
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
CONCISE INTERNATIONAL CHEMICAL ASSESSMENT DOCUMENT NO. 5
LIMONENE
INTER-ORGANIZATION PROGRAMME FOR THE SOUND MANAGEMENT OF CHEMICALS
A cooperative agreement among UNEP, ILO, FAO, WHO, UNIDO, UNITAR and
OECD
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
First draft prepared by
Dr A. Falk Filipsson, National Institute for Working Life, Solna,
Sweden,
Mr J. Bard, Aseda, Sweden, and
Ms S. Karlsson, National Chemicals Inspectorate, Solna, Sweden
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization, and produced within the framework of the
Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization Geneva, 1998
The International Programme on Chemical Safety (IPCS),
established in 1980, is a joint venture of the United Nations
Environment Programme (UNEP), the International Labour Organisation
(ILO), and the World Health Organization (WHO). The overall
objectives of the IPCS are to establish the scientific basis for
assessment of the risk to human health and the environment from
exposure to chemicals, through international peer review processes, as
a prerequisite for the promotion of chemical safety, and to provide
technical assistance in strengthening national capacities for the
sound management of chemicals.
The Inter-Organization Programme for the Sound Management of
Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and
Agriculture Organization of the United Nations, WHO, the United
Nations Industrial Development Organization, and the Organisation for
Economic Co-operation and Development (Participating Organizations),
following recommendations made by the 1992 UN Conference on
Environment and Development to strengthen cooperation and increase
coordination in the field of chemical safety. The purpose of the IOMC
is to promote coordination of the policies and activities pursued by
the Participating Organizations, jointly or separately, to achieve the
sound management of chemicals in relation to human health and the
environment.
WHO Library Cataloguing in Publication Data
Limonene.
(Concise international chemical assessment document ; 5)
1.Terpenes - toxicity 2.Environmental exposure
3.Food contamination I.International Programme on Chemical
Safety II.Series
ISBN 92 4 153005 7 (NLM Classification: QV 633)
ISSN 1020-6167
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1998
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city, or area
or of its authorities, or concerning the delimitation of its frontiers
or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
TABLE OF CONTENTS
FOREWORD
1. EXECUTIVE SUMMARY
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
3. ANALYTICAL METHODS
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1. Environmental levels
6.2. Human exposure
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1. Single exposure
8.2. Irritation and sensitization
8.3. Short-term exposure
8.4. Long-term exposure
8.4.1. Subchronic exposure
8.4.2. Chronic exposure and carcinogenicity
8.5. Genotoxicity and related end-points
8.6. Reproductive and developmental toxicity
8.7. Immunological and neurological effects
9. EFFECTS ON HUMANS
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10.1. Aquatic environment
10.2. Terrestrial environment
11. EFFECTS EVALUATION
11.1. Evaluation of health effects
11.1.1. Hazard identification and dose-response assessment
11.1.2. Criteria for setting guidance values for limonene
11.1.3. Sample risk characterization
11.2. Evaluation of environmental effects
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
13.1. Human health hazards
13.2. Advice to physicians
13.3. Storage
13.4. Spillage
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
INTERNATIONAL CHEMICAL SAFETY CARD
REFERENCES
APPENDIX 1 - SOURCE DOCUMENT
APPENDIX 2 - CICAD PEER REVIEW
APPENDIX 3 - CICAD FINAL REVIEW BOARD
RÉSUMÉ D'ORIENTATION
RESUMEN DE ORIENTACION
FOREWORD
Concise International Chemical Assessment Documents (CICADs) are
the latest in a family of publications from the International
Programme on Chemical Safety (IPCS) - a cooperative programme of the
World Health Organization (WHO), the International Labour Organisation
(ILO), and the United Nations Environment Programme (UNEP). CICADs
join the Environmental Health Criteria documents (EHCs) as
authoritative documents on the risk assessment of chemicals.
CICADs are concise documents that provide summaries of the
relevant scientific information concerning the potential effects of
chemicals upon human health and/or the environment. They are based on
selected national or regional evaluation documents or on existing
EHCs. Before acceptance for publication as CICADs by IPCS, these
documents have undergone extensive peer review by internationally
selected experts to ensure their completeness, accuracy in the way in
which the original data are represented, and the validity of the
conclusions drawn.
The primary objective of CICADs is characterization of hazard and
dose-response from exposure to a chemical. CICADs are not a summary
of all available data on a particular chemical; rather, they include
only that information considered critical for characterization of the
risk posed by the chemical. The critical studies are, however,
presented in sufficient detail to support the conclusions drawn. For
additional information, the reader should consult the identified
source documents upon which the CICAD has been based.
Risks to human health and the environment will vary considerably
depending upon the type and extent of exposure. Responsible
authorities are strongly encouraged to characterize risk on the basis
of locally measured or predicted exposure scenarios. To assist the
reader, examples of exposure estimation and risk characterization are
provided in CICADs, whenever possible. These examples cannot be
considered as representing all possible exposure situations, but are
provided as guidance only. The reader is referred to EHC 1701 for
advice on the derivation of health-based guidance values.
1 International Programme on Chemical Safety (1994) Assessing
human health risks of chemicals: derivation of guidance values for
health-based exposure limits. Geneva, World Health Organization
(Environmental Health Criteria 170).
While every effort is made to ensure that CICADs represent the
current status of knowledge, new information is being developed
constantly. Unless otherwise stated, CICADs are based on a search of
the scientific literature to the date shown in the executive summary.
In the event that a reader becomes aware of new information that would
change the conclusions drawn in a CICAD, the reader is requested to
contact the IPCS to inform it of the new information.
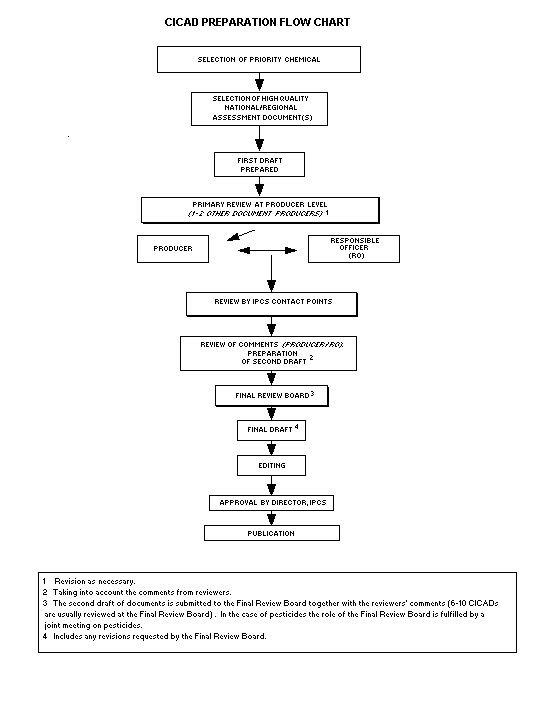
Procedures
The flow chart shows the procedures followed to produce a CICAD.
These procedures are designed to take advantage of the expertise that
exists around the world - expertise that is required to produce the
high-quality evaluations of toxicological, exposure, and other data
that are necessary for assessing risks to human health and/or the
environment.
The first draft is based on an existing national, regional, or
international review. Authors of the first draft are usually, but not
necessarily, from the institution that developed the original review.
A standard outline has been developed to encourage consistency in
form. The first draft undergoes primary review by IPCS and one or
more experienced authors of criteria documents to ensure that it meets
the specified criteria for CICADs.
The second stage involves international peer review by scientists
known for their particular expertise and by scientists selected from
an international roster compiled by IPCS through recommendations from
IPCS national Contact Points and from IPCS Participating Institutions.
Adequate time is allowed for the selected experts to undertake a
thorough review. Authors are required to take reviewers' comments
into account and revise their draft, if necessary. The resulting
second draft is submitted to a Final Review Board together with the
reviewers' comments.
The CICAD Final Review Board has several important functions:
- to ensure that each CICAD has been subjected to an appropriate
and thorough peer review;
- to verify that the peer reviewers' comments have been addressed
appropriately;
- to provide guidance to those responsible for the preparation of
CICADs on how to resolve any remaining issues if, in the opinion
of the Board, the author has not adequately addressed all
comments of the reviewers; and
- to approve CICADs as international assessments.
Board members serve in their personal capacity, not as representatives
of any organization, government, or industry. They are selected
because of their expertise in human and environmental toxicology or
because of their experience in the regulation of chemicals. Boards
are chosen according to the range of expertise required for a meeting
and the need for balanced geographic representation.
Board members, authors, reviewers, consultants, and advisers who
participate in the preparation of a CICAD are required to declare any
real or potential conflict of interest in relation to the subjects
under discussion at any stage of the process. Representatives of
nongovernmental organizations may be invited to observe the
proceedings of the Final Review Board. Observers may participate in
Board discussions only at the invitation of the Chairperson, and they
may not participate in the final decision-making process.
 1. EXECUTIVE SUMMARY
This CICAD on limonene (d-limonene, l-limonene, and
d/l-limonene) was based primarily on a review prepared in 1993 for
the Nordic Expert Group (Karlberg & Lindell, 1993). A second review
produced under the auspices of the Nordic Council of Ministers
(Josefsson, 1993), a preliminary, non-peer-reviewed information source
on environmental exposure and effects (US EPA, 1994), and searches of
relevant databases covering the years 1993-1995 were used for the
identification of additional data for the assessment of limonene. In
a final search of the literature from 1996 to 1997, no data that would
change the conclusions made in the CICAD were identified. Information
concerning the nature and availability of the source document is
presented in Appendix 1. Information on the peer review of this CICAD
is presented in Appendix 2. This CICAD was approved for publication
at a meeting of the Final Review Board, held in Brussels, Belgium, on
18-20 November 1996. Participants at the Final Review Board meeting
are listed in Appendix 3. The International Chemical Safety Card
(ICSC 0918) for d-limonene, produced by the International Programme
on Chemical Safety (IPCS, 1993), has also been reproduced in this
document. Emphasis was given to d-limonene owing to the large
amount of available data on this isomeric form.
Limonene occurs naturally in certain trees and bushes. Limonene
and other monoterpenes are released in large amounts mainly to the
atmosphere, from both biogenic and anthropogenic sources. Limonene is
used as a solvent in degreasing metals prior to industrial painting,
for cleaning in the electronic and printing industries, and in paint
as a solvent. Limonene is also used as a flavour and fragrance
additive in food, household cleaning products, and perfumes.
Limonene is a skin irritant in both experimental animals and
humans. In rabbits, d-limonene was found to be an eye irritant.
Studies in guinea-pigs revealed that air-oxidized d-limonene, but
not d-limonene itself, induced contact allergy. Because d- and
l-limonene are enantiomers, this could also be true for l-limonene
and dipentene (the mixture). Handling and purity of the chemical, and
possibly addition of antioxidants, may thus be crucial for the
allergenic capacity of limonene.
The critical organ in animals (except for male rats), following
peroral or intraperitoneal administration, is the liver. Studies in
which experimental animals were exposed by inhalation to limonene have
not been identified. Exposure to limonene affects the amount and
activity of different liver enzymes, liver weight, cholesterol levels,
and bile flow. These changes have been observed in mice, rats, and
dogs. Available data are insufficient to determine the critical organ
in humans.
In male rats, exposure to d-limonene causes damage to the
kidneys and renal tumours. The male rat specific protein
alpha2µ-globulin is considered to play a crucial role in the
development of neoplastic as well as non-neoplastic kidney lesions.
Thus, these kidney lesions are considered not relevant for human risk
assessment. d-Limonene has been studied in a battery of short-term
in vitro tests and found to be non-genotoxic. There is no evidence
that limonene has teratogenic or embryotoxic effects in the absence of
maternal toxicity. In general, d-limonene could be considered (with
the exception of its irritative and sensitizing properties) to be a
chemical with fairly low toxicity.
Food is the principal source of exposure to limonene, based on
available data. A guidance value for the ingestion of limonene was
calculated to be 0.1 mg/kg body weight per day. At current estimated
levels of exposure, limonene in foodstuffs does not appear to
represent a significant risk to human health.
In the atmosphere, limonene and other terpenes react rapidly with
photochemically produced hydroxyl and nitrate radicals and ozone. The
oxidation of terpenes such as limonene contributes to aerosol and
photochemical smog formation. In soil, limonene is expected to have
low mobility; in the aquatic environment, it is expected to bind
strongly to sediment. Limonene is resistant to hydrolysis.
Biodegradation occurs under aerobic, but not anaerobic, conditions.
Terrestrial organisms are most likely exposed to limonene via the
air. The few studies on terrestrial species (i.e. insects) using
vapour exposure revealed effects of limonene at parts per million
levels. Measured environmental concentrations are typically around
0.1-2 ppb (0.6-11 µg/m3). At polluted sites, limonene concentrations
in soil may exceed effect levels of soil-living organisms (e.g.
earthworms). In the aquatic environment, limonene shows high acute
toxicity to fish and Daphnia. Limonene concentrations in surface
waters are generally much lower than experimentally determined acute
toxicity levels, and therefore it is likely that limonene poses a low
risk for acute toxic effects on aquatic organisms. No studies were
found on chronic effects.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
Limonene is a colourless liquid at room temperature. The
structural formula for limonene is given below. The chemical exists
as two optical isomers, d- and l-limonene, and the racemic mixture
dipentene. The purity of commercial d-limonene is about 90-98%.
Physical and chemical data on limonene presented in Table 1 were
taken from Karlberg & Lindell (1993), unless otherwise stated.
Impurities are mainly other monoterpenes, such as myrcene
(7-methyl-3-methylene-1,6-octadiene), alpha-pinene
(2,6,6-trimethyl-bicyclo[3.1.1]hept-2-ene), alpha-pinene
(6,6-dimethyl-2-methylene-bicyclo[3.1.1]heptane), sabinene
(2-methyl-5-(1-methylethyl)-bicyclo[3.1.0]hexan-2-ol), and
Gamma3-carene ((1S-cis)-3,7,7-trimethyl-bicyclo[4.1.0]hept-2-ene).
The vapour pressure of limonene is high and its solubility in water is
low, giving a high value of the Henry's law constant, which predicts a
high rate of vaporization of limonene.
1. EXECUTIVE SUMMARY
This CICAD on limonene (d-limonene, l-limonene, and
d/l-limonene) was based primarily on a review prepared in 1993 for
the Nordic Expert Group (Karlberg & Lindell, 1993). A second review
produced under the auspices of the Nordic Council of Ministers
(Josefsson, 1993), a preliminary, non-peer-reviewed information source
on environmental exposure and effects (US EPA, 1994), and searches of
relevant databases covering the years 1993-1995 were used for the
identification of additional data for the assessment of limonene. In
a final search of the literature from 1996 to 1997, no data that would
change the conclusions made in the CICAD were identified. Information
concerning the nature and availability of the source document is
presented in Appendix 1. Information on the peer review of this CICAD
is presented in Appendix 2. This CICAD was approved for publication
at a meeting of the Final Review Board, held in Brussels, Belgium, on
18-20 November 1996. Participants at the Final Review Board meeting
are listed in Appendix 3. The International Chemical Safety Card
(ICSC 0918) for d-limonene, produced by the International Programme
on Chemical Safety (IPCS, 1993), has also been reproduced in this
document. Emphasis was given to d-limonene owing to the large
amount of available data on this isomeric form.
Limonene occurs naturally in certain trees and bushes. Limonene
and other monoterpenes are released in large amounts mainly to the
atmosphere, from both biogenic and anthropogenic sources. Limonene is
used as a solvent in degreasing metals prior to industrial painting,
for cleaning in the electronic and printing industries, and in paint
as a solvent. Limonene is also used as a flavour and fragrance
additive in food, household cleaning products, and perfumes.
Limonene is a skin irritant in both experimental animals and
humans. In rabbits, d-limonene was found to be an eye irritant.
Studies in guinea-pigs revealed that air-oxidized d-limonene, but
not d-limonene itself, induced contact allergy. Because d- and
l-limonene are enantiomers, this could also be true for l-limonene
and dipentene (the mixture). Handling and purity of the chemical, and
possibly addition of antioxidants, may thus be crucial for the
allergenic capacity of limonene.
The critical organ in animals (except for male rats), following
peroral or intraperitoneal administration, is the liver. Studies in
which experimental animals were exposed by inhalation to limonene have
not been identified. Exposure to limonene affects the amount and
activity of different liver enzymes, liver weight, cholesterol levels,
and bile flow. These changes have been observed in mice, rats, and
dogs. Available data are insufficient to determine the critical organ
in humans.
In male rats, exposure to d-limonene causes damage to the
kidneys and renal tumours. The male rat specific protein
alpha2µ-globulin is considered to play a crucial role in the
development of neoplastic as well as non-neoplastic kidney lesions.
Thus, these kidney lesions are considered not relevant for human risk
assessment. d-Limonene has been studied in a battery of short-term
in vitro tests and found to be non-genotoxic. There is no evidence
that limonene has teratogenic or embryotoxic effects in the absence of
maternal toxicity. In general, d-limonene could be considered (with
the exception of its irritative and sensitizing properties) to be a
chemical with fairly low toxicity.
Food is the principal source of exposure to limonene, based on
available data. A guidance value for the ingestion of limonene was
calculated to be 0.1 mg/kg body weight per day. At current estimated
levels of exposure, limonene in foodstuffs does not appear to
represent a significant risk to human health.
In the atmosphere, limonene and other terpenes react rapidly with
photochemically produced hydroxyl and nitrate radicals and ozone. The
oxidation of terpenes such as limonene contributes to aerosol and
photochemical smog formation. In soil, limonene is expected to have
low mobility; in the aquatic environment, it is expected to bind
strongly to sediment. Limonene is resistant to hydrolysis.
Biodegradation occurs under aerobic, but not anaerobic, conditions.
Terrestrial organisms are most likely exposed to limonene via the
air. The few studies on terrestrial species (i.e. insects) using
vapour exposure revealed effects of limonene at parts per million
levels. Measured environmental concentrations are typically around
0.1-2 ppb (0.6-11 µg/m3). At polluted sites, limonene concentrations
in soil may exceed effect levels of soil-living organisms (e.g.
earthworms). In the aquatic environment, limonene shows high acute
toxicity to fish and Daphnia. Limonene concentrations in surface
waters are generally much lower than experimentally determined acute
toxicity levels, and therefore it is likely that limonene poses a low
risk for acute toxic effects on aquatic organisms. No studies were
found on chronic effects.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
Limonene is a colourless liquid at room temperature. The
structural formula for limonene is given below. The chemical exists
as two optical isomers, d- and l-limonene, and the racemic mixture
dipentene. The purity of commercial d-limonene is about 90-98%.
Physical and chemical data on limonene presented in Table 1 were
taken from Karlberg & Lindell (1993), unless otherwise stated.
Impurities are mainly other monoterpenes, such as myrcene
(7-methyl-3-methylene-1,6-octadiene), alpha-pinene
(2,6,6-trimethyl-bicyclo[3.1.1]hept-2-ene), alpha-pinene
(6,6-dimethyl-2-methylene-bicyclo[3.1.1]heptane), sabinene
(2-methyl-5-(1-methylethyl)-bicyclo[3.1.0]hexan-2-ol), and
Gamma3-carene ((1S-cis)-3,7,7-trimethyl-bicyclo[4.1.0]hept-2-ene).
The vapour pressure of limonene is high and its solubility in water is
low, giving a high value of the Henry's law constant, which predicts a
high rate of vaporization of limonene.
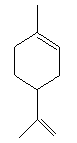 Table 1: Physical/chemical properties of limonene.a
d-Limonene l-Limonene Dipentene
CAS no. 5989-27-5 5989-54-8 138-86-3
Chemical name (R)-1-methyl-4-(1-methylethenyl) (S)-1-methyl-4-(1-methylethenyl) 1-methyl-4-(1-methylethenyl)
cyclohexene cyclohexene cyclohexene
Empirical formula C10H16 C10H16 C10H16
Molecular weight 136.23 136.23 136.23
Melting point (°C) -74.35 -74.35 -95.9
Boiling point (°C) 175.5-176.0 175.5-176.0 175.5-176.0
Density (g/cm3 at 20°C) 0.8411 0.8422 0.8402
Vapour pressure (Pa at 20°C) 190 190 190
Water solubility (mg/litre at 25°C) 13.8b - -
Henry's law constant (kPa m3/mol at 25°C) 34.8c - -
Log Kow 4.23d - 4.83e (limonene)
a Conversion factors: 1 ppm = 5.56 mg/m3; 1 mg/m3 = 0.177 ppm.
b Massaldi & King, 1973; Assessment Tool for the Evaluation of Risk (ASTER) database, Environmental Research Laboratory, US
Environmental Protection Agency, Duluth, MN, 1991.
c Calculated value (ENVIROFATE database, Office of Toxic Substances, US Environmental Protection Agency, and Syracuse Research
Corporation [SRC], New York, NY, 1995).
d Calculated value (US EPA, 1990a, 1994).
e Calculated value (US EPA, 1994; Log Octanol-Water Partition Coefficient Program [LOGKOW], Syracuse Research Corporation [SRC],
New York, NY).
3. ANALYTICAL METHODS
Airborne limonene may be collected by charcoal tube sampling
followed by desorption with carbon disulfide (Searle, 1989) or
alternatively on Tenax (Janson & Kristensson, 1991) or on multisorbent
sampling tubes (Chan et al., 1990) followed by thermal desorption.
Limonene is usually analysed by gas chromatography with flame
ionization detection or mass spectrometry. For limonene in blood,
liquids, and tissues, a head-space technique could be used. The
detection limit in air is 5 µg/m3 (Searle, 1989) and in blood,
1.4 µg/litre (Falk Filipsson et al., 1993). As limonene is easily
oxidized in air, it is also important to analyse the oxidation
products. Hydroperoxides of d-limonene can be analysed by gas
chromatography if the sample is injected on-column (Karlberg et al.,
1994). A high-performance liquid chromatography method for limonene
has also been developed (Nilsson et al., 1996).
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
Limonene, like other monoterpenes, occurs naturally in certain
trees and bushes. It is found in peel from citrus fruits, in dill,
caraway, fennel, and celery, and in turpentine. Typical
concentrations of monoterpenes in air in conifer forests are 1-10
µg/m3, but variations are large (Strömvall, 1992). Mean emission
rates of limonene from different plant species (i.e. lemon, orange,
pistachio, and walnut) in the Central Valley of California ranged from
0.4 to 2.5 mg/g dry leaf weight per hour (Arey et al., 1991).
Monoterpenes are released in significant amounts mainly to the
atmosphere. Biogenic emissions are in the order of, or may exceed,
those from anthropogenic sources (Dimitriades, 1981; Altshuller, 1983;
Lamb et al., 1987). Global annual emissions of biogenic monoterpenes
range from 147 to 827 million tonnes (Fehsenfeld et al., 1992).
Limonene is used as a substitute for chlorinated hydrocarbons,
chlorofluorocarbons, and other solvents. It is used in degreasing
metals (30% limonene) prior to industrial painting, for cleaning in
the electronic industry (50-100% limonene), for cleaning in the
printing industry (30-100% limonene), and in paint as a solvent.
Limonene is also used as a solvent in histological laboratories and as
a flavour and fragrance additive in food, household cleaning products,
and perfumes. d-Limonene has been used as a gallstone solubilizer in
humans (Igimi et al., 1976, 1991).
The annual worldwide production of d-limonene and orange
oil/essence oil (95% d-limonene) in 1991 was approximately 45 kt
(Florida Chemical Co., 1991). Citrus plantings under way are expected
to increase that figure to 73 kt annually within a decade (IARC,
1993). Production volumes in Japan were about 40 kt in each of 1992
and 1993 (Chemical Daily, 1994, 1995). In 1984, the US consumption of
d-limonene was 250 t.1 The number of industrial plants in the USA
handling d-limonene in 1983 was 87, and the estimated number of
employees exposed to the chemical was 140 000.2 The corresponding
numbers of industrial plants and exposed employees were 2 and 1843 for
l-limonene and 103 and 185 000 for dipentene, respectively. In
1974, the corresponding numbers for dipentene were 70 and 45 000,
respectively. The increased use of dipentene has probably continued
after 1983, especially because of its use as a substitute for
chlorinated hydrocarbons, chlorofluorocarbons, and other solvents, but
no production data were identified. According to the product register
set up by the Swedish National Chemicals Inspectorate, between 69 and
1 Source: Environmental Chemicals Data and Information Network
(ECDIN). Ispra, Italy, CEC Joint Research Centre (1993).
2 Source: Registry of Toxic Effects of Chemical Substances (RTECS).
US Department of Health and Human Services, National Institute of
Occupational Safety and Health (NIOSH) (1994).
80 t of d-limonene in 48 products (15 for consumers) were used
during 1994 in Sweden. The corresponding numbers for dipentene were
74-88 t in 106 products (26 for consumers). No use of l-limonene
was reported.
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Monoterpenes such as limonene are released in large amounts
mainly to the atmosphere. The chemical and physical properties of
limonene also indicate that the substance will be distributed mainly
to air.
When released to ground, limonene is expected to have low to very
low mobility in soil, based on its physical/chemical properties. The
soil adsorption coefficient (Koc), calculated on the basis of the
solubility (13.8 mg/litre at 25°C) and the log octanol/water partition
coefficient (4.232), ranges from 1030 to 4780.3 The Henry's law
constant indicates that limonene will rapidly volatilize from both dry
and moist soil; however, its strong adsorption to soil may slow this
process.3
In the aquatic environment, limonene is expected to adsorb to
sediment and suspended organic matter and to rapidly volatilize to the
atmosphere, based on its physical/chemical properties.3 The
estimated half-life for volatilization of limonene from a model river
(1 m deep, flow 1 m/s, and wind speed 3 m/s) is 3.4 hours.3 The
bioconcentration factor, calculated on the basis of water solubility
and the log octanol/water partition coefficient, is 246-262,3
suggesting that limonene may bioaccumulate in fish and other aquatic
organisms.
Limonene does not have functional groups for hydrolysis, and its
cyclohexene ring and ethylene group are known to be resistant to
hydrolysis (US EPA, 1994). Therefore, hydrolysis of limonene is not
expected, neither in terrestrial nor in aquatic environments. The
hydrolytic half-life of d-limonene has been estimated to be >1000
days.4 Biotic degradation of limonene has been shown with some
species of microorganisms, such as Penicillium digitatum,
Corynespora cassiicola, Diplodia gossypina (Abraham et al., 1985),
and a soil strain of Pseudomonas sp. (PL strain) (Dhavalikar &
Bhattacharayya, 1966; Shulka & Bhattacharayya, 1968). As these
studies were not designed to determine the biodegradability of
limonene, the results provided only indications of possible
biodegradation. However, limonene was readily biodegradable (41-98%
degradation by biochemical oxygen demand in 14 days) under aerobic
conditions in a standard test (OECD 301 C "Modified MITI Test (I)";
OECD, 1981) (MITI, 1992). Also, in a test simulating aerobic sewage
treatment (OECD 303 A "Simulation Test - Aerobic Sewage Treatment:
Coupled Units Test"; OECD, 1981), limonene disappeared almost
completely (>93.8%) during 14 days of incubation (Schwartz et al.,
3 Source: Hazardous Substances Data Bank. Bethesda, MD, National
Library of Medicine (1995).
4 Source: ASTER (Assessment Tool for the Evaluation of Risk)
database. Duluth, MN, US Environmental Protection Agency,
Environmental Research Laboratory.
1990). However, this test was not suitable for such a volatile
substance as limonene. The disappearance of limonene was likely due
in part to volatilization, but it could not be determined to what
extent the removal was due to biodegradation and sorption compared
with volatilization.
Biodegradation has also been assessed under anaerobic conditions.
In a test on methanogenic degradation (batch bioassay inoculated with
granular sludge, 30°C), there was no indication of any metabolism of
limonene, possibly because of toxicity to the microorganisms
Sierra-Alvarez et al., 1990). Complex chlorinated terpenes, similar to
toxaphene (a persistent, mobile, and toxic insecticide, with global
distribution) and its degradation products, were produced by
photo-initiated reactions in an aqueous system initially containing
limonene and other monoterpenes, simulating pulp bleaching conditions
(Larson & Marley, 1988).
In the atmosphere, limonene is expected to rapidly undergo
gas-phase reactions with photochemically produced hydroxyl radicals,
ozone, and nitrate radicals (Table 2). Based on experimentally
determined rate constants, calculated lifetimes for the reaction of
d-limonene with photochemically produced hydroxyl radicals range
from 0.3 to 2 hours (Winer et al., 1976, 1984; Atkinson & Carter,
1984; Atkinson et al., 1984; Atkinson, 1990). The corresponding
lifetimes for the reaction with ozone are in the range of 0.2-2.6
hours (Atkinson & Carter, 1984; Atkinson et al., 1984, 1990; Winer et
al., 1984; Klöpffer et al., 1988; Nolting & Zetzsch, 1988; Atkinson,
1990). Based on experimentally determined rate constants, calculated
lifetimes for the nighttime reaction of d-limonene with nitrate
radicals range from 0.9 to 9 minutes (Atkinson & Carter, 1984;
Atkinson et al., 1984; Winer et al., 1984; Atkinson, 1990). The
daytime atmospheric lifetime of d-limonene has been estimated to
range from 12 to 48 minutes, depending upon the local hydroxyl radical
and ozone concentrations (Altshuller, 1983).
Products formed from the hydroxyl radical reaction with limonene
are 4-acetyl-1-methylcyclohexene (Arey et al., 1990; Grosjean et al.,
1992; Hakola et al., 1994), a keto-aldehyde (Arey et al., 1990; Hakola
et al., 1994), formaldehyde, 3-oxobutanal, glyoxal, and a C10
dicarbonyl (Grosjean et al., 1992). The same carbonyls, along with
formic acid and C8 and C9 carboxylic acids, may also form in
reactions with ozone (Grosjean et al., 1992). Ozonolysis of limonene
may also result in bis(hydroxymethyl)peroxide, a precursor to
hydroxymethyl hydroperoxide (Gäb et al., 1985), and hydrogen peroxide
(Becker et al., 1990). Hydroxymethyl hydroperoxide,
bis(hydroxymethyl)peroxide, and hydrogen peroxide have various toxic
effects on plant cells and enzymes (Gäb et al., 1985; Becker et al.,
1990). The reaction of d-limonene with ozone in the dark results in
the formation of 4-acetyl-1-methylcyclohexene and formaldehyde
(Grosjean et al., 1993). Reactions with nitrogen oxides produce
aerosol formation as well as lower molecular weight products, such as
formaldehyde, acetaldehyde, formic acid, acetone, and peroxyacetyl
nitrate (Altshuller, 1983).
Table 2: Rate constants and lifetimes of d-limonene in gas-phase reactions with hydroxyl radicals (OH),
ozone (O3), and nitrate radicals (NO3).
Concentration Lifetime Rate constant
Substance (molecules/cm3)a (hours) (cm3 molecule-1 s-1) Reference
OH 1x106 (0.04 ppt) 0.32 9.0x10-10 Winer et al., 1976
4x106 (0.16 ppt) 0.5 1.4x10-10 Atkinson et al., 1984; Winer et al., 1984
1x106 (0.04 ppt) 1.6 1.7x10-10 Atkinson, 1990
1x106 (0.04 ppt) 2 1.4x10-10 Atkinson & Carter, 1984
1x106 (0.04 ppt) 2 1.4x10-10 Atkinson et al., 1984; Winer et al., 1984
O3 200 ppb 0.18 6.4x10-16 Atkinson et al., 1984; Winer et al., 1984
7x1011 0.5b 5.4x10-16 Klöpffer et al., 1988
30 ppb 0.6 6.4x10-16 Atkinson et al., 1984; Winer et al., 1984
7x1011 0.62 6.4x10-16 Atkinson, 1990
7x1011 0.67 6.0x10-16 Atkinson & Carter, 1984
7x1011 1.9 2.09x10-16 Atkinson et al., 1990
7x1011 2.6 1.53x10-16 Nolting & Zetzsch, 1988
NO3 100 ppt 0.015 7.7x10-12 Atkinson et al., 1984; Winer et al., 1984
(0.9 min)
2.4x108 0.08 1.4x10-11 Atkinson & Carter, 1984
(5 min)
2.4x108 0.09 1.3x10-11 Atkinson, 1990
(5.3 min)
10 ppt 0.15 7.7x10-12 Atkinson et al., 1984; Winer et al., 1984
(9 min)
a Unless otherwise indicated.
b Half-life (in hours).
Terpenes such as limonene contribute to aerosol and photochemical
smog formation (Gäb et al., 1985; Sekiya et al., 1988). Emissions of
biogenic hydrocarbons such as limonene and other terpenes to the
atmosphere may either decrease ozone concentrations when nitrogen
oxide concentrations are low or, if emissions take place in polluted
air (i.e. containing high nitrogen oxide levels), lead to an increase
in ozone concentrations (Altshuller, 1983; Fehsenfeld et al., 1992).
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
Data on environmental levels of limonene are presented in Table
3. The concentrations of limonene and other monoterpenes in air vary
considerably. Recorded concentrations in rural areas depend on many
factors, such as the type of vegetation, temperature, time of the day,
and time of the year (Strömvall, 1992). Biogenic monoterpene
emissions are assumed to be very low in the late autumn and winter
months compared with summer (Altshuller, 1983). Measured
concentrations (between 1979 and 1992) of limonene in the air of rural
forest areas in Europe, Canada, the USA, Nepal, the Republic of
Georgia, and Japan ranged from 1.6 × 10-4 to 2.2 ppb (0.9 ng/m3 to
12.2 µg/m3) (Shaw et al., 1983; Hutte et al., 1984; Roberts et al.,
1985; Jüttner, 1986, 1988; Petersson, 1988; Helmig et al., 1989;
Clement et al., 1990; Janson & Kristensson, 1991; Ciccoioli et al.,
1992, 1993; Helmig & Arey, 1992; Peters et al., 1994). Based upon
these data, typical concentrations of limonene in air from rural areas
range from 0.1 to 0.2 ppb (0.6-1.1 µg/m3).
On the basis of measured concentrations (between 1973 and 1990)
of limonene in the air from urban or suburban areas in Europe, the
USA, and Russia that ranged from not detectable to 5.7 ppb (31.7
µg/m3) (Bertsch et al., 1974; Ioffe et al., 1977, 1979; Hutte et al.,
1984; De Bortoli et al., 1986; Jüttner, 1988; Ciccoioli et al., 1992;
Helmig & Arey, 1992), typical concentrations of limonene in
urban/suburban air are likely to range from 0.1 to 2 ppb (0.6-11.1
µg/m3). Concentrations of limonene in air emissions from kraft pulp
industries, stone groundwood production, and various waste and
landfill sites have ranged from approximately 0.3 to 41 000 ppb (1.7
µg/m3 to 240 mg/m3) (Young & Parker, 1983, 1984; Koe & Ng, 1987;
Strömvall, 1992; Eitzer, 1995).
Limonene has been detected in groundwater and surface waters,
ice, sediments, and soil. Mean limonene concentrations in two
polluted Spanish rivers were 590 and 1600 ng/litre (Gomez-Belinchon et
al., 1991). Samples of water collected from the Gulf of Mexico
contained limonene at a concentration of 2-40 ng/litre (Sauer, 1981).
Limonene has also been detected at Terra Nova Bay, Antarctica; water
and pack ice samples contained limonene at concentrations up to 20 and
15 ng/litre, respectively (Desideri et al., 1991). Limonene
concentrations up to 920 µg/g in soil and from 1 to 130 µg/litre in
groundwater were measured in a polluted area at a former site for the
production of charcoal and pine tar products in Florida (McCreary et
al., 1983). Limonene was also detected but not quantified in fish
(i.e. carp) collected from Las Vegas Wash, Nevada (Hiatt, 1983).
Table 3: Concentrations of limonene in various media.
Medium Concentration Location and sampling date Reference
Air, rural 0.036 µg/m3 (6.4x10-3 ppb) Whitaker's Forest, Sierra Nevada Mountains, California,
June 1990 Helmig & Arey, 1992
0.49 ng/litre (8.7x10-2 ppb) Monte Cimini, Italy, (forest site) Ciccoioli et al., 1992
detected Eggegebirge, North Rhine-Westfalia, Germany, 1988
(forest site) Helmig et al., 1989
40 ppbCa (25 µg/m3)a Forest site in Republic of Georgia, July 1979 Shaw et al., 1983
0.030 ppb Rocky Mountains, Colorado, average day July-Dec. 1982 Roberts et al., 1985
0.072 ppb Rocky Mountains, Colorado, average night July-Dec. 1982 Roberts et al., 1985
0.002-0.13 ppb Rocky Mountains, Colorado, range night July-Dec. 1982 Roberts et al., 1985
detected Western Colorado Hutte et al., 1984
0.34 µg/m3 (6.0x10-2 ppb) Eastern Germany, July (forest site) Ciccoioli et al., 1993
1.16 µg/m3 (0.20 ppb) Nepal, September-October, 1991 Ciccoioli et al., 1993
1.3-7.3 µg/m3 (0.23-1.3 ppb) Forest, Jönköping, Sweden, night June-July, 1983 Petersson, 1988
0.1-2.2 ppb (0.6-12.2 µg/m3) Forest, Northwest Qubec, Canada, July 1989 Clement et al., 1990
detected Southern Black Forest, Germany, Nov.-Jan. (1984-1985) Jüttner, 1986
0.9-89 ng/m3 (1.6x10-4 - 1.6x10-2 ppb) Southern Black Forest, Germany, March-Dec. 1985 Jüttner, 1988
<0.05-0.25 ng/litre
(<8.8x10-3 - 4.4 x10-2 ppb) Speulderbos Forest, Netherlands, summer 1992 Peters et al., 1994
0-0.5 ppb Järllsa Sweden, June 1989 Janson & Kristensson,
1991
Air, urban/ ndb-0.36 µg/m3 (nd-6.4x10-2 ppb) Urban Riverside, California, June 1990 Helmig & Arey, 1992
suburban 0.14 ng/litre (2.5x10-2 ppb) Montelibretti, Italy (suburban site) Ciccoioli et al., 1992
0-5.7 ppb (0-31.7 µg/m3) Houston, Texas Bertsch et al., 1974
<1-11 µg/m3 (<0.2-1.9 ppb) Rural, suburban and urban sites in Northern Italy, 1983-1984
(mean 1 µg/m3, or 0.2 ppb) De Bortoli et al., 1986
detected Leningrad, Russia, summer-autumn, 1976 Ioffe et al., 1977
detected Denver, Colorado, USA, Jan.-Feb. 1984 Hutte et al., 1984
nd-2.0 ng/m3 (nd-3.5x10-4 ppb) Tübingen, Germany, March-April 1985 (suburban) Jüttner, 1988
detected Six larger citiesc in USSR, 1977 Ioffe et al., 1979
Table 3 (continued)
Medium Concentration Location and sampling date Reference
Air, emissions 1.7-10 100 µg/m3 (0.3-1.8x103 ppb) 8 municipal solid waste composting facilities, USA Eitzer, 1995
2-240 mg/m3 (3.5x102 - 4.1x104 ppb) 8 landfill sites, UK (mean approx. 101 mg/m3, or 1.8x104 ppb) Young & Parker, 1983,
1984
detected Refuse waste, Singapore Koe & Ng, 1987
1.9-14 µg/m3 (0.34-2.5 ppb) Emission plumes from kraft pulp industries, Sweden Strömvall, 1992
3.8-39 µg/m3 (0.67-6.9 ppb) Ambient air downwind from stone groundwood production,
Sweden, 1989 Strömvall, 1992
Water, sea 2-40 ng/litre Gulf of Mexico,d 1977 Sauer, 1981
0.55 ng/litre (mean) Barcelona, Mediterranean Sea, Spain, 1986 Gomez-Belinchon et
al., 1991
4.4 ng/litre (mean) Vilanova-Sitges, Mediterranean Sea, Spain, 1986 Gomez-Belinchon et
al., 1991
nd-20 ng/litre Terra Nova Bay, Antarctica, 1988-1989, seawater
(mean 5.4 ng/litre) Desideri et al., 1991
nd-82 ng/litre Terra Nova Bay, Antarctica, 1988-1989, particulate Desideri et al., 1991
84 ng/litre Resurrection Bay, Alaska, June 1985 Button & Jüttner, 1989
0.47 ng/litre Resurrection Bay, Alaska, June 1986 Button & Jüttner, 1989
Water, river 590 ng/litre (mean) Llobregat River, Barcelona, Spain, 1985-1986 Gomez-Belinchon et
al., 1991
1600 ng/litre (mean) Besós River, Barcelona, Spain, 1985-1986 Gomez-Belinchon et
al., 1991
detected Black Warrior River, Tuscaloosa, USA, 1975 Bertsch et al., 1975
detected River Lee, London, UK Waggott, 1981
detected River Glatt, Switzerland, 1975 Zürcher & Giger, 1976
Water, estuary 25-633 ng/litre Southampton Water estuary, UK Bianchi et al., 1991
Table 3 (continued)
Medium Concentration Location and sampling date Reference
Water, 70 ng/litre (max.) Otis Air Base, Massachusetts (sewage-contaminated water) Barber et al., 1988
groundwater 1-130 µg/litre Former site for production of charcoal and pine tar
products, Gainsville, Florida McCreary et al., 1983
Water, 0.03 µg/litre 13 cities in USA (detected in 1 of 13 cities) Keith et al., 1976
drinking-water detected UK (detected in 5 of 14 samples) Fielding et al., 1981
187 µg/kg (1.87x105 ng/litre) Canada, bottled drinking-water (detected in 1 of 182 samples) Page et al., 1993
Water, nd-20 µg/litre Influent waste water, sewage works, Göteborg, Sweden, Paxéus et al., 1992
1989-1991
wastewater, 10-220 ppb (10x103 - 220x103 ng/litre) Kraft mill aerated lagoons, USA Wilson & Hrutfiord, 1975
and landfill nd Effluent wastewater, sewage works, Göteborg, Sweden, Paxéus et al., 1992
1989-1991
leachate detected Industrial landfill leachate, USA Venkataramani & Ahlert,
1984
Ice 4-15 ng/litre Terra Nova Bay, Antarctica, 1988-1989, pack ice, Desideri et al., 1991
(mean 8 ng/litre)
Sediment 105-807 ng/kg Southampton Water estuary, UK Bianchi et al., 1991
Soil nd-920 µg/g Former site for production of charcoal and pine tar McCreary et al., 1983
products, Gainsville, Florida, USA
Litter 4.0 µg/g (mean) Litter of single leaf pinyon woodlands, Western Great Wilt et al., 1988
Basin, USA
Fish detected Carp from Las Vegas Wash, USA Hiatt, 1983
nd Rainbow trout from Colorado River, USA Hiatt, 1983
a Average concentration of terpenes, on a particulate carbon basis.
b Not detected.
c Baku, Kemerovo, Leningrad, Murmansk, Tashkent, and Tblisi.
d Near the mouth of the Mississippi River and on the Louisiana Shelf.
