
PHOSALONE
First draft prepared by
T.C. Marrs
Department of Health, London, United Kingdom
EXPLANATION
Phosalone was previously evaluated by the Joint Meeting in
1972, when an ADI of 0-0.006 mg/kg bw was allocated (Annex I,
reference 18). This monograph summarizes new or not previously
reviewed data on phosalone, as well as relevant data from the
previous monograph that was published on this pesticide.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution and excretion
Mice
Unlabelled phosalone (50 mg/kg bw) orally administered to mice
disappeared rapidly from the plasma, 7% remaining after 8 hours and
< 1% after 24 hours (Desmoras & Fournel, 1968).
Rats
Male and female Sprague-Dawley rats (Crl:CD(SD)BR) were dosed
by gavage with 14C ring-labelled phosalone in PEG 200. Single
doses of 1 mg/kg bw or 50 mg/kg bw were used. Muscle tremor, poor
coordination and lethargy were seen at the high dose. At the low
dose, peak blood radioactivity occurred at 1 hour in males and 0.5-2
hours in females. Excretion of label in the urine and faeces was
rapid and recovery after 72 hours was 102.3% and 101.3% for males
and females, respectively. Almost all the label was excreted in 24
hours. Total urinary excretion after 72 hours was 67.6% and 66.3%,
while total faecal excretion was 21.7% and 18.5% in males and
females, respectively. The remainder was found in cage washings.
Maximum tissue concentrations occurred 0.5 hours after dosing. Pre-
treatment with phosalone for 14 days made very little difference
(total urinary excretion over 72 hours was 69.5% in males and 66.3%
in females while faecal excretion was 18.4% and 15.3%,
respectively); tissue concentrations were marginally increased. At
the higher dose, peak blood concentration of label was delayed and
variable (3-6 hours) in males; 2 peaks occurred in females at 3 and
24 hours. About 95% and 94% of the label was recovered in 72 hours
for males and females, respectively (65.3% and 61.9% for males and
females in the urine, and 22.6% and 20.8% for males and females in
the faeces, the remainder being found in cage washings). In males,
the vast majority of label was excreted within 24 hours, but in
females urinary elimination was delayed, 39.6% being excreted in the
urine in the first 24 hours and 21.8% in the next 24 hours. Peak
tissue concentrations were seen at 1.25 and 5 hours in males and 3
and 9 hours in females; highest concentrations being found in the
gastrointestinal tract (Hopkins et al., 1991).
Cows
14C-Ring-labelled phosalone was given intraruminally to a
lactating Holstein cow with an external ruminal fistula. The label
was absorbed and eliminated rapidly, 79% being recovered within 48
hours. After 100 hours, 94% was recovered in the urine, 6.1% in the
faeces and 0.3% in milk (Craine, 1974a). In a second study, the
same author used labelled phosalone of greater purity and higher
specific activity and a different cow (Craine, 1974b). The results
were similar to the earlier study.
Pigs and rabbits
14C-Ring-labelled phosalone was poorly absorbed from the skin
of a pig, 73.5% still being present on the surface of the skin after
48 hours (Craine, 1974c). However, Safonov (1968) reported the
material to be well absorbed from the skin of depilated rabbits.
Biotransformation
Mice
After oral administration to mice, the sulfoxide and sulfone
were observed in tissues, while phosphorothioates and the sulfoxide
were seen in the excreta: phosalone oxon was not observed (Desmoras
& Fournel, 1968; Desmoras, 1979).
Rats
Phosalone was extensively metabolized in the rat, the
metabolites being mainly excreted in the urine. Many were not
identified, although small amounts of the sulfoxide and sulfone were
found. The main radioactive component found in faeces was
phosalone. Tissues such as brain, liver, fat and skeletal muscle
contained high proportions of the sulfoxide, together with the
sulfide, sulfone and unchanged phosalone (Hopkins et al., 1991).
The putative intermediate, the mercapto compound 2-oxo 3-
mercaptomethyl 6-chlorobenzoxazole, was not identified in rats.
These results are consistent with earlier studies of Desmoras &
Fournel (1968) and Desmoras (1979) using non-radioactive oral
phosalone. In the latter study, the sulfone and sulfoxide were
observed but not the oxon.
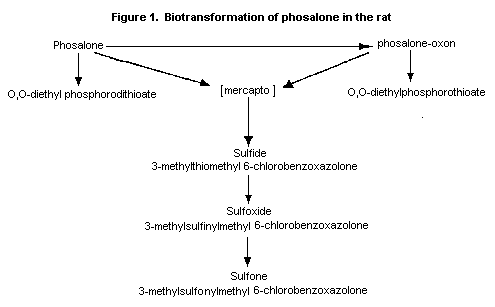
The putative pathway for metabolism in the rat is presumably
hydrolysis to O,O-diethylphosphorodithioate and the mercapto
compound 3-thiomethyl 6-chlorobenzoxazolone, which has not been
identified in vivo. This is subsequently converted to the
sulfide, which is oxidized to the sulfoxide and to the sulfone
(Figure 1).
Studies in vivo and in vitro of the formation of the oxon
of phosalone were carried out in rats. Small quantities of the oxon
were formed in the liver. Moreover, studies using human serum in
vitro showed that the addition of guinea-pig liver slices,
considerably increased the anticholinesterase activity of phosalone
(Dubost et al., 1963). Hence a further mammalian metabolic
pathway is likely to be hepatic desulfuration of phosalone to
phosalone-oxon and hydrolysis of the oxon to O,O-
diethylphosphorothioate. The other product of hydrolysis would be
the mercapto compound, with subsequent formation of the sulfide,
sulfoxide and sulfone.
Rabbits
In rabbits after percutaneous administration the excreta
contained phosalone and probably the sulfoxide and sulfone
(Desmoras, 1979). Metabolic pathways in mice and rabbits are
therefore similar to those in rats.
Cows
In two studies in lactating cows, the major route of
elimination was the urine (90%) while 6% was excreted in the faeces.
Only 0.3% of the label was excreted in the milk. Biotransformation
was extensive and only 2% of the label was excreted as phosalone or
its oxon. Numerous labelled metabolites were detected in the urine.
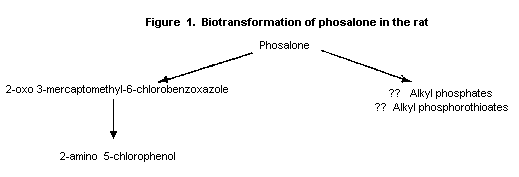
The main metabolic pathway appeared to be hydrolysis to the marcapto
compound, 2-oxo 3-mercaptomethyl 6-chlorobenzoxazole, which was
detected despite being unstable, and presumably alkyl phosphates and
alkyl phosphorothioates (see Figure 2). 2-Oxo 3-mercaptomethyl-6-
chlorobenzoxazole was converted via a metabolite which could not be
identified to 2-amino 5-chlorophenol in a reaction involving
cleavage of the benzoxazole ring. Less than 10% of the label found
in the milk was present as phosalone or the oxon. Numerous
components were present in the milk, including 2-oxo 3-
mercaptomethyl-6-chlorobenzoxazole and 2-amino 5-chlorophenol
(Craine, 1974a,b, 1975).
Residue studies in dairy cows were supportive of the above
metabolic scheme (Rhône-Poulenc Inc, 1979, 1980a,b,c).
Effects on enzymes and other biochemical parameters
In early studies, phosalone was shown to be a weak
cholinesterase inhibitor in vitro (Dubost et al., 1963; Rhône-
Poulenc, 1968) and it inhibited rat whole blood cholinesterase in
vivo (Dubost et al., 1963). In in vitro systems,
phosaloneoxon is approximately 1000 times more powerful as an
anticholinesterase than phosalone (Desmoras & Fournel, 1968). In
vivo in rats, orally administered phosalone is 4-9 times less
effective in inhibiting plasma or red cell cholinesterase than
phosaloneoxon (Fournel et al., 1968). Presumably phosalone exerts
mammalian anticholinesterase activity only after conversion to its
oxon, like other phosphorothioates and phosphorodithioates of the
P=S type (WHO, 1986). The cholinesterase inhibitory potential of
 technical grade phosalone is probably due to small amounts of oxon
present (up to 0.5% of the present technical material). The reason
that phosalone oxon did not inhibit rat brain cholinesterase at
single doses up to 9 mg/kg bw, whereas phosalone did at 27 mg/kg bw
(surprising in view of the much higher acute toxicity of the former)
is obscure (Fournel et al., 1968). Inhibition of brain
cholinesterase by phosalone was also observed by Pasquet et al.
(1976) with an I50 of 31 mg/kg bw p.o. Phosalone was reported to
shift to the right the oxygen dissociation curve in rats (Reddy et
al., 1992).
Toxicological studies
Acute toxicity studies
A summary of acute toxicity data for phosalone is shown in
Table 1. Phosalone has been classified as moderately hazardous by
WHO (WHO, 1992).
Short-term toxicity studies
Rats
A dose of 50 mg/kg bw/day administered by gavage (vehicle
unstated) to white rats for 14 days was lethal (Safonov, 1968).
Phosalone was administered as an oil solution to male rats
(10/dose) (strain unstated) at doses of 7.5 or 15 mg/kg bw/day for 5
weeks. The only death seen in the study was a control rat and no
abnormal clinical signs were observed. Slight reductions in body-
weight gain noted at both dose levels were not statistically
significant. No major differences between test and control groups
were seen in haematology or clinical chemistry except in
cholinesterase activity. Depression of blood cholinesterase
activity (> 20%) was observed at both doses. Brain cholinesterase
activity was reduced at the high dose only. No statistically
significant differences in organ weights or pathological findings
were seen between test groups and controls. The NOAEL was 7.5 mg/kg
bw/day, based on brain cholinesterase inhibition (Dubost et al.,
1963).
In an 8-week study, groups of 10 Crl:CD(SD) rats/sex/dose
received 10, 100, 300, 600 or 1200 ppm phosalone (94.1% pure) in the
diet. After 5 weeks, the doses administered to the 100 and 300 ppm
groups were increased to 2400 and 4800 ppm, respectively, in order
to establish the maximum tolerated dose. At 300/4800 ppm, one
female died and all remainder animals were sacrificed in extremis.
At 100/2400 ppm all females had to be sacrificed. Males at both
these doses survived but showed reduced body weight and food
Table 1. Acute toxicity of phosalone
Species Strain Sex Route LD50 (mg/kg bw) References
Mouse CD-1(COBS) M p.o. 118 (97-144) Fournel et al. (1968)
Mouse CD-1(COBS) F p.o. 93 (74-116) Fournel et al. (1968)
Mouse ? ? p.o. 180* 435** Dubost et al. (1963)
Mouse ? ? p.o. 205 Dubost et al. (1963)
Mouse ? ? p.o. 180 Safonov (1968)
Rat ? M p.o. 120 Dubost et al. (1963)
Rat CD(COBS) M p.o. 125 (102-152) Fournel et al. (1968)
Rat ? F p.o. 170 Dubost et al. (1963)
Rat ? F p.o. 135 Dubost et al. (1963)
Rat CD(COBS) F p.o. 90 (68-119) Fournel et al. (1968)
Rat ? ? p.o. 50 Hayward (1969)
Rat ? ? p.o. 135 Safonov (1968)
Rat CD(COBS) M p.o. 130 (104-163) Pasquet et al. (1976)
Rat CD(COBS) F p.o. 110 (87-139) Pasquet et al. (1976)
Rat ? F p.c. 390 Dubost et al. (1963)
Rat CD(COBS) F p.c. 700 (480-1015) Pasquet (1971)
Rat CD(COBS) F p.c. 1530 Mazuret (1971)
Rat ? F p.c. > 2560 Pasquet & Mazuret (1973a)
Rat CD(COBS) F p.c. 1530 Pasquet et al. (1976)
Rabbit ? ? p.c. > 1000 Dubost et al. (1963)
Guinea-pig ? ? p.o. 150 Dubost et al. (1963)
Guinea-pig ? ? p.o. 82 Dubost et al. (1963)
Hens Rhode Island red F s.c. 350 Heath et al (1967)
* Aqueous Suspension
** Oil solution
consumption. Body weight and cumulative body-weight gain were
significantly lower in females receiving 1200 ppm. No changes were
seen in the eyes. Significantly lower plasma and erythrocyte
cholinesterase were seen at 100 ppm in both sexes. Brain
cholinesterase activity was decreased at 600 ppm to about 80% and
50% of controls in males and females, respectively. Other changes
in clinical chemistry parameters were seen at higher doses,
including lower blood glucose, total protein and albumen and higher
cholesterol and blood urea. No treatment-related changes were seen
in organ weights or in organs on macroscopic or microscopic
examination. The NOAEL was 10 ppm, equal to 0.87 mg/kg bw/day in
males and 0.93 mg/kg bw/day in females, based on brain
cholinesterase inhibition (Kehoe, 1990).
Rabbits
A 3-week percutaneous study was undertaken in rabbits using
phosalone at doses of 0.4, 2.0 or 10 mg/kg bw/day
acetone/ethanol/arachid oil. There were no deaths. A 20%
depression in brain cholinesterase occurred in the highest dose
females. Some irritancy was observed (Kynoch et al., 1979).
Dogs
Phosalone was administered orally to dogs in oil solution for 1
month at dose levels of 7.5, 15 or 30 mg/kg bw/day. One animal of
each sex was used at each dose without controls. Neither clinical
signs nor significant weight changes were noted. Albumen/globulin
ratios in both animals treated with phosalone at 7.5 mg/kg bw/day
were depressed; however, this was not seen in the 15 mg/kg bw/day
animals. Significant plasma and erythrocyte cholinesterase
depression were seen in all animals except in females at 7.5 mg/kg
bw/day. A NOAEL could be not be determined in this study (Dubost
et al., 1963).
In another one-month study, groups of 4 beagle dogs/sex/dose
were administered phosalone (100% pure) at dietary concentrations of
0, 12.5, 25 or 37.5 ppm. No deaths were observed nor were there
abnormal clinical signs. There was no adverse effect on body
weight. Plasma cholinesterase activity was depressed by > 20% in
the high-dose group. Erythrocyte and brain cholinesterase activity
were not affected. At terminal autopsy a mauve discoloration was
observed in the bladder of all high- and mid-dose animals and in one
female at the low dose. Since the bladder discoloration was not seen
in any other dog study, the NOAEL was the highest dose of 37.5 ppm,
equal to 0.81 mg/kg bw/day (Noel et al., 1970).
In a 6-month study, groups of beagle dogs (4/sex/dose) received
phosalone at 0, 10 or 25 ppm in the diet. No haematological,
biochemical or histological changes attributable to phosalone were
observed except in erythrocyte and plasma cholinesterase. Brain
cholinesterase activity was not depressed. Depression of plasma
cholinesterase activity of about 40% and 25% was observed in the 25
ppm and 10 ppm groups, respectively. Decreased erythrocyte
cholinesterase activity was seen only at 25 ppm towards the end of
the study. The NOAEL was the highest dose (25 ppm, equivalent to
0.63 mg/kg bw/day) (Fournel et al,. 1966).
In a 107-week study, phosalone (purity unknown) was
administered in the diet to groups of 4 beagle dogs/sex/dose at
concentrations of 0, 100, 200 or 1000 ppm. One female at 1000 ppm
died during the study. Although marked plasma and erythrocyte
cholinesterase depression were observed, the immediate cause of
death seemed to be an infection. Body-weight loss was seen at 100
ppm, and cholinergic symptomatology was seen in two animals at the
highest dose and in one animal at 100 ppm. Abnormal ECGs (increased
T-waves) were seen at 1000 ppm. Biochemical and haematological
investigations demonstrated no changes that appeared to be compound-
related apart from elevations in ALAT in 4 animals at 1000 ppm.
Reductions in plasma cholinesterase > 20% were observed at 100 ppm
and above, and in erythrocyte cholinesterase at 200 ppm and above.
The degree of brain cholinesterase inhibition was variable, with a
mean in the 1000 ppm group of 79% of controls. Inhibition at
termination of the study was considerable in 3/8 animals and almost
complete in 2 animals. The NOAEL was 200 ppm, equivalent to 5 mg/kg
bw/day, based on clinical signs, body-weight loss, elevated ALAT and
brain cholinesterase depression at 1000 ppm (Donoso et al., 1967).
Groups of 6 beagle dogs (6/sex/dose) received 0, 5, 25 or 300
ppm phosalone (94.5% pure) in the diet for 52 weeks. Three deaths
in different groups were observed. During week 43, all animals
receiving 300 ppm vomited and lost weight and 5 showed reduced food
consumption. Some recovery in weight occurred towards the end of
the study. No changes of toxicological importance occurred on
ophthalmological, haematological, biochemical or urinary examination
except for cholinesterase activity. Reduction in plasma
cholinesterase > 20% compared to controls occurred in the 25 and
300 ppm groups (both sexes and combined and at all time points after
dosing commenced). At 5 ppm, significant reductions occurred in
males only at 4, 26, and 52 weeks. These reductions were just under
20%. Red cell cholinesterase was significantly reduced at 300 ppm
at all time points. At 25 ppm, this enzyme was reduced compared to
the control group in males at 26 weeks and in both sexes at 52
weeks. The reduction in this group compared to pre-dosing levels
was < 15%. Brain cholinesterase was significantly reduced in both
sexes at 300 ppm to about 60% of control values. No significant
differences between groups were observed in organ weights or after
macroscopic or histopathological examination of organs. The NOAEL
was 25 ppm, equal to 0.89 mg/kg bw/day (males) and 0.97 mg/kg bw/day
(females), based on depression of brain cholinesterase activity and
clinical effects at 300 ppm (Barker at al., 1992).
