 This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organization, or the World Health Organization.
This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organization, or the World Health Organization.
Concise International Chemical Assessment Document 48
First draft prepared by Drs A. Boehncke, J. Kielhorn, G. Könnecker, C. Pohlenz-Michel, and I. Mangelsdorf, Fraunhofer Institute of Toxicology and Aerosol Research, Drug Research and Clinical Inhalation, Hanover, Germany
Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organization, and the World Health Organization, and produced within the framework of the Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 2003
The International Programme on Chemical Safety (IPCS), established in 1980, is a joint venture of the United Nations Environment Programme (UNEP), the International Labour Organization (ILO), and the World Health Organization (WHO). The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals.
The Inter-Organization Programme for the Sound Management of Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and Agriculture Organization of the United Nations, WHO, the United Nations Industrial Development Organization, the United Nations Institute for Training and Research, and the Organisation for Economic Co-operation and Development (Participating Organizations), following recommendations made by the 1992 UN Conference on Environment and Development to strengthen cooperation and increase coordination in the field of chemical safety. The purpose of the IOMC is to promote coordination of the policies and activities pursued by the Participating Organizations, jointly or separately, to achieve the sound management of chemicals in relation to human health and the environment.
WHO Library Cataloguing-in-Publication Data
4-Chloroaniline.
(Concise international chemical assessment document ; 48)
1.Aniline compounds - adverse effects 2.Risk assessment 3.Environmental exposure
4.Occupational exposure I.International Programme on Chemical Safety II.Series
ISBN 92 4 153048 0 (NLM Classification: QV 632)
ISSN 1020-6167
©World Health Organization 2003
All rights reserved. Publications of the World Health Organization can be obtained from Marketing and Dissemination, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel: +41 22 791 2476; fax: +41 22 791 4857; email: bookorders@who.int). Requests for permission to reproduce or translate WHO publications — whether for sale or for noncommercial distribution — should be addressed to Publications, at the above address (fax: +41 22 791 4806; email: permissions@who.int).
The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement.
The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters.
The World Health Organization does not warrant that the information contained in this publication is complete and correct and shall not be liable for any damages incurred as a result of its use.
The Federal Ministry for the Environment, Nature Conservation and Nuclear Safety, Germany, provided financial support for the printing of this publication.
TABLE OF CONTENTS
Concise International Chemical Assessment Documents (CICADs) are the latest in a family of publications from the International Programme on Chemical Safety (IPCS) — a cooperative programme of the World Health Organization (WHO), the International Labour Organization (ILO), and the United Nations Environment Programme (UNEP). CICADs join the Environmental Health Criteria documents (EHCs) as authoritative documents on the risk assessment of chemicals.
International Chemical Safety Cards on the relevant chemical(s) are attached at the end of the CICAD, to provide the reader with concise information on the protection of human health and on emergency action. They are produced in a separate peer-reviewed procedure at IPCS. They may be complemented by information from IPCS Poison Information Monographs (PIM), similarly produced separately from the CICAD process.
CICADs are concise documents that provide summaries of the relevant scientific information concerning the potential effects of chemicals upon human health and/or the environment. They are based on selected national or regional evaluation documents or on existing EHCs. Before acceptance for publication as CICADs by IPCS, these documents undergo extensive peer review by internationally selected experts to ensure their completeness, accuracy in the way in which the original data are represented, and the validity of the conclusions drawn.
The primary objective of CICADs is characterization of hazard and dose–response from exposure to a chemical. CICADs are not a summary of all available data on a particular chemical; rather, they include only that information considered critical for characterization of the risk posed by the chemical. The critical studies are, however, presented in sufficient detail to support the conclusions drawn. For additional information, the reader should consult the identified source documents upon which the CICAD has been based.
Risks to human health and the environment will vary considerably depending upon the type and extent of exposure. Responsible authorities are strongly encouraged to characterize risk on the basis of locally measured or predicted exposure scenarios. To assist the reader, examples of exposure estimation and risk characterization are provided in CICADs, whenever possible. These examples cannot be considered as representing all possible exposure situations, but are provided as guidance only. The reader is referred to EHC 170.1
While every effort is made to ensure that CICADs represent the current status of knowledge, new information is being developed constantly. Unless otherwise stated, CICADs are based on a search of the scientific literature to the date shown in the executive summary. In the event that a reader becomes aware of new information that would change the conclusions drawn in a CICAD, the reader is requested to contact IPCS to inform it of the new information.Procedures
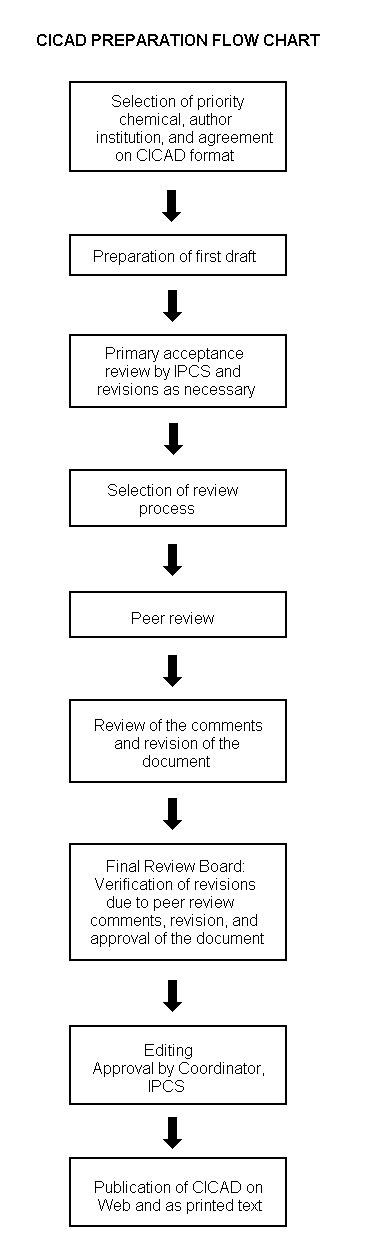
The flow chart on page 2 shows the procedures followed to produce a CICAD. These procedures are designed to take advantage of the expertise that exists around the world — expertise that is required to produce the high-quality evaluations of toxicological, exposure, and other data that are necessary for assessing risks to human health and/or the environment. The IPCS Risk Assessment Steering Group advises the Coordinator, IPCS, on the selection of chemicals for an IPCS risk assessment based on the following criteria:
Thus, it is typical of a priority chemical that

|
Advice from Risk Assessment Steering Group Criteria of priority:
Thus, it is typical of a priority chemical that
Special emphasis is placed on avoiding duplication of effort by WHO and other international organizations. A prerequisite of the production of a CICAD is the availability of a recent high-quality national/regional risk assessment document = source document. The source document and the CICAD may be produced in parallel. If the source document does not contain an environmental section, this may be produced de novo, provided it is not controversial. If no source document is available, IPCS may produce a de novo risk assessment document if the cost is justified. Depending on the complexity and extent of controversy of the issues involved, the steering group may advise on different levels of peer review:
|
The second stage involves international peer review by scientists known for their particular expertise and by scientists selected from an international roster compiled by IPCS through recommendations from IPCS national Contact Points and from IPCS Participating Institutions. Adequate time is allowed for the selected experts to undertake a thorough review. Authors are required to take reviewers’ comments into account and revise their draft, if necessary. The resulting second draft is submitted to a Final Review Board together with the reviewers’ comments. At any stage in the international review process, a consultative group may be necessary to address specific areas of the science.
The CICAD Final Review Board has several important functions:
Board members serve in their personal capacity, not as representatives of any organization, government, or industry. They are selected because of their expertise in human and environmental toxicology or because of their experience in the regulation of chemicals. Boards are chosen according to the range of expertise required for a meeting and the need for balanced geographic representation.
Board members, authors, reviewers, consultants, and advisers who participate in the preparation of a CICAD are required to declare any real or potential conflict of interest in relation to the subjects under discussion at any stage of the process. Representatives of nongovernmental organizations may be invited to observe the proceedings of the Final Review Board. Observers may participate in Board discussions only at the invitation of the Chairperson, and they may not participate in the final decision-making process.
This CICAD on 4-chloroaniline (p-chloroaniline) was prepared by the Fraunhofer Institute of Toxicology and Aerosol Research, Hanover, Germany. It is based on reports prepared by the German Advisory Committee on Existing Chemicals of Environmental Relevance (BUA, 1995), the German MAK Commission (MAK, 1992), and the US National Toxicology Program (NTP, 1989). A comprehensive literature search of relevant databases was conducted in March 2001 to identify any relevant references published subsequent to those incorporated in these reports. Information on the preparation and peer review of the source documents is presented in Appendix 1. Information on the peer review of this CICAD is presented in Appendix 2. This CICAD was approved as an international assessment at a meeting of the Final Review Board, held in Ottawa, Canada, on 29 October – 1 November 2001. Participants at the Final Review Board meeting are listed in Appendix 3. The International Chemical Safety Card on 4-chloroaniline (ICSC 0026), produced by the International Programme on Chemical Safety (IPCS, 1999), has also been reproduced in this document.
Anilines chlorinated at the 2, 3, and 4 (ortho, meta, and para) positions have the same use patterns. All chloroaniline isomers are haematotoxic and show the same pattern of toxicity in rats and mice, but in all cases 4-chloroaniline shows the most severe effects. 4-Chloroaniline is genotoxic in various systems (see below), while the results for 2- and 3-chloroaniline are inconsistent and indicate weak or no genotoxic effects. This CICAD therefore focuses only on 4-chloroaniline as the most toxic of the chlorinated anilines.
4-Chloroaniline (in the following called PCA) (CAS No.
water partition coefficient. It decomposes in the presence of light and air and at elevated temperatures.
PCA is used as an intermediate in the production of a number of products, including agricultural chemicals, azo dyes and pigments, cosmetics, and pharmaceutical products. Thus, releases of PCA into the environment may occur from a number of industrial sources (e.g., production, processing, dyeing/printing industry).
The main environmental target compartment of the chemical can be predicted from its use pattern to be the hydrosphere. Measured concentrations in, for example, the river Rhine and its tributaries are roughly between 0.1 and 1 µg/litre. In the hydrosphere, PCA is rapidly degraded under the influence of light (measured half-lives 2–7 h). The calculated half-life of the chemical in air for the reaction with hydroxyl radicals is 3.9 h. Numerous studies on the biodegradation of PCA indicate it to be inherently biodegradable in water under aerobic conditions, whereas no significant mineralization was detected under anaerobic conditions.
Soil sorption coefficients in a variety of soil types determined according to the Freundlich sorption isotherm indicate only a low potential for soil sorption. In most experiments, soil sorption increased with increasing organic matter and decreasing pH values. As a consequence, under conditions unfavourable for abiotic and biotic degradation, leaching of PCA from soil into groundwater, particularly in soils with a low organic matter content and elevated pH levels, may occur. The available experimental bioconcentration data as well as the measured n-octanol/water partition coefficients indicate no bioaccumulation potential for PCA in aquatic organisms.
PCA is rapidly absorbed and metabolized. The main metabolic pathways of PCA are as follows: a) C-hydroxylation in the ortho position to yield 2-amino-5-chlorophenol followed by sulfate conjugation to 2-amino-5-chlorophenyl sulfate, which is excreted per se or after N-acetylation to N-acetyl-2-amino-5-chlorophenyl sulfate; b) N-acetylation to 4-chloroacetanilide (found mainly in blood), which is further transformed to 4-chloroglycolanilide and then to 4-chlorooxanilic acid (found in the urine); or c) N-oxidation to 4-chlorophenylhydroxylamine and further to 4-chloronitrosobenzene (in erythrocytes).
Reactive metabolites of PCA bind covalently to haemoglobin and to proteins of liver and kidney. In humans, haemoglobin adducts are detectable as early as 30 min after accidental exposure, with a maximum level at 3 h. Slow acetylating individuals have a higher potency to form haemoglobin adducts compared with fast acetylators.
Excretion in animals or humans occurs primarily via the urine, with PCA and its conjugates appearing as early as 30 min after exposure. Excretion takes place mainly during the first 24 h and is almost complete within 72 h.
Oral LD50 values of 300–420 mg/kg body weight for rats, 228–500 mg/kg body weight for mice, and 350 mg/kg body weight for guinea-pigs are reported. Similar values have been found for intraperitoneal and dermal application of PCA to rats, rabbits, and cats. An LC50 value for rats was given as 2340 mg/m3. The prominent toxic effect is methaemoglobin formation. PCA is a more potent and faster methaemoglobin inducer than aniline. PCA also exhibits a nephrotoxic and hepatotoxic potential.
PCA was found to be non-irritating to rabbit skin and slightly irritating to rabbit eyes. A weak sensitizing potential was demonstrated with several test systems.
Repeated exposure to PCA leads to cyanosis and methaemoglobinaemia, followed by effects in blood, liver, spleen, and kidneys, manifested as changes in haematological parameters, splenomegaly, and moderate to heavy haemosiderosis in spleen, liver, and kidney, partially accompanied by extramedullary haematopoiesis. These effects occur secondary to excessive compound-induced haemolysis and are consistent with a regenerative anaemia. The lowest-observed-adverse-effect levels (LOAELs; no-observed-effect levels, or NOELs, are not derivable) for a significant increase in methaemoglobin levels in rats and mice are, respectively, 5 and 7.5 mg/kg body weight per day for a 13-week oral administration of PCA by gavage (5 days/week) and 2 mg/kg body weight per day for rats administered PCA by gavage (5 days/week) at 26, 52, 78, and 103 weeks’ exposure. Fibrotic changes of the spleen were observed in male rats, with a LOAEL of 2 mg/kg body weight per day, and hyperplasia of bone marrow was observed in female rats, with a LOAEL of 6 mg/kg body weight per day (103-week gavage).
PCA is carcinogenic in male rats, with the induction of unusual and rare tumours of the spleen (fibrosarcomas and osteosarcomas), which is typical for aniline and related substances. In female rats, the precancerous stages of the spleen tumours are increased in frequency. Increased incidences of pheochromocytoma of the adrenal gland in male and female rats may have been related to PCA administration. There was some evidence of carcinogenicity in male mice, indicated by hepatocellular tumours and haemangiosarcoma.
PCA shows transforming activity in cell transformation assays. A variety of in vitro genotoxicity tests (e.g., Salmonella mutagenicity test, mouse lymphoma assay, chromosomal aberration test, induction of sister chromatid exchange) indicate that PCA is possibly genotoxic, although results are sometimes conflicting. Due to lack of data, it is impossible to make any conclusion about PCA’s in vivo genotoxicity.
No studies are available on reproductive toxicity.
Data on occupational exposure of humans to PCA are mostly from a few older reports of severe intoxications after accidental exposure to PCA during production. Symptoms include increased methaemoglobin and sulfhaemoglobin levels, cyanosis, the development of anaemia, and changes due to anoxia. PCA has a strong tendency to form haemoglobin adducts, and their determination can be used in biomonitoring of employees exposed to 4-chloroaniline in the workplace.
There are reports of severe methaemoglobinaemia in neonates from neonatal intensive care units in two countries where premature babies were exposed to PCA as a breakdown product of chlorohexidine; the chlorohexidine, which had been inadvertently used in the humidifying fluid, broke down to PCA upon heating in a new type of incubator. Three neonates in one report (14.5–43.5% methaemoglobin) and 33 of 415 neonates in another report (6.5–45.5% methaemoglobin during the 8-month screening period) were found to be methaemoglobin positive. A prospective clinical study showed that immaturity, severe illness, time exposed to PCA, and low concentrations of NADH reductase probably contributed to the condition.
From valid test results available on the toxicity of PCA to various aquatic organisms, PCA can be classified as moderately to highly toxic in the aquatic compartment. The lowest no-observed-effect concentration (NOEC) found in long-term studies with freshwater organisms (Daphnia magna, 21-day NOEC 0.01 mg/litre) was 10 times higher than maximum levels determined in the river Rhine and its tributaries during the 1980s and 1990s. Therefore, a possible risk to aquatic organisms, particularly benthic species, cannot be completely ruled out, particularly in waters where significant amounts of particulate matter inhibit rapid photomineralization. The only benthic species tested, however, exhibited no significant sensitivity (48-h EC50 43 mg/litre), and experiments with Daphnia magna revealed significantly reduced toxicity with increasing concentrations of dissolved humic materials in the medium, possibly caused by reduced bioavailability of PCA from adsorption to dissolved humic materials. Furthermore, bioaccumulation in aquatic species was reported to be very low. Therefore, from the available data, a significant risk associated with exposure of aquatic organisms to PCA is not to be expected.
The data available on microorganisms and plants indicate only a moderate toxicity potential of PCA in the terrestrial environment. There is a 1000-fold safety margin between reported effects and concentrations in soil.
Assessment of consumer exposure to PCA via a number of possible routes leads to total exposure of a maximum 300 ng/kg body weight per day, assuming only 1% penetration by clothes. Considering only non-neoplastic effects (i.e., methaemoglobinaemia), these possible human exposures are within an order of magnitude of the calculated tolerable daily intake of 2 µg/kg body weight per day. Acute incidental exposure to high concentrations of PCA can be fatal.
Further effects that give reason for concern are carcinogenicity and possibly skin sensitization.
Residual levels of PCA in consumer products should be further reduced or entirely eliminated.
4-Chloroaniline (CAS No.
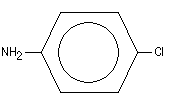
Figure 1: Molecular structure of 4-chloroaniline
The water solubility of PCA is given as 2.6 g/litre at 20 °C (Scheunert, 1981) and 3.9 g/litre (Kilzer et al., 1979). PCA dissociates in water, as it is a weak acid (measured pKa 4.1–4.2 at 20 °C; BUA, 1995). The chemical is furthermore readily soluble in most organic solvents (BUA, 1995). Numerous measured data on vapour pressure are available. The vapour pressure is 0.5 Pa at 10 °C and between 1.4 and 2.1 Pa at 20 °C (BUA, 1995). The n-octanol/water partition coefficients measured by high-performance liquid chromatographic (HPLC) or gas chromatographic (GC) standard methods are 1.83 and 2.05, respectively (Kishida & Otori, 1980; Kotzias, 1981; Garst & Wilson, 1984). From its water solubility and vapour pressure at 20 °C, a Henry’s law constant of about 0.1 Pa·m3/mol (air/water partition coefficient = 4.1 × 10–5) can be calculated for PCA.
PCA decomposes in the presence of light and air and at elevated temperatures (decomposition temperature 250–300 °C; BUA, 1995). Very vigorous reactions may occur with strong oxidants (Hommel, 1985). The decomposition in the presence of light is due to direct photolysis. In ethanolic solution, PCA shows a strong absorption maximum at about 300 nm (concentration not given; absorption coefficient log epsilon [from graphical presentation] = 3.3; Kharkharov, 1954).
The conversion factors2 for PCA in air (20 °C, 101.3 kPa) are as follows:
1 mg/m3 = 0.189 ppm
1 ppm = 5.30 mg/m3
Additional physicochemical properties for PCA are presented in the International Chemical Safety Card (ICSC 0026) reproduced in this document.
PCA can be analysed by either GC or HPLC methods. Analysis by GC (usually capillary columns) is frequently combined with a preceding derivatization step (e.g., diazotation/azo coupling, bromination). All common detectors (flame ionization, phosphorus–nitrogen, thermoionic, and electron capture) are used, with the electron capture detector showing the highest sensitivity. HPLC is in general carried out with reversed phases, ultraviolet (UV) detection being most important. The photodiode-array detector and the electrochemical detector are also applied. For GC and HPLC, mass spectrometric detection can be used for the identification of PCA. Thin-layer chromatographic methods (e.g., for screening purposes) are also described (see, for example, Ramachandran & Gupta, 1993). A detailed compilation of common detection methods is given in BUA (1995). Furthermore, the analytical methods that are described for the isomers 2-chloroaniline and 3-chloroaniline (BUA, 1991) may be used for the detection of PCA.
PCA has been shown to adsorb to several laboratory plastics (silicone, 44%; soft polyvinyl chloride, 30%; rubber, 25%; and polyvinyl acetate, 33%) with a test period of 4 h and a starting concentration of 50 mg/litre (Janicke, 1984). This may affect the recovery rates and sensitivity of the analytical determination of PCA.
The number of methods for the determination of PCA in air is small. Some methods for workplace surveillance (GC and HPLC) are described. However, a thorough validation of these methods is lacking (BIA, 1992; OSHA, 1992). The detection limit is given as 1.1 mg/m3 (OSHA, 1992).
Numerous methods are available for analysis of PCA in the water compartment (BUA, 1995; Holm et al., 1995; Börnick et al., 1996; Götz et al., 1998). Micro-extraction methods are also described (Müller et al., 1997; Fattore et al., 1998). Very low detection limits of up to 0.002 µg/litre can be achieved, especially with GC methods. With HPLC methods, the detection limits are in the range of 0.04–100 µg/litre. The recovery rates are in general near 100%. Also, some methods are available for the determination of PCA in wastewater (Riggin et al., 1983; Gurka, 1985; Onuska et al., 2000). Although no validated chromatographic method is available for the determination of PCA in drinking-water samples, the methods used for groundwater and surface water should be applicable.
For the detection of PCA in soil, a very sensitive GC method was reported (detection limit 1 µg/kg, recovery rate >90%; Wegman et al., 1984).
Combined with an appropriate enrichment technique (e.g., acid hydrolysis), the HPLC and GC methods can also be used in the determination of PCA in biological material such as urine (Lores et al., 1980; Hargesheimer et al., 1981). Recovery rates between 93 and 104% and a detection limit of <5 µg/litre were determined (Lores et al., 1980).
Some HPLC and GC methods are available for the detection of PCA in consumer products such as handwash and mouthwash products and dyed papers and textiles (Kohlbecker, 1989; Gavlick, 1992; Gavlick & Davis, 1994; BGVV, 1996). The method of BGVV (1996) is the official method in Germany for controlling the PCA content in dyed textiles and papers. Recovery rates of about 97% and detection limits up to 43 µg/kg are reported.
There are no known natural sources of PCA.
PCA is produced by low-pressure hydrogenation of 4-chloronitrobenzene in the liquid phase in the presence of noble metal and/or noble metal sulfide catalysts. The addition of metal oxides helps to avoid dehalogenation. The yield is about 98% (Kahl et al., 2000). The two German manufacturers produce PCA either continuously or in a batch process at temperatures of 50–60 °C, at pressures between 1000 and 10 000 kPa, and with toluene or isopropanol as solvent. The raw product is purified by distillation. Catalyst and solvent are recycled into the reactor (BUA, 1995).
In 1988, the global annual production figure was 3500 tonnes (Srour, 1989). A more recent global production figure is not available. In 1990, about 1350 tonnes of PCA were manufactured in the former Federal Republic of Germany, of which about 350 tonnes were exported; about 850 tonnes were processed by the manufacturers themselves. France has registered PCA in the high production volume chemicals programme of the Organisation for Economic Co-operation and Development (OECD), which indicates that the produced amount in this country is >1000 tonnes/year (OECD, 1997). For 1995, combined Western European and Japanese PCA production is given as 3000–3300 tonnes. In India and China, another 800–1300 tonnes/year are produced (Srour, 1996). For 1991, the annual US production quantity is estimated to be 45–450 tonnes (IARC, 1993). More recent data are not available.
PCA is used as an intermediate in the production of several urea herbicides and insecticides (e.g., monuron, diflubenzuron, monolinuron), azo dyes and pigments (e.g., Acid Red 119:1, Pigment Red 184, Pigment Orange 44), and pharmaceutical and cosmetic products (e.g., chlorohexidine, triclocarban [3,4,4'-trichlorocarbanilid], 4-chlorophenol) (Srour, 1989; BUA, 1995; Herbst & Hunger, 1995; Hunger et al., 2000; IFOP, 2001). In 1988, about 65% of the global annual production was processed to pesticides (Srour, 1989). In Germany, in 1990, about 7.5% was used as dye precursors, 20% as intermediates in the cosmetics industry, and 60% as pesticide intermediates. The use for the remaining 12.5% of the production quantity was not specified (BUA, 1995). More recent data on the use pattern of PCA are not available.
The PCA-based azo dyes and pigments are especially used for the dyeing and printing of textiles (Herbst & Hunger, 1995; Hunger et al., 2000). Triclocarban is a bactericide in deodorant soaps, sticks, sprays, and roll-ons (Srour, 1995), and chlorohexidine is used in mouthwashes (BUA, 1995) and spray antiseptics. 4-Chlorophenol is also listed as an antimicrobial agent for cosmetic products in the European Inventory of Cosmetic Ingredients (EC, 2001). However, no information is available on the products in which it is used. All of these products may contain residual PCA, or PCA may emerge during their degradation (see sections 6 and 11).
The marketing and use of products containing PCA-based azo dyes were recently banned by the European Union (EU) (EC, 2000).
The global release of PCA cannot be estimated with the available data.
The releases from the production of PCA at the German manufacturers in 1990 were as follows: <20 g/tonne produced released into air at each site (derived from the registry limit of the emission declaration of 25 kg/year), and 13 g/tonne produced released into surface water. The annual wastes are estimated to be a maximum of 400 g PCA/tonne produced. These wastes are disposed of in special company incinerators (BUA, 1995).
The releases from the processing of PCA at the German manufacturers in 1990 were as follows (assuming a processed quantity of approximately 1000 tonnes/
year): <25 g/tonne processed released into air at each site (derived from the registry limit of the emission declaration of 25 kg/year), and 240 g/tonne processed released into surface water (estimated degradation in industrial wastewater treatment plant about 85%). The annual wastes are estimated to be a maximum of 695 g PCA/tonne processed. These wastes are disposed of in special company incinerators (BUA, 1995).
Total releases of PCA for 1995, 1998, and 1999 in the USA were given as 500, 2814, and 212 kg, respectively (US Toxics Release Inventory, 1999).
In wastepaper and wastewater samples from the industrial wastepaper de-inking process in Germany, PCA (seven samples each) was not detected before or after the bleaching process at a detection limit of 1 mg/kg for solid and 1 mg/litre for liquid samples (Hamm & Putz, 1997). Data on releases from other countries or other industries (dyeing, printing) are not available.
PCA releases into the hydrosphere may furthermore result from the use of pesticides that contain residual PCA and/or form PCA as a degradation product. In some laboratory experiments in the anaerobic water layer over aquarium soil and in water samples of pasture, both being treated with diflubenzuron, PCA concentrations of 0.1 to about 4 µg/litre were detected a few days after the application (Booth & Ferrell, 1977; Schaefer et al., 1980). However, PCA was not detected in the drainage and in the groundwater after the use of diflubenzuron under field conditions in a Finnish forest area (Mutanen et al., 1988).
PCA could, in principle, be released into surface waters from the use of dyed textiles and printed papers. A residual PCA content of <100 mg/kg is reported for German dye products (BUA, 1995). A quantification of the releases from this source is not possible with the available data. However, as noted above, the marketing and use of PCA-based azo dyes have recently been restricted in the EU (EC, 2000).
