
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
CONCISE INTERNATIONAL CHEMICAL ASSESSMENT DOCUMENT NO. 3
1,1,2,2-TETRACHLOROETHANE
INTER-ORGANIZATION PROGRAMME FOR THE SOUND MANAGEMENT OF CHEMICALS
A cooperative agreement among UNEP, ILO, FAO, WHO, UNIDO, UNITAR and
OECD
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
First draft prepared by Ms K. Hughes and Ms M.E. Meek,
Environmental Health Directorate,
Health Canada
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization, and produced within the framework of the
Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization Geneva, 1998
The International Programme on Chemical Safety (IPCS),
established in 1980, is a joint venture of the United Nations
Environment Programme (UNEP), the International Labour Organisation
(ILO), and the World Health Organization (WHO). The overall
objectives of the IPCS are to establish the scientific basis for
assessment of the risk to human health and the environment from
exposure to chemicals, through international peer review processes, as
a prerequisite for the promotion of chemical safety, and to provide
technical assistance in strengthening national capacities for the
sound management of chemicals.
The Inter-Organization Programme for the Sound Management of
Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and
Agriculture Organization of the United Nations, WHO, the United
Nations Industrial Development Organization, and the Organisation for
Economic Co-operation and Development (Participating Organizations),
following recommendations made by the 1992 UN Conference on
Environment and Development to strengthen cooperation and increase
coordination in the field of chemical safety. The purpose of the IOMC
is to promote coordination of the policies and activities pursued by
the Participating Organizations, jointly or separately, to achieve the
sound management of chemicals in relation to human health and the
environment.
WHO Library Cataloguing in Publication Data
1,1,2,2-Tetrachloroethane.
(Concise international chemical assessment document ; 3)
1.Tetrachloroethylene - toxicity 2.Environmental exposure
I.International Programme on Chemical Safety II.Series
ISBN 92 4 153003 0 (NLM Classification: QV 253)
ISSN 1020-6167
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1998
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city, or area
or of its authorities, or concerning the delimitation of its frontiers
or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
TABLE OF CONTENTS
FOREWORD
1. EXECUTIVE SUMMARY
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
3. ANALYTICAL METHODS
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1. Environmental levels
6.2. Human exposure
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1. Single exposure
8.2. Irritation and sensitization
8.3. Short-term exposure
8.4. Long-term exposure
8.4.1. Subchronic exposure
8.4.2. Chronic exposure and carcinogenicity
8.5. Genotoxicity and related end-points
8.6. Reproductive and developmental toxicity
8.7. Immunological and neurological effects
9. EFFECTS ON HUMANS
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10.1. Aquatic environment
10.2. Terrestrial environment
11. EFFECTS EVALUATION
11.1. Evaluation of health effects
11.1.1. Hazard identification and dose-response assessment
11.1.2. Criteria for setting guidance values for 1,1,2,2-tetrachloroethane
11.1.3. Sample risk characterization
11.2. Evaluation of environmental effects
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
13.1. Advice to physicians
13.2. Health surveillance advice
13.3. Prevention
13.4. Spillage
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
INTERNATIONAL CHEMICAL SAFETY CARD
REFERENCES
APPENDIX 1 - SOURCE DOCUMENTS
APPENDIX 2 - CICAD PEER REVIEW
APPENDIX 3 - CICAD FINAL REVIEW BOARD
RÉSUMÉ D'ORIENTATION
RESUMEN DE ORIENTACION
FOREWORD
Concise International Chemical Assessment Documents (CICADs) are
the latest in a family of publications from the International
Programme on Chemical Safety (IPCS) - a cooperative programme of the
World Health Organization (WHO), the International Labour Organisation
(ILO), and the United Nations Environment Programme (UNEP). CICADs
join the Environmental Health Criteria documents (EHCs) as
authoritative documents on the risk assessment of chemicals.
CICADs are concise documents that provide summaries of the
relevant scientific information concerning the potential effects of
chemicals upon human health and/or the environment. They are based on
selected national or regional evaluation documents or on existing
EHCs. Before acceptance for publication as CICADs by IPCS, these
documents have undergone extensive peer review by internationally
selected experts to ensure their completeness, accuracy in the way in
which the original data are represented, and the validity of the
conclusions drawn.
The primary objective of CICADs is characterization of hazard and
dose-response from exposure to a chemical. CICADs are not a summary
of all available data on a particular chemical; rather, they include
only that information considered critical for characterization of the
risk posed by the chemical. The critical studies are, however,
presented in sufficient detail to support the conclusions drawn. For
additional information, the reader should consult the identified
source documents upon which the CICAD has been based.
Risks to human health and the environment will vary considerably
depending upon the type and extent of exposure. Responsible
authorities are strongly encouraged to characterize risk on the basis
of locally measured or predicted exposure scenarios. To assist the
reader, examples of exposure estimation and risk characterization are
provided in CICADs, whenever possible. These examples cannot be
considered as representing all possible exposure situations, but are
provided as guidance only. The reader is referred to EHC 1701 for
advice on the derivation of health-based guidance values.
1 International Programme on Chemical Safety (1994) Assessing
human health risks of chemicals: derivation of guidance values
for health-based exposure limits. Geneva, World Health Organization
(Environmental Health Criteria 170).
While every effort is made to ensure that CICADs represent the
current status of knowledge, new information is being developed
constantly. Unless otherwise stated, CICADs are based on a search of
the scientific literature to the date shown in the executive summary.
In the event that a reader becomes aware of new information that would
change the conclusions drawn in a CICAD, the reader is requested to
contact the IPCS to inform it of the new information.
Procedures
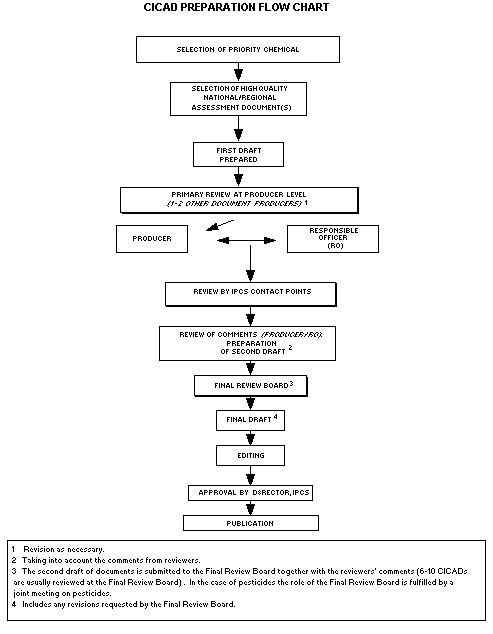
The flow chart shows the procedures followed to produce a CICAD.
These procedures are designed to take advantage of the expertise that
exists around the world - expertise that is required to produce the
high-quality evaluations of toxicological, exposure, and other data
that are necessary for assessing risks to human health and/or the
environment.
The first draft is based on an existing national, regional, or
international review. Authors of the first draft are usually, but not
necessarily, from the institution that developed the original review.
A standard outline has been developed to encourage consistency in
form. The first draft undergoes primary review by IPCS and one or
more experienced authors of criteria documents to ensure that it meets
the specified criteria for CICADs.
The second stage involves international peer review by scientists
known for their particular expertise and by scientists selected from
an international roster compiled by IPCS through recommendations from
IPCS national Contact Points and from IPCS Participating Institutions.
Adequate time is allowed for the selected experts to undertake a
thorough review. Authors are required to take reviewers' comments
into account and revise their draft, if necessary. The resulting
second draft is submitted to a Final Review Board together with the
reviewers' comments.
The CICAD Final Review Board has several important functions:
- to ensure that each CICAD has been subjected to an appropriate
and thorough peer review;
- to verify that the peer reviewers' comments have been addressed
appropriately;
- to provide guidance to those responsible for the preparation of
CICADs on how to resolve any remaining issues if, in the opinion
of the Board,
the author has not adequately addressed all comments of the
reviewers; and
- to approve CICADs as international assessments.
Board members serve in their personal capacity, not as representatives
of any organization, government, or industry. They are selected
because of their expertise in human and environmental toxicology or
because of their experience in the regulation of chemicals. Boards
are chosen according to the range of expertise required for a meeting
and the need for balanced geographic representation.
Board members, authors, reviewers, consultants, and advisers who
participate in the preparation of a CICAD are required to declare any
real or potential conflict of interest in relation to the subjects
under discussion at any stage of the process. Representatives of
nongovernmental organizations may be invited to observe the
proceedings of the Final Review Board. Observers may participate in
Board discussions only at the invitation of the Chairperson, and they
may not participate in the final decision-making process.
 1. EXECUTIVE SUMMARY
This CICAD on 1,1,2,2-tetrachloroethane was prepared by the
Environmental Health Directorate of Health Canada and was based
principally on a review prepared by the Government of Canada (1993) to
assess the potential effects on human health of indirect exposure to
1,1,2,2-tetrachloroethane in the general environment and the
chemical's environmental effects, as well as a review prepared by the
Agency for Toxic Substances and Disease Registry (ATSDR, 1994)
intended to characterize information on adverse health effects and
public exposure. Data identified as of September 1992 were considered
in the Government of Canada (1993) review. A comprehensive literature
search of several on-line databases was conducted in August 1995 to
identify any references published subsequent to those incorporated in
this review. Information on the nature of the peer review and the
availability of the source documents is presented in Appendix 1.
Information on the peer review of this CICAD is presented in Appendix
2. This CICAD was approved for publication at a meeting of the Final
Review Board, held in Brussels, Belgium, on 18-20 November 1996.
Participants at the Final Review Board meeting are listed in Appendix
3. The International Chemical Safety Card (ICSC 0332) for
1,1,2,2-tetrachloroethane, produced by the International Programme on
Chemical Safety (IPCS, 1993), has also been reproduced in this
document.
1,1,2,2-Tetrachloroethane (CAS no. 79-34-5) is a volatile
synthetic chemical that is used principally as an intermediate in the
synthesis of other chlorinated hydrocarbons, although use of this
substance has declined significantly. Releases to the environment are
primarily in emissions to ambient air, where the chemical is likely to
remain for several weeks. 1,1,2,2-Tetrachloroethane is not expected
to contribute to the depletion of stratospheric ozone or to global
warming. It is rapidly removed from aquatic systems and is unlikely
to bioaccumulate. Human exposure to 1,1,2,2-tetrachloroethane is
principally via inhalation.
Very few data are available on the effects of exposure to
1,1,2,2-tetrachloroethane in humans. The toxicological profile of
1,1,2,2-tetrachloroethane has also not been well characterized;
because of the chemical's declining use, available data are confined
primarily to early limited studies. The acute toxicity of 1,1,2,2-
tetrachloroethane in experimental animals is slight to moderate. Based
on the results of principally limited short-term and subchronic
studies, the liver appears to be the most sensitive target organ.
Although most of the available studies are inadequate to allow a no-
or lowest-observed-(adverse)-effect level [NO(A)EL or LO(A)EL] for
hepatotoxicity to be determined with confidence, minimal effects on
the liver (reversible increase in lipid content) and other end-points
(an increase in levels of adrenocorticotropic hormone and reversible
alterations in haematological parameters) have been observed in rats
exposed to 13.3 mg/m3 for up to 9 months. Based on limited,
primarily range-finding studies and early investigations, reproductive
and developmental effects have been observed in experimental animals
only at doses that caused reductions in body weight.
Long-term ingestion of 1,1,2,2-tetrachloroethane resulted in an
increased incidence of liver tumours in both male and female B6C3F1
mice. However, similar exposure was not associated with a significant
increase in tumours at any site in Osborne-Mendel rats, although both
species were exposed only for up to 78 weeks. Based on the results of
available in vivo and in vitro assays, 1,1,2,2-tetrachloroethane
has, at most, weak genotoxic potential. 1,1,2,2-Tetrachloroethane was
a potent promoter, but not an initiator, of gamma-glutamyl-
transpeptidase-positive foci in the liver of rats. The profile for
tumour induction by 1,1,2,2-tetrachloroethane is similar to that of
dichloroacetic acid, its primary metabolite. Information on the
mechanism of tumour induction by 1,1,2,2-tetrachloroethane is
incomplete; for several of its metabolites, it has been suggested that
tumours are likely induced by mechanisms for which there is a
threshold.
Exposure to 1,1,2,2-tetrachloroethane has been demonstrated to
inhibit the activities of environmental bacteria (the lowest reported
IC50 was 1.4 mg/litre) and cause immobilization in Daphnia magna
(48-hour EC50 values of 23 mg/litre and above). In freshwater fish
species, the lowest reported LC50 (96 hours) was 18.5 mg/litre in
flagfish (Jordanella floridae), whereas the lowest-observed-effect
concentration (LOEC) for longer-term exposure was 7.2 mg/litre, which
resulted in reduced larval survival in the same species. No data were
identified on the effects of this substance on terrestrial organisms.
In order to provide guidance to relevant authorities, sample
guidance values have been determined on the basis of the potency of
1,1,2,2-tetrachloroethane to induce liver tumours in mice, as this is
the toxicological end-point for which the dose-response relationship
is best characterized. It is noted, however, that observed increases
in tumour incidence are currently restricted to one species and that
there are suggestive but incomplete data indicating that tumours may
be induced by a non-genotoxic mechanism. The potency, expressed as
the dose associated with a 5% increase in tumours, ranged from 5.8 to
28 mg/kg body weight per day. Sample guidance values for air (the
principal source of human exposure), calculated on the basis of
division of this potency range by 5000 or 50 000, are 3.4-16 µg/m3
and 0.34-1.6 µg/m3. These values correspond to those considered by
some agencies to represent "essentially negligible" risk (i.e. 10-5
to 10-6) for a genotoxic carcinogen; it should be noted, however,
that a smaller margin may also be appropriate in view of the
suggestive but incomplete evidence for an epigenetic mechanism of
tumour induction. Corresponding values for ingestion are 1.2-5.6
µg/kg body weight per day and 0.12-0.56 µg/kg body weight per day.
Based on a sample estimate of exposure, indirect exposure in the
general environment is less than these values, which are considered to
be conservative in view of the suggestive but incomplete evidence that
1,1,2,2-tetrachloroethane may induce tumours through a threshold
mechanism.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
1,1,2,2-Tetrachloroethane (CAS no. 79-34-5; Cl2CHCHCl2;
acetylene tetrachloride, sym-tetrachlorethane; see structural
diagram below) is a synthetic chemical that is a colourless, non-
flammable liquid at room temperature. It is highly volatile, with a
vapour pressure of 0.65 kPa at 20°C and water solubility of 2900
mg/litre at 20°C. The log octanol/water partition coefficient for
1,1,2,2-tetrachloroethane is about 2.5, whereas its Henry's law
constant was determined to range from 0.0003 to 0.0009 m3.atm/mol
(Tse et al., 1992; Government of Canada, 1993; Nichols et al., 1993).
Additional physical/chemical properties are presented in the
International Chemical Safety Card (ICSC 0332) reproduced in this
document.
Cl Cl
' '
Cl - C - C - Cl
' '
H H
3. ANALYTICAL METHODS
Analysis of 1,1,2,2-tetrachloroethane in air usually involves
preconcentration on a sorbent tube followed by thermal or solvent
desorption or collection in a cryogenically cooled trap followed by
gas chromatography (flame ionization or electron capture detection).
Detection limits range from 0.7 ng/m3 to 0.3 mg/m3 (ATSDR, 1994).
Purge and trap methods followed by gas chromatography (flame
ionization, electron capture electrolytic conductivity, or
microcoulometric detection) are generally used for water as well as
sediment, soil, or other solid samples. Reported detection limits
range from 0.001 to 5 µg/litre for water and from 1 to 5 µg/kg for
soil and sediment samples (ATSDR, 1994). Detection limits of 0.01
µg/litre and 0.06 ppbv (0.4 µg/m3) have been reported for solid-phase
microextraction coupled with gas chromatography/ion trap mass
spectrometry analysis for water and air samples, respectively (Arthur
et al., 1992; Chai & Pawliszyn, 1995). Gas chromatography, often in
combination with mass spectrometry, is commonly used for quantifying
1,1,2,2-tetrachloroethane in biological samples, with detection limits
of 400 µg/kg in tissues and 5-500 ng/litre in blood (ATSDR, 1994).
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
There are no known natural sources of 1,1,2,2-tetrachloroethane.
The principal use of 1,1,2,2-tetrachloroethane is as an intermediate
in the manufacture of other chlorinated hydrocarbons, such as vinyl
chloride, 1,2-dichloroethane, trichloroethylene, and
tetrachloroethylene; in the past, it was also used as an industrial
solvent and as a pesticide. Use, and hence production, of
1,1,2,2-tetrachloroethane has declined significantly; no recent data
on production were identified. Releases to the atmosphere through its
use as a chemical intermediate in Canada in 1990 were estimated to be
approximately 246 kg (Government of Canada, 1993), whereas 64 251
pounds (29 144 kg) were estimated to be emitted to air from reporting
industries in the USA in 1991 (ATSDR, 1994). In 1991, 953 kg of
1,1,2,2-tetrachloroethane were discharged to water from reporting
facilities in the USA (ATSDR, 1994).
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
1,1,2,2-Tetrachloroethane is released to the environment
primarily in emissions to ambient air. Based on its vapour pressure,
it is not likely to be transferred to other compartments. The
atmospheric lifetime for 1,1,2,2-tetrachloroethane reacting with
hydroxyl radicals from moderately polluted areas is estimated to be
between 43 and 100 days, based on estimated and measured reaction
rates, respectively (Government of Canada, 1993). The half-life in
the troposphere is estimated to be in excess of 800 days, and
diffusion into the stratosphere is expected to be slow.1 Based on
these estimates, there is significant potential for long-range
transport of 1,1,2,2-tetrachloroethane. In the stratosphere,
1,1,2,2-tetrachloroethane undergoes photolysis to produce chlorine
radicals, which may subsequently react with ozone; however, the ozone
depletion potential for 1,1,2,2-tetrachloroethane is very much less
than 0.001 relative to the standard CFC-11 (trichlorofluoromethane),
based on the method developed by Nimitz & Skaggs (1992).
1,1,2,2-Tetrachloroethane released to the aquatic environment is
rapidly removed via volatilization, with an estimated half-life of 6.2
hours from running water and 3.5 days from still water.1 Hydrolysis
and biodegradation are the principal routes of removal from
groundwater. The hydrolysis half-life in subsurface sediment at 25°C
was determined to be 29 days (Haag & Mill, 1988). Neutral and
base-catalysed hydrolyses of 1,1,2,2-tetrachloroethane in pure water
yielded trichloroethylene as essentially the sole degradation product
(Haag & Mill, 1988). The products of anaerobic biodegradation of
1,1,2,2-tetrachloroethane were determined in a 6-week study to be (in
decreasing order) cis-1,2-dichloroethylene,
trans-1,2-dichloroethylene, trichloroethylene,
1,1,2-trichloroethane, 1,1-dichloroethylene, and vinyl chloride
(Hallen et al., 1986).
1,1,2,2-Tetrachloroethane is not expected to bioaccumulate in
aquatic species, based on low measured and calculated bioconcentration
factors in fish (Government of Canada, 1993).
1 Source: Hazardous Substances Data Bank, National Library of
Medicine, Bethesda, MD, 1996.
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
Data considered to be most representative of current levels of
1,1,2,2-tetrachloroethane in environmental media are presented in
Table 1. Mean concentrations of 1,1,2,2-tetrachloroethane in recent
surveys of ambient air in cities in Canada ranged from <0.1 to 0.25
µg/m3. Maximum concentrations of up to 79 µg/m3 have been detected
in the vicinity of waste sites in the USA (ATSDR, 1994).
Although data are limited, levels of 1,1,2,2-tetrachloroethane in
surface waters in Canada, the USA, and Germany generally range from
<0.005 to 4 µg/litre, from <10 µg/litre to a maximum reported value
of 180 µg/litre, and from <0.03 to 10 µg/litre, respectively; the
chemical was not detected (detection limits 0.001-0.05 µg/litre) in
surface waters in Japan.
1,1,2,2-Tetrachloroethane was not detected in sediment in Japan
in 1976 (detection limits ranged from 0.05 to 1 µg/g dry weight).
6.2 Human exposure
Exposure of the general population to 1,1,2,2-tetrachloroethane
in environmental media may be estimated based on concentrations
determined in various media and reference values for body weight and
consumption patterns. Owing to the paucity of relevant data from
other countries, particularly for recent years, exposure has been
estimated here based primarily on data from North America, as an
example. However, countries are encouraged to estimate total exposure
on the basis of local data, possibly in a manner similar to that
outlined here.
Mean levels in residential indoor air in Canada and the USA are
generally below the limits of detection (i.e. <0.1 µg/m3; see Table
1). Based on a daily inhalation volume for adults of 22 m3, a mean
body weight for males and females of 64 kg, the assumption that 4 of
24 hours are spent outdoors (IPCS, 1994), and the range of mean levels
of 1,1,2,2-tetrachloroethane in ambient air in recent surveys in
Canada of <0.1-0.25 µg/m3, the mean intake of
1,1,2,2-tetrachloroethane from ambient air for the general population
is estimated to range from <0.006 to 0.01 µg/kg body weight per day.
Average intake of 1,1,2,2-tetrachloroethane from indoor air, based on
the assumption that 20 of 24 hours are spent indoors (IPCS, 1994) and
the mean concentration in residential indoor air in Canada and the USA
of <0.1 µg/m3, is estimated to be <0.03 µg/kg body weight per day.
In a survey of 1159 household products in the USA,
1,1,2,2-tetrachloroethane was not detected above the limit of
detection of 0.1% (see Table 1).
1,1,2,2-Tetrachloroethane has not been detected in recent surveys
of drinking-water in Canada and has been only extremely rarely
detected (<0.03%) in recent surveys in the USA (detection limits
0.05-1.0 µg/litre; see Table 1), although it was detected in
groundwater near landfill sites in Finland at levels ranging from
<0.1 to 2.5 µg/litre (Assmuth & Strandberg, 1993). Similarly, it has
not been detected in three surveys of foodstuffs in Canada and the USA
(detection limits were 1 µg/litre for liquids and 5-50 µg/kg for
solids; see Table 1). No data were identified on levels of
1,1,2,2-tetrachloroethane in human breast milk. Drinking-water and
food probably do not represent significant sources of exposure to
1,1,2,2-tetrachloroethane, based on its volatility and low potential
for bioaccumulation.
Therefore, the principal media of exposure to
1,1,2,2-tetrachloroethane for the general population are likely indoor
and outdoor air, with negligible amounts being contributed by food and
drinking-water.
Although data on levels of 1,1,2,2-tetrachloroethane in the
workplace were not identified, workers may be exposed to the substance
via inhalation or dermal contact in "business services" (not further
specified) as well as the chemical and allied products industries
(ATSDR, 1994).
Table 1: Levels of 1,1,2,2-tetrachloroethane in various media.
Medium Location Year Concentrations Reference
Ambient air Canada 1989-1990 <0.1-0.25 µg/m3 (means) Environment Canada,
unpublished data, 1992
Ambient air USA pre-1987 0.7 µg/m3 (mean) Shah & Heyerdahl, 1988
Indoor air Canada 1991 <0.1 µg/m3 (mean) Fellin et al., 1992
Indoor air USA pre-1987 0.098 µg/m3 (mean) Shah & Heyerdahl, 1988
Drinking-water Canada 1988-1991 <0.05 µg/litre P. Lachmaniuk, personal
communication, 1991
1990 <1.0 µg/litre Ecobichon & Allen, 1990
Drinking-water USA pre-1986 <0.5 µg/litre ATSDR, 1994
1984-1992 NDa-5.8 µg/litre Storm, 1994
Surface water Canada 1985 <1.0-4.0 µg/litre COARGLWQ, 1986
1981 <0.005-0.06 µg/litre Kaiser & Comba, 1983
Surface water USA 1980-1988 <10-180 µg/litre ATSDR, 1994
Surface water Japan 1976 <0.001, <0.002, Environment Agency Japan, 1976
<0.05 µg/litre
Surface water Germany 1989-1990 <0.03-10 µg/litre Wittsiepe, 1990
Food (34 groups) Canada 1991 <50 µg/kg (solids), Enviro-Test Laboratories, 1991
<1 µg/litre (liquids)
1992 <5 µg/kg (solids), Enviro-Test Laboratories, 1992
<1 µg/litre (liquids)
Food (231 items) USA <13 µg/kg, <20 µg/kg Daft, 1988
Consumer products USA <0.1% Sack et al., 1992
(1159 items)
Sediment Japan 1976 <0.05 µg/g, <1 µg/g Environment Agency Japan, 1976
a Detection limit not specified.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND
HUMANS
1,1,2,2-Tetrachloroethane is readily absorbed following
inhalation, ingestion, and dermal exposure and is likely distributed
throughout the body, although relevant data are limited. Based on
data on the metabolism of 1,1,2,2-tetrachloroethane in mice, Yllner
(1971) suggested that the principal pathway of degradation involves
stagewise hydrolytic cleavage of the carbon-chlorine bonds and
oxidation to dichloroacetaldehyde hydrate, dichloroacetic acid (the
major metabolite), and eventually glyoxylic acid. The glyoxylic acid
is then metabolized to oxalic acid, glycine, formic acid, and carbon
dioxide. A small proportion of the parent compound is probably
non-enzymatically dehydrochlorinated to trichloroethylene, which is
further converted to trichloroacetic acid and trichloroethanol. In
addition, a minor amount of 1,1,2,2-tetrachloroethane may be oxidized
to tetrachloroethylene, which, in turn, is metabolized to
trichloroacetic acid and oxalic acid. It has also been proposed that
1,1,2,2-tetrachloroethane may be metabolized via cytochrome P-450 to
dichloroacetyl chloride, which is hydrolysed to dichloroacetic acid
(Halpert, 1982). In addition to the liver, metabolism may also occur
in the epithelia of the respiratory tract and upper alimentary tract
(Eriksson & Brittebo, 1991). The metabolites of
1,1,2,2-tetrachloroethane are eliminated in the urine, faeces, skin,
and expired air.
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
The acute toxicity of 1,1,2,2-tetrachloroethane in experimental
animals is slight to moderate. Exposure to concentrations of around
1000 ppm (6980 mg/m3) for 4 or 6 hours or about 5000-6000 ppm
(34 900-41 880 mg/m3) (duration not specified) caused deaths in rats
and mice, respectively. Oral LD50s of 250-330 and 1000 mg/kg body
weight for 1,1,2,2-tetrachloroethane in rats have been reported. The
dermal LD50 (24 hours) in rabbits was 6360 mg/kg body weight (Kennedy
& Graepel, 1991; ATSDR, 1994).
8.2 Irritation and sensitization
Epidermal and dermal changes were reported in rabbits following
cutaneous exposure to 1,1,2,2-tetrachloroethane (Smyth et al., 1969).
Exposure to 580 ppm (4050 mg/m3) 1,1,2,2-tetrachloroethane caused
ocular irritation in guinea-pigs (Price et al., 1978). No information
on the sensitization potential of this substance was identified.
