
Concise International Chemical Assessment Document 14
TRIBUTYLTIN OXIDE
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Concise International Chemical Assessment Document 14
TRIBUTYLTIN OXIDE
First draft prepared by Dr Robert Benson, United States Environmental
Protection Agency, Denver, Colorado, USA
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization, and produced within the framework of the
Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 1999
The International Programme on Chemical Safety (IPCS),
established in 1980, is a joint venture of the United Nations
Environment Programme (UNEP), the International Labour Organisation
(ILO), and the World Health Organization (WHO). The overall objectives
of the IPCS are to establish the scientific basis for assessment of
the risk to human health and the environment from exposure to
chemicals, through international peer review processes, as a
prerequisite for the promotion of chemical safety, and to provide
technical assistance in strengthening national capacities for the
sound management of chemicals.
The Inter-Organization Programme for the Sound Management of
Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and
Agriculture Organization of the United Nations, WHO, the United
Nations Industrial Development Organization, the United Nations
Institute for Training and Research, and the Organisation for Economic
Co-operation and Development (Participating Organizations), following
recommendations made by the 1992 UN Conference on Environment and
Development to strengthen cooperation and increase coordination in the
field of chemical safety. The purpose of the IOMC is to promote
coordination of the policies and activities pursued by the
Participating Organizations, jointly or separately, to achieve the
sound management of chemicals in relation to human health and the
environment.
WHO Library Cataloguing-in-Publication Data
Tributyltin oxide.
(Concise international chemical assessment document ; 14)
1.Trialkyltin compounds - adverse effects 2.Trialkyltin
compounds - toxicity
3.Environmental exposure 4.Maximum permissible exposure level
I.International Programme on Chemical Safety II.Series
ISBN 92 4 153014 6 (NLM classification: QV 290)
ISSN 1020-6167
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1999
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city, or area
or of its authorities, or concerning the delimitation of its frontiers
or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
TABLE OF CONTENTS
FOREWORD
1. EXECUTIVE SUMMARY
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
3. ANALYTICAL METHODS
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1. Environmental levels
6.2. Human exposure
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1. Single exposure
8.2. Irritation and sensitization
8.3. Short-term exposure
8.4. Long-term exposure
8.4.1. Subchronic exposure
8.4.2. Chronic exposure and carcinogenicity
8.5. Genotoxicity and related end-points
8.6. Reproductive and developmental toxicity
8.7. Immunological and neurological effects
8.7.1. Immunotoxicity
8.7.2. Neurotoxicity
9. EFFECTS ON HUMANS
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10.1. Aquatic environment
10.2. Terrestrial environment
11. EFFECTS EVALUATION
11.1. Evaluation of health effects
11.1.1. Hazard identification and dose-response assessment
11.1.2. Criteria for setting guidance values for TBTO
11.1.3. Sample risk characterization
11.2. Evaluation of environmental effects
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
13.1. Human health hazards
13.2. Advice to physicians
13.3. Health surveillance advice
13.4. Spillage
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
INTERNATIONAL CHEMICAL SAFETY CARD
REFERENCES
APPENDIX 1 -- SOURCE DOCUMENTS
APPENDIX 2 -- CICAD PEER REVIEW
APPENDIX 3 -- CICAD FINAL REVIEW BOARD
RÉSUMÉ D'ORIENTATION
RESUMEN DE ORIENTACION
FOREWORD
Concise International Chemical Assessment Documents (CICADs) are
the latest in a family of publications from the International
Programme on Chemical Safety (IPCS) -- a cooperative programme of the
World Health Organization (WHO), the International Labour Organisation
(ILO), and the United Nations Environment Programme (UNEP). CICADs
join the Environmental Health Criteria documents (EHCs) as
authoritative documents on the risk assessment of chemicals.
CICADs are concise documents that provide summaries of the
relevant scientific information concerning the potential effects of
chemicals upon human health and/or the environment. They are based on
selected national or regional evaluation documents or on existing
EHCs. Before acceptance for publication as CICADs by IPCS, these
documents undergo extensive peer review by internationally selected
experts to ensure their completeness, accuracy in the way in which the
original data are represented, and the validity of the conclusions
drawn.
The primary objective of CICADs is characterization of hazard and
dose-response from exposure to a chemical. CICADs are not a summary of
all available data on a particular chemical; rather, they include only
that information considered critical for characterization of the risk
posed by the chemical. The critical studies are, however, presented in
sufficient detail to support the conclusions drawn. For additional
information, the reader should consult the identified source documents
upon which the CICAD has been based.
Risks to human health and the environment will vary considerably
depending upon the type and extent of exposure. Responsible
authorities are strongly encouraged to characterize risk on the basis
of locally measured or predicted exposure scenarios. To assist the
reader, examples of exposure estimation and risk characterization are
provided in CICADs, whenever possible. These examples cannot be
considered as representing all possible exposure situations, but are
provided as guidance only. The reader is referred to EHC 1701 for
advice on the derivation of health-based guidance values.
While every effort is made to ensure that CICADs represent the
current status of knowledge, new information is being developed
constantly. Unless otherwise stated, CICADs are based on a search of
the scientific literature to the date shown in the executive summary.
In the event that a reader becomes aware of new information that would
change the conclusions drawn in a CICAD, the reader is requested to
contact IPCS to inform it of the new information.
1 International Programme on Chemical Safety (1994)
Assessing human health risks of chemicals: deriviation of guidance
values for health-based exposure limits. Geneva, World Health
Organization (Environmental Health Criteria 170).
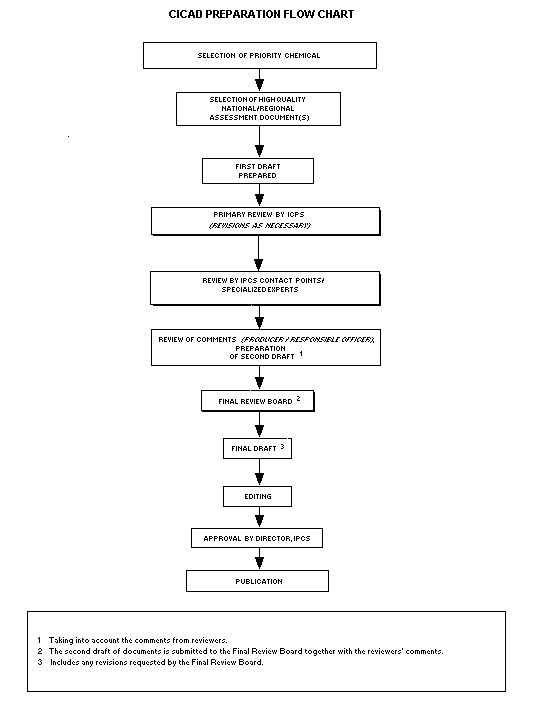
Procedures
The flow chart shows the procedures followed to produce a CICAD.
These procedures are designed to take advantage of the expertise that
exists around the world -- expertise that is required to produce the
high-quality evaluations of toxicological, exposure, and other data
that are necessary for assessing risks to human health and/or the
environment.
The first draft is based on an existing national, regional, or
international review. Authors of the first draft are usually, but not
necessarily, from the institution that developed the original review.
A standard outline has been developed to encourage consistency in
form. The first draft undergoes primary review by IPCS to ensure that
it meets the specified criteria for CICADs.
The second stage involves international peer review by scientists
known for their particular expertise and by scientists selected from
an international roster compiled by IPCS through recommendations from
IPCS national Contact Points and from IPCS Participating Institutions.
Adequate time is allowed for the selected experts to undertake a
thorough review. Authors are required to take reviewers' comments into
account and revise their draft, if necessary. The resulting second
draft is submitted to a Final Review Board together with the
reviewers' comments.
The CICAD Final Review Board has several important functions:
- to ensure that each CICAD has been subjected to an appropriate
and thorough peer review;
- to verify that the peer reviewers' comments have been addressed
appropriately;
- to provide guidance to those responsible for the preparation of
CICADs on how to resolve any remaining issues if, in the opinion
of the Board, the author has not adequately addressed all
comments of the reviewers; and
- to approve CICADs as international assessments.
Board members serve in their personal capacity, not as representatives
of any organization, government, or industry. They are selected
because of their expertise in human and environmental toxicology or
because of their experience in the regulation of chemicals. Boards are
chosen according to the range of expertise required for a meeting and
the need for balanced geographic representation.
Board members, authors, reviewers, consultants, and advisers who
participate in the preparation of a CICAD are required to declare any
real or potential conflict of interest in relation to the subjects
under discussion at any stage of the process. Representatives of
nongovernmental organizations may be invited to observe the
proceedings of the Final Review Board. Observers may participate in
Board discussions only at the invitation of the Chairperson, and they
may not participate in the final decision-making process.
 1. EXECUTIVE SUMMARY
This CICAD on tributyltin oxide (TBTO) was prepared by the United
States Environmental Protection Agency (US EPA) and is based on an
International Programme on Chemical Safety Environmental Health
Criteria document on tributyltin compounds (IPCS, 1990) and on the US
EPA's Toxicological review on tributyltin oxide (US EPA, 1997). Data
identified as of 1989 and 1996, respectively, were considered in these
reviews. Additional information identified as of June 1998 has been
included in this document. Information on the nature of the review
processes and the availability of the source documents is presented in
Appendix 1. Information on the peer review of this CICAD is presented
in Appendix 2. This CICAD was approved as an international assessment
at a meeting of the Final Review Board, held in Tokyo, Japan, on 30
June - 2 July 1998. Participants at the Final Review Board meeting are
listed in Appendix 3. The International Chemical Safety Card (ICSC
1282) for TBTO, produced by the International Programme on Chemical
Safety (IPCS, 1996), has also been reproduced in this document.
In this document, the term TBTO is used when that specific
chemical is intended. In the environment, however, tributyltin
compounds are expected to exist mainly as tributyltin hydroxide,
tributyltin chloride, and tributyltin carbonate. In those cases or
when the identity of the specific chemical is not clear, the general
term tributyltin is used.
TBTO is an effective biocidal preservative for wood, cotton
textiles, paper, and paints and stains for residential homes. It is
added as an antifouling agent in numerous formulations of marine
paints. Tributyltin is present in most of these antifouling
formulations as an organometallic copolymer. Tributyltin is slowly
released from the painted surface as the polymer is hydrolysed in
seawater, providing protection against encrustations for as long as
4-5 years.
As a result of its low water solubility and lipophilic character,
tributyltin adsorbs readily onto particles. Its half-life in the water
column ranges from a few days to weeks. Tributyltin may persist in
sediments for several years. It bioaccumulates in organisms, with the
highest concentrations found in liver and kidney. Uptake from food is
more important than uptake directly from water.
No information is available on the toxicity of TBTO in humans
following long-term exposure. Some data and case reports indicate that
TBTO is a severe dermal and respiratory irritant. The data, however,
are not adequate to characterize the exposure-response relationships.
Some studies have quantified human exposure to tributyltin from the
diet in Japan.
TBTO is moderately to highly acutely toxic to laboratory mammals
in short-term studies. In numerous well-conducted studies, both short
term and long term, the critical effect of TBTO is immunotoxicity
(depression of immune functions dependent on the thymus). The
no-observed-adverse-effect level (NOAEL) for immunosuppression in rats
following long-term exposure is 0.025 mg/kg body weight per day.
Benchmark dose analysis shows that the exposure corresponding to the
lower confidence limit (95%) on dose for a 10% decrease in
immunoglobulin (Ig) E titre in rats is 0.034 mg/kg body weight per
day. In a carcinogenicity study in rats, there was an increased
incidence of some tumours in some endocrine tissues. These tumours
occur spontaneously with variable incidence in the strain of rat used
in the study and are of unknown significance for a human health risk
assessment. TBTO is not carcinogenic in mice. The weight of evidence
shows that TBTO is not genotoxic. There is no indication that
reproductive or developmental effects occur at an exposure below that
identified as the NOAEL for immunotoxicity. Effects on reproduction
and development occur only at exposures near those causing maternal
toxicity. Data show that TBTO is a severe respiratory tract and skin
irritant. Based on the NOAEL for immunotoxicity and an uncertainty
factor of 100, a guidance value for oral exposure is 0.0003 mg/kg body
weight per day. No adequate data are available to derive a guidance
value for inhalation exposure.
TBTO is extremely hazardous to some aquatic organisms. It is an
endocrine disruptor in some organisms. The concentration of
tributyltin in some coastal waters is above a concentration causing
severe adverse effects. Adverse effects have been sufficiently severe
to lead to reproductive failure and population decline in some areas.
The general hazard to the terrestrial environment is likely to be low.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
Tributyltin oxide (CAS No. 56-35-9; C24H54OSn2;
bis-[tri- n-butyltin]-oxide; tri- n-butyltin oxide; TBTO; hexabutyl
distannoxane) has the structural formula
(CH3CH2CH2CH2)3Sn-O-Sn(CH2CH2CH2CH3)3. It is flammable but
does not form explosive mixtures with air. TBTO is a mild oxidizing
agent. In the presence of oxygen, light, or heat, slow breakdown
occurs. The solubility of TBTO in water ranges from <1 to >100 mg/
litre, depending on temperature and pH. TBTO is soluble in lipids and
very soluble in a number of organic solvents (e.g., ethanol, ether,
halogenated hydrocarbons). Its octanol/water partition coefficient
(log Kow) lies between 3.19 and 3.84 for distilled water and is
3.54 for seawater. Additional physical/chemical properties are
presented in the International Chemical Safety Card reproduced in this
document.
3. ANALYTICAL METHODS
Several methods are used for measuring tributyltin derivatives in
water, sediment, and biota (IPCS, 1990). Atomic absorption
spectrometry is the most common method used for all media. Flame
atomic absorption spectrometry has a detection limit of 0.1 mg/litre
in water. Flameless atomic absorption spectrometry, using atomization
in an electric furnace with graphite, is more sensitive and allows
detection limits of between 0.1 and 1.0 µg/litre. Recent modifications
using a gas chromatograph equipped with a flame photometric detector
allow a detection limit of 1 ng/litre (Tolosa et al., 1996).
Tributyltin can be separated from the sample matrix by capillary
supercritical fluid chromatography and determined by inductively
coupled plasma mass spectrometry. A detection limit of 12.5 pg was
obtained (Vela & Caruso, 1993). There are several different methods of
extracting tributyltin from sediment and biota and forming volatile
derivatives. The detection limits are 0.5 and 5.0 µg/kg for sediment
and biota, respectively (Vela & Caruso, 1993).
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
Tributyltin compounds have been registered as molluscicides; as
antifoulants on boats, ships, quays, buoys, crab pots, fish nets, and
cages; as wood preservatives; as slimicides on masonry; as
disinfectants; and as biocides for cooling systems, power station
cooling towers, pulp and paper mills, breweries, and leather
processing and textile mills. Tributyltin in antifouling paints was
first marketed in a form that allowed free release of the compound.
More recently, controlled-release paints, in which the tributyltin is
incorporated in a copolymer matrix, have become available. Rubber
matrices have also been developed to give long-term slow release and
lasting effectiveness for antifouling paints and molluscicides.
Government restrictions have decreased the global use of tributyltin
compounds in antifouling paints on small boats.
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
As a result of its low water solubility and lipophilic character,
tributyltin adsorbs readily onto particles (IPCS, 1990). Between 10%
and 95% of TBTO introduced into water is estimated to undergo
adsorption onto particulate matter. Progressive disappearance of
adsorbed TBTO is due to degradation, not desorption. The degree of
adsorption depends on the salinity of the water, the nature and size
of particles in suspension, the amount of suspended matter,
temperature, and the presence of dissolved organic matter.
The degradation of TBTO involves the splitting of the carbon-tin
bond (IPCS, 1990). This can result from various processes -- both
physicochemical (hydrolysis and photodegradation) and biological
(degradation by microorganisms and metabolism by higher organisms)
-- occurring simultaneously in the environment. Although the
hydrolysis of organotin compounds occurs under conditions of extreme
pH, it is barely evident under normal environmental conditions.
Photodegradation occurs during laboratory exposures of solutions to
ultraviolet light at 300 nm (and to a lesser extent at 350 nm). Under
natural conditions, photolysis is limited by the wavelength range of
sunlight and by the limited penetration of ultraviolet light into
water. The presence of photosensitizing substances can accelerate
photodegradation. Biodegradation depends on environmental conditions
such as temperature, oxygenation, pH, the level of mineral elements,
the presence of easily biodegradable organic substances for
co-metabolism, and the nature of the microflora and its capacity for
adaptation. It also depends on whether the TBTO concentration is lower
or higher than the lethal or inhibitory threshold for the
microorganisms. As with abiotic degradation, biotic breakdown of
tributyltin is a progressive oxidative debutylization founded on the
splitting of the carbon-tin bond. Dibutyltin derivatives, which are
more readily degraded than tributyltin, are formed. Monobutyltins are
mineralized slowly. Although anaerobic degradation occurs, there is a
lack of agreement as to its importance; some consider it to be slow,
whereas others believe that it is more rapid than aerobic degradation.
Species of bacteria, algae, and wood-degrading fungi have been
identified that can degrade TBTO. Estimates of the half-life of
tributyltin in the environment vary widely. The half-life in the water
column ranges from a few days to weeks. Tributyltin can persist in
sediments for several years.
Bioconcentration factors (BCFs) of up to 7000 have been reported
in laboratory investigations with molluscs and fish, and higher values
have been reported in field studies (IPCS, 1990). Bioaccumulation in
bivalves is especially high because of the low capacity for
metabolism. In molluscs, uptake from food is more important than
uptake directly from water. Higher BCFs in microorganisms (between 100
and 30 000) may reflect adsorption rather than uptake into cells
(IPCS, 1990). A recent publication reported a range of BCFs in the
Pacific oyster ( Crassostrea gigas) of 2400-7800 (Li et al., 1997).
Another recent publication reported a range of biomagnification
factors in marine mammals of 0.6-6.0 (Madhusree et al., 1997).
Although it has been suggested that tributyltin accumulates in
organisms because of its solubility in fat (IPCS, 1990), recent work
suggests that this might not be the case. Although tributyltin
residues in blubber of marine mammals have been reported (Iwata et
al., 1994, 1995, 1997), levels were considerably higher in other
tissues, notably liver (Iwata et al., 1994, 1995, 1997; Kannan et al.,
1996, 1997, 1998; Kim et al., 1996a,b; Madhusree et al., 1997; Tanabe,
1998; Tanabe et al., 1998). Comparison of patterns of tributyltin
residues with those of fat-soluble organochlorines in marine mammals
showed marked differences. Unlike the organochlorines, tributyltin
residues were the same in both sexes and remained constant after
animals reached maturity. It has been suggested that transfer through
milk to offspring, a marked trend with the organochlorines, does not
occur with tributyltin. Cetaceans showed greater bioaccumulation than
pinnipeds (Kim et al., 1996c). There has also been a report of
accumulation in liver and kidney of seabirds (Guruge et al., 1997).
Stäb et al. (1996) recently determined organotin compounds in the food
web of a shallow freshwater lake; in birds in the food web, the
highest concentrations of organotin compounds were also in liver and
kidney, not in subcutaneous fat. The various authors cited suggest
protein binding in liver to be the major mechanism of bioaccumulation.
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
Tributyltin compounds have been found in water, sediment, and
biota in areas close to pleasure boating activity, especially in or
near marinas, boat yards, and dry docks; in fish nets and cages
treated with antifouling paints; and in areas near cooling systems
(IPCS, 1990). The degree of tidal flushing and the turbidity of the
water influence tributyltin concentrations. As reported in IPCS
(1990), tributyltin levels have been found to reach 1.58 µg/litre in
seawater and estuaries; 7.1 µg/litre in fresh water; 26.3 mg/kg in
coastal sediments; 3.7 mg/kg in freshwater sediments; 6.39 mg/kg in
bivalves; 1.92 mg/kg in gastropods; and 11 mg/kg in fish. However,
these maximum concentrations of tributyltin should not be taken as
representative, because a number of factors, such as paint particles
in water and sediment samples, may give rise to anomalously high
values. It has been found that measured tributyltin concentrations in
the surface microlayer of both fresh water and seawater are up to two
orders of magnitude above those measured just below the surface.
However, it should be noted that recorded levels of tributyltin in
surface microlayers may be highly affected by the method of sampling.
More recent data (collected up to the mid-1990s) have documented
a decline in tributyltin levels in the environment, presumably due to
the restrictions placed on the use of antifouling paints on vessels
(CEFIC, 1994; Ruiz et al., 1996; Stronkhorst, 1996; Tolosa et al.,
1996; NIVA, 1997; dela Cruz & Molander, 1998). Recent data have also
documented a seasonal variation in the concentration of tributyltin in
a freshwater marina; the concentration was highest in late spring and
showed a progressive decline until winter (Fent & Hunn, 1991). This
same study also documented that the tributyltin concentration in
sediment decreased progressively with depth. In areas where the
tributyltin concentrations in water and sediment have been monitored
in the same location, the concentration of tributyltin in the water
has declined more rapidly than the concentration of tributyltin in
sediment (Stronkhorst, 1996). The range of concentrations reported in
coastal waters and estuaries is 1-10 ng/litre; the range reported for
water in marinas and major ports is 20-460 ng/litre. Most of the
sediment samples analysed contained less than 100 µg/kg, although some
samples exceeded 1000 µg/kg. The highest value reported for a sediment
sample obtained from a port in Sweden was 10 940 µg/kg. The range of
concentrations reported in biota is 0.01-3 mg/kg (dela Cruz &
Molander, 1998).
There are a number of reports on the occurrence of tributyltin
residues in marine organisms. Levels of total butyltin residues (the
sum of detected tributyltin, dibutyltin, and monobutyltin) of 5-230
ng/g in muscle of fish (Kannan et al., 1995, 1996, 1997), 300 ng/g in
liver and kidney of marine birds (Guruge et al., 1997), and 13-395
ng/g in muscle of marine mammals have been reported (Iwata et al.,
1994, 1995, 1997; Kannan et al., 1997). In marine mammals, much higher
total butyltin residues were reported for blubber (48-744 ng/g),
kidney (25-3210 ng/g), and liver (40-11 340 ng/g) (Iwata et al., 1994,
1995, 1997; Kannan et al., 1996, 1997, 1998; Kim et al., 1996a,b,c;
Madhusree et al., 1997; Tanabe, 1998; Tanabe et al., 1998).
Geographical comparisons showed greater accumulation of residues close
to coasts compared with the open sea and in the vicinity of developed
compared with developing countries.
6.2 Human exposure
Information on tributyltin concentrations in various media that
are relevant to estimation of human exposure is extremely limited,
being restricted to data from Japan.1 It is unknown if this
information is representative of other areas, and additional
investigation is desirable.
Tsuda et al. (1995) investigated the daily intakes of tributyltin
compounds from meals in Shiga Prefecture, Japan. Daily intakes of TBTO
determined by the duplicate-portion method were 4.7 + 7.0 µg/day in
1991 ( n = 39) and 2.2 + 2.2 µg/day in 1992 ( n = 40). Using the
market basket method, the daily intake was estimated at 6-9 µg/day in
1991 and 6-7 µg/day in 1992. The TBTO was found mostly in seafood.
Market basket studies in 10 local regions in Japan have shown
that the national average daily intake of tributyltin (expressed as
tributyltin chloride) was 3.7, 9.9, 5.4, 3.6, 2.9, 1.6, 1.5, and 2.3
µg/day in 1990, 1991, 1992, 1993, 1994, 1995, 1996, and 1997,
respectively.1 Variation among the local regions reflects differences
in food intake patterns as well as differences in tributyltin levels
in local fisheries.
Recent preliminary data (Takahashi et al., 1998) suggest the
potential for non-food sources of exposure -- for example, consumer
products such as rubber gloves and baking sheets.
1 Dr J. Sekizawa's (National Institute of Health Sciences, Tokyo,
Japan) review of unpublished data.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
Little definitive information is available on the
pharmacokinetics of TBTO (see IPCS, 1990, and references therein).
TBTO is absorbed from the gut (20-50%, depending on the vehicle) and
via the skin of mammals (approximately 10%). Other data suggest
absorption in the 1-5% range via the skin.1 TBTO can be transferred
across the blood-brain barrier and from the placenta to the fetus.
Following 14 days of oral administration, steady-state levels in
tissue are reached after 3-4 weeks. Absorbed material is rapidly and
widely distributed among tissues (principally the liver and kidney).
Metabolism in mammals is rapid; metabolites are detectable in the
blood within 3 h of TBTO administration. The principal metabolite
appears to be the hydroxybutyl compound, which is unstable and rapidly
splits to form the dibutyl derivative and butanol. In in vitro
studies, it has been shown that TBTO is a substrate for mixed-function
oxidases, but these enzymes are inhibited by very high concentrations
of TBTO. The rate of TBTO loss differs with different tissues. TBTO
and its metabolites are eliminated principally via the bile. The
calculated half-time for elimination of TBTO residues in mice is 29
days (Brown et al., 1977).
Tributyltin metabolism also occurs in lower organisms, but it is
slower, particularly in molluscs, than in mammals. The capacity for
bioaccumulation is, therefore, much greater in lower organisms than in
mammals.
There are some recent preliminary data (Takahashi et al., 1998)
on the occurrence of total butyltin residues in human liver. The
average concentration in four samples was 84 ng/g wet weight (range
59-96 ng/g). The concentration of tributyltin was less than the
detection limit of 2 ng/g. The concentration of dibutyltin was 79% of
the total.
1 Letter and attachments from J.A. Jonker, Elf Atochem, to D.J.
Stenhouse, Health and Safety Executive, United Kingdom, dated
3 February 1997.
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
Extensive data are available on the toxicity of TBTO. Detailed
descriptions of studies critical to the evaluation of TBTO follow; all
other studies are described in US EPA (1997) or IPCS (1990).
Collectively, these data establish that immunotoxicity is the critical
effect of TBTO. A detailed evaluation of these effects is found in
section 8.7. All studies involving repeated oral exposure are listed
in Table 1.
8.1 Single exposure
TBTO is moderately to highly acutely toxic to laboratory mammals.
Acute oral LD50 values range from 127 to 234 mg/kg body weight for
the rat and average 85 mg/kg body weight for the mouse (IPCS, 1990).
TBTO exhibits greater lethal potential when administered parenterally
(20 and 16 mg/kg body weight in the rat and mouse, respectively) as
opposed to orally, probably as a result of only partial absorption
from the gut. Single-exposure studies using 100 mg TBTO/kg body weight
by oral gavage (Funahashi et al., 1980) demonstrated a transient
increase in adrenal weight shortly after exposure (returning to normal
within 2 days) and a transient effect on thyroid follicles (distension
with flat epithelial cells). In addition, there were reversible
effects on the pituitary and on levels of adrenocorticotrophic
hormones, thyroid-stimulating hormone, thyroxine, and serum cortisol.
The acute dermal LD50 in rabbits is about 9000 mg/kg body weight.
Truhaut et al. (1979) exposed mice to an aerosol of TBTO in olive
oil for either a single 1-h period or seven 1-h periods on successive
days, using TBTO concentrations in air ranging between 50 and 400
mg/m3. Exploratory behaviour was scored over 5-min periods 2 h after
the single exposure was complete or 24 h after the last of the seven
exposure periods. The lower two exposures caused a significant
increase in exploratory behaviour (17% and 5% for 42 and 84 mg/kg body
weight, respectively), whereas the higher exposures reduced
exploratory behaviour (-18% and -38% for 170 and 340 mg/kg body
weight, respectively).
Schweinfurth & Gunzel (1987) summarized the results of several
inhalation studies in laboratory animals. After a single 4-h exposure
of rats to aerosols of TBTO, signs of irritation (nasal discharge,
lung oedema, and congestion of the pulmonary circulation) and
enteritis were observed. The LC50 was 77 mg/m3 (total particles) or
65 mg/m3 (particles with a diameter <10 µm). In guinea-pigs exposed
to aerosols of TBTO in olive oil at 200 mg/m3 and above, death
occurred within 1 h of exposure. Ten male and 10 female rats were
exposed to almost saturated vapours of TBTO (concentration not
specified) without a single death occurring during exposure for 7 h or
the following 14-day observation period. Only minor clinical signs
(slight nasal discharge directly after exposure) were noted. For this
study, the authors reported no information on particle size or the
end-points evaluated.
Table 1: Summary of oral toxicity studies on TBTO.
