contain
high levels of nitrates are used, appropriate food processing
precautions should be instituted. Nitrates and nitrites should
not be added to baby foods.
(c) The use of nitrates and nitrites in foods as preservatives
should be reduced to the minimum level that provides protection
against botulism. This applies particularly to cured and canned
meats and to fish. The use of nitrates and nitrites on fresh
meats or fish should be avoided.
(d) Nitrate levels in public drinking water should comply with,
or preferably be lower than, the tentative limit of
45 mg/litre recommended in the International Standards for
Drinking Water (WHO, 1971).
With respect to the carcinogenic risk from exposure to
N-nitroso compounds, it is prudent to assume that any exposure may
involve some degree of risk, and that exposure should therefore be
kept as low as practically achievable. This may not be an easy task in
many instances, since these compounds may occur in the environment in
concentrations of the order of parts per billion, as a result of a
variety of natural and technological processes, and, moreover, they
may be formed in vivo from nitrates, amines, and amides, which
are ubiquitous. Obviously the recommendations (a) to (d) will also
contribute to the reduction of carcinogenic risk related to
N-nitroso compounds.
REFERENCES
ACHTZEHN, M. K. & HAWAT, H. (1969) [Nitrate accumulation in vegetables
a possibility of nitrate intoxication in infants.] Die Nahrung,
13: 667-676 (in German).
ACHTZEHN, M. K. & HAWAT, H. (1970) [On nitrate formation in vegetables
and vegetable products. Part I. Raw spinach.] Die Nahrung,
14: 383-394 (in German).
ADRIANO, C. C., PRATT, D. F., & BISHOP, S. E. (1971) Fate of inorganic
forms of nitrogen and salt from land disposed manures from
dairies. In: Livestock waste management and pollution
abatement. St. Joseph, MI (Am. Soc. Agric. Eng. Proc. No. 271).
ALAM, B. S., SAPORESCHETZ, I. B., & EPSTEIN, S.S. (1971a) Formation of
N-nitroso-piperidine from piperidine and sodium nitrite in the
stomach and the isolated intestinal loop of the rat. Nature
(Lond.), 232: 116-118.
ALAM, B. S., SAPORESCHETZ, I. B., & EPSTEIN, S.S. (1971b) Synthesis of
nitrosopiperidine from nitrate and piperidine in the gastro-
intestinai tract of the rat. Nature (Lond.), 232: 199-200.
ALARIF, A. & EPSTEIN, S.S. (1974) The uptake and metabolism of
14 C-labelled nitrosomethylurethane and nitrosomethylurea in
guinea pigs and their in vitro metabolism in the guinea pig
and human pancreas. IARC Sci. Publ. No. 9, pp. 215-219.
ALEXANDROV, V. A. (1967) [On the mechanism of carcinogenicity of
N-nitrosodimethylamine in connection with peculiarities of
its effect on embryogenesis] Vop. Onkol., 13: 87-92
(in Russian).
ALEXANDROV, V. A. (1968a) Blastomogenic effect of dimethylnitrosamine
on pregnant rats and their offspring. Nature (Lond.),
218: 280-281.
ALEXANDROV, V. A. (1968b) [Effect of N-nitroso- N-methylaniline and
N-nitroso- N-ethylaniline on the rat embryo] Vop. Onkol.,
14: 37-38 (in Russian).
ALEXANDROV, V. A. (1969a) [Transplacental blastomogenic action of
N-nitrosomethylurea in rat offspring] Vop. Onkol.;
15: 55-61 (in Russian).
ALEXANDROV, V. A. (1969b) Uterine, vaginal and mammary tumors induced
by nitrosoureas in pregnant rats. Nature (Lond.),
222: 1064-1065.
ALEXANDROV, V. A. & JANISCH, W. (1971) Teratogenic effect of ethylurea
and nitrite in rats. Experientia (Basel), 27: 538-539.
ALLISTON, T. G., COX, G. B., & KIRK, R. S. (1972)The determination of
steam-volatile N-nitrosamines in foodstuffs by formation of
electron-capturing derivatives from electrochemically derived
amines. Analyst, 97: 915-920.
ALTHOFF, J., WILSON, R., & MOHR, U. (1971) Diethylnitrosamine-induced
alterations in the tracheobronchial system of Syrian golden
hamsters. J. Natl Cancer Inst., 46: 1067-1071.
ALTHORPE, J., GODDARD, D. A., & SISSONS, D. J., TELLING, G. M. (1970)
The gas chromatographic determination of nitrosamines at the
picogram level by conversion to their corresponding nitramines.
J. Chromatogr., 53: 371-373.
ARENA, J. M. (1970) Poisoning, toxicology, symptoms and treatment.
2nd ed., Springfield, I, Bannerstone House, p. 9.
ARISON, R. N. & FEUDALE, E. L. (1967) Induction of renal tumour by
streptozotoc n in rats. Nature (Lond.), 214: 1254-1255.
ASAHINA, S., FRIEDMAN, M. A., ARNOLD, E., MILLAR, G. N., MISHKIN, M.,
BISHOP, Y., & EPSTEIN, S.S. (1971) Acute synergistic toxicity and
hepatic necrosis following oral administration of sodium nitrite
and secondary amines to mice. Cancer Res., 31: 1201-1205.
ASATOOR, A.M. & SIMENHOFF, M. L. (1965)The origin of urinary
dimethylamine. Biochim. Biophys. Acta, 111: 384-392.
ASHTON, M. R. (1970) The occurrence of nitrates and nitrites in foods.
BFMIRA lit. surv. No 7, pp. 32.
BADER, G., STILLER, D., & ULLRICH, K. (1971) [Ultrastructural changes
in toxically injured and proliferating liver cells due to long-
term application of diethylnitrosamine] Arch.
Geschwulstforsch., 37: 327-343 (in German).
BAILEY, W. P. (1966) Methemoglobinemia-acute nitrate poisoning in
infants: Second report. J. Am. Osteopath. Assoc.,
66: 431-434.
BAKSHI, S. P., FAHEY, J. L., & PIERCE, L. E. (1967) Sausage cyanosis--
Acquired methemoglobinemia nitrite poisoning.
N. Eng. J. Med. 277: 1072.
BALSAMO, P., HARDY, W. R., & SCOTT, E. M. (1964) Hereditary
methaemoglobinaemia due to diaphorase deficiency in Navajo
Indians. J. Pediat., 65: 928-931.
BANNASCH, P. (1968) In: The cytoplasm of hepatocytes during
carcinogenesis. New York, Springer Verlag.
BARNES, J. M. & MAGEE, P.M. (1954) Some toxic properties of
dimethylnitrosamine. Br. J. ind. Med., 11: 167-174.
BARTOS, H. R., DESFORGES, J. F., & CLARK, N. (1966) Erythrocyte DPNH
dependent diaphorase levels in infants. Pediatrics, 37: 991-993.
BEHROOZI, K., GUTTMAN, R., GRUENER, N., & SHUVAL, H. I. (1971) Changes
in the motor activity of mice given sodium nitrite drinking
solution. In: Proceedings of a Symposium on Environmental
Physiology, Beersheba, December, 1971.
BNEMANSKIJ, V. V. & LITVINOV, N. N. (1969) [Some data on the histo-
and morphogenesis of experimental tumours of the kidneys in
animals.] Arkh. Pat., 31: 79-84 (in Russian).
BERTRAM, J. S. & CRAIG, A. W. (1972) Specific induction of bladder
cancer in mice by butyl- (4-hydroxybutyl) -nitrosamine and the
effects of hormonal modifications on the sex difference in
response. Eur. J. Cancer, 8(6): 587-594.
BERTRAM, J. S. & CRAIG, A. W. (1973) Induction of bladder tumours in
mice with dibutylnitrosamine. Br. J. Cancer, 24
(2): 352-359.
BETKE, K. (1953) [Comparative examination of the oxidation of fetal
and adult oxyhaemoglobin by sodium nitrite.] Naturwiss., 40: 60
(in German).
BHATHAL, P.S. & HURLEY, J. V. (1973) An electron microscope study of
the production of ascites in acute dimethylnitrosamine (NDMA)-
induced liver injury. J. Pathol., 111: 103-116.
BIOCEV, IV. & POCINKOVA, Z. (1972) [Effect of applied artificial
fertilizers on the soil, drinking water and foodstuffs of
vegetable origin. Report on the International Congress of
Country Hygiene, Varna] (in Russian).
BLATTMANN, L. & PREUSSMAN, R. (1973) [The structure of metabolites of
carcinogenic dialkylnitrosamine in the urine of rats]
Z. Krebsforsch., 79: 3-5 (in German).
BLATTMANN, L. & PREUSSMAN, R. (1974a). [The biotransformation of
carcinogenic dialkylnitrosamines. Further urine metabolites of
di-n-butyl- and di-n-pentylnitrosamines.] Z. Krebsforsch.,
81: 75-78 (in German).
BLATTMANN, L., JOSWIG, N., & PREUSSMAN, T. (1974b). [The structure of
the metabolites of the carcinogenic methyl-n-butyl-nitrosamine in
the urine of rats.] Z. Krebsforsch., 81: 71-73 (in German).
BODIPHALA, T. & ORMROD, D. P. (1971) Factors affecting the nitrate
content of vegetable and fruit foods. Can. Inst. Food Technol.,
J., 4(1): 6-8.
BOGOVSKI, P. & WALKER, E. A., ed. (1974) N-nitroso compounds in the
environment. Proceedings of a Working Conference, the
International Agency for Research on Cancer, Lyons, France,
17-20 October, 1973, Lyons, International Agency for Research on
Cancer, pp. 243.
BOGGYSKI, P., PREUSSMANN, R., & WALKER, E. A., ed. (1972a) N-nitroso
compounds analysis and formation. Proceedings of a Working
Conference, Deutsches Krebsforschungszentrum, Heidelberg,
Federal Republic of Germany, 13-15 October, 1971, Lyons,
International Agency for Research on Cancer, pp. 140.
BOGGYSKI, P., CASTEGNARO, M., PIGNETTELI, B., & WALKER, E. A. (1972b)
The inhibiting effects of tannins on the formation of
nitrosamines. IARC Sci. Publ. No. 3, pp. 127-129.
BOLIN, B. & ARRHENIUS, E., ed. (1977) An essential life factor and a
growing environmental hazard. Report from Nobel Symposium No. 38,
Ambio, 6 (2-3): 96-105.
BOSCH, H. M., ROSENFIELD, A. B., HUSTON, R., SHIPMAN, H. R., &
WOODWARD, F. L. (1950) Methemogiobinemia and Minnesota well
supplies. J. Am. Water Assoc., 42: 161-170.
BOYLAND, E. & WALKER, S. A. (1974) Effect of thiocyanate on
nitrosation of amines. Nature (Lond.), 248: 601-602.
BOYLAND, E., CARTER, R. L., GORROD, J. W., & ROE, F. J. C. (1968)
Carcinogenic properties of certain rubber additives. Eur. J.
Cancer, 4: 233-239.
BRESLER, S. E., KALININ, V. L., & PERUMOV, D. A. (1968) Inactivation
and mutagenesis on isolated DNA. IV Possibility of integration of
lethal damage into the chromosome of Bacillus subtills during
transformation. Mutat. Res., 5: 329-341.
BRETSCHNEIDER, K. & MATZ, J. (1973) [Nitrosamines (NA) in the
atmospheric air and in the air at the workplace.] Arch.
Geschwulstforsch., 42: 36-41 (in German).
BRETSCHNEIDER, K. & MATZ, J. (1976) Occurrence and analysis of
nitrosamines in air. IARC Sci. Publ. No. 14, pp. 395-399.
British Medical Journal (1966), 1: 250-251. Spinach--a risk to
babies.
BROWN, J. R. & SMITH, G. E. (1967) Nitrate accumulation in vegetable
crops as influenced by soil fertility practises. Columbia,
University of Missouri, pp. 43 (Res. Bull. No. 920).
BRUNI, C. (1973) Distinctive cells similar to fetal hepatocytes
associated with liver carcinogenesis by diethylnitrosamine.
Electron microscopic study. J. Natl Cancer Inst.,
50: 1513-1528.
BUGLASS, A. J., CHALLIS, B.C., & OSBORNE, M. R. (1974)
Transnitrosation and decomposition of N- nitrosamines.
N- nitroso compounds in the environment. IARC Sci. Publ., No. 9,
pp. 94-100.
BURDEN, E. H. W. J. (1961) The toxicology of nitrates and nitrites
with particular reference to the potability of water supplies.
Analyst, 86 (1024): 429-433.
BURNS, R. C. & HARDY, R. W. F. (1975) Nitrogen fixation in bacteria
and higher plants. Berlin, Springer-Verlag.
BURRELL, R. J. W., ROACH, W. A., & SHADWELL, A. (1966) Esophageal
cancer in the Bantu of the Transkei associated with mineral
deficiency in garden plants. J. Natl Cancer Inst.,
36: 201-214.
BUTLER, W. H. & HARD, G. C. (1971) Hepatoxicity of dimethylnitrosamine
in the rat with special reference to veno-occlusive disease.
Exp. mol. Pathol., 15: 209-219.
CALAFAT, J., DEN ENGELSE, L, & EMMELOT, P. (1970) Studies on lung
tumours. II. Morphological alterations induced by
dimethylnitrosamine in mouse lung and liver and their relevance
to tumourigenesis. Chem. biol. Interact.,
2: 309-320.
CARDESA, A., MIRVlSH, S.S., HAVEN, G. T., & SHUBIK, P. (1974)
Inhibitory effect of ascorbic acid on the acute toxicity of
dimethylamine plus nitrite in the rat. Proc. Soc. Exp. Biol.
Med., 145: 124-128.
CARTER, R. L., PERCIVAL, W. H., & ROE, F. J. C. (1969) Exceptional
sensitivity of mink to the hepatotoxic effects of
dimethylnitrosamine. J. Pathol., 97 (1): 79-88.
CASEY, H. (1975) Variation in chemical composition of the River Frome,
England from 1965 to 1972. Freshwater Biol.,
5: 507-514.
CASTEGNARO, M., PIGNATELLI, B., & WALKER, E. A. (1972) N-Nitroso
compounds: Analysis and formation. IARC Sci. Publ, No. 3,
pp. 87-89.
CAVETT, J. J. (1962) The microbiology of vacuum-packed sliced bacon.
J. appl. Bact., 25: 282-289.
CHALLIS, B.C. (1973) Rapid nitrosation of phenols anti its
implications for health hazards from dietary nitrites. Nature
(Load.), 244: 466.
CHALLIS, B.C. & BARTLETT, C. D. (1975) Possible cocarcinogenic effects
of coffee constituents. Nature (Lond.),
254: 532-533.
CHALLIS, B.C. & CHALLIS, J. A. (1970) Reactions of the carboxamide
group. In: Zabicky, J., ed. The chemistry of amides, London,
Interscience, pp. 733-848.
CIAVAGLIA, F. & THOMPSON, R. P. (1969) Infant methemoglobinemia.
Absence in areas with low concentrations of nitrate-nitrogen in
water supply. N.Y. State J. Med., 69: 3128-3129.
CLAPP, N. K. & TOYA, R. E. (1970) Effect of cumulative dose and dose
rate on dimethylnitrosamine oncogenesis in RF mice.
J. Natl Cancer Inst., 45: 495-498.
CLAPP, N. K., TYNDALL, R. L., & OTTEN, J. A. (1971) Differences in
tumor types and organ susceptibility in BALB-C and RF mice
following dimethylnitrosamine and diethyinitrosamine. Cancer
Res., 31: 196-198.
CLIFFORD, P. (1970) On the epidemiology of nasopharyngeal carcinoma.
Int. J. Cancer, 5: 287-309.
COLLINS-THOMPSON, D. L., SEN, N. P., ARIS, B., & SCHWENGHAMER, L.
(1972) Non-enzymic in vitro formation of nitrosamines by
bacteria isolated from meat products. Can. J. Microbiol,
18 (12): 1968-1971.
COMLY, H. H. (1945) Cyanosis in infants caused by nitrates in well
water. J. Am. Med. Assoc., 129: 112-116.
COMMITTEE ON NITRATE ACCUMULATION (1972) Accumulation of nitrate.
Washington, DC, National Academy of Sciences. p. 48.
COMMONER, B. (1970) Threats to the integrity of the nitrogen cycle.
In: Singer, F. S., ed. Global effects of environmental
pollution, NY, Springer Verlag, pp. 70-95.
COMMONER, B., SHEARER, G., & KOHL, D. (1972) A study of certain
ecological, public and economic consequences of the use of
inorganic nitrogen fertilizer, St. Louis, Washington
University. (1st year Progress Report NSF Grant No. GI29926x).
COOKE, G. E. & WILTIAMS, R. J. B. (1970) Losses of nitrogen and
phosphorus from agricultural land. Water Treat. Exam.,
19 (3): 253-276.
COORDINATING GROUP FOR RESEARCH ON THE ETIOLOGY OF ESOPHAGEAL CANCER
OF NORTH CHINA (1974) The epidemiology of esophageal cancer in
North China and preliminary results in the investigation of its
etiological factors. Abstract, 11th International Cancer
Congress, Florence, Italy.
CORREA, P., HAENSZEL, W., CUELLO, C., TANNENBAUM, S., & ARCHER, M.
(1975) A model for gastric cancer epidemiology, Lancet, July 12,
pp. 58-59.
COUCH, D. B. & FRIEDMAN, M. A. (1975) Interactive mutagenicity of
sodium nitrite, dimethylamine, methylurea and ethylurea. Mutat.
res., 31: 109-114.
CRADDOCK, V. M. (1971) Liver carcinomas induced in rats by single
administration of dimethylnitrosamine after partial hepatectomy.
J. Natl Cancer IMNST, 47: 899-907.
CROSBY, N. T., FOREMAN, K. K., PALFRAMEN, J. F., & SAWYER, R. (1972)
Determination of volatile nitrosamines in food products at the
µg/kg level. IARC Sci. Publ. No. 3, pp. 38-42.
DAIBER, D. (1966) [Methods for the quantitative and qualitative
estimation of organic N- nitroso compounds.] Freiburg (Thesis)
(in German).
DAY, N. E. (1975) Some aspects of the epidemiology of esophageal
cancer. Cancer Res., 35 (2): 3304-3307.
DELWICHE, C. C. (1970) The nitrogen cycle. Sci. Am., 223: 137-146.
DISKALENKO, A. P. (1968) Methemoglobinemia of water-nitrate origin in
the Moldavian SSR. Gig. i Sanit., 33: 32-37.
DISKALENKO, A. P. (1969) [Methaemoglobinaemia caused by nitrates in
water and its prophylaxis.] Kisinev (in Russian).
DISKALENKO, A. P. & DOBRJANSKAJA, E. V. (1972) [Changes in redox
processes in hepatic and cerebral tissues as a result of the
ingestion of nitrates with drinking water. In: Topical
questions of hygiene and epidemiology] Kisinev, pp. 22-23 (in
Russian).
DISKALENKO, A. P. & TROFIMENKO, JU. N. (1972) [Changes in the activity
of the glutathione-ascorbic acid system and respiration in
erythrocytes as a result of the ingestion of nitrites with
drinking water. In: Topical questions of hygiene and
epidemiology Kisinev, pp. 23-25 (in Russian).
DOWNS, E. F. (1950) Cyanosis of infants caused by nitrate
concentrations in rural water-supplies. Bull. World Health
Organ., 3: 165-169.
DRUCKREY, H. & PREUSSMANN, R. (1962) [The production of lung cancer in
rats by subcutaneous injection of diamylnitrosamine.]
Naturwiss., 49: 111-112 (in German).
DRUCKREY, H., PREUSSMAN, R., SCHMÄHL, D., & MÜLLER, M. (1961) [The
chemical constitution and carcinogenic effects of the
nitrosamines.] Naturwiss., 48: 134-135 (in German).
DRUCKREY, H., STEINHOFF, D., BEUTHNER, H., SCHNEIDER, H., & KLÄRNER,
P. (1963a) [Screening of nitrite for chronic toxicity in rats.]
Arzneimittel-Forsch., 13: 320-323 (in German).
DRUCKREY, H., SCHILDBACH, A., SCHMAHL, D., PREUSSMANN, R., &
IVANKOVIC, S. (1963b) [Quantitative analysis of the carcinogenic
action of diethylnitrosamine.] Arzneimittel-Forsch,
13: 841-851 (in German).
DRUCKREY, H., IVANKOVlC, S., & PREUSSMANN, R. (1966) Teratogenic and
carcinogenic effects in the offspring after single injection of
ethylnitrosourea in pregnant rats. Nature (Lond.),
210: 1378-1379.
DRUCKREY, H., PREUSSMANN, R., IVANKOVIC, S., & SCHMÄHL, D. (1967)
[Organotropic carcinogenic effects of 65 different N-nitroso-
compounds in BD-rats.] Z. Krebsforsch, 69: 103-201 (in German).
DRUCKREY, H., LANDSCHÜTZ, CH., & PREUSSMANN, R. (1968) [Oesophageal
carcinoma due to inhalation of methylbutylnitrosamine (MBNA) in
rats.] Z. Krebsforsch., 71: 135-139 (in German).
DRUCKREY, H., LANDSCHOTZ, CH., & IVANKOVlC, S. (1970a) [Transplacental
production of malignant tumours in the nervous system II.
Ethylnitroso urea in 10 genetically defined rat strains.] Z.
Krebsforsch., 73: 371-386 (in German).
DUBROW, H. & KABISCH, W. (1960) [Investigations of the evidence of
nitrates and nitrites in cheese.] Milchwiss., 15 (11): 543-549
(in German).
DURFOR, C. N. & BECKER, E. (1965) Public water supplies of the 100
largest cities in the United States. Washington DC, US Dept
Interior (Geological Survey Water Supply Paper 1812).
DUTTON, A. H. & HEATH, D. F. (1956) Demethylation of
dimethylnitrosamine in rats and mice. Nature (Lond.),
178: 644.
EISENBRAND, G. (1972) Determination of volatile nitrosamines at low
levels in food by acid-catalysed denitrosation and formation of
derivatives from the resulting amines. IARC Sci. Publ. No. 3,
pp. 64-71.
EISENBRAND, G. (1973) Determination of volatile nitrosamines: a
review. In: Proceedings of the International Symposium on
Nitrite in Meat Products. Zeist, pp. 45-52.
EISENBRAND, G. & PREUSSMANN, R. (1970) [A new method for the
colorimetric determination of nitrosamines following cleavage of
the N-nitroso group with hydrogen bromide in glacial acetic
acid.] Arzneimittel-Forsch., 20: 1513-1517 (in German).
EISENBRAND, G., JANZOWSKI, C., & PREUSSMANN, R. (1975) Gas
chromatographic determination of N-nitrosoamino acids by
trimethylsilylation and single-ion mass fragmentography.
J. Chromatogr., 115: 602-606.
EISENBRAND, G., VON RAPPARD, E., ZAPPE, R., & PREUSSMANN, R. (1976)
Trace analysis of volatile nitrosamines by a modified nitrogen
specific detector in pyrolytic mode and by ion-specific
determination of heptafiuorobutyrantides in a GC/MS system.
IARC Sci. Publ. No. 14, pp. 65-75.
EISENSTARK, A. & ROSNER, J. L. (1964) Chemically induced reversions in
the cysC region of Salmonella typhimurium. Genetics,
49: 343-355.
ELESPURU, R. K. & LIJINSKY, W. (1973) The formation of carcinogenic
nitroso compounds from nitrite and some types of agricultural
chemicals. Food Cosmet. Toxicol., 11 (3): 807-811.
ELESPURU, R., LIJINSKY, W., & SETLOW, J. K. (1974) Nitrosocarbaryl as
a potent mutagen of environmental significance. Nature (Lond.),
247: 386-387.
ENDER, F. & CEH, L. (1967) Occurrence and determination of
nitrosamines in foodstuffs for human and animal nutrition.
Alkylierend Wirkend Verbindungen, Second Conference on Tobacco
Research., Freiburg, 1960, pp. 83-91.
ENDER, F. & CEH, L. Z. (1971) Conditions and chemical reaction
mechanisms by which nitrosamines may be formed in biological
products with reference to their possible occurrence in food
products. Z. Lebensm. Unters. Forsch., 145 (3): 133-142.
ENDER, F., HAVRE, G., HELGEBOSTAD, A., KOPPANG, N., MADSEN, R., & CEH,
L. (1964) Isolation and identification of a hepatoxic factor in
herring meal produced from sodium nitrite preserved herring.
Naturwiss., 51: 637-638.
ENDER, F., HAVRE, G., MADSEN, R., CEH, L., & HELGEBOSTAD, A. (1967)
[Studies on conditions under which N-nitrosodime-thylamine is
formed in herring meal produced from nitrite-preserved herring.]
Z. Tierphysiol. Tierernähr. Futtermittelk., 22: 181-189 (in
German).
ENDO, H., TAKAHESHI, K., & AOYAGI, H. (1973) Screening of compounds
structurally and functionally related to N-methyl- N-nitroso
guanidine, a gastric carcinogen. Gann, 65: 45-54.
EPPSON, H. F., GLENN, M. W., ELLIS, W. W., & GILBERT, C. S. (1960)
Nitrate in the diet of pregnant ewes. JAVMA, 137 (10): 611-614.
EPSTEIN, S. (1972) In vivo studies on interactions between secondary
amines and nitrites or nitrates. IARC Sci. Publ. No. 3,
pp. 109-115.
FAN, T-Y. & TANNENBAUM, S. R. (1972) Automatic colorimetric
determination of N-nitroso compounds. J. agric. Food Chem.,
19: 1267-1269.
FAN, T-Y. & TANNENBAUM, S. R. (1973) Factors influencing the rate of
formation of nitrosomorpholine from morpholine and nitrite:
acceleration by thiocyanate and other anions
J. agric. Food Chem, 21: 237-240.
FAO/IAEA Panel of Experts (1974) Effects of agricultural production
on nitrates in food and water with particular reference to
isotope studies. Proceedings and Report of a Panel of Experts,
Vienna, 4-8 June 1973, Vienna, International Atomic Energy
Agency, pp. 158.
FASSETT, D. W. (1966) Nitrates and nitrites. In: Toxicants occurring
naturally in foods. Washington, DC, National Academy of
Sciences, pp. 250-449 (Publ. No. 1354).
FAZIO, T., DAMICO, J. N., HOWARD, J. W., WHITE, R. H., & WATTS, J. O.
(1971a) Gas chromatographic determination and mass spectrometric
confirmation of N-nitro-sodimethylamine in smoke-processed
marine fish. J. agric. Food Chem,
19(2): 250-253.
FAZlO, T., WHITE, R. H., & HOWARD, J. W. (1971b) Analysis of nitrite-
and/or nitrate-processed meats for
N-nitrosodimethylamine. J. Assoc. Off Anal. Chem.,
54(5): 1157-1159.
FAZIO, T., HOWARD, J. W., & WHITE, R. (1972) Multidetection method
for analysis of volatile nitrosamines in foods. IARC Sci. Publ.
No. 3, pp. 16-24.
FAZIO, T., WHITE, R. H., DUSOLD, L. R., & HOWARD, J. W. (1973)
Nitrosopyrrolidine in cooked bacon. J. Assoc. Off. Anal. Chem.,
56 (4): 919-921.
FERGUSON, J. H., MYSLIWY, T. J., & ARCHER, M. C. (1974) The
nitrosation of spermidine and spermine. IARC Sci. Publ. No. 9,
pp. 90-93.
FERON, V. J., EMMELOT, P., & VOSSENAAR, T. (1972) Lower respiratory
tract tumors in Syrian golden hamsters after intratracheal
instillations of diethylnitrosamine alone and with ferric oxide.
Eur. J. Cancer, 8: 445-449.
FIDDLER, W. (1975) The occurrence and determination of N-nitroso
compounds. Toxicoi. appl. Pharmacol., 31: 352-360.
FIDDLER, W., DOERR, R. C., ERTEL, J. R., & WASSERMAN, A. E. (1971)
Gas-liquid chromatographic determination of
N-nitrosodimethylamine in ham. J. Assoc. Off Anal. Chem, 54
(5): 1160-1163.
FIDDLER, W., PENSABENE, J. W., DOERR, R. C., & WASSERMAN, A. E. (1972)
Formation of N-nitrosodimethylamine from naturally occurring
quaternary ammonium compounds and tertiary amines. Nature
(Lond.), 236: 307.
FIDDLER, W., PENSABENE, J. W., PIOTROWSKI, E.G., DOERR, R. C., &
WASSERMAN, A. E. (1973) Use of sodium ascorbate or erythorbate to
inhibit formation of N-Nitroso-dimethylamine in frankfurters.
J. Food Sci., 38: 1084.
FIDDLER, W., PENSABENE, J. W., FAGAN, J. C., THORN, E. J., PIOTROWSKA,
E.G., & WASSERMAN, A. (1974) The role of lean and adipose tissue
on the formation of nitrosopyrrolidine in fried bacon. J. Food
Sci., 39: 1070-1071.
FIESER, L. F. & FIESER, M. (1967) Reagents for organic synthesis.
New York, Wiley, pp. 747-749.
FINE, D. H. & RUFFEH, F. (1974) Description of the thermal energy
analyser for N- nitroso compounds. IARC Sci. Publ. No. 9,
pp. 40-44.
FINE, D. H., RUFEH, F., & LIEB, D. (1974) Group analysis of volatile
and non-volatile N-nitroso compounds. Nature (Lond.),
247: 309-310.
FINE, D. H., HUFFMAN, F., ROUNBEHLER, D. P., & BETCHER, N.M. (1976a)
Analysis of N-nitroso compounds by combined high- performance
liquid chromatography and thermal energy analysis. IARC Sci.
Publ. No. 14, pp. 43-50.
FINE, D. H., ROUNBEHLER, D. P., BELCHER, N.M., & EPSTEIN, S.S. (1976b)
N-nitroso compounds in air and water. IARC Sci. Publ. No. 14,
pp. 401-408.
FLAKS, A., HAM LTON, J. M., CLAYSON, D. B., & BURCH, P. R. J. (1973)
The combined effect of radiation and chemical carcinogens in
female A x 1F mice. Br. J. cancer, 28: 227-231.
FONG, Y. Y. & CHAN, W. C. (1973a) Dimethylnitrosamine in Chinese
marine salt fish. Food Cosmet. Toxicol., 11: 841-845.
FONG, Y. Y. & CHAN, W. C. (1973b) Bacterial production of
dimethylnitrosamine in salted fish. Nature (Lond.),
243: 421-422.
FONG, Y. Y. & WALSH, E. O'F. (1971) Carcinogenic nitrosamines in
Cantonese salt-dried fish. Lancet, 2: 1032.
FOOD STANDARDS COMMITTEE (1959) Preservatives in food. London, Her
Majesty's Stationery Office.
FOSTER, S.S. D. & CREASE, R. I. (1974) Nitrate pollution of chalk
groundwater in east Yorkshire -- a hydrological appraisal. J.
Inst. Water Eng., 28: 178-194.
FREIMURTH, U. & GLASER, E. (1970) [The appearance of nitrosamines in
food.] Die Nahrung., 14 (5): 357-361 (in German).
FREUND, H. A. (1937) Clinical manifestations and studies in
parenchymatous hepatitis. Ann. int. Med., 10: 1144-1155.
FRIDMAN, A. L., MUKHAMETSIN, F. M., & NOMIKOV, S.S. (1971) [Advances
in the chemistry of aliphatic nitrosamines.] Russ. chem. Rev.,
40 (1): 34-50 (in Russian).
FRIEDMAN, M. A., GREENE, E. J., & EPSTEIN, S.S. (1972) Rapid gastric
absorption of sodium nitrite in mice. J. pharm. Sci.,
61 (9): 1492-1494.
GARBIDGE, M. G. & LEGATOR, M. S. (1969) A host-mediated microbic assay
for the detection of mutagenic contents. Proc. Soc. Exp. Biol.
Med., 130: 831-834.
GARBUZ, A.M. (1968) [The effect of symptomless methaemoglo-binaemia
on some bodily functions.] Author's summary of a thesis for the
degree of Candidate of Medical Sciences, Leningrad (in Russian).
GARBUZ, A.M. (1971) [Changes in the body's functional condition in
symptomless methaemoglobinaemia. In: Methaemoglobinaemia caused
by various factors and methods of preventing it.] Summaries of
papers read at the First Scientific Conference on
Methaemoglobinaemia, Leningrad, pp. 18-21 (in Russian).
GEIL, J. H., STENGER, R. J., BEHKI, R. M., & MORGAN, W. S. (1968)
Hepatotoxic and carcinogenic effects of dimethylnitrosamine in
low dosage. Light and electron microscopic study.
J. Natl Cancer Inst., 40: 713-730.
GELPERIN, A., JACOBS, E. E., & KLETKE, L. S. (1971) The development of
methemoglobin in mothers and newborn infants from nitrate in
water supplies. Ill. med. J., 140: 42-44.
GERRITSEN, G. A. & DE WILLIGEN, A. H. A. (1969) [The nitrite content
of potato and corn starch.] Die Starke, 21: 101-105 (in French).
GILBERTSON, C. B., MCCALLA, T. M., ELLIS, J. R., CROSS, O. E., &
WOODS, W. R. (1970) The effect of animal density and surface
slope on characteristics of runoff solid waste, and nitrate
movement on unpaved feedlots. Lincoln, Univ. Nebraska Coll.
Agric. and Home Econ.
GOBBI, A., SOVERINI, R., & GRISLER, R. (1974) [Normal
methemoglobinemia values observed in 500 inhabitants of the City
of Milan.] Med. Lavoro, 65: 306-310 (in Italian).
GOLDSMITH, J. R., ROKAW, S. N., & SHEARER, L. N. (1975) Distributions
of percentage methemoglobin in several population groups in
California. Int. J. Epidem., 4: 207-212.
GOTO, M. (1973) Inorganic chemicals in the environment -- with special
reference to the pollution problems in Japan. Environ. Qual.
Saf., 2: 72-77.
GROENEN, P. J., JONK, C., VAN INGEN, C., & TEN NOEVER DE BRAUW, M. C.
(1976) Determination of eight volatile nitrosamines in thirty
cured meat products with capillary gas chromatography-high
resolution mass spectrometry: the presence of
nitrosodiethylamine and the absence of nitrosopyrrolidine. IARC
Sci. Publ., No. 14, pp. 321-331.
GREENBERG, R. A. (1974) Ascorbate and nitrosamine formation in cured
meats. In: Proceedings of the International Symposium on
Nitrite in Meat Products, Zeist, 10-14 September, 1973,
Wageningen, Centre for Agricultural Publishing and Documentation,
pp. 179-188.
GREENBLATT, M. (1973) Ascorbic acid blocking of aminopyrine
nitrosation in NZO-Bl mice. J. Natl Cancer Inst.,
50: 1055-1056.
GREENBLATT, M. & MIRVISH, S.S. (1973) Dose-response studies with
concurrent administration of piperazine and sodium nitrite to
strain A mice. J. Natl Cancer Inst., 50 (1): 119-124.
GREENBLATT, M. & RIJHSINGHANI, K. (1969) Comparative cytopathologic
alterations induced by alkylnitrosamines in nasal epithelium of
the Syrian hamster. J. Natl Cancer Inst., 42: 421-433.
GREENBLATT, M., MIRVISH, S., & SO, B. T. (1971) Nitrosamine studies:
Induction of lung adenomas by concurrent administration of sodium
nitrite and secondary amines in Swiss mice. J. Natl Cancer
Inst., 46: 1029-1034.
GREENBLATT, M., KOMMINENI, V., CONRAD, E., WALLCAVE, L., & LIHNSKY, W.
(1972) In vivo conversion of phenmetrazine into its N-nitroso
derivative. Nature (Lond.), 236
(62): 25-26.
GREENBLATT, M., KOMMINENI, V. R. C., & LIJINSKY, W. (1973) Null effect
of concurrent feeding of sodium nitrite and amino acids to MRC
rats. J. Natl Cancer Inst., 50: 799-802.
GRUENER, N. & SHUVAL, H. I. (1970) Health aspects of nitrates in
drinking water. In: Shuval, H. I., ed. Developments in water
quality research. Ann Arbor, Humphrey Science Publ., pp. 89-106.
GRUNTHAL, D., HELLENBROICH, D. O., SANGER, P., & MAAS, H. (1970)[The
effect of partial hepatectomies on the hepatoma rate after
administration of diethyl-nitrosamine.]
Z. Naturforsch., 25: 1277-1281 (in German).
HADJIOLOV, D. (1971) [The inhibition of dimethylnitrosamine
carcinogenesis in rat liver by aminoacetonitrile.]
Z. Krebsforsch., 76: 91-92 (in German).
HAENSZEL, W. & CORREA, P. (1975) - Developments in the epidemiology of
stomach cancer over the past decade. Cancer Res.,
35: 3452-3459.
HANSEN, H. H. & MUGGIA, F. M. (1971) Treatment of malignant brain
tumours with nitrosoureas. Cancer Chemother. Rep. Part 1, 55
(2): 99-100.
HARD, G. C. & BUTLER, W. H. (1970a) Cellular analysis of renal
neoplasia. Induction of renal tumors in dietary-conditioned rats
by dimethylnitrosamine, with a reappraisal of morphological
characteristics. Cancer Res., 30: 2796-2805.
HARD, G. C. & BUTLER, W. H. (1970b) Cellular analysis of renal
neoplasia. Light microscope study of the development of
interstitial lesions induced in the rat kidney by a single
carcinogenic dose of dimethylnitrosamine. Cancer Res.,
30: 2806-2815.
HARD, G. C. & BUTLER, W. H. (1970c) Toxicity of dimethylni-trosamine
for the rat testis. J. Pathol., 102: 201-207.
HARD, G. C. & BUTLER, W. H. (1971a) Ultrastructural aspects of renal
adenocarcinoma induced in the rat by dimethylnitrosamine. Cancer
Res., 31: 366-372.
HARD, G. C. & BUTLER, W. H. (1971b) Ultrastructural study of the
development of interstitial lesions leading to mesenchymal
neoplasia induced in the rat renal cortex by dimethylnitrosamine.
Cancer Res., 31: 337-347.
HARD, G. C. & BUTLER, W. H. (1971c) Morphogenesis of epithelial
neoplasms induced in the rat kidney by dimethylnitrosamine.
Cancer Res., 31: 1496-1505.
HARD, G. C., BORLAND, R., & BUTLER, W. H. (1971) Altered morphology
and behavior of kidney fibroblasts in-vitro following in-vivo
treatment of rats with a carcinogenic dose of
dimethylnitrosamine. Experientia (Basel), 27: 1208-1209.
HARMOZDIAN, H., DAY, N. E., ARAMESH, B., & MAHBOUBI, E. (1975) Dietary
factors and esophageal cancer in the Caspian littoral of Iran.
Cancer Res., 35: 3493-3498.
HASHIMOTO, Y., SUZUKI, E., & OKADA, M. (1972) [Induction of urinary
bladder tumors in ACI/N rats by butyl (3-carboxypropyl)
nitrosamine, a major urinary metabolite of butyl-(4-hydroxbutyl)
nitrosamine.] Gann, 63: 637-638 (in Japanese).
HAWKSWORTH, G. & HILL, M. J. (1971a) The formation of nitrosamines by
human intestinal bacteria. Biochem. J., 122: 28p-29p.
HAWKSWORTH, G. & HILL, M. J. (1971b) Bacteria and the
N-nitrosation of secondary amines. Br. J. Cancer,
25: 520-526.
HAWKSWORTH, G., HILL, M. J., GORDILL, G., & CUELLO, C. (1974)
Possible relationship between nitrates, nitrosamines and
gastric cancer in S.U. Columbia. IARC Sci. Publ. No. 9,
pp. 229-234.
HEATH, D. F. (1962) The decomposition and toxicity of
dialkylnitrosamines in rats. Blochem. J., 85: 72-91.
HEDLER, L. & MARQUARDT, P. (1968) Occurrence of diethynitrosamine in
some samples of food. Food Cosmet. Toxicol, 6: 341-348.
HEDLIN, R. A. (1971) Nitrate contamination of ground water in the
Neepawa-Langruth area of Manitoba. Can. J. Soil Sci,
51: 75-84.
HEIN, G. E. (1963) Reaction of tertiary amines with nitrous acid,
J. chem. Educ, 40 (4): 181-184.
HENDERSON, W. R. & RASKIN, N.H. (1972) "Hot-Dog" headache: Individual
susceptibility to nitrite. Lancet, 2: 1162-1163.
HEPPEL, L. A. & PORTERFIELD, V. T. (1949) Metabolism of inorganic
nitrite and nitrate esters I. The coupled oxidation of nitrite by
peroxide-forming systems and catalase. J. biol. Chem, No. 178,
pp. 549-556.
HERMANN, H. (1960) [P. Methylnitrosaminebenzaldehyde, a metabolic
product of Clytocybe suaveolens.] Naturwiss., 47: 162
(in German).
HEYNS, K. (1973) [On nitrosamines in nutrients.] Getreide Mehl Brot,
27: 249-253 (in German).
HICKS, R. M., WAKEFIELD, J. ST. J., & CHOWANIEC, J. (1973)
Co-carcinogenic action of saccharin in the chemical induction of
bladder cancer. Nature (Lond.), 243: 347-349.
HILDRUM, K. I., SCANLAN, R. A., & LIBBEY, L. M. (1975) Identification
of gamma-butenyl-(beta-propenyl) nitrosamine, the principal
volatile nitrosamine formed in the nitrosation of spermidine or
sperminc. J. agric. Food Chem., 23
(1): 34-37.
HILL, M. J., HAWKSWORTH, G., & TATTERSALL, G. (1973) Bacteria,
nitrosamines and cancer of the stomach. Br. J. Cancer,
28 (6): 562-567.
HMSO (1973) The determination of nitrate-nitrogen in herbage. London,
Her Majesty's Stationery Office, pp. 3 (Tech. Bull. 27).
HMSO (1974) Annual Report 1972, London, Her Majesty's Stationery
Office, pp. 206.
HOCH-LIGETI, C., ARGUS, M. F., & ARCOS, J. C. (1968) Combined
carcinogenic effects of dimethylnitrosamine and 3-methyl-
cholanthrene in the rat. J. Natl Cancer Inst., 40: 535-549.
HOFFMAN, D., HECHT, S.S., ORNAF, R. M., & WYDNER, E. L. (1974)
N-nitrosonornicotine in tobacco. Science, 186: 265-267.
HÖLSCHER, P.M. & NATZSCHKA, J. (1964) [Methaemoglobinaemia in young
children due to the nitrite contents of spinach.] Dtsch Med.
Wschr, 89: 1751-1754 (in German).
HORN, E. E. & HERRIOT, R. M. (1962) The mutagenic action of nitrous
acid on "single-stranded" (denatured) haemophilus transforming
DNA. Proc. Natl Acad. Sci. US, 48: 1409-1416.
HORWlTZ, W. (1975) Association of Official Analytical Chemists,
Official methods of analysis. 12th ed. Washington, DC,
pp. 1094.
HOWARD, J. W., FAZlO, T., & WATTS, J. O. (1970) Extraction and gas
chromatographic determination of N-nitrosodimethy-lamine in
smoked fish application to smoked nitrite-treated chub. J.
Assoc. Off. Anal. Chem., 53(2): 269-274.
INGRAM, M. (1974) The microbiological effects of nitrite. In:
Proceedings of the International Symposium on Nitrite in Meat
Products, Zeist, 10-14 September, 1973, Wageningen, Centre for
Agricultural Publishing and Documentation, pp. 63-75.
IRETON, H. J., McGIVEN, A. R., & DAVIES, D. J. (1972) Renal
mesenchymal tumours induced in rats by dimethylnitrosamine. Light
and electron-microscope studies. J. Pathol., 108: 181-185.
ISSENBERG, P. & TANNENBAUM, S. (1972) Approaches to the
determination of volatile and non volatile N- nitroso
compounds in foods and beverages. IARC Sci. Publ., No. 3,
pp. 31-37.
ITO, N., HIASA, Y., TAMAI, A., OKAJIMA, E., & KITAMORA, H. (1969)
Histogenesis of urinary bladder tumors induced by N-butyl- N-
(4-hydroxybutyl) nitrosamine in rats. Gann, 60: 401-410.
ITO, N., MAKIURA, S., YOKOTA, Y., KAMAMOTO, Y., HIASA, Y., & SUGIHARA,
S. (1971) Effect of unilateral ureter ligation on development of
tumors in the urinary system of rats treated with N-butyl- N-
(4-hydroxybutyl) nitrosamine. Gann, 62: 359-365.
IVANKOVIC, S. & DRUCKREY, H. (1968) [Transplacental induction of
malignant tumours of the nervous system. I Ethylnitrosourea (ENU)
in BD-IX-rats.] Z. Krebsforsch., 71: 320-360 (in German).
IVANKOVIC, S. & PREUSSMANN, R. (1970) [Transplacental induction of
malignant tumours by the oral administration of ethylurea and
nitrite in rats.] Naturwiss., 57: 460-461 (in German).
IVANKOVIC, S., PREUSSMANN, R., SCHMÄHL, D., & gELLER, J. (1973)
[Prevention of nitrosamide induced hydrocephali by ascorbic acid
after prenatal administration of ethylurea and nitrite to rats.]
Z. Krebsforsch, 79: 145-147 (in German).
JACKSON, W. A., STEEL, J. S., & BOSWELL, V. R. (1967) Nitrates in
edible vegetables and vegetable products. Proc. Am. Soc. Hort.
Sci., 90: 349-352.
JAFFÉ, E. R. & HELLER, P. (1964) Methemoglobinemia in man. In: Moore,
C. V. & Brown, E. B., ed. Progress in hematology, vol. IV, New
York, Grune & Stratton, pp. 48-71.
JAPAN ENVIRONMENTAL SANITATION CENTER (1974) [Report of 1973 results
of the analysis for suspended particulate matter in the National
Air Sampling Network.] Kawasaki, Japan, pp. 14, 24 (in Japanese).
JASMIN, G. & CHA, J. W. (1969) Renal adenomas induced in rats by
dimethylnitrosamine. An electron microscopic study. Arch.
Pathol., 87: 267-278.
JENISSEN, H., HOSHINO, J., & KROGER, H. (1971) [The influence of
carcinogenic nitrosamines on the induction of serine dehydratase
in the rat liver.] Z. Krebsforsch., 75: 246-252 (in German).
JOHNSON, J. H. (1966) Internal can corrosion due to high nitrate
content of canned vegetables. Proc. Fla. State Hort. Sci.,
79: 239-242.
JUSKIEWICZ, T. & KOWALSKI, B. (1974) Passage of nitrosamines from
rumen into milk in goats. IARC Sci. Publ. No. 9, pp. 173-176.
KAMM, L., MCKEOWN, G. G., & SMITH, D. M. (1965) New colorimetric
method for the determination of the nitrate and nitrite content
of baby foods. J. Assoc. Off. Agric. Chem., 48 (5): 892-897.
KAMM, J. J., DASHMAN, T., CONNEY, A. H., & BURNS, J. J. (1973)
Protective effect of ascorbic acid on hepatoxicity caused by
sodium nitrite and aminopyrin. Proc. Natl Acad. Sci.,
70: 747-749.
KAPELLER-ADLER, R. & KRAEL, J. (1930a) [Research on nitrogen
distribution in the muscle of different animal species I,]
Biochem. Z., 221: 437-460 (in German).
KAPELLER-ADLER, R. & KRAEL, J. (1930b) [Research on nitrogen
distribution in the muscle of different animal species II.]
Biochem. Z., 224: 364-377 (in German).
KAUDEWITZ, F. (1959) Production of bacterial mutants with nitrous
acid. Nature (Lond.), 183: 1829-1830.
KAWABATA, T. (1974) Addendum, N- nitroso compounds: Recent studies
in Japan. IARC Sci. Publ. No. 9, pp. 154-158.
KEATING, J.P., LELL, M. E., STRAUSS, A. W., ZARKOWSKY, H., & SMITH, G.
E. (1973) Infantile methemoglobinemia caused by carrot juice. N.
Eng. J. Med., 288: 824-826.
KEOHANE, K. W. & METCALF, W. K. (1960) An investigation of the
differing sensitivity of juvenile and adult erythrocytes to
methemoglobinization. Phys. med. Biol., 5: 27-35.
KILGORE, L., STASCH, A. R. & BARRENTINE, B. F. (1963) Nitrate content
of beets, collards, turnip greens. J. Am. Diet. Assoc.,
43: 39-42.
KING, E. J. (1959) Qualitative analysis and electrolytic solutions.
New York, Harcourt, Brace & Co., pp. 519.
KIRKLAND, D. J. & PICK, C. R. (1973) The histological appearance of
tumours derived from rat embryo cells transformed in vitro
spontaneously and after treatment with nitrosomethylurea. Br.
J. Cancer, 28: 440-452.
KLUBES, P. & JONDORF, W. R. (1971) Dimethyl nitrosamine formation from
sodium nitrite and dimethylamine by bacterial flora of rat
intestine. Res. Commun. chem. Pathol. Pharmacol,
2 (1): 24-34.
KNELSON, J. H. & LEE, R. E. (1977) Oxides of nitrogen in the
atmosphere: Origin, fate and public health implications. Ambio,
6 (2-3): 126-130.
KNOTEK, Z. & SCHMIDT, P. (1964) Pathogenesis, incidence and
possibilities of preventing alimentary nitrate methemoglobinemia
in infants. Pediatrics, 34: 78-83.
KOCIBA, R. J. & SLEIGHT, S. D. (1970) Nitrite toxicosis in the
ascorbic acid-deficient guinea pig. Toxicol. appl. Pharmacol.
16: 424-429.
KOHL, D. H., SHEARER, G. B., & COMMONER, B. (1971) Fertilizer
nitrogen: Contribution to nitrate in surface water in a corn belt
watershed. Science, 174: 1331-1334.
KOPPANG, N. (1964) An outbreak of toxic liver injury in ruminants.
Nord. vet. Med., 16: 305-322.
KORDAC, V., SCHON, E., & BRAUN, A. (1969) Hepatocancerogenesis in mice
with simultaneous administration of diethylnitrosamine and the
virus Motol. Neoplasma, 16: 485-490.
KOYAMA, T., HANDA, J., HANDA, H., & MATSUMOTO, S. (1970)
Methylnitrosourea-induced malformations of brain in SD-JCL rat.
Arch. Neurol., 22: 342-347.
KRAVITZ, H., ELEGANT, L. D., KAISER, E., & KAGAN, B. M. (1956)
Methemoglobin values in premature and mature infants and
children. A.M.A.J. Dis. Child., 91: 1-5.
KRÖLLER, E. (1967) [Investigations of the evidence of nitrosamines in
tobacco smoke and food.] Dtsch. Lebensm. Rundsch., 63(10):
303-305 (in German).
KRÜGER, F. W. (1972) [On the biochemistry of carcinogenic
N-nitroso compounds.] Ärztliche Forsch., 26 (6): 197-202 (in
German).
KRÜGER, F. W. (1973) Metabolism of nitrosamines in vivo. II. On the
methylation of nucleic acids by aliphatic di-n-alkylnitrosamines
in vivo, caused by beta-oxidation: The increased formation of
7-methyl-guanine after application of beta-hydroxypropyl-
nitrosamine compared to that after application of di- N-propyl-
nitrosamine. Z. Krebsforsch., 79: 90-97.
KÜBLER, W. (1958) [The importance of the nitrate content of vegetables
in child nutrition.] Z. Kinderheilk, 81: 405-416 (in German).
KÜBLER, W. (1965) [Methaemoglobinaemia in infants after feeding with
spinach.] Dtsch. med. Wschr., 90: 1881-1882 (in German).
KUNZ, W., SCHAUDE, G., & THOMAS, C. (1969) [The effect of
phenobarbital and halogenated hydrocarbons on nitrosamine
carcinogenesis.] Z. Krebsforsch., 72: 291-304 (in German).
KÜNZER, W. & SCHNEIDER, D. (1953) [The activity of the reducing enzyme
system in erythrocytes of young infants.] Acta haemat., 9: 346
(in German).
KUWAHARA, A., OTSUKA, H., & NAGMATSU, A. (1972) Induction of
hemangiomatous lesions with dimethylnitrosamine. Influence of
route of administration and strain of mice. Gann,
63: 499-502.
LACASSAGNE, A., BUU-HOI, N., GIAO, N. B., & FERRANDO, R. (1968)
Retarding effect of resirpine on the cancerization of rat liver
induced by diethylnitrosamine. Bull. Cancer,
55 (1): 87-89.
LEE, D. H. K. (1970a) Nitrates, nitrites and methemoglobinemia.
Environ. Res., 3: 484-511.
LEE, G. F. (1970b) Eutrophication. Occasional Paper No. 2,
University of Wisconsin, Water Resources Center, Madison,
pp. 12-13.
LEE, K. Y. & LIJINSKY, W. (1966) Alkylation of rat liver RNA by cyclic
N-nitrosamines in vivo, J. Natl Cancer Inst., 37
(A): 401-407.
LEHMAN, A. J. (1958) Quarterly report to the editor on topics of
current interest. Nitrates and nitrites in meat products. Assoc.
Food Drug Off. US Q. Bull., 22: 136-138.
LEMBKE, A. & MOEBUS, O. (1970) Is there a connection between the
occurrence of nitrite-producing microorganisms in cheese and the
formation of nitrosamine? Proceedings of the 18th
International Dairy Congress A.2.3, p. 139.
LIJINSKY, W. (1974) Reaction of drugs with nitrous acid as a source of
carcinogenic nitrosamines. Cancer Res., 34: 255-258.
LIJINSKY, W. & EPSTEIN, S.S. (1970) Nitrosamines as environmental
carcinogens. Nature (Lond.), 225: 21-23.
LIJINSKY, W. & GREENBLATT, M. (1972) Carcinogen dimethylnitrosamine
produced in vivo from nitrite and aminopyrine. Nature (New
Biology), 236: 177-178.
LIJINSKY, W. & SINGER, G. (1974) Formation of nitrosamines from
tertiary amines and nitrous acid. IARC Sci. Publ. No. 9,
pp. 111-114.
LIJINSKY, W., TOMATIS, L, & WENGEN, C. E. M. (1969) Lung tumors in
rats treated with N-nitrosoheptamethyleneimine and
N-nitrosooctamethyleneimine. Proc. Soc. Exp. Biol. Med.,
130: 945-949.
LIJINSKY, W., FERRERO, A., MONTESANO, R., & WENYON, C. E. M. (1970)
Tumorigenecity of cyclic nitrosamines in Syrian golden hamsters.
Z. Krebsforsch., 74: 185-189.
LIJINSKY, W., CONRAD, E., & VAN DE BOGART, R. (1972a) Carcinogenic
nitrosamines formed by drug/nitrite interactions. Nature
(Lond.), 239: 165-167.
LIJINSKY, W., CONRAD, E., & VAN DE BOGART, R. (1972b) Formation of
carcinogenic nitrosamines by interaction of drugs with nitrite.
IARC Sci. Publ. No. 3, pp. 130-133.
LIJINSKY, W., KEEFER, L., CONRAD, E., & VAN DE BOGART, R. (1972c)
Nitrosation of tertiary amines and some biologic implications.
J. Natl Cancer Inst., 49 (5): 1239-1249.
LIJINSKY, W., GREENBLATT, M., & KOMMINENI, C. (1973a) Brief
communication: Feeding studies of nitrilotriacetic acid and
derivatives in rats. J. Natl Cancer Inst., 50: 1061-1063.
LIJINSKY, W., KEEFER, L., LOO, J., & ROSS, A. E. (1973b) Studies of
alkylation of nucleic acids in rats by cyclic nitrosamines.
Cancer Res., 33: 1634-1641.
LIJINSKY, W., TAYLOR, H. W., SNYDER, C., & NETTESHEIM, P. (1973c)
Malignant tumors of liver and lung in rats fed aminopyrine or
heptamethyleneimine together with nitrite. Nature (Lond.),
244: 176-178.
LOVELESS, A. & HAMPTON, C. (1969) Inactivation and mutation of
coliphage T2 by N-methyl- and N-ethyl- N-nitrosourea.
Murat. Res., 7: 1-12.
LUHRS, C. E. (1973) Nitrogenous materials in the environment. Draft
report. US Environmental Protection Agency, Hazardous Materials
Advisory Committee.
MAEKAWA, H. & ODASHIMA, S. (1975) Induction of tumours of the nervous
system in the HCI/N rat with N-buyl-I-nitrosourea administered
transplacentally, neonatally or via maternal milk. Gann,
66: 175-183.
MAGEE, P. N. (1956) Toxic liver injury. The metabolism of
dimethylnitrosamine. Biochem. J., 64: 676-682.
MAGEE, P. N. (1972) Possibilities of hazard from nitrosamines in
industry. Ann. occup. Hyg., 15: 19-22.
MAGEE, P. N. & BARNES, J. M. (1962) Induction of kidney tumours in the
rat with dimethyl ( N-nitrosodimethylamine) nitrosamine.
J. Pathol. Bacteriol., 84: 19-31.
MAGEE, P. N. & BARNES, J. M. (1967) Carcinogenic nitroso compounds.
Adv. Cancer Res., 10: 163-246.
MAGEE, P. N., MONTESANO, R., & PREUSSMANN, R. (1976) N-nitroso
compounds and related carcinogens. In: Searle, E. ed., Chemical
carcinogens. ACS monograph No. 173, pp 491-625.
MAKIURA, S., KAMMAMOTO, Y., SUGIHARA, S., HIRAO, K., HIASA, Y., ARAI,
M., & ITO, N. (1973) Effect of 1-naphthyl isothiocyanate and
3-methylcholanthrene on hepato-carcinogenesis in rats treated with
diethylnitrosamine. Gann, 64: 101-104.
MALINS, D.C., ROUBAL, W. T., & ROBISCH, P. A. (1970) The possible
nitrosation of amines in smoked chub. J. agric. Food. Chem.,
18(4): 740-741.
MALLING, H. V. (1966) Mutagenicity of two potent carcinogens
dimethylnitrosamine and diethylnitrosamine in Neurospora
crassa. Mutat. Res., 3: 537-540.
MANNING, P. B., COULTRER, S. T., & JENNESS, R. (1968) Determination of
nitrate and nitrite in milk and dry milk products. J. Dairy
Sci., 51: (11): 1725-1730.
MARCULESCU, V. (1917) Determination of nitrates in water. Stud.
Prot. Epurarea Apelor., 15: 127-161
MARRIOTT, W. M., HARTMANN, A. F., & SENN, M. J. E. (1933) Observations
on the nature and treatment of diarrhea and the associated
systemic disturbances. J. Pediatr., 3: 181-191.
MCLEAN, A. E. M. & MAGEE, P. N. (1970) Increased renal carcinogenesis
by dimethylnitrosamine in protein deficient rats. Br. J. exp.
Pathol., 51: 587-590.
MCLEAN, A. E. & VERSCHUUREN, H. G. (1969) Effects of diet and
microsomal enzyme induction on the toxicity of
dimethylnitrosamine. Br. J. exp. Pathol., 50: 22-25.
METCALF, W. K. (1961) The sensitivity of haemoglobin to oxidation in
various conditions. Nature (Lond.), 190: 543-544.
MIRNA, A. (1974) Determination of free and bound nitrite. In:
Proceedings of the International Symposium on Nitrite in Meat
Products, Ziest, 10-14 September 1973, Wageningen, Centre for
Agricultural Publishing and Documentation, pp. 21-28.
MIRVISH, S.S. (1970) Kinetics of dimethylamine nitrosation in relation
to nitrosamine carcinogenesis. J. Natl Cancer Inst., 44
(3): 633-639.
MIRVISH, S.S. (1971) Kinetics of nitrosamide formation from
alkylureas, N-alkylurethanes and alkylguanidines: possible
implications for the etiology of human gastric cancer. J. Natl
Cancer Inst., 46: 1183-1193.
MIRVISH, S.S. (1975) Formation of N-nitroso compounds: chemistry,
kinetics, and in vivo occurrence. Toxicol. appl. Pharmacol,
31: 325-351.
MIRVISH, S.S. (in press) N-nitroso compounds, nitrite and nitrate:
possible implications for the causation of human cancer. J.
Water Pollut.
MIRVISH, S., WALLCAVE, L., EAGEN, M., & SHUBIK, P. (1972) Ascorbate-
nitrite reaction; possible means of blocking the formation of
carcinogenic N-nitroso compounds. Science, 177: 65-68.
MIRVISH, S.S., SAMS, J., FAN, T. Y., & TANNENBAUM, S. R. (1973a)
Kinetics of nitrosation of the amino acids proline hydroxyproline
and sarcosine. J. Natl Cancer Inst., 51: 1833-1840.
MIRVISH, S.S., CARDESA, A., WALLCAVE, L., & SHUBIK, P. (1973b) Effect
on sodium ascorabte on lung adenoma induction by amines plus
nitrite. Proc. Am. Assoc. Cancer Res., 14: 102.
MIRVISH, S.S., ARNOLD, S., CONRAD, E., GHADIRIAN, P., KOMMINENI, V. R.
C., & SAMS, J. (1974) The formation of N- nitroso compounds,
preparation of heptafiuorobutyryl derivatives of ureas, and the
fate of nitrite in the rat stomach. IARC Sci. Publ. No. 9,
pp. 67-70.
MIRVISH, S.S., CARDESA, A., WALLCAVE, L., & SHUBIK, P. (1975)
Induction of mouse lung adenomas by amines or ureas plus nitrite
and by N-nitroso compounds: effects of ascorbates, gallic acid,
thyocyanate, and caffeine. J. Natl Cancer Inst., 55: 633-636.
MIRVISH, S.S., ISSENBERG, P., & SORNSON, H. C. (1976) Air-water and
ether-water distribution of N-nitroso compounds: implications
for laboratory safety, analytical methodology, and
carcinogenicity for the rat esophagus, nose, and liver. J. Natl
Cancer Inst., 56 (6): 1125-1129.
MÖHLER, K. & MAYRHOFER, O. L. (1968) [Evidence and determination of
nitrosamines in food.] Z. Lebensm. Unter-Forsch., 135 (6):
313-318 (in German).
MÖHLER, K. & MAYRHOFER, O. L. (1969) [Effect of various factors on the
formation of nitrosamines in meat products.] 15th European
Meeting of the Meat Research Workers, Helsinki, 17-24 August
1969, pp. 302-304 (in German).
MOHR, U. & HILFRICH, J. (1972) Brief communication: Effect of a single
dose of N-diethylnitrosamine on the rat kidney. J. Natl
Cancer Inst., 49: 1729-1731.
MONTESANG, R. (1970) Systemic carcinogens ( N-nitroso compounds) and
synergistic or additive effects of respiratory carcinogenesis.
Tumori, 56: 335-344.
MONTESANO, R. & BARTCH, H. (1976) Mutagenic and carcinogenic
N-nitroso compounds: possible environmental hazards. Mutat.
Res., 32: 179-228.
MONTESANO, R. & SAFFIOTTI, U. (1968) Carcinogenic response of the
respiratory tract of Syrian golden hamsters to different doses of
diethylnitrosamine. Cancer Res., 28: 2197-2210.
MOTYLEV, V. D. (1969) [The question of age-related methaemoglobinaemia
caused by nitrates and nitrites and its prophylaxis.] Author's
summary of a thesis for the degree of Candidate of Medical
Sciences, Leningrad, (in Russian).
MURTHY, Y. K. S., THIEMANN, J. E., CORONELLI, C. I., & SENSl, P.
(1966) Alanosine, a new antiviral and antitumour agent isolated
from a streptomyces. Nature (Lond.), 211: 1198-1199.
MYSLIWY, T. S., WICK, E. L., ARCHER, M. C., SHANK, R. C., & NEWBERNE,
P.M. (1974) Formation of N-nitrosopyrrolidine in a dog's
stomach. Br. J. Cancer, 30: 279-283.
NAGAWA, Y. & MIRNA, A. (1974) [Influence exerted by processing upon
the formation of nitrosamines in meat products.]
Fleischwirtschaft., 1781-1788 (in German).
NAPALKOV, N. P. (1971) [Experimentation on transplacental
blastomogenesis as a tool for study of etiopathogenesis of
tumours in children.] Vop. Onkol., 17 (8): 3-15 (in Russian).
NASHED, N. & JUBBUR, G. (1966) A genetic and functional
characterization of adenine mutants induced in yeast by
1-nitroso-imidazolidone-2 and nitrous acid. Z. Verer-bungsl,
98: 106-110.
NEURATH, H. (1972) Nitrosamine formation from precursors in tobacco
smoke. IARC Sci. Publ. No. 3, pp. 134-136.
NEURATH, G. & SCHREIBER, O. (1974) Investigations on amines in the
human environment. Sci. Publ. No. 9, pp. 211-214.
NEURATH, G., PIRMANN, B., & WICHERNOTT, (1964) [On the question of
nitroso compounds in tobacco smoke.] Beitr. Tabakforsch.,
2(7): 311-319 (in German).
NEWBERNE, P.M. & SHANK, R. C. (1973) Induction of liver and lung
tumors in rats by the simultaneous administration of sodium
nitrite and morpholine. Food Cosmet. Toxicol., 11: 819-825.
NEWELL, J. E. & SISKEN, H. R. (1972) Determination of nitrosodi-
methylamine in the low parts per billion. J. agric. Food Chem.,
20 (3): 711-713.
NIEHS (1970) Nitrates, nitrites and methemoglobinemia. North
Carolina, National Institute of Environmental Health Sciences,
pp. 43 (Rep. No. 2).
NOROHNA, R. F. & GOODALL, C. M. (1972) Nasal tumors in starved rats
injected once with dimethylnitrosamine. N. Z. med. J.,
75: 374-375.
NYE, J. C. (1973) Nitrogenous compounds in the environment. Draft
Report, Washington, DC, US Environmental Protection Agency,
Hazardous Materials Advisory Committee, pp. 95-109.
O'CONNOR, P. J., CAPPS, M. J., & CRAIG, A. W. (1973) Comparative
studies of the hepatocarcinogen N,N-dimethylnitrosamine in
vivo: reaction sites in rat liver DNA and the significance of
their relative stabilities. Br. J. Cancer, 27: 153-166.
ODASHIMA, S. (1969) Experimental carcinoma of the glandular stomach in
rats. I. Effect of 7, 12-dimethylbenz-[a]anthracene or
4-nitroquinolene 1-oxide placed on glandular stomach combined with
oral administration of N,N1-(2,7-fluorenylene) bisacetamide
or N-nitrosodiethylamine. Gann, 60: 211-222.
OEHME, F. W. (1975) Vetinary toxicology. In: Caserett, L. J. & Doulle,
J., ed. Toxicology, the basic science of poisons. New York,
Macmillan Publishing Co., Inc., pp. 701-727.
OKADA, M. & SUZUKI, E. (1972) Metabolism of butyl (4-hydroxybutyl)
nitrosamine in rats. Gann, 63 (3): 391-392.
OKADA, M., SUZUKI, E., ANJYO, T., & MOCHIZUKI, M. (1975) Mutagenicity
of gamma-acetoxy-dialkylnitrosamines: model compounds for an
ultimate carcinogen. Gann, 66 (4): 457-458.
OKAJIMA, E., HIRAMATSU, T., MOTOMIYA, Y., IRWA, K., IJUIN, M., & ITO,
N. (1971) Effect of DL-tryptophan on tumorigenesis in the urinary
bladder and liver of rats treated with N-nitrosodibutylamine.
Gann, 62: 163-169.
OLSON. O. E., NELSON, D. L., & EMERICK, R. J. (1963) Effect of nitrate
and some of its reduction products on carotine stability.
J. agric. Food Chem, 11: 140-143.
ORGERON, J. D., MARTIN, J. D., CARAWAY, C. T., MARTINE, R. M., &
HAUSER, G. H. (1957) Methemoglobinemia from eating meat with high
nitrite content. Public Health Rep., 72 (3): 189-193.
OSSKE, G., WARZOK, R., & SCHNEIDER, J. (1972) [Transplacental tumour
induction by endogenous N-ethyl- N-nitrosourea in the rat.]
J. Arch. Geschwulstforsch., 40: 244-247 (in German).
OWENS, M. (1970) Nutrient balances in rivers. Proc. Soc. Water
Treat. Exam., 19: 239-247.
PANALAKS, T., IYENGAR, J. R., & SEN, N. P. (1972) Nitrate, nitrite and
dimethylnitrosamine in cured meat products. J. Assoc. Off.
Anal. Chem, 56 (3): 621-625.
PANALAKS, T., IYENGAR, J. R., DONALDSON, B. A., MILES, W. F., & SEN.
N. P. (1974) Further survey of cured meat products for volatile
N-nitrosamines. J. Assoc. Off. Anal. Chem., 57 (4): 806-812.
PASTERNAK, L. (1962) [Mutagenic effects of dimethylnitrosamine with
Drosophila melanogaster.] Naturwiss., 16: 381 (in German).
PASTERNAK, L. (1964) Investigation into the mutagenic effect of
several nitrosamine and nitrosamide compounds. Arzneintittel-
Forsch., 14: 802-804.
