
MEVINPHOS
T. C. Marrs
Department of Health, Elephant and Castle, London, United Kingdom
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution, and excretion
Biotransformation
Toxicological studies
Acute toxicity
Short-term toxicity
Long-term toxicity and carcinogenicity
Reproductive toxicity
Developmental toxicity
Genotoxicity
Special studies
Dermal and ocular irritation and dermal sensitization
Neurotoxicity
Observations in humans
Comments
Toxicological evaluation
References
Explanation
Mevinphos was evaluated for toxicological effects by the JMPR in
1963 and 1965 (Annex 1, references 2 and 4); in neither case was an
ADI assigned. An ADI of 0-0.0015 mg/kg bw was established in 1972
(Annex 1, reference 18). The toxicology of the compound was reviewed
at the present Meeting within the CCPR periodic review programme. This
monograph summarizes data not previously reviewed and relevant data
from the previous monograph on mevinphos (Annex 1, reference 19).
Evaluation for acceptable daily intake
1. Biochemical aspects
Mevinphos consists of two cis/trans isomers of methyl
3-(dimethoxyphosphinyloxy)-2-butenoate. Commercial preparations vary
in the relative amounts of each, but most contain about 65-75% alpha
and 10-20% ß isomer (Figure 1).
Figure 1. Isomers of mevinphos
O
"
H3C C-OCH3
\ /
O C=C
" / \
CH3O-P-O H
'
OCH3
alpha-; E-; cis-mevinphos
H3C H
\ /
O C=C
" / \
CH3O-P-O C-OCH3
' "
OCH3 O
ß-; Z-; trans-mevinphos
(a) Absorption, distribution, and excretion
The penetration of 14C-mevinphos (purity, 96%; 73% alpha and
15% ß isomer) diluted with water across the nonoccluded skin of
Crl:CD(SD)BR rats was examined in vivo. The doses were 5, 30,
or 150 µg per animal (0.4, 2.5, and 12.5 µg/cm2 of skin). The
radiochemical purity of the labelled compound was 87%; this was
repurified to > 96%. Absorption, measured as the amount in the skin
and that penetrating the skin, was 47-48% of the applied dose; of
this, 13-17% crossed the skin (Jeffcoat & Coleman, 1993).
After a preliminary study in which it was found that substantial
amounts of label were expired, the absorption, distribution, and
excretion of mevinphos (75% alpha and 15% ß isomer) was studied in
groups of five male and five female Sprague-Dawley (Crl:CD BR) rats.
Either a single oral dose of 14C-mevinphos at 0.15 or 1.5 mg/kg bw;
multiple doses of 0.15 mg/kg bw, only the last (16th) dose being
radiolabelled; or a single intravenous dose of 0.15 mg/kg bw were
given. In all cases, the label was rapidly absorbed, transformed, and
excreted, predominantly by exhalation, with 77% in the breath of
males and 78% in the breath of females after the single oral dose of
0.15 mg/kg bw, and only 14% in the urine over 24 h, mostly within 8 h.
At the higher oral dose, 61-62% of the label appeared in the breath
and 23% in the urine. After the intravenous dose, about 71% of the
label appeared in the breath of animals of each sex and 16 and 17% in
the urine of males and females, respectively. After administration of
16 daily oral doses, 75% of the administered dose was found in the
expired air of males and 77% in that of females, urinary excretion
being 16 and 19%. In all these studies, almost all of the radiolabel
(85-96%) was eliminated within 24 h as carbon dioxide and in the
urine; < 1.3% was excreted in the faeces. Thus, mevinphos was almost
completely absorbed (Reddy et al., 1991).
(b) Biotransformation
In the study of Reddy et al. (1991), mevinphos was metabolized
primarily to carbon dioxide, and only traces of other volatile
materials were present. High-performance and thin-layer liquid
chromatography of the urine showed four major peaks, representing
unmetabolized alpha isomer of mevinphos and the alpha isomers of
mevinphos acid and desmethyl mevinphos. A fourth peak may have
represented mevinphos diacid.
The proposed metabolic pathway for mevinphos in Sprague-Dawley
rats is shown in Figure 2.
2. Toxicological studies
(a) Acute toxicity
The results of studies of the acute toxicity of mevinphos are
shown in Table 1.
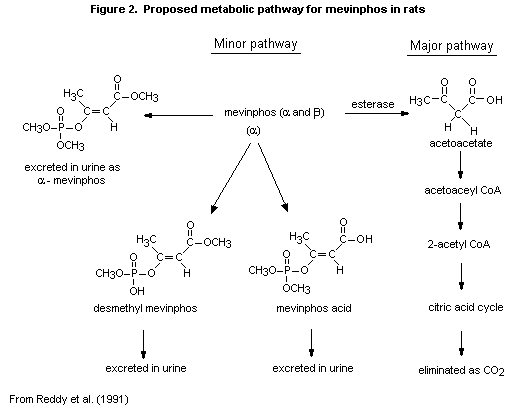 Table 1. Acute toxicity of mevinphos in experimental animals
Species Strain Sex Route Purity LD50 (mg/kg bw) Reference
(%) or LC50 (mg/litre)
(95% CI)
Mouse Swiss Webster Male Dermal 99 12 (6-24) Skinner & Kilgore
(feet) (1982)
Rat Sherman Male Oral NR 6.1 Gaines (1969)
Female 3.7
Rat Sprague-Dawley Male Oral NR 4.1 (3.5-5.0) Deenihan (1985)
Female 2.2 (1.8-2.6)
Rat Sprague-Dawley-derived CD Male Oral 97.7 3.5a Auletta (1988a)
Female 2.3 (1.0-3.6)
Rat Albino HSD:SD Male Oral Unknownb > 5 Kuhn (1994)
Female > 5
Rat Sherman Male Dermal NR 4.7 Gaines (1969)
Female 4.2
Rat Sprague-Dawley Crl:CD BR Male Dermal 90 >20 Trimmer (1989)
Female >20
Rat Sprague-Dawley CD Male Inhalationc 100 12 Hoffman (1988)
Female 7.3
Rabbit New Zealand white Male, Dermal NR 57 (38-86) Deenihan (1985)
female
Rabbit New Zealand white Male Dermal 99.3 51 (31-71) Auletta (1988b)
Female 60 (23-97)
Chicken White Leghorn pullets Female Oral approx. 97 11 (3.2-19) Barrett (1988)
CI, confidence interval; NR, not reported
a CI could not be calculated owing to distribution of deaths
b Phosdrin 4 EC
c 4-h exposure; only 3 h at highest dose as all animals had died
(b) Short-term toxicity
Mice
In a three-month range-finding study, groups of 10 male and 10
female CD-1 mice were fed diets containing mevinphos (66.5% alpha,
21.2% ß isomer) at 0, 0.5, 1, 2, or 10 ppm, equal to 0, 0.1, 0.2, 0.4,
and 2 mg/kg bw per day for males and 0, 0.1, 0.3, 0.5, and 2.7 mg/kg
bw per day for females. Clinical observations, body weight, and food
consumption were reported before treatment and weekly after the start
of exposure, and plasma and erythrocyte cholinesterase activities
were measured in five animals of each sex per group at week 7 and
at termination. After three months, the animals were killed and
autopsied, and brain acetylcholinesterase activity was measured in
five animals of each sex per group. Organs from controls and animals
at the high dose were examined grossly, and selected tissues were
examined microscopically. Body weight and food consumption were
similar in all groups, and no deaths occurred; clinical parameters
were similar in all groups. Plasma cholinesterase activity was
decreased by 37-49% in animals of each sex at 2 and 10 ppm at week 7.
At termination, similar findings were seen in males, but the decreased
plasma cholinesterase activity in females was confined to those at
10 ppm. Brain acetylcholinesterase activity was decreased by 16 and
22% in males and females at 10 ppm, respectively, in comparison with
controls. The NOAEL was 2 ppm, equal to 0.4 mg/kg bw per day, on the
basis of inhibition of brain acetylcholinesterase activity at 10 ppm
(Atkinson, 1990).
Rats
Groups of 10 Sprague-Dawley Crl:CD BR rats received mevinphos
(74.5% alpha, 15.1% ß isomer) by gavage for 90 days at doses of active
ingredient of 0.056, 0.56, 1.1, or 1.7 mg/kg bw per day for males and
0.011, 0.056, 0.56, or 0.84 mg/kg bw per day for females. Five males
at the two highest doses died, and the highest dose was decreased to
1.1 mg/kg bw per day on day 36; the groups at the two highest doses
were then combined for most statistical purposes. Additionally, one
female at 0.56 mg/kg bw per day died before scheduled termination.
Clinical signs, including pinpoint pupils, oral and ocular discharges,
and tremors were seen at doses > 0.56 mg/kg bw per day in animals
of each sex. No statistically significant differences in body weight
or food consumption were noted. Inhibition of plasma cholinesterase
activity > 15% was seen at week 7 and terminally in males at doses
> 0.56 mg/kg bw per days and in females at doses > 0.056 mg/kg
bw per day. Brain acetylcholinesterase activity was inhibited by
> 15% at termination in animals of each sex at doses > 0.56 mg/kg
bw per day. No significant depression of erythrocyte acetyl-
cholinesterase activity was seen. There was a dose-related increase
in mean cholesterol levels, which were statistically significantly
increased in males at the high dose. No statistically significant
differences in absolute or relative organ weights were seen between
groups, but a dose-related trend in increased relative liver weights
was seen in animals of each sex. Slight vacuolation in centrilobular
and midzonal hepatocytes was seen in two male rats at the highest
dose. The NOAEL was 0.056 mg/kg bw per day, on the basis of clinical
signs and depressed brain acetylcholinesterase activity at higher
doses (Keefe, 1992).
Dogs
Gelatine capsules containing mevinphos (75.1% alpha, 11.8% ß
isomer) in corn oil were given to groups of five male and five female
beagle dogs at doses of 0 or 0.5 mg/kg bw per day and to four males
and four females at 0.025 or 0.25 mg/kg bw per day for one year. No
deaths occurred during the study. The only clinical sign attributable
to the test material was a higher prevalence of vomiting in the dogs
at the high dose than in the controls. No significant differences in
body weight or food consumption were noted, and no treatment-related
changes were seen at ophthalmoscopic examination at weeks 12, 26, and
52. Blood was taken before treatment and at weeks 6, 12, 39, and 51
for laboratory analyses. No biologically significant haematological or
biochemical changes were seen, other than changes in cholinesterase
activity. Plasma and erythrocyte cholinesterase activities were
determined on blood samples taken before the start of treatment, 3 h
after dosing at 4, 12, 39, and 51 weeks, and before dosing at weeks
39 and 51. Brain acetylcholinesterase activity was measured at
termination of the study. At many of these time intervals, both
erythrocyte and plasma cholinesterase activities were depressed,
particularly at the two higher doses. In dogs at the lowest dose, a
depression > 15% was seen in erythrocyte enzyme activity at week 25
in males and females, at week 39 in males before treatment and in
females before and after treatment, and at week 51 in males before and
after treatment and in females after treatment. At this dose, plasma
cholinesterase activity was depressed by > 15% at 39 and 51 weeks in
females after treatment. Statistically and biologically significant
inhibition of brain acetylcholinesterase activity was observed only in
males at the highest dose, in which the activity was 77% of that in
concurrent controls. Higher absolute thyroid/parathyroid weights
were observed in females at the highest dose and higher relative
thyroid/parathyroid weights in females at the intermediate dose. No
associated histopathological abnormality was discerned, and these
findings are considered not to be significant. No treatment-related
pathological finding was seen at autopsy. The NOAEL was thus
0.25 mg/kg bw per day, on the basis of clinical signs and a reduction
in brain acetylcholinesterase activity at the highest dose (Kangas,
1995).
(c) Long-term toxicity and carcinogenicity
Mice
On the basis of a range-finding study, mevinphos (purity, 87.7%;
66.5% alpha, 21.2% ß isomer) was administered to four groups of 50
CD-1 mice of each sex at dietary concentrations providing doses of 0,
1, 10, or 25 ppm, equal to 0, 0.1, 1.5, and 3.7 mg/kg bw per day for
males and 0, 0.1, 1.9, and 4.8 mg/kg bw per day for females, for 18
months. Physical examinations were performed each week and body weight
and food consumption before the start of the study, weekly thereafter
for 13 weeks on alternate weeks until week 26, and thence monthly.
Blood smears for evaluating the differential leukocyte count and
erythrocyte morphology were taken from 10 animals of each sex per
group at 12 and 18 months. Treatment had few effects, but the survival
rate was somewhat reduced in males at the low dose, with only 20
survivors, in comparison with 27 male controls, 26 at the middle dose,
and 24 at the high dose. In females, the mortality rate was similar in
all groups (36-40%). During the first three months of the study, the
mean body weights of animals at the highest dose were lower than those
of controls; this effect lasted up to week 16 in males and week 12 in
females. Females at the two highest doses had lower leukocyte counts
than controls at 18 but not at 12 months. No treatment-related gross
or microscopic pathological changes were seen, nor were there
treatment-related changes in organ weights. Although tumours were
observed in a number of organs, especially the liver and lungs, there
was no evidence of treatment-related neoplasia in any treated group.
Cholinesterase activity was not measured in this study, as it had
been evaluated in the range-finding study, in which the NOAEL for
inhibition of brain acetylcholinesterase activity was 2 ppm. The NOAEL
in the main study was 25 ppm, equal to 3.7 mg/kg bw per day, as the
transient effect on body weight in mice at the high dose can be
ignored (Atkinson, 1989).
Rats
Groups of 80 male and 80 female Crl:CD BR rats were given
mevinphos (purity, 85.7%; 74.9% alpha, 10.8% ß isomer) by gavage in
water on five days per week for two years at doses of 0, 0.025, 0.35,
or 0.70 mg/kg bw per day. In females, the high dose was reduced to
0.60 mg/kg bw per day on day 83 of the study because of signs of
acute toxicity. Ten rats from each group were killed at 12 months for
histopathological examination, 10 rats from each group were used for
clinical chemical determinations, and a further 10 were used to
measure cholinesterase activity and were killed at 104 weeks. Clinical
examinations were conducted weekly, and body weights were recorded
before the start of the study, at the start of dosing, and thereafter
weekly for 13 weeks and every four weeks afterwards. Food consumption
was also measured weekly for 13 weeks and every four weeks thereafter.
Clinical chemical, haematological, and urinary analyses were undertaken
and plasma and erythrocyte cholinesterase activities were measured
before treatment, at 3, 6, 1,2 and 18 months, and at termination. Brain
acetylcholinesterase activity was measured at the interim kill and at
the end of the study, but there were too few survivors at the end of
the study for useful intergroup comparisons to be made. Ophthalmoscopic
examinations were done before the start of the study and before
termination. Full histopathological examinations were performed on the
10 animals from each group killed at 12 months and on the survivors at
two years. Selected organs were weighed and examined microscopically.
Less than 40% of the rats survived to termination at 104 weeks,
and there was a dose-related reduction in survival in males, the rate
being clearly reduced at the highest dose; there were no significant
differences in mortality among female rats. Clinical signs typical of
anticholinesterase poisoning, including tremors, were observed after
administration of both the medium and high doses. No treatment-related
changes in body weight, food consumption, or haematological, clinical
chemical, urinary, or ophthalmoscopic parameters were discerned,
except for cholinesterase activity. Plasma, erythrocyte, and brain
cholinesterase activity was diminished in the animals at the middle
and high doses but not in those at the low dose; decreases in
erythrocytic acetylcholinesterase activity > 20% were not observed
in animals of either sex at any dose, but a depression of 20% was
observed at six months in females treated with mevinphos at 0.60 mg/kg
bw per day. Although inhibition of plasma cholinesterase activity was
not seen at the low dose, reductions of 38-51% were seen in males
at the middle dose and 47-61% in males at the high dose; plasma
cholinesterase activity was depressed by 50-67% in females at the
middle dose and 66-71% at the high dose. Brain acetylcholinesterase
activity was depressed by 27% in males at the middle dose, 53% in
males at the high dose, 43% in females at the middle dose, and 55% in
females at the high dose at the interim kill. No treatment-related
effects were seen on histopathological examination. A low incidence of
combined incidental and fatal hepatocellular adenomas was seen (0/67,
1/67, 1/69, and 2/68 in males and 1/70, 0.66, 0/67, and 3/67 in
females), which gave a dose-response relationship. One female at
the high dose had a hepatocellular carcinoma, giving a significant
dose-response relationship for all hepatocellular tumours. The numbers
involved were small, there was no dose-response relationship for liver
carcinomas alone or for adenomas and carcinomas combined in males, and
the incidence was well within that of historical controls. Hence, it
was concluded that there was no evidence of any treatment-associated
tumours. The NOAEL was 0.025 mg/kg bw per day on the basis of
inhibition of brain acetylcholinesterase activity and clinical signs
at doses > 0.35 mg/kg bw per day (Plutnick, 1994).
(d) Reproductive toxicity
In a two-generation study, groups of 35 male and 35 female Crl:CD
BR (Sprague-Dawley) VAF/Plus rats, seven weeks of age, received
mevinphos (purity, 89.6%; 74.5% alpha, 15.1% ß isomer) in water by
gavage at doses of 0, 0.05, 0.1, or 0.5 mg/kg bw. Dosing was adjusted
to allow for the low purity of the material. The F0 rats were dosed
daily for 10 weeks before being assigned randomly, within the dose
groups, into mating pairs at least 11 weeks after the start of dosing.
Dosing was continued during mating, and the F0 females continued to
be treated during gestation and lactation until weaning of the F1
litter 21 days post partum. Offspring were counted, sexed, weighed
(excluding postnatal day 0), and examined externally on postnatal days
0, 1, 4, 7, 14, and 21; litters were culled to four males and four
females. The F1 litters were dosed from 28 days post partum for at
least 11 weeks before mating and also during the mating period. F1
females were dosed during gestation and lactation until weaning on day
21 after birth. Clinical observations were made and body weights
measured before selection of the F0 animals on the first day of
dosing and thereafter weekly in both generations. Observations were
made more frequently in the F0 generation during gestation (days 0,
7, 14, and 21) and on the same days post partum. Erythrocyte,
plasma, and brain cholinesterase activity was measured in all animals
that survived to termination. Necropsies were performed on all
mated animals, all decedent pups, and pups killed in extremis.
Histopathological examinations were performed only on the controls
and animals at the high dose, except that the ovaries of all F1
females were examined.
Treatment-related clinical signs were seen in F0 females at the
high dose, which included ataxia, fine or coarse tremors, pinpoint
pupils, and oral discharge. The growth of F1 animals of each sex was
significantly reduced, and some reduction in the body weights of F1
males and females at the high dose was observed before weaning on
postnatal days 4-21. A reduction in the body weights of F1 male
offspring at the high dose may represent continuation of the reduction
in the F0 males before weaning. The growth of F2 males at the high
dose was also reduced. Cholinesterase inhibition was observed in both
generations but was generally more severe in F1 animals. Significant
inhibition of brain acetylcholinesterase activity was observed in
animals of each sex at the high dose, while no significant inhibition
of erythrocyte cholinesterase activity was seen at any dose; inhibition
of plasma cholinesterase activity extended to animals at the middle
dose and to those at the low dose in the F1 generation. F1 animals
at the high dose showed a reduction in the absolute weight of the
testes or epididymides (12%) and in the relative ovarian weight
(17%). The former may be a reflection of the lower total body weight
of these animals, but the F1 males in all treated groups had lower
mean mating and fertility indices than controls; although these
effects were not statistically significant, they may be associated
with the lower testicular weight observed. The effect on the ovaries
may be a treatment-related effect, as some female rats at the highest
dose had fewer or absent corpora lutea. The frequency of this
pathological observation did not, however, attain statistical
significance, and there were no significant effects on female
fecundity or fertility indices; moreover, the live litter sizes of
the animals at the high dose and the controls were comparable. The
NOAEL was 0.1 mg/kg bw per day on the basis of the presence of clinical
signs and reduced brain acetylcholinesterase activity in animals at
the highest dose, at which impaired growth, impaired fertility
indices, and lower testicular weights were seen in males and lower
ovarian weights in females (Beyer, 1991b).
(e) Developmental toxicity
Rats
Mevinphos (65.5% alpha isomer) was administered to four groups of
24 mated CD rats by gavage in water. One group served as controls,
while the other groups were given mevinphos at daily doses of 0.2,
0.75, or 1.2 mg/kg bw per day on days 6-15 of gestation. A high
mortality rate (29.2%) was observed in rats at the high dose, which
was therefore terminated and a new group receiving 1 mg/kg bw per day
was added. Females were weighed and examined at regular intervals,
while food consumption was recorded on days 0-6, 6-10, 10-15, and
15-20 of gestation. The animals were killed on day 20 of gestation and
examined at autopsy; corpora lutea and uterine implantations were
counted, and fetuses were weighed, sexed, and examined grossly for
malformations. One-half of the fetuses in each litter were processed
for visceral examination and the remainder stained for evaluation of
skeletal changes. Deaths were observed only in the prematurely
terminated group at 1.2 mg/kg bw per day. A dose-related decrease in
mean weight gain was seen during days 6-15 of gestation, but none of
the decreases attained statistical significance. Clinical signs of
cholinergic poisoning (tremors) were seen in about half the animals at
the high dose, and excessive salivation was seen in four animals in
that group. No indication of maternal toxicity was seen in the other
groups, apart from tremor in two rats at the middle dose on day 15.
There were no adverse effects on uterine implantation, fetal weight,
fetal sex distribution, or the external appearance of fetuses, and
there were no visceral or skeletal abnormalities in any treated group.
The NOAEL for maternal toxicity was 0.75 mg/kg bw per day on the basis
of clinical signs at the next highest dose; the NOAEL for developmental
toxicity was 1.0 mg/kg bw per day, the highest dose tested (Schroeder &
Daly, 1987).
Rabbits
Mevinphos (purity, 89.6%; 74.5% alpha, 15.1% ß isomer) was
administered in water by gavage to groups of 20 pregnant New Zealand
white rabbits at doses of 0, 0.05, 0.5, or 1.5 mg/kg bw per day on
days 7-19 of gestation. Surviving animals were killed on day 29. Body
weights were measured on days 0 and 7 of gestation, every three days
until day 25, and on day 29 of gestation. Plasma and erythrocyte
cholinesterase activity was determined in blood taken from dams about
3 h after treatment on the last day. After the animals had been
killed, gross necropsy was carried out, and body weight and the weight
of the uterus plus ovary were determined. The uterine contents were
examined, live fetuses were killed, and all fetuses were weighed and
examined externally. The viscera and skeleton were examined for
malformations, and half of the fetuses from each litter were examined
for the presence of brain abnormalities. Brain acetylcholinesterase
activity was not measured. Maternal toxicity was seen in animals at
the high dose, resulting in one death; corrected body weights were
also decreased in that group on day 29 of gestation. Erythrocyte
acetylcholinesterase activity was statistically significantly reduced
in all three treated groups. The decreases were not biologically
significantly in those at the medium and low doses (13% and 6%), but
the reduction of 18% in animals at the high dose can be considered to
be marginally biologically significant. Plasma cholinesterase activity
was more sensitive to mevinphos, being significantly reduced at the
two higher doses. No significantly different findings among the groups
were made at autopsy. Mean fetal body weights and the crown-rump
lengths of the treated pups were similar to those of controls. An
increased incidence of accessory vessels was seen at 1.5 mg/kg bw
per day, which was not considered by the authors to be related to
treatment, as these are normal variations. Hypoplastic hyoids and
unossified forepaws were also seen at increasing incidence with dose,
but the difference in incidence between animals at the high dose and
the controls did not achieve clinical significance. The NOAEL was
0.5 mg/kg bw per day on the basis of a marginally biologically
significant decrease in erythrocyte acetylcholinesterase activity in
animals at the high dose (Beyer, 1991a).
(f) Genotoxicity
Studies on the genotoxicity of mevinphos in vitro and in vivo are
summarized in Table 2.
Table 4. Recent tests for the genotoxicity of mevinphos
End-point Test system Concentration Purity Results Reference
(%)
In vitro
Reverse mutation S. typhimurium TA98, 100-10 000 µg/plate 89.6 Negative in TA98 San & Schadly
TA100, TA1535, (74.5% alpha, with and without (1989)
TA1537, TA1538 15.1% ß) S9
Sister chromatid Chinese hamster 10-5-10-3 mol/litre NR Positive Hirka et al.
exchange ovary cells (1982)
Unscheduled DNA Rat primary hepatocytes 0.0003-0.3 µl/ml NR Negative Curren (1990)
synthesis
In vivo
Chromosomal CF-1 mouse, bone 1.5, 3 mg/kg bw per 70% alpha Negative Dean & Senner
aberration marrow day orally (1974)
Dominant lethal Mouse 1.5; 3, 6 mg/kg bw NR Negative Dean (1974)
mutation
NR, not reported
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Slight dermal irritation and erythema were observed in five of
six New Zealand white rabbits and oedema in the sixth. Most of the
effects had disappeared by 72 h. In the same study, the material
produced slight temporary ocular irritation in six New Zealand white
rabbits (Deenihan, 1985).
Groups of five male and five female New Zealand white rabbits
received applications of mevinphos (74.5% alpha, 15.1% ß isomer)
on the clipped unabraded skin of the back at doses of 0, 0.1, 1,
or 10 mg/kg bw per day, on five days per week for three weeks.
The material was not irritating. Males at the highest dose had a
significant increase in relative brain weight and a decrease in the
liver:brain and kidney:brain weight ratios. A dose-related decrease in
brain, erythrocyte, and plasma cholinesterase activity was also seen.