6.2 Human exposure
Examples of estimated exposure to limonene in the general and
occupational environments are presented here, on the basis of
identified data primarily from the USA and Sweden. Countries are
strongly encouraged, however, to estimate exposure on the basis of
local data, possibly in a manner similar to that outlined here.
The intake in food may be unavoidable, as limonene occurs
naturally in citrus fruits and spices and is used as a flavour and
fragrance additive. However, there is a considerable interindividual
variation in intake, owing to different diet patterns. Based on daily
US consumption of d-limonene per capita, the intake of d-limonene
from food for the general population was estimated to be 0.27 mg/kg
body weight per day (Flavor and Extract Manufacturers Association,
1991).
Indoor concentrations of limonene (no specification of
enantiomer) in northern Italy ranged from 10 to 480 µg/m3 (mean 140
µg/m3) (De Bortoli et al., 1986), whereas concentrations ranged from
1.6 to 78 µg/m3 (mean 18 µg/m3) in 17 residences in Ruston,
Washington (Montgomery & Kalman, 1989). In an investigation from Los
Angeles, California, the arithmetic mean limonene level in indoor air
was 40 µg/m3 (Wallace et al., 1991). In 754 randomly selected
residences in Canada, indoor concentrations of limonene ranged from 9
to 30 µg/m3 (Fellin & Otson, 1993); concentrations were higher during
the winter season when ventilation was lower.
The intake of limonene from indoor and outdoor air for the
general population is estimated to be 10 and 0.1 µg/kg body weight per
day, respectively. This is based on the daily inhalation volume for
adults of 22 m3, a mean body weight for males and females of 64 kg,
the assumption that 4 of 24 hours are spent outdoors (IPCS, 1994), and
arithmetic mean limonene levels in indoor and outdoor air of 0.04 and
0.002 mg/m3, respectively, in a study from Los Angeles (Wallace et
al., 1991).
Data on concentrations of limonene in drinking-water are limited.
However, the intake of limonene from drinking-water is likely to be
negligible owing to its low solubility. Dermal exposure to limonene
by the general population is mainly from contact with household
cleaning products in which limonene is a fragrance additive. The
dermal uptake of d-limonene by humans is likely to be low compared
with that via inhalation (Falk et al., 1991).
Inhalation is the principal route of occupational exposure to
limonene. According to the National Exposure Database in Norway,
concentrations of limonene between 1985 and 1992 in the occupational
environment ranged from 0 to 886 mg/m3 (mean 28 mg/m3) (Fjelstad &
Wolbæk, 1992). In a study from Sweden, occupational concentrations
ranged from 0.9 to 400 mg/m3 (Carlsson et al., 1991). There is also
a potential for dermal exposure to limonene in the occupational
environment, although quantitative data are not available.
The estimated intake of limonene from occupational exposure was
calculated on the same basis as for indoor and outdoor air, assuming
that 8 of 24 hours are spent in the workplace each day, with an air
concentration of 150 mg/m3, which is the occupational exposure limit
value in Sweden (National Board of Occupational Safety and Health,
1993). The intake of limonene associated with working at the
occupational exposure limit was estimated as 17 mg/kg body weight per
day.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS
AND HUMANS
d-Limonene has a high partition coefficient between blood and
air (lambdablood/air = 42) and is easily taken up in the blood at the
alveolus (Falk et al., 1990). The net uptake of d-limonene in
volunteers exposed to the chemical at concentrations of 450, 225, and
10 mg/m3 for 2 hours during light physical exercise averaged 65%
(Falk Filipsson et al., 1993). Orally administered d-limonene is
rapidly and almost completely taken up from the gastrointestinal tract
in humans as well as in animals (Igimi et al., 1974; Kodama et al.,
1976). Infusion of labelled d-limonene into the common bile duct of
volunteers revealed that the chemical was very poorly absorbed from
the biliary system (Igimi et al., 1991). In shaved mice, the dermal
absorption of [3H] d/l-limonene from bathing water was rapid,
reaching the maximum level in 10 minutes (von Schäfer & Schäfer,
1982). In one study (one hand exposed to 98% d-limonene for 2
hours), the dermal uptake of d-limonene in humans was reported to be
low compared with that by inhalation (Falk et al., 1991); however,
quantitative data were not provided.
d-Limonene is rapidly distributed to different tissues in the
body and is readily metabolized. Clearance from the blood was 1.1
litre/kg body weight per hour in males exposed for 2 hours to
d-limonene at 450 mg/m3 (Falk Filipsson et al., 1993). A high
oil/blood partition coefficient and a long half-life during the slow
elimination phase suggest high affinity to adipose tissues (Falk et
al., 1990; Falk Filipsson et al., 1993). In rats, the tissue
distribution of radioactivity was initially high in the liver,
kidneys, and blood after the oral administration of [14C] d-limonene
(Igimi et al., 1974); however, negligible amounts of radioactivity
were found after 48 hours. Differences between species regarding the
renal disposition and protein binding of d-limonene have been
observed. For rats, there is also a sex-related variation
(Lehman-McKeeman et al., 1989; Webb et al., 1989). The concentration
of d-limonene equivalents was about 3 times higher in male rats than
in females, and about 40% was reversibly bound to the male rat specific
protein, alpha2µ-globulin (Lehman-McKeeman et al., 1989;
Lehman-McKeeman & Caudill, 1992).
The biotransformation of d-limonene has been studied in many
species, with several possible pathways of metabolism (Figure 1).
Metabolic differences between species have been observed with respect
to the metabolites present in both plasma and urine. About 25-30% of
an oral dose of d-limonene in humans was found in urine as
d-limonene-8,9-diol and its glucuronide; about 7-11% was eliminated
as perillic acid (4-(1-methylethenyl)-1-cyclohexene-1-carboxylic acid)
and its metabolites (Smith et al., 1969; Kodama et al., 1976).
d-Limonene-8,9-diol is probably formed via d-limonene-8,9-epoxide
(Kodama et al., 1976; Watabe et al., 1981). In another study,
perillic acid was reported to be the principal metabolite in plasma in
both rats and humans (Crowell et al., 1992). Other reported pathways
of limonene metabolism involve ring hydroxylation and oxidation of the
methyl group (Kodama et al., 1976).
Following the inhalation exposure of volunteers to d-limonene
at 450 mg/m3 for 2 hours, three phases of elimination were observed
in the blood, with half-lives of about 3, 33, and 750 minutes,
respectively (Falk Filipsson et al., 1993). About 1% of the amount
taken up was eliminated unchanged in exhaled air, whereas about 0.003%
was eliminated unchanged in the urine. When male volunteers were
administered (per os) 1.6 g [14C] d-limonene, 50-80% of the
radioactivity was eliminated in the urine within 2 days (Kodama et
al., 1976). Limonene has been detected, but not quantified, in breast
milk of non-occupationally exposed mothers (Pellizzari et al., 1982).
Table 1: Physical/chemical properties of limonene.a
d-Limonene l-Limonene Dipentene
CAS no. 5989-27-5 5989-54-8 138-86-3
Chemical name (R)-1-methyl-4-(1-methylethenyl) (S)-1-methyl-4-(1-methylethenyl) 1-methyl-4-(1-methylethenyl)
cyclohexene cyclohexene cyclohexene
Empirical formula C10H16 C10H16 C10H16
Molecular weight 136.23 136.23 136.23
Melting point (°C) -74.35 -74.35 -95.9
Boiling point (°C) 175.5-176.0 175.5-176.0 175.5-176.0
Density (g/cm3 at 20°C) 0.8411 0.8422 0.8402
Vapour pressure (Pa at 20°C) 190 190 190
Water solubility (mg/litre at 25°C) 13.8b - -
Henry's law constant (kPa m3/mol at 25°C) 34.8c - -
Log Kow 4.23d - 4.83e (limonene)
a Conversion factors: 1 ppm = 5.56 mg/m3; 1 mg/m3 = 0.177 ppm.
b Massaldi & King, 1973; Assessment Tool for the Evaluation of Risk (ASTER) database, Environmental Research Laboratory, US
Environmental Protection Agency, Duluth, MN, 1991.
c Calculated value (ENVIROFATE database, Office of Toxic Substances, US Environmental Protection Agency, and Syracuse Research
Corporation [SRC], New York, NY, 1995).
d Calculated value (US EPA, 1990a, 1994).
e Calculated value (US EPA, 1994; Log Octanol-Water Partition Coefficient Program [LOGKOW], Syracuse Research Corporation [SRC],
New York, NY).
3. ANALYTICAL METHODS
Airborne limonene may be collected by charcoal tube sampling
followed by desorption with carbon disulfide (Searle, 1989) or
alternatively on Tenax (Janson & Kristensson, 1991) or on multisorbent
sampling tubes (Chan et al., 1990) followed by thermal desorption.
Limonene is usually analysed by gas chromatography with flame
ionization detection or mass spectrometry. For limonene in blood,
liquids, and tissues, a head-space technique could be used. The
detection limit in air is 5 µg/m3 (Searle, 1989) and in blood,
1.4 µg/litre (Falk Filipsson et al., 1993). As limonene is easily
oxidized in air, it is also important to analyse the oxidation
products. Hydroperoxides of d-limonene can be analysed by gas
chromatography if the sample is injected on-column (Karlberg et al.,
1994). A high-performance liquid chromatography method for limonene
has also been developed (Nilsson et al., 1996).
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
Limonene, like other monoterpenes, occurs naturally in certain
trees and bushes. It is found in peel from citrus fruits, in dill,
caraway, fennel, and celery, and in turpentine. Typical
concentrations of monoterpenes in air in conifer forests are 1-10
µg/m3, but variations are large (Strömvall, 1992). Mean emission
rates of limonene from different plant species (i.e. lemon, orange,
pistachio, and walnut) in the Central Valley of California ranged from
0.4 to 2.5 mg/g dry leaf weight per hour (Arey et al., 1991).
Monoterpenes are released in significant amounts mainly to the
atmosphere. Biogenic emissions are in the order of, or may exceed,
those from anthropogenic sources (Dimitriades, 1981; Altshuller, 1983;
Lamb et al., 1987). Global annual emissions of biogenic monoterpenes
range from 147 to 827 million tonnes (Fehsenfeld et al., 1992).
Limonene is used as a substitute for chlorinated hydrocarbons,
chlorofluorocarbons, and other solvents. It is used in degreasing
metals (30% limonene) prior to industrial painting, for cleaning in
the electronic industry (50-100% limonene), for cleaning in the
printing industry (30-100% limonene), and in paint as a solvent.
Limonene is also used as a solvent in histological laboratories and as
a flavour and fragrance additive in food, household cleaning products,
and perfumes. d-Limonene has been used as a gallstone solubilizer in
humans (Igimi et al., 1976, 1991).
The annual worldwide production of d-limonene and orange
oil/essence oil (95% d-limonene) in 1991 was approximately 45 kt
(Florida Chemical Co., 1991). Citrus plantings under way are expected
to increase that figure to 73 kt annually within a decade (IARC,
1993). Production volumes in Japan were about 40 kt in each of 1992
and 1993 (Chemical Daily, 1994, 1995). In 1984, the US consumption of
d-limonene was 250 t.1 The number of industrial plants in the USA
handling d-limonene in 1983 was 87, and the estimated number of
employees exposed to the chemical was 140 000.2 The corresponding
numbers of industrial plants and exposed employees were 2 and 1843 for
l-limonene and 103 and 185 000 for dipentene, respectively. In
1974, the corresponding numbers for dipentene were 70 and 45 000,
respectively. The increased use of dipentene has probably continued
after 1983, especially because of its use as a substitute for
chlorinated hydrocarbons, chlorofluorocarbons, and other solvents, but
no production data were identified. According to the product register
set up by the Swedish National Chemicals Inspectorate, between 69 and
1 Source: Environmental Chemicals Data and Information Network
(ECDIN). Ispra, Italy, CEC Joint Research Centre (1993).
2 Source: Registry of Toxic Effects of Chemical Substances (RTECS).
US Department of Health and Human Services, National Institute of
Occupational Safety and Health (NIOSH) (1994).
80 t of d-limonene in 48 products (15 for consumers) were used
during 1994 in Sweden. The corresponding numbers for dipentene were
74-88 t in 106 products (26 for consumers). No use of l-limonene
was reported.
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Monoterpenes such as limonene are released in large amounts
mainly to the atmosphere. The chemical and physical properties of
limonene also indicate that the substance will be distributed mainly
to air.
When released to ground, limonene is expected to have low to very
low mobility in soil, based on its physical/chemical properties. The
soil adsorption coefficient (Koc), calculated on the basis of the
solubility (13.8 mg/litre at 25°C) and the log octanol/water partition
coefficient (4.232), ranges from 1030 to 4780.3 The Henry's law
constant indicates that limonene will rapidly volatilize from both dry
and moist soil; however, its strong adsorption to soil may slow this
process.3
In the aquatic environment, limonene is expected to adsorb to
sediment and suspended organic matter and to rapidly volatilize to the
atmosphere, based on its physical/chemical properties.3 The
estimated half-life for volatilization of limonene from a model river
(1 m deep, flow 1 m/s, and wind speed 3 m/s) is 3.4 hours.3 The
bioconcentration factor, calculated on the basis of water solubility
and the log octanol/water partition coefficient, is 246-262,3
suggesting that limonene may bioaccumulate in fish and other aquatic
organisms.
Limonene does not have functional groups for hydrolysis, and its
cyclohexene ring and ethylene group are known to be resistant to
hydrolysis (US EPA, 1994). Therefore, hydrolysis of limonene is not
expected, neither in terrestrial nor in aquatic environments. The
hydrolytic half-life of d-limonene has been estimated to be >1000
days.4 Biotic degradation of limonene has been shown with some
species of microorganisms, such as Penicillium digitatum,
Corynespora cassiicola, Diplodia gossypina (Abraham et al., 1985),
and a soil strain of Pseudomonas sp. (PL strain) (Dhavalikar &
Bhattacharayya, 1966; Shulka & Bhattacharayya, 1968). As these
studies were not designed to determine the biodegradability of
limonene, the results provided only indications of possible
biodegradation. However, limonene was readily biodegradable (41-98%
degradation by biochemical oxygen demand in 14 days) under aerobic
conditions in a standard test (OECD 301 C "Modified MITI Test (I)";
OECD, 1981) (MITI, 1992). Also, in a test simulating aerobic sewage
treatment (OECD 303 A "Simulation Test - Aerobic Sewage Treatment:
Coupled Units Test"; OECD, 1981), limonene disappeared almost
completely (>93.8%) during 14 days of incubation (Schwartz et al.,
3 Source: Hazardous Substances Data Bank. Bethesda, MD, National
Library of Medicine (1995).
4 Source: ASTER (Assessment Tool for the Evaluation of Risk)
database. Duluth, MN, US Environmental Protection Agency,
Environmental Research Laboratory.
1990). However, this test was not suitable for such a volatile
substance as limonene. The disappearance of limonene was likely due
in part to volatilization, but it could not be determined to what
extent the removal was due to biodegradation and sorption compared
with volatilization.
Biodegradation has also been assessed under anaerobic conditions.
In a test on methanogenic degradation (batch bioassay inoculated with
granular sludge, 30°C), there was no indication of any metabolism of
limonene, possibly because of toxicity to the microorganisms
Sierra-Alvarez et al., 1990). Complex chlorinated terpenes, similar to
toxaphene (a persistent, mobile, and toxic insecticide, with global
distribution) and its degradation products, were produced by
photo-initiated reactions in an aqueous system initially containing
limonene and other monoterpenes, simulating pulp bleaching conditions
(Larson & Marley, 1988).
In the atmosphere, limonene is expected to rapidly undergo
gas-phase reactions with photochemically produced hydroxyl radicals,
ozone, and nitrate radicals (Table 2). Based on experimentally
determined rate constants, calculated lifetimes for the reaction of
d-limonene with photochemically produced hydroxyl radicals range
from 0.3 to 2 hours (Winer et al., 1976, 1984; Atkinson & Carter,
1984; Atkinson et al., 1984; Atkinson, 1990). The corresponding
lifetimes for the reaction with ozone are in the range of 0.2-2.6
hours (Atkinson & Carter, 1984; Atkinson et al., 1984, 1990; Winer et
al., 1984; Klöpffer et al., 1988; Nolting & Zetzsch, 1988; Atkinson,
1990). Based on experimentally determined rate constants, calculated
lifetimes for the nighttime reaction of d-limonene with nitrate
radicals range from 0.9 to 9 minutes (Atkinson & Carter, 1984;
Atkinson et al., 1984; Winer et al., 1984; Atkinson, 1990). The
daytime atmospheric lifetime of d-limonene has been estimated to
range from 12 to 48 minutes, depending upon the local hydroxyl radical
and ozone concentrations (Altshuller, 1983).
Products formed from the hydroxyl radical reaction with limonene
are 4-acetyl-1-methylcyclohexene (Arey et al., 1990; Grosjean et al.,
1992; Hakola et al., 1994), a keto-aldehyde (Arey et al., 1990; Hakola
et al., 1994), formaldehyde, 3-oxobutanal, glyoxal, and a C10
dicarbonyl (Grosjean et al., 1992). The same carbonyls, along with
formic acid and C8 and C9 carboxylic acids, may also form in
reactions with ozone (Grosjean et al., 1992). Ozonolysis of limonene
may also result in bis(hydroxymethyl)peroxide, a precursor to
hydroxymethyl hydroperoxide (Gäb et al., 1985), and hydrogen peroxide
(Becker et al., 1990). Hydroxymethyl hydroperoxide,
bis(hydroxymethyl)peroxide, and hydrogen peroxide have various toxic
effects on plant cells and enzymes (Gäb et al., 1985; Becker et al.,
1990). The reaction of d-limonene with ozone in the dark results in
the formation of 4-acetyl-1-methylcyclohexene and formaldehyde
(Grosjean et al., 1993). Reactions with nitrogen oxides produce
aerosol formation as well as lower molecular weight products, such as
formaldehyde, acetaldehyde, formic acid, acetone, and peroxyacetyl
nitrate (Altshuller, 1983).
Table 2: Rate constants and lifetimes of d-limonene in gas-phase reactions with hydroxyl radicals (OH),
ozone (O3), and nitrate radicals (NO3).
Concentration Lifetime Rate constant
Substance (molecules/cm3)a (hours) (cm3 molecule-1 s-1) Reference
OH 1x106 (0.04 ppt) 0.32 9.0x10-10 Winer et al., 1976
4x106 (0.16 ppt) 0.5 1.4x10-10 Atkinson et al., 1984; Winer et al., 1984
1x106 (0.04 ppt) 1.6 1.7x10-10 Atkinson, 1990
1x106 (0.04 ppt) 2 1.4x10-10 Atkinson & Carter, 1984
1x106 (0.04 ppt) 2 1.4x10-10 Atkinson et al., 1984; Winer et al., 1984
O3 200 ppb 0.18 6.4x10-16 Atkinson et al., 1984; Winer et al., 1984
7x1011 0.5b 5.4x10-16 Klöpffer et al., 1988
30 ppb 0.6 6.4x10-16 Atkinson et al., 1984; Winer et al., 1984
7x1011 0.62 6.4x10-16 Atkinson, 1990
7x1011 0.67 6.0x10-16 Atkinson & Carter, 1984
7x1011 1.9 2.09x10-16 Atkinson et al., 1990
7x1011 2.6 1.53x10-16 Nolting & Zetzsch, 1988
NO3 100 ppt 0.015 7.7x10-12 Atkinson et al., 1984; Winer et al., 1984
(0.9 min)
2.4x108 0.08 1.4x10-11 Atkinson & Carter, 1984
(5 min)
2.4x108 0.09 1.3x10-11 Atkinson, 1990
(5.3 min)
10 ppt 0.15 7.7x10-12 Atkinson et al., 1984; Winer et al., 1984
(9 min)
a Unless otherwise indicated.
b Half-life (in hours).
Terpenes such as limonene contribute to aerosol and photochemical
smog formation (Gäb et al., 1985; Sekiya et al., 1988). Emissions of
biogenic hydrocarbons such as limonene and other terpenes to the
atmosphere may either decrease ozone concentrations when nitrogen
oxide concentrations are low or, if emissions take place in polluted
air (i.e. containing high nitrogen oxide levels), lead to an increase
in ozone concentrations (Altshuller, 1983; Fehsenfeld et al., 1992).
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
Data on environmental levels of limonene are presented in Table
3. The concentrations of limonene and other monoterpenes in air vary
considerably. Recorded concentrations in rural areas depend on many
factors, such as the type of vegetation, temperature, time of the day,
and time of the year (Strömvall, 1992). Biogenic monoterpene
emissions are assumed to be very low in the late autumn and winter
months compared with summer (Altshuller, 1983). Measured
concentrations (between 1979 and 1992) of limonene in the air of rural
forest areas in Europe, Canada, the USA, Nepal, the Republic of
Georgia, and Japan ranged from 1.6 × 10-4 to 2.2 ppb (0.9 ng/m3 to
12.2 µg/m3) (Shaw et al., 1983; Hutte et al., 1984; Roberts et al.,
1985; Jüttner, 1986, 1988; Petersson, 1988; Helmig et al., 1989;
Clement et al., 1990; Janson & Kristensson, 1991; Ciccoioli et al.,
1992, 1993; Helmig & Arey, 1992; Peters et al., 1994). Based upon
these data, typical concentrations of limonene in air from rural areas
range from 0.1 to 0.2 ppb (0.6-1.1 µg/m3).
On the basis of measured concentrations (between 1973 and 1990)
of limonene in the air from urban or suburban areas in Europe, the
USA, and Russia that ranged from not detectable to 5.7 ppb (31.7
µg/m3) (Bertsch et al., 1974; Ioffe et al., 1977, 1979; Hutte et al.,
1984; De Bortoli et al., 1986; Jüttner, 1988; Ciccoioli et al., 1992;
Helmig & Arey, 1992), typical concentrations of limonene in
urban/suburban air are likely to range from 0.1 to 2 ppb (0.6-11.1
µg/m3). Concentrations of limonene in air emissions from kraft pulp
industries, stone groundwood production, and various waste and
landfill sites have ranged from approximately 0.3 to 41 000 ppb (1.7
µg/m3 to 240 mg/m3) (Young & Parker, 1983, 1984; Koe & Ng, 1987;
Strömvall, 1992; Eitzer, 1995).
Limonene has been detected in groundwater and surface waters,
ice, sediments, and soil. Mean limonene concentrations in two
polluted Spanish rivers were 590 and 1600 ng/litre (Gomez-Belinchon et
al., 1991). Samples of water collected from the Gulf of Mexico
contained limonene at a concentration of 2-40 ng/litre (Sauer, 1981).
Limonene has also been detected at Terra Nova Bay, Antarctica; water
and pack ice samples contained limonene at concentrations up to 20 and
15 ng/litre, respectively (Desideri et al., 1991). Limonene
concentrations up to 920 µg/g in soil and from 1 to 130 µg/litre in
groundwater were measured in a polluted area at a former site for the
production of charcoal and pine tar products in Florida (McCreary et
al., 1983). Limonene was also detected but not quantified in fish
(i.e. carp) collected from Las Vegas Wash, Nevada (Hiatt, 1983).
Table 3: Concentrations of limonene in various media.
Medium Concentration Location and sampling date Reference
Air, rural 0.036 µg/m3 (6.4x10-3 ppb) Whitaker's Forest, Sierra Nevada Mountains, California,
June 1990 Helmig & Arey, 1992
0.49 ng/litre (8.7x10-2 ppb) Monte Cimini, Italy, (forest site) Ciccoioli et al., 1992
detected Eggegebirge, North Rhine-Westfalia, Germany, 1988
(forest site) Helmig et al., 1989
40 ppbCa (25 µg/m3)a Forest site in Republic of Georgia, July 1979 Shaw et al., 1983
0.030 ppb Rocky Mountains, Colorado, average day July-Dec. 1982 Roberts et al., 1985
0.072 ppb Rocky Mountains, Colorado, average night July-Dec. 1982 Roberts et al., 1985
0.002-0.13 ppb Rocky Mountains, Colorado, range night July-Dec. 1982 Roberts et al., 1985
detected Western Colorado Hutte et al., 1984
0.34 µg/m3 (6.0x10-2 ppb) Eastern Germany, July (forest site) Ciccoioli et al., 1993
1.16 µg/m3 (0.20 ppb) Nepal, September-October, 1991 Ciccoioli et al., 1993
1.3-7.3 µg/m3 (0.23-1.3 ppb) Forest, Jönköping, Sweden, night June-July, 1983 Petersson, 1988
0.1-2.2 ppb (0.6-12.2 µg/m3) Forest, Northwest Qubec, Canada, July 1989 Clement et al., 1990
detected Southern Black Forest, Germany, Nov.-Jan. (1984-1985) Jüttner, 1986
0.9-89 ng/m3 (1.6x10-4 - 1.6x10-2 ppb) Southern Black Forest, Germany, March-Dec. 1985 Jüttner, 1988
<0.05-0.25 ng/litre
(<8.8x10-3 - 4.4 x10-2 ppb) Speulderbos Forest, Netherlands, summer 1992 Peters et al., 1994
0-0.5 ppb Järllsa Sweden, June 1989 Janson & Kristensson,
1991
Air, urban/ ndb-0.36 µg/m3 (nd-6.4x10-2 ppb) Urban Riverside, California, June 1990 Helmig & Arey, 1992
suburban 0.14 ng/litre (2.5x10-2 ppb) Montelibretti, Italy (suburban site) Ciccoioli et al., 1992
0-5.7 ppb (0-31.7 µg/m3) Houston, Texas Bertsch et al., 1974
<1-11 µg/m3 (<0.2-1.9 ppb) Rural, suburban and urban sites in Northern Italy, 1983-1984
(mean 1 µg/m3, or 0.2 ppb) De Bortoli et al., 1986
detected Leningrad, Russia, summer-autumn, 1976 Ioffe et al., 1977
detected Denver, Colorado, USA, Jan.-Feb. 1984 Hutte et al., 1984
nd-2.0 ng/m3 (nd-3.5x10-4 ppb) Tübingen, Germany, March-April 1985 (suburban) Jüttner, 1988
detected Six larger citiesc in USSR, 1977 Ioffe et al., 1979
Table 3 (continued)
Medium Concentration Location and sampling date Reference
Air, emissions 1.7-10 100 µg/m3 (0.3-1.8x103 ppb) 8 municipal solid waste composting facilities, USA Eitzer, 1995
2-240 mg/m3 (3.5x102 - 4.1x104 ppb) 8 landfill sites, UK (mean approx. 101 mg/m3, or 1.8x104 ppb) Young & Parker, 1983,
1984
detected Refuse waste, Singapore Koe & Ng, 1987
1.9-14 µg/m3 (0.34-2.5 ppb) Emission plumes from kraft pulp industries, Sweden Strömvall, 1992
3.8-39 µg/m3 (0.67-6.9 ppb) Ambient air downwind from stone groundwood production,
Sweden, 1989 Strömvall, 1992
Water, sea 2-40 ng/litre Gulf of Mexico,d 1977 Sauer, 1981
0.55 ng/litre (mean) Barcelona, Mediterranean Sea, Spain, 1986 Gomez-Belinchon et
al., 1991
4.4 ng/litre (mean) Vilanova-Sitges, Mediterranean Sea, Spain, 1986 Gomez-Belinchon et
al., 1991
nd-20 ng/litre Terra Nova Bay, Antarctica, 1988-1989, seawater
(mean 5.4 ng/litre) Desideri et al., 1991
nd-82 ng/litre Terra Nova Bay, Antarctica, 1988-1989, particulate Desideri et al., 1991
84 ng/litre Resurrection Bay, Alaska, June 1985 Button & Jüttner, 1989
0.47 ng/litre Resurrection Bay, Alaska, June 1986 Button & Jüttner, 1989
Water, river 590 ng/litre (mean) Llobregat River, Barcelona, Spain, 1985-1986 Gomez-Belinchon et
al., 1991
1600 ng/litre (mean) Besós River, Barcelona, Spain, 1985-1986 Gomez-Belinchon et
al., 1991
detected Black Warrior River, Tuscaloosa, USA, 1975 Bertsch et al., 1975
detected River Lee, London, UK Waggott, 1981
detected River Glatt, Switzerland, 1975 Zürcher & Giger, 1976
Water, estuary 25-633 ng/litre Southampton Water estuary, UK Bianchi et al., 1991
Table 3 (continued)
Medium Concentration Location and sampling date Reference
Water, 70 ng/litre (max.) Otis Air Base, Massachusetts (sewage-contaminated water) Barber et al., 1988
groundwater 1-130 µg/litre Former site for production of charcoal and pine tar
products, Gainsville, Florida McCreary et al., 1983
Water, 0.03 µg/litre 13 cities in USA (detected in 1 of 13 cities) Keith et al., 1976
drinking-water detected UK (detected in 5 of 14 samples) Fielding et al., 1981
187 µg/kg (1.87x105 ng/litre) Canada, bottled drinking-water (detected in 1 of 182 samples) Page et al., 1993
Water, nd-20 µg/litre Influent waste water, sewage works, Göteborg, Sweden, Paxéus et al., 1992
1989-1991
wastewater, 10-220 ppb (10x103 - 220x103 ng/litre) Kraft mill aerated lagoons, USA Wilson & Hrutfiord, 1975
and landfill nd Effluent wastewater, sewage works, Göteborg, Sweden, Paxéus et al., 1992
1989-1991
leachate detected Industrial landfill leachate, USA Venkataramani & Ahlert,
1984
Ice 4-15 ng/litre Terra Nova Bay, Antarctica, 1988-1989, pack ice, Desideri et al., 1991
(mean 8 ng/litre)
Sediment 105-807 ng/kg Southampton Water estuary, UK Bianchi et al., 1991
Soil nd-920 µg/g Former site for production of charcoal and pine tar McCreary et al., 1983
products, Gainsville, Florida, USA
Litter 4.0 µg/g (mean) Litter of single leaf pinyon woodlands, Western Great Wilt et al., 1988
Basin, USA
Fish detected Carp from Las Vegas Wash, USA Hiatt, 1983
nd Rainbow trout from Colorado River, USA Hiatt, 1983
a Average concentration of terpenes, on a particulate carbon basis.
b Not detected.
c Baku, Kemerovo, Leningrad, Murmansk, Tashkent, and Tblisi.
d Near the mouth of the Mississippi River and on the Louisiana Shelf.
6.2 Human exposure
Examples of estimated exposure to limonene in the general and
occupational environments are presented here, on the basis of
identified data primarily from the USA and Sweden. Countries are
strongly encouraged, however, to estimate exposure on the basis of
local data, possibly in a manner similar to that outlined here.
The intake in food may be unavoidable, as limonene occurs
naturally in citrus fruits and spices and is used as a flavour and
fragrance additive. However, there is a considerable interindividual
variation in intake, owing to different diet patterns. Based on daily
US consumption of d-limonene per capita, the intake of d-limonene
from food for the general population was estimated to be 0.27 mg/kg
body weight per day (Flavor and Extract Manufacturers Association,
1991).
Indoor concentrations of limonene (no specification of
enantiomer) in northern Italy ranged from 10 to 480 µg/m3 (mean 140
µg/m3) (De Bortoli et al., 1986), whereas concentrations ranged from
1.6 to 78 µg/m3 (mean 18 µg/m3) in 17 residences in Ruston,
Washington (Montgomery & Kalman, 1989). In an investigation from Los
Angeles, California, the arithmetic mean limonene level in indoor air
was 40 µg/m3 (Wallace et al., 1991). In 754 randomly selected
residences in Canada, indoor concentrations of limonene ranged from 9
to 30 µg/m3 (Fellin & Otson, 1993); concentrations were higher during
the winter season when ventilation was lower.
The intake of limonene from indoor and outdoor air for the
general population is estimated to be 10 and 0.1 µg/kg body weight per
day, respectively. This is based on the daily inhalation volume for
adults of 22 m3, a mean body weight for males and females of 64 kg,
the assumption that 4 of 24 hours are spent outdoors (IPCS, 1994), and
arithmetic mean limonene levels in indoor and outdoor air of 0.04 and
0.002 mg/m3, respectively, in a study from Los Angeles (Wallace et
al., 1991).
Data on concentrations of limonene in drinking-water are limited.
However, the intake of limonene from drinking-water is likely to be
negligible owing to its low solubility. Dermal exposure to limonene
by the general population is mainly from contact with household
cleaning products in which limonene is a fragrance additive. The
dermal uptake of d-limonene by humans is likely to be low compared
with that via inhalation (Falk et al., 1991).
Inhalation is the principal route of occupational exposure to
limonene. According to the National Exposure Database in Norway,
concentrations of limonene between 1985 and 1992 in the occupational
environment ranged from 0 to 886 mg/m3 (mean 28 mg/m3) (Fjelstad &
Wolbæk, 1992). In a study from Sweden, occupational concentrations
ranged from 0.9 to 400 mg/m3 (Carlsson et al., 1991). There is also
a potential for dermal exposure to limonene in the occupational
environment, although quantitative data are not available.
The estimated intake of limonene from occupational exposure was
calculated on the same basis as for indoor and outdoor air, assuming
that 8 of 24 hours are spent in the workplace each day, with an air
concentration of 150 mg/m3, which is the occupational exposure limit
value in Sweden (National Board of Occupational Safety and Health,
1993). The intake of limonene associated with working at the
occupational exposure limit was estimated as 17 mg/kg body weight per
day.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS
AND HUMANS
d-Limonene has a high partition coefficient between blood and
air (lambdablood/air = 42) and is easily taken up in the blood at the
alveolus (Falk et al., 1990). The net uptake of d-limonene in
volunteers exposed to the chemical at concentrations of 450, 225, and
10 mg/m3 for 2 hours during light physical exercise averaged 65%
(Falk Filipsson et al., 1993). Orally administered d-limonene is
rapidly and almost completely taken up from the gastrointestinal tract
in humans as well as in animals (Igimi et al., 1974; Kodama et al.,
1976). Infusion of labelled d-limonene into the common bile duct of
volunteers revealed that the chemical was very poorly absorbed from
the biliary system (Igimi et al., 1991). In shaved mice, the dermal
absorption of [3H] d/l-limonene from bathing water was rapid,
reaching the maximum level in 10 minutes (von Schäfer & Schäfer,
1982). In one study (one hand exposed to 98% d-limonene for 2
hours), the dermal uptake of d-limonene in humans was reported to be
low compared with that by inhalation (Falk et al., 1991); however,
quantitative data were not provided.
d-Limonene is rapidly distributed to different tissues in the
body and is readily metabolized. Clearance from the blood was 1.1
litre/kg body weight per hour in males exposed for 2 hours to
d-limonene at 450 mg/m3 (Falk Filipsson et al., 1993). A high
oil/blood partition coefficient and a long half-life during the slow
elimination phase suggest high affinity to adipose tissues (Falk et
al., 1990; Falk Filipsson et al., 1993). In rats, the tissue
distribution of radioactivity was initially high in the liver,
kidneys, and blood after the oral administration of [14C] d-limonene
(Igimi et al., 1974); however, negligible amounts of radioactivity
were found after 48 hours. Differences between species regarding the
renal disposition and protein binding of d-limonene have been
observed. For rats, there is also a sex-related variation
(Lehman-McKeeman et al., 1989; Webb et al., 1989). The concentration
of d-limonene equivalents was about 3 times higher in male rats than
in females, and about 40% was reversibly bound to the male rat specific
protein, alpha2µ-globulin (Lehman-McKeeman et al., 1989;
Lehman-McKeeman & Caudill, 1992).
The biotransformation of d-limonene has been studied in many
species, with several possible pathways of metabolism (Figure 1).
Metabolic differences between species have been observed with respect
to the metabolites present in both plasma and urine. About 25-30% of
an oral dose of d-limonene in humans was found in urine as
d-limonene-8,9-diol and its glucuronide; about 7-11% was eliminated
as perillic acid (4-(1-methylethenyl)-1-cyclohexene-1-carboxylic acid)
and its metabolites (Smith et al., 1969; Kodama et al., 1976).
d-Limonene-8,9-diol is probably formed via d-limonene-8,9-epoxide
(Kodama et al., 1976; Watabe et al., 1981). In another study,
perillic acid was reported to be the principal metabolite in plasma in
both rats and humans (Crowell et al., 1992). Other reported pathways
of limonene metabolism involve ring hydroxylation and oxidation of the
methyl group (Kodama et al., 1976).
Following the inhalation exposure of volunteers to d-limonene
at 450 mg/m3 for 2 hours, three phases of elimination were observed
in the blood, with half-lives of about 3, 33, and 750 minutes,
respectively (Falk Filipsson et al., 1993). About 1% of the amount
taken up was eliminated unchanged in exhaled air, whereas about 0.003%
was eliminated unchanged in the urine. When male volunteers were
administered (per os) 1.6 g [14C] d-limonene, 50-80% of the
radioactivity was eliminated in the urine within 2 days (Kodama et
al., 1976). Limonene has been detected, but not quantified, in breast
milk of non-occupationally exposed mothers (Pellizzari et al., 1982).
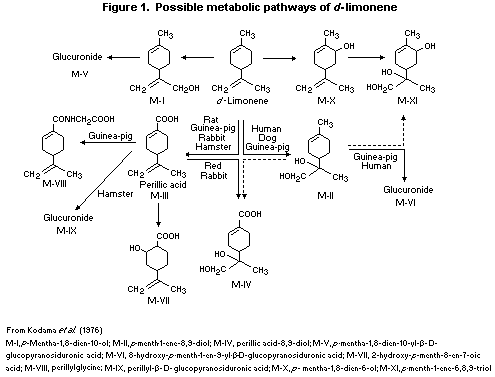 8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
The acute toxicity of d-limonene in rodents is fairly low after
oral, intraperitoneal, subcutaneous, and intravenous administration,
based on the magnitude of the LD50 values (Table 4). LD50 values
were approximately 5 g/kg body weight for the oral administration of
d-limonene or d/l-limonene to rats and for dermal application of
d/l-limonene to rabbits and 6 g/kg body weight for oral
administration to mice (Tsuji et al., 1974, 1975b; Opdyke, 1978).