PENSABENE, J. W., FIDDLER, W., GATES, R. A., FAGAN, J. C., &
WASSERMAN, A. E. (1974) Effect of frying and other cooking
conditions on nitrosopyrrolidine formation in bacon.
J. Food Sci., 39: 314-136.
PERRY, T. L., SHAW, K. N. F., WALKER, D., & REDLICH, D. (1962) Urinary
excretion of amines in normal children. Pediatrics, 30: 576-584.
PETUKHOV, N. I., RYVKIN, A. I., GAINULLEN, G. G., & LANDYSHEVA, V. I.
(1972) [Methaemoglobinaemia in children and adults caused by
nitrates in water] Gig. i Sanit., 3: 14-17 (in Russian).
PETUKHOV, N. I. & IVANOV, A. V. (1970) Investigation of certain
psychophysiological reactions in children suffering from
methemoglobinemia due to nitrates in water. Gig. i Sanit.,
35 (1): 26-28.
PHILLIPS, J. C., LAKE, B. G., HEADING, C. E., GANGOLLI, S. D., &
LLOYD, A. G. (1975a) Studies on the metabolism of dimethyl-
nitrosamine in the rat. I. Effect of dose, route of
administration and sex. Food Cosmet. Toxicol., 13: 203-209.
PHILLIPS, J. C., HEADING, C. E., LAKE, B. O., GANGOLLI, S. D., &
LLOYD, A. G. (1975b) Studies on the metabolism of
dimethylnitrosamine in the rat. II. The effects of phenobarbitone
and 20-methylcholanthrene on the in vitro and in vivo
metabolism and acute toxicity of dimethylnitrosamine in young
and mature rats. Food Cosmet. Toxicol., 13: 611-617.
PHILLIPS, W. E. J. (1966) Effect of dietary nitrite on the liver
storage of vitamin A in the rat. Can. J. Blochem.,
44 (1): 1-7.
PHILLIPS, W. E. J. (1968a) Changes in the nitrate and nitrite contents
of fresh and processed spinach during storage. J. agric. Food
Chem., 16 (1): 88-91.
PHILLIPS, W. E. J. (1968b) Nitrate content of foods -- public health
implications. Can. Inst. Food Technol. J., 1 (3): 98-103.
PHILLIPS, W. E. J. (1969) Lack of nitrite accumulation in partially
consumed jars of baby food. Can. Inst. Food Technol. J.,
2 (4): 160-161.
PHILLIPS, W. E. J. (1971) Naturally occurring nitrate and nitrite in
foods in relation to infant methemoglobinemia. Food Cosmet.
Toxicol., 9: 219-228.
PIELSTICKER, K. (1967) Absence of malformations following
diethylnitrosamine in the golden hamster. Naturwiss.,
54: 340-341.
PISCIOTTA, A. V., EBBE, S. N., & HINZ, J. E. (1959) Clinical and
laboratory features of two variants of methemoglobin M disease.
J. Lab. clin. Med, 54: 73-87.
PITTS, J. N. Jr. & LLOYD, A. C. (1973) Discharges into the atmosphere.
In: Nitrogenous compounds in the environment. Washington, US
Environmental Protection Agency, pp. 43-65 (Rep No. EPA-SAB-73-
001).
PLATT, D. & HERING, F. J. (1973) The effect of Calciparin on
diethylnitrosamine-induced liver tumors in the rat.
Arzneimitteljbrsch., 23 (7): 956-961.
POUND, A, W. LAWSON, T. A., & HORN, L. (1973) Increased carcinogenic
action of dimethylnitrosamine after prior administration of
carbon tetrachloride. Br. J. Cancer, 27: 451-459.
PREDA, N., POPA, L., GALEA, V., & SIMU, G. (1976) N-nitroso compound
formation by chiordiazepoxide and nitrite interaction in vitro
and in vivo: protective action of ascorbic acid. IARC Sci.
Publ. No. 14, pp. 301-304.
PREUSSMAN, R. (1975) [Carcinogenic chemicals in the human
environment.] Handbuch der Allg. Pathol., VI/6/11. 448-482 (in
German).
PURCHASE, I. F. H., TUSTIN, R. C., & van RENSBERG, S. J. (1975)
Biological testing of food grown in the Transkei. Food Cosmet.
Toxicol, 13: 639-647.
QUISPEL, A., ed. (1974) -- The biology of nitrogen fixation.
Amsterdam, North Holland Publishing Company,
RABES, H., HARTENSTEIN, R., & SCHOLZE, P. (1970) Specific stages of
cellular response to homeostatic control during
diethylnitrosamine-induced liver carcinogenesis. Experientia
(Basel), 26: 1356-1359.
RABES, H., HARTENSTEZN, R., & GMINDER, J. (1971) Kidney neoplasms
induced by diethylnitrosamine in partially hepatectomized rats.
Naturwiss., 58: 102-103.
RAMMELL, C. G. & JOERIN, M. N. (1972) Determination of nitrite in
cheese and other dairy products. J. Dairy Res., 39: 89-94.
REDDY, B. S. & THOMAS, J. W. (1962) Interrelationship between thyroid
status and nitrate on carotine conversion. J. anim. Sci.,
21: 1010.
REEVES, M. J., BIRTLES, A. B., COURCHE, R. C., & ALDRICK, R. J. (1974)
Groundwater resources of the Vale of York. London, Water
Resources Board Report, pp. 90.
RHOADES, J. W. & JOHNSON, D. E. (1970) Gas-chromatography and
selective detection of N-nitrosamines. J. Chromatogr. Sci.,
8: 616-618.
RICHARDSON, W. D. (1907) Nitrates in vegetable foods in cured meats
and elsewhere. J. Am. Chem. Soc, 29: 1757-1767.
RIDDER, W. E. & OEHME, F. W. (1974) Nitrates as an environmental,
animal, and human hazard. Clin. Toxicol 7 (2): 145-159.
RIEDEL, B. & PIPER, R. (1973) Early changes of the rat bladder mucosa
after experimental cancerogenesis with N-butyl- N-butanol
(4)-nitrosamine.] (author's transl.). Urol. Int, 28: 322-327.
ROBERTS, J. D. & CASERIO, M. C. (1964) Basic Principles of Organic
Chemistry, New York, Benjamin, pp. 656-666.
ROBERTS, W. K. & SELL, J. L. (1963) Vitamin A destruction by nitrite
in vitro and in vivo. J. anim. Sci., 22: 1081-1085.
ROBERTSON, H. E. & DRAYCOTT, M. E. (1948) Nitrate poisoning of
infants by contaminated drinking water. Abstracts of papers
presented at the fifteenth Annual Christmas Meeting of the
Laboratory Section, Canadian Public Health Association, London,
Ontario, 13-14 December, p. 30.
ROBERTSON, H. E. & RIDDELL, W. A. (1949) Cyanosis of infants produced
by high nitrate concentration in rural waters of Saskatchewan.
Can. J. Public Health, 40: 72-77.
ROBINSON, E. & ROBBINS, R. C. (1972) Emissions, concentrations and
fate of gaseous atmospheric pollutants. In: Strauss, W., ed. Air
pollution control Part II, New York, Wiley-Interscience,
pp. 1-93,
ROLLER, P. P. & KEEFER, L. K. (1974) Catalysis of nitrosation
reactions by electrophilic species. IARC Sci. Publ. No. 9,
pp. 86-89.
ROSS, J. D. (1963) Deficient activity of DPNH-dependent methemoglobin
diaphorase in cord blood erythrocytes. Blood, 21 (1): 51-62.
ROSS, J. D. & DESFORGES, J. F. (1959) Reduction of methemoglobin by
erythrocytes from cord blood. Pediatrics, 23: 718-726.
SAKSHAUG, J., SÖGNEN, E., HANSEN, M. A., & KOPPANG, N. (1965)
Dimethylnitrosamine; its hepatotoxic effect in sheep and its
occurrence in toxic batches of herring meal. Nature (Lond.),
206: 1261-1262.
SANDER, J. (1971a) [Investigations on the formation of carcinogenic
nitroso compounds in the stomach of experimental animals and its
significance for man. I] Arzneimittel-Forsch., 21: 1572-1580
(in German).
SANDER, J. (1971b) [Investigations on the formation of carcinogenic
nitroso compounds in the stomach of experimental animals and its
significance for man.] Arzneimittel-Forsch., 21: 1703-1707 (in
German).
SANDER, J. (1971c)[Further experiments on tumour induction by oral
administration of small doses of N-methylbenzylamine and sodium
nitrite.] Z. Krebsforsch., 76: 93-96 (in German).
SANDER, J. & BÜRKLE, G. (1969) [Induction of malignant tumors in rats
by simultaneous feeding of nitrite and secondary amines.]
Z. Krebsforsch., 73: 54-66 (in German).
SANDER, J. & SEIF, F. (1969) [Bacterial reduction of nitrate in the
stomach of man as a cause of nitrosamine formation.]
Arzneimittel-Forsch, 19: 1091-1093 (in German).
SANDER, J. & SCHWEINSBERG, F. (1972) In vivo and in vitro
experiments on the formation of N- nitroso compounds from
amines or amides and nitrate or nitrite. IARC Sci. Publ. No. 3,
pp. 97-103.
SANDER, J. & SCHWEZNSBERG, F. (1973) [Tumor induction in mice with
methylbenzylnitrosamine in low doses.] Z. Krebsforsch,
79: 157-161 (in German).
SANDER, J., SCHWEINSBERG, F., & MENZ, H. P. (1968) [Investigations on
the formation of carcinogenic nitrosamine in the stomach.]
Hoppe-Seyler's Z. physiol. Chem, 349: 1691-1697 (in German).
SANDER, J., BURKLE, G., FLOHE, L., & AEIKENS, B. (1971) [ In vitro
investigations on the possibility of the formation of
carcinogenic nitrosamide in the stomach.] Arzneim-Forsch.,
(Drug Res.), 21 (3): 411-414 (in German).
SANDER, J., LABAR, J., LADENSTEIN, M., & SCHWEINSBERG, F. (1974a)
Quantitative measurement of in vivo nitrosamine formation.
IARC Sci. Publ. No. 9, pp. 123-131.
SANDER, J., LABAR, J., LADENSTEIN, M., & SCHWEINSBERG, F. (1974b)
Experiments on the degradation of nitrosamines in plants. IARC
Sci. Publ. No. 9, pp. 205-210.
SATTELMACHER, P. G. (1962) [Methemoglobinemia from nitrates in
drinking water.] Schriflenr. Ver. Wasser Boden. Lufthyg., No. 21
(in German).
SAWYER, C. N. (1947) Fertilization of lakes by agricultural and urban
drainage. J. New England Water Works Assoc., 109-127.
SCANLAN, R. A. (1975) Nitrosamines in food. CRC Critical Reviews in
Food Technology, April 1975, pp. 357-402.
SCHERER, E., HOFFMAN, M., EMMELOT, P., & FRIEDRICH-FRESKA, M. (1972)
Quantitative study on foci of altered liver cells induced in the
rat by a single dose of diethylnitrosamine and partial
hepatectomy. J. Natl Cancer Inst., 49: 93-106.
SCHEUNIG, O. & ZIEBARTH, D. (1976) Formation of nitrosamines by
interaction of some drugs with nitrite in human gastric juice.
IARC Sci. Publ. No. 14, pp. 269-277.
SCHMÄHL, D. (1963) [The origin, growth and chemotherapy of malignant
tumours.] Arzneimittei-Forsch., 13 (Supplement): 612 (in
German).
SCHMÄHL, D. (1970) [Experimental studies on syncarcinogenesis VI: The
addition of minimal doses of four different hepatotropic
carcinogens in liver cancer induction in the rat.]
Z. Krebsforsch., 74: 457-466 (in German).
SCHMÄHL, D. & STACKELBERG, S. YON (1968)[The influence of lactoflavin,
nicotinamide or dipyridamole on the carcinogenic activity of
diethylnitrosamine in rats.] Arzneimittel-Forsch., 18: 318-320
(in German).
SCHMÄHL, D. & THOMAS, C. (1962) [Acute nitrosomethylurethane poisoning
in rats following oral application.] Arzneimittel-Forsch.,
12: 585-587 (in German).
SCHMÄHL, D., THOMAS, C., & KÖNIG, K. (1963) [Studies of
syncarcinogenesis: I. Experiments on the induction of cancer in
rats by the simultaneous administration of diethyinitrosamine and
4-dimethylamino-azobenzene.] Z. Krebsforsch., 65: 351-377
(in German).
SCHMÄHL, D., THOMAS, C., SATTLER, W., & SCHIELD, G. F. (1965)
[Experimental studies of syncarcinogenesis: III. Attempts to
induce cancer in rats by administering diethylnitrosamine and
CC14 (or ethyl alcohol) simultaneously. In addition, an
experimental contribution regarding "alcoholic cirrhosis".]
Z. Krebsforsch., 66: 526-532 (in German).
SCHMÄHL, D., STUTZ, E., & THOMAS, C. (1966) [Experimental studies on
syncarcinogenesis. V. Experiments on cancer generation in rats
with the simultaneous application of X-ray radiation and
diethylnitrosamine or 4-dimethyl-aminobiphenyl.]
Z. Krebsforsch., 68: 68-72 (in German).
SCHMÄHL, D., WAGNER, R., & SCHERF, H. R. (1971) [Influence of
immunosuppressive drugs on cancer formation in rat liver by
diethyinitrosamine and on the induction of fibrosarcoma by
3,4-benzopyrene.] Arzneimittel-Forsch, 21 (3): 403-404
(in German).
SCHMIDT, P. & KNOTEK, Z. (1970) Epidemiological evaluation of
nitrates as ground water contaminants in Czechoslovakia (Paper
presented to the Sixth International Water Pollution Research
Conference, San Francisco).
SCHMID-RUPPIN, K. H. & PAPADOUPOULU, G. (1972) [Effect of
diethylnitrosamine (DENA) and influenza viruses on the induction
of lung carcinomas in mice.] Z. Krebsforsch., 77: 150-154
(in German).
SCHMITZ-MOORMAN, P., GEDIGK, P., & DHARAMADHACH, A. (1972) [Early
histological and histochemical changes during experimental
production of liver carcinoma by diethylnitrosamine.]
Z. Krebsforsch, 77: 9-16 (in German).
SCHNEIDER, N. R. & YEARY, R. A. (1973) Measurement of nitrite and
nitrate in blood. Am. J. vet. Res., 34 (1): 133-136.
SCHOENTAL, R. (1960) Carcinogenic action of diagomethane and of
nitroso- N-methylurethane. Nature (Lond.), 188: 420-421.
SCHREIBER, H., NETTESHEIM, P., LIJINSKY, W., RICHTER, C. B., &
WALBURO, H. E. (1972) Induction of lung cancer in germfree,
specific-pathogen-free and infected rats by
N-nitrosoheptamethyleneimine. Enhancement by respiratory
infection. J. Natl Cancer Inst., 49: 1107-1114.
SCHUPHAN, W. (1965) [The nitrate content of spinach (Spinacia
oleracea, L) in relation to methaemoglobinaemia in children.]
Z. Ernähr. Wiss., 5 (3-4): 207-209 (in German).
SCHUPHAN, W. (1969) [The formation of nitrate and nitrite in plant
metabolism.] Nutritio Dieta, 11: 120-132 (in German).
SCHWEINSBERG, F. & SANDER, J. (1972) [Carcinogenic nitrosamine from
simple aliphatic tertiary amines and nitrite.] Hoppe-Seyler's
Z. physiol. Chem., 353: 1671-1676 (in German).
SEBRANEK, J. G., CASSENS, R. G., & HOEKSTRA, W. G. (1974) Fate of
added nitrite. In: Proceedings of the International Symposium
on Nitrite in Meat Products, Ziest, 10-14 September, 1973,
Wageningen, Centre for Agricultural Publishing and
Documentation, pp. 139-147.
SEHGAL, O. P. & KRAUSE, G. F. (1968) Efficiency of nitrous acid as an
inactivating and mutagenic agent of intact tobacco mosaic virus
and its isolated nucleic acid. J. Virol., 2 (10): 966-971.
SELENKA, F. (1970) Formation and reduction of nitrite in infant
nutrients containing nitrate. Archiv. Hyg., 154 (4): 336-345.
SELL, J. L. & ROBERTS, W. K. (1963) Effects of dietary nitrite on the
chick: Growth, liver vitamin A stores and thyroid weight. J.
Nutr, 79: 171-178.
SEN, N. P. (1970) Gas-liquid chromatographic determination of
dimethylnitrosamine as dimethylnitramine at picogram levels.
J. Chromatogr, 51: 301-304.
SEN, N. P. (1972) The evidence for the presence of dimethylnitrosamine
in meat products. Food Cosmet. Toxicol., 10: 219-223.
SEN, N. P. (1974) Nitrosamines in toxic constituents in animal
foodstuffs. New York, Academic Press Inc., pp. 131.
SEN, N. P. & DALPE, C. (1972) A simple thin-layer chromatographic
technique for the semi-quantitative determination of volatile
nitrosamines in alcoholic beverages. Analyst,
97: 216-220.
SEN, N. P. & DONALDSON, B. (1974) The effect of ascorbic acid and
glutathiane on the formation of nitrosopiperazines from
piperazine adipate and nitrite. IARC Sci. Publ.
No. 9, pp. 103-106.
SEN, N. P., SMITH, D.C., SCHWINGHAMER, L., & MARLEAU, J. J. (1969a)
Diethylnitrosamine and other N-nitrosamines in foods. J.
Assoc. Off. Anal. Chem., 52 (1): 47-52.
SEN, N. P., SMITH, D.C., & SCHWINGHAMER, L. (1969b) Formation of
N-nitrosamines from secondary amines and nitrite in human and
animal gastric juice. Food Cosmet. Toxicol., 7: 301-307.
SEN, N. P., SMITH, D.C., SCHWINGHAMER, L., & HOWSAM, B. (1970)
Formation of nitrosamines in nitrite-treated fish. Can. Inst.
Food Technol. J., 3 (2): 66-69.
SEN, N. P., SCHWINGHAMER, L. A., DONALDSON, B. A., & MILES, W. F.
(1972) N-nitrosodimethylamine in fish meal. J. agric. Food
Chem., 20 (6): 1280-1281.
SEN, N. P., DONALDSON, B., IYENGAR, J. R., & PANALAKS, T. (1973a)
Nitrosopyrrolidine and dimethylnitrosamine in bacon. Nature
(Lond.), 241: 473-474.
SEN, N. P., MILES, W. F., DONALDSON, B., PANALAKS, T., & IYENGAR, J.
R. (1973b) Formation of nitrosamines in a meat curing mixture.
Nature (Lond.), 245: 104-105.
SEN, N. P., IYENGAR, J. R., DONALDSON, B. A., & PANALAKS, T. (1974a)
Effect of sodium nitrite concentration on the formation of
nitrosopyrrolidine and dimethylnitrosamine in fried bacon. J.
agric. Food Chem, 22 (3): 540-541.
SEN, N. P., DONALDSON, B., CHARBONNEAU, C., & MILES, W. F. (1974b)
Effect of additives on the formation of nitrosamines in meat
curing mixtures containing spices and nitrite. J. agric. Food
Chem., 22 (6): 1125-1130.
SEN, N. P., IYENGAR, J. R., MILES, W. F., & PANALAKS, T. (1976)
Nitrosamines in cured meat products. IARC Sci. Publ.
No. 14, pp. 333-342.
SERFONTEIN, W. J. & HURTER, P. (1966) Nitrosamines as environmental
carcinogens. II. Evidence for the presence of nitrosamines in
tobacco smoke condensate. Cancer Res., 26: 575-579.
DE SERRES, F. J., BROCKMAN, H. E., BARNETT, W. E., & KOLMARK, H. G.
(1967) Allelic complementation among nitrous acid-induced ad-3B
mutants of Neurospora crassa. Mutat. Res., 4: 415-424.
SHANK, R. C. (1975) Toxicology of N-nitroso compounds. Toxicol.
appl. Pharmacol., 31: 361-368.
SHANK, R. C. & NEWnERNE, P.M. (1972) Nitrite-morpholine induced
hepatomas. Food Cosmet. Toxicol., 10: 887-888.
SHEARER, L. A., GOLDSMITH, J. R., YOUNG, C., KEARNS, O. A., & TAMPLIN,
B. R. (1972) Methaemoglobin levels in infants in an area with
high nitrate water supply. Am. J. Public Health, 62
(9): 1174-1180.
SCHECHTER, H., GARDNER, N., & SHUVAL, H. I. (1972) A micromethod for
the determination of nitrite in blood. Anal. Chem. Acta.,
60: 93-99.
SHUVAL, H. I. & GRUENER, N. (1972) Epidemiological and toxicological
aspects of nitrates and nitrites in the environment. Am. J.
Public Health, 62: 1045-1052.
SIBAY, T. M. & HAYES, J. A. (1969) Potential carcinogenic effect of
streptozotocin. Lancet, II: 912.
SIBERT, D. & EISENBRAND, G. (1974) Induction of mitotic gene
conversion in Saccharomyces cerevisae by N-nitrosated
pesticides. Mutat. Res., 22: 121-126.
SIDDIQI, O. H. (1962) Mutagenic action of nitrous acid on
Asperigillus nidulans. Genet. Res., 3 (2): 303-314.
SIMON, C. (1966) Nitrite poisoning from spinach. Lancet, I
(7442): 872.
SIMON, C., MANZKE, H., KAY, H., & MROWETZ, G. (1964) On the
occurrence, pathogenesis, and possibilities for prophylaxis of
the methemoglobinemia caused by nitrite. Z. Kinderheilkunde,
91: 124-138.
SIMONEIT, B. R. & BURLINGAME, A. L. (1971) Organic analyses of
selected areas of Surveyor III recovered on the Apollo 12
mission. Nature (Lond.), 234: 210-211.
SINGLEY, T. L. (1962) Secondary methemoglobineamia due to the
adulteration of fish with sodium nitrite. Ann. internal Med.,
57 (5): 800-803.
SINHA, D. P. & SLEIGHT, S. D. (1971) Pathogenesis of abortion in acute
nitrite toxicosis in guinea pigs. Toxicol. appl. Pharmacol.,
18 (2): 340-347.
SINIOS VON, A. & WODSAK, W. (1965) [Spinach toxicity in children.]
Dtsch Med. Wochenschr, 90: 1856-1863 (in German).
SKRIVAN, J. (1971) Methemoglobinemia in pregnancy. Acta
Universitatis Carolinae Medica, 17: 123-160.
SLEIGHT, S. D. & ATALLAH, O. A. (1968) Reproduction in the guinea pig
as affected by chronic administration of potassium nitrate and
potassium nitrite. Toxicol. appl. Pharmacol., 12: 179-185.
SMITH, C. H. (1972) Polycythemia, methemogiobinemia,
sulfhemoglobinemia, and miscellaneous anemias In: Blood
diseases of infancy and childhood, 3rd ed., Saint Louis, The C.
V. Mosby Company, pp. 450-454.
SMITH, G. E. (1964) Nitrate problems in plants and water supplies in
Missouri. In: 2nd Annual Symposium on Relation of Geology and
Trace Elements to Nutrition.
SMITH, G. E. (1966) Causes of nitrate accumulation in plants and water
supplies. In: 18th Annual Midwest Fertilizer Conference,
Chicago, Illinois.
SMITH, J. E. & BEUTLER, E. (1966) Methemoglobin formation and
reduction in man and various animals species. Am. J. Physiol.,
210: 347-350.
SMITH, P. A. S. & LOEPPKY, R. N. (1967) Nitrosative cleavage of
tertiary amines. J. Am. Chem. Soc., 89 (5): 1147-1157.
SMITH, R. P. (1969) The significance of methaemoglobinaemia in
toxicology. In: Blood, F. R., ed. Essays in toxicology Volume
I, New York and London, Academic Press, pp. 84-113.
SMITH, R. P. (1975) Toxicology of the formed elements of the blood.
In: Casseret, L. J. & Doull, J., ed. Toxicology the basic
science of poisons. New York, Macmillan Publishing Co., Inc.,
pp. 225-243.
SPENCER, R. (1967) A study of factors affecting the quality and
shelf life of vacuum packed bacon, and of the behaviour of
Wiltshire cooked bacon packed and stored under controlled
conditions. (BFMIRA Res. Rep. No. 136).
SPIEGELHALDER, B., EISENBRAND, G., & PREUSSMAN, R. (1976) The
influence of dietary intake of nitrate on the nitrite content in
human saliva: a factor of possible relevance for in vivo
formation of N-nitroso compounds. Food Cosmet. Toxicol.,
14 (6): 545-548.
STANDFORD, G., ENGLAND, C. B., & TAYLOR, A. W. (1969) Fertilizer use
and Water quality, US Department of Agriculture, ARS,
pp. 41-168.
STEINBERG, R. A. & THOM, C. (1940) Mutations and reversions in
reproductivity of Aspergilli with nitrite, colchicine and
d-lysine. Proc. Natl Acad. Sci., 26 (6): 363-366.
STENBACK, F. G., FERRERO, A., & SHUBIK, P. (1973) Synergistic effects
of diethylnitrosamine and different dusts on respiratory
carcinogenesis in hamsters. Cancer Res., 33: 2209-2214.
STEPHANY, R. W., FREUDENTHAL, J., & SCHULLER, P. L. (1976)
Quantitative and qualitative determination of some volatile
nitrosamines in various meat products, IARC Sci. Publ.
No. 14, pp. 343-354.
STEWART, B. W., SWANN, P. F., HOLSMAN, J. W., & MAGEE, P. N. (1974)
Cellular injury and carcinogenesis. Evidence for the alkylation
of rat liver nucleic acids in vivo by N-nitrosomorpholine. Z.
Krebsforsch., 82 (1): 1-12.
STOEWSAND, G. S. (1973) Nitrite-induced methemoglobinemia in guinea
pigs: Influence of diets containing beets with varying amounts of
nitrate, and the effect of ascorbic acid, and methionine. J.
Nutr., 103 (3): 419-424.
STONE, I. M., LASCHIVER, C., & SALETAN, L. T. (1968) Nitrates in
worts, beers and brewing. Waller Stein Lab. Communications, 31
(106): 193-201.
STRACK, H. B., FREESE, E. B., & FREESE, E. (1964) Comparison of
mutation and inactivation rates induced in bacteriophage and
transforming DNA by various mutagens. Mutat. Res.,
1: 10-21.
STRACKS W. & FERON, V. J. (1973) Ultrastructure of pulmonary adenomas
induced by intratracheal instillation of diethylnitrosamine in
Syrian golden hamsters. Eur. J. Cancer, 9: 359-362.
SVOBODA, D. & HIGGINSON, J. (1968) A comparison of ultrastructural
changes in rat liver due to chemical carcinogens. Cancer Res.,
28: 1703-1733.
SWANN, P. F. & KAUFMAN, D. G. (1973) The dose response for the
induction of kidney tumors in the rat by a single dose of
dimethylnitrosamine. Br. J. Cancer, 28: 83-84.
SWANN, P. F. & MAGEE, P. W. (1971) Nitrosamine-induced carcinogenesis:
The alkylation of N-7 of guanine of nucleic acids of the rat by
diethylnitrosamine, N-ethyl- N-nitrosourea and
ethylmethanesulphonate. Biochem. J., 125: 841-847.
SWENBERG, J. A., KOESTNER, A., WESCHLER, W., & DENLINGER, R. H. (1972)
Quantitative aspects of transplacentai tumor induction with
ethylnitrosourea in rats. Cancer Res., 32: 2656-2660.
SYLVESTER, R. O. (1961) Nutrient content of drainage water forested,
urban and agricultural areas. In: Algae and Metropolitan
Wastes. Transactions of the 1960 Seminar, Robert A. Taft Sanitary
Engineering Center, pp. 80-87, (Technical Report W61-3).
TAKAYAMA, S. (1968) The histological and autoradiographical studies of
mouse liver during the course of carcinogenesis by
dimethylnitrosamine. Z. Krebsforsch., 71: 246-254.
TAKAYAMA, S. & IMAIZUMI, T. (1969) Sequential effects of chemically
different carcinogens, dimethylnitrosamine and 4-dimethylaminoazo-
benzene, on hepatocarcinogenesis in rats. Int. J. Cancer,
4: 373-383.
TAKAYAMA, S. & MURAMATSU, M. (1969) Incorporation of tritiated
dimethylnitrosamine into subcellular fractions of mouse liver
after long-term administration of dimethylnitrosamine. Z.
Krebsforsch, 73: 172-179.
TANNENBAUM, S. R., SINSKEY, A. J., WEISMAN, M., & BISHOP, W. (1974)
Nitrite in human saliva. Its possible relationship to nitrosamine
formation. J. Natl Cancer Inst., 53 (1): 79-84.
TANNENBAUM, S. R., WEISMAN, M., & FELT, D. (1976) The effect of
nitrate intake on nitrite formation in human saliva. Food
Cosmet. Toxicol., 14 (6): 549-552.
TAYLOR, H. W. & LIJINSKY, W. (1975) Tumour induction in rats by
feeding heptamethyleneimine and nitrite in water. Cancer Res.,
35: 812-815.
TELLING G. M. (1972) A gas-liquid chromatographic procedure for the
detection of volatile N-nitrosamines at the 10 parts per
billion level in foodstuffs after conversion to their
corresponding nitramines. J. Chromatogr., 73: 79-87.
TELLING, G. M., BRYCE, T. A., & ALTHORPE, J. (1971) Use of vacuum
distillation and gas chromatography mass spectrometry for
determination of low levels of volatile nitrosamines in meat
products. J. agric. Food Chem., 19(5): 937-940.
TELLING G. M., BRICE, T. A., HOAR, D., OSBORNE, D., & WELTI, D. (1974)
Progress in the analysis of volatile N-nitroso compounds.
IARC Sci. Publ. No. 9, pp. 12-17.
TERRACINI, B., MAGEE, P. N., & BARNES, J. M. (1967) Hepatic pathology
in rats on low dietary levels of dimethylnitrosamine. Br. J.
Cancer, 21: 559-565.
THOMAS, J. F. J. (1948) Water Survey Report No. 2, Ottawa river
drainage basin. Canada Department of Mines and Technical
Surveys, pp. 11-23 (Publ. No. 834).
TOMATIS, L. & CEFIS, F. (1967) The effects of multiple and single
administration of dimethylnitrosamine to hamsters. Tumori,
53: 447-451.
TOMATIS, L. (1977) Prenatal exposure to chemical carcinogens and its
effect on subsequent generations. In: Conference on Perinatal
Carcinogenesis. Washington, DC, National Cancer Institute
Monograph.
TOMLINSON, T. E. (1970) Trends in nitrate concentrations in English
rivers in relation to fertilizer. Water Treat. Exam.,
pp. 277-293.
TOUSSAINT, W. & SELENKA, F. (1970) Methemoglobin formation in young
infants. A contribution to drinking water hygiene on Rheinhessen.
Mschr. Kinderheilk., 118(6): 282-284.
UNGERER, O., EISENBRAND, G., & PREUSSMANN, R. (1974) The reaction of
nitrite with pesticides. Formation, chemical properties and
carcinogenic activity of the N-nitroso compounds of the
herbicide N-methyl-N1-(2-benzothiazolyl)-urea. Z.
Krebsforsch., 81 (3-4): 217-242.
UNITED NATIONS (1976) Statistical Yearbook, 1975, New York, p. 298.
US DEPARTMENT OF AGRICULTURE (1965) Agricultural Information
Bulletin, No. 299, Washington, DC.
US DEPARTMENT OF HEALTH, EDUCATION AND WELFARE (1966) Air Quality
Data from the National Air Sampling Networks and Contributing
State and Local Networks, 1964-1965, Cincinnati, OH, USDHEW
Public Health Service Division of Air Pollution, pp. 125.
US DEPARTMENT OF HEALTH, EDUCATION AND WELFARE (1970) Nitrates,
nitrites and methaemoglobinaemia, Research Triangle Park, N.C.,
National Institute of Environmental Health Sciences, pp. 43
(Environ. Rev. No. 2).
VAN LOGTEN, M. J., DEN TONKELAAR, E. M., KROES, R. BERKVENS, J. M., &
VAN ESCH, G. J. (1972) Long-term experiment with canned meat
treated with sodium nitrate and glucono-delta-lactone in rats.
Food Cosmet. Toxicol., 10: 475-488.
VAN RENSBURG, S. J. (1972) Failure of low protein and zinc intakes to
influence nitrosamine-induced oesophageal carcinogenesis. S.
Afr. reed. J, 46: 1137-1138.
VAVRA, J. J., DE BOER, C., DIETZ, A., HANKA, L. J., & SOKOLSKI, W. T.
(1960) Antibiotics Annual 1959-1960, NY, Antiobiotica Inc,
pp. 230.
VERLY, W. G., BARBASON, H., DUSART, J, & PETITPAS-DEWANDRE, A. (1967)
A comparative study of the action of ethyl methane sulphonate and
HNO2 on the mutation to streptomycin resistance of Escherichia
coil K 12. Biochim. Biophys. Acta, 145: 752-762.
VESSELINOVITCH, S. D. (1969) The sex-dependent difference in the
development of liver tumors in mice administered
dimethylnitrosamine. Cancer Res., 29: 1024-1027.
VIETS, F. B. & ALDRICH, S. R. (1973) Nitrogenous materials in the
environment, Draft Report, US Environmental Protection Agency,
Hazardous Materials Advisory Committee.
VIGIL, J., WARBURTON, S., HAYNES, W. A., & KAISER, L. R. (1965)
Nitrates in municipal water supply cause methemoglobinemia in
infant. Public Health Rep., 80 (12): 1119-1121.
VON KREYBIG, T. (1965a) [The effect of teratogenic compounds on the
early stages of prenatal development in the rat.] Naunyn-
Schmiederbergs Arch: exp. Path. Pharmacol., 252: 196-204
(in German).
VON KREYBIG, T. (1965b) [The effect of carcinogen methylnitrosourea
doses on the embryonal development in the rat.] Z. Krebsforsch.,
67: 46-50 (in German).
VON KREYBIG, T. (1968) [Experimental prenatal toxicology.]
Arzneimittel-Forsch., Suppl. 17, pp. 1-211 (in German).
VON KREYBIG, T. & SCHMIDT, W. (1966) [On chemical teratogenesis in the
rat.] ArzneimitteI-Forsch., 8: 989-1001 (in German).
VON KREYBIG, T. & SCHMIDT, W. (1967) [Chemically induced fetopathies
in the rat.] Arzneimittel-Forsch., 9: 1093-1100 (in German)
VOOGT, P. (1906) The determination of nitrate with a nitrate selective
electrode. Dtsch Lebensm. Rundschau., 65: 196-198.
WALKER, E. A., PIGNATELLI, B., & CASTEGNARO, M. (1975) Effects of
gallic acid on nitrosamine formation. Nature (Lond.),
258: 176.
WALKER, E. A., BOGOVSKI, P., & GRICUITE, L., ed. (1976) Environmental
N-nitroso compounds analysis and formation. Proceedings of a
Working Conference, the Polytechnical Institute, Tallinin,
Estonian SSR, 1-3 October, 1975, Lyons, International Agency for
Research on Cancer, pp. 512.
WALTERS, C. L. (1971) The detection and estimation of trace amounts of
N-nitrosamines in a food matrix. Lab. Pract., 20: 574-578.
WALTON, G. (1951) Survey of literature relating to infant
methemoglobinemia due to nitrate-contaminated water. Am. J.
Public Health, 41: 986-996.
WASSERMAN, A. E., FIDDLER, W., DOERR, R. C., OSMAN, S. F., & DOOLEY,
C. J. (1972) Dimethylnitrosamine in frankfurters. Food Cosmet.
Toxicol., 10: 681-684.
WATER RESEARCH CENTRE (1974) Notes on water pollution, No. 66,
pp. 4.
WATROUS, R. M. (1947) Health hazards of the pharmaceutical industry.
Br. J. ind. Med., 4: 111-125.
WEAST, R. C. ed. (1976) Handbook of chemistry and physics, 57th
edition, Cleveland, OH, CRC Press, pp. C-81-C-685.
WESCHLER, W. (1970) Oncogenic, teratogenic and mutagenic effects of
methyl- and ethylnitrosourea. In: 6th International Congress of
Neuropathology, Paris. Masson, pp. 128-129.
WESCHLER, W. (1971) Teratogenic effects of the neurotropic resorptive
carcinogens methyl-and ethylnitrosourea in rats. J. Neuropath.
exp. Neurol., 30: 120-121.
WESCHLER, W. (1973) Carcinogenic and teratogenic effects of
ethylnitrosourea and methylnitrosourea during pregnancy in the
rat. IARC Sci. Publ. No. 4, pp. 127-142.
WEGNER, T. N. (1972) Simple and sensitive procedure for determining
nitrate and nitrite in mixtures in biological fluids. J. Dairy
Sci., 55 (5): 642-644.
WEIL, L. & QUENTIN, L.-E. (1973) Nitrogen compounds in water and
nitrate determination with ion-sensitive electrode. Vom Wasser,
40: 125-133.
WEISBERGER, J. H. & RAINERI, R. (1975) Dietary factors and the
etiology of gastric cancer. Cancer Res., 35: 3469-3474.
WEISBERGER, E. K., WARD, J. M., & BROWN, C. (1974) Dibenamine:
Selective protection against diethylnitrosamine-induced hepatic
carcinogenesis but not oral, pharyngeal and esophageal
carcinogenesis. Toxicol. appl. Pharmacol., 28: 477-484.
WELLS, B. E., WALKER, R., & WALTERS, C. L. (1974) Interactions between
sodium nitrite and foodstuffs under gastric conditions. J. Sci.
Food Agric., 25: 1048.
WHO (1971) International standards for drinking water. Third ed,
Geneva, World Health Organization, pp. 70.
WHO (1977) Environmental health criteria 4--Oxides of nitrogen,
Geneva, World Health Organization, pp. 81.
WILLIAMS, A. O. (1970) Ultrastructure of liver cell carcinoma in
Macaca mulata monkey. Exp. mol. Pathol., 13: 359-369.
WINTON, E. F., TARBIFF, R. G., & MCCABE, L. J. (1971) Nitrate in
drinking water. J. Am. Water Works Assoc., 63: 95-98.
WORKSHOP ON GLOBAL ECOLOGY (1971) Man in the living environment, The
Institute of Ecology Report of the workshop on global ecological
problems, Chicago, IL, Field Museum of Natural History, pp. 267.
WRIGHT, A. J. & DAVIDSON, K. L. (1964) Nitrate accumulation in crops
and nitrate poisoning in animals. Advan. Agron., 16: 197-247.
WRIGLEY, F. (1948) Toxic effects of nitroso-methyl urethane.
Br. J. ind. Med., 5: 26-27.
YANG, K. W. & BROWN, E. V. (1972) A sensitive analysis for
nitrosamines. Anal. Lett., 5 (5): 293-304.
ZALDIVAR & WETTERSTRAND (1975) Further evidence of a positive
correlation between exposure to nitrate fertilizers (NaNO3 and
KNO3) and gastric cancer death rates: Nitrites and
nitrosamines. Experientia (Basel), 31: 1354-1355.
ZIEBARTH, D. & SCHEUNIG, G. (1976) Effects of some inhibitors on the
nitrosation of drugs in human gastric juice. IARC Sci. Publ. No.
14, pp. 279-290.
ZIMMERMAN, F. K. & SCHWAIER, R. (1967) Induction of a mitotic gene
conversion with nitrous acid, 1-methyl-3-nitro-1-nitrosoguanidine
and other alkylating agents in Saccharomyces cerevisiae. Mol.
gen. Genet., 100: 63-76.
ZIMMERMAN, F. K., SCHWAIER, R., & LAER, U. V. (1966) Mitotic
recombination induced in Saccharomyces cerevisiae with nitrous
acid, diethylsulphate and carcinogenic, alkylating nitrosamides.
Z. Vererbungsl., 98: 230-246.
 artificial fertilizers are ultimately used for the synthesis of
biological molecules, particularly proteins. Plants and animal waste
and dead tissues return fixed nitrogen to the soils, where part of it
is recycled and part returned to the atmosphere, thus completing the
nitrogen cycle. According to Delwiche (1970), nitrogen fixation on a
world basis may exceed denitrification by about 10%. The increased use
of industrial fertilizers has resulted in some areas in increased
concentrations of nitrates in bodies of water, resulting, in some
cases, in eutrophication.
4.2 Transformation in Foods
4.2.1 Reduction of nitrates to nitrites
Because of the ability of spinach to accumulate large quantities
of nitrates and reported cases of intoxication associated with the
consumption of this vegetable, several studies have been undertaken on
the conversion of nitrates to nitrites in spinach.
Data presented by Phillips (1968a) indicated that initial
nitrite-N contents of fresh, frozen, canned, and baby-food spinach
were generally less than 1 mg/kg fresh weight. However, several
authors have reported a rapid fall in nitrate levels and increase in
nitrite levels in fresh spinach during the first 4 days of storage at
room temperature (Achtzehn & Hawat, 1970; Phillips, 1968a; Schuphan,
1965). Higher nitrite levels occurred in spinach from fertilized
ground (Brown & Smith, 1967) and these could reach exceptionally high
values (3600 mg/kg dry weight) with excessive fertilization (Schuphan,
1965).
Under refrigeration, the nitrite-N contents of fresh spinach
increased very gradually throughout a storage period of 28 days
(Phillips, 1968a). Significant increases in nitrite-N levels did not
occur during the storage of frozen, canned, or baby-food spinach, but
increased concentrations were found in frozen spinach, that had been
left to thaw at room temperature for an excessively long period (39 h)
(Phillips, 1968a).
There was also a slight rise in nitrite levels when partially
consumed jars of commercial baby foods containing nitrates were stored
for 7 days at room temperature instead of under refrigeration
(Phillips, 1969). Selenka (1970) noted that nitrite formation in baby
foods was rapid in the presence of Escherichia coli and Pseudomonas
fluorescens, less rapid with Bacillus subtilis, and very slow with
Staphylococcus albus.
When foods consisted of a solid immersed in a liquid (e.g. canned
foods or frozen foods after thawing) nitrates were partially
transferred to the liquid portion or into the water in which the food
was cooked (Bodiphala & Ormrod, 1971). When large volumes of water and
long cooking times were employed (Kilgore et al., 1963), and when
canned vegetables were blanched in hot water instead of steam
(Johnson, 1966), significant amounts of nitrates were leached out of
the foods. Nitrate reductase (NADPH) (1.6.6.3) activity was rapidly
destroyed during cooking, thereby greatly diminishing further
conversion of nitrates to nitrites (Bodiphala & Ormrod, 1971). It is
also well known that the sterilization treatments necessary for
canning destroy microorganisms that could convert nitrates to
nitrites.
Conversion of nitrates to nitrites occurred more slowly in
vacuum-packed bacon than in unpackaged bacon, presumably due to the
low reducing ability of anaerobes (Cavett, 1962). Spencer (1967) found
that the nitrite content of vacuum-packed bacon decreased slowly on
storage. It has also been reported by Sebranek et al. (1974) that
nitrite levels in meat, determined 2 days after processing, were less
than half those originally added to frozen samples and samples
processed at 71°C, and that they decreased further during storage.
Frying, grilling, or boiling bacon or ham reduced the nitrites content
by 20-90% (Food Standards Committee, 1959).
When direct gas firing of spray dryers was employed, the nitrate-
N contents of dried milk products increased by 1-3 mg/kg compared with
those obtained using indirectly heated sprayers but nitrite-N levels
were unaffected (Manning et al., 1968). Air drying of potato and corn
starch led to the formation of only trace amounts of nitrite
(Gerritsen & De Willingen, 1969).
Nitrates may be reduced to nitrites when cooking is carried out
in aluminium utensils (Osteryoung, unpublished data)a. This
observation appears to be significant since some countries use
aluminium utensils for boiling milk and water, a practice which could
lead to the formation of sizeable quantities of nitrites. This effect
of aluminium should be investigated further.
4.2.2 Formation and degradation of N-nitroso compounds
The conditions under which various amines, amino acids, and
proteins in food could react with nitrite to form nitrosamines were
studied by Ender & Ceh (1971) and by Sen et al. (1970) who showed that
when cod, herring, hake, halibut, mackerel, or salmon were treated
with sodium nitrite at 200 mg/kg and cooked at 110°C for 60-70 min
there were only trace amounts (2.5-25 µg/kg) of DMN in the cooked
product. The highest levels were found in mackerel and hake, both of
which contain large amounts of DMA and TMA. Samples without added
nitrite did not contain any detectable nitrosamines.
a Nitrates as human and animal health hazards, Paper presented at
the Second Conference on Environmental Chemicals, Colorado State
University, 1973.
The formation of DMN was studied in aqueous model systems
containing methyl amines and sodium nitrite under conditions which
were more severe than those employed in the commercial processing of
nitrite-treated smoked chub (a fresh water fish containing small
amounts of TMA, TMAO, and DMA). The results of the studies showed that
not more than 10 µg of DMN per kg of the final product would be formed
during the smoking process (Malins et al., 1970).
Recently, Sen et al. (1973b) suggested that a major source of
nitrosamines in cured meat might arise from an interaction between the
nitrites and spices, such as black pepper and paprika, that are
present in curing mixtures. Nitrosopyrrolidine, nitrosopiperidine, and
DMN were found in a curing mixture used by a meat manufacturer in
Canada. The same authors (Sen et al. 1974a) have also studied the
effect of sodium nitrite concentration on the formation of
nitrosopyrrolidine and DMN in fried bacon. Bacon samples prepared with
sodium nitrite at 0, 50, 100, 150, or 200 mg/kg were analyzed for
nitrosopyrrolidine and DMN. No nitrosamine was detected in samples
prepared without nitrite but all treated samples contained 2-20 µg/kg
of nitrosamines. The level of nitrosopyrrolidine was related to the
initial concentration of nitrite in the bacon. It has also been shown
that nitrosamine formation in bacon increases with increasing
temperature and time of frying and that whereas baking, broiling, or
frying produce variable amounts of nitrosamines none is produced with
cooking in a microwave oven (Pensabene et al., 1974).
After deliberate nitrosation of eggs and meat with unusually
large amounts (1%) of sodium nitrite, some N-nitroso compounds
appeared to have been formed but the chemical nature of the compounds
detected was not clear (Walters, 1971).
No systematic studies on the formation of N-nitroso compounds
in cheese have been performed, although some types of cheese are known
to be processed with nitrate and nitrite.
There are few data on the fate of N-nitroso compounds during
the cooking, processing, or storage of food but some studies have
demonstrated that the volatile nitrosamine DMN and nitrosopyrrolidine
may be lost during the frying of bacon (Sen et al., 1973a).
4.3 Formation of N-nitroso Compounds from Drugs and Pesticides
Reaction with nitrite to form nitrosamines is not restricted to
food components. Lijinsky & Greenblatt (1972) and Lijinsky (1974)
reported that some antibiotics and other drugs that are widely used
can react with nitrites to form nitrosamines in alarmingly high
quantities. The drugs examined included oxytetracycline,
aminophenazone (aminopyrine), disulfiram, N,N-diethyl-3-
pyridinecarboxamide (nikethamide), tolazamide, and (E,E)-1-[5-(1,3-
benzodioxol-5-yl)-1-oxo-2,4-pentadienyl] piperidine (piperine).
Optimum conditions of temperature, pH, and concentration for these
reactions have been reported by Lijinsky et al. (1972a, 1972b, 1972c)
who more recently (Lijinsky, 1974) studied the reactions of
aminopyrine and other commonly used drugs with nitrous acid at rather
low concentrations to assess the magnitude of the hazard to man from
such interactions. The topic has been reviewed by Mirvish (1975) who
has listed 41 drugs and pesticides that have been nitrosated.
Pesticides listed include atrazine, simazine, ziram, and thiram.
4.4 Formation of N-nitroso Compounds in Animal Organisms
4.4.1 Formation of N-nitroso compounds in simulated gastric juice
Formation of DEN was demonstrated when DEA and nitrite were
incubated in the gastric juice of the rat, rabbit, cat, dog, and man.
More DEN was formed in human and rabbit gastric juices (pH 1-2 in both
cases) than in rat gastric juice (pH 4-5). (Sen et al., 1969a, 1969b).
The formation of nitrosamines by the interaction of some drugs
with nitrite in the presence of human gastric juice have been studied
by Scheunig & Ziebarth (1976). At 37°C and a pH = 2, for 1 h,
aminopyrine, sodium [(2,3 dihydro-1,5-dimethyl-3-oxo-2-phenyl-IH-
pyrazol-4-yl)methylamino] methanesulfonate (analgin), and piperazine
gave nitrosamine yields (calculated on the basis of nitrite used) of
69%, 11%, and 74.8% respectively.
In vitro studies have been carried out (Wells et al., 1974) in
which several foods (pork, egg, bread, milk, and cheese) were
incubated under simulated gastric conditions with concentrations of
nitrite similar to those used as food preservatives. The effect of the
thiocyanate ion as a catalyst for nitrosation was also studied since
it is secreted in saliva. Of the foods studied, only cheese produced
detectable amounts of volatile nitrosamines. The identity of the
nitrosamines was not indicated.
4.4.2 Formation of N-nitroso compounds in vivo
When DEA and nitrite were fed to cats and rabbits, considerable
amounts of DEN were detected in the stomach of the experimental
animals (Sen et al, 1969a, 1969b). Similar results were reported by
Sander & Sief (1969). Epstein (1972) reported the formation of
nitrosopiperidine in the gastrointestinal tract of rats treated with
nitrite and piperidine hydrochloride. When the nitrite concentration
was constant, nitrosopiperidine formation in the small intestine
increased with increasing concentrations of piperidine.
Nitrosopiperidine was also found in the stomach. Recently, Sander et
al. (1974a) demonstrated the formation of N-N'-dinitrosopiperazine,
DMN, and N-nitroso- N-methylbenzylamine in the stomach contents of
rats given the parent amines combined with nitrite. Considerable
individual variation in the degree of synthesis of N-N'-
dinitrosopiperazine was noted in the animals. In another recent
report, N-nitrosopyrrolidine was formed very rapidly in the stomach
of dogs from sodium nitrite and pyrrolidine (within 2-6 min) but after
30 min nearly all of it had disappeared, presumably due to its rapid
absorption (Mysliwy et al., 1974).
Indirect evidence of in vivo formation of N-nitroso compounds
has also been provided by some toxicity studies. Thus, hepatic
lesions, formed following administration of nitrite and some amines,
were similar to those produced by DMN or N-nitroso- N-
methylbenzylamine (Asahina et al., 1971). Similar effects were noted
where nitrite was administered up to 3 h after DMA, but the effect was
markedly reduced if the nitrite was given prior to the amine.
4.5 Formation of N-nitroso Compounds by Microorganisms
Studies conducted by Hawksworth & Hill (1971a), Klubes & Jondorf
(1971), and Sander & Sief (1969) suggested that nitrosamines could be
synthesized from secondary amines and nitrates or nitrites by
Escherichia coli and some species of streptococci. Fong & Chan
(1973b) demonstrated that homogenized Chinese salt fish inoculated
with Staphylococcus aureus (a nitrate-reducing bacterium) produced
considerable amounts of DMN.
Formation of nitrosamines in the presence of bacteria is unlikely
to occur in the large intestine, but the infected bladder and
achlorhydric stomach are likely sites (Hawksworth et al., 1974).
The ultimate mechanism of bacterial production of nitrosamines
remains to be ascertained. According to Hawksworth et al. (1974),
certain bacteria do reduce nitrate to nitrite but the formation of the
nitrosamine may be nonenzymatic and involve some heat-resistant
metabolite.
4.6 The Effects of Other Chemicals on the Formation of N-nitroso
Compounds
Fiddler et al. (1973) and Greenberg (1974) showed that high
levels of ascorbic acid reduced nitrosamine formation in frankfurter
sausages and in fried bacon. On the other hand, Nagata & Mirna (1974)
reported an increase in nitrosamine formation in meat products in the
presence of ascorbic acid. Other studies conducted on the inhibition
of nitrosamine formation by various compounds include a report by Sen
& Donaldson (1974) in which nitrosamine formation in human saliva was
inhibited by ascorbic acid. Ziebarth & Scheunig (1976) tested a number
of substances and beverages for the inhibition of the nitrosation of
several drugs under simulated gastric conditions. Of all the
substances investigated, ascorbic acid was regarded as the best
inhibitor because of its pronounced activity at pH values occurring in
the stomach and because it was not toxic in the amounts used.
5. ENVIRONMENTAL LEVELS AND EXPOSURES
5.1 Nitrates and Nitrites
5.1.1 Ambient air
Nitrate aerosols are the final stage in the atmospheric oxidation
of gaseous oxides of nitrogen, and substantial amounts of particulate
nitrates may be formed in urban areas affected by photochemical
pollution (Pitts & Lloyd, 1973). The concentration of nitrates in air
may range from about 1 to 40 µg/m3, depending on the sampling and
averaging periods. For example, the estimated annual mean values
(1968-1972) in Chattanooga, TN, USA, were between 1 and 6 µg/m3
(French et al., unpublished)a. The daily mean concentrations of
airborne nitrates in the central part of Tokyo ranged, in 1973, from
0.9 to 41.8 µg/m3 with an annual mean of 8.2 µg/m3. On the other
hand, in a small city with few industries (Matsue City) the daily
means were in the range of 1.1 -- 9.2 µg/m3, with an annual mean of
2.6 µg/m3 (Japan Environmental Sanitation Center, 1974).
5.1.2 Water
The concentrations of nitrates and nitrites in surface and ground
waters vary within wide limits, depending on geochemical conditions,
human and animal waste management practices, the extent to which
nitrogen-containing agriculture fertilizers are used locally, and on
industrial discharges of nitrogen compounds (section 3.2.1.).
In general, surface waters do not usually contain nitrate in
concentrations higher than 10 mg/litre, and nitrite concentrations
rarely exceed 1 mg/litre. However, a steady upward trend of nitrate
levels has been reported in recent years in some countries, both in
surface and ground waters. Thus, for example, in the River Thames,
England, nitrate concentrations increased from an average of
4 mg/litre in 1968 to an average of 9 mg/litre for the last quarter of
1973 (Water Research Centre, 1974). Similar increases have been
observed in several other English rivers (Casey, 1975; Owens, 1970;
Tomlinson, 1970). The nitrate concentrations are increasing in some
rivers that drain the great agricultural section of the centre of the
USA, and in selected small rivers the 45 mg/litre limit is sometimes
exceeded (Viets & Aldrich, 1973). A small increase in the nitrate
concentration of the Tamagawa River, Tokyo, Japan has also been
reported. From 1951-1965, the nitrate ion concentration rose from
7.9 mg/litre to 9.1 mg/litre. During the same period, the nitrite
concentration increased from 0.049 mg/litre to 0.53 mg/litre, i.e by a
factor of about 10 (Goto, 1973).
a French, J. G., Hasselblad, V., & Johnson, R. Aggravation of
asthma by air pollutants. 1971 -72 Southeastern CHESS studies.
Studies of 991 settlements in Bulgaria indicated that only 64
towns and villages had drinking water levels of nitrates between
30 mg/litre (Bulgarian standard) and 50 mg/litre. In 20 settlements,
situated in areas with intensive agriculture and stock breeding, the
nitrate concentrations exceeded 50 mg/litre. The reportb points out
that such problems did not exist some 10 years ago when smaller
quantities of nitrogen fertilizers were used in agriculture.
Much higher concentrations of nitrates are sometimes found in
ground water, particularly in water derived from dug wells. A survey
of over 2000 rural wells in Saskatchewan, Canada, revealed that 18.8%
contained nitrate concentrations of more than 50 mg/litre and 5.3% had
nitrate levels exceeding 300 mg/litre (Robertson & Draycott, 1948).
Hedlin (1971) also reported levels above 45 mg/litre in some wells in
a rural area of Manitoba, Canada. In many farm wells in central USA,
nitrate concentrations may range from 45-450 mg/litre. This problem is
neither new nor local, since such conditions have been recorded from
1895 to 1970 in Illinois, in 1939 in Iowa, and in 1970 in Minnesota
(Viets & Aldrich, 1973). The mean nitrate concentration in ground
water consumed by children affected by methaemoglobinaemia in
Czechoslovakia ranged from 18-257 mg/litre (Schmidt & Knotek, 1970).
According to Gruenar & Shuval (1970), about 180 wells for community
water supplies in the densely populated central and southern coastal
plain in Israel had nitrate concentrations exceeding 45 mg/litre. In
England, nitrate concentrations in some ground waters have been
reported to range from 12 mg/litre (Foster & Crease, 1974) to over
22 mg/litre (Reeves et al., 1974). Nitrate concentrations exceeding
45 mg/litre have not been reported in centralized water supplies in
the USSRc. However, high concentrations have been found from time
to time in dug wells, for example, 310-400 mg/litre in Leningrad
Oblast (Motylev, 1969), 110-200 mg/litre in the Tatar SSR (Petukhov
et al., 1972) and up to 430 mg/litre in the Moldvian SSR (Diskalenko,
1969).
5.1.3 Selected foods
According to the data compiled by the National Institute of
Environmental Health Sciences (NIEHS, 1970), the levels of nitrates in
vegetables vary considerably. The highest levels were found in beets,
egg plant, kale, and spinach and the lowest in tomatoes and peas;
similar findings were obtained in the German Democratic Republic by
Achtzehn & Hawat (1969). It is of interest to note that the nitrate
levels in vegetables reported by Jackson in 1967 were similar to those
reported by Richardson in 1907, when manure was used instead of
chemical fertilizers.
b Contribution to the WHO environmental health criteria document on
nitrates, nitrites and nitrosamines, Sofia, 1974.
c Contribution to the WHO environmental health criteria document on
nitrates, nitrites and nitrosamines, Moscow, 1974.
Nitrate contents vary not only between vegetable species but also
widely within a given species. This variation within a species may be
accounted for by such factors as temperature, sunlight, soil moisture,
and the level of available nitrogen in the soil (US Department of
Agriculture, 1965). A relationship between nitrate accumulation in
spinach and levels of fertilizer applied to the soil has been
demonstrated by a number of authors (Brown & Smith, 1967; Phillips,
1971). Furthermore, Schuphan (1965) reported exceptionally high levels
of nitrites (3600 mg/kg dry weight) in excessively fertilized fresh
spinach stored at room temperature.
A survey of the nitrate contents of fruits in the German
Democratic Republic, revealed that they were high in bananas and
strawberries but could not be detected in the other fruits examined
(Achtzehn & Hawat, 1969).
Cow's milk contained nitrate levels of 0-0.5 mg/litre (Simon et
al., 1964).
The levels of nitrates and nitrites in baby foods are of special
concern since infants are considerably more sensitive to the toxic
effects of nitrates than adults. Kamm et al. (1965) studied 194
prepared infant foods and found that, on average, fruits, dairy
products, puddings, egg products, meats, dry and concentrated food
supplements, and precooked cereal products contained nitrate levels of
less than 90 mg/kg. Vegetables, however, had a wide range of nitrate
contents varying from 0.9 to 2165 mg/kg, but nitrite levels never
exceeded 7 mg/kg. In studies on a number of canned foods, baby foods,
frozen foods, and vegetables, several varieties of fruit, spinach, and
beets generally had the highest nitrate contents (Bodiphala & Ormrod,
1971). Additional information may be found in articles by NIEHS (1970)
and Ashton (1970).
In a survey of various cured meats (Table 1, Ashton, 1970), the
highest nitrate content of 370-511 mg/kg was found in ham. In
analysing 197 samples of cured meat products, Panalaks et al. (1972)
found that the levels of nitrates and nitrites ranged from
0-3467 mg/kg and 0-252 mg/kg, respectively.
Dubrow & Kakisch (1960) analysed 338 samples of cheese and
reported that all were free of nitrites (less than 1 mg/kg). However,
40% of the samples contained nitrate levels of more than 1 mg/kg.
Rammell & Joerin (1972) also found low nitrite levels in cheese.
Table 1. Nitrate and nitrite contents of cured meats
Meat type Nitrate (mg/kg) Nitrite (mg/kg)
silverside 133-303 9-26
ham 370-511 7-150
luncheon meat 59-214 3.1-47
chopped ham & pork 53-101 22-62
corned beef 118-135 18-208
frankfurter sausage 119-141 8.5-10.3
From: Ashton (1970)
5.1.4 Estimate of general population exposure
One of the important sources of exposure to nitrates for man is
water. The level of nitrates in water may vary from practically nil to
over 200 mg/litre. In water from municipal supplies, however, it is
likely to be under 10 mg/litre. Thus, assuming an intake of 2 litres
of water per day, the daily intake of nitrates from this source would
normally be less than 20 mg, but with extremes of 0 and over 400 mg.
The other main sources of nitrates and nitrites are certain
vegetables and meat products. The intake from these sources is even
more variable because of marked differences not only in levels in
these foods but also in dietary patterns. However, Ashton (1970)
estimated the weekly intake of nitrates for a member of the general
population in the USA to be about 400 mg including 210 mg from
vegetables, 110 mg from meat products, and 85 mg from water (7 litres
per week). The estimate of Hill et al. (1973) for a member of the
general population in England included 225 mg from vegetables, 110 mg
from meat, and 105 mg from water in "control towns" and 645 mg from
water in Worksop, England. However these figures cannot be applied
generally because of variations in feeding habits and nitrate levels
in environmental media. Since the intake of nitrites is even more
variable, no estimates have been reported.
5.2 N-nitroso Compounds
5.2.1 Ambient air
The occurrence of N-nitroso compounds in urban air was reported
first by Bretschneider & Matz (1973) and confirmed by Fine et al.
(1976b) who found DMN at concentrations of about 1.2-3.5 µg/m3
(0.33-0.96 ppb) in an industrial district in Baltimore, MD, USA, and
about 0.06-0.17 µg/m3 (0.014 ppb - 0.051 ppb) in Belle, WV, USA.
N-nitroso compounds may be present in air, either due to their
formation in the air from secondary amines and oxides of nitrogen
(Neurath, 1972) or due to industrial omissions as in the instances
referred to.
5.2.2 Water
There are few reports on the occurrence of N-nitroso compounds
in water. Fine et al. (1976b) analysed samples from the Mississippi
river (New Orleans, LA) and from 3 water treatment plants in
Louisiana. Using the new N-nitroso compound-specific thermal energy
analyser (TEA) interfaced to both a gas chromatograph (GC) and a high
performance liquid chromatograph (LC), several peaks were tentatively
identified as belonging to N-nitroso derivatives of some pesticides.
The estimated concentrations were of the order of 0.1 µg/kg.
5.2.3 Selected foods
A summary of the reported occurrences of nitrosamines in meat and
fish products, adapted from Sen (1974) and updated, is presented in
Table 2. Only the results that were confirmed by mass spectroscopy are
quoted in this table. It was noted that the majority of some 50
publications dealt with the determination of nitrosamines,
particularly DMN, in processed pork meat. The methods employed for
analysis mainly involved gas chromatography. A few results were
confirmed by mass spectroscopic techniques. However, mass spectroscopy
confirmation is currently being employed more frequently than in the
past.
Table 2. Levels of nitrosamines in various meat and fish productsa
N-nitroso
Meat Country compounds Levels (c) Reference
or area foundb
dry sausage Canada DMN 10-20 µg/kg Sen (1972)
uncooked salami sausage Canada DMN 20-80 µg/kg Sen (1972)
salami sausage Netherlands DMN 0.3 µg/kg(e) Stephany et al. (1976)
DMN 0.1(e) Stephany et al. (1976)
NDBA 1.1(e) Stephany et al. (1976)
Netherlands NPY 0.4(e) Stephany et al. (1976)
NPIP 0.3(e) Stephany et al. (1976)
bacon Canada NPY 4-25 µg/kg Sen et al. (1973a)
Canada NPY 25-40 µg/kg Sen et al. (1974a)
Netherlands DMN 0.8 µg/kg(e) Stephany et al. (1976)
bacon DEN 0.2(g)
Netherlands NDBA 0.6(g) Stephany et al. (1976)
NPY 0.4(g)
Netherlands NPIP 0.6(g) Stephany et al. (1976)
USA NPY 7-35 µg/kg Pensabene et al. (1974)
bacon USA NPY 2, 28, 13 Fiddler et al. 1974
uncooked bacon Canada DMN 30 µg/kg Sen et al. (1973a)
fried bacon Netherlands DMN 2.4 µg/kg Groenen et al. (1976)
DEN 4.43 µg/kg
Netherlands DMN 1.1 µg/kg(e) Stephany et al. (1976)
DEN 0.2(g)
Netherlands NDBA 0.7(g) Stephany et al. (1976)
NPY 16.4(g)
Table 2 (Cont'd)
N-nitroso
Meat Country compounds Levels (c) Reference
or area foundb
Netherlands NPIP 3.9(g) Stephany et al. (1976)
UK NPY 1-40 µg/kg Crosby et al. (1972)
USA NPY 20-207 µg/kg Fazio et al. (1973)
smoked meat Netherlands DEN 7.91 µg/kg Groenen et al. (1976)
Netherlands DMN 3 Groenen et al. (1976)
smoked horse and Netherlands DMN 7.3 µg/kg(d) Stephany et al. (1976)
beef meat DEN 0.6(d) Stephany et al. (1976)
Netherlands NDBA 0.4(d) Stephany et al. (1976)
NPY 0.1(d) Stephany et al. (1976)
Netherlands NPIP 0.1(d) Stephany et al. (1976)
ham with layer Germany DMN 3 µg/kg Eisenbrand et al. (1975)
of pepper grains (Federal Republic of)
on the outside.
Only fat portion.
ham with layer Germany NPIP 6 µg/kg Eisenbrand et al. (1975)
of pepper grains (Federal Republic of)
on the outside.
Whole product Germany NPY 6
homogenized. (Federal Republic of)
ham, fried Germany DMN 1 µg/kg Eisenbrand et al. (1975)
(Federal Republic of) NPIP 8
NPY 19
Table 2 (Cont'd)
N-nitroso
Meat Country compounds Levels (c) Reference
or area foundb
ham with layer of Germany NPIP 4 µg/kg Eisenbrand et al. (1975)
pepper grains (Federal Republic of)
on the outside. Germany NPY 9
Whole product (Federal Republic of)
homogenized.
German type of Germany DMN 1 µg/kg Eisenbrand et al. (1975)
bacon, raw (Federal Republic of)
German type of Germany DMN 1 µg/kg Eisenbrand et al. (1975)
bacon, fried (Federal Republic of) NPIP 5 Eisenbrand et al. (1975)
Germany NPY 19 Eisenbrand et al. (1975)
(Federal Republic of)
smoked raw meat Germany DMN 2 µg/kg Eisenbrand et al. (1975)
(Federal Republic of)
smoked ham Germany DMN 8 µg/kg Eisenbrand et al. (1975)
(Federal Republic of)
smoked ham USA DMN 5 µg/kg Fazio et al. (1971b)
smoked ham USA DMN 5 µg/kg Fiddler et al. (1974)
cooked and smoked Netherlands DMN 0.4 µg/kg(f) Stephany et al. (1976)
ham DEN 0.6(f) Stephany et al. (1976)
Table 2 (Cont'd)
N-nitroso
Meat Country compounds Levels (c) Reference
or area foundb
Netherlands NDBA 0.4(f) Stephany et al. (1976)
NPY 0.3(f) Stephany et al. (1976)
Netherlands NPIP 0.4(f) Stephany et al. (1976)
cooked ham Netherlands DMN 6 µg/kg Groenen et al. (1976)
Bologna sausage Canada DEN 25 µg/kg Panalaks et al. (1974)
Canada NPY 20,100,105 Panalaks et al. (1974)
frankfurter sausage USA NPIP 50, 50, 60 µg/kg Wasserman et al. (1972)
spiced meat Canada DMN 5-48 µg/kg Sen et al. (1976)
products DEN 6-16
Canada NPIP 14-50 Sen et al. (1976)
Canada NPY 7-33 Sen et al. (1976)
fish meal Canada DMN 0.35-0.5 mg/kg Sen et al. (1972)
smoked, nitrate/ USA DMN 4-26 µg/kg Fazio at el. (1971a)
or nitrite treated
sable, salmon shad
fresh, smoked or UK DMN 1-9 µg/kg Crosby et al. (1972)
salted fish
salted white Hong Kong DMN 40-100 µg/kg Fong & Chan (1973a, 1973b)
herring
Table 2 (Cont'd)
N-nitroso
Meat Country compounds Levels (c) Reference
or area foundb
salted yellow Hong Kong DMN 10-60 µg/kg Fong & Chan (1973a, 1973b)
croakers
crude salt salted Hong Kong DMN 400 µg/kg Fong & Chan (1973a, 1973b)
white herring
crude salt salted Hong Kong DMN 200 µg/kg Fong & Chan (1973a, 1973b)
yellow croakers
prime salt salted Hong Kong DMN 10 µg/kg Fong & Chan (1973a, 1973b)
white herring
prime salt salted Hong Kong DMN 5 µg/kg Fong & Chan (1973a, 1973b)
yellow croakers
salted anchovies Hong Kong DMN 20 µg/kg Fong & Chan (1973a, 1973b)
whole herring meal Hong Kong DMN 300 µg/kg Fong & Chan (1973a, 1973b)
a Adapted from Sen (1974)
b DMN -- N-methyl- N-nitrosomethanamine c All values confirmed by mass spectroscopy
DEN -- N-ethyl- N nitrosoethanamine d mean of 4 samples
NDBA -- Nitroso- N-butylamine e mean of 5 samples
NPY -- Nitrosopyrrolidine f mean of 6 samples
NPIP -- Nitrosopiperidine g mean of 10 samples
Several authors who detected nitrosamines in foods by screening
methods but did not confirm these results by mass spectrometry include
Ender et al. (1964, 1967), Ender & Ceh (1967), Fong & Walsh (1971),
Freimuth & Glaser (1970), Hedler & Marquardt (1968), Kröller (1967),
Lembke & Moebus (1970), Möhler & Mayrhofer (1968, 1969), and Sakshaug
et al. (1965). Nevertheless, results by screening methods should not
be entirely ignored.