8.3 Short-term exposure
In general, dose-response relationships have not been well
characterized in available short-term studies in experimental animals
owing to limitations of the studies, including use of only one level
of exposure or inadequate description of protocol or results. Hepatic
effects, including increased organ weight, congestion, fatty
degeneration, histological changes, alterations in levels of enzymes,
and elevated DNA synthesis (the degree of which increased with dose),
have been observed in rodents following short-term inhalation of
1,1,2,2-tetrachloroethane at concentrations as low as 13.3 mg/m3 (for
2-10 days) and ingestion of the chemical at doses as low as 75 mg/kg
body weight per day (for 4 days) in the few available, principally
limited, studies (Horiuchi et al., 1962; Gohlke & Schmidt, 1972;
Schmidt et al., 1972; Hanley et al., 1988; NTP, 1996). In a limited
account of a study in rats, Ulanova et al. (1984) reported effects on
the nervous system and kidneys to be similar following continuous or
intermittent exposure for 4-27 days to comparable
time-weighted-average concentrations of 1,1,2,2-tetrachloroethane
(235 and 250 mg/m3).
8.4 Long-term exposure
8.4.1 Subchronic exposure
Only a few limited studies have been identified on the effects in
experimental animals following subchronic exposure to
1,1,2,2-tetrachloroethane (see Table 2).1 Ingestion of up to 316
mg/kg body weight per day had no effects on body weight gain or
mortality in groups of five male or female B6C3F1 mice, whereas doses
of 100 (females) or 178 (males) mg/kg body weight per day and above
resulted in decreased body weight gain in groups of five male or
female Osborne-Mendel rats in subchronic studies preliminary to
longer-term bioassays (no other end-points appear to have been
examined) (NCI, 1978). Histopathological damage (including chronic
inflammation, necrosis, or atrophy) was observed in the liver, kidney,
testicles, and thyroid gland of rats ( n = 10 per group) administered
oral 1,1,2,2-tetrachloroethane doses of 3.2-50 mg/kg body weight per
day for periods ranging from 2 to 150 days (Gohlke et al., 1977),
although the limited documentation of results in this study precludes
validation of an effect level.
Exposure to 1,1,2,2-tetrachloroethane at 50 mg/m3 for
approximately 5 weeks resulted in alterations in biochemical
parameters and organ weights in male rats (strain and number not
specified), although no "morphological changes" were noted upon
examination (the nature and extent of the histopathological
examination were unspecified) (Schmidt et al., 1975). Depressed
agglutinin formation was observed in rabbits exposed to 100 mg/m3,
3-4 hours/day, for 4-6 weeks (Navrotskiy et al., 1971) (no other
effects were noted in this study, for which only secondary accounts
were available). Hepatic effects, including a transient increase in
DNA synthesis, reversible histopathological changes (cytoplasmic
vacuolization and hyperplasia), and an increase in relative liver
weight, were observed in female Sprague-Dawley rats ( n = 55) exposed
for 15 weeks to 560 ml/m3 (reported by ATSDR [1994] to be equivalent
to 130 ppm [907 mg/m3], although information on the exposure level
presented in the original paper was unclear) (Truffert et al., 1977).
8.4.2 Chronic exposure and carcinogenicity
The chronic toxicity of 1,1,2,2-tetrachloroethane has not been
extensively investigated; available studies are not adequate to allow
the confident determination of an "effect level" for non-neoplastic
effects. A reversible decrease in body weight and a reversible
1 Subchronic studies have been completed for the National Toxicology
Program (NTP) in which groups of 10 male or female F344 rats and
B6C3F1 mice were administered microencapsulated
1,1,2,2-tetrachloroethane in feed at doses equivalent to 18-300 mg/kg
body weight per day and 88-1400 mg/kg body weight per day,
respectively, for 13 weeks. The results of these studies are
currently being reviewed by the NTP's Pathology Working Group.
increase in lipid content of the liver were observed in male rats
exposed to 1,1,2,2-tetrachloroethane by inhalation at 13.3 mg/m3 for
110 or 265 days or for 265 days with a 60-day recovery period (seven
rats were killed at each interval); there were also reversible
alterations in haematological parameters, which were statistically
significantly different from controls only at one point in time during
the study, and increased adrenocorticotropic hormone activity in the
hypophysis (Schmidt et al., 1972). However, histopathological effects
were not described in the published account of the investigation. In
a study for which only secondary accounts were available, early signs
of liver degeneration were observed in rabbits exposed to 100 mg/m3
for 7-11 months (Navrotskiy et al., 1971) (no further details were
provided).
An increase in the incidence of hepatocellular carcinomas was
observed in groups of 50 ( n = 20 in controls) male and female
B6C3F1 mice administered technical-grade 1,1,2,2-tetrachloroethane in
corn oil by gavage at time-weighted-average daily doses of 142 or 284
mg/kg body weight for 78 weeks (1/18, 13/50, and 44/49 in males, and
0/20, 30/48, and 43/47 in females, in the vehicle controls, low-dose
group, and high-dose group, respectively). These tumours also
appeared earlier in mice administered the higher dose. Slightly
decreased body weight gain and increased mortality were also observed
in exposed mice; there were no increases in the incidences of non-
neoplastic lesions (NCI, 1978).
There were no significant increases in the incidence of any type
of neoplastic or non-neoplastic lesion in groups of 50 ( n = 20 in
controls) male or female Osborne-Mendel rats similarly administered
technical-grade 1,1,2,2-tetrachloroethane in corn oil by gavage at
time-weighted-average doses of 62 or 108 mg/kg body weight per day
(males) and 43 or 76 mg/kg body weight per day (females) for 78 weeks,
although there were two males with hepatocellular carcinomas and one
with a hepatic neoplastic nodule in the high-dose group. There were
also reversible dose-related decreases in body weight gain and
increased mortality in exposed rats (NCI, 1978).
In a limited bioassay designed to investigate the potential of
1,1,2,2-tetrachloroethane to induce pulmonary adenomas in a sensitive
strain of mice, there was no increase in the number of these tumours
in a group of 20 strain A mice intraperitoneally administered the
chemical for 24 weeks; however, mortality was high in this study
(Theiss et al., 1977; Stoner, 1991).
In an initiation/promotion assay, 1,1,2,2-tetrachloroethane did
not initiate formation of gamma-glutamyltranspeptidase-positive foci
in the liver (a putative preneoplastic indicator) in groups of 10 male
Osborne-Mendel rats administered an oral dose of 100 mg/kg body weight
followed by exposure to phenobarbital for 7 weeks, although it acted
as a potent promoter in rats initiated with a single dose of
diethylnitrosamine followed by exposure to 1,1,2,2-tetrachloroethane
by gavage for 7 weeks at 100 mg/kg body weight per day (Story et al.,
1986; Milman et al., 1988).
Table 2: Investigations of non-neoplastic effects of 1,1,2,2-tetrachloroethane.
Study design Effects Effect level Comments Reference
INHALATION
Male rats exposed to 50 mg/m3, 4 Neurological effects; alterations in Effects at 50 Strain and number of rats not Schmidt et al.,
hours/day, 5 days/week, for 5 weeks; biochemical parameters and organ mg/m3 specified; nature and extent of 1975
or to 130 mg/m3 for 15 minutes, 5 weights (although within ranges histopathological examination
times/day, separated by 40-minute observed in controls); no not specified
intervals, for 5 weeks "morphological changes"
Fifty-five female Sprague-Dawley Transient increase in hepatic DNA Effects at 560 One exposure group only; Truffert et
rats exposed to 560 ml/m3 for 5 or synthesis, reversible ml/m3 uncertainty concerning exposure al., 1977
6 hours/day, 5 days/week, for 15 histopathological changes in the (equivalent to level based on unclear
weeks; liver, kidneys, lungs, liver; increase in relative liver 130 ppm or information in article (note:
ovaries, uterus, and adrenal weight 907 mg/m3, concentration more than
glands histopathologically examined based on ATSDR approximately 10-fold higher
[1994] than that at which effects
conversion were reported in other
studies, no matter how converted)
Male rats exposed to 13.3 mg/m3 Increased adrenocorticotropic Minimal Exposure pattern (e.g. number Schmidt et
(probably for 4 hours/day) for 110 hormone activity in the hypophysis effects at of exposed days per week) not al., 1972
or 265 days; one group exposed for greatest at 4 months and lessened 13.3 mg/m3 clearly specified;
265 days and allowed to recover towards the end of the study; histopathological effects not
until day 325; seven rats reversible decrease in body weight; described in published account
sacrificed after each interval reversible increase in lipid of study
content of liver and reversible
alterations in haematological
parameters, which were significantly
different from controls only at one
point in time during the study
Table 2 (continued)
Study design Effects Effect level Comments Reference
Rabbits exposed to 100 mg/m3 3-4 Depressed agglutinin formation Effects at Secondary accounts available Navrotskiy et
hours/day for 4-6 weeks or exposed after 4-6 weeks; early signs of 100 mg/m3 only; strain, number, and sex al., 1971
for 7-11 months (different liver degeneration after 7-11 not specified; may be only
protocols noted in two secondary months one exposure level; no other
accounts) effects noted
Chinchilla rabbits exposed to 0, Decrease in titres of typhoid Effects at Sex and number of animals per Shmuter, 1977
2, 10, or 100 mg/m3, 3 hours/day, antibodies, an increase in the 10 mg/m3; no exposed group not specified
6 days/ week, for 8 months electrophoretic mobility of effects at 2
(n = 50 for controls) antibodies towards ß- and mg/m3
alpha-globulin fractions, and
a decrease in the level of
"normal" haemolysins to the
Forsman's antigen of sheep
erythrocytes
Six rabbits were exposed to Decreased levels of Effects at Isomer not specified; no Kulinskaya &
10 mg/m3, 3 hours/day, for acetylcholine and 10 mg/m3 end-points other than Verlinskaya,
approximately 8 months; 15 rabbits acetylcholinesterase in the cholinergic indices were 1972
were used as controls; animals blood investigated
were immunized at 1.5 and 4.5
months with typhoid vaccine
Table 2 (continued)
Study design Effects Effect level Comments Reference
INGESTION
Groups of 10 rats administered Damage to liver, kidney, testicles, Effects at Inadequate documentation of Gohlke et
3.2, 8.0, 20, or 50 mg/kg body and thyroid gland (determined by 3.2 mg/kg protocol and results; no al., 1977
weight per day by gavage for histological, enzyme histochemical, body weight quantitative data; not reported
2-150 days and histoautoradiographic per day in which dose groups effects
techniques) were observed (some groups were
also concomitantly exposed to
high temperatures); not possible
to verify effect level
Five male or female B6C3F1 mice No effects on body weight gain No effects at No end-points other than body NCI, 1978
administered 0, 32, 56, 100, or mortality highest dose weight and mortality appear to
178, or 316 mg/kg body weight of 316 mg/kg have been examined
per day by gavage, 5 days/week, body weight
for 6 weeks, followed by 2 per day
weeks of observation
Five male or female Osborne-Mendel Decrease in body weight gain in Effects at No end-points other than body NCI, 1978
rats administered 0, 56, 100, 178, males at 178 mg/kg body weight 100 mg/kg weight and mortality appear to
316, or 562 mg/kg body weight per per day and in females at 100 body weight have been examined; no data
day by gavage, 5 days/week, for and 178 mg/kg body weight per day; per day; no presented on effects on body
6 weeks, followed by 2 weeks of all females exposed to 316 mg/kg effects at 56 weight gain at two highest
observation body weight per day died; one male mg/kg body doses or on mortality for other
exposed to 100 mg/kg body weight weight per dose groups
per day died day
Table 2 (continued)
Study design Effects Effect level Comments Reference
Fifty male or female B6C3F1 mice Slight dose-related decreases in Significance of decrease in NCI, 1978
(n = 20 in controls) administered body weight gain; dose-related body weight gain not presented;
time-weighted-average doses of 0, increases in mortality; no no end-points other than body
142, or 284 mg/kg body weight per increases in incidences of weight, mortality, or
day by gavage, 5 days/week, for non-neoplastic lesions histopathology were examined
78 weeks, followed by 12 weeks
of observation
Fifty male or female Osborne-Mendel Reversible dose-related decreases Significance of decrease in NCI, 1978
rats (n = 20 in controls) in body weight gain; increased body weight gain not presented;
administered time-weighted-average mortality at higher dose; no no end-points other than body
doses of 0, 62, or 108 mg/kg body increases in incidences of weight, mortality, or
weight per day (male) or 0, 43, or non-neoplastic lesions histopathology were examined
76 mg/kg body weight per day
(female) by gavage, 5 days/week,
for 78 weeks, followed by 32 weeks
of observation
Little information on the mechanism(s) of liver tumour induction
in mice exposed to 1,1,2,2-tetrachloroethane has been identified.
Several of the metabolites of 1,1,2,2-tetrachloroethane, including
trichloroethylene, tetrachloroethylene, trichloroacetic acid, and
dichloroacetic acid, have been hepatocarcinogenic in experimental
animals (e.g. NCI, 1977; Maltoni et al., 1986, 1988; NTP, 1986, 1990;
Herren-Freund et al., 1987; Bull et al., 1990; DeAngelo et al., 1991).
Indeed, the toxicological profile for 1,1,2,2-tetrachloroethane is
very similar to that for dichloroacetic acid, the principal
metabolite.
8.5 Genotoxicity and related end-points
Results of identified in vitro studies are summarized in Table
3. Predominantly negative results have been reported for the
induction of gene mutation in prokaryotic systems with and without
metabolic activation, whereas both positive and negative results have
been observed for gene conversions in yeast and fungi.
1,1,2,2-Tetrachloroethane induced sister chromatid exchange but not
chromosomal aberrations, DNA repair, or unscheduled synthesis of DNA
in mammalian cells in vitro.
Exposure to 1,1,2,2-tetrachloroethane at 349 mg/m3 for 5 days
did not induce dominant lethal mutations in rats, and results for
chromosomal aberrations in rat bone marrow cells were equivocal;
however, this concentration did not induce cytotoxicity (McGregor,
1980). 1,1,2,2-Tetrachloroethane did not induce unscheduled DNA
synthesis in hepatocytes of mice exposed to doses of up to 1000 mg/kg
body weight by gavage, whereas results for the induction of S-phase
synthesis were negative and equivocal (Mirsalis et al., 1989).
1,1,2,2-Tetrachloroethane has also been reported to bind to
cellular macromolecules, including DNA, RNA, and proteins of several
organs in rodents, following in vivo exposure (Mitoma et al., 1985;
Colacci et al., 1987; Eriksson & Brittebo, 1991). Results for cell
transformation in mammalian cells have been mixed, with positive
results being reported by only one of four investigators (Little,
1983; Tu et al., 1985; Milman et al., 1988; Colacci et al., 1990,
1992, 1993).
1,1,2,2-Tetrachloroethane did not induce sex-linked recessive
lethal mutations or mitotic recombination in Drosophila
melanogaster in three studies (McGregor, 1980; Woodruff et al.,
1985; Vogel & Nivard, 1993).
With the possible exception of the equivocal results for
chromosomal aberrations observed in female rats following inhalation
(McGregor, 1980), the weight of evidence overall indicates that
1,1,2,2-tetrachloroethane is not genotoxic or that it is only weakly
genotoxic, acting through a mechanism that results in gene conversion
and induction of sister chromatid exchange.
Table 3: Genotoxicity of 1,1,2,2-tetrachloroethane in vitro.
Result
With Without
Species (test system) End-point activation activation Reference
Saccharomyces cerevisiae D7 Mitotic gene conversion nt + Callen et al., 1980
Recombination nt +
Saccharomyces cerevisiae D7 Gene conversion and reversion Nestmann and Lee, 1983
XV185-14C nt -
nt -
Salmonella typhimurium Reverse mutations Brem et al., 1974
TA1530 nt +
TA1535 nt +
TA1538 nt -
Salmonella typhimurium Reverse mutations Nestmann et al., 1980
TA1535 - -
TA100 - -
TA1537 - -
TA1538 - -
TA98 - -
Salmonella typhimurium Reverse mutations Milman et al., 1988
TA1535 - -
TA1537 - -
TA98 - -
TA100 - -
Salmonella typhimurium Reverse mutations Haworth et al., 1983
TA1535 - -
TA1537 - -
TA98 - -
TA100 - -
Table 3 (continued)
Result
With Without
Species (test system) End-point activation activation Reference
Salmonella typhimurium TA100 Reverse mutations - - Warner et al., 1988
Salmonella typhimurium Reverse mutations Mersch-Sundermann et al.,
TA97 + - 1989a
TA98 + -
TA100 - -
TA102 - -
Salmonella typhimurium Forward mutations - - Roldan-Arjona et al., 1991
BA13/BAL13
Escherichia coli (polymerase DNA damage nt + Brem et al., 1974
deficient pol A+/pol A-)
Escherichia coli PQ37 Gene mutation - - Mersch-Sundermann et al.,
1989b
Escherichia coli Induction of prophage lambda + - DeMarini & Brooks, 1992
Aspergillus nidulans Mitotic malsegregation nt + Crebelli et al., 1988
Chinese hamster ovary cells Chromosomal aberrations - - Galloway et al., 1987
Chinese hamster ovary cells Sister chromatid exchange + + Galloway et al., 1987
BALB/c3T3 cells (mouse) Sister chromatid exchange + + Colacci et al., 1992
Mouse hepatocytes DNA growth, repair, or synthesis nt - Williams, 1983
Table 3 (continued)
Result
With Without
Species (test system) End-point activation activation Reference
Mouse hepatocytes DNA repair nt - Milman et al., 1988
Rat hepatocytes DNA growth, repair, or synthesis nt - Williams, 1983
Rat hepatocytes DNA repair nt - Milman et al., 1988
Human embryonic intestinal cells Unscheduled DNA synthesis - - McGregor, 1980
nt = not tested
8.6 Reproductive and developmental toxicity
Although available data are limited, reproductive and
developmental effects have been observed only in experimental animals
exposed orally or by inhalation to levels of 1,1,2,2-tetrachloroethane
that are also associated with decreases in body weight. Effects on
reproductive parameters, including decreases in testicular,
epididymal, and caudal weights, decreased epididymal sperm motility,
and altered estrous cycles, were observed in pilot studies in rats and
mice orally exposed for 90 days to doses that also caused significant
decreases in body weight (NTP, 1993). Although histological changes
in the testes have been observed in rats administered
1,1,2,2-tetrachloroethane doses of 8 mg/kg body weight per day in
peanut oil by gavage for 150 days (Gohlke et al., 1977), no effects on
reproductive organs were reported in the long-term studies in which
rats and mice were administered much higher doses for 78 weeks (NCI,
1978) (see section 8.4.2) or in inhalation studies in rats (Gohlke &
Schmidt, 1972; Schmidt et al., 1972) or a single monkey (Horiuchi et
al., 1962). No effect on male fertility or viability and no
macroscopic changes in offspring were observed in male rats exposed to
13.3 mg/m3 for 258 days (Schmidt et al., 1972). Small, but
statistically significant, increases in one type of sperm abnormality
were observed in rats exposed to 349 mg/m3 for 5 days, although the
authors considered this effect to be of questionable biological
significance (McGregor, 1980). Decreased fetal body weight and/or
increased resorptions were observed in range-finding studies in rats
and mice exposed to 1,1,2,2-tetrachloroethane in the feed during
gestation at doses greater than those that induced maternal toxicity
(increased mortality or decreased body weight gain) (NTP, 1991a,b).
8.7 Immunological and neurological effects
Immunological effects have been observed in limited studies in
rabbits exposed to 1,1,2,2-tetrachloroethane by inhalation. For
example, Shmuter (1977) reported a decrease in the titres of typhoid
antibodies, an increase in the electrophoretic mobility of antibodies
towards ß- and alpha-globulin fractions, and a decrease in the level
of "normal" haemolysins to the Forsman's antigen of sheep erythrocytes
in animals exposed to 1,1,2,2-tetrachloroethane at 10 mg/m3 and above
for 8 months, whereas alterations in levels of acetylcholine and
acetylcholinesterase in the blood have been observed at 10 mg/m3
(Kulinskaya & Verlinskaya, 1972).
Neurological effects have been observed in several species
following acute or short-term exposure to 1,1,2,2-tetrachloroethane
(e.g. at concentrations as low as 200 ppm [1396 mg/m3] for 6 hours
[Horvath & Frantik, 1973] or 50 mg/m3 for approximately 5 weeks
[Schmidt et al., 1975]). A single oral dose of 50 mg/kg body weight
increased levels of several neurotransmitters in the brain of rats
(Kanada et al., 1994).
9. EFFECTS ON HUMANS
Death has been reported following suicidal ingestion of doses of
1,1,2,2-tetrachloroethane estimated to range from 285 to 6000 mg/kg
body weight (ATSDR, 1994). Hepatic effects and death have also been
reported following accidental poisoning with
1,1,2,2-tetrachloroethane. Other effects noted in earlier reports of
workers or volunteers exposed to 1,1,2,2-tetrachloroethane
concentrations ranging up to 1800 mg/m3 include respiratory failure,
mucosal irritation, unconsciousness, gastrointestinal and neurological
distress, jaundice, liver enlargement or degeneration, headache,
tremors, dizziness, numbness, and drowsiness (ATSDR, 1994).
No statistically significant increase in mortality due to any
specific cause was noted in a limited epidemiological investigation in
a population of 1099 men exposed to unknown concentrations of
"tetrachloroethane" (Norman et al., 1981). The prevalence of nervous
symptoms, including tremors, headaches, and vertigo, was reported to
increase with airborne concentration of 1,1,2,2-tetrachloroethane (up
to 98 ppm [684 mg/m3]) in a group of 380 workers in India exposed for
varying durations, although no information was presented on the
prevalence of these signs in an unexposed group. Exposed workers also
reported loss of appetite, nausea, vomiting, and abdominal pain (Lobo-
Mendonca, 1963). Similar symptoms (i.e. loss of appetite, bad taste
in the mouth, epigastric pain, sensation of pressure in the liver
area, headaches, general debility, lack of stamina, loss of body
weight, and occasional painful prurigo) were observed in employees of
a penicillin plant exposed to concentrations of
1,1,2,2-tetrachloroethane ranging from 10 to 1700 mg/m3. The
prevalence of symptoms decreased with the implementation of
improvements in working conditions, and most workers were reported to
be free of symptoms when maximum levels were below 250 mg/m3 (Jeney
et al., 1957).
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10.1 Aquatic environment
Bioassays were conducted by Blum & Speece (1991) on three groups
of bacteria: methanogens (anaerobes from an enrichment culture
maintained for >10 years), aerobic heterotrophic bacteria, and
Nitrosomonas obtained from the mixed liquor of an activated sludge
wastewater treatment plant. Inhibition of gas production by
methanogens, inhibition of oxygen uptake by aerobic heterotrophic
bacteria, and inhibition of ammonia oxidation by Nitrosomonas were
the end-points used in this study to evaluate toxicity. Varying
degrees of sensitivities were exhibited; however, Nitrosomonas, with
an IC50 value of 1.4 mg/litre, was more sensitive than methanogens
(IC50 value of 4.1 mg/litre) and significantly more sensitive than
aerobic heterotrophs (IC50 value of 130 mg/litre).
Based on bioluminescence, the 5-minute LC50 for
1,1,2,2-tetrachloroethane was 5.4 mg/litre in a Microtox test using
Photobacterium phosphoreum (Blum & Speece, 1991).
Unfed and fed Daphnia magna (first instar, <24 hours old) had
similar measured 48-hour LC50 values of 62 and 57 mg/litre,
respectively, under static test conditions (Richter et al., 1983).
With complete immobilization as the end-point, the 48-hour EC50
values were 23 and 25 mg/litre for unfed and fed D. magna,
respectively. LeBlanc (1980) conducted a similar test with
D. magna at 22°C and reported nominal 24-hour and 48-hour LC50
values of 18 and 9.3 mg/litre, respectively. Pawlisz & Peters (1995)
reported that prior exposure of D. magna to sublethal concentrations
of 1,1,2,2-tetrachloroethane (6.3-50% of the 48-hour LC50 of 0.095
mmol/litre) for 24 hours did not influence the body burden required to
narcoticize the animals upon subsequent exposure to the chemical at
the LC50.
The measured 28-day LOEC and no-observed-effect concentration
(NOEC) for reproductive impairment in D. magna were 14.4 and 6.9
mg/litre, respectively, under flow-through conditions (Richter et al.,
1983).
Numerous acute toxicity studies have been conducted on a variety
of freshwater fish species; in general, 96-hour LC50 values were very
similar. Under flow-through conditions, the measured 96-hour LC50s
for 30-day-old fathead minnows (Pimephales promelas) were 20.3 and
20.4 mg/litre (Veith et al., 1983; Walbridge et al., 1983). In
juvenile (2- to 4-month-old) flagfish, the measured 96-hour LC50 for
1,1,2,2-tetrachloroethane in the flow-through toxicity test was 18.5
mg/litre; the nominal LC50 value in a static-renewal 96-hour toxicity
test was 26.8 mg/litre (ATRG, 1988; Smith et al., 1991). No adequate
acute toxicity studies of marine fish were identified.
Chronic toxicity studies under flow-through test conditions were
conducted on the early life stages of flagfish by ATRG (1988) and
Smith et al. (1991). Egg hatchability was unaffected at a measured
1,1,2,2-tetrachloroethane concentration of 22.0 mg/litre, the highest
concentration tested in both studies. The measured LOECs for reduced
10-day larval survival were 10.6 and 7.2 mg/litre, whereas the LOECs
for 28-day juvenile survival were 11.7 and 8.5 mg/litre (ATRG, 1988;
Smith et al., 1991). There were no statistically significant effects
on the growth of 1-week-old fry over a 28-day exposure period, even at
the highest concentration of 1,1,2,2-tetrachloroethane tested (11.7
mg/litre).
Ninety-day carcinogenicity studies were conducted under
flow-through conditions on 2-day-old guppy (Poecilia reticulata) and
6-day-old Japanese medaka (Oryzias latipes) exposed continuously to
1,1,2,2-tetrachloroethane at 4 mg/litre, exposed once per week (24
hours) to 8 mg/litre, or exposed once per week (24 hours) to 15
mg/litre. Histopathological examination of all exposed groups at 90
days did not reveal any evidence of carcinogenicity (Hawkins, 1991).
10.2 Terrestrial environment
No studies were identified on the effects of
1,1,2,2-tetrachloroethane on terrestrial organisms.
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
Owing to the significant decline in the use of this substance,
the toxicological profile of 1,1,2,2-tetrachloroethane has not been
well characterized, with the available data being confined primarily
to early limited studies.
Based on the results of studies in experimental animals, the
acute toxicity of 1,1,2,2-tetrachloroethane is slight to moderate.
The chemical may induce skin, eye, and mucosal irritation. Owing to
the limitations of the available data in humans on effects associated
with longer-term exposure to 1,1,2,2-tetrachloroethane, it is
necessary to rely on information obtained from the limited studies in
animals for determination of the critical effects and associated
effect levels.
The results of available studies on the non-neoplastic effects of
1,1,2,2-tetrachloroethane in experimental animals exposed by ingestion
or inhalation indicate that the liver is the principal target organ.
However, the majority of subchronic and chronic studies are too
limited to allow a confident determination of a NO(A)EL or LO(A)EL for
hepatic or other effects, because of either the lack of information
presented in the published accounts or the limitations of the study
designs (e.g. small numbers of animals per experimental group, lack of
histopathological examination, etc.).
