
This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organization, or the World Health Organization.
Concise International Chemical Assessment Document 70
First draft prepared by Drs J. Kielhorn, S. Schmidt, and I. Mangelsdorf, Fraunhofer Institute of Toxicology and Experimental Medicine, Hanover, Germany; and Dr P. Howe, Centre for Ecology & Hydrology, Monks Wood, United Kingdom
Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organization, and the World Health Organization, and produced within the framework of the Inter-Organization Programme for the Sound Management of Chemicals.
The International Programme on Chemical Safety (IPCS), established in 1980, is a joint venture of the United Nations Environment Programme (UNEP), the International Labour Organization (ILO), and the World Health Organization (WHO). The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals.
The Inter-Organization Programme for the Sound Management of Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and Agriculture Organization of the United Nations, WHO, the United Nations Industrial Development Organization, the United Nations Institute for Training and Research, and the Organisation for Economic Co-operation and Development (Participating Organizations), following recommendations made by the 1992 UN Conference on Environment and Development to strengthen cooperation and increase coordination in the field of chemical safety. The purpose of the IOMC is to promote coordination of the policies and activities pursued by the Participating Organizations, jointly or separately, to achieve the sound management of chemicals in relation to human health and the environment.
WHO Library Cataloguing-in-Publication Data
Heptachlor.
(Concise international chemical assessment document ; 70)
First draft prepared by J. Kielhorn, S. Schmidt and I. Mangelsdorf.
1. Heptachlor - adverse effects. 2. Heptachlor - toxicity. 3. Environmental exposure.
4. Risk assessment. I. Kielhorn, Janet. II. Schmidt, S. III. Mangelsdorf, Inge. IV. World
Health Organization. V. International Programme on Chemical Safety, VI. Series.
ISBN 92 4 153070 7 (NLM Classification: WA 240)
ISBN 978 92 4 153070 5
©World Health Organization 2006
All rights reserved. Publications of the World Health Organization can be obtained from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel: +41 22 791 3264; fax: +41 22 791 4857; email: bookorders@who.int). Requests for permission to reproduce or translate WHO publications — whether for sale or for noncommercial distribution — should be addressed to WHO Press, at the above address (fax: +41 22 791 4806; email: permissions@who.int).
The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement.
The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters.
All reasonable precautions have been taken by WHO to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either express or implied. The responsibility for the interpretation and use of the material lies with the reader. In no event shall the World Health Organization be liable for damages arising from its use.
Risk assessment activities of the International Programme on Chemical Safety, including the production of Concise International Chemical Assessment Documents, are supported financially by the Department of Health and Department for Environment, Food & Rural Affairs, UK, Environmental Protection Agency, Food and Drug Administration, and National Institute of Environmental Health Sciences, USA, European Commission, German Federal Ministry of Environment, Nature Conservation and Nuclear Safety, Health Canada, Japanese Ministry of Health, Labour and Welfare, and Swiss Agency for Environment, Forests and Landscape.
Concise International Chemical Assessment Documents (CICADs) are published by the International Programme on Chemical Safety (IPCS) — a cooperative programme of the World Health Organization (WHO), the International Labour Organization (ILO), and the United Nations Environment Programme (UNEP). CICADs have been developed from the Environmental Health Criteria documents (EHCs), more than 200 of which have been published since 1976 as authoritative documents on the risk assessment of chemicals.
International Chemical Safety Cards on the relevant chemical(s) are attached at the end of the CICAD, to provide the reader with concise information on the protection of human health and on emergency action. They are produced in a separate peer-reviewed procedure at IPCS. They may be complemented by information from IPCS Poison Information Monographs (PIM), similarly produced separately from the CICAD process.
CICADs are concise documents that provide summaries of the relevant scientific information concerning the potential effects of chemicals upon human health and/or the environment. They are usually based on selected national or regional evaluation documents or on existing EHCs. Before acceptance for publication as CICADs by IPCS, these documents undergo extensive peer review by internationally selected experts to ensure their completeness, accuracy in the way in which the original data are represented, and the validity of the conclusions drawn.
The primary objective of CICADs is characterization of hazard and dose–response from exposure to a chemical. CICADs are not a summary of all available data on a particular chemical; rather, they include only that information considered critical for characterization of the risk posed by the chemical. The critical studies are, however, presented in sufficient detail to support the conclusions drawn. For additional information, the reader should consult the identified source documents upon which the CICAD has been based.
Risks to human health and the environment will vary considerably depending upon the type and extent of exposure. Responsible authorities are strongly encouraged to characterize risk on the basis of locally measured or predicted exposure scenarios. To assist the reader, examples of exposure estimation and risk characterization are provided in CICADs, whenever possible. These examples cannot be considered as representing all possible exposure situations, but are provided as guidance only. The reader is referred to EHC 170.1
While every effort is made to ensure that CICADs represent the current status of knowledge, new information is being developed constantly. Unless otherwise stated, CICADs are based on a search of the scientific literature to the date shown in the executive summary. In the event that a reader becomes aware of new information that would change the conclusions drawn in a CICAD, the reader is requested to contact IPCS to inform it of the new information.
Procedures
The flow chart shows the procedures followed to produce a CICAD. These procedures are designed to take advantage of the expertise that exists around the world — expertise that is required to produce the high-quality evaluations of toxicological, exposure, and other data that are necessary for assessing risks to human health and/or the environment. The IPCS Risk Assessment Steering Group advises the Coordinator, IPCS, on the selection of chemicals for an IPCS risk assessment based on the following criteria:
Thus, it is typical of a priority chemical that:

|
Advice from Risk Assessment Steering Group Criteria of priority:
Thus, it is typical of a priority chemical that
Special emphasis is placed on avoiding duplication of effort by WHO and other international organizations. A prerequisite of the production of a CICAD is the availability of a recent high-quality national/regional risk assessment document = source document. The source document and the CICAD may be produced in parallel. If the source document does not contain an environmental section, this may be produced de novo, provided it is not controversial. If no source document is available, IPCS may produce a de novo risk assessment document if the cost is justified. Depending on the complexity and extent of controversy of the issues involved, the steering group may advise on different levels of peer review:
|
The Steering Group will also advise IPCS on the appropriate form of the document (i.e. a standard CICAD or a de novo CICAD) and which institution bears the responsibility of the document production, as well as on the type and extent of the international peer review.
The first draft is usually based on an existing national, regional, or international review. When no appropriate source document is available, a CICAD may be produced de novo. Authors of the first draft are usually, but not necessarily, from the institution that developed the original review. A standard outline has been developed to encourage consistency in form. The first draft undergoes primary review by IPCS to ensure that it meets the specified criteria for CICADs.
The second stage involves international peer review by scientists known for their particular expertise and by scientists selected from an international roster compiled by IPCS through recommendations from IPCS national Contact Points and from IPCS Participating Institutions. Adequate time is allowed for the selected experts to undertake a thorough review. Authors are required to take reviewers’ comments into account and revise their draft, if necessary. The resulting second draft is submitted to a Final Review Board together with the reviewers’ comments. At any stage in the international review process, a consultative group may be necessary to address specific areas of the science. When a CICAD is prepared de novo, a consultative group is normally convened.
The CICAD Final Review Board has several important functions:
Board members serve in their personal capacity, not as representatives of any organization, government, or industry. They are selected because of their expertise in human and environmental toxicology or because of their experience in the regulation of chemicals. Boards are chosen according to the range of expertise required for a meeting and the need for balanced geographic representation.
Board members, authors, reviewers, consultants, and advisers who participate in the preparation of a CICAD are required to declare any real or potential conflict of interest in relation to the subjects under discussion at any stage of the process. Representatives of nongovernmental organizations may be invited to observe the proceedings of the Final Review Board. Observers may participate in Board discussions only at the invitation of the Chairperson, and they may not participate in the final decision-making process.
This CICAD2 on heptachlor was prepared by the Fraunhofer Institute of Toxicology and Experimental Medicine, Hanover, Germany. It is an update of the Environmental Health Criteria document on heptachlor (IPCS, 1984) and includes data from the IARC (2001) and JMPR (1992) reports. A comprehensive literature search of relevant databases was conducted from 2000 up to February 2004 to identify any relevant references published subsequent to those incorporated in these reports. Information on the source documents is presented in Appendix 2. Information on the peer review of this CICAD is presented in Appendix 3. This CICAD was considered and approved as an international assessment at a meeting of the Final Review Board, held in Hanoi, Viet Nam, on 28 September – 1 October 2004. Participants at the Final Review Board meeting are presented in Appendix 4. The International Chemical Safety Card on heptachlor (ICSC 0743), produced by the International Programme on Chemical Safety (IPCS, 2003), has also been reproduced in this document.
Heptachlor (CAS No.
Heptachlor released into the environment can be transformed by abiotic processes, such as the transformation by photochemically produced hydroxyl radicals, and it is transformed in the presence of water to compounds such as 1-hydroxychlordene or heptachlor epoxide (such as, for example, in moist soils). In addition, it can be removed to some extent from aquatic systems by evaporation and has a limited potential to leach from soil into groundwater due to its elevated soil sorption coefficient. It is not readily biodegraded, but it is transformed biologically (i.e. by bacteria, fungi, plants, animals), mainly to the stable heptachlor epoxide. The data available on the bioconcentration potential of this lipophilic chlorinated hydrocarbon indicate that it and its stable epoxide will bioaccumulate, which can be shown from the extent of heptachlor/heptachlor epoxide still detected in environmental samples.
The main exposure routes for heptachlor are probably via application-related inhalation or skin penetration, from extended exposure to dusts containing heptachlor in, for example, homes treated with this compound to control termites, and indirectly by uptake from food contaminated with heptachlor from crops or from other foods via the food-chain. However, heptachlor is a component of technical chlordane as well as a metabolite of chlordane, and thus identification of heptachlor or heptachlor epoxide does not always signify unequivocally that the primary exposure was to heptachlor (or heptachlor epoxide) per se.
A survey of recent studies shows that heptachlor and/or heptachlor epoxide are found in all environmental compartments — air, water, soil, and sediment — as well as in plants (vegetables), fish and other aquatic organisms, amphibians and reptiles, birds and bird eggs, and aquatic and terrestrial mammals. They are found particularly in adipose tissues, where they accumulate. They pass up the food-chain. They are detected in human serum, adipose tissue, including breast tissue, and human breast milk.
Heptachlor is readily absorbed via all routes of exposure and is readily metabolized. The major faecal metabolites include heptachlor epoxide, 1-hydroxychlordene, and 1-hydroxy-2,3-epoxychlordene. In liver microsomes incubated with heptachlor, 85.8% was metabolized to heptachlor epoxide in rats, but only 20.4% in humans. Other metabolites identified in the human liver microsome system were 1-hydroxy-2,3-epoxychlordene (5%), 1-hydroxychlordene (4.8%), and 1,2-dihydroxydihydrochlordene (0.1%). Heptachlor epoxide is metabolized slowly and is the most persistent metabolite; it is stored mainly in adipose tissue, but also in liver, kidney, and muscle. Females appear to store more heptachlor epoxide than males. A period of 12 weeks was required for complete disappearance from the fat after discontinuing heptachlor feeding in rats.
In humans and laboratory animals, placental transfer of heptachlor and/or heptachlor epoxide has been shown.
Acute oral LD50s for heptachlor for the rat and mouse are 40–162 and 68–90 mg/kg body weight, respectively. The acute toxicity of heptachlor in animals is associated with central nervous system disturbances, such as hyperexcitability, tremors, convulsions, and paralysis. The acute toxicity of heptachlor epoxide is greater than that of heptachlor, whereas that of the other metabolites is much less.
In animals fed heptachlor/heptachlor epoxide by diet, gavage, or subcutaneous injection, there is a sharp dose–response curve for mortality. Usually, no marked differences were seen between the treated animals and the controls with respect to body weights and food consumption. However, liver enlargement has been described, associated with accentuated lobulation, and histopathological findings showed enlargement of centrilobular and midzonal hepatocytes.
Fertility studies in rats injected with heptachlor subcutaneously resulted in LOAELs of 5 mg/kg body weight per day for suppression of reproductive hormone levels, disruptions in female cyclicity, and delays in mating behaviour.
In developmental toxicity studies, there were usually no clinical signs of maternal toxicity (dose-related alterations in weight gain) until mortality occurred [NOAEL for maternal toxicity = 3 mg/kg body weight per day]. In one study, reduced litter sizes were noted, but postnatal mortality of the pups was the most obvious finding [NOAEL for pre- or postnatal survival of pups = 6 mg/kg body weight per day]. No teratological effects were observed.
There is accumulating evidence that the nervous system and its development are influenced by cyclodiene pesticides. The profile of effects produced by repeated heptachlor administration to female rats consisted of altered activity, hyperexcitability, and autonomic effects [NOAEL = 2 mg/kg body weight per day]. Neurotoxicological studies on perinatal heptachlor exposure in the rat (0.03, 0.3, or 3 mg/kg body weight per day) suggested developmental delays, alterations in GABAergic neurotransmission, and neurobehavioural changes, including cognitive deficits at all doses.
Immunological studies in rats indicate the suppression of the primary IgM and secondary IgG anti-sheep red blood cell responses following perinatal exposure to all tested doses (0.03, 0.3, or 3 mg/kg body weight per day) of heptachlor.
Heptachlor, technical-grade heptachlor, heptachlor epoxide, and a mixture of heptachlor and heptachlor epoxide have been tested for carcinogenicity by oral administration in several strains of mice and rats. Heptachlor/heptachlor epoxide and technical-grade heptachlor were shown to be carcinogenic in male and female mice but not in rats. In an initiation–promotion assay, heptachlor was active as a promoter after initiation by .-nitrosodiethylamine.
Heptachlor shows mostly negative responses in in vitro and in vivo genotoxicity testing. Heptachlor causes in vitro inhibition of gap junctional intercellular communication, also suggesting a non-genotoxic carcinogenic mechanism.
Available epidemiological data do not show a clear relationship between adverse health effects and exposure to heptachlor. A tolerable intake was therefore developed from experimental studies. As hepatic tumours induced by heptachlor in mice are likely to be induced by a non-genotoxic mechanism and as non-neoplastic effects were observed at doses 1/20th of those inducing tumours, non-neoplastic effects (i.e. histopathological effects in the liver, neurotoxicological effects, and immunotoxicological effects) were used to derive the tolerable intake. The NOAEL for hepatic effects observed in dogs was 25 µg/kg body weight per day, and that for neurotoxicity and immunotoxicity observed in studies in rats was 30 µg/kg body weight per day. Applying an uncertainty factor of 10 for each of inter- and intraspecies variation and an additional factor of 2 for inadequacy of the database to the NOAEL in dogs gives a tolerable intake of 0.1 µg/kg body weight per day for the non-neoplastic effects.
Daily dietary intakes of heptachlor and heptachlor epoxide in Poland were estimated at 0.51–0.58 µg per person (about 0.01 µg/kg body weight, assuming a mean weight of 64 kg). This value is 10-fold less than the tolerable intake of 0.1 µg/kg body weight. However, if food is contaminated with heptachlor, such as fish from contaminated rivers (e.g. concentrations in fish in the 0.1–1 mg/kg range reported recently in some areas), vegetables from fields contaminated with heptachlor (up to 16 mg/kg), or contaminated milk (e.g. in the microgram per kilogram to milligram per kilogram range in some regions), then the dietary intake of this chemical would be much higher, and there would be a likely health risk if the contaminated food is ingested for a long period of time. For breast-fed children, taking the highest reported values for heptachlor epoxide in human breast milk and assuming a daily milk consumption of 150 g/kg body weight and an average milk fat content of 3.1%, a mean intake of 1.5 µg/kg body weight can be calculated. This value is more than 10-fold higher than the tolerable intake of 0.1 µg/kg body weight per day and, if the concentrations reported are correct, should be a cause of concern.
The acute toxicity of heptachlor was tested using a variety of aquatic species from different trophic levels. Heptachlor was shown to be toxic to fish and other aquatic species. However, there is a great deal of variability in the levels of toxicity reported, possibly due to evaporation of heptachlor, thereby reducing the actual concentration of the test compound from the nominal test concentration over time.
For the freshwater environment, 23 toxicity values were chosen to derive a guidance value. A guidance value for heptachlor, based on the species sensitivity distribution, for the protection of 99% of species with 50% confidence was derived at 10 ng/l. In many locations, freshwater heptachlor concentrations exceed the guidance value; the highest reported heptachlor concentration measured in fresh surface water, 62 000 ng/l, exceeds it more than 1000-fold.
For the marine environment, 18 toxicity values were chosen to derive a guidance value. A guidance value for heptachlor, based on the species sensitivity distribution, for the protection of 99% of species with 50% confidence was derived at 5 ng/l. For seawater, the highest reliable value for heptachlor present is about 0.15 ng/l, so the guidance value is not exceeded, suggesting a low risk for the marine environment.
From the few data available, heptachlor appears to exhibit moderate toxic effects upon terrestrial vertebrates. None of the studies appears reliable enough to serve as a basis for a quantitative risk characterization. It should be remembered that heptachlor is used as a termiticide.
In studies on rats, heptachlor has been shown to be neurotoxic and immunotoxic at 0.03 mg/kg body weight per day. The Göksu Delta, Turkey, which is one of the most important breeding and wintering areas for birds in the world, is contaminated by organochlorine pesticides from soils from agricultural areas that have been transported to the delta by the Göksu River. From this region, levels of heptachlor/heptachlor epoxide have been detected in birds and bird eggs in the lower milligram per kilogram range. The effect of such concentrations of heptachlor on the bird populations can only be speculated at present due to lack of data; however, there is a potential risk for the terrestrial environment in this location.
Heptachlor (1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-tetrahydro-1.-4,7-methanoindene; CAS No. 76-44-8; C10H5Cl7) is a chlorinated dicyclopentadiene belonging to the so-called persistent organic pollutants, which were restricted by the Stockholm treaty in 2001 (http:// www.chem.unep.ch/pops/). In its pure form at room temperature, it appears as a white or light tan, crystalline solid with a mild camphor- or cedar-like odour, exhibiting a melting point of about 95–96 °C. However, the technical product is a soft wax and has a melting point of about 46–74 °C, depending on its composition.
The technical product usually contains about 72% heptachlor and about 28% related compounds, such as trans-chlordane (20–22%) and trans-nonachlor (4–8%) (NCI, 1977).
The environmentally relevant physicochemical properties of heptachlor and its stable and recalcitrant transformation product heptachlor epoxide (2,3-epoxy-1,4,5,6,7,8,8-heptachloro-2,3,3a,4,7,7a-hexahydro-4,7-endomethanoindane; CAS No. 1024-57-3; C10H5Cl7O) are summarized in Table 1. Additional physical and chemical properties are presented in the International Chemical Safety Card (ICSC 0743) reproduced in this document. Their structures are shown in Figure 1.
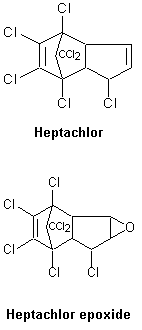
Fig. 1: Structure of heptachlor and heptachlor epoxide.
Table 1: Physicochemical properties of heptachlor and its epoxide.
|
Property |
Value |
Reference |
|
Heptachlor |
||
|
Relative molecular mass |
373.32 |
|
|
Vapour pressure (kPa) |
3.99 × 10−5 at 25 °C |
Verschueren (1996) |
|
Log .-octanol/water partition coefficient (log .ow) |
6.13 (measured) 6.1 (measured) |
MITI (1992) Simpson et al. (1995) |
|
Water solubility (g/l) |
0.000 10 at 15 °C 0.000 18 at 25 °C |
Biggar & Riggs (1974) |
|
Henry’s law constant (Pa·m3/mol) |
29.75 |
Thomas (1990) |
|
Henry’s law constant (dimensionless) |
1.2 × 10−2 at 25 °C |
Altschuh et al. (1999) |
|
Conversion factors at 20 °C |
1 mg/m3 = 0.0644 ppm 1 ppm = 15.5 mg/m3 |
|
|
Heptachlor epoxide |
||
|
Relative molecular mass |
389.32 |
|
|
Vapour pressure (kPa) |
3.46 × 10−7 at 20 °C |
Verschueren (1996) |
|
Log n-octanol/water partition coefficient (log .ow) |
5.1 (calculated) |
Meador et al. (1997) |
|
Water solubility (g/l) |
0.000 11 at 15 °C 0.000 20 at 25 °C |
Biggar & Riggs (1974) |
|
Henry’s law constant (dimensionless) |
8.6 × 10−4 at 25 °C |
Altschuh et al. (1999) |
A few selected examples of the analytical methods for the detection and quantification of heptachlor and its epoxide in different matrices are presented below. Further detailed information is available in IARC (2001).
Residues of heptachlor and its epoxide in solid materials such as animal tissues or sediments can be determined by GC using various detection methods, usually following appropriate cleanup and/or extraction procedures. For example, Lu & Wang (2002) employed Soxhlet extraction of ground rainbow trout tissue followed by column purification and GC/ECD. Diop et al. (1999) used solvent extraction followed by column purification and HRGC/ECD for plant material, whereas lyophilized sediment has been analysed by GC/ECD following Soxhlet extraction and column purification (González-Farias et al., 2002). Analysis of heptachlor in air usually involves sorption of the organochlorine insecticide onto a solid matrix followed by thermal or solvent desorption prior to HRGC/MS (e.g. Buser & Müller, 1993).
There are no known natural sources of heptachlor. However, its epoxide is not produced commercially but rather is formed by abiotic or biotic transformation of heptachlor in the environment.
The Stockholm treaty of 2001 restricted or banned the use of heptachlor, as this compound was recognized as a so-called persistent organic pollutant (http:// www.chem.unep.ch/pops/).
Heptachlor is a pesticide, and the technical grade contains structurally related compounds (see section 2). Heptachlor also occurs as a component of technical chlordane, which is also used as a pesticide (IARC, 2001). Chlordane consists of four major components — heptachlor (22%), 1,2-dichlorochlordane (13.2%), trans-chlordane (27.5%), and cis-chlordane (11.9%) — and seven minor components (Tsushimoto et al., 1983). Schmitt et al. (1999) give <10% heptachlor in chlordane.
The main use of heptachlor by farmers is to kill termites, ants, and soil insects in seed grains and on crops and by exterminators and home owners to kill termites in wooden structures. Agricultural consumption of heptachlor in the USA was about 550 tonnes per year in the early 1970s (Fendick et al., 1990). However, most applications of this insecticide were banned or at least severely restricted in the early 1980s in many countries. The only permitted commercial use of heptachlor products in the USA since 1987 is for fire ant control in power transformers and in underground cable television and telephone cable boxes (ATSDR, 1993). However, the pesticides heptachlor and chlordane were still produced for export. In 1997, Velsicol ceased production in the USA. United States customs data show that at least 2028 tonnes of technical chlordane (containing about 446 tonnes heptachlor) and 2584 tonnes of heptachlor were exported from the USA between 1991 and 1994 (therefore about 760 tonnes per year), and these figures are thought to greatly underestimate the actual figures (PANNA, 1997). In the Republic of Korea, about 560 tonnes of heptachlor were imported and employed in agriculture from 1961 until its use came to an end in 1980 (Kim & Smith, 2001). According to Bouwman (2003), South Africa currently still imports about 180–270 tonnes of heptachlor per year but will probably phase out its registration. However, use of chlordane (containing heptachlor) is still permitted for protecting buildings from termites. As in many other countries, stocks of obsolete pesticides have been accumulating for various reasons, and their disposal is a worldwide problem (FAO, 1999).
According to a survey on sources of persistent organic pollutants, performed by the United Nations Environment Programme, the global import figures of heptachlor in 1993 and 1994, based on the response of 61 countries, were 389 and 435 tonnes, respectively, with South America and Oceania being the largest consumers (UNEP, 1996). In those countries where use is restricted, but may continue, the applications are restricted to seed treatment, structural termite control, or wood treatment. In tropical and subtropical countries that have resumed use of heptachlor for seed treatment or preplanting agricultural use, heptachlor is restricted to crops that form edible portions above ground and, in particular, to crops with long growing seasons that undergo processing before consumption (FAO/UNEP, 1996; IARC, 2001).
Buildings constructed in temperate regions may contain added pesticides in materials. Therefore, their destruction or demolition may induce local environmental contamination (Offenberg et al., 2004).
Heptachlor has also been used as a component in plywood glues — e.g. two Finnish products contained 17–25% heptachlor and 6–9% chlordane (Mussalo-Rauhamaa et al., 1991).
Several recent monitoring studies have shown evidence of recent heptachlor usage (see section 6 and Appendix 5).
Heptachlor and heptachlor epoxide are subject to long-range transport and removal from the atmosphere by wet and dry deposition. Heptachlor epoxide was present in 20 of 21 snowpack samples collected in the Northwest Territories, Canada (1985–1986), at a mean concentration of 0.18 ng/l. No known local sources for heptachlor in Canadian Arctic snow were identified (Gregor & Gummer, 1989).
The environmental transport and distribution of heptachlor are reflected in the data shown in the tables in Appendix 5; heptachlor has been found in samples in various matrices all over the world where no local sources are to be found (e.g. in mammal tissue in the Arctic, etc.; Table A5-9).
The predominant target compartments for heptachlor in the environment are soil and sediment (about 43% and 55%) and, to a lesser extent, water (about 2%; Level III fugacity calculation, using EPI suite 3.10). Due to its experimentally determined water solubility of about 180 µg/l at 25 °C, only a limited proportion of this compound is expected to be removed from the atmosphere by wet deposition (dissolution in clouds, rainfall, etc.). According to Thomas (1990), the measured Henry’s law constant of 29.75 Pa·m3/mol indicates a moderate volatility of heptachlor from the aqueous phase.
In general, agricultural drains are a means of transporting the pesticides far from their original place of use (González-Farias et al., 2002).
Under environmental conditions, heptachlor will not be prone to wash out from soil, as its .oc value of about 16 000 (Johnson, 1991) indicates a low mobility in soil; together with its limited water solubility, this would restrict its potential for leaching to groundwater. Therefore, heptachlor was characterized as a non-leacher pesticide (Johnson, 1991). In principle, however, a long residence time may nevertheless result in an appreciable movement of heptachlor and its stable metabolite heptachlor epoxide, posing a potential risk for groundwater.
Heptachlor is moderately persistent in soil, where it is mainly transformed into its epoxide. It may undergo significant photolysis, oxidation, and volatilization (ATSDR, 1993).
Application-related release of heptachlor to soil surfaces can result in volatilization from the surface, but volatilization of heptachlor incorporated into soil will be slower (Fendick et al., 1990). In water and in moist soils, the reaction of heptachlor with water can give rise to the formation of 1-hydroxychlordene (Bowman et al., 1965) and, to a lesser extent, heptachlor epoxide (Miles et al., 1969). Chapman & Cole (1982) reported half-lives of heptachlor dissolved in phosphate-buffered sterile water–ethanol (99:1) of 0.77 (pH 4.5), 0.62 (pH 5), 0.62 (pH 6), 0.64 (pH 7), and 0.43 (pH 8) weeks, whereas Johnson (1991) reported a half-life of 180 days.
Assuming a hydroxyl radical concentration of 1.5 × 106 molecules per cubic centimetre and a 12-h day, half-lives of about 2.1 h for heptachlor and of about 24 h for the stable epoxide in the atmosphere (applying the USEPA program AOPWIN v. 1.9) can be calculated for indirect photochemical transformation by hydroxyl radicals in the atmosphere. Further, although heptachlor is apparently stable in the light (Fendick et al., 1990), Mansour & Parlar (1978) reported a phototransformation of heptachlor and its epoxide to photoisomerization products. The caged photoisomer derived from heptachlor by intramolecular cycloaddition upon UV irradiation (Buser & Müller, 1993) is known to be even more toxic and persistent than heptachlor and heptachlor epoxide (Zhu et al., 1995). Podowski et al. (1979) reported that heptachlor is converted into its photoisomer on exposure to low-intensity (longwave) UV light. This photolysis can also occur on plant leaves in the presence of sunlight or UV light. Photoheptachlor is about 20 times more toxic to rats than heptachlor.
In aerobic biodegradation tests performed according to OECD guideline 301C, heptachlor was not readily biodegradable (BOD 0% of theoretical oxygen demand, incubation for 28 days) (MITI, 1992). In an earlier report, Leigh (1969) demonstrated that there was no significant difference between abiotic and biological removal (as BOD5) of heptachlor. However, in contrast to the desired aerobic biodegradation (i.e. mineralization), which does not take place to any considerable extent, the simple and rapid biotransformation of heptachlor to transformation products such as the heptachlor epoxide was reported for typical soil bacteria (Miles et al., 1969; Bourquin et al., 1972; Beeman & Matsumura, 1981), soil fungi (Miles et al., 1969; Iyengar & Rao, 1973), higher fungi, such as the white rot fungus (Phanerochaete chrysosporium) (Kennedy et al., 1990; Arisoy, 1998), mixed microbial cultures from soil (Miles et al., 1971; Bourquin et al., 1972), plants (i.e. cabbage and wheat; Weisgerber et al., 1972), and animals such as water flea (Daphnia magna) (Feroz et al., 1990) and goldfish (Carassius auratus) (Feroz & Khan, 1979). Under anaerobic conditions, both heptachlor and its epoxide showed only limited conversion (Hill & McCarty, 1967). Johnson (1991) cited an aerobic biotransformation half-life for heptachlor of 2000 days and an anaerobic biotransformation half-life of 39 days. It is interesting to note that the presence of heptachlor epoxide in ambient air samples is due to the emission of this stable metabolite from soil where it is formed from heptachlor by microbial activity rather than to the photooxidation of heptachlor in the atmosphere (Bidleman et al., 1998a,b).
The experimentally determined bioconcentration factors (measured corresponding to OECD guideline 305C) in the range of 2000–17 000 (MITI, 1992) strongly indicate a high bioaccumulation potential. Bioconcentration may nevertheless be limited in the aquatic environment by the rapid transformation of heptachlor in water to hydroxychlordene and the sorption of heptachlor to sediment or particulate matter. However, as heptachlor epoxide is more stable in water, it is bioconcentrated extensively (Lu & Wang, 2002), with a measured concentration of about 5000 µg/kg (on a wet weight basis) after 20 days’ exposure of rainbow trout to heptachlor epoxide present in water at a concentration of about 1.5 µg/l.
Log .ow values for heptachlor and heptachlor epoxide of 6.1 and 5.1, respectively, suggest a high potential for bioaccumulation and biomagnification. An example illustrating the way in which heptachlor might enter the food-chain to end up as heptachlor epoxide in dairy milk for human consumption is provided by the report describing an application to control ants on pineapple plantations in Hawaii, USA (Baker et al., 1991). In this instance, chopped leaves (from the pineapple plants) were fed to dairy cattle, with subsequent excretion of metabolically formed epoxide into the milk.
Organochlorinated pesticides such as heptachlor, owing to their chemical stability, low aqueous solubility, and high lipophilicity, become concentrated along the food-chain, reaching higher concentrations at higher trophic levels. They reach the human body in the daily diet and are deposited and accumulated in adipose tissues.
Several recent monitoring studies have shown evidence of recent heptachlor usage, including, for example, elevated levels of heptachlor in air in Africa and the USA (Jantunen et al., 2000; Karlsson et al., 2000a), its presence in polluted rivers (e.g. Ayas et al., 1997), and some high residue levels found in vegetables (Bakore et al., 2002). Further, heptachlor and/or its epoxide are still present in sediment and microflora and are bioavailable to plants/crops (CU, 1999; Miglioranza et al., 2003) and thereafter biomagnify further up the food-chain. In an extensive data collection, Osibanjo (2003) showed the presence of heptachlor in the Nigerian environment (water, soil, sediment, etc.) and the food-chain (vegetables, meat, drinking-water).
Data from recent studies on levels of heptachlor and heptachlor epoxide in the environment are given in Appendix 5, Tables A5-1–A5-14.
Table A5-1 gives an overview of some recent studies on concentrations of heptachlor and heptachlor epoxide in ambient and indoor air. Ambient air concentrations are generally in the low picogram per cubic metre range. Higher values are to be found in agricultural areas where heptachlor was previously used as a pesticide and where the pesticide is still being released from the soil; for example, Meijer et al. (2003) detected heptachlor epoxide at 550 pg/m3 3 cm above the soil at a soya bean site and at 25 pg/m3 at 150 cm above the soil.
In houses where heptachlor was used against termites, indoor concentrations in the nanogram per cubic metre range have been measured; for example, Anderson & Hites (1989) reported heptachlor concentrations ranging from about 4 to 110 ng/m3 for basement levels and from about 3 to 66 ng/m3 for first-floor levels. More recently, Leone et al. (2000) found heptachlor concentrations in homes in the corn belt region in the USA ranging from not detectable to 79 ng/m3.
Heptachlor might enter the hydrosphere due to its use and application, mainly by drift or surface runoff. However, it can evaporate from the aqueous phase to some extent, and it can be converted in the presence of water (e.g. to yield hydroxychlordene); thus, the concentration of heptachlor in the hydrosphere may decrease.
Table A5-2 gives an overview of some recent studies on heptachlor and heptachlor epoxide concentrations in water samples.
Although the use of heptachlor has been banned in Europe for several years, there are reports that this pesticide is still present in rainwater. Heptachlor epoxide was detected in rainwater (wet and dry precipitation) in Poland (10 different sampling stations in the Gdansk region) at average concentrations ranging from 0.09 ng/l up to 0.58 ng/l (Grynkiewicz et al., 2001). A follow-up study (Polkowska et al., 2002) demonstrated the presence of heptachlor epoxide in the runoff water collected from roofs in an urban region (Gdansk, Poland) at concentrations up to 1.49 ng/l. In the Netherlands, while both heptachlor and its epoxide were detected in rainwater collected in typical agricultural locations (intense greenhouse horticulture and flower bulb production) at maximum concentrations of 3 ng/l (heptachlor) and 7 ng/l (heptachlor epoxide), only heptachlor was detected (maximum concentration 3 ng/l) in corresponding background locations (Hamers et al., 2003). Similar results were found by van Maanen et al. (2001).
In an intense monitoring programme in 21 counties in California, USA, 332 wells were sampled for heptachlor and 335 wells were sampled for heptachlor epoxide. Neither compound was detected (CEPA, 2000). However, in a state-wide survey (December 1985 to February 1986), heptachlor was detected in 1% of about 100 wells tested in Kansas, USA, at an average concentration of about 0.025 µg/l (Steichen et al., 1988). In New York City, USA, from 1989 to 1993, heptachlor was detected in influent and effluent of municipal treatment facilities at concentrations ranging from 0.021 to 0.35 µg/l and from 0.02 to 0.45 µg/l, respectively (Stubin et al., 1996).
The concentrations of heptachlor/heptachlor epoxide vary greatly from, for example, the lower picogram per litre range in surface water from Lake Malawi, southern Africa, to a mean value of 19 000 ng/l for heptachlor epoxide in water from around Göksu Delta, Turkey (Ayas et al., 1997) and a maximum value of 27 800 ng/l for heptachlor epoxide in water samples from canals and drains, El-Haram Giza, Arab Republic of Egypt (El-Kabbany et al., 2000). Göksu Delta is one of the most important breeding and wintering areas for birds in the world, so it is of concern that the environment is contaminated by organochlorine pesticides from soils from agricultural areas that have been transported by the Göksu River to the delta (González-Farias et al., 2002).
Jantunen & Bidleman (1998) reported the presence of the stable heptachlor epoxide in water samples from the western Arctic Ocean at an average concentration of 14.8 pg/l. However, the origin of the stable epoxide in polar water is not known. Water samples from the Bering and Chukchi seas contained heptachlor epoxide at concentrations up to 223 pg/l (Yao et al., 2002). Seawater surface samples from the Straits of Jahore (Singapore/Malaysia) apparently contained heptachlor at concentrations up to 233 ng/l (Basheer et al., 2002).
Heptachlor epoxide was detected in all samples of raw wastewater (82–1100 ng/l; median 200 ng/l) and secondary sedimentation effluent (6–120 ng/l; median 13 ng/l) at the municipal wastewater treatment plant in Thessaloniki, northern Greece (Katsoyiannis & Samara, 2004).
Several studies on sediment show concentrations of heptachlor/heptachlor epoxide in the lower microgram per kilogram (nanogram per gram) range (see Table A5-3). González-Farias et al. (2002) found heptachlor in single agricultural drains at high concentrations (49 and 65 ng/g dry weight for heptachlor and its epoxide, respectively), although officially the pesticide was not being used. In other studies, much higher concentrations were found; for example, means of 1377 and 244 ng/g for heptachlor and heptachlor epoxide, respectively, were measured in the Göksu Delta (Ayas et al., 1997). It should be noted that in all these studies, other organochlorine pesticides are also present at a 10-fold or higher concentration than heptachlor and heptachlor epoxide, and other pollutants are present as well. The study of Zhang et al. (2003) (Tables A5-3 and A5-4) shows how heptachlor accumulates in sediment, leaches out of sediment into the sediment porewater, and then transfers to surface water by processes such as diffusion.
Recent measurements of heptachlor and heptachlor epoxide in soil samples are given in Table A5-4. Kim & Smith (2001) reported soil levels of heptachlor up to 2.8 ng/g and of heptachlor epoxide up to 48 ng/g for top soil samples (mainly rice and vegetable farming) analysed in the Republic of Korea. Heptachlor was found not only in agricultural soil in Argentina but also in the surface of natural soil on highland hills where heptachlor was never directly applied but was probably blown by winds. The heptachlor was then metabolized in the soil to heptachlor epoxide. The majority of the heptachlor/heptachlor epoxide is found in the top layers of soil and does not penetrate to any great extent to lower layers (Miglioranza et al., 2003). In the study on soil from the Göksu Delta, heptachlor concentrations ranged from a maximum of 9616 ng/g (mean 4777 ng/g) in the agricultural area to a mean of 32 ng/g in the dune area (Ayas et al., 1997).
Tables A5-5 and A5-6 give an overview of concentrations of heptachlor and heptachlor epoxide in fish and other aquatic organisms, respectively.
Fish from several areas had heptachlor concentrations below 1 ng/g wet weight or in the low ng/g lipid weight range. Much higher values (in the 100–1000 ng/g range) were reported from Ganges Estuary, Bangladesh, from Bay of Bengal, and from Göksu Delta, Turkey (see Table A5-5 for details).
Concentrations of heptachlor/heptachlor epoxide in crabs, mussels, and whelks were below 1 ng/g lipid (from White Sea; Muir et al., 2003) or just above 1 ng/g lipid (Gulf of Gdansk: Falandysz et al., 2001) or 1 ng/g dry weight (marine [coastal] Pacific and Caribbean coasts: Castillo et al., 1997). Higher values were reported in estuarine Nicoya Gulf (Castillo et al., 1997), New Bedford Harbor, Massachusetts, USA (Hofelt & Shea, 1997), in the Karachi Coast area (Munshi et al., 2001), and in Australia (Connell et al., 2002). Concentrations in zooplankton were 0.02–0.55 ng/g lipid weight in the White Sea (Muir et al., 2003).
