
Concise International Chemical Assessment Document 18
CUMENE
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Concise International Chemical Assessment Document 18
CUMENE
First draft prepared by Dr Gary Foureman, National Center for
Environmental Assessment, US Environmental Protection Agency, Research
Triangle Park, NC, USA
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization, and produced within the framework of the
Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 1999
The International Programme on Chemical Safety (IPCS),
established in 1980, is a joint venture of the United Nations
Environment Programme (UNEP), the International Labour Organisation
(ILO), and the World Health Organization (WHO). The overall objectives
of the IPCS are to establish the scientific basis for assessment of
the risk to human health and the environment from exposure to
chemicals, through international peer review processes, as a
prerequisite for the promotion of chemical safety, and to provide
technical assistance in strengthening national capacities for the
sound management of chemicals.
The Inter-Organization Programme for the Sound Management of
Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and
Agriculture Organization of the United Nations, WHO, the United
Nations Industrial Development Organization, the United Nations
Institute for Training and Research, and the Organisation for Economic
Co-operation and Development (Participating Organizations), following
recommendations made by the 1992 UN Conference on Environment and
Development to strengthen cooperation and increase coordination in the
field of chemical safety. The purpose of the IOMC is to promote
coordination of the policies and activities pursued by the
Participating Organizations, jointly or separately, to achieve the
sound management of chemicals in relation to human health and the
environment.
WHO Library Cataloguing-in-Publication Data
Cumene.
(Concise international chemical assessment document ; 18)
1.Benzene derivatives - chemistry 2.No-observed-adverse-effect
level
3.Risk assessment 4.Environmental exposure I.International
Programme on Chemical Safety II.Series
ISBN 92 4 153018 9 (NLM Classification: QV 633)
ISSN 1020-6167
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1999
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city, or area
or of its authorities, or concerning the delimitation of its frontiers
or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
TABLE OF CONTENTS
FOREWORD
1. EXECUTIVE SUMMARY
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
3. ANALYTICAL METHODS
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1. Environmental levels
6.2. Human exposure
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1. Single exposure
8.2. Irritation and sensitization
8.3. Short-term exposure
8.4. Long-term exposure
8.4.1. Subchronic exposure
8.4.2. Chronic exposure and carcinogenicity
8.5. Genotoxicity and related end-points
8.6. Reproductive and developmental toxicity
8.7. Immunological and neurological effects
9. EFFECTS ON HUMANS
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
11. EFFECTS EVALUATION
11.1. Evaluation of health effects
11.1.1. Hazard identification and dose-response assessment
11.1.2. Criteria for setting guidance values for cumene
11.1.3. Sample risk characterization
11.2. Evaluation of environmental effects
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
13.1. Human health hazards
13.2. Advice to physicians
13.3. Health surveillance advice
13.4. Spillage
13.5. Storage
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
INTERNATIONAL CHEMICAL SAFETY CARD
REFERENCES
APPENDIX 1 -- SOURCE DOCUMENTS
APPENDIX 2 -- CICAD PEER REVIEW
APPENDIX 3 -- CICAD FINAL REVIEW BOARD
RÉSUMÉ D'ORIENTATION
RESUMEN DE ORIENTACION
FOREWORD
Concise International Chemical Assessment Documents (CICADs) are
the latest in a family of publications from the International
Programme on Chemical Safety (IPCS) -- a cooperative programme of the
World Health Organization (WHO), the International Labour Organisation
(ILO), and the United Nations Environment Programme (UNEP). CICADs
join the Environmental Health Criteria documents (EHCs) as
authoritative documents on the risk assessment of chemicals.
CICADs are concise documents that provide summaries of the
relevant scientific information concerning the potential effects of
chemicals upon human health and/or the environment. They are based on
selected national or regional evaluation documents or on existing
EHCs. Before acceptance for publication as CICADs by IPCS, these
documents undergo extensive peer review by internationally selected
experts to ensure their completeness, accuracy in the way in which the
original data are represented, and the validity of the conclusions
drawn.
The primary objective of CICADs is characterization of hazard and
dose-response from exposure to a chemical. CICADs are not a summary of
all available data on a particular chemical; rather, they include only
that information considered critical for characterization of the risk
posed by the chemical. The critical studies are, however, presented in
sufficient detail to support the conclusions drawn. For additional
information, the reader should consult the identified source documents
upon which the CICAD has been based.
Risks to human health and the environment will vary considerably
depending upon the type and extent of exposure. Responsible
authorities are strongly encouraged to characterize risk on the basis
of locally measured or predicted exposure scenarios. To assist the
reader, examples of exposure estimation and risk characterization are
provided in CICADs, whenever possible. These examples cannot be
considered as representing all possible exposure situations, but are
provided as guidance only. The reader is referred to EHC 1701 for
advice on the derivation of health-based guidance values.
While every effort is made to ensure that CICADs represent the
current status of knowledge, new information is being developed
constantly. Unless otherwise stated, CICADs are based on a search of
the scientific literature to the date shown in the executive summary.
In the event that a reader becomes aware of new information that would
change the conclusions drawn in a CICAD, the reader is requested to
contact IPCS to inform it of the new information.
1 International Programme on Chemical Safety (1994)
Assessing human health risks of chemicals: deriviation of
guidance values for health-based exposure limits. Geneva, World
Health Organization (Environmental Health Criteria 170).
Procedures
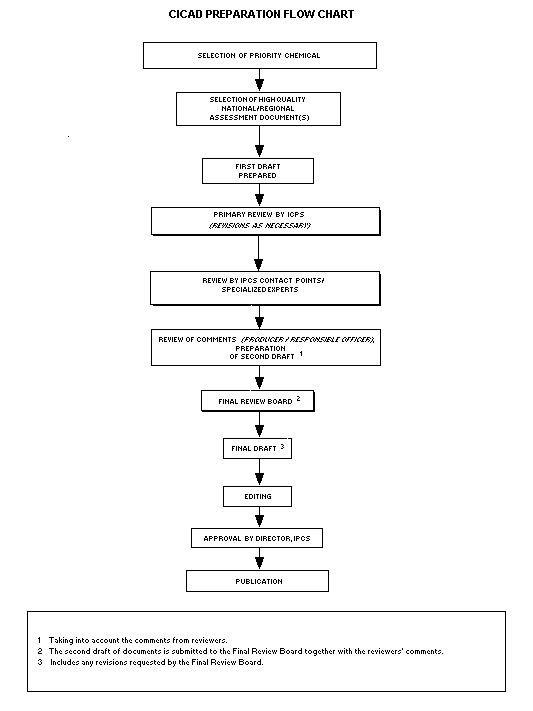
The flow chart shows the procedures followed to produce a CICAD.
These procedures are designed to take advantage of the expertise that
exists around the world -- expertise that is required to produce the
high-quality evaluations of toxicological, exposure, and other data
that are necessary for assessing risks to human health and/or the
environment.
The first draft is based on an existing national, regional, or
international review. Authors of the first draft are usually, but not
necessarily, from the institution that developed the original review.
A standard outline has been developed to encourage consistency in
form. The first draft undergoes primary review by IPCS to ensure that
it meets the specified criteria for CICADs.
The second stage involves international peer review by scientists
known for their particular expertise and by scientists selected from
an international roster compiled by IPCS through recommendations from
IPCS national Contact Points and from IPCS Participating Institutions.
Adequate time is allowed for the selected experts to undertake a
thorough review. Authors are required to take reviewers' comments into
account and revise their draft, if necessary. The resulting second
draft is submitted to a Final Review Board together with the
reviewers' comments.
The CICAD Final Review Board has several important functions:
- to ensure that each CICAD has been subjected to an appropriate
and thorough peer review;
- to verify that the peer reviewers' comments have been addressed
appropriately;
- to provide guidance to those responsible for the preparation of
CICADs on how to resolve any remaining issues if, in the opinion
of the Board, the author has not adequately addressed all
comments of the reviewers; and
- to approve CICADs as international assessments.
Board members serve in their personal capacity, not as representatives
of any organization, government, or industry. They are selected
because of their expertise in human and environmental toxicology or
because of their experience in the regulation of chemicals. Boards are
chosen according to the range of expertise required for a meeting and
the need for balanced geographic representation.
Board members, authors, reviewers, consultants, and advisers who
participate in the preparation of a CICAD are required to declare any
real or potential conflict of interest in relation to the subjects
under discussion at any stage of the process. Representatives of
nongovernmental organizations may be invited to observe the
proceedings of the Final Review Board. Observers may participate in
Board discussions only at the invitation of the Chairperson, and they
may not participate in the final decision-making process.
 1. EXECUTIVE SUMMARY
This CICAD on cumene was prepared by the US Environmental
Protection Agency (EPA) and is based on the US EPA's Health and
environmental effects document for cumene (US EPA, 1987), the US
EPA's Integrated Risk Information System (IRIS) file on cumene (US
EPA, 1997), and the United Kingdom's Environmental hazard assessment
(EHA): Cumene (UK DOE, 1994), supplemented by a literature search on
the ecology-based AQUIRE (Aquatic Toxicity Information Retrieval)
database. The literature search for the IRIS file was through November
1996 and for the AQUIRE database through April 1998. Information on
the nature of the peer review and the availability of the source
documents is presented in Appendix 1. Information on the peer review
of this CICAD is presented in Appendix 2. This CICAD was approved as
an international assessment at a meeting of the Final Review Board,
held in Washington, DC, USA, on 8-11 December 1998. Participants at
the Final Review Board meeting are listed in Appendix 3. The
International Chemical Safety Card (ICSC 0170) for cumene, produced by
the International Programme on Chemical Safety (IPCS, 1993), has also
been reproduced in this document.
Cumene (CAS no. 98-82-8) is a water-insoluble petrochemical used
in the manufacture of several chemicals, including phenol and acetone.
It readily volatilizes into the atmosphere from water and dry soil.
Cumene is expected to adsorb moderately to strongly to soil/sediments
and to undergo biodegradation in water and soil.
Cumene is metabolized primarily to the secondary alcohol,
2-phenyl-2-propanol, in both humans and animals. This alcohol and its
conjugates are readily excreted by both rodents and humans.
Increases in organ weights, primarily kidney weights, are the
most prominent effects observed in rodents repeatedly exposed to
cumene by either the oral or inhalation route. No adverse effects were
observed in rat or rabbit fetuses whose mothers had been exposed to
cumene during fetal development. Although no multigenerational
reproductive studies have been performed using cumene, its rapid
metabolism and excretion, coupled with lack of effects on sperm
morphology in a subchronic study, suggest that it has a low potential
for reproductive toxicity. A guidance value for oral exposure of 0.1
mg/kg body weight per day has been derived, based on the
no-observed-adverse-effect level (NOAEL) of 154 mg/kg body weight per
day for increased kidney weight in female rats in a 6- to 7-month oral
study; the NOAEL was adjusted for the dosing schedule, and a total
uncertainty factor of 1000 was applied. Guidance values for the
general population of 0.4 mg/m3 and 0.09 mg/m3 were derived for
inhalation exposure, based on alternative NOAELs derived from the same
subchronic inhalation study; again, the NOAELs were adjusted to a
continuous exposure, and a total uncertainty factor of 1000 was
applied.
No data are available with which to quantify human exposure to
cumene.
It is not possible to assess cumene's potential for
carcinogenicity in humans, because long-term carcinogenicity studies
with cumene have not been performed. Most genotoxicity test data with
cumene are negative.
Inadequate data, especially measured exposure information, exist
to allow a quantitative evaluation of the risk to populations of
aquatic or terrestrial organisms from exposure to cumene. Based on
existing data, however, cumene is anticipated to be of relatively low
risk. Values indicate a slight potential for bioconcentration of
cumene in fish. There are no data on bioaccumulation through food
chains (biomagnification).
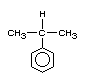
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
Cumene (CAS no. 98-82-8; C9H12; 2-phenyl-propane,
isopropylbenzene, (1-methylethyl)-benzene) is a volatile, colourless
liquid at room temperature with a characteristic sharp, penetrating,
aromatic odour (Ward, 1979). It is nearly insoluble in water but is
soluble in alcohol and many other organic solvents (Windholz, 1983).
Structurally, cumene is a member of the alkyl aromatic family of
hydrocarbons, which also includes toluene (methylbenzene) and
ethylbenzene. Its structural diagram is given below.
1. EXECUTIVE SUMMARY
This CICAD on cumene was prepared by the US Environmental
Protection Agency (EPA) and is based on the US EPA's Health and
environmental effects document for cumene (US EPA, 1987), the US
EPA's Integrated Risk Information System (IRIS) file on cumene (US
EPA, 1997), and the United Kingdom's Environmental hazard assessment
(EHA): Cumene (UK DOE, 1994), supplemented by a literature search on
the ecology-based AQUIRE (Aquatic Toxicity Information Retrieval)
database. The literature search for the IRIS file was through November
1996 and for the AQUIRE database through April 1998. Information on
the nature of the peer review and the availability of the source
documents is presented in Appendix 1. Information on the peer review
of this CICAD is presented in Appendix 2. This CICAD was approved as
an international assessment at a meeting of the Final Review Board,
held in Washington, DC, USA, on 8-11 December 1998. Participants at
the Final Review Board meeting are listed in Appendix 3. The
International Chemical Safety Card (ICSC 0170) for cumene, produced by
the International Programme on Chemical Safety (IPCS, 1993), has also
been reproduced in this document.
Cumene (CAS no. 98-82-8) is a water-insoluble petrochemical used
in the manufacture of several chemicals, including phenol and acetone.
It readily volatilizes into the atmosphere from water and dry soil.
Cumene is expected to adsorb moderately to strongly to soil/sediments
and to undergo biodegradation in water and soil.
Cumene is metabolized primarily to the secondary alcohol,
2-phenyl-2-propanol, in both humans and animals. This alcohol and its
conjugates are readily excreted by both rodents and humans.
Increases in organ weights, primarily kidney weights, are the
most prominent effects observed in rodents repeatedly exposed to
cumene by either the oral or inhalation route. No adverse effects were
observed in rat or rabbit fetuses whose mothers had been exposed to
cumene during fetal development. Although no multigenerational
reproductive studies have been performed using cumene, its rapid
metabolism and excretion, coupled with lack of effects on sperm
morphology in a subchronic study, suggest that it has a low potential
for reproductive toxicity. A guidance value for oral exposure of 0.1
mg/kg body weight per day has been derived, based on the
no-observed-adverse-effect level (NOAEL) of 154 mg/kg body weight per
day for increased kidney weight in female rats in a 6- to 7-month oral
study; the NOAEL was adjusted for the dosing schedule, and a total
uncertainty factor of 1000 was applied. Guidance values for the
general population of 0.4 mg/m3 and 0.09 mg/m3 were derived for
inhalation exposure, based on alternative NOAELs derived from the same
subchronic inhalation study; again, the NOAELs were adjusted to a
continuous exposure, and a total uncertainty factor of 1000 was
applied.
No data are available with which to quantify human exposure to
cumene.
It is not possible to assess cumene's potential for
carcinogenicity in humans, because long-term carcinogenicity studies
with cumene have not been performed. Most genotoxicity test data with
cumene are negative.
Inadequate data, especially measured exposure information, exist
to allow a quantitative evaluation of the risk to populations of
aquatic or terrestrial organisms from exposure to cumene. Based on
existing data, however, cumene is anticipated to be of relatively low
risk. Values indicate a slight potential for bioconcentration of
cumene in fish. There are no data on bioaccumulation through food
chains (biomagnification).
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
Cumene (CAS no. 98-82-8; C9H12; 2-phenyl-propane,
isopropylbenzene, (1-methylethyl)-benzene) is a volatile, colourless
liquid at room temperature with a characteristic sharp, penetrating,
aromatic odour (Ward, 1979). It is nearly insoluble in water but is
soluble in alcohol and many other organic solvents (Windholz, 1983).
Structurally, cumene is a member of the alkyl aromatic family of
hydrocarbons, which also includes toluene (methylbenzene) and
ethylbenzene. Its structural diagram is given below.
 Some relevant physical and chemical properties of cumene are
listed in Table 1. Additional physical/chemical properties are
presented in the International Chemical Safety Card (ICSC 0170)
reproduced in this document.
Table 1: Physical/chemical properties of cumene.
Property Value Reference
Molecular weight 120.2 g/mol
Boiling point 152.39 °C Ward, 1979
Vapour pressure, 611 Pa Mackay &
25 °C Shiu, 1981
Water solubility, 50 mg/litre Mackay &
25 °C Shiu, 1981
Log Kow 3.66 Hansch &
Leo, undated
Density, 20 °C 0.8619 g/cm3 Ward, 1979
Flashpoint (tag closed-cup) 35 °C Ward, 1965
Odour threshold limit 0.088 ppm (v/v) Amoore &
value (TLV) 0.43 mg/m3 Hautala, 1983
Table 1 (continued)
Conversion factor, 1 ppm = 5.2 mg/m3
20 °C, 101.3 kPa 1 mg/m3 = 0.19 ppm
Partition coefficients Sato &
Oil/air 6215 Nakajima, 1979
Oil/water 4316
Water/air 1.44
Human blood/air 37
3. ANALYTICAL METHODS
For sampling and measurement of cumene in air, Method 1501 of the
US National Institute for Occupational Safety and Health (NIOSH, 1994)
includes use of a solid sorbent tube (coconut shell charcoal) sampler
with a gas chromatography/flame ionization detector measurement
technique. The detection limit of this method is 1 mg/m3 (0.2 ppm).
US EPA (1996) methods for detecting cumene in media other than
air include the use of gas chromatography using photoionization Method
8021B, which is applicable to nearly all types of samples, regardless
of water content. The method detection limit for cumene is 0.05
µg/litre, and the applicable concentration range for this method is
approximately 0.1-200 µg/litre. The standard recovery using this
method is 98%, with a standard deviation of 0.9%. Another commonly
used gas chromatographic assay for volatiles including cumene is
Method 8260B (US EPA, 1996), with a general estimated quantitation
limit of approximately 5 µg/kg wet weight for soil/sediment samples,
0.5 mg/kg wet weight for wastes, and 5 µg/litre for groundwater.
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
Cumene is a naturally occurring constituent of crude oil and may
be released to the environment from a number of anthropogenic sources,
including processed hydrocarbon fuels. Crude oils typically contain
approximately 0.1 wt% of cumene, but concentrations as high as 1.0 wt%
have been reported.1 Measurements of various grades of petrol
revealed that cumene concentrations range from 0.14 to 0.51 vol% and
that the average cumene concentration was 0.3 vol%. Premium diesel
fuel contains 0.86 wt% of cumene; furnace oil (no. 2) contains 0.60
wt%.1
Primary sources of release of cumene include losses in wastewater
and fugitive emissions from manufacturing and use facilities and
petrochemical refineries, accidental spills of finished fuel products
during transport or processing, and emissions from petrol stations and
motor vehicles (US EPA, 1987). Cigarette tobacco also releases cumene
(Johnstone et al., 1962). Cumene release from all these sources is
estimated to be 9500 tonnes annually (US EPA, 1988). Other,
unquantifiable anthropogenic cumene releases include the rubber
vulcanization process (Cocheo et al., 1983), building materials
(Moelhave, 1979), jet engine exhaust (Katzman & Libby, 1975), outboard
motor operation (Montz et al., 1982), solvent uses (Levy, 1973),
pharmaceutical production, and textile plants (Gordon & Gordon, 1981).
Cumene is also released to the environment from leather tanning, iron
and steel manufacturing, paving and roofing, paint and ink
formulation, printing and publishing, ore mining, coal mining,
organics and plastics manufacturing, pesticide manufacturing,
electroplating, and pulp and paper production (Shackelford et al.,
1983).
SRI International (1986) reported the 1985 Western European
cumene production levels (in tonnes) for the following producer
countries:
Federal Republic of Germany 438 000
Finland 70 000
France 370 000
Italy 335 000
Netherlands 240 000
Spain 120 000
United Kingdom 220 000
This total 1985 production of 1 793 000 tonnes may be compared with
production in the USA, which was reported as 2 775 000 tonnes in 1997
(Anon., 1998).
1 Letter and attachment from W.F. O'Keefe, American Petroleum
Institute, to M. Greif, Toxic Substances Control Act (TSCA)
Interagency Testing Committee, US Environmental Protection
Agency, Washington, DC (TS-792).
The use pattern for cumene in the early 1970s in the USA was as
follows (Anon., 1984): oxidation for phenol/acetone production, 98%;
polymerization of alpha-methylstyrene, 1.8%; and exports, 0.2%. Cumene
is also used captively for the production of phenol and
alpha-methyl-styrene (SRI International, 1986).
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
In the atmosphere, cumene is expected to exist almost entirely in
the vapour phase (Eisenreich et al., 1981). Cumene does not absorb
ultraviolet light at wavelengths greater than 290 nm (US EPA, 1987),
which suggests that cumene would not be susceptible to direct
photolysis. In one study, the estimated half-life of cumene in the
atmosphere from photolysis alone was approximately 1500 years (Parlar
et al., 1983). Cumene is not susceptible to oxidation by ozone in the
atmosphere (US EPA, 1987). Thus, reaction with ozone and direct
photolysis are not expected to be important removal processes. Rather,
reaction with photochemically generated hydroxyl radicals appears to
be the primary degradation pathway (t´ 1-2 days) (Lloyd et al.,
1976; Ravishankara et al., 1978). Small amounts of cumene may be
removed from the atmosphere during precipitation. Cumene has been
assigned a Photochemical Ozone Creation Potential (POCP) value of 35
relative to ethylene at 100 (Derwent & Jenkin, 1990). POCP values
represent the ability of a substance to form ground-level ozone as a
result of its atmospheric degradation reactions.
In water, important fate and transport processes are expected to
be volatilization (t´ 4 h from a typical river) and aerobic
biodegradation (Kappeler & Wuhrmann, 1978; Sasaki, 1978; Van der
Linden, 1978). Chemical hydrolysis, oxidation, photolysis, and
reaction with hydroxyl radicals are not expected to be important fate
processes in water (Mill et al., 1978, 1979, 1980). Using an aerobic
freshwater sediment/water test system, Williams et al. (1993)
demonstrated that 10 days after addition of radiolabelled cumene (2.5
mg/litre) to the system, 46.9% was trapped as radiolabelled carbon
dioxide and another 21.8% was recovered as radiolabelled organics, the
overall recovery of cumene ranging from 56.8% to 88.3%. The
disappearance half-life based on these results was 2.5 days. During a
20-day incubation of cumene at 10 mg/litre under aerobic conditions in
either fresh water or salt water, Price et al. (1974) observed 70%
degradation in fresh water but only about 2% degradation in seawater.
Cumene was, however, observed to be degraded to a significant extent
by microorganisms isolated from ocean sediment samples incubated in
seawater, as Walker et al. (1976) noted decreases in cumene (gas
chromatographic analysis) ranging from 37% to 60% of initial amounts
over a period of 21 days in three separate incubations with seawater
and microorganisms isolated from Atlantic Ocean sediments. On the
other hand, cumene was found to be essentially non-biodegradable under
anaerobic conditions by Battersby & Wilson (1989), who noted that
cumene produced only about 2% of theoretical gas production when
incubated at 50 mg carbon/litre sludge for 60 days at 35°C under
anaerobic conditions; compounds at 80% of theoretical gas production
under these conditions were assumed to represent complete degradation,
whereas compounds at less than 30% production were considered
persistent.
In soil, it appears that cumene might biodegrade fairly rapidly
under aerobic conditions, because a number of microorganisms capable
of degrading cumene have been isolated (Yamada et al., 1965; Jamison
et al., 1970; Omori et al., 1975). Regression equations based on the
limit of cumene water solubility (50 mg/litre) predicted Koc (soil
sorption coefficient standardized to organic carbon) values ranging
from 513 to 1622. For equations based instead on log octanol/water
partition coefficients (log Kow) for cumene, predicted Koc
values were in a similar range, from 589 to 3890 (Lyman et al., 1982).
Other estimates of Koc values at 884 (Jeng et al., 1992) and 2800
(US EPA, 1987) were also in this range. These Koc values indicate
that cumene is expected to adsorb moderately to strongly to soil and
have only slight mobility. The relatively high vapour pressure of
cumene suggests that volatilization of this compound from dry soil
surfaces would be significant.
Measured and estimated bioconcentration factors (BCFs) suggest a
slight potential for cumene to bioconcentrate in fish species. A BCF
of 36 for cumene in goldfish ( Carassius auratus) has been measured
(Ogata et al., 1984), and a BCF of 356 was estimated from the log
Kow and a linear regression correlation equation (log BCF = 0.76
log Kow - 0.23) by the US EPA (1987). This value was concordant
with the BCF of 316 calculated for fish species in general exposed to
cumene (Sabljic, 1987). Cumene was detected at levels of 0.5-1.4 ng/g
wet weight (detection limit 0.5 ng/g wet weight by gas chromatography/
mass spectrometry) in 12 of 138 sampled fish (various species) from
several locations near a potential emission source (Japan Environment
Agency, 1987). Cumene has been detected in "oakmoss" ( Evernia
prunastri (L.) Ach.) (Gavin et al., 1978) and marsh grass (Mody et
al., 1974a,b).
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
Cumene has been found as a contaminant in various industrial
effluents and in groundwaters. Significant levels of cumene have been
recorded in groundwater near chemical plants (1581 µg/litre, Botta et
al., 1984; 360 µg/litre, Teply & Dressler, 1980; 11 µg/litre,
Pellizzari et al., 1979), around outboard motor operations (700
µg/litre, Montz et al., 1982), near coal gasification facilities (up
to 54 µg/litre, Steurmer et al., 1982), and around petroleum plants
and petroleum refineries (5 µg/litre, quantification method not clear;
Snider & Manning, 1982). Cumene was detected in 8 of 135 samples of
surface water (detection limit 0.03 µg/litre with gas
chromatography/mass spectrometry) at concentrations ranging from 0.09
to 0.44 µg/litre in several locations near a potential emission source
in the 1986 monitoring of the general environment in Japan (Japan
Environment Agency, 1987). Cumene levels in sediments and biota in
Puget Sound, Washington, USA, ranged from 0.02 to 19 µg/g, with a mean
concentration of 2.3 µg/g (Brown et al., 1979). A cumene level of
140 µg/litre was found in seawater near an offshore drilling platform
in the Gulf of Mexico (Sauer, 1981). Cumene was detected in 6 of 111
sediment samples at concentrations ranging from 0.58 to 11 ng/g dry
weight (detection limit 0.5 ng/g with gas chromatography/mass
spectrometry) in several locations near a potential emission source
(Japan Environment Agency, 1987).
Reports of air sampling in the USA indicate the mean
concentration of cumene to be about 14.7 µg/m3 (3 ppb) in urban
settings and as high as 2.5 µg/m3 (0.5 ppb) in rural settings.
Samples taken in Los Angeles, California, in 1966 averaged 14.7 µg/m3
(3 ppb) (Lonneman et al., 1968), and samples taken in Houston, Texas,
in 1973-1974 averaged 12.15 µg/m3 (2.48 ppb) (Lonneman et al., 1979).
The US EPA (1987) reported a mean concentration of 16.7 µg cumene/m3
(3.4 ppb) in undated samples from Los Angeles. In samples taken in the
fall of 1981 in Los Angeles, Grosjean & Fung (1984) did not detect
cumene, although a minimum detection level of 9.8 µg/m3 (2 ppb) was
reported. Although a number of sampling attempts in rural and remote
areas reported no detectable levels of cumene in air (detection limit
<0.05 µg/m3 [<0.01 ppb]), two attempts were positive: Seila (1979)
reported mean levels of 2.5 µg/m3 (0.5 ppb) in samples taken in a
rural area near Houston, Texas, in 1978, and Arnts & Meeks (1980,
1981) reported 0.25 µg/m3 (0.05 ppb) in samples taken near campfires
in the Great Smokey Mountains, USA, in 1978.
Average atmospheric concentrations of cumene in Europe are
reported to be somewhat less than those in the USA, although
concentrations in urban areas are also consistently much higher than
those in rural areas. Isodorov et al. (1983) recorded an average
cumene level of 8.3 µg/m3 (1.7 ppb) in the urban atmosphere of
Leningrad, USSR, in 1977-1979, with a maximum of 11.8 µg/m3 (2.4
ppb). Ambient air concentrations for the Netherlands in 1980 were
reported to average 0.5-1.0 µg/m3 (0.1-0.2 ppb), with maxima ranging
up to 34.8 µg/m3 (7.1 ppb) (Guicherit & Schulting, 1985). An annual
average of 1.6 µg/m3 (0.3 ppb) (maximum 3.9 µg/m3 [0.8 ppb]) was
reported from the Grenoble area in France in 1987 (Foster et al.,
1991).
6.2 Human exposure
Humans can be exposed to cumene via industrial emissions, petrol
station or motor vehicle emissions, accidental releases, food,
cigarette smoke, and drinking-water (US EPA, 1987).
In condensates of cigarette smoke, Johnstone et al. (1962)
recorded yields of cumene ranging from 7 to 14 µg/cigarette. Holzer et
al. (1976) detected cumene at 10 µg/m3 (2 ppb) in air samples taken
from a room immediately after a single cigarette had been smoked. No
further specifics, such as indication of a median value or minimum
detection level, are given.
Brugnone et al. (1989) reported cumene as measurable in all
alveolar air samples collected (single breath; range 1-81 µg/m3
[0.2-17 ppb], method detection limit not given) from among two groups
of workers ( n = 86, gender not specified) exposed to <0.1 mg
cumene/m3 (<0.02 ppm) through the work shift. These authors analysed
for but were unable to detect any significant differences in cumene
concentrations between smokers and non-smokers in either alveolar air
or blood samples. In another study, gases collected from 60 min of
normal continuous respiration from each of eight male volunteers
(three smokers) were analysed for trace organic constituents (Conkle
et al., 1975). Cumene was listed as detected in one of the three
smokers (expressed as 21 µg/h) and in one of the five non-smokers
(expressed as 0.13 µg/h). Krotoszynski & O'Neill (1982) also
identified cumene in expired air from non-smokers.
The presence of cumene in foods can be biogenic or due to
environmental contamination (US EPA, 1987). Although the detection
limit of cumene in various foods was not specified, the US EPA (1987)
noted that cumene has been detected but not quantified in foods as
diverse as tomatoes, Concord grapes, cooked rice, fried chicken,
bacon, Beaufort cheese, and dried legumes.
Only two reports of cumene quantification in drinking-water were
found in the available literature. Coleman et al. (1984) detected
cumene in Cincinnati, Ohio, USA, drinking-water at a level of 0.014
µg/litre (quantification method not clear). Keith et al. (1976)
reported 0.01 µg cumene/litre drinking-water in Terrebonne-Parish,
Louisiana, USA, but found none in the drinking-water of nine other
cities across the USA. These concentrations are considerably below the
0.5 µg/litre detection limit reported by Westrick et al. (1984), who
found no cumene in 945 US drinking-water systems, 479 of which were
selected because of known contamination problems. Burmaster (1982) and
Burnham et al. (1972) reported unquantified levels of cumene/
alkylbenzenes in drinking-water obtained from groundwater. Based on
the results of these studies, it may be concluded that cumene
contamination above 0.5 µg/litre is uncommon in drinking-water in the
USA.
One industrial hygiene survey (US EPA, 1988) reported that
approximately 739 US workers were occupationally exposed to cumene.
Personal exposure data in this report consisted of 1487 air samples
taken over the course of 12 years (1973-1984), of which 6 were in the
range of 20-150 mg/m3 (4-30 ppm), 4 in the range of 15-20 mg/m3 (3-4
ppm), and 25 in the range of 5-10 mg/m3 (1-2 ppm), with the remaining
samples below 5 mg/m3 (1 ppm) (US EPA, 1988).
Based on available monitoring data, it appears that the general
population would be exposed to cumene primarily by inhalation,
although occupational populations may be reasonably anticipated to be
exposed by the dermal route. Minor exposure may result from contact
with refined petroleum products and ingestion of contaminated foods
and possibly drinking-water.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND
HUMANS
Cumene has been shown to be absorbed after inhalation exposure in
humans and after inhalation, oral, and dermal exposure in animals
(Senczuk & Litewka, 1976; Research Triangle Institute, 1989). Tests
conducted in humans indicate that cumene is absorbed readily via the
inhalation route, that it is metabolized efficiently to water-soluble
metabolites within the body, and that these metabolites are excreted
efficiently into the urine with no evidence of long-term retention
within the body; these results concur with the results of animal
studies.
Senczuk & Litewka (1976) exposed human volunteers (five men and
five women) head only to one of three different concentrations of
cumene vapours (240, 480, or 720 mg/m3 [49, 98, or 147 ppm]) for 8 h
every 10 days. Exhaled breath samples (10 cm3) were collected near
the beginning and at the end of the exposure from a tube placed in the
breathing zone. The total amount of cumene absorbed during exposure,
calculated from retention, ventilation, and exposure duration, was
nearly twice as high at all exposure levels in the males (466-1400 mg)
as in the females (270-789 mg). The respiratory tract absorption
ranged from 45% to 64% depending on the time of exposure, with the
overall mean retention estimated at 50%. In rats, inhalation studies
(nose only for 6 h at 510, 2420, or 5850 mg/m3 [104, 494, or 1194
ppm]) indicate rapid absorption, with detectable levels of cumene
appearing in the blood within 5 min of the beginning of exposure at
all three exposure levels (Research Triangle Institute, 1989). Gavage
studies in rats showed that cumene was absorbed readily via this
route, with maximum levels in blood occurring at the earliest time
point sampled (4 h) for a lower dose (33 mg/kg body weight) and at
8-16 h for a higher dose (1350 mg/kg body weight) (Research Triangle
Institute, 1989). Dermal absorption of cumene was demonstrated in rats
and rabbits (Monsanto Co., 1984).
The human data reported by Brugnone et al. (1989) regarding
cumene distribution suggest that the cumene concentration was about 40
times higher in blood than in alveolar air, a figure concordant with
the reported human blood/air partition coefficient of 37 (Sato &
Nakajima, 1979; Table 1). Cumene was widely distributed in rats, and
distribution, presumably determined immediately after exposure, was
independent of administration route (inhalation, oral, or
intraperitoneal in 10% aqueous Emulphor). Adipose, liver, and kidney
were all shown to have elevated tissue/blood ratios of cumene
following all doses and routes of exposure (Research Triangle
Institute, 1989). Fabre et al. (1955) demonstrated that after rats
inhaled cumene vapour for up to 150 days, cumene was distributed to
the endocrine organs, central nervous system, bone marrow, spleen, and
liver.
The patterns of cumene disappearance (as total radioactivity)
from the blood in the nose-only inhalation studies were fitted with a
monoexponential model, with the half-lives increasing with dose, from
3.9 h at 490 mg/m3 (100 ppm) to 6.6 h at 5880 mg/m3 (1200 ppm). The
half-life of cumene in the blood in gavage studies with rats was
calculated to be between 9 and 16 h.
Metabolism of cumene by cytochrome P-450 is extensive and takes
place within hepatic and extrahepatic tissues, including lung (Sato &
Nakajima, 1987), with the secondary alcohol 2-phenyl-2-propanol being
a principal metabolite. Metabolites excreted in urine of rats and
rabbits include 2-phenyl-2-propanol and its glucuronide or sulfate
conjugates, conjugates of 2-phenyl-1,2-propanediol, and an unknown
metabolite, possibly the dicarboxylic acid that would result from
complete oxidation of the 1- and 3-alkyl carbons of phenylmalonic acid
(Research Triangle Institute, 1989; Ishida & Matsumoto, 1992; MAK,
1996).
Senczuk & Litewka (1976) also conducted excretion studies with
human volunteers exposed to cumene vapours (240, 480, or 720 mg/m3
[49, 98, or 147 ppm]) for 8 h every 10 days. These authors reported
excretion of the metabolite 2-phenyl-2-propanol in the urine as
biphasic, with a rapid early phase (t´ 2 h) and a slower later phase
(t´ 10 h); excretion of this metabolite in the urine (about 35% of
the calculated absorbed dose) was maximal after 6-8 h of exposure and
approached zero at 40 h post-exposure. With rats, the extent of
elimination across routes of administration (inhalation, oral, or
intraperitoneal) and exposure concentrations was very similar, with
urine being the major route of elimination, about 70% in all cases
(Research Triangle Institute, 1989). Total body clearance in the rats
was rapid and complete, with less than 1% of the absorbed fraction
being present in the body 72 h after the highest exposure regime
examined (5880 mg/m3 [1200 ppm] for 6 h). Following oral
administration of cumene in rabbits, 90% was recovered as metabolites
in the urine within 24 h (Robinson et al., 1955).
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
Cumene is not highly toxic to laboratory animals by inhalation,
oral, or dermal routes of exposure. An LC50 of 9800 mg cumene/m3
(2000 ppm) in mice has been reported (MAK, 1996). A 4-h inhalation
LC50 of 39 200 mg/m3 (8000 ppm) in rats was reported by several
investigators (Smyth et al., 1951; Koch Refining Co., 1984; Union
Carbide Corp., 1985). Acute oral LD50 values for rats range from 1400
to 2900 mg/kg body weight (Smyth et al., 1951; Koch Refining Co.,
1984; Monsanto Co., 1984; Ciba-Geigy Co., 1985; Union Carbide Corp.,
1985). Tanii et al. (1995) reported an intraperitoneal LD50 in male
mice in the same range, 2000 mg/kg body weight (16.9 mmol/kg).
Clinical signs of toxicity reported in rats in acute oral studies
include weakness, ocular discharge, collapse, and death; pathological
findings in animals that died were haemorrhagic lungs, liver
discolorations, and acute gastrointestinal inflammation (Monsanto Co.,
1984). The character of the dose-response for these effects is,
however, unclear.
Acute dermal LD50s for cumene applied undiluted to rabbit skin
range from >3160 mg/kg body weight (Monsanto Co., 1984) to >10 000
mg/kg body weight (Ciba-Geigy Co., 1985). Pathological findings in
animals that died were similar to those in animals that died after a
single oral exposure (Monsanto Co., 1984).
8.2 Irritation and sensitization
Undiluted cumene applied to the skin of New Zealand albino
rabbits (0.5 ml) according to standardized guidelines caused slight
defatting with skin flaking, a symptom not generally classified as
relating to primary skin irritancy (Monsanto Co., 1984). A study
conducted by Ciba-Geigy Co. (1985) reported a similar low level of
irritation.
Cumene is an ocular irritant. Ocular irritation, including
immediate discomfort followed by "erythema" (redness of the
conjunctiva) and copious discharge, was observed after the
instillation of undiluted cumene to rabbit, with these effects being
reversible within 120 h (Monsanto Co., 1984). Ciba-Geigy Co. (1985)
judged eye irritation as slight when cumene was applied to rabbit
eyes. However, a study by Union Carbide Corp. (1985) reported that
cumene was harmless to rabbit eyes when applied undiluted.
Observations of lacrimation (Tegeris & Balster, 1994) and periocular
swelling and blepharospasm (Cushman et al., 1995) also indicate that
cumene may exhibit ocular irritancy at high airborne concentrations.
The concentration of cumene causing a 50% reduction in the
respiratory rate in mice after 30 min of exposure was determined to be
10 084 mg/m3 (2058 ppm) (Kristiansen et al., 1986). This
concentration is quite high and in the range where repeated exposure
caused death and morbidity in rats (Gulf Oil Corp., 1985; Chemical
Manufacturers Association, 1989) and rabbits (Darmer et al., 1997).
No skin sensitization reactions were noted among a group of 20
female guinea-pigs treated with cumene in a Magnusson-Kligman
maximization test conducted in accordance with Organisation for
Economic Co-operation and Development (OECD) Guideline 406 (Hüls,
1988). No data were available on respiratory sensitization to cumene.
8.3 Short-term exposure
In a study by Monsanto Co. (1986), male and female Sprague-Dawley
rats (10 per sex per group) were exposed whole body to cumene vapour
concentrations of 0, 515, 1470, or 2935 mg/m3 (0, 105, 300, or
599 ppm) for 6 h/day, 5 days/week, for approximately 4 weeks (minimum
exposure, 20 days). Cage-side observations included
concentration-related increases in side-to-side head movements in both
males and females in all dose groups, head tilt in all dose groups,
and arched back in one female in the high-dose group. Increases in
mean absolute left and right kidney weights were observed in high-dose
males, as were increases in mean absolute left kidney weight in
low- and mid-dose males. In high-dose females, the mean absolute
weight of left kidneys was greater than in controls. This study
confirms that renal weight changes occur in females and corroborates
similar effects reported by Cushman et al. (1995). It should be noted
that the effects associated with central nervous system perturbation
(i.e., head movements) were not noted in several other longer-term
studies, including that of Cushman et al. (1995), in which
neurotoxicity was specifically assessed. If it is assumed that the
renal changes among the males were associated with male rat-specific
nephropathy (see section 8.4.1), the cage-side observations of head
tilt and head movements become the critical effects for this
short-term study.
Although not statistically significant, leukocytosis was noted in
a group of rats ( n = 15, mixed sex) exposed to cumene at 1200 mg/m3
(245 ppm) for 8 h/day, 5 days/week, for 30 exposures (Jenkins et al.,
1970).
Other short-term toxicity studies are described in section 8.7.
8.4 Long-term exposure
8.4.1 Subchronic exposure
In an inhalation exposure study by Jenkins et al. (1970), groups
of squirrel monkeys ( n = 2), beagle dogs ( n = 2),
Princeton-derived guinea-pigs ( n = 15), and Sprague-Dawley and
Long-Evans rats ( n = 15) were exposed whole body to cumene at
concentrations of 0, 18, or 147 mg/m3 (0, 4, or 30 ppm) continuously
for 90 days. Initial and terminal body weights, haematological and
clinical chemistry parameters, and histopathological data were
collected. No toxicologically significant effects were noted in the
monkeys, dogs, or guinea-pigs. The only effect noted in the rats was a
slight degree of leukocytosis at both concentrations.