Studies on the acute inhalation toxicity of limonene were not
identified.
Effects observed following the acute exposure of rodents to
limonene include increased bile flow at 85 mg/kg body weight (Kodama
et al., 1976), inhibition of S-3-hydroxy-3-methylglutaryl-CoA
reductase activity at 409 mg/kg body weight (Clegg et al., 1980),
enzyme induction at 600 and 1200 mg/kg body weight (Ariyoshi et al.,
1975), and decreased motor activity, hypothermia, and potentiation of
hexobarbital-induced sleep at 3 ml/kg body weight (Tsuji et al.,
1974).
8.2 Irritation and sensitization
d-Limonene is considered a skin irritant (Cronin, 1980;
Fischer, 1986). The skin irritancy of limonene in guinea-pigs (Klecak
et al., 1977) and rabbits (Lacy et al., 1987; Okabe et al., 1990) is
considered moderate and low, respectively. In an in vivo study of
rabbit skin irritation, d-limonene was ranked 3.5 of 8 on the basis
of the primary irritation index (Bagley et al., 1996); effects were
graded according to OECD Test Guideline 404 (OECD, 1993). In a study
in rabbits, d-limonene caused irritation to the eyes (Tsuji et al.,
1974).
Although d-limonene was once considered the main allergen in
citrus fruits, data from more recent studies in animals have revealed
air-oxidized d-limonene, rather than unoxidized d-limonene, to be
the sensitizing agent. When limonene (unspecified form and unknown
purity of the test material) was tested in four different
sensitization assays with guinea-pigs (Open Epicutaneous Test,
Maximization Test, Draize's Test, and a test with Freund's Complete
Adjuvant), it was sensitizing in all but Draize's Test (Klecak et al.,
1977). In another study in mice, d-limonene did not induce
sensitization (Maisey & Miller, 1986). Hydroperoxides and other
oxidation products of d-limonene formed on exposure to the air have
proved to be potent contact allergens when tested with Freund's
Complete Adjuvant in guinea-pigs, whereas unoxidized d-limonene did
not cause any sensitization (Karlberg et al., 1991, 1992).
Table 4: Acute toxicity of limonene.
Species (sex) Route of administration Type of limonene LD50 (g/kg body weight) Reference
rabbit dermal d/l >5 Opdyke, 1978
rat oral d/l 5.3 Opdyke, 1978
rat (m/f) oral d 4.4/5.1 Tsuji et al., 1975b
rat (m/f) intraperitoneal d 3.6/4.5 Tsuji et al., 1975b
rat (m/f) intravenous d 0.125/0.11 Tsuji et al., 1975b
mouse (m/f) oral d 5.6/6.6 Tsuji et al., 1975b
mouse (m/f) oral, 7 days d 5.3/6.8a Tsuji et al., 1974
mouse (m/f) intraperitoneal, 3 days d 3.1/3.0a Tsuji et al., 1974
mouse (m + f) intraperitoneal d 1.3 Tsuji et al., 1975b
mouse (m/f) intraperitoneal, 10 days d 0.59/0.50a Tsuji et al., 1974
mouse (m + f) subcutaneous d >41.5 Tsuji et al., 1975b
mouse (m + f) subcutaneous, 7 days d >21.5 Tsuji et al., 1974
a Calculated from ml/kg body weight.
8.3 Short-term exposure
Increases in hepatic cytochrome P-450 content have been observed
in female rats administered limonene (isomer unspecified; 40 mg/kg
body weight per day for 3 days) by intraperitoneal injection (Austin
et al., 1988) and in rats administered 5% d-limonene in the diet for
2 weeks (Maltzman et al., 1991). Increased epoxide hydratase activity
was observed in rats administered 1% or 5% d-limonene in the diet
for 2 weeks (Maltzman et al., 1991). Increases in phase II enzymes
(glutathionyltransferase and UDP-glucuronyltransferase) during the
exposure of rats to 5% limonene in food have also been described
(Maltzman, 1991). Increased relative liver weight (from 5 to 20
times) has been observed in rats administered d-limonene at a dose
of 75-300 mg/kg body weight; at 300 mg/kg body weight, the increase
was significant (Kanerva et al., 1987b). In cats, infusion of 97%
d-limonene into the bile system to dissolve gallstones caused acute
and chronic inflammatory changes (Schenk et al., 1980).
8.4 Long-term exposure
8.4.1 Subchronic exposure
Peroral administration of d-limonene to rats at a dose of 400
mg/kg body weight for 30 days resulted in a 20-30% increase in the
amount and activity of different liver enzymes (cytochrome P-450,
cytochrome b5, aminopyrine demethylase, and aniline hydroxylase),
increased relative liver weight, and decreased cholesterol levels
(Ariyoshi et al., 1975). Administration of d-limonene (0, 2, 5, 10,
30, and 75 mg/kg body weight per day) by gavage to groups of 10 male
rats, 5 days/week for 13 weeks (Webb et al., 1989), resulted in the
pathological formation of granular casts at the outer zone of the
renal medulla. The no-observed-effect level (NOEL), based upon
histological examination of the kidneys, was considered to be 5 mg/kg
body weight per day. The LOEL for increased liver and kidney weight
was 75 mg/kg body weight per day, the highest dose tested. The NOEL
for effects in the liver was 10 mg/kg body weight; the
no-observed-adverse-effect level (NOAEL) for effects in the liver was
30 mg/kg body weight per day. Linear regression analysis revealed a
dose-related trend in the increased relative weights of the kidney and
liver at 30 and 75 mg/kg body weight per day. No histopathological
changes were observed in the liver in these two studies. The amount
and activity of different liver enzymes were not investigated, and
thus the increase in relative liver weight may be due to enzyme
induction.
8.4.2 Chronic exposure and carcinogenicity
The oral administration of d-limonene (0.4, 1.2, or 3.6 ml/kg
body weight per day) to dogs for 6 months caused nausea and vomiting
(Tsuji et al., 1975a). A 35% increase in alkaline phosphatase and
cholesterol in serum and slightly increased total and relative liver
weights were observed in dogs after peroral administration of
d-limonene at a dose of 1.2 ml/kg body weight per day for 6 months
(about 1000 mg/kg body weight per day) (Webb et al., 1990).
In a 2-year study, d-limonene was administered (per os) 5
days/week to groups of 50 F344/N rats (0, 75, or 150 mg/kg body weight
per day to males, and 0, 300, or 600 mg/kg body weight per day to
females) and B6C3F1 mice (0, 250, or 500 mg/kg body weight per day to
males, and 0, 500, or 1000 mg/kg body weight per day to females) (NTP,
1990). Slightly lower body weights were observed for rats in the
high-dose groups and female mice in the high-dose group; however, no
clinical symptoms could be related to the administration of
d-limonene. For female rats in the high-dose group, survival was
reduced after 39 weeks (NTP, 1990). There was clear evidence of
carcinogenic activity of d-limonene in male rats, based upon a
dose-related increase in the incidence of hyperplasia and adenoma/
adenocarcinoma in renal tubular cells. However, there was no evidence
of carcinogenicity in female rats or in male and female mice. The
carcinogenic response in the kidney of male rats has been linked to a
unique renal perturbation involving alpha2µ-globulin.
To determine whether d-limonene would cause a sustained
increase in renal cell proliferation and exhibit promoting activity
for the development of renal adenomas in male F344 rats, the animals
were administered (by stomach tube) d-limonene (150 mg/kg body
weight per day) as a promoter 5 days/week for 30 weeks (Dietrich &
Swenberg, 1991). N-ethyl- N-hydroxyethylnitrosamine (500 ppm) was
used as an initiator in the drinking-water for 2 weeks. In addition,
male alpha2µ-globulin-deficient rats were exposed in the same manner
to determine if the male rat specific urinary protein alpha2µ-globulin
is required for d-limonene to cause these effects. Exposure to
d-limonene alone caused a significant increase in the number of
atypical tubules and atypical hyperplasias in F344 rats, compared with
vehicle controls. There was no increase in the incidence of tumours
or preneoplastic lesions in the alpha2µ-globulin-deficient rats
exposed to d-limonene, whereas a 10-fold increase in the incidence
of renal adenoma and atypical hyperplasia was observed in F344 rats
exposed to d-limonene, compared with controls. There was a
significant decrease in the incidence of liver tumours in animals
exposed to N-ethyl- N-hydroxyethylnitrosamine and d-limonene,
compared with N-ethyl- N-hydroxyethylnitrosamine exposure alone.
8.5 Genotoxicity and related end-points
On the basis of available data, there is no evidence that
d-limonene or its metabolites are genotoxic or mutagenic. Limonene
and its epoxides were not mutagenic when tested at concentrations of
0.3-3333 µg/plate in in vitro assays using different strains of
Salmonella typhimurium, in the presence or absence of metabolic
activation (Florin et al., 1980; Watabe et al., 1981; Haworth et al.,
1983; Connor et al., 1985; NTP, 1990). d-Limonene did not increase
the frequency of forward mutation at the TK+/- locus in mouse L5178Y
cells (NTP, 1990), induce cytogenetic damage in Chinese hamster ovary
cells (Anderson et al., 1990), or malignantly transform Syrian hamster
embryo cells (Pienta, 1980). In one in vitro study, following
exposure with benzo (a)pyrene, d-limonene (21.9 µmol/litre)
inhibited the formation of transformed cell colonies in tracheal
epithelium isolated from rats (Steele et al., 1990).
No evidence of mutagenicity was reported in an in vivo spot
test with mice, involving the intraperitoneal injection of limonene at
215 mg/kg body weight per day on days 9-11 during gestation (Fahrig,
1984).
8.6 Reproductive and developmental toxicity
Studies on the reproductive toxicity of limonene were not
identified. There is no evidence that limonene has teratogenic or
embryotoxic effects in the absence of maternal toxicity. In rats, the
oral administration of d-limonene (2869 mg/kg body weight per day)
on days 9-15 of gestation resulted in decreased body weight and deaths
among the dams. Delayed ossification and decreased total body and
organ weights (thymus, spleen, and ovary) were observed in the
offspring (Tsuji et al., 1975b). In mice, the oral administration of
d-limonene (2869 mg/kg body weight per day) on days 7-12 of
gestation resulted in reduced growth in the mothers and a
significantly increased incidence of skeletal anomalies and delayed
ossification in the offspring (Kodama et al., 1977a). The oral
administration of d-limonene (250, 500, or 1000 mg/kg body weight
per day) to rabbits on days 6-18 of gestation had no dose-related
effects on the offspring. At the highest dose, there were some deaths
and reduced weight gain among the dams; at the intermediate dose,
growth was decreased (Kodama et al., 1977b).
8.7 Immunological and neurological effects
Reports relating limonene to type I allergy were not identified.
In a study designed to assess the immunological effects of
d-limonene on B- and T-cell responses, BALB/c mice were administered
(by forced intragastric feeding) d-limonene (0.1 ml) daily for 9
weeks (Evans et al., 1987). Mice given keyhole limpet haemocyanin
prior to exposure to d-limonene had suppressed primary and secondary
anti-keyhole limpet haemocyanin responses. Mice exposed to
d-limonene prior to the administration of keyhole limpet haemocyanin
had significantly increased antibody and mitogen-induced proliferative
responses. However, the purity of the d-limonene in this study was
not checked, and oxidation products may have been the active
substances.
Effects on the central nervous system following exposure to
limonene have been reported in experimental studies with animals;
however, it is difficult to ascertain whether these effects were the
result of general intoxication or a more direct effect of the
chemical. The peroral administration of d-limonene (3 ml) to rats
and mice resulted in decreased motor activity (Tsuji et al., 1974). A
similar effect was also observed in mice orally administered a
limonene dose of 1000 mg/kg body weight per day for 13 weeks (NTP,
1990).
9. EFFECTS ON HUMANS
Case reports or epidemiological studies on the effects of
limonene on human health were not identified. Available data have
been derived from studies with volunteers. In older investigations,
multiple exposures and confounding factors such as mechanical damage,
irritation, other allergens, and infections due to wet work (Beerman
et al., 1938; Schwartz, 1938; Birmingham et al., 1951) may have
contributed to the effects reported following exposure to limonene.
None of eight subjects reported any discomfort, irritation, or
symptoms related to central nervous system effects during a 2-hour
inhalation exposure to d-limonene at 10, 225, or 450 mg/m3;
however, a slight decline in vital capacity was observed following
exposure to the highest concentration (Falk Filipsson et al., 1993).
In a study in which the sensitivity of four patch testing systems
(Finn chamber, Hill Top patch, Van der Bend chamber, and Webril patch)
was evaluated in volunteers, d-limonene (perfume-grade) reacted
strongly in all types of patches within 10-15 minutes of exposure
(York et al., 1995). Skin irritation was assessed before application,
as well as immediately and 1, 24, 48, and 72 hours after removal of
the patch, using a scoring system based broadly on that used for
rabbit irritation studies (OECD, 1993), but modified to account for
the nature of reactions on human skin. There was evidence of sensory
effects and urticarial responses on removal of the patches.
Significant irritation persisted for 24 hours, and these reactions
persisted for 48 and 72 hours in many volunteers (York et al., 1995).
Dermal exposure to d-limonene (98%) for 2 hours in one subject
caused burning, itching, aching, and a long-lasting purpuric rash
(Falk et al., 1991).
d-Limonene infused directly into the bile system of human
volunteers to dissolve gallstones caused pain in the upper abdomen,
nausea, vomiting, and diarrhoea, as well as increases in serum
aminotransferases and alkaline phosphatase (Igimi et al., 1976, 1991).
The oral administration of 20 g d-limonene to volunteers resulted in
diarrhoea, painful constrictions, and proteinuria, but no biochemical
changes (total protein, bilirubin, cholesterol, aspartate
aminotransferase, alanine aminotransferase, alkaline phosphatase) in
the liver (Igimi et al., 1976). Reports of contact allergy to
dipentene have appeared (Calnan, 1979; Rycroft, 1980). In one
investigation, 15 of 22 people with an allergy to oil of turpentine
also reacted to dipentene (Cachao et al., 1986). Patch testing in
consecutive dermatitis patients from Sweden and Belgium revealed
positive reactions in 1.5-2% of the subjects tested with oxidized
d-limonene, a finding similar to that observed with other common
sensitizers, such as formaldehyde (A.-T. Karlberg, personal
communication, 1996). d-Limonene reduced non-immunological contact
urticaria caused by cinnamic aldehyde, with competitive receptor
inhibition suggested as the mechanism of suppression (Guin et al.,
1984). No sensitizing effect was observed when 25 volunteers were
exposed to d-limonene in a Human Maximization Test (Grief, 1967).
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10.1 Aquatic environment
The acute toxicity of d-limonene ranges from slight to high for
aquatic organisms (Table 5). The lowest acute toxicity values (EC50
or LC50) identified were approximately 0.4 mg/litre for Daphnia (US
EPA, 1990b) and 0.7 mg/litre for fish (US EPA, 1990a,b). The
no-observed-effect concentration (NOEC) for green algae is
approximately 4 mg/litre (US EPA, 1990a). The acute toxicity (EC50
or LC50) of dipentene to Daphnia and fish is about 50-70 times
lower than that for d-limonene (US EPA, 1990b). No studies were
identified on the chronic toxicity of limonene to aquatic organisms.
10.2 Terrestrial environment
The toxicity of limonene has been studied in various terrestrial
organisms (Table 6). Limonene generally has moderate acute toxicity
in insects and mites. The acute toxicity of d-limonene to
earthworms (Eisenia foetida Savigny) was high (LC50 = 6.0 ppm;
mg/kg) (Karr et al., 1990). Sublethal effects (i.e. abnormal
rebounding of medial giant fibre pathway [MGF] impulses and
spontaneous lateral giant fibre pathway [LGF] spiking) were observed
following exposure of earthworms to 4.2 ppm (mg/kg) limonene (Karr et
al., 1990). Limonene has low subacute toxicity to bobwhite quail
(Colinus virginianus) exposed via the diet (LC50 > 5620 ppm;
mg/kg) (US EPA, 1994).
Table 5: Toxicity of limonene to aquatic organisms.
Species End-point; exposure Results (mg/litre) Reference
Algae
Green algaea 96-h NOEC; static 4.08 US EPA, 1990a
Crustaceans
Water flea (Daphnia magna)b 48-h LC50; flow-through 0.577 (0.496-0.672) US EPA, 1990b
48-h EC50; flow-through 0.421
Water flea (D. magna)c acute LC50 39 ppm US EPA, 1994
Water flea (D. magna)a 48-h LC50; flow-through 31 (27.5-34.8) US EPA, 1990b
48-h EC50; flow-through 28.2
Water flea (Daphnia pulex)b 48-h EC50; flow-through 0.730 US EPA, 1990a
Water flea (D. pulex)c 48-h EC50; static 69.6 Passino & Smith, 1987
Daphniab 21-d NOEC; structure-activity 0.15 US EPA, 1990a
relationship (SAR) analysis
Fish
Fathead minnow (Pimephales promelas)b 96-h LC50; flow-through 0.702 (0.619-0.796) US EPA, 1990b
Fathead minnow (P. promelas)b 96-h LC50; flow-through 0.720 (0.618-0.839) US EPA, 1990b
96-h EC50; flow-through 0.688 (0.606-0.782)
Fathead minnow (P. promelas)a 96-h LC50; flow-through 38.5 (35.4-41.8) US EPA, 1990a,b
96-h EC50; flow-through 28.2
Fishc acute LC50 80 ppm US EPA, 1994
Fishb 96-h LC50; flow-through 0.711 US EPA, 1990a
Golden orfe (Leuciscus idus)a 48-h LC50 32 Roth, 1990
Table 5 (continued)
Species End-point; exposure Results (mg/litre) Reference
Insects
Water hyacinth weevil (Neochetina Mortality (73%, range 40-100%), 50% limonene Haag, 1986
eichhorniae, 60%, N. bruchi, 40%)b weevils were dipped in limonene
Mosquito fly (Culex quinquefasciatus)c 2nd-instar larvae (23-33°C), 6.6-26.1 ppm Mohsen et al., 1989
72-h LC50; static
4th-instar larvae (23-33°C), 7.8-30.6 ppm Mohsen et al., 1989
72-h LC50; static
a d/l-Limonene.
b d-Limonene.
c Optical isomer not specified.
Table 6: Toxicity of limonene to terrestrial organisms.
Species End-point; exposure Results Reference
Insects
Cat flea Adult LD50; contact 160 (157-163) µg/cm2 Hink & Fee, 1986
(Ctenocephalides felis)a,b Adult LD50; vapour 259 (234-281) µg/cm2
Pupae LD50; contact 376 (259-468) µg/cm2
Larvae LD50; contact 226 (221-231) µg/cm2
Eggs; lethal to all eggs; contact 65 µg/cm2
Variegated cutworm Larvae; significant inhibition of pupation; dietary
(Peridroma saucia)b exposure 0.2% limonene in artificial feed Harwood et al., 1990
German cockroach Adult 24-h LD50; topical 700 (610-810) µg/insect Karr & Coats, 1988
(Blattella germanica L.)b Adult 24-h LC50; fumigation 23.3 (17.5-31.0) ppm
Adult; no mortality; oral 25% limonene in feed
Nymph; no mortality; oral 25% limonene in feed
Adult; no mortality; 72-h contact with treated limonene (conc. not given)
surface
German cockroach Effect on growth rate; diet 1-25% limonene in diet Karr & Coats, 1992
(B. germanica L.)b EC50, oothecae yielding young; topical exposure 0.68 mg/ootheca
No effect on reproduction; via diet 25% limonene in diet
Topical exposure 0.84 mg/cockroach
Vapour exposure 5 mg/litre in air
Rice weevil (Sitophilus Adult 24-h LC50; fumigation 19.0 (13.2-27.3) ppm Karr & Coats, 1988
oryzae L.)b
House fly (Musca 25-h LD50; topical 90 (70-130) µg/insect Karr & Coats, 1988
domestica L.)b
Western corn rootworm Egg 72-h LC50; contact with treated substrate 1.8 (0.8-2.9)% limonene Karr & Coats, 1988
(Diabrotica virgifera Larvae 72-h LC50; contact with treated soil 12.2 (4.5-32.6) ppm
virgifera LeConte)b
Table 6 (continued)
Species End-point; exposure Results Reference
Spiders and allies
Spruce spider mite 24-h LC50; vapour 24.5 ppm Cook, 1992
(Oligonychus ununguis Significant decrease in oviposition 5 ppm
(Jacobi)),c adult female
Segmented worms
Earthworm (Eisenia 48-h LC50 6.0 (5.1-7.1) ppm Karr et al., 1990
foetida Savigny)b Sublethal effects 4.2 ppm
Birds
Bobwhite quail (Colinus Subacute LC50; dietary exposure >5 620 ppm US EPA, 1994
virginianus)d
a Fleas were exposed to filter papers treated with limonene, either directly or to vapours from the filter papers.
b d-Limonene.
c l-Limonene.
d Optical isomers not specified.
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
Limonene is a skin irritant in experimental animals and humans.
d-Limonene is an eye irritant in rabbits. Studies in guinea-pigs
have revealed that air-oxidized d-limonene, but not d-limonene
itself, induced contact allergy. Similar results are likely with
l-limonene and dipentene.
The critical organ in animals (except for male rats) following
peroral or intraperitoneal administration is the liver. Exposure to
limonene affects the amount and activity of different liver enzymes,
liver weight, cholesterol levels, and bile flow, with effects having
been observed in mice, rats, and dogs. In male rats, exposure to
d-limonene results in damage to the kidneys and an increased
incidence of renal tumours. As the male rat specific protein
alpha2µ-globulin is considered to play a crucial role in the
development of the neoplastic and non-neoplastic kidney lesions, they
are considered not relevant for human risk assessment.
A dose-related nephropathy was observed in the kidneys of male
rats after oral administration of d-limonene (NTP, 1990). This
lesion, consisting of degeneration of epithelial cells in the
convoluted tubules, granular casts in the outer stripe of the outer
medulla, and epithelial regeneration, is characteristic of hyaline
droplet nephropathy associated with the accumulation of
alpha2µ-globulin in the cytoplasm of tubular cells (Alden et al.,
1984; Halder et al., 1985) in response to a variety of hydrocarbon
compounds (Swenberg et al., 1992). Some compounds fit deeply into a
hydrophobic pocket of alpha2µ-globulin. When hydrogen bonding between
the chemical and protein occurs, the digestibility of alpha2µ-globulin
by proteases is inhibited, leading to accumulation of the male rat
specific protein in lysosomes of the P2 segment of the nephron
(Lehman-McKeeman et al., 1990). Although such chemicals fall into
rather diverse classes, molecular modelling studies have demonstrated
a strong structure-activity relationship with respect to
alpha2µ-globulin binding (Borghoff et al., 1991). The accumulation of
alpha2µ-globulin is cytotoxic, resulting in single-cell necrosis
(Dietrich & Swenberg, 1991). The exfoliated renal epithelium is
restored by compensatory cell proliferation. The increase in cell
proliferation associated with alpha2µ-globulin is reversible. Damage
of this type has not been observed in female rats, male rats that do
not produce alpha2µ-globulin, or other mammals, such as mice,
hamsters, guinea-pigs, dogs, and monkeys (Alden, 1986; Kanerva &
Alden, 1987a; Swenberg et al., 1989; Webb et al., 1989, 1990; NTP,
1990; Ridder et al., 1990; Dietrich & Swenberg, 1991). The processes
leading to nephropathy and the development of renal cancer by such
compounds are among the best understood for non-genotoxic chemicals
and strongly indicate that it is a male rat specific process. Acute
and chronic renal effects induced in male rats by limonene will be
unlikely to occur in any species not producing alpha2µ-globulin or a
very closely related protein in the large quantities typically seen in
the male rat (US EPA, 1991; Swenberg, 1993).
d-Limonene has been studied in a variety of short-term
in vitro tests and has been found to be non-genotoxic. There is no
evidence that limonene has teratogenic or embryotoxic effects in the
absence of maternal toxicity.
11.1.2 Criteria for setting guidance values for limonene
In numerous experimental studies, exposure to limonene has been
shown to affect the liver. Owing to a lack of data on d-limonene
exposure in humans, this organ cannot with certainty be stated as the
critical organ in humans. Based on available data, food is believed
to be the principal source of exposure (96%) to limonene; the
contribution from ambient air is approximately 4%. The dermal uptake
of limonene has not been estimated.
To calculate a tolerable intake for humans, the animal study was
chosen in which effects on the liver were observed at the lowest
exposure level (Webb et al., 1989). In this study, gavage
administration of d-limonene (5 days/week for 13 weeks) to rats
caused increased relative liver weight at 30 and 75 mg/kg body weight
per day. The NOEL for the liver was considered to be 10 mg/kg body
weight per day. Using uncertainty factors of 10 for intraspecies
differences and 10 for interspecies differences, a tolerable intake
for ingestion of d-limonene by humans of 0.1 mg/kg body weight per
day may be calculated from the NOEL. A guidance value for inhalation
exposure to d-limonene was not developed, as inhalation is an
insignificant route of exposure compared with ingestion.
11.1.3 Sample risk characterization
Exposure estimates vary as a function of use patterns, and the
risk characterization presented here is provided only as an example,
primarily for illustrative purposes. In general, d-limonene could
be considered (with the exception of its irritative and sensitizing
properties) to be a chemical with fairly low toxicity. The calculated
tolerable intake of 0.1 mg/kg body weight per day is of a similar
magnitude as the estimated daily US consumption of d-limonene of
0.27 mg/kg body weight per day (Flavor and Extract Manufacturers
Association, 1991).
11.2 Evaluation of environmental effects
Limonene and other terpenes are released in large amounts mainly
to the atmosphere. When released to soil or water, limonene is
expected to evaporate to air to a significant extent, owing to its
high volatility. Thus, the atmosphere is the predominant
environmental sink of limonene, where it is expected to rapidly
undergo gas-phase reactions with photochemically produced hydroxyl
radicals, ozone, and nitrate radicals. The oxidation of terpenes,
such as limonene, contributes to aerosol and photochemical smog
formation. Ozonolysis of limonene may also lead to the formation of
hydrogen peroxide and organic peroxides, which have various toxic
effects on plant cells and may be part of the damage to forests
observed in the last decades (Peters et al., 1994). Emissions of
biogenic hydrocarbons such as limonene and other terpenes to the
atmosphere may either decrease ozone concentrations when nitrogen
oxide concentrations are low or, if emissions take place in polluted
air (i.e. containing high nitrogen oxide levels), lead to an increase
in ozone concentrations.
Terrestrial organisms are most likely to be exposed to limonene
via the air. The few studies on terrestrial species (i.e. insects)
using vapour exposure reveal effects of limonene at parts per million
levels. Measured environmental concentrations are typically around
0.1-2 ppb (0.6-11 µg/m3), indicating a low risk for acute toxic
effects on terrestrial organisms from direct exposure to limonene in
air. At polluted sites, limonene concentrations in soil (up to 920
mg/kg soil) may exceed effect levels of soil-living organisms (e.g.
earthworm, acute LC50 = 6.0 ppm; mg/kg).
In the aquatic environment, limonene exhibits high acute toxicity
to fish and Daphnia. It may also bioaccumulate. The lowest acute
toxicity value identified was 0.4 mg/litre (48-hour EC50 for
Daphnia). Because concentrations of limonene in surface waters of
"polluted" and "unpolluted" areas are at least about 250 and 20 000
times lower than this acute toxicity value, respectively, it is likely
that limonene poses a low risk for acute toxic effects on aquatic
organisms. No studies were identified on chronic effects, and
therefore risks associated with chronic exposures of aquatic organisms
to limonene in "polluted" waters cannot be determined.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The International Agency for Research on Cancer (IARC, 1993) has
classified d-limonene in Group 3 (not classifiable as to its
carcinogenicity to humans) based on a lack of available data on
carcinogenicity to humans and limited evidence for carcinogenicity in
experimental animals.
The 41st meeting of the Joint FAO/WHO Expert Committee on Food
Additives (JECFA, 1993b) withdrew the existing acceptable daily intake
for d-limonene of 0-1.5 mg/kg body weight per day (JECFA, 1993a) and
in its place allocated "not specified." On the basis of the available
data, the total daily intake of the chemical arising from its use at
the levels necessary to achieve the desired effect and from its
acceptable background levels in food did not, in the opinion of the
Committee, represent a health hazard. For that reason, and for the
reasons stated in the individual evaluations, the establishment of an
acceptable daily intake expressed in numerical form was not deemed
necessary.
Information on international hazard classification and labelling
is included in the International Chemical Safety Card reproduced in
this document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventative and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 0918) reproduced in this
document.
13.1 Human health hazards
Limonene is flammable but essentially non-toxic. Repeated or
prolonged contact with the oxidized chemical causes skin
sensitization.
13.2 Advice to physicians
In case of poisoning, the treatment is supportive. Like other
volatile oils, if the patient lives for 48 hours, complete recovery is
likely; laboratory evidence of renal damage may persist for several
months (Dreisbach & Robertson, 1987).
13.3 Storage
Limonene is flammable, with a flash point of 45°C. Keep the
container in a cool, dry, well ventilated area, out of direct
sunlight. Keep the container tightly closed to prevent oxidation of
the chemical.
13.4 Spillage
In the case of a large spill, emergency personnel need to use
non-sparking tools to avoid fire and explosion hazards.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards is
available from the International Register of Potentially Toxic
Chemicals (IRPTC) legal file.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
D-LIMONENE ICSC:0918
D-LIMONENE
(R)-4-Isopropenyl-1-methylcyclohexene
(+)-Limonene
C10H16
Molecular mass: 136.23
CAS # 5989-27-5
RTECS # GW6360000
ICSC # 0918
TYPES OF ACUTE HAZARDS/ PREVENTION FIRST AID/
HAZARD/ SYMPTOMS FIRE FIGHTING
EXPOSURE
FIRE Flammable. No open flames, NO sparks, Powder, AFFF, foam, carbon dioxide.
and NO smoking.
EXPLOSION Above 48°C explosive Above 48°C use a closed In case of fire: keep drums,
vapour/air mixtures may be system, ventilation, and etc., cool by spraying with
formed. explosion-proof electrical water.
equipment.
EXPOSURE STRICT HYGIENE!
* INHALATION Ventilation. Fresh air, rest.
* SKIN Redness. Protective gloves. Protective Remove contaminated clothes.
clothing. Rinse and then wash skin with
water and soap.
* EYES Redness. Safety spectacles. First rinse with plenty of
water for several minutes
(remove contact lenses if
easily possible), then take
to a doctor.
* INGESTION Do not eat, drink, or smoke Rinse mouth.
during work.
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
SPILLAGE DISPOSAL STORAGE PACKAGING & LABELLING
Collect leaking and spilled liquid in Fireproof. Cool. Well closed.
sealable containers as far as
possible. Absorb remaining liquid in
sand or inert absorbent and remove to
safe place.
IMPORTANT DATA PHYSICAL STATE; APPEARANCE: EFFECTS OF SHORT-TERM EXPOSURE:
COLOURLESS LIQUID, WITH CHARACTERISTIC ODOUR. The substance may irritate slightly the eyes
and the skin.
CHEMICAL DANGERS: EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
Reacts violently with a mixture of iodine Repeated or prolonged contact may cause skin
pentafluoride and tetrafluoroethylene, causing sensitization if the substance has been
fire and explosion hazard. oxidized.
OCCUPATIONAL EXPOSURE LIMITS (OELs):
TLV not established.
ROUTES OF EXPOSURE:
The substance can be absorbed into the body by
inhalation of its vapour, through the skin and
by ingestion.
INHALATION RISK:
No indication can be given about the rate in
which a harmful concentration in the air is
reached on evaporation of this substance at
20°C.
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
PHYSICAL Boiling point: 178°C
PROPERTIES Melting -75°C
Relative density (water = 1): 0.84
Solubility in water: none
Vapour pressure, kPa at 14.4°C: 0.4
Relative vapour density (air = 1): 4.7
Flash point: 48°C
Octanol/water partition coefficient
as log Pow: 4.2
ENVIRONMENTAL
DATA
NOTES
ICSC: 0918 1.1 Transport Emergency Card: TEC (R)-75
NFPA Code: H2; F3; R2
REFERENCES
Abraham WR, Hoffmann HMR, Kieslich K, Reng G, Stumpf B (1985)
Microbial transformation of some monoterpenoids and sesquiterpenoids.
Ciba Foundation symposium, III:146-160.
Alden CL (1986) A review of unique male rat hydrocarbon nephropathy.
Toxicologic pathology, 14:109-111.
Alden CL, Kanerva RL, Ridder G, Stone LC (1984) Pathogenesis of the
nephrotoxicity of volatile hydrocarbons in the male rat. In:
Advances in modern environmental toxicology. Vol. VII. Princeton,
NJ, Princeton Scientific Publ., pp. 107-120.
Altshuller AP (1983) Review: Natural volatile organic substances and
their effect on air quality in the United States. Atmospheric
environment, 17(11):2131-2165.
Anderson BE, Zeiger E, Shelby MD, Resnick MA, Gulati DK, Ivett JL,
Loveday KS (1990) Chromosome aberration and sister chromatid exchange
test results with 42 chemicals. Environmental and molecular
mutagenesis, 16:55-137.
Arey J, Atkinson R, Aschmann SM (1990) Product study of the gas-phase
reactions of monoterpenes with the OH radical in the presence of NOx.
Journal of geophysical research, 95(D11):18539-18546.
Arey J, Winer AM, Atkinson R, Aschmann SM, Long WD, Morrison CL,
Olszyk DM (1991) Terpenes emitted from agricultural species found in
California's Central Valley. Journal of geophysical research,
96(D5):9329-9336.
Ariyoshi T, Arakaki M, Ideguchi K, Ishizuka Y, Noda K, Ide H (1975)
Studies on the metabolism of d-limonene (p-mentha-1,8-diene). III.
Effects of d-limonene on the lipids and drug metabolizing enzymes in
rat livers. Xenobiotica, 5:33-38.
Atkinson R (1990) Gas-phase tropospheric chemistry of organic
compounds: a review. Atmospheric environment, 24A(1):1-41.
Atkinson R, Carter WPL (1984) Kinetics and mechanisms of gas-phase
reactions of ozone with organic compounds under atmospheric
conditions. Chemical reviews, 84:437-470.
Atkinson R, Aschmann SM, Winer AM, Pitts JN Jr (1984) Kinetics of the
gas-phase reactions of NO3 radicals with a series of dialkenes,
cycloalkenes, and monoterpenes at 295 ± 1 K. Environmental science
and technology, 18:370-375.
Atkinson R, Hasegawa D, Aschmann SM (1990) Rate constants for the
gas-phase reactions of O3 with a series of monoterpenes and related
compounds at 296 ± 2 K. International journal of chemical kinetics,
22:871-887.
Austin CA, Shepard EA, Pike SF, Rabin BR, Phillips IR (1988) The
effect of terpenoid compounds on cytochrome P-450 levels in rat liver.
Biochemical pharmacology, 37:2223-2229.
Bagley DM, Gardner JR, Holland G, Lewis RW, Regnier J-F, Stringer DA,
Walker AP (1996) Skin irritation: Reference Chemicals Data Bank.
Toxicology in vitro, 10:1-6.
Barber LB, Thurman EM, Schroeder MP (1988) Long-term fate of organic
micropollutants in sewage-contaminated groundwater. Environmental
science and technology, 22:205-211.
Becker KH, Brockmann KJ, Bechara J (1990) Production of hydrogen
peroxide in forest air by reaction of ozone with terpenes. Nature,
346:256-258.
Beerman H, Fondé GH, Callaway JL (1938) Citrus fruit dermatoses.
Archives of dermatology and syphilology (Chicago), 38:225-234.
Bertsch W, Chang RC, Zlatkis A (1974) The determination of organic
volatiles in air pollution studies: characterization of profiles.
Journal of chromatographic science, 12:175-182.
Bertsch W, Anderson E, Holzer G (1975) Trace analysis of organic
volatiles in water by gas chromatography-mass spectrometry with glass
capillary columns. Journal of chromatography, 122:701-718.
Bianchi AP, Varney MS, Phillips J (1991) Analysis of volatile organic
compounds in estuarine sediments using dynamic head space and gas
chromatography-mass spectrometry. Journal of chromatography,
542:413-450.
Birmingham DJ, Campbell PC Jr, Doyle HN, McDonald JM (1951)
Investigation of occupational dermatoses in the citrus fruit canning
industry. Archives of industrial hygiene and occupational medicine,
3:57-63.
Borghoff SJ, Miller AB, Bowen P, Swenberg JA (1991) Characteristics of
chemical binding to alpha2µ-globulin in vitro - evaluating
structure-activity relationships. Toxicology and applied
pharmacology, 107:228-238.
Button DK, Jüttner F (1989) Terpenes in Alaskan waters:
concentrations, sources, and the microbial kinetics used in their
prediction. Marine chemistry, 26:57-66.
Cachao P, Menezes Brandao F, Carmo M, Frazao S, Silva M (1986) Allergy
to oil of turpentine in Portugal. Contact dermatitis, 14:205-208.
Calnan CD (1979) Allergy to dipentene in paint thinner. Contact
dermatitis, 5:123-124.
Carlsson H, Andersson-Sköld Y, Antonsson A-B, Solyom P (1991)
[ Limonene - a solution of the environmental problems.] Swedish
Environmental Research Institute (Report B 1030) (in Swedish, with
English summary).
Chan CC, Vainer L, Martin JW, Williams DT (1990) Determination of
organic contaminants in residential indoor air using an
adsorption-thermal desorption technique. Journal of the Air & Waste
Management Association, 40:62-67.
Chemical Daily (1994) [ 12394 chemical products.] pp. 1214-1215 (in
Japanese).
Chemical Daily (1995) [ 12695 chemical products.] p. 1245 (in
Japanese).
Ciccoioli P, Cecinato A, Brancaleoni E, Frattoni M (1992)
Identification and quantitative evaluation of C4-C14 volatile
organic compounds in some urban, suburban and forest sites in Italy.
Fresenius environmental bulletin, 1:73-78.
Ciccoioli P, Brancaleoni E, Cecinato A, Sparapani R, Frattoni M (1993)
Identification and determination of volatile organic compounds in
forest areas of Northern and Southern Europe and remote sites of the
Himalaya region by high-resolution gas chromatography-mass
spectrometry. Journal of chromatography, 643(1/2):55-69.
Clegg RJ, Middleton B, Duncan Bell G, White DA (1980) Inhibition of
hepatic cholesterol synthesis and S-3-hydroxy-3-methylglutaryl-CoA
reductase by mono and bicyclic monoterpenes administered in vivo.
Biochemical pharmacology, 29:2125-2127.
Clement B, Riba ML, Leduc R, Haziza M, Torres L (1990) Concentration
of monoterpenes in a maple forest in Quebec. Atmospheric
environment, 24A(9):2513-2516.
Connor TH, Theiss JC, Hanna HA, Monteith DK, Matney TS (1985)
Genotoxicity of organic chemicals frequently found in the air of
mobile homes. Toxicology letters, 25:33-40.
Cook SP (1992) Influence of monoterpene vapors on spruce spider mite,
Oligonychus ununguis, adult females. Journal of chemical ecology,
18(9):1497-1504.
Cronin E, ed. (1980) Contact dermatitis. London, Churchill
Livingstone, pp. 532-534.
Crowell PL, Elegbede JA, Elson CE, Lin S, Vedejs E, Cunningham D,
Bailey HH, Gould MN (1992) Human metabolism of orally administered
d-limonene. Proceedings of the American Association for Cancer
Research, 33:524.
De Bortoli M, Knöppel H, Pecchio E, Peil A, Rogora L, Schauenburg H,
Schlitt H, Vissers H (1986) Concentrations of selected organic
pollutants in indoor and outdoor air in Northern Italy.
Environment international, 12:343-350.
