
This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organization, or the World Health Organization.
Concise International Chemical Assessment Document 71
First draft prepared by Drs S. Hahn, J. Kielhorn, J. Koppenhöfer, A. Wibbertmann, and I. Mangelsdorf, Fraunhofer Institute of Toxicology and Experimental Medicine, Hanover, Germany
Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organization, and the World Health Organization, and produced within the framework of the Inter-Organization Programme for the Sound Management of Chemicals.
The International Programme on Chemical Safety (IPCS), established in 1980, is a joint venture of the United Nations Environment Programme (UNEP), the International Labour Organization (ILO), and the World Health Organization (WHO). The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals.
The Inter-Organization Programme for the Sound Management of Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and Agriculture Organization of the United Nations, WHO, the United Nations Industrial Development Organization, the United Nations Institute for Training and Research, and the Organisation for Economic Co-operation and Development (Participating Organizations), following recommendations made by the 1992 UN Conference on Environment and Development to strengthen cooperation and increase coordination in the field of chemical safety. The purpose of the IOMC is to promote coordination of the policies and activities pursued by the Participating Organizations, jointly or separately, to achieve the sound management of chemicals in relation to human health and the environment.
WHO Library Cataloguing-in-Publication Data
Resorcinol.
(Concise international chemical assessment document ; 71)
First draft prepared by S. Hahn, J. Koppenhöfer, A. Wibbertmann, and I. Mangelsdorf.
1. Resorcinols - adverse effects. 2. Resorcinols - toxicity. 3. Environmental exposure.
4. Risk assessment. I. Hahn, S.K. II. World Health Organization. III. International
Programme on Chemical Safety, IV. Series.
ISBN 92 4 153071 5 (NLM Classification: QV 223)
ISBN 978 92 4 153071 2
©World Health Organization 2006
All rights reserved. Publications of the World Health Organization can be obtained from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel: +41 22 791 3264; fax: +41 22 791 4857; email: bookorders@who.int). Requests for permission to reproduce or translate WHO publications — whether for sale or for noncommercial distribution — should be addressed to WHO Press, at the above address (fax: +41 22 791 4806; email: permissions@who.int).
The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement.
The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters.
All reasonable precautions have been taken by WHO to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either express or implied. The responsibility for the interpretation and use of the material lies with the reader. In no event shall the World Health Organization be liable for damages arising from its use.
The named editors alone are responsible for the views expressed in this publication.
Risk assessment activities of the International Programme on Chemical Safety, including the production of Concise International Chemical Assessment Documents, are supported financially by the Department of Health and Department for Environment, Food & Rural Affairs, UK, Environmental Protection Agency, Food and Drug Administration, and National Institute of Environmental Health Sciences, USA, European Commission, German Federal Ministry of Environment, Nature Conservation and Nuclear Safety, Health Canada, Japanese Ministry of Health, Labour and Welfare, and Swiss Agency for Environment, Forests and Landscape.
Concise International Chemical Assessment Documents (CICADs) are published by the International Programme on Chemical Safety (IPCS) — a cooperative programme of the World Health Organization (WHO), the International Labour Organization (ILO), and the United Nations Environment Programme (UNEP). CICADs have been developed from the Environmental Health Criteria documents (EHCs), more than 200 of which have been published since 1976 as authoritative documents on the risk assessment of chemicals.
International Chemical Safety Cards on the relevant chemical(s) are attached at the end of the CICAD, to provide the reader with concise information on the protection of human health and on emergency action. They are produced in a separate peer-reviewed procedure at IPCS. They may be complemented by information from IPCS Poison Information Monographs (PIM), similarly produced separately from the CICAD process.
CICADs are concise documents that provide summaries of the relevant scientific information concerning the potential effects of chemicals upon human health and/or the environment. They are usually based on selected national or regional evaluation documents or on existing EHCs. Before acceptance for publication as CICADs by IPCS, these documents undergo extensive peer review by internationally selected experts to ensure their completeness, accuracy in the way in which the original data are represented, and the validity of the conclusions drawn.
The primary objective of CICADs is characterization of hazard and dose–response from exposure to a chemical. CICADs are not a summary of all available data on a particular chemical; rather, they include only that information considered critical for characterization of the risk posed by the chemical. The critical studies are, however, presented in sufficient detail to support the conclusions drawn. For additional information, the reader should consult the identified source documents upon which the CICAD has been based.
Risks to human health and the environment will vary considerably depending upon the type and extent of exposure. Responsible authorities are strongly encouraged to characterize risk on the basis of locally measured or predicted exposure scenarios. To assist the reader, examples of exposure estimation and risk characterization are provided in CICADs, whenever possible. These examples cannot be considered as representing all possible exposure situations, but are provided as guidance only. The reader is referred to EHC 170.1
While every effort is made to ensure that CICADs represent the current status of knowledge, new information is being developed constantly. Unless otherwise stated, CICADs are based on a search of the scientific literature to the date shown in the executive summary. In the event that a reader becomes aware of new information that would change the conclusions drawn in a CICAD, the reader is requested to contact IPCS to inform it of the new information.
Procedures
The flow chart shows the procedures followed to produce a CICAD. These procedures are designed to take advantage of the expertise that exists around the world — expertise that is required to produce the high-quality evaluations of toxicological, exposure, and other data that are necessary for assessing risks to human health and/or the environment. The IPCS Risk Assessment Steering Group advises the Coordinator, IPCS, on the selection of chemicals for an IPCS risk assessment based on the following criteria:
Thus, it is typical of a priority chemical that:

|
Advice from Risk Assessment Steering Group Criteria of priority:
Thus, it is typical of a priority chemical that
Special emphasis is placed on avoiding duplication of effort by WHO and other international organizations. A prerequisite of the production of a CICAD is the availability of a recent high-quality national/regional risk assessment document = source document. The source document and the CICAD may be produced in parallel. If the source document does not contain an environmental section, this may be produced de novo, provided it is not controversial. If no source document is available, IPCS may produce a de novo risk assessment document if the cost is justified. Depending on the complexity and extent of controversy of the issues involved, the steering group may advise on different levels of peer review:
|
The Steering Group will also advise IPCS on the appropriate form of the document (i.e. a standard CICAD or a de novo CICAD) and which institution bears the responsibility of the document production, as well as on the type and extent of the international peer review.
The first draft is usually based on an existing national, regional, or international review. When no appropriate source document is available, a CICAD may be produced de novo. Authors of the first draft are usually, but not necessarily, from the institution that developed the original review. A standard outline has been developed to encourage consistency in form. The first draft undergoes primary review by IPCS to ensure that it meets the specified criteria for CICADs.
The second stage involves international peer review by scientists known for their particular expertise and by scientists selected from an international roster compiled by IPCS through recommendations from IPCS national Contact Points and from IPCS Participating Institutions. Adequate time is allowed for the selected experts to undertake a thorough review. Authors are required to take reviewers’ comments into account and revise their draft, if necessary. The resulting second draft is submitted to a Final Review Board together with the reviewers’ comments. At any stage in the international review process, a consultative group may be necessary to address specific areas of the science. When a CICAD is prepared de novo, a consultative group is normally convened.
The CICAD Final Review Board has several important functions:
Board members serve in their personal capacity, not as representatives of any organization, government, or industry. They are selected because of their expertise in human and environmental toxicology or because of their experience in the regulation of chemicals. Boards are chosen according to the range of expertise required for a meeting and the need for balanced geographic representation.
Board members, authors, reviewers, consultants, and advisers who participate in the preparation of a CICAD are required to declare any real or potential conflict of interest in relation to the subjects under discussion at any stage of the process. Representatives of nongovernmental organizations may be invited to observe the proceedings of the Final Review Board. Observers may participate in Board discussions only at the invitation of the Chairperson, and they may not participate in the final decision-making process.
This CICAD2 on resorcinol was prepared by the Fraunhofer Institute of Toxicology and Experimental Medicine, Hanover, Germany. It is based on the BUA (1993) report, the German MAK Commission report (MAK, 2003), the Health Council of the Netherlands (2004) report, and a preliminary IUCLID for the USEPA HPV Challenge Program (INDSPEC, 2004). Information on the source documents and their peer review is presented in Appendix 2. A comprehensive literature search of relevant databases was conducted up to February 2005 to identify any relevant references published subsequent to those incorporated in these reports. Information on the peer review of this CICAD is presented in Appendix 3. This CICAD was considered and approved as an international assessment at a meeting of the 13th Final Review Board, held in Nagpur, India, on 31 October – 3 November 2005. Participants at the Final Review Board meeting are presented in Appendix 4. The International Chemical Safety Card for resorcinol (ICSC 1033), produced by IPCS (2003), has also been reproduced in this document. At the time of approval of the CICAD on resorcinol, an assessment of the chemical was also being undertaken as part of the HPV Chemicals Programme of the OECD. Peer review of this CICAD was extended to OECD Member countries during August and September 2005. As part of ongoing cooperation, any new information provided in the course of the OECD assessment will be provided by the OECD to IPCS.
Resorcinol (CAS No.
The resorcinol moiety has been found in a wide variety of natural products, and resorcinol is a monomeric by-product of the reduction, oxidation, and microbial degradation of humic substances.
The largest user of resorcinol is the rubber industry (about 50%). Resorcinol is also used for high-quality wood bonding applications (about 25%) and is an important chemical intermediate in the manufacture of speciality chemicals. Other uses include the manufacture of dyestuffs, pharmaceuticals, flame retardants, agricultural chemicals, fungicidal creams and lotions, and hair dye formulations.
Resorcinol is released into the environment from a number of anthropogenic sources, including production, processing, and consumer uses, especially from hair dyes and pharmaceuticals. In addition, localized high concentrations can appear in coal conversion wastewater or wastewater in regions with oil shale mining.
Calculations predict the hydrosphere to be the main target compartment of resorcinol. Data indicate that resorcinol is essentially non-volatile from aqueous solution.
In the hydrosphere, hydrolysis is not expected to occur. However, in aqueous solution, autoxidation of resorcinol takes place, and it can be assumed that resorcinol reacts in water bodies with hydroxyl and peroxyl radicals. Resorcinol is readily biodegradable under aerobic conditions and is likely to be biodegraded under anaerobic conditions.
In the upper atmosphere, resorcinol is rapidly degraded (half-life about 2 h) by reaction with photochemically produced hydroxyl radicals.
Experimental data using silty loam indicate a very low soil sorption of resorcinol, leading to a high potential for mobility. Bioaccumulation is not to be expected, based on the calculated BCF.
Localized concentrations are available only for coal conversion wastewater or wastewater in oil shale regions. These values are unsuitable for a risk assessment of the emissions from anthropogenic sources, because they are not representative of the background or local concentrations. Therefore, estimates of environmental concentrations were made for Europe using the software EUSES 2.0.3.
The results of the calculations show that the highest concentrations are expected at local point sources, such as at sites where hair dyes are formulated or rubber products are manufactured. These estimated concentrations in water are 1 order of magnitude higher than the local concentrations resulting from emissions from the use of consumer products containing resorcinol, which are released on a continental scale.
The results of pharmacokinetic studies in rats, rabbits, and humans suggest that resorcinol is absorbed by the oral, dermal, and subcutaneous routes, rapidly metabolized, and excreted principally as glucuronide conjugates in the urine. The available studies give no indication of bioaccumulation. There is a limited potential for absorption of resorcinol through intact skin using a hydroalcoholic vehicle.
In animal studies, the toxicological effects reported to be caused by administration of resorcinol include thyroid dysfunction, skin irritation, CNS effects, and altered relative adrenal gland weights. In some studies, decreases in body weight gain and decreased survival were noted.
Acute lethal toxicity data in experimental animals showed resorcinol to be of low toxicity following inhalation and dermal exposure but of higher toxicity after oral, intraperitoneal, or subcutaneous administration. Resorcinol is irritating to eyes and skin and may cause sensitization by skin contact.
Short-term (17 days) oral exposure studies via gavage in F344 rats and B6C3F1 mice dosed 5 days/ week resulted in NOAELs of 27.5 mg/kg body weight and 75 mg/kg body weight, respectively, for clinical signs such as hyperexcitability, tachypnoea, and tremors, which were most probably caused by an acute effect of resorcinol on the CNS. No gross or microscopic lesions were seen.
In a 13-week study in F344 rats and B6C3F1 mice, LOAELs for adrenal gland weight were in the range of 28–32 mg/kg body weight and the NOAEL for liver weight was 32 mg/kg body weight (dosing 5 days/week), without a clear dose–response. The highest dose levels (420–520 mg/kg body weight) caused tremors and increased mortality. No differences were seen in haematology or clinical chemistry, and no gross or microscopic lesions in dosed animals were found.
No signs of carcinogenicity were seen in male F344 rats and B6C3F1 mice of both sexes dosed with 0–225 mg/kg body weight and female rats exposed to 0–150 mg/kg body weight for 5 days/week for 104 weeks (NTP, 1992). Clinical signs of ataxia and tremors were noted at about 100 mg/kg body weight, but no differences in haematology, clinical chemistry, or other clinical pathology parameters were seen. There was a NOAEL of 50 mg/kg body weight for acute clinical signs indicative of effects on the CNS. A study with transgenic CB6F1-Tg rasH2 mice gavaged with 0 or 225 mg/kg body weight 5 days/week for 24–26 weeks showed only a slight, non-significant increased incidence of adenomas in the lungs. Negative results were mostly reported in the initiation–promotion studies performed using several species. However, three studies using nitrosamines as the initiator showed increased tumour incidences.
In bacterial mutagenicity assays, resorcinol showed mostly negative results. However, it induced mutations in the TK locus in mouse lymphoma cells. Resorcinol did not induce unscheduled DNA synthesis in hepatic cells or single-strand DNA breaks in mammalian cells in vitro. Studies for SCE and chromosomal aberrations in vitro in isolated cells and cell lines gave both negative and positive results. Cytogenetic studies in vivo (micronuclei in bone marrow in rats and two strains of mice; SCE in male and female rats) gave consistently negative results.
In a dose range-finding drinking-water study in male and female rats dosed continuously with resorcinol up to 360 mg/l for a minimum of 28 consecutive days prior to mating, no adverse effects concerning reproductive performance, mortality, and body or organ weights were observed (RTF, 2003). In the following two-generation drinking-water study, doses of 0, 120, 360, 1000, or 3000 mg/l were administered. A NOEL of 1000 mg/l and a NOAEL of 3000 mg/l for parental systemic and reproductive toxicity as well as neonatal toxicity were derived. When expressed on a body weight basis (average of F0 and F1 animals), the NOAEL corresponded to approximately 233 mg/kg body weight per day for males over the entire generation, 304 mg/kg body weight per day for females during premating and gestation, and 660 mg/kg body weight per day for females during lactation (RTF, 2005). A battery of neurotoxicological tests was included in the reproductive dose range-finding study, but no effects in tests other than the locomotor activity test in male offspring were observed.
Earlier studies with pregnant rats and rabbits had also shown no effects on developmental toxicity. Dosing of rats via gavage at up to 500 mg/kg body weight on gestation days 6–15 caused no embryotoxicity and no adverse effects on mean numbers of corpora lutea, total implantations, viable fetuses, or mean fetal body weights. There was also no increase in fetal anomalies or malformations. Slight maternal toxicity (weight loss at 24 h with decrease in maternal weight gain at 72 h) was seen in rats in a further study at doses of >667 mg/kg body weight.
Effects on the thyroid gland have been described in 30-day and 12-week drinking-water studies in rats at a dose of 5 mg/kg body weight per day. No histopathological changes in the thyroid were found in subacute, subchronic, or chronic studies performed via gavage in rats or mice; however, T3/T4 levels were not determined, with the exception of the 0 and 130 mg/kg body weight dose groups in the 13-week rat study. In the long-term study (104 weeks), NOAELs for thyroid effects were 150–520 mg/kg body weight per day (5 days/week); however, these studies were not designed to investigate this end-point. In a one-generation dose range-finding drinking-water study, male and female rats were dosed continuously with resorcinol at up to 360 mg/l (males: 1, 4, 13, or 37 mg/kg body weight per day; females: 1, 5, 16, or 47 mg/kg body weight per day). Some effects on the thyroid gland were reported, but they were inconsistent, not statistically significant, and not dose related (RTF, 2003). In the two-generation drinking-water study (RTF, 2005), no statistically significant resorcinol-related changes in the mean concentrations of T3, T4, or TSH were observed in the F0 and F1 parental animals or in the F1 and F2 pups selected for analysis (PND 4 or PND 21). Higher TSH values were noted in the F0 males at scheduled necropsy, but these were not considered as resorcinol-related effects in the absence of effects on T3 or T4, organ weights, or adverse macroscopic or microscopic findings. Test article-related decreased colloid within the thyroid glands of the 3000 mg/l F0 males was not considered to be adverse due to a lack of associated functional effects.
Resorcinol administered at high doses to rodents can disrupt thyroid synthesis and produce goitrogenic effects. There are species-specific differences in synthesis, binding, and transport of thyroid hormones that complicate interpretation of goitrogenesis.
In vitro studies indicate that the anti-thyroidal activity observed following resorcinol exposure is due to the inhibition of thyroid peroxidase enzymes, as evidenced by disruption of thyroid hormone synthesis and changes in the thyroid gland consistent with goitrogenesis.
In humans, exposure to resorcinol has been associated with thyroid effects, CNS disturbances, and red blood cell changes. Dermal sensitization to resorcinol has been well documented, but in practice it is rare; the available data do not allow assessment of the sensitization potency.
There are two toxicological effects that could be used for deriving a tolerable intake: thyroidal and neurological effects. Both these effects have been reported in human case-reports from dermal application of high concentrations (up to 50%) of resorcinol in ointments for ulcers and in peelings, as well as in rodent studies at high concentrations. There is no rodent study covering both end-points adequately.
The human data describing thyroidal and neurological effects were case-reports giving only estimates of exposure and are therefore inadequate to provide a tolerable intake.
For this reason, the study chosen to derive a tolerable intake was the long-term NTP (1992) study in which a NOAEL of 50 mg/kg body weight per day (about 36 mg/kg body weight per day after correcting for 5 days/week dosing) for neurological effects (acute clinical signs) was derived. No histopathological changes were seen in the thyroid. There was no measurement of T3/T4 ratio. Application of uncertainty factors for interspecies (10) and intraspecies (10) differences results in a tolerable intake of 0.4 mg/kg body weight per day.
In a worst-case exposure study in human volunteers using 2% anti-acne cream, no thyroidal effects (i.e. no alterations in T3/T4/T7/TSH levels) were seen at a dermal dose of 12 mg/kg body weight per day (estimated systemic dose levels of 0.4 mg/kg body weight per day).
Therefore, the tolerable intake of 0.4 mg/kg body weight per day derived from the NTP (1992) study would be protective for both neurological and thyroidal effects.
From valid test results available on the toxicity of resorcinol to various aquatic organisms, resorcinol can be classified as being of low to high toxicity in the aquatic compartment. The lowest NOEC was determined for Daphnia magna in a full life cycle toxicity test based on measured concentrations (21-day NOEC = 172 µg/l). However, higher concentrations were not tested, so the actual NOEC is likely to be higher. Nethertheless, a PNECaqua of 3.4 µg/l can be derived using an assessment factor of 50 according to the EU Technical Guidance Document (EC, 2003a), as results from chronic studies from two trophic levels (fish and daphnia) are available.
Using this PNEC value and PEC values for surface water, the risk (PEC/PNEC) from resorcinol for the aquatic environment (surface water) was estimated.
For regional surface waters, calculations showed a low risk. The rubber industry is the largest consumer of resorcinol. The PEC/PNEC value indicates a risk for surface waters, assuming that the wastewater of the rubber production sites is connected to a wastewater treatment plant. If this is not the case, the calculated risk from rubber industry effluent would be increased.
Applications as hair dyes and pharmaceuticals result in a low probability for negative effects on the surface water ecosystem. In contrast, at local point sources, such as at sites where hair dyes are formulated, a risk cannot be excluded using the conservative approach. However, in sewage treatment plants, as indicated by a simulation test, there is a higher removal of resorcinol, which would result in a reduced calculated risk.
In conclusion, there may be a risk from resorcinol in the aquatic environment from sites where hair dyes are formulated and from rubber production plants.
The data availability for toxicity to terrestrial organisms is not sufficient for a quantitative risk assessment. However, an estimation of risk using the equilibrium partitioning method can be made. Using this method, a low risk was found for the regional soil compartment, but a risk at local point sources cannot be excluded.
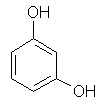
Resorcinol (CAS No. 108-46-3) is a white crystalline compound with a weak odour and a bittersweet taste (Schmiedel & Decker, 2000). It has the chemical formula C6H6O2, and its relative molecular mass is 110.11. The IUPAC name is 1,3-dihydroxybenzene; other names are 1,3-benzenediol, .-benzenediol, .-dihydroxybenzene, .-hydroquinone, 3-hydroxyphenol, and resorcin. Resorcinol can be regarded as a phenol derivative in which a hydrogen atom is substituted by a hydroxyl group in the meta.position to the OH. Its chemical structure is shown in Figure 1.
Resorcinol exists in at least two crystalline modifications (phases) (Kofler, 1943). At normal pressure, the alpha-phase is stable below about 71 °C, whereas the beta-phase is stable above that temperature up to the melting point (Schmiedel & Decker, 2000). Crystalline resorcinol turns pale red in the presence of air and light (Kirk-Othmer, 1981; O’Neil, 2001) and is hygroscopic (Health Council of the Netherlands, 2004). The water solubility data indicate that resorcinol is almost completely miscible in water. The pKa values of 9.32 and 9.81 (at 25 °C) indicate that resorcinol is present almost entirely in the protonated form under environmental conditions (pH 5–8). At pH 8, less than 2% of resorcinol is ionized; at pH 5, less than 0.1% is ionized.
Technical-grade resorcinol is available with a purity of a minimum of 99.5% and contains phenol, catechol, .-cresol, .-/.-cresol, and 3-mercaptophenol (maximum 0.1% each) as impurities (Schmiedel & Decker, 2000). In older studies, two commercial products were mentioned: flaked and industrial. This distinction is no longer made.
The physicochemical properties of resorcinol are summarized in Table 1. Additional physicochemical properties for resorcinol are presented in the International Chemical Safety Card (ICSC 1033) reproduced in this document. Conversion factors3 at 101.3 kPa and 20 °C are as follows: 1 ppm = 4.57 mg/mł; 1 mg/mł = 0.219 ppm.
Table 1: Physical and chemical properties of resorcinol.
|
Property |
Value/range |
Reference |
|
Melting point (°C) |
109–111 |
O’Neil (2001) |
|
110 |
Kirk-Othmer (1981) |
|
|
Boiling point (°C at 101.3 kPa) |
277 |
Kirk-Othmer (1981) |
|
280 |
O’Neil (2001) |
|
|
Density, solid (g/cm3 at 20 °C) |
1.272 |
O’Neil (2001) |
|
alpha-phase: 1.278 |
Schmiedel & Decker (2000) |
|
|
beta-phase: 1.33 |
Kirk-Othmer (1981) |
|
|
Vapour pressure |
0.065 (extrapolated) |
Yaws (1997) |
|
0.027 (measured) |
Hoyer & Peperle (1958) |
|
|
Water solubility |
717 g/l (at 25 °C) |
Yalkowsky & Dannenfelser (1992) |
|
141 g/100 g water (at 20 °C) |
Schmiedel & Decker (2000) |
|
|
1 g/0.9 g water |
O’Neil (2001) |
|
|
Henry’s law constant |
4.96 × 10−9 a |
Staudinger & Roberts (1996) |
|
4.21 × 10−9 a |
Fh-ITEM (2005b) |
|
|
Log octanol/water partition coefficient (log .ow) |
0.8 (measured) |
Hansch et al. (1995) |
|
0.93 (measured at 20 °C) |
Beezer et al. (1980) |
|
|
0.85 (measured at 25 °C) |
Beezer et al. (1980) |
|
|
Soil sorption coefficient (.oc) |
10.36 (measured) |
Boyd (1982) |
|
pKa1 (at 25 °C) |
9.32 |
Serjeant & Dempsey (1979) |
|
9.81 |
Lide (1995) |
|
a |
Calculated from vapour pressure/water solubility estimations, according to EC (2003a). This method is limited to substances of low water solubility. For water-miscible compounds, direct measurement is recommended. However, direct measurements were not available. |
In general, dihydroxybenzenes can be determined by gas chromatography using a capillary column and by liquid chromatography. Semiquantitative determination of dihydroxybenzenes by thin-layer chromatography gives detection limits of 0.008–4 µg, depending on which reagent spray is used (Kirk-Othmer, 1981). For quantitative analysis of resorcinol, high-performance liquid chromatography and gas chromatography are suitable (Dressler, 1994). Curtis & Ward (1981) used the direct photometric method for phenol described in APHA et al. (1976) for measuring the concentration in aquatic toxicity tests.
Table 2 summarizes the most commonly used methods to quantify resorcinol in environmental and biological samples.
Table 2: Determination of resorcinol in environmental and biological samples.
|
Sample matrix |
Sample preparation |
Separation/ |
Limit of detection |
References |
|
Environmental samples |
||||
|
Air |
Sampler: XAD-7 OVS tube, glass fibre filter |
GC/FID |
2 µg/sample (estimated) |
Eide (1994); NIOSH (1998) |
|
Water |
Filtration, extraction (methyl isobutyl ketone), derivatization (trimethylsilylation) |
GC/FID |
0.1 mg/l |
Cooper & Wheatstone (1973) |
|
Water (e.g. leachate) |
Filtration (0.45 µm); extraction (diethyl ether); dissolved in acetonitrile |
HPLC/UV-VIS |
4.3 ng injected (UV) |
Sooba et al. (1997) |
|
Water (leachate, wastewater) |
No data |
HPLC |
No data |
Kahru et al. (1998, 1999) |
|
Soil (water-extractable compounds) |
Aqueous extract |
HPLC/ECD |
0.002 mg/kg |
Kahru et al. (2002) |
|
Soil (water-extractable compounds) |
Aqueous extract |
HPLC |
No data |
Põllumaa et al. (2001) |
|
Soil |
Centrifugation, filtration of the aqueous phase |
HPLC/UV-VIS |
<3 mg/l |
Boyd (1982) |
|
Soil (soil–plant) |
Aqueous soil–plant mixture, filtration, centrifugation, extraction (ether), concentrate, dissolved in ethanol |
Paper chromatography, TLC, GC/FID |
No data |
Chou & Patrick (1976) |
|
Food (ground roast barley) |
Extraction with 50% aqueous methanol; purification through column chromatography, trimethylsilylation |
GC/MS; main peaks of GC further purified by column chromatography and TLC |
No data |
Shimizu et al. (1970) |
|
Food (molasses) |
Fractionation; trimethylsilylation |
GC |
No data |
Hashizume et al. (1967) |
|
Biological samples |
||||
|
Urine, plasma |
Extraction with diethyl ether, concentrate, trimethylsilylation (for GC/MS) |
HPLC/UV-VIS |
HPLC: 0.5 mg/l |
Yeung et al. (1981, 1983) |
ECD, electron capture detection; FID, flame ionization detection; GC, gas chromatography; HPLC, high-performance liquid chromatography; MS, mass spectrometry; TLC, thin-layer chromatography; UV, ultraviolet; UV-VIS, ultraviolet-visible spectrum detection
The resorcinol moiety has been found in a wide variety of natural products. In particular, the plant phenolics, of which resorcinol ring-containing constituents are a part, are ubiquitous in nature and are well documented. Resorcinol itself has been found in the broad bean (Vicia faba), detected as a flavour-forming compound in the honey mushroom (Armillaria mellea) (Dressler, 1994), and found in exudates of seedlings of the yellow pond lily (Nuphar lutea) (Sütfeld et al., 1996). Resorcinol has also been found in extracts of tobacco leaves (Dressler, 1994) and is a component of tobacco smoke (see section 6). In terms of resorcinol derivatives, resorcinol ethers are components of fragrance agents, and there is considerable literature on long-chain alk(en)yl resorcinols in plants and bacteria (Dressler, 1994).
Resorcinol is a monomeric by-product of the reduction, oxidation, and microbial degradation of humic substances. Humic substances are also present in coals, shales, and possibly other carbonaceous sedimentary rocks. This occurrence may explain the detection of resorcinol in wastewater effluents of coal conversion processes due to thermal breakdown (Cooksey et al., 1985). Chou & Patrick (1976) found resorcinol in some samples as a decomposition product of corn residues in soil.
Resorcinol is produced commercially worldwide in only a few specialized plants. All of these plants use benzene as the main feedstock, and only two production routes are used commercially on a large scale. Resorcinol is produced either via sulfonation of benzene under conditions promoting disubstitution in the meta position followed by fusion with anhydrous caustic ("classical" route via 1,3-benzenedisulfonic acid) or via hydroperoxidation of 1,3-diisopropylbenzene (Dressler, 1994; Schmiedel & Decker, 2000; CEH, 2001). Resorcinol is also a by-product of meta-amino phenol manufacture, as produced from metanilic acid fused with sodium hydroxide (T. Chakrabati, personal communication).
In Japan, resorcinol is produced in two plants (Sumitomo Chemical and Mitsui Petrochemical) via 1,3-diisopropylbenzene. The United States produces it in one plant (INDSPEC Chemical Corporation), using the "classical" route via 1,3-benzenedisulfonic acid (Dressler, 1994; Schmiedel & Decker, 2000; CEH, 2001). The same route was used by Hoechst AG (Germany), although production ceased in 1991 (Hoechst AG, 1992; CEH, 2001). According to CEH (2001), there are also three small-capacity plants located in China and four in India.
The total worldwide consumption of resorcinol was given as about 40 000 tonnes in 1990 (Schmiedel & Decker, 2000) and 44 800 tonnes in 2000 (see Table 3; CEH, 2001; EC, 2002), suggesting a slight increase over the decade. The total imports into Western Europe for 2000 are estimated to be 14 800 tonnes, with 1100 tonnes being re-exported, and the consumption was given as 13 500 tonnes. The projection for consumption in 2005 for Western Europe was approximately 12 700 tonnes (CEH, 2001; EC, 2002).
Table 3: Annual consumption of resorcinol by application and region in 2000.a
|
Application |
Annual consumption (tonnes) |
% |
||||
|
Western Europe |
United States |
Japan |
Other regions |
Total |
||
|
Rubber products |
6 480 |
10 271 |
1 598 |
5 470 |
23 820 |
53.2 |
|
Wood adhesives |
2 700 |
1 820 |
572 |
2 280 |
7 373 |
16.5 |
|
Flame retardants |
2 100 |
1 222 |
250 |
500 |
4 072 |
9.1 |
|
UV stabilizers |
1 000 |
588 |
120 |
200 |
1 908 |
4.3 |
|
Dyes |
300 |
350 |
230 |
750 |
1 630 |
3.6 |
|
Meta-amino phenols |
0 |
0 |
1 880 |
0 |
1 880 |
4.2 |
|
Hair dyes |
150b |
150 |
75 |
75 |
450 |
1.0 |
|
Pharmaceuticals |
75 |
75 |
50 |
25 |
225 |
0.5 |
|
Others |
695 |
323 |
875 |
1 550 |
3 443 |
7.7 |
|
Total |
13 500 |
14 799 |
5 650 |
10 850 |
44 801 |
100 |
|
a |
From EC (2002), adapted from CEH (2001) and producer sources. |
|
b |
This figure has recently been corrected to 90 tonnes (Resorcinol Task Force, personal communication, 2005). |
A detailed description of the uses of resorcinol is given in Dressler (1994). The largest user of resorcinol is the rubber industry (about 50%). Resorcinol is the essential component of an adhesive system, together with formaldehyde and synthetic rubber latex, used in the manufacture of tyres for passenger cars, trucks, off-road equipment, and other fibre-reinforced rubber mechanical goods, such as conveyor and driving belts. Resorcinol is also used for high-quality wood bonding applications (about 25%) in adhesives formulated from resorcinol–formaldehyde resins or phenol-modified resorcinol–formaldehyde resins for use, for example, under conditions of extreme heat or moisture. Resorcinol is an important chemical intermediate in the manufacture of speciality chemicals, such as hexylresorcinol, .-aminosalicylic acid, and light screening agents for the protection of plastics from exposure to UV light. Other uses include the manufacture of dyestuffs, pharmaceuticals, flame retardants, agricultural chemicals, fungicidal creams and lotions, explosive primers, antioxidants, a chain extender for urethane elastomers, and a treatment to improve the mechanical and chemical resistance of paper machine fabrics (Schmiedel & Decker, 2000; CEH, 2001).
Although of comparatively low tonnage, the use of resorcinol in oxidative hair dyes and anti-acne creams and peeling agents is relevant for consumer exposure. A total of 150 tonnes of resorcinol was used in oxidative hair dyes by the cosmetics industry in the year 2000 (COLIPA survey, cited in HCTS, 2002). In oxidative hair dyes, resorcinol is regulated to 5% or below (EC, 2003b); in practice, however, many manufacturers limit the level of free resorcinol in oxidative hair dyes to 1.25% (EC, 2002). Resorcinol is limited to 0.5% in shampoos and hair lotions (EC, 2003b). Resorcinol is used in pharmaceutical preparations for the topical treatment of skin conditions such as acne, seborrhoeic dermatitis, eczema, psoriasis, corns, and warts. Resorcinol is usually present in anti-acne preparations at a maximum concentration of 2%. The concentration of resorcinol can be much higher in peels, in some cases around 50% (Karam, 1993; Hernández-Pérez & Carpio, 1995; Hernández-Pérez, 1997, 2002; Hernández-Pérez & Jáurez-Arce, 2000; see also sections 6 and 9). Jessner’s solution (resorcinol in ethyl alcohol, 14% w/v; lactic acid, 14%; and salicylic acid, 14%) is commonly used in chemical peeling.4 A specialized medical use of resorcinol is in biological glues (gelatin–resorcinol–formaldehyde glue) for cardiovascular surgery, in particular aortic operations (Bachet & Guilmet, 1999; Kazui et al., 2001; von Oppell et al., 2002).
Resorcinol is released into the environment during production and processing. It will also be released directly during uses and disposal of resorcinol-containing consumer and professional products. Furthermore, resorcinol can appear as a degradation intermediate of other anthropogenic environmental contaminants, especially resorcinol derivatives. For example, resorcinol was detected as an intermediate in the anaerobic degradation of .-methoxyphenol (Boyd et al., 1983) and as an irradiation product of 3-chlorophenol in aqueous solution (Boule et al., 1982).
Owing to the low vapour pressure and high water solubility of resorcinol, the releases during production, formulation, and use of resorcinol are mainly via the hydrosphere (see section 5). Release into air via dust can occur during the life cycle steps of production or industrial use (e.g. as an intermediate) and is relevant only for occupational exposure, owing to resorcinol’s short half-life in air (indirect photochemical degradation).
No measurements of resorcinol releases during production, use, and disposal or recent resorcinol concentrations in the effluent of wastewater treatment plants are available. Thus, the emissions of resorcinol primarily into the hydrosphere and atmosphere during the life cycle steps of production or industrial use have to be estimated.
Production plants are point sources for releases of resorcinol, which is produced in only a few specialized plants. Although no quantifications exist, releases from production processes of less than 0.05% would be expected (RTF, 2002). Using this estimate of 0.05% and annual consumption of 44 800 tonnes, the global releases would be 22.4 tonnes per year, with a European contribution of 6.75 tonnes per year. At least some manufacturers operate a "no release" policy for aqueous waste streams. According to the generic tables of the EU Technical Guidance Document (EC, 2003a), for chemicals with a production volume of >1000 tonnes per year, the fraction of the wastewater released during production is estimated at 0.3%. The release of resorcinol into air is 0% and into soil 0.01%. For Germany, estimated releases into wastewater during production were 33 tonnes in 1991 (Hoechst AG, 1992; BUA, 1993).
The Resorcinol Task Force estimated the releases of resorcinol during its life cycle steps, and the results were published in EC (2002). The figures, which illustrate the releases per use pattern and compartment, are reproduced as Figures 2 and 3. As a result of this estimation, the uses in the rubber industry and as a wood adhesive are the most relevant for air releases. For the water compartment, releases from the use of resorcinol in hair dyes and pharmaceuticals are the most important.
