
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
CONCISE INTERNATIONAL CHEMICAL ASSESSMENT DOCUMENT NO. 9
N-PHENYL-1-NAPHTHYLAMINE
INTER-ORGANIZATION PROGRAMME FOR THE SOUND MANAGEMENT OF CHEMICALS
A cooperative agreement among UNEP, ILO, FAO, WHO, UNIDO, UNITAR and
OECD
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
First draft prepared by Dr G. Koennecker, Dr I. Mangelsdorf, and Dr A.
Wibbertmann, Fraunhofer Institute for Toxicology and Aerosol Research,
Hanover, Germany
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization, and produced within the framework of the
Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 1998
The International Programme on Chemical Safety (IPCS),
established in 1980, is a joint venture of the United Nations
Environment Programme (UNEP), the International Labour Organisation
(ILO), and the World Health Organization (WHO). The overall
objectives of the IPCS are to establish the scientific basis for
assessment of the risk to human health and the environment from
exposure to chemicals, through international peer review processes, as
a prerequisite for the promotion of chemical safety, and to provide
technical assistance in strengthening national capacities for the
sound management of chemicals.
The Inter-Organization Programme for the Sound Management of
Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and
Agriculture Organization of the United Nations, WHO, the United
Nations Industrial Development Organization, the United Nations
Institute for Training and Research, and the Organisation for Economic
Co-operation and Development (Participating Organizations), following
recommendations made by the 1992 UN Conference on Environment and
Development to strengthen cooperation and increase coordination in the
field of chemical safety. The purpose of the IOMC is to promote
coordination of the policies and activities pursued by the
Participating Organizations, jointly or separately, to achieve the
sound management of chemicals in relation to human health and the
environment.
WHO Library Cataloguing in Publication Data
N-phenyl-1-naphthylamine.
(Concise international chemical assessment document ; 9)
First draft prepared by G. Koennecker, I. Mangelsdorf and
A. Wibbertmann.
1.1-Naphthylamine - adverse effects 2.1-Naphthylamine -
toxicity 3.Environmental exposure I.Koennecker, G. II.Series
ISBN 92 4 153009 X (NLM Classification: QD 305.A8)
ISSN 1020-6167
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1998
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city, or area
or of its authorities, or concerning the delimitation of its frontiers
or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
The Federal Ministry for the Environment, Nature Conservation and
Nuclear Safety, Germany, provided financial support for the printing
of this publication.
TABLE OF CONTENTS
FOREWORD
1. EXECUTIVE SUMMARY
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
3. ANALYTICAL METHODS
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1. Environmental levels
6.2. Human exposure
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1. Single exposure
8.2. Irritation and sensitization
8.3. Short-term exposure
8.4. Long-term exposure
8.4.1. Subchronic exposure
8.4.2. Chronic exposure and carcinogenicity
8.5. Genotoxicity and related end-points
8.6. Reproductive and developmental toxicity
8.7. Immunological and neurological effects
9. EFFECTS ON HUMANS
9.1. Case reports
9.2. Epidemiological studies
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10.1. Aquatic environment
10.2. Terrestrial environment
11. EFFECTS EVALUATION
11.1. Evaluation of health effects
11.1.1. Hazard identification and dose-response assessment
11.1.2. Criteria for setting guidance values for N-phenyl-1-naphthylamine
11.1.3. Sample risk characterization
11.2. Evaluation of environmental effects
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
13.1. Human health hazards
13.2. Advice to physicians
13.3. Spillage
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
INTERNATIONAL CHEMICAL SAFETY CARD
REFERENCES
APPENDIX 1 - SOURCE DOCUMENT
APPENDIX 2 - CICAD PEER REVIEW
APPENDIX 3 - CICAD FINAL REVIEW BOARD
RÉSUMÉ D'ORIENTATION
RESUMEN DE ORIENTACION
FOREWORD
Concise International Chemical Assessment Documents (CICADs) are
the latest in a family of publications from the International
Programme on Chemical Safety (IPCS) - a cooperative programme of the
World Health Organization (WHO), the International Labour Organisation
(ILO), and the United Nations Environment Programme (UNEP). CICADs
join the Environmental Health Criteria documents (EHCs) as
authoritative documents on the risk assessment of chemicals.
CICADs are concise documents that provide summaries of the
relevant scientific information concerning the potential effects of
chemicals upon human health and/or the environment. They are based on
selected national or regional evaluation documents or on existing
EHCs. Before acceptance for publication as CICADs by IPCS, these
documents undergo extensive peer review by internationally selected
experts to ensure their completeness, accuracy in the way in which the
original data are represented, and the validity of the conclusions
drawn.
The primary objective of CICADs is characterization of hazard and
dose-response from exposure to a chemical. CICADs are not a summary
of all available data on a particular chemical; rather, they include
only that information considered critical for characterization of the
risk posed by the chemical. The critical studies are, however,
presented in sufficient detail to support the conclusions drawn. For
additional information, the reader should consult the identified
source documents upon which the CICAD has been based.
Risks to human health and the environment will vary considerably
depending upon the type and extent of exposure. Responsible
authorities are strongly encouraged to characterize risk on the basis
of locally measured or predicted exposure scenarios. To assist the
reader, examples of exposure estimation and risk characterization are
provided in CICADs, whenever possible. These examples cannot be
considered as representing all possible exposure situations, but are
provided as guidance only. The reader is referred to EHC 1701 for
advice on the derivation of health-based guidance values.
While every effort is made to ensure that CICADs represent the
current status of knowledge, new information is being developed
constantly. Unless otherwise stated, CICADs are based on a search of
the scientific literature to the date shown in the executive summary.
In the event that a reader becomes aware of new information that would
change the conclusions drawn in a CICAD, the reader is requested to
contact the IPCS to inform it of the new information.
1 International Programme on Chemical Safety (1994) Assessing
human health risks of chemicals: derivation of guidance values for
health-based exposure limits. Geneva, World Health Organization
(Environmental Health Criteria 170).
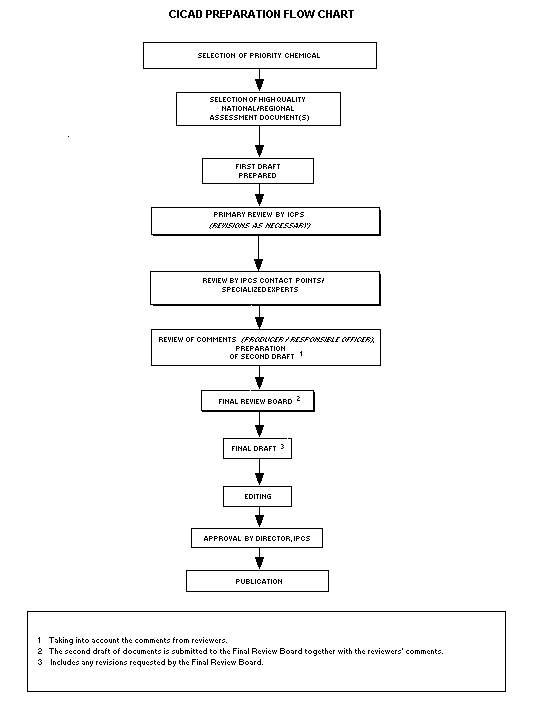
Procedures
The flow chart shows the procedures followed to produce a CICAD.
These procedures are designed to take advantage of the expertise that
exists around the world - expertise that is required to produce the
high-quality evaluations of toxicological, exposure, and other data
that are necessary for assessing risks to human health and/or the
environment.
The first draft is based on an existing national, regional, or
international review. Authors of the first draft are usually, but not
necessarily, from the institution that developed the original review.
A standard outline has been developed to encourage consistency in
form. The first draft undergoes primary review by IPCS to ensure that
it meets the specified criteria for CICADs.
The second stage involves international peer review by scientists
known for their particular expertise and by scientists selected from
an international roster compiled by IPCS through recommendations from
IPCS national Contact Points and from IPCS Participating Institutions.
Adequate time is allowed for the selected experts to undertake a
thorough review. Authors are required to take reviewers' comments
into account and revise their draft, if necessary. The resulting
second draft is submitted to a Final Review Board together with the
reviewers' comments.
The CICAD Final Review Board has several important functions:
- to ensure that each CICAD has been subjected to an appropriate
and thorough peer review;
- to verify that the peer reviewers' comments have been addressed
appropriately;
- to provide guidance to those responsible for the preparation of
CICADs on how to resolve any remaining issues if, in the opinion
of the Board, the author has not adequately addressed all
comments of the reviewers; and
- to approve CICADs as international assessments.
Board members serve in their personal capacity, not as representatives
of any organization, government, or industry. They are selected
because of their expertise in human and environmental toxicology or
because of their experience in the regulation of chemicals. Boards
are chosen according to the range of expertise required for a meeting
and the need for balanced geographic representation.
Board members, authors, reviewers, consultants, and advisers who
participate in the preparation of a CICAD are required to declare any
real or potential conflict of interest in relation to the subjects
under discussion at any stage of the process. Representatives of
nongovernmental organizations may be invited to observe the
proceedings of the Final Review Board. Observers may participate in
Board discussions only at the invitation of the Chairperson, and they
may not participate in the final decision-making process.
 1. EXECUTIVE SUMMARY
This CICAD on N-phenyl-1-naphthylamine was based principally on
a review prepared by the Fraunhofer Institute for Toxicology and
Aerosol Research, Hanover, Germany, for the German Advisory Committee
on Existing Chemicals of Environmental Relevance (BUA, 1993). This
review assesses the potential effects of N-phenyl-1-naphthylamine on
the environment and on human health. Data identified up to 1992 were
considered in the BUA report. A comprehensive literature search of
several on-line databases was conducted in 1997 to identify any
relevant references published subsequent to those incorporated in the
BUA report. Information on the preparation and peer review of the
source document is presented in Appendix 1. Information on the peer
review of this CICAD is presented in Appendix 2. This CICAD was
approved as an international assessment at a meeting of the Final
Review Board, held in Berlin, Germany, on 26-28 November 1997.
Participants at the Final Review Board meeting are listed in Appendix
3. The International Chemical Safety Card (ICSC 1113) for
N-phenyl-1-naphthylamine, produced by the International Programme on
Chemical Safety (IPCS, 1993), has also been reproduced in this
document.
N-Phenyl-1-naphthylamine (CAS no. 90-30-2) is a lipophilic
crystalline solid that is used as an antioxidant in various
lubrication oils and as a protective agent and antioxidant in rubber
and rubber mixtures for various products, including tyres. Between
1986 and 1990, the estimated worldwide production capacity of
N-phenyl-1-naphthylamine was 3000 tonnes per year. One German
company is the sole producer of N-phenyl-1-naphthylamine within the
European Union.
Based upon its physical/chemical properties, the distribution of
N-phenyl-1-naphthylamine in the environment, predicted on the basis
of a Level II fugacity model, was approximately 36% to soil, 34% to
sediment, 29% to water, and less than 1% each to air, suspended
sediment, and biota. Quantitative data on releases of
N-phenyl-1-naphthylamine into the environment from production,
processing, and use are not available. Indirect discharges to soil
and surface waters from the leakage of lubrication oils or leaching
from decaying tyres and rubber products may occur; however,
quantitative data are not available. Although data were not
identified, N-phenyl-1-naphthylamine may be emitted to the
atmosphere in exhaust gases during its production and processing and
during the vulcanization of rubber mixtures. The use of
N-phenyl-1-naphthylamine-containing lubrication oils should not
result in the introduction of this substance into the atmosphere, as
these oils are applied in closed systems. Overall, owing to its
limited production capacity and the application of emission reduction
techniques, the amount of N-phenyl-1-naphthylamine released into the
environment is expected to be low.
Laboratory studies yielded half-lives for the photochemical
degradation of N-phenyl-1-naphthylamine in water of 8.4 and 5.7 min.
Photolysis may lead to the preliminary breakdown of
N-phenyl-1-naphthylamine under favourable environmental conditions,
but further degradation is unlikely. The substance is stable to
hydrolysis under environmental conditions, and removal by
biodegradation in water and soil is slow. Owing to its moderate to
high potential for sorption to organic soil constituents and its
limited mineralization in soil, N-phenyl-1-naphthylamine is presumed
to have geoaccumulation potential. The probability of infiltration
into groundwater is low. Based upon studies with Daphnia and fish
and its measured log Kow of 4.2, N-phenyl-1-naphthylamine is
expected to have a moderate potential for bioaccumulation.
Nevertheless, secondary poisoning of higher trophic levels via the
aquatic food-chain seems unlikely in view of the chemical's metabolism
and extensive excretion. The acute toxicity of
N-phenyl-1-naphthylamine in fish and Daphnia is high, with lowest
reported no-observed-effect concentrations (NOECs) of 0.11 mg/litre
(192 h) and 0.02 mg/litre (21 days), respectively. Despite limited
hydrolytic or biotic degradation, the bioavailability of this chemical
in water is expected to be considerably reduced by sorption and
photochemical degradation.
Identified data on concentrations of N-phenyl-1-naphthylamine
in environmental media were limited to older studies from the USA, in
which the chemical was detected in river water (2-7 µg/litre) and
sediment (1-5 mg/kg) near a small speciality chemicals manufacturing
plant. Available data were inadequate to allow the assessment of
human exposure or the prediction of concentrations using fugacity
modelling.
Based upon studies conducted with laboratory animals,
N-phenyl-1-naphthylamine is well absorbed and extensively excreted
after ingestion. Following ingestion by rats, 60% of the administered
dose was excreted in the faeces and 35% in the urine within 72 h.
Several unidentified metabolites of N-phenyl-1-naphthylamine have
been detected in the urine of exposed rats. On the basis of
in vitro studies, metabolism likely occurs primarily via
hydroxylation.
The acute oral toxicity of N-phenyl-1-naphthylamine in
laboratory animals is low. In standard tests with rabbits,
N-phenyl-1-naphthylamine was reported to be neither a skin irritant
nor an eye irritant. However, the skin sensitizing properties of
N-phenyl-1-naphthylamine were revealed in the guinea-pig
maximization test as well as in humans exposed to greases or rubber
materials containing this chemical.
Limited data indicate that the kidneys and liver are the main
target organs following ingestion. Adequate studies with which to
derive putative effect levels were not identified. The potential
carcinogenicity of N-phenyl-1-naphthylamine could not be fully
evaluated, as none of the available studies was performed according to
currently accepted standard protocols.
N-Phenyl-1-naphthylamine was not mutagenic in bacterial cells,
nor were the frequencies of gene mutation (mouse lymphoma assay) or
chromosomal aberrations (in vitro metaphase analysis in Chinese
hamster ovary cells or Chinese hamster lung cells) increased in these
cell types exposed in vitro. A marginally positive result in a
sister chromatid exchange assay conducted with Chinese hamster ovary
cells in the presence of metabolic activation has been reported.
Unscheduled DNA synthesis was increased in exposed human lung (WI-38)
cells; however, the effects were not clearly concentration dependent.
N-Phenyl-1-naphthylamine was negative in a dominant lethal test
conducted in mice. Based upon the available data,
N-phenyl-1-naphthylamine does not appear to be genotoxic. Data on
the reproductive/developmental toxicity and on immunological or
neurological effects of N-phenyl-1-naphthylamine were not
identified.
An increased rate of cancer was observed in one epidemiological
study of N-phenyl-1-naphthylamine-exposed workers; however, owing to
the small number of excess deaths and concomitant exposure to other
substances, it is not possible to attribute this effect solely to
N-phenyl-1-naphthylamine. Although data are inadequate to allow a
more detailed characterization of the potential health risks of
N-phenyl-1-naphthylamine, dermal contact with the chemical should be
avoided because of its sensitizing properties.
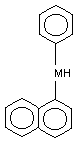
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
N-Phenyl-1-naphthylamine (CAS no. 90-30-2; C16H13N;
1-anilinonaphthaline; phenyl-[naphthyl-(1)] amine;
phenyl-alpha-naphthylamine) in its pure form crystallizes into lemon
yellow prisms or needles (melting point 62-63°C). The chemical is
marketed in the form of brown to dark violet crystals or light brown
to light violet granules. The vapour pressure (1.06 10-6 kPa) and
the water solubility of N-phenyl-1-naphthylamine at 20°C (3.0
mg/litre) are quite low. With a measured n-octanol/water partition
coefficient (log Kow) of 4.2, N-phenyl-1-naphthylamine is
characterized as a lipophilic substance. Additional properties are
presented in the International Chemical Safety Card reproduced in this
document. N-Phenyl-1-naphthylamine decomposes upon heating or
burning, producing irritating or toxic fumes or gases (nitrogen
oxides). The conversion for N-phenyl-1-naphthylamine is 1 ppm =
9.114 mg/m3 (at 101.3 kPa and 20°C). The structural formula for
N-phenyl-1-naphthylamine is:
1. EXECUTIVE SUMMARY
This CICAD on N-phenyl-1-naphthylamine was based principally on
a review prepared by the Fraunhofer Institute for Toxicology and
Aerosol Research, Hanover, Germany, for the German Advisory Committee
on Existing Chemicals of Environmental Relevance (BUA, 1993). This
review assesses the potential effects of N-phenyl-1-naphthylamine on
the environment and on human health. Data identified up to 1992 were
considered in the BUA report. A comprehensive literature search of
several on-line databases was conducted in 1997 to identify any
relevant references published subsequent to those incorporated in the
BUA report. Information on the preparation and peer review of the
source document is presented in Appendix 1. Information on the peer
review of this CICAD is presented in Appendix 2. This CICAD was
approved as an international assessment at a meeting of the Final
Review Board, held in Berlin, Germany, on 26-28 November 1997.
Participants at the Final Review Board meeting are listed in Appendix
3. The International Chemical Safety Card (ICSC 1113) for
N-phenyl-1-naphthylamine, produced by the International Programme on
Chemical Safety (IPCS, 1993), has also been reproduced in this
document.
N-Phenyl-1-naphthylamine (CAS no. 90-30-2) is a lipophilic
crystalline solid that is used as an antioxidant in various
lubrication oils and as a protective agent and antioxidant in rubber
and rubber mixtures for various products, including tyres. Between
1986 and 1990, the estimated worldwide production capacity of
N-phenyl-1-naphthylamine was 3000 tonnes per year. One German
company is the sole producer of N-phenyl-1-naphthylamine within the
European Union.
Based upon its physical/chemical properties, the distribution of
N-phenyl-1-naphthylamine in the environment, predicted on the basis
of a Level II fugacity model, was approximately 36% to soil, 34% to
sediment, 29% to water, and less than 1% each to air, suspended
sediment, and biota. Quantitative data on releases of
N-phenyl-1-naphthylamine into the environment from production,
processing, and use are not available. Indirect discharges to soil
and surface waters from the leakage of lubrication oils or leaching
from decaying tyres and rubber products may occur; however,
quantitative data are not available. Although data were not
identified, N-phenyl-1-naphthylamine may be emitted to the
atmosphere in exhaust gases during its production and processing and
during the vulcanization of rubber mixtures. The use of
N-phenyl-1-naphthylamine-containing lubrication oils should not
result in the introduction of this substance into the atmosphere, as
these oils are applied in closed systems. Overall, owing to its
limited production capacity and the application of emission reduction
techniques, the amount of N-phenyl-1-naphthylamine released into the
environment is expected to be low.
Laboratory studies yielded half-lives for the photochemical
degradation of N-phenyl-1-naphthylamine in water of 8.4 and 5.7 min.
Photolysis may lead to the preliminary breakdown of
N-phenyl-1-naphthylamine under favourable environmental conditions,
but further degradation is unlikely. The substance is stable to
hydrolysis under environmental conditions, and removal by
biodegradation in water and soil is slow. Owing to its moderate to
high potential for sorption to organic soil constituents and its
limited mineralization in soil, N-phenyl-1-naphthylamine is presumed
to have geoaccumulation potential. The probability of infiltration
into groundwater is low. Based upon studies with Daphnia and fish
and its measured log Kow of 4.2, N-phenyl-1-naphthylamine is
expected to have a moderate potential for bioaccumulation.
Nevertheless, secondary poisoning of higher trophic levels via the
aquatic food-chain seems unlikely in view of the chemical's metabolism
and extensive excretion. The acute toxicity of
N-phenyl-1-naphthylamine in fish and Daphnia is high, with lowest
reported no-observed-effect concentrations (NOECs) of 0.11 mg/litre
(192 h) and 0.02 mg/litre (21 days), respectively. Despite limited
hydrolytic or biotic degradation, the bioavailability of this chemical
in water is expected to be considerably reduced by sorption and
photochemical degradation.
Identified data on concentrations of N-phenyl-1-naphthylamine
in environmental media were limited to older studies from the USA, in
which the chemical was detected in river water (2-7 µg/litre) and
sediment (1-5 mg/kg) near a small speciality chemicals manufacturing
plant. Available data were inadequate to allow the assessment of
human exposure or the prediction of concentrations using fugacity
modelling.
Based upon studies conducted with laboratory animals,
N-phenyl-1-naphthylamine is well absorbed and extensively excreted
after ingestion. Following ingestion by rats, 60% of the administered
dose was excreted in the faeces and 35% in the urine within 72 h.
Several unidentified metabolites of N-phenyl-1-naphthylamine have
been detected in the urine of exposed rats. On the basis of
in vitro studies, metabolism likely occurs primarily via
hydroxylation.
The acute oral toxicity of N-phenyl-1-naphthylamine in
laboratory animals is low. In standard tests with rabbits,
N-phenyl-1-naphthylamine was reported to be neither a skin irritant
nor an eye irritant. However, the skin sensitizing properties of
N-phenyl-1-naphthylamine were revealed in the guinea-pig
maximization test as well as in humans exposed to greases or rubber
materials containing this chemical.
Limited data indicate that the kidneys and liver are the main
target organs following ingestion. Adequate studies with which to
derive putative effect levels were not identified. The potential
carcinogenicity of N-phenyl-1-naphthylamine could not be fully
evaluated, as none of the available studies was performed according to
currently accepted standard protocols.
N-Phenyl-1-naphthylamine was not mutagenic in bacterial cells,
nor were the frequencies of gene mutation (mouse lymphoma assay) or
chromosomal aberrations (in vitro metaphase analysis in Chinese
hamster ovary cells or Chinese hamster lung cells) increased in these
cell types exposed in vitro. A marginally positive result in a
sister chromatid exchange assay conducted with Chinese hamster ovary
cells in the presence of metabolic activation has been reported.
Unscheduled DNA synthesis was increased in exposed human lung (WI-38)
cells; however, the effects were not clearly concentration dependent.
N-Phenyl-1-naphthylamine was negative in a dominant lethal test
conducted in mice. Based upon the available data,
N-phenyl-1-naphthylamine does not appear to be genotoxic. Data on
the reproductive/developmental toxicity and on immunological or
neurological effects of N-phenyl-1-naphthylamine were not
identified.
An increased rate of cancer was observed in one epidemiological
study of N-phenyl-1-naphthylamine-exposed workers; however, owing to
the small number of excess deaths and concomitant exposure to other
substances, it is not possible to attribute this effect solely to
N-phenyl-1-naphthylamine. Although data are inadequate to allow a
more detailed characterization of the potential health risks of
N-phenyl-1-naphthylamine, dermal contact with the chemical should be
avoided because of its sensitizing properties.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
N-Phenyl-1-naphthylamine (CAS no. 90-30-2; C16H13N;
1-anilinonaphthaline; phenyl-[naphthyl-(1)] amine;
phenyl-alpha-naphthylamine) in its pure form crystallizes into lemon
yellow prisms or needles (melting point 62-63°C). The chemical is
marketed in the form of brown to dark violet crystals or light brown
to light violet granules. The vapour pressure (1.06 10-6 kPa) and
the water solubility of N-phenyl-1-naphthylamine at 20°C (3.0
mg/litre) are quite low. With a measured n-octanol/water partition
coefficient (log Kow) of 4.2, N-phenyl-1-naphthylamine is
characterized as a lipophilic substance. Additional properties are
presented in the International Chemical Safety Card reproduced in this
document. N-Phenyl-1-naphthylamine decomposes upon heating or
burning, producing irritating or toxic fumes or gases (nitrogen
oxides). The conversion for N-phenyl-1-naphthylamine is 1 ppm =
9.114 mg/m3 (at 101.3 kPa and 20°C). The structural formula for
N-phenyl-1-naphthylamine is:
 The commercial product has a typical purity of >99%. Named
impurities from three manufacturers are 1-naphthylamine (<100-500
mg/kg), 2-naphthylamine (<3-50 mg/kg), aniline (<100-2500 mg/kg),
1-naphthol (<5000 mg/kg), 1,1-dinaphthylamine (<1000 mg/kg), and
N-phenyl-2-naphthylamine (500-<5000 mg/kg) (BUA, 1993; Union
Carbide, 1996).
3. ANALYTICAL METHODS
N-Phenyl-1-naphthylamine is quantified in environmental media
either by high-performance liquid chromatography in combination with
ultraviolet absorption or by gas chromatography combined with
thermionic or mass spectrometric detection, flame ionization
detection, or electron capture detection. A method for the
determination of secondary amines in air is suitable for the detection
of N-phenyl-1-naphthylamine.
The following enrichment techniques are used for various types of
samples: solid-phase adsorption (silica gel, silica gel/glass fibre)
with liquid extraction (ethanol, acetic acid/2-propanol) for samples
of air (NIOSH, 1984a,b); liquid/liquid extraction (acetonitrile,
diethyl ether), stripping with helium, and solid adsorption (Tenax GC)
or alkaline extraction (dichloromethane) for samples of water
(Jungclaus et al., 1978; Lopez-Avila & Hites, 1980; Sikka et al.,
1981; Rosenberg, 1983); liquid extraction (isopropanol) for sediment
(Jungclaus et al., 1978; Lopez-Avila & Hites, 1980); and liquid
extraction (methanol) for fish, tissue, and serum (Sikka et al.,
1981). Detection limits range from 0.1 to 1 µg/litre for water and
from 50 to 100 µg/kg for sediment; detection limits for biological
materials were not identified.
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
There are no known natural sources of N-phenyl-1-naphthylamine.
Other than the information derived from the national source document,
additional data on the production, use patterns, and release of this
chemical were not identified.
Within the European Union, one German company is the sole
producer of N-phenyl-1-naphthylamine. Between 1986 and 1990, the
estimated worldwide production capacity of N-phenyl-1-naphthylamine
was 3000 tonnes per year. Over the same period, estimated production
capacities in Western Europe, the People's Republic of China, the USA,
and Japan were approximately 1000-1500 tonnes per year, 1000 tonnes
per year, 500 tonnes per year, and 300 tonnes per year, respectively.
Between 1986 and 1990, the consumption of N-phenyl-1-naphthylamine
in Germany was estimated to be approximately 300-450 tonnes per year,
although its use is now declining. Approximately 50-100 tonnes were
covered by imports; exports amounted to approximately 750-1150 tonnes
per year (BUA, 1993).
N-Phenyl-1-naphthylamine is used as an antioxidant in gear,
hydraulic, lubrication, and bearing oils and as a protective agent and
antioxidant in rubbers and rubber mixtures. In Germany,
N-phenyl-1-naphthylamine consumption is divided equally among these
two uses. In these products, the chemical acts as a radical scavenger
in the auto-oxidation of polymers and mineral lubricants. Average
concentrations of N-phenyl-1-naphthylamine in the final products are
<1% w/w (BUA, 1993). In the rubber industry, approximately 75% of
the N-phenyl-1-naphthylamine is used in products such as drums,
buffers, conveyor belts, flexible tubes, gaskets, and footwear
components. The remaining 25% is used in tyres (sidewalls or carcass,
but not treads) (BUA, 1993).