Cushman et al. (1995; also reported as Bushy Run Research Center,
1989a) conducted two successive subchronic whole-body inhalation
toxicity studies with cumene vapours (>99.9% pure) on Fischer-344
rats. In the first study, groups (21 per sex) were exposed to cumene
vapour at 0, 490, 2430, or 5890 mg/m3 (0, 100, 496, or 1202 ppm) 6
h/day, 5 days/week, for 13 weeks. The second study was a repeat of the
first, except that the group size was decreased to 15 per sex and an
additional group (at 245 mg/m3 [50 ppm]) and a 4-week post-exposure
period were added. Parameters monitored included clinical signs of
toxicity, auditory brain stem responses, ophthalmology, sperm count
and morphology, and histopathological examination of all respiratory
tract tissues (lungs and nasal turbinates) and the perfused nervous
system. Evaluations of neurological function (functional observation
battery and motor activity) were conducted in both studies. Light
microscopic evaluation of the perfused-fixed nervous system tissues
(six rats per sex per group) was conducted in the first study only.
In the first study, transient, reversible cage-side observations
during exposure periods included hypoactivity, blepharospasm, and a
delayed or absent startle reflex at the highest concentration. Rats
exposed to 2430 mg/m3 were reported as being hypoactive during
exposure, although no further specifics were given. Statistically
significant ( P < 0.05) exposure-related decreases in motor activity
(total) were observed in male rats exposed to the two highest
concentrations of cumene, but these results were not observed in the
second study in either sex. There were no exposure-related changes
noted in the functional observation battery in this or the subsequent
study. No effects were observed in the neurohistopathological
examinations. Cataracts were reported in males at all exposure
concentrations in this study. However, these results were not observed
in the second study in which a more comprehensive protocol for eye
examination was employed. Evaluation of the auditory brain stem
responses revealed no meaningful changes in the auditory function of
the exposed animals. The only gross histopathology noted was
periocular swelling, which occurred in animals at the two highest
concentrations (and for which neither incidence nor severity was
reported). Both absolute and relative weights were increased
significantly (>10%) in the kidneys, adrenal glands, and livers of
both sexes at the highest concentration. These changes were also noted
in the liver at the next lower concentration (2430 mg/m3) for both
females and males. Kidney lesions described in male rats at the two
highest exposure concentrations were considered to be closely related
to male rat-specific nephropathy (i.e., lesions were limited to males,
and tubular proteinosis, hypertrophy, and hyperplasia as well as
hyaline droplet formation were noted, although the identity of the
protein in the droplets was not confirmed) and are of questionable
relevance to human toxicity, principally because renal lesions
characteristic of this type of nephropathy have not been observed in
humans (US EPA, 1991a; Hard et al., 1993). Chronic progressive
nephropathy, a common spontaneous renal disease of Fischer-344 male
rats that occurs as early as 5 months of age (Montgomery & Seely,
1990), may also contribute to these renal lesions. Water consumption
was significantly increased (about 40%) in male rats above control
values at both 2430 and 5890 mg/m3. Several haematological and serum
measures were also changed in a statistically significant dose-related
manner at both 2430 and 5890 mg/m3: leukocytes (both sexes),
platelets (both sexes), lymphocytes (males only), glucose (females
only), and calcium/phosphorus (males only).
The results of the second study, with a 4-week post-exposure
period, indicated limited reversibility of the organ weight
alterations, because significant mean weight increases were still
present in female liver and female adrenals of the highest exposure
group. In males, only relative kidney weights (significant at 6%) and
absolute liver weights remained increased significantly. Blood and
serum parameters were not reported in this study. Morphological
evaluation of epididymal and testicular sperm showed no cumene-related
differences in count, morphology, or stages of spermatogenesis,
although one high-dose rat did have diffuse testicular atrophy.
The weight alterations in the male and female adrenals and female
kidney are considered potentially adverse, as the persistence noted
indicates limited reversibility and engenders uncertainty about the
progression and fate of these alterations under chronic exposure. The
increased water consumption noted may also indicate potential for
renal effects, although this effect was present at the next to highest
dose level at which renal weights were not altered. Although the
progression of these weight alterations from continued exposure cannot
be ascertained from this subchronic study, data from the second
(post-exposure) study indicate limited reversibility of effects on the
adrenals, at least in females. The liver weight alterations are not
viewed as adverse, because increase in liver weight without
accompanying pathology is a trait of common microsomal enzyme inducing
agents, although it should be noted that induction of hepatic
microsomal enzymes may influence the metabolism of other substances
and may either increase or decrease their toxicity (Sipes & Gandolfi,
1991). The altered haematological and serum parameters noted at the
two highest concentrations may be considered as significant, although
all are within normal ranges (Mitruka & Rawnsley, 1981). Based on the
lowest dose at which both relative and absolute weight alterations in
adrenal tissues of both sexes and in the kidneys of females are
statistically ( P < 0.05) and biologically (>10%) significant, 5890
mg/m3 may be considered as a lowest-observed-adverse-effect level
(LOAEL), and 2430 mg/m3 the corresponding NOAEL. Based on
consideration of the various measures in the first study (motor
effects, increased water consumption in males, haematological and
serum parameters, sporadic weight increases in male adrenals and
female kidneys) as significant, 2430 mg/m3 may be considered as a
LOAEL and 490 mg/m3 as the corresponding NOAEL. It should be noted
here that a LOAEL of 2391 mg/m3 (488 ppm) and a NOAEL of 485 mg/m3
(99 ppm) were noted for maternal toxicity in the short-term
developmental study in rats by Darmer et al. (1997), discussed in
section 8.6.
8.4.2 Chronic exposure and carcinogenicity
There are no long-term in vivo bioassays addressing the issue
of cancer. No data exist to support any quantitative cancer
assessment.
Wolf et al. (1956) conducted a study involving groups of 10
female Wistar rats administered cumene by gavage in olive oil at 154,
462, or 769 mg/kg body weight per day, 5 days/week, over a 194-day
(6- to 7-month) period, equivalent to 110, 331, or 551 mg/kg body
weight per day, adjusted for daily exposure. Rats given olive oil
served as controls ( n = 20). A pronounced increase in average kidney
weight, noted as a "moderate effect," occurred at 769 mg/kg body
weight per day, although no quantitative data are presented. An
increase in average kidney weight was noted as a "slight effect" at
462 mg/kg body weight per day. It is stated in the report that at 154
mg/kg body weight per day, no evidence of ill effects, as determined
by gross appearance, growth, periodic blood counts, analysis for blood
urea nitrogen, average final body and organ weights, and bone marrow
counts, was noted. The LOAEL is 462 mg/kg body weight per day, and the
NOAEL is 154 mg/kg body weight per day. These results are consistent
with those observed in more recent, better-reported studies described
elsewhere in this document.
In an inhalation study by Fabre et al. (1955), Wistar rats were
exposed (whole body) to cumene vapour at 2500 mg/m3 (510 ppm), and
rabbits were exposed to 6500 mg/m3 (1327 ppm), for 8 h/day, 6
days/week, for up to 180 days. Histological effects reported were
"passive congestion" in the lungs, liver, spleen, kidney, and adrenals
and the presence of haemorrhagic zones in the lung, haemosiderosis in
the spleen, and lesions from epithelial nephritis "in some cases." It
was not clear from the study if these effects occurred in rats or
rabbits, or both.
8.5 Genotoxicity and related end-points
In general, negative results have been obtained in a relatively
complete battery of in vivo and in vitro mutagenicity tests,
including gene mutation, chromosomal aberration, and primary DNA
damage (US EPA, 1997). Cumene was tested at concentrations up to
2000 µg/plate in a Salmonella typhimurium reverse mutation assay
(modified Ames test); negative results were observed with and without
metabolic activation (Lawlor & Wagner, 1987). Cumene was negative in
an Ames assay at concentrations up to 3606 µg/plate with
S. typhimurium strains TA98, TA100, TA1535, and TA1537 (Florin et
al., 1980). Cumene also tested negative, with and without metabolic
activation, in a set of HGPRT assays (using Chinese hamster ovary
cells) at cumene concentrations of 100-125 µg/ml, at which the
relative cloning efficiencies (a measure of cytotoxicity) ranged from
29% to 110% (Gulf Life Sciences Center, 1985a; Yang, 1987). A
micronucleus assay performed in mice given up to 1 g cumene/kg body
weight by gavage was negative (Gulf Life Sciences Center, 1985b).
Micronucleus assays done in Fischer-344 rats, however, gave values
that were weakly positive, although little dose-response was seen, and
deaths occurred at the highest dose (5 of 10 animals at 2.5 g/kg body
weight intraperitoneally; NTP, 1996). The positive control used in the
micronucleus tests, cyclophosphamide, produced strong positive
responses in all assays.
Cumene failed to induce significant rates of transformation in
BALB/3T3 cells (without activation) at concentrations up to 500 µg/ml
(Putnam, 1987) but tested positive in an earlier cell transformation
test also using BALB/3T3 cells, in which an increase in
transformations was observed at 60 µg/ml (Gulf Oil Corp., 1984a).
Results from an unscheduled DNA synthesis assay in rat hepatocytes
conducted by Gulf Oil Corp. (1984b) indicated positive results at
doses of 16 and 32 µg cumene/ml (with 128 µg/ml noted as toxic to the
hepatocytes). However, apparent technical difficulties with this test
(US EPA, 1988) prompted a repeat of the unscheduled DNA synthesis
assay in rat hepatocytes, the results of which showed cumene to be
clearly negative at doses up to 24 µg/ml, with doses above 24 µg/ml
noted as being too toxic for evaluation of unscheduled DNA synthesis
(Curren, 1987; US EPA, 1988).
8.6 Reproductive and developmental toxicity
No multigeneration reproductive study exists for this compound by
either the oral or inhalation route. There are no data concerning
cumene exposure of females prior to mating, from conception to
implantation, or during late gestation, parturition, or lactation.
The first subchronic inhalation study of Cushman et al. (1995),
however, conducted morphological evaluation of epididymal and
testicular sperm in rats exposed for 13 weeks to cumene vapours (see
section 8.4.1). No cumene-related differences in count, morphology, or
stages of spermatogenesis were noted, although one high-dose rat did
have diffuse testicular atrophy. No alterations (weight changes,
histopathology) were noted in the female reproductive organs that were
examined at the termination of this same study.
In an inhalation study (Darmer et al., 1997; also reported as
Bushy Run Research Center, 1989b), Sprague-Dawley rats (25 per group)
were exposed whole body to 0, 485, 2391, or 5934 mg cumene/m3 (0, 99,
488, or 1211 ppm) for 6 h/day on days 6 through 15 of gestation.
Perioral wetness and encrustation, a significant ( P < 0.01)
decrease in body weight gain on gestation days 6 through 9
(accompanied by a significant decrease in food consumption), and a
slight increase (7.7%) in relative liver weight were observed in dams
at the high dose only. Hypoactivity, blepharospasm, and significantly
( P < 0.05) decreased food consumption were observed in the dams at
the next highest concentration. There were no statistically
significant adverse effects on reproductive parameters or fetal
development. For this study, 5934 mg/m3 is a developmental NOAEL, and
485 mg/m3 is a maternal NOAEL.
In another inhalation study (Darmer et al., 1997; also reported
as Bushy Run Research Centre, 1989c), New Zealand white rabbits (15
per group) were exposed whole body to 0, 2411, 5909, or 11 255 mg
cumene/m3 (0, 492, 1206, or 2297 ppm) for 6 h/day on days 6 through
18 of gestation. Two does died and one aborted at the highest exposure
concentration. There were significant ( P < 0.01) reductions in body
weight gain (178 g lost compared with 31 g gained in the control
group) and food consumption at the highest exposure level during the
treatment period. Significantly reduced food consumption was also
observed in the 2411 and 5909 mg/m3 exposure groups, but it was not
accompanied by any decrease in weight gain. Clinical signs of toxicity
observed in the does included significant ( P < 0.01) increases in
perioral and perinasal wetness and blepharospasm at the highest
concentration. At necropsy, there were colour changes in the lungs of
33% of the does exposed to 11 255 mg/m3. Relative liver weight was
significantly ( P < 0.01) elevated (16.8% over control weight) at
the highest exposure level. There were no statistically significant
effects on gestation parameters; however, there were non-significant
increases in non-viable implants and early resorptions and a
non-significant decrease in the percentage of live fetuses concurrent
with maternal toxicity at 11 255 mg/m3. Apparent increases in
ecchymosis (haemorrhagic areas of the skin) of the head were shown to
be within the ranges observed for the historical controls of this test
facility (US EPA, 1991b). The highest exposure level resulted in
maternal mortality. The next lower dose of 5909 mg/m3, at which the
only effect noted was reduced food consumption without accompanying
weight loss, is considered the NOAEL of the study.
8.7 Immunological and neurological effects
No studies were located that examined immunotoxicity in animals
after exposure to cumene by any route.
Cumene appears to be similar to many solvents, such as alcohol,
that are known central nervous system depressants. The occurrence of
neurological effects from inhalation exposure to cumene has been
confirmed in several studies. These studies are acute exposures that
show neurotoxicological effects only at quite high concentrations
(>2450 mg/m3 [>500 ppm]). Neurotoxicological effects were not
observed in the longer-term inhalation study by Cushman et al. (1995),
which included complete batteries of functional and motor activity
tests and neurohistopathology and in which the highest exposure
concentration was 5890 mg/m3 (1202 ppm).
Cumene was tested at 0, 9800, 19 600, or 39 200 mg/m3 (0, 2000,
4000, or 8000 ppm) and produced a short-lived profile of
neurobehavioural effects in mice that indicated central nervous system
depressant activity (Tegeris & Balster, 1994). Effects noted from
brief (20-min) whole-body exposures to cumene included those on
central nervous system activity (decreased arousal and rearing at 9800
mg/m3), muscle tone/equilibrium (changes in grip strength and
mobility at 19 600 mg/m3), and sensorimotor activity (including
decreased tail pinch and touch response at 19 600 mg/m3).
In an acute experiment accompanying the subchronic exposures (see
section 8.4.1), Cushman et al. (1995) exposed Fischer-344 rats (whole
body) once to 0, 490, 2430, or 5890 mg/m3 (0, 100, 496, or 1202 ppm)
for 6 h and conducted functional observations 1 h post-exposure. Gait
abnormalities and decreased rectal temperatures were noted for both
sexes at the highest exposure level only. Decreased activity levels
were noted for both sexes at the highest level and for females only at
the next highest level (2430 mg/m3) of exposure. Males, but not
females, from the highest exposure group had decreased response to toe
pinch at 6 h post-exposure.
In a 5-day inhalation study, Fischer-344 rats exposed whole body
to 9800 or 24 500 mg cumene vapour/m3 (2000 or 5000 ppm) for 6 h/day
showed toxic effects from exposure (Gulf Oil Corp., 1985). All rats in
the high-exposure group died after 2 days. At the lower dose, females
demonstrated central nervous system effects (hypothermia and
staggering). Similar, but more severe, symptoms were observed in the
high-exposure animals before they died.
Fischer-344 rats (10 per sex per group) were exposed whole body
to cumene at 0, 1230, 2680, 5130, or 6321 mg/m3 (0, 251, 547, 1047,
or 1290 ppm) for 6 h/day, 5 days/week, for 2 weeks (Chemical
Manufacturers Association, 1989). Initial exposures to 9800 mg/m3
(2000 ppm) for 1-2 days resulted in such severe neurological and
respiratory effects that the concentration levels were reduced to
those given above. During the remainder of the 2-week exposure period,
clinical observations (ocular discharge, decreased motor activity or
hyperactivity, and ataxia) were noted sporadically at all levels
except 1230 mg/m3. For females in the two highest dose groups, the
average relative kidney weight and relative and absolute adrenal
weights were increased significantly over control values. These data
provide corroboration for these same effects reported in the study of
Cushman et al. (1995).
9. EFFECTS ON HUMANS
No information was located regarding the toxicity of cumene in
humans following acute, subchronic, or chronic exposure (US EPA,
1997). The minimum lethal human exposure to this agent has not been
delineated. No epidemiology, case reports, or clinical controls of
humans were located for this compound. There are no epidemiological or
occupational studies examining the carcinogenicity of cumene in humans
(US EPA, 1997).
No information was located regarding dermal irritation and
sensitization in humans following exposure to cumene.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
The available environmental effects studies are inadequate to
allow a quantitative assessment of the acute toxicity of cumene to
environmental organisms owing to the variability of the data and
flawed experimental designs. For example, 24-h toxicity values for
water fleas ranged from an EC50 of 91 mg/litre (Bringmann & Kuhn,
1982) down to an IC50 of 0.6 mg/litre (Abernathy et al., 1986).
Further, many of the reported toxicity values for aquatic
invertebrates exceed the water solubility of cumene at 50 mg/litre,
with Glickman et al. (1995) noting that actual measured concentrations
of cumene were only about 10% of nominal concentrations. The lowest
reported toxic concentration was 0.012 mg/litre, the toxicity
threshold for the protozoan Colpidium colpoda (Rogerson et al.,
1983). Concentrations of up to 50 mg/litre did not affect the growth
of the larvae of the mussel Mytilis edulis during a 27-day exposure
(Le Roux, 1977). Selected data demonstrating effect concentrations are
shown in Table 2. It should be noted that the high volatility and
biodegradability of cumene may further reduce the hazard to the
aquatic environment, especially for chronic exposure conditions.
Table 2: Acute toxicity of cumene to organisms other than laboratory mammals.
Species End-point Concentration
(effect) (mg/litre) Reference
Algae
Green alga 3-h EC50 21 Hutchinson et al.,
(Chlorella vulgaris) (photosynthetic 1980
inhibition)
Green alga 3-h EC50 9 Hutchinson et al.,
(Chlamydomonas angulosa) (photosynthetic 1980
inhibition)
Green alga 72-h EC50 2.6 Galassi et al.,
(Selenastrum capricornutum) (growth 1988
inhibition)
Green algae 72-h static EC50 2.0 Hüls, 1998a
(Scenedesmus subspicatus) (growth
inhibition)
Invertebrates
Water flea (Daphnia magna) 24-h EC50 91 Bringmann & Kuhn,
(immobilization) 1982
Water flea (Daphnia magna) 24-h LC50 4.8 Glickman et al.,
1995
Water flea (Daphnia magna) 21-day static EC50 1.5 Hüls, 1998b
Water flea (Daphnia magna) 24-h IC50a 1.4 Galassi et al.,
1988
Table 2 (continued)
Water flea (Daphnia magna) 24-h IC50 0.6 Abernathy et al.,
1986
Mysid shrimp (Mysidopsis bahia) 96-h flow LC50 1.3 Glickman et al.,
1995
Mysid shrimp (Mysidopsis bahia) 96-h flow LC50 1.2 Chemical Manufacturers
Association, 1990
Ciliate protozoan "toxicity threshold" 0.012 Rogerson et al.,
(Colpidium colpoda) (NR)b 1983
Vertebrates
Rainbow trout 96-h LC50 4.8 Glickman et al.,
(Oncorhynchus mykiss) 1995
Rainbow trout no observed effect 1.9 Glickman et al.,
(Oncorhynchus mykiss) 1995
Sheepshead minnow 96-h flow LC50 4.7 Glickman et al.,
(Cyprinodon variegatus) 1995
Sheepshead minnow no observed effect <2.9 Glickman et al.,
(Cyprinodon variegatus) 1995
a IC50 = immobilization concentration for 50% of the organisms.
b NR = not reported.
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
Kinetic analysis shows that there is rapid and complete clearance
of cumene and its metabolites from the body, indicating little
potential for accumulation. No human toxicity data are available from
exposure to cumene. Short-term exposures of animals to high
concentrations (>2450 mg/m3 [>500 ppm]) demonstrate that cumene,
like other solvents, may be considered harmful, inducing transient
reversible central nervous system effects. However, neurotoxicity,
portal-of-entry effects, developmental effects, and markedly adverse
systemic toxicity were not observed after long-term repeated-dose
studies conducted in animals at lower concentrations (<2450 mg/m3
[<500 ppm]). Cumene has caused dermal and ocular irritation in
animals in one study, but it had no such effects in others. A single
study indicates that cumene does not elicit dermal sensitization in
animals.
Increases in organ weights (most notably kidney) are the most
prominent and consistent effects observed in rodents exposed for 6-7
months by the oral route (Wolf et al., 1956) or for 3 months by the
inhalation route (Cushman et al., 1995). No adverse effects were
observed in rat or rabbit fetuses whose mothers had been exposed to
airborne cumene during fetal development.
The sparsity of long-term repeated-dose toxicity data and the
absence of any human toxicity data both constitute areas of scientific
uncertainty. The only repeated-dose toxicity studies of any
appreciable duration are the oral study of Wolf et al. (1956), at
about 7 months, and the 3-month subchronic inhalation study of Cushman
et al. (1995). Both of these studies are concurrent in indicating
kidneys of female rats as the target organ, regardless of exposure
route. Although neither of these studies is sufficient in duration to
reveal the fate of the observed alterations in organ weights from
lifetime chronic exposure, the subchronic study of Cushman et al.
(1995) is more scientifically comprehensive in its analyses than the
study of Wolf et al. (1956) and offers much more extensive data
reporting on more animals (both genders). The study of Cushman et al.
(1995) is therefore chosen as the pivotal study.
No multigeneration reproductive studies have been performed for
cumene. The rapid metabolism and excretion of cumene, coupled with the
lack of effects on sperm morphology reported by Cushman et al. (1995),
indicate that cumene has low potential for reproductive toxicity.
However, this lack of concern must be weighed against the fact that
kinetic studies indicate extensive and wide distribution of cumene,
including to reproductive organs, and the fact that the consequences
of long-term repeated/continuous exposure on either organs or
reproductive function have not been evaluated.
There are no data in humans or animals concerning the development
of cancer following exposure to cumene. The potential hazard for
carcinogenicity of cumene to humans has not been determined, although
the predominant evidence suggests that this compound is not likely to
produce a carcinogenic response (i.e., numerous genotoxic tests,
including gene mutation, chromosomal aberration, and primary DNA
damage tests, were conducted, all but one of which were negative or
not reproducible). No highly reactive chemical species are known to be
generated during the metabolism of cumene.
11.1.2 Criteria for setting guidance values for cumene
For oral exposures, the NOAEL for increased average kidney weight
in female rats following subchronic (139/194 days) oral (gavage)
exposure is 154 mg/kg body weight per day, which was adjusted, based
on the dosing schedule, to 110 mg/kg body weight per day (Wolf et al.,
1956). These data were not amenable to benchmark dose analysis. For
purposes of quantitative assessment, the quality of the principal oral
study is marginal, because the group sizes were minimal, the groups
comprised females only, and little quantitative information was
presented. Full uncertainty factors of 10 each are applied for
interindividual and interspecies variations. A partial uncertainty
factor (100.5) for extrapolation from subchronic to chronic duration
is applied, as the study was intermediate between chronic and
subchronic duration. Another partial uncertainty factor (100.5) is
also used owing to lack of a full-scale multigeneration reproductive
study. The total uncertainty factor applied was 1000 (10 × 10 × 100.5
× 100.5). This yields a guidance value for oral exposure of 0.1 mg/kg
body weight per day. This guidance value is meant to provide
information for risk managers to enable them in making decisions
concerning the protection of human health.
Interpretation of the effects reported in the subchronic
inhalation study of Cushman et al. (1995) allows for consideration of
either the 490 mg/m3 (100 ppm) (MAK, 1996) or the 2430 mg/m3 (496
ppm) (US EPA, 1997) exposure level as a defensible NOAEL. Whereas the
motor effects, organ weight changes, and clinical effects reported at
2430 mg/m3 (496 ppm) may be regarded as non-adverse indicators of
exposure (in other words, as a NOAEL), these same effects may be
regarded alternatively as potentially adverse indicators of
toxicologically significant effects apparent at the next highest
exposure level (in other words, a LOAEL). Consideration of both these
interpretations may be justified in derivation of an inhalation
guidance value for cumene. The experimental exposure scenario of the
NOAEL (either 490 or 2430 mg/m3 [100 or 496 ppm]) is first adjusted
to a continuous exposure scenario for the general population by
factoring the NOAEL by the hours exposed as a fraction of the day
(6/24 hours) and the number of days exposed as a fraction of the week
(5/7), resulting in the figure of 436 mg/m3 (89 ppm) for the 2430
mg/m3 (496 ppm) experimental exposure level and 88 mg/m3 (18 ppm)
for the 490 mg/m3 (100 ppm) experimental exposure level. Full
uncertainty factors of 10 each were applied for subchronic to chronic
extrapolation and for interindividual variations. A partial
uncertainty factor (100.5) is applied to account for the toxicodynamic
component of the interspecies extrapolation. In long-term inhalation
exposures, the blood/air partition coefficient ( Hb/a) is a principal
factor determining the amount of compound reaching a systemic tissue
(such as kidney). For a given external concentration and similar
exposure conditions, the smaller the Hb/a values, the less compound
in the blood and at the tissue. The blood/air partition coefficient
has been determined with human blood (Sato & Nakajima, 1979, 1987),
but not for rats. Information available on compounds structurally
related to cumene (xylenes and benzene; Gargas et al., 1989) indicates
that human Hb/a values are nearly always smaller than rat Hb/a
values, such that, for a given external concentration, human tissues
would receive less compound than would rat tissues. Thus, use of the
rat in a long-term repeated-dose study with cumene obviates the need
for the toxicokinetic component of the animal to human extrapolation.
An additional partial uncertainty factor (100.5) is used for database
deficiencies, owing principally to lack of a full-scale
multigeneration reproductive study, as discussed above. The total
uncertainty factor would be 1000 (10 × 10 × 100.5 × 100.5).
Application of this factor would result in guidance values of 0.4
mg/m3 (0.08 ppm) for the NOAEL of 436 mg/m3 (89 ppm), adjusted for
continuous exposure from 2430 mg/m3 (496 ppm), and 0.09 mg/m3
(0.02 ppm) for the NOAEL of 88 mg/m3 (18 ppm), adjusted for
continuous exposure from 490 mg/m3 (100 ppm).
The carcinogenic potential of cumene cannot be determined because
no adequate data, such as well-conducted long-term animal studies or
reliable human epidemiological studies, are available with which to
perform an assessment.
11.1.3 Sample risk characterization
The scenario chosen as an example is continuous lifetime exposure
for the general population.
No human data are available with which to characterize the
toxicity of cumene directly. The reported ambient cumene concentration
of 0.0147 mg/m3 (0.003 ppm) is appreciably below either of the
guidance values of 0.4 mg/m3 (0.08 ppm) (27-fold) or 0.09 mg/m3
(0.02 ppm) (6-fold). The upper limit of ambient cumene concentrations
reported in rural air, 2.5 µg/m3 (0.5 ppb), is even further below the
guidance values (36- to 160-fold). Other data presented in this
report, such as estimates from cigarette smoke, suggest that humans
would primarily be exposed through inhalation, although ingestion
through food may occur. Exposure via drinking-water is probably
unlikely.
The critical effect in the principal study for the oral
assessment is increased kidney weight in female rats and, although
poorly reported, is corroborated by inhalation studies with cumene.
Increased organ weights have been found in other toxicity studies with
cumene and have been observed across routes of exposure. Insufficient
data on oral exposure exist to apply the guidance value of 0.1 mg/kg
body weight per day derived above.
Following inhalation exposure, the effects observed included
increased kidney and adrenal weights and central nervous system,
haematological, and clinical biochemical alterations, which were
observed in rats. The critical effect was observed across species and
was observed in several studies. These results partially corroborate
and reinforce the significance of similar results seen in the
long-term oral study of cumene.
The potential hazard for carcinogenicity of cumene in humans
cannot be determined. Studies have indicated that cumene has low, if
any, genotoxicity.
Neither chronic nor multigeneration reproductive studies are
available for this substance.
Data are not available to determine whether young or aged animals
are more susceptible than adult animals (e.g., 2-year-old rats) to the
effects of cumene, and there is no evidence to suggest that this would
be so in young or aged humans. There is also no convincing evidence to
suggest that gender differences in susceptibility to cumene toxicity
would exist in humans.
11.2 Evaluation of environmental effects
Cumene is a volatile liquid and exists mainly in the vapour phase
in the atmosphere. It degrades in the atmosphere via reaction with
hydroxyl radicals. Although small amounts of cumene may be removed
from the atmosphere by precipitation, cumene is not expected to react
with ozone or directly with light. In water, cumene can be
volatilized, undergo biodegradation, or adsorb to sediments. In soil,
it is expected to biodegrade rapidly under aerobic conditions; as in
water, it can readily adsorb to soil or volatilize.
BCFs suggest a slight potential for cumene to bioconcentrate in
fish species. No data were available on the bioconcentration of cumene
in terrestrial organisms. Although the existing toxicological database
and limited exposure data do not permit a quantitative risk
assessment, the available information suggests that cumene will not
adversely affect populations or communities of terrestrial or aquatic
organisms based on its low availability (volatility, rapid
degradation).
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
No previous evaluations by international bodies were identified.
Information on international hazard classification and labelling
is included in the International Chemical Safety Card reproduced in
this document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventive and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 0170) reproduced in this
document.
13.1 Human health hazards
Cumene is flammable. Exposure could cause central nervous system
effects and at high concentrations could result in unconsciousness.
13.2 Advice to physicians
In the event of poisoning, treatment is supportive.
13.3 Health surveillance advice
For workers exposed to cumene, a health surveillance programme
should include surveillance of kidney function.
13.4 Spillage
In the event of spillage, measures should be taken to prevent
cumene from reaching drains and watercourses, owing to the potential
for hazardous effects on aquatic organisms.
13.5 Storage
Cumene should be stored away from acids and strong oxidants.
Long-term storage could result in the formation of explosive
peroxides. Proper safety and handling procedures must be used.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards
may be obtained from UNEP Chemicals (IRPTC), Geneva.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
ISOPROPYLBENZENE ICSC:0170
October 1994
CAS # 98-82-8 Cumene
RTECS# GR8575000 (1-Methylethyl)benzene
UN # 1918 2-Phenylpropane
EC # 601-024-00-X C9H12/C6H5CH(CH3)2
Molecular Mass
TYPES OF HAZARD/ ACUTE HAZARDS/ PREVENTION FIRST AID/
EXPOSURE SYMPTOMS FIRE FIGHTING
FIRE Flammable NO open flames, Powder, AFFF,
NO sparks, and foam, carbon dioxide
NO smoking.
EXPLOSION Above 31°C explosive Above 31°C use a In case of fire: keep
vapour/air mixtures closed system, drums, etc., cool by
may be formed. ventilation, and spraying with water.
explosion-proof
electrical equipment.
Prevent build-up of
electrostatic charges
(e.g., by grounding).
EXPOSURE PREVENT GENERATION
OF MISTS!
Inhalation Ataxia. Cough. Ventilation, local Fresh air, rest.
Dizziness. Drowsiness. exhaust, or breathing Half-upright position.
Headache. Sore throat. protection. Refer for medical
Unconsciousness. attention.
Skin Dry skin. Protective gloves. Remove contaminated
Protective clothing. clothes. Rinse and
then wash skin with
water and soap.
Eyes Redness. Pain. Safety spectacles. First rinse with
plenty of water for
several minutes
(remove contact lenses
if easily possible),
then take to a doctor.
Ingestion (further see inhalation). Do not eat, drink, or Rinse mouth. Do NOT
smoke during work. induce vomiting. Refer
for medical attention.
SPILLAGE DISPOSAL PACKAGING & LABELLING
Collect leaking and spilled liquid in Marine Pollutant
sealable containers as far as possible. EU Classification
Absorb remaining liquid in sand or inert Symbol: Xi
absorbent and remove to safe place. R: 10-37
Do NOT let this chemical enter the S: (2-)
environment (extra personal protection: Note: C
A/P2 filter respirator for organic vapour UN Classification
and harmful dust.) UN Hazard Class: 3
UN Pack Group: III
EMERGENCY RESPONSE STORAGE
Transport Emergency Card: TEC (R)-594 NFPA Fireproof. Separated from strong oxidants,
Code: H2; F3; R0 acids. Cool. Keep in the dark. Store only
if stabilized.
IMPORTANT DATA
PHYSICAL STATE; APPEARANCE ROUTES OF EXPOSURE:
COLOURLESS LIQUID, WITH CHARACTERISTIC The substance can be absorbed into the
ODOUR. the body by inhalation and through the
skin.
PHYSICAL DANGERS: INHALATION RISK:
As a result of flow, agitation, etc., A harmful contamination of the air will be
electrostatic charges can be generated. reached rather slowly on evaporation of this
substance at 20°C.
CHEMICAL DANGERS: EFFECTS OF SHORT TERM EXPOSURE:
The substance can form explosive peroxides. The substance irritates the eyes and the
Reacts violently with acids and strong respiratory tract. Swallowing the liquid may
oxidants causing fire and explosion hazards. cause aspiration into the lungs with the risk
of chemical pneumonitis. The substance may
cause effects on the central nervous system.
Exposure far above the OEL may result in
unconsciousness.
OCCASIONAL EXPOSURE LIMITS: EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
TLV: 50 ppm; 246 mg/m3 (skin) Repeated or prolonged contact with skin
(ACGIH 1996). may cause dermatitis.
PHYSICAL PROPERTIES
Boiling point: 152 °C Flash point: 31°C
Melting point: -96 °C Auto-ignition temperature: 420°C
Relative density (water = 1): 0.90 Explosive limits, vol% in air: 0.9-6.5
Solubility in water: none Octanol/water partition coefficient as
Vapour Pressure, Pa at 20°C: 427 log Pow: 3.66
Relative Vapour density (air = 1): 4.2
Relative density of the vapour/air-mixture
at 20°C (air = 1): 1.01
ENVIRONMENTAL DATA
This substance may be hazardous to the environment; special attention should be given to
water organisms, and birds.
NOTES
An added stabilizer or inhibitor can influence the toxicological properties of this
substance, consult an expert. Check for peroxides prior to distillation; eliminate if
found.
ADDITIONAL INFORMATION
LEGAL NOTICE Neither the CEC nor the IPCS nor any person acting on behalf of
the CEC or the IPCS is responsible for the use which might be
made of this information.
REFERENCES
Abernathy S, Bobra AM, Shiu WY, Wells PG, Mackay D (1986) Acute lethal
toxicity of hydrocarbons and chlorinated hydrocarbons to two
planktonic crustaceans: the key role of organism-water partitioning.
Aquatic toxicology, 8:163-174.
Amoore JE, Hautala E (1983) Odor as an aid to chemical safety: Odor
thresholds compared with threshold limit values and volatilities for
214 industrial chemicals in air and water dilution.
Journal of applied toxicology, 3(6):272-290.
Anon. (1984) Chemical profile: Cumene. Chemical Marketing Reporter,
July 23, 1984. New York, NY, Schnell Publishing Co., Inc.
Anon. (1998) Organic chemicals manufactured. Chemical and
engineering news, 78(26):43.
Arnts RR, Meeks SA (1980) Biogenic hydrocarbon contribution to the
ambient air of selected areas: Tulsa, Great Smokey Mountains, Rio
Blanco County, CO. Research Triangle Park, NC, US Environmental
Protection Agency, Office of Research and Development
(EPA 600/3-80-023).
Arnts RR, Meeks SA (1981) Biogenic hydrocarbon contribution to the
ambient air of selected areas. Atmospheric environment,
15(9):1643-1651.
Battersby NS, Wilson V (1989) Survey of the anaerobic biodegradation
potential of organic chemicals in digesting sludge. Applied
environmental microbiology, 55(2):433-439.
Botta D, Castellani Pirri L, Mantica E (1984) Ground water pollution
by organic solvents and their microbial degradation products. In:
Angeletti G, Bjorseth A, eds. Analysis of organic micropollutants in
water: Proceedings of the 3rd European Symposium, September 1983,
Oslo, Norway. Boston, MA, D. Reidel Publishing Co., pp. 261-275
(Report EUR 8518).
Bringmann G, Kuhn R (1982) Results of toxic action of water pollutants
on Daphnia magna straus tested by an improved standardized
procedure. Zeitschrift für Wasser und Abwasser Forschung, 15(1):1-6.
Brown DW, Friedman AJ, Burrows DG, Snyder GR, Patten BG (1979)
Investigation of petroleum in the marine environs of the Strait of
Juan de Fuca and northern Puget Sound. Washington, DC, US
Environmental Protection Agency (EPA 60/7-79-164).
Brugnone F, Perbellini L, Faccini GB, Pasini F, Romeo L, Gobbi M,
Zedde A (1989) Breath and blood levels of benzene, toluene, cumene and
styrene in non-occupational exposure. International archives of
occupational and environmental health, 61:303-311.
Burmaster DE (1982) The new pollution: groundwater contamination.
Environment, 24(2):7-36.
Burnham AK, Calder GV, Fritz JS, Junk GA, Svec HJ, Willis R (1972)
Identification and estimation of neutral organic contaminants in
potable water. Analytical chemistry, 44(1):139-142.
Bushy Run Research Center (1989a) Cumene fourteen-week vapor
inhalation study in rats with neurotoxicity evaluation (part 1-2),
with attached studies and cover letter dated 7 December 1989. Export,
PA. Washington, DC, US Environmental Protection Agency, Office of
Toxic Substances (Final Project Report 52-628; TSCA section 4
submission 40-8992172).
Bushy Run Research Center (1989b) Developmental toxicity study of
inhaled cumene vapor in CD (Sprague-Dawley) rats. Export, PA.
Washington, DC, US Environmental Protection Agency, Office of Toxic
Substances (Final Project Report 52-621; TSCA section 4 submission
40-8992172).
Bushy Run Research Center (1989c) Developmental toxicity study of
inhaled cumene vapor in New Zealand white rabbits. Export, PA.
Washington, DC, US Environmental Protection Agency, Office of Toxic
Substances (Final Project Report 52-622; TSCA section 4 submission
40-8992172).
Chemical Manufacturers Association (1989) A two-week pilot
inhalation toxicity study of cumene vapors in rats, with attachments
and cover letter dated 7 September 1989. Washington, DC. Washington,
DC, US Environmental Protection Agency, Office of Toxic Substances
(TSCA section 4 submission 40-8992168).
Chemical Manufacturers Association (1990) Cumene (isopropylbenzene):
Acute toxicity to mysid shrimp (Mysidopsis bahia ) under
flow-through conditions. Washington, DC. Washington, DC, US
Environmental Protection Agency, Office of Toxic Substances (TSCA
section 4 submission 40-9092188).
Ciba-Geigy Co. (1985) Toxicology data summary sheet. Washington, DC,
US Environmental Protection Agency, Office of Toxic Substances (TSCA
section 8(d) submission 878215034).
Cocheo V, Bellomo ML, Bombi GG (1983) Rubber manufucture: sampling and
identification of volatile pollutants. American Industrial Hygiene
Association journal, 44(7):521-527.
Coleman WE, Munch JW, Striecher RP, Ringhand HP, Kopfler FC (1984) The
identification and measurement of components in gasoline, kerosene,
and No. 2 fuel oil that partition into the aqueous phase after mixing.
Archives of environmental contamination and toxicology, 13:171-178.
Conkle JP, Camp BJ, Welch BE (1975) Trace composition of human
respiratory gas. Archives of environmental health, 30(6):290-295.
Curren RD (1987) Unscheduled DNA synthesis in rat primary
hepatocytes -- test article: Cumene. Bethesda, MD, Microbiological
Associates, Inc. Washington, DC, US Environmental Protection Agency,
Office of Toxic Substances (Study No. T4786.380005; TSCA section 4
submission 40-8792124).
Cushman JR, Norris JC, Dodd DE, Darmer KI, Morris CR (1995) Subchronic
inhalation toxicity assessment of cumene in Fischer 344 rats.
Journal of the American College of Toxicology, 14(2):129-147.
Darmer KI, Neeper-Bradley TL, Cushman JR, Morris CR, Francis BO (1997)
Developmental toxicity of cumene vapor in CD rats and New Zealand
white rabbits. International journal of toxicology, 16:119-139.
Derwent RG, Jenkin ME (1990) Hydrocarbon involvement in
photochemical ozone formation in Europe. Harwell, United Kingdom
Atomic Energy Authority (Report No. AERE R 13736).
Eisenreich SJ, Looney BB, Thornton JD (1981) Airborne organic
contaminants of the Great Lakes ecosystem. Environmental science and
technology, 15(1):30-38.
Fabre R, Truhaut R, Bernuchon J, Loisillier F (1955) Toxicologic
studies of solvents to replace benzene. III. Study of isopropyl
benzene or cumene. Archives des maladies professionnelles de
médecine du travail et de sécurité sociale, 16(4):285-299 (in
French).
Florin I, Rutberg L, Curvall M, Enzell CR (1980) Screening of tobacco
smoke constituents for mutagenicity using the Ames test. Toxicology,
18:219-232.
Foster P, Laffond M, Baussand P, Jacob V, Denis I (1991) VOC
measurements in the Grenoble area and study of the benzaldehyde
behaviour in a simulation chamber. Pollution atmosphérique,
19:175-191 (in French).
Galassi S, Mingazzini M, Vigano I, Cesareo D, Tosato MI (1988)
Approaches to modeling toxic responses of aquatic organisms to
aromatic hydrocarbons. Ecotoxicology and environmental safety,
16:158-169.
Gargas MJ, Burgess RJ, Voisard DE, Cason GH, Andersen ME (1989)
Partition coefficients of low-molecular-weight volatile chemicals in
various liquids and tissues. Toxicology and applied pharmacology,
98:87-99.
Gavin J, Nicollier G, Tabacchi R (1978) Volatile constituents of
"oakmoss" ( Evernia prunastri (L.)). Part 3. Helvetica Chimica
Acta, 61(1):352-357.
Glickman AH, Alexander HC, Buccafusco RJ, Morris CR, Francis BO,
Suprenant DC, Ward TJ (1995) An evaluation of the aquatic hazard of
cumene (isopropyl benzene). Ecotoxicology and environmental safety,
31:287-289.
Gordon AW, Gordon M (1981) Analysis of volatile organic compounds in a
textile finishing plant effluent. Transactions of the Kentucky
Academy of Science, 42(3-4):149-157.
Grosjean D, Fung K (1984) Hydrocarbons and carbonyls in Los Angeles
air. Journal of the Air Pollution Control Association, 34:537-543.
Guicherit R, Schulting RL (1985) The occurrence of organic chemicals
in the atmosphere of The Netherlands. The science of the total
environment, 43:193-219.
Gulf Life Sciences Center (1985a) CHO/HGPRT test of cumene.