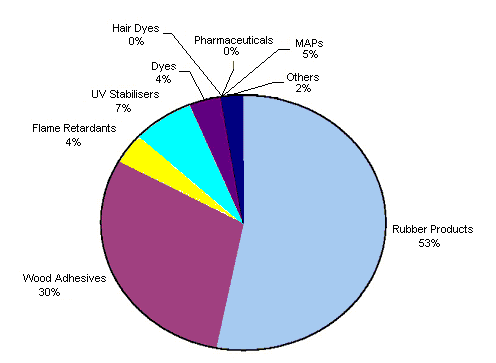
Fig. 2: Resorcinol losses to air for Western Europe (total 2.8 tonnes per year, 0.02% of the total yearly consumption)
(EC, 2002).
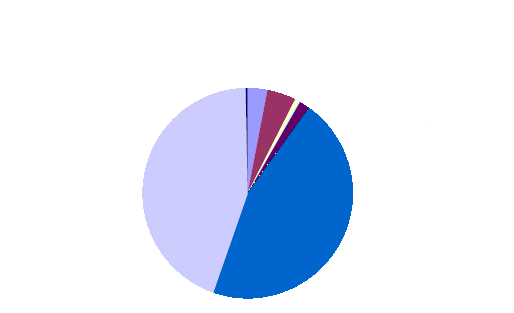
Fig. 3: Resorcinol losses to water for Western Europe (total 168.7 tonnes per year, 1.25% of the total yearly consumption)
(EC, 2002).
In the rubber industry, which consumes the highest tonnage of resorcinol, the percentage loss of resorcinol during production of tyres is around 0.1%. Most of the resorcinol lost in the processing of tyres is removed from the extraction air by water-based scrubbers (resorcinol is highly soluble) and then treated off site at wastewater treatment plants. Assuming that the scrubbers are at least 80% effective, the total amount of resorcinol reaching European wastewater plants from this source would be around 5 tonnes annually, with a further 1.5 tonnes possibly reaching the atmosphere (EC, 2002). According to the OECD emission scenario document on additives in the rubber industry (OECD, 2004), the percentage of processing aids (bonding agents) remaining in the rubber product is 99.9%. Thus, the release into wastewater can be estimated to be 0.1% (equal to 6.48 tonnes per year). However, for the releases into air and soil, the A-Tables of the EU Technical Guidance Document (EC, 2003a) can be consulted (IC11 "Polymer industry") according to OECD (2004), resulting in releases into air of 0.1% (equal to 6.48 tonnes per year) and into soil of 0.05% (equal to 3.24 tonnes per year). Further releases are the result of tyre abrasion and emissions from leachates of landfills. No resorcinol has been detected in leachates of cured rubber and at further extraction works. Although work continues on this issue, it is impossible to identify any meaningful mechanism for the release of resorcinol from cured rubber. Accordingly, no emissions can currently be ascribed to in-use or end-of-life phases of resorcinol in rubber tyres (EC, 2002).
Although the percentage use as hair dyes and pharmaceuticals from the total tonnage is only 1% and 0.5%, respectively (see Table 3), these releases seem to be the most relevant. Since hair dyes are manufactured in a closed process under vacuum, there are no losses to the atmosphere. However, losses in aqueous wastewater resulting from batch processing can amount to 1% because of the relatively small batch sizes used (EC, 2002). This represents 1.5 tonnes of the 150 tonnes used by the industry annually in Western Europe.
Concerning consumer usage of hair dyes, approximately all non-reacted resorcinol is rinsed off to the wastewater after the 30-min period of typical use as hair dyes. Estimates of non-reacted resorcinol range from 52% to 72% (Tsomi & Kalopissis, 1982; EC, 2002; HCTS, 2002). In addition, the amount of residual in the packages, which is disposed of with waste or wastewater, has to be considered. According to the cosmetics industry, the amount that may enter the wastewater can be estimated to be approximately 70–80 tonnes per year for Western Europe (EC, 2002; HCTS, 2002).
For pharmaceutical applications such as topical ointments, it is assumed, as a worst case, that 100% of the resorcinol (75 tonnes for Western Europe) reaches the wastewater stream, either directly or from the output of domestic landfills (EC, 2002).
Disposal methods include complete incineration, land (soil) farming, and decomposition in activated sludge-type wastewater treatment plants. All disposal practices should be carefully evaluated for compliance with applicable local, state, and federal regulations (Dressler, 1994). Specific waste data for production in Germany or use as intermediates are available in BUA (1993).
Using the model calculation Mackay Level I (distribution of a substance in a unit world under steady-state conditions), the following distribution of resorcinol in different environmental compartments was predicted: air, <0.01%; water, 99.9%; sediment, 0.05%; soil, 0.05%; biota, <0.01% (Fh-ITEM, 2005a).5 According to the calculation, the hydrosphere is predicted to be the main target compartment.
Based on the calculated dimensionless Henry’s law constant of 4.21 × 10−9 (Fh-ITEM, 2005b), resorcinol can be classified as essentially non-volatile from aqueous solution, according to the scheme of Thomas (1990).
Soil sorption studies on resorcinol (5–50 mg/l) using silty loam (organic matter 5.1%; pH 5.7; temperature 20 °C) revealed a measured organic carbon-normalized partition coefficient (.oc) of 10.36 (Boyd, 1982). According to Litz (1990), a very low soil sorption is to be expected.
Experimental data on the phototransformation of resorcinol in air are not available. However, crystalline resorcinol turns pale red in the presence of air and light (O’Neil, 2001). A direct photodegradation of resorcinol is not to be expected, as the substance does not absorb sunlight at wavelengths above 295 nm to a significant extent (lambdamax = 274 nm; epsilonmax = 2000 l/mol·cm3; Perbet et al., 1979). The indirect photochemical degradation in air by hydroxyl radicals was calculated via AOPWIN v.1.91 to have a half-life of about 2 h using 500 000 hydroxyl radicals/cm3 as a 24-h average (Fh-ITEM, 2004).
Owing to the type of chemical structure of resorcinol, it is not possible to calculate the hydrolysis rate constant via HYDROWIN v.1.67 (Fh-ITEM, 2004). However, resorcinol possesses no functional groups susceptible to hydrolysis under environmentally relevant conditions, so hydrolysis is not expected to occur (Harris, 1990).
Photolysis and photo-oxidation of resorcinol take place in dilute aqueous solution by reaction with oxygen (Perbet et al., 1979). Trihydroxybenzene and hydroxybenzoquinone were identified as reaction products. In the presence of ozone, resorcinol can be degraded in aqueous solution via pyrogallol (1,2,3-trihydroxybenzene) and 3-hydroxybenzoquinone to glyoxalic acid, glyoxal, oxalic acid, carbon dioxide, and water (Leszczynska & Kowoal, 1980). Moussavi (1979) determined a half-life of 1612 h (= 67 days) for the autoxidation of resorcinol in aqueous solution at 25 °C and pH 9. By analogy with other phenolic compounds (resorcinol can be regarded as a derivative of phenol; see section 2), resorcinol should react in water bodies with hydroxyl and peroxyl radicals. For phenol and hydroquinone, half-lives of 100 and 20 h, respectively, with hydroxyl radicals as sensitizer and half-lives of 19 and 0.2 h, respectively, with peroxyl radicals were determined (Mill & Mabey, 1985). Shen & Lin (2003) studied the decomposition of resorcinol by 254-nm UV direct photolysis and by the UV–hydrogen peroxide process in aqueous solution. The light absorbance and photolytic properties were highly dependent on solution pH. In acidic and neutral solution (pH 3–7), resorcinol was predominantly decomposed by reaction with hydroxyl radicals; the contribution of this degradation path was about 99% of the total decomposition. Direct photolysis was relevant only at pH values >9. Based on the experimentally determined rate constant (kOH = 1.4862/min at 25 °C and pH 7), a half-life of 0.5 min can be calculated.
The relevant studies for the assessment of the biodegradation are summarized in Table 4. Resorcinol proved to be biodegradable under aerobic and anaerobic conditions.
Table 4: Aerobic and anaerobic biodegradation of resorcinol.
|
Procedure |
Inoculum/test substance |
Result |
Reference |
|
Aerobic degradation |
|||
|
OECD TG 301C "Ready Biodegradability: Modified MITI Test I" |
Activated sludge, 30 mg/l (suspended solids) per 100 mg resorcinol/l |
66.7% degradation after 14 days |
MITI (1992) |
|
OECD TG 302B "Inherent Biodegradability: Modified Zahn-Wellens Test" |
Activated sludge, adapted 1.1 g/l (dry weight) per 50–400 mg DOC/l or 200–1000 mg COD/l |
97% degradation after 4 days |
Wellens (1990) |
|
Similar to OECD TG 302B "Inherent Biodegradability: Modified Zahn-Wellens Test" |
Activated sludge, adapted 100 mg/l (dry matter) per 200 mg COD/l |
90% after 5 days |
Pitter (1976) |
|
OECD TG 302B "Inherent Biodegradability: Modified Zahn-Wellens Test" |
Activated sludge, adapted |
>90% after 15 days |
Hoechst AG (1992) |
|
Wastewater treatment plant simulation test |
Initial resorcinol concentration: 138 mg/l and 500 mg/l, hydraulic retention time 3 h |
138 mg/l: 95–100% (based on DOC) |
Gubser (1969) |
|
Anaerobic degradation |
|||
|
Serum bottle test (Biochemical Methane Potential) |
Anaerobic sludge, adapted, 500 mg resorcinol/l |
36% degradation after 196 daysa |
Blum et al. (1986) |
|
Serum bottle test (Biochemical Methane Potential) |
Anaerobic sludge, phenol-enriched culture, 500 mg resorcinol/l |
83% after 245 daysb |
Blum et al. (1986) |
|
Serum bottle test |
Anaerobic sludge from two municipal wastewater treatment plants, 100 ml (10% sludge) per 50 mg C/l |
a. 98% degradation after 21 days |
Horowitz et al. (1982) |
|
Submerged anaerobic upflow filter and 2–10 days hydraulic retention times |
Anaerobic sludge, acetate-enriched culture, 90 mg resorcinol/l |
95% degradation after 110 days of acclimation |
Chou et al. (1979) |
|
C, carbon; COD, chemical oxygen demand; DOC, dissolved organic carbon |
|
|
a |
At concentrations of 1000 and 2000 mg/l, no degradation observed. |
|
b |
At a concentration of 1000 mg/l, 4% was degraded after 245 days; no degradation was observed at 2000 mg/l. |
Based on the results obtained in an aerobic biodegradation test conducted according to OECD TG 301C, resorcinol can be classified as readily biodegradable. After 14 days, a mineralization of 66.7% was measured (MITI, 1992). Furthermore, several studies on inherent biodegradability are available. Elimination rates of >90% were observed after 4–15 days in guideline studies (OECD TG 302B) and modifications thereof (Pitter, 1976; Wellens, 1990; Hoechst AG, 1992). In a wastewater treatment plant simulation test (modified German detergents test), degradation rates of 95–100% were observed based on DOC measurements at an initial resorcinol concentration of 138 mg/l and a hydraulic retention time of 3 h. For an initial concentration of 500 mg/l, the time for adaptation increases; afterwards, the decomposition is >60% (Gubser, 1969).
Resorcinol is likely to be biodegraded under anaerobic conditions. However, the results of the studies are not consistent. Using adapted anaerobic sludge and initial resorcinol concentrations of up to 500 mg/l, degradation rates of 36%, 83%, and 95% were determined, whereas no degradation was observed at concentrations of >1000 mg/l. Degradation with sludge from municipal wastewater treatment plants was 98% or 0% in the same test system, obviously depending on the origin of the used inoculum (Chou et al., 1979; Horowitz et al., 1982; Blum et al., 1986). The potential biodegradability of resorcinol under anaerobic conditions has been confirmed by studies using fixed film–fixed bed reactors or by fermentation (Tschech & Schink, 1985; Latkar & Chakrabarti, 1994).
Resorcinol in aqueous medium can be metabolized by bacteria and fungi via hydroxyhydroquinone (1,2,4-trihydroxybenzene) and maleyl acetate to beta-ketoadipate and via hydroxyhydroquinone and acetyl pyruvate to formic, acetic, and pyruvic acids (Chapman & Ribbons, 1976; Gaal & Neujahr, 1979; Ingle et al., 1985). Another potential pathway is via pyrogallol (Groseclose & Ribbons, 1981). Anaerobic degradation of resorcinol is catalysed by resorcinol reductase and hydratase. The products are 1,3-dioxocyclohexane, which is immediately hydrolysed to 5-oxohexanoate, and 5-oxohex-2-enecarboxylate, respectively. Further degradation probably proceeds via beta-oxidation (Heider & Fuchs, 1997).
The distribution in a sewage treatment plant can be calculated using the model "SimpleTreat", implemented in EUSES 2.0.3 (RIVM, 1996; EC, 2004). The model provides information on how much resorcinol that enters the sewage treatment plant goes to air, surface water, and sewage sludge and how much is degraded. Hence, the log octanol/water partition coefficient and the Henry’s law constant are needed, as well as the rate constant for degradation. The results are presented in Table 5.
Table 5: Distribution of resorcinol in sewage treatment plants (results from "SimpleTreat").a
|
Parameter |
Value |
|
Fraction directed to air by STP (%) |
<10−5 |
|
Fraction directed to water by STP (%) |
12.6 |
|
Fraction directed to sludge by STP (%) |
0.0977 |
|
Fraction degraded by STP (%) |
87.3 |
|
Total removal by STP (%) |
87.4 |
|
STP = sewage treatment plant; log octanol/water partition coefficient (.ow) = 0.8, dimensionless Henry’s law constant (H) = 4.21 × 10−9, rate constant for degradation (kbiostp) = 1/h |
|
|
a |
From Fh-ITEM (2005b). |
The percentage of biodegradation in a sewage treatment plant resulting from "SimpleTreat" is a conservative worst-case value. In reality, the fraction of degradation will be significantly higher, indicated by the result from the wastewater treatment plant simulation test (95–100% for a relatively high concentration of 138 mg/l; Gubser, 1969) listed in Table 4.
Experimental test results on bioaccumulation are not available. Based on a log octanol/water partition coefficient of <1 and an estimated BCF of 3.2 (log BCF = 0.5; BCFWIN v.2.15; Fh-ITEM, 2004), a low bioaccumulation is to be expected.
Resorcinol is a monomeric by-product of the reduction, oxidation, and microbial degradation of humic substances (Cooksey et al., 1985). Chou & Patrick (1976) examined the decomposition products of corn and rye residues in soil (incubation at 22–23 °C for 30 days). The soil was sampled in the fall at the Horticulture Experiment Station, Vineland Station, Ontario, Canada. The authors identified, among others, resorcinol at a concentration of <5 µg/g soil via paper chromatography, TLC, and GC/FID at an initial ratio of 400 g soil to 400 g chopped corn. At other ratios of soil to corn and in the decomposition study with rye, no resorcinol was found.
Resorcinol is one of the important pollutants in the effluent waters of chemical, fertilizer, and dye industries (Ingle et al., 1985) and is a typical constituent of coal conversion wastewater. It was identified at the milligram per litre level in the wastewater from a coal liquefaction plant in the United States by UV analysis (Jolley et al., 1975). It was quantified in the range of 176–272 mg/l at the Lurgi gasification facility in Westfield, Scotland, and at levels of 2000 mg/l in an aqueous process stream from the product scrubber of a bench-scale hydrocarbonization coal liquefaction operation (USEPA, 1978a). The typical concentration of resorcinol in coal conversion wastewater is given as 1000 mg/l (USEPA, 1978a; Blum et al., 1986). Resorcinol was detected at concentrations of 7–22 mg/l in the ammoniacal liquid of two typical coking ovens; a resorcinol concentration of 150 mg/l was found in a low-temperature carbonization ammoniacal liquor. However, no resorcinol was detected in the condensate of one oven’s waste gas or the drainage water (Cooper & Wheatstone, 1973).
Phenolic compounds like resorcinol, phenol, cresol, and dimethylphenols have been considered as major pollutants in the oil shale semi-coke dump leachates that contaminate the surrounding soils (Sooba et al., 1997; Kahru et al., 1998, 1999). Resorcinol has been determined in leachate samples associated with the oil shale industry from the north-east of Estonia at concentrations up to 8.7 mg/l (Sooba et al., 1997; Kahru et al., 1998). In wastewater samples from the same region, resorcinol concentrations up to 4.1 mg/l (total phenols 0.7–195 mg/l) have been found (Kahru et al., 1999). However, the amount of water-extractable phenols in the surrounding soils was very low (up to 0.7 mg/kg; Põllumaa et al., 2001). In a selected soil sample (polluted by leachates) with a relatively high concentration of steam-distillable phenols (43 mg/kg), the amount of resorcinol was quantified as <0.04 mg/kg and thus could be considered negligible (Kahru et al., 2002).
There are only a few recently measured concentrations of resorcinol in air, water, sediment, and soil, and concentrations in drinking-water or food are not available. However, the concentrations can be estimated using the emission values given in section 4 and a Mackay Level III fugacity model. In the following, the results of such a calculation are presented using EUSES 2.0.3 containing "SimpleTreat" and "SimpleBox" (http://ecb.jrc.it/existing-chemicals/); for further details (e.g. input parameters), see Appendix 5.
For calculating the regional and continental PECs, the model "SimpleBox" (EC, 2004; RIVM, 2004) is used. On the basis of the estimated emissions during the manufacture of rubber products, during the formulation and use of hair dyes, as well as during the use of pharmaceuticals (see Appendix 5), the regional PECs are:
|
PECregionalair |
= 0.458 pg/m3 |
|
PECregionalwater |
= 0.129 µg/l |
|
PECregionalsoil, ind. |
= 0.583 µg/kg dry weight |
Resorcinol is used in the production of rubber products as a bonding agent. Using the specific emission scenario document composed by OECD (2004), releases of 1.1 kg/day to both wastewater and air at the production site can be estimated (see Appendix 5). Considering the connection to a sewage treatment plant, the PECs for air and water are:
|
PEClocalair |
= Clocalair + PECregionalair |
|
= 0.247 µg/m3 |
|
|
PEClocalwater |
= Clocalwater + PECregionalwater |
|
= 7.09 µg/l |
During the formulation of hair dyes, releases of resorcinol to wastewater up to 3.5 kg/day can occur (see Appendix 5), resulting in the following PEC for surface water:
|
PEClocalwater |
= Clocalwater + PECregionalwater |
|
= 22.3 µg/l |
Taking into account a higher removal during sewage treatment (95%), indicated by a simulation test, in the calculation of Clocalwater, the local PEC for surface water is:
|
PEClocalwater |
= Clocalwater + PECregionalwater |
|
= 8.88 µg/l |
Hair dyes and pharmaceuticals are used by professional and private consumers. The worst-case releases are 0.0814 kg/day for hair dyes and 0.0411 kg/day for pharmaceuticals, which are disposed of to the same municipal sewage treatment plant. Hence, the local concentrations are summed, and a combined PEC for surface water is calculated:
|
PEClocalwater |
= Clocalwater, use hair dyes + Clocalwater, use pharmaceutical + PECregionalwater |
|
= 0.904 µg/l |
The results of the calculations show that the highest concentrations are expected to be at local point sources such as at sites where hair dyes are formulated or rubber products are manufactured. These estimated concentrations in water are 1 order of magnitude higher than the local concentrations resulting from emissions from the use of consumer products containing resorcinol, which are released on a continental scale.
There are very few data on occupational exposure.
Concentrations up to 45 mg/m3 (the occupational TWA limit in many countries) were reported in a production plant in the United States from sampling records ranging from 3.5 to 30 min (Flickinger, 1976). From a plant producing resorcinol by sulfonation of benzene — and also producing beta-resorcylic acid, resorcinol–formaldehyde resins, sulfites, and sulfates — 8-h TWA values are available from personal and area measurements for grinders, flaker operators, and operators making pharmaceutical-grade resorcinol. Workers in these groups were exposed primarily to resorcinol, but the exposure to other agents was not measured, and there were also no measurements of resorcinol in other areas of the plant. For 20 samples, the resorcinol concentrations were in a range of 0.6–66 mg/m3, and the distribution of exposure concentrations was given as follows: grinders 2–45 mg/m3 (four personal samples) and 2–66 mg/m3 (four area samples); flaker operators 0.6–2 mg/m3 (four personal samples) and 1–53 mg/m3 (four area samples); operators making pharmaceutical-grade resorcinol 0.7–2 mg/m3 (four personal samples) (Flickinger, 1978).
In a study on rubber workers, exposure to resorcinol was less than 0.3 mg/m3 (Gamble et al., 1976). In the tyre industry, occupational exposure to resorcinol occurs in the weighing, mixing, and preparation areas. Here, typical airborne concentrations are less than 0.1 mg/m3 and remain below 5 mg/m3 (8-h TWA) (EC, 2002).
Hairdressers using oxidative hair dyes are exposed to resorcinol. In a study of skin exposure to resorcinol, it was found that hairdressers do not always use gloves for hair dying, especially when only strands of hair are being dyed. Hair that had been rinsed after dyeing still contained traces of resorcinol, and the hairdressers had hand contact with the hair during cutting and styling of the hair. Resorcinol was found in hand rinse samples (22–738 nmol per hand) in 20 out of 29 hairdressers after cutting newly dyed hair (Lind et al., 2005).
There is a lack of quantitative data on the concentrations of resorcinol in food and drinking-water. Resorcinol and its derivatives are to be found in trace amounts in many natural products and foods to which the consumer is exposed. For example, Japanese mugi-cha tea is made by roasting of barley seeds. Resorcinol has been detected in roast barley (Shimizu et al., 1970), in cane molasses (Hashizume et al., 1967), and as a coffee flavouring compound (Walter & Weidemann, 1968).
Resorcinol has been detected in the mainstream of cigarette smoke at levels ranging from 0.8 to 8 µg per cigarette (Commins & Lindsey, 1956; Rustemeier et al., 2002).
Resorcinol is used in oxidative hair dyestuffs, anti-acne creams, and peels, and these seem to be the most relevant sources of consumer exposure to the compound (see also section 4).
1) Exposure estimate for resorcinol in hair dyes
Oxidation dyes are used in hair dyeing preparations composed of .- or .-phenylenediamines or aminophenols as the precursor (developer) and a dihydroxybenzene such as resorcinol as the coupler. On addition of the oxidant, usually hydrogen peroxide solutions, azine- and oxazine-type dyes are formed (Dressler, 1994, 1999). A resorcinol concentration of 5% is permitted in oxidative hair dyes (Cosmetic Ingredient Review, 2004); in practice, however, many manufacturers limit the level of free resorcinol in oxidative hair dyes to 1.25% (RTF, 2002). In vivo and in vitro studies suggest that only a small amount of resorcinol penetrates the skin during the actual process of hair colouring, but that some of the free compound is retained in the stratum corneum and is made slowly available to the systemic circulation, the time required for 50% excretion of the total excreted dose being 31 h in a human volunteer study (Wolfram & Maibach, 1985) (see section 7). The percentage of total dose excreted over 4 days was 0.076%. Exposure to resorcinol through hair dyeing would be about 30 min every 4 weeks.
Based on a usage volume of 100 ml (50 ml hair dye cream with 5% resorcinol and 50 ml developer), the exposure estimate for resorcinol in hair dyes can be calculated as follows:
|
Maximum content of resorcinol after mixing with developer |
2.5% |
|
Maximum amount of resorcinol applied (in 100 ml) |
2500 mg |
|
Dermal penetration (Wolfram & Maibach, 1985) |
0.076% |
|
Dermal absorption per treatment (2500 mg × 0.076%) |
1.9 mg |
|
Typical body weight of human (IPCS, 1994) |
64 kg |
|
Systemic exposure (1.9/64) |
0.03 mg/kg body weight |
2) Exposure estimate for resorcinol in anti-acne cream
Resorcinol in anti-acne preparations is usually 2%. Anti-acne creams are likely to be used twice a day for an unlimited period, and the preparation remains on the skin and is not washed off, as in the case of hair dyes.
In a human volunteer study to measure absorption and metabolic disposition, 2% resorcinol (800 mg resorcinol per day, a maximal exaggerated-use level) was applied topically in a hydroalcoholic vehicle over an application area of 2600 cm2 twice a day, 6 days a week, for 4 weeks to three male volunteers with one control volunteer (Yeung et al., 1983). Determination of resorcinol conjugates in the 24-h urine samples after 14 days of continuous product treatment showed that a maximal 23 mg (2.87%) of the daily dose was excreted. Assuming a body weight of 64 kg (IPCS, 1994) gives an exposure estimate of 0.4 mg/kg body weight.
In a report based on consumer research data (Gans, 1980), under reasonable maximum-use conditions (i.e. less than 1% of users), topical applications of resorcinol-containing ointments to treat acne would result in exposures of up to 1.2 mg/kg body weight per day (i.e. 77 mg resorcinol per day, assuming a body weight of 64 kg). More usual-use conditions would result in exposures of about 0.2 mg/kg body weight per day. Further details were not given. These figures agree well with the above exposure estimate based on the Yeung et al. (1983) study.
However, it should be considered that acned skin may be damaged due either to scratching or to the blemishes themselves. Therefore, the uptake may be higher than this, with up to 100% absorption in limited small areas, which would increase the daily systemic exposure.
As is well known in dermal absorption studies, the nature of the vehicle has a great influence on the absorption of a compound. Resorcinol seems to be absorbed much better from anti-acne preparations than from the hair dye preparations under normal usage conditions.
3) Exposure estimate for resorcinol in peels
The situation is even more critical in the case of resorcinol used in peels. Although resorcinol is not used or permitted in cosmetic surgery in many countries, it is still used in others, as can be seen from recent publications (Karam, 1993; Hernández-Pérez, 2002). In peeling, the compound, in concentrations up to about 50% alone or in combination with other agents, is purposely used to wound and disrupt the epidermis (Coleman, 2001). Although the application time is short (30 s to 10 min) and the "peel" is removed immediately, resorcinol could be 100% absorbed in this time. In some procedures, a series of 6–10 sessions 1 or 2 weeks apart are performed (Hernández-Pérez, 2002).
Assuming that 1000 mg is applied (SCCNFP, 2003), the exposure estimate for resorcinol with peeling can be calculated as follows:
|
Amount of peel applied per treatment |
1000 mg |
|
Amount of resorcinol in peel or treatment |
500 mg resorcinol per treatment |
|
Typical body weight of human (IPCS, 1994) |
64 kg |
|
Systemic exposure assuming 100% absorption |
7.8 mg/kg body weight |
A summary of these exposure scenarios is given in Table 6.
Table 6. Summary of estimated human exposure to resorcinol from cosmetic and hair dye products.
|
Product |
Reference of study used |
Amount of product applied |
Resorcinol content in product (%) |
Maximum amount of resorcinol applied (mg) |
Estimated % absorption |
Estimated exposure (mg/kg body weight) |
Estimated duration; frequency of application |
|
Hair dye |
Wolfram & Maibach (1985) |
100 ml |
2.5 |
2500 |
0.076 |
0.03 |
30 min; once a month |
|
Anti-acne cream |
Yeung et al. (1983) |
40 ml (worst case) |
2 |
800 |
2.87 |
0.4 |
Every day |
|
Peels |
Hernández-Pérez (2002) |
1 g (estimated) |
50 (worst case) |
500 |
100 |
7.8 |
30 s to 10 min; maximum 10 sessions 2 weeks apart |
In three rabbits dosed orally with resorcinol at 100 mg/kg body weight, 13.5% of the applied dose was excreted as monosulfate, 52% as monoglucuronide, and 11.4% as free resorcinol via urine within 24 h. Trihydroxybenzenes were not detected (Garton & Williams, 1949).
In F344 rats (. = 3 per sex), resorcinol with a purity of 97% was readily absorbed, rapidly metabolized, and excreted after single oral dosing with [14C]resorcinol at 112 mg/kg body weight. Within 24 h, most of the applied dose was excreted via urine (90.8–92.8%) and faeces (1.5–2.1%). The remaining 14C activity in blood and main tissues such as liver, skin, fat, muscle, large intestine content and also thyroid gland gave no indication of bioaccumulation. There were no significant sex differences. At least 50% of the excreted dose undergoes enterohepatic circulation, to be eventually excreted via urine. The major metabolite (about 65%) was a glucuronide conjugate, and minor metabolites included a monosulfate conjugate, a mixed sulfate–glucuronide conjugate, and a diglucuronide conjugate. In females, a greater proportion was excreted as sulfate conjugate, whereas males excreted a higher proportion of a diconjugate (both sulfate and glucuronide groups). From these data, the authors concluded that male rats have a higher capacity for glucuronidation than females. After dosing with 225 mg/kg body weight or daily doses of 225 mg/kg body weight for 5 consecutive days, comparable results were obtained (Kim & Matthews, 1987).
After single subcutaneous dosing of male Sprague-Dawley rats with [14C]resorcinol at 10, 50, or 100 mg/kg body weight, the 14C activity in plasma decreased rapidly (approximately 90% clearance within the first 2 h post-administration). The elimination was biphasic, with half-lives of 18–21 min and 8.6–10.5 h. Within 24 h after dosing with 10 mg/kg body weight, 98% of the applied dose was excreted via urine and 1% via faeces, mainly as glucuronide conjugate (84%). The 14C activity was rapidly distributed in major tissues such as muscles, kidneys, and liver, without indication of bioaccumulation (Merker et al., 1982).
In one female patient with leg ulcers treated dermally for 13 years with large amounts (~500 g/week) of an ointment containing 12.5% resorcinol, 2.1% of the applied dose was found in urine as glucuronide and monosulfate metabolites (Thomas & Gisburn, 1961).
Yeung et al. (1983) studied the absorption and metabolism of resorcinol in three male human volunteers after topical application. Twenty millilitres of 2% resorcinol in a hydroalcoholic vehicle were applied twice daily to face, shoulders, upper chest, and upper back areas on 6 days/week over 4 weeks (150 µg/cm2 per application to 2600 cm2 of body surface; daily dose: 12 mg/kg body weight). In 24-h urine, about 0.5–2.9% of the applied dose was detected as glucuronide or sulfate conjugates, and flux was calculated as 0.37 µg/cm2 per hour. In plasma, levels of free resorcinol or its conjugates were below the detection limit of 0.1 µg/ml. There was no information given on the remaining part of the dose. Measured thyroid functions (T3/T4/T7/TSH) gave no significant changes. In an in vitro.test with excised full-thickness human skin (application of 390 µg/cm2), the flux was 0.86 µg/cm2 per hour.
In an investigation in three human volunteers using conditions similar to those used in hair dyeing, 14C-ring-labelled 1.2% resorcinol was mixed with 6% hydrogen peroxide, and the mixture (approximately 100 g) was worked into dry hair for 5–8 min and left on the hair for a further 20 min followed by rinsing. Only 0.076% of the total dose was excreted. The urinary excretion was found to follow first-order kinetics, and the time required for 50% excretion was 31 h. This suggests that only a small amount of resorcinol penetrates the skin during the actual process of hair colouring. The bulk of the urine-recovered dye must have been taken up into the horny layer of the skin and then slowly released into the circulation. A cumulative 4-day absorption (assuming 700 cm2 of scalp) was given as 0.46 µg/cm2 (Wolfram & Maibach, 1985).
In in vitro human skin studies, resorcinol was evaluated from a representative hair dye formulation that contained 0.61% resorcinol (total dyes 2.7%) after dilution with developer. Mean data for 3 donors and 16 replicates indicate a plateau in receptor fluid concentration between 24 and 48 h, as reflected by cumulative absorption values of 1.17 and 1.30 µg/cm2 (average 1.23 µg/cm2) (Dressler, 1999).
In in vitro permeability studies using human skin testing 10% w/v resorcinol, resorcinol showed a long lag time (80 min). A steady-state permeability coefficient (.p) of 0.000 24 cm/h was calculated (Roberts et al., 1977).
For male albino rats (strain not given; . = 5 per group) dosed with resorcinol (flake grade) by gavage, an LD50 of 980 mg/kg body weight was reported. Animals that died showed hyperaemia and distension of stomach and intestine, while there were no gross lesions at necropsy in survivors (Flickinger, 1976).
In another study with CFY rats (. = 5 per sex and group), an LD50 of 370 mg/kg body weight was obtained. Dosed animals showed lethargy and piloerection, whereas no adverse findings were reported at sacrifice (14 days) (Lloyd et al., 1977).
For rats (sex and strain not given), an LD50 value of 301 mg/kg body weight was reported by Koppers Company (1970). Signs of intoxication included fibrillation, tremors, convulsions, salivation, dyspnoea, sedation, and emaciation. Gross autopsy of survivors gave no adverse effects, whereas haemorrhages of the lungs, inflammation of the gastrointestinal tract, and hyperaemia in livers were noted in animals that died.
For female Wistar rats, an LD50 of 202 mg/kg body weight was reported by Hoechst AG (1979). Signs of intoxication included motor difficulties, decubitus position, passivity, shivering, twitching, tonic–clonic seizures, cyanosis, and breathing difficulties. Sacrificed animals showed brown-dyed stomach walls and filling of stomach and small intestine with a dark-brown to orange substance. These findings were not noted in survivors.
In rabbits (giant chinchilla), doses of <500 mg/kg body weight caused no apparent toxic effects, whereas after dosing with 600 mg/kg body weight, temporary muscular twitching and increased respiration rate were noted (Garton & Williams, 1949).
The acute dermal toxicity of resorcinol was studied in male albino rabbits (Koppers Company, 1962). For flaked resorcinol, the LD50 was given as 3360 mg/kg body weight. Dosing with 1000 mg/kg body weight caused slight hyperkeratosis and moderate to severe irritation after 24 h, body weight loss, but no gross lesions. At >2000 mg/kg body weight, skin necrosis was seen. For industrial resorcinol, the LD50 was 2830 mg/kg body weight. Dosing with 1000 mg/kg body weight caused no irritation, body weight loss, but no gross lesions. In both studies, the treatment with >2000 mg/kg body weight caused skin necrosis.
In another study with rabbits (sex and strain not given), an LD50 of 3830 mg/kg body weight was obtained by Koppers Company (1970). Signs of intoxication included salivation, tremors, and convulsions, and treated skin areas showed slight erythema and extreme dryness. The gross autopsy of survivors gave no significant findings, whereas haemorrhages of the gastrointestinal tract were noted in the dead animals.
In Harlan-Wistar rats (. = 6 females per group) exposed to resorcinol–water solutions (approximately >1 µm size), no deaths were seen at concentrations up to 7800 mg/m3 (1 h) or up to 2800 mg/m3 (8 h). Survivors showed no exposure-related lesions at necropsy after 14 days (Flickinger, 1976).
For male rats (strain not given), a 1-h LC50 of >160 mg/m3 was reported by Koppers Company (1970). There were no signs of intoxication, and gross autopsy showed haemorrhages of the lungs.
In mice (. = 6 per group), the LD50 after subcutaneous injection was given as 213 mg/kg body weight. Immediately after dosing, the animals showed tremor, asphyxia, and cramps (Marquardt et al., 1947).
Angel & Rogers (1972) gave the dose applied intraperitoneally causing myoclonic convulsions in 50% of urethane-anaesthetized male albino mice (Sheffield strain) as 0.92 mmol (101 mg/kg body weight).
After subcutaneous daily administration of resorcinol at 2 × 50 mg/kg body weight given 6 h apart to male Sprague-Dawley rats over 14 and 30 days, no adverse effects concerning body or organ weights (liver, kidneys, brain, spleen, and testes), haematological parameters, serum T3/T4 levels, or microscopic appearance of the thyroid gland, spinal cord, or brain were reported. After subcutaneous injection of 55, 88, 140, 220, or 350 mg/kg body weight in male CD(SD) rats (n = 5 per group), slight tremors progressing from moderate to marked tonic–clonic convulsions were seen within 10 min at >140 mg/kg body weight. Complete recovery in all animals occurred within 1–1.5 h after dosing (Merker et al., 1982). A NOAEL of 100 mg/kg body weight was chosen..
After subcutaneous injection of 70–180 mg/kg body weight as aqueous solution to groups of four rats each, the 131I uptake by the thyroid gland 2 h after dosing was about 14–24% when compared with controls (Arnott & Doniach, 1952).