Plasma, erythrocyte, and brain cholinesterase activities at termination
were inhibited in males and females at the highest dose by > 15% in
comparison with concurrent controls. Erythrocyte acetylcholinesterase
activity was the least inhibited and brain acetylcholinesterase
activity the most inhibited in animals of each sex. The NOAEL for
transdermal exposure was thus 1 mg/kg bw per day (Trimmer, 1990b).
Mevinphos (purity, 93.6%) did not have dermal sensitization
potential in guinea-pigs (Auletta, 1988c).
(ii) Neurotoxicity
Hens
Ten Leghorn hens (Gallus gallus domesticus) were given 12.5 mg/kg
bw mevinphos (purity, approx. 97%), this dose being slightly greater
than the oral LD50 for hens. Antidotal treatment was required, and
0.625 mg/kg bw atropine was given as clinically indicated; each hen
was also treated with 10 mg/kg bw pralidoxime chloride. Three of the
treated hens died, despite antidotal therapy; the remainder were
treated again on day 21 of the study as they had shown no signs of
neurotoxicity. Four positive controls were treated with tri- ortho-
tolylphosphate (90% mixture of isomers), but, when no signs of
neurotoxicity were seen, material from a different supplier was used
to treat the hens again at 21 days. Hens that survived to 42 days
were injected with sodium pentobarbital and perfused with neutral
buffered formalin. Brain, including the medulla, spinal cord, sciatic
nerve, and the proximal parts of the peroneal and tibial nerves, were
examined histopathologically. The treated birds had slight weight
loss, presumably due to the cholinergic effect of mevinphos, but no
evidence of mevinphos-induced delayed polyneuropathy was seen, either
clinically or histopathologically, whereas characteristic changes were
seen in the positive controls. Neuropathy target esterase was not
measured in this study (Barrett, 1988).
A single dose of mevinphos (76% alpha, 11% ß isomer) was given by
gavage to groups of 27 male and 27 female Sprague-Dawley Crl:CD BR
rats at doses of 0, 0.025 (17 rats of each sex), 0.1, 2, or 3.5
mg/kg bw. Seven animals of each sex per group were allocated for
neuropathological examination and underwent a functional observation
battery and tests for locomotor activity before treatment, at the time
of the peak effect (about 45 min after dosing), and on days 7 and 14.
These animals were killed at 15 days, and their brains were perfused
in situ and weighed; neuropathological examination was then carried
out on five rats of each sex in the control group and those at the
highest dose. The other 20 animals in each group (10 at 0.025 mg/kg
bw) were allocated for determinations of cholinesterase activity;
viability, clinical signs, and body weight were recorded, and the
functional observation battery and tests for locomotor activity were
conducted on five rats of each sex per group. Plasma, erythrocyte, and
brain (including regional) cholinesterase activities were measured
before treatment, at the time of the peak effect (about 45 min after
dosing), and on days 7 and 15 in five animals of each sex per group,
except for those at the lowest dose, in which activity was measured
only at the time of peak effect and on day 15. The numbers of animals
available for determination of cholinesterase activity were also
reduced in the group at the high dose because of mortality (see
below).
One male and five females at the highest dose died, and clinical
signs, including gait alterations, tremors, salivation, and
lacrimation, were observed in those at 2 or 3.5 mg/kg bw only on the
day of dosing. Major disturbances in the locomotor test battery
were observed in animals at these doses but not in controls. The
alterations included lacrimation, salivation, impaired mobility and
gait, clonic and tonic convulsions, tremors, bizarre behaviour, and
altered reflexes. No treatment-related signs were seen in animals
at 0.025 or 0.1 mg/kg bw. On the first day of the study, plasma
cholinesterase activity was reduced by 36-39% in rats at 2 mg/kg bw
and by 41-50% in those at 3.5 mg/kg bw in comparison with concurrent
controls. Erythrocyte acetylcholinesterase activity was unaffected,
while reductions of 20-25% were seen in acetylcholinesterase activity
in the brain stem and/or cerebral cortex, hippocampus, and olfactory
region of animals at 2 mg/kg bw and 19-36% in those at 3.5 mg/kg bw.
The only statistically significant reductions in activity were those
in the brain stem and cortex in males and in the hippocampus and brain
stem in females. Plasma and brain cholinesterase activities were
normal on days 7 and 14. No adverse effects were seen on body or brain
weight. All signs of neurotoxicity had reversed by the end of day 1,
and no treatment-related neuropathological signs were seen at any
dose. The NOAEL was thus 0.1 mg/kg bw, on the basis of clinical
effects and reduced brain acetylcholinesterase activity at higher
doses (Lamb, 1993).
Mevinphos was given by gavage in water to groups of 25 male and
25 female Sprague-Dawley Crl:CD BR rats for 91 days. The initial doses
for all animals were 0.025, 0.035, or 0.70 mg/kg bw per day, but the
dose for females at the high dose was reduced to 0.60 mg/kg bw per day
on day 32 because of the deaths of two animals. Controls received
the vehicle only. Viability, clinical signs, body weights, and
food consumption were recorded for all animals, and a functional
observational battery and locomotor activity tests were conducted on
10 animals of each sex per group. Necropsy was performed on all
animals that died during the study. Neuropathological changes were
evaluated in five animals of each sex in the control group and those
at the high dose, and, because of the mortality at the high dose, in
the females at the intermediate dose. The remaining 20 animals in each
group were evaluated for plasma, erythrocyte, and brain cholinesterase
activity in groups of five animals during weeks 3, 7, and 12 of the
study.
The main clinical sign was tremor in animals at the high dose
45 min after treatment, even after the dose for the females had been
lowered. Excessive salivation was seen in animals of each sex at this
dose, and lacrimation and râles were seen in females. Treatment-
related declines of 32-56% were seen in erythrocyte and plasma
cholinesterase activities in animals of each sex at the intermediate
and high doses; the decreases in erythrocyte acetylcholinesterase
activity were slightly greater (34-62%). Regional brain acetyl-
cholinesterase activity showed some variation, with decreases of 9-21%
in animals at the intermediate and high doses. If decreases of 15% are
biologically significant, the decreased acetylcholinesterase activity
of 21% in the mid-brain of males at the middle dose and of 17% in
females would result in a NOAEL at 0.025 mg/kg bw per day. No
treatment-related neuropathological change was seen (Lamb, 1994).
Administration of a single dose of mevinphos (purity, 89.6%)
to Crl:CD Br rats transdermally did not induce statistically or
biologically significant depressions in erythrocyte acetyl-
cholinesterase activity. A significant depression of plasma
cholinesterase activity occurred at 20 mg/kg bw (29% of control value)
and a marginal fall at 5 mg/kg bw (11% of control). Significant
depressions of brain acetylcholinesterase activity occurred at
20 mg/kg bw (18% of control) (Trimmer, 1989, 1990a).
The effects of mevinphos on flash-evoked potentials were studied
in gerbils, pigeons, cats, and squirrel monkeys, through electrodes
placed in the superior colliculus. Mevinphos reduced the inhibitory
pause in pigeons at doses of 0.1-0.15 mg/kg bw. This effect could not
be prevented by pretreatment with atropine methylnitrite (Revzin,
1978). Electroretinograms of Swiss white mice were examined
after injection of organophosphates, including mevinphos at an
intraperitoneal dose of 0.015 mg. Mevinphos was found to interfere
with retinal function by a direct action on the photoreceptor, by
inhibition of cholinesterase at synapses, and possibly by damaging the
bipolar and/or ganglion cells (Carricaburu et al., 1980).
3. Observations in humans
A number of incidents of poisoning with mevinphos have been
reported through the US pesticide incident monitoring scheme (Hodgson
& Smith, 1993). Poisoning with this compound is reported to be
amenable to treatment with oximes (Bismuth et al., 1993).
After administration of 25 µg/kg bw per day mevinphos to eight
male volunteers for 28 days, plasma and erythrocyte cholinesterase
activity fell throughout the study, with mean decreases of 12.6 and
19% of the respective pretreatment levels. Plasma cholinesterase
activity had returned almost to pretreatment levels within seven days
after the end of treatment, whereas the levels of erythrocyte activity
showed no sign of recovery at follow-up to 14 days. A decrease in
slow fibre motor conduction was also seen; there was an increase in
Achilles tendon reflex force but no effect on neuromuscular
transmission (Verbeck, 1977; Verbeck & Sallé, 1977).
Mevinphos was given to groups of five male volunteers at doses
of 1, 1.5, 2, or 2.5 mg per day for 30 days. Plasma cholinesterase
activity was slightly affected by the highest dose, while the mean
erythrocyte acetylcholinesterase activity was depressed by 20% on one
occasion each at 1.5 and 2 mg per day. Consistent, significant
inhibition of cholinesterase activity was seen only at the highest
dose, at which the erythrocyte acetylcholinesterase fell consistently
to a minimum of 25% at 27 days (Rider et al., 1975).
In agricultural workers with cholinergic symptoms and with mean
inhibition of plasma and erythrocyte cholinesterase activity of 15.6
and 5.6%, respectively, cholinesterase activity recovered at a daily
rate of 1.2 and 0.43% of the respective final levels of activity of
the enzyme over the 14 days after cessation of exposure (Coye et
al., 1986). Higher rates of recovery were observed during the first
11 days after poisoning with a mixture of mevinphos and phosphamidon:
the plasma activity increased by 3% per day and erythrocyte activity
by 0.8% per day. The workers were, however, treated with pralidoxime.
As noted previously, inhibition of plasma cholinesterase activity was
greater than that of erythrocytic enzyme (Midtling et al., 1985).
Human erythrocyte acetylcholinesterase is susceptible to
reactivation in vitro by a variety of pyridinium oximes, including
pralidoximes and obidoxime (Woreck et al., 1996).
Comments
Mevinphos is almost completely absorbed when administered orally
to rats; a large proportion of the absorbed material is biotransformed
to carbon dioxide. Both metabolites and unchanged mevinphos are
observed in the urine but very little in the faeces. Mevinphos
depresses cholinesterase activity in the plasma mre than in the
erythrocytes in experimental animals.
The oral LD50 values for mevinphos in laboratory rodents are
2-12 mg/kg bw. WHO has classified mevinphos as 'extremely hazardous'
(WHO, 1996).
In a three-month range-finding study, mice were fed diets
containing mevinphos at concentrations of 0, 0.5, 1, 2, or 10 ppm.
The NOAEL was 2 ppm, equal to 0.4 mg/kg bw per day, on the basis of
inhibition of brain acetylcholinesterase activity at 10 ppm.
In a 90-day study of toxicity, rats were given mevinphos by
gavage at doses of 0, 0.056, 0.56, 1.1, or 1.7 mg/kg bw per day were
used in males (the highest dose was decreased to 1.1 mg/kg bw per day
at day 36 because of high mortality) and 0, 0.011, 0.056, 0.56, or
0.84 mg/kg bw per day in females. The NOAEL was 0.056 mg/kg bw per
day, on the basis of clinical signs and depressed brain acetyl-
cholinesterase activity at higher doses. Dose-related increases in
mean cholesterol levels and increased relative liver weights were also
observed.
In a one-year study in dogs, mevinphos was administered in corn
oil in gelatine capsules at doses of 0, 0.025, 0.25 or 0.5 mg/kg bw
per day. The NOAEL was 0.25 mg/kg bw per day on the basis of clinical
signs and a reduction in brain acetylcholinesterase activity at the
highest dose.
In an 18-month study of toxicity and carcinogenicity, mice were
fed dietary concentrations of 0, 1, 10, or 25 ppm; cholinesterase
activity was not measured. There was no evidence of carcinogenicity.
In a two-year study of toxicity and carcinogenicity, rats were
given mevinphos by gavage in water on five days per week at doses of
0, 0.025, 0.35, or 0.70 mg/kg bw per day. On day 83 of the study, the
high dose of the females was reduced to 0.60 mg/kg bw per day because
of signs of toxicity. The NOAEL was 0.025 mg/kg bw per day on the
basis of inhibition of brain acetylcholinesterase activity and clinical
signs at higher doses. There was no evidence of carcinogenicity.
A two-generation study of reproductive toxicity was carried out
in which rats were treated by gavage at doses of 0, 0.05, 0.1, or
0.5 mg/kg bw mevinphos in water. The NOAEL was 0.1 mg/kg bw per day on
the basis of clinical signs and reduced brain acetylcholinesterase
activity at the highest dose. This dose also impaired growth and
fertility indices and lowered testicular weights in males and ovarian
weights in females.
In a study of developmental toxicity in rats, groups were given
mevinphos at daily doses of 0, 0.2, 0.75, or 1.2 mg/kg bw per day on
days 6-15 of gestation. High mortality (29%) was observed in the
high-dose group, which was therefore terminated; accordingly, a new
high-dose group at 1 mg/kg bw per day was added. There were no adverse
effects on uterine implantation or on fetal weight, sex distribution,
external appearance, or visceral or skeletal malformations in any
group. It was concluded that mevinphos is not embryotoxic, fetotoxic,
or teratogenic at doses < 1.0 mg/kg bw per day. The NOAEL for
maternal toxicity was 0.75 mg/kg bw per day on the basis of clinical
signs at higher doses.
In a study of developmental toxicity, mevinphos was administered
by gavage to pregnant rabbits at doses of 0, 0.05, 0.5, or 1.5 mg/kg
bw per day on days 7-19 of gestation; surviving animals were killed.
The NOAEL was 0.5 mg/kg bw per day, on the basis of maternal toxicity.
Mevinphos was neither teratogenic nor fetotoxic.
There was some evidence of genotoxic potential in vitro, but
the limited studies available indicate that such potential is not
exhibited in vivo.
In a study in hens, the oral dose of 12 mg/kg bw that was
administered was slightly greater than the oral LD50 value, and
antidotal treatment was required. There was no evidence of delayed
polyneuropathy, either clinically or histopathologically, whereas
characteristic changes were seen in positive controls. Neurotoxic
target esterase was not measured during this study.
Two studies of humans were available. In male volunteers given a
dose of 0.025 mg/kg bw per day, plasma and erythrocyte cholinesterase
activity decreased throughout the 28 days of the study to 13% and 19%
less than the respective pre-dose levels. In the second study, daily
doses of 1, 1.5, 2 or 2.5 mg were given to male volunteers for 30
days, and an NOAEL of 1 mg/day, equivalent to 0.016 mg/kg bw per day,
was derived; however, only five people, all men, per dose were
studied.
An ADI of 0-0.0008 mg/kg bw was established on the basis of the
NOAEL of 0.016 mg/kg bw per day in the 30-day study in volunteers,
using a 20-fold safety factor because of the small numbers in each
group. This ADI is supported by the LOAEL in rats of 0.35 mg/kg bw per
day and the NOAELs of 0.5 mg/kg bw per day in rabbits and 0.25 mg/kg
bw per day in dogs.
An acute reference dose for humans was derived from the 28-day
study in volunteers, on the basis of a dose of 0.025 mg/kg bw per day,
using a 10-fold safety factor.
Toxicological evaluation
Levels that cause no toxicological effect
Mouse: 2 ppm, equal to 0.4 mg/kg bw per day (inhibition of
brain acetylcholinesterase activity in a three-month
study of toxicity)
Rat: 0.025 mg/kg bw per day (two-year study of toxicity and
carcinogenicity)
0.1 mg/kg bw per day (study of reproductive toxicity)
Rabbit: 0.5 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
Dog: 0.25 mg/kg bw per day (one-year study of toxicity)
Human: 1 mg per day, equivalent to 0.016 mg/kg bw per day
(inhibition of cholinesterase activity in a 30-day
study of toxicity)
Estimate of acceptable daily intake for humans
0-0.0008 mg/kg bw
Acute reference dose
0.003 mg/kg bw
Studies that would provide information useful for continued evaluation
of the compound
Study of micronucleus formation in mice in vivo
Toxicological criteria for estimating guidance values for dietary and non-dietary exposure to mevinphos
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Oral, toxicity, rat LD50 = 2.2-6.1 mg/kg bw
Dermal, toxicity, rat LD50 > 20 mg/kg bw
Inhalation, 4 h, toxicity, rat LD50 = 7.3-12 mg/m3
Dermal, irritation, rabbit Slightly irritating
Ocular, irritation, rabbit Slightly irritating
Dermal, sensitization, guinea-pig Not sensitizing
Medium-term (1-26 weeks) Repeated oral, 3 months, mouse NOAEL = 0.4 mg/kg bw per day, inhibition of brain
acetylcholinesterase activity
Repeated oral, 90 days, rat NOAEL = 0.056 mg/kg bw per day
Repeated dermal, 21 days, rabbit NOAEL = 1.0 mg/kg bw per day
Repeated oral, reproductive toxicity, rat NOAEL = 0.1 mg/kg bw per day, maternal and
reproductive toxicity
Repeated oral, developmental toxicity, rat NOAEL = 0.75 mg/kg bw per day, maternal toxicity;
no developmental toxicity
Repeated oral, developmental toxicity, rabbit NOAEL = 0.5 mg/kg bw per day, maternal toxicity;
no developmental toxicity
Long-term (> 1 year) Repeated oral, 2 years, rat NOAEL = 0.025 mg/kg bw per day; inhibition of
brain acetylcholinesterase activity
Repeated oral, 1 year, dog NOAEL = 0.25 mg/kg bw per day; inhibition of
brain acetylcholinesterase activity
References
Atkinson, J.E. (1989) An eighteen month oncogenicity feeding study in
mice with mevinphos. Project No 86-300. Unpublished report dated 23
February 1989 from BioDynamics Inc., East Millstone, NJ, USA.
Submitted to WHO by Amvac Chemical Corp., City of Commerce, CA, USA.
Atkinson, J.E. (1990) A three month dietary range-finding study in
mice with mevinphos technical. Project No. 85-3025. Unpublished report
in West, J. & Roberts, K. (1990) Supplement to an eighteen month
oncogenicity study in mice with mevinphos. Project ID JCODE 1562.1.
Unpublished report dated 31 August 1990 from Jelinek Schwartz Connolly
and Freshman Inc., Washington DC, USA. Submitted to WHO by Amvac
Chemical Corp., City of Commerce, CA, USA.
Auletta, C.S. (1988a) Acute oral toxicity study in rats with
mevinphos. Unpublished report dated 23 March 1988, Project No. 4644-87
from BioDynamics Inc., East Millstone, NJ, USA. Submitted to WHO by
Amvac Chemical Corp., City of Commerce, CA, USA.
Auletta, C.S. (1988b) Acute dermal toxicity study in rabbits with
mevinphos. Unpublished report dated 23 March 1988, Project No. 4645-87
from BioDynamics Inc., East Millstone, NJ, USA. Submitted to WHO by
Amvac Chemical Corp., City of Commerce, CA, USA.
Auletta, C.S. (1988c) Dermal sensitization study in guinea pigs with
mevinphos. Unpublished report dated 23 March 1988, Project No. 4648-87
from BioDynamics Inc., East Millstone, NJ, USA. Submitted to WHO by
Amvac Chemical Corp., City of Commerce, CA, USA.
Barrett, D.S. (1988) Acute delayed neurotoxicity study in mature hens
with mevinphos. Unpublished report dated 26 July 1988, Project No
4685-87 from BioDynamics Inc., East Millstone, NJ, USA. Submitted to
WHO by Amvac Chemical Corp., City of Commerce, CA, USA.
Beyer, B.K. (1991a) Teratology study in rabbits. Study No. MRD-88-331.
Unpublished report dated 22 February 1991 from Exxon Biomedical
Sciences Inc, East Millstone, NJ, USA. Submitted to WHO by Amvac
Chemical Corp., City of Commerce, CA, USA.
Beyer BK (1991b) Multigeneration rat reproduction study. Study No.
MRD-88-331. Unpublished report dated 26th November 1991 from Exxon
Biomedical Sciences Inc., East Millstone, NJ, USA. Submitted to WHO by
Amvac Chemical Corp., City of Commerce, CA, USA.
Bismuth, C., Inns, R.H. & Marrs, T.C. (1993) Efficacy, toxicity and
clinical use of oximes in anticholinesterase poisoning. In:
Ballantyne, B. & Marrs, T.C, eds, Clinical and Experimental Toxicology
of Organophosphates and Carbamates, Oxford, Butterworths, pp. 555-577.
Carricaburu, P., Lacroix, R. & Lacroix, J. (1980) Electroretinographic
study of the white mouse intoxicated by organo-phosphorus: mevinphos
and malathion. Toxicol. Environ. Res., 3, 87-91.
Coye, M.J., Barnett, P.G. & Midtling, J.E. (1986) Clinical confirmation
of organophosphate poisoning of agricultural workers. Am. J. Ind. Med.,
10, 399-409.
Curren, R.D. (1990) Unscheduled DNA synthesis in rat primary
hepatocytes with mevinphos. Unpublished report dated 25 January 1990,
Study No. T8858.380 from Microbiological Associates, Inc., Rockville,
MD, USA. Submitted to WHO by Amvac Chemical Corp., City of Commerce,
CA, USA [full report not available to the Meeting].
Dean, B.J. (1974) Toxicity studies with phosdrin. Dominant lethal
assay in male mice after a single oral dose of phosdrin. Unpublished
report dated July 1974 Group research phosdrin, No. TLGR.0031.74 from
Shell Research Centre, Tunstall, Kent, United Kingdom. Submitted to
WHO by Amvac Chemical Corp., City of Commerce, CA, USA [full report
not available to the Meeting].
Dean, B.J. & Senner, K. (1974) Toxicity studies with phosdrin:
Chromosome studies on bone marrow cells of mice after two daily oral
doses of phosdrin. Group research report No. TLGR.0008.74. Unpublished
report dated February 1974 from Shell Research Centre, Tunstall, Kent,
United Kingdom. Submitted to WHO by Amvac Chemical Corp., City of
Commerce, CA, USA.
Deenihan, M.J. (1985) Mevinphos insecticide acute oral toxicity, acute
dermal toxicity, primary skin irritation, primary eye irritation.
NVP report No. X5A027G. Unpublished report dated 10 April 1985 from
Northview Pacific Laboratories Inc., Berkeley, CA, USA. Submitted to
WHO by Amvac Chemical Corp., City of Commerce, CA, USA.
Gaines, T. (1969) Acute toxicity of pesticides. Toxicol. Appl.
Pharmacol., 14, 515-534.
Hirka, G., Béres, E. & Schmidt, K. (1982) Use of cell and tissue
cultures in genetic toxicology. Mutat. Res., 97, 192.
Hodgson, M.J. & Smith, A.D. (1993) Commercial and residential
poisoning with anticholinesterases. In: Ballantyne, B. & Marrs, T.C.,
eds, Clinical and Experimental Toxicology of Organophosphates and
Carbamates, Oxford, Butterworths, pp. 352-363.
Hoffman, G.M. (1988) An acute inhalation toxicity study of mevinphos
in the rat. Unpublished report and letter dated 12 April 1988 from
BioDynamics Inc., East Millstone, NJ, USA. Submitted to WHO by Amvac
Chemical Corp., City of Commerce, CA, USA.
Jeffcoat A.R. & Coleman, D.P. (1993) Dermal absorption of mevinphos in
rats at three dose levels. RTI Study No. 64C-5494. Unpublished report
dated 31 August 1993 from Research Triangle Institute, Research
Triangle Park, NC, USA. Submitted to WHO by Amvac Chemical Corp., City
of Commerce, CA, USA.
Kangas, L. (1995) A 52-week oral (capsule) toxicity study of mevinphos
in the beagle dog. Laboratory ID 85746. Unpublished report dated
9 June 1995 from Bio-research Laboratories Ltd, Senneville, Quebec,
Canada. Submitted to WHO by Amvac Chemical Corp, City of Commerce, CA,
USA.
Keefe, R.T. (1992) 90-Day subchronic oral toxicity study in rats with
mevinphos (MRD-88-331) Laboratory ID 233170B. Unpublished report dated
4 November 1992 from Exxon Biomedical Sciences Inc., East Millstone,
NJ, USA. Submitted to WHO by Amvac Chemical Corp., City of Commerce,
CA, USA.
Kuhn, J.O. (1994) Acute oral toxicity study in rats (DOT). Laboratory
study number 0946-94. Unpublished report dated 28 March 1994 from
Stillmeadow Inc, Sugar Land, TX, USA. Submitted to WHO by Amvac
Chemical Corp,, City of Commerce, CA, USA.
Lamb, I.C. (1993) An acute neurotoxicity study of mevinphos in rats.
Study No. WIL-188006. Unpublished report dated 13 October 1993 from
WIL Research Laboratories Inc., Ashland, OH, USA. Submitted to WHO by
Amvac Chemical Corp., City of Commerce, CA, USA.
Lamb, I.C. (1994) A subchronic (13 week) neurotoxicity study of
mevinphos in rats. Study No. WIL-188007. Unpublished report dated
17 June 1994 from WIL Research Laboratories Inc., Ashland, OH, USA.
Submitted to WHO by Amvac Chemical Corp., City of Commerce, CA, USA.
Midtling, J.E., Barnett, P.G., Coye, M.J., Velasco, A.R., Romero, P.,
Clements, C.L., O'Malley, M.A., Tobin, M.W., Rose, T.G. & Monosson,
I.H. (1985) Clinical management of field worker organophosphate
poisoning. West. J. Med., 142, 514-518.
Plutnick, R.T. (1994) 2-Year chronic toxicity/oncogenicity study in
rats with mevinphos (MRD-88-331). Laboratory ID 233170C. Unpublished
report dated 3 January 1994 from Exxon Biomedical Sciences Inc., East
Millstone, NJ, USA. Submitted to WHO by Amvac Chemical Corp., City of
Commerce, CA, USA.