The releases of PCA into the hydrosphere from the use of pharmaceuticals and cosmetics (e.g., chlorohexidine-based mouthwashes, triclocarban-containing soaps) with residual PCA are also not quantifiable with the available data. The residual PCA content in chlorohexidine is given as <500 mg/kg (<0.05%)3; in triclocarban, it is given as <100 mg/kg (<0.01%).4 If solutions of chlorohexidine are stored for prolonged periods (2 years or more) at high (tropical) ambient temperatures or if they are inadvertently heat sterilized, the PCA content may reach 2000 mg/litre (0.2%) (Scott & Eccleston, 1967; Hjelt et al., 1995).
In 1985, 6.1 tonnes of monochloroanilines (sum of 2-, 3-, and 4-chloroaniline), coming completely from industrial processes, were estimated to be released to the river Rhine (IAWR, 1998).
The application of pesticides (mainly phenylureas) may lead to releases of PCA into soils. Monolinuron is reported to contain an average of 0.1% PCA. The insecticide diflubenzuron and the herbicides monolinuron, buturon, propanil, chlorofenprop-methyl, benzoylpropmethyl, chloroaniformmethane, chlorobromuron, neburon, and oxadiazon can release PCA as a degradation product; this has been confirmed in laboratory experiments with some of these pesticides (diflubenzuron, monolinuron) using radioactive labelling. However, the reported concentrations vary widely. PCA can be released from 3,4-dichloroaniline only under anaerobic conditions. Under aerobic conditions, a complete mineralization without the intermediate synthesis of PCA was observed (BUA, 1995). In general, the results from laboratory tests are supported by a field study on PCA concentrations in agricultural soils in Germany. In 54 of 354 soil samples, PCA was detected with a maximum concentration of 968 µg/kg (Lepschy & Müller, 1991) (see section 6.1). An estimation of the total amount of PCA released from the use of pesticides cannot be made with the available data. In Germany, phenylurea pesticides, which may form PCA during their degradation, are no longer marketed.
Releases of PCA into the biosphere from the application of pesticides also appear to be possible. However, PCA concentrations in wild mushrooms, blueberries, and cranberries were below the detection limit of 10–20 µg/kg after diflubenzuron treatment in a forest area in Finland in 1984 (Mutanen et al., 1988). PCA was also not detected in spinach plants that were cultivated in soil treated with monolinuron or in the subsequently cultivated cultures of cress and potatoes (Schuphan & Ebing, 1978). In contrast, PCA concentrations between 0.9 and 1.3 µg/kg were detected in tissue samples of the bluegill (Lepomis macrochirus) 19 days after the application of diflubenzuron to an artificial pond (detection limit 0.8 µg/kg; calculated diflubenzuron concentration in pond 200 µg/litre; Schaefer et al., 1980).
PCA has a moderate vapour pressure (see section 2). From this, significant adsorption of the substance onto airborne particles is not to be expected. Releases of the chemical into air may, however, be washed out of the atmosphere by wet deposition (e.g., fog, rain, snow). Measured data on this are not available.
From PCA’s water solubility and vapour pressure, a Henry’s law constant of about 0.1 Pa·m3/mol can be calculated (see also section 2), suggesting a low volatility from aqueous solutions (Thomas, 1990). The measured half-life of PCA in water, measured according to a draft OECD Guideline from 1980 (unspecified; probably the Test Guideline for the Determination of the Volatility from Aqueous Solution of February 1980), is 151 days at a water depth of 1 m and a temperature of 20 °C (Scheunert, 1981). From this and its use pattern (see section 4), the hydrosphere is expected to be the main target compartment for PCA.
Evaporation from soil was found to be in the range of 0.11–3.65% of applied PCA, depending on soil type and sorption capacity (Fuchsbichler, 1977; Kilzer et al., 1979).
PCA is hydrolytically stable, as measured according to OECD Guideline A-79.74 D (25 °C; pH 3, pH 7, pH 9) (Lahaniatis, 1981). This is confirmed by measurements at 55 °C and pH 3, pH 7, and pH 11, which gave a half-life of about 3 years (initial concentration 129 mg/litre; Ekici et al., 2001).
From the UV spectrum of PCA (see section 2), direct photolysis of the chemical in air and water appears to be likely. However, as far as the atmosphere is concerned, the main degradation pathway is the reaction of PCA with hydroxyl radicals. The rate constant for this reaction was measured in flash photolysis/resonance fluorescence model experiments to be 8.2 ± 0.4 × 10–11 cm3/molecule per second (Wahner & Zetzsch, 1983). Calculated figures are in the same order of magnitude (BUA, 1995). Assuming a mean global hydroxyl radical concentration of 6 × 105 molecules/cm3 (BUA, 1993), the half-life of PCA in the troposphere can be calculated to be 3.9 h. From this, long-distance transport of PCA in ambient air is assumed to be negligible.
Irradiation of an aqueous PCA solution with light of wavelengths >290 nm (emission maximum 360 nm) led to a half-life of 7.25 h (Kondo et al., 1988) or to total disappearance of the substance after 6 h (Miller & Crosby, 1983). 4-Chloronitrobenzene and 4-chloronitrosobenzene were detected as primary degradation products. 4-Chloronitrobenzene was stable over the irradiation period of 20 h (Miller & Crosby, 1983). Furthermore, very short half-lives of 2 h (summer, 25 °C) and 4 h (winter, 15 °C) were measured in irradiation model experiments with sterilized natural river water (Hwang et al., 1987). From this, it can be concluded that PCA in aqueous solutions is rapidly degraded by direct photolysis.
Numerous tests have been performed on the biodegradability of PCA in various media. Tests performed according to internationally accepted standard procedures under aerobic conditions are summarized in Table 1. Whereas no degradation of PCA was found in tests on ready biodegradability (closed bottle test), >60% removal was observed in most tests on inherent biodegradability. In two of the latter tests (e.g., Zahn- Wellens test), however, nearly half of the elimination could be attributed to adsorption (Rott, 1981b; Haltrich, 1983). In non-standardized tests using spiked test material, 14.5 and 23% of the applied PCA concentration of 25 g/litre were mineralized by activated sludge within 5 days (Rott et al., 1982; Freitag et al., 1985). Thus, under conditions not particularly favouring abiotic removal during sewage treatment, PCA may be applied to agricultural soils in sludges.
Table 1: Elimination of PCA in standard biodegradation tests under aerobic conditions.a
|
Test |
Concentrationb (mg/litre) |
Additional carbon source |
Test duration (days) |
Removal (%) |
Remarks |
Reference |
|
Tests on ready biodegradability |
||||||
|
Closed bottle test |
2 |
No |
28 |
0 |
Rott (1981a) |
|
|
28 |
0–7 |
Haltrich (1983) |
||||
|
30 |
0 |
Janicke & Hilge (1980) |
||||
|
Tests on inherent biodegradability |
||||||
|
Modified OECD screening test |
17.5 DOC |
No |
28 |
10 |
Rott et al. (1982) |
|
|
20 DOC |
No |
28 |
9–80 |
Haltrich (1983) |
||
|
Zahn-Wellens test |
50–400 DOC |
No |
14 |
97 |
Wellens (1990) |
|
|
21 |
68 |
Adsorption 46% after 3 h |
Rott (1981b) |
|||
|
28 |
87 |
Adsorption 46% after 3 h |
Haltrich (1983) |
|||
|
28 |
78 |
|||||
|
28 |
29 |
|||||
|
Modified SCAS test |
20 TOC |
Yes |
17 |
>90 |
Marquart et al. (1984) |
|
|
34/31 |
>96 |
Scheubel (1984) |
||||
|
17 |
100 |
Rott (1984) |
||||
|
12 |
100 |
Fabig et al. (1984) |
||||
|
Confirmatory test |
20 |
Yes |
38 |
96.5 |
Lag phase: 10–16 days |
Janicke & Hilge (1980) |
|
54 |
97 |
|||||
|
a |
The data on methods were taken from Wagner (1988), insofar as they did not originate from the original studies. |
|
b |
DOC = dissolved organic carbon; TOC = total organic carbon. |
Non-standardized experiments with mixed microbial inocula from natural surface water (eutrophic pond, river estuary) showed no significant microbial decomposition of PCA. Observed elimination could be attributed to photodegradation, evaporation, and/or autoxidation (Lyons et al., 1985; Hwang et al., 1987). Thus, under conditions not favouring biotic or abiotic removal in surface waters, adsorption of PCA to sediment particles can be expected.
In several experiments with microbial cultures from soil, removal of PCA was in the range of 0–17% when non-acclimated inocula were used (Alexander & Lustigman, 1966; Fuchsbichler, 1977; Bollag et al., 1978; Süß et al., 1978; Kloskowski et al., 1981a; Cheng et al., 1983). Significant removal of >50% was observed after incubation periods exceeding 8 days only when cultures had been previously cultivated with the herbicide propham (McClure, 1974).
In tests on the incorporation and metabolism of PCA applied to cell suspension cultures of monocotyledon and dicotyledon plants, Harms & Langebartels (1986a,b) observed the formation of considerable amounts of polar metabolites in the cell extracts of soybean (13.5%) and wheat (6.1%).
Under anaerobic conditions, no significant biodegradation was found in sludge (US EPA, 1981; Wagner & Bräutigam, 1981) or aquifer samples (Kuhn & Suflita, 1989).
Under aerobic conditions, PCA released to soil may covalently bind to soil particles, particularly in the presence of high amounts of organic material and/or clay and under low pH levels. However, soil sorption coefficients in a variety of soil types, determined according to the Freundlich sorption isotherm, were in the range of 1.5–50.4, with the highest levels found in soils containing the highest amounts of organic carbon (Fuchsbichler, 1977; van Bladel & Moreale, 1977; Müller-Wegener, 1982; Rippen et al., 1982; Quast, 1984; Scheubel, 1984; Gawlik et al., 1998). In most experiments, soil sorption increased with increasing organic matter and decreasing pH values (Fuchsbichler, 1977; van Bladel & Moreale, 1977). Sorption to the clay fraction was less pronounced (Worobey & Webster, 1982). As a consequence, under conditions unfavourable for abiotic and biotic degradation, leaching of PCA from soil into groundwater, particularly in soils with a low organic matter content and elevated pH levels, may be expected.
Accumulation factors determined for PCA in various aquatic species are summarized in Table 2. For activated sludge and the green alga Chlorella fusca, levels were in the range of 240–280 when based on fresh weight, whereas factors based on dry weight were reported to be up to 1300. However, considering the sorption behaviour of PCA observed in biodegradation tests, the accumulation factors found in sludge and algae are expected to be caused by adsorption to surfaces rather than by bioaccumulation. Bioconcentration factors determined for fish in static and semistatic test systems were considerably lower, ranging from 4 to 20, even at exposure concentrations up to 5 mg/litre. Dissolved humic materials added to the exposure medium had no significant influence on the bioconcentration of PCA in Daphnia magna (Steinberg et al., 1993).
The available experimental bioconcentration data as well as the measured n-octanol/water partition coefficients (1.83 and 2.05) indicate no bioaccumulation potential for PCA in aquatic organisms.
Table 2: Bioaccumulation of PCA in aquatic species.a
|
Species |
Exposure system |
PCA concentration |
Accumulation factor based on fresh or dry weightb |
Determination under equilibrium conditions |
Reference |
|
Activated sludge |
Static |
50 |
280 (fw) |
n.s. |
Freitag et al. (1985) |
|
Activated sludge |
n.s. |
50 |
1300 (dw) |
n.s. |
Korte et al. (1978) |
|
Green algae |
Static |
50 |
260 (fw) |
n.s. |
Geyer et al. (1981) |
|
Green algae |
Static |
n.s. |
240 (fw) |
n.s. |
Kotzias et al. (1980) |
|
Green algae |
Static |
50 |
1200 (dw) |
n.s. |
Korte et al. (1978) |
|
Golden orfe |
Static |
52 |
<10 (fw) |
n.s. |
Freitag et al. (1985) |
|
Golden orfe |
Static |
52 |
<20 (fw) |
n.s. |
Korte et al. (1978) |
|
Zebra fish |
Semistatic |
1000 |
7 (fw) |
yes |
Ballhorn (1984) |
|
Zebra fish |
Static |
25.5 |
8.1 (fw) |
yes |
Kalsch et al. (1991) |
|
Guppy |
Flow-through |
198 |
13.4 (fw) |
yes |
De Wolf et al. (1994) |
|
a |
n.s. = not specified. |
|
b |
fw = fresh weight; dw = dry weight. |
The uptake and incorporation of PCA from soil into cultivated plants have been shown in several experiments (Fuchsbichler, 1977; Kloskowski et al., 1981a,b; Freitag et al., 1984; Harms & Langebartels, 1986a,b; Harms, 1996). PCA was predominantly incorporated in the roots. A translocation into shoots was also detected, with the amount mainly depending on the applied PCA concentration and the growth stage of the exposed plants (Fuchsbichler, 1977). In tests on the incorporation of PCA in cell suspension cultures of monocotyledon plants (corn, wheat), Pawlizki & Pogany (1988) found residual PCA and its metabolites mainly bound to the cell wall in the lignin and pectin fractions; in dicotyledon cell cultures (tomato), it was detected in the starch, protein, and pectin fractions.
Data on the concentrations of PCA in ambient and indoor air are not available.
The concentrations of PCA in surface water from the German and Dutch parts of the river Rhine and its tributaries in the 1980s and 1990s were reviewed in BUA (1995); the levels were roughly between 0.1 and 1 µg/litre. A temporal trend cannot be derived from the data, as the measured concentrations are in the range of the detection limit. In the same period, measured PCA concentrations in surface water in Japan were between 0.024 and 0.39 µg/litre, the chemical being detected in 9 of 128 samples (Office of Health Studies, 1985). In 1992, PCA was not detected at a detection limit of 0.002 µg/litre at two sampling sites in the river Elbe upstream and downstream of Hamburg harbour (Götz et al., 1998). In 1995, the PCA concentrations in the river Rhine and its main tributaries were below the detection limit of 0.5 µg/litre. In the river Emscher, a concentration of 0.84 µg/litre was measured in 1995 (LUA, 1997). More recent data are not available.
In the 1980s and 1990s, PCA was also found in German drinking-water samples at concentrations between 0.007 and 0.013 µg/litre (BUA, 1995). More recent data are not available.
PCA was not detected (detection limit 0.2 µg/litre) in groundwater after the application of the insecticide diflubenzuron in 1984 in Finland (Mutanen et al., 1988). In the groundwater below a Danish landfill site containing domestic wastes and wastes from pharmaceutical production, PCA was detected at concentrations between <10 µg/litre (depth 5.5 m) and 50 µg/litre (depth 8.5 m) (Holm et al., 1995). It is supposed by Holm et al. (1995) that PCA was formed from the wastes from pharmaceutical production (e.g., sulfonamides). In 1995–96, PCA was found in groundwater from three sites in an industrialized area near Milan, Italy, at concentrations between 0.01 and 0.06 µg/litre (positive results in four of seven wells) (Fattore et al., 1998).
In a German agricultural soil measurement programme detecting the degradation products from phenylurea herbicide application, including PCA (see also section 4.4), the following concentrations were found (Lepschy & Müller, 1991):
|
<5 µg/kg (detection limit) |
300 samples |
|
5–10 µg/kg |
18 samples |
|
10–30 µg/kg |
26 samples |
|
30–50 µg/kg |
6 samples |
|
>200 µg/kg |
2 samples (968 µg/kg maximum) |
A maximum of 30 µg/kg was detected in the upper soil horizons of meadows not being treated with phenylurea herbicides (BUA, 1995).
In 1976, PCA was detected in 39 of 121 Japanese sediment samples at concentrations between 1 and 270 µg/kg (detection limit 0.5–1200 µg/kg; Office of Health Studies, 1985). More recent data are not available.
PCA was not detected (detection limit 1000 µg/kg) in two not further specified Japanese fish samples from 1976 (Office of Health Studies, 1985). More recent data concerning the occurrence of PCA in biological material are not available.
Workplace exposure to PCA may occur during production and processing and in industrial dyeing and printing processes. The exposure can be by inhalation of dust containing PCA or by dermal contact with either PCA itself or products containing residual PCA.
For the production of PCA, only some older exposure data from a Hungarian production facility are given by Pacséri et al. (1958), with average values of 58 (range 37–89) and 63 (range 46–70) mg/m3 (two sites measured in one facility). More recent data were not available.
For the processing of PCA, only some older data from a Russian monuron manufacturing facility are available, with concentrations ranging from 0.2 to 2.0 mg/m3 (Levina et al., 1966). More recent data on inhalation exposure were not available.
Studies in a number of US textile dyeing factories, especially during weighing/mixing operations, gave concentration ranges of the colorant between 0.007 and 0.56 mg/m3 (personal sampling of 24 textile weighers; 95th percentile 0.27 mg/m3) for long-term measurements (8-h time-weighted average; US EPA, 1990). Assuming a PCA content in the corresponding dyes and pigments of <100 mg/kg (see section 4.4), PCA levels in the air of these workplaces are estimated to be below 27 ng/m3. Assuming an uptake by inhalation of 100%, an 8-h inhalation volume of about 10 m3, and a body weight of 64 kg (IPCS, 1994), a workshift inhalation intake of PCA of <4 ng/kg body weight per day can be calculated.
Measured or estimated data on dermal exposure to PCA at the different workplaces are not available.
The general public may be exposed to PCA from the use of PCA-based dyed/printed textiles and papers and cosmetic and pharmaceutical products. The exposure can result from residual PCA in the commercial product or from degradation of this product to PCA during use. It can be dermal (wearing of clothes, use of soaps or mouthwashes), oral (small children sucking clothes and other materials, use of mouthwashes), or by direct entry into the bloodstream (e.g., through breakdown products of chlorohexidine in spray antiseptics).
Estimates of exposure to PCA from the wearing of dyed textiles can be carried out on the basis of estimates made by the United Kingdom Laboratory of the Government Chemist (LGC, 1998). For direct dyes, for example, a dye weight of 0.5 g/m2, a weight fraction of 0.8 at 4% depth of shade, and a migration rate of 0.01%/h are assumed. If it is also assumed that the exposure time is 10 h/day, the exposed surface area is 1.7 m2, the percutaneous penetration of the dye is 1%, and the extent of azo cleavage via metabolism is 30% (according to Collier et al., 1993), the effective dermal body dose for PCA can be calculated to be 27 ng/kg body weight per day (average body weight 64 kg; see IPCS, 1994). Assuming 100% percutaneous penetration of the dye, the body dose is estimated at 2.7 µg/kg body weight per day.
The sucking of dyed clothes by small children may lead to oral exposure to PCA. This can also be estimated according to LGC (1998) for, for example, a direct dye (for dyeing and migration assumptions, see above). Assuming an exposure time of 6 h/day, a sucked area of 0.001 m2, and five sucking bursts per minute, with three sucks per burst, oral doses between about 1 µg/kg body weight per day (1% azo cleavage) and 130 µg/kg body weight per day (100% azo cleavage) can be calculated (body weight of young child is 10 kg, according to LGC, 1998).
In contrast, dermal and oral exposure to PCA due to the metabolism of azo compounds on printed textiles can be assumed to be negligible, as pigments are used. The bioavailability of these water-insoluble products is assumed to be low. Therefore, only the exposure from residual PCA has to be considered. Pigment dispersions for textile printing contain between 25 and 50% pigment (Koch & Nordmeyer, 2000). As no further data are available on the pigment application during textile printing, an estimation of dermal exposure from this source is not possible at this time.
From the use of triclocarban-containing deodorant products (see section 4), dermal exposure to residual PCA concentrations can be estimated as follows. In the EU, the maximum permitted amount of triclocarban in cosmetic products is 0.2% (EC, 1999). According to a German manufacturer, commercial triclocarban contains <100 mg PCA/kg.5 This would result in about 0.2 mg PCA/kg cosmetic product at a maximum. Assuming, for example, one application of a triclocarban-containing roll-on antiperspirant per day, a total amount of 0.5 g antiperspirant per application (SCCNFP, 1999), and a dermal absorption of PCA of 100%, this would result in a body dose of 1.6 ng/kg body weight per day at a maximum (average body weight 64 kg; see IPCS, 1994).
From the use of chlorohexidine-containing mouthwashes (see section 4), the following oral and dermal (via mucous membrane) exposure concentrations can be estimated. In the EU, the maximum permitted amount of chlorohexidine in cosmetic products is 0.3% (EC, 1999). According to a German manufacturer, chlorohexidine contains <500 mg PCA/kg,6 resulting in about 1.5 mg PCA/litre commercial chlorohexidine solution at a maximum. PCA concentrations between 0.5 and 2.4 mg/litre were detected in chlorohexidine preparations (chlorohexidine content 0.2%). Assuming two mouthwashes per day with 10 ml of this chlorohexidine solution each, the mucous membrane is exposed to between 10 and 48 µg PCA (Kohlbecker, 1989). About 30% of the chlorohexidine is retained in the oral cavity, and about 4% is swallowed (Bonesvoll et al., 1974). Therefore, uptake of PCA from mouthwash is from 50 to 255 ng/kg body weight (average body weight 64 kg, according to IPCS, 1994).
As no data are available on the use of 4-chlorophenol in cosmetics (see section 4), a quantitative exposure assessment concerning residual or degradation product PCA is not possible.
There is some evidence that PCA may be formed during the chlorination of drinking-water (Stiff & Wheatland, 1984). On the basis of the drinking-water concentrations measured in Germany in the 1980s and 1990s (see section 6.1), body doses of about 0.2–0.4 ng/kg body weight per day can be calculated (drinking-water consumed per day, 2 litres; average body weight, 64 kg; IPCS, 1994).
PCA is rapidly absorbed from the gastrointestinal tract. After nasogastric intubation of rhesus monkeys (20 mg [14C]PCA/kg body weight), the maximal 14C plasma concentration was reached within 0.5–1 h (Ehlhardt & Howbert, 1991).
Acute toxicity studies in rats show that PCA is well absorbed through the skin. LD50 values (see section 8.1; BUA, 1995) as well as methaemoglobin levels (see Table 3; Scott & Eccleston, 1967) are similar for oral, dermal, and intraperitoneal administration. In rats, dermal uptake of PCA seems to be greater than uptake via inhalation exposure (see section 8.1; Kondrashov, 1969b). Dermal absorption also plays a predominant role for humans (Linch, 1974).
Table 3: Methaemoglobin formation after a single dose of PCA.a
|
Species, strain, sex |
Exposure |
Dose, mg/kg body weight (mmol/kg body weight) |
Time after administration |
% methaemoglobin after PCA |
% methaemoglobin after aniline |
Remarks |
Reference |
|
Mouse |
Intraperitoneal |
63.8 |
10 min |
61.5 |
11.2 |
Sulfhaemoglobin formation: PCA significantly |
Nomura (1975) |
|
Wistar rat |
Oral, gavage |
76.5 |
15 min |
26 |
|
Birner & Neumann (1988) |
|
|
Wistar rat |
Oral |
13 |
All 60–90 min |
3.2 |
Cyanosis from 40 mg/kg body weight; methaemoglobin formation reversible within 18–48 h post-administration |
Scott & Eccleston (1967) |
|
|
Dermal |
13–40 |
All 60–90 min |
Comparable to oral administration |
||||
|
Wistar rat |
Intraperitoneal |
1.28 |
5 h |
10.0 |
15.1 |
No information on timepoint of maximal reaction |
Watanabe et al. (1976) |
|
Wistar rat |
Intraperitoneal |
128 |
n.g. |
4.9 |
1.9 |
Methaemoglobin of untreated control: 1.1% |
Yoshida et al. (1989) |
|
New Zealand White rabbit |
Intravenous |
3.2 |
10 min (max) |
3 |
<1 |
Smith et al. (1978) |
|
|
Cat |
Oral |
8.0 |
1 h |
17.1 |
25.4 |
McLean et al. (1969) |
|
|
Cat |
Oral, gavage |
10 |
3 h (max) |
28 |
Heinz bodies: 10 mg/kg body weight: max 39% at 7 h; higher doses up to 100%; mortality: 50 mg/kg body weight 1/2, 100 mg/kg body weight 1/1 |
Bayer AG (1984) |
|
|
Beagle dog |
Oral |
10 |
11–12 |
Methaemoglobinaemia and cyanosis after 1–2 h |
Scott & Eccleston (1967) |
||
|
Monkey |
Oral |
54 |
13.6 |
Methaemoglobinaemia and cyanosis after 1–2 h |
Scott & Eccleston (1967) |
a max = maximal reaction; n.g. = not given; f = female; m = male.
Percutaneous absorption of PCA was studied using in vivo microdialysis in female hairless mutant rats. Dialysates collected from the dermis and the jugular veins were analysed by HPLC, and PCA was found in both dialysates. The highest concentration was found 3 h after the topical application and gradually decreased with a half-life of about 20 h, showing that PCA was able to cross the skin (El Marbouh et al., 2000).
This confirmed results from in vitro studies with PCA using hairless rat skin, which showed that PCA was able to penetrate rat skin to a much greater extent than other aromatic amines tested (Levillain et al., 1998). Another in vitro study had previously shown the penetration of human skin by PCA (Marty & Wepierre, 1979).
After a single intravenous administration of 3 mg [14C]PCA/kg body weight to rats, most of the radioactivity was found within 15 min post-administration in the following tissues (% of dose): liver (8%), muscle (34%), fat (14%), skin (12%), blood (7%), and small intestine and kidney (each about 3%). These tissue levels decreased to less than 0.5% within 72 h.
Elimination from all tissues followed bi-exponential kinetics, with initial elimination half-lives between 1.5 and 4 h (Perry et al., 1981; NTP, 1989). The red blood cell to plasma ratio was 2:1 at 2 h, 20:1 at 12 h, and 74:1 after 2 days, indicating that PCA metabolites were rapidly bound to erythrocytes. After 7 days, radioactivity was found only in the erythrocytes (0.85–2.3% of dose).
After intraperitoneal application of 0.5 or 1.0 mmol [14C]PCA/kg body weight to male Fischer 344 rats, radioactivity was measured in blood, spleen, kidney, and liver. For the low-dose animals at 3 h post-application, the highest tissue concentrations were measured in liver and kidney medulla, followed by spleen (11.04, 9.05, and 4.19 µmol/g tissue, respectively), with 94% of the total dose located in liver. Doubling of the dose to 1.0 mol/kg body weight increased these tissue levels by 65, 83, and 50% to 18.19, 16.61, and 6.26 µmol/g tissue, whereas the percentage of the total dose located in these tissues decreased (e.g., 85% in liver) at 3 h post-application. However, 21 h later, the tissue distribution had changed: tissue concentrations were highest in kidney medulla, followed by spleen and then liver (25.55, 16.7, and 14.91 µmol/g tissue, respectively, corresponding to 3.16, 2.63, and 70% of the total dosage). In the kidney, the concentrations were lower in the cortex than in the medulla. The plasma concentration tended to increase with dose and post-application time, whereas the erythrocyte concentration showed minor changes. Subcellular distribution studies in the renal cortex demonstrated a preferential localization in the cytosolic compartment; in liver, distinct amounts were also found in the microsomal and nuclear fraction. Covalent binding of radioactivity to microsomal and cytosolic proteins of kidney and liver was shown, with little influence of time or dose, however (Dial et al., 1998).