Doniach & Fraser (1950) dosed single female Lister rats subcutaneously with >5 mg/kg body weight and noted a decreased uptake of iodine by the thyroid gland of 11–20% of normal values measured 2 h post-dosing. This effect could not be increased with higher dosing (up to 300 mg/kg body weight). Dosing with >50 mg/kg body weight caused severe tremors for the first half-hour post-application.
Of the following studies, the repeated-dose toxicity studies that were deemed to be most relevant to the risk assessment are summarized in Appendix 6.
In a study performed by NTP (1992), five male and female F344 rats were dosed with resorcinol in deionized water via gavage at 0, 27.5, 55, 110, 225, or 450 mg/kg body weight once daily on 5 days/week over 17 days (12 doses total). Hyperexcitability and tachypnoea were observed in males receiving 225 and 450 mg/kg body weight. Females receiving doses of 55 mg/kg body weight and greater showed hyperexcitability, and those receiving 110 and 450 mg/kg body weight showed tachypnoea. High-dose females had significantly decreased absolute and relative thymus weights. No other biologically significant differences in organ weights were observed. There were no gross or microscopic lesions attributable to resorcinol administration. The NOAEL was 27.5 mg/kg body weight (NTP, 1992).
In a parallel study, five male and female B6C3F1 mice were dosed with resorcinol in deionized water via gavage at 0, 37.5, 75, 150, 300, or 600 mg/kg body weight once daily on 5 days/week over 17 days (12 doses total). At 600 mg/kg body weight, 5/5 females and 4/5 males died on the first day, whereas 1/5 males dosed with 300 mg/kg body weight died before study termination. Clinical findings, including prostration and tremors, were recorded among males receiving >150 mg/kg body weight and among females receiving >300 mg/kg body weight. In both sexes, no gross or microscopic lesions were noted. The NOAEL was 75 mg/kg body weight (NTP, 1992).
The exposure of female Wistar Crl:(WI) BR rats fed on a low-iodine, low-protein diet to 0 or 9 µmol resorcinol per day via drinking-water (about 5–10 mg/kg body weight) over 30 days caused an enlargement of the thyroid gland and decreased ability of their thyroids to incorporate 125I into the active thyroid hormones T3 and T4 (see also section 8.8; Cooksey et al., 1985).
The oral dosing of male F344 rats with 0.8% resorcinol via diet (approximately 480 mg/kg body weight per day) over 8 weeks caused no adverse effects concerning mortality, body weight gain, or food and water consumption, and the examination of the forestomach/glandular stomach gave no increased incidence of hyperplasia or labelling indices (Shibata et al., 1990).
To investigate the effect of resorcinol as a peeling agent, 1% or 3% resorcinol in vaselinum flavum or Unguentum Cordes was applied onto the ears or shaven flanks of male guinea-pigs, once per day over 14 days. Resorcinol showed a concentration-dependent increase in labelling index ([3H]thymidine), acanthosis, and papillomatosis. The mode of peeling is therefore via proliferation hyperkeratosis (Windhager & Plewig, 1977).
After application of an ointment containing 12.5% resorcinol onto the shaved bellies of six albino rats of both sexes (and six controls) for 0.25 h twice daily over 3 weeks (about 300 mg/kg body weight per day), no significant changes in thyroid gland weights were found (see also section 8.8; Doniach & Logothetopoulos, 1953).
To study the goitrogenic activity of resorcinol, female Wistar rats were treated twice daily for 28 days by rubbing 6 g of an ointment containing 12.5% resorcinol (about 750 mg/kg body weight per day) onto shaved (. = 6) or shaved and scarified skin (. = 4 with and without resorcinol). Increased thyroid gland weights (2.5–4 times) and histological alterations were seen, indicating a goitrogenic effect (see also section 8.8; Samuel, 1955).
The exposure of rats, rabbits, and guinea-pigs to resorcinol at a concentration of 34 mg/m3 for 6 h/day over 2 weeks gave no evidence for toxic effects (lung or trachea damage, allergic reaction in the respiratory tract). Animals were maintained for several months with periodic sacrifices (Flickinger, 1976).
In a throat spray test, groups of guinea-pigs and rats (sex and strain not given) received three daily throat sprayings with 1% resorcinol in water over 2 weeks. The animals were then examined weekly for 10 additional weeks. During application, the throats of the animals showed signs of irritation, which was reversible after termination of the exposure. There was no gross evidence for respiratory damage, and the histopathological examination of the lungs revealed no adverse effects when compared with controls (water spray) (Flickinger, 1976).
Of the following studies, the repeated-dose toxicity studies that were deemed to be most relevant to the risk assessment are summarized in Appendix 6.
In a study performed by NTP (1992), 10 F344 rats and 10 B6C3F1 mice of both sexes were dosed with resorcinol by gavage in deionized water (rats: 0, 32, 65, 130, 260, or 520 mg/kg body weight; mice: 0, 28, 56, 112, 225, or 420 mg/kg body weight) on 5 days/week over 13 weeks. In high-dosed groups, 10/10 female rats and 8/10 male rats as well as 8/10 mice of both sexes died. In male rats dosed with 130–260 mg/kg body weight and in female rats dosed with 65–260 mg/kg body weight, significantly increased absolute and relative liver weights were found. Absolute/relative adrenal gland weights were significantly increased in all surviving males without clear dose–response. In high-dosed male mice, final mean body weights were significantly less than controls, while final mean body weights and changes in mean body weights of all other mice were comparable with controls. In high-dosed mice, clinical signs of intoxication included dyspnoea, prostration, and tremors, which generally appeared within 30 min after dosing. In male mice dosed with 28–225 mg/kg body weight, significantly decreased absolute/relative adrenal gland weights were seen. In both species, there were no adverse effects on final mean body weights, haematology, or clinical chemistry parameters, and no chemical-related gross or microscopic lesions were observed.
In another study, rats were exposed to 0 or 0.004% resorcinol via drinking-water (about 5 mg/kg body weight) over 12 weeks, and effects on the thyroid gland (increased mean follicular epithelial cell height, decreased mean follicle diameters, and decreased follicle epithelium indices) were observed (see also section 8.8; Seffner et al., 1995). No thyroid hormone measurements were performed.
Groups of 25 male and 25 female HLA-SD rats were exposed to resorcinol at about 1000 mg/m3 as atomized mist on 8 h/day on 60 days (over more than 4 months) or 90 days (over more than 5.75 months) (Koppers Company, 1977). Two groups of 5 males and 5 females (pair fed and normal controls) were used as controls. Because of high mortality (20% in males; 28% in females), the exposure was temporarily terminated after 64 weeks. Fifty per cent of survivors were sacrificed 1 week later, and blood and urine samples were taken. After a 2-week pasture period, the remaining animals were further exposed (total of 90 exposures). Apart from effects such as altered relative organ weights (liver, kidneys, spleen, adrenals) or changes in haematological parameters, the most important changes were seen in the thyroid gland: 39% (15/38 animals) showed a hyperplasia, which was not seen in controls. Although the data are limited due to interruption of the exposure, this study gives an indication of systemic effects after uptake via inhalation.
Of the following studies, the repeated-dose toxicity studies that were deemed to be most relevant to the risk assessment are summarized in Appendix 6.
In one study performed with male Syrian golden hamsters, which focused only on effects on the forestomach, pyloric region, and urinary bladder, the animals were dosed with 0 or 0.25% resorcinol via the diet (about 375 mg/kg body weight) over 20 weeks (Hirose et al., 1986). A mild hyperplasia of the forestomach was noted, but this was not statistically different from the controls. No other adverse effects were described.
Eastin et al. (1998) dosed heterozygote p53def (C57BL/6) mice with 0 or 225 mg/kg body weight via gavage, 5 times per week over 24 weeks, and found no increased incidence of neoplastic or non-neoplastic lesions (no further information available). The p53def (p53+/−) mouse model is heterozygous for the wild-type tumour suppressor gene Trp53, and the loss of p53 tumour suppressor function is associated with progression of tumours to malignancy.
In an interlaboratory study (two laboratories), transgenic CB6F1-Tg rasH2 mice or non-transgenic littermates were dosed with resorcinol in deionized water via gavage at 0 or 225 mg/kg body weight, 5 times per week, and sacrificed after 24–26 weeks. The histopathological examination of the lungs and spleen gave a slightly (non-significant) increased incidence of adenomas in the lung and no increase in haemangiosarcomas in the spleen (Maronpot et al., 2000). Lung tumours and splenic tumours are typically seen tumours in this transgenic mice strain, and therefore the study focused mainly on these tumour types (tumours in other organs were not investigated).
In a study performed by NTP (1992), F344 rats and B6C3F1 mice of both sexes were dosed with 0, 112, and 225 mg/kg body weight (male rats, male and female mice) or with 0, 50, 100, and 150 mg/kg body weight (female rats) on 5 days/week over 104 weeks. In both species, clinical signs such as ataxia and tremors were noted at about 100 mg/kg body weight. An interim sacrifice at 15 months gave no difference in haematology, clinical chemistry, or other clinical pathology parameters and no increased incidence of neoplasms or non-neoplastic lesions. Also, at final sacrifice, there was no evidence of carcinogenic activity in males or females of both species. The NOAEL was 50 mg/kg body weight (adjusted to 36 mg/kg body weight per day for 5 days/ week dosing).
Two long-term studies with Swiss mice or NZW rabbits, where the animals were treated with 0.02 ml of 5, 25, or 50% resorcinol dissolved in acetone on the shaved dorsal skin, 2 times per week over 110 weeks (mice; Stenbäck & Shubik, 1974), or with 0.02 ml of 5, 10, or 50% resorcinol dissolved in acetone onto the interior left ear, 2 times per week over 180 weeks (rabbits; Stenbäck, 1977), gave no evidence for systemic or carcinogenic effects.
In contrast to these findings, Eastin et al. (1998) observed a statistically increased incidence of squamous cell papillomas at the site of application in both male (10/15) and female (12/15) hemizygote Tg.AC (FVB/N) mice treated with resorcinol at 225 mg/kg body weight in acetone on the clipped skin 5 times per week over 24 weeks versus controls (males 2/30 and females 0/30). Other skin effects were hyperplasia, hyperkeratosis, inflammation, and sebaceous gland hyperplasia, whereas there were no systemic treatment-related lesions.
With resorcinol, several initiation–promotion studies with hamsters (Maruyama et al., 1991) and rats (Miyata et al., 1985; Hirose et al., 1989; Stenius et al., 1989; Yamaguchi et al., 1989; Hasegawa et al., 1990; Kurata et al., 1990; Hasegawa & Ito, 1992; Okazaki et al., 1993) have been performed.
Female Syrian golden hamsters (10–20 per group) were treated with two subcutaneous injections of .-nitrosobis(2-oxopropyl)amine at 70 mg/kg body weight within 2 weeks followed by 0 or 1.5% resorcinol via diet (approximately 2250 mg/kg body weight) over 16 weeks (Maruyama et al., 1991). In the forestomach/glandular stomach, an increased epithelial hyperplasia was noted (no further data), while there was no increase in neoplastic lesions such as papilloma, adenoma, or carcinoma.
In a study performed by Hasegawa et al. (1990), male F344/DuCrj rats were dosed with 0.1% .-bis(2-hydroxypropyl)nitrosamine via drinking-water over 2 weeks followed by 0 or 0.8% resorcinol (about 480 mg/kg body weight) via diet over 30 weeks. In the thyroid gland, an increased incidence of adenomas (6/20; control without resorcinol: 1/20) and carcinomas (5/20; control without resorcinol: 4/20) was found.
In another study, male F344 rats were treated with three intraperitoneal injections of 3-methyl-.-amylnitrosamine at 25 mg/kg body weight at 1-week intervals and, after a 1-week pause, dosed with 0 or 0.8% resorcinol (about 480 mg/kg body weight) via diet over 49 weeks. This treatment caused a significantly increased incidence of carcinomas in the oesophagus (7/12; control without resorcinol: 0/11) and papillomas of the tongue (6/12; control without resorcinol: 1/11) (Yamaguchi et al., 1989).
In the other initiation–promotion studies, negative results were obtained. Details are given in MAK (2003).
A dose of 10 mg resorcinol administered on 3 days weekly starting together with or 14 days after treatment with 150 µg benzo[.]pyrene gave no indication of tumour-promoting properties in ICR/Ha Swiss mice over 368–440 days (Van Duuren & Goldschmidt, 1976).
Overall, resorcinol showed mostly negative results in bacterial mutagenicity assays. However, it induced mutations in the TK locus in mouse lymphoma cells. Resorcinol did not induce unscheduled DNA synthesis in hepatic cells or single-stranded DNA breaks in mammalian cells in vitro. Studies for SCE and chromosomal aberrations in vitro in isolated cells and cell lines gave both negative and positive results. Cytogenetic studies in vivo (micronuclei in bone marrow in rats and two strains of mice; SCE in male and female rats) gave consistently negative results.
Resorcinol tested negative for mutagenicity in various strains of Salmonella typhimurium as well as in Escherichia coli both with and without metabolic activation (Florin et al., 1980; Shahin et al., 1980; Bracher et al., 1981; Crebelli et al., 1981, 1984, 1985; Probst et al., 1981; Haworth et al., 1983). In two studies, positive results were also reported. Gocke et al. (1981) found an increase in S. typhimurium TA 1535 (with S9) and TA 100 (without S9) when a so-called ZLM medium was used, but not on a Vogel-Bonner medium. Hosono et al. (1991) reported a pH-dependent response in TA 98 (SD 510 strain) and in E. coli B/r WP2 trp−hcr−. Mutagenicity was highest at pH 3.0, whereas there was little to no mutagenicity above pH 5.0. Resorcinol did not induce an SOS response in S. typhimurium TA 1535/pSK1002 with or without S9 (Nakamura et al., 1987).
In tests performed with mammalian cells, resorcinol gave no indication for increases in SCE in CHO cells with or without S9 (Darroudi & Natarajan, 1983) or without metabolic activation in hamster V79 cells (Wild et al., 1981) or in human blood lymphocytes (Darroudi & Natarajan, 1983; Jansson et al., 1986). However, in CHO cells, positive results were obtained both with and without S9 (NTP, 1992). Resorcinol induced a significant increase in the number of trifluorothymidine-resistant colonies in L5178Y TK+/− mouse lymphoma cells (McGregor et al., 1988; NTP, 1992). In tests for induction of chromosomal aberrations, resorcinol was positive in CHL and CHO cells with and without S9 (Stich et al., 1981; Sakano et al., 1985; NTP, 1992) as well as in human blood lymphocytes and human amnion cells without S9 (Schulz et al., 1982; Darroudi & Natarajan, 1983). In contrast, negative results were obtained with CHO cells with or without S9 (Darroudi & Natarajan, 1983) and human diploid fibroblasts without S9 (Darroudi & Natarajan, 1983).
Resorcinol also tested negative in an unscheduled DNA synthesis test with primary rat hepatocytes without S9 (Probst et al., 1981) and failed to induce DNA strand breaks in mammalian cells or in isolated DNA (Yamada et al., 1985; Kawanishi et al., 1989; Miura et al., 2000).
Different studies performed with rats and mice gave no increase in the micronucleus formation in bone marrow cells. In NMRI mice (. = 2 males and 2 females per group) dosed twice within 24 h with intraperitoneal injections of resorcinol at 0, 55, 110, or 220 mg/kg body weight 24 h apart, there was no increase in micronucleus formation in bone marrow cells. Bone marrow smears were prepared at 30 h, and 1000 polychromatic erythrocytes per animal were scored (Gocke et al., 1981). In male CBA mice (. = 4 per group) dosed once with an intraperitoneal injection of resorcinol at 0, 37.5, 75, 150, or 300 mg/kg body weight, there was no increase in micronucleus formation in bone marrow cells. Bone marrow smears were prepared at 24 or 48 h, and 1000 polychromatic erythrocytes were scored (Darroudi & Natarajan, 1983). In another study performed by Paschin et al. (1986), male and female (CBA × C57BL/6J)F1 mice (n = 5 per group) were dosed once with an intraperitoneal injection of resorcinol (analytical grade) at 0 or 75 mg/kg body weight. Bone marrow smears were prepared at 24–96 h, and 1000 polychromatic erythrocytes from each smear were scored for the incidence of micronucleated cells. There was also no indication of mutagenic potential when male and female Sprague-Dawley rats (CFY; n = 5 per group) were orally dosed with 2 × 250 mg/kg body weight within 24 h and examined 6 h after last dosing. Two thousand polychromatic erythrocytes per rat were scored (Hossack & Richardson, 1977).
In an SCE test, male and female Sprague-Dawley rats were dosed orally or intraperitoneally with 0–100 mg/kg body weight or epicutaneously with 0–300 mg/kg body weight, and negative results were obtained (Bracher et al., 1981).
Resorcinol was also tested for SLRL mutations in Drosophila melanogaster. In a study performed by NTP (1992), no mutations were induced after feeding of 11 000 mg/kg, whereas equivocal results were obtained after injection of 11 940 mg/l. In another study, no SLRL mutations were found after feeding resorcinol at 50 mmol/l (Gocke et al., 1981).
In mice, no inhibition of testicular DNA synthesis was seen after single oral dosing with 100 mg/kg body weight (Seiler, 1977). There was also no indication for adverse effects in a sperm head abnormality test with mice after intraperitoneal injections of 0.5–2.0 mmol/kg body weight (55–220 mg/kg body weight) (Wild et al., 1981).
In a dose range-finding one-generation reproductive toxicity study, groups of male and female Crl:CDÒ (SD)IGS BR (now called Crl:CDÒ (SD)) rats (14 per sex per group) were dosed continuously via drinking-water containing resorcinol at 0, 10, 40, 120, or 360 mg/l (males: 0, 1, 4, 13, and 37 mg/kg body weight per day; females: 0, 1, 5, 16, and 47 mg/kg body weight per day) for a minimum of 28 consecutive days prior to mating. F0 animals were about 8 weeks of age at the beginning of the study, and F1 pups selected for exposure (one pup per sex per litter) were dosed starting at weaning on PND 21. Dosing of the F0 generation continued during mating and throughout gestation and lactation (for F0 females) until euthanasia, and F1 pups not selected for behavioural evaluation were dosed until euthanasia on PND 28 (RTF, 2003).
The animals were observed twice daily for appearance and behaviour, and clinical observations, body weights, and water and food consumption were recorded at appropriate intervals. F0 females were allowed to deliver and rear their pups until weaning on lactation day 21. For reduction of variability among the litters, 10 pups per litter (equal sex distribution) were selected on PND 4. One F1 pup per sex per litter was selected for exposure until PND 28. With three F1 pups per sex per litter selected for behavioural testing, several developmental landmarks (balanopreputial separation and vaginal patency) as well as behavioural evaluations (see section 8.7) were performed, while the rest of the F1 pups were necropsied on PND 21. Surviving F0 parental and F1 (following selection) rats underwent complete gross necropsy following the breeding period (seven F0 males per group), following completion of weaning of F1 pups (F0 females), following scheduled necropsy of F0 females (remaining seven F0 males per group), on PND 28 (F1 exposed pups), or on PND 30 or 70 (F1 pups selected for behavioural testing). Several organs were weighed, and hormone analyses (TSH/T3/T4) were performed (see section 8.8) at scheduled necropsy on all F0 parents, on PND 28 for exposed F1 pups, and on PND 4 for all culled F1 pups. Thyroid glands from all surviving F0 parents were examined microscopically, and brain measurements were performed on all exposed F1 pups and those F1 pups selected for behavioural testing. In controls and the high-dose group, a qualitative histopathological analysis of the brain (forebrain, midbrain, and hindbrain) was also performed for the aforementioned animals (RTF, 2003). There were no adverse effects concerning reproductive performance, mortality, and body or organ weights. For other effects, see the respective sections.
Based on these data, a two-generation reproductive toxicity study with drinking-water doses of 0, 120, 360, 1000, or 3000 mg/l for the F0 and F1 generations was performed (RTF, 2005) (see Appendix 7). Groups of male and female Crl:CDÒ (SD)IGS BR rats (30 per sex per group) were dosed continuously via drinking-water for a minimum of 70 consecutive days prior to mating. F0 animals were about 6 weeks of age at the beginning of the study. The F0 and F1 males continued to receive the resorcinol throughout mating and through the day of euthanasia. The F0 and F1 females continued to receive the resorcinol throughout mating, gestation, and lactation and through the day of euthanasia (for study design, see Appendix 7).
There were no F0 and F1 parental resorcinol-related deaths or clinical findings during the weekly detailed physical examinations. Reproductive performance (estrous cycles, mating and fertility indices, number of days between pairing and coitus, and gestation length) and parturition in the F0 and F1 animals were unaffected by administration of resorcinol. No effect of the test substance was found for spermatogenic end-points (mean testicular and epididymal sperm numbers and sperm production rate, motility, progressive motility, and morphology) in the F0 and F1 males. No resorcinol-related effects on F0 and F1 pup survival or the general physical condition of the pups during the pre-weaning period were observed. No resorcinol-related macroscopic findings, organ weight, or adverse microscopic target organ effects were observed in the F0 and F1 parental animals.
In F0 animals dosed with 3000 mg/l, decreased mean cumulative body weight gains without clear trends were noted during the premating period (females) or the entire generation (males). However, in F0 females, mean body weights were decreased by up to 6.3% from study days 56 to 70 and also during the first week of gestation (up to 5.5%), throughout lactation (up to 8.4%), and after the lactation period (6.3%).
In F1 males dosed with 3000 mg/l, decreased mean cumulative body weight gains without clear trends were noted during the entire generation, but mean body weights were decreased by up to 7.1% during the entire generation. In high-dosed F1 females, there was also a decrease in mean body weights during lactation (up to 6.1%) and after the lactation period (up to 7.0%). The mean water consumption was decreased in parental F0 and F1 rats dosed with 3000 mg/l during the premating period (females) or the entire generation (males) and also in F1 pups gang-housed by litter from PND 21 to PND 28. A decreased water consumption was also often seen in rats dosed with 1000 mg/l, but this was less severe and the onset was later than in high-dosed rats. Owing to the lack of associated effects on food intake and food utilization, this effect was not considered as adverse. The observed decreased water consumption was probably due to unpalatability of the resorcinol in the water.
No adverse effects were seen at the highest dose tested; however, at this dose (3000 mg/l), decreased colloid in the thyroid was observed (although this was not statistically significant). When expressed on a body weight basis (average of F0 and F1 animals), the highest dose corresponded to approximately 233 mg/kg body weight per day for males over the entire generation, 304 mg/kg body weight per day for females during premating and gestation, and 660 mg/kg body weight per day for females during lactation (RTF, 2005).
In an in vitro study, Saito et al. (1999) investigated possible estrogenic and anti-estrogenic activities of resorcinol using a mammalian cell-based luciferase reporter gene assay and a yeast two-hybrid assay for detection of estrogenic and anti-estrogenic effects on human estrogen receptor-mediated transactivation. In both assays, no estrogenic or anti-estrogenic effects were detected at concentrations of 10−3 to 10 µmol/l (0.11–1101 µg/l), whereas marked effects were found in positive controls (estradiol and the anti-estrogen 4-hydroxytamoxifen).
Sprague-Dawley rats (. = 13 per group) were dosed with resorcinol dissolved in propylene glycol at 0, 125, 250, or 500 mg/kg body weight via gavage on gestation days 6–15 and sacrificed on gestation day 20. In dams, only a slight decrease in maternal weight gain was seen on gestation days 6–16 at 500 mg/kg body weight. Resorcinol caused no embryotoxicity and no adverse effects on mean numbers of corpora lutea, total implantations, viable fetuses, or mean fetal body weights. There was also no increase in fetal anomalies or malformations (DiNardo et al., 1985).
Resorcinol caused no maternal toxicity or embryotoxicity/teratogenicity in rats dosed via gavage with 0, 40, 80, or 250 mg/kg body weight on gestation days 6–15 (sacrifice on gestation day 19) or in rabbits dosed via gavage with 0, 25, 50, or 100 mg/kg body weight on gestation days 6–18 (sacrifice on gestation day 28) (Spengler et al., 1986).
In Sprague-Dawley rats (. = 15–20 per group) dosed by oral intubation once on gestation day 11 with 0, 333, 667, or 1000 mg/kg body weight, only slight maternal toxicity (weight loss within 24 h after dosing with decrease in maternal weight gain at 72 h) was seen at >667 mg/kg body weight. In the offspring, no developmental toxicity was observed (Kavlock, 1990).
In the RTF (2005) two-generation study (see section 8.6.1), no neonatal toxicity was observed.
In several studies, effects on the CNS have been reported (e.g. NTP, 1992; see Appendix 6).
In a fertility dose range-finding study with male and female Crl:CDÒ (SD)IGS BR rats (for dosing regimen and further details, see section 8.6.1; RTF, 2003), behavioural testing (balanopreputial separation and vaginal patency, functional observational battery evaluations, locomotor activity, acoustic startle response, and Biel maze swimming trials) was performed with three F1 pups per sex per litter. Locomotor activity for F1 males and females was unaffected on PND 21. When locomotor activity was evaluated as these same animals approached sexual maturity (PND 61), generally statistically significant increases in cumulative total (34–41%) and/or ambulatory (37–53%) counts were noted for F1 males in the 40, 120, and 360 mg/l groups. Of the functional domains, the locomotor activity values for the F1 males and females were increased compared with controls as well as laboratory historical control data. However, owing to missing correlating histopathological changes in the three levels of the brain examined and in the absence of a dose–response relationship, other indicators of developmental delay, or other changes in CNS function, these effects were not considered as conclusive evidence of a change in CNS function.
In the subsequent two-generation reproductive toxicity study (RTF, 2005), these end-points were not investigated.
The individual results of the mostly older studies concerning effects on the thyroid gland are summarized in Table 7. Doniach & Logothetopoulos (1953) and Lynch (2002) observed that the goitrogenic activity of resorcinol in rodents occurs only after administration in a manner that allows for continued systemic exposure (e.g. via diet, drinking-water, or subcutaneous administration in hydrophobic vehicles).
Table 7: NOAELs/LOAELs for thyroid effects in animals.
|
Species |
Route/duration |
Dose (mg/kg body weight per day) |
NOAEL/LOAEL for thyroid effects (mg/kg body weight per day) |
Thyroid effect |
Reference |
|
Rat |
Dermal |
~300 |
NOAEL: ~300 |
None |
Doniach & Logothetopoulos (1953) |
|
Rat |
Dermal |
~750 |
LOAEL: ~750 |
Increased thyroid gland weight and histological changes; no data on TSH or T3/T4 levels |
Samuel (1955) |
|
Rabbit |
s.c. (in 0.9% saline) |
50 over 4 days and 75 over 15 days |
NOAEL: 75 |
None |
Klein et al. (1950) |
|
Rat |
s.c. (in aqueous base) |
50 |
NOAEL: 50 |
None |
Cheymol et al. (1951) |
|
Rat |
s.c. (in arachis oil) |
~154 |
LOAEL: ~154 |
>47 days: increased thyroid gland weights with goitre-like histology |
Doniach & Logothetopoulos (1953) |
|
Rat |
s.c. (in peanut oil) |
I. ~154 |
NOAEL: ~154 |
I. none; II. enlarged thyroid gland and histological changes; no data on TSH or T3/T4 levels |
Samuel (1955) |
|
Rat |
s.c. (in peanut oil) |
~400 |
LOAEL: ~400 |
Increased thyroid gland weight and histological changes; no data on TSH or T3/T4 levels |
Samuel (1955) |
|
Rat |
Oral via diet |
5% (~3000 mg/kg body weight per day) |
LOAEL: ~3000 |
Increased thyroid gland weights |
Berthezéne et al. (1979) |
|
Rat |
Oral via drinking-water |
~5–10 |
LOAEL: ~5–10 |
Increased thyroid gland weights (~2.5 vs 1.2 mg/kg in controls) |
Cooksey et al. (1985) |
|
Rat |
Oral via drinking-water |
0.004% (~5 mg/kg body weight per day) |
LOAEL: ~5 |
Mean follicular epithelial cell height incr.; mean follicle diameter decr.; follicle epithelium index decr.; no data on TSH or T3/T4 levels |
Seffner et al. (1995) |
|
Rat |
Oral via gavage |
32, 65, 130, 260, or 520 |
NOAEL: 520 |
No histopathological changes; T3/T4 levels (determined only at 0 and 130 mg/kg body weight): 118/5 µg/dl vs 112/4 µg/dl in controls |
NTP (1992) |
|
Mouse |
Oral via gavage |
28, 56, 122, 225, or 420 |
NOAEL: 420 |
No histopathological changes; T3/T4 levels not determined |
NTP (1992) |
|
Rat |
Oral via gavage 104 weeks |
112 or 225 (m) 50, 100, or 150 (f) |
NOAEL: 150–225 |
No histopathological changes; T3/T4 levels not determined |
NTP (1992) |
|
Mouse |
Oral via gavage |
112 or 225 |
NOAEL: 225 |
No histopathological changes; T3/T4 levels not determined |
NTP (1992) |
|
Rat |
Oral via drinking-water (one-generation study) |
10, 40, 120, or 360 mg/l: 1, 4, 13, 37 (m); 1, 5, 16, 47 (f) |
NOAEL: 37 (m); 47 (f) |
No effect on thyroid gland weights 360 mg/l: follicular cell hyperplasia in 6/7 F0 males (interim sacrifice; statistically not significant; 3/7 in controls); follicular cell hyperplasia in 5/7 F0 males (scheduled sacrifice; statistically not significant; 3/7 in controls); follicular cell hyperplasia in 7/14 F0 females (scheduled sacrifice; statistically not significant; 3/13 in controls) Mean total T3 values (ng/dl) for F0 males (interim sacrifice): 90.1, 88.1, 87.8, 86.6, 96.9 Mean total T4 values (µg/dl) for F0 males (interim sacrifice): 5.8, 6.7, 5.6, 6.2, 6.1 Mean TSH values (ng/ml) for F0 males (interim sacrifice): 12, 13.7, 16.6, 15.6, 17 Mean total T3 values (ng/dl) for F0 males (scheduled sacrifice): 136.9, 142.9, 117.2, 130.9, 131.1 Mean total T4 values (µg/dl) for F0 males (scheduled sacrifice): 6.2, 5.9, 5.9, 5.9, 5.4 Mean TSH values (ng/ml) for F0 males (scheduled sacrifice): 17.5, 14.3, 20.2, 15.3, 20 Mean total T3 values (ng/dl) for F0 females (scheduled sacrifice): 69.3, 73.6, 72.3, 80.8, 87.9 Mean total T4 values (µg/dl) for F0 females (scheduled sacrifice): 3.9, 3.7, 4, 4.1, 4.1 Mean TSH values (ng/ml) for F0 females (scheduled sacrifice): 14.1, 15.3, 13.2, 14.6, 15.2 Mean total T3 values (ng/dl) for F1 pups (PND 4): 30.8, 28.5, 31.2, 33.3, 37.2 Mean total T4 values (µg/dl) for F1 pups (PND 4): 1.1, 1.2, 1.3, 1.5, 1.4 Mean TSH values (ng/ml) for F1 pups (PND 4): 7.9, 8, 7.7, 9.5, 8 Mean total T3 values (ng/dl) for F1 pups (PND 28): 121.9, 128.9, 130.4, 133.4, 136.7 Mean total T4 values (µg/dl) for F1 pups (PND 28): 3.5, 3.4, 3.4, 3.7, 3.3 Mean TSH values (ng/ml) for F1 pups (PND 28): 6.4, 6, 6.7, 6.8, 6.9 |
RTF (2003) |
|
Rat |
Oral via drinking-water (two-generation study) |
120, 360, 1000, or 3000 mg/l: up to 233 (m) or 304 (f) |
NOAEL: 233 (m); 304 (f) |
No effect on thyroid gland weights Decreased colloid in F0 males (scheduled sacrifice): 2/28, –, –, 2/30, 7/30 Mean total T3 values (ng/dl) for F0 males (scheduled sacrifice): 131.4, 133.3, 124.8, 143.3, 147.2 Mean total T4 values (µg/dl) for F0 males (scheduled sacrifice): 6.1, 6.2, 5.9, 5.9, 5.5 Mean TSH values (ng/ml) for F0 males (scheduled sacrifice): 9.3, 9.6, 11.3, 11.8, 12.6 Mean total T3 values (ng/dl) for F0 females (scheduled sacrifice): 136.7, 141.1, 133, 146.3, 138.3 Mean total T4 values (µg/dl) for F0 females (scheduled sacrifice): 4.8, 4.8, 4.8, 4.7, 4.2 Mean TSH values (ng/ml) for F0 females (scheduled sacrifice): 9.4, 8.3, 9.4, 7.9, 9.8 |
RTF (2005) |
Effects on the thyroid gland, such as increased thyroid gland weights and decreased ability of the thyroid to incorporate 125I into the active thyroid hormones T3 and T4, were reported in female rats fed a low-iodine, low-protein diet after oral dosing via drinking-water with resorcinol at about 5–10 mg/kg body weight over 30 days (Cooksey et al., 1985; see section 8.2.1). Changes in thyroid histopathology (increased mean follicular epithelial cell height, decreased mean follicle diameters, and decreased follicle epithelium indices) were noted over 12 weeks with resorcinol at about 5 mg/kg body weight (assuming 35 ml of 0.004% solution/day and 0.275 kg body weight) in drinking-water (Seffner et al., 1995; section 8.3.1).
No histopathological changes in the thyroid gland were found at higher dose levels in subacute, subchronic, or chronic studies performed via gavage in rats or mice (NTP, 1992). However, these studies were not designed to investigate this end-point, and T3/T4 levels were not determined, with the exception of the 0 and 130 mg/kg body weight dose groups in the 13-week rat study.
A truncated one-generation study with an abbreviated prebreeding exposure period was conducted as a dose range-finding study for a subsequent guideline-compliant two-generation study with male and female Crl:CDÒ (SD)IGS BR rats (continuous dosing with resorcinol at 0, 10, 40, 120, or 360 mg/l [0, 1, 4, 13, and 37 mg/kg body weight per day for makes; 0, 1, 5, 16, and 47 mg/kg body weight per day for females]; see also section 8.6.1). Effects on the thyroid gland were investigated, such as weight changes and histopathological effects as well as hormone analyses (TSH/T3/T4) at scheduled necropsy on all F0 parents, on PND 28 for exposed F1 pups, and on PND 4 for all culled F1 pups. Thyroid glands from all surviving F0 parents were examined microscopically. For the thyroid, a non-statistically significant increase in TSH levels was reported for interim males, but not at scheduled necropsy. In high-dosed females, T3 levels were increased, while there was no effect on T3 or T4 levels in males. The microscopic examination of the thyroid gave minimal changes (follicular hyperplasia), which were statistically not significant between controls and individual dose groups (RTF, 2003).
In a two-generation study with doses of 0, 120, 360, 1000, or 3000 mg/l, which also focused on thyroidal effects (RTF, 2005; see section 8.6 and Appendix 7), no statistically significant resorcinol-related changes in the mean concentrations of T3, T4, or TSH were observed in the F0 and F1 parental animals or in the F1 and F2 pups selected for analysis (PND 4 or PND 21). Higher TSH values were noted in the F0 males at scheduled necropsy, but these were not considered as resorcinol-related effects in the absence of effects on T3 or T4, organ weights, or adverse macroscopic or microscopic findings. Test article-related decreased colloid within the thyroid glands of the 3000 mg/l F0 males was not considered to be adverse due to a lack of associated functional effects. A resorcinol exposure level of 3000 mg/l was given as the NOAEL when the resorcinol was offered continuously in the drinking-water to parental rats. When expressed on a body weight basis (average of F0 and F1 animals), this concentration corresponded to approximately 233 mg/kg body weight per day for males over the entire generation and 304 mg/kg body weight per day for females during premating and gestation.
In vitro studies have been performed to assess the effects of resorcinol on thyroid function. In porcine thyroid gland slices, resorcinol concentrations of 2 × 10−7 to 5 × 10−5 mol/l caused a significant decrease of 125I uptake and its incorporation into tyrosine to form iodotyrosines. A concentration of 0.3 µmol/l caused a 50% inhibition of thyroid peroxidase (Cooksey et al., 1985).