Reddy, V., Freeman, T., Little, L. & Cannon, M. (1991) Disposition
and metabolism of 14C-labeled mevinphos in rats (preliminary and
definitive study). Laboratory ID MRI 9485B. Unpublished report dated
4 April 1991 from Midwest Research Institute, Kansas City, MI, USA.
Submitted to WHO by Amvac Chemical Corp., City of Commerce, CA, USA.
Revzin, A.M. (1978) Effects of organophosphate pesticides and alcohol
on visual mechanisms. In: Merigan, W.H. & Weiss, B., eds, Neurotoxicity
of the Visual System, New York, Raven Press, pp. 255-268.
Rider, J.A., Puletti, E.J. & Swader, J.I. (1975) The minimal oral
toxicity level for mevinphos in man. Toxicol. Appl. Pharmacol., 32,
97-100.
San, R.H.C. & Schadly, M.B. (1989) Salmonella/mammalian microsome
plate incorporation mutagenicity assay (Ames test) with a confirmatory
assay with mevinphos. Study No. T8858.501014. Unpublished report dated
23 October 1989 from Microbiological Associates Inc., Rockville, MD,
USA. Submitted to WHO by Amvac Chemical Corp., City of Commerce, CA,
USA.
Schroeder, R.E. & Daly, I.W. (1987) Mevinphos - A teratology study in
rats with mevinphos. Laboratory ID 85-3009. Unpublished report dated
2 March 1987 from BioDynamics Inc., East Millstone, NJ, USA. Submitted
to WHO by Amvac Chemical Corp., City of Commerce, CA, USA.
Skinner, C.S. & Kilgore, W.W. (1982) Acute dermal toxicities of various
organophosphate insecticides in mice. J. Toxicol. Environ. Health,
9, 491-497.
Trimmer, G.W. (1989) Acute dermal toxicity study in the rat phase 1
(MRD-88-331: mevinphos). Laboratory ID 233106. Unpublished report
dated 23 August 1989 from Exxon Biomedical Sciences Inc., East
Millstone, NJ, USA. Submitted to WHO by Amvac Chemical Corp., City of
Commerce, CA, USA.
Trimmer, G.W. (1990a) Acute dermal toxicity study in the rat phase II
cholinesterase determination (MRD-88-331: mevinphos). Laboratory ID
233177A. Unpublished report dated 20 July 1990 from Exxon Biomedical
Sciences Inc., East Millstone, NJ, USA. Submitted to WHO by Amvac
Chemical Corp., City of Commerce, CA, USA.
Trimmer, G.W. (1990b) 21-Day repeated dermal study in the rabbit
(MRD-88-331-mevinphos). Laboratory ID 233109. Unpublished report dated
4 April 1990 from Exxon Biomedical Sciences Inc., East Millstone, NJ,
USA. Submitted to WHO by Amvac Chemical Corp., City of Commerce, CA,
USA.
Verberk, M.M. (1977) Incipient cholinesterase inhibition in volunteers
ingesting monocrotophos or mevinphos for one month. Toxicol. Appl.
Pharmacol.,42, 345-350.
Verberk, M.M. & Sallé, H.J.A. (1977) Effects on nervous function of
volunteers ingesting mevinphos for 1 month. Toxicol. Appl. Pharmacol.,
42, 351-358.
WHO (1996) The WHO Recommended Classification of Pesticides by Hazard
and Guidelines to Classification 1996-1997 (WHO/PCS/96.3), International
Programme on Chemical Safety, Geneva.
Worek, F., Kirchner, T., Bäcker, M. & Szinicz, L. (1996) Reactivation
by various oximes of humans erythrocyte acetylcholinesterase inhibited
by different organophosphorus compounds. Arch. Toxicol., 70,
497-503.
Table 1. Acute toxicity of mevinphos in experimental animals
Species Strain Sex Route Purity LD50 (mg/kg bw) Reference
(%) or LC50 (mg/litre)
(95% CI)
Mouse Swiss Webster Male Dermal 99 12 (6-24) Skinner & Kilgore
(feet) (1982)
Rat Sherman Male Oral NR 6.1 Gaines (1969)
Female 3.7
Rat Sprague-Dawley Male Oral NR 4.1 (3.5-5.0) Deenihan (1985)
Female 2.2 (1.8-2.6)
Rat Sprague-Dawley-derived CD Male Oral 97.7 3.5a Auletta (1988a)
Female 2.3 (1.0-3.6)
Rat Albino HSD:SD Male Oral Unknownb > 5 Kuhn (1994)
Female > 5
Rat Sherman Male Dermal NR 4.7 Gaines (1969)
Female 4.2
Rat Sprague-Dawley Crl:CD BR Male Dermal 90 >20 Trimmer (1989)
Female >20
Rat Sprague-Dawley CD Male Inhalationc 100 12 Hoffman (1988)
Female 7.3
Rabbit New Zealand white Male, Dermal NR 57 (38-86) Deenihan (1985)
female
Rabbit New Zealand white Male Dermal 99.3 51 (31-71) Auletta (1988b)
Female 60 (23-97)
Chicken White Leghorn pullets Female Oral approx. 97 11 (3.2-19) Barrett (1988)
CI, confidence interval; NR, not reported
a CI could not be calculated owing to distribution of deaths
b Phosdrin 4 EC
c 4-h exposure; only 3 h at highest dose as all animals had died
(b) Short-term toxicity
Mice
In a three-month range-finding study, groups of 10 male and 10
female CD-1 mice were fed diets containing mevinphos (66.5% alpha,
21.2% ß isomer) at 0, 0.5, 1, 2, or 10 ppm, equal to 0, 0.1, 0.2, 0.4,
and 2 mg/kg bw per day for males and 0, 0.1, 0.3, 0.5, and 2.7 mg/kg
bw per day for females. Clinical observations, body weight, and food
consumption were reported before treatment and weekly after the start
of exposure, and plasma and erythrocyte cholinesterase activities
were measured in five animals of each sex per group at week 7 and
at termination. After three months, the animals were killed and
autopsied, and brain acetylcholinesterase activity was measured in
five animals of each sex per group. Organs from controls and animals
at the high dose were examined grossly, and selected tissues were
examined microscopically. Body weight and food consumption were
similar in all groups, and no deaths occurred; clinical parameters
were similar in all groups. Plasma cholinesterase activity was
decreased by 37-49% in animals of each sex at 2 and 10 ppm at week 7.
At termination, similar findings were seen in males, but the decreased
plasma cholinesterase activity in females was confined to those at
10 ppm. Brain acetylcholinesterase activity was decreased by 16 and
22% in males and females at 10 ppm, respectively, in comparison with
controls. The NOAEL was 2 ppm, equal to 0.4 mg/kg bw per day, on the
basis of inhibition of brain acetylcholinesterase activity at 10 ppm
(Atkinson, 1990).
Rats
Groups of 10 Sprague-Dawley Crl:CD BR rats received mevinphos
(74.5% alpha, 15.1% ß isomer) by gavage for 90 days at doses of active
ingredient of 0.056, 0.56, 1.1, or 1.7 mg/kg bw per day for males and
0.011, 0.056, 0.56, or 0.84 mg/kg bw per day for females. Five males
at the two highest doses died, and the highest dose was decreased to
1.1 mg/kg bw per day on day 36; the groups at the two highest doses
were then combined for most statistical purposes. Additionally, one
female at 0.56 mg/kg bw per day died before scheduled termination.
Clinical signs, including pinpoint pupils, oral and ocular discharges,
and tremors were seen at doses > 0.56 mg/kg bw per day in animals
of each sex. No statistically significant differences in body weight
or food consumption were noted. Inhibition of plasma cholinesterase
activity > 15% was seen at week 7 and terminally in males at doses
> 0.56 mg/kg bw per days and in females at doses > 0.056 mg/kg
bw per day. Brain acetylcholinesterase activity was inhibited by
> 15% at termination in animals of each sex at doses > 0.56 mg/kg
bw per day. No significant depression of erythrocyte acetyl-
cholinesterase activity was seen. There was a dose-related increase
in mean cholesterol levels, which were statistically significantly
increased in males at the high dose. No statistically significant
differences in absolute or relative organ weights were seen between
groups, but a dose-related trend in increased relative liver weights
was seen in animals of each sex. Slight vacuolation in centrilobular
and midzonal hepatocytes was seen in two male rats at the highest
dose. The NOAEL was 0.056 mg/kg bw per day, on the basis of clinical
signs and depressed brain acetylcholinesterase activity at higher
doses (Keefe, 1992).
Dogs
Gelatine capsules containing mevinphos (75.1% alpha, 11.8% ß
isomer) in corn oil were given to groups of five male and five female
beagle dogs at doses of 0 or 0.5 mg/kg bw per day and to four males
and four females at 0.025 or 0.25 mg/kg bw per day for one year. No
deaths occurred during the study. The only clinical sign attributable
to the test material was a higher prevalence of vomiting in the dogs
at the high dose than in the controls. No significant differences in
body weight or food consumption were noted, and no treatment-related
changes were seen at ophthalmoscopic examination at weeks 12, 26, and
52. Blood was taken before treatment and at weeks 6, 12, 39, and 51
for laboratory analyses. No biologically significant haematological or
biochemical changes were seen, other than changes in cholinesterase
activity. Plasma and erythrocyte cholinesterase activities were
determined on blood samples taken before the start of treatment, 3 h
after dosing at 4, 12, 39, and 51 weeks, and before dosing at weeks
39 and 51. Brain acetylcholinesterase activity was measured at
termination of the study. At many of these time intervals, both
erythrocyte and plasma cholinesterase activities were depressed,
particularly at the two higher doses. In dogs at the lowest dose, a
depression > 15% was seen in erythrocyte enzyme activity at week 25
in males and females, at week 39 in males before treatment and in
females before and after treatment, and at week 51 in males before and
after treatment and in females after treatment. At this dose, plasma
cholinesterase activity was depressed by > 15% at 39 and 51 weeks in
females after treatment. Statistically and biologically significant
inhibition of brain acetylcholinesterase activity was observed only in
males at the highest dose, in which the activity was 77% of that in
concurrent controls. Higher absolute thyroid/parathyroid weights
were observed in females at the highest dose and higher relative
thyroid/parathyroid weights in females at the intermediate dose. No
associated histopathological abnormality was discerned, and these
findings are considered not to be significant. No treatment-related
pathological finding was seen at autopsy. The NOAEL was thus
0.25 mg/kg bw per day, on the basis of clinical signs and a reduction
in brain acetylcholinesterase activity at the highest dose (Kangas,
1995).
(c) Long-term toxicity and carcinogenicity
Mice
On the basis of a range-finding study, mevinphos (purity, 87.7%;
66.5% alpha, 21.2% ß isomer) was administered to four groups of 50
CD-1 mice of each sex at dietary concentrations providing doses of 0,
1, 10, or 25 ppm, equal to 0, 0.1, 1.5, and 3.7 mg/kg bw per day for
males and 0, 0.1, 1.9, and 4.8 mg/kg bw per day for females, for 18
months. Physical examinations were performed each week and body weight
and food consumption before the start of the study, weekly thereafter
for 13 weeks on alternate weeks until week 26, and thence monthly.
Blood smears for evaluating the differential leukocyte count and
erythrocyte morphology were taken from 10 animals of each sex per
group at 12 and 18 months. Treatment had few effects, but the survival
rate was somewhat reduced in males at the low dose, with only 20
survivors, in comparison with 27 male controls, 26 at the middle dose,
and 24 at the high dose. In females, the mortality rate was similar in
all groups (36-40%). During the first three months of the study, the
mean body weights of animals at the highest dose were lower than those
of controls; this effect lasted up to week 16 in males and week 12 in
females. Females at the two highest doses had lower leukocyte counts
than controls at 18 but not at 12 months. No treatment-related gross
or microscopic pathological changes were seen, nor were there
treatment-related changes in organ weights. Although tumours were
observed in a number of organs, especially the liver and lungs, there
was no evidence of treatment-related neoplasia in any treated group.
Cholinesterase activity was not measured in this study, as it had
been evaluated in the range-finding study, in which the NOAEL for
inhibition of brain acetylcholinesterase activity was 2 ppm. The NOAEL
in the main study was 25 ppm, equal to 3.7 mg/kg bw per day, as the
transient effect on body weight in mice at the high dose can be
ignored (Atkinson, 1989).
Rats
Groups of 80 male and 80 female Crl:CD BR rats were given
mevinphos (purity, 85.7%; 74.9% alpha, 10.8% ß isomer) by gavage in
water on five days per week for two years at doses of 0, 0.025, 0.35,
or 0.70 mg/kg bw per day. In females, the high dose was reduced to
0.60 mg/kg bw per day on day 83 of the study because of signs of
acute toxicity. Ten rats from each group were killed at 12 months for
histopathological examination, 10 rats from each group were used for
clinical chemical determinations, and a further 10 were used to
measure cholinesterase activity and were killed at 104 weeks. Clinical
examinations were conducted weekly, and body weights were recorded
before the start of the study, at the start of dosing, and thereafter
weekly for 13 weeks and every four weeks afterwards. Food consumption
was also measured weekly for 13 weeks and every four weeks thereafter.
Clinical chemical, haematological, and urinary analyses were undertaken
and plasma and erythrocyte cholinesterase activities were measured
before treatment, at 3, 6, 1,2 and 18 months, and at termination. Brain
acetylcholinesterase activity was measured at the interim kill and at
the end of the study, but there were too few survivors at the end of
the study for useful intergroup comparisons to be made. Ophthalmoscopic
examinations were done before the start of the study and before
termination. Full histopathological examinations were performed on the
10 animals from each group killed at 12 months and on the survivors at
two years. Selected organs were weighed and examined microscopically.
Less than 40% of the rats survived to termination at 104 weeks,
and there was a dose-related reduction in survival in males, the rate
being clearly reduced at the highest dose; there were no significant
differences in mortality among female rats. Clinical signs typical of
anticholinesterase poisoning, including tremors, were observed after
administration of both the medium and high doses. No treatment-related
changes in body weight, food consumption, or haematological, clinical
chemical, urinary, or ophthalmoscopic parameters were discerned,
except for cholinesterase activity. Plasma, erythrocyte, and brain
cholinesterase activity was diminished in the animals at the middle
and high doses but not in those at the low dose; decreases in
erythrocytic acetylcholinesterase activity > 20% were not observed
in animals of either sex at any dose, but a depression of 20% was
observed at six months in females treated with mevinphos at 0.60 mg/kg
bw per day. Although inhibition of plasma cholinesterase activity was
not seen at the low dose, reductions of 38-51% were seen in males
at the middle dose and 47-61% in males at the high dose; plasma
cholinesterase activity was depressed by 50-67% in females at the
middle dose and 66-71% at the high dose. Brain acetylcholinesterase
activity was depressed by 27% in males at the middle dose, 53% in
males at the high dose, 43% in females at the middle dose, and 55% in
females at the high dose at the interim kill. No treatment-related
effects were seen on histopathological examination. A low incidence of
combined incidental and fatal hepatocellular adenomas was seen (0/67,
1/67, 1/69, and 2/68 in males and 1/70, 0.66, 0/67, and 3/67 in
females), which gave a dose-response relationship. One female at
the high dose had a hepatocellular carcinoma, giving a significant
dose-response relationship for all hepatocellular tumours. The numbers
involved were small, there was no dose-response relationship for liver
carcinomas alone or for adenomas and carcinomas combined in males, and
the incidence was well within that of historical controls. Hence, it
was concluded that there was no evidence of any treatment-associated
tumours. The NOAEL was 0.025 mg/kg bw per day on the basis of
inhibition of brain acetylcholinesterase activity and clinical signs
at doses > 0.35 mg/kg bw per day (Plutnick, 1994).
(d) Reproductive toxicity
In a two-generation study, groups of 35 male and 35 female Crl:CD
BR (Sprague-Dawley) VAF/Plus rats, seven weeks of age, received
mevinphos (purity, 89.6%; 74.5% alpha, 15.1% ß isomer) in water by
gavage at doses of 0, 0.05, 0.1, or 0.5 mg/kg bw. Dosing was adjusted
to allow for the low purity of the material. The F0 rats were dosed
daily for 10 weeks before being assigned randomly, within the dose
groups, into mating pairs at least 11 weeks after the start of dosing.
Dosing was continued during mating, and the F0 females continued to
be treated during gestation and lactation until weaning of the F1
litter 21 days post partum. Offspring were counted, sexed, weighed
(excluding postnatal day 0), and examined externally on postnatal days
0, 1, 4, 7, 14, and 21; litters were culled to four males and four
females. The F1 litters were dosed from 28 days post partum for at
least 11 weeks before mating and also during the mating period. F1
females were dosed during gestation and lactation until weaning on day
21 after birth. Clinical observations were made and body weights
measured before selection of the F0 animals on the first day of
dosing and thereafter weekly in both generations. Observations were
made more frequently in the F0 generation during gestation (days 0,
7, 14, and 21) and on the same days post partum. Erythrocyte,
plasma, and brain cholinesterase activity was measured in all animals
that survived to termination. Necropsies were performed on all
mated animals, all decedent pups, and pups killed in extremis.
Histopathological examinations were performed only on the controls
and animals at the high dose, except that the ovaries of all F1
females were examined.
Treatment-related clinical signs were seen in F0 females at the
high dose, which included ataxia, fine or coarse tremors, pinpoint
pupils, and oral discharge. The growth of F1 animals of each sex was
significantly reduced, and some reduction in the body weights of F1
males and females at the high dose was observed before weaning on
postnatal days 4-21. A reduction in the body weights of F1 male
offspring at the high dose may represent continuation of the reduction
in the F0 males before weaning. The growth of F2 males at the high
dose was also reduced. Cholinesterase inhibition was observed in both
generations but was generally more severe in F1 animals. Significant
inhibition of brain acetylcholinesterase activity was observed in
animals of each sex at the high dose, while no significant inhibition
of erythrocyte cholinesterase activity was seen at any dose; inhibition
of plasma cholinesterase activity extended to animals at the middle
dose and to those at the low dose in the F1 generation. F1 animals
at the high dose showed a reduction in the absolute weight of the
testes or epididymides (12%) and in the relative ovarian weight
(17%). The former may be a reflection of the lower total body weight
of these animals, but the F1 males in all treated groups had lower
mean mating and fertility indices than controls; although these
effects were not statistically significant, they may be associated
with the lower testicular weight observed. The effect on the ovaries
may be a treatment-related effect, as some female rats at the highest
dose had fewer or absent corpora lutea. The frequency of this
pathological observation did not, however, attain statistical
significance, and there were no significant effects on female
fecundity or fertility indices; moreover, the live litter sizes of
the animals at the high dose and the controls were comparable. The
NOAEL was 0.1 mg/kg bw per day on the basis of the presence of clinical
signs and reduced brain acetylcholinesterase activity in animals at
the highest dose, at which impaired growth, impaired fertility
indices, and lower testicular weights were seen in males and lower
ovarian weights in females (Beyer, 1991b).
(e) Developmental toxicity
Rats
Mevinphos (65.5% alpha isomer) was administered to four groups of
24 mated CD rats by gavage in water. One group served as controls,
while the other groups were given mevinphos at daily doses of 0.2,
0.75, or 1.2 mg/kg bw per day on days 6-15 of gestation. A high
mortality rate (29.2%) was observed in rats at the high dose, which
was therefore terminated and a new group receiving 1 mg/kg bw per day
was added. Females were weighed and examined at regular intervals,
while food consumption was recorded on days 0-6, 6-10, 10-15, and
15-20 of gestation. The animals were killed on day 20 of gestation and
examined at autopsy; corpora lutea and uterine implantations were
counted, and fetuses were weighed, sexed, and examined grossly for
malformations. One-half of the fetuses in each litter were processed
for visceral examination and the remainder stained for evaluation of
skeletal changes. Deaths were observed only in the prematurely
terminated group at 1.2 mg/kg bw per day. A dose-related decrease in
mean weight gain was seen during days 6-15 of gestation, but none of
the decreases attained statistical significance. Clinical signs of
cholinergic poisoning (tremors) were seen in about half the animals at
the high dose, and excessive salivation was seen in four animals in
that group. No indication of maternal toxicity was seen in the other
groups, apart from tremor in two rats at the middle dose on day 15.
There were no adverse effects on uterine implantation, fetal weight,
fetal sex distribution, or the external appearance of fetuses, and
there were no visceral or skeletal abnormalities in any treated group.
The NOAEL for maternal toxicity was 0.75 mg/kg bw per day on the basis
of clinical signs at the next highest dose; the NOAEL for developmental
toxicity was 1.0 mg/kg bw per day, the highest dose tested (Schroeder &
Daly, 1987).
Rabbits
Mevinphos (purity, 89.6%; 74.5% alpha, 15.1% ß isomer) was
administered in water by gavage to groups of 20 pregnant New Zealand
white rabbits at doses of 0, 0.05, 0.5, or 1.5 mg/kg bw per day on
days 7-19 of gestation. Surviving animals were killed on day 29. Body
weights were measured on days 0 and 7 of gestation, every three days
until day 25, and on day 29 of gestation. Plasma and erythrocyte
cholinesterase activity was determined in blood taken from dams about
3 h after treatment on the last day. After the animals had been
killed, gross necropsy was carried out, and body weight and the weight
of the uterus plus ovary were determined. The uterine contents were
examined, live fetuses were killed, and all fetuses were weighed and
examined externally. The viscera and skeleton were examined for
malformations, and half of the fetuses from each litter were examined
for the presence of brain abnormalities. Brain acetylcholinesterase
activity was not measured. Maternal toxicity was seen in animals at
the high dose, resulting in one death; corrected body weights were
also decreased in that group on day 29 of gestation. Erythrocyte
acetylcholinesterase activity was statistically significantly reduced
in all three treated groups. The decreases were not biologically
significantly in those at the medium and low doses (13% and 6%), but
the reduction of 18% in animals at the high dose can be considered to
be marginally biologically significant. Plasma cholinesterase activity
was more sensitive to mevinphos, being significantly reduced at the
two higher doses. No significantly different findings among the groups
were made at autopsy. Mean fetal body weights and the crown-rump
lengths of the treated pups were similar to those of controls. An
increased incidence of accessory vessels was seen at 1.5 mg/kg bw
per day, which was not considered by the authors to be related to
treatment, as these are normal variations. Hypoplastic hyoids and
unossified forepaws were also seen at increasing incidence with dose,
but the difference in incidence between animals at the high dose and
the controls did not achieve clinical significance. The NOAEL was
0.5 mg/kg bw per day on the basis of a marginally biologically
significant decrease in erythrocyte acetylcholinesterase activity in
animals at the high dose (Beyer, 1991a).
(f) Genotoxicity
Studies on the genotoxicity of mevinphos in vitro and in vivo are
summarized in Table 2.
Table 4. Recent tests for the genotoxicity of mevinphos
End-point Test system Concentration Purity Results Reference
(%)
In vitro
Reverse mutation S. typhimurium TA98, 100-10 000 µg/plate 89.6 Negative in TA98 San & Schadly
TA100, TA1535, (74.5% alpha, with and without (1989)
TA1537, TA1538 15.1% ß) S9
Sister chromatid Chinese hamster 10-5-10-3 mol/litre NR Positive Hirka et al.
exchange ovary cells (1982)
Unscheduled DNA Rat primary hepatocytes 0.0003-0.3 µl/ml NR Negative Curren (1990)
synthesis
In vivo
Chromosomal CF-1 mouse, bone 1.5, 3 mg/kg bw per 70% alpha Negative Dean & Senner
aberration marrow day orally (1974)
Dominant lethal Mouse 1.5; 3, 6 mg/kg bw NR Negative Dean (1974)
mutation
NR, not reported
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Slight dermal irritation and erythema were observed in five of
six New Zealand white rabbits and oedema in the sixth. Most of the
effects had disappeared by 72 h. In the same study, the material
produced slight temporary ocular irritation in six New Zealand white
rabbits (Deenihan, 1985).
Groups of five male and five female New Zealand white rabbits
received applications of mevinphos (74.5% alpha, 15.1% ß isomer)
on the clipped unabraded skin of the back at doses of 0, 0.1, 1,
or 10 mg/kg bw per day, on five days per week for three weeks.
The material was not irritating. Males at the highest dose had a
significant increase in relative brain weight and a decrease in the
liver:brain and kidney:brain weight ratios. A dose-related decrease in
brain, erythrocyte, and plasma cholinesterase activity was also seen.
Plasma, erythrocyte, and brain cholinesterase activities at termination
were inhibited in males and females at the highest dose by > 15% in
comparison with concurrent controls. Erythrocyte acetylcholinesterase
activity was the least inhibited and brain acetylcholinesterase
activity the most inhibited in animals of each sex. The NOAEL for
transdermal exposure was thus 1 mg/kg bw per day (Trimmer, 1990b).
Mevinphos (purity, 93.6%) did not have dermal sensitization
potential in guinea-pigs (Auletta, 1988c).
(ii) Neurotoxicity
Hens
Ten Leghorn hens (Gallus gallus domesticus) were given 12.5 mg/kg
bw mevinphos (purity, approx. 97%), this dose being slightly greater
than the oral LD50 for hens. Antidotal treatment was required, and
0.625 mg/kg bw atropine was given as clinically indicated; each hen
was also treated with 10 mg/kg bw pralidoxime chloride. Three of the
treated hens died, despite antidotal therapy; the remainder were
treated again on day 21 of the study as they had shown no signs of
neurotoxicity. Four positive controls were treated with tri- ortho-
tolylphosphate (90% mixture of isomers), but, when no signs of
neurotoxicity were seen, material from a different supplier was used
to treat the hens again at 21 days. Hens that survived to 42 days
were injected with sodium pentobarbital and perfused with neutral
buffered formalin. Brain, including the medulla, spinal cord, sciatic
nerve, and the proximal parts of the peroneal and tibial nerves, were
examined histopathologically. The treated birds had slight weight
loss, presumably due to the cholinergic effect of mevinphos, but no
evidence of mevinphos-induced delayed polyneuropathy was seen, either
clinically or histopathologically, whereas characteristic changes were
seen in the positive controls. Neuropathy target esterase was not
measured in this study (Barrett, 1988).