Pittsburgh, PA. Washington, DC, US Environmental Protection Agency,
Office of Toxic Substances (Gulf Project No. 84-2128; TSCA section
8(d) submission 878216011).
Gulf Life Sciences Center (1985b) Micronucleus test of cumene.
Pittsburgh, PA. Washington, DC, US Environmental Protection Agency,
Office of Toxic Substances (Gulf Project No. 84-2129; TSCA section
8(d) submission 878216015).
Gulf Oil Corp. (1984a) Cell transformation test of cumene. Houston,
TX. Washington, DC, US Environmental Protection Agency, Office of
Toxic Substances (Gulf Project No. 84-2131; TSCA section 8(e)
submission 888500694).
Gulf Oil Corp. (1984b) Hepatocyte primary culture/DNA repair test of
cumene. Houston, TX. Washington, DC, US Environmental Protection
Agency, Office of Toxic Substances (Gulf Project No. 84-2130; TSCA
section 4 submission 888500694).
Gulf Oil Corp. (1985) Five-day repeated dose inhalation toxicity
study in rats of cumene, with cover letter. Houston, TX. Washington,
DC, US Environmental Protection Agency, Office of Toxic Substances
(Gulf Project No. 84-2132; TSCA section 8(d) submission 878216016).
Hansch C, Leo AJ (undated) The Pomona College Medicinal Chemistry
Project. Claremont, CA, Pomona College.
Hard GC, Rodgers IS, Baetcke KP, Richards WL, McGaughy RE, Valcovic LR
(1993) Hazard evaluation of chemicals that cause accumulation of
alpha-2µ-globulin, hyaline droplet nephropathy, and tubular neoplasia
in the kidneys of male rats. Environmental health perspectives,
99:313-349.
Holzer G, Oró J, Bertsch W (1976) Gas chromatographic-mass
spectrometric evaluation of exhaled tobacco smoke. Journal of
chromatography, 126:771-785.
Hüls (1988) Prüfung auf hautsensibilisierende Wirkung am
Meerschweinchen von Cumol. Unveröffentlichte Untersuchung (Bericht
Nr. 1219).
Hüls (1998a) Determination of the effect of cumene on the growth of
Scenedesmus subspicatus 86.81.SAG (Algal growth inhibition test
complying with Directive 92/69/EEC). Marl, Hüls Infracor,
Prüfinstitute für Analytik, Biologie und Toxikologie (Final Report
AW-469).
Hüls (1998b) Determination of the effects of cumene on the
reproduction rate of Daphnia magna (as specified by OECD 211/draft
of December 1996). Marl, Hüls Infracor, Prüfinstitute für Analytik,
Biologie und Toxikologie (Final Report DL-172).
Hutchinson TC, Hellebust JA, Tam D, Mackay D, Mascarenhas RA, Shiu WY
(1980) The correlation of the toxicity to algae of hydrocarbons and
halogenated hydrocarbons with their physical-chemical properties.
Environmental science research, 16:577-586.
IPCS (1993) International Chemical Safety Card -- Isopropylbenzene.
Geneva, World Health Organization, International Programme on Chemical
Safety (ICSC 0170).
Ishida T, Matsumoto T (1992) Enantioselective metabolism of cumene.
Xenobiotica, 22:1291-1298.
Isidorov VA, Zenkevich IG, Ioffe BV (1983) Methods and results of gas
chromatographic-mass spectrometric determination of volatile organic
substances in an urban atmosphere. Atmospheric environment,
17(7):1347-1353.
Jamison VW, Raymond R, Hudson JO (1970) Hydrocarbon co-oxidation by
Nocardia corallina strain V-49. Developments in industrial
microbiology, 12:99-105.
Japan Environment Agency (1987) Chemicals in the environment, a
report of 1987 survey. Tokyo, Japan Environment Agency,
Environmental Health Department, Office of Health Studies.
Jeng CY, Chen DH, Yaws CL (1992) Data compilation for soil sorption
coefficient. Pollution engineering, 24(12):54-60.
Jenkins LJ, Jr, Jones RA, Siegel J (1970) Long-term inhalation
screening studies of benzene, toluene, o-xylene, and cumene on
experimental animals. Toxicology and applied pharmacology,
16(3):818-823.
Johnstone RAW, Quan PM, Carruthers W (1962) Composition of cigarette
smoke: some low-boiling components. Nature, 195:1267-1269.
Kappeler T, Wuhrmann K (1978) Microbial degradation of the
water-soluble fraction of gas oil -- I. Water research, 12:327-334.
Katzman H, Libby WF (1975) Hydrocarbon emissions from jet engines
operated at simulated high-altitude supersonic flight conditions.
Atmospheric environment, 9(9):839-842.
Keith LH, Garrison AW, Allen FR, Carter MH, Floyd TL, Pope JD,
Thruston AD, Jr (1976) Identification of organic compounds in drinking
water from thirteen U.S. cities. In: Keith LH, ed. Identification
and analysis of organic pollutants in water. Ann Arbor, MI, Ann
Arbor Science, pp. 329-373.
Koch Refining Co. (1984) Material safety data sheet: Cumene. Corpus
Christi, TX.
Kristiansen U, Hansen L, Nielsen GD, Holst D (1986) Sensory irritation
and pulmonary irritation of cumene and n-propanol: Mechanisms of
receptor activation and desensitization. Acta Pharmacologica et
Toxicologica, 59:60-72.
Krotoszynski BK, O'Neill HJ (1982) Involuntary bioaccumulation of
environmental pollutants in nonsmoking heterogeneous human
population. Journal of environmental science and health. Part A:
Environmental science and engineering, 17(6):855-883.
Lawlor TE, Wagner VO (1987) Salmonella /mammalian-microsome
preincubation in mutagenicity assay (Ames test); test article:
Cumene. Bethesda, MD, Microbiological Associates, Inc. Washington,
DC, US Environmental Protection Agency, Office of Toxic Substances
(Study No. T4786.502009; TSCA section 4 submission 40-8792121).
Le Roux S (1977) The toxicity of pure hydrocarbons to mussel larvae.
Rapports et procès-verbaux des réunions, Conseil International pour
l'Exploration de la Mer, 171:189-190 (in French with English
abstract).
Levy A (1973) The photochemical smog reactivity of organic solvents.
Advances in chemistry series, 124:70-94.
Lloyd AC, Darnall KR, Winer AM, Pitts JN, Jr (1976) Relative rate
constants for reaction of the hydroxyl radical with a series of
alkanes, alkenes, and aromatic hydrocarbons. Journal of physical
chemistry, 80(8):789-794.
Lonneman WA, Bellar TA, Altshuller AP (1968) Aromatic hydrocarbons in
the atmosphere of the Los Angeles basin. Environmental science and
technology, 2(11):1017-1020.
Lonneman WA, Namie GR, Bufalini JJ (1979) Hydrocarbons in Houston
air. Research Triangle Park, NC, US Environmental Protection Agency,
Office of Research and Development (EPA 600/3-79-018).
Lyman WJ, Reehl WF, Rosenblatt DH (1982) Handbook of chemical
property estimation methods. Environmental behaviour of organic
compounds. New York, NY, McGraw-Hill Book Company.
Mackay D, Shiu WY (1981) A critical review of Henry's law constants
for chemicals of environmental interest. Journal of physical and
chemical reference data, 19:1175-1199.
MAK (1996) Deutsche Forschungsgemeinschaft, MAK-Begründung:
Isopropylbenzol (cumol), 22. Lieferung.
Mill T, Richardson H, Hendry DG (1978) Oxidation of organic compounds
in aquatic systems: The free radical oxidation of cumene. In:
Hutzinger O, Van Lelyveld IH, Zoeteman BCJ, eds. Aquatic pollutants:
Transformation and biological effects. Proceedings of the 2nd
International Symposium on Aquatic Pollutants, Amsterdam, 26-28
September 1977. New York, NY, Pergamon Press, pp. 223-236.
Mill T, Hendry DG, Richardson H, Baraze A (1979) Chemical oxidation
processes in aquatic systems: Oxidation of cumene, pyridine, and
p -cresol. Menlo Park, CA, SRI International, 73 pp. (National
Science Foundation Grant No. ENV76-11153; NSF/RA-790085; NTIS
PB-298088).
Mill T, Hendry DG, Richardson H (1980) Free-radical oxidants in
natural waters. Science, 207:886-887.
Mills A, Foureman GL (1998) U.S. EPA's IRIS pilot program:
establishing IRIS as a centralized, peer-review database with agency
consensus. Toxicology, 127:85-95.
Mitruka BM, Rawnsley HM (1981) Clinical biochemical and
hematological reference values in normal experimental animals and
normal humans, 2nd ed. New York, NY, Masson Publishing.
Mody NV, Parish EJ, Bhattacharyya J, Miles DH (1974a) Constituents of
marsh grass. IV. Essential oil in Scirpus americanus.
Phytochemistry, 13(9):2027-2029.
Mody NV, Bhattacharyya J, Miles DH (1974b) Constituents of marsh
grass. II. Survey of the essential oil in Spartina cynosuroides.
Phytochemistry, 13(7):1175-1178.
Moelhave L (1979) Indoor air pollution due to building materials. In:
Fanger PO, Valbjrn O, eds. Indoor climate: effects on human comfort,
performance, and health in residential, commercial, and
light-industry buildings. Proceedings of the 1st International
Indoor Climate Symposium, Copenhagen, Denmark, August/September 1978,
pp. 89-110.
Monsanto Co. (1984) Cumene health study Y-78-213: Acute toxicity,
eye and skin irritation. St. Louis, MO. Washington, DC, US
Environmental Protection Agency, Office of Toxic Substances (TSCA
section 8(d) submission 878215096).
Monsanto Co. (1986) One-month study of cumene vapor administered to
male and female Sprague-Dawley rats by inhalation. St. Louis, MO.
Washington, DC, US Environmental Protection Agency, Office of Toxic
Substances (TSCA section 8(d) submission 86870000044).
Montgomery CA, Jr, Seely JC (1990) Kidney. In: Boorman GA, Eustis SL,
Elwell MR, Montgomery CA, Jr, MacKenzie WF, eds. Pathology of the
Fischer rat, reference and atlas. New York, NY, Academic Press, pp.
127-153.
Montz WE, Puyear RL, Brammer JD (1982) Identification and
quantification of water-soluble hydrocarbons generated by two-cycle
outboard motors. Archives of environmental contamination and
toxicology, 11:561-565.
NIOSH (1994) Method 1501. In: NIOSH manual of analytical methods
(NMAM), 4th ed. Cincinnati, OH, National Institute for Occupational
Safety and Health.
NTP (1996) In-vivo cytogenetics testing results for cumene,
micronucleus induction results. Available from National Toxicology
Program, National Institute for Environmental and Health Sciences,
Research Triangle Park, NC.
Ogata M, Fujisawa K, Ogino Y, Mano E (1984) Partition coefficients as
a measure of bioconcentration potential of crude oil compounds in fish
and shellfish. Bulletin of environmental contamination and
toxicology, 33(5):561-567.
Omori T, Jigami Y, Minoda Y (1975) Isolation, identification and
substrate assimilation specificity of some aromatic hydrocarbons
utilizing bacteria. Agricultural and biological chemistry,
39:1775-1779.
Parlar H, Coelhan M, Vaughan D, Czermak P, Kohler U, Korte F (1983)
Gas phase-mass analyzer system for the determination of the
photostability of organic compounds under simulated tropospheric
conditions. Fresenius' Zeitschrift für Analytische Chemie,
315(7):605-609.
Pellizzari ED, Castilo NP, Willis S, Smith D, Bursey JT (1979)
Identification of organic components in aqueous effluents from
energy-related processes. In: Van Hall CE, ed. Measurement of
organic pollutants in water and wastewater: Symposium on Water,
June 1978, Denver, CO. Philadelphia, PA, American Society for
Testing and Materials, ASTM Committee D19, pp. 256-274 (ASTM Special
Technical Publication 686).
Price KS, Waggy GT, Conway RA (1974) Brine shrimp bioassay and
seawater BOD of petrochemicals. Journal of the Water Pollution
Control Federation, 46(1):63-77.
Putnam DL (1987) Chromosome aberrations in Chinese hamster ovary
(CHO) cells -- test article: Cumene. Bethesda, MD, Microbiological
Associates, Inc. Washington, DC, US Environmental Protection Agency,
Office of Toxic Substances (Study No. T4786.337012; TSCA section 4
submission 40-8792123).
Ravishankara AR, Wagner S, Fischer S, Smith G, Schiff R, Watson RT,
Tesi G, Davis DD (1978) A kinetics study of the reaction of OH with
several aromatic and olefinic compounds. International journal of
chemical kinetics, 10:783-804.
Research Triangle Institute (1989) Metabolism, disposition and
pharmacokinetics of cumene in F-344 rats following oral, IV
administration or nose-only inhalation exposure. Research Triangle
Park, NC. Washington, DC, US Environmental Protection Agency, Office
of Toxic Substances (Study No. 3543; TSCA section 4 submission
40-8992171).
Robinson D, Smith JN, Williams RT (1955) Studies in detoxication: the
metabolism of alkylbenzenes isopropylbenzene (cumene) and derivatives
of hydrotropic acid. Biochemical journal, 59:153-159.
Rogerson A, Shiu WY, Huang GL, Mackay D, Berger J (1983) Determination
and interpretation of hydrocarbon toxicity to ciliate protozoa.
Aquatic toxicology, 3(3):215-228.
Sabljic A (1987) The prediction of fish bioconcentration factors of
organic pollutants from the molecular connectivity
model. Zeitschrift für die Gesamte Hygiene und Ihre Grenzgebiete,
33(10):493-496 (in German).
Sasaki S (1978) The scientific aspects of chemical substances control
law in Japan. In: Hutzinger O, Van Letyoeld LH, Zoeteman BCJ, eds.
Aquatic pollutants: Transformation and biological effects. Oxford,
Pergamon Press, pp. 282-298.
Sato A, Nakajima T (1979) Partition coefficients of some aromatic
hydrocarbons and ketones in water, blood and oil. British journal of
industrial medicine, 36(3):231-234.
Sato A, Nakajima T (1987) Pharmacokinetics of organic solvent vapors
in relation to their toxicity. Scandinavian journal of work,
environment & health, 13(2):81-93.
Sauer TC, Jr (1981) Volatile liquid hydrocarbon characterization of
underwater hydrocarbon vents and formation waters from offshore
production operations. Environmental science and technology,
15(8):917-923.
Seila RL (1979) Nonurban hydrocarbon concentrations in ambient air
north of Houston, TX. Research Triangle Park, NC, US Environmental
Protection Agency, Office of Research and Development
(EPA 600/3-79-101; NTIS PB 298-227).
Senczuk W, Litewka B (1976) Absorption of cumene through the
respiratory tract and excretion of dimethylphenylcarbinol in urine.
British journal of industrial medicine, 33:100-105.
Shackelford WM, Cline DM, Faas L, Kurth G (1983) An evaluation of
automated spectrum matching for survey identification of wastewater
components by gas chromatography-mass spectrometry. Analytica
Chimica Acta, 146:15-27.
Sipes IG, Gandolfi AJ (1991) Biotransformation of toxicants. In:
Klassen CD, Amdur MO, Doull J, eds. Casarett and Doull's toxicology,
4th ed. New York, NY, McGraw-Hill, pp. 88-126.
Smyth HF, Carpenter CP, Weil CS (1951) Range finding toxicity data:
List IV. American Medical Association archives of industrial hygiene
and occupational medicine, 4:119-122.
Snider EH, Manning FS (1982) A survey of pollutant emission levels in
waste waters and residuals from the petroleum refining industry.
Environment international, 7(4):237-258.
SRI International (1986) 1986 directory of chemical producers:
United States of America. Menlo Park, CA, pp. 568-569.
Steurmer DH, Ng DJ, Morris CJ (1982) Organic contaminants in
groundwater near an underground coal gasification site in northeastern
Wyoming. Environmental science and technology, 16(9):582-587.
Tanii H, Huang J, Hashimoto K (1995) Structure-acute toxicity
relationship of aromatic hydrocarbons in mice. Toxicology letters,
76:27-31.
Tegeris JS, Balster RL (1994) A comparison of the acute behavioral
effects of alkylbenzenes using a functional observational battery in
mice. Fundamental and applied toxicology, 22:240-250.
Teply J, Dressler M (1980) Direct determination of organic compounds
in water using steam-solid chromatography. Journal of
chromatography, 191:221-229.
UK DOE (1994) Environmental hazard assessment (EHA): Cumene.
Garston, United Kingdom Department of the Environment, Directorate for
Air, Climate, and Toxic Substances, Toxic Substances Division.
Union Carbide Corp. (1985) Range finding tests on cumene
(isopropylbenzene). Unpublished data. Washington, DC, US
Environmental Protection Agency, Office of Toxic Substances
(Industrial fellowship 274-12; TSCA section 8(d) submission
878214980).
US EPA (1987) Health and environmental effects document for cumene.
Prepared by the Office of Health and Environmental Assessment,
Environmental Criteria and Assessment Office, US Environmental
Protection Agency, Cincinnati, OH, for the Office of Solid Waste and
Emergency Response, US Environmental Protection Agency, Washington,
DC, dated August 1987.
US EPA (1988) Cumene; final test rule. Federal register,
53(144):28195-28206.
US EPA (1991a) Alpha 2µ-micro-globulin: Association with chemically
induced renal toxicity and neoplasia in the rat. US Environmental
Protection Agency, September 1991 (EPA/625/3-91/019F).
US EPA (1991b) Increased incidence of ecchymosis in a developmental
toxicity study of inhaled cumene vapor in New Zealand white rabbits.
Memorandum dated November 23, 1991, from J. Seed, Health and
Environmental Review Division, US Environmental Protection Agency, to
G.E. Timm, Chemical Testing Branch, Existing Chemical Assessment
Division, US Environmental Protection Agency, Washington, DC.
Washington, DC, US Environmental Protection Agency, Office of Toxic
Substances (TSCA section 4 submission 40-8992172).
US EPA (1996) Test methods for evaluating solid waste, physical and
chemical methods, Final Update III, 3rd ed. Washington, DC, US
Environmental Protection Agency (SW-846).
US EPA (1997) Integrated Risk Information System (IRIS) online at
http://www.epa.gov/iris (including the Toxicological review of
cumene in support of summary IRIS information, National Center for
Environmental Assessment, Cincinnati, OH). Washington, DC, US
Environmental Protection Agency.
Van der Linden AC (1978) Degradation of oil in the marine environment.
Developments in biodegradation of hydrocarbons, 1:165-200.
Walker JD, Calomiris JJ, Herbert TL, Colwell RR (1976) Petroleum
hydrocarbons: degradation and growth potential for Atlantic Ocean
sediment bacteria. Marine biology, 34:1-9.
Ward DJ (1965) Cumene. In: Standen A, ed. Kirk-Othmer encyclopedia
of chemical technology, 2nd ed. Vol. 6. New York, NY, John Wiley
and Sons, pp. 543-546.
Ward DJ (1979) Cumene. In: Standen A, ed. Kirk-Othmer encyclopedia
of chemical technology, 3rd ed. Vol. 7. New York, NY, John Wiley
and Sons, pp. 286-290.
Westrick JJ, Mello JW, Thomas RF (1984) The groundwater supply
survey. Journal of the American Water Works Association, 76:52-59.
Williams RT, Ziegenfuss PS, Lee CM (1993) Aerobic biodegradation of
cumene: an ecocore study. Environmental toxicology and chemistry,
12:484-492.
Windholz M, ed. (1983) The Merck index, 10th ed. Rahway, NJ, Merck
and Co., Inc., p. 375.
Wolf MA, Rowe VK, McCollister DD, Hollingsworth RL, Oyen F (1956)
Toxicological studies of certain alkylated benzenes and benzene.
Archives of industrial health, 14:387-398.
Yamada K, Horiguchi S, Takahashi J (1965) The utilization of
hydrocarbons by microorganisms. VI. Screening of aromatic
hydrocarbon-assimilating microorganisms and cumic acid formation from
p-cumene. Agricultural and biological chemistry, 29(10):943-948.
Yang LL (1987) CHO/HGPRT mutation assay; test article: Cumene.
Bethesda, MD, Microbiological Associates, Inc. Washington, DC, US
Environmental Protection Agency, Office of Toxic Substances (Study No.
T4786.332010; TSCA section 4 submission 40-8792124).
APPENDIX 1 -- SOURCE DOCUMENTS
US EPA (1997): Integrated Risk Information System (IRIS) online at
http://www.epa.gov/iris (including the Toxicological review of
cumene in support of summary IRIS information, National Center for
Environmental Assessment, Cincinnati, OH), US Environmental Protection
Agency, Washington, DC
The peer review process that this and other recent (post-1996)
IRIS assessments undergo includes internal (i.e., US Environmental
Protection Agency) and external review rounds. Comments of and
responses to the external reviewers are a matter of record in the
Toxicological review. Other aspects of the IRIS review process are
explained in Mills & Foureman (1998).
US EPA (1987): Health and environmental effects document for cumene,
August 1987, National Center for Environmental Assessment, Office of
Health and Environmental Assessment, US Environmental Protection
Agency
The US EPA (1987) report is used as an expanded reference source
for the IRIS document.
UK DOE (1994): Environmental hazard assessment (EHA): Cumene, Toxic
Substances Division, Directorate for Air, Climate, and Toxic
Substances, United Kingdom Department of the Environment, Garston
The Environmental hazard assessment (EHA): Cumene document was
drafted by the Building Research Establishment (United Kingdom
Department of the Environment) and the Institute of Terrestrial
Ecology (United Kingdom Natural Environment Research Council), with
I.R. Nielsen, J. Diment, and S. Dobson as the authors. The draft
document was peer reviewed both within the United Kingdom and
internationally. Comments and additional material were received from
A.L. Barton (US Environmental Protection Agency), C.B. Buckley (South
Western Water Services, United Kingdom), J.H. Duffus (Heriot-Watt
University, Edinburgh, United Kingdom), D. Keating (Health & Safety
Executive, United Kingdom), S. Killeen (National Rivers Authority,
United Kingdom), J.S. Lawson (ICI Chemicals, United Kingdom), P.
Matthiessen (Ministry of Agriculture, Fisheries and Food, United
Kingdom), H.A. Painter (Freshfield Analysis Ltd.), N. Passant
(Department of Trade and Industry, United Kingdom), T. Sheils
(Department of the Environment, United Kingdom), and G. Thom (US
Environmental Protection Agency) and were incorporated into the final
document. The document was published in 1994 and covers published and
unpublished material up to 1993.
APPENDIX 2 -- CICAD PEER REVIEW
The draft CICAD on cumene was sent for review to institutions and
organizations identified by IPCS after contact with IPCS national
Contact Points and Participating Institutions, as well as to
identified experts. Comments were received from:
Commission of the European Communities, Directorate-General,
Luxembourg
Federal Institute for Health Protection of Consumers & Veterinary
Medicine (BgVV), Berlin, Germany
GSF-Forschungszentrum für Umwelt und Gesundheit GmbH, Institut
für Toxikologie, Oberscheissheim, Germany
Health & Safety Executive, Merseyside, United Kingdom
Institut de Recherches en Santé et Sécurité du Travail du Québec,
Montreal, Canada
Institute of Occupational Medicine, Chinese Academy of Preventive
Medicine, Ministry of Health, Beijing, People's Republic of China
Institute of Terrestrial Ecology, Cambridgeshire, United Kingdom
Joint Food Safety and Standards Group, London, United Kingdom
National Chemicals Inspectorate, Solna, Sweden
National Industrial Chemicals Notification and Assessment Scheme,
Sydney, Australia
National Institute of Health Sciences, Tokyo, Japan
National Institute of Occupational Health, Budapest, Hungary
National Institute of Public Health, Czech Republic
United States Department of Health and Human Services (National
Institute of Environmental Health Sciences)
United States Environmental Protection Agency (National Center
for Environmental Assessment; Region VIII)
APPENDIX 3 -- CICAD FINAL REVIEW BOARD
Washington, DC, USA, 8-11 December 1998
Members
Dr T. Berzins, National Chemicals Inspectorate (KEMI), Solna, Sweden
( Vice-Chairperson)
Mr R. Cary, Toxicology Unit, Health Directorate, Health and Safety
Executive, Bootle, Merseyside, United Kingdom ( Rapporteur)
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr O. Faroon, Agency for Toxic Substances and Disease Registry,
Centers for Disease Control and Prevention, Atlanta, GA, USA
Dr G. Foureman, National Center for Environmental Assessment, US
Environmental Protection Agency, Research Triangle Park, NC, USA
Dr H. Gibb, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA ( Chairperson)
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Research, Hanover,
Germany
Dr A. Nishikawa, Division of Pathology, National Institute of Health
Sciences, Tokyo, Japan
Dr E.V. Ohanian, Office of Water/Office of Science and Technology,
Health and Ecological Criteria Division, US Environmental Protection
Agency, Washington, DC, USA
Dr J. Sekizawa, Division of Chem-Bio Informatics, National Institute
of Health Sciences, Tokyo, Japan
Professor P. Yao, Institute of Occupational Medicine, Chinese Academy
of Preventive Medicine, Ministry of Health, Beijing, People's Republic
of China
Observers
Dr K. Austin, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
Dr I. Daly (ICCA representative), Regulatory and Technical Associates,
Lebanon, NJ, USA
Ms K.L. Lang (CEFIC, European Chemical Industry Council,
representative), Shell International, London, United Kingdom
Ms K. Roberts (ICCA representative), Chemical Self-funded Technical
Advocacy and Research (CHEMSTAR), Chemical Manufacturers Association,
Arlington, VA, USA
Dr W. Snellings (ICCA representative), Union Carbide Corporation,
Danbury, CN, USA
Dr M. Sweeney, Document Development Branch, National Institute for
Occupational Safety and Health, Cincinnati, OH, USA
Dr K. Ziegler-Skylakakis, GSF-Forschungszentrum für Umwelt und
Gesundheit GmbH, Institut für Toxikologie, Oberschleissheim, Germany
Secretariat
Dr M. Baril, Institut de Recherches en Santé et Sécurité du Travail du
Québec (IRSST), Montreal, Quebec, Canada
Dr H. Galal-Gorchev, Chevy Chase, MD, USA
Ms M. Godden, Health and Safety Executive, Bootle, Merseyside, United
Kingdom
Dr R.G. Liteplo, Environmental Health Directorate, Health Canada,
Ottawa, Ontario, Canada
Ms L. Regis, Programme for the Promotion of Chemical Safety, World
Health Organization, Geneva, Switzerland
Mr A. Strawson, Health and Safety Executive, London, United Kingdom
Dr P. Toft, Programme for the Promotion of Chemical Safety, World
Health Organization, Geneva, Switzerland
RÉSUMÉ D'ORIENTATION
Ce CICAD relatif au cumène a été préparé par l'Environmental
Protection Agency (EPA) des Etats-Unis sur la base d'une de ses
publications intitulée Health and environmental effects document
for cumene (US EPA, 1987), du dossier cumène provenant de son
système intégré d'information sur les risques (IRIS) (US EPA, 1997) et
d'un document du Royaume Uni paru sous le titre de Environmental
hazard assessment (EHA): Cumene (UK DOE, 1994), complétés par une
étude bibliographique à partir de la base de données écologiques
AQUIRE (Aquatic Toxicity Information Retrieval). Les recherches
bibliographiques effectuée pour l'établissement du dossier IRIS vont
jusqu'à novembre 1996 et celles qui ont été effectuées à partir de la
base de données AQUIRE, jusqu'à avril 1998. On trouvera à l'appendice
1 des indications sur le mode d'examen par des pairs ainsi que sur les
sources documentaires utilisées. Les renseignements concernant
l'examen du CICAD par les pairs font l'objet de l'appendice 2. Ce
CICAD a été approuvé en tant qu'évaluation internationale lors de la
réunion du Comité d'évaluation finale qui s'est tenue à Washington du
8 au 11 décembre 1998. La liste des participants à cette réunion
figure à l'appendice 3. La fiche d'information internationale sur la
sécurité chimique (ICSC 0170) relative au cumène, établie par le
Programme internationale sur la sécurité chimique (IPCS, 1993) est
également reproduite dans ce document.
Le cumène (CAS No 98-82-8) est un produit pétrochimique insoluble
dans l'eau utilisé dans la préparation d'un certain nombre d'autres
substances chimiques, notamment le phénol et l'acétone. Il se
volatilise facilement dans l'atmosphère à partir de l'eau et des sols
secs. Il devrait en principe n'être que modérément adsorbé au
particules du sol et aux sédiments et subir une décomposition dans
l'eau et le sol.
Le métabolisme du cumène donne principalement naissance, chez
l'Homme comme chez l'animal, à un alcool secondaire le
2-phényl-2-propanol. Cet alcool et ses conjugués sont rapidement
excrétés chez les rongeurs comme chez l'Homme.
Les effets les plus marqués observés chez des rongeurs exposés de
façon répétée au cumène par la voie orale ou respiratoire, consistent
en une augmentation du poids de certains organes, mais plus
particulièrement du rein. Aucun effet indésirable n'a été relevé chez
des foetus de rats et de lapins dont la mère avait été exposée à ce
produit au cours du développement foetal. Il n'y a pas eu d'étude de
reproduction portant sur plusieurs générations, mais la métabolisation
et l'excrétion rapides du composé et le fait qu'une étude subchronique
n'ait pas mis en évidence d'effets sur les spermatozoïdes, semblent
indiquer que le cumène est dépourvu de toxicité génésique. On a établi
une valeur-guide de 0,1 mg/kg par jour en se basant sur la dose sans
effet nocif observable (NOAEL) de 154 mg/kg p.c. obtenue après avoir
fait ingérer du cumène à des rats pendant 6 à 7 mois, le critère
retenu étant l'hypertrophie rénale chez les femelles. Cette valeur de
la dose a été corrigée pour tenir compte du programme d'administration
et on a appliqué un facteur d'incertitude de 1000. D'autres valeurs de
la NOAEL tirées d'une même étude d'inhalation en mode subchronique ont
abouti à des valeurs-guides respectivement égales à 0,4 mg/m3 et 0,09
mg/m3 pour la population générale; dans ce cas également, on a
corrigé la valeur de la NOAEL pour tenir compte d'une exposition en
mode continu et on a appliqué un facteur global d'incertitude égal à
1000.
On ne possède pas de données qui permettent d'évaluer
quantitativement l'exposition humaine au cumène.
Il n'est pas possible d'évaluer le pouvoir cancérogène du cumène
chez l'Homme en raison de l'absence d'études de cancérogénicité à long
terme. La plupart des études de génotoxicité ont donné des résultats
négatifs.
Les données qui permettraient une évaluation du risque encouru
par les organismes aquatiques et terrestres sont insuffisantes,
notamment en ce qui concerne la mesure de l'exposition à ce composé.
Toutefois, si l'on se base sur les données existantes, on peut penser
que ce risque est relativement faible. Les valeurs disponibles
indiquent une légère tendance à la bioconcentration chez les poissons.
On dispose d'aucune donnée sur la bioaccumulation du cumène le long
des diverses chaînes alimentaires (bioamplification).
RESUMEN DE ORIENTACION
Este CICAD sobre el cumeno, preparado por la Agencia para la
Protección del Medio Ambiente de los Estados Unidos (EPA), se basa en
un documento de la EPA sobre los efectos sanitarios y medioambientales
del cumeno (US EPA, 1987), en un archivo sobre el cumeno del sistema
integrado de información sobre riesgos (IRIS) de la EPA de los Estados
Unidos (US EPA, 1997) y en un documento del Reino Unido sobre la
evaluación de los riesgos medioambientales del cumeno (UK DOE, 1994),
con el complemento de una búsqueda bibliográfica en la base de datos
AQUIRE (Aquatic Toxicity Information Retrieval), especializada en
ecología. La búsqueda bibliográfica en el archivo del IRIS se realizó
hasta noviembre de 1996 y en la base de datos AQUIRE hasta abril de
1998. La información relativa al carácter del examen colegiado y a la
disponibilidad de los documentos originales figura en el apéndice 1.
La información sobre el examen colegiado de este CICAD aparece en el
apéndice 2. Este CICAD se aprobó como evaluación internacional en una
reunión de la Junta de Evaluación Final celebrada en Washington, DC,
Estados Unidos, los días 8-11 de diciembre de 1998. La lista de
participantes en esta reunión figura en el apéndice 3. La ficha
internacional de seguridad química (ICSC 0170) para el cumeno,
preparada por el Programa Internacional de Seguridad de las Sustancias
Químicas (IPCS, 1993), también se reproduce en el presente documento.
El cumeno (CAS No 98-82-8) es un producto petroquímico insoluble
en agua que se utiliza en la fabricación de varias sustancias
químicas, entre ellas el fenol y la acetona. Se volatiliza fácilmente
a la atmósfera a partir del agua y del suelo seco. Se supone que se
adsorbe al suelo/sedimentos con una intensidad entre moderada y fuerte
y que se biodegrada en el agua y en el suelo.
El cumeno se metaboliza fundamentalmente al alcohol secundario
2-fenil-2-propanol, tanto en el ser humano como en los animales. Los
roedores y las personas excretan con facilidad este alcohol y sus
conjugados.
Los efectos más notables observados en los roedores expuestos a
dosis repetidas de cumeno por vía oral o por inhalación son un aumento
del peso de los órganos, en particular de los riñones. No se
detectaron efectos adversos en los fetos de rata o de conejo cuyas
madres habían estado expuestas al cumeno durante el desarrollo fetal.
Si bien no se han realizado estudios de reproducción multigeneracional
con exposición al cumeno, la rapidez de su metabolismo y su excreción,
junto con la falta de efectos en la morfología del esperma en un
estudio subcrónico, parecen indicar un potencial bajo de toxicidad
reproductiva. Se ha obtenido un valor guía para la exposición oral de
0,1 mg/kg de peso corporal al día, basado en una concentración sin
efectos adversos observados (NOAEL) de 154 mg/kg de peso corporal al
día para el aumento del peso del riñón en ratas hembras en un estudio
de administración oral de 6 a 7 meses de duración; la NOAEL se ajustó
para un calendario de dosificación y se aplicó un factor de
incertidumbre de 1 000. Con respecto a la exposición por inhalación,
se obtuvieron valores guía para la población general de 0,4 mg/m3 y
0,09 mg/m3, basados en otras NOAEL derivadas del mismo estudio de
inhalación subcrónica; en este caso también se ajustaron las NOAEL
para una exposición continua y se aplicó un factor de incertidumbre
total de 1 000.
No hay datos disponibles para cuantificar la exposición humana al
cumeno.
No es posible evaluar el potencial de carcinogenicidad del cumeno
en el ser humano, debido a que no se han realizado estudios de larga
duración con esta sustancia. La mayor parte de los datos obtenidos en
pruebas genotóxicas son negativos.
Son insuficientes los datos, especialmente de información de la
exposición medida, para poder realizar una evaluación cuantitativa del
riesgo de la exposición al cumeno para las poblaciones de organismos
acuáticos o terrestres. Sin embargo, teniendo en cuenta los datos
existentes, se prevé para el cumeno un riesgo relativamente bajo. Los
valores indican un ligero potencial de bioconcentración del cumeno en
los peces. No hay datos acerca de la bioacumulación a través de la
cadena alimentaria (bioamplificación).
Some relevant physical and chemical properties of cumene are
listed in Table 1. Additional physical/chemical properties are
presented in the International Chemical Safety Card (ICSC 0170)
reproduced in this document.
Table 1: Physical/chemical properties of cumene.
Property Value Reference
Molecular weight 120.2 g/mol
Boiling point 152.39 °C Ward, 1979
Vapour pressure, 611 Pa Mackay &
25 °C Shiu, 1981
Water solubility, 50 mg/litre Mackay &
25 °C Shiu, 1981
Log Kow 3.66 Hansch &
Leo, undated
Density, 20 °C 0.8619 g/cm3 Ward, 1979
Flashpoint (tag closed-cup) 35 °C Ward, 1965
Odour threshold limit 0.088 ppm (v/v) Amoore &
value (TLV) 0.43 mg/m3 Hautala, 1983
Table 1 (continued)
Conversion factor, 1 ppm = 5.2 mg/m3
20 °C, 101.3 kPa 1 mg/m3 = 0.19 ppm
Partition coefficients Sato &
Oil/air 6215 Nakajima, 1979
Oil/water 4316
Water/air 1.44
Human blood/air 37
3. ANALYTICAL METHODS
For sampling and measurement of cumene in air, Method 1501 of the
US National Institute for Occupational Safety and Health (NIOSH, 1994)
includes use of a solid sorbent tube (coconut shell charcoal) sampler
with a gas chromatography/flame ionization detector measurement
technique. The detection limit of this method is 1 mg/m3 (0.2 ppm).
US EPA (1996) methods for detecting cumene in media other than
air include the use of gas chromatography using photoionization Method
8021B, which is applicable to nearly all types of samples, regardless
of water content. The method detection limit for cumene is 0.05
µg/litre, and the applicable concentration range for this method is
approximately 0.1-200 µg/litre. The standard recovery using this
method is 98%, with a standard deviation of 0.9%. Another commonly
used gas chromatographic assay for volatiles including cumene is
Method 8260B (US EPA, 1996), with a general estimated quantitation
limit of approximately 5 µg/kg wet weight for soil/sediment samples,
0.5 mg/kg wet weight for wastes, and 5 µg/litre for groundwater.
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
Cumene is a naturally occurring constituent of crude oil and may
be released to the environment from a number of anthropogenic sources,
including processed hydrocarbon fuels. Crude oils typically contain
approximately 0.1 wt% of cumene, but concentrations as high as 1.0 wt%
have been reported.1 Measurements of various grades of petrol
revealed that cumene concentrations range from 0.14 to 0.51 vol% and
that the average cumene concentration was 0.3 vol%. Premium diesel
fuel contains 0.86 wt% of cumene; furnace oil (no. 2) contains 0.60
wt%.1
Primary sources of release of cumene include losses in wastewater
and fugitive emissions from manufacturing and use facilities and
petrochemical refineries, accidental spills of finished fuel products
during transport or processing, and emissions from petrol stations and
motor vehicles (US EPA, 1987). Cigarette tobacco also releases cumene
(Johnstone et al., 1962). Cumene release from all these sources is
estimated to be 9500 tonnes annually (US EPA, 1988). Other,
unquantifiable anthropogenic cumene releases include the rubber
vulcanization process (Cocheo et al., 1983), building materials
(Moelhave, 1979), jet engine exhaust (Katzman & Libby, 1975), outboard
motor operation (Montz et al., 1982), solvent uses (Levy, 1973),
pharmaceutical production, and textile plants (Gordon & Gordon, 1981).
Cumene is also released to the environment from leather tanning, iron
and steel manufacturing, paving and roofing, paint and ink
formulation, printing and publishing, ore mining, coal mining,
organics and plastics manufacturing, pesticide manufacturing,
electroplating, and pulp and paper production (Shackelford et al.,
1983).
SRI International (1986) reported the 1985 Western European
cumene production levels (in tonnes) for the following producer
countries:
Federal Republic of Germany 438 000
Finland 70 000
France 370 000
Italy 335 000
Netherlands 240 000
Spain 120 000
United Kingdom 220 000
This total 1985 production of 1 793 000 tonnes may be compared with
production in the USA, which was reported as 2 775 000 tonnes in 1997
(Anon., 1998).
1 Letter and attachment from W.F. O'Keefe, American Petroleum
Institute, to M. Greif, Toxic Substances Control Act (TSCA)
Interagency Testing Committee, US Environmental Protection
Agency, Washington, DC (TS-792).
The use pattern for cumene in the early 1970s in the USA was as
follows (Anon., 1984): oxidation for phenol/acetone production, 98%;
polymerization of alpha-methylstyrene, 1.8%; and exports, 0.2%. Cumene
is also used captively for the production of phenol and
alpha-methyl-styrene (SRI International, 1986).
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
In the atmosphere, cumene is expected to exist almost entirely in
the vapour phase (Eisenreich et al., 1981). Cumene does not absorb
ultraviolet light at wavelengths greater than 290 nm (US EPA, 1987),
which suggests that cumene would not be susceptible to direct
photolysis. In one study, the estimated half-life of cumene in the
atmosphere from photolysis alone was approximately 1500 years (Parlar
et al., 1983). Cumene is not susceptible to oxidation by ozone in the
atmosphere (US EPA, 1987). Thus, reaction with ozone and direct
photolysis are not expected to be important removal processes. Rather,
reaction with photochemically generated hydroxyl radicals appears to
be the primary degradation pathway (t´ 1-2 days) (Lloyd et al.,
1976; Ravishankara et al., 1978). Small amounts of cumene may be
removed from the atmosphere during precipitation. Cumene has been
assigned a Photochemical Ozone Creation Potential (POCP) value of 35
relative to ethylene at 100 (Derwent & Jenkin, 1990). POCP values
represent the ability of a substance to form ground-level ozone as a
result of its atmospheric degradation reactions.
In water, important fate and transport processes are expected to
be volatilization (t´ 4 h from a typical river) and aerobic
biodegradation (Kappeler & Wuhrmann, 1978; Sasaki, 1978; Van der
Linden, 1978). Chemical hydrolysis, oxidation, photolysis, and
reaction with hydroxyl radicals are not expected to be important fate
processes in water (Mill et al., 1978, 1979, 1980). Using an aerobic
freshwater sediment/water test system, Williams et al. (1993)
demonstrated that 10 days after addition of radiolabelled cumene (2.5
mg/litre) to the system, 46.9% was trapped as radiolabelled carbon
dioxide and another 21.8% was recovered as radiolabelled organics, the
overall recovery of cumene ranging from 56.8% to 88.3%. The
disappearance half-life based on these results was 2.5 days. During a
20-day incubation of cumene at 10 mg/litre under aerobic conditions in
either fresh water or salt water, Price et al. (1974) observed 70%
degradation in fresh water but only about 2% degradation in seawater.