Desideri P, Lepri L, Checchini L (1991) Organic compounds in seawater
and pack ice in Terra Nova Bay (Antarctica). Annales de Chimie
(Paris), 81:395-416.
Dhavalikar RS, Bhattacharayya PK (1966) Microbial transformation of
terpenes: Part VIII - Fermentation of limonene by a soil pseudomonad.
Indian journal of biochemistry, 3:144-157.
Dietrich DR, Swenberg JA (1991) The presence of alpha2µ-globulin is
necessary for d-limonene promotion of male rat kidney tumors.
Cancer research, 51:3512-3521.
Dimitriades B (1981) The role of natural organics in photochemical air
pollution - issues and research needs. Journal of the Air Pollution
Association, 31(3):229-235.
Dreisbach RH, Robertson WO (1987) Handbook of poisoning:
prevention, diagnosis & treatment, 12th ed. Norwalk, CT, Appleton &
Lange, 589 pp.
Eitzer BD (1995) Emissions of volatile organic chemicals from
municipal solid waste composting facilities. Environmental science
and technology, 29:896-902.
Evans DL, Miller DM, Jacobsen KL, Bush PB (1987) Modulation of immune
responses in mice by d-limonene. Journal of toxicology and
environmental health, 20:51-66.
Fahrig R (1984) Genetic mode of action of cocarcinogens and tumor
promoters in yeast and mice. Molecular & general genetics, 194:7-14.
Falk A, Gullstrand E, Löf A, Wigaeus Hjelm E (1990) Liquid/air
partition coefficients of four terpenes. British journal of
industrial medicine, 47:62-64.
Falk A, Fischer T, Hagberg M (1991) Purpuric rash caused by dermal
exposure to d-limonene. Contact dermatitis, 25:198-199.
Falk Filipsson A, Löf A, Hagberg M, Wigaeus Hjelm E, Wang Z (1993)
d-Limonene exposure to humans by inhalation: Uptake, distribution,
elimination, and effects on the pulmonary function. Journal of
toxicology and environmental health, 38:77-88.
Fehsenfeld F, Calvert J, Fall R, Goldan J, Guenther AB, Hewitt CN,
Lamb B, Liu S, Trainer M, Westberg H, Zimmerman P (1992) Emissions of
volatile organic compounds from vegetation and implications for
atmospheric chemistry. Global geochemical cycles, 6(4):389-430.
Fellin P, Otson R (1993) Seasonal trends of volatile organic compounds
(VOCs) in Canadian homes. In: Saarela K, Kalliokoski P, Seppänen O,
eds. Indoor air '93. Vol. 2. Chemicals in indoor air, material
emissions. Proceedings of the 6th International Conference on Indoor
Air Quality, held in Helsinki, 4-8 July 1993, pp. 117-122.
Fielding M, Gibson TM, James HA, McLoughlin K, Steel CP (1981)
Organic micropollutants in drinking water. Medmenham, Water Research
Centre, Environmental Protection (Technical Report TR 159).
Fischer A, ed. (1986) Contact dermatitis, 3rd ed. London, Churchill
Livingstone, pp. 421-422, 582-583.
Fjelstad PE, Wolbaek T (1992) A national exposure database. In: Brown
RH, Curtis M, Saunders KJ, Vandendriessche S, eds. Clean air at
work, New trends in assessment and measurement for the 1990s.
Cambridge, Royal Society of Chemistry, pp. 303-310.
Flavor and Extract Manufacturers Association (1991) d-Limonene
monograph. Washington, DC, pp. 1-4.
Florida Chemical Co. (1991) Marketing data sheet: d-Limonene. Lake
Alfred, FL.
Florin I, Rutberg L, Curvall M, Enzell CR (1980) Screening tobacco
smoke constituents for mutagenicity using the Ames test.
Toxicology, 18:219-232.
Gäb S, Hellpointner E, Turner WV, Korte F (1985) Hydroxymethyl
hydroperoxide and bis(hydroxymethyl) peroxide from gas-phase
ozonolysis of naturally occurring alkenes. Nature, 316:535-536.
Gomez-Belinchon JI, Grimalt JO, Albaigès J (1991) Volatile organic
compounds in two polluted rivers in Barcelona (Catalonia, Spain).
Water research, 25(5):577-589.
Grief N (1967) Cutaneous safety of fragrance material as measured by
the Maximization Test. American perfumer and cosmetics, 82:54-57.
Grosjean D, Williams EL, Seinfeld JH (1992) Atmospheric oxidation of
selected terpenes and related carbonyls: gas-phase carbonyl products.
Environmental science and technology, 26(8):1526-1533.
Grosjean D, Williams EL, Grosjean E, Andino JM, Seinfeld JH (1993)
Atmospheric oxidation of biogenic hydrocarbons: reaction of ozone with
beta-pinene, d-limone and trans-caryophyllene. Environmental
science and technology, 27(13):2754-2758.
Guin J, Meyer B, Drake R, Haffley P (1984) The effect of quenching
agents on contact urticaria caused by cinnamic aldehyde. Journal
of the American Academy of Dermatology, 1:45-51.
Haag KH (1986) Effects of herbicide application on mortality and
dispersive behaviour of the water hyacinth weevils, Neochetina
eichhorniae and Neochetina bruchi (Coleoptera: Curculionidae).
Environmental entomology, 15:1192-1198.
Hakola H, Arey J, Aschmann SM, Atkinson R (1994) Product formation
from the gas-phase reactions of OH radicals and O3 with a series of
monoterpenes. Journal of atmospheric chemistry, 18:75-102.
Halder CA, Holdsworth CE, Cockrell BY, Piccirillo VJ (1985)
Hydrocarbon nephropathy in male rats: Identification of the
nephrotoxic components of unleaded gasoline. Toxicology and
industrial health, 1:67-87.
Harwood SH, Moldenke AF, Berry RE (1990) Toxicity of peppermint
monoterpenes to the variegated cutworm (Lepidoptera: Noctuidae).
Journal of economic entomology, 83(5):1761-1767.
Haworth S, Lawlor T, Mortelmans K, Speck W, Zeiger E (1983)
Salmonella mutagenicity test results for 250 chemicals.
Environmental mutagenesis, 1:3-142.
Helmig D, Arey J (1992) Organic chemicals in the air at Whitaker's
Forest/Sierra Nevada Mountains, California. Science of the total
environment, 112:233-250.
Helmig D, Müller J, Klein W (1989) Volatile organic substances in a
forest atmosphere. Chemosphere, 19(8/9):1399-1412.
Hiatt MH (1983) Determination of volatile organic compounds in fish
samples by vacuum distillation and fused silica capillary gas
chromatography/mass spectrometry. Analytical chemistry, 55:506-516.
Hink WF, Fee BJ (1986) Toxicity of d-limonene, the major component
of citrus peel oil, to all life stages of the cat flea
Ctenocephalides felis (Siphonaptera: Pulicidae). Journal of
medical entomology, 23(4):400-404.
Hutte RS, Williams EJ, Staehelin J, Hawthorne SB, Barkley RM, Sievers
RE (1984) Chromatographic analysis of organic compounds in the
atmosphere. Journal of chromatography, 302:173-179.
IARC (1993) Some naturally occurring substances: food items and
constituents, heterocyclic aromatic amines and mycotoxins. Lyon,
International Agency for Research on Cancer, pp. 135-162 (IARC
Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol.
56).
Igimi H, Nishimura M, Kodama R, Ide H (1974) Studies on the metabolism
of d-limonene (p-mentha-1,8-diene). I. The absorption,
distribution and excretion of d-limonene in rats. Xenobiotica,
4:77-84.
Igimi H, Hisatsugu T, Nishimura M (1976) The use of d-limonene
preparation as a dissolving agent of gallstones. Digestive diseases
and science, 21:926-939.
Igimi H, Tamura R, Toraishi K, Yamamoto F, Kataoka A, Ikejiri Y,
Hisatsugu T, Shimura H (1991) Medical dissolution of gallstones.
Clinical experience of d-limonene as a simple, safe and effective
solvent. Digestive diseases and science, 36:200-208.
Ioffe BV, Isidorov VA, Zenkevich IG (1977) Gas chromatographic-mass
spectrometric determination of volatile organic compounds in an urban
atmosphere. Journal of chromatography, 142:787-795.
Ioffe BV, Isidorov VA, Zenkevich IG (1979) Certain regularities in the
composition of volatile organic pollutants in the urban atmosphere.
Environmental science and technology, 13(7):864-868.
IPCS (1993) International Chemical Safety Card - d-Limonene. Geneva,
World Health Organization, International Programme on Chemical Safety
(No. 0918).
IPCS (1994) Assessing health risks of chemicals: Derivation of
guidance values for health-based exposure limits. Geneva, World
Health Organization, International Programme on Chemical Safety
(Environmental Health Criteria 170).
Janson R, Kristensson J (1991) Sampling and analysis of atmospheric
monoterpenes. Stockholm, Stockholm University (Report CM-79).
JECFA (1993a) Limonene. In: Toxicological evaluation of certain
food additives and naturally occurring toxicants. Geneva, World
Health Organization, Joint FAO/WHO Expert Committee on Food Additives,
pp. 35-79 (WHO Food Additives Series 30).
JECFA (1993b) Limonene. In: Toxicological evaluation of certain
food additives and contaminants. Annex 4. Geneva, World Health
Organization, Joint FAO/WHO Expert Committee on Food Additives, pp.
299-301 (WHO Food Additives Series 32).
Josefsson C (1993) Limonene. In: Health effects of selected
chemicals. Vol. 2. Copenhagen, Nordic Council of Ministers, pp.
105-135 (Nord 29).
Jüttner F (1986) Analysis of organic compounds (VOC) in the forest air
of the southern Black Forest. Chemosphere, 15(8):985-992.
Jüttner F (1988) Quantitative analysis of monoterpenes and volatile
organic pollution products (VOC) in forest air of the southern Black
Forest. Chemosphere, 17(2):309-317.
Kanerva RL, Alden CL (1987a) Review of kidney sections from a
subchronic d-limonene oral dosing study conducted by the National
Cancer Institute. Food and chemical toxicology, 25:355-358.
Kanerva RL, Ridder GM, Lefever FR, Alden CL (1987b) Comparison of
short-term renal effects due to oral administration of decalin or
d-limonene in young adult male Fischer-344 rats. Food and
chemical toxicology, 25:345-353.
Karlberg A-T, Lindell B (1993) Limonene. In: Beije B, Lundberg P, eds.
Criteria documents from the Nordic Expert Group 1993. Solna,
National Institute of Occupational Health, Nordic Council of
Ministers, pp. 207-247 (Arbete och Hälsa 35).
Karlberg A-T, Boman A, Melin B (1991) Animal experiments on the
allergenicity of d-limonene - the citrus solvent. Annals of
occupational hygiene, 35:419-426.
Karlberg A-T, Magnusson K, Nilsson U (1992) Air oxidation of
d-limonene (the citrus solvent) creates potent allergens.
Contact dermatitis, 26:332-340.
Karlberg A-T, Shao LP, Nilsson U, Gäfvert E, Nilsson JLG (1994)
Hydroperoxides in oxidized d-limonene identified as potent contact
allergens. Archives for dermatological research, 286:97-103.
Karr LL, Coats JR (1988) Insecticidal properties of d-limonene.
Journal of pesticide science, 13:287-290.
Karr LL, Coats JR (1992) Effect of four monoterpenoids on growth and
reproduction of the German cockroach (Blattodea: Blattellidae).
Journal of economic entomology, 85(2):425-429.
Karr LL, Drewes CD, Coats JR (1990) Toxic effects of d-limonene in
the earthworm Eisenia foetida (Savigny). Pesticide biochemistry
and physiology, 36:175-186.
Keith LH, Garrison AW, Allen FR, Carter MH, Floyd TL, Pope JD,
Thruston AD Jr (1976) Identification of organic compounds in drinking
water from thirteen U.S. cities. In: Keith LH, ed. Identification
and analysis of organic pollutants in water. Ann Arbor, MI, Ann
Arbor Science Publ., pp. 329-373.
Klecak G, Geleick H, Noda K, Ide H (1977) Screening of fragrance
materials for allergenicity in the guinea pig. I. Comparison of four
testing methods. Journal of the Society of Cosmetic Chemists,
28:53-54.
Klöpffer W, Haag F, Kohl E-G, Frank R (1988) Testing of the abiotic
degradation of chemicals in the atmosphere: The smog chamber approach.
Ecotoxicology and environmental safety, 15:298-319.
Kodama R, Yano T, Furukawa K, Noda K, Ide H (1976) Studies on the
metabolism of d-limonene (p-mentha-1,8-diene). IV. Isolation and
characterization of new metabolites and species differences in
metabolism. Xenobiotica, 6:377-389.
Kodama R, Okubo A, Araki E, Noda K, Ide H, Ikeda T (1977a) Studies on
d-limonene as a gallstone solubilizer (VII). Effects on development
of mouse fetuses and offspring. (Japan) Oyo Yakuri, 13:863-873.
Kodama R, Okubo A, Sato K, Araki E, Noda K, Ide H, Ikeda T (1977b)
Studies on d-limonene as a gallstone solubilizer (IX). Effects on
development of rabbit fetuses and offspring. (Japan) Oyo Yakuri,
13:885-898.
Koe LCC, Ng WJ (1987) Identification of odorous gases originating from
refuse waste. Water, air, and soil pollution, 33:199-204.
Lacy MJ, Kent CR, Voss EW (1987) d-Limonene: An effective
vasodilator for use in collecting rabbit blood. Laboratory animal
science, 37(4):485-487.
Lamb B, Guenther A, Gay D, Westberg H (1987) A national inventory of
biogenic hydrocarbon emissions. Atmospheric environment,
21(8):1695-1705.
Larson RA, Marley KA (1988) Sunlight photochlorination and dark
chlorination of monoterpenes. Science of the total environment,
77:245-252.
Lehman-McKeeman LD, Caudill D (1992) Biochemical basis for mouse
resistance to hyaline droplet nephropathy: lack of relevance of the
alpha2µ-globulin protein superfamily in this male rat specific
syndrome. Toxicology and applied pharmacology, 112:214-221.
Lehman-McKeeman LD, Rodriguez PA, Takigiku R, Caudill D, Fey ML (1989)
d-Limonene induced male rat specific nephrotoxicity: Evaluation of
the association between d-limonene and alpha2µ-globulin.
Toxicology and applied pharmacology, 99:250-259.
Lehman-McKeeman LD, Rodriguez MI, Caudill D (1990) Lysosomal
degradation of alpha2µ-globulin and alpha2µ-globulin-xenobiotic
conjugates. Toxicology and applied pharmacology, 103:539-548.
Maisey J, Miller K (1986) Assessment of the ability of mice fed on
vitamin A diet to respond to a variety of potential contact
sensitizers. Contact dermatitis, 15:17-23.
Maltzman TH (1991) Anticarcinogenic mechanism of action of dietary
limonene during the initiation stage of DMBA-induced mammary
carcinogenesis. Dissertation abstracts international, 51:4283B.
Maltzman TH, Cristou M, Gould MN, Jefcoate CR (1991) Effects on
monoterpenoids on in vivo DMBA-DNA adduct formation and on phase I
hepatic metabolizing enzymes. Carcinogenesis, 12:2081-2087.
Massaldi HA, King CJ (1973) Simple technique to determine solubilities
of sparingly soluble organics: solubility and activity coefficients of
d-limonene, n-butylbenzene, and n-hexyl acetate in water and
sucrose solutions. Journal of chemical and engineering data,
18(4):393-397.
McCreary JJ, Jackson JG, Zoltec J (1983) Toxic chemicals in an
abandoned phenolic waste site. Chemosphere, 12 (11/12):1619-1632.
MITI (1992) Biodegradation and bioaccumulation data of existing
chemicals based on the CSCL Japan compiled under the supervision
of Chemical Products Safety Division, Basic Industries Bureau,
Ministry of International Trade and Industry, Japan. Ministry of
International Trade and Industry, Chemicals Inspection & Testing
Institute (ISBN 4-89074-101-1).
Mohsen ZH, Al-Chalabi BM, Kassir JT (1989). Factors influencing the
larvicidal activity of limonene against Culex quinquefasciatus Say
(Dipt., Culicidae). Journal of applied entomology, 108:107-110.
Montgomery D, Kalman DA (1989) Indoor/outdoor air quality: Reference
pollutant concentrations in complaint-free residences. Applied
industrial hygiene, 4(1):17-20.
National Board of Occupational Safety and Health (1993)
Occupational exposure limit value. Solna (Ordinance AFS, Vol. 3).
Nilsson U, Bergh M, Shao LP, Karlberg A-T (1996) Analysis of contact
allergenic compounds in oxidized d-limonene. Chromatographia,
42(3/4):199-205.
Nolting F, Zetzsch C (1988) Das ozon-bildungspotential des biogenen
alfa-pinens in vergleich mit anthropogenen kohlenwasserstoffen. In:
GSF-Bericht 17/88, International Symposium "Verteilung Wirkung
Photooxidantien Alpenraum." Neuherberg, Forschungszentrum für Umwelt
und Gesundheit, pp. 623-628.
NTP (1990) Toxicology and carcinogenesis studies of d-limonene in
F344/N rats and B6C3F1 mice. Springfield, VA, US Department of
Health and Human Services, National Institutes of Health, National
Toxicology Program (NTP Technical Report No. 347).
OECD (1981) OECD guidelines for testing of chemicals. Paris,
Organisation for Economic Co-operation and Development.
OECD (1993) Guidelines for testing of chemicals. Guideline 404,
Acute dermal irritation/corrosion. Adopted 1981, upgraded 1992.
Paris, Organisation for Economic Co-operation and Development.
Okabe H, Obata Y, Takayama K, Nagai T (1990) Percutaneous absorption
enhancing effect and skin irritation of monocyclic monoterpenes.
Drug design and delivery, 6:229-238.
Opdyke DLJ (1978) Monographs on fragrance raw materials. Food and
cosmetics toxicology, Special issue IV, 16 (Suppl.):809.
Page BD, Conacher HBS, Salminen J (1993) Survey of bottled drinking
water sold in Canada. Part 2. Selected volatile organic compounds.
Journal of the Association of Official Analytical Chemists
International, 76(1):26-31.
Passino DRM, Smith SB (1987) Acute bioassays and hazard evaluation of
representative contaminants detected in Great Lakes fish.
Environmental toxicology and chemistry, 6:901-907.
Paxéus N, Robinson P, Balmér P (1992) Study of organic pollutants in
municipal wastewater in Göteborg, Sweden. Water science and
technology, 25(11):249-256.
Pellizzari ED, Hartwell TD, Harris BSH III, Wadell RD, Whitaker DA,
Erickson MD (1982) Purgeable organic compounds in mother's milk.
Bulletin of environmental contamination and toxicology, 28:322-328.
Peters RJB, Duivenbode JAD, Duyzer JH, Verhagen HLM (1994) The
determination of terpenes in forest air. Atmospheric environment,
28(15):2413-2419.
Petersson G (1988) High ambient concentrations of monoterpenes in a
Scandinavian pine forest. Atmospheric environment, 22(11):2617-2619.
Pienta RJ (1980) Evaluation and relevance of the Syrian hamster embryo
cell system. Applied methods in oncology, 3:149-169.
Ridder GM, von Bargen EC, Alden CL, Parker RD (1990) Increased hyaline
droplet formation in male rats exposed to decalin is dependent on the
presence of alpha-2µ-globulin. Fundamental and applied toxicology,
15:732-743.
Roberts JM, Hahn CJ, Fehsenfeld FC, Warnock JM, Albritton DL, Sievers
RE (1985) Monoterpene hydrocarbons in the nighttime troposphere.
Environmental science and technology, 19(4):364-369.
Roth L (1990) Wassergefährende Stoffe (WGK). Landsberg, Ecomed
Verlag mbh.
Rycroft RJG (1980) Allergic contact dermatitis from dipentene in
honing oil. Contact dermatitis, 6:325-329.
Sauer TC Jr (1981) Volatile organic compounds in open ocean and
coastal surface waters. Organic geochemistry, 3:91-101.
Schenk J, Däbränte Z, Koch H, Stolte M (1980) Untersuchungen zur
Geweberträglichkeit von D-Limonen als einem Läsungsmittel
cholesterinreicher Gallensteine. Zeitschrift für Gastroenterologie,
18:389-394.
Schwartz L (1938) Cutaneous hazards in the citrus fruit industry.
Archives of dermatology, 37:631-649.
Schwartz SM, Boethling RS, Leighton T (1990) Fate of the terpenes
anethole and dipentene in a bench-scale activated sludge system.
Chemosphere, 21(10/11):1153-1160.
Searle E (1989) Determination of airborne limonene vapour by charcoal
tube sampling and gas-liquid chromatographic analysis. Analyst,
114:113-114.
Sekiya T, Yabuzaki T, Kitano M, Ogawa T (1988) Photochemical aerosol
formation from organic gases. Colloid and polymer science,
266:1037-1041.
Shaw RW, Crittenden AL, Stevens RK, Crohn DR, Titov VS (1983) Ambient
concentrations of hydrocarbons from conifers in atmospheric gases and
aerosol particles measured in Soviet Georgia. Environmental science
and technology, 17:389-395.
Shulka OP, Bhattacharyya PK (1968) Microbial transformation of
terpenes: Part XI - Pathways of degradation of alpha & beta pinenes in
a soil pseudomonad (PL-strain). Indian journal of biochemistry,
5(3): 92-101.
Sierra-Alvarez R, Kato M, Lettinga G (1990) The anaerobic
biodegradability of paper mill wastewater constituents.
Environmental technology, 11:891-898.
Smith OW, Wade AP, Dean FM (1969) Uroterpenol, a pettenkofer chromogen
of dietary origin and a common constituent of human urine. Journal
of endocrinology, 45:17-28.
Steele VE, Kelloff GJ, Wilkinson BP, Arnold JT (1990) Inhibition of
transformation in cultured rat tracheal epithelial cells by potential
chemopreventive agents. Cancer research, 50:2068-2074.
Strömvall AM (1992) Terpenes emitted to air from forestry and the
forest industry. Göteborg, Chalmers University of Technology.
Swenberg JA (1993) alpha2µ-globulin nephropathy: Review of the
cellular and molecular mechanisms involved and their implications for
human risk assessment. Environmental health perspectives, 101
(Suppl. 6):39-44.
Swenberg JA, Short B, Borghoff S, Strasser J, Charbonneau M (1989) The
comparative pathobiology of alpha2µ-globulin nephropathy.
Toxicology and applied pharmacology, 97:35-46.
Swenberg JA, Dietrich DR, McClain RM, Cohen SM (1992) Species-specific
mechanisms of carcinogenesis. In: Vaino H, Magee PN, McGregor DB,
McMichael AJ, eds. Mechanisms of carcinogenesis in risk
identification. Lyon, International Agency for Research on Cancer,
pp. 477-500 (IARC Monograph No. 116).
Tsuji M, Fujisaki Y, Yamachika K, Nakagami K, Fujisaka F, Mito M, Aoki
T, Kinoshita S, Okubo A, Watanabe I (1974) Studies on d-limonene, as
gallstone solubilizer (I): General pharmacological studies. (Japan)
Oyo Yakuri, 8:1439-1459.
Tsuji M, Fujisaki Y, Arikawa Y, Masuda S, Tanaka T, Sato K, Noda K,
Ide H, Kikuchi M (1975a) Studies on d-limonene, as gallstone
solubilizer (IV): Chronic toxicity in dogs. (Japan) Oyo Yakuri,
9:775-808.
Tsuji M, Fujisaki Y, Okubo A, Arikawa Y, Noda K, Ide H, Ikeda T
(1975b) Studies on d-limonene, as gallstone solubilizer (V): Effects
on development of rat fetuses and offspring. (Japan) Oyo Yakuri,
10:179-186.
US EPA (1990a) Terpene hazard assessment. US Environmental
Protection Agency, Office of Toxic Substances.
US EPA (1990b) Toxicity of eight terpenes to fathead minnows
(Pimephales promelas), daphnids (Daphnia magna), and algae
(Selenastrum capricornutum). AScI Corporation and US Environmental
Protection Agency, Environmental Research Laboratory-Duluth.
US EPA (1991) Alpha-2µ-globulin: Association with chemically
induced renal toxicity and neoplasia in the male rat. Washington,
DC, US Environmental Protection Agency, Risk Assessment Forum
(EPA-625-3-91-019F).
US EPA (1994) Reregistration Eligibility Decision (RED) Limonene. US
Environmental Protection Agency, Prevention, Pesticides and Toxic
Substances (Report No. EPA-738-R-94-034).
Venkataramani ES, Ahlert RC (1984) Rapid aerobic biostabilization of
high-strength industrial landfill leachate. Journal of the Water
Pollution Control Federation, 56(11):1178-1184.
von Schäfer R, Schäfer W (1982) Die perkutane Resorption verschiedener
Terpene-Menthol, Campher, Limonen, Isobornylacetat, alpha-pinen-aus
badezusätzen. Drug research, 32:56-58.
Waggott A (1981) Trace organic substances in the river Lee. In: Cooper
WJ, ed. Chemistry in water reuse. Ann Arbor, MI, Ann Arbor Science
Publ., pp. 55-99.
Wallace L, Nelson W, Zeigenfus R, Pellizzari E, Michael L, Whitmore R,
Zelon H, Hartwell T, Perritt R, Westerdahl D (1991) The Los Angeles
Team study: Personal exposures, indoor-outdoor air concentrations, and
breath concentrations of 25 volatile compounds. Journal of exposure
analysis and environmental epidemiology, 2:157-192.
Watabe T, Hiratsuka A, Osawa N, Isobe M (1981) A comparative study on
the metabolism of d-limonene and 4-vinylcyclohex-1-ene by hepatic
microsomes. Xenobiotica, 11:333-344.
Webb DR, Ridder GM, Alden CL (1989) Acute and subchronic
nephrotoxicity of d-limonene in Fischer 344 rats. Food and
chemical toxicology, 27:639-649.
Webb DR, Kanerva LL, Hysell DK, Alden CL, Lehman-McKeeman LD (1990)
Assessment of the subchronic oral toxicity of d-limonene in dogs.
Food and chemical toxicology, 28:669-675.
Wilson D, Hrutfiord B (1975) The fate of turpentine in aerated
lagoons. Pulp and paper magazine of Canada, 76(6):91-93.
Wilt FM, Miller GC, Everett RL (1988) Monoterpene concentrations in
litter and soil of single-leaf pinyon woodlands of the Western Great
Basin. The Great Basin naturalist, 48(2):228-231.
Winer AM, Lloyd AC, Darnall KR, Pitts JN Jr (1976) Relative rate
constants for the reaction of the hydroxyl radical with selected
ketones, chlorethenes, and monoterpene hydrocarbons. Journal of
physical chemistry, 80(14):1635-1639.
Winer AM, Atkinson R, Pitts JN Jr (1984) Gaseous nitrate radical:
Possible nighttime atmospheric sink for biogenic organic compounds.
Science, 224:156-159.
York M, Basketter Cuthbert JA, Neilson L (1995) Skin irritation in man
for hazard assessment - evaluation of four patch systems. Human and
experimental toxicology, 14:729-734.
Young PJ, Parker A (1983) The identification and possible
environmental impact of trace gases and vapours in landfill gas.
Waste management research, 1:231-226.
Young PJ, Parker A (1984) Vapors, odors, and toxic gases from
landfills. In: Jackson LP, Rohlik AR, Conway RA, eds. Hazardous
and industrial waste management and testing: Third symposium.
Philadelphia, PA, American Society for Testing and Materials, pp.
24-41 (ASTM Special Technical Publication 851).
Zürcher F, Giger W (1976) Flüchtige organiche spurenkomponenten in der
Glatt. Vom Wasser, 47:37-55 (in German with an English summary).
APPENDIX 1 - SOURCE DOCUMENT
Karlberg A-T, Lindell B (1993) Limonene. In: Beije B, Lundberg P, eds.
Criteria documents from the Nordic Expert Group 1993. Solna,
National Institute of Occupational Health, Nordic Council of
Ministers, pp. 207-246 (Arbete och Hälsa 35).
Copies of the Arbete och Hälsa document on limonene (ISSN:
0346-7821; ISBN: 91-7045-240-7), prepared by the Nordic Expert Group,
may be obtained from:
National Institute for Working Life
Publications Department
S-171 84 Solna
Sweden
In the peer review procedure of documents prepared in the series
Criteria documents from the Nordic Expert Group (focused on human
health effects only), one member of the Nordic Expert Group serves as
primary reviewer for the first draft. A second draft is forwarded to
all members of the Nordic Expert Group, who in turn consult
appropriate specialists to review the document. The specialists are
chosen either because they have an extended knowledge of the substance
itself or because they are specialists in the critical effect area of
the substance evaluated. The second review is performed by a review
board, including the Nordic Expert Group participants with the ad
hoc specialists. After revision, the document is checked again by
the members of the Nordic Expert Group and the ad hoc experts for
further comments. The review board meeting is repeated if necessary.
APPENDIX 2 - CICAD PEER REVIEW
The draft CICAD on limonene was sent for review to institutions
and organizations identified by IPCS after contact with IPCS national
Contact Points and Participating Institutions, as well as to
identified experts. Comments were received from:
Department of Health, London, United Kingdom
Department of Public Health, Albert Szent-Gyorgyi University
Medical School, Szeged, Hungary
Direccion General de Salud Ambiental, Subsecretario de Regulacion
y Fomento, Sanitario, San Luis Potosi, Mexico
Environmental Health Directorate, Health Canada, Ottawa, Canada
International Agency for Research on Cancer, Lyon, France
Ministry of Health, National Centre of Hygiene, Medical Ecology
and Nutrition, Sofia, Bulgaria
Ministry of Health and Welfare, International Affairs Division,
Government of Japan, Tokyo, Japan
National Institute for Working Life, Solna, Sweden
National Institute of Public Health, Oslo, Norway
United States Department of Health and Human Services (National
Institute of Environmental Health Sciences)
United States Environmental Protection Agency (Office of
Pollution Prevention and Toxics; Office of Drinking Water)
APPENDIX 3 - CICAD FINAL REVIEW BOARD
Brussels, Belgium, 18-20 November 1996
Members
Dr A. Aitio, Institute of Occupational Health, Helsinki, Finland
Dr K. Bentley, Director, Environment Policy Section, Commonwealth
Department of Human Services and Health, Canberra, Australia
Mr R. Cary, Toxicology and Existing Substances Regulation Unit, Health
and Safety Executive, Merseyside, United Kingdom
Dr J. de Fouw, National Institute of Public Health and Environmental
Protection, Bilthoven, The Netherlands
Dr C. DeRosa, Director, Division of Toxicology, Agency for Toxic
Substances and Disease Registry, Atlanta, GA, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr W. Farland, Director, National Center for Environmental Assessment,
Office of Research and Development, US Environmental Protection
Agency, Washington, DC, USA (Chairperson)
Dr T.I. Fortoul, Depto. Biologia Celular y Tisular, National
University of Mexico and Environmental Health Directorate of the
Health Ministry, Mexico D.F., Mexico
Dr H. Gibb, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany
Mr J.R. Hickman, Environmental Health Directorate, Health Canada,
Ottawa, Ontario, Canada
Dr T. Lakhanisky, Head, Division of Toxicology, Institute of Hygiene
and Epidemiology, Brussels, Belgium (Vice-Chairperson)
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Sciences, Hanover,
Germany
Ms E. Meek, Head, Priority Substances Section, Environmental Health
Directorate, Health Canada, Ottawa, Ontario, Canada
Dr K. Paksy, National Institute of Occupational Health, Budapest,
Hungary
Mr D. Renshaw, Department of Health, London, United Kingdom
Dr J. Sekizawa, Division of Chemo-Bio Informatics, National Institute
of Hygienic Sciences, Tokyo, Japan
Dr H. Sterzl-Eckert, GSF-Forschungszentrum für Umwelt und Gesundheit
GmbH, Institut für Toxikologie, Oberschleissheim, Germany
Professor S. Tarkowski, Department of Environmental Health Hazards,
The Nofer Institute of Occupational Medicine, Lodz, Poland
Dr M. Wallen, National Chemicals Inspectorate (KEMI), Solna, Sweden
Observers
Professor F.M.C. Carpanini,1 Director, Centre for Ecotoxicology and
Toxicology of Chemicals (ECETOC), Brussels, Belgium
Mr R. Haigh,1 Head of Unit, Health and Safety Directorate, European
Commission, Luxembourg
Mr B.U. Hildebrandt, Federal Ministry for the Environment, Nature
Conservation and Nuclear Safety, Bonn, Germany
Mr P. Hurst,1 Chemical and Consumer Policy Officer, Conservation
Policy Division, World Wide Fund for Nature, Gland, Switzerland
Dr A. Lombard (Representative of CEFIC), ELF-ATOCHEM, Paris, France
Dr P. McCutcheon,1 Environment, Consumer Protection and Nuclear
Safety, European Commission, Brussels, Belgium
Dr R. Montaigne, Counsellor, Technical Affairs Department, European
Chemical Industry Council (CEFIC), Brussels, Belgium
Dr M. Pemberton, ICI Acrylics, Lancashire, United Kingdom
Dr A. Smith, Organisation for Economic Co-operation and Development,
Environment Division, Paris, France
1 Invited but unable to attend.
Secretariat
Dr M. Baril, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Dr L. Harrison, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr M. Mercier, Director, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr P. Toft, Associate Director, International Programme on Chemical
Safety, World Health Organization, Geneva, Switzerland
RÉSUMÉ D'ORIENTATION
Les informations contenues dans le présent CICAD (document
international succinct sur l'évaluation des risques chimiques) sur le
limonène (d-limonène, l-limonène et d/l limonène) proviennent
principalement d'une étude effectuée en 1993 pour le Nordic Expert
Group (Karlberg & Lindell, 1993). D'autres données pour l'évaluation
du limonène ont été rassemblées à partir d'une deuxième étude menée
sous les auspices du Conseil des Ministres des Pays nordiques
(Josefsson, 1993), d'un rapport préliminaire (qui n'a pas fait l'objet
d'une évaluation par les pairs) sur la présence et les effets du
limonène dans l'environnement (US EPA, 1994), et de recherches
effectuées dans les bases de données pertinentes pour les années
1993-1995. Une dernière recherche bibliographique portant sur les
années 1996-1997 n'a pas fourni d'éléments de nature à modifier les
conclusions formulées dans le CICAD. L'appendice 1 donne des
informations sur la nature et la disponibilité des sources
documentaires. Des informations concernant l'examen par les pairs du
présent CICAD figurent à l'appendice 2. La publication de ce CICAD a
été approuvée à une réunion du Comité d'Evaluation finale qui s'est
tenue à Bruxelles (Belgique) du 18 au 20 novembre 1996. La liste des
participants à cette réunion figure à l'appendice 3. La fiche
internationale de sécurité chimique concernant le d-limonène (ICSC
0918), établie par le Programme international sur la sécurité chimique
(IPCS, 1993), est également reproduite dans le présent document.
L'accent a été mis sur le d-limonène en raison de l'abondance des
données disponibles sur cet isomère.
Le limonène se trouve à l'état naturel dans certains arbres et
arbustes. Le limonène et d'autres monoterpènes sont libérés en
grandes quantités, principalement dans l'atmosphère, à partir de
sources biologiques naturelles et de sources artificielles. Le
limonène est utilisé comme solvant dans le dégraissage des métaux
avant peinture, comme agent nettoyant dans les industries de
l'électronique et de l'imprimerie et comme solvant pour peintures. Il
est également employé comme aromatisant dans les aliments, les
produits d'entretien ménagers et les parfums.
Le limonène est un irritant de la peau, tant pour l'homme que
pour les animaux de laboratoire. Chez le lapin, le d-limonène est
un irritant oculaire. Des études effectuées sur des cobayes ont
montré que le d-limonène oxydé par l'air, mais non le d-limonène
lui-même, induisait des allergies de contact. Étant donné que le
d-limonène et le l-limonène sont des énantiomères, il pourrait en
être de même pour le l-limonène et le dipentène (mélange des deux).
Le caractère allergène du limonène pourrait donc dépendre dans une
large mesure des manipulations qu'il a subies, de sa pureté et de
l'adjonction éventuelle d'antioxygènes.
L'organe critique chez l'animal (sauf chez le rat mâle), après
administration par voie orale ou intrapéritonéale, est le foie. On
n'a pas trouvé d'études au cours desquelles des animaux auraient été
exposés au limonène par inhalation. L'exposition au limonène a des
effets sur la quantité et l'activité de diverses enzymes hépatiques,
ainsi que sur le poids du foie, la cholestérolémie et la sécrétion
biliaire. Ces effets ont été observés chez la souris, le rat et le
chien. Les données disponibles sont insuffisantes pour déterminer
quel est l'organe critique chez l'homme.
Chez le rat mâle, l'exposition au d-limonène provoque des
lésions et des tumeurs rénales. Il est admis qu'une protéine
spécifique du rat mâle, l'alpha2µ-globuline, joue un rôle crucial dans
le développement des lésions rénales néoplasiques ou non néoplasiques
chez cet animal, de sorte que leur étude n'est pas jugée pertinente
pour l'évaluation du risque chez l'homme. Le d-limonène a été
soumis à une série d'épreuves in vitro à court terme dans lesquelles
il s'est révélé non génotoxique. Il n'y a pas de preuve que le
limonène exerce des effets tératogènes ou embryotoxiques en l'absence
de toxicité maternelle. De façon générale, on peut admettre que le
d-limonène est un composé de toxicité relativement faible (si l'on
excepte ses propriétés irritantes et sensibilisantes).
D'après les données disponibles, les aliments sont la principale
source d'exposition au limonène. Une valeur guide de 0,1 mg/kg de
poids corporel par jour a été établie pour l'ingestion. D'après les
estimations actuelles concernant le niveau d'exposition, la présence
de limonène dans les aliments ne semble pas présenter un risque
significatif pour la santé humaine.
Dans l'atmosphère, le limonène et d'autres terpènes réagissent
rapidement avec les radicaux hydroxyle et nitrate résultant de
réactions photochimiques, ainsi qu'avec l'ozone. L'oxydation des
terpènes, dont le limonène, contribue à la formation d'aérosols et de
smogs photochimiques. Dans le sol, la mobilité du limonène devrait
théoriquement être faible; dans le milieu aquatique, il devrait se
lier fortement aux sédiments. Le limonène est résistant à
l'hydrolyse. Il est biodégradable en conditions d'aérobiose, mais non
en milieu anaérobie.
Pour les organismes terrestres, l'air est la voie d'exposition la
plus probable. Les quelques études effectuées sur des espèces
terrestres (des insectes) exposées aux vapeurs de limonène ont révélé
que celui-ci produisait des effets à des concentrations de l'ordre de
quelques parties par million. Les concentrations mesurées dans
l'environnement sont généralement de l'ordre de 0,1-2 ppb (0,6-
11 µg/m3). En cas de pollution, les concentrations dans le sol
peuvent dépasser les niveaux pour lesquels on constate des effets sur
les organisme vivants (par exemple, les vers de terre). En milieu
aquatique, des signes de toxicité aiguë peuvent être observés sur les
poissons et les daphnies. Les concentrations de limonène dans les
eaux de surface sont généralement bien inférieures aux doses toxiques
aiguës déterminées expérimentalement; il est donc peu probable que le
limonène présente un risque de toxicité aiguë pour les organismes
aquatiques. Aucune étude n'a été découverte sur ses effets
chroniques.
RESUMEN DE ORIENTACION
Esta reseña de la evaluación química internacional del limoneno
(d-limoneno, l-limoneno y d/ l-limoneno) se basa principalmente
en un examen preparado en 1993 para el Grupo Nórdico de Expertos
(Karlberg & Lindell, 1993). Un segundo examen efectuado bajo los
auspicios del Consejo Nórdico de Ministros (Josefsson, 1993), una
fuente de información preliminar, no revisada por expertos, sobre la
exposición y los efectos ambientales (EPA de los Estados Unidos, 1994)
y múltiples consultas en las bases de datos pertinentes sobre los años
1993 a 1995 permitieron identificar datos adicionales para evaluar el
limoneno. En una última consulta de las publicaciones aparecidas en
1996 y 1997 no se hallaron datos que modificaran las conclusiones
enunciadas en la reseña de la evaluación química internacional. En el
apéndice 1 figura información sobre la naturaleza y disponibilidad del
documento de base. En el apéndice 2 se da información sobre el examen
colegiado de la presente reseña. Esta reseña fue aprobada para su
publicación en una reunión del Comité de Revisión Final celebrada en
Bruselas (Bélgica) del 18 al 20 de noviembre de 1996. Los
participantes en la reunión figuran en el apéndice 3. Se ha
reproducido asimismo la ficha internacional de seguridad química (ICSC
0918) del d-limoneno, elaborada por el Programa Internacional de
Seguridad de las Sustancias Químicas (IPCS, 1993). La atención
especial prestada al d-limoneno obedece a la gran cantidad de datos
disponibles sobre esta forma isomérica.