A single dose of mevinphos (76% alpha, 11% ß isomer) was given by
gavage to groups of 27 male and 27 female Sprague-Dawley Crl:CD BR
rats at doses of 0, 0.025 (17 rats of each sex), 0.1, 2, or 3.5
mg/kg bw. Seven animals of each sex per group were allocated for
neuropathological examination and underwent a functional observation
battery and tests for locomotor activity before treatment, at the time
of the peak effect (about 45 min after dosing), and on days 7 and 14.
These animals were killed at 15 days, and their brains were perfused
in situ and weighed; neuropathological examination was then carried
out on five rats of each sex in the control group and those at the
highest dose. The other 20 animals in each group (10 at 0.025 mg/kg
bw) were allocated for determinations of cholinesterase activity;
viability, clinical signs, and body weight were recorded, and the
functional observation battery and tests for locomotor activity were
conducted on five rats of each sex per group. Plasma, erythrocyte, and
brain (including regional) cholinesterase activities were measured
before treatment, at the time of the peak effect (about 45 min after
dosing), and on days 7 and 15 in five animals of each sex per group,
except for those at the lowest dose, in which activity was measured
only at the time of peak effect and on day 15. The numbers of animals
available for determination of cholinesterase activity were also
reduced in the group at the high dose because of mortality (see
below).
One male and five females at the highest dose died, and clinical
signs, including gait alterations, tremors, salivation, and
lacrimation, were observed in those at 2 or 3.5 mg/kg bw only on the
day of dosing. Major disturbances in the locomotor test battery
were observed in animals at these doses but not in controls. The
alterations included lacrimation, salivation, impaired mobility and
gait, clonic and tonic convulsions, tremors, bizarre behaviour, and
altered reflexes. No treatment-related signs were seen in animals
at 0.025 or 0.1 mg/kg bw. On the first day of the study, plasma
cholinesterase activity was reduced by 36-39% in rats at 2 mg/kg bw
and by 41-50% in those at 3.5 mg/kg bw in comparison with concurrent
controls. Erythrocyte acetylcholinesterase activity was unaffected,
while reductions of 20-25% were seen in acetylcholinesterase activity
in the brain stem and/or cerebral cortex, hippocampus, and olfactory
region of animals at 2 mg/kg bw and 19-36% in those at 3.5 mg/kg bw.
The only statistically significant reductions in activity were those
in the brain stem and cortex in males and in the hippocampus and brain
stem in females. Plasma and brain cholinesterase activities were
normal on days 7 and 14. No adverse effects were seen on body or brain
weight. All signs of neurotoxicity had reversed by the end of day 1,
and no treatment-related neuropathological signs were seen at any
dose. The NOAEL was thus 0.1 mg/kg bw, on the basis of clinical
effects and reduced brain acetylcholinesterase activity at higher
doses (Lamb, 1993).
Mevinphos was given by gavage in water to groups of 25 male and
25 female Sprague-Dawley Crl:CD BR rats for 91 days. The initial doses
for all animals were 0.025, 0.035, or 0.70 mg/kg bw per day, but the
dose for females at the high dose was reduced to 0.60 mg/kg bw per day
on day 32 because of the deaths of two animals. Controls received
the vehicle only. Viability, clinical signs, body weights, and
food consumption were recorded for all animals, and a functional
observational battery and locomotor activity tests were conducted on
10 animals of each sex per group. Necropsy was performed on all
animals that died during the study. Neuropathological changes were
evaluated in five animals of each sex in the control group and those
at the high dose, and, because of the mortality at the high dose, in
the females at the intermediate dose. The remaining 20 animals in each
group were evaluated for plasma, erythrocyte, and brain cholinesterase
activity in groups of five animals during weeks 3, 7, and 12 of the
study.
The main clinical sign was tremor in animals at the high dose
45 min after treatment, even after the dose for the females had been
lowered. Excessive salivation was seen in animals of each sex at this
dose, and lacrimation and râles were seen in females. Treatment-
related declines of 32-56% were seen in erythrocyte and plasma
cholinesterase activities in animals of each sex at the intermediate
and high doses; the decreases in erythrocyte acetylcholinesterase
activity were slightly greater (34-62%). Regional brain acetyl-
cholinesterase activity showed some variation, with decreases of 9-21%
in animals at the intermediate and high doses. If decreases of 15% are
biologically significant, the decreased acetylcholinesterase activity
of 21% in the mid-brain of males at the middle dose and of 17% in
females would result in a NOAEL at 0.025 mg/kg bw per day. No
treatment-related neuropathological change was seen (Lamb, 1994).
Administration of a single dose of mevinphos (purity, 89.6%)
to Crl:CD Br rats transdermally did not induce statistically or
biologically significant depressions in erythrocyte acetyl-
cholinesterase activity. A significant depression of plasma
cholinesterase activity occurred at 20 mg/kg bw (29% of control value)
and a marginal fall at 5 mg/kg bw (11% of control). Significant
depressions of brain acetylcholinesterase activity occurred at
20 mg/kg bw (18% of control) (Trimmer, 1989, 1990a).
The effects of mevinphos on flash-evoked potentials were studied
in gerbils, pigeons, cats, and squirrel monkeys, through electrodes
placed in the superior colliculus. Mevinphos reduced the inhibitory
pause in pigeons at doses of 0.1-0.15 mg/kg bw. This effect could not
be prevented by pretreatment with atropine methylnitrite (Revzin,
1978). Electroretinograms of Swiss white mice were examined
after injection of organophosphates, including mevinphos at an
intraperitoneal dose of 0.015 mg. Mevinphos was found to interfere
with retinal function by a direct action on the photoreceptor, by
inhibition of cholinesterase at synapses, and possibly by damaging the
bipolar and/or ganglion cells (Carricaburu et al., 1980).
3. Observations in humans
A number of incidents of poisoning with mevinphos have been
reported through the US pesticide incident monitoring scheme (Hodgson
& Smith, 1993). Poisoning with this compound is reported to be
amenable to treatment with oximes (Bismuth et al., 1993).
After administration of 25 µg/kg bw per day mevinphos to eight
male volunteers for 28 days, plasma and erythrocyte cholinesterase
activity fell throughout the study, with mean decreases of 12.6 and
19% of the respective pretreatment levels. Plasma cholinesterase
activity had returned almost to pretreatment levels within seven days
after the end of treatment, whereas the levels of erythrocyte activity
showed no sign of recovery at follow-up to 14 days. A decrease in
slow fibre motor conduction was also seen; there was an increase in
Achilles tendon reflex force but no effect on neuromuscular
transmission (Verbeck, 1977; Verbeck & Sallé, 1977).
Mevinphos was given to groups of five male volunteers at doses
of 1, 1.5, 2, or 2.5 mg per day for 30 days. Plasma cholinesterase
activity was slightly affected by the highest dose, while the mean
erythrocyte acetylcholinesterase activity was depressed by 20% on one
occasion each at 1.5 and 2 mg per day. Consistent, significant
inhibition of cholinesterase activity was seen only at the highest
dose, at which the erythrocyte acetylcholinesterase fell consistently
to a minimum of 25% at 27 days (Rider et al., 1975).
In agricultural workers with cholinergic symptoms and with mean
inhibition of plasma and erythrocyte cholinesterase activity of 15.6
and 5.6%, respectively, cholinesterase activity recovered at a daily
rate of 1.2 and 0.43% of the respective final levels of activity of
the enzyme over the 14 days after cessation of exposure (Coye et
al., 1986). Higher rates of recovery were observed during the first
11 days after poisoning with a mixture of mevinphos and phosphamidon:
the plasma activity increased by 3% per day and erythrocyte activity
by 0.8% per day. The workers were, however, treated with pralidoxime.
As noted previously, inhibition of plasma cholinesterase activity was
greater than that of erythrocytic enzyme (Midtling et al., 1985).
Human erythrocyte acetylcholinesterase is susceptible to
reactivation in vitro by a variety of pyridinium oximes, including
pralidoximes and obidoxime (Woreck et al., 1996).
Comments
Mevinphos is almost completely absorbed when administered orally
to rats; a large proportion of the absorbed material is biotransformed
to carbon dioxide. Both metabolites and unchanged mevinphos are
observed in the urine but very little in the faeces. Mevinphos
depresses cholinesterase activity in the plasma mre than in the
erythrocytes in experimental animals.
The oral LD50 values for mevinphos in laboratory rodents are
2-12 mg/kg bw. WHO has classified mevinphos as 'extremely hazardous'
(WHO, 1996).
In a three-month range-finding study, mice were fed diets
containing mevinphos at concentrations of 0, 0.5, 1, 2, or 10 ppm.
The NOAEL was 2 ppm, equal to 0.4 mg/kg bw per day, on the basis of
inhibition of brain acetylcholinesterase activity at 10 ppm.
In a 90-day study of toxicity, rats were given mevinphos by
gavage at doses of 0, 0.056, 0.56, 1.1, or 1.7 mg/kg bw per day were
used in males (the highest dose was decreased to 1.1 mg/kg bw per day
at day 36 because of high mortality) and 0, 0.011, 0.056, 0.56, or
0.84 mg/kg bw per day in females. The NOAEL was 0.056 mg/kg bw per
day, on the basis of clinical signs and depressed brain acetyl-
cholinesterase activity at higher doses. Dose-related increases in
mean cholesterol levels and increased relative liver weights were also
observed.
In a one-year study in dogs, mevinphos was administered in corn
oil in gelatine capsules at doses of 0, 0.025, 0.25 or 0.5 mg/kg bw
per day. The NOAEL was 0.25 mg/kg bw per day on the basis of clinical
signs and a reduction in brain acetylcholinesterase activity at the
highest dose.
In an 18-month study of toxicity and carcinogenicity, mice were
fed dietary concentrations of 0, 1, 10, or 25 ppm; cholinesterase
activity was not measured. There was no evidence of carcinogenicity.
In a two-year study of toxicity and carcinogenicity, rats were
given mevinphos by gavage in water on five days per week at doses of
0, 0.025, 0.35, or 0.70 mg/kg bw per day. On day 83 of the study, the
high dose of the females was reduced to 0.60 mg/kg bw per day because
of signs of toxicity. The NOAEL was 0.025 mg/kg bw per day on the
basis of inhibition of brain acetylcholinesterase activity and clinical
signs at higher doses. There was no evidence of carcinogenicity.
A two-generation study of reproductive toxicity was carried out
in which rats were treated by gavage at doses of 0, 0.05, 0.1, or
0.5 mg/kg bw mevinphos in water. The NOAEL was 0.1 mg/kg bw per day on
the basis of clinical signs and reduced brain acetylcholinesterase
activity at the highest dose. This dose also impaired growth and
fertility indices and lowered testicular weights in males and ovarian
weights in females.
In a study of developmental toxicity in rats, groups were given
mevinphos at daily doses of 0, 0.2, 0.75, or 1.2 mg/kg bw per day on
days 6-15 of gestation. High mortality (29%) was observed in the
high-dose group, which was therefore terminated; accordingly, a new
high-dose group at 1 mg/kg bw per day was added. There were no adverse
effects on uterine implantation or on fetal weight, sex distribution,
external appearance, or visceral or skeletal malformations in any
group. It was concluded that mevinphos is not embryotoxic, fetotoxic,
or teratogenic at doses < 1.0 mg/kg bw per day. The NOAEL for
maternal toxicity was 0.75 mg/kg bw per day on the basis of clinical
signs at higher doses.
In a study of developmental toxicity, mevinphos was administered
by gavage to pregnant rabbits at doses of 0, 0.05, 0.5, or 1.5 mg/kg
bw per day on days 7-19 of gestation; surviving animals were killed.
The NOAEL was 0.5 mg/kg bw per day, on the basis of maternal toxicity.
Mevinphos was neither teratogenic nor fetotoxic.
There was some evidence of genotoxic potential in vitro, but
the limited studies available indicate that such potential is not
exhibited in vivo.
In a study in hens, the oral dose of 12 mg/kg bw that was
administered was slightly greater than the oral LD50 value, and
antidotal treatment was required. There was no evidence of delayed
polyneuropathy, either clinically or histopathologically, whereas
characteristic changes were seen in positive controls. Neurotoxic
target esterase was not measured during this study.
Two studies of humans were available. In male volunteers given a
dose of 0.025 mg/kg bw per day, plasma and erythrocyte cholinesterase
activity decreased throughout the 28 days of the study to 13% and 19%
less than the respective pre-dose levels. In the second study, daily
doses of 1, 1.5, 2 or 2.5 mg were given to male volunteers for 30
days, and an NOAEL of 1 mg/day, equivalent to 0.016 mg/kg bw per day,
was derived; however, only five people, all men, per dose were
studied.
An ADI of 0-0.0008 mg/kg bw was established on the basis of the
NOAEL of 0.016 mg/kg bw per day in the 30-day study in volunteers,
using a 20-fold safety factor because of the small numbers in each
group. This ADI is supported by the LOAEL in rats of 0.35 mg/kg bw per
day and the NOAELs of 0.5 mg/kg bw per day in rabbits and 0.25 mg/kg
bw per day in dogs.
An acute reference dose for humans was derived from the 28-day
study in volunteers, on the basis of a dose of 0.025 mg/kg bw per day,
using a 10-fold safety factor.
Toxicological evaluation
Levels that cause no toxicological effect
Mouse: 2 ppm, equal to 0.4 mg/kg bw per day (inhibition of
brain acetylcholinesterase activity in a three-month
study of toxicity)
Rat: 0.025 mg/kg bw per day (two-year study of toxicity and
carcinogenicity)
0.1 mg/kg bw per day (study of reproductive toxicity)
Rabbit: 0.5 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
Dog: 0.25 mg/kg bw per day (one-year study of toxicity)
Human: 1 mg per day, equivalent to 0.016 mg/kg bw per day
(inhibition of cholinesterase activity in a 30-day
study of toxicity)
Estimate of acceptable daily intake for humans
0-0.0008 mg/kg bw
Acute reference dose
0.003 mg/kg bw
Studies that would provide information useful for continued evaluation
of the compound
Study of micronucleus formation in mice in vivo
Toxicological criteria for estimating guidance values for dietary and non-dietary exposure to mevinphos
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Oral, toxicity, rat LD50 = 2.2-6.1 mg/kg bw
Dermal, toxicity, rat LD50 > 20 mg/kg bw
Inhalation, 4 h, toxicity, rat LD50 = 7.3-12 mg/m3
Dermal, irritation, rabbit Slightly irritating
Ocular, irritation, rabbit Slightly irritating
Dermal, sensitization, guinea-pig Not sensitizing
Medium-term (1-26 weeks) Repeated oral, 3 months, mouse NOAEL = 0.4 mg/kg bw per day, inhibition of brain
acetylcholinesterase activity
Repeated oral, 90 days, rat NOAEL = 0.056 mg/kg bw per day
Repeated dermal, 21 days, rabbit NOAEL = 1.0 mg/kg bw per day
Repeated oral, reproductive toxicity, rat NOAEL = 0.1 mg/kg bw per day, maternal and
reproductive toxicity
Repeated oral, developmental toxicity, rat NOAEL = 0.75 mg/kg bw per day, maternal toxicity;
no developmental toxicity
Repeated oral, developmental toxicity, rabbit NOAEL = 0.5 mg/kg bw per day, maternal toxicity;
no developmental toxicity
Long-term (> 1 year) Repeated oral, 2 years, rat NOAEL = 0.025 mg/kg bw per day; inhibition of
brain acetylcholinesterase activity
Repeated oral, 1 year, dog NOAEL = 0.25 mg/kg bw per day; inhibition of
brain acetylcholinesterase activity
References
Atkinson, J.E. (1989) An eighteen month oncogenicity feeding study in
mice with mevinphos. Project No 86-300. Unpublished report dated 23
February 1989 from BioDynamics Inc., East Millstone, NJ, USA.
Submitted to WHO by Amvac Chemical Corp., City of Commerce, CA, USA.
Atkinson, J.E. (1990) A three month dietary range-finding study in
mice with mevinphos technical. Project No. 85-3025. Unpublished report
in West, J. & Roberts, K. (1990) Supplement to an eighteen month
oncogenicity study in mice with mevinphos. Project ID JCODE 1562.1.
Unpublished report dated 31 August 1990 from Jelinek Schwartz Connolly
and Freshman Inc., Washington DC, USA. Submitted to WHO by Amvac
Chemical Corp., City of Commerce, CA, USA.
Auletta, C.S. (1988a) Acute oral toxicity study in rats with
mevinphos. Unpublished report dated 23 March 1988, Project No. 4644-87
from BioDynamics Inc., East Millstone, NJ, USA. Submitted to WHO by
Amvac Chemical Corp., City of Commerce, CA, USA.
Auletta, C.S. (1988b) Acute dermal toxicity study in rabbits with
mevinphos. Unpublished report dated 23 March 1988, Project No. 4645-87
from BioDynamics Inc., East Millstone, NJ, USA. Submitted to WHO by
Amvac Chemical Corp., City of Commerce, CA, USA.
Auletta, C.S. (1988c) Dermal sensitization study in guinea pigs with
mevinphos. Unpublished report dated 23 March 1988, Project No. 4648-87
from BioDynamics Inc., East Millstone, NJ, USA. Submitted to WHO by
Amvac Chemical Corp., City of Commerce, CA, USA.
Barrett, D.S. (1988) Acute delayed neurotoxicity study in mature hens
with mevinphos. Unpublished report dated 26 July 1988, Project No
4685-87 from BioDynamics Inc., East Millstone, NJ, USA. Submitted to
WHO by Amvac Chemical Corp., City of Commerce, CA, USA.
Beyer, B.K. (1991a) Teratology study in rabbits. Study No. MRD-88-331.
Unpublished report dated 22 February 1991 from Exxon Biomedical
Sciences Inc, East Millstone, NJ, USA. Submitted to WHO by Amvac
Chemical Corp., City of Commerce, CA, USA.
Beyer BK (1991b) Multigeneration rat reproduction study. Study No.
MRD-88-331. Unpublished report dated 26th November 1991 from Exxon
Biomedical Sciences Inc., East Millstone, NJ, USA. Submitted to WHO by
Amvac Chemical Corp., City of Commerce, CA, USA.
Bismuth, C., Inns, R.H. & Marrs, T.C. (1993) Efficacy, toxicity and
clinical use of oximes in anticholinesterase poisoning. In:
Ballantyne, B. & Marrs, T.C, eds, Clinical and Experimental Toxicology
of Organophosphates and Carbamates, Oxford, Butterworths, pp. 555-577.
Carricaburu, P., Lacroix, R. & Lacroix, J. (1980) Electroretinographic
study of the white mouse intoxicated by organo-phosphorus: mevinphos
and malathion. Toxicol. Environ. Res., 3, 87-91.
Coye, M.J., Barnett, P.G. & Midtling, J.E. (1986) Clinical confirmation
of organophosphate poisoning of agricultural workers. Am. J. Ind. Med.,
10, 399-409.
Curren, R.D. (1990) Unscheduled DNA synthesis in rat primary
hepatocytes with mevinphos. Unpublished report dated 25 January 1990,
Study No. T8858.380 from Microbiological Associates, Inc., Rockville,
MD, USA. Submitted to WHO by Amvac Chemical Corp., City of Commerce,
CA, USA [full report not available to the Meeting].
Dean, B.J. (1974) Toxicity studies with phosdrin. Dominant lethal
assay in male mice after a single oral dose of phosdrin. Unpublished
report dated July 1974 Group research phosdrin, No. TLGR.0031.74 from
Shell Research Centre, Tunstall, Kent, United Kingdom. Submitted to
WHO by Amvac Chemical Corp., City of Commerce, CA, USA [full report
not available to the Meeting].
Dean, B.J. & Senner, K. (1974) Toxicity studies with phosdrin:
Chromosome studies on bone marrow cells of mice after two daily oral
doses of phosdrin. Group research report No. TLGR.0008.74. Unpublished
report dated February 1974 from Shell Research Centre, Tunstall, Kent,
United Kingdom. Submitted to WHO by Amvac Chemical Corp., City of
Commerce, CA, USA.
Deenihan, M.J. (1985) Mevinphos insecticide acute oral toxicity, acute
dermal toxicity, primary skin irritation, primary eye irritation.
NVP report No. X5A027G. Unpublished report dated 10 April 1985 from
Northview Pacific Laboratories Inc., Berkeley, CA, USA. Submitted to
WHO by Amvac Chemical Corp., City of Commerce, CA, USA.
Gaines, T. (1969) Acute toxicity of pesticides. Toxicol. Appl.
Pharmacol., 14, 515-534.
Hirka, G., Béres, E. & Schmidt, K. (1982) Use of cell and tissue
cultures in genetic toxicology. Mutat. Res., 97, 192.
Hodgson, M.J. & Smith, A.D. (1993) Commercial and residential
poisoning with anticholinesterases. In: Ballantyne, B. & Marrs, T.C.,
eds, Clinical and Experimental Toxicology of Organophosphates and
Carbamates, Oxford, Butterworths, pp. 352-363.
Hoffman, G.M. (1988) An acute inhalation toxicity study of mevinphos
in the rat. Unpublished report and letter dated 12 April 1988 from
BioDynamics Inc., East Millstone, NJ, USA. Submitted to WHO by Amvac
Chemical Corp., City of Commerce, CA, USA.
Jeffcoat A.R. & Coleman, D.P. (1993) Dermal absorption of mevinphos in
rats at three dose levels. RTI Study No. 64C-5494. Unpublished report
dated 31 August 1993 from Research Triangle Institute, Research
Triangle Park, NC, USA. Submitted to WHO by Amvac Chemical Corp., City
of Commerce, CA, USA.
Kangas, L. (1995) A 52-week oral (capsule) toxicity study of mevinphos
in the beagle dog. Laboratory ID 85746. Unpublished report dated
9 June 1995 from Bio-research Laboratories Ltd, Senneville, Quebec,
Canada. Submitted to WHO by Amvac Chemical Corp, City of Commerce, CA,
USA.
Keefe, R.T. (1992) 90-Day subchronic oral toxicity study in rats with
mevinphos (MRD-88-331) Laboratory ID 233170B. Unpublished report dated
4 November 1992 from Exxon Biomedical Sciences Inc., East Millstone,
NJ, USA. Submitted to WHO by Amvac Chemical Corp., City of Commerce,
CA, USA.
Kuhn, J.O. (1994) Acute oral toxicity study in rats (DOT). Laboratory
study number 0946-94. Unpublished report dated 28 March 1994 from
Stillmeadow Inc, Sugar Land, TX, USA. Submitted to WHO by Amvac
Chemical Corp,, City of Commerce, CA, USA.
Lamb, I.C. (1993) An acute neurotoxicity study of mevinphos in rats.
Study No. WIL-188006. Unpublished report dated 13 October 1993 from
WIL Research Laboratories Inc., Ashland, OH, USA. Submitted to WHO by
Amvac Chemical Corp., City of Commerce, CA, USA.
Lamb, I.C. (1994) A subchronic (13 week) neurotoxicity study of
mevinphos in rats. Study No. WIL-188007. Unpublished report dated
17 June 1994 from WIL Research Laboratories Inc., Ashland, OH, USA.
Submitted to WHO by Amvac Chemical Corp., City of Commerce, CA, USA.
Midtling, J.E., Barnett, P.G., Coye, M.J., Velasco, A.R., Romero, P.,
Clements, C.L., O'Malley, M.A., Tobin, M.W., Rose, T.G. & Monosson,
I.H. (1985) Clinical management of field worker organophosphate
poisoning. West. J. Med., 142, 514-518.
Plutnick, R.T. (1994) 2-Year chronic toxicity/oncogenicity study in
rats with mevinphos (MRD-88-331). Laboratory ID 233170C. Unpublished
report dated 3 January 1994 from Exxon Biomedical Sciences Inc., East
Millstone, NJ, USA. Submitted to WHO by Amvac Chemical Corp., City of
Commerce, CA, USA.
Reddy, V., Freeman, T., Little, L. & Cannon, M. (1991) Disposition
and metabolism of 14C-labeled mevinphos in rats (preliminary and
definitive study). Laboratory ID MRI 9485B. Unpublished report dated
4 April 1991 from Midwest Research Institute, Kansas City, MI, USA.
Submitted to WHO by Amvac Chemical Corp., City of Commerce, CA, USA.
Revzin, A.M. (1978) Effects of organophosphate pesticides and alcohol
on visual mechanisms. In: Merigan, W.H. & Weiss, B., eds, Neurotoxicity
of the Visual System, New York, Raven Press, pp. 255-268.
Rider, J.A., Puletti, E.J. & Swader, J.I. (1975) The minimal oral
toxicity level for mevinphos in man. Toxicol. Appl. Pharmacol., 32,
97-100.
San, R.H.C. & Schadly, M.B. (1989) Salmonella/mammalian microsome
plate incorporation mutagenicity assay (Ames test) with a confirmatory
assay with mevinphos. Study No. T8858.501014. Unpublished report dated
23 October 1989 from Microbiological Associates Inc., Rockville, MD,
USA. Submitted to WHO by Amvac Chemical Corp., City of Commerce, CA,
USA.