Toxicity/ Study length End-point LOAEL NOAEL Reference
species (mg/kg body (mg/kg body
weight per day) weight per day)
General
Monkey 22 weeks Decreased leukocyte 0.14 - Karrer et
counts al., 1992
Rat 24 months Decreased survival, 2.1 0.19 Wester et al.,
changes in kidney 1987, 1988, 1990
and organ weights,
increased serum
IgA and IgM
Mouse 18 months Decreased survival 0.7 (frank - Daly, 1992
effect level)
Reproductive
Rat 2 generations Reproduction - 4.42 Schroeder, 1990
Decreased pup 3.43 0.34
weight
Developmental
Rat gestation days Decreased maternal 9 5 Schroeder, 1981
weight
6-19 Increased 5 -
ossification
variations
Rat gestation days Decreased maternal 10 5 Crofton et al., 1989
weight
6-20 Decreased pup 10 5
weight and survival
Table 1 (continued)
Toxicity/ Study length End-point LOAEL NOAEL Reference
species (mg/kg body (mg/kg body
weight per day) weight per day)
Mouse gestation days Decreased maternal 11.7 5.8 Davis et al., 1987
weight
6-15 Decreased fetal 23.4 11.7
weight; increased
skeletal
abnormalities
Mouse gestation days Decreased maternal 40 20 Baroncelli et al., 1990
weight
6-15 Increased 40 20
resorptions;
decreased fetal
weight
Mouse gestation days Haematology - 20 Karrer et al., 1995
6-15
Immunological
Rat 28 days Thymus-dependent 5 0.5 Verdier et al., 1991
immunity
Rat 4 weeks Lymph node 0.5 - Krajnc et al., 1984;
haemorrhage Vos et al., 1984
Rat 1 week; Lymph node 0.4 - Bressa et al., 1991
4 weeks haemorrhage
Rat 6 weeks Virus titres 2 - Garssen et al.,
1995
Table 1 (continued)
Toxicity/ Study length End-point LOAEL NOAEL Reference
species (mg/kg body (mg/kg body
weight per day) weight per day)
Rat 6 weeks Reduced thymus 8 2 Van Loveren et al.,
weight 1990
Rat 6 weeks Reduced 2 - Vos et al., 1984
thymus-dependent
immunity and
non-specific
resistance
Rat 6 weeks Decreased IL+2R 0.5 - Vandebriel et al.,
alpha mRNA; 1998
reduced CD25
expression
Rat 13-26 weeks Reduced thymus 3 - Funahashi et al.,
weight 1980
Rat 18 weeks Reduced thymus 16 - Carthew et al.,
weight 1992
Rat, aged 5 months Thymus-dependent 2.5 0.25 Vos et al., 1990
immunity
Rat, weanling 4.5 or Thymus-dependent 0.25 0.025 Vos et al., 1990
18 months immunity
Mouse gestation days Humoral and 0.1 - Buckiova et al.,
4-17 or 11-17 cell-mediated 1992
immunity
Rat 10 doses to Depressed mitogen 5 2.5 Smialowicz et al.,
pre-weanlings response 1989
8.2 Irritation and sensitization
TBTO is a potent skin irritant and an extreme eye irritant (IPCS,
1990). It is not a skin sensitizer (IPCS, 1990). Poitou et al. (1978)
investigated the skin-sensitizing potential of TBTO in guinea-pigs
using the Magnussen-Kligman method. The concentrations used for
sensitization were 1% (intradermal phase) and 5% (topical phase).
Using challenge concentrations of 0.25% and 0.1%, no sensitizing
action was demonstrated in the 20 test animals. It is not clear from
IPCS (1990) whether these challenge concentrations represented the
maximum non-irritant concentrations or what positive control
substances were used to verify the sensitivity of the assay. A recent
study (Stringer et al., 1991) demonstrated contact sensitivity in the
mouse.
8.3 Short-term exposure
Short-term studies focusing on effects on the immune system
following oral exposure are listed in Table 1.
In the only study involving repeated inhalation exposure that
reported effects in the respiratory tract, rats (10 males and 10
females per dose) were exposed in "nose-only" chambers for 4 h to TBTO
doses of 0, 0.03 (vapour), 0.16 (vapour), or 2.8 (aerosol) mg/m3, 5
days/week, for a total of 21-24 treatments (Schweinfurth & Gunzel,
1987). At the highest dose, severe toxic effects were produced.
Mortality was 5/10 in males and 6/10 in females. In addition,
inflammatory reactions (not further specified) in the total
respiratory tract and histological changes (not further specified) in
the lymphatic organs were observed. No local or systemic changes were
observed at the lower doses. The authors did not, however, report what
end-points were evaluated.
8.4 Long-term exposure
8.4.1 Subchronic exposure
A large number of well-conducted subchronic studies have been
conducted in rats focusing on toxicity to the immune system. These
studies and their NOAELs and lowest-observed-adverse-effect levels
(LOAELs) are listed in Table 1 and summarized in section 8.7.
Effects of TBTO (purity 96%) on haematology and serum chemistry
were assessed in groups of three and four adult male cynomolgus
monkeys that ingested doses of 0 or 0.160 mg/kg body weight per day,
respectively, 6 days/week for 22 weeks (0 and 0.14 mg/kg body weight
per day, actual intake) (Karrer et al., 1992). The TBTO was dissolved
in vegetable oil and added to Tween 80-augmented pear juice, which the
monkeys drank. Study end-points consisted of clinical observations,
body weight, and standard haematology and clinical chemistry indices,
including serum immunoglobulin (IgM and IgG) levels. A progressive
decrease in total leukocyte counts occurred during the first 10 weeks
of exposure (significantly [ P < 0.05] lower than controls at weeks
8 and 10; 67% of control value at week 10). Leukocytes subsequently
increased and were similar to controls between weeks 10 and 16, but
decreased again between weeks 16 and 20 (61.5% of control value at
week 20; P < 0.05). No significant alterations in differential
leukocyte count, serum immunoglobulins, or other study parameters were
observed. Based on decreased total leukocyte levels, 0.14 mg/kg body
weight per day (the only dose tested) is a LOAEL in monkeys.
8.4.2 Chronic exposure and carcinogenicity
Well-conducted studies are available in rats and mice. A study in
dogs (Schuh, 1992) is fatally flawed and is not reported. Long-term
studies assessing effects on the immune system are reported in section
8.7 and listed in Table 1.
In a chronic toxicity/carcinogenicity study, groups of 60 male
and 60 female Wistar rats were exposed to dietary TBTO (0.5, 5, and 50
mg/kg diet) for 2 years (Wester et al., 1987, 1988, 1990). Based on
estimates of average body weight and food consumption from reported
data, ingested dosages were approximately 0.019, 0.19, or 2.1 mg/kg
body weight per day in males and 0.025, 0.25, or 2.5 mg/kg body weight
per day in females. End-points that were evaluated included clinical
abnormalities, survival, body weight, food and water consumption, and
the incidence of neoplastic lesions. Haematology, urinalysis, clinical
chemistry (including immunoglobulins IgG, IgM, and IgA), and
endocrinology (total thyroxine and free thyroxine, thyrotropin,
luteinizing hormone, follicle-stimulating hormone, insulin) were
evaluated in 10 rats per sex per dose after approximately 3, 12, and
24 months (endocrinology not assessed at 3 months). Organ weights and
histology were evaluated in 10 rats per sex per dose after 12 and 24
months, and histology was also evaluated in all moribund rats as well
as rats surviving until 24 months.
No treatment-related adverse changes were found in males or
females at the lowest dose. Serum immunoglobulin levels were
significantly ( P < 0.05, Student's t-test) increased in the
high-dose group. Concentrations of IgA were increased in both sexes
after 12 and 24 months; at 24 months, levels of IgA were 508% of the
control value in males ( P < 0.001) and 294% of the control value in
females ( P < 0.01). Concentrations of IgG were significantly
( P < 0.01) reduced in females after 3 months (42% of the standard
serum value compared with 69-71% in controls and other treated groups)
and 12 months (80% compared with 124-127%), but not after 24 months or
in males. Concentrations of IgM were increased in both sexes after 3,
12, and 24 months; at 24 months, the IgM level was 258% of the
standard serum value in males ( P < 0.01) and 240% of the standard
value in females ( P < 0.01).
Other effects occurred predominantly in high-dose rats, including
decreased survival, decreased body weight, and changes in organ
weights. At termination, survival in females in the high-dose group
was 54% versus 74% in controls; survival in males in the high-dose
group was 40% versus 60% in controls. Terminal body weights at this
dose were approximately 13% (male) and 9% (female) lower than
controls. Absolute liver, kidney, adrenal gland (male only), and heart
(male only) weights were increased and thyroid weight (female only)
was decreased in high-dose rats at study termination; relative organ
weights were not reported. The liver weight was increased 36% and 29%
in males and females, respectively; the kidney weight was increased
29% and 33% in males and females, respectively; the adrenal weight in
males and females was increased 630% and 44%, respectively; the heart
weight in males was increased 13%; and the thyroid weight in females
was decreased 26%.
Treatment-related non-neoplastic histological changes occurred in
the liver, spleen, and thyroid of high-dose males and females.
Histological effects after 12 months included slight bile duct changes
(characterized by hyperplasia, cellular hypertrophy, and minimal
infiltration of mononuclear cells or by cholangiofibrosis), decreased
haemosiderin content in spleen (qualitative analysis only), and
decreased thyroid follicular epithelial cell height. Examination after
24 months showed that only the histological changes in the thyroid
persisted. There were no accompanying significant changes in
concentrations of serum thyroid hormones. The incidence and severity
of age-related degenerative changes in the kidney (nephrosis and
vacuolation and pigmentation of the proximal tubular epithelium,
suggestive of iron and/or lipofuscin) were increased in high-dose
males and females after 24 months.
Neoplastic lesions were examined in the control and high-dose
groups; if differences were observed, the intermediate-dose groups
were also examined for those tumour types. Increased incidences of
benign pituitary tumours, pheochromocytomas in the adrenal medulla,
and parathyroid adenomas were noted. These data are shown in Table 2.
There are increases in the incidence of some benign spontaneous
tumours at the high dose in some endocrine tissues. According to the
authors, these tumours normally occur in this strain of rats with high
and variable background incidence (Kroes et al., 1981; Wester et al.,
1985). In the two data sets available for these tumour types in the
strain of Wistar rats used by Wester et al. (1990), the reported
background occurrence of pituitary tumours (adenomas plus carcinomas)
in females was 52% and 55% and in males was 34% and 87%; the reported
background incidence of pheochromocytomas (benign plus malignant) in
females was 8% and 16% and in males was 22% and 58%. The authors
reported no data on the background occurrence of parathyroid tumours.
There was no significant endocrine imbalance documented in the
study. No significant change was observed in the serum levels of
thyroid-stimulating hormone, luteinizing hormone, follicle-stimulating
hormone, insulin, total thyroxine, or free thyroxine. There was,
however, a decrease in the free thyroxine: total thyroxine ratio for
both sexes at 12 and 24 months in the high-dose group and after 12
months in the mid-dose group. Although the pituitary tumours stained
for the presence of prolactin, there was no correlation between the
serum level of prolactin or the occurrence of hyperplastic or
neoplastic mammary tissue and the presence of pituitary tumour.
Based on the constellation of changes (increased mortality,
increased serum immunoglobulins, changes in organ weight, and
histopathological changes) observed at the highest dose, the LOAEL is
2.1 mg/kg body weight per day and the NOAEL is 0.19 mg/kg body weight
per day (Wester et al., 1987, 1988, 1990).
TBTO (purity 97.1%) was fed to groups of 50 male and 50 female
CD-1 mice in dietary concentrations of 0, 5, 25, or 50 mg/kg for 18
months in a study primarily designed to assess carcinogenicity (Daly,
1992). Based on food consumption and body weight data, mean compound
intake was reported to be 0, 0.7, 3.7, or 7.7 mg/kg body weight per
day in males and 0, 0.9, 4.8, or 9.2 mg/kg body weight per day in
females.
Statistically significant decreases in survival occurred in
treated mice of both sexes. In males, survival after 18 months was 67,
52, 42, and 42% in the control, low-, mid-, and high-dose groups,
respectively ( P < 0.05, all doses). Survival in females at 18
months was 59, 48, 40, and 27% in the control, low-, mid-, and
high-dose groups, respectively ( P < 0.05 except for low-dose
group). No information on cause of death was available. Other
treatment-related effects included significantly decreased food
consumption and increased absolute and relative liver weights in
females at the high dose. Incidences of gross liver enlargement and
discoloration were slightly increased in both sexes in all dose
groups. The gross liver changes are not considered biologically
significant because of the slight changes and absence of hepatic
histopathological alterations. Increased incidences of common
spontaneous non-neoplastic lesions, particularly
glomerular/interstitial amyloidosis of the kidney, were found.
Incidences of renal amyloidosis were increased in females in all dose
groups (50, 67.7, and 78.4%, respectively, compared with 34.8% in
controls) but not in males. The progression of this lesion appeared to
be more rapid in both sexes at the two highest doses, indicating a
compound-related effect. There were no statistically significant
increases in the incidence of any tumours or groups of tumours in
males or females. TBTO was not carcinogenic in this study in mice.
This study identified an effect level for mortality at 0.7 mg/kg body
weight per day (the lowest dose tested) (Daly, 1992).
Table 2: Neoplastic lesions in rats.a
Incidence of Incidence of Incidence of
pituitary tumoursb adrenal parathyroid
pheochromocytomasb adenomas
Concentration Female Male Female Male Female Male
of TBTO
(mg/kg diet)
0 22/50 34/50 3/50 16/50 0/64 0/39
0.5 32/50* 39/50* 3/50 13/50 0/4 42/50
5 22/50 29/50 3/50 14/50 1/40 1/51
50 35/50** 43/50*** 34/50*** 33/50*** 1/44 6/43c
a From Wester et al. (1990).
b Statistical analysis was carried out according to Peto et al. (1980), one tailed. Values marked
with asterisks differ significantly from the corresponding control values (* P < 0.05; ** P < 0.01;
*** P < 0.001).
c The value differs significantly (chi-square test) from the corresponding control (P < 0.01).
8.5 Genotoxicity and related end-points
The genetic effects of TBTO were evaluated in multiple in vivo
and in vitro short-term tests (Davis et al., 1987). TBTO was not
mutagenic in the rec assay in Bacillus subtilis, did not induce
reverse mutations in Klebsiella pneumoniae, and did not produce
point mutations in Salmonella typhimurium strains TA1530, TA1535,
TA1538, TA97, TA98, or TA100 in the presence or absence of a rat liver
activation system (Davis et al., 1987). TBTO was mutagenic in
S. typhimurium strain TA100 in a fluctuation test, but only in the
presence of rat liver S9 (Aroclor-induced) and at cytotoxic
concentrations. TBTO did not induce gene mutations in
Schizosaccharomyces pombe, mitotic gene conversions in
Saccharomyces cerevisiae, or sister chromatid exchange in Chinese
hamster ovary cells in the presence or absence of rat or mouse liver
S9. Structural chromosomal aberrations, endoreduplicated and polyploid
cells, were observed in Chinese hamster ovary cells. The aberrations
were observed only at 8 h after treatment (not at 15 or 24 h) and only
at the highest concentration tested in the presence of S9.
Cytotoxicity was also observed at this concentration. TBTO did not
induce gene mutations in V79 Chinese hamster cells or in mouse
lymphoma cells. It did not induce recessive lethal mutations in adult
male Drosophila melanogaster by either feeding or injection. Doses
of 0.37 or 0.74 mmol/litre did not increase the number of X-linked
recessive mutations. An increased number of micronuclei was observed
in polychromatic erythrocytes of male BALB/c mice 48 h after a single
oral dose of TBTO (60 mg/kg body weight). A reanalysis of the slides
from the high-dose group, however, failed to confirm the increase in
micronuclei (IPCS, 1990). A lower dose (30 mg/kg body weight) was
ineffective. Neither dose induced micronuclei 30 h after treatment.
One report demonstrated that TBTO and triphenyltin chloride are
co-clastogens in a whole mammalian system (Yamada & Sasaki, 1993). The
frequency of micronuclei induced by mitomycin C in mouse peripheral
reticulocytes was enhanced approximately 50% when 50 mg TBTO/kg body
weight and 100 mg triphenyltin chloride/kg body weight were given
orally to mice. No effect was observed when the chemicals were
administered separately.
In aggregate, despite the limited positive findings at cytotoxic
concentrations, the weight of evidence shows that TBTO is not
genotoxic. This conclusion remains consistent with the previous
evaluation by IPCS (1990).
8.6 Reproductive and developmental toxicity
Several well-conducted studies are available that investigated
the effects of TBTO on the reproductive system and fetal development
in rats and mice. The results of these studies are listed in Table 1.
These studies show no evidence that TBTO is a significant reproductive
or developmental toxicant in rodents. The developmental effects noted
in the various studies occur at or near the exposure that also causes
maternal toxicity (depressed body weight or impaired weight gain
during pregnancy). The LOAELs for maternal toxicity in rats and mice
are approximately 10 mg/kg body weight per day, with NOAELs of
approximately 5 mg/kg body weight per day.
8.7 Immunological and neurological effects
8.7.1 Immunotoxicity
A large number of well-conducted studies have shown that TBTO
causes depression of immune functions dependent on the thymus. Results
from a number of short-term studies are listed in Table 1. Subchronic
and chronic studies (Vos et al., 1990) are summarized in detail below
and are listed in Table 1. The chronic study conducted by Vos et al.
(1990) shows effects on thymus-dependent immune responses at a dose
lower than that at which any other toxic effects have been observed.
The Vos et al. (1990) study also establishes that weanling animals are
more sensitive than adults to the effects of TBTO. For example,
following subchronic exposure, the LOAEL in weanling rats was 0.25
mg/kg body weight per day, whereas the LOAEL in aged rats was 2.5
mg/kg body weight per day. The NOAELs were 0.025 and 0.25 mg/kg body
weight per day, respectively. Data from Buckiova et al. (1992) and
Smialowicz et al. (1989) also show that exposure of mice in utero
and exposure of rat pups prior to weaning cause effects at exposures
lower than those required for the same effects in adult animals.
In a subchronic immunotoxicity study (Vos et al., 1990; a
companion to the chronic study summarized below), aged (1-year-old)
male Wistar rats were exposed for 5 months to the same diets used in
the principal study. Based on the authors' statement from the chronic
study (see below), estimated compound intake was 0, 0.025, 0.25, or
2.5 mg/kg body weight per day. End-points were the same as some of
those evaluated in the chronic study.
Compound-related effects occurred only in the high-dose group and
consisted of significantly decreased thymus weight (39% lower than
controls, P < 0.01), impaired resistance to Trichinella spiralis
(indicated by increased recovery of adult worms from the small
intestine [780% higher than controls; P < 0.01] and number of
larvae in muscle [80% higher; P < 0.001]), and impaired resistance
to Listeria monocytogenes (indicated by approximately 300% increased
splenic bacterial count; P < 0.05).
Subchronic and chronic immunotoxicity studies were conducted in
which weanling SPF-derived Riv:TOX Wistar rats were fed TBTO (purity
95.3%) at concentrations of 0, 0.5, 5, or 50 mg/kg. Male rats (females
not tested) were evaluated following exposure to TBTO for up to 18
months (Krajnc et al., 1987; Vos et al., 1990). The authors reported
the 5 mg/kg dietary concentration to be equivalent to a dose of 0.25
mg/kg body weight per day, indicating that estimated test doses were
0.025, 0.25, and 2.5 mg/kg body weight per day. Body weight, absolute
thymus weight, and absolute spleen weight were measured in groups of
18, 12, and 12 rats, respectively, following exposure for 4.5 months.
Immunological function studies for specific and non-specific
resistance were performed in 9-12 rats per group after 4-6 or 15-17
months of exposure. Antigen-specific functional assays evaluated IgM
and IgG responses to sheep red blood cells (immunized after
16 months); IgM and IgG responses to ovalbumin and delayed-type
hypersensitivity (24-, 48-, and 72-h) responses to ovalbumin and
Mycobacterium tuberculosis (immunized after 6 or 15 months of
exposure); and resistance to oral infection by T. spiralis larvae
(infected after 5.5 or 16.5 months).
Non-specific resistance was assessed by splenic clearance of
intravenously injected L. monocytogenes bacteria (after 5 or 17
months of exposure) and natural cell-mediated cytotoxicity of spleen
cells (after 4.5 or 16 months of exposure) and peritoneal cells (after
4.5 months of exposure only) using a 4-h 51Cr-release assay with
YAC-lymphoma target cells. Non-specific end-points included the
numbers of viable nucleated thymus and spleen cells and responses of
thymus and spleen cells to T-cell and/or B-cell mitogens
(phytohaemagglutinin, concanavalin A, pokeweed mitogen, and/or
Escherichia coli lipopolysaccharide) after exposure for 4.5 months
(thymus and spleen) or 16 months (spleen only); and numbers of viable
nucleated mesenteric lymph node cells with cell surface marker
analysis (after 6 and 18 months of exposure; low-dose group not tested
in this assay).
No significant effects were observed in the IgM or IgG responses
to sheep red blood cells, the IgM or IgG responses to T. spiralis,
the IgM or IgG responses to ovalbumin, or the delayed-type
hypersensitivity responses to ovalbumin and M. tuberculosis.
Thymus weight was significantly reduced in the high-dose group
(17% lower than controls, P < 0.05), although the response of
thymocytes to T-cell mitogens was unaltered. No significant
alterations in spleen weight, response of spleen cells to T- and
B-cell mitogens, or body weight were found at any dose. Statistically
significant changes occurred in the percentage of mesenteric lymph
node T-lymphocytes in the high-dose group (20% lower than controls
after 18 months of exposure) and B-lymphocytes in the mid-dose (60%
higher than controls after 18 months) and high-dose (48% higher than
controls after 18 months) groups; however, the absolute number of
T-lymphocytes and B-lymphocytes per lymph node was not significantly
altered. The low-dose group was not tested with these assays. The
B-cell increase was an increase in the percentage of B-cells, but the
interpretation of these data is equivocal, because they are
counter-intuitive when viewed in context with the other effects,
especially the IgE titres.
In vivo clearance of injected L. monocytogenes was impaired
in rats exposed to the high dose for 17 months, as shown by the
approximately sevenfold increase in number of viable bacteria per
spleen, indicating that macrophage function was reduced. Resistance to
infection by T. spiralis was suppressed in rats exposed to the mid
or high dose, as shown by significantly reduced serum IgE titres (50
and 47% lower than controls after 16.5 months of exposure), increased
numbers of larvae in muscle 42 days after infection (56% and 306%
higher than controls after 16.5 months), and moderately reduced
inflammatory reaction around cysts in parasitized musculature
(qualitative assessment only).
There was no significant reduction in the activity of natural
killer cells isolated from the peritoneal cavity following exposure of
weanling or aged (1-year-old) rats to TBTO for 4.5 months. Also, there
was no significant reduction in the activity of natural killer cells
isolated from the spleen following exposure of weanling rats for 4.5
months. In contrast, the activity of natural killer cells isolated
from the spleen was suppressed when weanling rats were exposed to all
doses of TBTO for 16 months (31, 25, and 36% lower than controls,
respectively, at an effector to target cell ratio of 100, and 32, 18,
and 30% lower, respectively, at an effector to target cell ratio of
50). Based on these data, the effect did not progress significantly
with dose. Because the authors considered these data equivocal in this
experiment and because there was no clear treatment-related effect,
the suppression of natural killer cell activity in this study is not
considered biologically significant.
Essentially identical results on the immune system were observed
when weanling rats were exposed for 4.5 or 16.5 months. Based on the
depression of IgE titres and an increase in T. spiralis larvae in
muscle, the LOAEL for immunotoxicity is 0.25 mg/kg body weight per
day. The NOAEL is 0.025 mg/kg body weight per day (Krajnc et al.,
1987; Vos et al., 1990).
Some recent studies suggest that the mechanism of the immunotoxic
effects is related to induction of apoptosis (programmed cell death)
within the thymus. Raffray & Cohen (1991) demonstrated that thymocytes
in culture showed cellular changes consistent with apoptosis at
concentrations of TBTO that did not affect cell viability. Raffray et
al. (1993) showed that these effects occur independently of a
requirement for protein synthesis and do not require fully conserved
energetics (i.e., the effects occur despite depression of ATP levels
to less than 20% of control values). Raffray & Cohen (1993)
demonstrated a correlation between reduction of thymus weight in
animals given a single oral dose of TBTO and evidence of apoptosis
(increased DNA fragmentation) in thymic cell isolates (principally
thymocytes) isolated from the animals during the period of thymic
involution. These workers also showed that dibutyltin, the major
metabolite of tributyltin, is less effective in inducing apoptosis
in vitro, suggesting that the in vivo toxicity is directly
attributable to tributyltin.
A study comparing immunotoxic effects in pre-weanlings and adult
rats shows that some responses of the developing immune system are
more sensitive to TBTO (Smialowicz et al., 1989). Adult (9 weeks old)
male Fischer rats or pre-weanling (3-24 days old) rats were dosed by
oral gavage 3 times per week for a total of 10 doses. The adults were
dosed with 5, 10, or 20 mg/kg body weight per dose; the pre-weanlings
were dosed with 2.5, 5, or 10 mg/kg body weight per dose. Reductions
in mitogen responses were observed in adults at 10 and 20 mg/kg body
weight and in pre-weanlings at 5 and 10 mg/kg body weight. The mixed
lymphocyte reaction was suppressed in adults at 20 mg/kg body weight
and in pre-weanlings at 10 mg/kg body weight. Finally, natural killer
cell activity was suppressed only in pre-weanlings at 10 mg/kg body
weight. In this study, the lowest LOAEL is 5 mg/kg body weight per
day, and the lowest NOAEL is 2.5 mg/kg body weight per day.
Pregnant ICR mice were treated with TBTO in Tween
80:ethanol:saline (1:2:97) by gavage at 0.1 mg/kg body weight per day
on gestation days 4-17 or 11-17 (Buckiova et al., 1992). Humoral and
cell-mediated immune responses in offspring were assessed 4 and
8 weeks after birth. At 0.1 mg/kg body weight per day, the only dose
tested, effects in the offspring included suppressed primary antibody
responses to sheep red blood cells, ovalbumin, and lipopolysaccharide
and increased number of leukocytes. Suppressed delayed-type
hypersensitivity to sheep red blood cells and unspecified alterations
in polyclonal proliferative responses of thymocytes and splenocytes
were also observed. The significance of the LOAEL (0.1 mg/kg body
weight per day), however, is unclear, because a full publication of
the results is not available.
8.7.2 Neurotoxicity
Triethyltin and trimethyltin compounds have been shown to cause
severe neurotoxicity (for a summary, see Boyer, 1989). Triethyltin
causes interstitial oedema throughout the white matter in the spinal
cord and various regions of the brain; less marked damage occurs in
the peripheral nervous system. Trimethyltin also causes severe and
permanent damage to the central nervous system. In this case, however,
the effect is neuronal necrosis, rather than oedema. TBTO, in
contrast, causes no severe neurological signs or morphological or
histopathological changes in brain tissue. In a 4-week study, rats fed
a dietary concentration of 320 mg/kg (equivalent to 30 mg/kg body
weight per day) exhibited ptosis or enophthalmia and slight ataxia
(Krajnc et al., 1984). One chronic study in dogs (Schuh, 1992) also
gave a slight suggestion of neurotoxicity (atactic gait and apathy).
As noted above, however, this study is significantly flawed.
Crofton et al. (1989) measured brain weight and motor activity in
developmental studies. There was some suggestion of neurotoxicity
(based on decreased brain weight in pups) at exposures in excess of 10
mg/kg body weight per day, but no reported effects at 5 mg/kg body
weight per day.
Organotin compounds, including tributyltin, have recently been
shown to induce apoptosis in immortalized neuronal cell lines
(Thompson et al., 1996) and in pheochromocytoma PC12 cells (Viviani et
al., 1995). Although TBTO induces apoptosis in neural cells in
vitro, it does not cause neurotoxicity in whole animals.
Although the potential for neurotoxicity has not been completely
investigated with focused studies, there is no suggestion that
neurotoxicity is likely a critical or co-critical effect.
9. EFFECTS ON HUMANS
No information was located regarding the toxicity of TBTO in
humans following long-term exposure. Human data summarized by Boyer
(1989) suggest that TBTO is a potent non-allergenic dermal irritant.
There are several case reports claiming irritation of the respiratory
tract following acute inhalation exposure of people to TBTO (Anon.,
1991; Hay & Singer, 1991; Shelton et al., 1992; Wax & Dockstader,
1995). None of these reports, however, contains sufficient information
to characterize the exposure-response relationship for the reported
effects.
No epidemiological studies on TBTO were located in the
literature.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
Tributyltin in the environment is very toxic to most taxa (see
data cited in IPCS, 1990). Bivalves and gastropods are especially
sensitive, and larval stages are more vulnerable than adults. The
lowest reported effect concentrations for tributyltin are 2.4-4.8
ng/litre for induction of shell deformities in Pacific oyster and
imposex1 development in dogwhelk ( Nucella lapillus). Other low
toxicity values reported are no-observed-effect concentrations (NOECs)
of 80 ng/litre for Daphnia magna, 40 ng/litre for reduced viability
of mussel ( Mytilus edulis) larvae, and 10 ng/litre for reduced
growth of hard-shell clams ( Mercenaria mercenaria) and reduced egg
production in a marine copepod ( Acartia tonsa).
In the field, mainly the effects on oysters and prosobranchs have
been studied. Most studies on imposex have examined populations of
N. lapillus. It has been suggested that other prosobranchs are even
more sensitive, and other species have been suggested as indicator
species (Matthiessen & Gibbs, 1998).
Matthiessen & Gibbs (1998) reviewed the evidence that
TBTO-induced imposex and intersex in molluscs are the result of
endocrine disruption. The effect is most likely the result of elevated
testosterone titres that masculinize tributyltin-exposed females. The
precise mechanism has not been fully described, but the weight of
evidence suggests that TBTO acts as a competitive inhibitor of
cytochrome P-450-mediated aromatase, leading to increased testosterone
levels. Additional support for this mechanism has been presented by
Bettin et al. (1996). Testosterone addition (500 ng/litre) induces
faster and more intensive imposex development in N. lapillus than
that induced by tributyltin. Simultaneous exposure to tributyltin and
to the antiandrogen cyproterone acetate suppresses imposex development
completely in N. lapillus and reduces it in Hinia reticulata.
Furthermore, tributyltin-induced imposex development can be suppressed
by adding estrogens. Inhibition of the cytochrome P-450-dependent
aromatase using SH 489 (1-methyl-1,4-androstadiene-3,17-dione) and
flavone induces development of imposex. Some recent data suggest that
TBTO may also inhibit the formation of sulfur conjugates of
testosterone and its active metabolites, thus interfering with its
excretion.
There is a vast literature on the environmental effects of
tributyltin. Most of the information below was condensed from IPCS
(1990). Additional data published since this evaluation have also been
included.
