
This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organization, or the World Health Organization.
Concise International Chemical Assessment Document 44
First draft prepared by Mr P.D. Howe and Dr S. Dobson, Centre for Ecology and Hydrology, Monks Wood, United Kingdom
Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organization, and the World Health Organization, and produced within the framework of the Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 2002
The International Programme on Chemical Safety (IPCS), established in 1980, is a joint venture of the United Nations Environment Programme (UNEP), the International Labour Organization (ILO), and the World Health Organization (WHO). The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals.
The Inter-Organization Programme for the Sound Management of Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and Agriculture Organization of the United Nations, WHO, the United Nations Industrial Development Organization, the United Nations Institute for Training and Research, and the Organisation for Economic Co-operation and Development (Participating Organizations), following recommendations made by the 1992 UN Conference on Environment and Development to strengthen cooperation and increase coordination in the field of chemical safety. The purpose of the IOMC is to promote coordination of the policies and activities pursued by the Participating Organizations, jointly or separately, to achieve the sound management of chemicals in relation to human health and the environment.
WHO Library Cataloguing-in-Publication Data
Silver and silver compounds: environmental aspects.
(Concise international chemical assessment document ; 44)
1. Silver - adverse effects 2. Water pollutants, Chemical
3. Risk assessment 4. Environmental exposure I. International Programme on Chemical Safety II.Series
ISBN 92 4 153044 8 (NLM Classification: QV 297)
ISSN 1020-6167
The World Health Organization welcomes requests for permission to reproduce or translate its publications, in part or in full. Applications and enquiries should be addressed to the Office of Publications, World Health Organization, Geneva, Switzerland, which will be glad to provide the latest information on any changes made to the text, plans for new editions, and reprints and translations already available.
©World Health Organization 2002
Publications of the World Health Organization enjoy copyright protection in accordance with the provisions of Protocol 2 of the Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the World Health Organization concerning the legal status of any country, territory, city, or area or of its authorities, or concerning the delimitation of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters.
The Federal Ministry for the Environment, Nature Conservation and Nuclear Safety, Germany, provided financial support for the printing of this publication.
|
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION |
Concise International Chemical Assessment Documents (CICADs) are the latest in a family of publications from the International Programme on Chemical Safety (IPCS) — a cooperative programme of the World Health Organization (WHO), the International Labour Organization (ILO), and the United Nations Environment Programme (UNEP). CICADs join the Environmental Health Criteria documents (EHCs) as authoritative documents on the risk assessment of chemicals.
International Chemical Safety Cards on the relevant chemical(s) are attached at the end of the CICAD, to provide the reader with concise information on the protection of human health and on emergency action. They are produced in a separate peer-reviewed procedure at IPCS.
CICADs are concise documents that provide summaries of the relevant scientific information concerning the potential effects of chemicals upon human health and/or the environment. They are based on selected national or regional evaluation documents or on existing EHCs. Before acceptance for publication as CICADs by IPCS, these documents undergo extensive peer review by internationally selected experts to ensure their completeness, accuracy in the way in which the original data are represented, and the validity of the conclusions drawn.
The primary objective of CICADs is characterization of hazard and dose–response from exposure to a chemical. CICADs are not a summary of all available data on a particular chemical; rather, they include only that information considered critical for characterization of the risk posed by the chemical. The critical studies are, however, presented in sufficient detail to support the conclusions drawn. For additional information, the reader should consult the identified source documents upon which the CICAD has been based.
Risks to human health and the environment will vary considerably depending upon the type and extent of exposure. Responsible authorities are strongly encouraged to characterize risk on the basis of locally measured or predicted exposure scenarios. To assist the reader, examples of exposure estimation and risk characterization are provided in CICADs, whenever possible. These examples cannot be considered as representing all possible exposure situations, but are provided as guidance only. The reader is referred to EHC 170.1
While every effort is made to ensure that CICADs represent the current status of knowledge, new information is being developed constantly. Unless otherwise stated, CICADs are based on a search of the scientific literature to the date shown in the executive summary. In the event that a reader becomes aware of new information that would change the conclusions drawn in a CICAD, the reader is requested to contact IPCS to inform it of the new information.
Procedures
The flow chart on page 2 shows the procedures followed to produce a CICAD. These procedures are designed to take advantage of the expertise that exists around the world — expertise that is required to produce the high-quality evaluations of toxicological, exposure, and other data that are necessary for assessing risks to human health and/or the environment. The IPCS Risk Assessment Steering Group advises the Coordinator, IPCS, on the selection of chemicals for an IPCS risk assessment based on the following criteria:
Thus, a priority chemical typically
The Steering Group will also advise IPCS on the appropriate form of the document (i.e., EHC or CICAD) and which institution bears the responsibility of the document production, as well as on the type and extent of the international peer review.
The first draft is based on an existing national, regional, or international review. Authors of the first draft are usually, but not necessarily, from the institution that developed the original review. A standard outline has been developed to encourage consistency in form. The first draft undergoes primary review by IPCS to ensure that it meets the specified criteria for CICADs.
The second stage involves international peer review by scientists known for their particular expertise and by scientists selected from an international roster compiled by IPCS through recommendations from IPCS national Contact Points and from IPCS Participating Institutions. Adequate time is allowed for the selected experts to undertake a thorough review. Authors are required to take reviewers’ comments into account and revise their draft, if necessary. The resulting second draft is submitted to a Final Review Board together with the reviewers’ comments. At any stage in the international review process, a consultative group may be necessary to address specific areas of the science.

|
Advice from Risk Assessment Steering Group Criteria of priority:
Thus, it is typical of a priority chemical that
Special emphasis is placed on avoiding duplication of effort by WHO and other international organizations. A prerequisite of the production of a CICAD is the availability of a recent high-quality national/regional risk assessment document = source document. The source document and the CICAD may be produced in parallel. If the source document does not contain an environmental section, this may be produced de novo, provided it is not controversial. If no source document is available, IPCS may produce a de novo risk assessment document if the cost is justified. Depending on the complexity and extent of controversy of the issues involved, the steering group may advise on different levels of peer review:
|
The CICAD Final Review Board has several important functions:
Board members serve in their personal capacity, not as representatives of any organization, government, or industry. They are selected because of their expertise in human and environmental toxicology or because of their experience in the regulation of chemicals. Boards are chosen according to the range of expertise required for a meeting and the need for balanced geographic representation.
Board members, authors, reviewers, consultants, and advisers who participate in the preparation of a CICAD are required to declare any real or potential conflict of interest in relation to the subjects under discussion at any stage of the process. Representatives of nongovernmental organizations may be invited to observe the proceedings of the Final Review Board. Observers may participate in Board discussions only at the invitation of the Chairperson, and they may not participate in the final decision-making process.
This CICAD on silver and silver compounds (environmental aspects) was prepared by the Centre for Ecology and Hydrology, Monks Wood, United Kingdom. It is based on the US Department of the Interior’s Contaminant Hazard Reviews report entitled Silver hazards to fish, wildlife, and invertebrates: A synoptic review (Eisler, 1997), updated with reference to Eisler (2000) and supplemented by a literature search (April 2001). This document does not cover human health effects of silver; a review of human health aspects can be found in ATSDR (1990). Information on the nature of the peer review and availability of the source document is presented in Appendix 1. Information on the peer review of this CICAD is presented in Appendix 2. This CICAD was approved as an international assessment at a meeting of the Final Review Board, held in Ottawa, Canada, on 29 October – 1 November 2001. Participants at the Final Review Board meeting are listed in Appendix 3. The International Chemical Safety Cards for silver (ICSC 0810) and silver nitrate (ICSC 1116), produced by the International Programme on Chemical Safety (IPCS, 1999a, 1999b), have also been reproduced in this document.
Silver is a rare but naturally occurring metal, often found deposited as a mineral ore in association with other elements. Emissions from smelting operations, manufacture and disposal of certain photographic and electrical supplies, coal combustion, and cloud seeding are some of the anthropogenic sources of silver in the biosphere. The global biogeochemical movements of silver are characterized by releases to the atmosphere, water, and land by natural and anthropogenic sources, long-range transport of fine particles in the atmosphere, wet and dry deposition, and sorption to soils and sediments.
The most recent measurements of silver in rivers, lakes, and estuaries using clean techniques show levels of about 0.01 µg/litre for pristine, unpolluted areas and 0.01–0.1 µg/litre in urban and industrialized areas. Silver concentrations reported prior to the implementation of ultra-clean metal sampling, which began in the late 1980s, should be treated with caution. Maximum concentrations of total silver recorded during the 1970s and 1980s in selected non-biological materials were 36.5 ng/m3 in air near a smelter; 2.0 µg/m3 in atmospheric dust; 0.1 µg/litre in oil well brines; 4.5 µg/litre in precipitation from clouds seeded with silver iodide; 6.0 µg/litre in groundwater near a hazardous waste site; 8.9 µg/litre in seawater from Galveston Bay, USA; 260 µg/litre near photographic manufacturing waste discharges; 300 µg/litre in steam wells; 300 µg/litre in treated photoprocessing wastewaters; 31 mg/kg in soils; 43 mg/litre in water from certain hot springs; 50 mg/kg in granite; as much as 100 mg/kg in crude oils; and 150 mg/kg in river sediments. It should be noted that levels of silver in the environment have declined; for example, in the Lower Genesee River, USA, near a photographic manufacturing plant, levels declined from 260 µg/litre in the 1970s to below the detection limit (<10 µg/litre) in the 1990s. It should also be noted that only a small portion of the total silver in each of the environmental compartments is biologically available.
The ability to accumulate dissolved silver varies widely between species. Some reported bioconcentration factors for marine organisms (calculated as milligrams of silver per kilogram fresh weight organism divided by milligrams of silver per litre of medium) are 210 in diatoms, 240 in brown algae, 330 in mussels, 2300 in scallops, and 18 700 in oysters, whereas bioconcentration factors for freshwater organisms have been reported to range from negligible in bluegills (Lepomis macrochirus) to 60 in daphnids; these values represent uptake of bioavailable silver in laboratory experiments. Laboratory studies with the less toxic silver compounds, such as silver sulfide and silver chloride, reveal that accumulation of silver does not necessarily lead to adverse effects. At concentrations normally encountered in the environment, food-chain biomagnification of silver in aquatic systems is unlikely. Elevated silver concentrations in biota occur in the vicinities of sewage outfalls, electroplating plants, mine waste sites, and silver iodide-seeded areas. Maximum concentrations recorded in field collections, in milligrams total silver per kilogram dry weight (tissue), were 1.5 in marine mammals (liver) (except Alaskan beluga whales Delphinapterus leucas, which had concentrations 2 orders of magnitude higher than those of other marine mammals), 6 in fish (bone), 14 in plants (whole), 30 in annelid worms (whole), 44 in birds (liver), 110 in mushrooms (whole), 185 in bivalve molluscs (soft parts), and 320 in gastropods (whole).
In general, silver ion was less toxic to freshwater aquatic organisms under conditions of low dissolved silver ion concentration and increasing water pH, hardness, sulfides, and dissolved and particulate organic loadings; under static test conditions, compared with flow-through regimens; and when animals were adequately nourished instead of being starved. Silver ions are very toxic to microorganisms. However, there is generally no strong inhibitory effect on microbial activity in sewage treatment plants because of reduced bioavailability due to rapid complexation and adsorption. Free silver ion was lethal to representative species of sensitive aquatic plants, invertebrates, and teleosts at nominal water concentrations of 1–5 µg/litre. Adverse effects occur on development of trout at concentrations as low as 0.17 µg/litre and on phytoplankton species composition and succession at 0.3–0.6 µg/litre.
A knowledge of the speciation of silver and its consequent bioavailability is crucial to understanding the potential risk of the metal. Measurement of free ionic silver is the only direct method that can be used to assess the likely effects of the metal on organisms. Speciation models can be used to assess the likely proportion of the total silver measured that is bioavailable to organisms. Unlike some other metals, background freshwater concentrations in pristine and most urban areas are well below concentrations causing toxic effects. Levels in most industrialized areas border on the effect concentration, assuming that conditions favour bioavailability. On the basis of available toxicity test results, it is unlikely that bioavailable free silver ions would ever be at sufficiently high concentrations to cause toxicity in marine environments.
In general, accumulation of silver by terrestrial plants from soils is low, even if the soil is amended with silver-containing sewage sludge or the plants are grown on tailings from silver mines, where silver accumulates mainly in the root systems. No data were found on effects of silver on wild birds or mammals. Silver was harmful to poultry (tested as silver nitrate) at concentrations as low as 100 mg total silver/litre in drinking-water or 200 mg total silver/kg in diets. Sensitive laboratory mammals were adversely affected at total silver concentrations (added as silver nitrate) as low as 250 µg/litre in drinking-water (brain histopathology), 6 mg/kg in diet (high accumulations in kidneys and liver), or 13.9 mg/kg body weight (lethality).
Silver is a white, ductile metal occurring naturally in the pure form and in ores. Some silver compounds are extremely photosensitive and are stable in air and water, except for tarnishing readily when exposed to sulfur compounds. Metallic silver is insoluble in water, but many silver salts, such as silver nitrate (AgNO3), are soluble. In the natural environment, silver occurs primarily in the form of the sulfide (Ag2S) or is intimately associated with other metal sulfides, especially those of lead, copper, iron, and gold, which are all essentially insoluble (ATSDR, 1990). The colloidal phase, composed of organic and inorganic materials, is the most important component of aqueous silver (Bell & Kramer, 1999). Monovalent silver ion (Ag+) is rare or negligible in the natural environment. Silver readily forms compounds with antimony, arsenic, selenium, and tellurium (Smith & Carson, 1977). Silver has two stable isotopes (107Ag and 109Ag) and 20 radioisotopes; none of the radioisotopes of silver occurs naturally. Several compounds of silver are potential explosion hazards: silver oxalate (Ag2C2O4) decomposes explosively when heated; silver acetylide (Ag2C2) is sensitive to detonation on contact; and silver azide (AgN3) detonates spontaneously under certain circumstances (Smith & Carson, 1977).
Selected physicochemical properties of silver and its salts are summarized in Table 1. Additional properties for silver (ICSC 0810) and silver nitrate (ICSC 1116) are presented in the International Chemical Safety Cards reproduced in this document.
Table 1: Physicochemical properties of silver and some of its salts.
|
Properties |
Silver |
Silver nitrate |
Silver sulfide |
Silver chloride |
|
Alternative names |
Argentum; argentum crede Cl 77820; shell silver; silver atom; silver colloidal; silflake; silpowder; silber |
Lunar caustic fused silver nitrate; moulded silver nitrate argenti; nitras; nitric acid silver (I) salt; nitric acid silver (1+) salt; silver (1+) nitrate |
Acanthite; argentous sulfide |
Silver (I) chloride; silver monochloride |
|
Chemical Abstracts Service number |
|
|
|
|
|
Chemical formula |
Ag |
AgNO3 |
Ag2S |
AgCl |
|
Molecular mass |
107.87 |
169.89 |
247.80 |
143.34 |
|
Physical state |
Solid metal |
Solid crystalline |
Grey-black solid |
White solid |
|
Boiling point |
2212 °C |
Decomposes at 440 °C |
Decomposes at 810 °C |
1550 °C |
|
Solubility in water |
Insoluble |
2160 g/litre |
Insoluble |
1.93 mg/litre |
|
Solubility in organic solvents/acids |
Soluble in nitric acid but not sulfuric acid |
Soluble in ethanol and acetone |
|
|
|
Density |
10.5 g/cm3 |
4.35 g/cm3 |
7.33 g/cm3 |
5.56 g/cm3 |
A variety of spectrographic, colorimetric, polarographic, and other analytical techniques are used for routine measurement of silver in biological and abiotic samples. Silver concentrations reported prior to the implementation of ultra-clean metal sampling, which began in the late 1980s, should be treated with caution.
Atomic absorption and plasma emission spectroscopy are perhaps the most widely used analytical techniques for the determination of silver levels in air, soil, and water. Rains et al. (1984) employed atomic absorption spectroscopy with flame atomization and direct current plasma–atomic emission spectroscopy to determine silver levels in solid waste leachate. Detection limits of silver in leachate samples by the two techniques were 0.473 µg/ml and 0.38 µg/litre, respectively.
Inductively coupled argon plasma with atomic emission spectroscopy has been recommended for determining silver in air and for analysing dissolved, suspended, or total silver in drinking-water, surface water, and domestic and industrial wastewaters. Detection limits were 26 ng/ml in air and 7.0 µg/litre in water (ATSDR, 1990). Inductively coupled plasma–mass spectrometry is used to measure silver in environmental media at a detection limit of 0.4 ng/litre.
Sensitive voltammetric techniques using anodic stripping have been developed to measure free silver ion in solution at concentrations as low as 0.1 µg/litre (Schildkraut, 1993; Song & Osteryoung, 1993; Schildkraut et al., 1998). However, the anodic stripping voltammetric method does not work well with natural samples containing large amounts of organic matter, such as those found in sewage treatment plants (Ownby et al., 1997).
Trace levels of silver (10–6 to 10–9 g/g of sample) can be accurately determined in biological samples by several different analytical techniques. These methods include high-frequency plasma torch–atomic emission spectroscopy, neutron activation analysis, graphite furnace (flameless) atomic absorption spectroscopy, flame atomic absorption spectroscopy, and micro-cup atomic absorption spectroscopy. Atomic absorption spectroscopy equipped with various atomizers is the most prevalent analytical method used. Graphite furnace atomic absorption spectroscopy offers high detectability (subnanogram per gram sample) and requires relatively small samples for analysis of biological tissues (DiVincenzo et al., 1985). Detection limts of 0.02 µg/g, 0.2 µg/g, 0.005 µg/litre, and 0.5 µg/100 ml were achieved for hair, faeces, urine, and blood, respectively. Starkey et al. (1987) modified the graphite furnace atomic absorption spectroscopy technique for determining trace levels of silver in blood samples and reported a detection limit of 15 ng/100 ml.
Analytical techniques to measure trace levels of silver are extremely complex, and, unless the proper sample containers are used, the silver in a solution can be lost to container walls within hours after collection (Wen et al., 1997). Wen et al. (1997) found that ultraviolet irradiation of the sample water consistently achieved 100% recovery. They also found that the ultraviolet irradiation method was required for silver analysis in concentrated colloidal solutions. The presence of organic matter and sulfide clusters has been shown to enhance the loss of silver in natural water.