In Germany, N-phenyl-1-naphthylamine-containing distillation
residues from production facilities (approximately 20 tonnes per
year), like most gear and hydraulic oil, are disposed of in
chemical/physical/biological treatment plants or hazardous waste
incinerators (BUA, 1993). About 30% of used tyres are disposed of in
landfill sites, approximately 37% are used for the production of
energy in the cement industry, an estimated 22% are recycled, and
approximately 11% are exported (BUA, 1993). Exhaust gases may be
emitted during the production and processing of
N-phenyl-1-naphthylamine and during the vulcanization of rubber
mixtures at elevated temperatures; in Germany, however, emission
reduction techniques are applied, and releases of
N-phenyl-1-naphthylamine are therefore presumed to be low (<25 kg
per year). Data on levels of N-phenyl-1-naphthylamine in exhaust
gases from vulcanization processes are not available. The use of
N-phenyl-1-naphthylamine-containing lubrication oils should not
result in the introduction of the substance into the atmosphere, as
the oils are applied in closed systems (BUA, 1993).
Data on releases of N-phenyl-1-naphthylamine in effluents from
production and processing facilities were not identified. Indirect
discharges from leaching or the decay of tyres and other rubber
products are to be expected in the long term; however, estimation of
amounts was not possible (BUA, 1993). The release of
N-phenyl-1-naphthylamine into the geosphere from the leakage of
lubrication oils and discarded rubber products and tyres in landfill
sites may occur; however, estimation of the amounts was not possible
with the available data (BUA, 1993). Information on the occurrence of
N-phenyl-1-naphthylamine in plants or animals was not identified.
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Based on its physical/chemical properties, the distribution of
N-phenyl-1-naphthylamine in the environment was predicted, using a
Level II fugacity model (Mackay, 1991), to be 36.3% to soil, 33.9% to
sediment, 28.9% to water, 0.8% to air, 0.06% to suspended sediment,
and 0.02% to biota. Input data for the Level II fugacity model were
as follows: temperature, 298°K; atmospheric volume, 6 109 m3; soil
density, 1.5 g/cm3; density of biota, 1 g/cm3; carbon content of
soil, 2%; carbon content of sediment, 4%; water depth, 1000 cm; water
portion, 70%; soil depth, 15 cm; sediment depth, 3 cm; suspended
sediment portion, 5 ppm; biota portion, 1 ppm; molar mass, 219 g/mol;
water solubility, 3 mg/litre; vapour pressure, 1.06 mPa;
n-octanol/water partition coefficient, 15 850; soil sorption
coefficient, 6510; bioaccumulation factor, 760; half-lives (days) in
air (0), water (365), soil (29.2), sediment (29.2), suspended sediment
(29.2), and biota (0). Owing to the lack of relevant data, it was not
possible to predict the concentrations of N-phenyl-1-naphthylamine
in various media using a Level III fugacity model. Based on its
calculated Henry's law constant (7.748 10-2 Pa.m3/mol; 20°C) and
other information (Thomas, 1990), the volatility of
N-phenyl-1-naphthylamine from aqueous solution is expected to be
low.
Based on its ultraviolet absorption spectrum, direct
photochemical degradation of N-phenyl-1-naphthylamine in air is
expected (BUA, 1993). Data concerning the photo-oxidative degradation
of N-phenyl-1-naphthylamine in air are not available. Measured
half-lives for the photochemical degradation of the chemical in water
have been reported at 8.4 and 5.7 min (Sikka et al., 1981). In this
experiment, sealed tubes containing aqueous solutions of
N-phenyl-1-naphthylamine at approximately 1 mg/litre (water
unspecified but assumed to be distilled) were exposed to sunlight.
The experiment was conducted in May and repeated in June in Syracuse,
NY (USA); no information was provided on light intensity. A further
experiment using a lamp at 300 nm (Rayonette Model RNR-400 mini
photochemical reactor; no information provided on intensity)
demonstrated that the photodegradation product was produced rapidly
and was itself photostable. Given the lack of detail in the report,
the importance of photodegradation in the environment is difficult to
assess. There is also insufficient information to allow the
photodegradation product to be fully characterized, but the authors
suggest that it incorporates the basic phenylnaphthylamine skeleton.
It can therefore be concluded that photolysis may lead to preliminary
breakdown of N-phenyl-1-naphthylamine under favourable environmental
conditions, but that further degradation is unlikely. From
experiments conducted in aqueous solution, hydrolysis of
N-phenyl-1-naphthylamine under environmental conditions is expected
to be of limited importance (Sikka et al., 1981).
Two standard tests on biodegradation performed according to
guideline 301C of the Organisation for Economic Co-operation and
Development (OECD) (modified MITI-I test) reported no degradation of
N-phenyl-1-naphthylamine (100 mg/litre initial concentration) within
14 and 28 days, using non-adapted activated sludge (Bayer AG, 1990;
CITI, 1992). In tests with conditions favouring biodegradation,
N-phenyl-1-naphthylamine was degraded with a half-life ranging from
4 to 11 days (inocula: domestic sewage and lake water, respectively).
Additional substrates accelerated degradation (Sikka et al., 1981;
Rosenberg, 1983). Laboratory results indicate that
N-phenyl-1-naphthylamine is inherently biodegradable in the aquatic
compartment.
Mineralization of N-phenyl-1-naphthylamine (measured by the
evolution of [14C]carbon dioxide) was 17% in soil and 35% in a soil
suspension in buffered salt solution. In contrast to the aquatic
studies, the addition of degradable substrates reduced rather than
accelerated degradation. It was suggested that the organic materials
increased sorption of the N-phenyl-1-naphthylamine. The reported
lower degradation in soil may therefore reflect reduced
bioavailability of the N-phenyl-1-naphthylamine (Rosenberg, 1983).
Measured soil sorption coefficients (Koc) are not available. Using
the regression equations of Kenaga (1980) and Kenaga & Goring (1980),
Koc values of 2400 and 4600, respectively, were calculated for
N-phenyl-1-naphthylamine. Thus, soil sorption is predicted to be
moderate to high. From this expected sorption to organic soil
constituents and its limited mineralization in soil,
N-phenyl-1-naphthylamine is presumed to have geoaccumulation
potential. The probability of infiltration into groundwater is low
(BUA, 1993).
Considering its measured log Kow of 4.2 (Ozeki & Tejima, 1979)
and data from laboratory tests with Daphnia and freshwater fish,
N-phenyl-1-naphthylamine is classified as a substance with moderate
bioaccumulation potential (Sikka et al., 1981; CITI, 1992). For
Daphnia magna, a mean bioconcentration factor (related to
radioactivity) of 637 was calculated following exposure to
[14C] N-phenyl-1-naphthylamine in a static test (solubilizer:
acetone; steady state after 12 h). About 50% of the accumulated
radioactivity had been eliminated after 53 h in clean water (Sikka et
al., 1981). Bioconcentration factors ranging from 432 to 1285
(related to radioactivity) and from 233 to 694 (related to
N-phenyl-1-naphthylamine) were determined in a flow-through system
(sublethal N-phenyl-1-naphthylamine concentration) for the bluegill
sunfish (Lepomis macrochirus) at steady state. Depuration was
biphasic, with an elimination of [14C] N-phenyl-1-naphthylamine of
>90% after 8 days; radioactivity could still be detected 32 days
after treatment (Sikka et al., 1981). Bioconcentration factors for
N-phenyl-1-naphthylamine in common carp (Cyprinus carpio),
measured in a flow-through system after 8 weeks, were on the same
order of magnitude (427-2730) (CITI, 1992).
N-Phenyl-1-naphthylamine is metabolized by terrestrial and aquatic
microorganisms and by fish to at least two or three unidentified
metabolites (Sikka et al., 1981; Rosenberg, 1983).
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
In older studies from the USA, N-phenyl-1-naphthylamine was
detected in river water (2-7 µg/litre) and sediment (1-5 mg/kg) near a
small speciality chemicals manufacturing plant (Jungclaus et al.,
1978; Lopez-Avila & Hites, 1980). Additional data on levels of
N-phenyl-1-naphthylamine in environmental media were not identified.
Based upon the use patterns of N-phenyl-1-naphthylamine, the
presence of this substance in soil and sediment in some
source-dominated areas appears possible; however, quantitative data
are not available.
6.2 Human exposure
Owing to its low vapour pressure and use patterns, the ingestion
or inhalation of N-phenyl-1-naphthylamine is expected to be minor.
Dermal contact with oils and rubber articles containing
N-phenyl-1-naphthylamine may occur in the workplace. Data on
occupational exposure were not available from industries in Germany
involved in the manufacture or use of N-phenyl-1-naphthylamine.1
Dermal contact may also be a source of exposure for the general
population, although this should be of minor importance because of the
small quantities of N-phenyl-1-naphthylamine produced and present in
various products. Data on concentrations of
N-phenyl-1-naphthylamine in media relevant to assessing exposure of
the general population were not identified. Moreover, available data
were insufficient to allow an estimation of human exposure based upon
concentrations predicted from fugacity modelling.
1 Personal communications concerning 1) BUA report on
N-phenyl-1-naphthylamine, Heidelberg, Berufsgenossenschaft der
chemischen Industrie (BG Chemie), 27 August 1992; and 2) exposure data
for N-phenyl-1-naphthylamine, Bundesanstalt für Arbeitsschutz,
Dortmund, 1992.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND
HUMANS
Studies providing quantitative information on the absorption or
distribution of N-phenyl-1-naphthylamine in humans were not
identified. From the limited information available from studies
conducted with laboratory animals, it can be concluded that
N-phenyl-1-naphthylamine is well absorbed after ingestion and is
readily excreted. In vitro studies have demonstrated that the
metabolism of N-phenyl-1-naphthylamine occurs primarily via
hydroxylation.
In male Sprague-Dawley rats administered a single oral dose of
160 mg [14C] N-phenyl-1-naphthylamine/kg body weight, the chemical
was well absorbed, metabolized almost completely, and excreted
primarily in the faeces. Radioactivity was detected in plasma within
60 min, with the maximum concentration measured after 4 h. After
24 h, 20% of the radioactivity was found in the gastrointestinal tract
(including contents), 2.4% in fatty tissue, 0.4% in the liver, and
0.1% in the kidneys. Ninety per cent of the administered
radioactivity was excreted within 48 h; 95% was excreted within 72 h
(60% in the faeces and 35% in the urine). In the ether extract of the
urine, at least five radioactive metabolites were detected but not
identified. The elimination half-lives were reported as 1.68 h for
the fast elimination and 33 h for the slow elimination (Sikka et al.,
1981).
In a study in which male rats were administered
N-phenyl-1-naphthylamine orally, only small quantities of unchanged
N-phenyl-1-naphthylamine were excreted in the faeces and urine (0.4
and 0.01% of the applied dose, respectively). Large amounts of
glucuronide and sulfate conjugates, which were not identified further,
were detected in the urine. Small quantities of
N-phenyl-1-naphthylamine were distributed in fatty tissue after
single or multiple (6 day) oral administration, whereas the
distribution of unchanged N-phenyl-1-naphthylamine in liver,
kidneys, spleen, heart, and lung was extremely low (Miyazaki et al.,
1987).
Mono- and dihydroxy-derivatives of N-phenyl-1-naphthylamine
have been identified in in vitro metabolic studies conducted with
rat liver microsomes (Sikka et al., 1981; Xuanxian & Wolff, 1992).
Sikka et al. (1981) suggested that the hydroxyl group in the
mono-hydroxy derivative is in the naphthalene moiety at a
para-position to the amino group, whereas at least one hydroxyl group
in the dihydroxy-derivative is at the available para-position in the
naphthyl ring. Pretreatment of male rats with phenobarbital or
3-methylcholanthrene increased the rate of microsomal metabolism,
indicating that more than one P-450 enzyme is involved in the
metabolism of N-phenyl-1-naphthylamine (Xuanxian & Wolff, 1992).
In studies conducted with human volunteers or laboratory animals,
the isomer N-phenyl-2-naphthylamine (CAS no. 135-88-6) was partially
metabolized to the known human carcinogen 2-naphthylamine following
ingestion or inhalation (NIOSH, 1976). Although data concerning the
formation of this metabolite are not available for
N-phenyl-1-naphthylamine, it should be noted that, based on its
chemical structure, it is unlikely that N-phenyl-1-naphthylamine is
metabolized to 2-naphthylamine.
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
In most of the toxicity studies, information on the purity of
N-phenyl-1-naphthylamine was not provided. As discussed in
section 2, N-phenyl-1-naphthylamine with a typical purity of >99%
contains numerous contaminants, and therefore the observed effects may
not be solely attributable to N-phenyl-1-naphthylamine. Owing to
the limited available toxicity data on N-phenyl-1-naphthylamine,
information on the isomer N-phenyl-2-naphthylamine (a known
contaminant of commercially available N-phenyl-1-naphthylamine) has
been included to aid in the identification of potential target organs.
There is limited evidence to suggest that the kidneys and liver are
the main target organs following ingestion of
N-phenyl-1-naphthylamine; this has also been demonstrated for the
isomer N-phenyl-2-naphthylamine.
8.1 Single exposure
The acute oral toxicity of N-phenyl-1-naphthylamine is low.
Studies performed according to standard protocols yielded LD50s in
male and female Wistar rats of >5000 mg/kg body weight (Bayer AG,
1978a,b). LD50s for male CFE rats and male CF-1 mice are
>1625 mg/kg body weight (MacEwen & Vernot, 1974; Vernot et al.,
1977) and 1231 mg/kg body weight (MacEwen & Vernot, 1974),
respectively. No specific signs of toxicity were reported.
Slight fatty degeneration in the liver of rabbits was observed 3
months after a single subcutaneous injection of 200 mg
N-phenyl-1-naphthylamine/kg body weight (Bayer AG, 1931). Like
other aromatic amines, N-phenyl-1-naphthylamine induces the
formation of methaemoglobin. In mice, a slightly increased
methaemoglobin level (4.1% versus 0.4% in controls) was noted within
10 min of a single intraperitoneal administration; the increase was
still detectable up to 24 h later (Nomura, 1977). Mice are less
sensitive than humans to methaemoglobin induction, and this small
increase in methaemoglobin level may be of importance to human health.
Data on effects related to acute exposure to
N-phenyl-1-naphthylamine via the inhalation route were not
identified.
8.2 Irritation and sensitization
Three studies assessed skin irritation by
N-phenyl-1-naphthylamine using the Draize method in rabbits. In one
study, no effects were observed within 72 h of application (no further
information was reported) (MacEwen & Vernot, 1974). In a study
performed according to US Food and Drug Administration (FDA)
standards, N-phenyl-1-naphthylamine was classified as a very slight
skin irritant (3/6 animals with intact skin and 2/6 animals with
abraded skin showed a slight positive reaction) (van Beek, 1977). In
a test conducted according to OECD guideline 404,
N-phenyl-1-naphthylamine was not considered to be a skin irritant.
Slight erythema and oedema reactions in 1/3 rabbits were observed 1 h
after removal of the test substance, whereas no effects were noted
after 24 or 72 h (Ciba-Geigy Corp., 1987b). Studies conducted
according to US FDA standards or OECD guideline 405 did not consider
N-phenyl-1-naphthylamine to be an eye irritant. The observed
effects in some animals (slight conjunctivitis or swelling of the
eyelid) were reversible within a maximum of 10 days (van Beek, 1977;
Ciba-Geigy Corp., 1987a).
In a guinea-pig maximization test (Magnusson & Kligman, 1970) and
in a test performed according to OECD guideline 406,
N-phenyl-1-naphthylamine was shown to be a strong sensitizer
(positive reaction in 15/20 and 18/20 animals, respectively) (Boman et
al., 1980; Ciba-Geigy Corp., 1987c). In the modified Landsteiner's
guinea-pig sensitization test (no further information available),
which is not a standard method, N-phenyl-1-naphthylamine did not
exhibit sensitizing potential (MacEwen & Vernot, 1974).
8.3 Short-term exposure
In five female Sprague-Dawley rats (two untreated controls), no
adverse clinical signs or effects on body weight gain were noted after
daily oral (gavage) administration of 2000 mg
N-phenyl-1-naphthylamine/kg body weight, 5 days per week for 2
weeks. Data concerning the purity of the test substance or the
formulation administered were not provided. Gross morphological
observations (no histopathological examination) made at necropsy
revealed no evidence of exposure-related effects (Mobil Oil Corp.,
1989).
Older studies (Bayer AG, 1931) performed with small numbers of
rabbits, although inadequate to serve as a basis for the determination
of putative effect levels, may provide some useful information on
toxicity and target organs. The oral administration of 200 mg
N-phenyl-1-naphthylamine/kg body weight per day, 5 days per week for
6 weeks, resulted in diarrhoea, proteinuria, slight irritation of the
kidneys, and a fatty degeneration of the liver. After the
subcutaneous administration (42 times in 7 weeks) of 50 or 200 mg
N-phenyl-1-naphthylamine/kg body weight per day, a fatty
degeneration of the liver and single proliferation of connective
tissue were noted 3 months after the cessation of exposure. Dermal
application of a 5% solution of N-phenyl-1-naphthylamine to the ear
(28 times within 5 weeks) produced slight skin erythema, proteinuria,
and anorexia. Death occurred 5 days after the 27th application, and
necropsy revealed fatty degeneration of the liver (Bayer AG, 1931).
In mice (sex and number not specified), the intraperitoneal
administration of 219 mg N-phenyl-1-naphthylamine/kg body weight for
3 days resulted in increased methaemoglobin levels (1.6% versus 0.4%
in controls) 48 h after treatment; methaemoglobin concentrations were
not elevated after intraperitoneal administration of 109 mg/kg body
weight for 9 days (Nomura, 1977).
8.4 Long-term exposure
8.4.1 Subchronic exposure
There are no studies available concerning subchronic exposure to
N-phenyl-1-naphthylamine. In an oral 13-week study with the isomer
N-phenyl-2-naphthylamine (approximately 98% pure, containing <1 mg
2-naphthylamine/kg), relative liver weight in F344/N rats and B6C3F1
mice increased in a dose-dependent fashion. A chemical-related
nephropathy was observed in rats, characterized by renal tubular
epithelial degeneration and hyperplasia (NTP, 1988).
8.4.2 Chronic exposure and carcinogenicity
Long-term toxicity or carcinogenicity studies performed according
to currently accepted standard protocols using physiologically
relevant routes of exposure were not identified. The inhalation
exposure of four rabbits (number of controls not provided, approximate
dose 100 mg/day) for several months (no additional information
provided) resulted in progressive anaemia, leucopenia, lymphocytosis,
pneumonia, nephritis, nephrosis, formation of lung abscesses, fatty
degeneration of the liver after 3-5 months, and death within 6-24
months (Schär, 1930). This study is characterized by the limited
number of animals, methodological deficiencies, and insufficient
documentation of results.
Bladder tumours were not observed in a long-term study in which
three dogs were orally administered 290 mg N-phenyl-1-naphthylamine
5 days per week for up to 3.5 years (DuPont, 1945; Gehrmann et al.,
1948; Haskell Laboratory, 1971). Owing to the limited number of
animals and the examination for tumours only in the bladder, this
study is inadequate for evaluation of the carcinogenic potential of
N-phenyl-1-naphthylamine following oral administration.
Wang et al. (1984) observed an increased incidence of malignant
tumours in male ICR and TA-1 mice following repeated subcutaneous
administration of N-phenyl-1-naphthylamine (technical grade or pure;
no additional data provided). In ICR mice, there was a statistically
significant increase (p < 0.05) in the incidence of lung carcinoma
(5/30 versus 0/24 in controls administered vehicle alone) after the
administration of 16 mg technical-grade N-phenyl-1-naphthylamine per
animal 27 times over 9 weeks (total dose 432 mg per animal). The
incidence of kidney haemangiosarcomas was 1/30 and 0/24 in the exposed
and control groups, respectively; however, the combined incidence of
liver, kidney, and lung haemangiosarcomas was significantly (p <
0.05) increased in the exposed animals (5/30 versus 0/24 in unexposed
controls). The same dosing regimen using purified
N-phenyl-1-naphthylamine produced a significant (p < 0.05)
increase in the incidence of kidney haemangiosarcomas (4/23 versus
0/24 in controls); the numbers of animals with lung carcinoma were
3/23 and 0/24 in the exposed and control groups, respectively. The
administration of 5.3 mg purified N-phenyl-1-naphthylamine per
animal, 27 times over 9 weeks (total dose 143 mg per animal), produced
a significant (p < 0.05) increase in the number of animals with
lung carcinomas (6/25 versus 0/24 in controls). The incidence of
kidney haemangiosarcomas was not elevated (1/25 and 0/24 in the
exposed and control groups, respectively); however, there was a
significant increase (p < 0.05) in the combined incidence of kidney
and lung haemangiosarcomas (4/25 versus 0/24 in the controls). All
animals in the study were sacrificed after 10 months.
In TA-1 mice administered a total dose of 328 mg technical-grade
N-phenyl-1-naphthylamine per animal subcutaneously over a period of
12 weeks (48 mg peranimal over 3 weeks, followed by 280 mg per animal
over 9 weeks), there was a significant increase (p < 0.05) in the
incidence of kidney haemangiosarcomas (7/19 compared with 0/18 in
unexposed controls). The incidence of renal tumours was also
significantly (p < 0.05) elevated in unilaterally nephrectomized
TA-1 mice (one kidney was removed 1 week prior to treatment)
subcutaneously administered a total dose of 328 mg of either purified
or technical-grade N-phenyl-1-naphthylamine per animal over a period
of 12 weeks (48 mg per animal over 3 weeks, followed by 280 mg per
animal over 9 weeks). The incidence of kidney haemangiosarcomas in
controls and the unilaterally nephrectomized animals administered
either the pure or technical-grade material was 0/18, 12/16, and
13/13, respectively (Wang et al., 1984). Evaluation of this report is
difficult owing to the number of individual experiments. These
studies are characterized by the use of small numbers of animals of a
single sex, limited dose groups, the absence of data on mortality and
morbidity, use of a non-physiologically relevant route of exposure,
and insufficient characterization of the substance tested.
N-Phenyl-2-naphthylamine, which had effects comparable to those
of N-phenyl-1-naphthylamine in the above-mentioned study by Wang et
al. (1984), has been tested in a 2-year carcinogenicity bioassay (NTP,
1988). There was no evidence of carcinogenic activity in male or
female F344/N rats administered diets containing 2500 or 5000 ppm
(mg/kg) N-phenyl-2-naphthylamine (estimated daily intakes of 100 and
225 mg/kg body weight for males and 120 and 260 mg/kg body weight for
females, respectively). The lack of carcinogenicity in rats may be
related to an inability to metabolize N-phenyl-2-naphthylamine to
the known animal and human carcinogen 2-naphthylamine (NTP, 1988).
There was no evidence of carcinogenic activity in male B6C3F1 mice
administered diets containing 2500 or 5000 ppm (mg/kg)
N-phenyl-2-naphthylamine (estimated daily intakes of 500 or
1000 mg/kg body weight, respectively). However, in female mice
receiving these diets (estimated daily intakes of 450 or 900 mg/kg
body weight, respectively), there was equivocal evidence of
carcinogenic activity, based upon the occurrence of rare kidney
neoplasms in two high-dose animals (one tubular cell adenoma and one
tubular cell adenocarcinoma). For non-neoplastic effects, the kidney
was the principal target organ. Mineralization, necrosis of the renal
papilla, epithelial hyperplasia, calculi of the kidney pelvis,
hydronephrosis, atrophy, fibrosis, and chronic focal inflammation of
the kidney were observed in the high-dose female rats. In male rats
of both dose groups and in the high-dose female rats, cysts and acute
suppurative inflammation of the kidney were also noted. Nuclear
enlargement of renal tubular epithelial cells and nephropathy were
observed in the high-dose female mice (NTP, 1988).
In a dermal carcinogenicity study, approximately 0.75 mg
N-phenyl-1-naphthylamine/kg body weight (dissolved in 50 µl toluene)
was applied to the skin of 50 male C3H mice twice per week for 80
weeks. Data concerning the purity of the test substance or the
formulation applied were not provided. No adverse effects on survival
or increased incidence of skin tumours were observed; however,
pigmentation, fibrosis, scar formation, acanthosis, and hyperkeratosis
were noted. Histopathological examinations of organs other than the
skin were not performed (Mobil Oil Corp., 1985).
8.5 Genotoxicity and related end-points
The results of experiments on the genotoxicity of
N-phenyl-1-naphthylamine are summarized in Table 1.
N-Phenyl-1-naphthylamine was not mutagenic in bacterial tests
conducted in the presence or absence of metabolic activation. In
mammalian cells, neither gene mutations (mouse lymphoma assay) nor
chromosomal aberrations (in vitro metaphase analysis in Chinese
hamster ovary cells or Chinese hamster lung cells) were induced by
N-phenyl-1-naphthylamine. A sister chromatid exchange assay in
Chinese hamster ovary cells was marginally positive in the presence of
metabolic activation. An unscheduled DNA synthesis assay with human
lung (WI-38) cells yielded positive results, although the effects were
not clearly concentration dependent. A number of non-validated
short-term tests yielded conflicting results on the transforming
potential of N-phenyl-1-naphthylamine (BUA, 1993). Based upon the
weight of evidence from in vitro studies, N-phenyl-1-naphthylamine
does not appear to be genotoxic.
No in vivo somatic cell mutation tests were identified. In a
dominant lethal test, 10 male ICR mice were intraperitoneally
administered 0, 50, 166, or 500 mg N-phenyl-1-naphthylamine/kg body
weight per day for 5 consecutive days followed by 2 days without
exposure. Each male was then caged with two virgin females 5 days per
week, and the sequence was repeated weekly with two new females each
week for 8 weeks. Examination of females 14 days from the mid-week in
which they were caged with the males yielded negative results (Brusick
& Matheson, 1976, 1977).
8.6 Reproductive and developmental toxicity
Data on the reproductive and developmental toxicity of
N-phenyl-1-naphthylamine were not identified.
8.7 Immunological and neurological effects
Data on immunological and neurological effects of
N-phenyl-1-naphthylamine in laboratory animals were not identified.
Table 1: In vitro genotoxicity studies on N-phenyl-1-naphthylamine.