Long-term exposure to 1,1,2,2-tetrachloroethane resulted in a
significantly increased incidence of hepatocellular carcinomas in both
male and female mice. However, no significant increases in tumours
were observed in similarly exposed rats, although there was a non-
statistically significant increase at the highest dose tested (which
was lower, on a time-weighted-average basis, than the lowest dose to
which mice were exposed), and both species were exposed only for up to
78 weeks. 1,1,2,2-Tetrachloroethane was a potent promoter, but did
not act as an initiator, in an initiation/promotion assay. The weight
of evidence of available in vitro and in vivo assays suggests that
this substance is not genotoxic or that it is, at most, weakly
genotoxic. Although available data are incomplete, it has been
proposed that the liver tumours may be induced by mechanisms that may
not be relevant to humans, for which humans are less susceptible, or
for which there may be a threshold of exposure. In addition, it has
been hypothesized that the carcinogenicity of
1,1,2,2-tetrachloroethane may be associated with the formation of free
radicals, lipid peroxidation, or hepatic damage (such as focal
necrosis associated with intense cellular proliferation) (Hanley et
al., 1988; Larson & Bull, 1992; Paolini et al., 1992). Therefore, on
the basis of data currently available, it is not possible to draw any
firm conclusions with respect to the potential carcinogenicity of
1,1,2,2-tetrachloroethane in humans.
Owing to the limitations of available studies on the potential
toxicological effects associated with exposure to
1,1,2,2-tetrachloroethane, it is not possible to confidently determine
a NO(A)EL or LO(A)EL for non-neoplastic effects. The toxicological
end-point for which the dose-response relationship is best
characterized is the increase in hepatocellular carcinomas observed in
the long-term bioassay in mice (NCI, 1978). It is noted, however,
that the observed increases in tumour incidence are restricted to one
species and that the weight of available data indicates that
1,1,2,2-tetrachloroethane is, at most, weakly genotoxic.
Based on multistage modelling of the incidence of hepatocellular
carcinomas in male or female mice exposed to time-weighted-average
doses of 0, 142, or 284 mg/kg body weight per day for up to 78 weeks,
adjusted for continuous exposure for a standard duration of 104 weeks
and corrected for the expected rate of increase in tumour formation in
rodents in a standard bioassay of 104 weeks, the doses associated with
a 5% increase in tumour incidence (TD0.05) range from 5.8 to 28 mg/kg
body weight per day.
11.1.2 Criteria for setting guidance values for
1,1,2,2-tetrachloroethane
As noted in section 11.1.1, the toxicological end-point for which
the dose-response relationship is best characterized, and which might
provide the basis for derivation of limits of exposure or for
judgement of the quality of environmental media by relevant
authorities, is the increase in hepatocellular carcinomas observed in
the long-term bioassay in mice (NCI, 1978).
A value, for example, 5000 or 50 000 times less than the TD0.05s
derived above might be considered conservative as a guidance value.
This margin (5000-50 000) affords protection similar to that
associated with the range for low-dose risk estimates generally
considered by various agencies to be "essentially negligible" (i.e.
10-5 to 10-6). As, on the basis of available data,
1,1,2,2-tetrachloroethane is, at most, weakly genotoxic, a smaller
margin (e.g. 1000) might also be considered appropriate. As available
data indicate that air is the principal medium of human exposure, the
most conservative of these approaches result in, for example, a range
of airborne concentrations of 3.4-16 µg/m3 or 0.34-1.6 µg/m3,
respectively. Corresponding values for ingestion are 1.2-5.6 µg/kg
body weight per day or 0.12-0.56 µg/kg body weight per day. It should
be noted, however, that these possible guidance values for air have
been extrapolated directly from a study in which the chemical was
administered orally to experimental animals. Although there may be
substantial variations in toxicokinetics following exposure to
1,1,2,2-tetrachloroethane by different routes, available data are
inadequate to quantitatively account for these differences in the
derivation of guidance values.
It is noteworthy that a provisional tolerable concentration
derived on the basis of the minimal non-neoplastic effects observed in
rats exposed to 13.3 mg/m3 (Schmidt et al., 1972) would fall within
the range of the values presented here.
11.1.3 Sample risk characterization
Although data are insufficient to allow the confident
determination of a LO(A)EL or NO(A)EL for 1,1,2,2-tetrachloroethane,
minimal effects in rodents have been observed only at levels more than
50 000 times greater than those in the principal medium of exposure
(air) in the general environment.
Based on a sample estimate of exposure, indirect exposure in the
general environment is 14 to >160 or 1.4 to >16 times less than
guidance values that might be derived on the basis of available data
on the dose-response relationship for liver tumour induction in mice
(i.e. the TD0.05s divided by 5000 or 50 000, or 3.4-16 µg/m3 or
0.34-1.6 µg/m3, respectively). It should also be noted, however,
that indirect exposure in the general environment is likely
overestimated here, as it is based on the range of mean concentrations
for detected values, although 1,1,2,2-tetrachloroethane was detected
in only approximately 50% of samples.
11.2 Evaluation of environmental effects
1,1,2,2-Tetrachloroethane is released to the environment
principally in emissions to ambient air, where it is moderately
persistent. Because of its volatility, rapid photo-oxidation in the
atmosphere, and an atmospheric ozone-depleting potential of less than
0.001 relative to CFC-11, 1,1,2,2-tetrachloroethane is not expected to
contribute significantly either to the depletion of the stratospheric
ozone layer or to global warming.
Terrestrial organisms have the greatest potential for exposure to
1,1,2,2-tetrachloroethane in ambient air in the environment. However,
no data were identified on the effects of 1,1,2,2-tetrachloroethane in
terrestrial species. Therefore, it is not possible to characterize
the risk to these organisms associated with levels of
1,1,2,2-tetrachloroethane present in the environment.
Although 1,1,2,2-tetrachloroethane may be released to surface
waters in industrial effluents, it is rapidly removed by
volatilization. Based on the results of several studies in aquatic
bacteria, invertebrates, and fish, effect levels are generally greater
than 1 mg/litre. Although data are limited, concentrations of
1,1,2,2-tetrachloroethane in surface waters are generally much less
than this value (at least two orders of magnitude). Therefore, it is
likely that 1,1,2,2-tetrachloroethane does not pose significant risk
to aquatic organisms.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The International Agency for Research on Cancer (IARC, 1987) has
classified 1,1,2,2-tetrachloroethane in group 3 (not classifiable as
to its carcinogenicity to humans), based on inadequate evidence of
carcinogenicity in humans and limited evidence in animals.
Information on international hazard classification and labelling
is included in the International Chemical Safety Card reproduced in
this document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventative and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 0332) reproduced in this
document.
13.1 Advice to physicians
In case of emergency, it is important to wash skin with soap and
water after removing contaminated clothing. Like others of this
class, 1,1,2,2-tetrachloroethane could generate some hyperexcitability
of the heart. The prognosis following intoxication with this chemical
is that rapid progression of jaundice indicates a poor outcome. In
some instances, mild symptoms will persist up to 3 months and then
progress to acute yellow atrophy and death. Anuria may persist for as
long as 2 weeks and still be followed by complete recovery.
13.2 Health surveillance advice
Annual blood counts and monitoring of both liver and kidney
function should be included in a health surveillance programme of
individuals exposed to 1,1,2,2-tetrachloroethane.
13.3 Prevention
Because 1,1,2,2-tetrachloroethane decomposes on burning,
maintenance workers must wait until all liquid and vapour have been
cleared from the container or piping before performing any duty that
generates heat.
Fire-fighters need to wear chemical-resistant clothing and
positive self-contained breathing apparatus.
13.4 Spillage
It is very important in the case of spillage to use full
protection, including respiratory protection, because
1,1,2,2-tetrachloroethane passes through the skin and, under the
influence of air, moisture, and ultraviolet light, will decompose,
producing toxic and corrosive gases, such as hydrogen chloride and
phosgene.
The IDLH (Immediately Dangerous to Life or Health) value for this
substance is very low, at 100 ppm (698 mg/m3) (NIOSH, 1994).
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards is
available from the International Register of Potentially Toxic
Chemicals (IRPTC) legal file.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
1,1,2,2-TETRACHLOROETHANE ICSC:0332
1,1,2,2-TETRACHLOROETHANE
Acetylene tetrachloride
Symmetrical-tetrachlorethane
S-tetrachlorethane
CHCl2CHCl2
Molecular mass: 167.9
CAS # 79-34-5
RTECS # KI8575000
ICSC # 0332
UN # 1702
EC # 602-015-00-3
TYPES OF ACUTE HAZARDS/ PREVENTION FIRST AID/
HAZARD/ SYMPTOMS FIRE FIGHTING
EXPOSURE
FIRE Not flammable. Gives off NO open flames. In case of fire in the
irritating or toxic fumes (or surroundings: all
gases) in a fire. extinguishing agents allowed.
EXPLOSION In case of fire: keep drums,
etc., cool by spraying with
water.
EXPOSURE STRICT HYGIENE!
* INHALATION Abdominal pain. Cough. Ventilation, local exhaust, Fresh air, rest. Artificial
Dizziness. Headache. Nausea. or breathing protection. respiration if indiciated.
Sore throat. Vomiting. Refer for medical attention.
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
TYPES OF ACUTE HAZARDS/ PREVENTION FIRST AID/
HAZARD/ SYMPTOMS FIRE FIGHTING
EXPOSURE
* SKIN MAY BE ABSORBED! Dry skin. Protective gloves. Protective Remove contaminated clothes.
Tremors (further see clothing. Rinse skin with plenty of
Inhalation). water or shower. Refer for
medical attention.
* EYES Redness. Pain. Face shield or eye protection First rinse with plenty of
in combination with breathing water for several minutes
protection. (remove contact lenses if
easily possible), then take
to a doctor.
* INGESTION Abdominal pain. Nausea. Do not eat, drink, or smoke Do NOT induce vomiting. Rest.
Vomiting (further see during work. Refer for medical attention.
Inhalation).
SPILLAGE DISPOSAL STORAGE PACKAGING & LABELLING
Ventilation. Collect leaking and Separated from strong bases, food and Airtight. Do not transport with food
spilled liquid in sealable containers feedstuffs. Cool. Keep in the dark. and feedstuffs.
as far as possible. Absorb remaining Well closed. Keep in a well-ventilated
liquid in sand or inert absorbent and room. T+ symbol
remove to safe place. Do NOT let this
chemical enter the environment (extra R: 26/27-51/53
personal protection: complete S: (1/2-)38-45-61
protective clothing including
self-contained breathing apparatus). UN Hazard Class: 6.1
UN Packing Group: II
Marine pollutant.
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
IMPORTANT DATA PHYSICAL STATE; APPEARANCE: EFFECTS OF SHORT-TERM EXPOSURE:
COLOURLESS LIQUID, WITH CHARACTERISTIC ODOUR. A harmful contamination of the air can be
reached rather quickly on evaporation of this
substance at 20°C.
PHYSICAL DANGERS: EFFECTS OF SHORT-TERM EXPOSURE:
The vapour is heavier than air. The substance irritates the eyes and the
respiratory tract. The substance may cause
CHEMICAL DANGERS: effects on the central nervous system, kidneys
The substance decomposes on burning under and liver, resulting in depression of the
influence of air, moisture and UV light, central nervous system, kidney impairment and
producing toxic and corrosive gases including liver impairment. Exposure may result in
hydrogen chloride and phosgene. Reacts unconsciousness. Exposure may result in death.
violently with alkali metals, strong bases and
many powdered metals producing toxic and
explosive gases. Attacks plastic and rubber.
OCCUPATIONAL EXPOSURE LIMITS (OELs): EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
TLV: 1 ppm; 6.9 mg/m3 (as TWA) (skin) The liquid defats the skin. The substance may
(ACGIH 1994-1995). have effects on the central nervous system and
MAK: 1 ppm; 7 mg/m3; skin, 8 (1992). liver, resulting in impaired functions.
ROUTES OF EXPOSURE:
The substance can be absorbed into the body by
inhalation of its vapour, through the skin and
by ingestion.
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
PHYSICAL Boiling point: 146°C Relative vapour density (air = 1): 5.8
PROPERTIES Melting -44°C Relative density of the vapour/
Relative density (water = 1): 1.6 air-mixture at 20°C (air = 1): 1.031
Solubility in water, g/100 ml Octanol/water partition coefficient
at 20°C: 0.29 as log Pow: 2.39
Vapour pressure, kPa at 20°C: 0.647
Vapour pressure, Pa at 25°C: 780
ENVIRONMENTAL The substance is toxic to aquatic organisms.
DATA This substance may be hazardous to the environment; special attention should be given to its impact on the
ozone layer.
NOTES
Use of alcoholic beverages enhances the harmful effect.
The odour warning when the exposure limit value is exceeded is insufficient.
Do NOT use in the vicinity of a fire or a hot surface, or during welding.
ICSC: 0332 1.1 Transport Emergency Card: TEC (R)-719
1,1,2,2-TETRACHLOROETHANE
REFERENCES
Arthur CL, Pratt K, Motlagh S, Pawliszyn J (1992) Environmental
analysis of organic compounds in water using solid phase micro
extraction. Journal of high resolution chromatography,
15(11):741-744.
Assmuth TW, Strandberg T (1993) Groundwater contamination at Finnish
landfills. Water, air, and soil pollution, 69:179-199.
ATRG (1988) Aquatic toxicity of multiple organic compounds, Part
II: Chlorinated ethanes and chlorinated ethylenes, summary report
(interim). Prepared by the Aquatic Toxicity Research Group for the
Ontario Ministry of the Environment, Lakehead University, Thunder Bay,
Ontario, 95 pp.
ATSDR (1994) Toxicological profile for 1,1,2,2-tetrachloroethane
(draft). Atlanta, GA, US Department of Health and Human Services,
Agency for Toxic Substances and Disease Registry.
Blum DJW, Speece RE (1991) A database of chemical toxicity to
environmental bacteria and its use in interspecies comparisons and
correlations. Research journal of the Water Pollution Control
Federation, 63(3):198-207.
Brem H, Stein AB, Rosenkranz HS (1974) The mutagenicity and
DNA-modifying effect of haloalkanes. Cancer research, 34:2576-2579.
Bull RJ, Sanchez IM, Nelson MA, Larson JL, Lansing AJ (1990) Liver
tumor induction in B6C3F1 mice by dichloroacetate and
trichloroacetate. Toxicology, 63:341-359.
Callen DF, Wolf CR, Philpot RM (1980) Cytochrome P-450 mediated
genetic activity and cytotoxicity of seven halogenated aliphatic
hydrocarbons in Saccharomyces cerevisiae. Mutation research,
77:55-63.
Chai M, Pawliszyn J (1995) Analysis of environmental air samples by
solid-phase microextraction and gas chromatography/ion trap mass
spectrometry. Environmental science and technology, 29(3):693-701.
COARGLWQ (1986) St. Clair River pollution investigation (Sarnia
area). Canada-Ontario Agreement Respecting Great Lakes Water
Quality.
Colacci A, Grilli S, Lattanzi G, Prodi G, Turina MP, Forti GC,
Mazzullo M (1987) The covalent binding of 1,1,2,2-tetrachloroethane to
macromolecules of rat and mouse organs. Teratogenesis,
carcinogenesis, and mutagenesis, 7:465-474.
Colacci A, Perocco P, Vaccari M, Mazzullo M, Albini A, Parodi S,
Taningher M, Grilli S (1990) In vitro transformation of BALB/c 3T3
cells by 1,1,2,2-tetrachloroethane. Japanese journal of cancer
research, 81:786-792.
Colacci A, Perocco P, Bartoli S, Da Via C, Silingardi P, Vaccari M,
Grilli S (1992) Initiating activity of 1,1,2,2-tetrachloroethane in
two-stage BALB/c 3T3 cell transformation. Cancer letters,
64:145-153.
Colacci A, Albini A, Melchiori A, Nanni P, Nicoletti G, Noonan D,
Parodi S, Grilli S (1993) Induction of malignant phenotype in BALB/c
3T3 cells by 1,1,2,2-tetrachloroethane. International journal of
oncology, 2:937-945.
Crebelli R, Benigni R, Franekic J, Conti G, Conti L, Carere A (1988)
Induction of chromosome malsegregation by halogenated organic solvents
in Aspergillus nidulans: unspecific or specific mechanism?
Mutation research, 201:401-411.
Daft JL (1988) Rapid determination of fumigant and industrial chemical
residues in food. Journal of the Association of Official Analytical
Chemists, 71(4):748-760.
DeAngelo AB, Daniel FB, Stober JA, Olson GR (1991) The carcinogenicity
of dichloroacetic acid in the male B6C3F1 mouse. Fundamental and
applied toxicology, 16:337-347.
DeMarini DM, Brooks HG (1992) Induction of prophage lambda by
chlorinated organics: detection of some single-species/single-site
carcinogens. Environmental and molecular mutagenesis, 19:98-111.
Ecobichon DJ, Allen MC (1990) New Brunswick - Water Quality
Surveillance Program, New Brunswick public water supplies,
data summary report 1990. New Brunswick Department of Health and
Community Services.
Environment Agency Japan (1976) Chemicals in the environment. Tokyo,
p. 55.
Enviro-Test Laboratories (1991) Cayley background study: analysis
of food products for target organic and inorganic parameters.
Edmonton, Alberta. Prepared for Health and Welfare Canada, Ottawa,
Ontario (Series: 91-E1208).
Enviro-Test Laboratories (1992) Windsor area background study:
analysis of food products for target organic and inorganic
parameters. Edmonton, Alberta. Prepared for Health and Welfare
Canada, Ottawa, Ontario (Series: 92-E1052).
Eriksson C, Brittebo EB (1991) Epithelial binding of
1,1,2,2-tetrachloroethane in the respiratory and upper alimentary
tract. Archives of toxicology, 65:10-14.
Fellin P, Barnett SE, Tran QA (1992) Results of a national pilot
survey of airborne volatile organic compounds in Canadian
residences. Prepared by Concord Environmental Corporation for Health
and Welfare Canada, Ottawa, Ontario.
Galloway SM, Armstrong MJ, Reuben C, Colman S, Brown B, Cannon C,
Bloom AD, Nakamura F, Ahmed M, Duk S, Rimpo J, Margolin BH, Resnick
MA, Anderson B, Zeiger E (1987) Chromosome aberrations and sister
chromatid exchange in Chinese hamster ovary cells: evaluations of 108
chemicals. Environmental and molecular mutagenesis, 10 (Suppl.
10):1-175.
Gohlke R, Schmidt P (1972) [Subacute action of low concentrations of
chlorinated ethanes with and without additional ethanol treatment in
the rat.] Internationales Archiv für Arbeitsmedizin, 30:299-312 (in
German).
Gohlke R, Schmidt P, Bahmann H (1977) [1,1,2,2-Tetrachloroethane and
heat stress in animal experiment. Morphological results.]
Zeitschrift für die Gesamte Hygiene unde Ihre Grenzgebiete,
20:278-282 (in German).
Government of Canada (1993) Canadian Environmental Protection Act
Priority Substances List assessment report -
1,1,2,2-Tetrachloroethane. Ottawa, Ontario, Environment
Canada/Health and Welfare Canada.
Haag WR, Mill T (1988) Effect of a subsurface sediment on hydrolysis
of haloalkanes and epoxides. Environmental science and technology,
22:658-663.
Hallen RT, Pyne JW, Moulton PM (1986) Transformation of chlorinated
ethanes and ethenes by anaerobic microorganisms. In: Extended
abstracts, 192nd National Meeting of the American Chemical
Society, Anaheim, CA. Washington, DC, American Chemical Society.
Halpert J (1982) Cytochrome P-450-dependent covalent binding of
1,1,2,2-tetrachloroethane in vitro. Drug metabolism and
disposition, 10(5):465-468.
Hanley TR Jr, Quast JF, Schumann AM (1988) The metabolism and
hepatic macromolecular interactions of 1,1,2,2-tetrachloroethane
(TE) in mice and rats. Unpublished study performed by Mammalian and
Environmental Toxicology Research Laboratory, Health and Environmental
Sciences, The Dow Chemical Company, Midland, MI.
Hawkins WE (1991) Development of carcinogenesis bioassay models:
response of small fish species to various classes of carcinogens.
Ocean Springs, MS, Gulf Coast Research Laboratory, 119 pp. (NTIS
AD-A277-157).
Haworth S, Lawlor T, Mortelmans K, Speck W, Zeiger E (1983)
Salmonella mutagenicity test results for 250 chemicals.
Environmental mutagenesis, Suppl. 1:3-142.
Herren-Freund SL, Pereira MA, Khoury MD, Olson G (1987) The
carcinogenicity of trichloroethylene and its metabolites,
trichloroacetic acid and dichloroacetic acid, in mouse liver.
Toxicology and applied pharmacology, 90:183-189.
Horiuchi K, Horiguchi S, Hashimoto K, Kadowaki K, Aratake K (1962)
Studies on the industrial tetrachloroethane poisoning. Osaka
City medical journal, 8:29-38.
Horvath M, Frantik E (1973) Relative sensitivity of nervous function
and behavior to nonspecific effects of foreign substances.
Activitas Nervosa Superior, 15:25-27 [cited in ATSDR, 1994].
IARC (1987) Overall evaluations of carcinogenicity: an updating
of IARC monographs Volumes 1 to 42. Lyon, International Agency for
Research on Cancer (IARC Monographs on the Evaluation of Carcinogenic
Risks to Humans, Suppl. 7).
IPCS (1993) International Chemical Safety Card -
1,1,2,2-Tetrachloroethane. Geneva, World Health Organization,
International Programme on Chemical Safety (No. 0332).
IPCS (1994) Assessing human health risks of chemicals: derivation
of guidance values for health-based exposure limits. Geneva, World
Health Organization, International Programme on Chemical Safety
(Environmental Health Criteria 170).
Jeney E, Bartha F, Kondor L, Szendrei S (1957) Adatok az iizemi
tetrakloretan-mergezesek megelozesehez. [Prevention of industrial
tetrachloroethane intoxication - Part III.] Egeszsegtudomany,
1:142-164.
Kaiser KLE, Comba ME (1983) Volatile contaminants in the Welland River
watershed. Journal of Great Lakes research, 9(2):274-280.
Kanada M, Miyagawa M, Sato M, Hasegawa H, Honma T (1994) Neurochemical
profile of effects of 28 neurotoxic chemicals on the central nervous
system in rats. (1) Effects of oral administration on brain contents
of biogenic amines and metabolites. Industrial health, 32:145-164.
Kennedy GL Jr, Graepel GJ (1991) Acute toxicity in the rat following
either oral or inhalation exposure. Toxicology letters, 56:317-326.
Kulinskaya IL, Verlinskaya RV (1972) [Comparative effect of low
concentration of di-, tetra- and pentachloroethane on the blood
acetylcholine system.] Gigiena Truda i Professional'nye
Zabolevaniya, 16:56-58 (in Russian).
Larson JL, Bull RJ (1992) Metabolism and lipoperoxidative activity of
trichloroacetate and dichloroacetate in rats and mice. Toxicology
and applied pharmacology, 115:268-277.
LeBlanc GA (1980) Acute toxicity of priority pollutants to water flea
(Daphnia magna). Bulletin of environmental contamination and
toxicology, 24:684-691.
Little AD (1983) Cell transformation assays of 11 chlorinated
hydrocarbon analogs (final report). US Environmental Protection
Agency, Office of Toxic Substances (ICAIR Work Assignment No. 10;
Document No. 40+8324457) [cited in ATSDR, 1994].
Lobo-Mendonca R (1963) Tetrachloroethane - a survey. British
journal of industrial medicine, 20:50-56 [cited in ATSDR, 1994].
Maltoni C, Lefemine G, Cotti G (1986) Experimental research on
trichloroethylene carcinogenesis. Archives of research on
industrial carcinogenesis, Vol. V. Princeton, NJ, Princeton
Scientific Publishing, 393 pp.
Maltoni C, Lefemine G, Cotti G, Perino G (1988) Long-term
carcinogenicity bioassays on trichloroethylene administered by
inhalation to Sprague-Dawley rats and Swiss and B6C3F1 mice.
Annals of the New York Academy of Sciences, 534:316-342.
McGregor DB (1980) Tier II mutagenic screening of 13 NIOSH
priority compounds, individual compound report,
1,1,2,2-tetrachloroethane. Report prepared by Inveresk Research
International Limited, Musselburgh, for the National Institute for
Occupational Safety and Health, Cincinnati, OH (Report No. 26).
Mersch-Sundermann V (1989a) [The mutagenicity of organic
microcontamination in the environment. II. The mutagenicity of
volatile organic halogens in the Salmonella microsome test (Ames
test) with regard to the contamination of groundwater and
drinking-water.] Zentralblatt für Bakteriologie und Mikrobiologie,
Hygiene B, 187:230-243 (in German).
Mersch-Sundermann V (1989b) [Examination of mutagenicity of organic
microcontamination of the environment. IV. Communication: The
mutagenicity of halogenated aliphatic hydrocarbons with the
SOS-chromotest.] Zentralblatt für Bakteriologie und
Mikrobiologie, Hygiene B, 189:266-271 (in German).
Milman HA, Story DL, Riccio ES, Sivak A, Tu AS, Williams GM, Tong C,
Tyson CA (1988) Rat liver foci and in vitro assays to detect
initiating and promoting effects of chlorinated ethanes and ethylenes.
Annals of the New York Academy of Sciences, 534:521-530.
Mirsalis JC, Tyson CK, Steinmetz KL, Loh EK, Hamilton CM, Bakke JP,
Spalding JW (1989) Measurement of unscheduled DNA synthesis and
S-phase synthesis in rodent hepatocytes following in vivo treatment;
testing of 24 compounds. Environmental and molecular mutagenesis,
14:155-164.
Mitoma C, Steeger T, Jackson SE, Wheeler KP, Rogers JH, Milman HA
(1985) Metabolic disposition study of chlorinated hydrocarbons in rats
and mice. Drug chemistry and toxicology, 8(3):183-194.
Navrotskiy VK, Kashin LM, Kulinskoya IL (1971) [Comparative assessment
of the toxicity of a number of industrial poisons when inhaled in low
concentrations for prolonged periods.] Trudy Szed Gigiena
Ukrainskoi, 8:224-226 (in Russian) [cited in ATSDR, 1994].
NCI (1977) Bioassay of tetrachloroethylene for possible
carcinogenicity. Bethesda, MD, US Department of Health, Education
and Welfare, Public Health Services, National Institutes of Health,
National Cancer Institute (DHEW/PUB/NIH-77-813).
NCI (1978) Bioassay of 1,1,2,2-tetrachloroethane for possible
carcinogenicity. Bethesda, MD, US Department of Health, Education
and Welfare, Public Health Services, National Institutes of Health,
National Cancer Institute (NTIS PB277 4537GA; DHEW/PUB/NIH-78-827).
Nestmann ER, Lee EG-H (1983) Mutagenicity of constituents of pulp and
paper mill effluent in growing cells of Saccharomyces cerevisiae.
Mutation research, 119:273-280.
Nestmann ER, Lee EG-H, Matula TI, Douglas GR, Mueller JC (1980)
Mutagenicity of constituents identified in pulp and paper mill
effluents using the Salmonella mammalian-microsome assay.
Mutation research, 79:203-212.
Nichols JW, McKim JM, Lien GJ, Hoffman AD, Bertelsen SL, Gallinat CA
(1993) Physiologically-based toxicokinetic modelling of three
waterborne chloroethanes in channel catfish, Ictalurus punctatus.
Aquatic toxicology, 27:83-112.
Nimitz JS, Skaggs SR (1992) Estimating tropospheric lifetimes and
ozone-depletion potentials of one- and two-carbon hydrofluorocarbons
and hydrochlorofluorocarbons. Environmental science and technology,
26(4):739-744.