Silver is a rare but naturally occurring metal, often found deposited as a mineral ore in association with other elements (ATSDR, 1990). Argentite is the main ore from which silver is extracted by cyanide, zinc reduction, or electrolytic processes (Fowler & Nordberg, 1986). Silver is frequently recovered as a by-product from smelting of nickel ores in Canada, from lead-zinc and porphyry copper ores in the USA, and from platinum and gold deposits in South Africa (Smith & Carson, 1977). About 12–14% of the domestic silver output is recovered from lead ores, and about 4% from zinc ores. Secondary sources of silver include new scrap generated in the manufacture of silver-containing products; coin and bullion; and old scrap from electrical products, old film and photoprocessing wastes, batteries, jewellery, silverware, and bearings (Smith & Carson, 1977).
World production of silver increased from 7.4 million kilograms in 1964 to 9.1 million kilograms in 1972 and to 9.7 million kilograms in 1982 (Fowler & Nordberg, 1986). In 1986, 13.06 million kilograms of silver were produced globally; the USA produced 1.1 million kilograms in 1986 but consumed 3.9 million kilograms (ATSDR, 1990). In 1990, the estimated world mine production of silver was 14.6 million kilograms. Major producers were Mexico, with 17% of the total; the USA, with 14%; Peru, with 12%; the former Soviet Union, with 10%; and Canada, with 9% (Eisler, 1997). Ratte (1998) gives a world production figure of 14.9 million kilograms for 1995, whereas the World Silver Survey 2000 states that mine production of silver for 1999 was 15.5 million kilograms (Silver Institute, 2000).
Emissions from smelting operations, manufacture and disposal of certain photographic and electrical supplies, coal combustion, and cloud seeding are some of the anthropogenic sources of silver in the biosphere (Eisler, 1997). Fallout from cloud seeding with silver iodide (AgI) is not always confined to local precipitation; silver residuals have been detected several hundred kilometres downwind of seeding events (Freeman, 1979).
In 1978, the estimated loss of silver to the environment in the USA was 2.5 million kilograms, mostly to terrestrial and aquatic ecosystems; the photographic industry alone accounted for about 47% of all silver discharged into the environment from anthropogenic sources (Smith & Carson, 1977). Purcell & Peters (1998) cited a report by Scow et al. (1981) that stated that the estimated loss of silver to the environment in the USA in 1978 ranged from 2.4 to 2.5 million kilograms. Twenty-nine per cent was released to the aquatic environment, whereas 68% was released to land as solid waste. It was reported that 30% of the total release originated from natural sources and 30% from photographic developing and manufacture. In 1999, the estimated release to the environment in the USA via emissions, discharges, and waste disposal from sites listed in the Toxic Release Inventory were 270 000 kg for silver and 1.7 million kilograms for silver compounds. Releases to land amounted to 90% for silver compounds and 40% for silver, whereas nearly 60% of the silver releases were via off-site waste disposal (TRI, 1999).
Most of the silver lost to the environment enters terrestrial ecosystems, where it is immobilized in the form of minerals, metal, or alloys; agricultural lands may receive as much as 80 000 kg of silver per year from photoprocessing wastes in sewage sludge. An estimated 150 000 kg of silver enter the aquatic environment every year from the photographic industry, mine tailings, and electroplating (Smith & Carson, 1977). The atmosphere receives 300 000 kg of silver each year from a variety of sources.
Silver has been used for ornaments and utensils for almost 5000 years and as a precious metal, a monetary medium, and a basis of wealth for more than 2000 years.
In 1990, about 50% of the refined silver consumed in the USA was used to manufacture photographic and X-ray products; 25% in electrical and electronic products; 10% in electroplated ware, sterlingware, and jewellery; 5% in brazing alloys; and 10% in other uses (Eisler, 1997).
Because of their bacteriostatic properties, silver compounds are used in filters and other equipment to purify swimming pool water and drinking-water and in the processing of foods, drugs, and beverages (ATSDR, 1990).
Silver nitrate has been used for many years as an eyedrop treatment to prevent ophthalmia neonatorum (ATSDR, 1990). Several silver-containing pharmaceuticals have been used topically on skin or mucous membranes to assist in healing burn patients and to combat skin ulcers. Oral medicines containing silver include antismoking lozenges containing silver acetate (AgC2H3O2), breath mints coated with silver, and silver nitrate solutions for treating gum disease (ATSDR, 1990). The widespread medical use of silver compounds for topical application to mucous membranes and for internal use has become nearly obsolete in the past 50 years because of the fear of argyria and the development of sulfonamide and antibiotic microbials (Smith & Carson, 1977).
The global biogeochemical movements of silver are characterized by releases to the atmosphere, water, and land by natural and anthropogenic sources, long-range transport of fine particles in the atmosphere, wet and dry deposition, and sorption to soils and sediments (ATSDR, 1990). The chief source of silver contamination of water is silver thiosulfate complexes in photographic developing solutions that photofinishers discard directly to sewers (Smith & Carson, 1977). Secondary waste treatment converts most of the silver thiosulfate complex to insoluble silver sulfide and forms some metallic silver (Lytle, 1984). About 95% of the total silver is removed in publicly owned treatment works from inputs containing municipal sewage and commercial photoprocessing effluents, and effluents contain less than 0.07 µg ionic silver/litre; concentrations were independent of the influent silver concentration (Lytle, 1984; Shafer et al., 1998). Silver in sewage treatment plant effluents may be associated with suspended particles or be present as thiosulfate complex, colloidal silver complex, colloidal silver chloride (AgCl), silver sulfide, or soluble organic complexes (Smith & Carson, 1977). Silver on suspended matter and in colloidal forms and insoluble salts ultimately settles out in the sediments. At the water treatment plant, most of the silver is precipitated after treatment with lime or adsorbed after treatment with alum flocculant. Chlorination converts some silver to silver chloride or to a soluble silver chloride complex (Smith & Carson, 1977). Aerobic biodegradation of a photoprocessing wastewater containing 1.85 mg total silver/litre did not adversely affect the activated sludge process (Pavlostathis & Maeng, 1998). Practically all silver became associated with the sludge solids at 1840 mg silver/kg mixed liquor suspended solids. When fresh sludge and aerobically digested sludge solids were subjected to leaching procedures, the resulting silver concentration was at least 40 times lower than the regulatory limit of 5 mg/litre (Pavlostathis & Maeng, 1998).
Forms of silver in atmospheric emissions are probably silver sulfide, silver sulfate (Ag2SO4), silver carbonate (Ag2CO3), silver halides, and metallic silver (Smith & Carson, 1977). About 50% of the silver released into the atmosphere from industrial operations is transported more than 100 km and is eventually deposited in precipitation (ATSDR, 1990).
Emissions of silver from coal-fired power plants may lead to accumulations in nearby soils (Fowler & Nordberg, 1986). Silver in soils is largely immobilized by precipitation to insoluble salts and by complexation or adsorption by organic matter, clays, and manganese and iron oxides (Smith & Carson, 1977).
Silver occurs naturally in several oxidation states, usually as Ag0 and Ag+; other possible oxidation states of silver are Ag2+ and Ag3+ (ATSDR, 1990). In this report, "silver ion" refers to Ag+, unless stated otherwise. In surface fresh water, silver may be found as the monovalent ion; in combination with sulfide, bicarbonate, or sulfate; as part of more complex ions with chlorides and sulfates; and adsorbed onto particulate matter (ATSDR, 1990). In the aqueous phase, silver at the lowest concentrations exists either as a simple silver sulfhydrate (AgSH) or as a simple polymer HS-Ag-S-Ag-SH (Bell & Kramer, 1999). At higher concentrations, colloidal silver sulfide or polysulfide complexes are formed. Silver ion binds strongly with sulfide ion in inorganic and organic species, resulting in nanogram per litre aqueous dissolved concentrations (Bell & Kramer, 1999). Trace levels of dissolved silver in the presence of ferric sulfide are rapidly adsorbed, and silver remaining in solution remains as silver sulfide; however, silver thiolate complexes can be the dominant dissolved species in highly contaminated waters near urban centres or in waters with high levels of natural organic matter (Adams & Kramer, 1998). The most important and crucial aspect of silver thiolate chemistry is the rapid exchange of silver ion among thiolates, whereby silver ion can transfer onto, or off, particulate materials or the cells of an organism. Silver thiolates also react rapidly with hydrogen sulfide or HS– as ligands to form silver sulfide, although the reverse process is slow (Bell & Kramer, 1999). The monovalent ion does not hydrolyse appreciably in solution and is considered to be a mild oxidizing agent (Smith & Carson, 1977). Hypervalent silver species, such as Ag2+ and Ag3+, are significantly more effective as oxidizing agents than Ag0 and Ag+ (Kouadio et al., 1990; Sun et al., 1991) but unstable in aqueous environments, especially at water temperatures near 100 °C (Smith & Carson, 1977).
In fresh water and soils, the primary silver compounds under oxidizing conditions are bromides, chlorides, and iodides; under reducing conditions, the free metal and silver sulfide predominate (ATSDR, 1990). In river water, one study showed silver present as the monovalent ion at 53–71% of the total silver, as silver chloride at 28–45%, and as silver chloride ion (AgCl–) at 0.6–2.0% (Whitlow & Rice, 1985). Increasing salinity of brackish and marine waters increases concentrations of silver-chloro complexes (AgCl0, AgCl2–, AgCl32–, AgCl43–); these chloro complexes retain some silver in dissolved form, and relatively small anthropogenic quantities can substantially enrich the environment (Luoma, 1994). In the open ocean, the principal dissolved form of silver is AgCl2–, but the most bioavailable form may be the neutral monochloro complex silver chloride (Bryan & Langston, 1992).
Sorption is the dominant process that controls the partitioning of silver in water and its movement in soils and sediments (US EPA, 1980; ATSDR, 1990). Silver may leach from soils into groundwater, the leaching rate increasing with decreasing pH and increasing drainage (ATSDR, 1990). Silver adsorbs to manganese dioxide, ferric compounds, and clay minerals, and these compounds are involved in silver deposition into sediments; sorption by manganese dioxide and precipitation with halides reduce the concentration of dissolved silver, resulting in higher concentrations in sediments than in the water column (US EPA, 1980). Under reducing conditions, adsorbed silver in sediments may be released and subsequently reduced to metallic silver, or it may combine with reduced sulfur to form the insoluble silver sulfide (US EPA, 1980). In wastewaters, inorganic ligands were found to be important for silver binding. These inorganic ligands probably consist of metal sulfides, which are stable for hours and days in oxic waters. When complexed to these strong-affinity ligands, Ag(I) is protected from photoreduction to zero-valent silver (Adams & Kramer, 1999a). Partitioning of silver into size fractions showed that a significant proportion of silver is in the colloidal (30–35%) and dissolved phases (15–20%). Dissolved-phase concentrations were relatively constant in treatment plant effluent and receiving waters, suggesting that silver is strongly complexed by ligands that are not significantly affected by aggregation or sorption processes (Adams & Kramer, 1999b). However, depending on the conditions, sediments may be a significant source of silver to the water column. In one study, anoxic sediments containing 1–27 g silver/kg dry weight and 10 mmol of acid volatile sulfide/kg dry weight were resuspended in oxygenated seawater for several hours to days. The seawater in contact with sediment containing 10.8 g silver/kg had 20 µg silver/litre; seawater in contact with sediments containing 27 g silver/kg had about 2000 µg silver/litre, which seems to be the solubility of silver in seawater (Eisler, 1997).
The availability of free silver in marine environments is strongly controlled by salinity, because of the affinity of silver for the chloride ion (Sanders et al., 1991). Silver sorbs readily to phytoplankton and to suspended sediments. As salinity increases, the degree of sorption decreases. Nearly 80% of silver sorbed to suspended sediments at low salinities desorbs at higher salinities, but desorption does not occur when silver is associated with phytoplankton. Thus, silver incorporation in or on cellular material increases the retention of silver in the estuary, reducing the rate of transport (Sanders & Abbe, 1987).
In California, USA, anthropogenic sources contributed 50% more silver to sediments of coastal basins than did natural sources, as judged by sedimentary basin fluxes of 0.09 µg/cm2 in anthropogenic sources of silver and 0.06 µg/cm2 in natural sources (Bruland et al., 1974). Silver is tightly bound by sewage sludge, and elevated silver concentrations in sediments are often characteristic of areas near sewage outfalls. In the absence of sewage, silver in oxidized sediments is associated with oxides of iron and with humic substances (Bryan & Langston, 1992).
Riverine transport of silver to the ocean is considerable; suspended materials in the Susquehanna River, Pennsylvania, USA, containing as much as 25 mg silver/kg, result in an estimated transport of 4.5 tonnes of silver to the ocean each year (US EPA, 1980).
Sensitive marine algae accumulated silver from water containing as little as 2 µg silver/litre (as silver nitrate) to whole-cell burdens as high as 58 mg silver/kg dry weight (Sanders & Abbe, 1987). Dissolved silver speciation and bioavailability were important in deter-mining uptake and retention by aquatic plants (Connell et al., 1991). Silver availability was controlled by the concentrations of free silver ion and of other silver complexes, such as silver chloride (Sanders & Abbe, 1989). Silver uptake by phytoplankton was rapid, proportional to silver concentration, and inversely proportional to water salinity. Silver incorporated by phytoplankton was not lost as the salinity increased, and silver associated with cellular material was largely retained in the estuary (Sanders & Abbe, 1989). Diatoms (Thalassiosira sp.), for example, readily accumulated silver from the medium. Once incorporated, silver was tightly bound to the cell membrane, even after the cells were mechanically disrupted (Connell et al., 1991). Generally, silver accumulation is primarily attributed to the bioavailability of the free silver ion; however, Reinfelder & Chang (1999) demonstrated that in euryhaline marine microalgae (Thalassiosira weissflogii), AgCl(aq) is the principal bioavailable species of inorganic silver. In short-term (<1 h) experiments on freshwater green algae (Chlamydomonas reinhardtii) at low free silver ion concentrations (8 nmol/litre), silver uptake increases markedly (up to 4 times) as a function of the chloride ion concentration (5 µmol/litre to 4 mmol/litre) (Fortin & Campbell, 2000).
The ability to accumulate dissolved silver varies widely between species. Some reported bioaccumulation factors (calculated as milligrams of silver per kilogram fresh weight organism divided by milligrams of silver per litre of medium) are 210 in diatoms, 240 in brown algae, 330 in mussels, 2300 in scallops, and 18 700 in oysters (US EPA, 1980). Silver is the most strongly accumulated of all trace metals by marine bivalve molluscs (Luoma, 1994). Studies with 110mAg suggest that the half-time persistence of silver is 27 days in mussels, 44–80 days in clams, and more than 180 days in oysters (Fisher et al., 1994). In oysters and other bivalve molluscs, the major pathway of silver accumulation was from dissolved silver; uptake was negligible from silver adsorbed onto suspended sediments or algal cells, and oysters eliminated adsorbed silver in the faeces (Abbe & Sanders, 1990; Sanders et al., 1990). Benthic bivalve molluscs can accumulate silver from certain sediments. Sediment-bound silver was taken up by the Baltic clam (Macoma balthica) at 3.6–6.1 times the concentration in calcite sediments, but less than 0.85 times from manganous, ferrous, and biogenic calcium carbonate sediments (US EPA, 1980). In oysters, silver associated with food was unavailable for incorporation, which may be due to the ability of silver to adsorb rapidly to cell surfaces and to remain tightly bound despite changes in pH or enzymatic activity (Connell et al., 1991). Silver concentrations in American oysters (Crassostrea virginica) held in seawater solutions containing 1.0 mg silver/litre for 96 h rose from 6.1 to 14.9 mg/kg fresh weight soft parts; in gills, these values were 5.9 and 33.9 mg/kg, respectively (Thurberg et al., 1974). A similar pattern was evident in common mussels (Mytilus edulis) and quahog clams (Mercenaria mercenaria) (Thurberg et al., 1974). Adult surf clams (Spisula solidissima) immersed for 96 h in seawater containing 10 µg silver/litre had 1.0 mg silver/kg fresh weight soft tissues compared with 0.08 mg/kg in controls (Thurberg et al., 1974). Uptake of dissolved silver by oysters was higher at elevated temperatures in the range of 15–25 °C (Abbe & Sanders, 1990). American oysters maintained near a nuclear power plant in Maryland, USA, that discharged radionuclides on a daily basis into Chesapeake Bay accumulated 110mAg; accumulations were higher in summer and autumn than in winter and spring (Rose et al., 1988).
Juvenile Pacific oysters (Crassostrea gigas) exposed for 2 weeks to solutions containing 20 µg silver/litre had high silver accumulations in tissues and a reduced capacity to store glycogen; however, after 30 days of depuration, glycogen storage capacity was restored and 80% of the soluble silver and 27% of the insoluble forms were eliminated, suggesting recovery to a normal physiological state. About 70% of the insoluble silver in Pacific oysters was sequestered as silver sulfide, a stable mineral form that is not degradable, thereby limiting the risk of silver transfer through the food-chain. Most (69–89%) of the silver accumulated in soft tissues of oysters and clams was sequestered in amoebocytes and basement membranes; in scallops and mussels, silver was stored in basement membranes and the pericardial gland. In all species of bivalve mollusc, sequestered silver was in the form of silver sulfide. American oysters excreted about 60% of their accumulated silver in soft tissues within 30 days of transfer to silver-free seawater; soluble forms were preferentially eliminated, and insoluble forms retained (Berthet et al., 1992). Interspecies differences in ability to retain silver among bivalve molluscs are large, even among closely related species of Crassostreid oysters. For example, the half-time persistence of silver was about 149 days in American oysters but only 26 days in Pacific oysters (ATSDR, 1990).
Marine gastropods exposed to concentrations as low as 1 µg silver/litre for as long as 24 months showed accumulations as high as 34 mg silver/kg fresh weight soft parts; higher exposure concentrations of 5 and 10 µg silver/litre were associated with whole-body burdens as high as 87 mg silver/kg fresh weight (Nelson et al., 1983).
Among arthropods, grass shrimp (Palaemonetes pugio) rapidly incorporate silver dissolved in brackish water in proportion to its concentration, but not from planktonic or detrital food sources containing elevated silver burdens (Connell et al., 1991). Variations in the ability of decapod crustaceans to accumulate 110mAg from seawater are large, as judged by bioconcentration factors that ranged from 70 to 4000 (Pouvreau & Amiard, 1974). The reasons for this variability are unknown but may be associated with hepatopancreas morphology (Eisler, 1997). It is generally acknowledged that the hepatopancreas or digestive gland is the major repository of silver in decapods (Greig, 1975; Greig et al., 1977a, 1977b), although silver sequestered there may have less effect than in more sensitive tissues. Aquatic insects concentrate silver in relative proportion to environmental levels (Nehring, 1976) and more efficiently than most fish species (Diamond et al., 1990). Whole-body bioconcentration factors of silver in three species of aquatic insect (calculated as milligrams total silver per kilogram fresh weight tissue divided by milligrams total silver per litre of medium) ranged from 21 to 240 in water containing 30–65 mg calcium carbonate/litre during exposure of 3–15 days; in bluegill (Lepomis macrochirus), this value was less than 1 after exposure for 28 days (US EPA, 1980).