A study was conducted (see Table A5-7) during the period of October 1991 to October 1993 to determine organochlorine pesticide residues (including heptachlor) in waterbirds — Eurasian coot (Fulica atra), mallard (Anas platyrhynchos), and little egret (Egretta garzetta) — at Göksu Delta–Tasucu, which is an internationally important wetland for the waterbirds (Ayas et al., 1997). The results showed that environs and organisms were contaminated by 13 different organochlorine pesticides. Heptachlor and heptachlor epoxide generally accumulated in adipose tissue (e.g. mean heptachlor epoxide concentration of 2744 ng/g in mallards) and in the eggs of waterbirds (e.g. mean heptachlor concentration of 980 ng/g in eggs of the little egret). In comparison, heptachlor epoxide was detected in eggs of great black-backed gulls (Larus marinus) in Lake Ontario in North America at 90–140 ng/g wet weight (Weseloh et al., 2002), in an egg pool of black-footed albatross (Phoebastria nigripes) from Midway Atoll, Pacific, at 3.4 ng/g wet weight (Muir et al., 2002), in great blue heron (Ardea herodias) eggs, Upper Mississippi River, USA, at 20–100 ng/g wet weight, mean of 10 colonies (Custer et al., 1997a), and in eggs of Eurasian buzzard (Buteo buteo), La Segarra, north-eastern Spain, at 1–233 ng/g wet weight (Manosa et al., 2003).
Table A5-8 summarizes concentrations of heptachlor and heptachlor epoxide in amphibians and reptiles.
A survey performed in a Costa Rican tropical conservation area showed the presence of heptachlor in amphibians. The highest detected mean level of about 32 ng/g wet weight was observed in Mexican giant leopard frog (Rana forreri). Heptachlor was detected in turtles (Rhinoclemmys pulcherrima) at the highest detected mean level of about 17 ng/g wet weight (Klemens et al., 2003).
Levels of heptachlor in mammals are given in Table A5-9. Northwest Atlantic pilot whales (Globicephala melas) stranded in Massachusetts, USA, between 1990 and 1996 showed mean concentrations of heptachlor and heptachlor epoxide of 39 and 56 ng/g lipid weight, respectively (Weisbrod et al., 2000a). Higher concentrations (up to a mean of 1287 ng/g lipid weight in winter) were found in right whales (Eubalaena glacialis) that migrate in early spring from southern waters to the Bay of Fundy in Canada following warming water temperatures and plankton blooms and migrate back south in winter. Biopsies collected during winter had lower concentrations of lipid than biopsies collected during summer (Weisbrod et al., 2000b).
Several recent studies have measured the concentrations of heptachlor/heptachlor epoxide in seal blubber and polar bear fat in the Arctic (see Table A5-9). With a few exceptions, mean concentrations were up to 50 ng/g lipid weight in seal blubber and up to 475 ng/g lipid weight in polar bears.
Table A5-10 gives an overview of concentrations of heptachlor and heptachlor epoxide in food.
Fruits and vegetables sampled from different markets in Dakar, Senegal, showed the presence of heptachlor at concentrations ranging from 9 µg/kg (mandarins) up to 42 µg/kg (tomatoes) (Diop et al., 1999). Similarly, medicinal plants examined by these authors contained heptachlor, with concentrations ranging from 2.25 up to 17 µg/kg. However, heptachlor and its epoxide were not detected in different herbal formulations used as dietary supplements from South-East Asia (detection limit 0.03 ng/g; Hwang & Lee, 2000). Nakamura et al. (1994) failed to detect heptachlor or its epoxide in a range of agricultural products, such as rice, cabbage, and cucumber, in Japan (detection limit 1 ng/g).
Vegetables from Jaipur City, Rajasthan, India, analysed at the end of the season, contained much higher levels of heptachlor plus heptachlor epoxide: up to about 15.9 (tomatoes), 16 (cabbage), 9.3 (okra), 9.4 (spinach), and 1.5 (cauliflower) mg of heptachlor plus heptachlor epoxide per kilogram (Bakore et al., 2002). Dairy milk as well as buffalo milk sampled from 1993 to 1996 in Jaipur City were shown to contain heptachlor and its epoxide in milligram per litre amounts (John et al., 2001). Furthermore, concentrations of heptachlor in blood of breast cancer patients and controls from Jaipur City were also very high (Mathur et al., 2002). It is not known whether these extremely high values in vegetables, cow milk, and human samples are correct (which would indicate extreme pollution with organic pesticides) or whether this is a measurement or calculation error.
Heptachlor epoxide was reported in dairy milk for human consumption as a result of the feeding of chopped leaves from the pineapple plants to dairy cattle, with subsequent excretion of metabolically formed epoxide into the milk (Baker et al., 1991). The concentrations of heptachlor epoxide were 0.12 µg/g fat basis (October 1980 – April 1981); 1.20–5.00 µg/g (April 1981 – April 1982), and <0.30 µg/g (April 1982 – December 1982) (Le Marchand et al., 1986).
Heptachlor and/or heptachlor epoxide were not detected in samples of dairy milk retailed in China (detection limit 0.002 µg/g; Zhong et al., 2003).
Heptachlor has been reported in infant food from Nigeria and Italy at concentrations of 0.09 (ND–0.87) ng/g and 9.80 (ND–72) ng/g, respectively (Osibanjo, 2003).
Approximately 200 plywood workers in Finland were reported to be exposed to heptachlor, either through working with sizings that contain heptachlor or through stacking plywood for heat pressure treatment. Residue levels of heptachlor, heptachlor epoxide, and other chlordane compounds were determined in sera from 74 Finnish plywood workers and 52 controls. Concentrations of heptachlor epoxide in plywood workers varied from below the detection limit of 0.1 ng/g to 19.2 ng/g serum (1 ng/g = 0.98 µg/l); the mean and standard deviation were 3.2 and 3.9 ng/g, respectively. Heptachlor epoxide values in controls varied from below the detection limit to 1.2 ng/g serum. The exposure time (e.g. the number of years spent working with plywood sizings that contained heptachlor) correlated with the residue levels of heptachlor epoxide that were measured in serum samples obtained from employees at two companies (. = 0.03) (Mussalo-Rauhamaa et al., 1991).
Consumption of foods containing pesticides such as heptachlor leads to an accumulation of these compounds in human tissues. Concentrations of heptachlor and heptachlor epoxide in various human tissues and fluids from recent studies are given in Appendix 5: Table A5-11, serum and blood samples; Table A5-12, human breast milk; Table A5-13, adipose tissue; and Table A5-14, breast adipose tissue.
The most significant source of exposure of infants to heptachlor and its metabolites appears to be breast milk, in which the concentrations can be much higher than those found in dairy milk. A large international survey performed in the 1970s found that the mean concentrations of heptachlor and heptachlor epoxide in human breast milk ranged from 2 to 720 ng/g of fat (IPCS, 1984). Concentrations of heptachlor epoxide in breast milk from women in a number of countries are given in Table A5-12. Although heptachlor has not been found in all samples, it has been found in at least some samples in most surveys, showing that this organochlorine compound is a ubiquitous contaminant. Mean concentrations from recent reports are much below 100 ng/g on a milk fat basis; however, studies in some countries give noticeably higher values — e.g. Jordan (mean of 600 ng of heptachlor epoxide per gram of milk fat; Alawi & Khalil 2002) and Thailand (360 ng of heptachlor epoxide per gram of milk fat; Stuetz et al., 2001).
In a breast milk study in Victoria, Australia, a correlation was found between the levels of heptachlor epoxide in breast milk and the use of heptachlor as a termiticide (Sim et al., 1998).
Heptachlor that had been formerly sprayed on pineapple plants to control ants responsible for the spread of mealy bugs remained on the leaves that were chopped and fed to dairy cows on Oahu, Hawaii, USA. Heptachlor-contaminated "green chop" led to contamination of the commercial milk supply on Oahu for as long as 15 months during 1981–1982, at levels as high as 1.2 µg/g fat basis (Baker et al., 1991; Allen et al., 1997). Lactating women who consumed dairy products and beef from local sources had average levels of 123 ng of heptachlor epoxide per gram of fat, with a maximum concentration of >250 ng/g of fat (Baker et al., 1991). Heptachlor is usually only one of the organochlorine pesticides present in breast milk.
A compilation of data from the 1960s and 1970s indicated that the mean concentration of heptachlor epoxide in adipose tissue from the general population ranged from 10 to 460 ng/g of fat (IARC, 1991; Kutz et al., 1991). Data from more recent years still fall in this range. However, one report from Jordan gave values above 1000 ng/g of fat (Alawi et al., 1999; see Table A5-13).
The main exposure routes for heptachlor are probably via application-related inhalation or skin penetration, from extended exposure to dusts containing heptachlor in, for example, homes treated with this compound to control termites, and indirectly by uptake from food contaminated with heptachlor from crops or from other foods via the food-chain.
A study of the exposure of applicators and residents to heptachlor when used for subterranean termite control in homes indicated that applicator exposure by the dermal route to hands, forearms, and lower legs was 71.3, 29.3, and 17.1 µg/region per hour, respectively, and by the respiratory route in the breathing zone, 33.4 (range 2–176) µg/m3. Exposure of residents to heptachlor in ambient air during termiticide treatments was a maximum of 5 µg/m3, which decreased to 2.86 µg/m3 after 24 h (Kamble et al., 1992).
The most likely route of exposure of the general population to heptachlor is via food-related uptake. This is due to the fact that heptachlor will bioaccumulate in lipid phases. In particular, foods such as milk, fish, and meats, which have a higher fat content, and vegetables directly contaminated with the pesticide are likely to contain higher concentrations of heptachlor.
The estimated dietary intake of heptachlor epoxide in the 1960s and 1970s in the USA was about 0.3–2 µg/day (Duggan & Corneliussen, 1972; Peirano, 1980; IPCS, 1984). The average daily intake of heptachlor found in the United States Food and Drug Administration Total Diet Study (1986–1998) analysis was <0.0001 µg/kg body weight per day for all age/sex groups (i.e. 6–11 months; 2 years; 14–16 years, females, and 14–16 years, males; 25–30 years, females, and 25–30 years, males; 60–65 years, females, and 60–65 years, males) (Gunderson, 1995). Based on a total diet study conducted in the period April 1982 – April 1984, the estimated daily intakes of heptachlor and heptachlor epoxide for men aged 25–30 were 0.007 µg and 0.184 µg, respectively (Gunderson, 1988).
The intake of heptachlor epoxide in a Basque population in Spain in 1990–1991 was estimated to be <0.1 µg/day on average (Urieta et al., 1996).
For several countries, Kannan et al. (1997) summarized the average daily uptake values (in micrograms per person) for heptachlor and its epoxide from the available data as follows: 0.07 (India, 1989, heptachlor only), 0.08 (Thailand, 1980), 0.06 (Japan, 1992–1993), 1.1 (Australia, 1990–1992), 8.4 (Italy, 1971–1972), 0.18 (Italy, 1978–1984), 0.49 (Finland, 1983), 0.1 (USA, 1987), and 0.04 (USA, 1990).
Estimated heptachlor and heptachlor epoxide exposure from food items in Poland in 1970–1996 was calculated by multiplying annualized mean consumption rates by residue concentrations in the food. The daily dietary intakes of heptachlor and heptachlor epoxide were from 0.51 to 0.58 µg per person (Falandysz, 2003) (about 0.01 µg/kg body weight, assuming a mean weight of 64 kg). In this study, the main sources of heptachlor and heptachlor epoxide were thought to be meat, meat products, and animal fats.
However, if fish from contaminated rivers (e.g. concentrations in fish in the 0.1–1 mg/kg range reported from Ganges Estuary, Bangladesh, Bay of Bengal, and Göksu Delta, Turkey) were ingested, a much higher daily intake would be likely. This would also be the case for vegetables ingested from fields contaminated with heptachlor or contaminated milk (e.g. in the microgram per kilogram range in Dakar, Senegal; in the milligram per kilogram range in Jaipur City, Rajasthan, India).
The daily intake of heptachlor by breast-fed babies in Jordan calculated from the data of Alawi & Khalil (2002), assuming a daily milk consumption of 150 g/kg body weight and an average milk fat content of 3.1%, was a mean of 0.67 µg/kg body weight. The corresponding daily intake of heptachlor epoxide by babies in 2000 was a mean of 1.5 µg/kg body weight (Alawi & Khalil, 2002). In contrast, Rogan & Ragan (1994) calculated, on the basis of the 90th percentile of 100 ng/g fat (Table A5-12; Rogan et al., 1991), an average daily heptachlor epoxide dose of 0.003 µg/kg body weight.
Heptachlor is readily absorbed via all routes of exposure and is readily metabolized. The results in excretion studies on orally administered [14C]heptachlor in rats show that about 72% of the radioactivity was eliminated by the faeces in the form of metabolites and 26.2% as the parent compound by day 10. The major faecal metabolites include heptachlor epoxide, 1-hydroxychlordene, and 1-hydroxy-2,3-epoxychlordene (Tashiro & Matsumura, 1978). The metabolite 1-exo-hydroxychlordene epoxide was detected only in the urine of treated rats (Klein et al., 1968). In liver microsomes incubated with heptachlor, 85.8% was metabolized to heptachlor epoxide in rats, but only 20.4% in humans. Other metabolites identified in the human liver microsome system were 1-hydroxy-2,3-epoxychlordene (5%), 1-hydroxychlordene (4.8%), and 1,2-dihydroxydihydrochlordene (0.1%) (Tashiro & Matsumura, 1978). A scheme for the metabolism in rats is given in Figure 2.
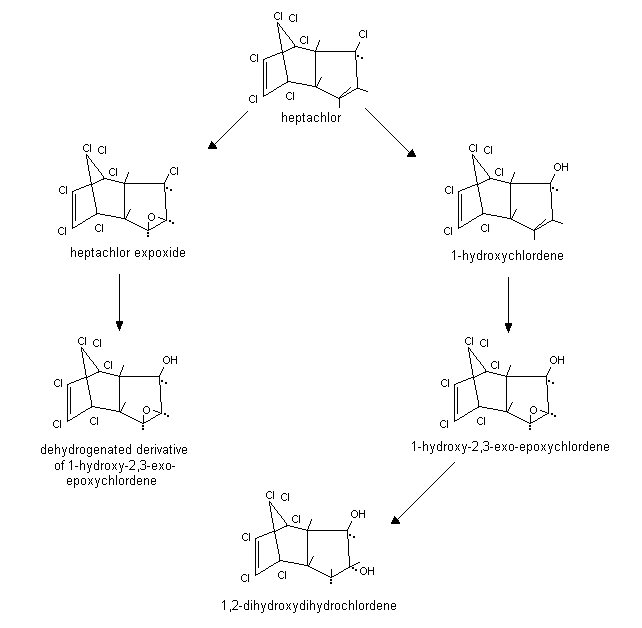
Fig. 2: Metabolic scheme for heptachlor in rats [1-hydroxychlordene
is also designated as 1-exohydroxychlordene; 1-hydroxy-2,3-exo-epoxychlordene
is also designated as 1-exohydroxy-2,3-exo-epoxychlordene]
(from Tashiro & Matsumura, 1978).
Heptachlor epoxide is metabolized slowly and is the most persistent metabolite; it is stored mainly in adipose tissue, but also in liver, kidney, and muscle (Radomski & Davidow, 1953). The concentrations of heptachlor epoxide in serum, adipose tissue, bile, and gallstone of a pesticide worker were 3, 400, 1, and 1 ng/g, respectively (Paschal et al., 1974). The mean ratio between concentrations of heptachlor epoxide in fat and blood was 880 for 10 workers at a technical heptachlor plant (Nisbet, 1986). The bioaccumulation factors (mg/g in fat / mg/g in diet) in the tissues of rats were 1.0 for males and 5.0 for females (Adams et al., 1974). Studies in male dogs indicated an average bioaccumulation factor for heptachlor epoxide of 22 (Radomski & Davidow, 1953). After prolonged exposure to heptachlor epoxide, cattle had bioaccumulation factors of 5 or more in males and 10–25 in females (Nisbet, 1986).
Heptachlor epoxide accumulated rapidly in the fat of rats fed heptachlor at a level of 30 mg/kg in the diet for 12 weeks. The maximum concentration in the fat was reached in 2–4 weeks. A period of 12 weeks was required for complete disappearance from the fat after discontinuing heptachlor feeding. The concentration of heptachlor epoxide in the fat bears a relationship to the concentration of heptachlor in the diet (Radomski & Davidow, 1953). In female rats, the amount of heptachlor and heptachlor epoxide found in milk, blood, fat, and tissues was proportional to the dose of heptachlor administered (Smialowicz et al., 2001). Heptachlor was not detected in rat pup tissues, but heptachlor epoxide was detected in fat, brain, liver, and plasma at concentrations proportional to the dose administered (Moser et al., 2001).
The presence of heptachlor epoxide in the adipose tissue of stillborn infants (Wassermann et al., 1974) and in the cord blood of newborns (D’Ercole et al., 1976) demonstrates placental transfer of heptachlor and/or heptachlor epoxide.
Acute toxicity studies of heptachlor and heptachlor epoxide in several animal species are reviewed in IPCS (1984) and FAO/WHO (1967) and summarized in JMPR (1992). Acute oral LD50s for heptachlor for the rat and mouse are 40–162 and 68–90 mg/kg body weight, respectively. Dermal LD50s for heptachlor for the rat are 119–250 mg/kg body weight (IPCS, 1984). The symptoms associated with acute heptachlor toxicity include hyperexcitability, tremors, convulsions, and paralysis.
The acute toxicity of heptachlor epoxide is greater than that of heptachlor; for example, the intravenous lethal doses for heptachlor and heptachlor epoxide are 40 and 10 mg/kg body weight, respectively (IPCS, 1984). However, four other heptachlor metabolites (chlordane, 3-chlordene, 1-hydroxychlordene, and chlordane epoxide) were found to have a much lower toxicity, with acute oral LD50 values of greater than 4600 mg/kg body weight (Mastri et al., 1969
Groups of 10 Charles River CD-1 mice per sex per dose were fed a mixture of heptachlor/heptachlor epoxide (1:3) at dietary concentrations of 1, 5, 10, 25, and 50 mg/kg for 30 days (Wazeter et al., 1971a). Nine males and eight females of the highest dose group and one female in the 25 mg/kg group died. No marked differences were seen between the treated animals and the controls with respect to body weights and food consumption. Liver enlargement in the 10, 25, and 50 mg/kg groups in both sexes was associated with accentuated lobulation. Histopathological findings showed enlargement of centrilobular and midzonal hepatocytes in these groups and in the 5 mg/kg female dose group. The NOAEL was given as 1 mg of heptachlor per kilogram diet [NOAEL = 0.13 mg/kg body weight per day].
In a study to investigate the effect of heptachlor on the development of the reproductive system (see section 8.5), pregnant Sprague-Dawley rats were administered heptachlor by oral gavage at doses of 0, 0.5, and 5.0 mg/kg body weight per day from GD 8 through PND 21, which was the day of weaning (. = 7–8 per group). Two of the dams in the 5.0 mg/kg body weight per day group died. Pups in the highest dose group weighed significantly less than those in the other two groups on PND 0. All but one litter of the 5.0 mg/kg body weight per day group died within the first 4 postnatal days (Lawson & Luderer, 2004).
Groups of eight female Fischer 344 rats were dosed daily (0, 2, or 7 mg/kg body weight per day) by oral gavage for 14 consecutive days. Hepatocytomegaly was seen at 2 mg/kg body weight per day in two out of eight rats and all seven surviving rats in the 7 mg/kg body weight per day group. These doses also increased liver weight and decreased thymus weight (Berman et al., 1995; see also studies on reproductive effects [Narotsky & Kavlock, 1995] in section 8.5 and on neurobehavioural effects [Moser et al., 1995] in section 8.6) [LOAEL = 2 mg/kg body weight per day].
Two dogs given heptachlor dissolved in corn oil orally at 5 mg/kg body weight per day died within 21 days, whereas three of four dogs given heptachlor dissolved in corn oil orally at 1 mg/kg body weight per day died within 424 days (Lehman, 1952).
Three dogs given heptachlor epoxide orally in dosages of 2, 4, and 8 mg/kg body weight per day for 5 days per week died after 22, 10, and 3 weeks, respectively. Daily oral doses of 0.25 and 0.5 mg/kg body weight did not cause illness during 52 weeks, but 0.25 mg/kg body weight, estimated to be equivalent to 6 mg/kg in the diet, was reported as the minimal dose producing a pathological effect (Velsicol Corporation, 1959).
Technical-grade heptachlor (72 ± 3% heptachlor, 18% trans-chlordane, 2% cis-chlordane, 2% nonachlor, 1% chlordene, 0.2% hexachlorobutadiene, and 10–15 other compounds) was tested for carcinogenicity using both sexes of Osborne-Mendel rats and B6C3F1 mice (NCI, 1977). Heptachlor was administered in the diet for 80 weeks using two separate dose levels for each sex and species. Owing to the observable toxic effects, the dose levels were changed during the course of the study. The time-weighted average doses3 were 39 and 78 mg/kg for male rats and 26 and 51 mg/kg for female rats. The average body weights of rats treated with high doses were consistently lower than those of untreated controls, while body weights of low-dose rats were unaffected. Mortality was dose related for female rats but not for male rats. No hepatic tumours were observed in the rats.
For mice, the time-weighted average doses were 6 and 14 mg/kg for the males and 9 and 18 mg/kg for the females. Mortality was dose related for female mice but not for male mice. A review of the liver samples from this study by the panel of the United States National Academy of Sciences (NAS, 1977) indicated a significant increase in the combined incidence of hepatocellular carcinoma and "nodular changes" in males and females receiving the higher concentration (see Table 2).
Table 2: Tumour incidence in B6C3F1 mice treated with technical-grade heptachlor.a
|
Treatment |
Males |
Females |
||
|
Hepatocellular carcinomas |
Hepatocellular carcinomas and nodules |
Hepatocellular carcinomas |
Hepatocellular carcinomas and nodules |
|
|
Controls |
2/19 |
5/19 |
0/10 |
1/10 |
|
Heptachlor (low dose) |
3/45 |
14/45 |
0/44 |
3/44 |
|
Heptachlor (high dose) |
2/45 |
24/45 (. = 0.042)b |
2/42 |
21/42 (. = 0.022)a |
|
a |
From NAS (1977); IARC (2001). |
|
b |
Armitage’s test for linear trend. |
An oral carcinogenicity study on heptachlor and its epoxide was carried out by the United States Food and Drug Administration in 1965 and was later published in a summarized form (Epstein, 1976). Three groups of 100 male and 100 female C3H mice were fed diets containing heptachlor at 0 or 10 mg/kg or heptachlor epoxide at 10 mg/kg for 24 months. A review of the histopathology of liver samples by the United States National Academy of Sciences (NAS, 1977) indicated a significant increase in the incidence of hepatocellular carcinomas in females but not in males given heptachlor and in both males and females given heptachlor epoxide. There was also a significant increase in the combined incidence of carcinomas and nodules in both males and females given heptachlor or heptachlor epoxide (IARC, 2001; see Table 3).
Table 3: Tumour incidence in C3H mice treated with heptachlor or heptachlor epoxide.a
|
Treatment |
Males |
Females |
||
|
Hepatocellular carcinomas |
Hepatocellular carcinomas and nodules |
Hepatocellular carcinomas |
Hepatocellular carcinomas and nodules |
|
|
Control |
29/77 |
48/77 |
5/53 |
11/53 |
|
Heptachlor (10 mg/kg of diet) |
35/85 |
72/85 (. = 0.001) |
18/80 (. = 0.04) |
61/80 (. < 0.001) |
|
Heptachlor epoxide (10 mg/kg of diet) |
42/78 (. = 0.031) |
71/78 (. < 0.001) |
34/83 (. < 0.001) |
75/83 (. < 0.001) |
a From Epstein (1976); NAS (1977); IARC (2001).
In a further study on heptachlor and its epoxide conducted by the International Research and Development Corporation in 1973 and later published in a summarized form (Epstein, 1976), groups of 100 male and 100 female CD-1 mice were fed diets containing a mixture of 75% heptachlor epoxide and 25% heptachlor at a concentration of 0, 1, 5, or 10 mg/kg for 18 months. After exclusion of 10 animals from each group that were killed for interim study at 6 months, the mortality rate at 18 months was 34–49%, with the exception of the group receiving the highest dose, for which the rate was about 70%. A review of the histopathology of liver samples from this study by the United States National Academy of Sciences (NAS, 1977) indicated a significant increase in the combined incidence of hepatocellular carcinomas and nodules in the groups at the highest concentration (Table 4).
Table 4: Tumour incidence in CD-1 mice treated with a mixture of heptachlor and heptachlor epoxide (25%:75%).a
|
Concentration (mg/kg of diet) |
Males |
Females |
||
|
Hepatocellular carcinomas |
Hepatocellular carcinomas and nodules |
Hepatocellular carcinomas |
Hepatocellular carcinomas and nodules |
|
|
0 (controls) |
1/59 |
2/59 |
1/74 |
1/74 |
|
1 |
1/58 |
1/58 |
0/71 |
0/71 |
|
5 |
2/66 |
4/66 |
1/65 |
3/65 |
|
10 |
1/73 |
27/73 (. < 0.001) |
4/52 |
16/52 (. < 0.001) |
a From NAS (1977); IARC (2001).
Groups of male B6C3F1 mice, 8 weeks of age, were given drinking-water containing the tumour initiator .-nitrosodiethylamine at 0 or 20 mg/l for 14 weeks. After 4 weeks with no treatment, mice received diets containing technical heptachlor at 0, 5, or 10 mg/kg for 25 weeks. All surviving animals were killed at 43 weeks; five mice from each group were sacrificed after 8 and 16 weeks’ administration of heptachlor. The effect of .-nitrosodiethylamine was assessed by the presence of altered foci displaying abnormalities in glucose-6-phosphatase. The incidence of hepatocellular adenomas and carcinomas was significantly increased with heptachlor compared with .-nitrosodiethylamine alone (Williams & Numoto, 1984; see Table 5). Heptachlor showed tumour promoter activity.
Table 5: Preneoplastic and neoplastic liver lesions in B6C3F1 mice treated with heptachlor in the diet after initiation with .-nitrosodiethylamine (NDEA).
|
Exposure |
Foci, glucose-6-phosphatase-deficient |
Liver cell neoplasms |
|||
|
Number/cm2 |
Area (mm2/cm2) |
Incidence |
Number of adenomas |
Number of carcinomas |
|
|
Control |
0.04 ± 0.11 |
21 ± 0 |
3/28 |
2 |
1 |
|
NDEA |
1.27 ± 1.07 |
10.2 ± 12.5 |
8/20 |
11 |
2 |
|
NDEA + 5 mg heptachlor/kg |
1.81 ± 1.14 |
27.3 ± 40.7 |
16/21b |
24 |
9 |
|
NDEA + 10 mg heptachlor/kg |
2.29 ± 1.70 |
31.0 ± 38.7 |
20/26b |
34 |
9 |
|
a |
From Williams & Numoto (1984). |
|
b |
Significantly different from group given NDEA alone at . < 0.05. |
Groups of beagle dogs (four per sex per dose) were fed heptachlor epoxide at concentrations of 0, 1, 3, 5, 7, and 10 mg/kg in the diet for 2 years (Wazeter et al., 1971b). After this time, two dogs per sex per dose were sacrificed and necropsied, while the other two dogs per sex per dose were maintained on the control diet for an additional 6 months. In addition, the test animals were also mated during the study and employed as the P1 parental animals for a two-generation reproduction and teratology study. Neither deaths nor compound-related behavioural changes were seen during the study. No marked differences were seen between the treated animals and the controls with respect to body weights and food consumption. There were increases in alkaline phosphatase activities in males and females at the 3 mg/kg dose and above; these increases, in some dogs, were more marked towards the end of the treatment period and tended to persist through the recovery period. The serum albumin and total protein levels were slightly decreased in 10 mg/kg dose male and female dogs during the treatment, extending into the recovery period. After 1 year of treatment, the animals in the 7 mg/kg group also showed an increase in the alanine aminotransferase level, which lasted into the recovery period.
There was an increase in liver weights in the 10 mg/kg male and female dogs relative to those of the controls, and this increase persisted with a slight attenuation during the recovery period.
Histopathological examination of the dogs (two dogs per sex per dose) sacrificed at the end of the treatment period showed an increase in the incidence of liver changes (e.g. enlargement and vacuolation of groups of centrilobular hepatocytes) in animals at 3 mg/kg or above. These changes were also noted in the dogs at 3 mg/kg and above after 6 months of recovery. No compound-related histopathological changes were seen in the 1 mg/kg dogs. Based upon changes in biochemical parameters and the histological changes in the liver, the NOAEL was given as 1 mg/kg diet [NOAEL = 0.025 mg/kg body weight per day] (Wazeter et al., 1971b).
The genotoxicity of heptachlor and heptachlor epoxide has been reviewed by IARC (1991, 2001), where further details of the studies are to be found (see also Appendix 6).
Heptachlor did not induce DNA damage or point mutations in bacteria (Simmon et al., 1977; Griffin & Hill, 1978; Probst et al., 1981; Gentile et al., 1982; Moriya et al., 1983; Rashid & Mumma, 1986; Zeiger et al., 1987; Mersch-Sundermann et al., 1988; Matsui et al., 1989), gene conversion in Saccharomyces cerevisiae (Gentile et al., 1982), or sex-linked recessive lethal mutations in Drosophila melanogaster (Benes & Šram, 1969). It did not induce unscheduled DNA synthesis in cultured rat, mouse, or hamster hepatocytes in the absence of metabolic activation (Maslansky & Williams, 1981; Probst et al., 1981; Williams et al., 1989); however, there was a statistically significant increase in unscheduled DNA synthesis in human fibroblasts in the presence of metabolic activation (Ahmed et al., 1977). Heptachlor induced gene mutations at the Tk (McGregor et al., 1988) but not at the Hprt locus in rodent cells (Telang et al., 1982). It inhibited gap junctional intercellular communication in cultured rodent and human cells (Telang et al., 1982; Ruch et al., 1990; Nomata et al., 1996).
IARC (2001) noted that it is aware of unpublished studies by the United States National Toxicology Program on the effect of heptachlor on sister chromatid exchange (weak positive with rat liver S9) and chromosomal aberrations (negative).
Heptachlor did not induce dominant lethal mutations in mice in vivo (Epstein et al., 1972; Arnold et al., 1977). It showed negative results for lacI mutations in liver DNA in C57BL/6 (Big Blue®) transgenic mice (Gunz et al., 1993).
Heptachlor epoxide did not induce forward mutation, mitotic crossing-over, or aneuploidy in Aspergillus (Crebelli et al., 1986) or reverse mutation in Salmonella typhimurium (Marshall et al., 1976). However, there was a statistically significant increase in unscheduled DNA synthesis in human fibroblasts in vitro in the presence of metabolic activation (Ahmed et al., 1977), and heptachlor epoxide inhibited gap junctional intercellular communication in rat liver (Matesic et al., 1994) and human breast epithelial cells in vitro (Nomata et al., 1996), without metabolic activation.
Male Sprague-Dawley rats (five per group) were injected subcutaneously every other day with heptachlor in corn oil at 5, 10, 15, 20, or 25 mg/kg body weight per day for 2 weeks. The rats did not show any adverse clinical signs throughout the treatment period. There was no clear dose-related effect of heptachlor on body weight. Plasma testosterone levels were suppressed (. < 0.05) and the plasma luteinizing hormone level was elevated (. < 0.01) at all doses, but the changes were not dose related. Cortisol levels were significantly elevated (. < 0.02) compared with control rats. The testes in the group treated with 25 mg/kg body weight per day showed some pathological changes (Wango et al., 1997).
Adult female Sprague-Dawley rats (10 in each dose group, 20 controls) were injected subcutaneously with corn oil or heptachlor solution at 5 or 20 mg/kg body weight every other day for up to 18 days (Oduma et al., 1995a). Animals in the lower dose group showed some disruptions in cyclicity, but the effects were more pronounced with 20 mg/kg body weight. Disruptions became more marked with continued treatment. Heptachlor affected body weight gain in a dose-related manner. In the second part of the study, female rats (10 per dose group) were injected with saline, corn oil, or heptachlor at 5 or 20 mg/kg body weight as above. Heptachlor caused a delay in mating behaviour in a dose-related manner. In summary, effects including disruptions in cyclicity and delayed mating behaviour were seen at all tested doses >5 mg/kg body weight.
Adult female Sprague-Dawley rats (10 per group) were injected subcutaneously with corn oil or heptachlor solution at 5, 20, 25, or 30 mg/kg body weight every other day for up to 18 days (total of nine injections). The stage of the estrous cycle was determined by vaginal smears a day following the last injection, and the rats were sacrificed. Blood samples were taken by cardiac puncture and assayed for progesterone and estrogen. A suppression of blood progesterone and estradiol levels was observed, depending on the dose and stage of the estrous cycle. Ovarian cells from rats treated with low doses of heptachlor (5 mg/kg body weight) showed an increased production of progesterone, whereas high doses (>20 mg/kg body weight) suppressed production (Oduma et al., 1995b). In summary, effects were seen at all tested doses >5 mg/kg body weight.
Prenatal toxicity studies were also carried out in albino CFT Wistar rats dosed by gavage at 0, 45.3, or 90.5 mg/kg body weight for 70 days prior to mating for males and at 25 or 50 mg/kg body weight for 14 days prior to mating for females (Amita Rani et al., 1992, 1993; Amita-Rani & Krishnakumari, 1995). However, the doses chosen resulted in high morbidity, and the studies are not described further here.
Groups of 6- to 9-month-old beagle dogs (four per sex per dose) were fed heptachlor epoxide at dietary concentrations of 0, 1, 3, 5, 7, and 10 mg/kg for 2 years (Wazeter et al., 1971c; JMPR, 1992; see also Wazeter et al., 1971b, in section 8.3.3). When the female dogs reached the age of 14 months, they were mated twice with male dogs from the same dose group. The females were allowed to deliver and to nurse their pups. For the F2 generation, four female and two male pups of the F1 generation were selected from each dose level to be the parental animals (P2) of the F2 generation. At an approximate age of 14 months, the animals were mated, and the pregnant females were allowed to deliver and to nurse their pups to 6 weeks of age, when the females and their pups were sacrificed. Neither deaths nor compound-related behavioural changes were seen in treated P1 or P2 animals or their offspring during the study. No marked differences were seen between the treated animals and the controls with respect to body weights and food consumption. Owing to the small number of animals and the limited data, it could not be determined whether heptachlor epoxide produced any reproductive effects.
In the beagle dog study described in the previous section (Wazeter et al., 1971c), there was a significant increase in the mortality rate of F1 pups in the 10 mg/kg group. Only one male pup survived to scheduled sacrifice, and no female was available to serve as a P2 parental animal. There were slight increases in death rates of F2 pups at 3 and 7 mg/kg relative to the controls. The 5 mg/kg group had no pups. The limited necropsy data showed that in F2 pups, 1/4 females at 7 mg/kg and 3/10 males and 3/7 females at 10 mg/kg had pale or greasy liver. This observation was compound related. Based on mortality rate, the NOAEL was given as 1 mg/kg diet (Wazeter et al., 1971c; JMPR, 1992).
Three reproduction studies in rats were evaluated by JMPR in 1966 and 1970 (FAO/WHO, 1967, 1971). In a three-generation reproduction study, a group of 80 rats was given heptachlor at 6 mg/kg in the diet daily for 3 months prior to mating. The only effect on reproduction was a decrease in the litter size (Mestitzova, 1966, 1967). In two other reproduction studies in rats, dietary levels of heptachlor ranging from 0.3 to 10 mg/kg were tested. No adverse effects on fertility or reproduction were seen at 10 mg/kg diet. A slight increase in postnatal mortality of pups was seen at 10 mg/kg (FAO/WHO, 1967).
Pregnant female Sprague-Dawley rats were dosed by gavage with heptachlor (0, 0.03, 0.3, or 3 mg/kg body weight per day) from GD 12 to PND 7, followed by direct dosing of the pups with heptachlor through PND 42. The doses were set so that the low dose, 0.03 mg/kg body weight per day, produced heptachlor and heptachlor epoxide levels in rat dam milk that matched the 95th percentile of human milk values on Oahu, Hawaii, USA, in 1981 (Siegel, 1988). Evaluation of the effects on the reproductive system included the following: monitoring the development of the reproductive system in males and females (i.e. anogenital distance at birth, age at vaginal opening, age at preputial separation); vaginal cytology monitoring over a 2-week period to assess cyclicity; two mating trials with untreated mates; and a necropsy, including organ weights and histology, measures of epididymal sperm motility and count, and testicular spermatid counts. Necropsies were also performed at the end of dosing (PND 46), and again in the adults, to assess organ weight and histology (liver, kidneys, adrenals, thymus, spleen, ovaries, uterus/vagina, testes, epididymides, seminal vesicles/coagulating glands, and ventral and dorsolateral prostate (Smialowicz et al., 2001).
There were no effects on the number or survival of pups born to heptachlor-exposed dams or to pups exposed postnatally. There were no effects on the number of treated dams delivering litters or on litter size, nor were there any effects on any of the reproductive end-points examined in the F0 or F1 rats. There was no detectable histopathology in any tissue examined. There were no changes in the fertility of adult males or females when mated to untreated partners. Body weights and somatic and reproductive organ weights of males and females exposed to heptachlor were unchanged at terminal necropsy (Smialowicz et al., 2001) [NOAEL = 3 mg/kg body weight per day, the highest dose tested].
Pregnant Fischer-344 rats were treated by gavage with vehicle or heptachlor (0, 4.5, or 6 mg/kg body weight per day) on GD 6–19 (Narotsky & Kavlock, 1995). The dams were allowed to deliver, and their litters were examined on PND 1, 3, 6, and 21. Litter weights were determined on PND 1, 6, and 21. Implants were also counted to determine prenatal loss. Heptachlor caused reduced maternal weight gains (reduced extrauterine weight gains) at both dose levels. Heptachlor at these doses had no effect on either pre- or postnatal survival. Pup weights were significantly reduced at both dose levels on PND 6 only but were comparable among treatment groups at PND 21 [maternal LOAEL for weight gain = 4.5 mg/kg body weight per day; NOAEL for pre- or postnatal survival of pups = 6 mg/kg body weight per day].
In another study in Fischer-344 rats (Narotsky et al., 1995), heptachlor was tested at doses of 0, 5.1, 6.8, 9.0, and 12.0 mg/kg body weight per day by gavage on GD 6–15. There were no clinical signs of maternal toxicity (dose-related alterations in weight gain) up to 9.0 mg/kg body weight per day (at 12.0 mg/kg body weight per day, 5 of 13 treated died), only a slight potential for developmental toxicity (reduced postnatal growth at 6.8 mg/kg body weight per day and above), and dramatically increased postnatal mortality (at 9.0 and 12.0 mg/kg body weight per day) [maternal NOAEL for weight gain = 6.8 mg/kg body weight per day for 9 days; NOAEL for postnatal growth = 5.1 mg/kg body weight per day (note postnatal mortality at 9.0 and 12.0 mg/kg body weight per day)].
Sprague-Dawley rats received oral doses of heptachlor during different developmental periods: 0, 4.2, and 8.4 mg/kg body weight per day for perinatal and gestational studies, and 0, 0.3, 1.0, and 3.0 mg/kg body weight per day for perinatal/adolescent studies (Purkerson-Parker et al., 2001). There were dose-related decreases in maternal weight gain and pup survival as well as delayed righting reflex at heptachlor doses >3.0 mg/kg body weight per day (see also section 8.6).