Schroeder, R.E. & Daly, I.W. (1987) Mevinphos - A teratology study in
rats with mevinphos. Laboratory ID 85-3009. Unpublished report dated
2 March 1987 from BioDynamics Inc., East Millstone, NJ, USA. Submitted
to WHO by Amvac Chemical Corp., City of Commerce, CA, USA.
Skinner, C.S. & Kilgore, W.W. (1982) Acute dermal toxicities of various
organophosphate insecticides in mice. J. Toxicol. Environ. Health,
9, 491-497.
Trimmer, G.W. (1989) Acute dermal toxicity study in the rat phase 1
(MRD-88-331: mevinphos). Laboratory ID 233106. Unpublished report
dated 23 August 1989 from Exxon Biomedical Sciences Inc., East
Millstone, NJ, USA. Submitted to WHO by Amvac Chemical Corp., City of
Commerce, CA, USA.
Trimmer, G.W. (1990a) Acute dermal toxicity study in the rat phase II
cholinesterase determination (MRD-88-331: mevinphos). Laboratory ID
233177A. Unpublished report dated 20 July 1990 from Exxon Biomedical
Sciences Inc., East Millstone, NJ, USA. Submitted to WHO by Amvac
Chemical Corp., City of Commerce, CA, USA.
Trimmer, G.W. (1990b) 21-Day repeated dermal study in the rabbit
(MRD-88-331-mevinphos). Laboratory ID 233109. Unpublished report dated
4 April 1990 from Exxon Biomedical Sciences Inc., East Millstone, NJ,
USA. Submitted to WHO by Amvac Chemical Corp., City of Commerce, CA,
USA.
Verberk, M.M. (1977) Incipient cholinesterase inhibition in volunteers
ingesting monocrotophos or mevinphos for one month. Toxicol. Appl.
Pharmacol.,42, 345-350.
Verberk, M.M. & Sallé, H.J.A. (1977) Effects on nervous function of
volunteers ingesting mevinphos for 1 month. Toxicol. Appl. Pharmacol.,
42, 351-358.
WHO (1996) The WHO Recommended Classification of Pesticides by Hazard
and Guidelines to Classification 1996-1997 (WHO/PCS/96.3), International
Programme on Chemical Safety, Geneva.
Worek, F., Kirchner, T., Bäcker, M. & Szinicz, L. (1996) Reactivation
by various oximes of humans erythrocyte acetylcholinesterase inhibited
by different organophosphorus compounds. Arch. Toxicol., 70,
497-503.
 Table 1. Acute toxicity of mevinphos in experimental animals
Species Strain Sex Route Purity LD50 (mg/kg bw) Reference
(%) or LC50 (mg/litre)
(95% CI)
Mouse Swiss Webster Male Dermal 99 12 (6-24) Skinner & Kilgore
(feet) (1982)
Rat Sherman Male Oral NR 6.1 Gaines (1969)
Female 3.7
Rat Sprague-Dawley Male Oral NR 4.1 (3.5-5.0) Deenihan (1985)
Female 2.2 (1.8-2.6)
Rat Sprague-Dawley-derived CD Male Oral 97.7 3.5a Auletta (1988a)
Female 2.3 (1.0-3.6)
Rat Albino HSD:SD Male Oral Unknownb > 5 Kuhn (1994)
Female > 5
Rat Sherman Male Dermal NR 4.7 Gaines (1969)
Female 4.2
Rat Sprague-Dawley Crl:CD BR Male Dermal 90 >20 Trimmer (1989)
Female >20
Rat Sprague-Dawley CD Male Inhalationc 100 12 Hoffman (1988)
Female 7.3
Rabbit New Zealand white Male, Dermal NR 57 (38-86) Deenihan (1985)
female
Rabbit New Zealand white Male Dermal 99.3 51 (31-71) Auletta (1988b)
Female 60 (23-97)
Chicken White Leghorn pullets Female Oral approx. 97 11 (3.2-19) Barrett (1988)
CI, confidence interval; NR, not reported
a CI could not be calculated owing to distribution of deaths
b Phosdrin 4 EC
c 4-h exposure; only 3 h at highest dose as all animals had died
(b) Short-term toxicity
Mice
In a three-month range-finding study, groups of 10 male and 10
female CD-1 mice were fed diets containing mevinphos (66.5% alpha,
21.2% ß isomer) at 0, 0.5, 1, 2, or 10 ppm, equal to 0, 0.1, 0.2, 0.4,
and 2 mg/kg bw per day for males and 0, 0.1, 0.3, 0.5, and 2.7 mg/kg
bw per day for females. Clinical observations, body weight, and food
consumption were reported before treatment and weekly after the start
of exposure, and plasma and erythrocyte cholinesterase activities
were measured in five animals of each sex per group at week 7 and
at termination. After three months, the animals were killed and
autopsied, and brain acetylcholinesterase activity was measured in
five animals of each sex per group. Organs from controls and animals
at the high dose were examined grossly, and selected tissues were
examined microscopically. Body weight and food consumption were
similar in all groups, and no deaths occurred; clinical parameters
were similar in all groups. Plasma cholinesterase activity was
decreased by 37-49% in animals of each sex at 2 and 10 ppm at week 7.
At termination, similar findings were seen in males, but the decreased
plasma cholinesterase activity in females was confined to those at
10 ppm. Brain acetylcholinesterase activity was decreased by 16 and
22% in males and females at 10 ppm, respectively, in comparison with
controls. The NOAEL was 2 ppm, equal to 0.4 mg/kg bw per day, on the
basis of inhibition of brain acetylcholinesterase activity at 10 ppm
(Atkinson, 1990).
Rats
Groups of 10 Sprague-Dawley Crl:CD BR rats received mevinphos
(74.5% alpha, 15.1% ß isomer) by gavage for 90 days at doses of active
ingredient of 0.056, 0.56, 1.1, or 1.7 mg/kg bw per day for males and
0.011, 0.056, 0.56, or 0.84 mg/kg bw per day for females. Five males
at the two highest doses died, and the highest dose was decreased to
1.1 mg/kg bw per day on day 36; the groups at the two highest doses
were then combined for most statistical purposes. Additionally, one
female at 0.56 mg/kg bw per day died before scheduled termination.
Clinical signs, including pinpoint pupils, oral and ocular discharges,
and tremors were seen at doses > 0.56 mg/kg bw per day in animals
of each sex. No statistically significant differences in body weight
or food consumption were noted. Inhibition of plasma cholinesterase
activity > 15% was seen at week 7 and terminally in males at doses
> 0.56 mg/kg bw per days and in females at doses > 0.056 mg/kg
bw per day. Brain acetylcholinesterase activity was inhibited by
> 15% at termination in animals of each sex at doses > 0.56 mg/kg
bw per day. No significant depression of erythrocyte acetyl-
cholinesterase activity was seen. There was a dose-related increase
in mean cholesterol levels, which were statistically significantly
increased in males at the high dose. No statistically significant
differences in absolute or relative organ weights were seen between
groups, but a dose-related trend in increased relative liver weights
was seen in animals of each sex. Slight vacuolation in centrilobular
and midzonal hepatocytes was seen in two male rats at the highest
dose. The NOAEL was 0.056 mg/kg bw per day, on the basis of clinical
signs and depressed brain acetylcholinesterase activity at higher
doses (Keefe, 1992).
Dogs
Gelatine capsules containing mevinphos (75.1% alpha, 11.8% ß
isomer) in corn oil were given to groups of five male and five female
beagle dogs at doses of 0 or 0.5 mg/kg bw per day and to four males
and four females at 0.025 or 0.25 mg/kg bw per day for one year. No
deaths occurred during the study. The only clinical sign attributable
to the test material was a higher prevalence of vomiting in the dogs
at the high dose than in the controls. No significant differences in
body weight or food consumption were noted, and no treatment-related
changes were seen at ophthalmoscopic examination at weeks 12, 26, and
52. Blood was taken before treatment and at weeks 6, 12, 39, and 51
for laboratory analyses. No biologically significant haematological or
biochemical changes were seen, other than changes in cholinesterase
activity. Plasma and erythrocyte cholinesterase activities were
determined on blood samples taken before the start of treatment, 3 h
after dosing at 4, 12, 39, and 51 weeks, and before dosing at weeks
39 and 51. Brain acetylcholinesterase activity was measured at
termination of the study. At many of these time intervals, both
erythrocyte and plasma cholinesterase activities were depressed,
particularly at the two higher doses. In dogs at the lowest dose, a
depression > 15% was seen in erythrocyte enzyme activity at week 25
in males and females, at week 39 in males before treatment and in
females before and after treatment, and at week 51 in males before and
after treatment and in females after treatment. At this dose, plasma
cholinesterase activity was depressed by > 15% at 39 and 51 weeks in
females after treatment. Statistically and biologically significant
inhibition of brain acetylcholinesterase activity was observed only in
males at the highest dose, in which the activity was 77% of that in
concurrent controls. Higher absolute thyroid/parathyroid weights
were observed in females at the highest dose and higher relative
thyroid/parathyroid weights in females at the intermediate dose. No
associated histopathological abnormality was discerned, and these
findings are considered not to be significant. No treatment-related
pathological finding was seen at autopsy. The NOAEL was thus
0.25 mg/kg bw per day, on the basis of clinical signs and a reduction
in brain acetylcholinesterase activity at the highest dose (Kangas,
1995).
(c) Long-term toxicity and carcinogenicity
Mice
On the basis of a range-finding study, mevinphos (purity, 87.7%;
66.5% alpha, 21.2% ß isomer) was administered to four groups of 50
CD-1 mice of each sex at dietary concentrations providing doses of 0,
1, 10, or 25 ppm, equal to 0, 0.1, 1.5, and 3.7 mg/kg bw per day for
males and 0, 0.1, 1.9, and 4.8 mg/kg bw per day for females, for 18
months. Physical examinations were performed each week and body weight
and food consumption before the start of the study, weekly thereafter
for 13 weeks on alternate weeks until week 26, and thence monthly.
Blood smears for evaluating the differential leukocyte count and
erythrocyte morphology were taken from 10 animals of each sex per
group at 12 and 18 months. Treatment had few effects, but the survival
rate was somewhat reduced in males at the low dose, with only 20
survivors, in comparison with 27 male controls, 26 at the middle dose,
and 24 at the high dose. In females, the mortality rate was similar in
all groups (36-40%). During the first three months of the study, the
mean body weights of animals at the highest dose were lower than those
of controls; this effect lasted up to week 16 in males and week 12 in
females. Females at the two highest doses had lower leukocyte counts
than controls at 18 but not at 12 months. No treatment-related gross
or microscopic pathological changes were seen, nor were there
treatment-related changes in organ weights. Although tumours were
observed in a number of organs, especially the liver and lungs, there
was no evidence of treatment-related neoplasia in any treated group.
Cholinesterase activity was not measured in this study, as it had
been evaluated in the range-finding study, in which the NOAEL for
inhibition of brain acetylcholinesterase activity was 2 ppm. The NOAEL
in the main study was 25 ppm, equal to 3.7 mg/kg bw per day, as the
transient effect on body weight in mice at the high dose can be
ignored (Atkinson, 1989).
Rats
Groups of 80 male and 80 female Crl:CD BR rats were given
mevinphos (purity, 85.7%; 74.9% alpha, 10.8% ß isomer) by gavage in
water on five days per week for two years at doses of 0, 0.025, 0.35,
or 0.70 mg/kg bw per day. In females, the high dose was reduced to
0.60 mg/kg bw per day on day 83 of the study because of signs of
acute toxicity. Ten rats from each group were killed at 12 months for
histopathological examination, 10 rats from each group were used for
clinical chemical determinations, and a further 10 were used to
measure cholinesterase activity and were killed at 104 weeks. Clinical
examinations were conducted weekly, and body weights were recorded
before the start of the study, at the start of dosing, and thereafter
weekly for 13 weeks and every four weeks afterwards. Food consumption
was also measured weekly for 13 weeks and every four weeks thereafter.
Clinical chemical, haematological, and urinary analyses were undertaken
and plasma and erythrocyte cholinesterase activities were measured
before treatment, at 3, 6, 1,2 and 18 months, and at termination. Brain
acetylcholinesterase activity was measured at the interim kill and at
the end of the study, but there were too few survivors at the end of
the study for useful intergroup comparisons to be made. Ophthalmoscopic
examinations were done before the start of the study and before
termination. Full histopathological examinations were performed on the
10 animals from each group killed at 12 months and on the survivors at
two years. Selected organs were weighed and examined microscopically.
Less than 40% of the rats survived to termination at 104 weeks,
and there was a dose-related reduction in survival in males, the rate
being clearly reduced at the highest dose; there were no significant
differences in mortality among female rats. Clinical signs typical of
anticholinesterase poisoning, including tremors, were observed after
administration of both the medium and high doses. No treatment-related
changes in body weight, food consumption, or haematological, clinical
chemical, urinary, or ophthalmoscopic parameters were discerned,
except for cholinesterase activity. Plasma, erythrocyte, and brain
cholinesterase activity was diminished in the animals at the middle
and high doses but not in those at the low dose; decreases in
erythrocytic acetylcholinesterase activity > 20% were not observed
in animals of either sex at any dose, but a depression of 20% was
observed at six months in females treated with mevinphos at 0.60 mg/kg
bw per day. Although inhibition of plasma cholinesterase activity was
not seen at the low dose, reductions of 38-51% were seen in males
at the middle dose and 47-61% in males at the high dose; plasma
cholinesterase activity was depressed by 50-67% in females at the
middle dose and 66-71% at the high dose. Brain acetylcholinesterase
activity was depressed by 27% in males at the middle dose, 53% in
males at the high dose, 43% in females at the middle dose, and 55% in
females at the high dose at the interim kill. No treatment-related
effects were seen on histopathological examination. A low incidence of
combined incidental and fatal hepatocellular adenomas was seen (0/67,
1/67, 1/69, and 2/68 in males and 1/70, 0.66, 0/67, and 3/67 in
females), which gave a dose-response relationship. One female at
the high dose had a hepatocellular carcinoma, giving a significant
dose-response relationship for all hepatocellular tumours. The numbers
involved were small, there was no dose-response relationship for liver
carcinomas alone or for adenomas and carcinomas combined in males, and
the incidence was well within that of historical controls. Hence, it
was concluded that there was no evidence of any treatment-associated
tumours. The NOAEL was 0.025 mg/kg bw per day on the basis of
inhibition of brain acetylcholinesterase activity and clinical signs
at doses > 0.35 mg/kg bw per day (Plutnick, 1994).
(d) Reproductive toxicity
In a two-generation study, groups of 35 male and 35 female Crl:CD
BR (Sprague-Dawley) VAF/Plus rats, seven weeks of age, received
mevinphos (purity, 89.6%; 74.5% alpha, 15.1% ß isomer) in water by
gavage at doses of 0, 0.05, 0.1, or 0.5 mg/kg bw. Dosing was adjusted
to allow for the low purity of the material. The F0 rats were dosed
daily for 10 weeks before being assigned randomly, within the dose
groups, into mating pairs at least 11 weeks after the start of dosing.
Dosing was continued during mating, and the F0 females continued to
be treated during gestation and lactation until weaning of the F1
litter 21 days post partum. Offspring were counted, sexed, weighed
(excluding postnatal day 0), and examined externally on postnatal days
0, 1, 4, 7, 14, and 21; litters were culled to four males and four
females. The F1 litters were dosed from 28 days post partum for at
least 11 weeks before mating and also during the mating period. F1
females were dosed during gestation and lactation until weaning on day
21 after birth. Clinical observations were made and body weights
measured before selection of the F0 animals on the first day of
dosing and thereafter weekly in both generations. Observations were
made more frequently in the F0 generation during gestation (days 0,
7, 14, and 21) and on the same days post partum. Erythrocyte,
plasma, and brain cholinesterase activity was measured in all animals
that survived to termination. Necropsies were performed on all
mated animals, all decedent pups, and pups killed in extremis.
Histopathological examinations were performed only on the controls
and animals at the high dose, except that the ovaries of all F1
females were examined.
Treatment-related clinical signs were seen in F0 females at the
high dose, which included ataxia, fine or coarse tremors, pinpoint
pupils, and oral discharge. The growth of F1 animals of each sex was
significantly reduced, and some reduction in the body weights of F1
males and females at the high dose was observed before weaning on
postnatal days 4-21. A reduction in the body weights of F1 male
offspring at the high dose may represent continuation of the reduction
in the F0 males before weaning. The growth of F2 males at the high
dose was also reduced. Cholinesterase inhibition was observed in both
generations but was generally more severe in F1 animals. Significant
inhibition of brain acetylcholinesterase activity was observed in
animals of each sex at the high dose, while no significant inhibition
of erythrocyte cholinesterase activity was seen at any dose; inhibition
of plasma cholinesterase activity extended to animals at the middle
dose and to those at the low dose in the F1 generation. F1 animals
at the high dose showed a reduction in the absolute weight of the
testes or epididymides (12%) and in the relative ovarian weight
(17%). The former may be a reflection of the lower total body weight
of these animals, but the F1 males in all treated groups had lower
mean mating and fertility indices than controls; although these
effects were not statistically significant, they may be associated
with the lower testicular weight observed. The effect on the ovaries
may be a treatment-related effect, as some female rats at the highest
dose had fewer or absent corpora lutea. The frequency of this
pathological observation did not, however, attain statistical
significance, and there were no significant effects on female
fecundity or fertility indices; moreover, the live litter sizes of
the animals at the high dose and the controls were comparable. The
NOAEL was 0.1 mg/kg bw per day on the basis of the presence of clinical
signs and reduced brain acetylcholinesterase activity in animals at
the highest dose, at which impaired growth, impaired fertility
indices, and lower testicular weights were seen in males and lower
ovarian weights in females (Beyer, 1991b).
(e) Developmental toxicity
Rats
Mevinphos (65.5% alpha isomer) was administered to four groups of
24 mated CD rats by gavage in water. One group served as controls,
while the other groups were given mevinphos at daily doses of 0.2,
0.75, or 1.2 mg/kg bw per day on days 6-15 of gestation. A high
mortality rate (29.2%) was observed in rats at the high dose, which
was therefore terminated and a new group receiving 1 mg/kg bw per day
was added. Females were weighed and examined at regular intervals,
while food consumption was recorded on days 0-6, 6-10, 10-15, and
15-20 of gestation. The animals were killed on day 20 of gestation and
examined at autopsy; corpora lutea and uterine implantations were
counted, and fetuses were weighed, sexed, and examined grossly for
malformations. One-half of the fetuses in each litter were processed
for visceral examination and the remainder stained for evaluation of
skeletal changes. Deaths were observed only in the prematurely
terminated group at 1.2 mg/kg bw per day. A dose-related decrease in
mean weight gain was seen during days 6-15 of gestation, but none of
the decreases attained statistical significance. Clinical signs of
cholinergic poisoning (tremors) were seen in about half the animals at
the high dose, and excessive salivation was seen in four animals in
that group. No indication of maternal toxicity was seen in the other
groups, apart from tremor in two rats at the middle dose on day 15.
There were no adverse effects on uterine implantation, fetal weight,
fetal sex distribution, or the external appearance of fetuses, and
there were no visceral or skeletal abnormalities in any treated group.
The NOAEL for maternal toxicity was 0.75 mg/kg bw per day on the basis
of clinical signs at the next highest dose; the NOAEL for developmental
toxicity was 1.0 mg/kg bw per day, the highest dose tested (Schroeder &
Daly, 1987).
Rabbits
Mevinphos (purity, 89.6%; 74.5% alpha, 15.1% ß isomer) was
administered in water by gavage to groups of 20 pregnant New Zealand
white rabbits at doses of 0, 0.05, 0.5, or 1.5 mg/kg bw per day on
days 7-19 of gestation. Surviving animals were killed on day 29. Body
weights were measured on days 0 and 7 of gestation, every three days
until day 25, and on day 29 of gestation. Plasma and erythrocyte
cholinesterase activity was determined in blood taken from dams about
3 h after treatment on the last day. After the animals had been
killed, gross necropsy was carried out, and body weight and the weight
of the uterus plus ovary were determined. The uterine contents were
examined, live fetuses were killed, and all fetuses were weighed and
examined externally. The viscera and skeleton were examined for
malformations, and half of the fetuses from each litter were examined
for the presence of brain abnormalities. Brain acetylcholinesterase
activity was not measured. Maternal toxicity was seen in animals at
the high dose, resulting in one death; corrected body weights were
also decreased in that group on day 29 of gestation. Erythrocyte
acetylcholinesterase activity was statistically significantly reduced
in all three treated groups. The decreases were not biologically
significantly in those at the medium and low doses (13% and 6%), but
the reduction of 18% in animals at the high dose can be considered to
be marginally biologically significant. Plasma cholinesterase activity
was more sensitive to mevinphos, being significantly reduced at the
two higher doses. No significantly different findings among the groups
were made at autopsy. Mean fetal body weights and the crown-rump
lengths of the treated pups were similar to those of controls. An
increased incidence of accessory vessels was seen at 1.5 mg/kg bw
per day, which was not considered by the authors to be related to
treatment, as these are normal variations. Hypoplastic hyoids and
unossified forepaws were also seen at increasing incidence with dose,
but the difference in incidence between animals at the high dose and
the controls did not achieve clinical significance. The NOAEL was
0.5 mg/kg bw per day on the basis of a marginally biologically
significant decrease in erythrocyte acetylcholinesterase activity in
animals at the high dose (Beyer, 1991a).
(f) Genotoxicity
Studies on the genotoxicity of mevinphos in vitro and in vivo are
summarized in Table 2.
Table 4. Recent tests for the genotoxicity of mevinphos
End-point Test system Concentration Purity Results Reference
(%)
In vitro
Reverse mutation S. typhimurium TA98, 100-10 000 µg/plate 89.6 Negative in TA98 San & Schadly
TA100, TA1535, (74.5% alpha, with and without (1989)
TA1537, TA1538 15.1% ß) S9
Sister chromatid Chinese hamster 10-5-10-3 mol/litre NR Positive Hirka et al.
exchange ovary cells (1982)
Unscheduled DNA Rat primary hepatocytes 0.0003-0.3 µl/ml NR Negative Curren (1990)
synthesis
In vivo
Chromosomal CF-1 mouse, bone 1.5, 3 mg/kg bw per 70% alpha Negative Dean & Senner
aberration marrow day orally (1974)
Dominant lethal Mouse 1.5; 3, 6 mg/kg bw NR Negative Dean (1974)
mutation
NR, not reported
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Slight dermal irritation and erythema were observed in five of
six New Zealand white rabbits and oedema in the sixth. Most of the
effects had disappeared by 72 h. In the same study, the material
produced slight temporary ocular irritation in six New Zealand white
rabbits (Deenihan, 1985).
Groups of five male and five female New Zealand white rabbits
received applications of mevinphos (74.5% alpha, 15.1% ß isomer)
on the clipped unabraded skin of the back at doses of 0, 0.1, 1,
or 10 mg/kg bw per day, on five days per week for three weeks.
The material was not irritating. Males at the highest dose had a
significant increase in relative brain weight and a decrease in the
liver:brain and kidney:brain weight ratios. A dose-related decrease in
brain, erythrocyte, and plasma cholinesterase activity was also seen.
Plasma, erythrocyte, and brain cholinesterase activities at termination
were inhibited in males and females at the highest dose by > 15% in
comparison with concurrent controls. Erythrocyte acetylcholinesterase
activity was the least inhibited and brain acetylcholinesterase
activity the most inhibited in animals of each sex. The NOAEL for
transdermal exposure was thus 1 mg/kg bw per day (Trimmer, 1990b).
Mevinphos (purity, 93.6%) did not have dermal sensitization
potential in guinea-pigs (Auletta, 1988c).
(ii) Neurotoxicity
Hens
Ten Leghorn hens (Gallus gallus domesticus) were given 12.5 mg/kg
bw mevinphos (purity, approx. 97%), this dose being slightly greater
than the oral LD50 for hens. Antidotal treatment was required, and
0.625 mg/kg bw atropine was given as clinically indicated; each hen
was also treated with 10 mg/kg bw pralidoxime chloride. Three of the
treated hens died, despite antidotal therapy; the remainder were
treated again on day 21 of the study as they had shown no signs of
neurotoxicity. Four positive controls were treated with tri- ortho-
tolylphosphate (90% mixture of isomers), but, when no signs of
neurotoxicity were seen, material from a different supplier was used
to treat the hens again at 21 days. Hens that survived to 42 days
were injected with sodium pentobarbital and perfused with neutral
buffered formalin. Brain, including the medulla, spinal cord, sciatic
nerve, and the proximal parts of the peroneal and tibial nerves, were
examined histopathologically. The treated birds had slight weight
loss, presumably due to the cholinergic effect of mevinphos, but no
evidence of mevinphos-induced delayed polyneuropathy was seen, either
clinically or histopathologically, whereas characteristic changes were
seen in the positive controls. Neuropathy target esterase was not
measured in this study (Barrett, 1988).
A single dose of mevinphos (76% alpha, 11% ß isomer) was given by
gavage to groups of 27 male and 27 female Sprague-Dawley Crl:CD BR
rats at doses of 0, 0.025 (17 rats of each sex), 0.1, 2, or 3.5
mg/kg bw. Seven animals of each sex per group were allocated for
neuropathological examination and underwent a functional observation
battery and tests for locomotor activity before treatment, at the time
of the peak effect (about 45 min after dosing), and on days 7 and 14.