Lindsay et al. (1992) studied potential anti-thyroid activities of aqueous coal and shale extracts and of compounds identified in aqueous effluents from coal conversion processes in thyroid peroxidase and thyroid slice systems. These substances were found to be potent inhibitors of thyroid peroxidase or 125I organification. Resorcinol as well as 2- and 5-methylresorcinol were reported to be 26.7, 22.5, or 7.2 times more potent than the anti-thyroid drug 6-propylthiouracil.
Divi & Doerge (1994) showed that the mechanism of action for the enzymes lactoperoxidase and thyroid peroxidase is different from that of the classical model peroxidase enzyme (horseradish peroxidase), as lactoperoxidase and thyroid peroxidase are inhibited irreversibly via a suicide inactivation by resorcinol. At an iodide concentration of 0.1 mmol/l, the inactivation of the enzyme and the binding of resorcinol to the enzyme were increased, while an iodide concentration of 5 mmol/l decreased the binding of resorcinol to the enzyme (about 25%) but increased the enzyme activity from 6.2 to 44.7% (rate of iodination of tyrosine).
Koppers Company (1962) studied possible skin irritating effects of flaked or industrial resorcinol in male albino rabbits (500 mg under occlusive conditions over 24 h, scoring after 24 and 72 h). Flaked resorcinol caused no perceptible to moderate irritation (intact skin) or no perceptible necrosis (abraded skin). The effects were more pronounced at 72 h. The irritation index was given as 4.4. Industrial resorcinol caused slight to severe irritation (intact skin) or severe irritation to necrosis (abraded skin), and the irritation index was given as 5.4. In both studies, the effects were more pronounced at 72 h.
In a Draize test with rabbits (application of 500 mg dry powder moistened with water), a skin irritation score of 0.5/8 was obtained (observation period 24–72 h) (Koppers Company, 1970).
In three NZW rabbits, the dermal application of a 2.5% (w/v) solution of resorcinol to intact or abraded skin according to the United States Code of Federal Regulations (Federal Hazardous Substances Act Regulations 16: Section 1500.41, Method of testing primary irritant substances) caused no irritating effects during a 72-h observation period (Primary Irritation Index = 0) (Lloyd et al., 1977).
In a study with six rabbits conducted according to United States Food and Drug Administration guidelines (500 mg over 24 h under occlusive conditions with scoring after 24, 48, and 72 h), an irritation score of 2.8/8 (slightly irritating) was obtained (Hoechst AG, 1979).
In a screening study with guinea-pigs, the application of an aqueous solution of resorcinol (0.1–10%) caused no skin irritation (no further information available) (Springborn Institute for Bioresearch, Inc., 1984).
Resorcinol was extremely irritating (score 105/110) in six rabbits after a single application of 100 mg as dissolved or semisolid material upon the cornea and into the conjunctival sac. The exposed eyes were not rinsed, and the observation period was 24–72 h (Koppers Company, 1962).
In a Draize test with six rabbits (application of 100 mg dry powder), irritation scores of 56.3/110, 45/110, and 39.9/110 were obtained after 24, 48, or 72 h. The total irritation was given as 56.3/110 (Koppers Company, 1970).
In three NZW rabbits, the application of a 2.5% (w/v) solution of resorcinol into the eyes (rinsing 10 s after application) according to the United States Code of Federal Regulations (Federal Hazardous Substances Act Regulations 16: Section 1500.42, Test for eye irritants) caused only mild conjunctival inflammation, disappearing within 24 h post-application (Lloyd et al., 1977).
In a study with six rabbits conducted according to United States Food and Drug Administration guidelines (100 mg into the conjunctival sac, rinsing after 24 h, scoring after 1–72 h), a severely irritating effect (index 70/110 after 48 h) was noted (Hoechst AG, 1979).
Resorcinol tested negative in a local lymph node assay with female CBA/Ca mice at concentrations of 5, 10, or 25% (Basketter et al., 1994).
A positive result was obtained in a maximization test with guinea-pigs comparable to OECD TG 406 (but lower number of animals in study: 5 controls and 10 treated). The purity of resorcinol was 99.9%, and the concentrations for intradermal induction were 2% in 0.9% sodium chloride solution and 25% in 0.9% sodium chloride solution for dermal induction and challenge, respectively (Hoechst AG, 1989).
In a study concerning photoallergenicity, resorcinol (0.3 ml of a 10% aqueous solution) was applied to guinea-pigs 6 times for induction (occlusively for 2 h on alternate days). After a 9-day non-treatment period, provocation treatment was carried out (also occlusively for 2 h) with 0.3 ml of a 2% or 10% aqueous solution. Resorcinol gave no indication for sensitizing or photosensitizing effects (10 J/cm; UV 320–400 nm) in animals treated with 2% for provocation, whereas 2/20 animals treated with 10% for provocation reacted positive. UV irradiation caused no increased skin reactions. One additional animal reacted positively when separately treated areas were exposed to UV and challenged with 10% resorcinol (Springborn Institute for Bioresearch, Inc., 1984).
Thyroid effects are given in section 8.8.
Doniach & Logothetopoulos (1953) observed that the goitrogenic activity of resorcinol in rodents occurs only after administration in a manner that allows for continued systemic exposure (e.g. via diet, drinking-water, or subcutaneous administration in hydrophobic vehicles).
In.vitro and in vivo data indicate that the anti-thyroidal activity of resorcinol is caused by inhibition of thyroid peroxidase enzymes, resulting in decreased thyroid hormone production and increased proliferation due to an increase in the secretion of TSH (see section 8.8). The iodination process is catalysed by a haem-containing enzyme. Resorcinol is known to form covalent bonds with haem (Sessler et al., 1988).
Effects on the thyroid gland have been reported both in animal studies and in case-reports in humans. However, there are species differences in the susceptibility to goitrogens. Especially in rats, long-term perturbations of the pituitary–thyroid axis caused by xenobiotics or physiological alterations (e.g. iodine deficiency or natural goitrogens in diet) are more likely to predispose a higher rate of proliferative lesions such as hyperplasia in response to chronic TSH stimulation than in the human thyroid. In addition, male rats have higher circulating levels of TSH than females. In rodents, the higher sensitivity is also related to a shorter plasma half-life of T4 than in humans due to considerable differences between species in the transport proteins for thyroid hormones. In plasma, the T4 half-life is about 12–24 h in rats vs 5–9 days in humans, and the serum levels of TSH are about 25 times higher in rodents than in humans. Rats require about a 10-fold higher production of T4 than do humans. In humans, the circulating T4 is bound primarily to thyroxine-binding globulin, whereas this protein is not present in rodents. The binding affinity of thyroxine-binding globulin for T4 is about 1000 times higher than for prealbumin. Therefore, more free T4 is transported in the blood of rodents, and there are higher levels of metabolism and excretion of T4 than in humans. T3 is bound to thyroxine-binding globulin and albumin in humans, but only to albumin in rodents (Döhler et al., 1979; Capen et al., 1991; Curran & DeGroot, 1991; Alison et al., 1994; McClain, 1994, 1995; Hard, 1998; Dybing & Sanner, 1999; Lynch et al., 2002).
Resorcinol in animals and humans is reported to affect the CNS. The only investigation into this end-point is that from the dose range-finding study reported in sections 8.6.1, 8.7, and 8.8.1 (RTF, 2003). Significant increases in locomotor activity were noted for F1 males in the 40, 120, and 360 mg/l groups (4, 13, and 37 mg/kg body weight). However, owing to missing correlating histopathological changes in the three levels of the brain examined and in the absence of a dose–response relationship, other indicators of developmental delay, or other changes in CNS function, these effects were not considered as conclusive evidence of a change in CNS function.
There are at present no indications of the significance of the differences in adrenal gland/liver weights suggested in the NTP (1992) studies.
In three male human volunteers, the topical application of an average daily resorcinol dose of 12 mg/kg body weight (applied as 2% resorcinol in a hydroalcoholic vehicle) twice daily on 6 days/week over 4 weeks gave no indication for altered thyroid function (T3/T4/T7/ TSH) (see also section 7.2) (Yeung et al., 1983).
Resorcinol has now been used for about 100 years in human medicine as an antiseptic and in keratolytic topical medications in low concentrations of 1–2%. Sometimes much higher concentrations (up to 50%) have been used in peeling agents or in pastes for the treatment of leg ulcers. One factor increasing potential toxic effects is the application of resorcinol to injured skin (Cassano et al., 1999).
In Graham & Tisdall (1922), Becker (1933), and Cunningham (1956), several single-case poisoning reports, especially in infants, with sometimes fatal outcome have been reported. In most cases, ointments or pastes containing resorcinol up to 50% were applied dermally over varying time intervals, but oral uptake also cannot be excluded. The observed symptoms included burning sensation or convulsions. Additionally, CNS disturbances, such as dizziness, vertigo, confusion, disorientation, amnesia, or tremors, or red blood cell changes, such as methaemoglobinaemia, haemolytic anaemia, haemoglubinuria, or cyanosis (Graham & Tisdall, 1922; Wüthrich et al., 1970; Bontemps et al., 1995; Tush & Kuhn, 1996; Hernández-Pérez, 2002; Duran et al., 2004), are described. In most cases, these effects disappeared within several days after discontinuing the resorcinol treatment.
Bull & Fraser (1950) reported the clinical signs (enlarged thyroid glands, hypoactivity) in three case-reports, where ointments containing resorcinol (up to 12%) were applied onto the skin (leg ulcers) over long time periods.
In one female patient with leg ulcers treated dermally for 13 years with an ointment containing 12.5% resorcinol, 2.1% of a mixture of resorcinol glucuronide and monosulfate was found in urine; the clinical examination gave an enlarged thyroid gland (Thomas & Gisburn, 1961).
Katin et al. (1977) reported one case of hypothyroidism in a 70-year-old male patient caused by long-term dermal application (about 3 months) of large amounts of a paste containing 2% resorcinol. After stopping the use of resorcinol, free T4 and TSH were within normal limits within 2 weeks.
Only about 10 cases of hypothyroidism linked to resorcinol have been reported, and these have mainly been in conjunction with its use in treating persistent skin ulcers involving dermal exposures of about 34–122 mg/kg body weight per day for many days or years (Gans, 1980).
Although the above are mainly older reports, there are more recent case-reports showing the acute effects of resorcinol. In some patients, symptoms of systemic absorption (mild and transitory dizziness) or contact dermatitis have been described directly after application of the peel (53% resorcinol in combination with Jessner’s solution) (Hernández-Pérez, 2002). In a case of poisoning in a pregnant woman accidentally given 50 g resorcinol orally instead of glucose at 30 weeks of pregnancy, the major systemic effects were unconsciousness, drowsiness, respiratory failure, tonic–clonic seizures, and hypothermia. Laboratory findings were leukocytosis, high bilirubin levels, severe metabolic acidosis, and green-coloured urine. The fetus, delivered by caesarean section, was considered dead after 24 h, but the mother recovered (Duran et al., 2004). It is not known whether the fetal demise was caused primarily by the marked maternal toxicity or through a direct fetotoxic effect.
In a study in a plant producing resorcinol by sulfonation of benzene — and also producing beta-resorcylic acid, resorcinol–formaldehyde resins, sulfites, and sulfates — workers were exposed primarily to resorcinol, but the exposure to other agents was not measured (for exposure levels, see section 6.2). In this plant, three cross-sectional studies were carried out between 1978 and 1984 (Flickinger, 1976).
In 1978, medical examinations, chest X-rays, pulmonary function, haematology, and clinical chemistry were performed with 281 of 329 persons actively employed at a production plant in Pennsylvania, USA. About 60% were under 40 years of age, and about 50% had worked at this plant for at least 10 years. Data concerning the different job categories were not provided. The prevalence of medical findings possibly consistent with subclinical hypothyroidism (low T4 and/or high TSH) was 5/280 (1.8%), and the prevalence of possible goitre was 2/280 (0.7%). One person showed a palpable thyroid with normal T4 and TSH values (TOMA, 1978).
In 1980, medical examinations (see above) and thyroid assessments were performed with 247 of 387 presumably active plant workers (214 men and 33 women). About 60% were under 40 years of age, and 153 of these subjects were tested for total T4 and TSH. Five of 153 (3.3%) showed signs of clinical/subclinical hypothyroidism, but in 3 of these 5 cases, other reasons, such as treatment with radioiodine, were given as causes for the thyroid abnormalities (TOMA, 1981).
The third study, performed in 1984, included 192 of 312 active workers. In 188 subjects (175 men, 13 women) with a mean age of 37 years, both laboratory and other tests, including medical examination, were done; no abnormal thyroid glands or changes in T4 values were found in any of the subjects when compared with normal values (Bauer, 1985).
The above data are limited due to small study sizes, lack of comparison groups, missing current and historical control data, and missing information concerning potential exposure categories.
In 52 rubber workers exposed to a hexamethylene tetramine–resorcinol adhesive system in a plant in the United States, no adverse effects were noted after prolonged exposure to resorcinol at concentrations below 0.3 mg/m3 (Gamble et al., 1976).
Roberts et al. (1990) investigated four cases of clinical overt hypothyroidism over a 6-year period among 539 subjects working in a textile factory. In the finishing departments, both thiourea and resorcinol were used, and measurements taken at the inlet of the local exhaust ventilation of stenters gave concentrations of 5 µg/m3 for thiourea and <20 µg/m3 for resorcinol. About 44% of the total workforce (189 men and 48 women) participated. One hundred and fifteen persons were process workers, and 122 worked in management, office, and laboratory jobs. Thyroid function tests, including TSH and antimicrosomal/antithyroglobulin antibodies, were conducted, and participants also filled out a questionnaire. The study found 15 new cases of thyroid abnormalities: one case of thyroid hyperactivity and 14 cases of hypothyroidism. Of these 14, 1 had inherited pituitary hypothyroidism and 1 had a partial thyroidectomy. In the remaining 12 cases (7 males [age distribution 26–60 years]; 5 females [age distribution 18–58 years]), 2 males and 2 females (3 of them had minor symptoms of hypothyroidism) showed slightly increased TSH values. The other 8 persons had normal TSH values and no symptoms, but had increased circulating thyroid antibodies. In this limited study (data concerning individual exposure levels are missing), an association between exposure to resorcinol (and/or thiourea) and hypothyroidism could not be excluded. The interpretation of the data is difficult, since thiourea is also a goitrogenic agent.
Workers in a resorcinol production plant were exposed over decades, at exposure levels up to 45 mg/m3, without any signs of irritation or discomfort (Flickinger, 1976). As mentioned in section 9.4 below, Abbate et al. (1989) examined 42 out of 268 workers from a tyre factory, and all subjects showed clinical signs of dermatitis after predominantly dermal contact with resorcinol. Complete healing was noted after about 1 week of absence from work.
Possible skin sensitization effects of resorcinol have been studied in several patch tests with large collectives (up to 10 892 persons) (Meneghini et al., 1971; Baer et al., 1973; Fräki et al., 1979; Frosch, 1990; Tarvainen, 1995; IVDK, 2001). In general, less than 2% of the collectives tested positive when the concentration of resorcinol was <2%. However, with increasing resorcinol concentrations, there was also an increase in the number of persons who tested positive. Barbaud et al. (1996, 2001) noted a cross-sensitivity between resorcinol and resorcinol monobenzoate and also pyrocatechol. However, in contrast to these studies, Kligman (1966) observed no sensitization in a maximization test with 22 healthy adults when resorcinol was tested at concentrations of 15% for induction and 5% for challenge.
Resorcinol was also tested in hairdressers, who may have been in contact with resorcinol-containing hair dyes. Apart from one positive single case-study (Vilaplana et al., 1991), several other studies with groups up to 354 persons gave no indication for a significant increase of positive results (test concentration: <2%; Guerra et al., 1992a, 1992b; Frosch et al., 1993; Peters et al., 1994; Katsarou et al., 1995).
Several case-reports described dermal sensitization caused by resorcinol due to the therapeutic use of Castellani’s paint (Carbol-Fuchsin: antiseptic and drying agent; Cronin, 1973; Dave, 1973; Marks et al., 1978; Fisher, 1982; Langeland & Braathen, 1987; Pecegueiro, 1992; Köhn, 1993; Erdmann et al., 1997) or for the treatment of acne and/or psoriasis (Waddell & Finn, 1981; Nakagawa et al., 1992; Serrano et al., 1992; Massone et al., 1993). In these cases, the tested concentration of resorcinol was 0.1–8%.
Abbate et al. (1989) examined 42 out of 268 workers from a tyre factory, and all subjects showed signs of dermatosis that was weakly irritating after predominantly dermal contact with resorcinol. Complete healing was noted after about 1 week of absence from work.
The key studies on the effects of resorcinol on aquatic and terrestrial organisms have been taken from BUA Report 99 on resorcinol (BUA, 1993), from the Study on the scientific evaluation of 12 substances in the context of endocrine disrupter priority list of actions (EC, 2002), and from the report for the USEPA HPV Challenge Program (INDSPEC, 2004). The data are summarized in Table 8.
Table 8: Summary of the ecotoxicity of resorcinol.
|
Species |
Guideline |
Test conditions |
Result |
Reference |
|
Acute toxicity to fish |
|
|
|
|
|
Rainbow trout (Oncorhynchus mykiss) |
USEPA, 1974 |
5–10 fish used per concentration; temperature: 14 °C; pH 7.9–8.3; dissolved oxygen: 3.9–8.0 mg/l; flow-through test with analytical monitoring |
96-h LC50 > 100 mg/l |
DeGraeve et al. (1980) |
|
Fathead minnow |
USEPA, 1974 |
5–10 fish used per concentration; temperature: 14 °C; pH 7.9–8.3; dissolved oxygen: 3.9–8.0 mg/l; flow-through test with analytical monitoring |
96-h LC50 = 100 mg/l |
DeGraeve et al. (1980) |
|
Fathead minnow (Pimephales promelas) |
USEPA, 1975, 1978b |
10 fish used per concentration step; temperature: 18–21 °C; pH 6.9–7.8; dissolved oxygen: 8.2–10.6 mg/l; 5 concentrations tested in duplicate; flow-through test with analytical monitoring |
96-h LC50 = 26.8 mg/l |
Koppers Company (1981) |
|
Acute toxicity to aquatic invertebrates |
|
|
|
|
|
Water flea |
–a |
Daphnids <24 h old; 10 animals/10 ml and concentration; temperature: 23 °C; pH 7.5; river water used for dilution; static test without analytical monitoring |
48-h EC50 < 0.8 mg/l |
Bringmann & Kühn (1959) |
|
Water flea |
–a |
Daphnids: 2–4 days old; temperature: 20 °C; pH 7.5; test conducted in duplicate; static test; no data on analytical monitoring |
48-h EC50 = 1.28 mg/l |
Herbes & Beauchamp (1977) |
|
Water flea (Daphnia magna) |
No information |
6–24 h old; temperature: 22 °C; static test; 10 animals/25 ml; 6 concentrations; reconstituted hard water; no data on analytical monitoring |
24-h EC50 =15.6 mg/l (pH 6) |
Cronin et al. (2000) |
|
Grass shrimp (Palaemonetes pugio) |
USEPA, 1975 |
10 organisms per concentration used; synthetic seawater; salinity of 25‰; temperature: 22 °C; pH 8.3–8.7; study performed in duplicate |
96-h LC50 = 32.7 mg/l (measured value) |
Curtis et al. (1979) |
|
Acute toxicity multispecies test |
|
|
|
|
|
Pillbug (Asellus intermedius) |
–a |
10 juvenile organisms each; biological loading <0.5 g wet weight/l; temperature: 20 °C; dilution water taken from Lake Ontario; pH 6.5–8.5; photoperiod 16 h light; static test; nominal concentrations; hardness value of 130 mg/l as CaCO3 |
96-h LC50 > 100 mg/l |
Ewell et al. (1986) |
|
Water flea (Daphnia magna) |
|
|
96-h LC50 = 0.25 mg/l |
|
|
Flatworm (Dugesia tigrina) |
|
|
96-h LC50 > 100 mg/l |
|
|
Sideswimmer |
|
|
96-h LC50 > 100 mg/l |
|
|
Ramshorn snail |
|
|
96-h LC50 > 100 mg/l |
|
|
Segmented worm (Lumbriculus variegatus) |
|
|
96-h LC50 > 100 mg/l |
|
|
Fathead minnow (Pimephales promelas) |
|
|
96-h LC50 = 40 mg/l |
|
|
Toxicity to aquatic plants |
|
|
|
|
|
Green algae (Chlorella pyrenoidosa) |
–a |
12 h light/12 h dark; 6400 lux; temperature: 21 °C; measurement of cell density via haemocytometer |
72 h EC0 = 1.1 mg/l |
Stauber & Florence (1987) |
|
Green algae |
–a |
Initial cell density: ~7.5 × 106/ml; temperature: 36.5 °C; light: 28 W/m˛ |
6-h EC50 = 605 |
Kramer & Trümper (1986) |
|
Green algae (Scenedesmus quadricauda) |
–a |
Continuous lighting; temperature: 24 °C; pH 7.5; river water for dilution; measurement of turbidity |
96-h TTC = 60 mg/l |
Bringmann & Kühn (1959) |
|
Lesser duckweed |
–a |
Cultivation at 24 °C; 800 lux and 9 h light/15 h dark; solvent: tap water; every 24 h exchange of media; analytical monitoring |
12-day EC50 = 165 mg/l |
Stom & Roth (1981) |
|
Acute toxicity to microorganisms |
|
|
|
|
|
Bacteria (Pseudomonas fluorescens) |
–a |
River water for dilution; temperature: 25 °C; pH 7.5–7.8 |
16-h TTC (EC10) = 200 mg/l |
Bringmann & Kühn (1960) |
|
Protozoa (Tetrahymena pyriformis) |
–a |
According to Schultz (1983); no further information provided |
48-h EC50 = 543 mg/l |
Schultz (1987) |
|
Fungi (Fusarium oxysporum) |
–a |
Incubation at 25 °C; measurement of colony diameter |
6-day EC50 = 1100 mg/l |
Soni & Bhatia (1980) |
|
Chronic toxicity |
|
|
|
|
|
Rainbow trout (Oncorhynchus mykiss) |
Early life stage test (OECD TG 210) |
100 eggs exposed in 15-litre aquaria to 10 litres of test solution; temperature: 10 °C; hardness: 50 mg/l as CaCO3; pH 7.7; oxygen concentration: 10.8 mg/l; study carried out in duplicate |
60-day LC50 = 320 mg/l (embryolethality) |
Van Leeuwen et al. (1990) |
|
Water flea |
OECD TG 211 |
Daphnids <24 h old at the beginning; temperature: 19–21 °C; pH 8.0–8.2; flow-through test with analytical monitoring |
21-day NOEC = 0.172 mg/l (measured value, highest concentration tested) |
Lima (2004) |
|
Toxicity to terrestrial organisms |
|
|
|
|
|
Earthworm |
–a |
2 hatchlings <10 mg wet weight/petri dish; 30 g sludge placed over silt loam; temperature: 24 °C; 25 dishes with 5 replicates of 5 concentrations |
42-day LOEC = 10 000 mg/kg soil dry weight (weight) |
Hartenstein (1982) |
|
Lettuce |
–a |
10 lettuce seeds; incubation at 21–22 °C; 7 concentrations tested in duplicate |
3-day EC50 ~200 mg/l (radicle growth) |
Chou & Patrick (1976) |
TTC, toxicity threshold concentration
a No guideline study.
Based on the available results from laboratory tests with fish, invertebrates, plants, and a multispecies test system, resorcinol is classified as being of low toxicity to most aquatic organisms except the water flea (Daphnia magna). Resorcinol is very toxic to Daphnia magna, the most sensitive organism tested in both short-term and long-term test systems, according to the classification criteria described in the EU directive on dangerous substances.
There are several studies available on acute toxicity to different fish species. In general, the 96-h LC50 values for resorcinol were in the range between 26.8 and >100 mg/l. In flow-through tests and analytical monitoring, DeGraeve et al. (1980) determined a 96-h LC50 of 100 mg/l with fathead minnow (Pimephales promelas) and a 96-h LC50 of >100 mg/l with rainbow trout (Oncorhynchus mykiss). In another flow-through test with fathead minnow conducted in duplicate, 96-h LC50 values of 26.8 and 29.5 mg/l were found. The test was performed according to USEPA guidelines with analytical monitoring (Koppers Company, 1981).
Among the aquatic invertebrates, Daphnia magna shows the highest sensitivity to resorcinol exposure. In static tests without analytical monitoring, Bringmann & Kühn (1959) observed a 48-h EC50 of <0.8 mg/l (damaging effect on 50% of test organisms), and Herbes & Beauchamp (1977) obtained a 48-h EC50 of 1.28 mg/l. In the frame of a full life cycle toxicity test (OECD TG 211), Lima (2004) observed no adverse effects after 48 h of exposure at the highest concentration tested, 0.172 mg/l (measured concentration). Furthermore, Cronin et al. (2000) reported the dependency of toxicity on pH, with 24-h EC50s of 15.6, 28.3, and 34.8 mg/l at pH 6, 7.8, and 9, respectively. Curtis et al. (1979) determined a 96-h LC50 of 32.7 mg/l for the saltwater grass shrimp (Palaemonetes pugio).
Ewell et al. (1986) elaborated a method for simultaneous testing of seven juvenile aquatic species from five phyla. The fish and pond snails were placed directly into the aquaria, while the remaining five species were segregated in different baskets and suspended in the aquaria. The organisms were exposed to the test concentrations for 96 h. Biological observations were performed daily. For Asellus intermedius (pillbug), Dugesia tigrina (flatworm), Gammarus fasciatus (sideswimmer), Helisoma trivolis (ramshorn snail), and Lumbriculus variegatus (segmented worm), the 96-h LC50 values were >100 mg/l each. Daphnia magna (96-h LC50 = 0.25 mg/l) and fathead minnow (96-h LC50 = 40 mg/l) proved to be the most sensitive species in this test.
There is no guideline study on toxicity for aquatic plants available. However, Stauber & Florence (1987) showed that resorcinol at a concentration of 1.1 mg/l had no effect on the 72-h cell division rate (growth rate) of the freshwater green microalga Chlorella pyrenoidosa. As only one concentration was tested, the study cannot be used in the risk evaluation. Kramer & Trümper (1986) conducted growth inhibition tests with Chlorella vulgaris. They determined 6-h EC50 values of 605 and 835 mg/l in relation to biomass. Bringmann & Kühn (1959) observed a 96-h toxicity threshold concentration of 60 mg/l in a cell multiplication inhibition test; in the study, there were no indications as to whether the cultures were in the exponential growth phase. Stom & Roth (1981) determined, among others, a 12-day EC50 of 165 mg/l for Lemna minor in respect to plant multiplication. Florence & Stauber (1986) also found no significant effect of resorcinol at one concentration (1.1 mg/l) on the 72-h cell division rate of the marine alga Nitzschia closterium.
In several toxicity tests of microorganisms, EC50 values of >100 mg of resorcinol per litre were found. For example, Bringmann & Kühn (1960) determined a 16-h toxicity threshold concentration (EC10) of 200 mg/l for the bacterium Pseudomonas fluorescens in respect to inhibition of glucose degradation. Schultz (1987) observed a 48-h EC50 of 543 mg/l for Tetrahymena pyriformis (protozoa) in a cell multiplication inhibition test. For the inhibition of growth of the fungus Fusarium oxysporum, a 6-day EC50 of 1100 mg/l was found (Soni & Bhatia, 1980).
Van Leeuwen et al. (1990) investigated the toxicity of resorcinol to rainbow trout. In the early life stage test (OECD TG 210), a 60-day LC50 of 320 mg/l (in respect to embryolethality) and a 60-day EC50 of 260 mg/l (in respect to total embryotoxicity: lethality and malformation) were obtained in a semistatic test system using soft water. Using the end-point decrease in growth rate compared with control (measured by the wet weight of the fish), a 60-day LOEC of 32 mg/l was determined. A NOEC is not described in this study, but may be estimated at 10 mg/l, being the next lowest concentration of resorcinol. There is no indication as to whether the teratogenic effects were endocrine mediated or not (EC, 2003c).
Lima (2004) conducted a full life cycle toxicity test with Daphnia magna according to internationally accepted guidelines (OECD TG 211). At the highest concentration tested (measured value of 172 µg/l), no adverse effects on survival, growth, or reproduction were observed. The LOEC was given to be >0.172 mg/l. Because a LOEC could not be determined, the real 21-day NOEC value for the species Daphnia magna is probably higher than stated in this study.
Earthworms (Eisenia foetida) exposed to resorcinol in artificial soil (sludge) showed a significant reduction in weight after 42 days of exposure to 10 000 mg of resorcinol per kilogram soil dry weight (LOEC = 10 000 mg/kg soil dry weight). A resorcinol concentration of 40 000 mg/kg soil dry weight caused 100% mortality after 42 days of exposure (Hartenstein, 1982).
After incubation for 3 days at 21–22 °C, radicle growth of lettuce (Lactuca sativa) was reduced by 50% at a resorcinol concentration of approximately 200 mg/l (Chou & Patrick, 1976).
In humans, dermal exposure to resorcinol has been reported to be associated with thyroid effects, CNS disturbances, red blood cell changes, and a low incidence of skin sensitization.
Thyroid effects (enlarged thyroid glands, hyperactivity) have been reported after application of keratolytic topical medications containing high concentrations of resorcinol (up to 50%) or large amounts of such medication with lower (2%) resorcinol content for long time periods.
After topical use of high concentrations of resorcinol, CNS disturbances, such as dizziness, vertigo, confusion, disorientation, amnesia, or tremors, or red blood cell changes, such as methaemoglobinaemia, haemolytic anaemia, haemoglubinuria, or cyanosis, have been reported. In most cases, these effects disappeared within several days after discontinuing the resorcinol treatment. In some single case-reports, after exposure to high dermal/oral concentrations, fatal outcomes have been reported. One factor increasing potential toxic effects is the application of resorcinol to injured skin.
In three male volunteers, topical application of resorcinol at 12 mg/kg body weight (applied as 2% resorcinol in a hydroalcoholic vehicle) twice daily on 6 days/week over 4 weeks gave no indication for altered thyroid function (T3/T4/T7/TSH levels) (Yeung et al., 1983).
From its use in topical medication and from limited data from occupational studies, resorcinol does not appear to be irritating to the skin in the concentrations reported.
Several case-reports describe sensitization to resorcinol through the use of medicinal products and anti-acne cream. In several patch test studies with large collectives, the prevalence of sensitivity to resorcinol was less than 2% when tested at resorcinol concentrations of <2%. With increasing resorcinol concentrations, there was an increase in the number of persons who tested positive. As no information is available on the levels of exposure in the studied populations, no estimate of the sensitizing potency of resorcinol can be made. In practice, however, the incidence of sensitization seems to be low.
In animal studies, the toxicological effects reported to be caused by administration of resorcinol include thyroid dysfunction, irritation to skin and eyes, CNS effects, and altered adrenal gland relative weights. In high-dose groups, a decrease in body weight and decreased survival were noted.
Owing to concern about the thyroid effects of resorcinol, which have been shown in human studies to occur at high doses, this end-point in particular has been the point of focus in several studies. In most of the older studies, the effects of resorcinol exposure on the thyroid gland are conflicting. It has been suggested that thyroid effects seem to be dependent on an administration route that allows for continued systemic exposure (e.g. continuous exposure via diet, drinking-water, or subcutaneous administration in hydrophobic vehicles). Effects on the thyroid gland, such as increased thyroid gland weights and changes in T3/T4 levels, were seen after oral dosing of rats via drinking-water with about 5–10 mg/kg body weight over 30 days with a low-iodine and low-protein diet (Cooksey et al., 1985) or over 12 weeks at about 5 mg/kg body weight (Seffner et al., 1995). However, these studies are single-dose studies and have not been confirmed by subsequent studies.
In a one-generation dose range-finding study with male and female Crl:CDÒ (SD)IGS BR rats (continuously dosed with resorcinol in drinking-water at 0, 10, 40, 120, or 360 mg/l; about 0, 1, 5, 15, and 40 mg/kg body weight per day), there were some thyroid effects, including minimal changes of the follicular hyperplasia at the higher doses, but they were not consistent or dose related (RTF, 2003).
In the subsequent main two-generation drinking-water study using 0, 120, 360, 1000, or 3000 mg/l, which also focused on thyroid effects (RTF, 2005), no statistically significant resorcinol-related changes in the mean concentrations of T3, T4, or TSH were observed in the F0 and F1 parental animals or in the F1 and F2 pups selected for analysis. Higher TSH values were noted in the F0 males at scheduled necropsy, but these were not considered to be resorcinol-related effects in the absence of effects on T3 or T4, organ weights, or adverse macroscopic or microscopic findings. Resorcinol-related decreased colloid within the thyroid glands of the 3000 mg/l F0 males was not considered to be adverse due to a lack of associated functional effects. An exposure level of 3000 mg/l was given as the NOAEL when resorcinol was offered continuously in the drinking-water to parental rats. When expressed on a body weight basis (average of F0 and F1 animals), this concentration corresponded to approximately 233 mg/kg body weight per day for males over the entire generation and 304 mg/kg body weight per day for females during premating and gestation.
No histopathological changes in the thyroid were found in subacute, subchronic, or chronic studies performed via gavage in rats or mice (NTP, 1992); however, T3/T4 levels were not determined, with the exception of the 0 and 130 mg/kg body weight dose groups in the 13-week rat study where no effect was reported. In the long-term study (104 weeks), NOAELs for thyroid effects were 150–520 mg/kg body weight per day (5 days/week); however, these studies were not designed to investigate this end-point (NTP, 1992).
Resorcinol caused no adverse effects in several reproduction and developmental toxicity studies in rats and rabbits.
Resorcinol is not considered to be genotoxic. In in vitro genotoxicity tests, resorcinol showed mostly negative results. Results from all reported in vivo tests for genotoxicity were negative.
Long-term carcinogenicity studies in male F344 rats and B6C3F1 mice of both sexes dosed at 0–225 mg/kg body weight per day and female rats exposed to 0–150 mg/kg body weight per day, 5 days/week, for 104 weeks were negative. Mostly negative results were reported in the initiation–promotion studies performed using several species.
However, in the above carcinogenicity study, clinical signs such as ataxia and tremors were noted at about 100 mg/kg body weight in both species (NOAEL for acute clinical signs indicative of an effect on the CNS = 50 mg/kg body weight; NOAEL adjusted for 5 days/week dosing = 36 mg/kg body weight per day).
There are two toxicological effects that could be used for deriving a tolerable intake: thyroid and neurological effects. Both these effects have been reported in human case-reports from dermal application of high concentrations (up to 50%) of resorcinol in ointments for ulcers and in peelings, as well as in rodent studies at high concentrations (see Table 7). There is no rodent study covering both end-points adequately.
The human data describing thyroidal and neurological effects were case-reports giving only estimates of exposure and are therefore inadequate to provide a tolerable intake. There are differences in thyroid physiology between humans and animals. The weight of evidence suggests that rodents are more sensitive to thyroid effects than humans. There were no published animal studies showing thyroidal effects with a dose–response relationship that could be used for a tolerable intake.
For this reason, the study chosen to derive a tolerable intake was the long-term NTP (1992) study in rats in which a NOAEL of 50 mg/kg body weight for neurological effects (acute clinical signs) was derived (equivalent to 36 mg/kg body weight after adjusting for 5 days/week dosing). No histopathological changes were seen in the thyroid. There was no measurement of T3/T4 ratios. Application of uncertainty factors for interspecies (10) and intraspecies (10) differences results in a tolerable intake of 0.5 mg/kg body weight per day (0.4 corrected for 5 days/week dosing).
In section 6.2, three scenarios are presented for consumers: dermal exposure to resorcinol in hair dyes, in anti-acne creams, and in peeling (see Table 6). The estimated exposure (mg/kg body weight) and the estimated duration and frequency of application were considered. Exposure to resorcinol by the use of peeling agents presents an acute exposure scenario where the person is exposed to a high concentration (7.8 mg/kg body weight) for a short time (30 s to 10 min; maximum 10 sessions 2 weeks apart). Acute effects have been described under these conditions. From the scarce data available, the use of resorcinol in hair dyes (0.03 mg/kg body weight) for 30 min once a month does not appear to be a cause of concern. The use of anti-acne cream is taken as the scenario of most concern due to the likelihood of continuous exposure. Based on the study of Yeung et al. (1983), a worst-case systemic exposure of 0.4 mg/kg body weight was calculated.