In experiments on rainbow trout (Oncorhynchus mykiss), the uptake of silver has been shown to be via a sodium ion channel situated on the branchial apical membrane of the gills (Bury & Wood, 1999). Uptake of silver by basolateral membrane vesicles was via a carrier-mediated process, which was ATP-dependent, reached equilibrium over time, and followed Michaelis-Menten kinetics. A P-type ATPase present in the basolateral membrane of the gills can actively transport silver (Bury et al., 1999b).
Forsythe et al. (1996) exposed green algae (Selenastrum capricornutum) and daphnids (Daphnia magna) to silver nitrate concentrations of 1 and 0.5 µg silver/litre, respectively, and reported bioconcentration factors of 4.8 and 61. No significant accumulation was found in bluegills exposed to 0.5 µg silver/litre. Coleman & Cearley (1974) found that largemouth bass (Micropterus salmoides) and bluegills accumulated silver; accumulations increased with increasing concentrations of ionic silver and increasing duration of exposure. Bioconcentration factors of 110mAg for various species of teleosts were as high as 40 after 98 days (Pouvreau & Amiard, 1974). However, plaice (Pleuronectes platessa; a marine flounder) and thornback rays (Raja clavata) fed nereid polychaete worms labelled with 110mAg retained about 4.2% of the ingested dose after 3 days (Pentreath, 1977), which suggests that the high silver bioconcentration factors reported by Pouvreau & Amiard (1974) may have been due to loosely bound adsorbed silver. Flounders (Pleuronectes sp.) held for 2 months in seawater solutions containing 40 µg silver/litre had elevated silver concentrations in the gut (0.49 mg silver/kg fresh weight) but less than 0.05 mg/kg in all other examined tissues (Pentreath, 1977). Similarly, exposed rays (Raja sp.) contained 1.5 mg silver/kg fresh weight in liver, 0.6 mg/kg in gut, 0.2 mg/kg in heart, and 0.005–0.18 mg/kg in spleen, kidney, and gill filament (Pentreath, 1977); liver is usually considered the major repository of silver in teleosts (Garnier et al., 1990). After a 21-day exposure to 14.5 µg silver/litre, silver levels increased 2- to 20-fold in most tissues of four marine teleosts and two marine elasmobranchs, with the highest concentrations occurring in the livers of teleosts and the gills of elasmobranchs. In sculpins (Oligocottus maculosus), salinity markedly affected silver accumulation, with tissue-specific levels approximately 6-fold higher at 18‰ than at 30‰. At lower salinities, a neutral AgClaq complex exists in the water, allowing for increased bioaccumulation, whereas at higher salinities, only less bioavailable, negatively charged AgCln1–n complexes (AgCl2–, AgCl32–, AgCl43–) are present (Webb & Wood, 2000).
Hogstrand et al. (1996) found that rainbow trout exposed to silver nitrate, silver thiosulfate, and silver chloride accumulated silver in the liver. Highest silver levels were found in the livers of trout exposed to 164 mg/litre as silver thiosulfate. In these fish, the hepatic silver concentration was increased 335 times from the control value. The metallothionein (a protein that sequesters metals, reducing their toxicity in tissues) levels in gills and liver increased with the water silver concentration, and the highest level of metallothionein was found in liver of fish exposed to silver thiosulfate. The studies revealed that whereas trout accumulated higher silver levels in the liver from high silver thiosulfate and silver chloride exposures, these compounds were much less toxic than silver nitrate. The authors suggest that the increased induction of metallothionein might explain the apparent lack of toxicity from the high hepatic silver loads in fish exposed to silver thiosulfate and silver chloride.
At concentrations normally encountered in the environment, food-chain biomagnification of silver in aquatic systems is unlikely (Connell et al., 1991; Ratte, 1999). Silver, as thiosulfate-complexed silver at nominal concentrations of 500 or 5000 µg silver/litre, was concentrated and magnified over a 10-week period in freshwater food-chains of algae, daphnids, mussels, and fathead minnows (Pimephales promelas) (Terhaar et al., 1977), although the mechanisms of accumulation in this study were imperfectly understood. Sediments contaminated with silver sulfide, however, do not seem to pose a major route of entry into the aquatic food web. Aquatic oligochaetes (Lumbriculus variegatus) held on sediments containing 444 mg silver/kg dry weight, as silver sulfide, for 28 days had a low bioconcentration factor of 0.18 (Hirsch, 1998b). Fisher & Wang (1998) showed that trophic transfer of silver in marine herbivores, especially mussels, was dependent on the silver assimilation efficiency from ingested food particles, feeding rate, and silver efflux rate. Silver assimilation efficiency is usually less than 30% and lower for sediment than for phytoplankton. Silver assimilation efficiency and distribution from ingested phytoplankton particles are modified by gut passage time, extracellular and intracellular digestion rates, and metal desorption at lowered pH. The kinetic model of Fisher & Wang (1998) for mussels predicts that either the solute or the particulate pathway can dominate, depending on the silver partition coefficients for suspended particles and silver assimilation efficiency.
Silver is comparatively rare in the Earth’s crust — 67th in order of natural abundance of elements. The crustal abundance is an estimated 0.07 mg/kg and is predominantly concentrated in basalt (0.1 mg/kg) and igneous rocks (0.07 mg/kg). Silver concentrations tend to be naturally elevated in crude oil and in water from hot springs and steam wells. Anthropogenic sources associated with the elevated concentrations of silver in non-living materials include smelting, hazardous waste sites, cloud seeding with silver iodide, metal mining, sewage outfalls, and especially the photoprocessing industry. Silver concentrations in biota were greater in organisms near sewage outfalls, electroplating plants, mine wastes, and silver iodide-seeded areas than in conspecifics from more distant sites (Eisler, 1997). Silver concentrations reported prior to the implementation of ultra-clean metal sampling, which began in the late 1980s, should be treated with caution.
Maximum concentrations of total silver that have been recorded in selected non-biological materials are 36.5 ng/m3 in air near a smelter in Idaho, USA (ATSDR, 1990); 2.0 µg/m3 in atmospheric dust (Freeman, 1979); 0.1 µg/litre in oil well brines (US EPA, 1980); 4.5 µg/litre in precipitation from clouds seeded with silver iodide; 6.0 µg/litre in groundwater near a hazardous waste site (ATSDR, 1990); 8.9 µg/litre in seawater from Galveston Bay, USA (Morse et al., 1993); 260 µg/litre in the Genesee River, New York, USA — the recipient of photographic manufacturing wastes; 300 µg/litre in steam wells (US EPA, 1980); 300 µg/litre in treated photoprocessing wastewaters; 31 mg/kg in some Idaho, USA, soils (ATSDR, 1990); 43 mg/litre in water from certain hot springs (US EPA, 1980); 50 mg/kg in granite (Fowler & Nordberg, 1986); as much as 100 mg/kg in crude oils; and 150 mg/kg in some Genesee River, USA, sediments (US EPA, 1980). It should be noted that only a small portion of the total silver in each of these compartments is biologically available. More recent monitoring studies revealed that levels in the Genesee River, USA, had decreased to below the detection limit (<10 µg/litre) in water samples and that maximum sediment values were 55 mg/kg (DEC, 1993). Gill et al. (1997) monitored surface waters and municipal and industrial discharge effluents in Colorado, USA, using ultra-clean sampling protocols. Silver measurements in unfiltered samples spanned more than 4 orders of magnitude, from a high of 33 µg/litre in industrial effluent to a low of 2 ng/litre. In general, upstream of the industrial discharges, unfiltered silver concentrations ranged from 3 to 20 ng/litre; downstream concentrations ranged from 3 ng/litre to 1 µg/litre.
Silver is usually found in extremely low concentrations in natural waters because of its low crustal abundance and low mobility in water (US EPA, 1980). One of the highest silver concentrations recorded in fresh water, 38 µg/litre, occurred in the Colorado River, USA, downstream of an abandoned gold–copper–silver mine, an oil shale extraction plant, a gasoline and coke refinery, and a uranium processing facility (US EPA, 1980).
In general, silver concentrations in surface waters in the USA were lower during 1975–1979 than during 1970–1974 (ATSDR, 1990). About 30–70% of the silver in surface waters may be ascribed to suspended particles (Smith & Carson, 1977), depending on water hardness and salinity. The most recent measurements of silver in rivers, lakes, and estuaries using clean techniques show levels of about 0.01 µg/litre for pristine, unpolluted areas and 0.01–0.1 µg/litre in urban and industrialized areas (Ratte, 1999).
Silver can remain attached to oceanic sediments for about 100 years under conditions of high pH, high salinity, and high sediment concentrations of iron, manganese oxide, and organics (Wingert-Runge & Andren, 1994). Estuarine sediments that receive metals, mining wastes, or sewage usually have higher silver concentrations (>0.1 mg/kg dry weight) than do non-contaminated sediments. Sediments in Puget Sound, Washington, USA, were significantly enriched in silver, in part from human activities; concentrations were higher in fine-grained particles (Bloom & Crecilius, 1987). Silver levels in sediments from San Francisco Bay, USA, declined from approximately 1.6 mg/kg (15 nmol/g dry weight) in the late 1970s to 0.2 mg/kg (1.8 nmol/g) in the late 1990s (Hornberger et al., 1999). Marine annelids and clams accumulate dissolved and sediment-bound forms of silver. Uptake of silver from sediments by marine polychaete annelids decreased in sediments with high concentrations of humic substances or copper but increased in sediments with elevated concentrations of manganese or iron (Bryan & Langston, 1992).
Maximum concentrations of total silver recorded in field collections of living organisms, in milligrams of silver per kilogram dry weight, were 1.5 in liver of marine mammals (Szefer et al., 1994), 2 in liver and 6 in bone of trout from ecosystems receiving precipitation from silver iodide-seeded clouds (Freeman, 1979), 7 in kidneys and 44 in liver of birds from a metals-contaminated area (Lande, 1977), 14 in marine algae and macrophytes (Eisler, 1981), 30 in whole annelid worms (Bryan & Hummerstone, 1977), 110 in whole mushrooms (Falandysz & Danisiewicz, 1995), 133–185 in soft parts of clams and mussels near sewage and mining waste outfalls (Luoma & Phillips, 1988; ATSDR, 1990), and 320 in whole gastropods from South San Francisco Bay (Luoma & Phillips, 1988). Silver concentrations in conspecifics from areas remote from anthropogenic contamination were usually lower by 1 or more orders of magnitude. The accumulation of silver by benthic organisms from marine sediment is attributed, in part, to the formation of stable complexes of silver with chlorine, which, in turn, favours the distribution and accumulation of silver (Ratte, 1999).
Silver is a normal trace constituent of many organisms (Smith & Carson, 1977). In terrestrial plants, silver concentrations are usually less than 1.0 mg/kg ash weight (equivalent to less than 0.1 mg/kg dry weight) and are higher in trees, shrubs, and other plants near regions of silver mining; seeds, nuts, and fruits usually contain higher silver concentrations than do other plant parts (US EPA, 1980). Silver accumulations in marine algae (maximum 14.1 mg/kg dry weight) are due mainly to adsorption rather than uptake; bioconcentration factors of 13 000–66 000 are not uncommon (ATSDR, 1990; Ratte, 1999).
Silver concentrations in molluscs vary widely between closely related species and among conspecifics from different areas (Bryan, 1973; Eisler, 1981). The highest silver concentrations in all examined species of molluscs were in the internal organs, especially in the digestive gland and kidneys (Eisler, 1981; Miramand & Bentley, 1992). Elevated concentrations of silver (5.3 mg/kg dry weight) in shells of limpets from uncontaminated sites suggest that silver may actively participate in carbonate mineral formation (Navrot et al., 1974), but this needs verification (Eisler, 1997). In general, silver concentrations were elevated in molluscs collected near port cities and in the vicinities of river discharges (Fowler & Oregioni, 1976; Berrow, 1991), electroplating plant outfalls (Eisler et al., 1978; Stephenson & Leonard, 1994), ocean dump sites (Greig, 1979), and urban point sources, including sewage outfalls (Alexander & Young, 1976; Smith & Carson, 1977; Martin et al., 1988; Anderlini, 1992; Crecelius, 1993), and from calcareous sediments rather than detrital organic or iron oxide sediments (Luoma & Jenne, 1977). Season of collection (Fowler & Oregioni, 1976; Sanders et al., 1991) and latitude (Anderlini, 1974) also influence silver accumulations. Seasonal variations in silver concentrations of Baltic clams were associated with seasonal variations in soft tissue weight and frequently reflected the silver content in the sediments (Cain & Luoma, 1990). Oysters from the Gulf of Mexico vary considerably in whole-body concentrations of silver and other trace metals. Variables that modify silver concentrations in oyster tissues include age, size, sex, reproductive stage, general health, and metabolism of the animal; water temperature, salinity, dissolved oxygen, and turbidity; natural and anthropogenic inputs to the biosphere; and chemical species and interactions with other compounds (Presley et al., 1990). Silver concentrations in whole American oysters from Chesapeake Bay were reduced in summer, reduced at increasing salinities, and elevated near sites of human activity; chemical forms of silver taken up by oysters included the free monovalent ion and the uncharged AgCl0 (Sanders et al., 1991; Daskalakis, 1996). Declines in tissue silver concentrations of the California mussel (Mytilus californicus) were significant between 1977 and 1990; body burdens decreased from 10–70 mg/kg dry weight to less than 2 mg/kg dry weight, apparently related to the termination of metal plating facilities in 1974 and decreased mass emission rates by wastewater treatment facilities (Stephenson & Leonard, 1994).
Among arthropods, pyrophosphate granules isolated from barnacles have the capability to bind and effectively detoxify silver and other metals under natural conditions (Pullen & Rainbow, 1991). In a Colorado, USA, alpine lake, silver concentrations in caddisflies and chironomid larvae usually reflected silver concentrations in sediments; seston, however, showed a high correlation with lakewater silver concentrations from 20 days earlier (Freeman, 1979).
In other studies, silver concentrations in fish muscles rarely exceeded 0.2 mg/kg dry weight and usually were less than 0.1 mg/kg fresh weight; livers contained as much as 0.8 mg/kg fresh weight, although values greater than 0.3 mg/kg fresh weight were unusual; and whole fish contained as much as 0.2 mg/kg fresh weight. Livers of Atlantic cod (Gadus morhua) contained significantly more silver than muscles or ovaries; a similar pattern was evident in other species of marine teleosts (Hellou et al., 1992; Szefer et al., 1993). Accumulation of silver in offshore populations of teleosts is unusual, even among fish collected near dumping sites impacted by substantial quantities of silver and other metals. For example, of seven species of marine fish from a disposal site in the New York Bight, USA, that were examined for silver content, concentrations were highest (0.15 mg/kg fresh weight) in muscle of blue hake (Antimora rostrata; Greig et al., 1976). Similarly, the elevated silver concentration of 0.8 mg/kg fresh weight in liver of winter flounder (Pleuronectes americanus) was from a specimen from the same general area (Greig & Wenzloff, 1977). The similarity between laboratory experiments with snow crab (Chionoecetes opilio) and American plaice (Hippoglossoides platessoides) and field data from an estuary receiving significant inputs of anthropogenic silver suggests that predation is the major transfer route of silver to marine benthic predators (Rouleau et al., 2000).
Silver concentrations were lower in muscles of Antarctic birds (0.01 mg/kg dry weight) than in livers (0.02–0.46 mg/kg dry weight) or faeces (0.18 mg/kg dry weight; Szefer et al., 1993). Silver concentrations in avian tissues, especially in livers, were elevated in the vicinity of metal-contaminated areas and in diving ducks from San Francisco Bay, USA.
The concentration of silver in three species of seal collected in the Antarctic during 1989 was highest in the liver (1.55 mg/kg dry weight) and lowest in muscle (0.01 mg/kg dry weight); intermediate values were found in kidney (0.29 mg/kg dry weight) and stomach contents (0.24 mg/kg dry weight; Szefer et al., 1993). The mean concentration of silver in livers from normal female California sea lions (Zalophus californicus), with normal pups, was 0.5 mg/kg dry weight (Martin et al., 1976). Silver concentrations in tissues of Antarctic seals were related to, and possibly governed by, concentrations of other metals (Szefer et al., 1993). In muscle, silver was inversely correlated with zinc; in liver, silver was positively correlated with nickel, copper, and zinc; and in kidney, correlations between silver and zinc and between silver and cadmium were negative (Szefer et al., 1993). Saeki et al. (2001) analysed samples from three species of pinniped from the North Pacific Ocean. In northern fur seals (Callorhinus ursinus), relatively high concentrations of silver were found in the liver and body hair, with some 70% of the body burden located in the liver. Hepatic silver concentrations were significantly correlated with age in northern fur seals and Steller sea lions (Eumetopias jubatus). Silver concentrations (milligrams per kilogram wet weight) were 0.04–0.55 for northern fur seals, 0.1–1.04 for Steller sea lions, and 0.03–0.83 for harbour seals (Phoca vitulina). Becker et al. (1995) reported silver levels in Alaskan beluga whale (Delphinapterus leucas) liver to be 2 orders of magnitude higher than for any other marine mammals, although no adverse effects were reported.
In solution, ionic silver is extremely toxic to aquatic plants and animals (Nehring, 1976; Nelson et al., 1976; Calabrese et al., 1977a; Gould & MacInnes, 1977; Smith & Carson, 1977; US EPA, 1980; Buhl & Hamilton, 1991; Bryan & Langston, 1992), and aqueous concentrations of 1–5 µg/litre killed sensitive species of aquatic organisms, including representative species of insects, daphnids, amphipods, trout, flounder, and dace. At nominal water concentrations of 0.5–4.5 µg/litre, accumulations in most species of exposed organisms were high and had adverse effects on growth in algae, clams, oysters, snails, daphnids, amphipods, and trout; moulting in mayflies; and histopathology in mussels (Eisler, 1997).
The acute toxicity of silver to aquatic species varies drastically by the chemical form and correlates with the availability of free ionic silver (Wood et al., 1994). In natural aquatic systems, ionic silver is rapidly complexed and sorbed by dissolved and suspended materials that are usually present. Complexed and sorbed silver species in natural waters are at least 1 order of magnitude less toxic to aquatic organisms than the free silver ion (Rodgers et al., 1994; Ratte, 1999). Thus, silver nitrate, which is strongly dissociated, is extremely toxic to rainbow trout; the 7-day LC50 value is 9.1 µg/litre. Silver thiosulfate, silver chloride, and silver sulfide were relatively benign (7-day LC50 values >100 000 µg/litre), presumably due to the abilities of the anions to remove ionic silver from solution (Wood et al., 1994, 1996b; Hogstrand et al., 1996). The toxicity of silver compounds to aquatic species is summarized in Table 2.