Resultsa (with /
without metabolic
Cell type (end-point) Test concentration activation) Remarks References
Salmonella typhimurium TA98, 0.5-500 µl/plate with and - / - Brusick & Matheson,
TA100, TA1535, TA1537, without metabolic activation 1976, 1977
TA1538; Escherichia coli
WP2uvrA-
(gene mutation)
S. typhimurium TA98, TA100, 0.01-1000 µg/plate with and - / - Baden et al., 1978
TA1535, TA1537; E. coli WP2 without metabolic activation
(gene mutation)
S. typhimurium TA97, TA98, 0.3-666 µg/plate with and - / - Zeiger et al., 1988
TA100, TA1535, TA1537 without metabolic activation
(gene mutation)
S. typhimurium TA98, TA100, 0.2-1000 µg/plate with and - / - JETOC, 1996
TA1537, TA1538 without metabolic activation
(gene mutation)
E. coli WP2uvrA 20-5000 µg/plate with and - / - JETOC, 1996
(gene mutation) without metabolic activation
S. typhimurium TA98, TA100, Not provided - / - Rannug et al., 1984
TA1535, TA1537, TA1538
(gene mutation)
Saccharomyces cerevisiae D4 0.5-500 µl/plate with and - / - Brusick & Matheson,
(gene mutation) without metabolic activation 1976, 1977
Table 1 (continued)
Resultsa (with /
without metabolic
Cell type (end-point) Test concentration activation) Remarks References
Mouse lymphoma (L5178Y) 0.005-0.1 µg/ml with - / - Brusick & Matheson,
cells metabolic activation 1976, 1977
(gene mutation) 0.5-25 µg/ml without
metabolic activation
Human lung (WI-38) cells 5, 10, or 50 µg/ml with - / (+) Weak positive response Brusick & Matheson,
(DNA repair [unscheduled metabolic activation at 50 µg/ml and toxic at 1976, 1977
DNA synthesis]) 10, 50, or 100 µg/ml without 100 µg/ml without
metabolic activation metabolic activation;
effects not clearly
concentration related
Human lung (WI-38) cells 5, 10, or 50 µg/ml with and (+) / (+) Positive response at 10 Brusick & Matheson,
(DNA repair [unscheduled without metabolic activation µg/ml with metabolic 1976, 1977
DNA synthesis] ) activation; positive
response at 5 and 50
µg/ml without metabolic
activation; effects not
clearly concentration
related
Chinese hamster ovary cells 0.6-19.9 µg/ml with (+) / - Marginally positive with NTP, 1987; Loveday
(sister chromatid exchange) metabolic activation metabolic activation et al., 1990
1.8-18.2 µg/ml without metabolic activation
Chinese hamster ovary cells 1.49-19.9 µg/ml with - / - NTP, 1987; Loveday
(chromosomal aberrations) metabolic activation et al., 1990
2.99-29.9 µg/ml without
metabolic activation
Table 1 (continued)
Resultsa (with /
without metabolic
Cell type (end-point) Test concentration activation) Remarks References
Chinese hamster lung cells 15.6 µg/ml with - / - Sofuni et al., 1990
(chromosomal aberrations) metabolic activation
30 µg/ml without metabolic
activation
a - = negative result; (+) = weak positive result.
9. EFFECTS ON HUMANS
9.1 Case reports
N-Phenyl-1-naphthylamine, mixed with oil (Bayer AG, 1931) or
water (Haskell Laboratory, 1971), was not irritating when applied to
the skin of volunteers (no data on concentrations available). Skin
eczema in workers has been attributed to repeated exposure to high
levels of N-phenyl-1-naphthylamine, possibly in combination with
other substances. Reportedly, the content of
N-phenyl-1-naphthylamine in a special antirust oil had to be lowered
from 2% to 0.5% because of skin problems. Workers, who did not wear
gloves, were exposed during the packaging of bearing rings covered
with antirust-oil containing N-phenyl-1-naphthylamine (Järvholm &
Lavenius, 1981).
N-Phenyl-1-naphthylamine was also reported to have sensitizing
properties in humans. Case-studies on patients with contact
dermatitis, potentially associated with occupational exposure to
N-phenyl-1-naphthylamine in greases or oils, have been identified.
The majority of these patients also had a positive reaction to other
substances in the test series, such as mercaptobenzothiazole or
p-phenylenediamine. Lower incidences were reported in patients with
past exposure to rubber materials (Blank & Miller, 1952; Schultheiss,
1959; Nater, 1975; Te Lintum & Nater, 1979; Boman et al., 1980;
Järvholm & Lavenius, 1981; Kantoh et al., 1985; Kalimo et al., 1989;
Carmichael & Foulds, 1990). Because of the chemical's incorporation
into the polymer matrix, exposure to N-phenyl-1-naphthylamine in
rubber materials is assumed to be lower than exposure from greases or
oils.
9.2 Epidemiological studies
An increased occurrence of cancers in a small packaging unit in a
Swedish engineering company was reported in a cohort study (Järvholm &
Lavenius, 1981). Between 1954 and 1957, a special anticorrosive oil,
which contained 0.5% N-phenyl-1-naphthylamine in addition to other
chemicals, had been used in this unit. In 12 of 78 women in this unit
(group A: 78 women/20 men), cancers were diagnosed between 1964 and
1973 in several organs (predominantly the uterus and ovary). The
staff performing the actual packaging, and thus in contact with the
oil, were mainly women. Morbidity and mortality from cancer were 3.1-
and 3.5-fold higher, respectively, than expected, based upon
age-specific and sex-specific data from the Swedish Cancer Register
(the standard cancer rates for the period 1974-1976 were estimated on
the basis of the 1973 rate). In the males of group A, no significant
differences were established. In another unit (reference group B: 25
women/8 men) where anticorrosive oil without
N-phenyl-1-naphthylamine had been used, morbidity and mortality from
cancer were not elevated. This was also true for reference group C (8
women/23 men), from units that had been in contact with
N-phenyl-1-naphthylamine-containing anticorrosive oil for a short
period of time only, because they had demonstrated allergic reactions.
The authors concluded that, apart from exposure to
N-phenyl-1-naphthylamine, the formation of
N-nitroso- N-phenyl-1-naphthylamine from sodium nitrite originating
from the packaging paper used may be a possible explanation for the
increased frequency of cancer in group A.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10.1 Aquatic environment
Valid results from laboratory tests with ciliates, Daphnia, and
fish indicate that N-phenyl-1-naphthylamine is highly toxic to
aquatic species. An EC50 (48 h) of 2 mg
N-phenyl-1-naphthylamine/litre (nominal concentration; static;
solubilizer: acetone) was measured for the inhibition of cell
proliferation of freshwater ciliates (Tetrahymena pyriformis)
(Epstein et al., 1967). Forty-eight-hour LC50s from static and
semi-static acute toxicity tests with young and adult Daphnia magna
were in the range of 0.30-0.68 mg N-phenyl-1-naphthylamine/litre
(nominal concentration; solubilizer: ethanol). The lowest reported
21-day LC50 from long-term semi-static tests was 0.06 mg/litre; the
lowest reported NOEC was 0.02 mg/litre (nominal concentration;
solubilizer: ethanol) (Sikka et al., 1981).
Acute toxicity tests in semi-static (daily renewal of test
medium) and flow-through systems yielded 96-h LC50s in the range of
0.44-0.74 mg N-phenyl-1-naphthylamine/litre for rainbow trout
(Oncorhynchus mykiss) and >0.57-0.82 mg/litre for bluegill sunfish
(solubilizers: ethanol and acetone, respectively; nominal
concentrations); the lowest reported NOEC (192 h) was 0.11 mg/litre
(Sikka et al., 1981). Sublethal N-phenyl-1-naphthylamine
concentrations of approximately 5.2 and 5.6 mg/litre had teratogenic
effects on embryos and larvae, respectively, of the clawed frog
(Xenopus laevis). Concentrations above 6.2 mg/litre were lethal
(100% death of larvae within 24 h) for both (Greenhouse, 1976a,b).
During neurulation, an EC50 of 4.57 mg/litre for teratogenic effects
was established. For larvae, a 48-h LC50 of 2.3 mg/litre was
determined (Greenhouse, 1977). For larvae of the leopard frog
(Rana pipiens), a 48-h LC100 of 5 mg/litre was reported; no effects
occurred after 24 h of exposure (Greenhouse, 1976b).
Data on chronic effects of N-phenyl-1-naphthylamine in the
aquatic environment are not available.
10.2 Terrestrial environment
Data on toxic effects of N-phenyl-1-naphthylamine on
terrestrial microorganisms, plants, animals, and ecosystems are not
available.
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
N-Phenyl-1-naphthylamine is well absorbed and readily excreted
following ingestion; accumulation in the body is not expected. The
acute oral toxicity of N-phenyl-1-naphthylamine in laboratory
animals is low. Based on the results of tests performed according to
OECD guidelines, the substance is not considered to be a skin or eye
irritant. N-Phenyl-1-naphthylamine has been observed to be a skin
sensitizer in laboratory animals and humans.
A no-observed-effect level could not be derived from the
available toxicological studies. There is limited evidence to suggest
that the kidneys and liver are the main target organs following oral
exposure to N-phenyl-1-naphthylamine, a finding comparable to that
observed for its isomer, N-phenyl-2-naphthylamine. As indicated
above, useful data on the toxicity of N-phenyl-1-naphthylamine are
limited, and therefore additional data on its isomer,
N-phenyl-2-naphthylamine, have been included to assist in the
identification of potential target organs. Available carcinogenicity
studies on N-phenyl-1-naphthylamine have not been performed
according to currently accepted standard protocols, and therefore the
potential carcinogenicity of this chemical cannot be fully evaluated.
However, in a 2-year carcinogenicity bioassay conducted with
N-phenyl-2-naphthylamine in rats and mice, there was no evidence of
carcinogenic activity in male or female rats or male mice and
equivocal evidence of carcinogenic activity in female mice.
N-Phenyl-1-naphthylamine was not mutagenic in bacterial test
systems. In tests with mammalian cells, some investigations yielded
marginally positive or questionably positive results. Based upon the
available evidence, N-phenyl-1-naphthylamine does not appear to be
genotoxic. It is worth noting, however, that several aromatic amines
(the chemical class to which N-phenyl-1-naphthylamine belongs),
while yielding negative or weakly positive results in mutagenicity
assays, are carcinogenic.
An increased occurrence of cancers was observed in one limited
epidemiological study of occupationally exposed individuals; however,
because of the small number of excess deaths and concomitant exposure
to other chemicals, it is not possible to attribute this finding
solely to N-phenyl-1-naphthylamine. Information on the reproductive
or developmental toxicity of N-phenyl-1-naphthylamine was not
available.
11.1.2 Criteria for setting guidance values for
N-phenyl-1-naphthylamine
Data are inadequate to allow the derivation of a
no-observed-effect level or the performance of a risk estimation for
carcinogenicity. Dermal contact with N-phenyl-1-naphthylamine
should be avoided because of its sensitizing properties.
11.1.3 Sample risk characterization
Owing to the lack of available data with which to derive a
suitable guidance value as well as the lack of information on
exposure, a sample quantitative risk characterization could not be
performed. At the workplace, there is a risk of dermal sensitization
from exposure to greases and antirust oils containing
N-phenyl-1-naphthylamine. The risk from exposure to rubber
materials may be much lower, owing to the low concentrations of
N-phenyl-1-naphthylamine in such materials; a risk to the general
population from exposure to products containing
N-phenyl-1-naphthylamine cannot be excluded. Although quantitative
information on the leaching of N-phenyl-1-naphthylamine from rubber
products is not available, the extent of such leaching is expected to
be low. Any leached material is expected to be degraded faster than
it is leached. Indirect exposure of humans to
N-phenyl-1-naphthylamine leached from rubber products into the soil
is unlikely.
11.2 Evaluation of environmental effects
Overall, releases of N-phenyl-1-naphthylamine to the
environment from production and processing (e.g. vulcanization of
rubber mixtures) are expected to be small in view of the chemical's
low production. Based upon the chemical's physical and chemical
properties, it is predicted that soil and sediment will be affected
indirectly by the leaching of N-phenyl-1-naphthylamine from decaying
tyres and rubber products; however, the amounts of
N-phenyl-1-naphthylamine introduced into the environment via this
route could not be quantified. Data on the occurrence of
N-phenyl-1-naphthylamine in environmental media were available only
from some older studies for highly polluted river water and sediment
samples; recent measurements on levels in water, soil, or biota were
not identified. Data on geoaccumulation or on the toxic effects of
N-phenyl-1-naphthylamine on terrestrial microorganisms, plants,
animals, and ecosystems were unavailable.
As data on effect levels for terrestrial organisms or on current
concentrations in environmental media were not available, a
quantitative risk assessment for the main target compartments, water
and soil, could not be carried out; however, some qualitative
statements can be made. Owing to its moderate to high potential for
sorption to organic soil constituents and its limited mineralization
in soil, N-phenyl-1-naphthylamine released to this environmental
compartment is presumed to have geoaccumulation potential. The
probability of its infiltration into groundwater is low. In
laboratory experiments, the acute toxicity of
N-phenyl-1-naphthylamine in fish and Daphnia was high, with lowest
reported NOECs of 0.11 mg/litre (192 h) and 0.02 mg/litre (21 days),
respectively. Although considerable bioconcentration factors were
measured in fish and Daphnia, biomagnification and secondary
poisoning of higher trophic levels via the aquatic food-chain seem
unlikely in view of the metabolism and extensive excretion of
N-phenyl-1-naphthylamine. Biodegradation is expected to be the
predominant route of environmental breakdown; in water, it is aided by
the presence of other degradable substrates, but it is reduced in soil
by sorption. Available N-phenyl-1-naphthylamine is likely to be
biodegraded in both compartments with half-lives of days to weeks.
Photolysis may lead to initial degradation under favourable conditions
but is not considered important in the mineralization of
N-phenyl-1-naphthylamine. Hydrolysis is of very limited or no
importance in the environment.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Previous evaluations of N-phenyl-1-naphthylamine by
international bodies were not identified. Information on
international hazard classification and labelling is included in the
International Chemical Safety Card reproduced in this document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventative and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 1113) reproduced in this
document.
13.1 Human health hazards
N-Phenyl-1-naphthylamine has sensitizing properties.
13.2 Advice to physicians
In case of intoxication, the treatment is supportive. Some
chemicals of this class induce methaemoglobinaemia.
13.3 Spillage
Because N-phenyl-1-naphthylamine is classified as a sensitizer,
emergency crews need to wear proper equipment to prevent contact with
the skin.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards
can be found in the International Register of Potentially Toxic
Chemicals (IRPTC), available from UNEP Chemicals (IRPTC), Geneva.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
N-PHENYL-1-NAPHTHYLAMINE ICSC: 1113
24.03.1998
CAS # 90-30-2
RTECS # QM4500000
UN #
N-(1-Naphthyl)aniline
N-Phenyl-alpha-naphthylamine
C16H13N/C10H7NHC6H5
Molecular mass: 219.30
TYPES OF HAZARD/ ACUTE HAZARDS/ PREVENTION FIRST AID/FIRE FIGHTING
EXPOSURE SYMPTOMS
FIRE Combustible. Gives off irritating or NO open flames. Powder, water spray, foam, carbon dioxide.
toxic fumes (or gases) in a fire.
EXPLOSION In case of fire: keep drums, etc., cool by
spraying with water.
electrical equipment and lighting.
EXPOSURE AVOID ALL CONTACT!
Inhalation Blue lips or finger nails. Blue skin. Local exhaust or breathing protection Fresh air, rest. Refer for medical
Confusion. Convulsions. Dizziness. attention.
Headache. Nausea.
Unconsciousness.
Skin MAY BE ABSORBED! Protective gloves. Protective Remove contaminated clothes. Rinse skin
clothing. with plenty of water or shower.
Eyes Face shield. First rinse with plenty of water for
several minutes (remove contact lenses if
protection. easily possible), then take to a doctor.
Ingestion (See Inhalation). Do not eat, drink, or smoke during Rinse mouth. Refer for medical attention.
work. Wash hands before eating.
(continued)
SPILLAGE DISPOSAL PACKAGING & LABELLING
Sweep spilled substance into sealable containers. Carefully collect Symbol
remainder, then remove to safe place. Do NOT let this chemical enter R:
the environment (extra personal protection: P2 filter respirator for S:
harmful particles). UN Hazard Class:
UN Subsidiary Risks:
UN Pack Group:
EMERGENCY RESPONSE STORAGE
Well closed.
IMPORTANT DATA
PHYSICAL STATE: APPEARANCE ROUTES OF EXPOSURE:
WHITE TO SLIGHT YELLOWISH CRYSTALS The substance can be absorbed into the body by inhalation of its
aerosol, through the skin and by ingestion.
CHEMICAL DANGERS: INHALATION RISK:
The substance decomposes on burning producing toxic fumes No indication can be given about the rate in which a harmful
including nitrogen oxides. concentration in the air is reached on evaporation of this
substance at 20°C.
OCCUPATIONAL EXPOSURE LIMITS: EFFECTS OF SHORT-TERM EXPOSURE:
TLV not established. The substance may cause effects on the blood, resulting in
formation of methaemoglobin. The effects may be delayed.
Medical observation is indicated.
EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
Repeated or prolonged contact may cause skin sensitization.
(continued)
PHYSICAL PROPERTIES
Melting point: 62-63°C
Relative density
(water =1): 1.2
Solubility in water: none
Octanol/water partition
coefficient as log Pow: 4.2
ENVIRONMENTAL DATA
The substance is very toxic to aquatic organisms. In the food chain important to humans, bioaccumulation takes place, especially in fish.
NOTES
Depending on the degree of exposure, periodic medical examination is indicated. Specific treatment is necessary in case of poisoning with
this substance; the appropriate means with instructions must be available.
REFERENCES
Baden JM, Kelley M, Simmon VF, Rice SA, Mazze RI (1978) Fluroxene
mutagenicity. Mutation research, 58:183-191.
Bayer AG (1931) Physiologische Eigenschaften von
Phenyl-alpha-naphthylamin und Phenyl--naphthylamin. Ludwigshafen,
I.G. Farben (not available in print).
Bayer AG (1978a) Akute orale Toxizität von Additin 30
(Phenyl-alpha-naphthylamin) bei weiblichen Wistar Ratten. Wuppertal,
Bayer AG (not available in print).
Bayer AG (1978b) Akute orale Toxizität von Additin 30
(Phenyl-alpha-naphthylamin) bei männlichen Wistar Ratten. Wuppertal,
Bayer AG (not available in print).
Bayer AG (1990) Biologischer Abbau von Vulkanox PAN (OECD Guideline
301C; modifiziert). Prüfbericht vom 05.12.1990. Leverkusen, Bayer AG
(not available in print).
Blank IH, Miller OG (1952) A study of rubber adhesives in shoes as the
cause of dermatitis of the feet. Journal of the American Medical
Association, 149:1371-1374.
Boman A, Hagelthorn G, Jeansson I, Karlberg A-T, Rystedt I, Wahlberg
JE (1980) Phenyl-alpha-naphthylamine - case report and guinea pig
studies. Contact dermatitis, 6:299-300.
Brusick D, Matheson DW (1976) Mutagen and oncogen study on
N-phenyl-alpha-naphthylamine - final report (Litton Bionetics, Inc.,
Kensington, MD). Wright-Patterson Air Force Base, OH, Aerospace
Medical Research Laboratory (Report No. AMRL-TR-76-79).
Brusick D, Matheson D (1977) Mutagenic evaluation of
1,1-dimethylhydrazine, methylhydrazine and
N-phenyl-alpha-naphthylamine. In: Proceedings of the 7th annual
conference on environmental toxicology, 13-15 October 1976.
Wright-Patterson Air Force Base, OH, Aerospace Medical Research
Laboratory, pp. 108-129 (Report No. AMRL-TR-76-125).
BUA (1993) BUA-Stoffbericht N-phenyl-1-naphthylamin. Beratergremium
fuer Umweltrelevante Altstoffe. Weinheim, VCH VerlagsGmbH (Report No.
113; April 1993).
Carmichael AJ, Foulds IS (1990) Isolated naphthylamine allergy to
phenyl-alpha-naphthylamine. Contact dermatitis, 22:298-299.
Ciba-Geigy Corp. (1987a) N-Phenyl-1-naphthylamine: Acute dermal
irritation/corrosion study in the rabbit (final report) - with cover
letter dated 10/02/91. Hawthorne, NY, US Environmental Protection
Agency, Office of Toxic Substances (EPA Report OTS 0533599; Doc.
ID 86-920000033).
Ciba-Geigy Corp. (1987b) N-Phenyl-1-naphthylamine: Acute eye
irritation/corrosion study in the rabbit (final report) - with cover
letter dated 10/02/91. Hawthorne, NY, US Environmental Protection
Agency, Office of Toxic Substances (EPA Report OTS 0533600; Doc.
ID 86-920000034).
Ciba-Geigy Corp. (1987c) N-Phenyl-1-naphthylamine: Skin
sensitization test in the guinea pig modified maximization test
(final report) - with cover letter dated 10/02/91. Hawthorne, NY, US
Environmental Protection Agency, Office of Toxic Substances (EPA
Report OTS 0533601; Doc. ID 86-920000035s).
CITI (1992) Biodegradation and bioaccumulation. Data of existing
chemicals based on the CSCL Japan. Tokyo, Japan Chemical Industry
Ecology-Toxicology & Information Center, Chemicals Inspection &
Testing Institute.
DuPont (1945) Aniline tumours of the bladder. Studies of urinary
bladder tumours. Wilmington, DE, E.I. DuPont de Nemours & Co., 7 pp.
Epstein SS, Saporoschetz IB, Hutner SO (1967) Toxicity of antioxidants
to Tetrahymena pyriformis. Journal of protozoology, 14:238-244.
Gehrmann GH, Foulger JH, Fleming AJ (1948) Occupational tumours of the
bladder. Proceedings of the 9th international congress on
industrial medicine (Chairperson: JM MacAlpine), Tudor Room, Caxton
Hall, pp. 472-475.
Greenhouse GA (1976a) The evaluation of toxic effects of chemicals in
fresh water by using frog embryos and larvae. Environmental
pollution, 11:303-315.
Greenhouse G (1976b) Effects of pollutants on eggs, embryos and
larvae of amphibian species. Wright-Patterson Air Force Base, OH,
Aerospace Medical Research Laboratory, 24 pp. (Report No.
AMRL-TR-76-31).
Greenhouse GA (1977) Toxicity of N-phenyl-alpha-naphthylamine and
hydrazine to Xenopus laevis embryos and larvae. Bulletin of
environmental contamination and toxicology, 18:503-511.
Haskell Laboratory (1971) Memorandum to customers. Wilmington, DE,
E.I. DuPont de Nemours & Co. [cited in McCormick WE (undated),
Environmental health control for the rubber industry, Part II, p.
634.]
IPCS (1993) International Chemical Safety Card -
N-phenyl-1-naphthylamine. Geneva, World Health Organization,
International Programme on Chemical Safety (No. 1113).
Järvholm B, Lavenius B (1981) A cohort study on cancer among workers
exposed to an antirust oil. Scandinavian journal of work,
environment & health, 7:179-184.
JETOC (1996) Mutagenicity test data of existing chemical substances
based on the toxicity investigation system of the industrial safety
and health law. Tokyo, Japan Chemical Industry Ecology-Toxicology &
Information Center.
Jungclaus GA, Lopez-Avila V, Hites RA (1978) Organic compounds in an
industrial wastewater: a case study of their environmental impact.
Environmental science and technology, 12:88-96.
Kalimo K, Jolanki R, Estlander T, Kanerva L (1989) Contact allergy to
antioxidants in industrial greases. Contact dermatitis, 20:151-152.
Kantoh H, Ishihara M, Itoh M, Hosono K, Nishimura M (1985) Allergens
in rubber products. HIFU (Skin research), 27:501-509 (in Japanese,
with English summary).
Kenaga EE (1980) Predicted bioconcentration factors and soil sorption
coefficients of pesticides and other chemicals. Ecotoxicology and
environmental safety, 4:26-38.
Kenaga EE, Goring CAI (1980) Relationship between water solubility,
soil sorption, octanol-water partitioning, and concentration of
chemicals in biota. In: Eaten JG, Parrish PR, Hendricks AC, eds.
Aquatic toxicology. Philadelphia, PA, American Society for Testing
and Materials, pp. 78-115 (ASTM Special Technical Publication 707).
Lopez-Avila V, Hites RA (1980) Organic compounds in an industrial
wastewater. Their transport into sediments. Environmental science
and technology, 14:1382-1390.
Loveday KS, Anderson BE, Resnick MA, Zeiger E (1990) Chromosome
aberration and sister chromatid exchange tests in Chinese hamster
ovary cells in vitro. V: Results with 46 chemicals. Environmental
and molecular mutagenesis, 16:272-303.
MacEwen JD, Vernot EH (1974) Toxic hazards research unit annual
technical report: 1974 (University of California). Wright-Patterson
Air Force Base, OH, Aerospace Medical Research Laboratory (Report No.
AMRL-TR-74-78).
Mackay D (1991) Multimedia models: the fugacity approach. Chelsea,
MI, Lewis Publishers.
Magnusson B, Kligman AM (1970) Allergic contact dermatitis in the
guinea pig. Identification of contact allergens. Springfield, IL,
Charles C. Thomas.
Miyazaki K, Kawai S, Sasayama T, Iseki K, Arita T (1987) Absorption,
metabolism and excretion of N-phenyl-1-naphthylamine in rat.
Yakuzaigaku, 47:17-22 (in Japanese, with English summary).
Mobil Oil Corp. (1985) Dermal carcinogenicity in mice - with cover
letter dated 12/17/91. Princeton, NJ, US Environmental Protection
Agency, Office of Toxic Substances (EPA Report OTS 0533828; Doc.
ID 86-920000547S).
Mobil Oil Corp. (1989) Two-week oral toxicity study in female rats
(final report) - with cover letter dated 12/17/91. Princeton, NJ, US
Environmental Protection Agency, Office of Toxic Substances (EPA
Report OTS 0533821; Doc. ID 86-920000540S).
Nater JP (1975) Überempfindlichkeit gegen Gummi. Berufsdermatosen,
23:161-168.
NIOSH (1976) Metabolic precursors of a known human carcinogen,
beta-naphthylamine. Cincinnati, OH, US Department of Health and
Human Services, National Institute of Occupational Safety and Health
(Current Intelligence Bulletin 16; Publication No. 78-127).
NIOSH (1984a) NIOSH manual of analytical methods, 3rd ed. Vol. 1.
Cincinnati, OH, US Department of Health and Human Services, National
Institute of Occupational Safety and Health, pp. 2002-1 - 2002-6
(Publication No. 84-100).
NIOSH (1984b) NIOSH manual of analytical methods, 3rd ed. Vol. 2.
Cincinnati, OH, US Department of Health and Human Services, National
Institute of Occupational Safety and Health, pp. 5518-1 - 5518-4
(Publication No. 84-100).
Nomura A (1977) Studies of sulfhemoglobin formation by various drugs.
Folia Pharmacologica Japonica, 73:793-802 (in Japanese, with English
summary).
NTP (1987) National Toxicology Program, fiscal year 1987, annual
plan. Research Triangle Park, NC, US Department of Health and Human
Services, National Institutes of Health, National Toxicology Program,
p. 87.
NTP (1988) NTP technical report on the toxicology and
carcinogenesis studies of N-phenyl-2-naphthylamine (CAS no.
135-88-6) in F344/N rats and B6C3F1 mice (feed studies). Research
Triangle Park, NC, US Department of Health and Human Services,
National Institutes of Health, National Toxicology Program, 4 pp. (NTP
TR 333).
Ozeki S, Tejima K (1979) Drug interactions. V. Binding of basic
compounds to bovine serum albumin by fluorescent probe technique.
Chemical and pharmaceutical bulletin, 27:638-646.
Rannug A, Rannug U, Ramel C (1984) Genotoxic effects of additives in
synthetic elastomers with special consideration to the mechanism of
action of thiurames and dithiocarbamates. In: Industrial hazards of
plastics and synthetic elastomers. New York, NY, Alan R. Liss Inc.,
pp. 407-419.
Rosenberg A (1983) Microbial metabolism of N-phenyl-1-naphthylamine
in soil, soil suspensions, and aquatic ecosystems. Chemosphere,
12:1517-1523.
Schär W (1930) Experimentelle Erzeugung von Blasentumoren. Die Wirkung
langdauernder Inhalation von aromatischen Aminoverbindungen.
Deutsche Zeitschrift für Chirurgie, 226:81-97.
Schultheiss E (1959) Gummi und Ekzem (3. Mitteilung), Kasuistik.
Berufsdermatosen, 5:76-96.
Sikka HC, Pack EJ, Sugatt RH, Banerjee S, Rosenberg A, Simpson BW
(1981) Environmental fate and effects of N -phenyl-1-naphthylamine
and its disposition and metabolism in the rat. Syracuse, NY,
Syracuse Research Co., 106 pp. (Report No. AFOSR-TR-81-0703).