Thyroid effects have been reported in human case-reports at crude estimates of dermal exposure of 34–122 mg/kg body weight per day (assuming that 2.87% of the dose is systemically available = 1–3.5 mg/kg body weight per day) (Gans, 1980). In contrast, in a worst-case exposure study using 2% anti-acne cream (Yeung et al., 1983), no thyroid effects (i.e. no alterations in T3/T4/T7/TSH levels) were seen at a dermal dose of 12 mg/kg body weight per day (estimated systemic dose level of 0.4 mg/kg body weight per day).
The tolerable intake of 0.4 mg/kg body weight per day derived from the NTP (1992) study would therefore be protective for both neurological and thyroid effects.
The key study (NTP, 1992) gave a NOAEL of 50 mg/kg body weight (36 mg/kg body weight per day corrected for 5 days/week dosing) based on the clinical signs observed after administration of doses of 100 mg/kg body weight (71 mg/kg body weight per day, corrected for 5 days/week dosing) and more. However, it should be noted that 100 mg/kg body weight when administered by drinking-water showed no effects on the CNS. It is therefore possible that these neurological effects are due to the acute effect of the gavage administration. CNS effects in humans and animals have been associated with acute exposure to resorcinol.
Although thyroid effects are a significant end-point in both human case-studies and animal studies, there is a lack of consistency in the results in the animal studies. Further, due to the lack of comparative toxicokinetic and toxicodynamic data between animals and humans for this end-point, it is not possible to extrapolate from animal studies to humans.
The key study for the exposure assessment (Yeung et al., 1983) was based on healthy volunteers and not acne patients. Acned skin may be damaged due to either scratching or the blemishes themselves. Therefore, the uptake may be higher than estimated in the scenario, with up to 100% absorption in limited small areas, which would increase the daily systemic exposure. However, the exposed area (2600 cm2) was greatly in excess of the average area of skin usually treated for acne.
Formulations tested in the key studies (anti-acne cream and hair dye) have probably changed over the last 20 or more years.
Resorcinol enters the environment mainly during its usage in consumer products (hair dyes and pharmaceuticals). In addition, localized high concentrations can appear in coal conversion wastewater or in wastewater in regions with oil shale mining.
Calculations predict the hydrosphere to be the main target compartment of resorcinol. Furthermore, the calculated Henry’s law constant indicates that resorcinol is essentially non-volatile from aqueous solution.
In the atmosphere, resorcinol is rapidly degraded (half-life about 2 h) by reaction with photochemically produced hydroxyl radicals.
In the hydrosphere, hydrolysis is not expected to occur. However, in aqueous solution, autoxidation of resorcinol takes place, and it can be assumed that resorcinol reacts in water bodies with hydroxyl and peroxyl radicals. Under aerobic conditions, resorcinol proved to be readily biodegradable in a test conducted according to OECD TG 301C, and it is likely to be biodegraded under anaerobic conditions.
Experimental data on soil sorption using silty loam indicate a very low potential for geoaccumulation. Bioaccumulation is also not to be expected based on the calculated BCF.
A risk characterization may be performed by calculating the ratio between a (local or regional) PEC (based on a measured or model calculation) and a PNEC (EC, 2003a). Localized concentrations are available only for coal conversion wastewater or wastewater in oil shale regions. These values are unsuitable for a risk assessment of the emissions from anthropogenic sources because they are not representative of concentrations infiltrating surface waters or groundwaters. However, modelled concentrations (see section 6 and Appendix 5) can be taken as a first approach.
The results of the calculations show that the highest concentrations are expected to be at local point sources, such as sites where hair dyes are formulated or rubber products are manufactured. These estimated concentrations in water are 1 order of magnitude higher than the local concentrations resulting from emissions from the use of consumer products containing resorcinol, which are released on a continental scale.
Results from tests with different aquatic species from different trophic levels are available for the acute toxicity of resorcinol to aquatic organisms. Furthermore, chronic studies with fish and Daphnia were conducted. For the toxicity to aquatic plants, no guideline study is available. However, taking into account the available studies for toxicity to aquatic plants, the NOEC for algae can be assumed to be higher than that for Daphnia magna. Overall, the studies are not sufficient for a statistical extrapolation technique according to the EU Technical Guidance Document (EC, 2003a). As a first approach, a PNEC can be calculated by applying an assessment factor to the NOEC for the most sensitive species. According to the results obtained, Daphnia magna is the most sensitive organism, and resorcinol can be classified as acutely toxic only to Daphnia. The lowest NOEC was determined for Daphnia magna in a full life cycle toxicity test based on measured concentrations (21-day NOEC = 172 µg/l). However, this value is questionable for use as a limit value in risk assessment (this is the highest concentration tested in this study), and the NOEC could be significantly higher than stated in the study. Without taking into account the drawback of this study, a PNECaqua = 3.44 µg/l can be derived using an assessment factor of 50 according to the EU Technical Guidance Document (EC, 2003a), as results from chronic studies from two trophic levels (fish and daphnia) are available.
Using this PNEC value and PEC values for surface water derived in section 6, the risk (PEC/PNEC) from resorcinol in the aquatic environment (surface water) was estimated (see Table 9).
Table 9. Risk characterization for surface water.
|
PECa (µg/l) |
PEC/PNECb |
|
|
Regional |
0.129 |
0.038 |
|
Local (rubber industry) |
7.09 |
2.06 |
|
Local (formulation of hair dyes)c |
22.3c / 8.88d |
6.5c / 2.6d |
|
Local (use as hair dyes and pharmaceuticals) |
0.904 |
0.26 |
|
a |
Estimated values; for calculation, see Appendix 5. |
|
b |
PNECaqua = 3.44 µg/l. |
|
c |
Conservative removal in sewage treatment plant ("SimpleTreat"). |
|
d |
Taking into account the simulation test for removal in sewage treatment plant. |
For the regional surface waters, calculations showed a low risk (i.e. PEC/PNEC < 1). The rubber industry is the largest consumer of resorcinol. The PEC/PNEC value of 2.06 indicates a risk for surface waters. This is assuming that the wastewater is connected to a wastewater treatment plant. If this is not the case, there is an increased calculated risk from rubber industry effluent.
The applications as hair dyes and pharmaceuticals result in a low probability of negative effects on surface waters (PEC/PNEC = 0.26). In contrast, at local point sources, such as at sites where hair dyes are formulated, a risk cannot be excluded using the conservative approach ("SimpleTreat") (PEC/PNEC = 6.5). In sewage treatment plants, there is actually a higher removal of resorcinol, as indicated by a simulation test; therefore, in hair dye formulation sites using sewage treatment plants, the calculated risk would be reduced (PEC/PNEC = 2.6).
In conclusion, there may be a risk from resorcinol in the aquatic environment from sites where hair dyes are formulated and from rubber production plants.
Owing to the fact that the data for toxicity to terrestrial organisms are not sufficient, a PNECsoil = 5.86 µg/kg dry weight was calculated from PNECaqua using the equilibrium partitioning method according to EC (2003a).
Resorcinol is released to soil during production of rubber products. Because of its low potential for adsorption to organic matter, resorcinol leaches out of the soil and is distributed to the hydrosphere. A local PEC has not been calculated; only a value for the regional industrial soil has been calculated (PECregionalsoil, ind. = 0.583 µg/kg dry weight; see Appendix 5). The conservative quotient for regional industrial soil is therefore PECsoil/PNECsoil = 0.099. A low risk is estimated for the regional soil compartment. However, a risk at local point sources cannot be excluded.
None of the measured concentrations of resorcinol in the environment (see section 6) are suitable for the risk characterization. However, environmental concentrations have been estimated using information on emissions and a Mackay Level III fugacity model. In such a model, the distribution and absolute concentrations may depend greatly upon the compartment of entry. Furthermore, the calculation is based only on the estimated emissions of selected applications (rubber industry, hair dyes, and pharmaceuticals). At local point sources, relatively high emissions are also possible from other applications.
Resorcinol is found in a wide variety of natural products and is a degradation product of humic substances. There are no data available on environmental monitoring in water except for coal conversion wastewater and leachate samples from the oil shale industry. It is therefore not possible to assess the consequences of these background levels of resorcinol on the environment.
The biodegradation rate used to estimate the environmental concentration is calculated using the model "SimpleTreat" (implemented in EUSES 2.0.3), resulting in a conservative, worst-case value. The percentage of biodegradation in a given wastewater treatment plant could be significantly higher, as indicated by a simulation test, so that the environmental concentration might be significantly lower. Hence, an improved biodegradation rate results in reduced risks for the environment.
Regarding the effects of resorcinol on aquatic species, the toxicity data set includes a variety of species from different trophic levels. Most of the studies are of sufficient quality and acceptable for risk characterization purposes. The estimated PNEC values represent a worst-case approach due to the uncertainty of the 21-day NOEC for Daphnia magna. For the benthic and terrestrial compartments, the available toxicity data cannot be regarded as adequate for a quantitative risk characterization. However, these compartments are less relevant for resorcinol, because of marginal releases and emissions to these compartments as well as a very low potential for adsorption of resorcinol to organic matter. However, an estimation of risk using the equilibrium partitioning method can be made.
IARC (1999) evaluated the carcinogenicity of resorcinol in 1998 and concluded that there are no epidemiological data relevant to the carcinogenicity of resorcinol in humans and that the evidence of its carcinogenicity in animals is inadequate; thus, resorcinol is not classifiable as to its carcinogenicity to humans.
JECFA (2001) assessed the hazards from the use of resorcinol as a food flavouring agent and concluded that this use is of no safety concern.
At the time of adoption of the CICAD, an assessment of resorcinol was under way as part of the HPV Chemical Programme of the OECD. It is intended that the results of this CICAD be shared to the maximal extent with the OECD; to this end, the peer review of the draft CICAD was extended to all OECD Member countries. Any new information being provided in the course of the OECD assessment will be provided to the IPCS so that an addendum can be prepared for consideration.
Abbate C, Polito I, Puglisi A, Brecciaroli R, Tanzariello A, Germano D (1989) Dermatosis from resorcinal in tyre makers. British Journal of Industrial Medicine, 46:212–214.
Alison RH, Capen CC, Prentice DE (1994) Neoplastic lesions of questionable significance to humans. Toxicologic Pathology, 22:179–186.
Angel A, Rogers KJ (1972) An analysis of the convulsant activity of substituted benzenes in the mouse. Toxicology and Applied Pharmacology, 21:214–229.
APHA, AWWA, WPCA (1976) Standard methods for the examination of water and wastewater, 14th ed. Washington, DC, American Public Health Association, American Water Works Association, and Water Pollution Control Federation, 1193 pp.
Arnott DG, Doniach I (1952) The effect of compounds allied to resorcinol upon the uptake of radioactive iodine (131I) by the thyroid of the rat. Biochemical Journal, 50:473–479.
Bachet J, Guilmet D (1999) The use of biological glue in aortic surgery. Cardiology Clinics, 17(4):779–796.
Baer RL, Ramsey DL, Biondi E (1973) The most common contact allergens 1968–1970. Archives of Dermatology, 108:74–78.
Barbaud A, Modiano P, Cocciale M, Reichert S, Schmutz J-L (1996) The topical application of resorcinol can provoke a systemic allergic reaction. British Journal of Dermatology, 135:1014–1015.
Barbaud A, Reichert-Penetrat S, Trechot P, Granel F, Schmutz J-L (2001) [Sensitization to resorcinol in a prescription verrucide preparation: unusual systemic clinical features and prevalence.] Annales de dermatologie et de vénéréologie, 128(5):615–618 (in French).
Basketter DA, Scholes EW, Kimber I (1994) The performance of the local lymph node assay with chemicals identified as contact allergens in the human maximization test. Food and Chemical Toxicology, 32(6):543–547.
Bauer (1985) Personal communication [cited in CANTOX, 2000].
Becker J (1933) Resorzin-Salbe verursacht tödliche Vergiftung bei einem Säugling. In: Fühner F, ed. Sammlung von Vergiftungsfällen. Vol. 4. Berlin, Verlag von F.C.W. Vogel in Berlin, pp. 7–8.
Beezer AE, Hunter WH, Storey DE (1980) Quantitative structure–activity relationships: the Van’t Hoff heats of transfer of resorcinol monoethers from water to .-octanol. Journal of Pharmacy and Pharmacology, 32:815–819.
Berthezéne F, Perrot L, Munary Y, Ponsin G (1979) Multiple effects of resorcinol on thyroid function. Annals of Endocrinology, 40:67–68.
Blum DJW, Heergenroeder R, Parkin GF, Speece RE (1986) Anaerobic treatment of coal conversion wastewater constituents: biodegradability and toxicity. Journal of the Water Pollution Control Federation, 58:122–131.
Bontemps H, Mallaret M, Besson G, Bochaton H, Carpentier F (1995) Confusion after topical use of resorcinol. Archives of Dermatology, 131(1):112.
Boule P, Guyon C, Lemaire J (1982) Photochemistry and environment IV — Photochemical behaviour of monochlorophenols in dilute aqueous solution. Chemosphere, 11(12):1179–1188.
Boyd SA (1982) Adsorption of substituted phenols by soil. Soil Science, 134(5):337–343.
Boyd SA, Shelton DR, Berry D, Tiedje JM (1983) Anaerobic biodegradation of phenolic compounds in digested sludge. Applied Environmental Microbiology, 46:50–54.
Bracher M, Wisak J, Noser F (1981) Studies on the potential in vivo induction of sister chromatid exchanges in rat bone marrow by resorcinol. Mutation Research, 91:363–369.
Bringmann G, Kühn R (1959) Vergleichende wasser-toxikologische Untersuchungen an Bakterien, Algen und Kleinkrebsen. Gesundheits-Ingenieur, 80:115–120.
Bringmann G, Kühn R (1960) Vergleichende toxikologische Befunde an Wasser-Bakterien. Gesundheits-Ingenieur, 81:337–339.
BUA (1993) Resorcinol (1,3-Dihydroxybenzol). Beratergremium für umweltrelevante Altstoffe; Stuttgart, S. Hirzel Wissenschaftliche Verlagsgesellschaft, pp. 1–80 (BUA Report 99).
Bull GM, Fraser R (1950) Myxoedema from resorcinol ointment applied to leg ulcers. Lancet, 1(18):851–855.
CANTOX (2000) Resorcinol: toxicology review and risk assessment with emphasis on thyroidal effects. Bridgewater, NJ, CANTOX Health Sciences International, pp. 1–29.
Capen CC, Roland RA, Yarrington JT (1991) Endocrine system. In: Haschek WM, Rousseaux CG, Wallig MA, eds. Handbook of toxicologic pathology, 2nd ed. Vol. 2. San Diego, CA, Academic Press, pp. 681–783.
Cassano N, Alessandrini G, Mastrolonardo M, Vena GA (1999) Peeling agents: toxicological and allergological aspects. Journal of the European Academy of Dermatology and Venereology, 13(1):14–23.
CEH (2001) Chemical economics handbook: Resorcinol — product review. Menlo Park, CA, SRI International, pp. 1–58 (691.7000A).
Chapman PJ, Ribbons DW (1976) Metabolism of resorcinylic compounds by bacteria: alternative pathways for resorcinol catabolism in Pseudomonas putida. Journal of Bacteriology, 125(3):985–998.
Cheymol J, Gay Y, Laveden JP (1951) [Antithyroid action of diphenols.] Comptes Rendus des Séances de la Société de la Biologie et de ses Filiales,.145:999–1001 (in French) [cited in CANTOX, 2000].
Chou C-H, Patrick ZA (1976) Identification and phytotoxic activity of compounds produced during decomposition of corn and rye residues in soil. Journal of Chemical Ecology, 2(3):369–387.
Chou WL, Speece RE, Siddiqi RH (1979) Acclimation and degradation of petrochemical wastewater components by methane fermentation. Biotechnology and Bioengineering Symposium, 8:391–414.
Coleman WP (2001) Dermal peels. Dermatology Clinics, 19(3):405–411.
Commins BT, Lindsey AJ (1956) The determination of phenols by chromatography and spectrophotometry of their methyl ethers. IV. The determination of phenols in cigarette smoke. Analytica Chimica Acta, 15:557–558.
Cooksey RC, Gaitan E, Lindsay RH, Hill HB, Kelly K (1985) Humic substances, a possible source of environmental goitrogens. Organic Geochemistry, 8(1):77–80.
Cooper RL, Wheatstone KC (1973) The determination of phenols in aqueous effluents. Water Research, 7:1375–1384.
Cosmetic Ingredient Review (1986) Final report on the safety assessment of 2-methylresorcinol and resorcinol. Journal of the American College of Toxicology, 3:167–203.
Cosmetic Ingredient Review (2004) Hair dye ingredients. Washington, DC, Cosmetic Ingredient Review (http://www.cir-safety.org/findings.shtml).
Crebelli R, Conti L, Carere A, Zito R (1981) Mutagenicity of commercial .-phenylenediamine and of an oxidation mixture of .-phenylenediamine and resorcinol in Salmonella typhimurium TA98. Food and Cosmetics Toxicology, 19:79–84.
Crebelli R, Falcone E, Aquilina G, Carere A (1984) Mutagenicity studies in a tyre plant: in vitro activity of urine concentrates and rubber chemicals. IARC Scientific Publications, 59:289–295.
Crebelli R, Paoletti A, Falcone E, Aquilina G, Fabri G, Carere A (1985) Mutagenicity studies in a tyre plant: in vitro activity of workers’ urinary concentrates and raw materials. British Journal of Industrial Medicine, 42:481–487.
Cronin E (1973) Resorcin in Castellani’s paint. Contact Dermatitis Newsletter, 14:401.
Cronin MTD, Zhao YH, Yu RL (2000) pH-dependence and QSAR analysis of the toxicity of phenols and anilines to Daphnia magna. Environmental Toxicology, 15(2):140–148.
Cunningham AA (1956) Resorcin poisoning. Archives of Disease in Childhood, 31:173–176.
Curran PG, DeGroot LJ (1991) The effect of hepatic enzyme-inducing drugs on thyroid hormones and the thyroid gland. Endocrine Reviews, 12(2):135–150.
Curtis M, Ward C (1981) Aquatic toxicity of forty industrial chemicals: testing in support of hazardous substance spill prevention regulation. Journal of Hydrology, 51:359–367.
Curtis MW, Copeland TL, Ward CH (1979) Acute toxicity of 12 industrial chemicals to freshwater and saltwater organisms. Water Research, 13:137–141.
Darroudi F, Natarajan AT (1983) Cytogenetic analysis of human peripheral blood lymphocytes (in vitro) treated with resorcinol. Mutation Research, 124:179–189.
Dave VK (1973) Contact dermatitis due to resorcin in Castellani’s paint. Contact Dermatitis Newsletter, 13:384.
DeGraeve GM, Geiger DL, Meyer JS, Bergman HL (1980) Acute and embryo–larval toxicity of phenolic compounds to aquatic biota. Archives of Environmental Contamination and Toxicology, 9:557–568.
Delzell E (2000) Clinical and epidemiologic research on resorcinol. Submitted to the Resorcinol Task Force [Appendix A in CANTOX, 2000].
DiNardo JC, Picciano JC, Schnetzinger RW, Morris WE, Wolf BA (1985) Teratological assessment of five oxidative hair dyes in the rat. Toxicology and Applied Pharmacology, 78:163–166.
Divi RL, Doerge DR (1994) Mechanism-based inactivation of lactoperoxidase and thyroid peroxidase by resorcinol derivatives. Biochemistry, 33:9668–9674.
Döhler K-D, Wong CC, von zur Mühlen A (1979) The rat as a model for the study of drug effects on thyroid function: consideration of methodological problems. Pharmacology & Therapeutics, 5:305–318.
Doniach I, Fraser R (1950) Effect of resorcinol on the thyroid uptake of I131 in rats. Lancet, 1(18):855–856.
Doniach I, Logothetopoulos J (1953) The goitrogenic action of resorcinol in rats. British Journal of Experimental Pathology, 34(2):146–151.
Dressler H (1994) Resorcinol, its uses and derivatives. New York, NY, Plenum Press.
Dressler WE (1999) Hair dye absorption. In: Bronaugh RL, Maibach HI, eds. Percutaneous absorption: drugs–cosmetics–mechanism–methodology, 3rd ed., revised and expanded. New York, NY, Marcel Dekker, pp. 685–716 (Drugs and the Pharmaceutical Sciences Vol. 97).
Duran B, Gursoy S, Cetin M, Demirkoprulu N, Demirel Y, Gurelik B (2004) The oral toxicity of resorcinol during pregnancy: a case report. Journal of Toxicology, Clinical Toxicology, 42(5):663–666.
Dybing E, Sanner T (1999) Species differences in chemical carcinogenesis of the thyroid gland, kidney and urinary bladder. IARC Scientific Publications, 147:15–32.
Eastin WC, Haseman JK, Mahler JF, Bucher JR (1998) The National Toxicology Program evaluation of geneticallly altered mice as predictive models for identifying carcinogens. Toxicologic Pathology, 26(4):461–473.
EC (2002) Study on the scientific evaluation of 12 substances in the context of endocrine disrupter priority list of actions. Produced by WRc-NSF, Medmenham, for the European Commission, 613 pp. (WRc-NSF Reference: UC 6052; http:// europa.eu.int/comm/environment/endocrine/documents/ wrc_report.pdf).
EC (2003a) Technical guidance document in support of Commission Directive 93/67/EEC on risk assessment for new notified substances, Commission Regulation (EC) No. 1488/94 on risk assessment for existing substances (Parts I, II, III and IV) and Directive 98/8/EC of the European Parliament and the Council concerning the placing of biocidal products on the market. Ispra, Italy, European Commission, European Chemicals Bureau, Joint Research Centre (http://ecb.jrc.it/home.php?CONTENU=/tgdoc/ sommaire.php).
EC (2003b) Council Directive 76/768/EEC of 27 July 1976 on the approximation of the laws of the Member States relating to cosmetic products. European Communities, pp. 1–83 (CONSLEG: 1976L0768-15/10/2003; http://europa.eu.int/ scadplus/leg/en/lvb/ l21191.htm).
EC (2003c) Opinion of the Scientific Committee on Toxicity, Ecotoxicity and the Environment (CSTEE) on: "Two study reports on endocrine disrupters by WRc-NSF and BKH Consulting Engineers". Brussels, European Commission, pp. 1–20 (http://europa.eu.int/comm/health/ph_risk/committees/sct/ documents/out208_en.pdf).
EC (2004) European Union System for the Evaluation of Substances 2.0 (EUSES 2.0). Prepared for the European Chemicals Bureau by the National Institute of Public Health and the Environment (RIVM), Bilthoven (RIVM Report No. 601900005). Available via the European Chemicals Bureau (http://ecb.jrc.it).
Eide ME (1994) Resorcinol. In: OSHA stopgap method. Salt Lake City, UT, Occupational Safety and Health Administration Technical Center, Organic Service Branch, 10 pp.
Erdmann S, Dickel H, Merk H-F (1997) Allergische Kontaktdermatitis durch Resorcin nach Anwendung von Solutio Castellani. Zeitschrift für Hautkrankheiten, 72:926–927.
Ewell WS, Gorsuch JW, Kringle RO, Robillard KA, Spiegel RC (1986) Simultaneous evaluation of the acute effects of chemicals on seven aquatic species. Environmental Toxicology and Chemistry, 5:831–840.
Fh-ITEM (2004) Estimation of environmental/chemical properties for 1,3-benzenediol (CAS: 108-46-3), Epi Suite Version 3.12. Hanover, Fraunhofer Institute of Toxicology and Experimental Medicine, 10 pp.
Fh-ITEM (2005a) Calculation of the distribution in the environment using the fugacity-based Environmental Equilibrium Partitioning Model Level I, Version 3.00. Hanover, Fraunhofer Institute of Toxicology and Experimental Medicine, 4 pp.
Fh-ITEM (2005b) Estimation of the environmental concentrations and the risk characterisation of resorcinol using EUSES 2.0.3 incl. SimpleTreat 3.0. Full study report. Hanover, Fraunhofer Institute of Toxicology and Experimental Medicine, 35 pp.
Fisher AA (1982) Resorcinol — a rare sensitizer. Cutis, 29:331–332, 338.
Flickinger CW (1976) The benzenediols: catechol, resorcinol and hydroquinone — a review of the industrial toxicology and current industrial exposure limits. American Industrial Hygiene Association Journal, 37:596–606.
Flickinger CW (1978) 1978 status report of industrial hygiene monitoring at the Petrolia, Pennsylvania, Plant, Chemical Division, Organic Material Group [unpublished study cited in Delzell, 2000].
Florence TM, Stauber JL (1986) Toxicity of copper complexes to the marine diatom Nitzschia closterium. Aquatic Toxicology, 8(1):11–26.
Florin I, Rutberg L, Curvall M, Enzell CR (1980) Screening of tobacco smoke constituents for mutagenicity using the Ames’ test. Toxicology, 18:219–232.
Fräki JE, Peltonen L, Hopsu-Havu VK (1979) Allergy to various components of topical preparations in stasis dermatitis and leg ulcer. Contact Dermatitis, 5:97–100.
Frosch PJ (1990) Aktuelle Kontaktallergene. Der Hautarzt, 41 (Suppl.10):129–133.
Frosch PJ, Burrows D, Camarasa JG, Dooms-Goossens A, Ducombs G, Lahti A, Menné T, Rycroft RJG, Shaw S, White IR, Wilkinson JD (1993) Allergic reactions to a hairdressers’ series: results from 9 European centres. Contact Dermatitis, 28:180–183.
Gaal A, Neujahr HY (1979) Metabolism of phenol and resorcinol in Trichosporon cutaneum. Journal of Bacteriology, 137(1):13–21.
Gamble JF, McMicheal AJ, Williams T, Battigelli M (1976) Respiratory function and symptoms: an environmental–epidemiological study of rubber workers exposed to a phenol–formaldehyde type resin. American Industrial Hygiene Association Journal, 37(5):499–513.
Gans EH (1980) Personal communication (unpublished letter) [cited in Lynch et al., 2002].
Garton GA, Williams RT (1949) Studies in detoxication: the fates of quinol and resorcinol in the rabbit in relation to the metabolism of benzene. Biochemical Journal, 44:234–238.
Gocke E, King MT, Eckhardt K, Wild D (1981) Mutagenicity of cosmetics ingredients licensed by the European Communities. Mutation Research, 90:91–109.
Graham SG, Tisdall FF (1922) Poisoning from external use of resorcin. Canadian Medical Association Journal, 12:730–732.
Groseclose EE, Ribbons DW (1981) Metabolism of resorcinylic compounds by bacteria: new pathway for resorcinol catabolism in Azotobacter vinelandii. Journal of Bacteriology, 146(2):460–466.
Gubser H (1969) Probleme bei der Reinigung von Chemieabody weightässern. Gas Wasser Abwasser, 49:175–181.
Guerra L, Bardazzi F, Tosti A (1992a) Contact dermatitis in hairdressers’ clients. Contact Dermatitis, 26:108–111.
Guerra L, Tosti A, Bardazzi F, Pigatto P, Lisi P, Santucci B, Valsecchi R, Schena D, Angelini G, Sertoli A, Ayala F, Kokelj F (1992a) Contact dermatitis in hairdressers: the Italian experience. Gruppo Italiano Ricerca Dermatiti da Contatto e Ambientali. Contact Dermatitis, 26:101–107.
Hansch C, Leo A, Hoekman D (1995) .-Dihydroxybenzene (CAS 108-46-3). In: Exploring QSAR. Hydrophobic, electronic, and steric constants. Washington, DC, American Chemical Society, p. 20.
Hard GC (1998) Recent developments in the investigation of thyroid regulation and thyroid carcinogenesis. Environmental Health Perspectives, 106(8):427–457.
Harris J (1990) Rate of hydrolysis. In: Lymann WJ, Reehla WF, Rosenblatt DH, eds. Handbook of chemical property estimation methods. Washington, DC, American Chemical Society, pp. 7-1–7-48.
Hartenstein R (1982) Effect of aromatic compounds, humic acids and lignins on growth of the earthworm Eisenia foetida. Soil Biology and Biochemistry, 14:595–599.
Hasegawa R, Ito N (1992) Liver medium-term bioassay in rats for screening of carcinogens and modifying factors in hepatocarcinogenesis. Food and Chemical Toxicology, 30(11):979–992.
Hasegawa R, Furukawa F, Toyoda K, Takahashi M, Hayashi Y, Hirose M, Ito N (1990) Inhibitory effects of antioxidants on .-bis(2-hydroxypropyl)nitrosamine-induced lung carcinogenesis in rats. Japanese Journal of Cancer Research, 81(9):871–877.
Hashizume T, Yamagami T, Sasaki Y (1967) Constituents of cane molasses. Part II. Separation and identification of the phenolic compounds. Agricultural and Biological Chemistry, 31(3):324–329.
Haworth S, Lawlor T, Mortelmans K, Speck W, Zeiger E (1983) Salmonella mutagenicity test results for 250 chemicals. Environmental Mutagenesis, 5(Suppl. 1):1–142.
HCTS (2002) Resorcinol. Use in the cosmetic industry assessment of exposure scenarios. Hair Colouring Technical Secretariat (Europe), pp. 1–12.
Health Council of the Netherlands (2004) Resorcinol (CAS No. 108-46-3). Health-based reassessment of administrative occupational exposure limits. The Hague, Health Council of the Netherlands, Committee on Updating of Occupational Exposure Limits, pp. 1–28 (No. 2000/15OSH/139; http://www.gr.nl/ pdf.php?ID=1099&p=1).
Heider J, Fuchs G (1997) Anaerobic metabolism of aromatic compounds. European Journal of Biochemistry, 243(3):577–596.
Herbes SE, Beauchamp JJ (1977) Toxic interaction of mixtures of two coal conversion effluent components (resorcinol and 6-methylquinoline) to Daphnia magna. Bulletin of Environmental Contamination and Toxicology, 17(1):25–32.
Hernández-Pérez E (1997) Resorcinol peel as a part of a facial rejuvenation program. American Journal of Cosmetic Surgery, 14:35–40.
Hernández-Pérez E (2002) The versatile golden peel: when less is more. International Journal of Cosmetic Surgery and Aesthetic Dermatology, 4(1):27–32.
Hernández-Pérez E, Carpio E (1995) Resorcinol peels: gross and microscopic study. American Journal of Cosmetic Surgery, 12:337–340.
Hernández-Pérez E, Jáurez-Arce V (2000) Gross and microscopic findings with a combination of Jessner solution plus 53% resorcinol paste in chemical peels. American Journal of Cosmetic Surgery, 17:85–89.
Hirose M, Inoue T, Asamoto M, Tagawa Y (1986) Comparison of the effects of 13 phenolic compounds in induction of proliferative lesions of the forestomach and increase in the labelling indices of the glandular stomach and urinary bladder epithelium of Syrian golden hamsters. Carcinogenesis, 7(8):1285–1289.
Hirose M, Yamaguchi S, Fukushima S, Hasegawa R, Takahashi S, Ito N (1989) Promotion by dihydroxybenzene derivatives of .-methyl-.’-nitro-.-nitrosoguanidine-induced F344 rat forestomach and glandular stomach carcinogenesis. Cancer Research, 49:5143–5147.
Hoechst AG (1979) Haut- und Schleimhautverträglichkeit von Nako-Braun 3G an Kaninchen. Frankfurt am Main, Hoechst AG, pp. 1–6 (172/79).
Hoechst AG (1989) Resorcin DS Hoechst. Prüfung auf sensibilisierende Eigenschaften an Pirbright-White-Meerschweinchen im Maximierungstest. Frankfurt am Main, Hoechst AG, pp. 1–21 (89.0483).
Hoechst AG (1992) Bericht über die biologische Abbaubarkeit und weitere ökologische Daten von Resorcin. Frankfurt am Main, Hoechst AG, 28 August 1992.
Horowitz A, Shelton DR, Cornell CP, Tiedje JM (1982) Anaerobic degradation of aromatic compounds in sediments and digest sludge. Developments in Industrial Microbiology, 23:435–444.
Hosono A, Makino K, Otani H (1991) Mutagenicity of resorcinol formed by the reaction of .-phenylenediamine with sodium nitrite. Journal of Agricultural and Food Chemistry, 39:1817–1819.
Hossack DJN, Richardson JC (1977) Examination of the potential mutagenicity of hair dye constituents using the micronucleus test. Experientia, 33:377–378.
Hoyer H, Peperle W (1958) Dampfdruckmessung an organischen Substanzen und ihre Sublimationswärmen. Zeitschrift für Elektrochemie, 62:61–66.
HSDB (2002) Hazardous Substances Data Bank. Bethesda, MD, United States Department of Health and Human Services, National Institutes of Health, National Library of Medicine (http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB; accessed 2005).
IARC (1999) Resorcinol. In: Re-evaluation of some organic chemicals, hydrazine and hydrogen peroxide. Part 3. Lyon, International Agency for Research on Cancer, p. 1119 (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 71).
INDSPEC (2004) Robust summaries & test plans: Resorcinol. Submitted by Huntingdon Life Sciences on behalf of INDSPEC Chemical Corporation, Pittsburgh, PA. Washington, DC, United States Environmental Protection Agency, High Production Volume Challenge Program (http://www.epa.gov/chemrtk/ resorcnl/c15385tc.htm).
Ingle AO, Purohit HJ, Daginawala HF (1985) Studies on the biodegradation of resorcinol by indigenously isolated bacteria, mold and yeast. Journal of Environmental Biology, 6(1):45–55.
IPCS (1994) Assessing human health risks of chemicals: derivation of guidance values for health-based exposure limits. Geneva, World Health Organization, International Programme on Chemical Safety (Environmental Health Criteria 170; http://www.who.int/pcs/).
IPCS (2003) International Chemical Safety Card — Resorcinol. Geneva, World Health Organization, International Programme on Chemical Safety (ICSC 1033; http://www.ilo.org/public/ english/protection/safework/cis/products/icsc/dtasht/_icsc10/ icsc1033.pdf).
IVDK (2001) [Analysis of the data collected by the IVDK between 1992 and 2000.] Informationsverbund Dermatologisher Kliniken (in German) [cited in MAK, 2003].
Jansson T, Curvalll M, Hedin A, Enzell CR (1986) In vitro studies of biological effects of cigarette smoke condensate. II. Induction of sister-chromatid exchanges in human lymphocytes by weakly acidic, semivolatile constituents. Mutation Research, 169:129–139.
JECFA (2001) Safety evaluation of certain food additives and contaminants. Geneva, World Health Organization, Joint FAO/WHO Expert Committee on Food Additives (WHO Food Additives Series 46).
Jolley RL, Pitt WW, Scott CD, Jones G, Thompson JE (1975) Analysis of soluble organic constituents in natural and process waters by high-pressure liquid chromatography. Environmental Health Perspectives, 9:247–253.
Jørgensen SE, Bendoricchio G (2001) Fundamentals of ecological modelling, 3rd ed. Amsterdam, Elsevier, 530 pp.
Kahru A, Reiman R, Ratsep A (1998) The efficiency of different phenol-degrading bacteria and activated sludges in detoxification of phenolic leachates. Chemosphere, 37:301–318.
Kahru A, Põllumaa L, Reiman R, Rätsep A (1999) Predicting the toxicity of oil-shale industry wastewater by its phenolic composition. Alternatives to Laboratory Animals, 27:359–366.
Kahru A, Maloverjan A, Sillak H, Põllumaa L (2002) The toxicity and fate of phenolic pollutants in the contaminated soils associated with the oil-shale industry. Environmental Science & Pollution Research International,.Special Issue 1:27–33.
Karam PG (1993) 50% resorcinol peel. International Journal of Dermatology, 32(8):569–574.
Katin MJ, Teehan BP, Sigler MH, Schleifer CR, Gilgore CS (1977) Resorcinol-induced hypothyroidism in a patient on chronic hemodialysis. Annals of Internal Medicine, 86(4):447–449.