PCA is rapidly metabolized. The main metabolic pathways of PCA are as follows (see Figure 2): a) C-hydroxylation in the ortho position to yield 2-amino-5-chlorophenol followed by sulfate conjugation to 2-amino-5-chlorophenyl sulfate, which is excreted per se or after N-acetylation to N-acetyl-2-amino-5-chlorophenyl sulfate; b) N-acetylation to 4-chloroacetanilide (found mainly in blood), which is further transformed to 4-chloroglycolanilide and then to 4-chlorooxanilic acid (found in the urine); or c) N-oxidation to 4-chlorophenylhydroxylamine and further to 4-chloronitrosobenzene (in erythrocytes). After a single intravenous administration of 3 mg [14C]PCA/kg body weight to rats, PCA in most tissues (except adipose tissue and small intestine) disappeared with bi-exponential decay kinetics, with initial half-lives of about 8 min and terminal half-lives of 3–4 h. However, the PCA concentration was higher at 1 h post-administration in blood, muscle, fat, and skin than at other time points. PCA is rapidly N-acetylated to 4-chloroacetanilide. The highest levels of this metabolite are found in muscle, skin, fat, liver, and blood, but it is not excreted in the urine. 4-Chloroacetanilide had an appearance half-life of approximately 10 min and an elimination half-life of 3 h (NTP, 1989).
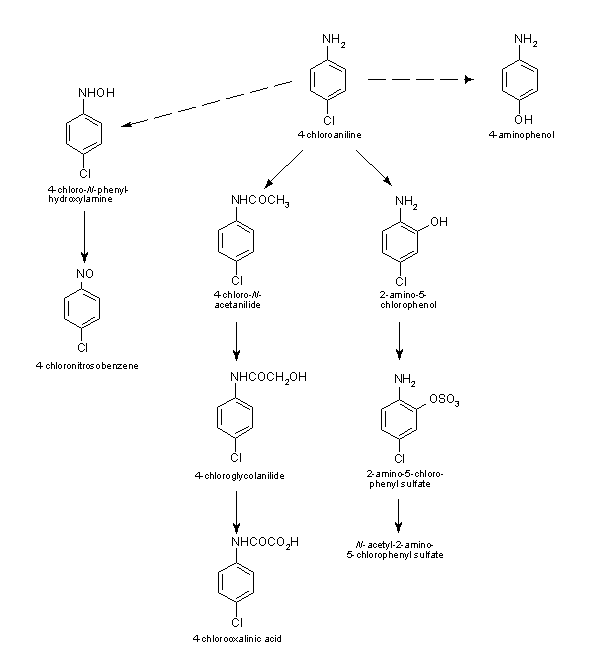
Figure 2: Scheme of metabolic pathways for 4-chloroaniline (MAK, 1992)
In parallel studies in which 14C-labelled PCA at a concentration of 20 mg/kg body weight was administered to three male Fischer rats and six female C3H mice (dosed intragastrically) and two male rhesus monkeys (dosed by nasogastric intubation), 2-amino-5-chlorophenyl sulfate was by far the main excretion product (54, 49, and 36% for rat, mouse, and monkey, respectively) in the 24-h urine, followed by 4-chlorooxanilic acid (11, 6.6, and 1.0%). The parent compound accounted for 0.2, 1.7, and 2.5%, respectively, of the radioactivity in urine. Minor urinary metabolites were N-acetyl-2-amino-5-chlorophenyl sulfate (7.0, <0.1, and 2.0%) and 4-chloroglycolanilide (<1%), whereas 4-chloroacetanilide was not detected in urine. Unknown metabolites accounted for 22, 14, and 14%. Total radioactivity was 80, 88, and 56%, respectively, in 0- to 24-h urine and 4, 7, and 1% in the faeces. In 24- to 48-h urine, 2, 3, and 16% of the radioactivity were found for mouse, rat, and monkey. In the monkey, 6% and 5% of the radioactivity were detected in the urine between 48 and 72 h and between 72 and 96 h, respectively, whereas no more notable amounts of radioactivity were excreted by rat and mouse after 48 h. Therefore, the monkey retains PCA metabolites in the body much longer than the mouse and rat (Ehlhardt & Howbert, 1991).
In a separate study in the monkeys dosed as above (Ehlhardt & Howbert, 1991), the major circulating metabolite in plasma at 1 h post-administration was 2-amino-5-chlorophenyl sulfate (27% of plasma radiocarbon). 4-Chloroacetanilide, which was the major circulating metabolite in the rat (Perry et al., 1981; NTP, 1989), appeared more slowly in monkey plasma, accounting for 26% of the circulating radiolabel at 1 h post-administration, but was the major plasma metabolite (>90%) at 24 h (Ehlhardt & Howbert, 1991).
A study on the disposition of 14C-labelled PCA or its hydrochloride in F344 rats, mongrel dogs, and A/J and Swiss Webster mice (route not given) showed that the initial decay constants for PCA clearance from whole blood in both strains of mice were 10 times greater than those in dogs and rats. The PCA clearance in mice was too rapid to permit calculation of kinetic parameters (NTP, 1989).
Early studies in rabbits given a single oral dose of 100 mg/kg body weight reported the presence of 2-amino-5-chlorophenol in urine (Bray et al., 1956). After a single intraperitoneal administration of 50 mg PCA/kg body weight to rabbits, the metabolites 4-chloroglycolanilide and 4-chlorooxanilic acid (only analysed metabolites) were quantified in equal amounts of 3% of the dosage in the 24-h urine. These metabolites are the excretion products of the primary metabolite 4-chloroacetanilide, as had been demonstrated by direct administration of this compound (Kiese & Lenk, 1971). In similar experiments with pigs (intraperitoneal injection of 20–50 mg PCA/kg body weight), 4-chlorooxanilic acid was not detectable in the urine of pigs (Kiese & Lenk, 1971).
From a case of acute PCA poisoning in humans (no details of exposure/dose), PCA (0.5% free, 62% total), 2-amino-5-chlorophenol (36%), and 2,4-dichloroaniline (1.7%; not reported in other studies), all in free and conjugated form, were detected (using HPLC) as excretory products in the urine (Yoshida et al., 1991). The biphasic elimination of the metabolites 2-amino-5-chlorophenol and 2,4-dichloroaniline was faster (half-lives of 1.7 h for both metabolites in the first phase [T1] and 3.3 and 3.8 h for the two metabolites, respectively, in the second phase [T2]) than that of PCA (all forms: half-lives T1 2.4 h, T2 4.5 h). PCA and 2-amino-5-chlorophenol were still detectable in the urine on days 3 and 4 (Yoshida et al., 1992a,b).
4-Chloronitrosobenzene (plus 4-chlorophenylhydroxylamine) was detected in the blood of dogs after a single intravenous injection of 25 or 100 mg PCA (Kiese, 1963). The relationships between rate of haemoglobin formation, the concentration of 4-chloronitrosobenzene, and time after injection were similar for both doses.
PCA undergoes N-oxidation to 4-chlorophenylhydroxylamine and 4-chloronitrosobenzene in rat liver microsomal preparations (Ping Pan et al., 1979). Further in vitro studies demonstrated that besides the microsomal monooxygenase, haemoglobin, prostaglandin synthetase, and products of lipid peroxidation can also be involved in this reaction (BUA, 1995). Of the possible metabolic reactions of PCA that could be catalysed by the cytochrome P450-dependent monooxygenase system, C2- and N-hydroxylation with 2-amino-5-chlorophenol and 4-chlorophenylhydroxylamine as products are favoured in vitro (values for apparent Vmax 0.54, 2.93, and 4.35 nmol product/min per nanomole cytochrome P450, respectively). Dechlorination followed by C-hydroxylation to 4-aminophenol plays a minor role (Cnubben et al., 1995).
It has been shown for aniline that the N-oxidation pathway is inconsequential in rat liver, as liver rapidly reduces N-oxidized metabolites back to the parent compound. In the erythrocytes, however, N-phenylhydroxylamine is rapidly oxidized by oxyhaemoglobin to nitrosobenzene, with concurrent formation of methaemoglobin (Bus & Popp, 1987). The mechanism and pattern of erythrocyte toxicity of PCA, an analogue of aniline, could be similar (NTP, 1989). It should be remembered that radioactivity was rapidly bound to erythrocytes of rats (Perry et al., 1981).
The methaemoglobin can be reduced to haemoglobin in mammalian species by an NADH-dependent methaemoglobin diaphorase located in the erythrocytes. Enzymatic activity in rat and mouse erythrocytes is 5 and 10 times higher, respectively, than that in human erythrocytes (Smith, 1986), suggesting that humans are more susceptible to this toxic effect.
Besides covalent binding of PCA to proteins of kidney and liver (see section 7.2; Dial et al., 1998), the formation of haemoglobin adducts has been investigated in both rats and exposed humans.
Among 12 chloro- or methyl-substituted anilines, PCA had the strongest potential to bind covalently to haemoglobin. The haemoglobin binding index was 569 in female Wistar rats and 132 in female B6C3F1 mice (dosages: 0.6 and 1 mmol/kg body weight, respectively, by gavage), compared with values of 22 and 2.2 for aniline (dosages: 0.47 and 2 mmol/kg body weight, respectively) (Birner & Neumann, 1987, 1988). The active metabolite responsible for covalent binding is 4-chloronitrosobenzene, which forms a hydrolysable sulfinic acid amide adduct (93% of total haemoglobin adducts). It is predominantly formed in the erythrocytes, as the ratio of the covalent binding index to haemoglobin and plasma proteins is 29.3 (Neumann et al., 1993).
Covalent binding of PCA to haemoglobin was detected in humans with accidental exposure to PCA and aniline (no information on exposure conditions or dose) as early as 30 min after exposure. Maximum haemoglobin adduct levels of PCA were detected 3 h after the accident, which also correlated with the time course of methaemoglobinaemia, whereas the maximum was delayed to 16 h for aniline adducts. For both substances, haemoglobin adducts were detectable up to 7 days post-administration and fell below the detection limit of 10 µg/litre within 12 days (Lewalter & Korallus, 1985). Biomonitoring of workers employed in the synthesis and processing of aniline and PCA (no information on dose or exposure conditions, dermal absorption assumed to prevail) and grouped for smoking habits and acetylator status showed that haemoglobin adduct levels of PCA (22–26 workers) were mostly higher than those of aniline (45 workers). For PCA, haemoglobin adduct levels were not significantly different for smokers (mean 975 ng/litre; range 500–1700 ng/litre) and non-smokers (mean 1340 ng/litre; range 500–2500 ng/litre). However, slow acetylators showed a significantly increased adduct level compared with fast acetylators for the whole group (1443 vs. 663 ng/litre; P = 0.0001) as well as in the subgroup of smokers (1575 vs. 725 ng/litre; P = 0.0052). There was no correlation between haemoglobin adduct level and urinary excretion (see section 7.5) of PCA (Riffelmann et al., 1995).
Excretion of PCA takes place primarily via the urine. Within 24 h, the urinary excretion of rats and mice (intragastric application) and monkeys (nasogastric intubation) of a 20 mg [14C]PCA/kg body weight dosage accounted for 93, 84, and 50–60% of the radiocarbon, respectively, and faecal excretion accounted for 6.9, 4.5, and 0.5–1.0%, respectively. Elimination was complete in the rat (98%) within 48 h and in the mouse (89%; 91% recovered in total) within 72 h, whereas the monkey still excreted considerable amounts, 3.1–5.8%, from 72 to 96 h post-administration (Ehlhardt & Howbert, 1991).
After oral application (gavage) of doses of 0.3, 3, or 30 mg [14C]PCA/kg body weight to rats, 77% of the radiocarbon was excreted in the urine and 10% in the faeces within 24 h, independent of dose. Within 72 h, excretion was almost complete. Consequently, doses up to 30 mg/kg body weight apparently did not saturate the metabolic and excretory pathways (Perry et al., 1981; NTP, 1989).
After intraperitoneal application of 0.5 or 1.0 mmol [14C]PCA/kg body weight to male Fischer 344 rats, excretion was primarily via the urine (5.2 and 1.2% of dose, respectively, within 3 h) compared with the faeces (0.01% for both doses). Within 24 h, urinary excretion rose to 30% of the injected dose. It was argued that excretion could have been retarded relative to oral administration because the parent compound enters the general circulation first before it is metabolized in the liver (Dial et al., 1998).
In humans with accidental exposure to PCA and aniline (no information on exposure conditions or dose), urinary elimination of PCA and aniline in free and conjugated form reached its maximum as early as 30 min after exposure. Detection was possible up to 16 h post-administration (50 µg PCA/g creatinine, 100 µg aniline/g creatinine), but not after 3 days (<10 µg/g) (Lewalter & Korallus, 1985).
Among 22–26 workers employed in the synthesis and processing of aniline and PCA (no information on dose or exposure conditions, dermal absorption assumed to prevail), urinary excretion of PCA (free and conjugated) was similar for smokers and non-smokers, whereas it tended to be higher for slow acetylators than for fast acetylators (no significant increase). There was no correlation between haemoglobin adduct level (see section 7.4) and urinary excretion (Riffelmann et al., 1995).
The LC50 for rats (4-h inhalation, head-only exposure) was calculated as 2340 mg PCA/m3 (vapour/aerosol mixture: respirable fraction 57–95%) (for further details of the study, see Table 4) (BUA, 1995).
Table 4: Acute toxicity of PCA (for methaemoglobin induction, see Table 3).
|
Species |
Exposure |
Dose |
Effectsa |
Remarks |
Reference |
|
Crl:CD rat |
Inhalation, head-only; 4-h exposure, post-observation 14 days |
1690, 1810, 1920, 2101, 2380, 2660 mg/m3; PCA vapour aerosol mixture: respirable fraction 57–95% |
All concentrations: cyanosis and lethargy for up to 24 h; weight loss 7–23%; clouding of the cornea for up to 14 days; mortality: LC50 2340 mg/m3 (2200–2570 mg/m3) |
Other effects not further specified in review |
Du Pont (1981) |
|
Fischer 344 rat |
Intraperitoneal |
51.2, 128, 191 mg/kg body weight (0.4, 1, 1.5 mmol/kg body weight) |
From 128 mg/kg body weight: food and water intake |
Urine volume: no uniform effect ( |
Rankin et al. (1986) |
|
Fischer 344 rat |
Intraperitoneal; post-observation up to 48 h |
191 mg/kg body weight (1.5 mmol/kg body weight) |
Urine: volume significantly |
Food and water intake nearly completely depressed on days 1 and 2 |
Rankin et al. (1996) |
|
Fischer 344 rat |
Intraperitoneal |
128, 191 mg/kg body weight (1–1.5 mmol/kg body weight) |
Dose-dependent |
Impairment of kidney and liver function within 24 h |
Valentovic et al. (1993) |
|
Fischer 344 rat |
Intraperitoneal |
128 mg/kg body weight (1 mmol/kg body weight) |
Urine: volume significantly Plasma: no effect on urea nitrogen Histopathology: renal tubules mild swelling of epithelial cells (1/5) |
Yoshida et al. (1989) |
|
a |
ALT: alanine aminotransferase; BUN: blood urea nitrogen; γ-GTP: γ-glutamyltranspeptidase; |
Mice, cats, and albino rats were exposed by inhalation for 4 h to PCA, and an increase of Heinz bodies in the erythrocytes was observed. The lowest doses at which an increase was seen were 22.5, 21.4, and 36 mg/m3, respectively (Kondrashov, 1969a). In a further inhalation study in albino rats, which applied an experimental device allowing exposure of either the head or the back half of the body (shaved skin) to a PCA-containing atmosphere, the lowest doses at which an increase of Heinz bodies in the erythrocytes was observed were 22 mg/m3 for "body-only" and 36 mg/m3 for head-only exposure, showing that dermal absorption of PCA contributes to the body burden to a greater extent than absorption by the lung (Kondrashov, 1969b).
Compilations of LD50 values are given in BUA (1995) and NTP (1998). Oral LD50 values of 300–420 mg/kg body weight for rats, 228–500 mg/kg body weight for mice, and 350 mg/kg body weight for guinea-pigs are reported. Similar values have been found for intraperitoneal and dermal application of PCA to rats, rabbits, and cats. Signs of intoxication included excitation, tremors, spasms, and shortness of breath (BUA, 1995). Cyanosis, methaemoglobinaemia, and mild hepatotoxic and nephrotoxic changes were reported after acute exposure to PCA (see Tables 3 and 4).
PCA is a more potent methaemoglobin inducer than aniline, as demonstrated in mice, rats, and cats (compilation of studies in Table 3). Very high methaemoglobin formation (60–70% methaemoglobin) occurs as early as 10 min after intraperitoneal application of 64 mg/kg body weight to mice (Nomura, 1975). After oral and dermal administration to rats and after dosing of pregnant rats, similar methaemoglobin levels of 3–15% after a 13–40 mg/kg body weight dose were induced. Rats and monkeys were of comparable sensitivity, whereas dogs were much more sensitive (similar methaemoglobin levels with 16% of the rat dose). Also, in an in vitro assay with whole blood, the dog was the most sensitive species (dog >> human and monkey > rat) (Scott & Eccleston, 1967). For comparison, significant increases of methaemoglobin have been reported for medium-term exposure by gavage, with LOAELs of 5 mg/kg body weight in rats and 7.5 mg/kg body weight in mice (lowest investigated doses) (see Table 5; NTP, 1989).
After intraperitoneal application of PCA to rats in dosages of 128–191 mg/kg body weight, a nephrotoxic and hepatotoxic potential was derived from changes in urine volume and urine and blood chemistry and from slight morphological alterations. However, these dosages lie in the range of 50% of the LD50 and were reported to cause a severe depression of water and food intake (for details, see Table 4).
Table 5: Toxicity studies with repeated oral administration of PCA.a
|
Species, sex, number |
Duration |
Application, dose per day |
Effects |
Reference |
|
Fischer 344 rat |
Exposure: 16 days, 5 days/week, 12 doses in total |
Gavage (as 4-chloroaniline hydrochloride in water) |
Cyanosis: no dose level given |
NTP (1989) |
|
Fischer 344 rat |
Exposure: 4 weeks, |
Diet |
Body weight gain: |
NCI (1979) |
|
Fischer 344 rat |
Exposure: 13 weeks, 5 days/week, 64–65 doses in total |
Gavage (as 4-chloroaniline hydrochloride in water) |
Survival: 10/10, except 80 mg/kg body weight: 9/10 f |
Chhabra et al. (1986, 1990); NTP (1989) |
|
Wistar rat |
3 months |
Diet |
Cyanosis |
Scott & Eccleston (1967) |
|
Albino rat |
3 months |
Gavage (in sunflower oil) |
Cyanosis, reduced movement |
Khamuev (1967) |
|
Fischer 344 rat |
Exposure: 78 weeks, 24 weeks post-observation period |
Diet |
From 15 mg/kg body weight: histology: non-neoplastic proliferative and fibrotic splenic capsular and parenchymal lesions |
NCI (1979) |
|
Fischer 344 rat |
Exposure: 103 weeks, 5 days/week |
Gavage (as 4-chloroaniline hydrochloride in water) |
Body weight gain: 4–6% |
NTP (1989) |
|
Evaluation of time-dependent haematological changes in blood samples: |
||||
|
After 26 weeks From 2 mg/kg body weight:  of mean corpuscular volume, methaemoglobin
of mean corpuscular volume, methaemoglobin From 6 mg/kg body weight:  of haemoglobin, erythrocytes, haematocrit;
of haemoglobin, erythrocytes, haematocrit;  of mean corpuscular haemoglobin
of mean corpuscular haemoglobinFrom 18 mg/kg body weight:  of nucleated erythrocytes, mean corpuscular haemoglobin concentration
of nucleated erythrocytes, mean corpuscular haemoglobin concentration |
||||
|
After 52 weeks From 2 mg/kg body weight:  of reticulocytes
of reticulocytes From 6 mg/kg body weight:  of leukocytes, mean corpuscular volume, segmented neutrophiles, nucleated erythrocytes, methaemoglobin;
of leukocytes, mean corpuscular volume, segmented neutrophiles, nucleated erythrocytes, methaemoglobin;  of lymphocytes
of lymphocytes From 18 mg/kg body weight:  of erythrocytes
of erythrocytes |
||||
|
After 78 weeks From 2 mg/kg body weight:  of reticulocytes, methaemoglobin
of reticulocytes, methaemoglobinFrom 6 mg/kg body weight:  of haemoglobin, haematocrit, erythrocytes;
of haemoglobin, haematocrit, erythrocytes;  of leukocytes, mean corpuscular volume, nucleated erythrocytes, mean corpuscular haemoglobin
of leukocytes, mean corpuscular volume, nucleated erythrocytes, mean corpuscular haemoglobin |
||||
|
After 103 weeks’ exposure followed by 11–14 days without |
||||
|
Data after necropsy at 103 weeks From 6 mg/kg body weight: cyanosis (blue extremities); f only: histology: bone marrow: femoral hyperplasia, femoral reticular cell hyperplasia; anterior pituitary gland: cysts of pars distalis 
18 mg/kg body weight: histology: spleen: fibrosis, fatty metaplasia; bone marrow: femoral hyperplasia  ; m only: liver haemosiderosis; f only: adrenal medulla: hyperplasia
; m only: liver haemosiderosis; f only: adrenal medulla: hyperplasia  ; for neoplastic changes, see Table 7
; for neoplastic changes, see Table 7 |
||||
|
B6C3F1 mouse |
Exposure: 16 days, 5 days/week, 12 doses in total |
Gavage (as 4-chloroaniline hydrochloride in water) |
Cyanosis: no dose level given |
NTP (1989) |
|
B6C3F1 mouse |
Exposure: 4 weeks, 2 weeks post-observation |
Diet |
Body weight gain: slightly |
NCI (1979) |
|
B6C3F1 mouse |
Exposure: 13 weeks, 5 days/week, 66–67 doses in total |
Gavage (as 4-chloroaniline hydrochloride in water) |
Survival partly |
Chhabra et al. (1986, 1990); NTP (1989) |
|
B6C3F1 mouse |
Exposure: 78 weeks, 13 weeks post-observation period |
Diet |
Moderate to heavy haemosiderosis of spleen, liver, kidney |
NCI (1979) |
|
B6C3F1 mouse |
Exposure: 103 weeks, 5 days/week |
Gavage (as 4-chloroaniline hydrochloride in water) |
Body weight gain: up to 5% |
NTP (1989) |
|
Guinea-pig |
7 months |
Gavage (in sunflower oil) |
From 0.5 mg/kg body weight: dystrophic changes of liver and kidneys |
Khamuev (1967) |
|
Beagle dog |
3 months |
Diet |
Cyanosis |
Scott & Eccleston (1967) |
|
a |
f = female; m = male; n.g. = no further information. |
|
b |
1 ppm in diet corresponding to 0.1 mg/kg body weight. |
|
c |
NTP (1989). |
|
d |
1 ppm in diet corresponding to 0.15 mg/kg body weight. |
In OECD guideline studies, PCA was found to be non-irritating to rabbit skin and slightly irritating to rabbit eyes (grade 1–2 effects). Older studies reported an absence of irritant effects on rat skin in contrast to inflammatory skin reactions of rabbits and cats, whereas effects on mucous membranes in these studies were characterized as slight to severe (limited validity of data because of insufficient documentation) (for details, see BUA, 1995).
In guinea-pigs, PCA was tested by three different testing procedures with the following classifications: moderate sensitizer in the maximization test (50% positive response), very weak sensitizer in the single injection adjuvant test (30% positive response), and no sensitizing potential in a modified Draize test (0% positive response) (Goodwin et al., 1981). Another group compared the sensitizing potential of PCA in the guinea-pig maximization test according to Magnusson & Kligman (1969) and the local lymph node assay (Kimber et al., 1986). In the guinea-pig maximization test (vehicle ethanol, intradermal induction with 0.3% PCA, topical induction with 10.0% PCA, and challenge with 2.5%), 50–60% of the animals responded with a positive reaction, so that PCA was classified as moderately sensitizing. Test results of the local lymph node assay from four independent laboratories (test concentrations 2.5, 5.0, and 10.0% in acetone–olive oil 4:1) pointed to a sensitizing potential, as test results in one laboratory were slightly positive and were confirmed by concentration-dependent increases (suggestive of sensitization) in two other laboratories. It was speculated that positive responses would have been obtained if testing with higher concentrations had been possible, but the high toxicity of PCA was limiting (Basketter & Scholes, 1992; Scholes et al., 1992). A weak sensitizing potential was reported in a further maximization test (BUA, 1995). From these data, PCA can be considered to be a skin sensitizer.
A 2-week inhalation exposure of rats from the lowest concentration of 12 mg PCA/m3 induced methaemoglobinaemia and increased haemolysis, which was apparent from decreased red blood cell count and both haemosiderosis and haematopoiesis in the spleen. Cyanosis appeared from 53 mg/m3 (for details, see Table 6; Du Pont, 1982).
Table 6: Toxicity studies with repeated inhalation of PCA.a
|
Species, sex, number |
Duration |
Exposure concentration |
Effects |
Reference |
|
Crl:CD rat |
Exposure: 2 weeks, 5 days/week, 6 h/day, 2 weeks post-observation |
0, 12, 53, 120 mg/m3 |
From 12 mg/m3: haematology: red blood cell count |
Du Pont (1982) |
|
Rat (n.g.) |
Exposure: 4 months, 6 days/week, 4 h/day, 1 month post-observation |
0, 1.0, 9.5 mg/m3 |
1.0 mg/m3: after 4 months: severe aggression; haematology: haemoglobin, erythrocytes significantly |
Kondrashov (1969b) |
|
Rat (n.g.) |
Exposure: 3 months |
0.15 mg/m3 |
Concentrations analytically controlled |
Zvezdaj (1970) |
|
Exposure: 6 months |
0, 1.5, 15 mg/m3 |
|||
|
Cat (n.g.) |
Exposure: 4 months, 6 days/week, 4 h/day, 1 month post-observation |
0, 1.04, 6.9 mg/m3 |
1.04 mg/m3: from 2 months: significant |
Kondrashov (1969b) |
a m = male; n.g. = not given.
Oral administration of 25–400 mg PCA/kg body weight (12 gavages in 16 days) to rats and mice led to cyanosis (from 100 mg/kg body weight), signs of intoxication (from 25 mg/kg body weight), and haemosiderosis (from 100 mg/kg body weight) (see Table 5; NTP, 1989).
Rats inhaling PCA for 3–6 months in concentrations ranging from 0.15 to 15 mg/m3 showed haematological disorders from 1.0 mg/m3 and slight methaemoglobinaemia from 1.5 mg/m3 (for details, see Table 6; Kondrashov, 1969b; Zvezdaj, 1970). From these insufficiently documented studies, a reliable no-observed-adverse- effect concentration/level (NOAEC/L) cannot be derived.