In a study to investigate the effect of heptachlor on the development of the reproductive system, pregnant Sprague-Dawley rats were administered heptachlor by oral gavage at doses of 0, 0.5, and 5.0 mg/kg body weight per day from GD 8 through PND 21, which was the day of weaning (. = 7–8 per group). Litters were standardized to four males and four females on the day of birth. Two of the dams in the 5.0 mg/kg body weight per day group died. Pups in the highest dose group weighed significantly less than those in the other two groups on PND 0. All but one litter of the 5.0 mg/kg body weight per day group died within the first 4 postnatal days. Age at eye opening was delayed with increasing heptachlor dose. There was no effect of treatment on pup weight gain in the surviving litters. There was no effect of treatment on anogenital distance, age at puberty, nipple retention past the infantile period in males, estrous cycling, serum sex steroid concentrations, reproductive organ weights, or testicular or ovarian histology, suggesting that heptachlor exposure during gestation and lactation does not disrupt development of the reproductive system in rats (Lawson & Luderer, 2004).
In the study by Oduma et al. (1995a) described above (section 8.5.1) in which female rats were injected subcutaneously every other day with heptachlor at 5 or 20 mg/kg body weight before mating, the weights of all pregnant animals increased with age of gestation but were not significantly different from the controls. Animals treated with 20 mg/kg body weight had longer mean gestational lengths of 25 ± 2.9 days, which were significantly different from those of the corn oil–treated controls (. < 0.05) of 22.7 ± 0.5 days, but not significantly different from rats treated with heptachlor at 5 mg/kg body weight. All pups were born alive, but the proportion of pups still alive by weaning time was lower for rats treated with 20 mg/kg body weight than for the other groups [NOAEL for litter size and gestational length = 5 mg/kg body weight (note postnatal mortality)].
Similar results were found in a dietary study on the reproductive function in mink (Mustela vison) (Crum et al., 1993). Maternal mortality was 0, 8, 67, and 100% for doses of 0, 6.25, 12.5, and 25 mg/kg in diet. Survival of kits was adversely affected in the two upper dose groups. Even at the lowest dose (6.25 mg/kg in diet), kit body weights were significantly less than for the control kits.
There is accumulating evidence that nervous system development is influenced by cyclodiene pesticides. Cyclodiene pesticides have been shown to act on the GABAA receptor, by binding at the chloride channel portion of the receptor and thereby blocking the inhibitory actions of the transmitter GABA. Heptachlor epoxide was more potent than heptachlor in the inhibition of the GABA-induced 36Cl− influx in rat brain (Gant et al., 1987). Acute actions of cyclodiene pesticides (e.g. heptachlor) include excitation, hyperstimulation, and convulsions (see section 8.1).
In a study on the neurobehavioural effects of heptachlor, female Fischer 344 rats (eight per dose level) were orally administered doses of 7, 23, 69, and 129 mg/kg body weight (Moser et al., 1995). No lethality occurred with single acute dosing. With repeated dosing, however (doses of 2, 7, 23, and 69 mg/kg body weight per day for 14 days), all rats receiving the two highest doses died during dosing, and one rat in the next lowest dose (7 mg/kg body weight per day) died shortly after the last dose. A neurobehavioural screening battery consisting of a functional observational battery and automated motor activity measurements was used to evaluate the neurotoxic potential of these chemicals. The magnitude of acute heptachlor effects on activity and excitability was greatest 4 h after dosing, and excitability changes were also observed at 24 h. The profile of effects produced by repeated heptachlor administration consisted of altered activity, hyperexcitability, and autonomic effects [NOAEL = 2 mg/kg body weight per day for a 14-day exposure].
In a study to investigate the neurological effects of perinatal heptachlor exposure, doses were set so that the low dose (0.03 mg/kg body weight per day) produced heptachlor and heptachlor epoxide levels in rat dam milk that matched the 95th percentile of human milk values on Oahu, Hawaii, USA, in 1981 (Siegel, 1988). Pregnant Sprague-Dawley dams were dosed by gavage from GD 12 to PND 7, whereupon the rat pups were dosed directly by gavage until PND 21 (group A) or PND 42 (group B). Dose levels were 0, 0.03, 0.3, or 3 mg/kg body weight per day (Smialowicz et al., 2001; see section 8.5). For the neurotoxicological evaluations, one male and one female from each of 10 litters were used for each dose group. The studies included screening evaluations (functional observational battery, motor activity, development of righting reflex), cognitive tests (associative and non-associative learning, spatial learning and memory, and working memory), and measures of GABAA receptor function and expression (Moser et al., 2001). The neurotoxicological outcomes of perinatal heptachlor exposure in the rat suggested developmental delays, alterations in GABAergic neurotransmission, and neurobehavioural changes, including cognitive deficits. Females were somewhat more affected, as were rats dosed until PND 42. Heptachlor had the most profound effects on cognitive function (slowed acquisition of the spatial task and impaired recall during probe trials), and these effects on probe trials were significant even at the lowest dose level [LOAEL = 0.03 mg/kg body weight per day].
There is increasing concern that environmental factors such as exposure to pesticides, and in particular to cyclodienes, may play a role in the onset of Parkinson’s disease (Priyadarshi et al., 2000). Changes in biochemical status of nerve terminals in the corpus striatum, one of the primary brain regions affected in Parkinson’s disease, were studied in groups of retired breeder male C57BL/6 mice treated by intraperitoneal injection of heptachlor 3 times over a 2-week period at doses of 3, 6, 12, 25, 50, or 100 mg/kg body weight (Kirby et al., 2001). No outward signs of intoxication or death were observed in mice given heptachlor at <25 mg/kg body weight. However, some mice treated at heptachlor doses of 50 or 100 mg/kg body weight showed hyperexcitability and became convulsive, which resulted in the death of some animals. The dopamine transporter normally functions to allow rapid uptake of dopamine and is therefore important in regulating dopamine actions. Dopamine transporter binding decreases with age and is additionally decreased due to neuronal loss in Parkinson’s disease (e.g. Fischman et al., 1998). On average, the maximal rate of striatal dopamine uptake increased >2-fold in mice treated at heptachlor doses of 6 mg/kg body weight and 1.7-fold at 12 mg/kg body weight, which was attributed to the induction of dopamine transporter. At higher dose levels, no increase in maximal dopamine intake was seen, which was suggested to be due to the toxic effects of heptachlor epoxide (Kirby et al., 2001). The dopaminergic system seems to be specifically sensitive to heptachlor, because no effects on serotonergic pathways were seen.
In an earlier study, retired breeder male C57BL/6 mice (8–10 months old) were treated intraperitoneally with heptachlor (0, 3, 6, 9, or 12 mg/kg body weight) administered 3 times over a 2-week period (Miller et al., 1999). An increase in both plasma membrane dopamine transporter as well as the vesicular monoamine transporter was reported in the striatum of the mice.
The dopamine transporter may also play an important role in neuronal development, appearing first at GD 14 (Fauchey et al., 2000). Therefore, changes in dopamine transporter number and dopamine levels may affect neuronal development early on and may play a role in the etiology of Parkinson’s disease later in life. To investigate this, Sprague-Dawley rats received oral doses of heptachlor during different developmental periods: 0, 4.2, or 8.4 mg/kg body weight per day for perinatal and gestational studies, and 0, 0.3, 1.0, or 3.0 mg/kg body weight per day for perinatal/adolescent studies (Purkerson-Parker et al., 2001). There were dose-related decreases in maternal weight gain and pup survival as well as delayed righting reflex at heptachlor doses >3.0 mg/kg body weight per day. Gestational, perinatal, and/or adolescent exposure to heptachlor produced an increase in dopamine transporter binding in the striatum as early as PND 10, and this change persisted into adulthood.
In a study on the effects of perinatal/juvenile heptachlor exposure (0, 0.03, 0.3, or 3 mg/kg body weight per day) in rats (see section 8.5; Smialowicz et al., 2001), heptachlor-exposed offspring were evaluated for a variety of innate and specific immune function end-points at 8 weeks of age (i.e. 2 weeks after cessation of dosing) and older. Perinatal/juvenile exposure of male and female rats to heptachlor did not alter spleen weight or cellularity or thymus weight, nor did it affect ex vivo immune function tests (i.e. splenic lymphoproliferative responses to mitogens of allogeneic cells and splenic natural killer cell activity) in a mixed lymphocyte response assay at 8 weeks of age. Further, in vivo delayed-type hypersensitivity and contact hypersensitivity were not affected by heptachlor exposure at 10 or 17 weeks of age, respectively. However, the primary IgM response to anti-sheep red blood cells was suppressed in male, but not in female, rats in a dose-related manner at 8 weeks of age. The percentage of B lymphocytes (OX12+OX19−) in spleen was also reduced in high-dose males. This suppression of antibody responses persisted for up to 20 weeks after the last exposure to heptachlor at a total heptachlor exposure of approximately 1.5 mg/kg body weight per rat. At 26 weeks of age, the secondary IgG antibody response to sheep red blood cells was suppressed in all of the heptachlor-exposed males but not females. The T cell–dependent antibody response to sheep red blood cells has been demonstrated to be one of the most commonly affected and most sensitive functional parameters in animals exposed to chemical immunosuppressants, requiring the interaction of three major immune cell types (i.e. macrophage, T-helper cell, and B cell; Luster et al., 1992).
Immunomodulatory effects have been shown by heptachlor in peripheral blood mononuclear cells from blood samples from male rhesus monkeys. Heptachlor at 80 µmol/l completely suppressed the proliferation and IL-2 release of the monkey lymphocytes (Chuang et al., 1992). Heptachlor inhibited the chemokine-induced chemotaxis of monkey neutrophils and monocytes at concentrations as low as 10−14–10−5 mol/l (Miyagi et al. 1998). This migration of neutrophils and monocytes towards chemokines normally plays a profound role in the body’s innate immune response towards infections (Chuang et al., 1999).
In rodent studies, heptachlor has a sharp dose–response curve for mortality (see section 8.2) without showing outward clinical signs of toxicity (with the exception of neurotoxic effects). At much lower doses, immunological and neurological effects have been shown. Heptachlor is a non-genotoxic carcinogen. Heptachlor shows tumour-promoting characteristics and down-regulates the tumour suppresser gene p53. There is increasing evidence that heptachlor in vitro causes inhibition of gap junctional intercellular communication. The following are recent studies into the mode of action of heptachlor.
Heptachlor triggered significant proliferation in quiescent rat hepatocytes (Okoumassoun et al., 2003). The key kinases that are a part of the signalling pathways known to be involved in cell proliferation were investigated. Exposure to heptachlor led to activation of protein kinase C MAPKs (Okoumassoun et al., 2003). This supports earlier studies that showed that chlordane stimulated protein kinase C activity in the rat brain (Bagchi et al., 1997).
Heptachlor (80 µmol/l) has been shown to decrease ras expression in human myeloblastic leukaemia (ML-1) cells (Chuang & Chuang, 1991). Studies on the signal transduction pathway using cultured human lymphocytes have shown that heptachlor down-regulated the tumour suppressor retinoblastoma (Bb) protein (Rought et al., 1999) and down-regulated p53 gene expression (Rought et al., 1998). Heptachlor exposure reduced the cellular levels of MAPK cascade proteins, which are important intermediates in the signal transduction pathway of immune cells (Chuang & Chuang, 1998). The activation of MAPKs may be one of the pathways used by heptachlor to exert its mitogenic action (resulting, for example, in cancer promotion).
Studies were undertaken to link cell cycle events and signal transduction pathways within heptachlor-treated cells. Heptachlor was found to block the cell cycle by preventing progression into S phase with a concomitant accumulation of cells in G1 phase; this is associated with a decrease (deactivation) in cyclin-dependent kinase cdk2 and dephosphorylation (activation) of cyclin-dependent kinase cdc2. The altered cell cycle progression may trigger the cell’s apoptotic potential, as indicated by the reduced amount of the anti-apoptotic protein Bcl-2 synthesized inside heptachlor-treated cells (Chuang et al., 1999).
Heptachlor strongly inhibited transforming growth factor beta–induced apoptosis and cytochrome c release into the cytosol in quiescent rat hepatocytes. The levels of Bcl-2 were also increased in the presence of heptachlor (Okoumassoun et al., 2003).
Two biochemical entities thought to be associated with promotion of liver cancer are the phosphoinositide signal transduction pathway and AP-1 nuclear transcription factors, of which protein kinase C is a critical enzyme. In vivo exposure of B6C3F1 male mice to heptachlor epoxide at 1, 10, or 20 mg/kg in the diet has been shown to selectively down-regulate particulate novel protein kinase C epsilon in B6C3F1 male mouse liver tissue, while persistently up-regulating AP-1. DNA binding activity (a critical factor in tumour promotion) was substantially increased at 3 and 6 h with 3.7 mg/kg intraperitoneal heptachlor epoxide and at 3 and 10 days with 20 mg/kg dietary heptachlor epoxide (Hansen & Matsumura, 2001a). Studies into the effects of heptachlor epoxide in mouse 1c1c7 hepatoma cells showed that many hepatocellular effects or changes occurred, suggesting a cellular programme shift. The tyrosine kinase growth factor receptor pathway seemed to be the probable critical pathway for heptachlor-induced tumour promotion, with the critical target most likely being upstream of PLCgamma1 and AP-1 (Hansen & Matsumura, 2001b).
Rought et al. (2000) reported that heptachlor by itself was able to stimulate apoptosis protease CPP32 at relatively high concentrations. When combined with the chemotherapeutic agent doxorubicin, a known CPP32 activator, a dual effect was noted. Low concentrations of heptachlor (5–10 µmol/l) suppressed doxorubicin-induced CPP32 activity, and high concentrations of heptachlor (80–120 µmol/l) augmented it. Rought et al. (2000) demonstrated that heptachlor has tumour promoting–like effects at lower concentrations and at higher concentrations induces apoptosis as a mechanism of toxicity.
Only a few of the studies that were analysed by IARC (2001) on the effects of chlordane and heptachlor mentioned or included exposure to heptachlor or heptachlor epoxide. These studies are summarized below. Further details are given in IARC (2001).
In a study on 74 Finnish plywood workers (and 52 controls), symptoms of headache, dizziness, and eye irritation were found to be not related to serum levels of heptachlor or chlordane compounds. Concentrations of heptachlor epoxide in sera ranged from below the detection limit of 0.1 ng/g to 19.2 ng/g serum (Mussalo-Rauhamaa et al., 1991).
Deaths among workers at a heptachlor-producing plant in the USA were analysed in a series of studies (Wang & MacMahon, 1979; Ditraglia et al., 1981; Brown, 1992). Production began in 1951, and there was co-exposure to other chemicals, such as endrin, chlorine, chlorendic anhydride, hexachlorocyclopentadiene, and vinyl chloride. The most recent investigation (Brown, 1992) included 305 white men employed for at least 6 months before 1965, with follow-up to the end of 1987 (one was lost to follow-up). The expected number of deaths was calculated from the mortality rates of white males in the USA. There were no historical exposure measurements available for this study. The results from all tumour sites are shown in Table 6.
Table 6: Cancer mortality study of workers exposed to heptachlor in a heptachlor/endrin manufacturing plant, USA.a
|
Cancer |
No. of deaths |
SMR |
95% CI |
|
All tumour sites |
18 |
1.0 |
0.60–1.6 |
|
Stomach |
2 |
2.8 |
0.34–10 |
|
Respiratory system |
6 |
0.88 |
0.32–1.9 |
|
Bladder |
3 |
7.1 |
1.5–21 |
|
Lymphatic/ haematopoietic |
1 |
0.58 |
0.01–3.2 |
a Brown (1992), adapted from IARC (2001).
Pesticide applicators, in particular termite control operators, were the group of workers primarily exposed occupationally to chlordane and, to a lesser extent, heptachlor. A cohort of 16 124 male pesticide applicators in the USA (exposed to a variety of pesticides) was matched with Social Security Administration and National Death Index files, with a follow-up from 1967 to December 31, 1984. In all, 1082 deaths were ascertained, of which 446 observed deaths occurred among termite control operators. There were no estimates of exposure available. For termite control operators, no excess of lung cancer deaths (30) was observed, with 31 expected deaths (SMR = 0.97; CI = 0.7–1.3); for other pesticide operators, the lung cancer SMR was 1.58, whereas SMRs for skin cancer (1.2) and bladder cancer (1.3) were comparable with those of the rest of the cohort (MacMahon et al., 1988; see Table 7).
Table 7: Cancer mortality study of workers (pesticide and termite control operators) exposed to heptachlor in the USA.a
|
Cancer |
No. of deaths |
SMR |
95% CI |
|
Entire cohort of applicators (mixed exposure, including heptachlor) |
|||
|
Lung |
108 |
1.4 |
1.1–1.6 |
|
Skin |
9 |
1.3 |
0.65–2.2 |
|
Bladder |
5 |
1.2 |
0.50–2.5 |
|
Lymphatic/haematopoietic |
25 |
0.97 |
0.67–1.4 |
|
Termite control operators only (exposure mainly to chlordane and heptachlor) |
|||
|
Lung |
30 |
0.97 |
0.7–1.3 |
|
Skin |
3 |
1.2 |
0.4–2.9 |
|
Bladder |
2 |
1.3 |
0.3–3.9 |
|
Buccal cavity and pharynx |
5 |
1.4 |
0.5–3.3 |
|
Stomach |
5 |
1.1 |
0.4–2.5 |
|
Colon |
11 |
1.1 |
0.6–2.0 |
|
Liver |
2 |
1.1 |
0.1–4.0 |
|
Pancreas |
6 |
1.0 |
0.4–2.2 |
|
Larynx |
4 |
2.4 |
0.7–6.2 |
a MacMahon et al. (1988), adapted from IARC (2001).
In a case–control study of non-Hodgkin’s lymphoma in the USA, an association with self-reported agricultural exposure to heptachlor and other organochlorine pesticides was reported (Cantor et al., 1992). Cases were identified through the Iowa State Health Registry and a special surveillance of Minnesota hospital and pathology laboratory records. A further study showed no evidence of an association between non-Hodgkin’s lymphoma risk and serum levels of any individual organochlorine or of the summed chlordane-related compounds (trans-nonachlor, heptachlor, heptachlor epoxide, oxychlordane) (Cantor et al., 2003).4
In a further study of men in Iowa and Minnesota, USA, 578 cases of leukaemia (340 living and 238 deceased) and 1245 controls were analysed (Brown et al., 1990). There was an association between occupation as a farmer and use of heptachlor (OR = 0.7, 95% CI 0.3–1.6), although the estimates were not adjusted for other possible confounding agricultural exposures. It was concluded that there was no evidence of increasing risk of leukaemia with increasing frequency of use of heptachlor (or chlordane) on crops (IARC, 2001).
Recent studies suggest an association between occupational exposure to organochlorine pesticides and PCBs and prostate cancer, but not to heptachlor in particular (Mills & Yang, 2003; Ritchie et al., 2003). Hispanic farm workers in California, USA, with relatively high levels of exposure to organochlorine pesticides (lindane and heptachlor), organophosphate pesticides (dichlorvos), fumigants (methyl bromide), or triazine herbicides (simazine), experienced an elevated risk of prostate cancer compared with workers with lower levels of exposure (use during 1987–1999) (Mills & Yang, 2003). The highest ORs were reported for heptachlor (1.35), dichlorvos (1.35), simazine (1.53), and lindane (1.32). However, due to the multiexposure, the role of heptachlor is unclear. In the study by Ritchie et al. (2003), 99 controls were frequency matched by age in 5-year increments to 58 prostate cancer patients. There was no difference in self-reported exposure between patients and controls. Heptachlor epoxide could be detected in 14 patients (24%) and 34 controls (34%). Adjusted ORs of 0.58 and 0.33 were reported for patients with lipid-adjusted serum levels of heptachlor epoxide of 0.006–0.02 µg/g and >0.021 µg/g, respectively.
There has recently been concern that the observed increase in breast cancer could correlate with the accumulation of organochlorine pesticides in human breast adipose tissues or serum, although there is little evidence at present to support this (Høyer et al., 1998, 2000; Ward et al., 2000; Zheng et al., 2000).
In several studies, heptachlor epoxide concentrations in breast adipose tissue samples from women with mammary carcinoma were compared with samples from women with benign disease. The results of two of those studies (Mussalo-Rauhamaa et al., 1990; Falck et al., 1992) suggest a possible correlation between heptachlor epoxide and breast cancer; in both studies, however, there were other pesticides present at much higher concentrations that correlated better than heptachlor with the occurrence of breast cancer.
Other studies have compared the serum or blood levels of heptachlor and/or heptachlor epoxide with breast cancer (see Table A5-11). Two recent studies show higher serum/blood concentrations of heptachlor in breast cancer patients than in the controls (Dello Iacovo et al., 1999; Mathur et al., 2002).
A temporal and geographical analysis of incidence rates for 23 major congenital malformations, derived from hospital-generated data collected by the Birth Defects Monitoring Program of the United States Centers for Disease Control and Prevention, failed to show any remarkable rate increase on Oahu, Hawaii, in 1981–1983 (the period when the milk supply was contaminated by the insecticide heptachlor; see section 6.2.1). This was based on comparisons with the rates for previous periods on this island and with the rates for the other Hawaiian islands, where no contamination occurred, and the total USA. A rise in the rates of cardiovascular malformations and hip dislocation was apparent but antedated the exposure. Over the 2-year period 1981–1982, 19 159 births (68% of those occurring on Oahu and all [8663] of those occurring on the other Hawiian islands) were reported to the Birth Defects Monitoring Program. Limitations to the study include a possible misclassification of exposure status, as many individuals on Oahu may not have actually been exposed to heptachlor (e.g. non-milk drinkers), therefore minimizing the effect of exposure (Le Marchand et al., 1986).
Two further studies examined this cohort exposed to heptachlor in Oahu. Burch (1983) examined trends in birth outcomes using birth, death, and fetal death certificates for 1968–1982. There was no significant change in outcome in 1982 compared with previous years. Grafton-Wasserman (1988) used medical charts to compare perinatal outcomes of births at two medical centres during 1982 with those during 1978 and 1979. There was no apparent change in birth weight, gestational age, or sex ratio. All these studies are limited by lack of assessment of individual exposure.
Hoffman (1985) reported a longitudinal evaluation of 120 infants born in Oahu, Hawaii, USA, during 1982 who were potentially exposed to heptachlor epoxide in utero and via breast milk (Baker et al., 1991). The mean heptachlor epoxide level in milk from 69 mothers was 123 ng/g milk fat. A significant association was found between breast milk heptachlor epoxide level and infant low birth weight, gestational age, and jaundice. Physical growth was not associated with any exposure measure. However, a slower acquisition of behaviours at 4 and 8 months, but not at 18 and 36 months, was noted (Hoffman, 1985; no further details were available).
A neurobehavioural study was performed on 332 randomly selected high school students who were in utero on Oahu at the time of the milk contamination and 113 students (comparison population) who were not born on Oahu but lived on the island since the first grade. The groups were frequency matched by age, school, primary language spoken at home, and ethnicity. The mothers’ reported milk consumption was used as an indicator of the past heptachlor epoxide exposure. To assess neurobehavioural function and academic achievement, a variety of tests were administered, school records were reviewed, and teacher interviews were conducted. Multivariate analyses controlling for potential confounding factors indicated that gestational heptachlor epoxide exposure was associated with lower neurological performance, especially abstract concept formation, visual perception, and motor planning, and with more reported behavioural problems. There were no strong associations for school-based performance measures, such as cumulative grade point average (Baker et al., 2001, 2004; further details were not available).
Hertz-Picciotto et al. (2003, 2004) described a study of 399 children born in 1964–1967 in the San Francisco Bay, California, USA, area who were tested 5 years later using the Raven’s Progressive Matrices, a test for non-verbal perceptual reasoning, and the Peabody Picture Vocabulary Test, a test of language development. Adjustment was made for a variety of confounders. The results of the testing were compared with the heptachlor epoxide concentrations in the stored maternal sera samples of the second or third trimester from the children’s mothers. The concentration of heptachlor epoxide was significantly associated with lower scores of the Raven’s Progressive Matrices test but not with scores on the Peabody Picture Vocabulary Test. In the Raven’s test, inclusion of PCBs in the model reduced precision considerably, suggesting that any association between heptachlor epoxide and cognitive deficits is probably due to other co-exposures, to either PCBs or unidentified confounders (Hertz-Picciotto et al., 2004).
Babies fed on breast milk contaminated by heptachlor/heptachlor epoxide and pregnant mothers and children drinking cow milk contaminated by heptachlor/heptachlor epoxide are suspected as being particularly sensitive populations. Recent research on the Oahu population (see above) and recent data from animal studies on neurotoxicological, immunotoxicological, and reproduction end-points (see section 8) point to heptachlor as being a chemical acting primarily at these sensitive stages in life.
In some regions, comparatively high levels of heptachlor in breast milk are still being reported (see section 6.2.1 and Table A5-12). Further, there are reports of high levels of heptachlor in vegetables and fish in some areas (see section 6.2.1). There is concern that developing fetuses, babies, and small children could be at risk of neurological or immunological effects through this exposure.
Several tests have been performed to establish the acute toxicity of heptachlor for aquatic biota (freshwater and marine species) representing different trophic levels, and a limited selection of some of these is shown in Tables 8 and 9. All of the studies in Tables 8 and 9 were chosen as key studies by the CICAD authors. Additional data are summarized and partly commented on in the extensive reviews by USEPA (1980), IPCS (1984), Fendick et al. (1990), and ECETOC (2003). However, many of these studies are only of limited value, because initial concentrations of heptachlor, or the technical formulations containing other compounds as well as heptachlor, were not measured; chemical purity of the heptachlor used was not clearly specified (i.e. technical formulations with varying content of heptachlor and accompanying compounds); abiotic and biotic losses of heptachlor were not taken into account over the tests’ duration; or concentrations employed in the test exceeded heptachlor’s water solubility many-fold. Therefore, it is difficult to decide whether the considerable variation existing among species might in many cases be due to test variability rather than to different species sensitivities.
Table 8: Toxicity of heptachlor in freshwater aquatic organisms.
|
Species tested |
End-point (effect other than survival) |
Concentrationa (mg/l) |
Reference |
|
Green algae |
|||
|
Pseudokirchneriella subcapitata (old name Selenastrum capricornutum) |
96-h EC50 (growth inhibition) |
0.027–0.04b |
USEPA (1980) |
|
Invertebrates |
|||
|
Midge (Tanytarsus dissimilis) |
48-h LC50 |
>2.5, as no mortality was observed (nominal)c |
Holcombe et al. (1983) |
|
Mosquito larvae (Culex pipiens quinquefasciatus) |
LC50d |
0.005 (nominal) |
Lu et al. (1975) |
|
Water flea (Daphnia magna) |
48-h LC50 |
0.08 (nominal) |
Macek et al. (1976) |
|
Water flea (Simocephalus serrulatus) |
48-h LC50 |
0.047 |
Mayer & Ellersieck (1986) |
|
48-h LC50 |
0.08 |
Mayer & Ellersieck (1986) |
|
|
Amphipod (Gammarus fasciatus) |
24-h LC50 96-h LC50 |
0.18 0.056 |
Mayer & Ellersieck (1986) |
|
24-h LC50 96-h LC50 |
0.14 0.04 |
Mayer & Ellersieck (1986) |
|
|
Amphipod (Gammarus lacustris) |
24-h LC50 96-h LC50 |
0.15 0.029 |
Mayer & Ellersieck (1986) |
|
Crayfish (Orconectes nais) |
24-h LC50 96-h LC50 |
0.0026 0.0005 |
Mayer & Ellersieck (1986) |
|
Grass shrimp (Palaemonetes kadiakensis) |
24-h LC50 96-h LC50 |
0.03 0.0018 |
Mayer & Ellersieck (1986) |
|
Stonefly (Claassenia sabulosa) |
24-h LC50 96-h LC50 |
0.009 0.0028 |
Mayer & Ellersieck (1986) |
|
Stonefly (Pteronarcella badia) |
24-h LC50 96-h LC50 |
0.006 0.0009 |
Mayer & Ellersieck (1986) |
|
Stonefly (Pteronarcys californica) |
24-h LC50 96-h LC50 |
0.008 0.0011 |
Mayer & Ellersieck (1986) |
|
Snail (Aplexa hypnorum) |
96-h LC50 |
1.45 (nominal)c |
Holcombe et al. (1983) |
|
Fish |
|||
|
Japanese medaka (Oryzias latipes) |
48-h LC50 |
0.014 (nominal) |
MITI (1992) |
|
Bluegill sunfish (Lepomis macrochirus) |
24-h LC50 48-h LC50 96-h LC50 |
0.030 (nominal)c 0.023 (nominal)c 0.019 (nominal)c |
Henderson et al. (1959) |
|
Bluegill sunfish (Lepomis macrochirus) |
24-h LC50 96-h LC50 |
0.083 (nominal) 0.013 (nominal) |
Schoettger (1970) |
|
Guppy (Poecilia reticulata) |
24-h LC50 48-h LC50 96-h LC50 |
0.16 (nominal)c 0.11 (nominal)c 0.11 (nominal)c |
Henderson et al. (1959) |
|
Fathead minnow (Pimephales promelas) |
24-h LC50e 48-h LC50e 96-h LC50e 24-h LC50 48-h LC50 96-h LC50 |
0.060 (nominal)c 0.058 (nominal)c 0.056 (nominal)c 0.097 (nominal)c 0.094 (nominal)c 0.094 (nominal)c |
Henderson et al. (1959) |
|
Fathead minnow (Pimephales promelas) |
240-h LC50 60-day NOEC (growth) |
0.007 (nominal) 0.000 86 (nominal) |
Macek et al. (1976) |
|
Goldfish (Carassius auratus) |
24-h LC50 48-h LC50 96-h LC50 |
0.52 (nominal)c 0.28 (nominal)c 0.23 (nominal)c |
Henderson et al. (1959) |
|
Rainbow trout (Oncorhynchus mykiss) |
24-h LC50 96-h LC50 |
0.032 0.032 |
Mayer & Ellersieck (1986) |
|
24-h LC50 96-h LC50 |
0.044 0.043 |
Mayer & Ellersieck (1986) |
|
|
24-h LC50 96-h LC50 |
0.019 0.007 |
Mayer & Ellersieck (1986) |
|
|
24-h LC50 96-h LC50 |
0.011 0.0071 |
Mayer & Ellersieck (1986) |
|
|
24-h LC50 96-h LC50 |
0.011 0.0074 |
Mayer & Ellersieck (1986) |
|
|
24-h LC50 96-h LC50 |
0.0098 0.008 |
Mayer & Ellersieck (1986) |
|
|
Northern pike (Esox lucius) |
24-h LC50 96-h LC50 |
0.008 0.0062 |
Mayer & Ellersieck (1986) |
|
Redear sunfish (Lepomis microlophus) |
24-h LC50 96-h LC50 |
0.034 0.017 |
Mayer & Ellersieck (1986) |
|
Fathead minnow (Pimephales promelas) |
24-h LC50 96-h LC50 |
0.063 0.023 |
Mayer & Ellersieck (1986) |
|
Channel catfish (Ictalurus punctatus) |
24-h LC50 96-h LC50 |
0.028 0.025 |
Mayer & Ellersieck (1986) |
|
Black bullhead (Ictalurus melas) |
24-h LC50 96-h LC50 |
0.086 0.063 |
Mayer & Ellersieck (1986) |
|
Largemouth bass (Micropterus salmoides) |
24-h LC50 96-h LC50 |
0.019 0.010 |
Mayer & Ellersieck (1986) |
|
Amphibians |
|||
|
Fowler’s toad (Bufo woodhousei fowleri) |
24-h LC50 96-h LC50 |
0.844 0.435 |
Mayer & Ellersieck (1986) |
|
a |
As heptachlor can evaporate to some extent from and react in the aqueous phase and therefore reduce the actual concentration of the test compound over time, we specified (in parentheses) whether the values given were based upon nominal (initial experimental concentrations) or effective (loss of compound checked and accounted for) concentrations if such information was provided. |
|
b |
Range of values due to different proportions of test substance abiotically transformed to hydroxychlordene in water. |
|
c |
Technical heptachlor (containing 65%, 72%, or 74% active ingredient) was employed, and given values were adjusted correspondingly to account for the amount of active ingredient present. |
|
d |
Time not specified. |
|
e |
Data obtained using hard water (calcium carbonate concentration of 400 mg/l instead of 20 mg/l). |
Table 9: Toxicity of heptachlor in marine aquatic organisms.
|
Species tested |
Salinity (‰) |
End-point (effect other than survival) |
Concentrationa (mg/l) |
Reference |
|
Green algae |
||||
|
Dunaliella tertiolecta |
96-h EC50 (growth inhibition via biomass) |
2.26 (nominal) |
USEPA (1980) |
|
|
Skeletonema costatum |
96-h EC50 (growth inhibition via biomass) |
0.093 (nominal) |
USEPA (1980) |
|
|
Porphyridium cruentum |
96-h EC50 (growth inhibition via biomass) |
0.27 (nominal) |
USEPA (1980) |
|
|
Invertebrates |
||||
|
Korean shrimp (Palaemon macrodactylus) |
26 |
96-h LC50 |
0.015 (nominal) |
Schoettger (1970) |
|
Pink shrimp (Penaeus duorarum) |
20–22 |
96-h LC50 |
0.000 11 (measured)b |
Schimmel et al. (1976) |
|
Pink shrimp (Penaeus duorarum) |
26–30 |
96-h LC50 |
0.000 03 (measured) |
Schimmel et al. (1976) |
|
Sand shrimp (Crangon septemspinosa) |
24 |
24-h LC50 48-h LC50 96-h LC50 |
0.11 (nominal) 0.028 (nominal) 0.008 (nominal) |
Eisler (1969) |
|
Grass shrimp (Palaemonetes vulgaris) |
24 |
24-h LC50 48-h LC50 96-h LC50 |
>6.5 (nominal) 3.3 (nominal) 0.44 (nominal) |
Eisler (1969) |
|
Hermit crab (Pagurus longicarpus) |
24 |
24-h LC50 48-h LC50 96-h LC50 |
0.47 (nominal) 0.10 (nominal) 0.06 (nominal) |
Eisler (1969) |
|
Eastern oyster (Crassostrea virginica) |
21 |
96-h EC50 (shell growth) |
0.021 (nominal) |
Mayer (1987) |
|
23 |
96-h EC50 (shell growth) |
0.017 (nominal) |
Mayer (1987) |
|
|
26 |
96-h EC50 (shell growth) |
0.0015 (nominal) |
Mayer (1987) |
|
|
Blue crab (Callinectes sapidus) |
27 |
48-h EC50 (immobilization) |
0.068 (nominal) |
Mayer (1987) |
|
Mysid shrimp (Mysidopsis bahia) |
23 |
96-h LC50 |
0.0034 (measured) |
Mayer (1987) |
|
Grass shrimp (Palaemonetes vulgaris) |
26 |
96-h LC50 |
0.0011 (measured) |
Mayer (1987) |
|
Fish |
||||
|
Striped bass (Morone saxatilis) |
28 |
24-h LC50 96-h LC50 |
0.023 (nominal) 0.003 (nominal) |
Schoettger (1970) |
|
Spot croaker (Leiostomus xanthurus) |
21 |
96-h LC50 |
0.000 85 (measured)b |
Schimmel et al. (1976) |
|
Spot croaker (Leiostomus xanthurus) |
20–22 |
96-h LC50 |
0.000 86 (measured) |
Schimmel et al. (1976) |
|
American eel (Anguilla rostrata) |
24 |
24-h LC50 48-h LC50 96-h LC50 |
0.071 (nominal) 0.049 (nominal) 0.010 (nominal) |
Eisler (1970) |
|
Northern puffer (Sphoeroides maculatus) |
24 |
24-h LC50 48-h LC50 96-h LC50 |
0.24 (nominal) 0.19 (nominal) 0.19 (nominal) |
Eisler (1970) |
|
Pinfish (Lagodon rhomboides) |
25–31 |
96-h LC50 |
0.004 (measured)b |
Schimmel et al. (1976) |
|
Sheepshead minnow (Cyprinodon variegatus variegatus) |
21–25 |
96-h LC50 |
0.004 (measured)b |
Schimmel et al. (1976) |
|
Sheepshead minnow (Cyprinodon variegatus variegatus) |
96-h LC50 18-week LOEC |
0.011 (measured)b 0.003 (measured)b |
Hansen & Parrish (1977) |
|
|
Striped mullet (Mugil cephalus) |
24 |
48-h LC50 |
0.0033 (nominal) |
Mayer (1987) |
|
a |
As heptachlor can evaporate to some extent from and react in the aqueous phase and therefore reduce the actual concentration of the test compound over time, we specified (in parentheses) whether the values given were based upon nominal (initial experimental concentrations) or effective (loss of compound checked and accounted for) concentrations if such information was provided. |
|
b |
Technical heptachlor (containing 65%, 72%, or 74% active ingredient) was employed, and given values were adjusted correspondingly to account for the amount of active ingredient present. |
According to Leigh (1969), heptachlor does not negatively affect microbial communities. However, guideline tests to ascertain that heptachlor is not interfering with microbial activity are not available.
Heptachlor is clearly toxic to a range of both freshwater and marine species, including algae, invertebrates, and fish (see Tables 8 and 9 and data reviewed in USEPA, 1980; IPCS, 1984; Fendick et al., 1990; and ECETOC, 2003).
Growth of both freshwater and marine/estuarine algal species was inhibited by heptachlor. However, the limited data available seem to indicate that saltwater algal species might be less sensitive than the one freshwater species (Pseudokirchneriella subcapitata) tested (see Tables 8 and 9).
Invertebrate species from both fresh water and seawater are clearly adversely influenced by heptachlor. Again, the reported values span a broad range, with the lowest LC50 (96 h) value of 0.03 µg/l reported by Schimmel et al. (1976) for the shrimp Penaeus duorarum at 26–30‰ salinity.
In toxicity studies conducted with several fish species (see Tables 8 and 9), the most recently reported LC50 value (48-h incubation) was established for Japanese medaka (Oryzias latipes) as 14 µg of heptachlor per litre (MITI, 1992). Earlier data by Schoettger (1970) show a similar LC50 value (96-h incubation) of about 13 µg/l for bluegill (Lepomis macrochirus), whereas Henderson et al. (1959) reported an LC50 (48 h) of 23 µg/l and an LC50 (96 h) of 19 µg/l for the same organism. Macek et al. (1976) reported an LC50 value (10 days) of 7 µg/l using fathead minnow (Pimephales promelas). In Macek et al. (1976), a 60-day NOEC value of 0.86 µg/l was estimated. Both Suter & Tsao (1996) and ECETOC (2003) used the data reported by Macek et al. (1976) for their analyses.
Under saline conditions, the lowest values reported are those for spot croaker (Leiostomus xanthurus), with a measured LC50 (96 h) value of 0.86 µg/l (Schimmel et al., 1976). However, Hansen & Parrish (1977) reported an acute LC50 (96 h) value of 11 µg/l in sheepshead minnow (Cyprinodon variegatus variegatus); by employing an 18-week partial life cycle test starting with juvenile fish, a corresponding LOEC value of 3 µg/l was reported.
In a more recent report, Okoumassoun et al. (2002) established an EC50 of about 45.5 mg/l (i.e. 122 µmol/l) for heptachlor by using rainbow trout (Oncorhynchus mykiss) hepatocytes. The value given refers to the relative effective concentration that caused 50% inhibition of binding of 17beta-estradiol to cytosolic estrogen receptors. As heptachlor was not able to induce vitellogenin production, it showed a lack of estrogenicity. However, co-exposure experiments (17beta-estradiol together with heptachlor) indicated that heptachlor inhibited the hepatocytes’ response to 17beta-estradiol, albeit not being a potent competitor, as it failed to displace 17beta-estradiol from the corresponding receptor to a significant degree. The authors therefore proposed that heptachlor hampers with the proper interaction of 17beta-estradiol with the receptor. By doing so, heptachlor could impair the estrogenic process in fish.