1 Imposex is the development of male characteristics by female
gastropods.
10.1 Aquatic environment
As tributyltin is used commercially to control bacteria and
fungi, the substance is toxic to these taxa. The reported minimum
inhibitory concentrations (MICs) range from 20 to 300 000 µg/litre.
The MIC for sludge from municipal sewage treatment plants was reported
as 25 µg/litre (IPCS, 1990). A recent study conducted in yeast
suggests that the target for TBTO action is the mitochondrial ATPase
(Veiga et al., 1997). TBTO reduced the respiratory capacity when
vanillic or benzoic acid was the energy source. The ATP level of the
cell was severely affected at a concentration of 1.19 mg/litre. The
mitochondrial ATPase was strongly inhibited at a concentration of 0.3
mg/litre, whereas the activity of the plasma membrane ATPase was not
affected by a concentration up to 17.9 mg/litre.
In the laboratory, effective concentrations for freshwater algae
ranged from 5 µg/litre (4-h IC50 for growth, Ankistrodesmus
falcatus) to 64 µg/litre (96-h EC50, Scenedesmus pannonicus). A
4-h IC50 for primary production of 3 µg/litre was reported for a
natural community from Lake Ontario (IPCS, 1990). For marine and
estuarine algae, most reported IC50 or EC50/LC50 values range from
0.1 to 15 µg/litre (IPCS, 1990). For motile spores of the green
macroalga Enteromorpha intestinalis, a 5-day EC50 of 0.001 µg/litre
for spore development and inhibition of settling was indicated (IPCS,
1990). Effects on community metabolism and nutrient dynamics in
bladderwrack ( Fucus vesiculosus) have been shown at 0.6 µg
tributyltin/litre and above (Lindblad et al., 1989). Studies on pure
cultures of marine algae show that these organisms do not adapt to
tributyltin; the same EC50 values were obtained for cultures exposed
for 12 weeks as for naive cells (IPCS, 1990).
For Lemna and Elodea species, reduction in growth was
observed from 0.06 µg/litre following 10 days of exposure to TBTO. For
the angiosperm Zostera marina, a NOEC of 0.1 mg/kg sediment was
reported. The lethal concentration for the salt-marsh species Aster
tripholium was 10 µg/kg mud (dry weight) (IPCS, 1990).
For Daphnia magna, the 48-h LC50 was 2.3 µg/litre; the NOEC
has been estimated to be 0.5 µg/litre based on reversal of normal
response to light (IPCS, 1990). The reported long-term toxicity value
(21-day NOEC) for Daphnia magna is 0.19 µg/litre; the 96-h LC50 for
Tubifex tubifex is 0.1 µg/litre (Fargasova, 1997).
For target snail adults, the 24-h LC50 was 30-400 µg/litre. The
sensitivity of snails decreases with age, but eggs are more resistant
than both young and adults. The lowest-observed-effect concentration
(LOEC) for reproduction for Biomphalaria and Bulinus is 0.001
µg/litre; the long-term NOEL for Lymnaea stagnalis is 0.32 µg/litre
(IPCS, 1990).
Several field studies on the effects of tributyltin used as a
molluscicide for schistosomiasis control in tropical areas have been
reported (IPCS, 1990). For schistosome larvae in the aquatic stages,
the LC50 was calculated to be 16.8 µg/litre for a 1-h exposure. The
dose causing 99-100% suppression of cercarial infectivity of mice was
between 2 and 6 µg/litre (IPCS, 1990).
Among marine aquatic invertebrates, larval stages are
considerably more sensitive than adults. For example, the 48-h LC50
for the Pacific oyster is 1.6 µg/litre for larvae and 1800 µg/litre
for adults; for the mussel M. edulis, the same values are 23 and 300
µg/litre, respectively. The larvae of brown shrimp ( Crangon crangon)
are also more sensitive than adults, the 96-h LC50s being 1.5 and 41
µg/litre, respectively (IPCS, 1990).
For subadults of the copepod Eurytemora affinis, the 72-h LC50
was reported as 0.6 µg/litre. For the mysid shrimp ( Acanthomysis
sculpta), the 96-h LC50 was reported as 0.41 µg/litre. For larvae
of the lugworm ( Arenicola cristata), the 96-h LC100 was reported as
4 µg/litre (IPCS, 1990).
The 144-day EC50 for morbidity and mortality of the copepod
Acartia tonsa was 0.4 µg/litre (IPCS, 1990). A 6-day NOEC and LOEC
for A. tonsa have been reported as 0.011 and 0.023 µg/litre,
respectively (Kusk & Peterson, 1997). Concentrations of 5-10 µg/litre
killed all larvae of lobster ( Homarus americanus) within 5-6 days,
and metamorphosis was affected at 1 µg/litre (IPCS, 1990). A 15-day
LC50 of mussel ( M. edulis) larvae has been reported as
approximately 0.1 µg/litre (IPCS, 1990).
A 22-day LC100 for adult polychaetes Nereis diversicolor was 4
µg/litre (IPCS, 1990). In another polychaete,
Sabellastarte sanctijosephi, mortality occurred at 0.04-1.0 µg/litre
(Langston, 1995).
Deformities in regenerated limbs of fiddler crab ( Uca
pugilator) were observed at 0.5 µg/litre and above. Inhibition of
the regeneration of arms of brittle star ( Ophioderma brevispina) was
observed at 0.1 and 0.5 µg/litre (IPCS, 1990).
It has been suggested that TBTO inhibits calcification of Pacific
oysters below 2 ng tin/litre (Alzieu, 1991; Langston, 1995). These
effects have also been observed in the field. In the early 1980s, a
good correlation was found between field observations of occurrence of
shell thickening and proximity of ports with large numbers of boats
(IPCS, 1990).
Reduced growth of Pacific oyster spat has been shown at all
concentrations above 20 ng TBTO/litre (IPCS, 1990). Recently
metamorphosed European oysters ( Ostrea edulis) showed a severe
reduction in growth rate over 10 days in 0.06 µg/litre. In spats of
C. gigas, M. edulis, and carpet shell ( Venerupis ducussata),
growth was reduced at 0.24 µg/litre over 45 days. For adult mussels
( M. edulis), shell length was reduced at 0.31 µg/litre following 66
days of exposure, and juvenile growth was reduced at 0.07 µg/litre. In
a study of hard-shell clam ( Mercenaria mercenaria) exposed from
fertilization to metamorphosis (approximately 14 days), growth was
reduced at 10 ng/litre and above.
In the laboratory, all female dogwhelks exposed to TBTO showed
imposex development at 1-2 ng tin/litre and above (IPCS, 1990). Some
of the females retained their breeding capacity at the lowest
concentration, but virtually all females were sterilized at 3-5 ng
tin/litre. From field observations, the NOEL has been set at less than
1 ng tributyltin/litre. Imposex has been observed in a number of other
species in the field. These include Ocenebra erinacea,
Ocinebrina aciculata, Hexaplex trunculus, Buccinum undatum,
Littorina littorea, and Nassarius reticulatus (Oehlmann et al.,
1996; Matthiessen & Gibbs, 1998).
In oysters ( O. edulis), severe effects on reproduction occurred
at 0.24 and 2.6 µg/litre; no larvae were released, gonads were
undifferentiated, and no females developed (IPCS, 1990). At 0.01
µg/litre, egg production in exposed A. tonsa was significantly
reduced (IPCS, 1990).
No effect on survival of grass shrimp ( Palaemonetes pugio) was
found after 96 h of exposure at 1 or 10 mg tributyltin/kg sediment,
but exposure via water alone resulted in a 96-h LC50 of 20 µg/litre
(IPCS, 1990). In sediments containing tributyltin, an LC50 of 1-10
mg/kg sediment was determined for Amphioxus (IPCS, 1990). No effects
on survival of the mole crab ( Emerita talpoida) were observed
following 7 days of exposure at 10 µg/litre seawater and 4.5 mg/kg
sand (IPCS, 1990). No mortality was observed in mysid shrimp
( Acanthomysis sculpta), worms ( Neanthes arenaceodentata), or clams
( Macoma nasuta) exposed to concentrations in sediment of 155-610
µg/kg and concentrations in overlaying water of 0.2 µg/litre over
10-20 days (IPCS, 1990).
The reported short-term LC50 values for TBTO in freshwater fish
obtained under static conditions range from 13 to 240 µg/litre (IPCS,
1990). The NOEL for the guppy ( Poecilia reticulata) was estimated to
be 0.01 µg/litre based on thymus atrophy, liver vacuolation, and
hyperplasia of the haematopoietic tissue (Wester & Canton, 1987).
The toxicity of TBTO to marine fish is highly variable; 96-h
LC50 values range between 1.5 and 36 µg/litre, with larval stages
being more sensitive than adults (IPCS, 1990). Data have been reported
for bleak ( Alburnus alburnus), sole ( Solea solea), armed bullhead
( Agonus cataphractus), girella ( Girella punctata), salt water goby
( Chasmichthys dolichograthus), and chinook salmon ( Oncorhynchus
tshawytscha). There are indications that marine fish avoid TBTO
concentrations of 1 µg/litre or more (IPCS, 1990). A recent study in
flounder ( Platichthys flesus) showed that TBTO at 17.3 µg/litre
caused mortality after 7-12 days, decreased the condition factor,
resulted in gill lesions, and induced significant reduction of
non-specific resistance. However, no marked effects on the relative
thymus volume or on the specific immune system were noted (Grinwis et
al., 1997).
Japanese medaka ( Oryzias latipes) fed daily for 3 weeks with
food containing tributyltin, polychlorinated biphenyls, or a
combination of the two at 1 mg/kg body weight showed slight
synergistic effects on reproduction, resulting in reduced spawning
frequency, number of eggs, and proportion of fertile eggs (Oshima et
al., 1998).
No effect on survival was found when eggs and larvae of frog
( Rana temporaria) were exposed to TBTO concentrations of 3 µg/litre
or less; at 30 µg/litre, however, significant mortality was observed
(IPCS, 1990).
10.2 Terrestrial environment
Although the exposure of terrestrial organisms to tributyltin
results primarily from its use as a wood preservative, tributyltin
compounds are toxic to insects exposed topically or via feeding on
treated wood (IPCS, 1990). The LD50 values for tributyltin compounds
applied topically to the thorax of newly emerged insects range from
0.48% to 0.72% (dilutions with acetone) for the house fly
( Musca domestica), from 0.29% to 0.69% for the mosquito
( Anophelese stephensi), and from 0.52% to 0.87% for the cotton
stainer ( Dysdercus cingulatus). TBTO is toxic to honey bees
( Apis mellifera) housed in hives made from TBTO-treated wood
(1.9 kg/m3). TBTO is toxic to bats ( Pipistrellus pipistrellus)
housed in roosting cages treated with TBTO, but this result was not
statistically significant, owing to high mortality in controls. The
acute toxicity of TBTO to wild mice (deer mice
[ Peromyscus maniculatus] and house mice [ Mus musculus]) is
moderate. The estimated dietary LC50 value, based on consumption of
treated seeds used in repellency tests, is 200 mg/kg diet per day.
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
A large number of studies have been conducted showing that TBTO
causes depression of immune functions dependent on the thymus. These
effects occur at doses lower than those that cause other toxicity (see
Table 1). Accordingly, the critical effect for TBTO is immunotoxicity.
Based on the study of Vos et al. (1990), the critical effect is
immunosuppression (reduced IgE titres and increase in T. spiralis
larvae in muscle). The LOAEL is 0.25 mg/kg body weight per day, and
the NOAEL is 0.025 mg/kg body weight per day. These values were based
on the authors' report that 5 mg/kg in the diet is equivalent to 0.25
mg/kg body weight per day. This study tested male animals only. Other
studies show no evidence of gender differences in the toxic responses
to TBTO. There is some evidence that a child might be more sensitive
to the toxic effects of TBTO. For example, Smialowicz et al. (1989)
showed that pre-weanling rats were more sensitive than adult rats. In
addition, the principal study (Vos et al., 1990) showed that
immunotoxic effects were observed when weanling rats were dosed for
4.5 or 16.5 months, whereas a companion study (Vos et al., 1990)
showed that these effects were absent or occurred at a higher dose
when adult (1-year-old) rats were dosed for 5 months.
Adequate data are not available to determine a no-effect or
effect level following long-term inhalation exposure. The inhalation
studies that are available document irritation to the respiratory
system. There are no pharmacokinetic studies available with which to
conduct a route-to-route extrapolation for extra-respiratory effects.
TBTO might cause immunosuppression following chronic exposure by
inhalation.
Cancer bioassays following oral exposure have been conducted in
rats and mice. The bioassay in rats shows increases in benign
pituitary tumours, in pheochromocytomas, and in parathyroid tumours at
the highest dose tested. The significance of these tumours, which
normally occur in this strain of rat with variable incidence, is
unclear. The bioassay in mice showed no increase in tumours at any
site. The weight of evidence shows that TBTO is not genotoxic.
11.1.2 Criteria for setting guidance values for TBTO
The no-effect level for immunosuppression (decrease in serum IgE
titre) following long-term oral exposure in rats is 0.025 mg/kg body
weight per day. Benchmark dose analysis shows that the exposure
corresponding to the lower confidence limit (95%) on dose for a 10%
decrease in serum IgE titre is 0.034 mg/kg body weight per day (US
EPA, 1997). Application of uncertainty factors of 10 each for
extrapolation from a laboratory animal species to humans and to
protect sensitive humans gives a guidance value for oral exposure of
0.0003 mg/kg body weight per day (rounded from 0.00025 for the
no-effect level or 0.00034 for the benchmark dose). No appropriate
data are available to develop a guidance value for inhalation exposure
or to estimate cancer risk.
11.1.3 Sample risk characterization
No human data are available to characterize the toxicity of TBTO,
but a wealth of data from oral exposure in laboratory animals is
available. The principal study and a variety of supporting studies
convincingly demonstrate that the critical effect for TBTO is
immunotoxicity. Some evidence indicates that young animals are more
sensitive than adults to the immunotoxic effects. TBTO is not a
reproductive or developmental toxicant. Insufficient data are
available to determine the critical effect for TBTO following exposure
by inhalation. Several case reports document severe irritation of the
human respiratory system following acute inhalation exposure. The
potential human hazard for carcinogenicity for TBTO cannot be
determined. The weight of evidence shows that TBTO is not genotoxic.
Dietary exposure to tributyltin has been assessed in Japan.
Consumption of aquatic organisms is the major route of human exposure.
Data from market basket surveys from 1990 to 1997 estimated the
average daily intake of tributyltin (expressed as tributyltin
chloride) at 3.9 µg/day. Using these data, correcting the exposure
estimate to TBTO by multiplying by the ratio of the molecular weights
(596/325), and assuming a body weight of 50 kg, the estimated daily
exposure to TBTO in Japan is 0.00014 mg/kg body weight per day. This
value is 47% of the guidance value.
Because of the limited and possibly unrepresentative information
on human exposure available to the author and reviewers of the CICAD
and the recent preliminary report (Takahashi et al., 1998) of a
relatively high burden of tributyltin residues in liver resulting,
perhaps, from non-food sources, additional investigation is warranted.
11.2 Evaluation of environmental effects
Because of their physical/chemical properties, tributyltin
compounds concentrate in the surface microlayer and in sediments.
Abiotic degradation does not appear to be a major mechanism of removal
under environmental conditions. Although TBTO is biodegradable in the
water column, this process is not rapid enough to prevent the
occurrence of elevated tributyltin levels in some areas. The half-life
in the water column ranges from a few days to weeks. Tributyltin may
persist in sediment for several years. Bioaccumulation occurs in most
aquatic organisms.
Tributyltin compounds are extremely hazardous to some aquatic
organisms because of their toxicity at very low concentrations in
water. Such concentrations seem to be prevalent in many coastal areas.
Adverse effects on non-target invertebrates, particularly molluscs,
have been reported in field studies, and these have been sufficiently
severe to lead to reproductive failure and population decline. Adverse
effects on the commercial production of shellfish have been
successfully reversed by restrictions on the use of antifouling paints
in some areas, and these restrictions are also leading to the reversal
of imposex effects in gastropod populations. However, the
concentrations of tributyltin measured in some coastal waters are
still above those that induce severe effects in some gastropods. The
effects on farmed fish indicate that tributyltin-containing paints
should not be used on restraining nets.
The general hazard to the terrestrial environment is likely to be
low. Tributyltin-treated wood could pose a hazard to terrestrial
organisms living in close contact with it.
The enhancement of tributyltin concentrations in the surface
microlayer may present a hazard to littoral organisms, neustonic
species (including benthic invertebrate and fish larvae), and
surface-feeding seabirds and wildfowl. Accumulation and low
biodegradation of tributyltin in sediment may pose a hazard to aquatic
organisms when these polluted sediments are disturbed by natural
processes or dredging activities.
The general decline in tributyltin concentrations in the
environment has been attributed to the restrictions placed on the use
of antifouling paints on vessels. However, it should be noted that in
some locations the concentration of tributyltin in the water is above
that necessary to elicit severe adverse effects in some sensitive
species.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Tributyltin compounds were reviewed by the World Health
Organization in 1989 (IPCS, 1990).
Information on international hazard classification and labelling
is included in the International Chemical Safety Card reproduced in
this document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventative and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 1282) reproduced in this
document.
13.1 Human health hazards
Effects on the immune system may be observed following acute or
repeated exposure to tributyltin.
13.2 Advice to physicians
In case of poisoning, treatment is supportive. Following
inhalation of aerosol, symptoms may not be noticeable until a few
hours have passed. Therefore, rest and medical observation are
essential.
13.3 Health surveillance advice
Periodic medical examination of the immune system should be
included in a health surveillance programme.
13.4 Spillage
TBTO is severely irritating to the skin and eyes. In case of
spillage, therefore, emergency crew must wear proper equipment,
including eye protection in combination with breathing protection. The
compound should not be allowed to enter drains or watercourses.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Many countries have restricted the use of TBTO. Information on
national regulations, guidelines, and standards may be obtained from
UNEP Chemicals (IRPTC), Geneva.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
TRIBUTYLTIN OXIDE ICSC: 1282
March 1998
CAS # 56-35-9 Hexabutyldistannoxane
RTECS # JN8750000 Tri-n-butylin oxide
UN # 3020 TBTO
EC # 050-008-00-3 C24H54OSn2
Molecular mass: 596.07
TYPES OF HAZARD/EXPOSURE ACUTE HAZARDS/SYMPTOMS PREVENTION FIRST AID/FIRE FIGHTING
FIRE Combustible NO open flames In case of fire in the
surroundings: all
extinguishing agents allowed.
EXPLOSION
EXPOSURE PREVENT GENERATION
OF MISTS! STRICT
HYGIENE!
Inhalation Abdominal cramps. Cough. Ventilation, local Fresh air, rest.
Diarrhoea. Laboured exhaust, or Half-upright position.
breathing. Nausea. Sore breathing protection Refer for medical
throat. Vomiting. attention.
Symptoms may be delayed
(See Notes).
Skin MAY BE ABSORBED! Redness. Protective gloves. Rinse and then wash skin
After delay skin burns. Protective clothing. with water and soap. Refer
for medical attention.
Eyes Redness. Pain. Safety spectacles, First rinse with plenty of
face shield, or eye water for several minutes
protection in (remove contact lenses if
combination with easily possible), then take
breathing protection. to a doctor.
Ingestion Abdominal cramps. Do not eat, drink or Induce vomiting (ONLY IN
Diarrhoea. Nausea. smoke during work. CONSCIOUS PERSONS!) Give
Vomiting. Wash hands before plenty of water to drink.
eating. Refer for medical attention.
SPILLAGE DISPOSAL PACKAGING & LABELLING
Do NOT wash away into sewer. Carefully collect Severe marine pollutant.
remainder, then remove to safe place. Do NOT let this EU Classification
chemical enter the environment. Chemical protection Symbol: T
suit including self-contained breathing apparatus. R: 21-25-36/38-48/23/25
S: (1/2)-35-36/37/39-45
Note: A
UN Classification
UN Hazard Class: 6.1
UN Pack Group: II
EMERGENCY RESPONSE STORAGE
Transport Emergency Card: TEC (R)-61G43b Provision to contain effluent from fire
extinguishing.
IMPORTANT DATA
PHYSICAL STATE; APPEARANCE: ROUTES OF EXPOSURE:
LIQUID The substance can be absorbed into the
body by inhalation of its aerosol, through
the skin and by ingestion.
CHEMICAL DANGERS: INHALATION RISK:
The substance decomposes on burning Evaporation at 20°C is neglible; a harmful
producing toxic fumes. concentration of airborne particles can,
however, be reached quickly.
OCCUPATIONAL EXPOSURE LIMITS: EFFECTS OF SHORT-TERM EXPOSURE:
TLV (as tin): ppm: 0.1 mg/m3 A4. The substance irritates severely the eyes, the skin.
STEL 0.2 mg/m3 A4 (skin) (ACGIH 1997). Inhalation of the aerosol may cause lung oedema (see
Notes). The substance may cause effects on the thymus,
resulting in depression of the immune function.
PHYSICAL PROPERTIES
Boiling point: 173°C
Melting point: <-45°C
Relative density (water = 1): 1.17 at 20°C
Solubility in water: poor
Vapour pressure, Pa at 20°C: 0.001
Flash point: 190°C c.c.
Octanol/water partition coefficient as log Pow: 3.19
ENVIRONMENTAL DATA
The substance is very toxic to aquatic organisms. In the food chain important to humans,
bioaccumulation takes place, specifically in fish and molluscs. Avoid release to
the environment in circumstances different to normal use.
NOTES
The symptoms of lung oedema often do not manifest until a few hours have passed and they are
aggravated by physical effort. Rest and medical observation is therefore essential. Immediate
administration of an appropriate spray, by a doctor or a person authorized by him/her,
should be considered.
ADDITIONAL INFORMATION
LEGAL NOTICE Neither the CEC nor the IPCS nor any person acting on behalf of the CEC or
the IPCS is responsible for the use which might be made of this information.
REFERENCES
Alzieu C (1991) Environmental problems caused by TBT in France:
Assessment, regulations, prospects. Marine environmental research,
32:7-17.
Anon. (1991) Acute effect of indoor exposure to paint containing
bis(tributyltin)oxide -- Wisconsin, 1991. Morbidity and mortality
weekly report, 40:280-281.
Baroncelli S, Karrer D, Turillazzi PG (1990) Embryotoxic evaluation of
bis(tri- n-butyltin)oxide (TBTO) in mice. Toxicology letters,
50:257-262.
Bettin C, Ohelmann J, Stroben E (1996) TBT-induced imposex in marine
neogastropods is mediated by an increasing androgen level.
Helgolander Meeresunersuchungen, 50:299-317.
Boyer IJ (1989) Toxicity of dibutyltin, tributyltin and other
organotin compounds to humans and to experimental animals.
Toxicology, 55:253-298.
Bressa G, Hinton RH, Price SC, Isbir M, Ahmed RS, Grasso P (1991)
Immunotoxicity of tri- n-butyltin oxide (TBTO) and tri- n-butyltin
chloride (TBTC) in the rat. Journal of applied toxicology,
11:397-402.
Brown RA, Nazario CM, de Tirado RS, Castrillón J, Agard ET (1977) A
comparison of the half-life of inorganic and organic tin in the mouse.
Environmental research, 13:56-61.
Buckiova D, Dostal M, Hofmannova V (1992) Embryotoxicity of organotins
[abstract]. Reproductive toxicology, 6:178-179.
Carthew P, Edwards RE, Dorman BM (1992) The immunotoxicity of
tributyltin oxide (TBTO) does not increase the susceptibility of rats
to experimental respiratory infection. Human and experimental
toxicology, 11:71-75.
CEFIC (1994) Use of triorganotin compounds in anti-fouling paints.
Results of TBT monitoring studies. Paper submitted by the European
Chemical Industry Council to the International Marine Organization
Marine Environment Protection Committee, 35th session, January 1994.
Crofton KM, Dean KF, Boncek VM, Rosen MB, Sheets LP, Chernoff N,
Reiter LW (1989) Prenatal or postnatal exposure to
bis(tri- n-butyltin)oxide in the rat: postnatal evaluation of
teratology and behavior. Toxicology and applied pharmacology,
97:113-123.
Daly IW (1992) An eighteen month oncogenicity feeding study in mice
with bis(tri-n -butyltin)oxide (TBTO). Unpublished report prepared
by Bio/dynamics, Inc., for TBTO Consortium (MRID No. 422650-01).
Davis A, Barale R, Brun G, Forster R, Günther T, Hautefeuille H, van
der Heijden CA, Knaap AGAC, Krowke R, Kuroki T, Loprieno N, Malaveille
C, Merker HJ, Monaco M, Mosesso P, Neubert D, Norppa H, Sorsa M, Vogel
E, Voogd CE, Umeda M, Bartsch H (1987) Evaluation of the genetic and
embryotoxic effects of bis(tri- n-butyltin)oxide (TBTO), a
broad-spectrum pesticide, in multiple in vivo and in vitro
short-term tests. Mutation research, 188:65-95.
dela Cruz MAT, Molander S (1998) Butyltins in marine sediments from
the Swedish west coast. Report to Swedish National Chemicals
Inspectorate. Gothenburg, Chalmers University of Technology.
Fargasova A (1997) Comparative study of ecotoxicological effect of
triorganotin compounds on various biological subjects. Ecotoxicology
and environmental safety, 36:38-42.
Fent K, Hunn J (1991) Phenyltins in water, sediment, and biota of
freshwater marinas (Lake Lucern, Switzerland). Environmental science
and technology, 25:956-963.
Funahashi N, Iwasaki I, Ide G (1980) Effects of
bis(tri- n-butyltin)-oxide on endocrine and lymphoid organs of male
rats. Acta Pathologica Japonica, 30:955-966.
Garssen J, Van der Vliet H, De Klerk A, Goettsch W, Dormans JAMA,
Bruggeman CA, Osterhaus ADME, Van Loveren H (1995) A rat
cytomegalovirus infection model as a tool for immunotoxicity testing.
European journal of pharmacology, 292:223-231.
Grinwis GCM, van den Brandhof EJ, Dormans JAMA, Engelsma M, Kuiper R,
Leewis R, van Loveren H, Wester PW, Vaal MA, Vethaak AD, Vos JG (1997)
Short term toxicity of bis(tri-n- butyltin)oxide in flounder
(Platichtys flesus): pathology and immune function. Bilthoven,
National Institute of Public Health and Environmental Protection (RIVM
Report No. 732501 002).
Guruge KS, Iwata H, Tanaka H, Tanabe S (1997) Butyltin accumulation in
the liver and kidney of seabirds. Marine environmental research,
44:191-199.
Hay A, Singer CR (1991) Wood preservatives, solvents, and
thrombocytopenic purpura [letter]. Lancet, 338:766.
IPCS (1990) Tributyltin compounds. Geneva, World Health
Organization, International Programme on Chemical Safety
(Environmental Health Criteria 116).
IPCS (1996) International Chemical Safety Card -- Tributyltin oxide.
Geneva, World Health Organization, International Programme on Chemical
Safety (ICSC 1282).
Iwata H, Tanabe S, Miyazaki N, Tatsukawa R (1994) Detection of
butyltin compound residues in the blubber of marine mammals. Marine
pollution bulletin, 28:607-612.
Iwata H, Tanabe S, Mizuno T, Tatsukawa R (1995) High accumulation of
toxic butyltins in marine mammals from Japanese coastal waters.
Environmental science and technology, 29:2959-2962.
Iwata H, Tanabe S, Mizuno T, Tatsukawa R (1997) Bioaccumulation of
butyltin compounds in marine mammals: The specific tissue distribution
and composition. Applied organometallic chemistry, 11:257-264.
Kannan K, Tanabe S, Tatsukawa R (1995) Occurrence of butyltin residues
in certain foodstuffs. Bulletin of environmental contamination and
toxicology, 55:510-516.
Kannan K, Corsolini S, Focardi S, Tanabe S, Tatsukawa R (1996)
Accumulation pattern of butyltin compounds in dolphin, tuna and shark
collected from Italian coastal waters. Archives of environmental
contamination and toxicology, 31:19-23.
Kannan K, Senthilkumar K, Loganathan BG, Takahashi S, Odell DK, Tanabe
S (1997) Elevated accumulation of tributyltin and its breakdown
products in bottlenose dolphins ( Tursiops truncatus) found stranded
along the US Atlantic and Gulf coasts. Environmental science and
technology, 31:296-301.
Kannan K, Guruge KS, Thomas NJ, Tanabe S, Giesy JP (1998) Butyltin
residues in southern sea otters ( Enhydra lutris nereis) found dead
along the California coastal waters. Environmental science and
technology, 32:1169-1175.
Karrer D, Baroncelli S, Ciaralli L, Turillazzi PG (1992) Effect of
subchronic bis(tri- n-butyltin)oxide (TBTO) oral administration on
haematological parameters in monkeys: a preliminary report. Food and
chemical toxicology, 30:715-718.
Karrer D, Baroncelli S, Turillazzi PG (1995) Oral
bis(tri- n-butyltin) oxide in pregnant mice. II. Alterations in
hematological parameters. Journal of toxicology and environmental
health, 46:369-377.
Kim GB, Lee JS, Tanabe S, Iwata H, Tatsukawa R, Shimazaki K (1996a)
Specific accumulation and distribution of butyltin compounds in
various organs and tissues of the stellar sea lion ( Eumetopias
jubatus): Comparison with organochlorine accumulation pattern.
Marine pollution bulletin, 32:558-563.