El limoneno está presente en forma natural en algunos árboles y
arbustos. Fuentes biógenas y antropógenas liberan limoneno y otros
monoterpenos en grandes cantidades, principalmente en la atmósfera.
El limoneno se utiliza como disolvente para desengrasar los metales
antes de la pintura industrial, para la limpieza en la industria
electrónica y de la imprenta, y como disolvente en la pintura. Se
emplea asimismo como aditivo de sabor y aroma en alimentos, productos
de limpieza de uso doméstico y perfumes.
El limoneno es un irritante de la piel, tanto en animales de
experimentación como en seres humanos. Se ha comprobado que en los
conejos el d-limoneno irrita los ojos. Estudios efectuados con
cobayos han revelado que el d-limoneno oxidado por el aire, pero no
propiamente el d-limoneno, produce dermatitis de contacto. Como el
d-limoneno y el l-limoneno son enantiómeros, lo anterior podría
ser cierto también para el l-limoneno y el dipenteno (la forma
racémica). La manipulación y la pureza de la sustancia química, y
posiblemente la adición de antioxidantes, pueden influir por tanto de
manera crucial en la alergenicidad del limoneno.
El órgano crítico en los animales (excepto en las ratas macho),
tras la administración oral o intraperitoneal de la sustancia, es el
hígado. No se sabe de estudios en que los animales de experimentación
hayan sido expuestos al limoneno por inhalación. La exposición al
limoneno altera la cantidad y la actividad de distintas enzimas
hepáticas, el peso del hígado, los niveles de colesterol y la
secreción biliar. Se han observado esos cambios en ratones, ratas y
perros. No se dispone de suficientes datos para determinar cuál es el
órgano crítico en el ser humano.
En las ratas macho, la exposición al d-limoneno causa lesiones
y tumores renales. Se cree que una proteína específica de la rata
macho, la alpha2µ-globulina, cumple una función crucial en el
desarrollo de lesiones renales tanto neoplásicas como no neoplásicas.
Por consiguiente, esas lesiones renales se consideran sin importancia
para la evaluación del riesgo en el ser humano. El d-limoneno se
estudió en una batería de pruebas in vitro de corta duración
demostrándose que no es genotóxico. No hay constancia de que el
limoneno tenga efectos teratogénicos o embriotóxicos en ausencia de
toxicidad materna. En general, el d-limoneno puede considerarse una
sustancia química con una toxicidad bastante baja (salvo por sus
propiedades irritantes y sensibilizantes).
Según los datos de que se dispone, los alimentos son la principal
fuente de exposición al limoneno. El valor de orientación calculado
para la ingestión de limoneno es de 0,1 mg por kg de peso corporal
diarios. A los actuales niveles de exposición estimados, el limoneno
presente en los productos alimenticios no parece constituir un riesgo
significativo para la salud humana.
En la atmósfera, el limoneno y otros terpenos reaccionan
rápidamente con los radicales hidroxilo y nitrato y con el ozono
producidos fotoquímicamente. La oxidación de los terpenos como el
limoneno contribuye a la formación de aerosoles y de niebla
fotoquímica. En principio, en el suelo el limoneno tiene poca
movilidad, y en el medio acuático se une fuertemente al sedimento. El
limoneno es resistente a la hidrólisis. La biodegradación se produce
en condiciones aerobias, pero no en condiciones anaerobias.
La vía de exposición al limoneno más probable en los organismos
terrestres es el aire. Los pocos estudios realizados sobre especies
terrestres (esto es, insectos) mediante exposición al vapor han
revelado efectos en niveles de partes por millón. Las concentraciones
ambientales medidas oscilan generalmente entre 0,1 y 2 ppmm (0,6-11
µg/m3). En lugares contaminados, las concentraciones de limoneno en
el suelo pueden sobrepasar los niveles por encima de los cuales
aparecen efectos en los organismos que viven en el suelo (por ejemplo,
los gusanos). En el medio acuático, el limoneno presenta una elevada
toxicidad aguda para los peces y para Daphnia. Las concentraciones
de limoneno en aguas superficiales suelen ser mucho más bajas que los
niveles de toxicidad aguda determinados experimentalmente, por lo que
es probable que el limoneno plantee un bajo riesgo de efectos tóxicos
agudos para los organismos acuáticos. No se conocen estudios sobre
los efectos crónicos.
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
The acute toxicity of d-limonene in rodents is fairly low after
oral, intraperitoneal, subcutaneous, and intravenous administration,
based on the magnitude of the LD50 values (Table 4). LD50 values
were approximately 5 g/kg body weight for the oral administration of
d-limonene or d/l-limonene to rats and for dermal application of
d/l-limonene to rabbits and 6 g/kg body weight for oral
administration to mice (Tsuji et al., 1974, 1975b; Opdyke, 1978).
Studies on the acute inhalation toxicity of limonene were not
identified.
Effects observed following the acute exposure of rodents to
limonene include increased bile flow at 85 mg/kg body weight (Kodama
et al., 1976), inhibition of S-3-hydroxy-3-methylglutaryl-CoA
reductase activity at 409 mg/kg body weight (Clegg et al., 1980),
enzyme induction at 600 and 1200 mg/kg body weight (Ariyoshi et al.,
1975), and decreased motor activity, hypothermia, and potentiation of
hexobarbital-induced sleep at 3 ml/kg body weight (Tsuji et al.,
1974).
8.2 Irritation and sensitization
d-Limonene is considered a skin irritant (Cronin, 1980;
Fischer, 1986). The skin irritancy of limonene in guinea-pigs (Klecak
et al., 1977) and rabbits (Lacy et al., 1987; Okabe et al., 1990) is
considered moderate and low, respectively. In an in vivo study of
rabbit skin irritation, d-limonene was ranked 3.5 of 8 on the basis
of the primary irritation index (Bagley et al., 1996); effects were
graded according to OECD Test Guideline 404 (OECD, 1993). In a study
in rabbits, d-limonene caused irritation to the eyes (Tsuji et al.,
1974).
Although d-limonene was once considered the main allergen in
citrus fruits, data from more recent studies in animals have revealed
air-oxidized d-limonene, rather than unoxidized d-limonene, to be
the sensitizing agent. When limonene (unspecified form and unknown
purity of the test material) was tested in four different
sensitization assays with guinea-pigs (Open Epicutaneous Test,
Maximization Test, Draize's Test, and a test with Freund's Complete
Adjuvant), it was sensitizing in all but Draize's Test (Klecak et al.,
1977). In another study in mice, d-limonene did not induce
sensitization (Maisey & Miller, 1986). Hydroperoxides and other
oxidation products of d-limonene formed on exposure to the air have
proved to be potent contact allergens when tested with Freund's
Complete Adjuvant in guinea-pigs, whereas unoxidized d-limonene did
not cause any sensitization (Karlberg et al., 1991, 1992).
Table 4: Acute toxicity of limonene.
Species (sex) Route of administration Type of limonene LD50 (g/kg body weight) Reference
rabbit dermal d/l >5 Opdyke, 1978
rat oral d/l 5.3 Opdyke, 1978
rat (m/f) oral d 4.4/5.1 Tsuji et al., 1975b
rat (m/f) intraperitoneal d 3.6/4.5 Tsuji et al., 1975b
rat (m/f) intravenous d 0.125/0.11 Tsuji et al., 1975b
mouse (m/f) oral d 5.6/6.6 Tsuji et al., 1975b
mouse (m/f) oral, 7 days d 5.3/6.8a Tsuji et al., 1974
mouse (m/f) intraperitoneal, 3 days d 3.1/3.0a Tsuji et al., 1974
mouse (m + f) intraperitoneal d 1.3 Tsuji et al., 1975b
mouse (m/f) intraperitoneal, 10 days d 0.59/0.50a Tsuji et al., 1974
mouse (m + f) subcutaneous d >41.5 Tsuji et al., 1975b
mouse (m + f) subcutaneous, 7 days d >21.5 Tsuji et al., 1974
a Calculated from ml/kg body weight.
8.3 Short-term exposure
Increases in hepatic cytochrome P-450 content have been observed
in female rats administered limonene (isomer unspecified; 40 mg/kg
body weight per day for 3 days) by intraperitoneal injection (Austin
et al., 1988) and in rats administered 5% d-limonene in the diet for
2 weeks (Maltzman et al., 1991). Increased epoxide hydratase activity
was observed in rats administered 1% or 5% d-limonene in the diet
for 2 weeks (Maltzman et al., 1991). Increases in phase II enzymes
(glutathionyltransferase and UDP-glucuronyltransferase) during the
exposure of rats to 5% limonene in food have also been described
(Maltzman, 1991). Increased relative liver weight (from 5 to 20
times) has been observed in rats administered d-limonene at a dose
of 75-300 mg/kg body weight; at 300 mg/kg body weight, the increase
was significant (Kanerva et al., 1987b). In cats, infusion of 97%
d-limonene into the bile system to dissolve gallstones caused acute
and chronic inflammatory changes (Schenk et al., 1980).
8.4 Long-term exposure
8.4.1 Subchronic exposure
Peroral administration of d-limonene to rats at a dose of 400
mg/kg body weight for 30 days resulted in a 20-30% increase in the
amount and activity of different liver enzymes (cytochrome P-450,
cytochrome b5, aminopyrine demethylase, and aniline hydroxylase),
increased relative liver weight, and decreased cholesterol levels
(Ariyoshi et al., 1975). Administration of d-limonene (0, 2, 5, 10,
30, and 75 mg/kg body weight per day) by gavage to groups of 10 male
rats, 5 days/week for 13 weeks (Webb et al., 1989), resulted in the
pathological formation of granular casts at the outer zone of the
renal medulla. The no-observed-effect level (NOEL), based upon
histological examination of the kidneys, was considered to be 5 mg/kg
body weight per day. The LOEL for increased liver and kidney weight
was 75 mg/kg body weight per day, the highest dose tested. The NOEL
for effects in the liver was 10 mg/kg body weight; the
no-observed-adverse-effect level (NOAEL) for effects in the liver was
30 mg/kg body weight per day. Linear regression analysis revealed a
dose-related trend in the increased relative weights of the kidney and
liver at 30 and 75 mg/kg body weight per day. No histopathological
changes were observed in the liver in these two studies. The amount
and activity of different liver enzymes were not investigated, and
thus the increase in relative liver weight may be due to enzyme
induction.
8.4.2 Chronic exposure and carcinogenicity
The oral administration of d-limonene (0.4, 1.2, or 3.6 ml/kg
body weight per day) to dogs for 6 months caused nausea and vomiting
(Tsuji et al., 1975a). A 35% increase in alkaline phosphatase and
cholesterol in serum and slightly increased total and relative liver
weights were observed in dogs after peroral administration of
d-limonene at a dose of 1.2 ml/kg body weight per day for 6 months
(about 1000 mg/kg body weight per day) (Webb et al., 1990).
In a 2-year study, d-limonene was administered (per os) 5
days/week to groups of 50 F344/N rats (0, 75, or 150 mg/kg body weight
per day to males, and 0, 300, or 600 mg/kg body weight per day to
females) and B6C3F1 mice (0, 250, or 500 mg/kg body weight per day to
males, and 0, 500, or 1000 mg/kg body weight per day to females) (NTP,
1990). Slightly lower body weights were observed for rats in the
high-dose groups and female mice in the high-dose group; however, no
clinical symptoms could be related to the administration of
d-limonene. For female rats in the high-dose group, survival was
reduced after 39 weeks (NTP, 1990). There was clear evidence of
carcinogenic activity of d-limonene in male rats, based upon a
dose-related increase in the incidence of hyperplasia and adenoma/
adenocarcinoma in renal tubular cells. However, there was no evidence
of carcinogenicity in female rats or in male and female mice. The
carcinogenic response in the kidney of male rats has been linked to a
unique renal perturbation involving alpha2µ-globulin.
To determine whether d-limonene would cause a sustained
increase in renal cell proliferation and exhibit promoting activity
for the development of renal adenomas in male F344 rats, the animals
were administered (by stomach tube) d-limonene (150 mg/kg body
weight per day) as a promoter 5 days/week for 30 weeks (Dietrich &
Swenberg, 1991). N-ethyl- N-hydroxyethylnitrosamine (500 ppm) was
used as an initiator in the drinking-water for 2 weeks. In addition,
male alpha2µ-globulin-deficient rats were exposed in the same manner
to determine if the male rat specific urinary protein alpha2µ-globulin
is required for d-limonene to cause these effects. Exposure to
d-limonene alone caused a significant increase in the number of
atypical tubules and atypical hyperplasias in F344 rats, compared with
vehicle controls. There was no increase in the incidence of tumours
or preneoplastic lesions in the alpha2µ-globulin-deficient rats
exposed to d-limonene, whereas a 10-fold increase in the incidence
of renal adenoma and atypical hyperplasia was observed in F344 rats
exposed to d-limonene, compared with controls. There was a
significant decrease in the incidence of liver tumours in animals
exposed to N-ethyl- N-hydroxyethylnitrosamine and d-limonene,
compared with N-ethyl- N-hydroxyethylnitrosamine exposure alone.
8.5 Genotoxicity and related end-points
On the basis of available data, there is no evidence that
d-limonene or its metabolites are genotoxic or mutagenic. Limonene
and its epoxides were not mutagenic when tested at concentrations of
0.3-3333 µg/plate in in vitro assays using different strains of
Salmonella typhimurium, in the presence or absence of metabolic
activation (Florin et al., 1980; Watabe et al., 1981; Haworth et al.,
1983; Connor et al., 1985; NTP, 1990). d-Limonene did not increase
the frequency of forward mutation at the TK+/- locus in mouse L5178Y
cells (NTP, 1990), induce cytogenetic damage in Chinese hamster ovary
cells (Anderson et al., 1990), or malignantly transform Syrian hamster
embryo cells (Pienta, 1980). In one in vitro study, following
exposure with benzo (a)pyrene, d-limonene (21.9 µmol/litre)
inhibited the formation of transformed cell colonies in tracheal
epithelium isolated from rats (Steele et al., 1990).
No evidence of mutagenicity was reported in an in vivo spot
test with mice, involving the intraperitoneal injection of limonene at
215 mg/kg body weight per day on days 9-11 during gestation (Fahrig,
1984).
8.6 Reproductive and developmental toxicity
Studies on the reproductive toxicity of limonene were not
identified. There is no evidence that limonene has teratogenic or
embryotoxic effects in the absence of maternal toxicity. In rats, the
oral administration of d-limonene (2869 mg/kg body weight per day)
on days 9-15 of gestation resulted in decreased body weight and deaths
among the dams. Delayed ossification and decreased total body and
organ weights (thymus, spleen, and ovary) were observed in the
offspring (Tsuji et al., 1975b). In mice, the oral administration of
d-limonene (2869 mg/kg body weight per day) on days 7-12 of
gestation resulted in reduced growth in the mothers and a
significantly increased incidence of skeletal anomalies and delayed
ossification in the offspring (Kodama et al., 1977a). The oral
administration of d-limonene (250, 500, or 1000 mg/kg body weight
per day) to rabbits on days 6-18 of gestation had no dose-related
effects on the offspring. At the highest dose, there were some deaths
and reduced weight gain among the dams; at the intermediate dose,
growth was decreased (Kodama et al., 1977b).
8.7 Immunological and neurological effects
Reports relating limonene to type I allergy were not identified.
In a study designed to assess the immunological effects of
d-limonene on B- and T-cell responses, BALB/c mice were administered
(by forced intragastric feeding) d-limonene (0.1 ml) daily for 9
weeks (Evans et al., 1987). Mice given keyhole limpet haemocyanin
prior to exposure to d-limonene had suppressed primary and secondary
anti-keyhole limpet haemocyanin responses. Mice exposed to
d-limonene prior to the administration of keyhole limpet haemocyanin
had significantly increased antibody and mitogen-induced proliferative
responses. However, the purity of the d-limonene in this study was
not checked, and oxidation products may have been the active
substances.
Effects on the central nervous system following exposure to
limonene have been reported in experimental studies with animals;
however, it is difficult to ascertain whether these effects were the
result of general intoxication or a more direct effect of the
chemical. The peroral administration of d-limonene (3 ml) to rats
and mice resulted in decreased motor activity (Tsuji et al., 1974). A
similar effect was also observed in mice orally administered a
limonene dose of 1000 mg/kg body weight per day for 13 weeks (NTP,
1990).
9. EFFECTS ON HUMANS
Case reports or epidemiological studies on the effects of
limonene on human health were not identified. Available data have
been derived from studies with volunteers. In older investigations,
multiple exposures and confounding factors such as mechanical damage,
irritation, other allergens, and infections due to wet work (Beerman
et al., 1938; Schwartz, 1938; Birmingham et al., 1951) may have
contributed to the effects reported following exposure to limonene.
None of eight subjects reported any discomfort, irritation, or
symptoms related to central nervous system effects during a 2-hour
inhalation exposure to d-limonene at 10, 225, or 450 mg/m3;
however, a slight decline in vital capacity was observed following
exposure to the highest concentration (Falk Filipsson et al., 1993).
In a study in which the sensitivity of four patch testing systems
(Finn chamber, Hill Top patch, Van der Bend chamber, and Webril patch)
was evaluated in volunteers, d-limonene (perfume-grade) reacted
strongly in all types of patches within 10-15 minutes of exposure
(York et al., 1995). Skin irritation was assessed before application,
as well as immediately and 1, 24, 48, and 72 hours after removal of
the patch, using a scoring system based broadly on that used for
rabbit irritation studies (OECD, 1993), but modified to account for
the nature of reactions on human skin. There was evidence of sensory
effects and urticarial responses on removal of the patches.
Significant irritation persisted for 24 hours, and these reactions
persisted for 48 and 72 hours in many volunteers (York et al., 1995).
Dermal exposure to d-limonene (98%) for 2 hours in one subject
caused burning, itching, aching, and a long-lasting purpuric rash
(Falk et al., 1991).
d-Limonene infused directly into the bile system of human
volunteers to dissolve gallstones caused pain in the upper abdomen,
nausea, vomiting, and diarrhoea, as well as increases in serum
aminotransferases and alkaline phosphatase (Igimi et al., 1976, 1991).
The oral administration of 20 g d-limonene to volunteers resulted in
diarrhoea, painful constrictions, and proteinuria, but no biochemical
changes (total protein, bilirubin, cholesterol, aspartate
aminotransferase, alanine aminotransferase, alkaline phosphatase) in
the liver (Igimi et al., 1976). Reports of contact allergy to
dipentene have appeared (Calnan, 1979; Rycroft, 1980). In one
investigation, 15 of 22 people with an allergy to oil of turpentine
also reacted to dipentene (Cachao et al., 1986). Patch testing in
consecutive dermatitis patients from Sweden and Belgium revealed
positive reactions in 1.5-2% of the subjects tested with oxidized
d-limonene, a finding similar to that observed with other common
sensitizers, such as formaldehyde (A.-T. Karlberg, personal
communication, 1996). d-Limonene reduced non-immunological contact
urticaria caused by cinnamic aldehyde, with competitive receptor
inhibition suggested as the mechanism of suppression (Guin et al.,
1984). No sensitizing effect was observed when 25 volunteers were
exposed to d-limonene in a Human Maximization Test (Grief, 1967).
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10.1 Aquatic environment
The acute toxicity of d-limonene ranges from slight to high for
aquatic organisms (Table 5). The lowest acute toxicity values (EC50
or LC50) identified were approximately 0.4 mg/litre for Daphnia (US
EPA, 1990b) and 0.7 mg/litre for fish (US EPA, 1990a,b). The
no-observed-effect concentration (NOEC) for green algae is
approximately 4 mg/litre (US EPA, 1990a). The acute toxicity (EC50
or LC50) of dipentene to Daphnia and fish is about 50-70 times
lower than that for d-limonene (US EPA, 1990b). No studies were
identified on the chronic toxicity of limonene to aquatic organisms.
10.2 Terrestrial environment
The toxicity of limonene has been studied in various terrestrial
organisms (Table 6). Limonene generally has moderate acute toxicity
in insects and mites. The acute toxicity of d-limonene to
earthworms (Eisenia foetida Savigny) was high (LC50 = 6.0 ppm;
mg/kg) (Karr et al., 1990). Sublethal effects (i.e. abnormal
rebounding of medial giant fibre pathway [MGF] impulses and
spontaneous lateral giant fibre pathway [LGF] spiking) were observed
following exposure of earthworms to 4.2 ppm (mg/kg) limonene (Karr et
al., 1990). Limonene has low subacute toxicity to bobwhite quail
(Colinus virginianus) exposed via the diet (LC50 > 5620 ppm;
mg/kg) (US EPA, 1994).
Table 5: Toxicity of limonene to aquatic organisms.
Species End-point; exposure Results (mg/litre) Reference
Algae
Green algaea 96-h NOEC; static 4.08 US EPA, 1990a
Crustaceans
Water flea (Daphnia magna)b 48-h LC50; flow-through 0.577 (0.496-0.672) US EPA, 1990b
48-h EC50; flow-through 0.421
Water flea (D. magna)c acute LC50 39 ppm US EPA, 1994
Water flea (D. magna)a 48-h LC50; flow-through 31 (27.5-34.8) US EPA, 1990b
48-h EC50; flow-through 28.2
Water flea (Daphnia pulex)b 48-h EC50; flow-through 0.730 US EPA, 1990a
Water flea (D. pulex)c 48-h EC50; static 69.6 Passino & Smith, 1987
Daphniab 21-d NOEC; structure-activity 0.15 US EPA, 1990a
relationship (SAR) analysis
Fish
Fathead minnow (Pimephales promelas)b 96-h LC50; flow-through 0.702 (0.619-0.796) US EPA, 1990b
Fathead minnow (P. promelas)b 96-h LC50; flow-through 0.720 (0.618-0.839) US EPA, 1990b
96-h EC50; flow-through 0.688 (0.606-0.782)
Fathead minnow (P. promelas)a 96-h LC50; flow-through 38.5 (35.4-41.8) US EPA, 1990a,b
96-h EC50; flow-through 28.2
Fishc acute LC50 80 ppm US EPA, 1994
Fishb 96-h LC50; flow-through 0.711 US EPA, 1990a
Golden orfe (Leuciscus idus)a 48-h LC50 32 Roth, 1990
Table 5 (continued)
Species End-point; exposure Results (mg/litre) Reference
Insects
Water hyacinth weevil (Neochetina Mortality (73%, range 40-100%), 50% limonene Haag, 1986
eichhorniae, 60%, N. bruchi, 40%)b weevils were dipped in limonene
Mosquito fly (Culex quinquefasciatus)c 2nd-instar larvae (23-33°C), 6.6-26.1 ppm Mohsen et al., 1989
72-h LC50; static
4th-instar larvae (23-33°C), 7.8-30.6 ppm Mohsen et al., 1989
72-h LC50; static
a d/l-Limonene.
b d-Limonene.
c Optical isomer not specified.
Table 6: Toxicity of limonene to terrestrial organisms.
Species End-point; exposure Results Reference
Insects
Cat flea Adult LD50; contact 160 (157-163) µg/cm2 Hink & Fee, 1986
(Ctenocephalides felis)a,b Adult LD50; vapour 259 (234-281) µg/cm2
Pupae LD50; contact 376 (259-468) µg/cm2
Larvae LD50; contact 226 (221-231) µg/cm2
Eggs; lethal to all eggs; contact 65 µg/cm2
Variegated cutworm Larvae; significant inhibition of pupation; dietary
(Peridroma saucia)b exposure 0.2% limonene in artificial feed Harwood et al., 1990
German cockroach Adult 24-h LD50; topical 700 (610-810) µg/insect Karr & Coats, 1988
(Blattella germanica L.)b Adult 24-h LC50; fumigation 23.3 (17.5-31.0) ppm
Adult; no mortality; oral 25% limonene in feed
Nymph; no mortality; oral 25% limonene in feed
Adult; no mortality; 72-h contact with treated limonene (conc. not given)
surface
German cockroach Effect on growth rate; diet 1-25% limonene in diet Karr & Coats, 1992
(B. germanica L.)b EC50, oothecae yielding young; topical exposure 0.68 mg/ootheca
No effect on reproduction; via diet 25% limonene in diet
Topical exposure 0.84 mg/cockroach
Vapour exposure 5 mg/litre in air
Rice weevil (Sitophilus Adult 24-h LC50; fumigation 19.0 (13.2-27.3) ppm Karr & Coats, 1988
oryzae L.)b
House fly (Musca 25-h LD50; topical 90 (70-130) µg/insect Karr & Coats, 1988
domestica L.)b
Western corn rootworm Egg 72-h LC50; contact with treated substrate 1.8 (0.8-2.9)% limonene Karr & Coats, 1988
(Diabrotica virgifera Larvae 72-h LC50; contact with treated soil 12.2 (4.5-32.6) ppm
virgifera LeConte)b
Table 6 (continued)
Species End-point; exposure Results Reference
Spiders and allies
Spruce spider mite 24-h LC50; vapour 24.5 ppm Cook, 1992
(Oligonychus ununguis Significant decrease in oviposition 5 ppm
(Jacobi)),c adult female
Segmented worms
Earthworm (Eisenia 48-h LC50 6.0 (5.1-7.1) ppm Karr et al., 1990
foetida Savigny)b Sublethal effects 4.2 ppm
Birds
Bobwhite quail (Colinus Subacute LC50; dietary exposure >5 620 ppm US EPA, 1994
virginianus)d
a Fleas were exposed to filter papers treated with limonene, either directly or to vapours from the filter papers.
b d-Limonene.
c l-Limonene.
d Optical isomers not specified.
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
Limonene is a skin irritant in experimental animals and humans.
d-Limonene is an eye irritant in rabbits. Studies in guinea-pigs
have revealed that air-oxidized d-limonene, but not d-limonene
itself, induced contact allergy. Similar results are likely with
l-limonene and dipentene.
The critical organ in animals (except for male rats) following
peroral or intraperitoneal administration is the liver. Exposure to
limonene affects the amount and activity of different liver enzymes,
liver weight, cholesterol levels, and bile flow, with effects having
been observed in mice, rats, and dogs. In male rats, exposure to
d-limonene results in damage to the kidneys and an increased
incidence of renal tumours. As the male rat specific protein
alpha2µ-globulin is considered to play a crucial role in the
development of the neoplastic and non-neoplastic kidney lesions, they
are considered not relevant for human risk assessment.
A dose-related nephropathy was observed in the kidneys of male
rats after oral administration of d-limonene (NTP, 1990). This
lesion, consisting of degeneration of epithelial cells in the
convoluted tubules, granular casts in the outer stripe of the outer
medulla, and epithelial regeneration, is characteristic of hyaline
droplet nephropathy associated with the accumulation of
alpha2µ-globulin in the cytoplasm of tubular cells (Alden et al.,
1984; Halder et al., 1985) in response to a variety of hydrocarbon
compounds (Swenberg et al., 1992). Some compounds fit deeply into a
hydrophobic pocket of alpha2µ-globulin. When hydrogen bonding between
the chemical and protein occurs, the digestibility of alpha2µ-globulin
by proteases is inhibited, leading to accumulation of the male rat
specific protein in lysosomes of the P2 segment of the nephron
(Lehman-McKeeman et al., 1990). Although such chemicals fall into
rather diverse classes, molecular modelling studies have demonstrated
a strong structure-activity relationship with respect to
alpha2µ-globulin binding (Borghoff et al., 1991). The accumulation of
alpha2µ-globulin is cytotoxic, resulting in single-cell necrosis
(Dietrich & Swenberg, 1991). The exfoliated renal epithelium is
restored by compensatory cell proliferation. The increase in cell
proliferation associated with alpha2µ-globulin is reversible. Damage
of this type has not been observed in female rats, male rats that do
not produce alpha2µ-globulin, or other mammals, such as mice,
hamsters, guinea-pigs, dogs, and monkeys (Alden, 1986; Kanerva &
Alden, 1987a; Swenberg et al., 1989; Webb et al., 1989, 1990; NTP,
1990; Ridder et al., 1990; Dietrich & Swenberg, 1991). The processes
leading to nephropathy and the development of renal cancer by such
compounds are among the best understood for non-genotoxic chemicals
and strongly indicate that it is a male rat specific process. Acute
and chronic renal effects induced in male rats by limonene will be
unlikely to occur in any species not producing alpha2µ-globulin or a
very closely related protein in the large quantities typically seen in
the male rat (US EPA, 1991; Swenberg, 1993).
d-Limonene has been studied in a variety of short-term
in vitro tests and has been found to be non-genotoxic. There is no
evidence that limonene has teratogenic or embryotoxic effects in the
absence of maternal toxicity.
11.1.2 Criteria for setting guidance values for limonene
In numerous experimental studies, exposure to limonene has been
shown to affect the liver. Owing to a lack of data on d-limonene
exposure in humans, this organ cannot with certainty be stated as the
critical organ in humans. Based on available data, food is believed
to be the principal source of exposure (96%) to limonene; the
contribution from ambient air is approximately 4%. The dermal uptake
of limonene has not been estimated.
To calculate a tolerable intake for humans, the animal study was
chosen in which effects on the liver were observed at the lowest
exposure level (Webb et al., 1989). In this study, gavage
administration of d-limonene (5 days/week for 13 weeks) to rats
caused increased relative liver weight at 30 and 75 mg/kg body weight
per day. The NOEL for the liver was considered to be 10 mg/kg body
weight per day. Using uncertainty factors of 10 for intraspecies
differences and 10 for interspecies differences, a tolerable intake
for ingestion of d-limonene by humans of 0.1 mg/kg body weight per
day may be calculated from the NOEL. A guidance value for inhalation
exposure to d-limonene was not developed, as inhalation is an
insignificant route of exposure compared with ingestion.
11.1.3 Sample risk characterization
Exposure estimates vary as a function of use patterns, and the
risk characterization presented here is provided only as an example,
primarily for illustrative purposes. In general, d-limonene could
be considered (with the exception of its irritative and sensitizing
properties) to be a chemical with fairly low toxicity. The calculated
tolerable intake of 0.1 mg/kg body weight per day is of a similar
magnitude as the estimated daily US consumption of d-limonene of
0.27 mg/kg body weight per day (Flavor and Extract Manufacturers
Association, 1991).
11.2 Evaluation of environmental effects
Limonene and other terpenes are released in large amounts mainly
to the atmosphere. When released to soil or water, limonene is
expected to evaporate to air to a significant extent, owing to its
high volatility. Thus, the atmosphere is the predominant
environmental sink of limonene, where it is expected to rapidly
undergo gas-phase reactions with photochemically produced hydroxyl
radicals, ozone, and nitrate radicals. The oxidation of terpenes,
such as limonene, contributes to aerosol and photochemical smog
formation. Ozonolysis of limonene may also lead to the formation of
hydrogen peroxide and organic peroxides, which have various toxic
effects on plant cells and may be part of the damage to forests
observed in the last decades (Peters et al., 1994). Emissions of
biogenic hydrocarbons such as limonene and other terpenes to the
atmosphere may either decrease ozone concentrations when nitrogen
oxide concentrations are low or, if emissions take place in polluted
air (i.e. containing high nitrogen oxide levels), lead to an increase
in ozone concentrations.
Terrestrial organisms are most likely to be exposed to limonene
via the air. The few studies on terrestrial species (i.e. insects)
using vapour exposure reveal effects of limonene at parts per million
levels. Measured environmental concentrations are typically around
0.1-2 ppb (0.6-11 µg/m3), indicating a low risk for acute toxic
effects on terrestrial organisms from direct exposure to limonene in
air. At polluted sites, limonene concentrations in soil (up to 920
mg/kg soil) may exceed effect levels of soil-living organisms (e.g.
earthworm, acute LC50 = 6.0 ppm; mg/kg).
In the aquatic environment, limonene exhibits high acute toxicity
to fish and Daphnia. It may also bioaccumulate. The lowest acute
toxicity value identified was 0.4 mg/litre (48-hour EC50 for
Daphnia). Because concentrations of limonene in surface waters of
"polluted" and "unpolluted" areas are at least about 250 and 20 000
times lower than this acute toxicity value, respectively, it is likely
that limonene poses a low risk for acute toxic effects on aquatic
organisms. No studies were identified on chronic effects, and
therefore risks associated with chronic exposures of aquatic organisms
to limonene in "polluted" waters cannot be determined.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The International Agency for Research on Cancer (IARC, 1993) has
classified d-limonene in Group 3 (not classifiable as to its
carcinogenicity to humans) based on a lack of available data on
carcinogenicity to humans and limited evidence for carcinogenicity in
experimental animals.
The 41st meeting of the Joint FAO/WHO Expert Committee on Food
Additives (JECFA, 1993b) withdrew the existing acceptable daily intake
for d-limonene of 0-1.5 mg/kg body weight per day (JECFA, 1993a) and
in its place allocated "not specified." On the basis of the available
data, the total daily intake of the chemical arising from its use at
the levels necessary to achieve the desired effect and from its
acceptable background levels in food did not, in the opinion of the
Committee, represent a health hazard. For that reason, and for the
reasons stated in the individual evaluations, the establishment of an
acceptable daily intake expressed in numerical form was not deemed
necessary.
Information on international hazard classification and labelling
is included in the International Chemical Safety Card reproduced in
this document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventative and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 0918) reproduced in this
document.
13.1 Human health hazards
Limonene is flammable but essentially non-toxic. Repeated or
prolonged contact with the oxidized chemical causes skin
sensitization.
13.2 Advice to physicians
In case of poisoning, the treatment is supportive. Like other
volatile oils, if the patient lives for 48 hours, complete recovery is
likely; laboratory evidence of renal damage may persist for several
months (Dreisbach & Robertson, 1987).
13.3 Storage
Limonene is flammable, with a flash point of 45°C. Keep the
container in a cool, dry, well ventilated area, out of direct
sunlight. Keep the container tightly closed to prevent oxidation of
the chemical.
13.4 Spillage
In the case of a large spill, emergency personnel need to use
non-sparking tools to avoid fire and explosion hazards.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards is
available from the International Register of Potentially Toxic
Chemicals (IRPTC) legal file.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
D-LIMONENE ICSC:0918
D-LIMONENE
(R)-4-Isopropenyl-1-methylcyclohexene
(+)-Limonene
C10H16
Molecular mass: 136.23
CAS # 5989-27-5
RTECS # GW6360000
ICSC # 0918
TYPES OF ACUTE HAZARDS/ PREVENTION FIRST AID/
HAZARD/ SYMPTOMS FIRE FIGHTING
EXPOSURE
FIRE Flammable. No open flames, NO sparks, Powder, AFFF, foam, carbon dioxide.
and NO smoking.
EXPLOSION Above 48°C explosive Above 48°C use a closed In case of fire: keep drums,
vapour/air mixtures may be system, ventilation, and etc., cool by spraying with
formed. explosion-proof electrical water.
equipment.
EXPOSURE STRICT HYGIENE!
* INHALATION Ventilation. Fresh air, rest.
* SKIN Redness. Protective gloves. Protective Remove contaminated clothes.
clothing. Rinse and then wash skin with
water and soap.
* EYES Redness. Safety spectacles. First rinse with plenty of
water for several minutes
(remove contact lenses if
easily possible), then take
to a doctor.
* INGESTION Do not eat, drink, or smoke Rinse mouth.
during work.
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
SPILLAGE DISPOSAL STORAGE PACKAGING & LABELLING
Collect leaking and spilled liquid in Fireproof. Cool. Well closed.
sealable containers as far as
possible. Absorb remaining liquid in
sand or inert absorbent and remove to
safe place.
IMPORTANT DATA PHYSICAL STATE; APPEARANCE: EFFECTS OF SHORT-TERM EXPOSURE:
COLOURLESS LIQUID, WITH CHARACTERISTIC ODOUR. The substance may irritate slightly the eyes
and the skin.
CHEMICAL DANGERS: EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
Reacts violently with a mixture of iodine Repeated or prolonged contact may cause skin
pentafluoride and tetrafluoroethylene, causing sensitization if the substance has been
fire and explosion hazard. oxidized.
OCCUPATIONAL EXPOSURE LIMITS (OELs):
TLV not established.
ROUTES OF EXPOSURE:
The substance can be absorbed into the body by
inhalation of its vapour, through the skin and
by ingestion.
INHALATION RISK:
No indication can be given about the rate in
which a harmful concentration in the air is
reached on evaporation of this substance at
20°C.
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
PHYSICAL Boiling point: 178°C
PROPERTIES Melting -75°C
Relative density (water = 1): 0.84
Solubility in water: none
Vapour pressure, kPa at 14.4°C: 0.4
Relative vapour density (air = 1): 4.7
Flash point: 48°C
Octanol/water partition coefficient
as log Pow: 4.2
ENVIRONMENTAL
DATA
NOTES
ICSC: 0918 1.1 Transport Emergency Card: TEC (R)-75
NFPA Code: H2; F3; R2
REFERENCES
Abraham WR, Hoffmann HMR, Kieslich K, Reng G, Stumpf B (1985)
Microbial transformation of some monoterpenoids and sesquiterpenoids.
Ciba Foundation symposium, III:146-160.
Alden CL (1986) A review of unique male rat hydrocarbon nephropathy.
Toxicologic pathology, 14:109-111.
Alden CL, Kanerva RL, Ridder G, Stone LC (1984) Pathogenesis of the
nephrotoxicity of volatile hydrocarbons in the male rat. In:
Advances in modern environmental toxicology. Vol. VII. Princeton,
NJ, Princeton Scientific Publ., pp. 107-120.
Altshuller AP (1983) Review: Natural volatile organic substances and
their effect on air quality in the United States. Atmospheric
environment, 17(11):2131-2165.
Anderson BE, Zeiger E, Shelby MD, Resnick MA, Gulati DK, Ivett JL,
Loveday KS (1990) Chromosome aberration and sister chromatid exchange
test results with 42 chemicals. Environmental and molecular
mutagenesis, 16:55-137.
Arey J, Atkinson R, Aschmann SM (1990) Product study of the gas-phase
reactions of monoterpenes with the OH radical in the presence of NOx.
Journal of geophysical research, 95(D11):18539-18546.
Arey J, Winer AM, Atkinson R, Aschmann SM, Long WD, Morrison CL,
Olszyk DM (1991) Terpenes emitted from agricultural species found in
California's Central Valley. Journal of geophysical research,
96(D5):9329-9336.
Ariyoshi T, Arakaki M, Ideguchi K, Ishizuka Y, Noda K, Ide H (1975)
Studies on the metabolism of d-limonene (p-mentha-1,8-diene). III.
Effects of d-limonene on the lipids and drug metabolizing enzymes in
rat livers. Xenobiotica, 5:33-38.
Atkinson R (1990) Gas-phase tropospheric chemistry of organic
compounds: a review. Atmospheric environment, 24A(1):1-41.
Atkinson R, Carter WPL (1984) Kinetics and mechanisms of gas-phase
reactions of ozone with organic compounds under atmospheric
conditions. Chemical reviews, 84:437-470.
Atkinson R, Aschmann SM, Winer AM, Pitts JN Jr (1984) Kinetics of the
gas-phase reactions of NO3 radicals with a series of dialkenes,
cycloalkenes, and monoterpenes at 295 ± 1 K. Environmental science
and technology, 18:370-375.
Atkinson R, Hasegawa D, Aschmann SM (1990) Rate constants for the
gas-phase reactions of O3 with a series of monoterpenes and related
compounds at 296 ± 2 K. International journal of chemical kinetics,
22:871-887.
Austin CA, Shepard EA, Pike SF, Rabin BR, Phillips IR (1988) The
effect of terpenoid compounds on cytochrome P-450 levels in rat liver.
Biochemical pharmacology, 37:2223-2229.