Long-term toxicity/carcinogenicity studies
Mice
In a 2-year study in CD-1 mice (60-65/sex/group), phosalone
(95.3% pure) was administered at dietary concentrations of 0, 15, 50
or 150 ppm. Five mice of each sex were sacrificed at 6 weeks and at
study termination at 105 weeks for plasma, erythrocyte and brain
cholinesterase determinations. Plasma and erythrocyte
cholinesterase were reduced by at least 20% in the 150 ppm group at
both 6 and 105 weeks. In some cases, particularly with plasma
cholinesterase, the reduction was much greater. Brain
cholinesterase was not reduced compared to controls. Statistically
significant increases in adrenal weights were found at all dose
levels and in both sexes, with the exception of females at the
lowest dose. These increases were both in relative and absolute
weights and were significant except for relative weight at 15 ppm in
males. Decrease in absolute brain weight was significant at 50 ppm
and 150 ppm in males. Relative increases in testicular weights (50
ppm), kidney weights in females only (15 ppm) and relative and
absolute increases in pituitary weights (males: 15 ppm) were also
significant. No neoplastic or non-neoplastic histopathological
findings were attributable to phosalone. There were adrenal weight
changes, which in the absence of histopathological changes, were
considered to be adaptive, and changes in testicular weights at 50
ppm and kidney weight in one sex at 15 ppm which were likely to be
insignificant in view of a lack of dose-response. The decrease in
absolute brain weight in males was not dose-related. The NOAEL was
thus the highest level of 150 ppm (equal to 23 and 31 mg/kg bw/day
in males and females, respectively), based on the lack of depression
of brain cholinesterase activity at this level (Nelson et al.,
1980).
Rats
In a 2-year study, groups of 30 rats/sex/dose (albino: strain
not stated) received phosalone of unknown purity at dietary
concentrations of 0, 25, 50 or 250 ppm (half of these concentrations
for the first four weeks). Mortality during the course of the study
was similar in all groups. There was some depression in weight gain
in the highest-dose animals compared to controls, especially in
males at 103 weeks. No haematological changes attributable to the
test material were observed. There were marked (up to three-fold)
differences in group mean plasma cholinesterase activities between
sexes, females having much higher activity; this raised some doubt
about the reliability of these assays. Inhibition of erythrocyte
and plasma cholinesterase activity was seen at 250 ppm. Inhibition
of brain cholinesterase activity > 20% was seen at 250 ppm in
males. No histopathological changes related to the test material
were seen. Although a few instances of testicular activity were
seen, there was no indication of any relationship between occurrence
of this finding and administration of the test material. The NOAEL
was 50 ppm, equivalent to 2.5 mg/kg bw/day, based on brain
cholinesterase inhibition at the next highest dose (Woodard Research
Corporation, 1967).
In a second 2-year study, Sprague-Dawley CD rats (50/sex/dose)
were given phosalone (purity 94.5%) at doses of 0, 5, 50 or 1000
ppm, the highest dose being reduced to 500 ppm at week 27. These
concentrations were equal to 0, 0.2, 1.8 or 20 mg/kg bw/day for
males and 0, 0.2, 2.5 or 31 mg/kg bw/day for females, on the basis
of consumption data from week 27. Satellite groups of animals
(15/sex/dose), were killed at 52 weeks. There was no effect on
mortality and clinical effects were mainly confined to the high-dose
group. Depression of plasma and erythrocyte cholinesterase was found
at the high and medium concentrations and depression of brain
cholinesterase activity was found in the highest dose group. There
were some treatment-related changes in the adrenals. A dose-response
across all groups in the prevalence of testicular atrophy was
observed (1/27, 3/34, 6/30 10/42 in survivors to the terminal kill).
Reductions in testis weight were dose-related, but were
statistically significant only at the high and mid-dose levels (5.03
g, 4.66 g, 4.51 g and 4.22 g, for the control, low, medium and high-
dose groups, respectively). The NOAEL was < 5 ppm, equal to 0.2
mg/kg bw/day (Barker & Sortwell, 1993).
Reproduction studies
Rats
In a three-generation reproduction study, groups of albino rats
(10 males and 20 females/group) were administered phosalone (purity
unknown) at dietary concentrations of 0, 25 or 50 ppm. Only half
the nominal dietary concentration was given during the initial three
weeks of the diet, on the grounds that young rats eat about twice as
much daily during this period; this procedure was repeated in each
generation. When the rats had received phosalone for 74 days, 10
females in each group were paired with the 10 males from the same
group for 10 days, after which the other 10 females were mated with
the 10 males. The animals from these litters (F1a) were sacrificed
after weaning, weighed and examined. Microscopic histopathological
examination was not carried out. The F0 rats were then remated as
different pairs to produce the F1b litters. Litters were examined
at weaning and representative male and female pups were placed on
control diet to constitute the next study groups. Within 21 days of
weaning, the F1b generation received 25 ppm or 50 ppm phosalone,
while controls continued to be fed control diet. The F1b rats were
mated in the same way as the F0 generation, F2a pups being
sacrificed and examined as above, while the F1b parents were also
killed and examined. Uteruses from females failing to bear two
litters were removed and examined for implantation sites. Rats were
selected from each F2b litter to give 10 males and 20 females in
each group, the remaining F2b pups were sacrificed and examined as
before without microscopic pathology. The retained F2b pups in the
two test groups received the test diet within 24 days of weaning.
After 51 days on the diet, the males and 10 females were paired for
10 days, the males being paired with the other 10 females after they
had all been on the diet for 71 days. The F3a pups were sacrificed
after weaning and examined as above. Within 10 days, F2b females
were re-mated with different males to produce the F3b litters.
After weaning, the pups were sacrificed and the main organs
examined. Tissues from one male and one female in each F3b litter
were examined histopathologically. The F2b parents were mated for
a third time to produce F3c pups litters, which were treated in the
same way as the F3b pups.
The test groups of rats were similar to the controls throughout
the study. Three F0 females died in the 50 ppm group and one in
the 25 ppm group while none died in the control group. Test
substance-related effects on fetal resorption were not observed and
malformations were observed only in 3 pups from one litter of the 25
ppm group. The three pups exhibited flaccid paralysis of the hind
limbs. Organs from the test and control weanlings from the F3b and
F3c groups did not differ significantly in weight or
histopathology. No adverse effect was determined in the study and
the NOAEL was > 50 ppm, equivalent to 2.5 mg/kg bw/day (Jones et
al., 1967a).
In a two-generation reproduction study, groups of 32 male and
32 female rats (6 week-old Crl: CD(SD)VSAF/Plus BR) were given
phosalone (96.8% pure) at dietary concentrations of 0, 10, 50 or 400
ppm. Phosalone was administered for 10 weeks, after which the rats
were mated for 20 days. The dams were allowed to rear their young
and from these litters, 21 days postpartum, 28 rats/sex/group were
selected as the F1 generation. F1 animals were selected and
treated from weaning until 16 weeks when they were mated. After
weaning of their litters the F1 parents were sacrificed as were the
F2 pups.
At 400 ppm, there was a lower body weight from conception to
day 17 in the F0 generation, and lower mean body-weights of the F1
males and females. There were 2 total litter losses post-partum in
the F0 generation and three in the F1 generation. There was a
reduced litter size in the F0 animals and increased pup mortality
to day 4 in both generations. Retarded pup growth and marked plasma
and erythrocyte cholinesterase depression were noted in both
generations. At 50 ppm, there was marked plasma and erythrocyte
cholinesterase depression in both generations, while at 10 ppm
cholinesterase reduction was < 20%. Brain cholinesterase activity
was not measured. The NOAEL was 50 ppm, equivalent to 2.5 mg/kg
bw/day, based on retarded pup growth and erythrocyte cholinesterase
depression (Bryson et al., 1991).
Special studies on embryotoxicity/teratogenicity
A study was carried out on chick embryos (white Leghorn) at
doses of 0.2, 0.6 or 1.8 mg/egg, using 30 eggs/group. Two further
groups of 30 eggs served as solvent (dimethylsulfoxide) and
untreated controls. At 18 days, the surviving embryos were removed,
weighed and examined. Phosalone had no significant effect on
mortality, weight or structure (Pasquet, 1969).
Groups of 25 female rats (Wistar HAN) were treated with 0, 2,
10 or 20 mg/kg bw/day of phosalone by gavage, from days 6 through 15
of gestation. Controls received distilled water with 4% CMC. The
animals were killed 21 days after coitus and the fetuses were
removed by caesarean section. Dams and fetuses were examined
macroscopically and microscopically. Oral administration of
phosalone at the highest dose produced clinical signs (chewing
movements, piloerection and dyspnea). Additionally, reduced food
consumption, weight loss, reduction in litter size, due to increased
resorptions and a slight increase in mean fetal body weight, were
observed. There was no evidence of fetal abnormality. At 2 and 10
mg/kg bw/day, maternal and fetal parameters were similar to
controls. The NOAEL was 10 mg/kg bw/day for both maternal toxicity
and fetotoxicity (Allen et al., 1989a).
Groups of 25 Fauve de Bourgogne rabbits were orally treated by
capsules with undiluted phosalone (purity unstated) from days 6
through 16 of gestation with doses of 0, 2, 6 or 18 mg/kg bw/day. A
positive control group was treated with thalidomide. The does were
sacrificed at 28 days. The percentage of animals becoming pregnant,
the number of resorptions, the number of fetuses recovered alive
after caesarian section and their weight did not differ
significantly from the untreated controls. The incidence of
malformed fetuses was low in all groups and the malformations were
trivial. By contrast malformations were observed in the thalidomide
group. The NOAEL was 18 mg/kg bw/day, the highest dose tested
(Pasquet, 1969).
Phosalone (93.5% pure) was administered in
carboxymethylcellulose (4%) by gavage to Chinchilla KFM/CHIN rabbits
(16/dose) at doses of 0, 1, 10 or 20 mg/kg bw/day from days 6
through 18 post-coitum. Clinical signs of cholinergic intoxication,
particularly dyspnea and extensor spasms, reduced food consumption,
loss of body weight and increased incidence of resorption of embryos
were observed at the highest dose. Post-implantation loss was
increased at 10 and 20 mg/kg bw/day compared to concurrent controls
but was within the range of historical controls. No adverse effect
was seen on fetuses at any dose, or on maternal parameters at doses
of 1 or 10 mg/kg bw/day. The NOAEL was 10 mg/kg bw/day, based on
both fetal and maternal toxicity (Allen et al., 1989b; Allen,
1989).
Special studies on genotoxicity
Based on the results of genotoxicity assays given in Table 2,
the Meeting concluded that phosalone was not genotoxic.
Special studies on neurotoxicity
Groups of white Leghorn hens (10/group/dose) were given dietary
doses of 0, 50, 163 or 500 ppm phosalone or TOCP at 500 ppm for 45
days. Hens were examined daily during the study. At termination,
brain, three levels of spinal cord and a portion of sciatic nerve
were taken from the controls, the TOCP-treated birds and the highest
dose of phosalone-treated hens. Although some signs of cholinergic
toxicity occurred, no phosalone-treated birds showed any indication
of organophosphate-induced delayed neuropathy. TOCP-treated hens
showed paralysis from 13 days onwards, and the only deaths seen,
were amongst the TOCP-treated fowl. Histopathological appearances
in tissues taken from the control and phosalone-treated animals were
within normal limits. Although there was some demyelination in the
TOCP group of animals, particularly in the sciatic nerve, this was
not significant (Woodard et al., 1966).
In another study, 10 Rhode Island red hens were dosed with 350
mg/kg bw phosalone (=LD50) as a 35% formulation, while protected by
atropine and pralidoxime methanesulfonate (P2S). The dose was
repeated after 3 weeks. There were 4 untreated controls and 4
positive controls treated with a neuropathic dose of mipafox. One
phosalone-treated hen died at 4 days, and another at 29 days. No
pathological changes were seen in the spinal cord. The positive
controls became severely ataxic at 13 days and showed serious
effects in the spinal cord. The untreated controls were clinically
and pathologically normal (Heath et al., 1967).
Groups of 20 New Hampshire red hens received TOCP (750 mg/kg bw
p.o., positive control), or 0 or 600 mg/kg bw p.o., phosalone.
Dosed and positive control animals also received atropine 15 min
before dosing. The vehicle control and phosalone group were redosed
at day 22 and sacrificed at day 41. Birds from the first positive
control group were killed on day 22 of the study at which time the
10 birds from the second positive control group were dosed and
sacrificed 19 days later. No significant clinical signs were seen
in the vehicle control fowl. Neuropathological examination was
performed on central and peripheral nervous tissue from all terminal
sacrificed animals and seven decedents in the phosalone group.
Table 2. Results of genotoxicity assays on phosalone
Test Object Concentration phosalone Purity Results Reference
In vitro
Ames test (1) S. typhimurium 1-1000 µg/plate ? Negative Benezet & Cartier, 1980
(strains TA98, 100, 125-1000 µg/plate
1535, 1537)
Ames test (1) S. typhimurium 250-10 000 94% Negative Haworth & Lawlor, 1989
(strains TA98, 100, µg/plate
1535, 1537, 1538)
HPRT test (1) CHO/K1 cells 0.1-50 µg/ml 95% Negative Cordier & Bonneau, 1985
Chromosomal CHO/K1 cells 4, 40, 75 µg/ml 95% Negative Cordier & Fournier, 1989
aberration in
CHO cells (1)
Chromosomal CHO/WBL cells 16.7, 500 µg/ml 94% Negative Murli, 1989
aberration in (also 5 µg/ml without
CHO cells (1) metabolic activation)
UDS in vitro Rat primary 0.005-252 µg/ml 94% weak Cifone, 1989
hepatocyte positive
In vivo
Micronucleus CD-1 mice 10, 20, 40 mg/kg bw/day 99.5% Negative Pasquet & Fournier, 1980
Dominant lethal CD-1 mice 10, 30, 75 mg/kg 95.3% Negative Goldenthal et al.,
test 1978
(1) With and without metabolic activation.
Tissues examined were spinal cord (cervical, thoracic and
lumbosacral), medulla, sciatic and tibial nerves. In the positive
control groups, all except one bird showed clinical signs of delayed
neuropathy, appearing from days 13-24. In both positive controls,
histopathological evidence of delayed neuropathy was found. In the
phosalone group, 4 birds showed signs of cholinergic toxicity after
the first dose and two birds died. On day 22 after the second dose,
six hens showed acute toxicity. Seven hens died on days 23-24.
Histopathological evidence of nerve degeneration was not more
frequent in this group than in the vehicle controls (Morris, 1983).
The Meeting concluded that there is no evidence that phosalone
has potential to cause organophosphate-induced delayed neuropathy.
Antidote studies of phosalone intoxication
Ten groups of 10 mice each were given phosalone orally followed
immediately by i.p. administration of combinations of pralidoxime
methylsulfate (Contrathion) at 0, 25, 50, 100 or 200 mg/kg bw and
atropine at 0, 10, 20 or 40 mg/kg bw. Both antidotes were effective
in preventing death although pralidoxime methylsulfate at 100 and
200 mg/kg bw appeared to be toxic. Some aspects of the results were
difficult to interpret since doses of pralidoxime methylsulfate used
were high compared to the recommended human dose of 5-6 mg/kg bw
(SERB, 1988). Moreover the i.p. LD50 of a similar pralidoxime salt
is 125-250 mg/kg bw in the mouse (Marrs, 1991). Doses of atropine
used in this study were also high (Dubost et al., 1963).
In another study using orally administered phosalone (vehicle
unstated), groups of rats (9-10/group) were given 50 or 100 mg/kg bw
phosalone. Phosalone (100 mg/kg bw) was administered to another
group of 10 rats, followed by pralidoxime methanesulfonate (50 mg/kg
bw s.c.) and atropine (17.5 mg/kg bw s.c.) 15-20 minutes after
clinical signs of anticholinesterase poisoning developed. All
animals in the 100 mg/kg bw group not treated with the antidotes
showed considerably reduced erythrocyte anticholinesterase activity
and died within 24 hours. Five out of 10 rats survived 24 hours in
the 50 mg/kg bw group and their cholinesterase activity was less
depressed. When treated with the antidotal regime, 9/10 rats dosed
with phosalone at 100 mg/kg bw survived and cholinesterase activity
was less depressed than that of animals treated with 50 mg/kg bw
phosalone but untreated with antidotes (Hayward, 1969).
In a study of the antidotal effects of bis-pyridinium oxime
(obidoxime), groups of mice (4/sex) were administered phosalone by
gavage at a dose of 250 mg/kg bw, a dose well above the LD50.
Immediately afterwards, obidoxime and atropine were injected
intraperitoneally at obidoxime doses of 0, 25, 50 or 100 mg/kg bw
and at atropine doses of 0, 10, 20 or 40 mg/kg bw in various
combinations. When no antidote was given, phosalone was invariably
fatal whereas survival was considerably improved with combinations
of antidote except at the highest dose of atropine and obidoxime.
Obidoxime alone was clearly more beneficial than atropine alone.
Synergism was not observed, because of the study design (Pasquet &
Masuret, 1970).
It was concluded from these studies that atropine is an
effective antidote to phosalone. Pralidoxime (either methylsulfate
or methanesulfonate) reactivated phosalone-inhibited rat erythrocyte
cholinesterase and was an effective antidote. Obidoxime was also an
effective antidote but the usual synergism of atropine and
pralidoxime salts or obidoxime was not noted probably due to the
design of the studies.