Cumene was, however, observed to be degraded to a significant extent
by microorganisms isolated from ocean sediment samples incubated in
seawater, as Walker et al. (1976) noted decreases in cumene (gas
chromatographic analysis) ranging from 37% to 60% of initial amounts
over a period of 21 days in three separate incubations with seawater
and microorganisms isolated from Atlantic Ocean sediments. On the
other hand, cumene was found to be essentially non-biodegradable under
anaerobic conditions by Battersby & Wilson (1989), who noted that
cumene produced only about 2% of theoretical gas production when
incubated at 50 mg carbon/litre sludge for 60 days at 35°C under
anaerobic conditions; compounds at 80% of theoretical gas production
under these conditions were assumed to represent complete degradation,
whereas compounds at less than 30% production were considered
persistent.
In soil, it appears that cumene might biodegrade fairly rapidly
under aerobic conditions, because a number of microorganisms capable
of degrading cumene have been isolated (Yamada et al., 1965; Jamison
et al., 1970; Omori et al., 1975). Regression equations based on the
limit of cumene water solubility (50 mg/litre) predicted Koc (soil
sorption coefficient standardized to organic carbon) values ranging
from 513 to 1622. For equations based instead on log octanol/water
partition coefficients (log Kow) for cumene, predicted Koc
values were in a similar range, from 589 to 3890 (Lyman et al., 1982).
Other estimates of Koc values at 884 (Jeng et al., 1992) and 2800
(US EPA, 1987) were also in this range. These Koc values indicate
that cumene is expected to adsorb moderately to strongly to soil and
have only slight mobility. The relatively high vapour pressure of
cumene suggests that volatilization of this compound from dry soil
surfaces would be significant.
Measured and estimated bioconcentration factors (BCFs) suggest a
slight potential for cumene to bioconcentrate in fish species. A BCF
of 36 for cumene in goldfish ( Carassius auratus) has been measured
(Ogata et al., 1984), and a BCF of 356 was estimated from the log
Kow and a linear regression correlation equation (log BCF = 0.76
log Kow - 0.23) by the US EPA (1987). This value was concordant
with the BCF of 316 calculated for fish species in general exposed to
cumene (Sabljic, 1987). Cumene was detected at levels of 0.5-1.4 ng/g
wet weight (detection limit 0.5 ng/g wet weight by gas chromatography/
mass spectrometry) in 12 of 138 sampled fish (various species) from
several locations near a potential emission source (Japan Environment
Agency, 1987). Cumene has been detected in "oakmoss" ( Evernia
prunastri (L.) Ach.) (Gavin et al., 1978) and marsh grass (Mody et
al., 1974a,b).
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
Cumene has been found as a contaminant in various industrial
effluents and in groundwaters. Significant levels of cumene have been
recorded in groundwater near chemical plants (1581 µg/litre, Botta et
al., 1984; 360 µg/litre, Teply & Dressler, 1980; 11 µg/litre,
Pellizzari et al., 1979), around outboard motor operations (700
µg/litre, Montz et al., 1982), near coal gasification facilities (up
to 54 µg/litre, Steurmer et al., 1982), and around petroleum plants
and petroleum refineries (5 µg/litre, quantification method not clear;
Snider & Manning, 1982). Cumene was detected in 8 of 135 samples of
surface water (detection limit 0.03 µg/litre with gas
chromatography/mass spectrometry) at concentrations ranging from 0.09
to 0.44 µg/litre in several locations near a potential emission source
in the 1986 monitoring of the general environment in Japan (Japan
Environment Agency, 1987). Cumene levels in sediments and biota in
Puget Sound, Washington, USA, ranged from 0.02 to 19 µg/g, with a mean
concentration of 2.3 µg/g (Brown et al., 1979). A cumene level of
140 µg/litre was found in seawater near an offshore drilling platform
in the Gulf of Mexico (Sauer, 1981). Cumene was detected in 6 of 111
sediment samples at concentrations ranging from 0.58 to 11 ng/g dry
weight (detection limit 0.5 ng/g with gas chromatography/mass
spectrometry) in several locations near a potential emission source
(Japan Environment Agency, 1987).
Reports of air sampling in the USA indicate the mean
concentration of cumene to be about 14.7 µg/m3 (3 ppb) in urban
settings and as high as 2.5 µg/m3 (0.5 ppb) in rural settings.
Samples taken in Los Angeles, California, in 1966 averaged 14.7 µg/m3
(3 ppb) (Lonneman et al., 1968), and samples taken in Houston, Texas,
in 1973-1974 averaged 12.15 µg/m3 (2.48 ppb) (Lonneman et al., 1979).
The US EPA (1987) reported a mean concentration of 16.7 µg cumene/m3
(3.4 ppb) in undated samples from Los Angeles. In samples taken in the
fall of 1981 in Los Angeles, Grosjean & Fung (1984) did not detect
cumene, although a minimum detection level of 9.8 µg/m3 (2 ppb) was
reported. Although a number of sampling attempts in rural and remote
areas reported no detectable levels of cumene in air (detection limit
<0.05 µg/m3 [<0.01 ppb]), two attempts were positive: Seila (1979)
reported mean levels of 2.5 µg/m3 (0.5 ppb) in samples taken in a
rural area near Houston, Texas, in 1978, and Arnts & Meeks (1980,
1981) reported 0.25 µg/m3 (0.05 ppb) in samples taken near campfires
in the Great Smokey Mountains, USA, in 1978.
Average atmospheric concentrations of cumene in Europe are
reported to be somewhat less than those in the USA, although
concentrations in urban areas are also consistently much higher than
those in rural areas. Isodorov et al. (1983) recorded an average
cumene level of 8.3 µg/m3 (1.7 ppb) in the urban atmosphere of
Leningrad, USSR, in 1977-1979, with a maximum of 11.8 µg/m3 (2.4
ppb). Ambient air concentrations for the Netherlands in 1980 were
reported to average 0.5-1.0 µg/m3 (0.1-0.2 ppb), with maxima ranging
up to 34.8 µg/m3 (7.1 ppb) (Guicherit & Schulting, 1985). An annual
average of 1.6 µg/m3 (0.3 ppb) (maximum 3.9 µg/m3 [0.8 ppb]) was
reported from the Grenoble area in France in 1987 (Foster et al.,
1991).
6.2 Human exposure
Humans can be exposed to cumene via industrial emissions, petrol
station or motor vehicle emissions, accidental releases, food,
cigarette smoke, and drinking-water (US EPA, 1987).
In condensates of cigarette smoke, Johnstone et al. (1962)
recorded yields of cumene ranging from 7 to 14 µg/cigarette. Holzer et
al. (1976) detected cumene at 10 µg/m3 (2 ppb) in air samples taken
from a room immediately after a single cigarette had been smoked. No
further specifics, such as indication of a median value or minimum
detection level, are given.
Brugnone et al. (1989) reported cumene as measurable in all
alveolar air samples collected (single breath; range 1-81 µg/m3
[0.2-17 ppb], method detection limit not given) from among two groups
of workers ( n = 86, gender not specified) exposed to <0.1 mg
cumene/m3 (<0.02 ppm) through the work shift. These authors analysed
for but were unable to detect any significant differences in cumene
concentrations between smokers and non-smokers in either alveolar air
or blood samples. In another study, gases collected from 60 min of
normal continuous respiration from each of eight male volunteers
(three smokers) were analysed for trace organic constituents (Conkle
et al., 1975). Cumene was listed as detected in one of the three
smokers (expressed as 21 µg/h) and in one of the five non-smokers
(expressed as 0.13 µg/h). Krotoszynski & O'Neill (1982) also
identified cumene in expired air from non-smokers.
The presence of cumene in foods can be biogenic or due to
environmental contamination (US EPA, 1987). Although the detection
limit of cumene in various foods was not specified, the US EPA (1987)
noted that cumene has been detected but not quantified in foods as
diverse as tomatoes, Concord grapes, cooked rice, fried chicken,
bacon, Beaufort cheese, and dried legumes.
Only two reports of cumene quantification in drinking-water were
found in the available literature. Coleman et al. (1984) detected
cumene in Cincinnati, Ohio, USA, drinking-water at a level of 0.014
µg/litre (quantification method not clear). Keith et al. (1976)
reported 0.01 µg cumene/litre drinking-water in Terrebonne-Parish,
Louisiana, USA, but found none in the drinking-water of nine other
cities across the USA. These concentrations are considerably below the
0.5 µg/litre detection limit reported by Westrick et al. (1984), who
found no cumene in 945 US drinking-water systems, 479 of which were
selected because of known contamination problems. Burmaster (1982) and
Burnham et al. (1972) reported unquantified levels of cumene/
alkylbenzenes in drinking-water obtained from groundwater. Based on
the results of these studies, it may be concluded that cumene
contamination above 0.5 µg/litre is uncommon in drinking-water in the
USA.
One industrial hygiene survey (US EPA, 1988) reported that
approximately 739 US workers were occupationally exposed to cumene.
Personal exposure data in this report consisted of 1487 air samples
taken over the course of 12 years (1973-1984), of which 6 were in the
range of 20-150 mg/m3 (4-30 ppm), 4 in the range of 15-20 mg/m3 (3-4
ppm), and 25 in the range of 5-10 mg/m3 (1-2 ppm), with the remaining
samples below 5 mg/m3 (1 ppm) (US EPA, 1988).
Based on available monitoring data, it appears that the general
population would be exposed to cumene primarily by inhalation,
although occupational populations may be reasonably anticipated to be
exposed by the dermal route. Minor exposure may result from contact
with refined petroleum products and ingestion of contaminated foods
and possibly drinking-water.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND
HUMANS
Cumene has been shown to be absorbed after inhalation exposure in
humans and after inhalation, oral, and dermal exposure in animals
(Senczuk & Litewka, 1976; Research Triangle Institute, 1989). Tests
conducted in humans indicate that cumene is absorbed readily via the
inhalation route, that it is metabolized efficiently to water-soluble
metabolites within the body, and that these metabolites are excreted
efficiently into the urine with no evidence of long-term retention
within the body; these results concur with the results of animal
studies.
Senczuk & Litewka (1976) exposed human volunteers (five men and
five women) head only to one of three different concentrations of
cumene vapours (240, 480, or 720 mg/m3 [49, 98, or 147 ppm]) for 8 h
every 10 days. Exhaled breath samples (10 cm3) were collected near
the beginning and at the end of the exposure from a tube placed in the
breathing zone. The total amount of cumene absorbed during exposure,
calculated from retention, ventilation, and exposure duration, was
nearly twice as high at all exposure levels in the males (466-1400 mg)
as in the females (270-789 mg). The respiratory tract absorption
ranged from 45% to 64% depending on the time of exposure, with the
overall mean retention estimated at 50%. In rats, inhalation studies
(nose only for 6 h at 510, 2420, or 5850 mg/m3 [104, 494, or 1194
ppm]) indicate rapid absorption, with detectable levels of cumene
appearing in the blood within 5 min of the beginning of exposure at
all three exposure levels (Research Triangle Institute, 1989). Gavage
studies in rats showed that cumene was absorbed readily via this
route, with maximum levels in blood occurring at the earliest time
point sampled (4 h) for a lower dose (33 mg/kg body weight) and at
8-16 h for a higher dose (1350 mg/kg body weight) (Research Triangle
Institute, 1989). Dermal absorption of cumene was demonstrated in rats
and rabbits (Monsanto Co., 1984).
The human data reported by Brugnone et al. (1989) regarding
cumene distribution suggest that the cumene concentration was about 40
times higher in blood than in alveolar air, a figure concordant with
the reported human blood/air partition coefficient of 37 (Sato &
Nakajima, 1979; Table 1). Cumene was widely distributed in rats, and
distribution, presumably determined immediately after exposure, was
independent of administration route (inhalation, oral, or
intraperitoneal in 10% aqueous Emulphor). Adipose, liver, and kidney
were all shown to have elevated tissue/blood ratios of cumene
following all doses and routes of exposure (Research Triangle
Institute, 1989). Fabre et al. (1955) demonstrated that after rats
inhaled cumene vapour for up to 150 days, cumene was distributed to
the endocrine organs, central nervous system, bone marrow, spleen, and
liver.
The patterns of cumene disappearance (as total radioactivity)
from the blood in the nose-only inhalation studies were fitted with a
monoexponential model, with the half-lives increasing with dose, from
3.9 h at 490 mg/m3 (100 ppm) to 6.6 h at 5880 mg/m3 (1200 ppm). The
half-life of cumene in the blood in gavage studies with rats was
calculated to be between 9 and 16 h.
Metabolism of cumene by cytochrome P-450 is extensive and takes
place within hepatic and extrahepatic tissues, including lung (Sato &
Nakajima, 1987), with the secondary alcohol 2-phenyl-2-propanol being
a principal metabolite. Metabolites excreted in urine of rats and
rabbits include 2-phenyl-2-propanol and its glucuronide or sulfate
conjugates, conjugates of 2-phenyl-1,2-propanediol, and an unknown
metabolite, possibly the dicarboxylic acid that would result from
complete oxidation of the 1- and 3-alkyl carbons of phenylmalonic acid
(Research Triangle Institute, 1989; Ishida & Matsumoto, 1992; MAK,
1996).
Senczuk & Litewka (1976) also conducted excretion studies with
human volunteers exposed to cumene vapours (240, 480, or 720 mg/m3
[49, 98, or 147 ppm]) for 8 h every 10 days. These authors reported
excretion of the metabolite 2-phenyl-2-propanol in the urine as
biphasic, with a rapid early phase (t´ 2 h) and a slower later phase
(t´ 10 h); excretion of this metabolite in the urine (about 35% of
the calculated absorbed dose) was maximal after 6-8 h of exposure and
approached zero at 40 h post-exposure. With rats, the extent of
elimination across routes of administration (inhalation, oral, or
intraperitoneal) and exposure concentrations was very similar, with
urine being the major route of elimination, about 70% in all cases
(Research Triangle Institute, 1989). Total body clearance in the rats
was rapid and complete, with less than 1% of the absorbed fraction
being present in the body 72 h after the highest exposure regime
examined (5880 mg/m3 [1200 ppm] for 6 h). Following oral
administration of cumene in rabbits, 90% was recovered as metabolites
in the urine within 24 h (Robinson et al., 1955).
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
Cumene is not highly toxic to laboratory animals by inhalation,
oral, or dermal routes of exposure. An LC50 of 9800 mg cumene/m3
(2000 ppm) in mice has been reported (MAK, 1996). A 4-h inhalation
LC50 of 39 200 mg/m3 (8000 ppm) in rats was reported by several
investigators (Smyth et al., 1951; Koch Refining Co., 1984; Union
Carbide Corp., 1985). Acute oral LD50 values for rats range from 1400
to 2900 mg/kg body weight (Smyth et al., 1951; Koch Refining Co.,
1984; Monsanto Co., 1984; Ciba-Geigy Co., 1985; Union Carbide Corp.,
1985). Tanii et al. (1995) reported an intraperitoneal LD50 in male
mice in the same range, 2000 mg/kg body weight (16.9 mmol/kg).
Clinical signs of toxicity reported in rats in acute oral studies
include weakness, ocular discharge, collapse, and death; pathological
findings in animals that died were haemorrhagic lungs, liver
discolorations, and acute gastrointestinal inflammation (Monsanto Co.,
1984). The character of the dose-response for these effects is,
however, unclear.
Acute dermal LD50s for cumene applied undiluted to rabbit skin
range from >3160 mg/kg body weight (Monsanto Co., 1984) to >10 000
mg/kg body weight (Ciba-Geigy Co., 1985). Pathological findings in
animals that died were similar to those in animals that died after a
single oral exposure (Monsanto Co., 1984).
8.2 Irritation and sensitization
Undiluted cumene applied to the skin of New Zealand albino
rabbits (0.5 ml) according to standardized guidelines caused slight
defatting with skin flaking, a symptom not generally classified as
relating to primary skin irritancy (Monsanto Co., 1984). A study
conducted by Ciba-Geigy Co. (1985) reported a similar low level of
irritation.
Cumene is an ocular irritant. Ocular irritation, including
immediate discomfort followed by "erythema" (redness of the
conjunctiva) and copious discharge, was observed after the
instillation of undiluted cumene to rabbit, with these effects being
reversible within 120 h (Monsanto Co., 1984). Ciba-Geigy Co. (1985)
judged eye irritation as slight when cumene was applied to rabbit
eyes. However, a study by Union Carbide Corp. (1985) reported that
cumene was harmless to rabbit eyes when applied undiluted.
Observations of lacrimation (Tegeris & Balster, 1994) and periocular
swelling and blepharospasm (Cushman et al., 1995) also indicate that
cumene may exhibit ocular irritancy at high airborne concentrations.
The concentration of cumene causing a 50% reduction in the
respiratory rate in mice after 30 min of exposure was determined to be
10 084 mg/m3 (2058 ppm) (Kristiansen et al., 1986). This
concentration is quite high and in the range where repeated exposure
caused death and morbidity in rats (Gulf Oil Corp., 1985; Chemical
Manufacturers Association, 1989) and rabbits (Darmer et al., 1997).
No skin sensitization reactions were noted among a group of 20
female guinea-pigs treated with cumene in a Magnusson-Kligman
maximization test conducted in accordance with Organisation for
Economic Co-operation and Development (OECD) Guideline 406 (Hüls,
1988). No data were available on respiratory sensitization to cumene.
8.3 Short-term exposure
In a study by Monsanto Co. (1986), male and female Sprague-Dawley
rats (10 per sex per group) were exposed whole body to cumene vapour
concentrations of 0, 515, 1470, or 2935 mg/m3 (0, 105, 300, or
599 ppm) for 6 h/day, 5 days/week, for approximately 4 weeks (minimum
exposure, 20 days). Cage-side observations included
concentration-related increases in side-to-side head movements in both
males and females in all dose groups, head tilt in all dose groups,
and arched back in one female in the high-dose group. Increases in
mean absolute left and right kidney weights were observed in high-dose
males, as were increases in mean absolute left kidney weight in
low- and mid-dose males. In high-dose females, the mean absolute
weight of left kidneys was greater than in controls. This study
confirms that renal weight changes occur in females and corroborates
similar effects reported by Cushman et al. (1995). It should be noted
that the effects associated with central nervous system perturbation
(i.e., head movements) were not noted in several other longer-term
studies, including that of Cushman et al. (1995), in which
neurotoxicity was specifically assessed. If it is assumed that the
renal changes among the males were associated with male rat-specific
nephropathy (see section 8.4.1), the cage-side observations of head
tilt and head movements become the critical effects for this
short-term study.
Although not statistically significant, leukocytosis was noted in
a group of rats ( n = 15, mixed sex) exposed to cumene at 1200 mg/m3
(245 ppm) for 8 h/day, 5 days/week, for 30 exposures (Jenkins et al.,
1970).
Other short-term toxicity studies are described in section 8.7.
8.4 Long-term exposure
8.4.1 Subchronic exposure
In an inhalation exposure study by Jenkins et al. (1970), groups
of squirrel monkeys ( n = 2), beagle dogs ( n = 2),
Princeton-derived guinea-pigs ( n = 15), and Sprague-Dawley and
Long-Evans rats ( n = 15) were exposed whole body to cumene at
concentrations of 0, 18, or 147 mg/m3 (0, 4, or 30 ppm) continuously
for 90 days. Initial and terminal body weights, haematological and
clinical chemistry parameters, and histopathological data were
collected. No toxicologically significant effects were noted in the
monkeys, dogs, or guinea-pigs. The only effect noted in the rats was a
slight degree of leukocytosis at both concentrations.
Cushman et al. (1995; also reported as Bushy Run Research Center,
1989a) conducted two successive subchronic whole-body inhalation
toxicity studies with cumene vapours (>99.9% pure) on Fischer-344
rats. In the first study, groups (21 per sex) were exposed to cumene
vapour at 0, 490, 2430, or 5890 mg/m3 (0, 100, 496, or 1202 ppm) 6
h/day, 5 days/week, for 13 weeks. The second study was a repeat of the
first, except that the group size was decreased to 15 per sex and an
additional group (at 245 mg/m3 [50 ppm]) and a 4-week post-exposure
period were added. Parameters monitored included clinical signs of
toxicity, auditory brain stem responses, ophthalmology, sperm count
and morphology, and histopathological examination of all respiratory
tract tissues (lungs and nasal turbinates) and the perfused nervous
system. Evaluations of neurological function (functional observation
battery and motor activity) were conducted in both studies. Light
microscopic evaluation of the perfused-fixed nervous system tissues
(six rats per sex per group) was conducted in the first study only.
In the first study, transient, reversible cage-side observations
during exposure periods included hypoactivity, blepharospasm, and a
delayed or absent startle reflex at the highest concentration. Rats
exposed to 2430 mg/m3 were reported as being hypoactive during
exposure, although no further specifics were given. Statistically
significant ( P < 0.05) exposure-related decreases in motor activity
(total) were observed in male rats exposed to the two highest
concentrations of cumene, but these results were not observed in the
second study in either sex. There were no exposure-related changes
noted in the functional observation battery in this or the subsequent
study. No effects were observed in the neurohistopathological
examinations. Cataracts were reported in males at all exposure
concentrations in this study. However, these results were not observed
in the second study in which a more comprehensive protocol for eye
examination was employed. Evaluation of the auditory brain stem
responses revealed no meaningful changes in the auditory function of
the exposed animals. The only gross histopathology noted was
periocular swelling, which occurred in animals at the two highest
concentrations (and for which neither incidence nor severity was
reported). Both absolute and relative weights were increased
significantly (>10%) in the kidneys, adrenal glands, and livers of
both sexes at the highest concentration. These changes were also noted
in the liver at the next lower concentration (2430 mg/m3) for both
females and males. Kidney lesions described in male rats at the two
highest exposure concentrations were considered to be closely related
to male rat-specific nephropathy (i.e., lesions were limited to males,
and tubular proteinosis, hypertrophy, and hyperplasia as well as
hyaline droplet formation were noted, although the identity of the
protein in the droplets was not confirmed) and are of questionable
relevance to human toxicity, principally because renal lesions
characteristic of this type of nephropathy have not been observed in
humans (US EPA, 1991a; Hard et al., 1993). Chronic progressive
nephropathy, a common spontaneous renal disease of Fischer-344 male
rats that occurs as early as 5 months of age (Montgomery & Seely,
1990), may also contribute to these renal lesions. Water consumption
was significantly increased (about 40%) in male rats above control
values at both 2430 and 5890 mg/m3. Several haematological and serum
measures were also changed in a statistically significant dose-related
manner at both 2430 and 5890 mg/m3: leukocytes (both sexes),
platelets (both sexes), lymphocytes (males only), glucose (females
only), and calcium/phosphorus (males only).
The results of the second study, with a 4-week post-exposure
period, indicated limited reversibility of the organ weight
alterations, because significant mean weight increases were still
present in female liver and female adrenals of the highest exposure
group. In males, only relative kidney weights (significant at 6%) and
absolute liver weights remained increased significantly. Blood and
serum parameters were not reported in this study. Morphological
evaluation of epididymal and testicular sperm showed no cumene-related
differences in count, morphology, or stages of spermatogenesis,
although one high-dose rat did have diffuse testicular atrophy.
The weight alterations in the male and female adrenals and female
kidney are considered potentially adverse, as the persistence noted
indicates limited reversibility and engenders uncertainty about the
progression and fate of these alterations under chronic exposure. The
increased water consumption noted may also indicate potential for
renal effects, although this effect was present at the next to highest
dose level at which renal weights were not altered. Although the
progression of these weight alterations from continued exposure cannot
be ascertained from this subchronic study, data from the second
(post-exposure) study indicate limited reversibility of effects on the
adrenals, at least in females. The liver weight alterations are not
viewed as adverse, because increase in liver weight without
accompanying pathology is a trait of common microsomal enzyme inducing
agents, although it should be noted that induction of hepatic
microsomal enzymes may influence the metabolism of other substances
and may either increase or decrease their toxicity (Sipes & Gandolfi,
1991). The altered haematological and serum parameters noted at the
two highest concentrations may be considered as significant, although
all are within normal ranges (Mitruka & Rawnsley, 1981). Based on the
lowest dose at which both relative and absolute weight alterations in
adrenal tissues of both sexes and in the kidneys of females are
statistically ( P < 0.05) and biologically (>10%) significant, 5890
mg/m3 may be considered as a lowest-observed-adverse-effect level
(LOAEL), and 2430 mg/m3 the corresponding NOAEL. Based on
consideration of the various measures in the first study (motor
effects, increased water consumption in males, haematological and
serum parameters, sporadic weight increases in male adrenals and
female kidneys) as significant, 2430 mg/m3 may be considered as a
LOAEL and 490 mg/m3 as the corresponding NOAEL. It should be noted
here that a LOAEL of 2391 mg/m3 (488 ppm) and a NOAEL of 485 mg/m3
(99 ppm) were noted for maternal toxicity in the short-term
developmental study in rats by Darmer et al. (1997), discussed in
section 8.6.
8.4.2 Chronic exposure and carcinogenicity
There are no long-term in vivo bioassays addressing the issue
of cancer. No data exist to support any quantitative cancer
assessment.
Wolf et al. (1956) conducted a study involving groups of 10
female Wistar rats administered cumene by gavage in olive oil at 154,
462, or 769 mg/kg body weight per day, 5 days/week, over a 194-day
(6- to 7-month) period, equivalent to 110, 331, or 551 mg/kg body
weight per day, adjusted for daily exposure. Rats given olive oil
served as controls ( n = 20). A pronounced increase in average kidney
weight, noted as a "moderate effect," occurred at 769 mg/kg body
weight per day, although no quantitative data are presented. An
increase in average kidney weight was noted as a "slight effect" at
462 mg/kg body weight per day. It is stated in the report that at 154
mg/kg body weight per day, no evidence of ill effects, as determined
by gross appearance, growth, periodic blood counts, analysis for blood
urea nitrogen, average final body and organ weights, and bone marrow
counts, was noted. The LOAEL is 462 mg/kg body weight per day, and the
NOAEL is 154 mg/kg body weight per day. These results are consistent
with those observed in more recent, better-reported studies described
elsewhere in this document.
In an inhalation study by Fabre et al. (1955), Wistar rats were
exposed (whole body) to cumene vapour at 2500 mg/m3 (510 ppm), and
rabbits were exposed to 6500 mg/m3 (1327 ppm), for 8 h/day, 6
days/week, for up to 180 days. Histological effects reported were
"passive congestion" in the lungs, liver, spleen, kidney, and adrenals
and the presence of haemorrhagic zones in the lung, haemosiderosis in
the spleen, and lesions from epithelial nephritis "in some cases." It
was not clear from the study if these effects occurred in rats or
rabbits, or both.
8.5 Genotoxicity and related end-points
In general, negative results have been obtained in a relatively
complete battery of in vivo and in vitro mutagenicity tests,
including gene mutation, chromosomal aberration, and primary DNA
damage (US EPA, 1997). Cumene was tested at concentrations up to
2000 µg/plate in a Salmonella typhimurium reverse mutation assay
(modified Ames test); negative results were observed with and without
metabolic activation (Lawlor & Wagner, 1987). Cumene was negative in
an Ames assay at concentrations up to 3606 µg/plate with
S. typhimurium strains TA98, TA100, TA1535, and TA1537 (Florin et
al., 1980). Cumene also tested negative, with and without metabolic
activation, in a set of HGPRT assays (using Chinese hamster ovary
cells) at cumene concentrations of 100-125 µg/ml, at which the
relative cloning efficiencies (a measure of cytotoxicity) ranged from
29% to 110% (Gulf Life Sciences Center, 1985a; Yang, 1987). A
micronucleus assay performed in mice given up to 1 g cumene/kg body
weight by gavage was negative (Gulf Life Sciences Center, 1985b).
Micronucleus assays done in Fischer-344 rats, however, gave values
that were weakly positive, although little dose-response was seen, and
deaths occurred at the highest dose (5 of 10 animals at 2.5 g/kg body
weight intraperitoneally; NTP, 1996). The positive control used in the
micronucleus tests, cyclophosphamide, produced strong positive
responses in all assays.
Cumene failed to induce significant rates of transformation in
BALB/3T3 cells (without activation) at concentrations up to 500 µg/ml
(Putnam, 1987) but tested positive in an earlier cell transformation
test also using BALB/3T3 cells, in which an increase in
transformations was observed at 60 µg/ml (Gulf Oil Corp., 1984a).
Results from an unscheduled DNA synthesis assay in rat hepatocytes
conducted by Gulf Oil Corp. (1984b) indicated positive results at
doses of 16 and 32 µg cumene/ml (with 128 µg/ml noted as toxic to the
hepatocytes). However, apparent technical difficulties with this test
(US EPA, 1988) prompted a repeat of the unscheduled DNA synthesis
assay in rat hepatocytes, the results of which showed cumene to be
clearly negative at doses up to 24 µg/ml, with doses above 24 µg/ml
noted as being too toxic for evaluation of unscheduled DNA synthesis
(Curren, 1987; US EPA, 1988).
8.6 Reproductive and developmental toxicity
No multigeneration reproductive study exists for this compound by
either the oral or inhalation route. There are no data concerning
cumene exposure of females prior to mating, from conception to
implantation, or during late gestation, parturition, or lactation.
The first subchronic inhalation study of Cushman et al. (1995),
however, conducted morphological evaluation of epididymal and
testicular sperm in rats exposed for 13 weeks to cumene vapours (see
section 8.4.1). No cumene-related differences in count, morphology, or
stages of spermatogenesis were noted, although one high-dose rat did
have diffuse testicular atrophy. No alterations (weight changes,
histopathology) were noted in the female reproductive organs that were
examined at the termination of this same study.
In an inhalation study (Darmer et al., 1997; also reported as
Bushy Run Research Center, 1989b), Sprague-Dawley rats (25 per group)
were exposed whole body to 0, 485, 2391, or 5934 mg cumene/m3 (0, 99,
488, or 1211 ppm) for 6 h/day on days 6 through 15 of gestation.
Perioral wetness and encrustation, a significant ( P < 0.01)
decrease in body weight gain on gestation days 6 through 9
(accompanied by a significant decrease in food consumption), and a
slight increase (7.7%) in relative liver weight were observed in dams
at the high dose only. Hypoactivity, blepharospasm, and significantly
( P < 0.05) decreased food consumption were observed in the dams at
the next highest concentration. There were no statistically
significant adverse effects on reproductive parameters or fetal
development. For this study, 5934 mg/m3 is a developmental NOAEL, and
485 mg/m3 is a maternal NOAEL.
In another inhalation study (Darmer et al., 1997; also reported
as Bushy Run Research Centre, 1989c), New Zealand white rabbits (15
per group) were exposed whole body to 0, 2411, 5909, or 11 255 mg
cumene/m3 (0, 492, 1206, or 2297 ppm) for 6 h/day on days 6 through
18 of gestation. Two does died and one aborted at the highest exposure
concentration. There were significant ( P < 0.01) reductions in body
weight gain (178 g lost compared with 31 g gained in the control
group) and food consumption at the highest exposure level during the
treatment period. Significantly reduced food consumption was also
observed in the 2411 and 5909 mg/m3 exposure groups, but it was not
accompanied by any decrease in weight gain. Clinical signs of toxicity
observed in the does included significant ( P < 0.01) increases in
perioral and perinasal wetness and blepharospasm at the highest
concentration. At necropsy, there were colour changes in the lungs of
33% of the does exposed to 11 255 mg/m3. Relative liver weight was
significantly ( P < 0.01) elevated (16.8% over control weight) at
the highest exposure level. There were no statistically significant
effects on gestation parameters; however, there were non-significant
increases in non-viable implants and early resorptions and a
non-significant decrease in the percentage of live fetuses concurrent
with maternal toxicity at 11 255 mg/m3. Apparent increases in
ecchymosis (haemorrhagic areas of the skin) of the head were shown to
be within the ranges observed for the historical controls of this test
facility (US EPA, 1991b). The highest exposure level resulted in
maternal mortality. The next lower dose of 5909 mg/m3, at which the
only effect noted was reduced food consumption without accompanying
weight loss, is considered the NOAEL of the study.
8.7 Immunological and neurological effects
No studies were located that examined immunotoxicity in animals
after exposure to cumene by any route.
Cumene appears to be similar to many solvents, such as alcohol,
that are known central nervous system depressants. The occurrence of
neurological effects from inhalation exposure to cumene has been
confirmed in several studies. These studies are acute exposures that
show neurotoxicological effects only at quite high concentrations
(>2450 mg/m3 [>500 ppm]). Neurotoxicological effects were not
observed in the longer-term inhalation study by Cushman et al. (1995),
which included complete batteries of functional and motor activity
tests and neurohistopathology and in which the highest exposure
concentration was 5890 mg/m3 (1202 ppm).
Cumene was tested at 0, 9800, 19 600, or 39 200 mg/m3 (0, 2000,
4000, or 8000 ppm) and produced a short-lived profile of
neurobehavioural effects in mice that indicated central nervous system
depressant activity (Tegeris & Balster, 1994). Effects noted from
brief (20-min) whole-body exposures to cumene included those on
central nervous system activity (decreased arousal and rearing at 9800
mg/m3), muscle tone/equilibrium (changes in grip strength and
mobility at 19 600 mg/m3), and sensorimotor activity (including
decreased tail pinch and touch response at 19 600 mg/m3).
In an acute experiment accompanying the subchronic exposures (see
section 8.4.1), Cushman et al. (1995) exposed Fischer-344 rats (whole
body) once to 0, 490, 2430, or 5890 mg/m3 (0, 100, 496, or 1202 ppm)
for 6 h and conducted functional observations 1 h post-exposure. Gait
abnormalities and decreased rectal temperatures were noted for both
sexes at the highest exposure level only. Decreased activity levels
were noted for both sexes at the highest level and for females only at
the next highest level (2430 mg/m3) of exposure. Males, but not
females, from the highest exposure group had decreased response to toe
pinch at 6 h post-exposure.
In a 5-day inhalation study, Fischer-344 rats exposed whole body
to 9800 or 24 500 mg cumene vapour/m3 (2000 or 5000 ppm) for 6 h/day
showed toxic effects from exposure (Gulf Oil Corp., 1985). All rats in
the high-exposure group died after 2 days. At the lower dose, females
demonstrated central nervous system effects (hypothermia and
staggering). Similar, but more severe, symptoms were observed in the
high-exposure animals before they died.
Fischer-344 rats (10 per sex per group) were exposed whole body
to cumene at 0, 1230, 2680, 5130, or 6321 mg/m3 (0, 251, 547, 1047,
or 1290 ppm) for 6 h/day, 5 days/week, for 2 weeks (Chemical
Manufacturers Association, 1989). Initial exposures to 9800 mg/m3
(2000 ppm) for 1-2 days resulted in such severe neurological and
respiratory effects that the concentration levels were reduced to
those given above. During the remainder of the 2-week exposure period,
clinical observations (ocular discharge, decreased motor activity or
hyperactivity, and ataxia) were noted sporadically at all levels
except 1230 mg/m3. For females in the two highest dose groups, the
average relative kidney weight and relative and absolute adrenal
weights were increased significantly over control values. These data
provide corroboration for these same effects reported in the study of
Cushman et al. (1995).
9. EFFECTS ON HUMANS
No information was located regarding the toxicity of cumene in
humans following acute, subchronic, or chronic exposure (US EPA,
1997). The minimum lethal human exposure to this agent has not been
delineated. No epidemiology, case reports, or clinical controls of
humans were located for this compound. There are no epidemiological or
occupational studies examining the carcinogenicity of cumene in humans
(US EPA, 1997).
No information was located regarding dermal irritation and
sensitization in humans following exposure to cumene.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
The available environmental effects studies are inadequate to
allow a quantitative assessment of the acute toxicity of cumene to
environmental organisms owing to the variability of the data and
flawed experimental designs. For example, 24-h toxicity values for
water fleas ranged from an EC50 of 91 mg/litre (Bringmann & Kuhn,
1982) down to an IC50 of 0.6 mg/litre (Abernathy et al., 1986).
Further, many of the reported toxicity values for aquatic
invertebrates exceed the water solubility of cumene at 50 mg/litre,
with Glickman et al. (1995) noting that actual measured concentrations
of cumene were only about 10% of nominal concentrations. The lowest
reported toxic concentration was 0.012 mg/litre, the toxicity
threshold for the protozoan Colpidium colpoda (Rogerson et al.,
1983). Concentrations of up to 50 mg/litre did not affect the growth
of the larvae of the mussel Mytilis edulis during a 27-day exposure
(Le Roux, 1977). Selected data demonstrating effect concentrations are
shown in Table 2. It should be noted that the high volatility and
biodegradability of cumene may further reduce the hazard to the
aquatic environment, especially for chronic exposure conditions.
Table 2: Acute toxicity of cumene to organisms other than laboratory mammals.
Species End-point Concentration
(effect) (mg/litre) Reference
Algae
Green alga 3-h EC50 21 Hutchinson et al.,
(Chlorella vulgaris) (photosynthetic 1980
inhibition)
Green alga 3-h EC50 9 Hutchinson et al.,
(Chlamydomonas angulosa) (photosynthetic 1980
inhibition)
Green alga 72-h EC50 2.6 Galassi et al.,
(Selenastrum capricornutum) (growth 1988
inhibition)
Green algae 72-h static EC50 2.0 Hüls, 1998a
(Scenedesmus subspicatus) (growth
inhibition)
Invertebrates
Water flea (Daphnia magna) 24-h EC50 91 Bringmann & Kuhn,
(immobilization) 1982
Water flea (Daphnia magna) 24-h LC50 4.8 Glickman et al.,
1995
Water flea (Daphnia magna) 21-day static EC50 1.5 Hüls, 1998b
Water flea (Daphnia magna) 24-h IC50a 1.4 Galassi et al.,
1988
Table 2 (continued)
Water flea (Daphnia magna) 24-h IC50 0.6 Abernathy et al.,
1986
Mysid shrimp (Mysidopsis bahia) 96-h flow LC50 1.3 Glickman et al.,
1995
Mysid shrimp (Mysidopsis bahia) 96-h flow LC50 1.2 Chemical Manufacturers
Association, 1990
Ciliate protozoan "toxicity threshold" 0.012 Rogerson et al.,
(Colpidium colpoda) (NR)b 1983
Vertebrates
Rainbow trout 96-h LC50 4.8 Glickman et al.,
(Oncorhynchus mykiss) 1995
Rainbow trout no observed effect 1.9 Glickman et al.,
(Oncorhynchus mykiss) 1995
Sheepshead minnow 96-h flow LC50 4.7 Glickman et al.,
(Cyprinodon variegatus) 1995
Sheepshead minnow no observed effect <2.9 Glickman et al.,
(Cyprinodon variegatus) 1995
a IC50 = immobilization concentration for 50% of the organisms.
b NR = not reported.
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
Kinetic analysis shows that there is rapid and complete clearance
of cumene and its metabolites from the body, indicating little
potential for accumulation. No human toxicity data are available from
exposure to cumene. Short-term exposures of animals to high
concentrations (>2450 mg/m3 [>500 ppm]) demonstrate that cumene,
like other solvents, may be considered harmful, inducing transient
reversible central nervous system effects. However, neurotoxicity,
portal-of-entry effects, developmental effects, and markedly adverse
systemic toxicity were not observed after long-term repeated-dose
studies conducted in animals at lower concentrations (<2450 mg/m3
[<500 ppm]). Cumene has caused dermal and ocular irritation in
animals in one study, but it had no such effects in others. A single
study indicates that cumene does not elicit dermal sensitization in
animals.
Increases in organ weights (most notably kidney) are the most
prominent and consistent effects observed in rodents exposed for 6-7
months by the oral route (Wolf et al., 1956) or for 3 months by the
inhalation route (Cushman et al., 1995). No adverse effects were
observed in rat or rabbit fetuses whose mothers had been exposed to
airborne cumene during fetal development.
The sparsity of long-term repeated-dose toxicity data and the
absence of any human toxicity data both constitute areas of scientific
uncertainty. The only repeated-dose toxicity studies of any
appreciable duration are the oral study of Wolf et al. (1956), at
about 7 months, and the 3-month subchronic inhalation study of Cushman
et al. (1995). Both of these studies are concurrent in indicating
kidneys of female rats as the target organ, regardless of exposure
route. Although neither of these studies is sufficient in duration to
reveal the fate of the observed alterations in organ weights from
lifetime chronic exposure, the subchronic study of Cushman et al.
(1995) is more scientifically comprehensive in its analyses than the
study of Wolf et al. (1956) and offers much more extensive data
reporting on more animals (both genders). The study of Cushman et al.
(1995) is therefore chosen as the pivotal study.
No multigeneration reproductive studies have been performed for
cumene. The rapid metabolism and excretion of cumene, coupled with the
lack of effects on sperm morphology reported by Cushman et al. (1995),
indicate that cumene has low potential for reproductive toxicity.
However, this lack of concern must be weighed against the fact that
kinetic studies indicate extensive and wide distribution of cumene,
including to reproductive organs, and the fact that the consequences
of long-term repeated/continuous exposure on either organs or
reproductive function have not been evaluated.
There are no data in humans or animals concerning the development
of cancer following exposure to cumene. The potential hazard for
carcinogenicity of cumene to humans has not been determined, although
the predominant evidence suggests that this compound is not likely to
produce a carcinogenic response (i.e., numerous genotoxic tests,
including gene mutation, chromosomal aberration, and primary DNA
damage tests, were conducted, all but one of which were negative or
not reproducible). No highly reactive chemical species are known to be
generated during the metabolism of cumene.
11.1.2 Criteria for setting guidance values for cumene
For oral exposures, the NOAEL for increased average kidney weight
in female rats following subchronic (139/194 days) oral (gavage)
exposure is 154 mg/kg body weight per day, which was adjusted, based
on the dosing schedule, to 110 mg/kg body weight per day (Wolf et al.,
1956). These data were not amenable to benchmark dose analysis. For
purposes of quantitative assessment, the quality of the principal oral
study is marginal, because the group sizes were minimal, the groups
comprised females only, and little quantitative information was
presented. Full uncertainty factors of 10 each are applied for
interindividual and interspecies variations. A partial uncertainty
factor (100.5) for extrapolation from subchronic to chronic duration
is applied, as the study was intermediate between chronic and
subchronic duration. Another partial uncertainty factor (100.5) is
also used owing to lack of a full-scale multigeneration reproductive
study. The total uncertainty factor applied was 1000 (10 × 10 × 100.5
× 100.5). This yields a guidance value for oral exposure of 0.1 mg/kg
body weight per day. This guidance value is meant to provide
information for risk managers to enable them in making decisions
concerning the protection of human health.