Bagley DM, Gardner JR, Holland G, Lewis RW, Regnier J-F, Stringer DA,
Walker AP (1996) Skin irritation: Reference Chemicals Data Bank.
Toxicology in vitro, 10:1-6.
Barber LB, Thurman EM, Schroeder MP (1988) Long-term fate of organic
micropollutants in sewage-contaminated groundwater. Environmental
science and technology, 22:205-211.
Becker KH, Brockmann KJ, Bechara J (1990) Production of hydrogen
peroxide in forest air by reaction of ozone with terpenes. Nature,
346:256-258.
Beerman H, Fondé GH, Callaway JL (1938) Citrus fruit dermatoses.
Archives of dermatology and syphilology (Chicago), 38:225-234.
Bertsch W, Chang RC, Zlatkis A (1974) The determination of organic
volatiles in air pollution studies: characterization of profiles.
Journal of chromatographic science, 12:175-182.
Bertsch W, Anderson E, Holzer G (1975) Trace analysis of organic
volatiles in water by gas chromatography-mass spectrometry with glass
capillary columns. Journal of chromatography, 122:701-718.
Bianchi AP, Varney MS, Phillips J (1991) Analysis of volatile organic
compounds in estuarine sediments using dynamic head space and gas
chromatography-mass spectrometry. Journal of chromatography,
542:413-450.
Birmingham DJ, Campbell PC Jr, Doyle HN, McDonald JM (1951)
Investigation of occupational dermatoses in the citrus fruit canning
industry. Archives of industrial hygiene and occupational medicine,
3:57-63.
Borghoff SJ, Miller AB, Bowen P, Swenberg JA (1991) Characteristics of
chemical binding to alpha2µ-globulin in vitro - evaluating
structure-activity relationships. Toxicology and applied
pharmacology, 107:228-238.
Button DK, Jüttner F (1989) Terpenes in Alaskan waters:
concentrations, sources, and the microbial kinetics used in their
prediction. Marine chemistry, 26:57-66.
Cachao P, Menezes Brandao F, Carmo M, Frazao S, Silva M (1986) Allergy
to oil of turpentine in Portugal. Contact dermatitis, 14:205-208.
Calnan CD (1979) Allergy to dipentene in paint thinner. Contact
dermatitis, 5:123-124.
Carlsson H, Andersson-Sköld Y, Antonsson A-B, Solyom P (1991)
[ Limonene - a solution of the environmental problems.] Swedish
Environmental Research Institute (Report B 1030) (in Swedish, with
English summary).
Chan CC, Vainer L, Martin JW, Williams DT (1990) Determination of
organic contaminants in residential indoor air using an
adsorption-thermal desorption technique. Journal of the Air & Waste
Management Association, 40:62-67.
Chemical Daily (1994) [ 12394 chemical products.] pp. 1214-1215 (in
Japanese).
Chemical Daily (1995) [ 12695 chemical products.] p. 1245 (in
Japanese).
Ciccoioli P, Cecinato A, Brancaleoni E, Frattoni M (1992)
Identification and quantitative evaluation of C4-C14 volatile
organic compounds in some urban, suburban and forest sites in Italy.
Fresenius environmental bulletin, 1:73-78.
Ciccoioli P, Brancaleoni E, Cecinato A, Sparapani R, Frattoni M (1993)
Identification and determination of volatile organic compounds in
forest areas of Northern and Southern Europe and remote sites of the
Himalaya region by high-resolution gas chromatography-mass
spectrometry. Journal of chromatography, 643(1/2):55-69.
Clegg RJ, Middleton B, Duncan Bell G, White DA (1980) Inhibition of
hepatic cholesterol synthesis and S-3-hydroxy-3-methylglutaryl-CoA
reductase by mono and bicyclic monoterpenes administered in vivo.
Biochemical pharmacology, 29:2125-2127.
Clement B, Riba ML, Leduc R, Haziza M, Torres L (1990) Concentration
of monoterpenes in a maple forest in Quebec. Atmospheric
environment, 24A(9):2513-2516.
Connor TH, Theiss JC, Hanna HA, Monteith DK, Matney TS (1985)
Genotoxicity of organic chemicals frequently found in the air of
mobile homes. Toxicology letters, 25:33-40.
Cook SP (1992) Influence of monoterpene vapors on spruce spider mite,
Oligonychus ununguis, adult females. Journal of chemical ecology,
18(9):1497-1504.
Cronin E, ed. (1980) Contact dermatitis. London, Churchill
Livingstone, pp. 532-534.
Crowell PL, Elegbede JA, Elson CE, Lin S, Vedejs E, Cunningham D,
Bailey HH, Gould MN (1992) Human metabolism of orally administered
d-limonene. Proceedings of the American Association for Cancer
Research, 33:524.
De Bortoli M, Knöppel H, Pecchio E, Peil A, Rogora L, Schauenburg H,
Schlitt H, Vissers H (1986) Concentrations of selected organic
pollutants in indoor and outdoor air in Northern Italy.
Environment international, 12:343-350.
Desideri P, Lepri L, Checchini L (1991) Organic compounds in seawater
and pack ice in Terra Nova Bay (Antarctica). Annales de Chimie
(Paris), 81:395-416.
Dhavalikar RS, Bhattacharayya PK (1966) Microbial transformation of
terpenes: Part VIII - Fermentation of limonene by a soil pseudomonad.
Indian journal of biochemistry, 3:144-157.
Dietrich DR, Swenberg JA (1991) The presence of alpha2µ-globulin is
necessary for d-limonene promotion of male rat kidney tumors.
Cancer research, 51:3512-3521.
Dimitriades B (1981) The role of natural organics in photochemical air
pollution - issues and research needs. Journal of the Air Pollution
Association, 31(3):229-235.
Dreisbach RH, Robertson WO (1987) Handbook of poisoning:
prevention, diagnosis & treatment, 12th ed. Norwalk, CT, Appleton &
Lange, 589 pp.
Eitzer BD (1995) Emissions of volatile organic chemicals from
municipal solid waste composting facilities. Environmental science
and technology, 29:896-902.
Evans DL, Miller DM, Jacobsen KL, Bush PB (1987) Modulation of immune
responses in mice by d-limonene. Journal of toxicology and
environmental health, 20:51-66.
Fahrig R (1984) Genetic mode of action of cocarcinogens and tumor
promoters in yeast and mice. Molecular & general genetics, 194:7-14.
Falk A, Gullstrand E, Löf A, Wigaeus Hjelm E (1990) Liquid/air
partition coefficients of four terpenes. British journal of
industrial medicine, 47:62-64.
Falk A, Fischer T, Hagberg M (1991) Purpuric rash caused by dermal
exposure to d-limonene. Contact dermatitis, 25:198-199.
Falk Filipsson A, Löf A, Hagberg M, Wigaeus Hjelm E, Wang Z (1993)
d-Limonene exposure to humans by inhalation: Uptake, distribution,
elimination, and effects on the pulmonary function. Journal of
toxicology and environmental health, 38:77-88.
Fehsenfeld F, Calvert J, Fall R, Goldan J, Guenther AB, Hewitt CN,
Lamb B, Liu S, Trainer M, Westberg H, Zimmerman P (1992) Emissions of
volatile organic compounds from vegetation and implications for
atmospheric chemistry. Global geochemical cycles, 6(4):389-430.
Fellin P, Otson R (1993) Seasonal trends of volatile organic compounds
(VOCs) in Canadian homes. In: Saarela K, Kalliokoski P, Seppänen O,
eds. Indoor air '93. Vol. 2. Chemicals in indoor air, material
emissions. Proceedings of the 6th International Conference on Indoor
Air Quality, held in Helsinki, 4-8 July 1993, pp. 117-122.
Fielding M, Gibson TM, James HA, McLoughlin K, Steel CP (1981)
Organic micropollutants in drinking water. Medmenham, Water Research
Centre, Environmental Protection (Technical Report TR 159).
Fischer A, ed. (1986) Contact dermatitis, 3rd ed. London, Churchill
Livingstone, pp. 421-422, 582-583.
Fjelstad PE, Wolbaek T (1992) A national exposure database. In: Brown
RH, Curtis M, Saunders KJ, Vandendriessche S, eds. Clean air at
work, New trends in assessment and measurement for the 1990s.
Cambridge, Royal Society of Chemistry, pp. 303-310.
Flavor and Extract Manufacturers Association (1991) d-Limonene
monograph. Washington, DC, pp. 1-4.
Florida Chemical Co. (1991) Marketing data sheet: d-Limonene. Lake
Alfred, FL.
Florin I, Rutberg L, Curvall M, Enzell CR (1980) Screening tobacco
smoke constituents for mutagenicity using the Ames test.
Toxicology, 18:219-232.
Gäb S, Hellpointner E, Turner WV, Korte F (1985) Hydroxymethyl
hydroperoxide and bis(hydroxymethyl) peroxide from gas-phase
ozonolysis of naturally occurring alkenes. Nature, 316:535-536.
Gomez-Belinchon JI, Grimalt JO, Albaigès J (1991) Volatile organic
compounds in two polluted rivers in Barcelona (Catalonia, Spain).
Water research, 25(5):577-589.
Grief N (1967) Cutaneous safety of fragrance material as measured by
the Maximization Test. American perfumer and cosmetics, 82:54-57.
Grosjean D, Williams EL, Seinfeld JH (1992) Atmospheric oxidation of
selected terpenes and related carbonyls: gas-phase carbonyl products.
Environmental science and technology, 26(8):1526-1533.
Grosjean D, Williams EL, Grosjean E, Andino JM, Seinfeld JH (1993)
Atmospheric oxidation of biogenic hydrocarbons: reaction of ozone with
beta-pinene, d-limone and trans-caryophyllene. Environmental
science and technology, 27(13):2754-2758.
Guin J, Meyer B, Drake R, Haffley P (1984) The effect of quenching
agents on contact urticaria caused by cinnamic aldehyde. Journal
of the American Academy of Dermatology, 1:45-51.
Haag KH (1986) Effects of herbicide application on mortality and
dispersive behaviour of the water hyacinth weevils, Neochetina
eichhorniae and Neochetina bruchi (Coleoptera: Curculionidae).
Environmental entomology, 15:1192-1198.
Hakola H, Arey J, Aschmann SM, Atkinson R (1994) Product formation
from the gas-phase reactions of OH radicals and O3 with a series of
monoterpenes. Journal of atmospheric chemistry, 18:75-102.
Halder CA, Holdsworth CE, Cockrell BY, Piccirillo VJ (1985)
Hydrocarbon nephropathy in male rats: Identification of the
nephrotoxic components of unleaded gasoline. Toxicology and
industrial health, 1:67-87.
Harwood SH, Moldenke AF, Berry RE (1990) Toxicity of peppermint
monoterpenes to the variegated cutworm (Lepidoptera: Noctuidae).
Journal of economic entomology, 83(5):1761-1767.
Haworth S, Lawlor T, Mortelmans K, Speck W, Zeiger E (1983)
Salmonella mutagenicity test results for 250 chemicals.
Environmental mutagenesis, 1:3-142.
Helmig D, Arey J (1992) Organic chemicals in the air at Whitaker's
Forest/Sierra Nevada Mountains, California. Science of the total
environment, 112:233-250.
Helmig D, Müller J, Klein W (1989) Volatile organic substances in a
forest atmosphere. Chemosphere, 19(8/9):1399-1412.
Hiatt MH (1983) Determination of volatile organic compounds in fish
samples by vacuum distillation and fused silica capillary gas
chromatography/mass spectrometry. Analytical chemistry, 55:506-516.
Hink WF, Fee BJ (1986) Toxicity of d-limonene, the major component
of citrus peel oil, to all life stages of the cat flea
Ctenocephalides felis (Siphonaptera: Pulicidae). Journal of
medical entomology, 23(4):400-404.
Hutte RS, Williams EJ, Staehelin J, Hawthorne SB, Barkley RM, Sievers
RE (1984) Chromatographic analysis of organic compounds in the
atmosphere. Journal of chromatography, 302:173-179.
IARC (1993) Some naturally occurring substances: food items and
constituents, heterocyclic aromatic amines and mycotoxins. Lyon,
International Agency for Research on Cancer, pp. 135-162 (IARC
Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol.
56).
Igimi H, Nishimura M, Kodama R, Ide H (1974) Studies on the metabolism
of d-limonene (p-mentha-1,8-diene). I. The absorption,
distribution and excretion of d-limonene in rats. Xenobiotica,
4:77-84.
Igimi H, Hisatsugu T, Nishimura M (1976) The use of d-limonene
preparation as a dissolving agent of gallstones. Digestive diseases
and science, 21:926-939.
Igimi H, Tamura R, Toraishi K, Yamamoto F, Kataoka A, Ikejiri Y,
Hisatsugu T, Shimura H (1991) Medical dissolution of gallstones.
Clinical experience of d-limonene as a simple, safe and effective
solvent. Digestive diseases and science, 36:200-208.
Ioffe BV, Isidorov VA, Zenkevich IG (1977) Gas chromatographic-mass
spectrometric determination of volatile organic compounds in an urban
atmosphere. Journal of chromatography, 142:787-795.
Ioffe BV, Isidorov VA, Zenkevich IG (1979) Certain regularities in the
composition of volatile organic pollutants in the urban atmosphere.
Environmental science and technology, 13(7):864-868.
IPCS (1993) International Chemical Safety Card - d-Limonene. Geneva,
World Health Organization, International Programme on Chemical Safety
(No. 0918).
IPCS (1994) Assessing health risks of chemicals: Derivation of
guidance values for health-based exposure limits. Geneva, World
Health Organization, International Programme on Chemical Safety
(Environmental Health Criteria 170).
Janson R, Kristensson J (1991) Sampling and analysis of atmospheric
monoterpenes. Stockholm, Stockholm University (Report CM-79).
JECFA (1993a) Limonene. In: Toxicological evaluation of certain
food additives and naturally occurring toxicants. Geneva, World
Health Organization, Joint FAO/WHO Expert Committee on Food Additives,
pp. 35-79 (WHO Food Additives Series 30).
JECFA (1993b) Limonene. In: Toxicological evaluation of certain
food additives and contaminants. Annex 4. Geneva, World Health
Organization, Joint FAO/WHO Expert Committee on Food Additives, pp.
299-301 (WHO Food Additives Series 32).
Josefsson C (1993) Limonene. In: Health effects of selected
chemicals. Vol. 2. Copenhagen, Nordic Council of Ministers, pp.
105-135 (Nord 29).
Jüttner F (1986) Analysis of organic compounds (VOC) in the forest air
of the southern Black Forest. Chemosphere, 15(8):985-992.
Jüttner F (1988) Quantitative analysis of monoterpenes and volatile
organic pollution products (VOC) in forest air of the southern Black
Forest. Chemosphere, 17(2):309-317.
Kanerva RL, Alden CL (1987a) Review of kidney sections from a
subchronic d-limonene oral dosing study conducted by the National
Cancer Institute. Food and chemical toxicology, 25:355-358.
Kanerva RL, Ridder GM, Lefever FR, Alden CL (1987b) Comparison of
short-term renal effects due to oral administration of decalin or
d-limonene in young adult male Fischer-344 rats. Food and
chemical toxicology, 25:345-353.
Karlberg A-T, Lindell B (1993) Limonene. In: Beije B, Lundberg P, eds.
Criteria documents from the Nordic Expert Group 1993. Solna,
National Institute of Occupational Health, Nordic Council of
Ministers, pp. 207-247 (Arbete och Hälsa 35).
Karlberg A-T, Boman A, Melin B (1991) Animal experiments on the
allergenicity of d-limonene - the citrus solvent. Annals of
occupational hygiene, 35:419-426.
Karlberg A-T, Magnusson K, Nilsson U (1992) Air oxidation of
d-limonene (the citrus solvent) creates potent allergens.
Contact dermatitis, 26:332-340.
Karlberg A-T, Shao LP, Nilsson U, Gäfvert E, Nilsson JLG (1994)
Hydroperoxides in oxidized d-limonene identified as potent contact
allergens. Archives for dermatological research, 286:97-103.
Karr LL, Coats JR (1988) Insecticidal properties of d-limonene.
Journal of pesticide science, 13:287-290.
Karr LL, Coats JR (1992) Effect of four monoterpenoids on growth and
reproduction of the German cockroach (Blattodea: Blattellidae).
Journal of economic entomology, 85(2):425-429.
Karr LL, Drewes CD, Coats JR (1990) Toxic effects of d-limonene in
the earthworm Eisenia foetida (Savigny). Pesticide biochemistry
and physiology, 36:175-186.
Keith LH, Garrison AW, Allen FR, Carter MH, Floyd TL, Pope JD,
Thruston AD Jr (1976) Identification of organic compounds in drinking
water from thirteen U.S. cities. In: Keith LH, ed. Identification
and analysis of organic pollutants in water. Ann Arbor, MI, Ann
Arbor Science Publ., pp. 329-373.
Klecak G, Geleick H, Noda K, Ide H (1977) Screening of fragrance
materials for allergenicity in the guinea pig. I. Comparison of four
testing methods. Journal of the Society of Cosmetic Chemists,
28:53-54.
Klöpffer W, Haag F, Kohl E-G, Frank R (1988) Testing of the abiotic
degradation of chemicals in the atmosphere: The smog chamber approach.
Ecotoxicology and environmental safety, 15:298-319.
Kodama R, Yano T, Furukawa K, Noda K, Ide H (1976) Studies on the
metabolism of d-limonene (p-mentha-1,8-diene). IV. Isolation and
characterization of new metabolites and species differences in
metabolism. Xenobiotica, 6:377-389.
Kodama R, Okubo A, Araki E, Noda K, Ide H, Ikeda T (1977a) Studies on
d-limonene as a gallstone solubilizer (VII). Effects on development
of mouse fetuses and offspring. (Japan) Oyo Yakuri, 13:863-873.
Kodama R, Okubo A, Sato K, Araki E, Noda K, Ide H, Ikeda T (1977b)
Studies on d-limonene as a gallstone solubilizer (IX). Effects on
development of rabbit fetuses and offspring. (Japan) Oyo Yakuri,
13:885-898.
Koe LCC, Ng WJ (1987) Identification of odorous gases originating from
refuse waste. Water, air, and soil pollution, 33:199-204.
Lacy MJ, Kent CR, Voss EW (1987) d-Limonene: An effective
vasodilator for use in collecting rabbit blood. Laboratory animal
science, 37(4):485-487.
Lamb B, Guenther A, Gay D, Westberg H (1987) A national inventory of
biogenic hydrocarbon emissions. Atmospheric environment,
21(8):1695-1705.
Larson RA, Marley KA (1988) Sunlight photochlorination and dark
chlorination of monoterpenes. Science of the total environment,
77:245-252.
Lehman-McKeeman LD, Caudill D (1992) Biochemical basis for mouse
resistance to hyaline droplet nephropathy: lack of relevance of the
alpha2µ-globulin protein superfamily in this male rat specific
syndrome. Toxicology and applied pharmacology, 112:214-221.
Lehman-McKeeman LD, Rodriguez PA, Takigiku R, Caudill D, Fey ML (1989)
d-Limonene induced male rat specific nephrotoxicity: Evaluation of
the association between d-limonene and alpha2µ-globulin.
Toxicology and applied pharmacology, 99:250-259.
Lehman-McKeeman LD, Rodriguez MI, Caudill D (1990) Lysosomal
degradation of alpha2µ-globulin and alpha2µ-globulin-xenobiotic
conjugates. Toxicology and applied pharmacology, 103:539-548.
Maisey J, Miller K (1986) Assessment of the ability of mice fed on
vitamin A diet to respond to a variety of potential contact
sensitizers. Contact dermatitis, 15:17-23.
Maltzman TH (1991) Anticarcinogenic mechanism of action of dietary
limonene during the initiation stage of DMBA-induced mammary
carcinogenesis. Dissertation abstracts international, 51:4283B.
Maltzman TH, Cristou M, Gould MN, Jefcoate CR (1991) Effects on
monoterpenoids on in vivo DMBA-DNA adduct formation and on phase I
hepatic metabolizing enzymes. Carcinogenesis, 12:2081-2087.
Massaldi HA, King CJ (1973) Simple technique to determine solubilities
of sparingly soluble organics: solubility and activity coefficients of
d-limonene, n-butylbenzene, and n-hexyl acetate in water and
sucrose solutions. Journal of chemical and engineering data,
18(4):393-397.
McCreary JJ, Jackson JG, Zoltec J (1983) Toxic chemicals in an
abandoned phenolic waste site. Chemosphere, 12 (11/12):1619-1632.
MITI (1992) Biodegradation and bioaccumulation data of existing
chemicals based on the CSCL Japan compiled under the supervision
of Chemical Products Safety Division, Basic Industries Bureau,
Ministry of International Trade and Industry, Japan. Ministry of
International Trade and Industry, Chemicals Inspection & Testing
Institute (ISBN 4-89074-101-1).
Mohsen ZH, Al-Chalabi BM, Kassir JT (1989). Factors influencing the
larvicidal activity of limonene against Culex quinquefasciatus Say
(Dipt., Culicidae). Journal of applied entomology, 108:107-110.
Montgomery D, Kalman DA (1989) Indoor/outdoor air quality: Reference
pollutant concentrations in complaint-free residences. Applied
industrial hygiene, 4(1):17-20.
National Board of Occupational Safety and Health (1993)
Occupational exposure limit value. Solna (Ordinance AFS, Vol. 3).
Nilsson U, Bergh M, Shao LP, Karlberg A-T (1996) Analysis of contact
allergenic compounds in oxidized d-limonene. Chromatographia,
42(3/4):199-205.
Nolting F, Zetzsch C (1988) Das ozon-bildungspotential des biogenen
alfa-pinens in vergleich mit anthropogenen kohlenwasserstoffen. In:
GSF-Bericht 17/88, International Symposium "Verteilung Wirkung
Photooxidantien Alpenraum." Neuherberg, Forschungszentrum für Umwelt
und Gesundheit, pp. 623-628.
NTP (1990) Toxicology and carcinogenesis studies of d-limonene in
F344/N rats and B6C3F1 mice. Springfield, VA, US Department of
Health and Human Services, National Institutes of Health, National
Toxicology Program (NTP Technical Report No. 347).
OECD (1981) OECD guidelines for testing of chemicals. Paris,
Organisation for Economic Co-operation and Development.
OECD (1993) Guidelines for testing of chemicals. Guideline 404,
Acute dermal irritation/corrosion. Adopted 1981, upgraded 1992.
Paris, Organisation for Economic Co-operation and Development.
Okabe H, Obata Y, Takayama K, Nagai T (1990) Percutaneous absorption
enhancing effect and skin irritation of monocyclic monoterpenes.
Drug design and delivery, 6:229-238.
Opdyke DLJ (1978) Monographs on fragrance raw materials. Food and
cosmetics toxicology, Special issue IV, 16 (Suppl.):809.
Page BD, Conacher HBS, Salminen J (1993) Survey of bottled drinking
water sold in Canada. Part 2. Selected volatile organic compounds.
Journal of the Association of Official Analytical Chemists
International, 76(1):26-31.
Passino DRM, Smith SB (1987) Acute bioassays and hazard evaluation of
representative contaminants detected in Great Lakes fish.
Environmental toxicology and chemistry, 6:901-907.
Paxéus N, Robinson P, Balmér P (1992) Study of organic pollutants in
municipal wastewater in Göteborg, Sweden. Water science and
technology, 25(11):249-256.
Pellizzari ED, Hartwell TD, Harris BSH III, Wadell RD, Whitaker DA,
Erickson MD (1982) Purgeable organic compounds in mother's milk.
Bulletin of environmental contamination and toxicology, 28:322-328.
Peters RJB, Duivenbode JAD, Duyzer JH, Verhagen HLM (1994) The
determination of terpenes in forest air. Atmospheric environment,
28(15):2413-2419.
Petersson G (1988) High ambient concentrations of monoterpenes in a
Scandinavian pine forest. Atmospheric environment, 22(11):2617-2619.
Pienta RJ (1980) Evaluation and relevance of the Syrian hamster embryo
cell system. Applied methods in oncology, 3:149-169.
Ridder GM, von Bargen EC, Alden CL, Parker RD (1990) Increased hyaline
droplet formation in male rats exposed to decalin is dependent on the
presence of alpha-2µ-globulin. Fundamental and applied toxicology,
15:732-743.
Roberts JM, Hahn CJ, Fehsenfeld FC, Warnock JM, Albritton DL, Sievers
RE (1985) Monoterpene hydrocarbons in the nighttime troposphere.
Environmental science and technology, 19(4):364-369.
Roth L (1990) Wassergefährende Stoffe (WGK). Landsberg, Ecomed
Verlag mbh.
Rycroft RJG (1980) Allergic contact dermatitis from dipentene in
honing oil. Contact dermatitis, 6:325-329.
Sauer TC Jr (1981) Volatile organic compounds in open ocean and
coastal surface waters. Organic geochemistry, 3:91-101.
Schenk J, Däbränte Z, Koch H, Stolte M (1980) Untersuchungen zur
Geweberträglichkeit von D-Limonen als einem Läsungsmittel
cholesterinreicher Gallensteine. Zeitschrift für Gastroenterologie,
18:389-394.
Schwartz L (1938) Cutaneous hazards in the citrus fruit industry.
Archives of dermatology, 37:631-649.
Schwartz SM, Boethling RS, Leighton T (1990) Fate of the terpenes
anethole and dipentene in a bench-scale activated sludge system.
Chemosphere, 21(10/11):1153-1160.
Searle E (1989) Determination of airborne limonene vapour by charcoal
tube sampling and gas-liquid chromatographic analysis. Analyst,
114:113-114.
Sekiya T, Yabuzaki T, Kitano M, Ogawa T (1988) Photochemical aerosol
formation from organic gases. Colloid and polymer science,
266:1037-1041.
Shaw RW, Crittenden AL, Stevens RK, Crohn DR, Titov VS (1983) Ambient
concentrations of hydrocarbons from conifers in atmospheric gases and
aerosol particles measured in Soviet Georgia. Environmental science
and technology, 17:389-395.
Shulka OP, Bhattacharyya PK (1968) Microbial transformation of
terpenes: Part XI - Pathways of degradation of alpha & beta pinenes in
a soil pseudomonad (PL-strain). Indian journal of biochemistry,
5(3): 92-101.
Sierra-Alvarez R, Kato M, Lettinga G (1990) The anaerobic
biodegradability of paper mill wastewater constituents.
Environmental technology, 11:891-898.
Smith OW, Wade AP, Dean FM (1969) Uroterpenol, a pettenkofer chromogen
of dietary origin and a common constituent of human urine. Journal
of endocrinology, 45:17-28.
Steele VE, Kelloff GJ, Wilkinson BP, Arnold JT (1990) Inhibition of
transformation in cultured rat tracheal epithelial cells by potential
chemopreventive agents. Cancer research, 50:2068-2074.
Strömvall AM (1992) Terpenes emitted to air from forestry and the
forest industry. Göteborg, Chalmers University of Technology.
Swenberg JA (1993) alpha2µ-globulin nephropathy: Review of the
cellular and molecular mechanisms involved and their implications for
human risk assessment. Environmental health perspectives, 101
(Suppl. 6):39-44.
Swenberg JA, Short B, Borghoff S, Strasser J, Charbonneau M (1989) The
comparative pathobiology of alpha2µ-globulin nephropathy.
Toxicology and applied pharmacology, 97:35-46.
Swenberg JA, Dietrich DR, McClain RM, Cohen SM (1992) Species-specific
mechanisms of carcinogenesis. In: Vaino H, Magee PN, McGregor DB,
McMichael AJ, eds. Mechanisms of carcinogenesis in risk
identification. Lyon, International Agency for Research on Cancer,
pp. 477-500 (IARC Monograph No. 116).
Tsuji M, Fujisaki Y, Yamachika K, Nakagami K, Fujisaka F, Mito M, Aoki
T, Kinoshita S, Okubo A, Watanabe I (1974) Studies on d-limonene, as
gallstone solubilizer (I): General pharmacological studies. (Japan)
Oyo Yakuri, 8:1439-1459.
Tsuji M, Fujisaki Y, Arikawa Y, Masuda S, Tanaka T, Sato K, Noda K,
Ide H, Kikuchi M (1975a) Studies on d-limonene, as gallstone
solubilizer (IV): Chronic toxicity in dogs. (Japan) Oyo Yakuri,
9:775-808.
Tsuji M, Fujisaki Y, Okubo A, Arikawa Y, Noda K, Ide H, Ikeda T
(1975b) Studies on d-limonene, as gallstone solubilizer (V): Effects
on development of rat fetuses and offspring. (Japan) Oyo Yakuri,
10:179-186.
US EPA (1990a) Terpene hazard assessment. US Environmental
Protection Agency, Office of Toxic Substances.
US EPA (1990b) Toxicity of eight terpenes to fathead minnows
(Pimephales promelas), daphnids (Daphnia magna), and algae
(Selenastrum capricornutum). AScI Corporation and US Environmental
Protection Agency, Environmental Research Laboratory-Duluth.
US EPA (1991) Alpha-2µ-globulin: Association with chemically
induced renal toxicity and neoplasia in the male rat. Washington,
DC, US Environmental Protection Agency, Risk Assessment Forum
(EPA-625-3-91-019F).
US EPA (1994) Reregistration Eligibility Decision (RED) Limonene. US
Environmental Protection Agency, Prevention, Pesticides and Toxic
Substances (Report No. EPA-738-R-94-034).
Venkataramani ES, Ahlert RC (1984) Rapid aerobic biostabilization of
high-strength industrial landfill leachate. Journal of the Water
Pollution Control Federation, 56(11):1178-1184.
von Schäfer R, Schäfer W (1982) Die perkutane Resorption verschiedener
Terpene-Menthol, Campher, Limonen, Isobornylacetat, alpha-pinen-aus
badezusätzen. Drug research, 32:56-58.
Waggott A (1981) Trace organic substances in the river Lee. In: Cooper
WJ, ed. Chemistry in water reuse. Ann Arbor, MI, Ann Arbor Science
Publ., pp. 55-99.
Wallace L, Nelson W, Zeigenfus R, Pellizzari E, Michael L, Whitmore R,
Zelon H, Hartwell T, Perritt R, Westerdahl D (1991) The Los Angeles
Team study: Personal exposures, indoor-outdoor air concentrations, and
breath concentrations of 25 volatile compounds. Journal of exposure
analysis and environmental epidemiology, 2:157-192.
Watabe T, Hiratsuka A, Osawa N, Isobe M (1981) A comparative study on
the metabolism of d-limonene and 4-vinylcyclohex-1-ene by hepatic
microsomes. Xenobiotica, 11:333-344.
Webb DR, Ridder GM, Alden CL (1989) Acute and subchronic
nephrotoxicity of d-limonene in Fischer 344 rats. Food and
chemical toxicology, 27:639-649.
Webb DR, Kanerva LL, Hysell DK, Alden CL, Lehman-McKeeman LD (1990)
Assessment of the subchronic oral toxicity of d-limonene in dogs.
Food and chemical toxicology, 28:669-675.
Wilson D, Hrutfiord B (1975) The fate of turpentine in aerated
lagoons. Pulp and paper magazine of Canada, 76(6):91-93.
Wilt FM, Miller GC, Everett RL (1988) Monoterpene concentrations in
litter and soil of single-leaf pinyon woodlands of the Western Great
Basin. The Great Basin naturalist, 48(2):228-231.
Winer AM, Lloyd AC, Darnall KR, Pitts JN Jr (1976) Relative rate
constants for the reaction of the hydroxyl radical with selected
ketones, chlorethenes, and monoterpene hydrocarbons. Journal of
physical chemistry, 80(14):1635-1639.
Winer AM, Atkinson R, Pitts JN Jr (1984) Gaseous nitrate radical:
Possible nighttime atmospheric sink for biogenic organic compounds.
Science, 224:156-159.
York M, Basketter Cuthbert JA, Neilson L (1995) Skin irritation in man
for hazard assessment - evaluation of four patch systems. Human and
experimental toxicology, 14:729-734.
Young PJ, Parker A (1983) The identification and possible
environmental impact of trace gases and vapours in landfill gas.
Waste management research, 1:231-226.
Young PJ, Parker A (1984) Vapors, odors, and toxic gases from
landfills. In: Jackson LP, Rohlik AR, Conway RA, eds. Hazardous
and industrial waste management and testing: Third symposium.
Philadelphia, PA, American Society for Testing and Materials, pp.
24-41 (ASTM Special Technical Publication 851).
Zürcher F, Giger W (1976) Flüchtige organiche spurenkomponenten in der
Glatt. Vom Wasser, 47:37-55 (in German with an English summary).
APPENDIX 1 - SOURCE DOCUMENT
Karlberg A-T, Lindell B (1993) Limonene. In: Beije B, Lundberg P, eds.
Criteria documents from the Nordic Expert Group 1993. Solna,
National Institute of Occupational Health, Nordic Council of
Ministers, pp. 207-246 (Arbete och Hälsa 35).
Copies of the Arbete och Hälsa document on limonene (ISSN:
0346-7821; ISBN: 91-7045-240-7), prepared by the Nordic Expert Group,
may be obtained from:
National Institute for Working Life
Publications Department
S-171 84 Solna
Sweden
In the peer review procedure of documents prepared in the series
Criteria documents from the Nordic Expert Group (focused on human
health effects only), one member of the Nordic Expert Group serves as
primary reviewer for the first draft. A second draft is forwarded to
all members of the Nordic Expert Group, who in turn consult
appropriate specialists to review the document. The specialists are
chosen either because they have an extended knowledge of the substance
itself or because they are specialists in the critical effect area of
the substance evaluated. The second review is performed by a review
board, including the Nordic Expert Group participants with the ad
hoc specialists. After revision, the document is checked again by
the members of the Nordic Expert Group and the ad hoc experts for
further comments. The review board meeting is repeated if necessary.
APPENDIX 2 - CICAD PEER REVIEW
The draft CICAD on limonene was sent for review to institutions
and organizations identified by IPCS after contact with IPCS national
Contact Points and Participating Institutions, as well as to
identified experts. Comments were received from:
Department of Health, London, United Kingdom
Department of Public Health, Albert Szent-Gyorgyi University
Medical School, Szeged, Hungary
Direccion General de Salud Ambiental, Subsecretario de Regulacion
y Fomento, Sanitario, San Luis Potosi, Mexico
Environmental Health Directorate, Health Canada, Ottawa, Canada
International Agency for Research on Cancer, Lyon, France
Ministry of Health, National Centre of Hygiene, Medical Ecology
and Nutrition, Sofia, Bulgaria
Ministry of Health and Welfare, International Affairs Division,
Government of Japan, Tokyo, Japan
National Institute for Working Life, Solna, Sweden
National Institute of Public Health, Oslo, Norway
United States Department of Health and Human Services (National
Institute of Environmental Health Sciences)
United States Environmental Protection Agency (Office of
Pollution Prevention and Toxics; Office of Drinking Water)
APPENDIX 3 - CICAD FINAL REVIEW BOARD
Brussels, Belgium, 18-20 November 1996
Members
Dr A. Aitio, Institute of Occupational Health, Helsinki, Finland
Dr K. Bentley, Director, Environment Policy Section, Commonwealth
Department of Human Services and Health, Canberra, Australia
Mr R. Cary, Toxicology and Existing Substances Regulation Unit, Health
and Safety Executive, Merseyside, United Kingdom
Dr J. de Fouw, National Institute of Public Health and Environmental
Protection, Bilthoven, The Netherlands
Dr C. DeRosa, Director, Division of Toxicology, Agency for Toxic
Substances and Disease Registry, Atlanta, GA, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr W. Farland, Director, National Center for Environmental Assessment,
Office of Research and Development, US Environmental Protection
Agency, Washington, DC, USA (Chairperson)
Dr T.I. Fortoul, Depto. Biologia Celular y Tisular, National
University of Mexico and Environmental Health Directorate of the
Health Ministry, Mexico D.F., Mexico
Dr H. Gibb, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany
Mr J.R. Hickman, Environmental Health Directorate, Health Canada,
Ottawa, Ontario, Canada
Dr T. Lakhanisky, Head, Division of Toxicology, Institute of Hygiene
and Epidemiology, Brussels, Belgium (Vice-Chairperson)
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Sciences, Hanover,
Germany
Ms E. Meek, Head, Priority Substances Section, Environmental Health
Directorate, Health Canada, Ottawa, Ontario, Canada
Dr K. Paksy, National Institute of Occupational Health, Budapest,
Hungary
Mr D. Renshaw, Department of Health, London, United Kingdom
Dr J. Sekizawa, Division of Chemo-Bio Informatics, National Institute
of Hygienic Sciences, Tokyo, Japan
Dr H. Sterzl-Eckert, GSF-Forschungszentrum für Umwelt und Gesundheit
GmbH, Institut für Toxikologie, Oberschleissheim, Germany
Professor S. Tarkowski, Department of Environmental Health Hazards,
The Nofer Institute of Occupational Medicine, Lodz, Poland
Dr M. Wallen, National Chemicals Inspectorate (KEMI), Solna, Sweden
Observers
Professor F.M.C. Carpanini,1 Director, Centre for Ecotoxicology and
Toxicology of Chemicals (ECETOC), Brussels, Belgium
Mr R. Haigh,1 Head of Unit, Health and Safety Directorate, European
Commission, Luxembourg
Mr B.U. Hildebrandt, Federal Ministry for the Environment, Nature
Conservation and Nuclear Safety, Bonn, Germany
Mr P. Hurst,1 Chemical and Consumer Policy Officer, Conservation
Policy Division, World Wide Fund for Nature, Gland, Switzerland
Dr A. Lombard (Representative of CEFIC), ELF-ATOCHEM, Paris, France
Dr P. McCutcheon,1 Environment, Consumer Protection and Nuclear
Safety, European Commission, Brussels, Belgium
Dr R. Montaigne, Counsellor, Technical Affairs Department, European
Chemical Industry Council (CEFIC), Brussels, Belgium
Dr M. Pemberton, ICI Acrylics, Lancashire, United Kingdom
Dr A. Smith, Organisation for Economic Co-operation and Development,
Environment Division, Paris, France
1 Invited but unable to attend.
Secretariat
Dr M. Baril, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Dr L. Harrison, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr M. Mercier, Director, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr P. Toft, Associate Director, International Programme on Chemical
Safety, World Health Organization, Geneva, Switzerland
RÉSUMÉ D'ORIENTATION
Les informations contenues dans le présent CICAD (document
international succinct sur l'évaluation des risques chimiques) sur le
limonène (d-limonène, l-limonène et d/l limonène) proviennent
principalement d'une étude effectuée en 1993 pour le Nordic Expert
Group (Karlberg & Lindell, 1993). D'autres données pour l'évaluation
du limonène ont été rassemblées à partir d'une deuxième étude menée
sous les auspices du Conseil des Ministres des Pays nordiques
(Josefsson, 1993), d'un rapport préliminaire (qui n'a pas fait l'objet
d'une évaluation par les pairs) sur la présence et les effets du
limonène dans l'environnement (US EPA, 1994), et de recherches
effectuées dans les bases de données pertinentes pour les années
1993-1995. Une dernière recherche bibliographique portant sur les
années 1996-1997 n'a pas fourni d'éléments de nature à modifier les
conclusions formulées dans le CICAD. L'appendice 1 donne des
informations sur la nature et la disponibilité des sources
documentaires. Des informations concernant l'examen par les pairs du
présent CICAD figurent à l'appendice 2. La publication de ce CICAD a
été approuvée à une réunion du Comité d'Evaluation finale qui s'est
tenue à Bruxelles (Belgique) du 18 au 20 novembre 1996. La liste des
participants à cette réunion figure à l'appendice 3. La fiche
internationale de sécurité chimique concernant le d-limonène (ICSC
0918), établie par le Programme international sur la sécurité chimique
(IPCS, 1993), est également reproduite dans le présent document.
L'accent a été mis sur le d-limonène en raison de l'abondance des
données disponibles sur cet isomère.
Le limonène se trouve à l'état naturel dans certains arbres et
arbustes. Le limonène et d'autres monoterpènes sont libérés en
grandes quantités, principalement dans l'atmosphère, à partir de
sources biologiques naturelles et de sources artificielles. Le
limonène est utilisé comme solvant dans le dégraissage des métaux
avant peinture, comme agent nettoyant dans les industries de
l'électronique et de l'imprimerie et comme solvant pour peintures. Il
est également employé comme aromatisant dans les aliments, les
produits d'entretien ménagers et les parfums.