NIOSH (1994) NIOSH pocket guide to chemical hazards. US Department
of Health and Human Services, Public Health Service, Center for
Disease Control and Prevention, National Institute for Occupational
Safety and Health, June.
Norman JE, Robinette CD, Fraumeni JF (1981) The mortality experience
of army World War II chemical processing companies. Journal of
occupational medicine, 23(12):818-822.
NTP (1986) Toxicology and carcinogenesis studies of
tetrachloroethylene (perchloroethylene) (CAS No. 127-18-4) in
F344/N rats and B6C3F1 mice (inhalation studies). Research Triangle
Park, NC, US Department of Health and Human Services, National
Institutes of Health, National Toxicology Program (NTP-TR-311).
NTP (1990) Carcinogenesis studies of trichloroethylene (without
epichlorohydrin) (CAS No. 79-01-6) in F344/N rats and B6C3F1
mice (gavage studies). Research Triangle Park, NC, US Department of
Health and Human Services, National Institutes of Health, National
Toxicology Program (NTP TR 243).
NTP (1991a) Range finding studies: developmental toxicity -
1,1,2,2-tetrachloroethane when administered via feed in CD
Sprague-Dawley rats. Research Triangle Park, NC, US Department of
Health and Human Services, National Institutes of Health, National
Toxicology Program (NTP-91-RF/DT-017).
NTP (1991b) Range finding studies: developmental toxicity -
1,1,2,2-tetrachloroethane (repeat) when administered via feed
in Swiss CD-1 mice. Research Triangle Park, NC, US Department of
Health and Human Services, National Institutes of Health, National
Toxicology Program (NTP-91-RF/DT-020).
NTP (1993) 1,1,2,2-Tetrachloroethane. C: C3554. Sperm motility
vaginal cytology evaluation in rodents. Research Triangle Park, NC,
US Department of Health and Human Services, National Institutes of
Health, National Toxicology Program (SMVCE-93-192).
NTP (1996) NTP technical report on renal toxicity studies of
selected halogenated ethanes administered by gavage to F344/N
rats. Research Triangle Park, NC, US Department of Health and Human
Services, National Institutes of Health, National Toxicology Program
(Toxicity Report Series Number 45).
Paolini M, Sapigni E, Mesirca R, Pedulli GF, Corongiu FP, Dessi MA,
Cantelli-Forti G (1992) On the hepatotoxicity of
1,1,2,2-tetrachloroethane. Toxicology, 73:101-115.
Pawlisz AV, Peters RH (1995) Effects of sublethal exposure on lethal
body burdens of narcotic organic chemicals in Daphnia magna.
Environmental science and technology, 29(3):613-621.
Price NH, Allen SD, Daniels AU, Yates WG (1978) Toxicity data for
establishing "immediately dangerous to life or health" (IDLH)
values (NTIS PB87-163531) [cited in ATSDR, 1994].
Richter JE, Peterson SF, Kleiner CF (1983) Acute and chronic toxicity
of some chlorinated benzenes, chlorinated ethanes, and
tetrachloroethylene to Daphnia magna. Archives of environmental
contamination and toxicology, 12:679-684.
Roldan-Arjona T, Garcia-Pedrajas MD, Luque-Romero FL, Hera C, Pueyo C
(1991) An association between mutagenicity of the Ara test of
Salmonella typhimurium and carcinogenicity in rodents for 16
halogenated aliphatic hydrocarbons. Mutagenesis, 6(3):199-205.
Sack TM, Steele DH, Hammerstrom K, Remmers J (1992) A survey of
household products for volatile organic compounds. Atmospheric
environment, 26A(6):1063-1070.
Schmidt P, Binnevies S, Gohlke R, Rothe R (1972) [Subacute action of
low concentration of chlorinated ethanes on rats with and without
additional ethanol treatment. I. Biochemical and toxicometrical
aspects, especially results in subacute and chronic toxicity studies
with 1,1,2,2-tetrachloroethane.] Internationales Archiv für
Arbeitsmedizin, 30:283-298 (in German).
Schmidt P, Ulanova IP, Avilova GG, Binnevis SM (1975) [Comparison of
the processes of adaptation of the organism to monotonic and
intermittent action of 1,1,2,2-tetrachloroethane.] Gigiena Truda
i Professional'nye Zabolevaniya, 2:30-34 (in Russian).
Shah JJ, Heyerdahl EK (1988) National ambient volatile organic
compounds (VOCs) database update. Report prepared by Nero and
Associates, Inc., Portland, OR, for Office of Research and
Development, US Environmental Protection Agency, Research Triangle
Park, NC (EPA/600/3-88-010a; NTIS No. PB88-195631).
Shmuter LM (1977) [The effect of chronic exposure to low concentration
of ethane series chlorinated hydrocarbons on specific and nonspecific
immunological reactivity in animal experiments.] Gigiena Truda i
Professional'nye Zabolevaniya, 8:38-43 (in Russian).
Smith AD, Bharath A, Mallard C, Orr D, Smith K, Sutton JA, Vukmanich
J, McCarty LS, Ozburn GW (1991) The acute and chronic toxicity of ten
chlorinated organic compounds to the American flagfish (Jordanella
floridae). Archives of environmental contamination and toxicology,
20:94-102.
Smyth HF Jr, Carpenter CP, Weil CS, Pozzani UC, Striegel JA, Nycum JS
(1969) Range-finding toxicity data - List VII. American Industrial
Hygiene Association journal, 30:470-476.
Stoner GD (1991) Lung tumors in strain A mice as a bioassay for
carcinogenicity of environmental chemicals. Experimental lung
research, 17:405-423.
Storm DL (1994) Chemical monitoring of California's public drinking
water sources: public exposures and health impacts. In: Wang RGM, ed.
Water contamination and health. Integration of exposure assessment,
toxicology, and risk assessment. New York, NY, Marcel Dekker, Inc.,
pp. 67-124.
Story DL, Meierhenry EF, Tyson CA, Milman HA (1986) Differences in rat
liver enzyme-altered foci produced by chlorinated aliphatics and
phenobarbital. Toxicology and industrial health, 2(4):351-362.
Theiss JC, Stoner GD, Shimkin MB, Weisburger EK (1977) Test for
carcinogenicity of organic contaminants of United States drinking
waters by pulmonary tumor response in strain A mice. Cancer
research, 37(8):2717-2720.
Truffert L, Girard-Wallon C, Emmerich E, Neauport C, Ripault J (1977)
[Early experimental demonstration of the hepatotoxicity of some
chlorinated solvents by the study of the synthesis of hepatic DNA.]
Archives des maladies professionnelles de medecine du travail et
de securité sociale (Paris), 38:261-263 (in French).
Tse G, Orbey H, Sandler SI (1992) Infinite dilution activity
coefficients and Henry's law coefficients of some priority water
pollutants determined by a relative gas chromatographic method.
Environmental science and technology, 26(1):2017-2022.
Tu AS, Murray TA, Hatch KM, Sivak A, Milman HA (1985) In vitro
transformation of BALB/c-3T3 cells by chlorinated ethanes and
ethylenes. Cancer letters, 28:85-92.
Ulanova IP, Khalepo AI, Avilova GG (1984) Intermittent exposure to
toxic compounds in the working-zone atmosphere viewed from the aspect
of hygienic standardization. Journal of hygiene, epidemiology,
microbiology, and immunology, 29(3):243-251.
Veith GD, Call DJ, Brooke LT (1983) Structure-toxicity relationships
for the fathead minnow, Pimephales promelas: Narcotic industrial
chemicals. Canadian journal of fisheries and aquatic sciences,
40:743-748.
Vogel EW, Nivard MJM (1993) Performance of 181 chemicals in a
Drosophila assay predominantly monitoring interchromosomal mitotic
recombination. Mutagenesis, 8(1):57-81.
Walbridge CT, Fiandt JT, Phipps GL, Holcombe GW (1983) Acute toxicity
of ten chlorinated aliphatic hydrocarbons to the fathead minnow
(Pimephales promelas). Archives of environmental contamination and
toxicology, 12:661-666.
Warner JR, Hughes TJ, Claxton LD (1988) Mutagenicity of 16 volatile
organic chemicals in a vaporization technique with Salmonella
typhimurium TA100. Environmental and molecular mutagenesis, 11
(Suppl. 11):111.
Williams G (1983) DNA repair tests of 11 chlorinated hydrocarbon
analogs final report EPA contract. US Environmental Protection
Agency, Office of Toxic Substances (Document No. 40+8324292) [cited in
ATSDR, 1994].
Wittsiepe J (1990) Occurrence of vinyl chloride and other halogenated
C1- and C2-hydrocarbons in German surface water. Organohalogen
compounds, 4:425-434.
Woodruff RC, Mason JM, Valencia R, Zimmering S (1985) Chemical
mutagenesis testing in Drosophila. 5. Results of 53 coded compounds
tested for the National Toxicology Program. Environmental
mutagenesis, 7:677-702.
Yllner S (1971) Metabolism of 1,1,2,2-tetrachloroethane-14C in the
mouse. Acta Pharmacologica et Toxicologica, 29:499-512.
APPENDIX 1 - SOURCE DOCUMENTS
Government of Canada (1993)
Copies of the Canadian Environmental Protection Act (CEPA)
Priority Substances List Assessment Report for
1,1,2,2-tetrachloroethane (Government of Canada, 1993) may be obtained
from the:
Commercial Chemicals Branch
Environment Canada
14th Floor, Place Vincent Massey
351 St. Joseph Blvd.
Hull, Quebec
Canada K1A 0H3
Environmental Health Centre
Health Canada
Address Locator: 0801A
Tunney's Pasture
Ottawa, Ontario
Canada K1A 0L2
Copies of the unpublished Supporting Documentation related to
human health effects that formed the basis for preparation of the
above-mentioned report may be obtained from the Environmental Health
Centre at the address noted above.
Initial drafts of the Supporting Documentation and Assessment
Report for 1,1,2,2-tetrachloroethane were prepared by staff of Health
Canada and Environment Canada. The environmental sections were
reviewed externally by Dr P. Cammer (Cammer and Associates), Dr D.
Muir (Department of Fisheries and Oceans), Dr D. Singleton (National
Research Council of Canada), and Dr K. Woodburn (Dow Chemical Canada
Inc.). Sections related to the assessment of human exposure and
health effects were peer reviewed by Dr J. Domoradzki (Dow Chemical
Company, USA, Supporting Documentation only), Dr R. Bull (Washington
State University, USA), and the Information Department of BIBRA
Toxicology International, UK, and subsequently approved by the
Standards and Guidelines Rulings Committee of the Bureau of Chemical
Hazards of Health Canada. The final Assessment Report was reviewed
and approved by the Environment Canada/Health Canada CEPA Management
Committee.
Agency for Toxic Substances and Disease Registry (ATSDR, 1994)
Copies of the draft ATSDR profile for 1,1,2,2-tetrachloroethane
(ATSDR, 1994) may be obtained from:
Agency for Toxic Substances and Disease Registry
Division of Toxicology/Toxicology Information Branch
1600 Clifton Road, NE, E-29
Atlanta, Georgia 30333
USA
The profile has undergone the following ATSDR internal reviews:
Green Border Review, Health Effects Review, Minimal Risk Level Review,
and Quality Assurance Review. In addition, a peer review panel, which
included Dr Martin Alexander (Cornell University, USA), Mr Lyman Skory
(private consultant, USA), and Dr James Withey (Health Canada), was
assembled.
APPENDIX 2 - CICAD PEER REVIEW
The draft CICAD on 1,1,2,2-tetrachloroethane was sent for review
to institutions and organizations identified by IPCS after contact
with IPCS national Contact Points and Participating Institutions, as
well as to identified experts. Comments were received from:
Department of Health, London, United Kingdom
Department of Public Health, Albert Szent-Gyorgyi University
Medical School, Szeged, Hungary
Direccion General de Salud Ambiental, Subsecretario de Regulacion
y Fomento Sanitario, San Luis Potosi, Mexico
Finnish Institute for Occupational Health, Helsinki, Finland
International Agency for Research on Cancer, Lyon, France
Ministry of Health and Welfare, International Affairs Division,
Government of Japan, Tokyo, Japan
National Institute for Working Life, Solna, Sweden
United States Department of Health and Human Services (Agency for
Toxic Substances and Disease Registry; National Institute of
Environmental Health Sciences)
United States Environmental Protection Agency (Office of
Pollution Prevention and Toxics; National Center for
Environmental Assessment, Office of Research and Development;
Office of Drinking Water)
APPENDIX 3 - CICAD FINAL REVIEW BOARD
Brussels, Belgium, 18-20 November 1996
Members
Dr A. Aitio, Institute of Occupational Health, Helsinki, Finland
Dr K. Bentley, Director, Environment Policy Section, Commonwealth
Department of Human Services and Health, Canberra, Australia
Mr R. Cary, Toxicology and Existing Substances Regulation Unit, Health
and Safety Executive, Merseyside, United Kingdom
Dr J. de Fouw, National Institute of Public Health and Environmental
Protection, Bilthoven, The Netherlands
Dr C. DeRosa, Director, Division of Toxicology, Agency for Toxic
Substances and Disease Registry, Atlanta, GA, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr W. Farland, Director, National Center for Environmental Assessment,
Office of Research and Development, US Environmental Protection
Agency, Washington, DC, USA (Chairperson)
Dr T.I. Fortoul, Depto. Biologia Celular y Tisular, National
University of Mexico and Environmental Health Directorate of the
Health Ministry, Mexico D.F., Mexico
Dr H. Gibb, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany
Mr J.R. Hickman, Environmental Health Directorate, Health Canada,
Ottawa, Ontario, Canada
Dr T. Lakhanisky, Head, Division of Toxicology, Institute of Hygiene
and Epidemiology, Brussels, Belgium (Vice-Chairperson)
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Sciences, Hanover,
Germany
Ms E. Meek, Head, Priority Substances Section, Environmental Health
Directorate, Health Canada, Ottawa, Ontario, Canada
Dr K. Paksy, National Institute of Occupational Health, Budapest,
Hungary
Mr D. Renshaw, Department of Health, London, United Kingdom
Dr J. Sekizawa, Division of Chemo-Bio Informatics, National Institute
of Hygienic Sciences, Tokyo, Japan
Dr H. Sterzl-Eckert, GSF-Forschungszentrum für Umwelt und Gesundheit
GmbH, Institut für Toxikologie, Oberschleissheim, Germany
Professor S. Tarkowski, Department of Environmental Health Hazards,
The Nofer Institute of Occupational Medicine, Lodz, Poland
Dr M. Wallen, National Chemicals Inspectorate (KEMI), Solna, Sweden
Observers
Professor F.M.C. Carpanini,1 Director, Centre for Ecotoxicology and
Toxicology of Chemicals (ECETOC), Brussels, Belgium
Mr R. Haigh,1 Head of Unit, Health and Safety Directorate, European
Commission, Luxembourg
Mr B.U. Hildebrandt, Federal Ministry for the Environment, Nature
Conservation and Nuclear Safety, Bonn, Germany
Mr P. Hurst,1 Chemical and Consumer Policy Officer, Conservation
Policy Division, World Wide Fund for Nature, Gland, Switzerland
Dr A. Lombard (Representative of CEFIC), ELF-ATOCHEM, Paris, France
Dr P. McCutcheon,1 Environment, Consumer Protection and Nuclear
Safety, European Commission, Brussels, Belgium
Dr R. Montaigne, Counsellor, Technical Affairs Department, European
Chemical Industry Council (CEFIC), Brussels, Belgium
Dr M. Pemberton, ICI Acrylics, Lancashire, United Kingdom
Dr A. Smith, Organisation for Economic Co-operation and Development,
Environment Division, Paris, France
1 Invited but unable to attend.
Secretariat
Dr M. Baril, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Dr L. Harrison, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr M. Mercier, Director, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr P. Toft, Associate Director, International Programme on Chemical
Safety, World Health Organization, Geneva, Switzerland
RÉSUMÉ D'ORIENTATION
La Direction de l'Hygiène du Milieu de Santé Canada a rédigé ce
CICAD (document international succinct sur l'évaluation des risques
chimiques) sur le 1,1,2,2-tétrachloréthane en s'inspirant d'un
document du Gouvernement du Canada (1993) qui évaluait les
conséquences potentielles pour la santé humaine d'une exposition
indirecte au 1,1,2,2-tétrachloréthane et les effets de cette substance
sur l'environnement, ainsi que d'une étude entreprise par l'Agence
pour les substances toxiques et l'enregistrement des maladies (ATSDR,
1994) en vue de caractériser les informations disponibles sur ses
effets sanitaires indésirables et l'exposition du public. L'examen du
Gouvernement canadien a pris en considération les données disponibles
en septembre 1992. Une recherche bibliographique détaillée a été
effectuée en août 1995 dans plusieurs bases de données en ligne pour
identifier les références publiées postérieurement. L'appendice 1
donne des informations sur la nature du processus d'évaluation par les
pairs et sur la disponibilité des sources documentaires. Les
informations concernant l'examen par les pairs du présent CICAD
figurent à l'appendice 2. La publication de ce CICAD a été approuvée
à une réunion du Comité d'évaluation finale qui s'est tenue à
Bruxelles (Belgique) du 18 au 20 novembre 1996. La liste des
participants à cette réunion figure à l'appendice 3. La fiche
internationale de sécurité chimique (ICSC 0332) concernant le
1,1,2,2-tétrachloréthane, établie par le Programme international sur
la sécurité chimique (IPCS, 1993) est également reproduite dans le
présent document.
Le 1,1,2,2-tétrachloréthane (CAS n° 79-34-5) est un produit
synthétique volatil utilisé principalement comme intermédiaire dans la
synthèse d'autres hydrocarbures chlorés, bien que cette utilisation
ait diminué considérablement. Sa présence dans l'environnement
résulte principalement d'émissions dans l'atmosphère, où il persiste
probablement plusieurs semaines. Il ne semble pas que le
1,1,2,2-tétrachloréthane contribue à la destruction de l'ozone
atmosphérique ni au réchauffement mondial. Il est rapidement éliminé
des systèmes aquatiques et son potentiel de bioaccumulation est
faible. L'inhalation est la principale voie d'exposition pour l'homme.
On dispose de très peu de données concernant les effets sur
l'homme de l'exposition au 1,1,2,2-tétrachloréthane. Son profil
toxicologique n'a pas non plus été caractérisé de façon précise.
Étant donné qu'il est de moins en moins utilisé, la majorité des
données disponibles proviennent d'études limitées relativement
anciennes. La toxicité aiguë du 1,1,2,2-tétrachloréthane chez les
animaux de laboratoire est considérée comme légère à modérée. D'après
les résultats d'études de toxicité à court terme et subchronique, pour
la plupart limitées, le foie semble être l'organe cible le plus
sensible. La plupart des études disponibles ne permettent pas
d'établir de NO(A)EL [dose sans effet (indésirable) observé] ni de
LO(A)EL [dose minimale suivie d'un effet (indésirable) observé] fiable
pour ce qui est de l'hépatotoxicité. Cependant, des effets minimes
sur le foie (augmentation réversible de la teneur en lipides), ainsi
que d'autres effets (augmentation de la concentration
d'adrénocorticotropine et altérations réversibles des paramètres
hématologiques), ont été observés chez des rats exposés à 13,3 mg/m3
pendant 9 mois. D'après les résultats d'études limitées et pour la
plupart anciennes, des effets n'ont été observés sur la reproduction
et le développement des animaux de laboratoire qu'à des doses qui
provoquaient une diminution de poids.
L'ingestion sur une longue période de 1,1,2,2-tétrachloréthane a
provoqué une augmentation de l'incidence des tumeurs du foie chez des
souris B6C3F1 mâles et femelles. Toutefois, une exposition analogue
n'a pas été suivie d'une augmentation significative du nombre des
tumeurs, quel que soit leur site, chez des rats Osborne-Mendel, mais
l'exposition a été limitée à 78 semaines pour les deux espèces.
D'après les résultats disponibles in vivo et in vitro, le
1,1,2,2-tétrachloréthane aurait tout au plus une faible activité
génotoxique. Il ne déclenche pas la formation de foyers positifs à la
gamma-glutamyltranspeptidase dans le foie des rats, mais il la
favorise fortement. Le profil d'induction des tumeurs par le
1,1,2,2-tétrachloréthane est semblable à celui de l'acide
dichloracétique, son principal métabolite. Le mécanisme d'induction
des tumeurs par le 1,1,2,2-tétrachloréthane est encore mal connu; pour
plusieurs de ses métabolites, on a émis l'hypothèse qu'il existerait
un seuil.
Il a été démontré que l'exposition au 1,1,2,2-tétrachloréthane
inhibe l'activité de certaines bactéries présentes dans
l'environnement (la CI50 la plus faible était de 1,4 mg/litre) et
qu'il provoque l'immobilisation de Daphnia magna (CE50 à 48 heures
> 23 mg/litre). Chez les poissons d'eau douce, la CL50 la plus
faible qui ait été signalée (96 heures) était de 18,5 mg/litre
(Jordanella floridae), tandis que la concentration minimale suivie
d'effet (LOEC) à plus long terme (réduction de la survie des larves
chez la même espèce) était de 7,2 mg/litre. Aucun renseignement n'a
été découvert concernant les effets de cette substance sur les
organismes terrestres.
Des valeurs guides à l'intention des autorités compétentes ont
été établies sur la base de la capacité du 1,1,2,2-tétrachloréthane à
induire la formation de tumeurs du foie chez la souris, étant donné
qu'il s'agit du critère toxicologique pour lequel la relation dose-
réponse est la mieux établie. Il faut cependant noter que
l'augmentation observée de l'incidence des tumeurs se limite
actuellement à une espèce et qu'il existe des données qui, bien
qu'incomplètes, laissent supposer que les tumeurs pourraient être
induites par un mécanisme non génotoxique. Les doses associées à une
augmentation de 5 % de l'incidence des tumeurs se situent entre 5,8 et
28 mg/kg de poids corporel par jour. Dans le cas de l'air (principale
source d'exposition pour l'homme), si l'on divise ces doses par 5000
ou 50 000, on obtient des valeurs guides de 3,4-16 µg/m3 et
0,34-1,6 µg/m3 respectivement. Ces valeurs correspondent à ce que
certains organismes considèrent comme un risque «pratiquement
négligeable» (c'est-à-dire 10-5 à 10-6) pour un cancérogène
génotoxique; il faut cependant noter qu'une marge plus faible serait
peut-être appropriée compte tenu des arguments en faveur d'un
mécanisme épigénétique d'induction des tumeurs. Les valeurs
correspondantes pour l'ingestion sont respectivement de 1,2-5,6 et
0,12-0,56 µg/kg de poids corporel par jour. D'après une des
estimations qui ont été faites, l'exposition indirecte dans un
environnement normal est inférieure à ces valeurs, qui apportent déjà
une marge de sécurité considérable, compte tenu des arguments selon
lesquels le mécanisme d'induction des tumeurs par le tétrachloréthane
pourrait impliquer un seuil.
RESUMEN DE ORIENTACION
Esta reseña de la evaluación química internacional del
1,1,2,2-tetracloroetano ha sido preparada por la Dirección de Higiene
del Medio de Health Canadá, principalmente sobre la base de un informe
preparado por el Gobierno del Canadá (1993) para evaluar los efectos
potenciales en la salud humana de la exposición directa al
1,1,2,2-tetracloroetano en el medio ambiente general y los efectos
ambientales de esta sustancia, así como un estudio preparado por la
Agencia para el Registro de Sustancias Tóxicas y Enfermedades (ATSDR,
1994) con objeto de caracterizar información sobre los efectos
adversos en la salud y la exposición del público. En el estudio del
Gobierno del Canadá (1993) se examinaron datos identificados hasta
septiembre de 1992. En agosto de 1995 se hizo una búsqueda exhaustiva
de la bibliografía existente en varias bases de datos en línea con
objeto de identificar toda referencia publicada con posterioridad a
los trabajos incorporados en este estudio. En el apéndice 1 se
presenta información sobre la naturaleza de la revisión científica y
la disponibilidad de las fuentes documentales. En el apéndice 2 se
presenta información sobre la revisión científica de esta reseña. La
presente reseña fue aprobada para publicación en una reunión de la
Junta de Revisión Final, celebrada en Bruselas (Bélgica), del 18 al 20
de noviembre de 1996. Los participantes en la reunión de la Junta de
Revisión Final figuran en el apéndice 3. En el presente documento
también se reproduce la ficha internacional de seguridad química
(ICSC 0332) del 1,1,2,2-tetracloroetano, producida por el Programa
Internacional de Seguridad de las Sustancias Químicas (IPCS, 1993).
El 1,1,2,2-tetracloroetano (N° CAS 79-34-5) es una sustancia
química sintética volátil que se utiliza principalmente como precursor
en la síntesis de otros hidrocarburos clorados, aunque la utilización
de esta sustancia ha disminuido significativamente. La liberación en
el medio ambiente ocurre principalmente en forma de emisiones en el
aire ambiente, donde la sustancia química probablemente permanezca
durante varias semanas. No se prevé que el 1,1,2,2-tetracloroetano
contribuya al agotamiento del ozono estratosférico ni al calentamiento
de la atmósfera. Se elimina rápidamente de los sistemas acuáticos y
probablemente no sea objeto de bioacumulación. La exposición humana
al 1,1,2,2-tetracloroetano se hace principalmente por inhalación.
Se dispone de muy pocos datos sobre los efectos de la exposición
al 1,1,2,2-tetracloroetano en el ser humano. El perfil toxicológico
del 1,1,2,2-tetracloroetano tampoco se ha caracterizado bien; como la
utilización de la sustancia química se halla en disminución, los datos
disponibles se limitan principalmente a estudios iniciales limitados.
La toxicidad aguda del 1,1,2,2-tetracloroetano en animales de
laboratorio es de leve a moderada. Sobre la base de los resultados de
estudios principalmente limitados a corto plazo y subcrónicos, parece
que el hígado es el órgano diana más sensible. Si bien la mayor parte
de los estudios disponibles son insuficientes para determinar con
confianza un nivel sin efectos (adversos) observados [NO(A)EL] o el
nivel más bajo con efectos (adversos) observados [LO(A)EL] de
hepatotoxicidad, se han observado efectos mínimos en el hígado
(aumento reversible del contenido de lípidos) y otros parámetros
(aumento de los niveles de hormona adrenocorticotrópica y alteraciones
reversibles en los parámetros hematológicos) en ratas expuestas a 13,3
mg/m3 durante no más de nueve meses. Sobre la base de estudios
limitados, principalmente de determinación de la dosis e
investigaciones iniciales, se han observado efectos en la reproducción
y en el desarrollo en animales experimentales solamente con dosis que
ocasionaban reducción del peso corporal.
La ingestión prolongada de 1,1,2,2-tetracloroetano hizo aumentar
la incidencia de tumores del hígado en ratones B6C3F1, tanto machos
como hembras. Sin embargo, una exposición semejante no estuvo
asociada a un aumento significativo de tumores en ningún lugar en
ratas Osborne-Mendel, aunque ambas especies sólo estuvieron expuestas
durante un máximo de 78 semanas. Sobre la base de los resultados de
valoraciones disponibles in vivo e in vitro el
1,1,2,2-tetracloroetano tiene, como máximo, un potencial genotóxico
débil. El 1,1,2,2-tetracloroetano fue un potente promotor, pero no un
iniciador, de focos positivos a la gamma-glutamiltranspeptidasa en el
hígado de ratas. El perfil de inducción de tumores por el
1,1,2,2-tetracloroetano es semejante al del ácido dicloroacético, su
metabolito principal. La información existente sobre el mecanismo de
inducción de tumores por el 1,1,2,2-tetracloroetano es incompleta; con
respecto a varios de sus metabolitos, se ha sugerido que los tumores
probablemente estén inducidos por mecanismos para los cuales existe un
umbral.