These animals were killed at 15 days, and their brains were perfused
in situ and weighed; neuropathological examination was then carried
out on five rats of each sex in the control group and those at the
highest dose. The other 20 animals in each group (10 at 0.025 mg/kg
bw) were allocated for determinations of cholinesterase activity;
viability, clinical signs, and body weight were recorded, and the
functional observation battery and tests for locomotor activity were
conducted on five rats of each sex per group. Plasma, erythrocyte, and
brain (including regional) cholinesterase activities were measured
before treatment, at the time of the peak effect (about 45 min after
dosing), and on days 7 and 15 in five animals of each sex per group,
except for those at the lowest dose, in which activity was measured
only at the time of peak effect and on day 15. The numbers of animals
available for determination of cholinesterase activity were also
reduced in the group at the high dose because of mortality (see
below).
One male and five females at the highest dose died, and clinical
signs, including gait alterations, tremors, salivation, and
lacrimation, were observed in those at 2 or 3.5 mg/kg bw only on the
day of dosing. Major disturbances in the locomotor test battery
were observed in animals at these doses but not in controls. The
alterations included lacrimation, salivation, impaired mobility and
gait, clonic and tonic convulsions, tremors, bizarre behaviour, and
altered reflexes. No treatment-related signs were seen in animals
at 0.025 or 0.1 mg/kg bw. On the first day of the study, plasma
cholinesterase activity was reduced by 36-39% in rats at 2 mg/kg bw
and by 41-50% in those at 3.5 mg/kg bw in comparison with concurrent
controls. Erythrocyte acetylcholinesterase activity was unaffected,
while reductions of 20-25% were seen in acetylcholinesterase activity
in the brain stem and/or cerebral cortex, hippocampus, and olfactory
region of animals at 2 mg/kg bw and 19-36% in those at 3.5 mg/kg bw.
The only statistically significant reductions in activity were those
in the brain stem and cortex in males and in the hippocampus and brain
stem in females. Plasma and brain cholinesterase activities were
normal on days 7 and 14. No adverse effects were seen on body or brain
weight. All signs of neurotoxicity had reversed by the end of day 1,
and no treatment-related neuropathological signs were seen at any
dose. The NOAEL was thus 0.1 mg/kg bw, on the basis of clinical
effects and reduced brain acetylcholinesterase activity at higher
doses (Lamb, 1993).
Mevinphos was given by gavage in water to groups of 25 male and
25 female Sprague-Dawley Crl:CD BR rats for 91 days. The initial doses
for all animals were 0.025, 0.035, or 0.70 mg/kg bw per day, but the
dose for females at the high dose was reduced to 0.60 mg/kg bw per day
on day 32 because of the deaths of two animals. Controls received
the vehicle only. Viability, clinical signs, body weights, and
food consumption were recorded for all animals, and a functional
observational battery and locomotor activity tests were conducted on
10 animals of each sex per group. Necropsy was performed on all
animals that died during the study. Neuropathological changes were
evaluated in five animals of each sex in the control group and those
at the high dose, and, because of the mortality at the high dose, in
the females at the intermediate dose. The remaining 20 animals in each
group were evaluated for plasma, erythrocyte, and brain cholinesterase
activity in groups of five animals during weeks 3, 7, and 12 of the
study.
The main clinical sign was tremor in animals at the high dose
45 min after treatment, even after the dose for the females had been
lowered. Excessive salivation was seen in animals of each sex at this
dose, and lacrimation and râles were seen in females. Treatment-
related declines of 32-56% were seen in erythrocyte and plasma
cholinesterase activities in animals of each sex at the intermediate
and high doses; the decreases in erythrocyte acetylcholinesterase
activity were slightly greater (34-62%). Regional brain acetyl-
cholinesterase activity showed some variation, with decreases of 9-21%
in animals at the intermediate and high doses. If decreases of 15% are
biologically significant, the decreased acetylcholinesterase activity
of 21% in the mid-brain of males at the middle dose and of 17% in
females would result in a NOAEL at 0.025 mg/kg bw per day. No
treatment-related neuropathological change was seen (Lamb, 1994).
Administration of a single dose of mevinphos (purity, 89.6%)
to Crl:CD Br rats transdermally did not induce statistically or
biologically significant depressions in erythrocyte acetyl-
cholinesterase activity. A significant depression of plasma
cholinesterase activity occurred at 20 mg/kg bw (29% of control value)
and a marginal fall at 5 mg/kg bw (11% of control). Significant
depressions of brain acetylcholinesterase activity occurred at
20 mg/kg bw (18% of control) (Trimmer, 1989, 1990a).
The effects of mevinphos on flash-evoked potentials were studied
in gerbils, pigeons, cats, and squirrel monkeys, through electrodes
placed in the superior colliculus. Mevinphos reduced the inhibitory
pause in pigeons at doses of 0.1-0.15 mg/kg bw. This effect could not
be prevented by pretreatment with atropine methylnitrite (Revzin,
1978). Electroretinograms of Swiss white mice were examined
after injection of organophosphates, including mevinphos at an
intraperitoneal dose of 0.015 mg. Mevinphos was found to interfere
with retinal function by a direct action on the photoreceptor, by
inhibition of cholinesterase at synapses, and possibly by damaging the
bipolar and/or ganglion cells (Carricaburu et al., 1980).
3. Observations in humans
A number of incidents of poisoning with mevinphos have been
reported through the US pesticide incident monitoring scheme (Hodgson
& Smith, 1993). Poisoning with this compound is reported to be
amenable to treatment with oximes (Bismuth et al., 1993).
After administration of 25 µg/kg bw per day mevinphos to eight
male volunteers for 28 days, plasma and erythrocyte cholinesterase
activity fell throughout the study, with mean decreases of 12.6 and
19% of the respective pretreatment levels. Plasma cholinesterase
activity had returned almost to pretreatment levels within seven days
after the end of treatment, whereas the levels of erythrocyte activity
showed no sign of recovery at follow-up to 14 days. A decrease in
slow fibre motor conduction was also seen; there was an increase in
Achilles tendon reflex force but no effect on neuromuscular
transmission (Verbeck, 1977; Verbeck & Sallé, 1977).
Mevinphos was given to groups of five male volunteers at doses
of 1, 1.5, 2, or 2.5 mg per day for 30 days. Plasma cholinesterase
activity was slightly affected by the highest dose, while the mean
erythrocyte acetylcholinesterase activity was depressed by 20% on one
occasion each at 1.5 and 2 mg per day. Consistent, significant
inhibition of cholinesterase activity was seen only at the highest
dose, at which the erythrocyte acetylcholinesterase fell consistently
to a minimum of 25% at 27 days (Rider et al., 1975).
In agricultural workers with cholinergic symptoms and with mean
inhibition of plasma and erythrocyte cholinesterase activity of 15.6
and 5.6%, respectively, cholinesterase activity recovered at a daily
rate of 1.2 and 0.43% of the respective final levels of activity of
the enzyme over the 14 days after cessation of exposure (Coye et
al., 1986). Higher rates of recovery were observed during the first
11 days after poisoning with a mixture of mevinphos and phosphamidon:
the plasma activity increased by 3% per day and erythrocyte activity
by 0.8% per day. The workers were, however, treated with pralidoxime.
As noted previously, inhibition of plasma cholinesterase activity was
greater than that of erythrocytic enzyme (Midtling et al., 1985).
Human erythrocyte acetylcholinesterase is susceptible to
reactivation in vitro by a variety of pyridinium oximes, including
pralidoximes and obidoxime (Woreck et al., 1996).
Comments
Mevinphos is almost completely absorbed when administered orally
to rats; a large proportion of the absorbed material is biotransformed
to carbon dioxide. Both metabolites and unchanged mevinphos are
observed in the urine but very little in the faeces. Mevinphos
depresses cholinesterase activity in the plasma mre than in the
erythrocytes in experimental animals.
The oral LD50 values for mevinphos in laboratory rodents are
2-12 mg/kg bw. WHO has classified mevinphos as 'extremely hazardous'
(WHO, 1996).
In a three-month range-finding study, mice were fed diets
containing mevinphos at concentrations of 0, 0.5, 1, 2, or 10 ppm.
The NOAEL was 2 ppm, equal to 0.4 mg/kg bw per day, on the basis of
inhibition of brain acetylcholinesterase activity at 10 ppm.
In a 90-day study of toxicity, rats were given mevinphos by
gavage at doses of 0, 0.056, 0.56, 1.1, or 1.7 mg/kg bw per day were
used in males (the highest dose was decreased to 1.1 mg/kg bw per day
at day 36 because of high mortality) and 0, 0.011, 0.056, 0.56, or
0.84 mg/kg bw per day in females. The NOAEL was 0.056 mg/kg bw per
day, on the basis of clinical signs and depressed brain acetyl-
cholinesterase activity at higher doses. Dose-related increases in
mean cholesterol levels and increased relative liver weights were also
observed.
In a one-year study in dogs, mevinphos was administered in corn
oil in gelatine capsules at doses of 0, 0.025, 0.25 or 0.5 mg/kg bw
per day. The NOAEL was 0.25 mg/kg bw per day on the basis of clinical
signs and a reduction in brain acetylcholinesterase activity at the
highest dose.
In an 18-month study of toxicity and carcinogenicity, mice were
fed dietary concentrations of 0, 1, 10, or 25 ppm; cholinesterase
activity was not measured. There was no evidence of carcinogenicity.
In a two-year study of toxicity and carcinogenicity, rats were
given mevinphos by gavage in water on five days per week at doses of
0, 0.025, 0.35, or 0.70 mg/kg bw per day. On day 83 of the study, the
high dose of the females was reduced to 0.60 mg/kg bw per day because
of signs of toxicity. The NOAEL was 0.025 mg/kg bw per day on the
basis of inhibition of brain acetylcholinesterase activity and clinical
signs at higher doses. There was no evidence of carcinogenicity.
A two-generation study of reproductive toxicity was carried out
in which rats were treated by gavage at doses of 0, 0.05, 0.1, or
0.5 mg/kg bw mevinphos in water. The NOAEL was 0.1 mg/kg bw per day on
the basis of clinical signs and reduced brain acetylcholinesterase
activity at the highest dose. This dose also impaired growth and
fertility indices and lowered testicular weights in males and ovarian
weights in females.
In a study of developmental toxicity in rats, groups were given
mevinphos at daily doses of 0, 0.2, 0.75, or 1.2 mg/kg bw per day on
days 6-15 of gestation. High mortality (29%) was observed in the
high-dose group, which was therefore terminated; accordingly, a new
high-dose group at 1 mg/kg bw per day was added. There were no adverse
effects on uterine implantation or on fetal weight, sex distribution,
external appearance, or visceral or skeletal malformations in any
group. It was concluded that mevinphos is not embryotoxic, fetotoxic,
or teratogenic at doses < 1.0 mg/kg bw per day. The NOAEL for
maternal toxicity was 0.75 mg/kg bw per day on the basis of clinical
signs at higher doses.
In a study of developmental toxicity, mevinphos was administered
by gavage to pregnant rabbits at doses of 0, 0.05, 0.5, or 1.5 mg/kg
bw per day on days 7-19 of gestation; surviving animals were killed.
The NOAEL was 0.5 mg/kg bw per day, on the basis of maternal toxicity.
Mevinphos was neither teratogenic nor fetotoxic.
There was some evidence of genotoxic potential in vitro, but
the limited studies available indicate that such potential is not
exhibited in vivo.
In a study in hens, the oral dose of 12 mg/kg bw that was
administered was slightly greater than the oral LD50 value, and
antidotal treatment was required. There was no evidence of delayed
polyneuropathy, either clinically or histopathologically, whereas
characteristic changes were seen in positive controls. Neurotoxic
target esterase was not measured during this study.
Two studies of humans were available. In male volunteers given a
dose of 0.025 mg/kg bw per day, plasma and erythrocyte cholinesterase
activity decreased throughout the 28 days of the study to 13% and 19%
less than the respective pre-dose levels. In the second study, daily
doses of 1, 1.5, 2 or 2.5 mg were given to male volunteers for 30
days, and an NOAEL of 1 mg/day, equivalent to 0.016 mg/kg bw per day,
was derived; however, only five people, all men, per dose were
studied.
An ADI of 0-0.0008 mg/kg bw was established on the basis of the
NOAEL of 0.016 mg/kg bw per day in the 30-day study in volunteers,
using a 20-fold safety factor because of the small numbers in each
group. This ADI is supported by the LOAEL in rats of 0.35 mg/kg bw per
day and the NOAELs of 0.5 mg/kg bw per day in rabbits and 0.25 mg/kg
bw per day in dogs.
An acute reference dose for humans was derived from the 28-day
study in volunteers, on the basis of a dose of 0.025 mg/kg bw per day,
using a 10-fold safety factor.
Toxicological evaluation
Levels that cause no toxicological effect
Mouse: 2 ppm, equal to 0.4 mg/kg bw per day (inhibition of
brain acetylcholinesterase activity in a three-month
study of toxicity)
Rat: 0.025 mg/kg bw per day (two-year study of toxicity and
carcinogenicity)
0.1 mg/kg bw per day (study of reproductive toxicity)
Rabbit: 0.5 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
Dog: 0.25 mg/kg bw per day (one-year study of toxicity)
Human: 1 mg per day, equivalent to 0.016 mg/kg bw per day
(inhibition of cholinesterase activity in a 30-day
study of toxicity)
Estimate of acceptable daily intake for humans
0-0.0008 mg/kg bw
Acute reference dose
0.003 mg/kg bw
Studies that would provide information useful for continued evaluation
of the compound
Study of micronucleus formation in mice in vivo
Toxicological criteria for estimating guidance values for dietary and non-dietary exposure to mevinphos
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Oral, toxicity, rat LD50 = 2.2-6.1 mg/kg bw
Dermal, toxicity, rat LD50 > 20 mg/kg bw
Inhalation, 4 h, toxicity, rat LD50 = 7.3-12 mg/m3
Dermal, irritation, rabbit Slightly irritating
Ocular, irritation, rabbit Slightly irritating
Dermal, sensitization, guinea-pig Not sensitizing
Medium-term (1-26 weeks) Repeated oral, 3 months, mouse NOAEL = 0.4 mg/kg bw per day, inhibition of brain
acetylcholinesterase activity
Repeated oral, 90 days, rat NOAEL = 0.056 mg/kg bw per day
Repeated dermal, 21 days, rabbit NOAEL = 1.0 mg/kg bw per day
Repeated oral, reproductive toxicity, rat NOAEL = 0.1 mg/kg bw per day, maternal and
reproductive toxicity
Repeated oral, developmental toxicity, rat NOAEL = 0.75 mg/kg bw per day, maternal toxicity;
no developmental toxicity
Repeated oral, developmental toxicity, rabbit NOAEL = 0.5 mg/kg bw per day, maternal toxicity;
no developmental toxicity
Long-term (> 1 year) Repeated oral, 2 years, rat NOAEL = 0.025 mg/kg bw per day; inhibition of
brain acetylcholinesterase activity
Repeated oral, 1 year, dog NOAEL = 0.25 mg/kg bw per day; inhibition of
brain acetylcholinesterase activity
References
Atkinson, J.E. (1989) An eighteen month oncogenicity feeding study in
mice with mevinphos. Project No 86-300. Unpublished report dated 23
February 1989 from BioDynamics Inc., East Millstone, NJ, USA.
Submitted to WHO by Amvac Chemical Corp., City of Commerce, CA, USA.
Atkinson, J.E. (1990) A three month dietary range-finding study in
mice with mevinphos technical. Project No. 85-3025. Unpublished report
in West, J. & Roberts, K. (1990) Supplement to an eighteen month
oncogenicity study in mice with mevinphos. Project ID JCODE 1562.1.
Unpublished report dated 31 August 1990 from Jelinek Schwartz Connolly
and Freshman Inc., Washington DC, USA. Submitted to WHO by Amvac
Chemical Corp., City of Commerce, CA, USA.
Auletta, C.S. (1988a) Acute oral toxicity study in rats with
mevinphos. Unpublished report dated 23 March 1988, Project No. 4644-87
from BioDynamics Inc., East Millstone, NJ, USA. Submitted to WHO by
Amvac Chemical Corp., City of Commerce, CA, USA.
Auletta, C.S. (1988b) Acute dermal toxicity study in rabbits with
mevinphos. Unpublished report dated 23 March 1988, Project No. 4645-87
from BioDynamics Inc., East Millstone, NJ, USA. Submitted to WHO by
Amvac Chemical Corp., City of Commerce, CA, USA.
Auletta, C.S. (1988c) Dermal sensitization study in guinea pigs with
mevinphos. Unpublished report dated 23 March 1988, Project No. 4648-87
from BioDynamics Inc., East Millstone, NJ, USA. Submitted to WHO by
Amvac Chemical Corp., City of Commerce, CA, USA.
Barrett, D.S. (1988) Acute delayed neurotoxicity study in mature hens
with mevinphos. Unpublished report dated 26 July 1988, Project No
4685-87 from BioDynamics Inc., East Millstone, NJ, USA. Submitted to
WHO by Amvac Chemical Corp., City of Commerce, CA, USA.
Beyer, B.K. (1991a) Teratology study in rabbits. Study No. MRD-88-331.
Unpublished report dated 22 February 1991 from Exxon Biomedical
Sciences Inc, East Millstone, NJ, USA. Submitted to WHO by Amvac
Chemical Corp., City of Commerce, CA, USA.
Beyer BK (1991b) Multigeneration rat reproduction study. Study No.
MRD-88-331. Unpublished report dated 26th November 1991 from Exxon
Biomedical Sciences Inc., East Millstone, NJ, USA. Submitted to WHO by
Amvac Chemical Corp., City of Commerce, CA, USA.
Bismuth, C., Inns, R.H. & Marrs, T.C. (1993) Efficacy, toxicity and
clinical use of oximes in anticholinesterase poisoning. In:
Ballantyne, B. & Marrs, T.C, eds, Clinical and Experimental Toxicology
of Organophosphates and Carbamates, Oxford, Butterworths, pp. 555-577.
Carricaburu, P., Lacroix, R. & Lacroix, J. (1980) Electroretinographic
study of the white mouse intoxicated by organo-phosphorus: mevinphos
and malathion. Toxicol. Environ. Res., 3, 87-91.
Coye, M.J., Barnett, P.G. & Midtling, J.E. (1986) Clinical confirmation
of organophosphate poisoning of agricultural workers. Am. J. Ind. Med.,
10, 399-409.
Curren, R.D. (1990) Unscheduled DNA synthesis in rat primary
hepatocytes with mevinphos. Unpublished report dated 25 January 1990,
Study No. T8858.380 from Microbiological Associates, Inc., Rockville,
MD, USA. Submitted to WHO by Amvac Chemical Corp., City of Commerce,
CA, USA [full report not available to the Meeting].
Dean, B.J. (1974) Toxicity studies with phosdrin. Dominant lethal
assay in male mice after a single oral dose of phosdrin. Unpublished
report dated July 1974 Group research phosdrin, No. TLGR.0031.74 from
Shell Research Centre, Tunstall, Kent, United Kingdom. Submitted to
WHO by Amvac Chemical Corp., City of Commerce, CA, USA [full report
not available to the Meeting].
Dean, B.J. & Senner, K. (1974) Toxicity studies with phosdrin:
Chromosome studies on bone marrow cells of mice after two daily oral
doses of phosdrin. Group research report No. TLGR.0008.74. Unpublished
report dated February 1974 from Shell Research Centre, Tunstall, Kent,
United Kingdom. Submitted to WHO by Amvac Chemical Corp., City of
Commerce, CA, USA.
Deenihan, M.J. (1985) Mevinphos insecticide acute oral toxicity, acute
dermal toxicity, primary skin irritation, primary eye irritation.
NVP report No. X5A027G. Unpublished report dated 10 April 1985 from
Northview Pacific Laboratories Inc., Berkeley, CA, USA. Submitted to
WHO by Amvac Chemical Corp., City of Commerce, CA, USA.
Gaines, T. (1969) Acute toxicity of pesticides. Toxicol. Appl.
Pharmacol., 14, 515-534.
Hirka, G., Béres, E. & Schmidt, K. (1982) Use of cell and tissue
cultures in genetic toxicology. Mutat. Res., 97, 192.
Hodgson, M.J. & Smith, A.D. (1993) Commercial and residential
poisoning with anticholinesterases. In: Ballantyne, B. & Marrs, T.C.,
eds, Clinical and Experimental Toxicology of Organophosphates and
Carbamates, Oxford, Butterworths, pp. 352-363.
Hoffman, G.M. (1988) An acute inhalation toxicity study of mevinphos
in the rat. Unpublished report and letter dated 12 April 1988 from
BioDynamics Inc., East Millstone, NJ, USA. Submitted to WHO by Amvac
Chemical Corp., City of Commerce, CA, USA.
Jeffcoat A.R. & Coleman, D.P. (1993) Dermal absorption of mevinphos in
rats at three dose levels. RTI Study No. 64C-5494. Unpublished report
dated 31 August 1993 from Research Triangle Institute, Research
Triangle Park, NC, USA. Submitted to WHO by Amvac Chemical Corp., City
of Commerce, CA, USA.
Kangas, L. (1995) A 52-week oral (capsule) toxicity study of mevinphos
in the beagle dog. Laboratory ID 85746. Unpublished report dated
9 June 1995 from Bio-research Laboratories Ltd, Senneville, Quebec,
Canada. Submitted to WHO by Amvac Chemical Corp, City of Commerce, CA,
USA.
Keefe, R.T. (1992) 90-Day subchronic oral toxicity study in rats with
mevinphos (MRD-88-331) Laboratory ID 233170B. Unpublished report dated
4 November 1992 from Exxon Biomedical Sciences Inc., East Millstone,
NJ, USA. Submitted to WHO by Amvac Chemical Corp., City of Commerce,
CA, USA.
Kuhn, J.O. (1994) Acute oral toxicity study in rats (DOT). Laboratory
study number 0946-94. Unpublished report dated 28 March 1994 from
Stillmeadow Inc, Sugar Land, TX, USA. Submitted to WHO by Amvac
Chemical Corp,, City of Commerce, CA, USA.
Lamb, I.C. (1993) An acute neurotoxicity study of mevinphos in rats.
Study No. WIL-188006. Unpublished report dated 13 October 1993 from
WIL Research Laboratories Inc., Ashland, OH, USA. Submitted to WHO by
Amvac Chemical Corp., City of Commerce, CA, USA.
Lamb, I.C. (1994) A subchronic (13 week) neurotoxicity study of
mevinphos in rats. Study No. WIL-188007. Unpublished report dated
17 June 1994 from WIL Research Laboratories Inc., Ashland, OH, USA.
Submitted to WHO by Amvac Chemical Corp., City of Commerce, CA, USA.
Midtling, J.E., Barnett, P.G., Coye, M.J., Velasco, A.R., Romero, P.,
Clements, C.L., O'Malley, M.A., Tobin, M.W., Rose, T.G. & Monosson,
I.H. (1985) Clinical management of field worker organophosphate
poisoning. West. J. Med., 142, 514-518.
Plutnick, R.T. (1994) 2-Year chronic toxicity/oncogenicity study in
rats with mevinphos (MRD-88-331). Laboratory ID 233170C. Unpublished
report dated 3 January 1994 from Exxon Biomedical Sciences Inc., East
Millstone, NJ, USA. Submitted to WHO by Amvac Chemical Corp., City of
Commerce, CA, USA.
Reddy, V., Freeman, T., Little, L. & Cannon, M. (1991) Disposition
and metabolism of 14C-labeled mevinphos in rats (preliminary and
definitive study). Laboratory ID MRI 9485B. Unpublished report dated
4 April 1991 from Midwest Research Institute, Kansas City, MI, USA.
Submitted to WHO by Amvac Chemical Corp., City of Commerce, CA, USA.
Revzin, A.M. (1978) Effects of organophosphate pesticides and alcohol
on visual mechanisms. In: Merigan, W.H. & Weiss, B., eds, Neurotoxicity
of the Visual System, New York, Raven Press, pp. 255-268.
Rider, J.A., Puletti, E.J. & Swader, J.I. (1975) The minimal oral
toxicity level for mevinphos in man. Toxicol. Appl. Pharmacol., 32,
97-100.
San, R.H.C. & Schadly, M.B. (1989) Salmonella/mammalian microsome
plate incorporation mutagenicity assay (Ames test) with a confirmatory
assay with mevinphos. Study No. T8858.501014. Unpublished report dated
23 October 1989 from Microbiological Associates Inc., Rockville, MD,
USA. Submitted to WHO by Amvac Chemical Corp., City of Commerce, CA,
USA.
Schroeder, R.E. & Daly, I.W. (1987) Mevinphos - A teratology study in
rats with mevinphos. Laboratory ID 85-3009. Unpublished report dated
2 March 1987 from BioDynamics Inc., East Millstone, NJ, USA. Submitted
to WHO by Amvac Chemical Corp., City of Commerce, CA, USA.
Skinner, C.S. & Kilgore, W.W. (1982) Acute dermal toxicities of various
organophosphate insecticides in mice. J. Toxicol. Environ. Health,
9, 491-497.
Trimmer, G.W. (1989) Acute dermal toxicity study in the rat phase 1
(MRD-88-331: mevinphos). Laboratory ID 233106. Unpublished report
dated 23 August 1989 from Exxon Biomedical Sciences Inc., East
Millstone, NJ, USA. Submitted to WHO by Amvac Chemical Corp., City of
Commerce, CA, USA.
Trimmer, G.W. (1990a) Acute dermal toxicity study in the rat phase II
cholinesterase determination (MRD-88-331: mevinphos). Laboratory ID
233177A. Unpublished report dated 20 July 1990 from Exxon Biomedical
Sciences Inc., East Millstone, NJ, USA. Submitted to WHO by Amvac
Chemical Corp., City of Commerce, CA, USA.