The targets of PCA-induced changes are the blood, liver, spleen, and kidneys. Changes in haematological parameters, splenomegaly, and moderate to heavy haemosiderosis in spleen, liver, and kidney, partially accompanied by extramedullary haematopoiesis, occur, indicating excessive compound-induced haemolysis. These effects are reported from the lowest tested dose of 5 mg/kg body weight in rats and 7.5 mg/kg body weight in mice after 13 weeks of gavage (for details, see Table 5; Chhabra et al., 1986, 1990; NTP, 1989), as well as after feeding of daily doses of 50 mg/kg body weight to rats or 5 mg/kg body weight to dogs (for details, see Table 5; Scott & Eccleston, 1967). Methaemoglobin levels are significantly increased from 5 mg/kg body weight in rats (males: 0.59%, females: 1.35%; controls: 0.08%, 0.46%) and from 7.5 and 15 mg/kg body weight in male and female mice, respectively (NTP, 1989).
Time-dependent haematological changes were investigated after gavage of rats with 4-chloroaniline hydrochloride in water (dosage 2, 6, or 18 mg/kg body weight) for up to 103 weeks. Minimal to moderate dose-dependent changes were found after 23–78 weeks. They were reversible to a minimal grade within 11–14 days in rats exposed for 103 weeks, allowing the conclusion that the direct haematological effects of PCA are transient. The most sensitive parameters, affected by a dose of 2 mg/kg body weight (the LOAEL), were methaemoglobin level, number of reticulocytes, and mean corpuscular volume. The observed changes are consistent with a regenerative anaemia secondary to a decreased erythrocyte mass. The increases in methaemoglobin concentration indicate a haemolytic mechanism through the oxidation and subsequent denaturation of haemoglobin (for details, see Table 5; NTP, 1989). Furthermore, haemosiderosis in spleen, liver, and kidney is indicative of excessive haemolysis (NCI, 1979; NTP, 1989). Cyanosis was reported from a dose of 6 mg/kg body weight (NTP, 1989).
Histological examination of rats exposed to PCA for 78 weeks by feeding showed non-neoplastic proliferative and fibrotic lesions of the spleen from the lowest dose of 15 mg/kg body weight (for details, see Table 5; NCI, 1979). In the gavage study, fibrotic splenic changes were observed in male rats from 2 mg/kg body weight (LOAEL). Hyperplasia in bone marrow was reported for female rats from 6 mg/kg body weight (LOAEL) and for male rats from 18 mg/kg body weight (for details, see Table 5; NTP, 1989).
The carcinogenicity of PCA in Fischer 344 rats and B6C3F1 mice was examined in a 78-week feeding study (375 and 750 mg/kg body weight; NCI, 1979) and in a 103-week gavage study (2, 6, and 18 mg/kg body weight; NTP, 1989; Chhabra et al., 1991). In the NCI (1979) study, rare splenic neoplasms were found in dosed male rats, but the number of neoplasms was considered to be insufficient to allow the carcinogenicity to be clearly established. Furthermore, PCA is unstable in feed, and the animals may have received the chemical at less than the targeted concentration. The details of the NTP (1989) study are given in Tables 7 and 8.
In the NTP (1989) study, PCA was found to be carcinogenic in male rats, based on the increased incidence of splenic sarcoma, osteosarcoma, and haemangiosarcoma in high-dose (18 mg/kg body weight) rats, but the dose–response was non-linear. At 2 and 6 mg/kg body weight, the incidence of sarcomas was marginal. Fibrosis of the spleen, which is a potential preneoplastic lesion that may progress to fibrosarcomas, was seen in all dose groups (Goodman et al., 1984; NTP, 1989). It should be noted that in historical control male rat studies (NTP, 1989), the splenic fibrosis incidence is 12/299 animals, with a mean of 4% and a maximum incidence of 5/50 or 10%. This markedly decreases the potential difference between the control and low-dose animals.
In females, splenic neoplasms were seen only in one mid-dose rat and one high-dose rat. The induction of unusual and rare tumours of the spleen is typical for aniline and structurally related substances. It is sex-specific, occurring mainly in male rats, and exhibits a non-linear response. Increased incidences of pheochromocytoma of the adrenal gland in male and female rats (see Table 7) may have been related to PCA administration. Marginal increases of common interstitial cell adenomas of the testes were not considered to be substance related. The incidence of mononuclear cell leukaemia was significantly depressed in dosed rats (NTP, 1989).
Table 7: Carcinogenicity studies with PCA in rats.a
|
Incidence at following doses (mg/kg body weight) |
||||||||||
|
Males |
Females |
|||||||||
|
0 |
2 |
6 |
18 |
0 |
2 |
6 |
18 |
|||
|
Spleen |
Fibrosis |
3/49 |
11/50 |
12/50 |
41/50 |
1/50 |
2/50 |
3/50 |
42/50 |
|
|
Tumours |
Fibroma |
0/49 |
0/50 |
0/50 |
2/50 |
|||||
|
|
Fibrosarcomab (1) |
0/49 |
1/50 |
2/50 |
17/50c |
0/50 |
0/50 |
1/50 |
0/50 |
|
|
Osteosarcomab (2) |
0/49 |
0/50 |
1/50 |
19/50c |
0/50 |
0/50 |
0/50 |
1/50 |
||
|
Haemangiosarcomad (3) |
0/49 |
0/50 |
0/50 |
4/50 |
||||||
|
(1), (2), or (3) |
0/49 |
1/50 |
3/50 |
36/50c |
||||||
|
Adrenal medulla |
Hyperplasia |
15/49 |
21/48 |
15/48 |
17/49 |
4/50 |
4/50 |
7/50 |
24/50 |
|
|
Tumours |
Pheochromocytoma (4) |
13/49 |
14/48 |
14/48 |
25/49c |
2/50 |
3/50 |
1/50 |
6/50 |
|
|
Malignant pheochromocytoma (5) |
1/49 |
0/48 |
1/48 |
1/49 |
||||||
|
(4) or (5) |
13/49 |
14/48 |
15/48 |
26/49e |
||||||
|
Haematopoietic system |
Mononuclear cell leukaemia |
21/49 |
3/50 |
2/50 |
3/50 |
10/50 |
2/50 |
1/50 |
1/50 |
|
|
Testis |
Interstitial cell adenomas |
36/49 |
44/46e |
44/50 |
46/50f |
|||||
|
a |
Experimental details are as follows: |
|
|
Reference: |
NTP, 1989; Chhabra et al., 1991 |
|
|
Substance: |
4-Chloroaniline hydrochloride, technical grade (purity 99.1%) |
|
|
Species: |
Fischer 344 rat: 50 m and 50 f/group |
|
|
Administration route: |
Oral, gavage (vehicle: water containing hydrogen chloride) |
|
|
Dose: |
0, 2, 6, 18 mg/kg body weight |
|
|
Duration: |
5 days/week, 103 weeks |
|
|
Toxicity: |
Body weight gain: 4–6% |
|
|
Remarks: |
Clear evidence of carcinogenicity for male rats indicated by increased incidence of splenic sarcomas; possible association for induction of pheochromocytomas; equivocal evidence of carcinogenic activity for female rats indicated by uncommon sarcomas of the spleen and increased pheochromocytomas |
|
|
b |
Historical incidence of all sarcomas (no fibrosarcomas or osteosarcomas observed): male rats: water vehicle 1/298 (0.3%); untreated control animals 8/1906 (0.4%); female rats: water vehicle 0/297; untreated control animals 1/1961 (0.05%). |
|
|
c |
Significant in Fisher Exact Test and Cochran-Armitage test: P < 0.001. |
|
|
d |
Historical incidence of haemangiosarcomas or haemangiomas (for all organs): water vehicle 2/300 (0.7%); untreated control animals 12/1936 (0.6%). |
|
|
e |
Significant in Fisher Exact Test: P < 0.01. |
|
|
f |
Significant in Fisher Exact Test: P < 0.05. |
|
No splenic fibrosarcomas or osteosarcomas were observed in mice of either sex (see Table 8). There was some evidence of carcinogenicity in male mice, indicated by hepatocellular tumours and haemangiosarcoma.
Table 8: Carcinogenicity studies with PCA in mice.a
|
Tumours |
Incidence at following doses (mg/kg body weight) |
|||||||||
|
Males |
Females |
|||||||||
|
0 |
3 |
10 |
30 |
0 |
3 |
10 |
30 |
|||
|
Liver |
Adenoma |
9/50 |
15/49 |
10/50 |
4/50 |
|||||
|
Carcinomab |
3/50 |
7/49 |
11/50c |
17/50d |
||||||
|
Adenoma or carcinomae |
11/50 |
21/49c |
20/50c |
21/50c |
6/50 |
9/50 |
8/50 |
11/50 |
||
|
Haemangiosarcoma |
2/50 |
2/49 |
1/50 |
6/50 |
||||||
|
Spleen |
Haemangiosarcoma |
3/50 |
2/49 |
0/50 |
5/50 |
|||||
|
All sites |
Haemangiosarcomaf |
4/50 |
4/49 |
1/50 |
10/50 |
|||||
|
Haematopoietic system |
Malignant lymphomas |
10/50 |
3/49 |
9/50 |
3/50 |
19/50 |
12/50 |
5/50 |
10/50 |
|
|
a |
Experimental details are as follows: |
|
|
Reference: |
NTP, 1989; Chhabra et al., 1991 |
|
|
Substance: |
4-Chloroaniline hydrochloride, technical grade |
|
|
Species: |
B6C3F1 mouse: 50 m and 50 f/group |
|
|
Administration route: |
Oral, gavage (vehicle: water containing hydrogen chloride) |
|
|
Dose: |
0, 3, 10, 30 mg/kg body weight |
|
|
Duration: |
5 days/week, 103 weeks |
|
|
Toxicity: |
Body weight gain: up to 5% |
|
|
Remarks: |
Some evidence of carcinogenicity for male mice indicated by hepatocellular tumours and haemangiosarcomas; no evidence of carcinogenicity for female mice |
|
|
b |
Historical incidence of liver carcinomas: water vehicle 56/347 (16%); untreated control animals 379/2032 (19%). |
|
|
c |
Significant in Fisher Exact Test: P < 0.05. |
|
|
d |
Significant in Fisher Exact Test: P < 0.001. |
|
|
e |
Historical incidence of liver adenomas and carcinomas: water vehicle 106/347 (31%); untreated control animals 609/2032 (30%). |
|
|
f |
Historical incidence of haemangiosarcomas or haemangiomas (for all organs): water vehicle 11/350 (3%); untreated control animals 98/2040 (5%). |
|
In the mouse studies, induction of liver tumours was related to PCA exposure, as has also been demonstrated for other aromatic amines. None of the aromatic amines studied for their carcinogenic potential by the US National Cancer Institute/National Toxicology Program caused increased incidences of splenic tumours in mice, although liver tumours were often induced (NTP, 1989). There was no evidence of carcinogenicity in female mice (NTP, 1989).
The final conclusion of NTP (1989) was that there was clear evidence of carcinogenicity in male rats, equivocal evidence of carcinogenicity in female rats, some evidence of carcinogenicity in male mice, and no evidence of carcinogenicity in female mice.
In the Strain A mouse pulmonary tumour test, PCA showed no tumorigenic activity. When PCA was given intraperitoneally at doses of 25, 57.5, or 60 mg/kg body weight (vehicle tricaprylin), 3 times per week for 8 weeks, to 10 mice of each sex per dose group, only 1 male in the low-dose group developed a lung adenoma. Survival rates of females were unaffected, whereas those of males were 10/10, 4/10, and 9/10, respectively (Maronpot et al., 1986).
The in vitro genotoxicity of 4-chloroaniline has been studied in a variety of studies (compilation of data in Table 9). These concentrated on the investigation of the mutagenic potential in bacteria and mammalian cells and of unscheduled DNA synthesis in primary rat hepatocytes, whereas only single studies exist on other end-points, such as mitotic recombination, induction of DNA strand breaks, chromosomal aberrations, and sister chromatid exchange.
Table 9: In vitro genotoxicity of PCA.
|
Test system |
Strain/cell type |
Concentrations tested |
Resulta |
Remarks |
Reference |
|
|
–S9 |
+S9 |
|||||
|
Salmonella mutagenicity test |
Salmonella typhimurium TA 1538 |
50–100 µg/plate |
– |
– |
Plate incorporation assay |
Garner & Nutman (1977) |
|
Salmonella mutagenicity est |
S. typhimurium TA 98, TA 100, TA 1530, TA 1532, TA 1535, TA 1537, TA 1538, TA 1950, TA 1975, TA 1978, G 46 |
1–2000 µg/plate |
– |
– |
Plate incorporation assay and fluctuation test in aerobic and anaerobic conditions |
Gilbert et al. (1980) |
|
Salmonella mutagenicity test |
S. typhimurium TA 98 |
33–5000 µg/plate |
– |
+ |
Plate incorporation assay |
Dunkel & Simmon (1980) |
|
Salmonella mutagenicity test |
S. typhimurium TA 98, TA 1538 |
0.3–5000 µg/plate |
– |
+ |
Plate incorporation assay; no cytotoxicity |
Dunkel et al. (1985); NTP (1989) |
|
S. typhimurium TA 100, TA 1535, TA 1537 |
– |
– |
||||
|
Salmonella mutagenicity test |
S. typhimurium TA 100 |
1–2000 µg/plate |
n.t. |
+ |
Plate incorporation assay; 90–100% survival |
McGregor et al. (1984) |
|
Salmonella mutagenicity test |
S. typhimurium TA 97, TA 100, TA 1535, TA 1537 |
10–3333 µg/plate |
– |
– |
Preincubation assay; cytotoxicity from 1666 µg/plate |
Mortelmans et al. (1986) |
|
S. typhimurium TA 98 |
33–1666 µg/plate |
– |
+ |
|||
|
Salmonella mutagenicity test |
S. typhimurium TA 100 |
1–2000 µg/plate |
n.t. |
+ |
Plate incorporation assay; 90–100% survival |
McGregor et al. (1984) |
|
Salmonella mutagenicity test |
S. typhimurium TA 98, TA 100, TA 1535 |
0.1–1000 µg/plate |
– |
n.t. |
Plate incorporation assay |
Pai et al. (1985) |
|
Salmonella mutagenicity test |
S. typhimurium TA 1535, TA 1538 |
250 µg/plate |
– |
– |
Plate incorporation assay |
Rosenkranz & Poirier (1979) |
|
Salmonella mutagenicity test |
S. typhimurium TA 98, TA 100, TA 1535, TA 1537, TA 1538, C 3076, D 3052, G 46 |
–1000 µg/ml |
– |
– |
Plate incorporation assay |
Thompson et al. (1983) |
|
Salmonella mutagenicity test |
S. typhimurium TA 98, TA 100 |
1–1000 µg/plate |
– |
– |
Plate incorporation assay |
Rashid et al. (1987) |
|
Salmonella mutagenicity test |
S. typhimurium TA 100 |
Not given |
n.t. |
– |
Plate incorporation assay |
Zimmer et al. (1980) |
|
Salmonella mutagenicity test |
S. typhimurium TA 98, TA 100, TA 1535, TA 1537, TA 1538 |
–1000 µg/plate |
– |
– |
Plate incorporation assay |
Simmon (1979a) |
|
Salmonella mutagenicity test |
S. typhimurium TA 98, TA 100, TA 1535, TA 1537, TA 1538 |
1000 µg/plate |
Insufficient documentation of method and results |
Seuferer et al. (1979) |
||
|
Salmonella mutagenicity test |
S. typhimurium TA 98, TA 100, TA 1535, TA 1537, TA 1538 |
10, 20, 50 µg/ml |
– |
– |
Spot test |
van der Bijl et al. (1984) |
|
100–5000 µg/plate |
– |
+ |
Plate incorporation assay: positive only for TA 98 in the presence of S9 from Aroclor 1254-induced rats |
|||
|
Salmonella mutagenicity test |
S. typhimurium TA 98 |
10–2000 µg/plate |
– |
+ |
Preincubation assay |
Zeiger (1990) |
|
S. typhimurium TA 100 |
– |
(+) |
||||
|
S. typhimurium TA 97, TA 1535, TA 1537 |
– |
– |
||||
|
Umu-test |
S. typhimurium TA 1535/pSK1002 |
100 µg/ml |
– |
– |
Phenobarbital-induced S9 from rat |
Ono et al. (1992) |
|
Umu-test |
S. typhimurium TA 1535/pSK1002 |
–800 µg/ml |
– |
– |
Sakagami et al. (1988) |
|
|
E. coli mutagenicity test |
Escherichia coli WP2 uvrA |
0.3–5000 µg/plate |
– |
– |
Tested with rat, mouse, and hamster S9, no cytotoxicity |
Dunkel et al. (1985) |
|
E. coli mutagenicity test |
E. coli WP2 uvrA |
0.1–1000 µg/plate |
– |
n.t. |
Plate incorporation assay |
Pai et al. (1985) |
|
E. coli WP2 uvrA(P) |
0.075–0.3 mmol/litre |
– |
n.t. |
Fluctuation test |
||
|
E. coli mutagenicity test |
E. coli WP2, WP2 uvrA– |
–1000 µg/ml |
– |
– |
Plate incorporation assay |
Thompson et al. (1983) |
|
Pol A test |
E. coli pol A+, pol A– |
5 µg/ml |
+ |
+ |
S9 from uninduced rats |
Rosenkranz & Poirier (1979) |
|
Mitotic recombination |
Saccharomyces cerevisiae D3 |
2000 µg/ml |
– |
– |
S9 from Aroclor 1254-induced rats |
Simmon (1979b) |
|
Mutagenicity test in Aspergillus nidulans |
Aspergillus nidulans auxotroph for methionine |
200 µg/ml |
+ |
n.t. |
55% survival |
Prasad (1970) |
|
Mouse lymphoma mutagenicity assay |
L5178Y mouse lymphoma cells |
–500 µg/ml (–S9) |
+ |
+ |
Parallel tests in two laboratories |
Caspary et al. (1988) |
|
–200 µg/ml (+S9) |
||||||
|
Mouse lymphoma mutagenicity assay |
L5178Y mouse lymphoma cells |
–500 µg/ml (–S9) |
(+) |
(+) |
Cytotoxicity at 60 µg/ml with S9 |
Mitchell et al. (1988) |
|
15–60 µg/ml (+S9) |
||||||
|
Mouse lymphoma mutagenicity assay |
L5178Y mouse lymphoma cells |
31–1000 µg/ml |
+ |
+ |
Cytotoxicity from 400 µg/ml without S9 |
Myhr & Caspary (1988) |
|
7.8–200 µg/ml (+S9) |
||||||
|
Mouse lymphoma mutagenicity assay |
L5178Y mouse lymphoma cells |
31–1000 µg/ml |
+ |
+ |
Parallel tests in two laboratories |
NTP (1989); Myhr et al. (1990) |
|
7.8–400 µg/ml (+S9) |
||||||
|
Mouse lymphoma mutagenicity assay |
L5178Y mouse lymphoma cells |
0.5–2.5 mmol/litre |
+ |
n.t. |
Wangenheim & Bolcsfoldi (1988) |
|
|
DNA strand breaks |
L5178Y mouse lymphoma cells |
0.5–3.0 mmol/litre |
– |
n.t. |
20% relative toxicity at 3.0 mmol/litre |
Garberg et al. (1988) |
|
Chromosome aberration test |
Chinese hamster ovary cells |
400–1000 µg/ml |
– |
+ |
Inconsistent results in parallel tests in two laboratories |
NTP (1989); Anderson et al. (1990) |
|
1.6–800 µg/ml |
+ |
– |
||||
|
Sister chromatid exchange |
Chinese hamster ovary cells |
16.7–1200 µg/ml |
+ |
+ |
Inconsistent results in parallel tests in two laboratories |
NTP (1989); Anderson et al. (1990) |
|
0.5–1600 µg/ml |
(+) |
– |
||||
|
Unscheduled DNA synthesis |
Primary rat hepatocytes |
5–50 µg/ml |
+ |
n.a. |
Toxicity at 50 µg/ml |
Williams et al. (1982) |
|
Unscheduled DNA synthesis |
Primary rat hepatocytes |
0.5–1000 nmol/ml (0.08–160 µg/ml) |
– |
n.a. |
Thompson et al. (1983) |
|
a
n.a. = not applicable; n.t. = not tested; + = positive; – = negative; (+) = weakly positive.
The results of the many Salmonella mutagenicity tests were inconsistent. However, weak mutagenic activity was repeatedly shown in the presence of S9, presumably due to optimized test conditions. Single tests demonstrated the absence of mutagenic activity in the umu-test or in tests with Escherichia coli and the absence of mitotic recombination in Saccharomyces cerevisiae. PCA induced DNA damage in the Pol A test and gene mutations in Aspergillus nidulans. However, several mouse lymphoma assays uniformly found mutagenic activity of PCA in both the presence and absence of metabolic activation. In contrast, test results for the induction of chromosomal aberrations and sister chromatid exchange in Chinese hamster ovary cells as well as for the induction of DNA repair in primary rat hepatocytes again were conflicting for different laboratories. Overall, the screening tests indicate a possibility of mutagenicity.
PCA (14.5 and 19.0 µg/52 000 cells) was evaluated as positive in a cell transformation assay in Rauscher leukaemia virus-infected rat embryo cells measuring acquisition of attachment independence (Traul et al., 1981). Conflicting test results have been reported by one working group on the transforming activity of PCA in Syrian hamster embryo cells in identical test concentrations ranging from 0.01 to 100 µg/ml. In the first detailed publication of test results, no transformed colonies were identified at all (Pienta et al., 1977); in the later publication, however, test results were summarized as positive in the entire concentration range tested (Pienta & Kawalek, 1981). In an interlaboratory evaluation, both laboratories reported PCA as having transforming activity in the C3H/10T˝ cell transformation assay in non-cytotoxic concentrations ranging from 0.8 to 100 µg/ml (Dunkel et al., 1988).
In a wing somatic mutation and recombination test in Drosophila melanogaster, exposure was by 6-h feeding of 7.84 mmol PCA/litre. PCA was genotoxic in both repair-proficient and repair-defective larvae of the mei-9 cross, which indicates a potential to induce point mutations, chromosome breakages, and mitotic recombina-tions (Graf et al., 1990).
In a micronucleus test in the bone marrow of CFLP mice, the maximum tolerated dose of 180 mg PCA/kg body weight (single dose given by gavage as a suspension in tragacanth) caused no significant elevation of micronuclei in the time range 24–72 h post-administration (BUA, 1995). In another study, B6C3F1 mice were dosed with 0, 25, 50, 100, 200, or 300 mg PCA/kg body weight dissolved in phosphate-buffered saline 3 times at 24-h intervals, and bone marrow was collected 24 h after the third treatment. The micronucleus frequency at the highest dose (300 mg/kg body weight) was significantly increased (P < 0.001) over the corresponding control value.
No studies are available on the reproductive toxicity of PCA, nor were conclusive fertility studies available for aniline.
In an in vitro assay with renal cortical slices from untreated rats, 4-chloroaniline showed a weak nephrotoxic potential. Altered cellular function was inferred from decreased gluconeogenesis (27% with 0.1 mmol PCA/litre and 42% with 0.5–2 mmol PCA/litre), whereas there was obviously no cytotoxic effect (absence of increased lactate dehydrogenase release) (Hong et al., 2000).
An in vitro test system, the migration-inhibition test with sheep peripheral blood lymphocytes, demonstrated the cytotoxic activity of PCA at concentrations ranging from 0.1 to 1 mg/ml and its immunotoxic activity at concentrations ranging from 0.001 to 0.01 mg/ml; the NOEC for immunotoxicity was 0.0001 mg/ml. The immunotoxic effect was detected as a decreased ability of leukocytes to respond to the mitogenic stimulus of lipopolysaccharide (polyclonal activator of B lymphocytes), concanavaline A, and phytohaemagglutinin (polyclonal activators of T lymphocytes) (Kačmár et al., 1995).
The unusual and rare tumours of the spleen induced by rats administered aniline and related compounds have led to studies into the pathogenesis of splenic lesions. Fibrosis of the spleen was seen in all dose groups with PCA. Goodman et al. (1984) proposed that methaemoglobin bound with aniline compounds (e.g., PCA) or their reactive metabolites is broken down in the red pulp of the spleen and the reactive metabolites are released, binding to splenic mesenchymal tissues and resulting in fibrosis, which progresses to the formation of splenic tumours. Bus & Popp (1987) suggested that the splenic tumours are a result of erythrocyte toxicity. The damaged erythrocytes are scavenged by the spleen, where they cause vascular congestion, hyperplasia, fibrosis, and tumours.
Whether the mechanism of carcinogenesis is mediated through genotoxic or non-genotoxic events is unresolved. PCA is genotoxic in vitro but appears to be dependent on metabolism for its full expression. There is one positive study in vivo (micronucleus test), but this was positive only at a dose level in the range of the LD50.
It is suggested that species differences between rats and mice with regard to splenic and liver neoplasms could be due to differences in the metabolism and disposition of PCA. The NTP (1989) report points out that the initial decay constants for PCA clearance from the two strains of mice tested (A/J and Swiss Webster) were 10 times greater than those in mongrel dogs and rats. The PCA clearance in mice was too rapid to permit the calculation of kinetic parameters (Perry et al., 1981).
For aniline, it was found that there was a larger concentration of the putative reactive metabolite N- phenylhydroxylamine in rats than in mice. There were also higher levels of methaemoglobin reductase in mice than in rats.
Confirming the results of animal studies (see section 8.1.2), PCA in humans is a more potent cyanogenic agent than aniline. Dermal absorption plays a predominant role in intoxication (Linch, 1974). Severity of adverse effects may increase with concomitant intake of ethanol (BUA, 1995).
Three cases of severe intoxication have been reported after occupational exposure to PCA (Betke, 1926; Scotti & Tomasini, 1966; Faivre et al., 1971). One of these cases (Betke, 1926) resulted in death. Severe methaemoglobinaemia was reported in all three cases. The fatal case was accidentally sprayed in the face and on the upper part of his clothing with hot PCA. One of the cases (Faivre et al., 1971) handled powdered PCA. The third case (Scotti & Tomasini, 1966) was likewise exposed to dust while grinding PCA.
An insufficiently documented study reported an inhalation exposure to 44 mg PCA/m3 as being "severely toxic" within 1 min, whereas "signs of illness" appeared after prolonged inhalation of 22 mg/m3 (no further information) (Goldblatt, 1955). In one plant, average workplace air concentrations at two sites in PCA production were 58 mg PCA/m3 (range 37–89 mg/m3) and 63 mg PCA/m3 (range 46–70 mg/m3), respectively. Inhalation and simultaneous dermal absorption resulted in cyanosis, increased methaemoglobin and sulfhaemoglobin levels, the development of anaemia (2 of 6 workers within 4 weeks), and acute intoxication (1/6, who had to discontinue working) (Pacséri et al., 1958). In another plant producing PCA from 4-chloronitrobenzene, 14 workers showed a significant fall in haemoglobin and significant increases in methaemoglobin, which did not correlate with the air concentrations of PCA (values not given in study report) (Monsanto, 1986).
For comparison, in otherwise healthy patients, levels of methaemoglobin in excess of 30% may cause fatigue, headache, dyspnoea, nausea, and tachycardia. Lethargy and stupor, as well as deteriorating consciousness, occur as levels approach 55%. Higher levels may cause cardiac arrhythmias, circulatory failure, and neurological depression. Methaemoglobin levels higher than 70% are usually fatal (Coleman & Coleman, 1996).