Se ha demostrado que la exposición al 1,1,2,2-tetracloroetano
inhibe la actividad de las bacterias ambientales (la CI50 más baja
comunicada ha sido de 1,4 mg/litro) y ocasiona inmovilización en
Daphnia magna (valores de CE50 a las 48 horas de 23 mg/litro y
superiores). En las especies ictícolas de agua dulce, la CL50 más
baja comunicada (a las 96 horas) ha sido de 18,5 mg/litro en
Jordanella floridae, mientras que la concentración más baja con
efectos observados (LOEC) tras la exposición a más largo plazo ha sido
de 7,2 mg/litro; ésta dio lugar a una reducción de la supervivencia de
las larvas en las mismas especies mencionadas más arriba. No se
identificaron datos sobre los efectos de esta sustancia en organismos
terrestres.
A fin de facilitar orientación a las autoridades pertinentes, se
han determinado valores de orientación de muestra sobre la base del
potencial del 1,1,2,2-tetracloroetano para inducir tumores hepáticos
en ratones, porque éste es el parámetro toxicológico con respecto al
cual se caracteriza mejor la relación de respuesta a la dosis. Sin
embargo, es de señalar que los aumentos observados en la incidencia de
tumores se limitan actualmente a una especie y hay datos, si bien
incompletos, que sugieren que los tumores tal vez estén inducidos por
un mecanismo no genotóxico. El potencial, expresado como la dosis
asociada a un aumento del 5% en la incidencia de tumores, oscilaba
entre 5,8 y 28 mg/kg de peso corporal por día. Los valores de
orientación de muestra correspondientes al aire (la principal fuente
de exposición humana), que se han calculado dividiendo esos márgenes
de variación del potencial por 5000 ó 50 000, son de 3,4-16 µg/m3 y
0,34-1,6 µg/m3. Estos valores corresponden a los considerados por
algunas autoridades como indicativos de riesgo «esencialmente
insignificante» (es decir 10-5 a 10-6) para un carcinógeno
genotóxico; sin embargo, es de señalar que tal vez sea apropiado
considerar un margen más estrecho en vista de las indicaciones, si
bien incompletas, que sugieren un mecanismo epigenético de inducción
de tumores. Los valores correspondientes para la ingestión son de
1,2-5,6 µg/kg de peso corporal por día y 0,12-0,56 µg/kg de peso
corporal por día. La exposición indirecta en el medio ambiente
general, calculada sobre la base de una estimación de muestra de la
exposición, es inferior a esos valores, que se consideran moderados en
vista de los indicios, si bien incompletos, que sugieren que el
1,1,2,2-tetracloroetano tal vez induzca tumores por un mecanismo de
umbral.
1. EXECUTIVE SUMMARY
This CICAD on 1,1,2,2-tetrachloroethane was prepared by the
Environmental Health Directorate of Health Canada and was based
principally on a review prepared by the Government of Canada (1993) to
assess the potential effects on human health of indirect exposure to
1,1,2,2-tetrachloroethane in the general environment and the
chemical's environmental effects, as well as a review prepared by the
Agency for Toxic Substances and Disease Registry (ATSDR, 1994)
intended to characterize information on adverse health effects and
public exposure. Data identified as of September 1992 were considered
in the Government of Canada (1993) review. A comprehensive literature
search of several on-line databases was conducted in August 1995 to
identify any references published subsequent to those incorporated in
this review. Information on the nature of the peer review and the
availability of the source documents is presented in Appendix 1.
Information on the peer review of this CICAD is presented in Appendix
2. This CICAD was approved for publication at a meeting of the Final
Review Board, held in Brussels, Belgium, on 18-20 November 1996.
Participants at the Final Review Board meeting are listed in Appendix
3. The International Chemical Safety Card (ICSC 0332) for
1,1,2,2-tetrachloroethane, produced by the International Programme on
Chemical Safety (IPCS, 1993), has also been reproduced in this
document.
1,1,2,2-Tetrachloroethane (CAS no. 79-34-5) is a volatile
synthetic chemical that is used principally as an intermediate in the
synthesis of other chlorinated hydrocarbons, although use of this
substance has declined significantly. Releases to the environment are
primarily in emissions to ambient air, where the chemical is likely to
remain for several weeks. 1,1,2,2-Tetrachloroethane is not expected
to contribute to the depletion of stratospheric ozone or to global
warming. It is rapidly removed from aquatic systems and is unlikely
to bioaccumulate. Human exposure to 1,1,2,2-tetrachloroethane is
principally via inhalation.
Very few data are available on the effects of exposure to
1,1,2,2-tetrachloroethane in humans. The toxicological profile of
1,1,2,2-tetrachloroethane has also not been well characterized;
because of the chemical's declining use, available data are confined
primarily to early limited studies. The acute toxicity of 1,1,2,2-
tetrachloroethane in experimental animals is slight to moderate. Based
on the results of principally limited short-term and subchronic
studies, the liver appears to be the most sensitive target organ.
Although most of the available studies are inadequate to allow a no-
or lowest-observed-(adverse)-effect level [NO(A)EL or LO(A)EL] for
hepatotoxicity to be determined with confidence, minimal effects on
the liver (reversible increase in lipid content) and other end-points
(an increase in levels of adrenocorticotropic hormone and reversible
alterations in haematological parameters) have been observed in rats
exposed to 13.3 mg/m3 for up to 9 months. Based on limited,
primarily range-finding studies and early investigations, reproductive
and developmental effects have been observed in experimental animals
only at doses that caused reductions in body weight.
Long-term ingestion of 1,1,2,2-tetrachloroethane resulted in an
increased incidence of liver tumours in both male and female B6C3F1
mice. However, similar exposure was not associated with a significant
increase in tumours at any site in Osborne-Mendel rats, although both
species were exposed only for up to 78 weeks. Based on the results of
available in vivo and in vitro assays, 1,1,2,2-tetrachloroethane
has, at most, weak genotoxic potential. 1,1,2,2-Tetrachloroethane was
a potent promoter, but not an initiator, of gamma-glutamyl-
transpeptidase-positive foci in the liver of rats. The profile for
tumour induction by 1,1,2,2-tetrachloroethane is similar to that of
dichloroacetic acid, its primary metabolite. Information on the
mechanism of tumour induction by 1,1,2,2-tetrachloroethane is
incomplete; for several of its metabolites, it has been suggested that
tumours are likely induced by mechanisms for which there is a
threshold.
Exposure to 1,1,2,2-tetrachloroethane has been demonstrated to
inhibit the activities of environmental bacteria (the lowest reported
IC50 was 1.4 mg/litre) and cause immobilization in Daphnia magna
(48-hour EC50 values of 23 mg/litre and above). In freshwater fish
species, the lowest reported LC50 (96 hours) was 18.5 mg/litre in
flagfish (Jordanella floridae), whereas the lowest-observed-effect
concentration (LOEC) for longer-term exposure was 7.2 mg/litre, which
resulted in reduced larval survival in the same species. No data were
identified on the effects of this substance on terrestrial organisms.
In order to provide guidance to relevant authorities, sample
guidance values have been determined on the basis of the potency of
1,1,2,2-tetrachloroethane to induce liver tumours in mice, as this is
the toxicological end-point for which the dose-response relationship
is best characterized. It is noted, however, that observed increases
in tumour incidence are currently restricted to one species and that
there are suggestive but incomplete data indicating that tumours may
be induced by a non-genotoxic mechanism. The potency, expressed as
the dose associated with a 5% increase in tumours, ranged from 5.8 to
28 mg/kg body weight per day. Sample guidance values for air (the
principal source of human exposure), calculated on the basis of
division of this potency range by 5000 or 50 000, are 3.4-16 µg/m3
and 0.34-1.6 µg/m3. These values correspond to those considered by
some agencies to represent "essentially negligible" risk (i.e. 10-5
to 10-6) for a genotoxic carcinogen; it should be noted, however,
that a smaller margin may also be appropriate in view of the
suggestive but incomplete evidence for an epigenetic mechanism of
tumour induction. Corresponding values for ingestion are 1.2-5.6
µg/kg body weight per day and 0.12-0.56 µg/kg body weight per day.
Based on a sample estimate of exposure, indirect exposure in the
general environment is less than these values, which are considered to
be conservative in view of the suggestive but incomplete evidence that
1,1,2,2-tetrachloroethane may induce tumours through a threshold
mechanism.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
1,1,2,2-Tetrachloroethane (CAS no. 79-34-5; Cl2CHCHCl2;
acetylene tetrachloride, sym-tetrachlorethane; see structural
diagram below) is a synthetic chemical that is a colourless, non-
flammable liquid at room temperature. It is highly volatile, with a
vapour pressure of 0.65 kPa at 20°C and water solubility of 2900
mg/litre at 20°C. The log octanol/water partition coefficient for
1,1,2,2-tetrachloroethane is about 2.5, whereas its Henry's law
constant was determined to range from 0.0003 to 0.0009 m3.atm/mol
(Tse et al., 1992; Government of Canada, 1993; Nichols et al., 1993).
Additional physical/chemical properties are presented in the
International Chemical Safety Card (ICSC 0332) reproduced in this
document.
Cl Cl
' '
Cl - C - C - Cl
' '
H H
3. ANALYTICAL METHODS
Analysis of 1,1,2,2-tetrachloroethane in air usually involves
preconcentration on a sorbent tube followed by thermal or solvent
desorption or collection in a cryogenically cooled trap followed by
gas chromatography (flame ionization or electron capture detection).
Detection limits range from 0.7 ng/m3 to 0.3 mg/m3 (ATSDR, 1994).
Purge and trap methods followed by gas chromatography (flame
ionization, electron capture electrolytic conductivity, or
microcoulometric detection) are generally used for water as well as
sediment, soil, or other solid samples. Reported detection limits
range from 0.001 to 5 µg/litre for water and from 1 to 5 µg/kg for
soil and sediment samples (ATSDR, 1994). Detection limits of 0.01
µg/litre and 0.06 ppbv (0.4 µg/m3) have been reported for solid-phase
microextraction coupled with gas chromatography/ion trap mass
spectrometry analysis for water and air samples, respectively (Arthur
et al., 1992; Chai & Pawliszyn, 1995). Gas chromatography, often in
combination with mass spectrometry, is commonly used for quantifying
1,1,2,2-tetrachloroethane in biological samples, with detection limits
of 400 µg/kg in tissues and 5-500 ng/litre in blood (ATSDR, 1994).
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
There are no known natural sources of 1,1,2,2-tetrachloroethane.
The principal use of 1,1,2,2-tetrachloroethane is as an intermediate
in the manufacture of other chlorinated hydrocarbons, such as vinyl
chloride, 1,2-dichloroethane, trichloroethylene, and
tetrachloroethylene; in the past, it was also used as an industrial
solvent and as a pesticide. Use, and hence production, of
1,1,2,2-tetrachloroethane has declined significantly; no recent data
on production were identified. Releases to the atmosphere through its
use as a chemical intermediate in Canada in 1990 were estimated to be
approximately 246 kg (Government of Canada, 1993), whereas 64 251
pounds (29 144 kg) were estimated to be emitted to air from reporting
industries in the USA in 1991 (ATSDR, 1994). In 1991, 953 kg of
1,1,2,2-tetrachloroethane were discharged to water from reporting
facilities in the USA (ATSDR, 1994).
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
1,1,2,2-Tetrachloroethane is released to the environment
primarily in emissions to ambient air. Based on its vapour pressure,
it is not likely to be transferred to other compartments. The
atmospheric lifetime for 1,1,2,2-tetrachloroethane reacting with
hydroxyl radicals from moderately polluted areas is estimated to be
between 43 and 100 days, based on estimated and measured reaction
rates, respectively (Government of Canada, 1993). The half-life in
the troposphere is estimated to be in excess of 800 days, and
diffusion into the stratosphere is expected to be slow.1 Based on
these estimates, there is significant potential for long-range
transport of 1,1,2,2-tetrachloroethane. In the stratosphere,
1,1,2,2-tetrachloroethane undergoes photolysis to produce chlorine
radicals, which may subsequently react with ozone; however, the ozone
depletion potential for 1,1,2,2-tetrachloroethane is very much less
than 0.001 relative to the standard CFC-11 (trichlorofluoromethane),
based on the method developed by Nimitz & Skaggs (1992).
1,1,2,2-Tetrachloroethane released to the aquatic environment is
rapidly removed via volatilization, with an estimated half-life of 6.2
hours from running water and 3.5 days from still water.1 Hydrolysis
and biodegradation are the principal routes of removal from
groundwater. The hydrolysis half-life in subsurface sediment at 25°C
was determined to be 29 days (Haag & Mill, 1988). Neutral and
base-catalysed hydrolyses of 1,1,2,2-tetrachloroethane in pure water
yielded trichloroethylene as essentially the sole degradation product
(Haag & Mill, 1988). The products of anaerobic biodegradation of
1,1,2,2-tetrachloroethane were determined in a 6-week study to be (in
decreasing order) cis-1,2-dichloroethylene,
trans-1,2-dichloroethylene, trichloroethylene,
1,1,2-trichloroethane, 1,1-dichloroethylene, and vinyl chloride
(Hallen et al., 1986).
1,1,2,2-Tetrachloroethane is not expected to bioaccumulate in
aquatic species, based on low measured and calculated bioconcentration
factors in fish (Government of Canada, 1993).
1 Source: Hazardous Substances Data Bank, National Library of
Medicine, Bethesda, MD, 1996.
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
Data considered to be most representative of current levels of
1,1,2,2-tetrachloroethane in environmental media are presented in
Table 1. Mean concentrations of 1,1,2,2-tetrachloroethane in recent
surveys of ambient air in cities in Canada ranged from <0.1 to 0.25
µg/m3. Maximum concentrations of up to 79 µg/m3 have been detected
in the vicinity of waste sites in the USA (ATSDR, 1994).
Although data are limited, levels of 1,1,2,2-tetrachloroethane in
surface waters in Canada, the USA, and Germany generally range from
<0.005 to 4 µg/litre, from <10 µg/litre to a maximum reported value
of 180 µg/litre, and from <0.03 to 10 µg/litre, respectively; the
chemical was not detected (detection limits 0.001-0.05 µg/litre) in
surface waters in Japan.
1,1,2,2-Tetrachloroethane was not detected in sediment in Japan
in 1976 (detection limits ranged from 0.05 to 1 µg/g dry weight).
6.2 Human exposure
Exposure of the general population to 1,1,2,2-tetrachloroethane
in environmental media may be estimated based on concentrations
determined in various media and reference values for body weight and
consumption patterns. Owing to the paucity of relevant data from
other countries, particularly for recent years, exposure has been
estimated here based primarily on data from North America, as an
example. However, countries are encouraged to estimate total exposure
on the basis of local data, possibly in a manner similar to that
outlined here.
Mean levels in residential indoor air in Canada and the USA are
generally below the limits of detection (i.e. <0.1 µg/m3; see Table
1). Based on a daily inhalation volume for adults of 22 m3, a mean
body weight for males and females of 64 kg, the assumption that 4 of
24 hours are spent outdoors (IPCS, 1994), and the range of mean levels
of 1,1,2,2-tetrachloroethane in ambient air in recent surveys in
Canada of <0.1-0.25 µg/m3, the mean intake of
1,1,2,2-tetrachloroethane from ambient air for the general population
is estimated to range from <0.006 to 0.01 µg/kg body weight per day.
Average intake of 1,1,2,2-tetrachloroethane from indoor air, based on
the assumption that 20 of 24 hours are spent indoors (IPCS, 1994) and
the mean concentration in residential indoor air in Canada and the USA
of <0.1 µg/m3, is estimated to be <0.03 µg/kg body weight per day.
In a survey of 1159 household products in the USA,
1,1,2,2-tetrachloroethane was not detected above the limit of
detection of 0.1% (see Table 1).
1,1,2,2-Tetrachloroethane has not been detected in recent surveys
of drinking-water in Canada and has been only extremely rarely
detected (<0.03%) in recent surveys in the USA (detection limits
0.05-1.0 µg/litre; see Table 1), although it was detected in
groundwater near landfill sites in Finland at levels ranging from
<0.1 to 2.5 µg/litre (Assmuth & Strandberg, 1993). Similarly, it has
not been detected in three surveys of foodstuffs in Canada and the USA
(detection limits were 1 µg/litre for liquids and 5-50 µg/kg for
solids; see Table 1). No data were identified on levels of
1,1,2,2-tetrachloroethane in human breast milk. Drinking-water and
food probably do not represent significant sources of exposure to
1,1,2,2-tetrachloroethane, based on its volatility and low potential
for bioaccumulation.
Therefore, the principal media of exposure to
1,1,2,2-tetrachloroethane for the general population are likely indoor
and outdoor air, with negligible amounts being contributed by food and
drinking-water.
Although data on levels of 1,1,2,2-tetrachloroethane in the
workplace were not identified, workers may be exposed to the substance
via inhalation or dermal contact in "business services" (not further
specified) as well as the chemical and allied products industries
(ATSDR, 1994).
Table 1: Levels of 1,1,2,2-tetrachloroethane in various media.
Medium Location Year Concentrations Reference
Ambient air Canada 1989-1990 <0.1-0.25 µg/m3 (means) Environment Canada,
unpublished data, 1992
Ambient air USA pre-1987 0.7 µg/m3 (mean) Shah & Heyerdahl, 1988
Indoor air Canada 1991 <0.1 µg/m3 (mean) Fellin et al., 1992
Indoor air USA pre-1987 0.098 µg/m3 (mean) Shah & Heyerdahl, 1988
Drinking-water Canada 1988-1991 <0.05 µg/litre P. Lachmaniuk, personal
communication, 1991
1990 <1.0 µg/litre Ecobichon & Allen, 1990
Drinking-water USA pre-1986 <0.5 µg/litre ATSDR, 1994
1984-1992 NDa-5.8 µg/litre Storm, 1994
Surface water Canada 1985 <1.0-4.0 µg/litre COARGLWQ, 1986
1981 <0.005-0.06 µg/litre Kaiser & Comba, 1983
Surface water USA 1980-1988 <10-180 µg/litre ATSDR, 1994
Surface water Japan 1976 <0.001, <0.002, Environment Agency Japan, 1976
<0.05 µg/litre
Surface water Germany 1989-1990 <0.03-10 µg/litre Wittsiepe, 1990
Food (34 groups) Canada 1991 <50 µg/kg (solids), Enviro-Test Laboratories, 1991
<1 µg/litre (liquids)
1992 <5 µg/kg (solids), Enviro-Test Laboratories, 1992
<1 µg/litre (liquids)
Food (231 items) USA <13 µg/kg, <20 µg/kg Daft, 1988
Consumer products USA <0.1% Sack et al., 1992
(1159 items)
Sediment Japan 1976 <0.05 µg/g, <1 µg/g Environment Agency Japan, 1976
a Detection limit not specified.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND
HUMANS
1,1,2,2-Tetrachloroethane is readily absorbed following
inhalation, ingestion, and dermal exposure and is likely distributed
throughout the body, although relevant data are limited. Based on
data on the metabolism of 1,1,2,2-tetrachloroethane in mice, Yllner
(1971) suggested that the principal pathway of degradation involves
stagewise hydrolytic cleavage of the carbon-chlorine bonds and
oxidation to dichloroacetaldehyde hydrate, dichloroacetic acid (the
major metabolite), and eventually glyoxylic acid. The glyoxylic acid
is then metabolized to oxalic acid, glycine, formic acid, and carbon
dioxide. A small proportion of the parent compound is probably
non-enzymatically dehydrochlorinated to trichloroethylene, which is
further converted to trichloroacetic acid and trichloroethanol. In
addition, a minor amount of 1,1,2,2-tetrachloroethane may be oxidized
to tetrachloroethylene, which, in turn, is metabolized to
trichloroacetic acid and oxalic acid. It has also been proposed that
1,1,2,2-tetrachloroethane may be metabolized via cytochrome P-450 to
dichloroacetyl chloride, which is hydrolysed to dichloroacetic acid
(Halpert, 1982). In addition to the liver, metabolism may also occur
in the epithelia of the respiratory tract and upper alimentary tract
(Eriksson & Brittebo, 1991). The metabolites of
1,1,2,2-tetrachloroethane are eliminated in the urine, faeces, skin,
and expired air.
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
The acute toxicity of 1,1,2,2-tetrachloroethane in experimental
animals is slight to moderate. Exposure to concentrations of around
1000 ppm (6980 mg/m3) for 4 or 6 hours or about 5000-6000 ppm
(34 900-41 880 mg/m3) (duration not specified) caused deaths in rats
and mice, respectively. Oral LD50s of 250-330 and 1000 mg/kg body
weight for 1,1,2,2-tetrachloroethane in rats have been reported. The
dermal LD50 (24 hours) in rabbits was 6360 mg/kg body weight (Kennedy
& Graepel, 1991; ATSDR, 1994).
8.2 Irritation and sensitization
Epidermal and dermal changes were reported in rabbits following
cutaneous exposure to 1,1,2,2-tetrachloroethane (Smyth et al., 1969).
Exposure to 580 ppm (4050 mg/m3) 1,1,2,2-tetrachloroethane caused
ocular irritation in guinea-pigs (Price et al., 1978). No information
on the sensitization potential of this substance was identified.
8.3 Short-term exposure
In general, dose-response relationships have not been well
characterized in available short-term studies in experimental animals
owing to limitations of the studies, including use of only one level
of exposure or inadequate description of protocol or results. Hepatic
effects, including increased organ weight, congestion, fatty
degeneration, histological changes, alterations in levels of enzymes,
and elevated DNA synthesis (the degree of which increased with dose),
have been observed in rodents following short-term inhalation of
1,1,2,2-tetrachloroethane at concentrations as low as 13.3 mg/m3 (for
2-10 days) and ingestion of the chemical at doses as low as 75 mg/kg
body weight per day (for 4 days) in the few available, principally
limited, studies (Horiuchi et al., 1962; Gohlke & Schmidt, 1972;
Schmidt et al., 1972; Hanley et al., 1988; NTP, 1996). In a limited
account of a study in rats, Ulanova et al. (1984) reported effects on
the nervous system and kidneys to be similar following continuous or
intermittent exposure for 4-27 days to comparable
time-weighted-average concentrations of 1,1,2,2-tetrachloroethane
(235 and 250 mg/m3).
8.4 Long-term exposure
8.4.1 Subchronic exposure
Only a few limited studies have been identified on the effects in
experimental animals following subchronic exposure to
1,1,2,2-tetrachloroethane (see Table 2).1 Ingestion of up to 316
mg/kg body weight per day had no effects on body weight gain or
mortality in groups of five male or female B6C3F1 mice, whereas doses
of 100 (females) or 178 (males) mg/kg body weight per day and above
resulted in decreased body weight gain in groups of five male or
female Osborne-Mendel rats in subchronic studies preliminary to
longer-term bioassays (no other end-points appear to have been
examined) (NCI, 1978). Histopathological damage (including chronic
inflammation, necrosis, or atrophy) was observed in the liver, kidney,
testicles, and thyroid gland of rats ( n = 10 per group) administered
oral 1,1,2,2-tetrachloroethane doses of 3.2-50 mg/kg body weight per
day for periods ranging from 2 to 150 days (Gohlke et al., 1977),
although the limited documentation of results in this study precludes
validation of an effect level.
Exposure to 1,1,2,2-tetrachloroethane at 50 mg/m3 for
approximately 5 weeks resulted in alterations in biochemical
parameters and organ weights in male rats (strain and number not
specified), although no "morphological changes" were noted upon
examination (the nature and extent of the histopathological
examination were unspecified) (Schmidt et al., 1975). Depressed
agglutinin formation was observed in rabbits exposed to 100 mg/m3,
3-4 hours/day, for 4-6 weeks (Navrotskiy et al., 1971) (no other
effects were noted in this study, for which only secondary accounts
were available). Hepatic effects, including a transient increase in
DNA synthesis, reversible histopathological changes (cytoplasmic
vacuolization and hyperplasia), and an increase in relative liver
weight, were observed in female Sprague-Dawley rats ( n = 55) exposed
for 15 weeks to 560 ml/m3 (reported by ATSDR [1994] to be equivalent
to 130 ppm [907 mg/m3], although information on the exposure level
presented in the original paper was unclear) (Truffert et al., 1977).
8.4.2 Chronic exposure and carcinogenicity
The chronic toxicity of 1,1,2,2-tetrachloroethane has not been
extensively investigated; available studies are not adequate to allow
the confident determination of an "effect level" for non-neoplastic
effects. A reversible decrease in body weight and a reversible
1 Subchronic studies have been completed for the National Toxicology
Program (NTP) in which groups of 10 male or female F344 rats and
B6C3F1 mice were administered microencapsulated
1,1,2,2-tetrachloroethane in feed at doses equivalent to 18-300 mg/kg
body weight per day and 88-1400 mg/kg body weight per day,
respectively, for 13 weeks. The results of these studies are
currently being reviewed by the NTP's Pathology Working Group.
increase in lipid content of the liver were observed in male rats
exposed to 1,1,2,2-tetrachloroethane by inhalation at 13.3 mg/m3 for
110 or 265 days or for 265 days with a 60-day recovery period (seven
rats were killed at each interval); there were also reversible
alterations in haematological parameters, which were statistically
significantly different from controls only at one point in time during
the study, and increased adrenocorticotropic hormone activity in the
hypophysis (Schmidt et al., 1972). However, histopathological effects
were not described in the published account of the investigation. In
a study for which only secondary accounts were available, early signs
of liver degeneration were observed in rabbits exposed to 100 mg/m3
for 7-11 months (Navrotskiy et al., 1971) (no further details were
provided).
An increase in the incidence of hepatocellular carcinomas was
observed in groups of 50 ( n = 20 in controls) male and female
B6C3F1 mice administered technical-grade 1,1,2,2-tetrachloroethane in
corn oil by gavage at time-weighted-average daily doses of 142 or 284
mg/kg body weight for 78 weeks (1/18, 13/50, and 44/49 in males, and
0/20, 30/48, and 43/47 in females, in the vehicle controls, low-dose
group, and high-dose group, respectively). These tumours also
appeared earlier in mice administered the higher dose. Slightly
decreased body weight gain and increased mortality were also observed
in exposed mice; there were no increases in the incidences of non-
neoplastic lesions (NCI, 1978).
There were no significant increases in the incidence of any type
of neoplastic or non-neoplastic lesion in groups of 50 ( n = 20 in
controls) male or female Osborne-Mendel rats similarly administered
technical-grade 1,1,2,2-tetrachloroethane in corn oil by gavage at
time-weighted-average doses of 62 or 108 mg/kg body weight per day
(males) and 43 or 76 mg/kg body weight per day (females) for 78 weeks,
although there were two males with hepatocellular carcinomas and one
with a hepatic neoplastic nodule in the high-dose group. There were
also reversible dose-related decreases in body weight gain and
increased mortality in exposed rats (NCI, 1978).
In a limited bioassay designed to investigate the potential of
1,1,2,2-tetrachloroethane to induce pulmonary adenomas in a sensitive
strain of mice, there was no increase in the number of these tumours
in a group of 20 strain A mice intraperitoneally administered the
chemical for 24 weeks; however, mortality was high in this study
(Theiss et al., 1977; Stoner, 1991).