Le limonène est un irritant de la peau, tant pour l'homme que
pour les animaux de laboratoire. Chez le lapin, le d-limonène est
un irritant oculaire. Des études effectuées sur des cobayes ont
montré que le d-limonène oxydé par l'air, mais non le d-limonène
lui-même, induisait des allergies de contact. Étant donné que le
d-limonène et le l-limonène sont des énantiomères, il pourrait en
être de même pour le l-limonène et le dipentène (mélange des deux).
Le caractère allergène du limonène pourrait donc dépendre dans une
large mesure des manipulations qu'il a subies, de sa pureté et de
l'adjonction éventuelle d'antioxygènes.
L'organe critique chez l'animal (sauf chez le rat mâle), après
administration par voie orale ou intrapéritonéale, est le foie. On
n'a pas trouvé d'études au cours desquelles des animaux auraient été
exposés au limonène par inhalation. L'exposition au limonène a des
effets sur la quantité et l'activité de diverses enzymes hépatiques,
ainsi que sur le poids du foie, la cholestérolémie et la sécrétion
biliaire. Ces effets ont été observés chez la souris, le rat et le
chien. Les données disponibles sont insuffisantes pour déterminer
quel est l'organe critique chez l'homme.
Chez le rat mâle, l'exposition au d-limonène provoque des
lésions et des tumeurs rénales. Il est admis qu'une protéine
spécifique du rat mâle, l'alpha2µ-globuline, joue un rôle crucial dans
le développement des lésions rénales néoplasiques ou non néoplasiques
chez cet animal, de sorte que leur étude n'est pas jugée pertinente
pour l'évaluation du risque chez l'homme. Le d-limonène a été
soumis à une série d'épreuves in vitro à court terme dans lesquelles
il s'est révélé non génotoxique. Il n'y a pas de preuve que le
limonène exerce des effets tératogènes ou embryotoxiques en l'absence
de toxicité maternelle. De façon générale, on peut admettre que le
d-limonène est un composé de toxicité relativement faible (si l'on
excepte ses propriétés irritantes et sensibilisantes).
D'après les données disponibles, les aliments sont la principale
source d'exposition au limonène. Une valeur guide de 0,1 mg/kg de
poids corporel par jour a été établie pour l'ingestion. D'après les
estimations actuelles concernant le niveau d'exposition, la présence
de limonène dans les aliments ne semble pas présenter un risque
significatif pour la santé humaine.
Dans l'atmosphère, le limonène et d'autres terpènes réagissent
rapidement avec les radicaux hydroxyle et nitrate résultant de
réactions photochimiques, ainsi qu'avec l'ozone. L'oxydation des
terpènes, dont le limonène, contribue à la formation d'aérosols et de
smogs photochimiques. Dans le sol, la mobilité du limonène devrait
théoriquement être faible; dans le milieu aquatique, il devrait se
lier fortement aux sédiments. Le limonène est résistant à
l'hydrolyse. Il est biodégradable en conditions d'aérobiose, mais non
en milieu anaérobie.
Pour les organismes terrestres, l'air est la voie d'exposition la
plus probable. Les quelques études effectuées sur des espèces
terrestres (des insectes) exposées aux vapeurs de limonène ont révélé
que celui-ci produisait des effets à des concentrations de l'ordre de
quelques parties par million. Les concentrations mesurées dans
l'environnement sont généralement de l'ordre de 0,1-2 ppb (0,6-
11 µg/m3). En cas de pollution, les concentrations dans le sol
peuvent dépasser les niveaux pour lesquels on constate des effets sur
les organisme vivants (par exemple, les vers de terre). En milieu
aquatique, des signes de toxicité aiguë peuvent être observés sur les
poissons et les daphnies. Les concentrations de limonène dans les
eaux de surface sont généralement bien inférieures aux doses toxiques
aiguës déterminées expérimentalement; il est donc peu probable que le
limonène présente un risque de toxicité aiguë pour les organismes
aquatiques. Aucune étude n'a été découverte sur ses effets
chroniques.
RESUMEN DE ORIENTACION
Esta reseña de la evaluación química internacional del limoneno
(d-limoneno, l-limoneno y d/ l-limoneno) se basa principalmente
en un examen preparado en 1993 para el Grupo Nórdico de Expertos
(Karlberg & Lindell, 1993). Un segundo examen efectuado bajo los
auspicios del Consejo Nórdico de Ministros (Josefsson, 1993), una
fuente de información preliminar, no revisada por expertos, sobre la
exposición y los efectos ambientales (EPA de los Estados Unidos, 1994)
y múltiples consultas en las bases de datos pertinentes sobre los años
1993 a 1995 permitieron identificar datos adicionales para evaluar el
limoneno. En una última consulta de las publicaciones aparecidas en
1996 y 1997 no se hallaron datos que modificaran las conclusiones
enunciadas en la reseña de la evaluación química internacional. En el
apéndice 1 figura información sobre la naturaleza y disponibilidad del
documento de base. En el apéndice 2 se da información sobre el examen
colegiado de la presente reseña. Esta reseña fue aprobada para su
publicación en una reunión del Comité de Revisión Final celebrada en
Bruselas (Bélgica) del 18 al 20 de noviembre de 1996. Los
participantes en la reunión figuran en el apéndice 3. Se ha
reproducido asimismo la ficha internacional de seguridad química (ICSC
0918) del d-limoneno, elaborada por el Programa Internacional de
Seguridad de las Sustancias Químicas (IPCS, 1993). La atención
especial prestada al d-limoneno obedece a la gran cantidad de datos
disponibles sobre esta forma isomérica.
El limoneno está presente en forma natural en algunos árboles y
arbustos. Fuentes biógenas y antropógenas liberan limoneno y otros
monoterpenos en grandes cantidades, principalmente en la atmósfera.
El limoneno se utiliza como disolvente para desengrasar los metales
antes de la pintura industrial, para la limpieza en la industria
electrónica y de la imprenta, y como disolvente en la pintura. Se
emplea asimismo como aditivo de sabor y aroma en alimentos, productos
de limpieza de uso doméstico y perfumes.
El limoneno es un irritante de la piel, tanto en animales de
experimentación como en seres humanos. Se ha comprobado que en los
conejos el d-limoneno irrita los ojos. Estudios efectuados con
cobayos han revelado que el d-limoneno oxidado por el aire, pero no
propiamente el d-limoneno, produce dermatitis de contacto. Como el
d-limoneno y el l-limoneno son enantiómeros, lo anterior podría
ser cierto también para el l-limoneno y el dipenteno (la forma
racémica). La manipulación y la pureza de la sustancia química, y
posiblemente la adición de antioxidantes, pueden influir por tanto de
manera crucial en la alergenicidad del limoneno.
El órgano crítico en los animales (excepto en las ratas macho),
tras la administración oral o intraperitoneal de la sustancia, es el
hígado. No se sabe de estudios en que los animales de experimentación
hayan sido expuestos al limoneno por inhalación. La exposición al
limoneno altera la cantidad y la actividad de distintas enzimas
hepáticas, el peso del hígado, los niveles de colesterol y la
secreción biliar. Se han observado esos cambios en ratones, ratas y
perros. No se dispone de suficientes datos para determinar cuál es el
órgano crítico en el ser humano.
En las ratas macho, la exposición al d-limoneno causa lesiones
y tumores renales. Se cree que una proteína específica de la rata
macho, la alpha2µ-globulina, cumple una función crucial en el
desarrollo de lesiones renales tanto neoplásicas como no neoplásicas.
Por consiguiente, esas lesiones renales se consideran sin importancia
para la evaluación del riesgo en el ser humano. El d-limoneno se
estudió en una batería de pruebas in vitro de corta duración
demostrándose que no es genotóxico. No hay constancia de que el
limoneno tenga efectos teratogénicos o embriotóxicos en ausencia de
toxicidad materna. En general, el d-limoneno puede considerarse una
sustancia química con una toxicidad bastante baja (salvo por sus
propiedades irritantes y sensibilizantes).
Según los datos de que se dispone, los alimentos son la principal
fuente de exposición al limoneno. El valor de orientación calculado
para la ingestión de limoneno es de 0,1 mg por kg de peso corporal
diarios. A los actuales niveles de exposición estimados, el limoneno
presente en los productos alimenticios no parece constituir un riesgo
significativo para la salud humana.
En la atmósfera, el limoneno y otros terpenos reaccionan
rápidamente con los radicales hidroxilo y nitrato y con el ozono
producidos fotoquímicamente. La oxidación de los terpenos como el
limoneno contribuye a la formación de aerosoles y de niebla
fotoquímica. En principio, en el suelo el limoneno tiene poca
movilidad, y en el medio acuático se une fuertemente al sedimento. El
limoneno es resistente a la hidrólisis. La biodegradación se produce
en condiciones aerobias, pero no en condiciones anaerobias.
La vía de exposición al limoneno más probable en los organismos
terrestres es el aire. Los pocos estudios realizados sobre especies
terrestres (esto es, insectos) mediante exposición al vapor han
revelado efectos en niveles de partes por millón. Las concentraciones
ambientales medidas oscilan generalmente entre 0,1 y 2 ppmm (0,6-11
µg/m3). En lugares contaminados, las concentraciones de limoneno en
el suelo pueden sobrepasar los niveles por encima de los cuales
aparecen efectos en los organismos que viven en el suelo (por ejemplo,
los gusanos). En el medio acuático, el limoneno presenta una elevada
toxicidad aguda para los peces y para Daphnia. Las concentraciones
de limoneno en aguas superficiales suelen ser mucho más bajas que los
niveles de toxicidad aguda determinados experimentalmente, por lo que
es probable que el limoneno plantee un bajo riesgo de efectos tóxicos
agudos para los organismos acuáticos. No se conocen estudios sobre
los efectos crónicos.
 1. EXECUTIVE SUMMARY
This CICAD on limonene (d-limonene, l-limonene, and
d/l-limonene) was based primarily on a review prepared in 1993 for
the Nordic Expert Group (Karlberg & Lindell, 1993). A second review
produced under the auspices of the Nordic Council of Ministers
(Josefsson, 1993), a preliminary, non-peer-reviewed information source
on environmental exposure and effects (US EPA, 1994), and searches of
relevant databases covering the years 1993-1995 were used for the
identification of additional data for the assessment of limonene. In
a final search of the literature from 1996 to 1997, no data that would
change the conclusions made in the CICAD were identified. Information
concerning the nature and availability of the source document is
presented in Appendix 1. Information on the peer review of this CICAD
is presented in Appendix 2. This CICAD was approved for publication
at a meeting of the Final Review Board, held in Brussels, Belgium, on
18-20 November 1996. Participants at the Final Review Board meeting
are listed in Appendix 3. The International Chemical Safety Card
(ICSC 0918) for d-limonene, produced by the International Programme
on Chemical Safety (IPCS, 1993), has also been reproduced in this
document. Emphasis was given to d-limonene owing to the large
amount of available data on this isomeric form.
Limonene occurs naturally in certain trees and bushes. Limonene
and other monoterpenes are released in large amounts mainly to the
atmosphere, from both biogenic and anthropogenic sources. Limonene is
used as a solvent in degreasing metals prior to industrial painting,
for cleaning in the electronic and printing industries, and in paint
as a solvent. Limonene is also used as a flavour and fragrance
additive in food, household cleaning products, and perfumes.
Limonene is a skin irritant in both experimental animals and
humans. In rabbits, d-limonene was found to be an eye irritant.
Studies in guinea-pigs revealed that air-oxidized d-limonene, but
not d-limonene itself, induced contact allergy. Because d- and
l-limonene are enantiomers, this could also be true for l-limonene
and dipentene (the mixture). Handling and purity of the chemical, and
possibly addition of antioxidants, may thus be crucial for the
allergenic capacity of limonene.
The critical organ in animals (except for male rats), following
peroral or intraperitoneal administration, is the liver. Studies in
which experimental animals were exposed by inhalation to limonene have
not been identified. Exposure to limonene affects the amount and
activity of different liver enzymes, liver weight, cholesterol levels,
and bile flow. These changes have been observed in mice, rats, and
dogs. Available data are insufficient to determine the critical organ
in humans.
In male rats, exposure to d-limonene causes damage to the
kidneys and renal tumours. The male rat specific protein
alpha2µ-globulin is considered to play a crucial role in the
development of neoplastic as well as non-neoplastic kidney lesions.
Thus, these kidney lesions are considered not relevant for human risk
assessment. d-Limonene has been studied in a battery of short-term
in vitro tests and found to be non-genotoxic. There is no evidence
that limonene has teratogenic or embryotoxic effects in the absence of
maternal toxicity. In general, d-limonene could be considered (with
the exception of its irritative and sensitizing properties) to be a
chemical with fairly low toxicity.
Food is the principal source of exposure to limonene, based on
available data. A guidance value for the ingestion of limonene was
calculated to be 0.1 mg/kg body weight per day. At current estimated
levels of exposure, limonene in foodstuffs does not appear to
represent a significant risk to human health.
In the atmosphere, limonene and other terpenes react rapidly with
photochemically produced hydroxyl and nitrate radicals and ozone. The
oxidation of terpenes such as limonene contributes to aerosol and
photochemical smog formation. In soil, limonene is expected to have
low mobility; in the aquatic environment, it is expected to bind
strongly to sediment. Limonene is resistant to hydrolysis.
Biodegradation occurs under aerobic, but not anaerobic, conditions.
Terrestrial organisms are most likely exposed to limonene via the
air. The few studies on terrestrial species (i.e. insects) using
vapour exposure revealed effects of limonene at parts per million
levels. Measured environmental concentrations are typically around
0.1-2 ppb (0.6-11 µg/m3). At polluted sites, limonene concentrations
in soil may exceed effect levels of soil-living organisms (e.g.
earthworms). In the aquatic environment, limonene shows high acute
toxicity to fish and Daphnia. Limonene concentrations in surface
waters are generally much lower than experimentally determined acute
toxicity levels, and therefore it is likely that limonene poses a low
risk for acute toxic effects on aquatic organisms. No studies were
found on chronic effects.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
Limonene is a colourless liquid at room temperature. The
structural formula for limonene is given below. The chemical exists
as two optical isomers, d- and l-limonene, and the racemic mixture
dipentene. The purity of commercial d-limonene is about 90-98%.
Physical and chemical data on limonene presented in Table 1 were
taken from Karlberg & Lindell (1993), unless otherwise stated.
Impurities are mainly other monoterpenes, such as myrcene
(7-methyl-3-methylene-1,6-octadiene), alpha-pinene
(2,6,6-trimethyl-bicyclo[3.1.1]hept-2-ene), alpha-pinene
(6,6-dimethyl-2-methylene-bicyclo[3.1.1]heptane), sabinene
(2-methyl-5-(1-methylethyl)-bicyclo[3.1.0]hexan-2-ol), and
Gamma3-carene ((1S-cis)-3,7,7-trimethyl-bicyclo[4.1.0]hept-2-ene).
The vapour pressure of limonene is high and its solubility in water is
low, giving a high value of the Henry's law constant, which predicts a
high rate of vaporization of limonene.
1. EXECUTIVE SUMMARY
This CICAD on limonene (d-limonene, l-limonene, and
d/l-limonene) was based primarily on a review prepared in 1993 for
the Nordic Expert Group (Karlberg & Lindell, 1993). A second review
produced under the auspices of the Nordic Council of Ministers
(Josefsson, 1993), a preliminary, non-peer-reviewed information source
on environmental exposure and effects (US EPA, 1994), and searches of
relevant databases covering the years 1993-1995 were used for the
identification of additional data for the assessment of limonene. In
a final search of the literature from 1996 to 1997, no data that would
change the conclusions made in the CICAD were identified. Information
concerning the nature and availability of the source document is
presented in Appendix 1. Information on the peer review of this CICAD
is presented in Appendix 2. This CICAD was approved for publication
at a meeting of the Final Review Board, held in Brussels, Belgium, on
18-20 November 1996. Participants at the Final Review Board meeting
are listed in Appendix 3. The International Chemical Safety Card
(ICSC 0918) for d-limonene, produced by the International Programme
on Chemical Safety (IPCS, 1993), has also been reproduced in this
document. Emphasis was given to d-limonene owing to the large
amount of available data on this isomeric form.
Limonene occurs naturally in certain trees and bushes. Limonene
and other monoterpenes are released in large amounts mainly to the
atmosphere, from both biogenic and anthropogenic sources. Limonene is
used as a solvent in degreasing metals prior to industrial painting,
for cleaning in the electronic and printing industries, and in paint
as a solvent. Limonene is also used as a flavour and fragrance
additive in food, household cleaning products, and perfumes.
Limonene is a skin irritant in both experimental animals and
humans. In rabbits, d-limonene was found to be an eye irritant.
Studies in guinea-pigs revealed that air-oxidized d-limonene, but
not d-limonene itself, induced contact allergy. Because d- and
l-limonene are enantiomers, this could also be true for l-limonene
and dipentene (the mixture). Handling and purity of the chemical, and
possibly addition of antioxidants, may thus be crucial for the
allergenic capacity of limonene.
The critical organ in animals (except for male rats), following
peroral or intraperitoneal administration, is the liver. Studies in
which experimental animals were exposed by inhalation to limonene have
not been identified. Exposure to limonene affects the amount and
activity of different liver enzymes, liver weight, cholesterol levels,
and bile flow. These changes have been observed in mice, rats, and
dogs. Available data are insufficient to determine the critical organ
in humans.
In male rats, exposure to d-limonene causes damage to the
kidneys and renal tumours. The male rat specific protein
alpha2µ-globulin is considered to play a crucial role in the
development of neoplastic as well as non-neoplastic kidney lesions.
Thus, these kidney lesions are considered not relevant for human risk
assessment. d-Limonene has been studied in a battery of short-term
in vitro tests and found to be non-genotoxic. There is no evidence
that limonene has teratogenic or embryotoxic effects in the absence of
maternal toxicity. In general, d-limonene could be considered (with
the exception of its irritative and sensitizing properties) to be a
chemical with fairly low toxicity.
Food is the principal source of exposure to limonene, based on
available data. A guidance value for the ingestion of limonene was
calculated to be 0.1 mg/kg body weight per day. At current estimated
levels of exposure, limonene in foodstuffs does not appear to
represent a significant risk to human health.
In the atmosphere, limonene and other terpenes react rapidly with
photochemically produced hydroxyl and nitrate radicals and ozone. The
oxidation of terpenes such as limonene contributes to aerosol and
photochemical smog formation. In soil, limonene is expected to have
low mobility; in the aquatic environment, it is expected to bind
strongly to sediment. Limonene is resistant to hydrolysis.
Biodegradation occurs under aerobic, but not anaerobic, conditions.
Terrestrial organisms are most likely exposed to limonene via the
air. The few studies on terrestrial species (i.e. insects) using
vapour exposure revealed effects of limonene at parts per million
levels. Measured environmental concentrations are typically around
0.1-2 ppb (0.6-11 µg/m3). At polluted sites, limonene concentrations
in soil may exceed effect levels of soil-living organisms (e.g.
earthworms). In the aquatic environment, limonene shows high acute
toxicity to fish and Daphnia. Limonene concentrations in surface
waters are generally much lower than experimentally determined acute
toxicity levels, and therefore it is likely that limonene poses a low
risk for acute toxic effects on aquatic organisms. No studies were
found on chronic effects.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
Limonene is a colourless liquid at room temperature. The
structural formula for limonene is given below. The chemical exists
as two optical isomers, d- and l-limonene, and the racemic mixture
dipentene. The purity of commercial d-limonene is about 90-98%.
Physical and chemical data on limonene presented in Table 1 were
taken from Karlberg & Lindell (1993), unless otherwise stated.
Impurities are mainly other monoterpenes, such as myrcene
(7-methyl-3-methylene-1,6-octadiene), alpha-pinene
(2,6,6-trimethyl-bicyclo[3.1.1]hept-2-ene), alpha-pinene
(6,6-dimethyl-2-methylene-bicyclo[3.1.1]heptane), sabinene
(2-methyl-5-(1-methylethyl)-bicyclo[3.1.0]hexan-2-ol), and
Gamma3-carene ((1S-cis)-3,7,7-trimethyl-bicyclo[4.1.0]hept-2-ene).
The vapour pressure of limonene is high and its solubility in water is
low, giving a high value of the Henry's law constant, which predicts a
high rate of vaporization of limonene.
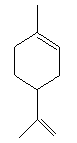 Table 1: Physical/chemical properties of limonene.a
d-Limonene l-Limonene Dipentene
CAS no.
Table 1: Physical/chemical properties of limonene.a
d-Limonene l-Limonene Dipentene
CAS no.  8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
The acute toxicity of d-limonene in rodents is fairly low after
oral, intraperitoneal, subcutaneous, and intravenous administration,
based on the magnitude of the LD50 values (Table 4). LD50 values
were approximately 5 g/kg body weight for the oral administration of
d-limonene or d/l-limonene to rats and for dermal application of
d/l-limonene to rabbits and 6 g/kg body weight for oral
administration to mice (Tsuji et al., 1974, 1975b; Opdyke, 1978).
Studies on the acute inhalation toxicity of limonene were not
identified.
Effects observed following the acute exposure of rodents to
limonene include increased bile flow at 85 mg/kg body weight (Kodama
et al., 1976), inhibition of S-3-hydroxy-3-methylglutaryl-CoA
reductase activity at 409 mg/kg body weight (Clegg et al., 1980),
enzyme induction at 600 and 1200 mg/kg body weight (Ariyoshi et al.,
1975), and decreased motor activity, hypothermia, and potentiation of
hexobarbital-induced sleep at 3 ml/kg body weight (Tsuji et al.,
1974).
8.2 Irritation and sensitization
d-Limonene is considered a skin irritant (Cronin, 1980;
Fischer, 1986). The skin irritancy of limonene in guinea-pigs (Klecak
et al., 1977) and rabbits (Lacy et al., 1987; Okabe et al., 1990) is
considered moderate and low, respectively. In an in vivo study of
rabbit skin irritation, d-limonene was ranked 3.5 of 8 on the basis
of the primary irritation index (Bagley et al., 1996); effects were
graded according to OECD Test Guideline 404 (OECD, 1993). In a study
in rabbits, d-limonene caused irritation to the eyes (Tsuji et al.,
1974).
Although d-limonene was once considered the main allergen in
citrus fruits, data from more recent studies in animals have revealed
air-oxidized d-limonene, rather than unoxidized d-limonene, to be
the sensitizing agent. When limonene (unspecified form and unknown
purity of the test material) was tested in four different
sensitization assays with guinea-pigs (Open Epicutaneous Test,
Maximization Test, Draize's Test, and a test with Freund's Complete
Adjuvant), it was sensitizing in all but Draize's Test (Klecak et al.,
1977). In another study in mice, d-limonene did not induce
sensitization (Maisey & Miller, 1986). Hydroperoxides and other
oxidation products of d-limonene formed on exposure to the air have
proved to be potent contact allergens when tested with Freund's
Complete Adjuvant in guinea-pigs, whereas unoxidized d-limonene did
not cause any sensitization (Karlberg et al., 1991, 1992).
Table 4: Acute toxicity of limonene.
Species (sex) Route of administration Type of limonene LD50 (g/kg body weight) Reference
rabbit dermal d/l >5 Opdyke, 1978
rat oral d/l 5.3 Opdyke, 1978
rat (m/f) oral d 4.4/5.1 Tsuji et al., 1975b
rat (m/f) intraperitoneal d 3.6/4.5 Tsuji et al., 1975b
rat (m/f) intravenous d 0.125/0.11 Tsuji et al., 1975b
mouse (m/f) oral d 5.6/6.6 Tsuji et al., 1975b
mouse (m/f) oral, 7 days d 5.3/6.8a Tsuji et al., 1974
mouse (m/f) intraperitoneal, 3 days d 3.1/3.0a Tsuji et al., 1974
mouse (m + f) intraperitoneal d 1.3 Tsuji et al., 1975b
mouse (m/f) intraperitoneal, 10 days d 0.59/0.50a Tsuji et al., 1974
mouse (m + f) subcutaneous d >41.5 Tsuji et al., 1975b
mouse (m + f) subcutaneous, 7 days d >21.5 Tsuji et al., 1974
a Calculated from ml/kg body weight.
8.3 Short-term exposure
Increases in hepatic cytochrome P-450 content have been observed
in female rats administered limonene (isomer unspecified; 40 mg/kg
body weight per day for 3 days) by intraperitoneal injection (Austin
et al., 1988) and in rats administered 5% d-limonene in the diet for
2 weeks (Maltzman et al., 1991). Increased epoxide hydratase activity
was observed in rats administered 1% or 5% d-limonene in the diet
for 2 weeks (Maltzman et al., 1991). Increases in phase II enzymes
(glutathionyltransferase and UDP-glucuronyltransferase) during the
exposure of rats to 5% limonene in food have also been described
(Maltzman, 1991). Increased relative liver weight (from 5 to 20
times) has been observed in rats administered d-limonene at a dose
of 75-300 mg/kg body weight; at 300 mg/kg body weight, the increase
was significant (Kanerva et al., 1987b). In cats, infusion of 97%
d-limonene into the bile system to dissolve gallstones caused acute
and chronic inflammatory changes (Schenk et al., 1980).
8.4 Long-term exposure
8.4.1 Subchronic exposure
Peroral administration of d-limonene to rats at a dose of 400
mg/kg body weight for 30 days resulted in a 20-30% increase in the
amount and activity of different liver enzymes (cytochrome P-450,
cytochrome b5, aminopyrine demethylase, and aniline hydroxylase),
increased relative liver weight, and decreased cholesterol levels
(Ariyoshi et al., 1975). Administration of d-limonene (0, 2, 5, 10,
30, and 75 mg/kg body weight per day) by gavage to groups of 10 male
rats, 5 days/week for 13 weeks (Webb et al., 1989), resulted in the
pathological formation of granular casts at the outer zone of the
renal medulla. The no-observed-effect level (NOEL), based upon
histological examination of the kidneys, was considered to be 5 mg/kg
body weight per day. The LOEL for increased liver and kidney weight
was 75 mg/kg body weight per day, the highest dose tested. The NOEL
for effects in the liver was 10 mg/kg body weight; the
no-observed-adverse-effect level (NOAEL) for effects in the liver was
30 mg/kg body weight per day. Linear regression analysis revealed a
dose-related trend in the increased relative weights of the kidney and
liver at 30 and 75 mg/kg body weight per day. No histopathological
changes were observed in the liver in these two studies. The amount
and activity of different liver enzymes were not investigated, and
thus the increase in relative liver weight may be due to enzyme
induction.
8.4.2 Chronic exposure and carcinogenicity
The oral administration of d-limonene (0.4, 1.2, or 3.6 ml/kg
body weight per day) to dogs for 6 months caused nausea and vomiting
(Tsuji et al., 1975a). A 35% increase in alkaline phosphatase and
cholesterol in serum and slightly increased total and relative liver
weights were observed in dogs after peroral administration of
d-limonene at a dose of 1.2 ml/kg body weight per day for 6 months
(about 1000 mg/kg body weight per day) (Webb et al., 1990).
In a 2-year study, d-limonene was administered (per os) 5
days/week to groups of 50 F344/N rats (0, 75, or 150 mg/kg body weight
per day to males, and 0, 300, or 600 mg/kg body weight per day to
females) and B6C3F1 mice (0, 250, or 500 mg/kg body weight per day to
males, and 0, 500, or 1000 mg/kg body weight per day to females) (NTP,
1990). Slightly lower body weights were observed for rats in the
high-dose groups and female mice in the high-dose group; however, no
clinical symptoms could be related to the administration of
d-limonene. For female rats in the high-dose group, survival was
reduced after 39 weeks (NTP, 1990). There was clear evidence of
carcinogenic activity of d-limonene in male rats, based upon a
dose-related increase in the incidence of hyperplasia and adenoma/
adenocarcinoma in renal tubular cells. However, there was no evidence
of carcinogenicity in female rats or in male and female mice. The
carcinogenic response in the kidney of male rats has been linked to a
unique renal perturbation involving alpha2µ-globulin.
To determine whether d-limonene would cause a sustained
increase in renal cell proliferation and exhibit promoting activity
for the development of renal adenomas in male F344 rats, the animals
were administered (by stomach tube) d-limonene (150 mg/kg body
weight per day) as a promoter 5 days/week for 30 weeks (Dietrich &
Swenberg, 1991). N-ethyl- N-hydroxyethylnitrosamine (500 ppm) was
used as an initiator in the drinking-water for 2 weeks. In addition,
male alpha2µ-globulin-deficient rats were exposed in the same manner
to determine if the male rat specific urinary protein alpha2µ-globulin
is required for d-limonene to cause these effects. Exposure to
d-limonene alone caused a significant increase in the number of
atypical tubules and atypical hyperplasias in F344 rats, compared with
vehicle controls. There was no increase in the incidence of tumours
or preneoplastic lesions in the alpha2µ-globulin-deficient rats
exposed to d-limonene, whereas a 10-fold increase in the incidence
of renal adenoma and atypical hyperplasia was observed in F344 rats
exposed to d-limonene, compared with controls. There was a
significant decrease in the incidence of liver tumours in animals
exposed to N-ethyl- N-hydroxyethylnitrosamine and d-limonene,
compared with N-ethyl- N-hydroxyethylnitrosamine exposure alone.
8.5 Genotoxicity and related end-points
On the basis of available data, there is no evidence that
d-limonene or its metabolites are genotoxic or mutagenic. Limonene
and its epoxides were not mutagenic when tested at concentrations of
0.3-3333 µg/plate in in vitro assays using different strains of
Salmonella typhimurium, in the presence or absence of metabolic
activation (Florin et al., 1980; Watabe et al., 1981; Haworth et al.,
1983; Connor et al., 1985; NTP, 1990). d-Limonene did not increase
the frequency of forward mutation at the TK+/- locus in mouse L5178Y
cells (NTP, 1990), induce cytogenetic damage in Chinese hamster ovary
cells (Anderson et al., 1990), or malignantly transform Syrian hamster
embryo cells (Pienta, 1980). In one in vitro study, following
exposure with benzo (a)pyrene, d-limonene (21.9 µmol/litre)
inhibited the formation of transformed cell colonies in tracheal
epithelium isolated from rats (Steele et al., 1990).
No evidence of mutagenicity was reported in an in vivo spot
test with mice, involving the intraperitoneal injection of limonene at
215 mg/kg body weight per day on days 9-11 during gestation (Fahrig,
1984).
8.6 Reproductive and developmental toxicity
Studies on the reproductive toxicity of limonene were not
identified. There is no evidence that limonene has teratogenic or
embryotoxic effects in the absence of maternal toxicity. In rats, the
oral administration of d-limonene (2869 mg/kg body weight per day)
on days 9-15 of gestation resulted in decreased body weight and deaths
among the dams. Delayed ossification and decreased total body and
organ weights (thymus, spleen, and ovary) were observed in the
offspring (Tsuji et al., 1975b). In mice, the oral administration of
d-limonene (2869 mg/kg body weight per day) on days 7-12 of
gestation resulted in reduced growth in the mothers and a
significantly increased incidence of skeletal anomalies and delayed
ossification in the offspring (Kodama et al., 1977a). The oral
administration of d-limonene (250, 500, or 1000 mg/kg body weight
per day) to rabbits on days 6-18 of gestation had no dose-related
effects on the offspring. At the highest dose, there were some deaths
and reduced weight gain among the dams; at the intermediate dose,
growth was decreased (Kodama et al., 1977b).
8.7 Immunological and neurological effects
Reports relating limonene to type I allergy were not identified.
In a study designed to assess the immunological effects of
d-limonene on B- and T-cell responses, BALB/c mice were administered
(by forced intragastric feeding) d-limonene (0.1 ml) daily for 9
weeks (Evans et al., 1987). Mice given keyhole limpet haemocyanin
prior to exposure to d-limonene had suppressed primary and secondary
anti-keyhole limpet haemocyanin responses. Mice exposed to
d-limonene prior to the administration of keyhole limpet haemocyanin
had significantly increased antibody and mitogen-induced proliferative
responses. However, the purity of the d-limonene in this study was
not checked, and oxidation products may have been the active
substances.
Effects on the central nervous system following exposure to
limonene have been reported in experimental studies with animals;
however, it is difficult to ascertain whether these effects were the
result of general intoxication or a more direct effect of the
chemical. The peroral administration of d-limonene (3 ml) to rats
and mice resulted in decreased motor activity (Tsuji et al., 1974). A
similar effect was also observed in mice orally administered a
limonene dose of 1000 mg/kg body weight per day for 13 weeks (NTP,
1990).
9. EFFECTS ON HUMANS
Case reports or epidemiological studies on the effects of
limonene on human health were not identified. Available data have
been derived from studies with volunteers. In older investigations,
multiple exposures and confounding factors such as mechanical damage,
irritation, other allergens, and infections due to wet work (Beerman
et al., 1938; Schwartz, 1938; Birmingham et al., 1951) may have
contributed to the effects reported following exposure to limonene.
None of eight subjects reported any discomfort, irritation, or
symptoms related to central nervous system effects during a 2-hour
inhalation exposure to d-limonene at 10, 225, or 450 mg/m3;
however, a slight decline in vital capacity was observed following
exposure to the highest concentration (Falk Filipsson et al., 1993).
In a study in which the sensitivity of four patch testing systems
(Finn chamber, Hill Top patch, Van der Bend chamber, and Webril patch)
was evaluated in volunteers, d-limonene (perfume-grade) reacted
strongly in all types of patches within 10-15 minutes of exposure
(York et al., 1995). Skin irritation was assessed before application,
as well as immediately and 1, 24, 48, and 72 hours after removal of
the patch, using a scoring system based broadly on that used for
rabbit irritation studies (OECD, 1993), but modified to account for
the nature of reactions on human skin. There was evidence of sensory
effects and urticarial responses on removal of the patches.
Significant irritation persisted for 24 hours, and these reactions
persisted for 48 and 72 hours in many volunteers (York et al., 1995).
Dermal exposure to d-limonene (98%) for 2 hours in one subject
caused burning, itching, aching, and a long-lasting purpuric rash
(Falk et al., 1991).
d-Limonene infused directly into the bile system of human
volunteers to dissolve gallstones caused pain in the upper abdomen,
nausea, vomiting, and diarrhoea, as well as increases in serum
aminotransferases and alkaline phosphatase (Igimi et al., 1976, 1991).
The oral administration of 20 g d-limonene to volunteers resulted in
diarrhoea, painful constrictions, and proteinuria, but no biochemical
changes (total protein, bilirubin, cholesterol, aspartate
aminotransferase, alanine aminotransferase, alkaline phosphatase) in
the liver (Igimi et al., 1976). Reports of contact allergy to
dipentene have appeared (Calnan, 1979; Rycroft, 1980). In one
investigation, 15 of 22 people with an allergy to oil of turpentine
also reacted to dipentene (Cachao et al., 1986). Patch testing in
consecutive dermatitis patients from Sweden and Belgium revealed
positive reactions in 1.5-2% of the subjects tested with oxidized
d-limonene, a finding similar to that observed with other common
sensitizers, such as formaldehyde (A.-T. Karlberg, personal
communication, 1996). d-Limonene reduced non-immunological contact
urticaria caused by cinnamic aldehyde, with competitive receptor
inhibition suggested as the mechanism of suppression (Guin et al.,
1984). No sensitizing effect was observed when 25 volunteers were
exposed to d-limonene in a Human Maximization Test (Grief, 1967).
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10.1 Aquatic environment
The acute toxicity of d-limonene ranges from slight to high for
aquatic organisms (Table 5). The lowest acute toxicity values (EC50
or LC50) identified were approximately 0.4 mg/litre for Daphnia (US
EPA, 1990b) and 0.7 mg/litre for fish (US EPA, 1990a,b). The
no-observed-effect concentration (NOEC) for green algae is
approximately 4 mg/litre (US EPA, 1990a). The acute toxicity (EC50
or LC50) of dipentene to Daphnia and fish is about 50-70 times
lower than that for d-limonene (US EPA, 1990b). No studies were
identified on the chronic toxicity of limonene to aquatic organisms.
10.2 Terrestrial environment
The toxicity of limonene has been studied in various terrestrial
organisms (Table 6). Limonene generally has moderate acute toxicity
in insects and mites. The acute toxicity of d-limonene to
earthworms (Eisenia foetida Savigny) was high (LC50 = 6.0 ppm;
mg/kg) (Karr et al., 1990). Sublethal effects (i.e. abnormal
rebounding of medial giant fibre pathway [MGF] impulses and
spontaneous lateral giant fibre pathway [LGF] spiking) were observed
following exposure of earthworms to 4.2 ppm (mg/kg) limonene (Karr et
al., 1990). Limonene has low subacute toxicity to bobwhite quail
(Colinus virginianus) exposed via the diet (LC50 > 5620 ppm;
mg/kg) (US EPA, 1994).
Table 5: Toxicity of limonene to aquatic organisms.
Species End-point; exposure Results (mg/litre) Reference
Algae
Green algaea 96-h NOEC; static 4.08 US EPA, 1990a
Crustaceans
Water flea (Daphnia magna)b 48-h LC50; flow-through 0.577 (0.496-0.672) US EPA, 1990b
48-h EC50; flow-through 0.421
Water flea (D. magna)c acute LC50 39 ppm US EPA, 1994
Water flea (D. magna)a 48-h LC50; flow-through 31 (27.5-34.8) US EPA, 1990b
48-h EC50; flow-through 28.2
Water flea (Daphnia pulex)b 48-h EC50; flow-through 0.730 US EPA, 1990a
Water flea (D. pulex)c 48-h EC50; static 69.6 Passino & Smith, 1987
Daphniab 21-d NOEC; structure-activity 0.15 US EPA, 1990a
relationship (SAR) analysis
Fish
Fathead minnow (Pimephales promelas)b 96-h LC50; flow-through 0.702 (0.619-0.796) US EPA, 1990b
Fathead minnow (P. promelas)b 96-h LC50; flow-through 0.720 (0.618-0.839) US EPA, 1990b
96-h EC50; flow-through 0.688 (0.606-0.782)
Fathead minnow (P. promelas)a 96-h LC50; flow-through 38.5 (35.4-41.8) US EPA, 1990a,b
96-h EC50; flow-through 28.2
Fishc acute LC50 80 ppm US EPA, 1994
Fishb 96-h LC50; flow-through 0.711 US EPA, 1990a
Golden orfe (Leuciscus idus)a 48-h LC50 32 Roth, 1990
Table 5 (continued)
Species End-point; exposure Results (mg/litre) Reference
Insects
Water hyacinth weevil (Neochetina Mortality (73%, range 40-100%), 50% limonene Haag, 1986
eichhorniae, 60%, N. bruchi, 40%)b weevils were dipped in limonene
Mosquito fly (Culex quinquefasciatus)c 2nd-instar larvae (23-33°C), 6.6-26.1 ppm Mohsen et al., 1989
72-h LC50; static
4th-instar larvae (23-33°C), 7.8-30.6 ppm Mohsen et al., 1989
72-h LC50; static
a d/l-Limonene.
b d-Limonene.
c Optical isomer not specified.
Table 6: Toxicity of limonene to terrestrial organisms.