Considerable variations have been found in the levels of volatile
N-nitroso compounds in fried and grilled bacon. Attempts to
correlate these levels with levels of nitrates and nitrites did not
reveal any definite pattern. Telling et al. (1974) studied the effect
of various cooking temperatures on the levels of N-nitroso compounds
in grilled bacon. The results indicated that the levels of DMN
remained fairly constant as the cooking temperature was raised but
those of nitrosopyrrolidine increased.
Lipid soluble nitrosamines have an affinity for the fatty
portions of food (Sen et al. 1973a).
5.2.4 Tobacco and tobacco smoke
Since precursors for the formation of nitrosamines occur in
tobacco, Druckrey & Preussman (1962) thought it likely that tobacco or
tobacco smoke might contain trace amounts of nitrosamines. Initially,
studies on the nitrosamine content of tobacco products were hampered
due to interference from other compounds. Later, evidence suggesting
the presence of DMN, nitrosopyrrolidine, methylbutylnitrosamine, and
nitrosopiperidine in tobacco smoke was obtained (Kröller, 1967;
Neurath et al., 1964; Neurath, 1972, Serfontein & Hurter, 1966).
Although anabasine and nornicotine are constituents of tobacco smoke,
the corresponding nitroso derivatives were not detected by Neurath
(1972). Recently, however, Hoffman et al. (1974) reported the presence
of N-nitrosonornicotine at levels of up to 88 mg/kg in unburned
tobacco.
5.2.5 Estimate of general population exposure
The N-nitroso compounds that have been identified and
determined in meat and fish are listed in Table 2; consumption of
these foods constitutes a definite exposure of the general population
to these chemicals. However, at present, no estimate can be made of
the human exposure from these and other sources because insufficient
samples have been analysed and because the relevant food consumption
surveys have not been made.
5.2.6 Occupational exposure to N-nitroso compounds
The potential occupational hazards associated with the use or
manufacture of N-nitroso compounds in industry have been pointed out
by Magee & Barnes (1967).
Only a few quantitative data are available, however, on the
concentration of N-nitroso compounds in the work environment. In a
study by Bretschneider & Matz, (1976) DMN levels in the air in a
factory manufacturing DMA were roughly estimated to range from 0.001
to 0.43 µg/m3.
6. METABOLISM OF NITRATES, NITRITES, AND N-NITROSO COMPOUNDS
6.1 Gastrointestinal Absorption
6.1.1 Nitrates and nitrites
A part of ingested nitrates is readily absorbed and a part may be
metabolized by the microflora in the gastrointestinal tract (Ridder &
Oehme, 1974). Nitrites (NO2-), oxides of nitrogen (N2O5, NO2,
NO), hydroxylamine (NH2 OH), and ammonia (NH3) can be formed
depending upon the organisms present, the pH, and the available
nutrients (trace elements and carbohydrates), and may be absorbed.
Friedman et al. (1972) gave mice a single oral dose of 150 µg of
sodium nitrite. Measurement of the rate of disappearance showed that
the compound was rapidly absorbed and that food in the stomach had
little effect on absorption. Results using animals with a ligated
gastro-duodenal junction suggested that the major absorption site was
the gastric mucosa. Studies by Mirvish et al. (1974) on rats fed a
diet containing nitrite supported the previous observations on mice.
Experiments with food containing phenol red showed that a decrease in
nitrite levels in the stomach contents, especially in the glandular
part, that occurred within 5 h of feeding, was significantly greater
than that due to direct faecal elimination. This was attributed to
decomposition and other acid-catalyzed reactions of nitrite and to
direct absorption from the stomach.
6.1.2 N-nitroso compounds
Although a considerable degree of absorption of nitrosamines can
be inferred from the nature of their toxic effects following oral
administration, few reports could be found giving quantitative
information on absorption. Alarif & Epstein (1974) gave 3H-labelled
nitrosomethylurea and nitrosomethylurethane by gavage to groups of
pregnant guineapigs at doses of 2 mg/kg, and 5 mg/kg body weight,
respectively. The animals were killed 1 h after dosing. When the
uptake by maternal and fetal tissues was measured by liquid
scintillation counting and DNA determination, maternal levels were
generally higher than fetal levels. In studies by Juszkiewicz &
Kowalski (1974) DMN, DEN, and nitro-propylamine, administered orally
to goats at 20-30 mg/kg, appeared in the blood within “ h, and later
in the milk. The concentration in the milk, 2 h after administration
of DEN at 30 mg/kg, was 14 mg/litre; after 24 h only traces could be
found. Phillips et al. (1975a) examined the disappearance of DMN from
the stomach and small intestine of rats as an index of absorption, and
found that, while very little was absorbed from the stomach, DMN was
rapidly absorbed from the small intestine.
6.2 Biotransformation and Elimination
6.2.1 Nitrates and nitrites
In a study on 4 rats, 42-90% of nitrates, administered by stomach
tube, was excreted in the urine within 8 h of administration. Nitrites
were not detected in the urine either before or after administration
(Hawksworth & Hill, 1971b). The same authors carried out a study on
122 samples of human urine, and found that urinary nitrate
concentration was related to the amount of nitrate ingested.
The excretion of nitrates and nitrites in the saliva was studied
by Spiegelhalder et al. (1976) in 11 volunteers given various
vegetable juices with nitrate concentrations ranging from 30 to
550 mg/litre. The levels of nitrates and nitrites excreted were
proportional to the amounts of nitrates ingested. After ingesting
100 mg of nitrates, nitrite concentrations in the saliva increased, on
average, by 20 mg/litre.
6.2.2 N-nitroso compounds
Magee & Barnes (1967) have reviewed available information on the
metabolism and elimination of N-nitroso compounds. Magee (1956)
measured recovery of DMN from the whole body of the mouse and noted
that 97% of the total dose (0.05 mg/kg) could be recovered immediately
after oral administration and that the amounts recovered decreased
with time until at 4 h no DMN was recovered. Similar results were
obtained with the rat given DMN orally at 50 mg/kg, the concentration
fell rapidly with increasing time after injection so that only 30% of
the dose was recovered at 8 h and none at 24 h.
Metabolic transformation of DMN was demonstrated by Dutton &
Heath (1956) using 14C-labelled DMN. In both the mouse and the rat,
the main radioactive product was expired carbon dioxide. In the mouse,
65% of the injected 14C was recovered as expired carbon dioxide, 6 h
after a subcutaneous injection of DMN at 50 mg per kg body weight. In
the rat, about 40% of the radioactive 14C was recovered as expired
carbon dioxide, 8 h after the injection. At the end of the experiment,
the remainder of the 14C was fairly evenly distributed in the
tissues, apart from about 7% that was excreted in the urine.
Heath (1962) found that, while part of each of a number of
nitrosamines studied was excreted unchanged in urine and in expired
air, the greater part was decomposed. From the rates of expiration of
labelled carbon dioxide it was shown that decomposition of dimethyl-,
diethyl-, and N-butylmethylni-trosamine obeyed Michaelis-Menten
kinetics. The rate of decomposition was dose-dependent.
When the metabolic transformation of dialkylnitrosamines,
especially of the bladder carcinogen di- N-butylnitrosamine was
investigated, major urinary metabolites with retained N-nitroso
structure were identified (Blattmann & Preussman, 1973, 1974;
Blattmann et al., 1974; Okada & Suzuki, 1972; Okada et al., 1975).
Hydroxylation, particularly at the terminal CH3 group, has been
observed as well as chain shortening. Butyl (3-carboxypropyl)
nitrosamine (BCPN), a major metabolite of butyl (4-hydroxy butyl)-
nitrosamine (BBN) was an equally potent and selective bladder
carcinogen (Okada & Suzuki, 1972). Ring-opening was observed during
metabolism of nitrosomorpholine (Stewart et al., 1974).
7. EXPERIMENTAL STUDIES ON THE EFFECTS OF NITRATES, NITRITES, AND
N-NITROSO COMPOUNDS
7.1 Nitrates and Nitrites
7.1.1 Acute and subacute toxicity studies
Acute nitrate poisoning was first recognized in cattlea by Mayo
as early as 1895 (Wright & Davidson, 1964), while Comly (1945) was the
first to report nitrate poisoning from well water in infants in the
USA. As a result of these and other reports, several studies have been
conducted on the toxicity of nitrates in a wide variety of animal
species. They are mainly centred on the formation of methaemoglobin
that accompanies excessive exposure to nitrates and nitrites. The
acute toxicity of nitrates and nitrites was recently reviewed by the
Committee on Nitrate Accumulation (1972).
Although the outstanding feature of nitrate toxicity is the
development of methaemoglobinaemia, nitrates may also cause
vasodilation which aggravates the effects of the methaemoglobinaemia.
The nitrite ion formed by reduction of nitrates, oxidizes the iron in
the haemoglobin molecule from the ferrous to the ferric state. The
resultant methaemoglobin is incapable of reversibly binding oxygen
(Bosch et al., 1950). Clinical signs of nitrate toxicity, attributable
to hypoxia appear when methaemoglobin values exceed about 20% (section
8.1). Oxidation of haemoglobin to methaemoglobin by the nitrite ion
occurs at different rates for each animal species, but there is little
difference between individuals of the same species (Smith & Beutler,
1966). Similarly, the reduction of methaemoglobin in erythrocytes
mainly by the enzyme system, NADH--methaemoglobin reductase, is
characteristically different for each animal species. The chemical
induction of methaemoglobinaemia has recently been reviewed by Smith
(1969, 1975). These physiological processes appear to be related, even
though there is a large variation in the rate of formation of
methaemoglobin and its subsequent reduction. This may help to explain
the difference in species susceptibility and the variation in signs
seen in nitrate poisoning.
In an effort to develop sensitive tests for the detection of the
possible effects of subclinical methaemoglobinaemia, behavioural
studies with mice were undertaken by Behroozi et al., 1971. Groups of
57 black, 6J, male mice were given nitrites in their drinking water at
doses aimed at producing methaemoglobin levels varying from slightly
above normal to 15%, which can be considered to be in the subclinical
a For further discussion of nitrate intoxication in livestock,
that may involve significant economic loss, see Oehme (1975).
range. Sodium nitrite doses in water were 100, 1000, 1500, or
2000 mg/litre. The results showed a significant reduction of overall
motor activity in the groups receiving the highest levels of
nitrites. There was a significant inverse relationship between the
methaemoglobin level and motor activity, with a coefficient of
correlation of 0.65. An effort to counteract the methaemoglobinaemia
was made by giving ascorbic acid to the group receiving the highest
level of nitrites (2000 mg/litre). (See section 7.1.5). The effect
was to reduce the methaemoglobin levels to almost normal but the
motor activity level of the group so treated remained low and about
equal to the equivalent group that had not received an antidote.
These experiments seemed to indicate that the nitrites had some form
of sedative effect on the treated mice, that was not necessarily
associated with the development of methaemoglobinaemia.
Experiments on rabbits have shown that methaemoglobinaemia caused
by nitrates in water also affects cardiac activity by increasing the
number of cardiac contractions to an extent directly proportional to
the increase in methaemoglobin levels. At the 10-15% methaemoglobin
level, the electrocardiogram shows a shortening of the Q-T interval
and a reduction in the T wave, which may even become negative (Garbuz,
1968, 1971).
7.1.2 Chronic toxicity and carcinogenicity studies
In a study conducted by Shuval & Gruener (1972), groups of 8 male
rats (3 months old) were given tap water (control) or 100, 1000, 2000,
or 3000 mg of sodium nitrite per litre of drinking water. After 24
months, there were no significant differences in growth and
development, mortality, and total haemoglobin levels between the
control and treated groups. However, the methaemoglobin levels in the
groups receiving sodium nitrite at 1000, 2000, or 3000 mg/litre were
raised significantly throughout the study and averaged 5%, 12%, and
22% of total haemoglobin respectively. The methaemoglobin levels in
the group receiving 100 mg/litre were slightly above those of the
control group for the first 60 days only. There were some changes in
the liver and spleen of treated animals but the main pathological
changes occurred in the heart and lungs. In the heart, small foci of
cells and fibrosis were seen in some animals with pronounced
degenerative foci in animals receiving the highest concentrations of
nitrites. The coronary arteries were thin and dilated. The bronchi
were frequently dilated with the walls infiltrated by lymphocytes and
the mucosa and muscle were often atrophied. Emphysema was the rule.
These changes, which were present in 1 or 2 control rats and in a
small number of those receiving nitrite levels of 100 mg/litre, were
found with increasing frequency and severity in the 3 highest dose
groups.
Druckery et al. (1963a) did not observe extensive methaemoglobin
formation in rats given 100 mg sodium nitrite per kg body weight in
the drinking water, but there was a slight reduction in the life span.
Studies by Van Logten et al. (1972) in which groups of 30 male and 30
female rats received sodium nitrite at concentrations of 0, 0.02, or
0.05% with or without glucono--lactone (GDL) in the diet for 29
months did not demonstrate any significant haematological or
biochemical effects. Carcinogenic action, which could be related to
the administration of sodium nitrite with or without DEA or GDL, was
not observed in either of these studies.
It has been reported that prolonged administration to rats of
1/20 of the LD50 of calcium and sodium nitrates disturbed the energy
conversion processes such as glycolysis and the pentose phosphate
cycle, changed the activity of the glutathione-ascorbic acid system in
the blood and in the hepatic and cerebral tissues, raised the levels
of methaemoglobin and of NADH-methaemoglobin reductase activity, and
reduced the haemoglobin levels (Diskalenko & Dobrjanskaja, 1972;
Diskalenko & Trofimenko, 1972).
Greenblatt & Mirvish (1972) gave 3 groups of about 40, 7-9 week
old, male, strain A mice, 1 or 2 g of sodium nitrite or 12.3 g of
sodium nitrate/litre of water respectively, for 20 weeks and did not
observe any increase in lung tumour incidence in comparison with
untreated controls. Lijinsky et al. (1973a) reported similar negative
results when groups of 30 rats were given sodium nitrate at 5 g/litre
or sodium nitrite at 2 g/litre in their drinking water at a daily rate
of 20 ml/rat throughout most of their lifetime. Other studies by
Lijinsky et al. (1973c) on groups of 30 rats given 20 ml of sodium
nitrite at 2 g/litre daily, for 5 days a week, also produced negative
results. Taylor & Lijinsky (1975) reported that tumour formation in 57
Sprague-Dawley rats, exposed for 2 years to a drinking solution
containing sodium nitrite at 2 g/litre, was no greater than in the
controls.
7.1.3 Embryotoxicity
A study has been reported by Shuval & Gruener (1972) in which 2
groups of 12 pregnant rats were given 2000 or 3000 mg of sodium
nitrite per litre of drinking water, respectively. A control group did
not receive any treatment. Pregnant rats developed anaemia and had
higher methaemoglobin levels than nonpregnant rats receiving similar
doses. There was a pronounced increase in mortality among the newborn
rats of treated dams compared with those of untreated controls,
particularly in the 3-week period before weaning. Mortality in the
offspring was 6% in controls, 30% in those given 2000 mg/litre and
53% in those given 3000 mg/litre. Birthweights were similar in all
groups but growth was markedly reduced in pups of treated dams. Such
pups had thin hair coats. Treatment of 2 groups of 10 and 15 pregnant
rats with 1% and 0.3%, respectively, of sodium nitrate in the diet did
not result in any embryotoxic or teratogenic effects on the 9th and
10th days of gestation (Alexandrov & Jänisch, 1971).
Sleight & Atallah (1968) conducted a study in which 46 female
guineapigs, divided into 12 groups each containing at least 1 male,
were given potassium nitrate in doses ranging from 300 to 10 000 mg/kg
body weight in the water and potassium nitrite in amounts ranging from
300 to 10 000 mg/kg for periods ranging from 100-240 days.
Reproduction in the female was grossly impaired in the high nitrate
group. Fetal losses were 100% in females given 5000 or 10 000 mg/kg of
the nitrite and one female died. Reproduction was maintained at lower
levels of treatment. Apparently male fertility was not impaired, since
conception took place at all levels of treatment. Food and water
consumption and weight gains of treated animals were normal except for
a diminished rate of gain at a nitrite level of 10 000 mg/kg body
weight. Uterine and cervical inflammatory lesions and degenerative
placental lesions were present in females in which the fetuses had
been aborted, mummified, or absorbed. Sinha & Sleight (1971) reported
studies in which 4 pregnant guineapigs, given sodium nitrite at 50 mg
per kg of body weight, subcutaneously, underwent normal parturition.
However, fetal deaths followed by abortion, occurred in 3 guineapigs
given sodium nitrite at 60 mg/kg. The fetal deaths occurred
approximately 1 h after nitrite administration, when the maternal and
fetal methaemoglobin levels were highest. At the time of death, there
were no noticeable changes in the placenta; pathological changes
developed after the death of the fetuses. There were lower blood pO2
values in the fetuses of the guineapigs treated with nitrite at
60 mg/kg than in those of the controls. Fetal death did not occur in
pregnant animals given sodium nitrite at 60 mg/kg combined with
simultaneous intraperitoneal treatment with 10 mg of methylene blue
per kg body weight. The data suggest that fetal death resulted from
hypoxia, mainly induced by maternal methaemoglobinaemia.
Studies by Shuval & Gruener (1972) indicated that
methaemoglobinaemia might be induced transplacentally and that the
observed limit for transplacentally induced methaemoglobinemia was a
sodium nitrite dose of 2.5 mg/kg body weight. A steep increase in
effect occurred with increasing doses of sodium nitrite.
7.1.4 Mutagenicity
The mutagenic potential of nitrates and nitrites has not been
studied extensively; in fact, no data are available on their mutagenic
action in the mammalian systems. The mutagenicity of nitrates and
nitrites with respect to the transformation of DNA has been reported
(Bressler et al., 1968; Horn & Herriot, 1962; Strack et al., 1964) and
nitrous acid has been shown to be mutagenic in bacterial systems such
as Escherichia coli (Kaudewitz, 1959; Verly et al., 1967) and
Salmonella typhimurium (de Serres et al., 1967). Positive results in
mutagenic studies have been reported with the yeast Saccharamyces
cerevisiae (Nashed & Jabbur, 1966; Zimmerman & Schwaier, 1967;
Zimmerman et al., 1966), as well as with Aspergillus nidulans
(Siddiqi, 1962), A. niger, and A. amstelodami (Steinberg & Thom,
1940), and tobacco mosaic Virus (Sehgal & Krause, 1968).
7.1.5 Interaction with nutritional factors
Studies by Kociba & Sleight (1970) showed that maternal blood
levels of methaemoglobin were significantly higher in 12 ascorbic
acid-deficient, pregnant guineapigs, following the subcutaneous
administration of sodium nitrite at 40 mg/kg body weight, than in
those on a normal diet. Following subcutaneous administration of
sodium nitrite at 50 mg/kg, there was a higher percentage of fetal
death in the ascorbic acid-deficient guineapigs.
Experiments were conducted by Stoewsand (1973) with young, male,
guineapigs (number of animals not stated) to investigate the influence
of feeding beets with naturally occurring low and high amounts of
nitrates and nitrites, and the influence of dietary supplementation
with ascorbic acid and methionine on methaemoglobinaemia, induced by
orally administered sodium nitrite at doses of 25 or 50 mg/kg body
weight. Low-nitrate beet diets seemed to "protect" guineapigs from
nitrite intoxication. In addition, a 1% diet containing L-ascorbic
acid and 1% methione reduced nitrite-induced methaemoglobin blood
levels.
Sell & Roberts (1963) showed that ad libitum feeding of diets
containing 0.4% potassium nitrite to 6 groups of 50 chicks depressed
growth, irrespective of the amount of vitamin A administered. Also,
all the test animals, except those given massive injections of vitamin
A, exhibited reduced liver stores of the vitamin and enlarged thyroid
glands.
Studies by Phillips (1966) demonstrated that the liver vitamin A
contents of rats fed 1% potassium nitrite in diets containing carotene
or vitamin A, were less than those of control rats. It was suggested
that the dietary nitrite degraded the carotene and vitamin A in the
digestive tract before their absorption.
7.2. N-nitroso Compounds
7.2.1 Acute and subacute toxicity studies
The acute toxicity of N-nitroso compounds is not of great
toxicological significance because there is no relationship between
acute toxicity and the carcinogenic potential of this class of
compounds (Druckrey et al., 1967, Magee & Barnes, 1967). Because DMN
was reported to cause cirrhosis and other toxic effects in industrial
workers, Barnes & Magee (1954) examined the toxicity of this compound.
Doses of 20-40 mg/kg body weight given to rats, dogs, rabbits, and
guineapigs produced severe hepatic damage. A single dose of DMN given
to rats orally or by intravenous, intraperitoneal, or subcutaneous
injection, produced centrilobular necrosis accompanied by haemmorhages
in the liver. In rats given 20 mg DMN/kg body weight the liver cells
in the centrilobular and mid-zonal regions became pale and, after
18 h, the cytoplasm was amorphous and vacuolated; the nuclei were pale
and irregular in outline. By 24 h, the cells were necrotic and
confluent areas became haemorrhagic. Haemorrhage was usually more
pronounced after 48 h but after 72 h the recovery process had begun
and was almost complete in 3 weeks (Barnes & Magee, 1954; Magee &
Barnes, 1962). A detailed study by light and electronmicroscopy of
changes in the liver cytoplasm of rats treated with
N-nitrosomorpholine has been reported by Bannasch (1968).
The acute toxicity of acyl-alkyl-nitrosamides and diazoalkanes
was reported by Druckery et al. (1967), Magee & Barnes (1967), and
Shank (1975). The acute toxicity (LD50) of the nitroso compounds
varied widely; some were only mildly toxic while others produced
highly destructive lesions. N-nitroso- N-methylurethane, for
example, when given orally, produced severe necrotic lesions in the
stomach, congestion of the lungs, and periportal necrosis of the liver
(Schmähl & Thomas, 1962; Schoental, 1960). N-nitroso- N-methylurea
also produced inflammatory haemorrhagic lesions of the stomach,
intestine, and pancreas and a reduction in the bone marrow when given
orally to rats, (Druckrey et al., 1961, 1967).
The acute toxicity of some N-nitroso compounds, e.g. nitroso-
piperidine and nitroso-morpholine, was not manifested as liver damage
but as neurotoxic effects, e.g. convulsions (Lee & Lijinsky, 1966).
The acute toxicity of nitrosomines formed in vivo was studied
by Asahina et al. (1971). Sodium nitrite was administered by gavage to
mice at 100 or 150 mg/kg bodyweight alone or in combination with DMA
and methylbenzylamine at doses of 500-2500 mg/kg and 800-1600 mg/kg,
respectively. Combinations of the amines and nitrite produced hepatic
lesions similar to those produced by DMN or nitrosome-thylbenzylamine.
Similar effects were noted when nitrite was administered up to 3 h
after DMA but these effects were markedly reduced if the nitrite was
given prior to the amine. Lijinsky & Greenblatt (1972) reported
hepatic necrosis following coadministration of aminopyrine and sodium
nitrite.
In a number of studies, ascorbic acid has been shown to have an
inhibitory effect on: a) nitrosamine formation in the stomach and
small intestine of rats treated with 7-chloro-2-methylamino-5-phenyl-
3H-1, 4-benzodiazepine-4-oxide, (chlordiazepoxide, Librium) and sodium
nitrite (Preda et al., 1976); b) hepatotoxicity induced by the
combined administration of sodium nitrite and aminopyrine in rats
(Kamm et al., 1973) and mice (Greenblatt, 1973); c) liver necrosis
produced by DMA and nitrite administered to rats by gavage (Cardesa et
al., 1974); d) the teratogenic and transplacental carcinogenic effects
produced in rats by treatment with alkylurea and nitrite (Ivankovic et
al., 1973); and e) the induction of lung adenomas in mice by prolonged
treatment with morpholine, piperazine, and methylurea plus nitrite
(Mirvish et al., 1975).
Species differences exist with respect to the toxic effects of
N-nitroso compounds. Mink appear to be especially sensitive to DMN,
and as in other species, the effects are seen primarily in the liver.
Carter et al. (1969) noted widespread liver degeneration and necrosis
of hepatocytes in mink given DMN at 2.5 or 5.0 mg/kg in the diet for
7-11 days. The liver lesions were accompanied by bile-duct
proliferation, ascites, and haemorrhage of the gastrointestinal tract.
Sheep and cattle are also more sensitive to nitrosamines than
laboratory animals (Sakshaug et al, 1965; Koppang, 1964). Sheep, given
a single dose of DMN at 5 mg/kg body weight or 12 doses of 0.5 mg/kg,
died or were severely affected displaying anoxia, lack of rumination,
ataxia, and respiratory difficulties, while cattle given DMN at
0.1 mg/kg body weight showed pronounced hepatotoxic effects in 1-6 months.
Pathological and biochemical effects, observed in the liver of a
number of animal species including the rat following continuous
administration of nitrosamides, have been reviewed by Magee & Barnes
(1967) and Magee et al. (1976); the main effect is inhibition of
protein synthesis which might be a result of an accelerated breakdown
of messenger ribonucleic acid (RNA).
7.2.2 Carcinogenicity
The carcinogenic activity of N-nitroso compounds has been
summarized in several reviews (Druckrey, et al., 1967; Magee & Barnes,
1967; Magee et al., 1976). Various animal species including mammals,
birds, fish, and amphibia have been shown to be susceptible to the
carcinogenic action of nitrosamines. At present, some 80 nitrosamines
and 23 nitrosamides have been tested in rats and about 80% of the
nitrosamines and practically all the nitrosamides have proved to be
carcinogenic (Montesano & Bartch, 1976). These carcinogens show a
marked organ specificity as shown in Table 3.
Nitrosamines produce a carcinogenic effect in the liver,
oesophagus, respiratory system, and kidney, whereas nitrosamides
affect the peripheral and central nervous systems, and the gastro-
intestinal tract organs.
The dose schedule seems to play an important role in this organ
specificity. For example, in rats, long-term exposure to relatively
low doses of DMN induced mainly liver tumours, whereas a single or a
few high doses over a short period induced mainly kidney tumours
(Magee & Barnes, 1962). Similar responses in relation to the liver and
oesophagus were observed with DEN in rats (Druckey et al., 1967). The
route of administration does not seem to play an important role in the
carcinogenicity of this chemical. It is worthwhile pointing out that
many of these chemicals were carcinogenic following a single
administration and that, furthermore, exposure of rats to a single
dose during pregnancy resulted in carcinogenesis in the immediate
descendants and also in the two succeeding generations (Tomatis et
al., 1977).
Nitrosamines exert their adverse biological effects after being
metabolically activated by microsomal mixed function oxidases to form
reactive intermediates. On the other hand, nitrosamides decompose
enzymatically to reactive, and in most cases, alkylating derivatives.
The importance of hydroxilation of the alpha-hydrogen atoms of the
nitrosamines is demonstrated by the lack of carcinogenicity of
compounds, such as diphenylnitrosamine, which do not have this alpha-
hydrogen (Magee et al., 1976).
Table 3. Localization of tumours induced by N-nitroso
compounds in ratsa
Number of N-nitroso compounds
affecting target organ
Target organ Nitrosamines Nitrosamides
liver 35 2
oesophagus-pharynx 32 3
nasal cavities 18 -
respiratory tract 10 1
kidney 8 9
tongue 8 -
forestomach 7 11
bladder 4 1
central and peripheral 2 9
nervous system
ear duct 2 1
testis 1 -
ovary 1 2
mammary glands 1 1
sites of injection 3 4
intestine - 7
glandular stomach - 6
skin - 3
jaw - 1
uterus - 2
vagina - 1
haemopoietic system - 2
a From: Montesano & Bartch (1976)
7.2.2.1 Interspecies variation in response
Several N-nitroso compounds have been tested in different
animal species. DEN, tested in more than 20 species including
primates, induced tumours of the liver in all of them, together with
various tumours of other organs. In some cases, there have been marked
differences between species in response to N-nitroso compounds. For
example, nitrosoheptamethyleneimine produced squamous carcinoma of the
lung in rats (Lijinsky et al., 1969), the same effects in European
hamsters, but tumours of the forestomach and the lung in Syrian
hamsters (Lijinsky et al., 1970). In all three species, this compound
also induced oesophageal tumours. Nitrosomethylurethane induced
tumours of the pancreas in guineapigs (Druckrey et al., 1968) and
forestomach carcinomas in rats. Bis-2-hydroxypropyl-nitrosamine
produced pancreatic tumours in Syrian hamsters. These results show
the difficulty of attributing, with certainty, any particular tumour
response of man to a particular N-nitroso compound.
7.2.2.2 Intraspecies variation in response
Kuwahra et al. (1972) administered DMN orally or by subcutaneous
or intraperitoneal injections, to 8 to 12-week-old, male and female
mice of the DDD, BALB/C, and SJL/J strains. Tumours were found mainly
in the retroperitoneum and abdominal cavity and the incidence and
distribution were little affected by the strain of mouse but markedly
by the route of administration. Clapp et al. (1971) noted that DEN
induced forestomach and oesophageal squamous cell carcinomas in both
BALB/C and RF/Un strains of mice but induced liver haemangiosarcomas
in the former and hepatomas in the latter. In addition, DEN induced a
high incidence of lung tumours in RF mice but a very low incidence in
BALB/C mice. In both strains, DMN induced lung adenomas and liver
haemangiosarcomas. Tissue sensitivity did not appear to be related to
the spontaneous tumour incidence of the strain.
7.2.2.3 Dose-response relationships of N-nitroso compounds
Druckrey et al. (1963b) were the first to study the dose-response
relationships of N-nitroso compounds in relation to minimum effect
doses. They concluded, from studies in rats given DEN, that the
carcinogenic effect was related to dose and induction time in such a
way that d.t2.3 = constant, where d represents the daily dose and
t the induction time. Even a low dose of 0.15 mg/kg body weight
resulted in liver carcinomas in 27 out of 30 surviving animals. The
average induction time was 609 ± 38 days. The lowest dose studied
(0.075 mg/kg body weight) produced liver and oesophageal tumours in
all four surviving animals with an induction time of 830 days.
Mohr & Hilfrich (1972) gave a single intravenous injection of DEN
to rats at 8 dose levels between 1.25 and 160 mg/kg body weight. A
dose-response relationship was noted; at the lowest dose of 1.25
mg/kg, only one kidney tumour was observed in 20 treated rats, but, at
higher doses, the incidence of these tumours increased. Single dose
experiments with ethylnitrosourea in a transplacental carcinogenesis
experiment also showed that the incidence of induced tumours (mainly
of the brain and nervous system in the progeny) was directly
proportional to the dose of the carcinogen. Four doses between 1 and
50 mg/kg were used and even in the lowest dose group 36/41 animals in
the progeny died with tumours (Swenberg et al., 1972). Terracini et
al. (1967) fed rats concentrations of DMN ranging from 2 to 50 mg/kg
diet. At 2 and 5 mg/kg the incidence of liver tumours in the survivors
was 1/26 and 8/74, respectively, at 60 weeks. At 20 and 50 mg/kg,
liver tumours were observed in more than 60% of the test animals.
From this study, it can be concluded that a dietary level of DMN of
5 mg/kg is still carcinogenic in the rat.
A current dose-response study on rats receiving oral doses of
N-nitrosopyrrolidine has been reported by Preussmann (unpublished
data).a Liver tumours were induced in groups receiving 10, 3, and
1 mg/kg body weight per day, respectively, but not in a group
receiving 0.3 mg/kg per day.
Clapp & Toya (1970) gave male, RF mice DMN in the drinking water
for various lengths of time, with cumulative doses ranging from 87 to
243 mg/kg body weight. The incidence of lung adenomas in all treated
groups was higher than that in the untreated control groups. Whereas,
apparently, the incidence of hepatocellular tumours was not affected,
the incidence of liver haemangiosarcomas increased in the higher dose
groups and reached a maximum of 96%. Bertram & Craig (1973) using 2
groups of 100, C57BL/6 mice found that the incidence of bladder
tumours fell from 80% in animals given 30 mg of nitrosodi- N-
butylamine per kg/body weight per day to 36% at 7.5 mg/kg body weight
per day. Sander & Schweinsberg (1973) noted that an increasing
incidence of tumours of the oesophagus and forestomach was induced in
NMRI mice by adding a given dose of methylbenzylnitrosamine to the
drinking water at various times to provide total doses ranging from
1.4 to 44 mg/kg body weight.
a Paper presented at the Proceedings of the Second International
Symposium on Nitrite in Meat Products, Zeist, September 1976.
In studies conducted by Vesselinovitch (1969), DMN was
administered repeatedly by intraperitoneal injection to 73 male and 54
female C57BL x C3H mice starting at 7 days of age. The injections were
given at 3 day intervals for a total of six doses of 1, 2, or 4 mg/kg
body weight. Mice killed at 66 weeks of age showed an increase in the
incidence of hepatomas, hepatocarcinomas, lung adenomas, and
haemangiomas as the dose increased. The incidence of liver tumours was
higher in males (75%) than in females (28%).
Tomatis & cefis (1967) gave a dose of 3.6 mg of DMN by stomach
tube to one group of 30 Syrian golden hamsters in 3 administrations of
1.6 mg, 1.0 mg, and 1.0 mg over a 5-week period. A second group
received a single dose of 1.6 mg. Both dosing regimes produced liver
cell carcinomas and a few cholangiomatous lesions but no kidney
tumours. Montesano & Saffiotti (1968) administered 12, weekly,
subcutaneous injections of DEN at 0.5, 1.0, 2.0, or 4.0 mg to 4 groups
of 36 Syrian golden hamsters. The results demonstrated a positive
dose-response relationship for tumour induction in the upper
respiratory tract (nasal cavities, larynx, and trachea). The incidence
of nasal cavity tumours, which developed early, varied from 6/35 to
27/36. The incidence of tumours of the larynx varied from 6/35 to
26/36 and that of tumours of the trachea from 29/35 to 35/36.
7.2.2.4 Tumour induction by combined administration of nitrites
and amines or amides
Carcinogenic effects following the combined administration of
secondary and tertiary amines or amides with nitrite have been
reported. Tumours were not observed when sodium nitrite or the amine
or amide were given singly. Tumours of the same type occurred at the
same site when the N-nitroso compound, believed to be formed from
the nitrite and amine or amide, was given as a positive control. In
some of the experiments, the nitrite was given in the drinking water
and the amine or amide in the food. In others, the compounds were
combined in the same medium (drinking water or food).
Preussman (1975) reviewed studies in which tumours that had been
produced at certain sites by the oral administration of combinations
of amines and nitrite, were compared with the tumours induced by the
corresponding N-nitroso compounds. Table 4 has been adapted from
Preussman (1975) and updated.
Table 4. In vivo formation of N-nitroso compounds following oral administration of sodium nitrite and amino
compounds as demonstrated by specific carcinogenesisa
sodium nitrite + amine Expected tumour Reference
oral administration Observed tumour site for
amino compounds site corresponding
N-nitroso compound
Rat
diethylamine -- liver Druckrey et al.
(1963b)
Sander et al.
(1968)
Sander (1971a)
triethylamine -- liver (for Schweinsberg &
diethylnitrosamine Sander (1972)
morpholine liver, kidney, lung liver Sander & Burkle
N-methylbenzylamine oesophagus oesophagus (1969), Sander
(1971c)
piperidine -- oesophagus
liver
N-methylanaline oesophagus, nasal oesophagus
cavity
N-methylcyclohexylamine oesophagus oesophagus Sander (1971a)
Table 4 (Cont'd)
sodium nitrite + amine Expected tumour Reference
oral administration Observed tumour site for
amino compounds site corresponding
N-nitroso compound
N-methylbenzylamine oesophagus oesophagus
phenylbenzylamine negative untested
N,N'-dibenzylethylenediamine negative untested
indole negative untested
morpholine liver (kidney) liver (kidney) Shank & Newberne
(1972)
aminopyrine liver liver (for Lijinsky et al.
(Pyramidon) dimethylnitrosamine) (1973c)
heptamethyleneamine lung lung
oesophagus oesophagus
aminopyrine liver liver Taylor &
(for DMN) Lijinsky
(1975)
oxytetracycline liver (for DMN) liver Greenblatt et al. (1973)
proline negative untested
Table 4 (Cont'd)
sodium nitrite + amine Expected tumour Reference
oral administration Observed tumour site for
amino compounds site corresponding
N-nitroso compound
hydroxyproline negative untested
arginine negative untested
N-methylacetamide negative forestomach
N-methylurethane negative forestomach
N-ethylurethane negative (carcinogenic Sander (1971b)
following
i.v. administration)
acetanilide negative untested
glycylglycine negative untested
N-methyluracil negative untested
N-methylguanidine negative untested
N-phenylurea negative (carcinogenic Sander (1971b)
following
s.c. administration)
N-methylthiourea negative untested
N-methylurea brain, nervous brain, nervous
system, kidney system, kidney
Table 4 (Cont'd)
sodium nitrite + amine Expected tumour Reference
oral administration Observed tumour site for
amino compounds site corresponding
N-nitroso compound
N-ethylurea brain, nervous brain, nervous
system, kidney system, kidney
N'N'-dimethylurea brain, nervous brain, nervous Sander (1971b)
system, kidney system, kidney
imidazolidinone kidney (carcinogenic Sander & Burkle
following (1971)
s.c. administration)
ethylurea (during brain, nervous brain, nervous Ivankovic &
pregnancy, system system (in Preussmann
transplacental) (in descendants) descendants) (1970); Osske
et al. (1972)
Mouse
dimethylamine lung (adenoma)
piperazine lung (adenoma) lung (adenoma) Greenblatt et al. (1971)
morpholine lung (adenoma) lung (adenoma)
N-methylaniline lung (adenoma) lung (adenoma)
morpholine lung (adenoma) lung (adenoma)
Table 4 (Cont'd)
sodium nitrite + amine Expected tumour Reference
oral administration Observed tumour site for
amino compounds site corresponding
N-nitroso compound
N-methylbenzylamine oesophagus oesophagus Sander (1971a)
forestomach forestomach
piperazine lung (adenoma) lung (adenoma) Greenblatt &
Mirvish (1972)
N-methylurea lung (adenoma) lung (adenoma) Mirvish et al. (1973b)
N-ethylurea lung (adenoma) lung (adenoma)
a Adapted from Preussmann (1975)
7.2.2.5 Dose-response relationship for combinations of nitrite
and amines
Greenblatt & Mirvish (1972) conducted a study in which groups of
40, male, strain A mice, given 0.69-18.75 g of piperazine per kg of
food and 0.05-2.0 g of sodium nitrite per litre of drinking water for
20-25 weeks, were killed 10-13 weeks later. The yield of lung adenomas
was statistically significantly greater than in untreated controls,
when doses as low as 0.69 g piperazine/kg plus 1.0 g sodium nitrite/
litre, or 6.25 g piperazine/kg plus 0.25 g sodium nitrite/litre were
given. In the animals given 6.25 g piperazine per kg of food, plus
2.0 g sodium nitrite per litre of water, 39 out of 40 had adenomas,
in contrast with 5 out of 39 controls. A progressive decrease in the
incidence of adenomas was seen with reduction in the nitrite level.
On the other hand, when the nitrite level was maintained at
1.0 g/litre, the tumour incidence increased marginally when the dose
of piperazine was increased from 0.69 g to 18.75 g/kg of food.
Nitrite and piperazine administered alone yielded negative results.
When various concentrations of nitrite and morpholine (up to
1000 mg/kg) were fed to groups of Sprague-Dawley rats, the
development of hepatocellular carcinomas and angiosarcomas identical
to those produced by N-nitrosomorpholine was noted (Newberne &
Shank, 1973).
A 2-3% incidence of hepatomas was induced with only 5 mg of
morpholine plus 1000 mg of sodium nitrite per kg of food, or 1000 mg
of morpholine plus 50 mg sodium nitrite/kg. The tumour incidence was
98% when the concentration of both components was 1000 mg/kg, and 0%
when diet alone was given.
7.2.2.6 Transplacental carcinogenesis
Induction of neoplasms in offspring as a result of prenatal
exposure to various N-nitroso compounds and related substances has
been reported in different animal species including the rat, mouse,
golden hamster, guineapig, rabbit, dog, and monkey (Magee et al.,
1976). The various routes of administration (subcutaneous,
intraperitoneal, intravenous, oral, and inhalation) were equally
effective. However, a critical factor was the time of treatment during
gestation.
Since many of these substances appeared to be metabolically
activated to exert their carcinogenic action, the lack of an adequate
metabolic system during the first period of pregnancy may explain the
failure to observe tumours, when exposure was limited to this period.
DMN induced tumours in the offspring only when administered during the
last days of pregnancy (Alexandrov, 1968a). The data of Magee (1972)
are in keeping with these findings; the formation of 7-methylguanine
in the nucleic acids of fetal rat tissues was detected following
treatment with DMN on the 21st day of pregnancy but not on the
15th day.
When considering the effect of different acyl alkyl nitrosamides,
N-ethyl- N-nitrosourea was found to be more active than its methyl
analogue (Alexandrov, 1969b; Ivankovic & Druckrey, 1968).
The sensitivity of the nervous system at various stages of
prenatal development was examined in rats and golden hamsters by
Ivankovic & Druckrey (1968) by means of single intravenous injections
of N-ethyl- N-nitrosourea on different days during gestation. A
high incidence of tumours was observed after treatment on the 18th day
or shortly before delivery (21st day) but none developed when the
mother animals were treated before the 12th day. A positive dose-
response relationship was obtained with a single dose in the range of
5 to 80 mg/kg bodyweight on the 15th day. A single dose as low as
2 mg/kg was sufficient to produce malignant, neurogenic tumours in
2/25 newborn whereas in adult animals an effect was produced in 50%
of the animals by a single dose at 160 mg/kg. Thus a 50-times higher
sensitivity was demonstrated for the fetal nervous system. Similar
results were obtained by Swenberg et al. (1972) in Sprague-Dawley and
Fisher rats using a dose of N-ethyl- N-nitrosourea as low as
1 mg/kg. In a recent study on rats, Maekawa & Odashima (1975) explored
the effects of subcutaneous injections of N-butyl-1-nitrosourea on
embryonal, fetal, and newborn nervous systems. Treatment during early
gestation led only to the death of the embryo. When the compound was
given in the middle of the pregnancy (8-14 days), a high incidence of
nervous system tumours and pituitary tumours was found in the
offspring. Treatment late in pregnancy (15-21 days) led to the
development of nervous system tumours in 33/36 offspring.
When lactating animals were given the compounds, tumours were
induced in 19/39 of the offspring, the target organs being mainly the
testes and uterus.
Tomatis (1977) reported an increased incidence of cancer in the
descendants (second and third generation) of transplacentally treated
rats. In certain cases, the tumour-producing doses were lower than
those for adult animals. The target organs were usually similar in
fetal, newborn, and adult animals but the nervous system has been
shown to be highly sensitive to certain compounds, notably the
nitrosoureas. The induction time for transplacental tumours may be
shorter than that in adults.
7.2.2.7 Morphological studies
Morphological events associated with the development of hepatic
tumours, following exposure to N-nitroso compounds, were studied by
Rabes et al. (1970), Scherer et al. (1972), and Schmitz-Moorman et al.
(1972). Schmitz-Moorman et al. (1972) followed the carcinogenesis
induced by DMN in rat liver, using histological and histochemical
procedures. In the initial phase, there were vacuolar changes
accompanied by a decrease in the RNA and glycogen contents. In the
second phase, glycogen storage was noted while in the third phase,
basophilic cells with atypical nuclei were observed and could not be
distinguished from microcarcinomas. The RNA content of these cells was
substantially higher. The activities of acid phosphatase (3.1.3.2),
succinic dehydrogenase (1.3.99.1) and glucose-6-phosphatase (3.1.3.9)
substantially decreased. In the last carcinogenic stage, dramatic
changes were noted in several enzymes (Jennissen et al., 1971).
Early changes in the lung tissue of mice exposed to carcinogenic
doses of DMN were reported by Calafat et al. (1970) and DEN-induced
alterations in the respiratory tract of hamsters were reported by
Althoff et al. (1971). Greenblatt & Rijhsinghani (1969) compared the
cytopathological changes induced by DEN and DMN in the nasal
epithelium of hamsters; other light microscopic studies have been
published by Bertram & Craig (1972), Boyland et al. (1968), Hicks et
al. (1973), and Terracini et al., (1967). The histological appearance
of tumours under the electron microscope has been described by
Kirkland & Pick (1973). Veno-occlusive disease in the liver of rats
given DMN was reported by Butler & Hard (1971) who had also studied
the effects of this compound on rat testes (Hard & Butler, 1970c).
Other studies, by light microscopy, to observe early changes
related to the formation of neoplasms induced in the kidneys of rats
treated with DMN, were reported by Benemanski & Litvinov (1969), Hard
& Butler (1970a, 1970b), and Hard et al. (1971).
Electron microscopic studies of DMN-induced liver and kidney
tumours have been reported in rats (Bhathal & Hurley, 1973; Geil et
al., 1968; Hard & Butler, 1971a, 1971b, 1971c; Ireton et al., 1972;
Jasmin & Cha, 1969; Svoboda & Higginson, 1968) and in mice (Takayama,
1968). Treatment with DEN resulted in ultrastructural changes in the
lungs of hamsters (Stracks & Feron, 1973) and in the liver of monkeys
(Williams, 1970) and rats (Bader et al., 1971; Bruni, 1973). Early
morphological changes in the bladder of rats exposed to N-butyl- N-
butanol-4-nitrosamine were reported by Riedel & Piper (1973).
7.2.2.8 Biochemical mechanisms
Extensive work on the biochemical mechanisms of carcinogenesis
produced by N-nitroso compounds has been reviewed by Magee & Barnes
(1967) and more recently by Magee et al. (1976). Alkylation of nucleic
acids by N-nitroso compounds or their metabolites has been
investigated extensively and has been suggested as the mechanism of
carcinogenicity (Krüger, 1972, 1973; Lijinsky et al., 1973b; Swann &
Magee, 1971; Takayama & Muramatsu, 1969). In early studies, it was
thought that the 7 position of guanine was the significant site of the
reaction.
Following the important discovery by Loveless and Hampton (1969)
of the O,6-alkylation of deoxyguanosine by nitrosomethylurea (NMU)
and the implications of this reaction in terms of carcinogenicity,
O'Connor et al. (1973) estimated the amount of methylation at the
O,6-position of guanine DNA isolated from animals treated with DMN.
This base accounted for 4-6% of the methylation after DMN treatment.
O,6-methylguanine was lost (by "excision") from DNA with a half life
of approximately 13 h. The excision of the abnormal components of DNA,
O,6-methylguanine, and the unstable acid-labile products, may be
important processes in liver carcinogenesis. O'Connor et al. (1973)
suggested that events leading to the development of tumours may be
related to the efficiency of the cellular excision system for certain
products of alkylation rather than to the level of alkylation obtained
at a particular site.
7.2.2.9 Interaction with various chemical factors
Several studies are available on the combined effects of
N-nitroso compounds and other carcinogens. Schmähl et al. (1963)
showed that combined oral administration of the hepatocarcinogens DEN
and N,N-dimethyl-4-(phenylazo)benzenamine (4-dimethyl-
aminoazobenzene) significantly reduced the time for tumour induction
and that only 66% of the total dose administered when single compounds
were used, was necessary for tumour induction. Takayama & Imaizumi
(1969) also demonstrated synergism with the combined administration of
DMN and 4-dimethylaminoazo-benzene. Liver tumours were induced in rats
by sequential administration of the two carcinogens in doses that did
not induce tumours when each carcinogen was given alone and the tumour
induction time was reduced. The syncarcinogenic effects of a single
dose of a combination of DEN and carbon tetrachloride were observed by
both Pound et al. (1973) and Schmähl et al. (1965). The incidence of
liver cancer increased and the induction time decreased in combined
treatments compared with treatment with the individual compounds.
Schmähl (1970) administered hepatocarcinogens (DMN, DEN,
nitrosomorpholine, and 4-dimethylaminoazobenzene) to rats in such low
daily doses that the administration of any one of the compounds alone
did not lead to tumours during the lifetime of the animals. However,
combined administration induced tumours in 43% of the treated animals.
Combined administration to rats of DMN plus 1,2-dihydro-3-methyl-
benz[j]aceanthrylene (3-methylcholanthrene) did not increase the
number of liver tumours compared with that induced by DMN alone but
resulted in tumours in the lungs, that were not seen in the treatment
with DMN alone (Hoch-Ligeti et al., 1968).
1-Isothiocyanatonaphthalene (1-napthyl-isothiocyanate) and
3-methyl-cholanthrene failed to inhibit the hepatocarcinogenic
effects of DEN (Makiura et al., 1973). Similarly, local treatment of
the glandular mucosa of the stomach of rats with 4-nitroquinoline-
1-oxide or p-dimethylaminoazobenzene did not have any effect on
the production of liver or oesophagal tumours due to oral
administration of DEN (Odashima, 1969). It has been reported that
ionizing radiation did not increase tumour incidence in animals
exposed to nitrosamines (Flaks et al., 1973; Schmähl et al., 1966).
The effect of many other substances on nitrosamine carcinogenesis
has been studied. For example, various dusts including aluminium (III)
oxide, magnesium (II) oxide, and carbon had little effect on the
respiratory carcinogenesis of DEN in hamsters (Stenback et al., 1973)
but other studies on hamsters demonstrated that iron (III) oxide
markedly increased the incidence of respiratory tract tumours induced
by DEN (Feron et al., 1972). The synergistic effects of various
substances on respiratory carcinogenesis in hamsters given N-nitroso
compounds were reviewed by Montesano (1970).
The effect of noncarcinogenic chemicals on the incidence of other
tumours induced by N-nitroso compounds has also been studied. In
rats given DEN, liver tumour incidence was decreased by the
administration of calcium heparin (Platt & Hering, 1973),
aminoacetonitrile (Hadjiolov, 1971), and reserpine (Lacassagne et al.,
1968). Lactoflavin, nicotinamide, or 2,6-bis-(diethanolamino)-4,8-
dipiperidino-pyrimido [5,4-d] pyrimidine (dipyridamole) (Schmähl &
Stackelberg, 1968) and hydrocortisone (Schmähl et al., 1971) did not
influence the incidence of liver tumours induced in rats by DEN.
Tryptophan was shown to inhibit the production of liver tumours, but
not bladder tumours in rats exposed to N-nitrosodibutylamine
(Okajima et al., 1971). N-(2-chloroethyl)- N (phenylmethyl)
benzenemethanamine (dibenamine) did not have any effect on the
incidence of oral, pharyngeal, or oesophageal tumours in DEN-treated
rats, but did significantly reduce the number and severity of hepatic
neoplasms (Weisburger et al., 1974). In mice, treatment with
phenobarbital decreased the toxicity and carcinogenicity of DEN (Kunz
et al., 1969).
7.2.2.10 Miscellaneous modifying factors
Nasal infection of mice with the influenza viruses PR8/FIK and
A2/Bethesda 10/63 followed by treatment with DEN significantly
increased lung tumour incidence in comparison with that in mice
treated only with DEN (Schmidt-Ruppin & Papadoupulu, 1972). The Motol
virus has been shown to increase hepatic carcinoma in mice given DEN
(Kordac et al., 1969). Rats with chronic respiratory disease showed an
increased lung tumour incidence when given nitrosoheptamethylenemine
compared with germ-free or specific pathogen-free animals (Schreiber
et al., 1972).
Feeding with a protein-deficient diet protected rats against the
lethal and hepatotoxic effects of DMN (McLean & Verschuuren, 1969) but
increased the incidence of renal carcinomas (McLean & Magee, 1970).
Low protein diets and low zinc intake failed to influence the
incidence of oesophageal tumours in rats treated with N-methyl- N-
nitroso-'pentyl' amine (Van Rensburg, 1972). A single intraperitoneal
injection of DMA induced nasal tumours in rats, previously starved for
48 h (Noronha & Goodall, 1972).
The carcinogenic activity of nitroso- N-(hydroxybutyl)
butanamine (butyl-( N-hydroxybutyl) nitrosamine) appears to be
influenced by sex hormones (Bertram & Craig, 1972). Tumours developed
much earlier in male, than in female mice. This difference was
abolished, however, if the males were castrated or, conversely, if the
females were treated with testosterone.
Surgical manipulation of experimental animals may influence the
induction of tumours by N-nitroso compounds. This has been shown for
partial hepatectomy (Craddock, 1971; Grunthal et al., 1970; Rabes et
al., 1971) unilateral nephrectomy (Ito et al., 1969) and ureter
ligation (Ito et al., 1971).
7.2.3 Embryotoxicity and teratogenicity
Whereas nitrosamines are reported to have toxic and lethal
effects on the embryo, usually at dose levels that are toxic to the
mother animals, nitrosamides ( N-ethyl-and N'methyl urea) bring about
malformations of several of the organs and systems of the developing
organisms at levels not toxic to the pregnant animal. Animal
experiments have shown that immature tissues are especially sensitive
to these compounds. Dose-response relationships have been established
and the existence of a noneffect level has been indicated.
DMN, administered orally at 30 mg/kg to pregnant rats, produced
increased prenatal mortality (Alexandrov, 1967). Toxic effects on the
embryo were noted following intravenous or intraperitoneal
administration of DMN at various times during gestation but no
teratological abnormalities were observed. Similar results were
observed with DEN and with nitroso- N,N-bis butanamine (nitrosodi-
N-butylamine) in rats (Alexandrov 1967), and with DEN in hamsters
(Pielsticker, 1967). The aromatic nitroso compounds such as
nitrosomethylbenzenamine (nitrosomethylaniline) were toxic to the
embryo and had a mild teratogenic action when given to rats at the
maximum tolerated doses (Alexandrov, 1968a, 1968b).
Treatment of pregnant rats with a single dose of 10-30mg of
nitrosomethylurea per kg body weight on day 9 of gestation produced
anophthalmia, hydrocephaly, exencephaly, and occasionally spina bifida
while treatment on days 12-15 produced microcephaly (Alexandrov,
1969a; Koyama et al., 1970; Napaikov, 1971; Von Kreybig, 1965a, 1965b;
Von Kreybig & Schmidt, 1966, 1967).
The teratogenic action of nitrosoethylurea in rats was described
by Druckrey et al. (1966) and Ivankovic & Druckrey (1968) who noted
that oligodactylia and syndactylia of the fore and hind limbs were
dose-related. Von Kreybig & Schmidt (1967) and von Kreybig (1968)
confirmed these effects and also found brain anomalies. Napalkov
(1971) and Wechsler (1970, 1971) reported similar results. The
teratogenic and embryotoxic effects of N-nitroso compounds including
the pathogenesis of brain lesions were reviewed by Wechsler (1973).
7.2.4 Mutagenicity
The mutagenicity of N-nitroso compounds was reviewed by Magee &
Barnes (1967) and more recently by Montesano & Bartch (1976). Until
recently, the data available were mainly concerned with the
nitrosamides, the data on nitrosamines being limited to test systems
which do not take into consideration the metabolic activation of these
compounds by mammalian enzymes. As with other biological effects,
there is a clear distinction between the mutagenic action of
nitrosamides and nitrosamines. Nitrosamides were found to be mutagenic
to almost all genetic indicators; this was attributed to the
nonenzymatic formation of alkylating reactants.
On the other hand, nitrosamines have been reported, prior to
1970, to have a much more limited range of mutagenic activity; they
were found to be mutagenic in tests with Drosophila melanogaster
(Pasternak, 1964) but no such activity was observed in assays in
which bacteria, yeast, or fungi were used. Malling (1966) showed that
DMN and DEN were mutagenic for Neurospora crassa when the conidia
were suspended in Udenfriend's hydroxylating model system and the
treatment carried out under conditions believed to give rise to the
same metabolic products as those formed by the action of liver enzymes
in rat and mouse. Garbidge & Legator (1969), using the host-mediated
assay, were the first to show that DMN, administered to mice, induced
mutations in Salmonella O,phimurium, which had been injected
beforehand and was then reisolated from the peritoneal cavity. Most of
the mutagenicity assays were carried out using bacteria or fungi as
genetic indicators in the presence or absence of a microsomal
activation system. Only a few data are available on the mutagenicity
of DMN in mammalian systems such as the dominant lethal test or
cytogenetic studies. However, in general, the last two systems have a
very low sensitivity and false negatives can easily result with
N-nitroso compounds. The early observation of Pasternak (1962)
that DMN induced lethal mutations in Drosophila melanogaster
demonstrated the possible value of this system for testing the
mutagenicity of compounds that require metabolic activation.
The mutagenicity of certain nitrosated pesticides, herbicides,
and primary amines has been examined. The N-nitrosated derivatives
of the pesticides propoxur and carbaryl, which are aryl- N-methyl
carbamates, and of the herbicide benzothiazuron, a methylurea
derivative, induced mitotic gene conversion in Saccharomyces
cerevisiae (Sibert & Eisenbrand, 1974). N-nitrosocarbaryl was
also found to be mutagenic in Haemophilus infiuenzae (Elespuru
et al., 1974). Endo et al. (1973) screened a number of
N-nitrosated guanidine derivatives for their ability to induce
base pair substitutions in Salmonella typhimurium and a mutagenic
effect was observed for many of these compounds; nitrosated
methylguanidine was the most potent.
The mutagenicity of sodium nitrite, DMA, methylurea, and
ethylurea given orally to mice, has been demonstrated in a host-
mediated assay using a strain of Salmonella typhimurium as an
indicator (Couch & Friedman, 1975). When combined with sodium nitrite,
both ethylurea and methylurea had a greater effect than DMA.
Other properties of N-nitroso compounds such as cell
transformation in vitro and their influence on DNA repair mechanisms
are being investigated, but as yet, there are not sufficient data for
their evaluation.
8. EFFECTS OF NITRATES, NITRITES AND N-NITROSO COMPOUNDS ON MAN
8.1 Nitrates and Nitrites
In the erythrocytes of healthy individuals, the process of
methaemoglobin formation and reduction is continuous. The mean content
of methaemoglobin in healthy populations is usually reported to be
below 2% of the total haemoglobin concentration (Committee on Nitrate
Accumulation, 1972; Gobbi et al., 1974; Smith, 1972). However,
Goldsmith et al. (1975) recently found mean levels in Californian
populations ranging up to 2.11% with 1% of the adults and 8% of
infants having methaemoglobin levels exceeding 4%. Higher values are
found in premature than in full-term infants and levels in infants are
higher than those in older children and adults (Kravitz et al., 1956).
At a level of about 10%, methaemoglobinaemia may produce symptomless
cyanosis, whereas levels of 20-50% are associated with conspicuous
cyanosis accompanied by hypoxic signs and symptoms, such as weakness,
exertional dyspnoea, headaches, tachycardia, and loss of consciousness
(Arena, 1970; Committee of Nitrate Accumulation, 1972; Jaffé & Heller,
1964). The lethal concentration of methaemoglobin is not known, but
death may occur at levels exceeding 50% (Committee of Nitrate
Accumulation, 1972).
8.1.1 Epidemiologicai studies
The National Academy of Sciences, USA (Committee on Nitrate
Accumulation, 1972) recently reviewed about 350 cases of
methaemoglobinaemia in the USA and about 1000 cases in Europe that
were reported to be associated with the intake of nitrates in well
water or in food. There were 41 fatalities in the USA and about 80 in
Europe. Only one case of infant methaemoglobinaemia resulting from the
consumption of water from a municipal water supply was reported in the
USA (Vigil et al., 1965).
8.1.1.1 Exposure through water
The toxicity to man of nitrates in water was first reported by
Comly (1945). He noted high levels of methaemoglobin and the
associated signs of nitrate toxicity in 2 infants who had consumed
water containing high concentrations of nitrates (619 and
388 mg/litre). Since then, several epidemiological and case studies
have been carried out in various parts of the world, particularly in
areas with naturally high nitrate levels in water.
Robertson & Riddell (1949) reported 10 cases in which infants
receiving powdered milk preparations made with well waters with
nitrate concentrations exceeding 75 mg/litre had blood levels of
methaemoglobin ranging from 5 to 50% of total haemoglobin. Two of
the infants, whose dried milk preparations had been reconstituted
with well waters containing nitrate levels of 1200-1300mg/litre, had
methaemoglobin levels of 25% and 44% of total haemoglobin,
respectively; both cyanosed rapidly and died before therapy could be
applied.
In the state of Minnesota (USA), Bosch et al. (1950) reported 139
cases of infant methaemoglobinaemia due to the ingestion of well water
with a high nitrate content (over 89 mg/litre); the mortality rate was
10%. In Kansas, 13 cases of infant methaemoglobinaemia including 3
deaths caused by drinking well water, were reported between the early
1940s and 1950 (Walton, 1951). Many other cases in the USA have been
reported by this author. On the other hand, infant methaemoglobinaemia
was absent in urban areas of New York and the province of Ontario,
Canada where nitrate levels in water were low (Ciavaglia & Thompson,
1969). Knotek & Schmidt (1964) reported 115 cases of methaemo-
globinaemia from a total of 5800 children born in central
Czechoslovakia between 1953 and 1960. Of these, 8% were fatal, 52%
were severe, and 40% were mild. Most deaths were associated with the
consumption of water containing nitrate concentrations ranging from
70 to 250 mg/litre. In these cases, methaemoglobinaemia was
invariably associated with the consumption of infant milk
preparations, which contained microorganisms such as B. subtills,
capable of reducing nitrates to nitrites. These workers reported the
inhibitory effect of buttermilk on this conversion. They attributed
this effect to the presence of Streptococcus lactis which
produces the antibiotic nisin and is capable of preventing the growth
of the spores of B. subtilis.
Shuval & Gruener (1972) studied communities with various
concentrations of nitrates in the drinking water, in Israel. Infants
from rural communities with reported nitrate levels of 50 --
90 mg/litre in the water supplies were compared with controls where
the average level was 5 mg/litre. They did not find any definite cases
of methaemoglobinaemia nor were there any significant differences in
the methaemoglobin levels. However, there was widespread consumption
of citrus juices and the water consumption in infant milk preparations
was low (94% of the infants studied were breast fed or received whole
cow's milk). Soviet literature contains information concerning a
comparatively small number of cases of symptomless methaemoglobinaemia
caused by nitrates in water (Diskalenko, 1969; Motylev, 1969). In
children whose drinking water contained a high level of nitrates, the
methaemoglobin level did not usually exceed 10% although higher levels
were found occasionally. Sattelmacher (1962) and Simon et al. (1964)
compiled 1060 and 745 cases, respectively, of infant
methaemoglobinaemia due to nitrate-contaminated water in the Federal
Republic of Germany. Most of the cases were associated with water
from private wells and in 84-90% of the cases the water contained
nitrate concentrations exceeding 100 mg/litre (although a few cases
of methaemoglobinaemia were reported with water containing less than
50 mg/litre).
Commoner et al. (1972) found small, but statistically
significant, subclinical elevations in methaemoglobin levels in adults
exposed to high intakes of nitrates in a rural area when compared with
an urban control population. There is also a small amount of data
showing that under similar conditions, pregnant women in rural areas
have higher methaemoglobin levels than those in urban areas. This is
of particular interest since previous workers have reported an
increased susceptibility to nitrates in pregnant women. (Skrivan,
1971). Thus, there is concern over the effects on the fetus of the
general lowering of oxygen tension. Gelperin et al. (1971) recently
reported the presence of methaemoglobinaemia in a newborn infant,
presumably exposed to nitrates transplacentally. In this study, 72
mothers and infants tested were exposed to water with nitrate
concentrations ranging from 28 to 45 mg/litre over a 2-month period.
During the 2 weeks of maximum concentration (45 mg/litre), the average
methaemoglobin levels were 1.18% for mothers and 1.91% for newborns
with those of one mother and one infant rising to 6.39% and 5.87%,
respectively.
Children aged 12-14 years who drank water with a nitrate level of
105 mg/litre were noted to have slightly delayed reactions to light
and sound stimuli combined with a mean methaemoglobin level of 5.3% in
comparison with control children drinking water with a nitrate level
of 8 mg/litre whose methaemoglobin levels average 0.75% (Petukhov &
Ivanov, 1970).
8.1.1.2 Exposure through vegetables
Cases of methaemoglobinaemia, some resulting in death, have been
observed following the consumption of spinach. Hölscher & Natzschka
(1964) reported 2 cases in young infants (aged 2 and 3.5 months) who
had eaten spinach purée. Fresh spinach from the same source contained
only traces of nitrates but had nitrite ion levels of 2180 mg/kg.
Fourteen further cases of methaemoglobinaemia in infants (aged 2-10
months) were reported from the Federal Republic of Germany (Sinios &
Wodsak, 1965). Unprocessed spinach was used almost exclusively in the
preparation of the infants' meals and preparation took place at least
24 h before the mealtime. Since in most cases some of the same spinach
had been eaten 24 and 48 h before without causing illness, the authors
assumed that the nitrites were formed within the final 24 h of
storage. Information on 7 cases showed that the mother tasted the
spinach before feeding the child and no change in taste was apparent.
None of the children refused the meal which caused the poisoning.
Conversion of nitrates to nitrites in fresh spinach was demonstrated
by Schuphan (1965) (section 4.2.1). Keating et al. (1973) reported a
case of methaemoglobinaemia in an infant given carrot juice. Other
cases of food-induced methaemoglobinaemia have recently been reviewed
by Luhrs (1973).
8.1.1.3 High accidental exposures through food
Certain meat products contain nitrates and nitrites. Normally, no
adverse effects result from consumption of such products. However, in
1955, an outbreak of 10 cases of methaemoglobinaemia in children
occurred in New Orleans, USA, attributed to the consumption of large
amounts of nitrites in sausage meats (Orgeron et al., 1957). Further
studies revealed that the meats had nitrite concentrations of more
than 200 mg/kg and that some had levels as high as 6570 mg/kg. Singley
(1962) reported 3 cases of methaemoglobinaemia resulting from the
consumption of fish, that had been adulterated with sodium nitrite.
One patient died and it was assessed that he had consumed
approximately 33 mg sodium nitrite/kg body weight. Other cases of
poisoning involving the consumption of nitrite-treated sausage and
frankfurters were reported by Bakshi et al. (1967) and Henderson &
Raskin (1972).
8.1.1.4 Ambient air exposures
Effects on man of nitrate aerosols in ambient air were not
considered by the Task Group. However, because of the recent concern
with the role of nitrates in urban air pollution, it may be of
interest to briefly summarize the current information on this problem.
Airborne nitrates may act as respiratory irritants (Knelson & Lee,
1977) and a recent study conducted by the US Environmental Protection
Agency in the New York-New Jersey metropolitan area showed that
increased asthmatic attacks were significantly associated with
elevated levels of suspended nitrates in six of the seven communities
studied. No such effect was observed with nitrogen dioxide. Another
study in two south-eastern communities in the USA showed some evidence
that a combination of suspended nitrates and suspended sulfates
increased the risk of asthma attacks more than either pollutant did
alone. Because of the present difficulties in measuring suspended
nitrates, these results should be considered as qualitative instead of
quantitative (French et al., unpublished data)a.
8.1.2 Factors involved in susceptibility to nitrates
The work of Marriott et al. (1933), who investigated the acidity
and bacterial flora of 200 infants suffering from diarrhoea, supports
the hypothesis that lack of acidity in the gastric juices of newborn
infants might permit the growth of nitrate-reducing organisms in the
upper gastrointestinal tract and, thus, the reduction of nitrates to
nitrites before the former could be completely absorbed. The pH of the
a French, J. G., Hasselblad, V., & Johnson, R. Aggravation of
asthma by air pollutants. 1971-72 Southeastern CHESS studies.
stomach contents of healthy infants varied from 2.0 to 5.0 (average
3.7) and that for infants suffering from bacillary dysentery ranged
from 2.0 to 5.0 (average 3.0). However, for infants with nonspecified
diarrhoea the pH varied from 4.6 to 6.5 (average 5.6).
According to estimates made by Burden (1961), the water intake of
young infants is nearly 10 times higher than that of adults on a per
unit body weight basis.