Special studies on metabolites and intermediates
A number of acute toxicity and genotoxicity studies have been
conducted with phosalone metabolites and intermediates. The results
are summarized in Tables 3-6.
Other studies
3-Hydroxymethyl 6-chlorobenzoxolone was non-irritant to the
intact rabbit skin but very irritant to the scarified skin (Pasquet
& Mazuret, 1973b). 3-Chloromethyl 6-chlorobenzoxolone was non-
irritant to the intact rabbit skin (Pasquet et al., 1983b).
In a 13-week study, groups of 15 male and 15 female rats
(Sprague-Dawley-derived Crl:CD(SD)BR) were given 6-chlorobenzoxolone
at dietary concentrations of 0,5, 15 or 45 mg/kg bw/day for 13
weeks. Groups of 10 male and female controls and high-dose animals
underwent a subsequent 28-day treatment-free period. The only
effect of the treatment was suppression of weight gain in all
treated males and in the high-dose females. A compensatory weight
gain was observed in the animals that underwent the treatment-free
period (Owens, 1985).
A study was performed to determine the potential of Di-syston
(disulfoton) to potentiate the activity of phosalone. Groups of 8
beagle dogs were fed diets containing phosalone at concentrations of
7.5, 15 or 25 ppm for 19 weeks. In each case, Di-syston was added
at a concentration of 1 ppm from week 14 to week 19. There were no
changes in physical appearance, haematology or pathology that could
be ascribed to the treatment. No treatment-related changes in
plasma or erythrocyte cholinesterase were seen (Jones et al.,
1967b).
Table 3. Acute toxicity of phosalone metabolites
Species Strain Sex Route LD50 References
(mg/kg bw)
Phosalone-oxon
Mouse CD-1(COBS) M PO 35 Fournel et al., 1968
(30-41)
Mouse CD-1(COBS) F PO 40 Fournel et al., 1968
(33-49)
Mouse ? Mix PO 32 Rhône-Poulenc, 1968
Rat CD(COBS) M PO 36 Fournel et al., 1968
(29-44)
Rat CD(COBS) F PO 21 Pasquet et al., 1976
(12-28)
Rat CD(COBS) F PO 20 Fournel et al., 1968
(15-29)
Rat CD(COBS) F PC 380 Pasquet & Mazuret, 1973a
(240-610)
3-methylthiomethyl 6-chlorobenzoxazolone
Mouse OF1 M PO 590 Pasquet & Mazuret, 1980
(499-698)
OF1 F PO 837 Pasquet & Mazuret, 1980
(706-991)
3-methylsulfinylmethyl 6-chlorobenzoxazolone
Mouse OF1 M PO 405 Pasquet & Mazuret (1980)
OF1 F PO 315 Pasquet & Mazuret (1980)
(256-386)
Table 3 (contd)
Species Strain Sex Route LD50 References
(mg/kg bw)
3-methylsulfonylmethyl 6-chlorobenzoxazolone
Mouse OF1 M PO > 5000 Pasquet & Mazuret 1980
OF1 F PO > 5000 Pasquet & Mazuret 1980
Table 4. Acute toxicity of intermediates and soil metabolites
Species Strain Sex Route LD50 References
(mg/kg bw)
3-hydroxymethyl 6-chlorobenzoxolone
Mouse CD-1(COBS) Mix p.o. 730 Pasquet & Mazuret, 1973b
(557-956)
Benzoxolone
Mouse CD-1(COBS) Mix p.o. 580 Pasquet & Mazuret, 1973b
3-chloromethyl 6-chlorobenzoxolone
Rat CD Sprague- Mix i.p. 305 Pasquet et al., 1983a
Dawley
Table 5. Genotoxicity of phosalone intermediates
Compound Test object Concentration Purity Result References
intermediate
Unknown intermediate
Ames test (1) Salmonella typhimurium 0.005-0.5 µg/plate ? -ve Weill, 1988
TA98, TA100, TA1535,
TA1537, TA1538
Table 6. Genotoxicity of phosalone soil metabolites
Compound Test object Concentration Purity Result Reference
intermediate
3-amino 7-chloro 2-phenoxazone
Ames test (1,2) Salmonella typhimurium 10-100 µg/plate (-S9) 95% +ve Vanrel et al., 1989
TA98, TA100. TA1535, 25-125 µg/plate (+S9)
TA1537, TA1538
(1) With and without metabolic activation.
(2) Positive with metabolic activation and in TA1537, and TA100 only.
Phosalone (96% pure) was applied to the skin of rabbits (New
Zeeland white) for 21 days at dose levels of 0.4, 2 or 10 mg/kg
bw/day. Azinphosethyl was applied at 2 mg/kg bw/day. Both
chemicals were applied to intact or abraded skin. Inhibition of
brain cholinesterase > 20% was not noted in any phosalone group,
but marked depression was seen with azinphosethyl. Phosalone did
not cause red blood cell or plasma cholinesterase depression (Kynoch
et al., 1979).
In studies to elicit delayed sensitization in guinea-pigs
(Pasquet & Mazuret, 1977; Cunny & Siglin, 1989) no sensitization was
seen. Phosalone was moderately irritant to the eye in New Zeeland
white rabbits (Siglin et al., 1989). Irritancy to the rabbit eye
was also observed by Safonov (1968).
Observations in humans
In a study performed on 14 male volunteers to establish a re-
entry period after applying phosalone in citrus groves, dislodgeable
residues up to 3.6 µg/cm2 (leaf) produced no adverse effect other
than some plasma cholinesterase depression (Knaak et al., 1978).
In 1987, several cases of illness resembling anticholinesterase
poisoning were reported in California. On investigation by the
California Department of Food and Agriculture, it was found that the
illness had occurred in workers harvesting grapes from vineyards
treated with a preparation containing phosalone. A study was
therefore carried out in which grapes were treated with phosalone (3
lb/acre) twice, 24 days apart. Fourteen days after the second
treatment, 30 volunteers harvested the crop for 6 consecutive days
while 22 volunteers harvested a crop from an untreated vineyard.
Assignment to the treated or control group was random. Blood was
collected for plasma and erythrocyte cholinesterase activity in the
afternoon of days 4, 3 and 2 pre-harvest, daily during harvest, and
three days thereafter. Samples were transported cooled in ice and
assayed within six hours by a modified Ellman method. Urinary
diethylphosphates were assayed on early morning urine samples
collected on days 6, 5, 4 and 1 before harvest, daily during
harvest, and days 1 and 2 thereafter. No symptoms or clinical signs
were recorded that could be attributed to anticholinesterase
effects. There was a gradual decline in plasma cholinesterase
levels during the harvest, which recovered after the end of the
harvest. This effect was not seen in controls nor in red blood cell
cholinesterase level in either test or control groups. Ethyl
phosphate metabolites were found in the urine of the test group
(Baugher, 1989). A similar study was carried out on French apple
pickers (Vogel, 1991). No clinical signs, symptoms suggestive of
anticholinesterase toxicity, or significant depression of plasma or
erythrocyte cholinesterase were observed.
In 1966-1979, eleven pesticide incidents involving the
agricultural use of phosalone were reported to the USEPA's Pesticide
Incident Monitoring System (Hodgson & Smith, 1992). The antidote
dietixim (a cholinesterase reactivator) was effective in human
phosalone poisoning (Kundiev & Kagan, 1992).
COMMENTS
After oral administration, phosalone was moderately well
absorbed, 15-25% appearing in the faeces. Phosalone was extensively
metabolized to phosphorothioates, phosphorodithioates and
3-methylthiomethyl 6-chloro-benzoxazolone, the last of which is
subsequently metabolized ultimately to 3-methylsulfonylmethyl 6-
chlorobenzoxazolone.
Pure phosalone is almost certainly not a cholinesterase
inhibitor, but acquires inhibitory activity after conversion to
phosalone oxon in vivo.
The acute oral toxicity varies with species, but is in the
region of 100-200 mg/kg bw in rodents. Phosalone has been
classified as moderately hazardous by WHO.
There were two short-term studies in rats which could be used
to give NOAELs. In a five-week oral gavage study in rats at doses
of 0, 7.5 or 15 mg/kg bw/day, the NOAEL or 7.5 mg/kg bw/day based on
brain cholinesterase inhibition. In an eight-week study in rats
using dietary concentrations of 0, 10, 100, 300, 600 or 1200 ppm,
the NOAEL was 10 ppm (equal to 0.87 mg/kg bw/day), based on brain
cholinesterase inhibition. It is possible that NOAELs could have
been established at 100 or 300 ppm, but the dose rates for those
groups were increased to 2400 and 4800 ppm, respectively, after 5
weeks to establish a maximum tolerated dose.
Five studies were carried out in dogs. In a one-month oral
dosing study in dogs, a NOAEL could not be determined as plasma and
erythrocyte cholinesterase depression were seen at the lowest dose
(7.5 mg/kg bw/day). In another one-month study using dietary
concentrations of 0, 12.5, 25 or 37.5 ppm, the NOAEL was the highest
level, which was equal to 0.81 mg/kg bw/day; although plasma
cholinesterase depression was seen in the study, neither erythrocyte
nor brain cholinesterase activity was depressed. In a 6-month study
in dogs using dietary concentrations of 0, 10 or 25 ppm, although
plasma and erythrocyte cholinesterase were depressed, the brain
enzyme was not, so the NOAEL was the highest dose (equivalent to
0.63 mg/kg bw/day). In a two-year study in beagle dogs, males and
females were fed phosalone in the diet at concentrations of 0, 100,
200 or 1000 ppm. The NOAEL was 200 ppm, equivalent to 5 mg/kg
bw/day, based on brain cholinesterase depression, body-weight loss
and elevated alanine aminotransferase at the highest dose. In a
more recent one-year study in dogs using dietary concentrations of
0, 5, 25 or 300 ppm phosalone, the NOAEL was 25 ppm (equal to 0.89
mg/kg bw/day), based on brain cholinesterase depression at 300 ppm.
The overall NOAEL for dogs was considered to be 200 ppm, in view of
the spacing of the doses in the most recent study.
In a lifetime carcinogenicity study in mice, phosalone was
given at dietary concentrations of 0, 15, 50 or 150 ppm. The NOAEL
was 150 ppm, equal to 23 mg/kg bw/day, based on the lack of
depression of brain cholinesterase activity, although plasma and red
blood cell cholinesterase depression were seen at this level. There
was no evidence of carcinogenicity.
In a two-year study in rats using concentrations of 0, 25, 50
or 250 ppm phosalone in the diet, the NOAEL was 50 ppm, equivalent
to 2.5 mg/kg bw/day, based on brain cholinesterase depression at the
highest dose. In a second 2-year study in rats, dietary
concentrations of 0, 5, 50 or 1000 ppm were used, the highest dose
being reduced to 500 ppm later in the study. There was a
statistically significant increase in the prevalence of testicular
atrophy and reduction in testicular weight in both the high and mid-
dose groups and a dose response across all groups for both effects.
The Meeting concluded that the NOAEL was < 5 ppm, equal to 0.2
mg/kg bw/day.
In a teratogenicity study in rats at doses of 0, 2, 10 or 20
mg/kg bw/day, the NOAEL was 10 mg/kg bw/day for both maternal
toxicity and fetotoxicity. In a study in rabbits using doses of 0,
2, 6 or 18 mg/kg bw/day, the NOAEL was the highest dose. In another
study in rabbits, using doses of 0, 1, 10 or 20 mg/kg bw/day, the
NOAEL was 10 mg/kg bw/day based on maternal toxicity. Phosalone was
not teratogenic in either the rat or rabbit.
Two multigeneration reproduction studies in rats were reviewed.
In the first study, using dietary concentrations of phosalone of 25
or 50 ppm, no adverse effects were observed. The NOAEL was > 50
ppm, equivalent to 2.5 mg/kg bw/day. In the second study, in which
phosalone was administered at dietary concentrations of 0, 10, 50 or
400 ppm, the NOAEL was 50 ppm (equivalent to 2.5 mg/kg bw/day) based
on retarded pup growth and plasma and erythrocyte cholinesterase
depression.
Phosalone has been adequately tested in a series of in vitro
and in vivo genotoxicity assays. The Meeting concluded that
phosalone was not genotoxic.
There is no evidence that phosalone has potential to cause
organophosphate-induced delayed neuropathy.
Pralidoxime salts and obidoxime are both effective in
experimental phosalone poisoning.
No human study was available from which a NOAEL could be
derived.
An ADI of 0-0.001 mg/kg bw was established based on the lowest
dose (0.2 mg/kg bw/day) in the recent two-year study in rats. A
200-fold safety factor was used because of concerns that the trend
for the occurrence of testicular atrophy and reduction in testis
weight existed across all groups.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 150 ppm, equal to 23 mg/kg bw/day (two-year study)
Rat: < 5 ppm, equal to 0.2 mg/kg bw/day
(two-year study)
50 ppm, equivalent to 2.5 mg/kg bw/day
(multigeneration reproduction study)
Rabbit: 10 mg/kg bw/day (teratology study)
Dog: 200 ppm, equivalent to 5 mg/kg bw/day
(several studies)
Estimate of acceptable daily intake for humans
0-0.001 mg/kg bw
Studies which will provide information valuable in the continued
evaluation of the compound
Explanation of the testicular atrophy seen in the recent study
in rats.
Observations in humans.
REFERENCES
Allen, P.A. (1989) Unpublished study. Additional evaluation of the
incidence of resorptions in the studies performed by RCC on the
effects of phosalone technical on embryonic and fetal development in
the rabbit. Project 083002, 204423, 082991. RCC Research and
Consulting Company AG, PO Box, CH 4452 Itingen, Switzerland.
Supplied to WHO by Rhône-Poulenc.
Allen, P.A., Frei, D., Mladenovic, P. & Terrier C. (1989a)
Unpublished study. Embryotoxicity study (including teratogenicity)
with phosalone technical in the rat. Project 082980. RCC Research
and Consulting Company AG, PO Box, CH 4452 Itingen, Switzerland.
Supplied to WHO by Rhône-Poulenc.
Allen, P.A., Mladenovic, P. & Terrier, C. (1989b) Unpublished study.
Embryotoxicity study (including teratogenicity) with phosalone
technical in the rabbit. Project 083002. RCC Research and Consulting
Company AG, PO Box, CH 4452 Itingen, Switzerland. Supplied to WHO by
Rhône-Poulenc.
Barker, M.H., Smith, T.G., Buist, D.P., Crook, D., Morrow, J. &
Gopinath, C. (1992) Unpublished study. Phosalone dietary toxicity
study in beagle dogs. HRC report No. RNP 336/91907. Huntingdon
Research Centre Ltd, Wooley, Huntingdon, PE18 6ES, England. Supplied
to WHO by Rhône-Poulenc.
Barker, M.H. & Sortwell, R.J. (1993) Unpublished study. Phosalone
(11974 RP). Potential tumorigenic and toxic effects in prolonged
dietary administration to rats Volume 1. Huntingdon Research Centre
Ltd, PO Box 2, Huntingdon, Cambridgeshire PE18 6ES. Supplied to WHO
by Rhône-Poulenc. England
Baugher (1989). Unpublished study. Exposure of harvesters to Zolone
(R) EC brand insecticide applied to grapes in California, 1988.
Project no 29088. Orius Associates, Frederick, Maryland, USA.
Supplied to WHO by Rhône-Poulenc.
Benezet, F. & Cartier, J.R. (1980) Unpublished study. Phosalone (11
974 RP) mutagenicity study on Salmonella typhimurium. Report
RP/RD/CNG No. 19 234 E of 19th January 1978. Centre Nicolas Grillet,
94400 Vitry-sur-Seine, France. Supplied to WHO by Rhône-Poulenc.
Bryson, A.M., Parker, C.A., Offer, J.M. & Farmer, H. (1991)
Unpublished study. A study of the effect of phosalone on
reproductive function of two generations in the rat. Report No. RNP
326/91277. Huntingdon Research Centre Ltd, PO Box 2, Huntingdon,
Cambridgeshire, PE18 6ES England. Supplied to WHO by Rhône-Poulenc.
Cifone, M.A. (1989) Unpublished study. Mutagenicity test on
phosalone technical in the rat primary hepatocyte unscheduled DNA
synthesis test. HLA study No. 10754-0-447, Hazleton Laboratories
America Inc 5518 Nicholson Lane, Suite 400, Kensington Maryland
20895, USA. Supplied to WHO by Rhône-Poulenc.
Cordier, A. & Bonneau, D. (1985) Unpublished study. Phosalone (11
974 RP) CHO/HPRT test. Report. ST/CRV/Tox. No. 22 363-E of 10th May
1985. Rhône-Poulenc Santé, Centre de Recherches de Vitry, 94403
Vitry-sur-Seine Cedex, France. Supplied to WHO by Rhône-Poulenc.
Cordier, A. & Fournier, E. (1985) Unpublished study. Phosalone (11
974 RP) chromosome aberration test in Chinese hamster ovary cells
(CHO). Report ST/CRV/Tox. No. 435-E of 28th August 1985. Rhône-
Poulenc Santé, Centre de Recherches de Vitry, 94403 Vitry-sur-Seine
Cedex, France. Supplied to WHO by Rhône-Poulenc.
Craine, E.M. (1974a) Unpublished study. Disposition of phosalone-
14C in a lactating cow. Research report No. EMC 74:17 of the 29th
April 1974. Hess and Clark Research Department, Ashland, Ohio, USA.
Supplied to WHO by Rhône-Poulenc.