Cyanosis and methaemoglobinaemia (14.5–43.5% methaemoglobin; normal range <2.3%) in three premature neonates (gestational age 25–27 weeks) were reported to be associated with PCA contamination of the incubators (no information on PCA concentration) in Amsterdam. Exposure was by percutaneous absorption or by inhalation of PCA-containing vapour produced by the inadvertent use of chlorohexidine gluconate (0.25 g/litre) as a humidifying agent, which decomposed on heating to produce PCA (van der Vorst et al., 1990).
A further report describes the same scenario in a neonatal intensive care unit in Copenhagen, showing that premature neonates developed severe methaemoglobinaemia when exposed to even small amounts of PCA formed from the inadvertent use of a 0.02% chlorohexidine solution as a humidifier in new incubators. The authors estimated that the maximum amount of PCA that the neonate could be exposed to was 0.3 mg/day, provided that all PCA produced was absorbed by the neonate. Thirty-three of 415 neonates (8%) were found to be methaemoglobin positive (mean methaemoglobin concentration 19%; range 6.5–45.5%) during the 8-month screening period. Of those patients with a gestational age of less than 31 weeks, 40% were positive; 15 out of 25 neonates (60%) with a birth weight <1000 g proved to be positive. All the methaemoglobin-positive cases started when the neonate was in the new incubator.
A prospective clinical study showed that immaturity, severe illness, the time exposed to PCA, and low concentrations of NADH reductase probably contributed to the condition. Fetal haemoglobin is more easily oxidized than adult haemoglobin; further, the delicate skin of the premature neonate is more permeable (Hjelt et al., 1995).
Data on the metabolism of PCA, biomonitoring of haemoglobin adducts, and urinary excretion of PCA in humans are presented in sections 7.3–7.5.
Numerous tests have been performed on the toxicity of PCA to all trophic levels of aquatic organisms. Experimental test results for the most sensitive species are summarized in Table 10. Additional data on the toxicity of PCA to aquatic organisms are cited in the BUA (1995) report. Acute and long-term tests are available for invertebrates and fish. Among all tested organisms, green algae (Scenedesmus subspicatus) and water fleas (Daphnia magna) proved to be the most sensitive freshwater species in a short-term cell multiplication inhibition test and an immobilization test, respectively. EC50 values of 2.1 mg/litre for Scenedesmus subspicatus and 0.31 mg/litre for Daphnia magna were determined. The test on fluorescence inhibition of Scenedesmus subspicatus, which resulted in the lowest reported EC10, is not considered reliable, as the test method is not validated and the environmental relevance of the results is questionable. In the only available valid long-term test with Daphnia magna, a 21-day NOEC of 0.01 mg/litre was established for reproduction (Kühn et al., 1988, 1989b). The shrimp Crangon septemspinosa was the more sensitive of two tested marine invertebrate species, exhibiting a 96-h lethal threshold concentration of 12.5 mg/litre (McLeese et al., 1979). With respect to freshwater fish, the lowest short-term (96-h) LC50 value of 2.4 mg/litre was determined for the bluegill (Lepomis macrochirus) (Julin & Sanders, 1978). After long-term exposure, a 3-week NOEC of 1.8 mg/litre was reported for zebra fish (Brachydanio rerio) (BUA, 1995).
Table 10: Aquatic toxicity of PCA.
|
Most sensitive species |
End-point |
Concentration |
Reference |
|
Bacteria |
|||
|
Bioluminescent bacterium Photobacterium phosphoreum |
30-min EC10 |
5.1 |
Ribo & Kaiser (1984) |
|
Fungi |
|||
|
Yeast Pichia sp. |
24-h EC50 |
78.7 |
Kwasniewska & Kaiser (1984) |
|
Protozoa |
|||
|
Ciliated protozoan Tetrahymena pyriformis |
24-h EC50 |
10 |
Yoshioka et al. (1985) |
|
Algae |
|||
|
Green alga Scenedesmus subspicatus |
168-h EC10 |
0.02 |
Schmidt & Schnabl (1988); Schmidt (1989) |
|
Scenedesmus subspicatus |
EC10 |
0.003 |
Schmidt (1989) |
|
Invertebrates |
|||
|
Water flea Daphnia magna |
48-h EC0 |
0.013 |
Kühn (1989); Kühn et al. (1989a) |
|
Daphnia magna |
21-day NOEC |
0.01 |
Kühn et al. (1988); Kühn et al. (1989b) |
|
Midge Chironomus plumosus larvae |
48-h EC50 |
43 |
Julin & Sanders (1978) |
|
Fish |
|||
|
Bluegill Lepomis macrochirus |
96-h LC50 |
2.4 |
Julin & Sanders (1978) |
|
Medaka Oryzias latipes |
28-day MATCb |
<2.25 |
Holcombe et al. (1995) |
|
Zebra fish Brachydanio rerio |
3-week NOEC |
1.8 |
Adolphi et al. (1984) |
|
a |
Determination of fluorescence after a light pulse (0.5 s). |
|
b |
Maximum acceptable toxicant concentration. |
The chronic effects of PCA on growth and reproduction of zebra fish were further studied in a three-generation test (flow-through system). Whereas the F0 generation remained unaffected by PCA concentrations of 0.04, 0.2, and 1 mg/litre, the F1 and F2 generations showed abdominal swelling, spinal deformations, reduced number of eggs, and reduced fertilization from the age of 5 weeks at the exposure concentration of 1 mg/litre (Bresch et al., 1990). In the same tests, Braunbeck et al. (1990) studied the effect of the applied PCA on hepatic cells of zebra fish and additionally exposed rainbow trout (Oncorhynchus mykiss). For both species, exposure to PCA concentrations of 0.2 and 1 mg/litre resulted in significant ultrastructural changes of hepatic cells. In later studies on zebra fish with prolonged exposure (31 days), hepatocytes and gills of exposed fish exhibited dose-dependent alterations at PCA concentrations of >0.05 and >0.5 mg/litre, respectively. Pathological symptoms in both liver and gills disappeared almost completely within a regeneration period of 14 days (Burkhardt-Holm et al., 1999).
Dissolved humic materials in the exposure media may have a marked influence on the toxicity of PCA to aquatic species. Lee et al. (1993) found significantly reduced toxicity for cell multiplication inhibition of Daphnia magna (48-h EC50) with increasing dissolved humic material concentrations, whereas the LC50 for zebra fish remained unaffected in the presence of dissolved humic materials. Among several explanations, the authors discussed reduced bioavailability by sorption of PCA to dissolved humic materials as a possible cause of this effect.
Several experiments have been conducted on the influence of PCA on soil microbial activity. In general, PCA exhibits low toxicity. None of the applied test procedures is validated, and degradation as well as adsorption to soil particles, which may significantly influence bioavailability, were considered in only one of these studies. Therefore, only the results of the latter study are summarized here. Welp & Brümmer (1999) determined effective doses (ED10) in the range of 85–1000 mg/kg for the Fe(III) reduction by the natural microflora in upper soil material (A horizon) of six different soil types. The authors found a close correlation between effect concentrations and the amount of organic carbon, emphasizing the importance of sorption and solubility for the bioavailability of the chemical. They concluded that the variability of the determined effective dose values could mainly be attributed to the differing ability of soil particles to withdraw organic chemicals from the liquid phase via sorption.
The toxicity of PCA to higher plants was tested according to OECD Guideline 208 with oats (Avena sativa) and turnip (Brassica rapa). After exposure of seeds to different test substance concentrations, 14-day EC50 values of 140 mg/kg soil (oats) and 66.5 mg/kg soil (turnip) were determined for reduced fresh weight of grown shoots (Scheunert, 1984).
In a test conducted according to OECD Guideline 207, the adverse effects of PCA on earthworms (Eisenia fetida) were examined by Viswanathan (1984). After exposure in an artificial soil mixture, a 28-day LC50 value of 540 mg/kg dry soil was established. The minimum weight of test animals as prescribed by the guideline was not reached in 32% of the tests, thus leading to a limited validity of the study. Furthermore, the environmental relevance of the earthworm test seems questionable, as the factors determining the bioavailability of the applied chemical remain completely unconsidered.
Valid studies on the toxicity of PCA to terrestrial vertebrates or on its effects on ecosystems are not available.
The results available on microorganisms and plants indicate a low toxicity potential of PCA in the terrestrial environment.
Repeated exposure to PCA in rodents leads to cyanosis and methaemoglobinaemia, followed by effects in blood, liver, spleen, and kidneys, manifested as changes in haematological parameters, splenomegaly, and moderate to heavy haemosiderosis in spleen, liver, and kidney, partially accompanied by extramedullary haematopoiesis. These effects occur secondary to excessive compound-induced haemolysis and are consistent with a regenerative anaemia. The LOAELs (lowest tested dosages; NOELs not derivable) for significant increases in methaemoglobin levels have been reported for a 13-week oral (gavage) exposure as 5 mg/kg body weight in rats and 7.5 mg/kg body weight in mice and 2 mg/kg body weight per day for 26–103 weeks’ oral (gavage) application to rats. Fibrotic changes of the spleen were observed in male rats, with a LOAEL of 2 mg/kg body weight per day, and hyperplasia of bone marrow was seen in female rats, with a LOAEL of 6 mg/kg body weight per day (103-week gavage) (Table 5; NTP, 1989; Chhabra et al., 1991).
There seem to be species differences not only in rates of metabolic clearance, but also in the levels of crucial enzymes (e.g., NADH-dependent methaemoglobin reductase located in the erythrocytes). Enzymatic activity in rat and mouse erythrocytes is 5 and 10 times higher, respectively, than that in human erythrocytes, suggesting that humans are more susceptible to this toxic effect. This is also reported for aniline, where the oral dose for acute methaemoglobinaemia seems to be 10–100 times less than that for rats or dogs, calculated on a body weight basis (EU, 2002). However, available data are inadequate to quantify these species differences for PCA.
Regarding intraspecies differences, in the study on haemoglobin adducts in workers occupationally exposed to PCA, it could be shown that workers who were slow acetylators showed a significantly increased haemoglobin adduct level compared with fast acetylators. (About 50% of the European population, for example, are slow acetylators as a result of a genetically caused lower activity of N-acetyltransferase, with increased sensitivity to compounds such as PCA.)
The scarce data on the effects on humans of occupational exposure to PCA are mostly from a few older reports of severe intoxications after accidental exposure during production, with symptoms of cyanosis and methaemoglobinaemia. No dose–effect correlations can be derived because of the absence of characterizations of the exposure or the body burden. Haemoglobin adducts were observed after PCA exposure and have been used in the biomonitoring of workers employed in the synthesis and processing of PCA.
There are reports of premature neonates being inadvertently exposed to PCA as a breakdown product of chlorohexidine. Three neonates in one report (14.5–43.5% methaemoglobin) and 33 of 415 neonates in another report (6.5–45.5% methaemoglobin, mean 19%, during the 8-month screening period) were found to be methaemoglobin positive. A prospective clinical study showed that immaturity, severe illness, time exposed to PCA, and low concentrations of NADH reductase probably contributed to the condition. Fetal haemoglobin is more easily oxidized than adult haemoglobin; further, the delicate skin of the premature neonate is more permeable (see section 9).
PCA is carcinogenic in male rats, with the induction of unusual and rare tumours of the spleen (fibrosarcomas and osteosarcomas), which is typical for aniline (EU, 2002) and related substances. In the NTP (1989) study, PCA was found to be carcinogenic in male rats, based on the increased incidence of splenic sarcoma, osteosarcoma, and haemangiosarcoma in high-dose (18 mg/kg body weight) rats. The dose–response was non-linear, the incidence of sarcomas at 2 and 6 mg/kg body weight being marginal.
In female rats, the precancerous stages of the spleen tumours are increased in frequency. Increased incidences of pheochromocytoma of the adrenal gland in male and female rats may have been related to PCA administration.
PCA induced hepatocellular tumours in male mice and a marginal to significant increase in the incidences of haemangiosarcoma in male and female mice (Table 9; NTP, 1989; Chhabra et al., 1991).
Whether the mechanism of carcinogenesis is mediated through genotoxic or non-genotoxic events is unresolved. PCA is genotoxic in vitro but appears to be dependent on metabolism for its full expression.
There were no data available concerning carcinogenicity in humans associated with PCA exposure.
As available data indicate that PCA is a carcinogen, exposure should be reduced to the extent possible.
A tolerable intake for non-neoplastic effects is based on the significant increases in methaemoglobin levels in rats and mice and fibrotic changes of the spleen in male rats.
The LOAELs (lowest tested dosages, NOELs not derivable) for significant increases of methaemoglobin have been reported for a 13-week oral exposure by gavage of 5 mg/kg body weight in rats and 7.5 mg/kg body weight in mice and 2 mg/kg body weight per day for 26–103 weeks’ oral (gavage) application to rats.
In male rats, fibrotic changes of the spleen were described at the lowest dose tested (LOAEL of 2 mg/kg body weight per day). This is the same dose as the LOAEL in rats for significant increases in methaemoglobin level. A NOEL was not available.
If one applied the uncertainty factors 10 (for use of a LOAEL rather than a NOEL) × 10 (for interspecies extrapolation) × 10 (for interindividual variability) to the LOAEL of 2 mg/kg body weight per day, one could derive a tolerable intake (IPCS, 1994) of 2 µg/kg body weight per day.
In premature babies (mean birth weight about 1.2 kg), an exposure to 0.3 mg/day (equivalent to 0.25 mg/kg body weight per day) (dermal/inhalation) caused a mean methaemoglobin concentration of 19% (6.5–45.5%). Therefore, the NOAEL must have been much lower. Further, the exposure was comparatively short, there being 1–18 methaemoglobin-positive days (mean 6 days). It is clear that premature babies are much more susceptible than healthy workers. First, the reducing capacity of NADH reductase in neonates is downgraded and not fully developed. Further, fetal haemoglobin is more easily oxidized than the adult form, and the delicate skin of the premature neonate is more permeable.
Workers may be exposed to PCA via inhalation and skin contact during the production and processing of PCA and in the printing and dyeing industries where PCA-based azo dyes and pigments are used.
Recent data on PCA concentrations at the workplace during production are not available for a risk characterization. It is to be hoped that the cited older figures of 58 and 63 mg/m3 (which resulted in toxic effects) and even of 0.2–2.0 mg/m3 are no longer found in any countries producing and processing PCA.
From studies at dyeing factories, especially during weighing/mixing operations, a workshift inhalation intake of PCA of <4 ng/kg body weight per day can be calculated (see section 6.2.1).
There is a large potential for consumer exposure to PCA from the use of dyed/printed textiles and papers and cosmetic and pharmaceutical products. The exposure can result from residual PCA in the commercial product and/or from hydrolytic or metabolic degradation of the product during use. It can be dermal (wearing of clothes, use of deodorant products or mouthwashes) or oral (small children sucking clothes and other materials, use of mouthwashes).
Sample estimates of maximal consumer exposure (details given in section 6.2.2) are as given in Table 11.
Table 11: Sample estimates of maximal consumer exposure.
|
Exposure |
Estimate (ng/kg body weight per day) |
|
Dyed textiles (containing certain azo dyes) |
27a |
|
Deodorant products (containing triclocarban) |
16 |
|
Mouthwashes (containing chlorohexidine) |
50–255 |
|
Total, assuming all possible consumer exposures |
Maximum 300 |
|
a |
Assuming the dyed textiles are worn next to the skin 24 h/day and assuming 1% percutaneous penetration of the dye. If one assumes 100% penetration, the body dose is estimated at 2.7 µg/kg body weight per day. |
In addition, for young children, an oral exposure from the sucking of dyed textiles may occur, which could be in the range of several micrograms per kilogram body weight per day. Although each estimate in itself is not certain enough to be used for a quantitative risk characterization for each individual route of exposure, it can be seen that consumers are exposed via a number of possible routes, leading in total to exposure concentrations of about 0.1–0.3 µg/kg body weight per day, assuming only 1% penetration by clothes.
Considering only non-neoplastic effects (i.e., methaemoglobinaemia), these possible human exposures are within an order of magnitude of the calculated tolerable intake of 2 µg/kg body weight per day (see section 11.1.2). Incidental exposure to high concentrations of PCA can be fatal.
Further effects of concern are carcinogenicity and possibly skin sensitization.
There are no reliable data on occupational exposure levels PCA or on exposure of the general population to PCA.
Therefore, it was not possible to make a risk estimate for PCA production workers. Methaemoglobinaemia in premature babies as a result of PCA exposure is reported, but it is recognized that neonates are much more susceptible than healthy workers, and it is therefore difficult to make an evaluation using these data.
Data were not available to determine a NOAEL instead of a LOAEL for methaemoglobin formation and fibrotic changes of the spleen.
The effect of PCA on the reproductive system cannot be evaluated due to lack of data.
11.2.1 Evaluation of effects in surface waters
The main environmental target compartment of PCA from its industrial use as an intermediate in the production of pesticides, azo dyes and pigments, and cosmetic products is the hydrosphere.
In surface waters, PCA is rapidly degraded by direct photolysis (half-life 2–7 h), whereas biodegradation is expected to be of minor importance. Elimination observed in experiments with different microbial inocula could be largely attributed to abiotic processes (e.g., photodegradation or adsorption). Thus, under conditions not favouring biotic or abiotic removal, adsorption of PCA to sediment particles in surface waters can be expected, as can application of PCA to agricultural soils in sewage sludges.
The available experimental bioconcentration data, as well as the measured n-octanol/water partition coefficients, indicate no bioaccumulation potential for PCA in aquatic organisms.
A sample risk characterization with respect to the aquatic environment may be performed by calculating the ratio between a (local or regional) predicted environmental concentration (PEC; based on measured or model concentrations) and a predicted no-effect concentration (PNEC) (EC, 1996).
A quantification of PCA releases from all the different industrial sources is not possible with the available data. As a first approach, however, the measured concentrations in the river Rhine and its tributaries can be taken as a basis for a sample risk characterization, as this area is one of the most heavily industrialized areas in the EU. There, the concentration range is between about 0.1 and 1 µg/litre. The higher concentration was taken as the PEC.
A PNEC for surface waters may be calculated by dividing the lowest valid NOEC by an appropriate uncertainty factor:
|
PNEC |
= (10 µg/litre) / 10 |
|
= 1 µg/litre |
where:
10 µg/litre is the lowest NOEC value from a long-term study with Daphnia magna. The lower EC10 value of 3 µg/litre found for fluorescence inhibition after a light pulse in the alga Scenedesmus subspicatus is not regarded as relevant and appropriate for risk characterization purposes.
10 is chosen as the uncertainty factor. According to EC (1996), this factor should be applied when long-term toxicity NOECs are available from at least three species across three trophic levels (e.g., fish, daphnia, algae).
Therefore, based upon the highest measured PCA concentrations in the river Rhine and its tributaries, the PEC/PNEC ratio is 1. In EU jurisdiction, for substances exhibiting ratios of less than or equal to 1, further information and/or testing as well as risk reduction measures beyond those that are already being applied are not required.
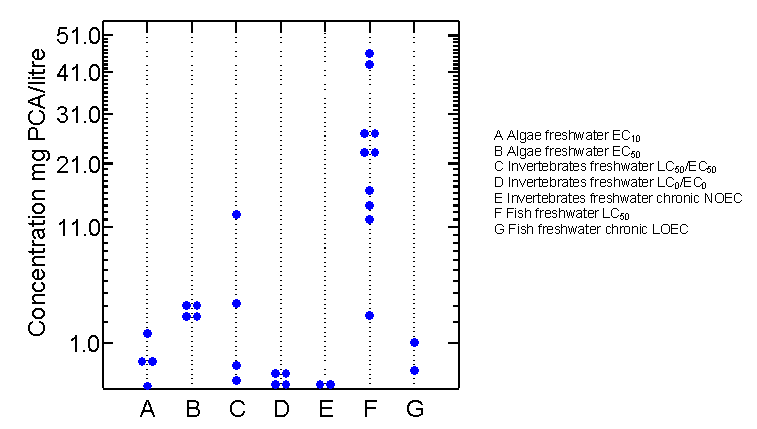
Figure 3: Plot of reported toxicity values for PCA in aquatic organisms.
The range of values for the adverse effects of PCA on aquatic species of different trophic levels, experimentally determined in short- and long-term tests, is given in Figure 3.
PCA released to surface water that contains low amounts of organic matter is expected to undergo rapid photodegradation. In waters with significant amounts of particulate matter, however, photooxidation may be reduced, and adsorption to organic material may increase. Therefore, a possible risk for aquatic organisms, particularly benthic species, cannot be completely ruled out. The only benthic species tested (Chironomus plumosus larvae) exhibited no significant sensitivity (48-h EC50 of 43 mg/litre for immobilization). However, the test medium used was reconstituted water and contained no elevated levels of organic matter. Moreover, experiments with Daphnia magna revealed significantly reduced toxicity with increasing concentrations of dissolved humic materials in the medium, possibly caused by reduced bioavailability of PCA from adsorption to dissolved humic materials. Furthermore, bioaccumulation in aquatic species was reported to be very low. Therefore, from the available data, a significant risk associated with exposure of aquatic organisms to PCA is not to be expected.
The use of phenylurea herbicides or insecticides may lead to the contamination of agricultural soils with PCA. A measurement programme on agricultural soils in Bavaria, Germany, gave about 15% positive samples. The concentration range in the positive samples was between 5 and 50 µg/kg (BUA, 1995). It cannot be judged whether these data are representative for other regions of the world. However, due to the lack of other data, they are used for the risk characterization.
The measured data on PCA concentrations in biota due to the use of pesticides are somewhat conflicting. In wild (mushrooms, berries) and cultured plants (spinach, cress, potatoes), PCA was not detected after insecticide application, whereas it was found in tissue samples of fish in concentrations of about 1 mg/kg. As this is a single finding, a proper sample risk characterization is not possible due to the high degree of uncertainty.
Soil sorption coefficients determined in a variety of soil types indicate only a low potential for soil sorption. In most experiments, soil sorption increased with increasing organic matter and decreasing pH values. As a consequence, under conditions unfavourable for abiotic and biotic degradation, leaching of PCA from soil into groundwater, particularly in soils with a low organic matter content and elevated pH levels, may occur.
For the terrestrial compartment, toxicity tests on microbial activity, higher plants, and earthworms are available. None of the studies seems appropriate to serve as a basis for a quantitative risk characterization. The lowest effect value reported for turnip (Brassica rapa) was 66.5 mg/kg and exceeds the soil concentrations measured in agricultural soils (Bavaria, Germany; cited above) by a factor of approximately 1000. Therefore, PCA is not expected to pose a significant risk for the tested terrestrial species. Valid studies on the toxicity of PCA to terrestrial vertebrates or on effects of PCA on ecosystems are not available.
Regarding the effects of PCA on aquatic species, the toxicity data set includes a variety of species from different trophic levels. Most studies are of good quality and acceptable for risk characterization purposes. Only one test is available for benthic species that may be exposed to PCA adsorbed to organic matter. However, this test has been performed in reconstituted water without elevated levels of particulate matter. For the terrestrial compartment, the available toxicity studies cannot be regarded as adequate for a quantitative risk characterization.
The International Agency for Research on Cancer (IARC, 1993) has classified 4-chloroaniline in Group 2B (possibly carcinogenic to humans) based on inadequate evidence in humans and sufficient evidence in experimental animals for the carcinogenicity of 4-chloroaniline.
Adolphi H, Müller H-G, Munk R, Neu H-J, Pagga U (1984) 14-Tage-Fischtest mit Brachydanio rerio. Überprüfung der Durchführbarkeit von Prüfungsvorschriften und der Aussagekraft der Stufe I und II ChemG. Ludwigshafen, BASF AG (Forschungsbericht 106 04 011/03).
Alexander M, Lustigman BK (1966) Effect of chemical structure on microbial degradation of substituted benzenes. Journal of Agricultural and Food Chemistry, 14:410–413.
Anderson BE, Zeiger E, Shelby MD, Resnick MA, Gulati DK, Ivett JL, Loveday KS (1990) Chromosome aberration and sister chromatid exchange test results with 42 chemicals. Environmental and Molecular Mutagenesis, 16(Suppl. 18):55–137.
Ballhorn L (1984) Semistatischer Fischtest. In: Korte F, Freitag D, eds. Überprüfung der Durchfühbarkeit von Prüfungsvorschriften und der Aussagekraft der Stufe I und II des E. Chem. G. Neuherberg, Gesellschaft für Strahlen- und Umweltforschung München mbH, pp. 44–58 (Research Report 106 04 011/02).
Basketter DA, Scholes EW (1992) Comparison of the local lymph node assay with the guinea pig maximization test for the detection of a range of contact allergens. Food and Chemical Toxicology, 30:65–69.
Bayer AG (1984) p-Chloroanilin, Untersuchungen zur akuten oralen Toxizität an der Katze. Einfluß auf Met-Hämoglobingehalt und Zahl der Heinz-Innenkörper im peripheren Blut. Leverkusen, Bayer AG (unpublished report) [cited in BUA, 1995].
BGVV (1996) Untersuchung von Bedarfsgegenständen. Nachweis der Verwendung verbotener Azofarbstoffe auf gefärbten, textile Bedarfsgegenständen. Amtliche Sammlung von Untersuchungsverfahren nach § 35 LMBG. Berlin, Federal Institute for Health Protection of Consumers and Veterinary Medicine.
Betke [initial not given] (1926) Jahresbericht des Gewerbemedizinalrats für den Aufsichtsbezirk Wiesbaden über das Geschäftsjahr 1922. Veröffentlichungen aus dem Gebiete der Medizinalverwaltung, 578:212–230.
BIA (1992) BUA-Stoffdossier p-chloranilin - Schreiben vom 31.07.92. Sankt Augustin, Berufsgenossenschaftliches Institut für Arbeitssicherheit.
Birner G, Neumann HG (1987) Biomonitoring of aromatic amines. Binding of substituted anilines to rat hemoglobin. Naunyn-Schmiedeberg’s Archiv für Pharmakologie, 335:R19 (Abstract No. 73).
Birner G, Neumann HG (1988) Biomonitoring of aromatic amines. II: Hemoglobin binding of some monocyclic aromatic amines. Archives of Toxicology, 62:110–115.
Bollag JM, Blattmann P, Laanio T (1978) Adsorption and transformation of four substituted anilines in soil. Journal of Agricultural and Food Chemistry, 26:1302–1306.
Bonesvoll P, Lökken P, Rölla G, Paus PN (1974) Retention of chlorhexidine in the human oral cavity after mouth rinses. Archives of Oral Biology, 19:209.
Booth GM, Ferrell D (1977) Degradation of dimilin by aquatic foodwebs. Environmental Science Research, 10:221–243.
Börnick H, Hultsch V, Grischek T, Lienig D, Worch E (1996) Aromatic amines in the Elbe river — determination and behaviour during drinking water treatment. Vom Wasser, 87:305–326.
Braunbeck T, Storch V, Bresch H (1990) Species-specific reaction of liver ultrastructure in zebrafish (Brachydanio rerio) and trout (Salmo gairdneri) after prolonged exposure to 4-chloroaniline. Archives of Environmental Contamination and Toxicology, 19:405–418.