Interpretation of the effects reported in the subchronic
inhalation study of Cushman et al. (1995) allows for consideration of
either the 490 mg/m3 (100 ppm) (MAK, 1996) or the 2430 mg/m3 (496
ppm) (US EPA, 1997) exposure level as a defensible NOAEL. Whereas the
motor effects, organ weight changes, and clinical effects reported at
2430 mg/m3 (496 ppm) may be regarded as non-adverse indicators of
exposure (in other words, as a NOAEL), these same effects may be
regarded alternatively as potentially adverse indicators of
toxicologically significant effects apparent at the next highest
exposure level (in other words, a LOAEL). Consideration of both these
interpretations may be justified in derivation of an inhalation
guidance value for cumene. The experimental exposure scenario of the
NOAEL (either 490 or 2430 mg/m3 [100 or 496 ppm]) is first adjusted
to a continuous exposure scenario for the general population by
factoring the NOAEL by the hours exposed as a fraction of the day
(6/24 hours) and the number of days exposed as a fraction of the week
(5/7), resulting in the figure of 436 mg/m3 (89 ppm) for the 2430
mg/m3 (496 ppm) experimental exposure level and 88 mg/m3 (18 ppm)
for the 490 mg/m3 (100 ppm) experimental exposure level. Full
uncertainty factors of 10 each were applied for subchronic to chronic
extrapolation and for interindividual variations. A partial
uncertainty factor (100.5) is applied to account for the toxicodynamic
component of the interspecies extrapolation. In long-term inhalation
exposures, the blood/air partition coefficient ( Hb/a) is a principal
factor determining the amount of compound reaching a systemic tissue
(such as kidney). For a given external concentration and similar
exposure conditions, the smaller the Hb/a values, the less compound
in the blood and at the tissue. The blood/air partition coefficient
has been determined with human blood (Sato & Nakajima, 1979, 1987),
but not for rats. Information available on compounds structurally
related to cumene (xylenes and benzene; Gargas et al., 1989) indicates
that human Hb/a values are nearly always smaller than rat Hb/a
values, such that, for a given external concentration, human tissues
would receive less compound than would rat tissues. Thus, use of the
rat in a long-term repeated-dose study with cumene obviates the need
for the toxicokinetic component of the animal to human extrapolation.
An additional partial uncertainty factor (100.5) is used for database
deficiencies, owing principally to lack of a full-scale
multigeneration reproductive study, as discussed above. The total
uncertainty factor would be 1000 (10 × 10 × 100.5 × 100.5).
Application of this factor would result in guidance values of 0.4
mg/m3 (0.08 ppm) for the NOAEL of 436 mg/m3 (89 ppm), adjusted for
continuous exposure from 2430 mg/m3 (496 ppm), and 0.09 mg/m3
(0.02 ppm) for the NOAEL of 88 mg/m3 (18 ppm), adjusted for
continuous exposure from 490 mg/m3 (100 ppm).
The carcinogenic potential of cumene cannot be determined because
no adequate data, such as well-conducted long-term animal studies or
reliable human epidemiological studies, are available with which to
perform an assessment.
11.1.3 Sample risk characterization
The scenario chosen as an example is continuous lifetime exposure
for the general population.
No human data are available with which to characterize the
toxicity of cumene directly. The reported ambient cumene concentration
of 0.0147 mg/m3 (0.003 ppm) is appreciably below either of the
guidance values of 0.4 mg/m3 (0.08 ppm) (27-fold) or 0.09 mg/m3
(0.02 ppm) (6-fold). The upper limit of ambient cumene concentrations
reported in rural air, 2.5 µg/m3 (0.5 ppb), is even further below the
guidance values (36- to 160-fold). Other data presented in this
report, such as estimates from cigarette smoke, suggest that humans
would primarily be exposed through inhalation, although ingestion
through food may occur. Exposure via drinking-water is probably
unlikely.
The critical effect in the principal study for the oral
assessment is increased kidney weight in female rats and, although
poorly reported, is corroborated by inhalation studies with cumene.
Increased organ weights have been found in other toxicity studies with
cumene and have been observed across routes of exposure. Insufficient
data on oral exposure exist to apply the guidance value of 0.1 mg/kg
body weight per day derived above.
Following inhalation exposure, the effects observed included
increased kidney and adrenal weights and central nervous system,
haematological, and clinical biochemical alterations, which were
observed in rats. The critical effect was observed across species and
was observed in several studies. These results partially corroborate
and reinforce the significance of similar results seen in the
long-term oral study of cumene.
The potential hazard for carcinogenicity of cumene in humans
cannot be determined. Studies have indicated that cumene has low, if
any, genotoxicity.
Neither chronic nor multigeneration reproductive studies are
available for this substance.
Data are not available to determine whether young or aged animals
are more susceptible than adult animals (e.g., 2-year-old rats) to the
effects of cumene, and there is no evidence to suggest that this would
be so in young or aged humans. There is also no convincing evidence to
suggest that gender differences in susceptibility to cumene toxicity
would exist in humans.
11.2 Evaluation of environmental effects
Cumene is a volatile liquid and exists mainly in the vapour phase
in the atmosphere. It degrades in the atmosphere via reaction with
hydroxyl radicals. Although small amounts of cumene may be removed
from the atmosphere by precipitation, cumene is not expected to react
with ozone or directly with light. In water, cumene can be
volatilized, undergo biodegradation, or adsorb to sediments. In soil,
it is expected to biodegrade rapidly under aerobic conditions; as in
water, it can readily adsorb to soil or volatilize.
BCFs suggest a slight potential for cumene to bioconcentrate in
fish species. No data were available on the bioconcentration of cumene
in terrestrial organisms. Although the existing toxicological database
and limited exposure data do not permit a quantitative risk
assessment, the available information suggests that cumene will not
adversely affect populations or communities of terrestrial or aquatic
organisms based on its low availability (volatility, rapid
degradation).
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
No previous evaluations by international bodies were identified.
Information on international hazard classification and labelling
is included in the International Chemical Safety Card reproduced in
this document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventive and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 0170) reproduced in this
document.
13.1 Human health hazards
Cumene is flammable. Exposure could cause central nervous system
effects and at high concentrations could result in unconsciousness.
13.2 Advice to physicians
In the event of poisoning, treatment is supportive.
13.3 Health surveillance advice
For workers exposed to cumene, a health surveillance programme
should include surveillance of kidney function.
13.4 Spillage
In the event of spillage, measures should be taken to prevent
cumene from reaching drains and watercourses, owing to the potential
for hazardous effects on aquatic organisms.
13.5 Storage
Cumene should be stored away from acids and strong oxidants.
Long-term storage could result in the formation of explosive
peroxides. Proper safety and handling procedures must be used.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards
may be obtained from UNEP Chemicals (IRPTC), Geneva.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
ISOPROPYLBENZENE ICSC:0170
October 1994
CAS # 98-82-8 Cumene
RTECS# GR8575000 (1-Methylethyl)benzene
UN # 1918 2-Phenylpropane
EC # 601-024-00-X C9H12/C6H5CH(CH3)2
Molecular Mass
TYPES OF HAZARD/ ACUTE HAZARDS/ PREVENTION FIRST AID/
EXPOSURE SYMPTOMS FIRE FIGHTING
FIRE Flammable NO open flames, Powder, AFFF,
NO sparks, and foam, carbon dioxide
NO smoking.
EXPLOSION Above 31°C explosive Above 31°C use a In case of fire: keep
vapour/air mixtures closed system, drums, etc., cool by
may be formed. ventilation, and spraying with water.
explosion-proof
electrical equipment.
Prevent build-up of
electrostatic charges
(e.g., by grounding).
EXPOSURE PREVENT GENERATION
OF MISTS!
Inhalation Ataxia. Cough. Ventilation, local Fresh air, rest.
Dizziness. Drowsiness. exhaust, or breathing Half-upright position.
Headache. Sore throat. protection. Refer for medical
Unconsciousness. attention.
Skin Dry skin. Protective gloves. Remove contaminated
Protective clothing. clothes. Rinse and
then wash skin with
water and soap.
Eyes Redness. Pain. Safety spectacles. First rinse with
plenty of water for
several minutes
(remove contact lenses
if easily possible),
then take to a doctor.
Ingestion (further see inhalation). Do not eat, drink, or Rinse mouth. Do NOT
smoke during work. induce vomiting. Refer
for medical attention.
SPILLAGE DISPOSAL PACKAGING & LABELLING
Collect leaking and spilled liquid in Marine Pollutant
sealable containers as far as possible. EU Classification
Absorb remaining liquid in sand or inert Symbol: Xi
absorbent and remove to safe place. R: 10-37
Do NOT let this chemical enter the S: (2-)
environment (extra personal protection: Note: C
A/P2 filter respirator for organic vapour UN Classification
and harmful dust.) UN Hazard Class: 3
UN Pack Group: III
EMERGENCY RESPONSE STORAGE
Transport Emergency Card: TEC (R)-594 NFPA Fireproof. Separated from strong oxidants,
Code: H2; F3; R0 acids. Cool. Keep in the dark. Store only
if stabilized.
IMPORTANT DATA
PHYSICAL STATE; APPEARANCE ROUTES OF EXPOSURE:
COLOURLESS LIQUID, WITH CHARACTERISTIC The substance can be absorbed into the
ODOUR. the body by inhalation and through the
skin.
PHYSICAL DANGERS: INHALATION RISK:
As a result of flow, agitation, etc., A harmful contamination of the air will be
electrostatic charges can be generated. reached rather slowly on evaporation of this
substance at 20°C.
CHEMICAL DANGERS: EFFECTS OF SHORT TERM EXPOSURE:
The substance can form explosive peroxides. The substance irritates the eyes and the
Reacts violently with acids and strong respiratory tract. Swallowing the liquid may
oxidants causing fire and explosion hazards. cause aspiration into the lungs with the risk
of chemical pneumonitis. The substance may
cause effects on the central nervous system.
Exposure far above the OEL may result in
unconsciousness.
OCCASIONAL EXPOSURE LIMITS: EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
TLV: 50 ppm; 246 mg/m3 (skin) Repeated or prolonged contact with skin
(ACGIH 1996). may cause dermatitis.
PHYSICAL PROPERTIES
Boiling point: 152 °C Flash point: 31°C
Melting point: -96 °C Auto-ignition temperature: 420°C
Relative density (water = 1): 0.90 Explosive limits, vol% in air: 0.9-6.5
Solubility in water: none Octanol/water partition coefficient as
Vapour Pressure, Pa at 20°C: 427 log Pow: 3.66
Relative Vapour density (air = 1): 4.2
Relative density of the vapour/air-mixture
at 20°C (air = 1): 1.01
ENVIRONMENTAL DATA
This substance may be hazardous to the environment; special attention should be given to
water organisms, and birds.
NOTES
An added stabilizer or inhibitor can influence the toxicological properties of this
substance, consult an expert. Check for peroxides prior to distillation; eliminate if
found.
ADDITIONAL INFORMATION
LEGAL NOTICE Neither the CEC nor the IPCS nor any person acting on behalf of
the CEC or the IPCS is responsible for the use which might be
made of this information.
REFERENCES
Abernathy S, Bobra AM, Shiu WY, Wells PG, Mackay D (1986) Acute lethal
toxicity of hydrocarbons and chlorinated hydrocarbons to two
planktonic crustaceans: the key role of organism-water partitioning.
Aquatic toxicology, 8:163-174.
Amoore JE, Hautala E (1983) Odor as an aid to chemical safety: Odor
thresholds compared with threshold limit values and volatilities for
214 industrial chemicals in air and water dilution.
Journal of applied toxicology, 3(6):272-290.
Anon. (1984) Chemical profile: Cumene. Chemical Marketing Reporter,
July 23, 1984. New York, NY, Schnell Publishing Co., Inc.
Anon. (1998) Organic chemicals manufactured. Chemical and
engineering news, 78(26):43.
Arnts RR, Meeks SA (1980) Biogenic hydrocarbon contribution to the
ambient air of selected areas: Tulsa, Great Smokey Mountains, Rio
Blanco County, CO. Research Triangle Park, NC, US Environmental
Protection Agency, Office of Research and Development
(EPA 600/3-80-023).
Arnts RR, Meeks SA (1981) Biogenic hydrocarbon contribution to the
ambient air of selected areas. Atmospheric environment,
15(9):1643-1651.
Battersby NS, Wilson V (1989) Survey of the anaerobic biodegradation
potential of organic chemicals in digesting sludge. Applied
environmental microbiology, 55(2):433-439.
Botta D, Castellani Pirri L, Mantica E (1984) Ground water pollution
by organic solvents and their microbial degradation products. In:
Angeletti G, Bjorseth A, eds. Analysis of organic micropollutants in
water: Proceedings of the 3rd European Symposium, September 1983,
Oslo, Norway. Boston, MA, D. Reidel Publishing Co., pp. 261-275
(Report EUR 8518).
Bringmann G, Kuhn R (1982) Results of toxic action of water pollutants
on Daphnia magna straus tested by an improved standardized
procedure. Zeitschrift für Wasser und Abwasser Forschung, 15(1):1-6.
Brown DW, Friedman AJ, Burrows DG, Snyder GR, Patten BG (1979)
Investigation of petroleum in the marine environs of the Strait of
Juan de Fuca and northern Puget Sound. Washington, DC, US
Environmental Protection Agency (EPA 60/7-79-164).
Brugnone F, Perbellini L, Faccini GB, Pasini F, Romeo L, Gobbi M,
Zedde A (1989) Breath and blood levels of benzene, toluene, cumene and
styrene in non-occupational exposure. International archives of
occupational and environmental health, 61:303-311.
Burmaster DE (1982) The new pollution: groundwater contamination.
Environment, 24(2):7-36.
Burnham AK, Calder GV, Fritz JS, Junk GA, Svec HJ, Willis R (1972)
Identification and estimation of neutral organic contaminants in
potable water. Analytical chemistry, 44(1):139-142.
Bushy Run Research Center (1989a) Cumene fourteen-week vapor
inhalation study in rats with neurotoxicity evaluation (part 1-2),
with attached studies and cover letter dated 7 December 1989. Export,
PA. Washington, DC, US Environmental Protection Agency, Office of
Toxic Substances (Final Project Report 52-628; TSCA section 4
submission 40-8992172).
Bushy Run Research Center (1989b) Developmental toxicity study of
inhaled cumene vapor in CD (Sprague-Dawley) rats. Export, PA.
Washington, DC, US Environmental Protection Agency, Office of Toxic
Substances (Final Project Report 52-621; TSCA section 4 submission
40-8992172).
Bushy Run Research Center (1989c) Developmental toxicity study of
inhaled cumene vapor in New Zealand white rabbits. Export, PA.
Washington, DC, US Environmental Protection Agency, Office of Toxic
Substances (Final Project Report 52-622; TSCA section 4 submission
40-8992172).
Chemical Manufacturers Association (1989) A two-week pilot
inhalation toxicity study of cumene vapors in rats, with attachments
and cover letter dated 7 September 1989. Washington, DC. Washington,
DC, US Environmental Protection Agency, Office of Toxic Substances
(TSCA section 4 submission 40-8992168).
Chemical Manufacturers Association (1990) Cumene (isopropylbenzene):
Acute toxicity to mysid shrimp (Mysidopsis bahia ) under
flow-through conditions. Washington, DC. Washington, DC, US
Environmental Protection Agency, Office of Toxic Substances (TSCA
section 4 submission 40-9092188).
Ciba-Geigy Co. (1985) Toxicology data summary sheet. Washington, DC,
US Environmental Protection Agency, Office of Toxic Substances (TSCA
section 8(d) submission 878215034).
Cocheo V, Bellomo ML, Bombi GG (1983) Rubber manufucture: sampling and
identification of volatile pollutants. American Industrial Hygiene
Association journal, 44(7):521-527.
Coleman WE, Munch JW, Striecher RP, Ringhand HP, Kopfler FC (1984) The
identification and measurement of components in gasoline, kerosene,
and No. 2 fuel oil that partition into the aqueous phase after mixing.
Archives of environmental contamination and toxicology, 13:171-178.
Conkle JP, Camp BJ, Welch BE (1975) Trace composition of human
respiratory gas. Archives of environmental health, 30(6):290-295.
Curren RD (1987) Unscheduled DNA synthesis in rat primary
hepatocytes -- test article: Cumene. Bethesda, MD, Microbiological
Associates, Inc. Washington, DC, US Environmental Protection Agency,
Office of Toxic Substances (Study No. T4786.380005; TSCA section 4
submission 40-8792124).
Cushman JR, Norris JC, Dodd DE, Darmer KI, Morris CR (1995) Subchronic
inhalation toxicity assessment of cumene in Fischer 344 rats.
Journal of the American College of Toxicology, 14(2):129-147.
Darmer KI, Neeper-Bradley TL, Cushman JR, Morris CR, Francis BO (1997)
Developmental toxicity of cumene vapor in CD rats and New Zealand
white rabbits. International journal of toxicology, 16:119-139.
Derwent RG, Jenkin ME (1990) Hydrocarbon involvement in
photochemical ozone formation in Europe. Harwell, United Kingdom
Atomic Energy Authority (Report No. AERE R 13736).
Eisenreich SJ, Looney BB, Thornton JD (1981) Airborne organic
contaminants of the Great Lakes ecosystem. Environmental science and
technology, 15(1):30-38.
Fabre R, Truhaut R, Bernuchon J, Loisillier F (1955) Toxicologic
studies of solvents to replace benzene. III. Study of isopropyl
benzene or cumene. Archives des maladies professionnelles de
médecine du travail et de sécurité sociale, 16(4):285-299 (in
French).
Florin I, Rutberg L, Curvall M, Enzell CR (1980) Screening of tobacco
smoke constituents for mutagenicity using the Ames test. Toxicology,
18:219-232.
Foster P, Laffond M, Baussand P, Jacob V, Denis I (1991) VOC
measurements in the Grenoble area and study of the benzaldehyde
behaviour in a simulation chamber. Pollution atmosphérique,
19:175-191 (in French).
Galassi S, Mingazzini M, Vigano I, Cesareo D, Tosato MI (1988)
Approaches to modeling toxic responses of aquatic organisms to
aromatic hydrocarbons. Ecotoxicology and environmental safety,
16:158-169.
Gargas MJ, Burgess RJ, Voisard DE, Cason GH, Andersen ME (1989)
Partition coefficients of low-molecular-weight volatile chemicals in
various liquids and tissues. Toxicology and applied pharmacology,
98:87-99.
Gavin J, Nicollier G, Tabacchi R (1978) Volatile constituents of
"oakmoss" ( Evernia prunastri (L.)). Part 3. Helvetica Chimica
Acta, 61(1):352-357.
Glickman AH, Alexander HC, Buccafusco RJ, Morris CR, Francis BO,
Suprenant DC, Ward TJ (1995) An evaluation of the aquatic hazard of
cumene (isopropyl benzene). Ecotoxicology and environmental safety,
31:287-289.
Gordon AW, Gordon M (1981) Analysis of volatile organic compounds in a
textile finishing plant effluent. Transactions of the Kentucky
Academy of Science, 42(3-4):149-157.
Grosjean D, Fung K (1984) Hydrocarbons and carbonyls in Los Angeles
air. Journal of the Air Pollution Control Association, 34:537-543.
Guicherit R, Schulting RL (1985) The occurrence of organic chemicals
in the atmosphere of The Netherlands. The science of the total
environment, 43:193-219.
Gulf Life Sciences Center (1985a) CHO/HGPRT test of cumene.
Pittsburgh, PA. Washington, DC, US Environmental Protection Agency,
Office of Toxic Substances (Gulf Project No. 84-2128; TSCA section
8(d) submission 878216011).
Gulf Life Sciences Center (1985b) Micronucleus test of cumene.
Pittsburgh, PA. Washington, DC, US Environmental Protection Agency,
Office of Toxic Substances (Gulf Project No. 84-2129; TSCA section
8(d) submission 878216015).
Gulf Oil Corp. (1984a) Cell transformation test of cumene. Houston,
TX. Washington, DC, US Environmental Protection Agency, Office of
Toxic Substances (Gulf Project No. 84-2131; TSCA section 8(e)
submission 888500694).
Gulf Oil Corp. (1984b) Hepatocyte primary culture/DNA repair test of
cumene. Houston, TX. Washington, DC, US Environmental Protection
Agency, Office of Toxic Substances (Gulf Project No. 84-2130; TSCA
section 4 submission 888500694).
Gulf Oil Corp. (1985) Five-day repeated dose inhalation toxicity
study in rats of cumene, with cover letter. Houston, TX. Washington,
DC, US Environmental Protection Agency, Office of Toxic Substances
(Gulf Project No. 84-2132; TSCA section 8(d) submission 878216016).
Hansch C, Leo AJ (undated) The Pomona College Medicinal Chemistry
Project. Claremont, CA, Pomona College.
Hard GC, Rodgers IS, Baetcke KP, Richards WL, McGaughy RE, Valcovic LR
(1993) Hazard evaluation of chemicals that cause accumulation of
alpha-2µ-globulin, hyaline droplet nephropathy, and tubular neoplasia
in the kidneys of male rats. Environmental health perspectives,
99:313-349.
Holzer G, Oró J, Bertsch W (1976) Gas chromatographic-mass
spectrometric evaluation of exhaled tobacco smoke. Journal of
chromatography, 126:771-785.
Hüls (1988) Prüfung auf hautsensibilisierende Wirkung am
Meerschweinchen von Cumol. Unveröffentlichte Untersuchung (Bericht
Nr. 1219).
Hüls (1998a) Determination of the effect of cumene on the growth of
Scenedesmus subspicatus 86.81.SAG (Algal growth inhibition test
complying with Directive 92/69/EEC). Marl, Hüls Infracor,
Prüfinstitute für Analytik, Biologie und Toxikologie (Final Report
AW-469).
Hüls (1998b) Determination of the effects of cumene on the
reproduction rate of Daphnia magna (as specified by OECD 211/draft
of December 1996). Marl, Hüls Infracor, Prüfinstitute für Analytik,
Biologie und Toxikologie (Final Report DL-172).
Hutchinson TC, Hellebust JA, Tam D, Mackay D, Mascarenhas RA, Shiu WY
(1980) The correlation of the toxicity to algae of hydrocarbons and
halogenated hydrocarbons with their physical-chemical properties.
Environmental science research, 16:577-586.
IPCS (1993) International Chemical Safety Card -- Isopropylbenzene.
Geneva, World Health Organization, International Programme on Chemical
Safety (ICSC 0170).
Ishida T, Matsumoto T (1992) Enantioselective metabolism of cumene.
Xenobiotica, 22:1291-1298.
Isidorov VA, Zenkevich IG, Ioffe BV (1983) Methods and results of gas
chromatographic-mass spectrometric determination of volatile organic
substances in an urban atmosphere. Atmospheric environment,
17(7):1347-1353.
Jamison VW, Raymond R, Hudson JO (1970) Hydrocarbon co-oxidation by
Nocardia corallina strain V-49. Developments in industrial
microbiology, 12:99-105.
Japan Environment Agency (1987) Chemicals in the environment, a
report of 1987 survey. Tokyo, Japan Environment Agency,
Environmental Health Department, Office of Health Studies.
Jeng CY, Chen DH, Yaws CL (1992) Data compilation for soil sorption
coefficient. Pollution engineering, 24(12):54-60.
Jenkins LJ, Jr, Jones RA, Siegel J (1970) Long-term inhalation
screening studies of benzene, toluene, o-xylene, and cumene on
experimental animals. Toxicology and applied pharmacology,
16(3):818-823.
Johnstone RAW, Quan PM, Carruthers W (1962) Composition of cigarette
smoke: some low-boiling components. Nature, 195:1267-1269.
Kappeler T, Wuhrmann K (1978) Microbial degradation of the
water-soluble fraction of gas oil -- I. Water research, 12:327-334.
Katzman H, Libby WF (1975) Hydrocarbon emissions from jet engines
operated at simulated high-altitude supersonic flight conditions.
Atmospheric environment, 9(9):839-842.
Keith LH, Garrison AW, Allen FR, Carter MH, Floyd TL, Pope JD,
Thruston AD, Jr (1976) Identification of organic compounds in drinking
water from thirteen U.S. cities. In: Keith LH, ed. Identification
and analysis of organic pollutants in water. Ann Arbor, MI, Ann
Arbor Science, pp. 329-373.
Koch Refining Co. (1984) Material safety data sheet: Cumene. Corpus
Christi, TX.
Kristiansen U, Hansen L, Nielsen GD, Holst D (1986) Sensory irritation
and pulmonary irritation of cumene and n-propanol: Mechanisms of
receptor activation and desensitization. Acta Pharmacologica et
Toxicologica, 59:60-72.
Krotoszynski BK, O'Neill HJ (1982) Involuntary bioaccumulation of
environmental pollutants in nonsmoking heterogeneous human
population. Journal of environmental science and health. Part A:
Environmental science and engineering, 17(6):855-883.
Lawlor TE, Wagner VO (1987) Salmonella /mammalian-microsome
preincubation in mutagenicity assay (Ames test); test article:
Cumene. Bethesda, MD, Microbiological Associates, Inc. Washington,
DC, US Environmental Protection Agency, Office of Toxic Substances
(Study No. T4786.502009; TSCA section 4 submission 40-8792121).
Le Roux S (1977) The toxicity of pure hydrocarbons to mussel larvae.
Rapports et procès-verbaux des réunions, Conseil International pour
l'Exploration de la Mer, 171:189-190 (in French with English
abstract).
Levy A (1973) The photochemical smog reactivity of organic solvents.
Advances in chemistry series, 124:70-94.
Lloyd AC, Darnall KR, Winer AM, Pitts JN, Jr (1976) Relative rate
constants for reaction of the hydroxyl radical with a series of
alkanes, alkenes, and aromatic hydrocarbons. Journal of physical
chemistry, 80(8):789-794.
Lonneman WA, Bellar TA, Altshuller AP (1968) Aromatic hydrocarbons in
the atmosphere of the Los Angeles basin. Environmental science and
technology, 2(11):1017-1020.
Lonneman WA, Namie GR, Bufalini JJ (1979) Hydrocarbons in Houston
air. Research Triangle Park, NC, US Environmental Protection Agency,
Office of Research and Development (EPA 600/3-79-018).
Lyman WJ, Reehl WF, Rosenblatt DH (1982) Handbook of chemical
property estimation methods. Environmental behaviour of organic
compounds. New York, NY, McGraw-Hill Book Company.
Mackay D, Shiu WY (1981) A critical review of Henry's law constants
for chemicals of environmental interest. Journal of physical and
chemical reference data, 19:1175-1199.
MAK (1996) Deutsche Forschungsgemeinschaft, MAK-Begründung:
Isopropylbenzol (cumol), 22. Lieferung.
Mill T, Richardson H, Hendry DG (1978) Oxidation of organic compounds
in aquatic systems: The free radical oxidation of cumene. In:
Hutzinger O, Van Lelyveld IH, Zoeteman BCJ, eds. Aquatic pollutants:
Transformation and biological effects. Proceedings of the 2nd
International Symposium on Aquatic Pollutants, Amsterdam, 26-28
September 1977. New York, NY, Pergamon Press, pp. 223-236.
Mill T, Hendry DG, Richardson H, Baraze A (1979) Chemical oxidation
processes in aquatic systems: Oxidation of cumene, pyridine, and
p -cresol. Menlo Park, CA, SRI International, 73 pp. (National
Science Foundation Grant No. ENV76-11153; NSF/RA-790085; NTIS
PB-298088).
Mill T, Hendry DG, Richardson H (1980) Free-radical oxidants in
natural waters. Science, 207:886-887.
Mills A, Foureman GL (1998) U.S. EPA's IRIS pilot program:
establishing IRIS as a centralized, peer-review database with agency
consensus. Toxicology, 127:85-95.
Mitruka BM, Rawnsley HM (1981) Clinical biochemical and
hematological reference values in normal experimental animals and
normal humans, 2nd ed. New York, NY, Masson Publishing.
Mody NV, Parish EJ, Bhattacharyya J, Miles DH (1974a) Constituents of
marsh grass. IV. Essential oil in Scirpus americanus.
Phytochemistry, 13(9):2027-2029.
Mody NV, Bhattacharyya J, Miles DH (1974b) Constituents of marsh
grass. II. Survey of the essential oil in Spartina cynosuroides.
Phytochemistry, 13(7):1175-1178.
Moelhave L (1979) Indoor air pollution due to building materials. In:
Fanger PO, Valbjrn O, eds. Indoor climate: effects on human comfort,
performance, and health in residential, commercial, and
light-industry buildings. Proceedings of the 1st International
Indoor Climate Symposium, Copenhagen, Denmark, August/September 1978,
pp. 89-110.
Monsanto Co. (1984) Cumene health study Y-78-213: Acute toxicity,
eye and skin irritation. St. Louis, MO. Washington, DC, US
Environmental Protection Agency, Office of Toxic Substances (TSCA
section 8(d) submission 878215096).
Monsanto Co. (1986) One-month study of cumene vapor administered to
male and female Sprague-Dawley rats by inhalation. St. Louis, MO.
Washington, DC, US Environmental Protection Agency, Office of Toxic
Substances (TSCA section 8(d) submission 86870000044).
Montgomery CA, Jr, Seely JC (1990) Kidney. In: Boorman GA, Eustis SL,
Elwell MR, Montgomery CA, Jr, MacKenzie WF, eds. Pathology of the
Fischer rat, reference and atlas. New York, NY, Academic Press, pp.
127-153.
Montz WE, Puyear RL, Brammer JD (1982) Identification and
quantification of water-soluble hydrocarbons generated by two-cycle
outboard motors. Archives of environmental contamination and
toxicology, 11:561-565.
NIOSH (1994) Method 1501. In: NIOSH manual of analytical methods
(NMAM), 4th ed. Cincinnati, OH, National Institute for Occupational
Safety and Health.
NTP (1996) In-vivo cytogenetics testing results for cumene,
micronucleus induction results. Available from National Toxicology
Program, National Institute for Environmental and Health Sciences,
Research Triangle Park, NC.
Ogata M, Fujisawa K, Ogino Y, Mano E (1984) Partition coefficients as
a measure of bioconcentration potential of crude oil compounds in fish
and shellfish. Bulletin of environmental contamination and
toxicology, 33(5):561-567.
Omori T, Jigami Y, Minoda Y (1975) Isolation, identification and
substrate assimilation specificity of some aromatic hydrocarbons
utilizing bacteria. Agricultural and biological chemistry,
39:1775-1779.
Parlar H, Coelhan M, Vaughan D, Czermak P, Kohler U, Korte F (1983)
Gas phase-mass analyzer system for the determination of the
photostability of organic compounds under simulated tropospheric
conditions. Fresenius' Zeitschrift für Analytische Chemie,
315(7):605-609.
Pellizzari ED, Castilo NP, Willis S, Smith D, Bursey JT (1979)
Identification of organic components in aqueous effluents from
energy-related processes. In: Van Hall CE, ed. Measurement of
organic pollutants in water and wastewater: Symposium on Water,
June 1978, Denver, CO. Philadelphia, PA, American Society for
Testing and Materials, ASTM Committee D19, pp. 256-274 (ASTM Special
Technical Publication 686).
Price KS, Waggy GT, Conway RA (1974) Brine shrimp bioassay and
seawater BOD of petrochemicals. Journal of the Water Pollution
Control Federation, 46(1):63-77.
Putnam DL (1987) Chromosome aberrations in Chinese hamster ovary
(CHO) cells -- test article: Cumene. Bethesda, MD, Microbiological
Associates, Inc. Washington, DC, US Environmental Protection Agency,
Office of Toxic Substances (Study No. T4786.337012; TSCA section 4
submission 40-8792123).
Ravishankara AR, Wagner S, Fischer S, Smith G, Schiff R, Watson RT,
Tesi G, Davis DD (1978) A kinetics study of the reaction of OH with
several aromatic and olefinic compounds. International journal of
chemical kinetics, 10:783-804.
Research Triangle Institute (1989) Metabolism, disposition and
pharmacokinetics of cumene in F-344 rats following oral, IV
administration or nose-only inhalation exposure. Research Triangle
Park, NC. Washington, DC, US Environmental Protection Agency, Office
of Toxic Substances (Study No. 3543; TSCA section 4 submission
40-8992171).
Robinson D, Smith JN, Williams RT (1955) Studies in detoxication: the
metabolism of alkylbenzenes isopropylbenzene (cumene) and derivatives
of hydrotropic acid. Biochemical journal, 59:153-159.
Rogerson A, Shiu WY, Huang GL, Mackay D, Berger J (1983) Determination
and interpretation of hydrocarbon toxicity to ciliate protozoa.
Aquatic toxicology, 3(3):215-228.
Sabljic A (1987) The prediction of fish bioconcentration factors of
organic pollutants from the molecular connectivity
model. Zeitschrift für die Gesamte Hygiene und Ihre Grenzgebiete,
33(10):493-496 (in German).
Sasaki S (1978) The scientific aspects of chemical substances control
law in Japan. In: Hutzinger O, Van Letyoeld LH, Zoeteman BCJ, eds.
Aquatic pollutants: Transformation and biological effects. Oxford,
Pergamon Press, pp. 282-298.
Sato A, Nakajima T (1979) Partition coefficients of some aromatic
hydrocarbons and ketones in water, blood and oil. British journal of
industrial medicine, 36(3):231-234.
Sato A, Nakajima T (1987) Pharmacokinetics of organic solvent vapors
in relation to their toxicity. Scandinavian journal of work,
environment & health, 13(2):81-93.
Sauer TC, Jr (1981) Volatile liquid hydrocarbon characterization of
underwater hydrocarbon vents and formation waters from offshore
production operations. Environmental science and technology,
15(8):917-923.
Seila RL (1979) Nonurban hydrocarbon concentrations in ambient air
north of Houston, TX. Research Triangle Park, NC, US Environmental
Protection Agency, Office of Research and Development
(EPA 600/3-79-101; NTIS PB 298-227).
Senczuk W, Litewka B (1976) Absorption of cumene through the
respiratory tract and excretion of dimethylphenylcarbinol in urine.
British journal of industrial medicine, 33:100-105.
Shackelford WM, Cline DM, Faas L, Kurth G (1983) An evaluation of
automated spectrum matching for survey identification of wastewater
components by gas chromatography-mass spectrometry. Analytica
Chimica Acta, 146:15-27.
Sipes IG, Gandolfi AJ (1991) Biotransformation of toxicants. In:
Klassen CD, Amdur MO, Doull J, eds. Casarett and Doull's toxicology,
4th ed. New York, NY, McGraw-Hill, pp. 88-126.
Smyth HF, Carpenter CP, Weil CS (1951) Range finding toxicity data:
List IV. American Medical Association archives of industrial hygiene
and occupational medicine, 4:119-122.
Snider EH, Manning FS (1982) A survey of pollutant emission levels in
waste waters and residuals from the petroleum refining industry.
Environment international, 7(4):237-258.
SRI International (1986) 1986 directory of chemical producers:
United States of America. Menlo Park, CA, pp. 568-569.
Steurmer DH, Ng DJ, Morris CJ (1982) Organic contaminants in
groundwater near an underground coal gasification site in northeastern
Wyoming. Environmental science and technology, 16(9):582-587.
Tanii H, Huang J, Hashimoto K (1995) Structure-acute toxicity
relationship of aromatic hydrocarbons in mice. Toxicology letters,
76:27-31.
Tegeris JS, Balster RL (1994) A comparison of the acute behavioral
effects of alkylbenzenes using a functional observational battery in
mice. Fundamental and applied toxicology, 22:240-250.
Teply J, Dressler M (1980) Direct determination of organic compounds
in water using steam-solid chromatography. Journal of
chromatography, 191:221-229.
UK DOE (1994) Environmental hazard assessment (EHA): Cumene.
Garston, United Kingdom Department of the Environment, Directorate for
Air, Climate, and Toxic Substances, Toxic Substances Division.
Union Carbide Corp. (1985) Range finding tests on cumene
(isopropylbenzene). Unpublished data. Washington, DC, US
Environmental Protection Agency, Office of Toxic Substances
(Industrial fellowship 274-12; TSCA section 8(d) submission
878214980).
US EPA (1987) Health and environmental effects document for cumene.
Prepared by the Office of Health and Environmental Assessment,
Environmental Criteria and Assessment Office, US Environmental
Protection Agency, Cincinnati, OH, for the Office of Solid Waste and
Emergency Response, US Environmental Protection Agency, Washington,
DC, dated August 1987.
US EPA (1988) Cumene; final test rule. Federal register,
53(144):28195-28206.
US EPA (1991a) Alpha 2µ-micro-globulin: Association with chemically
induced renal toxicity and neoplasia in the rat. US Environmental
Protection Agency, September 1991 (EPA/625/3-91/019F).
US EPA (1991b) Increased incidence of ecchymosis in a developmental
toxicity study of inhaled cumene vapor in New Zealand white rabbits.
Memorandum dated November 23, 1991, from J. Seed, Health and
Environmental Review Division, US Environmental Protection Agency, to
G.E. Timm, Chemical Testing Branch, Existing Chemical Assessment
Division, US Environmental Protection Agency, Washington, DC.
Washington, DC, US Environmental Protection Agency, Office of Toxic
Substances (TSCA section 4 submission 40-8992172).
US EPA (1996) Test methods for evaluating solid waste, physical and
chemical methods, Final Update III, 3rd ed. Washington, DC, US
Environmental Protection Agency (SW-846).
US EPA (1997) Integrated Risk Information System (IRIS) online at
http://www.epa.gov/iris (including the Toxicological review of
cumene in support of summary IRIS information, National Center for
Environmental Assessment, Cincinnati, OH). Washington, DC, US
Environmental Protection Agency.
Van der Linden AC (1978) Degradation of oil in the marine environment.
Developments in biodegradation of hydrocarbons, 1:165-200.
Walker JD, Calomiris JJ, Herbert TL, Colwell RR (1976) Petroleum
hydrocarbons: degradation and growth potential for Atlantic Ocean
sediment bacteria. Marine biology, 34:1-9.
Ward DJ (1965) Cumene. In: Standen A, ed. Kirk-Othmer encyclopedia
of chemical technology, 2nd ed. Vol. 6. New York, NY, John Wiley
and Sons, pp. 543-546.
Ward DJ (1979) Cumene. In: Standen A, ed. Kirk-Othmer encyclopedia
of chemical technology, 3rd ed. Vol. 7. New York, NY, John Wiley
and Sons, pp. 286-290.
Westrick JJ, Mello JW, Thomas RF (1984) The groundwater supply
survey. Journal of the American Water Works Association, 76:52-59.
Williams RT, Ziegenfuss PS, Lee CM (1993) Aerobic biodegradation of
cumene: an ecocore study. Environmental toxicology and chemistry,
12:484-492.
Windholz M, ed. (1983) The Merck index, 10th ed. Rahway, NJ, Merck
and Co., Inc., p. 375.
Wolf MA, Rowe VK, McCollister DD, Hollingsworth RL, Oyen F (1956)
Toxicological studies of certain alkylated benzenes and benzene.
Archives of industrial health, 14:387-398.
Yamada K, Horiguchi S, Takahashi J (1965) The utilization of
hydrocarbons by microorganisms. VI. Screening of aromatic
hydrocarbon-assimilating microorganisms and cumic acid formation from
p-cumene. Agricultural and biological chemistry, 29(10):943-948.
Yang LL (1987) CHO/HGPRT mutation assay; test article: Cumene.
Bethesda, MD, Microbiological Associates, Inc. Washington, DC, US
Environmental Protection Agency, Office of Toxic Substances (Study No.
T4786.332010; TSCA section 4 submission 40-8792124).
APPENDIX 1 -- SOURCE DOCUMENTS
US EPA (1997): Integrated Risk Information System (IRIS) online at
http://www.epa.gov/iris (including the Toxicological review of
cumene in support of summary IRIS information, National Center for
Environmental Assessment, Cincinnati, OH), US Environmental Protection
Agency, Washington, DC
The peer review process that this and other recent (post-1996)
IRIS assessments undergo includes internal (i.e., US Environmental
Protection Agency) and external review rounds. Comments of and
responses to the external reviewers are a matter of record in the
Toxicological review. Other aspects of the IRIS review process are
explained in Mills & Foureman (1998).
US EPA (1987): Health and environmental effects document for cumene,
August 1987, National Center for Environmental Assessment, Office of
Health and Environmental Assessment, US Environmental Protection
Agency
The US EPA (1987) report is used as an expanded reference source
for the IRIS document.
UK DOE (1994): Environmental hazard assessment (EHA): Cumene, Toxic
Substances Division, Directorate for Air, Climate, and Toxic
Substances, United Kingdom Department of the Environment, Garston
The Environmental hazard assessment (EHA): Cumene document was
drafted by the Building Research Establishment (United Kingdom
Department of the Environment) and the Institute of Terrestrial
Ecology (United Kingdom Natural Environment Research Council), with
I.R. Nielsen, J. Diment, and S. Dobson as the authors. The draft
document was peer reviewed both within the United Kingdom and
internationally. Comments and additional material were received from
A.L. Barton (US Environmental Protection Agency), C.B. Buckley (South
Western Water Services, United Kingdom), J.H. Duffus (Heriot-Watt
University, Edinburgh, United Kingdom), D. Keating (Health & Safety
Executive, United Kingdom), S. Killeen (National Rivers Authority,
United Kingdom), J.S. Lawson (ICI Chemicals, United Kingdom), P.
Matthiessen (Ministry of Agriculture, Fisheries and Food, United
Kingdom), H.A. Painter (Freshfield Analysis Ltd.), N. Passant
(Department of Trade and Industry, United Kingdom), T. Sheils
(Department of the Environment, United Kingdom), and G. Thom (US
Environmental Protection Agency) and were incorporated into the final
document. The document was published in 1994 and covers published and
unpublished material up to 1993.