Kim GB, Tanabe S, Tatsukawa R, Loughlin TR, Shimazaki K (1996b)
Characteristics of butyltin accumulation and its biomagnification in
stellar sea lion ( Eumetopias jubatus). Environmental toxicology
and chemistry, 15:2043-2048.
Kim GB, Tanabe S, Iwakiri R, Tatsukawa R, Amano M, Miyazaki N, Tanaka
H (1996c) Accumulation of butyltin compounds in Risso's dolphin
( Grampus griseus) from the Pacific coast of Japan: Comparison with
organochlorine residue pattern. Environmental science and
technology, 30:2620-2625.
Krajnc EI, Wester PW, Loeber JG, van Leeuwen FXR, Vos JG, Vaessen
HAMG, van der Heijden CA (1984) Toxicity of bis(tri- n-butyltin)oxide
(TBTO) in the rat. I. Short-term effects on general parameters and on
the endocrine and lymphoid systems. Toxicology and applied
pharmacology, 75:363-386.
Krajnc EI, Vos JG, Wester PW, Loeber JG, van der Heijden CA (1987)
Toxicity of bis(tri-n -butyltin)oxide (TBTO) in rats. Unpublished
report submitted to the Office of Toxic Substances, US Environmental
Protection Agency, with cover letter dated 18 May 1987 (Document
Control No. FYI-OTS-0687-0550 Sequence A).
Kroes R, Garbis-Berkyens JM, deVries T, van Nellesrooy JHJ (1981)
Histopathological profile of a Wistar rat stock including a survey of
the literature. Journal of gerontology, 36:259-279.
Kusk KO, Peterson S (1997) Acute and chronic toxicity of tributyltin
and linear alkylbenzene sulfonate to the marine copepod Acartia
tonsa. Environmental toxicology and chemistry, 16:1629-1633.
Langston WJ (1995) Tributyl tin in the marine environment: A review of
past and present risks. Pesticide outlook, 6:18-24.
Li Q, Osada M, Takahashi K, Matsutani T, Mori K (1997) Accumulation
and depuration of tributyltin oxide and its effect on the
fertilization and embryonic development in the Pacific oyster,
Crassostrea gigas. Bulletin of environmental contamination and
toxicology, 58:489-496.
Lindblad C, Kautsky U, Andre C, Kautsky N, Tedengren M (1989)
Functional response of Fucus vesiculosus communities to tributyltin
measured in an in situ continuous flow-through system.
Hydrobiologia, 188/189:277-283.
Madhusree B, Tanabe S, Ozturk AA, Tatsukawa R, Miyazaki N, Ozdamar E,
Raral O, Samsun O, Ozturk B (1997) Contamination by butyltin compounds
in harbour porpoise ( Phocoena phocoena) from the Black Sea.
Fresenius journal of analytical chemistry, 359:244-248.
Matthiessen P, Gibbs PE (1998) Critical appraisal of the evidence for
tributyltin-mediated endocrine disruption in mollusks. Environmental
toxicology and chemistry, 17:37-43.
NIVA (1997) Levels and environmental effects of TBT in marine
organisms and sediments from the Norwegian coast. A summary report.
Oslo, Norwegian State Pollution Control Authority, Norwegian Institute
for Water Research.
Oehlmann J, Fioroni P, Stroben E, Markert B (1996) Tributyltin (TBT)
effects on Ocinebrina aciculata (Gastropoda: Muricidae): imposex
development, sterilization, sex change and population decline. The
science of the total environment, 188:205-223.
Oshima Y, Nirmala K, Yokota Y, Shimazaki Y, Go J, Imada N, Honjo T,
Kobayashi K (1998) High accumulation of tributyltin in blood of fish
and its transgenerational and synergistic toxicity with PCB on the
reproductive process of fish. Japanese journal of environmental
toxicology, 1:26-35.
Peto R, Pike MC, Day NE, Gray RG, Lee PN (1980) Guidelines for
simple, sensitive significance tests for carcinogenic effects in
long-term animal experiments. Long-term and short-term screening
assays for carcinogens: a critical appraisal. Lyon, International
Agency for Research on Cancer, pp. 311-426 (IARC Monographs on the
Evaluation of Carcinogenic Risks of Chemicals to Humans, Supplement
2).
Poitou P, Marignac B, Certin C, Gradiski D (1978) Étude de l'effet sur
le système nerveux central et du pouvoir sensibilisant de l'oxyde de
tributylétain. Annales Pharmaceutiques Françaises, 36:569-572 [cited
in IPCS, 1990].
Raffray M, Cohen GM (1991) Bis(tri- n-butyltin)oxide induces
programmed cell death (apoptosis) in immature rat thymocytes.
Archives of toxicology, 65:135-139.
Raffray M, Cohen GM (1993) Thymocyte apoptosis as a mechanism for
tributyltin-induced thymic atrophy in vivo. Archives of
toxicology, 67:231-236.
Raffray M, McCarthy D, Snowden RT, Cohen GM (1993) Apoptosis as a
mechanism of tributyltin cytotoxicity to thymocytes: relationship of
apoptotic markers to biochemical and cellular effects. Toxicology
and applied pharmacology, 119:122-130.
Ruiz JM, Bachelet G, Caumette P, Donard OFX (1996) Three decades of
tributyltin in the coastal environment with emphasis on Arcachon Bay,
France. Environmental pollution, 93:195-203.
Schroeder RE (1981) A teratology study in rats with
bis(tri-n -butyl-tin)oxide. Unpublished report prepared by
Bio/dynamics, Inc., for Elf Atochem (MRID No. 00137158, 92172005,
92172016; HED Document No. 003914, 004691, 010916).
Schroeder RE (1990) A two-generation reproduction study in rats with
bis(tri-n -butyltin)oxide. Unpublished report prepared by
Bio/dynamics, Inc., for Schering AG and M&T Chemicals, Inc. (MRID No.
416938-01).
Schuh W (1992) One year chronic feeding study in beagle dogs.
Unpublished report prepared by Schering AG Laboratories for Elf
Atochem North America, Inc., Aceto Chemicals, and Schering Berlin
Polymers (MRID No. 425498).
Schweinfurth HA, Gunzel P (1987) The tributyltins: mammalian toxicity
and risk evaluation for humans. Oceans '87: The ocean, "an
international workplace." Proceedings of the International Organotin
Symposium, 4:1421-1431.
Shelton D, Urch B, Tarlo SM (1992) Occupational asthma induced by a
carpet fungicide -- tributyltin oxide. Journal of allergy and
clinical immunology, 90:274-275.
Smialowicz RJ, Riddle MM, Rogers RR, Leubke RW, Copeland CB (1989)
Immunotoxicity of tributyltin oxide in rats exposed as adults or
pre-weanlings. Toxicology, 57:97-111.
Stäb JA, Traas TP, Stromberg G, van Kesteren J, Leonards P, van Hattum
B, Brinkman UAT, Cofino WP (1996) Determination of organotin compounds
in the foodweb of a shallow freshwater lake in the Netherlands.
Archives of environmental contamination and toxicology, 31:319-328.
Stringer CP, Hicks R, Botham PA (1991) Contact sensitivity (allergic
contact dermatitis) to bis(tri- n-butyltin)oxide in mice. Contact
dermatitis, 24:210-215.
Stronkhorst J (1996) Contamination and toxicity of sediments: a
persistent problem. Lecture 7 in: The present status of
TBT-copolymer anti-fouling paints. Proceedings from an international
one-day symposium on anti-fouling paints for ocean-going vessels, The
Hague, February 1996.
Takahashi M, Mukai H, Tanabe S, Sakayama N, Masuno H (1998)
Accumulation of butyltin compounds in humans and some terrestrial
mammals and investigation of potential source of pollution.
Proceedings of the Fourth Joint Meeting of the Association for
Bioassay Research and the Association of Environmental Toxicology,
Kusatsu, Japan, 10 September 1998, pp. 50-51 (in Japanese).
Tanabe S (1998) Butyltin contamination in marine mammals. Japanese
journal of environmental toxicology, 1:14-25.
Tanabe S, Prudente M, Mizuno T, Hasegawa J, Miyazaki N (1998) Butyltin
contamination in marine mammals from North Pacific and Asian coastal
waters. Environmental science and technology, 32:193-198.
Thompson TA, Lewis JM, Dejneka NS, Severs WR, Polavarapu R,
Billingsley ML (1996) Induction of apoptosis by organotin compounds
in vitro: neuronal protection with antisense oligonucleotides
directed against stannin. Journal of pharmacology and experimental
therapeutics, 276:1201-1214.
Tolosa J, Readman JW, Blaevoet A, Ghilini S, Bartocci J, Horvat M
(1996) Contamination of Mediterranean (Cote d'Azur) coastal waters by
organotins and Irgarol 1051 used in anti-fouling paints. Marine
pollution bulletin, 32:335-341.
Truhaut R, Anger JP, Reymann JM, Chauvel Y, Van Den Driessche J (1979)
Influence de l'oxyde de tributylétain (OTBE) en aérosol sur le
comportement exploratoire chez la souris. Toxicological European
research, 11:181-186 [cited in IPCS, 1990].
Tsuda T, Inoue DT, Kojima M, Aoki S (1995) Daily intakes of
tributyltin and triphenyltin compounds from meals. Journal of the
Association of Official Analytical Chemists International,
78:941-943.
US EPA (1997) Toxicological review on tributyltin oxide. Available
from the US Environmental Protection Agency's Risk Assessment Hotline
(513-569-7254 [phone], 513-569-7159 [fax], rih.iris@epamail.epa.gov
[Internet address], or the IRIS Website at www.epa.gov/iris).
Vandebriel RJ, Meredith C, Scott MO, Rohall PJ, van Loveren H (1998)
Effects of in vivo exposure to bis(tri- n-butyltin)oxide,
hexa-chlorobenzene, and benzo(a)pyrene on cytokine (receptor) mRNA
levels in cultured rat splenocytes and on IL-2 receptor protein
levels. Toxicology and applied pharmacology, 148:126-136.
Van Loveren H, Krajnc EI, Rombout PJA, Blommaert FA, Vos JG (1990)
Effects of ozone, hexachlorobenzene, and bis(tri- n-butyltin)oxide on
natural killer activity in the rat lung. Toxicology and applied
pharmacology, 102:21-33.
Veiga A, Pinto AF, Loureiro-Dias MC (1997) Tributyltin oxide affects
energy production in the yeast Rhodotorula ferulica, a utilizer of
phenolic compounds. Canadian journal of microbiology, 43:683-687.
Vela NP, Caruso JA (1993) Comparison of flame ionization and
inductively coupled plasma mass spectrometry for the detection of
organometallics separated by capillary supercritical fluid
chromatography. Journal of chromatography, 6741:337-345.
Verdier F, Virat M, Schweinfurth H, Descotes J (1991) Immunotoxicity
of bis(tri- n-butyltin) oxide in the rat. Journal of toxicology and
environmental health, 32:307-319.
Viviani B, Ross AD, Chow SC, Nicotera P (1995) Organotin compounds
induce calcium overload and apoptosis in PC12 cells.
Neurotoxicology, 16:19-26.
Vos JG, DeKlerk A, Krajnc EI, Kruizinga W, Van Ommen B, Rozing J
(1984) Toxicity of bis(tri- n-butyltin) oxide in the rat. II.
Suppression of thymus-dependent immune responses and of parameters of
nonspecific resistance after short-term exposure. Toxicology and
applied pharmacology, 75:387-408.
Vos JG, DeKlerk A, Krajnc EI, Van Loveren V, Rozing J (1990)
Immunotoxicity of bis(tri- n-butyltin)oxide in the rat: effects on
thymus-dependent immunity and on nonspecific resistance following
long-term exposure in young versus aged rats. Toxicology and applied
pharmacology, 105:144-155.
Wax PM, Dockstader L (1995) Tributyltin use in interior paints: a
continuing health hazard. Clinical toxicology, 33:239-241.
Wester PM, Canton JH (1987) Histopathological study of Poecilia
reticulata (guppy) after long-term exposure to
bis(tri- n-butyltin)oxide (TBTO) and di- n-butyltindichloride
(DBTC). Aquatic toxicology, 10:143-163.
Wester PW, van der Heijden CA, Bisschop A, van Esch GJ (1985)
Carcinogenicity study with epichlorohydrin (CEP) by gavage in rats.
Toxicology, 36:325-339.
Wester PW, Krajnc EI, van der Heijden CA (1987) Chronic toxicity and
carcinogenicity study with bis(tri-n -butyltin)oxide (TBTO) in
rats. Unpublished report submitted to the Office of Toxic
Substances, US Environmental Protection Agency, with cover letter
dated 18 May 1987 (Document Control No. FYI-OTS-0687-0550 Sequence A).
Wester PW, Krajnc EI, van Leeuwen FXR, Loeber JG, van der Heijden CA,
Vaessen HAMG, Helleman PW (1988) Two year feeding study in rats with
bis(tri-n -butyltin)oxide (TBTO). Unpublished report, National
Institute of Public Health and Environmental Hygiene, Bilthoven.
Wester PW, Krajnc EI, van Leeuwen FXR, Loeber JG, van der Heijden CA,
Vaessen HAMG, Helleman PW (1990) Chronic toxicity and carcinogenicity
of bis(tri- n-butyltin)oxide (TBTO) in the rat. Food and chemical
toxicology, 28:179-196.
Yamada H, Sasaki YF (1993) Organotins are co-clastogens in a whole
mammalian system. Mutation research, 301:195-200.
APPENDIX 1 -- SOURCE DOCUMENTS
IPCS (1990): Tributyltin compounds
(Environmental Health Criteria 116)
A WHO Task Group meeting on Environmental Health Criteria for
Tributyltin Compounds was held at the Institute of Terrestrial
Ecology, Monks Wood, United Kingdom, from 11 to 15 September 1989. The
Task Group reviewed and revised the draft criteria document and made
an evaluation of the risks for human health and the environment from
exposure to tributyltin compounds.
Copies of this document may be obtained from:
International Programme on Chemical Safety
World Health Organization
Geneva, Switzerland
US EPA (1997): Toxicological review on tributyltin oxide
This document received internal peer review by EPA scientists, an
external peer review by three well-qualified nongovernment scientists,
and consensus review by EPA Program Offices and the 10 Regional
Offices. Summaries of significant comments from external peer
reviewers are included in an appendix to the document.
Copies of this document may be obtained from:
EPA Risk Assessment Hotline
513-569-7254 (phone)
513-569-7159 (fax)
rih.iris@epamail.epa.gov (Internet address)
www.epa.gov/iris (Website)
APPENDIX 2 -- CICAD PEER REVIEW
The draft CICAD on TBTO was sent for review to institutions and
organizations identified by IPCS after contact with IPCS national
Contact Points and Participating Institutions, as well as to
identified experts. Comments were received from:
Department of Health, London, United Kingdom
Health and Safety Executive, Bootle, United Kingdom
Health Canada, Ottawa, Canada
International Agency for Research on Cancer, Lyon, France
International Council on Metals and the Environment, Ottawa,
Canada
József Fodor National Center of Public Health, Budapest, Hungary
Karolinska Institute, Stockholm, Sweden
National Chemicals Inspectorate (KEMI), Solna, Sweden
National Institute for Working Life, Solna, Sweden
National Institute of Public Health and Environmental Protection,
Bilthoven, The Netherlands
United States Department of Health and Human Services (National
Institute of Environmental Health Sciences, Research Triangle
Park, USA)
United States Environmental Protection Agency (Office of Research
and Development, National Center for Environmental Assessment,
Washington, DC, USA)
World Health Organization, International Programme on Chemical
Safety, Geneva, Switzerland
APPENDIX 3 -- CICAD FINAL REVIEW BOARD
Tokyo, Japan, 30 June - 2 July 1998
Members
Dr R. Benson, Drinking Water Program, United States Environmental
Protection Agency, Denver, CO, USA
Dr T. Berzins, National Chemicals Inspectorate (KEMI), Solna, Sweden
Mr R. Cary, Health Directorate, Health and Safety Executive,
Merseyside, United Kingdom
Dr C. DeRosa, Agency for Toxic Substances and Disease Registry, Center
for Disease Control and Prevention, Atlanta, GA, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Cambridgeshire, United
Kingdom
Dr H. Gibb, National Center for Environmental Assessment, United
States Environmental Protection Agency, Washington, DC, USA
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Research, Hanover,
Germany
Ms M.E. Meek, Environmental Health Directorate, Health Canada, Ottawa,
Ontario, Canada ( Chairperson)
Dr J. Sekizawa, Division of Chem-Bio Informatics, National Institute
of Health Sciences, Tokyo, Japan ( Vice-Chairperson)
Professor S.A. Soliman, Department of Pesticide Chemistry, Alexandria
University, Alexandria, Egypt
Ms D. Willcocks, Chemical Assessment Division, Worksafe Australia,
Camperdown, Australia ( Rapporteur)
Professor P. Yao, Chinese Academy of Preventive Medicine, Institute of
Occupational Medicine, Beijing, People's Republic of China
Observers
Professor F.M.C. Carpanini,1 Secretary-General, ECETOC (European
Centre for Ecotoxicology and Toxicology of Chemicals), Brussels,
Belgium
Dr M. Ema, Division of Biological Evaluation, National Institute of
Health Sciences, Osakai, Japan
Mr R. Green,1 International Federation of Chemical, Energy, Mine and
General Workers' Unions, Brussels, Belgium
Dr B. Hansen,1 European Chemicals Bureau, European Commission,
Ispra, Italy
Mr T. Jacob,1 Dupont, Washington, DC, USA
Dr H. Koeter, Organisation for Economic Co-operation and Development,
Paris, France
Mr H. Kondo, Chemical Safety Policy Office, Ministry of International
Trade and Industry, Tokyo, Japan
Ms J. Matsui, Chemical Safety Policy Office, Ministry of International
Trade and Industry, Tokyo, Japan
Mr R. Montaigne,1 European Chemical Industry Council (CEFIC),
Brussels, Belgium
Dr A. Nishikawa, Division of Pathology, National Institute of Health
Sciences, Tokyo, Japan
Dr H. Nishimura, Environmental Health Science Laboratory, National
Institute of Health Sciences, Osaka, Japan
Ms C. Ohtake, Chem-Bio Informatics, National Institute of Health
Sciences, Tokyo, Japan
Dr T. Suzuki, Division of Food, National Institute of Health Sciences,
Tokyo, Japan
Dr K. Takeda, Mitsubishikagaku Institute of Toxicological and
Environmental Sciences, Yokohama, Japan
Dr K. Tasaka, Department of Chemistry, International Christian
University, Tokyo, Japan
Dr H. Yamada, Environment Conservation Division, National Research
Institute of Fisheries Science, Kanagawa, Japan
1 Invited but unable to attend.
Dr M. Yamamoto, Chem-Bio Informatics, National Institute of Health
Sciences, Tokyo, Japan
Dr M. Yasuno, School of Environmental Science, The University of Shiga
Prefecture, Hikone, Japan
Dr K. Ziegler-Skylakakis, GSF-Forschungszentrum für Umwelt und
Gesundheit GmbH, Institut für Toxikologie, Oberschleissheim, Germany
Secretariat
Ms L. Regis, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Mr A. Strawson, Health and Safety Executive, London, United Kingdom
Dr P. Toft, Associate Director, International Programme on Chemical
Safety, World Health Organization, Geneva, Switzerland
RÉSUMÉ D'ORIENTATION
Ce CICAD relatif à l'oxyde de tributyétain (TBTO) a été préparé
par l'Agence américaine pour la protection de l'environnement (United
States Environmental Protection Agency, US EPA) à partir d'un document
sur les dérivés du tributylétain publié par le Programme international
sur la Sécurité chimique dans la série Critères d'Hygiène de
l'Environnement (IPCS, 1990) et d'un document de l'US EPA intitulé
Toxicological review on tributyltin oxide (US EPA, 1997). Ces deux
mises au point étaient basées sur des bibliographies arrêtées
respectivement en 1989 et 1996. Des informations complémentaires dont
les dernières remontent à juin 1998 ont été incluses dans le présent
document. On trouvera à l'appendice 1 des indications sur les sources
documentaires utilisées ainsi que sur leur mode de dépouillement. Les
renseignements concernant l'examen du CICAD par des pairs font l'objet
de l'appendice 2. Ce CICAD a été aprouvé en tant qu'évaluation
internationale lors d'une réunion du Comité d'évaluation finale qui
s'est tenue à Tokyo (Japon) du 30 juin au 2 juillet 1998. La liste des
participants à cette réunion figure à l'appendice 3. La fiche
d'information internationale sur la sécurité chimique (ICSC 1282)
établie pour l'oxyde de tributylétain par le Programme international
sur la Sécurité chimique (IPCS, 1996) est également reproduite dans ce
document.
Dans le présent document, on utilise le terme d'oxyde de
tributylétain chaque fois qu'il est question de ce composé en
particulier. Toutefois dans l'environnement, les dérivés du
tributylétain existent selon toute probabilité principalement sous la
forme d'hydroxyde, de chlorure et de carbonate de tributylétain. Dans
ce cas et lorsque l'identité du composé est douteuse, on utilise le
terme général de tributylétain.
L'oxyde de tributylétain protège efficacement le bois, les
cotonnades, le papier et les peintures murales contre l'attaque de la
vermine. Il entre dans la composition de nombreuses peintures marines
auxquelles on l'ajoute comme agent antisalissures. Il est présent dans
ces produits sous la forme de copolymère organométallique. Lorsque le
copolymère est hydrolysé par l'eau de mer, le tributylétain est
lentement libéré de la surface peinte qu'il protège des incrustations
pendant des durées pouvant aller jusqu'à 4 ou 5 ans.
Du fait de sa faible solubilité dans l'eau et de son caractère
lipophile, le tributylétain s'adsorbe facilement sur les particules.
Sa demi-vie dans la colonne d'eau va de quelques jours à plusieurs
semaines. Il peut subsister dans les sédiments pendant plusieurs
années. Il s'accumule dans l'organisme des animaux, ses organes
d'élection étant le rein et le foie. L'absorption s'effectue davantage
à partir des denrées alimentaires que directement à partir de l'eau.
On ne dispose d'aucun renseignement sur la toxicité du
tributylétain chez l'Homme à la suite d'une exposition de longue
durée. D'après un certain nombre de données et d'observations, le
tributylétain serait fortement irritant pour la peau et les voies
respiratoires. Ces données ne se prêtent toutefois pas à
l'établissement de relations dose-réponse bien caractérisées. Des
études effectuées au Japon ont permis une évaluation quantitative de
l'exposition au tributylétain présent dans les denrées alimentaires.
Les études à court terme montrent que l'oxyde de tributylétain
est modérément à fortement toxique pour les mammifères de laboratoire.
Des études nombreuses et bien conduites, tant à court qu'à long terme,
montrent que l'effet essentiel de l'oxyde de tributylétain réside dans
son immunotoxicité (dépression des fonctions immunitaires
thymo-dépendantes). La dose sans effet nocif observable (NOAEL) chez
le rat est de 0,025 mg/kg de poids corporel par jour, le critère
retenu étant une immunodépression après exposition de longue durée.
Une analyse des doses de référence montre que l'exposition
correspondant à la limite inférieure de confiance (95 %) pour une
réduction de 10 % du titre des anticorps IgE chez le rat est égale à
0,034 mg/kg de poids corporel par jour. Lors d'une étude de
cancérogénicité chez le rat, on a constaté une augmentation de
l'incidence de certaines tumeurs endocriniennes. Ces tumeurs se
produisent spontanément chez les rats appartenant à l'espèce utilisée
dans cette étude et on ignore dans quelle mesure on peut les prendre
en compte dans une évaluation du risque chez l'Homme. L'oxyde de
tributylétain n'est pas cancérogène pour la souris. L'expérience
montre qu'il n'est pas non plus génotoxique. Rien n'indique qu'il
exerce des effets nocifs sur la fonction de reproduction et le
développement à des doses inférieures à la dose sans effet
immunotoxique observable. De tels effets ne se produisent que lorsque
la dose est voisine de celle qui est toxique pour la mère. Comme on
l'a indiqué plus haut, les données révèlent un effet irritant prononcé
sur la peau et les voies respiratoires. En s'appuyant sur la valeur de
la dose sans effet immunotoxique observable et en appliquant un
coefficient de sécurité de 100, on peut donner une valeur-guide de
0,0003 mg/kg p.c. par jour pour l'exposition par la voie buccale. On
ne dispose pas de données suffisantes pour établir une valeur-guide
dans le cas d'une exposition par inhalation.
L'oxyde de tributylétain est extrêmement dangereux pour certains
organismes aquatiques. Dans certains cas, il bloque les fonctions
endocrines. Dans les eaux littorales de quelques régions, il est
présent à une concentration supérieure à celle qui produit de graves
effets nocifs. Dans certaines régions, les effets constatés ont été
suffisamment graves pour faire chuter la fécondité et réduire
l'effectif de la population touchée. Le risque global pour
l'environnement terrestre est vraisemblablement faible.
RESUMEN DE ORIENTACION
Este CICAD sobre el óxido de tributilestaño (TBTO), preparado por
la Agencia para la Protección del Medio Ambiente de los Estados Unidos
(EPA), se basa en un documento sobre los Criterios de Salud Ambiental
del Programa Internacional de Seguridad de las Sustancias Químicas
relativo a los compuestos de tributilestaño (IPCS, 1990) y en el
Examen toxicológico sobre el óxido de tributilestaño de la EPA de
los Estados Unidos (US EPA, 1997). En estos exámenes se analizaron los
datos identificados hasta 1989 y 1996, respectivamente. En el presente
documento aparece también la información adicional obtenida hasta
1998. La información relativa a las características de los proceso de
examen y la disponibilidad de los documentos originales figura en el
apéndice 1. La información acerca del examen colegiado de este CICAD
se presenta en el apéndice 2. Su aprobación tuvo lugar como evaluación
internacional en una reunión de la Junta de Evaluación Final,
celebrada en Tokio, Japón, del 30 de junio al 2 de julio de 1998. La
lista de participantes en esta reunión de la Junta de Evaluación Final
aparece en el apéndice 3. La Ficha internacional de seguridad química
(ICSC 1282), preparada por el Programa Internacional de Seguridad de
las Sustancias Químicas (IPCS, 1996), también se reproduce en el
presente documento.
En este documento, el término de óxido de tributilestaño se
aplica específicamente a este producto químico. Sin embargo, en el
medio ambiente es más probable que los compuestos de tributilestaño se
encuentren como hidróxido, cloruro o carbonato. En esos casos o cuando
la identidad del producto químico específico no está clara, se utiliza
el término general de tributilestaño.
El óxido de tributilestaño es un conservante biocida eficaz de la
madera, los textiles de algodón, el papel y las pinturas y colorantes
domésticos. Se añade como agente antiincrustante en numerosas
formulaciones de pinturas marinas. El tributilestaño está presente en
la mayoría de esas formulaciones antiincrustantes como copolímero
organometálico. Se libera lentamente de la superficie pintada a medida
que el polímero se hidroliza en el agua de mar, proporcionando una
protección prolongada contra las incrustaciones de hasta cuatro o
cinco años.
Debido a su baja solubilidad en agua y a su carácter lipófilo, el
tributilestaño se adsorbe fácilmente en las partículas. Su semivida en
la columna de agua oscila entre unos días y varias semanas. Puede
persistir en los sedimentos durante varios años. Se bioacumula en los
organismos, alcanzando las concentraciones más altas en el hígado y el
riñón. La absorción a partir de los alimentos es más importante que la
procedente directamente del agua.
No se dispone de información sobre la toxicidad del óxido de
tributilestaño en el ser humano tras una exposición prolongada.
Algunos datos e informes de casos ponen de manifiesto que produce
irritación cutánea y respiratoria grave. Sin embargo, los datos no son
suficientes para caracterizar la relación exposición-respuesta. En
algunos estudios realizados en el Japón se ha cuantificado la
exposición humana al tributilestaño procedente de los alimentos.
En estudios de corta duración con mamíferos de laboratorio, la
toxicidad aguda del óxido de tributilestaño es entre moderada y alta.
En numerosos estudios bien realizados, tanto de corta duración como
prolongados, su efecto más importante es la inmunotoxicidad (depresión
de las funciones inmunitarias dependientes del timo). La concentración
sin efectos adversos observados (NOAEL) para la inmunosupresión en
ratas tras una exposición prolongada es de 0,025 mg/kg de peso
corporal al día. El análisis de las dosis de referencia pone de
manifiesto que la exposición correspondiente al límite de confianza
más bajo (95%) de la dosis que produce una disminución del 10% en la
concentración de la inmunoglobulina (Ig) E en ratas es de 0,034 mg/kg
de peso corporal al día. En un estudio de carcinogenicidad en ratas,
se observó un aumento en la incidencia de algunos tumores en
determinados tejidos endocrinos. Estos tumores se producen
espontáneamente, con una incidencia variable en la estirpe de ratas
utilizada en el estudio, y se desconoce su importancia en la
evaluación del riesgo para la salud humana. El óxido de tributilestaño
no es carcinogénico para los ratones. Las pruebas ponen de manifiesto
que no es genotóxico. No hay indicios de que se produzcan efectos
reproductivos o en el desarrollo con una exposición inferior a la
establecida como NOAEL para la inmunotoxicidad. Estos efectos aparecen
solamente con exposiciones próximas a las que causan toxicidad
materna. Los datos demuestran que el óxido de tributilestaño produce
una irritación cutánea y respiratoria grave. Teniendo en cuenta la
NOAEL para la inmunotoxicidad y un factor de incertidumbre de 100, el
valor guía para la exposición oral es de 0,0003 mg/kg de peso corporal
al día. No se dispone de datos adecuados para extrapolar un valor guía
aplicable a la exposición por inhalación.