The susceptibility of infants to methaemoglobinaemia during the
first six months of life, and especially during the first trimester
(Bailey, 1966; Kübler, 1965) can be explained by various mechanisms,
none of which is fully understood at present. It has been reported
that fetal haemoglobin, which in newborn infants makes up to 60-80% of
the total haemoglobin decreasing to about 20-30% in 3 months (British
Medical Journal, 1966; Kübler, 1965), is more readily oxidized to
methaemoglobin than adult haemoglobin (Betke, 1953; Künzer &
Schneider, 1953). This might explain why premature infants, who
frequently have a higher percentage of fetal haemoglobin than full-
term infants are more susceptible to methaemoglobinaemia. Keohane &
Metcalfe (1960) reported that the sensitivity of erythrocytes to
oxidation to methaemoglobin on exposure to nitrites gradually declined
during childhood until the age of puberty, after which it decreased
rapidly. This decline in sensitivity was not related to the
disappearance of fetal haemoglobin and the authors suggested that some
other factor might be responsible. The susceptibility of newborn and
young infants to develop methaemoglobinaemia could also be attributed
to the incomplete development of the NADH methaemoglobin reductase
system. Several studies have shown that the erythrocytes of newborn
and young infants have a lower capacity to reduce methaemoglobin than
those of older children and adults and that the erythrocytes of
premature newborns have a lower reduction capacity than those of full-
term infants (Bartos et al., 1966; Ross, 1963; Ross & Desforges,
1959). Except in rare cases of hereditary enzyme deficiency (Balsamo
et al., 1964), this deficiency in the NADH methaemoglobin reductase
system seems to disappear after the first 3-4 months of life (Bartos
et al., 1966: Künzer & Schneider, 1953).
The haemoglobin of pregnant women and that of patients suffering
from carcinomata has also been reported to be sensitive to oxidation
to methaemoglobin. However, this sensitivity disappeared in the first
instance after delivery and in the second, after radical extirpation
of the neoplasms (Metcalf, 1961).
8.1.3 Dose-response relationships for nitrates and nitrites
Estimates of the effective exposure of the general population to
nitrates and nitrites have been discussed briefly in section 5.1.4.
However, this information is not sufficient to relate environmental
concentrations to actual intake of nitrates or nitrites in cases of
observed methaemoglobinaemia. Retrospective epidemiological studies
discussed in section 8.1.1 provide little information on intake
because in most cases either the data concerning concentrations in
water or food were not reliable enough, or the amounts of water or
food consumed had not been measured. However, a study reported by
Winton et al. (1971) considered the nitrate intake from water in some
detail. The study was conducted in southern California and central
Illinois, USA, and involved 111 infants whose ages ranged from less
than two weeks to six months. The mother of each infant was asked
about the fluid intake of the infant in the previous 24 h, the method
of formula preparation, and the possible inclusion of any other source
of nitrate in the diet (e.g. vegetables) or administration of
methaemoglobin-forming medicines. The variables that determined the
nitrate dose were the daily fluid intake per body weight (which might
increase in hot and arid climates, or with fever), the fraction of the
total daily fluid intake taken in the form of water, and the
concentration of nitrates in the water. Using this method, it was
possible to estimate that a daily dose of 10 mg/kg body weight could
be obtained from water containing a nitrate concentration of
50 mg/litre. The daily water intake varied from 10% of the total daily
fluid intake for breast-fed infants or for those receiving ready-to-
feed preparations to 90% for infants who were fed preparations made
with powder. This shows that no generalization is possible, at
present, about the relationship between the nitrate concentration in
drinking water and the dose of nitrate, and that estimates of nitrate
dose from nitrate concentrations in water or food would have to be
made for individual cases, taking into account local conditions and
dietary habits.
The relationship between the doses of nitrate and methaemoglobin
levels is even more difficult to establish because of large individual
differences in response, depending on age and many other host
variables. For example, using the method described in the previous
paragraph, Winton et al. (1971) found that in a group of 111 infants,
63 received a nitrate dose of less than 1 mg/kg body weight, 23 were
exposed to 1-4.9 mg/kg, 20 to 5.0-9.9 mg/kg, and 5 infants to
10-15.5 mg/kg. However, only 3 infants appeared to have methaemoglobin
levels above normal (0-2.9%) and they were the youngest of the five
who had received more than 10 mg/kg. The highest methaemoglobin level
(5.3%) was found in 30-day-old baby who had received 15.5 mg/kg.
Another possible approach is to analyse the available retrospective
epidemiological data. This has been done by the Committee on Nitrate
Accumulation (1972) and by Diskalenko (1968) who concluded that only
very crude estimates of correlations between nitrate concentration in
water and methaemoglobin levels could be obtained, probably because of
the delay between the analyses of water and blood, difficulties in
identifying the sources of water consumed, and the heterogeneity of
the population samples studied, particularly with respect to age. The
analyses performed by the Committee on Nitrate Accumulation (1972)
showed, for example, that an increase in the nitrate concentration in
water from 0-49 mg/litre to 49-98 mg/litre, increased the
methaemoglobin level, on average, from 1.0% to 1.3% in infants aged
0-3 months, but did not affect the methaemoglobin level (0.8%) in
infants aged 3-6 months (Simon et al., 1964). Methaemoglobin and
haemoglobin levels, measured in 96 infants from 22 localities in
Rheinhessen, Federal Republic of Germany, during official maternal
counselling, were correlated with the nitrate concentrations of the
drinking water in the localities concerned by Würkert (1974,
unpublished data)a. Nitrate concentrations of 0-5 mg/litre,
31-50 mg/litre, and over 100 mg/litre corresponded to methaemoglobin
levels of 1.65%, 2.44%, and 6.59%, respectively. Higher methaemoglobin
levels were found in the presence of infections and in infants given
tea to drink or vegetable nutrients.
The last question pertains to the relation between the level of
methaemoglobin and clinical signs and symptoms of methaemoglobinaemia.
Normally, methaemoglobin is present in the blood at a concentration of
less than 2% of total haemoglobin.
Subclinical methaemoglobinaemia (less than 10% methaemoglobin)
has not been considered to be of direct health significance, but
Petukhov & Ivanov (1970) reported behavioural effects at these levels.
Clinical signs of methaemoglobinaemia such as cyanosis become
apparent at a methaemoglobin level of about 10%. Hypoxic signs and
symptoms may develop at levels exceeding 20% and death may occur at
levels of 50% or more.
In conclusion, the available information does not permit the
establishment of a quantitative dose-response relationship for human
exposure to nitrates in water or food.
8.2 N-nitroso Compounds
Freund (1937) first described acute intoxication by DMN. Barnes &
Magee (1954) described 2 cases of industrial intoxication due to DMN
in which one individual had hepatic cirrhosis at death; the other
survived but, 6 months later, was shown to have a hard liver suspected
of being cirrhotic. Watrous (1947) and Wrigley (1948) reported cases
of accidental exposure to N-nitroso-methylurethane. Reddening of the
conjunctiva and erythema of the face and feet developed quickly, and a
respiratory disorder developed later.
No reports are available concerning carcinogenesis in industrial
or other workers exposed to N-nitroso compounds, nor have
relationships been established, from epidemiological and analytical
data, that link cancer in man with exposure to N-nitroso compounds or
their possible precursors such as nitrates, nitrites, and compounds
a Thesis reported in the contributed of the Federal Republic of
Germany to the WHO environmental health critera document on
Nitrates, nitrites and N-nitroso compounds.
that can be nitrosated, occurring as food components, drugs, and
pesticides. A recent review of these data is available (Mirvish,
1976).
Some reports have been concerned with the possible etiological
role of N-nitroso compounds in nasopharyngeal cancer in south-east
Asia (Clifford, 1970; Fong & Chan, 1973a) and oesophageal cancer in
South Africa, Iran, and China (Burrell et al., 1966; Coordinating
Group for Research on the Etiology of Esophageal Cancer of North
China, 1974; Day, 1975; Harmozdian et al., 1975). However, no
relationship has been established; nitrosamines were detected in food
from these areas but this was not confirmed by mass spectroscopy
(Eisenbrand et al., 1976; Purchase et al., 1975).
The epidemiology of stomach cancer has been discussed from a
similar point of view by Correa et al. (1975), Endo et al. (1973),
Haenzel & Correa (1975), Hill et al. (1973), Mirvish (1971), and
Weisburger & Raineri (1975). It was suggested that nitrosamides might
be formed in the stomach from amides occurring in the diet, and might
then act locally on this organ. Relationships have been sought between
the occurrence of stomach cancer and the nitrate contents of the soil
or water in Chile, Colombia, and the United Kingdom (Hawksworth et
al., 1974; Hill et al., 1973; Zaldivar & Wetterstrand, 1975) but none
was established.
9. EVALUATION OF HEALTH RISKS TO MAN FROM EXPOSURE TO NITRATES,
NITRITES, AND N-NITROSO COMPOUNDS
9.1 Nitrates and Nitrites
9.1.1 General considerations
Man is exposed to nitrates and nitrites mainly through water and
food. Nitrate concentrations may be particularly high in drinking
water derived from dug wells. Nitrates in food may occur naturally or
may be added for various technological or even public health reasons
(e.g. addition of nitrates and nitrites to certain meat products to
protect against botulism). Although intake of very large doses of
nitrates can be fatal to man, such intake is not likely to occur
through environmental exposure, except in the case of infants and very
young children who are high risk groups because of their
susceptibility to nitrates and nitrites. Weekly intakes of nitrates by
members of the general population are difficult to evaluate but rough
estimates are available for England and the USA giving values of about
400-450 mg/week (85-105 mg from water; 210-225 mg from vegetables, and
about 110 mg from meat products).
These figures cannot be applied generally and a separate
estimation of the nitrate intake from food and water should be made
for each case, especially when the subjects are infants or young
children (section 8.1.3). Exposure to nitrates can also occur through
the inhalation of polluted air.
The assessment of health risks to man (section 9.1.2) has been
based on epidemiological studies and clinical evidence. The animal
data discussion in section 7.1, confirm the findings in man that
methaemoglobinaemia is the main toxic effect of nitrate and nitrite
ingestion. Methaemoglobinaemia is caused by nitrites, the reduction
products of nitrates. The reduction usually occurs through microbial
action either in the environment or in the body. The health risks from
exposure to nitrates is therefore related not only to their
concentration in drinking water and food, but also to the presence or
absence of conditions conducive to their reduction to nitrites. Young
infants constitute the most vulnerable group for the following
reasons:
(1) Lower acidity in their stomach allows the growth of certain
microbes that contain enzymes capable of reducing nitrates
to nitrites;
(2) Fetal haemoglobin, which constitutes a considerable
proportion of the haemoglobin of the young infant, and the
erythrocytes during childhood may be more susceptible to
conversion to methaemoglobin by the action of nitrites;
(3) The enzyme system capable of reducing methaemoglobin to
haemoglobin is deficient in the young infant; and
(4) The fluid intake of the young infant is higher than that of
the adult in relation to the body weight.
9.1.2 Assessment of health risks
Precise dose-response relationships could not be established by
the Task Group because of the existence of various strongly modifying
factors and the lack of accurate quantitative data. However, on the
basis of the available information, the Task Group reached the
following conclusions:
(a) General population -- The prevailing levels of nitrates and
nitrites in water and food do not seem to have any harmful
effects in adults and older children, although there are reports
of susceptible individuals who have been affected by meat treated
with nitrites, and of cases of poisoning resulting from the
ingestion of certain foods accidently containing excessive
amounts of nitrites (8.1.1.3). Subclinical methaemoglobinaemia
may also be found in individuals consuming water containing high
levels of nitrates (8.1.3).
(b) Susceptible group -- Infants less than 6 months old and
especially those under 3 months of age are particularly
susceptible to methaemoglobinaemia caused by intake of water
containing elevated levels of nitrates, especially when they are
fed with preparations made from dried milk of low acidity. While
a few cases of methaemoglobinaemia have been reported associated
with water nitrate levels of less than 50 mg/litre, most cases
occur with nitrate levels of 90 mg/litre or more. Nitrates in
water may cause death of the infant, but the lowest level that
may be fatal cannot be estimated at present.
The ingestion of vegetables (e.g. spinach, carrots) containing
elevated nitrate and/or nitrite levels may also cause
methaemoglobinaemia in infants, especially in those aged between 6
months and 1 year. Storage of vegetables, other than in the frozen or
canned state, is likely to increase the nitrite level and hence the
risk.
(c) Effects of airborne nitrates -- A few recent studies have
indicated that airborne nitrates may act as respiratory irritants
but, at present, adequate quantitative data are not available and
the Task Group did not consider this exposure in their health
risk evaluation.
9.2 N-nitroso Compounds
9.2.1 General considerations
Nitrites (and indirectly nitrates) can react with amines and
amides to form nitrosamines and nitrosamides. The precursors of these
N-nitroso compounds are widely distributed in various environmental
media. Information concerning the presence of N-nitroso compounds
per se is limited although they have been identified in certain
foods such as luncheon meats and in air and water samples. The
conditions under which N-nitroso compounds can be formed are
outlined in sections 4.2-4.6.
More than 80% of over one hundred N-nitroso compounds tested
proved to be carcinogenic in animal experiments giving rise to tumours
in many organs and also producing tumours transplacentally.
N-nitroso compounds are carcinogenic in a wide range of animal
species; most are mutagenic in test systems and some have been shown
to be teratogenic to animals.
The possible health hazard from N-nitroso compounds is not
confined to those present in the environment. Their formation, from a
variety of precursors in the body of animals, has been demonstrated,
and this may also occur in man.
9.2.2 Assessment of health risks
A dose-response relationship has been shown to exist in different
species of rodents for some carcinogenic N-nitroso compounds. As the
dose is reduced, the tumour incidence decreases and the time for
tumour induction increases and may exceed the life span of the animals.
Although there is no clinical or epidemiological evidence, it is
highly probable that these compounds are also carcinogenic to man.
However, present limitations concerning available dose-response data
in animals and their interpretation, and inadequate knowledge of the
biomechanism of cancer induction preclude a quantitative estimation of
the carcinogenic risk to man that may be associated with different
exposures to N-nitroso compounds.
9.3 Reduction of Exposure
The assessments of health risk given in sections 9.1.2
[(a) and (b)] and 9.2.2 lead to a number of practical conclusions
concerning the need to reduce the exposure to nitrates, nitrites, and
N-nitroso compounds.
As regards the exposure to nitrates and nitrites, the Task Group
made the following specific recommendations:
(a) Infant dried milk preparations should be reconstituted only
with water containing low levels of nitrates. If such water is
not available, breast feeding or the use of cow's milk should be
encouraged.
(b) Only vegetables with a low nitrate content should be used in
the preparation of baby foods. If vegetables known to contain
high levels of nitrates are used, appropriate food processing
precautions should be instituted. Nitrates and nitrites should
not be added to baby foods.
(c) The use of nitrates and nitrites in foods as preservatives
should be reduced to the minimum level that provides protection
against botulism. This applies particularly to cured and canned
meats and to fish. The use of nitrates and nitrites on fresh
meats or fish should be avoided.
(d) Nitrate levels in public drinking water should comply with,
or preferably be lower than, the tentative limit of
45 mg/litre recommended in the International Standards for
Drinking Water (WHO, 1971).
With respect to the carcinogenic risk from exposure to
N-nitroso compounds, it is prudent to assume that any exposure may
involve some degree of risk, and that exposure should therefore be
kept as low as practically achievable. This may not be an easy task in
many instances, since these compounds may occur in the environment in
concentrations of the order of parts per billion, as a result of a
variety of natural and technological processes, and, moreover, they
may be formed in vivo from nitrates, amines, and amides, which
are ubiquitous. Obviously the recommendations (a) to (d) will also
contribute to the reduction of carcinogenic risk related to
N-nitroso compounds.
REFERENCES
ACHTZEHN, M. K. & HAWAT, H. (1969) [Nitrate accumulation in vegetables
a possibility of nitrate intoxication in infants.] Die Nahrung,
13: 667-676 (in German).
ACHTZEHN, M. K. & HAWAT, H. (1970) [On nitrate formation in vegetables
and vegetable products. Part I. Raw spinach.] Die Nahrung,
14: 383-394 (in German).
ADRIANO, C. C., PRATT, D. F., & BISHOP, S. E. (1971) Fate of inorganic
forms of nitrogen and salt from land disposed manures from
dairies. In: Livestock waste management and pollution
abatement. St. Joseph, MI (Am. Soc. Agric. Eng. Proc. No. 271).
ALAM, B. S., SAPORESCHETZ, I. B., & EPSTEIN, S.S. (1971a) Formation of
N-nitroso-piperidine from piperidine and sodium nitrite in the
stomach and the isolated intestinal loop of the rat. Nature
(Lond.), 232: 116-118.
ALAM, B. S., SAPORESCHETZ, I. B., & EPSTEIN, S.S. (1971b) Synthesis of
nitrosopiperidine from nitrate and piperidine in the gastro-
intestinai tract of the rat. Nature (Lond.), 232: 199-200.
ALARIF, A. & EPSTEIN, S.S. (1974) The uptake and metabolism of
14 C-labelled nitrosomethylurethane and nitrosomethylurea in
guinea pigs and their in vitro metabolism in the guinea pig
and human pancreas. IARC Sci. Publ. No. 9, pp. 215-219.
ALEXANDROV, V. A. (1967) [On the mechanism of carcinogenicity of
N-nitrosodimethylamine in connection with peculiarities of
its effect on embryogenesis] Vop. Onkol., 13: 87-92
(in Russian).
ALEXANDROV, V. A. (1968a) Blastomogenic effect of dimethylnitrosamine
on pregnant rats and their offspring. Nature (Lond.),
218: 280-281.
ALEXANDROV, V. A. (1968b) [Effect of N-nitroso- N-methylaniline and
N-nitroso- N-ethylaniline on the rat embryo] Vop. Onkol.,
14: 37-38 (in Russian).
ALEXANDROV, V. A. (1969a) [Transplacental blastomogenic action of
N-nitrosomethylurea in rat offspring] Vop. Onkol.;
15: 55-61 (in Russian).
ALEXANDROV, V. A. (1969b) Uterine, vaginal and mammary tumors induced
by nitrosoureas in pregnant rats. Nature (Lond.),
222: 1064-1065.
ALEXANDROV, V. A. & JANISCH, W. (1971) Teratogenic effect of ethylurea
and nitrite in rats. Experientia (Basel), 27: 538-539.
ALLISTON, T. G., COX, G. B., & KIRK, R. S. (1972)The determination of
steam-volatile N-nitrosamines in foodstuffs by formation of
electron-capturing derivatives from electrochemically derived
amines. Analyst, 97: 915-920.
ALTHOFF, J., WILSON, R., & MOHR, U. (1971) Diethylnitrosamine-induced
alterations in the tracheobronchial system of Syrian golden
hamsters. J. Natl Cancer Inst., 46: 1067-1071.
ALTHORPE, J., GODDARD, D. A., & SISSONS, D. J., TELLING, G. M. (1970)
The gas chromatographic determination of nitrosamines at the
picogram level by conversion to their corresponding nitramines.
J. Chromatogr., 53: 371-373.
ARENA, J. M. (1970) Poisoning, toxicology, symptoms and treatment.
2nd ed., Springfield, I, Bannerstone House, p. 9.
ARISON, R. N. & FEUDALE, E. L. (1967) Induction of renal tumour by
streptozotoc n in rats. Nature (Lond.), 214: 1254-1255.
ASAHINA, S., FRIEDMAN, M. A., ARNOLD, E., MILLAR, G. N., MISHKIN, M.,
BISHOP, Y., & EPSTEIN, S.S. (1971) Acute synergistic toxicity and
hepatic necrosis following oral administration of sodium nitrite
and secondary amines to mice. Cancer Res., 31: 1201-1205.
ASATOOR, A.M. & SIMENHOFF, M. L. (1965)The origin of urinary
dimethylamine. Biochim. Biophys. Acta, 111: 384-392.
ASHTON, M. R. (1970) The occurrence of nitrates and nitrites in foods.
BFMIRA lit. surv. No 7, pp. 32.
BADER, G., STILLER, D., & ULLRICH, K. (1971) [Ultrastructural changes
in toxically injured and proliferating liver cells due to long-
term application of diethylnitrosamine] Arch.
Geschwulstforsch., 37: 327-343 (in German).
BAILEY, W. P. (1966) Methemoglobinemia-acute nitrate poisoning in
infants: Second report. J. Am. Osteopath. Assoc.,
66: 431-434.
BAKSHI, S. P., FAHEY, J. L., & PIERCE, L. E. (1967) Sausage cyanosis--
Acquired methemoglobinemia nitrite poisoning.
N. Eng. J. Med. 277: 1072.
BALSAMO, P., HARDY, W. R., & SCOTT, E. M. (1964) Hereditary
methaemoglobinaemia due to diaphorase deficiency in Navajo
Indians. J. Pediat., 65: 928-931.
BANNASCH, P. (1968) In: The cytoplasm of hepatocytes during
carcinogenesis. New York, Springer Verlag.
BARNES, J. M. & MAGEE, P.M. (1954) Some toxic properties of
dimethylnitrosamine. Br. J. ind. Med., 11: 167-174.
BARTOS, H. R., DESFORGES, J. F., & CLARK, N. (1966) Erythrocyte DPNH
dependent diaphorase levels in infants. Pediatrics, 37: 991-993.
BEHROOZI, K., GUTTMAN, R., GRUENER, N., & SHUVAL, H. I. (1971) Changes
in the motor activity of mice given sodium nitrite drinking
solution. In: Proceedings of a Symposium on Environmental
Physiology, Beersheba, December, 1971.
BNEMANSKIJ, V. V. & LITVINOV, N. N. (1969) [Some data on the histo-
and morphogenesis of experimental tumours of the kidneys in
animals.] Arkh. Pat., 31: 79-84 (in Russian).
BERTRAM, J. S. & CRAIG, A. W. (1972) Specific induction of bladder
cancer in mice by butyl- (4-hydroxybutyl) -nitrosamine and the
effects of hormonal modifications on the sex difference in
response. Eur. J. Cancer, 8(6): 587-594.
BERTRAM, J. S. & CRAIG, A. W. (1973) Induction of bladder tumours in
mice with dibutylnitrosamine. Br. J. Cancer, 24
(2): 352-359.
BETKE, K. (1953) [Comparative examination of the oxidation of fetal
and adult oxyhaemoglobin by sodium nitrite.] Naturwiss., 40: 60
(in German).
BHATHAL, P.S. & HURLEY, J. V. (1973) An electron microscope study of
the production of ascites in acute dimethylnitrosamine (NDMA)-
induced liver injury. J. Pathol., 111: 103-116.
BIOCEV, IV. & POCINKOVA, Z. (1972) [Effect of applied artificial
fertilizers on the soil, drinking water and foodstuffs of
vegetable origin. Report on the International Congress of
Country Hygiene, Varna] (in Russian).
BLATTMANN, L. & PREUSSMAN, R. (1973) [The structure of metabolites of
carcinogenic dialkylnitrosamine in the urine of rats]
Z. Krebsforsch., 79: 3-5 (in German).
BLATTMANN, L. & PREUSSMAN, R. (1974a). [The biotransformation of
carcinogenic dialkylnitrosamines. Further urine metabolites of
di-n-butyl- and di-n-pentylnitrosamines.] Z. Krebsforsch.,
81: 75-78 (in German).
BLATTMANN, L., JOSWIG, N., & PREUSSMAN, T. (1974b). [The structure of
the metabolites of the carcinogenic methyl-n-butyl-nitrosamine in
the urine of rats.] Z. Krebsforsch., 81: 71-73 (in German).
BODIPHALA, T. & ORMROD, D. P. (1971) Factors affecting the nitrate
content of vegetable and fruit foods. Can. Inst. Food Technol.,
J., 4(1): 6-8.
BOGOVSKI, P. & WALKER, E. A., ed. (1974) N-nitroso compounds in the
environment. Proceedings of a Working Conference, the
International Agency for Research on Cancer, Lyons, France,
17-20 October, 1973, Lyons, International Agency for Research on
Cancer, pp. 243.
BOGGYSKI, P., PREUSSMANN, R., & WALKER, E. A., ed. (1972a) N-nitroso
compounds analysis and formation. Proceedings of a Working
Conference, Deutsches Krebsforschungszentrum, Heidelberg,
Federal Republic of Germany, 13-15 October, 1971, Lyons,
International Agency for Research on Cancer, pp. 140.
BOGGYSKI, P., CASTEGNARO, M., PIGNETTELI, B., & WALKER, E. A. (1972b)
The inhibiting effects of tannins on the formation of
nitrosamines. IARC Sci. Publ. No. 3, pp. 127-129.
BOLIN, B. & ARRHENIUS, E., ed. (1977) An essential life factor and a
growing environmental hazard. Report from Nobel Symposium No. 38,
Ambio, 6 (2-3): 96-105.
BOSCH, H. M., ROSENFIELD, A. B., HUSTON, R., SHIPMAN, H. R., &
WOODWARD, F. L. (1950) Methemogiobinemia and Minnesota well
supplies. J. Am. Water Assoc., 42: 161-170.
BOYLAND, E. & WALKER, S. A. (1974) Effect of thiocyanate on
nitrosation of amines. Nature (Lond.), 248: 601-602.
BOYLAND, E., CARTER, R. L., GORROD, J. W., & ROE, F. J. C. (1968)
Carcinogenic properties of certain rubber additives. Eur. J.
Cancer, 4: 233-239.
BRESLER, S. E., KALININ, V. L., & PERUMOV, D. A. (1968) Inactivation
and mutagenesis on isolated DNA. IV Possibility of integration of
lethal damage into the chromosome of Bacillus subtills during
transformation. Mutat. Res., 5: 329-341.
BRETSCHNEIDER, K. & MATZ, J. (1973) [Nitrosamines (NA) in the
atmospheric air and in the air at the workplace.] Arch.
Geschwulstforsch., 42: 36-41 (in German).
BRETSCHNEIDER, K. & MATZ, J. (1976) Occurrence and analysis of
nitrosamines in air. IARC Sci. Publ. No. 14, pp. 395-399.
British Medical Journal (1966), 1: 250-251. Spinach--a risk to
babies.
BROWN, J. R. & SMITH, G. E. (1967) Nitrate accumulation in vegetable
crops as influenced by soil fertility practises. Columbia,
University of Missouri, pp. 43 (Res. Bull. No. 920).
BRUNI, C. (1973) Distinctive cells similar to fetal hepatocytes
associated with liver carcinogenesis by diethylnitrosamine.
Electron microscopic study. J. Natl Cancer Inst.,
50: 1513-1528.
BUGLASS, A. J., CHALLIS, B.C., & OSBORNE, M. R. (1974)
Transnitrosation and decomposition of N- nitrosamines.
N- nitroso compounds in the environment. IARC Sci. Publ., No. 9,
pp. 94-100.
BURDEN, E. H. W. J. (1961) The toxicology of nitrates and nitrites
with particular reference to the potability of water supplies.
Analyst, 86 (1024): 429-433.
BURNS, R. C. & HARDY, R. W. F. (1975) Nitrogen fixation in bacteria
and higher plants. Berlin, Springer-Verlag.
BURRELL, R. J. W., ROACH, W. A., & SHADWELL, A. (1966) Esophageal
cancer in the Bantu of the Transkei associated with mineral
deficiency in garden plants. J. Natl Cancer Inst.,
36: 201-214.
BUTLER, W. H. & HARD, G. C. (1971) Hepatoxicity of dimethylnitrosamine
in the rat with special reference to veno-occlusive disease.
Exp. mol. Pathol., 15: 209-219.
CALAFAT, J., DEN ENGELSE, L, & EMMELOT, P. (1970) Studies on lung
tumours. II. Morphological alterations induced by
dimethylnitrosamine in mouse lung and liver and their relevance
to tumourigenesis. Chem. biol. Interact.,
2: 309-320.
CARDESA, A., MIRVlSH, S.S., HAVEN, G. T., & SHUBIK, P. (1974)
Inhibitory effect of ascorbic acid on the acute toxicity of
dimethylamine plus nitrite in the rat. Proc. Soc. Exp. Biol.
Med., 145: 124-128.
CARTER, R. L., PERCIVAL, W. H., & ROE, F. J. C. (1969) Exceptional
sensitivity of mink to the hepatotoxic effects of
dimethylnitrosamine. J. Pathol., 97 (1): 79-88.
CASEY, H. (1975) Variation in chemical composition of the River Frome,
England from 1965 to 1972. Freshwater Biol.,
5: 507-514.
CASTEGNARO, M., PIGNATELLI, B., & WALKER, E. A. (1972) N-Nitroso
compounds: Analysis and formation. IARC Sci. Publ, No. 3,
pp. 87-89.
CAVETT, J. J. (1962) The microbiology of vacuum-packed sliced bacon.
J. appl. Bact., 25: 282-289.
CHALLIS, B.C. (1973) Rapid nitrosation of phenols anti its
implications for health hazards from dietary nitrites. Nature
(Load.), 244: 466.
CHALLIS, B.C. & BARTLETT, C. D. (1975) Possible cocarcinogenic effects
of coffee constituents. Nature (Lond.),
254: 532-533.
CHALLIS, B.C. & CHALLIS, J. A. (1970) Reactions of the carboxamide
group. In: Zabicky, J., ed. The chemistry of amides, London,
Interscience, pp. 733-848.
CIAVAGLIA, F. & THOMPSON, R. P. (1969) Infant methemoglobinemia.
Absence in areas with low concentrations of nitrate-nitrogen in
water supply. N.Y. State J. Med., 69: 3128-3129.
CLAPP, N. K. & TOYA, R. E. (1970) Effect of cumulative dose and dose
rate on dimethylnitrosamine oncogenesis in RF mice.
J. Natl Cancer Inst., 45: 495-498.
CLAPP, N. K., TYNDALL, R. L., & OTTEN, J. A. (1971) Differences in
tumor types and organ susceptibility in BALB-C and RF mice
following dimethylnitrosamine and diethyinitrosamine. Cancer
Res., 31: 196-198.
CLIFFORD, P. (1970) On the epidemiology of nasopharyngeal carcinoma.
Int. J. Cancer, 5: 287-309.
COLLINS-THOMPSON, D. L., SEN, N. P., ARIS, B., & SCHWENGHAMER, L.
(1972) Non-enzymic in vitro formation of nitrosamines by
bacteria isolated from meat products. Can. J. Microbiol,
18 (12): 1968-1971.
COMLY, H. H. (1945) Cyanosis in infants caused by nitrates in well
water. J. Am. Med. Assoc., 129: 112-116.
COMMITTEE ON NITRATE ACCUMULATION (1972) Accumulation of nitrate.
Washington, DC, National Academy of Sciences. p. 48.
COMMONER, B. (1970) Threats to the integrity of the nitrogen cycle.
In: Singer, F. S., ed. Global effects of environmental
pollution, NY, Springer Verlag, pp. 70-95.
COMMONER, B., SHEARER, G., & KOHL, D. (1972) A study of certain
ecological, public and economic consequences of the use of
inorganic nitrogen fertilizer, St. Louis, Washington
University. (1st year Progress Report NSF Grant No. GI29926x).
COOKE, G. E. & WILTIAMS, R. J. B. (1970) Losses of nitrogen and
phosphorus from agricultural land. Water Treat. Exam.,
19 (3): 253-276.
COORDINATING GROUP FOR RESEARCH ON THE ETIOLOGY OF ESOPHAGEAL CANCER
OF NORTH CHINA (1974) The epidemiology of esophageal cancer in
North China and preliminary results in the investigation of its
etiological factors. Abstract, 11th International Cancer
Congress, Florence, Italy.
CORREA, P., HAENSZEL, W., CUELLO, C., TANNENBAUM, S., & ARCHER, M.
(1975) A model for gastric cancer epidemiology, Lancet, July 12,
pp. 58-59.
COUCH, D. B. & FRIEDMAN, M. A. (1975) Interactive mutagenicity of
sodium nitrite, dimethylamine, methylurea and ethylurea. Mutat.
res., 31: 109-114.
CRADDOCK, V. M. (1971) Liver carcinomas induced in rats by single
administration of dimethylnitrosamine after partial hepatectomy.
J. Natl Cancer IMNST, 47: 899-907.
CROSBY, N. T., FOREMAN, K. K., PALFRAMEN, J. F., & SAWYER, R. (1972)
Determination of volatile nitrosamines in food products at the
µg/kg level. IARC Sci. Publ. No. 3, pp. 38-42.
DAIBER, D. (1966) [Methods for the quantitative and qualitative
estimation of organic N- nitroso compounds.] Freiburg (Thesis)
(in German).
DAY, N. E. (1975) Some aspects of the epidemiology of esophageal
cancer. Cancer Res., 35 (2): 3304-3307.
DELWICHE, C. C. (1970) The nitrogen cycle. Sci. Am., 223: 137-146.
DISKALENKO, A. P. (1968) Methemoglobinemia of water-nitrate origin in
the Moldavian SSR. Gig. i Sanit., 33: 32-37.
DISKALENKO, A. P. (1969) [Methaemoglobinaemia caused by nitrates in
water and its prophylaxis.] Kisinev (in Russian).
DISKALENKO, A. P. & DOBRJANSKAJA, E. V. (1972) [Changes in redox
processes in hepatic and cerebral tissues as a result of the
ingestion of nitrates with drinking water. In: Topical
questions of hygiene and epidemiology] Kisinev, pp. 22-23 (in
Russian).
DISKALENKO, A. P. & TROFIMENKO, JU. N. (1972) [Changes in the activity
of the glutathione-ascorbic acid system and respiration in
erythrocytes as a result of the ingestion of nitrites with
drinking water. In: Topical questions of hygiene and
epidemiology Kisinev, pp. 23-25 (in Russian).
DOWNS, E. F. (1950) Cyanosis of infants caused by nitrate
concentrations in rural water-supplies. Bull. World Health
Organ., 3: 165-169.
DRUCKREY, H. & PREUSSMANN, R. (1962) [The production of lung cancer in
rats by subcutaneous injection of diamylnitrosamine.]
Naturwiss., 49: 111-112 (in German).
DRUCKREY, H., PREUSSMAN, R., SCHMÄHL, D., & MÜLLER, M. (1961) [The
chemical constitution and carcinogenic effects of the
nitrosamines.] Naturwiss., 48: 134-135 (in German).
DRUCKREY, H., STEINHOFF, D., BEUTHNER, H., SCHNEIDER, H., & KLÄRNER,
P. (1963a) [Screening of nitrite for chronic toxicity in rats.]
Arzneimittel-Forsch., 13: 320-323 (in German).
DRUCKREY, H., SCHILDBACH, A., SCHMAHL, D., PREUSSMANN, R., &
IVANKOVIC, S. (1963b) [Quantitative analysis of the carcinogenic
action of diethylnitrosamine.] Arzneimittel-Forsch,
13: 841-851 (in German).
DRUCKREY, H., IVANKOVlC, S., & PREUSSMANN, R. (1966) Teratogenic and
carcinogenic effects in the offspring after single injection of
ethylnitrosourea in pregnant rats. Nature (Lond.),
210: 1378-1379.
DRUCKREY, H., PREUSSMANN, R., IVANKOVIC, S., & SCHMÄHL, D. (1967)
[Organotropic carcinogenic effects of 65 different N-nitroso-
compounds in BD-rats.] Z. Krebsforsch, 69: 103-201 (in German).
DRUCKREY, H., LANDSCHÜTZ, CH., & PREUSSMANN, R. (1968) [Oesophageal
carcinoma due to inhalation of methylbutylnitrosamine (MBNA) in
rats.] Z. Krebsforsch., 71: 135-139 (in German).
DRUCKREY, H., LANDSCHOTZ, CH., & IVANKOVlC, S. (1970a) [Transplacental
production of malignant tumours in the nervous system II.
Ethylnitroso urea in 10 genetically defined rat strains.] Z.
Krebsforsch., 73: 371-386 (in German).
DUBROW, H. & KABISCH, W. (1960) [Investigations of the evidence of
nitrates and nitrites in cheese.] Milchwiss., 15 (11): 543-549
(in German).
DURFOR, C. N. & BECKER, E. (1965) Public water supplies of the 100
largest cities in the United States. Washington DC, US Dept
Interior (Geological Survey Water Supply Paper 1812).
DUTTON, A. H. & HEATH, D. F. (1956) Demethylation of
dimethylnitrosamine in rats and mice. Nature (Lond.),
178: 644.
EISENBRAND, G. (1972) Determination of volatile nitrosamines at low
levels in food by acid-catalysed denitrosation and formation of
derivatives from the resulting amines. IARC Sci. Publ. No. 3,
pp. 64-71.
EISENBRAND, G. (1973) Determination of volatile nitrosamines: a
review. In: Proceedings of the International Symposium on
Nitrite in Meat Products. Zeist, pp. 45-52.
EISENBRAND, G. & PREUSSMANN, R. (1970) [A new method for the
colorimetric determination of nitrosamines following cleavage of
the N-nitroso group with hydrogen bromide in glacial acetic
acid.] Arzneimittel-Forsch., 20: 1513-1517 (in German).
EISENBRAND, G., JANZOWSKI, C., & PREUSSMANN, R. (1975) Gas
chromatographic determination of N-nitrosoamino acids by
trimethylsilylation and single-ion mass fragmentography.
J. Chromatogr., 115: 602-606.
EISENBRAND, G., VON RAPPARD, E., ZAPPE, R., & PREUSSMANN, R. (1976)
Trace analysis of volatile nitrosamines by a modified nitrogen
specific detector in pyrolytic mode and by ion-specific
determination of heptafiuorobutyrantides in a GC/MS system.
IARC Sci. Publ. No. 14, pp. 65-75.
EISENSTARK, A. & ROSNER, J. L. (1964) Chemically induced reversions in
the cysC region of Salmonella typhimurium. Genetics,
49: 343-355.
ELESPURU, R. K. & LIJINSKY, W. (1973) The formation of carcinogenic
nitroso compounds from nitrite and some types of agricultural
chemicals. Food Cosmet. Toxicol., 11 (3): 807-811.
ELESPURU, R., LIJINSKY, W., & SETLOW, J. K. (1974) Nitrosocarbaryl as
a potent mutagen of environmental significance. Nature (Lond.),
247: 386-387.
ENDER, F. & CEH, L. (1967) Occurrence and determination of
nitrosamines in foodstuffs for human and animal nutrition.
Alkylierend Wirkend Verbindungen, Second Conference on Tobacco
Research., Freiburg, 1960, pp. 83-91.
ENDER, F. & CEH, L. Z. (1971) Conditions and chemical reaction
mechanisms by which nitrosamines may be formed in biological
products with reference to their possible occurrence in food
products. Z. Lebensm. Unters. Forsch., 145 (3): 133-142.
ENDER, F., HAVRE, G., HELGEBOSTAD, A., KOPPANG, N., MADSEN, R., & CEH,
L. (1964) Isolation and identification of a hepatoxic factor in
herring meal produced from sodium nitrite preserved herring.
Naturwiss., 51: 637-638.
ENDER, F., HAVRE, G., MADSEN, R., CEH, L., & HELGEBOSTAD, A. (1967)
[Studies on conditions under which N-nitrosodime-thylamine is
formed in herring meal produced from nitrite-preserved herring.]
Z. Tierphysiol. Tierernähr. Futtermittelk., 22: 181-189 (in
German).
ENDO, H., TAKAHESHI, K., & AOYAGI, H. (1973) Screening of compounds
structurally and functionally related to N-methyl- N-nitroso
guanidine, a gastric carcinogen. Gann, 65: 45-54.
EPPSON, H. F., GLENN, M. W., ELLIS, W. W., & GILBERT, C. S. (1960)
Nitrate in the diet of pregnant ewes. JAVMA, 137 (10): 611-614.
EPSTEIN, S. (1972) In vivo studies on interactions between secondary
amines and nitrites or nitrates. IARC Sci. Publ. No. 3,
pp. 109-115.
FAN, T-Y. & TANNENBAUM, S. R. (1972) Automatic colorimetric
determination of N-nitroso compounds. J. agric. Food Chem.,
19: 1267-1269.
FAN, T-Y. & TANNENBAUM, S. R. (1973) Factors influencing the rate of
formation of nitrosomorpholine from morpholine and nitrite:
acceleration by thiocyanate and other anions
J. agric. Food Chem, 21: 237-240.
FAO/IAEA Panel of Experts (1974) Effects of agricultural production
on nitrates in food and water with particular reference to
isotope studies. Proceedings and Report of a Panel of Experts,
Vienna, 4-8 June 1973, Vienna, International Atomic Energy
Agency, pp. 158.
FASSETT, D. W. (1966) Nitrates and nitrites. In: Toxicants occurring
naturally in foods. Washington, DC, National Academy of
Sciences, pp. 250-449 (Publ. No. 1354).
FAZIO, T., DAMICO, J. N., HOWARD, J. W., WHITE, R. H., & WATTS, J. O.
(1971a) Gas chromatographic determination and mass spectrometric
confirmation of N-nitro-sodimethylamine in smoke-processed
marine fish. J. agric. Food Chem,
19(2): 250-253.
FAZlO, T., WHITE, R. H., & HOWARD, J. W. (1971b) Analysis of nitrite-
and/or nitrate-processed meats for
N-nitrosodimethylamine. J. Assoc. Off Anal. Chem.,
54(5): 1157-1159.
FAZIO, T., HOWARD, J. W., & WHITE, R. (1972) Multidetection method
for analysis of volatile nitrosamines in foods. IARC Sci. Publ.
No. 3, pp. 16-24.
FAZIO, T., WHITE, R. H., DUSOLD, L. R., & HOWARD, J. W. (1973)
Nitrosopyrrolidine in cooked bacon. J. Assoc. Off. Anal. Chem.,
56 (4): 919-921.
FERGUSON, J. H., MYSLIWY, T. J., & ARCHER, M. C. (1974) The
nitrosation of spermidine and spermine. IARC Sci. Publ. No. 9,
pp. 90-93.
FERON, V. J., EMMELOT, P., & VOSSENAAR, T. (1972) Lower respiratory
tract tumors in Syrian golden hamsters after intratracheal
instillations of diethylnitrosamine alone and with ferric oxide.
Eur. J. Cancer, 8: 445-449.
FIDDLER, W. (1975) The occurrence and determination of N-nitroso
compounds. Toxicoi. appl. Pharmacol., 31: 352-360.
FIDDLER, W., DOERR, R. C., ERTEL, J. R., & WASSERMAN, A. E. (1971)
Gas-liquid chromatographic determination of
N-nitrosodimethylamine in ham. J. Assoc. Off Anal. Chem, 54
(5): 1160-1163.
FIDDLER, W., PENSABENE, J. W., DOERR, R. C., & WASSERMAN, A. E. (1972)
Formation of N-nitrosodimethylamine from naturally occurring
quaternary ammonium compounds and tertiary amines. Nature
(Lond.), 236: 307.
FIDDLER, W., PENSABENE, J. W., PIOTROWSKI, E.G., DOERR, R. C., &
WASSERMAN, A. E. (1973) Use of sodium ascorbate or erythorbate to
inhibit formation of N-Nitroso-dimethylamine in frankfurters.
J. Food Sci., 38: 1084.
FIDDLER, W., PENSABENE, J. W., FAGAN, J. C., THORN, E. J., PIOTROWSKA,
E.G., & WASSERMAN, A. (1974) The role of lean and adipose tissue
on the formation of nitrosopyrrolidine in fried bacon. J. Food
Sci., 39: 1070-1071.
FIESER, L. F. & FIESER, M. (1967) Reagents for organic synthesis.
New York, Wiley, pp. 747-749.
FINE, D. H. & RUFFEH, F. (1974) Description of the thermal energy
analyser for N- nitroso compounds. IARC Sci. Publ. No. 9,
pp. 40-44.
FINE, D. H., RUFEH, F., & LIEB, D. (1974) Group analysis of volatile
and non-volatile N-nitroso compounds. Nature (Lond.),
247: 309-310.
FINE, D. H., HUFFMAN, F., ROUNBEHLER, D. P., & BETCHER, N.M. (1976a)
Analysis of N-nitroso compounds by combined high- performance
liquid chromatography and thermal energy analysis. IARC Sci.
Publ. No. 14, pp. 43-50.
FINE, D. H., ROUNBEHLER, D. P., BELCHER, N.M., & EPSTEIN, S.S. (1976b)
N-nitroso compounds in air and water. IARC Sci. Publ. No. 14,
pp. 401-408.
FLAKS, A., HAM LTON, J. M., CLAYSON, D. B., & BURCH, P. R. J. (1973)
The combined effect of radiation and chemical carcinogens in
female A x 1F mice. Br. J. cancer, 28: 227-231.
FONG, Y. Y. & CHAN, W. C. (1973a) Dimethylnitrosamine in Chinese
marine salt fish. Food Cosmet. Toxicol., 11: 841-845.
FONG, Y. Y. & CHAN, W. C. (1973b) Bacterial production of
dimethylnitrosamine in salted fish. Nature (Lond.),
243: 421-422.
FONG, Y. Y. & WALSH, E. O'F. (1971) Carcinogenic nitrosamines in
Cantonese salt-dried fish. Lancet, 2: 1032.
FOOD STANDARDS COMMITTEE (1959) Preservatives in food. London, Her
Majesty's Stationery Office.
FOSTER, S.S. D. & CREASE, R. I. (1974) Nitrate pollution of chalk
groundwater in east Yorkshire -- a hydrological appraisal. J.
Inst. Water Eng., 28: 178-194.
FREIMURTH, U. & GLASER, E. (1970) [The appearance of nitrosamines in
food.] Die Nahrung., 14 (5): 357-361 (in German).
FREUND, H. A. (1937) Clinical manifestations and studies in
parenchymatous hepatitis. Ann. int. Med., 10: 1144-1155.
FRIDMAN, A. L., MUKHAMETSIN, F. M., & NOMIKOV, S.S. (1971) [Advances
in the chemistry of aliphatic nitrosamines.] Russ. chem. Rev.,
40 (1): 34-50 (in Russian).
FRIEDMAN, M. A., GREENE, E. J., & EPSTEIN, S.S. (1972) Rapid gastric
absorption of sodium nitrite in mice. J. pharm. Sci.,
61 (9): 1492-1494.
GARBIDGE, M. G. & LEGATOR, M. S. (1969) A host-mediated microbic assay
for the detection of mutagenic contents. Proc. Soc. Exp. Biol.
Med., 130: 831-834.
GARBUZ, A.M. (1968) [The effect of symptomless methaemoglo-binaemia
on some bodily functions.] Author's summary of a thesis for the
degree of Candidate of Medical Sciences, Leningrad (in Russian).
GARBUZ, A.M. (1971) [Changes in the body's functional condition in
symptomless methaemoglobinaemia. In: Methaemoglobinaemia caused
by various factors and methods of preventing it.] Summaries of
papers read at the First Scientific Conference on
Methaemoglobinaemia, Leningrad, pp. 18-21 (in Russian).
GEIL, J. H., STENGER, R. J., BEHKI, R. M., & MORGAN, W. S. (1968)
Hepatotoxic and carcinogenic effects of dimethylnitrosamine in
low dosage. Light and electron microscopic study.
J. Natl Cancer Inst., 40: 713-730.
GELPERIN, A., JACOBS, E. E., & KLETKE, L. S. (1971) The development of
methemoglobin in mothers and newborn infants from nitrate in
water supplies. Ill. med. J., 140: 42-44.
GERRITSEN, G. A. & DE WILLIGEN, A. H. A. (1969) [The nitrite content
of potato and corn starch.] Die Starke, 21: 101-105 (in French).
GILBERTSON, C. B., MCCALLA, T. M., ELLIS, J. R., CROSS, O. E., &
WOODS, W. R. (1970) The effect of animal density and surface
slope on characteristics of runoff solid waste, and nitrate
movement on unpaved feedlots. Lincoln, Univ. Nebraska Coll.
Agric. and Home Econ.
GOBBI, A., SOVERINI, R., & GRISLER, R. (1974) [Normal
methemoglobinemia values observed in 500 inhabitants of the City
of Milan.] Med. Lavoro, 65: 306-310 (in Italian).
GOLDSMITH, J. R., ROKAW, S. N., & SHEARER, L. N. (1975) Distributions
of percentage methemoglobin in several population groups in
California. Int. J. Epidem., 4: 207-212.
GOTO, M. (1973) Inorganic chemicals in the environment -- with special
reference to the pollution problems in Japan. Environ. Qual.
Saf., 2: 72-77.
GROENEN, P. J., JONK, C., VAN INGEN, C., & TEN NOEVER DE BRAUW, M. C.
(1976) Determination of eight volatile nitrosamines in thirty
cured meat products with capillary gas chromatography-high
resolution mass spectrometry: the presence of
nitrosodiethylamine and the absence of nitrosopyrrolidine. IARC
Sci. Publ., No. 14, pp. 321-331.
GREENBERG, R. A. (1974) Ascorbate and nitrosamine formation in cured
meats. In: Proceedings of the International Symposium on
Nitrite in Meat Products, Zeist, 10-14 September, 1973,
Wageningen, Centre for Agricultural Publishing and Documentation,
pp. 179-188.
GREENBLATT, M. (1973) Ascorbic acid blocking of aminopyrine
nitrosation in NZO-Bl mice. J. Natl Cancer Inst.,
50: 1055-1056.
GREENBLATT, M. & MIRVISH, S.S. (1973) Dose-response studies with
concurrent administration of piperazine and sodium nitrite to
strain A mice. J. Natl Cancer Inst., 50 (1): 119-124.
GREENBLATT, M. & RIJHSINGHANI, K. (1969) Comparative cytopathologic
alterations induced by alkylnitrosamines in nasal epithelium of
the Syrian hamster. J. Natl Cancer Inst., 42: 421-433.
GREENBLATT, M., MIRVISH, S., & SO, B. T. (1971) Nitrosamine studies:
Induction of lung adenomas by concurrent administration of sodium
nitrite and secondary amines in Swiss mice. J. Natl Cancer
Inst., 46: 1029-1034.
GREENBLATT, M., KOMMINENI, V., CONRAD, E., WALLCAVE, L., & LIHNSKY, W.
(1972) In vivo conversion of phenmetrazine into its N-nitroso
derivative. Nature (Lond.), 236
(62): 25-26.
GREENBLATT, M., KOMMINENI, V. R. C., & LIJINSKY, W. (1973) Null effect
of concurrent feeding of sodium nitrite and amino acids to MRC
rats. J. Natl Cancer Inst., 50: 799-802.
GRUENER, N. & SHUVAL, H. I. (1970) Health aspects of nitrates in
drinking water. In: Shuval, H. I., ed. Developments in water
quality research. Ann Arbor, Humphrey Science Publ., pp. 89-106.
GRUNTHAL, D., HELLENBROICH, D. O., SANGER, P., & MAAS, H. (1970)[The
effect of partial hepatectomies on the hepatoma rate after
administration of diethyl-nitrosamine.]
Z. Naturforsch., 25: 1277-1281 (in German).
HADJIOLOV, D. (1971) [The inhibition of dimethylnitrosamine
carcinogenesis in rat liver by aminoacetonitrile.]
Z. Krebsforsch., 76: 91-92 (in German).
HAENSZEL, W. & CORREA, P. (1975) - Developments in the epidemiology of
stomach cancer over the past decade. Cancer Res.,
35: 3452-3459.
HANSEN, H. H. & MUGGIA, F. M. (1971) Treatment of malignant brain
tumours with nitrosoureas. Cancer Chemother. Rep. Part 1, 55
(2): 99-100.
HARD, G. C. & BUTLER, W. H. (1970a) Cellular analysis of renal
neoplasia. Induction of renal tumors in dietary-conditioned rats
by dimethylnitrosamine, with a reappraisal of morphological
characteristics. Cancer Res., 30: 2796-2805.
HARD, G. C. & BUTLER, W. H. (1970b) Cellular analysis of renal
neoplasia. Light microscope study of the development of
interstitial lesions induced in the rat kidney by a single
carcinogenic dose of dimethylnitrosamine. Cancer Res.,
30: 2806-2815.
HARD, G. C. & BUTLER, W. H. (1970c) Toxicity of dimethylni-trosamine
for the rat testis. J. Pathol., 102: 201-207.
HARD, G. C. & BUTLER, W. H. (1971a) Ultrastructural aspects of renal
adenocarcinoma induced in the rat by dimethylnitrosamine. Cancer
Res., 31: 366-372.
HARD, G. C. & BUTLER, W. H. (1971b) Ultrastructural study of the
development of interstitial lesions leading to mesenchymal
neoplasia induced in the rat renal cortex by dimethylnitrosamine.
Cancer Res., 31: 337-347.
HARD, G. C. & BUTLER, W. H. (1971c) Morphogenesis of epithelial
neoplasms induced in the rat kidney by dimethylnitrosamine.
Cancer Res., 31: 1496-1505.
HARD, G. C., BORLAND, R., & BUTLER, W. H. (1971) Altered morphology
and behavior of kidney fibroblasts in-vitro following in-vivo
treatment of rats with a carcinogenic dose of
dimethylnitrosamine. Experientia (Basel), 27: 1208-1209.
HARMOZDIAN, H., DAY, N. E., ARAMESH, B., & MAHBOUBI, E. (1975) Dietary
factors and esophageal cancer in the Caspian littoral of Iran.
Cancer Res., 35: 3493-3498.
HASHIMOTO, Y., SUZUKI, E., & OKADA, M. (1972) [Induction of urinary
bladder tumors in ACI/N rats by butyl (3-carboxypropyl)
nitrosamine, a major urinary metabolite of butyl-(4-hydroxbutyl)
nitrosamine.] Gann, 63: 637-638 (in Japanese).
HAWKSWORTH, G. & HILL, M. J. (1971a) The formation of nitrosamines by
human intestinal bacteria. Biochem. J., 122: 28p-29p.
HAWKSWORTH, G. & HILL, M. J. (1971b) Bacteria and the
N-nitrosation of secondary amines. Br. J. Cancer,
25: 520-526.
HAWKSWORTH, G., HILL, M. J., GORDILL, G., & CUELLO, C. (1974)
Possible relationship between nitrates, nitrosamines and
gastric cancer in S.U. Columbia. IARC Sci. Publ. No. 9,
pp. 229-234.
HEATH, D. F. (1962) The decomposition and toxicity of
dialkylnitrosamines in rats. Blochem. J., 85: 72-91.
HEDLER, L. & MARQUARDT, P. (1968) Occurrence of diethynitrosamine in
some samples of food. Food Cosmet. Toxicol, 6: 341-348.
HEDLIN, R. A. (1971) Nitrate contamination of ground water in the
Neepawa-Langruth area of Manitoba. Can. J. Soil Sci,
51: 75-84.
HEIN, G. E. (1963) Reaction of tertiary amines with nitrous acid,
J. chem. Educ, 40 (4): 181-184.
HENDERSON, W. R. & RASKIN, N.H. (1972) "Hot-Dog" headache: Individual
susceptibility to nitrite. Lancet, 2: 1162-1163.
HEPPEL, L. A. & PORTERFIELD, V. T. (1949) Metabolism of inorganic
nitrite and nitrate esters I. The coupled oxidation of nitrite by
peroxide-forming systems and catalase. J. biol. Chem, No. 178,
pp. 549-556.
HERMANN, H. (1960) [P. Methylnitrosaminebenzaldehyde, a metabolic
product of Clytocybe suaveolens.] Naturwiss., 47: 162
(in German).
HEYNS, K. (1973) [On nitrosamines in nutrients.] Getreide Mehl Brot,
27: 249-253 (in German).
HICKS, R. M., WAKEFIELD, J. ST. J., & CHOWANIEC, J. (1973)
Co-carcinogenic action of saccharin in the chemical induction of
bladder cancer. Nature (Lond.), 243: 347-349.
HILDRUM, K. I., SCANLAN, R. A., & LIBBEY, L. M. (1975) Identification
of gamma-butenyl-(beta-propenyl) nitrosamine, the principal
volatile nitrosamine formed in the nitrosation of spermidine or
sperminc. J. agric. Food Chem., 23
(1): 34-37.
HILL, M. J., HAWKSWORTH, G., & TATTERSALL, G. (1973) Bacteria,
nitrosamines and cancer of the stomach. Br. J. Cancer,
28 (6): 562-567.
HMSO (1973) The determination of nitrate-nitrogen in herbage. London,
Her Majesty's Stationery Office, pp. 3 (Tech. Bull. 27).
HMSO (1974) Annual Report 1972, London, Her Majesty's Stationery
Office, pp. 206.
HOCH-LIGETI, C., ARGUS, M. F., & ARCOS, J. C. (1968) Combined
carcinogenic effects of dimethylnitrosamine and 3-methyl-
cholanthrene in the rat. J. Natl Cancer Inst., 40: 535-549.
HOFFMAN, D., HECHT, S.S., ORNAF, R. M., & WYDNER, E. L. (1974)
N-nitrosonornicotine in tobacco. Science, 186: 265-267.
HÖLSCHER, P.M. & NATZSCHKA, J. (1964) [Methaemoglobinaemia in young
children due to the nitrite contents of spinach.] Dtsch Med.
Wschr, 89: 1751-1754 (in German).
HORN, E. E. & HERRIOT, R. M. (1962) The mutagenic action of nitrous
acid on "single-stranded" (denatured) haemophilus transforming
DNA. Proc. Natl Acad. Sci. US, 48: 1409-1416.
HORWlTZ, W. (1975) Association of Official Analytical Chemists,
Official methods of analysis. 12th ed. Washington, DC,
pp. 1094.
HOWARD, J. W., FAZlO, T., & WATTS, J. O. (1970) Extraction and gas
chromatographic determination of N-nitrosodimethy-lamine in
smoked fish application to smoked nitrite-treated chub. J.
Assoc. Off. Anal. Chem., 53(2): 269-274.
INGRAM, M. (1974) The microbiological effects of nitrite. In:
Proceedings of the International Symposium on Nitrite in Meat
Products, Zeist, 10-14 September, 1973, Wageningen, Centre for
Agricultural Publishing and Documentation, pp. 63-75.
IRETON, H. J., McGIVEN, A. R., & DAVIES, D. J. (1972) Renal
mesenchymal tumours induced in rats by dimethylnitrosamine. Light
and electron-microscope studies. J. Pathol., 108: 181-185.
ISSENBERG, P. & TANNENBAUM, S. (1972) Approaches to the
determination of volatile and non volatile N- nitroso
compounds in foods and beverages. IARC Sci. Publ., No. 3,
pp. 31-37.
ITO, N., HIASA, Y., TAMAI, A., OKAJIMA, E., & KITAMORA, H. (1969)
Histogenesis of urinary bladder tumors induced by N-butyl- N-
(4-hydroxybutyl) nitrosamine in rats. Gann, 60: 401-410.
ITO, N., MAKIURA, S., YOKOTA, Y., KAMAMOTO, Y., HIASA, Y., & SUGIHARA,
S. (1971) Effect of unilateral ureter ligation on development of
tumors in the urinary system of rats treated with N-butyl- N-
(4-hydroxybutyl) nitrosamine. Gann, 62: 359-365.
IVANKOVIC, S. & DRUCKREY, H. (1968) [Transplacental induction of
malignant tumours of the nervous system. I Ethylnitrosourea (ENU)
in BD-IX-rats.] Z. Krebsforsch., 71: 320-360 (in German).
IVANKOVIC, S. & PREUSSMANN, R. (1970) [Transplacental induction of
malignant tumours by the oral administration of ethylurea and
nitrite in rats.] Naturwiss., 57: 460-461 (in German).
IVANKOVIC, S., PREUSSMANN, R., SCHMÄHL, D., & gELLER, J. (1973)
[Prevention of nitrosamide induced hydrocephali by ascorbic acid
after prenatal administration of ethylurea and nitrite to rats.]
Z. Krebsforsch, 79: 145-147 (in German).
JACKSON, W. A., STEEL, J. S., & BOSWELL, V. R. (1967) Nitrates in
edible vegetables and vegetable products. Proc. Am. Soc. Hort.
Sci., 90: 349-352.
JAFFÉ, E. R. & HELLER, P. (1964) Methemoglobinemia in man. In: Moore,
C. V. & Brown, E. B., ed. Progress in hematology, vol. IV, New
York, Grune & Stratton, pp. 48-71.
JAPAN ENVIRONMENTAL SANITATION CENTER (1974) [Report of 1973 results
of the analysis for suspended particulate matter in the National
Air Sampling Network.] Kawasaki, Japan, pp. 14, 24 (in Japanese).
JASMIN, G. & CHA, J. W. (1969) Renal adenomas induced in rats by
dimethylnitrosamine. An electron microscopic study. Arch.
Pathol., 87: 267-278.
JENISSEN, H., HOSHINO, J., & KROGER, H. (1971) [The influence of
carcinogenic nitrosamines on the induction of serine dehydratase
in the rat liver.] Z. Krebsforsch., 75: 246-252 (in German).
JOHNSON, J. H. (1966) Internal can corrosion due to high nitrate
content of canned vegetables. Proc. Fla. State Hort. Sci.,
79: 239-242.
JUSKIEWICZ, T. & KOWALSKI, B. (1974) Passage of nitrosamines from
rumen into milk in goats. IARC Sci. Publ. No. 9, pp. 173-176.
KAMM, L., MCKEOWN, G. G., & SMITH, D. M. (1965) New colorimetric
method for the determination of the nitrate and nitrite content
of baby foods. J. Assoc. Off. Agric. Chem., 48 (5): 892-897.
KAMM, J. J., DASHMAN, T., CONNEY, A. H., & BURNS, J. J. (1973)
Protective effect of ascorbic acid on hepatoxicity caused by
sodium nitrite and aminopyrin. Proc. Natl Acad. Sci.,
70: 747-749.
KAPELLER-ADLER, R. & KRAEL, J. (1930a) [Research on nitrogen
distribution in the muscle of different animal species I,]
Biochem. Z., 221: 437-460 (in German).
KAPELLER-ADLER, R. & KRAEL, J. (1930b) [Research on nitrogen
distribution in the muscle of different animal species II.]
Biochem. Z., 224: 364-377 (in German).
KAUDEWITZ, F. (1959) Production of bacterial mutants with nitrous
acid. Nature (Lond.), 183: 1829-1830.
KAWABATA, T. (1974) Addendum, N- nitroso compounds: Recent studies
in Japan. IARC Sci. Publ. No. 9, pp. 154-158.
KEATING, J.P., LELL, M. E., STRAUSS, A. W., ZARKOWSKY, H., & SMITH, G.
E. (1973) Infantile methemoglobinemia caused by carrot juice. N.
Eng. J. Med., 288: 824-826.
KEOHANE, K. W. & METCALF, W. K. (1960) An investigation of the
differing sensitivity of juvenile and adult erythrocytes to
methemoglobinization. Phys. med. Biol., 5: 27-35.
KILGORE, L., STASCH, A. R. & BARRENTINE, B. F. (1963) Nitrate content
of beets, collards, turnip greens. J. Am. Diet. Assoc.,
43: 39-42.
KING, E. J. (1959) Qualitative analysis and electrolytic solutions.
New York, Harcourt, Brace & Co., pp. 519.
KIRKLAND, D. J. & PICK, C. R. (1973) The histological appearance of
tumours derived from rat embryo cells transformed in vitro
spontaneously and after treatment with nitrosomethylurea. Br.
J. Cancer, 28: 440-452.
KLUBES, P. & JONDORF, W. R. (1971) Dimethyl nitrosamine formation from
sodium nitrite and dimethylamine by bacterial flora of rat
intestine. Res. Commun. chem. Pathol. Pharmacol,
2 (1): 24-34.
KNELSON, J. H. & LEE, R. E. (1977) Oxides of nitrogen in the
atmosphere: Origin, fate and public health implications. Ambio,
6 (2-3): 126-130.
KNOTEK, Z. & SCHMIDT, P. (1964) Pathogenesis, incidence and
possibilities of preventing alimentary nitrate methemoglobinemia
in infants. Pediatrics, 34: 78-83.
KOCIBA, R. J. & SLEIGHT, S. D. (1970) Nitrite toxicosis in the
ascorbic acid-deficient guinea pig. Toxicol. appl. Pharmacol.
16: 424-429.
KOHL, D. H., SHEARER, G. B., & COMMONER, B. (1971) Fertilizer
nitrogen: Contribution to nitrate in surface water in a corn belt
watershed. Science, 174: 1331-1334.
KOPPANG, N. (1964) An outbreak of toxic liver injury in ruminants.
Nord. vet. Med., 16: 305-322.
KORDAC, V., SCHON, E., & BRAUN, A. (1969) Hepatocancerogenesis in mice
with simultaneous administration of diethylnitrosamine and the
virus Motol. Neoplasma, 16: 485-490.
KOYAMA, T., HANDA, J., HANDA, H., & MATSUMOTO, S. (1970)
Methylnitrosourea-induced malformations of brain in SD-JCL rat.
Arch. Neurol., 22: 342-347.
KRAVITZ, H., ELEGANT, L. D., KAISER, E., & KAGAN, B. M. (1956)
Methemoglobin values in premature and mature infants and
children. A.M.A.J. Dis. Child., 91: 1-5.
KRÖLLER, E. (1967) [Investigations of the evidence of nitrosamines in
tobacco smoke and food.] Dtsch. Lebensm. Rundsch., 63(10):
303-305 (in German).
KRÜGER, F. W. (1972) [On the biochemistry of carcinogenic
N-nitroso compounds.] Ärztliche Forsch., 26 (6): 197-202 (in
German).
KRÜGER, F. W. (1973) Metabolism of nitrosamines in vivo. II. On the
methylation of nucleic acids by aliphatic di-n-alkylnitrosamines
in vivo, caused by beta-oxidation: The increased formation of
7-methyl-guanine after application of beta-hydroxypropyl-
nitrosamine compared to that after application of di- N-propyl-
nitrosamine. Z. Krebsforsch., 79: 90-97.
KÜBLER, W. (1958) [The importance of the nitrate content of vegetables
in child nutrition.] Z. Kinderheilk, 81: 405-416 (in German).
KÜBLER, W. (1965) [Methaemoglobinaemia in infants after feeding with
spinach.] Dtsch. med. Wschr., 90: 1881-1882 (in German).
KUNZ, W., SCHAUDE, G., & THOMAS, C. (1969) [The effect of
phenobarbital and halogenated hydrocarbons on nitrosamine
carcinogenesis.] Z. Krebsforsch., 72: 291-304 (in German).
KÜNZER, W. & SCHNEIDER, D. (1953) [The activity of the reducing enzyme
system in erythrocytes of young infants.] Acta haemat., 9: 346
(in German).
KUWAHARA, A., OTSUKA, H., & NAGMATSU, A. (1972) Induction of
hemangiomatous lesions with dimethylnitrosamine. Influence of
route of administration and strain of mice. Gann,
63: 499-502.
LACASSAGNE, A., BUU-HOI, N., GIAO, N. B., & FERRANDO, R. (1968)
Retarding effect of resirpine on the cancerization of rat liver
induced by diethylnitrosamine. Bull. Cancer,
55 (1): 87-89.
LEE, D. H. K. (1970a) Nitrates, nitrites and methemoglobinemia.
Environ. Res., 3: 484-511.
LEE, G. F. (1970b) Eutrophication. Occasional Paper No. 2,
University of Wisconsin, Water Resources Center, Madison,
pp. 12-13.
LEE, K. Y. & LIJINSKY, W. (1966) Alkylation of rat liver RNA by cyclic
N-nitrosamines in vivo, J. Natl Cancer Inst., 37
(A): 401-407.
LEHMAN, A. J. (1958) Quarterly report to the editor on topics of
current interest. Nitrates and nitrites in meat products. Assoc.
Food Drug Off. US Q. Bull., 22: 136-138.
LEMBKE, A. & MOEBUS, O. (1970) Is there a connection between the
occurrence of nitrite-producing microorganisms in cheese and the
formation of nitrosamine? Proceedings of the 18th
International Dairy Congress A.2.3, p. 139.
LIJINSKY, W. (1974) Reaction of drugs with nitrous acid as a source of
carcinogenic nitrosamines. Cancer Res., 34: 255-258.
LIJINSKY, W. & EPSTEIN, S.S. (1970) Nitrosamines as environmental
carcinogens. Nature (Lond.), 225: 21-23.
LIJINSKY, W. & GREENBLATT, M. (1972) Carcinogen dimethylnitrosamine
produced in vivo from nitrite and aminopyrine. Nature (New
Biology), 236: 177-178.
LIJINSKY, W. & SINGER, G. (1974) Formation of nitrosamines from
tertiary amines and nitrous acid. IARC Sci. Publ. No. 9,
pp. 111-114.
LIJINSKY, W., TOMATIS, L, & WENGEN, C. E. M. (1969) Lung tumors in
rats treated with N-nitrosoheptamethyleneimine and
N-nitrosooctamethyleneimine. Proc. Soc. Exp. Biol. Med.,
130: 945-949.
LIJINSKY, W., FERRERO, A., MONTESANO, R., & WENYON, C. E. M. (1970)
Tumorigenecity of cyclic nitrosamines in Syrian golden hamsters.