In an initiation/promotion assay, 1,1,2,2-tetrachloroethane did
not initiate formation of gamma-glutamyltranspeptidase-positive foci
in the liver (a putative preneoplastic indicator) in groups of 10 male
Osborne-Mendel rats administered an oral dose of 100 mg/kg body weight
followed by exposure to phenobarbital for 7 weeks, although it acted
as a potent promoter in rats initiated with a single dose of
diethylnitrosamine followed by exposure to 1,1,2,2-tetrachloroethane
by gavage for 7 weeks at 100 mg/kg body weight per day (Story et al.,
1986; Milman et al., 1988).
Table 2: Investigations of non-neoplastic effects of 1,1,2,2-tetrachloroethane.
Study design Effects Effect level Comments Reference
INHALATION
Male rats exposed to 50 mg/m3, 4 Neurological effects; alterations in Effects at 50 Strain and number of rats not Schmidt et al.,
hours/day, 5 days/week, for 5 weeks; biochemical parameters and organ mg/m3 specified; nature and extent of 1975
or to 130 mg/m3 for 15 minutes, 5 weights (although within ranges histopathological examination
times/day, separated by 40-minute observed in controls); no not specified
intervals, for 5 weeks "morphological changes"
Fifty-five female Sprague-Dawley Transient increase in hepatic DNA Effects at 560 One exposure group only; Truffert et
rats exposed to 560 ml/m3 for 5 or synthesis, reversible ml/m3 uncertainty concerning exposure al., 1977
6 hours/day, 5 days/week, for 15 histopathological changes in the (equivalent to level based on unclear
weeks; liver, kidneys, lungs, liver; increase in relative liver 130 ppm or information in article (note:
ovaries, uterus, and adrenal weight 907 mg/m3, concentration more than
glands histopathologically examined based on ATSDR approximately 10-fold higher
[1994] than that at which effects
conversion were reported in other
studies, no matter how converted)
Male rats exposed to 13.3 mg/m3 Increased adrenocorticotropic Minimal Exposure pattern (e.g. number Schmidt et
(probably for 4 hours/day) for 110 hormone activity in the hypophysis effects at of exposed days per week) not al., 1972
or 265 days; one group exposed for greatest at 4 months and lessened 13.3 mg/m3 clearly specified;
265 days and allowed to recover towards the end of the study; histopathological effects not
until day 325; seven rats reversible decrease in body weight; described in published account
sacrificed after each interval reversible increase in lipid of study
content of liver and reversible
alterations in haematological
parameters, which were significantly
different from controls only at one
point in time during the study
Table 2 (continued)
Study design Effects Effect level Comments Reference
Rabbits exposed to 100 mg/m3 3-4 Depressed agglutinin formation Effects at Secondary accounts available Navrotskiy et
hours/day for 4-6 weeks or exposed after 4-6 weeks; early signs of 100 mg/m3 only; strain, number, and sex al., 1971
for 7-11 months (different liver degeneration after 7-11 not specified; may be only
protocols noted in two secondary months one exposure level; no other
accounts) effects noted
Chinchilla rabbits exposed to 0, Decrease in titres of typhoid Effects at Sex and number of animals per Shmuter, 1977
2, 10, or 100 mg/m3, 3 hours/day, antibodies, an increase in the 10 mg/m3; no exposed group not specified
6 days/ week, for 8 months electrophoretic mobility of effects at 2
(n = 50 for controls) antibodies towards ß- and mg/m3
alpha-globulin fractions, and
a decrease in the level of
"normal" haemolysins to the
Forsman's antigen of sheep
erythrocytes
Six rabbits were exposed to Decreased levels of Effects at Isomer not specified; no Kulinskaya &
10 mg/m3, 3 hours/day, for acetylcholine and 10 mg/m3 end-points other than Verlinskaya,
approximately 8 months; 15 rabbits acetylcholinesterase in the cholinergic indices were 1972
were used as controls; animals blood investigated
were immunized at 1.5 and 4.5
months with typhoid vaccine
Table 2 (continued)
Study design Effects Effect level Comments Reference
INGESTION
Groups of 10 rats administered Damage to liver, kidney, testicles, Effects at Inadequate documentation of Gohlke et
3.2, 8.0, 20, or 50 mg/kg body and thyroid gland (determined by 3.2 mg/kg protocol and results; no al., 1977
weight per day by gavage for histological, enzyme histochemical, body weight quantitative data; not reported
2-150 days and histoautoradiographic per day in which dose groups effects
techniques) were observed (some groups were
also concomitantly exposed to
high temperatures); not possible
to verify effect level
Five male or female B6C3F1 mice No effects on body weight gain No effects at No end-points other than body NCI, 1978
administered 0, 32, 56, 100, or mortality highest dose weight and mortality appear to
178, or 316 mg/kg body weight of 316 mg/kg have been examined
per day by gavage, 5 days/week, body weight
for 6 weeks, followed by 2 per day
weeks of observation
Five male or female Osborne-Mendel Decrease in body weight gain in Effects at No end-points other than body NCI, 1978
rats administered 0, 56, 100, 178, males at 178 mg/kg body weight 100 mg/kg weight and mortality appear to
316, or 562 mg/kg body weight per per day and in females at 100 body weight have been examined; no data
day by gavage, 5 days/week, for and 178 mg/kg body weight per day; per day; no presented on effects on body
6 weeks, followed by 2 weeks of all females exposed to 316 mg/kg effects at 56 weight gain at two highest
observation body weight per day died; one male mg/kg body doses or on mortality for other
exposed to 100 mg/kg body weight weight per dose groups
per day died day
Table 2 (continued)
Study design Effects Effect level Comments Reference
Fifty male or female B6C3F1 mice Slight dose-related decreases in Significance of decrease in NCI, 1978
(n = 20 in controls) administered body weight gain; dose-related body weight gain not presented;
time-weighted-average doses of 0, increases in mortality; no no end-points other than body
142, or 284 mg/kg body weight per increases in incidences of weight, mortality, or
day by gavage, 5 days/week, for non-neoplastic lesions histopathology were examined
78 weeks, followed by 12 weeks
of observation
Fifty male or female Osborne-Mendel Reversible dose-related decreases Significance of decrease in NCI, 1978
rats (n = 20 in controls) in body weight gain; increased body weight gain not presented;
administered time-weighted-average mortality at higher dose; no no end-points other than body
doses of 0, 62, or 108 mg/kg body increases in incidences of weight, mortality, or
weight per day (male) or 0, 43, or non-neoplastic lesions histopathology were examined
76 mg/kg body weight per day
(female) by gavage, 5 days/week,
for 78 weeks, followed by 32 weeks
of observation
Little information on the mechanism(s) of liver tumour induction
in mice exposed to 1,1,2,2-tetrachloroethane has been identified.
Several of the metabolites of 1,1,2,2-tetrachloroethane, including
trichloroethylene, tetrachloroethylene, trichloroacetic acid, and
dichloroacetic acid, have been hepatocarcinogenic in experimental
animals (e.g. NCI, 1977; Maltoni et al., 1986, 1988; NTP, 1986, 1990;
Herren-Freund et al., 1987; Bull et al., 1990; DeAngelo et al., 1991).
Indeed, the toxicological profile for 1,1,2,2-tetrachloroethane is
very similar to that for dichloroacetic acid, the principal
metabolite.
8.5 Genotoxicity and related end-points
Results of identified in vitro studies are summarized in Table
3. Predominantly negative results have been reported for the
induction of gene mutation in prokaryotic systems with and without
metabolic activation, whereas both positive and negative results have
been observed for gene conversions in yeast and fungi.
1,1,2,2-Tetrachloroethane induced sister chromatid exchange but not
chromosomal aberrations, DNA repair, or unscheduled synthesis of DNA
in mammalian cells in vitro.
Exposure to 1,1,2,2-tetrachloroethane at 349 mg/m3 for 5 days
did not induce dominant lethal mutations in rats, and results for
chromosomal aberrations in rat bone marrow cells were equivocal;
however, this concentration did not induce cytotoxicity (McGregor,
1980). 1,1,2,2-Tetrachloroethane did not induce unscheduled DNA
synthesis in hepatocytes of mice exposed to doses of up to 1000 mg/kg
body weight by gavage, whereas results for the induction of S-phase
synthesis were negative and equivocal (Mirsalis et al., 1989).
1,1,2,2-Tetrachloroethane has also been reported to bind to
cellular macromolecules, including DNA, RNA, and proteins of several
organs in rodents, following in vivo exposure (Mitoma et al., 1985;
Colacci et al., 1987; Eriksson & Brittebo, 1991). Results for cell
transformation in mammalian cells have been mixed, with positive
results being reported by only one of four investigators (Little,
1983; Tu et al., 1985; Milman et al., 1988; Colacci et al., 1990,
1992, 1993).
1,1,2,2-Tetrachloroethane did not induce sex-linked recessive
lethal mutations or mitotic recombination in Drosophila
melanogaster in three studies (McGregor, 1980; Woodruff et al.,
1985; Vogel & Nivard, 1993).
With the possible exception of the equivocal results for
chromosomal aberrations observed in female rats following inhalation
(McGregor, 1980), the weight of evidence overall indicates that
1,1,2,2-tetrachloroethane is not genotoxic or that it is only weakly
genotoxic, acting through a mechanism that results in gene conversion
and induction of sister chromatid exchange.
Table 3: Genotoxicity of 1,1,2,2-tetrachloroethane in vitro.
Result
With Without
Species (test system) End-point activation activation Reference
Saccharomyces cerevisiae D7 Mitotic gene conversion nt + Callen et al., 1980
Recombination nt +
Saccharomyces cerevisiae D7 Gene conversion and reversion Nestmann and Lee, 1983
XV185-14C nt -
nt -
Salmonella typhimurium Reverse mutations Brem et al., 1974
TA1530 nt +
TA1535 nt +
TA1538 nt -
Salmonella typhimurium Reverse mutations Nestmann et al., 1980
TA1535 - -
TA100 - -
TA1537 - -
TA1538 - -
TA98 - -
Salmonella typhimurium Reverse mutations Milman et al., 1988
TA1535 - -
TA1537 - -
TA98 - -
TA100 - -
Salmonella typhimurium Reverse mutations Haworth et al., 1983
TA1535 - -
TA1537 - -
TA98 - -
TA100 - -
Table 3 (continued)
Result
With Without
Species (test system) End-point activation activation Reference
Salmonella typhimurium TA100 Reverse mutations - - Warner et al., 1988
Salmonella typhimurium Reverse mutations Mersch-Sundermann et al.,
TA97 + - 1989a
TA98 + -
TA100 - -
TA102 - -
Salmonella typhimurium Forward mutations - - Roldan-Arjona et al., 1991
BA13/BAL13
Escherichia coli (polymerase DNA damage nt + Brem et al., 1974
deficient pol A+/pol A-)
Escherichia coli PQ37 Gene mutation - - Mersch-Sundermann et al.,
1989b
Escherichia coli Induction of prophage lambda + - DeMarini & Brooks, 1992
Aspergillus nidulans Mitotic malsegregation nt + Crebelli et al., 1988
Chinese hamster ovary cells Chromosomal aberrations - - Galloway et al., 1987
Chinese hamster ovary cells Sister chromatid exchange + + Galloway et al., 1987
BALB/c3T3 cells (mouse) Sister chromatid exchange + + Colacci et al., 1992
Mouse hepatocytes DNA growth, repair, or synthesis nt - Williams, 1983
Table 3 (continued)
Result
With Without
Species (test system) End-point activation activation Reference
Mouse hepatocytes DNA repair nt - Milman et al., 1988
Rat hepatocytes DNA growth, repair, or synthesis nt - Williams, 1983
Rat hepatocytes DNA repair nt - Milman et al., 1988
Human embryonic intestinal cells Unscheduled DNA synthesis - - McGregor, 1980
nt = not tested
8.6 Reproductive and developmental toxicity
Although available data are limited, reproductive and
developmental effects have been observed only in experimental animals
exposed orally or by inhalation to levels of 1,1,2,2-tetrachloroethane
that are also associated with decreases in body weight. Effects on
reproductive parameters, including decreases in testicular,
epididymal, and caudal weights, decreased epididymal sperm motility,
and altered estrous cycles, were observed in pilot studies in rats and
mice orally exposed for 90 days to doses that also caused significant
decreases in body weight (NTP, 1993). Although histological changes
in the testes have been observed in rats administered
1,1,2,2-tetrachloroethane doses of 8 mg/kg body weight per day in
peanut oil by gavage for 150 days (Gohlke et al., 1977), no effects on
reproductive organs were reported in the long-term studies in which
rats and mice were administered much higher doses for 78 weeks (NCI,
1978) (see section 8.4.2) or in inhalation studies in rats (Gohlke &
Schmidt, 1972; Schmidt et al., 1972) or a single monkey (Horiuchi et
al., 1962). No effect on male fertility or viability and no
macroscopic changes in offspring were observed in male rats exposed to
13.3 mg/m3 for 258 days (Schmidt et al., 1972). Small, but
statistically significant, increases in one type of sperm abnormality
were observed in rats exposed to 349 mg/m3 for 5 days, although the
authors considered this effect to be of questionable biological
significance (McGregor, 1980). Decreased fetal body weight and/or
increased resorptions were observed in range-finding studies in rats
and mice exposed to 1,1,2,2-tetrachloroethane in the feed during
gestation at doses greater than those that induced maternal toxicity
(increased mortality or decreased body weight gain) (NTP, 1991a,b).
8.7 Immunological and neurological effects
Immunological effects have been observed in limited studies in
rabbits exposed to 1,1,2,2-tetrachloroethane by inhalation. For
example, Shmuter (1977) reported a decrease in the titres of typhoid
antibodies, an increase in the electrophoretic mobility of antibodies
towards ß- and alpha-globulin fractions, and a decrease in the level
of "normal" haemolysins to the Forsman's antigen of sheep erythrocytes
in animals exposed to 1,1,2,2-tetrachloroethane at 10 mg/m3 and above
for 8 months, whereas alterations in levels of acetylcholine and
acetylcholinesterase in the blood have been observed at 10 mg/m3
(Kulinskaya & Verlinskaya, 1972).
Neurological effects have been observed in several species
following acute or short-term exposure to 1,1,2,2-tetrachloroethane
(e.g. at concentrations as low as 200 ppm [1396 mg/m3] for 6 hours
[Horvath & Frantik, 1973] or 50 mg/m3 for approximately 5 weeks
[Schmidt et al., 1975]). A single oral dose of 50 mg/kg body weight
increased levels of several neurotransmitters in the brain of rats
(Kanada et al., 1994).
9. EFFECTS ON HUMANS
Death has been reported following suicidal ingestion of doses of
1,1,2,2-tetrachloroethane estimated to range from 285 to 6000 mg/kg
body weight (ATSDR, 1994). Hepatic effects and death have also been
reported following accidental poisoning with
1,1,2,2-tetrachloroethane. Other effects noted in earlier reports of
workers or volunteers exposed to 1,1,2,2-tetrachloroethane
concentrations ranging up to 1800 mg/m3 include respiratory failure,
mucosal irritation, unconsciousness, gastrointestinal and neurological
distress, jaundice, liver enlargement or degeneration, headache,
tremors, dizziness, numbness, and drowsiness (ATSDR, 1994).
No statistically significant increase in mortality due to any
specific cause was noted in a limited epidemiological investigation in
a population of 1099 men exposed to unknown concentrations of
"tetrachloroethane" (Norman et al., 1981). The prevalence of nervous
symptoms, including tremors, headaches, and vertigo, was reported to
increase with airborne concentration of 1,1,2,2-tetrachloroethane (up
to 98 ppm [684 mg/m3]) in a group of 380 workers in India exposed for
varying durations, although no information was presented on the
prevalence of these signs in an unexposed group. Exposed workers also
reported loss of appetite, nausea, vomiting, and abdominal pain (Lobo-
Mendonca, 1963). Similar symptoms (i.e. loss of appetite, bad taste
in the mouth, epigastric pain, sensation of pressure in the liver
area, headaches, general debility, lack of stamina, loss of body
weight, and occasional painful prurigo) were observed in employees of
a penicillin plant exposed to concentrations of
1,1,2,2-tetrachloroethane ranging from 10 to 1700 mg/m3. The
prevalence of symptoms decreased with the implementation of
improvements in working conditions, and most workers were reported to
be free of symptoms when maximum levels were below 250 mg/m3 (Jeney
et al., 1957).
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10.1 Aquatic environment
Bioassays were conducted by Blum & Speece (1991) on three groups
of bacteria: methanogens (anaerobes from an enrichment culture
maintained for >10 years), aerobic heterotrophic bacteria, and
Nitrosomonas obtained from the mixed liquor of an activated sludge
wastewater treatment plant. Inhibition of gas production by
methanogens, inhibition of oxygen uptake by aerobic heterotrophic
bacteria, and inhibition of ammonia oxidation by Nitrosomonas were
the end-points used in this study to evaluate toxicity. Varying
degrees of sensitivities were exhibited; however, Nitrosomonas, with
an IC50 value of 1.4 mg/litre, was more sensitive than methanogens
(IC50 value of 4.1 mg/litre) and significantly more sensitive than
aerobic heterotrophs (IC50 value of 130 mg/litre).
Based on bioluminescence, the 5-minute LC50 for
1,1,2,2-tetrachloroethane was 5.4 mg/litre in a Microtox test using
Photobacterium phosphoreum (Blum & Speece, 1991).
Unfed and fed Daphnia magna (first instar, <24 hours old) had
similar measured 48-hour LC50 values of 62 and 57 mg/litre,
respectively, under static test conditions (Richter et al., 1983).
With complete immobilization as the end-point, the 48-hour EC50
values were 23 and 25 mg/litre for unfed and fed D. magna,
respectively. LeBlanc (1980) conducted a similar test with
D. magna at 22°C and reported nominal 24-hour and 48-hour LC50
values of 18 and 9.3 mg/litre, respectively. Pawlisz & Peters (1995)
reported that prior exposure of D. magna to sublethal concentrations
of 1,1,2,2-tetrachloroethane (6.3-50% of the 48-hour LC50 of 0.095
mmol/litre) for 24 hours did not influence the body burden required to
narcoticize the animals upon subsequent exposure to the chemical at
the LC50.
The measured 28-day LOEC and no-observed-effect concentration
(NOEC) for reproductive impairment in D. magna were 14.4 and 6.9
mg/litre, respectively, under flow-through conditions (Richter et al.,
1983).
Numerous acute toxicity studies have been conducted on a variety
of freshwater fish species; in general, 96-hour LC50 values were very
similar. Under flow-through conditions, the measured 96-hour LC50s
for 30-day-old fathead minnows (Pimephales promelas) were 20.3 and
20.4 mg/litre (Veith et al., 1983; Walbridge et al., 1983). In
juvenile (2- to 4-month-old) flagfish, the measured 96-hour LC50 for
1,1,2,2-tetrachloroethane in the flow-through toxicity test was 18.5
mg/litre; the nominal LC50 value in a static-renewal 96-hour toxicity
test was 26.8 mg/litre (ATRG, 1988; Smith et al., 1991). No adequate
acute toxicity studies of marine fish were identified.
Chronic toxicity studies under flow-through test conditions were
conducted on the early life stages of flagfish by ATRG (1988) and
Smith et al. (1991). Egg hatchability was unaffected at a measured
1,1,2,2-tetrachloroethane concentration of 22.0 mg/litre, the highest
concentration tested in both studies. The measured LOECs for reduced
10-day larval survival were 10.6 and 7.2 mg/litre, whereas the LOECs
for 28-day juvenile survival were 11.7 and 8.5 mg/litre (ATRG, 1988;
Smith et al., 1991). There were no statistically significant effects
on the growth of 1-week-old fry over a 28-day exposure period, even at
the highest concentration of 1,1,2,2-tetrachloroethane tested (11.7
mg/litre).
Ninety-day carcinogenicity studies were conducted under
flow-through conditions on 2-day-old guppy (Poecilia reticulata) and
6-day-old Japanese medaka (Oryzias latipes) exposed continuously to
1,1,2,2-tetrachloroethane at 4 mg/litre, exposed once per week (24
hours) to 8 mg/litre, or exposed once per week (24 hours) to 15
mg/litre. Histopathological examination of all exposed groups at 90
days did not reveal any evidence of carcinogenicity (Hawkins, 1991).
10.2 Terrestrial environment
No studies were identified on the effects of
1,1,2,2-tetrachloroethane on terrestrial organisms.
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
Owing to the significant decline in the use of this substance,
the toxicological profile of 1,1,2,2-tetrachloroethane has not been
well characterized, with the available data being confined primarily
to early limited studies.
Based on the results of studies in experimental animals, the
acute toxicity of 1,1,2,2-tetrachloroethane is slight to moderate.
The chemical may induce skin, eye, and mucosal irritation. Owing to
the limitations of the available data in humans on effects associated
with longer-term exposure to 1,1,2,2-tetrachloroethane, it is
necessary to rely on information obtained from the limited studies in
animals for determination of the critical effects and associated
effect levels.
The results of available studies on the non-neoplastic effects of
1,1,2,2-tetrachloroethane in experimental animals exposed by ingestion
or inhalation indicate that the liver is the principal target organ.
However, the majority of subchronic and chronic studies are too
limited to allow a confident determination of a NO(A)EL or LO(A)EL for
hepatic or other effects, because of either the lack of information
presented in the published accounts or the limitations of the study
designs (e.g. small numbers of animals per experimental group, lack of
histopathological examination, etc.).
Long-term exposure to 1,1,2,2-tetrachloroethane resulted in a
significantly increased incidence of hepatocellular carcinomas in both
male and female mice. However, no significant increases in tumours
were observed in similarly exposed rats, although there was a non-
statistically significant increase at the highest dose tested (which
was lower, on a time-weighted-average basis, than the lowest dose to
which mice were exposed), and both species were exposed only for up to
78 weeks. 1,1,2,2-Tetrachloroethane was a potent promoter, but did
not act as an initiator, in an initiation/promotion assay. The weight
of evidence of available in vitro and in vivo assays suggests that
this substance is not genotoxic or that it is, at most, weakly
genotoxic. Although available data are incomplete, it has been
proposed that the liver tumours may be induced by mechanisms that may
not be relevant to humans, for which humans are less susceptible, or
for which there may be a threshold of exposure. In addition, it has
been hypothesized that the carcinogenicity of
1,1,2,2-tetrachloroethane may be associated with the formation of free
radicals, lipid peroxidation, or hepatic damage (such as focal
necrosis associated with intense cellular proliferation) (Hanley et
al., 1988; Larson & Bull, 1992; Paolini et al., 1992). Therefore, on
the basis of data currently available, it is not possible to draw any
firm conclusions with respect to the potential carcinogenicity of
1,1,2,2-tetrachloroethane in humans.
Owing to the limitations of available studies on the potential
toxicological effects associated with exposure to
1,1,2,2-tetrachloroethane, it is not possible to confidently determine
a NO(A)EL or LO(A)EL for non-neoplastic effects. The toxicological
end-point for which the dose-response relationship is best
characterized is the increase in hepatocellular carcinomas observed in
the long-term bioassay in mice (NCI, 1978). It is noted, however,
that the observed increases in tumour incidence are restricted to one
species and that the weight of available data indicates that
1,1,2,2-tetrachloroethane is, at most, weakly genotoxic.
Based on multistage modelling of the incidence of hepatocellular
carcinomas in male or female mice exposed to time-weighted-average
doses of 0, 142, or 284 mg/kg body weight per day for up to 78 weeks,
adjusted for continuous exposure for a standard duration of 104 weeks
and corrected for the expected rate of increase in tumour formation in
rodents in a standard bioassay of 104 weeks, the doses associated with
a 5% increase in tumour incidence (TD0.05) range from 5.8 to 28 mg/kg
body weight per day.
11.1.2 Criteria for setting guidance values for
1,1,2,2-tetrachloroethane
As noted in section 11.1.1, the toxicological end-point for which
the dose-response relationship is best characterized, and which might
provide the basis for derivation of limits of exposure or for
judgement of the quality of environmental media by relevant
authorities, is the increase in hepatocellular carcinomas observed in
the long-term bioassay in mice (NCI, 1978).
A value, for example, 5000 or 50 000 times less than the TD0.05s
derived above might be considered conservative as a guidance value.
This margin (5000-50 000) affords protection similar to that
associated with the range for low-dose risk estimates generally
considered by various agencies to be "essentially negligible" (i.e.
10-5 to 10-6). As, on the basis of available data,
1,1,2,2-tetrachloroethane is, at most, weakly genotoxic, a smaller
margin (e.g. 1000) might also be considered appropriate. As available
data indicate that air is the principal medium of human exposure, the
most conservative of these approaches result in, for example, a range
of airborne concentrations of 3.4-16 µg/m3 or 0.34-1.6 µg/m3,
respectively. Corresponding values for ingestion are 1.2-5.6 µg/kg
body weight per day or 0.12-0.56 µg/kg body weight per day. It should
be noted, however, that these possible guidance values for air have
been extrapolated directly from a study in which the chemical was
administered orally to experimental animals. Although there may be
substantial variations in toxicokinetics following exposure to
1,1,2,2-tetrachloroethane by different routes, available data are
inadequate to quantitatively account for these differences in the
derivation of guidance values.
It is noteworthy that a provisional tolerable concentration
derived on the basis of the minimal non-neoplastic effects observed in
rats exposed to 13.3 mg/m3 (Schmidt et al., 1972) would fall within
the range of the values presented here.
11.1.3 Sample risk characterization
Although data are insufficient to allow the confident
determination of a LO(A)EL or NO(A)EL for 1,1,2,2-tetrachloroethane,
minimal effects in rodents have been observed only at levels more than
50 000 times greater than those in the principal medium of exposure
(air) in the general environment.
Based on a sample estimate of exposure, indirect exposure in the
general environment is 14 to >160 or 1.4 to >16 times less than
guidance values that might be derived on the basis of available data
on the dose-response relationship for liver tumour induction in mice
(i.e. the TD0.05s divided by 5000 or 50 000, or 3.4-16 µg/m3 or
0.34-1.6 µg/m3, respectively). It should also be noted, however,
that indirect exposure in the general environment is likely
overestimated here, as it is based on the range of mean concentrations
for detected values, although 1,1,2,2-tetrachloroethane was detected
in only approximately 50% of samples.
11.2 Evaluation of environmental effects
1,1,2,2-Tetrachloroethane is released to the environment
principally in emissions to ambient air, where it is moderately
persistent. Because of its volatility, rapid photo-oxidation in the
atmosphere, and an atmospheric ozone-depleting potential of less than
0.001 relative to CFC-11, 1,1,2,2-tetrachloroethane is not expected to
contribute significantly either to the depletion of the stratospheric
ozone layer or to global warming.
Terrestrial organisms have the greatest potential for exposure to
1,1,2,2-tetrachloroethane in ambient air in the environment. However,
no data were identified on the effects of 1,1,2,2-tetrachloroethane in
terrestrial species. Therefore, it is not possible to characterize
the risk to these organisms associated with levels of
1,1,2,2-tetrachloroethane present in the environment.