Craine, E.M (1974b) Unpublished study. Disposition of phosalone-14C
in a lactating cow. Research report No. EMC 74:84 of the 25th
November 1974. Hess and Clark Research Department, Ashland, Ohio,
USA. Supplied to WHO by Rhône-Poulenc.
Craine, E.M. (1974c) Unpublished study. The disposition of
phosalone-14C applied to the skin of a pig. Research report No. EMC
74:30 of the 14th May 1974. Hess and Clark Research Department,
Ashland, Ohio, USA. Supplied to WHO by Rhône-Poulenc.
Craine, E.M. (1975) Unpublished study. The metabolism of phosalone-
14C in dairy cows. Research report No. EMC 75:51 of the 2nd July
1975. Hess and Clark Research Department, Ashland, Ohio, USA.
Supplied to WHO by Rhône-Poulenc.
Cunny, H. & Siglin, J.C. (19789) Unpublished study. Delayed contact
hypersensitivity study in guinea-pigs with phosalone technical.
Study No. 3147.37. Springborn Life Sciences Inc. Spencerville, Ohio
45887, USA. Supplied to WHO by Rhône-Poulenc.
Desmoras, M.J. (1979). Unpublished study. Studies on the metabolism
of phosalone (11974 R.P.), in the mouse, the rat and the rabbit.
Note RP/RD/CNG No 20 234 of the 20 August 1979. Supplied to WHO by
Rhône-Poulenc.
Desmoras, M.J. & Fournel, J. (1968) Unpublished study. Studies on
the degradation of phosalone (11974 R.P.), in mammals. Note RP -
DSPh of the 1st March 1968. Rhône-Poulenc, Laboratoires de
Recherches, 22 Avenue Montaigne, 75 Paris 8, France. Supplied to WHO
by Rhône-Poulenc.
Donoso, J., Woodard, M.W. & Woodard, G. (1967) Unpublished study.
Phosalone safety evaluation by repeated oral administration to dogs
for 107 weeks. Report dated 7th September 1967. Woodard Research
Corporation, Herndon, Virginia 22070 USA. Supplied to WHO by Rhône-
Poulenc.
Dubost, P. Fournel, J., Ganter, P., Julou, L., Koenig, F. & Myon, J.
(1963). Unpublished study. Acute toxicity, local tolerance,
anticholinesterase activity and chronic toxicity in rats and dogs.
Note DSPh 9, 203 of the 29th March 1963. Centre Nicolas Grillet,
94400 Vitry-sur-Seine, France. Supplied to WHO by Rhône-Poulenc.
Fournel, J., Ganter, P., Julou, L., Populaire, P., Myon, J., Pascal,
S. & Pasquet, J. (1966) Unpublished study. Phosalone (11 974 RP)
Sub-acute (6-month) toxicity study in the dog. Note RP-DSPh 11 330 E
of the 14th June 1966. Société des Usines Chimiques Rhône-Poulenc,
Direction Scientifiques, Laboratoires de Recherche, Centre Nicolas
Grillet, 94400 Vitry-sur-Seine, France. Supplied to WHO by Rhône-
Poulenc.
Fournel, J., Julou, L. & Pasquet, J. (1968) 12244 R.P. Unpublished
study. Acute toxicity in mice rats and in vivo anti-cholinesterase
activity in rats. Note RP - DSPh 12663 (=12665E) of the 1st March
1968. Société des Usines Chimiques Rhône-Poulenc, Direction
Scientifiques, Laboratoires de Recherche, 22 Avenue Montaigne, 75
Paris 8,France. Supplied to WHO by Rhône-Poulenc.
Goldenthal, E.I., Jessup, D.C. & Rodwell, D.E. (1978) Unpublished
study. Dominant lethal study in mice. Study carried out for Rhône-
Poulenc dated the 18th December 1978. International Research and
Development Corporation, Mattawan, Michigan, 49071, USA.
Haworth, L. & Lawlor, T.E. (1989) Unpublished study. Mutagenicity
test on phosalone technical in the Ames Salmonella/microsome
reverse mutation assay. HLA study No. 10754-0-401, Hazleton
Laboratories America Inc., 5518 Nicholson Lane, Suite 400,
Kensington Maryland 20895, USA. Supplied to WHO by Rhône-Poulenc.
Heath, S.A.B., Rivett, K.F. & Woolf, N. (1967) Unpublished study.
Phosalone test for neurotoxicity. Study No. 230857, May and Baker
Ltd, Dagenham, Essex, England. Supplied to WHO by Rhône-Poulenc.
Hayward, A. (1969). Unpublished study. Insecticides: a study of the
effects of "Cyanox" (S-4084) and "phosalone" on rat erythrocyte
cholinesterase. Report No. PRG/584 (=274359). May and Baker Ltd,
Ongar, Essex, England. Supplied to WHO by Rhône-Poulenc.
Hodgson, H.J. & Smith, A.D. (1992). Commercial and residential
poisoning with anticholinesterases. In: Ballantyne B and Marrs TC
(ed) Clinical and experimental toxicology of organophosphates and
carbamates, pp. 352-363. Butterworth-Heinemann, Oxford, England.
Hopkins, R., Lewis, C.J. & Lewsley, R. (1991). Unpublished study.
(14C)-phosalone: a study of the absorption, distribution,
metabolism and excretion in the rat. Report No. 5759-68/93R.
Hazleton UK, Harrogate, England. Supplied to WHO by Rhône-Poulenc.
Jones, E.M., Post, K.F., Scott, W.J., Woodyard, M.W., Woodyard, G. &
Cronin, M.T.I. (1967a) Unpublished study. Phosalone three generation
reproduction study in the rat. Report dated September 11th 1967.
Woodyard Research Corporation and Chipman Chemical Company. Supplied
to WHO by Rhône-Poulenc.
Jones, M.E., Woodard, M.W., Imming, R.J., Woodard, G. & Cronin,
M.T.I. (1967b) Unpublished study. Phosalone followed by a
determination of potentiation activity with the compound Di-syston.
Report dated August 10th, 1967. Woodyard Research Corporation and
Chipman Chemical Company. Supplied to WHO by Rhône-Poulenc.
Kehoe, D.F. (1990) Unpublished study. 8-week dietary toxicity study
with phosalone in rats. HLA study No. 6224-149, Hazleton
Laboratories America Inc 3301 Kinsman Boulevard, Madison, Wisconsin
53704, USA. Supplied to WHO by Rhône-Poulenc.
Knaak, J.B., Maddy, K.T., Gallo, M.A., Lillie, D.T., Craine, E.M &
Serat, W.F. (1978) Worker re-entry study involving phosalone
application to citrus groves. Toxicol Appl. Pharmacol., 46 363-
374.
Kundiev, Y.I. & Kagan, Y.S. (1992) Anticholinesterses used in the
USSR: poisoning, treatment and preventative measures. In: Ballantyne
B and Marrs TC (ed) Clinical and experimental toxicology of
organophosphates and carbamates.pp 494-501. Butterworth-Heinemann,
Oxford, England.
Kynoch, S.R., Lloyd, G.K., Mallard, J.R., Street, A.E., Gibson,
W.A., Wadsworth, P.F. & Prentice, D.E. (1979) Unpublished study. The
effect of repeated applications of phosalone 11974 RP to the skin of
rabbits for 21 days. Report No. RNP 125/79335. Huntingdon Research
Centre Ltd, PO Box 2, Huntingdon, Cambridgeshire, PE18 6ES, England.
Supplied to WHO by Rhône-Poulenc.
Marrs, T.C. (1991). Toxicology of oximes used in the treatment of
organophosphate poisoning. Adverse drug reactions and Toxicology
Reviews, 10: 61-72.
Mazuret, A. (1971) Unpublished study. Phosalone,methyl-azinphos and
parathion acute toxicity in the rat. Report No. RP -DSPh No. 15544-
E. Rhône-Poulenc Santé, Centre de Recherches de Vitry, 94403 Vitry-
sur-Seine Cedex, France. Supplied to WHO by Rhône-Poulenc.
Morris, J.M. (1983) Unpublished study. Acute delayed neurotoxicity
study in hens with phosalone. GSRI project No. 411-B51-40, Gulf
South Research Institute, PO Box 1177, New Iberia, Louisiana 70560,
USA. Supplied to WHO by Rhône-Poulenc.
Murli, H. (1989) Unpublished study. Mutagenicity test on phosalone
technical in an in vitro cytogenetic assay measuring chromosomal
aberration frequencies in Chinese hamster ovary (CHO) cells. HLA
study No. 10754-0-437, Hazleton Laboratories America Inc., 5518
Nicholson Lane, Suite 400, Kensington, Maryland 20895, USA. Supplied
to WHO by Rhône-Poulenc.
Nelson, L.W., Geil, R.G. & Spicer, E.J. (1980) Unpublished study.
Phosalone lifetime oncogenicity study in mice. International
Research and Development Corporation, Mattawan, Michigan 49071, USA.
Report 347-009, submitted June 23rd, 1980. Supplied to WHO by Rhône-
Poulenc.
Noel, P.R., Rivett, K.F., Osborne, B.E. & Street, A.E. (1970)
Unpublished study. Phosalone dietary intake in beagle dogs for four
weeks. Report No. 3619/70/431. Huntingdon Research Centre Ltd, PO
Box 2, Huntingdon, Cambridgeshire, PE18 6ES, England. Supplied to
WHO by Rhône-Poulenc.
Owen, P.E. (1985) Unpublished study.6-chlorobenzoxolone: 13 week
oral (dietary administration) study in the rat with a 28-day
treatment-free period. Report No. 4229-198/14. Hazleton UK, Otley
Road, Harrogate, England. Supplied to WHO by Rhône-Poulenc.
Pasquet, J. (1969) Unpublished study. Study of the teratogenic
activity of phosalone (11974 RP) on the chick embryo and on the
rabbit. Note RP - DSPh 13447E of the 7th February 1969 (translation
no 2707). Centre Nicolas Grillet, 94400 Vitry-sur-Seine, France.
Supplied to WHO by Rhône-Poulenc.
Pasquet, J. (1971) Unpublished study. Phosalone (11 974 R.P.)
toxicité aigüe chez la ratte par voie percutanée. Note RP - DSPh
15481 of the 28th June 1971. Centre Nicolas Grillet, 94400 Vitry-
sur-Seine, France. Supplied to WHO by Rhône-Poulenc.
Pasquet, J. & Fournier, E. (1980) Unpublished study. Phosalone (11
974 RP) micronucleus test in the mouse. Report.RP/RD/CNG No 557-E of
8th April 1980. Rhône-Poulenc Santé, Centre de Recherches de Vitry,
94403 Vitry-sur-Seine Cedex, France. Supplied to WHO by Rhône-
Poulenc.
Pasquet, J. & Mazuret, A. (1970) Unpublished study. Obidoxime
chloride (Toxogonin R Merck = 24,590 R.P. chloride) acute
intraperitoneal toxicity in the mouse and antidotal effect on the
acute oral toxicity in the mouse of phosalone (11974 RP) RP - DSPh
No. 14514-E. Rhône-Poulenc Santé, Centre de Recherches de Vitry,
94403 Vitry-sur-Seine Cedex, France. Supplied to WHO by Rhône-
Poulenc.
Pasquet, J., Mazuret, A., Vaissere, J. & Gaildrat (1983a)
Unpublished study. Chloromethyl-3 chloro-6 benzoxolone (16891 R.P.)
- acute toxicity in the rat by the intraperitoneal route. Report
ST.CRV/Tox No. 21971. Rhône-Poulenc Santé, Centre de Recherches de
Vitry, 94407 Vitry-sur-Seine Cedex, France. Supplied to WHO by
Rhône-Poulenc.
Pasquet, J., Mazuret, A. & Vaissere, J. (1983b) Unpublished study.
Chlorométhyl-3 chloro-6 benzoxolone (16891 R.P.) - eau de lavage de
la solution chlorométhyléique - irritation cutanée primaire chez le
lapin. Note No ST.CRV/Tox. No. 21972. Rhône-Poulenc Santé, Centre de
Recherches de Vitry, 94407 Vitry-sur-Seine Cedex, France. Supplied
to WHO by Rhône-Poulenc.
Pasquet, J. & Mazuret, A. (1973a). Unpublished study. Phosalone
oxygen analog (12244 R.P.) acute percutaneous toxicity in the rat.
SUCRP - DSPh No.16979. Rhône-Poulenc,Laboratoires de Recherches, 22
Avenue Montaigne, 75 Paris 8, France. Supplied to WHO by Rhône-
Poulenc.
Pasquet, J. & Mazuret, A. (1973b). Unpublished study. Hydroxymethyl-
3 chloro-6 benzoxolone (16 236 RP) toxicité et tolérance locale. RP)
acute percutaneous toxicity in the rat. Note SUCRP - DSPh No.
17144.Rhône-Poulenc Santé, Centre de Recherches de Vitry, 94403
Vitry-sur-Seine Cedex, France. Supplied to WHO by Rhône-Poulenc.
Supplied to WHO by Rhône-Poulenc.
Pasquet, J. & Mazuret, A. (1977). Unpublished study. Phosalone (11
974 RP) guinea-pig skin sensitization study. Report RP/RD/CNG No.
19117 of 27th April 1977. Centre Nicolas Grillet, 94400 Vitry-sur-
Seine, France. Supplied to WHO by Rhône-Poulenc.
Pasquet, J. & Mazuret, A. (1980). Unpublished study. Metabolites of
phosalone (11 974 RP) : compounds 19889 RP, 19889 RP and 19914 RP.
Acute oral toxicity in the mouse. Report C.R. Vitry/CNG. No. 20 736-
E of 12th November 1980. Centre Nicolas Grillet, 94400 Vitry-sur-
Seine, France. Supplied to WHO by Rhône-Poulenc.
Pasquet, J., Mazuret, A., Fournel, M. & Koenig, M. (1976)
Unpublished study. Acute oral and percutaneous toxicity of phosalone
(R.P.11974) in the rat, in comparison with azinphosmethyl and
parathion. Note RP/RD/CNG No. 18 550 EP/LR of 27th February 1976.
Centre Nicolas Grillet, 94400 Vitry-sur-Seine, France. Supplied to
WHO by Rhône-Poulenc.
Reddy, S.J., Reddy, B.V. & Ramamurthy, R. (1992). Impact of chronic
phosalone toxicity on Bohr factor and oxygen equilibrium curves of
rat. Biochem Inter. 26: 171-179.
Rhône-Poulenc (1968) Unpublished study. 12244 R.P. oxygen analog of
phosalone acute oral toxicity and anticholinesterasic activity in
vitro. Note RP - DSPh 12664 of the 20th February 1968. Société des
Usines Chimiques Rhône-Poulenc, Direction Scientifiques,
Laboratoires de Recherche, 22 Avenue Montaigne, 75 Paris 8, France.
Supplied to WHO by Rhône-Poulenc.
Rhône-Poulenc Inc. (1979) Unpublished study. Phosalone dairy cow
feeding study. ADC project #475A, B of the 6th July 1968. Rhône-
Poulenc Inc., Agricultural Division, PO Box 125, Black Horse Lane,
Monmouth Junction, New Jersey 08852, USA. Supplied to WHO by Rhône-
Poulenc.
Rhône-Poulenc Inc. (1980a) Unpublished study. Residue determination
of phosalone and its oxygen analog in the milk and tissues of dairy
cattle by electron capture gas chromatography. ADC project #475D of
the 22nd September 1968. Rhône-Poulenc Inc., Agricultural division,
PO Box 125, Black Horse Lane, Monmouth Junction New Jersey 08852,
USA. Supplied to WHO by Rhône-Poulenc.
Rhône-Poulenc Inc. (1980b) Unpublished study. Residue determination
of metabolites containing the chlorobenzoxazole and
chloroaminoiphenol moieties in the milk and tissues from dairy
cattle fed phosalone. ADC project #475D1 of the 22nd September 1968.
Rhône-Poulenc Inc., Agricultural division, PO Box 125, Black Horse
Lane, Monmouth Junction, New Jersey 08852, USA. Supplied to WHO by
Rhône-Poulenc.
Rhône-Poulenc Inc. (1980c) Unpublished study. Validation of
analytical methods of phosalone and its metabolites in milk and
animal tissues. ADC project #496 of the 22nd September 1968. Rhône-
Poulenc Inc., Agricultural Division, PO Box 125, Black Horse Lane,
Monmouth Junction, New Jersey 08852, USA. Supplied to WHO by Rhône-
Poulenc.
Safonov, N.M. (1968) (Toxicological characteristics of a new
organophosphorus insecticide: phosalone preliminary results).
Gigiena truda professional, nye zabolevanija, 12, 43 - Translation
supplied to WHO by Rhône-Poulenc.
SERB (1988) Contrathion, pralidoxime, pralidoxima, Data Sheet.
Laboratoires SERB, 53 Rue de l'Isle-Adam, 75020, Paris, France.
Siglin, J.C., Jenkins, P.K., Rush, R.E., Liao, J.T. & Rodwell, D.E.
(1979) Unpublished study. Primary eye irritation study of phosalone
technical in rabbits with washout (EPA). Study No. 3147.36.
Springborn Life Sciences, Inc., Spencerville, Ohio 45887, USA.
Supplied to WHO by Rhône-Poulenc.
Vanrel, B., Thenaisie, S., Gascoin, M.N., Vanrell, B., Glomot, R. &
Le Bigot, J.F. (1989) Unpublished study. Metabolite of phosalone in
soil reverse mutation assay in vitro Ames test. Study No. 5137
MMO. Rhône-Poulenc Agrochimie, Lyon, France. Supplied to WHO by
Rhône-Poulenc.