Heptachlor is moderately toxic to bird species, with dietary LC50 values ranging from 90 to about 500 mg/kg body weight (see examples in Table 10). Podowski et al. (1979) reported a nominal 24-h LD50 of 71 mg/kg body weight for a typical rodent such as the rat. However, LD50 values reported for other rodents such as mice or rabbits as representative vertebrates present in the terrestrial environment range from 40 to about 162 mg/kg body weight, with purity of the test substance in many cases not specified and only limited numbers of animals tested (see section 8). However, Okoumassoun et al. (2003) only recently demonstrated the cytotoxicity of heptachlor using hepatocytes from the rat. By using the lactate dehydrogenase assay, they showed a clear cytotoxic effect in the concentration range from 50 to 100 µmol/l (i.e. about 19–37 mg/l, 24- and 48-h incubation).
Table 10: Toxicity of heptachlor for birds.
|
Species tested |
End-point |
Concentration (mg/kg body weight) |
Reference |
|
Mallard (Anas platyrhynchos) |
Dietarya LC50 |
480 (nominal) |
USFWS (1975) |
|
Ring-necked pheasant (Phasianus colchicus) |
Dietarya LC50 |
224 (nominal) |
USFWS (1975) |
|
Japanese quail (Coturnix japonica) |
Dietarya LC50 |
93 (nominal) |
USFWS (1975) |
|
a |
Dietary LC50 values based on active ingredient present in technical-grade heptachlor in ad libitum diet calculated to produce 50% mortality in 8 days (5 days of diet containing heptachlor followed by 3 days of normal diet). |
Heptachlor is well absorbed through the mammalian gastrointestinal tract and metabolized mainly to heptachlor epoxide and minor metabolites. Heptachlor epoxide has been shown to accumulate in adipose tissue and to cross the placenta. Furthermore, heptachlor and heptachlor epoxide are detected in breast milk.
Heptachlor shows mostly negative responses in in vitro and in vivo genotoxicity testing. There is increasing evidence that heptachlor in vitro causes inhibition of gap junctional intercellular communication.
Acute exposure in animal studies causes neurotoxicological disturbances and death. In both short- and long-term studies in dogs, rats, and mice, the liver was found to be the target organ.
In a 30-day feeding study in mice fed a mixture of heptachlor and heptachlor epoxide (1:3) at doses of 1, 5, 10, 25, and 50 mg/kg, the NOAEL was given as 1 mg/kg for dose-related histopathological liver changes (enlargement of centrilobular and midzonal hepatocytes) (Wazeter et al., 1971a) [NOAEL = 0.13 mg/kg body weight per day].
In a 2-year feeding study in dogs, heptachlor epoxide was administered in the diet at concentrations of 0, 1, 3, 5, 7, or 10 mg/kg; the NOAEL was given as 1 mg/kg for dose-related histopathological liver changes (Wazeter et al., 1971b, 1971c) [NOAEL = 0.025 mg/kg body weight per day].
In reproduction studies in rats, there were usually no clinical signs of maternal toxicity (dose-related alterations in weight gain) (Smialowicz et al., 2001) [NOAEL for maternal toxicity = 3 mg/kg body weight per day]. In one study, reduced litter sizes were noted. In all studies, postnatal mortality of the pups was the most obvious finding [NOAEL for pre- or postnatal survival of pups = 6 mg/kg body weight per day]. There were no histological examinations of the liver, so the NOAELs would probably have been much lower.
No teratological effects were observed.
There is accumulating evidence that nervous system development is influenced by cyclodiene pesticides. The profile of effects produced by repeated heptachlor administration to female rats consisted of altered activity, hyperexcitability, and autonomic effects (Moser et al., 1995) [NOAEL = 2 mg/kg body weight per day]. In another study, neurotoxicological studies on perinatal heptachlor exposure in the rat (0.03, 0.3, or 3 mg/kg body weight per day) suggested developmental delays, alterations in GABAergic neurotransmission, and neurobehavioural changes, including cognitive deficits, at all doses (Moser et al., 2001). Statistics were not given, but the LOAEL was probably 0.03 mg/kg body weight per day.
Immunological studies indicate the suppression of the primary and secondary response to sheep red blood cells following perinatal exposure (0, 0.03, 0.3, or 3 mg/kg body weight per day) to heptachlor.
There was a highly significant dose-related incidence of hepatocellular carcinoma in both male and female B6C3F1 mice fed technical-grade heptachlor in the diet. The time-weighted average doses for the male mice were 6 and 14 mg/kg and for the female mice were 9 and 18 mg/kg (NCI, 1977). No hepatic tumours were observed in Osborne-Mendel rats at time-weighted average doses for male rats of 39 and 78 mg/kg and for female rats of 26 and 51 mg/kg (NCI, 1977) [NOAEL = 39 mg/kg (= 2 mg/kg body weight) for males and 26 mg/kg (= 1.3 mg/kg body weight) for females]. Deficiencies in this study precluded proper evaluation of the carcinogenic potential of heptachlor in rats (JMPR, 1992).
There was a significant increase in the incidence of hepatocellular carcinomas in female but not in male C3H mice given heptachlor and in both males and females given heptachlor epoxide (10 mg/kg [= 0.5 mg/kg body weight]) in the diet for 2 years (United States Food and Drug Administration study performed in 1965, summarized in Epstein, 1976).
In a study with CD-1 mice fed a mixture of heptachlor and heptachlor epoxide for 18 months, a significant increase in the combined incidence of hepatocellular carcinomas and nodules was found in the male and female 10 mg/kg (in the diet) dose groups [= 0.5 mg/kg body weight] (IRDC, 1973).
In initiation–promotion studies in mice, administration of heptachlor after .-nitrosodiethylamine resulted in increased incidences of hepatocellular tumours.
Available epidemiological studies were not able to show a clear relationship between any effects in humans and exposure to heptachlor.
There are inadequate data on the effects of heptachlor on humans. A tolerable intake is therefore based on animal data.
Heptachlor was found to be carcinogenic to mice at doses of 0.5 mg/kg body weight and above. It was not carcinogenic in rats. It acts as a tumour promoter in mouse liver. It is unlikely that tumours in mouse liver are induced through a genotoxic mechanism. Therefore, non-neoplastic effects (histopathological liver changes, neurotoxicological effects, and immunotoxicological effects) were chosen as the basis for the derivation of a tolerable intake; they have been reported at about 1/20th of the concentration at which carcinogenic effects were seen.
In animals fed heptachlor/heptachlor epoxide by diet, gavage, or subcutaneous injection, there is a sharp dose–response curve for mortality.
The lowest NOAEL for histopathological liver changes is from dog studies (Wazeter et al., 1971b, 1971c) (1 mg of heptachlor per kilogram of diet = 0.025 mg/kg body weight per day, calculated from standard table). Recent developmental neurotoxicity and immunotoxicological studies in rats give 0.03 mg/kg body weight per day as the NOAEL or LOAEL.
Application of a safety factor of 200 — 10 for interspecies variability, 10 for intraspecies extrapolation, and 2 for inadequacy of the database — to the lowest NOAEL of 0.025 mg/kg body weight per day gives a tolerable intake of 0.0001 mg/kg body weight (0.1 µg/kg body weight) for humans for non-carcinogenic effects.
There have been several studies giving estimates of human dietary intake of heptachlor/heptachlor epoxide in the past decades (see section 6). Recently, estimates include those, for example, for Poland, giving daily dietary intakes of heptachlor and heptachlor epoxide ranging from 0.51 to 0.58 µg per person (Falandysz, 2003) (about 0.01 µg/kg body weight, assuming a mean weight of 64 kg). This value is 10-fold less than the tolerable intake of 0.1 µg/kg body weight. In this study, the main sources of heptachlor and heptachlor epoxide were thought to be meat, meat products, and animal fats. However, if food is contaminated with heptachlor, such as fish from contaminated rivers (e.g. concentrations in fish in the 0.1–1 mg/kg range were reported from Ganges Estuary, Bangladesh, Bay of Bengal, and Göksu Delta, Turkey), vegetables from fields contaminated with heptachlor (up to 16 mg/kg), or contaminated milk (e.g. in the microgram per kilogram range in Dakar, Senegal; in the milligram per kilogram range in Jaipur City, Rajasthan, India), then the dietary intake of this chemical would be much higher; if the contaminated food is ingested for a long period of time, there is likely to be a health risk.
The daily intake of heptachlor by breast-fed babies in Jordan (highest values taken), calculated from the data of Alawi & Khalil (2002), assuming a daily milk consumption of 150 g/kg body weight and an average milk fat content of 3.1%, was a mean of 0.67 µg/kg body weight. The corresponding daily intake of heptachlor epoxide by babies in 2000 was a mean of 1.5 µg/kg body weight (Alawi & Khalil, 2002). These values are more than 10-fold higher than the tolerable intake and, if the concentrations reported are correct, should be a cause of concern.
There are inadequate data on the effects of heptachlor on humans.
The key animal studies were not recent studies conducted under Good Laboratory Practice. They were older, mostly inadequate unpublished reports not available to the authors.
Recent neurotoxicological and immunotoxicological studies in rats have shown that there are effects at the lowest doses tested.
In some of the environmental samples reported, it is not certain whether the values given are truly due to a contamination with heptachlor or due to an experimental or calculational error.
The tolerable intake is calculated for one substance. From the results of many studies, it has been shown that usually there are several persistent compounds (e.g. other organochlorine pesticides) present at much higher values than heptachlor and heptachlor epoxide. Therefore, an estimation of health risks cannot be carried out in isolation, but must include the range of compounds found in that particular food or tissue.
Heptachlor and heptachlor epoxide are persistent and are found in all environmental compartments — air, water, soil, and sediment — as well as in plants (vegetables), fish and other aquatic organisms, amphibians and reptiles, birds and bird eggs, and aquatic and terrestrial mammals. They are found particularly in adipose tissues, where they accumulate. They pass up the food-chain.
The main environmental target compartments of heptachlor are soil and sediment, where this compound is slowly transformed by abiotic reactions and poorly transformed aerobically by microorganisms. Therefore, conditions not accelerating biotic or abiotic removal might lead to the presence of detectable concentrations of this persistent organic pollutant in these two compartments. However, a reliable quantification of heptachlor (the application of which is restricted in most countries) currently released from all sources is impossible with the data available.
The experimentally determined bioconcentration data as well as the measured log .ow values indicate a high bioaccumulation potential for both heptachlor and its epoxide. The determined soil sorption coefficients indicate a high potential for soil sorption. Therefore, heptachlor may not easily leach into groundwater, and its bioavailability might in aquatic environments be rather limited in the presence of particulate or dissolved organic matter.
Suggestive guidance values for heptachlor toxicity in the marine and freshwater environments can be derived using a probabilistic approach, since the data set is sufficiently large to warrant it. Appendix 7 details the methodology used.
For the freshwater environment, 23 data points were chosen to derive a guidance value. The criteria for choosing the toxicity values and the actual values are presented in Appendix 7. These acute values have been converted to chronic values using an acute:chronic ratio of 10. The guidance value for the protection of 99% of species with 50% confidence was derived at 10 ng of heptachlor per litre (see Figure A7-2, Appendix 7).
For the marine environment, 18 toxicity values were used in the derivation in the same way. For further details, refer to Appendix 7 and Table A7-2. An overall guidance value for the protection of 99% of species with 50% confidence was derived at 5 ng of heptachlor per litre (see Figure A7-3, Appendix 7).
A sample risk characterization with respect to the aquatic environment might be performed by calculating the ratio between a local heptachlor concentration (measured concentration) and the guidance value. Due to the highly varying data concerning the appearance of heptachlor in the environment, Table 11 compares some possible sample risk characterizations based upon upper and lower values of reported concentrations in the aquatic environment (rather than a specific value) and the relevant guidance value.
Table 11: Sample risk characterizations for different aquatic environments.
|
Measured concentration (MC) rangea (ng/l) |
Guidance value (GV)b (ng/l) |
MC/GV ratio range |
|
Surface water 0.0001–62 000 |
10 |
0.000 01–6200 |
|
Seawater 0.025–0.15 |
5 |
0.005–0.03 |
|
a |
Included are data from 1996 onwards, with lowest and highest reported values determining the range (see Table A5-2 in Appendix 5 for data). The extreme outlier for seawater (Straits of Jahore between Singapore and Malaysia with 233 ng/l; Basheer et al., 2002) is not included. |
|
b |
Guidance value obtained from species sensitivity distributions for the protection of 99% of species with 50% confidence (see Appendix 7). |
Taking surface water from freshwater environments as an example, the highest reported value for such water samples is 62 000 ng/l (see Appendix 5, Table A5-2; Ayas et al., 1997), which can be employed as the local worst-case measured concentration. The species sensitivity distribution guidance value was 10 ng/l, giving a measured concentration to guidance value ratio of 6200, well above 1. Therefore, for some surface waters in several countries (see Table A5-2), there might be a severe risk for the aquatic environment in such highly polluted locations. Measured concentrations for rainwater are an indicator of the global distribution of heptachlor in the atmosphere. The range of values (0.073–4 ng/l) falls within that of surface water values.
Although marine species appear to be more sensitive to heptachlor than freshwater species, the lower concentrations of heptachlor in seawater reduce the overall risk. For seawater, the highest reliable measured concentration for heptachlor is about 0.15 ng/l (Bering Sea; Yao et al., 2002). Using the species sensitivity distribution derived guidance value of 5 ng/l, the measured concentration to guidance value ratio is 0.03 (i.e. <1), suggesting low risk.
Heptachlor will preferably partition into soil (about 43%) and sediment (about 55%) and only to a lesser extent into water (about 2%). For the terrestrial compartment, a few toxicity tests on rodents and birds are available. None of the studies appears reliable enough to serve as a basis for a quantitative risk characterization. It should be remembered that heptachlor is used as a termiticide.
In studies on rats, heptachlor has been shown to be neurotoxic and immunotoxic at 0.03 mg/kg body weight per day (see sections 8.6 and 8.7). The Göksu Delta, Turkey, which is one of the most important breeding and wintering areas for birds in the world, is contaminated by organochlorine pesticides from soils from agricultural areas that have been transported to the delta by the Göksu River. From this region, levels of heptachlor/heptachlor epoxide have been detected in birds and bird eggs in the lower milligram per kilogram range. The effect of such concentrations of heptachlor on the bird populations can only be speculated at present.
Heptachlor can evaporate from and react with water, and (especially) long-term studies not stating the effective concentration of the test compound might therefore be unreliable.
There are no data available to predict the effect (additive, antagonistic, synergistic) of other organochlorine pesticides usually accompanying heptachlor in polluted environments.
There is a lack of recent data on the toxicity of heptachlor in the environment. In addition, in many of the available studies, test procedures are clearly varying (biological variation, methodological variation), and thus a reliable comparison of data obtained even for related species is a difficult task given the data available.
There is a lack of valid data concerning the toxicity of heptachlor to microorganisms.
There is a lack of reliable data on its epoxide.
For the terrestrial compartment, the available toxicity studies are not sufficient to enable a quantitative risk characterization of heptachlor. Further, there is an apparent lack of data concerning the difference between the degradation of heptachlor in soils of moderate and tropical regions. Heptachlor (and the epoxide) will be prone to ageing and sequestration in soil, and therefore the bioavailability will be impacted by different soil types.
In some of the environmental samples reported, it is not certain whether the measured values given are truly due to a contamination with heptachlor or due to an experimental or calculational error.
IARC (2001) evaluated the carcinogenicity of chlordane and heptachlor in 2000 and concluded that there is inadequate evidence in humans and sufficient evidence in experimental animals for the carcinogenicity of heptachlor; the overall evaluation was that heptachlor is possibly carcinogenic to humans (Group 2B). Heptachlor was considered by previous IARC working groups in 1978 (IARC, 1979), 1987 (IARC, 1987), and 1990 (IARC, 1991).
Heptachlor was evaluated by JMPR in 1966, 1970, and 1991 (FAO/WHO, 1967, 1971; JMPR, 1992). The Committee recommended that heptachlor should not be used directly on food crops and that its use in the production of food commodities should be phased out. An ADI of 0–0.0005 mg/kg body weight was allocated in 1966 (FAO/WHO, 1967), which was changed to 0–0.0001 mg/kg body weight in 1992 (JMPR, 1992), when the Committee increased the safety factor to 200, recognizing the inadequacy of the database.
Extraneous residue limits were established by the Codex Alimentarius Commission (1997) for the sum of heptachlor and heptachlor epoxide (fat-soluble residue) in or on the following commodities (in milligrams per kilogram): 0.5 for soya bean oil (crude); 0.2 for carrots, meat (fat), and poultry meat (fat); 0.05 for eggs and vegetables (except carrots, soya beans, sugar beets, and tomatoes); 0.02 for cereal grains, cottonseed, tomatoes, soya beans (immature seeds), and soya bean oil (refined); 0.01 for citrus fruit and pineapples; and 0.006 for milk (fat soluble) (Codex Alimentarius Commission, 1997).
Heptachlor is subject to the Prior Informed Consent procedure code under the Rotterdam Convention (see http://www.pic.int/), which entered into force on 24 February 2004 (see also FAO/UNEP, 1996).
Heptachlor is prohibited or severely restricted by the Stockholm Convention on Persistent Organic Pollutants, which entered into force on 17 May 2004 (see http:// www.pops.int/).
Abou-Arab AAK, Abou Donia MA (2001) Pesticide residues in some Egyptian spices and medicinal plants as affected by processing. Food Chemistry, 72(4):439–445.
Abou-Arab AAK, Gomaa MNE, Badawy A, Nagui BK (1995) Distribution of organochlorine pesticides in the Egyptian aquatic ecosystem. Food Chemistry, 54:141–146.
Adams M, Coon FB, Poline CE (1974) Insecticides in the tissue of four generations of rats fed different dietary fats containing a mixture of chlorinated hydrocarbon insecticides. Journal of Agricultural and Food Chemistry, 22(1):69–75 [cited in Adeshina & Todd, 1991].
Adeshina F, Todd EL (1990) Organochlorine compounds in human adipose tissue from north Texas. Journal of Toxicology and Environmental Health, 29:147–156.
Adeshina F, Todd EL (1991) Application of biological data in cancer risk estimations of chlordane and heptachlor. Regulatory Toxicology and Pharmacology, 14(1):59–77.
Ahmed FE, Hart RW, Lewis JJ (1977) Pesticide induced DNA damage and its repair in cultured human cells. Mutation Research, 42:161–174.
Aigner JE, Leone DA, Falconer LR (1998) Concentrations and enantiomeric ratios of organochlorine pesticides in soils from the U.S. corn belt. Environmental Science & Technology, 32:1162–1168.
Alawi MA, Khalil MW (2002) Regional distribution of organochlorine pesticides in human breast milk from Jordan. Fresenius Environmental Bulletin, 11(11):1023–1029.
Alawi MA, Ammari N, Al-Shuraiki Y (1992) Organochlorine pesticide contaminations in human milk samples from women living in Amman, Jordan. Archives of Environmental Contamination and Toxicology, 23:235–239.
Alawi MA, Tamimi S, Jaghabir M (1999) Storage of organochlorine pesticides in human adipose tissues of Jordanian males and females. Chemosphere, 38:2865–2873.
Aldenberg T, Slob W (1993) Confidence limits for hazardous concentrations based on logistically distributed NOEC toxicity data. Ecotoxicology and Environmental Safety, 25:48–63.
Alegria HA, Bidleman TF, Shaw TJ (2000) Organochlorine pesticides in ambient air of Belize, Central America. Environmental Science & Technology, 34(10):1953–1958.
Allen RH, Gottlieb M, Clute E, Pongsiri MJ, Sherman J, Obrams GI (1997) Breast cancer and pesticides in Hawaii: the need for further study. Environmental Health Perspectives, 105(Suppl. 3):679–683.
Al-Saleh I, Echeverria-Quevedo A, Al-Dgaither S, Faris R (1998) Residue levels of organochlorinated insecticides in breast milk: a preliminary report from Al-Kharj, Saudi Arabia. Journal of Environmental Pathology, Toxicology and Oncology, 17(1):37–50.
Altschuh J, Brüggemann R, Santl H, Eichinger G, Piringer OG (1999) Henry’s law constants for a diverse set of organic chemicals: Experimental determination and comparison of estimation methods. Chemosphere, 39(11):1871–1887.
Amita-Rani BE, Krishnakumari MK (1995) Prenatal toxicity of heptachlor in albino rats. Pharmacology & Toxicology, 76(2):112–114.
Amita Rani BE, Karanth NGK, Krishnakumari MK (1992) Accumulation and embryotoxicity of heptachlor in the albino rat (Rattus norvegicus). Journal of Environmental Biology, 13(2):95–100.
Amita Rani BE, Krishnakumari MK, Karanth NGK (1993) Tissue burden of heptachlor metabolites in the albino rats fed heptachlor (Rattus norvegicus). Journal of Environmental Biology, 14(1):77–87.
Anderson DJ, Hites RA (1989) Indoor air: spatial variations of chlorinated pesticides. Atmospheric Environment, 23(9):2063–2066.
Anthony RG, Miles AK, Estes JA, Isaacs FB (1999) Productivity, diets, and environmental contaminants in nesting bald eagles from the Aleutian archipelago. Environmental Toxicology and Chemistry, 18(9):2054–2062.
ANZECC/ARMCANZ (2000) Australian and New Zealand guidelines for fresh and marine water quality. National Water Quality Management Strategy, Australian and New Zealand Environment Conservation Council, and Agriculture and Resource Management Council of Australia and New Zealand.
Arisoy M (1998) Biodegradation of chlorinated organic compounds by white-rot fungi. Bulletin of Environmental Contamination and Toxicology, 60:872–876.
Armenta-Arteaga G, Elizalde-González MP (2003) Contamination by PAHs, PCPs and heavy metals in the Mecoacan Lake estuarine water and sediments after oil spilling. Journal of Soils and Sediments, 3(1):35–40.
Armishaw P, Majewski MJ, McLay JP, Millar GR (1998) Development and certification of reference materials for residues of organochlorine and organophosphorus pesticides in beef fat ACSL CRM 1 and 2. Fresenius Journal of Analytical Chemistry, 360:630–639.
Arnold DW, Kennedy GL, Keplinger ML, Calandra JC, Calo CJ (1977) Dominant lethal studies with technical chlordane, HCS-3260, and heptachlor:heptachlor epoxide. Journal of Toxicology and Environmental Health, 2:547–555.
ATSDR (1993) Toxicological profile for heptachlor/heptachlor epoxide. Atlanta, GA, United States Department of Health and Human Services, Agency for Toxic Substances and Disease Registry, April (http://www.atsdr.cdc.gov/toxprofiles).
Ayas Z, Barlas NE, Kolankaya D (1997) Determination of organochlorine pesticide residues in various environments and organisms in Göksu Delta, Turkey. Aquatic Toxicology, 39:171–181.
Bagchi D, Bagchi M, Tang L, Stohs SJ (1997) Comparative in vitro and in vivo protein kinase C activation by selected pesticides and transition metal salts. Toxicology Letters, 91:31–37.
Baker DB, Loo S, Barker J (1991) Evaluation of human exposure to the heptachlor epoxide contamination of milk in Hawaii. Hawaii Medical Journal, 50(3):108–112.
Baker D, Yang H, Kojaku S, Inn K, Crinella F (2001) Latent neurobehavioral effects of gestational exposure to heptachlor epoxide. Epidemiology, 12(4):S96.
Baker DB, Yang H, Crinella F (2004) Neurobehavioural study of 18 year olds exposed to heptachlor epoxide during gestation. Neurotoxicology, 25(4):700 (Abstract 91).
Bakore N, John PJ, Bhatnagar P (2002) Evaluation of organochlorine insecticide residue levels in locally marketed vegetables of Jaipur City, Rajasthan, India. Journal of Environmental Biology, 23(3):247–252.
Basheer C, Lee HK, Obbard JP (2002) Determination of organochlorine pesticides in seawater using liquid-phase hollow fibre membrane microextraction and gas chromatography–mass spectrometry. Journal of Chromatography A, 968:191–199.
Baudino OM, Suero EA, Augusto M, Gimenez ME, Flores N (2003) Monitoring organochlorine pesticides in surface and ground water in San Juan Argentina. Journal of the Chilean Chemical Society, 48(2):7–12.
Beeman RW, Matsumura F (1981) Metabolism of cis- and trans-chlordane by a soil microorganism. Journal of Agricultural and Food Chemistry, 29:84–89.
Benes V, Šram R (1969) Mutagenic activity of some pesticides in Drosophila melanogaster. Industrial Medicine & Surgery, 38:442–444.
Berman E, Schlicht M, Moser V, MacPhail R (1995) A multidisciplinary approach to toxicological screening: 1. Systemic toxicity. Journal of Toxicology and Environmental Health, 45:127–143.
Bidleman TF, Jantunen LM, Harner T, Wiberg K, Wideman JL, Brice K, Su K, Falconer RL, Aigner EJ, Leone AD, Ridal JJ, Kerman B, Finizio A, Alegria H, Parkhurst WJ, Szeto SY (1998a) Chiral pesticides as tracers of air–surface exchange. Environmental Pollution, 102:43–49.
Bidleman TF, Jantunen LMM, Wiberg K, Harner T, Brice KA, Su K, Falconer RL, Leone AD, Aigner EJ, Parkhurst WJ (1998b) Soil as a source of atmospheric heptachlor epoxide. Environmental Science & Technology, 32(10):1546–1548.
Biggar JW, Riggs RL (1974) Apparent solubility of organochlorine insecticides in water at various temperatures. Hilgardia, 42(10):383–391.
Birch GF, Taylor SE (2000) Distribution and possible sources of organochlorine residues in sediments of a large urban estuary, Port Jackson, Sydney. Australian Journal of Earth Science, 47(4):749–756.
Black RW, Haggland AL, Voss FD (2000) Predicting the probability of detecting organochlorine pesticides and polychlorinated biphenyls in stream systems on the basis of land use in the Pacific northwest, USA. Environmental Toxicology and Chemistry, 19(4):1044–1054.
Bourquin AW, Alexander SK, Speidel HK, Mann JE, Fair JF (1972) Microbial interactions with cyclodiene pesticides. Developments in Industrial Microbiology, 13:264–276.
Bouwman H (2003) POPs in southern Africa. In: The handbook of environmental chemistry. Vol. 3. Part O. Persistent organic pollutants. Berlin-Heidelberg, Springer, pp. 287–320.
Bowman MC, Schechter MS, Carter RL (1965) Behavior of chlorinated insecticides in a broad spectrum of soil types. Journal of Agricultural and Food Chemistry, 13:360–365.
Brown DP (1992) Mortality of workers employed at organochlorine pesticide manufacturing plants — An update. Scandinavian Journal of Work, Environment and Health, 18:155–161.
Brown L, Blair A, Gibson R, Everett GD, Cantor KP, Schuman LM, Burmeister LF, Van-Lier SF, Dick F (1990) Pesticide exposures and other agricultural risk factors for leukemia among men in Iowa and Minnesota. Cancer Research, 50:6585–6591.
Burch TA (1983) An investigation of the possible effect of heptachlor contamination of milk on pregnancy outcome of Oahu women residents. Honolulu, HI, Hawaii State Department of Health, Research and Statistics Office (R & S Report, Vol. 46) [cited in Baker et al., 1991].
Buser H-R, Müller MD (1993) Enantioselective determination of chlordane components, metabolites, and photoconversion products in environmental samples using chiral high-resolution gas chromatography and mass spectrometry. Environmental Science & Technology, 27:1211–1220.
Cañas JE, Anderson TA (2002) Organochlorine contaminants in eggs: the influence of contaminated nest material. Chemosphere, 47:585–589.
Cantor KP, Blair A, Everett G, Gibson R, Burmeister LF, Brown LF, Schuman L, Dick FR (1992) Pesticides and other agricultural risk factors for non-Hodgkin’s lymphoma among men in Iowa and Minnesota. Cancer Research, 52:2447–2455.
Cantor KP, Strickland PT, Brock JW, Bush D, Helzlsouer K, Needham LL, Hoar Zahm S, Comstock GW, Rothman N (2003) Risk of non-Hodgkin’s lymphoma and prediagnostic serum organochlorines: beta-hexachlorocyclohexane, chlordane/ heptachlor-related compounds, dieldrin, and hexachlorobenzene. Environmental Health Perspectives, 111(2):179–183.
Carvalho FP, Montenegro-Guillen S, Villeneuve J-P, Cattini C, Bartocci J, Lacayo M, Cruz A (1999) Chlorinated hydrocarbons in coastal lagoons of the Pacific coast of Nicaragua. Archives of Environmental Contamination and Toxicology, 36:132–139.
Castillo JE, De La Cruz E, Ruepert C (1997) Ecotoxicology and pesticides in tropical aquatic ecosystems of central America. Environmental Toxicology and Chemistry, 16(1):41–51.
CEPA (2000) Sampling for pesticide residues in California well water — 1998 update of the well inventory database. Sacramento, CA, California Environmental Protection Agency, Department of Pesticide Regulation, pp. 1–68 (EH00-03).
Chapman P, Fairbrother A, Brown D (1998) A critical evaluation of safety (uncertainty) factors for ecological risk assessment. Environmental Toxicology and Chemistry, 17:99–108.
Chapman RA, Cole CM (1982) Observations on the influence of water and soil pH on the persistence of insecticides. Journal of Environmental Science and Health, Part B, 17(5):487–504.
Chuang LF, Chuang RY (1991) The effects of the insecticide heptachlor on ras proto-oncogene expression in human myeloblastic leukemia (ML-I) cells. Toxicology, 70:283–292.
Chuang LF, Chuang RY (1998) Heptachlor and the mitogen-activated protein kinase module in human lymphocytes. Toxicology, 128:17–23.
Chuang LF, Liu Y, Killam K, Chuang RY (1992) Modulation by the insecticides heptachlor and chlordane of the cell-mediated immune proliferative responses of rhesus monkeys. In Vivo, 6:29–32.
Chuang LF, Rought SE, Chuang RY (1999) Differential regulation of the major cyclin-dependent kinases, cdk2 and cdc2, during cell cycle progression in human lymphocytes exposed to heptachlor. In Vivo, 13:455–461.
Codex Alimentarius Commission (1997) Codex maximum limits for pesticide residues. Rome, Food and Agriculture Organization of the United Nations.
Cok I, Bilgili A, Özdemir M, Özbek H, Bilgili N, Burgaz S (1997) Organochlorine pesticide residues in human breast milk from agricultural regions of Turkey. Bulletin of Environmental Contamination and Toxicology, 59:577–582.
Cok I, Bilgili A, Yarsan E, Bagci C, Burgaz S (1998) Organochlorine pesticide residue levels in human adipose tissue of residents of Manisa (Turkey), 1995–1996. Bulletin of Environmental Contamination and Toxicology, 61:311–316.
Connell DW, Miller G, Anderson S (2002) Chlorohydrocarbon pesticides in the Australian marine environment after banning in the period from the 1970s to 1980s. Marine Pollution Bulletin, 45:78–83.
Crebelli R, Bellincampi D, Conti G, Conti L, Morpurgo G, Carere A (1986) A comparative study on selected chemical carcinogens for chromosome malsegregation, mitotic crossing-over and forward mutation induction in Aspergillus nidulans. Mutation Research, 172:139–149.
Crum JA, Bursian SJ, Aulerich RJ, Polin D, Braselton WE (1993) The reproductive effects of dietary heptachlor in mink (Mustela vison). Archives of Environmental Contamination and Toxicology, 24:156–164.
CU (1999) Do you know what you’re eating? An analysis of U.S. Government data on pesticides in foods. Yonkers, NY, Consumers Union of United States, Inc., February, pp. 1–78 (http://www.consumersunion.org/food/do_you_know2.htm).
Custer TW, Hines RK, Melancon MJ, Hoffman DJ, Wickliffe JK, Bickham JW, Martin JW, Henshel DS (1997a) Contaminant concentrations and biomarker response in great blue heron eggs from 10 colonies on the upper Mississippi river, USA. Environmental Toxicology and Chemistry, 16(2):260–271.
Custer TW, Custer CM, Stromborg KL (1997b) Distribution of organochlorine contaminants in double-crested cormorant eggs and sibling embryos. Environmental Toxicology and Chemistry, 16(8):1646–1649.
Das B, Kahn YSA, Das P, Shaheen SM (2002) Organochlorine pesticide residues in catfish, Tachysrus thalassinus (Ruppel, 1835) from the South Patches of the Bay of Bengal. Environmental Pollution, 120(2):255–259.
Dello Iacovo R, Celentano E, Strollo AM, Iazzetta G, Capasso I, Randazzo G (1999) Organochlorines and breast cancer. A study on Neapolitan women. Advances in Experimental Medicine and Biology, 472:57–66.
D’Ercole AJ, Arthur RD, Cain JD, Barrentine BF (1976) Insecticide exposure of mothers and newborns in a rural agricultural area. Pediatrics, 57(6):869–874.
de Solla SR, Bishop CA, Brooks RJ (2002) Sexually dimorphic morphology of hatchling snapping turtles (Chelydra serpentina) from contaminated and reference sites in the Great Lakes and St. Lawrence River basin, North America. Environmental Toxicology and Chemistry, 21(5):922–929.
Diop YM, Diouf A, Fall M, Thiam A, Ndiaye B, Ciss M, Ba D (1999) Pesticide bioaccumulation: measurement and levels of organochlorine residues in products of vegetable origin. Dakar Médical, 44(2):153–157.
Ditraglia D, Brown DP, Namekata T, Iverson N (1981) Mortality study of workers employed at organochlorine pesticide manufacturing plants. Scandinavian Journal of Work, Environment and Health, 7(Suppl. 4):140–146.
Dorgan JF, Brock JW, Rothman N, Needham LL, Miller R, Stephenson HE, Schussler N, Taylor PR (1999) Serum organochlorine pesticides and PCBs and breast cancer risk: results from a prospective analysis (USA). Cancer Causes and Control, 10(1):1–11.
Duggan RE, Corneliussen PE (1972) Dietary intake of pesticide chemicals in the United States (III), June 1968 – April 1970. Pesticides Monitoring Journal, 5:331–341.
Eaton HJ, Lydy MJ (2000) Assessment of water quality in Wichita, Kansas, using an index of biotic integrity and analysis of bed sediment and fish tissue for organochlorine insecticides. Archives of Environmental Contamination and Toxicology, 9(4):531–540.
ECETOC (2003) Aquatic hazard assessment II. Brussels, European Centre for Ecotoxicology and Toxicology of Chemicals (Technical Report No. 91).
Eisler R (1969) Acute toxicities of insecticides to marine decapod crustaceans. Crustaceana, 16(3):302–310.
Eisler R (1970) Acute toxicities of organochlorine and organophosphorus insecticides to estuarine fishes. Washington, DC, United States Department of the Interior, Bureau of Sport Fisheries, pp. 3–20 (Wildlife Technical Paper No. 45).
El-Kabbany S, Rashed MM, Zayed MA (2000) Monitoring of the pesticide levels in some water supplies and agricultural land, in El-Haram, Giza (ARE). Journal of Hazardous Materials, 72(1):11–21.
Epstein SS (1976) Carcinogenicity of heptachlor and chlordane. Science of the Total Environment, 6:103–154.
Epstein SS, Arnold E, Andrea J, Bass W, Bishop Y (1972) Detection of chemical mutagens by the dominant lethal assay in the mouse. Toxicology and Applied Pharmacology, 23:288–325.
Falandysz J (2003) [The use amount of chlordane, heptachlor and heptachlor epoxide in Poland.] Roczniki Panstwowego Zakladu Higieny, 54(1):49–64.
Falandysz J, Strandberg L, Puzyn T, Gucia M (2001) Chlorinated cyclodiene pesticide residues in blue mussel, crab, and fish in the Gulf of Gdansk, Baltic Sea. Environmental Science & Technology, 35:4163–4169.
Falck F, Ricci A, Wolff MS, Godbold J, Deckers P (1992) Pesticides and polychlorinated biphenyl residues in human breast lipids and their relation to breast cancer. Archives of Environmental Health, 47(2):143–146.
FAO (1999) Obsolete pesticides: Problems, prevention and disposal. Rome, Food and Agriculture Organization of the United Nations.
FAO/UNEP (1996) Prior informed consent decision guidance documents: Heptachlor. Geneva, Food and Agriculture Organization of the United Nations and United Nations Environment Programme, Rotterdam Convention on the Prior Informed Consent Procedures for Certain Hazardous Chemicals and Pesticides in International Trade (http://www.fao.org/ag/agp/ agpp/pesticid).
FAO/WHO (1967) Heptachlor. In: Evaluation of some pesticide residues in food. Report of a Joint Meeting of the FAO Working Party and the WHO Expert Committee on Pesticide Residues. Rome, Food and Agriculture Organization of the United Nations, pp. 1–13 (FAO Agricultural Studies 066; http://www.inchem.org/ documents/jmpr/jmpmono/v66apr11.htm).
FAO/WHO (1971) Heptachlor. In: 1970 evaluations of some pesticide residues in food. Joint Meeting of the FAO Working Party of Experts and the WHO Expert Group on Pesticide Residues. Rome, Food and Agriculture Organization of the United Nations, pp. 1–2 (AGP:1970/M/12/1; INCHEM No. 193; http://www.inchem.org/documents/jmpr/jmpmono/v070pr16.htm).
Fauchey V, Jaber M, Bloch BLM (2000) Dopamine control of striatal gene expression during development: Relevance to knockout mice for the dopamine transporter. European Journal of Neuroscience, 12:3415–3425.
Fendick EA, Mather-Mihaich E, Houck KA, St Clair MB, Faust JB, Rockwell CH, Owens M (1990) Ecological toxicology and human health effects of heptachlor. Reviews in Environmental Contamination and Toxicology, 111:61–142.
Feroz M, Khan MAQ (1979) Metabolism of 14C-heptachlor in goldfish (Carassius auratus). Archives of Environmental Contamination and Toxicology, 8:519–531.
Feroz M, Podowski AA, Khan MAQ (1990) Oxidative dehydrochlorination of heptachlor by Daphnia magna. Pesticide Biochemistry and Physiology, 36:101–105.
Fischman AJ, Bonab AA, Babich JW, Palmer EP, Alpert NM, Elmaleh DR, Callahan RJ, Barrow SA, Graham W, Melzer PC, Hanson RN, Madras BK (1998) Rapid detection of Parkinson’s disease by SPECT with altropane: A selective ligand for dopamine transporters. Synapse, 29(2):128–141.
Fisk TA, Holst M, Hobson AK, Duffe J, Moisey J, Norstrom JR (2002) Persistent organochlorine contaminants and enantiomeric signature of chiral pollutants in ringed seals (Phoca hispida) collected on the east and west side of the Northwater Polynya, Canadian Arctic. Archives of Environmental Contamination and Toxicology, 42:118–126.
Gant DB, Eldefrawi ME, Eldefrawi AT (1987) Cyclodiene insecticides inhibit GABAA receptor-regulated chloride transport. Toxicology and Applied Pharmacology, 88:313–321.
Gentile JM, Gentile G, Bultman J, Sechriest R, Wagner E, Plewa M (1982) An evaluation of the genotoxic properties of insecticides following plant and animal activation. Mutation Research, 101:19–29.
Gladen BC, Shkiryak-Nyzhnyk ZA, Chyslovska N, Zadorozhnaja TD, Little RE (2003) Persistent organochlorine compounds and birth weight. Annals of Epidemiology, 13(3):151–157.