APPENDIX 2 -- CICAD PEER REVIEW
The draft CICAD on cumene was sent for review to institutions and
organizations identified by IPCS after contact with IPCS national
Contact Points and Participating Institutions, as well as to
identified experts. Comments were received from:
Commission of the European Communities, Directorate-General,
Luxembourg
Federal Institute for Health Protection of Consumers & Veterinary
Medicine (BgVV), Berlin, Germany
GSF-Forschungszentrum für Umwelt und Gesundheit GmbH, Institut
für Toxikologie, Oberscheissheim, Germany
Health & Safety Executive, Merseyside, United Kingdom
Institut de Recherches en Santé et Sécurité du Travail du Québec,
Montreal, Canada
Institute of Occupational Medicine, Chinese Academy of Preventive
Medicine, Ministry of Health, Beijing, People's Republic of China
Institute of Terrestrial Ecology, Cambridgeshire, United Kingdom
Joint Food Safety and Standards Group, London, United Kingdom
National Chemicals Inspectorate, Solna, Sweden
National Industrial Chemicals Notification and Assessment Scheme,
Sydney, Australia
National Institute of Health Sciences, Tokyo, Japan
National Institute of Occupational Health, Budapest, Hungary
National Institute of Public Health, Czech Republic
United States Department of Health and Human Services (National
Institute of Environmental Health Sciences)
United States Environmental Protection Agency (National Center
for Environmental Assessment; Region VIII)
APPENDIX 3 -- CICAD FINAL REVIEW BOARD
Washington, DC, USA, 8-11 December 1998
Members
Dr T. Berzins, National Chemicals Inspectorate (KEMI), Solna, Sweden
( Vice-Chairperson)
Mr R. Cary, Toxicology Unit, Health Directorate, Health and Safety
Executive, Bootle, Merseyside, United Kingdom ( Rapporteur)
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr O. Faroon, Agency for Toxic Substances and Disease Registry,
Centers for Disease Control and Prevention, Atlanta, GA, USA
Dr G. Foureman, National Center for Environmental Assessment, US
Environmental Protection Agency, Research Triangle Park, NC, USA
Dr H. Gibb, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA ( Chairperson)
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Research, Hanover,
Germany
Dr A. Nishikawa, Division of Pathology, National Institute of Health
Sciences, Tokyo, Japan
Dr E.V. Ohanian, Office of Water/Office of Science and Technology,
Health and Ecological Criteria Division, US Environmental Protection
Agency, Washington, DC, USA
Dr J. Sekizawa, Division of Chem-Bio Informatics, National Institute
of Health Sciences, Tokyo, Japan
Professor P. Yao, Institute of Occupational Medicine, Chinese Academy
of Preventive Medicine, Ministry of Health, Beijing, People's Republic
of China
Observers
Dr K. Austin, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
Dr I. Daly (ICCA representative), Regulatory and Technical Associates,
Lebanon, NJ, USA
Ms K.L. Lang (CEFIC, European Chemical Industry Council,
representative), Shell International, London, United Kingdom
Ms K. Roberts (ICCA representative), Chemical Self-funded Technical
Advocacy and Research (CHEMSTAR), Chemical Manufacturers Association,
Arlington, VA, USA
Dr W. Snellings (ICCA representative), Union Carbide Corporation,
Danbury, CN, USA
Dr M. Sweeney, Document Development Branch, National Institute for
Occupational Safety and Health, Cincinnati, OH, USA
Dr K. Ziegler-Skylakakis, GSF-Forschungszentrum für Umwelt und
Gesundheit GmbH, Institut für Toxikologie, Oberschleissheim, Germany
Secretariat
Dr M. Baril, Institut de Recherches en Santé et Sécurité du Travail du
Québec (IRSST), Montreal, Quebec, Canada
Dr H. Galal-Gorchev, Chevy Chase, MD, USA
Ms M. Godden, Health and Safety Executive, Bootle, Merseyside, United
Kingdom
Dr R.G. Liteplo, Environmental Health Directorate, Health Canada,
Ottawa, Ontario, Canada
Ms L. Regis, Programme for the Promotion of Chemical Safety, World
Health Organization, Geneva, Switzerland
Mr A. Strawson, Health and Safety Executive, London, United Kingdom
Dr P. Toft, Programme for the Promotion of Chemical Safety, World
Health Organization, Geneva, Switzerland
RÉSUMÉ D'ORIENTATION
Ce CICAD relatif au cumène a été préparé par l'Environmental
Protection Agency (EPA) des Etats-Unis sur la base d'une de ses
publications intitulée Health and environmental effects document
for cumene (US EPA, 1987), du dossier cumène provenant de son
système intégré d'information sur les risques (IRIS) (US EPA, 1997) et
d'un document du Royaume Uni paru sous le titre de Environmental
hazard assessment (EHA): Cumene (UK DOE, 1994), complétés par une
étude bibliographique à partir de la base de données écologiques
AQUIRE (Aquatic Toxicity Information Retrieval). Les recherches
bibliographiques effectuée pour l'établissement du dossier IRIS vont
jusqu'à novembre 1996 et celles qui ont été effectuées à partir de la
base de données AQUIRE, jusqu'à avril 1998. On trouvera à l'appendice
1 des indications sur le mode d'examen par des pairs ainsi que sur les
sources documentaires utilisées. Les renseignements concernant
l'examen du CICAD par les pairs font l'objet de l'appendice 2. Ce
CICAD a été approuvé en tant qu'évaluation internationale lors de la
réunion du Comité d'évaluation finale qui s'est tenue à Washington du
8 au 11 décembre 1998. La liste des participants à cette réunion
figure à l'appendice 3. La fiche d'information internationale sur la
sécurité chimique (ICSC 0170) relative au cumène, établie par le
Programme internationale sur la sécurité chimique (IPCS, 1993) est
également reproduite dans ce document.
Le cumène (CAS No 98-82-8) est un produit pétrochimique insoluble
dans l'eau utilisé dans la préparation d'un certain nombre d'autres
substances chimiques, notamment le phénol et l'acétone. Il se
volatilise facilement dans l'atmosphère à partir de l'eau et des sols
secs. Il devrait en principe n'être que modérément adsorbé au
particules du sol et aux sédiments et subir une décomposition dans
l'eau et le sol.
Le métabolisme du cumène donne principalement naissance, chez
l'Homme comme chez l'animal, à un alcool secondaire le
2-phényl-2-propanol. Cet alcool et ses conjugués sont rapidement
excrétés chez les rongeurs comme chez l'Homme.
Les effets les plus marqués observés chez des rongeurs exposés de
façon répétée au cumène par la voie orale ou respiratoire, consistent
en une augmentation du poids de certains organes, mais plus
particulièrement du rein. Aucun effet indésirable n'a été relevé chez
des foetus de rats et de lapins dont la mère avait été exposée à ce
produit au cours du développement foetal. Il n'y a pas eu d'étude de
reproduction portant sur plusieurs générations, mais la métabolisation
et l'excrétion rapides du composé et le fait qu'une étude subchronique
n'ait pas mis en évidence d'effets sur les spermatozoïdes, semblent
indiquer que le cumène est dépourvu de toxicité génésique. On a établi
une valeur-guide de 0,1 mg/kg par jour en se basant sur la dose sans
effet nocif observable (NOAEL) de 154 mg/kg p.c. obtenue après avoir
fait ingérer du cumène à des rats pendant 6 à 7 mois, le critère
retenu étant l'hypertrophie rénale chez les femelles. Cette valeur de
la dose a été corrigée pour tenir compte du programme d'administration
et on a appliqué un facteur d'incertitude de 1000. D'autres valeurs de
la NOAEL tirées d'une même étude d'inhalation en mode subchronique ont
abouti à des valeurs-guides respectivement égales à 0,4 mg/m3 et 0,09
mg/m3 pour la population générale; dans ce cas également, on a
corrigé la valeur de la NOAEL pour tenir compte d'une exposition en
mode continu et on a appliqué un facteur global d'incertitude égal à
1000.
On ne possède pas de données qui permettent d'évaluer
quantitativement l'exposition humaine au cumène.
Il n'est pas possible d'évaluer le pouvoir cancérogène du cumène
chez l'Homme en raison de l'absence d'études de cancérogénicité à long
terme. La plupart des études de génotoxicité ont donné des résultats
négatifs.
Les données qui permettraient une évaluation du risque encouru
par les organismes aquatiques et terrestres sont insuffisantes,
notamment en ce qui concerne la mesure de l'exposition à ce composé.
Toutefois, si l'on se base sur les données existantes, on peut penser
que ce risque est relativement faible. Les valeurs disponibles
indiquent une légère tendance à la bioconcentration chez les poissons.
On dispose d'aucune donnée sur la bioaccumulation du cumène le long
des diverses chaînes alimentaires (bioamplification).
RESUMEN DE ORIENTACION
Este CICAD sobre el cumeno, preparado por la Agencia para la
Protección del Medio Ambiente de los Estados Unidos (EPA), se basa en
un documento de la EPA sobre los efectos sanitarios y medioambientales
del cumeno (US EPA, 1987), en un archivo sobre el cumeno del sistema
integrado de información sobre riesgos (IRIS) de la EPA de los Estados
Unidos (US EPA, 1997) y en un documento del Reino Unido sobre la
evaluación de los riesgos medioambientales del cumeno (UK DOE, 1994),
con el complemento de una búsqueda bibliográfica en la base de datos
AQUIRE (Aquatic Toxicity Information Retrieval), especializada en
ecología. La búsqueda bibliográfica en el archivo del IRIS se realizó
hasta noviembre de 1996 y en la base de datos AQUIRE hasta abril de
1998. La información relativa al carácter del examen colegiado y a la
disponibilidad de los documentos originales figura en el apéndice 1.
La información sobre el examen colegiado de este CICAD aparece en el
apéndice 2. Este CICAD se aprobó como evaluación internacional en una
reunión de la Junta de Evaluación Final celebrada en Washington, DC,
Estados Unidos, los días 8-11 de diciembre de 1998. La lista de
participantes en esta reunión figura en el apéndice 3. La ficha
internacional de seguridad química (ICSC 0170) para el cumeno,
preparada por el Programa Internacional de Seguridad de las Sustancias
Químicas (IPCS, 1993), también se reproduce en el presente documento.
El cumeno (CAS No 98-82-8) es un producto petroquímico insoluble
en agua que se utiliza en la fabricación de varias sustancias
químicas, entre ellas el fenol y la acetona. Se volatiliza fácilmente
a la atmósfera a partir del agua y del suelo seco. Se supone que se
adsorbe al suelo/sedimentos con una intensidad entre moderada y fuerte
y que se biodegrada en el agua y en el suelo.
El cumeno se metaboliza fundamentalmente al alcohol secundario
2-fenil-2-propanol, tanto en el ser humano como en los animales. Los
roedores y las personas excretan con facilidad este alcohol y sus
conjugados.
Los efectos más notables observados en los roedores expuestos a
dosis repetidas de cumeno por vía oral o por inhalación son un aumento
del peso de los órganos, en particular de los riñones. No se
detectaron efectos adversos en los fetos de rata o de conejo cuyas
madres habían estado expuestas al cumeno durante el desarrollo fetal.
Si bien no se han realizado estudios de reproducción multigeneracional
con exposición al cumeno, la rapidez de su metabolismo y su excreción,
junto con la falta de efectos en la morfología del esperma en un
estudio subcrónico, parecen indicar un potencial bajo de toxicidad
reproductiva. Se ha obtenido un valor guía para la exposición oral de
0,1 mg/kg de peso corporal al día, basado en una concentración sin
efectos adversos observados (NOAEL) de 154 mg/kg de peso corporal al
día para el aumento del peso del riñón en ratas hembras en un estudio
de administración oral de 6 a 7 meses de duración; la NOAEL se ajustó
para un calendario de dosificación y se aplicó un factor de
incertidumbre de 1 000. Con respecto a la exposición por inhalación,
se obtuvieron valores guía para la población general de 0,4 mg/m3 y
0,09 mg/m3, basados en otras NOAEL derivadas del mismo estudio de
inhalación subcrónica; en este caso también se ajustaron las NOAEL
para una exposición continua y se aplicó un factor de incertidumbre
total de 1 000.
No hay datos disponibles para cuantificar la exposición humana al
cumeno.
No es posible evaluar el potencial de carcinogenicidad del cumeno
en el ser humano, debido a que no se han realizado estudios de larga
duración con esta sustancia. La mayor parte de los datos obtenidos en
pruebas genotóxicas son negativos.
Son insuficientes los datos, especialmente de información de la
exposición medida, para poder realizar una evaluación cuantitativa del
riesgo de la exposición al cumeno para las poblaciones de organismos
acuáticos o terrestres. Sin embargo, teniendo en cuenta los datos
existentes, se prevé para el cumeno un riesgo relativamente bajo. Los
valores indican un ligero potencial de bioconcentración del cumeno en
los peces. No hay datos acerca de la bioacumulación a través de la
cadena alimentaria (bioamplificación).
 1. EXECUTIVE SUMMARY
This CICAD on cumene was prepared by the US Environmental
Protection Agency (EPA) and is based on the US EPA's Health and
environmental effects document for cumene (US EPA, 1987), the US
EPA's Integrated Risk Information System (IRIS) file on cumene (US
EPA, 1997), and the United Kingdom's Environmental hazard assessment
(EHA): Cumene (UK DOE, 1994), supplemented by a literature search on
the ecology-based AQUIRE (Aquatic Toxicity Information Retrieval)
database. The literature search for the IRIS file was through November
1996 and for the AQUIRE database through April 1998. Information on
the nature of the peer review and the availability of the source
documents is presented in Appendix 1. Information on the peer review
of this CICAD is presented in Appendix 2. This CICAD was approved as
an international assessment at a meeting of the Final Review Board,
held in Washington, DC, USA, on 8-11 December 1998. Participants at
the Final Review Board meeting are listed in Appendix 3. The
International Chemical Safety Card (ICSC 0170) for cumene, produced by
the International Programme on Chemical Safety (IPCS, 1993), has also
been reproduced in this document.
Cumene (CAS no.
1. EXECUTIVE SUMMARY
This CICAD on cumene was prepared by the US Environmental
Protection Agency (EPA) and is based on the US EPA's Health and
environmental effects document for cumene (US EPA, 1987), the US
EPA's Integrated Risk Information System (IRIS) file on cumene (US
EPA, 1997), and the United Kingdom's Environmental hazard assessment
(EHA): Cumene (UK DOE, 1994), supplemented by a literature search on
the ecology-based AQUIRE (Aquatic Toxicity Information Retrieval)
database. The literature search for the IRIS file was through November
1996 and for the AQUIRE database through April 1998. Information on
the nature of the peer review and the availability of the source
documents is presented in Appendix 1. Information on the peer review
of this CICAD is presented in Appendix 2. This CICAD was approved as
an international assessment at a meeting of the Final Review Board,
held in Washington, DC, USA, on 8-11 December 1998. Participants at
the Final Review Board meeting are listed in Appendix 3. The
International Chemical Safety Card (ICSC 0170) for cumene, produced by
the International Programme on Chemical Safety (IPCS, 1993), has also
been reproduced in this document.
Cumene (CAS no.  Some relevant physical and chemical properties of cumene are
listed in Table 1. Additional physical/chemical properties are
presented in the International Chemical Safety Card (ICSC 0170)
reproduced in this document.
Table 1: Physical/chemical properties of cumene.
Property Value Reference
Molecular weight 120.2 g/mol
Boiling point 152.39 °C Ward, 1979
Vapour pressure, 611 Pa Mackay &
25 °C Shiu, 1981
Water solubility, 50 mg/litre Mackay &
25 °C Shiu, 1981
Log Kow 3.66 Hansch &
Leo, undated
Density, 20 °C 0.8619 g/cm3 Ward, 1979
Flashpoint (tag closed-cup) 35 °C Ward, 1965
Odour threshold limit 0.088 ppm (v/v) Amoore &
value (TLV) 0.43 mg/m3 Hautala, 1983
Table 1 (continued)
Conversion factor, 1 ppm = 5.2 mg/m3
20 °C, 101.3 kPa 1 mg/m3 = 0.19 ppm
Partition coefficients Sato &
Oil/air 6215 Nakajima, 1979
Oil/water 4316
Water/air 1.44
Human blood/air 37
3. ANALYTICAL METHODS
For sampling and measurement of cumene in air, Method 1501 of the
US National Institute for Occupational Safety and Health (NIOSH, 1994)
includes use of a solid sorbent tube (coconut shell charcoal) sampler
with a gas chromatography/flame ionization detector measurement
technique. The detection limit of this method is 1 mg/m3 (0.2 ppm).
US EPA (1996) methods for detecting cumene in media other than
air include the use of gas chromatography using photoionization Method
8021B, which is applicable to nearly all types of samples, regardless
of water content. The method detection limit for cumene is 0.05
µg/litre, and the applicable concentration range for this method is
approximately 0.1-200 µg/litre. The standard recovery using this
method is 98%, with a standard deviation of 0.9%. Another commonly
used gas chromatographic assay for volatiles including cumene is
Method 8260B (US EPA, 1996), with a general estimated quantitation
limit of approximately 5 µg/kg wet weight for soil/sediment samples,
0.5 mg/kg wet weight for wastes, and 5 µg/litre for groundwater.
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
Cumene is a naturally occurring constituent of crude oil and may
be released to the environment from a number of anthropogenic sources,
including processed hydrocarbon fuels. Crude oils typically contain
approximately 0.1 wt% of cumene, but concentrations as high as 1.0 wt%
have been reported.1 Measurements of various grades of petrol
revealed that cumene concentrations range from 0.14 to 0.51 vol% and
that the average cumene concentration was 0.3 vol%. Premium diesel
fuel contains 0.86 wt% of cumene; furnace oil (no. 2) contains 0.60
wt%.1
Primary sources of release of cumene include losses in wastewater
and fugitive emissions from manufacturing and use facilities and
petrochemical refineries, accidental spills of finished fuel products
during transport or processing, and emissions from petrol stations and
motor vehicles (US EPA, 1987). Cigarette tobacco also releases cumene
(Johnstone et al., 1962). Cumene release from all these sources is
estimated to be 9500 tonnes annually (US EPA, 1988). Other,
unquantifiable anthropogenic cumene releases include the rubber
vulcanization process (Cocheo et al., 1983), building materials
(Moelhave, 1979), jet engine exhaust (Katzman & Libby, 1975), outboard
motor operation (Montz et al., 1982), solvent uses (Levy, 1973),
pharmaceutical production, and textile plants (Gordon & Gordon, 1981).
Cumene is also released to the environment from leather tanning, iron
and steel manufacturing, paving and roofing, paint and ink
formulation, printing and publishing, ore mining, coal mining,
organics and plastics manufacturing, pesticide manufacturing,
electroplating, and pulp and paper production (Shackelford et al.,
1983).
SRI International (1986) reported the 1985 Western European
cumene production levels (in tonnes) for the following producer
countries:
Federal Republic of Germany 438 000
Finland 70 000
France 370 000
Italy 335 000
Netherlands 240 000
Spain 120 000
United Kingdom 220 000
This total 1985 production of 1 793 000 tonnes may be compared with
production in the USA, which was reported as 2 775 000 tonnes in 1997
(Anon., 1998).
1 Letter and attachment from W.F. O'Keefe, American Petroleum
Institute, to M. Greif, Toxic Substances Control Act (TSCA)
Interagency Testing Committee, US Environmental Protection
Agency, Washington, DC (TS-792).
The use pattern for cumene in the early 1970s in the USA was as
follows (Anon., 1984): oxidation for phenol/acetone production, 98%;
polymerization of alpha-methylstyrene, 1.8%; and exports, 0.2%. Cumene
is also used captively for the production of phenol and
alpha-methyl-styrene (SRI International, 1986).
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
In the atmosphere, cumene is expected to exist almost entirely in
the vapour phase (Eisenreich et al., 1981). Cumene does not absorb
ultraviolet light at wavelengths greater than 290 nm (US EPA, 1987),
which suggests that cumene would not be susceptible to direct
photolysis. In one study, the estimated half-life of cumene in the
atmosphere from photolysis alone was approximately 1500 years (Parlar
et al., 1983). Cumene is not susceptible to oxidation by ozone in the
atmosphere (US EPA, 1987). Thus, reaction with ozone and direct
photolysis are not expected to be important removal processes. Rather,
reaction with photochemically generated hydroxyl radicals appears to
be the primary degradation pathway (t´ 1-2 days) (Lloyd et al.,
1976; Ravishankara et al., 1978). Small amounts of cumene may be
removed from the atmosphere during precipitation. Cumene has been
assigned a Photochemical Ozone Creation Potential (POCP) value of 35
relative to ethylene at 100 (Derwent & Jenkin, 1990). POCP values
represent the ability of a substance to form ground-level ozone as a
result of its atmospheric degradation reactions.
In water, important fate and transport processes are expected to
be volatilization (t´ 4 h from a typical river) and aerobic
biodegradation (Kappeler & Wuhrmann, 1978; Sasaki, 1978; Van der
Linden, 1978). Chemical hydrolysis, oxidation, photolysis, and
reaction with hydroxyl radicals are not expected to be important fate
processes in water (Mill et al., 1978, 1979, 1980). Using an aerobic
freshwater sediment/water test system, Williams et al. (1993)
demonstrated that 10 days after addition of radiolabelled cumene (2.5
mg/litre) to the system, 46.9% was trapped as radiolabelled carbon
dioxide and another 21.8% was recovered as radiolabelled organics, the
overall recovery of cumene ranging from 56.8% to 88.3%. The
disappearance half-life based on these results was 2.5 days. During a
20-day incubation of cumene at 10 mg/litre under aerobic conditions in
either fresh water or salt water, Price et al. (1974) observed 70%
degradation in fresh water but only about 2% degradation in seawater.
Cumene was, however, observed to be degraded to a significant extent
by microorganisms isolated from ocean sediment samples incubated in
seawater, as Walker et al. (1976) noted decreases in cumene (gas
chromatographic analysis) ranging from 37% to 60% of initial amounts
over a period of 21 days in three separate incubations with seawater
and microorganisms isolated from Atlantic Ocean sediments. On the
other hand, cumene was found to be essentially non-biodegradable under
anaerobic conditions by Battersby & Wilson (1989), who noted that
cumene produced only about 2% of theoretical gas production when
incubated at 50 mg carbon/litre sludge for 60 days at 35°C under
anaerobic conditions; compounds at 80% of theoretical gas production
under these conditions were assumed to represent complete degradation,
whereas compounds at less than 30% production were considered
persistent.
In soil, it appears that cumene might biodegrade fairly rapidly
under aerobic conditions, because a number of microorganisms capable
of degrading cumene have been isolated (Yamada et al., 1965; Jamison
et al., 1970; Omori et al., 1975). Regression equations based on the
limit of cumene water solubility (50 mg/litre) predicted Koc (soil
sorption coefficient standardized to organic carbon) values ranging
from 513 to 1622. For equations based instead on log octanol/water
partition coefficients (log Kow) for cumene, predicted Koc
values were in a similar range, from 589 to 3890 (Lyman et al., 1982).
Other estimates of Koc values at 884 (Jeng et al., 1992) and 2800
(US EPA, 1987) were also in this range. These Koc values indicate
that cumene is expected to adsorb moderately to strongly to soil and
have only slight mobility. The relatively high vapour pressure of
cumene suggests that volatilization of this compound from dry soil
surfaces would be significant.
Measured and estimated bioconcentration factors (BCFs) suggest a
slight potential for cumene to bioconcentrate in fish species. A BCF
of 36 for cumene in goldfish ( Carassius auratus) has been measured
(Ogata et al., 1984), and a BCF of 356 was estimated from the log
Kow and a linear regression correlation equation (log BCF = 0.76
log Kow - 0.23) by the US EPA (1987). This value was concordant
with the BCF of 316 calculated for fish species in general exposed to
cumene (Sabljic, 1987). Cumene was detected at levels of 0.5-1.4 ng/g
wet weight (detection limit 0.5 ng/g wet weight by gas chromatography/
mass spectrometry) in 12 of 138 sampled fish (various species) from
several locations near a potential emission source (Japan Environment
Agency, 1987). Cumene has been detected in "oakmoss" ( Evernia
prunastri (L.) Ach.) (Gavin et al., 1978) and marsh grass (Mody et
al., 1974a,b).
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
Cumene has been found as a contaminant in various industrial
effluents and in groundwaters. Significant levels of cumene have been
recorded in groundwater near chemical plants (1581 µg/litre, Botta et
al., 1984; 360 µg/litre, Teply & Dressler, 1980; 11 µg/litre,
Pellizzari et al., 1979), around outboard motor operations (700
µg/litre, Montz et al., 1982), near coal gasification facilities (up
to 54 µg/litre, Steurmer et al., 1982), and around petroleum plants
and petroleum refineries (5 µg/litre, quantification method not clear;
Snider & Manning, 1982). Cumene was detected in 8 of 135 samples of
surface water (detection limit 0.03 µg/litre with gas
chromatography/mass spectrometry) at concentrations ranging from 0.09
to 0.44 µg/litre in several locations near a potential emission source
in the 1986 monitoring of the general environment in Japan (Japan
Environment Agency, 1987). Cumene levels in sediments and biota in
Puget Sound, Washington, USA, ranged from 0.02 to 19 µg/g, with a mean
concentration of 2.3 µg/g (Brown et al., 1979). A cumene level of
140 µg/litre was found in seawater near an offshore drilling platform
in the Gulf of Mexico (Sauer, 1981). Cumene was detected in 6 of 111
sediment samples at concentrations ranging from 0.58 to 11 ng/g dry
weight (detection limit 0.5 ng/g with gas chromatography/mass
spectrometry) in several locations near a potential emission source
(Japan Environment Agency, 1987).
Reports of air sampling in the USA indicate the mean
concentration of cumene to be about 14.7 µg/m3 (3 ppb) in urban
settings and as high as 2.5 µg/m3 (0.5 ppb) in rural settings.
Samples taken in Los Angeles, California, in 1966 averaged 14.7 µg/m3
(3 ppb) (Lonneman et al., 1968), and samples taken in Houston, Texas,
in 1973-1974 averaged 12.15 µg/m3 (2.48 ppb) (Lonneman et al., 1979).
The US EPA (1987) reported a mean concentration of 16.7 µg cumene/m3
(3.4 ppb) in undated samples from Los Angeles. In samples taken in the
fall of 1981 in Los Angeles, Grosjean & Fung (1984) did not detect
cumene, although a minimum detection level of 9.8 µg/m3 (2 ppb) was
reported. Although a number of sampling attempts in rural and remote
areas reported no detectable levels of cumene in air (detection limit
<0.05 µg/m3 [<0.01 ppb]), two attempts were positive: Seila (1979)
reported mean levels of 2.5 µg/m3 (0.5 ppb) in samples taken in a
rural area near Houston, Texas, in 1978, and Arnts & Meeks (1980,
1981) reported 0.25 µg/m3 (0.05 ppb) in samples taken near campfires
in the Great Smokey Mountains, USA, in 1978.
Average atmospheric concentrations of cumene in Europe are
reported to be somewhat less than those in the USA, although
concentrations in urban areas are also consistently much higher than
those in rural areas. Isodorov et al. (1983) recorded an average
cumene level of 8.3 µg/m3 (1.7 ppb) in the urban atmosphere of
Leningrad, USSR, in 1977-1979, with a maximum of 11.8 µg/m3 (2.4
ppb). Ambient air concentrations for the Netherlands in 1980 were
reported to average 0.5-1.0 µg/m3 (0.1-0.2 ppb), with maxima ranging
up to 34.8 µg/m3 (7.1 ppb) (Guicherit & Schulting, 1985). An annual
average of 1.6 µg/m3 (0.3 ppb) (maximum 3.9 µg/m3 [0.8 ppb]) was
reported from the Grenoble area in France in 1987 (Foster et al.,
1991).
6.2 Human exposure
Humans can be exposed to cumene via industrial emissions, petrol
station or motor vehicle emissions, accidental releases, food,
cigarette smoke, and drinking-water (US EPA, 1987).
In condensates of cigarette smoke, Johnstone et al. (1962)
recorded yields of cumene ranging from 7 to 14 µg/cigarette. Holzer et
al. (1976) detected cumene at 10 µg/m3 (2 ppb) in air samples taken
from a room immediately after a single cigarette had been smoked. No
further specifics, such as indication of a median value or minimum
detection level, are given.
Brugnone et al. (1989) reported cumene as measurable in all
alveolar air samples collected (single breath; range 1-81 µg/m3
[0.2-17 ppb], method detection limit not given) from among two groups
of workers ( n = 86, gender not specified) exposed to <0.1 mg
cumene/m3 (<0.02 ppm) through the work shift. These authors analysed
for but were unable to detect any significant differences in cumene
concentrations between smokers and non-smokers in either alveolar air
or blood samples. In another study, gases collected from 60 min of
normal continuous respiration from each of eight male volunteers
(three smokers) were analysed for trace organic constituents (Conkle
et al., 1975). Cumene was listed as detected in one of the three
smokers (expressed as 21 µg/h) and in one of the five non-smokers
(expressed as 0.13 µg/h). Krotoszynski & O'Neill (1982) also
identified cumene in expired air from non-smokers.
The presence of cumene in foods can be biogenic or due to
environmental contamination (US EPA, 1987). Although the detection
limit of cumene in various foods was not specified, the US EPA (1987)
noted that cumene has been detected but not quantified in foods as
diverse as tomatoes, Concord grapes, cooked rice, fried chicken,
bacon, Beaufort cheese, and dried legumes.
Only two reports of cumene quantification in drinking-water were
found in the available literature. Coleman et al. (1984) detected
cumene in Cincinnati, Ohio, USA, drinking-water at a level of 0.014
µg/litre (quantification method not clear). Keith et al. (1976)
reported 0.01 µg cumene/litre drinking-water in Terrebonne-Parish,
Louisiana, USA, but found none in the drinking-water of nine other
cities across the USA. These concentrations are considerably below the
0.5 µg/litre detection limit reported by Westrick et al. (1984), who
found no cumene in 945 US drinking-water systems, 479 of which were
selected because of known contamination problems. Burmaster (1982) and
Burnham et al. (1972) reported unquantified levels of cumene/
alkylbenzenes in drinking-water obtained from groundwater. Based on
the results of these studies, it may be concluded that cumene
contamination above 0.5 µg/litre is uncommon in drinking-water in the
USA.
One industrial hygiene survey (US EPA, 1988) reported that
approximately 739 US workers were occupationally exposed to cumene.
Personal exposure data in this report consisted of 1487 air samples
taken over the course of 12 years (1973-1984), of which 6 were in the
range of 20-150 mg/m3 (4-30 ppm), 4 in the range of 15-20 mg/m3 (3-4
ppm), and 25 in the range of 5-10 mg/m3 (1-2 ppm), with the remaining
samples below 5 mg/m3 (1 ppm) (US EPA, 1988).
Based on available monitoring data, it appears that the general
population would be exposed to cumene primarily by inhalation,
although occupational populations may be reasonably anticipated to be
exposed by the dermal route. Minor exposure may result from contact
with refined petroleum products and ingestion of contaminated foods
and possibly drinking-water.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND
HUMANS
Cumene has been shown to be absorbed after inhalation exposure in
humans and after inhalation, oral, and dermal exposure in animals
(Senczuk & Litewka, 1976; Research Triangle Institute, 1989). Tests
conducted in humans indicate that cumene is absorbed readily via the
inhalation route, that it is metabolized efficiently to water-soluble
metabolites within the body, and that these metabolites are excreted
efficiently into the urine with no evidence of long-term retention
within the body; these results concur with the results of animal
studies.
Senczuk & Litewka (1976) exposed human volunteers (five men and
five women) head only to one of three different concentrations of
cumene vapours (240, 480, or 720 mg/m3 [49, 98, or 147 ppm]) for 8 h
every 10 days. Exhaled breath samples (10 cm3) were collected near
the beginning and at the end of the exposure from a tube placed in the
breathing zone. The total amount of cumene absorbed during exposure,
calculated from retention, ventilation, and exposure duration, was
nearly twice as high at all exposure levels in the males (466-1400 mg)
as in the females (270-789 mg). The respiratory tract absorption
ranged from 45% to 64% depending on the time of exposure, with the
overall mean retention estimated at 50%. In rats, inhalation studies
(nose only for 6 h at 510, 2420, or 5850 mg/m3 [104, 494, or 1194
ppm]) indicate rapid absorption, with detectable levels of cumene
appearing in the blood within 5 min of the beginning of exposure at
all three exposure levels (Research Triangle Institute, 1989). Gavage
studies in rats showed that cumene was absorbed readily via this
route, with maximum levels in blood occurring at the earliest time
point sampled (4 h) for a lower dose (33 mg/kg body weight) and at
8-16 h for a higher dose (1350 mg/kg body weight) (Research Triangle
Institute, 1989). Dermal absorption of cumene was demonstrated in rats
and rabbits (Monsanto Co., 1984).
The human data reported by Brugnone et al. (1989) regarding
cumene distribution suggest that the cumene concentration was about 40
times higher in blood than in alveolar air, a figure concordant with
the reported human blood/air partition coefficient of 37 (Sato &
Nakajima, 1979; Table 1). Cumene was widely distributed in rats, and
distribution, presumably determined immediately after exposure, was
independent of administration route (inhalation, oral, or
intraperitoneal in 10% aqueous Emulphor). Adipose, liver, and kidney
were all shown to have elevated tissue/blood ratios of cumene
following all doses and routes of exposure (Research Triangle
Institute, 1989). Fabre et al. (1955) demonstrated that after rats
inhaled cumene vapour for up to 150 days, cumene was distributed to
the endocrine organs, central nervous system, bone marrow, spleen, and
liver.
The patterns of cumene disappearance (as total radioactivity)
from the blood in the nose-only inhalation studies were fitted with a
monoexponential model, with the half-lives increasing with dose, from
3.9 h at 490 mg/m3 (100 ppm) to 6.6 h at 5880 mg/m3 (1200 ppm). The
half-life of cumene in the blood in gavage studies with rats was
calculated to be between 9 and 16 h.
Metabolism of cumene by cytochrome P-450 is extensive and takes
place within hepatic and extrahepatic tissues, including lung (Sato &
Nakajima, 1987), with the secondary alcohol 2-phenyl-2-propanol being
a principal metabolite. Metabolites excreted in urine of rats and
rabbits include 2-phenyl-2-propanol and its glucuronide or sulfate
conjugates, conjugates of 2-phenyl-1,2-propanediol, and an unknown
metabolite, possibly the dicarboxylic acid that would result from
complete oxidation of the 1- and 3-alkyl carbons of phenylmalonic acid
(Research Triangle Institute, 1989; Ishida & Matsumoto, 1992; MAK,
1996).
Senczuk & Litewka (1976) also conducted excretion studies with
human volunteers exposed to cumene vapours (240, 480, or 720 mg/m3
[49, 98, or 147 ppm]) for 8 h every 10 days. These authors reported
excretion of the metabolite 2-phenyl-2-propanol in the urine as
biphasic, with a rapid early phase (t´ 2 h) and a slower later phase
(t´ 10 h); excretion of this metabolite in the urine (about 35% of
the calculated absorbed dose) was maximal after 6-8 h of exposure and
approached zero at 40 h post-exposure. With rats, the extent of
elimination across routes of administration (inhalation, oral, or
intraperitoneal) and exposure concentrations was very similar, with
urine being the major route of elimination, about 70% in all cases
(Research Triangle Institute, 1989). Total body clearance in the rats
was rapid and complete, with less than 1% of the absorbed fraction
being present in the body 72 h after the highest exposure regime
examined (5880 mg/m3 [1200 ppm] for 6 h). Following oral
administration of cumene in rabbits, 90% was recovered as metabolites
in the urine within 24 h (Robinson et al., 1955).
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
Cumene is not highly toxic to laboratory animals by inhalation,
oral, or dermal routes of exposure. An LC50 of 9800 mg cumene/m3
(2000 ppm) in mice has been reported (MAK, 1996). A 4-h inhalation
LC50 of 39 200 mg/m3 (8000 ppm) in rats was reported by several
investigators (Smyth et al., 1951; Koch Refining Co., 1984; Union
Carbide Corp., 1985). Acute oral LD50 values for rats range from 1400
to 2900 mg/kg body weight (Smyth et al., 1951; Koch Refining Co.,
1984; Monsanto Co., 1984; Ciba-Geigy Co., 1985; Union Carbide Corp.,
1985). Tanii et al. (1995) reported an intraperitoneal LD50 in male
mice in the same range, 2000 mg/kg body weight (16.9 mmol/kg).
Clinical signs of toxicity reported in rats in acute oral studies
include weakness, ocular discharge, collapse, and death; pathological
findings in animals that died were haemorrhagic lungs, liver
discolorations, and acute gastrointestinal inflammation (Monsanto Co.,
1984). The character of the dose-response for these effects is,
however, unclear.
Acute dermal LD50s for cumene applied undiluted to rabbit skin
range from >3160 mg/kg body weight (Monsanto Co., 1984) to >10 000
mg/kg body weight (Ciba-Geigy Co., 1985). Pathological findings in
animals that died were similar to those in animals that died after a
single oral exposure (Monsanto Co., 1984).
8.2 Irritation and sensitization
Undiluted cumene applied to the skin of New Zealand albino
rabbits (0.5 ml) according to standardized guidelines caused slight
defatting with skin flaking, a symptom not generally classified as
relating to primary skin irritancy (Monsanto Co., 1984). A study
conducted by Ciba-Geigy Co. (1985) reported a similar low level of
irritation.
Cumene is an ocular irritant. Ocular irritation, including
immediate discomfort followed by "erythema" (redness of the
conjunctiva) and copious discharge, was observed after the
instillation of undiluted cumene to rabbit, with these effects being
reversible within 120 h (Monsanto Co., 1984). Ciba-Geigy Co. (1985)
judged eye irritation as slight when cumene was applied to rabbit
eyes. However, a study by Union Carbide Corp. (1985) reported that
cumene was harmless to rabbit eyes when applied undiluted.
Observations of lacrimation (Tegeris & Balster, 1994) and periocular
swelling and blepharospasm (Cushman et al., 1995) also indicate that
cumene may exhibit ocular irritancy at high airborne concentrations.
The concentration of cumene causing a 50% reduction in the
respiratory rate in mice after 30 min of exposure was determined to be
10 084 mg/m3 (2058 ppm) (Kristiansen et al., 1986). This
concentration is quite high and in the range where repeated exposure
caused death and morbidity in rats (Gulf Oil Corp., 1985; Chemical
Manufacturers Association, 1989) and rabbits (Darmer et al., 1997).
No skin sensitization reactions were noted among a group of 20
female guinea-pigs treated with cumene in a Magnusson-Kligman
maximization test conducted in accordance with Organisation for
Economic Co-operation and Development (OECD) Guideline 406 (Hüls,
1988). No data were available on respiratory sensitization to cumene.
8.3 Short-term exposure
In a study by Monsanto Co. (1986), male and female Sprague-Dawley
rats (10 per sex per group) were exposed whole body to cumene vapour
concentrations of 0, 515, 1470, or 2935 mg/m3 (0, 105, 300, or
599 ppm) for 6 h/day, 5 days/week, for approximately 4 weeks (minimum
exposure, 20 days). Cage-side observations included
concentration-related increases in side-to-side head movements in both
males and females in all dose groups, head tilt in all dose groups,
and arched back in one female in the high-dose group. Increases in
mean absolute left and right kidney weights were observed in high-dose
males, as were increases in mean absolute left kidney weight in
low- and mid-dose males. In high-dose females, the mean absolute
weight of left kidneys was greater than in controls. This study
confirms that renal weight changes occur in females and corroborates
similar effects reported by Cushman et al. (1995). It should be noted
that the effects associated with central nervous system perturbation
(i.e., head movements) were not noted in several other longer-term
studies, including that of Cushman et al. (1995), in which
neurotoxicity was specifically assessed. If it is assumed that the
renal changes among the males were associated with male rat-specific
nephropathy (see section 8.4.1), the cage-side observations of head
tilt and head movements become the critical effects for this
short-term study.
Although not statistically significant, leukocytosis was noted in
a group of rats ( n = 15, mixed sex) exposed to cumene at 1200 mg/m3
(245 ppm) for 8 h/day, 5 days/week, for 30 exposures (Jenkins et al.,
1970).
Other short-term toxicity studies are described in section 8.7.
8.4 Long-term exposure
8.4.1 Subchronic exposure
In an inhalation exposure study by Jenkins et al. (1970), groups
of squirrel monkeys ( n = 2), beagle dogs ( n = 2),
Princeton-derived guinea-pigs ( n = 15), and Sprague-Dawley and
Long-Evans rats ( n = 15) were exposed whole body to cumene at
concentrations of 0, 18, or 147 mg/m3 (0, 4, or 30 ppm) continuously
for 90 days. Initial and terminal body weights, haematological and
clinical chemistry parameters, and histopathological data were
collected. No toxicologically significant effects were noted in the
monkeys, dogs, or guinea-pigs. The only effect noted in the rats was a
slight degree of leukocytosis at both concentrations.
Cushman et al. (1995; also reported as Bushy Run Research Center,
1989a) conducted two successive subchronic whole-body inhalation
toxicity studies with cumene vapours (>99.9% pure) on Fischer-344
rats. In the first study, groups (21 per sex) were exposed to cumene
vapour at 0, 490, 2430, or 5890 mg/m3 (0, 100, 496, or 1202 ppm) 6
h/day, 5 days/week, for 13 weeks. The second study was a repeat of the
first, except that the group size was decreased to 15 per sex and an
additional group (at 245 mg/m3 [50 ppm]) and a 4-week post-exposure
period were added. Parameters monitored included clinical signs of
toxicity, auditory brain stem responses, ophthalmology, sperm count
and morphology, and histopathological examination of all respiratory
tract tissues (lungs and nasal turbinates) and the perfused nervous
system. Evaluations of neurological function (functional observation
battery and motor activity) were conducted in both studies. Light
microscopic evaluation of the perfused-fixed nervous system tissues
(six rats per sex per group) was conducted in the first study only.