Trimmer, G.W. (1990b) 21-Day repeated dermal study in the rabbit
(MRD-88-331-mevinphos). Laboratory ID 233109. Unpublished report dated
4 April 1990 from Exxon Biomedical Sciences Inc., East Millstone, NJ,
USA. Submitted to WHO by Amvac Chemical Corp., City of Commerce, CA,
USA.
Verberk, M.M. (1977) Incipient cholinesterase inhibition in volunteers
ingesting monocrotophos or mevinphos for one month. Toxicol. Appl.
Pharmacol.,42, 345-350.
Verberk, M.M. & Sallé, H.J.A. (1977) Effects on nervous function of
volunteers ingesting mevinphos for 1 month. Toxicol. Appl. Pharmacol.,
42, 351-358.
WHO (1996) The WHO Recommended Classification of Pesticides by Hazard
and Guidelines to Classification 1996-1997 (WHO/PCS/96.3), International
Programme on Chemical Safety, Geneva.
Worek, F., Kirchner, T., Bäcker, M. & Szinicz, L. (1996) Reactivation
by various oximes of humans erythrocyte acetylcholinesterase inhibited
by different organophosphorus compounds. Arch. Toxicol., 70,
497-503.
Table 1. Acute toxicity of mevinphos in experimental animals
Species Strain Sex Route Purity LD50 (mg/kg bw) Reference
(%) or LC50 (mg/litre)
(95% CI)
Mouse Swiss Webster Male Dermal 99 12 (6-24) Skinner & Kilgore
(feet) (1982)
Rat Sherman Male Oral NR 6.1 Gaines (1969)
Female 3.7
Rat Sprague-Dawley Male Oral NR 4.1 (3.5-5.0) Deenihan (1985)
Female 2.2 (1.8-2.6)
Rat Sprague-Dawley-derived CD Male Oral 97.7 3.5a Auletta (1988a)
Female 2.3 (1.0-3.6)
Rat Albino HSD:SD Male Oral Unknownb > 5 Kuhn (1994)
Female > 5
Rat Sherman Male Dermal NR 4.7 Gaines (1969)
Female 4.2
Rat Sprague-Dawley Crl:CD BR Male Dermal 90 >20 Trimmer (1989)
Female >20
Rat Sprague-Dawley CD Male Inhalationc 100 12 Hoffman (1988)
Female 7.3
Rabbit New Zealand white Male, Dermal NR 57 (38-86) Deenihan (1985)
female
Rabbit New Zealand white Male Dermal 99.3 51 (31-71) Auletta (1988b)
Female 60 (23-97)
Chicken White Leghorn pullets Female Oral approx. 97 11 (3.2-19) Barrett (1988)
CI, confidence interval; NR, not reported
a CI could not be calculated owing to distribution of deaths
b Phosdrin 4 EC
c 4-h exposure; only 3 h at highest dose as all animals had died
(b) Short-term toxicity
Mice
In a three-month range-finding study, groups of 10 male and 10
female CD-1 mice were fed diets containing mevinphos (66.5% alpha,
21.2% ß isomer) at 0, 0.5, 1, 2, or 10 ppm, equal to 0, 0.1, 0.2, 0.4,
and 2 mg/kg bw per day for males and 0, 0.1, 0.3, 0.5, and 2.7 mg/kg
bw per day for females. Clinical observations, body weight, and food
consumption were reported before treatment and weekly after the start
of exposure, and plasma and erythrocyte cholinesterase activities
were measured in five animals of each sex per group at week 7 and
at termination. After three months, the animals were killed and
autopsied, and brain acetylcholinesterase activity was measured in
five animals of each sex per group. Organs from controls and animals
at the high dose were examined grossly, and selected tissues were
examined microscopically. Body weight and food consumption were
similar in all groups, and no deaths occurred; clinical parameters
were similar in all groups. Plasma cholinesterase activity was
decreased by 37-49% in animals of each sex at 2 and 10 ppm at week 7.
At termination, similar findings were seen in males, but the decreased
plasma cholinesterase activity in females was confined to those at
10 ppm. Brain acetylcholinesterase activity was decreased by 16 and
22% in males and females at 10 ppm, respectively, in comparison with
controls. The NOAEL was 2 ppm, equal to 0.4 mg/kg bw per day, on the
basis of inhibition of brain acetylcholinesterase activity at 10 ppm
(Atkinson, 1990).
Rats
Groups of 10 Sprague-Dawley Crl:CD BR rats received mevinphos
(74.5% alpha, 15.1% ß isomer) by gavage for 90 days at doses of active
ingredient of 0.056, 0.56, 1.1, or 1.7 mg/kg bw per day for males and
0.011, 0.056, 0.56, or 0.84 mg/kg bw per day for females. Five males
at the two highest doses died, and the highest dose was decreased to
1.1 mg/kg bw per day on day 36; the groups at the two highest doses
were then combined for most statistical purposes. Additionally, one
female at 0.56 mg/kg bw per day died before scheduled termination.
Clinical signs, including pinpoint pupils, oral and ocular discharges,
and tremors were seen at doses > 0.56 mg/kg bw per day in animals
of each sex. No statistically significant differences in body weight
or food consumption were noted. Inhibition of plasma cholinesterase
activity > 15% was seen at week 7 and terminally in males at doses
> 0.56 mg/kg bw per days and in females at doses > 0.056 mg/kg
bw per day. Brain acetylcholinesterase activity was inhibited by
> 15% at termination in animals of each sex at doses > 0.56 mg/kg
bw per day. No significant depression of erythrocyte acetyl-
cholinesterase activity was seen. There was a dose-related increase
in mean cholesterol levels, which were statistically significantly
increased in males at the high dose. No statistically significant
differences in absolute or relative organ weights were seen between
groups, but a dose-related trend in increased relative liver weights
was seen in animals of each sex. Slight vacuolation in centrilobular
and midzonal hepatocytes was seen in two male rats at the highest
dose. The NOAEL was 0.056 mg/kg bw per day, on the basis of clinical
signs and depressed brain acetylcholinesterase activity at higher
doses (Keefe, 1992).
Dogs
Gelatine capsules containing mevinphos (75.1% alpha, 11.8% ß
isomer) in corn oil were given to groups of five male and five female
beagle dogs at doses of 0 or 0.5 mg/kg bw per day and to four males
and four females at 0.025 or 0.25 mg/kg bw per day for one year. No
deaths occurred during the study. The only clinical sign attributable
to the test material was a higher prevalence of vomiting in the dogs
at the high dose than in the controls. No significant differences in
body weight or food consumption were noted, and no treatment-related
changes were seen at ophthalmoscopic examination at weeks 12, 26, and
52. Blood was taken before treatment and at weeks 6, 12, 39, and 51
for laboratory analyses. No biologically significant haematological or
biochemical changes were seen, other than changes in cholinesterase
activity. Plasma and erythrocyte cholinesterase activities were
determined on blood samples taken before the start of treatment, 3 h
after dosing at 4, 12, 39, and 51 weeks, and before dosing at weeks
39 and 51. Brain acetylcholinesterase activity was measured at
termination of the study. At many of these time intervals, both
erythrocyte and plasma cholinesterase activities were depressed,
particularly at the two higher doses. In dogs at the lowest dose, a
depression > 15% was seen in erythrocyte enzyme activity at week 25
in males and females, at week 39 in males before treatment and in
females before and after treatment, and at week 51 in males before and
after treatment and in females after treatment. At this dose, plasma
cholinesterase activity was depressed by > 15% at 39 and 51 weeks in
females after treatment. Statistically and biologically significant
inhibition of brain acetylcholinesterase activity was observed only in
males at the highest dose, in which the activity was 77% of that in
concurrent controls. Higher absolute thyroid/parathyroid weights
were observed in females at the highest dose and higher relative
thyroid/parathyroid weights in females at the intermediate dose. No
associated histopathological abnormality was discerned, and these
findings are considered not to be significant. No treatment-related
pathological finding was seen at autopsy. The NOAEL was thus
0.25 mg/kg bw per day, on the basis of clinical signs and a reduction
in brain acetylcholinesterase activity at the highest dose (Kangas,
1995).
(c) Long-term toxicity and carcinogenicity
Mice
On the basis of a range-finding study, mevinphos (purity, 87.7%;
66.5% alpha, 21.2% ß isomer) was administered to four groups of 50
CD-1 mice of each sex at dietary concentrations providing doses of 0,
1, 10, or 25 ppm, equal to 0, 0.1, 1.5, and 3.7 mg/kg bw per day for
males and 0, 0.1, 1.9, and 4.8 mg/kg bw per day for females, for 18
months. Physical examinations were performed each week and body weight
and food consumption before the start of the study, weekly thereafter
for 13 weeks on alternate weeks until week 26, and thence monthly.
Blood smears for evaluating the differential leukocyte count and
erythrocyte morphology were taken from 10 animals of each sex per
group at 12 and 18 months. Treatment had few effects, but the survival
rate was somewhat reduced in males at the low dose, with only 20
survivors, in comparison with 27 male controls, 26 at the middle dose,
and 24 at the high dose. In females, the mortality rate was similar in
all groups (36-40%). During the first three months of the study, the
mean body weights of animals at the highest dose were lower than those
of controls; this effect lasted up to week 16 in males and week 12 in
females. Females at the two highest doses had lower leukocyte counts
than controls at 18 but not at 12 months. No treatment-related gross
or microscopic pathological changes were seen, nor were there
treatment-related changes in organ weights. Although tumours were
observed in a number of organs, especially the liver and lungs, there
was no evidence of treatment-related neoplasia in any treated group.
Cholinesterase activity was not measured in this study, as it had
been evaluated in the range-finding study, in which the NOAEL for
inhibition of brain acetylcholinesterase activity was 2 ppm. The NOAEL
in the main study was 25 ppm, equal to 3.7 mg/kg bw per day, as the
transient effect on body weight in mice at the high dose can be
ignored (Atkinson, 1989).
Rats
Groups of 80 male and 80 female Crl:CD BR rats were given
mevinphos (purity, 85.7%; 74.9% alpha, 10.8% ß isomer) by gavage in
water on five days per week for two years at doses of 0, 0.025, 0.35,
or 0.70 mg/kg bw per day. In females, the high dose was reduced to
0.60 mg/kg bw per day on day 83 of the study because of signs of
acute toxicity. Ten rats from each group were killed at 12 months for
histopathological examination, 10 rats from each group were used for
clinical chemical determinations, and a further 10 were used to
measure cholinesterase activity and were killed at 104 weeks. Clinical
examinations were conducted weekly, and body weights were recorded
before the start of the study, at the start of dosing, and thereafter
weekly for 13 weeks and every four weeks afterwards. Food consumption
was also measured weekly for 13 weeks and every four weeks thereafter.
Clinical chemical, haematological, and urinary analyses were undertaken
and plasma and erythrocyte cholinesterase activities were measured
before treatment, at 3, 6, 1,2 and 18 months, and at termination. Brain
acetylcholinesterase activity was measured at the interim kill and at
the end of the study, but there were too few survivors at the end of
the study for useful intergroup comparisons to be made. Ophthalmoscopic
examinations were done before the start of the study and before
termination. Full histopathological examinations were performed on the
10 animals from each group killed at 12 months and on the survivors at
two years. Selected organs were weighed and examined microscopically.
Less than 40% of the rats survived to termination at 104 weeks,
and there was a dose-related reduction in survival in males, the rate
being clearly reduced at the highest dose; there were no significant
differences in mortality among female rats. Clinical signs typical of
anticholinesterase poisoning, including tremors, were observed after
administration of both the medium and high doses. No treatment-related
changes in body weight, food consumption, or haematological, clinical
chemical, urinary, or ophthalmoscopic parameters were discerned,
except for cholinesterase activity. Plasma, erythrocyte, and brain
cholinesterase activity was diminished in the animals at the middle
and high doses but not in those at the low dose; decreases in
erythrocytic acetylcholinesterase activity > 20% were not observed
in animals of either sex at any dose, but a depression of 20% was
observed at six months in females treated with mevinphos at 0.60 mg/kg
bw per day. Although inhibition of plasma cholinesterase activity was
not seen at the low dose, reductions of 38-51% were seen in males
at the middle dose and 47-61% in males at the high dose; plasma
cholinesterase activity was depressed by 50-67% in females at the
middle dose and 66-71% at the high dose. Brain acetylcholinesterase
activity was depressed by 27% in males at the middle dose, 53% in
males at the high dose, 43% in females at the middle dose, and 55% in
females at the high dose at the interim kill. No treatment-related
effects were seen on histopathological examination. A low incidence of
combined incidental and fatal hepatocellular adenomas was seen (0/67,
1/67, 1/69, and 2/68 in males and 1/70, 0.66, 0/67, and 3/67 in
females), which gave a dose-response relationship. One female at
the high dose had a hepatocellular carcinoma, giving a significant
dose-response relationship for all hepatocellular tumours. The numbers
involved were small, there was no dose-response relationship for liver
carcinomas alone or for adenomas and carcinomas combined in males, and
the incidence was well within that of historical controls. Hence, it
was concluded that there was no evidence of any treatment-associated
tumours. The NOAEL was 0.025 mg/kg bw per day on the basis of
inhibition of brain acetylcholinesterase activity and clinical signs
at doses > 0.35 mg/kg bw per day (Plutnick, 1994).
(d) Reproductive toxicity
In a two-generation study, groups of 35 male and 35 female Crl:CD
BR (Sprague-Dawley) VAF/Plus rats, seven weeks of age, received
mevinphos (purity, 89.6%; 74.5% alpha, 15.1% ß isomer) in water by
gavage at doses of 0, 0.05, 0.1, or 0.5 mg/kg bw. Dosing was adjusted
to allow for the low purity of the material. The F0 rats were dosed
daily for 10 weeks before being assigned randomly, within the dose
groups, into mating pairs at least 11 weeks after the start of dosing.
Dosing was continued during mating, and the F0 females continued to
be treated during gestation and lactation until weaning of the F1
litter 21 days post partum. Offspring were counted, sexed, weighed
(excluding postnatal day 0), and examined externally on postnatal days
0, 1, 4, 7, 14, and 21; litters were culled to four males and four
females. The F1 litters were dosed from 28 days post partum for at
least 11 weeks before mating and also during the mating period. F1
females were dosed during gestation and lactation until weaning on day
21 after birth. Clinical observations were made and body weights
measured before selection of the F0 animals on the first day of
dosing and thereafter weekly in both generations. Observations were
made more frequently in the F0 generation during gestation (days 0,
7, 14, and 21) and on the same days post partum. Erythrocyte,
plasma, and brain cholinesterase activity was measured in all animals
that survived to termination. Necropsies were performed on all
mated animals, all decedent pups, and pups killed in extremis.
Histopathological examinations were performed only on the controls
and animals at the high dose, except that the ovaries of all F1
females were examined.
Treatment-related clinical signs were seen in F0 females at the
high dose, which included ataxia, fine or coarse tremors, pinpoint
pupils, and oral discharge. The growth of F1 animals of each sex was
significantly reduced, and some reduction in the body weights of F1
males and females at the high dose was observed before weaning on
postnatal days 4-21. A reduction in the body weights of F1 male
offspring at the high dose may represent continuation of the reduction
in the F0 males before weaning. The growth of F2 males at the high
dose was also reduced. Cholinesterase inhibition was observed in both
generations but was generally more severe in F1 animals. Significant
inhibition of brain acetylcholinesterase activity was observed in
animals of each sex at the high dose, while no significant inhibition
of erythrocyte cholinesterase activity was seen at any dose; inhibition
of plasma cholinesterase activity extended to animals at the middle
dose and to those at the low dose in the F1 generation. F1 animals
at the high dose showed a reduction in the absolute weight of the
testes or epididymides (12%) and in the relative ovarian weight
(17%). The former may be a reflection of the lower total body weight
of these animals, but the F1 males in all treated groups had lower
mean mating and fertility indices than controls; although these
effects were not statistically significant, they may be associated
with the lower testicular weight observed. The effect on the ovaries
may be a treatment-related effect, as some female rats at the highest
dose had fewer or absent corpora lutea. The frequency of this
pathological observation did not, however, attain statistical
significance, and there were no significant effects on female
fecundity or fertility indices; moreover, the live litter sizes of
the animals at the high dose and the controls were comparable. The
NOAEL was 0.1 mg/kg bw per day on the basis of the presence of clinical
signs and reduced brain acetylcholinesterase activity in animals at
the highest dose, at which impaired growth, impaired fertility
indices, and lower testicular weights were seen in males and lower
ovarian weights in females (Beyer, 1991b).
(e) Developmental toxicity
Rats
Mevinphos (65.5% alpha isomer) was administered to four groups of
24 mated CD rats by gavage in water. One group served as controls,
while the other groups were given mevinphos at daily doses of 0.2,
0.75, or 1.2 mg/kg bw per day on days 6-15 of gestation. A high
mortality rate (29.2%) was observed in rats at the high dose, which
was therefore terminated and a new group receiving 1 mg/kg bw per day
was added. Females were weighed and examined at regular intervals,
while food consumption was recorded on days 0-6, 6-10, 10-15, and
15-20 of gestation. The animals were killed on day 20 of gestation and
examined at autopsy; corpora lutea and uterine implantations were
counted, and fetuses were weighed, sexed, and examined grossly for
malformations. One-half of the fetuses in each litter were processed
for visceral examination and the remainder stained for evaluation of
skeletal changes. Deaths were observed only in the prematurely
terminated group at 1.2 mg/kg bw per day. A dose-related decrease in
mean weight gain was seen during days 6-15 of gestation, but none of
the decreases attained statistical significance. Clinical signs of
cholinergic poisoning (tremors) were seen in about half the animals at
the high dose, and excessive salivation was seen in four animals in
that group. No indication of maternal toxicity was seen in the other
groups, apart from tremor in two rats at the middle dose on day 15.
There were no adverse effects on uterine implantation, fetal weight,
fetal sex distribution, or the external appearance of fetuses, and
there were no visceral or skeletal abnormalities in any treated group.
The NOAEL for maternal toxicity was 0.75 mg/kg bw per day on the basis
of clinical signs at the next highest dose; the NOAEL for developmental
toxicity was 1.0 mg/kg bw per day, the highest dose tested (Schroeder &
Daly, 1987).
Rabbits
Mevinphos (purity, 89.6%; 74.5% alpha, 15.1% ß isomer) was
administered in water by gavage to groups of 20 pregnant New Zealand
white rabbits at doses of 0, 0.05, 0.5, or 1.5 mg/kg bw per day on
days 7-19 of gestation. Surviving animals were killed on day 29. Body
weights were measured on days 0 and 7 of gestation, every three days
until day 25, and on day 29 of gestation. Plasma and erythrocyte
cholinesterase activity was determined in blood taken from dams about
3 h after treatment on the last day. After the animals had been
killed, gross necropsy was carried out, and body weight and the weight
of the uterus plus ovary were determined. The uterine contents were
examined, live fetuses were killed, and all fetuses were weighed and
examined externally. The viscera and skeleton were examined for
malformations, and half of the fetuses from each litter were examined
for the presence of brain abnormalities. Brain acetylcholinesterase
activity was not measured. Maternal toxicity was seen in animals at
the high dose, resulting in one death; corrected body weights were
also decreased in that group on day 29 of gestation. Erythrocyte
acetylcholinesterase activity was statistically significantly reduced
in all three treated groups. The decreases were not biologically
significantly in those at the medium and low doses (13% and 6%), but
the reduction of 18% in animals at the high dose can be considered to
be marginally biologically significant. Plasma cholinesterase activity
was more sensitive to mevinphos, being significantly reduced at the
two higher doses. No significantly different findings among the groups
were made at autopsy. Mean fetal body weights and the crown-rump
lengths of the treated pups were similar to those of controls. An
increased incidence of accessory vessels was seen at 1.5 mg/kg bw
per day, which was not considered by the authors to be related to
treatment, as these are normal variations. Hypoplastic hyoids and
unossified forepaws were also seen at increasing incidence with dose,
but the difference in incidence between animals at the high dose and
the controls did not achieve clinical significance. The NOAEL was
0.5 mg/kg bw per day on the basis of a marginally biologically
significant decrease in erythrocyte acetylcholinesterase activity in
animals at the high dose (Beyer, 1991a).
(f) Genotoxicity
Studies on the genotoxicity of mevinphos in vitro and in vivo are
summarized in Table 2.
Table 4. Recent tests for the genotoxicity of mevinphos
End-point Test system Concentration Purity Results Reference
(%)
In vitro
Reverse mutation S. typhimurium TA98, 100-10 000 µg/plate 89.6 Negative in TA98 San & Schadly
TA100, TA1535, (74.5% alpha, with and without (1989)
TA1537, TA1538 15.1% ß) S9
Sister chromatid Chinese hamster 10-5-10-3 mol/litre NR Positive Hirka et al.
exchange ovary cells (1982)
Unscheduled DNA Rat primary hepatocytes 0.0003-0.3 µl/ml NR Negative Curren (1990)
synthesis
In vivo
Chromosomal CF-1 mouse, bone 1.5, 3 mg/kg bw per 70% alpha Negative Dean & Senner
aberration marrow day orally (1974)
Dominant lethal Mouse 1.5; 3, 6 mg/kg bw NR Negative Dean (1974)
mutation
NR, not reported
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Slight dermal irritation and erythema were observed in five of
six New Zealand white rabbits and oedema in the sixth. Most of the
effects had disappeared by 72 h. In the same study, the material
produced slight temporary ocular irritation in six New Zealand white
rabbits (Deenihan, 1985).
Groups of five male and five female New Zealand white rabbits
received applications of mevinphos (74.5% alpha, 15.1% ß isomer)
on the clipped unabraded skin of the back at doses of 0, 0.1, 1,
or 10 mg/kg bw per day, on five days per week for three weeks.
The material was not irritating. Males at the highest dose had a
significant increase in relative brain weight and a decrease in the
liver:brain and kidney:brain weight ratios. A dose-related decrease in
brain, erythrocyte, and plasma cholinesterase activity was also seen.
Plasma, erythrocyte, and brain cholinesterase activities at termination
were inhibited in males and females at the highest dose by > 15% in
comparison with concurrent controls. Erythrocyte acetylcholinesterase
activity was the least inhibited and brain acetylcholinesterase
activity the most inhibited in animals of each sex. The NOAEL for
transdermal exposure was thus 1 mg/kg bw per day (Trimmer, 1990b).
Mevinphos (purity, 93.6%) did not have dermal sensitization
potential in guinea-pigs (Auletta, 1988c).
(ii) Neurotoxicity
Hens
Ten Leghorn hens (Gallus gallus domesticus) were given 12.5 mg/kg
bw mevinphos (purity, approx. 97%), this dose being slightly greater
than the oral LD50 for hens. Antidotal treatment was required, and
0.625 mg/kg bw atropine was given as clinically indicated; each hen
was also treated with 10 mg/kg bw pralidoxime chloride. Three of the
treated hens died, despite antidotal therapy; the remainder were
treated again on day 21 of the study as they had shown no signs of
neurotoxicity. Four positive controls were treated with tri- ortho-
tolylphosphate (90% mixture of isomers), but, when no signs of
neurotoxicity were seen, material from a different supplier was used
to treat the hens again at 21 days. Hens that survived to 42 days
were injected with sodium pentobarbital and perfused with neutral
buffered formalin. Brain, including the medulla, spinal cord, sciatic
nerve, and the proximal parts of the peroneal and tibial nerves, were
examined histopathologically. The treated birds had slight weight
loss, presumably due to the cholinergic effect of mevinphos, but no
evidence of mevinphos-induced delayed polyneuropathy was seen, either
clinically or histopathologically, whereas characteristic changes were
seen in the positive controls. Neuropathy target esterase was not
measured in this study (Barrett, 1988).
A single dose of mevinphos (76% alpha, 11% ß isomer) was given by
gavage to groups of 27 male and 27 female Sprague-Dawley Crl:CD BR
rats at doses of 0, 0.025 (17 rats of each sex), 0.1, 2, or 3.5
mg/kg bw. Seven animals of each sex per group were allocated for
neuropathological examination and underwent a functional observation
battery and tests for locomotor activity before treatment, at the time
of the peak effect (about 45 min after dosing), and on days 7 and 14.
These animals were killed at 15 days, and their brains were perfused
in situ and weighed; neuropathological examination was then carried
out on five rats of each sex in the control group and those at the
highest dose. The other 20 animals in each group (10 at 0.025 mg/kg
bw) were allocated for determinations of cholinesterase activity;
viability, clinical signs, and body weight were recorded, and the
functional observation battery and tests for locomotor activity were
conducted on five rats of each sex per group. Plasma, erythrocyte, and
brain (including regional) cholinesterase activities were measured
before treatment, at the time of the peak effect (about 45 min after
dosing), and on days 7 and 15 in five animals of each sex per group,
except for those at the lowest dose, in which activity was measured
only at the time of peak effect and on day 15. The numbers of animals
available for determination of cholinesterase activity were also
reduced in the group at the high dose because of mortality (see
below).
One male and five females at the highest dose died, and clinical
signs, including gait alterations, tremors, salivation, and
lacrimation, were observed in those at 2 or 3.5 mg/kg bw only on the
day of dosing. Major disturbances in the locomotor test battery
were observed in animals at these doses but not in controls. The
alterations included lacrimation, salivation, impaired mobility and
gait, clonic and tonic convulsions, tremors, bizarre behaviour, and
altered reflexes. No treatment-related signs were seen in animals
at 0.025 or 0.1 mg/kg bw. On the first day of the study, plasma
cholinesterase activity was reduced by 36-39% in rats at 2 mg/kg bw
and by 41-50% in those at 3.5 mg/kg bw in comparison with concurrent
controls. Erythrocyte acetylcholinesterase activity was unaffected,
while reductions of 20-25% were seen in acetylcholinesterase activity
in the brain stem and/or cerebral cortex, hippocampus, and olfactory
region of animals at 2 mg/kg bw and 19-36% in those at 3.5 mg/kg bw.