Gonzalez M, Miglioranza KSB, Aizpún de Moreno JE, Moreno VJ (2003) Occurrence and distribution of organochlorine pesticides (OCPs) in tomato (Lycopersicon esculentum) crops from organic production. Journal of Agricultural and Food Chemistry, 51(5):1353–1359.
González-Farias F, Cisneros Estrada X, Fuentes Ruis C, Diaz González G, Botello AV (2002) Pesticides distribution in sediments of a tropical coastal lagoon adjacent to an irrigation district in northwest Mexico. Environmental Technology, 23(11):1247–1256.
Grafton-Wasserman DA (1988) The incidence of neonatal jaundice and abnormal pregnancy outcomes in relation to in utero heptachlor exposure [Doctoral dissertation]. University of Hawaii, Department of Epidemiology [cited in Baker et al., 1991].
Gregor DJ, Gummer WD (1989) Evidence of atmospheric transport and deposition of organochlorine pesticides and polychlorinated biphenyls in Canadian Arctic snow. Environmental Science & Technology, 23:561–565.
Griffin III DE, Hill WE (1978) In vitro breakage of plasmid DNA by mutagens and pesticides. Mutation Research, 52:161–169.
Grynkiewicz M, Polkowska Z, Gorecki T, Namiesnik J (2001) Pesticides in precipitation in the Gdansk region (Poland). Chemosphere, 43:303–312.
Gunderson EL (1988) FDA Total Diet Study, April 1982 – April 1984, dietary intakes of pesticides, selected elements, and other chemicals. Journal of the Association of Official Analytical Chemists, 71:1200–1209.
Gunderson EL (1995) FDA Total Diet Study, July 1986 – April 1991, dietary intakes of pesticides, selected elements, and other chemicals. Journal of AOAC International, 78:1353–1363.
Gunz D, Shephard SE, Lutz WK (1993) Can nongenotoxic carcinogens be detected with the lacI transgenic mouse mutation assay? Environmental and Molecular Mutagenesis, 21:209–211.
Hamers T, van den Brink PJ, Mos L, van der Linden SC, Legler J, Koeman JH, Murk AJ (2003) Estrogenic and esterase- inhibiting potency in rainwater in relation to pesticide concentrations, sampling season and location. Environmental Pollution, 123:47–65.
Hansen DJ, Parrish PR (1977) Suitability of sheepshead minnows (Cyprinodon variegatus) for life-cycle toxicity tests. In: Mayer FL, Hamelink JL, eds. Aquatic toxicology and hazard evaluation. Philadelphia, PA, American Society for Testing and Materials, pp. 117–126 (ASTM Special Technical Publication 634).
Hansen ME, Matsumura F (2001a) Down-regulation of particulate protein kinase C epsilon and up-regulation of nuclear activator protein-1 DNA binding in liver following in vivo exposure of B6C3F1 male mice to heptachlor epoxide. Journal of Biochemical and Molecular Toxicology, 15(1):1–14.
Hansen ME, Matsumura F (2001b) Effects of heptachlor epoxide on components of various signal transduction pathways important in tumor promotion in mouse hepatoma cells. Determination of the most sensitive tumor promoter related effect induced by heptachlor epoxide. Toxicology, 160(1–3):139–153.
Henderson C, Pickering QH, Tarzwell CM (1959) Relative toxicity of ten chlorinated hydrocarbon insecticides to four species of fish. Transactions of the American Fisheries Society, 88:23–32.
Hertz-Picciotto I, Greenfield T, Teplin S, James-Baker R, Charles MJF (2003) Prenatal heptachlor epoxide exposure and cognitive development. Epidemiology, 14(Suppl. 5):88S–89S (abstract).
Hertz-Picciotto I, Greenfield T, Teplin S, James-Baker R, Charles MJF (2004) Prenatal exposure to heptachlor epoxide and early childhood development. Neurotoxicology, 25(4):701 (Abstract 92).
Hill DW, McCarty PL (1967) Anaerobic degradation of selected chlorinated hydrocarbon pesticides. Journal of the Water Pollution Control Federation, 39(8):1259–1277.
Hofelt CS, Shea D (1997) Accumulation of the organochlorine pesticides and PCBs by semipermeable membrane devices and Mytilus edulis in New Bedford Harbor. Environmental Science & Technology, 31(1):154–159.
Hoffman JS (1985) The effects of prenatal heptachlor exposure on infant development [Doctoral dissertation]. University of Hawaii [cited in Baker et al., 1991].
Holcombe GW, Phipps GL, Fiandt JT (1983) Toxicity of selected priority pollutants to various aquatic organisms. Ecotoxicology and Environmental Safety, 7:400–409.
Høyer AP, Grandjean P, Jorgensen T, Brock JW, Hartvig HB (1998) Organochlorine exposure and risk of breast cancer. Lancet, 352:1816–1820.
Høyer AP, Jorgensen T, Grandjean P, Hartvig H (2000) Repeated measurements of organochlorine exposure and breast cancer risk (Denmark). Cancer Causes and Control, 11:177–184.
Huggett DB, Block DS, Khan IA, Allgood JC, Benson WH (2000) Environmental contaminants in the botanical dietary supplement ginseng and potential human risk. Human and Ecological Risk Assessment, 6(5):767–776.
Hung DQ, Thiemann W (2002) Contamination by selected chlorinated pesticides in surface waters in Hanoi, Vietnam. Chemosphere, 47:357–367.
Hwang B-H, Lee M-R (2000) Solid-phase microextraction for organochlorine pesticide residues analysis in Chinese herbal formulations. Journal of Chromatography A, 898:245–256.
IARC (1979) Some halogenated hydrocarbons. Lyon, International Agency for Research on Cancer, pp. 45–65 (IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Humans, Vol. 20).
IARC (1987) Overall evaluations of carcinogenicity: an updating of IARC monographs, Vols. 1 to 42. Lyon, International Agency for Research on Cancer, pp. 146–148 (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Supplement 7).
IARC (1991) Chlordane and heptachlor. In: Occupational exposures in insecticide application, and some pesticides. Lyon, International Agency for Research on Cancer, pp. 115–175 (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 53).
IARC (2001) Chlordane and heptachlor. In: Some thyrotropic agents. Lyon, International Agency for Research on Cancer, pp. 411–493 (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 79).
IPCS (1984) Heptachlor. Geneva, World Health Organization, International Programme on Chemical Safety, pp. 1–55 (Environmental Health Criteria 38).
IPCS (2003) International Chemical Safety Card — Heptachlor. Geneva, World Health Organization, International Programme on Chemical Safety (ICSC 0743).
IRDC (1973) Unpublished report by the International Research and Development Corporation under contract to the Velsicol Chemical Corporation [cited in Epstein, 1976].
Iyengar L, Rao AV (1973) Metabolism of chlordane and heptachlor by Aspergillus niger. Journal of General and Applied Microbiology, 19:321–324.
Jabber SA, Khan YSA, Rahman MS (2001) Levels of organochlorine pesticide residues in some of the Ganges perch, Lates calcifer, from the Ganges–Brahmaputra–Meghna Estuary, Bangladesh. Marine Pollution Bulletin, 42(12):1291–1296.
Jantunen LMM, Bidleman TF (1998) Organochlorine pesticides and enantiomers of chiral pesticides in Arctic Ocean water. Archives of Environmental Contamination and Toxicology, 35:218–228.
Jantunen LMM, Bidleman TF, Harner T, Parkhurst WJ (2000) Toxaphene, chlordane, and other organochlorine pesticides in Alabama air. Environmental Science & Technology, 34(24):5097–5105.
JMPR (1992) Heptachlor. In: Pesticide residues in food: 1991 evaluations. Part II — Toxicology. Geneva, World Health Organization, Joint FAO/WHO Meeting on Pesticide Residues, pp. 1–24 (WHO/PCS/92.52; INCHEM No. 829; http:// www.inchem.org/documents/jmpr/jmpmono/v91pr13.htm).
John PJ, Bakore N, Bhatnagar P (2001) Assessment of organochlorine pesticide residue levels in dairy milk and buffalo milk from Jaipur City, Rajasthan, India. Environment International, 26:231–236.
Johnson B (1991) Setting revised specific numerical values; persuant to the Pesticide Contamination Prevention Act. Sacramento, CA, State of California Department of Food and Agriculture, pp. 1–17 (PB 91-143 130).
Kajiwara N, Ueno D, Monirith I, Tanabe S, Pourkazemi M, Aubrey DG (2003) Contamination by organochlorine compounds in sturgeons from Caspian Sea during 2001 and 2002. Marine Pollution Bulletin, 46(6):741–747.
Kamarianos A, Karamanlis X, Goulas P, Theodosiadou E, Smokovitis A (2003a) The presence of environmental pollutants in the follicular fluid of farm animals (cattle, sheep, goats, and pigs). Reproductive Toxicology, 17(2):185–190.
Kamarianos A, Karamanlis X, Theodosiadou E, Goulas P, Smokovitis A (2003b) The presence of environmental pollutants in the semen of farm animals (bull, ram, goat, and boar). Reproductive Toxicology, 17(4):439–445.
Kamble ST, Ogg CL, Gold RE, Vance AD (1992) Exposure of applicators and residents to chlordane and heptachlor when used for subterranean termite control. Archives of Environmental Contamination and Toxicology, 22:253–259.
Kannan K, Tanabe S, Williams R, Tatsukawa R (1994) Persistent organochlorine residues in foodstuffs from Australia, Papua New Guinea and the Solomon Islands: Contamination levels and human exposure. Science of the Total Environment, 153:29–49.
Kannan K, Tanabe S, Giesy JP, Tatsukawa R (1997) Organochlorine pesticides and polychlorinated biphenyls in foodstuffs from Asian and Oceanic countries. Reviews in Environmental Contamination and Toxicology, 152:1–55.
Karlsson H, Muir DCG, Teixiera CF, Burniston DA, Strachan WMJ, Hecky RE, Mwita J, Bootsma HA, Grift NP, Kidd KA, Rosenberg B (2000a) Persistent chlorinated pesticides in air, water, and precipitation from the Lake Malawi area, southern Africa. Environmental Science & Technology, 34:4490–4495.
Karlsson H, Oehme M, Skopp S, Burkow IC (2000b) Enantiomer ratios of chlordane congeners are gender specific in cod (Gadus morhua) from the Barents Sea. Environmental Science & Technology, 34:2126–2130.
Kashimoto T, Takayama K, Mimura M, Miyata H, Murakami Y, Matsumoto H (1989) Eighth international symposium on chlorinated dioxins and related compounds, Umea, Sweden, August 21–26, 1988. Chemosphere, 19:921–926.
Katsoyiannis A, Samara C (2004) Persistent organic pollutants (POPs) in the sewage treatment plant of Thessaloniki, northern Greece: occurrence and removal. Water Research, 38(11):2685–2698.
Kennedy DW, Aust SD, Bumpus JA (1990) Comparative biodegradation of alkyl halide insecticides by the white-rot fungus Phanerochaete chrysosporium (BKM-F-1767). Applied Environmental Microbiology, 56:2347–2353.
Khaled A, El Nemr A, Said TO, El-Sikaily A, Abd-Alla AMA (2004) Polychlorinated biphenyls and chlorinated pesticides in mussels from the Egyptian Red Sea coast. Chemosphere, 54(10):1407–1412.
Kim J-H, Smith A (2001) Distribution of organochlorine pesticides in soils from South Korea. Chemosphere, 43(2):137–140.
Kirby ML, Barlow RL, Bloomquist JR (2001) Neurotoxicity of the organochlorine insecticide heptachlor to murine striatal dopaminergic pathways. Toxicological Sciences, 61(1):100–106.
Klein W, Korte F, Weisgerber I, Kaul R, Mueller W (1968) [The metabolism of endrin, heptachlor, and telodrin.] Qualitas Plantarum et Materiae Vegetabiles, 15:225–238 (in German).
Klemens JA, Wieland Ml, Flanagin VJ, Frick JA, Harper RG (2003) A cross-taxa survey of organochlorine pesticide contamination in a Costa Rican wildland. Environmental Pollution, 122:245–251.
Kucklick JR, Struntz WDJ, Becker PR, York GW, O’Hara TM, Bohonowych JE (2002) Persistent organochlorine pollutants in ringed seals and polar bears collected from northern Alaska. Science of the Total Environment, 287:45–59.
Kucklick JR, Baker EJ (1998) Organochlorines in Lake Superior’s food web. Environmental Science & Technology, 32:1192–1198.
Kurata M, Hirose K, Umeda M (1982) Inhibition of metabolic cooperation in Chinese hamster cells by organochlorine pesticides. Gann, 73(2):217–221.
Kutz FW, Wood PH, Bottimore DP (1991) Organochlorine pesticides and polychlorinated biphenyls in human adipose tissue. Reviews in Environmental Contamination and Toxicology, 120:1–82.
Lawson G, Luderer U (2004) Gestational and lactational exposure to heptachlor does not alter reproductive system development in rats. Veterinary and Human Toxicology, 46(3):113–118.
Lehman AJ (1952) Quarterly Bulletin of the Association of Food and Drug Officials of the United States, 16:126 [cited in FAO/WHO, 1967].
Leigh GM (1969) Degradation of selected chlorinated hydrocarbon insecticides. Journal of the Water Pollution Control Federation, 41:R450–R460.
Le Marchand L, Kolonel LN, Siegel BZ, Dendle WH (1986) Trends in birth defects for a Hawaiian population exposed to heptachlor and for the United States. Archives of Environmental Health, 41:145–148.
Leone AD, Ulrich EM, Bodnar CE, Falconer RL, Hites RA (2000) Organochlorine pesticide concentrations and enantiomer fractions for chlordane in indoor air from the US cornbelt. Atmospheric Environment, 34:4131–4138.
Lewis MA, Scott GI, Bearden DW, Quarles RL, Moore J, Strozier ED, Sivertsen SK, Dias AR, Sanders M (2002) Fish tissue quality in near-coastal areas of the Gulf of Mexico receiving point source discharges. Science of the Total Environment, 284(1–3):249–261.
Louie PK, Sin DW (2003) A preliminary investigation of persistent organic pollutants in ambient air in Hong Kong. Chemosphere, 52(9):1397–1403.
Lu P-Y, Metcalf RL, Hirwe AS, Williams JW (1975) Evaluation of environmental distribution and fate of hexachlorocyclopentadiene, chlordene, heptachlor and heptachlor epoxide in a laboratory model ecosystem. Journal of Agricultural and Food Chemistry, 23:967–973.
Lu Y, Wang Z (2002) Bioconcentration of trace organochlorine pesticides by the rainbow trout. Journal of Environmental Science and Health, Part A, 37(4):529–539.
Luster M, Portier C, Pait D, White K, Gennings C, Munson A, Rosenthal G (1992) Risk assessment in immunotoxicology — I. Sensitivity and predictability of immune tests. Fundamental and Applied Toxicology, 18:200–210.
Macek KJ, Lindberg MA, Sauter S, Buxton KS, Costa PA (1976) Toxicity of four pesticides to water fleas and fathead minnows: Acute and chronic toxicity of acrolein, heptachlor, endosulfan, and trifluralin to the water flea (Daphnia magna) and the fathead minnow (Pimephales promelas). Duluth, MN, United States Environmental Protection Agency (EPA-600/3-76-099).
MacMahon B, Monson RR, Wang HH, Zheng T (1988) A second follow-up of mortality in a cohort of pesticide applicators. Journal of Occupational Medicine, 30:429–432.
Manosa S, Mateo R, Freixa C, Guitart R (2003) Persistent organochlorine contaminants in eggs of northern goshawk and Eurasian buzzard from northeastern Spain: temporal trends related to changes in the diet. Environmental Pollution, 122(3):351–359.
Mansour M, Parlar H (1978) Gas chromatographic determination of several cyclodiene insecticides in the presence of polychlorinated biphenyls by photoisomerization reactions. Journal of Agricultural and Food Chemistry, 26:483–485.
Marshall TC, Dorough HW, Swim HE (1976) Screening of pesticides for mutagenic potential using Salmonella typhimurium mutants. Journal of Agricultural and Food Chemistry, 24(3):560–563.
Maslansky CJ, Williams GM (1981) Evidence for an epigenetic mode of action in organochlorine pesticide hepatocarcinogenicity: a lack of genotoxicity in rat, mouse and hamster hepatocytes. Journal of Toxicology and Environmental Health, 8:121–130.
Massone EH, Martinez ED, Cionchi LJ, Bocanegra E (1998) Suburban areas in developing countries and their relationship to groundwater pollution: A case study of Mar del Plata, Argentina. Environmental Management, 22(2):245–254.
Mastri C, Keplinger ML, Fancher OE (1969) Acute oral toxicity study on 4 chlordenes in albino rats. Report prepared by Industrial Bio-Test Laboratories for Velsicol Chemical Corporation, Rosemont, IL [cited in IPCS, 1984].
Matesic DF, Rupp HL, Bonney WJ, Ruch RJ, Trosko JE (1994) Changes in gap-junction permeability, phosphorylation, and number mediated by phorbol ester and non-phorbol-ester tumor promoters in rat liver epithelial cells. Molecular Carcinogenesis, 10:226–236.
Mathur V, Bhatnagar P, Sharma RG, Acharya V, Sexana R (2002) Breast cancer incidence and exposure to pesticides among women originating from Jaipur. Environment International, 28(5):331–336.
Matsui S, Yamamoto R, Yamada H (1989) The Bacillus subtilis/ microsome rec-assay for the detection of DNA damaging substances which may occur in chlorinated and ozonated waters. Water Science and Technology, 21:875–887.
Mayer FL (1987) Acute toxicity handbook of chemicals to estuarine organisms. Gulf Breeze, TX, United States Environmental Protection Agency, Office of Research and Development, Environmental Research Laboratory (EPA/600/8-87/017; PB87 188686).
Mayer FL, Ellersieck MR (1986) Manual of acute toxicity: Interpretation and data base for 410 chemicals and 66 species of freshwater animals. Washington, DC, United States Department of the Interior, Fish and Wildlife Service, 506 pp. (Resource Publication 160).
McGregor DB, Brown A, Cattanach P, Edwards I, McBride D, Riach C, Caspary WJ (1988) Responses of the L5178Y tk+/tk− mouse lymphoma cell forward mutation assay: III. 72 coded chemicals. Environmental and Molecular Mutagenesis, 12:85–154.
Meador JP, Adams NG, Casillas E, Bolton JL (1997) Comparative bioaccumulation of chlorinated hydrocarbons from sediment by two infaunal invertebrates. Archives of Environmental Contamination and Toxicology, 33:388–400.
Meijer SN, Shoeib M, Jantunen LMM, Jones KC, Harner T (2003) Air–soil exchange of organochlorine pesticides in agricultural soils. 1. Field measurements using a novel in situ sampling device. Environmental Science & Technology, 37(7):1292–1299.
Menone ML, Aizpun de Moreno JE, Moreno VJ, Lanfranchi AL, Metcalfe TL, Metcalfe CD (2001) Organochlorine pesticides and PCBs in a southern Atlantic coastal lagoon watershed, Argentina. Archives of Environmental Contamination and Toxicology, 40:355–362.
Mersch-Sundermann V, Dickgiesser N, Hablizel U, Gruber B (1988) [Examination of mutagenicity of organic microcontaminations on the environment. I. Communication: The mutagenicity of selected herbicides and insecticides with Salmonella-microsome-test (Ames-test) in consideration of the pathogenic potence of contaminated ground- and drinking-water.] Zentralblatt für Bakteriologie, Mikrobiologie und Hygiene B, 186:247–260 (in German).
Mes J, Davies DJ, Doucet J, Weber D, McMullen E (1993) Levels of chlorinated hydrocarbon residues in Canadian human milk and their relationship to some characteristics of the donors. Food Additives and Contaminants, 10(4):429–441.
Mestitzova M (1966) Wirkung des cyklodienischen Insektizids Heptachlor auf die Reproduktionsfähigkeit von weißen Ratten (C IX - 7). In: Proceedings of the XVth International Congress of Occupational Health, pp. 455–458.
Mestitzova M (1967) On reproduction studies and the occurrence of cataracts in rats after long-term feeding of the insecticide heptachlor. Experientia, 23:42–43.
Miglioranza KSB, Aizpun de Moreno JE, Moreno VJ (2003) Dynamics of organochlorine pesticides in soils from a southeastern region of Argentina. Environmental Toxicology and Chemistry, 22(4):712–717.
Miles JRW, Tu CM, Harris CR (1969) Metabolism of heptachlor and its degradation products by soil microorganisms. Journal of Economic Entomology, 62:1334–1338.
Miles JRW, Tu CM, Harris CR (1971) Degradation of heptachlor epoxide and heptachlor by a mixed culture of soil microorganisms. Journal of Economic Entomology, 64:839–841.
Miller GW, Kirby ML, Levey AI, Bloomquist JR (1999) Heptachlor alters expression and function of dopamine transporters. Neurotoxicology, 20:631–637.
Mills PK, Yang R (2003) Prostate cancer risk in California farm workers. Journal of Occupational and Environmental Medicine, 45(3):249–258.
MITI (1992) Biodegradation and bioaccumulation: Data of existing chemicals based on the CSCL Japan. Tokyo, Ministry of International Trade & Industry, October, pp. 2–19.
Miyagi T, Lam K, Chuang LF, Chuang RY (1998) Suppression of chemokine-induced chemotaxis of monkey neutrophils and monocytes by chlorinated hydrocarbon insecticides. In Vivo, 12(5):441–446.
Moriya M, Ohta T, Watanabe K, Miyazawa T, Kato K, Shirasu Y (1983) Further mutagenicity studies on pesticides in bacterial reversion assay systems. Mutation Research, 116:185–216.
Moser VC, Cheek BM, MacPhail RC (1995) A multidisciplinary approach to toxicological screening: III. Neurobehavioral toxicity. Journal of Toxicology and Environmental Health, 45:173–210.
Moser VC, Shafer TJ, Ward TR, Meacham CA, Harris MW, Chapin RE (2001) Neurotoxicological outcomes of perinatal heptachlor exposure in the rat. Toxicological Sciences, 60(2):315–326.
Mossing ML, Redetzke KA, Applegate HG (1985) Organochlorine pesticides in blood of persons from El Paso, Texas. Journal of Environmental Health, 47:312–313.
Muir DCG, Jones PD, Karlsson H, Koczansky K, Stern GA, Kannan K, Ludwig JP, Reid H, Robertson CJR, Giesy JP (2002) Toxaphene and other persistent organochlorine pesticides in three species of albatrosses from the North and South Pacific Ocean. Environmental Toxicology and Chemistry, 21(2):413–423.
Muir D, Savinova T, Savinov V, Alexeeva L, Potelov V, Svetochev V (2003) Bioaccumulation of PCB’s and chlorinated pesticides in seals, fishes and invertebrates from the White Sea, Russia. Science of the Total Environment, 306(1–3):111–131.
Munshi AB, Jamil K, Zuberi R (2001) Pesticide residues in food available for human consumption. Journal of the Chemical Society of Pakistan, 23(2):95–97.
Mussalo-Rauhamaa H, Hasanen E, Pyysalo H, Antervo K, Kauppila Pantzar P (1990) Occurrence of beta-hexachlorocyclohexane in breast cancer patients. Cancer, 66(10):2124–2128.
Mussalo-Rauhamaa H, Pyysalo H, Antervo K (1991) Heptachlor, heptachlor epoxide and other chlordane compounds in Finnish plywood workers. Archives of Environmental Health, 46(6):340–346.
Nakamura Y, Tonogai Y, Sekiguchi Y, Tsumura Y, Nishida N, Takakura K, Isechi M, Yuasa K, Nakamura M, Kifune N, Yamamoto K, Terasawa S, Oshima T, Miyata M, Kamakura K, Ito Y (1994) Multiresidue analysis of 48 pesticides in agricultural products by capillary gas chromatography. Journal of Agricultural and Food Chemistry, 42:2508–2518.
Narotsky MG, Kavlock RJ (1995) A multidisciplinary approach to toxicological screening: II. Developmental toxicity. Journal of Toxicology and Environmental Health, 45:145–171.
Narotsky MG, Weller EA, Chinchilli VM, Kavlock RJ (1995) Nonadditive developmental toxicity in mixtures of trichloroethylene, di(2-ethylhexyl) phthalate, and heptachlor in a 5×5×5 design. Fundamental and Applied Toxicology, 27(2):203–216.
NAS (1977) An evaluation of the carcinogenicity of chlordane and heptachlor. Washington, DC, National Academy of Sciences.
NCI (1977) Bioassay of heptachlor for possible carcinogenicity. CAS No.: 76-44-8. Bethesda, MD, National Institutes of Health, National Cancer Institute, pp. 1–109 (PB-271 967).
Nisbet ICT (1986) Human exposure to chlordane, heptachlor, and their metabolites. Washington, DC, United States Environmental Protection Agency [cited in Adeshina & Todd, 1991].
Nomata K, Kang K-S, Hayashi T, Matesic D, Lockwood L, Chang CC, Trosko JE (1996) Inhibition of gap junctional intercellular communication in heptachlor- and heptachlor epoxide–treated normal human breast epithelial cells. Cell Biology and Toxicology, 12:69–78.
Ntow WJ (2001) Organochlorine pesticides in water, sediment, crops, and human fluids in a farming community in Ghana. Archives of Environmental Contamination and Toxicology, 40:557–563.
Oduma JA, Wango EO, Makawiti DW, Einer-Jensen N, Oduor-Okelo D (1995a) Effects of graded doses of the pesticide heptachlor on body weight, mating success, oestrous cycle, gestation length and litter size in laboratory rats. Comparative Biochemistry and Physiology C, Comparative Pharmacology, 110(2):221–227.
Oduma JA, Wango EO, Oduor-Okelo D, Makawiti DW, Odongo H (1995b) In vivo and in vitro effects of graded doses of the pesticide heptachlor on female sex steroid hormone production in rats. Comparative Biochemistry and Physiology C, Comparative Pharmacology, 111(2):191–196.
OECD (1992) Report of the OECD workshop on the extrapolation of laboratory aquatic toxicity data to the real environment. Paris, Organisation for Economic Co-operation and Development, pp. 1–43 (OECD Environment Monograph No. 59; http://www.oecd.org).
OECD (1995) Guidance document for aquatic effects assessment. Paris, Organisation for Economic Co-operation and Development, pp. 1–118 (OECD Environment Monograph No. 92; http://www.oecd.org).
Offenberg JH, Eisenreich SJ, Gigliotti CL, Chen LC, Xiong JQ, Quan C, Lou X, Zhong M, Gorczynski J, Yiin L-M, Illacqua V, Lioy PJ (2004) Persistent organic polluntants in dust that settled indoors in lower Manhattan after September 11, 2001. Journal of Exposure Analysis and Environmental Epidemiology, 14:164–172.
Okoumassoun LE, Averill-Bates D, Gagne F, Marion M, Denizeau F (2002) Assessing the estrogenic potential of organochlorine pesticides in primary cultures of male rainbow trout (Oncorhynchus mykiss) hepatocytes using vitellogenin as a biomarker. Toxicology, 178(3):193–207.
Okoumassoun LE, Averill-Bates D, Marion M, Denizeau F (2003) Possible mechanisms underlying the mitogenic action of heptachlor in rat hepatocytes. Toxicology and Applied Pharmacology, 193(3):356–369.
Osibanjo O (2003) Organochlorines in Nigeria and Africa. In: The handbook of environmental chemistry. Vol. 3. Part O. Persistent organic pollutants. Berlin-Heidelberg, Springer, pp. 321–354.
Osuna-Flores I, Riva MC (2002) Organochlorine pesticide residue concentrations in shrimps, sediments, and surface water from Bay of Ohiura, Topolobampo, Sinaloa, Mexico. Bulletin of Environmental Contamination and Toxicology, 68:532–539.
PANNA (1997) PANUPS: Velsicol ceases production of chlordane and heptachlor. San Francisco, CA, Pesticide Action Network North America, 23 May 1997, pp. 1–2 (http:// panna.org/).
Paschal EH, Roan CC, Morgan DP (1974) Evidence of excretion of chlorinated hydrocarbon pesticides by the human liver. Bulletin of Environmental Contamination and Toxicology, 12(5):547–554.
Paumgartten FJ, Cruz CM, Chahoud I, Palavinskas R, Mathar W (2000) PCDDs, PCDFs, PCBs, and other organochlorine compounds in human milk from Rio de Janeiro, Brazil. Environmental Research, 83(3):293–297.
Peirano WB (1980) Heptachlor — maximum acceptable limit in drinking water (a criteria document prepared for the World Health Organization). Washington, DC, United States Environmental Protection Agency [cited in IPCS, 1984].
Podowski AA, Banerjee BC, Feroz M, Dudek MA, Willey RL, Khan MAQ (1979) Photolysis of heptachlor and cis-chlordane and toxicity of their photoisomers to animals. Archives of Environmental Contamination and Toxicology, 8:509–518.
Polkowska Z, Gorecki T, Namiesnik J (2002) Quality of roof runoff waters from an urban region (Gdansk, Poland). Chemosphere, 49:1275–1283.
Priyadarshi A, Khuder SA, Schaub EA, Shrivastava S (2000) A meta-analysis of Parkinson’s disease and exposure to pesticides. Neurotoxicology, 21:435–440.
Probst G, McMahon R, Hill L, Thompson C, Epp J, Neal S (1981) Chemically-induced unscheduled DNA synthesis in primary rat hepatocyte cultures: a comparison with bacterial mutagenicity using 218 compounds. Environmental Mutagenesis, 3:11–32.
Purkerson-Parker S, McDaniel KL, Moser VC (2001) Dopamine transporter binding in the rat striatum is increased by gestational, perinatal, and adolescent exposure to heptachlor. Toxicological Sciences, 64(2):216–223.
Quinsey PM, Donohue DC, Ahokas JT (1995) Persistence of organochlorines in breast milk of women in Victoria, Australia. Food and Chemical Toxicology, 33(1):49–56.
Quintana PJ, Delfino RJ, Korrick S, Ziogas A, Kutz FW, Jones EL, Laden F, Garshick E (2004) Adipose tissue levels of organochlorine pesticides and polychlorinated biphenyls and risk of non-Hodgkin’s lymphoma. Environmental Health Perspectives, 112(8):854–861.
Radomski JL, Davidow B (1953) The metabolite of heptachlor: its estimation, storage, and toxicity. Journal of Pharmacology and Experimental Therapeutics, 107(3):266–272.
Rashid K, Mumma R (1986) Screening pesticides for their ability to damage bacterial DNA. Journal of Environmental Science and Health, Part C, 21:319–334.
Ritchie JM, Vial SL, Fuortes LJ, Guo H, Reedy VE, Smith EM (2003) Organochlorines and risk of prostate cancer. Journal of Occupational and Environmental Medicine, 45(7):692–702.
Rogan WJ, Ragan NB (1994) Chemical contaminants, pharmacokinetics, and the lactating mother. Environmental Health Perspectives, 102(Suppl. 11):89–95.
Rogan WJ, Blanton P, Portier C, Stallard E (1991) Should the presence of carcinogens in breast milk discourage breast feeding? Regulatory Toxicology and Pharmacology, 13:228–240.
Romero MLL, Dorea JG, Granja ACC (2000) Concentrations of organochlorine pesticides in milk of Nicaraguan mothers. Archives of Environmental Health, 55(4):274–278.
Rought SE, Yau PM, Schnier JB, Chuang LF, Chuang RY (1998) The effects of heptachlor, a chlorinated hydrocarbon insecticide, on p53 tumor suppressor in human lymphocytes. Toxicology Letters, 94:29–36.
Rought SE, Yau PM, Chuang LF, Doi RH, Chuang RY (1999) Effects of the chlorinated hydrocarbons heptachlor, chlordane, and toxaphene on retinoblastoma tumor suppressor in human lymphocytes. Toxicology Letters, 104:127–135.
Rought SE, Yau PM, Guo XW, Chuang LF, Doi RH, Chuang RY (2000) Modulation of CPP 32 activity and induction of apoptosis in human CEMx174 lymphocytes by heptachlor, a chlorinated hydrocarbon insecticide. Journal of Biochemical and Molecular Toxicology, 14(1):42–50.
Ruch RJ, Fransson R, Flodstrom S, Warngard L, Klaunig JE (1990) Inhibition of hepatocyte gap junctional intercellular communication by endosulfan, chlordane and heptachlor. Carcinogenesis, 11(7):1097–1101.
Saeed T, Sawaya WN, Ahmad N, Rajagopal S, Dashti B, Al-Awadhi S (2000) Assessment of the levels of chlorinated pesticides in breast milk in Kuwait. Food Additives and Contaminants, 17(12):1013–1018.
Savage EP, Keefe TJ, Tessari JD, Wheeler HW, Applehans FM, Goes EA, Ford SA (1981) National study of chlorinated hydrocarbon insecticide residues in human milk, USA. I. Geographic distribution of dieldrin, heptachlor, heptachlor epoxide, chlordane, oxychlordane, and mirex. American Journal of Epidemiology, 113(4):413–422.
Schimmel SC, Patrick JM, Forester J (1976) Heptachlor: toxicity to and uptake by several estuarine organisms. Journal of Toxicology and Environmental Health, 1(6):955–965.
Schlenk D, Sapozhnikova Y, Baquirian JP, Mason A (2002) Predicting chemical contaminants in freshwater sediments through the use of historical biochemical endpoints in resident fish species. Environmental Toxicology and Chemistry, 21(10):2138–2145.
Schmitt CJ (2002) Organochlorine chemical residues in fish from the Mississippi River Basin, 1995. Archives of Environmental Contamination and Toxicology, 43:81–97.
Schmitt CJ, Zajicek JL, May TW, Cowman DF (1999) Organochlorine residues and elemental contaminants in U.S. freshwater fish, 1976–1986: National Contaminant Biomonitoring Program. Reviews in Environmental Contamination and Toxicology, 162:43–104.
Schoettger RA (1970) Acute toxicity of pesticides to freshwater invertebrates. Progress in Sport Fishery Research, 106:2–40.
Siegel BZ (1988) Heptachlor epoxide in mothers’ milk in Hawaii 1981–1984 and its relationship to the recall of contaminated dairy products on Oahu in 1982. Pesticide Hazard Assessment Project, completion report. Honolulu, HI, University of Hawaii, Pacific Biomedical Research Center [cited in Baker et al., 1991].
Sim M, Forbes A, McNeil J, Roberts G (1998) Termite control and other determinants of high body burden cyclodiene insecticides. Archives of Environmental Health, 53(2):114–121.
Simmon V, Kauhanen K, Tardiff R (1977) Mutagenic activity of chemicals identified in drinking water. Developments in Toxicology and Environmental Science, 2:249–258.
Simpson CD, Wilcock RJ, Smith TJ, Wilkins AL, Langdon AG (1995) Determination of octanol–water partition coefficients for the major components of technical chlordane. Bulletin of Environmental Contamination and Toxicology, 55:149–153.
Skopp S, Oehme M, Fürst P (2002) Enantiomer ratios, patterns and levels of toxaphene congeners in human milk from Germany. Journal of Environmental Monitoring, 4(3):389–394.
Smialowicz RJ, Williams WC, Copeland CB, Harris MW, Overstreet D, Davis BJ, Chapin RE (2001) The effects of perinatal/juvenile heptachlor exposure on adult immune and reproductive system function in rats. Toxicological Sciences, 61(1):164–175.
Stansley W, Roscoe DE (1999) Chlordane poisoning of birds in New Jersey, USA. Environmental Toxicology and Chemistry, 18(9):2095–2099.
Stansley W, Roscoe ED, Hawthorne E, Meyer R (2001) Food chain aspects of chlordane poisoning in birds and bats. Archives of Environmental Contamination and Toxicology, 40:285–291.
Steichen J, Koelliker J, Grosh D, Heiman A, Yearout R, Robbins V (1988) Contamination of farmstead wells by pesticides, volatile organics and inorganic chemicals in Kansas. Ground Water Monitoring Review, 8:153–160.
Strucinski P, Ludwicki JK, Góralczyk K, Czaja K, Olszewski W, Jethon J, Baranska J, Hernik A (2002) Levels of organochlorine insecticides in breast adipose tissue of Polish women, 1997–2001. Roczniki Panstwowego Zakladu Higieny, 53(3):221–230.
Stubin AI, Brosnan TM, Porter KD, Jimenez L, Lochan H (1996) Organic priority pollutants in New York City municipal wastewater: 1989–1993. Water Environment Research, 68:1037–1044.
Stuetz W, Prapamontol T, Erhardt JG, Classen HG (2001) Organochlorine pesticide residues in human milk of a Hmong hill tribe living in Northern Thailand. Science of the Total Environment, 273(1–3):53–60.
Suter GW, Tsao CL (1996) Toxicological benchmarks for screening of potential contaminants of concern for effects on aquatic biota on Oak Ridge Reservation: 1996 revision. Oak Ridge, TN, Oak Ridge National Laboratory (ES/ER/TM-96/R2).
Takahashi W, Saidin D, Takei G, Wong L (1981) Organochloride pesticide residues in human milk in Hawaii, 1979–80. Bulletin of Environmental Contamination and Toxicology, 27(4):506–511.
Tashiro S, Matsumura F (1978) Metabolism of trans-nonachlor and related chlordane components in rat and man. Archives of Environmental Contamination and Toxicology, 7:113–127.
Telang S, Tong C, Williams GM (1982) Epigenetic membrane effects of a possible tumor promoting type on cultured liver cells by the non-genotoxic organochlorine pesticides chlordane and heptachlor. Carcinogenesis, 3(10):1175–1178.
Teufel M, Niessen KH, Sartoris J, Brands W, Lochbuhler H, Waag K, Schweizer P, von Oelsnitz G (1990) Chlorinated hydrocarbons in fat tissue: analyses of residues in healthy children, tumor patients, and malformed children. Archives of Environmental Contamination and Toxicology, 19(5):646–652.
Thomas R (1990) Volatilization from water. In: Lyman WJ, Reehl WF, Rosenblatt DH, eds. Handbook of chemical property estimation methods: Environmental behavior of organic compounds. New York, McGraw-Hill Book Company, pp. 15.1–15.34.
Tsushimoto G, Chang CC, Trosko J, Matsumura F (1983) Cytotoxic, mutagenic and cell–cell communication inhibitory properties of DDT, lindane and chlordane on Chinese hamster cells in vitro. Archives of Environmental Contamination and Toxicology, 12:721–730.
Turgut C (2003) The contamination with organochlorine pesticides and heavy metals in surface water in Kücük Menderes River in Turkey, 2000–2002. Environment International, 29(1):29–32.
UNEP (1996) UNEP survey on sources of POPs: a report prepared for an IFCS expert meeting on persistent organic pollutants, Manila, Philippines, 17–19 June 1996. United Nations Environment Programme, pp. 1–25 (http:// www.chem.unep.ch/ pops/indxhtms/manexp3.html).
Urieta I, Jalon M, Eguileor I (1996) Food surveillance in the Basque country (Spain). II. Estimation of the dietary intake of organochlorine pesticides, heavy metals, arsenic, aflatoxin M1, iron and zinc through the total diet study, 1990/91. Food Additives and Contaminants, 13(1):29–52.
USEPA (1980) Ambient water quality criteria for heptachlor. Washington, DC, United States Environmental Protection Agency, Criteria and Standards Division, 115 pp. (PB81-117632; EPA-440/5-80-052).