Bray HG, James SP, Thorpe WV (1956) The metabolism of the monochloronitrobenzenes in the rabbit. Biochemical Journal, 64:38–44.
Bresch H, Beck H, Ehlermann D, Schlaszus H, Urbanek M (1990) A long-term toxicity test comprising reproduction and growth of zebrafish with 4-chloroaniline. Archives of Environmental Contamination and Toxicology, 19:419–427.
BUA (1991) o-Chloranilin, m-Chloranilin. Beratergremium für Umweltrelevante Altstoffe (BUA) der Gesellschaft Deutscher Chemiker. Weinheim, VCH, 269 pp. (BUA Report 57).
BUA (1993) OH-radicals in the troposphere — Concentration level and consequences. Weinheim, VCH, 163 pp. (BUA Report 100).
BUA (1995) p-Chloroaniline. Beratergremium für Umweltrelevante Altstoffe (BUA) der Gesellschaft Deutscher Chemiker. Weinheim, VCH, 171 pp. (BUA Report 153).
Burkhardt-Holm P, Oulmi Y, Schroeder A, Storch V, Braunbeck T (1999) Toxicity of 4-chloroaniline in early life stages of zebrafish (Danio rerio): II. Cytopathology and regeneration of liver and gills after prolonged exposure to waterborne 4-chloroaniline. Archives of Environmental Contamination and Toxicology, 37(1):85–102.
Bus JS, Popp JA (1987) Perspectives on the mechanism of action of the splenic toxicity of aniline and structurally-related compounds. Food and Chemical Toxicology, 25:619–626.
Caspary WJ, Datson DS, Myhr BC, Mitchell AD, Rudd CJ, Lee PS (1988) Evaluation of the L5178Y mouse lymphoma cell mutagenesis assay: interlaboratory reproducibility and assessment. Environmental and Molecular Mutagenesis, 12(Suppl. 13):195–229.
Cheng HH, Haider K, Harper SS (1983) Catechol and chlorocatechols in soil: degradation and extractability. Soil Biology and Biochemistry, 15:311–317.
Chhabra RS, Gerken DK, Wilson FD, Peters AC (1986) p-Chloroaniline subchronic toxicity studies in rats and mice. Toxicology Letters, 31:244 (Abstract P17-17).
Chhabra RS, Thompson M, Elwell MR, Gerken DK (1990) Toxicity of p-chloroaniline in rats and mice. Food and Chemical Toxicology, 28(10):717–722.
Chhabra RS, Huff JE, Haseman JK, Elwell MR (1991) Carcinogenicity of p-chloroaniline in rats and mice. Food and Chemical Toxicology, 29(2):119–124.
Cnubben NH, Vervoort J, Boersma MG, Rietjens IM (1995) The effect of varying halogen substituent patterns on the cytochrome P450 catalysed dehalogenation of 4-halogenated anilines to 4-aminophenol metabolites. Biochemical Pharmacology, 49(9):1235–1248.
Coleman MD, Coleman NA (1996) Drug-induced methaemoglobinaemia. Drug Safety, 14(6):394–405.
Collier SW, Storm JE, Bronaugh RL (1993) Reduction of azo dyes during in vitro percutaneous adsorption. Toxicology and Applied Pharmacology, 118:73–79.
De Wolf W, Mast B, Yedema ESE, Seinen W, Hermens JLM (1994) Kinetics of 4-chloraniline in guppy, Poecilia reticulata. Aquatic Toxicology, 28(1/2):65–78.
Dial LD, Anestis DK, Kennedy SR, Rankin GO (1998) Tissue distribution, subcellular localization and covalent binding of 2-chloroaniline and 4-chloroaniline in Fischer 344 rats. Toxicology, 131(2–3):109–119.
Dunkel VC, Simmon VF (1980) Mutagenic activity of chemicals previously tested for carcinogenicity in the National Cancer Institute bioassay program. IARC Scientific Publications, 27:283–302.
Dunkel VC, Zeiger E, Brusick D, McCoy E, McGregor D, Mortelmans K, Rosenkranz HS, Simmon VF (1985) Reproducibility of microbial mutagenicity assays: II. Testing of carcinogens and noncarcinogens in Salmonella typhimurium and Escherichia coli. Environmental Mutagenesis, 7(Suppl. 5):1–248.
Dunkel VC, Schechtman LM, Tu AS, Sivak A, Lubet RA, Cameron TP (1988) Intralaboratory evaluation of the C3H/1OT1/2 cell transformation assay. Environmental and Molecular Mutagenesis, 12:21–31.
Du Pont (1981) Benzenamine, 4-chloro, inhalation median lethal concentration (LC50). Wilmington, DE, Du Pont (Report No. 14050; NTIS/OTS 84003A) [cited in BUA, 1995].
Du Pont (1982) Benzenamine, 4-chloro, subacute inhalation study of p-chloroaniline in rats. Wilmington, DE, Du Pont (Report No. 14050; NTIS/OTS 84003A) [cited in BUA, 1995].
EC (1996) Technical guidance documents in support of the Commission Directive 93/67/EEC on risk assessment for new notified substances and the Commission Regulation (EC) 1488/94 on risk assessment for existing substances. Ispra, European Chemicals Bureau.
EC (1999) Cosmetics legislation. Cosmetic products. Volume 1. Brussels, European Commission, DG III Enterprise.
EC (2000) Amended proposal for a Directive of the European Parliament and of the Council amending for the nineteenth time Council Directive 76/769/EEC relating to restrictions on the marketing and use of certain dangerous substances and preparations (azocolourants). Brussels, European Commission, 8 pp.
EC (2001) Inventory of Cosmetics Ingredients (INCI). Brussels, European Commission, DG III Enterprise, at website http://dg3.eudra.org/F3/home.html.
Ehlhardt WJ, Howbert JJ (1991) Metabolism and disposition of p-chloraniline in rat, mouse and monkey. Drug Metabolism and Disposition, 19(2):366–369.
Ekici P, Leupold G, Parlar H (2001) Degradability of selected azo dye metabolites in activated sludge systems. Chemosphere, 44:721–728.
El Marbouh L, Arellano C, Philibert C, Evrard P, Poey J, Houin G (2000) In vivo study of percutaneous absorption of 4-chloroaniline using microdialysis in the rat. Arzneimittelforschung, 50(11):1033–1036.
EU (2002) EU risk assessment report aniline, CAS-No.
Fabig W, Bludau B, Görtz T, Rengshausen M (1984) Biodegradation. In: Evaluation of test guidelines for environmental chemicals. Schmallenberg-Grafschaft, Fraunhofer Institute of Environmental Chemistry and Ecotoxicology, pp. 71–88.
Faivre M, Armand J, Évreux JC, Duverneuil G, Colin C (1971) Méthhémoglobinémie toxique par des dérivés de l’aniline: parachloroaniline et paratoluidine. Archives des Maladies Professionnelles de Medecine du Travail et de Sécurité Sociale, 32:575–577.
Fattore E, Müller L, Davoli E, Castelli D, Benfenati E (1998) Industrial pollutants in ground waters from northern Milan. Chemosphere, 36(9):2007–2017.
Freitag D, Lay JP, Korte F (1984) Environmental hazard profile — test results as related to structures and translation into the environment. In: Kaiser KLE, ed. QSAR in environmental toxicology. Dordrecht, D. Reidel Publishing Company, pp. 111–136.
Freitag D, Ballhorn L, Geyer H, Korte F (1985) Environmental hazard profile of organic chemicals. An experimental method for the assessment of the behaviour of organic chemicals in the ecosphere by means of simple laboratory tests with 14C labelled chemicals. Chemosphere, 14:1589–1616.
Fuchsbichler G (1977) Sorptionsverhalten und Abbau von 4-Chloranilin und 3,4-Dichloranilin im Boden sowie deren Aufnahme in und Wirkung auf Kulturpflanzen. München, Bayrische Landesanstalt für Bodenkultur und Pflanzenbau, 148 pp.
Garberg P, Akerblom EL, Bolcsfoldi G (1988) Evaluation of a genotoxicity test measuring DNA-strand breaks in mouse lymphoma cells by alkaline unwinding and hydroxyapatite elution. Mutation Research, 203:155–176.
Garner RC, Nutman CA (1977) Testing of some azo dyes and their reduction products for mutagenicity using Salmonella typhimurium TA 1538. Mutation Research, 44:9–19.
Garst JE, Wilson WC (1984) Accurate, wide-range, automated, high-performance liquid chromatographic method for the estimation of octanol/water partition coefficients. I: Effect of chromatographic conditions and procedure variables on accuracy and reproducibility of the method. Journal of Pharmaceutical Sciences, 73:1616–1623.
Gavlick WK (1992) High-performance liquid chromatographic analysis of chlorhexidine and p-chloraniline using a specialty column and a photodiode-array detector. Journal of Chromatography, 623(2):375–380.
Gavlick WK, Davis PK (1994) Gas chromatographic determination of p-chloroaniline in a chlorhexidine digluconate-containing alcohol foam surgical scrub product. Journal of the Association of Official Analytical Chemists International, 77(3):583–586.
Gawlik BM, Feicht EA, Karcher W, Kettrup A, Muntau H (1998) Application of the European reference soil set (eurosoils) to a HPLC-screening method for the estimation of soil adsorption coefficients of organic compounds. Chemosphere, 36(14):2903–2919.
Geyer H, Viswanathan R, Freitag D, Korte F (1981) Relationship between water solubility of organic chemicals and their bioaccumulation by the alga Chlorella. Chemosphere, 10:1307–1313.
Gilbert P, Saint-Ruf G, Poncelet F, Mercier M (1980) Genetic effects of chlorinated anilines and azobenzenes on Salmonella typhimurium. Archives of Environmental Contamination and Toxicology, 9:533–541.
Goldblatt MW (1955) Research in industrial health in the chemical industry. British Journal of Industrial Medicine, 12:1–20.
Goodman DG, Ward JM, Reichardt WD (1984) Splenic fibrosis and sarcomas in F344 rats fed diets containing aniline hydrochloride, p-chloroaniline, azobenzene, o-toluidine hydrochloride, 4,4'-sulfonylaniline, or D&C Red No. 9. Journal of the National Cancer Institute, 73:265–273.
Goodwin BFJ, Crevel RWR, Johnson AW (1981) A comparison of three guinea-pig sensitization procedures for the detection of 19 reported human contact sensitizers. Contact Dermatitis, 7:248–258.
Götz R, Bauer OH, Friesel P, Roch K (1998) Organic trace compounds in the water of the river Elbe near Hamburg — part 1. Chemosphere, 36(9):2085–2101.
Graf U, Hall CB, van Schaik N (1990) On the use of excision repair defective cells in the wing somatic mutation and recombination test in Drosophila melanogaster. Environmental and Molecular Mutagenesis, 16:225–237.
Gurka DF (1985) Interim protocol for the automated analysis of semivolatile organic compounds by gas chromatography/Fourier transform infrared (GC/FT-IR) spectrometry. Applied Spectroscopy, 39:827–833.
Haltrich W (1983) Biotischer Abbau. In: Überprüfung der Durchführbarkeit von Prüfungsvorschriften und der Aussagekraft der Grundprüfung des Chem. G. - Berichteines Seminars vom 25. und 26. Februar 1982. München, Gesellschaft für Strahlen- und Umweltforschung mbH (GSF), pp. 124–138.
Hamm U, Putz HJ (1997) Untersuchungen zur möglichen Bildung aromatischer Amine bei der reduktiven Altpapierbleiche / Aromatische Amine. Darmstadt, Institut für Papierfabrikation, Technische Hochschule (INGEDE Projekt 4896 IfP).
Hargesheimer EE, Coutts RT, Pasutto FM (1981) Gas–liquid chromatographic determination of aniline metabolites of substituted urea and carbamate herbicides in aqueous solution. Journal of the Association of Official Analytical Chemists, 64:833–840.
Harms H (1996) Bioaccumulation and metabolic fate of sewage sludge derived organic xenobiotics in plants. The Science of the Total Environment, 185:83–92.
Harms H, Langebartels C (1986a) Pflanzliche Zellsuspensionskulturen als System zum Studium des Abbauverhaltens von Chemikalien in Pflanzen. In: VDLUFA-Schriftenreihe 16, Kongreßband 1985 Gießen "Bodenbewirtschaftung, Bodenfruchtbarkeit, Bodenschutz." Darmstadt, VDLUFA-Verlag, pp. 445–455.
Harms H, Langebartels C (1986b) Standardized plant cell suspension test systems for an ecotoxicologic evaluation of the metabolic fate of xenobiotics. Plant Science, 45:157–166.
Herbst W, Hunger K (1995) Disazopigmente. In: Industrielle organische Pigmente. Herstellung, Eigenschaften, Anwendung. Zweite, vollständig überarbeitete Auflage. Weinheim, VCH Verlagsgesellschaft mbH, pp. 244–269.
Hjelt K, Lund J, Scherling B, Bendixen S, Lundstrom S, Stovring S, Voldsgaard P, Linnet K (1995) Methaemoglobinaemia among neonates in a neonatal intensive care unit. Acta Paediatrica, 84:365–370.
Holcombe GW, Benoit DA, Hammermeister DE, Leonard EN, Johnson RD (1995) Acute and long-term effects of nine chemicals on the Japanese medaka (Oryzias latipes). Archives of Environmental Contamination and Toxicology, 28(3):287–297.
Holm JV, Rügge K, Bjerg PL, Christensen TH (1995) Occurrence and distribution of pharmaceutical organic compounds in the groundwater downgradient of a landfill (Grinsted, Denmark). Environmental Science and Technology, 29(5):1415–1420.
Hommel D (1985) Handbuch der gefährlichen Güter. Berlin, Springer-Verlag.
Hong SK, Anestis DK, Henderson TT, Rankin GO (2000) Haloaniline-induced in vitro nephrotoxicity: Effects of 4-haloanilines and 3,5-dihaloanilines. Toxicology Letters, 114(1–3):125–133.
Hunger K, Mischke P, Rieper W, Raue R (2000) Azo dyes. In: Ullmanns’s encyclopedia of industrial chemistry, 6th ed. Weinheim, Wiley VCH Verlag.
Hwang HM, Hodson RE, Lee RF (1987) Degradation of aniline and chloroanilines by sunlight and microbes in estuarine water. Water Research, 21:309–316.
IARC (1993) Para-chloroaniline. In: Occupational exposures of hairdressers and barbers and personal use of hair colourants; some hair dyes, cosmetic colourants, industrial dyestuffs and aromatic amines. Lyon, International Agency for Research on Cancer, pp. 305–321 (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Volume 57).
IAWR (1998) Rheinbericht 1996–1998. Jülich, Internationale Arbeitsgemeinschaft der Wasserwerke im Rheineinzugsgebiet.
IFOP (2001) List of azo dyes which can degrade to cancerogenic aromatic amines. Frankfurt, German Trade Association for Dyes and Organic Pigments (IFOP), at website http://www.vci.de.
IPCS (1994) Assessing human health risks of chemicals. Derivation of guidance values for health-based exposure limits. Geneva, World Health Organization, International Programme on Chemical Safety (Environmental Health Criteria 170).
IPCS (1999) International Chemical Safety Card — p-Chloroaniline. Geneva, World Health Organization, International Programme on Chemical Safety (ICSC 0026).
Janicke W (1984) Sorbtionswirkungen von Kunststoffen auf organische Wasserinhaltstoffe. Zeitschrift für Wasser- und Abwasser-Forschung, 17:7–11.
Janicke W, Hilge G (1980) Messung der Bioelimination von Chloranilinen. GWF-Wasser/Abwasser, 121:131–135.
Julin AM, Sanders HO (1978) Toxicity of the IGR, diflubenzuron, to freshwater invertebrates and fishes. Mosquito News, 38:256–259.
Kačmár P, Pistl J, Mikula I (1995) The effect of p-chloroaniline on leucocytes of sheep peripheral blood under the migration-inhibition test conditions. Immunopharmacology and Immunotoxicology, 17(3):577–584.
Kahl T, Schröder KW, Lawrence FR, Marshall WJ, Höke H, Jäckh R (2000) Aniline. In: Ullmann’s encyclopedia of industrial chemistry, 6th ed. Weinheim, Wiley VCH Verlag.
Kalsch W, Nagel R, Urich K (1991) Uptake, elimination, and bioconcentration of ten anilines in zebrafish (Brachydanio rerio). Chemosphere, 22:351–363.
Khamuev GD (1967) The maximum possible concentration of p-chloroaniline and m-chloroaniline in water bodies. Gigiena i Sanitariya, 32:15–21 [cited in BUA, 1995].
Kharkharov AA (1954) Absorption spectra and the structure of molecules. Communication 2. Spectral investigations of alcoholic solutions of chloro derivates of aniline. Izvestiya Akademii S S S R Otdelenie Tekhnicheskikh Nauk, pp. 739–741.
Kiese M (1963) The effect of certain substituents upon the N-oxidation of aniline in vivo. Naunyn Schmiedeberg’s Archiv für Experimentelle Pathologie und Pharmakologie, 244:387–404.
Kiese M, Lenk W (1971) Metabolites of 4-chloroaniline and chloroacetanilides produced by rabbits and pigs. Biochemical Pharmacology, 20:379–391.
Kilzer L, Scheunert I, Geyer H, Klein W, Korte F (1979) Laboratory screening of the volatilization rates of organic chemicals from water and soil. Chemosphere, 10:751–761.
Kimber I, Mitchell JA, Griffin AC (1986) Development of a murine local lymph node assay for the determination of sensitizing potential. Food and Chemical Toxicology, 24:585–586.
Kishida K, Otori T (1980) A quantitative study on the relationship between transcorneal permeability of drugs and their hydrophobicity. Japanese Journal of Ophthalmology, 24:251–259.
Kloskowski R, Scheunert I, Klein W, Korte F (1981a) Laboratory screening of distribution, conversion and mineralization of chemicals in the soil–plant system and comparison to outdoor experimental data. Chemosphere, 10:1089–1100.
Kloskowski R, Scheunert I, Korte F (1981b) Kurzzeit-Tests zur Verteilung und Umwandlung von Umweltchemikalien im System Pflanze-Boden und Vergleich der Ergebnisse mit Freilandversuchen. München, Gesellschaft für Strahlen- und Umweltforschung, Institut für Ökologische Chemie, pp. 161–171 (GSF Report O 599).
Koch R, Nordmeyer JH (2000) Textile printing. In: Ullmanns’s encyclopedia of industrial chemistry, 6th ed. Weinheim, Wiley VCH Verlag.
Kohlbecker G (1989) Toxic impurities in chlorhexidine digluconate. Deutsche Zahnarztliche Zeitschrift, 44:273–276.
Kondo M, Nishihara T, Shimamoto T, Ichikawa T, Fuji M (1988) Screening test for degradation of chemicals in water — degradation test by photoirradiation. Eisei-Kagaku, 34:41–47.
Kondrashov VA (1969a) The toxicology of chloraniline and aniline. Trudy, Gosudarstvennyi Institut Prikladnoi Khimii, 62:209–214.
Kondrashov VA (1969b) On the toxic action of chloraniline and aniline fumes on the organism through the intact skin exposed to them. Gigiena Truda i Professional’nye Zabolevaniya, 13:29–32 [cited in BUA, 1995].
Korte F, Freitag D, Geyer H, Klein W, Kraus AG, Lahaniatis E (1978) Ecotoxicologic profile analysis. A concept for establishing ecotoxicologic priority lists for chemicals. Chemosphere, 1:79–102.
Kotzias D (1981) Verteilungkoeffizient. In: Überprüfung der Durchführbarkeit von Prüfungsvorschriften und der Ausagekraft der Grundprüfung des E. Chem. G. München, Gesellschaft für Strahlen- und Umweltforschung (GSF) München, pp. 115–121.
Kotzias D, Geyer H, Viswanathan R, Kraus A, Freitag D, Klein W, Korte F (1980) An approach to the ecotoxicological evaluation of environmental chemicals. Presented at the 9th International Congress of the European Association of Poison Control Centers and the European Meeting of the International Association of Forensic Toxicologists, Thessaloniki, pp. 257–268.
Kuhn EP, Suflita JM (1989) Sequential reductive dehalogenation of chloroanilines by microorganisms from a methanogenic aquifer. Environmental Science and Technology, 23:848–852.
Kühn R (1989) Umweltchemikalien/Schadstoffwirkungen. Ergebnisse der Schadstoffwirkung von ausgewählten Stoffen (Aniline, Phenole, Aliphaten) gegen Daphnia magna. In: Chemikaliengesetz Heft 8, Prüfung und Bewertung von Stoffen auf ihre Umweltverträglichkeit. Berlin, Umweltbundesamt, pp. 138–152.
Kühn R, Pattard M, Pernak K-D, Winter A (1988) Schadstoffwirkungen von Umweltchemikalien im Daphnien- Reproduktionstest als Grundlage zur Bewertung der Umweltgefährlichkeit in aquatischen Systemen. Prepared by Institut für Wasser-, Boden- und Lufthygiene des Bundesgesundheitsamtes, Berlin, for Umweltbundesamt, 412 pp. (Report No. 10603052).
Kühn R, Pattard M, Pernak KD, Winter A (1989a) Results of the harmful effects of selected water pollutants (anilines, phenols, aliphatic compounds) to Daphnia magna. Water Research, 23:495–499.
Kühn R, Pattard M, Pernak KD, Winter A (1989b) Results of the harmful effects of water pollutants to Daphnia magna in the 21 day reproduction test. Water Research, 23:501–510.
Kwasniewska K, Kaiser KLE (1984) Toxicities of selected chloroanilines to four strains of yeast. In: Kaiser KLE, ed. QSAR environmental toxicology. Dordrecht, Reidel Publishing Company, pp. 223–233.
Lahaniatis E (1981) Hydrolyse in pH-Abhängigkeit. In: Überprüfung der Durchführbarkeit von Prüfungsvorschriften und der Ausagekraft der Grundprüfung des E. Chem. G. München, Gesellschaft für Strahlen- und Umweltforschung (GSF) München, pp. 127–130.
Lee SK, Freitag D, Steinberg C, Kettrup A, Kim YH (1993) Effects of dissolved humic materials on acute toxicity of some organic chemicals to aquatic organisms. Water Research, 27(2):199–204.
Lepschy J, Müller C (1991) Das Bodenbeobachtungsprogramm der LPB, erste Untersuchungsergebnisse über organische Schadstoffe in Böden. Teil I: Herbizide – extrahierbare Anteile. Schule und Beratung, Heft 1/91:III-9 – III-11.
Levillain F, Charasson V, El Marbouh L, Poey J, Houin G (1998) In vitro study of the percutaneous absorption of four aromatic amines using hairless rat skin. Drug Research, 48(9):948–951.
Levina MM, Kurando TB, Belyakov AA, Smirnova VG, Odlyzhko SL (1966) Questions of occupational hygiene and the status of employee health in the production of monuron. Gigiena Truda i Professional’nye Zabolevaniya, 11:54–56.
Lewalter J, Korallus U (1985) Blood protein conjugates and acetylation of aromatic amines. New findings on biological monitoring. International Archives of Occupational and Environmental Health, 56:179–196.
LGC (1998) The risk of cancer caused by textiles and leather goods coloured with azo dyes. Final report. Report prepared by Laboratory of the Government Chemist, London, for European Commission DG III.
Linch AC (1974) Biological monitoring for industrial exposure to cyanogenic aromatic nitro and amino compounds. American Industrial Hygiene Association Journal, 35:426–432.
Lores EM, Meekins FC, Moseman RF (1980) Determination of halogenated anilines in urine by high-performance liquid chromatography with an electrochemical detector. Journal of Chromatography, 188:412–416.
LUA (1997) Rheingütebericht NRW 1995. Essen, Landesumweltamt Nordrhein-Westfalen.
Lyons CD, Katz SE, Bartha R (1985) Persistence and mutagenic potential of herbicide-derived aniline residues in pond water. Bulletin of Environmental Contamination and Toxicology, 35:696–703.
Magnusson B, Kligman AM (1969) The identification of contact allergens by animal assay. The guinea pig maximisation test. Journal of Investigative Dermatology, 52:268–276.
MAK (1992) p-Chloroaniline. In: Occupational toxicants: critical data evaluation for MAK values and classification of carcinogens. Volume 3. Deutsche Forschungsgemeinschaft, Commission for the Investigation of Health Hazards of Chemical Compounds in the Work Area. Weinheim, VCH Publishers, pp. 45–61.
Maronpot RR, Shimkin MB, Witschi HP, Smith LH, Cline JM (1986) Strain A mouse pulmonary tumor test results for chemicals previously tested in the National Cancer Institute carcinogenicity tests. Journal of the National Cancer Institute, 76(6):1101–1112.
Marquart HW, Sewekow B, Hamburger B, Harzdorf C, Hellbusch HD (1984) Umweltforschungsplan des Bundesministers des Innern, Umweltchemikalien, Überprüfung der Durchführbarkeit von Prüfungsvorschriften und der Aussagekraft der Stufe 1 und 2 des ChemG - Teil II. Leverkusen, Bayer-AG, pp. 1–128.
Marty J-P, Wepierre J (1979) Évaluation de la toxicité d’agents d’activité cosmétique cas du trichlorocarbanilide. Labo-Pharma — Problèmes et Techniques, 286:306–310.
McClure GW (1974) Degradation of anilide herbicides by propham-adapted microorganism. Weed Science, 22:323–329.
McGregor D, Prentice RD, McConville M, Lee YJ, Caspary WJ (1984) Reduced mutant yield at high doses in the Salmonella/ activation assay: The cause is not always toxicity. Environmental Mutagenesis, 6:545–557.
McLean S, Starmer GA, Thomas J (1969) Methaemoglobin formation by aromatic amines. Journal of Pharmacy and Pharmacology, 21:441–450.
McLeese DW, Zitko V, Peterson MR (1979) Structure–lethality relationships for phenols, anilines and other aromatic compounds in shrimp and clams. Chemosphere, 8:53–57.
Miller GC, Crosby DG (1983) Photooxidation of 4-chloroaniline and N-(4-chlorophenyl)-benzenesulfonamide to nitroso- and nitro-products. Chemosphere, 12:1217–1228.
Mitchell AD, Rudd CJ, Caspary WJ (1988) Evaluation of the L5178Y mouse lymphoma cell mutagenesis assay: intralaboratory results for sixty-three coded chemicals tested at SRI International. Environmental and Molecular Mutagenesis, 12(Suppl.13):37–101.
Monsanto (1986) Evaluation of blood methemoglobin levels for workers involved in production of parachloroaniline. St. Louis, MO, Monsanto Company, Department of Medicine and Environmental Health (Report No. MC 86-9055; NTIS/OTS 0510320) [cited in BUA, 1995].
Mortelmans K, Haworth S, Lawlor T, Speck W, Tainer B, Zeiger E (1986) Salmonella mutagenicity tests: II. Results from the testing of 270 chemicals. Environmental Mutagenesis, 8(S7):1–119.
Müller L, Fattore E, Benfenati E (1997) Determination of aromatic amines by solid-phase microextraction and gas chromatography–mass spectrometry in water samples, 1997. Journal of Chromatography, 791(1–2):221–230.