Table 2: Toxicity of silver compounds to aquatic species.
|
Organism |
End-pointa |
Silver concentration (µg/litre)b |
Reference |
|
Microorganisms |
|||
|
Marine |
|||
|
Marine bacteria (isolated from tubes of deep-sea polychaete annelids) |
10-day EC50 (growth) |
3000 |
Jeanthon & Prieur (1990) |
|
Silver-resistant strains |
10-day NOEC |
20 000–40 000 |
Jeanthon & Prieur (1990) |
|
Marine algae (Prorocentrum mariae lebouriae) |
5-day EC50 (growth) at 7.5‰ salinity |
3.3 |
Sanders & Abbe (1989) |
|
5-day EC50 (growth) at 15–22.5‰ salinity |
6.7 |
Sanders & Abbe (1989) |
|
|
5-day EC50 (growth) at 30‰ salinity |
8.2 |
Sanders & Abbe (1989) |
|
|
Marine diatom (Skeletonema costatum) |
5-day EC50 (growth) at 7.5‰ salinity |
5.9 |
Sanders & Abbe (1989) |
|
5-day EC50 (growth) at 15‰ salinity |
15.4 |
Sanders & Abbe (1989) |
|
|
5-day EC50 (growth) at 22.5–30‰ salinity |
20 |
Sanders & Abbe (1989) |
|
|
96-h EC50 (cell numbers) |
130–170 |
US EPA (1980) |
|
|
Freshwater |
|||
|
Alga (Selenastrum capricornutum) |
7-day NOEC |
10 000c |
Ratte (1999) |
|
Alga (Scenedesmus sp.) |
EC100 (growth) |
100–200 |
US EPA (1980) |
|
Protozoan (Spirostomum ambiguum) |
24-h LC50 at 2.8 mg CaCO3/litre |
8.8 |
Nalecz-Jawecki et al. (1993) |
|
24-h LC50 at 250 mg CaCO3/litre |
15.3 |
Nalecz-Jawecki et al. (1993) |
|
|
Invertebrates |
|||
|
Marine |
|||
|
Bay scallop (Argopecten irradians) juvenile |
96-h LC50 |
33 |
Calabrese et al. (1977b) |
|
Scallop (Chlamys varia) adult |
115-h LC50 |
100 |
Berthet et al. (1992) |
|
Pacific oyster (Crassostrea gigas) adult |
209-h LC50 |
100 |
Berthet et al. (1992) |
|
American oyster (Crassostrea virginica) embryo |
48-h LC50 |
5.8 |
Calabrese et al. (1977b) |
|
American oyster juvenile |
12-day LC50 |
25 |
Calabrese et al. (1977b) |
|
Quahog clam (Mercenaria mercenaria) embryo |
48-h LC50 |
21 |
Calabrese et al. (1977b) |
|
Quahog clam juvenile |
10-day LC50 |
32.4 |
Calabrese et al. (1977b) |
|
Clam (Scrobicularia plana) adult |
96-h LC50 |
200 |
Berthet et al. (1992) |
|
250-h LC50 |
100 |
Berthet et al. (1992) |
|
|
Mussel (Mytilus edulis) |
21-month LOEC (histopathologyd) |
1 |
Calabrese et al. (1984) |
|
Mussel (Mytilus galloprovincialis) |
110-h LC50 |
100 |
Berthet et al. (1992) |
|
Freshwater |
|||
|
Asiatic clam (Corbicula fluminea) |
21-day NOEC (survival) |
7.8 |
Diamond et al. (1990) |
|
21-day NOEC (growth) |
2.6 |
Diamond et al. (1990) |
|
|
21-day LOEC (growth) |
7.8 |
Diamond et al. (1990) |
|
|
Flatworm (Dugesia dorotocephala) |
96-h LC50 |
30 |
Ratte (1999) |
|
96-h LC50 |
>1 000 000e |
Ratte (1999) |
|
|
96-h EC50 |
>1300c |
Ratte (1999) |
|
|
Oligochaete (Lumbriculus variegatus) |
96-h LC50 |
>1 000 000e |
Ratte (1999) |
|
Snail (Planorbella trivolis) |
96-h LC50 |
300 |
Ratte (1999) |
|
96-h LC50 |
>1 000 000e |
Ratte (1999) |
|
|
96-h LC50 |
>1300c |
Ratte (1999) |
|
|
Free-living nematode (Caernorhabditis elegans) |
96-h LC50 |
102 (10–4980) |
Williams & Dusenbery (1990) |
|
Copepod (Acartia tonsa) |
96-h LC50 |
36 |
US EPA (1980) |
|
Copepods (Acartia tonsa and A. hudsonica) |
48-h LC50 |
43 |
Hook & Fisher (2001) |
|
Amphipod (Ampelisca abdita) |
10-day LC50 |
20 |
Berry et al. (1999) |
|
Scud (Gammarus pseudolimnaeus) |
96-h LC50 at 44 mg CaCO3/litre |
4.5 (3.7–5.5) |
Lima et al. (1982) |
|
Amphipod (Hyalella azteca) |
96-h LC50 |
1.9 (1.4–2.3) |
Diamond et al. (1990) |
|
21-day NOEC (survival) |
0.95 |
Diamond et al. (1990) |
|
|
21-day LOEC (survival) |
1.9 |
Diamond et al. (1990) |
|
|
Daphnid (Daphnia magna) |
48-h EC50 |
0.9 |
Nebeker et al. (1983) |
|
96-h LC50 |
5 |
Ratte (1999) |
|
|
96-h LC50 |
20f |
Ratte (1999) |
|
|
96-h EC50 |
>1 000 000e |
Ratte (1999) |
|
|
96-h LC50 |
>1330c |
Ratte (1999) |
|
|
96-h LC50 at 38–75 mg CaCO3/litre |
0.4–15.0 |
US EPA (1980) |
|
|
96-h LC50 at 255 mg CaCO3/litre |
45–49 |
US EPA (1980) |
|
|
21-day EC50 (growth) |
3.5 |
Nebeker et al. (1983) |
|
|
Daphnids (Daphnia spp.) |
96-h LC50 |
10 (0.25–49.0) |
Williams & Dusenbery (1990) |
|
Cladocerans (Simocephalus sp. and Ceriodaphnia dubia) |
48-h LC50 at 16 mg CaCO3/litre |
27 |
Hook & Fisher (2001) |
|
Chironomid (Chironomus tentans) |
10-day LC50 |
57 |
Call et al. (1999) |
|
10-day LC50 |
1 170 000–2 750 000g |
Call et al. (1999) |
|
|
Mayfly (Ephemerella grandis) |
7- to 15-day LC50 |
4.0–8.8 |
US EPA (1980) |
|
Mayfly (Isonychia bicolor) |
96-h LC50 |
6.8 (5.5–7.8) |
Diamond et al. (1990) |
|
14-day NOEC (moult) |
0.3 |
Diamond et al. (1990) |
|
|
14-day LOEC (moult) |
1.6 |
Diamond et al. (1990) |
|
|
Mayfly (Stenonema sp.) |
96-h LC50 |
3.9 (2.5–5.7) |
Diamond et al. (1990) |
|
Stonefly (Leuctra sp.) |
96-h LC50 |
2.5 (1.7–3.2) |
Diamond et al. (1990) |
|
Fish |
|||
|
Marine |
|||
|
Rainbow trout (Oncorhynchus mykiss) |
96-h NOEC at 15–20‰ salinity; seawater acclimatized |
401 |
Ferguson & Hogstrand (1998) |
|
96-h LC50 at 25‰ salinity; seawater acclimatized |
401 |
Ferguson & Hogstrand (1998) |
|
|
Tidepool sculpin (Oligocottus maculosus) |
96-h LC50 |
331 (25‰ salinity) |
Shaw et al. (1998) |
|
168-h LC50 |
119 (25‰ salinity) |
Shaw et al. (1998) |
|
|
96-h LC50 |
664 (32‰ salinity) |
Shaw et al. (1998) |
|
|
168-h LC50 |
472 (32‰ salinity) |
Shaw et al. (1998) |
|
|
Sheepshead minnow (Cyprinodon variegatus) juvenile |
96-h LC50 |
1400 |
US EPA (1980) |
|
Freshwater |
|||
|
Mottled sculpin (Cottus bairdi) |
96-h LC50 at 30 mg CaCO3/litre |
5.3 |
US EPA (1980) |
|
96-h LC50 at 250 mg CaCO3/litre |
14 |
US EPA (1980) |
|
|
Mosquitofish (Gambusia affinis) juvenile |
96-h LC50 |
23.5 (17.2–27.0) |
Diamond et al. (1990) |
|
Flagfish (Jordanella floridae) |
96-h LC50 at 44 mg CaCO3/litre |
9.2 (8.0–10.7) |
Lima et al. (1982) |
|
Bluegill (Lepomis macrochirus) |
96-h LC50 |
31.7 (24.2–48.4) |
Diamond et al. (1990) |
|
Atlantic silverside (Menidia menidia) larvae |
96-h LC50 |
110 |
US EPA (1980) |
|
Atlantic silverside juvenile |
96-h LC50 |
400 |
US EPA (1980) |
|
Coho salmon (Oncorhynchus kisutch) alevin |
96-h LC50 |
11.1 (7.9–15.7) |
Buhl & Hamilton (1991) |
|
Coho salmon juvenile |
96-h LC50 |
12.5 (10.7–14.6) |
Buhl & Hamilton (1991) |
|
Rainbow trout (Oncorhynchus mykiss) eyed embryo to adult |
70-day LOEC (survival) at 20–31 mg CaCO3/litre |
1.2 |
Davies et al. (1978) |
|
18-month LOEC (survival) at 20–31 mg CaCO3/litre |
0.17 |
Davies et al. (1978) |
|
|
Rainbow trout juvenile |
144-h LC50 |
4.8 |
Diamond et al. (1990) |
|
Rainbow trout juvenile |
96-h LC50 at 20–31 mg CaCO3/litre |
5.3–8.1 |
Davies et al. (1978) |
|
96-h LC50 |
7.6–10.9 |
US EPA (1980); Nebeker et al. (1983) |
|
|
96-h LC50 |
11.8 |
Hogstrand et al. (1996) |
|
|
96-h LC50 |
161 000c |
Hogstrand et al. (1996) |
|
|
168-h LC50 |
9.1 |
Hogstrand et al. (1996) |
|
|
168-h LC50 |
137 000c |
Hogstrand et al. (1996) |
|
|
168-h LC50 |
>100 000h |
Hogstrand et al. (1996) |
|
|
96-h LC50 at 350 mg CaCO3/litre |
13 |
Davies et al. (1978) |
|
|
Rainbow trout adult |
96-h LC50 in soft, low-chloride (10 µmol/litre) water |
10.2 |
Grosell et al. (2000) |
|
Rainbow trout alevin |
96-h LC50 |
16.1 (12.8–20.2) |
Buhl & Hamilton (1991) |
|
Rainbow trout juvenile |
96-h LC50 |
19.2 (16–23.1) |
Buhl & Hamilton (1991) |
|
28-day LC50 at 93–105 mg CaCO3/litre |
10 |
US EPA (1980) |
|
|
96-h LC50 |
9.2 |
Nebeker et al. (1983) |
|
|
Rainbow trout eyed embryo |
96-h LC50 |
200 |
Rombough (1985) |
|
Rainbow trout embryo/larva |
60-day LOEC (growth) |
0.1 |
Nebeker et al. (1983) |
|
60-day LOEC (survival) |
0.5 |
Nebeker et al. (1983) |
|
|
Rainbow trout embryo |
32-day LOEC (survival) at 120 mg CaCO3/litre |
13.5 |
Guadagnolo et al. (2001) |
|
Summer flounder (Paralichthys dentatus) larvae |
96-h LC50 |
4.7 |
US EPA (1980) |
|
Summer flounder embryos |
96-h LC50 |
8.0–48.0 |
US EPA (1980) |
|
Fathead minnow (Pimephales promelas) |
96-h LC50 at 25–75 mg CaCO3/litre |
5.3–20.0 |
US EPA (1980) |
|
96-h LC50; flow-through tests |
5.6–7.4 |
Nebeker et al. (1983) |
|
|
96-h LC50; static tests |
9.4–9.7 |
Nebeker et al. (1983) |
|
|
96-h LC50 at 44 mg CaCO3/litre |
10.7 (10.6–10.8) |
Lima et al. (1982) |
|
|
96-h LC50 at 255 mg CaCO3/litre |
110–270 |
US EPA (1980) |
|
|
96-h LC50 |
16 (12–20) |
LeBlanc et al. (1984) |
|
|
96-h LC50 |
>280 000c |
LeBlanc et al. (1984) |
|
|
96-h LC50 |
>240 000e |
LeBlanc et al. (1984) |
|
|
Winter flounder (Pleuronectes americanus) embryo |
96-h LC50 |
200–450 |
US EPA (1980) |
|
Speckled dace (Rhinichthys osculus) |
96-h LC50 in soft water |
4.9 |
US EPA (1980) |
|
96-h LC50 in hard water |
14 |
US EPA (1980) |
|
|
Arctic grayling (Thymallus arcticus) alevin |
96-h LC50 |
6.7 (5.5–8.0) |
Buhl & Hamilton (1991) |
|
Arctic grayling juvenile |
96-h LC50 |
11.1 (9.2–13.4) |
Buhl & Hamilton (1991) |
|
European eel (Anguilla anguilla) |
96-h LC50 in soft, low-chloride (10 µmol/litre) water |
34.4 |
Grosell et al. (2000) |
|
Amphibians |
|||
|
Leopard frog (Rana pipiens) |
EC10 based on mortality or abnormal development of embryos and larvae |
0.7–0.8 |
Birge & Zuiderveen (1996) |
|
EC50 based on mortality or gross terata of embryos and larvae |
10 |
Birge & Zuiderveen (1996) |
|
|
a |
EC50 = median effective concentration; EC100 = effective concentration for 100% of the population; |
|
b |
Tests performed using silver nitrate, unless stated otherwise. |
|
c |
Silver thiosulfate. |
|
d |
Accumulations of yellowish-brown to black particulates in the basement membrane and connective tissue of the body organs; black particulate-laden macrophages were noted throughout the connective tissue and accumulated in large groups in the intertubular connective tissue of the digestive diverticula and the kidneys. |
|
e |
Silver sulfide. |
|
f |
Silver sulfate. |
|
g |
Sediments supplemented with silver nitrate, in µg silver/kg dry weight sediment. |
|
h |
Silver chloride. |
In general, silver ion was less toxic to freshwater aquatic organisms under conditions of low dissolved silver ion concentration and increasing water pH, hardness, sulfides, and dissolved and particulate organic loadings; under static test conditions compared with flow-through regimens; and when animals were adequately nourished instead of being starved (Erickson et al., 1998; Bury et al., 1999a, 1999c; Karen et al., 1999; Ratte, 1999; Wood et al., 1999). It is now agreed that increasing concentrations of dissolved organic carbon afford the highest protective effects (Berry et al., 1999; Karen et al., 1999). Among all tested species, the individuals most sensitive to silver were the poorly nourished and young and those exposed to low water hardness or salinity (Smith & Carson, 1977; US EPA, 1980; LeBlanc et al., 1984; Erickson et al., 1998; Shaw et al., 1998). In the case of seawater-acclimatized rainbow trout, silver-induced mortality was greater at higher salinities, but the increased toxicity with salinity was linked to an incomplete hypo-osmoregulatory ability and not to an increase in a more toxic AgCln species (Ferguson & Hogstrand, 1998). Sediment chemistry can affect the toxicity of silver to marine amphipods (Ampelisca abdita) exposed for 10 days to sediments supplemented with various concentrations of silver (Berry et al., 1999). In general, sediments with an excess of acid-volatile sulfide relative to simultaneously extracted metal were generally not toxic to marine amphipods. Sediments with an excess of simultaneously extracted metal relative to acid-volatile sulfide, and no measurable acid-volatile sulfide, were generally toxic. Sediments with measurable acid-volatile sulfide were not toxic (Berry et al., 1999).
Silver ions are very toxic to microorganisms (Ratte, 1999). However, there is generally no strong inhibitory effect on microbial activity in sewage treatment plants (NAPM, 1974; Bard et al., 1976) because of reduced bioavailability due to rapid complexation and adsorption. In photographic wastewaters, silver is present as a thiosulfate complex, which in sludge is transformed to insoluble silver sulfide. Neither silver thiosulfate nor silver sulfide shows the same inhibitory effects as free silver ions (NAPM, 1974; Bard et al., 1976; Pavlostathis & Maeng, 1998). Photoprocessing wastewater did not inhibit sludge respiration when (total) silver was present in concentrations of up to 100 mg/litre, but a concentration of 10 mg/litre present as free silver ions caused up to 84% inhibition of respiration (Leonhardt & Pfeiffer, 1985). Pavlostathis & Maeng (2000) found no significant effect of silver on the anaerobic digestive process. They found no significant difference between the ultimate biodegradability of silver-bearing waste activated sludge (5 g silver/kg sludge dry solids) and control (silver-free) sludge under anaerobic conditions. Addition of either silver nitrate or silver sulfide (100 mg silver/litre) to methanogenic, mixed cultures did not affect the rate and extent of methane production, and the inhibition of methanogenesis by silver thiosulfate was found to be due to excess thiosulfate accumulation.
Sensitive aquatic plants grew poorly at 3.3–8.2 µg silver/litre during exposure for 5 days and died at concentrations greater than 130 µg silver/litre. Some metals seem to protect aquatic plants against adverse effects of silver. Algae in small lakes that contained elevated concentrations of metals, especially copper and nickel, had higher tolerances to silver than conspecifics reared in the laboratory under conditions of depressed concentrations of heavy metals (US EPA, 1980). Phytoplankton communities in Chesapeake Bay, USA, were significantly altered in experimental ecosystems continuously stressed by low concentrations (0.3–0.6 µg/litre) of silver. At higher concentrations of 2–7 µg/litre for 3–4 weeks, silver inputs caused disappearance of Anacystis marina, a mat-forming blue-green algal species; increased dominance by Skeletonema costatum, a chain-forming centric diatom; and increased silver concentrations (to 8.6–43.7 mg silver/kg dry weight) in various species of phytoplankton (Sanders & Cibik, 1988; Sanders et al., 1990).