Z. Krebsforsch., 74: 185-189.
LIJINSKY, W., CONRAD, E., & VAN DE BOGART, R. (1972a) Carcinogenic
nitrosamines formed by drug/nitrite interactions. Nature
(Lond.), 239: 165-167.
LIJINSKY, W., CONRAD, E., & VAN DE BOGART, R. (1972b) Formation of
carcinogenic nitrosamines by interaction of drugs with nitrite.
IARC Sci. Publ. No. 3, pp. 130-133.
LIJINSKY, W., KEEFER, L., CONRAD, E., & VAN DE BOGART, R. (1972c)
Nitrosation of tertiary amines and some biologic implications.
J. Natl Cancer Inst., 49 (5): 1239-1249.
LIJINSKY, W., GREENBLATT, M., & KOMMINENI, C. (1973a) Brief
communication: Feeding studies of nitrilotriacetic acid and
derivatives in rats. J. Natl Cancer Inst., 50: 1061-1063.
LIJINSKY, W., KEEFER, L., LOO, J., & ROSS, A. E. (1973b) Studies of
alkylation of nucleic acids in rats by cyclic nitrosamines.
Cancer Res., 33: 1634-1641.
LIJINSKY, W., TAYLOR, H. W., SNYDER, C., & NETTESHEIM, P. (1973c)
Malignant tumors of liver and lung in rats fed aminopyrine or
heptamethyleneimine together with nitrite. Nature (Lond.),
244: 176-178.
LOVELESS, A. & HAMPTON, C. (1969) Inactivation and mutation of
coliphage T2 by N-methyl- and N-ethyl- N-nitrosourea.
Murat. Res., 7: 1-12.
LUHRS, C. E. (1973) Nitrogenous materials in the environment. Draft
report. US Environmental Protection Agency, Hazardous Materials
Advisory Committee.
MAEKAWA, H. & ODASHIMA, S. (1975) Induction of tumours of the nervous
system in the HCI/N rat with N-buyl-I-nitrosourea administered
transplacentally, neonatally or via maternal milk. Gann,
66: 175-183.
MAGEE, P. N. (1956) Toxic liver injury. The metabolism of
dimethylnitrosamine. Biochem. J., 64: 676-682.
MAGEE, P. N. (1972) Possibilities of hazard from nitrosamines in
industry. Ann. occup. Hyg., 15: 19-22.
MAGEE, P. N. & BARNES, J. M. (1962) Induction of kidney tumours in the
rat with dimethyl ( N-nitrosodimethylamine) nitrosamine.
J. Pathol. Bacteriol., 84: 19-31.
MAGEE, P. N. & BARNES, J. M. (1967) Carcinogenic nitroso compounds.
Adv. Cancer Res., 10: 163-246.
MAGEE, P. N., MONTESANO, R., & PREUSSMANN, R. (1976) N-nitroso
compounds and related carcinogens. In: Searle, E. ed., Chemical
carcinogens. ACS monograph No. 173, pp 491-625.
MAKIURA, S., KAMMAMOTO, Y., SUGIHARA, S., HIRAO, K., HIASA, Y., ARAI,
M., & ITO, N. (1973) Effect of 1-naphthyl isothiocyanate and
3-methylcholanthrene on hepato-carcinogenesis in rats treated with
diethylnitrosamine. Gann, 64: 101-104.
MALINS, D.C., ROUBAL, W. T., & ROBISCH, P. A. (1970) The possible
nitrosation of amines in smoked chub. J. agric. Food. Chem.,
18(4): 740-741.
MALLING, H. V. (1966) Mutagenicity of two potent carcinogens
dimethylnitrosamine and diethylnitrosamine in Neurospora
crassa. Mutat. Res., 3: 537-540.
MANNING, P. B., COULTRER, S. T., & JENNESS, R. (1968) Determination of
nitrate and nitrite in milk and dry milk products. J. Dairy
Sci., 51: (11): 1725-1730.
MARCULESCU, V. (1917) Determination of nitrates in water. Stud.
Prot. Epurarea Apelor., 15: 127-161
MARRIOTT, W. M., HARTMANN, A. F., & SENN, M. J. E. (1933) Observations
on the nature and treatment of diarrhea and the associated
systemic disturbances. J. Pediatr., 3: 181-191.
MCLEAN, A. E. M. & MAGEE, P. N. (1970) Increased renal carcinogenesis
by dimethylnitrosamine in protein deficient rats. Br. J. exp.
Pathol., 51: 587-590.
MCLEAN, A. E. & VERSCHUUREN, H. G. (1969) Effects of diet and
microsomal enzyme induction on the toxicity of
dimethylnitrosamine. Br. J. exp. Pathol., 50: 22-25.
METCALF, W. K. (1961) The sensitivity of haemoglobin to oxidation in
various conditions. Nature (Lond.), 190: 543-544.
MIRNA, A. (1974) Determination of free and bound nitrite. In:
Proceedings of the International Symposium on Nitrite in Meat
Products, Ziest, 10-14 September 1973, Wageningen, Centre for
Agricultural Publishing and Documentation, pp. 21-28.
MIRVISH, S.S. (1970) Kinetics of dimethylamine nitrosation in relation
to nitrosamine carcinogenesis. J. Natl Cancer Inst., 44
(3): 633-639.
MIRVISH, S.S. (1971) Kinetics of nitrosamide formation from
alkylureas, N-alkylurethanes and alkylguanidines: possible
implications for the etiology of human gastric cancer. J. Natl
Cancer Inst., 46: 1183-1193.
MIRVISH, S.S. (1975) Formation of N-nitroso compounds: chemistry,
kinetics, and in vivo occurrence. Toxicol. appl. Pharmacol,
31: 325-351.
MIRVISH, S.S. (in press) N-nitroso compounds, nitrite and nitrate:
possible implications for the causation of human cancer. J.
Water Pollut.
MIRVISH, S., WALLCAVE, L., EAGEN, M., & SHUBIK, P. (1972) Ascorbate-
nitrite reaction; possible means of blocking the formation of
carcinogenic N-nitroso compounds. Science, 177: 65-68.
MIRVISH, S.S., SAMS, J., FAN, T. Y., & TANNENBAUM, S. R. (1973a)
Kinetics of nitrosation of the amino acids proline hydroxyproline
and sarcosine. J. Natl Cancer Inst., 51: 1833-1840.
MIRVISH, S.S., CARDESA, A., WALLCAVE, L., & SHUBIK, P. (1973b) Effect
on sodium ascorabte on lung adenoma induction by amines plus
nitrite. Proc. Am. Assoc. Cancer Res., 14: 102.
MIRVISH, S.S., ARNOLD, S., CONRAD, E., GHADIRIAN, P., KOMMINENI, V. R.
C., & SAMS, J. (1974) The formation of N- nitroso compounds,
preparation of heptafiuorobutyryl derivatives of ureas, and the
fate of nitrite in the rat stomach. IARC Sci. Publ. No. 9,
pp. 67-70.
MIRVISH, S.S., CARDESA, A., WALLCAVE, L., & SHUBIK, P. (1975)
Induction of mouse lung adenomas by amines or ureas plus nitrite
and by N-nitroso compounds: effects of ascorbates, gallic acid,
thyocyanate, and caffeine. J. Natl Cancer Inst., 55: 633-636.
MIRVISH, S.S., ISSENBERG, P., & SORNSON, H. C. (1976) Air-water and
ether-water distribution of N-nitroso compounds: implications
for laboratory safety, analytical methodology, and
carcinogenicity for the rat esophagus, nose, and liver. J. Natl
Cancer Inst., 56 (6): 1125-1129.
MÖHLER, K. & MAYRHOFER, O. L. (1968) [Evidence and determination of
nitrosamines in food.] Z. Lebensm. Unter-Forsch., 135 (6):
313-318 (in German).
MÖHLER, K. & MAYRHOFER, O. L. (1969) [Effect of various factors on the
formation of nitrosamines in meat products.] 15th European
Meeting of the Meat Research Workers, Helsinki, 17-24 August
1969, pp. 302-304 (in German).
MOHR, U. & HILFRICH, J. (1972) Brief communication: Effect of a single
dose of N-diethylnitrosamine on the rat kidney. J. Natl
Cancer Inst., 49: 1729-1731.
MONTESANG, R. (1970) Systemic carcinogens ( N-nitroso compounds) and
synergistic or additive effects of respiratory carcinogenesis.
Tumori, 56: 335-344.
MONTESANO, R. & BARTCH, H. (1976) Mutagenic and carcinogenic
N-nitroso compounds: possible environmental hazards. Mutat.
Res., 32: 179-228.
MONTESANO, R. & SAFFIOTTI, U. (1968) Carcinogenic response of the
respiratory tract of Syrian golden hamsters to different doses of
diethylnitrosamine. Cancer Res., 28: 2197-2210.
MOTYLEV, V. D. (1969) [The question of age-related methaemoglobinaemia
caused by nitrates and nitrites and its prophylaxis.] Author's
summary of a thesis for the degree of Candidate of Medical
Sciences, Leningrad, (in Russian).
MURTHY, Y. K. S., THIEMANN, J. E., CORONELLI, C. I., & SENSl, P.
(1966) Alanosine, a new antiviral and antitumour agent isolated
from a streptomyces. Nature (Lond.), 211: 1198-1199.
MYSLIWY, T. S., WICK, E. L., ARCHER, M. C., SHANK, R. C., & NEWBERNE,
P.M. (1974) Formation of N-nitrosopyrrolidine in a dog's
stomach. Br. J. Cancer, 30: 279-283.
NAGAWA, Y. & MIRNA, A. (1974) [Influence exerted by processing upon
the formation of nitrosamines in meat products.]
Fleischwirtschaft., 1781-1788 (in German).
NAPALKOV, N. P. (1971) [Experimentation on transplacental
blastomogenesis as a tool for study of etiopathogenesis of
tumours in children.] Vop. Onkol., 17 (8): 3-15 (in Russian).
NASHED, N. & JUBBUR, G. (1966) A genetic and functional
characterization of adenine mutants induced in yeast by
1-nitroso-imidazolidone-2 and nitrous acid. Z. Verer-bungsl,
98: 106-110.
NEURATH, H. (1972) Nitrosamine formation from precursors in tobacco
smoke. IARC Sci. Publ. No. 3, pp. 134-136.
NEURATH, G. & SCHREIBER, O. (1974) Investigations on amines in the
human environment. Sci. Publ. No. 9, pp. 211-214.
NEURATH, G., PIRMANN, B., & WICHERNOTT, (1964) [On the question of
nitroso compounds in tobacco smoke.] Beitr. Tabakforsch.,
2(7): 311-319 (in German).
NEWBERNE, P.M. & SHANK, R. C. (1973) Induction of liver and lung
tumors in rats by the simultaneous administration of sodium
nitrite and morpholine. Food Cosmet. Toxicol., 11: 819-825.
NEWELL, J. E. & SISKEN, H. R. (1972) Determination of nitrosodi-
methylamine in the low parts per billion. J. agric. Food Chem.,
20 (3): 711-713.
NIEHS (1970) Nitrates, nitrites and methemoglobinemia. North
Carolina, National Institute of Environmental Health Sciences,
pp. 43 (Rep. No. 2).
NOROHNA, R. F. & GOODALL, C. M. (1972) Nasal tumors in starved rats
injected once with dimethylnitrosamine. N. Z. med. J.,
75: 374-375.
NYE, J. C. (1973) Nitrogenous compounds in the environment. Draft
Report, Washington, DC, US Environmental Protection Agency,
Hazardous Materials Advisory Committee, pp. 95-109.
O'CONNOR, P. J., CAPPS, M. J., & CRAIG, A. W. (1973) Comparative
studies of the hepatocarcinogen N,N-dimethylnitrosamine in
vivo: reaction sites in rat liver DNA and the significance of
their relative stabilities. Br. J. Cancer, 27: 153-166.
ODASHIMA, S. (1969) Experimental carcinoma of the glandular stomach in
rats. I. Effect of 7, 12-dimethylbenz-[a]anthracene or
4-nitroquinolene 1-oxide placed on glandular stomach combined with
oral administration of N,N1-(2,7-fluorenylene) bisacetamide
or N-nitrosodiethylamine. Gann, 60: 211-222.
OEHME, F. W. (1975) Vetinary toxicology. In: Caserett, L. J. & Doulle,
J., ed. Toxicology, the basic science of poisons. New York,
Macmillan Publishing Co., Inc., pp. 701-727.
OKADA, M. & SUZUKI, E. (1972) Metabolism of butyl (4-hydroxybutyl)
nitrosamine in rats. Gann, 63 (3): 391-392.
OKADA, M., SUZUKI, E., ANJYO, T., & MOCHIZUKI, M. (1975) Mutagenicity
of gamma-acetoxy-dialkylnitrosamines: model compounds for an
ultimate carcinogen. Gann, 66 (4): 457-458.
OKAJIMA, E., HIRAMATSU, T., MOTOMIYA, Y., IRWA, K., IJUIN, M., & ITO,
N. (1971) Effect of DL-tryptophan on tumorigenesis in the urinary
bladder and liver of rats treated with N-nitrosodibutylamine.
Gann, 62: 163-169.
OLSON. O. E., NELSON, D. L., & EMERICK, R. J. (1963) Effect of nitrate
and some of its reduction products on carotine stability.
J. agric. Food Chem, 11: 140-143.
ORGERON, J. D., MARTIN, J. D., CARAWAY, C. T., MARTINE, R. M., &
HAUSER, G. H. (1957) Methemoglobinemia from eating meat with high
nitrite content. Public Health Rep., 72 (3): 189-193.
OSSKE, G., WARZOK, R., & SCHNEIDER, J. (1972) [Transplacental tumour
induction by endogenous N-ethyl- N-nitrosourea in the rat.]
J. Arch. Geschwulstforsch., 40: 244-247 (in German).
OWENS, M. (1970) Nutrient balances in rivers. Proc. Soc. Water
Treat. Exam., 19: 239-247.
PANALAKS, T., IYENGAR, J. R., & SEN, N. P. (1972) Nitrate, nitrite and
dimethylnitrosamine in cured meat products. J. Assoc. Off.
Anal. Chem, 56 (3): 621-625.
PANALAKS, T., IYENGAR, J. R., DONALDSON, B. A., MILES, W. F., & SEN.
N. P. (1974) Further survey of cured meat products for volatile
N-nitrosamines. J. Assoc. Off. Anal. Chem., 57 (4): 806-812.
PASTERNAK, L. (1962) [Mutagenic effects of dimethylnitrosamine with
Drosophila melanogaster.] Naturwiss., 16: 381 (in German).
PASTERNAK, L. (1964) Investigation into the mutagenic effect of
several nitrosamine and nitrosamide compounds. Arzneintittel-
Forsch., 14: 802-804.
PENSABENE, J. W., FIDDLER, W., GATES, R. A., FAGAN, J. C., &
WASSERMAN, A. E. (1974) Effect of frying and other cooking
conditions on nitrosopyrrolidine formation in bacon.
J. Food Sci., 39: 314-136.
PERRY, T. L., SHAW, K. N. F., WALKER, D., & REDLICH, D. (1962) Urinary
excretion of amines in normal children. Pediatrics, 30: 576-584.
PETUKHOV, N. I., RYVKIN, A. I., GAINULLEN, G. G., & LANDYSHEVA, V. I.
(1972) [Methaemoglobinaemia in children and adults caused by
nitrates in water] Gig. i Sanit., 3: 14-17 (in Russian).
PETUKHOV, N. I. & IVANOV, A. V. (1970) Investigation of certain
psychophysiological reactions in children suffering from
methemoglobinemia due to nitrates in water. Gig. i Sanit.,
35 (1): 26-28.
PHILLIPS, J. C., LAKE, B. G., HEADING, C. E., GANGOLLI, S. D., &
LLOYD, A. G. (1975a) Studies on the metabolism of dimethyl-
nitrosamine in the rat. I. Effect of dose, route of
administration and sex. Food Cosmet. Toxicol., 13: 203-209.
PHILLIPS, J. C., HEADING, C. E., LAKE, B. O., GANGOLLI, S. D., &
LLOYD, A. G. (1975b) Studies on the metabolism of
dimethylnitrosamine in the rat. II. The effects of phenobarbitone
and 20-methylcholanthrene on the in vitro and in vivo
metabolism and acute toxicity of dimethylnitrosamine in young
and mature rats. Food Cosmet. Toxicol., 13: 611-617.
PHILLIPS, W. E. J. (1966) Effect of dietary nitrite on the liver
storage of vitamin A in the rat. Can. J. Blochem.,
44 (1): 1-7.
PHILLIPS, W. E. J. (1968a) Changes in the nitrate and nitrite contents
of fresh and processed spinach during storage. J. agric. Food
Chem., 16 (1): 88-91.
PHILLIPS, W. E. J. (1968b) Nitrate content of foods -- public health
implications. Can. Inst. Food Technol. J., 1 (3): 98-103.
PHILLIPS, W. E. J. (1969) Lack of nitrite accumulation in partially
consumed jars of baby food. Can. Inst. Food Technol. J.,
2 (4): 160-161.
PHILLIPS, W. E. J. (1971) Naturally occurring nitrate and nitrite in
foods in relation to infant methemoglobinemia. Food Cosmet.
Toxicol., 9: 219-228.
PIELSTICKER, K. (1967) Absence of malformations following
diethylnitrosamine in the golden hamster. Naturwiss.,
54: 340-341.
PISCIOTTA, A. V., EBBE, S. N., & HINZ, J. E. (1959) Clinical and
laboratory features of two variants of methemoglobin M disease.
J. Lab. clin. Med, 54: 73-87.
PITTS, J. N. Jr. & LLOYD, A. C. (1973) Discharges into the atmosphere.
In: Nitrogenous compounds in the environment. Washington, US
Environmental Protection Agency, pp. 43-65 (Rep No. EPA-SAB-73-
001).
PLATT, D. & HERING, F. J. (1973) The effect of Calciparin on
diethylnitrosamine-induced liver tumors in the rat.
Arzneimitteljbrsch., 23 (7): 956-961.
POUND, A, W. LAWSON, T. A., & HORN, L. (1973) Increased carcinogenic
action of dimethylnitrosamine after prior administration of
carbon tetrachloride. Br. J. Cancer, 27: 451-459.
PREDA, N., POPA, L., GALEA, V., & SIMU, G. (1976) N-nitroso compound
formation by chiordiazepoxide and nitrite interaction in vitro
and in vivo: protective action of ascorbic acid. IARC Sci.
Publ. No. 14, pp. 301-304.
PREUSSMAN, R. (1975) [Carcinogenic chemicals in the human
environment.] Handbuch der Allg. Pathol., VI/6/11. 448-482 (in
German).
PURCHASE, I. F. H., TUSTIN, R. C., & van RENSBERG, S. J. (1975)
Biological testing of food grown in the Transkei. Food Cosmet.
Toxicol, 13: 639-647.
QUISPEL, A., ed. (1974) -- The biology of nitrogen fixation.
Amsterdam, North Holland Publishing Company,
RABES, H., HARTENSTEIN, R., & SCHOLZE, P. (1970) Specific stages of
cellular response to homeostatic control during
diethylnitrosamine-induced liver carcinogenesis. Experientia
(Basel), 26: 1356-1359.
RABES, H., HARTENSTEZN, R., & GMINDER, J. (1971) Kidney neoplasms
induced by diethylnitrosamine in partially hepatectomized rats.
Naturwiss., 58: 102-103.
RAMMELL, C. G. & JOERIN, M. N. (1972) Determination of nitrite in
cheese and other dairy products. J. Dairy Res., 39: 89-94.
REDDY, B. S. & THOMAS, J. W. (1962) Interrelationship between thyroid
status and nitrate on carotine conversion. J. anim. Sci.,
21: 1010.
REEVES, M. J., BIRTLES, A. B., COURCHE, R. C., & ALDRICK, R. J. (1974)
Groundwater resources of the Vale of York. London, Water
Resources Board Report, pp. 90.
RHOADES, J. W. & JOHNSON, D. E. (1970) Gas-chromatography and
selective detection of N-nitrosamines. J. Chromatogr. Sci.,
8: 616-618.
RICHARDSON, W. D. (1907) Nitrates in vegetable foods in cured meats
and elsewhere. J. Am. Chem. Soc, 29: 1757-1767.
RIDDER, W. E. & OEHME, F. W. (1974) Nitrates as an environmental,
animal, and human hazard. Clin. Toxicol 7 (2): 145-159.
RIEDEL, B. & PIPER, R. (1973) Early changes of the rat bladder mucosa
after experimental cancerogenesis with N-butyl- N-butanol
(4)-nitrosamine.] (author's transl.). Urol. Int, 28: 322-327.
ROBERTS, J. D. & CASERIO, M. C. (1964) Basic Principles of Organic
Chemistry, New York, Benjamin, pp. 656-666.
ROBERTS, W. K. & SELL, J. L. (1963) Vitamin A destruction by nitrite
in vitro and in vivo. J. anim. Sci., 22: 1081-1085.
ROBERTSON, H. E. & DRAYCOTT, M. E. (1948) Nitrate poisoning of
infants by contaminated drinking water. Abstracts of papers
presented at the fifteenth Annual Christmas Meeting of the
Laboratory Section, Canadian Public Health Association, London,
Ontario, 13-14 December, p. 30.
ROBERTSON, H. E. & RIDDELL, W. A. (1949) Cyanosis of infants produced
by high nitrate concentration in rural waters of Saskatchewan.
Can. J. Public Health, 40: 72-77.
ROBINSON, E. & ROBBINS, R. C. (1972) Emissions, concentrations and
fate of gaseous atmospheric pollutants. In: Strauss, W., ed. Air
pollution control Part II, New York, Wiley-Interscience,
pp. 1-93,
ROLLER, P. P. & KEEFER, L. K. (1974) Catalysis of nitrosation
reactions by electrophilic species. IARC Sci. Publ. No. 9,
pp. 86-89.
ROSS, J. D. (1963) Deficient activity of DPNH-dependent methemoglobin
diaphorase in cord blood erythrocytes. Blood, 21 (1): 51-62.
ROSS, J. D. & DESFORGES, J. F. (1959) Reduction of methemoglobin by
erythrocytes from cord blood. Pediatrics, 23: 718-726.
SAKSHAUG, J., SÖGNEN, E., HANSEN, M. A., & KOPPANG, N. (1965)
Dimethylnitrosamine; its hepatotoxic effect in sheep and its
occurrence in toxic batches of herring meal. Nature (Lond.),
206: 1261-1262.
SANDER, J. (1971a) [Investigations on the formation of carcinogenic
nitroso compounds in the stomach of experimental animals and its
significance for man. I] Arzneimittel-Forsch., 21: 1572-1580
(in German).
SANDER, J. (1971b) [Investigations on the formation of carcinogenic
nitroso compounds in the stomach of experimental animals and its
significance for man.] Arzneimittel-Forsch., 21: 1703-1707 (in
German).
SANDER, J. (1971c)[Further experiments on tumour induction by oral
administration of small doses of N-methylbenzylamine and sodium
nitrite.] Z. Krebsforsch., 76: 93-96 (in German).
SANDER, J. & BÜRKLE, G. (1969) [Induction of malignant tumors in rats
by simultaneous feeding of nitrite and secondary amines.]
Z. Krebsforsch., 73: 54-66 (in German).
SANDER, J. & SEIF, F. (1969) [Bacterial reduction of nitrate in the
stomach of man as a cause of nitrosamine formation.]
Arzneimittel-Forsch, 19: 1091-1093 (in German).
SANDER, J. & SCHWEINSBERG, F. (1972) In vivo and in vitro
experiments on the formation of N- nitroso compounds from
amines or amides and nitrate or nitrite. IARC Sci. Publ. No. 3,
pp. 97-103.
SANDER, J. & SCHWEZNSBERG, F. (1973) [Tumor induction in mice with
methylbenzylnitrosamine in low doses.] Z. Krebsforsch,
79: 157-161 (in German).
SANDER, J., SCHWEINSBERG, F., & MENZ, H. P. (1968) [Investigations on
the formation of carcinogenic nitrosamine in the stomach.]
Hoppe-Seyler's Z. physiol. Chem, 349: 1691-1697 (in German).
SANDER, J., BURKLE, G., FLOHE, L., & AEIKENS, B. (1971) [ In vitro
investigations on the possibility of the formation of
carcinogenic nitrosamide in the stomach.] Arzneim-Forsch.,
(Drug Res.), 21 (3): 411-414 (in German).
SANDER, J., LABAR, J., LADENSTEIN, M., & SCHWEINSBERG, F. (1974a)
Quantitative measurement of in vivo nitrosamine formation.
IARC Sci. Publ. No. 9, pp. 123-131.
SANDER, J., LABAR, J., LADENSTEIN, M., & SCHWEINSBERG, F. (1974b)
Experiments on the degradation of nitrosamines in plants. IARC
Sci. Publ. No. 9, pp. 205-210.
SATTELMACHER, P. G. (1962) [Methemoglobinemia from nitrates in
drinking water.] Schriflenr. Ver. Wasser Boden. Lufthyg., No. 21
(in German).
SAWYER, C. N. (1947) Fertilization of lakes by agricultural and urban
drainage. J. New England Water Works Assoc., 109-127.
SCANLAN, R. A. (1975) Nitrosamines in food. CRC Critical Reviews in
Food Technology, April 1975, pp. 357-402.
SCHERER, E., HOFFMAN, M., EMMELOT, P., & FRIEDRICH-FRESKA, M. (1972)
Quantitative study on foci of altered liver cells induced in the
rat by a single dose of diethylnitrosamine and partial
hepatectomy. J. Natl Cancer Inst., 49: 93-106.
SCHEUNIG, O. & ZIEBARTH, D. (1976) Formation of nitrosamines by
interaction of some drugs with nitrite in human gastric juice.
IARC Sci. Publ. No. 14, pp. 269-277.
SCHMÄHL, D. (1963) [The origin, growth and chemotherapy of malignant
tumours.] Arzneimittei-Forsch., 13 (Supplement): 612 (in
German).
SCHMÄHL, D. (1970) [Experimental studies on syncarcinogenesis VI: The
addition of minimal doses of four different hepatotropic
carcinogens in liver cancer induction in the rat.]
Z. Krebsforsch., 74: 457-466 (in German).
SCHMÄHL, D. & STACKELBERG, S. YON (1968)[The influence of lactoflavin,
nicotinamide or dipyridamole on the carcinogenic activity of
diethylnitrosamine in rats.] Arzneimittel-Forsch., 18: 318-320
(in German).
SCHMÄHL, D. & THOMAS, C. (1962) [Acute nitrosomethylurethane poisoning
in rats following oral application.] Arzneimittel-Forsch.,
12: 585-587 (in German).
SCHMÄHL, D., THOMAS, C., & KÖNIG, K. (1963) [Studies of
syncarcinogenesis: I. Experiments on the induction of cancer in
rats by the simultaneous administration of diethyinitrosamine and
4-dimethylamino-azobenzene.] Z. Krebsforsch., 65: 351-377
(in German).
SCHMÄHL, D., THOMAS, C., SATTLER, W., & SCHIELD, G. F. (1965)
[Experimental studies of syncarcinogenesis: III. Attempts to
induce cancer in rats by administering diethylnitrosamine and
CC14 (or ethyl alcohol) simultaneously. In addition, an
experimental contribution regarding "alcoholic cirrhosis".]
Z. Krebsforsch., 66: 526-532 (in German).
SCHMÄHL, D., STUTZ, E., & THOMAS, C. (1966) [Experimental studies on
syncarcinogenesis. V. Experiments on cancer generation in rats
with the simultaneous application of X-ray radiation and
diethylnitrosamine or 4-dimethyl-aminobiphenyl.]
Z. Krebsforsch., 68: 68-72 (in German).
SCHMÄHL, D., WAGNER, R., & SCHERF, H. R. (1971) [Influence of
immunosuppressive drugs on cancer formation in rat liver by
diethyinitrosamine and on the induction of fibrosarcoma by
3,4-benzopyrene.] Arzneimittel-Forsch, 21 (3): 403-404
(in German).
SCHMIDT, P. & KNOTEK, Z. (1970) Epidemiological evaluation of
nitrates as ground water contaminants in Czechoslovakia (Paper
presented to the Sixth International Water Pollution Research
Conference, San Francisco).
SCHMID-RUPPIN, K. H. & PAPADOUPOULU, G. (1972) [Effect of
diethylnitrosamine (DENA) and influenza viruses on the induction
of lung carcinomas in mice.] Z. Krebsforsch., 77: 150-154
(in German).
SCHMITZ-MOORMAN, P., GEDIGK, P., & DHARAMADHACH, A. (1972) [Early
histological and histochemical changes during experimental
production of liver carcinoma by diethylnitrosamine.]
Z. Krebsforsch, 77: 9-16 (in German).
SCHNEIDER, N. R. & YEARY, R. A. (1973) Measurement of nitrite and
nitrate in blood. Am. J. vet. Res., 34 (1): 133-136.
SCHOENTAL, R. (1960) Carcinogenic action of diagomethane and of
nitroso- N-methylurethane. Nature (Lond.), 188: 420-421.
SCHREIBER, H., NETTESHEIM, P., LIJINSKY, W., RICHTER, C. B., &
WALBURO, H. E. (1972) Induction of lung cancer in germfree,
specific-pathogen-free and infected rats by
N-nitrosoheptamethyleneimine. Enhancement by respiratory
infection. J. Natl Cancer Inst., 49: 1107-1114.
SCHUPHAN, W. (1965) [The nitrate content of spinach (Spinacia
oleracea, L) in relation to methaemoglobinaemia in children.]
Z. Ernähr. Wiss., 5 (3-4): 207-209 (in German).
SCHUPHAN, W. (1969) [The formation of nitrate and nitrite in plant
metabolism.] Nutritio Dieta, 11: 120-132 (in German).
SCHWEINSBERG, F. & SANDER, J. (1972) [Carcinogenic nitrosamine from
simple aliphatic tertiary amines and nitrite.] Hoppe-Seyler's
Z. physiol. Chem., 353: 1671-1676 (in German).
SEBRANEK, J. G., CASSENS, R. G., & HOEKSTRA, W. G. (1974) Fate of
added nitrite. In: Proceedings of the International Symposium
on Nitrite in Meat Products, Ziest, 10-14 September, 1973,
Wageningen, Centre for Agricultural Publishing and
Documentation, pp. 139-147.
SEHGAL, O. P. & KRAUSE, G. F. (1968) Efficiency of nitrous acid as an
inactivating and mutagenic agent of intact tobacco mosaic virus
and its isolated nucleic acid. J. Virol., 2 (10): 966-971.
SELENKA, F. (1970) Formation and reduction of nitrite in infant
nutrients containing nitrate. Archiv. Hyg., 154 (4): 336-345.
SELL, J. L. & ROBERTS, W. K. (1963) Effects of dietary nitrite on the
chick: Growth, liver vitamin A stores and thyroid weight. J.
Nutr, 79: 171-178.
SEN, N. P. (1970) Gas-liquid chromatographic determination of
dimethylnitrosamine as dimethylnitramine at picogram levels.
J. Chromatogr, 51: 301-304.
SEN, N. P. (1972) The evidence for the presence of dimethylnitrosamine
in meat products. Food Cosmet. Toxicol., 10: 219-223.
SEN, N. P. (1974) Nitrosamines in toxic constituents in animal
foodstuffs. New York, Academic Press Inc., pp. 131.
SEN, N. P. & DALPE, C. (1972) A simple thin-layer chromatographic
technique for the semi-quantitative determination of volatile
nitrosamines in alcoholic beverages. Analyst,
97: 216-220.
SEN, N. P. & DONALDSON, B. (1974) The effect of ascorbic acid and
glutathiane on the formation of nitrosopiperazines from
piperazine adipate and nitrite. IARC Sci. Publ.
No. 9, pp. 103-106.
SEN, N. P., SMITH, D.C., SCHWINGHAMER, L., & MARLEAU, J. J. (1969a)
Diethylnitrosamine and other N-nitrosamines in foods. J.
Assoc. Off. Anal. Chem., 52 (1): 47-52.
SEN, N. P., SMITH, D.C., & SCHWINGHAMER, L. (1969b) Formation of
N-nitrosamines from secondary amines and nitrite in human and
animal gastric juice. Food Cosmet. Toxicol., 7: 301-307.
SEN, N. P., SMITH, D.C., SCHWINGHAMER, L., & HOWSAM, B. (1970)
Formation of nitrosamines in nitrite-treated fish. Can. Inst.
Food Technol. J., 3 (2): 66-69.
SEN, N. P., SCHWINGHAMER, L. A., DONALDSON, B. A., & MILES, W. F.
(1972) N-nitrosodimethylamine in fish meal. J. agric. Food
Chem., 20 (6): 1280-1281.
SEN, N. P., DONALDSON, B., IYENGAR, J. R., & PANALAKS, T. (1973a)
Nitrosopyrrolidine and dimethylnitrosamine in bacon. Nature
(Lond.), 241: 473-474.
SEN, N. P., MILES, W. F., DONALDSON, B., PANALAKS, T., & IYENGAR, J.
R. (1973b) Formation of nitrosamines in a meat curing mixture.
Nature (Lond.), 245: 104-105.
SEN, N. P., IYENGAR, J. R., DONALDSON, B. A., & PANALAKS, T. (1974a)
Effect of sodium nitrite concentration on the formation of
nitrosopyrrolidine and dimethylnitrosamine in fried bacon. J.
agric. Food Chem, 22 (3): 540-541.
SEN, N. P., DONALDSON, B., CHARBONNEAU, C., & MILES, W. F. (1974b)
Effect of additives on the formation of nitrosamines in meat
curing mixtures containing spices and nitrite. J. agric. Food
Chem., 22 (6): 1125-1130.
SEN, N. P., IYENGAR, J. R., MILES, W. F., & PANALAKS, T. (1976)
Nitrosamines in cured meat products. IARC Sci. Publ.
No. 14, pp. 333-342.
SERFONTEIN, W. J. & HURTER, P. (1966) Nitrosamines as environmental
carcinogens. II. Evidence for the presence of nitrosamines in
tobacco smoke condensate. Cancer Res., 26: 575-579.
DE SERRES, F. J., BROCKMAN, H. E., BARNETT, W. E., & KOLMARK, H. G.
(1967) Allelic complementation among nitrous acid-induced ad-3B
mutants of Neurospora crassa. Mutat. Res., 4: 415-424.
SHANK, R. C. (1975) Toxicology of N-nitroso compounds. Toxicol.
appl. Pharmacol., 31: 361-368.
SHANK, R. C. & NEWnERNE, P.M. (1972) Nitrite-morpholine induced
hepatomas. Food Cosmet. Toxicol., 10: 887-888.
SHEARER, L. A., GOLDSMITH, J. R., YOUNG, C., KEARNS, O. A., & TAMPLIN,
B. R. (1972) Methaemoglobin levels in infants in an area with
high nitrate water supply. Am. J. Public Health, 62
(9): 1174-1180.
SCHECHTER, H., GARDNER, N., & SHUVAL, H. I. (1972) A micromethod for
the determination of nitrite in blood. Anal. Chem. Acta.,
60: 93-99.
SHUVAL, H. I. & GRUENER, N. (1972) Epidemiological and toxicological
aspects of nitrates and nitrites in the environment. Am. J.
Public Health, 62: 1045-1052.
SIBAY, T. M. & HAYES, J. A. (1969) Potential carcinogenic effect of
streptozotocin. Lancet, II: 912.
SIBERT, D. & EISENBRAND, G. (1974) Induction of mitotic gene
conversion in Saccharomyces cerevisae by N-nitrosated
pesticides. Mutat. Res., 22: 121-126.
SIDDIQI, O. H. (1962) Mutagenic action of nitrous acid on
Asperigillus nidulans. Genet. Res., 3 (2): 303-314.
SIMON, C. (1966) Nitrite poisoning from spinach. Lancet, I
(7442): 872.
SIMON, C., MANZKE, H., KAY, H., & MROWETZ, G. (1964) On the
occurrence, pathogenesis, and possibilities for prophylaxis of
the methemoglobinemia caused by nitrite. Z. Kinderheilkunde,
91: 124-138.
SIMONEIT, B. R. & BURLINGAME, A. L. (1971) Organic analyses of
selected areas of Surveyor III recovered on the Apollo 12
mission. Nature (Lond.), 234: 210-211.
SINGLEY, T. L. (1962) Secondary methemoglobineamia due to the
adulteration of fish with sodium nitrite. Ann. internal Med.,
57 (5): 800-803.
SINHA, D. P. & SLEIGHT, S. D. (1971) Pathogenesis of abortion in acute
nitrite toxicosis in guinea pigs. Toxicol. appl. Pharmacol.,
18 (2): 340-347.
SINIOS VON, A. & WODSAK, W. (1965) [Spinach toxicity in children.]
Dtsch Med. Wochenschr, 90: 1856-1863 (in German).
SKRIVAN, J. (1971) Methemoglobinemia in pregnancy. Acta
Universitatis Carolinae Medica, 17: 123-160.
SLEIGHT, S. D. & ATALLAH, O. A. (1968) Reproduction in the guinea pig
as affected by chronic administration of potassium nitrate and
potassium nitrite. Toxicol. appl. Pharmacol., 12: 179-185.
SMITH, C. H. (1972) Polycythemia, methemogiobinemia,
sulfhemoglobinemia, and miscellaneous anemias In: Blood
diseases of infancy and childhood, 3rd ed., Saint Louis, The C.
V. Mosby Company, pp. 450-454.
SMITH, G. E. (1964) Nitrate problems in plants and water supplies in
Missouri. In: 2nd Annual Symposium on Relation of Geology and
Trace Elements to Nutrition.
SMITH, G. E. (1966) Causes of nitrate accumulation in plants and water
supplies. In: 18th Annual Midwest Fertilizer Conference,
Chicago, Illinois.
SMITH, J. E. & BEUTLER, E. (1966) Methemoglobin formation and
reduction in man and various animals species. Am. J. Physiol.,
210: 347-350.
SMITH, P. A. S. & LOEPPKY, R. N. (1967) Nitrosative cleavage of
tertiary amines. J. Am. Chem. Soc., 89 (5): 1147-1157.
SMITH, R. P. (1969) The significance of methaemoglobinaemia in
toxicology. In: Blood, F. R., ed. Essays in toxicology Volume
I, New York and London, Academic Press, pp. 84-113.
SMITH, R. P. (1975) Toxicology of the formed elements of the blood.
In: Casseret, L. J. & Doull, J., ed. Toxicology the basic
science of poisons. New York, Macmillan Publishing Co., Inc.,
pp. 225-243.
SPENCER, R. (1967) A study of factors affecting the quality and
shelf life of vacuum packed bacon, and of the behaviour of
Wiltshire cooked bacon packed and stored under controlled
conditions. (BFMIRA Res. Rep. No. 136).
SPIEGELHALDER, B., EISENBRAND, G., & PREUSSMAN, R. (1976) The
influence of dietary intake of nitrate on the nitrite content in
human saliva: a factor of possible relevance for in vivo
formation of N-nitroso compounds. Food Cosmet. Toxicol.,
14 (6): 545-548.
STANDFORD, G., ENGLAND, C. B., & TAYLOR, A. W. (1969) Fertilizer use
and Water quality, US Department of Agriculture, ARS,
pp. 41-168.
STEINBERG, R. A. & THOM, C. (1940) Mutations and reversions in
reproductivity of Aspergilli with nitrite, colchicine and
d-lysine. Proc. Natl Acad. Sci., 26 (6): 363-366.
STENBACK, F. G., FERRERO, A., & SHUBIK, P. (1973) Synergistic effects
of diethylnitrosamine and different dusts on respiratory
carcinogenesis in hamsters. Cancer Res., 33: 2209-2214.
STEPHANY, R. W., FREUDENTHAL, J., & SCHULLER, P. L. (1976)
Quantitative and qualitative determination of some volatile
nitrosamines in various meat products, IARC Sci. Publ.
No. 14, pp. 343-354.
STEWART, B. W., SWANN, P. F., HOLSMAN, J. W., & MAGEE, P. N. (1974)
Cellular injury and carcinogenesis. Evidence for the alkylation
of rat liver nucleic acids in vivo by N-nitrosomorpholine. Z.
Krebsforsch., 82 (1): 1-12.
STOEWSAND, G. S. (1973) Nitrite-induced methemoglobinemia in guinea
pigs: Influence of diets containing beets with varying amounts of
nitrate, and the effect of ascorbic acid, and methionine. J.
Nutr., 103 (3): 419-424.
STONE, I. M., LASCHIVER, C., & SALETAN, L. T. (1968) Nitrates in
worts, beers and brewing. Waller Stein Lab. Communications, 31
(106): 193-201.
STRACK, H. B., FREESE, E. B., & FREESE, E. (1964) Comparison of
mutation and inactivation rates induced in bacteriophage and
transforming DNA by various mutagens. Mutat. Res.,
1: 10-21.
STRACKS W. & FERON, V. J. (1973) Ultrastructure of pulmonary adenomas
induced by intratracheal instillation of diethylnitrosamine in
Syrian golden hamsters. Eur. J. Cancer, 9: 359-362.
SVOBODA, D. & HIGGINSON, J. (1968) A comparison of ultrastructural
changes in rat liver due to chemical carcinogens. Cancer Res.,
28: 1703-1733.
SWANN, P. F. & KAUFMAN, D. G. (1973) The dose response for the
induction of kidney tumors in the rat by a single dose of
dimethylnitrosamine. Br. J. Cancer, 28: 83-84.
SWANN, P. F. & MAGEE, P. W. (1971) Nitrosamine-induced carcinogenesis:
The alkylation of N-7 of guanine of nucleic acids of the rat by
diethylnitrosamine, N-ethyl- N-nitrosourea and
ethylmethanesulphonate. Biochem. J., 125: 841-847.
SWENBERG, J. A., KOESTNER, A., WESCHLER, W., & DENLINGER, R. H. (1972)
Quantitative aspects of transplacentai tumor induction with
ethylnitrosourea in rats. Cancer Res., 32: 2656-2660.
SYLVESTER, R. O. (1961) Nutrient content of drainage water forested,
urban and agricultural areas. In: Algae and Metropolitan
Wastes. Transactions of the 1960 Seminar, Robert A. Taft Sanitary
Engineering Center, pp. 80-87, (Technical Report W61-3).
TAKAYAMA, S. (1968) The histological and autoradiographical studies of
mouse liver during the course of carcinogenesis by
dimethylnitrosamine. Z. Krebsforsch., 71: 246-254.
TAKAYAMA, S. & IMAIZUMI, T. (1969) Sequential effects of chemically
different carcinogens, dimethylnitrosamine and 4-dimethylaminoazo-
benzene, on hepatocarcinogenesis in rats. Int. J. Cancer,
4: 373-383.
TAKAYAMA, S. & MURAMATSU, M. (1969) Incorporation of tritiated
dimethylnitrosamine into subcellular fractions of mouse liver
after long-term administration of dimethylnitrosamine. Z.
Krebsforsch, 73: 172-179.
TANNENBAUM, S. R., SINSKEY, A. J., WEISMAN, M., & BISHOP, W. (1974)
Nitrite in human saliva. Its possible relationship to nitrosamine
formation. J. Natl Cancer Inst., 53 (1): 79-84.
TANNENBAUM, S. R., WEISMAN, M., & FELT, D. (1976) The effect of
nitrate intake on nitrite formation in human saliva. Food
Cosmet. Toxicol., 14 (6): 549-552.
TAYLOR, H. W. & LIJINSKY, W. (1975) Tumour induction in rats by
feeding heptamethyleneimine and nitrite in water. Cancer Res.,
35: 812-815.
TELLING G. M. (1972) A gas-liquid chromatographic procedure for the
detection of volatile N-nitrosamines at the 10 parts per
billion level in foodstuffs after conversion to their
corresponding nitramines. J. Chromatogr., 73: 79-87.
TELLING, G. M., BRYCE, T. A., & ALTHORPE, J. (1971) Use of vacuum
distillation and gas chromatography mass spectrometry for
determination of low levels of volatile nitrosamines in meat
products. J. agric. Food Chem., 19(5): 937-940.
TELLING G. M., BRICE, T. A., HOAR, D., OSBORNE, D., & WELTI, D. (1974)
Progress in the analysis of volatile N-nitroso compounds.
IARC Sci. Publ. No. 9, pp. 12-17.
TERRACINI, B., MAGEE, P. N., & BARNES, J. M. (1967) Hepatic pathology
in rats on low dietary levels of dimethylnitrosamine. Br. J.
Cancer, 21: 559-565.
THOMAS, J. F. J. (1948) Water Survey Report No. 2, Ottawa river
drainage basin. Canada Department of Mines and Technical
Surveys, pp. 11-23 (Publ. No. 834).
TOMATIS, L. & CEFIS, F. (1967) The effects of multiple and single
administration of dimethylnitrosamine to hamsters. Tumori,
53: 447-451.
TOMATIS, L. (1977) Prenatal exposure to chemical carcinogens and its
effect on subsequent generations. In: Conference on Perinatal
Carcinogenesis. Washington, DC, National Cancer Institute
Monograph.
TOMLINSON, T. E. (1970) Trends in nitrate concentrations in English
rivers in relation to fertilizer. Water Treat. Exam.,
pp. 277-293.
TOUSSAINT, W. & SELENKA, F. (1970) Methemoglobin formation in young
infants. A contribution to drinking water hygiene on Rheinhessen.
Mschr. Kinderheilk., 118(6): 282-284.
UNGERER, O., EISENBRAND, G., & PREUSSMANN, R. (1974) The reaction of
nitrite with pesticides. Formation, chemical properties and
carcinogenic activity of the N-nitroso compounds of the
herbicide N-methyl-N1-(2-benzothiazolyl)-urea. Z.
Krebsforsch., 81 (3-4): 217-242.
UNITED NATIONS (1976) Statistical Yearbook, 1975, New York, p. 298.
US DEPARTMENT OF AGRICULTURE (1965) Agricultural Information
Bulletin, No. 299, Washington, DC.
US DEPARTMENT OF HEALTH, EDUCATION AND WELFARE (1966) Air Quality
Data from the National Air Sampling Networks and Contributing
State and Local Networks, 1964-1965, Cincinnati, OH, USDHEW
Public Health Service Division of Air Pollution, pp. 125.
US DEPARTMENT OF HEALTH, EDUCATION AND WELFARE (1970) Nitrates,
nitrites and methaemoglobinaemia, Research Triangle Park, N.C.,
National Institute of Environmental Health Sciences, pp. 43
(Environ. Rev. No. 2).
VAN LOGTEN, M. J., DEN TONKELAAR, E. M., KROES, R. BERKVENS, J. M., &
VAN ESCH, G. J. (1972) Long-term experiment with canned meat
treated with sodium nitrate and glucono-delta-lactone in rats.
Food Cosmet. Toxicol., 10: 475-488.
VAN RENSBURG, S. J. (1972) Failure of low protein and zinc intakes to
influence nitrosamine-induced oesophageal carcinogenesis. S.
Afr. reed. J, 46: 1137-1138.
VAVRA, J. J., DE BOER, C., DIETZ, A., HANKA, L. J., & SOKOLSKI, W. T.
(1960) Antibiotics Annual 1959-1960, NY, Antiobiotica Inc,
pp. 230.
VERLY, W. G., BARBASON, H., DUSART, J, & PETITPAS-DEWANDRE, A. (1967)
A comparative study of the action of ethyl methane sulphonate and
HNO2 on the mutation to streptomycin resistance of Escherichia
coil K 12. Biochim. Biophys. Acta, 145: 752-762.
VESSELINOVITCH, S. D. (1969) The sex-dependent difference in the
development of liver tumors in mice administered
dimethylnitrosamine. Cancer Res., 29: 1024-1027.
VIETS, F. B. & ALDRICH, S. R. (1973) Nitrogenous materials in the
environment, Draft Report, US Environmental Protection Agency,
Hazardous Materials Advisory Committee.
VIGIL, J., WARBURTON, S., HAYNES, W. A., & KAISER, L. R. (1965)
Nitrates in municipal water supply cause methemoglobinemia in
infant. Public Health Rep., 80 (12): 1119-1121.
VON KREYBIG, T. (1965a) [The effect of teratogenic compounds on the
early stages of prenatal development in the rat.] Naunyn-
Schmiederbergs Arch: exp. Path. Pharmacol., 252: 196-204
(in German).
VON KREYBIG, T. (1965b) [The effect of carcinogen methylnitrosourea
doses on the embryonal development in the rat.] Z. Krebsforsch.,
67: 46-50 (in German).
VON KREYBIG, T. (1968) [Experimental prenatal toxicology.]
Arzneimittel-Forsch., Suppl. 17, pp. 1-211 (in German).
VON KREYBIG, T. & SCHMIDT, W. (1966) [On chemical teratogenesis in the
rat.] ArzneimitteI-Forsch., 8: 989-1001 (in German).
VON KREYBIG, T. & SCHMIDT, W. (1967) [Chemically induced fetopathies
in the rat.] Arzneimittel-Forsch., 9: 1093-1100 (in German)
VOOGT, P. (1906) The determination of nitrate with a nitrate selective
electrode. Dtsch Lebensm. Rundschau., 65: 196-198.
WALKER, E. A., PIGNATELLI, B., & CASTEGNARO, M. (1975) Effects of
gallic acid on nitrosamine formation. Nature (Lond.),
258: 176.
WALKER, E. A., BOGOVSKI, P., & GRICUITE, L., ed. (1976) Environmental
N-nitroso compounds analysis and formation. Proceedings of a
Working Conference, the Polytechnical Institute, Tallinin,
Estonian SSR, 1-3 October, 1975, Lyons, International Agency for
Research on Cancer, pp. 512.
WALTERS, C. L. (1971) The detection and estimation of trace amounts of
N-nitrosamines in a food matrix. Lab. Pract., 20: 574-578.
WALTON, G. (1951) Survey of literature relating to infant
methemoglobinemia due to nitrate-contaminated water. Am. J.
Public Health, 41: 986-996.
WASSERMAN, A. E., FIDDLER, W., DOERR, R. C., OSMAN, S. F., & DOOLEY,
C. J. (1972) Dimethylnitrosamine in frankfurters. Food Cosmet.
Toxicol., 10: 681-684.
WATER RESEARCH CENTRE (1974) Notes on water pollution, No. 66,
pp. 4.
WATROUS, R. M. (1947) Health hazards of the pharmaceutical industry.
Br. J. ind. Med., 4: 111-125.
WEAST, R. C. ed. (1976) Handbook of chemistry and physics, 57th
edition, Cleveland, OH, CRC Press, pp. C-81-C-685.
WESCHLER, W. (1970) Oncogenic, teratogenic and mutagenic effects of
methyl- and ethylnitrosourea. In: 6th International Congress of
Neuropathology, Paris. Masson, pp. 128-129.
WESCHLER, W. (1971) Teratogenic effects of the neurotropic resorptive
carcinogens methyl-and ethylnitrosourea in rats. J. Neuropath.
exp. Neurol., 30: 120-121.
WESCHLER, W. (1973) Carcinogenic and teratogenic effects of
ethylnitrosourea and methylnitrosourea during pregnancy in the
rat. IARC Sci. Publ. No. 4, pp. 127-142.
WEGNER, T. N. (1972) Simple and sensitive procedure for determining
nitrate and nitrite in mixtures in biological fluids. J. Dairy
Sci., 55 (5): 642-644.
WEIL, L. & QUENTIN, L.-E. (1973) Nitrogen compounds in water and
nitrate determination with ion-sensitive electrode. Vom Wasser,
40: 125-133.
WEISBERGER, J. H. & RAINERI, R. (1975) Dietary factors and the
etiology of gastric cancer. Cancer Res., 35: 3469-3474.
WEISBERGER, E. K., WARD, J. M., & BROWN, C. (1974) Dibenamine:
Selective protection against diethylnitrosamine-induced hepatic
carcinogenesis but not oral, pharyngeal and esophageal
carcinogenesis. Toxicol. appl. Pharmacol., 28: 477-484.
WELLS, B. E., WALKER, R., & WALTERS, C. L. (1974) Interactions between
sodium nitrite and foodstuffs under gastric conditions. J. Sci.
Food Agric., 25: 1048.
WHO (1971) International standards for drinking water. Third ed,
Geneva, World Health Organization, pp. 70.
WHO (1977) Environmental health criteria 4--Oxides of nitrogen,
Geneva, World Health Organization, pp. 81.
WILLIAMS, A. O. (1970) Ultrastructure of liver cell carcinoma in
Macaca mulata monkey. Exp. mol. Pathol., 13: 359-369.
WINTON, E. F., TARBIFF, R. G., & MCCABE, L. J. (1971) Nitrate in
drinking water. J. Am. Water Works Assoc., 63: 95-98.
WORKSHOP ON GLOBAL ECOLOGY (1971) Man in the living environment, The
Institute of Ecology Report of the workshop on global ecological
problems, Chicago, IL, Field Museum of Natural History, pp. 267.
WRIGHT, A. J. & DAVIDSON, K. L. (1964) Nitrate accumulation in crops
and nitrate poisoning in animals. Advan. Agron., 16: 197-247.
WRIGLEY, F. (1948) Toxic effects of nitroso-methyl urethane.
Br. J. ind. Med., 5: 26-27.
YANG, K. W. & BROWN, E. V. (1972) A sensitive analysis for
nitrosamines. Anal. Lett., 5 (5): 293-304.
ZALDIVAR & WETTERSTRAND (1975) Further evidence of a positive
correlation between exposure to nitrate fertilizers (NaNO3 and
KNO3) and gastric cancer death rates: Nitrites and
nitrosamines. Experientia (Basel), 31: 1354-1355.
ZIEBARTH, D. & SCHEUNIG, G. (1976) Effects of some inhibitors on the
nitrosation of drugs in human gastric juice. IARC Sci. Publ. No.
14, pp. 279-290.
ZIMMERMAN, F. K. & SCHWAIER, R. (1967) Induction of a mitotic gene
conversion with nitrous acid, 1-methyl-3-nitro-1-nitrosoguanidine
and other alkylating agents in Saccharomyces cerevisiae. Mol.
gen. Genet., 100: 63-76.
ZIMMERMAN, F. K., SCHWAIER, R., & LAER, U. V. (1966) Mitotic
recombination induced in Saccharomyces cerevisiae with nitrous
acid, diethylsulphate and carcinogenic, alkylating nitrosamides.
Z. Vererbungsl., 98: 230-246.
artificial fertilizers are ultimately used for the synthesis of
biological molecules, particularly proteins. Plants and animal waste
and dead tissues return fixed nitrogen to the soils, where part of it
is recycled and part returned to the atmosphere, thus completing the
nitrogen cycle. According to Delwiche (1970), nitrogen fixation on a
world basis may exceed denitrification by about 10%. The increased use
of industrial fertilizers has resulted in some areas in increased
concentrations of nitrates in bodies of water, resulting, in some
cases, in eutrophication.
4.2 Transformation in Foods
4.2.1 Reduction of nitrates to nitrites
Because of the ability of spinach to accumulate large quantities
of nitrates and reported cases of intoxication associated with the
consumption of this vegetable, several studies have been undertaken on
the conversion of nitrates to nitrites in spinach.
Data presented by Phillips (1968a) indicated that initial
nitrite-N contents of fresh, frozen, canned, and baby-food spinach
were generally less than 1 mg/kg fresh weight. However, several
authors have reported a rapid fall in nitrate levels and increase in
nitrite levels in fresh spinach during the first 4 days of storage at
room temperature (Achtzehn & Hawat, 1970; Phillips, 1968a; Schuphan,
1965). Higher nitrite levels occurred in spinach from fertilized
ground (Brown & Smith, 1967) and these could reach exceptionally high
values (3600 mg/kg dry weight) with excessive fertilization (Schuphan,
1965).
Under refrigeration, the nitrite-N contents of fresh spinach
increased very gradually throughout a storage period of 28 days
(Phillips, 1968a). Significant increases in nitrite-N levels did not
occur during the storage of frozen, canned, or baby-food spinach, but
increased concentrations were found in frozen spinach, that had been
left to thaw at room temperature for an excessively long period (39 h)
(Phillips, 1968a).
There was also a slight rise in nitrite levels when partially
consumed jars of commercial baby foods containing nitrates were stored
for 7 days at room temperature instead of under refrigeration
(Phillips, 1969). Selenka (1970) noted that nitrite formation in baby
foods was rapid in the presence of Escherichia coli and Pseudomonas
fluorescens, less rapid with Bacillus subtilis, and very slow with
Staphylococcus albus.
When foods consisted of a solid immersed in a liquid (e.g. canned
foods or frozen foods after thawing) nitrates were partially
transferred to the liquid portion or into the water in which the food
was cooked (Bodiphala & Ormrod, 1971). When large volumes of water and
long cooking times were employed (Kilgore et al., 1963), and when
canned vegetables were blanched in hot water instead of steam
(Johnson, 1966), significant amounts of nitrates were leached out of
the foods. Nitrate reductase (NADPH) (1.6.6.3) activity was rapidly
destroyed during cooking, thereby greatly diminishing further
conversion of nitrates to nitrites (Bodiphala & Ormrod, 1971). It is
also well known that the sterilization treatments necessary for
canning destroy microorganisms that could convert nitrates to
nitrites.
Conversion of nitrates to nitrites occurred more slowly in
vacuum-packed bacon than in unpackaged bacon, presumably due to the
low reducing ability of anaerobes (Cavett, 1962). Spencer (1967) found
that the nitrite content of vacuum-packed bacon decreased slowly on
storage. It has also been reported by Sebranek et al. (1974) that
nitrite levels in meat, determined 2 days after processing, were less
than half those originally added to frozen samples and samples
processed at 71°C, and that they decreased further during storage.
Frying, grilling, or boiling bacon or ham reduced the nitrites content
by 20-90% (Food Standards Committee, 1959).
When direct gas firing of spray dryers was employed, the nitrate-
N contents of dried milk products increased by 1-3 mg/kg compared with
those obtained using indirectly heated sprayers but nitrite-N levels
were unaffected (Manning et al., 1968). Air drying of potato and corn
starch led to the formation of only trace amounts of nitrite
(Gerritsen & De Willingen, 1969).
Nitrates may be reduced to nitrites when cooking is carried out
in aluminium utensils (Osteryoung, unpublished data)a. This
observation appears to be significant since some countries use
aluminium utensils for boiling milk and water, a practice which could
lead to the formation of sizeable quantities of nitrites. This effect
of aluminium should be investigated further.
4.2.2 Formation and degradation of N-nitroso compounds
The conditions under which various amines, amino acids, and
proteins in food could react with nitrite to form nitrosamines were
studied by Ender & Ceh (1971) and by Sen et al. (1970) who showed that
when cod, herring, hake, halibut, mackerel, or salmon were treated
with sodium nitrite at 200 mg/kg and cooked at 110°C for 60-70 min
there were only trace amounts (2.5-25 µg/kg) of DMN in the cooked
product. The highest levels were found in mackerel and hake, both of
which contain large amounts of DMA and TMA. Samples without added
nitrite did not contain any detectable nitrosamines.
a Nitrates as human and animal health hazards, Paper presented at
the Second Conference on Environmental Chemicals, Colorado State
University, 1973.
The formation of DMN was studied in aqueous model systems
containing methyl amines and sodium nitrite under conditions which
were more severe than those employed in the commercial processing of
nitrite-treated smoked chub (a fresh water fish containing small
amounts of TMA, TMAO, and DMA). The results of the studies showed that
not more than 10 µg of DMN per kg of the final product would be formed
during the smoking process (Malins et al., 1970).
Recently, Sen et al. (1973b) suggested that a major source of
nitrosamines in cured meat might arise from an interaction between the
nitrites and spices, such as black pepper and paprika, that are
present in curing mixtures. Nitrosopyrrolidine, nitrosopiperidine, and
DMN were found in a curing mixture used by a meat manufacturer in
Canada. The same authors (Sen et al. 1974a) have also studied the
effect of sodium nitrite concentration on the formation of
nitrosopyrrolidine and DMN in fried bacon. Bacon samples prepared with
sodium nitrite at 0, 50, 100, 150, or 200 mg/kg were analyzed for
nitrosopyrrolidine and DMN. No nitrosamine was detected in samples
prepared without nitrite but all treated samples contained 2-20 µg/kg
of nitrosamines. The level of nitrosopyrrolidine was related to the
initial concentration of nitrite in the bacon. It has also been shown
that nitrosamine formation in bacon increases with increasing
temperature and time of frying and that whereas baking, broiling, or
frying produce variable amounts of nitrosamines none is produced with
cooking in a microwave oven (Pensabene et al., 1974).
After deliberate nitrosation of eggs and meat with unusually
large amounts (1%) of sodium nitrite, some N-nitroso compounds
appeared to have been formed but the chemical nature of the compounds
detected was not clear (Walters, 1971).
No systematic studies on the formation of N-nitroso compounds
in cheese have been performed, although some types of cheese are known
to be processed with nitrate and nitrite.
There are few data on the fate of N-nitroso compounds during
the cooking, processing, or storage of food but some studies have
demonstrated that the volatile nitrosamine DMN and nitrosopyrrolidine
may be lost during the frying of bacon (Sen et al., 1973a).
4.3 Formation of N-nitroso Compounds from Drugs and Pesticides
Reaction with nitrite to form nitrosamines is not restricted to
food components. Lijinsky & Greenblatt (1972) and Lijinsky (1974)
reported that some antibiotics and other drugs that are widely used
can react with nitrites to form nitrosamines in alarmingly high
quantities. The drugs examined included oxytetracycline,
aminophenazone (aminopyrine), disulfiram, N,N-diethyl-3-
pyridinecarboxamide (nikethamide), tolazamide, and (E,E)-1-[5-(1,3-
benzodioxol-5-yl)-1-oxo-2,4-pentadienyl] piperidine (piperine).
Optimum conditions of temperature, pH, and concentration for these
reactions have been reported by Lijinsky et al. (1972a, 1972b, 1972c)
who more recently (Lijinsky, 1974) studied the reactions of
aminopyrine and other commonly used drugs with nitrous acid at rather
low concentrations to assess the magnitude of the hazard to man from
such interactions. The topic has been reviewed by Mirvish (1975) who
has listed 41 drugs and pesticides that have been nitrosated.
Pesticides listed include atrazine, simazine, ziram, and thiram.
4.4 Formation of N-nitroso Compounds in Animal Organisms
4.4.1 Formation of N-nitroso compounds in simulated gastric juice
Formation of DEN was demonstrated when DEA and nitrite were
incubated in the gastric juice of the rat, rabbit, cat, dog, and man.
More DEN was formed in human and rabbit gastric juices (pH 1-2 in both
cases) than in rat gastric juice (pH 4-5). (Sen et al., 1969a, 1969b).
The formation of nitrosamines by the interaction of some drugs
with nitrite in the presence of human gastric juice have been studied
by Scheunig & Ziebarth (1976). At 37°C and a pH = 2, for 1 h,
aminopyrine, sodium [(2,3 dihydro-1,5-dimethyl-3-oxo-2-phenyl-IH-
pyrazol-4-yl)methylamino] methanesulfonate (analgin), and piperazine
gave nitrosamine yields (calculated on the basis of nitrite used) of
69%, 11%, and 74.8% respectively.
In vitro studies have been carried out (Wells et al., 1974) in
which several foods (pork, egg, bread, milk, and cheese) were
incubated under simulated gastric conditions with concentrations of
nitrite similar to those used as food preservatives. The effect of the
thiocyanate ion as a catalyst for nitrosation was also studied since
it is secreted in saliva. Of the foods studied, only cheese produced
detectable amounts of volatile nitrosamines. The identity of the
nitrosamines was not indicated.
4.4.2 Formation of N-nitroso compounds in vivo
When DEA and nitrite were fed to cats and rabbits, considerable
amounts of DEN were detected in the stomach of the experimental
animals (Sen et al, 1969a, 1969b). Similar results were reported by
Sander & Sief (1969). Epstein (1972) reported the formation of
nitrosopiperidine in the gastrointestinal tract of rats treated with
nitrite and piperidine hydrochloride. When the nitrite concentration
was constant, nitrosopiperidine formation in the small intestine
increased with increasing concentrations of piperidine.
Nitrosopiperidine was also found in the stomach. Recently, Sander et
al. (1974a) demonstrated the formation of N-N'-dinitrosopiperazine,
DMN, and N-nitroso- N-methylbenzylamine in the stomach contents of
rats given the parent amines combined with nitrite. Considerable
individual variation in the degree of synthesis of N-N'-
dinitrosopiperazine was noted in the animals. In another recent
report, N-nitrosopyrrolidine was formed very rapidly in the stomach
of dogs from sodium nitrite and pyrrolidine (within 2-6 min) but after
30 min nearly all of it had disappeared, presumably due to its rapid
absorption (Mysliwy et al., 1974).
Indirect evidence of in vivo formation of N-nitroso compounds
has also been provided by some toxicity studies. Thus, hepatic
lesions, formed following administration of nitrite and some amines,
were similar to those produced by DMN or N-nitroso- N-
methylbenzylamine (Asahina et al., 1971). Similar effects were noted
where nitrite was administered up to 3 h after DMA, but the effect was
markedly reduced if the nitrite was given prior to the amine.
4.5 Formation of N-nitroso Compounds by Microorganisms
Studies conducted by Hawksworth & Hill (1971a), Klubes & Jondorf
(1971), and Sander & Sief (1969) suggested that nitrosamines could be
synthesized from secondary amines and nitrates or nitrites by
Escherichia coli and some species of streptococci. Fong & Chan
(1973b) demonstrated that homogenized Chinese salt fish inoculated
with Staphylococcus aureus (a nitrate-reducing bacterium) produced
considerable amounts of DMN.
Formation of nitrosamines in the presence of bacteria is unlikely
to occur in the large intestine, but the infected bladder and
achlorhydric stomach are likely sites (Hawksworth et al., 1974).
The ultimate mechanism of bacterial production of nitrosamines
remains to be ascertained. According to Hawksworth et al. (1974),
certain bacteria do reduce nitrate to nitrite but the formation of the
nitrosamine may be nonenzymatic and involve some heat-resistant
metabolite.
4.6 The Effects of Other Chemicals on the Formation of N-nitroso
Compounds
Fiddler et al. (1973) and Greenberg (1974) showed that high
levels of ascorbic acid reduced nitrosamine formation in frankfurter
sausages and in fried bacon. On the other hand, Nagata & Mirna (1974)
reported an increase in nitrosamine formation in meat products in the
presence of ascorbic acid. Other studies conducted on the inhibition
of nitrosamine formation by various compounds include a report by Sen
& Donaldson (1974) in which nitrosamine formation in human saliva was
inhibited by ascorbic acid. Ziebarth & Scheunig (1976) tested a number
of substances and beverages for the inhibition of the nitrosation of
several drugs under simulated gastric conditions. Of all the
substances investigated, ascorbic acid was regarded as the best
inhibitor because of its pronounced activity at pH values occurring in
the stomach and because it was not toxic in the amounts used.
5. ENVIRONMENTAL LEVELS AND EXPOSURES
5.1 Nitrates and Nitrites
5.1.1 Ambient air
Nitrate aerosols are the final stage in the atmospheric oxidation
of gaseous oxides of nitrogen, and substantial amounts of particulate
nitrates may be formed in urban areas affected by photochemical
pollution (Pitts & Lloyd, 1973). The concentration of nitrates in air
may range from about 1 to 40 µg/m3, depending on the sampling and
averaging periods. For example, the estimated annual mean values
(1968-1972) in Chattanooga, TN, USA, were between 1 and 6 µg/m3
(French et al., unpublished)a. The daily mean concentrations of
airborne nitrates in the central part of Tokyo ranged, in 1973, from
0.9 to 41.8 µg/m3 with an annual mean of 8.2 µg/m3. On the other
hand, in a small city with few industries (Matsue City) the daily
means were in the range of 1.1 -- 9.2 µg/m3, with an annual mean of
2.6 µg/m3 (Japan Environmental Sanitation Center, 1974).
5.1.2 Water
The concentrations of nitrates and nitrites in surface and ground
waters vary within wide limits, depending on geochemical conditions,
human and animal waste management practices, the extent to which
nitrogen-containing agriculture fertilizers are used locally, and on
industrial discharges of nitrogen compounds (section 3.2.1.).
In general, surface waters do not usually contain nitrate in
concentrations higher than 10 mg/litre, and nitrite concentrations
rarely exceed 1 mg/litre. However, a steady upward trend of nitrate
levels has been reported in recent years in some countries, both in
surface and ground waters. Thus, for example, in the River Thames,
England, nitrate concentrations increased from an average of
4 mg/litre in 1968 to an average of 9 mg/litre for the last quarter of
1973 (Water Research Centre, 1974). Similar increases have been
observed in several other English rivers (Casey, 1975; Owens, 1970;
Tomlinson, 1970). The nitrate concentrations are increasing in some
rivers that drain the great agricultural section of the centre of the
USA, and in selected small rivers the 45 mg/litre limit is sometimes
exceeded (Viets & Aldrich, 1973). A small increase in the nitrate
concentration of the Tamagawa River, Tokyo, Japan has also been
reported. From 1951-1965, the nitrate ion concentration rose from
7.9 mg/litre to 9.1 mg/litre. During the same period, the nitrite
concentration increased from 0.049 mg/litre to 0.53 mg/litre, i.e by a
factor of about 10 (Goto, 1973).
a French, J. G., Hasselblad, V., & Johnson, R. Aggravation of
asthma by air pollutants. 1971 -72 Southeastern CHESS studies.