USFWS (1975) Lethal dietary toxicities of environmental pollutants to birds. Washington, DC, United States Department of the Interior, Fish and Wildlife Service, pp. 1–61 (Special Scientific Report – Wildlife No. 191).
van der Oost R, Opperhuizen A, Satumalay K, Heida H, Vermeulen NPE (1996) Biomonitoring aquatic pollution with feral eel (Anguilla anguilla). I. Biota–sediment ratios of PCBs, OCPs, PCDDs and PCDFs. Aquatic Toxicology, 35:21–46.
van Maanen JMS, de Vaan MAJ, Veldstra AWF, Hendrix WPAM (2001) Pesticides and nitrate in groundwater and rainwater in the province of Limburg in the Netherlands. Environmental Monitoring and Assessment, 72(1):95–114.
Vazquez-Moreno L, Langure A, Orantes C, Flores ME, Bermudez MC (1999) Incidence of pesticide residues in adipose tissue of beef, pork, and poultry from plants located in northwestern Mexico. Journal of Muscle Foods, 10(4):295–303.
Velsicol Corporation (1959) Unpublished report [cited in JMPR, 1992].
Verschueren K (1996) Handbook of environmental data on organic chemicals, 3rd ed. New York, John Wiley & Sons.
Wagman N, Strandberg B, Van Bavel B, Berqvist P-A, Öberg L, Rappe C (1999) Organochlorine pesticides and polychlorinated biphenyls in household composts and earthworms (Eisenia foetida). Environmental Toxicology and Chemistry, 18(6):1157–1163.
Waizenegger W, Kypke K, Feteroll B, Hahn J, Sohnius E, Feucht M (1998) Humanmilch-Untersuchungen 1980–1996: chlorganische Pestizide, polychlorierte Biphenyle und Nitromoschusverbindungen. Deutsche Lebensmittel-Rundschau: Zeitschrift für Lebensmittelkunde und Lebensmittelrecht, 94(4):120–122.
Walker JB, Seddon L, McMullen E, Houseman J, Tofflemire K, Corriveau A, Weber JP, Mills C, Smith S, van Oostdam J (2003) Organochlorine levels in maternal and umbilical cord blood plasma in Arctic Canada. Science of the Total Environment, 302(1–3):27–52.
Wallace JC, Brzuzy LP, Simonich SL, Visscher SM, Hites RA (1996) Case study of organochlorine pesticides in the indoor air of a home. Environmental Science & Technology, 30(9):2715–2718.
Wang HH, MacMahon B (1979) Mortality of workers employed in the manufacture of chlordane and heptachlor. Journal of Occupational Medicine, 21:745–748.
Wango EO, Onyango DW, Odongo H, Okindo E, Mugweru J (1997) In vitro production of testosterone and plasma levels of luteinising hormone, testosterone and cortisol in male rats treated with heptachlor. Comparative Biochemistry and Physiology C, Comparative Pharmacology, 118(3):381–386.
Wania F, Shen L, Lei YD, Teixeira C, Muir DCG (2003) Development and calibration of a resin-based passive sampling system for monitoring persistent organic pollutants in the atmosphere. Environmental Science & Technology, 37(7):1352–1359.
Ward E, Schulte P, Grajewski B, Anderson A, Patterson D, Turner W, Jellum E, Deddens J, Friedland J, Roeleveld N, Waters M, Butler M, DiPietro E, Needham L (2000) Serum organochlorine levels and breast cancer: a nested case–control study of Norwegian women. Cancer Epidemiology, Biomarkers & Prevention, 9(12):1357–1367.
Warne MS (1998) Critical review of methods to derive water quality guidelines for toxicants and a proposal for a new framework. Canberra, Environment Australia (Supervising Scientist Report 135).
Wassermann M, Tomatis L, Wassermann D, Day NE, Groner Y, Lazarovici S, Rosenfeld D (1974) Epidemiology of organochlorine insecticides in the adipose tissue of Israelis. Pesticides Monitoring Journal, 8:1–7.
Wassermann M, Nogueira DP, Tomatis L, Mirra AP, Shibata H, Arie G, Cucos S, Wassermann D (1976) Organochlorine compounds in neoplastic and adjacent apparently normal breast tissue. Bulletin of Environmental Contamination and Toxicology, 15:478–484.
Wazeter FX, Goldenthal EI, Geil RG, Howell DG, Dean WP (1971a) 30-day range-finding study in mice. Unpublished report, International Research and Development Corporation (submitted to WHO by Velsicol Chemical Corp., Rosemont, IL) [cited in JMPR, 1992].
Wazeter FX, Geil RG, Goldenthal EI, Cookson KM, Howell DG (1971b) Two-year oral study in beagle dogs. International Research and Development Corporation (Unpublished Report No. 163-048, submitted to WHO by Velsicol Chemical Corp., Rosemont, IL) [cited in JMPR, 1992].
Wazeter FX, Geil RG, Goldenthal EI, Howell DG (1971c) Two-generation reproduction and teratology study in beagle dogs. International Research and Development Corporation (Unpublished Report No. 163-048, submitted to WHO by Velsicol Chemical Corp., Rosemont, IL) [cited in JMPR, 1992].
Weisbrod AV, Shea D, Moore MJ, Stegeman JJ (2000a) Bioaccumulation patterns of polychlorinated biphenyls and chlorinated biphenyls and chlorinated pesticides in Northwest Atlantic pilot whales. Environmental Toxicology and Chemistry, 19(3):667–677.
Weisbrod AV, Shea D, Moore MJ, Stegeman JJ (2000b) Organochlorine exposure and bioaccumulation in the endangered Northwest Atlantic right whale (Eubalaena glacialis) population. Environmental Toxicology and Chemistry, 19(3):654–666.
Weisgerber I, Klein W, Korte F (1972) Beiträge zur ökologischen Chemie. XLII: Verteilung und Umwandlung von Heptachlor-14C in Weisskohl und Weizen. Chemosphere, 1(2):89–94.
Weseloh DV, Hughes KD, Ewins PJ, Best D, Kubiak T, Shieldcastle MC (2002) Herring gulls and great black-backed gulls as indicators of contaminants in bald eagles in Lake Ontario, Canada. Environmental Toxicology and Chemistry, 21(5):1015–1025.
Wiberg K, Letcher RJ, Sandau CD, Norstrom RJ, Tysklind M, Bidleman TF (2000) The enantioselective bioaccumulation of chiral chlordane and α-HCH contaminants in the polar bear food chain. Environmental Science & Technology, 34(13):2668–2674.
Wiberg K, Bergman A, Olsson M, Roos A, Blomkvist G, Haglund P (2002) Concentrations and enantiomer fractions of organochlorine compounds in Baltic species hit by reproductive impairment. Environmental Toxicology and Chemistry, 21(12):2542–2551.
Williams DT, LeBel GL, Junkins E (1988) Organohalogen residues in human adipose autopsy samples from six Ontario municipalities. Journal of the Association of Official Analytical Chemists, 71(2):410–414.
Williams GM, Numoto S (1984) Promotion of mouse liver neoplasms by the organochlorine pesticides chlordane and heptachlor in comparison to dichlorodiphenyltrichloroethane. Carcinogenesis, 5(12):1689–1696.
Williams GM, Mori H, McQueen CA (1989) Structure–activity relationship in the rat hepatocyte DNA-repair test for 300 chemicals. Mutation Research, 221:263–286.
Yamashita N, Urushigawa Y, Masunaga S, Walash MI, Miyazaki A (2000) Organochlorine pesticides in water, sediment and fish from the Nile River and Manzala Lake in Egypt. International Journal of Environmental Analytical Chemistry, 77(4):289–303.
Yao Z-W, Jiang GB, Xu HZ (2002) Distribution of organochlorine pesticides in seawater of the Bering and Chukchi sea. Environmental Pollution, 116(1):49–56.
Zeiger E, Anderson B, Haworth S, Lawlor T, Mortelmans K, Speck W (1987) Salmonella mutagenicity tests: III. Results from the testing of 255 chemicals. Environmental Mutagenesis, 9:1–110.
Zhang ZL, Hong HS, Zhou JL, Huang J, Yu G (2003) Fate and assessment of persistent organic pollutants in a water and sediment from Minjiang River Estuary, southeast China. Chemosphere, 52(9):1423–1430.
Zheng T, Hoford TR, Tessari J, Mayne ST, Zahm SH, Owens PH, Zhang B, Ward B, Carter D, Zhang Y, Zhang W, Dubrow R, Boyle P (2000) Oxychlordane and trans-nonachlor in breast adipose tissue and risk of female breast cancer. Journal of Epidemiology and Biostatistics, 5(3):153–160.
Zhong W, Xu D, Chai Z, Mao X (2003) 2001 survey of organochlorine pesticides in retail milk from Beijing, P. R. China. Food Additives and Contaminants, 20(3):254–258.
Zhu J, Norstrom RJ, Muir DCG, Ferron LA, Weber J-P, Dewailly E (1995) Persistent chlorinated cyclodiene compounds in ringed seal blubber, polar bear fat, and human plasma from northern Québec, Canada: Identification and concentrations of photoheptachlor. Environmental Science & Technology, 29:267–271.
|
ADI |
acceptable daily intake |
|
AOPWIN |
Atmospheric Oxidation Program for Microsoft Windows |
|
AP-1 |
activator protein-1 |
|
BOD |
biological oxygen demand |
|
BOD5 |
5-day biological oxygen demand |
|
CAS |
Chemical Abstracts Service |
|
CI |
confidence interval |
|
CICAD |
Concise International Chemical Assessment Document |
|
DNA |
deoxyribonucleic acid |
|
EC50 |
median effective concentration |
|
ECD |
electron capture detection |
|
EPI |
Estimation Programs Interface (suite of models) |
|
FAO |
Food and Agriculture Organization of the United Nations |
|
GABA |
gamma-aminobutyric acid |
|
GABAA |
gamma-aminobutyric acid type A |
|
GABAergic |
activated by GABA |
|
GC |
gas chromatography |
|
GD |
gestational day |
|
HCp |
hazardous concentration for p% of the species |
|
HC1(50) |
the hazardous concentration to protect 99% of species with 50% confidence |
|
HC5(50) |
the hazardous concentration to protect 95% of species with 50% confidence |
|
HRGC |
high-resolution gas chromatography |
|
IARC |
International Agency for Research on Cancer |
|
ICSC |
International Chemical Safety Card |
|
Ig |
immunoglobulin |
|
IOMC |
Inter-Organization Programme for the Sound Management of Chemicals |
|
IPCS |
International Programme on Chemical Safety |
|
JMPR |
Joint FAO/WHO Meeting on Pesticide Residues |
|
.oc |
soil sorption coefficient |
|
.ow |
octanol/water partition coefficient |
|
LC50 |
median lethal concentration |
|
LD50 |
median lethal dose |
|
LOAEL |
lowest-observed-adverse-effect level |
|
LOEC |
lowest-observed-effect concentration |
|
MAPK |
mitogen-activated protein kinase |
|
mRNA |
messenger ribonucleic acid |
|
MS |
mass spectrometry |
|
ND |
not detected |
|
NOAEL |
no-observed-adverse-effect level |
|
NOEC |
no-observed-effect concentration |
|
OECD |
Organisation for Economic Co-operation and Development |
|
OR |
odds ratio |
|
PCBs |
polychlorinated biphenyls |
|
PIC |
Prior Informed Consent |
|
PLCgamma1 |
phospholipase C gamma-1 |
|
PND |
postnatal day |
|
SMR |
standardized mortality ratio |
|
USA |
United States of America |
|
USEPA |
United States Environmental Protection Agency |
|
UV |
ultraviolet |
|
WHO |
World Health Organization |
IPCS (1984)
A WHO Task Group on Environmental Health Criteria for organochlorine pesticides other than DDT met in Geneva from 28 November to 2 December 1983. The Task Group reviewed and revised the draft criteria document on heptachlor and made an evaluation of the health risks of exposure to heptachlor. The drafts of this document were prepared by Dr D.C. Villeneuve of Canada and Dr S. Dobson of the United Kingdom.
The Environmental Health Criteria monograph on heptachlor is available on the Internet at: http://www.inchem.org/ documents/ehc/ehc/ehc38.htm.
IARC (2001)
Heptachlor (and chlordane) were evaluated by IARC in Volume 79 of the IARC Monographs on the Evaluation of Carcinogenic Risks to Humans.
A summary of the data reported and the evaluation are available on the Internet at: http://www-cie.iarc.fr/htdocs/ monographs/vol79/79-12.html and http://www.inchem.org/ documents/iarc/vol79/79-12.html.
Only the summary and evaluation (section 5) are available. The whole monograph, which includes other chemicals as well, is available for purchasing from the WHO bookshop.
JMPR (1992)
The JMPR monograph on heptachlor, published as part of the 1991 evaluations of pesticide residues in food, is available on the Internet at: http://www.inchem.org/documents/jmpr/ jmpmono/v91pr13.htm.
The draft CICAD on heptachlor was sent for review to IPCS national Contact Points and Participating Institutions, as well as to identified experts. Comments were received from:
M. Baril, Institut de recherche Robert Sauvé en santé et en sécurité du travail, Montreal, Canada
R. Benson, United States Environmental Protection Agency, Denver, CO, USA
J. Chapman, Department of Environment & Conservation, Lidcombe, New South Wales, Australia
R. Chhabra, National Institute of Environmental Health Sciences, Research Triangle Park, NC, USA
P. Copestake, Toxicology Advice & Consulting Ltd, Surrey, United Kingdom
I. Desi, University of Szeged, Szeged, Hungary
L. Fishbein, Fairfax, VA, USA
E. Frantik, Institute of Public Health, Prague, Czech Republic
H. Gibb, Sciences International, Alexandria, VA, USA
P. Howe, Centre for Ecology & Hydrology, Monks Wood, United Kingdom
L. Maltby, University of Sheffield, Western Bank, United Kingdom
H.V.T. Santonen, Institute of Occupational Health, Helsinki, Finland
H. Savolainen, Ministry of Social Affairs & Health, Tampere, Finland
P. Schulte, National Institute for Occupational Safety and Health, Cincinnati, OH, USA
R. Smith, Hydrobiology Pty Ltd, Brisbane, Australia
J.L. Stauber, CSIRO Energy Technology, Menai, New South Wales, Australia
U. Stenius, Karolinska Institute, Stockholm, Sweden
M.H. Sweeney, United States Embassy, Hanoi, Viet Nam
K. Ziegler-Skylakakis, European Commission, Luxembourg
Hanoi, Viet Nam
28 September – 1 October 2004
Members
Mr D.T. Bai, Centre of Environmental Protection & Chemical Safety, Institute of Industrial Chemistry, Hanoi, Viet Nam
Dr R. Chhabra, National Institute of Environmental Health Sciences, Research Triangle Park, NC, USA
Mr P. Copestake, Toxicology Advice & Consulting Ltd, Surrey, United Kingdom
Dr C. De Rosa, Agency for Toxic Substances and Disease Registry, Centres for Disease Control and Prevention, Atlanta, GA, USA
Dr S. Dobson, Centre for Ecology & Hydrology, Monks Wood, Abbots Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr G. Dura, National Institute of Environmental Health of József Fodor National Centre of Public Health, Budapest, Hungary
Ms C.W. Fang, National Institute of Occupational Safety and Health Malaysia, Selangor, Malaysia
Dr L. Fishbein, Fairfax, VA, USA
Dr L. Fruchtengarten, Poison Control Center of São Paulo, São Paulo, Brazil
Dr C.L. Geraci, Document Development Branch, Centers for Disease Control and Prevention / National Institute for Occupational Safety and Health, Cincinnati, OH, USA
Dr H. Gibb, Sciences International, Alexandria, VA, USA
Dr R.F. Hertel, Federal Institute for Risk Assessment, Berlin, Germany
Mr P. Howe, Centre for Ecology & Hydrology, Monks Wood, Abbots Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr S. Ishimitsu, Division of Safety Information on Drug, Food and Chemicals, National Institute of Health Sciences, Tokyo, Japan
Dr J. Kielhorn, Fraunhofer Institute of Toxicology and Experimental Medicine, Hanover, Germany
Dr S. Kunarattanapruke, Food & Drug Administration, Ministry of Public Health, Nonthaburi, Thailand
Dr Y. Liang, Department of Occupational Health, Fudan University School of Public Health, Shanghai, China
Ms M.E. Meek, Existing Substances Division, Environmental Health Directorate, Health Canada, Ottawa, Ontario, Canada
Mr F.K. Muchiri, Directorate of Occupational Health and Safety Services, Nairobi, Kenya
Dr O. Sabzevari, Food and Drug Quality Control Laboratories, Ministry of Health and Medical Education, Tehran, Islamic Republic of Iran
Dr J. Stauber, CSIRO Energy Technology, Menai, New South Wales, Australia
Dr M.H. Sweeney, United States Embassy, Hanoi, Viet Nam
Mr P. Watts, Toxicology Advice & Consulting Ltd, Surrey, United Kingdom
Ms D. Willcocks, National Industrial Chemicals Notification and Assessment Scheme, Sydney, New South Wales, Australia
Dr K. Ziegler-Skylakakis, European Commission, Luxembourg
Secretariat
Dr A. Aitio, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
The authors are aware that the tables included in this appendix are not a comprehensive listing of environmental levels of heptachlor and heptachlor epoxide, nor are all details (e.g. number of samples) recorded. The various isomers of heptachlor epoxide are sometimes measured separately in environmental sampling. For simplicity, the details are not given here. The intention was to gain an overview of the actual extent of environmental contamination with heptachlor and heptachlor epoxide one or two decades after their restriction of use in many countries. Only in a few tables was there any attempt to collect older data.
|
Overview of tables |
|
|
Table |
|
|
A5-1 |
Air |
|
A5-2 |
Water |
|
A5-3 |
Sediment |
|
A5-4 |
Soil |
|
A5-5 |
Fish |
|
A5-6 |
Other aquatic organisms |
|
A5-7 |
Birds and bird eggs |
|
A5-8 |
Amphibians and reptiles |
|
A5-9 |
Mammals |
|
A5-10 |
Food |
|
A5-11 |
Human blood, serum, and plasma |
|
A5-12 |
Breast milk |
|
A5-13 |
Human adipose tissue |
|
A5-14 |
Breast adipose tissue |
Table A5-1: Concentrations of heptachlor and heptachlor epoxide in ambient and indoor air.
|
Compound |
Where found |
Year |
Concentration (pg/m3) |
Reference |
|
Ambient air |
|
|
||
|
Heptachlor |
Ambient air, south Norwegian coast |
1991 |
1–7.4 |
Buser & Müller (1993) |
|
Heptachlor epoxide |
Ambient air, various locations, USA (Muscle Shoals, Alabama; Point Petre, Lake Ontario; Lake Superior) |
1992–1996 |
7.3–20 |
Bidleman et al. (1998b) |
|
Heptachlor |
Air samples, northern Alabama, USA |
January–October 1996 |
4–54 (mean 26) |
Jantunen et al. (2000) |
|
Heptachlor epoxide |
Air samples, northern Alabama, USA |
January–October 1996 |
4–43 (mean 16) |
Jantunen et al. (2000) |
|
Heptachlor |
Lake Superior, USA |
1996–1997 |
1 |
Jantunen et al. (2000) |
|
Heptachlor epoxide |
Lake Superior, USA |
1996–1997 |
7 |
Jantunen et al. (2000) |
|
Heptachlor |
Air, Senga Bay (Lake Malawi, southern Africa) |
1997–1998 |
44 |
Karlsson et al. (2000a) |
|
Heptachlor |
Ambient air, Hong Kong |
2000–2001 |
ND–85 |
Louie & Sin (2003) |
|
Heptachlor epoxide |
Ambient air, Hong Kong |
2000–2001 |
ND |
Louie & Sin (2003) |
|
Heptachlor |
Air above muck crop |
2000 |
38 (3 cm) |
Meijer et al. (2003) |
|
Heptachlor epoxide |
Air above soya bean crops |
2000 |
25 (150 cm) – 550 (3 cm) |
Meijer et al. (2003) |
|
Heptachlor epoxide |
Annual mean air concentration, Alert, Canada |
2000–2001 |
0.2 |
Wania et al. (2003) |
|
Heptachlor |
Ambient air, Belmopan, inland station |
December 1995 – August 1996 |
0.8–3.3 |
Alegria et al. (2000) |
|
Heptachlor epoxide |
Ambient air, Belmopan, inland station |
December 1995 – August 1996 |
2.1–4.8 |
Alegria et al. (2000) |
|
Heptachlor |
Ambient air, Belize City, coastal station |
December 1995 – August 1996 |
0.2–1.2 |
Alegria et al. (2000) |
|
Heptachlor epoxide |
Ambient air, Belize City, coastal station |
December 1995 – August 1996 |
2.0–5.9 |
Alegria et al. (2000) |
|
Indoor air |
|
|
||
|
Heptachlor |
Indoor air, Bloomington, Indiana, USA |
1979–1986 |
4–110 ng/mł (basement) 3–66 (first floor) |
Anderson & Hites (1989) |
|
Heptachlor |
Outdoor air, house, Bloomington, Indiana, USA |
1994–1995 |
<0.05 ng/mł |
Wallace et al. (1996) |
|
Heptachlor |
Indoor air, Bloomington, Indiana, USA |
1994–1995 |
0.5–3.3 ng/mł |
Wallace et al. (1996) |
|
Heptachlor |
Indoor air, USA (Pennsylvania, Ohio, Indiana, Illinois) |
1996–1997 |
ND–79 ng/mł |
Leone et al. (2000) |
|
Heptachlor epoxide + oxychlordane |
indoor air, USA (Pennsylvania, Ohio, Indiana, Illinois) |
1996–1997 |
ND–4.4 ng/mł |
Leone et al. (2000) |
ND: not detected, below detection limits
Table A5-2: Concentrations of heptachlor and heptachlor epoxide in water samples.
|
Compound |
Where found |
Year |
Concentration |
Reference |
|
Rainwater |
||||
|
Heptachlor |
Rainwater, Netherlands |
1998–1999 |
2–4 ng/l |
van Maanen et al. (2001); Hamers et al. (2003) |
|
Heptachlor epoxide |
Rainwater, Netherlands |
1998–1999 |
3–9 ng/l |
van Maanen et al. (2001); Hamers et al. (2003) |
|
Heptachlor epoxide |
Precipitation from various sites in the Gdansk region, Poland |
1998 |
0.05–3.28 ng/l 0.09–0.58 ng/l (range of means) |
Grynkiewicz et al. (2001) |
|
Heptachlor epoxide |
Runoff water from roofs in Gdansk, Poland |
November 1999 – April 2000 |
Up to 1.49 ng/l |
Polkowska et al. (2002) |
|
Heptachlor |
Precipitation in Senga Bay, Lake Malawi, southern Africa |
1997–1998 |
73 pg/l |
Karlsson et al. (2000a) |
|
Heptachlor epoxide |
Precipitation in Senga Bay, Lake Malawi, southern Africa |
1997–1998 |
11 pg/l |
Karlsson et al. (2000a) |
|
Drinking-water |
||||
|
Heptachlor |
332 wells, California, USA |
1997–1998 |
ND |
CEPA (2000) |
|
Heptachlor epoxide |
335 wells, California, USA |
1997–1998 |
ND |
CEPA (2000) |
|
Heptachlor |
1% of tested wells in Kansas, USA |
1985–1986 |
0.025 µg/l |
Steichen et al. (1988) |
|
Heptachlor |
New York City, NY, USA; municipal treatment facilities, influent and effluent |
1989–1993 |
0.02–0.5 µg/l |
Stubin et al. (1996) |
|
Surface water |
||||
|
Heptachlor |
Filtered water samples, Nile River and Manzala Lake, Arab Republic of Egypt |
1993–1994 |
ND–8 pg/l |
Yamashita et al. (2000) |
|
Heptachlor |
River Nile, Cairo, Arab Republic of Egypt |
1991 Winter Summer |
2.4–3.5 ng/l 5.9–10.3 ng/l |
Abou-Arab et al. (1995) |
|
Heptachlor |
Manzala Lake, Arab Republic of Egypt |
1991 Winter Summer |
6.0–15.6 ng/l 6.5–30.0 ng/l |
Abou-Arab et al. (1995) |
|
Heptachlor epoxide |
Surface water from Lake Malawi, southern Africa |
1996 |
0.1–11 pg/l |
Karlsson et al. (2000a) |
|
Heptachlor |
Surface waters in city, Ibadan |
1980s |
4–202 ng/l; mean 72 ng/l |
Osibanjo (2003) |
|
Other rivers in Nigeria |
ND–11 ng/l |
|||
|
Heptachlor |
Groundwater, Ibadan, Oyo State, Nigeria |
290–17 500 ng/l; mean 2256 ng/l |
Osibanjo (2003) |
|
|
Heptachlor |
Rivers and lakes, Hanoi, Viet Nam |
1998–1999 |
<0.025–50.8 ng/l |
Hung & Thiemann (2002) |
|
Heptachlor epoxide |
Rivers and lakes, Hanoi, Viet Nam |
1998–1999 |
<0.025–31.3 ng/l |
Hung & Thiemann (2002) |
|
Heptachlor |
Groundwater sample from Mar del Plata, Argentina |
n.g. |
15 ng/l |
Massone et al. (1998) |
|
Heptachlor epoxide |
Water samples from streams and public standpipe at Akumadan, Ghana |
n.g. |
15 ng/l |
Ntow (2001) |
|
Heptachlor epoxide |
Surface water, Bay of Ohuira, Mexico |
1995–1996 |
20–230 ng/l |
Osuna-Flores & Riva (2002) |
|
Heptachlor |
Surface water, Bay of Ohuira, Mexico |
1995–1996 |
10–1480 ng/l |
Osuna-Flores & Riva (2002) |
|
Heptachlor |
Surface water samples, Küçük Menderes River, West Turkey |
May 2000 – January 2002 |
ND–181 ng/l |
Turgut (2003) |
|
Heptachlor epoxide |
Surface water samples, Küçük Menderes River, West Turkey |
May 2000 |
ND–297 ng/l |
Turgut (2003) |
|
Heptachlor |
Minjiang River Estuary, south-east China; water and porewater |
1999 |
90 ng/l and 963 ng/l |
Zhang et al. (2003) |
|
Heptachlor epoxide |
Minjiang River Estuary, south-east China, water and porewater |
1999 |
33 ng/l and 67 ng/l |
Zhang et al. (2003) |
|
Heptachlor |
Surface water and groundwater, San Juan, Argentina |
1996–1997 |
968 ng/l (mean) |
Baudino et al. (2003) |
|
Heptachlor epoxide |
Surface water and groundwater, San Juan, Argentina |
1996–1997 |
378 ng/l |
Baudino et al. (2003) |
|
Heptachlor |
Samples of water from five different points around Göksu Delta, Turkey |
1991–1993 |
ND–62 000 ng/l; mean 15 000 ng/l |
Ayas et al. (1997) |
|
Heptachlor epoxide |
Samples of water from five different points around Göksu Delta, Turkey |
1991–1993 |
ND–52 000 ng/l; mean 19 000 ng/l |
Ayas et al. (1997) |
|
Heptachlor |
Water samples from canals and drains, El-Haram Giza, Arab Republic of Egypt |
1996 |
3600–12 110 ng/l |
El-Kabbany et al. (2000) |
|
Heptachlor epoxide |
Water samples from canals and drains, El-Haram Giza, Arab Republic of Egypt |
1996 |
10 700–27 800 ng/l |
El-Kabbany et al. (2000) |
|
Seawater |
||||
|
Heptachlor epoxide |
Chukchi Sea |
1993–1994 |
6.3 pg/l |
Jantunen & Bidleman (1998) |
|
Heptachlor epoxide |
Western Arctic Ocean |
1994 |
14.8 pg/l |
Jantunen & Bidleman (1998) |
|
Heptachlor epoxide |
Near Spitsbergen and Greenland Sea |
1994 |
6.6 pg/l |
Jantunen & Bidleman (1998) |
|
Heptachlor |
Bering and Chukchi seas |
1999 |
25–146 pg/l |
Yao et al. (2002) |
|
Heptachlor epoxide |
Bering and Chukchi seas |
1999 |
17–223 pg/l |
Yao et al. (2002) |
|
Heptachlor |
Straits of Jahore between Singapore and Malaysia |
n.g. |
233 ng/l |
Basheer et al. (2002) |
|
Wastewater |
||||
|
Heptachlor epoxide |
Raw wastewater, municipal wastewater treatment plant, Thessaloniki, Greece |
n.g. |
82–1100 ng/l; median 200 ng/l |
Katsoyiannis & Samara, 2004 |
|
Heptachlor epoxide |
Secondary sedimentation effluent, municipal wastewater treatment plant, Thessaloniki, Greece |
n.g. |
6–120 ng/l; median 13 ng/l |
Katsoyiannis & Samara, 2004 |
ND: not detected, below detection limits; n.g.: not given
Table A5-3: Concentrations of heptachlor and heptachlor epoxide in sediment.
|
Compound |
Where found |
Year |
Concentration (ng/g dry weight)a |
Reference |
|
Heptachlor |
Arizona, USA |
2000 |
Up to 6.3 |
Schlenk et al. (2002) |
|
Heptachlor |
North-western Mexico (coastal lagoons, agricultural drains) |
1998 |
1.16–4.25 (maximum 49) |
González-Farias et al. (2002) |
|
Heptachlor epoxide |
North-western Mexico (coastal lagoons, agricultural drains) |
1998 |
ND–20.1 (maximum 64.5) |
González-Farias et al. (2002) |
|
Heptachlor epoxide |
Lake sediments sampled in Mexico |
1996 |
3 (mean) |
Armenta-Arteaga & Elizalde-González (2003) |
|
Heptachlor |
Sediments, coastal station, northern Baltic Sea |
1991 |
<0.020–<0.030 |
Stansley & Roscoe (1999) |
|
Heptachlor epoxide |
Sediments from streams, and public standpipe, Akumadan, Ghana |
n.g. |
0.50 |
Ntow (2001) |
|
Heptachlor epoxide |
Sediment, Bay of Ohuira, Mexico |
1995–1996 |
20–132 |
Osuna-Flores & Riva (2002) |
|
Heptachlor |
Sediment, Bay of Ohuira, Mexico |
1995–1996 |
<20–57 |
Osuna-Flores & Riva (2002) |
|
Heptachlor |
Mean concentrations in sediments from Mar Chiquita lagoon tributaries, Argentina |
1995–1996 |
1 ng/g organic carbon |
Menone et al. (2001) |
|
Heptachlor epoxide |
Mean concentrations in sediments from Mar Chiquita lagoon tributaries, Argentina |
1995–1996 |
944 ng/g organic matter |
Menone et al. (2001) |
|
Heptachlor |
Minjiang River Estuary, south-east China, sediment |
1999 |
1.8 |
Zhang et al. (2003) |
|
Heptachlor epoxide |
Minjiang River Estuary, south-east China, sediment |
1999 |
1.3 |
Zhang et al. (2003) |
|
Heptachlor |
Sediments of Amsterdam freshwater sites |
1991 |
1–2 ng/g organic matter |
van der Oost et al. (1996) |
|
Heptachlor epoxide |
Sediments of Amsterdam freshwater sites |
1991 |
1–9 ng/g organic matter |
van der Oost et al. (1996) |
|
Heptachlor |
Sediments of coastal lagoons of the Pacific coast of Nicaragua |
1995 |
ND–65.4 |
Carvalho et al. (1999) |
|
Heptachlor |
Sediments, Port Jackson, Sydney, Australia |
1995 |
ND–24.4 |
Birch & Taylor (2000) |
|
Heptachlor epoxide |
Sediments, Port Jackson, Sydney, Australia |
1995 |
ND–14.8 |
Birch & Taylor (2000) |
|
Heptachlor |
Lekki Lagoon, Nigeria |
n.g. |
ND–1845; mean 64 |
Osibanjo (2003) |
|
Heptachlor |
Sediments from five different points around Göksu Delta, Turkey |
1991–1993 |
1377 (541–2612) |
Ayas et al. (1997) |
|
Heptachlor epoxide |
Sediments from five different points around Göksu Delta, Turkey |
1991–1993 |
244 (ND–718) |
Ayas et al. (1997) |
ND: not detected, below detection limits; n.g.: not given
a Unless otherwise given.
Table A5-4: Concentrations of heptachlor and heptachlor epoxide in soil.
|
Compound |
Where found |
Year |
Concentration (µg/kg = ng/g = ppb) |
Reference |
|
Heptachlor |
Corn belt, USA |
1995–1996 |
ND–56 |
Aigner et al. (1998) |
|
Heptachlor epoxide |
Corn belt, USA |
1995–1996 |
ND–40 |
Aigner et al. (1998) |
|
Heptachlor |
Agricultural area of the Göksu Delta, Turkey |
1991–1993 |
4777 (792–9616) |
Ayas et al. (1997) |
|
Heptachlor epoxide |
Agricultural area of the Göksu Delta, Turkey |
1991–1993 |
174 (ND–453) |
Ayas et al. (1997) |
|
Heptachlor |
Non-agricultural area of the Göksu Delta, Turkey |
1991–1993 |
735 (93–1823) |
Ayas et al. (1997) |
|
Heptachlor epoxide |
Non-agricultural area of the Göksu Delta, Turkey |
1991–1993 |
180 (70–444) |
Ayas et al. (1997) |
|
Heptachlor |
Dune area of the Göksu Delta, Turkey |
1991–1993 |
474 (216–912) |
Ayas et al. (1997) |
|
Heptachlor epoxide |
Dune area of the Göksu Delta, Turkey |
1991–1993 |
32 (ND–183) |
Ayas et al. (1997) |
|
Heptachlor |
Natural soil from south-eastern region of Argentina |
1999 |
4.42 (0–15 cm) 1.29 (15–30 cm) 1.51 (45–55 cm) |
Miglioranza et al. (2003) |
|
Heptachlor epoxide |
Natural soil from south-eastern region of Argentina |
1999 |
13.5 (0–15 cm) 0.08 (15–30 cm) ND (45–55 cm) |
Miglioranza et al. (2003) |
|
Heptachlor |
Agricultural soil from south-eastern region of Argentina |
1999 |
2.71 (0–15 cm) 0.74 (15–30 cm) 0.92 (45–55 cm) |
Miglioranza et al. (2003) |
|
Heptachlor epoxide |
Agricultural soil from south-eastern region of Argentina |
1999 |
0.06 (0–15 cm) ND (15–30 cm) ND (45–55 cm) |
Miglioranza et al. (2003) |
|
Heptachlor |
Abd-el-aal and El-Zomor Giza, El-Moheet drain, Kafer Hakim, Arab Republic of Egypt |
1996 |
ND |
El-Kabbany et al. (2000) |
|
Heptachlor epoxide |
El-Zomor Giza, Arab Republic of Egypt |
1996 |
2.5 |
El-Kabbany et al. (2000) |
|
Heptachlor epoxide |
Abd-el-aal (land side) and Kafer Hakim, Arab Republic of Egypt |
1996 |
ND |
El-Kabbany et al. (2000) |
|
Heptachlor epoxide |
Abd-el-aal (seaside), Arab Republic of Egypt |
1996 |
4.3 |
El-Kabbany et al. (2000) |
|
Heptachlor epoxide |
El-Moheet drain (El-Maryotia), Arab Republic of Egypt |
1996 |
13.0 |
El-Kabbany et al. (2000) |
|
Heptachlor |
Chulla North Province, Republic of Korea |
1996 |
<0.1–2.8 |
Kim & Smith (2001) |
|
Heptachlor epoxide |
Chulla North Province, Republic of Korea |
1996 |
1.38–48.0 |
Kim & Smith (2001) |
ND: not detected, below detection limits
Table A5-5: Concentrations of heptachlor and heptachlor epoxide in fish.
|
Compound |
Where found |
Year |
Concentration (ng/g wet weight = ppb)a |
Reference |
|
Heptachlor |
Various fish in USA |
1976–1977 1986 |
780 maximum 100 maximum |
Schmitt et al. (1999) |
|
Heptachlor epoxide |
Common carp, Mississippi River Basin, USA |
1995 |
80 maximum |
Schmitt (2002) |
|
Heptachlor/ heptachlor epoxide |
Fish in Australia |
1970 1980s 1990s |
ND 4900 maximum 59 maximum |
Connell et al. (2002) |
|
Heptachlor |
Adipose tissue of carp (Cyprinus carpio) from Göksu Delta, Turkey |
1991–1993 |
1867 mean (range: 1314–2446), lipid weight |
Ayas et al. (1997) |
|
Heptachlor epoxide |
Adipose tissue of carp (Cyprinus carpio) from Göksu Delta, Turkey |
1991–1993 |
615 mean (range: 431–1824), lipid weight |
Ayas et al. (1997) |
|
Heptachlor epoxide |
Sculpin tissue from the Puget Sound and Willamette basins |
1995 |
6.9 |
Black et al. (2000) |
|
Heptachlor |
Ganges perch (Lates calcifer) from the Ganges Estuary, Bangladesh |
1996–1997 |
ND–221 |
Jabber et al. (2001) |
|
Heptachlor |
Muscles of catfish, from the Bay of Bengal |
1997–1998 |
126–284 |
Das et al. (2002) |
|
Heptachlor epoxide |
Various fish, Gulf of Gdansk, Poland |
1992 |
0.09–0.17 |
Falandysz et al. (2001) |
|
Heptachlor epoxide |
Various fish, Gulf of Gdansk, Poland |
1992 |
1.1–4.3 ng/g lipid weight |
Falandysz et al. (2001) |
|
Heptachlor epoxide |
Various species of sturgeon, Caspian Sea, samples from Islamic Republic of Iran, Azerbaijan, Turkmenistan, Kazakhstan |
2001–2002 |
1.6–3.8 ng/g lipid weight |
Kajiwara et al. (2003) |
|
Heptachlor epoxide |
Cod (Gadus morhua), Barents Sea, Kvaløya, Norway |
1995 |
4.8–5.8 |
Karlsson et al. (2000b) |
|
Heptachlor |
Various fish, Lake Superior |
1994 |
<0.2–1.3 |
Kucklick & Baker (1998) |
|
Heptachlor epoxide |
Various fish, Lake Superior |
1994 |
<2–13.6 |
Kucklick & Baker (1998) |
|
Heptachlor |
Various fish, Karachi Coast area, Pakistan |
ca. 1998 |
ND–36.4 |
Munshi et al. (2001) |
|
Heptachlor |
Eel from six Amsterdam freshwater sites |
1991 |
6–36 ng/g lipid weight |
van der Oost et al. (1996) |
|
Heptachlor epoxide |
Eel from six Amsterdam freshwater sites |
1991 |
5–6 ng/g lipid weight |
van der Oost et al. (1996) |
|
Heptachlor epoxide |
Muscle of Baltic salmon, River Dalälven, Sweden |
1995 |
12 ng/g lipid weight |
Wiberg et al. (2002) |
|
Heptachlor epoxide |
Fish, urban area, Wichita, Kansas |
n.g. |
ND–0.8 |
Eaton & Lydy (2000) |
|
Heptachlor |
Fish, north-western Florida/south-western Alabama |
1996 |
0.65 |
Lewis et al. (2002) |
|
Heptachlor epoxide |
Fish, north-western Florida/south-western Alabama |
1996 |
<0.03 |
Lewis et al. (2002) |
|
Heptachlor |
Various fish, White Sea, Russian Federation |
1998–2001 |
0.03–0.79 |
Muir et al. (2003) |
|
Heptachlor epoxide |
Various fish, White Sea, Russian Federation |
1998–2001 |
<0.01–1.03 |
Muir et al. (2003) |
|
Heptachlor epoxide |
Arctic cod, Resolute Bay area of the Canadian Arctic |
1992–1993 |
13–16 ng/g lipid |
Wiberg et al. (2000) |
|
Heptachlor |
Fish samples from Nile River, Arab Republic of Egypt |
1993–1994 |
0.07–0.12 ng/g |
Yamashita et al. (2000) |
|
Heptachlor |
Fish sample from Manzala Lake, Arab Republic of Egypt |
1993–1994 |
ND–0.18 ng/g |
Yamashita et al. (2000) |
LOD: limit of detection; ND: not detected, below detection limits; n.g.: not given
a Unless otherwise given.