In freshwater sediments supplemented with silver nitrate, a high proportion of the dissolved silver fraction was not readily bioavailable to cause lethality to dipteran insect larvae (Call et al., 1999). Porewater concentrations of dissolved silver that killed 50% of the dipteran larvae were up to 275 times greater than the 10-day water-only LC50 value of 57 µg/litre, indicating that most of the dissolved fraction was not readily bioavailable to cause death. Concentrations of silver in these sediments that caused significant adverse effects (200–500 mg silver/kg dry weight) in dipteran larvae were markedly above silver concentrations usually reported in the environment (Call et al., 1999). Juvenile amphipods (Hyalella azteca) held on sediments containing as much as 753 mg silver/kg dry weight, as silver sulfide, for 10 days had normal growth and survival (Hirsch, 1998c). Similarly, no significant effect was observed on the growth of oligochaetes (Lumbriculus variegatus) exposed to laboratory-spiked sediments containing 444 mg silver/kg dry weight, as silver sulfide, for 28 days (Hirsch, 1998b).
For freshwater fish, the acute toxicity of silver is caused solely by silver ion interacting with the gills, inhibiting basolateral Na+/K+-ATPase activity. Disruption of this enzyme inhibits active sodium and chloride ion uptake and, therefore, osmoregulation by the fish (Wood et al., 1999). The primary toxic mechanism of silver in rainbow trout is the interruption of ionic regulation at the gills, stopping active sodium and chloride ion uptake without increasing passive efflux, thereby causing net ion loss (Webb & Wood, 1998). However, concentrations of silver in the gills of rainbow trout were not correlated with silver ion concentrations in the medium, and no correlation was found between gill silver levels and either sodium ion influx rates or gill Na+/K+-ATPase activity (Bury et al., 1999c). Morgan et al. (1996) suggested that the sites of action of silver toxicity in rainbow trout may be inside the cells of the gill epithelium rather than at the external surface and linked to carbonic anhydrase, a gill enzyme involved in sodium and chloride ion transport. Silver concentrations and metallothionein levels in gills and livers of rainbow trout increased with increasing exposure to silver; internal toxicity associated with increased silver accumulations may be lessened by the formation of silver-induced metallothioneins (Hogstrand et al., 1996). A key toxic effect of silver ion in fresh water is the inhibition of branchial Na+/K+-ATPase activity, which leads to the blockade of active sodium and chloride ions across the gills; increased metabolic ammonia production and internal buildup occur as part of this acute stress syndrome (Hogstrand & Wood, 1998). The probable cause of hyperventilation in rainbow trout exposed to silver nitrate was a severe metabolic acidosis manifested in decreased arterial plasma pH and bicarbonate ion levels. Lethality of ionic silver to trout is probably due to surface effects at the gills, disrupting sodium, chloride, and hydrogen ions and causing secondary fluid volume disturbance, haemoconcentration, and eventual cardiovascular collapse (Wood et al., 1994, 1996a, 1996b, 1996c). Acidosis in rainbow trout, due to a net uptake of acidic equivalents from the water, in the intracellular compartment accounts for the continual loss of potassium ion to the water in the absence of any change in plasma potassium ion concentration (Webb & Wood, 1998).
Silver nitrate is less toxic in seawater than in fresh water (Wood et al., 1996c, 1999). This difference is probably due to the low concentration of free silver ion (the toxic moiety in fresh water) in seawater, the high levels of chloride, and the predominance of negatively charged silver-chloro complexes. However, high levels of silver nitrate are toxic to marine invertebrates despite the absence of silver ion, and this is attributed to the bioavailability of stable silver-chloro complexes (Wood et al., 1996c; Ratte, 1999). In seawater, in contrast to fresh water, plasma sodium and chloride ion concentrations rise rather than fall, and death may result from the elevated sodium and chloride ion concentrations combined with dehydration (Hogstrand & Wood, 1998). Osmoregulatory failure occurs in marine teleosts exposed to high concentrations of silver ion, and the intestine is the main toxic site of action (Wood et al., 1999).
Silver ion was the most toxic chemical species of silver to fish. For fathead minnows, silver ion was 300 times more toxic than silver chloride, 15 000 times more toxic than silver sulfide, and more than 17 500 times more toxic than silver thiosulfate complex; in all cases, toxicity reflected the free silver ion content of tested compounds (LeBlanc et al., 1984); a similar pattern was noted in rainbow trout (Hogstrand et al., 1996). Silver was less toxic to fathead minnow under conditions of increasing water hardness between 50 and 250 mg calcium carbonate/litre, increasing pH between 7.2 and 8.6, and increasing concentrations of humic acid and copper; starved minnows were more sensitive to ionic silver than were minnows fed regularly (Brooke et al., 1994). Eggs of rainbow trout exposed continuously to silver concentrations as low as 0.17 µg/litre had increased embryotoxicity and hatched prematurely; resultant fry had a reduced growth rate (Davies et al., 1978). Removal of the egg capsule of eyed embryos of rainbow trout significantly lowered the resistance of the embryos to salts of silver, copper, and mercury, but not zinc and lead (Rombough, 1985). Silver accumulation in gills of juvenile rainbow trout exposed to 11 µg silver/litre for 2–3 h was significantly inhibited by various cations (calcium, sodium, hydrogen ions) and complexing agents (dissolved organic carbon, thiosulfate, chloride); these variables must be considered when constructing predictive models of silver binding to gills (Janes & Playle, 1995).
In tidewater sculpins (Oligocottus maculatus), ionic silver was more toxic at lower salinities, longer exposure durations, and increasing ammonia concentrations in the medium; however, there was no correlation between whole-body silver burden and toxicity at 25‰ salinity and no uptake at 32‰ salinity (Shaw et al., 1998).
Hook & Fisher (2001) fed marine copepods (Acartia tonsa and A. hudsonia) and freshwater cladocerans (Simocephalus sp. and Ceriodaphnia dubia) on algal food for 4 h and found significant effects on reproduction at 4 and 2 mg/kg dry weight, respectively. Algae had previously been maintained in water at silver concentrations of 0.1 and 0.05 µg/litre, respectively, for 4 days.
It has been demonstrated that silver inhibits enzymes for the phosphorus, sulfur, and nitrogen cycles of nitrifying bacteria in soil at concentrations ranging from 540 to 2700 mg silver/kg (Domsch, 1984).
In general, accumulation of silver by terrestrial plants from soils is low, even if the soil is amended with silver-containing sewage sludge or the plants are grown on tailings from silver mines, where silver accumulates mainly in the root systems (Ratte, 1999). Germination was the most sensitive stage for plants grown in solutions containing various concentrations of silver nitrate. Adverse effects on germination were expected at concentrations greater than 0.75 mg silver/litre (as silver nitrate) for lettuce and 7.5 mg/litre for ryegrass (Lolium perenne) and other plants tested (Ratte, 1999). Smith & Carson (1977) reported that sprays containing 9.8 mg dissolved silver/litre kill corn (Zea mays), and sprays containing 100–1000 mg dissolved silver/litre kill tomato (Lycopersicon esculentum) and bean (Phaseolus spp.) plants. Seeds of corn, lettuce (Lactuca sativa), oat (Avena sativa), turnip (Brassica rapa), soybean (Glycine max), spinach (Spinacia oleracea), and Chinese cabbage (Brassica campestris) were planted in soils amended with silver sulfide and sewage sludge to contain as much as 106 mg silver/kg dry weight soil (Hirsch et al., 1993; Hirsch, 1998a). All plants germinated, and most grew normally at the highest soil concentration of silver tested. Yields of lettuce, oat, turnip, and soybean were higher on soils amended with silver-laden, waste activated sludge than on control soils, but growth of Chinese cabbage and lettuce was adversely affected at 14 mg silver/kg dry weight soil and higher. Silver concentrations in edible portions from all plants at all soil levels of silver tested, except lettuce, were less than 80 µg/kg dry weight, suggesting that the availability of sludge-borne silver sulfide to most agricultural crops is negligible. Lettuce grown in soil containing 5 and 120 mg silver/kg dry weight had about 0.5 and as much as 2.7 mg silver/kg dry weight leaves, respectively, compared with 0.03 mg/kg dry weight in controls (Hirsch et al., 1993; Hirsch, 1998a).
Beglinger & Ruffing (1997) found no effect of 1600 mg silver/kg dry weight of soil (applied as silver sulfide) on mortality, burrowing time, appearance, or weight of earthworms (Lumbricus terrestris) exposed for up to 14 days.
Young turkeys (Meleagris gallopavo) on diets containing 900 mg silver/kg feed for 4 weeks had enlarged hearts and reduced growth, haemoglobin, and haematocrit (US EPA, 1980). Adverse effects of silver (given as silver nitrate) were reported in normal chicks fed diets containing 200 mg silver/kg ration (growth suppression) or given drinking-water containing 100 mg silver/litre (liver necrosis) (Smith & Carson, 1977). Chicks on copper-deficient diets had adverse effects at 10 mg silver/kg ration (reduced haemoglobin; reversible when fed copper-adequate diet) and at 50–100 mg silver/kg ration (growth suppression and increased mortality). Chicks that were deficient in vitamin E experienced reduced growth when given drinking-water containing 1500 mg silver/litre (Smith & Carson, 1977).
No data were found on effects of silver on wild mammals. Ionic silver (given as silver nitrate) is lethal to laboratory mice (Mus spp.) and rabbits (Oryctolagus spp.) at 13.9 and 20 mg/kg body weight, respectively, by intraperitoneal injection (US EPA, 1980; ATSDR, 1990), to dogs (Canis familiaris) at 50 mg/kg body weight by intravenous injection (Smith & Carson, 1977), and to rats (Rattus spp.) at 1586 mg/litre drinking-water for 37 weeks (ATSDR, 1990). Sublethal effects are reported in rabbits given silver (as silver nitrate) at concentrations of 250 µg/litre drinking-water (brain histopathology) (Smith & Carson, 1977), in rats given 400 µg/litre drinking-water for 100 days (kidney damage) (US EPA, 1980), in mice given 95 mg/litre drinking-water for 125 days (sluggishness), in guinea-pigs (Cavia spp.) given 81 mg/cm2 skin applied daily for 8 weeks (reduced growth) (ATSDR, 1990), and in rats given diets containing 6 mg/kg for 3 months (high accumulations in kidneys and liver) or 130–1110 mg/kg (liver necrosis) (Smith & Carson, 1977).
Silver is a rare but naturally occurring metal, often found deposited as a mineral ore in association with other elements. Emissions from smelting operations, manufacture and disposal of certain photographic and electrical supplies, coal combustion, and cloud seeding are some of the anthropogenic sources of silver in the biosphere. The global biogeochemical movements of silver are characterized by releases to the atmosphere, water, and land by natural and anthropogenic sources, long-range transport of fine particles in the atmosphere, wet and dry deposition, and sorption to soils and sediments.
The most recent measurements of silver in rivers, lakes, and estuaries using clean techniques show levels of about 0.01 µg/litre for pristine, unpolluted areas and 0.01–0.1 µg/litre in urban and industrialized areas. Silver concentrations reported prior to the implementation of ultra-clean metal sampling, which began in the late 1980s, should be treated with caution. Maximum concentrations of total silver recorded during the 1970s and 1980s in selected non-biological materials were 36.5 ng/m3 in air near a smelter; 2.0 µg/m3 in atmospheric dust; 0.1 µg/litre in oil well brines; 4.5 µg/litre in precipitation from clouds seeded with silver iodide; 6.0 µg/litre in groundwater near a hazardous waste site; 8.9 µg/litre in seawater from Galveston Bay, USA; 260 µg/litre near photographic manufacturing waste discharges; 300 µg/litre in steam wells; 300 µg/litre in treated photoprocessing wastewaters; 31 mg/kg in soils; 43 mg/litre in water from certain hot springs; 50 mg/kg in granite; as much as 100 mg/kg in crude oils; and 150 mg/kg in river sediments. It should be noted that levels of silver in the environment have declined; for example, in the Lower Genesee River, USA, near a photographic manufacturing plant, levels declined from 260 µg/litre in the 1970s to below the detection limit (<10 µg/litre) in the 1990s. It should also be noted that only a small portion of the total silver in each of the environmental compartments is biologically available.
The ability to accumulate dissolved silver varies widely between species. Some reported bioaccumulation factors for marine organisms (calculated as milligrams of silver per kilogram fresh weight organism divided by milligrams of silver per litre of medium) are 210 in diatoms, 240 in brown algae, 330 in mussels, 2300 in scallops, and 18 700 in oysters, whereas bioconcentration factors for freshwater organisms have been reported to range from negligible in bluegills to 60 in daphnids; these values represent uptake of bioavailable silver in laboratory experiments. Laboratory studies with the less toxic silver compounds, such as silver sulfide and silver chloride, reveal that accumulation of silver does not necessarily lead to adverse effects. At concentrations normally encountered in the environment, food-chain biomagnification of silver in aquatic systems is unlikely. Elevated silver concentrations in biota occur in the vicinities of sewage outfalls, electroplating plants, mine waste sites, and silver iodide-seeded areas. Maximum concentrations recorded in field collections, in milligrams total silver per kilogram dry weight (tissue), were 1.5 in marine mammals (liver) (except Alaskan beluga whales, which had concentrations 2 orders of magnitude higher than those of other marine mammals), 6 in fish (bone), 14 in plants (whole), 30 in annelid worms (whole), 44 in birds (liver), 110 in mushrooms (whole), 185 in bivalve molluscs (soft parts), and 320 in gastropods (whole).
In general, silver ion was less toxic to freshwater aquatic organisms under conditions of low dissolved silver ion concentration and increasing water pH, hardness, sulfides, and dissolved and particulate organic loadings; under static test conditions compared with flow-through regimens; and when animals were adequately nourished instead of being starved. Silver ions are very toxic to microorganisms. However, there is generally no strong inhibitory effect on microbial activity in sewage treatment plants because of reduced bioavailability due to rapid complexation and adsorption. The toxicity of silver to aquatic organisms is summarized in Figure 1; all values for silver nitrate from Table 2 in section 7 are included in this figure. Free silver ion was lethal to representative species of sensitive aquatic plants, invertebrates, and teleosts at nominal water concentrations of 1–5 µg/litre. Adverse effects occur on development of trout at concentrations as low as 0.17 µg/litre and on phytoplankton species composition and succession at 0.3–0.6 µg/litre.
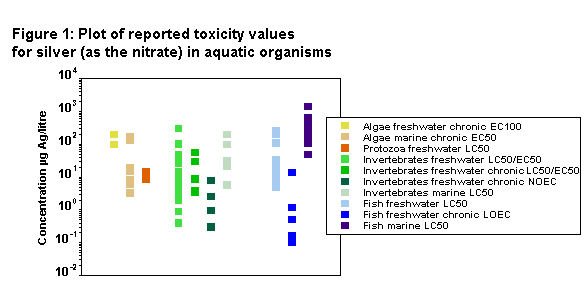
Note: Plotted values are from studies where silver was added to the medium as silver nitrate and the silver was likely to be present as the free ion (a scenario unlikely in the environment). A single study with silver sulfate gave an acute LC50 at 20 µg/litre for daphnids. Studies using other silver salts (sulfide, thiosulfate, and chloride) showed substantially lower toxicity, with acute values ranging from >1300 to >1 000 000 µg/litre.
Knowledge of the speciation of silver and its consequent bioavailability is crucial to understanding the potential risk of the metal. The only environmental compartment where a risk evaluation is possible is surface waters. The substantially lower toxicity reported from studies with marine/estuarine organisms (Figure 1) reflects the lower bioavailability in saline waters because of complexation with chloride ions. On the basis of available toxicity test results, it is unlikely that bioavailable free silver ions would ever be at sufficiently high concentrations to cause toxicity in marine environments. However, fewer marine studies than freshwater studies have been conducted. The values for marine organisms in Figure 1 reflect concentrations of total silver in the test medium rather than bioavailable silver. For freshwater organisms, most of the reported toxicity tests were conducted with silver nitrate, and the reported concentrations are taken to reflect free ion concentrations; there were few instances where this was actually measured. From Figure 1, chronic lowest- and no-observed-effect concentrations (LOECs and NOECs) for fish and invertebrates indicate effects at concentrations higher than about 0.1 µg free silver/litre.
Almost all values for silver concentrations in surface waters are expressed as total silver. These concentrations bear little relationship to the likely toxicity of the metal in surface waters. Measurement of free ionic silver is the only direct method that can be used to assess the likely effects of the metal on organisms. Speciation models could be used to assess the likely proportion of the total silver measured that was bioavailable to organisms. For example, the Biotic Ligand Model is being developed for use in evaluating how waterborne metal effect levels will vary with site water characteristics. Not only does the Biotic Ligand Model consider the effect of the dissolved metal concentration on toxicity, but it also considers metal speciation and hence bioavailability. Additionally, it incorporates the competitive interactions of metals and other cations with the organism at the site of action of toxicity. To date, the model has been used to predict the acute toxicity of silver in fathead minnows, rainbow trout, and daphnids to within a factor of 2 over a range of water quality conditions (Di Toro et al., 2001). McGeer et al. (2000) validated the Biotic Ligand Model for rainbow trout against 31 data sets in 10 studies and found it to be a good match with published acute silver nitrate toxicity data. Unlike some other metals, background concentrations in pristine and most urban areas (0.01 µg/litre; see above) are well below concentrations causing toxic effects. Levels in most industrialized areas, at around 0.1 µg/litre, border on the effect concentration, assuming that conditions favour bioavailability. The metal would be most bioavailable under conditions of low concentrations of anions (e.g., in soft waters), low concentrations of organic ligands, low suspended sediment, and lower pH. Point sources of release of silver could be seen as potentially exceeding toxic concentrations; however, actual toxic effects are highly dependent on the chemical form of the released silver and the local water chemistry of the receiving waters.
In general, accumulation of silver by terrestrial plants from soils is low, even if the soil is amended with silver-containing sewage sludge or the plants are grown on tailings from silver mines, where silver accumulates mainly in the root systems. Germination was the most sensitive stage for plants grown in culture solution; adverse effects on germination were expected at concentrations greater than 0.75 mg silver/litre (as silver nitrate) in the most sensitive species. In soils amended with silver sulfide and sewage sludge, the most sensitive plant species tested were adversely affected at 14 mg silver/kg dry weight soil. No data were found on effects of silver on wild birds or mammals. Silver was harmful to poultry (tested as silver nitrate) at concentrations as low as 100 mg total silver/litre in drinking-water or 200 mg total silver/kg in diets. Sensitive laboratory mammals were adversely affected at total silver concentrations as low as 250 µg/litre in drinking-water, 6 mg/kg in diets, or 13.9 mg/kg body weight. However, the significance of these LOECs is difficult to evaluate with regard to the natural environment, given uncertainties with respect to exposure and bioavailability.
No previous international evaluations of the environmental effects of silver or silver compounds were identified.
Abbe G, Sanders J (1990) Pathways of silver uptake and accumulation by the American oyster (Crassostrea virginica) in Chesapeake Bay. Estuarine, Coastal and Shelf Science, 31:113–123.