Müller-Wegener U (1982) Über die Adsorption umweltrelevanter Chemikalien in Böden. Chemie Erde, 41:175–181.
Mutanen R, Siltanen HT, Kuukka VP, Annila EA, Varama MM (1988) Residues of diflubenzuron and two of its metabolites in a forest ecosystem after control of the pine looper moth, Bupalus piniarius L. Pesticide Science, 23:131–140.
Myhr BC, Caspary WJ (1988) Evaluation of the L5178Y mouse lymphoma cell mutagenesis assay: intralaboratory results for sixty-three coded chemicals tested at Litton Bionetics, Inc. Environmental and Molecular Mutagenesis, 12(Suppl.13):103–194.
Myhr BC, McGregor D, Bowers L, Riach C, Brown AG, Edwards I, McBride D, Martin R, Caspary WJ (1990) L5178Y mouse lymphoma cell mutation assay results with 41 compounds. Environmental and Molecular Mutagenesis, 16(Suppl.18):138–167.
NCI (1979) Bioassay of p-chloroaniline for possible carcinogenicity, CAS No.
Neumann HG, Birner G, Kowallik P, Schütze D, Zwirner-Baier I (1993) Hemoglobin adducts of N-substituted aryl compounds in exposure control and risk assessment. Environmental Health Perspectives, 99:65–69.
Nomura A (1975) Studies on sulfhemoglobin formation by various drugs. Folia Pharmacologica Japonica, 71:351–365.
NTP (1989) Toxicology and carcinogenesis studies of para-chloroaniline hydrochloride (CAS No.
NTP (1998) NTP technical report on comparative toxicity studies of o-, m-, and p-chloroaniline (CAS Nos.
OECD (1997) The 1997 OECD list of high production volume chemicals. Paris, Organisation for Economic Co-operation and Development.
Office of Health Studies (1985) Chemicals in the environment. Report on environmental survey and wildlife monitoring in F.Y. 1982 and 1983. Tokyo, Environment Agency Japan, 9 pp.
Ono Y, Somiya I, Kawaguchi T (1992) Genotoxic evaluation on aromatic organochlorine compounds by using umu test. Water Science and Technology, 26(1–2):61–69.
Onuska FI, Terry KA, Maguire RJ (2000) Analysis of aromatic amines in industrial wastewater by capillary gas chromatography– mass spectrometry. Water Quality Research Journal of Canada, 35(2):245–261.
OSHA (1992) Chemical sampling information, p-Chloroaniline. Washington, DC, US Department of Labor, Occupational Safety and Health Administration, at website http://www.osha.gov/dts/chemicalsampling/data/CH_226936.html.
Pacséri I, Magos L, Batskor IA (1958) Threshold and toxic limits of some amino and nitro compounds. Archives of Industrial Health, 18:1–8.
Pai V, Bloomfield SF, Gorrod JW (1985) Mutagenicity of N-hydroxylamines and N-hydroxycarbamates towards strains of Escherichia coli and Salmonella typhimurium. Mutation Research, 151:201–207.
Pawlizki KH, Pogany E (1988) Charakterisierung zellwandgebundener Rückstände und ihr Verhalten im System Boden/Kulturpflanze. Gesunde Pflanzen, 40:390–395.
Perry DF, Carter DE, Sipes IG (1981) Distribution and excretion of 14C-parachloroaniline in the rat. Toxicologist, 1:4.
Pienta RJ, Kawalek JC (1981) Transformation of hamster embryo cells by aromatic amines. National Cancer Institute Monographs, 58:243–251.
Pienta RJ, Poiley JA, Lebherz WB III (1977) Morphological transformation of early passage golden Syrian hamster embryo cells derived from cryopreserved primary cultures as a reliable in vitro bioassay for identifying diverse carcinogens. International Journal of Cancer, 19:642–655.
Ping Pan H, Fouts JR, Devereux TR (1979) Hepatic microsomal N-hydroxylation of p-chloroaniline and p-chloro-N-methylaniline in red-winged blackbird compared with rat. Xenobiotica, 9(7):441–446.
Prasad I (1970) Mutagenic effects of the herbicide 3',4'-dichloropropionanilide and its degradation products. Canadian Journal of Microbiology, 16:369–372.
Quast I (1984) Adsorption/desorption. In: Ballhorn L, Freitag D, eds. Überprüfung der Durchführbarkeit von Prüfungsvorschriften und der Aussagekraft der Stufe I und II des E. Chem. G. Neuherberg, Gesellschaft für Strahlen- und Umweltforschung München mbH, pp. 15–43.
Ramachandran KN, Gupta VK (1993) New analytical technique for the simultaneous determination of aromatic amines. Fresenius Journal of Analytical Chemistry, 346(4):457.
Rankin GO, Yang DJ, Cressey-Veneziano K, Casto S, Wang RT, Brown PI (1986) In vivo and in vitro nephrotoxicity of aniline and its monochlorophenyl derivatives in the Fischer 344 rat. Toxicology, 38:269–283.
Rankin GO, Beers KW, Nicoll DW, Anestis DK, Hong SK, Hubbard JL, Ball JG, Valentovic MA, Brown PI (1996) Nephrotoxic potential of 2-amino-5-chlorophenol and 4-amino-3-chlorophenol in Fischer 344 rats: comparisons with 2- and 4-chloroaniline and 2- and 4-aminophenol. Toxicology, 108(1–2):109–123.
Rashid KA, Arjmand M, Sandermann H, Mumma RO (1987) Mutagenicity of chloroaniline/lignin metabolites in the Salmonella/microsome assay. Journal of Environmental Science and Health, B22(6):721–729.
Ribo JM, Kaiser KLE (1984) Toxicities of chloranilines to Photobacterium phosphoreum and their correlations with effects on other organisms and structural parameters. In: Kaiser KLE, ed. QSAR environmental toxicology. Dordrecht, Reidel Publishing Company, pp. 319–336.
Riffelmann M, Muller G, Schmieding W, Popp W, Norpoth K (1995) Biomonitoring of urinary aromatic amines and arylamine hemoglobin adducts in exposed workers and nonexposed control persons. International Archives of Occupational and Environmental Health, 68:36–43.
Riggin RM, Cole TF, Billits S (1983) Determination of aniline and substituted derivates in wastewater by gas and liquid chromatography. Analytical Chemistry, 55:1862–1869.
Rippen G, Ilgenstein M, Klöpffer W, Poremski HJ (1982) Screening of the adsorption behavior of new chemicals: Natural soils and model adsorbents. Ecotoxicology and Environmental Safety, 6:236–245.
Rosenkranz HS, Poirier LA (1979) Evaluation of the mutagenicity of DNA-modifying activity of carcinogens and noncarcinogens in microbial systems. Journal of the National Cancer Institute, 62(4):873–891.
Rott B (1981a) Closed bottle test (CBT). In: Überprüfung der Durchfühbarkeit von Prüfungsvorschriften und der Aussagekraft der Grundprüfung des E. Chem. G. München, Gesellschaft für Strahlen- und Umweltforschung (GSF) München, pp. 276–284.
Rott B (1981b) Zahn-Wellens-Test (ZWT). In: Überprüfung der Durchführbarkeit von Prüfungsvorschriften und der Aussagekraft der Grundprüfung des E. Chem. G. München, Gesellschaft für Strahlen- und Umweltforschung (GSF) München, pp. 263–275.
Rott B (1984) 21 Tage-Daphnientest. In: Ballhorn L, Freitag D, eds. Überprüfung der Durchführbarkeit von Prüfungsvorschriften und der Aussagekrsft der Stufe I und II des E. Chem. G. Neuherberg, Gesellschaft für Strahlen- und Umweltforschung München mbH, pp. 88–112.
Rott B, Viswanathan R, Freitag D, Korte F (1982) Vergleichende Untersuchung der Anwendbarkeit verschiedener Tests zur Überprüfung der Abbaubarkeit von Umweltchemikalien. Chemosphere, 11:531–538.
Sakagami Y, Yamazaki H, Ogasawara N, Yokoyama H, Ose Y, Sato T (1988) The evaluation of genotoxic activities of disinfectants and their metabolites by umu test. Mutation Research, 209:155–160.
SCCNFP (1999) Notes of guidance for testing of cosmetic ingredients for their safety evaluation. Brussels, The Scientific Committee on Cosmetic Products and Non-Food Products Intended for Consumers.
Schaefer CH, Colwell AE, Dupras EF (1980) The occurrence of p-chloroaniline and p-chlorophenylurea from the degradation of diflubenzuron in water and fish. In: Proceedings Papers of the 48th Annual Conference of the California Mosquito Vector Control Association. Fresno, CA, California Mosquito Vector Control Association, pp. 84–89.
Scheubel JB (1984) Überprüfung der Durchführbarkeit von Prüfungsvorschriften und der Aussagekraft der Stufe I und II des Chemikaliengesetzes (Teil III), Abschlußbericht. Prepared by Chemische Werke Hüls AG, Abt. Umweltschutz, Marl, for Umweltbundesamt, 83 pp. (Report 10604011/05).
Scheunert I (1981) Bestimmung der Votalität aus wäßriger Lösung. In: Überprüfung der Durchführbarkeit von Prüfungsvorschriften und der Aussagekraft der Grundprüfung des E. Chem. G. München, Gesellschaft für Strahlen- und Umweltforschung (GSF) München, pp. 165–241.
Scheunert I (1984) Wachstumstest mit höheren Pflanzen. In: Ballhorn L, Freitag D, eds. Überprüfung der Durchführbarkeit von Prüfungsvorschriften und der Aussagekraft der Stufe I und II des E. Chem. G. Neuherberg, Gesellschaft für Strahlen- und Umweltforschung München mbH, pp. 113–123,
Schmidt C (1989) Schadwirkung von Phenolen, Anilinen und Aliphaten auf Algen. In: Chemikaliengesetz Heft 8, Prüfung und Bewertung von Stoffen auf ihre Umweltverträglichkeit. Berlin, Umweltbundesamt, pp. 98–137.
Schmidt C, Schnabl H (1988) Stoffbezogene Struktur- Wirkungsbeziehungen bei Biotesten. Vom Wasser, 70:21–32.
Scholes EW, Basketter DA, Sarll AE, Kimber I, Evans CD, Miller K, Robbins MC, Harrison PTC, Waite SJ (1992) The local lymph node assay: results of a final inter-laboratory validation under field conditions. Journal of Applied Toxicology, 12(3):217–222.
Schuphan I, Ebing W (1978) Metabolism and balance studies of [14C]monolinuron after use in spinach followed by cress and potato cultures. Pesticide Biochemistry and Physiology, 38:107–118.
Scott AI, Eccleston E (1967) Investigations of the general toxic and haematological effects of para-chloraniline in several species. Proceedings of the European Society for the Study of Drug Toxicity, 8:195–204.
Scotti P, Tomasini M (1966) Su di un caso di grave intossicazione acuta da parachloroanilina con intensa metemoglobinemia e con transitorie alterazioni elettrocardiografiche. Medicina del Lavoro, 51(1):662–666.
Seuferer SL, Braymer HD, Dunn JJ (1979) Metabolism of diflubenzuron by soil microorganisms and mutagenicity of the metabolites. Pesticide Biochemistry and Physiology, 10:174–180.
Simmon VF (1979a) In vitro mutagenicity assays of chemical carcinogens and related compounds with Salmonella typhimurium. Journal of the National Cancer Institute, 62(4):893–900.
Simmon VF (1979b) In vitro assays for recombinogenic activity of chemical carcinogens and related compounds with Saccharomyces cerevisiae D3. Journal of the National Cancer Institute, 62:901–909.
Smith MR, Damani LA, Disley LG, Gorrod JW, Marsden JT, Patterson LH, Rhenius ST (1978) Ferrihaemoglobin formation by N-oxidation products of certain compounds in the rabbit. In: Gorrod JW, ed. Biological oxidation of nitrogen. Amsterdam, Elsevier, pp. 363–368.
Smith RP (1986) Toxic responses of the blood. In: Klaassen CD, Amdur MO, Doull J, eds. Casarett & Doull’s toxicology — The basic science of poisons, 3rd ed. New York, Macmillan, pp. 223–244.
Srour R (1989) Aromatic intermediates and derivatives. p-Chloroaniline. Chemical Consultant, Paris (formerly Bureau d’Études industrielles et de Coopération de L’Institute français de Pétrôle).
Srour R (1995) Aromatic intermediates and derivatives. Trichlorocarbanilide. Chemical Consultant, Paris (formerly Bureau d’Études industrielles et de Coopération de L’Institute français de Pétrôle).
Srour R (1996) Aromatic intermediates and derivatives. p-Chloroaniline. Chemical Consultant, Paris (formerly Bureau d’Études industrielles et de Coopération de L’Institute français de Pétrôle).
Steinberg CEW, Xu Y, Lee SK, Freitag D, Kettrup A (1993) Effect of dissolved humic material (DHM) on bioavailability of some organic xenobiotics to Daphnia magna. Chemical Speciation and Bioavailability, 5(1):1–9.
Stiff MJ, Wheatland AB (1984) Study of discharges of chloroanilines and chloronitrobenzenes into the aquatic environment and the best technical means for the reduction of water pollution from such discharges. Stevenage, Water Research Centre, pp. 29–52 (EEC Contract No. P.83.462).
Süß A, Fuchsbichler G, Eben C (1978) Abbau von Anilin, 4-Chloranilin und 3,4-Dichloranilin in verschiedenen Böden. Zeitschrift für Pflanzenernahrung und Bodenkunde, 141:57–66.
Thomas RG (1990) Volatilization from water. In: Lyman WJ, Reehl WF, Rosenblatt DH, eds. Handbook of chemical property estimation methods. Washington, DC, American Chemical Society.
Thompson CZ, Hill LE, Epp JK, Probst GS (1983) The induction of bacterial mutation and hepatocyte unscheduled DNA synthesis by monosubstituted anilines. Environmental Mutagenesis, 5:803–811.
Traul KA, Takayama K, Kachevsky V, Hink RJ, Wolff JS (1981) A rapid in vitro assay for carcinogenicity of chemical substances in mammalian cells utilizing an attachment-independence endpoint. Journal of Applied Toxicology, 1:190–195.
US EPA (1981) Development of tests for determining anaerobic biodegradation potential. Washington, DC, US Environmental Protection Agency, Department of Crop and Soil Science, 92 pp. (EPA-560/5-81-013; PB84-166495).
US EPA (1990) Textile dye weighing monitoring study. Washington, DC, US Environmental Protection Agency, Office of Toxic Substances (EPA 560/5-90-009).
US Toxics Release Inventory (1999) TRI database. Washington, DC, US Environmental Protection Agency, Toxics Release Inventory (TRI) Program, at website http://www.epa.gov/tri/.
Valentovic MA, Ball JG, Anestis D, Rankin GO (1993) Investigation of 4-chloroaniline toxicity in Fischer 344 (F344) rats. Toxicologist, 13(1):208.
van Bladel R, Moreale A (1977) Adsorption of herbicide-derived p-chloroaniline residues in soils: a predictive equation. Journal of Soil Science, 28:93–102.
van der Bijl P, Gelderblom WCA, Thiel PG (1984) On the mutagenicity of parachloroaniline, a breakdown product of chlorhexidine. Journal of the Dental Association of South Africa, 39:535–537.
van der Vorst MMJ, Tamminga P, Wijburg FA, Schutgens RBH (1990) Severe methaemoglobinaemia due to para-chloroaniline intoxication in premature neonates. European Journal of Pediatrics, 50:72–73.
Viswanathan R (1984) Regenwurmtest. In: Ballhorn L, Freitag D, eds. Überprüfung der Durchführbarkeit von Prüfungsvorschriften und der Aussagekraft der Stufe I und II des E. Chem. G. Neuherberg, Gesellschaft für Strahlen- und Umweltforschung München mbH, pp. 124–131.
Wagner R (1988) Methoden zur Prüfung der biochemischen Abbaubarkeit chemischer Substanzen. Weinheim (BRD), VCH Verlagsgesellschaft mbH.
Wagner R, Bräutigam HJ (1981) Entwicklung und Erprobung einer Methode zur Untersuchung des Abbauverhaltens von organischen Substanzen unter anaeroben Milieubedingungen. Stuttgart, Institut für Siedlungswasserbau, Wassergüte- und Abfallwirtschaft, pp. 21–41 (Report No. 03 7221).
Wahner A, Zetzsch C (1983) Rate constants for the addition of OH to aromatics (benzene, p-chloroaniline, and o-, m-, and p-dichlorobenzene) and the unimolecular decay of the adduct. Kinetics into a quasi-equilibrium. Journal of Physical Chemistry, 87:4945–4951.
Wangenheim J, Bolcsfoldi G (1988) Mouse lymphoma L5178Y thymidine kinase locus assay of 50 compounds. Mutagenesis, 3(3):193–205.
Watanabe T, Ishihara N, Ikeda M (1976) Toxicity of and biological monitoring for 1,3-diamino-2,4,6-trinitrobenzene and other nitro-amino derivates of benzene and chlorobenzene. International Archives of Occupational and Environmental Health, 37:157–168.
Wegman RCC, van den Broek HH, Hofstee AWM, Marsman JA (1984) Determination of triazines, organophosphorus containing pesticides and aromatic amines in soil samples. Mededelingen van de Faculteit Landbouwwetenschappen, Rijksuniversiteit Gent, 49/3b:1231–1239.
Wellens H (1990) Zur biologischen Abbaubarkeit mono- und disubstituierter Benzolderivate. Zeitschrift für Wasser- und Abwasser-Forschung, 23:85–98.
Welp G, Brümmer GW (1999) Effects of organic pollutants on soil microbial activity: the influence of sorption, solubility, and speciation. Ecotoxicology and Environmental Safety, 43:83–90.
Williams GM, Laspia MF, Dunkel VC (1982) Reliability of the hepatocyte primary culture/DNA repair test in testing of coded carcinogens and noncarcinogens. Mutation Research, 97:359–370.
Worobey BL, Webster GRB (1982) Pyrolytic release of tightly complexed 4-chloroaniline from soil and soil humic acids. Journal of Agricultural and Food Chemistry, 30:164–169.
Yoshida M, Yoshikawa H, Goto H, Hara I (1989) Evaluation of the nephrotoxicity of aromatic nitro-amino compounds by urinary enzyme activities. Journal of Toxicological Sciences, 14:257–268.
Yoshida T, Hirata M, Tabuchi T, Miyajima K (1991) Identification of urinary metabolites in a patient of acute poisoning by p-chloroaniline. Japanese Journal of Industrial Health, 33:501–508.
Yoshida T, Hirata M, Tabuchi T, Miyajima K (1992a) Excretion of p-chloroaniline metabolites into urine. Japanese Journal of Industrial Health, 34:3–9.
Yoshida T, Hirata M, Tabuchi T, Miyajima K, Andoh K (1992b) Amounts of urinary metabolites of p-chloroaniline and their half lives in a patient with acute poisoning. Japanese Journal of Industrial Health, 34:126–130.
Yoshioka Y, Ose Y, Sato T (1985) Testing for the toxicity of chemicals with Tetrahymena pyriformis. The Science of the Total Environment, 43:149–157.
Zeiger E (1990) Mutagenicity of 42 chemicals in Salmonella. Environmental and Molecular Mutagenesis, 16(Suppl. 18):32–54.
Zimmer D, Mazurek J, Petzhold G, Bhuyan BK (1980) Bacterial mutagenicity and mammalian cell DNA damage by several substituted anilines. Mutation Research, 77:317–326.
Zvezdaj VI (1970) Die Toxikologie von isomeren (para- und meta-) Chloranilinen und die experimentelle Begründung der maximal zulässigen Konzentrationen dieser Verbindungen in der Luft von Arbeitsräumen. Farmakologiya i Toksikologiya (Kiev), 5:145–148 [cited in BUA, 1995].
BUA (1995) p-Chloroaniline. Beratergremium für Umweltrelevante Altstoffe (BUA) der Gesellschaft Deutscher Chemiker. Weinheim, VCH, 171 pp. (BUA Report 153)
For the BUA review process, the company that is in charge of writing the report (usually the largest producer in Germany) prepares a draft report using literature from an extensive literature search as well as internal company studies.
In this BUA report, the toxicological sections were prepared by Berufsgenossenschaft der Chemischen Industrie (BG Chemie, Toxicological Evaluation No. 9). The draft document is subject to a peer review in several readings of a working group consisting of representatives from government agencies, the scientific community, and industry.
The authors of this BUA report were Drs A. Boehncke, J. Kielhorn, G. Könnecker, C. Pohlenz-Michel, and I. Mangelsdorf of the Fraunhofer Institute of Toxicology and Aerosol Research, Drug Research and Clinical Inhalation, Hanover, Germany.
The English version of the report was published in 1997.
MAK (1992) p-Chloroaniline. In: Occupational toxicants: critical data evaluation for MAK values and classification of carcinogens, Vol. 3. Deutsche Forschungsgemeinschaft, Commission for the Investigation of Health Hazards of Chemical Compounds in the Work Area (MAK Commission). Weinheim, VCH Publishers, pp. 45–61
The scientific documents of the German Commission for the Investigation of Health Hazards of Chemical Compounds in the Work Area (MAK Commission) are based on critical evaluations of the available toxicological and occupational medical data from extensive literature searches and of well documented industrial data. The evaluation documents involve a critical examination of the quality of the database, indicating inadequacy or doubtful validity of data and identification of data gaps. This critical evaluation and the classification of substances are the result of an extensive discussion process by the members of the Commission proceeding from a draft document prepared by members of the Commission, by ad hoc experts, or by the Scientific Secretariat of the Commission. Scientific expertise is guaranteed by the members of the Commission, consisting of experts from the scientific community, industry, and employers’ associations.
NTP (1989) Toxicology and carcinogenesis studies of para-chloroaniline hydrochloride (CAS No.
The members of the Peer Review Panel who evaluated the draft Technical Report on p-chloroaniline hydrochloride on 8 April 1988 are listed below. Panel members serve as independent scientists, not as representatives of any institution, company, or governmental agency. In this capacity, Panel members have five major responsibilities: (a) to ascertain that all relevant literature data have been adequately cited and interpreted, (b) to determine if the design and conditions of the NTP studies were appropriate, (c) to ensure that the Technical Report presents the experimental results and conclusions fully and clearly, (d) to judge the significance of the experimental results by scientific criteria, and (e) to assess the evaluation of the evidence of carcinogenicity and other observed toxic responses.
National Toxicology Program Board of Scientific Counselors
Technical Reports Review Subcommittee
Robert A. Scala (Chair), Exxon Corporation, East Millstone, NJ
Michael A. Gallo (Principal Reviewer), Department of Environmental and Community Medicine, Rutgers Medical School, Piscataway, NJ
Frederica Perera, School of Public Health, Columbia University, New York, NY (unable to attend)
Ad Hoc Subcommittee Panel of Experts
John Ashby, Imperial Chemical Industries, PLC, Alderley Park, England
Charles C. Capen, Department of Veterinary Pathobiology, Ohio State University, Columbus, OH
Vernon M. Chinchilli, Medical College of Virginia, Virginia Commonwealth University, Richmond, VA
Kim Hooper, Department of Health Services, State of California, Berkeley, CA
Donald H. Hughes (Principal Reviewer), Regulatory Services Division, The Procter and Gamble Company, Cincinnati, OH
William Lijinsky, Frederick Cancer Research Facility, Frederick, MD
Franklin E. Mirer, United Auto Workers, Detroit, MI (unable to attend)
James A. Popp, Chemical Industry Institute of Toxicology, Research Triangle Park, NC
Andrew Sivak (Principal Reviewer), Arthur D. Little, Inc., Cambridge, MA
The draft CICAD on 4-chloroaniline was sent for review to institutions and organizations identified by IPCS after contact with IPCS national Contact Points and Participating Institutions, as well as to identified experts. Comments were received from:
M. Baril, International Programme on Chemical Safety/Institut de Recherches en Santé et en Sécurité du Travail du Québec, Montreal, Quebec, Canada
R. Benson, Drinking Water Program, US Environmental Protection Agency, Denver, CO, USA
R Cary, Health and Safety Executive, Bootle, Merseyside, United Kingdom
R. Chhabra, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC, USA
S. Dobson, Centre for Ecology and Hydrology, Monks Wood, United Kingdom
H. Galal-Gorchev, US Environmental Protection Agency, Washington DC, USA
H. Gibb, National Center for Environmental Assessment, US Environmental Protection Agency, Washington, DC, USA
R.F. Hertel, Federal Institute for Health Protection of Consumers and Veterinary Medicine, Berlin, Germany
C. Hiremath, National Center for Environmental Assessment, US Environmental Protection Agency, Washington, DC, USA
S. Humphreys, Food and Drug Administration, Washington, DC, USA
H. Nagy, National Institute for Occupational Safety and Health, Cincinnati, OH, USA
H.G. Neumann, Würzberg University, Würzberg, Germany
D. Singh, National Center for Environmental Assessment, US Environmental Protection Agency, Washington, DC, USA
E. Soderlund, National Institute of Public Health, Oslo, Norway
K. Ziegler-Skylakakis, GSF-Forschungszentrum für Umvelt und Gesundheit, Neuherberg, Oberschleissheim, Germany
Ottawa, Canada,
29 October – 1 November 2001
Members
Mr R. Cary, Health and Safety Executive, Merseyside, United Kingdom
Dr T. Chakrabarti, National Environmental Engineering Research Institute, Nehru Marg, India
Dr B.-H. Chen, School of Public Health, Fudan University (formerly Shanghai Medical University), Shanghai, China
Dr R. Chhabra, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC, USA (teleconference participant)
Dr C. De Rosa, Agency for Toxic Substances and Disease Registry, Department of Health and Human Services, Atlanta, GA, USA (Chairman)
Dr S. Dobson, Centre for Ecology and Hydrology, Huntingdon, Cambridgeshire, United Kingdom (Vice-Chairman)
Dr O. Faroon, Agency for Toxic Substances and Disease Registry, Department of Health and Human Services, Atlanta, GA, USA
Dr H. Gibb, National Center for Environmental Assessment, US Environmental Protection Agency, Washington, DC, USA
Ms R. Gomes, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario, Canada
Dr M. Gulumian, National Centre for Occupational Health, Johannesburg, South Africa
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers and Veterinary Medicine, Berlin, Germany
Dr A. Hirose, National Institute of Health Sciences, Tokyo, Japan
Mr P. Howe, Centre for Ecology and Hydrology, Huntingdon, Cambridgeshire, United Kingdom (Co-Rapporteur)
Dr J. Kielhorn, Fraunhofer Institute of Toxicology and Aerosol Research, Hanover, Germany (Co-Rapporteur)
Dr S.-H. Lee, College of Medicine, The Catholic University of Korea, Seoul, Korea
Ms B. Meek, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario, Canada
Dr J.A. Menezes Filho, Faculty of Pharmacy, Federal University of Bahia, Salvador, Bahia, Brazil
Dr R. Rolecki, Nofer Institute of Occupational Medicine, Lodz, Poland
Dr J. Sekizawa, Division of Chem-Bio Informatics, National Institute of Health Sciences, Tokyo, Japan
Dr S.A. Soliman, Faculty of Agriculture, Alexandria University, Alexandria, Egypt
Dr M.H. Sweeney, Document Development Branch, Education and Information Division, National Institute for Occupational Safety and Health, Cincinnati, OH, USA
Dr J. Temmink, Department of Agrotechnology & Food Sciences, Wageningen University, Wageningen, The Netherlands
Ms D. Willcocks, National Industrial Chemicals Notification and Assessment Scheme (NICNAS), Sydney, Australia
Representative of the European Union
Dr K. Ziegler-Skylakakis, European Commission, DG Employment and Social Affairs, Luxembourg
Observers
Dr R.M. David, Eastman Kodak Company, Rochester, NY, USA
Dr R.J. Golden, ToxLogic LC, Potomac, MD, USA
Mr J.W. Gorsuch, Eastman Kodak Company, Rochester, NY, USA
Mr W. Gulledge, American Chemistry Council, Arlington, VA, USA
Mr S.B. Hamilton, General Electric Company, Fairfield, CN, USA
Dr J.B. Silkworth, GE Corporate Research and Development, Schenectady, NY, USA
Dr W.M. Snellings, Union Carbide Corporation, Danbury, CN, USA
Dr E. Watson, American Chemistry Council, Arlington, VA, USA
Secretariat
Dr A. Aitio, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
Mr T. Ehara, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
Dr P. Jenkins, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
Le présent CICAD consacré à la 4-chloroaniline (p-chloroaniline ) a été préparé par l'Institut Fraunhofer de toxicologie et de recherche sur les aérosols , de Hanovre (Allemagne). Il s'inspire de rapports établis par le Comité consultatif de la Société allemande de chimie sur les substances chimiques d'importance écologique (BUA, 1993a) , par la Commission allemande MAK (MAK,1992), par l'Union allemande des industries chimiques (BG Chemie,1994 ) et par le Programme toxicologique national des Etats-Unis (NTP, 1989). En mars 2001,il a été procédé à un dépouillement bibliographique exhaustif des bases de données correspondantes , afin de rechercher toute référence intéressante à des publications postérieures aux rapports en question. Des informations sur la préparation et l'examen par des pairs des sources documentaires utilisées sont données à l'appendice 1. L'appendice 2 fournit des renseignements sur l'examen par des pairs du présent CICAD. Ce CICAD a été approuvé en tant qu'évaluation internationale lors d'une réunion du Comité d'évaluation finale qui s'est tenue à Ottawa (Canada) du 29 octobre au 1er novembre 2001. La liste des participants à cette réunion figure à l'appendice 3. La fiche internationale sur la sécurité chimique de la 4-chloroaniline (ICSC 0026) , établie par le Programme international sur la sécurité chimique (IPCS, 2002) , est également reproduite dans le présent document.