Vogel, W.(1991). Unpublished study. Exposure of harvesters to
residues of Zolone(R) flo (Phosalone) insecticide applied to apples
in France in 1989. RCC project No 241683. RCC Umweltchemie AG, PO
Itingen CH 4452 Switzerland. Supplied to WHO by Rhône-Poulenc.
Woodard, M.W., Cockrell, K.O., Woodyard, G. & Cronin, M.T.I. (1966)
Unpublished study. Phosalone demyelination study in chickens. Report
submitted April 7th, 1966 to the Chipman Chemical Company. Woodard
Research Corporation. Supplied to WHO by Rhône-Poulenc.
Woodard Research Corporation (1967) Unpublished study. Phosalone
safety evaluation by repeated oral administration to rats for 103-
104 weeks. Report submitted April 7th, 1966 to the Chipman Chemical
Company. Supplied to WHO by Rhône-Poulenc.
Weill, N. (1988) Unpublished study. Ames test on Salmonella
typhimurium on 50221 RP. Report No. 803380. Hazleton France.
Supplied to WHO by Rhône-Poulenc.
WHO (1986) Environmental Health Criteria 63. Organophosphorus
insecticides: a general introduction. World Health Organization,
Geneva, p 63.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
technical grade phosalone is probably due to small amounts of oxon
present (up to 0.5% of the present technical material). The reason
that phosalone oxon did not inhibit rat brain cholinesterase at
single doses up to 9 mg/kg bw, whereas phosalone did at 27 mg/kg bw
(surprising in view of the much higher acute toxicity of the former)
is obscure (Fournel et al., 1968). Inhibition of brain
cholinesterase by phosalone was also observed by Pasquet et al.
(1976) with an I50 of 31 mg/kg bw p.o. Phosalone was reported to
shift to the right the oxygen dissociation curve in rats (Reddy et
al., 1992).
Toxicological studies
Acute toxicity studies
A summary of acute toxicity data for phosalone is shown in
Table 1. Phosalone has been classified as moderately hazardous by
WHO (WHO, 1992).
Short-term toxicity studies
Rats
A dose of 50 mg/kg bw/day administered by gavage (vehicle
unstated) to white rats for 14 days was lethal (Safonov, 1968).
Phosalone was administered as an oil solution to male rats
(10/dose) (strain unstated) at doses of 7.5 or 15 mg/kg bw/day for 5
weeks. The only death seen in the study was a control rat and no
abnormal clinical signs were observed. Slight reductions in body-
weight gain noted at both dose levels were not statistically
significant. No major differences between test and control groups
were seen in haematology or clinical chemistry except in
cholinesterase activity. Depression of blood cholinesterase
activity (> 20%) was observed at both doses. Brain cholinesterase
activity was reduced at the high dose only. No statistically
significant differences in organ weights or pathological findings
were seen between test groups and controls. The NOAEL was 7.5 mg/kg
bw/day, based on brain cholinesterase inhibition (Dubost et al.,
1963).
In an 8-week study, groups of 10 Crl:CD(SD) rats/sex/dose
received 10, 100, 300, 600 or 1200 ppm phosalone (94.1% pure) in the
diet. After 5 weeks, the doses administered to the 100 and 300 ppm
groups were increased to 2400 and 4800 ppm, respectively, in order
to establish the maximum tolerated dose. At 300/4800 ppm, one
female died and all remainder animals were sacrificed in extremis.
At 100/2400 ppm all females had to be sacrificed. Males at both
these doses survived but showed reduced body weight and food
Table 1. Acute toxicity of phosalone
Species Strain Sex Route LD50 (mg/kg bw) References
Mouse CD-1(COBS) M p.o. 118 (97-144) Fournel et al. (1968)
Mouse CD-1(COBS) F p.o. 93 (74-116) Fournel et al. (1968)
Mouse ? ? p.o. 180* 435** Dubost et al. (1963)
Mouse ? ? p.o. 205 Dubost et al. (1963)
Mouse ? ? p.o. 180 Safonov (1968)
Rat ? M p.o. 120 Dubost et al. (1963)
Rat CD(COBS) M p.o. 125 (102-152) Fournel et al. (1968)
Rat ? F p.o. 170 Dubost et al. (1963)
Rat ? F p.o. 135 Dubost et al. (1963)
Rat CD(COBS) F p.o. 90 (68-119) Fournel et al. (1968)
Rat ? ? p.o. 50 Hayward (1969)
Rat ? ? p.o. 135 Safonov (1968)
Rat CD(COBS) M p.o. 130 (104-163) Pasquet et al. (1976)
Rat CD(COBS) F p.o. 110 (87-139) Pasquet et al. (1976)
Rat ? F p.c. 390 Dubost et al. (1963)
Rat CD(COBS) F p.c. 700 (480-1015) Pasquet (1971)
Rat CD(COBS) F p.c. 1530 Mazuret (1971)
Rat ? F p.c. > 2560 Pasquet & Mazuret (1973a)
Rat CD(COBS) F p.c. 1530 Pasquet et al. (1976)
Rabbit ? ? p.c. > 1000 Dubost et al. (1963)
Guinea-pig ? ? p.o. 150 Dubost et al. (1963)
Guinea-pig ? ? p.o. 82 Dubost et al. (1963)
Hens Rhode Island red F s.c. 350 Heath et al (1967)
* Aqueous Suspension
** Oil solution
consumption. Body weight and cumulative body-weight gain were
significantly lower in females receiving 1200 ppm. No changes were
seen in the eyes. Significantly lower plasma and erythrocyte
cholinesterase were seen at 100 ppm in both sexes. Brain
cholinesterase activity was decreased at 600 ppm to about 80% and
50% of controls in males and females, respectively. Other changes
in clinical chemistry parameters were seen at higher doses,
including lower blood glucose, total protein and albumen and higher
cholesterol and blood urea. No treatment-related changes were seen
in organ weights or in organs on macroscopic or microscopic
examination. The NOAEL was 10 ppm, equal to 0.87 mg/kg bw/day in
males and 0.93 mg/kg bw/day in females, based on brain
cholinesterase inhibition (Kehoe, 1990).
Rabbits
A 3-week percutaneous study was undertaken in rabbits using
phosalone at doses of 0.4, 2.0 or 10 mg/kg bw/day
acetone/ethanol/arachid oil. There were no deaths. A 20%
depression in brain cholinesterase occurred in the highest dose
females. Some irritancy was observed (Kynoch et al., 1979).
Dogs
Phosalone was administered orally to dogs in oil solution for 1
month at dose levels of 7.5, 15 or 30 mg/kg bw/day. One animal of
each sex was used at each dose without controls. Neither clinical
signs nor significant weight changes were noted. Albumen/globulin
ratios in both animals treated with phosalone at 7.5 mg/kg bw/day
were depressed; however, this was not seen in the 15 mg/kg bw/day
animals. Significant plasma and erythrocyte cholinesterase
depression were seen in all animals except in females at 7.5 mg/kg
bw/day. A NOAEL could be not be determined in this study (Dubost
et al., 1963).
In another one-month study, groups of 4 beagle dogs/sex/dose
were administered phosalone (100% pure) at dietary concentrations of
0, 12.5, 25 or 37.5 ppm. No deaths were observed nor were there
abnormal clinical signs. There was no adverse effect on body
weight. Plasma cholinesterase activity was depressed by > 20% in
the high-dose group. Erythrocyte and brain cholinesterase activity
were not affected. At terminal autopsy a mauve discoloration was
observed in the bladder of all high- and mid-dose animals and in one
female at the low dose. Since the bladder discoloration was not seen
in any other dog study, the NOAEL was the highest dose of 37.5 ppm,
equal to 0.81 mg/kg bw/day (Noel et al., 1970).
In a 6-month study, groups of beagle dogs (4/sex/dose) received
phosalone at 0, 10 or 25 ppm in the diet. No haematological,
biochemical or histological changes attributable to phosalone were
observed except in erythrocyte and plasma cholinesterase. Brain
cholinesterase activity was not depressed. Depression of plasma
cholinesterase activity of about 40% and 25% was observed in the 25
ppm and 10 ppm groups, respectively. Decreased erythrocyte
cholinesterase activity was seen only at 25 ppm towards the end of
the study. The NOAEL was the highest dose (25 ppm, equivalent to
0.63 mg/kg bw/day) (Fournel et al,. 1966).
In a 107-week study, phosalone (purity unknown) was
administered in the diet to groups of 4 beagle dogs/sex/dose at
concentrations of 0, 100, 200 or 1000 ppm. One female at 1000 ppm
died during the study. Although marked plasma and erythrocyte
cholinesterase depression were observed, the immediate cause of
death seemed to be an infection. Body-weight loss was seen at 100
ppm, and cholinergic symptomatology was seen in two animals at the
highest dose and in one animal at 100 ppm. Abnormal ECGs (increased
T-waves) were seen at 1000 ppm. Biochemical and haematological
investigations demonstrated no changes that appeared to be compound-
related apart from elevations in ALAT in 4 animals at 1000 ppm.
Reductions in plasma cholinesterase > 20% were observed at 100 ppm
and above, and in erythrocyte cholinesterase at 200 ppm and above.
The degree of brain cholinesterase inhibition was variable, with a
mean in the 1000 ppm group of 79% of controls. Inhibition at
termination of the study was considerable in 3/8 animals and almost
complete in 2 animals. The NOAEL was 200 ppm, equivalent to 5 mg/kg
bw/day, based on clinical signs, body-weight loss, elevated ALAT and
brain cholinesterase depression at 1000 ppm (Donoso et al., 1967).
Groups of 6 beagle dogs (6/sex/dose) received 0, 5, 25 or 300
ppm phosalone (94.5% pure) in the diet for 52 weeks. Three deaths
in different groups were observed. During week 43, all animals
receiving 300 ppm vomited and lost weight and 5 showed reduced food
consumption. Some recovery in weight occurred towards the end of
the study. No changes of toxicological importance occurred on
ophthalmological, haematological, biochemical or urinary examination
except for cholinesterase activity. Reduction in plasma
cholinesterase > 20% compared to controls occurred in the 25 and
300 ppm groups (both sexes and combined and at all time points after
dosing commenced). At 5 ppm, significant reductions occurred in
males only at 4, 26, and 52 weeks. These reductions were just under
20%. Red cell cholinesterase was significantly reduced at 300 ppm
at all time points. At 25 ppm, this enzyme was reduced compared to
the control group in males at 26 weeks and in both sexes at 52
weeks. The reduction in this group compared to pre-dosing levels
was < 15%. Brain cholinesterase was significantly reduced in both
sexes at 300 ppm to about 60% of control values. No significant
differences between groups were observed in organ weights or after
macroscopic or histopathological examination of organs. The NOAEL
was 25 ppm, equal to 0.89 mg/kg bw/day (males) and 0.97 mg/kg bw/day
(females), based on depression of brain cholinesterase activity and
clinical effects at 300 ppm (Barker at al., 1992).
Long-term toxicity/carcinogenicity studies
Mice
In a 2-year study in CD-1 mice (60-65/sex/group), phosalone
(95.3% pure) was administered at dietary concentrations of 0, 15, 50
or 150 ppm. Five mice of each sex were sacrificed at 6 weeks and at
study termination at 105 weeks for plasma, erythrocyte and brain
cholinesterase determinations. Plasma and erythrocyte
cholinesterase were reduced by at least 20% in the 150 ppm group at
both 6 and 105 weeks. In some cases, particularly with plasma
cholinesterase, the reduction was much greater. Brain
cholinesterase was not reduced compared to controls. Statistically
significant increases in adrenal weights were found at all dose
levels and in both sexes, with the exception of females at the
lowest dose. These increases were both in relative and absolute
weights and were significant except for relative weight at 15 ppm in
males. Decrease in absolute brain weight was significant at 50 ppm
and 150 ppm in males. Relative increases in testicular weights (50
ppm), kidney weights in females only (15 ppm) and relative and
absolute increases in pituitary weights (males: 15 ppm) were also
significant. No neoplastic or non-neoplastic histopathological
findings were attributable to phosalone. There were adrenal weight
changes, which in the absence of histopathological changes, were
considered to be adaptive, and changes in testicular weights at 50
ppm and kidney weight in one sex at 15 ppm which were likely to be
insignificant in view of a lack of dose-response. The decrease in
absolute brain weight in males was not dose-related. The NOAEL was
thus the highest level of 150 ppm (equal to 23 and 31 mg/kg bw/day
in males and females, respectively), based on the lack of depression
of brain cholinesterase activity at this level (Nelson et al.,
1980).
Rats
In a 2-year study, groups of 30 rats/sex/dose (albino: strain
not stated) received phosalone of unknown purity at dietary
concentrations of 0, 25, 50 or 250 ppm (half of these concentrations
for the first four weeks). Mortality during the course of the study
was similar in all groups. There was some depression in weight gain
in the highest-dose animals compared to controls, especially in
males at 103 weeks. No haematological changes attributable to the
test material were observed. There were marked (up to three-fold)
differences in group mean plasma cholinesterase activities between
sexes, females having much higher activity; this raised some doubt
about the reliability of these assays. Inhibition of erythrocyte
and plasma cholinesterase activity was seen at 250 ppm. Inhibition
of brain cholinesterase activity > 20% was seen at 250 ppm in
males. No histopathological changes related to the test material
were seen. Although a few instances of testicular activity were
seen, there was no indication of any relationship between occurrence
of this finding and administration of the test material. The NOAEL
was 50 ppm, equivalent to 2.5 mg/kg bw/day, based on brain
cholinesterase inhibition at the next highest dose (Woodard Research
Corporation, 1967).
In a second 2-year study, Sprague-Dawley CD rats (50/sex/dose)
were given phosalone (purity 94.5%) at doses of 0, 5, 50 or 1000
ppm, the highest dose being reduced to 500 ppm at week 27. These
concentrations were equal to 0, 0.2, 1.8 or 20 mg/kg bw/day for
males and 0, 0.2, 2.5 or 31 mg/kg bw/day for females, on the basis
of consumption data from week 27. Satellite groups of animals
(15/sex/dose), were killed at 52 weeks. There was no effect on
mortality and clinical effects were mainly confined to the high-dose
group. Depression of plasma and erythrocyte cholinesterase was found
at the high and medium concentrations and depression of brain
cholinesterase activity was found in the highest dose group. There
were some treatment-related changes in the adrenals. A dose-response
across all groups in the prevalence of testicular atrophy was
observed (1/27, 3/34, 6/30 10/42 in survivors to the terminal kill).
Reductions in testis weight were dose-related, but were
statistically significant only at the high and mid-dose levels (5.03
g, 4.66 g, 4.51 g and 4.22 g, for the control, low, medium and high-
dose groups, respectively). The NOAEL was < 5 ppm, equal to 0.2
mg/kg bw/day (Barker & Sortwell, 1993).
Reproduction studies
Rats
In a three-generation reproduction study, groups of albino rats
(10 males and 20 females/group) were administered phosalone (purity
unknown) at dietary concentrations of 0, 25 or 50 ppm. Only half
the nominal dietary concentration was given during the initial three
weeks of the diet, on the grounds that young rats eat about twice as
much daily during this period; this procedure was repeated in each
generation. When the rats had received phosalone for 74 days, 10
females in each group were paired with the 10 males from the same
group for 10 days, after which the other 10 females were mated with
the 10 males. The animals from these litters (F1a) were sacrificed
after weaning, weighed and examined. Microscopic histopathological
examination was not carried out. The F0 rats were then remated as
different pairs to produce the F1b litters. Litters were examined
at weaning and representative male and female pups were placed on
control diet to constitute the next study groups. Within 21 days of
weaning, the F1b generation received 25 ppm or 50 ppm phosalone,
while controls continued to be fed control diet. The F1b rats were
mated in the same way as the F0 generation, F2a pups being
sacrificed and examined as above, while the F1b parents were also
killed and examined. Uteruses from females failing to bear two
litters were removed and examined for implantation sites. Rats were
selected from each F2b litter to give 10 males and 20 females in
each group, the remaining F2b pups were sacrificed and examined as
before without microscopic pathology. The retained F2b pups in the
two test groups received the test diet within 24 days of weaning.
After 51 days on the diet, the males and 10 females were paired for
10 days, the males being paired with the other 10 females after they
had all been on the diet for 71 days. The F3a pups were sacrificed
after weaning and examined as above. Within 10 days, F2b females
were re-mated with different males to produce the F3b litters.
After weaning, the pups were sacrificed and the main organs
examined. Tissues from one male and one female in each F3b litter
were examined histopathologically. The F2b parents were mated for
a third time to produce F3c pups litters, which were treated in the
same way as the F3b pups.
The test groups of rats were similar to the controls throughout
the study. Three F0 females died in the 50 ppm group and one in
the 25 ppm group while none died in the control group. Test
substance-related effects on fetal resorption were not observed and
malformations were observed only in 3 pups from one litter of the 25
ppm group. The three pups exhibited flaccid paralysis of the hind
limbs. Organs from the test and control weanlings from the F3b and
F3c groups did not differ significantly in weight or
histopathology. No adverse effect was determined in the study and
the NOAEL was > 50 ppm, equivalent to 2.5 mg/kg bw/day (Jones et
al., 1967a).