Katsarou A, Koufou B, Takou K, Kalogeromitros D, Papanayiotou G, Vareltzidis A (1995) Patch test results in hairdressers with contact dermatitis in Greece (1985–1994). Contact Dermatitis, 33:347–348.
Kavlock R (1990) Structure–activity relationships in the developmental toxicity of substituted phenols: in vivo effects. Teratology, 41:43–59.
Kawanishi S, Inoue S, Kawanashi M (1989) Human DNA damage induced by 1,2,4-benzenetriol, a benzene metabolite. Cancer Research, 49:164–168.
Kazui T, Washiyama N, Bashar AH, Terada H, Suzuki K, Yamashita K, Takinami M (2001) Role of biologic glue repair of proximal aortic dissection in the development of early and midterm redissection of the aortic root. Annals of Thoracic Surgery, 72(2):509–514.
Kim YC, Matthews HB (1987) Comparative metabolism and excretion of resorcinol in male and female F344 rats. Fundamental and Applied Toxicology, 9:409–414.
Kirk-Othmer (1981) Hydroquinone, resorcinol, and catechol. In: Kirk-Othmer encyclopedia of chemical technology, 3rd ed. Vol. 13. New York, NY, John Wiley & Sons, pp. 39–69.
Klein F, Ottis V, Velvari J (1950) Myxoedema from resorcinol ointment applied to leg ulcers. Lancet, 2:768.
Kligman A (1966) The identification of contact allergens by human assay. III. The maximization test: a procedure for screening and rating contact sensitizers. Journal of Investigative Dermatology, 47(5):393–409.
Kofler A (1943) Über die stabilen Modifikationen organischer Stoffe, die bei der üblichen Darstellung in einer total oder partiell instabilen Form kristallisieren. Archiv der Pharmazie und Berichte der Deutschen Pharmazeutischen Gesellschaft, 281:8–22.
Köhn R (1993) Allergisches Kontaktekzem durch Resorcinol nach Anwendung von Solutio Castellani. Aktuelle Dermatologie, 19:13–16.
Koppers Company (1962) Report on range-finding tests on flaked grade resorcinol, industrial grade resorcinol, and phenol. Pittsburgh, PA, Koppers Company, Inc., pp. 1–18.
Koppers Company (1970) Bio-Fax toxicity report resorcinol. Pittsburgh, PA, Koppers Company, Inc., pp. 1–3.
Koppers Company (1977) Final report to Koppers Company, Inc. on a ninety-day inhalation exposure to resorcinol. Pittsburgh, PA, Koppers Company, Inc., pp. 1–17.
Koppers Company (1981) Fish bioassay for resorcinol. Pittsburgh, PA, Koppers Company, Inc., pp. 1–11.
Kramer C-R, Trümper L (1986) Quantitative Struktur-Aktivitäts-Beziehungen für die Hemmung des autotrophen Wachstums synchroner Chlorella vulgaris-Suspensionen durch monosubstituierte Phenole. Biochemie und Physiologie der Pflanzen, 181:81–89.
Kurata Y, Fukushima S, Hasegawa R, Hirose M, Shibata M, Shirai T, Ito N (1990) Structure–activity relations in promotion of rat urinary bladder carcinogenesis by phenolic antioxidants. Japanese Journal of Cancer Research, 81(8):754–759.
Langeland T, Braathen LR (1987) Allergic contact dermatitis from resorcinol. Contact Dermatitis, 17(2):126.
Latkar M, Chakrabarti T (1994) Resorcinol, catechol and hydroquinone biodegradation in mono and binary substrate matrices in upflow anaerobic fixed-film fixed-bed reactors. Water Research, 28(3):599–607.
Leszczynska D, Kowoal AL (1980) Destruction of selected phenol compounds with ozone. Environment Protection Engineering, 6(4):465–480.
Lide DR (1995) Dissociation constants of organic acids and bases: resorcinol. In: CRC handbook of chemistry and physics, 76th ed. Boca Raton, FL, CRC Press, pp. 8–49.
Lima W (2004) Resorcinol — full life cycle toxicity test with water fleas, Daphnia magna under flow-through conditions. Submitted to Sumitomo Chemical Company, Ltd., by Springborn Smithers Laboratories, pp. 1–80 (13048.6404).
Lind ML, Boman A, Sollenberg J, Johnsson S, Hagelthorn G, Meding B (2005) Occupational dermal exposure to permanent hair dyes among hairdressers. Annals of Occupational Hygiene, 49(6):473–480.
Lindsay RH, Hill JB, Gaitan E, Cooksey RC, Jolley RL (1992) Antithyroid effects of coal-derived pollutants. Journal of Toxicology and Environmental Health, 37:467–481.
Litz N (1990) Schutz vor weiteren anthropogenen Organika-Einträgen. In: Blume H-P, ed. Handbuch des Bodenschutzes. Bodenökologie und -belastung. Vorbeugende und abody weightehrende Schutzmassnahmen. Landsberg/Lech, Ecomed, pp. 579–584.
Lloyd GK, Liggett SR, Kynoch SR, Devies R (1977) Assessment of the acute toxicity and potential irritancy of hair dye constituents. Food and Chemical Toxicology, 15:607–610.
Lynch BS, Delzell ES, Bechtel DH (2002) Toxicology review and risk assessment of resorcinol: thyroid effects. Regulatory Toxicology and Pharmacology, 36(2):198–210.
MAK (2003) Resorcinol. In: Deutsche Forschungsgemeinschaft (DFG), ed. Occupational toxicants. Critical data evaluation for MAK values and classification of carcinogens. Vol. 20. German Commission for the Investigation of Health Hazards of Chemical Compounds in the Work Area (MAK Commission). Weinheim, Wiley-VCH, pp. 239–273.
Marks JG, West JR, West GW (1978) Allergic contact dermatitis to radiotherapy dye. Contact Dermatitis, 4:1–2.
Maronpot RR, Mitsumori K, Mann P, Takaoka M, Yamamoto S, Usui T, Okamiya H, Nishikawa S, Nomura T (2000) Interlaboratory comparison of the CB6F1-Tg rasH2 rapid carcinogenicity testing model. Toxicology, 146(2–3):149–159.
Marquardt P, Koch R, Aubert J-P (1947) Die Toxizität der ein-, zwei- und dreiwertigen Phenole. Zeitschrift für die gesamte innere Medizin und ihre Grenzgebiete, 11/12:333–346.
Maruyama H, Amanuma T, Nakae D, Tsutsumi M, Kondo S, Tsujiuchi T, Denda A, Konishi Y (1991) Effects of catechol and its analogs on pancreatic carcinogenesis initiated by .-nitrosobis(2-oxopropyl)amine in Syrian hamsters. Carcinogenesis, 12(7):1331–1334.
Massone L, Anonide A, Borghi S, Usiglio D (1993) Contact dermatitis of the eyelids from resorcinol in an ophthalmic ointment. Contact Dermatitis, 28:49.
McClain RM (1994) Mechanistic considerations in the regulation and classification of chemical carcinogens. In: Kotsonis FN, Mackey M, Hjelle J, eds. Nutritional toxicology. New York, NY, Raven Press, pp. 273–304.
McClain RM (1995) Mechanistic considerations for the relevance of animal data on thyroid neoplasia to human risk assessment. Mutation Research, 333:131–142.
McGregor DB, Brown A, Cattanach P, Edwards I, McBride D, Caspary WJ (1988) Responses of the L5178Y tk+/tk− mouse lymphoma cell forward mutation assay II: 18 coded chemicals. Environmental and Molecular Mutagenesis, 11:91–118.
Meneghini CL, Rantuccio F, Lomuto M (1971) Additives, vehicles and active drugs of topical medicaments as causes of delayed-type allergic dermatitis. Dermatologica, 143:137–147.
Merker PC, Yeung D, Doughty D, Nacht S (1982) Pharmacokinetics of resorcinol in the rat. Research Communications in Chemical Pathology and Pharmacology, 33(3):367–388.
Mill T, Mabey W (1985) Photochemical transformations. In: Neely W, Blau G, eds. Environmental exposure from chemicals. Vol. 1. Boca Raton, FL, CRC Press, pp. 175–216.
MITI (1992) Data of existing chemicals based on the CSCL Japan. Tokyo, Ministry of International Trade and Industry, Chemicals Inspection & Testing Institute, Japan Chemical Industry Ecology-Toxicology & Information Center, pp. 3–19.
Miura T, Muraoka S, Fujimoto Y, Zhao K (2000) DNA damage induced by catechol derivatives. Chemico-Biological Interactions, 126:125–136.
Miyata Y, Fukushima S, Hirose M, Masui T, Ito N (1985) Short-term screening of promoters of bladder carcinogenesis in .-butyl-.-(4-hydroxybutyl)nitrosamine-initiated, unilaterally ureter-ligated rats. Japanese Journal of Cancer Research, 76(9):828–834.
Moussavi M (1979) Effect of polar substituents on autoxidation of phenols. Water Research, 13:1125–1128.
Nakagawa M, Kawai K, Kawai D (1992) Cross-sensitivity between resorcinol, resorcinol monobenzoate and phenyl salicylate. Contact Dermatitis, 27:199–200.
Nakamura S, Oda Y, Shimada T, Oki I, Sugimoto K (1987) SOS-inducing activity of chemical carcinogens and mutagens in Salmonella typhimurium TA1535/pSK1002: examination with 151 chemicals. Mutation Research, 192:239–246.
NIOSH (1998) Resorcinol: Method 5701. In: Eller PM, Cassinelli ME, eds. NIOSH manual of analytical methods (NMAM), 4th ed. Cincinnati, OH, United States Department of Health and Human Services, National Institute for Occupational Safety and Health, 4 pp. (DHHS (NIOSH) Publication No. 94-113).
NTP (1992) Toxicology and carcinogenesis studies of resorcinol (CAS No.108-46-3) in F344/N rats and B6C3F1 mice (gavage studies). Research Triangle Park, NC, United States Department of Health and Human Services, National Institutes of Health, National Toxicology Program, 235 pp. (NTP TR No. 403).
OECD (2004) Emission scenario document on additives in rubber industry. Paris, Organisation for Economic Co-operation and Development (OECD Series on Emission Scenario Documents No. 6; ENV/JM/MONO(2004)11; http://www.oecd.org/ ehs).
Okazaki S, Hoshiya T, Takahashi S, Futakuchi M, Saito K, Hirose M (1993) Modification of hepato- and renal carcinogenesis by catechol and its isomers in rats pretreated with N-ethyl-.-hydroxyethylnitrosamine. Teratogenesis, Carcinogenesis, Mutagenesis, 13(3):127–137.
O’Neil MJ (2001) Resorcinol. In: The Merck index, 13th ed. Whitehouse Station, NJ, Merck, p. 1462.
Paschin YV, Bakhitova LM, Benthen TI (1986) Increased antimutagenic activity of simple substituted phenols mixed with the hindered phenolic antioxidant dibunol. Food and Chemical Toxicology, 24(8):881–883.
Pecegueiro M (1992) Contact dermatitis due to resorcinol in a radiotherapy dye. Contact Dermatitis, 26:273.
Perbet G, Filiol C, Boule P, Lemaire J (1979) Photolyse et photo-oxydation des diphenols en solution aqueuse diluée. Journal de Chimie Physique et de Physico-Chimie Biologique, 76:89–96.
Peters K-P, Henseler T, Müller S, Przybilla B, Schulze-Dirks A, Stary A, Zimmermann J (1994) Typ IV-Allergien auf Friseurberufsstoffe. Dermatosen in Beruf und Umwelt, 42(2):50–57.
Pitter P (1976) Determination of biological degradability of organic substances. Water Research, 10:231–235.
Põllumaa L, Maloveryan A, Trapido M, Sillak H, Kahru A (2001) Study of the environmental hazard caused by the oil-shale industry solid waste. Alternatives to Laboratory Animals, 29:259–267.
Probst G, McMahon R, Hill L, Thompson C, Epp J, Neal S (1981) Chemically-induced unscheduled DNA synthesis in primary rat hepatocyte cultures: a comparison with bacterial mutagenicity using 218 compounds. Environmental Mutagenesis, 3:11–32.
RIVM (1996) SimpleTreat 3.0: a model to predict the distribution and elimination of chemicals by sewage treatment plants. Bilthoven, National Institute of Public Health and the Environment (RIVM) (Report No. 719101025).
RIVM (2004) Model parameters and equations used in SimpleBox 3.0. Bilthoven, National Institute of Public Health and the Environment, January (Report No. 601200003). Appendix to SimpleBox 3.0: A multimedia mass balance model for evaluating the environmental fate of chemicals. Bilthoven, National Institute of Public Health and the Environment (RIVM Report 601200003a / 2004).
Roberts FP, Wright AL, O’Hagan SA (1990) Hypothyroidism in textile workers. Journal of the Society of Occupational Medicine, 40(4):153–156.
Roberts MS, Anderson RA, Swarbrick J (1977) Permeability of human epidermis to phenolic compounds. Journal of Pharmaceutics and Pharmacology, 29:677–683.
RTF (2002) Anthropogenic resorcinol emissions to the environment. Personal communication. Resorcinol Task Force.
RTF (2003) A drinking water dose range-finding reproductive toxicity study of resorcinol in rats. Final report. Resorcinol Task Force, 2419 pp. (WIL-455002).
RTF (2005) A drinking water two-generation reproductive toxicity study of resorcinol in rats. Final report. Resorcinol Task Force, 4769 pp. (WIL-455003).
Rustemeier K, Stabbert R, Haussmann HJ, Roemer E, Carmines EL (2002) Evaluation of the potential effects of ingredients added to cigarettes. Part 2: Chemical composition of mainstream smoke. Food and Chemical Toxicology, 40(1):93–104.
Saito K, Isobe N, Kaneko H, Nakatsuka I (1999) In vitro studies for evaluating estrogenic and anti-estrogenic activities of resorcinol. Osaka, Sumitomo Chemical Company Ltd., Environmental Health Science Laboratory, pp. 1–16 (unpublished study).
Sakano Y, Tsuyoshi T, Kobayashi Y, Andoh H, Masamoto Y (1985) The role of oxygen free-radicals in the mutagenesis of divalent phenols. Mutation Research, 147:272–273.
Samuel KC (1955) Experimental production of goiter in rats: with resorcinol and its derivatives. Laboratory Investigation, 4:90–105.
SCCNFP (2003) The SCCNFP’s notes of guidance for testing of cosmetic ingredients and their safety evaluation, 5th revision, adopted by the SCCNFP during 25th plenary meeting of 20 October 2003. European Union, Scientific Committee on Cosmetic Products and Non-Food Products intended for consumers, pp. 1–102 (SCCNFP/0690/03, Final; http:// europa.eu.int/comm/health/ph_risk/committees/sccp/documents/out242_en.pdf).
Schmiedel KW, Decker D (2000) Resorcinol. In: Ullmann’s encyclopedia of industrial chemistry. Weinheim, Wiley-VCH Verlagsgesselschaft, pp. 1–14.
Schultz TW (1987) The use of the ionization constant (pKa) in selecting models of toxicity in phenols. Ecotoxicology and Environmental Safety, 14:178–183.
Schulz R, Schwanitz G, Winterhoff H (1982) [Investigations on the mutagenic and clastogenic activity of resorcin / cytogenetic findings from different types of human cells.] Arzneimittelforschung, 32(5):533–536 (in German).
Seffner W, Schiller F, Heinze R, Breng R (1995) Subchronic application of humic acids and associated compounds provokes histological changes of goitre in the rat. Experimental Toxicology and Pathology, 47:63–70.
Seiler J (1977) Inhibition of testicular DNA synthesis by chemical mutagens and carcinogens. Preliminary results in the validation of a novel short term test. Mutation Research, 46:305–310.
Serjeant EP, Dempsey B (1979) Ionisation constants of organic acids in aqueous solution. Oxford, Pergamon Press, p. 162.(IUPAC Chemical Data Series 23).
Serrano G, Fortea JM, Millan F, Botella R, Latasa JM (1992) Contact allergy to resorcinol in anti-acne medications: report of three cases. Journal of the American Academy of Dermatology, 26(3 pt 2):502–504.
Sessler JL, Johnson MR, Lin T-Y, Creager SE (1988) Quinone-substituted monometalated porphyrin dimers: models for photoinduced charge separation at fixed orientation and energy. Journal of the American Chemical Society, 110:3659–3661.
Shahin MM, Bugaut A, Gilard P, Kalopissis G (1980) Studies on the mutagenicity of resorcinol and hydroxy-3-(.-amino)anilino-6,.-[(.-amino)phenol] benzoquinone-monoimine-1,4 in Salmonella typhimurium. Mutation Research, 78:213–218.
Shen YS, Lin CC (2003) The effects of pH on the decomposition of hydrophenols in aqueous solutions by ultraviolet direct photolysis and the ultraviolet–hydrogen peroxide process. Water Environment Research, 75(1):54–60.
Shibata MA, Yamada M, Hirose M, Asakawa E, Tatematsu M, Ito N (1990) Early proliferative responses of forestomach and glandular stomach of rats treated with five different phenolic antioxidants. Carcinogenesis, 11(3):425–429.
Shimizu Y, Matsuto S, Mizunuma Y, Okada I (1970) Studies on the flavors of roast barley (mugi-cha). Part IV. Separation and identification of 5-hydroxymaltol, maltol, 5-methylcyclopent-2-en-2-ol-1-one and other compounds. Agricultural and Biological Chemistry, 34(6):845–853.
Soni G, Bhatia I (1980) Toxicity of phenolics and related compounds to Fusarium oxasporium Schlecht. and effect of pH on their toxicity. Indian Journal of Agricultural Science, 50(10):772–777.
Sooba E, Tenno T, Jáuregui O, Galceran MT (1997) Determination of phenols by liquid chromatography with electrochemical and UV detection. Oil Shale, 14(4 Special):544–553.
Spengler J, Osterburg I, Korte R (1986) Teratogenic evaluation of .-toluenediamine sulphate, resorcinol and .-aminophenol in rats and rabbits. Teratology, 33:31A.
Springborn Institute for Bioresearch, Inc. (1984) Photoallergic contact dermatitis by resorcinol in guinea pigs (Armstrong method). Submission of unpublished data by the Cosmetic, Toiletry, and Fragrance Association [cited in Cosmetic Ingredient Review, 1986].
Stauber JL, Florence TM (1987) Mechanism of toxicity of ionic copper and copper complexes to algae. Marine Biology, 94:511–519.
Staudinger J, Roberts PV (1996) A critical review of Henry’s law constants for environmental applications. Critical Reviews in Environmental Science and Technology, 26(3):205–297.
Stenbäck F (1977) Local and systemic effects of commonly used cutaneous agents: lifetime studies of 16 compounds in mice and rabbits. Acta Pharmacologica et Toxicologica, 41:417–431.
Stenbäck F, Shubik P (1974) Lack of toxicity and carcinogenicity of some commonly used cutaneous agents. Toxicology and Applied Pharmacology, 30:7–13.
Stenius U, Warholm M, Rannung A, Walles S, Lundberg I, Högberg J (1989) The role of GSH depletion and toxicity in hydroquinone-induced development of enzyme-altered foci. Carcinogenesis, 10(3):593–599.
Stich HF, Rosin MP, Wu CH, Powrie WD (1981) The action of transition metals on the genotoxicity of simple phenols, phenolic acids and cinnamic acids. Cancer Letters, 14:251–260.
Stom DI, Roth R (1981) Some effects of polyphenols on aquatic plants: I. Toxicity of phenols in aquatic plants. Bulletin of Environmental Contamination and Toxicology, 27:332–337.
Sütfeld R, Petereit F, Nahrstedt A (1996) Resorcinol in exudates of Nuphar lutea. Journal of Chemical Ecology, 22(12): 2221–2231.
Tarvainen K (1995) Analysis of patients with allergic patch test reactions to a plastics and glues series. Contact Dermatitis, 32:346–351.
Thomas AE, Gisburn MA (1961) Exogenous ochronosis and myxoedema from resorcinol. British Journal of Dermatology, 73:378–381.
Thomas RG (1990) Volatilization from water. In: Lyman WJ, Reehl WF, Rosenblatt DH, eds. Handbook of chemical property estimation methods, environmental behavior of organic compounds. New York, NY, McGraw-Hill Book Company, pp. 15-1–15-17.
TOMA (1978) 1978 cross-sectional health study of workers at the Petrolia, Pennsylvania, plant of Koppers Company Inc. Final report. Tabershaw Occupational Medicine Associates (unpublished) [cited in Delzell, 2000].
TOMA (1981) Occupational health evaluation of the Petrolia, Pennsylvania, plant of Koppers Company Inc. Final report. Tabershaw Occupational Medicine Associates (unpublished) [cited in Delzell, 2000].
Tschech A, Schink B (1985) Fermentation degradation of resorcinol and resorcylic acid. Archives of Microbiology, 143(1):52–59.
Tsomi V, Kalopissis G (1982) Cutaneous penetration of some hairdyes in the hairless rat. Toxicological European Research, 4(3):119–127.
Tush GM, Kuhn RJ (1996) Methemoglobinemia induced by an over-the-counter medication. Annals of Pharmacotherapy, 30(11):1251–1254.
USEPA (1974) Methods for acute toxicity tests with fish, macroinvertebrates, and amphibians. Corvallis, OR, United States Environmental Protection Agency, National Environmental Research Center.
USEPA (1975) Methods for acute toxicity tests with fish, macroinvertebrates, and amphibians. Duluth, MN, United States Environmental Protection Agency, Environmental Research Laboratory (EPA-660/3-75-009).
USEPA (1978a) Assessment of coal conversion wastewaters: characterization and preliminary biotreatability. Washington, DC, United States Environmental Protection Agency, 102 pp. (EPA-600/7-78-181; PB 294338).
USEPA (1978b) Methods for measuring acute toxicity of effluents to aquatic organisms, 2nd ed. Cincinnati, OH, United States Environmental Protection Agency, Environmental Monitoring and Support Laboratory (EPA-600/4-78-012).
Van Duuren BL, Goldschmidt BM (1976) Cocarcinogenic and tumor-promoting agents in tobacco carcinogenesis. Journal of the National Cancer Institute, 56(6):1237–1242.
Van Leeuwen CJ, Grootelaar EMM, Niebeek G (1990) Fish embryos as teratogenicity screens: a comparison of embryotoxicity between fish and birds. Ecotoxicology and Environmental Safety, 20:42–52.
Vilaplana J, Romaguera C, Grimalt F (1991) Contact dermatitis from resorcinol in a hair dye. Contact Dermatitis, 24:151–152.
von Oppell UO, Karani Z, Brooks A, Brink J (2002) Dissected aortic sinuses repaired with gelatin–resorcin–formaldehyde (GRF) glue are not stable on follow up. Journal of Heart Valve Disease, 11(2):249–257.
Waddell MM, Finn OA (1981) Sensitivity to resorcin. Contact Dermatitis, 7(4):216.
Walter W, Weidemann H-L (1968) Verbindungen des Kaffeearomas. Zeitschrift für Ernährungswissenschaft, 9(2/3):123–147.
Wellens H (1990) Zur biologischen Abbaubarkeit mono- und disubstituierter Benzolderivate. Zeitschrift für Wasser- und Abwasserforschung, 23:85–98.
Wild D, King MT, Eckhardt K, Gocke E (1981) Mutagenic activity of aminophenols and diphenols, and relations with chemical structure. Mutation Research, 85:456.
Windhager K, Plewig G (1977) [Effects of peeling agents (resorcinol, crystalline sulfur, salicylic acid) on the epidermis of guinea pig.] Archives of Dermatological Research, 259(2):187–198 (in German).
Wolfram LJ, Maibach HI (1985) Percutaneous penetration of hair dyes. Archives of Dermatological Research, 277:235–241.
Wüthrich B, Zabrodsky S, Storck H (1970) [Percutaneous poisoning by resorcinol, salicylic acid and white precipitated ointment.] Pharmaceutica Acta Helvetiae, 45:453–460 (in German).
Yalkowsky SH, Dannenfelser RM (1992) The aquasol database of aqueous solubility, 5th ed. Tucson, AZ, University of Arizona, College of Pharmacy [cited by HSDB, 2002].
Yamada K, Shirahata S, Murakami H, Nishiyama K, Shinohara K, Omura H (1985) DNA breakage by phenyl compounds. Agricultural and Biological Chemistry, 49(5):1423–1428.
Yamaguchi S, Hirose M, Fukushima S, Hasegawa R, Ito N (1989) Modification by catechol and resorcinol of upper digestive tract carcinogenesis in rats treated with methyl-.-amylnitrosamine. Cancer Research, 49:6015–6018.
Yaws CL (1997) Vapor pressure. In: Handbook of chemical compound data for process safety: comprehensive safety and health-related data for hydrocarbons and organic chemicals; selected data for inorganic chemicals. Houston, TX, Gulf Publishing, pp. 27–53.
Yeung D, Nacht S, Gans EH (1981) High-performance liquid chromatographic determination of free resorcinol in plasma and in urine. Journal of Chromatography, 224:513–518.
Yeung D, Kantor S, Nacht S, Gans EH (1983) Percutaneous absorption, blood levels and urinary excretion of resorcinol applied topically in humans. International Journal of Dermatology, 22(5):321–324.
|
BCF |
bioconcentration factor |
|
CAS |
Chemical Abstracts Service |
|
CFR |
Code of Federal Regulations (USA) |
|
CHL |
Chinese hamster lung |
|
CHO |
Chinese hamster ovary |
|
CICAD |
Concise International Chemical Assessment Document |
|
CNS |
central nervous system |
|
COD |
chemical oxygen demand |
|
COLIPA |
European Cosmetics, Toiletry and Perfumery Association |
|
DNA |
deoxyribonucleic acid |
|
DOC |
dissolved organic carbon |
|
EC50 |
median effective concentration |
|
ECD |
electron capture detection |
|
EU |
European Union |
|
EUSES |
European Union System for the Evaluation of Substances |
|
FID |
flame ionization detection |
|
HPLC |
high-performance liquid chromatography |
|
HPV |
high production volume |
|
GC |
gas chromatography |
|
IC |
industry category |
|
ICSC |
International Chemical Safety Card |
|
IOMC |
Inter-Organization Programme for the Sound Management of Chemicals |
|
IPCS |
International Programme on Chemical Safety |
|
IUCLID |
International Uniform Chemical Information Database |
|
IUPAC |
International Union of Pure and Applied Chemistry |
|
.oc |
soil sorption coefficient |
|
.ow |
octanol/water partition coefficient |
|
LC50 |
median lethal concentration |
|
LD50 |
median lethal dose |
|
LOAEL |
lowest-observed-adverse-effect level |
|
LOEC |
lowest-observed-effect concentration |
|
MAK |
German Commission for the Investigation of Health Hazards of Chemical Compounds in the Work Area (MAK Commission) |
|
MS |
mass spectrometry |
|
NOAEL |
no-observed-adverse-effect level |
|
NOEC |
no-observed-effect concentration |
|
NOEL |
no-observed-effect level |
|
OECD |
Organisation for Economic Co-operation and Development |
|
PEC |
predicted environmental concentration |
|
PND |
postnatal day |
|
PNEC |
predicted no-effect concentration |
|
S9 |
9000 × g supernatant of rat liver |
|
SCE |
sister chromatid exchange |
|
SI |
International System of Units (Système international d’unités) |
|
STP |
sewage treatment plant |
|
T3 |
triiodothyronine |
|
T4 |
thyroxine |
|
T7 |
T4 measurement by radioimmunoassay, which includes a resin T3 uptake measurement |
|
TG |
Test Guideline |
|
TLC |
thin-layer chromatography |
|
TSH |
thyroid stimulating hormone |
|
TTC |
threshold toxicity concentration |
|
TWA |
time-weighted average |
|
UC |
use category |
|
USA |
United States of America |
|
USEPA |
United States Environmental Protection Agency |
|
UV |
ultraviolet |
|
UV-VIS |
ultraviolet–visible spectrum detection |
|
w/v |
weight by volume |
BUA (1993)
For the BUA review process, the company that is in charge of writing the report (usually the largest producer in Germany) prepares a draft report using literature from an extensive literature search as well as internal company studies. This draft is subject to a peer review in several readings by a working group consisting of representatives from government agencies, the scientific community, and industry.
MAK (2003)
The scientific documentations of the German Commission for the Investigation of Health Hazards of Chemical Compounds in the Work Area (MAK Commission) are based on critical evaluations of the available toxicological and occupational medical data from extensive literature searches and of well documented industrial data. The evaluation documents involve a critical examination of the quality of the database, indicating inadequacy or doubtful validity of data and identifying data gaps. This critical evaluation and the classification of substances are the result of an extensive discussion process by the members of the Commission, proceeding from a draft documentation prepared by members of the Commission, by ad hoc experts, or by the Scientific Secretariat of the Commission. Scientific expertise is guaranteed by the members of the Commission, which consists of experts from the scientific community, industry, and employer associations.
Health Council of the Netherlands (2004)
This document contains the assessment of the health hazards of resorcinol by the Committee on Updating of Occupational Exposure Limits, a committee of the Health Council of the Netherlands. The first draft of this document was prepared by Dr C. de Heer (TNO Nutrition and Food Research, Zeist, The Netherlands).
The evaluation of the toxicity of resorcinol has been based on the review by the American Conference of Governmental Industrial Hygienists. Where relevant, the original publications were reviewed and evaluated, as indicated in the text. In addition, in December 1998, literature was retrieved from the online databases Medline, Toxline, and Chemical Abstracts starting from 1966, 1965, and 1990, respectively, and using the following key words: resorcinol, 3-hydroxyphenol, 1,3-benzenediol, and 108-46-3.
In July 2000, the President of the Health Council released a draft of the document for public review. Comments were received from the following individuals and organizations: P. Ashford (Resorcinol Task Force). An additional search in Toxline and Medline in September 2004 did not result in information changing the committee’s conclusions.
The document is available on the Internet at http:// www.gr.nl/ pdf.php?ID=1099&p=1.
INDSPEC (2004)
In May 2004, INDSPEC Chemical Corporation identified and submitted data on resorcinol from various sources as part of meeting its 2001 commitment to the United States HPV Challenge Program. These data include company proprietary files, peer-reviewed published literature, specific test reports, and/or calculated end-points using widely accepted computer modelling programs.
The USEPA has posted submissions made to the USEPA relating to the HPV Challenge Program on its web site for the purposes of making them more easily accessible to the public and inviting public comment on them. The USEPA has posted these submissions verbatim without editing them in any way. The USEPA has not evaluated the submissions on their merits prior to posting.
The submissions on resorcinol made by INDSPEC in May 2004 are available on the USEPA website at http:// www.epa.gov/chemrtk/resorcnl/c15385tc.htm.
The draft CICAD on resorcinol was sent for review to institutions and organizations identified by IPCS after contact with IPCS national Contact Points and Participating Institutions, as well as to identified experts. Comments were received from:
P. Ashford, Resorcinol Task Force, Gloucestershire, United Kingdom
M. Baril, Institut de recherche Robert Sauvé en santé et en sécurité du travail, Montreal, Canada
R. Benson, United States Environmental Protection Agency, Denver, CO, USA
N. Chen, National Industrial Chemicals Notification and Assessment Scheme, Sydney, New South Wales, Australia
R. Chhabra, National Institute of Environmental Health Sciences, Research Triangle Park, NC, USA
S. Dungey, Environment Agency, Wallingford, United Kingdom
L. Fishbein, Fairfax, Virginia, USA
E. Frantik, Institute of Public Health, Prague, Czech Republic
H. Gibb, Sciences International Inc., Alexandria, VA, USA
S.H. Henry, Center for Food Safety & Applied Nutrition, United States Food and Drug Administration, College Park, MD, USA
P. Howe, Centre for Ecology and Hydrology, Monks Wood, United Kingdom
G. Hsu, United States Environmental Protection Agency, Washington, DC, USA
T. Santonen, Finnish Institute of Occupational Health, Helsinki, Finland
H. Savolainen, Ministry of Social Affairs and Health, Department of Occupational Safety & Health, Tampere, Finland
E. Søderlund, Norwegian Institute of Public Health, Nydalen, Norway
J.L. Stauber, CSIRO Energy Technology, Bangor, New South Wales, Australia
T. Stedeford, United States Environmental Protection Agency, Washington, DC, USA
M.H. Sweeney, United States Health Attaché, United States Embassy, Hanoi, Viet Nam
S.P. Tucker, National Institute for Occupational Safety and Health, Cincinnati, OH, USA
G. Ungvary, József Fodor National Center for Public Health, Budapest, Hungary
K. Ziegler-Skylakakis, Secretariat of the Commission for the Investigation of Health Hazards of Chemical Compounds in the Workplace Area (MAK Commission), Freising-Weihenstephan, Germany
Nagpur, India
31 October – 3 November 2005
Members
Dr T. Chakrabarti, National Environmental Engineering Research Institute, Nagpur, India
Dr R. Chhabra, National Institute of Environmental Health Sciences, Research Triangle Park, NC, USA
Mr P. Copestake, Toxicology Advice & Consulting Ltd, Surrey, United Kingdom
Dr C. De Rosa, Agency for Toxic Substances and Disease Registry, Atlanta, GA, USA
Dr S. Dobson, Centre for Ecology and Hydrology, Monks Wood, United Kingdom
Dr L. Fishbein, Fairfax, VA, USA
Dr L. Fruchtengarten, Poison Control Center of São Paulo, São Paulo, Brazil
Dr H. Gibb, Sciences International Inc., Alexandria, VA, USA
Dr R.F. Hertel, Federal Institute for Risk Assessment (BfR), Berlin, Germany
Mr P. Howe, Centre for Ecology and Hydrology, Monks Wood, United Kingdom
Ms K. Hughes, Health Canada, Ottawa, Ontario, Canada
Dr D. Kanungo, Directorate General of Health Services, New Delhi, India
Dr J. Kielhorn, Fraunhofer Institute of Toxicology and Experimental Medicine, Hanover, Germany
Dr G. Kong, Hanyang University, Seoul, Republic of Korea
Dr J. Rischer, Agency for Toxic Substances and Disease Registry, Chamblee, GA, USA
Dr O. Sabzevari, Tehran University of Medical Sciences, Tehran, Islamic Republic of Iran
Dr R. Sonawane, National Center for Environmental Assessment, Environmental Protection Agency, Washington, DC, USA
Dr J. Stauber, CSIRO Energy Technology, Menai, New South Wales, Australia
Dr M.H. Sweeney, United States Embassy, Hanoi, Viet Nam
Ms D. Willcocks, National Industrial Chemicals Notification and Assessment Scheme, Sydney, New South Wales, Australia
Dr Y. Zheng, National Institute for Occupational Health & Poison Control, Beijing, People’s Republic of China
Dr K. Ziegler-Skylakakis, Secretariat of the Commission for the Investigation of Health Hazards of Chemical Compounds in the Workplace Area (MAK Commission), Freising-Weihenstephan, Germany
Observer
Mr P. Ashford, Resorcinol Task Force, Wotton-under-edge, Gloucestershire, United Kingdom
Secretariat
Dr A. Aitio, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
Ms L. Onyon, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
Mr M. Shibatsuji, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
EUSES 2.0.3 (http://ecb.jrc.it/existing-chemicals/) with default values according to the EU Technical Guidance Document (EC, 2003a) is used to calculate the distribution in the environment and the concentrations in the different environmental compartments (Fh-ITEM, 2005b).
Estimation of local concentration (Clocal) during production of rubber products
Resorcinol is used in the production of tyres as a bonding agent (processing aid). The IC/UC6 combination is IC11 "Polymer Industry" / UC49 "Stabilisers".