Sofuni T, Matsuoka A, Sawada M, Ishidate M, Zeiger E, Shelby MD (1990)
A comparison of chromosome aberration induction by 25 compounds tested
by two hamster cell (CHL and CHO) systems in culture. Mutation
research, 241:175-213.
Te Lintum JCA, Nater JP (1979) Allergic contact dermatitis caused by
rubber chemicals in dairy workers. Dermatologica, 148:42-44.
Thomas RG (1990) Volatilization from water. In: Lyman WJ, Reehl WF,
Rosenblatt DH, eds. Handbook of chemical property estimation
methods. Environmental behavior of organic compounds. New York, NY,
McGraw-Hill Book Co., pp. 15-34.
Union Carbide (1996) Material safety data sheet from 2/22/96. Union
Carbide Corporation, South Charleston Plant (USA), 8 pp.
van Beek L (1977) Primary skin and eye irritation tests with the
compound WTR 10 in albino rabbits. Zeist, TNO Central Institute on
Nutrition and Food Research, 10 pp. (Report No. R 5468).
Vernot EH, MacEwen JD, Haun CC, Kinkead ER (1977) Acute toxicity and
skin corrosion data for some organic and inorganic compounds and
aqueous solutions. Toxicology and applied pharmacology, 42:417-423.
Wang H, Wang D, Dzeng R (1984) Carcinogenicity of
N-phenyl-1-naphthylamine and N-phenyl-2-naphthylamine in mice.
Cancer research, 44:3098-3100.
Xuanxian X, Wolff T (1992) Metabolism of N-phenyl-2-naphthylamine
and N-phenyl-1-naphthylamine by rat hepatic microsomes and
hepatocytes. Journal of environmental science, 4:74-83.
Zeiger E, Anderson B, Haworth S, Lawlor T, Mortelmans K (1988)
Salmonella mutagenicity tests: IV. Results from the testing of 300
chemicals. Environmental and molecular mutagenesis, 12:1-158.
APPENDIX 1 - SOURCE DOCUMENT
BUA-Stoffbericht N-phenyl-1-naphthylamin. Beratergremium fuer
Umweltrelevante Altstoffe (Report No. 113; April 1993). VCH
VerlagsGmbH, Weinheim
For the BUA review process, the company that is in charge of
writing the report (usually the largest producer in Germany) prepares
a draft report using literature from an extensive literature search as
well as internal company studies. This draft is subject to a peer
review during several readings of a working group consisting of
representatives from government agencies, the scientific community,
and industry.
The English translation of the BUA report (BUA Report
N-phenyl-1-naphthylamine. GDCh-Advisory Committee on Existing
Chemicals of Environmental Relevance. VCH VerlagsGmbH, Weinheim) was
released in 1994.
APPENDIX 2 - CICAD PEER REVIEW
The draft CICAD on N-phenyl-1-naphthylamine was sent for review
to institutions and organizations identified by IPCS after contact
with IPCS national Contact Points and Participating Institutions, as
well as to identified experts. Comments were received from:
Department of Health, London, United Kingdom
Health and Safety Executive, Bootle, United Kingdom
Health Canada, Ottawa, Canada
National Chemicals Inspectorate (KEMI), Solna, Sweden
National Institute for Working Life, Solna, Sweden
National Institute of Occupational Health, Budapest, Hungary
National Institute of Public Health, Oslo, Norway
National Institute of Public Health and Environmental Protection,
Bilthoven, The Netherlands
United States Department of Health and Human Services (National
Institute for Occupational Safety and Health, Cincinnati, USA;
National Institute of Environmental Health Sciences, Research
Triangle Park, USA)
APPENDIX 3 - CICAD FINAL REVIEW BOARD
Berlin, Germany, 26-28 November 1997
Members
Dr H. Ahlers, Education and Information Division, National Institute
for Occupational Safety and Health, Cincinnati, OH, USA
Mr R. Cary, Health Directorate, Health and Safety Executive, Bootle,
United Kingdom
Dr S. Dobson, Institute of Terrestrial Ecology, Huntingdon, United
Kingdom
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany (Chairperson)
Mr J.R. Hickman, Health Protection Branch, Health Canada, Ottawa,
Ontario, Canada
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Research, Hanover,
Germany
Ms M.E. Meek, Environmental Health Directorate, Health Canada, Ottawa,
Ontario, Canada (Rapporteur)
Dr K. Paksy, Department of Reproductive Toxicology, National Institute
of Occupational Health, Budapest, Hungary
Mr V. Quarg, Ministry for the Environment, Nature Conservation &
Nuclear Safety, Bonn, Germany
Mr D. Renshaw, Department of Health, London, United Kingdom
Dr J. Sekizawa, Division of Chemo-Bio Informatics, National Institute
of Health Sciences, Tokyo, Japan
Prof. S. Soliman, Department of Pesticide Chemistry, Alexandria
University, Alexandria, Egypt (Vice-Chairperson)
Dr M. Wallen, National Chemicals Inspectorate (KEMI), Solna, Sweden
Ms D. Willcocks, Chemical Assessment Division, Worksafe Australia,
Camperdown, Australia
Dr M. Williams-Johnson, Division of Toxicology, Agency for Toxic
Substances and Disease Registry, Atlanta, GA, USA
Dr K. Ziegler-Skylakakis, Senatskommission der Deutschen
Forschungsgemeinschaft zuer Pruefung gesundheitsschaedlicher
Arbeitsstoffe, GSF-Institut fuer Toxikologie, Neuherberg,
Oberschleissheim, Germany
Observers
Mrs B. Dinham,1 The Pesticide Trust, London, United Kingdom
Dr R. Ebert, KSU Ps-Toxicology, Huels AG, Marl, Germany (representing
ECETOC, the European Centre for Ecotoxicology and Toxicology of
Chemicals)
Mr R. Green,1 International Federation of Chemical, Energy, Mine and
General Workers' Unions, Brussels, Belgium
Dr B. Hansen,1 European Chemicals Bureau, European Commission, Ispra,
Italy
Dr J. Heuer, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany
Mr T. Jacob,1 DuPont, Washington, DC, USA
Ms L. Onyon, Environment Directorate, Organisation for Economic
Co-operation and Development, Paris, France
Dr H.J. Weideli, Ciba Speciality Chemicals Inc., Basel, Switzerland
(representing CEFIC, the European Chemical Industry Council)
Secretariat
Dr M. Baril, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Dr R.G. Liteplo, Health Canada, Ottawa, Ontario, Canada
Ms L. Regis, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Mr A. Strawson, Health and Safety Executive, London, United Kingdom
Dr P. Toft, Associate Director, International Programme on Chemical
Safety, World Health Organization, Geneva, Switzerland
1 Invited but unable to attend.
RÉSUMÉ D'ORIENTATION
Ce CICAD relatif à la N-phényl-1-naphtylamine est fondé
principalement sur une étude menée par l'Institut Fraunhofer de
Toxicologie et de Recherche sur les Aérosols de Hanovre (Allemagne)
pour le compte du Comité consultatif allemand sur les substances
chimiques importantes pour l'environnement (BUA, 1993). Cette étude
évalue les effets potentiels de la N-phényl-1-naphtylamine sur
l'environnement et la santé humaine. Le rapport du BUA s'appuie sur
les données disponibles jusqu'en 1992. Une recherche bibliographique
approfondie a été menée en 1997 dans plusieurs bases de données en
ligne pour retrouver toutes les références publiées postérieurement au
rapport du BUA. Les informations relatives à la préparation du
document initial et à son examen par les pairs figurent à
l'appendice 1. Les renseignements concernant l'examen du CICAD par
les pairs font l'objet de l'appendice 2. Ce CICAD a été approuvé en
tant qu'évaluation internationale lors d'une réunion du Comité
d'évaluation finale qui s'est tenue à Berlin (Allemagne) du 26 au
28 novembre 1997. La liste des participants à cette réunion figure à
l'appendice 3. La fiche d'information sur la sécurité chimique de la
N-phényl-1-naphtylamine (ICSC 1113), établie par le Programme
international sur la Sécurité chimique (IPCS, 1993), est également
reproduite dans le présent document.
La N-phényl-1-naphtylamine (CAS No 90-30-2) est un solide
cristallin lipophile utilisé comme antioxygène dans diverses huiles de
graissage et comme agent protecteur et antioxygène dans le caoutchouc
et les mélanges à base de caoutchouc servant à la fabrication de
différents produits, notamment les pneumatiques. De 1986 à 1990, la
capacité mondiale de production de la N-phényl-1-naphtylamine a été
estimée à 3000 tonnes par an. Une entreprise allemande est la seule à
produire cette substance dans l'Union européenne.
Compte tenu des propriétés physico-chimiques de la
N-phényl-1-naphtylamine, sa distribution dans l'environnement,
prédite sur la base d'un modèle de fugacité de niveau II est
approximativement la suivante : 36 % dans le sol, 34 % dans les
sédiments, 29 % dans l'eau et moins de 1 % dans l'air, les sédiments
en suspension et les biotes. Il n'existe pas de données quantitatives
sur les rejets de N-phényl-1-naphtylamine dans l'environnement lors
de sa production, de sa transformation et de son utilisation. Des
rejets indirects sont possibles dans le sol et les eaux de surface en
cas de fuites d'huiles de graissage ou par suite d'un relargage lors
de la dégradation des pneus et des articles en caoutchouc, mais on ne
dispose pas de données quantitatives à ce sujet. La
N-phényl-1-naphtylamine peut être libérée dans l'atmosphère avec les
gaz émis lors de sa production et de son traitement ou à l'occasion de
la vulcanisation du caoutchouc, mais là encore, on ne dispose d'aucune
donnée. La présence de N-phényl-1-naphtylamine dans les huiles de
graissage ne devrait pas contribuer à sa libération dans l'atmosphère,
car ces huiles sont utilisées en circuit fermé. Globalement, compte
tenu des capacités de production limitées et de l'application de
techniques de réduction des émissions, la quantité de
N-phényl-1-naphtylamine libérée dans l'environnement devrait être
faible.
Selon des études de laboratoire, la N-phényl-1-naphtylamine
subit une dégradation photochimique dans l'eau avec une demi-vie de
8,4 ou 5,7 minutes. La photolyse peut conduire à une dégradation
préliminaire dans des conditions favorables, mais une dégradation
ultérieure est peu probable. Dans l'environnement, la substance
résiste à l'hydrolyse et sa biodégradation dans l'eau et le sol se
fait lentement. En raison d'un potentiel de sorption modéré à élevé
sur les constituants organiques du sol et d'une minéralisation limitée
dans le sol, on peut supposer que la N-phényl-1-naphtylamine
présente un potentiel de géoaccumulation. La probabilité
d'infiltration dans les eaux souterraines est faible. Les résultats
d'études effectuées sur des daphnies et des poissons et un log Ko/w
de 4,2 laissent supposer que la N-phényl-1-naphtylamine a un
potentiel de bioaccumulation modéré. Néanmoins, une contamination
secondaire des niveaux trophiques supérieurs par la chaîne alimentaire
aquatique semble peu probable compte tenu du fait que la substance est
métabolisée et excrétée dans des proportions importantes. La
N-phényl-1-naphtylamine est très toxique pour les poissons et les
daphnies, avec des concentrations sans effet observé (NOEC) de
0,11 mg/litre (192 h) et 0,02 mg/litre (21 jours), respectivement. En
dépit d'une dégradation hydrolytique ou biologique limitée, les
mécanismes de sorption et de dégradation photochimique devraient
contribuer à réduire considérablement la biodisponibilité de la
substance dans l'eau.
Les données connues sur les niveaux de N-phényl-1-naphtylamine
dans l'environnement se limitent aux résultats d'études relativement
anciennes menées aux États-Unis d'Amérique, selon lesquelles la
substance a été détectée dans l'eau (2-7 µg/litre) et les sédiments
(1-5 mg/kg) des cours d'eau à proximité d'une petite usine de
fabrication de produits chimiques. Les données disponibles n'ont pas
permis d'évaluer l'exposition humaine ni de prédire les concentrations
à l'aide d'un modèle de fugacité.
Selon des études menées sur des animaux de laboratoire, la
N-phényl-1-naphtylamine est bien absorbée et facilement excrétée
après ingestion. Chez le rat, 60 % de la dose ingérée ont été
excrétés dans les fèces et 35 % dans l'urine au cours des 72 heures
suivantes. Plusieurs métabolites non identifiées ont été détectées
dans l'urine de rats exposés à la substance. D'après des études
in vitro, il semble que le principal mécanisme de métabolisation de
la N-phényl-1-naphtylamine soit l'hydroxylation.
La toxicité aiguë par voie orale de la N-phényl-1-naphtylamine
chez les animaux de laboratoire est faible. La substance a été
soumise à des épreuves normalisées chez le lapin, qui ont montré
qu'elle n'était irritante ni pour la peau ni pour l'oeil. Toutefois,
un test de maximalisation sur cobayes a révélé que la
N-phényl-1-naphtylamine était un sensibilisant de la peau, ce qui a
été confirmé par des épreuves pratiquées sur des personnes exposées à
des graisses ou à des caoutchoucs contenant cette substance.
Des données limitées montrent que les principaux organes cibles
après ingestion sont les reins et le foie. On n'a trouvé aucune étude
permettant de déterminer les concentrations suivies d'un effet
présumé. Le potentiel cancérogène de la N-phényl-1-naphtylamine n'a
pu être pleinement évalué car aucune des études disponibles n'a été
menée conformément aux normes actuellement reconnues.
La N-phényl-1-naphtylamine ne s'est pas révélée mutagène sur
des cellules bactériennes et il n'y a pas eu augmentation de la
fréquence des mutations géniques (analyse des mutations du lymphome de
la souris) ni des aberrations chromosomiques (analyse in vitro de la
métaphase sur des cellules d'ovaires ou de poumons de hamsters
chinois) dans ce type de cellules à la suite d'une exposition
in vitro. On a signalé un résultat faiblement positif dans une
épreuve d'échange de chromatides soeurs sur des cellules d'ovaires de
hamsters chinois avec activation métabolique. Il y a eu augmentation
de la synthèse non programmée d'ADN dans des cellules de poumon humain
exposé (WI-38); toutefois, cet effet n'a pas semblé clairement lié à
la concentration. Un test de létalité dominante a donné un résultat
négatif chez la souris. D'après les données disponibles, la
N-phényl-1-naphtylamine ne semble pas génotoxique. Aucune donnée
n'a été trouvée sur la toxicité pour la reproduction ou le
développement ni sur les effets immunologiques ou neurologiques de la
N-phényl-1-naphtylamine.
Une étude épidémiologique a révélé une augmentation du taux de
cancers chez des ouvriers exposés à la N-phényl-1-naphtylamine;
toutefois, cet effet n'a pu être attribué exclusivement à la
N-phényl-1-naphtylamine en raison du petit nombre de décès
supplémentaires et de l'exposition concomitante à d'autres substances.
Bien que les données disponibles soient insuffisantes pour
caractériser exactement les risques potentiels pour la santé, il
convient d'éviter le contact de la N-phényl-1-naphtylamine avec la
peau en raison de ses propriétés sensibilisantes.
RESUMEN DE ORIENTACION
Este CICAD relativo a la N-fenil-1-naftilamina se basa
fundamentalmente en un examen preparado por el Instituto Fraunhofer de
Toxicología y de Investigación sobre los Aerosoles de Hannover,
Alemania, para el Comité Consultivo Alemán sobre las Sustancias
Químicas Importantes para el Medio Ambiente (BUA, 1993; versión
inglesa: BUA, 1994). Este estudio evalúa los efectos potenciales de
la N-fenil-1-naftilamina en el medio ambiente y la salud humana. El
informe del BUA se basa en los datos disponibles hasta 1992. En 1997
se realizó una investigación bibliográfica amplia de varias bases de
datos en línea para encontrar todas las referencias publicadas con
posterioridad al informe del BUA. La información sobre la preparación
del documento original y su examen colegiado figura en el Apéndice 1.
La información acerca del examen colegiado de este CICAD se presenta
en el Apéndice 2. Este CICAD se aprobó como evaluación internacional
en una reunión de la Junta de Evaluación Final celebrada en Berlín
(Alemania) los días 26-28 de noviembre de 1997. La lista de
participantes en esta reunión figura en el Apéndice 3. La Ficha
internacional de seguridad química (ICSC 1113) para la
N-fenil-1-naftilamina, preparada por el Programa Internacional de
Seguridad de las Sustancias Químicas (IPCS, 1993), también se
reproduce en el presente documento.
La N-fenil-1-naftilamina (CAS Nº 90-30-2) es una sustancia
cristalina lipófila utilizada como antioxidante en diversos aceites
lubricantes y como agente protector y antioxidante en el caucho y las
mezclas a base de caucho que se emplean en la fabricación de
diferentes productos, en particular los neumáticos. De 1986 a 1990,
la capacidad estimada de producción mundial de N-fenil-1-naftilamina
fue de 3000 toneladas al año. Una empresa alemana es la única
productora de esta sustancia en la Unión Europea.
Teniendo en cuenta las propiedades fisicoquímicas de la
N-fenil-1-naftilamina, su distribución en el medio ambiente,
prevista tomando como base el modelo de fugacidad de nivel II, es la
siguiente: 36% en el suelo, 34% en los sedimentos, 29% en el agua y
menos del 1% en el aire, los sedimentos en suspensión y la biota. No
existen datos cuantitativos de la liberación de
N-fenil-1-naftilamina en el medio ambiente a partir de su
producción, elaboración y uso. Se pueden producir vertidos indirectos
en el suelo y en las aguas superficiales en caso de derrames de
aceites lubricantes o por lixiviación a partir de neumáticos o
productos de caucho en descomposición, pero no se dispone de datos
cuantitativos a este respecto. La N-fenil-1-naftilamina se puede
liberar en la atmósfera con los gases de escape durante su producción
y tratamiento y a partir de la vulcanización de las mezclas de caucho.
El uso de aceites lubricantes con N-fenil-1-naftilamina no debería
contribuir a su liberación en la atmósfera, puesto que estos aceites
se aplican en sistemas cerrados. En conjunto, teniendo en cuenta la
capacidad de producción limitada y la aplicación de técnicas de
reducción de las emisiones, la cantidad de N-fenil-1-naftilamina
liberada en el medio ambiente debería ser baja.
Según los estudios de laboratorio, la N-fenil-1-naftilamina
sufre una degradación fotoquímica en el agua con una semivida de 8,4 y
5,7 minutos. La fotolisis puede dar lugar a una degradación
preliminar en condiciones favorables, pero es poco probable que se
produzca una degradación ulterior. En el medio ambiente, la sustancia
resiste la hidrólisis, y la eliminación por biodegradación en el agua
y el suelo es lenta. Habida cuenta de su potencial de sorción entre
moderado y alto en los constituyentes orgánicos del suelo y de su
mineralización limitada en el suelo, se supone que la
N-fenil-1-naftilamina tiene un potencial de geoacumulación. La
probabilidad de infiltración en el agua fréatica es baja. Los
resultados de los estudios efectuados con Daphnia y con peces y el
log Ko/w de 4,2 obtenido hacen suponer que la
N-fenil-1-naftilamina tiene un potencial de bioacumulación moderado.
No obstante, la contaminación secundaria de los niveles tróficos
superiores a través de la cadena alimentaria acuática parece poco
probable, teniendo en cuenta que la sustancia se metaboliza y excreta
en proporciones importantes. La N-fenil-1-naftilamina es muy tóxica
para los peces y para Daphnia, siendo la concentración sin efectos
observados (NOEC) más que baja que se ha notificado de 0,11 mg/litro
(192 h) y de 0,02 mg/litro (21 días), respectivamente. A pesar de
producirse una degradación hidrolítica o biológica limitada, los
mecanismos de sorción y de degradación fotoquímica deberían contribuir
a reducir considerablemente la disponibilidad de la sustancia en el
agua.
Los datos conocidos sobre los niveles de N-fenil-1-naftilamina
en el medio ambiente se limitan a los resultados de estudios
relativamente antiguos realizados en los Estados Unidos, según los
cuales la sustancia se detectó en el agua (2-7 µg/litro) y en los
sedimentos (1-5 mg/kg) de un río cerca de una pequeña fábrica de
productos químicos. Los datos disponibles no permiten evaluar la
exposición humana ni predecir las concentraciones utilizando un modelo
de fugacidad.
Según los estudios realizados en animales de laboratorio, la
N-fenil-1-naftilamina se absorbe bien y se excreta en su mayor parte
tras la ingestión. En el caso de la rata, el 60% de la dosis
administrada se excretó con las heces y el 35% con la orina en un
plazo de 72 horas. En la orina de ratas expuestas a la sustancia se
han detectado varios metabolitos no identificados. Teniendo en cuenta
los estudios in vitro, parece que el mecanismo principal de
metabolización de la N-fenil-1-naftilamina es la hidroxilación.
La toxicidad aguda por vía oral de la N-fenil-1-naftilamina en
animales de laboratorio es baja. En pruebas normalizadas realizadas
con conejos, se puso de manifiesto que la sustancia no era irritante
de la piel ni de los ojos. Sin embargo, en una prueba de maximización
realizada con cobayas se observó que la N-fenil-1-naftilamina tenía
propiedades de sensibilizante cutáneo, y también en seres humanos
expuestos a grasas o a materiales de caucho que contenían esta
sustancia.
Hay datos limitados que indican que los principales órganos
destinatarios tras la ingestión son los riñones y el hígado. No se ha
encontrado ningún estudio adecuado que permita establecer posibles
niveles de efectos. No se ha podido evaluar completamente la posible
carcinogenicidad de la N-fenil-1-naftilamina, puesto que ninguno de
los estudios disponibles se realizó de acuerdo con los protocolos
normalizados aceptados actualmente.
La N-fenil-1-naftilamina no fue mutagénica en células
bacterianas, ni produjo un aumento de la frecuencia de mutaciones
génicas (análisis de las mutaciones del linfoma de ratón) ni de las
aberraciones cromosómicas (análisis in vitro de la metafase en
células de los ovarios y de los pulmones de hámster chino) en este
tipo de células tras la exposición in vitro. Se ha notificado un
resultado débilmente positivo en un ensayo de intercambio de
cromátidas hermanas en células de ovario de hámster chino con
activación metabólica. Se observó un aumento de síntesis no
programada de ADN en células de pulmón humano expuestas (WI-38); sin
embargo, estos efectos no parecían depender de la concentración. El
resultado de un ensayo de letalidad dominante realizado con
N-fenil-1-naftilamina en ratones fue negativo. Según los datos
disponibles, esta sustancia no parece ser genotóxica. No se han
encontrado datos acerca de la toxicidad reproductiva y en el
desarrollo y de los efectos inmunológicos o neurológicos de la
N-fenil-1-naftilamina.
En un estudio epidemiológico se observó un aumento de la
frecuencia de cáncer en los trabajadores expuestos a la
N-fenil-1-naftilamina; sin embargo, este efecto no se puede atribuir
exclusivamente a la N-fenil-1-naftilamina, debido al pequeño aumento
en el número de fallecimientos y a la exposición concomitante a otras
sustancias. Aunque los datos disponibles sean inadecuados para
caracterizar exactamente los riesgos potenciales para la salud,
conviene evitar el contacto cutáneo con la N-fenil-1-naftilamina,
debido a sus propiedades sensibilizantes.
The commercial product has a typical purity of >99%. Named
impurities from three manufacturers are 1-naphthylamine (<100-500
mg/kg), 2-naphthylamine (<3-50 mg/kg), aniline (<100-2500 mg/kg),
1-naphthol (<5000 mg/kg), 1,1-dinaphthylamine (<1000 mg/kg), and
N-phenyl-2-naphthylamine (500-<5000 mg/kg) (BUA, 1993; Union
Carbide, 1996).
3. ANALYTICAL METHODS
N-Phenyl-1-naphthylamine is quantified in environmental media
either by high-performance liquid chromatography in combination with
ultraviolet absorption or by gas chromatography combined with
thermionic or mass spectrometric detection, flame ionization
detection, or electron capture detection. A method for the
determination of secondary amines in air is suitable for the detection
of N-phenyl-1-naphthylamine.
The following enrichment techniques are used for various types of
samples: solid-phase adsorption (silica gel, silica gel/glass fibre)
with liquid extraction (ethanol, acetic acid/2-propanol) for samples
of air (NIOSH, 1984a,b); liquid/liquid extraction (acetonitrile,
diethyl ether), stripping with helium, and solid adsorption (Tenax GC)
or alkaline extraction (dichloromethane) for samples of water
(Jungclaus et al., 1978; Lopez-Avila & Hites, 1980; Sikka et al.,
1981; Rosenberg, 1983); liquid extraction (isopropanol) for sediment
(Jungclaus et al., 1978; Lopez-Avila & Hites, 1980); and liquid
extraction (methanol) for fish, tissue, and serum (Sikka et al.,
1981). Detection limits range from 0.1 to 1 µg/litre for water and
from 50 to 100 µg/kg for sediment; detection limits for biological
materials were not identified.
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
There are no known natural sources of N-phenyl-1-naphthylamine.
Other than the information derived from the national source document,
additional data on the production, use patterns, and release of this
chemical were not identified.
Within the European Union, one German company is the sole
producer of N-phenyl-1-naphthylamine. Between 1986 and 1990, the
estimated worldwide production capacity of N-phenyl-1-naphthylamine
was 3000 tonnes per year. Over the same period, estimated production
capacities in Western Europe, the People's Republic of China, the USA,
and Japan were approximately 1000-1500 tonnes per year, 1000 tonnes
per year, 500 tonnes per year, and 300 tonnes per year, respectively.
Between 1986 and 1990, the consumption of N-phenyl-1-naphthylamine
in Germany was estimated to be approximately 300-450 tonnes per year,
although its use is now declining. Approximately 50-100 tonnes were
covered by imports; exports amounted to approximately 750-1150 tonnes
per year (BUA, 1993).
N-Phenyl-1-naphthylamine is used as an antioxidant in gear,
hydraulic, lubrication, and bearing oils and as a protective agent and
antioxidant in rubbers and rubber mixtures. In Germany,
N-phenyl-1-naphthylamine consumption is divided equally among these
two uses. In these products, the chemical acts as a radical scavenger
in the auto-oxidation of polymers and mineral lubricants. Average
concentrations of N-phenyl-1-naphthylamine in the final products are
<1% w/w (BUA, 1993). In the rubber industry, approximately 75% of
the N-phenyl-1-naphthylamine is used in products such as drums,
buffers, conveyor belts, flexible tubes, gaskets, and footwear
components. The remaining 25% is used in tyres (sidewalls or carcass,
but not treads) (BUA, 1993).
In Germany, N-phenyl-1-naphthylamine-containing distillation
residues from production facilities (approximately 20 tonnes per
year), like most gear and hydraulic oil, are disposed of in
chemical/physical/biological treatment plants or hazardous waste
incinerators (BUA, 1993). About 30% of used tyres are disposed of in
landfill sites, approximately 37% are used for the production of
energy in the cement industry, an estimated 22% are recycled, and
approximately 11% are exported (BUA, 1993). Exhaust gases may be
emitted during the production and processing of
N-phenyl-1-naphthylamine and during the vulcanization of rubber
mixtures at elevated temperatures; in Germany, however, emission
reduction techniques are applied, and releases of
N-phenyl-1-naphthylamine are therefore presumed to be low (<25 kg
per year). Data on levels of N-phenyl-1-naphthylamine in exhaust
gases from vulcanization processes are not available. The use of
N-phenyl-1-naphthylamine-containing lubrication oils should not
result in the introduction of the substance into the atmosphere, as
the oils are applied in closed systems (BUA, 1993).