Table A5-6: Concentrations of heptachlor and heptachlor epoxide in aquatic organisms other than fish.
|
Compound |
Where found |
Year |
Concentration |
Reference |
|
Heptachlor |
Anadara tuberculosa (mollusc). marine (estuarine), Nicoya Gulf, Pacific Ocean |
1988–1991 |
<0.01–29.9 ng/g dry weight |
Castillo et al. (1997) |
|
Heptachlor |
Several species of bivalve, marine (coastal), Pacific and Caribbean coasts |
1991 |
<0.01–1.75 ng/g dry weight |
Castillo et al. (1997) |
|
Heptachlor/ heptachlor epoxide |
Aquatic invertebrates in Australia |
1990s |
330 ng/g wet weight |
Connell et al. (2002) |
|
Heptachlor epoxide |
Blue mussel and crab from the Gulf of Gdansk, Baltic Sea |
1992 |
0.03 ng/g fresh weight |
Falandysz et al. (2001) |
|
Heptachlor epoxide |
Blue mussel and crab from the Gulf of Gdansk, Baltic Sea |
1992 |
0.9–2.6 ng/g lipids |
Falandysz et al. (2001) |
|
Heptachlor epoxide |
Brachiodontes mussel, Egyptian Red Sea coast |
2000 |
3-84 ng/g wet weight |
Khaled et al. (2004) |
|
Heptachlor epoxide |
Mytilus edulis in New Bedford Harbor, Massachusetts, USA |
n.g. |
ND–130 ng/g lipid |
Hofelt & Shea (1997) |
|
Heptachlor epoxide |
Shrimp from Bay of Ohuira, Mexico |
1995–1996 |
ND–58 ng/l |
Osuna-Flores & Riva (2002) |
|
Heptachlor |
Shrimp from Bay of Ohiura, Mexico |
1995–1996 |
18–127 ng/l |
Osuna-Flores & Riva (2002) |
|
Heptachlor |
Mussel, Karachi Coast area, Pakistan |
ca. 1998 |
3.1–18.2 ng/g |
Munshi et al. (2001) |
|
Heptachlor epoxide |
Spider crab (Hyas araneus), White Sea, Russian Federation |
1999–2000 |
0.59–0.73 ng/g lipid weight |
Muir et al. (2003) |
|
Heptachlor |
Spider crab (Hyas araneus), White Sea, Russian Federation |
1999–2000 |
0.05–0.30 ng/g lipid weight |
Muir et al. (2003) |
|
Heptachlor epoxide |
Whelk (Buccinum undatum), White Sea, Russian Federation |
1999–2000 |
<0.01 ng/g lipid weight |
Muir et al. (2003) |
|
Heptachlor |
Whelk (Buccinum undatum), White Sea, Russian Federation |
1999–2000 |
<0.01 ng/g lipid weight |
Muir et al. (2003) |
|
Heptachlor epoxide |
Isopod (Mesidothea entomon), White Sea, Russian Federation |
1999–2000 |
0.27 ng/g lipid weight |
Muir et al. (2003) |
|
Heptachlor |
Isopod (Mesidothea entomon), White Sea, Russian Federation |
1999–2000 |
0.13 ng/g lipid weight |
Muir et al. (2003) |
|
Heptachlor epoxide |
Zooplankton (Copepoda), White Sea, Russian Federation |
1999–2000 |
0.02–0.55 ng/g lipid weight |
Muir et al. (2003) |
|
Heptachlor |
Zooplankton (Copepoda), White Sea, Russian Federation |
1999–2000 |
<0.01–0.09 ng/g lipid weight |
Muir et al. (2003) |
ND: not detected, below detection limits; n.g.: not given
a Unless otherwise given.
Table A5-7: Concentrations of heptachlor and heptachlor epoxide in birds and bird eggs.
|
Compound |
Where found |
Year |
Concentration (µg/kg = ng/g = ppb) |
Reference |
|
|
Heptachlor |
Liver of coots (Fulica atra), Göksu Delta, Turkey |
1991–1993 |
107 (ND–217) |
Ayas et al. (1997) |
|
|
Heptachlor epoxide |
Liver of coots (Fulica atra), Göksu Delta, Turkey |
1991–1993 |
272 (ND–415) |
Ayas et al. (1997) |
|
|
Heptachlor |
Adipose tissue of coots (Fulica atra), Göksu Delta, Turkey |
1991–1993 |
1765 (ND–3176) |
Ayas et al. (1997) |
|
|
Heptachlor epoxide |
Adipose tissue of coots (Fulica atra), Göksu Delta, Turkey |
1991–1993 |
679 (217–1320) |
Ayas et al. (1997) |
|
|
Heptachlor |
Egg of coots (Fulica atra), Göksu Delta, Turkey |
1991–1993 |
218 (ND–596) |
Ayas et al. (1997) |
|
|
Heptachlor epoxide |
Egg of coots (Fulica atra), Göksu Delta, Turkey |
1991–1993 |
186 (ND–278) |
Ayas et al. (1997) |
|
|
Heptachlor |
Liver of mallards (Anas platyrhynchos), Göksu Delta, Turkey |
1991–1993 |
343 (ND–715) |
Ayas et al. (1997) |
|
|
Heptachlor epoxide |
Liver of mallards (Anas platyrhynchos), Göksu Delta, Turkey |
1991–1993 |
412 (ND–611) |
Ayas et al. (1997) |
|
|
Heptachlor |
Adipose tissue of mallards (Anas platyrhynchos), Göksu Delta, Turkey |
1991–1993 |
2545 (ND–5729) |
Ayas et al. (1997) |
|
|
Heptachlor epoxide |
Adipose tissue of mallards (Anas platyrhynchos), Göksu Delta, Turkey |
1991–1993 |
2744 (336–4622) |
Ayas et al. (1997) |
|
|
Heptachlor |
Egg of mallards (Anas platyrhynchos), Göksu Delta, Turkey |
1991–1993 |
95 (ND–122) |
Ayas et al. (1997) |
|
|
Heptachlor epoxide |
Egg of mallards (Anas platyrhynchos), Göksu Delta, Turkey |
1991–1993 |
119 (ND–322) |
Ayas et al. (1997) |
|
|
Heptachlor |
Liver of little egret (Egretta garzetta), Göksu Delta, Turkey |
1991–1993 |
222 (ND–445) |
Ayas et al. (1997) |
|
|
Heptachlor epoxide |
Liver of little egret (Egretta garzetta), Göksu Delta, Turkey |
1991–1993 |
428 (215–642) |
Ayas et al. (1997) |
|
|
Heptachlor |
Adipose tissue of little egret (Egretta garzetta), Göksu Delta, Turkey |
1991–1993 |
409 (ND–818) |
Ayas et al. (1997) |
|
|
Heptachlor epoxide |
Adipose tissue of little egret (Egretta garzetta), Göksu Delta, Turkey |
1991–1993 |
1247 (ND–2495) |
Ayas et al. (1997) |
|
|
Heptachlor |
Egg of little egret (Egretta garzetta), Göksu Delta, Turkey |
1991–1993 |
980 (ND–2621) |
Ayas et al. (1997) |
|
|
Heptachlor epoxide |
Egg of little egret (Egretta garzetta), Göksu Delta, Turkey |
1991–1993 |
406 (ND–1494) |
Ayas et al. (1997) |
|
|
Heptachlor epoxide |
Eggs of great black-backed gulls (Larus marinus), Lake Ontario, Canada |
1993–1994 |
90–140 (wet weight) |
Weseloh et al. (2002) |
|
|
cis-Heptachlor epoxide |
Fat of Laysan albatross (Phoebastria immutabilis), Sand and Eastern islands, Midway Atoll in central North Pacific |
1994–1995 |
24 (wet weight) |
Muir et al. (2002) |
|
|
Heptachlor |
Fat of Laysan albatross, Sand and Eastern islands, Midway Atoll in central North Pacific |
1994–1995 |
2.1 (wet weight) |
Muir et al. (2002) |
|
|
Heptachlor epoxide |
Fat of black-footed albatross (Phoebastria nigripes), Sand and Eastern islands, Midway Atoll in central north Pacific |
1994–1995 |
33.3 (wet weight) |
Muir et al. (2002) |
|
|
Heptachlor |
Fat of black-footed albatross (Phoebastria nigripes) |
1994–1995 |
2.2 (wet weight) |
Muir et al. (2002) |
|
|
Heptachlor epoxide |
Black-footed albatross (Phoebastria nigripes), egg pool |
1994–1995 |
3.4 (wet weight) |
Muir et al. (2002) |
|
|
Heptachlor epoxide |
Northern royal albatross (Diomedea sanfordi), eggs |
1994–1995 |
1.17 (wet weight) |
Muir et al. (2002) |
|
|
Heptachlor epoxide |
Brains of common grackles (Quiscalus quiscula), Scotch Plains, New Jersey, USA |
1998–1999 |
9–630 (wet weight) |
Stansley et al. (2001) |
|
|
Heptachlor epoxide |
Brains of various birds, New Jersey, USA |
1997 |
2500–8700 (wet weight) |
Stansley & Roscoe (1999) |
|
|
Heptachlor epoxide |
Great blue heron (Ardea herodias) eggs, 10 colonies, Upper Mississippi River, USA |
1993 |
20–100 (wet weight) |
Custer et al. (1997a) |
|
|
Heptachlor epoxide |
Double-crested cormorant (Phalacrocorax auritus) eggs and sibling embryos, Green Bay, Wisconsin, USA |
June 1995 |
50 (wet weight) |
Custer et al. (1997b) |
|
|
Heptachlor epoxide |
Eggs of bald eagles (Haliaeetus leucocephalus) from the Aleutian Islands, Alaska, USA |
1993–1994 |
<10–30 |
Anthony et al. (1999) |
|
|
Heptachlor epoxide |
Eggs of northern goshawk (Accipiter gentilis), La Segarra, north-eastern Spain |
1988–1999 |
ND–49 (wet weight) |
Manosa et al. (2003) |
|
|
Heptachlor epoxide |
Eggs of Eurasian buzzard (Buteo buteo), La Segarra, north-eastern Spain |
1988–1999 |
1–233 (wet weight) |
Manosa et al. (2003) |
|
ND: not detected, below detection limits; n.g.: not given
Table A5-8: Concentrations of heptachlor and heptachlor epoxide in amphibians, reptiles, and annelids.
|
Compound |
Where found |
Year |
Concentration (ng/g wet weight) |
Reference |
|
Heptachlor |
Frogs (Rana forreri), Costa Rican wildland |
1998 |
32 |
Klemens et al. (2003) |
|
Heptachlor |
Toads (Bufo marinus), Costa Rican wildland |
1998 |
6 |
Klemens et al. (2003) |
|
Heptachlor |
10 eggs of bullsnake (Pituophis melanoleucus) after 6 weeks in organochlorine-contaminated nest |
n.g. |
8.4 |
Cañas & Anderson (2002) |
|
Heptachlor |
Turtles (Kinosternon scorpioides), Costa Rican wildland |
1998 |
7 |
Klemens et al. (2003) |
|
Heptachlor |
Turtles (Rhinoclemmys pulcherrima), Costa Rican wildland |
1998 |
17 |
Klemens et al. (2003) |
|
Heptachlor epoxide |
Snapping turtle eggs (Chelydra serpentina), three sites, Canada |
1998 |
ND–3.5 |
de Solla et al. (2002) |
|
Heptachlor |
Earthworm (Eisenia foetida), Sweden |
n.g. |
<0.2–<0.6 |
Wagman et al. (1999) |
|
Heptachlor epoxide |
Earthworm (Eisenia foetida), Sweden |
n.g. |
<0.4–0.2 |
Wagman et al. (1999) |
ND: not detected, below detection limits; n.g.: not given
Table A5-9: Concentrations of heptachlor and heptachlor epoxide in mammals.
|
Compound |
Where found |
Year |
Concentration (ng/g lipid weight)a |
Reference |
|
Heptachlor |
Northwest Atlantic pilot whales (Globicephala melas); whales stranded in Massachusetts, USA |
1990–1996 |
39 (mean) |
Weisbrod et al. (2000a) |
|
Heptachlor epoxide |
Northwest Atlantic pilot whales (Globicephala melas); whales stranded in Massachusetts, USA |
1990–1996 |
56 (mean) |
Weisbrod et al. (2000a) |
|
Heptachlor |
Right whale (Eubalaena glacialis). Bay of Fundy, Canada; southern coast of Georgia; Cape Cod Bay, Massachusetts, USA |
Summer 1994 and 1996 |
236 (mean) |
Weisbrod et al. (2000b) |
|
Winter 1997 |
1287 (mean) |
|||
|
Heptachlor epoxide |
Right whale (Eubalaena glacialis); Bay of Fundy, Canada; southern coast of Georgia; Cape Cod Bay, Massachusetts, USA |
Summer 1994 and1996 |
177 (mean) |
Weisbrod et al. (2000b) |
|
Winter 1997 |
901 (mean) |
|||
|
Heptachlor epoxide |
Blubber of ring seals (Phoca hispida), in the Canadian Arctic |
1998 |
Mean 49 ng/g wet weight (90% lipid) |
Fisk et al. (2002) |
|
Heptachlor epoxide |
Harp seal (Phoca groenlandica) pups, White Sea, Russian Federation |
1993 |
53–126 |
Muir et al. (2003) |
|
Heptachlor epoxide |
Harp seal pups, White Sea, Russian Federation |
1998 |
20–48 |
Muir et al. (2003) |
|
Heptachlor epoxide |
Bearded seal (Eringnathus barbatus), males, White Sea, Russian Federation |
1998–2001 |
14–39 |
Muir et al. (2003) |
|
Heptachlor epoxide |
Harp seal (Phoca groenlandica), adult females, White Sea, Russian Federation |
1998–2001 |
9–40 |
Muir et al. (2003) |
|
Heptachlor epoxide |
Juvenile ringed seal (Phoca hispida), males and females, White Sea, Russian Federation |
1998 |
9–62 |
Muir et al. (2003) |
|
Heptachlor epoxide |
Juvenile/adult ringed seal, females, NW Onega Bay, White Sea, Russian Federation |
2001 |
<0.1 |
Muir et al. (2003) |
|
Heptachlor epoxide |
Juvenile/adult ringed seal, males, NW Onega Bay, White Sea, Russian Federation |
2001 |
<0.1 |
Muir et al. (2003) |
|
Heptachlor |
Ringed seal blubber adipose samples, northern Alaska, USA |
1996 |
<2.1 |
Kucklick et al. (2002) |
|
Heptachlor epoxide |
Tinged seal blubber adipose samples, northern Alaska, USA |
1996 |
29–187 |
Kucklick et al. (2002) |
|
Heptachlor epoxide |
Tinged seal blubber, Resolute Bay area, Canadian Arctic |
1992–1993 |
41–60 |
Wiberg et al. (2000) |
|
Heptachlor epoxide |
Polar bear fat, Resolute Bay area, Canadian Arctic |
1992–1993 |
140–300 |
Wiberg et al. (2000) |
|
Heptachlor epoxide |
Polar bear liver, Resolute Bay area, Canadian Arctic |
1992–1993 |
660–2200 |
Wiberg et al. (2000) |
|
Heptachlor epoxide |
Seal blubber, northern Quebec, Canada |
1990–1991 |
91 |
Zhu et al. (1995) |
|
Heptachlor epoxide |
Bear fat, northern Quebec, Canada |
1989–1990 |
475 (mean) |
Zhu et al. (1995) |
|
Heptachlor |
Polar bear adipose sample, northern Alaska, USA |
1996 |
<2 |
Kucklick et al. (2002) |
|
Heptachlor epoxide |
Polar bear adipose sample, northern Alaska, USA |
1996 |
61–164 |
Kucklick et al. (2002) |
|
Heptachlor epoxide |
Follicular fluid; cattle, sheep, goat, pig; Makedonia and Thessalia, Greece |
n.g. |
0.2–59 ng/ml |
Kamarianos et al. (2003a) |
|
Heptachlor epoxide |
Seminal plasma; boar, bull, ram, goat, Makedonia and Thessalia, Greece |
n.g. |
ND–0.4 ng/ml |
Kamarianos et al. (2003b) |
ND: not detected, below detection limits; n.g.: not given
a Unless otherwise given.
Table A5-10: Concentrations of heptachlor and heptachlor epoxide in food.
|
Compound |
Where found |
Year |
Concentration (µg/kg = ng/g = ppb)a |
Reference |
|
|
Heptachlor/ heptachlor epoxide |
Various herbs, collected in Arab Republic of Egypt |
n.g. |
13–124 |
Abou-Arab & Abou Donia (2001) |
|
|
Heptachlor epoxide |
Tea, collected in Arab Republic of Egypt |
n.g. |
68 |
Abou-Arab & Abou Donia (2001) |
|
|
Heptachlor |
Ginseng samples, bought in USA, Europe, and Asia |
n.g. |
N D–16.3 |
Huggett et al. (2000) |
|
|
Heptachlor epoxide |
Ginseng samples, bought in USA, Europe, and Asia |
n.g. |
ND–22.8 |
Huggett et al. (2000) |
|
|
Heptachlor |
Medicinal plants; markets in Dakar, Senegal |
September–November 1994 |
2.25–17 |
Diop et al. (1999) |
|
|
Heptachlor |
Oranges; mandarins; markets in Dakar, Senegal |
September–November 1994 |
10; 9 |
Diop et al. (1999) |
|
|
Heptachlor |
Tomatoes; markets in Dakar, Senegal |
September–November 1994 |
42 |
Diop et al. (1999) |
|
|
Sigma Heptachlor + heptachlor epoxide |
Cabbage, end of season, different markets of Jaipur City, Rajasthan, India |
1993–1996 |
16 076 |
Bakore et al. (2002) |
|
|
Sigma Heptachlor + heptachlor epoxide |
Spinach, end of season; markets of Jaipur City, Rajasthan, India |
1993–1996 |
9414 |
Bakore et al. (2002) |
|
|
Sigma Heptachlor + heptachlor epoxide |
Cauliflower at the end of the season; markets of Jaipur City, Rajasthan, India |
1993–1996 |
1524 |
Bakore et al. (2002) |
|
|
Sigma Heptachlor + heptachlor epoxide |
Okra at the end of the season; markets of Jaipur City, Rajasthan, India |
1993–1996 |
9298 |
Bakore et al. (2002) |
|
|
Sigma Heptachlor + heptachlor epoxide |
Tomatoes at the end of the season; markets of Jaipur City, Rajasthan, India |
1993–1996 |
15 856 |
Bakore et al. (2002) |
|
|
Sigma Heptachlor + heptachlor epoxide |
Potatoes at the end of the season; markets of Jaipur City, Rajasthan, India |
1993–1996 |
3812 |
Bakore et al. (2002) |
|
|
Heptachlor epoxide |
Winter squash, frozen, USA |
1997 |
9 |
CU (1999) |
|
|
Heptachlor epoxide |
Winter squash, fresh, USA |
1998 |
4 |
CU (1999) |
|
|
Heptachlor epoxide |
Strawberries, fresh, USA |
1998 |
2 |
CU (1999) |
|
|
Heptachlor epoxide |
Tomatoes from farms in Akumadan, Ghana |
1998–2000 |
1.7 |
Ntow (2001) |
|
|
Sigma Heptachlor + heptachlor epoxide |
Tomato, Buenos Aires province, Argentina |
2000–2001 |
2.6 |
Gonzalez et al. (2003) |
|
|
Heptachlor |
Lucin (vegetable), Karachi Coast area, Pakistan |
ca. 1998 |
2.4 |
Munshi et al. (2001) |
|
|
Heptachlor |
Tomato, Karachi Coast area, Pakistan |
ca. 1998 |
1.3 |
Munshi et al. (2001) |
|
|
Heptachlor |
Brinjal, Karachi Coast area, Pakistan |
ca. 1998 |
1.0 |
Munshi et al. (2001) |
|
|
Heptachlor |
Cow milk, Karachi Coast area, Pakistan |
ca. 1998 |
0.5 ng/g lipid |
Munshi et al. (2001) |
|
|
Heptachlor epoxide |
Cow milk, Oahu, Hawaii, USA |
1981–1982 |
Up to 1200 ng/g fat |
Baker et al. (1991) |
|
|
Up to 5000 |
Le Marchand et al. (1986) |
||||
|
Heptachlor |
Cow milk, Jaipur City, Rajasthan, India |
1993–1996 |
Seasonal range: 10–30 µg/g milk fat |
John et al. (2001) |
|
|
Heptachlor epoxide |
Cow milk, Jaipur City, Rajasthan, India |
1993–1996 |
1.4–12.4 µg/g milk fat |
John et al. (2001) |
|
|
Heptachlor |
Animal meat and fat, different locations in Australia |
1992 |
0.06–3.3 ng/g lipid |
Kannan et al. (1994) |
|
|
Heptachlor epoxide |
Animal meat and fat, different locations in Australia |
1992 |
1.4–160 ng/g lipid |
Kannan et al. (1994) |
|
|
Heptachlor epoxide |
Beef fat, Australia |
1993 |
194 |
Armishaw et al. (1998) |
|
|
Heptachlor |
Beef fat, north-western Mexico |
1996–1997 |
100–200 ng/g lipid |
Vazquez-Moreno et al. (1999) |
|
|
Heptachlor |
Poultry fat, north-western Mexico |
1996–1997 |
100 ng/g lipid |
Vazquez-Moreno et al. (1999) |
|
|
Heptachlor |
Infant food, Nigeria |
n.g. |
0.09 (ND–0.87) |
Osibanjo (2003) |
|
|
Heptachlor |
Infant food, Italy |
n.g. |
9.80 (ND–72) |
Osibanjo (2003) |
|
ND: not detected, below detection limits; n.g.: not given
a Unless otherwise given.
Table A5-11: Concentrations of heptachlor and heptachlor epoxide in human blood and plasma.
|
Compound |
Source |
Year |
Concentration |
Reference |
|
Heptachlor |
Detected in the blood of 19% of 112 residents from El Paso, Texas, USA |
1982–1983 |
Mean 3.1 ng/ml (range, ND–9.9 ng/ml) |
Mossing et al. (1985) |
|
Heptachlor |
Columbia, Missouri Breast Cancer Serum Bank, USA |
20% of 105 cases and 19.2% of 208 controls had >LOD of 0.33 ng/g |
Dorgan et al. (1999) |
|
|
Heptachlor epoxide |
Columbia, Missouri Breast Cancer Serum Bank, USA |
All 105 cases and all 2089 controls were <LOD of 0.29 ng/ml |
Dorgan et al. (1999) |
|
|
Heptachlor |
Serum, breast cancer study, Washington County, Maryland, USA; 74 cases, 147 controls |
1975–1994 |
Cases: 0 ng/g lipid (mean) Controls: 0 ng/g lipid (mean) |
Cantor et al. (2003) |
|
Heptachlor epoxide |
Serum, breast cancer study, Washington County, Maryland, USA; 74 cases, 147 controls |
1975–1994 |
Cases: 111.2 ng/g lipid (mean) Controls: 103.6 ng/g lipid (mean) |
Cantor et al. (2003) |
|
Heptachlor |
Serum, breast cancer study; Naples, Italy; 170 cases, 190 controls |
1997–1998 |
Cases: 2.86 ng/ml Controls: 1.16 ng/ml |
Dello Iacovo et al. (1999) |
|
Heptachlor |
Blood, breast cancer study; Jaipur City, India; 135 cases, 50 controls |
n.g. |
Cases: 274–677 ng/ml Controls: ND–164 ng/ml |
Mathur et al. (2002) |
|
Heptachlor epoxide |
Plasma, Canada (northern Quebec) |
51 ng/g (lipid weight) |
Zhu et al. (1995) |
|
|
Heptachlor epoxide |
Umbilical cord blood plasma, 400 children, Arctic Canada |
1994–1999 |
ND–0.20 ng/ml |
Walker et al. (2003) |
|
Heptachlor epoxide |
Maternal blood plasma, 385 women, Arctic Canada |
1994–1999 |
ND–0.70 ng/ml; mean 0.06 ng/ml |
Walker et al. (2003) |
LOD: limit of detection; ND: not detected, below detection limits; n.g.: not given
Table A5-12: Concentrations of heptachlor and heptachlor epoxide in human breast milk.
|
Compound |
Source |
Year |
Concentration (ng/g = ppb on milk fat basis)a |
Reference |
|
Heptachlor/ heptachlor epoxide |
International |
1970s |
2–720 |
IPCS (1984) |
|
Heptachlor epoxide |
USA total |
1979 |
91 ± 125 (63% of 1436 women) |
Savage et al. (1981) |
|
Heptachlor epoxide |
USA |
1991 |
10; 90th percentile = 100 |
Rogan et al. (1991) |
|
Heptachlor epoxide |
Oahu, Hawaii, USA |
1982 |
Maximum >250; mean 123 |
Baker et al. (1991) |
|
Heptachlor epoxide |
Women of Hawaii, USA, and neighbouring islands |
1979–1980 |
31–36 (mean) |
Takahashi et al. (1981) |
|
Heptachlor epoxide |
Canada |
1986 |
11 (mean) |
Mes et al. (1993) |
|
Heptachlor epoxide |
Former West Germany |
Since 1984 |
<20 (mean) |
Waizenegger et al. (1998) |
|
Heptachlor epoxide |
Al-Kharj, Saudi Arabia |
1995–1996 |
20 (mean) |
Al-Saleh et al. (1998) |
|
Heptachlor epoxide |
Victoria, Australia |
n.g. |
61 (mean) |
Quinsey et al. (1995) |
|
Heptachlor epoxide |
Victoria, Australia |
1991–1992 |
7 (median) 170 (maximum) |
Sim et al. (1998) |
|
Heptachlor epoxide |
Germany |
1998–1999 |
<2–12 |
Skopp et al. (2002) |
|
Heptachlor epoxide |
Turkey |
1995–1996 |
72 (mean) |
Cok et al. (1997) |
|
Heptachlor |
Nicaragua |
1994–1995 |
6 |
Romero et al. (2000) |
|
Heptachlor epoxide |
Nicaragua |
1994–1995 |
1 |
Romero et al. (2000) |
|
Heptachlor epoxide |
Kuwait |
Late 1990s |
0.6–9.7; 1.3 (mean) |
Saeed et al. (2000) |
|
Heptachlor |
Northern Thailand |
1998 |
4.3 ng/ml milk = 210 ng/g (mean) |
Stuetz et al. (2001) |
|
Heptachlor epoxide |
Northern Thailand |
1998 |
4.4 ng/ml milk = 360 ng/g (mean) |
Stuetz et al. (2001) |
|
Heptachlor |
Amman, Jordan |
1989–1990 |
700 (median) |
Alawi et al. (1992) |
|
Heptachlor |
Whole study, Jordan |
1993–1994 |
130 (mean) |
Alawi & Khalil (2002) |
|
Heptachlor |
Whole study, Jordan |
2000 |
230 (mean) |
Alawi & Khalil (2002) |
|
Heptachlor epoxide |
Amman, Jordan |
1989–1990 |
580 (median) |
Alawi et al. (1992) |
|
Heptachlor epoxide |
Several cities, Jordan |
1993–1994 |
600 (mean) |
Alawi & Khalil (2002) |
|
Heptachlor epoxide |
Several cities, Jordan |
2000 |
320 (mean) |
Alawi & Khalil (2002) |
|
Heptachlor |
Nigeria |
n.g. |
<10–380; mean 60 ng/g |
Osibanjo (2003) |
|
Heptachlor epoxide |
Rio de Janeiro County, Brazil |
1992 |
8 ng/g milk fat |
Paumgartten et al. (2000) |
|
Heptachlor epoxide |
Dniprodzerzhinsk and Kyiv, Ukraine |
1993–1994 |
<14–244 (median = 16) |
Gladen et al. (2003) |
n.g.: not given
a Unless otherwise given.
Table A5-13: Concentrations of heptachlor and heptachlor epoxide in human adipose tissue.
|
Compound |
Source |
Year |
Concentration (ng/g fat) |
Reference |
|
Heptachlor epoxide |
General population adipose tissue |
1960s to 1970s |
10–460 |
IARC (1991) |
|
Heptachlor epoxide |
Adipose tissue, six Ontario communities, Canada |
1984 |
2–150 (males) 3–107 (females) |
Williams et al. (1988) |
|
Heptachlor epoxide |
Adipose tissue, Osaka, Japan |
1986–1987 |
24–72 |
Kashimoto et al. (1989) |
|
Heptachlor |
183 children, Germany |
1985–1988 |
6 (mean) 87 (maximum) |
Teufel et al. (1990) |
|
Heptachlor epoxide |
183 healthy children, German |
1985–1988 |
4 (mean) 86 (maximum) |
Teufel et al. (1990) |
|
Heptachlor epoxide |
North Texas, USA |
1987–1988 |
37 (mean; age 21–40 years) 73 (mean; age 41–60 years) 142 (mean; 61+ years) |
Adeshina & Todd (1990) |
|
Heptachlor epoxide |
From cadavers, USEPA survey |
1969–1983 |
|
Quintana et al. (2004) |
|
175 Non-Hodgkin’s lymphoma cases |
Mean 120 for cases |
|||
|
481 controls |
Mean 103 for controls |
|||
|
Heptachlor epoxide |
From surgical operations, Manisa, Turkey |
1995–1996 |
Mean 121; range 30–316 |
Cok et al. (1998) |
|
Heptachlor |
50 patients (5–96 years old); males and females, Jordan |
1996 |
<0.5–1610 |
Alawi et al. (1999) |
|
Heptachlor epoxide |
50 patients (5–96 years old); males and females, Jordan |
1996 |
<0.5–1840 |
Alawi et al. (1999) |
Table A5-14: Concentrations of heptachlor and heptachlor epoxide in human breast adipose tissue.
|
Compound |
Source |
Year |
Mean concentration (ng/g adipose tissue) |
Reference |
|
Heptachlor epoxide |
Breast cancer study, Connecticut, USA (cases) |
1987 |
136 ± 53 (cases) 121 ± 53 (controls) |
Falck et al. (1992) |
|
Heptachlor epoxide |
Breast cancer study, Finland |
1985–1986 |
30 ± 20 (cases) 20 ± 20 (controls) |
Mussalo-Rauhamaa et al. (1990) |
|
Heptachlor epoxide |
Breast cancer study, São Paulo, Brazil |
n.g. |
274 (cases) 44 (controls) |
Wassermann et al. (1976) |
|
Heptachlor |
Poland; non-cancer patients aged 15–74 years (43% positive) |
1997–2001 |
Median 2.5; maximum 80 |
Strucinski et al. (2002) |
n.g. = not given
Table A6-1: Genetic and related effects of heptachlor.a
|
Test system |
Resultb |
Dosec (LED/HID) |
Reference |
|
|
Without exogenous metabolic system |
With exogenous metabolic system |
|||
|
In vitro |
||||
|
Salmonella typhimurium TA1538, TA1978, differential toxicity |
– |
NT |
2000 µg/disk |
Rashid & Mumma (1986) |
|
S. typhimurium TA100, TA1535, TA1537, TA1538, TA98, reverse mutation |
NT |
– |
5000 µg/plate |
Simmon et al. (1977) |
|
S. typhimurium TA100, TA1535, TA1537, TA1538, TA98, G46, C3076, D3052, reverse mutation |
– |
– |
NR |
Probst et al. (1981) |
|
S. typhimurium TA100, TA1535, TA98, reverse mutation |
– |
(+)d |
10 µg/plate |
Gentile et al. (1982) |
|
S. typhimurium TA100, TA1535, TA1537, TA1538, TA98, reverse mutation |
– |
– |
5000 µg/plate |
Moriya et al. (1983) |
|
S. typhimurium TA100, TA1535, TA1537, TA98, reverse mutation |
– |
– |
333 µg/plate |
Zeiger et al. (1987) |
|
S. typhimurium TA100, TA102, TA98, TA97, reverse mutation |
– |
– |
1000 µg/plate |
Mersch-Sundermann et al. (1988) |
|
S. typhimurium TA1535, TA1536, TA1537, TA1538, reverse mutation |
– |
– |
1000 µg/plate |
Marshall et al. (1976) |
|
Escherichia coli WP2, K12, differential toxicity |
– |
NT |
2000 µg/disc |
Rashid & Mumma (1986) |
|
E. coli WP2, WP2 uvrA−, reverse mutation |
– |
– |
NR |
Probst et al. (1981) |
|
E. coli WP2, hcr reverse mutation |
– |
– |
5000 µg/plate |
Moriya et al. (1983) |
|
ColE1 plasmid DNA strand breaks (from E. coli K12 ColE1) |
– |
NT |
100 |
Griffin & Hill (1978) |
|
Bacillus subtilis rec strains, differential toxicity |
– |
– |
356 µg/ml |
Matsui et al. (1989) |
|
Saccharomyces cerevisiae D4, gene conversion |
– |
– |
NR |
Gentile et al. (1982) |
|
Unscheduled DNA synthesis, rat, mouse, and Syrian hamster primary hepatocytes in vitro |
– |
NT |
3.7 |
Maslansky & Williams (1981) |
|
Unscheduled DNA synthesis, Fischer 344 rat primary hepatocytes in vitro |
– |
NT |
3.7 |
Probst et al. (1981) |
|
Unscheduled DNA synthesis, rat primary hepatocytes in vitro |
– |
NT |
3.7 |
Williams et al. (1989) |
|
Gene mutation, mouse lymphoma L5178Y cells, Tk locus in vitro |
+ |
NT |
25 |
McGregor et al. (1988) |
|
Gene mutation, rat liver epithelial ARL cells in vitro, Hprt locus |
– |
NT |
37 |
Telang et al. (1982) |
|
Unscheduled DNA synthesis, human VA-4 fibroblasts in vitro |
– |
+ |
37 |
Ahmed et al. (1977) |
|
Inhibition of intercellular communication, rat liver epithelial ARL cells in vitro |
+ |
NT |
0.37 |
Telang et al. (1982) |
|
Inhibition of intercellular communication, Chinese hamster V79 cells in vitro |
+ |
NT |
10 µg/ml |
Kurata et al. (1982) |
|
Inhibition of intercellular communication, male Fischer 344 rat primary hepatocytes in vitro |
+ |
NT |
18.7 |
Ruch et al. (1990) |
|
Inhibition of intercellular communication, male B6C3F1 mouse primary hepatocytes in vitro |
+ |
NT |
18.7 |
Ruch et al. (1990) |
|
Inhibition of intercellular communication, human breast epithelial cells in vitro |
+ |
NT |
10 |
Nomata et al. (1996) |
|
In vivo |
||||
|
Drosophila melanogaster, sex-linked recessive lethal mutations |
– |
1 ng, injection |
Benes & Šram (1969) |
|
|
Gene mutation, lacI transgenic mouse liver assay in vivo |
– |
20 mg/kg of diet, 120 days |
Gunz et al. (1993) |
|
|
Dominant lethal mutation, male ICR/Ha Swiss mice |
– |
24 ip × 1; 10 po × 5 |
Epstein et al. (1972) |
|
|
Dominant lethal mutation, CD-1 mice |
– |
7.5 and 15 |
Arnold et al. (1977)* |
|
|
HID: highest ineffective dose; ip: intraperitoneal; LED: lowest effective dose; NR: not reported; NT: not tested; po: oral |
|
|
a |
From IARC (2001) ; * from JMPR (1992). |
|
b |
+, positive; (+), weak positive; −, negative. |
|
c |
In vitro tests, µg/ml; in vivo tests, mg/kg body weight per day, unless otherwise noted. |
|
d |
Technical grade. |
Table A6-2: Genetic and related effects of heptachlor epoxide.a
|
Test system |
Resultb |
Dosec (LED/HID) |
Reference |
|
Without exogenous metabolic system |
With exogenous metabolic system |
|||
|
Aspergillus nidulans, forward mutation |
– |
NT |
10 450 |
Crebelli et al. (1986) |
|
A. nidulans, mitotic crossing-over |
– |
NT |
10 000 |
Crebelli et al. (1986) |
|
A. nidulans, aneuploidy |
– |
NT |
10 000 |
Crebelli et al. (1986) |
|
S. typhimurium TA1535, TA1536, TA1537, TA1538, reverse mutation |
– |
– |
1000 µg/plate |
Marshall et al. (1976) |
|
Unscheduled DNA synthesis, human VA-4 fibroblasts in vitro |
– |
+ |
3.9 |
Ahmed et al. (1977) |
|
Inhibition of intercellular communication (dye transfer), rat liver WB F344 cells in vitro |
+d |
NT |
10 |
Matesic et al. (1994) |
|
Inhibition of intercellular communication, human breast epithelial cells in vitro |
+d |
NT |
1 |
Nomata et al. (1996) |
|
HID: highest ineffective dose; LED: lowest effective dose; NT: not tested |
|
|
a |
From IARC (2001). |
|
b |
+, positive; −, negative. |
|
c |
In vitro tests, µg/ml; in vivo tests, mg/kg body weight per day. |
|
d |
Loss of intercellular communication was characterized by a substantial, sustained loss of connexin 43 immunostaining within 15–60 min of treatment; at least in human cells, there was no reduction of connexin 43 mRNA. |
Introduction
The traditional approach to using single-species toxicity data to protect field ecosystems has been to apply standardized assessment factors, safety factors, or application factors to the lowest toxicity figure for a particular chemical. The magnitude of these safety factors depends on whether acute or chronic toxicity figures are available and the degree of confidence that one has in whether the figures reflect the field situation. Most of the factors are multiples of 10, and larger factors are applied where there is less certainty in the data. For example, a factor of 1000 is generally used for acute data. This factor of 1000 includes a factor of 10 for extrapolating from laboratory to field, a further factor of 10 for a limited data set, and a factor of 10 for conversion of an acute end-point to a chronic end-point.
Concerns have often been raised as to the arbitrary nature of assessment factors (Chapman et al., 1998) and the fact that they do not conform to risk assessment principles. OECD (1992) recommended that assessment factors be used only when there are inadequate data to allow statistical extrapolation methods to be used.
The following sections briefly outline the statistical extrapolation method used to derive the heptachlor guidance values for the protection of freshwater and marine aquatic organisms for this CICAD. Much of the text is taken directly from the Australian and New Zealand Guidelines for Fresh and Marine Water Quality (ANZECC/ARMCANZ, 2000).
Use of statistical extrapolation methods
New methods using statistical risk-based approaches have been developed over the last decade for deriving guideline (trigger) values. These are based on calculations of a statistical distribution of laboratory ecotoxicity data and attempt to offer a predetermined level of protection, usually 95%. The approach of Aldenberg & Slob (1993) has been adopted in the Netherlands, Australia, and New Zealand for guideline derivation and is recommended for use by the OECD. It was chosen because of its theoretical basis, its ease of use, and the fact that it has been extensively evaluated. Warne (1998) compared in detail the risk-based and assessment factor approaches used in various countries.