In a two-generation reproduction study, groups of 32 male and
32 female rats (6 week-old Crl: CD(SD)VSAF/Plus BR) were given
phosalone (96.8% pure) at dietary concentrations of 0, 10, 50 or 400
ppm. Phosalone was administered for 10 weeks, after which the rats
were mated for 20 days. The dams were allowed to rear their young
and from these litters, 21 days postpartum, 28 rats/sex/group were
selected as the F1 generation. F1 animals were selected and
treated from weaning until 16 weeks when they were mated. After
weaning of their litters the F1 parents were sacrificed as were the
F2 pups.
At 400 ppm, there was a lower body weight from conception to
day 17 in the F0 generation, and lower mean body-weights of the F1
males and females. There were 2 total litter losses post-partum in
the F0 generation and three in the F1 generation. There was a
reduced litter size in the F0 animals and increased pup mortality
to day 4 in both generations. Retarded pup growth and marked plasma
and erythrocyte cholinesterase depression were noted in both
generations. At 50 ppm, there was marked plasma and erythrocyte
cholinesterase depression in both generations, while at 10 ppm
cholinesterase reduction was < 20%. Brain cholinesterase activity
was not measured. The NOAEL was 50 ppm, equivalent to 2.5 mg/kg
bw/day, based on retarded pup growth and erythrocyte cholinesterase
depression (Bryson et al., 1991).
Special studies on embryotoxicity/teratogenicity
A study was carried out on chick embryos (white Leghorn) at
doses of 0.2, 0.6 or 1.8 mg/egg, using 30 eggs/group. Two further
groups of 30 eggs served as solvent (dimethylsulfoxide) and
untreated controls. At 18 days, the surviving embryos were removed,
weighed and examined. Phosalone had no significant effect on
mortality, weight or structure (Pasquet, 1969).
Groups of 25 female rats (Wistar HAN) were treated with 0, 2,
10 or 20 mg/kg bw/day of phosalone by gavage, from days 6 through 15
of gestation. Controls received distilled water with 4% CMC. The
animals were killed 21 days after coitus and the fetuses were
removed by caesarean section. Dams and fetuses were examined
macroscopically and microscopically. Oral administration of
phosalone at the highest dose produced clinical signs (chewing
movements, piloerection and dyspnea). Additionally, reduced food
consumption, weight loss, reduction in litter size, due to increased
resorptions and a slight increase in mean fetal body weight, were
observed. There was no evidence of fetal abnormality. At 2 and 10
mg/kg bw/day, maternal and fetal parameters were similar to
controls. The NOAEL was 10 mg/kg bw/day for both maternal toxicity
and fetotoxicity (Allen et al., 1989a).
Groups of 25 Fauve de Bourgogne rabbits were orally treated by
capsules with undiluted phosalone (purity unstated) from days 6
through 16 of gestation with doses of 0, 2, 6 or 18 mg/kg bw/day. A
positive control group was treated with thalidomide. The does were
sacrificed at 28 days. The percentage of animals becoming pregnant,
the number of resorptions, the number of fetuses recovered alive
after caesarian section and their weight did not differ
significantly from the untreated controls. The incidence of
malformed fetuses was low in all groups and the malformations were
trivial. By contrast malformations were observed in the thalidomide
group. The NOAEL was 18 mg/kg bw/day, the highest dose tested
(Pasquet, 1969).
Phosalone (93.5% pure) was administered in
carboxymethylcellulose (4%) by gavage to Chinchilla KFM/CHIN rabbits
(16/dose) at doses of 0, 1, 10 or 20 mg/kg bw/day from days 6
through 18 post-coitum. Clinical signs of cholinergic intoxication,
particularly dyspnea and extensor spasms, reduced food consumption,
loss of body weight and increased incidence of resorption of embryos
were observed at the highest dose. Post-implantation loss was
increased at 10 and 20 mg/kg bw/day compared to concurrent controls
but was within the range of historical controls. No adverse effect
was seen on fetuses at any dose, or on maternal parameters at doses
of 1 or 10 mg/kg bw/day. The NOAEL was 10 mg/kg bw/day, based on
both fetal and maternal toxicity (Allen et al., 1989b; Allen,
1989).
Special studies on genotoxicity
Based on the results of genotoxicity assays given in Table 2,
the Meeting concluded that phosalone was not genotoxic.
Special studies on neurotoxicity
Groups of white Leghorn hens (10/group/dose) were given dietary
doses of 0, 50, 163 or 500 ppm phosalone or TOCP at 500 ppm for 45
days. Hens were examined daily during the study. At termination,
brain, three levels of spinal cord and a portion of sciatic nerve
were taken from the controls, the TOCP-treated birds and the highest
dose of phosalone-treated hens. Although some signs of cholinergic
toxicity occurred, no phosalone-treated birds showed any indication
of organophosphate-induced delayed neuropathy. TOCP-treated hens
showed paralysis from 13 days onwards, and the only deaths seen,
were amongst the TOCP-treated fowl. Histopathological appearances
in tissues taken from the control and phosalone-treated animals were
within normal limits. Although there was some demyelination in the
TOCP group of animals, particularly in the sciatic nerve, this was
not significant (Woodard et al., 1966).
In another study, 10 Rhode Island red hens were dosed with 350
mg/kg bw phosalone (=LD50) as a 35% formulation, while protected by
atropine and pralidoxime methanesulfonate (P2S). The dose was
repeated after 3 weeks. There were 4 untreated controls and 4
positive controls treated with a neuropathic dose of mipafox. One
phosalone-treated hen died at 4 days, and another at 29 days. No
pathological changes were seen in the spinal cord. The positive
controls became severely ataxic at 13 days and showed serious
effects in the spinal cord. The untreated controls were clinically
and pathologically normal (Heath et al., 1967).
Groups of 20 New Hampshire red hens received TOCP (750 mg/kg bw
p.o., positive control), or 0 or 600 mg/kg bw p.o., phosalone.
Dosed and positive control animals also received atropine 15 min
before dosing. The vehicle control and phosalone group were redosed
at day 22 and sacrificed at day 41. Birds from the first positive
control group were killed on day 22 of the study at which time the
10 birds from the second positive control group were dosed and
sacrificed 19 days later. No significant clinical signs were seen
in the vehicle control fowl. Neuropathological examination was
performed on central and peripheral nervous tissue from all terminal
sacrificed animals and seven decedents in the phosalone group.
Table 2. Results of genotoxicity assays on phosalone
Test Object Concentration phosalone Purity Results Reference
In vitro
Ames test (1) S. typhimurium 1-1000 µg/plate ? Negative Benezet & Cartier, 1980
(strains TA98, 100, 125-1000 µg/plate
1535, 1537)
Ames test (1) S. typhimurium 250-10 000 94% Negative Haworth & Lawlor, 1989
(strains TA98, 100, µg/plate
1535, 1537, 1538)
HPRT test (1) CHO/K1 cells 0.1-50 µg/ml 95% Negative Cordier & Bonneau, 1985
Chromosomal CHO/K1 cells 4, 40, 75 µg/ml 95% Negative Cordier & Fournier, 1989
aberration in
CHO cells (1)
Chromosomal CHO/WBL cells 16.7, 500 µg/ml 94% Negative Murli, 1989
aberration in (also 5 µg/ml without
CHO cells (1) metabolic activation)
UDS in vitro Rat primary 0.005-252 µg/ml 94% weak Cifone, 1989
hepatocyte positive
In vivo
Micronucleus CD-1 mice 10, 20, 40 mg/kg bw/day 99.5% Negative Pasquet & Fournier, 1980
Dominant lethal CD-1 mice 10, 30, 75 mg/kg 95.3% Negative Goldenthal et al.,
test 1978
(1) With and without metabolic activation.
Tissues examined were spinal cord (cervical, thoracic and
lumbosacral), medulla, sciatic and tibial nerves. In the positive
control groups, all except one bird showed clinical signs of delayed
neuropathy, appearing from days 13-24. In both positive controls,
histopathological evidence of delayed neuropathy was found. In the
phosalone group, 4 birds showed signs of cholinergic toxicity after
the first dose and two birds died. On day 22 after the second dose,
six hens showed acute toxicity. Seven hens died on days 23-24.
Histopathological evidence of nerve degeneration was not more
frequent in this group than in the vehicle controls (Morris, 1983).
The Meeting concluded that there is no evidence that phosalone
has potential to cause organophosphate-induced delayed neuropathy.
Antidote studies of phosalone intoxication
Ten groups of 10 mice each were given phosalone orally followed
immediately by i.p. administration of combinations of pralidoxime
methylsulfate (Contrathion) at 0, 25, 50, 100 or 200 mg/kg bw and
atropine at 0, 10, 20 or 40 mg/kg bw. Both antidotes were effective
in preventing death although pralidoxime methylsulfate at 100 and
200 mg/kg bw appeared to be toxic. Some aspects of the results were
difficult to interpret since doses of pralidoxime methylsulfate used
were high compared to the recommended human dose of 5-6 mg/kg bw
(SERB, 1988). Moreover the i.p. LD50 of a similar pralidoxime salt
is 125-250 mg/kg bw in the mouse (Marrs, 1991). Doses of atropine
used in this study were also high (Dubost et al., 1963).
In another study using orally administered phosalone (vehicle
unstated), groups of rats (9-10/group) were given 50 or 100 mg/kg bw
phosalone. Phosalone (100 mg/kg bw) was administered to another
group of 10 rats, followed by pralidoxime methanesulfonate (50 mg/kg
bw s.c.) and atropine (17.5 mg/kg bw s.c.) 15-20 minutes after
clinical signs of anticholinesterase poisoning developed. All
animals in the 100 mg/kg bw group not treated with the antidotes
showed considerably reduced erythrocyte anticholinesterase activity
and died within 24 hours. Five out of 10 rats survived 24 hours in
the 50 mg/kg bw group and their cholinesterase activity was less
depressed. When treated with the antidotal regime, 9/10 rats dosed
with phosalone at 100 mg/kg bw survived and cholinesterase activity
was less depressed than that of animals treated with 50 mg/kg bw
phosalone but untreated with antidotes (Hayward, 1969).
In a study of the antidotal effects of bis-pyridinium oxime
(obidoxime), groups of mice (4/sex) were administered phosalone by
gavage at a dose of 250 mg/kg bw, a dose well above the LD50.
Immediately afterwards, obidoxime and atropine were injected
intraperitoneally at obidoxime doses of 0, 25, 50 or 100 mg/kg bw
and at atropine doses of 0, 10, 20 or 40 mg/kg bw in various
combinations. When no antidote was given, phosalone was invariably
fatal whereas survival was considerably improved with combinations
of antidote except at the highest dose of atropine and obidoxime.
Obidoxime alone was clearly more beneficial than atropine alone.
Synergism was not observed, because of the study design (Pasquet &
Masuret, 1970).
It was concluded from these studies that atropine is an
effective antidote to phosalone. Pralidoxime (either methylsulfate
or methanesulfonate) reactivated phosalone-inhibited rat erythrocyte
cholinesterase and was an effective antidote. Obidoxime was also an
effective antidote but the usual synergism of atropine and
pralidoxime salts or obidoxime was not noted probably due to the
design of the studies.
Special studies on metabolites and intermediates
A number of acute toxicity and genotoxicity studies have been
conducted with phosalone metabolites and intermediates. The results
are summarized in Tables 3-6.
Other studies
3-Hydroxymethyl 6-chlorobenzoxolone was non-irritant to the
intact rabbit skin but very irritant to the scarified skin (Pasquet
& Mazuret, 1973b). 3-Chloromethyl 6-chlorobenzoxolone was non-
irritant to the intact rabbit skin (Pasquet et al., 1983b).
In a 13-week study, groups of 15 male and 15 female rats
(Sprague-Dawley-derived Crl:CD(SD)BR) were given 6-chlorobenzoxolone
at dietary concentrations of 0,5, 15 or 45 mg/kg bw/day for 13
weeks. Groups of 10 male and female controls and high-dose animals
underwent a subsequent 28-day treatment-free period. The only
effect of the treatment was suppression of weight gain in all
treated males and in the high-dose females. A compensatory weight
gain was observed in the animals that underwent the treatment-free
period (Owens, 1985).
A study was performed to determine the potential of Di-syston
(disulfoton) to potentiate the activity of phosalone. Groups of 8
beagle dogs were fed diets containing phosalone at concentrations of
7.5, 15 or 25 ppm for 19 weeks. In each case, Di-syston was added
at a concentration of 1 ppm from week 14 to week 19. There were no
changes in physical appearance, haematology or pathology that could
be ascribed to the treatment. No treatment-related changes in
plasma or erythrocyte cholinesterase were seen (Jones et al.,
1967b).
Table 3. Acute toxicity of phosalone metabolites
Species Strain Sex Route LD50 References
(mg/kg bw)
Phosalone-oxon
Mouse CD-1(COBS) M PO 35 Fournel et al., 1968
(30-41)
Mouse CD-1(COBS) F PO 40 Fournel et al., 1968
(33-49)
Mouse ? Mix PO 32 Rhône-Poulenc, 1968
Rat CD(COBS) M PO 36 Fournel et al., 1968
(29-44)
Rat CD(COBS) F PO 21 Pasquet et al., 1976
(12-28)
Rat CD(COBS) F PO 20 Fournel et al., 1968
(15-29)
Rat CD(COBS) F PC 380 Pasquet & Mazuret, 1973a
(240-610)
3-methylthiomethyl 6-chlorobenzoxazolone
Mouse OF1 M PO 590 Pasquet & Mazuret, 1980
(499-698)
OF1 F PO 837 Pasquet & Mazuret, 1980
(706-991)
3-methylsulfinylmethyl 6-chlorobenzoxazolone
Mouse OF1 M PO 405 Pasquet & Mazuret (1980)
OF1 F PO 315 Pasquet & Mazuret (1980)
(256-386)
Table 3 (contd)
Species Strain Sex Route LD50 References
(mg/kg bw)
3-methylsulfonylmethyl 6-chlorobenzoxazolone
Mouse OF1 M PO > 5000 Pasquet & Mazuret 1980
OF1 F PO > 5000 Pasquet & Mazuret 1980
Table 4. Acute toxicity of intermediates and soil metabolites
Species Strain Sex Route LD50 References
(mg/kg bw)
3-hydroxymethyl 6-chlorobenzoxolone
Mouse CD-1(COBS) Mix p.o. 730 Pasquet & Mazuret, 1973b
(557-956)
Benzoxolone
Mouse CD-1(COBS) Mix p.o. 580 Pasquet & Mazuret, 1973b
3-chloromethyl 6-chlorobenzoxolone
Rat CD Sprague- Mix i.p. 305 Pasquet et al., 1983a
Dawley
Table 5. Genotoxicity of phosalone intermediates
Compound Test object Concentration Purity Result References
intermediate
Unknown intermediate
Ames test (1) Salmonella typhimurium 0.005-0.5 µg/plate ? -ve Weill, 1988
TA98, TA100, TA1535,
TA1537, TA1538
Table 6. Genotoxicity of phosalone soil metabolites
Compound Test object Concentration Purity Result Reference
intermediate
3-amino 7-chloro 2-phenoxazone
Ames test (1,2) Salmonella typhimurium 10-100 µg/plate (-S9) 95% +ve Vanrel et al., 1989
TA98, TA100. TA1535, 25-125 µg/plate (+S9)
TA1537, TA1538
(1) With and without metabolic activation.
(2) Positive with metabolic activation and in TA1537, and TA100 only.
Phosalone (96% pure) was applied to the skin of rabbits (New
Zeeland white) for 21 days at dose levels of 0.4, 2 or 10 mg/kg
bw/day. Azinphosethyl was applied at 2 mg/kg bw/day. Both
chemicals were applied to intact or abraded skin. Inhibition of
brain cholinesterase > 20% was not noted in any phosalone group,
but marked depression was seen with azinphosethyl. Phosalone did
not cause red blood cell or plasma cholinesterase depression (Kynoch
et al., 1979).
In studies to elicit delayed sensitization in guinea-pigs
(Pasquet & Mazuret, 1977; Cunny & Siglin, 1989) no sensitization was
seen. Phosalone was moderately irritant to the eye in New Zeeland
white rabbits (Siglin et al., 1989). Irritancy to the rabbit eye
was also observed by Safonov (1968).
Observations in humans
In a study performed on 14 male volunteers to establish a re-
entry period after applying phosalone in citrus groves, dislodgeable
residues up to 3.6 µg/cm2 (leaf) produced no adverse effect other
than some plasma cholinesterase depression (Knaak et al., 1978).
In 1987, several cases of illness resembling anticholinesterase
poisoning were reported in California. On investigation by the
California Department of Food and Agriculture, it was found that the
illness had occurred in workers harvesting grapes from vineyards
treated with a preparation containing phosalone. A study was
therefore carried out in which grapes were treated with phosalone (3
lb/acre) twice, 24 days apart. Fourteen days after the second
treatment, 30 volunteers harvested the crop for 6 consecutive days
while 22 volunteers harvested a crop from an untreated vineyard.
Assignment to the treated or control group was random. Blood was
collected for plasma and erythrocyte cholinesterase activity in the
afternoon of days 4, 3 and 2 pre-harvest, daily during harvest, and
three days thereafter. Samples were transported cooled in ice and
assayed within six hours by a modified Ellman method. Urinary
diethylphosphates were assayed on early morning urine samples
collected on days 6, 5, 4 and 1 before harvest, daily during
harvest, and days 1 and 2 thereafter. No symptoms or clinical signs
were recorded that could be attributed to anticholinesterase
effects. There was a gradual decline in plasma cholinesterase
levels during the harvest, which recovered after the end of the
harvest. This effect was not seen in controls nor in red blood cell
cholinesterase level in either test or control groups. Ethyl
phosphate metabolites were found in the urine of the test group
(Baugher, 1989). A similar study was carried out on French apple
pickers (Vogel, 1991). No clinical signs, symptoms suggestive of
anticholinesterase toxicity, or significant depression of plasma or
erythrocyte cholinesterase were observed.