Studies of 991 settlements in Bulgaria indicated that only 64
towns and villages had drinking water levels of nitrates between
30 mg/litre (Bulgarian standard) and 50 mg/litre. In 20 settlements,
situated in areas with intensive agriculture and stock breeding, the
nitrate concentrations exceeded 50 mg/litre. The reportb points out
that such problems did not exist some 10 years ago when smaller
quantities of nitrogen fertilizers were used in agriculture.
Much higher concentrations of nitrates are sometimes found in
ground water, particularly in water derived from dug wells. A survey
of over 2000 rural wells in Saskatchewan, Canada, revealed that 18.8%
contained nitrate concentrations of more than 50 mg/litre and 5.3% had
nitrate levels exceeding 300 mg/litre (Robertson & Draycott, 1948).
Hedlin (1971) also reported levels above 45 mg/litre in some wells in
a rural area of Manitoba, Canada. In many farm wells in central USA,
nitrate concentrations may range from 45-450 mg/litre. This problem is
neither new nor local, since such conditions have been recorded from
1895 to 1970 in Illinois, in 1939 in Iowa, and in 1970 in Minnesota
(Viets & Aldrich, 1973). The mean nitrate concentration in ground
water consumed by children affected by methaemoglobinaemia in
Czechoslovakia ranged from 18-257 mg/litre (Schmidt & Knotek, 1970).
According to Gruenar & Shuval (1970), about 180 wells for community
water supplies in the densely populated central and southern coastal
plain in Israel had nitrate concentrations exceeding 45 mg/litre. In
England, nitrate concentrations in some ground waters have been
reported to range from 12 mg/litre (Foster & Crease, 1974) to over
22 mg/litre (Reeves et al., 1974). Nitrate concentrations exceeding
45 mg/litre have not been reported in centralized water supplies in
the USSRc. However, high concentrations have been found from time
to time in dug wells, for example, 310-400 mg/litre in Leningrad
Oblast (Motylev, 1969), 110-200 mg/litre in the Tatar SSR (Petukhov
et al., 1972) and up to 430 mg/litre in the Moldvian SSR (Diskalenko,
1969).
5.1.3 Selected foods
According to the data compiled by the National Institute of
Environmental Health Sciences (NIEHS, 1970), the levels of nitrates in
vegetables vary considerably. The highest levels were found in beets,
egg plant, kale, and spinach and the lowest in tomatoes and peas;
similar findings were obtained in the German Democratic Republic by
Achtzehn & Hawat (1969). It is of interest to note that the nitrate
levels in vegetables reported by Jackson in 1967 were similar to those
reported by Richardson in 1907, when manure was used instead of
chemical fertilizers.
b Contribution to the WHO environmental health criteria document on
nitrates, nitrites and nitrosamines, Sofia, 1974.
c Contribution to the WHO environmental health criteria document on
nitrates, nitrites and nitrosamines, Moscow, 1974.
Nitrate contents vary not only between vegetable species but also
widely within a given species. This variation within a species may be
accounted for by such factors as temperature, sunlight, soil moisture,
and the level of available nitrogen in the soil (US Department of
Agriculture, 1965). A relationship between nitrate accumulation in
spinach and levels of fertilizer applied to the soil has been
demonstrated by a number of authors (Brown & Smith, 1967; Phillips,
1971). Furthermore, Schuphan (1965) reported exceptionally high levels
of nitrites (3600 mg/kg dry weight) in excessively fertilized fresh
spinach stored at room temperature.
A survey of the nitrate contents of fruits in the German
Democratic Republic, revealed that they were high in bananas and
strawberries but could not be detected in the other fruits examined
(Achtzehn & Hawat, 1969).
Cow's milk contained nitrate levels of 0-0.5 mg/litre (Simon et
al., 1964).
The levels of nitrates and nitrites in baby foods are of special
concern since infants are considerably more sensitive to the toxic
effects of nitrates than adults. Kamm et al. (1965) studied 194
prepared infant foods and found that, on average, fruits, dairy
products, puddings, egg products, meats, dry and concentrated food
supplements, and precooked cereal products contained nitrate levels of
less than 90 mg/kg. Vegetables, however, had a wide range of nitrate
contents varying from 0.9 to 2165 mg/kg, but nitrite levels never
exceeded 7 mg/kg. In studies on a number of canned foods, baby foods,
frozen foods, and vegetables, several varieties of fruit, spinach, and
beets generally had the highest nitrate contents (Bodiphala & Ormrod,
1971). Additional information may be found in articles by NIEHS (1970)
and Ashton (1970).
In a survey of various cured meats (Table 1, Ashton, 1970), the
highest nitrate content of 370-511 mg/kg was found in ham. In
analysing 197 samples of cured meat products, Panalaks et al. (1972)
found that the levels of nitrates and nitrites ranged from
0-3467 mg/kg and 0-252 mg/kg, respectively.
Dubrow & Kakisch (1960) analysed 338 samples of cheese and
reported that all were free of nitrites (less than 1 mg/kg). However,
40% of the samples contained nitrate levels of more than 1 mg/kg.
Rammell & Joerin (1972) also found low nitrite levels in cheese.
Table 1. Nitrate and nitrite contents of cured meats
Meat type Nitrate (mg/kg) Nitrite (mg/kg)
silverside 133-303 9-26
ham 370-511 7-150
luncheon meat 59-214 3.1-47
chopped ham & pork 53-101 22-62
corned beef 118-135 18-208
frankfurter sausage 119-141 8.5-10.3
From: Ashton (1970)
5.1.4 Estimate of general population exposure
One of the important sources of exposure to nitrates for man is
water. The level of nitrates in water may vary from practically nil to
over 200 mg/litre. In water from municipal supplies, however, it is
likely to be under 10 mg/litre. Thus, assuming an intake of 2 litres
of water per day, the daily intake of nitrates from this source would
normally be less than 20 mg, but with extremes of 0 and over 400 mg.
The other main sources of nitrates and nitrites are certain
vegetables and meat products. The intake from these sources is even
more variable because of marked differences not only in levels in
these foods but also in dietary patterns. However, Ashton (1970)
estimated the weekly intake of nitrates for a member of the general
population in the USA to be about 400 mg including 210 mg from
vegetables, 110 mg from meat products, and 85 mg from water (7 litres
per week). The estimate of Hill et al. (1973) for a member of the
general population in England included 225 mg from vegetables, 110 mg
from meat, and 105 mg from water in "control towns" and 645 mg from
water in Worksop, England. However these figures cannot be applied
generally because of variations in feeding habits and nitrate levels
in environmental media. Since the intake of nitrites is even more
variable, no estimates have been reported.
5.2 N-nitroso Compounds
5.2.1 Ambient air
The occurrence of N-nitroso compounds in urban air was reported
first by Bretschneider & Matz (1973) and confirmed by Fine et al.
(1976b) who found DMN at concentrations of about 1.2-3.5 µg/m3
(0.33-0.96 ppb) in an industrial district in Baltimore, MD, USA, and
about 0.06-0.17 µg/m3 (0.014 ppb - 0.051 ppb) in Belle, WV, USA.
N-nitroso compounds may be present in air, either due to their
formation in the air from secondary amines and oxides of nitrogen
(Neurath, 1972) or due to industrial omissions as in the instances
referred to.
5.2.2 Water
There are few reports on the occurrence of N-nitroso compounds
in water. Fine et al. (1976b) analysed samples from the Mississippi
river (New Orleans, LA) and from 3 water treatment plants in
Louisiana. Using the new N-nitroso compound-specific thermal energy
analyser (TEA) interfaced to both a gas chromatograph (GC) and a high
performance liquid chromatograph (LC), several peaks were tentatively
identified as belonging to N-nitroso derivatives of some pesticides.
The estimated concentrations were of the order of 0.1 µg/kg.
5.2.3 Selected foods
A summary of the reported occurrences of nitrosamines in meat and
fish products, adapted from Sen (1974) and updated, is presented in
Table 2. Only the results that were confirmed by mass spectroscopy are
quoted in this table. It was noted that the majority of some 50
publications dealt with the determination of nitrosamines,
particularly DMN, in processed pork meat. The methods employed for
analysis mainly involved gas chromatography. A few results were
confirmed by mass spectroscopic techniques. However, mass spectroscopy
confirmation is currently being employed more frequently than in the
past.
Table 2. Levels of nitrosamines in various meat and fish productsa
N-nitroso
Meat Country compounds Levels (c) Reference
or area foundb
dry sausage Canada DMN 10-20 µg/kg Sen (1972)
uncooked salami sausage Canada DMN 20-80 µg/kg Sen (1972)
salami sausage Netherlands DMN 0.3 µg/kg(e) Stephany et al. (1976)
DMN 0.1(e) Stephany et al. (1976)
NDBA 1.1(e) Stephany et al. (1976)
Netherlands NPY 0.4(e) Stephany et al. (1976)
NPIP 0.3(e) Stephany et al. (1976)
bacon Canada NPY 4-25 µg/kg Sen et al. (1973a)
Canada NPY 25-40 µg/kg Sen et al. (1974a)
Netherlands DMN 0.8 µg/kg(e) Stephany et al. (1976)
bacon DEN 0.2(g)
Netherlands NDBA 0.6(g) Stephany et al. (1976)
NPY 0.4(g)
Netherlands NPIP 0.6(g) Stephany et al. (1976)
USA NPY 7-35 µg/kg Pensabene et al. (1974)
bacon USA NPY 2, 28, 13 Fiddler et al. 1974
uncooked bacon Canada DMN 30 µg/kg Sen et al. (1973a)
fried bacon Netherlands DMN 2.4 µg/kg Groenen et al. (1976)
DEN 4.43 µg/kg
Netherlands DMN 1.1 µg/kg(e) Stephany et al. (1976)
DEN 0.2(g)
Netherlands NDBA 0.7(g) Stephany et al. (1976)
NPY 16.4(g)
Table 2 (Cont'd)
N-nitroso
Meat Country compounds Levels (c) Reference
or area foundb
Netherlands NPIP 3.9(g) Stephany et al. (1976)
UK NPY 1-40 µg/kg Crosby et al. (1972)
USA NPY 20-207 µg/kg Fazio et al. (1973)
smoked meat Netherlands DEN 7.91 µg/kg Groenen et al. (1976)
Netherlands DMN 3 Groenen et al. (1976)
smoked horse and Netherlands DMN 7.3 µg/kg(d) Stephany et al. (1976)
beef meat DEN 0.6(d) Stephany et al. (1976)
Netherlands NDBA 0.4(d) Stephany et al. (1976)
NPY 0.1(d) Stephany et al. (1976)
Netherlands NPIP 0.1(d) Stephany et al. (1976)
ham with layer Germany DMN 3 µg/kg Eisenbrand et al. (1975)
of pepper grains (Federal Republic of)
on the outside.
Only fat portion.
ham with layer Germany NPIP 6 µg/kg Eisenbrand et al. (1975)
of pepper grains (Federal Republic of)
on the outside.
Whole product Germany NPY 6
homogenized. (Federal Republic of)
ham, fried Germany DMN 1 µg/kg Eisenbrand et al. (1975)
(Federal Republic of) NPIP 8
NPY 19
Table 2 (Cont'd)
N-nitroso
Meat Country compounds Levels (c) Reference
or area foundb
ham with layer of Germany NPIP 4 µg/kg Eisenbrand et al. (1975)
pepper grains (Federal Republic of)
on the outside. Germany NPY 9
Whole product (Federal Republic of)
homogenized.
German type of Germany DMN 1 µg/kg Eisenbrand et al. (1975)
bacon, raw (Federal Republic of)
German type of Germany DMN 1 µg/kg Eisenbrand et al. (1975)
bacon, fried (Federal Republic of) NPIP 5 Eisenbrand et al. (1975)
Germany NPY 19 Eisenbrand et al. (1975)
(Federal Republic of)
smoked raw meat Germany DMN 2 µg/kg Eisenbrand et al. (1975)
(Federal Republic of)
smoked ham Germany DMN 8 µg/kg Eisenbrand et al. (1975)
(Federal Republic of)
smoked ham USA DMN 5 µg/kg Fazio et al. (1971b)
smoked ham USA DMN 5 µg/kg Fiddler et al. (1974)
cooked and smoked Netherlands DMN 0.4 µg/kg(f) Stephany et al. (1976)
ham DEN 0.6(f) Stephany et al. (1976)
Table 2 (Cont'd)
N-nitroso
Meat Country compounds Levels (c) Reference
or area foundb
Netherlands NDBA 0.4(f) Stephany et al. (1976)
NPY 0.3(f) Stephany et al. (1976)
Netherlands NPIP 0.4(f) Stephany et al. (1976)
cooked ham Netherlands DMN 6 µg/kg Groenen et al. (1976)
Bologna sausage Canada DEN 25 µg/kg Panalaks et al. (1974)
Canada NPY 20,100,105 Panalaks et al. (1974)
frankfurter sausage USA NPIP 50, 50, 60 µg/kg Wasserman et al. (1972)
spiced meat Canada DMN 5-48 µg/kg Sen et al. (1976)
products DEN 6-16
Canada NPIP 14-50 Sen et al. (1976)
Canada NPY 7-33 Sen et al. (1976)
fish meal Canada DMN 0.35-0.5 mg/kg Sen et al. (1972)
smoked, nitrate/ USA DMN 4-26 µg/kg Fazio at el. (1971a)
or nitrite treated
sable, salmon shad
fresh, smoked or UK DMN 1-9 µg/kg Crosby et al. (1972)
salted fish
salted white Hong Kong DMN 40-100 µg/kg Fong & Chan (1973a, 1973b)
herring
Table 2 (Cont'd)
N-nitroso
Meat Country compounds Levels (c) Reference
or area foundb
salted yellow Hong Kong DMN 10-60 µg/kg Fong & Chan (1973a, 1973b)
croakers
crude salt salted Hong Kong DMN 400 µg/kg Fong & Chan (1973a, 1973b)
white herring
crude salt salted Hong Kong DMN 200 µg/kg Fong & Chan (1973a, 1973b)
yellow croakers
prime salt salted Hong Kong DMN 10 µg/kg Fong & Chan (1973a, 1973b)
white herring
prime salt salted Hong Kong DMN 5 µg/kg Fong & Chan (1973a, 1973b)
yellow croakers
salted anchovies Hong Kong DMN 20 µg/kg Fong & Chan (1973a, 1973b)
whole herring meal Hong Kong DMN 300 µg/kg Fong & Chan (1973a, 1973b)
a Adapted from Sen (1974)
b DMN -- N-methyl- N-nitrosomethanamine c All values confirmed by mass spectroscopy
DEN -- N-ethyl- N nitrosoethanamine d mean of 4 samples
NDBA -- Nitroso- N-butylamine e mean of 5 samples
NPY -- Nitrosopyrrolidine f mean of 6 samples
NPIP -- Nitrosopiperidine g mean of 10 samples
Several authors who detected nitrosamines in foods by screening
methods but did not confirm these results by mass spectrometry include
Ender et al. (1964, 1967), Ender & Ceh (1967), Fong & Walsh (1971),
Freimuth & Glaser (1970), Hedler & Marquardt (1968), Kröller (1967),
Lembke & Moebus (1970), Möhler & Mayrhofer (1968, 1969), and Sakshaug
et al. (1965). Nevertheless, results by screening methods should not
be entirely ignored.
Considerable variations have been found in the levels of volatile
N-nitroso compounds in fried and grilled bacon. Attempts to
correlate these levels with levels of nitrates and nitrites did not
reveal any definite pattern. Telling et al. (1974) studied the effect
of various cooking temperatures on the levels of N-nitroso compounds
in grilled bacon. The results indicated that the levels of DMN
remained fairly constant as the cooking temperature was raised but
those of nitrosopyrrolidine increased.
Lipid soluble nitrosamines have an affinity for the fatty
portions of food (Sen et al. 1973a).
5.2.4 Tobacco and tobacco smoke
Since precursors for the formation of nitrosamines occur in
tobacco, Druckrey & Preussman (1962) thought it likely that tobacco or
tobacco smoke might contain trace amounts of nitrosamines. Initially,
studies on the nitrosamine content of tobacco products were hampered
due to interference from other compounds. Later, evidence suggesting
the presence of DMN, nitrosopyrrolidine, methylbutylnitrosamine, and
nitrosopiperidine in tobacco smoke was obtained (Kröller, 1967;
Neurath et al., 1964; Neurath, 1972, Serfontein & Hurter, 1966).
Although anabasine and nornicotine are constituents of tobacco smoke,
the corresponding nitroso derivatives were not detected by Neurath
(1972). Recently, however, Hoffman et al. (1974) reported the presence
of N-nitrosonornicotine at levels of up to 88 mg/kg in unburned
tobacco.
5.2.5 Estimate of general population exposure
The N-nitroso compounds that have been identified and
determined in meat and fish are listed in Table 2; consumption of
these foods constitutes a definite exposure of the general population
to these chemicals. However, at present, no estimate can be made of
the human exposure from these and other sources because insufficient
samples have been analysed and because the relevant food consumption
surveys have not been made.
5.2.6 Occupational exposure to N-nitroso compounds
The potential occupational hazards associated with the use or
manufacture of N-nitroso compounds in industry have been pointed out
by Magee & Barnes (1967).
Only a few quantitative data are available, however, on the
concentration of N-nitroso compounds in the work environment. In a
study by Bretschneider & Matz, (1976) DMN levels in the air in a
factory manufacturing DMA were roughly estimated to range from 0.001
to 0.43 µg/m3.
6. METABOLISM OF NITRATES, NITRITES, AND N-NITROSO COMPOUNDS
6.1 Gastrointestinal Absorption
6.1.1 Nitrates and nitrites
A part of ingested nitrates is readily absorbed and a part may be
metabolized by the microflora in the gastrointestinal tract (Ridder &
Oehme, 1974). Nitrites (NO2-), oxides of nitrogen (N2O5, NO2,
NO), hydroxylamine (NH2 OH), and ammonia (NH3) can be formed
depending upon the organisms present, the pH, and the available
nutrients (trace elements and carbohydrates), and may be absorbed.
Friedman et al. (1972) gave mice a single oral dose of 150 µg of
sodium nitrite. Measurement of the rate of disappearance showed that
the compound was rapidly absorbed and that food in the stomach had
little effect on absorption. Results using animals with a ligated
gastro-duodenal junction suggested that the major absorption site was
the gastric mucosa. Studies by Mirvish et al. (1974) on rats fed a
diet containing nitrite supported the previous observations on mice.
Experiments with food containing phenol red showed that a decrease in
nitrite levels in the stomach contents, especially in the glandular
part, that occurred within 5 h of feeding, was significantly greater
than that due to direct faecal elimination. This was attributed to
decomposition and other acid-catalyzed reactions of nitrite and to
direct absorption from the stomach.
6.1.2 N-nitroso compounds
Although a considerable degree of absorption of nitrosamines can
be inferred from the nature of their toxic effects following oral
administration, few reports could be found giving quantitative
information on absorption. Alarif & Epstein (1974) gave 3H-labelled
nitrosomethylurea and nitrosomethylurethane by gavage to groups of
pregnant guineapigs at doses of 2 mg/kg, and 5 mg/kg body weight,
respectively. The animals were killed 1 h after dosing. When the
uptake by maternal and fetal tissues was measured by liquid
scintillation counting and DNA determination, maternal levels were
generally higher than fetal levels. In studies by Juszkiewicz &
Kowalski (1974) DMN, DEN, and nitro-propylamine, administered orally
to goats at 20-30 mg/kg, appeared in the blood within “ h, and later
in the milk. The concentration in the milk, 2 h after administration
of DEN at 30 mg/kg, was 14 mg/litre; after 24 h only traces could be
found. Phillips et al. (1975a) examined the disappearance of DMN from
the stomach and small intestine of rats as an index of absorption, and
found that, while very little was absorbed from the stomach, DMN was
rapidly absorbed from the small intestine.
6.2 Biotransformation and Elimination
6.2.1 Nitrates and nitrites
In a study on 4 rats, 42-90% of nitrates, administered by stomach
tube, was excreted in the urine within 8 h of administration. Nitrites
were not detected in the urine either before or after administration
(Hawksworth & Hill, 1971b). The same authors carried out a study on
122 samples of human urine, and found that urinary nitrate
concentration was related to the amount of nitrate ingested.
The excretion of nitrates and nitrites in the saliva was studied
by Spiegelhalder et al. (1976) in 11 volunteers given various
vegetable juices with nitrate concentrations ranging from 30 to
550 mg/litre. The levels of nitrates and nitrites excreted were
proportional to the amounts of nitrates ingested. After ingesting
100 mg of nitrates, nitrite concentrations in the saliva increased, on
average, by 20 mg/litre.
6.2.2 N-nitroso compounds
Magee & Barnes (1967) have reviewed available information on the
metabolism and elimination of N-nitroso compounds. Magee (1956)
measured recovery of DMN from the whole body of the mouse and noted
that 97% of the total dose (0.05 mg/kg) could be recovered immediately
after oral administration and that the amounts recovered decreased
with time until at 4 h no DMN was recovered. Similar results were
obtained with the rat given DMN orally at 50 mg/kg, the concentration
fell rapidly with increasing time after injection so that only 30% of
the dose was recovered at 8 h and none at 24 h.
Metabolic transformation of DMN was demonstrated by Dutton &
Heath (1956) using 14C-labelled DMN. In both the mouse and the rat,
the main radioactive product was expired carbon dioxide. In the mouse,
65% of the injected 14C was recovered as expired carbon dioxide, 6 h
after a subcutaneous injection of DMN at 50 mg per kg body weight. In
the rat, about 40% of the radioactive 14C was recovered as expired
carbon dioxide, 8 h after the injection. At the end of the experiment,
the remainder of the 14C was fairly evenly distributed in the
tissues, apart from about 7% that was excreted in the urine.
Heath (1962) found that, while part of each of a number of
nitrosamines studied was excreted unchanged in urine and in expired
air, the greater part was decomposed. From the rates of expiration of
labelled carbon dioxide it was shown that decomposition of dimethyl-,
diethyl-, and N-butylmethylni-trosamine obeyed Michaelis-Menten
kinetics. The rate of decomposition was dose-dependent.
When the metabolic transformation of dialkylnitrosamines,
especially of the bladder carcinogen di- N-butylnitrosamine was
investigated, major urinary metabolites with retained N-nitroso
structure were identified (Blattmann & Preussman, 1973, 1974;
Blattmann et al., 1974; Okada & Suzuki, 1972; Okada et al., 1975).
Hydroxylation, particularly at the terminal CH3 group, has been
observed as well as chain shortening. Butyl (3-carboxypropyl)
nitrosamine (BCPN), a major metabolite of butyl (4-hydroxy butyl)-
nitrosamine (BBN) was an equally potent and selective bladder
carcinogen (Okada & Suzuki, 1972). Ring-opening was observed during
metabolism of nitrosomorpholine (Stewart et al., 1974).
7. EXPERIMENTAL STUDIES ON THE EFFECTS OF NITRATES, NITRITES, AND
N-NITROSO COMPOUNDS
7.1 Nitrates and Nitrites
7.1.1 Acute and subacute toxicity studies
Acute nitrate poisoning was first recognized in cattlea by Mayo
as early as 1895 (Wright & Davidson, 1964), while Comly (1945) was the
first to report nitrate poisoning from well water in infants in the
USA. As a result of these and other reports, several studies have been
conducted on the toxicity of nitrates in a wide variety of animal
species. They are mainly centred on the formation of methaemoglobin
that accompanies excessive exposure to nitrates and nitrites. The
acute toxicity of nitrates and nitrites was recently reviewed by the
Committee on Nitrate Accumulation (1972).
Although the outstanding feature of nitrate toxicity is the
development of methaemoglobinaemia, nitrates may also cause
vasodilation which aggravates the effects of the methaemoglobinaemia.
The nitrite ion formed by reduction of nitrates, oxidizes the iron in
the haemoglobin molecule from the ferrous to the ferric state. The
resultant methaemoglobin is incapable of reversibly binding oxygen
(Bosch et al., 1950). Clinical signs of nitrate toxicity, attributable
to hypoxia appear when methaemoglobin values exceed about 20% (section
8.1). Oxidation of haemoglobin to methaemoglobin by the nitrite ion
occurs at different rates for each animal species, but there is little
difference between individuals of the same species (Smith & Beutler,
1966). Similarly, the reduction of methaemoglobin in erythrocytes
mainly by the enzyme system, NADH--methaemoglobin reductase, is
characteristically different for each animal species. The chemical
induction of methaemoglobinaemia has recently been reviewed by Smith
(1969, 1975). These physiological processes appear to be related, even
though there is a large variation in the rate of formation of
methaemoglobin and its subsequent reduction. This may help to explain
the difference in species susceptibility and the variation in signs
seen in nitrate poisoning.
In an effort to develop sensitive tests for the detection of the
possible effects of subclinical methaemoglobinaemia, behavioural
studies with mice were undertaken by Behroozi et al., 1971. Groups of
57 black, 6J, male mice were given nitrites in their drinking water at
doses aimed at producing methaemoglobin levels varying from slightly
above normal to 15%, which can be considered to be in the subclinical
a For further discussion of nitrate intoxication in livestock,
that may involve significant economic loss, see Oehme (1975).
range. Sodium nitrite doses in water were 100, 1000, 1500, or
2000 mg/litre. The results showed a significant reduction of overall
motor activity in the groups receiving the highest levels of
nitrites. There was a significant inverse relationship between the
methaemoglobin level and motor activity, with a coefficient of
correlation of 0.65. An effort to counteract the methaemoglobinaemia
was made by giving ascorbic acid to the group receiving the highest
level of nitrites (2000 mg/litre). (See section 7.1.5). The effect
was to reduce the methaemoglobin levels to almost normal but the
motor activity level of the group so treated remained low and about
equal to the equivalent group that had not received an antidote.
These experiments seemed to indicate that the nitrites had some form
of sedative effect on the treated mice, that was not necessarily
associated with the development of methaemoglobinaemia.
Experiments on rabbits have shown that methaemoglobinaemia caused
by nitrates in water also affects cardiac activity by increasing the
number of cardiac contractions to an extent directly proportional to
the increase in methaemoglobin levels. At the 10-15% methaemoglobin
level, the electrocardiogram shows a shortening of the Q-T interval
and a reduction in the T wave, which may even become negative (Garbuz,
1968, 1971).
7.1.2 Chronic toxicity and carcinogenicity studies
In a study conducted by Shuval & Gruener (1972), groups of 8 male
rats (3 months old) were given tap water (control) or 100, 1000, 2000,
or 3000 mg of sodium nitrite per litre of drinking water. After 24
months, there were no significant differences in growth and
development, mortality, and total haemoglobin levels between the
control and treated groups. However, the methaemoglobin levels in the
groups receiving sodium nitrite at 1000, 2000, or 3000 mg/litre were
raised significantly throughout the study and averaged 5%, 12%, and
22% of total haemoglobin respectively. The methaemoglobin levels in
the group receiving 100 mg/litre were slightly above those of the
control group for the first 60 days only. There were some changes in
the liver and spleen of treated animals but the main pathological
changes occurred in the heart and lungs. In the heart, small foci of
cells and fibrosis were seen in some animals with pronounced
degenerative foci in animals receiving the highest concentrations of
nitrites. The coronary arteries were thin and dilated. The bronchi
were frequently dilated with the walls infiltrated by lymphocytes and
the mucosa and muscle were often atrophied. Emphysema was the rule.
These changes, which were present in 1 or 2 control rats and in a
small number of those receiving nitrite levels of 100 mg/litre, were
found with increasing frequency and severity in the 3 highest dose
groups.
Druckery et al. (1963a) did not observe extensive methaemoglobin
formation in rats given 100 mg sodium nitrite per kg body weight in
the drinking water, but there was a slight reduction in the life span.
Studies by Van Logten et al. (1972) in which groups of 30 male and 30
female rats received sodium nitrite at concentrations of 0, 0.02, or
0.05% with or without glucono--lactone (GDL) in the diet for 29
months did not demonstrate any significant haematological or
biochemical effects. Carcinogenic action, which could be related to
the administration of sodium nitrite with or without DEA or GDL, was
not observed in either of these studies.
It has been reported that prolonged administration to rats of
1/20 of the LD50 of calcium and sodium nitrates disturbed the energy
conversion processes such as glycolysis and the pentose phosphate
cycle, changed the activity of the glutathione-ascorbic acid system in
the blood and in the hepatic and cerebral tissues, raised the levels
of methaemoglobin and of NADH-methaemoglobin reductase activity, and
reduced the haemoglobin levels (Diskalenko & Dobrjanskaja, 1972;
Diskalenko & Trofimenko, 1972).
Greenblatt & Mirvish (1972) gave 3 groups of about 40, 7-9 week
old, male, strain A mice, 1 or 2 g of sodium nitrite or 12.3 g of
sodium nitrate/litre of water respectively, for 20 weeks and did not
observe any increase in lung tumour incidence in comparison with
untreated controls. Lijinsky et al. (1973a) reported similar negative
results when groups of 30 rats were given sodium nitrate at 5 g/litre
or sodium nitrite at 2 g/litre in their drinking water at a daily rate
of 20 ml/rat throughout most of their lifetime. Other studies by
Lijinsky et al. (1973c) on groups of 30 rats given 20 ml of sodium
nitrite at 2 g/litre daily, for 5 days a week, also produced negative
results. Taylor & Lijinsky (1975) reported that tumour formation in 57
Sprague-Dawley rats, exposed for 2 years to a drinking solution
containing sodium nitrite at 2 g/litre, was no greater than in the
controls.
7.1.3 Embryotoxicity
A study has been reported by Shuval & Gruener (1972) in which 2
groups of 12 pregnant rats were given 2000 or 3000 mg of sodium
nitrite per litre of drinking water, respectively. A control group did
not receive any treatment. Pregnant rats developed anaemia and had
higher methaemoglobin levels than nonpregnant rats receiving similar
doses. There was a pronounced increase in mortality among the newborn
rats of treated dams compared with those of untreated controls,
particularly in the 3-week period before weaning. Mortality in the
offspring was 6% in controls, 30% in those given 2000 mg/litre and
53% in those given 3000 mg/litre. Birthweights were similar in all
groups but growth was markedly reduced in pups of treated dams. Such
pups had thin hair coats. Treatment of 2 groups of 10 and 15 pregnant
rats with 1% and 0.3%, respectively, of sodium nitrate in the diet did
not result in any embryotoxic or teratogenic effects on the 9th and
10th days of gestation (Alexandrov & Jänisch, 1971).
Sleight & Atallah (1968) conducted a study in which 46 female
guineapigs, divided into 12 groups each containing at least 1 male,
were given potassium nitrate in doses ranging from 300 to 10 000 mg/kg
body weight in the water and potassium nitrite in amounts ranging from
300 to 10 000 mg/kg for periods ranging from 100-240 days.
Reproduction in the female was grossly impaired in the high nitrate
group. Fetal losses were 100% in females given 5000 or 10 000 mg/kg of
the nitrite and one female died. Reproduction was maintained at lower
levels of treatment. Apparently male fertility was not impaired, since
conception took place at all levels of treatment. Food and water
consumption and weight gains of treated animals were normal except for
a diminished rate of gain at a nitrite level of 10 000 mg/kg body
weight. Uterine and cervical inflammatory lesions and degenerative
placental lesions were present in females in which the fetuses had
been aborted, mummified, or absorbed. Sinha & Sleight (1971) reported
studies in which 4 pregnant guineapigs, given sodium nitrite at 50 mg
per kg of body weight, subcutaneously, underwent normal parturition.
However, fetal deaths followed by abortion, occurred in 3 guineapigs
given sodium nitrite at 60 mg/kg. The fetal deaths occurred
approximately 1 h after nitrite administration, when the maternal and
fetal methaemoglobin levels were highest. At the time of death, there
were no noticeable changes in the placenta; pathological changes
developed after the death of the fetuses. There were lower blood pO2
values in the fetuses of the guineapigs treated with nitrite at
60 mg/kg than in those of the controls. Fetal death did not occur in
pregnant animals given sodium nitrite at 60 mg/kg combined with
simultaneous intraperitoneal treatment with 10 mg of methylene blue
per kg body weight. The data suggest that fetal death resulted from
hypoxia, mainly induced by maternal methaemoglobinaemia.
Studies by Shuval & Gruener (1972) indicated that
methaemoglobinaemia might be induced transplacentally and that the
observed limit for transplacentally induced methaemoglobinemia was a
sodium nitrite dose of 2.5 mg/kg body weight. A steep increase in
effect occurred with increasing doses of sodium nitrite.
7.1.4 Mutagenicity
The mutagenic potential of nitrates and nitrites has not been
studied extensively; in fact, no data are available on their mutagenic
action in the mammalian systems. The mutagenicity of nitrates and
nitrites with respect to the transformation of DNA has been reported
(Bressler et al., 1968; Horn & Herriot, 1962; Strack et al., 1964) and
nitrous acid has been shown to be mutagenic in bacterial systems such
as Escherichia coli (Kaudewitz, 1959; Verly et al., 1967) and
Salmonella typhimurium (de Serres et al., 1967). Positive results in
mutagenic studies have been reported with the yeast Saccharamyces
cerevisiae (Nashed & Jabbur, 1966; Zimmerman & Schwaier, 1967;
Zimmerman et al., 1966), as well as with Aspergillus nidulans
(Siddiqi, 1962), A. niger, and A. amstelodami (Steinberg & Thom,
1940), and tobacco mosaic Virus (Sehgal & Krause, 1968).
7.1.5 Interaction with nutritional factors
Studies by Kociba & Sleight (1970) showed that maternal blood
levels of methaemoglobin were significantly higher in 12 ascorbic
acid-deficient, pregnant guineapigs, following the subcutaneous
administration of sodium nitrite at 40 mg/kg body weight, than in
those on a normal diet. Following subcutaneous administration of
sodium nitrite at 50 mg/kg, there was a higher percentage of fetal
death in the ascorbic acid-deficient guineapigs.
Experiments were conducted by Stoewsand (1973) with young, male,
guineapigs (number of animals not stated) to investigate the influence
of feeding beets with naturally occurring low and high amounts of
nitrates and nitrites, and the influence of dietary supplementation
with ascorbic acid and methionine on methaemoglobinaemia, induced by
orally administered sodium nitrite at doses of 25 or 50 mg/kg body
weight. Low-nitrate beet diets seemed to "protect" guineapigs from
nitrite intoxication. In addition, a 1% diet containing L-ascorbic
acid and 1% methione reduced nitrite-induced methaemoglobin blood
levels.
Sell & Roberts (1963) showed that ad libitum feeding of diets
containing 0.4% potassium nitrite to 6 groups of 50 chicks depressed
growth, irrespective of the amount of vitamin A administered. Also,
all the test animals, except those given massive injections of vitamin
A, exhibited reduced liver stores of the vitamin and enlarged thyroid
glands.
Studies by Phillips (1966) demonstrated that the liver vitamin A
contents of rats fed 1% potassium nitrite in diets containing carotene
or vitamin A, were less than those of control rats. It was suggested
that the dietary nitrite degraded the carotene and vitamin A in the
digestive tract before their absorption.
7.2. N-nitroso Compounds
7.2.1 Acute and subacute toxicity studies
The acute toxicity of N-nitroso compounds is not of great
toxicological significance because there is no relationship between
acute toxicity and the carcinogenic potential of this class of
compounds (Druckrey et al., 1967, Magee & Barnes, 1967). Because DMN
was reported to cause cirrhosis and other toxic effects in industrial
workers, Barnes & Magee (1954) examined the toxicity of this compound.
Doses of 20-40 mg/kg body weight given to rats, dogs, rabbits, and
guineapigs produced severe hepatic damage. A single dose of DMN given
to rats orally or by intravenous, intraperitoneal, or subcutaneous
injection, produced centrilobular necrosis accompanied by haemmorhages
in the liver. In rats given 20 mg DMN/kg body weight the liver cells
in the centrilobular and mid-zonal regions became pale and, after
18 h, the cytoplasm was amorphous and vacuolated; the nuclei were pale
and irregular in outline. By 24 h, the cells were necrotic and
confluent areas became haemorrhagic. Haemorrhage was usually more
pronounced after 48 h but after 72 h the recovery process had begun
and was almost complete in 3 weeks (Barnes & Magee, 1954; Magee &
Barnes, 1962). A detailed study by light and electronmicroscopy of
changes in the liver cytoplasm of rats treated with
N-nitrosomorpholine has been reported by Bannasch (1968).
The acute toxicity of acyl-alkyl-nitrosamides and diazoalkanes
was reported by Druckery et al. (1967), Magee & Barnes (1967), and
Shank (1975). The acute toxicity (LD50) of the nitroso compounds
varied widely; some were only mildly toxic while others produced
highly destructive lesions. N-nitroso- N-methylurethane, for
example, when given orally, produced severe necrotic lesions in the
stomach, congestion of the lungs, and periportal necrosis of the liver
(Schmähl & Thomas, 1962; Schoental, 1960). N-nitroso- N-methylurea
also produced inflammatory haemorrhagic lesions of the stomach,
intestine, and pancreas and a reduction in the bone marrow when given
orally to rats, (Druckrey et al., 1961, 1967).
The acute toxicity of some N-nitroso compounds, e.g. nitroso-
piperidine and nitroso-morpholine, was not manifested as liver damage
but as neurotoxic effects, e.g. convulsions (Lee & Lijinsky, 1966).
The acute toxicity of nitrosomines formed in vivo was studied
by Asahina et al. (1971). Sodium nitrite was administered by gavage to
mice at 100 or 150 mg/kg bodyweight alone or in combination with DMA
and methylbenzylamine at doses of 500-2500 mg/kg and 800-1600 mg/kg,
respectively. Combinations of the amines and nitrite produced hepatic
lesions similar to those produced by DMN or nitrosome-thylbenzylamine.
Similar effects were noted when nitrite was administered up to 3 h
after DMA but these effects were markedly reduced if the nitrite was
given prior to the amine. Lijinsky & Greenblatt (1972) reported
hepatic necrosis following coadministration of aminopyrine and sodium
nitrite.
In a number of studies, ascorbic acid has been shown to have an
inhibitory effect on: a) nitrosamine formation in the stomach and
small intestine of rats treated with 7-chloro-2-methylamino-5-phenyl-
3H-1, 4-benzodiazepine-4-oxide, (chlordiazepoxide, Librium) and sodium
nitrite (Preda et al., 1976); b) hepatotoxicity induced by the
combined administration of sodium nitrite and aminopyrine in rats
(Kamm et al., 1973) and mice (Greenblatt, 1973); c) liver necrosis
produced by DMA and nitrite administered to rats by gavage (Cardesa et
al., 1974); d) the teratogenic and transplacental carcinogenic effects
produced in rats by treatment with alkylurea and nitrite (Ivankovic et
al., 1973); and e) the induction of lung adenomas in mice by prolonged
treatment with morpholine, piperazine, and methylurea plus nitrite
(Mirvish et al., 1975).
Species differences exist with respect to the toxic effects of
N-nitroso compounds. Mink appear to be especially sensitive to DMN,
and as in other species, the effects are seen primarily in the liver.
Carter et al. (1969) noted widespread liver degeneration and necrosis
of hepatocytes in mink given DMN at 2.5 or 5.0 mg/kg in the diet for
7-11 days. The liver lesions were accompanied by bile-duct
proliferation, ascites, and haemorrhage of the gastrointestinal tract.
Sheep and cattle are also more sensitive to nitrosamines than
laboratory animals (Sakshaug et al, 1965; Koppang, 1964). Sheep, given
a single dose of DMN at 5 mg/kg body weight or 12 doses of 0.5 mg/kg,
died or were severely affected displaying anoxia, lack of rumination,
ataxia, and respiratory difficulties, while cattle given DMN at
0.1 mg/kg body weight showed pronounced hepatotoxic effects in 1-6 months.
Pathological and biochemical effects, observed in the liver of a
number of animal species including the rat following continuous
administration of nitrosamides, have been reviewed by Magee & Barnes
(1967) and Magee et al. (1976); the main effect is inhibition of
protein synthesis which might be a result of an accelerated breakdown
of messenger ribonucleic acid (RNA).
7.2.2 Carcinogenicity
The carcinogenic activity of N-nitroso compounds has been
summarized in several reviews (Druckrey, et al., 1967; Magee & Barnes,
1967; Magee et al., 1976). Various animal species including mammals,
birds, fish, and amphibia have been shown to be susceptible to the
carcinogenic action of nitrosamines. At present, some 80 nitrosamines
and 23 nitrosamides have been tested in rats and about 80% of the
nitrosamines and practically all the nitrosamides have proved to be
carcinogenic (Montesano & Bartch, 1976). These carcinogens show a
marked organ specificity as shown in Table 3.
Nitrosamines produce a carcinogenic effect in the liver,
oesophagus, respiratory system, and kidney, whereas nitrosamides
affect the peripheral and central nervous systems, and the gastro-
intestinal tract organs.
The dose schedule seems to play an important role in this organ
specificity. For example, in rats, long-term exposure to relatively
low doses of DMN induced mainly liver tumours, whereas a single or a
few high doses over a short period induced mainly kidney tumours
(Magee & Barnes, 1962). Similar responses in relation to the liver and
oesophagus were observed with DEN in rats (Druckey et al., 1967). The
route of administration does not seem to play an important role in the
carcinogenicity of this chemical. It is worthwhile pointing out that
many of these chemicals were carcinogenic following a single
administration and that, furthermore, exposure of rats to a single
dose during pregnancy resulted in carcinogenesis in the immediate
descendants and also in the two succeeding generations (Tomatis et
al., 1977).
Nitrosamines exert their adverse biological effects after being
metabolically activated by microsomal mixed function oxidases to form
reactive intermediates. On the other hand, nitrosamides decompose
enzymatically to reactive, and in most cases, alkylating derivatives.
The importance of hydroxilation of the alpha-hydrogen atoms of the
nitrosamines is demonstrated by the lack of carcinogenicity of
compounds, such as diphenylnitrosamine, which do not have this alpha-
hydrogen (Magee et al., 1976).
Table 3. Localization of tumours induced by N-nitroso
compounds in ratsa
Number of N-nitroso compounds
affecting target organ
Target organ Nitrosamines Nitrosamides
liver 35 2
oesophagus-pharynx 32 3
nasal cavities 18 -
respiratory tract 10 1
kidney 8 9
tongue 8 -
forestomach 7 11
bladder 4 1
central and peripheral 2 9
nervous system
ear duct 2 1
testis 1 -
ovary 1 2
mammary glands 1 1
sites of injection 3 4
intestine - 7
glandular stomach - 6
skin - 3
jaw - 1
uterus - 2
vagina - 1
haemopoietic system - 2
a From: Montesano & Bartch (1976)
7.2.2.1 Interspecies variation in response
Several N-nitroso compounds have been tested in different
animal species. DEN, tested in more than 20 species including
primates, induced tumours of the liver in all of them, together with
various tumours of other organs. In some cases, there have been marked
differences between species in response to N-nitroso compounds. For
example, nitrosoheptamethyleneimine produced squamous carcinoma of the
lung in rats (Lijinsky et al., 1969), the same effects in European
hamsters, but tumours of the forestomach and the lung in Syrian
hamsters (Lijinsky et al., 1970). In all three species, this compound
also induced oesophageal tumours. Nitrosomethylurethane induced
tumours of the pancreas in guineapigs (Druckrey et al., 1968) and
forestomach carcinomas in rats. Bis-2-hydroxypropyl-nitrosamine
produced pancreatic tumours in Syrian hamsters. These results show
the difficulty of attributing, with certainty, any particular tumour
response of man to a particular N-nitroso compound.
7.2.2.2 Intraspecies variation in response
Kuwahra et al. (1972) administered DMN orally or by subcutaneous
or intraperitoneal injections, to 8 to 12-week-old, male and female
mice of the DDD, BALB/C, and SJL/J strains. Tumours were found mainly
in the retroperitoneum and abdominal cavity and the incidence and
distribution were little affected by the strain of mouse but markedly
by the route of administration. Clapp et al. (1971) noted that DEN
induced forestomach and oesophageal squamous cell carcinomas in both
BALB/C and RF/Un strains of mice but induced liver haemangiosarcomas
in the former and hepatomas in the latter. In addition, DEN induced a
high incidence of lung tumours in RF mice but a very low incidence in
BALB/C mice. In both strains, DMN induced lung adenomas and liver
haemangiosarcomas. Tissue sensitivity did not appear to be related to
the spontaneous tumour incidence of the strain.
7.2.2.3 Dose-response relationships of N-nitroso compounds
Druckrey et al. (1963b) were the first to study the dose-response
relationships of N-nitroso compounds in relation to minimum effect
doses. They concluded, from studies in rats given DEN, that the
carcinogenic effect was related to dose and induction time in such a
way that d.t2.3 = constant, where d represents the daily dose and
t the induction time. Even a low dose of 0.15 mg/kg body weight
resulted in liver carcinomas in 27 out of 30 surviving animals. The
average induction time was 609 ± 38 days. The lowest dose studied
(0.075 mg/kg body weight) produced liver and oesophageal tumours in
all four surviving animals with an induction time of 830 days.
Mohr & Hilfrich (1972) gave a single intravenous injection of DEN
to rats at 8 dose levels between 1.25 and 160 mg/kg body weight. A
dose-response relationship was noted; at the lowest dose of 1.25
mg/kg, only one kidney tumour was observed in 20 treated rats, but, at
higher doses, the incidence of these tumours increased. Single dose
experiments with ethylnitrosourea in a transplacental carcinogenesis
experiment also showed that the incidence of induced tumours (mainly
of the brain and nervous system in the progeny) was directly
proportional to the dose of the carcinogen. Four doses between 1 and
50 mg/kg were used and even in the lowest dose group 36/41 animals in
the progeny died with tumours (Swenberg et al., 1972). Terracini et
al. (1967) fed rats concentrations of DMN ranging from 2 to 50 mg/kg
diet. At 2 and 5 mg/kg the incidence of liver tumours in the survivors
was 1/26 and 8/74, respectively, at 60 weeks. At 20 and 50 mg/kg,
liver tumours were observed in more than 60% of the test animals.
From this study, it can be concluded that a dietary level of DMN of
5 mg/kg is still carcinogenic in the rat.
A current dose-response study on rats receiving oral doses of
N-nitrosopyrrolidine has been reported by Preussmann (unpublished
data).a Liver tumours were induced in groups receiving 10, 3, and
1 mg/kg body weight per day, respectively, but not in a group
receiving 0.3 mg/kg per day.
Clapp & Toya (1970) gave male, RF mice DMN in the drinking water
for various lengths of time, with cumulative doses ranging from 87 to
243 mg/kg body weight. The incidence of lung adenomas in all treated
groups was higher than that in the untreated control groups. Whereas,
apparently, the incidence of hepatocellular tumours was not affected,
the incidence of liver haemangiosarcomas increased in the higher dose
groups and reached a maximum of 96%. Bertram & Craig (1973) using 2
groups of 100, C57BL/6 mice found that the incidence of bladder
tumours fell from 80% in animals given 30 mg of nitrosodi- N-
butylamine per kg/body weight per day to 36% at 7.5 mg/kg body weight
per day. Sander & Schweinsberg (1973) noted that an increasing
incidence of tumours of the oesophagus and forestomach was induced in
NMRI mice by adding a given dose of methylbenzylnitrosamine to the
drinking water at various times to provide total doses ranging from
1.4 to 44 mg/kg body weight.
a Paper presented at the Proceedings of the Second International
Symposium on Nitrite in Meat Products, Zeist, September 1976.
In studies conducted by Vesselinovitch (1969), DMN was
administered repeatedly by intraperitoneal injection to 73 male and 54
female C57BL x C3H mice starting at 7 days of age. The injections were
given at 3 day intervals for a total of six doses of 1, 2, or 4 mg/kg
body weight. Mice killed at 66 weeks of age showed an increase in the
incidence of hepatomas, hepatocarcinomas, lung adenomas, and
haemangiomas as the dose increased. The incidence of liver tumours was
higher in males (75%) than in females (28%).
Tomatis & cefis (1967) gave a dose of 3.6 mg of DMN by stomach
tube to one group of 30 Syrian golden hamsters in 3 administrations of
1.6 mg, 1.0 mg, and 1.0 mg over a 5-week period. A second group
received a single dose of 1.6 mg. Both dosing regimes produced liver
cell carcinomas and a few cholangiomatous lesions but no kidney
tumours. Montesano & Saffiotti (1968) administered 12, weekly,
subcutaneous injections of DEN at 0.5, 1.0, 2.0, or 4.0 mg to 4 groups
of 36 Syrian golden hamsters. The results demonstrated a positive
dose-response relationship for tumour induction in the upper
respiratory tract (nasal cavities, larynx, and trachea). The incidence
of nasal cavity tumours, which developed early, varied from 6/35 to
27/36. The incidence of tumours of the larynx varied from 6/35 to
26/36 and that of tumours of the trachea from 29/35 to 35/36.
7.2.2.4 Tumour induction by combined administration of nitrites
and amines or amides
Carcinogenic effects following the combined administration of
secondary and tertiary amines or amides with nitrite have been
reported. Tumours were not observed when sodium nitrite or the amine
or amide were given singly. Tumours of the same type occurred at the
same site when the N-nitroso compound, believed to be formed from
the nitrite and amine or amide, was given as a positive control. In
some of the experiments, the nitrite was given in the drinking water
and the amine or amide in the food. In others, the compounds were
combined in the same medium (drinking water or food).
Preussman (1975) reviewed studies in which tumours that had been
produced at certain sites by the oral administration of combinations
of amines and nitrite, were compared with the tumours induced by the
corresponding N-nitroso compounds. Table 4 has been adapted from
Preussman (1975) and updated.
Table 4. In vivo formation of N-nitroso compounds following oral administration of sodium nitrite and amino
compounds as demonstrated by specific carcinogenesisa
sodium nitrite + amine Expected tumour Reference
oral administration Observed tumour site for
amino compounds site corresponding
N-nitroso compound
Rat
diethylamine -- liver Druckrey et al.
(1963b)
Sander et al.
(1968)
Sander (1971a)
triethylamine -- liver (for Schweinsberg &
diethylnitrosamine Sander (1972)
morpholine liver, kidney, lung liver Sander & Burkle
N-methylbenzylamine oesophagus oesophagus (1969), Sander
(1971c)
piperidine -- oesophagus
liver
N-methylanaline oesophagus, nasal oesophagus
cavity
N-methylcyclohexylamine oesophagus oesophagus Sander (1971a)
Table 4 (Cont'd)
sodium nitrite + amine Expected tumour Reference
oral administration Observed tumour site for
amino compounds site corresponding
N-nitroso compound
N-methylbenzylamine oesophagus oesophagus
phenylbenzylamine negative untested
N,N'-dibenzylethylenediamine negative untested
indole negative untested
morpholine liver (kidney) liver (kidney) Shank & Newberne
(1972)
aminopyrine liver liver (for Lijinsky et al.
(Pyramidon) dimethylnitrosamine) (1973c)
heptamethyleneamine lung lung
oesophagus oesophagus
aminopyrine liver liver Taylor &
(for DMN) Lijinsky
(1975)
oxytetracycline liver (for DMN) liver Greenblatt et al. (1973)
proline negative untested
Table 4 (Cont'd)
sodium nitrite + amine Expected tumour Reference
oral administration Observed tumour site for
amino compounds site corresponding
N-nitroso compound
hydroxyproline negative untested
arginine negative untested
N-methylacetamide negative forestomach
N-methylurethane negative forestomach
N-ethylurethane negative (carcinogenic Sander (1971b)
following
i.v. administration)
acetanilide negative untested
glycylglycine negative untested
N-methyluracil negative untested
N-methylguanidine negative untested
N-phenylurea negative (carcinogenic Sander (1971b)
following
s.c. administration)
N-methylthiourea negative untested
N-methylurea brain, nervous brain, nervous
system, kidney system, kidney
Table 4 (Cont'd)
sodium nitrite + amine Expected tumour Reference
oral administration Observed tumour site for
amino compounds site corresponding
N-nitroso compound
N-ethylurea brain, nervous brain, nervous
system, kidney system, kidney
N'N'-dimethylurea brain, nervous brain, nervous Sander (1971b)
system, kidney system, kidney
imidazolidinone kidney (carcinogenic Sander & Burkle
following (1971)
s.c. administration)
ethylurea (during brain, nervous brain, nervous Ivankovic &
pregnancy, system system (in Preussmann
transplacental) (in descendants) descendants) (1970); Osske
et al. (1972)
Mouse
dimethylamine lung (adenoma)
piperazine lung (adenoma) lung (adenoma) Greenblatt et al. (1971)
morpholine lung (adenoma) lung (adenoma)
N-methylaniline lung (adenoma) lung (adenoma)
morpholine lung (adenoma) lung (adenoma)
Table 4 (Cont'd)
sodium nitrite + amine Expected tumour Reference
oral administration Observed tumour site for
amino compounds site corresponding
N-nitroso compound
N-methylbenzylamine oesophagus oesophagus Sander (1971a)
forestomach forestomach
piperazine lung (adenoma) lung (adenoma) Greenblatt &
Mirvish (1972)
N-methylurea lung (adenoma) lung (adenoma) Mirvish et al. (1973b)
N-ethylurea lung (adenoma) lung (adenoma)
a Adapted from Preussmann (1975)
7.2.2.5 Dose-response relationship for combinations of nitrite
and amines
Greenblatt & Mirvish (1972) conducted a study in which groups of
40, male, strain A mice, given 0.69-18.75 g of piperazine per kg of
food and 0.05-2.0 g of sodium nitrite per litre of drinking water for
20-25 weeks, were killed 10-13 weeks later. The yield of lung adenomas
was statistically significantly greater than in untreated controls,
when doses as low as 0.69 g piperazine/kg plus 1.0 g sodium nitrite/
litre, or 6.25 g piperazine/kg plus 0.25 g sodium nitrite/litre were
given. In the animals given 6.25 g piperazine per kg of food, plus
2.0 g sodium nitrite per litre of water, 39 out of 40 had adenomas,
in contrast with 5 out of 39 controls. A progressive decrease in the
incidence of adenomas was seen with reduction in the nitrite level.
On the other hand, when the nitrite level was maintained at
1.0 g/litre, the tumour incidence increased marginally when the dose
of piperazine was increased from 0.69 g to 18.75 g/kg of food.
Nitrite and piperazine administered alone yielded negative results.
When various concentrations of nitrite and morpholine (up to
1000 mg/kg) were fed to groups of Sprague-Dawley rats, the
development of hepatocellular carcinomas and angiosarcomas identical
to those produced by N-nitrosomorpholine was noted (Newberne &
Shank, 1973).
A 2-3% incidence of hepatomas was induced with only 5 mg of
morpholine plus 1000 mg of sodium nitrite per kg of food, or 1000 mg
of morpholine plus 50 mg sodium nitrite/kg. The tumour incidence was
98% when the concentration of both components was 1000 mg/kg, and 0%
when diet alone was given.
7.2.2.6 Transplacental carcinogenesis
Induction of neoplasms in offspring as a result of prenatal
exposure to various N-nitroso compounds and related substances has
been reported in different animal species including the rat, mouse,
golden hamster, guineapig, rabbit, dog, and monkey (Magee et al.,
1976). The various routes of administration (subcutaneous,
intraperitoneal, intravenous, oral, and inhalation) were equally
effective. However, a critical factor was the time of treatment during
gestation.
Since many of these substances appeared to be metabolically
activated to exert their carcinogenic action, the lack of an adequate
metabolic system during the first period of pregnancy may explain the
failure to observe tumours, when exposure was limited to this period.
DMN induced tumours in the offspring only when administered during the
last days of pregnancy (Alexandrov, 1968a). The data of Magee (1972)
are in keeping with these findings; the formation of 7-methylguanine
in the nucleic acids of fetal rat tissues was detected following
treatment with DMN on the 21st day of pregnancy but not on the
15th day.
When considering the effect of different acyl alkyl nitrosamides,
N-ethyl- N-nitrosourea was found to be more active than its methyl
analogue (Alexandrov, 1969b; Ivankovic & Druckrey, 1968).
The sensitivity of the nervous system at various stages of
prenatal development was examined in rats and golden hamsters by
Ivankovic & Druckrey (1968) by means of single intravenous injections
of N-ethyl- N-nitrosourea on different days during gestation. A
high incidence of tumours was observed after treatment on the 18th day
or shortly before delivery (21st day) but none developed when the
mother animals were treated before the 12th day. A positive dose-
response relationship was obtained with a single dose in the range of
5 to 80 mg/kg bodyweight on the 15th day. A single dose as low as
2 mg/kg was sufficient to produce malignant, neurogenic tumours in
2/25 newborn whereas in adult animals an effect was produced in 50%
of the animals by a single dose at 160 mg/kg. Thus a 50-times higher
sensitivity was demonstrated for the fetal nervous system. Similar
results were obtained by Swenberg et al. (1972) in Sprague-Dawley and
Fisher rats using a dose of N-ethyl- N-nitrosourea as low as
1 mg/kg. In a recent study on rats, Maekawa & Odashima (1975) explored
the effects of subcutaneous injections of N-butyl-1-nitrosourea on
embryonal, fetal, and newborn nervous systems. Treatment during early
gestation led only to the death of the embryo. When the compound was
given in the middle of the pregnancy (8-14 days), a high incidence of
nervous system tumours and pituitary tumours was found in the
offspring. Treatment late in pregnancy (15-21 days) led to the
development of nervous system tumours in 33/36 offspring.
When lactating animals were given the compounds, tumours were
induced in 19/39 of the offspring, the target organs being mainly the
testes and uterus.
Tomatis (1977) reported an increased incidence of cancer in the
descendants (second and third generation) of transplacentally treated
rats. In certain cases, the tumour-producing doses were lower than
those for adult animals. The target organs were usually similar in
fetal, newborn, and adult animals but the nervous system has been
shown to be highly sensitive to certain compounds, notably the
nitrosoureas. The induction time for transplacental tumours may be
shorter than that in adults.
7.2.2.7 Morphological studies
Morphological events associated with the development of hepatic
tumours, following exposure to N-nitroso compounds, were studied by
Rabes et al. (1970), Scherer et al. (1972), and Schmitz-Moorman et al.
(1972). Schmitz-Moorman et al. (1972) followed the carcinogenesis
induced by DMN in rat liver, using histological and histochemical
procedures. In the initial phase, there were vacuolar changes
accompanied by a decrease in the RNA and glycogen contents. In the
second phase, glycogen storage was noted while in the third phase,
basophilic cells with atypical nuclei were observed and could not be
distinguished from microcarcinomas. The RNA content of these cells was
substantially higher. The activities of acid phosphatase (3.1.3.2),
succinic dehydrogenase (1.3.99.1) and glucose-6-phosphatase (3.1.3.9)
substantially decreased. In the last carcinogenic stage, dramatic
changes were noted in several enzymes (Jennissen et al., 1971).
Early changes in the lung tissue of mice exposed to carcinogenic
doses of DMN were reported by Calafat et al. (1970) and DEN-induced
alterations in the respiratory tract of hamsters were reported by
Althoff et al. (1971). Greenblatt & Rijhsinghani (1969) compared the
cytopathological changes induced by DEN and DMN in the nasal
epithelium of hamsters; other light microscopic studies have been
published by Bertram & Craig (1972), Boyland et al. (1968), Hicks et
al. (1973), and Terracini et al., (1967). The histological appearance
of tumours under the electron microscope has been described by
Kirkland & Pick (1973). Veno-occlusive disease in the liver of rats
given DMN was reported by Butler & Hard (1971) who had also studied
the effects of this compound on rat testes (Hard & Butler, 1970c).
Other studies, by light microscopy, to observe early changes
related to the formation of neoplasms induced in the kidneys of rats
treated with DMN, were reported by Benemanski & Litvinov (1969), Hard
& Butler (1970a, 1970b), and Hard et al. (1971).
Electron microscopic studies of DMN-induced liver and kidney
tumours have been reported in rats (Bhathal & Hurley, 1973; Geil et
al., 1968; Hard & Butler, 1971a, 1971b, 1971c; Ireton et al., 1972;
Jasmin & Cha, 1969; Svoboda & Higginson, 1968) and in mice (Takayama,
1968). Treatment with DEN resulted in ultrastructural changes in the
lungs of hamsters (Stracks & Feron, 1973) and in the liver of monkeys
(Williams, 1970) and rats (Bader et al., 1971; Bruni, 1973). Early
morphological changes in the bladder of rats exposed to N-butyl- N-
butanol-4-nitrosamine were reported by Riedel & Piper (1973).
7.2.2.8 Biochemical mechanisms
Extensive work on the biochemical mechanisms of carcinogenesis
produced by N-nitroso compounds has been reviewed by Magee & Barnes
(1967) and more recently by Magee et al. (1976). Alkylation of nucleic
acids by N-nitroso compounds or their metabolites has been
investigated extensively and has been suggested as the mechanism of
carcinogenicity (Krüger, 1972, 1973; Lijinsky et al., 1973b; Swann &
Magee, 1971; Takayama & Muramatsu, 1969). In early studies, it was
thought that the 7 position of guanine was the significant site of the
reaction.
Following the important discovery by Loveless and Hampton (1969)
of the O,6-alkylation of deoxyguanosine by nitrosomethylurea (NMU)
and the implications of this reaction in terms of carcinogenicity,
O'Connor et al. (1973) estimated the amount of methylation at the
O,6-position of guanine DNA isolated from animals treated with DMN.
This base accounted for 4-6% of the methylation after DMN treatment.
O,6-methylguanine was lost (by "excision") from DNA with a half life
of approximately 13 h. The excision of the abnormal components of DNA,
O,6-methylguanine, and the unstable acid-labile products, may be
important processes in liver carcinogenesis. O'Connor et al. (1973)
suggested that events leading to the development of tumours may be
related to the efficiency of the cellular excision system for certain
products of alkylation rather than to the level of alkylation obtained
at a particular site.
7.2.2.9 Interaction with various chemical factors
Several studies are available on the combined effects of
N-nitroso compounds and other carcinogens. Schmähl et al. (1963)
showed that combined oral administration of the hepatocarcinogens DEN
and N,N-dimethyl-4-(phenylazo)benzenamine (4-dimethyl-
aminoazobenzene) significantly reduced the time for tumour induction
and that only 66% of the total dose administered when single compounds
were used, was necessary for tumour induction. Takayama & Imaizumi
(1969) also demonstrated synergism with the combined administration of
DMN and 4-dimethylaminoazo-benzene. Liver tumours were induced in rats
by sequential administration of the two carcinogens in doses that did
not induce tumours when each carcinogen was given alone and the tumour
induction time was reduced. The syncarcinogenic effects of a single
dose of a combination of DEN and carbon tetrachloride were observed by
both Pound et al. (1973) and Schmähl et al. (1965). The incidence of
liver cancer increased and the induction time decreased in combined
treatments compared with treatment with the individual compounds.
Schmähl (1970) administered hepatocarcinogens (DMN, DEN,
nitrosomorpholine, and 4-dimethylaminoazobenzene) to rats in such low
daily doses that the administration of any one of the compounds alone
did not lead to tumours during the lifetime of the animals. However,
combined administration induced tumours in 43% of the treated animals.
Combined administration to rats of DMN plus 1,2-dihydro-3-methyl-
benz[j]aceanthrylene (3-methylcholanthrene) did not increase the
number of liver tumours compared with that induced by DMN alone but
resulted in tumours in the lungs, that were not seen in the treatment
with DMN alone (Hoch-Ligeti et al., 1968).
1-Isothiocyanatonaphthalene (1-napthyl-isothiocyanate) and
3-methyl-cholanthrene failed to inhibit the hepatocarcinogenic
effects of DEN (Makiura et al., 1973). Similarly, local treatment of
the glandular mucosa of the stomach of rats with 4-nitroquinoline-
1-oxide or p-dimethylaminoazobenzene did not have any effect on
the production of liver or oesophagal tumours due to oral
administration of DEN (Odashima, 1969). It has been reported that
ionizing radiation did not increase tumour incidence in animals
exposed to nitrosamines (Flaks et al., 1973; Schmähl et al., 1966).
The effect of many other substances on nitrosamine carcinogenesis
has been studied. For example, various dusts including aluminium (III)
oxide, magnesium (II) oxide, and carbon had little effect on the
respiratory carcinogenesis of DEN in hamsters (Stenback et al., 1973)
but other studies on hamsters demonstrated that iron (III) oxide
markedly increased the incidence of respiratory tract tumours induced
by DEN (Feron et al., 1972). The synergistic effects of various
substances on respiratory carcinogenesis in hamsters given N-nitroso
compounds were reviewed by Montesano (1970).
The effect of noncarcinogenic chemicals on the incidence of other
tumours induced by N-nitroso compounds has also been studied. In
rats given DEN, liver tumour incidence was decreased by the
administration of calcium heparin (Platt & Hering, 1973),
aminoacetonitrile (Hadjiolov, 1971), and reserpine (Lacassagne et al.,
1968). Lactoflavin, nicotinamide, or 2,6-bis-(diethanolamino)-4,8-
dipiperidino-pyrimido [5,4-d] pyrimidine (dipyridamole) (Schmähl &
Stackelberg, 1968) and hydrocortisone (Schmähl et al., 1971) did not
influence the incidence of liver tumours induced in rats by DEN.
Tryptophan was shown to inhibit the production of liver tumours, but
not bladder tumours in rats exposed to N-nitrosodibutylamine
(Okajima et al., 1971). N-(2-chloroethyl)- N (phenylmethyl)
benzenemethanamine (dibenamine) did not have any effect on the
incidence of oral, pharyngeal, or oesophageal tumours in DEN-treated
rats, but did significantly reduce the number and severity of hepatic
neoplasms (Weisburger et al., 1974). In mice, treatment with
phenobarbital decreased the toxicity and carcinogenicity of DEN (Kunz
et al., 1969).
7.2.2.10 Miscellaneous modifying factors
Nasal infection of mice with the influenza viruses PR8/FIK and
A2/Bethesda 10/63 followed by treatment with DEN significantly
increased lung tumour incidence in comparison with that in mice
treated only with DEN (Schmidt-Ruppin & Papadoupulu, 1972). The Motol
virus has been shown to increase hepatic carcinoma in mice given DEN
(Kordac et al., 1969). Rats with chronic respiratory disease showed an
increased lung tumour incidence when given nitrosoheptamethylenemine
compared with germ-free or specific pathogen-free animals (Schreiber
et al., 1972).
Feeding with a protein-deficient diet protected rats against the
lethal and hepatotoxic effects of DMN (McLean & Verschuuren, 1969) but
increased the incidence of renal carcinomas (McLean & Magee, 1970).
Low protein diets and low zinc intake failed to influence the
incidence of oesophageal tumours in rats treated with N-methyl- N-
nitroso-'pentyl' amine (Van Rensburg, 1972). A single intraperitoneal
injection of DMA induced nasal tumours in rats, previously starved for
48 h (Noronha & Goodall, 1972).
The carcinogenic activity of nitroso- N-(hydroxybutyl)
butanamine (butyl-( N-hydroxybutyl) nitrosamine) appears to be
influenced by sex hormones (Bertram & Craig, 1972). Tumours developed
much earlier in male, than in female mice. This difference was
abolished, however, if the males were castrated or, conversely, if the
females were treated with testosterone.
Surgical manipulation of experimental animals may influence the
induction of tumours by N-nitroso compounds. This has been shown for
partial hepatectomy (Craddock, 1971; Grunthal et al., 1970; Rabes et
al., 1971) unilateral nephrectomy (Ito et al., 1969) and ureter
ligation (Ito et al., 1971).
7.2.3 Embryotoxicity and teratogenicity
Whereas nitrosamines are reported to have toxic and lethal
effects on the embryo, usually at dose levels that are toxic to the
mother animals, nitrosamides ( N-ethyl-and N'methyl urea) bring about
malformations of several of the organs and systems of the developing
organisms at levels not toxic to the pregnant animal. Animal
experiments have shown that immature tissues are especially sensitive
to these compounds. Dose-response relationships have been established
and the existence of a noneffect level has been indicated.
DMN, administered orally at 30 mg/kg to pregnant rats, produced
increased prenatal mortality (Alexandrov, 1967). Toxic effects on the
embryo were noted following intravenous or intraperitoneal
administration of DMN at various times during gestation but no
teratological abnormalities were observed. Similar results were
observed with DEN and with nitroso- N,N-bis butanamine (nitrosodi-
N-butylamine) in rats (Alexandrov 1967), and with DEN in hamsters
(Pielsticker, 1967). The aromatic nitroso compounds such as
nitrosomethylbenzenamine (nitrosomethylaniline) were toxic to the
embryo and had a mild teratogenic action when given to rats at the
maximum tolerated doses (Alexandrov, 1968a, 1968b).