Les anilines chlorées en position 2-, 3-, et 4- (ortho-, méta- et para- ) ont sensiblement les mêmes utilisations. Ces trois isomères sont tous hémotoxiques et leurs propriétés toxiques sont identiques chez le rat et la souris, la 4-chloroaniline étant dans tous les cas celui des trois qui produit les effets les plus graves. La 4-chloroaniline se révèle génotoxique dans divers systèmes d'épreuve (voir plus loin) , alors que la 2- et la 3-chloroaniline donnent à cet égard des résultats irréguliers, qui correspondent à une génotoxicité faible ou nulle. C'est pourquoi le présent CICAD ne concerne que la 4-chloroaniline, c'est-à-dire à la plus toxique des chloranilines.
La 4-chloroaniline ou parachloroaniline (désignée dans la suite du texte par le sigle PCA) (No CAS
On utilise la PCA comme intermédiaire dans la fabrication d'un certain nombre de produits, notamment des produits agrochimiques, des colorants et des pigments azoïques, des cosmétiques et des produits pharmaceutiques. Les rejets de PCA dans l'atmosphère peuvent donc provenir de sources industrielles diverses (production, transformation, teinture, impression ).
Compte tenu des utilisations de la PCA, on peut s'attendre à ce que l'hydrosphère soit le principal compartiment de l'environnement où se retrouve ce composé. Dans le Rhin et ses affluents par exemple, on en trouve à une concentration qui se situe à peu près entre 0,1 et 1 μg/litre. Dans l'hydrosphère, la PCA se décompose rapidement sous l'action de la lumière (demi-vie mesurée : 2 à 7 h). Le calcul de la demi-vie du composé dans l'air en prenant en compte sa réaction avec les radicaux hydroxyle donne une valeur de 3,9 h . Les nombreuses études consacrées à la biodégradation de la PCA indiquent que ce composé est intrinsèquement biodégradable dans l'eau en aérobiose, aucune minéralisation importante n'étant par contre décelée en anaérobiose.
Les coefficients de sorption dans divers types de sols déterminés d'après l'isotherme de sorption de Freundlich indiquent que les possibilités sont limitées à cet égard. Dans la plupart des expériences, on a constaté que la sorption aux particules du sol était d'autant plus importante que la teneur en matières organiques était élevée et le pH faible. Il en résulte que lorsque les conditions ne favorisent pas une décomposition biotique ou abiotique, la PCA peut passer du sol dans les eaux souterraines par lessivage, lorsque le sol est pauvre en matières organiques et que son pH est élevé. Les données expérimentales dont on dispose au sujet de la bioconcentration du composé ainsi que les valeurs mesurées de son coefficient de partage entre le n-octanol et l'eau, indiquent que la PCA n'a pas tendance à s'accumuler dans les organismes aquatiques.
La PCA est rapidement résorbée et métabolisée. Les principales voies métaboliques de ce composé sont les suivantes : a) une C-hydroxylation en position ortho conduisant au 2-amino-5-chlorophénol, suivie d'une sulfoconjugaison en sulfate de 2-amino-5-chlorophényle, lequel est excrété tel quel ou après N-acétylation en sulfate de N-acétyl-2-amino-5-chlorophényle ; b) une N-acétylation en 4-chloroacétanilide (que l'on retrouve principalement dans le sang ) , qui est transformé à son tour en 4-chloroglycolanilide, puis en acide 4-chlorooxanilique (que l'on retrouve dans l'urine ) ; c) une N-oxydation en 4-chlorophénylhydroxylamine puis en 4-chloronitrosobenzène (présent dans les érythrocytes).
Les métabolites réactifs de la PCA forment des liaisons covalentes avec l'hémoglobine et les protéines du foie et du rein. Chez l'Homme , on peut peut déjà mettre en évidence des adduits à l'hémoglobine 30 minutes après une exposition accidentelle , leur concentration étant maximale au bout de 3 h. Les sujets qui sont des acétylateurs lents ont davantage tendance à former des adduits à l'hémoglobine que les acétylateurs rapides.
Chez l'Homme et l'animal , l'excrétion est essentiellement urinaire , la PCA et ses conjugués apparaissant déjà 30 minutes après l'exposition. L'excrétion se produit au cours des 24 premières heures et elle est presque complète au bout de 72 h.
En ce qui concerne la DL50 par voie orale , on indique des valeurs de 300 à 420 mg/kg de poids corporel pour le rat, de 228 à 500 mg/kg p.c. pour la souris et de 350 mg/kg p.c. pour le cobaye. Des valeurs du même ordre ont été obtenues pour la voie intrapéritonéale et cutanée chez le rat, le lapin et le chat. On a trouvé une CL50 de 2340mg/m3 chez le rat. L'effet toxique principal consiste dans la formation de méthémoglobine. A cet égard, la PCA est plus active et plus rapide que l'aniline. La PCA est également capable de produire des effets néphrotoxiques et hépatotoxiques.
Chez le lapin, la PCA ne provoque pas d'irritation cutanée , mais seulement une légère irritation de la muqueuse oculaire. On a mis en évidence un faible pouvoir sensibilisateur dans plusieurs systèmes d'épreuve.
Une exposition répétée à la PCA entraîne une cyanose et une méthémoglobinémie, suivies d'effets sanguins, hépatiques, spléniques et rénaux qui se manifestent par des anomalies hématologiques, une splénomégalie ainsi que par une hémosidérose modérée à forte au niveau de la rate, du foie et du rein, partiellement accompagnée d'une hémopoïèse extramédullaire. Ces effets sont consécutifs à une hémolyse excessive provoquée par ce composé et correspondent à une anémie régénérative. La valeur de la dose la plus faible provoquant un effet nocif observable (LOAEL ; il n'est pas possible d'obtenir une valeur pour la NOEL ou dose sans effet observable) , à savoir une augmentation sensible de la méthémoglobinémie chez le rat et la souris, est respectivement égale à 5 et 7,5 mg/kg p.c. par jour pour une durée d'administration de 13 semaines par gavage (à raison de 5 jours par semaines). Elle a été trouvée égale à 2 mg/kg p.c. par jour pour des rats qui avaient reçu de la PCA , également par gavage, à raison de 5 jours par semaine pendant des durées de 26 , 52 , 78 et 103 semaines. On a observé une fibrose de la rate chez les rats mâles pour une LOAEL de 2 mg/kg p.c.par jour et une hyperplasie de la moelle osseuse chez les femelles pour une LOAEL de 6 mg/kg p.c. par jour (administration par gavage sur une période de 103 semaines).
La PCA est cancérogène pour le rat mâle, chez lequel elle provoque la formation de tumeurs spléniques inhabituelles et rares (fibrosarcomes et ostéosarcomes) qui sont caractéristiques de l'aniline et des substances apparentées. Chez la ratte, on constate une augmentation de la fréquence des stades précancéreux des tumeurs spléniques. On pense également que l'augmentation de l'incidence des phéochromocytomes de la surrénale pourrait être due à l'administration de PCA. On a également relevé des signes de cancérogénicité chez la souris mâle , se traduisant par des tumeurs hépatocellulaires et des hémangiosarcomes.
Les tests de transformation cellulaire effectués sur PCA se révèlent positifs. Selon diverses épreuves de génotoxicité in vitro (test de mutagénicité sur salmonelles, test du lymphome de souris, test des aberrations chromosomiques , induction d'échanges entre chromatides soeurs ) , la PCA pourrait être génotoxique , mais les résultats obtenus sont parfois contradictoires. En raison du manque de données, il est impossible de se prononcer sur la génotoxicité in vivo de la PCA.
On ne dispose d'aucune étude relative à la toxicité de la PCA pour la fonction reproductrice (toxicité génésique).
Les données concernant l'exposition professionnelle humaine à la PCA sont pour l'essentiel tirées de quelques rapports médicaux anciens portant sur des cas graves d'intoxication consécutifs à une exposition accidentelle au composé pendant la production. Les symptômes observés sont notamment les suivants : augmentation de la méthémoglobinémie et de la sulfhémoglobinémie , cyanose, apparition d'une anémie et d'anomalies d'origine anoxique. La PCA a fortement tendance à former des adduits avec l'hémoglobine dont la recherche et le dosage peuvent être utiles pour la surveillance biologique des travailleurs exposés à la 4-chloraniline sur le lieu de travail.
On a fait état dans deux pays de cas de méthémoglobinémie grave chez des nouveau-nés provenant d'unités de soins néonatals intensifs où des prématurés s'étaient trouvés exposés à de la PCA issue de la décomposition de la chlorhexidine ; la chlorhexidine, ajoutée par inadvertance au liquide utilisé pour l'humidification, s'était en effet décomposée en parachloraniline sous l'effet de la chaleur produite par un incubateur d'un type nouveau. Selon un rapport, trois nouveau-nés (14,5-43,5 % de méthémoglobine ) et 33 sur 415 selon un autre rapport (6,5-45,5 % de méthémoglobine au cours de la période de dépistage de 8 mois ) se sont révélés porteurs de méthémoglobine. Une étude clinique prospective a montré que l'immaturité, une maladie grave , la durée d'exposition à la PCA et une faible concentration en NADH ont probablement contribué à cette pathologie.
Selon les résultats considérés comme valables des tests de toxicité effectués sur divers organismes aquatiques, on peut classer la PCA comme modérément à fortement toxique pour les biotes du compartiment aquatique. La concentration la plus faible sans effet observable (NOEC) qui ressort des études de longue durée sur des organismes dulçaquicoles (Daphnia magna, NOEC à 21 jours 0,01 mg/litre ) est dix fois plus forte que les valeurs maximales mesurées dans le Rhin et ses affluents au cours des années 1980 et 1990. On ne peut donc totalement exclure un risque pour les organismes aquatiques , notamment les espèces benthiques , en particulier dans les eaux où la présence d'une quantité importante de matières particulaires empêche une photominéralisation rapide. Toutefois la seule espèce benthique qui ait été étudié à cet égard ne présente pas de sensibilité particulière au composé (CE50 à 48 h , 43 mg/litre) et l'expérimentation sur la daphnie montre que la toxicité est sensiblement réduite lorsque la concentration en matières humiques dissoutes augmente dans le milieu, peut-être du fait que l'adsorption de la PCA sur ces matières en réduit la biodisponibilité. De plus, la bioaccumulation du composé par les espèces aquatiques semble très faible. Dans ces conditions, on peut conclure sur la base des données disponibles que la PCA ne représente pas un risque important pour les organismes aquatiques.
Selon les données relatives aux microorganismes et aux végétaux, la PCA n'est que modérément toxique pour le milieu terrestre. Il est existe une marge de sécurité correspondant à un facteur 1000 entre les effets toxiques signalés et les concentrations relevées dans le sol.
L'évaluation de l'exposition des consommateurs à la PCA par les diverses voies envisageables conduit à une exposition totale journalière ne dépassant pas 300 ng/kg de poids corporel, en supposant que la pénétration par les vêtements n'est que de 1 %. En ne prenant en compte que les effets non néoplasiques (c'est-à-dire la méthémoglobinémie), cette exposition humaine possible est inférieure d'un ordre de grandeur à la dose journalière tolérable calculée qui est égale à 2 μg /kg de poids corporel. Une exposition aiguë à une forte concentration de PCA peut être mortelle.
Les autres effets que l'on peut redouter sont la cancérogénicité et le pouvoir de sensibilisation cutanée de ce composé.
Les résidus de PCA qui subsistent dans les produits de consommation doivent encore être réduits, voire totalement éliminés.
El presente CICAD sobre la 4-cloroanilina (p-cloroanilina) fue preparado por el Instituto Fraunhofer de Toxicología y de Investigación sobre los Aerosoles, de Hannover (Alemania). Se basa en informes compilados por el Comité Consultivo Alemán sobre las Sustancias Químicas Importantes para el Medio Ambiente (BUA, 1993a), la Comisión Alemana MAK (MAK, 1992), la Unión Alemana de la Industria Química (BG Chemie, 1994) y el Programa Nacional de Toxicología de los Estados Unidos (NTP, 1989). En marzo de 2001 se realizó una búsqueda bibliográfica amplia de bases de datos pertinentes para localizar cualquier referencia de interés publicada después de las incorporadas a estos informes. La información relativa a la preparación y el examen colegiado de los documentos originales figura en el Apéndice 1. La información sobre el examen colegiado de este CICAD se presenta en el Apéndice 2. Este CICAD se aprobó en una reunión de la Junta de Evaluación Final celebrada en Ottawa (Canadá) del 29 de octubre al 1ş de noviembre de 2001. La lista de participantes en la Junta de Evaluación Final figura en el Apéndice 3. También se reproduce en este documento la Ficha internacional de seguridad química (ICSC 0026) para la 4-cloroanilina, preparada por el Programa Internacional de Seguridad de las Sustancias Químicas (IPCS, 2002).
Las anilinas cloradas en las posiciones 2-, 3- y 4- (orto-, meta- y para-) tienen los mismos tipos de aplicaciones. Todos los isómeros de la cloroanilina son hematotóxicos y muestran la misma pauta de toxicidad en ratas y ratones, pero en todos los casos cabe atribuir a la 4-cloroanilina los efectos más graves. La 4-cloroanilina es genotóxica en diversos sistemas (véase infra), mientras que los resultados para la 2- y la 3-cloroanilina no son uniformes e indican efectos genotóxicos débiles o nulos. Por consiguiente, este CICAD se concentra sólo en la 4-cloroanilina como la sustancia más tóxica de las anilinas cloradas.
La 4-cloroanilina (denominada en lo sucesivo PCA) (CAS Nş
La PCA se utiliza como intermediario en la fabricación de diversos productos, entre ellos sustancias químicas para la agricultura, colorantes y pigmentos azoicos, cosméticos y productos farmacéuticos. Así pues, se pueden producir emisiones de PCA a la atmósfera a partir de varias fuentes industriales (por ejemplo, la industria de producción, elaboración, teñido/impresión).
A partir de los tipos de aplicaciones se puede predecir que el principal compartimento destinatario de la sustancia química en el medio ambiente es la hidrosfera. Las concentraciones medidas, por ejemplo, en el Rin y sus afluentes son de alrededor de 0,1 y 1 µg/l. En la hidrosfera, la PCA se degrada con rapidez bajo la influencia de la luz (semividas medidas de 2 a 7 h). Se ha calculado una semivida de la sustancia química en el aire para la reacción con los radicales hidroxilo de 3,9 h. Numerosos estudios sobre la biodegradación de la PCA indican que se trata de una sustancia fundamentalmente biodegradable en el agua en condiciones aerobias, mientras que no se ha detectado una mineralización significativa en condiciones anaerobias.
Los coeficientes de sorción en diversos tipos de suelos, determinados de acuerdo con la isoterma de sorción de Freundlich, indican sólo un bajo potencial de sorción en el suelo. En numerosos experimentos, la sorción en el suelo aumentó con el incremento de la materia orgánica y la disminución de los valores del pH. Por consiguiente, en condiciones desfavorables para la degradación abiótica y biótica se puede producir lixiviación de la PCA desde el suelo hacia el agua freática, particularmente en suelos con un bajo contenido de materia orgánica y niveles elevados de pH. Los datos experimentales disponibles sobre bioconcentración, así como los coeficientes de reparto n-octanol/agua medidos, indican que la PCA no tiene potencial de bioacumulación en los organismos acuáticos.
La PCA se absorbe y metaboliza con rapidez. Sus principales vías metabólicas son las siguientes: a) C-hidroxilación en la posición orto para formar 2-amino-5-clorofenol, seguida de una conjugación con sulfato para producir sulfato de 2-amino-5-clorofenilo, que se excreta per se o tras una N-acetilación para dar sulfato de N-acetil- 2-amino-5-clorofenilo; b) N-acetilación para formar 4-cloroacetanilida (detectada principalmente en la sangre), que a continuación se transforma en 4-cloroglicolanilida y luego en ácido 4-clorooxanílico (encontrado en la orina); o c) N-oxidación para formar 4-clorofenilhidroxilamina y luego 4-cloronitrosobenceno (en los eritrocitos).
Los metabolitos reactivos de la PCA se unen por enlace covalente a la hemoglobina y a las proteínas del hígado y el riñón. En las personas son detectables aductos de hemoglobina ya a los 30 minutos de una exposición accidental, con un nivel máximo a las tres horas. La acetilación lenta tiene un mayor potencial de formación de aductos de hemoglobina, en comparación con los acetiladores rápidos.
La excreción en los animales o las personas se produce fundamentalmente en la orina, apareciendo ya PCA y sus conjugados a los 30 minutos de la exposición. La excreción se produce fundamentalmente durante las primeras 24 horas y es casi completa a las 72 horas.
Se han notificado valores de la DL50 por vía oral de 300-420 mg/kg de peso corporal para las ratas, 228-500 mg/kg de peso corporal para los ratones y 350 mg/kg de peso corporal para los cobayas. Se han obtenido valores semejantes para la administración intraperitoneal y cutánea de PCA a ratas, conejos y gatos. Se informó de un valor de la DL50 para ratas de 2340 mg/m3. El efecto tóxico principal es la formación de metahemoglobina. La PCA es un inductor más potente y rápido de metahemoglobina que la anilina. También muestra un potencial nefrotóxico y hepatotóxico.
Se ha comprobado que la PCA no es un irritante de la piel en el conejo, pero sí de los ojos en grado leve. Se ha demostrado un débil potencial de sensibilización mediante varios sistemas de pruebas.
La exposición repetida a la PCA produce cianosis y metahemoglobinemia, seguidas de efectos en la sangre, el hígado, el bazo y el riñón, que se manifiestan como cambios en los parámetros hematológicos, esplenomegalia y hemosiderosis de moderada a grave en el bazo, el hígado y el riñón, parcialmente acompañada de hematopoyesis extramedular. Estos efectos se producen tras una hemólisis excesiva inducida por el compuesto y son coherentes con una anemia regenerativa. Las concentraciones más bajas con efectos adversos observados o LOAEL (no son derivables las concentraciones sin efectos observados o NOEL) para un aumento significativo de la concentración de metahemoglobina en ratas y ratones son, respectivamente, de 5 y 7,5 mg/kg de peso corporal al día con una administración de PCA mediante sonda durante 13 semanas (5 días/semana) y de 2 mg/kg de peso corporal al día para las ratas que recibieron PCA por sonda (5 días/semana) con una exposición de 26, 52, 78 y 103 semanas. Se observaron cambios fibróticos del bazo en ratas macho, con una LOAEL de 2 mg/kg de peso corporal al día, e hiperplasia de la médula ósea en ratas hembra, con una LOAEL de 6 mg/kg de peso corporal al día (por sonda durante 103 semanas).
La PCA tiene actividad carcinogénica en ratas macho, con la inducción de tumores de bazo insólitos y raros (fibrosarcomas y osteosarcomas), que son típicos de la anilina y sustancias afines. En ratas hembra aumenta la frecuencia de los estados precancerosos de los tumores de bazo. Podría haber una relación entre la mayor incidencia de feocromocitoma de la glándula adrenal en ratas macho y hembra y la administración de PCA. Se encontraron algunas pruebas de carcinogenicidad en ratas macho, en forma de tumores hepatocelulares y hemangiosarcoma.
La PCA muestra actividad en valoraciones de transformación celular. Una serie de pruebas de genotoxicidad in vitro (por ejemplo, prueba de mutagenicidad de Salmonella, valoración del linfoma del ratón, prueba de aberración cromosómica, inducción del intercambio de cromátidas hermanas) indican que la PCA es posiblemente genotóxica, aunque los resultados son a veces contradictorios. Debido a la falta de datos, no es posible sacar conclusiones acerca de la genotoxicidad de la PCA in vivo.
No se dispone de estudios sobre toxicidad reproductiva.
Los datos sobre la exposición ocupacional de las personas a la PCA proceden fundamentalmente de un pequeño número de informes más antiguos de intoxicaciones graves tras la exposición accidental a esta sustancia durante la producción. Entre los síntomas cabe mencionar niveles más elevados de metahemoglobina y sulfohemoglobina, cianosis, aparición de anemia y cambios debidos a la anoxia. La PCA tiene una fuerte tendencia a formar aductos de hemoglobina y su determinación se puede utilizar en la biovigilancia de los empleados expuestos a la 4-cloroanilina en el lugar de trabajo.
Hay informes de metahemoglobinemia en neonatos, procedentes de unidades de cuidados intensivos neonatales de dos países donde los niños prematuros estuvieron expuestos a la PCA como producto de la descomposición de clorohexidina; ésta, que se había utilizado inadvertidamente en el fluido de humidificación, se descompuso para formar PCA por la acción del calor en un nuevo tipo de incubadora. En un informe se señalaron tres casos positivos a la metahemoglobina (14,5-43,5% de metahemoglobina) y en el otro lo fueron 33 de 415 neonatos (6,5-45,5% de metahemoglobina durante el período de detección sistemática de ocho meses). En un estudio clínico prospectivo se puso de manifiesto que probablemente contribuían a ello la inmadurez, una enfermedad grave, el tiempo de exposición a la PCA y concentraciones bajas de NADH reductasa.
Basándose en los resultados de pruebas válidas disponibles sobre la toxicidad de la PCA para diversos organismos acuáticos, esta sustancia se puede clasificar como entre moderada y muy tóxica en el compartimento acuático. La concentración más baja sin efectos adversos observados (NOEC) obtenida en estudios prolongados con organismos de agua dulce (Daphnia magna, NOEC de 0,01 mg/l en 21 días) fue 10 veces superior a las concentraciones máximas determinadas en el Rin y sus afluentes durante los años ochenta y noventa. Por consiguiente, no se puede excluir por completo un posible riesgo para los organismos acuáticos, en particular las especies bentónicas, sobre todo en aguas donde hay cantidades significativas de materia particulada que inhiben la fotomineralización rápida. Sin embargo, no se observó una sensibilidad significativa en la única especie bentónica sometida a prueba (CE50 de 43 mg/l a las 48 h); los experimentos con Daphnia magna pusieron de manifiesto una toxicidad muy reducida con concentraciones crecientes de materiales húmicos disueltos en el medio, posiblemente debido a la reducida biodisponibilidad de la PCA a partir de la adsorción a los materiales húmicos disueltos. Además, se informó de que la bioacumulación en las especies acuáticas era muy baja. Por consiguiente, de los datos disponibles no cabe prever un riesgo importante asociado con la exposición de los organismos acuáticos a la PCA.
Los datos disponibles sobre microorganismos y plantas indican sólo un potencial de toxicidad moderado de la PCA en el medio terrestre. Hay un margen de seguridad de 1000 veces entre los efectos notificados y las concentraciones detectadas en el suelo.
La evaluación de la exposición del consumidor a la PCA por una serie de posibles conductos da como resultado una exposición total de un máximo de 300 ng/kg de peso corporal al día, suponiendo la penetración a través de la ropa de sólo un 1%. Considerando sólo los efectos no neoplásicos (es decir, metahemoglobinemia), esta posible exposición de las personas está dentro de un orden de magnitud de la ingesta diaria tolerable calculada de 2 µg/kg de peso corporal al día. La exposición accidental aguda a concentraciones altas de PCA puede ser mortal.
Otros efectos que despiertan preocupación son la carcinogenicidad y la posible sensibilización cutánea.
Se deberían reducir o eliminar completamente las concentraciones residuales de la PCA en los productos de consumo.
ENDNOTES
|
International Programme on Chemical Safety (1994) Assessing human health risks of chemicals: derivation of guidance values for health-based exposure limits. Geneva, World Health Organization (Environmental Health Criteria 170) (also available at http://www.who.int/pcs/). |
|
|
In keeping with WHO policy, which is to provide measurements in SI units, all concentrations of gaseous chemicals in air will be given in SI units in the CICAD series. Where the original study or source document has provided concentrations in SI units, these will be cited here. Where the original study or source document has provided concentrations in volumetric units, conversions will be done using the conversion factors given here, assuming a temperature of 20 °C and a pressure of 101.3 kPa. Conversions are to no more than two significant digits. |
|
|
Unpublished data, Degussa-Hüls AG, Hanau, Germany, 2001. |
|
|
Unpublished data, Bayer AG, Leverkusen, Germany, 2001. |
|
|
Unpublished data, Degussa-Hüls AG, Hanau, Germany, 2001. |
|
|
Unpublished data, Bayer AG, Leverkusen, Germany, 2001. |
|
|
NTP, unpublished report; personal communication to Final Review Board Meeting, 2001. |
See Also:
Toxicological Abbreviations