In 1966-1979, eleven pesticide incidents involving the
agricultural use of phosalone were reported to the USEPA's Pesticide
Incident Monitoring System (Hodgson & Smith, 1992). The antidote
dietixim (a cholinesterase reactivator) was effective in human
phosalone poisoning (Kundiev & Kagan, 1992).
COMMENTS
After oral administration, phosalone was moderately well
absorbed, 15-25% appearing in the faeces. Phosalone was extensively
metabolized to phosphorothioates, phosphorodithioates and
3-methylthiomethyl 6-chloro-benzoxazolone, the last of which is
subsequently metabolized ultimately to 3-methylsulfonylmethyl 6-
chlorobenzoxazolone.
Pure phosalone is almost certainly not a cholinesterase
inhibitor, but acquires inhibitory activity after conversion to
phosalone oxon in vivo.
The acute oral toxicity varies with species, but is in the
region of 100-200 mg/kg bw in rodents. Phosalone has been
classified as moderately hazardous by WHO.
There were two short-term studies in rats which could be used
to give NOAELs. In a five-week oral gavage study in rats at doses
of 0, 7.5 or 15 mg/kg bw/day, the NOAEL or 7.5 mg/kg bw/day based on
brain cholinesterase inhibition. In an eight-week study in rats
using dietary concentrations of 0, 10, 100, 300, 600 or 1200 ppm,
the NOAEL was 10 ppm (equal to 0.87 mg/kg bw/day), based on brain
cholinesterase inhibition. It is possible that NOAELs could have
been established at 100 or 300 ppm, but the dose rates for those
groups were increased to 2400 and 4800 ppm, respectively, after 5
weeks to establish a maximum tolerated dose.
Five studies were carried out in dogs. In a one-month oral
dosing study in dogs, a NOAEL could not be determined as plasma and
erythrocyte cholinesterase depression were seen at the lowest dose
(7.5 mg/kg bw/day). In another one-month study using dietary
concentrations of 0, 12.5, 25 or 37.5 ppm, the NOAEL was the highest
level, which was equal to 0.81 mg/kg bw/day; although plasma
cholinesterase depression was seen in the study, neither erythrocyte
nor brain cholinesterase activity was depressed. In a 6-month study
in dogs using dietary concentrations of 0, 10 or 25 ppm, although
plasma and erythrocyte cholinesterase were depressed, the brain
enzyme was not, so the NOAEL was the highest dose (equivalent to
0.63 mg/kg bw/day). In a two-year study in beagle dogs, males and
females were fed phosalone in the diet at concentrations of 0, 100,
200 or 1000 ppm. The NOAEL was 200 ppm, equivalent to 5 mg/kg
bw/day, based on brain cholinesterase depression, body-weight loss
and elevated alanine aminotransferase at the highest dose. In a
more recent one-year study in dogs using dietary concentrations of
0, 5, 25 or 300 ppm phosalone, the NOAEL was 25 ppm (equal to 0.89
mg/kg bw/day), based on brain cholinesterase depression at 300 ppm.
The overall NOAEL for dogs was considered to be 200 ppm, in view of
the spacing of the doses in the most recent study.
In a lifetime carcinogenicity study in mice, phosalone was
given at dietary concentrations of 0, 15, 50 or 150 ppm. The NOAEL
was 150 ppm, equal to 23 mg/kg bw/day, based on the lack of
depression of brain cholinesterase activity, although plasma and red
blood cell cholinesterase depression were seen at this level. There
was no evidence of carcinogenicity.
In a two-year study in rats using concentrations of 0, 25, 50
or 250 ppm phosalone in the diet, the NOAEL was 50 ppm, equivalent
to 2.5 mg/kg bw/day, based on brain cholinesterase depression at the
highest dose. In a second 2-year study in rats, dietary
concentrations of 0, 5, 50 or 1000 ppm were used, the highest dose
being reduced to 500 ppm later in the study. There was a
statistically significant increase in the prevalence of testicular
atrophy and reduction in testicular weight in both the high and mid-
dose groups and a dose response across all groups for both effects.
The Meeting concluded that the NOAEL was < 5 ppm, equal to 0.2
mg/kg bw/day.
In a teratogenicity study in rats at doses of 0, 2, 10 or 20
mg/kg bw/day, the NOAEL was 10 mg/kg bw/day for both maternal
toxicity and fetotoxicity. In a study in rabbits using doses of 0,
2, 6 or 18 mg/kg bw/day, the NOAEL was the highest dose. In another
study in rabbits, using doses of 0, 1, 10 or 20 mg/kg bw/day, the
NOAEL was 10 mg/kg bw/day based on maternal toxicity. Phosalone was
not teratogenic in either the rat or rabbit.
Two multigeneration reproduction studies in rats were reviewed.
In the first study, using dietary concentrations of phosalone of 25
or 50 ppm, no adverse effects were observed. The NOAEL was > 50
ppm, equivalent to 2.5 mg/kg bw/day. In the second study, in which
phosalone was administered at dietary concentrations of 0, 10, 50 or
400 ppm, the NOAEL was 50 ppm (equivalent to 2.5 mg/kg bw/day) based
on retarded pup growth and plasma and erythrocyte cholinesterase
depression.
Phosalone has been adequately tested in a series of in vitro
and in vivo genotoxicity assays. The Meeting concluded that
phosalone was not genotoxic.
There is no evidence that phosalone has potential to cause
organophosphate-induced delayed neuropathy.
Pralidoxime salts and obidoxime are both effective in
experimental phosalone poisoning.
No human study was available from which a NOAEL could be
derived.
An ADI of 0-0.001 mg/kg bw was established based on the lowest
dose (0.2 mg/kg bw/day) in the recent two-year study in rats. A
200-fold safety factor was used because of concerns that the trend
for the occurrence of testicular atrophy and reduction in testis
weight existed across all groups.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 150 ppm, equal to 23 mg/kg bw/day (two-year study)
Rat: < 5 ppm, equal to 0.2 mg/kg bw/day
(two-year study)
50 ppm, equivalent to 2.5 mg/kg bw/day
(multigeneration reproduction study)
Rabbit: 10 mg/kg bw/day (teratology study)
Dog: 200 ppm, equivalent to 5 mg/kg bw/day
(several studies)
Estimate of acceptable daily intake for humans
0-0.001 mg/kg bw
Studies which will provide information valuable in the continued
evaluation of the compound
Explanation of the testicular atrophy seen in the recent study
in rats.
Observations in humans.
REFERENCES
Allen, P.A. (1989) Unpublished study. Additional evaluation of the
incidence of resorptions in the studies performed by RCC on the
effects of phosalone technical on embryonic and fetal development in
the rabbit. Project 083002, 204423, 082991. RCC Research and
Consulting Company AG, PO Box, CH 4452 Itingen, Switzerland.
Supplied to WHO by Rhône-Poulenc.
Allen, P.A., Frei, D., Mladenovic, P. & Terrier C. (1989a)
Unpublished study. Embryotoxicity study (including teratogenicity)
with phosalone technical in the rat. Project 082980. RCC Research
and Consulting Company AG, PO Box, CH 4452 Itingen, Switzerland.
Supplied to WHO by Rhône-Poulenc.
Allen, P.A., Mladenovic, P. & Terrier, C. (1989b) Unpublished study.
Embryotoxicity study (including teratogenicity) with phosalone
technical in the rabbit. Project 083002. RCC Research and Consulting
Company AG, PO Box, CH 4452 Itingen, Switzerland. Supplied to WHO by
Rhône-Poulenc.
Barker, M.H., Smith, T.G., Buist, D.P., Crook, D., Morrow, J. &
Gopinath, C. (1992) Unpublished study. Phosalone dietary toxicity
study in beagle dogs. HRC report No. RNP 336/91907. Huntingdon
Research Centre Ltd, Wooley, Huntingdon, PE18 6ES, England. Supplied
to WHO by Rhône-Poulenc.
Barker, M.H. & Sortwell, R.J. (1993) Unpublished study. Phosalone
(11974 RP). Potential tumorigenic and toxic effects in prolonged
dietary administration to rats Volume 1. Huntingdon Research Centre
Ltd, PO Box 2, Huntingdon, Cambridgeshire PE18 6ES. Supplied to WHO
by Rhône-Poulenc. England
Baugher (1989). Unpublished study. Exposure of harvesters to Zolone
(R) EC brand insecticide applied to grapes in California, 1988.
Project no 29088. Orius Associates, Frederick, Maryland, USA.
Supplied to WHO by Rhône-Poulenc.
Benezet, F. & Cartier, J.R. (1980) Unpublished study. Phosalone (11
974 RP) mutagenicity study on Salmonella typhimurium. Report
RP/RD/CNG No. 19 234 E of 19th January 1978. Centre Nicolas Grillet,
94400 Vitry-sur-Seine, France. Supplied to WHO by Rhône-Poulenc.
Bryson, A.M., Parker, C.A., Offer, J.M. & Farmer, H. (1991)
Unpublished study. A study of the effect of phosalone on
reproductive function of two generations in the rat. Report No. RNP
326/91277. Huntingdon Research Centre Ltd, PO Box 2, Huntingdon,
Cambridgeshire, PE18 6ES England. Supplied to WHO by Rhône-Poulenc.
Cifone, M.A. (1989) Unpublished study. Mutagenicity test on
phosalone technical in the rat primary hepatocyte unscheduled DNA
synthesis test. HLA study No. 10754-0-447, Hazleton Laboratories
America Inc 5518 Nicholson Lane, Suite 400, Kensington Maryland
20895, USA. Supplied to WHO by Rhône-Poulenc.
Cordier, A. & Bonneau, D. (1985) Unpublished study. Phosalone (11
974 RP) CHO/HPRT test. Report. ST/CRV/Tox. No. 22 363-E of 10th May
1985. Rhône-Poulenc Santé, Centre de Recherches de Vitry, 94403
Vitry-sur-Seine Cedex, France. Supplied to WHO by Rhône-Poulenc.
Cordier, A. & Fournier, E. (1985) Unpublished study. Phosalone (11
974 RP) chromosome aberration test in Chinese hamster ovary cells
(CHO). Report ST/CRV/Tox. No. 435-E of 28th August 1985. Rhône-
Poulenc Santé, Centre de Recherches de Vitry, 94403 Vitry-sur-Seine
Cedex, France. Supplied to WHO by Rhône-Poulenc.
Craine, E.M. (1974a) Unpublished study. Disposition of phosalone-
14C in a lactating cow. Research report No. EMC 74:17 of the 29th
April 1974. Hess and Clark Research Department, Ashland, Ohio, USA.
Supplied to WHO by Rhône-Poulenc.
Craine, E.M (1974b) Unpublished study. Disposition of phosalone-14C
in a lactating cow. Research report No. EMC 74:84 of the 25th
November 1974. Hess and Clark Research Department, Ashland, Ohio,
USA. Supplied to WHO by Rhône-Poulenc.
Craine, E.M. (1974c) Unpublished study. The disposition of
phosalone-14C applied to the skin of a pig. Research report No. EMC
74:30 of the 14th May 1974. Hess and Clark Research Department,
Ashland, Ohio, USA. Supplied to WHO by Rhône-Poulenc.
Craine, E.M. (1975) Unpublished study. The metabolism of phosalone-
14C in dairy cows. Research report No. EMC 75:51 of the 2nd July
1975. Hess and Clark Research Department, Ashland, Ohio, USA.
Supplied to WHO by Rhône-Poulenc.
Cunny, H. & Siglin, J.C. (19789) Unpublished study. Delayed contact
hypersensitivity study in guinea-pigs with phosalone technical.
Study No. 3147.37. Springborn Life Sciences Inc. Spencerville, Ohio
45887, USA. Supplied to WHO by Rhône-Poulenc.
Desmoras, M.J. (1979). Unpublished study. Studies on the metabolism
of phosalone (11974 R.P.), in the mouse, the rat and the rabbit.
Note RP/RD/CNG No 20 234 of the 20 August 1979. Supplied to WHO by
Rhône-Poulenc.
Desmoras, M.J. & Fournel, J. (1968) Unpublished study. Studies on
the degradation of phosalone (11974 R.P.), in mammals. Note RP -
DSPh of the 1st March 1968. Rhône-Poulenc, Laboratoires de
Recherches, 22 Avenue Montaigne, 75 Paris 8, France. Supplied to WHO
by Rhône-Poulenc.
Donoso, J., Woodard, M.W. & Woodard, G. (1967) Unpublished study.
Phosalone safety evaluation by repeated oral administration to dogs
for 107 weeks. Report dated 7th September 1967. Woodard Research
Corporation, Herndon, Virginia 22070 USA. Supplied to WHO by Rhône-
Poulenc.
Dubost, P. Fournel, J., Ganter, P., Julou, L., Koenig, F. & Myon, J.
(1963). Unpublished study. Acute toxicity, local tolerance,
anticholinesterase activity and chronic toxicity in rats and dogs.
Note DSPh 9, 203 of the 29th March 1963. Centre Nicolas Grillet,
94400 Vitry-sur-Seine, France. Supplied to WHO by Rhône-Poulenc.
Fournel, J., Ganter, P., Julou, L., Populaire, P., Myon, J., Pascal,
S. & Pasquet, J. (1966) Unpublished study. Phosalone (11 974 RP)
Sub-acute (6-month) toxicity study in the dog. Note RP-DSPh 11 330 E
of the 14th June 1966. Société des Usines Chimiques Rhône-Poulenc,
Direction Scientifiques, Laboratoires de Recherche, Centre Nicolas
Grillet, 94400 Vitry-sur-Seine, France. Supplied to WHO by Rhône-
Poulenc.
Fournel, J., Julou, L. & Pasquet, J. (1968) 12244 R.P. Unpublished
study. Acute toxicity in mice rats and in vivo anti-cholinesterase
activity in rats. Note RP - DSPh 12663 (=12665E) of the 1st March
1968. Société des Usines Chimiques Rhône-Poulenc, Direction
Scientifiques, Laboratoires de Recherche, 22 Avenue Montaigne, 75
Paris 8,France. Supplied to WHO by Rhône-Poulenc.
Goldenthal, E.I., Jessup, D.C. & Rodwell, D.E. (1978) Unpublished
study. Dominant lethal study in mice. Study carried out for Rhône-
Poulenc dated the 18th December 1978. International Research and
Development Corporation, Mattawan, Michigan, 49071, USA.
Haworth, L. & Lawlor, T.E. (1989) Unpublished study. Mutagenicity
test on phosalone technical in the Ames Salmonella/microsome
reverse mutation assay. HLA study No. 10754-0-401, Hazleton
Laboratories America Inc., 5518 Nicholson Lane, Suite 400,
Kensington Maryland 20895, USA. Supplied to WHO by Rhône-Poulenc.
Heath, S.A.B., Rivett, K.F. & Woolf, N. (1967) Unpublished study.
Phosalone test for neurotoxicity. Study No. 230857, May and Baker
Ltd, Dagenham, Essex, England. Supplied to WHO by Rhône-Poulenc.
Hayward, A. (1969). Unpublished study. Insecticides: a study of the
effects of "Cyanox" (S-4084) and "phosalone" on rat erythrocyte
cholinesterase. Report No. PRG/584 (=274359). May and Baker Ltd,
Ongar, Essex, England. Supplied to WHO by Rhône-Poulenc.
Hodgson, H.J. & Smith, A.D. (1992). Commercial and residential
poisoning with anticholinesterases. In: Ballantyne B and Marrs TC
(ed) Clinical and experimental toxicology of organophosphates and
carbamates, pp. 352-363. Butterworth-Heinemann, Oxford, England.
Hopkins, R., Lewis, C.J. & Lewsley, R. (1991). Unpublished study.
(14C)-phosalone: a study of the absorption, distribution,
metabolism and excretion in the rat. Report No. 5759-68/93R.
Hazleton UK, Harrogate, England. Supplied to WHO by Rhône-Poulenc.
Jones, E.M., Post, K.F., Scott, W.J., Woodyard, M.W., Woodyard, G. &
Cronin, M.T.I. (1967a) Unpublished study. Phosalone three generation
reproduction study in the rat. Report dated September 11th 1967.
Woodyard Research Corporation and Chipman Chemical Company. Supplied
to WHO by Rhône-Poulenc.
Jones, M.E., Woodard, M.W., Imming, R.J., Woodard, G. & Cronin,
M.T.I. (1967b) Unpublished study. Phosalone followed by a
determination of potentiation activity with the compound Di-syston.
Report dated August 10th, 1967. Woodyard Research Corporation and
Chipman Chemical Company. Supplied to WHO by Rhône-Poulenc.
Kehoe, D.F. (1990) Unpublished study. 8-week dietary toxicity study
with phosalone in rats. HLA study No. 6224-149, Hazleton
Laboratories America Inc 3301 Kinsman Boulevard, Madison, Wisconsin
53704, USA. Supplied to WHO by Rhône-Poulenc.
Knaak, J.B., Maddy, K.T., Gallo, M.A., Lillie, D.T., Craine, E.M &
Serat, W.F. (1978) Worker re-entry study involving phosalone
application to citrus groves. Toxicol Appl. Pharmacol., 46 363-
374.
Kundiev, Y.I. & Kagan, Y.S. (1992) Anticholinesterses used in the
USSR: poisoning, treatment and preventative measures. In: Ballantyne
B and Marrs TC (ed) Clinical and experimental toxicology of
organophosphates and carbamates.pp 494-501. Butterworth-Heinemann,
Oxford, England.