Treatment of pregnant rats with a single dose of 10-30mg of
nitrosomethylurea per kg body weight on day 9 of gestation produced
anophthalmia, hydrocephaly, exencephaly, and occasionally spina bifida
while treatment on days 12-15 produced microcephaly (Alexandrov,
1969a; Koyama et al., 1970; Napaikov, 1971; Von Kreybig, 1965a, 1965b;
Von Kreybig & Schmidt, 1966, 1967).
The teratogenic action of nitrosoethylurea in rats was described
by Druckrey et al. (1966) and Ivankovic & Druckrey (1968) who noted
that oligodactylia and syndactylia of the fore and hind limbs were
dose-related. Von Kreybig & Schmidt (1967) and von Kreybig (1968)
confirmed these effects and also found brain anomalies. Napalkov
(1971) and Wechsler (1970, 1971) reported similar results. The
teratogenic and embryotoxic effects of N-nitroso compounds including
the pathogenesis of brain lesions were reviewed by Wechsler (1973).
7.2.4 Mutagenicity
The mutagenicity of N-nitroso compounds was reviewed by Magee &
Barnes (1967) and more recently by Montesano & Bartch (1976). Until
recently, the data available were mainly concerned with the
nitrosamides, the data on nitrosamines being limited to test systems
which do not take into consideration the metabolic activation of these
compounds by mammalian enzymes. As with other biological effects,
there is a clear distinction between the mutagenic action of
nitrosamides and nitrosamines. Nitrosamides were found to be mutagenic
to almost all genetic indicators; this was attributed to the
nonenzymatic formation of alkylating reactants.
On the other hand, nitrosamines have been reported, prior to
1970, to have a much more limited range of mutagenic activity; they
were found to be mutagenic in tests with Drosophila melanogaster
(Pasternak, 1964) but no such activity was observed in assays in
which bacteria, yeast, or fungi were used. Malling (1966) showed that
DMN and DEN were mutagenic for Neurospora crassa when the conidia
were suspended in Udenfriend's hydroxylating model system and the
treatment carried out under conditions believed to give rise to the
same metabolic products as those formed by the action of liver enzymes
in rat and mouse. Garbidge & Legator (1969), using the host-mediated
assay, were the first to show that DMN, administered to mice, induced
mutations in Salmonella O,phimurium, which had been injected
beforehand and was then reisolated from the peritoneal cavity. Most of
the mutagenicity assays were carried out using bacteria or fungi as
genetic indicators in the presence or absence of a microsomal
activation system. Only a few data are available on the mutagenicity
of DMN in mammalian systems such as the dominant lethal test or
cytogenetic studies. However, in general, the last two systems have a
very low sensitivity and false negatives can easily result with
N-nitroso compounds. The early observation of Pasternak (1962)
that DMN induced lethal mutations in Drosophila melanogaster
demonstrated the possible value of this system for testing the
mutagenicity of compounds that require metabolic activation.
The mutagenicity of certain nitrosated pesticides, herbicides,
and primary amines has been examined. The N-nitrosated derivatives
of the pesticides propoxur and carbaryl, which are aryl- N-methyl
carbamates, and of the herbicide benzothiazuron, a methylurea
derivative, induced mitotic gene conversion in Saccharomyces
cerevisiae (Sibert & Eisenbrand, 1974). N-nitrosocarbaryl was
also found to be mutagenic in Haemophilus infiuenzae (Elespuru
et al., 1974). Endo et al. (1973) screened a number of
N-nitrosated guanidine derivatives for their ability to induce
base pair substitutions in Salmonella typhimurium and a mutagenic
effect was observed for many of these compounds; nitrosated
methylguanidine was the most potent.
The mutagenicity of sodium nitrite, DMA, methylurea, and
ethylurea given orally to mice, has been demonstrated in a host-
mediated assay using a strain of Salmonella typhimurium as an
indicator (Couch & Friedman, 1975). When combined with sodium nitrite,
both ethylurea and methylurea had a greater effect than DMA.
Other properties of N-nitroso compounds such as cell
transformation in vitro and their influence on DNA repair mechanisms
are being investigated, but as yet, there are not sufficient data for
their evaluation.
8. EFFECTS OF NITRATES, NITRITES AND N-NITROSO COMPOUNDS ON MAN
8.1 Nitrates and Nitrites
In the erythrocytes of healthy individuals, the process of
methaemoglobin formation and reduction is continuous. The mean content
of methaemoglobin in healthy populations is usually reported to be
below 2% of the total haemoglobin concentration (Committee on Nitrate
Accumulation, 1972; Gobbi et al., 1974; Smith, 1972). However,
Goldsmith et al. (1975) recently found mean levels in Californian
populations ranging up to 2.11% with 1% of the adults and 8% of
infants having methaemoglobin levels exceeding 4%. Higher values are
found in premature than in full-term infants and levels in infants are
higher than those in older children and adults (Kravitz et al., 1956).
At a level of about 10%, methaemoglobinaemia may produce symptomless
cyanosis, whereas levels of 20-50% are associated with conspicuous
cyanosis accompanied by hypoxic signs and symptoms, such as weakness,
exertional dyspnoea, headaches, tachycardia, and loss of consciousness
(Arena, 1970; Committee of Nitrate Accumulation, 1972; Jaffé & Heller,
1964). The lethal concentration of methaemoglobin is not known, but
death may occur at levels exceeding 50% (Committee of Nitrate
Accumulation, 1972).
8.1.1 Epidemiologicai studies
The National Academy of Sciences, USA (Committee on Nitrate
Accumulation, 1972) recently reviewed about 350 cases of
methaemoglobinaemia in the USA and about 1000 cases in Europe that
were reported to be associated with the intake of nitrates in well
water or in food. There were 41 fatalities in the USA and about 80 in
Europe. Only one case of infant methaemoglobinaemia resulting from the
consumption of water from a municipal water supply was reported in the
USA (Vigil et al., 1965).
8.1.1.1 Exposure through water
The toxicity to man of nitrates in water was first reported by
Comly (1945). He noted high levels of methaemoglobin and the
associated signs of nitrate toxicity in 2 infants who had consumed
water containing high concentrations of nitrates (619 and
388 mg/litre). Since then, several epidemiological and case studies
have been carried out in various parts of the world, particularly in
areas with naturally high nitrate levels in water.
Robertson & Riddell (1949) reported 10 cases in which infants
receiving powdered milk preparations made with well waters with
nitrate concentrations exceeding 75 mg/litre had blood levels of
methaemoglobin ranging from 5 to 50% of total haemoglobin. Two of
the infants, whose dried milk preparations had been reconstituted
with well waters containing nitrate levels of 1200-1300mg/litre, had
methaemoglobin levels of 25% and 44% of total haemoglobin,
respectively; both cyanosed rapidly and died before therapy could be
applied.
In the state of Minnesota (USA), Bosch et al. (1950) reported 139
cases of infant methaemoglobinaemia due to the ingestion of well water
with a high nitrate content (over 89 mg/litre); the mortality rate was
10%. In Kansas, 13 cases of infant methaemoglobinaemia including 3
deaths caused by drinking well water, were reported between the early
1940s and 1950 (Walton, 1951). Many other cases in the USA have been
reported by this author. On the other hand, infant methaemoglobinaemia
was absent in urban areas of New York and the province of Ontario,
Canada where nitrate levels in water were low (Ciavaglia & Thompson,
1969). Knotek & Schmidt (1964) reported 115 cases of methaemo-
globinaemia from a total of 5800 children born in central
Czechoslovakia between 1953 and 1960. Of these, 8% were fatal, 52%
were severe, and 40% were mild. Most deaths were associated with the
consumption of water containing nitrate concentrations ranging from
70 to 250 mg/litre. In these cases, methaemoglobinaemia was
invariably associated with the consumption of infant milk
preparations, which contained microorganisms such as B. subtills,
capable of reducing nitrates to nitrites. These workers reported the
inhibitory effect of buttermilk on this conversion. They attributed
this effect to the presence of Streptococcus lactis which
produces the antibiotic nisin and is capable of preventing the growth
of the spores of B. subtilis.
Shuval & Gruener (1972) studied communities with various
concentrations of nitrates in the drinking water, in Israel. Infants
from rural communities with reported nitrate levels of 50 --
90 mg/litre in the water supplies were compared with controls where
the average level was 5 mg/litre. They did not find any definite cases
of methaemoglobinaemia nor were there any significant differences in
the methaemoglobin levels. However, there was widespread consumption
of citrus juices and the water consumption in infant milk preparations
was low (94% of the infants studied were breast fed or received whole
cow's milk). Soviet literature contains information concerning a
comparatively small number of cases of symptomless methaemoglobinaemia
caused by nitrates in water (Diskalenko, 1969; Motylev, 1969). In
children whose drinking water contained a high level of nitrates, the
methaemoglobin level did not usually exceed 10% although higher levels
were found occasionally. Sattelmacher (1962) and Simon et al. (1964)
compiled 1060 and 745 cases, respectively, of infant
methaemoglobinaemia due to nitrate-contaminated water in the Federal
Republic of Germany. Most of the cases were associated with water
from private wells and in 84-90% of the cases the water contained
nitrate concentrations exceeding 100 mg/litre (although a few cases
of methaemoglobinaemia were reported with water containing less than
50 mg/litre).
Commoner et al. (1972) found small, but statistically
significant, subclinical elevations in methaemoglobin levels in adults
exposed to high intakes of nitrates in a rural area when compared with
an urban control population. There is also a small amount of data
showing that under similar conditions, pregnant women in rural areas
have higher methaemoglobin levels than those in urban areas. This is
of particular interest since previous workers have reported an
increased susceptibility to nitrates in pregnant women. (Skrivan,
1971). Thus, there is concern over the effects on the fetus of the
general lowering of oxygen tension. Gelperin et al. (1971) recently
reported the presence of methaemoglobinaemia in a newborn infant,
presumably exposed to nitrates transplacentally. In this study, 72
mothers and infants tested were exposed to water with nitrate
concentrations ranging from 28 to 45 mg/litre over a 2-month period.
During the 2 weeks of maximum concentration (45 mg/litre), the average
methaemoglobin levels were 1.18% for mothers and 1.91% for newborns
with those of one mother and one infant rising to 6.39% and 5.87%,
respectively.
Children aged 12-14 years who drank water with a nitrate level of
105 mg/litre were noted to have slightly delayed reactions to light
and sound stimuli combined with a mean methaemoglobin level of 5.3% in
comparison with control children drinking water with a nitrate level
of 8 mg/litre whose methaemoglobin levels average 0.75% (Petukhov &
Ivanov, 1970).
8.1.1.2 Exposure through vegetables
Cases of methaemoglobinaemia, some resulting in death, have been
observed following the consumption of spinach. Hölscher & Natzschka
(1964) reported 2 cases in young infants (aged 2 and 3.5 months) who
had eaten spinach purée. Fresh spinach from the same source contained
only traces of nitrates but had nitrite ion levels of 2180 mg/kg.
Fourteen further cases of methaemoglobinaemia in infants (aged 2-10
months) were reported from the Federal Republic of Germany (Sinios &
Wodsak, 1965). Unprocessed spinach was used almost exclusively in the
preparation of the infants' meals and preparation took place at least
24 h before the mealtime. Since in most cases some of the same spinach
had been eaten 24 and 48 h before without causing illness, the authors
assumed that the nitrites were formed within the final 24 h of
storage. Information on 7 cases showed that the mother tasted the
spinach before feeding the child and no change in taste was apparent.
None of the children refused the meal which caused the poisoning.
Conversion of nitrates to nitrites in fresh spinach was demonstrated
by Schuphan (1965) (section 4.2.1). Keating et al. (1973) reported a
case of methaemoglobinaemia in an infant given carrot juice. Other
cases of food-induced methaemoglobinaemia have recently been reviewed
by Luhrs (1973).
8.1.1.3 High accidental exposures through food
Certain meat products contain nitrates and nitrites. Normally, no
adverse effects result from consumption of such products. However, in
1955, an outbreak of 10 cases of methaemoglobinaemia in children
occurred in New Orleans, USA, attributed to the consumption of large
amounts of nitrites in sausage meats (Orgeron et al., 1957). Further
studies revealed that the meats had nitrite concentrations of more
than 200 mg/kg and that some had levels as high as 6570 mg/kg. Singley
(1962) reported 3 cases of methaemoglobinaemia resulting from the
consumption of fish, that had been adulterated with sodium nitrite.
One patient died and it was assessed that he had consumed
approximately 33 mg sodium nitrite/kg body weight. Other cases of
poisoning involving the consumption of nitrite-treated sausage and
frankfurters were reported by Bakshi et al. (1967) and Henderson &
Raskin (1972).
8.1.1.4 Ambient air exposures
Effects on man of nitrate aerosols in ambient air were not
considered by the Task Group. However, because of the recent concern
with the role of nitrates in urban air pollution, it may be of
interest to briefly summarize the current information on this problem.
Airborne nitrates may act as respiratory irritants (Knelson & Lee,
1977) and a recent study conducted by the US Environmental Protection
Agency in the New York-New Jersey metropolitan area showed that
increased asthmatic attacks were significantly associated with
elevated levels of suspended nitrates in six of the seven communities
studied. No such effect was observed with nitrogen dioxide. Another
study in two south-eastern communities in the USA showed some evidence
that a combination of suspended nitrates and suspended sulfates
increased the risk of asthma attacks more than either pollutant did
alone. Because of the present difficulties in measuring suspended
nitrates, these results should be considered as qualitative instead of
quantitative (French et al., unpublished data)a.
8.1.2 Factors involved in susceptibility to nitrates
The work of Marriott et al. (1933), who investigated the acidity
and bacterial flora of 200 infants suffering from diarrhoea, supports
the hypothesis that lack of acidity in the gastric juices of newborn
infants might permit the growth of nitrate-reducing organisms in the
upper gastrointestinal tract and, thus, the reduction of nitrates to
nitrites before the former could be completely absorbed. The pH of the
a French, J. G., Hasselblad, V., & Johnson, R. Aggravation of
asthma by air pollutants. 1971-72 Southeastern CHESS studies.
stomach contents of healthy infants varied from 2.0 to 5.0 (average
3.7) and that for infants suffering from bacillary dysentery ranged
from 2.0 to 5.0 (average 3.0). However, for infants with nonspecified
diarrhoea the pH varied from 4.6 to 6.5 (average 5.6).
According to estimates made by Burden (1961), the water intake of
young infants is nearly 10 times higher than that of adults on a per
unit body weight basis.
The susceptibility of infants to methaemoglobinaemia during the
first six months of life, and especially during the first trimester
(Bailey, 1966; Kübler, 1965) can be explained by various mechanisms,
none of which is fully understood at present. It has been reported
that fetal haemoglobin, which in newborn infants makes up to 60-80% of
the total haemoglobin decreasing to about 20-30% in 3 months (British
Medical Journal, 1966; Kübler, 1965), is more readily oxidized to
methaemoglobin than adult haemoglobin (Betke, 1953; Künzer &
Schneider, 1953). This might explain why premature infants, who
frequently have a higher percentage of fetal haemoglobin than full-
term infants are more susceptible to methaemoglobinaemia. Keohane &
Metcalfe (1960) reported that the sensitivity of erythrocytes to
oxidation to methaemoglobin on exposure to nitrites gradually declined
during childhood until the age of puberty, after which it decreased
rapidly. This decline in sensitivity was not related to the
disappearance of fetal haemoglobin and the authors suggested that some
other factor might be responsible. The susceptibility of newborn and
young infants to develop methaemoglobinaemia could also be attributed
to the incomplete development of the NADH methaemoglobin reductase
system. Several studies have shown that the erythrocytes of newborn
and young infants have a lower capacity to reduce methaemoglobin than
those of older children and adults and that the erythrocytes of
premature newborns have a lower reduction capacity than those of full-
term infants (Bartos et al., 1966; Ross, 1963; Ross & Desforges,
1959). Except in rare cases of hereditary enzyme deficiency (Balsamo
et al., 1964), this deficiency in the NADH methaemoglobin reductase
system seems to disappear after the first 3-4 months of life (Bartos
et al., 1966: Künzer & Schneider, 1953).
The haemoglobin of pregnant women and that of patients suffering
from carcinomata has also been reported to be sensitive to oxidation
to methaemoglobin. However, this sensitivity disappeared in the first
instance after delivery and in the second, after radical extirpation
of the neoplasms (Metcalf, 1961).
8.1.3 Dose-response relationships for nitrates and nitrites
Estimates of the effective exposure of the general population to
nitrates and nitrites have been discussed briefly in section 5.1.4.
However, this information is not sufficient to relate environmental
concentrations to actual intake of nitrates or nitrites in cases of
observed methaemoglobinaemia. Retrospective epidemiological studies
discussed in section 8.1.1 provide little information on intake
because in most cases either the data concerning concentrations in
water or food were not reliable enough, or the amounts of water or
food consumed had not been measured. However, a study reported by
Winton et al. (1971) considered the nitrate intake from water in some
detail. The study was conducted in southern California and central
Illinois, USA, and involved 111 infants whose ages ranged from less
than two weeks to six months. The mother of each infant was asked
about the fluid intake of the infant in the previous 24 h, the method
of formula preparation, and the possible inclusion of any other source
of nitrate in the diet (e.g. vegetables) or administration of
methaemoglobin-forming medicines. The variables that determined the
nitrate dose were the daily fluid intake per body weight (which might
increase in hot and arid climates, or with fever), the fraction of the
total daily fluid intake taken in the form of water, and the
concentration of nitrates in the water. Using this method, it was
possible to estimate that a daily dose of 10 mg/kg body weight could
be obtained from water containing a nitrate concentration of
50 mg/litre. The daily water intake varied from 10% of the total daily
fluid intake for breast-fed infants or for those receiving ready-to-
feed preparations to 90% for infants who were fed preparations made
with powder. This shows that no generalization is possible, at
present, about the relationship between the nitrate concentration in
drinking water and the dose of nitrate, and that estimates of nitrate
dose from nitrate concentrations in water or food would have to be
made for individual cases, taking into account local conditions and
dietary habits.
The relationship between the doses of nitrate and methaemoglobin
levels is even more difficult to establish because of large individual
differences in response, depending on age and many other host
variables. For example, using the method described in the previous
paragraph, Winton et al. (1971) found that in a group of 111 infants,
63 received a nitrate dose of less than 1 mg/kg body weight, 23 were
exposed to 1-4.9 mg/kg, 20 to 5.0-9.9 mg/kg, and 5 infants to
10-15.5 mg/kg. However, only 3 infants appeared to have methaemoglobin
levels above normal (0-2.9%) and they were the youngest of the five
who had received more than 10 mg/kg. The highest methaemoglobin level
(5.3%) was found in 30-day-old baby who had received 15.5 mg/kg.
Another possible approach is to analyse the available retrospective
epidemiological data. This has been done by the Committee on Nitrate
Accumulation (1972) and by Diskalenko (1968) who concluded that only
very crude estimates of correlations between nitrate concentration in
water and methaemoglobin levels could be obtained, probably because of
the delay between the analyses of water and blood, difficulties in
identifying the sources of water consumed, and the heterogeneity of
the population samples studied, particularly with respect to age. The
analyses performed by the Committee on Nitrate Accumulation (1972)
showed, for example, that an increase in the nitrate concentration in
water from 0-49 mg/litre to 49-98 mg/litre, increased the
methaemoglobin level, on average, from 1.0% to 1.3% in infants aged
0-3 months, but did not affect the methaemoglobin level (0.8%) in
infants aged 3-6 months (Simon et al., 1964). Methaemoglobin and
haemoglobin levels, measured in 96 infants from 22 localities in
Rheinhessen, Federal Republic of Germany, during official maternal
counselling, were correlated with the nitrate concentrations of the
drinking water in the localities concerned by Würkert (1974,
unpublished data)a. Nitrate concentrations of 0-5 mg/litre,
31-50 mg/litre, and over 100 mg/litre corresponded to methaemoglobin
levels of 1.65%, 2.44%, and 6.59%, respectively. Higher methaemoglobin
levels were found in the presence of infections and in infants given
tea to drink or vegetable nutrients.
The last question pertains to the relation between the level of
methaemoglobin and clinical signs and symptoms of methaemoglobinaemia.
Normally, methaemoglobin is present in the blood at a concentration of
less than 2% of total haemoglobin.
Subclinical methaemoglobinaemia (less than 10% methaemoglobin)
has not been considered to be of direct health significance, but
Petukhov & Ivanov (1970) reported behavioural effects at these levels.
Clinical signs of methaemoglobinaemia such as cyanosis become
apparent at a methaemoglobin level of about 10%. Hypoxic signs and
symptoms may develop at levels exceeding 20% and death may occur at
levels of 50% or more.
In conclusion, the available information does not permit the
establishment of a quantitative dose-response relationship for human
exposure to nitrates in water or food.
8.2 N-nitroso Compounds
Freund (1937) first described acute intoxication by DMN. Barnes &
Magee (1954) described 2 cases of industrial intoxication due to DMN
in which one individual had hepatic cirrhosis at death; the other
survived but, 6 months later, was shown to have a hard liver suspected
of being cirrhotic. Watrous (1947) and Wrigley (1948) reported cases
of accidental exposure to N-nitroso-methylurethane. Reddening of the
conjunctiva and erythema of the face and feet developed quickly, and a
respiratory disorder developed later.
No reports are available concerning carcinogenesis in industrial
or other workers exposed to N-nitroso compounds, nor have
relationships been established, from epidemiological and analytical
data, that link cancer in man with exposure to N-nitroso compounds or
their possible precursors such as nitrates, nitrites, and compounds
a Thesis reported in the contributed of the Federal Republic of
Germany to the WHO environmental health critera document on
Nitrates, nitrites and N-nitroso compounds.
that can be nitrosated, occurring as food components, drugs, and
pesticides. A recent review of these data is available (Mirvish,
1976).
Some reports have been concerned with the possible etiological
role of N-nitroso compounds in nasopharyngeal cancer in south-east
Asia (Clifford, 1970; Fong & Chan, 1973a) and oesophageal cancer in
South Africa, Iran, and China (Burrell et al., 1966; Coordinating
Group for Research on the Etiology of Esophageal Cancer of North
China, 1974; Day, 1975; Harmozdian et al., 1975). However, no
relationship has been established; nitrosamines were detected in food
from these areas but this was not confirmed by mass spectroscopy
(Eisenbrand et al., 1976; Purchase et al., 1975).
The epidemiology of stomach cancer has been discussed from a
similar point of view by Correa et al. (1975), Endo et al. (1973),
Haenzel & Correa (1975), Hill et al. (1973), Mirvish (1971), and
Weisburger & Raineri (1975). It was suggested that nitrosamides might
be formed in the stomach from amides occurring in the diet, and might
then act locally on this organ. Relationships have been sought between
the occurrence of stomach cancer and the nitrate contents of the soil
or water in Chile, Colombia, and the United Kingdom (Hawksworth et
al., 1974; Hill et al., 1973; Zaldivar & Wetterstrand, 1975) but none
was established.
9. EVALUATION OF HEALTH RISKS TO MAN FROM EXPOSURE TO NITRATES,
NITRITES, AND N-NITROSO COMPOUNDS
9.1 Nitrates and Nitrites
9.1.1 General considerations
Man is exposed to nitrates and nitrites mainly through water and
food. Nitrate concentrations may be particularly high in drinking
water derived from dug wells. Nitrates in food may occur naturally or
may be added for various technological or even public health reasons
(e.g. addition of nitrates and nitrites to certain meat products to
protect against botulism). Although intake of very large doses of
nitrates can be fatal to man, such intake is not likely to occur
through environmental exposure, except in the case of infants and very
young children who are high risk groups because of their
susceptibility to nitrates and nitrites. Weekly intakes of nitrates by
members of the general population are difficult to evaluate but rough
estimates are available for England and the USA giving values of about
400-450 mg/week (85-105 mg from water; 210-225 mg from vegetables, and
about 110 mg from meat products).
These figures cannot be applied generally and a separate
estimation of the nitrate intake from food and water should be made
for each case, especially when the subjects are infants or young
children (section 8.1.3). Exposure to nitrates can also occur through
the inhalation of polluted air.
The assessment of health risks to man (section 9.1.2) has been
based on epidemiological studies and clinical evidence. The animal
data discussion in section 7.1, confirm the findings in man that
methaemoglobinaemia is the main toxic effect of nitrate and nitrite
ingestion. Methaemoglobinaemia is caused by nitrites, the reduction
products of nitrates. The reduction usually occurs through microbial
action either in the environment or in the body. The health risks from
exposure to nitrates is therefore related not only to their
concentration in drinking water and food, but also to the presence or
absence of conditions conducive to their reduction to nitrites. Young
infants constitute the most vulnerable group for the following
reasons:
(1) Lower acidity in their stomach allows the growth of certain
microbes that contain enzymes capable of reducing nitrates
to nitrites;
(2) Fetal haemoglobin, which constitutes a considerable
proportion of the haemoglobin of the young infant, and the
erythrocytes during childhood may be more susceptible to
conversion to methaemoglobin by the action of nitrites;
(3) The enzyme system capable of reducing methaemoglobin to
haemoglobin is deficient in the young infant; and
(4) The fluid intake of the young infant is higher than that of
the adult in relation to the body weight.
9.1.2 Assessment of health risks
Precise dose-response relationships could not be established by
the Task Group because of the existence of various strongly modifying
factors and the lack of accurate quantitative data. However, on the
basis of the available information, the Task Group reached the
following conclusions:
(a) General population -- The prevailing levels of nitrates and
nitrites in water and food do not seem to have any harmful
effects in adults and older children, although there are reports
of susceptible individuals who have been affected by meat treated
with nitrites, and of cases of poisoning resulting from the
ingestion of certain foods accidently containing excessive
amounts of nitrites (8.1.1.3). Subclinical methaemoglobinaemia
may also be found in individuals consuming water containing high
levels of nitrates (8.1.3).
(b) Susceptible group -- Infants less than 6 months old and
especially those under 3 months of age are particularly
susceptible to methaemoglobinaemia caused by intake of water
containing elevated levels of nitrates, especially when they are
fed with preparations made from dried milk of low acidity. While
a few cases of methaemoglobinaemia have been reported associated
with water nitrate levels of less than 50 mg/litre, most cases
occur with nitrate levels of 90 mg/litre or more. Nitrates in
water may cause death of the infant, but the lowest level that
may be fatal cannot be estimated at present.
The ingestion of vegetables (e.g. spinach, carrots) containing
elevated nitrate and/or nitrite levels may also cause
methaemoglobinaemia in infants, especially in those aged between 6
months and 1 year. Storage of vegetables, other than in the frozen or
canned state, is likely to increase the nitrite level and hence the
risk.
(c) Effects of airborne nitrates -- A few recent studies have
indicated that airborne nitrates may act as respiratory irritants
but, at present, adequate quantitative data are not available and
the Task Group did not consider this exposure in their health
risk evaluation.
9.2 N-nitroso Compounds
9.2.1 General considerations
Nitrites (and indirectly nitrates) can react with amines and
amides to form nitrosamines and nitrosamides. The precursors of these
N-nitroso compounds are widely distributed in various environmental
media. Information concerning the presence of N-nitroso compounds
per se is limited although they have been identified in certain
foods such as luncheon meats and in air and water samples. The
conditions under which N-nitroso compounds can be formed are
outlined in sections 4.2-4.6.
More than 80% of over one hundred N-nitroso compounds tested
proved to be carcinogenic in animal experiments giving rise to tumours
in many organs and also producing tumours transplacentally.
N-nitroso compounds are carcinogenic in a wide range of animal
species; most are mutagenic in test systems and some have been shown
to be teratogenic to animals.
The possible health hazard from N-nitroso compounds is not
confined to those present in the environment. Their formation, from a
variety of precursors in the body of animals, has been demonstrated,
and this may also occur in man.
9.2.2 Assessment of health risks
A dose-response relationship has been shown to exist in different
species of rodents for some carcinogenic N-nitroso compounds. As the
dose is reduced, the tumour incidence decreases and the time for
tumour induction increases and may exceed the life span of the animals.
Although there is no clinical or epidemiological evidence, it is
highly probable that these compounds are also carcinogenic to man.
However, present limitations concerning available dose-response data
in animals and their interpretation, and inadequate knowledge of the
biomechanism of cancer induction preclude a quantitative estimation of
the carcinogenic risk to man that may be associated with different
exposures to N-nitroso compounds.
9.3 Reduction of Exposure
The assessments of health risk given in sections 9.1.2
[(a) and (b)] and 9.2.2 lead to a number of practical conclusions
concerning the need to reduce the exposure to nitrates, nitrites, and
N-nitroso compounds.
As regards the exposure to nitrates and nitrites, the Task Group
made the following specific recommendations:
(a) Infant dried milk preparations should be reconstituted only
with water containing low levels of nitrates. If such water is
not available, breast feeding or the use of cow's milk should be
encouraged.
(b) Only vegetables with a low nitrate content should be used in
the preparation of baby foods. If vegetables known to contain
high levels of nitrates are used, appropriate food processing
precautions should be instituted. Nitrates and nitrites should
not be added to baby foods.
(c) The use of nitrates and nitrites in foods as preservatives
should be reduced to the minimum level that provides protection
against botulism. This applies particularly to cured and canned
meats and to fish. The use of nitrates and nitrites on fresh
meats or fish should be avoided.
(d) Nitrate levels in public drinking water should comply with,
or preferably be lower than, the tentative limit of
45 mg/litre recommended in the International Standards for
Drinking Water (WHO, 1971).
With respect to the carcinogenic risk from exposure to
N-nitroso compounds, it is prudent to assume that any exposure may
involve some degree of risk, and that exposure should therefore be
kept as low as practically achievable. This may not be an easy task in
many instances, since these compounds may occur in the environment in
concentrations of the order of parts per billion, as a result of a
variety of natural and technological processes, and, moreover, they
may be formed in vivo from nitrates, amines, and amides, which
are ubiquitous. Obviously the recommendations (a) to (d) will also
contribute to the reduction of carcinogenic risk related to
N-nitroso compounds.
REFERENCES
ACHTZEHN, M. K. & HAWAT, H. (1969) [Nitrate accumulation in vegetables
a possibility of nitrate intoxication in infants.] Die Nahrung,
13: 667-676 (in German).
ACHTZEHN, M. K. & HAWAT, H. (1970) [On nitrate formation in vegetables
and vegetable products. Part I. Raw spinach.] Die Nahrung,
14: 383-394 (in German).
ADRIANO, C. C., PRATT, D. F., & BISHOP, S. E. (1971) Fate of inorganic
forms of nitrogen and salt from land disposed manures from
dairies. In: Livestock waste management and pollution
abatement. St. Joseph, MI (Am. Soc. Agric. Eng. Proc. No. 271).
ALAM, B. S., SAPORESCHETZ, I. B., & EPSTEIN, S.S. (1971a) Formation of
N-nitroso-piperidine from piperidine and sodium nitrite in the
stomach and the isolated intestinal loop of the rat. Nature
(Lond.), 232: 116-118.
ALAM, B. S., SAPORESCHETZ, I. B., & EPSTEIN, S.S. (1971b) Synthesis of
nitrosopiperidine from nitrate and piperidine in the gastro-
intestinai tract of the rat. Nature (Lond.), 232: 199-200.
ALARIF, A. & EPSTEIN, S.S. (1974) The uptake and metabolism of
14 C-labelled nitrosomethylurethane and nitrosomethylurea in
guinea pigs and their in vitro metabolism in the guinea pig
and human pancreas. IARC Sci. Publ. No. 9, pp. 215-219.
ALEXANDROV, V. A. (1967) [On the mechanism of carcinogenicity of
N-nitrosodimethylamine in connection with peculiarities of
its effect on embryogenesis] Vop. Onkol., 13: 87-92
(in Russian).
ALEXANDROV, V. A. (1968a) Blastomogenic effect of dimethylnitrosamine
on pregnant rats and their offspring. Nature (Lond.),
218: 280-281.
ALEXANDROV, V. A. (1968b) [Effect of N-nitroso- N-methylaniline and
N-nitroso- N-ethylaniline on the rat embryo] Vop. Onkol.,
14: 37-38 (in Russian).
ALEXANDROV, V. A. (1969a) [Transplacental blastomogenic action of
N-nitrosomethylurea in rat offspring] Vop. Onkol.;
15: 55-61 (in Russian).
ALEXANDROV, V. A. (1969b) Uterine, vaginal and mammary tumors induced
by nitrosoureas in pregnant rats. Nature (Lond.),
222: 1064-1065.
ALEXANDROV, V. A. & JANISCH, W. (1971) Teratogenic effect of ethylurea
and nitrite in rats. Experientia (Basel), 27: 538-539.
ALLISTON, T. G., COX, G. B., & KIRK, R. S. (1972)The determination of
steam-volatile N-nitrosamines in foodstuffs by formation of
electron-capturing derivatives from electrochemically derived
amines. Analyst, 97: 915-920.
ALTHOFF, J., WILSON, R., & MOHR, U. (1971) Diethylnitrosamine-induced
alterations in the tracheobronchial system of Syrian golden
hamsters. J. Natl Cancer Inst., 46: 1067-1071.
ALTHORPE, J., GODDARD, D. A., & SISSONS, D. J., TELLING, G. M. (1970)
The gas chromatographic determination of nitrosamines at the
picogram level by conversion to their corresponding nitramines.
J. Chromatogr., 53: 371-373.
ARENA, J. M. (1970) Poisoning, toxicology, symptoms and treatment.
2nd ed., Springfield, I, Bannerstone House, p. 9.
ARISON, R. N. & FEUDALE, E. L. (1967) Induction of renal tumour by
streptozotoc n in rats. Nature (Lond.), 214: 1254-1255.
ASAHINA, S., FRIEDMAN, M. A., ARNOLD, E., MILLAR, G. N., MISHKIN, M.,
BISHOP, Y., & EPSTEIN, S.S. (1971) Acute synergistic toxicity and
hepatic necrosis following oral administration of sodium nitrite
and secondary amines to mice. Cancer Res., 31: 1201-1205.
ASATOOR, A.M. & SIMENHOFF, M. L. (1965)The origin of urinary
dimethylamine. Biochim. Biophys. Acta, 111: 384-392.
ASHTON, M. R. (1970) The occurrence of nitrates and nitrites in foods.
BFMIRA lit. surv. No 7, pp. 32.
BADER, G., STILLER, D., & ULLRICH, K. (1971) [Ultrastructural changes
in toxically injured and proliferating liver cells due to long-
term application of diethylnitrosamine] Arch.
Geschwulstforsch., 37: 327-343 (in German).
BAILEY, W. P. (1966) Methemoglobinemia-acute nitrate poisoning in
infants: Second report. J. Am. Osteopath. Assoc.,
66: 431-434.
BAKSHI, S. P., FAHEY, J. L., & PIERCE, L. E. (1967) Sausage cyanosis--
Acquired methemoglobinemia nitrite poisoning.
N. Eng. J. Med. 277: 1072.
BALSAMO, P., HARDY, W. R., & SCOTT, E. M. (1964) Hereditary
methaemoglobinaemia due to diaphorase deficiency in Navajo
Indians. J. Pediat., 65: 928-931.
BANNASCH, P. (1968) In: The cytoplasm of hepatocytes during
carcinogenesis. New York, Springer Verlag.
BARNES, J. M. & MAGEE, P.M. (1954) Some toxic properties of
dimethylnitrosamine. Br. J. ind. Med., 11: 167-174.
BARTOS, H. R., DESFORGES, J. F., & CLARK, N. (1966) Erythrocyte DPNH
dependent diaphorase levels in infants. Pediatrics, 37: 991-993.
BEHROOZI, K., GUTTMAN, R., GRUENER, N., & SHUVAL, H. I. (1971) Changes
in the motor activity of mice given sodium nitrite drinking
solution. In: Proceedings of a Symposium on Environmental
Physiology, Beersheba, December, 1971.
BNEMANSKIJ, V. V. & LITVINOV, N. N. (1969) [Some data on the histo-
and morphogenesis of experimental tumours of the kidneys in
animals.] Arkh. Pat., 31: 79-84 (in Russian).
BERTRAM, J. S. & CRAIG, A. W. (1972) Specific induction of bladder
cancer in mice by butyl- (4-hydroxybutyl) -nitrosamine and the
effects of hormonal modifications on the sex difference in
response. Eur. J. Cancer, 8(6): 587-594.
BERTRAM, J. S. & CRAIG, A. W. (1973) Induction of bladder tumours in
mice with dibutylnitrosamine. Br. J. Cancer, 24
(2): 352-359.
BETKE, K. (1953) [Comparative examination of the oxidation of fetal
and adult oxyhaemoglobin by sodium nitrite.] Naturwiss., 40: 60
(in German).
BHATHAL, P.S. & HURLEY, J. V. (1973) An electron microscope study of
the production of ascites in acute dimethylnitrosamine (NDMA)-
induced liver injury. J. Pathol., 111: 103-116.
BIOCEV, IV. & POCINKOVA, Z. (1972) [Effect of applied artificial
fertilizers on the soil, drinking water and foodstuffs of
vegetable origin. Report on the International Congress of
Country Hygiene, Varna] (in Russian).
BLATTMANN, L. & PREUSSMAN, R. (1973) [The structure of metabolites of
carcinogenic dialkylnitrosamine in the urine of rats]
Z. Krebsforsch., 79: 3-5 (in German).
BLATTMANN, L. & PREUSSMAN, R. (1974a). [The biotransformation of
carcinogenic dialkylnitrosamines. Further urine metabolites of
di-n-butyl- and di-n-pentylnitrosamines.] Z. Krebsforsch.,
81: 75-78 (in German).
BLATTMANN, L., JOSWIG, N., & PREUSSMAN, T. (1974b). [The structure of
the metabolites of the carcinogenic methyl-n-butyl-nitrosamine in
the urine of rats.] Z. Krebsforsch., 81: 71-73 (in German).
BODIPHALA, T. & ORMROD, D. P. (1971) Factors affecting the nitrate
content of vegetable and fruit foods. Can. Inst. Food Technol.,
J., 4(1): 6-8.
BOGOVSKI, P. & WALKER, E. A., ed. (1974) N-nitroso compounds in the
environment. Proceedings of a Working Conference, the
International Agency for Research on Cancer, Lyons, France,
17-20 October, 1973, Lyons, International Agency for Research on
Cancer, pp. 243.
BOGGYSKI, P., PREUSSMANN, R., & WALKER, E. A., ed. (1972a) N-nitroso
compounds analysis and formation. Proceedings of a Working
Conference, Deutsches Krebsforschungszentrum, Heidelberg,
Federal Republic of Germany, 13-15 October, 1971, Lyons,
International Agency for Research on Cancer, pp. 140.
BOGGYSKI, P., CASTEGNARO, M., PIGNETTELI, B., & WALKER, E. A. (1972b)
The inhibiting effects of tannins on the formation of
nitrosamines. IARC Sci. Publ. No. 3, pp. 127-129.
BOLIN, B. & ARRHENIUS, E., ed. (1977) An essential life factor and a
growing environmental hazard. Report from Nobel Symposium No. 38,
Ambio, 6 (2-3): 96-105.
BOSCH, H. M., ROSENFIELD, A. B., HUSTON, R., SHIPMAN, H. R., &
WOODWARD, F. L. (1950) Methemogiobinemia and Minnesota well
supplies. J. Am. Water Assoc., 42: 161-170.
BOYLAND, E. & WALKER, S. A. (1974) Effect of thiocyanate on
nitrosation of amines. Nature (Lond.), 248: 601-602.
BOYLAND, E., CARTER, R. L., GORROD, J. W., & ROE, F. J. C. (1968)
Carcinogenic properties of certain rubber additives. Eur. J.
Cancer, 4: 233-239.
BRESLER, S. E., KALININ, V. L., & PERUMOV, D. A. (1968) Inactivation
and mutagenesis on isolated DNA. IV Possibility of integration of
lethal damage into the chromosome of Bacillus subtills during
transformation. Mutat. Res., 5: 329-341.
BRETSCHNEIDER, K. & MATZ, J. (1973) [Nitrosamines (NA) in the
atmospheric air and in the air at the workplace.] Arch.
Geschwulstforsch., 42: 36-41 (in German).
BRETSCHNEIDER, K. & MATZ, J. (1976) Occurrence and analysis of
nitrosamines in air. IARC Sci. Publ. No. 14, pp. 395-399.
British Medical Journal (1966), 1: 250-251. Spinach--a risk to
babies.
BROWN, J. R. & SMITH, G. E. (1967) Nitrate accumulation in vegetable
crops as influenced by soil fertility practises. Columbia,
University of Missouri, pp. 43 (Res. Bull. No. 920).
BRUNI, C. (1973) Distinctive cells similar to fetal hepatocytes
associated with liver carcinogenesis by diethylnitrosamine.
Electron microscopic study. J. Natl Cancer Inst.,
50: 1513-1528.
BUGLASS, A. J., CHALLIS, B.C., & OSBORNE, M. R. (1974)
Transnitrosation and decomposition of N- nitrosamines.
N- nitroso compounds in the environment. IARC Sci. Publ., No. 9,
pp. 94-100.
BURDEN, E. H. W. J. (1961) The toxicology of nitrates and nitrites
with particular reference to the potability of water supplies.
Analyst, 86 (1024): 429-433.
BURNS, R. C. & HARDY, R. W. F. (1975) Nitrogen fixation in bacteria
and higher plants. Berlin, Springer-Verlag.
BURRELL, R. J. W., ROACH, W. A., & SHADWELL, A. (1966) Esophageal
cancer in the Bantu of the Transkei associated with mineral
deficiency in garden plants. J. Natl Cancer Inst.,
36: 201-214.
BUTLER, W. H. & HARD, G. C. (1971) Hepatoxicity of dimethylnitrosamine
in the rat with special reference to veno-occlusive disease.
Exp. mol. Pathol., 15: 209-219.
CALAFAT, J., DEN ENGELSE, L, & EMMELOT, P. (1970) Studies on lung
tumours. II. Morphological alterations induced by
dimethylnitrosamine in mouse lung and liver and their relevance
to tumourigenesis. Chem. biol. Interact.,
2: 309-320.
CARDESA, A., MIRVlSH, S.S., HAVEN, G. T., & SHUBIK, P. (1974)
Inhibitory effect of ascorbic acid on the acute toxicity of
dimethylamine plus nitrite in the rat. Proc. Soc. Exp. Biol.
Med., 145: 124-128.
CARTER, R. L., PERCIVAL, W. H., & ROE, F. J. C. (1969) Exceptional
sensitivity of mink to the hepatotoxic effects of
dimethylnitrosamine. J. Pathol., 97 (1): 79-88.
CASEY, H. (1975) Variation in chemical composition of the River Frome,
England from 1965 to 1972. Freshwater Biol.,
5: 507-514.
CASTEGNARO, M., PIGNATELLI, B., & WALKER, E. A. (1972) N-Nitroso
compounds: Analysis and formation. IARC Sci. Publ, No. 3,
pp. 87-89.
CAVETT, J. J. (1962) The microbiology of vacuum-packed sliced bacon.
J. appl. Bact., 25: 282-289.
CHALLIS, B.C. (1973) Rapid nitrosation of phenols anti its
implications for health hazards from dietary nitrites. Nature
(Load.), 244: 466.
CHALLIS, B.C. & BARTLETT, C. D. (1975) Possible cocarcinogenic effects
of coffee constituents. Nature (Lond.),
254: 532-533.
CHALLIS, B.C. & CHALLIS, J. A. (1970) Reactions of the carboxamide
group. In: Zabicky, J., ed. The chemistry of amides, London,
Interscience, pp. 733-848.
CIAVAGLIA, F. & THOMPSON, R. P. (1969) Infant methemoglobinemia.
Absence in areas with low concentrations of nitrate-nitrogen in
water supply. N.Y. State J. Med., 69: 3128-3129.
CLAPP, N. K. & TOYA, R. E. (1970) Effect of cumulative dose and dose
rate on dimethylnitrosamine oncogenesis in RF mice.
J. Natl Cancer Inst., 45: 495-498.
CLAPP, N. K., TYNDALL, R. L., & OTTEN, J. A. (1971) Differences in
tumor types and organ susceptibility in BALB-C and RF mice
following dimethylnitrosamine and diethyinitrosamine. Cancer
Res., 31: 196-198.
CLIFFORD, P. (1970) On the epidemiology of nasopharyngeal carcinoma.
Int. J. Cancer, 5: 287-309.
COLLINS-THOMPSON, D. L., SEN, N. P., ARIS, B., & SCHWENGHAMER, L.
(1972) Non-enzymic in vitro formation of nitrosamines by
bacteria isolated from meat products. Can. J. Microbiol,
18 (12): 1968-1971.
COMLY, H. H. (1945) Cyanosis in infants caused by nitrates in well
water. J. Am. Med. Assoc., 129: 112-116.
COMMITTEE ON NITRATE ACCUMULATION (1972) Accumulation of nitrate.
Washington, DC, National Academy of Sciences. p. 48.
COMMONER, B. (1970) Threats to the integrity of the nitrogen cycle.
In: Singer, F. S., ed. Global effects of environmental
pollution, NY, Springer Verlag, pp. 70-95.
COMMONER, B., SHEARER, G., & KOHL, D. (1972) A study of certain
ecological, public and economic consequences of the use of
inorganic nitrogen fertilizer, St. Louis, Washington
University. (1st year Progress Report NSF Grant No. GI29926x).
COOKE, G. E. & WILTIAMS, R. J. B. (1970) Losses of nitrogen and
phosphorus from agricultural land. Water Treat. Exam.,
19 (3): 253-276.
COORDINATING GROUP FOR RESEARCH ON THE ETIOLOGY OF ESOPHAGEAL CANCER
OF NORTH CHINA (1974) The epidemiology of esophageal cancer in
North China and preliminary results in the investigation of its
etiological factors. Abstract, 11th International Cancer
Congress, Florence, Italy.
CORREA, P., HAENSZEL, W., CUELLO, C., TANNENBAUM, S., & ARCHER, M.
(1975) A model for gastric cancer epidemiology, Lancet, July 12,
pp. 58-59.
COUCH, D. B. & FRIEDMAN, M. A. (1975) Interactive mutagenicity of
sodium nitrite, dimethylamine, methylurea and ethylurea. Mutat.
res., 31: 109-114.
CRADDOCK, V. M. (1971) Liver carcinomas induced in rats by single
administration of dimethylnitrosamine after partial hepatectomy.
J. Natl Cancer IMNST, 47: 899-907.
CROSBY, N. T., FOREMAN, K. K., PALFRAMEN, J. F., & SAWYER, R. (1972)
Determination of volatile nitrosamines in food products at the
µg/kg level. IARC Sci. Publ. No. 3, pp. 38-42.
DAIBER, D. (1966) [Methods for the quantitative and qualitative
estimation of organic N- nitroso compounds.] Freiburg (Thesis)
(in German).
DAY, N. E. (1975) Some aspects of the epidemiology of esophageal
cancer. Cancer Res., 35 (2): 3304-3307.
DELWICHE, C. C. (1970) The nitrogen cycle. Sci. Am., 223: 137-146.
DISKALENKO, A. P. (1968) Methemoglobinemia of water-nitrate origin in
the Moldavian SSR. Gig. i Sanit., 33: 32-37.
DISKALENKO, A. P. (1969) [Methaemoglobinaemia caused by nitrates in
water and its prophylaxis.] Kisinev (in Russian).
DISKALENKO, A. P. & DOBRJANSKAJA, E. V. (1972) [Changes in redox
processes in hepatic and cerebral tissues as a result of the
ingestion of nitrates with drinking water. In: Topical
questions of hygiene and epidemiology] Kisinev, pp. 22-23 (in
Russian).
DISKALENKO, A. P. & TROFIMENKO, JU. N. (1972) [Changes in the activity
of the glutathione-ascorbic acid system and respiration in
erythrocytes as a result of the ingestion of nitrites with
drinking water. In: Topical questions of hygiene and
epidemiology Kisinev, pp. 23-25 (in Russian).
DOWNS, E. F. (1950) Cyanosis of infants caused by nitrate
concentrations in rural water-supplies. Bull. World Health
Organ., 3: 165-169.
DRUCKREY, H. & PREUSSMANN, R. (1962) [The production of lung cancer in
rats by subcutaneous injection of diamylnitrosamine.]
Naturwiss., 49: 111-112 (in German).
DRUCKREY, H., PREUSSMAN, R., SCHMÄHL, D., & MÜLLER, M. (1961) [The
chemical constitution and carcinogenic effects of the
nitrosamines.] Naturwiss., 48: 134-135 (in German).
DRUCKREY, H., STEINHOFF, D., BEUTHNER, H., SCHNEIDER, H., & KLÄRNER,
P. (1963a) [Screening of nitrite for chronic toxicity in rats.]
Arzneimittel-Forsch., 13: 320-323 (in German).
DRUCKREY, H., SCHILDBACH, A., SCHMAHL, D., PREUSSMANN, R., &
IVANKOVIC, S. (1963b) [Quantitative analysis of the carcinogenic
action of diethylnitrosamine.] Arzneimittel-Forsch,
13: 841-851 (in German).
DRUCKREY, H., IVANKOVlC, S., & PREUSSMANN, R. (1966) Teratogenic and
carcinogenic effects in the offspring after single injection of
ethylnitrosourea in pregnant rats. Nature (Lond.),
210: 1378-1379.
DRUCKREY, H., PREUSSMANN, R., IVANKOVIC, S., & SCHMÄHL, D. (1967)
[Organotropic carcinogenic effects of 65 different N-nitroso-
compounds in BD-rats.] Z. Krebsforsch, 69: 103-201 (in German).
DRUCKREY, H., LANDSCHÜTZ, CH., & PREUSSMANN, R. (1968) [Oesophageal
carcinoma due to inhalation of methylbutylnitrosamine (MBNA) in
rats.] Z. Krebsforsch., 71: 135-139 (in German).
DRUCKREY, H., LANDSCHOTZ, CH., & IVANKOVlC, S. (1970a) [Transplacental
production of malignant tumours in the nervous system II.
Ethylnitroso urea in 10 genetically defined rat strains.] Z.
Krebsforsch., 73: 371-386 (in German).
DUBROW, H. & KABISCH, W. (1960) [Investigations of the evidence of
nitrates and nitrites in cheese.] Milchwiss., 15 (11): 543-549
(in German).
DURFOR, C. N. & BECKER, E. (1965) Public water supplies of the 100
largest cities in the United States. Washington DC, US Dept
Interior (Geological Survey Water Supply Paper 1812).
DUTTON, A. H. & HEATH, D. F. (1956) Demethylation of
dimethylnitrosamine in rats and mice. Nature (Lond.),
178: 644.
EISENBRAND, G. (1972) Determination of volatile nitrosamines at low
levels in food by acid-catalysed denitrosation and formation of
derivatives from the resulting amines. IARC Sci. Publ. No. 3,
pp. 64-71.
EISENBRAND, G. (1973) Determination of volatile nitrosamines: a
review. In: Proceedings of the International Symposium on
Nitrite in Meat Products. Zeist, pp. 45-52.
EISENBRAND, G. & PREUSSMANN, R. (1970) [A new method for the
colorimetric determination of nitrosamines following cleavage of
the N-nitroso group with hydrogen bromide in glacial acetic
acid.] Arzneimittel-Forsch., 20: 1513-1517 (in German).
EISENBRAND, G., JANZOWSKI, C., & PREUSSMANN, R. (1975) Gas
chromatographic determination of N-nitrosoamino acids by
trimethylsilylation and single-ion mass fragmentography.
J. Chromatogr., 115: 602-606.
EISENBRAND, G., VON RAPPARD, E., ZAPPE, R., & PREUSSMANN, R. (1976)
Trace analysis of volatile nitrosamines by a modified nitrogen
specific detector in pyrolytic mode and by ion-specific
determination of heptafiuorobutyrantides in a GC/MS system.
IARC Sci. Publ. No. 14, pp. 65-75.
EISENSTARK, A. & ROSNER, J. L. (1964) Chemically induced reversions in
the cysC region of Salmonella typhimurium. Genetics,
49: 343-355.
ELESPURU, R. K. & LIJINSKY, W. (1973) The formation of carcinogenic
nitroso compounds from nitrite and some types of agricultural
chemicals. Food Cosmet. Toxicol., 11 (3): 807-811.
ELESPURU, R., LIJINSKY, W., & SETLOW, J. K. (1974) Nitrosocarbaryl as
a potent mutagen of environmental significance. Nature (Lond.),
247: 386-387.
ENDER, F. & CEH, L. (1967) Occurrence and determination of
nitrosamines in foodstuffs for human and animal nutrition.
Alkylierend Wirkend Verbindungen, Second Conference on Tobacco
Research., Freiburg, 1960, pp. 83-91.
ENDER, F. & CEH, L. Z. (1971) Conditions and chemical reaction
mechanisms by which nitrosamines may be formed in biological
products with reference to their possible occurrence in food
products. Z. Lebensm. Unters. Forsch., 145 (3): 133-142.
ENDER, F., HAVRE, G., HELGEBOSTAD, A., KOPPANG, N., MADSEN, R., & CEH,
L. (1964) Isolation and identification of a hepatoxic factor in
herring meal produced from sodium nitrite preserved herring.
Naturwiss., 51: 637-638.
ENDER, F., HAVRE, G., MADSEN, R., CEH, L., & HELGEBOSTAD, A. (1967)
[Studies on conditions under which N-nitrosodime-thylamine is
formed in herring meal produced from nitrite-preserved herring.]
Z. Tierphysiol. Tierernähr. Futtermittelk., 22: 181-189 (in
German).
ENDO, H., TAKAHESHI, K., & AOYAGI, H. (1973) Screening of compounds
structurally and functionally related to N-methyl- N-nitroso
guanidine, a gastric carcinogen. Gann, 65: 45-54.
EPPSON, H. F., GLENN, M. W., ELLIS, W. W., & GILBERT, C. S. (1960)
Nitrate in the diet of pregnant ewes. JAVMA, 137 (10): 611-614.
EPSTEIN, S. (1972) In vivo studies on interactions between secondary
amines and nitrites or nitrates. IARC Sci. Publ. No. 3,
pp. 109-115.
FAN, T-Y. & TANNENBAUM, S. R. (1972) Automatic colorimetric
determination of N-nitroso compounds. J. agric. Food Chem.,
19: 1267-1269.
FAN, T-Y. & TANNENBAUM, S. R. (1973) Factors influencing the rate of
formation of nitrosomorpholine from morpholine and nitrite:
acceleration by thiocyanate and other anions
J. agric. Food Chem, 21: 237-240.
FAO/IAEA Panel of Experts (1974) Effects of agricultural production
on nitrates in food and water with particular reference to
isotope studies. Proceedings and Report of a Panel of Experts,
Vienna, 4-8 June 1973, Vienna, International Atomic Energy
Agency, pp. 158.
FASSETT, D. W. (1966) Nitrates and nitrites. In: Toxicants occurring
naturally in foods. Washington, DC, National Academy of
Sciences, pp. 250-449 (Publ. No. 1354).
FAZIO, T., DAMICO, J. N., HOWARD, J. W., WHITE, R. H., & WATTS, J. O.
(1971a) Gas chromatographic determination and mass spectrometric
confirmation of N-nitro-sodimethylamine in smoke-processed
marine fish. J. agric. Food Chem,
19(2): 250-253.
FAZlO, T., WHITE, R. H., & HOWARD, J. W. (1971b) Analysis of nitrite-
and/or nitrate-processed meats for
N-nitrosodimethylamine. J. Assoc. Off Anal. Chem.,
54(5): 1157-1159.
FAZIO, T., HOWARD, J. W., & WHITE, R. (1972) Multidetection method
for analysis of volatile nitrosamines in foods. IARC Sci. Publ.
No. 3, pp. 16-24.
FAZIO, T., WHITE, R. H., DUSOLD, L. R., & HOWARD, J. W. (1973)
Nitrosopyrrolidine in cooked bacon. J. Assoc. Off. Anal. Chem.,
56 (4): 919-921.
FERGUSON, J. H., MYSLIWY, T. J., & ARCHER, M. C. (1974) The
nitrosation of spermidine and spermine. IARC Sci. Publ. No. 9,
pp. 90-93.
FERON, V. J., EMMELOT, P., & VOSSENAAR, T. (1972) Lower respiratory
tract tumors in Syrian golden hamsters after intratracheal
instillations of diethylnitrosamine alone and with ferric oxide.
Eur. J. Cancer, 8: 445-449.
FIDDLER, W. (1975) The occurrence and determination of N-nitroso
compounds. Toxicoi. appl. Pharmacol., 31: 352-360.
FIDDLER, W., DOERR, R. C., ERTEL, J. R., & WASSERMAN, A. E. (1971)
Gas-liquid chromatographic determination of
N-nitrosodimethylamine in ham. J. Assoc. Off Anal. Chem, 54
(5): 1160-1163.
FIDDLER, W., PENSABENE, J. W., DOERR, R. C., & WASSERMAN, A. E. (1972)
Formation of N-nitrosodimethylamine from naturally occurring
quaternary ammonium compounds and tertiary amines. Nature
(Lond.), 236: 307.
FIDDLER, W., PENSABENE, J. W., PIOTROWSKI, E.G., DOERR, R. C., &
WASSERMAN, A. E. (1973) Use of sodium ascorbate or erythorbate to
inhibit formation of N-Nitroso-dimethylamine in frankfurters.
J. Food Sci., 38: 1084.
FIDDLER, W., PENSABENE, J. W., FAGAN, J. C., THORN, E. J., PIOTROWSKA,
E.G., & WASSERMAN, A. (1974) The role of lean and adipose tissue
on the formation of nitrosopyrrolidine in fried bacon. J. Food
Sci., 39: 1070-1071.
FIESER, L. F. & FIESER, M. (1967) Reagents for organic synthesis.
New York, Wiley, pp. 747-749.
FINE, D. H. & RUFFEH, F. (1974) Description of the thermal energy
analyser for N- nitroso compounds. IARC Sci. Publ. No. 9,
pp. 40-44.
FINE, D. H., RUFEH, F., & LIEB, D. (1974) Group analysis of volatile
and non-volatile N-nitroso compounds. Nature (Lond.),
247: 309-310.
FINE, D. H., HUFFMAN, F., ROUNBEHLER, D. P., & BETCHER, N.M. (1976a)
Analysis of N-nitroso compounds by combined high- performance
liquid chromatography and thermal energy analysis. IARC Sci.
Publ. No. 14, pp. 43-50.
FINE, D. H., ROUNBEHLER, D. P., BELCHER, N.M., & EPSTEIN, S.S. (1976b)
N-nitroso compounds in air and water. IARC Sci. Publ. No. 14,
pp. 401-408.
FLAKS, A., HAM LTON, J. M., CLAYSON, D. B., & BURCH, P. R. J. (1973)
The combined effect of radiation and chemical carcinogens in
female A x 1F mice. Br. J. cancer, 28: 227-231.
FONG, Y. Y. & CHAN, W. C. (1973a) Dimethylnitrosamine in Chinese
marine salt fish. Food Cosmet. Toxicol., 11: 841-845.
FONG, Y. Y. & CHAN, W. C. (1973b) Bacterial production of
dimethylnitrosamine in salted fish. Nature (Lond.),
243: 421-422.
FONG, Y. Y. & WALSH, E. O'F. (1971) Carcinogenic nitrosamines in
Cantonese salt-dried fish. Lancet, 2: 1032.
FOOD STANDARDS COMMITTEE (1959) Preservatives in food. London, Her
Majesty's Stationery Office.
FOSTER, S.S. D. & CREASE, R. I. (1974) Nitrate pollution of chalk
groundwater in east Yorkshire -- a hydrological appraisal. J.
Inst. Water Eng., 28: 178-194.
FREIMURTH, U. & GLASER, E. (1970) [The appearance of nitrosamines in
food.] Die Nahrung., 14 (5): 357-361 (in German).
FREUND, H. A. (1937) Clinical manifestations and studies in
parenchymatous hepatitis. Ann. int. Med., 10: 1144-1155.
FRIDMAN, A. L., MUKHAMETSIN, F. M., & NOMIKOV, S.S. (1971) [Advances
in the chemistry of aliphatic nitrosamines.] Russ. chem. Rev.,
40 (1): 34-50 (in Russian).
FRIEDMAN, M. A., GREENE, E. J., & EPSTEIN, S.S. (1972) Rapid gastric
absorption of sodium nitrite in mice. J. pharm. Sci.,
61 (9): 1492-1494.
GARBIDGE, M. G. & LEGATOR, M. S. (1969) A host-mediated microbic assay
for the detection of mutagenic contents. Proc. Soc. Exp. Biol.
Med., 130: 831-834.
GARBUZ, A.M. (1968) [The effect of symptomless methaemoglo-binaemia
on some bodily functions.] Author's summary of a thesis for the
degree of Candidate of Medical Sciences, Leningrad (in Russian).
GARBUZ, A.M. (1971) [Changes in the body's functional condition in
symptomless methaemoglobinaemia. In: Methaemoglobinaemia caused
by various factors and methods of preventing it.] Summaries of
papers read at the First Scientific Conference on
Methaemoglobinaemia, Leningrad, pp. 18-21 (in Russian).
GEIL, J. H., STENGER, R. J., BEHKI, R. M., & MORGAN, W. S. (1968)
Hepatotoxic and carcinogenic effects of dimethylnitrosamine in
low dosage. Light and electron microscopic study.
J. Natl Cancer Inst., 40: 713-730.
GELPERIN, A., JACOBS, E. E., & KLETKE, L. S. (1971) The development of
methemoglobin in mothers and newborn infants from nitrate in
water supplies. Ill. med. J., 140: 42-44.
GERRITSEN, G. A. & DE WILLIGEN, A. H. A. (1969) [The nitrite content
of potato and corn starch.] Die Starke, 21: 101-105 (in French).
GILBERTSON, C. B., MCCALLA, T. M., ELLIS, J. R., CROSS, O. E., &
WOODS, W. R. (1970) The effect of animal density and surface
slope on characteristics of runoff solid waste, and nitrate
movement on unpaved feedlots. Lincoln, Univ. Nebraska Coll.
Agric. and Home Econ.
GOBBI, A., SOVERINI, R., & GRISLER, R. (1974) [Normal
methemoglobinemia values observed in 500 inhabitants of the City
of Milan.] Med. Lavoro, 65: 306-310 (in Italian).
GOLDSMITH, J. R., ROKAW, S. N., & SHEARER, L. N. (1975) Distributions
of percentage methemoglobin in several population groups in
California. Int. J. Epidem., 4: 207-212.
GOTO, M. (1973) Inorganic chemicals in the environment -- with special
reference to the pollution problems in Japan. Environ. Qual.
Saf., 2: 72-77.
GROENEN, P. J., JONK, C., VAN INGEN, C., & TEN NOEVER DE BRAUW, M. C.
(1976) Determination of eight volatile nitrosamines in thirty
cured meat products with capillary gas chromatography-high
resolution mass spectrometry: the presence of
nitrosodiethylamine and the absence of nitrosopyrrolidine. IARC
Sci. Publ., No. 14, pp. 321-331.
GREENBERG, R. A. (1974) Ascorbate and nitrosamine formation in cured
meats. In: Proceedings of the International Symposium on
Nitrite in Meat Products, Zeist, 10-14 September, 1973,
Wageningen, Centre for Agricultural Publishing and Documentation,
pp. 179-188.
GREENBLATT, M. (1973) Ascorbic acid blocking of aminopyrine
nitrosation in NZO-Bl mice. J. Natl Cancer Inst.,
50: 1055-1056.
GREENBLATT, M. & MIRVISH, S.S. (1973) Dose-response studies with
concurrent administration of piperazine and sodium nitrite to
strain A mice. J. Natl Cancer Inst., 50 (1): 119-124.
GREENBLATT, M. & RIJHSINGHANI, K. (1969) Comparative cytopathologic
alterations induced by alkylnitrosamines in nasal epithelium of
the Syrian hamster. J. Natl Cancer Inst., 42: 421-433.
GREENBLATT, M., MIRVISH, S., & SO, B. T. (1971) Nitrosamine studies:
Induction of lung adenomas by concurrent administration of sodium
nitrite and secondary amines in Swiss mice. J. Natl Cancer
Inst., 46: 1029-1034.
GREENBLATT, M., KOMMINENI, V., CONRAD, E., WALLCAVE, L., & LIHNSKY, W.
(1972) In vivo conversion of phenmetrazine into its N-nitroso
derivative. Nature (Lond.), 236
(62): 25-26.
GREENBLATT, M., KOMMINENI, V. R. C., & LIJINSKY, W. (1973) Null effect
of concurrent feeding of sodium nitrite and amino acids to MRC
rats. J. Natl Cancer Inst., 50: 799-802.
GRUENER, N. & SHUVAL, H. I. (1970) Health aspects of nitrates in
drinking water. In: Shuval, H. I., ed. Developments in water
quality research. Ann Arbor, Humphrey Science Publ., pp. 89-106.
GRUNTHAL, D., HELLENBROICH, D. O., SANGER, P., & MAAS, H. (1970)[The
effect of partial hepatectomies on the hepatoma rate after
administration of diethyl-nitrosamine.]
Z. Naturforsch., 25: 1277-1281 (in German).
HADJIOLOV, D. (1971) [The inhibition of dimethylnitrosamine
carcinogenesis in rat liver by aminoacetonitrile.]
Z. Krebsforsch., 76: 91-92 (in German).
HAENSZEL, W. & CORREA, P. (1975) - Developments in the epidemiology of
stomach cancer over the past decade. Cancer Res.,
35: 3452-3459.
HANSEN, H. H. & MUGGIA, F. M. (1971) Treatment of malignant brain
tumours with nitrosoureas. Cancer Chemother. Rep. Part 1, 55
(2): 99-100.
HARD, G. C. & BUTLER, W. H. (1970a) Cellular analysis of renal
neoplasia. Induction of renal tumors in dietary-conditioned rats
by dimethylnitrosamine, with a reappraisal of morphological
characteristics. Cancer Res., 30: 2796-2805.
HARD, G. C. & BUTLER, W. H. (1970b) Cellular analysis of renal
neoplasia. Light microscope study of the development of
interstitial lesions induced in the rat kidney by a single
carcinogenic dose of dimethylnitrosamine. Cancer Res.,
30: 2806-2815.
HARD, G. C. & BUTLER, W. H. (1970c) Toxicity of dimethylni-trosamine
for the rat testis. J. Pathol., 102: 201-207.
HARD, G. C. & BUTLER, W. H. (1971a) Ultrastructural aspects of renal
adenocarcinoma induced in the rat by dimethylnitrosamine. Cancer
Res., 31: 366-372.
HARD, G. C. & BUTLER, W. H. (1971b) Ultrastructural study of the
development of interstitial lesions leading to mesenchymal
neoplasia induced in the rat renal cortex by dimethylnitrosamine.
Cancer Res., 31: 337-347.
HARD, G. C. & BUTLER, W. H. (1971c) Morphogenesis of epithelial
neoplasms induced in the rat kidney by dimethylnitrosamine.
Cancer Res., 31: 1496-1505.
HARD, G. C., BORLAND, R., & BUTLER, W. H. (1971) Altered morphology
and behavior of kidney fibroblasts in-vitro following in-vivo
treatment of rats with a carcinogenic dose of
dimethylnitrosamine. Experientia (Basel), 27: 1208-1209.
HARMOZDIAN, H., DAY, N. E., ARAMESH, B., & MAHBOUBI, E. (1975) Dietary
factors and esophageal cancer in the Caspian littoral of Iran.
Cancer Res., 35: 3493-3498.
HASHIMOTO, Y., SUZUKI, E., & OKADA, M. (1972) [Induction of urinary
bladder tumors in ACI/N rats by butyl (3-carboxypropyl)
nitrosamine, a major urinary metabolite of butyl-(4-hydroxbutyl)
nitrosamine.] Gann, 63: 637-638 (in Japanese).
HAWKSWORTH, G. & HILL, M. J. (1971a) The formation of nitrosamines by
human intestinal bacteria. Biochem. J., 122: 28p-29p.
HAWKSWORTH, G. & HILL, M. J. (1971b) Bacteria and the
N-nitrosation of secondary amines. Br. J. Cancer,
25: 520-526.
HAWKSWORTH, G., HILL, M. J., GORDILL, G., & CUELLO, C. (1974)
Possible relationship between nitrates, nitrosamines and
gastric cancer in S.U. Columbia. IARC Sci. Publ. No. 9,
pp. 229-234.
HEATH, D. F. (1962) The decomposition and toxicity of
dialkylnitrosamines in rats. Blochem. J., 85: 72-91.
HEDLER, L. & MARQUARDT, P. (1968) Occurrence of diethynitrosamine in
some samples of food. Food Cosmet. Toxicol, 6: 341-348.