Adams N, Kramer J (1998) Reactivity of Ag+ ion with thiol ligands in the presence of iron sulfide. Environmental Toxicology and Chemistry, 17(4):625–629.
Adams N, Kramer J (1999a) Determination of silver speciation in wastewater and receiving waters by competitive ligand equilibration/
solvent extraction. Environmental Toxicology and Chemistry, 18(12):2674–2680.
Adams N, Kramer J (1999b) Silver speciation in wastewater effluent, surface waters, and pore waters. Environmental Toxicology and Chemistry, 18(12):2667–2673.
Alexander G, Young D (1976) Trace metals in southern California mussels. Marine Pollution Bulletin, 7:7–9.
Anderlini V (1974) The distribution of heavy metals in the red abalone, Haliotis rufescens, on the California coast. Archives of Environmental Contamination and Toxicology, 2:253–265.
Anderlini V (1992) The effect of sewage on trace metal concentrations and scope for growth in Mytilus edulis and Perna canaliculus from Wellington Harbour, New Zealand. Science of the Total Environment, 125:263–288.
ATSDR (1990) Toxicological profile for silver. Atlanta, GA, US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry (TP-90-24).
Bard C, Murphy J, Stone DL, Terhaar C (1976) Silver in photoprocessing effluents. Journal of the Water Pollution Control Federation, 48:389–394.
Becker PR, Mackey EA, Demiralp R, Suydam R, Early G, Koster BJ, Wise SA (1995) Relationship of silver with selenium and mercury in the liver of two species of toothed whales (Odontocetes). Marine Pollution Bulletin, 30(4):262–271.
Beglinger JM, Ruffing CJ (1997) Effects of silver sulfide on the terrestrial earthworm. In: Andren A, Bober T, eds. Transport, fate, and effects of silver in the environment. Proceedings of the 5th international conference. 28 September – 1 October 1997, Hamilton, Ontario. Madison, WI, University of Wisconsin Sea Grant Institute, pp. 313–314.
Bell R, Kramer J (1999) Structural chemistry and geochemistry of silver-sulfur compounds: Critical review. Environmental Toxicology and Chemistry, 18(1):9–22.
Berrow S (1991) Heavy metals in sediments and shellfish from Cork Harbour, Ireland. Marine Pollution Bulletin, 22:467–469.
Berry W, Cantwell M, Edwards P, Serbst J, Hansen D (1999) Predicting toxicity of sediments spiked with silver. Environmental Toxicology and Chemistry, 18(1):40–48.
Berthet B, Amiard J, Amiard-Triquet C, Martoja M, Jeantet A (1992) Bioaccumulation, toxicity and physico-chemical speciation of silver in bivalve molluscs: ecotoxicological and health consequences. The Science of the Total Environment, 125:97–122.
Birge W, Zuiderveen J (1996) The comparative toxicity of silver to aquatic biota. In: Andren W, Bober T, eds. Transport, fate and effects of silver in the environment. Abstracts of the 3rd international conference. 6–9 August 1995, Washington, DC. Madison, WI, University of Wisconsin Sea Grant Institute, pp. 79–85.
Bloom N, Crecilius E (1987) Distribution of silver, mercury, lead, copper and cadmium in central Puget Sound sediments. Marine Chemistry, 21:377–390.
Brooke L, Erickson R, Kahl M, Vande-Venter F, Harting S, Markee T, Stephan C, Spehar R (1994) Effects of laboratory test conditions on the toxicity of silver to aquatic organisms. In: Andren A, Bober T, eds. Transport, fate, and effects of silver in the environment. Proceedings of the 2nd international conference. 11–14 September 1994. Madison, WI, University of Wisconsin Sea Grant Institute, pp. 119–122.
Bruland K, Bertine K, Koide M, Goldberg E (1974) History of metal pollution in southern California coastal zone. Environmental Science and Technology, 8:425–432.
Bryan G (1973) The occurrence and seasonal variation of trace metals in the scallops Pecten maximus (L.) and Chlamys opercularis (L.). Journal of the Marine Biological Association of the United Kingdom, 53:145–166.
Bryan G, Hummerstone L (1977) Indicators of heavy metal contamination in the Looe estuary (Cornwall) with particular regard to silver and lead. Journal of the Marine Biological Association of the United Kingdom, 57:75–92.
Bryan G, Langston W (1992) Bioavailability, accumulation and effects of heavy metals in sediments with special reference to United Kingdom estuaries: a review. Environmental Pollution, 76:89–131.
Buhl K, Hamilton S (1991) Relative sensitivity of early life stages of Arctic grayling, coho salmon, and rainbow trout to nine inorganics. Ecotoxicology and Environmental Safety, 22:184–197.
Bury N, Wood C (1999) Mechanism of branchial apical silver uptake by rainbow trout is via the proton-coupled Na(+) channel. American Journal of Physiology — Regulatory Integrative and Comparative Physiology, 277(5):R1385–R1391.
Bury N, Galvez F, Wood C (1999a) Effects of chloride, calcium, and dissolved organic carbon on silver toxicity: Comparison between rainbow trout and fathead minnows. Environmental Toxicology and Chemistry, 18(1):56–62.
Bury N, Grosell M, Grover A, Wood C (1999b) ATP-dependent silver transport across the basolateral membrane of rainbow trout gills. Toxicology and Applied Pharmacology, 159(1):1–8.
Bury N, McGeer J, Wood C (1999c) Effects of altering freshwater chemistry on physiological responses of rainbow trout to silver exposure. Environmental Toxicology and Chemistry, 18(1):49–55.
Cain D, Luoma S (1990) Influence of seasonal growth, age, and environmental exposure on Cu and Ag in a bivalve indicator, Macoma balthica, in San Francisco Bay. Marine Ecology — Progress Series, 60:45–55.
Calabrese A, MacInnes J, Nelson D, Miller J (1977a) Survival and growth of bivalve larvae under heavy-metal stress. Marine Biology, 41:179–184.
Calabrese A, Thurberg F, Gould E (1977b) Effects of cadmium, mercury, and silver on marine animals. Marine Fisheries Review, 39:5–11.
Calabrese A, MacInnes J, Nelson D, Greig R, Yevich P (1984) Effects of long term exposure to silver or copper on growth, bioaccumulation and histopathology in the blue mussel Mytilus edulis. Marine Environmental Research, 11:253–274.
Call D, Polkinghorne C, Markee T, Brooke L, Geiger D, Gorsuch J, Robillard K (1999) Silver toxicity to Chironomus tentans in two freshwater sediments. Environmental Toxicology and Chemistry, 18(1):30–39.
Coleman R, Cearley J (1974) Silver toxicity and accumulation in largemouth bass and bluegill. Bulletin of Environmental Contamination and Toxicology, 12:53–61.
Connell D, Sanders J, Riedel G, Abbe G (1991) Pathways of silver uptake and trophic transfer in estuarine organisms. Environmental Science and Technology, 25:921–924.
Crecelius E (1993) The concentration of silver in mussels and oysters from NOAA National Status and Trends Mussel Watch sites. In: Andren A, Bober T, Crecelius E, Kramer J, Luoma S, Rodgers J, Sodergren A, eds. Transport, fate, and effects of silver in the environment. Proceedings of the 1st international conference. 8–10 August 1993. Madison, WI, University of Wisconsin Sea Grant Institute, pp. 65–66.
Daskalakis K (1996) Silver in oyster soft tissue: relations to site selection and sampling size. In: Andren A, Bober T, eds. Transport, fate, and effects of silver in the environment. Abstracts of the 3rd international conference. 6–9 August 1995, Washington, DC. Madison, WI, University of Wisconsin Sea Grant Institute.
Davies P, Goettl J, Sinley J (1978) Toxicity of silver to rainbow trout. Water Research, 12:113–117.
DEC (1993) Lower Genesee River Project. Phase 1 report — summary 1992 results. Albany, NY, New York State Department of Environmental Conservation, 147 pp.
Diamond J, Mackler D, Collins M, Gruber D (1990) Derivation of freshwater silver criteria for the New River, Virginia, using representative species. Environmental Toxicology and Chemistry, 9:1425–1434.
Di Toro DM, Kavvadas CD, Mathew R, Paquin PR, Winfield RP (2001) The persistence and availability of metals in aquatic environments. Ottawa, Ontario, International Council on Metals and the Environment, 67 pp.
DiVincenzo G, Giordano C, Schriever L (1985) Biological monitoring of workers exposed to silver. International Archives of Occupational and Environmental Health, 56:207–215.
Domsch K (1984) Effects of pesticides and heavy metals on biological processes in soil. Plant and Soil, 76: 367–378.
Eisler R (1981) Trace metal concentrations in marine organisms. New York, NY, Pergamon Press, 687 pp.
Eisler R (1997) Silver hazards to fish, wildlife and invertebrates: A synoptic review. Washington, DC, US Department of the Interior, National Biological Service, 44 pp. (Biological Report 32 and Contaminant Hazard Reviews Report 32).
Eisler R (2000) Silver. In: Handbook of chemical risk assessment. Health hazards to humans, plants, and animals. Volume 1. Metals. Boca Raton, FL, Lewis Publishers, pp. 499–550.
Eisler R, Barry M, Lapan R, Telek G, Davey E, Soper A (1978) Metal survey of the marine clam Pitar morrhuana collected near a Rhode Island (USA) electroplating plant. Marine Biology, 45:311–317.
Erickson R, Brooke L, Kahl M, Venter F, Harting S, Markee T, Spehar R (1998) Effects of laboratory test conditions on the toxicity of silver to aquatic organisms. Environmental Toxicology and Chemistry, 17(4):572–578.
Falandysz J, Danisiewicz D (1995) Bioconcentration factors (BCF) of silver in wild Agaricus campestris. Bulletin of Environmental Contamination and Toxicology, 55:122–129.
Ferguson E, Hogstrand C (1998) Acute silver toxicity to seawater-acclimated rainbow trout: Influence of salinity on toxicity and silver speciation. Environmental Toxicology and Chemistry, 17(4):589–593.
Fisher N, Wang W (1998) Trophic transfer of silver to marine herbivores: A review of recent studies. Environmental Toxicology and Chemistry, 17(4):562–571.
Fisher N, Wang W, Reinfelder J, Luoma S (1994) Bioaccumulation of silver in marine bivalves. In: Andren A, Bober T, eds. Transport, fate, and effects of silver in the environment. Proceedings of the 2nd international conference. 11–14 September 1994. Madison, WI, University of Wisconsin Sea Grant Institute, pp. 139–140.
Forsythe BL, Cobb GP, La Point TW, Klaine S (1996) The bioconcentration and bioaccumulation of silver in an experimental freshwater ecosystem. In: Andren A, Bober T, eds. Transport, fate, and effects of silver in the environment. Proceedings of the 4th international conference. 25–28 August 1996. Madison, WI, University of Wisconsin Sea Grant Institute, pp. 185–188.
Fortin C, Campbell P (2000) Silver uptake by the green alga Chlamydomonas reinhardtii in relation to chemical speciation: Influence of chloride. Environmental Toxicology and Chemistry, 19(11):2769–2778.
Fowler B, Nordberg G (1986) Silver. In: Friberg L, Nordberg G, Vouk V, eds. Handbook on the toxicology of metals. Volume II. Specific metals. New York, NY, Elsevier, pp. 521–530.
Fowler S, Oregioni B (1976) Trace metals in mussels from the N.W. Mediterranean. Marine Pollution Bulletin, 7:26–29.
Freeman R (1979) Ecological kinetics of silver in an alpine lake ecosystem. In: Marking L, Kimerle R, eds. Aquatic toxicology. Proceedings of the 2nd annual symposium on aquatic toxicology. Philadelphia, PA, American Society for Testing and Materials, pp. 342–358 (ASTM Special Technical Publication 667).
Garnier J, Baudin J, Foulquier L (1990) Accumulation from water and depuration of 110mAg by a freshwater fish, Salmo trutta L. Water Research, 24:1407–1414.
Gill GA, Wen L-S, Lehman R, Tang D, Santschi P (1997) Silver in Colorado watersheds. In: Andren A, Bober T, eds. Transport, fate, and effects of silver in the environment. Proceedings of the 5th international conference. 28 September – 1 October 1997, Hamilton, Ontario. Madison, WI, University of Wisconsin Sea Grant Institute, pp. 155–160.
Gould E, MacInnes J (1977) Short-term effects of two silver salts on tissue respiration and enzyme activity in the cunner (Tautoglolabrus adspersus). Bulletin of Environmental Contamination and Toxicology, 18:401–408.
Greig R (1975) Comparison of atomic absorption and neutron activation analyses for determination of silver, chromium, and zinc in various marine organisms. Analytical Chemistry, 47:1682–1684.
Greig R (1979) Trace metal uptake by three species of mollusks. Bulletin of Environmental Contamination and Toxicology, 22:643–647.
Greig R, Wenzloff D (1977) Trace metals in finfish from the New York Bight and Long Island Sound. Marine Pollution Bulletin, 8:198–200.
Greig RA, Wenzloff DR, Pearce JB (1976) Distribution and abundance of heavy metals in finfish, invertebrates, and sediments collected at a deepwater disposal site. Marine Pollution Bulletin, 7:185–187.
Greig R, Adams A, Wenzloff D (1977a) Trace metal content of plankton and zooplankton collected from the New York Bight and Long Island Sound. Bulletin of Environmental Contamination and Toxicology, 18:3–8.
Greig R, Wenzloff D, Nelson B, Shelpuk C (1977b) Trace metals in organisms from ocean disposal sites of the middle eastern United States. Archives of Environmental Contamination and Toxicology, 6:395–409.
Grosell M, Hogstrand C, Wood C, Hansen H (2000) A nose-to-nose comparison of the physiological effects of exposure to ionic silver versus silver chloride in the European eel (Anguilla anguilla) and the rainbow trout (Oncorhynchus mykiss). Aquatic Toxicology, 48(2–3):327–342.
Guadagnolo C, Brauner C, Wood C (2001) Chronic effects of silver exposure on ion levels, survival, and silver distribution within developing rainbow trout (Oncorhynchus mykiss) embryos. Environmental Toxicology and Chemistry, 20(3):553–560.
Hellou J, Warren W, Payne J, Belkhode S, Lobel P (1992) Heavy metals and other elements in three tissues of cod, Gadus morhua from the northwest Atlantic. Marine Pollution Bulletin, 24:452–458.
Hirsch M (1998a) Availability of sludge-borne silver to agricultural crops. Environmental Toxicology and Chemistry, 17(4):610–616.
Hirsch M (1998b) Bioaccumulation of silver from laboratory-spiked sediments in the oligochaete (Lumbriculus variegatus). Environmental Toxicology and Chemistry, 17(4):605–609.
Hirsch M (1998c) Toxicity of silver sulfide-spiked sediments to the freshwater amphipod (Hyalella azteca). Environmental Toxicology and Chemistry, 17(4):601–604.
Hirsch M, Ritter M, Roser K, Garrisi P, Forsythe S (1993) The effect of silver on plants grown in sludge-amended soils. In: Andren A, Bober T, Crecelius E, Kramer J, Luoma S, Rodgers J, Sodergren A, eds. Transport, fate, and effects of silver in the environment. Proceedings of the 1st international conference. 8–10 August 1993. Madison, WI, University of Wisconsin Sea Grant Institute, pp. 69–73.
Hogstrand C, Wood C (1998) Toward a better understanding of the bioavailability, physiology and toxicity of silver in fish: Implications for water quality criteria. Environmental Toxicology and Chemistry, 17(4):547–561.
Hogstrand C, Galvez F, Wood C (1996) Toxicity, silver accumulation and metallothionein induction in freshwater rainbow trout during exposure to different silver salts. Environmental Toxicology and Chemistry, 15:1102–1108.
Hook S, Fisher N (2001) Sublethal effects of silver in zooplankton: importance of exposure pathways and implications for toxicity testing. Environmental Toxicology and Chemistry, 20(3):568–574.
Hornberger M, Luoma MS, Cain D, Parchaso F, Brown C, Bouse R, Wellise C, Thompson J (1999) Bioaccumulation of metals by the bivalve Macoma balthica at a site in South San Francisco Bay between 1977 and 1997: long term trends and associated biological effects with changing pollutant loadings. US Geological Survey (USGS Open File Report 99-55) [cited in Luoma et al., 2001].
IPCS (1999a) International Chemical Safety Card — Silver. Geneva, World Health Organization, International Programme on Chemical Safety (ICSC 0810).
IPCS (1999b) International Chemical Safety Card — Silver nitrate. Geneva, World Health Organization, International Programme on Chemical Safety (ICSC 1116).
Janes N, Playle R (1995) Modeling silver binding to gills of rainbow trout (Oncorhynchus mykiss). Environmental Toxicology and Chemistry, 14:1847–1858.
Jeanthon C, Prieur D (1990) Susceptibility to heavy metals and characterization of heterotrophic bacteria isolated from two hydrothermal vent polychaete annelids, Alvinella pompejana and Alvinella caudata. Applied and Environmental Microbiology, 56:3308–3314.
Karen D, Ownby D, Forsythe B, Bills T, La Point T, Cobb G, Klaine S (1999) Influence of water quality on silver toxicity to rainbow trout (Oncorhynchus mykiss), fathead minnows (Pimephales promelas), and water fleas (Daphnia magna). Environmental Toxicology and Chemistry, 18(1):63–70.
Kouadio I, Kirschenbaum L, Mehrota R (1990) The oxidation of iodide by the tetrahydroxoargentate (III) ion in aqueous alkaline media. Journal of the Chemical Society, Dalton Transactions, 1990:1928–1933.
Lande E (1977) Heavy metal pollution in Trondheimsfjorden, Norway, and the recorded effects on the fauna and flora. Environmental Pollution, 12:187–198.
LeBlanc G, Mastone J, Paradice A, Wilson B, Lockhart H, Robillard K (1984) The influence of speciation on the toxicity of silver to fathead minnow (Pimephales promelas). Environmental Toxicology and Chemistry, 3:37–46.
Leonhardt K, Pfeiffer W (1985) Die Wirkung von Schwermetallen im Klarschlamm- Kupfer, Zink und Silber. Berichte – Wassergüte Wirtschaft und Gesundheitsingenieurwesen Technische Universitat Munchen, 62: 61–155.
Lima A, Curtis C, Hammermeister D, Call D, Felhaber T (1982) Acute toxicity of silver to selected fish and invertebrates. Bulletin of Environmental Contamination and Toxicology, 29:184–189.
Luoma S (1994) Fate, bioavailability and toxicity of silver in estuarine environments. In: Andren A, Bober T, eds. Transport, fate and effects of silver in the environment. Proceedings of the 2nd international conference. 11–14 September 1994. Madison, WI, University of Wisconsin Sea Grant Institute, pp. 151–155.