Data on releases of N-phenyl-1-naphthylamine in effluents from
production and processing facilities were not identified. Indirect
discharges from leaching or the decay of tyres and other rubber
products are to be expected in the long term; however, estimation of
amounts was not possible (BUA, 1993). The release of
N-phenyl-1-naphthylamine into the geosphere from the leakage of
lubrication oils and discarded rubber products and tyres in landfill
sites may occur; however, estimation of the amounts was not possible
with the available data (BUA, 1993). Information on the occurrence of
N-phenyl-1-naphthylamine in plants or animals was not identified.
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Based on its physical/chemical properties, the distribution of
N-phenyl-1-naphthylamine in the environment was predicted, using a
Level II fugacity model (Mackay, 1991), to be 36.3% to soil, 33.9% to
sediment, 28.9% to water, 0.8% to air, 0.06% to suspended sediment,
and 0.02% to biota. Input data for the Level II fugacity model were
as follows: temperature, 298°K; atmospheric volume, 6 109 m3; soil
density, 1.5 g/cm3; density of biota, 1 g/cm3; carbon content of
soil, 2%; carbon content of sediment, 4%; water depth, 1000 cm; water
portion, 70%; soil depth, 15 cm; sediment depth, 3 cm; suspended
sediment portion, 5 ppm; biota portion, 1 ppm; molar mass, 219 g/mol;
water solubility, 3 mg/litre; vapour pressure, 1.06 mPa;
n-octanol/water partition coefficient, 15 850; soil sorption
coefficient, 6510; bioaccumulation factor, 760; half-lives (days) in
air (0), water (365), soil (29.2), sediment (29.2), suspended sediment
(29.2), and biota (0). Owing to the lack of relevant data, it was not
possible to predict the concentrations of N-phenyl-1-naphthylamine
in various media using a Level III fugacity model. Based on its
calculated Henry's law constant (7.748 10-2 Pa.m3/mol; 20°C) and
other information (Thomas, 1990), the volatility of
N-phenyl-1-naphthylamine from aqueous solution is expected to be
low.
Based on its ultraviolet absorption spectrum, direct
photochemical degradation of N-phenyl-1-naphthylamine in air is
expected (BUA, 1993). Data concerning the photo-oxidative degradation
of N-phenyl-1-naphthylamine in air are not available. Measured
half-lives for the photochemical degradation of the chemical in water
have been reported at 8.4 and 5.7 min (Sikka et al., 1981). In this
experiment, sealed tubes containing aqueous solutions of
N-phenyl-1-naphthylamine at approximately 1 mg/litre (water
unspecified but assumed to be distilled) were exposed to sunlight.
The experiment was conducted in May and repeated in June in Syracuse,
NY (USA); no information was provided on light intensity. A further
experiment using a lamp at 300 nm (Rayonette Model RNR-400 mini
photochemical reactor; no information provided on intensity)
demonstrated that the photodegradation product was produced rapidly
and was itself photostable. Given the lack of detail in the report,
the importance of photodegradation in the environment is difficult to
assess. There is also insufficient information to allow the
photodegradation product to be fully characterized, but the authors
suggest that it incorporates the basic phenylnaphthylamine skeleton.
It can therefore be concluded that photolysis may lead to preliminary
breakdown of N-phenyl-1-naphthylamine under favourable environmental
conditions, but that further degradation is unlikely. From
experiments conducted in aqueous solution, hydrolysis of
N-phenyl-1-naphthylamine under environmental conditions is expected
to be of limited importance (Sikka et al., 1981).
Two standard tests on biodegradation performed according to
guideline 301C of the Organisation for Economic Co-operation and
Development (OECD) (modified MITI-I test) reported no degradation of
N-phenyl-1-naphthylamine (100 mg/litre initial concentration) within
14 and 28 days, using non-adapted activated sludge (Bayer AG, 1990;
CITI, 1992). In tests with conditions favouring biodegradation,
N-phenyl-1-naphthylamine was degraded with a half-life ranging from
4 to 11 days (inocula: domestic sewage and lake water, respectively).
Additional substrates accelerated degradation (Sikka et al., 1981;
Rosenberg, 1983). Laboratory results indicate that
N-phenyl-1-naphthylamine is inherently biodegradable in the aquatic
compartment.
Mineralization of N-phenyl-1-naphthylamine (measured by the
evolution of [14C]carbon dioxide) was 17% in soil and 35% in a soil
suspension in buffered salt solution. In contrast to the aquatic
studies, the addition of degradable substrates reduced rather than
accelerated degradation. It was suggested that the organic materials
increased sorption of the N-phenyl-1-naphthylamine. The reported
lower degradation in soil may therefore reflect reduced
bioavailability of the N-phenyl-1-naphthylamine (Rosenberg, 1983).
Measured soil sorption coefficients (Koc) are not available. Using
the regression equations of Kenaga (1980) and Kenaga & Goring (1980),
Koc values of 2400 and 4600, respectively, were calculated for
N-phenyl-1-naphthylamine. Thus, soil sorption is predicted to be
moderate to high. From this expected sorption to organic soil
constituents and its limited mineralization in soil,
N-phenyl-1-naphthylamine is presumed to have geoaccumulation
potential. The probability of infiltration into groundwater is low
(BUA, 1993).
Considering its measured log Kow of 4.2 (Ozeki & Tejima, 1979)
and data from laboratory tests with Daphnia and freshwater fish,
N-phenyl-1-naphthylamine is classified as a substance with moderate
bioaccumulation potential (Sikka et al., 1981; CITI, 1992). For
Daphnia magna, a mean bioconcentration factor (related to
radioactivity) of 637 was calculated following exposure to
[14C] N-phenyl-1-naphthylamine in a static test (solubilizer:
acetone; steady state after 12 h). About 50% of the accumulated
radioactivity had been eliminated after 53 h in clean water (Sikka et
al., 1981). Bioconcentration factors ranging from 432 to 1285
(related to radioactivity) and from 233 to 694 (related to
N-phenyl-1-naphthylamine) were determined in a flow-through system
(sublethal N-phenyl-1-naphthylamine concentration) for the bluegill
sunfish (Lepomis macrochirus) at steady state. Depuration was
biphasic, with an elimination of [14C] N-phenyl-1-naphthylamine of
>90% after 8 days; radioactivity could still be detected 32 days
after treatment (Sikka et al., 1981). Bioconcentration factors for
N-phenyl-1-naphthylamine in common carp (Cyprinus carpio),
measured in a flow-through system after 8 weeks, were on the same
order of magnitude (427-2730) (CITI, 1992).
N-Phenyl-1-naphthylamine is metabolized by terrestrial and aquatic
microorganisms and by fish to at least two or three unidentified
metabolites (Sikka et al., 1981; Rosenberg, 1983).
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
In older studies from the USA, N-phenyl-1-naphthylamine was
detected in river water (2-7 µg/litre) and sediment (1-5 mg/kg) near a
small speciality chemicals manufacturing plant (Jungclaus et al.,
1978; Lopez-Avila & Hites, 1980). Additional data on levels of
N-phenyl-1-naphthylamine in environmental media were not identified.
Based upon the use patterns of N-phenyl-1-naphthylamine, the
presence of this substance in soil and sediment in some
source-dominated areas appears possible; however, quantitative data
are not available.
6.2 Human exposure
Owing to its low vapour pressure and use patterns, the ingestion
or inhalation of N-phenyl-1-naphthylamine is expected to be minor.
Dermal contact with oils and rubber articles containing
N-phenyl-1-naphthylamine may occur in the workplace. Data on
occupational exposure were not available from industries in Germany
involved in the manufacture or use of N-phenyl-1-naphthylamine.1
Dermal contact may also be a source of exposure for the general
population, although this should be of minor importance because of the
small quantities of N-phenyl-1-naphthylamine produced and present in
various products. Data on concentrations of
N-phenyl-1-naphthylamine in media relevant to assessing exposure of
the general population were not identified. Moreover, available data
were insufficient to allow an estimation of human exposure based upon
concentrations predicted from fugacity modelling.
1 Personal communications concerning 1) BUA report on
N-phenyl-1-naphthylamine, Heidelberg, Berufsgenossenschaft der
chemischen Industrie (BG Chemie), 27 August 1992; and 2) exposure data
for N-phenyl-1-naphthylamine, Bundesanstalt für Arbeitsschutz,
Dortmund, 1992.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND
HUMANS
Studies providing quantitative information on the absorption or
distribution of N-phenyl-1-naphthylamine in humans were not
identified. From the limited information available from studies
conducted with laboratory animals, it can be concluded that
N-phenyl-1-naphthylamine is well absorbed after ingestion and is
readily excreted. In vitro studies have demonstrated that the
metabolism of N-phenyl-1-naphthylamine occurs primarily via
hydroxylation.
In male Sprague-Dawley rats administered a single oral dose of
160 mg [14C] N-phenyl-1-naphthylamine/kg body weight, the chemical
was well absorbed, metabolized almost completely, and excreted
primarily in the faeces. Radioactivity was detected in plasma within
60 min, with the maximum concentration measured after 4 h. After
24 h, 20% of the radioactivity was found in the gastrointestinal tract
(including contents), 2.4% in fatty tissue, 0.4% in the liver, and
0.1% in the kidneys. Ninety per cent of the administered
radioactivity was excreted within 48 h; 95% was excreted within 72 h
(60% in the faeces and 35% in the urine). In the ether extract of the
urine, at least five radioactive metabolites were detected but not
identified. The elimination half-lives were reported as 1.68 h for
the fast elimination and 33 h for the slow elimination (Sikka et al.,
1981).
In a study in which male rats were administered
N-phenyl-1-naphthylamine orally, only small quantities of unchanged
N-phenyl-1-naphthylamine were excreted in the faeces and urine (0.4
and 0.01% of the applied dose, respectively). Large amounts of
glucuronide and sulfate conjugates, which were not identified further,
were detected in the urine. Small quantities of
N-phenyl-1-naphthylamine were distributed in fatty tissue after
single or multiple (6 day) oral administration, whereas the
distribution of unchanged N-phenyl-1-naphthylamine in liver,
kidneys, spleen, heart, and lung was extremely low (Miyazaki et al.,
1987).
Mono- and dihydroxy-derivatives of N-phenyl-1-naphthylamine
have been identified in in vitro metabolic studies conducted with
rat liver microsomes (Sikka et al., 1981; Xuanxian & Wolff, 1992).
Sikka et al. (1981) suggested that the hydroxyl group in the
mono-hydroxy derivative is in the naphthalene moiety at a
para-position to the amino group, whereas at least one hydroxyl group
in the dihydroxy-derivative is at the available para-position in the
naphthyl ring. Pretreatment of male rats with phenobarbital or
3-methylcholanthrene increased the rate of microsomal metabolism,
indicating that more than one P-450 enzyme is involved in the
metabolism of N-phenyl-1-naphthylamine (Xuanxian & Wolff, 1992).
In studies conducted with human volunteers or laboratory animals,
the isomer N-phenyl-2-naphthylamine (CAS no. 135-88-6) was partially
metabolized to the known human carcinogen 2-naphthylamine following
ingestion or inhalation (NIOSH, 1976). Although data concerning the
formation of this metabolite are not available for
N-phenyl-1-naphthylamine, it should be noted that, based on its
chemical structure, it is unlikely that N-phenyl-1-naphthylamine is
metabolized to 2-naphthylamine.
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
In most of the toxicity studies, information on the purity of
N-phenyl-1-naphthylamine was not provided. As discussed in
section 2, N-phenyl-1-naphthylamine with a typical purity of >99%
contains numerous contaminants, and therefore the observed effects may
not be solely attributable to N-phenyl-1-naphthylamine. Owing to
the limited available toxicity data on N-phenyl-1-naphthylamine,
information on the isomer N-phenyl-2-naphthylamine (a known
contaminant of commercially available N-phenyl-1-naphthylamine) has
been included to aid in the identification of potential target organs.
There is limited evidence to suggest that the kidneys and liver are
the main target organs following ingestion of
N-phenyl-1-naphthylamine; this has also been demonstrated for the
isomer N-phenyl-2-naphthylamine.
8.1 Single exposure
The acute oral toxicity of N-phenyl-1-naphthylamine is low.
Studies performed according to standard protocols yielded LD50s in
male and female Wistar rats of >5000 mg/kg body weight (Bayer AG,
1978a,b). LD50s for male CFE rats and male CF-1 mice are
>1625 mg/kg body weight (MacEwen & Vernot, 1974; Vernot et al.,
1977) and 1231 mg/kg body weight (MacEwen & Vernot, 1974),
respectively. No specific signs of toxicity were reported.
Slight fatty degeneration in the liver of rabbits was observed 3
months after a single subcutaneous injection of 200 mg
N-phenyl-1-naphthylamine/kg body weight (Bayer AG, 1931). Like
other aromatic amines, N-phenyl-1-naphthylamine induces the
formation of methaemoglobin. In mice, a slightly increased
methaemoglobin level (4.1% versus 0.4% in controls) was noted within
10 min of a single intraperitoneal administration; the increase was
still detectable up to 24 h later (Nomura, 1977). Mice are less
sensitive than humans to methaemoglobin induction, and this small
increase in methaemoglobin level may be of importance to human health.
Data on effects related to acute exposure to
N-phenyl-1-naphthylamine via the inhalation route were not
identified.
8.2 Irritation and sensitization
Three studies assessed skin irritation by
N-phenyl-1-naphthylamine using the Draize method in rabbits. In one
study, no effects were observed within 72 h of application (no further
information was reported) (MacEwen & Vernot, 1974). In a study
performed according to US Food and Drug Administration (FDA)
standards, N-phenyl-1-naphthylamine was classified as a very slight
skin irritant (3/6 animals with intact skin and 2/6 animals with
abraded skin showed a slight positive reaction) (van Beek, 1977). In
a test conducted according to OECD guideline 404,
N-phenyl-1-naphthylamine was not considered to be a skin irritant.
Slight erythema and oedema reactions in 1/3 rabbits were observed 1 h
after removal of the test substance, whereas no effects were noted
after 24 or 72 h (Ciba-Geigy Corp., 1987b). Studies conducted
according to US FDA standards or OECD guideline 405 did not consider
N-phenyl-1-naphthylamine to be an eye irritant. The observed
effects in some animals (slight conjunctivitis or swelling of the
eyelid) were reversible within a maximum of 10 days (van Beek, 1977;
Ciba-Geigy Corp., 1987a).
In a guinea-pig maximization test (Magnusson & Kligman, 1970) and
in a test performed according to OECD guideline 406,
N-phenyl-1-naphthylamine was shown to be a strong sensitizer
(positive reaction in 15/20 and 18/20 animals, respectively) (Boman et
al., 1980; Ciba-Geigy Corp., 1987c). In the modified Landsteiner's
guinea-pig sensitization test (no further information available),
which is not a standard method, N-phenyl-1-naphthylamine did not
exhibit sensitizing potential (MacEwen & Vernot, 1974).
8.3 Short-term exposure
In five female Sprague-Dawley rats (two untreated controls), no
adverse clinical signs or effects on body weight gain were noted after
daily oral (gavage) administration of 2000 mg
N-phenyl-1-naphthylamine/kg body weight, 5 days per week for 2
weeks. Data concerning the purity of the test substance or the
formulation administered were not provided. Gross morphological
observations (no histopathological examination) made at necropsy
revealed no evidence of exposure-related effects (Mobil Oil Corp.,
1989).
Older studies (Bayer AG, 1931) performed with small numbers of
rabbits, although inadequate to serve as a basis for the determination
of putative effect levels, may provide some useful information on
toxicity and target organs. The oral administration of 200 mg
N-phenyl-1-naphthylamine/kg body weight per day, 5 days per week for
6 weeks, resulted in diarrhoea, proteinuria, slight irritation of the
kidneys, and a fatty degeneration of the liver. After the
subcutaneous administration (42 times in 7 weeks) of 50 or 200 mg
N-phenyl-1-naphthylamine/kg body weight per day, a fatty
degeneration of the liver and single proliferation of connective
tissue were noted 3 months after the cessation of exposure. Dermal
application of a 5% solution of N-phenyl-1-naphthylamine to the ear
(28 times within 5 weeks) produced slight skin erythema, proteinuria,
and anorexia. Death occurred 5 days after the 27th application, and
necropsy revealed fatty degeneration of the liver (Bayer AG, 1931).
In mice (sex and number not specified), the intraperitoneal
administration of 219 mg N-phenyl-1-naphthylamine/kg body weight for
3 days resulted in increased methaemoglobin levels (1.6% versus 0.4%
in controls) 48 h after treatment; methaemoglobin concentrations were
not elevated after intraperitoneal administration of 109 mg/kg body
weight for 9 days (Nomura, 1977).
8.4 Long-term exposure
8.4.1 Subchronic exposure
There are no studies available concerning subchronic exposure to
N-phenyl-1-naphthylamine. In an oral 13-week study with the isomer
N-phenyl-2-naphthylamine (approximately 98% pure, containing <1 mg
2-naphthylamine/kg), relative liver weight in F344/N rats and B6C3F1
mice increased in a dose-dependent fashion. A chemical-related
nephropathy was observed in rats, characterized by renal tubular
epithelial degeneration and hyperplasia (NTP, 1988).
8.4.2 Chronic exposure and carcinogenicity
Long-term toxicity or carcinogenicity studies performed according
to currently accepted standard protocols using physiologically
relevant routes of exposure were not identified. The inhalation
exposure of four rabbits (number of controls not provided, approximate
dose 100 mg/day) for several months (no additional information
provided) resulted in progressive anaemia, leucopenia, lymphocytosis,
pneumonia, nephritis, nephrosis, formation of lung abscesses, fatty
degeneration of the liver after 3-5 months, and death within 6-24
months (Schär, 1930). This study is characterized by the limited
number of animals, methodological deficiencies, and insufficient
documentation of results.
Bladder tumours were not observed in a long-term study in which
three dogs were orally administered 290 mg N-phenyl-1-naphthylamine
5 days per week for up to 3.5 years (DuPont, 1945; Gehrmann et al.,
1948; Haskell Laboratory, 1971). Owing to the limited number of
animals and the examination for tumours only in the bladder, this
study is inadequate for evaluation of the carcinogenic potential of
N-phenyl-1-naphthylamine following oral administration.
Wang et al. (1984) observed an increased incidence of malignant
tumours in male ICR and TA-1 mice following repeated subcutaneous
administration of N-phenyl-1-naphthylamine (technical grade or pure;
no additional data provided). In ICR mice, there was a statistically
significant increase (p < 0.05) in the incidence of lung carcinoma
(5/30 versus 0/24 in controls administered vehicle alone) after the
administration of 16 mg technical-grade N-phenyl-1-naphthylamine per
animal 27 times over 9 weeks (total dose 432 mg per animal). The
incidence of kidney haemangiosarcomas was 1/30 and 0/24 in the exposed
and control groups, respectively; however, the combined incidence of
liver, kidney, and lung haemangiosarcomas was significantly (p <
0.05) increased in the exposed animals (5/30 versus 0/24 in unexposed
controls). The same dosing regimen using purified
N-phenyl-1-naphthylamine produced a significant (p < 0.05)
increase in the incidence of kidney haemangiosarcomas (4/23 versus
0/24 in controls); the numbers of animals with lung carcinoma were
3/23 and 0/24 in the exposed and control groups, respectively. The
administration of 5.3 mg purified N-phenyl-1-naphthylamine per
animal, 27 times over 9 weeks (total dose 143 mg per animal), produced
a significant (p < 0.05) increase in the number of animals with
lung carcinomas (6/25 versus 0/24 in controls). The incidence of
kidney haemangiosarcomas was not elevated (1/25 and 0/24 in the
exposed and control groups, respectively); however, there was a
significant increase (p < 0.05) in the combined incidence of kidney
and lung haemangiosarcomas (4/25 versus 0/24 in the controls). All
animals in the study were sacrificed after 10 months.
In TA-1 mice administered a total dose of 328 mg technical-grade
N-phenyl-1-naphthylamine per animal subcutaneously over a period of
12 weeks (48 mg peranimal over 3 weeks, followed by 280 mg per animal
over 9 weeks), there was a significant increase (p < 0.05) in the
incidence of kidney haemangiosarcomas (7/19 compared with 0/18 in
unexposed controls). The incidence of renal tumours was also
significantly (p < 0.05) elevated in unilaterally nephrectomized
TA-1 mice (one kidney was removed 1 week prior to treatment)
subcutaneously administered a total dose of 328 mg of either purified
or technical-grade N-phenyl-1-naphthylamine per animal over a period
of 12 weeks (48 mg per animal over 3 weeks, followed by 280 mg per
animal over 9 weeks). The incidence of kidney haemangiosarcomas in
controls and the unilaterally nephrectomized animals administered
either the pure or technical-grade material was 0/18, 12/16, and
13/13, respectively (Wang et al., 1984). Evaluation of this report is
difficult owing to the number of individual experiments. These
studies are characterized by the use of small numbers of animals of a
single sex, limited dose groups, the absence of data on mortality and
morbidity, use of a non-physiologically relevant route of exposure,
and insufficient characterization of the substance tested.
N-Phenyl-2-naphthylamine, which had effects comparable to those
of N-phenyl-1-naphthylamine in the above-mentioned study by Wang et
al. (1984), has been tested in a 2-year carcinogenicity bioassay (NTP,
1988). There was no evidence of carcinogenic activity in male or
female F344/N rats administered diets containing 2500 or 5000 ppm
(mg/kg) N-phenyl-2-naphthylamine (estimated daily intakes of 100 and
225 mg/kg body weight for males and 120 and 260 mg/kg body weight for
females, respectively). The lack of carcinogenicity in rats may be
related to an inability to metabolize N-phenyl-2-naphthylamine to
the known animal and human carcinogen 2-naphthylamine (NTP, 1988).
There was no evidence of carcinogenic activity in male B6C3F1 mice
administered diets containing 2500 or 5000 ppm (mg/kg)
N-phenyl-2-naphthylamine (estimated daily intakes of 500 or
1000 mg/kg body weight, respectively). However, in female mice
receiving these diets (estimated daily intakes of 450 or 900 mg/kg
body weight, respectively), there was equivocal evidence of
carcinogenic activity, based upon the occurrence of rare kidney
neoplasms in two high-dose animals (one tubular cell adenoma and one
tubular cell adenocarcinoma). For non-neoplastic effects, the kidney
was the principal target organ. Mineralization, necrosis of the renal
papilla, epithelial hyperplasia, calculi of the kidney pelvis,
hydronephrosis, atrophy, fibrosis, and chronic focal inflammation of
the kidney were observed in the high-dose female rats. In male rats
of both dose groups and in the high-dose female rats, cysts and acute
suppurative inflammation of the kidney were also noted. Nuclear
enlargement of renal tubular epithelial cells and nephropathy were
observed in the high-dose female mice (NTP, 1988).
In a dermal carcinogenicity study, approximately 0.75 mg
N-phenyl-1-naphthylamine/kg body weight (dissolved in 50 µl toluene)
was applied to the skin of 50 male C3H mice twice per week for 80
weeks. Data concerning the purity of the test substance or the
formulation applied were not provided. No adverse effects on survival
or increased incidence of skin tumours were observed; however,
pigmentation, fibrosis, scar formation, acanthosis, and hyperkeratosis
were noted. Histopathological examinations of organs other than the
skin were not performed (Mobil Oil Corp., 1985).
8.5 Genotoxicity and related end-points
The results of experiments on the genotoxicity of
N-phenyl-1-naphthylamine are summarized in Table 1.
N-Phenyl-1-naphthylamine was not mutagenic in bacterial tests
conducted in the presence or absence of metabolic activation. In
mammalian cells, neither gene mutations (mouse lymphoma assay) nor
chromosomal aberrations (in vitro metaphase analysis in Chinese
hamster ovary cells or Chinese hamster lung cells) were induced by
N-phenyl-1-naphthylamine. A sister chromatid exchange assay in
Chinese hamster ovary cells was marginally positive in the presence of
metabolic activation. An unscheduled DNA synthesis assay with human
lung (WI-38) cells yielded positive results, although the effects were
not clearly concentration dependent. A number of non-validated
short-term tests yielded conflicting results on the transforming
potential of N-phenyl-1-naphthylamine (BUA, 1993). Based upon the
weight of evidence from in vitro studies, N-phenyl-1-naphthylamine
does not appear to be genotoxic.
No in vivo somatic cell mutation tests were identified. In a
dominant lethal test, 10 male ICR mice were intraperitoneally
administered 0, 50, 166, or 500 mg N-phenyl-1-naphthylamine/kg body
weight per day for 5 consecutive days followed by 2 days without
exposure. Each male was then caged with two virgin females 5 days per
week, and the sequence was repeated weekly with two new females each
week for 8 weeks. Examination of females 14 days from the mid-week in
which they were caged with the males yielded negative results (Brusick
& Matheson, 1976, 1977).
8.6 Reproductive and developmental toxicity
Data on the reproductive and developmental toxicity of
N-phenyl-1-naphthylamine were not identified.
8.7 Immunological and neurological effects
Data on immunological and neurological effects of
N-phenyl-1-naphthylamine in laboratory animals were not identified.
Table 1: In vitro genotoxicity studies on N-phenyl-1-naphthylamine.
Resultsa (with /
without metabolic
Cell type (end-point) Test concentration activation) Remarks References
Salmonella typhimurium TA98, 0.5-500 µl/plate with and - / - Brusick & Matheson,
TA100, TA1535, TA1537, without metabolic activation 1976, 1977
TA1538; Escherichia coli
WP2uvrA-
(gene mutation)
S. typhimurium TA98, TA100, 0.01-1000 µg/plate with and - / - Baden et al., 1978
TA1535, TA1537; E. coli WP2 without metabolic activation
(gene mutation)
S. typhimurium TA97, TA98, 0.3-666 µg/plate with and - / - Zeiger et al., 1988
TA100, TA1535, TA1537 without metabolic activation
(gene mutation)
S. typhimurium TA98, TA100, 0.2-1000 µg/plate with and - / - JETOC, 1996
TA1537, TA1538 without metabolic activation
(gene mutation)
E. coli WP2uvrA 20-5000 µg/plate with and - / - JETOC, 1996
(gene mutation) without metabolic activation
S. typhimurium TA98, TA100, Not provided - / - Rannug et al., 1984
TA1535, TA1537, TA1538
(gene mutation)
Saccharomyces cerevisiae D4 0.5-500 µl/plate with and - / - Brusick & Matheson,
(gene mutation) without metabolic activation 1976, 1977
Table 1 (continued)
Resultsa (with /
without metabolic
Cell type (end-point) Test concentration activation) Remarks References
Mouse lymphoma (L5178Y) 0.005-0.1 µg/ml with - / - Brusick & Matheson,
cells metabolic activation 1976, 1977
(gene mutation) 0.5-25 µg/ml without
metabolic activation
Human lung (WI-38) cells 5, 10, or 50 µg/ml with - / (+) Weak positive response Brusick & Matheson,
(DNA repair [unscheduled metabolic activation at 50 µg/ml and toxic at 1976, 1977
DNA synthesis]) 10, 50, or 100 µg/ml without 100 µg/ml without
metabolic activation metabolic activation;
effects not clearly
concentration related
Human lung (WI-38) cells 5, 10, or 50 µg/ml with and (+) / (+) Positive response at 10 Brusick & Matheson,
(DNA repair [unscheduled without metabolic activation µg/ml with metabolic 1976, 1977
DNA synthesis] ) activation; positive
response at 5 and 50
µg/ml without metabolic
activation; effects not
clearly concentration
related
Chinese hamster ovary cells 0.6-19.9 µg/ml with (+) / - Marginally positive with NTP, 1987; Loveday
(sister chromatid exchange) metabolic activation metabolic activation et al., 1990
1.8-18.2 µg/ml without metabolic activation
Chinese hamster ovary cells 1.49-19.9 µg/ml with - / - NTP, 1987; Loveday
(chromosomal aberrations) metabolic activation et al., 1990
2.99-29.9 µg/ml without
metabolic activation
Table 1 (continued)
Resultsa (with /
without metabolic
Cell type (end-point) Test concentration activation) Remarks References
Chinese hamster lung cells 15.6 µg/ml with - / - Sofuni et al., 1990
(chromosomal aberrations) metabolic activation
30 µg/ml without metabolic
activation
a - = negative result; (+) = weak positive result.