In the first study, transient, reversible cage-side observations
during exposure periods included hypoactivity, blepharospasm, and a
delayed or absent startle reflex at the highest concentration. Rats
exposed to 2430 mg/m3 were reported as being hypoactive during
exposure, although no further specifics were given. Statistically
significant ( P < 0.05) exposure-related decreases in motor activity
(total) were observed in male rats exposed to the two highest
concentrations of cumene, but these results were not observed in the
second study in either sex. There were no exposure-related changes
noted in the functional observation battery in this or the subsequent
study. No effects were observed in the neurohistopathological
examinations. Cataracts were reported in males at all exposure
concentrations in this study. However, these results were not observed
in the second study in which a more comprehensive protocol for eye
examination was employed. Evaluation of the auditory brain stem
responses revealed no meaningful changes in the auditory function of
the exposed animals. The only gross histopathology noted was
periocular swelling, which occurred in animals at the two highest
concentrations (and for which neither incidence nor severity was
reported). Both absolute and relative weights were increased
significantly (>10%) in the kidneys, adrenal glands, and livers of
both sexes at the highest concentration. These changes were also noted
in the liver at the next lower concentration (2430 mg/m3) for both
females and males. Kidney lesions described in male rats at the two
highest exposure concentrations were considered to be closely related
to male rat-specific nephropathy (i.e., lesions were limited to males,
and tubular proteinosis, hypertrophy, and hyperplasia as well as
hyaline droplet formation were noted, although the identity of the
protein in the droplets was not confirmed) and are of questionable
relevance to human toxicity, principally because renal lesions
characteristic of this type of nephropathy have not been observed in
humans (US EPA, 1991a; Hard et al., 1993). Chronic progressive
nephropathy, a common spontaneous renal disease of Fischer-344 male
rats that occurs as early as 5 months of age (Montgomery & Seely,
1990), may also contribute to these renal lesions. Water consumption
was significantly increased (about 40%) in male rats above control
values at both 2430 and 5890 mg/m3. Several haematological and serum
measures were also changed in a statistically significant dose-related
manner at both 2430 and 5890 mg/m3: leukocytes (both sexes),
platelets (both sexes), lymphocytes (males only), glucose (females
only), and calcium/phosphorus (males only).
The results of the second study, with a 4-week post-exposure
period, indicated limited reversibility of the organ weight
alterations, because significant mean weight increases were still
present in female liver and female adrenals of the highest exposure
group. In males, only relative kidney weights (significant at 6%) and
absolute liver weights remained increased significantly. Blood and
serum parameters were not reported in this study. Morphological
evaluation of epididymal and testicular sperm showed no cumene-related
differences in count, morphology, or stages of spermatogenesis,
although one high-dose rat did have diffuse testicular atrophy.
The weight alterations in the male and female adrenals and female
kidney are considered potentially adverse, as the persistence noted
indicates limited reversibility and engenders uncertainty about the
progression and fate of these alterations under chronic exposure. The
increased water consumption noted may also indicate potential for
renal effects, although this effect was present at the next to highest
dose level at which renal weights were not altered. Although the
progression of these weight alterations from continued exposure cannot
be ascertained from this subchronic study, data from the second
(post-exposure) study indicate limited reversibility of effects on the
adrenals, at least in females. The liver weight alterations are not
viewed as adverse, because increase in liver weight without
accompanying pathology is a trait of common microsomal enzyme inducing
agents, although it should be noted that induction of hepatic
microsomal enzymes may influence the metabolism of other substances
and may either increase or decrease their toxicity (Sipes & Gandolfi,
1991). The altered haematological and serum parameters noted at the
two highest concentrations may be considered as significant, although
all are within normal ranges (Mitruka & Rawnsley, 1981). Based on the
lowest dose at which both relative and absolute weight alterations in
adrenal tissues of both sexes and in the kidneys of females are
statistically ( P < 0.05) and biologically (>10%) significant, 5890
mg/m3 may be considered as a lowest-observed-adverse-effect level
(LOAEL), and 2430 mg/m3 the corresponding NOAEL. Based on
consideration of the various measures in the first study (motor
effects, increased water consumption in males, haematological and
serum parameters, sporadic weight increases in male adrenals and
female kidneys) as significant, 2430 mg/m3 may be considered as a
LOAEL and 490 mg/m3 as the corresponding NOAEL. It should be noted
here that a LOAEL of 2391 mg/m3 (488 ppm) and a NOAEL of 485 mg/m3
(99 ppm) were noted for maternal toxicity in the short-term
developmental study in rats by Darmer et al. (1997), discussed in
section 8.6.
8.4.2 Chronic exposure and carcinogenicity
There are no long-term in vivo bioassays addressing the issue
of cancer. No data exist to support any quantitative cancer
assessment.
Wolf et al. (1956) conducted a study involving groups of 10
female Wistar rats administered cumene by gavage in olive oil at 154,
462, or 769 mg/kg body weight per day, 5 days/week, over a 194-day
(6- to 7-month) period, equivalent to 110, 331, or 551 mg/kg body
weight per day, adjusted for daily exposure. Rats given olive oil
served as controls ( n = 20). A pronounced increase in average kidney
weight, noted as a "moderate effect," occurred at 769 mg/kg body
weight per day, although no quantitative data are presented. An
increase in average kidney weight was noted as a "slight effect" at
462 mg/kg body weight per day. It is stated in the report that at 154
mg/kg body weight per day, no evidence of ill effects, as determined
by gross appearance, growth, periodic blood counts, analysis for blood
urea nitrogen, average final body and organ weights, and bone marrow
counts, was noted. The LOAEL is 462 mg/kg body weight per day, and the
NOAEL is 154 mg/kg body weight per day. These results are consistent
with those observed in more recent, better-reported studies described
elsewhere in this document.
In an inhalation study by Fabre et al. (1955), Wistar rats were
exposed (whole body) to cumene vapour at 2500 mg/m3 (510 ppm), and
rabbits were exposed to 6500 mg/m3 (1327 ppm), for 8 h/day, 6
days/week, for up to 180 days. Histological effects reported were
"passive congestion" in the lungs, liver, spleen, kidney, and adrenals
and the presence of haemorrhagic zones in the lung, haemosiderosis in
the spleen, and lesions from epithelial nephritis "in some cases." It
was not clear from the study if these effects occurred in rats or
rabbits, or both.
8.5 Genotoxicity and related end-points
In general, negative results have been obtained in a relatively
complete battery of in vivo and in vitro mutagenicity tests,
including gene mutation, chromosomal aberration, and primary DNA
damage (US EPA, 1997). Cumene was tested at concentrations up to
2000 µg/plate in a Salmonella typhimurium reverse mutation assay
(modified Ames test); negative results were observed with and without
metabolic activation (Lawlor & Wagner, 1987). Cumene was negative in
an Ames assay at concentrations up to 3606 µg/plate with
S. typhimurium strains TA98, TA100, TA1535, and TA1537 (Florin et
al., 1980). Cumene also tested negative, with and without metabolic
activation, in a set of HGPRT assays (using Chinese hamster ovary
cells) at cumene concentrations of 100-125 µg/ml, at which the
relative cloning efficiencies (a measure of cytotoxicity) ranged from
29% to 110% (Gulf Life Sciences Center, 1985a; Yang, 1987). A
micronucleus assay performed in mice given up to 1 g cumene/kg body
weight by gavage was negative (Gulf Life Sciences Center, 1985b).
Micronucleus assays done in Fischer-344 rats, however, gave values
that were weakly positive, although little dose-response was seen, and
deaths occurred at the highest dose (5 of 10 animals at 2.5 g/kg body
weight intraperitoneally; NTP, 1996). The positive control used in the
micronucleus tests, cyclophosphamide, produced strong positive
responses in all assays.
Cumene failed to induce significant rates of transformation in
BALB/3T3 cells (without activation) at concentrations up to 500 µg/ml
(Putnam, 1987) but tested positive in an earlier cell transformation
test also using BALB/3T3 cells, in which an increase in
transformations was observed at 60 µg/ml (Gulf Oil Corp., 1984a).
Results from an unscheduled DNA synthesis assay in rat hepatocytes
conducted by Gulf Oil Corp. (1984b) indicated positive results at
doses of 16 and 32 µg cumene/ml (with 128 µg/ml noted as toxic to the
hepatocytes). However, apparent technical difficulties with this test
(US EPA, 1988) prompted a repeat of the unscheduled DNA synthesis
assay in rat hepatocytes, the results of which showed cumene to be
clearly negative at doses up to 24 µg/ml, with doses above 24 µg/ml
noted as being too toxic for evaluation of unscheduled DNA synthesis
(Curren, 1987; US EPA, 1988).
8.6 Reproductive and developmental toxicity
No multigeneration reproductive study exists for this compound by
either the oral or inhalation route. There are no data concerning
cumene exposure of females prior to mating, from conception to
implantation, or during late gestation, parturition, or lactation.
The first subchronic inhalation study of Cushman et al. (1995),
however, conducted morphological evaluation of epididymal and
testicular sperm in rats exposed for 13 weeks to cumene vapours (see
section 8.4.1). No cumene-related differences in count, morphology, or
stages of spermatogenesis were noted, although one high-dose rat did
have diffuse testicular atrophy. No alterations (weight changes,
histopathology) were noted in the female reproductive organs that were
examined at the termination of this same study.
In an inhalation study (Darmer et al., 1997; also reported as
Bushy Run Research Center, 1989b), Sprague-Dawley rats (25 per group)
were exposed whole body to 0, 485, 2391, or 5934 mg cumene/m3 (0, 99,
488, or 1211 ppm) for 6 h/day on days 6 through 15 of gestation.
Perioral wetness and encrustation, a significant ( P < 0.01)
decrease in body weight gain on gestation days 6 through 9
(accompanied by a significant decrease in food consumption), and a
slight increase (7.7%) in relative liver weight were observed in dams
at the high dose only. Hypoactivity, blepharospasm, and significantly
( P < 0.05) decreased food consumption were observed in the dams at
the next highest concentration. There were no statistically
significant adverse effects on reproductive parameters or fetal
development. For this study, 5934 mg/m3 is a developmental NOAEL, and
485 mg/m3 is a maternal NOAEL.
In another inhalation study (Darmer et al., 1997; also reported
as Bushy Run Research Centre, 1989c), New Zealand white rabbits (15
per group) were exposed whole body to 0, 2411, 5909, or 11 255 mg
cumene/m3 (0, 492, 1206, or 2297 ppm) for 6 h/day on days 6 through
18 of gestation. Two does died and one aborted at the highest exposure
concentration. There were significant ( P < 0.01) reductions in body
weight gain (178 g lost compared with 31 g gained in the control
group) and food consumption at the highest exposure level during the
treatment period. Significantly reduced food consumption was also
observed in the 2411 and 5909 mg/m3 exposure groups, but it was not
accompanied by any decrease in weight gain. Clinical signs of toxicity
observed in the does included significant ( P < 0.01) increases in
perioral and perinasal wetness and blepharospasm at the highest
concentration. At necropsy, there were colour changes in the lungs of
33% of the does exposed to 11 255 mg/m3. Relative liver weight was
significantly ( P < 0.01) elevated (16.8% over control weight) at
the highest exposure level. There were no statistically significant
effects on gestation parameters; however, there were non-significant
increases in non-viable implants and early resorptions and a
non-significant decrease in the percentage of live fetuses concurrent
with maternal toxicity at 11 255 mg/m3. Apparent increases in
ecchymosis (haemorrhagic areas of the skin) of the head were shown to
be within the ranges observed for the historical controls of this test
facility (US EPA, 1991b). The highest exposure level resulted in
maternal mortality. The next lower dose of 5909 mg/m3, at which the
only effect noted was reduced food consumption without accompanying
weight loss, is considered the NOAEL of the study.
8.7 Immunological and neurological effects
No studies were located that examined immunotoxicity in animals
after exposure to cumene by any route.
Cumene appears to be similar to many solvents, such as alcohol,
that are known central nervous system depressants. The occurrence of
neurological effects from inhalation exposure to cumene has been
confirmed in several studies. These studies are acute exposures that
show neurotoxicological effects only at quite high concentrations
(>2450 mg/m3 [>500 ppm]). Neurotoxicological effects were not
observed in the longer-term inhalation study by Cushman et al. (1995),
which included complete batteries of functional and motor activity
tests and neurohistopathology and in which the highest exposure
concentration was 5890 mg/m3 (1202 ppm).
Cumene was tested at 0, 9800, 19 600, or 39 200 mg/m3 (0, 2000,
4000, or 8000 ppm) and produced a short-lived profile of
neurobehavioural effects in mice that indicated central nervous system
depressant activity (Tegeris & Balster, 1994). Effects noted from
brief (20-min) whole-body exposures to cumene included those on
central nervous system activity (decreased arousal and rearing at 9800
mg/m3), muscle tone/equilibrium (changes in grip strength and
mobility at 19 600 mg/m3), and sensorimotor activity (including
decreased tail pinch and touch response at 19 600 mg/m3).
In an acute experiment accompanying the subchronic exposures (see
section 8.4.1), Cushman et al. (1995) exposed Fischer-344 rats (whole
body) once to 0, 490, 2430, or 5890 mg/m3 (0, 100, 496, or 1202 ppm)
for 6 h and conducted functional observations 1 h post-exposure. Gait
abnormalities and decreased rectal temperatures were noted for both
sexes at the highest exposure level only. Decreased activity levels
were noted for both sexes at the highest level and for females only at
the next highest level (2430 mg/m3) of exposure. Males, but not
females, from the highest exposure group had decreased response to toe
pinch at 6 h post-exposure.
In a 5-day inhalation study, Fischer-344 rats exposed whole body
to 9800 or 24 500 mg cumene vapour/m3 (2000 or 5000 ppm) for 6 h/day
showed toxic effects from exposure (Gulf Oil Corp., 1985). All rats in
the high-exposure group died after 2 days. At the lower dose, females
demonstrated central nervous system effects (hypothermia and
staggering). Similar, but more severe, symptoms were observed in the
high-exposure animals before they died.
Fischer-344 rats (10 per sex per group) were exposed whole body
to cumene at 0, 1230, 2680, 5130, or 6321 mg/m3 (0, 251, 547, 1047,
or 1290 ppm) for 6 h/day, 5 days/week, for 2 weeks (Chemical
Manufacturers Association, 1989). Initial exposures to 9800 mg/m3
(2000 ppm) for 1-2 days resulted in such severe neurological and
respiratory effects that the concentration levels were reduced to
those given above. During the remainder of the 2-week exposure period,
clinical observations (ocular discharge, decreased motor activity or
hyperactivity, and ataxia) were noted sporadically at all levels
except 1230 mg/m3. For females in the two highest dose groups, the
average relative kidney weight and relative and absolute adrenal
weights were increased significantly over control values. These data
provide corroboration for these same effects reported in the study of
Cushman et al. (1995).
9. EFFECTS ON HUMANS
No information was located regarding the toxicity of cumene in
humans following acute, subchronic, or chronic exposure (US EPA,
1997). The minimum lethal human exposure to this agent has not been
delineated. No epidemiology, case reports, or clinical controls of
humans were located for this compound. There are no epidemiological or
occupational studies examining the carcinogenicity of cumene in humans
(US EPA, 1997).
No information was located regarding dermal irritation and
sensitization in humans following exposure to cumene.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
The available environmental effects studies are inadequate to
allow a quantitative assessment of the acute toxicity of cumene to
environmental organisms owing to the variability of the data and
flawed experimental designs. For example, 24-h toxicity values for
water fleas ranged from an EC50 of 91 mg/litre (Bringmann & Kuhn,
1982) down to an IC50 of 0.6 mg/litre (Abernathy et al., 1986).
Further, many of the reported toxicity values for aquatic
invertebrates exceed the water solubility of cumene at 50 mg/litre,
with Glickman et al. (1995) noting that actual measured concentrations
of cumene were only about 10% of nominal concentrations. The lowest
reported toxic concentration was 0.012 mg/litre, the toxicity
threshold for the protozoan Colpidium colpoda (Rogerson et al.,
1983). Concentrations of up to 50 mg/litre did not affect the growth
of the larvae of the mussel Mytilis edulis during a 27-day exposure
(Le Roux, 1977). Selected data demonstrating effect concentrations are
shown in Table 2. It should be noted that the high volatility and
biodegradability of cumene may further reduce the hazard to the
aquatic environment, especially for chronic exposure conditions.
Table 2: Acute toxicity of cumene to organisms other than laboratory mammals.
Species End-point Concentration
(effect) (mg/litre) Reference
Algae
Green alga 3-h EC50 21 Hutchinson et al.,
(Chlorella vulgaris) (photosynthetic 1980
inhibition)
Green alga 3-h EC50 9 Hutchinson et al.,
(Chlamydomonas angulosa) (photosynthetic 1980
inhibition)
Green alga 72-h EC50 2.6 Galassi et al.,
(Selenastrum capricornutum) (growth 1988
inhibition)
Green algae 72-h static EC50 2.0 Hüls, 1998a
(Scenedesmus subspicatus) (growth
inhibition)
Invertebrates
Water flea (Daphnia magna) 24-h EC50 91 Bringmann & Kuhn,
(immobilization) 1982
Water flea (Daphnia magna) 24-h LC50 4.8 Glickman et al.,
1995
Water flea (Daphnia magna) 21-day static EC50 1.5 Hüls, 1998b
Water flea (Daphnia magna) 24-h IC50a 1.4 Galassi et al.,
1988
Table 2 (continued)
Water flea (Daphnia magna) 24-h IC50 0.6 Abernathy et al.,
1986
Mysid shrimp (Mysidopsis bahia) 96-h flow LC50 1.3 Glickman et al.,
1995
Mysid shrimp (Mysidopsis bahia) 96-h flow LC50 1.2 Chemical Manufacturers
Association, 1990
Ciliate protozoan "toxicity threshold" 0.012 Rogerson et al.,
(Colpidium colpoda) (NR)b 1983
Vertebrates
Rainbow trout 96-h LC50 4.8 Glickman et al.,
(Oncorhynchus mykiss) 1995
Rainbow trout no observed effect 1.9 Glickman et al.,
(Oncorhynchus mykiss) 1995
Sheepshead minnow 96-h flow LC50 4.7 Glickman et al.,
(Cyprinodon variegatus) 1995
Sheepshead minnow no observed effect <2.9 Glickman et al.,
(Cyprinodon variegatus) 1995
a IC50 = immobilization concentration for 50% of the organisms.
b NR = not reported.
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
Kinetic analysis shows that there is rapid and complete clearance
of cumene and its metabolites from the body, indicating little
potential for accumulation. No human toxicity data are available from
exposure to cumene. Short-term exposures of animals to high
concentrations (>2450 mg/m3 [>500 ppm]) demonstrate that cumene,
like other solvents, may be considered harmful, inducing transient
reversible central nervous system effects. However, neurotoxicity,
portal-of-entry effects, developmental effects, and markedly adverse
systemic toxicity were not observed after long-term repeated-dose
studies conducted in animals at lower concentrations (<2450 mg/m3
[<500 ppm]). Cumene has caused dermal and ocular irritation in
animals in one study, but it had no such effects in others. A single
study indicates that cumene does not elicit dermal sensitization in
animals.
Increases in organ weights (most notably kidney) are the most
prominent and consistent effects observed in rodents exposed for 6-7
months by the oral route (Wolf et al., 1956) or for 3 months by the
inhalation route (Cushman et al., 1995). No adverse effects were
observed in rat or rabbit fetuses whose mothers had been exposed to
airborne cumene during fetal development.
The sparsity of long-term repeated-dose toxicity data and the
absence of any human toxicity data both constitute areas of scientific
uncertainty. The only repeated-dose toxicity studies of any
appreciable duration are the oral study of Wolf et al. (1956), at
about 7 months, and the 3-month subchronic inhalation study of Cushman
et al. (1995). Both of these studies are concurrent in indicating
kidneys of female rats as the target organ, regardless of exposure
route. Although neither of these studies is sufficient in duration to
reveal the fate of the observed alterations in organ weights from
lifetime chronic exposure, the subchronic study of Cushman et al.
(1995) is more scientifically comprehensive in its analyses than the
study of Wolf et al. (1956) and offers much more extensive data
reporting on more animals (both genders). The study of Cushman et al.
(1995) is therefore chosen as the pivotal study.
No multigeneration reproductive studies have been performed for
cumene. The rapid metabolism and excretion of cumene, coupled with the
lack of effects on sperm morphology reported by Cushman et al. (1995),
indicate that cumene has low potential for reproductive toxicity.
However, this lack of concern must be weighed against the fact that
kinetic studies indicate extensive and wide distribution of cumene,
including to reproductive organs, and the fact that the consequences
of long-term repeated/continuous exposure on either organs or
reproductive function have not been evaluated.
There are no data in humans or animals concerning the development
of cancer following exposure to cumene. The potential hazard for
carcinogenicity of cumene to humans has not been determined, although
the predominant evidence suggests that this compound is not likely to
produce a carcinogenic response (i.e., numerous genotoxic tests,
including gene mutation, chromosomal aberration, and primary DNA
damage tests, were conducted, all but one of which were negative or
not reproducible). No highly reactive chemical species are known to be
generated during the metabolism of cumene.
11.1.2 Criteria for setting guidance values for cumene
For oral exposures, the NOAEL for increased average kidney weight
in female rats following subchronic (139/194 days) oral (gavage)
exposure is 154 mg/kg body weight per day, which was adjusted, based
on the dosing schedule, to 110 mg/kg body weight per day (Wolf et al.,
1956). These data were not amenable to benchmark dose analysis. For
purposes of quantitative assessment, the quality of the principal oral
study is marginal, because the group sizes were minimal, the groups
comprised females only, and little quantitative information was
presented. Full uncertainty factors of 10 each are applied for
interindividual and interspecies variations. A partial uncertainty
factor (100.5) for extrapolation from subchronic to chronic duration
is applied, as the study was intermediate between chronic and
subchronic duration. Another partial uncertainty factor (100.5) is
also used owing to lack of a full-scale multigeneration reproductive
study. The total uncertainty factor applied was 1000 (10 × 10 × 100.5
× 100.5). This yields a guidance value for oral exposure of 0.1 mg/kg
body weight per day. This guidance value is meant to provide
information for risk managers to enable them in making decisions
concerning the protection of human health.
Interpretation of the effects reported in the subchronic
inhalation study of Cushman et al. (1995) allows for consideration of
either the 490 mg/m3 (100 ppm) (MAK, 1996) or the 2430 mg/m3 (496
ppm) (US EPA, 1997) exposure level as a defensible NOAEL. Whereas the
motor effects, organ weight changes, and clinical effects reported at
2430 mg/m3 (496 ppm) may be regarded as non-adverse indicators of
exposure (in other words, as a NOAEL), these same effects may be
regarded alternatively as potentially adverse indicators of
toxicologically significant effects apparent at the next highest
exposure level (in other words, a LOAEL). Consideration of both these
interpretations may be justified in derivation of an inhalation
guidance value for cumene. The experimental exposure scenario of the
NOAEL (either 490 or 2430 mg/m3 [100 or 496 ppm]) is first adjusted
to a continuous exposure scenario for the general population by
factoring the NOAEL by the hours exposed as a fraction of the day
(6/24 hours) and the number of days exposed as a fraction of the week
(5/7), resulting in the figure of 436 mg/m3 (89 ppm) for the 2430
mg/m3 (496 ppm) experimental exposure level and 88 mg/m3 (18 ppm)
for the 490 mg/m3 (100 ppm) experimental exposure level. Full
uncertainty factors of 10 each were applied for subchronic to chronic
extrapolation and for interindividual variations. A partial
uncertainty factor (100.5) is applied to account for the toxicodynamic
component of the interspecies extrapolation. In long-term inhalation
exposures, the blood/air partition coefficient ( Hb/a) is a principal
factor determining the amount of compound reaching a systemic tissue
(such as kidney). For a given external concentration and similar
exposure conditions, the smaller the Hb/a values, the less compound
in the blood and at the tissue. The blood/air partition coefficient
has been determined with human blood (Sato & Nakajima, 1979, 1987),
but not for rats. Information available on compounds structurally
related to cumene (xylenes and benzene; Gargas et al., 1989) indicates
that human Hb/a values are nearly always smaller than rat Hb/a
values, such that, for a given external concentration, human tissues
would receive less compound than would rat tissues. Thus, use of the
rat in a long-term repeated-dose study with cumene obviates the need
for the toxicokinetic component of the animal to human extrapolation.
An additional partial uncertainty factor (100.5) is used for database
deficiencies, owing principally to lack of a full-scale
multigeneration reproductive study, as discussed above. The total
uncertainty factor would be 1000 (10 × 10 × 100.5 × 100.5).
Application of this factor would result in guidance values of 0.4
mg/m3 (0.08 ppm) for the NOAEL of 436 mg/m3 (89 ppm), adjusted for
continuous exposure from 2430 mg/m3 (496 ppm), and 0.09 mg/m3
(0.02 ppm) for the NOAEL of 88 mg/m3 (18 ppm), adjusted for
continuous exposure from 490 mg/m3 (100 ppm).
The carcinogenic potential of cumene cannot be determined because
no adequate data, such as well-conducted long-term animal studies or
reliable human epidemiological studies, are available with which to
perform an assessment.
11.1.3 Sample risk characterization
The scenario chosen as an example is continuous lifetime exposure
for the general population.
No human data are available with which to characterize the
toxicity of cumene directly. The reported ambient cumene concentration
of 0.0147 mg/m3 (0.003 ppm) is appreciably below either of the
guidance values of 0.4 mg/m3 (0.08 ppm) (27-fold) or 0.09 mg/m3
(0.02 ppm) (6-fold). The upper limit of ambient cumene concentrations
reported in rural air, 2.5 µg/m3 (0.5 ppb), is even further below the
guidance values (36- to 160-fold). Other data presented in this
report, such as estimates from cigarette smoke, suggest that humans
would primarily be exposed through inhalation, although ingestion
through food may occur. Exposure via drinking-water is probably
unlikely.
The critical effect in the principal study for the oral
assessment is increased kidney weight in female rats and, although
poorly reported, is corroborated by inhalation studies with cumene.
Increased organ weights have been found in other toxicity studies with
cumene and have been observed across routes of exposure. Insufficient
data on oral exposure exist to apply the guidance value of 0.1 mg/kg
body weight per day derived above.
Following inhalation exposure, the effects observed included
increased kidney and adrenal weights and central nervous system,
haematological, and clinical biochemical alterations, which were
observed in rats. The critical effect was observed across species and
was observed in several studies. These results partially corroborate
and reinforce the significance of similar results seen in the
long-term oral study of cumene.
The potential hazard for carcinogenicity of cumene in humans
cannot be determined. Studies have indicated that cumene has low, if
any, genotoxicity.
Neither chronic nor multigeneration reproductive studies are
available for this substance.
Data are not available to determine whether young or aged animals
are more susceptible than adult animals (e.g., 2-year-old rats) to the
effects of cumene, and there is no evidence to suggest that this would
be so in young or aged humans. There is also no convincing evidence to
suggest that gender differences in susceptibility to cumene toxicity
would exist in humans.
11.2 Evaluation of environmental effects
Cumene is a volatile liquid and exists mainly in the vapour phase
in the atmosphere. It degrades in the atmosphere via reaction with
hydroxyl radicals. Although small amounts of cumene may be removed
from the atmosphere by precipitation, cumene is not expected to react
with ozone or directly with light. In water, cumene can be
volatilized, undergo biodegradation, or adsorb to sediments. In soil,
it is expected to biodegrade rapidly under aerobic conditions; as in
water, it can readily adsorb to soil or volatilize.
BCFs suggest a slight potential for cumene to bioconcentrate in
fish species. No data were available on the bioconcentration of cumene
in terrestrial organisms. Although the existing toxicological database
and limited exposure data do not permit a quantitative risk
assessment, the available information suggests that cumene will not
adversely affect populations or communities of terrestrial or aquatic
organisms based on its low availability (volatility, rapid
degradation).
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
No previous evaluations by international bodies were identified.
Information on international hazard classification and labelling
is included in the International Chemical Safety Card reproduced in
this document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventive and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 0170) reproduced in this
document.
13.1 Human health hazards
Cumene is flammable. Exposure could cause central nervous system
effects and at high concentrations could result in unconsciousness.
13.2 Advice to physicians
In the event of poisoning, treatment is supportive.
13.3 Health surveillance advice
For workers exposed to cumene, a health surveillance programme
should include surveillance of kidney function.
13.4 Spillage
In the event of spillage, measures should be taken to prevent
cumene from reaching drains and watercourses, owing to the potential
for hazardous effects on aquatic organisms.
13.5 Storage
Cumene should be stored away from acids and strong oxidants.
Long-term storage could result in the formation of explosive
peroxides. Proper safety and handling procedures must be used.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards
may be obtained from UNEP Chemicals (IRPTC), Geneva.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
ISOPROPYLBENZENE ICSC:0170
October 1994
CAS #
Some relevant physical and chemical properties of cumene are
listed in Table 1. Additional physical/chemical properties are
presented in the International Chemical Safety Card (ICSC 0170)
reproduced in this document.
Table 1: Physical/chemical properties of cumene.
Property Value Reference
Molecular weight 120.2 g/mol
Boiling point 152.39 °C Ward, 1979
Vapour pressure, 611 Pa Mackay &
25 °C Shiu, 1981
Water solubility, 50 mg/litre Mackay &
25 °C Shiu, 1981
Log Kow 3.66 Hansch &
Leo, undated
Density, 20 °C 0.8619 g/cm3 Ward, 1979
Flashpoint (tag closed-cup) 35 °C Ward, 1965
Odour threshold limit 0.088 ppm (v/v) Amoore &
value (TLV) 0.43 mg/m3 Hautala, 1983
Table 1 (continued)
Conversion factor, 1 ppm = 5.2 mg/m3
20 °C, 101.3 kPa 1 mg/m3 = 0.19 ppm
Partition coefficients Sato &
Oil/air 6215 Nakajima, 1979
Oil/water 4316
Water/air 1.44
Human blood/air 37
3. ANALYTICAL METHODS
For sampling and measurement of cumene in air, Method 1501 of the
US National Institute for Occupational Safety and Health (NIOSH, 1994)
includes use of a solid sorbent tube (coconut shell charcoal) sampler
with a gas chromatography/flame ionization detector measurement
technique. The detection limit of this method is 1 mg/m3 (0.2 ppm).
US EPA (1996) methods for detecting cumene in media other than
air include the use of gas chromatography using photoionization Method
8021B, which is applicable to nearly all types of samples, regardless
of water content. The method detection limit for cumene is 0.05
µg/litre, and the applicable concentration range for this method is
approximately 0.1-200 µg/litre. The standard recovery using this
method is 98%, with a standard deviation of 0.9%. Another commonly
used gas chromatographic assay for volatiles including cumene is
Method 8260B (US EPA, 1996), with a general estimated quantitation
limit of approximately 5 µg/kg wet weight for soil/sediment samples,
0.5 mg/kg wet weight for wastes, and 5 µg/litre for groundwater.
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
Cumene is a naturally occurring constituent of crude oil and may
be released to the environment from a number of anthropogenic sources,
including processed hydrocarbon fuels. Crude oils typically contain
approximately 0.1 wt% of cumene, but concentrations as high as 1.0 wt%
have been reported.1 Measurements of various grades of petrol
revealed that cumene concentrations range from 0.14 to 0.51 vol% and
that the average cumene concentration was 0.3 vol%. Premium diesel
fuel contains 0.86 wt% of cumene; furnace oil (no. 2) contains 0.60
wt%.1
Primary sources of release of cumene include losses in wastewater
and fugitive emissions from manufacturing and use facilities and
petrochemical refineries, accidental spills of finished fuel products
during transport or processing, and emissions from petrol stations and
motor vehicles (US EPA, 1987). Cigarette tobacco also releases cumene
(Johnstone et al., 1962). Cumene release from all these sources is
estimated to be 9500 tonnes annually (US EPA, 1988). Other,
unquantifiable anthropogenic cumene releases include the rubber
vulcanization process (Cocheo et al., 1983), building materials
(Moelhave, 1979), jet engine exhaust (Katzman & Libby, 1975), outboard
motor operation (Montz et al., 1982), solvent uses (Levy, 1973),
pharmaceutical production, and textile plants (Gordon & Gordon, 1981).
Cumene is also released to the environment from leather tanning, iron
and steel manufacturing, paving and roofing, paint and ink
formulation, printing and publishing, ore mining, coal mining,
organics and plastics manufacturing, pesticide manufacturing,
electroplating, and pulp and paper production (Shackelford et al.,
1983).
SRI International (1986) reported the 1985 Western European
cumene production levels (in tonnes) for the following producer
countries:
Federal Republic of Germany 438 000
Finland 70 000
France 370 000
Italy 335 000
Netherlands 240 000
Spain 120 000
United Kingdom 220 000
This total 1985 production of 1 793 000 tonnes may be compared with
production in the USA, which was reported as 2 775 000 tonnes in 1997
(Anon., 1998).
1 Letter and attachment from W.F. O'Keefe, American Petroleum
Institute, to M. Greif, Toxic Substances Control Act (TSCA)
Interagency Testing Committee, US Environmental Protection
Agency, Washington, DC (TS-792).
The use pattern for cumene in the early 1970s in the USA was as
follows (Anon., 1984): oxidation for phenol/acetone production, 98%;
polymerization of alpha-methylstyrene, 1.8%; and exports, 0.2%. Cumene
is also used captively for the production of phenol and
alpha-methyl-styrene (SRI International, 1986).
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
In the atmosphere, cumene is expected to exist almost entirely in
the vapour phase (Eisenreich et al., 1981). Cumene does not absorb
ultraviolet light at wavelengths greater than 290 nm (US EPA, 1987),
which suggests that cumene would not be susceptible to direct
photolysis. In one study, the estimated half-life of cumene in the
atmosphere from photolysis alone was approximately 1500 years (Parlar
et al., 1983). Cumene is not susceptible to oxidation by ozone in the
atmosphere (US EPA, 1987). Thus, reaction with ozone and direct
photolysis are not expected to be important removal processes. Rather,
reaction with photochemically generated hydroxyl radicals appears to
be the primary degradation pathway (t´ 1-2 days) (Lloyd et al.,
1976; Ravishankara et al., 1978). Small amounts of cumene may be
removed from the atmosphere during precipitation. Cumene has been
assigned a Photochemical Ozone Creation Potential (POCP) value of 35
relative to ethylene at 100 (Derwent & Jenkin, 1990). POCP values
represent the ability of a substance to form ground-level ozone as a
result of its atmospheric degradation reactions.
In water, important fate and transport processes are expected to
be volatilization (t´ 4 h from a typical river) and aerobic
biodegradation (Kappeler & Wuhrmann, 1978; Sasaki, 1978; Van der
Linden, 1978). Chemical hydrolysis, oxidation, photolysis, and
reaction with hydroxyl radicals are not expected to be important fate
processes in water (Mill et al., 1978, 1979, 1980). Using an aerobic
freshwater sediment/water test system, Williams et al. (1993)
demonstrated that 10 days after addition of radiolabelled cumene (2.5
mg/litre) to the system, 46.9% was trapped as radiolabelled carbon
dioxide and another 21.8% was recovered as radiolabelled organics, the
overall recovery of cumene ranging from 56.8% to 88.3%. The
disappearance half-life based on these results was 2.5 days. During a
20-day incubation of cumene at 10 mg/litre under aerobic conditions in
either fresh water or salt water, Price et al. (1974) observed 70%
degradation in fresh water but only about 2% degradation in seawater.
Cumene was, however, observed to be degraded to a significant extent
by microorganisms isolated from ocean sediment samples incubated in
seawater, as Walker et al. (1976) noted decreases in cumene (gas
chromatographic analysis) ranging from 37% to 60% of initial amounts
over a period of 21 days in three separate incubations with seawater
and microorganisms isolated from Atlantic Ocean sediments. On the
other hand, cumene was found to be essentially non-biodegradable under
anaerobic conditions by Battersby & Wilson (1989), who noted that
cumene produced only about 2% of theoretical gas production when
incubated at 50 mg carbon/litre sludge for 60 days at 35°C under
anaerobic conditions; compounds at 80% of theoretical gas production
under these conditions were assumed to represent complete degradation,
whereas compounds at less than 30% production were considered
persistent.
In soil, it appears that cumene might biodegrade fairly rapidly
under aerobic conditions, because a number of microorganisms capable
of degrading cumene have been isolated (Yamada et al., 1965; Jamison
et al., 1970; Omori et al., 1975). Regression equations based on the
limit of cumene water solubility (50 mg/litre) predicted Koc (soil
sorption coefficient standardized to organic carbon) values ranging
from 513 to 1622. For equations based instead on log octanol/water
partition coefficients (log Kow) for cumene, predicted Koc
values were in a similar range, from 589 to 3890 (Lyman et al., 1982).
Other estimates of Koc values at 884 (Jeng et al., 1992) and 2800
(US EPA, 1987) were also in this range. These Koc values indicate
that cumene is expected to adsorb moderately to strongly to soil and
have only slight mobility. The relatively high vapour pressure of
cumene suggests that volatilization of this compound from dry soil
surfaces would be significant.
Measured and estimated bioconcentration factors (BCFs) suggest a
slight potential for cumene to bioconcentrate in fish species. A BCF
of 36 for cumene in goldfish ( Carassius auratus) has been measured
(Ogata et al., 1984), and a BCF of 356 was estimated from the log
Kow and a linear regression correlation equation (log BCF = 0.76
log Kow - 0.23) by the US EPA (1987). This value was concordant
with the BCF of 316 calculated for fish species in general exposed to
cumene (Sabljic, 1987). Cumene was detected at levels of 0.5-1.4 ng/g
wet weight (detection limit 0.5 ng/g wet weight by gas chromatography/
mass spectrometry) in 12 of 138 sampled fish (various species) from
several locations near a potential emission source (Japan Environment
Agency, 1987). Cumene has been detected in "oakmoss" ( Evernia
prunastri (L.) Ach.) (Gavin et al., 1978) and marsh grass (Mody et
al., 1974a,b).
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
Cumene has been found as a contaminant in various industrial
effluents and in groundwaters. Significant levels of cumene have been
recorded in groundwater near chemical plants (1581 µg/litre, Botta et
al., 1984; 360 µg/litre, Teply & Dressler, 1980; 11 µg/litre,
Pellizzari et al., 1979), around outboard motor operations (700
µg/litre, Montz et al., 1982), near coal gasification facilities (up
to 54 µg/litre, Steurmer et al., 1982), and around petroleum plants
and petroleum refineries (5 µg/litre, quantification method not clear;
Snider & Manning, 1982). Cumene was detected in 8 of 135 samples of
surface water (detection limit 0.03 µg/litre with gas
chromatography/mass spectrometry) at concentrations ranging from 0.09
to 0.44 µg/litre in several locations near a potential emission source
in the 1986 monitoring of the general environment in Japan (Japan
Environment Agency, 1987). Cumene levels in sediments and biota in
Puget Sound, Washington, USA, ranged from 0.02 to 19 µg/g, with a mean
concentration of 2.3 µg/g (Brown et al., 1979). A cumene level of
140 µg/litre was found in seawater near an offshore drilling platform
in the Gulf of Mexico (Sauer, 1981). Cumene was detected in 6 of 111
sediment samples at concentrations ranging from 0.58 to 11 ng/g dry
weight (detection limit 0.5 ng/g with gas chromatography/mass
spectrometry) in several locations near a potential emission source
(Japan Environment Agency, 1987).
Reports of air sampling in the USA indicate the mean
concentration of cumene to be about 14.7 µg/m3 (3 ppb) in urban
settings and as high as 2.5 µg/m3 (0.5 ppb) in rural settings.
Samples taken in Los Angeles, California, in 1966 averaged 14.7 µg/m3
(3 ppb) (Lonneman et al., 1968), and samples taken in Houston, Texas,
in 1973-1974 averaged 12.15 µg/m3 (2.48 ppb) (Lonneman et al., 1979).
The US EPA (1987) reported a mean concentration of 16.7 µg cumene/m3
(3.4 ppb) in undated samples from Los Angeles. In samples taken in the
fall of 1981 in Los Angeles, Grosjean & Fung (1984) did not detect
cumene, although a minimum detection level of 9.8 µg/m3 (2 ppb) was
reported. Although a number of sampling attempts in rural and remote
areas reported no detectable levels of cumene in air (detection limit
<0.05 µg/m3 [<0.01 ppb]), two attempts were positive: Seila (1979)
reported mean levels of 2.5 µg/m3 (0.5 ppb) in samples taken in a
rural area near Houston, Texas, in 1978, and Arnts & Meeks (1980,
1981) reported 0.25 µg/m3 (0.05 ppb) in samples taken near campfires
in the Great Smokey Mountains, USA, in 1978.
Average atmospheric concentrations of cumene in Europe are
reported to be somewhat less than those in the USA, although
concentrations in urban areas are also consistently much higher than
those in rural areas. Isodorov et al. (1983) recorded an average
cumene level of 8.3 µg/m3 (1.7 ppb) in the urban atmosphere of
Leningrad, USSR, in 1977-1979, with a maximum of 11.8 µg/m3 (2.4
ppb). Ambient air concentrations for the Netherlands in 1980 were
reported to average 0.5-1.0 µg/m3 (0.1-0.2 ppb), with maxima ranging
up to 34.8 µg/m3 (7.1 ppb) (Guicherit & Schulting, 1985). An annual
average of 1.6 µg/m3 (0.3 ppb) (maximum 3.9 µg/m3 [0.8 ppb]) was
reported from the Grenoble area in France in 1987 (Foster et al.,
1991).
6.2 Human exposure
Humans can be exposed to cumene via industrial emissions, petrol
station or motor vehicle emissions, accidental releases, food,
cigarette smoke, and drinking-water (US EPA, 1987).
In condensates of cigarette smoke, Johnstone et al. (1962)
recorded yields of cumene ranging from 7 to 14 µg/cigarette. Holzer et
al. (1976) detected cumene at 10 µg/m3 (2 ppb) in air samples taken
from a room immediately after a single cigarette had been smoked. No
further specifics, such as indication of a median value or minimum
detection level, are given.
Brugnone et al. (1989) reported cumene as measurable in all
alveolar air samples collected (single breath; range 1-81 µg/m3
[0.2-17 ppb], method detection limit not given) from among two groups
of workers ( n = 86, gender not specified) exposed to <0.1 mg
cumene/m3 (<0.02 ppm) through the work shift. These authors analysed
for but were unable to detect any significant differences in cumene
concentrations between smokers and non-smokers in either alveolar air
or blood samples. In another study, gases collected from 60 min of
normal continuous respiration from each of eight male volunteers
(three smokers) were analysed for trace organic constituents (Conkle
et al., 1975). Cumene was listed as detected in one of the three
smokers (expressed as 21 µg/h) and in one of the five non-smokers
(expressed as 0.13 µg/h). Krotoszynski & O'Neill (1982) also
identified cumene in expired air from non-smokers.