Luoma S, Jenne E (1977) The availability of sediment-bound cobalt, silver, and zinc to a deposit-feeding clam. In: Drucker H, Wildung R, eds. Biological implications of metals in the environment. Washington, DC, US Energy Research and Development Administration, pp. 213–230 (ERDA Symposium Series 42).
Luoma S, Phillips D (1988) Distribution, variability, and impacts of trace elements in San Francisco Bay. Marine Pollution Bulletin, 19:413–425.
Luoma SN, Hogstrand C, Bell RA, Bielmyer GK, Galvez F, LeBlanc GA, Lee BG, Purcell TW, Santore RC, Santschi PH, Shaw JR (2001) Biological processes. In: Andren A, Bober T, eds. Transport, fate, and effects of silver in the environment. Proceedings of the 6th international conference. 21–25 August 1999. Madison, WI, University of Wisconsin Sea Grant Institute, pp. 71–100.
Lytle P (1984) Fate and speciation of silver in publicly owned treatment works. Environmental Toxicology and Chemistry, 3:21–30.
Martin J, Elliot P, Anderlini V, Girvin D, Jacobs S, Risebrough R, Delong R, Gilmartin W (1976) Mercury–selenium–bromine imbalance in premature parturient California sea lions. Marine Biology, 35:91–104.
Martin M, Stephenson M, Smith D, Gitierrez-Galindo E, Munoz G (1988) Use of silver in mussels as a tracer of domestic wastewater discharge. Marine Pollution Bulletin, 19:512–520.
McGeer JC, Playle RC, Wood CM, Galvez F (2000) A physiologically based biotic ligand model for predicting the acute toxicity of waterborne silver to rainbow trout in freshwaters. Environmental Science and Technology, 34:4199–4207.
Miramand P, Bentley D (1992) Concentration and distribution of heavy metals in tissues of two cephalopods, Eledone cirrhosa and Sepia officinalis, from the French coast of the English Channel. Marine Biology, 114:407–414.
Morgan L, Galvez F, Munger R, Wood C, Henry R (1996) The physiological effects of acute silver exposure in rainbow trout (Oncorhynchus mykiss). In: Andren A, Bober T, eds. Transport, fate, and effects of silver in the environment. Abstracts of the 3rd international conference. 6–9 August 1995, Washington, DC. Madison, WI, University of Wisconsin Sea Grant Institute.
Morse J, Presley B, Taylor R, Benoit G, Santschi P (1993) Trace metal chemistry of Galveston Bay: water, sediment, and biota. Marine Environmental Research, 36:1–37.
Nalecz-Jawecki G, Demkowicz-Dobrzanski K, Sawicki J (1993) Protozoan Spirostomum ambiguum as a highly sensitive bioindicator for rapid and easy determination of water quality. The Science of the Total Environment, Supplement 2:1227–1234.
NAPM (1974) Environmental effect of photoprocessing chemicals. Volume 1. New York, NY, National Association of Photographic Manufacturers.
Navrot J, Amiel A, Kronfeld J (1974) Patella vulgata: a biological monitor of coastal metal pollution — a preliminary study. Environmental Pollution, 7:303–308.
Nebeker A, McAuliffe C, Mshar R, Stevens D (1983) Toxicity of silver to steelhead and rainbow trout, fathead minnows and Daphnia. Environmental Toxicology and Chemistry, 2:95–104.
Nehring R (1976) Aquatic insects as biological monitors of heavy metal pollution. Bulletin of Environmental Contamination and Toxicology, 15:147–154.
Nelson D, Calabrese A, Nelson B, MacInnes J, Wenzloff D (1976) Biological effects of heavy metals on juvenile bay scallops, Argopecten irradians, in short-term exposures. Bulletin of Environmental Contamination and Toxicology, 16:275–282.
Nelson D, Calabrese A, Greig R, Yevich P, Chang S (1983) Long-term silver effects on the marine gastropod Crepidula fornicata. Marine Ecology — Progress Series, 12:155–165.
Ownby DR, Karen DJ, Shupack DP, Day BS, La Point TW, Klaine SJ, Cobb GP (1997) Using spectroscopy and voltammetry to evaluate silver activity in aquatic toxicity evaluations. In: Andren A, Bober T, eds. Transport, fate, and effects of silver in the environment. Proceedings of the 5th international conference. 28 September – 1 October 1997, Hamilton, Ontario. Madison, WI, University of Wisconsin Sea Grant Institute, pp. 59–62.
Pavlostathis S, Maeng S (1998) Aerobic biodegradation of a silver-bearing photoprocessing wastewater. Environmental Toxicology and Chemistry, 17(4):617–624.
Pavlostathis S, Maeng S (2000) Fate and effect of silver on the anaerobic digestion process. Water Research, 34(16): 3957–3966.
Pentreath R (1977) The accumulation of 110mAg by the plaice, Pleuronectes platessa L. and the thornback ray, Raja clavata L. Journal of Experimental Marine Biology and Ecology, 29:315–325.
Pouvreau B, Amiard J (1974) Etude experimentale de l’accumulation de l’argent 110m chez divers organismes marine. Paris, Commissariat à l’Energie Atomique (Report CEA-R-4571).
Presley B, Taylor R, Boohe P (1990) Trace metals in Gulf of Mexico oysters. The Science of the Total Environment, 97/98:551–593.
Pullen J, Rainbow P (1991) The composition of pyrophosphate heavy metal detoxification granules in barnacles. Journal of Experimental Marine Biology and Ecology, 150:249–266.
Purcell TW, Peters JJ (1998) Sources of silver in the environment. Environmental Toxicology and Chemistry, 17(4): 539–546.
Rains T, Watters R, Epstein M (1984) Application of atomic absorption and plasma emission spectrometry for environmental analysis. Environment International, 10:163–168.
Ratte HT (1998) Silberverbindungen – Umweltverhalten, Bioakkumulation und Toxizität. Landsberg, Ecomed Vlg.
Ratte H (1999) Bioaccumulation and toxicity of silver compounds: A review. Environmental Toxicology and Chemistry, 18(1):89–108.
Reinfelder J, Chang S (1999) Speciation and microalgal bioavailability of inorganic silver. Environmental Science and Technology, 33(11):1860–1863.
Rodgers J, Deaver E, Rogers P (1994) Evaluations of the bioavailability and toxicity of silver in sediment. In: Andren A, Bober T, eds. Transport, fate, and effects of silver in the environment. Proceedings of the 2nd international conference. 11–14 September 1994. Madison, WI, University of Wisconsin Sea Grant Institute, pp. 131–137.
Rombough P (1985) The influence of the zona radiata on the toxicities of zinc, lead, mercury, copper and silver ions to embryos of steelhead trout Salmo gairdneri. Comparative Biochemistry and Physiology, 82C:115–117.
Rose K, Summers J, Mclean R, Domotor S (1988) Radiosilver (Ag-110m) concentrations in Chesapeake Bay oysters maintained near a nuclear powerplant: a statistical analysis. Environmental Monitoring and Assessment, 10:205–218.
Rouleau C, Gobeil C, Tjälve H (2000) Accumulation of silver from the diet in two marine benthic predators: the snow crab (Chionoecetes opilio) and the American plaice (Hippoglossoides platessoides). Environmental Toxicology and Chemistry, 19(3):631–637.
Saeki K, Nakajima M, Loughlin T, Calkins D, Baba N, Kiyota M, Tatsukawa R (2001) Accumulation of silver in the liver of three species of pinnipeds. Environmental Pollution, 112(1):19–25.
Sanders J, Abbe G (1987) The role of suspended sediments and phytoplankton in the partitioning and transport of silver in estuaries. Continental Shelf Research, 7:1357–1361.
Sanders J, Abbe G (1989) Silver transport and impact in estuarine and marine systems. In: Suter G, Lewis M, eds. Aquatic toxicology and environmental fate. Volume 11. Philadelphia, PA, American Society for Testing and Materials, pp. 5–18 (ASTM Special Technical Publication 1007).
Sanders J, Cibik S (1988) Response of Chesapeake Bay phytoplankton communities to low levels of toxic substances. Marine Pollution Bulletin, 19:439–444.
Sanders J, Abbe G, Riedel G (1990) Silver uptake and subsequent effects on growth and species composition in an estuarine community. The Science of the Total Environment, 97/98:761–769.
Sanders J, Riedel G, Abbe G (1991) Factors controlling the spatial and temporal variability of trace metal concentrations in Crassostrea virginica (Gmelin). In: Elliot M, Ducrotoy J, eds. Estuaries and coasts: spatial and temporal intercomparisons. Proceedings of the Estuarine and Coastal Sciences Association Symposium, 4–8 September 1989, University of Caen, France. Fredensborg, Olsen & Olsen, pp. 335–339 (ECSA Symposium 19).
Schildkraut D (1993) Application of analytical voltammetric methods to the determination of silver at sub ng/mL levels. In: Andren A, Bober T, Crecelius E, Kramer J, Luoma S, Rodgers J, Sodergren A, eds. Transport, fate, and effects of silver in the environment. Proceedings of the 1st international conference. 8–20 August 1993. Madison, WI, University of Wisconsin Sea Grant Institute, pp. 5–7.
Schildkraut D, Dao P, Twist J, Davis A, Robillard K (1998) Determination of silver ions at sub microgram-per-liter levels using anodic square-wave stripping voltammetry. Environmental Toxicology and Chemistry, 17(4):642–649.
Scow K, Goyer M, Nelken L et al. (1981) Exposure and risk assessment for silver. Technical report prepared for Office of Water Regulations and Standards, US Environmental Protection Agency, Washington, DC, by Arthur D. Little, Inc., Cambridge, Massachusetts (PB85-211993).
Shafer M, Overdier J, Armstrong D (1998) Removal, partitioning, and fate of silver and other metals in wastewater treatment plants and effluent-receiving streams. Environmental Toxicology and Chemistry, 17(4):630–641.
Shaw J, Wood C, Birge W, Hogstrand C (1998) Toxicity of silver to the marine teleost (Oligocottus maculosus): Effects of salinity and ammonia. Environmental Toxicology and Chemistry, 17(4):594–600.
Silver Institute (2000) World Silver Survey 2000. Washington, DC, The Silver Institute.
Smith I, Carson B (1977) Trace metals in the environment. Volume 2. Silver. Ann Arbor, MI, Ann Arbor Science Publishers, 469 pp.
Song S, Osteryoung J (1993) Determination of silver (I) ion by anodic tripping in flow injection system. In: Andren A, Bober T, Crecelius E, Kramer J, Luoma S, Rodgers J, Sodergren A, eds. Transport, fate, and effects of silver in the environment. Proceedings of the 1st international conference. 8–10 August 1993. Madison, WI, University of Wisconsin Sea Grant Institute, pp. 109–111.
Starkey B, Taylor A, Walker A (1987) Measurement of silver in blood by electrothermal atomic absorption spectrometry (ET-AAS). Annals of Clinical Biochemistry, 24:191–193.
Stephenson J, Leonard G (1994) Evidence for the decline of silver and lead and the increase of copper from 1977 to 1990 in the coastal marine waters of California. Marine Pollution Bulletin, 28:148–153.
Sun Y, Kirshenbaum L, Kouadio I (1991) Kinetics and mechanism of the multi-step oxidation of ethylenediaminetetracetate by [Ag(OH)4]– in alkaline media. Journal of the Chemical Society, Dalton Transactions, 1991:2311–2315.
Szefer P, Pempkowiak J, Skwarzec B, Bojanowski R, Holm E (1993) Concentration of selected metals in penguins and other representative fauna of the Antarctica. The Science of the Total Environment, 138:281–288.
Szefer P, Szefer K, Pempkowiak J, Skwarzec B, Bojanowski R, Holm E (1994) Distribution and coassociations of selected metals in seals of the Antarctic. Environmental Pollution, 83:341–349.
Terhaar C, Ewell W, Dziuba S, White W, Murphy P (1977) A laboratory model for evaluating the behavior of heavy metals in an aquatic environment. Water Research, 11:101–110.
Thurberg F, Calabrese A, Dawson M (1974) Effects of silver on oxygen consumption of bivalves at various salinities. In: Vernberg F, Vernberg W, eds. Pollution and physiology of marine organisms. New York, NY, Academic Press, pp. 67–78.
TRI (1999) Toxic Release Inventory. US Environmental Protection Agency (http://www.epa.gov/TRI).
US EPA (1980) Ambient water quality criteria for silver. Washington, DC, US Environmental Protection Agency (440/5-80-071).
Webb N, Wood C (1998) Physiological analysis of the stress response associated with acute silver nitrate exposure in freshwater rainbow trout (Oncorhynchus mykiss). Environmental Toxicology and Chemistry, 17(4):579–588.
Webb N, Wood C (2000) Bioaccumulation and distribution of silver in four marine teleosts and two marine elasmobranchs: influence of exposure duration, concentration, and salinity. Aquatic Toxicology, 49(1–2):111–129.
Wen L-S, Tang D, Lehman R, Gill G, Santschi P (1997) Dissolved and colloidal Ag in natural waters — analytical aspects. In: Andren A, Bober T, eds. Transport, fate, and effects of silver in the environment. Proceedings of the 5th international conference. 28 September – 1 October 1997, Hamilton, Ontario. Madison, WI, University of Wisconsin Sea Grant Institute, pp. 415–420.
Whitlow S, Rice D (1985) Silver complexation in river waters of central New York. Water Research, 19:619–626.
Williams P, Dusenbery D (1990) Aquatic toxicity testing using the nematode, Caernorhabditis elegans. Environmental Toxicology and Chemistry, 9:1285–1290.
Wingert-Runge B, Andren A (1994) Desorption behavior of silver from natural sediments under freshwater and marine conditions. In: Andren A, Bober T, eds. Transport, fate, and effects of silver in the environment. Proceedings of the 2nd international conference. 11–14 September 1994. Madison, WI, University of Wisconsin Sea Grant Institute, pp. 89–93.
Wood C, Munger S, Galvez F, Hogstrand C (1994) The physiology of silver toxicity in freshwater fish. In: Andren A, Bober T, eds. Transport, fate, and effects of silver in the environment. Proceedings of the 2nd international conference. 11–14 September 1994. Madison, WI, University of Wisconsin Sea Grant Institute, pp. 109–114.
Wood C, Hogstrand C, Galvez F, Munger R (1996a) The physiology of waterborne silver toxicity in freshwater rainbow trout (Oncorhynchus mykiss): 1. The effects of ionic Ag+. Aquatic Toxicology, 35:93–109.
Wood C, Hogstrand C, Galvez F, Munger R (1996b) The physiology of waterborne silver toxicity in freshwater rainbow trout (Oncorhynchus mykiss): 2. The effects of silver thiosulfate. Aquatic Toxicology, 35:111–125.
Wood C, Morgan I, Galvez F, Hogstrand C (1996c) The toxicity of silver in fresh and marine waters. In: Andren A, Bober T, eds. Transport, fate, and effects of silver in the environment. Abstracts of the 3rd international conference. 6–9 August 1995, Washington, DC. Madison, WI, University of Wisconsin Sea Grant Institute.
Wood C, Playle R, Hogstrand C (1999) Physiology and modeling of mechanisms of silver uptake and toxicity in fish. Environmental Toxicology and Chemistry, 18(1):71–83.
Eisler R (1997) Silver hazards to fish, wildlife, and invertebrates: A synoptic review. Washington, DC, US Department of the Interior, National Biological Service, 44 pp. (Biological Report 32 and Contaminant Hazard Reviews Report 32)
The source document was peer reviewed by three internal reviewers and three external reviewers. Evidence of satisfactory response by the author to comments by reviewers was approved by the Assistant Director of the US Geological Survey Patuxent Wildlife Research Center before the manuscript was officially approved for release.
The draft CICAD on silver and silver compounds was sent for review to institutions and organizations identified by IPCS after contact with IPCS national Contact Points and Participating Institutions, as well as to identified experts. Comments were received from:
R. Benson, Drinking Water Program, US Environmental Protection Agency, Denver, CO, USA
C. Cubbison, National Center for Environmental Assessment, US Environmental Protection Agency, Cincinnati, OH, USA
J.W. Gorsuch, Eastman Kodak Company, Rochester, NY, USA
C. Hiremath, National Center for Environmental Assessment, US Environmental Protection Agency, Washington, DC, USA
J. Kielhorn, Fraunhofer Institute of Toxicology and Aerosol Research, Hanover, Germany
G. Koennecker, Fraunhofer Institute of Toxicology and Aerosol Research, Hanover, Germany
S. Tao, Center for Food Safety and Applied Nutrition, Food and Drug Administration, College Park, MD, USA
J. Temmink, Wageningen University, Wageningen, The Netherlands
M. Vojtisek, National Institute of Public Health, Prague, Czech Republic
Ottawa, Canada,
29 October – 1 November 2001
Members
Mr R. Cary, Health and Safety Executive, Merseyside, United Kingdom
Dr T. Chakrabarti, National Environmental Engineering Research Institute, Nehru Marg, India
Dr B.-H. Chen, School of Public Health, Fudan University (formerly Shanghai Medical University), Shanghai, China
Dr R. Chhabra, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC, USA (teleconference participant)
Dr C. De Rosa, Agency for Toxic Substances and Disease Registry, Department of Health and Human Services, Atlanta, GA, USA (Chairman)
Dr S. Dobson, Centre for Ecology and Hydrology, Huntingdon, Cambridgeshire, United Kingdom (Vice-Chairman)
Dr O. Faroon, Agency for Toxic Substances and Disease Registry, Department of Health and Human Services, Atlanta, GA, USA
Dr H. Gibb, National Center for Environmental Assessment, US Environmental Protection Agency, Washington, DC, USA
Ms R. Gomes, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario, Canada
Dr M. Gulumian, National Centre for Occupational Health, Johannesburg, South Africa
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers and Veterinary Medicine, Berlin, Germany
Dr A. Hirose, National Institute of Health Sciences, Tokyo, Japan
Mr P. Howe, Centre for Ecology and Hydrology, Huntingdon, Cambridgeshire, United Kingdom (Co-Rapporteur)
Dr J. Kielhorn, Fraunhofer Institute of Toxicology and Aerosol Research, Hanover, Germany (Co-Rapporteur)
Dr S.-H. Lee, College of Medicine, The Catholic University of Korea, Seoul, Korea
Ms B. Meek, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario, Canada
Dr J.A. Menezes Filho, Faculty of Pharmacy, Federal University of Bahia, Salvador, Bahia, Brazil
Dr R. Rolecki, Nofer Institute of Occupational Medicine, Lodz, Poland
Dr J. Sekizawa, Division of Chem-Bio Informatics, National Institute of Health Sciences, Tokyo, Japan
Dr S.A. Soliman, Faculty of Agriculture, Alexandria University, Alexandria, Egypt
Dr M.H. Sweeney, Document Development Branch, Education and Information Division, National Institute for Occupational Safety and Health, Cincinnati, OH, USA
Dr J. Temmink, Department of Agrotechnology & Food Sciences, Wageningen University, Wageningen, The Netherlands
Ms D. Willcocks, National Industrial Chemicals Notification and Assessment Scheme (NICNAS), Sydney, Australia
Representative of the European Union
Dr K. Ziegler-Skylakakis, European Commission, DG Employment and Social Affairs, Luxembourg
Observers
Dr R.M. David, Eastman Kodak Company, Rochester, NY, USA
Dr R.J. Golden, ToxLogic LC, Potomac, MD, USA
Mr J.W. Gorsuch, Eastman Kodak Company, Rochester, NY, USA
Mr W. Gulledge, American Chemistry Council, Arlington, VA, USA
Mr S.B. Hamilton, General Electric Company, Fairfield, CN, USA
Dr J.B. Silkworth, GE Corporate Research and Development, Schenectady, NY, USA
Dr W.M. Snellings, Union Carbide Corporation, Danbury, CN, USA
Dr E. Watson, American Chemistry Council, Arlington, VA, USA
Secretariat
Dr A. Aitio, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
Mr T. Ehara, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
Dr P. Jenkins, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
Ce CICAD relatif à l’argent et à ses dérivés (aspects environnementaux) a été préparé par le Centre for Ecology and Hydrology (Centre d’écologie et d’hydrologie) de Monks Wood (Royaume-Uni). Il est basé sur un Contaminant Hazard Reviews document du Department of the Interior des Etats-Unis intitulé : Silver hazards to fish, wildlife, and invertebrates: A synoptic review (Eisler, 1997), mis à jour avec référence aux travaux d’Eisler (Eisler, 2000) et complété par une recherche bibliographique datée d’avril 2001. Ce document ne concerne pas les effets de l’argent sur la santé humaine; on pourra trouver une mise au point à ce sujet dans l’ATDSR (1990). Des renseignements sur la nature de l’examen par des pairs et la disponibilité du document de base sont donnés à l’appendice 1. Des informations concernant l’examen par des pairs du présent CICAD figurent à l’appendice 2. Ce CICAD a été approuvé en tant qu’évaluation internationale lors d’une réunion du Comité d’évaluation finale qui s’est tenue à Ottawa (Canada), du 29 octobre au 1er novembre 2001. Les fiches internationales sur la sécurité chimique de l’argent (ICSC 0810) et du nitrate d’argent (ICSC 1116) établies par le Programme international sur la sécurité chimique (IPCS, 1999a, 1999b) sont également reproduites dans le présent document.