9. EFFECTS ON HUMANS
9.1 Case reports
N-Phenyl-1-naphthylamine, mixed with oil (Bayer AG, 1931) or
water (Haskell Laboratory, 1971), was not irritating when applied to
the skin of volunteers (no data on concentrations available). Skin
eczema in workers has been attributed to repeated exposure to high
levels of N-phenyl-1-naphthylamine, possibly in combination with
other substances. Reportedly, the content of
N-phenyl-1-naphthylamine in a special antirust oil had to be lowered
from 2% to 0.5% because of skin problems. Workers, who did not wear
gloves, were exposed during the packaging of bearing rings covered
with antirust-oil containing N-phenyl-1-naphthylamine (Järvholm &
Lavenius, 1981).
N-Phenyl-1-naphthylamine was also reported to have sensitizing
properties in humans. Case-studies on patients with contact
dermatitis, potentially associated with occupational exposure to
N-phenyl-1-naphthylamine in greases or oils, have been identified.
The majority of these patients also had a positive reaction to other
substances in the test series, such as mercaptobenzothiazole or
p-phenylenediamine. Lower incidences were reported in patients with
past exposure to rubber materials (Blank & Miller, 1952; Schultheiss,
1959; Nater, 1975; Te Lintum & Nater, 1979; Boman et al., 1980;
Järvholm & Lavenius, 1981; Kantoh et al., 1985; Kalimo et al., 1989;
Carmichael & Foulds, 1990). Because of the chemical's incorporation
into the polymer matrix, exposure to N-phenyl-1-naphthylamine in
rubber materials is assumed to be lower than exposure from greases or
oils.
9.2 Epidemiological studies
An increased occurrence of cancers in a small packaging unit in a
Swedish engineering company was reported in a cohort study (Järvholm &
Lavenius, 1981). Between 1954 and 1957, a special anticorrosive oil,
which contained 0.5% N-phenyl-1-naphthylamine in addition to other
chemicals, had been used in this unit. In 12 of 78 women in this unit
(group A: 78 women/20 men), cancers were diagnosed between 1964 and
1973 in several organs (predominantly the uterus and ovary). The
staff performing the actual packaging, and thus in contact with the
oil, were mainly women. Morbidity and mortality from cancer were 3.1-
and 3.5-fold higher, respectively, than expected, based upon
age-specific and sex-specific data from the Swedish Cancer Register
(the standard cancer rates for the period 1974-1976 were estimated on
the basis of the 1973 rate). In the males of group A, no significant
differences were established. In another unit (reference group B: 25
women/8 men) where anticorrosive oil without
N-phenyl-1-naphthylamine had been used, morbidity and mortality from
cancer were not elevated. This was also true for reference group C (8
women/23 men), from units that had been in contact with
N-phenyl-1-naphthylamine-containing anticorrosive oil for a short
period of time only, because they had demonstrated allergic reactions.
The authors concluded that, apart from exposure to
N-phenyl-1-naphthylamine, the formation of
N-nitroso- N-phenyl-1-naphthylamine from sodium nitrite originating
from the packaging paper used may be a possible explanation for the
increased frequency of cancer in group A.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10.1 Aquatic environment
Valid results from laboratory tests with ciliates, Daphnia, and
fish indicate that N-phenyl-1-naphthylamine is highly toxic to
aquatic species. An EC50 (48 h) of 2 mg
N-phenyl-1-naphthylamine/litre (nominal concentration; static;
solubilizer: acetone) was measured for the inhibition of cell
proliferation of freshwater ciliates (Tetrahymena pyriformis)
(Epstein et al., 1967). Forty-eight-hour LC50s from static and
semi-static acute toxicity tests with young and adult Daphnia magna
were in the range of 0.30-0.68 mg N-phenyl-1-naphthylamine/litre
(nominal concentration; solubilizer: ethanol). The lowest reported
21-day LC50 from long-term semi-static tests was 0.06 mg/litre; the
lowest reported NOEC was 0.02 mg/litre (nominal concentration;
solubilizer: ethanol) (Sikka et al., 1981).
Acute toxicity tests in semi-static (daily renewal of test
medium) and flow-through systems yielded 96-h LC50s in the range of
0.44-0.74 mg N-phenyl-1-naphthylamine/litre for rainbow trout
(Oncorhynchus mykiss) and >0.57-0.82 mg/litre for bluegill sunfish
(solubilizers: ethanol and acetone, respectively; nominal
concentrations); the lowest reported NOEC (192 h) was 0.11 mg/litre
(Sikka et al., 1981). Sublethal N-phenyl-1-naphthylamine
concentrations of approximately 5.2 and 5.6 mg/litre had teratogenic
effects on embryos and larvae, respectively, of the clawed frog
(Xenopus laevis). Concentrations above 6.2 mg/litre were lethal
(100% death of larvae within 24 h) for both (Greenhouse, 1976a,b).
During neurulation, an EC50 of 4.57 mg/litre for teratogenic effects
was established. For larvae, a 48-h LC50 of 2.3 mg/litre was
determined (Greenhouse, 1977). For larvae of the leopard frog
(Rana pipiens), a 48-h LC100 of 5 mg/litre was reported; no effects
occurred after 24 h of exposure (Greenhouse, 1976b).
Data on chronic effects of N-phenyl-1-naphthylamine in the
aquatic environment are not available.
10.2 Terrestrial environment
Data on toxic effects of N-phenyl-1-naphthylamine on
terrestrial microorganisms, plants, animals, and ecosystems are not
available.
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
N-Phenyl-1-naphthylamine is well absorbed and readily excreted
following ingestion; accumulation in the body is not expected. The
acute oral toxicity of N-phenyl-1-naphthylamine in laboratory
animals is low. Based on the results of tests performed according to
OECD guidelines, the substance is not considered to be a skin or eye
irritant. N-Phenyl-1-naphthylamine has been observed to be a skin
sensitizer in laboratory animals and humans.
A no-observed-effect level could not be derived from the
available toxicological studies. There is limited evidence to suggest
that the kidneys and liver are the main target organs following oral
exposure to N-phenyl-1-naphthylamine, a finding comparable to that
observed for its isomer, N-phenyl-2-naphthylamine. As indicated
above, useful data on the toxicity of N-phenyl-1-naphthylamine are
limited, and therefore additional data on its isomer,
N-phenyl-2-naphthylamine, have been included to assist in the
identification of potential target organs. Available carcinogenicity
studies on N-phenyl-1-naphthylamine have not been performed
according to currently accepted standard protocols, and therefore the
potential carcinogenicity of this chemical cannot be fully evaluated.
However, in a 2-year carcinogenicity bioassay conducted with
N-phenyl-2-naphthylamine in rats and mice, there was no evidence of
carcinogenic activity in male or female rats or male mice and
equivocal evidence of carcinogenic activity in female mice.
N-Phenyl-1-naphthylamine was not mutagenic in bacterial test
systems. In tests with mammalian cells, some investigations yielded
marginally positive or questionably positive results. Based upon the
available evidence, N-phenyl-1-naphthylamine does not appear to be
genotoxic. It is worth noting, however, that several aromatic amines
(the chemical class to which N-phenyl-1-naphthylamine belongs),
while yielding negative or weakly positive results in mutagenicity
assays, are carcinogenic.
An increased occurrence of cancers was observed in one limited
epidemiological study of occupationally exposed individuals; however,
because of the small number of excess deaths and concomitant exposure
to other chemicals, it is not possible to attribute this finding
solely to N-phenyl-1-naphthylamine. Information on the reproductive
or developmental toxicity of N-phenyl-1-naphthylamine was not
available.
11.1.2 Criteria for setting guidance values for
N-phenyl-1-naphthylamine
Data are inadequate to allow the derivation of a
no-observed-effect level or the performance of a risk estimation for
carcinogenicity. Dermal contact with N-phenyl-1-naphthylamine
should be avoided because of its sensitizing properties.
11.1.3 Sample risk characterization
Owing to the lack of available data with which to derive a
suitable guidance value as well as the lack of information on
exposure, a sample quantitative risk characterization could not be
performed. At the workplace, there is a risk of dermal sensitization
from exposure to greases and antirust oils containing
N-phenyl-1-naphthylamine. The risk from exposure to rubber
materials may be much lower, owing to the low concentrations of
N-phenyl-1-naphthylamine in such materials; a risk to the general
population from exposure to products containing
N-phenyl-1-naphthylamine cannot be excluded. Although quantitative
information on the leaching of N-phenyl-1-naphthylamine from rubber
products is not available, the extent of such leaching is expected to
be low. Any leached material is expected to be degraded faster than
it is leached. Indirect exposure of humans to
N-phenyl-1-naphthylamine leached from rubber products into the soil
is unlikely.
11.2 Evaluation of environmental effects
Overall, releases of N-phenyl-1-naphthylamine to the
environment from production and processing (e.g. vulcanization of
rubber mixtures) are expected to be small in view of the chemical's
low production. Based upon the chemical's physical and chemical
properties, it is predicted that soil and sediment will be affected
indirectly by the leaching of N-phenyl-1-naphthylamine from decaying
tyres and rubber products; however, the amounts of
N-phenyl-1-naphthylamine introduced into the environment via this
route could not be quantified. Data on the occurrence of
N-phenyl-1-naphthylamine in environmental media were available only
from some older studies for highly polluted river water and sediment
samples; recent measurements on levels in water, soil, or biota were
not identified. Data on geoaccumulation or on the toxic effects of
N-phenyl-1-naphthylamine on terrestrial microorganisms, plants,
animals, and ecosystems were unavailable.
As data on effect levels for terrestrial organisms or on current
concentrations in environmental media were not available, a
quantitative risk assessment for the main target compartments, water
and soil, could not be carried out; however, some qualitative
statements can be made. Owing to its moderate to high potential for
sorption to organic soil constituents and its limited mineralization
in soil, N-phenyl-1-naphthylamine released to this environmental
compartment is presumed to have geoaccumulation potential. The
probability of its infiltration into groundwater is low. In
laboratory experiments, the acute toxicity of
N-phenyl-1-naphthylamine in fish and Daphnia was high, with lowest
reported NOECs of 0.11 mg/litre (192 h) and 0.02 mg/litre (21 days),
respectively. Although considerable bioconcentration factors were
measured in fish and Daphnia, biomagnification and secondary
poisoning of higher trophic levels via the aquatic food-chain seem
unlikely in view of the metabolism and extensive excretion of
N-phenyl-1-naphthylamine. Biodegradation is expected to be the
predominant route of environmental breakdown; in water, it is aided by
the presence of other degradable substrates, but it is reduced in soil
by sorption. Available N-phenyl-1-naphthylamine is likely to be
biodegraded in both compartments with half-lives of days to weeks.
Photolysis may lead to initial degradation under favourable conditions
but is not considered important in the mineralization of
N-phenyl-1-naphthylamine. Hydrolysis is of very limited or no
importance in the environment.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Previous evaluations of N-phenyl-1-naphthylamine by
international bodies were not identified. Information on
international hazard classification and labelling is included in the
International Chemical Safety Card reproduced in this document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventative and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 1113) reproduced in this
document.
13.1 Human health hazards
N-Phenyl-1-naphthylamine has sensitizing properties.
13.2 Advice to physicians
In case of intoxication, the treatment is supportive. Some
chemicals of this class induce methaemoglobinaemia.
13.3 Spillage
Because N-phenyl-1-naphthylamine is classified as a sensitizer,
emergency crews need to wear proper equipment to prevent contact with
the skin.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards
can be found in the International Register of Potentially Toxic
Chemicals (IRPTC), available from UNEP Chemicals (IRPTC), Geneva.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
N-PHENYL-1-NAPHTHYLAMINE ICSC: 1113
24.03.1998
CAS # 90-30-2
RTECS # QM4500000
UN #
N-(1-Naphthyl)aniline
N-Phenyl-alpha-naphthylamine
C16H13N/C10H7NHC6H5
Molecular mass: 219.30
TYPES OF HAZARD/ ACUTE HAZARDS/ PREVENTION FIRST AID/FIRE FIGHTING
EXPOSURE SYMPTOMS
FIRE Combustible. Gives off irritating or NO open flames. Powder, water spray, foam, carbon dioxide.
toxic fumes (or gases) in a fire.
EXPLOSION In case of fire: keep drums, etc., cool by
spraying with water.
electrical equipment and lighting.
EXPOSURE AVOID ALL CONTACT!
Inhalation Blue lips or finger nails. Blue skin. Local exhaust or breathing protection Fresh air, rest. Refer for medical
Confusion. Convulsions. Dizziness. attention.
Headache. Nausea.
Unconsciousness.
Skin MAY BE ABSORBED! Protective gloves. Protective Remove contaminated clothes. Rinse skin
clothing. with plenty of water or shower.
Eyes Face shield. First rinse with plenty of water for
several minutes (remove contact lenses if
protection. easily possible), then take to a doctor.
Ingestion (See Inhalation). Do not eat, drink, or smoke during Rinse mouth. Refer for medical attention.
work. Wash hands before eating.
(continued)
SPILLAGE DISPOSAL PACKAGING & LABELLING
Sweep spilled substance into sealable containers. Carefully collect Symbol
remainder, then remove to safe place. Do NOT let this chemical enter R:
the environment (extra personal protection: P2 filter respirator for S:
harmful particles). UN Hazard Class:
UN Subsidiary Risks:
UN Pack Group:
EMERGENCY RESPONSE STORAGE
Well closed.
IMPORTANT DATA
PHYSICAL STATE: APPEARANCE ROUTES OF EXPOSURE:
WHITE TO SLIGHT YELLOWISH CRYSTALS The substance can be absorbed into the body by inhalation of its
aerosol, through the skin and by ingestion.
CHEMICAL DANGERS: INHALATION RISK:
The substance decomposes on burning producing toxic fumes No indication can be given about the rate in which a harmful
including nitrogen oxides. concentration in the air is reached on evaporation of this
substance at 20°C.
OCCUPATIONAL EXPOSURE LIMITS: EFFECTS OF SHORT-TERM EXPOSURE:
TLV not established. The substance may cause effects on the blood, resulting in
formation of methaemoglobin. The effects may be delayed.
Medical observation is indicated.
EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
Repeated or prolonged contact may cause skin sensitization.
(continued)
PHYSICAL PROPERTIES
Melting point: 62-63°C
Relative density
(water =1): 1.2
Solubility in water: none
Octanol/water partition
coefficient as log Pow: 4.2
ENVIRONMENTAL DATA
The substance is very toxic to aquatic organisms. In the food chain important to humans, bioaccumulation takes place, especially in fish.
NOTES
Depending on the degree of exposure, periodic medical examination is indicated. Specific treatment is necessary in case of poisoning with
this substance; the appropriate means with instructions must be available.
REFERENCES
Baden JM, Kelley M, Simmon VF, Rice SA, Mazze RI (1978) Fluroxene
mutagenicity. Mutation research, 58:183-191.
Bayer AG (1931) Physiologische Eigenschaften von
Phenyl-alpha-naphthylamin und Phenyl--naphthylamin. Ludwigshafen,
I.G. Farben (not available in print).
Bayer AG (1978a) Akute orale Toxizität von Additin 30
(Phenyl-alpha-naphthylamin) bei weiblichen Wistar Ratten. Wuppertal,
Bayer AG (not available in print).
Bayer AG (1978b) Akute orale Toxizität von Additin 30
(Phenyl-alpha-naphthylamin) bei männlichen Wistar Ratten. Wuppertal,
Bayer AG (not available in print).
Bayer AG (1990) Biologischer Abbau von Vulkanox PAN (OECD Guideline
301C; modifiziert). Prüfbericht vom 05.12.1990. Leverkusen, Bayer AG
(not available in print).
Blank IH, Miller OG (1952) A study of rubber adhesives in shoes as the
cause of dermatitis of the feet. Journal of the American Medical
Association, 149:1371-1374.
Boman A, Hagelthorn G, Jeansson I, Karlberg A-T, Rystedt I, Wahlberg
JE (1980) Phenyl-alpha-naphthylamine - case report and guinea pig
studies. Contact dermatitis, 6:299-300.
Brusick D, Matheson DW (1976) Mutagen and oncogen study on
N-phenyl-alpha-naphthylamine - final report (Litton Bionetics, Inc.,
Kensington, MD). Wright-Patterson Air Force Base, OH, Aerospace
Medical Research Laboratory (Report No. AMRL-TR-76-79).
Brusick D, Matheson D (1977) Mutagenic evaluation of
1,1-dimethylhydrazine, methylhydrazine and
N-phenyl-alpha-naphthylamine. In: Proceedings of the 7th annual
conference on environmental toxicology, 13-15 October 1976.
Wright-Patterson Air Force Base, OH, Aerospace Medical Research
Laboratory, pp. 108-129 (Report No. AMRL-TR-76-125).
BUA (1993) BUA-Stoffbericht N-phenyl-1-naphthylamin. Beratergremium
fuer Umweltrelevante Altstoffe. Weinheim, VCH VerlagsGmbH (Report No.
113; April 1993).
Carmichael AJ, Foulds IS (1990) Isolated naphthylamine allergy to
phenyl-alpha-naphthylamine. Contact dermatitis, 22:298-299.
Ciba-Geigy Corp. (1987a) N-Phenyl-1-naphthylamine: Acute dermal
irritation/corrosion study in the rabbit (final report) - with cover
letter dated 10/02/91. Hawthorne, NY, US Environmental Protection
Agency, Office of Toxic Substances (EPA Report OTS 0533599; Doc.
ID 86-920000033).
Ciba-Geigy Corp. (1987b) N-Phenyl-1-naphthylamine: Acute eye
irritation/corrosion study in the rabbit (final report) - with cover
letter dated 10/02/91. Hawthorne, NY, US Environmental Protection
Agency, Office of Toxic Substances (EPA Report OTS 0533600; Doc.
ID 86-920000034).
Ciba-Geigy Corp. (1987c) N-Phenyl-1-naphthylamine: Skin
sensitization test in the guinea pig modified maximization test
(final report) - with cover letter dated 10/02/91. Hawthorne, NY, US
Environmental Protection Agency, Office of Toxic Substances (EPA
Report OTS 0533601; Doc. ID 86-920000035s).
CITI (1992) Biodegradation and bioaccumulation. Data of existing
chemicals based on the CSCL Japan. Tokyo, Japan Chemical Industry
Ecology-Toxicology & Information Center, Chemicals Inspection &
Testing Institute.
DuPont (1945) Aniline tumours of the bladder. Studies of urinary
bladder tumours. Wilmington, DE, E.I. DuPont de Nemours & Co., 7 pp.
Epstein SS, Saporoschetz IB, Hutner SO (1967) Toxicity of antioxidants
to Tetrahymena pyriformis. Journal of protozoology, 14:238-244.
Gehrmann GH, Foulger JH, Fleming AJ (1948) Occupational tumours of the
bladder. Proceedings of the 9th international congress on
industrial medicine (Chairperson: JM MacAlpine), Tudor Room, Caxton
Hall, pp. 472-475.
Greenhouse GA (1976a) The evaluation of toxic effects of chemicals in
fresh water by using frog embryos and larvae. Environmental
pollution, 11:303-315.
Greenhouse G (1976b) Effects of pollutants on eggs, embryos and
larvae of amphibian species. Wright-Patterson Air Force Base, OH,
Aerospace Medical Research Laboratory, 24 pp. (Report No.
AMRL-TR-76-31).
Greenhouse GA (1977) Toxicity of N-phenyl-alpha-naphthylamine and
hydrazine to Xenopus laevis embryos and larvae. Bulletin of
environmental contamination and toxicology, 18:503-511.
Haskell Laboratory (1971) Memorandum to customers. Wilmington, DE,
E.I. DuPont de Nemours & Co. [cited in McCormick WE (undated),
Environmental health control for the rubber industry, Part II, p.
634.]
IPCS (1993) International Chemical Safety Card -
N-phenyl-1-naphthylamine. Geneva, World Health Organization,
International Programme on Chemical Safety (No. 1113).
Järvholm B, Lavenius B (1981) A cohort study on cancer among workers
exposed to an antirust oil. Scandinavian journal of work,
environment & health, 7:179-184.
JETOC (1996) Mutagenicity test data of existing chemical substances
based on the toxicity investigation system of the industrial safety
and health law. Tokyo, Japan Chemical Industry Ecology-Toxicology &
Information Center.
Jungclaus GA, Lopez-Avila V, Hites RA (1978) Organic compounds in an
industrial wastewater: a case study of their environmental impact.
Environmental science and technology, 12:88-96.
Kalimo K, Jolanki R, Estlander T, Kanerva L (1989) Contact allergy to
antioxidants in industrial greases. Contact dermatitis, 20:151-152.
Kantoh H, Ishihara M, Itoh M, Hosono K, Nishimura M (1985) Allergens
in rubber products. HIFU (Skin research), 27:501-509 (in Japanese,
with English summary).
Kenaga EE (1980) Predicted bioconcentration factors and soil sorption
coefficients of pesticides and other chemicals. Ecotoxicology and
environmental safety, 4:26-38.
Kenaga EE, Goring CAI (1980) Relationship between water solubility,
soil sorption, octanol-water partitioning, and concentration of
chemicals in biota. In: Eaten JG, Parrish PR, Hendricks AC, eds.
Aquatic toxicology. Philadelphia, PA, American Society for Testing
and Materials, pp. 78-115 (ASTM Special Technical Publication 707).
Lopez-Avila V, Hites RA (1980) Organic compounds in an industrial
wastewater. Their transport into sediments. Environmental science
and technology, 14:1382-1390.
Loveday KS, Anderson BE, Resnick MA, Zeiger E (1990) Chromosome
aberration and sister chromatid exchange tests in Chinese hamster
ovary cells in vitro. V: Results with 46 chemicals. Environmental
and molecular mutagenesis, 16:272-303.
MacEwen JD, Vernot EH (1974) Toxic hazards research unit annual
technical report: 1974 (University of California). Wright-Patterson
Air Force Base, OH, Aerospace Medical Research Laboratory (Report No.
AMRL-TR-74-78).
Mackay D (1991) Multimedia models: the fugacity approach. Chelsea,
MI, Lewis Publishers.
Magnusson B, Kligman AM (1970) Allergic contact dermatitis in the
guinea pig. Identification of contact allergens. Springfield, IL,
Charles C. Thomas.
Miyazaki K, Kawai S, Sasayama T, Iseki K, Arita T (1987) Absorption,
metabolism and excretion of N-phenyl-1-naphthylamine in rat.
Yakuzaigaku, 47:17-22 (in Japanese, with English summary).
Mobil Oil Corp. (1985) Dermal carcinogenicity in mice - with cover
letter dated 12/17/91. Princeton, NJ, US Environmental Protection
Agency, Office of Toxic Substances (EPA Report OTS 0533828; Doc.
ID 86-920000547S).
Mobil Oil Corp. (1989) Two-week oral toxicity study in female rats
(final report) - with cover letter dated 12/17/91. Princeton, NJ, US
Environmental Protection Agency, Office of Toxic Substances (EPA
Report OTS 0533821; Doc. ID 86-920000540S).
Nater JP (1975) Überempfindlichkeit gegen Gummi. Berufsdermatosen,
23:161-168.
NIOSH (1976) Metabolic precursors of a known human carcinogen,
beta-naphthylamine. Cincinnati, OH, US Department of Health and
Human Services, National Institute of Occupational Safety and Health
(Current Intelligence Bulletin 16; Publication No. 78-127).
NIOSH (1984a) NIOSH manual of analytical methods, 3rd ed. Vol. 1.
Cincinnati, OH, US Department of Health and Human Services, National
Institute of Occupational Safety and Health, pp. 2002-1 - 2002-6
(Publication No. 84-100).
NIOSH (1984b) NIOSH manual of analytical methods, 3rd ed. Vol. 2.
Cincinnati, OH, US Department of Health and Human Services, National
Institute of Occupational Safety and Health, pp. 5518-1 - 5518-4
(Publication No. 84-100).
Nomura A (1977) Studies of sulfhemoglobin formation by various drugs.
Folia Pharmacologica Japonica, 73:793-802 (in Japanese, with English
summary).
NTP (1987) National Toxicology Program, fiscal year 1987, annual
plan. Research Triangle Park, NC, US Department of Health and Human
Services, National Institutes of Health, National Toxicology Program,
p. 87.
NTP (1988) NTP technical report on the toxicology and
carcinogenesis studies of N-phenyl-2-naphthylamine (CAS no.
135-88-6) in F344/N rats and B6C3F1 mice (feed studies). Research
Triangle Park, NC, US Department of Health and Human Services,
National Institutes of Health, National Toxicology Program, 4 pp. (NTP
TR 333).
Ozeki S, Tejima K (1979) Drug interactions. V. Binding of basic
compounds to bovine serum albumin by fluorescent probe technique.
Chemical and pharmaceutical bulletin, 27:638-646.
Rannug A, Rannug U, Ramel C (1984) Genotoxic effects of additives in
synthetic elastomers with special consideration to the mechanism of
action of thiurames and dithiocarbamates. In: Industrial hazards of
plastics and synthetic elastomers. New York, NY, Alan R. Liss Inc.,
pp. 407-419.
Rosenberg A (1983) Microbial metabolism of N-phenyl-1-naphthylamine
in soil, soil suspensions, and aquatic ecosystems. Chemosphere,
12:1517-1523.
Schär W (1930) Experimentelle Erzeugung von Blasentumoren. Die Wirkung
langdauernder Inhalation von aromatischen Aminoverbindungen.
Deutsche Zeitschrift für Chirurgie, 226:81-97.
Schultheiss E (1959) Gummi und Ekzem (3. Mitteilung), Kasuistik.
Berufsdermatosen, 5:76-96.
Sikka HC, Pack EJ, Sugatt RH, Banerjee S, Rosenberg A, Simpson BW
(1981) Environmental fate and effects of N -phenyl-1-naphthylamine
and its disposition and metabolism in the rat. Syracuse, NY,
Syracuse Research Co., 106 pp. (Report No. AFOSR-TR-81-0703).
Sofuni T, Matsuoka A, Sawada M, Ishidate M, Zeiger E, Shelby MD (1990)
A comparison of chromosome aberration induction by 25 compounds tested
by two hamster cell (CHL and CHO) systems in culture. Mutation
research, 241:175-213.
Te Lintum JCA, Nater JP (1979) Allergic contact dermatitis caused by
rubber chemicals in dairy workers. Dermatologica, 148:42-44.
Thomas RG (1990) Volatilization from water. In: Lyman WJ, Reehl WF,
Rosenblatt DH, eds. Handbook of chemical property estimation
methods. Environmental behavior of organic compounds. New York, NY,
McGraw-Hill Book Co., pp. 15-34.