The presence of cumene in foods can be biogenic or due to
environmental contamination (US EPA, 1987). Although the detection
limit of cumene in various foods was not specified, the US EPA (1987)
noted that cumene has been detected but not quantified in foods as
diverse as tomatoes, Concord grapes, cooked rice, fried chicken,
bacon, Beaufort cheese, and dried legumes.
Only two reports of cumene quantification in drinking-water were
found in the available literature. Coleman et al. (1984) detected
cumene in Cincinnati, Ohio, USA, drinking-water at a level of 0.014
µg/litre (quantification method not clear). Keith et al. (1976)
reported 0.01 µg cumene/litre drinking-water in Terrebonne-Parish,
Louisiana, USA, but found none in the drinking-water of nine other
cities across the USA. These concentrations are considerably below the
0.5 µg/litre detection limit reported by Westrick et al. (1984), who
found no cumene in 945 US drinking-water systems, 479 of which were
selected because of known contamination problems. Burmaster (1982) and
Burnham et al. (1972) reported unquantified levels of cumene/
alkylbenzenes in drinking-water obtained from groundwater. Based on
the results of these studies, it may be concluded that cumene
contamination above 0.5 µg/litre is uncommon in drinking-water in the
USA.
One industrial hygiene survey (US EPA, 1988) reported that
approximately 739 US workers were occupationally exposed to cumene.
Personal exposure data in this report consisted of 1487 air samples
taken over the course of 12 years (1973-1984), of which 6 were in the
range of 20-150 mg/m3 (4-30 ppm), 4 in the range of 15-20 mg/m3 (3-4
ppm), and 25 in the range of 5-10 mg/m3 (1-2 ppm), with the remaining
samples below 5 mg/m3 (1 ppm) (US EPA, 1988).
Based on available monitoring data, it appears that the general
population would be exposed to cumene primarily by inhalation,
although occupational populations may be reasonably anticipated to be
exposed by the dermal route. Minor exposure may result from contact
with refined petroleum products and ingestion of contaminated foods
and possibly drinking-water.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND
HUMANS
Cumene has been shown to be absorbed after inhalation exposure in
humans and after inhalation, oral, and dermal exposure in animals
(Senczuk & Litewka, 1976; Research Triangle Institute, 1989). Tests
conducted in humans indicate that cumene is absorbed readily via the
inhalation route, that it is metabolized efficiently to water-soluble
metabolites within the body, and that these metabolites are excreted
efficiently into the urine with no evidence of long-term retention
within the body; these results concur with the results of animal
studies.
Senczuk & Litewka (1976) exposed human volunteers (five men and
five women) head only to one of three different concentrations of
cumene vapours (240, 480, or 720 mg/m3 [49, 98, or 147 ppm]) for 8 h
every 10 days. Exhaled breath samples (10 cm3) were collected near
the beginning and at the end of the exposure from a tube placed in the
breathing zone. The total amount of cumene absorbed during exposure,
calculated from retention, ventilation, and exposure duration, was
nearly twice as high at all exposure levels in the males (466-1400 mg)
as in the females (270-789 mg). The respiratory tract absorption
ranged from 45% to 64% depending on the time of exposure, with the
overall mean retention estimated at 50%. In rats, inhalation studies
(nose only for 6 h at 510, 2420, or 5850 mg/m3 [104, 494, or 1194
ppm]) indicate rapid absorption, with detectable levels of cumene
appearing in the blood within 5 min of the beginning of exposure at
all three exposure levels (Research Triangle Institute, 1989). Gavage
studies in rats showed that cumene was absorbed readily via this
route, with maximum levels in blood occurring at the earliest time
point sampled (4 h) for a lower dose (33 mg/kg body weight) and at
8-16 h for a higher dose (1350 mg/kg body weight) (Research Triangle
Institute, 1989). Dermal absorption of cumene was demonstrated in rats
and rabbits (Monsanto Co., 1984).
The human data reported by Brugnone et al. (1989) regarding
cumene distribution suggest that the cumene concentration was about 40
times higher in blood than in alveolar air, a figure concordant with
the reported human blood/air partition coefficient of 37 (Sato &
Nakajima, 1979; Table 1). Cumene was widely distributed in rats, and
distribution, presumably determined immediately after exposure, was
independent of administration route (inhalation, oral, or
intraperitoneal in 10% aqueous Emulphor). Adipose, liver, and kidney
were all shown to have elevated tissue/blood ratios of cumene
following all doses and routes of exposure (Research Triangle
Institute, 1989). Fabre et al. (1955) demonstrated that after rats
inhaled cumene vapour for up to 150 days, cumene was distributed to
the endocrine organs, central nervous system, bone marrow, spleen, and
liver.
The patterns of cumene disappearance (as total radioactivity)
from the blood in the nose-only inhalation studies were fitted with a
monoexponential model, with the half-lives increasing with dose, from
3.9 h at 490 mg/m3 (100 ppm) to 6.6 h at 5880 mg/m3 (1200 ppm). The
half-life of cumene in the blood in gavage studies with rats was
calculated to be between 9 and 16 h.
Metabolism of cumene by cytochrome P-450 is extensive and takes
place within hepatic and extrahepatic tissues, including lung (Sato &
Nakajima, 1987), with the secondary alcohol 2-phenyl-2-propanol being
a principal metabolite. Metabolites excreted in urine of rats and
rabbits include 2-phenyl-2-propanol and its glucuronide or sulfate
conjugates, conjugates of 2-phenyl-1,2-propanediol, and an unknown
metabolite, possibly the dicarboxylic acid that would result from
complete oxidation of the 1- and 3-alkyl carbons of phenylmalonic acid
(Research Triangle Institute, 1989; Ishida & Matsumoto, 1992; MAK,
1996).
Senczuk & Litewka (1976) also conducted excretion studies with
human volunteers exposed to cumene vapours (240, 480, or 720 mg/m3
[49, 98, or 147 ppm]) for 8 h every 10 days. These authors reported
excretion of the metabolite 2-phenyl-2-propanol in the urine as
biphasic, with a rapid early phase (t´ 2 h) and a slower later phase
(t´ 10 h); excretion of this metabolite in the urine (about 35% of
the calculated absorbed dose) was maximal after 6-8 h of exposure and
approached zero at 40 h post-exposure. With rats, the extent of
elimination across routes of administration (inhalation, oral, or
intraperitoneal) and exposure concentrations was very similar, with
urine being the major route of elimination, about 70% in all cases
(Research Triangle Institute, 1989). Total body clearance in the rats
was rapid and complete, with less than 1% of the absorbed fraction
being present in the body 72 h after the highest exposure regime
examined (5880 mg/m3 [1200 ppm] for 6 h). Following oral
administration of cumene in rabbits, 90% was recovered as metabolites
in the urine within 24 h (Robinson et al., 1955).
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
Cumene is not highly toxic to laboratory animals by inhalation,
oral, or dermal routes of exposure. An LC50 of 9800 mg cumene/m3
(2000 ppm) in mice has been reported (MAK, 1996). A 4-h inhalation
LC50 of 39 200 mg/m3 (8000 ppm) in rats was reported by several
investigators (Smyth et al., 1951; Koch Refining Co., 1984; Union
Carbide Corp., 1985). Acute oral LD50 values for rats range from 1400
to 2900 mg/kg body weight (Smyth et al., 1951; Koch Refining Co.,
1984; Monsanto Co., 1984; Ciba-Geigy Co., 1985; Union Carbide Corp.,
1985). Tanii et al. (1995) reported an intraperitoneal LD50 in male
mice in the same range, 2000 mg/kg body weight (16.9 mmol/kg).
Clinical signs of toxicity reported in rats in acute oral studies
include weakness, ocular discharge, collapse, and death; pathological
findings in animals that died were haemorrhagic lungs, liver
discolorations, and acute gastrointestinal inflammation (Monsanto Co.,
1984). The character of the dose-response for these effects is,
however, unclear.
Acute dermal LD50s for cumene applied undiluted to rabbit skin
range from >3160 mg/kg body weight (Monsanto Co., 1984) to >10 000
mg/kg body weight (Ciba-Geigy Co., 1985). Pathological findings in
animals that died were similar to those in animals that died after a
single oral exposure (Monsanto Co., 1984).
8.2 Irritation and sensitization
Undiluted cumene applied to the skin of New Zealand albino
rabbits (0.5 ml) according to standardized guidelines caused slight
defatting with skin flaking, a symptom not generally classified as
relating to primary skin irritancy (Monsanto Co., 1984). A study
conducted by Ciba-Geigy Co. (1985) reported a similar low level of
irritation.
Cumene is an ocular irritant. Ocular irritation, including
immediate discomfort followed by "erythema" (redness of the
conjunctiva) and copious discharge, was observed after the
instillation of undiluted cumene to rabbit, with these effects being
reversible within 120 h (Monsanto Co., 1984). Ciba-Geigy Co. (1985)
judged eye irritation as slight when cumene was applied to rabbit
eyes. However, a study by Union Carbide Corp. (1985) reported that
cumene was harmless to rabbit eyes when applied undiluted.
Observations of lacrimation (Tegeris & Balster, 1994) and periocular
swelling and blepharospasm (Cushman et al., 1995) also indicate that
cumene may exhibit ocular irritancy at high airborne concentrations.
The concentration of cumene causing a 50% reduction in the
respiratory rate in mice after 30 min of exposure was determined to be
10 084 mg/m3 (2058 ppm) (Kristiansen et al., 1986). This
concentration is quite high and in the range where repeated exposure
caused death and morbidity in rats (Gulf Oil Corp., 1985; Chemical
Manufacturers Association, 1989) and rabbits (Darmer et al., 1997).
No skin sensitization reactions were noted among a group of 20
female guinea-pigs treated with cumene in a Magnusson-Kligman
maximization test conducted in accordance with Organisation for
Economic Co-operation and Development (OECD) Guideline 406 (Hüls,
1988). No data were available on respiratory sensitization to cumene.
8.3 Short-term exposure
In a study by Monsanto Co. (1986), male and female Sprague-Dawley
rats (10 per sex per group) were exposed whole body to cumene vapour
concentrations of 0, 515, 1470, or 2935 mg/m3 (0, 105, 300, or
599 ppm) for 6 h/day, 5 days/week, for approximately 4 weeks (minimum
exposure, 20 days). Cage-side observations included
concentration-related increases in side-to-side head movements in both
males and females in all dose groups, head tilt in all dose groups,
and arched back in one female in the high-dose group. Increases in
mean absolute left and right kidney weights were observed in high-dose
males, as were increases in mean absolute left kidney weight in
low- and mid-dose males. In high-dose females, the mean absolute
weight of left kidneys was greater than in controls. This study
confirms that renal weight changes occur in females and corroborates
similar effects reported by Cushman et al. (1995). It should be noted
that the effects associated with central nervous system perturbation
(i.e., head movements) were not noted in several other longer-term
studies, including that of Cushman et al. (1995), in which
neurotoxicity was specifically assessed. If it is assumed that the
renal changes among the males were associated with male rat-specific
nephropathy (see section 8.4.1), the cage-side observations of head
tilt and head movements become the critical effects for this
short-term study.
Although not statistically significant, leukocytosis was noted in
a group of rats ( n = 15, mixed sex) exposed to cumene at 1200 mg/m3
(245 ppm) for 8 h/day, 5 days/week, for 30 exposures (Jenkins et al.,
1970).
Other short-term toxicity studies are described in section 8.7.
8.4 Long-term exposure
8.4.1 Subchronic exposure
In an inhalation exposure study by Jenkins et al. (1970), groups
of squirrel monkeys ( n = 2), beagle dogs ( n = 2),
Princeton-derived guinea-pigs ( n = 15), and Sprague-Dawley and
Long-Evans rats ( n = 15) were exposed whole body to cumene at
concentrations of 0, 18, or 147 mg/m3 (0, 4, or 30 ppm) continuously
for 90 days. Initial and terminal body weights, haematological and
clinical chemistry parameters, and histopathological data were
collected. No toxicologically significant effects were noted in the
monkeys, dogs, or guinea-pigs. The only effect noted in the rats was a
slight degree of leukocytosis at both concentrations.
Cushman et al. (1995; also reported as Bushy Run Research Center,
1989a) conducted two successive subchronic whole-body inhalation
toxicity studies with cumene vapours (>99.9% pure) on Fischer-344
rats. In the first study, groups (21 per sex) were exposed to cumene
vapour at 0, 490, 2430, or 5890 mg/m3 (0, 100, 496, or 1202 ppm) 6
h/day, 5 days/week, for 13 weeks. The second study was a repeat of the
first, except that the group size was decreased to 15 per sex and an
additional group (at 245 mg/m3 [50 ppm]) and a 4-week post-exposure
period were added. Parameters monitored included clinical signs of
toxicity, auditory brain stem responses, ophthalmology, sperm count
and morphology, and histopathological examination of all respiratory
tract tissues (lungs and nasal turbinates) and the perfused nervous
system. Evaluations of neurological function (functional observation
battery and motor activity) were conducted in both studies. Light
microscopic evaluation of the perfused-fixed nervous system tissues
(six rats per sex per group) was conducted in the first study only.
In the first study, transient, reversible cage-side observations
during exposure periods included hypoactivity, blepharospasm, and a
delayed or absent startle reflex at the highest concentration. Rats
exposed to 2430 mg/m3 were reported as being hypoactive during
exposure, although no further specifics were given. Statistically
significant ( P < 0.05) exposure-related decreases in motor activity
(total) were observed in male rats exposed to the two highest
concentrations of cumene, but these results were not observed in the
second study in either sex. There were no exposure-related changes
noted in the functional observation battery in this or the subsequent
study. No effects were observed in the neurohistopathological
examinations. Cataracts were reported in males at all exposure
concentrations in this study. However, these results were not observed
in the second study in which a more comprehensive protocol for eye
examination was employed. Evaluation of the auditory brain stem
responses revealed no meaningful changes in the auditory function of
the exposed animals. The only gross histopathology noted was
periocular swelling, which occurred in animals at the two highest
concentrations (and for which neither incidence nor severity was
reported). Both absolute and relative weights were increased
significantly (>10%) in the kidneys, adrenal glands, and livers of
both sexes at the highest concentration. These changes were also noted
in the liver at the next lower concentration (2430 mg/m3) for both
females and males. Kidney lesions described in male rats at the two
highest exposure concentrations were considered to be closely related
to male rat-specific nephropathy (i.e., lesions were limited to males,
and tubular proteinosis, hypertrophy, and hyperplasia as well as
hyaline droplet formation were noted, although the identity of the
protein in the droplets was not confirmed) and are of questionable
relevance to human toxicity, principally because renal lesions
characteristic of this type of nephropathy have not been observed in
humans (US EPA, 1991a; Hard et al., 1993). Chronic progressive
nephropathy, a common spontaneous renal disease of Fischer-344 male
rats that occurs as early as 5 months of age (Montgomery & Seely,
1990), may also contribute to these renal lesions. Water consumption
was significantly increased (about 40%) in male rats above control
values at both 2430 and 5890 mg/m3. Several haematological and serum
measures were also changed in a statistically significant dose-related
manner at both 2430 and 5890 mg/m3: leukocytes (both sexes),
platelets (both sexes), lymphocytes (males only), glucose (females
only), and calcium/phosphorus (males only).
The results of the second study, with a 4-week post-exposure
period, indicated limited reversibility of the organ weight
alterations, because significant mean weight increases were still
present in female liver and female adrenals of the highest exposure
group. In males, only relative kidney weights (significant at 6%) and
absolute liver weights remained increased significantly. Blood and
serum parameters were not reported in this study. Morphological
evaluation of epididymal and testicular sperm showed no cumene-related
differences in count, morphology, or stages of spermatogenesis,
although one high-dose rat did have diffuse testicular atrophy.
The weight alterations in the male and female adrenals and female
kidney are considered potentially adverse, as the persistence noted
indicates limited reversibility and engenders uncertainty about the
progression and fate of these alterations under chronic exposure. The
increased water consumption noted may also indicate potential for
renal effects, although this effect was present at the next to highest
dose level at which renal weights were not altered. Although the
progression of these weight alterations from continued exposure cannot
be ascertained from this subchronic study, data from the second
(post-exposure) study indicate limited reversibility of effects on the
adrenals, at least in females. The liver weight alterations are not
viewed as adverse, because increase in liver weight without
accompanying pathology is a trait of common microsomal enzyme inducing
agents, although it should be noted that induction of hepatic
microsomal enzymes may influence the metabolism of other substances
and may either increase or decrease their toxicity (Sipes & Gandolfi,
1991). The altered haematological and serum parameters noted at the
two highest concentrations may be considered as significant, although
all are within normal ranges (Mitruka & Rawnsley, 1981). Based on the
lowest dose at which both relative and absolute weight alterations in
adrenal tissues of both sexes and in the kidneys of females are
statistically ( P < 0.05) and biologically (>10%) significant, 5890
mg/m3 may be considered as a lowest-observed-adverse-effect level
(LOAEL), and 2430 mg/m3 the corresponding NOAEL. Based on
consideration of the various measures in the first study (motor
effects, increased water consumption in males, haematological and
serum parameters, sporadic weight increases in male adrenals and
female kidneys) as significant, 2430 mg/m3 may be considered as a
LOAEL and 490 mg/m3 as the corresponding NOAEL. It should be noted
here that a LOAEL of 2391 mg/m3 (488 ppm) and a NOAEL of 485 mg/m3
(99 ppm) were noted for maternal toxicity in the short-term
developmental study in rats by Darmer et al. (1997), discussed in
section 8.6.
8.4.2 Chronic exposure and carcinogenicity
There are no long-term in vivo bioassays addressing the issue
of cancer. No data exist to support any quantitative cancer
assessment.
Wolf et al. (1956) conducted a study involving groups of 10
female Wistar rats administered cumene by gavage in olive oil at 154,
462, or 769 mg/kg body weight per day, 5 days/week, over a 194-day
(6- to 7-month) period, equivalent to 110, 331, or 551 mg/kg body
weight per day, adjusted for daily exposure. Rats given olive oil
served as controls ( n = 20). A pronounced increase in average kidney
weight, noted as a "moderate effect," occurred at 769 mg/kg body
weight per day, although no quantitative data are presented. An
increase in average kidney weight was noted as a "slight effect" at
462 mg/kg body weight per day. It is stated in the report that at 154
mg/kg body weight per day, no evidence of ill effects, as determined
by gross appearance, growth, periodic blood counts, analysis for blood
urea nitrogen, average final body and organ weights, and bone marrow
counts, was noted. The LOAEL is 462 mg/kg body weight per day, and the
NOAEL is 154 mg/kg body weight per day. These results are consistent
with those observed in more recent, better-reported studies described
elsewhere in this document.
In an inhalation study by Fabre et al. (1955), Wistar rats were
exposed (whole body) to cumene vapour at 2500 mg/m3 (510 ppm), and
rabbits were exposed to 6500 mg/m3 (1327 ppm), for 8 h/day, 6
days/week, for up to 180 days. Histological effects reported were
"passive congestion" in the lungs, liver, spleen, kidney, and adrenals
and the presence of haemorrhagic zones in the lung, haemosiderosis in
the spleen, and lesions from epithelial nephritis "in some cases." It
was not clear from the study if these effects occurred in rats or
rabbits, or both.
8.5 Genotoxicity and related end-points
In general, negative results have been obtained in a relatively
complete battery of in vivo and in vitro mutagenicity tests,
including gene mutation, chromosomal aberration, and primary DNA
damage (US EPA, 1997). Cumene was tested at concentrations up to
2000 µg/plate in a Salmonella typhimurium reverse mutation assay
(modified Ames test); negative results were observed with and without
metabolic activation (Lawlor & Wagner, 1987). Cumene was negative in
an Ames assay at concentrations up to 3606 µg/plate with
S. typhimurium strains TA98, TA100, TA1535, and TA1537 (Florin et
al., 1980). Cumene also tested negative, with and without metabolic
activation, in a set of HGPRT assays (using Chinese hamster ovary
cells) at cumene concentrations of 100-125 µg/ml, at which the
relative cloning efficiencies (a measure of cytotoxicity) ranged from
29% to 110% (Gulf Life Sciences Center, 1985a; Yang, 1987). A
micronucleus assay performed in mice given up to 1 g cumene/kg body
weight by gavage was negative (Gulf Life Sciences Center, 1985b).
Micronucleus assays done in Fischer-344 rats, however, gave values
that were weakly positive, although little dose-response was seen, and
deaths occurred at the highest dose (5 of 10 animals at 2.5 g/kg body
weight intraperitoneally; NTP, 1996). The positive control used in the
micronucleus tests, cyclophosphamide, produced strong positive
responses in all assays.
Cumene failed to induce significant rates of transformation in
BALB/3T3 cells (without activation) at concentrations up to 500 µg/ml
(Putnam, 1987) but tested positive in an earlier cell transformation
test also using BALB/3T3 cells, in which an increase in
transformations was observed at 60 µg/ml (Gulf Oil Corp., 1984a).
Results from an unscheduled DNA synthesis assay in rat hepatocytes
conducted by Gulf Oil Corp. (1984b) indicated positive results at
doses of 16 and 32 µg cumene/ml (with 128 µg/ml noted as toxic to the
hepatocytes). However, apparent technical difficulties with this test
(US EPA, 1988) prompted a repeat of the unscheduled DNA synthesis
assay in rat hepatocytes, the results of which showed cumene to be
clearly negative at doses up to 24 µg/ml, with doses above 24 µg/ml
noted as being too toxic for evaluation of unscheduled DNA synthesis
(Curren, 1987; US EPA, 1988).
8.6 Reproductive and developmental toxicity
No multigeneration reproductive study exists for this compound by
either the oral or inhalation route. There are no data concerning
cumene exposure of females prior to mating, from conception to
implantation, or during late gestation, parturition, or lactation.
The first subchronic inhalation study of Cushman et al. (1995),
however, conducted morphological evaluation of epididymal and
testicular sperm in rats exposed for 13 weeks to cumene vapours (see
section 8.4.1). No cumene-related differences in count, morphology, or
stages of spermatogenesis were noted, although one high-dose rat did
have diffuse testicular atrophy. No alterations (weight changes,
histopathology) were noted in the female reproductive organs that were
examined at the termination of this same study.
In an inhalation study (Darmer et al., 1997; also reported as
Bushy Run Research Center, 1989b), Sprague-Dawley rats (25 per group)
were exposed whole body to 0, 485, 2391, or 5934 mg cumene/m3 (0, 99,
488, or 1211 ppm) for 6 h/day on days 6 through 15 of gestation.
Perioral wetness and encrustation, a significant ( P < 0.01)
decrease in body weight gain on gestation days 6 through 9
(accompanied by a significant decrease in food consumption), and a
slight increase (7.7%) in relative liver weight were observed in dams
at the high dose only. Hypoactivity, blepharospasm, and significantly
( P < 0.05) decreased food consumption were observed in the dams at
the next highest concentration. There were no statistically
significant adverse effects on reproductive parameters or fetal
development. For this study, 5934 mg/m3 is a developmental NOAEL, and
485 mg/m3 is a maternal NOAEL.
In another inhalation study (Darmer et al., 1997; also reported
as Bushy Run Research Centre, 1989c), New Zealand white rabbits (15
per group) were exposed whole body to 0, 2411, 5909, or 11 255 mg
cumene/m3 (0, 492, 1206, or 2297 ppm) for 6 h/day on days 6 through
18 of gestation. Two does died and one aborted at the highest exposure
concentration. There were significant ( P < 0.01) reductions in body
weight gain (178 g lost compared with 31 g gained in the control
group) and food consumption at the highest exposure level during the
treatment period. Significantly reduced food consumption was also
observed in the 2411 and 5909 mg/m3 exposure groups, but it was not
accompanied by any decrease in weight gain. Clinical signs of toxicity
observed in the does included significant ( P < 0.01) increases in
perioral and perinasal wetness and blepharospasm at the highest
concentration. At necropsy, there were colour changes in the lungs of
33% of the does exposed to 11 255 mg/m3. Relative liver weight was
significantly ( P < 0.01) elevated (16.8% over control weight) at
the highest exposure level. There were no statistically significant
effects on gestation parameters; however, there were non-significant
increases in non-viable implants and early resorptions and a
non-significant decrease in the percentage of live fetuses concurrent
with maternal toxicity at 11 255 mg/m3. Apparent increases in
ecchymosis (haemorrhagic areas of the skin) of the head were shown to
be within the ranges observed for the historical controls of this test
facility (US EPA, 1991b). The highest exposure level resulted in
maternal mortality. The next lower dose of 5909 mg/m3, at which the
only effect noted was reduced food consumption without accompanying
weight loss, is considered the NOAEL of the study.
8.7 Immunological and neurological effects
No studies were located that examined immunotoxicity in animals
after exposure to cumene by any route.
Cumene appears to be similar to many solvents, such as alcohol,
that are known central nervous system depressants. The occurrence of
neurological effects from inhalation exposure to cumene has been
confirmed in several studies. These studies are acute exposures that
show neurotoxicological effects only at quite high concentrations
(>2450 mg/m3 [>500 ppm]). Neurotoxicological effects were not
observed in the longer-term inhalation study by Cushman et al. (1995),
which included complete batteries of functional and motor activity
tests and neurohistopathology and in which the highest exposure
concentration was 5890 mg/m3 (1202 ppm).
Cumene was tested at 0, 9800, 19 600, or 39 200 mg/m3 (0, 2000,
4000, or 8000 ppm) and produced a short-lived profile of
neurobehavioural effects in mice that indicated central nervous system
depressant activity (Tegeris & Balster, 1994). Effects noted from
brief (20-min) whole-body exposures to cumene included those on
central nervous system activity (decreased arousal and rearing at 9800
mg/m3), muscle tone/equilibrium (changes in grip strength and
mobility at 19 600 mg/m3), and sensorimotor activity (including
decreased tail pinch and touch response at 19 600 mg/m3).
In an acute experiment accompanying the subchronic exposures (see
section 8.4.1), Cushman et al. (1995) exposed Fischer-344 rats (whole
body) once to 0, 490, 2430, or 5890 mg/m3 (0, 100, 496, or 1202 ppm)
for 6 h and conducted functional observations 1 h post-exposure. Gait
abnormalities and decreased rectal temperatures were noted for both
sexes at the highest exposure level only. Decreased activity levels
were noted for both sexes at the highest level and for females only at
the next highest level (2430 mg/m3) of exposure. Males, but not
females, from the highest exposure group had decreased response to toe
pinch at 6 h post-exposure.
In a 5-day inhalation study, Fischer-344 rats exposed whole body
to 9800 or 24 500 mg cumene vapour/m3 (2000 or 5000 ppm) for 6 h/day
showed toxic effects from exposure (Gulf Oil Corp., 1985). All rats in
the high-exposure group died after 2 days. At the lower dose, females
demonstrated central nervous system effects (hypothermia and
staggering). Similar, but more severe, symptoms were observed in the
high-exposure animals before they died.
Fischer-344 rats (10 per sex per group) were exposed whole body
to cumene at 0, 1230, 2680, 5130, or 6321 mg/m3 (0, 251, 547, 1047,
or 1290 ppm) for 6 h/day, 5 days/week, for 2 weeks (Chemical
Manufacturers Association, 1989). Initial exposures to 9800 mg/m3
(2000 ppm) for 1-2 days resulted in such severe neurological and
respiratory effects that the concentration levels were reduced to
those given above. During the remainder of the 2-week exposure period,
clinical observations (ocular discharge, decreased motor activity or
hyperactivity, and ataxia) were noted sporadically at all levels
except 1230 mg/m3. For females in the two highest dose groups, the
average relative kidney weight and relative and absolute adrenal
weights were increased significantly over control values. These data
provide corroboration for these same effects reported in the study of
Cushman et al. (1995).
9. EFFECTS ON HUMANS
No information was located regarding the toxicity of cumene in
humans following acute, subchronic, or chronic exposure (US EPA,
1997). The minimum lethal human exposure to this agent has not been
delineated. No epidemiology, case reports, or clinical controls of
humans were located for this compound. There are no epidemiological or
occupational studies examining the carcinogenicity of cumene in humans
(US EPA, 1997).
No information was located regarding dermal irritation and
sensitization in humans following exposure to cumene.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
The available environmental effects studies are inadequate to
allow a quantitative assessment of the acute toxicity of cumene to
environmental organisms owing to the variability of the data and
flawed experimental designs. For example, 24-h toxicity values for
water fleas ranged from an EC50 of 91 mg/litre (Bringmann & Kuhn,
1982) down to an IC50 of 0.6 mg/litre (Abernathy et al., 1986).
Further, many of the reported toxicity values for aquatic
invertebrates exceed the water solubility of cumene at 50 mg/litre,
with Glickman et al. (1995) noting that actual measured concentrations
of cumene were only about 10% of nominal concentrations. The lowest
reported toxic concentration was 0.012 mg/litre, the toxicity
threshold for the protozoan Colpidium colpoda (Rogerson et al.,
1983). Concentrations of up to 50 mg/litre did not affect the growth
of the larvae of the mussel Mytilis edulis during a 27-day exposure
(Le Roux, 1977). Selected data demonstrating effect concentrations are
shown in Table 2. It should be noted that the high volatility and
biodegradability of cumene may further reduce the hazard to the
aquatic environment, especially for chronic exposure conditions.
Table 2: Acute toxicity of cumene to organisms other than laboratory mammals.
Species End-point Concentration
(effect) (mg/litre) Reference
Algae
Green alga 3-h EC50 21 Hutchinson et al.,
(Chlorella vulgaris) (photosynthetic 1980
inhibition)
Green alga 3-h EC50 9 Hutchinson et al.,
(Chlamydomonas angulosa) (photosynthetic 1980
inhibition)
Green alga 72-h EC50 2.6 Galassi et al.,
(Selenastrum capricornutum) (growth 1988
inhibition)
Green algae 72-h static EC50 2.0 Hüls, 1998a
(Scenedesmus subspicatus) (growth
inhibition)
Invertebrates
Water flea (Daphnia magna) 24-h EC50 91 Bringmann & Kuhn,
(immobilization) 1982
Water flea (Daphnia magna) 24-h LC50 4.8 Glickman et al.,
1995
Water flea (Daphnia magna) 21-day static EC50 1.5 Hüls, 1998b
Water flea (Daphnia magna) 24-h IC50a 1.4 Galassi et al.,
1988
Table 2 (continued)
Water flea (Daphnia magna) 24-h IC50 0.6 Abernathy et al.,
1986
Mysid shrimp (Mysidopsis bahia) 96-h flow LC50 1.3 Glickman et al.,
1995
Mysid shrimp (Mysidopsis bahia) 96-h flow LC50 1.2 Chemical Manufacturers
Association, 1990
Ciliate protozoan "toxicity threshold" 0.012 Rogerson et al.,
(Colpidium colpoda) (NR)b 1983
Vertebrates
Rainbow trout 96-h LC50 4.8 Glickman et al.,
(Oncorhynchus mykiss) 1995
Rainbow trout no observed effect 1.9 Glickman et al.,
(Oncorhynchus mykiss) 1995
Sheepshead minnow 96-h flow LC50 4.7 Glickman et al.,
(Cyprinodon variegatus) 1995
Sheepshead minnow no observed effect <2.9 Glickman et al.,
(Cyprinodon variegatus) 1995
a IC50 = immobilization concentration for 50% of the organisms.
b NR = not reported.
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
Kinetic analysis shows that there is rapid and complete clearance
of cumene and its metabolites from the body, indicating little
potential for accumulation. No human toxicity data are available from
exposure to cumene. Short-term exposures of animals to high
concentrations (>2450 mg/m3 [>500 ppm]) demonstrate that cumene,
like other solvents, may be considered harmful, inducing transient
reversible central nervous system effects. However, neurotoxicity,
portal-of-entry effects, developmental effects, and markedly adverse
systemic toxicity were not observed after long-term repeated-dose
studies conducted in animals at lower concentrations (<2450 mg/m3
[<500 ppm]). Cumene has caused dermal and ocular irritation in
animals in one study, but it had no such effects in others. A single
study indicates that cumene does not elicit dermal sensitization in
animals.
Increases in organ weights (most notably kidney) are the most
prominent and consistent effects observed in rodents exposed for 6-7
months by the oral route (Wolf et al., 1956) or for 3 months by the
inhalation route (Cushman et al., 1995). No adverse effects were
observed in rat or rabbit fetuses whose mothers had been exposed to
airborne cumene during fetal development.
The sparsity of long-term repeated-dose toxicity data and the
absence of any human toxicity data both constitute areas of scientific
uncertainty. The only repeated-dose toxicity studies of any
appreciable duration are the oral study of Wolf et al. (1956), at
about 7 months, and the 3-month subchronic inhalation study of Cushman
et al. (1995). Both of these studies are concurrent in indicating
kidneys of female rats as the target organ, regardless of exposure
route. Although neither of these studies is sufficient in duration to
reveal the fate of the observed alterations in organ weights from
lifetime chronic exposure, the subchronic study of Cushman et al.
(1995) is more scientifically comprehensive in its analyses than the
study of Wolf et al. (1956) and offers much more extensive data
reporting on more animals (both genders). The study of Cushman et al.
(1995) is therefore chosen as the pivotal study.
No multigeneration reproductive studies have been performed for
cumene. The rapid metabolism and excretion of cumene, coupled with the
lack of effects on sperm morphology reported by Cushman et al. (1995),
indicate that cumene has low potential for reproductive toxicity.
However, this lack of concern must be weighed against the fact that
kinetic studies indicate extensive and wide distribution of cumene,
including to reproductive organs, and the fact that the consequences
of long-term repeated/continuous exposure on either organs or
reproductive function have not been evaluated.
There are no data in humans or animals concerning the development
of cancer following exposure to cumene. The potential hazard for
carcinogenicity of cumene to humans has not been determined, although
the predominant evidence suggests that this compound is not likely to
produce a carcinogenic response (i.e., numerous genotoxic tests,
including gene mutation, chromosomal aberration, and primary DNA
damage tests, were conducted, all but one of which were negative or
not reproducible). No highly reactive chemical species are known to be
generated during the metabolism of cumene.
11.1.2 Criteria for setting guidance values for cumene
For oral exposures, the NOAEL for increased average kidney weight
in female rats following subchronic (139/194 days) oral (gavage)
exposure is 154 mg/kg body weight per day, which was adjusted, based
on the dosing schedule, to 110 mg/kg body weight per day (Wolf et al.,
1956). These data were not amenable to benchmark dose analysis. For
purposes of quantitative assessment, the quality of the principal oral
study is marginal, because the group sizes were minimal, the groups
comprised females only, and little quantitative information was
presented. Full uncertainty factors of 10 each are applied for
interindividual and interspecies variations. A partial uncertainty
factor (100.5) for extrapolation from subchronic to chronic duration
is applied, as the study was intermediate between chronic and
subchronic duration. Another partial uncertainty factor (100.5) is
also used owing to lack of a full-scale multigeneration reproductive
study. The total uncertainty factor applied was 1000 (10 × 10 × 100.5
× 100.5). This yields a guidance value for oral exposure of 0.1 mg/kg
body weight per day. This guidance value is meant to provide
information for risk managers to enable them in making decisions
concerning the protection of human health.
Interpretation of the effects reported in the subchronic
inhalation study of Cushman et al. (1995) allows for consideration of
either the 490 mg/m3 (100 ppm) (MAK, 1996) or the 2430 mg/m3 (496
ppm) (US EPA, 1997) exposure level as a defensible NOAEL. Whereas the
motor effects, organ weight changes, and clinical effects reported at
2430 mg/m3 (496 ppm) may be regarded as non-adverse indicators of
exposure (in other words, as a NOAEL), these same effects may be
regarded alternatively as potentially adverse indicators of
toxicologically significant effects apparent at the next highest
exposure level (in other words, a LOAEL). Consideration of both these
interpretations may be justified in derivation of an inhalation
guidance value for cumene. The experimental exposure scenario of the
NOAEL (either 490 or 2430 mg/m3 [100 or 496 ppm]) is first adjusted
to a continuous exposure scenario for the general population by
factoring the NOAEL by the hours exposed as a fraction of the day
(6/24 hours) and the number of days exposed as a fraction of the week
(5/7), resulting in the figure of 436 mg/m3 (89 ppm) for the 2430
mg/m3 (496 ppm) experimental exposure level and 88 mg/m3 (18 ppm)
for the 490 mg/m3 (100 ppm) experimental exposure level. Full
uncertainty factors of 10 each were applied for subchronic to chronic
extrapolation and for interindividual variations. A partial
uncertainty factor (100.5) is applied to account for the toxicodynamic
component of the interspecies extrapolation. In long-term inhalation
exposures, the blood/air partition coefficient ( Hb/a) is a principal
factor determining the amount of compound reaching a systemic tissue
(such as kidney). For a given external concentration and similar
exposure conditions, the smaller the Hb/a values, the less compound
in the blood and at the tissue. The blood/air partition coefficient
has been determined with human blood (Sato & Nakajima, 1979, 1987),
but not for rats. Information available on compounds structurally
related to cumene (xylenes and benzene; Gargas et al., 1989) indicates
that human Hb/a values are nearly always smaller than rat Hb/a
values, such that, for a given external concentration, human tissues
would receive less compound than would rat tissues. Thus, use of the
rat in a long-term repeated-dose study with cumene obviates the need
for the toxicokinetic component of the animal to human extrapolation.
An additional partial uncertainty factor (100.5) is used for database
deficiencies, owing principally to lack of a full-scale
multigeneration reproductive study, as discussed above. The total
uncertainty factor would be 1000 (10 × 10 × 100.5 × 100.5).
Application of this factor would result in guidance values of 0.4
mg/m3 (0.08 ppm) for the NOAEL of 436 mg/m3 (89 ppm), adjusted for
continuous exposure from 2430 mg/m3 (496 ppm), and 0.09 mg/m3
(0.02 ppm) for the NOAEL of 88 mg/m3 (18 ppm), adjusted for
continuous exposure from 490 mg/m3 (100 ppm).
The carcinogenic potential of cumene cannot be determined because
no adequate data, such as well-conducted long-term animal studies or
reliable human epidemiological studies, are available with which to
perform an assessment.
11.1.3 Sample risk characterization
The scenario chosen as an example is continuous lifetime exposure
for the general population.
No human data are available with which to characterize the
toxicity of cumene directly. The reported ambient cumene concentration
of 0.0147 mg/m3 (0.003 ppm) is appreciably below either of the
guidance values of 0.4 mg/m3 (0.08 ppm) (27-fold) or 0.09 mg/m3
(0.02 ppm) (6-fold). The upper limit of ambient cumene concentrations
reported in rural air, 2.5 µg/m3 (0.5 ppb), is even further below the
guidance values (36- to 160-fold). Other data presented in this
report, such as estimates from cigarette smoke, suggest that humans
would primarily be exposed through inhalation, although ingestion
through food may occur. Exposure via drinking-water is probably
unlikely.
The critical effect in the principal study for the oral
assessment is increased kidney weight in female rats and, although
poorly reported, is corroborated by inhalation studies with cumene.
Increased organ weights have been found in other toxicity studies with
cumene and have been observed across routes of exposure. Insufficient
data on oral exposure exist to apply the guidance value of 0.1 mg/kg
body weight per day derived above.
Following inhalation exposure, the effects observed included
increased kidney and adrenal weights and central nervous system,
haematological, and clinical biochemical alterations, which were
observed in rats. The critical effect was observed across species and
was observed in several studies. These results partially corroborate
and reinforce the significance of similar results seen in the
long-term oral study of cumene.
The potential hazard for carcinogenicity of cumene in humans
cannot be determined. Studies have indicated that cumene has low, if
any, genotoxicity.
Neither chronic nor multigeneration reproductive studies are
available for this substance.
Data are not available to determine whether young or aged animals
are more susceptible than adult animals (e.g., 2-year-old rats) to the
effects of cumene, and there is no evidence to suggest that this would
be so in young or aged humans. There is also no convincing evidence to
suggest that gender differences in susceptibility to cumene toxicity
would exist in humans.
11.2 Evaluation of environmental effects
Cumene is a volatile liquid and exists mainly in the vapour phase
in the atmosphere. It degrades in the atmosphere via reaction with
hydroxyl radicals. Although small amounts of cumene may be removed
from the atmosphere by precipitation, cumene is not expected to react
with ozone or directly with light. In water, cumene can be
volatilized, undergo biodegradation, or adsorb to sediments. In soil,
it is expected to biodegrade rapidly under aerobic conditions; as in
water, it can readily adsorb to soil or volatilize.
BCFs suggest a slight potential for cumene to bioconcentrate in
fish species. No data were available on the bioconcentration of cumene
in terrestrial organisms. Although the existing toxicological database
and limited exposure data do not permit a quantitative risk
assessment, the available information suggests that cumene will not
adversely affect populations or communities of terrestrial or aquatic
organisms based on its low availability (volatility, rapid
degradation).
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
No previous evaluations by international bodies were identified.
Information on international hazard classification and labelling
is included in the International Chemical Safety Card reproduced in
this document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventive and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 0170) reproduced in this
document.
13.1 Human health hazards
Cumene is flammable. Exposure could cause central nervous system
effects and at high concentrations could result in unconsciousness.
13.2 Advice to physicians
In the event of poisoning, treatment is supportive.
13.3 Health surveillance advice
For workers exposed to cumene, a health surveillance programme
should include surveillance of kidney function.
13.4 Spillage
In the event of spillage, measures should be taken to prevent
cumene from reaching drains and watercourses, owing to the potential
for hazardous effects on aquatic organisms.
13.5 Storage
Cumene should be stored away from acids and strong oxidants.
Long-term storage could result in the formation of explosive
peroxides. Proper safety and handling procedures must be used.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards
may be obtained from UNEP Chemicals (IRPTC), Geneva.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
ISOPROPYLBENZENE ICSC:0170
October 1994
CAS # 