El óxido de tributilestaño es enormemente peligroso para algunos
organismos acuáticos. Es un perturbador endocrino en algunos
organismos. La concentración de tributilestaño en determinadas aguas
costeras es superior a la que produce efectos adversos graves. Estos
efectos han sido suficientemente importantes para producir fracaso
reproductivo y disminución de la población en algunas zonas. El
peligro general para el medio ambiente terrestre es probablemente
bajo.
1. EXECUTIVE SUMMARY
This CICAD on tributyltin oxide (TBTO) was prepared by the United
States Environmental Protection Agency (US EPA) and is based on an
International Programme on Chemical Safety Environmental Health
Criteria document on tributyltin compounds (IPCS, 1990) and on the US
EPA's Toxicological review on tributyltin oxide (US EPA, 1997). Data
identified as of 1989 and 1996, respectively, were considered in these
reviews. Additional information identified as of June 1998 has been
included in this document. Information on the nature of the review
processes and the availability of the source documents is presented in
Appendix 1. Information on the peer review of this CICAD is presented
in Appendix 2. This CICAD was approved as an international assessment
at a meeting of the Final Review Board, held in Tokyo, Japan, on 30
June - 2 July 1998. Participants at the Final Review Board meeting are
listed in Appendix 3. The International Chemical Safety Card (ICSC
1282) for TBTO, produced by the International Programme on Chemical
Safety (IPCS, 1996), has also been reproduced in this document.
In this document, the term TBTO is used when that specific
chemical is intended. In the environment, however, tributyltin
compounds are expected to exist mainly as tributyltin hydroxide,
tributyltin chloride, and tributyltin carbonate. In those cases or
when the identity of the specific chemical is not clear, the general
term tributyltin is used.
TBTO is an effective biocidal preservative for wood, cotton
textiles, paper, and paints and stains for residential homes. It is
added as an antifouling agent in numerous formulations of marine
paints. Tributyltin is present in most of these antifouling
formulations as an organometallic copolymer. Tributyltin is slowly
released from the painted surface as the polymer is hydrolysed in
seawater, providing protection against encrustations for as long as
4-5 years.
As a result of its low water solubility and lipophilic character,
tributyltin adsorbs readily onto particles. Its half-life in the water
column ranges from a few days to weeks. Tributyltin may persist in
sediments for several years. It bioaccumulates in organisms, with the
highest concentrations found in liver and kidney. Uptake from food is
more important than uptake directly from water.
No information is available on the toxicity of TBTO in humans
following long-term exposure. Some data and case reports indicate that
TBTO is a severe dermal and respiratory irritant. The data, however,
are not adequate to characterize the exposure-response relationships.
Some studies have quantified human exposure to tributyltin from the
diet in Japan.
TBTO is moderately to highly acutely toxic to laboratory mammals
in short-term studies. In numerous well-conducted studies, both short
term and long term, the critical effect of TBTO is immunotoxicity
(depression of immune functions dependent on the thymus). The
no-observed-adverse-effect level (NOAEL) for immunosuppression in rats
following long-term exposure is 0.025 mg/kg body weight per day.
Benchmark dose analysis shows that the exposure corresponding to the
lower confidence limit (95%) on dose for a 10% decrease in
immunoglobulin (Ig) E titre in rats is 0.034 mg/kg body weight per
day. In a carcinogenicity study in rats, there was an increased
incidence of some tumours in some endocrine tissues. These tumours
occur spontaneously with variable incidence in the strain of rat used
in the study and are of unknown significance for a human health risk
assessment. TBTO is not carcinogenic in mice. The weight of evidence
shows that TBTO is not genotoxic. There is no indication that
reproductive or developmental effects occur at an exposure below that
identified as the NOAEL for immunotoxicity. Effects on reproduction
and development occur only at exposures near those causing maternal
toxicity. Data show that TBTO is a severe respiratory tract and skin
irritant. Based on the NOAEL for immunotoxicity and an uncertainty
factor of 100, a guidance value for oral exposure is 0.0003 mg/kg body
weight per day. No adequate data are available to derive a guidance
value for inhalation exposure.
TBTO is extremely hazardous to some aquatic organisms. It is an
endocrine disruptor in some organisms. The concentration of
tributyltin in some coastal waters is above a concentration causing
severe adverse effects. Adverse effects have been sufficiently severe
to lead to reproductive failure and population decline in some areas.
The general hazard to the terrestrial environment is likely to be low.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
Tributyltin oxide (CAS No. 56-35-9; C24H54OSn2;
bis-[tri- n-butyltin]-oxide; tri- n-butyltin oxide; TBTO; hexabutyl
distannoxane) has the structural formula
(CH3CH2CH2CH2)3Sn-O-Sn(CH2CH2CH2CH3)3. It is flammable but
does not form explosive mixtures with air. TBTO is a mild oxidizing
agent. In the presence of oxygen, light, or heat, slow breakdown
occurs. The solubility of TBTO in water ranges from <1 to >100 mg/
litre, depending on temperature and pH. TBTO is soluble in lipids and
very soluble in a number of organic solvents (e.g., ethanol, ether,
halogenated hydrocarbons). Its octanol/water partition coefficient
(log Kow) lies between 3.19 and 3.84 for distilled water and is
3.54 for seawater. Additional physical/chemical properties are
presented in the International Chemical Safety Card reproduced in this
document.
3. ANALYTICAL METHODS
Several methods are used for measuring tributyltin derivatives in
water, sediment, and biota (IPCS, 1990). Atomic absorption
spectrometry is the most common method used for all media. Flame
atomic absorption spectrometry has a detection limit of 0.1 mg/litre
in water. Flameless atomic absorption spectrometry, using atomization
in an electric furnace with graphite, is more sensitive and allows
detection limits of between 0.1 and 1.0 µg/litre. Recent modifications
using a gas chromatograph equipped with a flame photometric detector
allow a detection limit of 1 ng/litre (Tolosa et al., 1996).
Tributyltin can be separated from the sample matrix by capillary
supercritical fluid chromatography and determined by inductively
coupled plasma mass spectrometry. A detection limit of 12.5 pg was
obtained (Vela & Caruso, 1993). There are several different methods of
extracting tributyltin from sediment and biota and forming volatile
derivatives. The detection limits are 0.5 and 5.0 µg/kg for sediment
and biota, respectively (Vela & Caruso, 1993).
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
Tributyltin compounds have been registered as molluscicides; as
antifoulants on boats, ships, quays, buoys, crab pots, fish nets, and
cages; as wood preservatives; as slimicides on masonry; as
disinfectants; and as biocides for cooling systems, power station
cooling towers, pulp and paper mills, breweries, and leather
processing and textile mills. Tributyltin in antifouling paints was
first marketed in a form that allowed free release of the compound.
More recently, controlled-release paints, in which the tributyltin is
incorporated in a copolymer matrix, have become available. Rubber
matrices have also been developed to give long-term slow release and
lasting effectiveness for antifouling paints and molluscicides.
Government restrictions have decreased the global use of tributyltin
compounds in antifouling paints on small boats.
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
As a result of its low water solubility and lipophilic character,
tributyltin adsorbs readily onto particles (IPCS, 1990). Between 10%
and 95% of TBTO introduced into water is estimated to undergo
adsorption onto particulate matter. Progressive disappearance of
adsorbed TBTO is due to degradation, not desorption. The degree of
adsorption depends on the salinity of the water, the nature and size
of particles in suspension, the amount of suspended matter,
temperature, and the presence of dissolved organic matter.
The degradation of TBTO involves the splitting of the carbon-tin
bond (IPCS, 1990). This can result from various processes -- both
physicochemical (hydrolysis and photodegradation) and biological
(degradation by microorganisms and metabolism by higher organisms)
-- occurring simultaneously in the environment. Although the
hydrolysis of organotin compounds occurs under conditions of extreme
pH, it is barely evident under normal environmental conditions.
Photodegradation occurs during laboratory exposures of solutions to
ultraviolet light at 300 nm (and to a lesser extent at 350 nm). Under
natural conditions, photolysis is limited by the wavelength range of
sunlight and by the limited penetration of ultraviolet light into
water. The presence of photosensitizing substances can accelerate
photodegradation. Biodegradation depends on environmental conditions
such as temperature, oxygenation, pH, the level of mineral elements,
the presence of easily biodegradable organic substances for
co-metabolism, and the nature of the microflora and its capacity for
adaptation. It also depends on whether the TBTO concentration is lower
or higher than the lethal or inhibitory threshold for the
microorganisms. As with abiotic degradation, biotic breakdown of
tributyltin is a progressive oxidative debutylization founded on the
splitting of the carbon-tin bond. Dibutyltin derivatives, which are
more readily degraded than tributyltin, are formed. Monobutyltins are
mineralized slowly. Although anaerobic degradation occurs, there is a
lack of agreement as to its importance; some consider it to be slow,
whereas others believe that it is more rapid than aerobic degradation.
Species of bacteria, algae, and wood-degrading fungi have been
identified that can degrade TBTO. Estimates of the half-life of
tributyltin in the environment vary widely. The half-life in the water
column ranges from a few days to weeks. Tributyltin can persist in
sediments for several years.
Bioconcentration factors (BCFs) of up to 7000 have been reported
in laboratory investigations with molluscs and fish, and higher values
have been reported in field studies (IPCS, 1990). Bioaccumulation in
bivalves is especially high because of the low capacity for
metabolism. In molluscs, uptake from food is more important than
uptake directly from water. Higher BCFs in microorganisms (between 100
and 30 000) may reflect adsorption rather than uptake into cells
(IPCS, 1990). A recent publication reported a range of BCFs in the
Pacific oyster ( Crassostrea gigas) of 2400-7800 (Li et al., 1997).
Another recent publication reported a range of biomagnification
factors in marine mammals of 0.6-6.0 (Madhusree et al., 1997).
Although it has been suggested that tributyltin accumulates in
organisms because of its solubility in fat (IPCS, 1990), recent work
suggests that this might not be the case. Although tributyltin
residues in blubber of marine mammals have been reported (Iwata et
al., 1994, 1995, 1997), levels were considerably higher in other
tissues, notably liver (Iwata et al., 1994, 1995, 1997; Kannan et al.,
1996, 1997, 1998; Kim et al., 1996a,b; Madhusree et al., 1997; Tanabe,
1998; Tanabe et al., 1998). Comparison of patterns of tributyltin
residues with those of fat-soluble organochlorines in marine mammals
showed marked differences. Unlike the organochlorines, tributyltin
residues were the same in both sexes and remained constant after
animals reached maturity. It has been suggested that transfer through
milk to offspring, a marked trend with the organochlorines, does not
occur with tributyltin. Cetaceans showed greater bioaccumulation than
pinnipeds (Kim et al., 1996c). There has also been a report of
accumulation in liver and kidney of seabirds (Guruge et al., 1997).
Stäb et al. (1996) recently determined organotin compounds in the food
web of a shallow freshwater lake; in birds in the food web, the
highest concentrations of organotin compounds were also in liver and
kidney, not in subcutaneous fat. The various authors cited suggest
protein binding in liver to be the major mechanism of bioaccumulation.
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
Tributyltin compounds have been found in water, sediment, and
biota in areas close to pleasure boating activity, especially in or
near marinas, boat yards, and dry docks; in fish nets and cages
treated with antifouling paints; and in areas near cooling systems
(IPCS, 1990). The degree of tidal flushing and the turbidity of the
water influence tributyltin concentrations. As reported in IPCS
(1990), tributyltin levels have been found to reach 1.58 µg/litre in
seawater and estuaries; 7.1 µg/litre in fresh water; 26.3 mg/kg in
coastal sediments; 3.7 mg/kg in freshwater sediments; 6.39 mg/kg in
bivalves; 1.92 mg/kg in gastropods; and 11 mg/kg in fish. However,
these maximum concentrations of tributyltin should not be taken as
representative, because a number of factors, such as paint particles
in water and sediment samples, may give rise to anomalously high
values. It has been found that measured tributyltin concentrations in
the surface microlayer of both fresh water and seawater are up to two
orders of magnitude above those measured just below the surface.
However, it should be noted that recorded levels of tributyltin in
surface microlayers may be highly affected by the method of sampling.
More recent data (collected up to the mid-1990s) have documented
a decline in tributyltin levels in the environment, presumably due to
the restrictions placed on the use of antifouling paints on vessels
(CEFIC, 1994; Ruiz et al., 1996; Stronkhorst, 1996; Tolosa et al.,
1996; NIVA, 1997; dela Cruz & Molander, 1998). Recent data have also
documented a seasonal variation in the concentration of tributyltin in
a freshwater marina; the concentration was highest in late spring and
showed a progressive decline until winter (Fent & Hunn, 1991). This
same study also documented that the tributyltin concentration in
sediment decreased progressively with depth. In areas where the
tributyltin concentrations in water and sediment have been monitored
in the same location, the concentration of tributyltin in the water
has declined more rapidly than the concentration of tributyltin in
sediment (Stronkhorst, 1996). The range of concentrations reported in
coastal waters and estuaries is 1-10 ng/litre; the range reported for
water in marinas and major ports is 20-460 ng/litre. Most of the
sediment samples analysed contained less than 100 µg/kg, although some
samples exceeded 1000 µg/kg. The highest value reported for a sediment
sample obtained from a port in Sweden was 10 940 µg/kg. The range of
concentrations reported in biota is 0.01-3 mg/kg (dela Cruz &
Molander, 1998).
There are a number of reports on the occurrence of tributyltin
residues in marine organisms. Levels of total butyltin residues (the
sum of detected tributyltin, dibutyltin, and monobutyltin) of 5-230
ng/g in muscle of fish (Kannan et al., 1995, 1996, 1997), 300 ng/g in
liver and kidney of marine birds (Guruge et al., 1997), and 13-395
ng/g in muscle of marine mammals have been reported (Iwata et al.,
1994, 1995, 1997; Kannan et al., 1997). In marine mammals, much higher
total butyltin residues were reported for blubber (48-744 ng/g),
kidney (25-3210 ng/g), and liver (40-11 340 ng/g) (Iwata et al., 1994,
1995, 1997; Kannan et al., 1996, 1997, 1998; Kim et al., 1996a,b,c;
Madhusree et al., 1997; Tanabe, 1998; Tanabe et al., 1998).
Geographical comparisons showed greater accumulation of residues close
to coasts compared with the open sea and in the vicinity of developed
compared with developing countries.
6.2 Human exposure
Information on tributyltin concentrations in various media that
are relevant to estimation of human exposure is extremely limited,
being restricted to data from Japan.1 It is unknown if this
information is representative of other areas, and additional
investigation is desirable.
Tsuda et al. (1995) investigated the daily intakes of tributyltin
compounds from meals in Shiga Prefecture, Japan. Daily intakes of TBTO
determined by the duplicate-portion method were 4.7 + 7.0 µg/day in
1991 ( n = 39) and 2.2 + 2.2 µg/day in 1992 ( n = 40). Using the
market basket method, the daily intake was estimated at 6-9 µg/day in
1991 and 6-7 µg/day in 1992. The TBTO was found mostly in seafood.
Market basket studies in 10 local regions in Japan have shown
that the national average daily intake of tributyltin (expressed as
tributyltin chloride) was 3.7, 9.9, 5.4, 3.6, 2.9, 1.6, 1.5, and 2.3
µg/day in 1990, 1991, 1992, 1993, 1994, 1995, 1996, and 1997,
respectively.1 Variation among the local regions reflects differences
in food intake patterns as well as differences in tributyltin levels
in local fisheries.
Recent preliminary data (Takahashi et al., 1998) suggest the
potential for non-food sources of exposure -- for example, consumer
products such as rubber gloves and baking sheets.
1 Dr J. Sekizawa's (National Institute of Health Sciences, Tokyo,
Japan) review of unpublished data.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
Little definitive information is available on the
pharmacokinetics of TBTO (see IPCS, 1990, and references therein).
TBTO is absorbed from the gut (20-50%, depending on the vehicle) and
via the skin of mammals (approximately 10%). Other data suggest
absorption in the 1-5% range via the skin.1 TBTO can be transferred
across the blood-brain barrier and from the placenta to the fetus.
Following 14 days of oral administration, steady-state levels in
tissue are reached after 3-4 weeks. Absorbed material is rapidly and
widely distributed among tissues (principally the liver and kidney).
Metabolism in mammals is rapid; metabolites are detectable in the
blood within 3 h of TBTO administration. The principal metabolite
appears to be the hydroxybutyl compound, which is unstable and rapidly
splits to form the dibutyl derivative and butanol. In in vitro
studies, it has been shown that TBTO is a substrate for mixed-function
oxidases, but these enzymes are inhibited by very high concentrations
of TBTO. The rate of TBTO loss differs with different tissues. TBTO
and its metabolites are eliminated principally via the bile. The
calculated half-time for elimination of TBTO residues in mice is 29
days (Brown et al., 1977).
Tributyltin metabolism also occurs in lower organisms, but it is
slower, particularly in molluscs, than in mammals. The capacity for
bioaccumulation is, therefore, much greater in lower organisms than in
mammals.
There are some recent preliminary data (Takahashi et al., 1998)
on the occurrence of total butyltin residues in human liver. The
average concentration in four samples was 84 ng/g wet weight (range
59-96 ng/g). The concentration of tributyltin was less than the
detection limit of 2 ng/g. The concentration of dibutyltin was 79% of
the total.
1 Letter and attachments from J.A. Jonker, Elf Atochem, to D.J.
Stenhouse, Health and Safety Executive, United Kingdom, dated
3 February 1997.
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
Extensive data are available on the toxicity of TBTO. Detailed
descriptions of studies critical to the evaluation of TBTO follow; all
other studies are described in US EPA (1997) or IPCS (1990).
Collectively, these data establish that immunotoxicity is the critical
effect of TBTO. A detailed evaluation of these effects is found in
section 8.7. All studies involving repeated oral exposure are listed
in Table 1.
8.1 Single exposure
TBTO is moderately to highly acutely toxic to laboratory mammals.
Acute oral LD50 values range from 127 to 234 mg/kg body weight for
the rat and average 85 mg/kg body weight for the mouse (IPCS, 1990).
TBTO exhibits greater lethal potential when administered parenterally
(20 and 16 mg/kg body weight in the rat and mouse, respectively) as
opposed to orally, probably as a result of only partial absorption
from the gut. Single-exposure studies using 100 mg TBTO/kg body weight
by oral gavage (Funahashi et al., 1980) demonstrated a transient
increase in adrenal weight shortly after exposure (returning to normal
within 2 days) and a transient effect on thyroid follicles (distension
with flat epithelial cells). In addition, there were reversible
effects on the pituitary and on levels of adrenocorticotrophic
hormones, thyroid-stimulating hormone, thyroxine, and serum cortisol.
The acute dermal LD50 in rabbits is about 9000 mg/kg body weight.
Truhaut et al. (1979) exposed mice to an aerosol of TBTO in olive
oil for either a single 1-h period or seven 1-h periods on successive
days, using TBTO concentrations in air ranging between 50 and 400
mg/m3. Exploratory behaviour was scored over 5-min periods 2 h after
the single exposure was complete or 24 h after the last of the seven
exposure periods. The lower two exposures caused a significant
increase in exploratory behaviour (17% and 5% for 42 and 84 mg/kg body
weight, respectively), whereas the higher exposures reduced
exploratory behaviour (-18% and -38% for 170 and 340 mg/kg body
weight, respectively).
Schweinfurth & Gunzel (1987) summarized the results of several
inhalation studies in laboratory animals. After a single 4-h exposure
of rats to aerosols of TBTO, signs of irritation (nasal discharge,
lung oedema, and congestion of the pulmonary circulation) and
enteritis were observed. The LC50 was 77 mg/m3 (total particles) or
65 mg/m3 (particles with a diameter <10 µm). In guinea-pigs exposed
to aerosols of TBTO in olive oil at 200 mg/m3 and above, death
occurred within 1 h of exposure. Ten male and 10 female rats were
exposed to almost saturated vapours of TBTO (concentration not
specified) without a single death occurring during exposure for 7 h or
the following 14-day observation period. Only minor clinical signs
(slight nasal discharge directly after exposure) were noted. For this
study, the authors reported no information on particle size or the
end-points evaluated.
Table 1: Summary of oral toxicity studies on TBTO.
Toxicity/ Study length End-point LOAEL NOAEL Reference
species (mg/kg body (mg/kg body
weight per day) weight per day)
General
Monkey 22 weeks Decreased leukocyte 0.14 - Karrer et
counts al., 1992
Rat 24 months Decreased survival, 2.1 0.19 Wester et al.,
changes in kidney 1987, 1988, 1990
and organ weights,
increased serum
IgA and IgM
Mouse 18 months Decreased survival 0.7 (frank - Daly, 1992
effect level)
Reproductive
Rat 2 generations Reproduction - 4.42 Schroeder, 1990
Decreased pup 3.43 0.34
weight
Developmental
Rat gestation days Decreased maternal 9 5 Schroeder, 1981
weight
6-19 Increased 5 -
ossification
variations
Rat gestation days Decreased maternal 10 5 Crofton et al., 1989
weight
6-20 Decreased pup 10 5
weight and survival
Table 1 (continued)
Toxicity/ Study length End-point LOAEL NOAEL Reference
species (mg/kg body (mg/kg body
weight per day) weight per day)
Mouse gestation days Decreased maternal 11.7 5.8 Davis et al., 1987
weight
6-15 Decreased fetal 23.4 11.7
weight; increased
skeletal
abnormalities
Mouse gestation days Decreased maternal 40 20 Baroncelli et al., 1990
weight
6-15 Increased 40 20
resorptions;
decreased fetal
weight
Mouse gestation days Haematology - 20 Karrer et al., 1995
6-15
Immunological
Rat 28 days Thymus-dependent 5 0.5 Verdier et al., 1991
immunity
Rat 4 weeks Lymph node 0.5 - Krajnc et al., 1984;
haemorrhage Vos et al., 1984
Rat 1 week; Lymph node 0.4 - Bressa et al., 1991
4 weeks haemorrhage
Rat 6 weeks Virus titres 2 - Garssen et al.,
1995
Table 1 (continued)
Toxicity/ Study length End-point LOAEL NOAEL Reference
species (mg/kg body (mg/kg body
weight per day) weight per day)
Rat 6 weeks Reduced thymus 8 2 Van Loveren et al.,
weight 1990
Rat 6 weeks Reduced 2 - Vos et al., 1984
thymus-dependent
immunity and
non-specific
resistance
Rat 6 weeks Decreased IL+2R 0.5 - Vandebriel et al.,
alpha mRNA; 1998
reduced CD25
expression
Rat 13-26 weeks Reduced thymus 3 - Funahashi et al.,
weight 1980
Rat 18 weeks Reduced thymus 16 - Carthew et al.,
weight 1992
Rat, aged 5 months Thymus-dependent 2.5 0.25 Vos et al., 1990
immunity
Rat, weanling 4.5 or Thymus-dependent 0.25 0.025 Vos et al., 1990
18 months immunity
Mouse gestation days Humoral and 0.1 - Buckiova et al.,
4-17 or 11-17 cell-mediated 1992
immunity
Rat 10 doses to Depressed mitogen 5 2.5 Smialowicz et al.,
pre-weanlings response 1989
8.2 Irritation and sensitization
TBTO is a potent skin irritant and an extreme eye irritant (IPCS,
1990). It is not a skin sensitizer (IPCS, 1990). Poitou et al. (1978)
investigated the skin-sensitizing potential of TBTO in guinea-pigs
using the Magnussen-Kligman method. The concentrations used for
sensitization were 1% (intradermal phase) and 5% (topical phase).
Using challenge concentrations of 0.25% and 0.1%, no sensitizing
action was demonstrated in the 20 test animals. It is not clear from
IPCS (1990) whether these challenge concentrations represented the
maximum non-irritant concentrations or what positive control
substances were used to verify the sensitivity of the assay. A recent
study (Stringer et al., 1991) demonstrated contact sensitivity in the
mouse.
8.3 Short-term exposure
Short-term studies focusing on effects on the immune system
following oral exposure are listed in Table 1.
In the only study involving repeated inhalation exposure that
reported effects in the respiratory tract, rats (10 males and 10
females per dose) were exposed in "nose-only" chambers for 4 h to TBTO
doses of 0, 0.03 (vapour), 0.16 (vapour), or 2.8 (aerosol) mg/m3, 5
days/week, for a total of 21-24 treatments (Schweinfurth & Gunzel,
1987). At the highest dose, severe toxic effects were produced.
Mortality was 5/10 in males and 6/10 in females. In addition,
inflammatory reactions (not further specified) in the total
respiratory tract and histological changes (not further specified) in
the lymphatic organs were observed. No local or systemic changes were
observed at the lower doses. The authors did not, however, report what
end-points were evaluated.
8.4 Long-term exposure
8.4.1 Subchronic exposure
A large number of well-conducted subchronic studies have been
conducted in rats focusing on toxicity to the immune system. These
studies and their NOAELs and lowest-observed-adverse-effect levels
(LOAELs) are listed in Table 1 and summarized in section 8.7.
Effects of TBTO (purity 96%) on haematology and serum chemistry
were assessed in groups of three and four adult male cynomolgus
monkeys that ingested doses of 0 or 0.160 mg/kg body weight per day,
respectively, 6 days/week for 22 weeks (0 and 0.14 mg/kg body weight
per day, actual intake) (Karrer et al., 1992). The TBTO was dissolved
in vegetable oil and added to Tween 80-augmented pear juice, which the
monkeys drank. Study end-points consisted of clinical observations,
body weight, and standard haematology and clinical chemistry indices,
including serum immunoglobulin (IgM and IgG) levels. A progressive
decrease in total leukocyte counts occurred during the first 10 weeks
of exposure (significantly [ P < 0.05] lower than controls at weeks
8 and 10; 67% of control value at week 10). Leukocytes subsequently
increased and were similar to controls between weeks 10 and 16, but
decreased again between weeks 16 and 20 (61.5% of control value at
week 20; P < 0.05). No significant alterations in differential
leukocyte count, serum immunoglobulins, or other study parameters were
observed. Based on decreased total leukocyte levels, 0.14 mg/kg body
weight per day (the only dose tested) is a LOAEL in monkeys.
8.4.2 Chronic exposure and carcinogenicity
Well-conducted studies are available in rats and mice. A study in
dogs (Schuh, 1992) is fatally flawed and is not reported. Long-term
studies assessing effects on the immune system are reported in section
8.7 and listed in Table 1.
In a chronic toxicity/carcinogenicity study, groups of 60 male
and 60 female Wistar rats were exposed to dietary TBTO (0.5, 5, and 50
mg/kg diet) for 2 years (Wester et al., 1987, 1988, 1990). Based on
estimates of average body weight and food consumption from reported
data, ingested dosages were approximately 0.019, 0.19, or 2.1 mg/kg
body weight per day in males and 0.025, 0.25, or 2.5 mg/kg body weight
per day in females. End-points that were evaluated included clinical
abnormalities, survival, body weight, food and water consumption, and
the incidence of neoplastic lesions. Haematology, urinalysis, clinical
chemistry (including immunoglobulins IgG, IgM, and IgA), and
endocrinology (total thyroxine and free thyroxine, thyrotropin,
luteinizing hormone, follicle-stimulating hormone, insulin) were
evaluated in 10 rats per sex per dose after approximately 3, 12, and
24 months (endocrinology not assessed at 3 months). Organ weights and
histology were evaluated in 10 rats per sex per dose after 12 and 24
months, and histology was also evaluated in all moribund rats as well
as rats surviving until 24 months.
No treatment-related adverse changes were found in males or
females at the lowest dose. Serum immunoglobulin levels were
significantly ( P < 0.05, Student's t-test) increased in the
high-dose group. Concentrations of IgA were increased in both sexes
after 12 and 24 months; at 24 months, levels of IgA were 508% of the
control value in males ( P < 0.001) and 294% of the control value in
females ( P < 0.01). Concentrations of IgG were significantly
( P < 0.01) reduced in females after 3 months (42% of the standard
serum value compared with 69-71% in controls and other treated groups)
and 12 months (80% compared with 124-127%), but not after 24 months or
in males. Concentrations of IgM were increased in both sexes after 3,
12, and 24 months; at 24 months, the IgM level was 258% of the
standard serum value in males ( P < 0.01) and 240% of the standard
value in females ( P < 0.01).
Other effects occurred predominantly in high-dose rats, including
decreased survival, decreased body weight, and changes in organ
weights. At termination, survival in females in the high-dose group
was 54% versus 74% in controls; survival in males in the high-dose
group was 40% versus 60% in controls. Terminal body weights at this
dose were approximately 13% (male) and 9% (female) lower than
controls. Absolute liver, kidney, adrenal gland (male only), and heart
(male only) weights were increased and thyroid weight (female only)
was decreased in high-dose rats at study termination; relative organ
weights were not reported. The liver weight was increased 36% and 29%
in males and females, respectively; the kidney weight was increased
29% and 33% in males and females, respectively; the adrenal weight in
males and females was increased 630% and 44%, respectively; the heart
weight in males was increased 13%; and the thyroid weight in females
was decreased 26%.
Treatment-related non-neoplastic histological changes occurred in
the liver, spleen, and thyroid of high-dose males and females.
Histological effects after 12 months included slight bile duct changes
(characterized by hyperplasia, cellular hypertrophy, and minimal
infiltration of mononuclear cells or by cholangiofibrosis), decreased
haemosiderin content in spleen (qualitative analysis only), and
decreased thyroid follicular epithelial cell height. Examination after
24 months showed that only the histological changes in the thyroid
persisted. There were no accompanying significant changes in
concentrations of serum thyroid hormones. The incidence and severity
of age-related degenerative changes in the kidney (nephrosis and
vacuolation and pigmentation of the proximal tubular epithelium,
suggestive of iron and/or lipofuscin) were increased in high-dose
males and females after 24 months.