HEDLIN, R. A. (1971) Nitrate contamination of ground water in the
Neepawa-Langruth area of Manitoba. Can. J. Soil Sci,
51: 75-84.
HEIN, G. E. (1963) Reaction of tertiary amines with nitrous acid,
J. chem. Educ, 40 (4): 181-184.
HENDERSON, W. R. & RASKIN, N.H. (1972) "Hot-Dog" headache: Individual
susceptibility to nitrite. Lancet, 2: 1162-1163.
HEPPEL, L. A. & PORTERFIELD, V. T. (1949) Metabolism of inorganic
nitrite and nitrate esters I. The coupled oxidation of nitrite by
peroxide-forming systems and catalase. J. biol. Chem, No. 178,
pp. 549-556.
HERMANN, H. (1960) [P. Methylnitrosaminebenzaldehyde, a metabolic
product of Clytocybe suaveolens.] Naturwiss., 47: 162
(in German).
HEYNS, K. (1973) [On nitrosamines in nutrients.] Getreide Mehl Brot,
27: 249-253 (in German).
HICKS, R. M., WAKEFIELD, J. ST. J., & CHOWANIEC, J. (1973)
Co-carcinogenic action of saccharin in the chemical induction of
bladder cancer. Nature (Lond.), 243: 347-349.
HILDRUM, K. I., SCANLAN, R. A., & LIBBEY, L. M. (1975) Identification
of gamma-butenyl-(beta-propenyl) nitrosamine, the principal
volatile nitrosamine formed in the nitrosation of spermidine or
sperminc. J. agric. Food Chem., 23
(1): 34-37.
HILL, M. J., HAWKSWORTH, G., & TATTERSALL, G. (1973) Bacteria,
nitrosamines and cancer of the stomach. Br. J. Cancer,
28 (6): 562-567.
HMSO (1973) The determination of nitrate-nitrogen in herbage. London,
Her Majesty's Stationery Office, pp. 3 (Tech. Bull. 27).
HMSO (1974) Annual Report 1972, London, Her Majesty's Stationery
Office, pp. 206.
HOCH-LIGETI, C., ARGUS, M. F., & ARCOS, J. C. (1968) Combined
carcinogenic effects of dimethylnitrosamine and 3-methyl-
cholanthrene in the rat. J. Natl Cancer Inst., 40: 535-549.
HOFFMAN, D., HECHT, S.S., ORNAF, R. M., & WYDNER, E. L. (1974)
N-nitrosonornicotine in tobacco. Science, 186: 265-267.
HÖLSCHER, P.M. & NATZSCHKA, J. (1964) [Methaemoglobinaemia in young
children due to the nitrite contents of spinach.] Dtsch Med.
Wschr, 89: 1751-1754 (in German).
HORN, E. E. & HERRIOT, R. M. (1962) The mutagenic action of nitrous
acid on "single-stranded" (denatured) haemophilus transforming
DNA. Proc. Natl Acad. Sci. US, 48: 1409-1416.
HORWlTZ, W. (1975) Association of Official Analytical Chemists,
Official methods of analysis. 12th ed. Washington, DC,
pp. 1094.
HOWARD, J. W., FAZlO, T., & WATTS, J. O. (1970) Extraction and gas
chromatographic determination of N-nitrosodimethy-lamine in
smoked fish application to smoked nitrite-treated chub. J.
Assoc. Off. Anal. Chem., 53(2): 269-274.
INGRAM, M. (1974) The microbiological effects of nitrite. In:
Proceedings of the International Symposium on Nitrite in Meat
Products, Zeist, 10-14 September, 1973, Wageningen, Centre for
Agricultural Publishing and Documentation, pp. 63-75.
IRETON, H. J., McGIVEN, A. R., & DAVIES, D. J. (1972) Renal
mesenchymal tumours induced in rats by dimethylnitrosamine. Light
and electron-microscope studies. J. Pathol., 108: 181-185.
ISSENBERG, P. & TANNENBAUM, S. (1972) Approaches to the
determination of volatile and non volatile N- nitroso
compounds in foods and beverages. IARC Sci. Publ., No. 3,
pp. 31-37.
ITO, N., HIASA, Y., TAMAI, A., OKAJIMA, E., & KITAMORA, H. (1969)
Histogenesis of urinary bladder tumors induced by N-butyl- N-
(4-hydroxybutyl) nitrosamine in rats. Gann, 60: 401-410.
ITO, N., MAKIURA, S., YOKOTA, Y., KAMAMOTO, Y., HIASA, Y., & SUGIHARA,
S. (1971) Effect of unilateral ureter ligation on development of
tumors in the urinary system of rats treated with N-butyl- N-
(4-hydroxybutyl) nitrosamine. Gann, 62: 359-365.
IVANKOVIC, S. & DRUCKREY, H. (1968) [Transplacental induction of
malignant tumours of the nervous system. I Ethylnitrosourea (ENU)
in BD-IX-rats.] Z. Krebsforsch., 71: 320-360 (in German).
IVANKOVIC, S. & PREUSSMANN, R. (1970) [Transplacental induction of
malignant tumours by the oral administration of ethylurea and
nitrite in rats.] Naturwiss., 57: 460-461 (in German).
IVANKOVIC, S., PREUSSMANN, R., SCHMÄHL, D., & gELLER, J. (1973)
[Prevention of nitrosamide induced hydrocephali by ascorbic acid
after prenatal administration of ethylurea and nitrite to rats.]
Z. Krebsforsch, 79: 145-147 (in German).
JACKSON, W. A., STEEL, J. S., & BOSWELL, V. R. (1967) Nitrates in
edible vegetables and vegetable products. Proc. Am. Soc. Hort.
Sci., 90: 349-352.
JAFFÉ, E. R. & HELLER, P. (1964) Methemoglobinemia in man. In: Moore,
C. V. & Brown, E. B., ed. Progress in hematology, vol. IV, New
York, Grune & Stratton, pp. 48-71.
JAPAN ENVIRONMENTAL SANITATION CENTER (1974) [Report of 1973 results
of the analysis for suspended particulate matter in the National
Air Sampling Network.] Kawasaki, Japan, pp. 14, 24 (in Japanese).
JASMIN, G. & CHA, J. W. (1969) Renal adenomas induced in rats by
dimethylnitrosamine. An electron microscopic study. Arch.
Pathol., 87: 267-278.
JENISSEN, H., HOSHINO, J., & KROGER, H. (1971) [The influence of
carcinogenic nitrosamines on the induction of serine dehydratase
in the rat liver.] Z. Krebsforsch., 75: 246-252 (in German).
JOHNSON, J. H. (1966) Internal can corrosion due to high nitrate
content of canned vegetables. Proc. Fla. State Hort. Sci.,
79: 239-242.
JUSKIEWICZ, T. & KOWALSKI, B. (1974) Passage of nitrosamines from
rumen into milk in goats. IARC Sci. Publ. No. 9, pp. 173-176.
KAMM, L., MCKEOWN, G. G., & SMITH, D. M. (1965) New colorimetric
method for the determination of the nitrate and nitrite content
of baby foods. J. Assoc. Off. Agric. Chem., 48 (5): 892-897.
KAMM, J. J., DASHMAN, T., CONNEY, A. H., & BURNS, J. J. (1973)
Protective effect of ascorbic acid on hepatoxicity caused by
sodium nitrite and aminopyrin. Proc. Natl Acad. Sci.,
70: 747-749.
KAPELLER-ADLER, R. & KRAEL, J. (1930a) [Research on nitrogen
distribution in the muscle of different animal species I,]
Biochem. Z., 221: 437-460 (in German).
KAPELLER-ADLER, R. & KRAEL, J. (1930b) [Research on nitrogen
distribution in the muscle of different animal species II.]
Biochem. Z., 224: 364-377 (in German).
KAUDEWITZ, F. (1959) Production of bacterial mutants with nitrous
acid. Nature (Lond.), 183: 1829-1830.
KAWABATA, T. (1974) Addendum, N- nitroso compounds: Recent studies
in Japan. IARC Sci. Publ. No. 9, pp. 154-158.
KEATING, J.P., LELL, M. E., STRAUSS, A. W., ZARKOWSKY, H., & SMITH, G.
E. (1973) Infantile methemoglobinemia caused by carrot juice. N.
Eng. J. Med., 288: 824-826.
KEOHANE, K. W. & METCALF, W. K. (1960) An investigation of the
differing sensitivity of juvenile and adult erythrocytes to
methemoglobinization. Phys. med. Biol., 5: 27-35.
KILGORE, L., STASCH, A. R. & BARRENTINE, B. F. (1963) Nitrate content
of beets, collards, turnip greens. J. Am. Diet. Assoc.,
43: 39-42.
KING, E. J. (1959) Qualitative analysis and electrolytic solutions.
New York, Harcourt, Brace & Co., pp. 519.
KIRKLAND, D. J. & PICK, C. R. (1973) The histological appearance of
tumours derived from rat embryo cells transformed in vitro
spontaneously and after treatment with nitrosomethylurea. Br.
J. Cancer, 28: 440-452.
KLUBES, P. & JONDORF, W. R. (1971) Dimethyl nitrosamine formation from
sodium nitrite and dimethylamine by bacterial flora of rat
intestine. Res. Commun. chem. Pathol. Pharmacol,
2 (1): 24-34.
KNELSON, J. H. & LEE, R. E. (1977) Oxides of nitrogen in the
atmosphere: Origin, fate and public health implications. Ambio,
6 (2-3): 126-130.
KNOTEK, Z. & SCHMIDT, P. (1964) Pathogenesis, incidence and
possibilities of preventing alimentary nitrate methemoglobinemia
in infants. Pediatrics, 34: 78-83.
KOCIBA, R. J. & SLEIGHT, S. D. (1970) Nitrite toxicosis in the
ascorbic acid-deficient guinea pig. Toxicol. appl. Pharmacol.
16: 424-429.
KOHL, D. H., SHEARER, G. B., & COMMONER, B. (1971) Fertilizer
nitrogen: Contribution to nitrate in surface water in a corn belt
watershed. Science, 174: 1331-1334.
KOPPANG, N. (1964) An outbreak of toxic liver injury in ruminants.
Nord. vet. Med., 16: 305-322.
KORDAC, V., SCHON, E., & BRAUN, A. (1969) Hepatocancerogenesis in mice
with simultaneous administration of diethylnitrosamine and the
virus Motol. Neoplasma, 16: 485-490.
KOYAMA, T., HANDA, J., HANDA, H., & MATSUMOTO, S. (1970)
Methylnitrosourea-induced malformations of brain in SD-JCL rat.
Arch. Neurol., 22: 342-347.
KRAVITZ, H., ELEGANT, L. D., KAISER, E., & KAGAN, B. M. (1956)
Methemoglobin values in premature and mature infants and
children. A.M.A.J. Dis. Child., 91: 1-5.
KRÖLLER, E. (1967) [Investigations of the evidence of nitrosamines in
tobacco smoke and food.] Dtsch. Lebensm. Rundsch., 63(10):
303-305 (in German).
KRÜGER, F. W. (1972) [On the biochemistry of carcinogenic
N-nitroso compounds.] Ärztliche Forsch., 26 (6): 197-202 (in
German).
KRÜGER, F. W. (1973) Metabolism of nitrosamines in vivo. II. On the
methylation of nucleic acids by aliphatic di-n-alkylnitrosamines
in vivo, caused by beta-oxidation: The increased formation of
7-methyl-guanine after application of beta-hydroxypropyl-
nitrosamine compared to that after application of di- N-propyl-
nitrosamine. Z. Krebsforsch., 79: 90-97.
KÜBLER, W. (1958) [The importance of the nitrate content of vegetables
in child nutrition.] Z. Kinderheilk, 81: 405-416 (in German).
KÜBLER, W. (1965) [Methaemoglobinaemia in infants after feeding with
spinach.] Dtsch. med. Wschr., 90: 1881-1882 (in German).
KUNZ, W., SCHAUDE, G., & THOMAS, C. (1969) [The effect of
phenobarbital and halogenated hydrocarbons on nitrosamine
carcinogenesis.] Z. Krebsforsch., 72: 291-304 (in German).
KÜNZER, W. & SCHNEIDER, D. (1953) [The activity of the reducing enzyme
system in erythrocytes of young infants.] Acta haemat., 9: 346
(in German).
KUWAHARA, A., OTSUKA, H., & NAGMATSU, A. (1972) Induction of
hemangiomatous lesions with dimethylnitrosamine. Influence of
route of administration and strain of mice. Gann,
63: 499-502.
LACASSAGNE, A., BUU-HOI, N., GIAO, N. B., & FERRANDO, R. (1968)
Retarding effect of resirpine on the cancerization of rat liver
induced by diethylnitrosamine. Bull. Cancer,
55 (1): 87-89.
LEE, D. H. K. (1970a) Nitrates, nitrites and methemoglobinemia.
Environ. Res., 3: 484-511.
LEE, G. F. (1970b) Eutrophication. Occasional Paper No. 2,
University of Wisconsin, Water Resources Center, Madison,
pp. 12-13.
LEE, K. Y. & LIJINSKY, W. (1966) Alkylation of rat liver RNA by cyclic
N-nitrosamines in vivo, J. Natl Cancer Inst., 37
(A): 401-407.
LEHMAN, A. J. (1958) Quarterly report to the editor on topics of
current interest. Nitrates and nitrites in meat products. Assoc.
Food Drug Off. US Q. Bull., 22: 136-138.
LEMBKE, A. & MOEBUS, O. (1970) Is there a connection between the
occurrence of nitrite-producing microorganisms in cheese and the
formation of nitrosamine? Proceedings of the 18th
International Dairy Congress A.2.3, p. 139.
LIJINSKY, W. (1974) Reaction of drugs with nitrous acid as a source of
carcinogenic nitrosamines. Cancer Res., 34: 255-258.
LIJINSKY, W. & EPSTEIN, S.S. (1970) Nitrosamines as environmental
carcinogens. Nature (Lond.), 225: 21-23.
LIJINSKY, W. & GREENBLATT, M. (1972) Carcinogen dimethylnitrosamine
produced in vivo from nitrite and aminopyrine. Nature (New
Biology), 236: 177-178.
LIJINSKY, W. & SINGER, G. (1974) Formation of nitrosamines from
tertiary amines and nitrous acid. IARC Sci. Publ. No. 9,
pp. 111-114.
LIJINSKY, W., TOMATIS, L, & WENGEN, C. E. M. (1969) Lung tumors in
rats treated with N-nitrosoheptamethyleneimine and
N-nitrosooctamethyleneimine. Proc. Soc. Exp. Biol. Med.,
130: 945-949.
LIJINSKY, W., FERRERO, A., MONTESANO, R., & WENYON, C. E. M. (1970)
Tumorigenecity of cyclic nitrosamines in Syrian golden hamsters.
Z. Krebsforsch., 74: 185-189.
LIJINSKY, W., CONRAD, E., & VAN DE BOGART, R. (1972a) Carcinogenic
nitrosamines formed by drug/nitrite interactions. Nature
(Lond.), 239: 165-167.
LIJINSKY, W., CONRAD, E., & VAN DE BOGART, R. (1972b) Formation of
carcinogenic nitrosamines by interaction of drugs with nitrite.
IARC Sci. Publ. No. 3, pp. 130-133.
LIJINSKY, W., KEEFER, L., CONRAD, E., & VAN DE BOGART, R. (1972c)
Nitrosation of tertiary amines and some biologic implications.
J. Natl Cancer Inst., 49 (5): 1239-1249.
LIJINSKY, W., GREENBLATT, M., & KOMMINENI, C. (1973a) Brief
communication: Feeding studies of nitrilotriacetic acid and
derivatives in rats. J. Natl Cancer Inst., 50: 1061-1063.
LIJINSKY, W., KEEFER, L., LOO, J., & ROSS, A. E. (1973b) Studies of
alkylation of nucleic acids in rats by cyclic nitrosamines.
Cancer Res., 33: 1634-1641.
LIJINSKY, W., TAYLOR, H. W., SNYDER, C., & NETTESHEIM, P. (1973c)
Malignant tumors of liver and lung in rats fed aminopyrine or
heptamethyleneimine together with nitrite. Nature (Lond.),
244: 176-178.
LOVELESS, A. & HAMPTON, C. (1969) Inactivation and mutation of
coliphage T2 by N-methyl- and N-ethyl- N-nitrosourea.
Murat. Res., 7: 1-12.
LUHRS, C. E. (1973) Nitrogenous materials in the environment. Draft
report. US Environmental Protection Agency, Hazardous Materials
Advisory Committee.
MAEKAWA, H. & ODASHIMA, S. (1975) Induction of tumours of the nervous
system in the HCI/N rat with N-buyl-I-nitrosourea administered
transplacentally, neonatally or via maternal milk. Gann,
66: 175-183.
MAGEE, P. N. (1956) Toxic liver injury. The metabolism of
dimethylnitrosamine. Biochem. J., 64: 676-682.
MAGEE, P. N. (1972) Possibilities of hazard from nitrosamines in
industry. Ann. occup. Hyg., 15: 19-22.
MAGEE, P. N. & BARNES, J. M. (1962) Induction of kidney tumours in the
rat with dimethyl ( N-nitrosodimethylamine) nitrosamine.
J. Pathol. Bacteriol., 84: 19-31.
MAGEE, P. N. & BARNES, J. M. (1967) Carcinogenic nitroso compounds.
Adv. Cancer Res., 10: 163-246.
MAGEE, P. N., MONTESANO, R., & PREUSSMANN, R. (1976) N-nitroso
compounds and related carcinogens. In: Searle, E. ed., Chemical
carcinogens. ACS monograph No. 173, pp 491-625.
MAKIURA, S., KAMMAMOTO, Y., SUGIHARA, S., HIRAO, K., HIASA, Y., ARAI,
M., & ITO, N. (1973) Effect of 1-naphthyl isothiocyanate and
3-methylcholanthrene on hepato-carcinogenesis in rats treated with
diethylnitrosamine. Gann, 64: 101-104.
MALINS, D.C., ROUBAL, W. T., & ROBISCH, P. A. (1970) The possible
nitrosation of amines in smoked chub. J. agric. Food. Chem.,
18(4): 740-741.
MALLING, H. V. (1966) Mutagenicity of two potent carcinogens
dimethylnitrosamine and diethylnitrosamine in Neurospora
crassa. Mutat. Res., 3: 537-540.
MANNING, P. B., COULTRER, S. T., & JENNESS, R. (1968) Determination of
nitrate and nitrite in milk and dry milk products. J. Dairy
Sci., 51: (11): 1725-1730.
MARCULESCU, V. (1917) Determination of nitrates in water. Stud.
Prot. Epurarea Apelor., 15: 127-161
MARRIOTT, W. M., HARTMANN, A. F., & SENN, M. J. E. (1933) Observations
on the nature and treatment of diarrhea and the associated
systemic disturbances. J. Pediatr., 3: 181-191.
MCLEAN, A. E. M. & MAGEE, P. N. (1970) Increased renal carcinogenesis
by dimethylnitrosamine in protein deficient rats. Br. J. exp.
Pathol., 51: 587-590.
MCLEAN, A. E. & VERSCHUUREN, H. G. (1969) Effects of diet and
microsomal enzyme induction on the toxicity of
dimethylnitrosamine. Br. J. exp. Pathol., 50: 22-25.
METCALF, W. K. (1961) The sensitivity of haemoglobin to oxidation in
various conditions. Nature (Lond.), 190: 543-544.
MIRNA, A. (1974) Determination of free and bound nitrite. In:
Proceedings of the International Symposium on Nitrite in Meat
Products, Ziest, 10-14 September 1973, Wageningen, Centre for
Agricultural Publishing and Documentation, pp. 21-28.
MIRVISH, S.S. (1970) Kinetics of dimethylamine nitrosation in relation
to nitrosamine carcinogenesis. J. Natl Cancer Inst., 44
(3): 633-639.
MIRVISH, S.S. (1971) Kinetics of nitrosamide formation from
alkylureas, N-alkylurethanes and alkylguanidines: possible
implications for the etiology of human gastric cancer. J. Natl
Cancer Inst., 46: 1183-1193.
MIRVISH, S.S. (1975) Formation of N-nitroso compounds: chemistry,
kinetics, and in vivo occurrence. Toxicol. appl. Pharmacol,
31: 325-351.
MIRVISH, S.S. (in press) N-nitroso compounds, nitrite and nitrate:
possible implications for the causation of human cancer. J.
Water Pollut.
MIRVISH, S., WALLCAVE, L., EAGEN, M., & SHUBIK, P. (1972) Ascorbate-
nitrite reaction; possible means of blocking the formation of
carcinogenic N-nitroso compounds. Science, 177: 65-68.
MIRVISH, S.S., SAMS, J., FAN, T. Y., & TANNENBAUM, S. R. (1973a)
Kinetics of nitrosation of the amino acids proline hydroxyproline
and sarcosine. J. Natl Cancer Inst., 51: 1833-1840.
MIRVISH, S.S., CARDESA, A., WALLCAVE, L., & SHUBIK, P. (1973b) Effect
on sodium ascorabte on lung adenoma induction by amines plus
nitrite. Proc. Am. Assoc. Cancer Res., 14: 102.
MIRVISH, S.S., ARNOLD, S., CONRAD, E., GHADIRIAN, P., KOMMINENI, V. R.
C., & SAMS, J. (1974) The formation of N- nitroso compounds,
preparation of heptafiuorobutyryl derivatives of ureas, and the
fate of nitrite in the rat stomach. IARC Sci. Publ. No. 9,
pp. 67-70.
MIRVISH, S.S., CARDESA, A., WALLCAVE, L., & SHUBIK, P. (1975)
Induction of mouse lung adenomas by amines or ureas plus nitrite
and by N-nitroso compounds: effects of ascorbates, gallic acid,
thyocyanate, and caffeine. J. Natl Cancer Inst., 55: 633-636.
MIRVISH, S.S., ISSENBERG, P., & SORNSON, H. C. (1976) Air-water and
ether-water distribution of N-nitroso compounds: implications
for laboratory safety, analytical methodology, and
carcinogenicity for the rat esophagus, nose, and liver. J. Natl
Cancer Inst., 56 (6): 1125-1129.
MÖHLER, K. & MAYRHOFER, O. L. (1968) [Evidence and determination of
nitrosamines in food.] Z. Lebensm. Unter-Forsch., 135 (6):
313-318 (in German).
MÖHLER, K. & MAYRHOFER, O. L. (1969) [Effect of various factors on the
formation of nitrosamines in meat products.] 15th European
Meeting of the Meat Research Workers, Helsinki, 17-24 August
1969, pp. 302-304 (in German).
MOHR, U. & HILFRICH, J. (1972) Brief communication: Effect of a single
dose of N-diethylnitrosamine on the rat kidney. J. Natl
Cancer Inst., 49: 1729-1731.
MONTESANG, R. (1970) Systemic carcinogens ( N-nitroso compounds) and
synergistic or additive effects of respiratory carcinogenesis.
Tumori, 56: 335-344.
MONTESANO, R. & BARTCH, H. (1976) Mutagenic and carcinogenic
N-nitroso compounds: possible environmental hazards. Mutat.
Res., 32: 179-228.
MONTESANO, R. & SAFFIOTTI, U. (1968) Carcinogenic response of the
respiratory tract of Syrian golden hamsters to different doses of
diethylnitrosamine. Cancer Res., 28: 2197-2210.
MOTYLEV, V. D. (1969) [The question of age-related methaemoglobinaemia
caused by nitrates and nitrites and its prophylaxis.] Author's
summary of a thesis for the degree of Candidate of Medical
Sciences, Leningrad, (in Russian).
MURTHY, Y. K. S., THIEMANN, J. E., CORONELLI, C. I., & SENSl, P.
(1966) Alanosine, a new antiviral and antitumour agent isolated
from a streptomyces. Nature (Lond.), 211: 1198-1199.
MYSLIWY, T. S., WICK, E. L., ARCHER, M. C., SHANK, R. C., & NEWBERNE,
P.M. (1974) Formation of N-nitrosopyrrolidine in a dog's
stomach. Br. J. Cancer, 30: 279-283.
NAGAWA, Y. & MIRNA, A. (1974) [Influence exerted by processing upon
the formation of nitrosamines in meat products.]
Fleischwirtschaft., 1781-1788 (in German).
NAPALKOV, N. P. (1971) [Experimentation on transplacental
blastomogenesis as a tool for study of etiopathogenesis of
tumours in children.] Vop. Onkol., 17 (8): 3-15 (in Russian).
NASHED, N. & JUBBUR, G. (1966) A genetic and functional
characterization of adenine mutants induced in yeast by
1-nitroso-imidazolidone-2 and nitrous acid. Z. Verer-bungsl,
98: 106-110.
NEURATH, H. (1972) Nitrosamine formation from precursors in tobacco
smoke. IARC Sci. Publ. No. 3, pp. 134-136.
NEURATH, G. & SCHREIBER, O. (1974) Investigations on amines in the
human environment. Sci. Publ. No. 9, pp. 211-214.
NEURATH, G., PIRMANN, B., & WICHERNOTT, (1964) [On the question of
nitroso compounds in tobacco smoke.] Beitr. Tabakforsch.,
2(7): 311-319 (in German).
NEWBERNE, P.M. & SHANK, R. C. (1973) Induction of liver and lung
tumors in rats by the simultaneous administration of sodium
nitrite and morpholine. Food Cosmet. Toxicol., 11: 819-825.
NEWELL, J. E. & SISKEN, H. R. (1972) Determination of nitrosodi-
methylamine in the low parts per billion. J. agric. Food Chem.,
20 (3): 711-713.
NIEHS (1970) Nitrates, nitrites and methemoglobinemia. North
Carolina, National Institute of Environmental Health Sciences,
pp. 43 (Rep. No. 2).
NOROHNA, R. F. & GOODALL, C. M. (1972) Nasal tumors in starved rats
injected once with dimethylnitrosamine. N. Z. med. J.,
75: 374-375.
NYE, J. C. (1973) Nitrogenous compounds in the environment. Draft
Report, Washington, DC, US Environmental Protection Agency,
Hazardous Materials Advisory Committee, pp. 95-109.
O'CONNOR, P. J., CAPPS, M. J., & CRAIG, A. W. (1973) Comparative
studies of the hepatocarcinogen N,N-dimethylnitrosamine in
vivo: reaction sites in rat liver DNA and the significance of
their relative stabilities. Br. J. Cancer, 27: 153-166.
ODASHIMA, S. (1969) Experimental carcinoma of the glandular stomach in
rats. I. Effect of 7, 12-dimethylbenz-[a]anthracene or
4-nitroquinolene 1-oxide placed on glandular stomach combined with
oral administration of N,N1-(2,7-fluorenylene) bisacetamide
or N-nitrosodiethylamine. Gann, 60: 211-222.
OEHME, F. W. (1975) Vetinary toxicology. In: Caserett, L. J. & Doulle,
J., ed. Toxicology, the basic science of poisons. New York,
Macmillan Publishing Co., Inc., pp. 701-727.
OKADA, M. & SUZUKI, E. (1972) Metabolism of butyl (4-hydroxybutyl)
nitrosamine in rats. Gann, 63 (3): 391-392.
OKADA, M., SUZUKI, E., ANJYO, T., & MOCHIZUKI, M. (1975) Mutagenicity
of gamma-acetoxy-dialkylnitrosamines: model compounds for an
ultimate carcinogen. Gann, 66 (4): 457-458.
OKAJIMA, E., HIRAMATSU, T., MOTOMIYA, Y., IRWA, K., IJUIN, M., & ITO,
N. (1971) Effect of DL-tryptophan on tumorigenesis in the urinary
bladder and liver of rats treated with N-nitrosodibutylamine.
Gann, 62: 163-169.
OLSON. O. E., NELSON, D. L., & EMERICK, R. J. (1963) Effect of nitrate
and some of its reduction products on carotine stability.
J. agric. Food Chem, 11: 140-143.
ORGERON, J. D., MARTIN, J. D., CARAWAY, C. T., MARTINE, R. M., &
HAUSER, G. H. (1957) Methemoglobinemia from eating meat with high
nitrite content. Public Health Rep., 72 (3): 189-193.
OSSKE, G., WARZOK, R., & SCHNEIDER, J. (1972) [Transplacental tumour
induction by endogenous N-ethyl- N-nitrosourea in the rat.]
J. Arch. Geschwulstforsch., 40: 244-247 (in German).
OWENS, M. (1970) Nutrient balances in rivers. Proc. Soc. Water
Treat. Exam., 19: 239-247.
PANALAKS, T., IYENGAR, J. R., & SEN, N. P. (1972) Nitrate, nitrite and
dimethylnitrosamine in cured meat products. J. Assoc. Off.
Anal. Chem, 56 (3): 621-625.
PANALAKS, T., IYENGAR, J. R., DONALDSON, B. A., MILES, W. F., & SEN.
N. P. (1974) Further survey of cured meat products for volatile
N-nitrosamines. J. Assoc. Off. Anal. Chem., 57 (4): 806-812.
PASTERNAK, L. (1962) [Mutagenic effects of dimethylnitrosamine with
Drosophila melanogaster.] Naturwiss., 16: 381 (in German).
PASTERNAK, L. (1964) Investigation into the mutagenic effect of
several nitrosamine and nitrosamide compounds. Arzneintittel-
Forsch., 14: 802-804.
PENSABENE, J. W., FIDDLER, W., GATES, R. A., FAGAN, J. C., &
WASSERMAN, A. E. (1974) Effect of frying and other cooking
conditions on nitrosopyrrolidine formation in bacon.
J. Food Sci., 39: 314-136.
PERRY, T. L., SHAW, K. N. F., WALKER, D., & REDLICH, D. (1962) Urinary
excretion of amines in normal children. Pediatrics, 30: 576-584.
PETUKHOV, N. I., RYVKIN, A. I., GAINULLEN, G. G., & LANDYSHEVA, V. I.
(1972) [Methaemoglobinaemia in children and adults caused by
nitrates in water] Gig. i Sanit., 3: 14-17 (in Russian).
PETUKHOV, N. I. & IVANOV, A. V. (1970) Investigation of certain
psychophysiological reactions in children suffering from
methemoglobinemia due to nitrates in water. Gig. i Sanit.,
35 (1): 26-28.
PHILLIPS, J. C., LAKE, B. G., HEADING, C. E., GANGOLLI, S. D., &
LLOYD, A. G. (1975a) Studies on the metabolism of dimethyl-
nitrosamine in the rat. I. Effect of dose, route of
administration and sex. Food Cosmet. Toxicol., 13: 203-209.
PHILLIPS, J. C., HEADING, C. E., LAKE, B. O., GANGOLLI, S. D., &
LLOYD, A. G. (1975b) Studies on the metabolism of
dimethylnitrosamine in the rat. II. The effects of phenobarbitone
and 20-methylcholanthrene on the in vitro and in vivo
metabolism and acute toxicity of dimethylnitrosamine in young
and mature rats. Food Cosmet. Toxicol., 13: 611-617.
PHILLIPS, W. E. J. (1966) Effect of dietary nitrite on the liver
storage of vitamin A in the rat. Can. J. Blochem.,
44 (1): 1-7.
PHILLIPS, W. E. J. (1968a) Changes in the nitrate and nitrite contents
of fresh and processed spinach during storage. J. agric. Food
Chem., 16 (1): 88-91.
PHILLIPS, W. E. J. (1968b) Nitrate content of foods -- public health
implications. Can. Inst. Food Technol. J., 1 (3): 98-103.
PHILLIPS, W. E. J. (1969) Lack of nitrite accumulation in partially
consumed jars of baby food. Can. Inst. Food Technol. J.,
2 (4): 160-161.
PHILLIPS, W. E. J. (1971) Naturally occurring nitrate and nitrite in
foods in relation to infant methemoglobinemia. Food Cosmet.
Toxicol., 9: 219-228.
PIELSTICKER, K. (1967) Absence of malformations following
diethylnitrosamine in the golden hamster. Naturwiss.,
54: 340-341.
PISCIOTTA, A. V., EBBE, S. N., & HINZ, J. E. (1959) Clinical and
laboratory features of two variants of methemoglobin M disease.
J. Lab. clin. Med, 54: 73-87.
PITTS, J. N. Jr. & LLOYD, A. C. (1973) Discharges into the atmosphere.
In: Nitrogenous compounds in the environment. Washington, US
Environmental Protection Agency, pp. 43-65 (Rep No. EPA-SAB-73-
001).
PLATT, D. & HERING, F. J. (1973) The effect of Calciparin on
diethylnitrosamine-induced liver tumors in the rat.
Arzneimitteljbrsch., 23 (7): 956-961.
POUND, A, W. LAWSON, T. A., & HORN, L. (1973) Increased carcinogenic
action of dimethylnitrosamine after prior administration of
carbon tetrachloride. Br. J. Cancer, 27: 451-459.
PREDA, N., POPA, L., GALEA, V., & SIMU, G. (1976) N-nitroso compound
formation by chiordiazepoxide and nitrite interaction in vitro
and in vivo: protective action of ascorbic acid. IARC Sci.
Publ. No. 14, pp. 301-304.
PREUSSMAN, R. (1975) [Carcinogenic chemicals in the human
environment.] Handbuch der Allg. Pathol., VI/6/11. 448-482 (in
German).
PURCHASE, I. F. H., TUSTIN, R. C., & van RENSBERG, S. J. (1975)
Biological testing of food grown in the Transkei. Food Cosmet.
Toxicol, 13: 639-647.
QUISPEL, A., ed. (1974) -- The biology of nitrogen fixation.
Amsterdam, North Holland Publishing Company,
RABES, H., HARTENSTEIN, R., & SCHOLZE, P. (1970) Specific stages of
cellular response to homeostatic control during
diethylnitrosamine-induced liver carcinogenesis. Experientia
(Basel), 26: 1356-1359.
RABES, H., HARTENSTEZN, R., & GMINDER, J. (1971) Kidney neoplasms
induced by diethylnitrosamine in partially hepatectomized rats.
Naturwiss., 58: 102-103.
RAMMELL, C. G. & JOERIN, M. N. (1972) Determination of nitrite in
cheese and other dairy products. J. Dairy Res., 39: 89-94.
REDDY, B. S. & THOMAS, J. W. (1962) Interrelationship between thyroid
status and nitrate on carotine conversion. J. anim. Sci.,
21: 1010.
REEVES, M. J., BIRTLES, A. B., COURCHE, R. C., & ALDRICK, R. J. (1974)
Groundwater resources of the Vale of York. London, Water
Resources Board Report, pp. 90.
RHOADES, J. W. & JOHNSON, D. E. (1970) Gas-chromatography and
selective detection of N-nitrosamines. J. Chromatogr. Sci.,
8: 616-618.
RICHARDSON, W. D. (1907) Nitrates in vegetable foods in cured meats
and elsewhere. J. Am. Chem. Soc, 29: 1757-1767.
RIDDER, W. E. & OEHME, F. W. (1974) Nitrates as an environmental,
animal, and human hazard. Clin. Toxicol 7 (2): 145-159.
RIEDEL, B. & PIPER, R. (1973) Early changes of the rat bladder mucosa
after experimental cancerogenesis with N-butyl- N-butanol
(4)-nitrosamine.] (author's transl.). Urol. Int, 28: 322-327.
ROBERTS, J. D. & CASERIO, M. C. (1964) Basic Principles of Organic
Chemistry, New York, Benjamin, pp. 656-666.
ROBERTS, W. K. & SELL, J. L. (1963) Vitamin A destruction by nitrite
in vitro and in vivo. J. anim. Sci., 22: 1081-1085.
ROBERTSON, H. E. & DRAYCOTT, M. E. (1948) Nitrate poisoning of
infants by contaminated drinking water. Abstracts of papers
presented at the fifteenth Annual Christmas Meeting of the
Laboratory Section, Canadian Public Health Association, London,
Ontario, 13-14 December, p. 30.
ROBERTSON, H. E. & RIDDELL, W. A. (1949) Cyanosis of infants produced
by high nitrate concentration in rural waters of Saskatchewan.
Can. J. Public Health, 40: 72-77.
ROBINSON, E. & ROBBINS, R. C. (1972) Emissions, concentrations and
fate of gaseous atmospheric pollutants. In: Strauss, W., ed. Air
pollution control Part II, New York, Wiley-Interscience,
pp. 1-93,
ROLLER, P. P. & KEEFER, L. K. (1974) Catalysis of nitrosation
reactions by electrophilic species. IARC Sci. Publ. No. 9,
pp. 86-89.
ROSS, J. D. (1963) Deficient activity of DPNH-dependent methemoglobin
diaphorase in cord blood erythrocytes. Blood, 21 (1): 51-62.
ROSS, J. D. & DESFORGES, J. F. (1959) Reduction of methemoglobin by
erythrocytes from cord blood. Pediatrics, 23: 718-726.
SAKSHAUG, J., SÖGNEN, E., HANSEN, M. A., & KOPPANG, N. (1965)
Dimethylnitrosamine; its hepatotoxic effect in sheep and its
occurrence in toxic batches of herring meal. Nature (Lond.),
206: 1261-1262.
SANDER, J. (1971a) [Investigations on the formation of carcinogenic
nitroso compounds in the stomach of experimental animals and its
significance for man. I] Arzneimittel-Forsch., 21: 1572-1580
(in German).
SANDER, J. (1971b) [Investigations on the formation of carcinogenic
nitroso compounds in the stomach of experimental animals and its
significance for man.] Arzneimittel-Forsch., 21: 1703-1707 (in
German).
SANDER, J. (1971c)[Further experiments on tumour induction by oral
administration of small doses of N-methylbenzylamine and sodium
nitrite.] Z. Krebsforsch., 76: 93-96 (in German).
SANDER, J. & BÜRKLE, G. (1969) [Induction of malignant tumors in rats
by simultaneous feeding of nitrite and secondary amines.]
Z. Krebsforsch., 73: 54-66 (in German).
SANDER, J. & SEIF, F. (1969) [Bacterial reduction of nitrate in the
stomach of man as a cause of nitrosamine formation.]
Arzneimittel-Forsch, 19: 1091-1093 (in German).
SANDER, J. & SCHWEINSBERG, F. (1972) In vivo and in vitro
experiments on the formation of N- nitroso compounds from
amines or amides and nitrate or nitrite. IARC Sci. Publ. No. 3,
pp. 97-103.
SANDER, J. & SCHWEZNSBERG, F. (1973) [Tumor induction in mice with
methylbenzylnitrosamine in low doses.] Z. Krebsforsch,
79: 157-161 (in German).
SANDER, J., SCHWEINSBERG, F., & MENZ, H. P. (1968) [Investigations on
the formation of carcinogenic nitrosamine in the stomach.]
Hoppe-Seyler's Z. physiol. Chem, 349: 1691-1697 (in German).
SANDER, J., BURKLE, G., FLOHE, L., & AEIKENS, B. (1971) [ In vitro
investigations on the possibility of the formation of
carcinogenic nitrosamide in the stomach.] Arzneim-Forsch.,
(Drug Res.), 21 (3): 411-414 (in German).
SANDER, J., LABAR, J., LADENSTEIN, M., & SCHWEINSBERG, F. (1974a)
Quantitative measurement of in vivo nitrosamine formation.
IARC Sci. Publ. No. 9, pp. 123-131.
SANDER, J., LABAR, J., LADENSTEIN, M., & SCHWEINSBERG, F. (1974b)
Experiments on the degradation of nitrosamines in plants. IARC
Sci. Publ. No. 9, pp. 205-210.
SATTELMACHER, P. G. (1962) [Methemoglobinemia from nitrates in
drinking water.] Schriflenr. Ver. Wasser Boden. Lufthyg., No. 21
(in German).
SAWYER, C. N. (1947) Fertilization of lakes by agricultural and urban
drainage. J. New England Water Works Assoc., 109-127.
SCANLAN, R. A. (1975) Nitrosamines in food. CRC Critical Reviews in
Food Technology, April 1975, pp. 357-402.
SCHERER, E., HOFFMAN, M., EMMELOT, P., & FRIEDRICH-FRESKA, M. (1972)
Quantitative study on foci of altered liver cells induced in the
rat by a single dose of diethylnitrosamine and partial
hepatectomy. J. Natl Cancer Inst., 49: 93-106.
SCHEUNIG, O. & ZIEBARTH, D. (1976) Formation of nitrosamines by
interaction of some drugs with nitrite in human gastric juice.
IARC Sci. Publ. No. 14, pp. 269-277.
SCHMÄHL, D. (1963) [The origin, growth and chemotherapy of malignant
tumours.] Arzneimittei-Forsch., 13 (Supplement): 612 (in
German).
SCHMÄHL, D. (1970) [Experimental studies on syncarcinogenesis VI: The
addition of minimal doses of four different hepatotropic
carcinogens in liver cancer induction in the rat.]
Z. Krebsforsch., 74: 457-466 (in German).
SCHMÄHL, D. & STACKELBERG, S. YON (1968)[The influence of lactoflavin,
nicotinamide or dipyridamole on the carcinogenic activity of
diethylnitrosamine in rats.] Arzneimittel-Forsch., 18: 318-320
(in German).
SCHMÄHL, D. & THOMAS, C. (1962) [Acute nitrosomethylurethane poisoning
in rats following oral application.] Arzneimittel-Forsch.,
12: 585-587 (in German).
SCHMÄHL, D., THOMAS, C., & KÖNIG, K. (1963) [Studies of
syncarcinogenesis: I. Experiments on the induction of cancer in
rats by the simultaneous administration of diethyinitrosamine and
4-dimethylamino-azobenzene.] Z. Krebsforsch., 65: 351-377
(in German).
SCHMÄHL, D., THOMAS, C., SATTLER, W., & SCHIELD, G. F. (1965)
[Experimental studies of syncarcinogenesis: III. Attempts to
induce cancer in rats by administering diethylnitrosamine and
CC14 (or ethyl alcohol) simultaneously. In addition, an
experimental contribution regarding "alcoholic cirrhosis".]
Z. Krebsforsch., 66: 526-532 (in German).
SCHMÄHL, D., STUTZ, E., & THOMAS, C. (1966) [Experimental studies on
syncarcinogenesis. V. Experiments on cancer generation in rats
with the simultaneous application of X-ray radiation and
diethylnitrosamine or 4-dimethyl-aminobiphenyl.]
Z. Krebsforsch., 68: 68-72 (in German).
SCHMÄHL, D., WAGNER, R., & SCHERF, H. R. (1971) [Influence of
immunosuppressive drugs on cancer formation in rat liver by
diethyinitrosamine and on the induction of fibrosarcoma by
3,4-benzopyrene.] Arzneimittel-Forsch, 21 (3): 403-404
(in German).
SCHMIDT, P. & KNOTEK, Z. (1970) Epidemiological evaluation of
nitrates as ground water contaminants in Czechoslovakia (Paper
presented to the Sixth International Water Pollution Research
Conference, San Francisco).
SCHMID-RUPPIN, K. H. & PAPADOUPOULU, G. (1972) [Effect of
diethylnitrosamine (DENA) and influenza viruses on the induction
of lung carcinomas in mice.] Z. Krebsforsch., 77: 150-154
(in German).
SCHMITZ-MOORMAN, P., GEDIGK, P., & DHARAMADHACH, A. (1972) [Early
histological and histochemical changes during experimental
production of liver carcinoma by diethylnitrosamine.]
Z. Krebsforsch, 77: 9-16 (in German).
SCHNEIDER, N. R. & YEARY, R. A. (1973) Measurement of nitrite and
nitrate in blood. Am. J. vet. Res., 34 (1): 133-136.
SCHOENTAL, R. (1960) Carcinogenic action of diagomethane and of
nitroso- N-methylurethane. Nature (Lond.), 188: 420-421.
SCHREIBER, H., NETTESHEIM, P., LIJINSKY, W., RICHTER, C. B., &
WALBURO, H. E. (1972) Induction of lung cancer in germfree,
specific-pathogen-free and infected rats by
N-nitrosoheptamethyleneimine. Enhancement by respiratory
infection. J. Natl Cancer Inst., 49: 1107-1114.
SCHUPHAN, W. (1965) [The nitrate content of spinach (Spinacia
oleracea, L) in relation to methaemoglobinaemia in children.]
Z. Ernähr. Wiss., 5 (3-4): 207-209 (in German).
SCHUPHAN, W. (1969) [The formation of nitrate and nitrite in plant
metabolism.] Nutritio Dieta, 11: 120-132 (in German).
SCHWEINSBERG, F. & SANDER, J. (1972) [Carcinogenic nitrosamine from
simple aliphatic tertiary amines and nitrite.] Hoppe-Seyler's
Z. physiol. Chem., 353: 1671-1676 (in German).
SEBRANEK, J. G., CASSENS, R. G., & HOEKSTRA, W. G. (1974) Fate of
added nitrite. In: Proceedings of the International Symposium
on Nitrite in Meat Products, Ziest, 10-14 September, 1973,
Wageningen, Centre for Agricultural Publishing and
Documentation, pp. 139-147.
SEHGAL, O. P. & KRAUSE, G. F. (1968) Efficiency of nitrous acid as an
inactivating and mutagenic agent of intact tobacco mosaic virus
and its isolated nucleic acid. J. Virol., 2 (10): 966-971.
SELENKA, F. (1970) Formation and reduction of nitrite in infant
nutrients containing nitrate. Archiv. Hyg., 154 (4): 336-345.
SELL, J. L. & ROBERTS, W. K. (1963) Effects of dietary nitrite on the
chick: Growth, liver vitamin A stores and thyroid weight. J.
Nutr, 79: 171-178.
SEN, N. P. (1970) Gas-liquid chromatographic determination of
dimethylnitrosamine as dimethylnitramine at picogram levels.
J. Chromatogr, 51: 301-304.
SEN, N. P. (1972) The evidence for the presence of dimethylnitrosamine
in meat products. Food Cosmet. Toxicol., 10: 219-223.
SEN, N. P. (1974) Nitrosamines in toxic constituents in animal
foodstuffs. New York, Academic Press Inc., pp. 131.
SEN, N. P. & DALPE, C. (1972) A simple thin-layer chromatographic
technique for the semi-quantitative determination of volatile
nitrosamines in alcoholic beverages. Analyst,
97: 216-220.
SEN, N. P. & DONALDSON, B. (1974) The effect of ascorbic acid and
glutathiane on the formation of nitrosopiperazines from
piperazine adipate and nitrite. IARC Sci. Publ.
No. 9, pp. 103-106.
SEN, N. P., SMITH, D.C., SCHWINGHAMER, L., & MARLEAU, J. J. (1969a)
Diethylnitrosamine and other N-nitrosamines in foods. J.
Assoc. Off. Anal. Chem., 52 (1): 47-52.
SEN, N. P., SMITH, D.C., & SCHWINGHAMER, L. (1969b) Formation of
N-nitrosamines from secondary amines and nitrite in human and
animal gastric juice. Food Cosmet. Toxicol., 7: 301-307.
SEN, N. P., SMITH, D.C., SCHWINGHAMER, L., & HOWSAM, B. (1970)
Formation of nitrosamines in nitrite-treated fish. Can. Inst.
Food Technol. J., 3 (2): 66-69.
SEN, N. P., SCHWINGHAMER, L. A., DONALDSON, B. A., & MILES, W. F.
(1972) N-nitrosodimethylamine in fish meal. J. agric. Food
Chem., 20 (6): 1280-1281.
SEN, N. P., DONALDSON, B., IYENGAR, J. R., & PANALAKS, T. (1973a)
Nitrosopyrrolidine and dimethylnitrosamine in bacon. Nature
(Lond.), 241: 473-474.
SEN, N. P., MILES, W. F., DONALDSON, B., PANALAKS, T., & IYENGAR, J.
R. (1973b) Formation of nitrosamines in a meat curing mixture.
Nature (Lond.), 245: 104-105.
SEN, N. P., IYENGAR, J. R., DONALDSON, B. A., & PANALAKS, T. (1974a)
Effect of sodium nitrite concentration on the formation of
nitrosopyrrolidine and dimethylnitrosamine in fried bacon. J.
agric. Food Chem, 22 (3): 540-541.
SEN, N. P., DONALDSON, B., CHARBONNEAU, C., & MILES, W. F. (1974b)
Effect of additives on the formation of nitrosamines in meat
curing mixtures containing spices and nitrite. J. agric. Food
Chem., 22 (6): 1125-1130.
SEN, N. P., IYENGAR, J. R., MILES, W. F., & PANALAKS, T. (1976)
Nitrosamines in cured meat products. IARC Sci. Publ.
No. 14, pp. 333-342.
SERFONTEIN, W. J. & HURTER, P. (1966) Nitrosamines as environmental
carcinogens. II. Evidence for the presence of nitrosamines in
tobacco smoke condensate. Cancer Res., 26: 575-579.
DE SERRES, F. J., BROCKMAN, H. E., BARNETT, W. E., & KOLMARK, H. G.
(1967) Allelic complementation among nitrous acid-induced ad-3B
mutants of Neurospora crassa. Mutat. Res., 4: 415-424.
SHANK, R. C. (1975) Toxicology of N-nitroso compounds. Toxicol.
appl. Pharmacol., 31: 361-368.
SHANK, R. C. & NEWnERNE, P.M. (1972) Nitrite-morpholine induced
hepatomas. Food Cosmet. Toxicol., 10: 887-888.
SHEARER, L. A., GOLDSMITH, J. R., YOUNG, C., KEARNS, O. A., & TAMPLIN,
B. R. (1972) Methaemoglobin levels in infants in an area with
high nitrate water supply. Am. J. Public Health, 62
(9): 1174-1180.
SCHECHTER, H., GARDNER, N., & SHUVAL, H. I. (1972) A micromethod for
the determination of nitrite in blood. Anal. Chem. Acta.,
60: 93-99.
SHUVAL, H. I. & GRUENER, N. (1972) Epidemiological and toxicological
aspects of nitrates and nitrites in the environment. Am. J.
Public Health, 62: 1045-1052.
SIBAY, T. M. & HAYES, J. A. (1969) Potential carcinogenic effect of
streptozotocin. Lancet, II: 912.
SIBERT, D. & EISENBRAND, G. (1974) Induction of mitotic gene
conversion in Saccharomyces cerevisae by N-nitrosated
pesticides. Mutat. Res., 22: 121-126.
SIDDIQI, O. H. (1962) Mutagenic action of nitrous acid on
Asperigillus nidulans. Genet. Res., 3 (2): 303-314.
SIMON, C. (1966) Nitrite poisoning from spinach. Lancet, I
(7442): 872.
SIMON, C., MANZKE, H., KAY, H., & MROWETZ, G. (1964) On the
occurrence, pathogenesis, and possibilities for prophylaxis of
the methemoglobinemia caused by nitrite. Z. Kinderheilkunde,
91: 124-138.
SIMONEIT, B. R. & BURLINGAME, A. L. (1971) Organic analyses of
selected areas of Surveyor III recovered on the Apollo 12
mission. Nature (Lond.), 234: 210-211.
SINGLEY, T. L. (1962) Secondary methemoglobineamia due to the
adulteration of fish with sodium nitrite. Ann. internal Med.,
57 (5): 800-803.
SINHA, D. P. & SLEIGHT, S. D. (1971) Pathogenesis of abortion in acute
nitrite toxicosis in guinea pigs. Toxicol. appl. Pharmacol.,
18 (2): 340-347.
SINIOS VON, A. & WODSAK, W. (1965) [Spinach toxicity in children.]
Dtsch Med. Wochenschr, 90: 1856-1863 (in German).
SKRIVAN, J. (1971) Methemoglobinemia in pregnancy. Acta
Universitatis Carolinae Medica, 17: 123-160.
SLEIGHT, S. D. & ATALLAH, O. A. (1968) Reproduction in the guinea pig
as affected by chronic administration of potassium nitrate and
potassium nitrite. Toxicol. appl. Pharmacol., 12: 179-185.
SMITH, C. H. (1972) Polycythemia, methemogiobinemia,
sulfhemoglobinemia, and miscellaneous anemias In: Blood
diseases of infancy and childhood, 3rd ed., Saint Louis, The C.
V. Mosby Company, pp. 450-454.
SMITH, G. E. (1964) Nitrate problems in plants and water supplies in
Missouri. In: 2nd Annual Symposium on Relation of Geology and
Trace Elements to Nutrition.
SMITH, G. E. (1966) Causes of nitrate accumulation in plants and water
supplies. In: 18th Annual Midwest Fertilizer Conference,
Chicago, Illinois.
SMITH, J. E. & BEUTLER, E. (1966) Methemoglobin formation and
reduction in man and various animals species. Am. J. Physiol.,
210: 347-350.
SMITH, P. A. S. & LOEPPKY, R. N. (1967) Nitrosative cleavage of
tertiary amines. J. Am. Chem. Soc., 89 (5): 1147-1157.
SMITH, R. P. (1969) The significance of methaemoglobinaemia in
toxicology. In: Blood, F. R., ed. Essays in toxicology Volume
I, New York and London, Academic Press, pp. 84-113.
SMITH, R. P. (1975) Toxicology of the formed elements of the blood.
In: Casseret, L. J. & Doull, J., ed. Toxicology the basic
science of poisons. New York, Macmillan Publishing Co., Inc.,
pp. 225-243.
SPENCER, R. (1967) A study of factors affecting the quality and
shelf life of vacuum packed bacon, and of the behaviour of
Wiltshire cooked bacon packed and stored under controlled
conditions. (BFMIRA Res. Rep. No. 136).
SPIEGELHALDER, B., EISENBRAND, G., & PREUSSMAN, R. (1976) The
influence of dietary intake of nitrate on the nitrite content in
human saliva: a factor of possible relevance for in vivo
formation of N-nitroso compounds. Food Cosmet. Toxicol.,
14 (6): 545-548.
STANDFORD, G., ENGLAND, C. B., & TAYLOR, A. W. (1969) Fertilizer use
and Water quality, US Department of Agriculture, ARS,
pp. 41-168.
STEINBERG, R. A. & THOM, C. (1940) Mutations and reversions in
reproductivity of Aspergilli with nitrite, colchicine and
d-lysine. Proc. Natl Acad. Sci., 26 (6): 363-366.
STENBACK, F. G., FERRERO, A., & SHUBIK, P. (1973) Synergistic effects
of diethylnitrosamine and different dusts on respiratory
carcinogenesis in hamsters. Cancer Res., 33: 2209-2214.
STEPHANY, R. W., FREUDENTHAL, J., & SCHULLER, P. L. (1976)
Quantitative and qualitative determination of some volatile
nitrosamines in various meat products, IARC Sci. Publ.
No. 14, pp. 343-354.
STEWART, B. W., SWANN, P. F., HOLSMAN, J. W., & MAGEE, P. N. (1974)
Cellular injury and carcinogenesis. Evidence for the alkylation
of rat liver nucleic acids in vivo by N-nitrosomorpholine. Z.
Krebsforsch., 82 (1): 1-12.
STOEWSAND, G. S. (1973) Nitrite-induced methemoglobinemia in guinea
pigs: Influence of diets containing beets with varying amounts of
nitrate, and the effect of ascorbic acid, and methionine. J.
Nutr., 103 (3): 419-424.
STONE, I. M., LASCHIVER, C., & SALETAN, L. T. (1968) Nitrates in
worts, beers and brewing. Waller Stein Lab. Communications, 31
(106): 193-201.
STRACK, H. B., FREESE, E. B., & FREESE, E. (1964) Comparison of
mutation and inactivation rates induced in bacteriophage and
transforming DNA by various mutagens. Mutat. Res.,
1: 10-21.
STRACKS W. & FERON, V. J. (1973) Ultrastructure of pulmonary adenomas
induced by intratracheal instillation of diethylnitrosamine in
Syrian golden hamsters. Eur. J. Cancer, 9: 359-362.
SVOBODA, D. & HIGGINSON, J. (1968) A comparison of ultrastructural
changes in rat liver due to chemical carcinogens. Cancer Res.,
28: 1703-1733.
SWANN, P. F. & KAUFMAN, D. G. (1973) The dose response for the
induction of kidney tumors in the rat by a single dose of
dimethylnitrosamine. Br. J. Cancer, 28: 83-84.
SWANN, P. F. & MAGEE, P. W. (1971) Nitrosamine-induced carcinogenesis:
The alkylation of N-7 of guanine of nucleic acids of the rat by
diethylnitrosamine, N-ethyl- N-nitrosourea and
ethylmethanesulphonate. Biochem. J., 125: 841-847.
SWENBERG, J. A., KOESTNER, A., WESCHLER, W., & DENLINGER, R. H. (1972)
Quantitative aspects of transplacentai tumor induction with
ethylnitrosourea in rats. Cancer Res., 32: 2656-2660.
SYLVESTER, R. O. (1961) Nutrient content of drainage water forested,
urban and agricultural areas. In: Algae and Metropolitan
Wastes. Transactions of the 1960 Seminar, Robert A. Taft Sanitary
Engineering Center, pp. 80-87, (Technical Report W61-3).
TAKAYAMA, S. (1968) The histological and autoradiographical studies of
mouse liver during the course of carcinogenesis by
dimethylnitrosamine. Z. Krebsforsch., 71: 246-254.
TAKAYAMA, S. & IMAIZUMI, T. (1969) Sequential effects of chemically
different carcinogens, dimethylnitrosamine and 4-dimethylaminoazo-
benzene, on hepatocarcinogenesis in rats. Int. J. Cancer,
4: 373-383.
TAKAYAMA, S. & MURAMATSU, M. (1969) Incorporation of tritiated
dimethylnitrosamine into subcellular fractions of mouse liver
after long-term administration of dimethylnitrosamine. Z.
Krebsforsch, 73: 172-179.
TANNENBAUM, S. R., SINSKEY, A. J., WEISMAN, M., & BISHOP, W. (1974)
Nitrite in human saliva. Its possible relationship to nitrosamine
formation. J. Natl Cancer Inst., 53 (1): 79-84.
TANNENBAUM, S. R., WEISMAN, M., & FELT, D. (1976) The effect of
nitrate intake on nitrite formation in human saliva. Food
Cosmet. Toxicol., 14 (6): 549-552.
TAYLOR, H. W. & LIJINSKY, W. (1975) Tumour induction in rats by
feeding heptamethyleneimine and nitrite in water. Cancer Res.,
35: 812-815.
TELLING G. M. (1972) A gas-liquid chromatographic procedure for the
detection of volatile N-nitrosamines at the 10 parts per
billion level in foodstuffs after conversion to their
corresponding nitramines. J. Chromatogr., 73: 79-87.
TELLING, G. M., BRYCE, T. A., & ALTHORPE, J. (1971) Use of vacuum
distillation and gas chromatography mass spectrometry for
determination of low levels of volatile nitrosamines in meat
products. J. agric. Food Chem., 19(5): 937-940.
TELLING G. M., BRICE, T. A., HOAR, D., OSBORNE, D., & WELTI, D. (1974)
Progress in the analysis of volatile N-nitroso compounds.
IARC Sci. Publ. No. 9, pp. 12-17.
TERRACINI, B., MAGEE, P. N., & BARNES, J. M. (1967) Hepatic pathology
in rats on low dietary levels of dimethylnitrosamine. Br. J.
Cancer, 21: 559-565.
THOMAS, J. F. J. (1948) Water Survey Report No. 2, Ottawa river
drainage basin. Canada Department of Mines and Technical
Surveys, pp. 11-23 (Publ. No. 834).
TOMATIS, L. & CEFIS, F. (1967) The effects of multiple and single
administration of dimethylnitrosamine to hamsters. Tumori,
53: 447-451.
TOMATIS, L. (1977) Prenatal exposure to chemical carcinogens and its
effect on subsequent generations. In: Conference on Perinatal
Carcinogenesis. Washington, DC, National Cancer Institute
Monograph.
TOMLINSON, T. E. (1970) Trends in nitrate concentrations in English
rivers in relation to fertilizer. Water Treat. Exam.,
pp. 277-293.
TOUSSAINT, W. & SELENKA, F. (1970) Methemoglobin formation in young
infants. A contribution to drinking water hygiene on Rheinhessen.
Mschr. Kinderheilk., 118(6): 282-284.
UNGERER, O., EISENBRAND, G., & PREUSSMANN, R. (1974) The reaction of
nitrite with pesticides. Formation, chemical properties and
carcinogenic activity of the N-nitroso compounds of the
herbicide N-methyl-N1-(2-benzothiazolyl)-urea. Z.
Krebsforsch., 81 (3-4): 217-242.
UNITED NATIONS (1976) Statistical Yearbook, 1975, New York, p. 298.
US DEPARTMENT OF AGRICULTURE (1965) Agricultural Information
Bulletin, No. 299, Washington, DC.
US DEPARTMENT OF HEALTH, EDUCATION AND WELFARE (1966) Air Quality
Data from the National Air Sampling Networks and Contributing
State and Local Networks, 1964-1965, Cincinnati, OH, USDHEW
Public Health Service Division of Air Pollution, pp. 125.
US DEPARTMENT OF HEALTH, EDUCATION AND WELFARE (1970) Nitrates,
nitrites and methaemoglobinaemia, Research Triangle Park, N.C.,
National Institute of Environmental Health Sciences, pp. 43
(Environ. Rev. No. 2).
VAN LOGTEN, M. J., DEN TONKELAAR, E. M., KROES, R. BERKVENS, J. M., &
VAN ESCH, G. J. (1972) Long-term experiment with canned meat
treated with sodium nitrate and glucono-delta-lactone in rats.
Food Cosmet. Toxicol., 10: 475-488.
VAN RENSBURG, S. J. (1972) Failure of low protein and zinc intakes to
influence nitrosamine-induced oesophageal carcinogenesis. S.
Afr. reed. J, 46: 1137-1138.
VAVRA, J. J., DE BOER, C., DIETZ, A., HANKA, L. J., & SOKOLSKI, W. T.
(1960) Antibiotics Annual 1959-1960, NY, Antiobiotica Inc,
pp. 230.
VERLY, W. G., BARBASON, H., DUSART, J, & PETITPAS-DEWANDRE, A. (1967)
A comparative study of the action of ethyl methane sulphonate and
HNO2 on the mutation to streptomycin resistance of Escherichia
coil K 12. Biochim. Biophys. Acta, 145: 752-762.
VESSELINOVITCH, S. D. (1969) The sex-dependent difference in the
development of liver tumors in mice administered
dimethylnitrosamine. Cancer Res., 29: 1024-1027.
VIETS, F. B. & ALDRICH, S. R. (1973) Nitrogenous materials in the
environment, Draft Report, US Environmental Protection Agency,
Hazardous Materials Advisory Committee.
VIGIL, J., WARBURTON, S., HAYNES, W. A., & KAISER, L. R. (1965)
Nitrates in municipal water supply cause methemoglobinemia in
infant. Public Health Rep., 80 (12): 1119-1121.
VON KREYBIG, T. (1965a) [The effect of teratogenic compounds on the
early stages of prenatal development in the rat.] Naunyn-
Schmiederbergs Arch: exp. Path. Pharmacol., 252: 196-204
(in German).
VON KREYBIG, T. (1965b) [The effect of carcinogen methylnitrosourea
doses on the embryonal development in the rat.] Z. Krebsforsch.,
67: 46-50 (in German).
VON KREYBIG, T. (1968) [Experimental prenatal toxicology.]
Arzneimittel-Forsch., Suppl. 17, pp. 1-211 (in German).
VON KREYBIG, T. & SCHMIDT, W. (1966) [On chemical teratogenesis in the
rat.] ArzneimitteI-Forsch., 8: 989-1001 (in German).
VON KREYBIG, T. & SCHMIDT, W. (1967) [Chemically induced fetopathies
in the rat.] Arzneimittel-Forsch., 9: 1093-1100 (in German)
VOOGT, P. (1906) The determination of nitrate with a nitrate selective
electrode. Dtsch Lebensm. Rundschau., 65: 196-198.
WALKER, E. A., PIGNATELLI, B., & CASTEGNARO, M. (1975) Effects of
gallic acid on nitrosamine formation. Nature (Lond.),
258: 176.
WALKER, E. A., BOGOVSKI, P., & GRICUITE, L., ed. (1976) Environmental
N-nitroso compounds analysis and formation. Proceedings of a
Working Conference, the Polytechnical Institute, Tallinin,
Estonian SSR, 1-3 October, 1975, Lyons, International Agency for
Research on Cancer, pp. 512.
WALTERS, C. L. (1971) The detection and estimation of trace amounts of
N-nitrosamines in a food matrix. Lab. Pract., 20: 574-578.
WALTON, G. (1951) Survey of literature relating to infant
methemoglobinemia due to nitrate-contaminated water. Am. J.
Public Health, 41: 986-996.
WASSERMAN, A. E., FIDDLER, W., DOERR, R. C., OSMAN, S. F., & DOOLEY,
C. J. (1972) Dimethylnitrosamine in frankfurters. Food Cosmet.
Toxicol., 10: 681-684.
WATER RESEARCH CENTRE (1974) Notes on water pollution, No. 66,
pp. 4.
WATROUS, R. M. (1947) Health hazards of the pharmaceutical industry.
Br. J. ind. Med., 4: 111-125.
WEAST, R. C. ed. (1976) Handbook of chemistry and physics, 57th
edition, Cleveland, OH, CRC Press, pp. C-81-C-685.
WESCHLER, W. (1970) Oncogenic, teratogenic and mutagenic effects of
methyl- and ethylnitrosourea. In: 6th International Congress of
Neuropathology, Paris. Masson, pp. 128-129.
WESCHLER, W. (1971) Teratogenic effects of the neurotropic resorptive
carcinogens methyl-and ethylnitrosourea in rats. J. Neuropath.
exp. Neurol., 30: 120-121.
WESCHLER, W. (1973) Carcinogenic and teratogenic effects of
ethylnitrosourea and methylnitrosourea during pregnancy in the
rat. IARC Sci. Publ. No. 4, pp. 127-142.
WEGNER, T. N. (1972) Simple and sensitive procedure for determining
nitrate and nitrite in mixtures in biological fluids. J. Dairy
Sci., 55 (5): 642-644.
WEIL, L. & QUENTIN, L.-E. (1973) Nitrogen compounds in water and
nitrate determination with ion-sensitive electrode. Vom Wasser,
40: 125-133.
WEISBERGER, J. H. & RAINERI, R. (1975) Dietary factors and the
etiology of gastric cancer. Cancer Res., 35: 3469-3474.
WEISBERGER, E. K., WARD, J. M., & BROWN, C. (1974) Dibenamine:
Selective protection against diethylnitrosamine-induced hepatic
carcinogenesis but not oral, pharyngeal and esophageal
carcinogenesis. Toxicol. appl. Pharmacol., 28: 477-484.
WELLS, B. E., WALKER, R., & WALTERS, C. L. (1974) Interactions between
sodium nitrite and foodstuffs under gastric conditions. J. Sci.
Food Agric., 25: 1048.
WHO (1971) International standards for drinking water. Third ed,
Geneva, World Health Organization, pp. 70.
WHO (1977) Environmental health criteria 4--Oxides of nitrogen,
Geneva, World Health Organization, pp. 81.
WILLIAMS, A. O. (1970) Ultrastructure of liver cell carcinoma in
Macaca mulata monkey. Exp. mol. Pathol., 13: 359-369.
WINTON, E. F., TARBIFF, R. G., & MCCABE, L. J. (1971) Nitrate in
drinking water. J. Am. Water Works Assoc., 63: 95-98.
WORKSHOP ON GLOBAL ECOLOGY (1971) Man in the living environment, The
Institute of Ecology Report of the workshop on global ecological
problems, Chicago, IL, Field Museum of Natural History, pp. 267.
WRIGHT, A. J. & DAVIDSON, K. L. (1964) Nitrate accumulation in crops
and nitrate poisoning in animals. Advan. Agron., 16: 197-247.
WRIGLEY, F. (1948) Toxic effects of nitroso-methyl urethane.
Br. J. ind. Med., 5: 26-27.
YANG, K. W. & BROWN, E. V. (1972) A sensitive analysis for
nitrosamines. Anal. Lett., 5 (5): 293-304.
ZALDIVAR & WETTERSTRAND (1975) Further evidence of a positive
correlation between exposure to nitrate fertilizers (NaNO3 and
KNO3) and gastric cancer death rates: Nitrites and
nitrosamines. Experientia (Basel), 31: 1354-1355.
ZIEBARTH, D. & SCHEUNIG, G. (1976) Effects of some inhibitors on the
nitrosation of drugs in human gastric juice. IARC Sci. Publ. No.
14, pp. 279-290.
ZIMMERMAN, F. K. & SCHWAIER, R. (1967) Induction of a mitotic gene
conversion with nitrous acid, 1-methyl-3-nitro-1-nitrosoguanidine
and other alkylating agents in Saccharomyces cerevisiae. Mol.
gen. Genet., 100: 63-76.
ZIMMERMAN, F. K., SCHWAIER, R., & LAER, U. V. (1966) Mitotic
recombination induced in Saccharomyces cerevisiae with nitrous
acid, diethylsulphate and carcinogenic, alkylating nitrosamides.
Z. Vererbungsl., 98: 230-246.