Union Carbide (1996) Material safety data sheet from 2/22/96. Union
Carbide Corporation, South Charleston Plant (USA), 8 pp.
van Beek L (1977) Primary skin and eye irritation tests with the
compound WTR 10 in albino rabbits. Zeist, TNO Central Institute on
Nutrition and Food Research, 10 pp. (Report No. R 5468).
Vernot EH, MacEwen JD, Haun CC, Kinkead ER (1977) Acute toxicity and
skin corrosion data for some organic and inorganic compounds and
aqueous solutions. Toxicology and applied pharmacology, 42:417-423.
Wang H, Wang D, Dzeng R (1984) Carcinogenicity of
N-phenyl-1-naphthylamine and N-phenyl-2-naphthylamine in mice.
Cancer research, 44:3098-3100.
Xuanxian X, Wolff T (1992) Metabolism of N-phenyl-2-naphthylamine
and N-phenyl-1-naphthylamine by rat hepatic microsomes and
hepatocytes. Journal of environmental science, 4:74-83.
Zeiger E, Anderson B, Haworth S, Lawlor T, Mortelmans K (1988)
Salmonella mutagenicity tests: IV. Results from the testing of 300
chemicals. Environmental and molecular mutagenesis, 12:1-158.
APPENDIX 1 - SOURCE DOCUMENT
BUA-Stoffbericht N-phenyl-1-naphthylamin. Beratergremium fuer
Umweltrelevante Altstoffe (Report No. 113; April 1993). VCH
VerlagsGmbH, Weinheim
For the BUA review process, the company that is in charge of
writing the report (usually the largest producer in Germany) prepares
a draft report using literature from an extensive literature search as
well as internal company studies. This draft is subject to a peer
review during several readings of a working group consisting of
representatives from government agencies, the scientific community,
and industry.
The English translation of the BUA report (BUA Report
N-phenyl-1-naphthylamine. GDCh-Advisory Committee on Existing
Chemicals of Environmental Relevance. VCH VerlagsGmbH, Weinheim) was
released in 1994.
APPENDIX 2 - CICAD PEER REVIEW
The draft CICAD on N-phenyl-1-naphthylamine was sent for review
to institutions and organizations identified by IPCS after contact
with IPCS national Contact Points and Participating Institutions, as
well as to identified experts. Comments were received from:
Department of Health, London, United Kingdom
Health and Safety Executive, Bootle, United Kingdom
Health Canada, Ottawa, Canada
National Chemicals Inspectorate (KEMI), Solna, Sweden
National Institute for Working Life, Solna, Sweden
National Institute of Occupational Health, Budapest, Hungary
National Institute of Public Health, Oslo, Norway
National Institute of Public Health and Environmental Protection,
Bilthoven, The Netherlands
United States Department of Health and Human Services (National
Institute for Occupational Safety and Health, Cincinnati, USA;
National Institute of Environmental Health Sciences, Research
Triangle Park, USA)
APPENDIX 3 - CICAD FINAL REVIEW BOARD
Berlin, Germany, 26-28 November 1997
Members
Dr H. Ahlers, Education and Information Division, National Institute
for Occupational Safety and Health, Cincinnati, OH, USA
Mr R. Cary, Health Directorate, Health and Safety Executive, Bootle,
United Kingdom
Dr S. Dobson, Institute of Terrestrial Ecology, Huntingdon, United
Kingdom
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany (Chairperson)
Mr J.R. Hickman, Health Protection Branch, Health Canada, Ottawa,
Ontario, Canada
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Research, Hanover,
Germany
Ms M.E. Meek, Environmental Health Directorate, Health Canada, Ottawa,
Ontario, Canada (Rapporteur)
Dr K. Paksy, Department of Reproductive Toxicology, National Institute
of Occupational Health, Budapest, Hungary
Mr V. Quarg, Ministry for the Environment, Nature Conservation &
Nuclear Safety, Bonn, Germany
Mr D. Renshaw, Department of Health, London, United Kingdom
Dr J. Sekizawa, Division of Chemo-Bio Informatics, National Institute
of Health Sciences, Tokyo, Japan
Prof. S. Soliman, Department of Pesticide Chemistry, Alexandria
University, Alexandria, Egypt (Vice-Chairperson)
Dr M. Wallen, National Chemicals Inspectorate (KEMI), Solna, Sweden
Ms D. Willcocks, Chemical Assessment Division, Worksafe Australia,
Camperdown, Australia
Dr M. Williams-Johnson, Division of Toxicology, Agency for Toxic
Substances and Disease Registry, Atlanta, GA, USA
Dr K. Ziegler-Skylakakis, Senatskommission der Deutschen
Forschungsgemeinschaft zuer Pruefung gesundheitsschaedlicher
Arbeitsstoffe, GSF-Institut fuer Toxikologie, Neuherberg,
Oberschleissheim, Germany
Observers
Mrs B. Dinham,1 The Pesticide Trust, London, United Kingdom
Dr R. Ebert, KSU Ps-Toxicology, Huels AG, Marl, Germany (representing
ECETOC, the European Centre for Ecotoxicology and Toxicology of
Chemicals)
Mr R. Green,1 International Federation of Chemical, Energy, Mine and
General Workers' Unions, Brussels, Belgium
Dr B. Hansen,1 European Chemicals Bureau, European Commission, Ispra,
Italy
Dr J. Heuer, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany
Mr T. Jacob,1 DuPont, Washington, DC, USA
Ms L. Onyon, Environment Directorate, Organisation for Economic
Co-operation and Development, Paris, France
Dr H.J. Weideli, Ciba Speciality Chemicals Inc., Basel, Switzerland
(representing CEFIC, the European Chemical Industry Council)
Secretariat
Dr M. Baril, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Dr R.G. Liteplo, Health Canada, Ottawa, Ontario, Canada
Ms L. Regis, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Mr A. Strawson, Health and Safety Executive, London, United Kingdom
Dr P. Toft, Associate Director, International Programme on Chemical
Safety, World Health Organization, Geneva, Switzerland
1 Invited but unable to attend.
RÉSUMÉ D'ORIENTATION
Ce CICAD relatif à la N-phényl-1-naphtylamine est fondé
principalement sur une étude menée par l'Institut Fraunhofer de
Toxicologie et de Recherche sur les Aérosols de Hanovre (Allemagne)
pour le compte du Comité consultatif allemand sur les substances
chimiques importantes pour l'environnement (BUA, 1993). Cette étude
évalue les effets potentiels de la N-phényl-1-naphtylamine sur
l'environnement et la santé humaine. Le rapport du BUA s'appuie sur
les données disponibles jusqu'en 1992. Une recherche bibliographique
approfondie a été menée en 1997 dans plusieurs bases de données en
ligne pour retrouver toutes les références publiées postérieurement au
rapport du BUA. Les informations relatives à la préparation du
document initial et à son examen par les pairs figurent à
l'appendice 1. Les renseignements concernant l'examen du CICAD par
les pairs font l'objet de l'appendice 2. Ce CICAD a été approuvé en
tant qu'évaluation internationale lors d'une réunion du Comité
d'évaluation finale qui s'est tenue à Berlin (Allemagne) du 26 au
28 novembre 1997. La liste des participants à cette réunion figure à
l'appendice 3. La fiche d'information sur la sécurité chimique de la
N-phényl-1-naphtylamine (ICSC 1113), établie par le Programme
international sur la Sécurité chimique (IPCS, 1993), est également
reproduite dans le présent document.
La N-phényl-1-naphtylamine (CAS No 90-30-2) est un solide
cristallin lipophile utilisé comme antioxygène dans diverses huiles de
graissage et comme agent protecteur et antioxygène dans le caoutchouc
et les mélanges à base de caoutchouc servant à la fabrication de
différents produits, notamment les pneumatiques. De 1986 à 1990, la
capacité mondiale de production de la N-phényl-1-naphtylamine a été
estimée à 3000 tonnes par an. Une entreprise allemande est la seule à
produire cette substance dans l'Union européenne.
Compte tenu des propriétés physico-chimiques de la
N-phényl-1-naphtylamine, sa distribution dans l'environnement,
prédite sur la base d'un modèle de fugacité de niveau II est
approximativement la suivante : 36 % dans le sol, 34 % dans les
sédiments, 29 % dans l'eau et moins de 1 % dans l'air, les sédiments
en suspension et les biotes. Il n'existe pas de données quantitatives
sur les rejets de N-phényl-1-naphtylamine dans l'environnement lors
de sa production, de sa transformation et de son utilisation. Des
rejets indirects sont possibles dans le sol et les eaux de surface en
cas de fuites d'huiles de graissage ou par suite d'un relargage lors
de la dégradation des pneus et des articles en caoutchouc, mais on ne
dispose pas de données quantitatives à ce sujet. La
N-phényl-1-naphtylamine peut être libérée dans l'atmosphère avec les
gaz émis lors de sa production et de son traitement ou à l'occasion de
la vulcanisation du caoutchouc, mais là encore, on ne dispose d'aucune
donnée. La présence de N-phényl-1-naphtylamine dans les huiles de
graissage ne devrait pas contribuer à sa libération dans l'atmosphère,
car ces huiles sont utilisées en circuit fermé. Globalement, compte
tenu des capacités de production limitées et de l'application de
techniques de réduction des émissions, la quantité de
N-phényl-1-naphtylamine libérée dans l'environnement devrait être
faible.
Selon des études de laboratoire, la N-phényl-1-naphtylamine
subit une dégradation photochimique dans l'eau avec une demi-vie de
8,4 ou 5,7 minutes. La photolyse peut conduire à une dégradation
préliminaire dans des conditions favorables, mais une dégradation
ultérieure est peu probable. Dans l'environnement, la substance
résiste à l'hydrolyse et sa biodégradation dans l'eau et le sol se
fait lentement. En raison d'un potentiel de sorption modéré à élevé
sur les constituants organiques du sol et d'une minéralisation limitée
dans le sol, on peut supposer que la N-phényl-1-naphtylamine
présente un potentiel de géoaccumulation. La probabilité
d'infiltration dans les eaux souterraines est faible. Les résultats
d'études effectuées sur des daphnies et des poissons et un log Ko/w
de 4,2 laissent supposer que la N-phényl-1-naphtylamine a un
potentiel de bioaccumulation modéré. Néanmoins, une contamination
secondaire des niveaux trophiques supérieurs par la chaîne alimentaire
aquatique semble peu probable compte tenu du fait que la substance est
métabolisée et excrétée dans des proportions importantes. La
N-phényl-1-naphtylamine est très toxique pour les poissons et les
daphnies, avec des concentrations sans effet observé (NOEC) de
0,11 mg/litre (192 h) et 0,02 mg/litre (21 jours), respectivement. En
dépit d'une dégradation hydrolytique ou biologique limitée, les
mécanismes de sorption et de dégradation photochimique devraient
contribuer à réduire considérablement la biodisponibilité de la
substance dans l'eau.
Les données connues sur les niveaux de N-phényl-1-naphtylamine
dans l'environnement se limitent aux résultats d'études relativement
anciennes menées aux États-Unis d'Amérique, selon lesquelles la
substance a été détectée dans l'eau (2-7 µg/litre) et les sédiments
(1-5 mg/kg) des cours d'eau à proximité d'une petite usine de
fabrication de produits chimiques. Les données disponibles n'ont pas
permis d'évaluer l'exposition humaine ni de prédire les concentrations
à l'aide d'un modèle de fugacité.
Selon des études menées sur des animaux de laboratoire, la
N-phényl-1-naphtylamine est bien absorbée et facilement excrétée
après ingestion. Chez le rat, 60 % de la dose ingérée ont été
excrétés dans les fèces et 35 % dans l'urine au cours des 72 heures
suivantes. Plusieurs métabolites non identifiées ont été détectées
dans l'urine de rats exposés à la substance. D'après des études
in vitro, il semble que le principal mécanisme de métabolisation de
la N-phényl-1-naphtylamine soit l'hydroxylation.
La toxicité aiguë par voie orale de la N-phényl-1-naphtylamine
chez les animaux de laboratoire est faible. La substance a été
soumise à des épreuves normalisées chez le lapin, qui ont montré
qu'elle n'était irritante ni pour la peau ni pour l'oeil. Toutefois,
un test de maximalisation sur cobayes a révélé que la
N-phényl-1-naphtylamine était un sensibilisant de la peau, ce qui a
été confirmé par des épreuves pratiquées sur des personnes exposées à
des graisses ou à des caoutchoucs contenant cette substance.
Des données limitées montrent que les principaux organes cibles
après ingestion sont les reins et le foie. On n'a trouvé aucune étude
permettant de déterminer les concentrations suivies d'un effet
présumé. Le potentiel cancérogène de la N-phényl-1-naphtylamine n'a
pu être pleinement évalué car aucune des études disponibles n'a été
menée conformément aux normes actuellement reconnues.
La N-phényl-1-naphtylamine ne s'est pas révélée mutagène sur
des cellules bactériennes et il n'y a pas eu augmentation de la
fréquence des mutations géniques (analyse des mutations du lymphome de
la souris) ni des aberrations chromosomiques (analyse in vitro de la
métaphase sur des cellules d'ovaires ou de poumons de hamsters
chinois) dans ce type de cellules à la suite d'une exposition
in vitro. On a signalé un résultat faiblement positif dans une
épreuve d'échange de chromatides soeurs sur des cellules d'ovaires de
hamsters chinois avec activation métabolique. Il y a eu augmentation
de la synthèse non programmée d'ADN dans des cellules de poumon humain
exposé (WI-38); toutefois, cet effet n'a pas semblé clairement lié à
la concentration. Un test de létalité dominante a donné un résultat
négatif chez la souris. D'après les données disponibles, la
N-phényl-1-naphtylamine ne semble pas génotoxique. Aucune donnée
n'a été trouvée sur la toxicité pour la reproduction ou le
développement ni sur les effets immunologiques ou neurologiques de la
N-phényl-1-naphtylamine.
Une étude épidémiologique a révélé une augmentation du taux de
cancers chez des ouvriers exposés à la N-phényl-1-naphtylamine;
toutefois, cet effet n'a pu être attribué exclusivement à la
N-phényl-1-naphtylamine en raison du petit nombre de décès
supplémentaires et de l'exposition concomitante à d'autres substances.
Bien que les données disponibles soient insuffisantes pour
caractériser exactement les risques potentiels pour la santé, il
convient d'éviter le contact de la N-phényl-1-naphtylamine avec la
peau en raison de ses propriétés sensibilisantes.
RESUMEN DE ORIENTACION
Este CICAD relativo a la N-fenil-1-naftilamina se basa
fundamentalmente en un examen preparado por el Instituto Fraunhofer de
Toxicología y de Investigación sobre los Aerosoles de Hannover,
Alemania, para el Comité Consultivo Alemán sobre las Sustancias
Químicas Importantes para el Medio Ambiente (BUA, 1993; versión
inglesa: BUA, 1994). Este estudio evalúa los efectos potenciales de
la N-fenil-1-naftilamina en el medio ambiente y la salud humana. El
informe del BUA se basa en los datos disponibles hasta 1992. En 1997
se realizó una investigación bibliográfica amplia de varias bases de
datos en línea para encontrar todas las referencias publicadas con
posterioridad al informe del BUA. La información sobre la preparación
del documento original y su examen colegiado figura en el Apéndice 1.
La información acerca del examen colegiado de este CICAD se presenta
en el Apéndice 2. Este CICAD se aprobó como evaluación internacional
en una reunión de la Junta de Evaluación Final celebrada en Berlín
(Alemania) los días 26-28 de noviembre de 1997. La lista de
participantes en esta reunión figura en el Apéndice 3. La Ficha
internacional de seguridad química (ICSC 1113) para la
N-fenil-1-naftilamina, preparada por el Programa Internacional de
Seguridad de las Sustancias Químicas (IPCS, 1993), también se
reproduce en el presente documento.
La N-fenil-1-naftilamina (CAS Nº 90-30-2) es una sustancia
cristalina lipófila utilizada como antioxidante en diversos aceites
lubricantes y como agente protector y antioxidante en el caucho y las
mezclas a base de caucho que se emplean en la fabricación de
diferentes productos, en particular los neumáticos. De 1986 a 1990,
la capacidad estimada de producción mundial de N-fenil-1-naftilamina
fue de 3000 toneladas al año. Una empresa alemana es la única
productora de esta sustancia en la Unión Europea.
Teniendo en cuenta las propiedades fisicoquímicas de la
N-fenil-1-naftilamina, su distribución en el medio ambiente,
prevista tomando como base el modelo de fugacidad de nivel II, es la
siguiente: 36% en el suelo, 34% en los sedimentos, 29% en el agua y
menos del 1% en el aire, los sedimentos en suspensión y la biota. No
existen datos cuantitativos de la liberación de
N-fenil-1-naftilamina en el medio ambiente a partir de su
producción, elaboración y uso. Se pueden producir vertidos indirectos
en el suelo y en las aguas superficiales en caso de derrames de
aceites lubricantes o por lixiviación a partir de neumáticos o
productos de caucho en descomposición, pero no se dispone de datos
cuantitativos a este respecto. La N-fenil-1-naftilamina se puede
liberar en la atmósfera con los gases de escape durante su producción
y tratamiento y a partir de la vulcanización de las mezclas de caucho.
El uso de aceites lubricantes con N-fenil-1-naftilamina no debería
contribuir a su liberación en la atmósfera, puesto que estos aceites
se aplican en sistemas cerrados. En conjunto, teniendo en cuenta la
capacidad de producción limitada y la aplicación de técnicas de
reducción de las emisiones, la cantidad de N-fenil-1-naftilamina
liberada en el medio ambiente debería ser baja.
Según los estudios de laboratorio, la N-fenil-1-naftilamina
sufre una degradación fotoquímica en el agua con una semivida de 8,4 y
5,7 minutos. La fotolisis puede dar lugar a una degradación
preliminar en condiciones favorables, pero es poco probable que se
produzca una degradación ulterior. En el medio ambiente, la sustancia
resiste la hidrólisis, y la eliminación por biodegradación en el agua
y el suelo es lenta. Habida cuenta de su potencial de sorción entre
moderado y alto en los constituyentes orgánicos del suelo y de su
mineralización limitada en el suelo, se supone que la
N-fenil-1-naftilamina tiene un potencial de geoacumulación. La
probabilidad de infiltración en el agua fréatica es baja. Los
resultados de los estudios efectuados con Daphnia y con peces y el
log Ko/w de 4,2 obtenido hacen suponer que la
N-fenil-1-naftilamina tiene un potencial de bioacumulación moderado.
No obstante, la contaminación secundaria de los niveles tróficos
superiores a través de la cadena alimentaria acuática parece poco
probable, teniendo en cuenta que la sustancia se metaboliza y excreta
en proporciones importantes. La N-fenil-1-naftilamina es muy tóxica
para los peces y para Daphnia, siendo la concentración sin efectos
observados (NOEC) más que baja que se ha notificado de 0,11 mg/litro
(192 h) y de 0,02 mg/litro (21 días), respectivamente. A pesar de
producirse una degradación hidrolítica o biológica limitada, los
mecanismos de sorción y de degradación fotoquímica deberían contribuir
a reducir considerablemente la disponibilidad de la sustancia en el
agua.
Los datos conocidos sobre los niveles de N-fenil-1-naftilamina
en el medio ambiente se limitan a los resultados de estudios
relativamente antiguos realizados en los Estados Unidos, según los
cuales la sustancia se detectó en el agua (2-7 µg/litro) y en los
sedimentos (1-5 mg/kg) de un río cerca de una pequeña fábrica de
productos químicos. Los datos disponibles no permiten evaluar la
exposición humana ni predecir las concentraciones utilizando un modelo
de fugacidad.
Según los estudios realizados en animales de laboratorio, la
N-fenil-1-naftilamina se absorbe bien y se excreta en su mayor parte
tras la ingestión. En el caso de la rata, el 60% de la dosis
administrada se excretó con las heces y el 35% con la orina en un
plazo de 72 horas. En la orina de ratas expuestas a la sustancia se
han detectado varios metabolitos no identificados. Teniendo en cuenta
los estudios in vitro, parece que el mecanismo principal de
metabolización de la N-fenil-1-naftilamina es la hidroxilación.
La toxicidad aguda por vía oral de la N-fenil-1-naftilamina en
animales de laboratorio es baja. En pruebas normalizadas realizadas
con conejos, se puso de manifiesto que la sustancia no era irritante
de la piel ni de los ojos. Sin embargo, en una prueba de maximización
realizada con cobayas se observó que la N-fenil-1-naftilamina tenía
propiedades de sensibilizante cutáneo, y también en seres humanos
expuestos a grasas o a materiales de caucho que contenían esta
sustancia.
Hay datos limitados que indican que los principales órganos
destinatarios tras la ingestión son los riñones y el hígado. No se ha
encontrado ningún estudio adecuado que permita establecer posibles
niveles de efectos. No se ha podido evaluar completamente la posible
carcinogenicidad de la N-fenil-1-naftilamina, puesto que ninguno de
los estudios disponibles se realizó de acuerdo con los protocolos
normalizados aceptados actualmente.
La N-fenil-1-naftilamina no fue mutagénica en células
bacterianas, ni produjo un aumento de la frecuencia de mutaciones
génicas (análisis de las mutaciones del linfoma de ratón) ni de las
aberraciones cromosómicas (análisis in vitro de la metafase en
células de los ovarios y de los pulmones de hámster chino) en este
tipo de células tras la exposición in vitro. Se ha notificado un
resultado débilmente positivo en un ensayo de intercambio de
cromátidas hermanas en células de ovario de hámster chino con
activación metabólica. Se observó un aumento de síntesis no
programada de ADN en células de pulmón humano expuestas (WI-38); sin
embargo, estos efectos no parecían depender de la concentración. El
resultado de un ensayo de letalidad dominante realizado con
N-fenil-1-naftilamina en ratones fue negativo. Según los datos
disponibles, esta sustancia no parece ser genotóxica. No se han
encontrado datos acerca de la toxicidad reproductiva y en el
desarrollo y de los efectos inmunológicos o neurológicos de la
N-fenil-1-naftilamina.
En un estudio epidemiológico se observó un aumento de la
frecuencia de cáncer en los trabajadores expuestos a la
N-fenil-1-naftilamina; sin embargo, este efecto no se puede atribuir
exclusivamente a la N-fenil-1-naftilamina, debido al pequeño aumento
en el número de fallecimientos y a la exposición concomitante a otras
sustancias. Aunque los datos disponibles sean inadecuados para
caracterizar exactamente los riesgos potenciales para la salud,
conviene evitar el contacto cutáneo con la N-fenil-1-naftilamina,
debido a sus propiedades sensibilizantes.
 1. EXECUTIVE SUMMARY
This CICAD on N-phenyl-1-naphthylamine was based principally on
a review prepared by the Fraunhofer Institute for Toxicology and
Aerosol Research, Hanover, Germany, for the German Advisory Committee
on Existing Chemicals of Environmental Relevance (BUA, 1993). This
review assesses the potential effects of N-phenyl-1-naphthylamine on
the environment and on human health. Data identified up to 1992 were
considered in the BUA report. A comprehensive literature search of
several on-line databases was conducted in 1997 to identify any
relevant references published subsequent to those incorporated in the
BUA report. Information on the preparation and peer review of the
source document is presented in Appendix 1. Information on the peer
review of this CICAD is presented in Appendix 2. This CICAD was
approved as an international assessment at a meeting of the Final
Review Board, held in Berlin, Germany, on 26-28 November 1997.
Participants at the Final Review Board meeting are listed in Appendix
3. The International Chemical Safety Card (ICSC 1113) for
N-phenyl-1-naphthylamine, produced by the International Programme on
Chemical Safety (IPCS, 1993), has also been reproduced in this
document.
N-Phenyl-1-naphthylamine (CAS no.
1. EXECUTIVE SUMMARY
This CICAD on N-phenyl-1-naphthylamine was based principally on
a review prepared by the Fraunhofer Institute for Toxicology and
Aerosol Research, Hanover, Germany, for the German Advisory Committee
on Existing Chemicals of Environmental Relevance (BUA, 1993). This
review assesses the potential effects of N-phenyl-1-naphthylamine on
the environment and on human health. Data identified up to 1992 were
considered in the BUA report. A comprehensive literature search of
several on-line databases was conducted in 1997 to identify any
relevant references published subsequent to those incorporated in the
BUA report. Information on the preparation and peer review of the
source document is presented in Appendix 1. Information on the peer
review of this CICAD is presented in Appendix 2. This CICAD was
approved as an international assessment at a meeting of the Final
Review Board, held in Berlin, Germany, on 26-28 November 1997.
Participants at the Final Review Board meeting are listed in Appendix
3. The International Chemical Safety Card (ICSC 1113) for
N-phenyl-1-naphthylamine, produced by the International Programme on
Chemical Safety (IPCS, 1993), has also been reproduced in this
document.
N-Phenyl-1-naphthylamine (CAS no.  The commercial product has a typical purity of >99%. Named
impurities from three manufacturers are 1-naphthylamine (<100-500
mg/kg), 2-naphthylamine (<3-50 mg/kg), aniline (<100-2500 mg/kg),
1-naphthol (<5000 mg/kg), 1,1-dinaphthylamine (<1000 mg/kg), and
N-phenyl-2-naphthylamine (500-<5000 mg/kg) (BUA, 1993; Union
Carbide, 1996).
3. ANALYTICAL METHODS
N-Phenyl-1-naphthylamine is quantified in environmental media
either by high-performance liquid chromatography in combination with
ultraviolet absorption or by gas chromatography combined with
thermionic or mass spectrometric detection, flame ionization
detection, or electron capture detection. A method for the
determination of secondary amines in air is suitable for the detection
of N-phenyl-1-naphthylamine.
The following enrichment techniques are used for various types of
samples: solid-phase adsorption (silica gel, silica gel/glass fibre)
with liquid extraction (ethanol, acetic acid/2-propanol) for samples
of air (NIOSH, 1984a,b); liquid/liquid extraction (acetonitrile,
diethyl ether), stripping with helium, and solid adsorption (Tenax GC)
or alkaline extraction (dichloromethane) for samples of water
(Jungclaus et al., 1978; Lopez-Avila & Hites, 1980; Sikka et al.,
1981; Rosenberg, 1983); liquid extraction (isopropanol) for sediment
(Jungclaus et al., 1978; Lopez-Avila & Hites, 1980); and liquid
extraction (methanol) for fish, tissue, and serum (Sikka et al.,
1981). Detection limits range from 0.1 to 1 µg/litre for water and
from 50 to 100 µg/kg for sediment; detection limits for biological
materials were not identified.
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
There are no known natural sources of N-phenyl-1-naphthylamine.
Other than the information derived from the national source document,
additional data on the production, use patterns, and release of this
chemical were not identified.
Within the European Union, one German company is the sole
producer of N-phenyl-1-naphthylamine. Between 1986 and 1990, the
estimated worldwide production capacity of N-phenyl-1-naphthylamine
was 3000 tonnes per year. Over the same period, estimated production
capacities in Western Europe, the People's Republic of China, the USA,
and Japan were approximately 1000-1500 tonnes per year, 1000 tonnes
per year, 500 tonnes per year, and 300 tonnes per year, respectively.
Between 1986 and 1990, the consumption of N-phenyl-1-naphthylamine
in Germany was estimated to be approximately 300-450 tonnes per year,
although its use is now declining. Approximately 50-100 tonnes were
covered by imports; exports amounted to approximately 750-1150 tonnes
per year (BUA, 1993).