Neoplastic lesions were examined in the control and high-dose
groups; if differences were observed, the intermediate-dose groups
were also examined for those tumour types. Increased incidences of
benign pituitary tumours, pheochromocytomas in the adrenal medulla,
and parathyroid adenomas were noted. These data are shown in Table 2.
There are increases in the incidence of some benign spontaneous
tumours at the high dose in some endocrine tissues. According to the
authors, these tumours normally occur in this strain of rats with high
and variable background incidence (Kroes et al., 1981; Wester et al.,
1985). In the two data sets available for these tumour types in the
strain of Wistar rats used by Wester et al. (1990), the reported
background occurrence of pituitary tumours (adenomas plus carcinomas)
in females was 52% and 55% and in males was 34% and 87%; the reported
background incidence of pheochromocytomas (benign plus malignant) in
females was 8% and 16% and in males was 22% and 58%. The authors
reported no data on the background occurrence of parathyroid tumours.
There was no significant endocrine imbalance documented in the
study. No significant change was observed in the serum levels of
thyroid-stimulating hormone, luteinizing hormone, follicle-stimulating
hormone, insulin, total thyroxine, or free thyroxine. There was,
however, a decrease in the free thyroxine: total thyroxine ratio for
both sexes at 12 and 24 months in the high-dose group and after 12
months in the mid-dose group. Although the pituitary tumours stained
for the presence of prolactin, there was no correlation between the
serum level of prolactin or the occurrence of hyperplastic or
neoplastic mammary tissue and the presence of pituitary tumour.
Based on the constellation of changes (increased mortality,
increased serum immunoglobulins, changes in organ weight, and
histopathological changes) observed at the highest dose, the LOAEL is
2.1 mg/kg body weight per day and the NOAEL is 0.19 mg/kg body weight
per day (Wester et al., 1987, 1988, 1990).
TBTO (purity 97.1%) was fed to groups of 50 male and 50 female
CD-1 mice in dietary concentrations of 0, 5, 25, or 50 mg/kg for 18
months in a study primarily designed to assess carcinogenicity (Daly,
1992). Based on food consumption and body weight data, mean compound
intake was reported to be 0, 0.7, 3.7, or 7.7 mg/kg body weight per
day in males and 0, 0.9, 4.8, or 9.2 mg/kg body weight per day in
females.
Statistically significant decreases in survival occurred in
treated mice of both sexes. In males, survival after 18 months was 67,
52, 42, and 42% in the control, low-, mid-, and high-dose groups,
respectively ( P < 0.05, all doses). Survival in females at 18
months was 59, 48, 40, and 27% in the control, low-, mid-, and
high-dose groups, respectively ( P < 0.05 except for low-dose
group). No information on cause of death was available. Other
treatment-related effects included significantly decreased food
consumption and increased absolute and relative liver weights in
females at the high dose. Incidences of gross liver enlargement and
discoloration were slightly increased in both sexes in all dose
groups. The gross liver changes are not considered biologically
significant because of the slight changes and absence of hepatic
histopathological alterations. Increased incidences of common
spontaneous non-neoplastic lesions, particularly
glomerular/interstitial amyloidosis of the kidney, were found.
Incidences of renal amyloidosis were increased in females in all dose
groups (50, 67.7, and 78.4%, respectively, compared with 34.8% in
controls) but not in males. The progression of this lesion appeared to
be more rapid in both sexes at the two highest doses, indicating a
compound-related effect. There were no statistically significant
increases in the incidence of any tumours or groups of tumours in
males or females. TBTO was not carcinogenic in this study in mice.
This study identified an effect level for mortality at 0.7 mg/kg body
weight per day (the lowest dose tested) (Daly, 1992).
Table 2: Neoplastic lesions in rats.a
Incidence of Incidence of Incidence of
pituitary tumoursb adrenal parathyroid
pheochromocytomasb adenomas
Concentration Female Male Female Male Female Male
of TBTO
(mg/kg diet)
0 22/50 34/50 3/50 16/50 0/64 0/39
0.5 32/50* 39/50* 3/50 13/50 0/4 42/50
5 22/50 29/50 3/50 14/50 1/40 1/51
50 35/50** 43/50*** 34/50*** 33/50*** 1/44 6/43c
a From Wester et al. (1990).
b Statistical analysis was carried out according to Peto et al. (1980), one tailed. Values marked
with asterisks differ significantly from the corresponding control values (* P < 0.05; ** P < 0.01;
*** P < 0.001).
c The value differs significantly (chi-square test) from the corresponding control (P < 0.01).
8.5 Genotoxicity and related end-points
The genetic effects of TBTO were evaluated in multiple in vivo
and in vitro short-term tests (Davis et al., 1987). TBTO was not
mutagenic in the rec assay in Bacillus subtilis, did not induce
reverse mutations in Klebsiella pneumoniae, and did not produce
point mutations in Salmonella typhimurium strains TA1530, TA1535,
TA1538, TA97, TA98, or TA100 in the presence or absence of a rat liver
activation system (Davis et al., 1987). TBTO was mutagenic in
S. typhimurium strain TA100 in a fluctuation test, but only in the
presence of rat liver S9 (Aroclor-induced) and at cytotoxic
concentrations. TBTO did not induce gene mutations in
Schizosaccharomyces pombe, mitotic gene conversions in
Saccharomyces cerevisiae, or sister chromatid exchange in Chinese
hamster ovary cells in the presence or absence of rat or mouse liver
S9. Structural chromosomal aberrations, endoreduplicated and polyploid
cells, were observed in Chinese hamster ovary cells. The aberrations
were observed only at 8 h after treatment (not at 15 or 24 h) and only
at the highest concentration tested in the presence of S9.
Cytotoxicity was also observed at this concentration. TBTO did not
induce gene mutations in V79 Chinese hamster cells or in mouse
lymphoma cells. It did not induce recessive lethal mutations in adult
male Drosophila melanogaster by either feeding or injection. Doses
of 0.37 or 0.74 mmol/litre did not increase the number of X-linked
recessive mutations. An increased number of micronuclei was observed
in polychromatic erythrocytes of male BALB/c mice 48 h after a single
oral dose of TBTO (60 mg/kg body weight). A reanalysis of the slides
from the high-dose group, however, failed to confirm the increase in
micronuclei (IPCS, 1990). A lower dose (30 mg/kg body weight) was
ineffective. Neither dose induced micronuclei 30 h after treatment.
One report demonstrated that TBTO and triphenyltin chloride are
co-clastogens in a whole mammalian system (Yamada & Sasaki, 1993). The
frequency of micronuclei induced by mitomycin C in mouse peripheral
reticulocytes was enhanced approximately 50% when 50 mg TBTO/kg body
weight and 100 mg triphenyltin chloride/kg body weight were given
orally to mice. No effect was observed when the chemicals were
administered separately.
In aggregate, despite the limited positive findings at cytotoxic
concentrations, the weight of evidence shows that TBTO is not
genotoxic. This conclusion remains consistent with the previous
evaluation by IPCS (1990).
8.6 Reproductive and developmental toxicity
Several well-conducted studies are available that investigated
the effects of TBTO on the reproductive system and fetal development
in rats and mice. The results of these studies are listed in Table 1.
These studies show no evidence that TBTO is a significant reproductive
or developmental toxicant in rodents. The developmental effects noted
in the various studies occur at or near the exposure that also causes
maternal toxicity (depressed body weight or impaired weight gain
during pregnancy). The LOAELs for maternal toxicity in rats and mice
are approximately 10 mg/kg body weight per day, with NOAELs of
approximately 5 mg/kg body weight per day.
8.7 Immunological and neurological effects
8.7.1 Immunotoxicity
A large number of well-conducted studies have shown that TBTO
causes depression of immune functions dependent on the thymus. Results
from a number of short-term studies are listed in Table 1. Subchronic
and chronic studies (Vos et al., 1990) are summarized in detail below
and are listed in Table 1. The chronic study conducted by Vos et al.
(1990) shows effects on thymus-dependent immune responses at a dose
lower than that at which any other toxic effects have been observed.
The Vos et al. (1990) study also establishes that weanling animals are
more sensitive than adults to the effects of TBTO. For example,
following subchronic exposure, the LOAEL in weanling rats was 0.25
mg/kg body weight per day, whereas the LOAEL in aged rats was 2.5
mg/kg body weight per day. The NOAELs were 0.025 and 0.25 mg/kg body
weight per day, respectively. Data from Buckiova et al. (1992) and
Smialowicz et al. (1989) also show that exposure of mice in utero
and exposure of rat pups prior to weaning cause effects at exposures
lower than those required for the same effects in adult animals.
In a subchronic immunotoxicity study (Vos et al., 1990; a
companion to the chronic study summarized below), aged (1-year-old)
male Wistar rats were exposed for 5 months to the same diets used in
the principal study. Based on the authors' statement from the chronic
study (see below), estimated compound intake was 0, 0.025, 0.25, or
2.5 mg/kg body weight per day. End-points were the same as some of
those evaluated in the chronic study.
Compound-related effects occurred only in the high-dose group and
consisted of significantly decreased thymus weight (39% lower than
controls, P < 0.01), impaired resistance to Trichinella spiralis
(indicated by increased recovery of adult worms from the small
intestine [780% higher than controls; P < 0.01] and number of
larvae in muscle [80% higher; P < 0.001]), and impaired resistance
to Listeria monocytogenes (indicated by approximately 300% increased
splenic bacterial count; P < 0.05).
Subchronic and chronic immunotoxicity studies were conducted in
which weanling SPF-derived Riv:TOX Wistar rats were fed TBTO (purity
95.3%) at concentrations of 0, 0.5, 5, or 50 mg/kg. Male rats (females
not tested) were evaluated following exposure to TBTO for up to 18
months (Krajnc et al., 1987; Vos et al., 1990). The authors reported
the 5 mg/kg dietary concentration to be equivalent to a dose of 0.25
mg/kg body weight per day, indicating that estimated test doses were
0.025, 0.25, and 2.5 mg/kg body weight per day. Body weight, absolute
thymus weight, and absolute spleen weight were measured in groups of
18, 12, and 12 rats, respectively, following exposure for 4.5 months.
Immunological function studies for specific and non-specific
resistance were performed in 9-12 rats per group after 4-6 or 15-17
months of exposure. Antigen-specific functional assays evaluated IgM
and IgG responses to sheep red blood cells (immunized after
16 months); IgM and IgG responses to ovalbumin and delayed-type
hypersensitivity (24-, 48-, and 72-h) responses to ovalbumin and
Mycobacterium tuberculosis (immunized after 6 or 15 months of
exposure); and resistance to oral infection by T. spiralis larvae
(infected after 5.5 or 16.5 months).
Non-specific resistance was assessed by splenic clearance of
intravenously injected L. monocytogenes bacteria (after 5 or 17
months of exposure) and natural cell-mediated cytotoxicity of spleen
cells (after 4.5 or 16 months of exposure) and peritoneal cells (after
4.5 months of exposure only) using a 4-h 51Cr-release assay with
YAC-lymphoma target cells. Non-specific end-points included the
numbers of viable nucleated thymus and spleen cells and responses of
thymus and spleen cells to T-cell and/or B-cell mitogens
(phytohaemagglutinin, concanavalin A, pokeweed mitogen, and/or
Escherichia coli lipopolysaccharide) after exposure for 4.5 months
(thymus and spleen) or 16 months (spleen only); and numbers of viable
nucleated mesenteric lymph node cells with cell surface marker
analysis (after 6 and 18 months of exposure; low-dose group not tested
in this assay).
No significant effects were observed in the IgM or IgG responses
to sheep red blood cells, the IgM or IgG responses to T. spiralis,
the IgM or IgG responses to ovalbumin, or the delayed-type
hypersensitivity responses to ovalbumin and M. tuberculosis.
Thymus weight was significantly reduced in the high-dose group
(17% lower than controls, P < 0.05), although the response of
thymocytes to T-cell mitogens was unaltered. No significant
alterations in spleen weight, response of spleen cells to T- and
B-cell mitogens, or body weight were found at any dose. Statistically
significant changes occurred in the percentage of mesenteric lymph
node T-lymphocytes in the high-dose group (20% lower than controls
after 18 months of exposure) and B-lymphocytes in the mid-dose (60%
higher than controls after 18 months) and high-dose (48% higher than
controls after 18 months) groups; however, the absolute number of
T-lymphocytes and B-lymphocytes per lymph node was not significantly
altered. The low-dose group was not tested with these assays. The
B-cell increase was an increase in the percentage of B-cells, but the
interpretation of these data is equivocal, because they are
counter-intuitive when viewed in context with the other effects,
especially the IgE titres.
In vivo clearance of injected L. monocytogenes was impaired
in rats exposed to the high dose for 17 months, as shown by the
approximately sevenfold increase in number of viable bacteria per
spleen, indicating that macrophage function was reduced. Resistance to
infection by T. spiralis was suppressed in rats exposed to the mid
or high dose, as shown by significantly reduced serum IgE titres (50
and 47% lower than controls after 16.5 months of exposure), increased
numbers of larvae in muscle 42 days after infection (56% and 306%
higher than controls after 16.5 months), and moderately reduced
inflammatory reaction around cysts in parasitized musculature
(qualitative assessment only).
There was no significant reduction in the activity of natural
killer cells isolated from the peritoneal cavity following exposure of
weanling or aged (1-year-old) rats to TBTO for 4.5 months. Also, there
was no significant reduction in the activity of natural killer cells
isolated from the spleen following exposure of weanling rats for 4.5
months. In contrast, the activity of natural killer cells isolated
from the spleen was suppressed when weanling rats were exposed to all
doses of TBTO for 16 months (31, 25, and 36% lower than controls,
respectively, at an effector to target cell ratio of 100, and 32, 18,
and 30% lower, respectively, at an effector to target cell ratio of
50). Based on these data, the effect did not progress significantly
with dose. Because the authors considered these data equivocal in this
experiment and because there was no clear treatment-related effect,
the suppression of natural killer cell activity in this study is not
considered biologically significant.
Essentially identical results on the immune system were observed
when weanling rats were exposed for 4.5 or 16.5 months. Based on the
depression of IgE titres and an increase in T. spiralis larvae in
muscle, the LOAEL for immunotoxicity is 0.25 mg/kg body weight per
day. The NOAEL is 0.025 mg/kg body weight per day (Krajnc et al.,
1987; Vos et al., 1990).
Some recent studies suggest that the mechanism of the immunotoxic
effects is related to induction of apoptosis (programmed cell death)
within the thymus. Raffray & Cohen (1991) demonstrated that thymocytes
in culture showed cellular changes consistent with apoptosis at
concentrations of TBTO that did not affect cell viability. Raffray et
al. (1993) showed that these effects occur independently of a
requirement for protein synthesis and do not require fully conserved
energetics (i.e., the effects occur despite depression of ATP levels
to less than 20% of control values). Raffray & Cohen (1993)
demonstrated a correlation between reduction of thymus weight in
animals given a single oral dose of TBTO and evidence of apoptosis
(increased DNA fragmentation) in thymic cell isolates (principally
thymocytes) isolated from the animals during the period of thymic
involution. These workers also showed that dibutyltin, the major
metabolite of tributyltin, is less effective in inducing apoptosis
in vitro, suggesting that the in vivo toxicity is directly
attributable to tributyltin.
A study comparing immunotoxic effects in pre-weanlings and adult
rats shows that some responses of the developing immune system are
more sensitive to TBTO (Smialowicz et al., 1989). Adult (9 weeks old)
male Fischer rats or pre-weanling (3-24 days old) rats were dosed by
oral gavage 3 times per week for a total of 10 doses. The adults were
dosed with 5, 10, or 20 mg/kg body weight per dose; the pre-weanlings
were dosed with 2.5, 5, or 10 mg/kg body weight per dose. Reductions
in mitogen responses were observed in adults at 10 and 20 mg/kg body
weight and in pre-weanlings at 5 and 10 mg/kg body weight. The mixed
lymphocyte reaction was suppressed in adults at 20 mg/kg body weight
and in pre-weanlings at 10 mg/kg body weight. Finally, natural killer
cell activity was suppressed only in pre-weanlings at 10 mg/kg body
weight. In this study, the lowest LOAEL is 5 mg/kg body weight per
day, and the lowest NOAEL is 2.5 mg/kg body weight per day.
Pregnant ICR mice were treated with TBTO in Tween
80:ethanol:saline (1:2:97) by gavage at 0.1 mg/kg body weight per day
on gestation days 4-17 or 11-17 (Buckiova et al., 1992). Humoral and
cell-mediated immune responses in offspring were assessed 4 and
8 weeks after birth. At 0.1 mg/kg body weight per day, the only dose
tested, effects in the offspring included suppressed primary antibody
responses to sheep red blood cells, ovalbumin, and lipopolysaccharide
and increased number of leukocytes. Suppressed delayed-type
hypersensitivity to sheep red blood cells and unspecified alterations
in polyclonal proliferative responses of thymocytes and splenocytes
were also observed. The significance of the LOAEL (0.1 mg/kg body
weight per day), however, is unclear, because a full publication of
the results is not available.
8.7.2 Neurotoxicity
Triethyltin and trimethyltin compounds have been shown to cause
severe neurotoxicity (for a summary, see Boyer, 1989). Triethyltin
causes interstitial oedema throughout the white matter in the spinal
cord and various regions of the brain; less marked damage occurs in
the peripheral nervous system. Trimethyltin also causes severe and
permanent damage to the central nervous system. In this case, however,
the effect is neuronal necrosis, rather than oedema. TBTO, in
contrast, causes no severe neurological signs or morphological or
histopathological changes in brain tissue. In a 4-week study, rats fed
a dietary concentration of 320 mg/kg (equivalent to 30 mg/kg body
weight per day) exhibited ptosis or enophthalmia and slight ataxia
(Krajnc et al., 1984). One chronic study in dogs (Schuh, 1992) also
gave a slight suggestion of neurotoxicity (atactic gait and apathy).
As noted above, however, this study is significantly flawed.
Crofton et al. (1989) measured brain weight and motor activity in
developmental studies. There was some suggestion of neurotoxicity
(based on decreased brain weight in pups) at exposures in excess of 10
mg/kg body weight per day, but no reported effects at 5 mg/kg body
weight per day.
Organotin compounds, including tributyltin, have recently been
shown to induce apoptosis in immortalized neuronal cell lines
(Thompson et al., 1996) and in pheochromocytoma PC12 cells (Viviani et
al., 1995). Although TBTO induces apoptosis in neural cells in
vitro, it does not cause neurotoxicity in whole animals.
Although the potential for neurotoxicity has not been completely
investigated with focused studies, there is no suggestion that
neurotoxicity is likely a critical or co-critical effect.
9. EFFECTS ON HUMANS
No information was located regarding the toxicity of TBTO in
humans following long-term exposure. Human data summarized by Boyer
(1989) suggest that TBTO is a potent non-allergenic dermal irritant.
There are several case reports claiming irritation of the respiratory
tract following acute inhalation exposure of people to TBTO (Anon.,
1991; Hay & Singer, 1991; Shelton et al., 1992; Wax & Dockstader,
1995). None of these reports, however, contains sufficient information
to characterize the exposure-response relationship for the reported
effects.
No epidemiological studies on TBTO were located in the
literature.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
Tributyltin in the environment is very toxic to most taxa (see
data cited in IPCS, 1990). Bivalves and gastropods are especially
sensitive, and larval stages are more vulnerable than adults. The
lowest reported effect concentrations for tributyltin are 2.4-4.8
ng/litre for induction of shell deformities in Pacific oyster and
imposex1 development in dogwhelk ( Nucella lapillus). Other low
toxicity values reported are no-observed-effect concentrations (NOECs)
of 80 ng/litre for Daphnia magna, 40 ng/litre for reduced viability
of mussel ( Mytilus edulis) larvae, and 10 ng/litre for reduced
growth of hard-shell clams ( Mercenaria mercenaria) and reduced egg
production in a marine copepod ( Acartia tonsa).
In the field, mainly the effects on oysters and prosobranchs have
been studied. Most studies on imposex have examined populations of
N. lapillus. It has been suggested that other prosobranchs are even
more sensitive, and other species have been suggested as indicator
species (Matthiessen & Gibbs, 1998).
Matthiessen & Gibbs (1998) reviewed the evidence that
TBTO-induced imposex and intersex in molluscs are the result of
endocrine disruption. The effect is most likely the result of elevated
testosterone titres that masculinize tributyltin-exposed females. The
precise mechanism has not been fully described, but the weight of
evidence suggests that TBTO acts as a competitive inhibitor of
cytochrome P-450-mediated aromatase, leading to increased testosterone
levels. Additional support for this mechanism has been presented by
Bettin et al. (1996). Testosterone addition (500 ng/litre) induces
faster and more intensive imposex development in N. lapillus than
that induced by tributyltin. Simultaneous exposure to tributyltin and
to the antiandrogen cyproterone acetate suppresses imposex development
completely in N. lapillus and reduces it in Hinia reticulata.
Furthermore, tributyltin-induced imposex development can be suppressed
by adding estrogens. Inhibition of the cytochrome P-450-dependent
aromatase using SH 489 (1-methyl-1,4-androstadiene-3,17-dione) and
flavone induces development of imposex. Some recent data suggest that
TBTO may also inhibit the formation of sulfur conjugates of
testosterone and its active metabolites, thus interfering with its
excretion.
There is a vast literature on the environmental effects of
tributyltin. Most of the information below was condensed from IPCS
(1990). Additional data published since this evaluation have also been
included.
1 Imposex is the development of male characteristics by female
gastropods.
10.1 Aquatic environment
As tributyltin is used commercially to control bacteria and
fungi, the substance is toxic to these taxa. The reported minimum
inhibitory concentrations (MICs) range from 20 to 300 000 µg/litre.
The MIC for sludge from municipal sewage treatment plants was reported
as 25 µg/litre (IPCS, 1990). A recent study conducted in yeast
suggests that the target for TBTO action is the mitochondrial ATPase
(Veiga et al., 1997). TBTO reduced the respiratory capacity when
vanillic or benzoic acid was the energy source. The ATP level of the
cell was severely affected at a concentration of 1.19 mg/litre. The
mitochondrial ATPase was strongly inhibited at a concentration of 0.3
mg/litre, whereas the activity of the plasma membrane ATPase was not
affected by a concentration up to 17.9 mg/litre.
In the laboratory, effective concentrations for freshwater algae
ranged from 5 µg/litre (4-h IC50 for growth, Ankistrodesmus
falcatus) to 64 µg/litre (96-h EC50, Scenedesmus pannonicus). A
4-h IC50 for primary production of 3 µg/litre was reported for a
natural community from Lake Ontario (IPCS, 1990). For marine and
estuarine algae, most reported IC50 or EC50/LC50 values range from
0.1 to 15 µg/litre (IPCS, 1990). For motile spores of the green
macroalga Enteromorpha intestinalis, a 5-day EC50 of 0.001 µg/litre
for spore development and inhibition of settling was indicated (IPCS,
1990). Effects on community metabolism and nutrient dynamics in
bladderwrack ( Fucus vesiculosus) have been shown at 0.6 µg
tributyltin/litre and above (Lindblad et al., 1989). Studies on pure
cultures of marine algae show that these organisms do not adapt to
tributyltin; the same EC50 values were obtained for cultures exposed
for 12 weeks as for naive cells (IPCS, 1990).
For Lemna and Elodea species, reduction in growth was
observed from 0.06 µg/litre following 10 days of exposure to TBTO. For
the angiosperm Zostera marina, a NOEC of 0.1 mg/kg sediment was
reported. The lethal concentration for the salt-marsh species Aster
tripholium was 10 µg/kg mud (dry weight) (IPCS, 1990).
For Daphnia magna, the 48-h LC50 was 2.3 µg/litre; the NOEC
has been estimated to be 0.5 µg/litre based on reversal of normal
response to light (IPCS, 1990). The reported long-term toxicity value
(21-day NOEC) for Daphnia magna is 0.19 µg/litre; the 96-h LC50 for
Tubifex tubifex is 0.1 µg/litre (Fargasova, 1997).
For target snail adults, the 24-h LC50 was 30-400 µg/litre. The
sensitivity of snails decreases with age, but eggs are more resistant
than both young and adults. The lowest-observed-effect concentration
(LOEC) for reproduction for Biomphalaria and Bulinus is 0.001
µg/litre; the long-term NOEL for Lymnaea stagnalis is 0.32 µg/litre
(IPCS, 1990).
Several field studies on the effects of tributyltin used as a
molluscicide for schistosomiasis control in tropical areas have been
reported (IPCS, 1990). For schistosome larvae in the aquatic stages,
the LC50 was calculated to be 16.8 µg/litre for a 1-h exposure. The
dose causing 99-100% suppression of cercarial infectivity of mice was
between 2 and 6 µg/litre (IPCS, 1990).
Among marine aquatic invertebrates, larval stages are
considerably more sensitive than adults. For example, the 48-h LC50
for the Pacific oyster is 1.6 µg/litre for larvae and 1800 µg/litre
for adults; for the mussel M. edulis, the same values are 23 and 300
µg/litre, respectively. The larvae of brown shrimp ( Crangon crangon)
are also more sensitive than adults, the 96-h LC50s being 1.5 and 41
µg/litre, respectively (IPCS, 1990).
For subadults of the copepod Eurytemora affinis, the 72-h LC50
was reported as 0.6 µg/litre. For the mysid shrimp ( Acanthomysis
sculpta), the 96-h LC50 was reported as 0.41 µg/litre. For larvae
of the lugworm ( Arenicola cristata), the 96-h LC100 was reported as
4 µg/litre (IPCS, 1990).
The 144-day EC50 for morbidity and mortality of the copepod
Acartia tonsa was 0.4 µg/litre (IPCS, 1990). A 6-day NOEC and LOEC
for A. tonsa have been reported as 0.011 and 0.023 µg/litre,
respectively (Kusk & Peterson, 1997). Concentrations of 5-10 µg/litre
killed all larvae of lobster ( Homarus americanus) within 5-6 days,
and metamorphosis was affected at 1 µg/litre (IPCS, 1990). A 15-day
LC50 of mussel ( M. edulis) larvae has been reported as
approximately 0.1 µg/litre (IPCS, 1990).
A 22-day LC100 for adult polychaetes Nereis diversicolor was 4
µg/litre (IPCS, 1990). In another polychaete,
Sabellastarte sanctijosephi, mortality occurred at 0.04-1.0 µg/litre
(Langston, 1995).
Deformities in regenerated limbs of fiddler crab ( Uca
pugilator) were observed at 0.5 µg/litre and above. Inhibition of
the regeneration of arms of brittle star ( Ophioderma brevispina) was
observed at 0.1 and 0.5 µg/litre (IPCS, 1990).
It has been suggested that TBTO inhibits calcification of Pacific
oysters below 2 ng tin/litre (Alzieu, 1991; Langston, 1995). These
effects have also been observed in the field. In the early 1980s, a
good correlation was found between field observations of occurrence of
shell thickening and proximity of ports with large numbers of boats
(IPCS, 1990).
Reduced growth of Pacific oyster spat has been shown at all
concentrations above 20 ng TBTO/litre (IPCS, 1990). Recently
metamorphosed European oysters ( Ostrea edulis) showed a severe
reduction in growth rate over 10 days in 0.06 µg/litre. In spats of
C. gigas, M. edulis, and carpet shell ( Venerupis ducussata),
growth was reduced at 0.24 µg/litre over 45 days. For adult mussels
( M. edulis), shell length was reduced at 0.31 µg/litre following 66
days of exposure, and juvenile growth was reduced at 0.07 µg/litre. In
a study of hard-shell clam ( Mercenaria mercenaria) exposed from
fertilization to metamorphosis (approximately 14 days), growth was
reduced at 10 ng/litre and above.
In the laboratory, all female dogwhelks exposed to TBTO showed
imposex development at 1-2 ng tin/litre and above (IPCS, 1990). Some
of the females retained their breeding capacity at the lowest
concentration, but virtually all females were sterilized at 3-5 ng
tin/litre. From field observations, the NOEL has been set at less than
1 ng tributyltin/litre. Imposex has been observed in a number of other
species in the field. These include Ocenebra erinacea,
Ocinebrina aciculata, Hexaplex trunculus, Buccinum undatum,
Littorina littorea, and Nassarius reticulatus (Oehlmann et al.,
1996; Matthiessen & Gibbs, 1998).
In oysters ( O. edulis), severe effects on reproduction occurred
at 0.24 and 2.6 µg/litre; no larvae were released, gonads were
undifferentiated, and no females developed (IPCS, 1990). At 0.01
µg/litre, egg production in exposed A. tonsa was significantly
reduced (IPCS, 1990).
No effect on survival of grass shrimp ( Palaemonetes pugio) was
found after 96 h of exposure at 1 or 10 mg tributyltin/kg sediment,
but exposure via water alone resulted in a 96-h LC50 of 20 µg/litre
(IPCS, 1990). In sediments containing tributyltin, an LC50 of 1-10
mg/kg sediment was determined for Amphioxus (IPCS, 1990). No effects
on survival of the mole crab ( Emerita talpoida) were observed
following 7 days of exposure at 10 µg/litre seawater and 4.5 mg/kg
sand (IPCS, 1990). No mortality was observed in mysid shrimp
( Acanthomysis sculpta), worms ( Neanthes arenaceodentata), or clams
( Macoma nasuta) exposed to concentrations in sediment of 155-610
µg/kg and concentrations in overlaying water of 0.2 µg/litre over
10-20 days (IPCS, 1990).