Although 1,1,2,2-tetrachloroethane may be released to surface
waters in industrial effluents, it is rapidly removed by
volatilization. Based on the results of several studies in aquatic
bacteria, invertebrates, and fish, effect levels are generally greater
than 1 mg/litre. Although data are limited, concentrations of
1,1,2,2-tetrachloroethane in surface waters are generally much less
than this value (at least two orders of magnitude). Therefore, it is
likely that 1,1,2,2-tetrachloroethane does not pose significant risk
to aquatic organisms.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The International Agency for Research on Cancer (IARC, 1987) has
classified 1,1,2,2-tetrachloroethane in group 3 (not classifiable as
to its carcinogenicity to humans), based on inadequate evidence of
carcinogenicity in humans and limited evidence in animals.
Information on international hazard classification and labelling
is included in the International Chemical Safety Card reproduced in
this document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventative and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 0332) reproduced in this
document.
13.1 Advice to physicians
In case of emergency, it is important to wash skin with soap and
water after removing contaminated clothing. Like others of this
class, 1,1,2,2-tetrachloroethane could generate some hyperexcitability
of the heart. The prognosis following intoxication with this chemical
is that rapid progression of jaundice indicates a poor outcome. In
some instances, mild symptoms will persist up to 3 months and then
progress to acute yellow atrophy and death. Anuria may persist for as
long as 2 weeks and still be followed by complete recovery.
13.2 Health surveillance advice
Annual blood counts and monitoring of both liver and kidney
function should be included in a health surveillance programme of
individuals exposed to 1,1,2,2-tetrachloroethane.
13.3 Prevention
Because 1,1,2,2-tetrachloroethane decomposes on burning,
maintenance workers must wait until all liquid and vapour have been
cleared from the container or piping before performing any duty that
generates heat.
Fire-fighters need to wear chemical-resistant clothing and
positive self-contained breathing apparatus.
13.4 Spillage
It is very important in the case of spillage to use full
protection, including respiratory protection, because
1,1,2,2-tetrachloroethane passes through the skin and, under the
influence of air, moisture, and ultraviolet light, will decompose,
producing toxic and corrosive gases, such as hydrogen chloride and
phosgene.
The IDLH (Immediately Dangerous to Life or Health) value for this
substance is very low, at 100 ppm (698 mg/m3) (NIOSH, 1994).
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards is
available from the International Register of Potentially Toxic
Chemicals (IRPTC) legal file.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
1,1,2,2-TETRACHLOROETHANE ICSC:0332
1,1,2,2-TETRACHLOROETHANE
Acetylene tetrachloride
Symmetrical-tetrachlorethane
S-tetrachlorethane
CHCl2CHCl2
Molecular mass: 167.9
CAS # 79-34-5
RTECS # KI8575000
ICSC # 0332
UN # 1702
EC # 602-015-00-3
TYPES OF ACUTE HAZARDS/ PREVENTION FIRST AID/
HAZARD/ SYMPTOMS FIRE FIGHTING
EXPOSURE
FIRE Not flammable. Gives off NO open flames. In case of fire in the
irritating or toxic fumes (or surroundings: all
gases) in a fire. extinguishing agents allowed.
EXPLOSION In case of fire: keep drums,
etc., cool by spraying with
water.
EXPOSURE STRICT HYGIENE!
* INHALATION Abdominal pain. Cough. Ventilation, local exhaust, Fresh air, rest. Artificial
Dizziness. Headache. Nausea. or breathing protection. respiration if indiciated.
Sore throat. Vomiting. Refer for medical attention.
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
TYPES OF ACUTE HAZARDS/ PREVENTION FIRST AID/
HAZARD/ SYMPTOMS FIRE FIGHTING
EXPOSURE
* SKIN MAY BE ABSORBED! Dry skin. Protective gloves. Protective Remove contaminated clothes.
Tremors (further see clothing. Rinse skin with plenty of
Inhalation). water or shower. Refer for
medical attention.
* EYES Redness. Pain. Face shield or eye protection First rinse with plenty of
in combination with breathing water for several minutes
protection. (remove contact lenses if
easily possible), then take
to a doctor.
* INGESTION Abdominal pain. Nausea. Do not eat, drink, or smoke Do NOT induce vomiting. Rest.
Vomiting (further see during work. Refer for medical attention.
Inhalation).
SPILLAGE DISPOSAL STORAGE PACKAGING & LABELLING
Ventilation. Collect leaking and Separated from strong bases, food and Airtight. Do not transport with food
spilled liquid in sealable containers feedstuffs. Cool. Keep in the dark. and feedstuffs.
as far as possible. Absorb remaining Well closed. Keep in a well-ventilated
liquid in sand or inert absorbent and room. T+ symbol
remove to safe place. Do NOT let this
chemical enter the environment (extra R: 26/27-51/53
personal protection: complete S: (1/2-)38-45-61
protective clothing including
self-contained breathing apparatus). UN Hazard Class: 6.1
UN Packing Group: II
Marine pollutant.
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
IMPORTANT DATA PHYSICAL STATE; APPEARANCE: EFFECTS OF SHORT-TERM EXPOSURE:
COLOURLESS LIQUID, WITH CHARACTERISTIC ODOUR. A harmful contamination of the air can be
reached rather quickly on evaporation of this
substance at 20°C.
PHYSICAL DANGERS: EFFECTS OF SHORT-TERM EXPOSURE:
The vapour is heavier than air. The substance irritates the eyes and the
respiratory tract. The substance may cause
CHEMICAL DANGERS: effects on the central nervous system, kidneys
The substance decomposes on burning under and liver, resulting in depression of the
influence of air, moisture and UV light, central nervous system, kidney impairment and
producing toxic and corrosive gases including liver impairment. Exposure may result in
hydrogen chloride and phosgene. Reacts unconsciousness. Exposure may result in death.
violently with alkali metals, strong bases and
many powdered metals producing toxic and
explosive gases. Attacks plastic and rubber.
OCCUPATIONAL EXPOSURE LIMITS (OELs): EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
TLV: 1 ppm; 6.9 mg/m3 (as TWA) (skin) The liquid defats the skin. The substance may
(ACGIH 1994-1995). have effects on the central nervous system and
MAK: 1 ppm; 7 mg/m3; skin, 8 (1992). liver, resulting in impaired functions.
ROUTES OF EXPOSURE:
The substance can be absorbed into the body by
inhalation of its vapour, through the skin and
by ingestion.
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
PHYSICAL Boiling point: 146°C Relative vapour density (air = 1): 5.8
PROPERTIES Melting -44°C Relative density of the vapour/
Relative density (water = 1): 1.6 air-mixture at 20°C (air = 1): 1.031
Solubility in water, g/100 ml Octanol/water partition coefficient
at 20°C: 0.29 as log Pow: 2.39
Vapour pressure, kPa at 20°C: 0.647
Vapour pressure, Pa at 25°C: 780
ENVIRONMENTAL The substance is toxic to aquatic organisms.
DATA This substance may be hazardous to the environment; special attention should be given to its impact on the
ozone layer.
NOTES
Use of alcoholic beverages enhances the harmful effect.
The odour warning when the exposure limit value is exceeded is insufficient.
Do NOT use in the vicinity of a fire or a hot surface, or during welding.
ICSC: 0332 1.1 Transport Emergency Card: TEC (R)-719
1,1,2,2-TETRACHLOROETHANE
REFERENCES
Arthur CL, Pratt K, Motlagh S, Pawliszyn J (1992) Environmental
analysis of organic compounds in water using solid phase micro
extraction. Journal of high resolution chromatography,
15(11):741-744.
Assmuth TW, Strandberg T (1993) Groundwater contamination at Finnish
landfills. Water, air, and soil pollution, 69:179-199.
ATRG (1988) Aquatic toxicity of multiple organic compounds, Part
II: Chlorinated ethanes and chlorinated ethylenes, summary report
(interim). Prepared by the Aquatic Toxicity Research Group for the
Ontario Ministry of the Environment, Lakehead University, Thunder Bay,
Ontario, 95 pp.
ATSDR (1994) Toxicological profile for 1,1,2,2-tetrachloroethane
(draft). Atlanta, GA, US Department of Health and Human Services,
Agency for Toxic Substances and Disease Registry.
Blum DJW, Speece RE (1991) A database of chemical toxicity to
environmental bacteria and its use in interspecies comparisons and
correlations. Research journal of the Water Pollution Control
Federation, 63(3):198-207.
Brem H, Stein AB, Rosenkranz HS (1974) The mutagenicity and
DNA-modifying effect of haloalkanes. Cancer research, 34:2576-2579.
Bull RJ, Sanchez IM, Nelson MA, Larson JL, Lansing AJ (1990) Liver
tumor induction in B6C3F1 mice by dichloroacetate and
trichloroacetate. Toxicology, 63:341-359.
Callen DF, Wolf CR, Philpot RM (1980) Cytochrome P-450 mediated
genetic activity and cytotoxicity of seven halogenated aliphatic
hydrocarbons in Saccharomyces cerevisiae. Mutation research,
77:55-63.
Chai M, Pawliszyn J (1995) Analysis of environmental air samples by
solid-phase microextraction and gas chromatography/ion trap mass
spectrometry. Environmental science and technology, 29(3):693-701.
COARGLWQ (1986) St. Clair River pollution investigation (Sarnia
area). Canada-Ontario Agreement Respecting Great Lakes Water
Quality.
Colacci A, Grilli S, Lattanzi G, Prodi G, Turina MP, Forti GC,
Mazzullo M (1987) The covalent binding of 1,1,2,2-tetrachloroethane to
macromolecules of rat and mouse organs. Teratogenesis,
carcinogenesis, and mutagenesis, 7:465-474.
Colacci A, Perocco P, Vaccari M, Mazzullo M, Albini A, Parodi S,
Taningher M, Grilli S (1990) In vitro transformation of BALB/c 3T3
cells by 1,1,2,2-tetrachloroethane. Japanese journal of cancer
research, 81:786-792.
Colacci A, Perocco P, Bartoli S, Da Via C, Silingardi P, Vaccari M,
Grilli S (1992) Initiating activity of 1,1,2,2-tetrachloroethane in
two-stage BALB/c 3T3 cell transformation. Cancer letters,
64:145-153.
Colacci A, Albini A, Melchiori A, Nanni P, Nicoletti G, Noonan D,
Parodi S, Grilli S (1993) Induction of malignant phenotype in BALB/c
3T3 cells by 1,1,2,2-tetrachloroethane. International journal of
oncology, 2:937-945.
Crebelli R, Benigni R, Franekic J, Conti G, Conti L, Carere A (1988)
Induction of chromosome malsegregation by halogenated organic solvents
in Aspergillus nidulans: unspecific or specific mechanism?
Mutation research, 201:401-411.
Daft JL (1988) Rapid determination of fumigant and industrial chemical
residues in food. Journal of the Association of Official Analytical
Chemists, 71(4):748-760.
DeAngelo AB, Daniel FB, Stober JA, Olson GR (1991) The carcinogenicity
of dichloroacetic acid in the male B6C3F1 mouse. Fundamental and
applied toxicology, 16:337-347.
DeMarini DM, Brooks HG (1992) Induction of prophage lambda by
chlorinated organics: detection of some single-species/single-site
carcinogens. Environmental and molecular mutagenesis, 19:98-111.
Ecobichon DJ, Allen MC (1990) New Brunswick - Water Quality
Surveillance Program, New Brunswick public water supplies,
data summary report 1990. New Brunswick Department of Health and
Community Services.
Environment Agency Japan (1976) Chemicals in the environment. Tokyo,
p. 55.
Enviro-Test Laboratories (1991) Cayley background study: analysis
of food products for target organic and inorganic parameters.
Edmonton, Alberta. Prepared for Health and Welfare Canada, Ottawa,
Ontario (Series: 91-E1208).
Enviro-Test Laboratories (1992) Windsor area background study:
analysis of food products for target organic and inorganic
parameters. Edmonton, Alberta. Prepared for Health and Welfare
Canada, Ottawa, Ontario (Series: 92-E1052).
Eriksson C, Brittebo EB (1991) Epithelial binding of
1,1,2,2-tetrachloroethane in the respiratory and upper alimentary
tract. Archives of toxicology, 65:10-14.
Fellin P, Barnett SE, Tran QA (1992) Results of a national pilot
survey of airborne volatile organic compounds in Canadian
residences. Prepared by Concord Environmental Corporation for Health
and Welfare Canada, Ottawa, Ontario.
Galloway SM, Armstrong MJ, Reuben C, Colman S, Brown B, Cannon C,
Bloom AD, Nakamura F, Ahmed M, Duk S, Rimpo J, Margolin BH, Resnick
MA, Anderson B, Zeiger E (1987) Chromosome aberrations and sister
chromatid exchange in Chinese hamster ovary cells: evaluations of 108
chemicals. Environmental and molecular mutagenesis, 10 (Suppl.
10):1-175.
Gohlke R, Schmidt P (1972) [Subacute action of low concentrations of
chlorinated ethanes with and without additional ethanol treatment in
the rat.] Internationales Archiv für Arbeitsmedizin, 30:299-312 (in
German).
Gohlke R, Schmidt P, Bahmann H (1977) [1,1,2,2-Tetrachloroethane and
heat stress in animal experiment. Morphological results.]
Zeitschrift für die Gesamte Hygiene unde Ihre Grenzgebiete,
20:278-282 (in German).
Government of Canada (1993) Canadian Environmental Protection Act
Priority Substances List assessment report -
1,1,2,2-Tetrachloroethane. Ottawa, Ontario, Environment
Canada/Health and Welfare Canada.
Haag WR, Mill T (1988) Effect of a subsurface sediment on hydrolysis
of haloalkanes and epoxides. Environmental science and technology,
22:658-663.
Hallen RT, Pyne JW, Moulton PM (1986) Transformation of chlorinated
ethanes and ethenes by anaerobic microorganisms. In: Extended
abstracts, 192nd National Meeting of the American Chemical
Society, Anaheim, CA. Washington, DC, American Chemical Society.
Halpert J (1982) Cytochrome P-450-dependent covalent binding of
1,1,2,2-tetrachloroethane in vitro. Drug metabolism and
disposition, 10(5):465-468.
Hanley TR Jr, Quast JF, Schumann AM (1988) The metabolism and
hepatic macromolecular interactions of 1,1,2,2-tetrachloroethane
(TE) in mice and rats. Unpublished study performed by Mammalian and
Environmental Toxicology Research Laboratory, Health and Environmental
Sciences, The Dow Chemical Company, Midland, MI.
Hawkins WE (1991) Development of carcinogenesis bioassay models:
response of small fish species to various classes of carcinogens.
Ocean Springs, MS, Gulf Coast Research Laboratory, 119 pp. (NTIS
AD-A277-157).
Haworth S, Lawlor T, Mortelmans K, Speck W, Zeiger E (1983)
Salmonella mutagenicity test results for 250 chemicals.
Environmental mutagenesis, Suppl. 1:3-142.
Herren-Freund SL, Pereira MA, Khoury MD, Olson G (1987) The
carcinogenicity of trichloroethylene and its metabolites,
trichloroacetic acid and dichloroacetic acid, in mouse liver.
Toxicology and applied pharmacology, 90:183-189.
Horiuchi K, Horiguchi S, Hashimoto K, Kadowaki K, Aratake K (1962)
Studies on the industrial tetrachloroethane poisoning. Osaka
City medical journal, 8:29-38.
Horvath M, Frantik E (1973) Relative sensitivity of nervous function
and behavior to nonspecific effects of foreign substances.
Activitas Nervosa Superior, 15:25-27 [cited in ATSDR, 1994].
IARC (1987) Overall evaluations of carcinogenicity: an updating
of IARC monographs Volumes 1 to 42. Lyon, International Agency for
Research on Cancer (IARC Monographs on the Evaluation of Carcinogenic
Risks to Humans, Suppl. 7).
IPCS (1993) International Chemical Safety Card -
1,1,2,2-Tetrachloroethane. Geneva, World Health Organization,
International Programme on Chemical Safety (No. 0332).
IPCS (1994) Assessing human health risks of chemicals: derivation
of guidance values for health-based exposure limits. Geneva, World
Health Organization, International Programme on Chemical Safety
(Environmental Health Criteria 170).
Jeney E, Bartha F, Kondor L, Szendrei S (1957) Adatok az iizemi
tetrakloretan-mergezesek megelozesehez. [Prevention of industrial
tetrachloroethane intoxication - Part III.] Egeszsegtudomany,
1:142-164.
Kaiser KLE, Comba ME (1983) Volatile contaminants in the Welland River
watershed. Journal of Great Lakes research, 9(2):274-280.
Kanada M, Miyagawa M, Sato M, Hasegawa H, Honma T (1994) Neurochemical
profile of effects of 28 neurotoxic chemicals on the central nervous
system in rats. (1) Effects of oral administration on brain contents
of biogenic amines and metabolites. Industrial health, 32:145-164.
Kennedy GL Jr, Graepel GJ (1991) Acute toxicity in the rat following
either oral or inhalation exposure. Toxicology letters, 56:317-326.
Kulinskaya IL, Verlinskaya RV (1972) [Comparative effect of low
concentration of di-, tetra- and pentachloroethane on the blood
acetylcholine system.] Gigiena Truda i Professional'nye
Zabolevaniya, 16:56-58 (in Russian).
Larson JL, Bull RJ (1992) Metabolism and lipoperoxidative activity of
trichloroacetate and dichloroacetate in rats and mice. Toxicology
and applied pharmacology, 115:268-277.
LeBlanc GA (1980) Acute toxicity of priority pollutants to water flea
(Daphnia magna). Bulletin of environmental contamination and
toxicology, 24:684-691.
Little AD (1983) Cell transformation assays of 11 chlorinated
hydrocarbon analogs (final report). US Environmental Protection
Agency, Office of Toxic Substances (ICAIR Work Assignment No. 10;
Document No. 40+8324457) [cited in ATSDR, 1994].
Lobo-Mendonca R (1963) Tetrachloroethane - a survey. British
journal of industrial medicine, 20:50-56 [cited in ATSDR, 1994].
Maltoni C, Lefemine G, Cotti G (1986) Experimental research on
trichloroethylene carcinogenesis. Archives of research on
industrial carcinogenesis, Vol. V. Princeton, NJ, Princeton
Scientific Publishing, 393 pp.
Maltoni C, Lefemine G, Cotti G, Perino G (1988) Long-term
carcinogenicity bioassays on trichloroethylene administered by
inhalation to Sprague-Dawley rats and Swiss and B6C3F1 mice.
Annals of the New York Academy of Sciences, 534:316-342.
McGregor DB (1980) Tier II mutagenic screening of 13 NIOSH
priority compounds, individual compound report,
1,1,2,2-tetrachloroethane. Report prepared by Inveresk Research
International Limited, Musselburgh, for the National Institute for
Occupational Safety and Health, Cincinnati, OH (Report No. 26).
Mersch-Sundermann V (1989a) [The mutagenicity of organic
microcontamination in the environment. II. The mutagenicity of
volatile organic halogens in the Salmonella microsome test (Ames
test) with regard to the contamination of groundwater and
drinking-water.] Zentralblatt für Bakteriologie und Mikrobiologie,
Hygiene B, 187:230-243 (in German).
Mersch-Sundermann V (1989b) [Examination of mutagenicity of organic
microcontamination of the environment. IV. Communication: The
mutagenicity of halogenated aliphatic hydrocarbons with the
SOS-chromotest.] Zentralblatt für Bakteriologie und
Mikrobiologie, Hygiene B, 189:266-271 (in German).
Milman HA, Story DL, Riccio ES, Sivak A, Tu AS, Williams GM, Tong C,
Tyson CA (1988) Rat liver foci and in vitro assays to detect
initiating and promoting effects of chlorinated ethanes and ethylenes.
Annals of the New York Academy of Sciences, 534:521-530.
Mirsalis JC, Tyson CK, Steinmetz KL, Loh EK, Hamilton CM, Bakke JP,
Spalding JW (1989) Measurement of unscheduled DNA synthesis and
S-phase synthesis in rodent hepatocytes following in vivo treatment;
testing of 24 compounds. Environmental and molecular mutagenesis,
14:155-164.
Mitoma C, Steeger T, Jackson SE, Wheeler KP, Rogers JH, Milman HA
(1985) Metabolic disposition study of chlorinated hydrocarbons in rats
and mice. Drug chemistry and toxicology, 8(3):183-194.
Navrotskiy VK, Kashin LM, Kulinskoya IL (1971) [Comparative assessment
of the toxicity of a number of industrial poisons when inhaled in low
concentrations for prolonged periods.] Trudy Szed Gigiena
Ukrainskoi, 8:224-226 (in Russian) [cited in ATSDR, 1994].
NCI (1977) Bioassay of tetrachloroethylene for possible
carcinogenicity. Bethesda, MD, US Department of Health, Education
and Welfare, Public Health Services, National Institutes of Health,
National Cancer Institute (DHEW/PUB/NIH-77-813).
NCI (1978) Bioassay of 1,1,2,2-tetrachloroethane for possible
carcinogenicity. Bethesda, MD, US Department of Health, Education
and Welfare, Public Health Services, National Institutes of Health,
National Cancer Institute (NTIS PB277 4537GA; DHEW/PUB/NIH-78-827).
Nestmann ER, Lee EG-H (1983) Mutagenicity of constituents of pulp and
paper mill effluent in growing cells of Saccharomyces cerevisiae.
Mutation research, 119:273-280.
Nestmann ER, Lee EG-H, Matula TI, Douglas GR, Mueller JC (1980)
Mutagenicity of constituents identified in pulp and paper mill
effluents using the Salmonella mammalian-microsome assay.
Mutation research, 79:203-212.
Nichols JW, McKim JM, Lien GJ, Hoffman AD, Bertelsen SL, Gallinat CA
(1993) Physiologically-based toxicokinetic modelling of three
waterborne chloroethanes in channel catfish, Ictalurus punctatus.
Aquatic toxicology, 27:83-112.
Nimitz JS, Skaggs SR (1992) Estimating tropospheric lifetimes and
ozone-depletion potentials of one- and two-carbon hydrofluorocarbons
and hydrochlorofluorocarbons. Environmental science and technology,
26(4):739-744.
NIOSH (1994) NIOSH pocket guide to chemical hazards. US Department
of Health and Human Services, Public Health Service, Center for
Disease Control and Prevention, National Institute for Occupational
Safety and Health, June.
Norman JE, Robinette CD, Fraumeni JF (1981) The mortality experience
of army World War II chemical processing companies. Journal of
occupational medicine, 23(12):818-822.
NTP (1986) Toxicology and carcinogenesis studies of
tetrachloroethylene (perchloroethylene) (CAS No. 127-18-4) in
F344/N rats and B6C3F1 mice (inhalation studies). Research Triangle
Park, NC, US Department of Health and Human Services, National
Institutes of Health, National Toxicology Program (NTP-TR-311).
NTP (1990) Carcinogenesis studies of trichloroethylene (without
epichlorohydrin) (CAS No. 79-01-6) in F344/N rats and B6C3F1
mice (gavage studies). Research Triangle Park, NC, US Department of
Health and Human Services, National Institutes of Health, National
Toxicology Program (NTP TR 243).
NTP (1991a) Range finding studies: developmental toxicity -
1,1,2,2-tetrachloroethane when administered via feed in CD
Sprague-Dawley rats. Research Triangle Park, NC, US Department of
Health and Human Services, National Institutes of Health, National
Toxicology Program (NTP-91-RF/DT-017).
NTP (1991b) Range finding studies: developmental toxicity -
1,1,2,2-tetrachloroethane (repeat) when administered via feed
in Swiss CD-1 mice. Research Triangle Park, NC, US Department of
Health and Human Services, National Institutes of Health, National
Toxicology Program (NTP-91-RF/DT-020).
NTP (1993) 1,1,2,2-Tetrachloroethane. C: C3554. Sperm motility
vaginal cytology evaluation in rodents. Research Triangle Park, NC,
US Department of Health and Human Services, National Institutes of
Health, National Toxicology Program (SMVCE-93-192).
NTP (1996) NTP technical report on renal toxicity studies of
selected halogenated ethanes administered by gavage to F344/N
rats. Research Triangle Park, NC, US Department of Health and Human
Services, National Institutes of Health, National Toxicology Program
(Toxicity Report Series Number 45).
Paolini M, Sapigni E, Mesirca R, Pedulli GF, Corongiu FP, Dessi MA,
Cantelli-Forti G (1992) On the hepatotoxicity of
1,1,2,2-tetrachloroethane. Toxicology, 73:101-115.
Pawlisz AV, Peters RH (1995) Effects of sublethal exposure on lethal
body burdens of narcotic organic chemicals in Daphnia magna.
Environmental science and technology, 29(3):613-621.
Price NH, Allen SD, Daniels AU, Yates WG (1978) Toxicity data for
establishing "immediately dangerous to life or health" (IDLH)
values (NTIS PB87-163531) [cited in ATSDR, 1994].
Richter JE, Peterson SF, Kleiner CF (1983) Acute and chronic toxicity
of some chlorinated benzenes, chlorinated ethanes, and
tetrachloroethylene to Daphnia magna. Archives of environmental
contamination and toxicology, 12:679-684.
Roldan-Arjona T, Garcia-Pedrajas MD, Luque-Romero FL, Hera C, Pueyo C
(1991) An association between mutagenicity of the Ara test of
Salmonella typhimurium and carcinogenicity in rodents for 16
halogenated aliphatic hydrocarbons. Mutagenesis, 6(3):199-205.
Sack TM, Steele DH, Hammerstrom K, Remmers J (1992) A survey of
household products for volatile organic compounds. Atmospheric
environment, 26A(6):1063-1070.
Schmidt P, Binnevies S, Gohlke R, Rothe R (1972) [Subacute action of
low concentration of chlorinated ethanes on rats with and without
additional ethanol treatment. I. Biochemical and toxicometrical
aspects, especially results in subacute and chronic toxicity studies
with 1,1,2,2-tetrachloroethane.] Internationales Archiv für
Arbeitsmedizin, 30:283-298 (in German).
Schmidt P, Ulanova IP, Avilova GG, Binnevis SM (1975) [Comparison of
the processes of adaptation of the organism to monotonic and
intermittent action of 1,1,2,2-tetrachloroethane.] Gigiena Truda
i Professional'nye Zabolevaniya, 2:30-34 (in Russian).
Shah JJ, Heyerdahl EK (1988) National ambient volatile organic
compounds (VOCs) database update. Report prepared by Nero and
Associates, Inc., Portland, OR, for Office of Research and
Development, US Environmental Protection Agency, Research Triangle
Park, NC (EPA/600/3-88-010a; NTIS No. PB88-195631).
Shmuter LM (1977) [The effect of chronic exposure to low concentration
of ethane series chlorinated hydrocarbons on specific and nonspecific
immunological reactivity in animal experiments.] Gigiena Truda i
Professional'nye Zabolevaniya, 8:38-43 (in Russian).
Smith AD, Bharath A, Mallard C, Orr D, Smith K, Sutton JA, Vukmanich
J, McCarty LS, Ozburn GW (1991) The acute and chronic toxicity of ten
chlorinated organic compounds to the American flagfish (Jordanella
floridae). Archives of environmental contamination and toxicology,
20:94-102.
Smyth HF Jr, Carpenter CP, Weil CS, Pozzani UC, Striegel JA, Nycum JS
(1969) Range-finding toxicity data - List VII. American Industrial
Hygiene Association journal, 30:470-476.
Stoner GD (1991) Lung tumors in strain A mice as a bioassay for
carcinogenicity of environmental chemicals. Experimental lung
research, 17:405-423.