N-Phenyl-1-naphthylamine is used as an antioxidant in gear,
hydraulic, lubrication, and bearing oils and as a protective agent and
antioxidant in rubbers and rubber mixtures. In Germany,
N-phenyl-1-naphthylamine consumption is divided equally among these
two uses. In these products, the chemical acts as a radical scavenger
in the auto-oxidation of polymers and mineral lubricants. Average
concentrations of N-phenyl-1-naphthylamine in the final products are
<1% w/w (BUA, 1993). In the rubber industry, approximately 75% of
the N-phenyl-1-naphthylamine is used in products such as drums,
buffers, conveyor belts, flexible tubes, gaskets, and footwear
components. The remaining 25% is used in tyres (sidewalls or carcass,
but not treads) (BUA, 1993).
In Germany, N-phenyl-1-naphthylamine-containing distillation
residues from production facilities (approximately 20 tonnes per
year), like most gear and hydraulic oil, are disposed of in
chemical/physical/biological treatment plants or hazardous waste
incinerators (BUA, 1993). About 30% of used tyres are disposed of in
landfill sites, approximately 37% are used for the production of
energy in the cement industry, an estimated 22% are recycled, and
approximately 11% are exported (BUA, 1993). Exhaust gases may be
emitted during the production and processing of
N-phenyl-1-naphthylamine and during the vulcanization of rubber
mixtures at elevated temperatures; in Germany, however, emission
reduction techniques are applied, and releases of
N-phenyl-1-naphthylamine are therefore presumed to be low (<25 kg
per year). Data on levels of N-phenyl-1-naphthylamine in exhaust
gases from vulcanization processes are not available. The use of
N-phenyl-1-naphthylamine-containing lubrication oils should not
result in the introduction of the substance into the atmosphere, as
the oils are applied in closed systems (BUA, 1993).
Data on releases of N-phenyl-1-naphthylamine in effluents from
production and processing facilities were not identified. Indirect
discharges from leaching or the decay of tyres and other rubber
products are to be expected in the long term; however, estimation of
amounts was not possible (BUA, 1993). The release of
N-phenyl-1-naphthylamine into the geosphere from the leakage of
lubrication oils and discarded rubber products and tyres in landfill
sites may occur; however, estimation of the amounts was not possible
with the available data (BUA, 1993). Information on the occurrence of
N-phenyl-1-naphthylamine in plants or animals was not identified.
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Based on its physical/chemical properties, the distribution of
N-phenyl-1-naphthylamine in the environment was predicted, using a
Level II fugacity model (Mackay, 1991), to be 36.3% to soil, 33.9% to
sediment, 28.9% to water, 0.8% to air, 0.06% to suspended sediment,
and 0.02% to biota. Input data for the Level II fugacity model were
as follows: temperature, 298°K; atmospheric volume, 6 109 m3; soil
density, 1.5 g/cm3; density of biota, 1 g/cm3; carbon content of
soil, 2%; carbon content of sediment, 4%; water depth, 1000 cm; water
portion, 70%; soil depth, 15 cm; sediment depth, 3 cm; suspended
sediment portion, 5 ppm; biota portion, 1 ppm; molar mass, 219 g/mol;
water solubility, 3 mg/litre; vapour pressure, 1.06 mPa;
n-octanol/water partition coefficient, 15 850; soil sorption
coefficient, 6510; bioaccumulation factor, 760; half-lives (days) in
air (0), water (365), soil (29.2), sediment (29.2), suspended sediment
(29.2), and biota (0). Owing to the lack of relevant data, it was not
possible to predict the concentrations of N-phenyl-1-naphthylamine
in various media using a Level III fugacity model. Based on its
calculated Henry's law constant (7.748 10-2 Pa.m3/mol; 20°C) and
other information (Thomas, 1990), the volatility of
N-phenyl-1-naphthylamine from aqueous solution is expected to be
low.
Based on its ultraviolet absorption spectrum, direct
photochemical degradation of N-phenyl-1-naphthylamine in air is
expected (BUA, 1993). Data concerning the photo-oxidative degradation
of N-phenyl-1-naphthylamine in air are not available. Measured
half-lives for the photochemical degradation of the chemical in water
have been reported at 8.4 and 5.7 min (Sikka et al., 1981). In this
experiment, sealed tubes containing aqueous solutions of
N-phenyl-1-naphthylamine at approximately 1 mg/litre (water
unspecified but assumed to be distilled) were exposed to sunlight.
The experiment was conducted in May and repeated in June in Syracuse,
NY (USA); no information was provided on light intensity. A further
experiment using a lamp at 300 nm (Rayonette Model RNR-400 mini
photochemical reactor; no information provided on intensity)
demonstrated that the photodegradation product was produced rapidly
and was itself photostable. Given the lack of detail in the report,
the importance of photodegradation in the environment is difficult to
assess. There is also insufficient information to allow the
photodegradation product to be fully characterized, but the authors
suggest that it incorporates the basic phenylnaphthylamine skeleton.
It can therefore be concluded that photolysis may lead to preliminary
breakdown of N-phenyl-1-naphthylamine under favourable environmental
conditions, but that further degradation is unlikely. From
experiments conducted in aqueous solution, hydrolysis of
N-phenyl-1-naphthylamine under environmental conditions is expected
to be of limited importance (Sikka et al., 1981).
Two standard tests on biodegradation performed according to
guideline 301C of the Organisation for Economic Co-operation and
Development (OECD) (modified MITI-I test) reported no degradation of
N-phenyl-1-naphthylamine (100 mg/litre initial concentration) within
14 and 28 days, using non-adapted activated sludge (Bayer AG, 1990;
CITI, 1992). In tests with conditions favouring biodegradation,
N-phenyl-1-naphthylamine was degraded with a half-life ranging from
4 to 11 days (inocula: domestic sewage and lake water, respectively).
Additional substrates accelerated degradation (Sikka et al., 1981;
Rosenberg, 1983). Laboratory results indicate that
N-phenyl-1-naphthylamine is inherently biodegradable in the aquatic
compartment.
Mineralization of N-phenyl-1-naphthylamine (measured by the
evolution of [14C]carbon dioxide) was 17% in soil and 35% in a soil
suspension in buffered salt solution. In contrast to the aquatic
studies, the addition of degradable substrates reduced rather than
accelerated degradation. It was suggested that the organic materials
increased sorption of the N-phenyl-1-naphthylamine. The reported
lower degradation in soil may therefore reflect reduced
bioavailability of the N-phenyl-1-naphthylamine (Rosenberg, 1983).
Measured soil sorption coefficients (Koc) are not available. Using
the regression equations of Kenaga (1980) and Kenaga & Goring (1980),
Koc values of 2400 and 4600, respectively, were calculated for
N-phenyl-1-naphthylamine. Thus, soil sorption is predicted to be
moderate to high. From this expected sorption to organic soil
constituents and its limited mineralization in soil,
N-phenyl-1-naphthylamine is presumed to have geoaccumulation
potential. The probability of infiltration into groundwater is low
(BUA, 1993).
Considering its measured log Kow of 4.2 (Ozeki & Tejima, 1979)
and data from laboratory tests with Daphnia and freshwater fish,
N-phenyl-1-naphthylamine is classified as a substance with moderate
bioaccumulation potential (Sikka et al., 1981; CITI, 1992). For
Daphnia magna, a mean bioconcentration factor (related to
radioactivity) of 637 was calculated following exposure to
[14C] N-phenyl-1-naphthylamine in a static test (solubilizer:
acetone; steady state after 12 h). About 50% of the accumulated
radioactivity had been eliminated after 53 h in clean water (Sikka et
al., 1981). Bioconcentration factors ranging from 432 to 1285
(related to radioactivity) and from 233 to 694 (related to
N-phenyl-1-naphthylamine) were determined in a flow-through system
(sublethal N-phenyl-1-naphthylamine concentration) for the bluegill
sunfish (Lepomis macrochirus) at steady state. Depuration was
biphasic, with an elimination of [14C] N-phenyl-1-naphthylamine of
>90% after 8 days; radioactivity could still be detected 32 days
after treatment (Sikka et al., 1981). Bioconcentration factors for
N-phenyl-1-naphthylamine in common carp (Cyprinus carpio),
measured in a flow-through system after 8 weeks, were on the same
order of magnitude (427-2730) (CITI, 1992).
N-Phenyl-1-naphthylamine is metabolized by terrestrial and aquatic
microorganisms and by fish to at least two or three unidentified
metabolites (Sikka et al., 1981; Rosenberg, 1983).
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
In older studies from the USA, N-phenyl-1-naphthylamine was
detected in river water (2-7 µg/litre) and sediment (1-5 mg/kg) near a
small speciality chemicals manufacturing plant (Jungclaus et al.,
1978; Lopez-Avila & Hites, 1980). Additional data on levels of
N-phenyl-1-naphthylamine in environmental media were not identified.
Based upon the use patterns of N-phenyl-1-naphthylamine, the
presence of this substance in soil and sediment in some
source-dominated areas appears possible; however, quantitative data
are not available.
6.2 Human exposure
Owing to its low vapour pressure and use patterns, the ingestion
or inhalation of N-phenyl-1-naphthylamine is expected to be minor.
Dermal contact with oils and rubber articles containing
N-phenyl-1-naphthylamine may occur in the workplace. Data on
occupational exposure were not available from industries in Germany
involved in the manufacture or use of N-phenyl-1-naphthylamine.1
Dermal contact may also be a source of exposure for the general
population, although this should be of minor importance because of the
small quantities of N-phenyl-1-naphthylamine produced and present in
various products. Data on concentrations of
N-phenyl-1-naphthylamine in media relevant to assessing exposure of
the general population were not identified. Moreover, available data
were insufficient to allow an estimation of human exposure based upon
concentrations predicted from fugacity modelling.
1 Personal communications concerning 1) BUA report on
N-phenyl-1-naphthylamine, Heidelberg, Berufsgenossenschaft der
chemischen Industrie (BG Chemie), 27 August 1992; and 2) exposure data
for N-phenyl-1-naphthylamine, Bundesanstalt für Arbeitsschutz,
Dortmund, 1992.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND
HUMANS
Studies providing quantitative information on the absorption or
distribution of N-phenyl-1-naphthylamine in humans were not
identified. From the limited information available from studies
conducted with laboratory animals, it can be concluded that
N-phenyl-1-naphthylamine is well absorbed after ingestion and is
readily excreted. In vitro studies have demonstrated that the
metabolism of N-phenyl-1-naphthylamine occurs primarily via
hydroxylation.
In male Sprague-Dawley rats administered a single oral dose of
160 mg [14C] N-phenyl-1-naphthylamine/kg body weight, the chemical
was well absorbed, metabolized almost completely, and excreted
primarily in the faeces. Radioactivity was detected in plasma within
60 min, with the maximum concentration measured after 4 h. After
24 h, 20% of the radioactivity was found in the gastrointestinal tract
(including contents), 2.4% in fatty tissue, 0.4% in the liver, and
0.1% in the kidneys. Ninety per cent of the administered
radioactivity was excreted within 48 h; 95% was excreted within 72 h
(60% in the faeces and 35% in the urine). In the ether extract of the
urine, at least five radioactive metabolites were detected but not
identified. The elimination half-lives were reported as 1.68 h for
the fast elimination and 33 h for the slow elimination (Sikka et al.,
1981).
In a study in which male rats were administered
N-phenyl-1-naphthylamine orally, only small quantities of unchanged
N-phenyl-1-naphthylamine were excreted in the faeces and urine (0.4
and 0.01% of the applied dose, respectively). Large amounts of
glucuronide and sulfate conjugates, which were not identified further,
were detected in the urine. Small quantities of
N-phenyl-1-naphthylamine were distributed in fatty tissue after
single or multiple (6 day) oral administration, whereas the
distribution of unchanged N-phenyl-1-naphthylamine in liver,
kidneys, spleen, heart, and lung was extremely low (Miyazaki et al.,
1987).
Mono- and dihydroxy-derivatives of N-phenyl-1-naphthylamine
have been identified in in vitro metabolic studies conducted with
rat liver microsomes (Sikka et al., 1981; Xuanxian & Wolff, 1992).
Sikka et al. (1981) suggested that the hydroxyl group in the
mono-hydroxy derivative is in the naphthalene moiety at a
para-position to the amino group, whereas at least one hydroxyl group
in the dihydroxy-derivative is at the available para-position in the
naphthyl ring. Pretreatment of male rats with phenobarbital or
3-methylcholanthrene increased the rate of microsomal metabolism,
indicating that more than one P-450 enzyme is involved in the
metabolism of N-phenyl-1-naphthylamine (Xuanxian & Wolff, 1992).
In studies conducted with human volunteers or laboratory animals,
the isomer N-phenyl-2-naphthylamine (CAS no.
The commercial product has a typical purity of >99%. Named
impurities from three manufacturers are 1-naphthylamine (<100-500
mg/kg), 2-naphthylamine (<3-50 mg/kg), aniline (<100-2500 mg/kg),
1-naphthol (<5000 mg/kg), 1,1-dinaphthylamine (<1000 mg/kg), and
N-phenyl-2-naphthylamine (500-<5000 mg/kg) (BUA, 1993; Union
Carbide, 1996).
3. ANALYTICAL METHODS
N-Phenyl-1-naphthylamine is quantified in environmental media
either by high-performance liquid chromatography in combination with
ultraviolet absorption or by gas chromatography combined with
thermionic or mass spectrometric detection, flame ionization
detection, or electron capture detection. A method for the
determination of secondary amines in air is suitable for the detection
of N-phenyl-1-naphthylamine.
The following enrichment techniques are used for various types of
samples: solid-phase adsorption (silica gel, silica gel/glass fibre)
with liquid extraction (ethanol, acetic acid/2-propanol) for samples
of air (NIOSH, 1984a,b); liquid/liquid extraction (acetonitrile,
diethyl ether), stripping with helium, and solid adsorption (Tenax GC)
or alkaline extraction (dichloromethane) for samples of water
(Jungclaus et al., 1978; Lopez-Avila & Hites, 1980; Sikka et al.,
1981; Rosenberg, 1983); liquid extraction (isopropanol) for sediment
(Jungclaus et al., 1978; Lopez-Avila & Hites, 1980); and liquid
extraction (methanol) for fish, tissue, and serum (Sikka et al.,
1981). Detection limits range from 0.1 to 1 µg/litre for water and
from 50 to 100 µg/kg for sediment; detection limits for biological
materials were not identified.
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
There are no known natural sources of N-phenyl-1-naphthylamine.
Other than the information derived from the national source document,
additional data on the production, use patterns, and release of this
chemical were not identified.
Within the European Union, one German company is the sole
producer of N-phenyl-1-naphthylamine. Between 1986 and 1990, the
estimated worldwide production capacity of N-phenyl-1-naphthylamine
was 3000 tonnes per year. Over the same period, estimated production
capacities in Western Europe, the People's Republic of China, the USA,
and Japan were approximately 1000-1500 tonnes per year, 1000 tonnes
per year, 500 tonnes per year, and 300 tonnes per year, respectively.
Between 1986 and 1990, the consumption of N-phenyl-1-naphthylamine
in Germany was estimated to be approximately 300-450 tonnes per year,
although its use is now declining. Approximately 50-100 tonnes were
covered by imports; exports amounted to approximately 750-1150 tonnes
per year (BUA, 1993).
N-Phenyl-1-naphthylamine is used as an antioxidant in gear,
hydraulic, lubrication, and bearing oils and as a protective agent and
antioxidant in rubbers and rubber mixtures. In Germany,
N-phenyl-1-naphthylamine consumption is divided equally among these
two uses. In these products, the chemical acts as a radical scavenger
in the auto-oxidation of polymers and mineral lubricants. Average
concentrations of N-phenyl-1-naphthylamine in the final products are
<1% w/w (BUA, 1993). In the rubber industry, approximately 75% of
the N-phenyl-1-naphthylamine is used in products such as drums,
buffers, conveyor belts, flexible tubes, gaskets, and footwear
components. The remaining 25% is used in tyres (sidewalls or carcass,
but not treads) (BUA, 1993).
In Germany, N-phenyl-1-naphthylamine-containing distillation
residues from production facilities (approximately 20 tonnes per
year), like most gear and hydraulic oil, are disposed of in
chemical/physical/biological treatment plants or hazardous waste
incinerators (BUA, 1993). About 30% of used tyres are disposed of in
landfill sites, approximately 37% are used for the production of
energy in the cement industry, an estimated 22% are recycled, and
approximately 11% are exported (BUA, 1993). Exhaust gases may be
emitted during the production and processing of
N-phenyl-1-naphthylamine and during the vulcanization of rubber
mixtures at elevated temperatures; in Germany, however, emission
reduction techniques are applied, and releases of
N-phenyl-1-naphthylamine are therefore presumed to be low (<25 kg
per year). Data on levels of N-phenyl-1-naphthylamine in exhaust
gases from vulcanization processes are not available. The use of
N-phenyl-1-naphthylamine-containing lubrication oils should not
result in the introduction of the substance into the atmosphere, as
the oils are applied in closed systems (BUA, 1993).
Data on releases of N-phenyl-1-naphthylamine in effluents from
production and processing facilities were not identified. Indirect
discharges from leaching or the decay of tyres and other rubber
products are to be expected in the long term; however, estimation of
amounts was not possible (BUA, 1993). The release of
N-phenyl-1-naphthylamine into the geosphere from the leakage of
lubrication oils and discarded rubber products and tyres in landfill
sites may occur; however, estimation of the amounts was not possible
with the available data (BUA, 1993). Information on the occurrence of
N-phenyl-1-naphthylamine in plants or animals was not identified.
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Based on its physical/chemical properties, the distribution of
N-phenyl-1-naphthylamine in the environment was predicted, using a
Level II fugacity model (Mackay, 1991), to be 36.3% to soil, 33.9% to
sediment, 28.9% to water, 0.8% to air, 0.06% to suspended sediment,
and 0.02% to biota. Input data for the Level II fugacity model were
as follows: temperature, 298°K; atmospheric volume, 6 109 m3; soil
density, 1.5 g/cm3; density of biota, 1 g/cm3; carbon content of
soil, 2%; carbon content of sediment, 4%; water depth, 1000 cm; water
portion, 70%; soil depth, 15 cm; sediment depth, 3 cm; suspended
sediment portion, 5 ppm; biota portion, 1 ppm; molar mass, 219 g/mol;
water solubility, 3 mg/litre; vapour pressure, 1.06 mPa;
n-octanol/water partition coefficient, 15 850; soil sorption
coefficient, 6510; bioaccumulation factor, 760; half-lives (days) in
air (0), water (365), soil (29.2), sediment (29.2), suspended sediment
(29.2), and biota (0). Owing to the lack of relevant data, it was not
possible to predict the concentrations of N-phenyl-1-naphthylamine
in various media using a Level III fugacity model. Based on its
calculated Henry's law constant (7.748 10-2 Pa.m3/mol; 20°C) and
other information (Thomas, 1990), the volatility of
N-phenyl-1-naphthylamine from aqueous solution is expected to be
low.
Based on its ultraviolet absorption spectrum, direct
photochemical degradation of N-phenyl-1-naphthylamine in air is
expected (BUA, 1993). Data concerning the photo-oxidative degradation
of N-phenyl-1-naphthylamine in air are not available. Measured
half-lives for the photochemical degradation of the chemical in water
have been reported at 8.4 and 5.7 min (Sikka et al., 1981). In this
experiment, sealed tubes containing aqueous solutions of
N-phenyl-1-naphthylamine at approximately 1 mg/litre (water
unspecified but assumed to be distilled) were exposed to sunlight.
The experiment was conducted in May and repeated in June in Syracuse,
NY (USA); no information was provided on light intensity. A further
experiment using a lamp at 300 nm (Rayonette Model RNR-400 mini
photochemical reactor; no information provided on intensity)
demonstrated that the photodegradation product was produced rapidly
and was itself photostable. Given the lack of detail in the report,
the importance of photodegradation in the environment is difficult to
assess. There is also insufficient information to allow the
photodegradation product to be fully characterized, but the authors
suggest that it incorporates the basic phenylnaphthylamine skeleton.
It can therefore be concluded that photolysis may lead to preliminary
breakdown of N-phenyl-1-naphthylamine under favourable environmental
conditions, but that further degradation is unlikely. From
experiments conducted in aqueous solution, hydrolysis of
N-phenyl-1-naphthylamine under environmental conditions is expected
to be of limited importance (Sikka et al., 1981).
Two standard tests on biodegradation performed according to
guideline 301C of the Organisation for Economic Co-operation and
Development (OECD) (modified MITI-I test) reported no degradation of
N-phenyl-1-naphthylamine (100 mg/litre initial concentration) within
14 and 28 days, using non-adapted activated sludge (Bayer AG, 1990;
CITI, 1992). In tests with conditions favouring biodegradation,
N-phenyl-1-naphthylamine was degraded with a half-life ranging from
4 to 11 days (inocula: domestic sewage and lake water, respectively).
Additional substrates accelerated degradation (Sikka et al., 1981;
Rosenberg, 1983). Laboratory results indicate that
N-phenyl-1-naphthylamine is inherently biodegradable in the aquatic
compartment.
Mineralization of N-phenyl-1-naphthylamine (measured by the
evolution of [14C]carbon dioxide) was 17% in soil and 35% in a soil
suspension in buffered salt solution. In contrast to the aquatic
studies, the addition of degradable substrates reduced rather than
accelerated degradation. It was suggested that the organic materials
increased sorption of the N-phenyl-1-naphthylamine. The reported
lower degradation in soil may therefore reflect reduced
bioavailability of the N-phenyl-1-naphthylamine (Rosenberg, 1983).
Measured soil sorption coefficients (Koc) are not available. Using
the regression equations of Kenaga (1980) and Kenaga & Goring (1980),
Koc values of 2400 and 4600, respectively, were calculated for
N-phenyl-1-naphthylamine. Thus, soil sorption is predicted to be
moderate to high. From this expected sorption to organic soil
constituents and its limited mineralization in soil,
N-phenyl-1-naphthylamine is presumed to have geoaccumulation
potential. The probability of infiltration into groundwater is low
(BUA, 1993).
Considering its measured log Kow of 4.2 (Ozeki & Tejima, 1979)
and data from laboratory tests with Daphnia and freshwater fish,
N-phenyl-1-naphthylamine is classified as a substance with moderate
bioaccumulation potential (Sikka et al., 1981; CITI, 1992). For
Daphnia magna, a mean bioconcentration factor (related to
radioactivity) of 637 was calculated following exposure to
[14C] N-phenyl-1-naphthylamine in a static test (solubilizer:
acetone; steady state after 12 h). About 50% of the accumulated
radioactivity had been eliminated after 53 h in clean water (Sikka et
al., 1981). Bioconcentration factors ranging from 432 to 1285
(related to radioactivity) and from 233 to 694 (related to
N-phenyl-1-naphthylamine) were determined in a flow-through system
(sublethal N-phenyl-1-naphthylamine concentration) for the bluegill
sunfish (Lepomis macrochirus) at steady state. Depuration was
biphasic, with an elimination of [14C] N-phenyl-1-naphthylamine of
>90% after 8 days; radioactivity could still be detected 32 days
after treatment (Sikka et al., 1981). Bioconcentration factors for
N-phenyl-1-naphthylamine in common carp (Cyprinus carpio),
measured in a flow-through system after 8 weeks, were on the same
order of magnitude (427-2730) (CITI, 1992).
N-Phenyl-1-naphthylamine is metabolized by terrestrial and aquatic
microorganisms and by fish to at least two or three unidentified
metabolites (Sikka et al., 1981; Rosenberg, 1983).
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
In older studies from the USA, N-phenyl-1-naphthylamine was
detected in river water (2-7 µg/litre) and sediment (1-5 mg/kg) near a
small speciality chemicals manufacturing plant (Jungclaus et al.,
1978; Lopez-Avila & Hites, 1980). Additional data on levels of
N-phenyl-1-naphthylamine in environmental media were not identified.
Based upon the use patterns of N-phenyl-1-naphthylamine, the
presence of this substance in soil and sediment in some
source-dominated areas appears possible; however, quantitative data
are not available.
6.2 Human exposure
Owing to its low vapour pressure and use patterns, the ingestion
or inhalation of N-phenyl-1-naphthylamine is expected to be minor.
Dermal contact with oils and rubber articles containing
N-phenyl-1-naphthylamine may occur in the workplace. Data on
occupational exposure were not available from industries in Germany
involved in the manufacture or use of N-phenyl-1-naphthylamine.1
Dermal contact may also be a source of exposure for the general
population, although this should be of minor importance because of the
small quantities of N-phenyl-1-naphthylamine produced and present in
various products. Data on concentrations of
N-phenyl-1-naphthylamine in media relevant to assessing exposure of
the general population were not identified. Moreover, available data
were insufficient to allow an estimation of human exposure based upon
concentrations predicted from fugacity modelling.
1 Personal communications concerning 1) BUA report on
N-phenyl-1-naphthylamine, Heidelberg, Berufsgenossenschaft der
chemischen Industrie (BG Chemie), 27 August 1992; and 2) exposure data
for N-phenyl-1-naphthylamine, Bundesanstalt für Arbeitsschutz,
Dortmund, 1992.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND
HUMANS
Studies providing quantitative information on the absorption or
distribution of N-phenyl-1-naphthylamine in humans were not
identified. From the limited information available from studies
conducted with laboratory animals, it can be concluded that
N-phenyl-1-naphthylamine is well absorbed after ingestion and is
readily excreted. In vitro studies have demonstrated that the
metabolism of N-phenyl-1-naphthylamine occurs primarily via
hydroxylation.
In male Sprague-Dawley rats administered a single oral dose of
160 mg [14C] N-phenyl-1-naphthylamine/kg body weight, the chemical
was well absorbed, metabolized almost completely, and excreted
primarily in the faeces. Radioactivity was detected in plasma within
60 min, with the maximum concentration measured after 4 h. After
24 h, 20% of the radioactivity was found in the gastrointestinal tract
(including contents), 2.4% in fatty tissue, 0.4% in the liver, and
0.1% in the kidneys. Ninety per cent of the administered
radioactivity was excreted within 48 h; 95% was excreted within 72 h
(60% in the faeces and 35% in the urine). In the ether extract of the
urine, at least five radioactive metabolites were detected but not
identified. The elimination half-lives were reported as 1.68 h for
the fast elimination and 33 h for the slow elimination (Sikka et al.,
1981).
In a study in which male rats were administered
N-phenyl-1-naphthylamine orally, only small quantities of unchanged
N-phenyl-1-naphthylamine were excreted in the faeces and urine (0.4
and 0.01% of the applied dose, respectively). Large amounts of
glucuronide and sulfate conjugates, which were not identified further,
were detected in the urine. Small quantities of
N-phenyl-1-naphthylamine were distributed in fatty tissue after
single or multiple (6 day) oral administration, whereas the
distribution of unchanged N-phenyl-1-naphthylamine in liver,
kidneys, spleen, heart, and lung was extremely low (Miyazaki et al.,
1987).
Mono- and dihydroxy-derivatives of N-phenyl-1-naphthylamine
have been identified in in vitro metabolic studies conducted with
rat liver microsomes (Sikka et al., 1981; Xuanxian & Wolff, 1992).
Sikka et al. (1981) suggested that the hydroxyl group in the
mono-hydroxy derivative is in the naphthalene moiety at a
para-position to the amino group, whereas at least one hydroxyl group
in the dihydroxy-derivative is at the available para-position in the
naphthyl ring. Pretreatment of male rats with phenobarbital or
3-methylcholanthrene increased the rate of microsomal metabolism,
indicating that more than one P-450 enzyme is involved in the
metabolism of N-phenyl-1-naphthylamine (Xuanxian & Wolff, 1992).
In studies conducted with human volunteers or laboratory animals,
the isomer N-phenyl-2-naphthylamine (CAS no. 