The reported short-term LC50 values for TBTO in freshwater fish
obtained under static conditions range from 13 to 240 µg/litre (IPCS,
1990). The NOEL for the guppy ( Poecilia reticulata) was estimated to
be 0.01 µg/litre based on thymus atrophy, liver vacuolation, and
hyperplasia of the haematopoietic tissue (Wester & Canton, 1987).
The toxicity of TBTO to marine fish is highly variable; 96-h
LC50 values range between 1.5 and 36 µg/litre, with larval stages
being more sensitive than adults (IPCS, 1990). Data have been reported
for bleak ( Alburnus alburnus), sole ( Solea solea), armed bullhead
( Agonus cataphractus), girella ( Girella punctata), salt water goby
( Chasmichthys dolichograthus), and chinook salmon ( Oncorhynchus
tshawytscha). There are indications that marine fish avoid TBTO
concentrations of 1 µg/litre or more (IPCS, 1990). A recent study in
flounder ( Platichthys flesus) showed that TBTO at 17.3 µg/litre
caused mortality after 7-12 days, decreased the condition factor,
resulted in gill lesions, and induced significant reduction of
non-specific resistance. However, no marked effects on the relative
thymus volume or on the specific immune system were noted (Grinwis et
al., 1997).
Japanese medaka ( Oryzias latipes) fed daily for 3 weeks with
food containing tributyltin, polychlorinated biphenyls, or a
combination of the two at 1 mg/kg body weight showed slight
synergistic effects on reproduction, resulting in reduced spawning
frequency, number of eggs, and proportion of fertile eggs (Oshima et
al., 1998).
No effect on survival was found when eggs and larvae of frog
( Rana temporaria) were exposed to TBTO concentrations of 3 µg/litre
or less; at 30 µg/litre, however, significant mortality was observed
(IPCS, 1990).
10.2 Terrestrial environment
Although the exposure of terrestrial organisms to tributyltin
results primarily from its use as a wood preservative, tributyltin
compounds are toxic to insects exposed topically or via feeding on
treated wood (IPCS, 1990). The LD50 values for tributyltin compounds
applied topically to the thorax of newly emerged insects range from
0.48% to 0.72% (dilutions with acetone) for the house fly
( Musca domestica), from 0.29% to 0.69% for the mosquito
( Anophelese stephensi), and from 0.52% to 0.87% for the cotton
stainer ( Dysdercus cingulatus). TBTO is toxic to honey bees
( Apis mellifera) housed in hives made from TBTO-treated wood
(1.9 kg/m3). TBTO is toxic to bats ( Pipistrellus pipistrellus)
housed in roosting cages treated with TBTO, but this result was not
statistically significant, owing to high mortality in controls. The
acute toxicity of TBTO to wild mice (deer mice
[ Peromyscus maniculatus] and house mice [ Mus musculus]) is
moderate. The estimated dietary LC50 value, based on consumption of
treated seeds used in repellency tests, is 200 mg/kg diet per day.
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
A large number of studies have been conducted showing that TBTO
causes depression of immune functions dependent on the thymus. These
effects occur at doses lower than those that cause other toxicity (see
Table 1). Accordingly, the critical effect for TBTO is immunotoxicity.
Based on the study of Vos et al. (1990), the critical effect is
immunosuppression (reduced IgE titres and increase in T. spiralis
larvae in muscle). The LOAEL is 0.25 mg/kg body weight per day, and
the NOAEL is 0.025 mg/kg body weight per day. These values were based
on the authors' report that 5 mg/kg in the diet is equivalent to 0.25
mg/kg body weight per day. This study tested male animals only. Other
studies show no evidence of gender differences in the toxic responses
to TBTO. There is some evidence that a child might be more sensitive
to the toxic effects of TBTO. For example, Smialowicz et al. (1989)
showed that pre-weanling rats were more sensitive than adult rats. In
addition, the principal study (Vos et al., 1990) showed that
immunotoxic effects were observed when weanling rats were dosed for
4.5 or 16.5 months, whereas a companion study (Vos et al., 1990)
showed that these effects were absent or occurred at a higher dose
when adult (1-year-old) rats were dosed for 5 months.
Adequate data are not available to determine a no-effect or
effect level following long-term inhalation exposure. The inhalation
studies that are available document irritation to the respiratory
system. There are no pharmacokinetic studies available with which to
conduct a route-to-route extrapolation for extra-respiratory effects.
TBTO might cause immunosuppression following chronic exposure by
inhalation.
Cancer bioassays following oral exposure have been conducted in
rats and mice. The bioassay in rats shows increases in benign
pituitary tumours, in pheochromocytomas, and in parathyroid tumours at
the highest dose tested. The significance of these tumours, which
normally occur in this strain of rat with variable incidence, is
unclear. The bioassay in mice showed no increase in tumours at any
site. The weight of evidence shows that TBTO is not genotoxic.
11.1.2 Criteria for setting guidance values for TBTO
The no-effect level for immunosuppression (decrease in serum IgE
titre) following long-term oral exposure in rats is 0.025 mg/kg body
weight per day. Benchmark dose analysis shows that the exposure
corresponding to the lower confidence limit (95%) on dose for a 10%
decrease in serum IgE titre is 0.034 mg/kg body weight per day (US
EPA, 1997). Application of uncertainty factors of 10 each for
extrapolation from a laboratory animal species to humans and to
protect sensitive humans gives a guidance value for oral exposure of
0.0003 mg/kg body weight per day (rounded from 0.00025 for the
no-effect level or 0.00034 for the benchmark dose). No appropriate
data are available to develop a guidance value for inhalation exposure
or to estimate cancer risk.
11.1.3 Sample risk characterization
No human data are available to characterize the toxicity of TBTO,
but a wealth of data from oral exposure in laboratory animals is
available. The principal study and a variety of supporting studies
convincingly demonstrate that the critical effect for TBTO is
immunotoxicity. Some evidence indicates that young animals are more
sensitive than adults to the immunotoxic effects. TBTO is not a
reproductive or developmental toxicant. Insufficient data are
available to determine the critical effect for TBTO following exposure
by inhalation. Several case reports document severe irritation of the
human respiratory system following acute inhalation exposure. The
potential human hazard for carcinogenicity for TBTO cannot be
determined. The weight of evidence shows that TBTO is not genotoxic.
Dietary exposure to tributyltin has been assessed in Japan.
Consumption of aquatic organisms is the major route of human exposure.
Data from market basket surveys from 1990 to 1997 estimated the
average daily intake of tributyltin (expressed as tributyltin
chloride) at 3.9 µg/day. Using these data, correcting the exposure
estimate to TBTO by multiplying by the ratio of the molecular weights
(596/325), and assuming a body weight of 50 kg, the estimated daily
exposure to TBTO in Japan is 0.00014 mg/kg body weight per day. This
value is 47% of the guidance value.
Because of the limited and possibly unrepresentative information
on human exposure available to the author and reviewers of the CICAD
and the recent preliminary report (Takahashi et al., 1998) of a
relatively high burden of tributyltin residues in liver resulting,
perhaps, from non-food sources, additional investigation is warranted.
11.2 Evaluation of environmental effects
Because of their physical/chemical properties, tributyltin
compounds concentrate in the surface microlayer and in sediments.
Abiotic degradation does not appear to be a major mechanism of removal
under environmental conditions. Although TBTO is biodegradable in the
water column, this process is not rapid enough to prevent the
occurrence of elevated tributyltin levels in some areas. The half-life
in the water column ranges from a few days to weeks. Tributyltin may
persist in sediment for several years. Bioaccumulation occurs in most
aquatic organisms.
Tributyltin compounds are extremely hazardous to some aquatic
organisms because of their toxicity at very low concentrations in
water. Such concentrations seem to be prevalent in many coastal areas.
Adverse effects on non-target invertebrates, particularly molluscs,
have been reported in field studies, and these have been sufficiently
severe to lead to reproductive failure and population decline. Adverse
effects on the commercial production of shellfish have been
successfully reversed by restrictions on the use of antifouling paints
in some areas, and these restrictions are also leading to the reversal
of imposex effects in gastropod populations. However, the
concentrations of tributyltin measured in some coastal waters are
still above those that induce severe effects in some gastropods. The
effects on farmed fish indicate that tributyltin-containing paints
should not be used on restraining nets.
The general hazard to the terrestrial environment is likely to be
low. Tributyltin-treated wood could pose a hazard to terrestrial
organisms living in close contact with it.
The enhancement of tributyltin concentrations in the surface
microlayer may present a hazard to littoral organisms, neustonic
species (including benthic invertebrate and fish larvae), and
surface-feeding seabirds and wildfowl. Accumulation and low
biodegradation of tributyltin in sediment may pose a hazard to aquatic
organisms when these polluted sediments are disturbed by natural
processes or dredging activities.
The general decline in tributyltin concentrations in the
environment has been attributed to the restrictions placed on the use
of antifouling paints on vessels. However, it should be noted that in
some locations the concentration of tributyltin in the water is above
that necessary to elicit severe adverse effects in some sensitive
species.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Tributyltin compounds were reviewed by the World Health
Organization in 1989 (IPCS, 1990).
Information on international hazard classification and labelling
is included in the International Chemical Safety Card reproduced in
this document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventative and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 1282) reproduced in this
document.
13.1 Human health hazards
Effects on the immune system may be observed following acute or
repeated exposure to tributyltin.
13.2 Advice to physicians
In case of poisoning, treatment is supportive. Following
inhalation of aerosol, symptoms may not be noticeable until a few
hours have passed. Therefore, rest and medical observation are
essential.
13.3 Health surveillance advice
Periodic medical examination of the immune system should be
included in a health surveillance programme.
13.4 Spillage
TBTO is severely irritating to the skin and eyes. In case of
spillage, therefore, emergency crew must wear proper equipment,
including eye protection in combination with breathing protection. The
compound should not be allowed to enter drains or watercourses.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Many countries have restricted the use of TBTO. Information on
national regulations, guidelines, and standards may be obtained from
UNEP Chemicals (IRPTC), Geneva.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
TRIBUTYLTIN OXIDE ICSC: 1282
March 1998
CAS # 56-35-9 Hexabutyldistannoxane
RTECS # JN8750000 Tri-n-butylin oxide
UN # 3020 TBTO
EC # 050-008-00-3 C24H54OSn2
Molecular mass: 596.07
TYPES OF HAZARD/EXPOSURE ACUTE HAZARDS/SYMPTOMS PREVENTION FIRST AID/FIRE FIGHTING
FIRE Combustible NO open flames In case of fire in the
surroundings: all
extinguishing agents allowed.
EXPLOSION
EXPOSURE PREVENT GENERATION
OF MISTS! STRICT
HYGIENE!
Inhalation Abdominal cramps. Cough. Ventilation, local Fresh air, rest.
Diarrhoea. Laboured exhaust, or Half-upright position.
breathing. Nausea. Sore breathing protection Refer for medical
throat. Vomiting. attention.
Symptoms may be delayed
(See Notes).
Skin MAY BE ABSORBED! Redness. Protective gloves. Rinse and then wash skin
After delay skin burns. Protective clothing. with water and soap. Refer
for medical attention.
Eyes Redness. Pain. Safety spectacles, First rinse with plenty of
face shield, or eye water for several minutes
protection in (remove contact lenses if
combination with easily possible), then take
breathing protection. to a doctor.
Ingestion Abdominal cramps. Do not eat, drink or Induce vomiting (ONLY IN
Diarrhoea. Nausea. smoke during work. CONSCIOUS PERSONS!) Give
Vomiting. Wash hands before plenty of water to drink.
eating. Refer for medical attention.
SPILLAGE DISPOSAL PACKAGING & LABELLING
Do NOT wash away into sewer. Carefully collect Severe marine pollutant.
remainder, then remove to safe place. Do NOT let this EU Classification
chemical enter the environment. Chemical protection Symbol: T
suit including self-contained breathing apparatus. R: 21-25-36/38-48/23/25
S: (1/2)-35-36/37/39-45
Note: A
UN Classification
UN Hazard Class: 6.1
UN Pack Group: II
EMERGENCY RESPONSE STORAGE
Transport Emergency Card: TEC (R)-61G43b Provision to contain effluent from fire
extinguishing.
IMPORTANT DATA
PHYSICAL STATE; APPEARANCE: ROUTES OF EXPOSURE:
LIQUID The substance can be absorbed into the
body by inhalation of its aerosol, through
the skin and by ingestion.
CHEMICAL DANGERS: INHALATION RISK:
The substance decomposes on burning Evaporation at 20°C is neglible; a harmful
producing toxic fumes. concentration of airborne particles can,
however, be reached quickly.
OCCUPATIONAL EXPOSURE LIMITS: EFFECTS OF SHORT-TERM EXPOSURE:
TLV (as tin): ppm: 0.1 mg/m3 A4. The substance irritates severely the eyes, the skin.
STEL 0.2 mg/m3 A4 (skin) (ACGIH 1997). Inhalation of the aerosol may cause lung oedema (see
Notes). The substance may cause effects on the thymus,
resulting in depression of the immune function.
PHYSICAL PROPERTIES
Boiling point: 173°C
Melting point: <-45°C
Relative density (water = 1): 1.17 at 20°C
Solubility in water: poor
Vapour pressure, Pa at 20°C: 0.001
Flash point: 190°C c.c.
Octanol/water partition coefficient as log Pow: 3.19
ENVIRONMENTAL DATA
The substance is very toxic to aquatic organisms. In the food chain important to humans,
bioaccumulation takes place, specifically in fish and molluscs. Avoid release to
the environment in circumstances different to normal use.
NOTES
The symptoms of lung oedema often do not manifest until a few hours have passed and they are
aggravated by physical effort. Rest and medical observation is therefore essential. Immediate
administration of an appropriate spray, by a doctor or a person authorized by him/her,
should be considered.
ADDITIONAL INFORMATION
LEGAL NOTICE Neither the CEC nor the IPCS nor any person acting on behalf of the CEC or
the IPCS is responsible for the use which might be made of this information.
REFERENCES
Alzieu C (1991) Environmental problems caused by TBT in France:
Assessment, regulations, prospects. Marine environmental research,
32:7-17.
Anon. (1991) Acute effect of indoor exposure to paint containing
bis(tributyltin)oxide -- Wisconsin, 1991. Morbidity and mortality
weekly report, 40:280-281.
Baroncelli S, Karrer D, Turillazzi PG (1990) Embryotoxic evaluation of
bis(tri- n-butyltin)oxide (TBTO) in mice. Toxicology letters,
50:257-262.
Bettin C, Ohelmann J, Stroben E (1996) TBT-induced imposex in marine
neogastropods is mediated by an increasing androgen level.
Helgolander Meeresunersuchungen, 50:299-317.
Boyer IJ (1989) Toxicity of dibutyltin, tributyltin and other
organotin compounds to humans and to experimental animals.
Toxicology, 55:253-298.
Bressa G, Hinton RH, Price SC, Isbir M, Ahmed RS, Grasso P (1991)
Immunotoxicity of tri- n-butyltin oxide (TBTO) and tri- n-butyltin
chloride (TBTC) in the rat. Journal of applied toxicology,
11:397-402.
Brown RA, Nazario CM, de Tirado RS, Castrillón J, Agard ET (1977) A
comparison of the half-life of inorganic and organic tin in the mouse.
Environmental research, 13:56-61.
Buckiova D, Dostal M, Hofmannova V (1992) Embryotoxicity of organotins
[abstract]. Reproductive toxicology, 6:178-179.
Carthew P, Edwards RE, Dorman BM (1992) The immunotoxicity of
tributyltin oxide (TBTO) does not increase the susceptibility of rats
to experimental respiratory infection. Human and experimental
toxicology, 11:71-75.
CEFIC (1994) Use of triorganotin compounds in anti-fouling paints.
Results of TBT monitoring studies. Paper submitted by the European
Chemical Industry Council to the International Marine Organization
Marine Environment Protection Committee, 35th session, January 1994.
Crofton KM, Dean KF, Boncek VM, Rosen MB, Sheets LP, Chernoff N,
Reiter LW (1989) Prenatal or postnatal exposure to
bis(tri- n-butyltin)oxide in the rat: postnatal evaluation of
teratology and behavior. Toxicology and applied pharmacology,
97:113-123.
Daly IW (1992) An eighteen month oncogenicity feeding study in mice
with bis(tri-n -butyltin)oxide (TBTO). Unpublished report prepared
by Bio/dynamics, Inc., for TBTO Consortium (MRID No. 422650-01).
Davis A, Barale R, Brun G, Forster R, Günther T, Hautefeuille H, van
der Heijden CA, Knaap AGAC, Krowke R, Kuroki T, Loprieno N, Malaveille
C, Merker HJ, Monaco M, Mosesso P, Neubert D, Norppa H, Sorsa M, Vogel
E, Voogd CE, Umeda M, Bartsch H (1987) Evaluation of the genetic and
embryotoxic effects of bis(tri- n-butyltin)oxide (TBTO), a
broad-spectrum pesticide, in multiple in vivo and in vitro
short-term tests. Mutation research, 188:65-95.
dela Cruz MAT, Molander S (1998) Butyltins in marine sediments from
the Swedish west coast. Report to Swedish National Chemicals
Inspectorate. Gothenburg, Chalmers University of Technology.
Fargasova A (1997) Comparative study of ecotoxicological effect of
triorganotin compounds on various biological subjects. Ecotoxicology
and environmental safety, 36:38-42.
Fent K, Hunn J (1991) Phenyltins in water, sediment, and biota of
freshwater marinas (Lake Lucern, Switzerland). Environmental science
and technology, 25:956-963.
Funahashi N, Iwasaki I, Ide G (1980) Effects of
bis(tri- n-butyltin)-oxide on endocrine and lymphoid organs of male
rats. Acta Pathologica Japonica, 30:955-966.
Garssen J, Van der Vliet H, De Klerk A, Goettsch W, Dormans JAMA,
Bruggeman CA, Osterhaus ADME, Van Loveren H (1995) A rat
cytomegalovirus infection model as a tool for immunotoxicity testing.
European journal of pharmacology, 292:223-231.
Grinwis GCM, van den Brandhof EJ, Dormans JAMA, Engelsma M, Kuiper R,
Leewis R, van Loveren H, Wester PW, Vaal MA, Vethaak AD, Vos JG (1997)
Short term toxicity of bis(tri-n- butyltin)oxide in flounder
(Platichtys flesus): pathology and immune function. Bilthoven,
National Institute of Public Health and Environmental Protection (RIVM
Report No. 732501 002).
Guruge KS, Iwata H, Tanaka H, Tanabe S (1997) Butyltin accumulation in
the liver and kidney of seabirds. Marine environmental research,
44:191-199.
Hay A, Singer CR (1991) Wood preservatives, solvents, and
thrombocytopenic purpura [letter]. Lancet, 338:766.
IPCS (1990) Tributyltin compounds. Geneva, World Health
Organization, International Programme on Chemical Safety
(Environmental Health Criteria 116).
IPCS (1996) International Chemical Safety Card -- Tributyltin oxide.
Geneva, World Health Organization, International Programme on Chemical
Safety (ICSC 1282).
Iwata H, Tanabe S, Miyazaki N, Tatsukawa R (1994) Detection of
butyltin compound residues in the blubber of marine mammals. Marine
pollution bulletin, 28:607-612.
Iwata H, Tanabe S, Mizuno T, Tatsukawa R (1995) High accumulation of
toxic butyltins in marine mammals from Japanese coastal waters.
Environmental science and technology, 29:2959-2962.
Iwata H, Tanabe S, Mizuno T, Tatsukawa R (1997) Bioaccumulation of
butyltin compounds in marine mammals: The specific tissue distribution
and composition. Applied organometallic chemistry, 11:257-264.
Kannan K, Tanabe S, Tatsukawa R (1995) Occurrence of butyltin residues
in certain foodstuffs. Bulletin of environmental contamination and
toxicology, 55:510-516.
Kannan K, Corsolini S, Focardi S, Tanabe S, Tatsukawa R (1996)
Accumulation pattern of butyltin compounds in dolphin, tuna and shark
collected from Italian coastal waters. Archives of environmental
contamination and toxicology, 31:19-23.
Kannan K, Senthilkumar K, Loganathan BG, Takahashi S, Odell DK, Tanabe
S (1997) Elevated accumulation of tributyltin and its breakdown
products in bottlenose dolphins ( Tursiops truncatus) found stranded
along the US Atlantic and Gulf coasts. Environmental science and
technology, 31:296-301.
Kannan K, Guruge KS, Thomas NJ, Tanabe S, Giesy JP (1998) Butyltin
residues in southern sea otters ( Enhydra lutris nereis) found dead
along the California coastal waters. Environmental science and
technology, 32:1169-1175.
Karrer D, Baroncelli S, Ciaralli L, Turillazzi PG (1992) Effect of
subchronic bis(tri- n-butyltin)oxide (TBTO) oral administration on
haematological parameters in monkeys: a preliminary report. Food and
chemical toxicology, 30:715-718.
Karrer D, Baroncelli S, Turillazzi PG (1995) Oral
bis(tri- n-butyltin) oxide in pregnant mice. II. Alterations in
hematological parameters. Journal of toxicology and environmental
health, 46:369-377.
Kim GB, Lee JS, Tanabe S, Iwata H, Tatsukawa R, Shimazaki K (1996a)
Specific accumulation and distribution of butyltin compounds in
various organs and tissues of the stellar sea lion ( Eumetopias
jubatus): Comparison with organochlorine accumulation pattern.
Marine pollution bulletin, 32:558-563.
Kim GB, Tanabe S, Tatsukawa R, Loughlin TR, Shimazaki K (1996b)
Characteristics of butyltin accumulation and its biomagnification in
stellar sea lion ( Eumetopias jubatus). Environmental toxicology
and chemistry, 15:2043-2048.
Kim GB, Tanabe S, Iwakiri R, Tatsukawa R, Amano M, Miyazaki N, Tanaka
H (1996c) Accumulation of butyltin compounds in Risso's dolphin
( Grampus griseus) from the Pacific coast of Japan: Comparison with
organochlorine residue pattern. Environmental science and
technology, 30:2620-2625.
Krajnc EI, Wester PW, Loeber JG, van Leeuwen FXR, Vos JG, Vaessen
HAMG, van der Heijden CA (1984) Toxicity of bis(tri- n-butyltin)oxide
(TBTO) in the rat. I. Short-term effects on general parameters and on
the endocrine and lymphoid systems. Toxicology and applied
pharmacology, 75:363-386.
Krajnc EI, Vos JG, Wester PW, Loeber JG, van der Heijden CA (1987)
Toxicity of bis(tri-n -butyltin)oxide (TBTO) in rats. Unpublished
report submitted to the Office of Toxic Substances, US Environmental
Protection Agency, with cover letter dated 18 May 1987 (Document
Control No. FYI-OTS-0687-0550 Sequence A).
Kroes R, Garbis-Berkyens JM, deVries T, van Nellesrooy JHJ (1981)
Histopathological profile of a Wistar rat stock including a survey of
the literature. Journal of gerontology, 36:259-279.
Kusk KO, Peterson S (1997) Acute and chronic toxicity of tributyltin
and linear alkylbenzene sulfonate to the marine copepod Acartia
tonsa. Environmental toxicology and chemistry, 16:1629-1633.
Langston WJ (1995) Tributyl tin in the marine environment: A review of
past and present risks. Pesticide outlook, 6:18-24.
Li Q, Osada M, Takahashi K, Matsutani T, Mori K (1997) Accumulation
and depuration of tributyltin oxide and its effect on the
fertilization and embryonic development in the Pacific oyster,
Crassostrea gigas. Bulletin of environmental contamination and
toxicology, 58:489-496.
Lindblad C, Kautsky U, Andre C, Kautsky N, Tedengren M (1989)
Functional response of Fucus vesiculosus communities to tributyltin
measured in an in situ continuous flow-through system.
Hydrobiologia, 188/189:277-283.
Madhusree B, Tanabe S, Ozturk AA, Tatsukawa R, Miyazaki N, Ozdamar E,
Raral O, Samsun O, Ozturk B (1997) Contamination by butyltin compounds
in harbour porpoise ( Phocoena phocoena) from the Black Sea.
Fresenius journal of analytical chemistry, 359:244-248.
Matthiessen P, Gibbs PE (1998) Critical appraisal of the evidence for
tributyltin-mediated endocrine disruption in mollusks. Environmental
toxicology and chemistry, 17:37-43.
NIVA (1997) Levels and environmental effects of TBT in marine
organisms and sediments from the Norwegian coast. A summary report.
Oslo, Norwegian State Pollution Control Authority, Norwegian Institute
for Water Research.
Oehlmann J, Fioroni P, Stroben E, Markert B (1996) Tributyltin (TBT)
effects on Ocinebrina aciculata (Gastropoda: Muricidae): imposex
development, sterilization, sex change and population decline. The
science of the total environment, 188:205-223.
Oshima Y, Nirmala K, Yokota Y, Shimazaki Y, Go J, Imada N, Honjo T,
Kobayashi K (1998) High accumulation of tributyltin in blood of fish
and its transgenerational and synergistic toxicity with PCB on the
reproductive process of fish. Japanese journal of environmental
toxicology, 1:26-35.
Peto R, Pike MC, Day NE, Gray RG, Lee PN (1980) Guidelines for
simple, sensitive significance tests for carcinogenic effects in
long-term animal experiments. Long-term and short-term screening
assays for carcinogens: a critical appraisal. Lyon, International
Agency for Research on Cancer, pp. 311-426 (IARC Monographs on the
Evaluation of Carcinogenic Risks of Chemicals to Humans, Supplement
2).
Poitou P, Marignac B, Certin C, Gradiski D (1978) Étude de l'effet sur
le système nerveux central et du pouvoir sensibilisant de l'oxyde de
tributylétain. Annales Pharmaceutiques Françaises, 36:569-572 [cited
in IPCS, 1990].
Raffray M, Cohen GM (1991) Bis(tri- n-butyltin)oxide induces
programmed cell death (apoptosis) in immature rat thymocytes.
Archives of toxicology, 65:135-139.
Raffray M, Cohen GM (1993) Thymocyte apoptosis as a mechanism for
tributyltin-induced thymic atrophy in vivo. Archives of
toxicology, 67:231-236.
Raffray M, McCarthy D, Snowden RT, Cohen GM (1993) Apoptosis as a
mechanism of tributyltin cytotoxicity to thymocytes: relationship of
apoptotic markers to biochemical and cellular effects. Toxicology
and applied pharmacology, 119:122-130.
Ruiz JM, Bachelet G, Caumette P, Donard OFX (1996) Three decades of
tributyltin in the coastal environment with emphasis on Arcachon Bay,
France. Environmental pollution, 93:195-203.
Schroeder RE (1981) A teratology study in rats with
bis(tri-n -butyl-tin)oxide. Unpublished report prepared by
Bio/dynamics, Inc., for Elf Atochem (MRID No. 00137158, 92172005,
92172016; HED Document No. 003914, 004691, 010916).
Schroeder RE (1990) A two-generation reproduction study in rats with
bis(tri-n -butyltin)oxide. Unpublished report prepared by
Bio/dynamics, Inc., for Schering AG and M&T Chemicals, Inc. (MRID No.
416938-01).
Schuh W (1992) One year chronic feeding study in beagle dogs.
Unpublished report prepared by Schering AG Laboratories for Elf
Atochem North America, Inc., Aceto Chemicals, and Schering Berlin
Polymers (MRID No. 425498).
Schweinfurth HA, Gunzel P (1987) The tributyltins: mammalian toxicity
and risk evaluation for humans. Oceans '87: The ocean, "an
international workplace." Proceedings of the International Organotin
Symposium, 4:1421-1431.
Shelton D, Urch B, Tarlo SM (1992) Occupational asthma induced by a
carpet fungicide -- tributyltin oxide. Journal of allergy and
clinical immunology, 90:274-275.
Smialowicz RJ, Riddle MM, Rogers RR, Leubke RW, Copeland CB (1989)
Immunotoxicity of tributyltin oxide in rats exposed as adults or
pre-weanlings. Toxicology, 57:97-111.
Stäb JA, Traas TP, Stromberg G, van Kesteren J, Leonards P, van Hattum
B, Brinkman UAT, Cofino WP (1996) Determination of organotin compounds
in the foodweb of a shallow freshwater lake in the Netherlands.
Archives of environmental contamination and toxicology, 31:319-328.
Stringer CP, Hicks R, Botham PA (1991) Contact sensitivity (allergic
contact dermatitis) to bis(tri- n-butyltin)oxide in mice. Contact
dermatitis, 24:210-215.
Stronkhorst J (1996) Contamination and toxicity of sediments: a
persistent problem. Lecture 7 in: The present status of
TBT-copolymer anti-fouling paints. Proceedings from an international
one-day symposium on anti-fouling paints for ocean-going vessels, The
Hague, February 1996.
Takahashi M, Mukai H, Tanabe S, Sakayama N, Masuno H (1998)
Accumulation of butyltin compounds in humans and some terrestrial
mammals and investigation of potential source of pollution.
Proceedings of the Fourth Joint Meeting of the Association for
Bioassay Research and the Association of Environmental Toxicology,
Kusatsu, Japan, 10 September 1998, pp. 50-51 (in Japanese).
Tanabe S (1998) Butyltin contamination in marine mammals. Japanese
journal of environmental toxicology, 1:14-25.
Tanabe S, Prudente M, Mizuno T, Hasegawa J, Miyazaki N (1998) Butyltin
contamination in marine mammals from North Pacific and Asian coastal
waters. Environmental science and technology, 32:193-198.
Thompson TA, Lewis JM, Dejneka NS, Severs WR, Polavarapu R,
Billingsley ML (1996) Induction of apoptosis by organotin compounds
in vitro: neuronal protection with antisense oligonucleotides
directed against stannin. Journal of pharmacology and experimental
therapeutics, 276:1201-1214.