Storm DL (1994) Chemical monitoring of California's public drinking
water sources: public exposures and health impacts. In: Wang RGM, ed.
Water contamination and health. Integration of exposure assessment,
toxicology, and risk assessment. New York, NY, Marcel Dekker, Inc.,
pp. 67-124.
Story DL, Meierhenry EF, Tyson CA, Milman HA (1986) Differences in rat
liver enzyme-altered foci produced by chlorinated aliphatics and
phenobarbital. Toxicology and industrial health, 2(4):351-362.
Theiss JC, Stoner GD, Shimkin MB, Weisburger EK (1977) Test for
carcinogenicity of organic contaminants of United States drinking
waters by pulmonary tumor response in strain A mice. Cancer
research, 37(8):2717-2720.
Truffert L, Girard-Wallon C, Emmerich E, Neauport C, Ripault J (1977)
[Early experimental demonstration of the hepatotoxicity of some
chlorinated solvents by the study of the synthesis of hepatic DNA.]
Archives des maladies professionnelles de medecine du travail et
de securité sociale (Paris), 38:261-263 (in French).
Tse G, Orbey H, Sandler SI (1992) Infinite dilution activity
coefficients and Henry's law coefficients of some priority water
pollutants determined by a relative gas chromatographic method.
Environmental science and technology, 26(1):2017-2022.
Tu AS, Murray TA, Hatch KM, Sivak A, Milman HA (1985) In vitro
transformation of BALB/c-3T3 cells by chlorinated ethanes and
ethylenes. Cancer letters, 28:85-92.
Ulanova IP, Khalepo AI, Avilova GG (1984) Intermittent exposure to
toxic compounds in the working-zone atmosphere viewed from the aspect
of hygienic standardization. Journal of hygiene, epidemiology,
microbiology, and immunology, 29(3):243-251.
Veith GD, Call DJ, Brooke LT (1983) Structure-toxicity relationships
for the fathead minnow, Pimephales promelas: Narcotic industrial
chemicals. Canadian journal of fisheries and aquatic sciences,
40:743-748.
Vogel EW, Nivard MJM (1993) Performance of 181 chemicals in a
Drosophila assay predominantly monitoring interchromosomal mitotic
recombination. Mutagenesis, 8(1):57-81.
Walbridge CT, Fiandt JT, Phipps GL, Holcombe GW (1983) Acute toxicity
of ten chlorinated aliphatic hydrocarbons to the fathead minnow
(Pimephales promelas). Archives of environmental contamination and
toxicology, 12:661-666.
Warner JR, Hughes TJ, Claxton LD (1988) Mutagenicity of 16 volatile
organic chemicals in a vaporization technique with Salmonella
typhimurium TA100. Environmental and molecular mutagenesis, 11
(Suppl. 11):111.
Williams G (1983) DNA repair tests of 11 chlorinated hydrocarbon
analogs final report EPA contract. US Environmental Protection
Agency, Office of Toxic Substances (Document No. 40+8324292) [cited in
ATSDR, 1994].
Wittsiepe J (1990) Occurrence of vinyl chloride and other halogenated
C1- and C2-hydrocarbons in German surface water. Organohalogen
compounds, 4:425-434.
Woodruff RC, Mason JM, Valencia R, Zimmering S (1985) Chemical
mutagenesis testing in Drosophila. 5. Results of 53 coded compounds
tested for the National Toxicology Program. Environmental
mutagenesis, 7:677-702.
Yllner S (1971) Metabolism of 1,1,2,2-tetrachloroethane-14C in the
mouse. Acta Pharmacologica et Toxicologica, 29:499-512.
APPENDIX 1 - SOURCE DOCUMENTS
Government of Canada (1993)
Copies of the Canadian Environmental Protection Act (CEPA)
Priority Substances List Assessment Report for
1,1,2,2-tetrachloroethane (Government of Canada, 1993) may be obtained
from the:
Commercial Chemicals Branch
Environment Canada
14th Floor, Place Vincent Massey
351 St. Joseph Blvd.
Hull, Quebec
Canada K1A 0H3
Environmental Health Centre
Health Canada
Address Locator: 0801A
Tunney's Pasture
Ottawa, Ontario
Canada K1A 0L2
Copies of the unpublished Supporting Documentation related to
human health effects that formed the basis for preparation of the
above-mentioned report may be obtained from the Environmental Health
Centre at the address noted above.
Initial drafts of the Supporting Documentation and Assessment
Report for 1,1,2,2-tetrachloroethane were prepared by staff of Health
Canada and Environment Canada. The environmental sections were
reviewed externally by Dr P. Cammer (Cammer and Associates), Dr D.
Muir (Department of Fisheries and Oceans), Dr D. Singleton (National
Research Council of Canada), and Dr K. Woodburn (Dow Chemical Canada
Inc.). Sections related to the assessment of human exposure and
health effects were peer reviewed by Dr J. Domoradzki (Dow Chemical
Company, USA, Supporting Documentation only), Dr R. Bull (Washington
State University, USA), and the Information Department of BIBRA
Toxicology International, UK, and subsequently approved by the
Standards and Guidelines Rulings Committee of the Bureau of Chemical
Hazards of Health Canada. The final Assessment Report was reviewed
and approved by the Environment Canada/Health Canada CEPA Management
Committee.
Agency for Toxic Substances and Disease Registry (ATSDR, 1994)
Copies of the draft ATSDR profile for 1,1,2,2-tetrachloroethane
(ATSDR, 1994) may be obtained from:
Agency for Toxic Substances and Disease Registry
Division of Toxicology/Toxicology Information Branch
1600 Clifton Road, NE, E-29
Atlanta, Georgia 30333
USA
The profile has undergone the following ATSDR internal reviews:
Green Border Review, Health Effects Review, Minimal Risk Level Review,
and Quality Assurance Review. In addition, a peer review panel, which
included Dr Martin Alexander (Cornell University, USA), Mr Lyman Skory
(private consultant, USA), and Dr James Withey (Health Canada), was
assembled.
APPENDIX 2 - CICAD PEER REVIEW
The draft CICAD on 1,1,2,2-tetrachloroethane was sent for review
to institutions and organizations identified by IPCS after contact
with IPCS national Contact Points and Participating Institutions, as
well as to identified experts. Comments were received from:
Department of Health, London, United Kingdom
Department of Public Health, Albert Szent-Gyorgyi University
Medical School, Szeged, Hungary
Direccion General de Salud Ambiental, Subsecretario de Regulacion
y Fomento Sanitario, San Luis Potosi, Mexico
Finnish Institute for Occupational Health, Helsinki, Finland
International Agency for Research on Cancer, Lyon, France
Ministry of Health and Welfare, International Affairs Division,
Government of Japan, Tokyo, Japan
National Institute for Working Life, Solna, Sweden
United States Department of Health and Human Services (Agency for
Toxic Substances and Disease Registry; National Institute of
Environmental Health Sciences)
United States Environmental Protection Agency (Office of
Pollution Prevention and Toxics; National Center for
Environmental Assessment, Office of Research and Development;
Office of Drinking Water)
APPENDIX 3 - CICAD FINAL REVIEW BOARD
Brussels, Belgium, 18-20 November 1996
Members
Dr A. Aitio, Institute of Occupational Health, Helsinki, Finland
Dr K. Bentley, Director, Environment Policy Section, Commonwealth
Department of Human Services and Health, Canberra, Australia
Mr R. Cary, Toxicology and Existing Substances Regulation Unit, Health
and Safety Executive, Merseyside, United Kingdom
Dr J. de Fouw, National Institute of Public Health and Environmental
Protection, Bilthoven, The Netherlands
Dr C. DeRosa, Director, Division of Toxicology, Agency for Toxic
Substances and Disease Registry, Atlanta, GA, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr W. Farland, Director, National Center for Environmental Assessment,
Office of Research and Development, US Environmental Protection
Agency, Washington, DC, USA (Chairperson)
Dr T.I. Fortoul, Depto. Biologia Celular y Tisular, National
University of Mexico and Environmental Health Directorate of the
Health Ministry, Mexico D.F., Mexico
Dr H. Gibb, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany
Mr J.R. Hickman, Environmental Health Directorate, Health Canada,
Ottawa, Ontario, Canada
Dr T. Lakhanisky, Head, Division of Toxicology, Institute of Hygiene
and Epidemiology, Brussels, Belgium (Vice-Chairperson)
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Sciences, Hanover,
Germany
Ms E. Meek, Head, Priority Substances Section, Environmental Health
Directorate, Health Canada, Ottawa, Ontario, Canada
Dr K. Paksy, National Institute of Occupational Health, Budapest,
Hungary
Mr D. Renshaw, Department of Health, London, United Kingdom
Dr J. Sekizawa, Division of Chemo-Bio Informatics, National Institute
of Hygienic Sciences, Tokyo, Japan
Dr H. Sterzl-Eckert, GSF-Forschungszentrum für Umwelt und Gesundheit
GmbH, Institut für Toxikologie, Oberschleissheim, Germany
Professor S. Tarkowski, Department of Environmental Health Hazards,
The Nofer Institute of Occupational Medicine, Lodz, Poland
Dr M. Wallen, National Chemicals Inspectorate (KEMI), Solna, Sweden
Observers
Professor F.M.C. Carpanini,1 Director, Centre for Ecotoxicology and
Toxicology of Chemicals (ECETOC), Brussels, Belgium
Mr R. Haigh,1 Head of Unit, Health and Safety Directorate, European
Commission, Luxembourg
Mr B.U. Hildebrandt, Federal Ministry for the Environment, Nature
Conservation and Nuclear Safety, Bonn, Germany
Mr P. Hurst,1 Chemical and Consumer Policy Officer, Conservation
Policy Division, World Wide Fund for Nature, Gland, Switzerland
Dr A. Lombard (Representative of CEFIC), ELF-ATOCHEM, Paris, France
Dr P. McCutcheon,1 Environment, Consumer Protection and Nuclear
Safety, European Commission, Brussels, Belgium
Dr R. Montaigne, Counsellor, Technical Affairs Department, European
Chemical Industry Council (CEFIC), Brussels, Belgium
Dr M. Pemberton, ICI Acrylics, Lancashire, United Kingdom
Dr A. Smith, Organisation for Economic Co-operation and Development,
Environment Division, Paris, France
1 Invited but unable to attend.
Secretariat
Dr M. Baril, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Dr L. Harrison, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr M. Mercier, Director, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr P. Toft, Associate Director, International Programme on Chemical
Safety, World Health Organization, Geneva, Switzerland
RÉSUMÉ D'ORIENTATION
La Direction de l'Hygiène du Milieu de Santé Canada a rédigé ce
CICAD (document international succinct sur l'évaluation des risques
chimiques) sur le 1,1,2,2-tétrachloréthane en s'inspirant d'un
document du Gouvernement du Canada (1993) qui évaluait les
conséquences potentielles pour la santé humaine d'une exposition
indirecte au 1,1,2,2-tétrachloréthane et les effets de cette substance
sur l'environnement, ainsi que d'une étude entreprise par l'Agence
pour les substances toxiques et l'enregistrement des maladies (ATSDR,
1994) en vue de caractériser les informations disponibles sur ses
effets sanitaires indésirables et l'exposition du public. L'examen du
Gouvernement canadien a pris en considération les données disponibles
en septembre 1992. Une recherche bibliographique détaillée a été
effectuée en août 1995 dans plusieurs bases de données en ligne pour
identifier les références publiées postérieurement. L'appendice 1
donne des informations sur la nature du processus d'évaluation par les
pairs et sur la disponibilité des sources documentaires. Les
informations concernant l'examen par les pairs du présent CICAD
figurent à l'appendice 2. La publication de ce CICAD a été approuvée
à une réunion du Comité d'évaluation finale qui s'est tenue à
Bruxelles (Belgique) du 18 au 20 novembre 1996. La liste des
participants à cette réunion figure à l'appendice 3. La fiche
internationale de sécurité chimique (ICSC 0332) concernant le
1,1,2,2-tétrachloréthane, établie par le Programme international sur
la sécurité chimique (IPCS, 1993) est également reproduite dans le
présent document.
Le 1,1,2,2-tétrachloréthane (CAS n° 79-34-5) est un produit
synthétique volatil utilisé principalement comme intermédiaire dans la
synthèse d'autres hydrocarbures chlorés, bien que cette utilisation
ait diminué considérablement. Sa présence dans l'environnement
résulte principalement d'émissions dans l'atmosphère, où il persiste
probablement plusieurs semaines. Il ne semble pas que le
1,1,2,2-tétrachloréthane contribue à la destruction de l'ozone
atmosphérique ni au réchauffement mondial. Il est rapidement éliminé
des systèmes aquatiques et son potentiel de bioaccumulation est
faible. L'inhalation est la principale voie d'exposition pour l'homme.
On dispose de très peu de données concernant les effets sur
l'homme de l'exposition au 1,1,2,2-tétrachloréthane. Son profil
toxicologique n'a pas non plus été caractérisé de façon précise.
Étant donné qu'il est de moins en moins utilisé, la majorité des
données disponibles proviennent d'études limitées relativement
anciennes. La toxicité aiguë du 1,1,2,2-tétrachloréthane chez les
animaux de laboratoire est considérée comme légère à modérée. D'après
les résultats d'études de toxicité à court terme et subchronique, pour
la plupart limitées, le foie semble être l'organe cible le plus
sensible. La plupart des études disponibles ne permettent pas
d'établir de NO(A)EL [dose sans effet (indésirable) observé] ni de
LO(A)EL [dose minimale suivie d'un effet (indésirable) observé] fiable
pour ce qui est de l'hépatotoxicité. Cependant, des effets minimes
sur le foie (augmentation réversible de la teneur en lipides), ainsi
que d'autres effets (augmentation de la concentration
d'adrénocorticotropine et altérations réversibles des paramètres
hématologiques), ont été observés chez des rats exposés à 13,3 mg/m3
pendant 9 mois. D'après les résultats d'études limitées et pour la
plupart anciennes, des effets n'ont été observés sur la reproduction
et le développement des animaux de laboratoire qu'à des doses qui
provoquaient une diminution de poids.
L'ingestion sur une longue période de 1,1,2,2-tétrachloréthane a
provoqué une augmentation de l'incidence des tumeurs du foie chez des
souris B6C3F1 mâles et femelles. Toutefois, une exposition analogue
n'a pas été suivie d'une augmentation significative du nombre des
tumeurs, quel que soit leur site, chez des rats Osborne-Mendel, mais
l'exposition a été limitée à 78 semaines pour les deux espèces.
D'après les résultats disponibles in vivo et in vitro, le
1,1,2,2-tétrachloréthane aurait tout au plus une faible activité
génotoxique. Il ne déclenche pas la formation de foyers positifs à la
gamma-glutamyltranspeptidase dans le foie des rats, mais il la
favorise fortement. Le profil d'induction des tumeurs par le
1,1,2,2-tétrachloréthane est semblable à celui de l'acide
dichloracétique, son principal métabolite. Le mécanisme d'induction
des tumeurs par le 1,1,2,2-tétrachloréthane est encore mal connu; pour
plusieurs de ses métabolites, on a émis l'hypothèse qu'il existerait
un seuil.
Il a été démontré que l'exposition au 1,1,2,2-tétrachloréthane
inhibe l'activité de certaines bactéries présentes dans
l'environnement (la CI50 la plus faible était de 1,4 mg/litre) et
qu'il provoque l'immobilisation de Daphnia magna (CE50 à 48 heures
> 23 mg/litre). Chez les poissons d'eau douce, la CL50 la plus
faible qui ait été signalée (96 heures) était de 18,5 mg/litre
(Jordanella floridae), tandis que la concentration minimale suivie
d'effet (LOEC) à plus long terme (réduction de la survie des larves
chez la même espèce) était de 7,2 mg/litre. Aucun renseignement n'a
été découvert concernant les effets de cette substance sur les
organismes terrestres.
Des valeurs guides à l'intention des autorités compétentes ont
été établies sur la base de la capacité du 1,1,2,2-tétrachloréthane à
induire la formation de tumeurs du foie chez la souris, étant donné
qu'il s'agit du critère toxicologique pour lequel la relation dose-
réponse est la mieux établie. Il faut cependant noter que
l'augmentation observée de l'incidence des tumeurs se limite
actuellement à une espèce et qu'il existe des données qui, bien
qu'incomplètes, laissent supposer que les tumeurs pourraient être
induites par un mécanisme non génotoxique. Les doses associées à une
augmentation de 5 % de l'incidence des tumeurs se situent entre 5,8 et
28 mg/kg de poids corporel par jour. Dans le cas de l'air (principale
source d'exposition pour l'homme), si l'on divise ces doses par 5000
ou 50 000, on obtient des valeurs guides de 3,4-16 µg/m3 et
0,34-1,6 µg/m3 respectivement. Ces valeurs correspondent à ce que
certains organismes considèrent comme un risque «pratiquement
négligeable» (c'est-à-dire 10-5 à 10-6) pour un cancérogène
génotoxique; il faut cependant noter qu'une marge plus faible serait
peut-être appropriée compte tenu des arguments en faveur d'un
mécanisme épigénétique d'induction des tumeurs. Les valeurs
correspondantes pour l'ingestion sont respectivement de 1,2-5,6 et
0,12-0,56 µg/kg de poids corporel par jour. D'après une des
estimations qui ont été faites, l'exposition indirecte dans un
environnement normal est inférieure à ces valeurs, qui apportent déjà
une marge de sécurité considérable, compte tenu des arguments selon
lesquels le mécanisme d'induction des tumeurs par le tétrachloréthane
pourrait impliquer un seuil.
RESUMEN DE ORIENTACION
Esta reseña de la evaluación química internacional del
1,1,2,2-tetracloroetano ha sido preparada por la Dirección de Higiene
del Medio de Health Canadá, principalmente sobre la base de un informe
preparado por el Gobierno del Canadá (1993) para evaluar los efectos
potenciales en la salud humana de la exposición directa al
1,1,2,2-tetracloroetano en el medio ambiente general y los efectos
ambientales de esta sustancia, así como un estudio preparado por la
Agencia para el Registro de Sustancias Tóxicas y Enfermedades (ATSDR,
1994) con objeto de caracterizar información sobre los efectos
adversos en la salud y la exposición del público. En el estudio del
Gobierno del Canadá (1993) se examinaron datos identificados hasta
septiembre de 1992. En agosto de 1995 se hizo una búsqueda exhaustiva
de la bibliografía existente en varias bases de datos en línea con
objeto de identificar toda referencia publicada con posterioridad a
los trabajos incorporados en este estudio. En el apéndice 1 se
presenta información sobre la naturaleza de la revisión científica y
la disponibilidad de las fuentes documentales. En el apéndice 2 se
presenta información sobre la revisión científica de esta reseña. La
presente reseña fue aprobada para publicación en una reunión de la
Junta de Revisión Final, celebrada en Bruselas (Bélgica), del 18 al 20
de noviembre de 1996. Los participantes en la reunión de la Junta de
Revisión Final figuran en el apéndice 3. En el presente documento
también se reproduce la ficha internacional de seguridad química
(ICSC 0332) del 1,1,2,2-tetracloroetano, producida por el Programa
Internacional de Seguridad de las Sustancias Químicas (IPCS, 1993).
El 1,1,2,2-tetracloroetano (N° CAS 79-34-5) es una sustancia
química sintética volátil que se utiliza principalmente como precursor
en la síntesis de otros hidrocarburos clorados, aunque la utilización
de esta sustancia ha disminuido significativamente. La liberación en
el medio ambiente ocurre principalmente en forma de emisiones en el
aire ambiente, donde la sustancia química probablemente permanezca
durante varias semanas. No se prevé que el 1,1,2,2-tetracloroetano
contribuya al agotamiento del ozono estratosférico ni al calentamiento
de la atmósfera. Se elimina rápidamente de los sistemas acuáticos y
probablemente no sea objeto de bioacumulación. La exposición humana
al 1,1,2,2-tetracloroetano se hace principalmente por inhalación.
Se dispone de muy pocos datos sobre los efectos de la exposición
al 1,1,2,2-tetracloroetano en el ser humano. El perfil toxicológico
del 1,1,2,2-tetracloroetano tampoco se ha caracterizado bien; como la
utilización de la sustancia química se halla en disminución, los datos
disponibles se limitan principalmente a estudios iniciales limitados.
La toxicidad aguda del 1,1,2,2-tetracloroetano en animales de
laboratorio es de leve a moderada. Sobre la base de los resultados de
estudios principalmente limitados a corto plazo y subcrónicos, parece
que el hígado es el órgano diana más sensible. Si bien la mayor parte
de los estudios disponibles son insuficientes para determinar con
confianza un nivel sin efectos (adversos) observados [NO(A)EL] o el
nivel más bajo con efectos (adversos) observados [LO(A)EL] de
hepatotoxicidad, se han observado efectos mínimos en el hígado
(aumento reversible del contenido de lípidos) y otros parámetros
(aumento de los niveles de hormona adrenocorticotrópica y alteraciones
reversibles en los parámetros hematológicos) en ratas expuestas a 13,3
mg/m3 durante no más de nueve meses. Sobre la base de estudios
limitados, principalmente de determinación de la dosis e
investigaciones iniciales, se han observado efectos en la reproducción
y en el desarrollo en animales experimentales solamente con dosis que
ocasionaban reducción del peso corporal.
La ingestión prolongada de 1,1,2,2-tetracloroetano hizo aumentar
la incidencia de tumores del hígado en ratones B6C3F1, tanto machos
como hembras. Sin embargo, una exposición semejante no estuvo
asociada a un aumento significativo de tumores en ningún lugar en
ratas Osborne-Mendel, aunque ambas especies sólo estuvieron expuestas
durante un máximo de 78 semanas. Sobre la base de los resultados de
valoraciones disponibles in vivo e in vitro el
1,1,2,2-tetracloroetano tiene, como máximo, un potencial genotóxico
débil. El 1,1,2,2-tetracloroetano fue un potente promotor, pero no un
iniciador, de focos positivos a la gamma-glutamiltranspeptidasa en el
hígado de ratas. El perfil de inducción de tumores por el
1,1,2,2-tetracloroetano es semejante al del ácido dicloroacético, su
metabolito principal. La información existente sobre el mecanismo de
inducción de tumores por el 1,1,2,2-tetracloroetano es incompleta; con
respecto a varios de sus metabolitos, se ha sugerido que los tumores
probablemente estén inducidos por mecanismos para los cuales existe un
umbral.
Se ha demostrado que la exposición al 1,1,2,2-tetracloroetano
inhibe la actividad de las bacterias ambientales (la CI50 más baja
comunicada ha sido de 1,4 mg/litro) y ocasiona inmovilización en
Daphnia magna (valores de CE50 a las 48 horas de 23 mg/litro y
superiores). En las especies ictícolas de agua dulce, la CL50 más
baja comunicada (a las 96 horas) ha sido de 18,5 mg/litro en
Jordanella floridae, mientras que la concentración más baja con
efectos observados (LOEC) tras la exposición a más largo plazo ha sido
de 7,2 mg/litro; ésta dio lugar a una reducción de la supervivencia de
las larvas en las mismas especies mencionadas más arriba. No se
identificaron datos sobre los efectos de esta sustancia en organismos
terrestres.
A fin de facilitar orientación a las autoridades pertinentes, se
han determinado valores de orientación de muestra sobre la base del
potencial del 1,1,2,2-tetracloroetano para inducir tumores hepáticos
en ratones, porque éste es el parámetro toxicológico con respecto al
cual se caracteriza mejor la relación de respuesta a la dosis. Sin
embargo, es de señalar que los aumentos observados en la incidencia de
tumores se limitan actualmente a una especie y hay datos, si bien
incompletos, que sugieren que los tumores tal vez estén inducidos por
un mecanismo no genotóxico. El potencial, expresado como la dosis
asociada a un aumento del 5% en la incidencia de tumores, oscilaba
entre 5,8 y 28 mg/kg de peso corporal por día. Los valores de
orientación de muestra correspondientes al aire (la principal fuente
de exposición humana), que se han calculado dividiendo esos márgenes
de variación del potencial por 5000 ó 50 000, son de 3,4-16 µg/m3 y
0,34-1,6 µg/m3. Estos valores corresponden a los considerados por
algunas autoridades como indicativos de riesgo «esencialmente
insignificante» (es decir 10-5 a 10-6) para un carcinógeno
genotóxico; sin embargo, es de señalar que tal vez sea apropiado
considerar un margen más estrecho en vista de las indicaciones, si
bien incompletas, que sugieren un mecanismo epigenético de inducción
de tumores. Los valores correspondientes para la ingestión son de
1,2-5,6 µg/kg de peso corporal por día y 0,12-0,56 µg/kg de peso
corporal por día. La exposición indirecta en el medio ambiente
general, calculada sobre la base de una estimación de muestra de la
exposición, es inferior a esos valores, que se consideran moderados en
vista de los indicios, si bien incompletos, que sugieren que el
1,1,2,2-tetracloroetano tal vez induzca tumores por un mecanismo de
umbral.
 1. EXECUTIVE SUMMARY
This CICAD on 1,1,2,2-tetrachloroethane was prepared by the
Environmental Health Directorate of Health Canada and was based
principally on a review prepared by the Government of Canada (1993) to
assess the potential effects on human health of indirect exposure to
1,1,2,2-tetrachloroethane in the general environment and the
chemical's environmental effects, as well as a review prepared by the
Agency for Toxic Substances and Disease Registry (ATSDR, 1994)
intended to characterize information on adverse health effects and
public exposure. Data identified as of September 1992 were considered
in the Government of Canada (1993) review. A comprehensive literature
search of several on-line databases was conducted in August 1995 to
identify any references published subsequent to those incorporated in
this review. Information on the nature of the peer review and the
availability of the source documents is presented in Appendix 1.
Information on the peer review of this CICAD is presented in Appendix
2. This CICAD was approved for publication at a meeting of the Final
Review Board, held in Brussels, Belgium, on 18-20 November 1996.
Participants at the Final Review Board meeting are listed in Appendix
3. The International Chemical Safety Card (ICSC 0332) for
1,1,2,2-tetrachloroethane, produced by the International Programme on
Chemical Safety (IPCS, 1993), has also been reproduced in this
document.
1,1,2,2-Tetrachloroethane (CAS no.
1. EXECUTIVE SUMMARY
This CICAD on 1,1,2,2-tetrachloroethane was prepared by the
Environmental Health Directorate of Health Canada and was based
principally on a review prepared by the Government of Canada (1993) to
assess the potential effects on human health of indirect exposure to
1,1,2,2-tetrachloroethane in the general environment and the
chemical's environmental effects, as well as a review prepared by the
Agency for Toxic Substances and Disease Registry (ATSDR, 1994)
intended to characterize information on adverse health effects and
public exposure. Data identified as of September 1992 were considered
in the Government of Canada (1993) review. A comprehensive literature
search of several on-line databases was conducted in August 1995 to
identify any references published subsequent to those incorporated in
this review. Information on the nature of the peer review and the
availability of the source documents is presented in Appendix 1.
Information on the peer review of this CICAD is presented in Appendix
2. This CICAD was approved for publication at a meeting of the Final
Review Board, held in Brussels, Belgium, on 18-20 November 1996.
Participants at the Final Review Board meeting are listed in Appendix
3. The International Chemical Safety Card (ICSC 0332) for
1,1,2,2-tetrachloroethane, produced by the International Programme on
Chemical Safety (IPCS, 1993), has also been reproduced in this
document.
1,1,2,2-Tetrachloroethane (CAS no. 