The only statistically significant reductions in activity were those
in the brain stem and cortex in males and in the hippocampus and brain
stem in females. Plasma and brain cholinesterase activities were
normal on days 7 and 14. No adverse effects were seen on body or brain
weight. All signs of neurotoxicity had reversed by the end of day 1,
and no treatment-related neuropathological signs were seen at any
dose. The NOAEL was thus 0.1 mg/kg bw, on the basis of clinical
effects and reduced brain acetylcholinesterase activity at higher
doses (Lamb, 1993).
Mevinphos was given by gavage in water to groups of 25 male and
25 female Sprague-Dawley Crl:CD BR rats for 91 days. The initial doses
for all animals were 0.025, 0.035, or 0.70 mg/kg bw per day, but the
dose for females at the high dose was reduced to 0.60 mg/kg bw per day
on day 32 because of the deaths of two animals. Controls received
the vehicle only. Viability, clinical signs, body weights, and
food consumption were recorded for all animals, and a functional
observational battery and locomotor activity tests were conducted on
10 animals of each sex per group. Necropsy was performed on all
animals that died during the study. Neuropathological changes were
evaluated in five animals of each sex in the control group and those
at the high dose, and, because of the mortality at the high dose, in
the females at the intermediate dose. The remaining 20 animals in each
group were evaluated for plasma, erythrocyte, and brain cholinesterase
activity in groups of five animals during weeks 3, 7, and 12 of the
study.
The main clinical sign was tremor in animals at the high dose
45 min after treatment, even after the dose for the females had been
lowered. Excessive salivation was seen in animals of each sex at this
dose, and lacrimation and râles were seen in females. Treatment-
related declines of 32-56% were seen in erythrocyte and plasma
cholinesterase activities in animals of each sex at the intermediate
and high doses; the decreases in erythrocyte acetylcholinesterase
activity were slightly greater (34-62%). Regional brain acetyl-
cholinesterase activity showed some variation, with decreases of 9-21%
in animals at the intermediate and high doses. If decreases of 15% are
biologically significant, the decreased acetylcholinesterase activity
of 21% in the mid-brain of males at the middle dose and of 17% in
females would result in a NOAEL at 0.025 mg/kg bw per day. No
treatment-related neuropathological change was seen (Lamb, 1994).
Administration of a single dose of mevinphos (purity, 89.6%)
to Crl:CD Br rats transdermally did not induce statistically or
biologically significant depressions in erythrocyte acetyl-
cholinesterase activity. A significant depression of plasma
cholinesterase activity occurred at 20 mg/kg bw (29% of control value)
and a marginal fall at 5 mg/kg bw (11% of control). Significant
depressions of brain acetylcholinesterase activity occurred at
20 mg/kg bw (18% of control) (Trimmer, 1989, 1990a).
The effects of mevinphos on flash-evoked potentials were studied
in gerbils, pigeons, cats, and squirrel monkeys, through electrodes
placed in the superior colliculus. Mevinphos reduced the inhibitory
pause in pigeons at doses of 0.1-0.15 mg/kg bw. This effect could not
be prevented by pretreatment with atropine methylnitrite (Revzin,
1978). Electroretinograms of Swiss white mice were examined
after injection of organophosphates, including mevinphos at an
intraperitoneal dose of 0.015 mg. Mevinphos was found to interfere
with retinal function by a direct action on the photoreceptor, by
inhibition of cholinesterase at synapses, and possibly by damaging the
bipolar and/or ganglion cells (Carricaburu et al., 1980).
3. Observations in humans
A number of incidents of poisoning with mevinphos have been
reported through the US pesticide incident monitoring scheme (Hodgson
& Smith, 1993). Poisoning with this compound is reported to be
amenable to treatment with oximes (Bismuth et al., 1993).
After administration of 25 µg/kg bw per day mevinphos to eight
male volunteers for 28 days, plasma and erythrocyte cholinesterase
activity fell throughout the study, with mean decreases of 12.6 and
19% of the respective pretreatment levels. Plasma cholinesterase
activity had returned almost to pretreatment levels within seven days
after the end of treatment, whereas the levels of erythrocyte activity
showed no sign of recovery at follow-up to 14 days. A decrease in
slow fibre motor conduction was also seen; there was an increase in
Achilles tendon reflex force but no effect on neuromuscular
transmission (Verbeck, 1977; Verbeck & Sallé, 1977).
Mevinphos was given to groups of five male volunteers at doses
of 1, 1.5, 2, or 2.5 mg per day for 30 days. Plasma cholinesterase
activity was slightly affected by the highest dose, while the mean
erythrocyte acetylcholinesterase activity was depressed by 20% on one
occasion each at 1.5 and 2 mg per day. Consistent, significant
inhibition of cholinesterase activity was seen only at the highest
dose, at which the erythrocyte acetylcholinesterase fell consistently
to a minimum of 25% at 27 days (Rider et al., 1975).
In agricultural workers with cholinergic symptoms and with mean
inhibition of plasma and erythrocyte cholinesterase activity of 15.6
and 5.6%, respectively, cholinesterase activity recovered at a daily
rate of 1.2 and 0.43% of the respective final levels of activity of
the enzyme over the 14 days after cessation of exposure (Coye et
al., 1986). Higher rates of recovery were observed during the first
11 days after poisoning with a mixture of mevinphos and phosphamidon:
the plasma activity increased by 3% per day and erythrocyte activity
by 0.8% per day. The workers were, however, treated with pralidoxime.
As noted previously, inhibition of plasma cholinesterase activity was
greater than that of erythrocytic enzyme (Midtling et al., 1985).
Human erythrocyte acetylcholinesterase is susceptible to
reactivation in vitro by a variety of pyridinium oximes, including
pralidoximes and obidoxime (Woreck et al., 1996).
Comments
Mevinphos is almost completely absorbed when administered orally
to rats; a large proportion of the absorbed material is biotransformed
to carbon dioxide. Both metabolites and unchanged mevinphos are
observed in the urine but very little in the faeces. Mevinphos
depresses cholinesterase activity in the plasma mre than in the
erythrocytes in experimental animals.
The oral LD50 values for mevinphos in laboratory rodents are
2-12 mg/kg bw. WHO has classified mevinphos as 'extremely hazardous'
(WHO, 1996).
In a three-month range-finding study, mice were fed diets
containing mevinphos at concentrations of 0, 0.5, 1, 2, or 10 ppm.
The NOAEL was 2 ppm, equal to 0.4 mg/kg bw per day, on the basis of
inhibition of brain acetylcholinesterase activity at 10 ppm.
In a 90-day study of toxicity, rats were given mevinphos by
gavage at doses of 0, 0.056, 0.56, 1.1, or 1.7 mg/kg bw per day were
used in males (the highest dose was decreased to 1.1 mg/kg bw per day
at day 36 because of high mortality) and 0, 0.011, 0.056, 0.56, or
0.84 mg/kg bw per day in females. The NOAEL was 0.056 mg/kg bw per
day, on the basis of clinical signs and depressed brain acetyl-
cholinesterase activity at higher doses. Dose-related increases in
mean cholesterol levels and increased relative liver weights were also
observed.
In a one-year study in dogs, mevinphos was administered in corn
oil in gelatine capsules at doses of 0, 0.025, 0.25 or 0.5 mg/kg bw
per day. The NOAEL was 0.25 mg/kg bw per day on the basis of clinical
signs and a reduction in brain acetylcholinesterase activity at the
highest dose.
In an 18-month study of toxicity and carcinogenicity, mice were
fed dietary concentrations of 0, 1, 10, or 25 ppm; cholinesterase
activity was not measured. There was no evidence of carcinogenicity.
In a two-year study of toxicity and carcinogenicity, rats were
given mevinphos by gavage in water on five days per week at doses of
0, 0.025, 0.35, or 0.70 mg/kg bw per day. On day 83 of the study, the
high dose of the females was reduced to 0.60 mg/kg bw per day because
of signs of toxicity. The NOAEL was 0.025 mg/kg bw per day on the
basis of inhibition of brain acetylcholinesterase activity and clinical
signs at higher doses. There was no evidence of carcinogenicity.
A two-generation study of reproductive toxicity was carried out
in which rats were treated by gavage at doses of 0, 0.05, 0.1, or
0.5 mg/kg bw mevinphos in water. The NOAEL was 0.1 mg/kg bw per day on
the basis of clinical signs and reduced brain acetylcholinesterase
activity at the highest dose. This dose also impaired growth and
fertility indices and lowered testicular weights in males and ovarian
weights in females.
In a study of developmental toxicity in rats, groups were given
mevinphos at daily doses of 0, 0.2, 0.75, or 1.2 mg/kg bw per day on
days 6-15 of gestation. High mortality (29%) was observed in the
high-dose group, which was therefore terminated; accordingly, a new
high-dose group at 1 mg/kg bw per day was added. There were no adverse
effects on uterine implantation or on fetal weight, sex distribution,
external appearance, or visceral or skeletal malformations in any
group. It was concluded that mevinphos is not embryotoxic, fetotoxic,
or teratogenic at doses < 1.0 mg/kg bw per day. The NOAEL for
maternal toxicity was 0.75 mg/kg bw per day on the basis of clinical
signs at higher doses.
In a study of developmental toxicity, mevinphos was administered
by gavage to pregnant rabbits at doses of 0, 0.05, 0.5, or 1.5 mg/kg
bw per day on days 7-19 of gestation; surviving animals were killed.
The NOAEL was 0.5 mg/kg bw per day, on the basis of maternal toxicity.
Mevinphos was neither teratogenic nor fetotoxic.
There was some evidence of genotoxic potential in vitro, but
the limited studies available indicate that such potential is not
exhibited in vivo.
In a study in hens, the oral dose of 12 mg/kg bw that was
administered was slightly greater than the oral LD50 value, and
antidotal treatment was required. There was no evidence of delayed
polyneuropathy, either clinically or histopathologically, whereas
characteristic changes were seen in positive controls. Neurotoxic
target esterase was not measured during this study.
Two studies of humans were available. In male volunteers given a
dose of 0.025 mg/kg bw per day, plasma and erythrocyte cholinesterase
activity decreased throughout the 28 days of the study to 13% and 19%
less than the respective pre-dose levels. In the second study, daily
doses of 1, 1.5, 2 or 2.5 mg were given to male volunteers for 30
days, and an NOAEL of 1 mg/day, equivalent to 0.016 mg/kg bw per day,
was derived; however, only five people, all men, per dose were
studied.
An ADI of 0-0.0008 mg/kg bw was established on the basis of the
NOAEL of 0.016 mg/kg bw per day in the 30-day study in volunteers,
using a 20-fold safety factor because of the small numbers in each
group. This ADI is supported by the LOAEL in rats of 0.35 mg/kg bw per
day and the NOAELs of 0.5 mg/kg bw per day in rabbits and 0.25 mg/kg
bw per day in dogs.
An acute reference dose for humans was derived from the 28-day
study in volunteers, on the basis of a dose of 0.025 mg/kg bw per day,
using a 10-fold safety factor.
Toxicological evaluation
Levels that cause no toxicological effect
Mouse: 2 ppm, equal to 0.4 mg/kg bw per day (inhibition of
brain acetylcholinesterase activity in a three-month
study of toxicity)
Rat: 0.025 mg/kg bw per day (two-year study of toxicity and
carcinogenicity)
0.1 mg/kg bw per day (study of reproductive toxicity)
Rabbit: 0.5 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
Dog: 0.25 mg/kg bw per day (one-year study of toxicity)
Human: 1 mg per day, equivalent to 0.016 mg/kg bw per day
(inhibition of cholinesterase activity in a 30-day
study of toxicity)
Estimate of acceptable daily intake for humans
0-0.0008 mg/kg bw
Acute reference dose
0.003 mg/kg bw
Studies that would provide information useful for continued evaluation
of the compound
Study of micronucleus formation in mice in vivo
Toxicological criteria for estimating guidance values for dietary and non-dietary exposure to mevinphos
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Oral, toxicity, rat LD50 = 2.2-6.1 mg/kg bw
Dermal, toxicity, rat LD50 > 20 mg/kg bw
Inhalation, 4 h, toxicity, rat LD50 = 7.3-12 mg/m3
Dermal, irritation, rabbit Slightly irritating
Ocular, irritation, rabbit Slightly irritating
Dermal, sensitization, guinea-pig Not sensitizing
Medium-term (1-26 weeks) Repeated oral, 3 months, mouse NOAEL = 0.4 mg/kg bw per day, inhibition of brain
acetylcholinesterase activity
Repeated oral, 90 days, rat NOAEL = 0.056 mg/kg bw per day
Repeated dermal, 21 days, rabbit NOAEL = 1.0 mg/kg bw per day
Repeated oral, reproductive toxicity, rat NOAEL = 0.1 mg/kg bw per day, maternal and
reproductive toxicity
Repeated oral, developmental toxicity, rat NOAEL = 0.75 mg/kg bw per day, maternal toxicity;
no developmental toxicity
Repeated oral, developmental toxicity, rabbit NOAEL = 0.5 mg/kg bw per day, maternal toxicity;
no developmental toxicity
Long-term (> 1 year) Repeated oral, 2 years, rat NOAEL = 0.025 mg/kg bw per day; inhibition of
brain acetylcholinesterase activity
Repeated oral, 1 year, dog NOAEL = 0.25 mg/kg bw per day; inhibition of
brain acetylcholinesterase activity
References
Atkinson, J.E. (1989) An eighteen month oncogenicity feeding study in
mice with mevinphos. Project No 86-300. Unpublished report dated 23
February 1989 from BioDynamics Inc., East Millstone, NJ, USA.
Submitted to WHO by Amvac Chemical Corp., City of Commerce, CA, USA.
Atkinson, J.E. (1990) A three month dietary range-finding study in
mice with mevinphos technical. Project No. 85-3025. Unpublished report
in West, J. & Roberts, K. (1990) Supplement to an eighteen month
oncogenicity study in mice with mevinphos. Project ID JCODE 1562.1.
Unpublished report dated 31 August 1990 from Jelinek Schwartz Connolly
and Freshman Inc., Washington DC, USA. Submitted to WHO by Amvac
Chemical Corp., City of Commerce, CA, USA.
Auletta, C.S. (1988a) Acute oral toxicity study in rats with
mevinphos. Unpublished report dated 23 March 1988, Project No. 4644-87
from BioDynamics Inc., East Millstone, NJ, USA. Submitted to WHO by
Amvac Chemical Corp., City of Commerce, CA, USA.
Auletta, C.S. (1988b) Acute dermal toxicity study in rabbits with
mevinphos. Unpublished report dated 23 March 1988, Project No. 4645-87
from BioDynamics Inc., East Millstone, NJ, USA. Submitted to WHO by
Amvac Chemical Corp., City of Commerce, CA, USA.
Auletta, C.S. (1988c) Dermal sensitization study in guinea pigs with
mevinphos. Unpublished report dated 23 March 1988, Project No. 4648-87
from BioDynamics Inc., East Millstone, NJ, USA. Submitted to WHO by
Amvac Chemical Corp., City of Commerce, CA, USA.
Barrett, D.S. (1988) Acute delayed neurotoxicity study in mature hens
with mevinphos. Unpublished report dated 26 July 1988, Project No
4685-87 from BioDynamics Inc., East Millstone, NJ, USA. Submitted to
WHO by Amvac Chemical Corp., City of Commerce, CA, USA.
Beyer, B.K. (1991a) Teratology study in rabbits. Study No. MRD-88-331.
Unpublished report dated 22 February 1991 from Exxon Biomedical
Sciences Inc, East Millstone, NJ, USA. Submitted to WHO by Amvac
Chemical Corp., City of Commerce, CA, USA.
Beyer BK (1991b) Multigeneration rat reproduction study. Study No.
MRD-88-331. Unpublished report dated 26th November 1991 from Exxon
Biomedical Sciences Inc., East Millstone, NJ, USA. Submitted to WHO by
Amvac Chemical Corp., City of Commerce, CA, USA.
Bismuth, C., Inns, R.H. & Marrs, T.C. (1993) Efficacy, toxicity and
clinical use of oximes in anticholinesterase poisoning. In:
Ballantyne, B. & Marrs, T.C, eds, Clinical and Experimental Toxicology
of Organophosphates and Carbamates, Oxford, Butterworths, pp. 555-577.
Carricaburu, P., Lacroix, R. & Lacroix, J. (1980) Electroretinographic
study of the white mouse intoxicated by organo-phosphorus: mevinphos
and malathion. Toxicol. Environ. Res., 3, 87-91.
Coye, M.J., Barnett, P.G. & Midtling, J.E. (1986) Clinical confirmation
of organophosphate poisoning of agricultural workers. Am. J. Ind. Med.,
10, 399-409.
Curren, R.D. (1990) Unscheduled DNA synthesis in rat primary
hepatocytes with mevinphos. Unpublished report dated 25 January 1990,
Study No. T8858.380 from Microbiological Associates, Inc., Rockville,
MD, USA. Submitted to WHO by Amvac Chemical Corp., City of Commerce,
CA, USA [full report not available to the Meeting].
Dean, B.J. (1974) Toxicity studies with phosdrin. Dominant lethal
assay in male mice after a single oral dose of phosdrin. Unpublished
report dated July 1974 Group research phosdrin, No. TLGR.0031.74 from
Shell Research Centre, Tunstall, Kent, United Kingdom. Submitted to
WHO by Amvac Chemical Corp., City of Commerce, CA, USA [full report
not available to the Meeting].
Dean, B.J. & Senner, K. (1974) Toxicity studies with phosdrin:
Chromosome studies on bone marrow cells of mice after two daily oral
doses of phosdrin. Group research report No. TLGR.0008.74. Unpublished
report dated February 1974 from Shell Research Centre, Tunstall, Kent,
United Kingdom. Submitted to WHO by Amvac Chemical Corp., City of
Commerce, CA, USA.
Deenihan, M.J. (1985) Mevinphos insecticide acute oral toxicity, acute
dermal toxicity, primary skin irritation, primary eye irritation.
NVP report No. X5A027G. Unpublished report dated 10 April 1985 from
Northview Pacific Laboratories Inc., Berkeley, CA, USA. Submitted to
WHO by Amvac Chemical Corp., City of Commerce, CA, USA.
Gaines, T. (1969) Acute toxicity of pesticides. Toxicol. Appl.
Pharmacol., 14, 515-534.
Hirka, G., Béres, E. & Schmidt, K. (1982) Use of cell and tissue
cultures in genetic toxicology. Mutat. Res., 97, 192.
Hodgson, M.J. & Smith, A.D. (1993) Commercial and residential
poisoning with anticholinesterases. In: Ballantyne, B. & Marrs, T.C.,
eds, Clinical and Experimental Toxicology of Organophosphates and
Carbamates, Oxford, Butterworths, pp. 352-363.
Hoffman, G.M. (1988) An acute inhalation toxicity study of mevinphos
in the rat. Unpublished report and letter dated 12 April 1988 from
BioDynamics Inc., East Millstone, NJ, USA. Submitted to WHO by Amvac
Chemical Corp., City of Commerce, CA, USA.
Jeffcoat A.R. & Coleman, D.P. (1993) Dermal absorption of mevinphos in
rats at three dose levels. RTI Study No. 64C-5494. Unpublished report
dated 31 August 1993 from Research Triangle Institute, Research
Triangle Park, NC, USA. Submitted to WHO by Amvac Chemical Corp., City
of Commerce, CA, USA.
Kangas, L. (1995) A 52-week oral (capsule) toxicity study of mevinphos
in the beagle dog. Laboratory ID 85746. Unpublished report dated
9 June 1995 from Bio-research Laboratories Ltd, Senneville, Quebec,
Canada. Submitted to WHO by Amvac Chemical Corp, City of Commerce, CA,
USA.
Keefe, R.T. (1992) 90-Day subchronic oral toxicity study in rats with
mevinphos (MRD-88-331) Laboratory ID 233170B. Unpublished report dated
4 November 1992 from Exxon Biomedical Sciences Inc., East Millstone,
NJ, USA. Submitted to WHO by Amvac Chemical Corp., City of Commerce,
CA, USA.
Kuhn, J.O. (1994) Acute oral toxicity study in rats (DOT). Laboratory
study number 0946-94. Unpublished report dated 28 March 1994 from
Stillmeadow Inc, Sugar Land, TX, USA. Submitted to WHO by Amvac
Chemical Corp,, City of Commerce, CA, USA.
Lamb, I.C. (1993) An acute neurotoxicity study of mevinphos in rats.
Study No. WIL-188006. Unpublished report dated 13 October 1993 from
WIL Research Laboratories Inc., Ashland, OH, USA. Submitted to WHO by
Amvac Chemical Corp., City of Commerce, CA, USA.
Lamb, I.C. (1994) A subchronic (13 week) neurotoxicity study of
mevinphos in rats. Study No. WIL-188007. Unpublished report dated
17 June 1994 from WIL Research Laboratories Inc., Ashland, OH, USA.
Submitted to WHO by Amvac Chemical Corp., City of Commerce, CA, USA.
Midtling, J.E., Barnett, P.G., Coye, M.J., Velasco, A.R., Romero, P.,
Clements, C.L., O'Malley, M.A., Tobin, M.W., Rose, T.G. & Monosson,
I.H. (1985) Clinical management of field worker organophosphate
poisoning. West. J. Med., 142, 514-518.
Plutnick, R.T. (1994) 2-Year chronic toxicity/oncogenicity study in
rats with mevinphos (MRD-88-331). Laboratory ID 233170C. Unpublished
report dated 3 January 1994 from Exxon Biomedical Sciences Inc., East
Millstone, NJ, USA. Submitted to WHO by Amvac Chemical Corp., City of
Commerce, CA, USA.
Reddy, V., Freeman, T., Little, L. & Cannon, M. (1991) Disposition
and metabolism of 14C-labeled mevinphos in rats (preliminary and
definitive study). Laboratory ID MRI 9485B. Unpublished report dated
4 April 1991 from Midwest Research Institute, Kansas City, MI, USA.
Submitted to WHO by Amvac Chemical Corp., City of Commerce, CA, USA.
Revzin, A.M. (1978) Effects of organophosphate pesticides and alcohol
on visual mechanisms. In: Merigan, W.H. & Weiss, B., eds, Neurotoxicity
of the Visual System, New York, Raven Press, pp. 255-268.
Rider, J.A., Puletti, E.J. & Swader, J.I. (1975) The minimal oral
toxicity level for mevinphos in man. Toxicol. Appl. Pharmacol., 32,
97-100.
San, R.H.C. & Schadly, M.B. (1989) Salmonella/mammalian microsome
plate incorporation mutagenicity assay (Ames test) with a confirmatory
assay with mevinphos. Study No. T8858.501014. Unpublished report dated
23 October 1989 from Microbiological Associates Inc., Rockville, MD,
USA. Submitted to WHO by Amvac Chemical Corp., City of Commerce, CA,
USA.
Schroeder, R.E. & Daly, I.W. (1987) Mevinphos - A teratology study in
rats with mevinphos. Laboratory ID 85-3009. Unpublished report dated
2 March 1987 from BioDynamics Inc., East Millstone, NJ, USA. Submitted
to WHO by Amvac Chemical Corp., City of Commerce, CA, USA.
Skinner, C.S. & Kilgore, W.W. (1982) Acute dermal toxicities of various
organophosphate insecticides in mice. J. Toxicol. Environ. Health,
9, 491-497.
Trimmer, G.W. (1989) Acute dermal toxicity study in the rat phase 1
(MRD-88-331: mevinphos). Laboratory ID 233106. Unpublished report
dated 23 August 1989 from Exxon Biomedical Sciences Inc., East
Millstone, NJ, USA. Submitted to WHO by Amvac Chemical Corp., City of
Commerce, CA, USA.
Trimmer, G.W. (1990a) Acute dermal toxicity study in the rat phase II
cholinesterase determination (MRD-88-331: mevinphos). Laboratory ID
233177A. Unpublished report dated 20 July 1990 from Exxon Biomedical
Sciences Inc., East Millstone, NJ, USA. Submitted to WHO by Amvac
Chemical Corp., City of Commerce, CA, USA.
Trimmer, G.W. (1990b) 21-Day repeated dermal study in the rabbit
(MRD-88-331-mevinphos). Laboratory ID 233109. Unpublished report dated
4 April 1990 from Exxon Biomedical Sciences Inc., East Millstone, NJ,
USA. Submitted to WHO by Amvac Chemical Corp., City of Commerce, CA,
USA.
Verberk, M.M. (1977) Incipient cholinesterase inhibition in volunteers
ingesting monocrotophos or mevinphos for one month. Toxicol. Appl.
Pharmacol.,42, 345-350.
Verberk, M.M. & Sallé, H.J.A. (1977) Effects on nervous function of
volunteers ingesting mevinphos for 1 month. Toxicol. Appl. Pharmacol.,
42, 351-358.
WHO (1996) The WHO Recommended Classification of Pesticides by Hazard
and Guidelines to Classification 1996-1997 (WHO/PCS/96.3), International
Programme on Chemical Safety, Geneva.
Worek, F., Kirchner, T., Bäcker, M. & Szinicz, L. (1996) Reactivation
by various oximes of humans erythrocyte acetylcholinesterase inhibited
by different organophosphorus compounds. Arch. Toxicol., 70,
497-503.