Calculation of local emission to wastewater during production of tyres (formulation and processing of resorcinol) using the OECD Emission Scenario Document (OECD, 2004)
Amount of product type produced per day:
|
Qprod |
= 55 000 kg/day7 |
Parts of additives introduced:
|
Qadditive |
= 4 phr8 |
Recipe factor:
|
Frecipe |
= 2 |
Fraction of resorcinol remaining in product:
|
Fremaining |
= 0.999 |
Fraction of emission directed to water by sewage treatment plant (STP) (SimpleTreat Model; EC, 2003a):
|
Fstpwater |
= 0.126 |
Capacity of sewage treatment plant (STP) (EC, 2003a):
|
EFFLUENTstp |
= 2000 m3/day |
Dilution factor (EC, 2003a):
|
DILUTION |
= 10 |
Factor (1+Kp*SUSPwater) (EC, 2003a):
|
FACTOR |
= 1 |
Emission per day:
|
Elocalwater |
= Qprod * [Qadditive / (100 * Frecipe) ] * (1−Fremaining) |
|
|
= 1.1 kg/day |
Influent concentration:
|
Clocalinf. |
= Elocalwater / EFFLUENTstp |
|
|
= 0.55 mg/l |
Effluent concentration:
|
Clocaleff. |
= Clocalinf. * Fstpwater |
|
|
= 69.6 µg/l |
Concentration in surface water:
|
Clocalwater |
= Clocaleff. / (DILUTION * FACTOR) |
|
|
= 6.96 µg/l |
Calculation of local emission to air and soil during production of tyres (formulation and processing of resorcinol) using the OECD Emission Scenario Document (OECD, 2004)
Amount of product type produced per day:
|
Qprod |
= 55 000 kg/day9 |
Parts of additives introduced:
|
Qadditive |
= 4 phr |
Recipe factor:
|
Frecipe |
= 2 |
Emission factor to air:
|
Fair |
= 0.001 |
Emission factor to soil (industrial):
|
Fsoil |
= 0.0005 |
Concentration in air at source strength of 1 kg/day (EC, 2003a):
|
Cstdair |
= 0.278 µg/m3 |
Number of emission days per year (Table B3.9 in EC, 2003a):
|
Temission |
= 300 |
Emission per day:
|
Elocalair |
= Qprod * [Qadditive / (100 * Frecipe) ] * Fair |
|
|
= 1.1 kg/day |
||
|
Elocalsoil, ind. |
= Qprod * [Qadditive / (100 * Frecipe) ] * Fsoil |
|
|
= 0.55 kg/day10 |
Concentration in air:
|
Clocalair |
= Elocalair * Cstdair |
|
|
= 0.306 µg/m3 |
||
|
Clocalair,ann |
= Clocalair * Temission/365 |
|
|
= 0.251 µg/m3 |
Estimation of local concentration (Clocal) during formulation and use of hair dyes
Resorcinol is used as a component in hair dyes. The IC/UC combination is IC5 "Personal/domestic use" / UC10 "Colouring agent".
Calculation of emission to wastewater during formulation of hair dyes
Amount of resorcinol for formulation of hair dyes per year (EC, 2002):
|
Qsubst., form. |
= 150 tonnes/year |
Fraction of tonnage released to wastewater (EC, 2002):
|
Fwastewater |
= 0.01 |
Fraction of main local source (Table B2.3 in EC, 2003a):
|
Fmainsource |
= 0.7 |
Number of emission days (Table B2.3 in EC, 2003a):
|
Temission |
= 300 |
Fraction of emission directed to water by sewage treatment plant (STP), tier 1 (SimpleTreat Model, EC, 2003a):
|
Fstpwater |
= 0.126 |
Fraction of emission directed to water by sewage treatment plant (STP), tier 2 (Simulation test):
|
Fstpwater |
= 0.05 |
Capacity of sewage treatment plant (STP) (EC, 2003a):
|
EFFLUENTstp |
= 2000 m3/day |
Dilution factor (EC, 2003a):
|
DILUTION |
= 10 |
Factor (1+Kp*SUSPwater) (EC, 2003a):
|
FACTOR |
= 1 |
Emission per day:
|
Elocalwater |
= Qsubst., form. * Fwastewater * Fmainsource / Temission |
|
|
= 3.5 kg/day |
Influent concentration:
|
Clocalinf. |
= Elocalwater / EFFLUENTstp |
|
|
= 1.75 mg/l |
Effluent concentration:
|
Tier 1: Clocaleff. |
= Clocalinf. * Fstpwater |
|
|
= 221 µg/l |
||
|
Tier 2: Clocaleff. |
= Clocalinf. * Fstpwater |
|
|
= 87.5 µg/l |
Concentration in surface water:
|
Tier 1: Clocalwater |
= Clocaleff. / (DILUTION * FACTOR) |
|
|
= 22.1 µg/l |
||
|
Tier 2: Clocalwater |
= Clocaleff. / (DILUTION * FACTOR) |
|
|
= 8.75 µg/l |
Calculation of emission to wastewater during use (including disposal) of hair dyes
Amount of resorcinol for use of hair dyes per year:
|
Qsubst., use |
= 148.5 tonnes/year11 |
Fraction of tonnage for region (EC, 2003a):
|
Fregional |
= 0.1 |
Fraction of main local source (Table B4.1 in EC, 2003a):
|
Fmainsource |
= 0.002 |
Number of emission days (Table B4.1 in EC, 2003a):
|
Temission |
= 365 |
Fraction released to wastewater:
|
Fwastewater |
= 112 |
Fraction of emission directed to water by sewage treatment plant (STP) (SimpleTreat Model; EC, 2003a):
|
Fstpwater |
= 0.126 |
Capacity of sewage treatment plant (STP) (EC, 2003a):
|
EFFLUENTstp |
= 2000 m3/day |
Dilution factor (EC, 2003a):
|
DILUTION |
= 10 |
Factor (1+Kp*SUSPwater) (EC, 2003a):
|
FACTOR |
= 1 |
Emission per day:
|
Elocalwater |
= Qsubst., use * Fregional * Fwastewater * Fmainsource / Temission |
|
|
= 0.0814 kg/day |
Influent concentration:
|
Clocalinf. |
= Elocalwater / EFFLUENTstp |
|
|
= 0.0407 mg/l |
Effluent concentration:
|
Clocaleff. |
= Clocalinf. * Fstpwater |
|
|
= 5.15 µg/l |
Concentration in surface water:
|
Clocalwater |
= Clocaleff. / (DILUTION * FACTOR) |
|
|
= 0.515 µg/l |
Estimation of local concentration (Clocal) during use of pharmaceuticals
Resorcinol is used in pharmaceutical applications such as topical ointments. The IC/UC combination is IC5 "Personal/ domestic use" / UC41 "Pharmaceutical".
Calculation of emission to wastewater during use of pharmaceuticals
Amount of resorcinol for pharmaceuticals per year (EC, 2002):
|
Qsubst., use |
= 75 tonnes/year |
Fraction of tonnage for region (EC, 2003a):
|
Fregional |
= 0.1 |
Fraction of main local source (Table B4.1 in EC, 2003a):
|
Fmainsource |
= 0.002 |
Number of emission days (Table B4.1 in EC, 2003a):
|
Temission |
= 365 |
Fraction released to wastewater (EC, 2002):
|
Fwastewater |
= 1 |
Fraction of emission directed to water by sewage treatment plant (STP) (SimpleTreat Model; EC, 2003a):
|
Fstpwater |
= 0.126 |
Capacity of sewage treatment plant (STP) (EC, 2003a):
|
EFFLUENTstp |
= 2000 m3/day |
Dilution factor (EC, 2003a):
|
DILUTION |
= 10 |
Factor (1+Kp*SUSPwater) (EC, 2003a):
|
FACTOR |
= 1 |
Emission per day:
|
Elocalwater |
= Qsubst., use * Fregional * Fwastewater * Fmainsource / Temission |
|
|
= 0.0411 kg/day |
Influent concentration:
|
Clocalinf. |
= Elocalwater / EFFLUENTstp |
|
|
= 0.0205 mg/l |
Effluent concentration:
|
Clocaleff. |
= Clocalinf. * Fstpwater |
|
|
= 2.60 µg/l |
Concentration in surface water:
|
Clocalwater |
= Clocaleff. / (DILUTION * FACTOR) |
|
|
= 0.26 µg/l |
Estimation of regional and continental emissions
Calculation of regional emissions during production of tyres (formulation and processing of resorcinol)
Amount of resorcinol for tyres per year (EC, 2002):
|
Qsubst. |
= 6480 tonnes/year |
Fraction of tonnage released to air (OECD, 2004):
|
Fair |
= 0.001 |
Fraction of tonnage released to wastewater (OECD, 2004):
|
Fwastewater |
= 0.001 |
Fraction of tonnage released to soil (OECD, 2004):
|
Fsoil |
= 0.0005 |
|
|
Eregionalair |
= Qsubst. * Fair |
|
|
= 6.48 tonnes/year |
||
|
Eregionalwastewater |
= Qsubst. * Fwastewater |
|
|
= 6.48 tonnes/year |
||
|
Eregionalsoil |
= Qsubst. * Fsoil |
|
|
= 3.24 tonnes/year |
Calculation of regional emission during formulation of hair dyes
Amount of resorcinol for formulation of hair dyes per year (EC, 2002):
|
Qsubst., form. |
= 150 tonnes/year |
Fraction of tonnage released to wastewater (EC, 2002):
|
Fwastewater |
= 0.01 |
|
|
Eregionalwastewater |
= Qsubst., form. * Fwastewater |
|
|
= 1.5 tonnes/year |
Calculation of regional and continental emission during consumer use (including disposal) of hair dyes
Amount of resorcinol for use as hair dyes per year (EC, 2002):
|
Qsubst., use |
= 148.5 tonnes/year |
Fraction of tonnage for region (EC, 2003a):
|
Fregional |
= 0.1 |
Fraction of tonnage released to wastewater:
|
Fwastewater |
= 113 |
|
|
Eregionalwastewater |
= Qsubst., use * Fregional * Fwastewater |
|
|
= 14.9 tonnes/year |
||
|
Econtinentalwastewater |
= Qsubst., use * (1−Fregional) * Fwastewater |
|
|
= 134 tonnes/year |
Calculation of regional and continental emission during consumer use of pharmaceuticals
Amount of resorcinol for use as pharmaceutical per year (EC, 2002):
|
Qsubst., use |
= 75 tonnes/year |
Fraction of tonnage for region (EC, 2003a):
|
Fregional |
= 0.1 |
Fraction of tonnage released to wastewater (EC, 2002):
|
Fwastewater |
= 1 |
|
|
Eregionalwastewater |
= Qsubst., use * Fregional * Fwastewater |
|
|
= 7.5 tonnes/year |
||
|
Econtinentalwastewater |
= Qsubst., use * (1−Fregional) * Fwastewater |
|
|
= 67.5 tonnes/year |
Calculation of total regional and continental releases
A sewage treatment plant connection rate of 80% (EC, 2003a, chapter 3, Appendix XII) is assumed.
|
Eregionalair, total |
= Sigma Eregionalair |
|
|
= 6.48 tonnes/year |
||
|
Eregionalwastewater, total |
= 0.8 * Sigma Eregionalwastewater |
|
|
= 24.3 tonnes/year |
||
|
Eregionalsurfacewater, total |
= 0.2 * Sigma Eregionalwastewater |
|
|
= 6.06 tonnes/year |
||
|
Eregionalsoil, total |
= Sigma Eregionalsoil |
|
|
= 3.24 tonnes/year |
||
|
Econtinentalwastewater, total |
= 0.8 * Sigma Econtinentalwastewater |
|
|
= 161 tonnes/year |
||
|
Econtinentalsurfacewater, total |
= 0.2 * Sigma Econtinentalwastewater |
|
|
= 40.3 tonnes/year |
Predicted environmental concentrations (PEC)14
|
PECregionalair |
= 0.458 pg/m3 |
|
|
PECregionalwater |
= 0.129 µg/l |
|
|
PECregionalsoil, ind. |
= 0.583 µg/kg dry weight |
Rubber industry
|
PEClocalair |
= Clocalair + PECregionalair |
|
|
= 0.306 µg/m3 + 0.458 pg/m3 |
||
|
PEClocalwater |
= Clocalwater + PECregionalwater |
|
|
= 7.09 µg/l |
Formulation of hair dyes
|
Tier 1: PEClocalwater |
= Clocalwater + PECregionalwater |
|
|
= 22.3 µg/l |
||
|
Tier 2: PEClocalwater |
= Clocalwater + PECregionalwater |
|
|
= 8.88 µg/l |
Use of hair dyes and pharmaceuticals
|
PEClocalwater |
= Clocalwater, use hair dyes + Clocalwater, use pharmaceutical + PECregionalwater |
|
|
= 0.904 µg/l |
|
Species / strain / number of animals / sex |
Duration |
Dosage |
NOAEL |
LOAEL |
Results |
Reference |
|
|
Oral exposure |
|||||||
|
Rat |
14 days |
Oral feed 0 or 5% via diet (about 3000 mg/kg body weight per day) |
~3000 |
Thyroid gland: weight incr. (14.2 vs 11.5 mg in controls) Plasma T4 levels decr. (24 vs 51 µg/l in controls) Labelled T4 half-life decr. (13.4 vs 18.8 h in controls) |
Berthezéne et al. (1979) |
||
|
Rat F344/N 5 males and 5 females per dose group |
17 days 5 days/ week (12 doses total) |
Gavage 0, 27.5, 55, 110, 225, or 450 mg/kg body weight Purity: >99% |
27.5 |
55 |
225 or 450 mg/kg body weight: hyperexcitability and tachypnoea (m) >55 mg/kg body weight: hyperexcitability (f) 110 or 450 mg/kg body weight: tachypnoea (f) 450 mg/kg body weight: relative/absolute thymus weights decr. (f): 2.33 vs 2.71 mg/g body weight in controls or 344 vs 412 mg in controls No adverse effects on body weight gain. No gross or microscopic lesions. |
NTP (1992) |
|
|
Mouse B6C3F1 5 males and 5 females per dose group |
17 days 5 days/ week (12 doses total) |
Gavage 0, 37.5, 75, 150, 300, or 600 mg/kg body weight Purity: >99% |
75 |
150 |
>150 mg/kg body weight: prostration, tremors (m) >300 mg/kg body weight: prostration, tremors (f); mortality incr. (m: 1 [gavage accident]/0/0/0/1/4; f: 0/0/0/0/0/5) No adverse effects on body weight gain or organ weights. No gross or microscopic lesions. |
NTP (1992) |
|
|
Rat albino 10 males per dose group |
28 days |
Oral feed 0, 300, 1000, or 3000 mg/kg (about 0, 26, 87, or 260 mg/kg body weight) Purity: no data |
300 |
Adrenals: relative weights in all dosed rats incr. (0.12, 0.19, 0.23 or 0.2 mg%) No adverse effects concerning mortality or body weight gain. No signs of intoxication. No adverse findings at necropsy. |
Koppers Company (1970) |
||
|
Rat Wistar Crl:(WI) BR 5 females per dose group |
30 days |
Drinking-water 0 or 9 µmol/day (about 5–10 mg/kg body weight) Purity: reagent grade Low-iodine, low-protein diet |
5–10 |
Thyroid gland weight incr.: ~2.5 vs 1.2 mg/kg in controls T3/T4 levels decr.: ~1.5% vs 2.8% in controls |
Cooksey et al. (1985) |
||
|
Rat F344 5 males per dose group |
8 weeks |
Oral feed 0 or 0.8% via diet (about 480 mg/kg body weight); 100 mg/kg BrdU i.p. before sacrifice Purity: no data |
~480 |
No adverse effects concerning mortality, body weight gain, or food and water consumption Forestomach/glandular stomach: no increases in hyperplasia or labelling index |
Shibata et al. (1990) |
||
|
Rat F1 from 1.0 BD IX × 0.1 WELS/Fohm 10–13 males and 10–13 females per dose group |
12 weeks |
Drinking-water 0 or 0.004% (about 5 mg/kg body weight) Purity: no data |
~5 |
Thyroid gland: mean follicular epithelial cell height incr. (7.8–8.5 vs 6.9–7.0 µm in controls); mean follicle diameter decr. (16.1–20.3 vs 22.9–24.1 µm in controls); follicle epithelium index decr. (1.91–2.64 vs 3.32–3.53 in controls) No other effects were investigated. Iodine content in diet: 0.9 mg/kg (exceeding a requirement of 0.1–0.2 mg/kg) |
Seffner et al. (1995) |
||
|
Rat F344/N 10 males and 10 females per dose group |
13 weeks 5 days/ week |
Gavage 0, 32, 65, 130, 260, or 520 mg/kg body weight Purity: >99% |
32 |
130 or 260 mg/kg body weight: significantly increased relative/absolute liver weights (m): 11.75/11.74 vs 10.84 g in controls or 34.4/34.9 vs 32.0 mg/g body weight in controls 65–260 mg/kg body weight: significantly increased relative/absolute liver weights (f): 5.43/5.41/5.49 vs 4.77 g in controls or 29.7/28.8/30.2 vs 26.0 mg/g body weight in controls 32–260 mg/kg body weight: relative/absolute adrenal gland weights incr. (m): 5.42/5.48/5.21/5.74 vs 4.73 mg in controls or 0.16/0.16/0.15/0.17 vs 0.14 mg/g body weight in controls 520 mg/kg body weight: tremors; mortality incr. (m: 0/0/0/0/2[dosing error]/8; f: 0/0/0/0/4[dosing error]/10) No adverse effects on body weight gain. No differences in haematology or clinical chemistry. No gross or microscopic lesions. Iodine content in diet: 3.37 mg/kg (exceeding a requirement of 0.1–0.2 mg/kg) |
NTP (1992) |
||
|
Mouse B6C3F1 10 males and 10 females per dose group |
13 weeks 5 days/ week |
Gavage 0, 28, 56, 112, 225, or 420 mg/kg body weight Purity: >99% |
28 |
28–225 mg/kg body weight: relative/absolute adrenal gland weights decr. (m): 6.4/5.9/5.89/5.7 vs 8.3 mg in controls or 0.25/0.22/0.23/0.23 vs 0.31 mg/g body weight in controls 420 mg/kg body weight: dyspnoea, prostration, tremors; final mean body weights decr. (m); mortality incr. (m: 0/0/0/1[gavage accident]/0/8; f: 0/0/0/0/0/8) No differences in haematology or clinical chemistry. No gross or microscopic lesions. Iodine content in diet: 3.37 mg/kg (exceeding a requirement of 0.1–0.2 mg/kg) |
NTP (1992) |
||
|
Hamster Syrian golden 15 males per dose group |
20 weeks |
Oral feed 0 or 0.25% via diet (about 375 mg/kg body weight) 3 animals per group were dosed with 37 MBq [methyl-3H]thymidine)/kg body weight before sacrifice Purity: >99.5% |
No adverse effects on body and relative liver weights Forestomach: hyperplasia incr. (mild/moderate: 80/13% vs 47/7% in controls); no papillomatous lesions; no increased labelling index (forestomach/pyloric region) Urinary bladder: no increased labelling index Only effects on forestomach, pyloric region, and urinary bladder were described in detail. |
Hirose et al. (1986) |
|||
|
Mouse heterozygote p53def (C57BL/6) 15 males and 15 females 30 control animals per sex (single dose) |
24 weeks 5 times per week |
Gavage 0 or 225 mg/kg body weight Purity: no data |
No increased incidence of neoplastic or nonneoplastic lesions |
Eastin et al. (1998) |
|||
|
Mouse I. CB6F1-Tg rasH2 II. non-transgenic littermates 50 males and 55 females 24 control animals (single dose) |
24–26 weeks 5 days/ week |
Gavage 0 or 225 mg/kg body weight Purity: no data |
For evaluation purposes, two identical studies were performed at different locations (USA and Japan). 225 mg/kg body weight: hyperactivity and tremors (USA); mean body weights and body weight gain decr. (USA); mean body weights and body weight gain decr. (m; only wild type; Japan) Lung effects: I. adenomas: m: 4/50 vs 1/24 in controls; f: 4/55 vs 0/25 in controls; carcinomas: m: 0/50 vs 1/24 in controls; f: 2/55 vs 0/25 in controls II. adenomas: m: 3/51 vs 1/24 in controls; f: 1/56 vs 1/25 in controls; carcinomas: m: 0/51 vs 0/24 in controls; f: 0/56 vs 0/25 in controls Spleen effects (haemangiosarcomas): I. m: 0/50 vs 1/24 in controls; f: 0/55 vs 0/25 in controls II. m: 0/51 vs 0/24 in controls; f: 0/56 vs 0/25 in controls |
Maronpot et al. (2000) |
|||
|
Rat F344/N 60 males and 60 females per dose group |
104 weeks 5 days/ week Interim sacrifice: 10 males and 10 females after 15 months |
Gavage 0, 112, or 225 mg/kg body weight (males) 0, 50, 100, or 150 mg/kg body weight (females) Purity: >99% |
50 (female) |
>100 mg/kg body weight: ataxia, prostration, salivation, tremors 150–225 mg/kg body weight: mean body weights decr. (m: 10–15%; f: 11–14%); mortality incr. (m: 22/25/41; f: 16/17/22/26) Interim sacrifice: no differences in haematology, clinical chemistry, or other clinical pathology parameters; no increased incidence of neoplasms or non-neoplastic lesions Final sacrifice: no evidence of carcinogenic activity in males or females |
NTP (1992) |
||
|
Mouse B6C3F1 60 males and 60 females per dose group |
104 weeks 5 days/ week Interim sacrifice: 10 males and 10 females after 15 months |
Gavage 0, 112, or 225 mg/kg body weight Purity: >99% |
112 |
Survival rate: m: 37/43/34; f: 38/33/34 >112 mg/kg body weight: ataxia, recumbency, tremors 225 mg/kg body weight: mean body weights decr. (f: 10–15%) Interim sacrifice: no differences in organ weights, haematology, or other clinical parameters; no increased incidence of neoplasms or non-neoplastic lesions Final sacrifice: no evidence of carcinogenic activity in males or females |
NTP (1992) |
||
|
Rat Crl:CDÒ (SD) 14 males and 14 females per dose group |
One-generation dose-finding study |
Drinking-water 10, 40, 120, or 360 mg/l 1, 4, 13, or 37 mg/kg body weight (males) 1, 5, 16, or 47 mg/kg body weight (females) Purity: >99.8% |
37 (male); 47 (female) |
Minimal microscopic changes in the thyroid glands of the F0 generation exposed to 360 mg/l. However, no effects on thyroid hormones or thyroid weights at any dose. |
RTF (2003) |
||
|
Rat Crl:CDÒ (SD) 30 males and 30 females per dose group |
Two-generation study |
Drinking-water 120, 360, 1000, or 3000 mg/l Up to 233 mg/kg body weight (males) Up to 304 mg/kg body weight (females) Purity: >99.8% |
233 (male); 304 (female) |
No effect on thyroid gland weights; no effects on T3/T4, TSH levels; histopathological change (colloid) at highest dose (no adverse effect) |
RTF (2005) |
||
|
Dermal exposure |
|||||||
|
Guinea-pig Pirbright White 10 males per dose group Controls: 2 (solvent) 2 (untreated) |
14 days |
0, 1, or 3% onto the ears or shaven flanks Once per day Purity: "purest" |
2 animals each were treated on days 2, 4, 7, 11, or 14 with 0.1 ml 370 kBq [3H]thymidine (ears and flanks) and killed after 45 min >1%: flanks: labelling index, acanthosis, and hypergranulosis/hyperkeratosis decr. (concentration-dependent); ears: labelling index, acanthosis, hypergranulosis/hyperkeratosis, and papillomatosis decr. (concentration-dependent) |
Windhager & Plewig (1977) |
|||
|
Wistar rats I. 6 females (shaved skin) II. 2 females / 1 male (shaved and scarified skin) III. 3 females as controls |
28 days |
12.5% resorcinol ointment twice daily (about 750 mg/kg body weight per day) |
~750 |
Enlarged thyroid gland (~3 times) compared with controls; histological changes in the thyroid and the anterior lobe of the pituitary gland |
Samuel (1955) |
||
|
Mouse hemizygote Tg.AC (FVB/N) 15 males and 15 females 30 control animals per sex (single dose) |
24 weeks 5 times per week |
Dermal 0 or 225 mg/kg body weight in acetone onto the clipped skin Purity: no data |
No systemic treatment-related lesions Incidence of squamous cell papillomas incr. (m: 10/15 vs 3/30 in controls; f: 12/15 vs 1/30 in controls); hyperplasia incr. (m/f); hyperkeratosis, inflammation, and sebaceous gland hyperplasia incr. (m) |
Eastin et al. (1998) |
|||
|
Mouse Swiss 50 females per dose group Controls: 150 females (untreated) 50 females (solvent) 50 females (positive [DMBA]) |
110 weeks 2 times per week |
0.02 ml of 5, 25, or 50% dissolved in acetone onto shaved dorsal skin Purity: no data |
<50%: no systemic or carcinogenic effects (complete autopsies on all animals) Local skin lesions: ulceration, inflammation, and hyperplasia |
Stenbäck & Shubik (1974) |
|||
|
Rabbit NZW 5 (males and females) per dose group Controls: 19 (untreated) 15 (positive [DMBA]) |
180 weeks 2 times per week |
0.02 ml of 5, 10, or 50% dissolved in acetone onto interior left ear Purity: no data |
<50%: no systemic or carcinogenic effects (complete autopsies on all animals) |
Stenbäck (1977) |
|||
|
Inhalation exposure |
|||||||
|
Rat HLA-SD 25 males and 25 females Controls: 5 males and 5 females deprived of food and water on 8 h/day 5 males and 5 females were given food |
60 or 90 days (8 h/day) |
220 ppm = 1000 mg/m3 |
Exposure was temporarily terminated after 64 weeks due to high mortality (20% in m; 28% in f); 50% of survivors were sacrificed 1 week later, and blood and urine samples were taken. After a 2-week pasture period, the remaining animals were further exposed (total of 90 exposures). Reduced body weight gain due to decreased feed intake, changes in relative organ weights (liver, kidneys, spleen, adrenals), hyperplastic thyroid glands in 15/38 rats, mild albuminuria (probably due to decreased food/water consumption), some haematological changes |
Koppers Company (1977) |
|||
|
Exposure by other routes |
|||||||
|
Rat Sprague-Dawley males (no further data) |
14 or 30 days |
100 (2 × 50) mg/kg body weight Subcutaneous (injections 6 h apart) Purity: no data |
100 |
No overt toxic signs or adverse reactions concerning body weight gain or organ weights (liver, kidneys, brain, spleen, testes), thyroid function (serum T3/T4 levels), haematological parameters (red blood cell count, haemoglobin, haematocrit), or histopathology of thyroid, spinal cord, or brain |
Merker et al. (1982) |
||
|
One albino rat per dose |
10, 31, 47, or 69 days 3 controls: one killed on day 47 and 2 on day 69 |
1.4 mmol/kg body weight per day (about 154 mg/kg body weight) Subcutaneous injection |
>47 days: increased thyroid gland weights with goitre-like histology |
Doniach & Logothetopoulos (1953) |
|||
|
Wistar rats I. 2 females II. 3 females 3 females as controls |
I. 21–38 days II. 39–78 days |
I. 1.4 mmol/kg body weight per day twice daily (about 300 mg/kg body weight) II. 1.8 mmol/kg body weight per day twice daily (about 400 mg/kg body weight) Subcutaneous injection (peanut oil) |
I. No changes in the thyroid gland of two rats II. Enlarged thyroid gland (2 times) and histological changes in the thyroid and the anterior lobe of the pituitary gland |
Samuel (1955) |
|||
|
Wistar rats 5 females 3 females as controls |
21–79 days |
3.6 mmol/kg body weight per day twice daily (about 800 mg/kg body weight) Subcutaneous injection (beeswax, peanut oil) |
Enlarged thyroid gland (2–3 times) and histological changes in the thyroid and the anterior lobe of the pituitary gland |
Samuel (1955) |
|||
|
Rabbit Moravia Black 7 per group 5 controls |
19 days |
50 mg/kg body weight over 4 days and 75 mg/kg body weight over 15 days Subcutaneous injection |
Body weight loss (~5%); no changes in the thyroid gland |
Klein et al. (1950) |
|||
BrdU, bromodeoxyuridine; DMBA, dimethylbenzanthracene; f, female; i.p., intraperitoneally; m, male
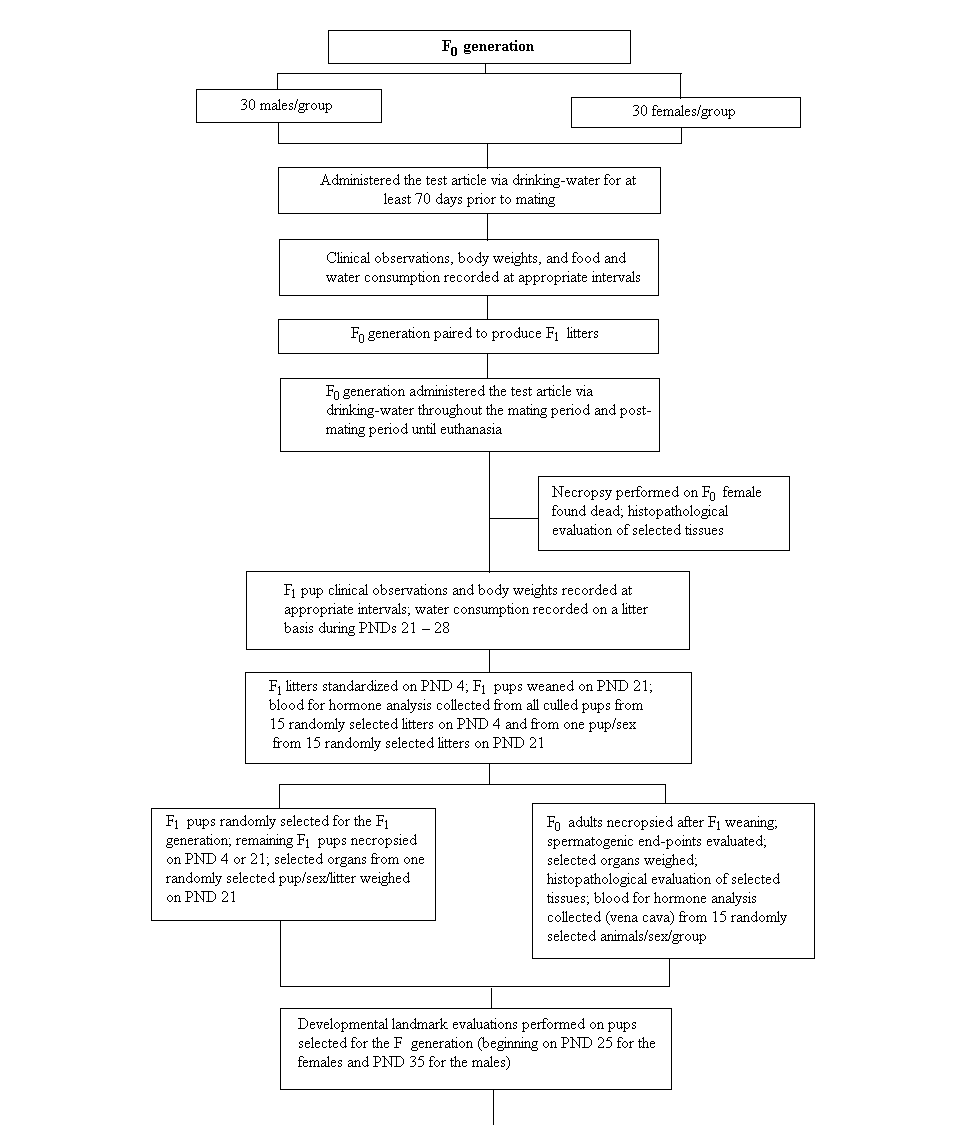
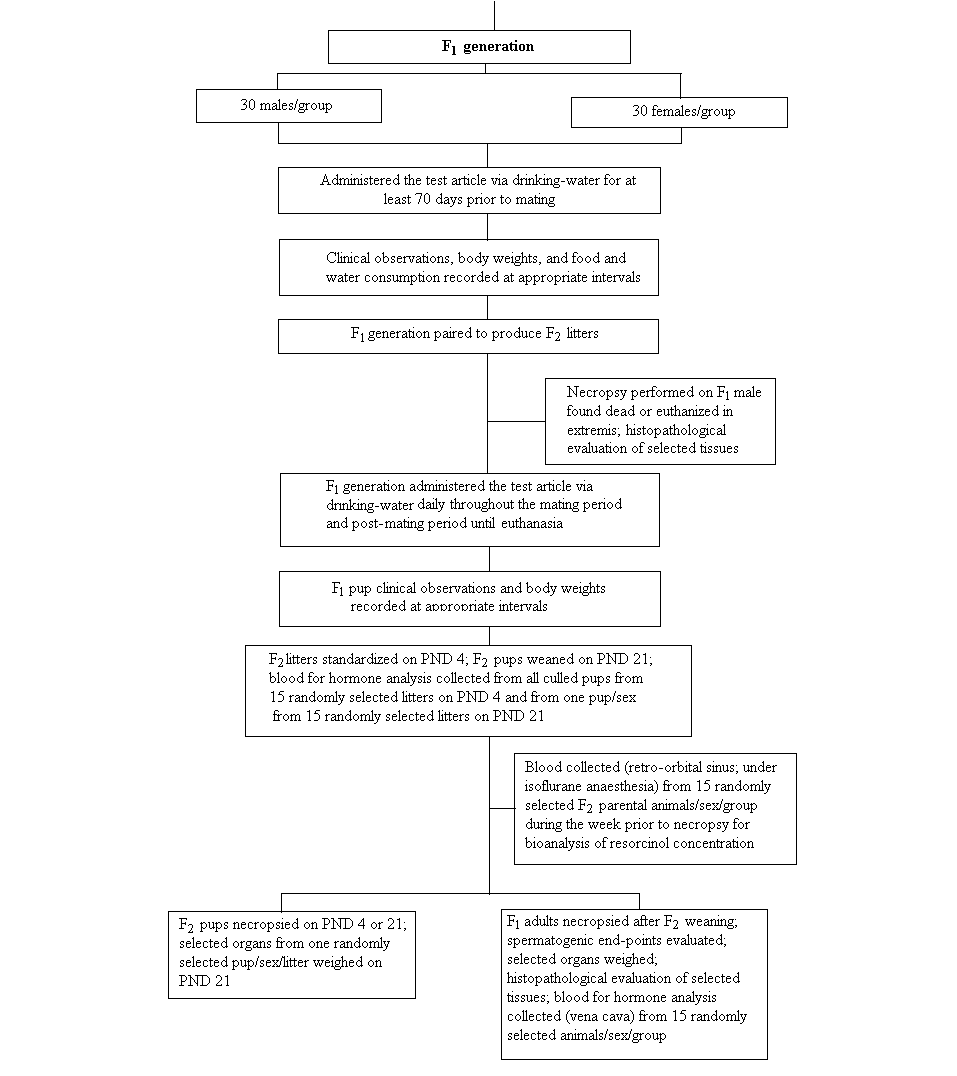
Le présent CICAD16 relatif au résorcinol a été préparé par l’Institut Fraunhofer de toxicologie et de médecine expérimentale de Hanovre (Allemagne). Il s’appuie sur les rapports respectifs de la BUA (1993) et de la Commission allemande MAK (MAK, 2003), sur celui du Conseil de la santé des Pays-Bas (2004) ainsi que sur la version préliminaire d’une base de données internationale uniforme pour l’information chimique (IUCLID) destinée au Programme HPV Challenge de l’USEPA (Agence des Etats-Unis pour l’environnement) (INDSPEC, 2004). L’appendice 2 donne des indications sur les sources bibliographiques et sur leur examen par des pairs. Une recherche bibliographique exhaustive a été effectuée jusqu’en février 2005 dans les bases de données pertinentes afin de retrouver toute référence intéressante postérieure à celles qui sont prises en compte dans les rapports précités. L’appendice 3 donne des renseignements sur l’examen par des pairs du présent CICAD. Ce CICAD a été examiné et approuvé en tant qu’évaluation internationale lors de la 13ème réunion du Comité d’évaluation finale qui s’est tenue à Nagpur (Inde) du 31 octobre au 3 novembre 2005. La liste des participants à cette réunion figure à l’appendice 4. La Fiche internationale sur la sécurité chimique du résorcinol (ICSC 1033) établie par le Programme international sur la sécurité chimique (IPCS, 2003) est également reproduite dans le présent document. Lors de l’approbation du CICAD relatif au résorcinol, cette substance a également fait l’objet d’une évaluation dans le cadre du Programme de l’OCDE sur les substances chimiques produites en grande quantité. Le résultat de l’examen par des pairs du présent CICAD a été communiqué aux Etats Membres de l’OCDE en août et septembre 2005. Dans le cadre de la coopération interinstitutionnelle en cours, toute information nouvelle produite lors de l’évaluation effectuée par l’OCDE sera communiquée par cette organisation à l’IPCS.