Species End-point; exposure Results Reference
Insects
Cat flea Adult LD50; contact 160 (157-163) µg/cm2 Hink & Fee, 1986
(Ctenocephalides felis)a,b Adult LD50; vapour 259 (234-281) µg/cm2
Pupae LD50; contact 376 (259-468) µg/cm2
Larvae LD50; contact 226 (221-231) µg/cm2
Eggs; lethal to all eggs; contact 65 µg/cm2
Variegated cutworm Larvae; significant inhibition of pupation; dietary
(Peridroma saucia)b exposure 0.2% limonene in artificial feed Harwood et al., 1990
German cockroach Adult 24-h LD50; topical 700 (610-810) µg/insect Karr & Coats, 1988
(Blattella germanica L.)b Adult 24-h LC50; fumigation 23.3 (17.5-31.0) ppm
Adult; no mortality; oral 25% limonene in feed
Nymph; no mortality; oral 25% limonene in feed
Adult; no mortality; 72-h contact with treated limonene (conc. not given)
surface
German cockroach Effect on growth rate; diet 1-25% limonene in diet Karr & Coats, 1992
(B. germanica L.)b EC50, oothecae yielding young; topical exposure 0.68 mg/ootheca
No effect on reproduction; via diet 25% limonene in diet
Topical exposure 0.84 mg/cockroach
Vapour exposure 5 mg/litre in air
Rice weevil (Sitophilus Adult 24-h LC50; fumigation 19.0 (13.2-27.3) ppm Karr & Coats, 1988
oryzae L.)b
House fly (Musca 25-h LD50; topical 90 (70-130) µg/insect Karr & Coats, 1988
domestica L.)b
Western corn rootworm Egg 72-h LC50; contact with treated substrate 1.8 (0.8-2.9)% limonene Karr & Coats, 1988
(Diabrotica virgifera Larvae 72-h LC50; contact with treated soil 12.2 (4.5-32.6) ppm
virgifera LeConte)b
Table 6 (continued)
Species End-point; exposure Results Reference
Spiders and allies
Spruce spider mite 24-h LC50; vapour 24.5 ppm Cook, 1992
(Oligonychus ununguis Significant decrease in oviposition 5 ppm
(Jacobi)),c adult female
Segmented worms
Earthworm (Eisenia 48-h LC50 6.0 (5.1-7.1) ppm Karr et al., 1990
foetida Savigny)b Sublethal effects 4.2 ppm
Birds
Bobwhite quail (Colinus Subacute LC50; dietary exposure >5 620 ppm US EPA, 1994
virginianus)d
a Fleas were exposed to filter papers treated with limonene, either directly or to vapours from the filter papers.
b d-Limonene.
c l-Limonene.
d Optical isomers not specified.
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
Limonene is a skin irritant in experimental animals and humans.
d-Limonene is an eye irritant in rabbits. Studies in guinea-pigs
have revealed that air-oxidized d-limonene, but not d-limonene
itself, induced contact allergy. Similar results are likely with
l-limonene and dipentene.
The critical organ in animals (except for male rats) following
peroral or intraperitoneal administration is the liver. Exposure to
limonene affects the amount and activity of different liver enzymes,
liver weight, cholesterol levels, and bile flow, with effects having
been observed in mice, rats, and dogs. In male rats, exposure to
d-limonene results in damage to the kidneys and an increased
incidence of renal tumours. As the male rat specific protein
alpha2µ-globulin is considered to play a crucial role in the
development of the neoplastic and non-neoplastic kidney lesions, they
are considered not relevant for human risk assessment.
A dose-related nephropathy was observed in the kidneys of male
rats after oral administration of d-limonene (NTP, 1990). This
lesion, consisting of degeneration of epithelial cells in the
convoluted tubules, granular casts in the outer stripe of the outer
medulla, and epithelial regeneration, is characteristic of hyaline
droplet nephropathy associated with the accumulation of
alpha2µ-globulin in the cytoplasm of tubular cells (Alden et al.,
1984; Halder et al., 1985) in response to a variety of hydrocarbon
compounds (Swenberg et al., 1992). Some compounds fit deeply into a
hydrophobic pocket of alpha2µ-globulin. When hydrogen bonding between
the chemical and protein occurs, the digestibility of alpha2µ-globulin
by proteases is inhibited, leading to accumulation of the male rat
specific protein in lysosomes of the P2 segment of the nephron
(Lehman-McKeeman et al., 1990). Although such chemicals fall into
rather diverse classes, molecular modelling studies have demonstrated
a strong structure-activity relationship with respect to
alpha2µ-globulin binding (Borghoff et al., 1991). The accumulation of
alpha2µ-globulin is cytotoxic, resulting in single-cell necrosis
(Dietrich & Swenberg, 1991). The exfoliated renal epithelium is
restored by compensatory cell proliferation. The increase in cell
proliferation associated with alpha2µ-globulin is reversible. Damage
of this type has not been observed in female rats, male rats that do
not produce alpha2µ-globulin, or other mammals, such as mice,
hamsters, guinea-pigs, dogs, and monkeys (Alden, 1986; Kanerva &
Alden, 1987a; Swenberg et al., 1989; Webb et al., 1989, 1990; NTP,
1990; Ridder et al., 1990; Dietrich & Swenberg, 1991). The processes
leading to nephropathy and the development of renal cancer by such
compounds are among the best understood for non-genotoxic chemicals
and strongly indicate that it is a male rat specific process. Acute
and chronic renal effects induced in male rats by limonene will be
unlikely to occur in any species not producing alpha2µ-globulin or a
very closely related protein in the large quantities typically seen in
the male rat (US EPA, 1991; Swenberg, 1993).
d-Limonene has been studied in a variety of short-term
in vitro tests and has been found to be non-genotoxic. There is no
evidence that limonene has teratogenic or embryotoxic effects in the
absence of maternal toxicity.
11.1.2 Criteria for setting guidance values for limonene
In numerous experimental studies, exposure to limonene has been
shown to affect the liver. Owing to a lack of data on d-limonene
exposure in humans, this organ cannot with certainty be stated as the
critical organ in humans. Based on available data, food is believed
to be the principal source of exposure (96%) to limonene; the
contribution from ambient air is approximately 4%. The dermal uptake
of limonene has not been estimated.
To calculate a tolerable intake for humans, the animal study was
chosen in which effects on the liver were observed at the lowest
exposure level (Webb et al., 1989). In this study, gavage
administration of d-limonene (5 days/week for 13 weeks) to rats
caused increased relative liver weight at 30 and 75 mg/kg body weight
per day. The NOEL for the liver was considered to be 10 mg/kg body
weight per day. Using uncertainty factors of 10 for intraspecies
differences and 10 for interspecies differences, a tolerable intake
for ingestion of d-limonene by humans of 0.1 mg/kg body weight per
day may be calculated from the NOEL. A guidance value for inhalation
exposure to d-limonene was not developed, as inhalation is an
insignificant route of exposure compared with ingestion.
11.1.3 Sample risk characterization
Exposure estimates vary as a function of use patterns, and the
risk characterization presented here is provided only as an example,
primarily for illustrative purposes. In general, d-limonene could
be considered (with the exception of its irritative and sensitizing
properties) to be a chemical with fairly low toxicity. The calculated
tolerable intake of 0.1 mg/kg body weight per day is of a similar
magnitude as the estimated daily US consumption of d-limonene of
0.27 mg/kg body weight per day (Flavor and Extract Manufacturers
Association, 1991).
11.2 Evaluation of environmental effects
Limonene and other terpenes are released in large amounts mainly
to the atmosphere. When released to soil or water, limonene is
expected to evaporate to air to a significant extent, owing to its
high volatility. Thus, the atmosphere is the predominant
environmental sink of limonene, where it is expected to rapidly
undergo gas-phase reactions with photochemically produced hydroxyl
radicals, ozone, and nitrate radicals. The oxidation of terpenes,
such as limonene, contributes to aerosol and photochemical smog
formation. Ozonolysis of limonene may also lead to the formation of
hydrogen peroxide and organic peroxides, which have various toxic
effects on plant cells and may be part of the damage to forests
observed in the last decades (Peters et al., 1994). Emissions of
biogenic hydrocarbons such as limonene and other terpenes to the
atmosphere may either decrease ozone concentrations when nitrogen
oxide concentrations are low or, if emissions take place in polluted
air (i.e. containing high nitrogen oxide levels), lead to an increase
in ozone concentrations.
Terrestrial organisms are most likely to be exposed to limonene
via the air. The few studies on terrestrial species (i.e. insects)
using vapour exposure reveal effects of limonene at parts per million
levels. Measured environmental concentrations are typically around
0.1-2 ppb (0.6-11 µg/m3), indicating a low risk for acute toxic
effects on terrestrial organisms from direct exposure to limonene in
air. At polluted sites, limonene concentrations in soil (up to 920
mg/kg soil) may exceed effect levels of soil-living organisms (e.g.
earthworm, acute LC50 = 6.0 ppm; mg/kg).
In the aquatic environment, limonene exhibits high acute toxicity
to fish and Daphnia. It may also bioaccumulate. The lowest acute
toxicity value identified was 0.4 mg/litre (48-hour EC50 for
Daphnia). Because concentrations of limonene in surface waters of
"polluted" and "unpolluted" areas are at least about 250 and 20 000
times lower than this acute toxicity value, respectively, it is likely
that limonene poses a low risk for acute toxic effects on aquatic
organisms. No studies were identified on chronic effects, and
therefore risks associated with chronic exposures of aquatic organisms
to limonene in "polluted" waters cannot be determined.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The International Agency for Research on Cancer (IARC, 1993) has
classified d-limonene in Group 3 (not classifiable as to its
carcinogenicity to humans) based on a lack of available data on
carcinogenicity to humans and limited evidence for carcinogenicity in
experimental animals.
The 41st meeting of the Joint FAO/WHO Expert Committee on Food
Additives (JECFA, 1993b) withdrew the existing acceptable daily intake
for d-limonene of 0-1.5 mg/kg body weight per day (JECFA, 1993a) and
in its place allocated "not specified." On the basis of the available
data, the total daily intake of the chemical arising from its use at
the levels necessary to achieve the desired effect and from its
acceptable background levels in food did not, in the opinion of the
Committee, represent a health hazard. For that reason, and for the
reasons stated in the individual evaluations, the establishment of an
acceptable daily intake expressed in numerical form was not deemed
necessary.
Information on international hazard classification and labelling
is included in the International Chemical Safety Card reproduced in
this document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventative and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 0918) reproduced in this
document.
13.1 Human health hazards
Limonene is flammable but essentially non-toxic. Repeated or
prolonged contact with the oxidized chemical causes skin
sensitization.
13.2 Advice to physicians
In case of poisoning, the treatment is supportive. Like other
volatile oils, if the patient lives for 48 hours, complete recovery is
likely; laboratory evidence of renal damage may persist for several
months (Dreisbach & Robertson, 1987).
13.3 Storage
Limonene is flammable, with a flash point of 45°C. Keep the
container in a cool, dry, well ventilated area, out of direct
sunlight. Keep the container tightly closed to prevent oxidation of
the chemical.
13.4 Spillage
In the case of a large spill, emergency personnel need to use
non-sparking tools to avoid fire and explosion hazards.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards is
available from the International Register of Potentially Toxic
Chemicals (IRPTC) legal file.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
D-LIMONENE ICSC:0918
D-LIMONENE
(R)-4-Isopropenyl-1-methylcyclohexene
(+)-Limonene
C10H16
Molecular mass: 136.23
CAS #
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
The acute toxicity of d-limonene in rodents is fairly low after
oral, intraperitoneal, subcutaneous, and intravenous administration,
based on the magnitude of the LD50 values (Table 4). LD50 values
were approximately 5 g/kg body weight for the oral administration of
d-limonene or d/l-limonene to rats and for dermal application of
d/l-limonene to rabbits and 6 g/kg body weight for oral
administration to mice (Tsuji et al., 1974, 1975b; Opdyke, 1978).
Studies on the acute inhalation toxicity of limonene were not
identified.
Effects observed following the acute exposure of rodents to
limonene include increased bile flow at 85 mg/kg body weight (Kodama
et al., 1976), inhibition of S-3-hydroxy-3-methylglutaryl-CoA
reductase activity at 409 mg/kg body weight (Clegg et al., 1980),
enzyme induction at 600 and 1200 mg/kg body weight (Ariyoshi et al.,
1975), and decreased motor activity, hypothermia, and potentiation of
hexobarbital-induced sleep at 3 ml/kg body weight (Tsuji et al.,
1974).
8.2 Irritation and sensitization
d-Limonene is considered a skin irritant (Cronin, 1980;
Fischer, 1986). The skin irritancy of limonene in guinea-pigs (Klecak
et al., 1977) and rabbits (Lacy et al., 1987; Okabe et al., 1990) is
considered moderate and low, respectively. In an in vivo study of
rabbit skin irritation, d-limonene was ranked 3.5 of 8 on the basis
of the primary irritation index (Bagley et al., 1996); effects were
graded according to OECD Test Guideline 404 (OECD, 1993). In a study
in rabbits, d-limonene caused irritation to the eyes (Tsuji et al.,
1974).
Although d-limonene was once considered the main allergen in
citrus fruits, data from more recent studies in animals have revealed
air-oxidized d-limonene, rather than unoxidized d-limonene, to be
the sensitizing agent. When limonene (unspecified form and unknown
purity of the test material) was tested in four different
sensitization assays with guinea-pigs (Open Epicutaneous Test,
Maximization Test, Draize's Test, and a test with Freund's Complete
Adjuvant), it was sensitizing in all but Draize's Test (Klecak et al.,
1977). In another study in mice, d-limonene did not induce
sensitization (Maisey & Miller, 1986). Hydroperoxides and other
oxidation products of d-limonene formed on exposure to the air have
proved to be potent contact allergens when tested with Freund's
Complete Adjuvant in guinea-pigs, whereas unoxidized d-limonene did
not cause any sensitization (Karlberg et al., 1991, 1992).
Table 4: Acute toxicity of limonene.
Species (sex) Route of administration Type of limonene LD50 (g/kg body weight) Reference
rabbit dermal d/l >5 Opdyke, 1978
rat oral d/l 5.3 Opdyke, 1978
rat (m/f) oral d 4.4/5.1 Tsuji et al., 1975b
rat (m/f) intraperitoneal d 3.6/4.5 Tsuji et al., 1975b
rat (m/f) intravenous d 0.125/0.11 Tsuji et al., 1975b
mouse (m/f) oral d 5.6/6.6 Tsuji et al., 1975b
mouse (m/f) oral, 7 days d 5.3/6.8a Tsuji et al., 1974
mouse (m/f) intraperitoneal, 3 days d 3.1/3.0a Tsuji et al., 1974
mouse (m + f) intraperitoneal d 1.3 Tsuji et al., 1975b
mouse (m/f) intraperitoneal, 10 days d 0.59/0.50a Tsuji et al., 1974
mouse (m + f) subcutaneous d >41.5 Tsuji et al., 1975b
mouse (m + f) subcutaneous, 7 days d >21.5 Tsuji et al., 1974
a Calculated from ml/kg body weight.
8.3 Short-term exposure
Increases in hepatic cytochrome P-450 content have been observed
in female rats administered limonene (isomer unspecified; 40 mg/kg
body weight per day for 3 days) by intraperitoneal injection (Austin
et al., 1988) and in rats administered 5% d-limonene in the diet for
2 weeks (Maltzman et al., 1991). Increased epoxide hydratase activity
was observed in rats administered 1% or 5% d-limonene in the diet
for 2 weeks (Maltzman et al., 1991). Increases in phase II enzymes
(glutathionyltransferase and UDP-glucuronyltransferase) during the
exposure of rats to 5% limonene in food have also been described
(Maltzman, 1991). Increased relative liver weight (from 5 to 20
times) has been observed in rats administered d-limonene at a dose
of 75-300 mg/kg body weight; at 300 mg/kg body weight, the increase
was significant (Kanerva et al., 1987b). In cats, infusion of 97%
d-limonene into the bile system to dissolve gallstones caused acute
and chronic inflammatory changes (Schenk et al., 1980).
8.4 Long-term exposure
8.4.1 Subchronic exposure
Peroral administration of d-limonene to rats at a dose of 400
mg/kg body weight for 30 days resulted in a 20-30% increase in the
amount and activity of different liver enzymes (cytochrome P-450,
cytochrome b5, aminopyrine demethylase, and aniline hydroxylase),
increased relative liver weight, and decreased cholesterol levels
(Ariyoshi et al., 1975). Administration of d-limonene (0, 2, 5, 10,
30, and 75 mg/kg body weight per day) by gavage to groups of 10 male
rats, 5 days/week for 13 weeks (Webb et al., 1989), resulted in the
pathological formation of granular casts at the outer zone of the
renal medulla. The no-observed-effect level (NOEL), based upon
histological examination of the kidneys, was considered to be 5 mg/kg
body weight per day. The LOEL for increased liver and kidney weight
was 75 mg/kg body weight per day, the highest dose tested. The NOEL
for effects in the liver was 10 mg/kg body weight; the
no-observed-adverse-effect level (NOAEL) for effects in the liver was
30 mg/kg body weight per day. Linear regression analysis revealed a
dose-related trend in the increased relative weights of the kidney and
liver at 30 and 75 mg/kg body weight per day. No histopathological
changes were observed in the liver in these two studies. The amount
and activity of different liver enzymes were not investigated, and
thus the increase in relative liver weight may be due to enzyme
induction.
8.4.2 Chronic exposure and carcinogenicity
The oral administration of d-limonene (0.4, 1.2, or 3.6 ml/kg
body weight per day) to dogs for 6 months caused nausea and vomiting
(Tsuji et al., 1975a). A 35% increase in alkaline phosphatase and
cholesterol in serum and slightly increased total and relative liver
weights were observed in dogs after peroral administration of
d-limonene at a dose of 1.2 ml/kg body weight per day for 6 months
(about 1000 mg/kg body weight per day) (Webb et al., 1990).
In a 2-year study, d-limonene was administered (per os) 5
days/week to groups of 50 F344/N rats (0, 75, or 150 mg/kg body weight
per day to males, and 0, 300, or 600 mg/kg body weight per day to
females) and B6C3F1 mice (0, 250, or 500 mg/kg body weight per day to
males, and 0, 500, or 1000 mg/kg body weight per day to females) (NTP,
1990). Slightly lower body weights were observed for rats in the
high-dose groups and female mice in the high-dose group; however, no
clinical symptoms could be related to the administration of
d-limonene. For female rats in the high-dose group, survival was
reduced after 39 weeks (NTP, 1990). There was clear evidence of
carcinogenic activity of d-limonene in male rats, based upon a
dose-related increase in the incidence of hyperplasia and adenoma/
adenocarcinoma in renal tubular cells. However, there was no evidence
of carcinogenicity in female rats or in male and female mice. The
carcinogenic response in the kidney of male rats has been linked to a
unique renal perturbation involving alpha2µ-globulin.
To determine whether d-limonene would cause a sustained
increase in renal cell proliferation and exhibit promoting activity
for the development of renal adenomas in male F344 rats, the animals
were administered (by stomach tube) d-limonene (150 mg/kg body
weight per day) as a promoter 5 days/week for 30 weeks (Dietrich &
Swenberg, 1991). N-ethyl- N-hydroxyethylnitrosamine (500 ppm) was
used as an initiator in the drinking-water for 2 weeks. In addition,
male alpha2µ-globulin-deficient rats were exposed in the same manner
to determine if the male rat specific urinary protein alpha2µ-globulin
is required for d-limonene to cause these effects. Exposure to
d-limonene alone caused a significant increase in the number of
atypical tubules and atypical hyperplasias in F344 rats, compared with
vehicle controls. There was no increase in the incidence of tumours
or preneoplastic lesions in the alpha2µ-globulin-deficient rats
exposed to d-limonene, whereas a 10-fold increase in the incidence
of renal adenoma and atypical hyperplasia was observed in F344 rats
exposed to d-limonene, compared with controls. There was a
significant decrease in the incidence of liver tumours in animals
exposed to N-ethyl- N-hydroxyethylnitrosamine and d-limonene,
compared with N-ethyl- N-hydroxyethylnitrosamine exposure alone.
8.5 Genotoxicity and related end-points
On the basis of available data, there is no evidence that
d-limonene or its metabolites are genotoxic or mutagenic. Limonene
and its epoxides were not mutagenic when tested at concentrations of
0.3-3333 µg/plate in in vitro assays using different strains of
Salmonella typhimurium, in the presence or absence of metabolic
activation (Florin et al., 1980; Watabe et al., 1981; Haworth et al.,
1983; Connor et al., 1985; NTP, 1990). d-Limonene did not increase
the frequency of forward mutation at the TK+/- locus in mouse L5178Y
cells (NTP, 1990), induce cytogenetic damage in Chinese hamster ovary
cells (Anderson et al., 1990), or malignantly transform Syrian hamster
embryo cells (Pienta, 1980). In one in vitro study, following
exposure with benzo (a)pyrene, d-limonene (21.9 µmol/litre)
inhibited the formation of transformed cell colonies in tracheal
epithelium isolated from rats (Steele et al., 1990).
No evidence of mutagenicity was reported in an in vivo spot
test with mice, involving the intraperitoneal injection of limonene at
215 mg/kg body weight per day on days 9-11 during gestation (Fahrig,
1984).
8.6 Reproductive and developmental toxicity
Studies on the reproductive toxicity of limonene were not
identified. There is no evidence that limonene has teratogenic or
embryotoxic effects in the absence of maternal toxicity. In rats, the
oral administration of d-limonene (2869 mg/kg body weight per day)
on days 9-15 of gestation resulted in decreased body weight and deaths
among the dams. Delayed ossification and decreased total body and
organ weights (thymus, spleen, and ovary) were observed in the
offspring (Tsuji et al., 1975b). In mice, the oral administration of
d-limonene (2869 mg/kg body weight per day) on days 7-12 of
gestation resulted in reduced growth in the mothers and a
significantly increased incidence of skeletal anomalies and delayed
ossification in the offspring (Kodama et al., 1977a). The oral
administration of d-limonene (250, 500, or 1000 mg/kg body weight
per day) to rabbits on days 6-18 of gestation had no dose-related
effects on the offspring. At the highest dose, there were some deaths
and reduced weight gain among the dams; at the intermediate dose,
growth was decreased (Kodama et al., 1977b).
8.7 Immunological and neurological effects
Reports relating limonene to type I allergy were not identified.
In a study designed to assess the immunological effects of
d-limonene on B- and T-cell responses, BALB/c mice were administered
(by forced intragastric feeding) d-limonene (0.1 ml) daily for 9
weeks (Evans et al., 1987). Mice given keyhole limpet haemocyanin
prior to exposure to d-limonene had suppressed primary and secondary
anti-keyhole limpet haemocyanin responses. Mice exposed to
d-limonene prior to the administration of keyhole limpet haemocyanin
had significantly increased antibody and mitogen-induced proliferative
responses. However, the purity of the d-limonene in this study was
not checked, and oxidation products may have been the active
substances.
Effects on the central nervous system following exposure to
limonene have been reported in experimental studies with animals;
however, it is difficult to ascertain whether these effects were the
result of general intoxication or a more direct effect of the
chemical. The peroral administration of d-limonene (3 ml) to rats
and mice resulted in decreased motor activity (Tsuji et al., 1974). A
similar effect was also observed in mice orally administered a
limonene dose of 1000 mg/kg body weight per day for 13 weeks (NTP,
1990).
9. EFFECTS ON HUMANS
Case reports or epidemiological studies on the effects of
limonene on human health were not identified. Available data have
been derived from studies with volunteers. In older investigations,
multiple exposures and confounding factors such as mechanical damage,
irritation, other allergens, and infections due to wet work (Beerman
et al., 1938; Schwartz, 1938; Birmingham et al., 1951) may have
contributed to the effects reported following exposure to limonene.
None of eight subjects reported any discomfort, irritation, or
symptoms related to central nervous system effects during a 2-hour
inhalation exposure to d-limonene at 10, 225, or 450 mg/m3;
however, a slight decline in vital capacity was observed following
exposure to the highest concentration (Falk Filipsson et al., 1993).
In a study in which the sensitivity of four patch testing systems
(Finn chamber, Hill Top patch, Van der Bend chamber, and Webril patch)
was evaluated in volunteers, d-limonene (perfume-grade) reacted
strongly in all types of patches within 10-15 minutes of exposure
(York et al., 1995). Skin irritation was assessed before application,
as well as immediately and 1, 24, 48, and 72 hours after removal of
the patch, using a scoring system based broadly on that used for
rabbit irritation studies (OECD, 1993), but modified to account for
the nature of reactions on human skin. There was evidence of sensory
effects and urticarial responses on removal of the patches.
Significant irritation persisted for 24 hours, and these reactions
persisted for 48 and 72 hours in many volunteers (York et al., 1995).
Dermal exposure to d-limonene (98%) for 2 hours in one subject
caused burning, itching, aching, and a long-lasting purpuric rash
(Falk et al., 1991).
d-Limonene infused directly into the bile system of human
volunteers to dissolve gallstones caused pain in the upper abdomen,
nausea, vomiting, and diarrhoea, as well as increases in serum
aminotransferases and alkaline phosphatase (Igimi et al., 1976, 1991).
The oral administration of 20 g d-limonene to volunteers resulted in
diarrhoea, painful constrictions, and proteinuria, but no biochemical
changes (total protein, bilirubin, cholesterol, aspartate
aminotransferase, alanine aminotransferase, alkaline phosphatase) in
the liver (Igimi et al., 1976). Reports of contact allergy to
dipentene have appeared (Calnan, 1979; Rycroft, 1980). In one
investigation, 15 of 22 people with an allergy to oil of turpentine
also reacted to dipentene (Cachao et al., 1986). Patch testing in
consecutive dermatitis patients from Sweden and Belgium revealed
positive reactions in 1.5-2% of the subjects tested with oxidized
d-limonene, a finding similar to that observed with other common
sensitizers, such as formaldehyde (A.-T. Karlberg, personal
communication, 1996). d-Limonene reduced non-immunological contact
urticaria caused by cinnamic aldehyde, with competitive receptor
inhibition suggested as the mechanism of suppression (Guin et al.,
1984). No sensitizing effect was observed when 25 volunteers were
exposed to d-limonene in a Human Maximization Test (Grief, 1967).
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10.1 Aquatic environment
The acute toxicity of d-limonene ranges from slight to high for
aquatic organisms (Table 5). The lowest acute toxicity values (EC50
or LC50) identified were approximately 0.4 mg/litre for Daphnia (US
EPA, 1990b) and 0.7 mg/litre for fish (US EPA, 1990a,b). The
no-observed-effect concentration (NOEC) for green algae is
approximately 4 mg/litre (US EPA, 1990a). The acute toxicity (EC50
or LC50) of dipentene to Daphnia and fish is about 50-70 times
lower than that for d-limonene (US EPA, 1990b). No studies were
identified on the chronic toxicity of limonene to aquatic organisms.
10.2 Terrestrial environment
The toxicity of limonene has been studied in various terrestrial
organisms (Table 6). Limonene generally has moderate acute toxicity
in insects and mites. The acute toxicity of d-limonene to
earthworms (Eisenia foetida Savigny) was high (LC50 = 6.0 ppm;
mg/kg) (Karr et al., 1990). Sublethal effects (i.e. abnormal
rebounding of medial giant fibre pathway [MGF] impulses and
spontaneous lateral giant fibre pathway [LGF] spiking) were observed
following exposure of earthworms to 4.2 ppm (mg/kg) limonene (Karr et
al., 1990). Limonene has low subacute toxicity to bobwhite quail
(Colinus virginianus) exposed via the diet (LC50 > 5620 ppm;
mg/kg) (US EPA, 1994).
Table 5: Toxicity of limonene to aquatic organisms.
Species End-point; exposure Results (mg/litre) Reference
Algae
Green algaea 96-h NOEC; static 4.08 US EPA, 1990a
Crustaceans
Water flea (Daphnia magna)b 48-h LC50; flow-through 0.577 (0.496-0.672) US EPA, 1990b
48-h EC50; flow-through 0.421
Water flea (D. magna)c acute LC50 39 ppm US EPA, 1994
Water flea (D. magna)a 48-h LC50; flow-through 31 (27.5-34.8) US EPA, 1990b
48-h EC50; flow-through 28.2
Water flea (Daphnia pulex)b 48-h EC50; flow-through 0.730 US EPA, 1990a
Water flea (D. pulex)c 48-h EC50; static 69.6 Passino & Smith, 1987
Daphniab 21-d NOEC; structure-activity 0.15 US EPA, 1990a
relationship (SAR) analysis
Fish
Fathead minnow (Pimephales promelas)b 96-h LC50; flow-through 0.702 (0.619-0.796) US EPA, 1990b
Fathead minnow (P. promelas)b 96-h LC50; flow-through 0.720 (0.618-0.839) US EPA, 1990b
96-h EC50; flow-through 0.688 (0.606-0.782)
Fathead minnow (P. promelas)a 96-h LC50; flow-through 38.5 (35.4-41.8) US EPA, 1990a,b
96-h EC50; flow-through 28.2
Fishc acute LC50 80 ppm US EPA, 1994
Fishb 96-h LC50; flow-through 0.711 US EPA, 1990a
Golden orfe (Leuciscus idus)a 48-h LC50 32 Roth, 1990
Table 5 (continued)
Species End-point; exposure Results (mg/litre) Reference
Insects
Water hyacinth weevil (Neochetina Mortality (73%, range 40-100%), 50% limonene Haag, 1986
eichhorniae, 60%, N. bruchi, 40%)b weevils were dipped in limonene
Mosquito fly (Culex quinquefasciatus)c 2nd-instar larvae (23-33°C), 6.6-26.1 ppm Mohsen et al., 1989
72-h LC50; static
4th-instar larvae (23-33°C), 7.8-30.6 ppm Mohsen et al., 1989
72-h LC50; static
a d/l-Limonene.
b d-Limonene.
c Optical isomer not specified.
Table 6: Toxicity of limonene to terrestrial organisms.
Species End-point; exposure Results Reference
Insects
Cat flea Adult LD50; contact 160 (157-163) µg/cm2 Hink & Fee, 1986
(Ctenocephalides felis)a,b Adult LD50; vapour 259 (234-281) µg/cm2
Pupae LD50; contact 376 (259-468) µg/cm2
Larvae LD50; contact 226 (221-231) µg/cm2
Eggs; lethal to all eggs; contact 65 µg/cm2
Variegated cutworm Larvae; significant inhibition of pupation; dietary
(Peridroma saucia)b exposure 0.2% limonene in artificial feed Harwood et al., 1990
German cockroach Adult 24-h LD50; topical 700 (610-810) µg/insect Karr & Coats, 1988
(Blattella germanica L.)b Adult 24-h LC50; fumigation 23.3 (17.5-31.0) ppm
Adult; no mortality; oral 25% limonene in feed
Nymph; no mortality; oral 25% limonene in feed
Adult; no mortality; 72-h contact with treated limonene (conc. not given)
surface
German cockroach Effect on growth rate; diet 1-25% limonene in diet Karr & Coats, 1992
(B. germanica L.)b EC50, oothecae yielding young; topical exposure 0.68 mg/ootheca
No effect on reproduction; via diet 25% limonene in diet
Topical exposure 0.84 mg/cockroach
Vapour exposure 5 mg/litre in air
Rice weevil (Sitophilus Adult 24-h LC50; fumigation 19.0 (13.2-27.3) ppm Karr & Coats, 1988
oryzae L.)b
House fly (Musca 25-h LD50; topical 90 (70-130) µg/insect Karr & Coats, 1988
domestica L.)b
Western corn rootworm Egg 72-h LC50; contact with treated substrate 1.8 (0.8-2.9)% limonene Karr & Coats, 1988
(Diabrotica virgifera Larvae 72-h LC50; contact with treated soil 12.2 (4.5-32.6) ppm
virgifera LeConte)b
Table 6 (continued)
Species End-point; exposure Results Reference
Spiders and allies
Spruce spider mite 24-h LC50; vapour 24.5 ppm Cook, 1992
(Oligonychus ununguis Significant decrease in oviposition 5 ppm
(Jacobi)),c adult female
Segmented worms
Earthworm (Eisenia 48-h LC50 6.0 (5.1-7.1) ppm Karr et al., 1990
foetida Savigny)b Sublethal effects 4.2 ppm
Birds
Bobwhite quail (Colinus Subacute LC50; dietary exposure >5 620 ppm US EPA, 1994
virginianus)d
a Fleas were exposed to filter papers treated with limonene, either directly or to vapours from the filter papers.
b d-Limonene.
c l-Limonene.
d Optical isomers not specified.
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
Limonene is a skin irritant in experimental animals and humans.
d-Limonene is an eye irritant in rabbits. Studies in guinea-pigs
have revealed that air-oxidized d-limonene, but not d-limonene
itself, induced contact allergy. Similar results are likely with
l-limonene and dipentene.
The critical organ in animals (except for male rats) following
peroral or intraperitoneal administration is the liver. Exposure to
limonene affects the amount and activity of different liver enzymes,
liver weight, cholesterol levels, and bile flow, with effects having
been observed in mice, rats, and dogs. In male rats, exposure to
d-limonene results in damage to the kidneys and an increased
incidence of renal tumours. As the male rat specific protein
alpha2µ-globulin is considered to play a crucial role in the
development of the neoplastic and non-neoplastic kidney lesions, they
are considered not relevant for human risk assessment.
A dose-related nephropathy was observed in the kidneys of male
rats after oral administration of d-limonene (NTP, 1990). This
lesion, consisting of degeneration of epithelial cells in the
convoluted tubules, granular casts in the outer stripe of the outer
medulla, and epithelial regeneration, is characteristic of hyaline
droplet nephropathy associated with the accumulation of
alpha2µ-globulin in the cytoplasm of tubular cells (Alden et al.,
1984; Halder et al., 1985) in response to a variety of hydrocarbon
compounds (Swenberg et al., 1992). Some compounds fit deeply into a
hydrophobic pocket of alpha2µ-globulin. When hydrogen bonding between
the chemical and protein occurs, the digestibility of alpha2µ-globulin
by proteases is inhibited, leading to accumulation of the male rat
specific protein in lysosomes of the P2 segment of the nephron
(Lehman-McKeeman et al., 1990). Although such chemicals fall into
rather diverse classes, molecular modelling studies have demonstrated
a strong structure-activity relationship with respect to
alpha2µ-globulin binding (Borghoff et al., 1991). The accumulation of
alpha2µ-globulin is cytotoxic, resulting in single-cell necrosis
(Dietrich & Swenberg, 1991). The exfoliated renal epithelium is
restored by compensatory cell proliferation. The increase in cell
proliferation associated with alpha2µ-globulin is reversible. Damage
of this type has not been observed in female rats, male rats that do
not produce alpha2µ-globulin, or other mammals, such as mice,
hamsters, guinea-pigs, dogs, and monkeys (Alden, 1986; Kanerva &
Alden, 1987a; Swenberg et al., 1989; Webb et al., 1989, 1990; NTP,
1990; Ridder et al., 1990; Dietrich & Swenberg, 1991). The processes
leading to nephropathy and the development of renal cancer by such
compounds are among the best understood for non-genotoxic chemicals
and strongly indicate that it is a male rat specific process. Acute
and chronic renal effects induced in male rats by limonene will be
unlikely to occur in any species not producing alpha2µ-globulin or a
very closely related protein in the large quantities typically seen in
the male rat (US EPA, 1991; Swenberg, 1993).
d-Limonene has been studied in a variety of short-term
in vitro tests and has been found to be non-genotoxic. There is no
evidence that limonene has teratogenic or embryotoxic effects in the
absence of maternal toxicity.
11.1.2 Criteria for setting guidance values for limonene
In numerous experimental studies, exposure to limonene has been
shown to affect the liver. Owing to a lack of data on d-limonene
exposure in humans, this organ cannot with certainty be stated as the
critical organ in humans. Based on available data, food is believed
to be the principal source of exposure (96%) to limonene; the
contribution from ambient air is approximately 4%. The dermal uptake
of limonene has not been estimated.
To calculate a tolerable intake for humans, the animal study was
chosen in which effects on the liver were observed at the lowest
exposure level (Webb et al., 1989). In this study, gavage
administration of d-limonene (5 days/week for 13 weeks) to rats
caused increased relative liver weight at 30 and 75 mg/kg body weight
per day. The NOEL for the liver was considered to be 10 mg/kg body
weight per day. Using uncertainty factors of 10 for intraspecies
differences and 10 for interspecies differences, a tolerable intake
for ingestion of d-limonene by humans of 0.1 mg/kg body weight per
day may be calculated from the NOEL. A guidance value for inhalation
exposure to d-limonene was not developed, as inhalation is an
insignificant route of exposure compared with ingestion.
11.1.3 Sample risk characterization
Exposure estimates vary as a function of use patterns, and the
risk characterization presented here is provided only as an example,
primarily for illustrative purposes. In general, d-limonene could
be considered (with the exception of its irritative and sensitizing
properties) to be a chemical with fairly low toxicity. The calculated
tolerable intake of 0.1 mg/kg body weight per day is of a similar
magnitude as the estimated daily US consumption of d-limonene of
0.27 mg/kg body weight per day (Flavor and Extract Manufacturers
Association, 1991).
11.2 Evaluation of environmental effects
Limonene and other terpenes are released in large amounts mainly
to the atmosphere. When released to soil or water, limonene is
expected to evaporate to air to a significant extent, owing to its
high volatility. Thus, the atmosphere is the predominant
environmental sink of limonene, where it is expected to rapidly
undergo gas-phase reactions with photochemically produced hydroxyl
radicals, ozone, and nitrate radicals. The oxidation of terpenes,
such as limonene, contributes to aerosol and photochemical smog
formation. Ozonolysis of limonene may also lead to the formation of
hydrogen peroxide and organic peroxides, which have various toxic
effects on plant cells and may be part of the damage to forests
observed in the last decades (Peters et al., 1994). Emissions of
biogenic hydrocarbons such as limonene and other terpenes to the
atmosphere may either decrease ozone concentrations when nitrogen
oxide concentrations are low or, if emissions take place in polluted
air (i.e. containing high nitrogen oxide levels), lead to an increase
in ozone concentrations.
Terrestrial organisms are most likely to be exposed to limonene
via the air. The few studies on terrestrial species (i.e. insects)
using vapour exposure reveal effects of limonene at parts per million
levels. Measured environmental concentrations are typically around
0.1-2 ppb (0.6-11 µg/m3), indicating a low risk for acute toxic
effects on terrestrial organisms from direct exposure to limonene in
air. At polluted sites, limonene concentrations in soil (up to 920
mg/kg soil) may exceed effect levels of soil-living organisms (e.g.
earthworm, acute LC50 = 6.0 ppm; mg/kg).
In the aquatic environment, limonene exhibits high acute toxicity
to fish and Daphnia. It may also bioaccumulate. The lowest acute
toxicity value identified was 0.4 mg/litre (48-hour EC50 for
Daphnia). Because concentrations of limonene in surface waters of
"polluted" and "unpolluted" areas are at least about 250 and 20 000
times lower than this acute toxicity value, respectively, it is likely
that limonene poses a low risk for acute toxic effects on aquatic
organisms. No studies were identified on chronic effects, and
therefore risks associated with chronic exposures of aquatic organisms
to limonene in "polluted" waters cannot be determined.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The International Agency for Research on Cancer (IARC, 1993) has
classified d-limonene in Group 3 (not classifiable as to its
carcinogenicity to humans) based on a lack of available data on
carcinogenicity to humans and limited evidence for carcinogenicity in
experimental animals.
The 41st meeting of the Joint FAO/WHO Expert Committee on Food
Additives (JECFA, 1993b) withdrew the existing acceptable daily intake
for d-limonene of 0-1.5 mg/kg body weight per day (JECFA, 1993a) and
in its place allocated "not specified." On the basis of the available
data, the total daily intake of the chemical arising from its use at
the levels necessary to achieve the desired effect and from its
acceptable background levels in food did not, in the opinion of the
Committee, represent a health hazard. For that reason, and for the
reasons stated in the individual evaluations, the establishment of an
acceptable daily intake expressed in numerical form was not deemed
necessary.
Information on international hazard classification and labelling
is included in the International Chemical Safety Card reproduced in
this document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventative and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 0918) reproduced in this
document.
13.1 Human health hazards
Limonene is flammable but essentially non-toxic. Repeated or
prolonged contact with the oxidized chemical causes skin
sensitization.
13.2 Advice to physicians
In case of poisoning, the treatment is supportive. Like other
volatile oils, if the patient lives for 48 hours, complete recovery is
likely; laboratory evidence of renal damage may persist for several
months (Dreisbach & Robertson, 1987).
13.3 Storage
Limonene is flammable, with a flash point of 45°C. Keep the
container in a cool, dry, well ventilated area, out of direct
sunlight. Keep the container tightly closed to prevent oxidation of
the chemical.
13.4 Spillage
In the case of a large spill, emergency personnel need to use
non-sparking tools to avoid fire and explosion hazards.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards is
available from the International Register of Potentially Toxic
Chemicals (IRPTC) legal file.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
D-LIMONENE ICSC:0918
D-LIMONENE
(R)-4-Isopropenyl-1-methylcyclohexene
(+)-Limonene
C10H16
Molecular mass: 136.23
CAS # 