L’argent est un métal rare, souvent présent dans la nature à l’état natif mais pouvant également accompagner d’autres métaux dans leurs minerais. Les émissions produites lors de la fusion ou lors de la fabrication et du rejet de certains produits photographiques et appareillages électriques, comptent parmi les sources anthropogéniques d’argent présentes dans la biosphère. Le cycle biogéochimique de l’argent se caractérise par des émissions et des décharges dans l’atmosphère, les eaux et le sol provenant de sources naturelles ou anthropogéniques, par le transport sur de longues distances de fines particules aéroportées, par des dépôts à sec ou par voie humide ou encore par la sorption des diverses espèces chimiques dans les sols et les sédiments.
Les dosages les plus récents effectués sur des eaux fluviales, lacustres et estuarielles en utilisant des techniques de prélèvement propres révèlent des teneurs de l’ordre de 0,01 µg/litre dans les zones préservées et non polluées et de 0,01 à 0,1 µg/litre dans zones urbaines et industrielles. Les résultats des dosages effectués avant que ne soient utilisées des techniques de prélèvement ultra-propres, c’est-à-dire avant la fin des années 1980, doivent être considérés avec prudence. La concentration maximale d’argent total mesurée au cours des années 1970 et 1980 dans divers échantillons non biologiques, s’établissait comme suit : 36,5 ng/m3 à proximité d’une fonderie, 2,0 µg/m3 dans des poussières aéroportées, 0,1 µg/litre dans des saumures de puits de pétrole, 4,5 µg/litre dans des précipitations provenant de nuages ensemencés avec de l’iodure d’argent, 6,0 µg/litre dans des eaux souterraines à proximité d’une décharge dangereuse, 8,9 µg/litre dans de l’eau de mer prélevée dans la baie de Galveston, aux Etats-Unis, 260 µg/litre près de décharges utilisées par une fabrique de produits photographiques, 300 µg/litre dans des puits de vapeur, 300 µg/litre dans des eaux résiduaires traitées provenant d’une installation de développement photographique, 31 mg/kg dans des sols, 43 mg/litre dans l’eau de certaines sources chaudes, 50 mg/kg dans du granit, jusqu’à 100 mg/kg dans du pétrole brut et 150 mg/kg dans les sédiments de cours d’eau. Il est à noter que la concentration de l’argent dans l’environnement a diminué. Par exemple, dans le cours inférieur de la rivière Genesee, aux Etats-Unis, à proximité d’une fabrique de produits photographiques, la concentration est tombée de 260 µg/litre au cours des années 1970 à une teneur inférieure à la limite de détection (<10 µg/litre) au cours des années 1990. On notera également que seule une petite fraction de l’argent total présent dans chacun des compartiments de l’environnement est biodisponible.
L’aptitude à accumuler l’argent en solution varie largement d’une espèce à l’autre. Dans le cas de certains organismes marins, on fait état de facteurs de bioconcentration (en milligrammes d’argent par kg de poids humide, le tout divisé par le nombre de milligrammes d’argent par litre de milieu) tels que 210 pour les diatomées, 240 pour les algues brunes, 330 pour les moules, 2300 pour les coquilles Saint-Jacques et 18 700 pour les huîtres. Pour les organismes d’eau douce, la valeur du facteur de bioconcentration va de "négligeable" pour le crapet arlequin (Lepomis macrochirus) à 60 pour la daphnie. Ces valeurs correspondent à la bioaccumulation de l’argent dans les conditions de laboratoire. Des études de laboratoire portant sur des dérivés de l’argent comme le sulfure et le chlorure, qui sont moins toxiques, montrent que l’accumulation de cet élément n’entraîne pas nécessairement d’effets nocifs. Aux concentrations présentes dans l’environnement aquatique, il est peu probable qu’il y ait bioamplification de l’argent le long de la chaîne alimentaire. C’est au voisinage d’émissaires d’égouts, d’ateliers de galvanoplastie, de décharges de déchets de mines et de zones où l’on a procédé à l’ensemencement de nuages à l’aide d’iodure d’argent, que l’on trouve d’importantes concentrations d’argent dans les biotes. Les concentrations maximales observées dans des échantillons prélevés sur le terrain et exprimées en milligrammes d’argent total par kg de tissus secs se présentent comme suit : 1,5 pour les mammifères marins (foie) (à l’exception des cétacés de l’espèce Delphinapterus leucas, chez lesquels la concentration est 100 fois plus élevée que chez les autres mammifères marins), 6 pour les poissons (os), 14 pour les végétaux (plante entière), 30 pour les annélidés (corps entier), 44 pour les oiseaux (foie), 110 pour les champignons (plante entière), 185 pour les mollusques bivalves (parties molles) et 320 pour les gastéropodes (corps entier).
D’une façon générale, l’ion argent est moins toxique pour les organismes dulçaquicoles lorsque la concentration des ions argent en solution est faible et lorsque le pH de l’eau, sa dureté, sa teneur en sulfures et en composés organiques dissous ou sous forme particulaires sont élevés. C’est également le cas dans des conditions statiques d’expérimentation, comparativement aux tests effectués dans un courant d’eau ou encore lorsque les animaux sont bien nourris au lieu d’être affamés. Les ions argent sont en revanche très toxiques pour les microorganismes. On ne constate cependant pas d’inhibition importante de l’activité microbienne dans les installations de traitement de l’eau car la complexation et l’adsorption rapides des ions argent réduit leur biodisponibilité. A des concentrations nominales de 1 à 5 µg/litre, l’ion argent libre se révèle mortel pour les espèces représentatives des plantes, invertébrés aquatiques et téléostéens aquatiques sensibles. A une concentration ne dépassant pas 0,17 µg/litre, des effets nocifs sur le développement sont constatés chez la truite, effets que l’on observe également sur la composition et la succession des espèces de phytoplancton à des concentration de 0,3 à 0,6 µg/litre.
Il est capital de déterminer la spéciation de l’argent et la biodisponibilité qui en résulte pour apprécier le risque que représente ce métal. La seule méthode directe utilisable pour déterminer la fraction de l’argent total qui est biodisponible consiste dans le dosage de l’argent sous forme ionisée libre. A l’inverse d’autres métaux, l’argent présent dans les zones préservées ou dans la plupart des agglomérations urbaines se trouve à des concentrations nettement inférieures au seuil de toxicité. Dans la plupart des zones industrialisées, la concentration de l’argent est à la limite de ce seuil, et encore, en supposant que les conditions en favorisent la biodisponibilité. A la lumière des données toxicologiques disponibles, il apparaît comme peu probable que les ions argent libres et biodisponibles existent à des concentrations suffisamment élevées pour produire des effets toxiques sur les organismes qui peuplent le milieu marin.
L’accumulation d’argent dans les végétaux terrestres est généralement faible, même lorsque le sol est amendé à l’aide de boues argentifères ou lorsque les végétaux sont cultivés sur des terrils de mines d’argent, cet élément s’accumulant alors dans la racine. On n’a pas trouvé de données concernant les effets de l’argent sur les oiseaux ou les mammifères sauvages. L’argent s’est révélé nocif pour des volailles à des concentrations ne dépassant pas 100 mg d’argent total par litre d’eau de boisson ou 200 mg par kg de nourriture. Des mammifères de laboratoire sensibles ont subi des effets indésirables à des concentrations d’argent total (ajouté sous forme de nitrate) ne dépassant pas 250 µg/litre dans leur eau de boisson (anomalies histologiques cérébrales), 6 mg/kg dans leur nourriture (forte accumulation dans le rein et le foie) ou encore 13,9 mg/kg de poids corporel (mortalité).
Este CICAD sobre la plata y sus compuestos (aspectos ecológicos) fue preparado por el Centro de Ecología e Hidrología de Monks Wood (Reino Unido). Se basa en la Contaminant Hazard Reviews [Examen del peligro de contaminación] publicación del Departamento del Interior de los Estados Unidos: Silver hazards to fish, wildlife, and invertebrates: A synoptic review [Peligros de la plata para los peces, la flora y fauna silvestres y los invertebrados: Examen sinóptico] (Eisler, 1997), actualizada con referencia a Eisler (2000) y complementada con una búsqueda bibliográfica (abril de 2001). El presente documento no se ocupa de los efectos de la plata en la salud humana; éstos se examinan en un informe de la ATSDR (1990). La información relativa al carácter del examen colegiado y a la disponibilidad del documento original se presenta en el apéndice 1. La información sobre el examen colegiado de este CICAD figura en el apéndice 2. Este CICAD se aprobó como evaluación internacional en una reunión de la Junta de Evaluación Final, celebrada en Ottawa (Canadá) del 29 de octubre al 1ş de noviembre de 2001. La lista de participantes en esta reunión figura en el apéndice 3. Las Fichas internacionales de seguridad química para la plata (ICSC 0810) y el nitrato de plata (ICSC 1116), preparadas por el Programa Internacional de Seguridad de las Sustancias Químicas (IPCS, 1999a, 1999b), también se reproducen en el presente documento.
La plata es un metal poco común, pero presente en la naturaleza, con frecuencia depositado como mineral en asociación con otros elementos. Las emisiones derivadas de las operaciones de fusión, la manufactura y la eliminación de ciertos suministros fotográficos y eléctricos, la combustión del carbón y la lluvia artificial son algunas de las fuentes humanas de plata en la biosfera. Los desplazamientos biogeoquímicos mundiales de la plata se caracterizan por emisiones a la atmósfera, el agua y el suelo a través de fuentes naturales y humanas, el transporte a larga distancia de partículas finas suspendidas en la atmósfera, la deposición húmeda y seca y la sorción a los suelos y los sedimentos.
En las mediciones más recientes de las concentraciones de plata en ríos, lagos y estuarios utilizando técnicas limpias se observan concentraciones de alrededor de 0,01 µg/l en zonas prístinas no contaminadas y de 0,01-0,1 µg/l en zonas urbanas e industrializadas. Las concentraciones de plata notificadas antes de la utilización de un sistema de muestreo de metales ultralimpio, que comenzó a aplicarse a finales de los años ochenta, se deben tomar con precaución. Las concentraciones máximas de plata total registradas durante los años setenta y ochenta en determinados materiales no biológicos fueron de 36,5 ng/m3 en el aire próximo a una fundición; 2,0 µg/m3 en el polvo suspendido en la atmósfera; 0,1 µg/l en las salmueras de los pozos petrolíferos; 4,5 µg/l en la lluvia de nubes creadas con yoduro de plata; 6,0 µg/l en el agua freática cercana a un vertedero de residuos peligrosos; 8,9 µg/l en el agua de mar de la bahía de Galveston, Estados Unidos; 260 µg/l cerca de zonas de eliminación de residuos de fabricación de material fotográfico; 300 µg/l en pozos de vapor; 300 µg/l en aguas residuales de procedimientos fototipográficos sometidas a tratamiento; 31 mg/kg en el suelo; 43 mg/l en el agua de ciertas fuentes termales; 50 mg/kg en el granito; hasta 100 mg/kg en el petróleo bruto; y 150 mg/kg en sedimentos fluviales. Hay que señalar que las concentraciones de plata en el medio ambiente han disminuido; por ejemplo, en la parte baja del río Genesee, Estados Unidos, cerca de una fábrica de material fotográfico, su concentración se redujo de 260 µg/l en los años setenta a un valor inferior al límite de detección (<10 µg/l) en los años noventa. Hay que señalar asimismo que en cada uno de los compartimentos del medio ambiente sólo hay biológicamente disponible una pequeña proporción de la concentración total de plata.
La capacidad para acumular plata disuelta varía ampliamente de unas especies otras. Algunos factores de bioconcentración notificados para organismos marinos (calculados como la razón entre los mg de plata por kg de organismo en peso fresco y los mg de plata por litro de medio) son 210 en las diatomeas, 240 en las algas pardas, 330 en los mejillones, 2300 en las vieiras y 18 700 en las ostras, mientras que se han notificado factores de bioconcentración para organismos de agua dulce que varían entre un valor insignificante en Lepomis macrochirus y 60 en los dáfnidos; estos valores representan la absorción de plata biodisponible en experimentos de laboratorio. Los estudios de laboratorio con los compuestos de plata menos tóxicos, como el sulfuro de plata y el cloruro de plata, ponen de manifiesto que la acumulación de plata no produce necesariamente efectos adversos. Con las concentraciones normales en el medio ambiente, es poco probable su bioamplificación en los sistemas acuáticos a través de la cadena trófica. Se producen concentraciones elevadas de plata en la biota en las proximidades de colectores de aguas residuales, instalaciones de galvanoplastia, vertederos de residuos mineros y zonas en las que se ha aplicado yoduro de plata. Las concentraciones máximas registradas en muestras recogidas sobre el terreno, en mg de plata total por kg de peso seco (tejido), fueron 1,5 en mamíferos marinos (hígado) (excepto en las ballenas beluga de Alaska, Delphinapterus leucas, cuyas concentraciones eran dos órdenes de magnitud más altas que las de otros mamíferos marinos), seis en peces (espina), 14 en plantas (enteras), 30 en anélidos (enteros), 44 en aves (hígado), 110 en champiñones (enteros), 185 en moluscos bivalvos (partes blandas) y 320 en gasterópodos (enteros).
En general, el ión plata era menos tóxico para los organismos de agua dulce cuando había una concentración baja del ión plata disuelto y valores crecientes del pH del agua, su dureza, la concentración de sulfuros y la materia orgánica disuelta y particulada; en condiciones de pruebas estáticas, en comparación con los sistemas de flujo continuo; y cuando los animales estaban nutridos adecuadamente en lugar de hambrientos. Los iones plata son muy tóxicos para los microorganismos. Sin embargo, no suele haber un efecto inhibitorio fuerte sobre la actividad microbiana en las instalaciones de tratamiento de aguas residuales, debido a la reducida biodisponibilidad que se deriva de la formación de complejos y la adsorción rápidas. El ión plata libre fue letal para especies representativas de plantas acuáticas, invertebrados y teleósteos sensibles en concentraciones nominales en el agua de 1-5 µg/l. Se observaron efectos adversos en el crecimiento de la trucha con concentraciones de sólo 0,17 µg/l y sobre la composición y sucesión de especies del fitoplancton con 0,3-0,6 µg/l.
La especiación de la plata y su consiguiente biodisponibilidad son fundamentales para comprender el riesgo potencial del metal. La medición de la plata iónica libre es el único método directo que se puede utilizar para evaluar los efectos probables del metal en los organismos. Se puede recurrir a modelos de especiación para evaluar la proporción probable de la plata total medida que está biodisponible para los organismos. A diferencia de algunos otros metales, las concentraciones de fondo en el agua dulce de zonas prístinas y de la mayor parte de las urbanas son muy inferiores a las que producen efectos tóxicos. Los niveles en las zonas más industrializadas se aproximan a la concentración con efectos, suponiendo que las condiciones favorezcan la biodisponibilidad. Basándose en los resultados disponibles de pruebas de toxicidad, es poco probable que los iones plata libres biodisponibles puedan alcanzar una concentración suficientemente alta para provocar toxicidad en los entornos marinos.
En general, la acumulación de plata por las plantas terrestres a partir del suelo es baja, incluso si el suelo está modificado con fangos cloacales que contienen plata o las plantas han crecido en escombreras de minas de plata, acumulándose ésta principalmente en los sistemas radiculares. No se encontraron datos sobre los efectos de la plata en las aves o los mamíferos silvestres. La plata era perjudicial para las aves de corral con concentraciones de sólo 100 mg de plata total/l de agua de bebida o 200 mg de plata total/kg de alimentos. Se observaron efectos adversos en mamíferos de laboratorio sensibles con concentraciones de plata total (añadida como nitrato de plata) de sólo 250 µg/l en el agua de bebida (histopatología cerebral), 6 mg/kg en los alimentos (acumulaciones elevadas en los riñones y el hígado) o 13,9 mg/kg de peso corporal (letalidad).
ENDNOTES
1. International Programme on Chemical Safety (1994) Assessing human health risks of chemicals: derivation of guidance values for health-based exposure limits. Geneva, World Health Organization (Environmental Health Criteria 170) (also available at http://www.who.int/pcs/).
See Also:
Toxicological Abbreviations