Tolosa J, Readman JW, Blaevoet A, Ghilini S, Bartocci J, Horvat M
(1996) Contamination of Mediterranean (Cote d'Azur) coastal waters by
organotins and Irgarol 1051 used in anti-fouling paints. Marine
pollution bulletin, 32:335-341.
Truhaut R, Anger JP, Reymann JM, Chauvel Y, Van Den Driessche J (1979)
Influence de l'oxyde de tributylétain (OTBE) en aérosol sur le
comportement exploratoire chez la souris. Toxicological European
research, 11:181-186 [cited in IPCS, 1990].
Tsuda T, Inoue DT, Kojima M, Aoki S (1995) Daily intakes of
tributyltin and triphenyltin compounds from meals. Journal of the
Association of Official Analytical Chemists International,
78:941-943.
US EPA (1997) Toxicological review on tributyltin oxide. Available
from the US Environmental Protection Agency's Risk Assessment Hotline
(513-569-7254 [phone], 513-569-7159 [fax], rih.iris@epamail.epa.gov
[Internet address], or the IRIS Website at www.epa.gov/iris).
Vandebriel RJ, Meredith C, Scott MO, Rohall PJ, van Loveren H (1998)
Effects of in vivo exposure to bis(tri- n-butyltin)oxide,
hexa-chlorobenzene, and benzo(a)pyrene on cytokine (receptor) mRNA
levels in cultured rat splenocytes and on IL-2 receptor protein
levels. Toxicology and applied pharmacology, 148:126-136.
Van Loveren H, Krajnc EI, Rombout PJA, Blommaert FA, Vos JG (1990)
Effects of ozone, hexachlorobenzene, and bis(tri- n-butyltin)oxide on
natural killer activity in the rat lung. Toxicology and applied
pharmacology, 102:21-33.
Veiga A, Pinto AF, Loureiro-Dias MC (1997) Tributyltin oxide affects
energy production in the yeast Rhodotorula ferulica, a utilizer of
phenolic compounds. Canadian journal of microbiology, 43:683-687.
Vela NP, Caruso JA (1993) Comparison of flame ionization and
inductively coupled plasma mass spectrometry for the detection of
organometallics separated by capillary supercritical fluid
chromatography. Journal of chromatography, 6741:337-345.
Verdier F, Virat M, Schweinfurth H, Descotes J (1991) Immunotoxicity
of bis(tri- n-butyltin) oxide in the rat. Journal of toxicology and
environmental health, 32:307-319.
Viviani B, Ross AD, Chow SC, Nicotera P (1995) Organotin compounds
induce calcium overload and apoptosis in PC12 cells.
Neurotoxicology, 16:19-26.
Vos JG, DeKlerk A, Krajnc EI, Kruizinga W, Van Ommen B, Rozing J
(1984) Toxicity of bis(tri- n-butyltin) oxide in the rat. II.
Suppression of thymus-dependent immune responses and of parameters of
nonspecific resistance after short-term exposure. Toxicology and
applied pharmacology, 75:387-408.
Vos JG, DeKlerk A, Krajnc EI, Van Loveren V, Rozing J (1990)
Immunotoxicity of bis(tri- n-butyltin)oxide in the rat: effects on
thymus-dependent immunity and on nonspecific resistance following
long-term exposure in young versus aged rats. Toxicology and applied
pharmacology, 105:144-155.
Wax PM, Dockstader L (1995) Tributyltin use in interior paints: a
continuing health hazard. Clinical toxicology, 33:239-241.
Wester PM, Canton JH (1987) Histopathological study of Poecilia
reticulata (guppy) after long-term exposure to
bis(tri- n-butyltin)oxide (TBTO) and di- n-butyltindichloride
(DBTC). Aquatic toxicology, 10:143-163.
Wester PW, van der Heijden CA, Bisschop A, van Esch GJ (1985)
Carcinogenicity study with epichlorohydrin (CEP) by gavage in rats.
Toxicology, 36:325-339.
Wester PW, Krajnc EI, van der Heijden CA (1987) Chronic toxicity and
carcinogenicity study with bis(tri-n -butyltin)oxide (TBTO) in
rats. Unpublished report submitted to the Office of Toxic
Substances, US Environmental Protection Agency, with cover letter
dated 18 May 1987 (Document Control No. FYI-OTS-0687-0550 Sequence A).
Wester PW, Krajnc EI, van Leeuwen FXR, Loeber JG, van der Heijden CA,
Vaessen HAMG, Helleman PW (1988) Two year feeding study in rats with
bis(tri-n -butyltin)oxide (TBTO). Unpublished report, National
Institute of Public Health and Environmental Hygiene, Bilthoven.
Wester PW, Krajnc EI, van Leeuwen FXR, Loeber JG, van der Heijden CA,
Vaessen HAMG, Helleman PW (1990) Chronic toxicity and carcinogenicity
of bis(tri- n-butyltin)oxide (TBTO) in the rat. Food and chemical
toxicology, 28:179-196.
Yamada H, Sasaki YF (1993) Organotins are co-clastogens in a whole
mammalian system. Mutation research, 301:195-200.
APPENDIX 1 -- SOURCE DOCUMENTS
IPCS (1990): Tributyltin compounds
(Environmental Health Criteria 116)
A WHO Task Group meeting on Environmental Health Criteria for
Tributyltin Compounds was held at the Institute of Terrestrial
Ecology, Monks Wood, United Kingdom, from 11 to 15 September 1989. The
Task Group reviewed and revised the draft criteria document and made
an evaluation of the risks for human health and the environment from
exposure to tributyltin compounds.
Copies of this document may be obtained from:
International Programme on Chemical Safety
World Health Organization
Geneva, Switzerland
US EPA (1997): Toxicological review on tributyltin oxide
This document received internal peer review by EPA scientists, an
external peer review by three well-qualified nongovernment scientists,
and consensus review by EPA Program Offices and the 10 Regional
Offices. Summaries of significant comments from external peer
reviewers are included in an appendix to the document.
Copies of this document may be obtained from:
EPA Risk Assessment Hotline
513-569-7254 (phone)
513-569-7159 (fax)
rih.iris@epamail.epa.gov (Internet address)
www.epa.gov/iris (Website)
APPENDIX 2 -- CICAD PEER REVIEW
The draft CICAD on TBTO was sent for review to institutions and
organizations identified by IPCS after contact with IPCS national
Contact Points and Participating Institutions, as well as to
identified experts. Comments were received from:
Department of Health, London, United Kingdom
Health and Safety Executive, Bootle, United Kingdom
Health Canada, Ottawa, Canada
International Agency for Research on Cancer, Lyon, France
International Council on Metals and the Environment, Ottawa,
Canada
József Fodor National Center of Public Health, Budapest, Hungary
Karolinska Institute, Stockholm, Sweden
National Chemicals Inspectorate (KEMI), Solna, Sweden
National Institute for Working Life, Solna, Sweden
National Institute of Public Health and Environmental Protection,
Bilthoven, The Netherlands
United States Department of Health and Human Services (National
Institute of Environmental Health Sciences, Research Triangle
Park, USA)
United States Environmental Protection Agency (Office of Research
and Development, National Center for Environmental Assessment,
Washington, DC, USA)
World Health Organization, International Programme on Chemical
Safety, Geneva, Switzerland
APPENDIX 3 -- CICAD FINAL REVIEW BOARD
Tokyo, Japan, 30 June - 2 July 1998
Members
Dr R. Benson, Drinking Water Program, United States Environmental
Protection Agency, Denver, CO, USA
Dr T. Berzins, National Chemicals Inspectorate (KEMI), Solna, Sweden
Mr R. Cary, Health Directorate, Health and Safety Executive,
Merseyside, United Kingdom
Dr C. DeRosa, Agency for Toxic Substances and Disease Registry, Center
for Disease Control and Prevention, Atlanta, GA, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Cambridgeshire, United
Kingdom
Dr H. Gibb, National Center for Environmental Assessment, United
States Environmental Protection Agency, Washington, DC, USA
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Research, Hanover,
Germany
Ms M.E. Meek, Environmental Health Directorate, Health Canada, Ottawa,
Ontario, Canada ( Chairperson)
Dr J. Sekizawa, Division of Chem-Bio Informatics, National Institute
of Health Sciences, Tokyo, Japan ( Vice-Chairperson)
Professor S.A. Soliman, Department of Pesticide Chemistry, Alexandria
University, Alexandria, Egypt
Ms D. Willcocks, Chemical Assessment Division, Worksafe Australia,
Camperdown, Australia ( Rapporteur)
Professor P. Yao, Chinese Academy of Preventive Medicine, Institute of
Occupational Medicine, Beijing, People's Republic of China
Observers
Professor F.M.C. Carpanini,1 Secretary-General, ECETOC (European
Centre for Ecotoxicology and Toxicology of Chemicals), Brussels,
Belgium
Dr M. Ema, Division of Biological Evaluation, National Institute of
Health Sciences, Osakai, Japan
Mr R. Green,1 International Federation of Chemical, Energy, Mine and
General Workers' Unions, Brussels, Belgium
Dr B. Hansen,1 European Chemicals Bureau, European Commission,
Ispra, Italy
Mr T. Jacob,1 Dupont, Washington, DC, USA
Dr H. Koeter, Organisation for Economic Co-operation and Development,
Paris, France
Mr H. Kondo, Chemical Safety Policy Office, Ministry of International
Trade and Industry, Tokyo, Japan
Ms J. Matsui, Chemical Safety Policy Office, Ministry of International
Trade and Industry, Tokyo, Japan
Mr R. Montaigne,1 European Chemical Industry Council (CEFIC),
Brussels, Belgium
Dr A. Nishikawa, Division of Pathology, National Institute of Health
Sciences, Tokyo, Japan
Dr H. Nishimura, Environmental Health Science Laboratory, National
Institute of Health Sciences, Osaka, Japan
Ms C. Ohtake, Chem-Bio Informatics, National Institute of Health
Sciences, Tokyo, Japan
Dr T. Suzuki, Division of Food, National Institute of Health Sciences,
Tokyo, Japan
Dr K. Takeda, Mitsubishikagaku Institute of Toxicological and
Environmental Sciences, Yokohama, Japan
Dr K. Tasaka, Department of Chemistry, International Christian
University, Tokyo, Japan
Dr H. Yamada, Environment Conservation Division, National Research
Institute of Fisheries Science, Kanagawa, Japan
1 Invited but unable to attend.
Dr M. Yamamoto, Chem-Bio Informatics, National Institute of Health
Sciences, Tokyo, Japan
Dr M. Yasuno, School of Environmental Science, The University of Shiga
Prefecture, Hikone, Japan
Dr K. Ziegler-Skylakakis, GSF-Forschungszentrum für Umwelt und
Gesundheit GmbH, Institut für Toxikologie, Oberschleissheim, Germany
Secretariat
Ms L. Regis, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Mr A. Strawson, Health and Safety Executive, London, United Kingdom
Dr P. Toft, Associate Director, International Programme on Chemical
Safety, World Health Organization, Geneva, Switzerland
RÉSUMÉ D'ORIENTATION
Ce CICAD relatif à l'oxyde de tributyétain (TBTO) a été préparé
par l'Agence américaine pour la protection de l'environnement (United
States Environmental Protection Agency, US EPA) à partir d'un document
sur les dérivés du tributylétain publié par le Programme international
sur la Sécurité chimique dans la série Critères d'Hygiène de
l'Environnement (IPCS, 1990) et d'un document de l'US EPA intitulé
Toxicological review on tributyltin oxide (US EPA, 1997). Ces deux
mises au point étaient basées sur des bibliographies arrêtées
respectivement en 1989 et 1996. Des informations complémentaires dont
les dernières remontent à juin 1998 ont été incluses dans le présent
document. On trouvera à l'appendice 1 des indications sur les sources
documentaires utilisées ainsi que sur leur mode de dépouillement. Les
renseignements concernant l'examen du CICAD par des pairs font l'objet
de l'appendice 2. Ce CICAD a été aprouvé en tant qu'évaluation
internationale lors d'une réunion du Comité d'évaluation finale qui
s'est tenue à Tokyo (Japon) du 30 juin au 2 juillet 1998. La liste des
participants à cette réunion figure à l'appendice 3. La fiche
d'information internationale sur la sécurité chimique (ICSC 1282)
établie pour l'oxyde de tributylétain par le Programme international
sur la Sécurité chimique (IPCS, 1996) est également reproduite dans ce
document.
Dans le présent document, on utilise le terme d'oxyde de
tributylétain chaque fois qu'il est question de ce composé en
particulier. Toutefois dans l'environnement, les dérivés du
tributylétain existent selon toute probabilité principalement sous la
forme d'hydroxyde, de chlorure et de carbonate de tributylétain. Dans
ce cas et lorsque l'identité du composé est douteuse, on utilise le
terme général de tributylétain.
L'oxyde de tributylétain protège efficacement le bois, les
cotonnades, le papier et les peintures murales contre l'attaque de la
vermine. Il entre dans la composition de nombreuses peintures marines
auxquelles on l'ajoute comme agent antisalissures. Il est présent dans
ces produits sous la forme de copolymère organométallique. Lorsque le
copolymère est hydrolysé par l'eau de mer, le tributylétain est
lentement libéré de la surface peinte qu'il protège des incrustations
pendant des durées pouvant aller jusqu'à 4 ou 5 ans.
Du fait de sa faible solubilité dans l'eau et de son caractère
lipophile, le tributylétain s'adsorbe facilement sur les particules.
Sa demi-vie dans la colonne d'eau va de quelques jours à plusieurs
semaines. Il peut subsister dans les sédiments pendant plusieurs
années. Il s'accumule dans l'organisme des animaux, ses organes
d'élection étant le rein et le foie. L'absorption s'effectue davantage
à partir des denrées alimentaires que directement à partir de l'eau.
On ne dispose d'aucun renseignement sur la toxicité du
tributylétain chez l'Homme à la suite d'une exposition de longue
durée. D'après un certain nombre de données et d'observations, le
tributylétain serait fortement irritant pour la peau et les voies
respiratoires. Ces données ne se prêtent toutefois pas à
l'établissement de relations dose-réponse bien caractérisées. Des
études effectuées au Japon ont permis une évaluation quantitative de
l'exposition au tributylétain présent dans les denrées alimentaires.
Les études à court terme montrent que l'oxyde de tributylétain
est modérément à fortement toxique pour les mammifères de laboratoire.
Des études nombreuses et bien conduites, tant à court qu'à long terme,
montrent que l'effet essentiel de l'oxyde de tributylétain réside dans
son immunotoxicité (dépression des fonctions immunitaires
thymo-dépendantes). La dose sans effet nocif observable (NOAEL) chez
le rat est de 0,025 mg/kg de poids corporel par jour, le critère
retenu étant une immunodépression après exposition de longue durée.
Une analyse des doses de référence montre que l'exposition
correspondant à la limite inférieure de confiance (95 %) pour une
réduction de 10 % du titre des anticorps IgE chez le rat est égale à
0,034 mg/kg de poids corporel par jour. Lors d'une étude de
cancérogénicité chez le rat, on a constaté une augmentation de
l'incidence de certaines tumeurs endocriniennes. Ces tumeurs se
produisent spontanément chez les rats appartenant à l'espèce utilisée
dans cette étude et on ignore dans quelle mesure on peut les prendre
en compte dans une évaluation du risque chez l'Homme. L'oxyde de
tributylétain n'est pas cancérogène pour la souris. L'expérience
montre qu'il n'est pas non plus génotoxique. Rien n'indique qu'il
exerce des effets nocifs sur la fonction de reproduction et le
développement à des doses inférieures à la dose sans effet
immunotoxique observable. De tels effets ne se produisent que lorsque
la dose est voisine de celle qui est toxique pour la mère. Comme on
l'a indiqué plus haut, les données révèlent un effet irritant prononcé
sur la peau et les voies respiratoires. En s'appuyant sur la valeur de
la dose sans effet immunotoxique observable et en appliquant un
coefficient de sécurité de 100, on peut donner une valeur-guide de
0,0003 mg/kg p.c. par jour pour l'exposition par la voie buccale. On
ne dispose pas de données suffisantes pour établir une valeur-guide
dans le cas d'une exposition par inhalation.
L'oxyde de tributylétain est extrêmement dangereux pour certains
organismes aquatiques. Dans certains cas, il bloque les fonctions
endocrines. Dans les eaux littorales de quelques régions, il est
présent à une concentration supérieure à celle qui produit de graves
effets nocifs. Dans certaines régions, les effets constatés ont été
suffisamment graves pour faire chuter la fécondité et réduire
l'effectif de la population touchée. Le risque global pour
l'environnement terrestre est vraisemblablement faible.
RESUMEN DE ORIENTACION
Este CICAD sobre el óxido de tributilestaño (TBTO), preparado por
la Agencia para la Protección del Medio Ambiente de los Estados Unidos
(EPA), se basa en un documento sobre los Criterios de Salud Ambiental
del Programa Internacional de Seguridad de las Sustancias Químicas
relativo a los compuestos de tributilestaño (IPCS, 1990) y en el
Examen toxicológico sobre el óxido de tributilestaño de la EPA de
los Estados Unidos (US EPA, 1997). En estos exámenes se analizaron los
datos identificados hasta 1989 y 1996, respectivamente. En el presente
documento aparece también la información adicional obtenida hasta
1998. La información relativa a las características de los proceso de
examen y la disponibilidad de los documentos originales figura en el
apéndice 1. La información acerca del examen colegiado de este CICAD
se presenta en el apéndice 2. Su aprobación tuvo lugar como evaluación
internacional en una reunión de la Junta de Evaluación Final,
celebrada en Tokio, Japón, del 30 de junio al 2 de julio de 1998. La
lista de participantes en esta reunión de la Junta de Evaluación Final
aparece en el apéndice 3. La Ficha internacional de seguridad química
(ICSC 1282), preparada por el Programa Internacional de Seguridad de
las Sustancias Químicas (IPCS, 1996), también se reproduce en el
presente documento.
En este documento, el término de óxido de tributilestaño se
aplica específicamente a este producto químico. Sin embargo, en el
medio ambiente es más probable que los compuestos de tributilestaño se
encuentren como hidróxido, cloruro o carbonato. En esos casos o cuando
la identidad del producto químico específico no está clara, se utiliza
el término general de tributilestaño.
El óxido de tributilestaño es un conservante biocida eficaz de la
madera, los textiles de algodón, el papel y las pinturas y colorantes
domésticos. Se añade como agente antiincrustante en numerosas
formulaciones de pinturas marinas. El tributilestaño está presente en
la mayoría de esas formulaciones antiincrustantes como copolímero
organometálico. Se libera lentamente de la superficie pintada a medida
que el polímero se hidroliza en el agua de mar, proporcionando una
protección prolongada contra las incrustaciones de hasta cuatro o
cinco años.
Debido a su baja solubilidad en agua y a su carácter lipófilo, el
tributilestaño se adsorbe fácilmente en las partículas. Su semivida en
la columna de agua oscila entre unos días y varias semanas. Puede
persistir en los sedimentos durante varios años. Se bioacumula en los
organismos, alcanzando las concentraciones más altas en el hígado y el
riñón. La absorción a partir de los alimentos es más importante que la
procedente directamente del agua.
No se dispone de información sobre la toxicidad del óxido de
tributilestaño en el ser humano tras una exposición prolongada.
Algunos datos e informes de casos ponen de manifiesto que produce
irritación cutánea y respiratoria grave. Sin embargo, los datos no son
suficientes para caracterizar la relación exposición-respuesta. En
algunos estudios realizados en el Japón se ha cuantificado la
exposición humana al tributilestaño procedente de los alimentos.
En estudios de corta duración con mamíferos de laboratorio, la
toxicidad aguda del óxido de tributilestaño es entre moderada y alta.
En numerosos estudios bien realizados, tanto de corta duración como
prolongados, su efecto más importante es la inmunotoxicidad (depresión
de las funciones inmunitarias dependientes del timo). La concentración
sin efectos adversos observados (NOAEL) para la inmunosupresión en
ratas tras una exposición prolongada es de 0,025 mg/kg de peso
corporal al día. El análisis de las dosis de referencia pone de
manifiesto que la exposición correspondiente al límite de confianza
más bajo (95%) de la dosis que produce una disminución del 10% en la
concentración de la inmunoglobulina (Ig) E en ratas es de 0,034 mg/kg
de peso corporal al día. En un estudio de carcinogenicidad en ratas,
se observó un aumento en la incidencia de algunos tumores en
determinados tejidos endocrinos. Estos tumores se producen
espontáneamente, con una incidencia variable en la estirpe de ratas
utilizada en el estudio, y se desconoce su importancia en la
evaluación del riesgo para la salud humana. El óxido de tributilestaño
no es carcinogénico para los ratones. Las pruebas ponen de manifiesto
que no es genotóxico. No hay indicios de que se produzcan efectos
reproductivos o en el desarrollo con una exposición inferior a la
establecida como NOAEL para la inmunotoxicidad. Estos efectos aparecen
solamente con exposiciones próximas a las que causan toxicidad
materna. Los datos demuestran que el óxido de tributilestaño produce
una irritación cutánea y respiratoria grave. Teniendo en cuenta la
NOAEL para la inmunotoxicidad y un factor de incertidumbre de 100, el
valor guía para la exposición oral es de 0,0003 mg/kg de peso corporal
al día. No se dispone de datos adecuados para extrapolar un valor guía
aplicable a la exposición por inhalación.
El óxido de tributilestaño es enormemente peligroso para algunos
organismos acuáticos. Es un perturbador endocrino en algunos
organismos. La concentración de tributilestaño en determinadas aguas
costeras es superior a la que produce efectos adversos graves. Estos
efectos han sido suficientemente importantes para producir fracaso
reproductivo y disminución de la población en algunas zonas. El
peligro general para el medio ambiente terrestre es probablemente
bajo.
 1. EXECUTIVE SUMMARY
This CICAD on tributyltin oxide (TBTO) was prepared by the United
States Environmental Protection Agency (US EPA) and is based on an
International Programme on Chemical Safety Environmental Health
Criteria document on tributyltin compounds (IPCS, 1990) and on the US
EPA's Toxicological review on tributyltin oxide (US EPA, 1997). Data
identified as of 1989 and 1996, respectively, were considered in these
reviews. Additional information identified as of June 1998 has been
included in this document. Information on the nature of the review
processes and the availability of the source documents is presented in
Appendix 1. Information on the peer review of this CICAD is presented
in Appendix 2. This CICAD was approved as an international assessment
at a meeting of the Final Review Board, held in Tokyo, Japan, on 30
June - 2 July 1998. Participants at the Final Review Board meeting are
listed in Appendix 3. The International Chemical Safety Card (ICSC
1282) for TBTO, produced by the International Programme on Chemical
Safety (IPCS, 1996), has also been reproduced in this document.
In this document, the term TBTO is used when that specific
chemical is intended. In the environment, however, tributyltin
compounds are expected to exist mainly as tributyltin hydroxide,
tributyltin chloride, and tributyltin carbonate. In those cases or
when the identity of the specific chemical is not clear, the general
term tributyltin is used.
TBTO is an effective biocidal preservative for wood, cotton
textiles, paper, and paints and stains for residential homes. It is
added as an antifouling agent in numerous formulations of marine
paints. Tributyltin is present in most of these antifouling
formulations as an organometallic copolymer. Tributyltin is slowly
released from the painted surface as the polymer is hydrolysed in
seawater, providing protection against encrustations for as long as
4-5 years.
As a result of its low water solubility and lipophilic character,
tributyltin adsorbs readily onto particles. Its half-life in the water
column ranges from a few days to weeks. Tributyltin may persist in
sediments for several years. It bioaccumulates in organisms, with the
highest concentrations found in liver and kidney. Uptake from food is
more important than uptake directly from water.
No information is available on the toxicity of TBTO in humans
following long-term exposure. Some data and case reports indicate that
TBTO is a severe dermal and respiratory irritant. The data, however,
are not adequate to characterize the exposure-response relationships.
Some studies have quantified human exposure to tributyltin from the
diet in Japan.
TBTO is moderately to highly acutely toxic to laboratory mammals
in short-term studies. In numerous well-conducted studies, both short
term and long term, the critical effect of TBTO is immunotoxicity
(depression of immune functions dependent on the thymus). The
no-observed-adverse-effect level (NOAEL) for immunosuppression in rats
following long-term exposure is 0.025 mg/kg body weight per day.
Benchmark dose analysis shows that the exposure corresponding to the
lower confidence limit (95%) on dose for a 10% decrease in
immunoglobulin (Ig) E titre in rats is 0.034 mg/kg body weight per
day. In a carcinogenicity study in rats, there was an increased
incidence of some tumours in some endocrine tissues. These tumours
occur spontaneously with variable incidence in the strain of rat used
in the study and are of unknown significance for a human health risk
assessment. TBTO is not carcinogenic in mice. The weight of evidence
shows that TBTO is not genotoxic. There is no indication that
reproductive or developmental effects occur at an exposure below that
identified as the NOAEL for immunotoxicity. Effects on reproduction
and development occur only at exposures near those causing maternal
toxicity. Data show that TBTO is a severe respiratory tract and skin
irritant. Based on the NOAEL for immunotoxicity and an uncertainty
factor of 100, a guidance value for oral exposure is 0.0003 mg/kg body
weight per day. No adequate data are available to derive a guidance
value for inhalation exposure.
TBTO is extremely hazardous to some aquatic organisms. It is an
endocrine disruptor in some organisms. The concentration of
tributyltin in some coastal waters is above a concentration causing
severe adverse effects. Adverse effects have been sufficiently severe
to lead to reproductive failure and population decline in some areas.
The general hazard to the terrestrial environment is likely to be low.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
Tributyltin oxide (CAS No.
1. EXECUTIVE SUMMARY
This CICAD on tributyltin oxide (TBTO) was prepared by the United
States Environmental Protection Agency (US EPA) and is based on an
International Programme on Chemical Safety Environmental Health
Criteria document on tributyltin compounds (IPCS, 1990) and on the US
EPA's Toxicological review on tributyltin oxide (US EPA, 1997). Data
identified as of 1989 and 1996, respectively, were considered in these
reviews. Additional information identified as of June 1998 has been
included in this document. Information on the nature of the review
processes and the availability of the source documents is presented in
Appendix 1. Information on the peer review of this CICAD is presented
in Appendix 2. This CICAD was approved as an international assessment
at a meeting of the Final Review Board, held in Tokyo, Japan, on 30
June - 2 July 1998. Participants at the Final Review Board meeting are
listed in Appendix 3. The International Chemical Safety Card (ICSC
1282) for TBTO, produced by the International Programme on Chemical
Safety (IPCS, 1996), has also been reproduced in this document.
In this document, the term TBTO is used when that specific
chemical is intended. In the environment, however, tributyltin
compounds are expected to exist mainly as tributyltin hydroxide,
tributyltin chloride, and tributyltin carbonate. In those cases or
when the identity of the specific chemical is not clear, the general
term tributyltin is used.
TBTO is an effective biocidal preservative for wood, cotton
textiles, paper, and paints and stains for residential homes. It is
added as an antifouling agent in numerous formulations of marine
paints. Tributyltin is present in most of these antifouling
formulations as an organometallic copolymer. Tributyltin is slowly
released from the painted surface as the polymer is hydrolysed in
seawater, providing protection against encrustations for as long as
4-5 years.
As a result of its low water solubility and lipophilic character,
tributyltin adsorbs readily onto particles. Its half-life in the water
column ranges from a few days to weeks. Tributyltin may persist in
sediments for several years. It bioaccumulates in organisms, with the
highest concentrations found in liver and kidney. Uptake from food is
more important than uptake directly from water.
No information is available on the toxicity of TBTO in humans
following long-term exposure. Some data and case reports indicate that
TBTO is a severe dermal and respiratory irritant. The data, however,
are not adequate to characterize the exposure-response relationships.
Some studies have quantified human exposure to tributyltin from the
diet in Japan.
TBTO is moderately to highly acutely toxic to laboratory mammals
in short-term studies. In numerous well-conducted studies, both short
term and long term, the critical effect of TBTO is immunotoxicity
(depression of immune functions dependent on the thymus). The
no-observed-adverse-effect level (NOAEL) for immunosuppression in rats
following long-term exposure is 0.025 mg/kg body weight per day.
Benchmark dose analysis shows that the exposure corresponding to the
lower confidence limit (95%) on dose for a 10% decrease in
immunoglobulin (Ig) E titre in rats is 0.034 mg/kg body weight per
day. In a carcinogenicity study in rats, there was an increased
incidence of some tumours in some endocrine tissues. These tumours
occur spontaneously with variable incidence in the strain of rat used
in the study and are of unknown significance for a human health risk
assessment. TBTO is not carcinogenic in mice. The weight of evidence
shows that TBTO is not genotoxic. There is no indication that
reproductive or developmental effects occur at an exposure below that
identified as the NOAEL for immunotoxicity. Effects on reproduction
and development occur only at exposures near those causing maternal
toxicity. Data show that TBTO is a severe respiratory tract and skin
irritant. Based on the NOAEL for immunotoxicity and an uncertainty
factor of 100, a guidance value for oral exposure is 0.0003 mg/kg body
weight per day. No adequate data are available to derive a guidance
value for inhalation exposure.
TBTO is extremely hazardous to some aquatic organisms. It is an
endocrine disruptor in some organisms. The concentration of
tributyltin in some coastal waters is above a concentration causing
severe adverse effects. Adverse effects have been sufficiently severe
to lead to reproductive failure and population decline in some areas.
The general hazard to the terrestrial environment is likely to be low.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
Tributyltin oxide (CAS No. 