The Aldenberg & Slob (1993) method uses a statistical approach to protect 95% of species with a predetermined level of confidence, provided there is an adequate data set. This approach uses available data from all tested species (not just the most sensitive species) and considers these data to be a subsample of the range of concentrations at which effects would occur in all species in the environment. The method may be applied if toxicity data, usually chronic NOEC values, are available for at least five different species from at least four taxonomic groups. Data are entered into a computer program and generally fitted to a log-logistic distribution. A hazardous concentration for . per cent of the species (HCp) is derived. HCp is a value such that the probability of selecting a species from the community with a NOEC lower than HCp is equal to . (e.g. 5%, HC5). HC5 is the estimated concentration that should protect 95% of species. A level of uncertainty is associated with this derived value, and so values with a given confidence level (e.g. 50% or 95%) are computed in the program by attaching a distribution to the error in the tail (Figure A7-1). The ANZECC/ ARMCANZ (2000) guidelines use the median of 50% confidence.
Fig. A7-1: The Dutch statistical approach for the derivation of
guidance (trigger) values (from Aldenberg & Slob, 1993).
HC5 is estimated by dividing the geometric mean of the NOEC values for . species by an extrapolation factor . (OECD, 1995), where:
. = exp(.m × .)
and where:
The Aldenberg & Slob (1993) extrapolation method is based on several critical assumptions, outlined below. Many of these are common to other statistical distribution methods:
Modification of the Aldenberg & Slob (1993) approach
The Aldenberg & Slob (1993) approach assumes the data are best fitted to a log-logistic distribution. For some data sets, however, a better fit is obtained with other models. By using a program developed by CSIRO Biometrics, the data are compared with a range of statistical distributions called the Burr family of distributions, of which the log-logistic distribution is one case. The program determines the distribution that best fits the available toxicity data and calculates the HC5 with 50% confidence (ANZECC/ARMCANZ, 2000); this method has been used to calculate the HC5 for heptachlor.
Application to the data set for heptachlor
For both the freshwater and marine risk assessments, acute LC50 values were each converted to chronic NOEC values using an acute to chronic ratio of 10 (ANZECC/ARMCANZ, 2000); it would be better to use experimentally derived acute to chronic conversion factors, but these were not available for heptachlor. It should be noted that the algal EC50 values were regarded as chronic. These chronic values were then each converted to chronic NOECs by applying a factor of 5, according to ANZECC/ARMCANZ (2000) guidelines, prior to the species sensitivity distribution being undertaken.
Freshwater guidance value
Twenty-three freshwater data were used from Table 8 (section 10.1), and from these data were developed calculated chronic NOECs (see Table A7-1). Non-standard test end-points such as total cell volume reduction and deformations were not included. Geometric means of multiple test results from the same species over the same time period were calculated.
Table A7-1: Toxicity end-points and calculated chronic NOECs used in the derivation of a freshwater guidance value for heptachlor.
|
Organism |
End-point |
Heptachlor concentration (µg/l) |
Calculated chronic NOEC (µg/l) |
|
Algae |
|||
|
Green alga (Pseudokirchneriella subcapitata) |
96-h EC50 (growth inhibition) |
27 |
5.4 |
Invertebrates |
|||
|
Water flea (Daphnia magna) |
48-h LC50 |
80 |
8 |
|
Water flea (Simocephalus serrulatus) |
48-h LC50 |
61.3a |
6.1 |
|
Amphipod (Gammarus fasciatus) |
96-h LC50 |
47.3a |
4.7 |
|
Amphipod (Gammarus lacustris) |
96-h LC50 |
29 |
2.9 |
|
Crayfish (Orconectes nais) |
96-h LC50 |
0.5 |
0.05 |
|
Grass shrimp (Palaemonetes kadiakensis) |
96-h LC50 |
1.8 |
0.2 |
|
Snail (Aplexa hypnorum) |
96-h LC50 |
1450 |
145 |
|
Stonefly (Claassenia sabulosa) |
96-h LC50 |
2.8 |
0.3 |
|
Stonefly (Pteronarcella badia) |
96-h LC50 |
0.9 |
0.09 |
|
Stonefly (Pteronarcys californica) |
96-h LC50 |
1.1 |
0.1 |
|
Fish |
|||
|
Rainbow trout (Oncorhynchus mykiss) |
96-h LC50 |
12.6a |
1.3 |
|
Northern pike (Esox lucius) |
96-h LC50 |
6.2 |
0.6 |
|
Japanese medaka (Oryzias latipes) |
48-h LC50 |
14 |
1.4 |
|
Bluegill sunfish (Lepomis macrochirus) |
96-h LC50 |
15.7a |
1.6 |
|
Redear sunfish (Lepomis microlophus) |
96-h LC50 |
17 |
1.7 |
|
Guppy (Poecilia reticulata) |
96-h LC50 |
110 |
11 |
|
Fathead minnow (Pimephales promelas) |
60-day NOEC |
0.86 |
0.86b |
|
Goldfish (Carassius auratus) |
96-h LC50 |
230 |
23 |
|
Channel catfish (Ictalurus punctatus) |
96-h LC50 |
25 |
2.5 |
|
Black bullhead (Ictalurus melas) |
96-h LC50 |
63 |
6.3 |
|
Largemouth bass (Micropterus salmoides) |
96-h LC50 |
10 |
1 |
|
Amphibians |
|||
|
Fowler’s toad (Bufo woodhousei fowleri) |
96-h LC50 |
435 |
43.5 |
|
a |
Geometric mean. |
|
b |
Taken directly and not calculated. |
Using the calculated chronic NOECs, the HC5(50) — i.e. the hazardous concentration to protect 95% of species with 50% confidence — was 0.08 µg of heptachlor per litre. However, heptachlor has a log .ow of greater than 4; therefore, it has the potential to bioaccumulate. To account for this, the HC1(50) value has been used to recalculate a moderate-reliability guidance value. Using the calculated chronic NOECs, the HC1(50) — i.e. the hazardous concentration to protect 99% of species with 50% confidence — was 0.01 µg of heptachlor per litre. This is a "safe" value to ensure protection against chronic toxicity for most species (see Figure A7-2).
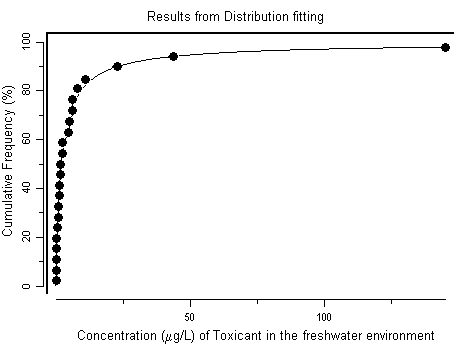
Fig. A7-2: Probability curve for heptachlor in the freshwater environment using derived data from Table A7-1.
Marine water guidance value
Eighteen marine data were used from Table 8 (section 10.1), and from these data chronic NOECs were estimated (Table A7-2). Geometric means of multiple test results from the same species over the same time period were calculated. A short-term 18-week fish test LOEC (mortality) was not included.
Using the calculated chronic NOECs, the HC5(50) — i.e. the hazardous concentration to protect 95% of species with 50% confidence — was 0.03 µg of heptachlor per litre. However, heptachlor has a log .ow of greater than 4; therefore, it has the potential to bioaccumulate. To account for this, the HC1(50) value has been used to recalculate a moderate-reliability guidance value. Using the calculated chronic NOECs, the HC1(50) — i.e. the hazardous concentration to protect 99% of species with 50% confidence — was 0.005 µg of heptachlor per litre. This is a "safe" value to ensure protection against chronic toxicity for most species (see Figure A7-3).
Table A7-2: Toxicity end-points and calculated chronic NOECs used in the derivation of a marine guidance value for heptachlor.
|
Organism |
End-point |
Heptachlor concentration (µg/l) |
Calculated chronic NOEC (µg/l) |
|
Algae |
|||
|
Green alga (Dunaliella tertiolecta) |
96-h EC50 (growth inhibition) |
2260 |
452 |
|
Green alga (Skeletonema costatum) |
96-h EC50 (growth inhibition) |
93 |
18.6 |
|
Green alga (Porphyridium cruentum) |
96-h EC50 (growth inhibition) |
270 |
54 |
|
Invertebrates |
|||
|
Eastern oyster (Crassostrea virginica) |
96-h EC50 (shell growth) |
8.1a |
0.8 |
|
Blue crab (Callinectes sapidus) |
48-h EC50 |
68 |
6.8 |
|
Mysid shrimp (Mysidopsis bahia) |
96-h LC50 |
3.4 |
0.3 |
|
Korean shrimp (Palaemon macrodactylus) |
96-h LC50 |
15 |
1.5 |
|
Pink shrimp (Penaeus duorarum) |
96-h LC50 |
0.06a |
0.006a |
|
Sand shrimp (Crangon septemspinosa) |
96-h LC50 |
8 |
0.8 |
|
Grass shrimp (Palaemonetes vulgaris) |
96-h LC50 |
22a |
2.2 |
|
Hermit crab (Pagurus longicarpus) |
96-h LC50 |
60 |
6.0 |
|
Fish |
|||
|
Striped bass (Morone saxatilis) |
96-h LC50 |
3 |
0.3 |
|
Spot croaker (Leiostomus xanthurus) |
96-h LC50 |
0.85a |
0.085 |
|
American eel (Anguilla rostrata) |
96-h LC50 |
10 |
1.0 |
|
Northern puffer (Sphoeroides maculatus) |
96-h LC50 |
190 |
19 |
|
Pinfish (Lagodon rhomboides) |
96-h LC50 |
4 |
0.4 |
|
Sheepshead minnow (Cyprinodon variegatus) |
96-h LC50 |
6.6a |
0.7 |
|
Striped mullet (Mugil cephalus) |
48-h LC50 |
3.3 |
0.3 |
a Geometric mean.
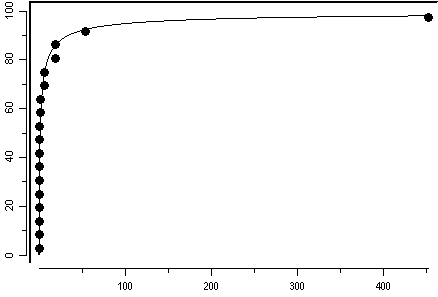
Fig. A7-3: Probability curve for heptachlor in the marine environment using derived data from Table A7-2.
Le présent CICAD5 relatif à l’heptachlore a été préparé par l’institut Fraunhofer de toxicologie et de médecine expérimentale de Hanovre (Allemagne). C’est une mise à jour du document de la série Critères d’hygiène de l’environnement consacré à ce composé (IPCS, 1984) qui comporte des données tirées de rapports du IARC (2001) et du Comité mixte FAO/OMS sur les résidus de pesticides (JMPR, 1992). Une recherche bibliographique exhaustive a été effectuée de 2000 jusqu’à février 2004 dans les bases de données appropriées, à la recherche de toute référence intéressante postérieure à celles qui sont prises en compte dans les rapports précités. Des renseignements sur les sources bibliographiques sont donnés à l’appendice 2. L’appendice 3 donne des indications sur l’examen par des pairs du présent CICAD. Ce CICAD a été examiné et approuvé en tant qu’évaluation internationale lors d’une réunion du Comité d’évaluation finale qui s’est tenue à Hanoi (Viet Nam) du 28 septembre au 1er octobre 2004. La liste des participants à cette réunion figure à l’appendice 4. La Fiche internationale sur la sécurité chimique de l’heptachlore (ICSC 0743) établie par le Programme international sur la sécurité chimique (IPCS, 2003) est également reproduite dans le présent document.
L’heptachlore (No CAS 76-44-8) est un insecticide dicyclopentadiénique chloré qui se révèle persistant dans l’environnement et s’accumule dans la chaîne alimentaire. Bien que depuis les années 1980 son usage soit interdit ou strictement réglementé dans de nombreux pays, on continue à le mettre en évidence comme contaminant de certaines denrées alimentaires. S’il est vrai que c’est là une conséquence de sa persistance dans l’environnement, on peut également penser que ce composé est encore utilisé illégalement – ou l’était encore il y a peu – ou même qu’il est encore autorisé dans quelques pays. Un certain nombre de pesticides organochlorés sont persistants dans l’environnement et l’heptachlore est l’un d’entre eux. La concentration totale de tous ces produits dans les tissus de l’organisme et dans l’environnement est plusieurs fois supérieure à celle de l’heptachlore ou de son époxyde (un métabolite persistant de l’heptachlore).
Une fois libéré dans l’environnement, l’heptachlore peut subir une transformation sous l’effet de processus abiotiques, par exemple de radicaux hydroxyles produits par voie photochimique et, en présence d’eau (notamment dans un sol humide), il peut être transformé en dérivés tels que le 1-hydroxychlordène ou l’époxyheptachlore. Par ailleurs, il est susceptible de s’évaporer dans une certaine mesure des milieux aquatiques et ne peut guère passer dans les eaux souterraines par lessivage du sol en raison de son coefficient élevé de sorption par les particules du sol. Il ne subit pas véritablement de biodégradation, mais une transformation biologique (c’est-à-dire par des bactéries, des champignons, des plantes ou des animaux), principalement sous forme d’un époxyde qui en constitue un métabolite stable. D’après les données dont on dispose au sujet de la capacité de bioconcentration de cet hydrocarbure chloré lipophile, il est – tout comme son époxyde stable – capable de bioaccumulation, comme le montre d’ailleurs la proportion d’heptachlore et d’époxyheptachlore que l’on décèle encore dans des échantillons environnementaux.
Les principales voies d’exposition à l’heptachlore sont, pour la voie directe, probablement l’inhalation lors d’opérations d’épandage ou la pénétration transcutanée par suite d’une exposition prolongée à des poussières contenant cet insecticide, par exemple à l’occasion de traitements domiciliaires contre les termites et, pour la voie indirecte, la consommation d’aliments contaminés provenant de cultures traitées par cet insecticide ou d’autres produits contaminés par l’intermédiaire de la chaîne alimentaire. Toutefois, comme l’heptachlore est présent dans le chlordane technique et qu’il est aussi un métabolite de ce composé, la mise en évidence de cet insecticide ou de son époxyde n’est pas toujours la preuve absolue qu’il y a eu au départ exposition à l’heptachlore ou à son époxyde.
Il ressort d’un bilan portant sur des études récentes que l’heptachlore ou son époxyde se retrouvent dans tous les compartiments de l’environnement – air, eau, sol et sédiments – ainsi que dans les végétaux (légumes), chez les poissons et autres organismes aquatiques, les amphibiens, les reptiles, les oiseaux et leurs śufs ainsi que chez les mammifères aquatiques et terrestres. Ils sont notamment présents dans les tissus adipeux où ils s’accumulent. Ils sont capables de progresser le long de la chaîne alimentaire. On en décèle la présence chez l’Homme dans le sérum, les tissus adipeux – y compris les tissus mammaires – ainsi que dans le lait maternel.
L’heptachlore est facilement résorbé et métabolisé quelle que soit la voie d’exposition. Ses principaux métabolites fécaux sont l’époxyheptachlore, le 1-hydroxychlordène et le 1-hydroxy-2,3-époxychlordène. On a constaté que des microsomes hépatiques incubés en présence d’heptachlore métabolisaient à 85,8 % ce composé en époxyheptachlore lorsqu’il s’agissait de microsomes de rat et à 20,4 % seulement dans le cas de microsomes humains. Les autres métabolites mis en évidence dans le système microsomique du foie humain étaient le 1-hydroxy-2,3-époxychlordène (5%), le 1-hydroxychlordène (4,8 %) et le 1,2-dihydroxydihydrochlordène (0,1 %). L’époxyheptachlore est lentement métabolisé et représente le métabolite le plus stable; il est principalement retenu dans les tissus adipeux, mais aussi dans le foie, les reins et les muscles. Les individus femelles accumulent apparemment davantage d’époxyheptachlore que les individus mâles. Il a fallu 12 semaines pour que l’heptachlore administré à des rats disparaisse complètement de leurs tissus graisseux, une fois l’administration interrompue.
On a montré que chez l’Homme comme chez les animaux de laboratoire, il y a transport transplacentaire de l’heptachlore et de son époxyde.
La DL50 aiguë par voie orale est de 40-162 mg/kg de poids corporel (p.c.) pour le rat et de 68-90 mg/kg p.c. pour la souris. Chez l’animal, une intoxication aiguë par l’heptachlore se traduit par des troubles du système nerveux central, tels qu’hyperexcitabilité, tremblements, convulsions et paralysie. La toxicité aiguë de l’époxyheptachlore est supérieure à celle du composé parent, alors que celle des autres métabolites est très inférieure.
Chez les animaux à qui l’on administre de l’heptachlore ou de l’époxyheptachlore dans leur nourriture, par gavage ou par injection sous-cutanée, la pente de la courbe donnant la mortalité en fonction de la dose est accentuée. En général, on ne constate aucune différence de poids corporel ou de consommation de nourriture entre les animaux traités et les témoins. On a toutefois fait état d’une hypertrophie du foie accompagnée d’une accentuation de la lobulation hépatique et l’examen histopathologique a révélé une hypertrophie des hépatocytes centrolobulaires et médiolobulaires.
Chez le rat, l’étude de la fécondité après injection d’heptachlore par voie sous-cutanée a montré que la LOAEL (dose la plus faible à laquelle un effet nocif a été observé) était égale à 5 mg/kg p.c. par jour, les effets observés étant l’effondrement du taux des hormones sexuelles, la perturbation du cycle oestral chez les femelles et des retards dans les accouplements.
Les études concernant les effets toxiques de ce pesticide sur le développement ne font généralement pas ressortir de signes cliniques d’intoxication maternelle (modification du gain de poids corporel liée à la dose) jusqu’à la mort de l’animal. La NOAEL (dose maximale pour laquelle aucun effet n’a été observé) en ce qui concerne les effets toxiques sur la mère a été trouvée égale à 3 mg/kg p.c. par jour. Selon une étude, il y a eu réduction de la taille des portées, la mortalité postnatale des ratons étant l’effet observé le plus évident (la NOAEL relative à la survie pré- ou postnatale des ratons était égale à 6 mg/kg p.c. par jour). Aucun effet tératogène n’a été observé.
On recueille de plus en plus de données selon lesquelles les pesticides cyclodiéniques agissent sur le système nerveux et son développement. La typologie des effets produits par une administration répétée d’heptachlore à des rats femelles se ramène à des troubles de l’activité, une hyperexcitabilité et des effets sur le système nerveux autonome (NOAEL égale à 2 mg/kg p.c. par jour). Selon des études neurotoxicologiques sur des rats exposés quotidiennement à de l’heptachlore pendant la période périnatale à raison de 0,03, 0,3 ou 3 mg de composé par kg de poids corporel, ce composé provoquerait des retards de développement, une modification de la neurotransmission GABAergique ainsi que des altérations de nature neurocomportementale consistant notamment en un déficit cognitif à toutes les doses.
Des études immunologiques sur le rat indiquent qu’après exposition périnatale à l’heptachlore, il y a suppression des réponses primaire en IgM et secondaire en IgG anti-hématies de mouton à toutes les doses utilisées (0,03, 0,3 et 3 mg/kg de poids corporel).
On a étudié la cancérogénicité de l’heptachlore, de l’heptachlore de qualité technique, de l’époxyheptachlore et d’un mélange d’heptachlore et d’époxyheptachlore en administrant ces produits par voie orale à plusieurs souches de rats et de souris. Il ressort de ces travaux que l’heptachlore, l’époxyheptachlore et l’heptachlore de qualité technique sont cancérogènes pour la souris mâle et femelle mais pas pour le rat. Lors d’une épreuve d’initiation-promotion, on a constaté que l’heptachlore se comportait comme un promoteur actif après initiation par la .-nitrosodiéthylamine.
En ce qui concerne les tests de génotoxicité in vivo et in vitro, l’heptachlore donne dans la plupart des cas un résultat négatif. In vitro, ce composé provoque l’inhibition des jonctions intercellulaires communicantes, ce qui incite également à penser que son pouvoir cancérogène ne repose pas sur un mécanisme génotoxique.
Au vu des données épidémiologiques disponibles, il n’y a pas de relation claire entre les effets indésirables sur la santé qui ont pu être observés et l’exposition à l’heptachlore. Dans ces conditions, c’est sur la base d’études expérimentales que l’on a établi la valeur de la dose tolérable. Comme c’est vraisemblablement un mécanisme non génotoxique qui est à l’origine des tumeurs hépatiques provoquées chez la souris par cet insecticide et qu’à des doses de l’ordre du vingtième de ces doses cancérogènes les effets observés ne sont pas de nature néoplasique, c’est en fonction de ces effets non néoplasiques (par ex. des anomalies histologiques au niveau du foie, des effets neurotoxiques ou encore immunotoxiques) que l’on a déterminé la dose tolérable. La NOAEL relative aux effets hépatiques observés chez le chien a été fixée à 25 µg/kg p.c. par jour et celle qui concerne les effets neurotoxiques et immunotoxiques observés chez le rat, à 30 µg/kg p.c. par jour. En appliquant un facteur d’incertitude de 10 pour tenir compte des variations inter- et intraspécifiques, plus un facteur supplémentaire de 2 dû au fait que la base de données utilisée dans la détermination de la NOAEL pour le chien n’était pas appropriée, on obtient une dose tolérable de 0,1 µg/kg p.c. par jour dans le cas des effets non néoplasiques.
En Pologne, on estime à 0,51-0,58 µg par personne l’apport quotidien d’heptachlore et d’époxyheptachlore par la voie alimentaire (soit environ 0,01 µg/kg p.c. pour un poids individuel moyen de 64 kg). Cette valeur est 10 fois plus petite que celle de la dose tolérable (0,1 µg/kg p.c.). Toutefois, si les denrées alimentaires consommées contiennent de l’heptachlore, comme par exemple du poisson pêché dans un cours d’eau pollué (la teneur du poisson en heptachlore serait, selon des rapports récents, comprise entre 0,1 et 1 mg/kg dans certaines zones), des légumes cultivés dans des champs contaminés par cet insecticide (jusqu’à 16 mg/kg) ou encore du lait contaminé (dans certaines régions, la contamination du lait va du microgramme par kilogramme au milligramme par kilogramme), l’apport d’heptachlore d’origine alimentaire sera beaucoup plus élevé, avec un risque probable pour la santé pour peu que les produits contaminés soient consommés pendant une longue période. Dans le cas des enfants nourris au sein, on arrive à un apport moyen de 1,5 µg/kg p.c. en prenant la valeur la plus élevée de la teneur en époxyheptachlore relevée dans le lait maternel et en supposant en outre que la consommation journalière de lait est de 150 g/kg de poids corporel et que la teneur moyenne du lait en lipides est de 3,1 %. Cette valeur est plus de 10 fois supérieure à celle de la dose tolérable (0,1 µg/kg p.c. par jour) et si les concentrations rapportées sont exactes, elle devrait donc être un sujet de préoccupation.
On a évalué la toxicité aiguë de l’heptachlore pour diverses espèces aquatiques à différents niveaux trophiques. Le composé s’est révélé toxique pour les poissons et d’autres espèces aquatiques. Toutefois, les niveaux de toxicité relevés sont très variables, peut-être en raison de l’évaporation de l’heptachlore qui a pour conséquence de réduire au cours du temps la concentration effective du produit étudié par rapport à la concentration nominale fixée pour l’essai.
En ce qui concerne les eaux douces, on a choisi 23 valeurs de la toxicité pour déterminer une valeur-guide. Ainsi, en se basant sur la distribution de la sensibilité par espèce et en optant pour la protection de 99 % des espèces avec un indice de confiance de 50 %, on a obtenu pour l’heptachlore une valeur-guide de 10 ng/l. Dans de nombreux endroits, la teneur des eaux douces en heptachlore dépasse la valeur-guide; la concentration la plus élevée mesurée dans des eaux douces superficielles est de 62 000 ng/l, c’est-à-dire plus de 1000 fois supérieure à la valeur-guide.
S’agissant du milieu marin, on a retenu 18 valeurs de la toxicité pour déterminer la valeur-guide. Ainsi, en se basant sur la distribution de la sensibilité par espèce et en optant pour la protection de 99 % des espèces avec un indice de confiance de 50 %, on a obtenu pour l’heptachlore une valeur-guide de 5 ng/l. Dans le cas du milieu marin, la valeur la plus élevée qui ait été mesurée de manière fiable est égale à environ 0,15 ng/l, soit une concentration inférieure à la valeur-guide, ce qui indique que, dans ce milieu, le risque est faible.
A la lumière des données existantes, qui sont peu nombreuses, l’heptachlore se révèle modérément toxique pour les vertébrés terrestres. Toutefois, aucune des études examinées ne se montre suffisamment fiable pour servir de base à une quantification du risque. On rappelle que cet insecticide est utilisé pour lutter contre les termites.
L’expérimentation sur le rat a montré que l’heptachlore est neurotoxique et immunotoxique à la dose de 0,03 mg/kg de poids corporel par jour. En Turquie, le delta du Göksu, qui est l’un des lieux de ponte et d’hivernage les plus importants du monde pour les oiseaux, est contaminé par des composés organochlorés contenus dans les alluvions amenés par le fleuve depuis les zones agricoles jusqu’au delta. Dans cette région, on a décelé chez des oiseaux et dans leurs śufs la présence d’heptachlore et d’époxyheptachlore à une concentration de quelques milligrammes par kg. Si l’effet d’une telle concentration d’heptachlore sur les populations aviaires reste à l’état d’hypothèse en raison du manque de données, le risque pour l’environnement terrestre ne peut néanmoins être écarté.
El presente CICAD6 sobre el heptacloro fue preparado por el Instituto Fraunhofer de Toxicología y Medicina Experimental de Hannover, Alemania. Es una actualización del documento sobre el heptacloro de los Criterios de Salud Ambiental (IPCS, 1984) e incluye datos de los informes del Centro Internacional de Investigaciones sobre el Cáncer (IARC, 2001) y de la Reunión Conjunta sobre Residuos de Plaguicidas (JMPR, 1992). Se realizó una búsqueda bibliográfica amplia de las bases de datos pertinentes desde 2000 hasta febrero de 2004 para identificar cualquier referencia publicada después de las incorporadas a estos informes. La información sobre los documentos originales se presenta en el apéndice 2. La información sobre el carácter colegiado de este CICAD figura en el apéndice 3. Este CICAD se examinó y aprobó como evaluación internacional en una reunión de la de la Junta de Evaluación Final, celebrada en Hanoi (Viet Nam) del 28 de septiembre al 1şde octubre de 2004. La lista de participantes en esta reunión aparece en el apéndice 4. También se reproduce en este documento la Ficha internacional de seguridad química para el heptacloro (ICSC 0743), preparada por el Programa Internacional de Seguridad de las Sustancias Químicas (IPCS, 2003).
El heptacloro (CAS Nş 76-44-8) es un insecticida diciclopentadieno clorado que persiste en el medio ambiente y se acumula en la cadena alimentaria. Aunque su utilización se ha prohibido o limitado rigurosamente en muchos países desde el decenio de 1980, se sigue detectando como contaminante en algunos productos alimenticios. Esto se debe a su persistencia, pero también indica el uso ilícito de este plaguicida recientemente o en la actualidad (o tal vez su uso permitido en algunos países). El heptacloro es uno de varios plaguicidas organoclorados persistentes en el medio ambiente. Las concentraciones de todos estos compuestos considerados en conjunto en los tejidos corporales y en el medio ambiente son varias veces superiores a los del heptacloro y/o el heptacloro epóxido (metabolito persistente del heptacloro) solos.
El heptacloro liberado en el medio ambiente se puede transformar mediante procesos abióticos, como la transformación por radicales hidroxilo de origen fotoquímico, y en presencia de agua forma compuestos como el 1-hidroxiclordeno o el heptacloro epóxido (por ejemplo en suelos húmedos). Además, puede desaparecer en cierto grado de los sistemas acuáticos por evaporación y tiene un potencial limitado para filtrarse del suelo al agua freática, debido a su elevado coeficiente de sorción en el suelo. No es fácilmente biodegradable, pero sufre trasformaciones biológicas (es decir, por bacterias, hongos, plantas, animales), principalmente para formar el heptacloro epóxido estable. Los datos disponibles sobre el potencial de bioconcentración de este hidrocarburo clorado lipofílico indican que éste y su epóxido estable se bioacumulan, lo cual se puede demostrar por la medida en que el heptacloro/heptacloro epóxido se siguen detectando en muestras del medio ambiente.
Las principales vías de exposición al heptacloro son probablemente la inhalación o la penetración cutánea relacionadas con la aplicación, a partir de exposiciones prolongadas a polvo que contiene heptacloro, por ejemplo en viviendas tratadas con este compuesto para combatir las termitas, y de manera indirecta mediante la ingesta de alimentos contaminados por heptacloro procedente de los cultivos o de otros alimentos a través de la cadena alimentaria. Sin embargo, el heptacloro es un componente del clordano técnico, así como un metabolito del clordano, por lo que la detección de heptacloro o de heptacloro epóxido no siempre significa de manera inequívoca que la exposición primaria fuera al propio heptacloro (o al heptacloro epóxido).
Un examen de estudios recientes pone de manifiesto que el heptacloro y/o el heptacloro epóxido se encuentran en todos los compartimentos del medio ambiente - aire, agua, suelo y sedimentos, - así como en las plantas (hortalizas), los peces y otros organismos acuáticos, los anfibios y reptiles, las aves y sus huevos y los mamíferos acuáticos y terrestres. Se encuentran sobre todo en el tejido adiposo, donde se acumulan, se incorporan a la cadena alimentaria y se detectan en el suero humano, el tejido adiposo, con inclusión del tejido mamario, y la leche humana.
El heptacloro se absorbe con facilidad por todas las vías de exposición y se metaboliza con rapidez. Los metabolitos fecales más importantes son el heptacloro epóxido, el 1-hidroxiclordeno y el 1-hidroxi-2,3-epoxiclordeno. En microsomas del hígado incubados con heptacloro se metabolizaba a heptacloro epóxido el 85,8% en ratas, pero sólo el 20,4% en las personas. Otros metabolitos identificados en el sistema de microsomas hepáticos humanos fueron el 1-hidroxi-2,3-epoxiclordeno (5%), el 1-hidroxiclordeno (4,8%) y el 1,2-dihidroxidihidroclordeno (0,1%). El heptacloro epóxido se metaboliza con lentitud y es el metabolito más persistente; se acumula principalmente en el tejido adiposo, pero también en el hígado, el riñón y el músculo. Las hembras parecen almacenar más heptacloro epóxido que los machos. Tras la interrupción del suministro de heptacloro a ratas con la alimentación, se necesitó un periodo de 12 semanas para lograr su desaparición completa de la grasa.
Se ha demostrado que en las personas y en los animales de laboratorio el heptacloro y/o el heptacloro epóxido atraviesan la placenta.
Las DL50 agudas por vía oral del heptacloro para la rata y el ratón son de 40–162 y 68–90 mg/kg de peso corporal, respectivamente. La toxicidad aguda del heptacloro en animales está asociada con perturbaciones del sistema nervioso central, tales como hiperexcitabilidad, temblores, convulsiones y parálisis. La toxicidad aguda del heptacloro epóxido es superior a la del heptacloro, mientras que la de los demás metabolitos es muy inferior.
En los animales que reciben heptacloro/heptacloro epóxido con los alimentos, por sonda o mediante inyección subcutánea, se registra una curva de dosis-respuesta pronunciada para la mortalidad. Normalmente no se han observado diferencias importantes entre los animales y los testigos con respecto al peso corporal y el consumo de alimentos. Sin embargo, se ha descrito un aumento del tamaño del hígado asociado con una lobulación acentuada y los resultados histopatológicos pusieron de manifiesto un aumento de los hepatocitos centrolobulares y de la zona intermedia.
En estudios de fecundidad en ratas a las que se inyectó heptacloro por vía subcutánea se obtuvieron LOAEL de 5 mg/kg de peso corporal al día para la supresión de los niveles de hormonas reproductivas, perturbaciones en el ciclo de las hembras y retrasos en el comportamiento de apareamiento.
En los estudios de toxicidad en el desarrollo normalmente no se observaron signos de toxicidad materna (alteraciones del aumento de peso relacionadas con la dosis) hasta que sobrevenía la muerte [NOAEL para la toxicidad materna = 3 mg/kg de peso corporal al día]. En un estudio se observó un tamaño reducido de las camadas, pero el resultado más evidente fue la mortalidad postnatal de las crías [NOAEL para la supervivencia prenatal o postnatal de las crías = 6 mg/kg de peso corporal al día]. No se detectaron efectos teratológicos.
Hay cada vez más pruebas de que los plaguicidas de ciclodieno afectan al sistema nervioso y su desarrollo. El perfil de los efectos producidos por la administración repetida de heptacloro a ratas hembras consistió en la alteración de la actividad, hiperexcitabilidad y efectos autonómicos [NOAEL = 2 mg/kg de peso corporal al día]. Los estudios neurotoxicológicos sobre la exposición perinatal al heptacloro en la rata (0,03, 0,3 ó 3 mg/kg de peso corporal al día) indicaron que había retrasos en el desarrollo, alteraciones en el sistema de neurotransmisión del ácido γ-aminobutirνco y cambios en el neurocomportamiento, con inclusión de deficiencias cognitivas con todas las dosis.
En estudios inmunológicos en las ratas se observó la supresión de las respuestas celulares antiglóbulos rojos de cordero de la Ig M primaria y la Ig G secundaria tras la exposición perinatal a todas las dosis de heptacloro (0,03, 0,3 ó 3 mg/kg de peso corporal al día) sometidas a prueba.
Se ha estudiado la carcinogenicidad del heptacloro, el heptacloro de calidad técnica, el heptacloro epóxido y una mezcla de heptacloro y heptacloro epóxido mediante administración oral en varias razas de ratones y ratas. Se demostró que el heptacloro/heptacloro epóxido y el heptacloro de calidad técnica eran carcinogénicos en ratones machos y hembras, pero no en ratas. En una valoración de iniciación-promoción, el heptacloro fue activo como promotor tras la iniciación mediante .-nitrosodietilamina.
El heptacloro da una respuesta fundamentalmente negativa en las pruebas de genotoxicidad in vitro e in vivo. Provoca inhibición in vitro de la comunicación por uniones intercelulares, lo que parece indicar asimismo un mecanismo carcinogénico no genotóxico.
Los datos epidemiológicos disponibles no muestran una relación clara entre los efectos adversos para la salud y la exposición al heptacloro. Por consiguiente, a partir de los estudios experimentales se preparó una ingesta tolerable. Dado que los tumores hepáticos inducidos por el heptacloro en ratones probablemente se deben a un mecanismo no genotóxico y que se observaron efectos no neoplásicos con dosis 20 veces inferiores a las que indujeron tumores, para obtener la ingesta tolerable se utilizaron los efectos no neoplásicos (es decir, efectos histopatológicos en el hígado, efectos neurotoxicológicos y efectos inmunotoxicológicos). La NOAEL para los efectos hepáticos observados en perros fue de 25 µg/kg de peso corporal al día y para la neurotoxicidad e inmunotoxicidad observada en estudios con ratas fue de 30 µg/kg de peso corporal al día. Aplicando a la NOAEL en perros un factor de incertidumbre de 10 para la variación interespecífica y el mismo para la intraespecífica y un factor adicional de 2 por la deficiencia de la base de datos, se obtiene una ingesta tolerable de 0,1 µg/kg de peso corporal al día para los efectos no neoplásicos.
La ingesta diaria de heptacloro y heptacloro epóxido con la alimentación en Polonia se estimó en 0,51–0,58 µg por persona (alrededor de 0,01 µg/kg de peso corporal, suponiendo un peso medio de 64 kg). Este valor es 10 veces inferior a la ingesta tolerable de 0,1 µg/kg de peso corporal. Sin embargo, si los alimentos están contaminados con heptacloro, como el pescado procedente de ríos contaminados (por ejemplo, recientemente se notificaron concentraciones del orden de 0,1–1 mg/kg en algunas zonas), las hortalizas de campos contaminados con heptacloro (hasta 16 mg/kg) o la leche contaminada (por ejemplo, del orden de µg/kg a mg/kg en algunas regiones), la ingesta de esta sustancia química con la alimentación sería mucho mayor, y si los alimentos contaminados se consumieran durante un largo periodo de tiempo habría un riesgo probable para la salud. En el caso de los lactantes, tomando los valores notificados más altos para el heptacloro epóxido en la leche humana y suponiendo un consumo diario de leche de 150 g/kg de peso corporal y un contenido medio de grasa en la leche del 3,1%, se puede calcular una ingesta media de 1,5 µg/kg de peso corporal al día. Este valor es más de 10 veces superior a la ingesta tolerable de 0,1 µg/kg de peso corporal al día, y si las cantidades notificadas son correctas debe ser motivo de preocupación.
Se examinó la toxicidad aguda del heptacloro utilizando diversas especies acuáticas de diferentes niveles tróficos. Se demostró que el heptacloro era tóxico para los peces y otras especies acuáticas. Sin embargo, hay una gran variabilidad en los niveles de toxicidad notificados, posiblemente debido a la evaporación del heptacloro, reduciéndose de esta manera la concentración real del compuesto con respecto a la concentración nominal de prueba con el paso del tiempo.
Para el entorno de agua dulce, se eligieron 23 valores de la toxicidad a fin de obtener un valor guía. Se derivó un valor guía para el heptacloro de 10 ng/l, basado en la distribución de la sensibilidad de las especies, para la protección del 99% de ellas con una confianza del 50%. En muchos lugares, las concentraciones de heptacloro en el agua dulce eran superiores al valor guía; la mayor concentración de heptacloro notificada medida en el agua dulce superficial, de 62 000 ng/l, era más de 1000 veces superior.
Para el medio marino, se eligieron 18 valores de la toxicidad a fin de obtener un valor guía. Se derivó un valor guía para el heptacloro de 5 ng/l, basado en la distribución de la sensibilidad de las especies, para la protección del 99% de ellas con una confianza del 50%. En el agua de mar, el valor fidedigno más alto para el heptacloro presente es de unos 0,15 ng/l, de manera que no supera el valor guía, indicando un nivel bajo para el medio marino.
De acuerdo con los escasos datos disponibles, el heptacloro parece presentar efectos tóxicos moderados para los vertebrados terrestres. Ninguno de los estudios parece suficientemente fidedigno como para utilizarlo en una caracterización cuantitativa del riesgo. Hay que recordar que el heptacloro se utiliza como termiticida.
En estudios con ratas, el heptacloro ha mostrado efectos neurotóxicos e inmunotóxicos con 0,03 mg/kg de peso corporal al día. El delta del Göksu, en Turquía, una de las más zonas importantes de cría e invernación de aves del mundo, está contaminado por plaguicidas organoclorados procedentes del suelo de las zonas agrícolas que ha arrastrado el río Göksu hasta el delta. En esta región se han detectado niveles de heptacloro/ heptacloro epóxido en las aves y sus huevos en la gama más baja de los mg/kg. En este momento sólo se puede especular sobre el efecto de estas concentraciones de heptacloro en las poblaciones de aves, debido a la falta de datos; sin embargo, en este lugar hay un riesgo potencial para el medio terrestre.
ENDNOTES:
See Also:
Toxicological Abbreviations
Heptachlor (EHC 38, 1984)
Heptachlor (HSG 14, 1988)
Heptachlor (ICSC)
Heptachlor (PIM 578)
Heptachlor (FAO Meeting Report PL/1965/10/1)
Heptachlor (FAO/PL:CP/15)
Heptachlor (FAO/PL:1967/M/11/1)
Heptachlor (FAO/PL:1968/M/9/1)
Heptachlor (FAO/PL:1969/M/17/1)
Heptachlor (AGP:1970/M/12/1)
Heptachlor (WHO Pesticide Residues Series 4)
Heptachlor (WHO Pesticide Residues Series 5)
Heptachlor (Pesticide residues in food: 1991 evaluations Part II Toxicology)