Le résorcinol (No CAS 108-46-3) se présente sous la forme d’un solide cristallin blanc. Il est soluble dans l’eau et sa tension de vapeur, de même que son coefficient de partage entre l’eau et le .-octanol, sont peu élevés.
De nombreux produits naturels contiennent un reste résorcinol et la réduction, l’oxydation et la dégradation microbienne des composés humiques conduisent notamment à la formation de résorcinol comme sous-produit monomère.
C’est l’industrie du caoutchouc qui est le plus gros utilisateur de résorcinol (à hauteur d’environ 50 %). On l’emploie également pour la fabrication de colles à bois de haute qualité (à hauteur d’environ 25 %) et il constitue un intermédiaire important dans la préparation de certains composés chimiques. Parmi les autres utilisations, on peut citer la fabrication de colorants, de produits pharmaceutiques, de retardateurs de flamme, de produits agrochimiques, de crèmes et de lotions fongicides ou encore de teintures capillaires.
Du résorcinol peut être libéré dans l’environnement à partir de diverses sources anthropogéniques, notamment lors de sa production, de sa transformation ou de son utilisation par le consommateur, en particulier sous la forme de teintures capillaires ou de produits pharmaceutiques. Par ailleurs, il peut être présent localement à forte concentration dans les eaux usées des installations de conversion de la houille ou dans celles des zones d’extraction de l’huile de schiste.
Le calcul montre que l’hydrosphère constitue le principal compartiment du milieu où aboutit le résorcinol. Selon les données disponibles, le résorcinol présent en solution aqueuse est essentiellement non volatil.
Le composé ne devrait pas subir d’hydrolyse dans l’hydrosphère. Toutefois, en solution aqueuse, il se produit une auto-oxydation et on peut supposer que dans les étendues d’eau, le résorcinol réagit avec les radicaux hydroxyles et peroxyles. Il est facilement biodégradable en aérobiose et peut probablement l’être aussi en anaérobiose.
Dans les couches supérieures de l’atmosphère, le résorcinol subit une décomposition rapide (demi-vie d’environ 2 h) sous l’action des radicaux hydroxyles produits par voie photochimique.
Les résultats expérimentaux obtenus sur sol limoneux indiquent une très faible sorption du résorcinol par les particules du sol, d’où la grande mobilité potentielle de ce composé. Le calcul du BCF (facteur de bioconcentration) montre que la bioaccumulation est peu probable.
On ne connaît la valeur des concentrations locales que dans le cas des eaux usées provenant d’installations de conversion de la houille ou de celles qui sont présentes dans les zones d’extraction de l’huile de schiste. Ces valeurs ne conviennent pas pour une évaluation du risque imputable aux émissions d’origine anthropogénique car elles ne sont pas représentatives de la concentration de fond ni des concentrations locales. C’est pourquoi l’estimation des concentrations présentes dans l’environnement en Europe a été effectuée à l’aide du logiciel EUSES 2.0.3.
Le calcul montre que les concentrations les plus élevées doivent être présentes au niveau des sources ponctuelles locales, par exemple là où des teintures capillaires sont préparées et des élastomères fabriqués. Selon ces estimations, ces concentrations présentes dans l’eau sont 10 fois plus élevées que les concentrations locales résultant de l’utilisation de produits de consommation contenant du résorcinol, qui sont libérés dans l’environnement à l’échelle du continent.
Les résultats des études pharmacocinétiques sur le rat, le lapin et l’Homme indiquent que l’absorption du résorcinol s’effectue par la voie orale, transcutanée ou sous-cutanée et que le composé est ensuite rapidement métabolisé puis excrété principalement dans l’urine sous forme de conjugués de type glucuronide. Selon les études existantes, il n’y a pas de signes de bioaccumulation. Sous forme de soluté hydroalcoolique, les possibilités de résorption du résorcinol par la peau intacte sont limitées.
Parmi les effets toxiques imputables à l’administration de résorcinol à des animaux de laboratoire on a notamment fait état de troubles de la fonction thyroïdienne, d’irritation cutanée, d’effets neurologiques centraux et d’un poids relatif anormal des surrénales. Dans un certain nombre d’études, on a constaté une réduction du gain de poids et une moindre survie.
Les études relatives à la toxicité aiguë du résorcinol chez les animaux de laboratoire montrent que ce composé est peu toxique après inhalation ou exposition par la voie cutanée, mais que sa toxicité augmente lorsqu’il est administré par voie intrapéritonéale ou sous-cutanée. Le résorcinol est irritant pour les yeux et la peau et peut provoquer une sensibilisation cutanée.
Après avoir exposé par gavage pendant une courte période (17 jours) des rats F344 et des souris B6C3F1 5 jours par semaine à du résorcinol, on a obtenu, pour des signes cliniques consistant en hyperexcitabilité, tachypnée et tremblements, des NOAEL (dose maximale pour laquelle aucun effet n’a été observé) respectivement égales à 27,5 mg/kg et à 75 mg/kg de poids corporel, les signes observés étant selon toute probabilité imputables à un effet aigu du composé sur le système nerveux central. Aucune lésion macroscopique ou microscopique n’a été observée.
Lors d’une étude de 13 semaines sur des rats F344 et des souris B6C3F1, on a constaté que les LOAEL (dose la plus faible à laquelle un effet nocif a été observé) relatives au poids des surrénales étaient comprises entre 28 et 32 mg/kg de poids corporel et les NOAEL relatives au poids du foie égales à 32 mg/kg de poids corporel (administration 5 jours par semaine) sans relation dose-réponse bien caractérisée. Aux doses les plus fortes (420 à 520 mg/kg de poids corporel), on a observé des tremblements et une augmentation de la mortalité. Aucune différence n’a été observée par rapport aux témoins en ce qui concerne les paramètres hématologiques et biochimiques et les animaux traités ne présentaient pas de lésions macroscopiques ou microscopiques.
Aucun signe de cancérogénicité n’a été constaté chez des rats mâles F344 et des souris B6C3F1 des deux sexes soumis à des doses de résorcinol allant de 0 à 225 mg/kg de poids corporel, ni chez des rattes exposées 5 jours par semaine pendant 104 semaines à ce même composé à raison de 0-150 mg/kg de poids corporel (NTP, 1992). Des signes cliniques d’ataxie ainsi que des tremblements ont été observés à la dose d’environ 100 mg/kg p.c., mais aucune différence concernant les paramètres hématologiques et biochimiques n’a été relevée par rapport aux témoins, ni d’ailleurs sur le plan anatomo-pathologique. La NOAEL pour les signes cliniques traduisant des effets aigus au niveau du SNC était de 50 mg/kg de poids corporel. Une étude portant sur des souris transgéniques CB6F1-Tg rasH2 recevant par gavage soit 0, soit 225 mg de résorcinol par kg de poids corporel 5 jours par semaine pendant 24 à 26 semaines n’a permis de mettre en évidence qu’une légère augmentation, non significative, de l’incidence des adénomes pulmonaires. Les expériences d’initiation-promotion pratiquées sur plusieurs espèces ont, dans la plupart des cas, donné des résultats négatifs. Cependant, dans trois études utilisant une nitrosamine comme initiateur, il y a eu augmentation de l’incidence des tumeurs.
Dans les épreuves de mutagénicité sur bactéries, le résorcinol a donné des résultats négatifs dans la plupart des cas. Le composé a toutefois provoqué des mutations dans des cellules lymphomateuses murines au niveau du locus TK. Il n’a pas entraîné de synthèse non programmée de l’ADN dans des cellules hépatiques ni de ruptures de l’ADN monocaténaire dans des cellules mammaliennes in vitro. La recherche d’échanges entre chromatides sśurs ou d’aberrations chromosomiques dans des cellules isolées in vitro a donné des résultats positifs et négatifs. Par contre, les résultats des études cytogénétiques in vivo (présence de micronoyaux dans la moelle osseuses de rats et de deux souches de souris; échanges entre chromatides sśurs chez des rats mâles et femelles) ont toujours été négatifs.
Lors d’une étude toxicologique portant sur les effets de différentes doses de résorcinol présentes dans de l’eau de boisson, des rats mâles et femelles ont reçu sans interruption pendant une durée minimale de 28 jours avant l’accouplement des doses de ce composé allant jusqu’à 360 mg/l sans que l’on puisse relever d’effets indésirables sur la capacité de reproduction, la mortalité ou encore le poids du corps et des organes (RTF, 2003). Dans l’étude bigénérationnelle qui a suivi, des doses respectives de 0, 120, 360, 1000 et 3000 mg/l ont été administrées aux animaux dans leur eau de boisson. On en a tiré une valeur de 1000 mg/l pour la NOEL (dose sans effet observé) et une valeur de 3000 mg/l pour la NOAEL, les critères retenus étant la toxicité systémique ou les effets toxiques sur la reproduction dans la génération parentale et la toxicité en général chez les rats nouveau-nés. Rapportée au poids corporel (valeur moyenne pour les animaux des générations F0 et F1), cette valeur de la NOAEL correspond à une dose journalière d’environ 233 mg/kg de poids corporel pour les mâles sur toute une génération, 304 mg/kg pour les femelles pendant la période avant l’accouplement et la gestation et 660 mg/kg pour les femelle pendant la période de lactation (RTF, 2005). L’étude relative aux effets d’une série de doses sur la reproduction comportait une batterie d’épreuves neurotoxicologiques, mais aucun effet n’a été observé dans ces tests sauf dans celui qui portait sur l’activité locomotrice de la progéniture mâle.
Des études antérieures sur des rattes et des lapines gravides n’ont pas non plus révélé d’effets toxiques sur le développement. Après administration de résorcinol à des rattes par gavage à des doses allant jusqu’à 500 mg/kg de poids corporel du 6ème au 15ème jour de la gestation, on n’a pas observé d’embryotoxicité ni d’effets indésirables sur le nombre moyen de corps jaunes, le nombre total de nidations, de fśtus viables ou encore sur le poids corporel moyen des fśtus. Il n’y avait pas non plus d’anomalies ou de malformations fśtales plus nombreuses. Une légère toxicité a été constatée chez les mères (perte de poids au bout de 24 h et réduction du gain de poids au bout de 72 h) lors d’une autre étude avec des doses > 667 mg/kg de poids corporel.
Les effets du résorcinol sur la thyroïde ont été décrits dans des études d’une durée respective de 30 jours et de 12 semaines au cours desquelles les animaux ont reçu quotidiennement le composé dans leur eau de boisson à raison de 5 mg/kg de poids corporel. Aucune modification histopathologique n’a été observée au niveau de la thyroïde lors d’études au cours desquelles des rats ou des souris ont reçu du résorcinol de manière subaiguë, subchronique ou chronique; les taux de T3/T4 (triiodothyronine/thyroxine) n’ont toutefois pas été déterminés, sauf dans une étude de 13 semaines sur le rat, chez les animaux des groupes recevant soit 0, soit 130 mg de résorcinol par kg de poids corporel. L’étude de longue durée (104 semaines) a permis de fixer à 150-520 mg/kg de poids corporel par jour la NOAEL correspondant aux effets thyroïdiens (administration 5 jours par semaine); ces études n’avaient toutefois pas été conçues pour une exploration de ce point d’aboutissement des effets toxiques du résorcinol. Lors d’une étude monogénérationnelle visant à déterminer les effets du résorcinol présent à différentes doses dans de l’eau de boisson, des rats mâles et femelles ont reçu sans interruption des doses de ce composé allant jusqu’à 360 mg/ kg de poids corporel (mâles : 1, 4, 13 ou 37 mg/kg de poids corporel par jour; femelles : 1, 5, 16 ou 47 mg/kg de poids corporel par jour). Certains effets ont été observés au niveau de la thyroïde, mais de façon sporadique, sans signification statistique et sans relation avec la dose administrée (RTF, 2003). Dans une étude bigénérationnelle consistant également à administrer de l’eau de boisson additionnée de résorcinol (RTF, 2005), aucune modification statistiquement significative attribuable au résorcinol n’a été observée dans la concentration de la T3, de la T4 ou de la TSH (thyréostimuline) chez les animaux des générations parentales F0 et F1 ni chez les animaux nouveau-nés des générations F1 et F2 sélectionnés en vue de cette analyse (4 jours ou 21 jours après la naissance). Des taux plus élevés de TSH ont été relevés chez les mâles F0 lors de l’examen nécropsique prévu, mais on a considéré que ces effets ne pouvaient être attribués au résorcinol en l’absence d’effets sur la T3, la T4, le poids des organes et faute d’autres anomalies microscopiques ou macroscopiques. La diminution, attribuable au produit à expertiser, de la substance colloïde thyroïdienne observée chez les mâles de la génération F0 qui recevaient une dose de 3000 mg/l n’a pas été considérée comme nocive du fait de l’absence d’effets fonctionnels concomittants.
Administré à forte dose à des rongeurs, le résorcinol peut inhiber l’activité de synthèse de la thyroïde et produire des effet goitrogènes. Les différences interspécifiques qui existent dans la synthèse, la fixation et le transport des hormones thyroïdiennes compliquent l’interprétation des effets goitrogènes.
Des études in vitro indiquent que l’activité antithyroïdienne constatée après exposition au résorcinol est due à l’inhibition des peroxydases thyroïdiennes, comme le prouve le blocage de la synthèse des hormones thyroïdiennes et les anomalies de la thyroïde correspondant aux effets goitrogènes.
Chez l’Homme, des effets thyroïdiens, des troubles du SNC et des anomalies érythrocytaires ont été imputés à une exposition au résorcinol. La sensibilisation cutanée au résorcinol est bien connue mais rare en pratique; les données disponibles ne permettent pas d’évaluer le pouvoir sensibilisateur de ce composé.
Deux types d’effets toxiques pourraient être utilisés pour déterminer la valeur de la dose tolérable : les effets thyroïdiens et les effets neurologiques. Ces deux types d’effets ont été signalés dans des rapports médicaux à la suite de l’utilisation de produits à forte teneur en résorcinol (jusqu’à 50 %) soit sous forme de pommades pour le traitement des ulcères, soit sous forme d’exfoliants cutanés. Ils ont également été notés lors d’études sur rongeurs utilisant de fortes concentrations de résorcinol. Aucune étude sur rongeur n’examine convenablement ces deux types d’effets.
Les données concernant les effets thyroïdiens et neurologiques chez des sujets humains proviennent de rapports médicaux qui ne donnent qu’une estimation de l’exposition et ne permettent donc pas d’établir la valeur de la dose tolérable.
C’est pour cette raison que l’étude retenue pour l’établissement de la dose tolérable est l’étude au long cours du NTP (Programme national de toxicologie des Etats-Unis) (1992). Cette étude a permis de fixer à 50 mg/kg de poids corporel par jour – soit environ 36 mg/kg p.c. par jour après correction pour tenir compte du fait que le composé était administré 5 jours par semaine – la NOAEL relative aux effets neurologiques (signes cliniques aigus). Aucune anomalie histopathologique n’a été relevée au niveau de la thyroïde et aucune mesure du rapport T3/T4 n’a été effectuée. En appliquant un facteur d’incertitude de 10 pour tenir compte des différences interspécifiques et un facteur supplémentaire également égal à 10 pour les différences intraspécifiques, on arrive à une dose tolérable de 0,4 mg/kg de poids corporel par jour.
Lors d’une étude sur des volontaires humains reproduisant les cas d’exposition les pires (utilisation d’une crème antiacnéique à 2 %), on n’a observé aucun effet sur la thyroïde (pas de modification constatée dans les dosages suivants : T3/T4/T7/TSH) pour une dose quotidienne de 12 mg/kg de poids corporel en application cutanée (dose systémique estimative égale à 0,4 mg/kg de poids corporel par jour).
On peut donc considérer que la valeur de 0,4 mg/kg de poids corporel par jour attribuée à la dose tolérable par l’étude du NTP (1992) garantit une protection à la fois contre les effets neurologiques et contre les effets thyroïdiens.
Les résultats des tests toxicologiques valables effectués sur divers organismes aquatiques permettent de considérer le résorcinol comme faiblement à fortement toxique dans le milieu aquatique. La concentration la plus faible sans effet observé (NOEC) a été déterminée sur la daphnie Daphnia magna par un test toxicologique consistant à mesurer les concentrations sur toute la durée du cycle biologique (NOEC à 21 jours = 172 μg/l). On n’a toutefois pas pratiqué de test à des concentrations plus fortes, si bien que la valeur réelle de la NOEC pourrait bien être plus élevée. Comme on dispose des résultats d’études longitudinales concernant deux niveaux trophiques (poissons et daphnies), il est néanmoins possible de fixer à 3,4 μg/l la valeur de la concentration prédite sans effet dans l’environnement aquatique (PNECaqua) en appliquant un facteur d’évaluation de 50 comme l’indique le document d’orientation technique de l’Union européenne (CE, 2003a).
En utilisant cette valeur de la PNEC et la valeur de la concentration prédite dans l’environnement (PEC) en ce qui concerne les eaux superficielles, on a pu estimer le risque que le résorcinol représente pour le milieu aquatique superficielle (PEC/PNEC).
Au niveau régional, le calcul indique un risque moindre pour les eaux superficielles. L’industrie du caoutchouc est le plus gros consommateur de résorcinol. La valeur du rapport PEC/PNEC donne une estimation du risque pour les eaux superficielle en prenant pour hypothèse que les sites de production d’élastomères sont reliés à une station de traitement des eaux usées. Si tel n’est pas le cas, le risque imputable aux effluents produits par l’industrie du caoutchouc sera plus élevé.
Il est peu probable que l’emploi de résorcinol dans les teintures capillaires et les produits pharmaceutiques ait des effets négatifs sur l’écosystème aquatique superficiel. En revanche, au niveau de certaines source locales ponctuelles, comme les sites de fabrication de teintures capillaires, la prudence incite à penser que tout risque ne peut être exclu. Un essai de simulation a par contre montré qu’en présence de stations de traitement des effluents, l’élimination du résorcinol est meilleure, de sorte que le risque estimatif est moindre.
On peut donc conclure que le résorcinol constitue un risque pour le milieu aquatique en présence d’installations de fabrication de teintures capillaires ou d’unités de production d’élastomères.
Les données dont on dispose au sujet de la toxicité du résorcinol pour les organismes terrestres ne sont pas suffisantes pour une évaluation quantitative du risque. On peut toutefois estimer ce risque par la méthode de partage à l’équilibre. L’application de cette méthode montre qu’au niveau régional le risque est faible pour les sols, mais qu’un risque plus élevé ne peut être exclu localement à proximité de sources ponctuelles.
El presente CICAD17 sobre el resorcinol fue preparado por el Instituto Fraunhofer de Toxicología y Medicina Experimental de Hannover (Alemania). Se basa en un informe del Comité Consultivo Alemán sobre las Sustancias Químicas Importantes para el Medio Ambiente (BUA, 1993), el informe de la Comisión MAK Alemana (MAK, 2003), el informe del Consejo de Salud de los Países Bajos (2004) y una Base de datos internacional sobre información química uniforme (IUCLID) de carácter preliminar para el Programa sobre los problemas de los productos químicos de alto volumen de producción de la Agencia de los Estados Unidos para la Protección del Medio Ambiente (USEPA) (INDSPEC, 2004). La información sobre los documentos originales y su examen colegiado se presenta en el apéndice 2. Se realizó una búsqueda bibliográfica amplia de las bases de datos pertinentes hasta febrero de 2005 para identificar cualquier referencia de interés que se hubiera publicado después de las incorporadas a estos informes. La información sobre el examen colegiado de este CICAD figura en el apéndice 3. Este CICAD se examinó y aprobó como evaluación internacional en una reunión de la 13Ş Junta de Evaluación Final, celebrada en Nagpur (India) del 31 de octubre al 3 de noviembre de 2005. La lista de participantes en esta reunión aparece en el apéndice 4. También se reproduce en este documento la Ficha internacional de seguridad química (ICSC 1033) para el resorcinol, preparada por el Programa Internacional de Seguridad de las Sustancias Químicas (IPCS, 2003). Cuando se aprobó el CICAD sobre el resorcinol también se estaba realizando una evaluación de esta sustancia como parte del Programa de la OCDE sobre productos químicos de alto volumen de producción. El examen colegiado de este CICAD se amplió durante los meses de agosto y septiembre de 2005 para incluir a los Estados Miembros de la OCDE. Como parte de la presente cooperación, cualquier nueva información que se obtenga en el curso de la evaluación de la OCDE se facilitará al IPCS.
El resorcinol (CAS Nş 108-46-3) es una sustancia cristalina blanca, soluble en agua y con una presión de vapor y un coeficiente de reparto .-octanol/agua bajos.
El grupo funcional del resorcinol se ha encontrado en una amplia variedad de productos naturales, siendo un subproducto monomérico de la reducción, oxidación y degradación microbiana de sustancias húmicas.
El destino más importante del resorcinol es la industria del caucho (alrededor del 50%). También se utiliza en aplicaciones de encolado de madera de calidad elevada (en torno al 25%) y es un intermediario importante en la fabricación de especialidades químicas. Entre otros usos cabe mencionar la fabricación de sustancias colorantes, productos farmacéuticos, pirorretardantes, productos químicos agrícolas, cremas y lociones fungicidas y tintes para el pelo.
El resorcinol se libera en el medio ambiente a partir de diversas fuentes antropogénicas, por ejemplo la producción, la elaboración y el consumo, en particular de los tintes para el pelo y los productos farmacéuticos. Además, puede aparecer en las aguas residuales de la transformación del carbón o de las regiones con minas de esquisto bituminoso.
Según los cálculos, el compartimento final más importante del resorcinol es la hidrosfera. Los datos indican que apenas se volatiliza a partir de soluciones acuosas.
En la hidrosfera no cabe esperar que se produzca hidrólisis. Sin embargo, en solución acuosa experimenta autooxidación y se puede suponer que en las masas de agua reacciona con radicales hidroxilo y peroxilo. El resorcinol es fácilmente biodegradable en condiciones aerobias y es probable que sufra biodegradación en condiciones anaerobias.
El resorcinol se degrada con rapidez en la capa superior de la atmósfera (semivida de unas 2 h) al reaccionar con los radicales hidroxilo que se forman por vía fotoquímica.
Los datos experimentales obtenidos utilizando marga limosa indican una sorción muy lenta del resorcinol en el suelo, con el consiguiente potencial elevado de movilidad. Basándose en el factor de bioconcentración calculado, no se prevé que haya bioacumulación.
Sólo se observan concentraciones localizadas en las aguas residuales de la transformación del carbón o de regiones con minas de esquisto bituminoso. Estos valores son insuficientes para una evaluación del riesgo de las emisiones procedentes de fuentes antropogénicas, porque no son representativos de las concentraciones de fondo o locales. Por consiguiente, la estimación de las concentraciones en el medio ambiente para Europa se realizaron utilizando el programa informático EUSES 2.0.3.
Los resultados de los cálculos ponen de manifiesto que las concentraciones más elevadas se supone que se encuentran en fuentes puntuales locales, como los lugares donde se preparan tintes para el pelo o se fabrican productos de caucho. Estas concentraciones estimadas en el agua son un orden de magnitud superiores a las concentraciones locales derivadas de las emisiones debidas a la utilización de productos de consumo que contienen resorcinol, que se liberan a nivel continental.
Los resultados de los estudios farmacocinéticos en ratas, conejos y personas parecen indicar que el resorcinol se absorbe por vía oral, cutánea o subcutánea, se metaboliza con rapidez y se excreta principalmente en la orina en forma de conjugados de glucurónido. En los estudios disponibles no hay indicios de bioacumulación. El potencial de absorción del resorcinol a través de la piel intacta utilizando un vehículo hidroalcohólico es limitado.
En estudios con animales, los efectos toxicológicos notificados debidos a la administración de resorcinol son los siguientes: disfunción del tiroides, irritación cutánea, efectos en el sistema nervioso central y peso relativo alterado de las glándulas adrenales. En algunos estudios se observó una disminución del aumento del peso corporal y una reducción de la supervivencia.
Los datos de la toxicidad letal aguda en animales de experimentación pusieron de manifiesto que el resorcinol tiene una toxicidad baja tras la inhalación y la exposición cutánea, pero más elevada tras la administración oral, intraperitoneal o subcutánea. Es irritante ocular y cutáneo y puede causar sensibilización por contacto.
Los estudios de exposición oral breve (17 días) con administración mediante sonda en ratas F344 y ratones B6C3F1 con tratamiento de cinco días/semana dieron valores de la NOAEL de 27,5 y 75 mg/kg de peso corporal, respectivamente, para signos clínicos como la hiperexcitabilidad, la taquipnea y los temblores, debido probablemente a un efecto agudo del resorcinol en el sistema nervioso central. No se observaron lesiones macroscópicas ni microscópicas.
En un estudio de 13 semanas en ratas F344 y ratones B6C3F1, los valores de la LOAEL para el peso de las glándulas adrenales fueron del orden de 28–32 mg/kg de peso corporal y el de la NOAEL para el peso del hígado de 32 mg/kg de peso corporal (tratamiento de cinco días/semana), sin una relación dosis-respuesta clara. La dosificación más alta (420–520 mg/kg de peso corporal) provocó temblores y un aumento de la mortalidad. No se observaron diferencias en la hematología o la química clínica y no se detectaron lesiones macroscópicas o microscópicas en los animales tratados.
No se observaron signos de carcinogenicidad en ratas F344 machos y en ratones B6C3F1 de ambos sexos con dosis de 0–225 mg/kg de peso corporal y en ratas hembras expuestas a 0–150 mg/kg de peso corporal cinco días/semana durante 104 semanas (NTP, 1992). Se detectaron signos clínicos de ataxia y temblores con unos 100 mg/kg de peso corporal, pero no se observaron diferencias en la hematología, la química clínica u otros parámetros de patología clínica. Se calculó una NOAEL de 50 mg/kg de peso corporal para signos clínicos agudos indicativos de efectos en el sistema nervioso central. En un estudio con ratones transgénicos CB6F1-Tg rasH2 tratados mediante sonda con concentraciones de 0 ó 225 mg/kg de peso corporal cinco días/semana durante 24–26 semanas sólo se puso de manifiesto un aumento ligero no significativo de la incidencia de adenomas en los pulmones. En los estudios de promoción de la iniciación realizados utilizando varias especies casi siempre se notificaron resultados negativos. Sin embargo, tres estudios utilizando nitrosaminas como iniciadoras mostraron una mayor incidencia de tumores.
En valoraciones de la mutagenicidad bacteriana, el resorcinol dio resultados en su mayor parte negativos. Sin embargo, en células de linfoma de ratón indujo mutaciones en el locus TK. No indujo in vitro síntesis de ADN no programada en células hepáticas o roturas de cadenas sencillas de ADN en células de mamíferos. Los estudios in vitro de intercambio de cromátidas hermanas y aberraciones cromosómicas en células aisladas y líneas de células dieron resultados tanto negativos como positivos. En estudios citogenéticos in vivo (micronúcleos de la médula ósea de ratas y dos estirpes de ratones; intercambio de cromátidas hermanas en ratas machos y hembras) se obtuvieron sistemáticamente resultados negativos.
En un estudio con agua de bebida para determinar la gama de dosis en ratas machos y hembras tratados de manera continua con concentraciones de resorcinol de hasta 360 mg/l durante un período mínimo de 28 días consecutivos antes del apareamiento, no se observaron efectos adversos en relación con el rendimiento reproductivo, la mortalidad y el peso corporal o de los órganos (RTF, 2003). En el siguiente estudio con agua de bebida en dos generaciones, se administraron dosis de 0, 120, 360, 1000 ó 3000 mg/l. Se derivaron una NOEL de 1000 mg/l y una NOAEL de 3000 mg/l para la toxicidad sistémica y reproductiva parenteral, así como para la toxicidad neonatal. Expresada en función del peso corporal (promedio de los animales F0 y F1), la NOAEL equivalía a unos 233 mg/kg de peso corporal al día para los machos de toda la generación, 304 mg/kg de peso corporal al día para las hembras durante el período previo al apareamiento y la gestación y 660 mg/kg de peso corporal al día para las hembras durante la lactación (RTF, 2005). En el estudio de la reproducción para determinar la gama de dosis se incluyó una serie de pruebas neurotoxicológicas, pero no se observaron efectos distintos de los detectados en la prueba de actividad locomotora en las crías machos.
En estudios anteriores con ratas y conejas preñadas tampoco se había puesto de manifiesto ningún efecto de toxicidad en el desarrollo. La administración a ratas mediante sonda de dosis de hasta 500 mg/kg de peso corporal durante los días 6–15 de la gestación no causó embriotoxicidad y no se observaron efectos adversos en el número medio de cuerpos lúteos, las implantaciones totales, los fetos viables o el peso corporal medio de los fetos. Tampoco se detectó un aumento de las anomalías o malformaciones fetales. En un nuevo estudio con dosis de > 667 mg/kg de peso corporal se observó una ligera toxicidad materna (pérdida de peso a las 24 h, con disminución del aumento del peso materno a las 72 h) en ratas.
Se han descrito en ratas efectos en el tiroides en estudios de 30 días y 12 semanas con agua de bebida utilizando dosis de 5 mg/kg de peso corporal al día. No se observaron cambios histopatológicos en el tiroides en estudios de toxicidad subaguda, subcrónica o crónica realizados con administración mediante sonda en ratas o ratones; sin embargo, en el estudio de 13 semanas en ratas no se determinaron niveles T3/T4, con la excepción de los grupos tratados con dosis de 0 y 130 mg/kg de peso corporal. En el estudio prolongado (104 semanas), las NOAEL para los efectos en el tiroides fueron de 150–520 mg/kg de peso corporal al día (cinco días/semana); sin embargo, estos estudios no tenían por objeto investigar este efecto final. En un estudio de una generación con agua de bebida para determinar la gama de dosis se administraron de manera continua a ratas machos y hembras concentraciones de resorcinol de hasta 360 mg/l (machos: 1, 4, 13 ó 37 mg/kg de peso corporal al día; hembras: 1, 5, 16 ó 47 mg/kg de peso corporal al día). Se notificaron algunos efectos en el tiroides, pero eran desiguales, no eran estadísticamente significativos y no guardaban relación con la dosis (RTF, 2003). En el estudio con el agua de bebida en dos generaciones (RTF, 2005) no se observaron cambios estadísticamente significativos relacionados con el resorcinol en las concentraciones medias de la T3, la T4, o la TSH en los animales parentales F0 y F1 o en las crías F1 y F2 seleccionadas para el análisis (día postnatal 4 ó 21). En una necropsia programada se detectaron valores más elevados de la TSH en los machos F0, pero dada la ausencia de efectos en la T3 ó la T4 o en el peso de los órganos o de resultados macroscópicos o microscópicos adversos, no se consideraron como efectos relacionados con el resorcinol. No se consideró que la disminución de los coloides relacionada con la sustancia de prueba en el tiroides de machos F0 con 3000 mg/l fuera un efecto adverso, debido a la falta de efectos funcionales asociados.
La administración de dosis altas de resorcinol a roedores puede causar trastornos en la actividad de síntesis del tiroides y producir efectos bociogénicos. Hay diferencias específicas de especies en la síntesis, la unión y el transporte de la hormona tiroidea que complican la interpretación de la bociogénesis.
Hay estudios in vitro que indican que la actividad antitiroidea observada tras la exposición al resorcinol se debe a la inhibición de las enzimas peroxidasas del tiroides, puesta de manifiesto por la alteración de la síntesis de la hormona tiroidea y los cambios en la glándula concordantes con la bociogénesis.
En las personas, la exposición al resorcinol ha estado asociada con efectos en el tiroides, alteraciones del sistema nervioso central y cambios en los glóbulos rojos. La sensibilización cutánea al resorcinol está bien documentada, pero en la práctica es rara; los datos disponibles no permiten evaluar el grado de sensibilización.
Hay dos efectos toxicológicos que se podrían utilizar para derivar una ingesta tolerable: tiroideos y neurológicos. Se han notificado ambos efectos en informes de casos humanos debido a la aplicación cutánea de concentraciones altas (hasta un 50%) de resorcinol en ungüentos para úlceras y en exfoliantes, así como en estudios de concentraciones altas en roedores. No hay ningún estudio en roedores que abarque suficientemente ambos efectos finales.
Por este motivo, el estudio elegido para derivar una ingesta tolerable fue el estudio prolongado del NTP (1992) en el que se obtuvo una NOAEL de 50 mg/kg de peso corporal al día (unos 36 mg/kg de peso corporal al día, tras ajustar la dosificación a cinco días/semana) para los efectos neurológicos (signos clínicos agudos). No se observaron cambios histopatológicos en el tiroides. No se efectuó una medición de la razón T3/T4. La aplicación de factores de incertidumbre para diferencias interespecíficas (10) e intraespecíficas (10) da lugar a una ingesta tolerable de 0,4 mg/kg de peso corporal al día.
En un estudio de exposición del peor de los casos en personas voluntarias utilizando crema antiacné al 2% no se observaron efectos en el tiroides (es decir, no se detectaron alteraciones en las concentraciones de T3/T4/T7/TSH) con una dosis cutánea de 12 mg/kg de peso corporal al día (con niveles estimados de dosificación sistémica de 0,4 mg/kg de peso corporal al día).
Por consiguiente, la ingesta tolerable de 0,4 mg/kg de peso corporal al día derivada del estudio del NTP (1992) tendría un carácter protector para los efectos tanto neurológicos como tiroideos.
De los resultados de pruebas válidas disponibles sobre la toxicidad del resorcinol para distintos organismos acuáticos, se deduce que su toxicidad en el compartimento acuático se puede clasificar entre baja y alta. Se determinó la NOEC más baja para Daphnia magna en una prueba de toxicidad del ciclo biológico completo basada en concentraciones medidas (NOEC a los 21 días = 172 µg/l). Sin embargo, no se sometieron a prueba concentraciones más elevadas, de manera que la NOEC real probablemente será más alta. No obstante, se puede derivar una PNECagua de 3,4 µg/l utilizando un factor de evaluación de 50, conforme al Documento de orientación técnica de la Unión Europea (CE, 2003a), puesto que se dispone de resultados procedentes de estudios crónicos de dos niveles tróficos (peces y Daphnia).
Utilizando este valor de la PNEC y los valores de las PEC para el agua superficial, se obtuvo una estimación del riesgo del resorcinol para el medio acuático en el agua superficial (PEC/PNEC).
Para el agua superficial regional, los cálculos pusieron de manifiesto un riesgo bajo. La industria del caucho es la consumidora más importante de resorcinol. El valor PEC/PNEC indica un riesgo para el agua superficial, suponiendo que las aguas residuales de las zonas de producción de caucho estén conectadas a una planta depuradora de aguas residuales. En caso contrario, el riesgo calculado a partir del efluente de la industria del caucho sería mayor.
Las aplicaciones como los tintes para el pelo y los productos farmacéuticos tienen una probabilidad baja de efectos negativos en el ecosistema del agua superficial. En cambio, utilizando un criterio prudente no se puede excluir un riesgo en fuentes puntuales locales, como los lugares donde se preparan los tintes para el pelo. Sin embargo, los resultados de una prueba de simulación indicaron que en las plantas depuradoras de aguas residuales hay una eliminación más elevada de resorcinol que daría lugar a un riesgo calculado menor.
En conclusión, el resorcinol puede representar un riesgo para el medio acuático en zonas donde se preparan tintes para el pelo y en instalaciones de producción de caucho.
Los datos disponibles de la toxicidad para los organismos terrestres no permiten realizar una evaluación cuantitativa del riesgo. Sin embargo, se puede realizar una estimación del riesgo utilizando el método de reparto en equilibrio. Utilizando este método, se encontró un riesgo bajo para el compartimento del suelo regional, pero no se puede excluir un riesgo en fuentes puntuales locales.
ENDNOTES:
See Also:
Toxicological Abbreviations
Resorcinol (ICSC)
RESORCINOL (JECFA Evaluation)
Resorcinol (IARC Summary & Evaluation, Volume 71, 1999)