Kynoch, S.R., Lloyd, G.K., Mallard, J.R., Street, A.E., Gibson,
W.A., Wadsworth, P.F. & Prentice, D.E. (1979) Unpublished study. The
effect of repeated applications of phosalone 11974 RP to the skin of
rabbits for 21 days. Report No. RNP 125/79335. Huntingdon Research
Centre Ltd, PO Box 2, Huntingdon, Cambridgeshire, PE18 6ES, England.
Supplied to WHO by Rhône-Poulenc.
Marrs, T.C. (1991). Toxicology of oximes used in the treatment of
organophosphate poisoning. Adverse drug reactions and Toxicology
Reviews, 10: 61-72.
Mazuret, A. (1971) Unpublished study. Phosalone,methyl-azinphos and
parathion acute toxicity in the rat. Report No. RP -DSPh No. 15544-
E. Rhône-Poulenc Santé, Centre de Recherches de Vitry, 94403 Vitry-
sur-Seine Cedex, France. Supplied to WHO by Rhône-Poulenc.
Morris, J.M. (1983) Unpublished study. Acute delayed neurotoxicity
study in hens with phosalone. GSRI project No. 411-B51-40, Gulf
South Research Institute, PO Box 1177, New Iberia, Louisiana 70560,
USA. Supplied to WHO by Rhône-Poulenc.
Murli, H. (1989) Unpublished study. Mutagenicity test on phosalone
technical in an in vitro cytogenetic assay measuring chromosomal
aberration frequencies in Chinese hamster ovary (CHO) cells. HLA
study No. 10754-0-437, Hazleton Laboratories America Inc., 5518
Nicholson Lane, Suite 400, Kensington, Maryland 20895, USA. Supplied
to WHO by Rhône-Poulenc.
Nelson, L.W., Geil, R.G. & Spicer, E.J. (1980) Unpublished study.
Phosalone lifetime oncogenicity study in mice. International
Research and Development Corporation, Mattawan, Michigan 49071, USA.
Report 347-009, submitted June 23rd, 1980. Supplied to WHO by Rhône-
Poulenc.
Noel, P.R., Rivett, K.F., Osborne, B.E. & Street, A.E. (1970)
Unpublished study. Phosalone dietary intake in beagle dogs for four
weeks. Report No. 3619/70/431. Huntingdon Research Centre Ltd, PO
Box 2, Huntingdon, Cambridgeshire, PE18 6ES, England. Supplied to
WHO by Rhône-Poulenc.
Owen, P.E. (1985) Unpublished study.6-chlorobenzoxolone: 13 week
oral (dietary administration) study in the rat with a 28-day
treatment-free period. Report No. 4229-198/14. Hazleton UK, Otley
Road, Harrogate, England. Supplied to WHO by Rhône-Poulenc.
Pasquet, J. (1969) Unpublished study. Study of the teratogenic
activity of phosalone (11974 RP) on the chick embryo and on the
rabbit. Note RP - DSPh 13447E of the 7th February 1969 (translation
no 2707). Centre Nicolas Grillet, 94400 Vitry-sur-Seine, France.
Supplied to WHO by Rhône-Poulenc.
Pasquet, J. (1971) Unpublished study. Phosalone (11 974 R.P.)
toxicité aigüe chez la ratte par voie percutanée. Note RP - DSPh
15481 of the 28th June 1971. Centre Nicolas Grillet, 94400 Vitry-
sur-Seine, France. Supplied to WHO by Rhône-Poulenc.
Pasquet, J. & Fournier, E. (1980) Unpublished study. Phosalone (11
974 RP) micronucleus test in the mouse. Report.RP/RD/CNG No 557-E of
8th April 1980. Rhône-Poulenc Santé, Centre de Recherches de Vitry,
94403 Vitry-sur-Seine Cedex, France. Supplied to WHO by Rhône-
Poulenc.
Pasquet, J. & Mazuret, A. (1970) Unpublished study. Obidoxime
chloride (Toxogonin R Merck = 24,590 R.P. chloride) acute
intraperitoneal toxicity in the mouse and antidotal effect on the
acute oral toxicity in the mouse of phosalone (11974 RP) RP - DSPh
No. 14514-E. Rhône-Poulenc Santé, Centre de Recherches de Vitry,
94403 Vitry-sur-Seine Cedex, France. Supplied to WHO by Rhône-
Poulenc.
Pasquet, J., Mazuret, A., Vaissere, J. & Gaildrat (1983a)
Unpublished study. Chloromethyl-3 chloro-6 benzoxolone (16891 R.P.)
- acute toxicity in the rat by the intraperitoneal route. Report
ST.CRV/Tox No. 21971. Rhône-Poulenc Santé, Centre de Recherches de
Vitry, 94407 Vitry-sur-Seine Cedex, France. Supplied to WHO by
Rhône-Poulenc.
Pasquet, J., Mazuret, A. & Vaissere, J. (1983b) Unpublished study.
Chlorométhyl-3 chloro-6 benzoxolone (16891 R.P.) - eau de lavage de
la solution chlorométhyléique - irritation cutanée primaire chez le
lapin. Note No ST.CRV/Tox. No. 21972. Rhône-Poulenc Santé, Centre de
Recherches de Vitry, 94407 Vitry-sur-Seine Cedex, France. Supplied
to WHO by Rhône-Poulenc.
Pasquet, J. & Mazuret, A. (1973a). Unpublished study. Phosalone
oxygen analog (12244 R.P.) acute percutaneous toxicity in the rat.
SUCRP - DSPh No.16979. Rhône-Poulenc,Laboratoires de Recherches, 22
Avenue Montaigne, 75 Paris 8, France. Supplied to WHO by Rhône-
Poulenc.
Pasquet, J. & Mazuret, A. (1973b). Unpublished study. Hydroxymethyl-
3 chloro-6 benzoxolone (16 236 RP) toxicité et tolérance locale. RP)
acute percutaneous toxicity in the rat. Note SUCRP - DSPh No.
17144.Rhône-Poulenc Santé, Centre de Recherches de Vitry, 94403
Vitry-sur-Seine Cedex, France. Supplied to WHO by Rhône-Poulenc.
Supplied to WHO by Rhône-Poulenc.
Pasquet, J. & Mazuret, A. (1977). Unpublished study. Phosalone (11
974 RP) guinea-pig skin sensitization study. Report RP/RD/CNG No.
19117 of 27th April 1977. Centre Nicolas Grillet, 94400 Vitry-sur-
Seine, France. Supplied to WHO by Rhône-Poulenc.
Pasquet, J. & Mazuret, A. (1980). Unpublished study. Metabolites of
phosalone (11 974 RP) : compounds 19889 RP, 19889 RP and 19914 RP.
Acute oral toxicity in the mouse. Report C.R. Vitry/CNG. No. 20 736-
E of 12th November 1980. Centre Nicolas Grillet, 94400 Vitry-sur-
Seine, France. Supplied to WHO by Rhône-Poulenc.
Pasquet, J., Mazuret, A., Fournel, M. & Koenig, M. (1976)
Unpublished study. Acute oral and percutaneous toxicity of phosalone
(R.P.11974) in the rat, in comparison with azinphosmethyl and
parathion. Note RP/RD/CNG No. 18 550 EP/LR of 27th February 1976.
Centre Nicolas Grillet, 94400 Vitry-sur-Seine, France. Supplied to
WHO by Rhône-Poulenc.
Reddy, S.J., Reddy, B.V. & Ramamurthy, R. (1992). Impact of chronic
phosalone toxicity on Bohr factor and oxygen equilibrium curves of
rat. Biochem Inter. 26: 171-179.
Rhône-Poulenc (1968) Unpublished study. 12244 R.P. oxygen analog of
phosalone acute oral toxicity and anticholinesterasic activity in
vitro. Note RP - DSPh 12664 of the 20th February 1968. Société des
Usines Chimiques Rhône-Poulenc, Direction Scientifiques,
Laboratoires de Recherche, 22 Avenue Montaigne, 75 Paris 8, France.
Supplied to WHO by Rhône-Poulenc.
Rhône-Poulenc Inc. (1979) Unpublished study. Phosalone dairy cow
feeding study. ADC project #475A, B of the 6th July 1968. Rhône-
Poulenc Inc., Agricultural Division, PO Box 125, Black Horse Lane,
Monmouth Junction, New Jersey 08852, USA. Supplied to WHO by Rhône-
Poulenc.
Rhône-Poulenc Inc. (1980a) Unpublished study. Residue determination
of phosalone and its oxygen analog in the milk and tissues of dairy
cattle by electron capture gas chromatography. ADC project #475D of
the 22nd September 1968. Rhône-Poulenc Inc., Agricultural division,
PO Box 125, Black Horse Lane, Monmouth Junction New Jersey 08852,
USA. Supplied to WHO by Rhône-Poulenc.
Rhône-Poulenc Inc. (1980b) Unpublished study. Residue determination
of metabolites containing the chlorobenzoxazole and
chloroaminoiphenol moieties in the milk and tissues from dairy
cattle fed phosalone. ADC project #475D1 of the 22nd September 1968.
Rhône-Poulenc Inc., Agricultural division, PO Box 125, Black Horse
Lane, Monmouth Junction, New Jersey 08852, USA. Supplied to WHO by
Rhône-Poulenc.
Rhône-Poulenc Inc. (1980c) Unpublished study. Validation of
analytical methods of phosalone and its metabolites in milk and
animal tissues. ADC project #496 of the 22nd September 1968. Rhône-
Poulenc Inc., Agricultural Division, PO Box 125, Black Horse Lane,
Monmouth Junction, New Jersey 08852, USA. Supplied to WHO by Rhône-
Poulenc.
Safonov, N.M. (1968) (Toxicological characteristics of a new
organophosphorus insecticide: phosalone preliminary results).
Gigiena truda professional, nye zabolevanija, 12, 43 - Translation
supplied to WHO by Rhône-Poulenc.
SERB (1988) Contrathion, pralidoxime, pralidoxima, Data Sheet.
Laboratoires SERB, 53 Rue de l'Isle-Adam, 75020, Paris, France.
Siglin, J.C., Jenkins, P.K., Rush, R.E., Liao, J.T. & Rodwell, D.E.
(1979) Unpublished study. Primary eye irritation study of phosalone
technical in rabbits with washout (EPA). Study No. 3147.36.
Springborn Life Sciences, Inc., Spencerville, Ohio 45887, USA.
Supplied to WHO by Rhône-Poulenc.
Vanrel, B., Thenaisie, S., Gascoin, M.N., Vanrell, B., Glomot, R. &
Le Bigot, J.F. (1989) Unpublished study. Metabolite of phosalone in
soil reverse mutation assay in vitro Ames test. Study No. 5137
MMO. Rhône-Poulenc Agrochimie, Lyon, France. Supplied to WHO by
Rhône-Poulenc.
Vogel, W.(1991). Unpublished study. Exposure of harvesters to
residues of Zolone(R) flo (Phosalone) insecticide applied to apples
in France in 1989. RCC project No 241683. RCC Umweltchemie AG, PO
Itingen CH 4452 Switzerland. Supplied to WHO by Rhône-Poulenc.
Woodard, M.W., Cockrell, K.O., Woodyard, G. & Cronin, M.T.I. (1966)
Unpublished study. Phosalone demyelination study in chickens. Report
submitted April 7th, 1966 to the Chipman Chemical Company. Woodard
Research Corporation. Supplied to WHO by Rhône-Poulenc.
Woodard Research Corporation (1967) Unpublished study. Phosalone
safety evaluation by repeated oral administration to rats for 103-
104 weeks. Report submitted April 7th, 1966 to the Chipman Chemical
Company. Supplied to WHO by Rhône-Poulenc.
Weill, N. (1988) Unpublished study. Ames test on Salmonella
typhimurium on 50221 RP. Report No. 803380. Hazleton France.
Supplied to WHO by Rhône-Poulenc.
WHO (1986) Environmental Health Criteria 63. Organophosphorus
insecticides: a general introduction. World Health Organization,
Geneva, p 63.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.

 technical grade phosalone is probably due to small amounts of oxon
present (up to 0.5% of the present technical material). The reason
that phosalone oxon did not inhibit rat brain cholinesterase at
single doses up to 9 mg/kg bw, whereas phosalone did at 27 mg/kg bw
(surprising in view of the much higher acute toxicity of the former)
is obscure (Fournel et al., 1968). Inhibition of brain
cholinesterase by phosalone was also observed by Pasquet et al.
(1976) with an I50 of 31 mg/kg bw p.o. Phosalone was reported to
shift to the right the oxygen dissociation curve in rats (Reddy et
al., 1992).
Toxicological studies
Acute toxicity studies
A summary of acute toxicity data for phosalone is shown in
Table 1. Phosalone has been classified as moderately hazardous by
WHO (WHO, 1992).
Short-term toxicity studies
Rats
A dose of 50 mg/kg bw/day administered by gavage (vehicle
unstated) to white rats for 14 days was lethal (Safonov, 1968).
Phosalone was administered as an oil solution to male rats
(10/dose) (strain unstated) at doses of 7.5 or 15 mg/kg bw/day for 5
weeks. The only death seen in the study was a control rat and no
abnormal clinical signs were observed. Slight reductions in body-
weight gain noted at both dose levels were not statistically
significant. No major differences between test and control groups
were seen in haematology or clinical chemistry except in
cholinesterase activity. Depression of blood cholinesterase
activity (> 20%) was observed at both doses. Brain cholinesterase
activity was reduced at the high dose only. No statistically
significant differences in organ weights or pathological findings
were seen between test groups and controls. The NOAEL was 7.5 mg/kg
bw/day, based on brain cholinesterase inhibition (Dubost et al.,
1963).
In an 8-week study, groups of 10 Crl:CD(SD) rats/sex/dose
received 10, 100, 300, 600 or 1200 ppm phosalone (94.1% pure) in the
diet. After 5 weeks, the doses administered to the 100 and 300 ppm
groups were increased to 2400 and 4800 ppm, respectively, in order
to establish the maximum tolerated dose. At 300/4800 ppm, one
female died and all remainder animals were sacrificed in extremis.
At 100/2400 ppm all females had to be sacrificed. Males at both
these doses survived but showed reduced body weight and food
Table 1. Acute toxicity of phosalone
Species Strain Sex Route LD50 (mg/kg bw) References
Mouse CD-1(COBS) M p.o. 118 (97-144) Fournel et al. (1968)
Mouse CD-1(COBS) F p.o. 93 (74-116) Fournel et al. (1968)
Mouse ? ? p.o. 180* 435** Dubost et al. (1963)
Mouse ? ? p.o. 205 Dubost et al. (1963)
Mouse ? ? p.o. 180 Safonov (1968)
Rat ? M p.o. 120 Dubost et al. (1963)
Rat CD(COBS) M p.o. 125 (102-152) Fournel et al. (1968)
Rat ? F p.o. 170 Dubost et al. (1963)
Rat ? F p.o. 135 Dubost et al. (1963)
Rat CD(COBS) F p.o. 90 (68-119) Fournel et al. (1968)
Rat ? ? p.o. 50 Hayward (1969)
Rat ? ? p.o. 135 Safonov (1968)
Rat CD(COBS) M p.o. 130 (104-163) Pasquet et al. (1976)
Rat CD(COBS) F p.o. 110 (87-139) Pasquet et al. (1976)
Rat ? F p.c. 390 Dubost et al. (1963)
Rat CD(COBS) F p.c. 700 (480-1015) Pasquet (1971)
Rat CD(COBS) F p.c. 1530 Mazuret (1971)
Rat ? F p.c. > 2560 Pasquet & Mazuret (1973a)
Rat CD(COBS) F p.c. 1530 Pasquet et al. (1976)
Rabbit ? ? p.c. > 1000 Dubost et al. (1963)
Guinea-pig ? ? p.o. 150 Dubost et al. (1963)
Guinea-pig ? ? p.o. 82 Dubost et al. (1963)
Hens Rhode Island red F s.c. 350 Heath et al (1967)
* Aqueous Suspension
** Oil solution
consumption. Body weight and cumulative body-weight gain were
significantly lower in females receiving 1200 ppm. No changes were
seen in the eyes. Significantly lower plasma and erythrocyte
cholinesterase were seen at 100 ppm in both sexes. Brain
cholinesterase activity was decreased at 600 ppm to about 80% and
50% of controls in males and females, respectively. Other changes
in clinical chemistry parameters were seen at higher doses,
including lower blood glucose, total protein and albumen and higher
cholesterol and blood urea. No treatment-related changes were seen
in organ weights or in organs on macroscopic or microscopic
examination. The NOAEL was 10 ppm, equal to 0.87 mg/kg bw/day in
males and 0.93 mg/kg bw/day in females, based on brain
cholinesterase inhibition (Kehoe, 1990).
Rabbits
A 3-week percutaneous study was undertaken in rabbits using
phosalone at doses of 0.4, 2.0 or 10 mg/kg bw/day
acetone/ethanol/arachid oil. There were no deaths. A 20%
depression in brain cholinesterase occurred in the highest dose
females. Some irritancy was observed (Kynoch et al., 1979).
Dogs
Phosalone was administered orally to dogs in oil solution for 1
month at dose levels of 7.5, 15 or 30 mg/kg bw/day. One animal of
each sex was used at each dose without controls. Neither clinical
signs nor significant weight changes were noted. Albumen/globulin
ratios in both animals treated with phosalone at 7.5 mg/kg bw/day
were depressed; however, this was not seen in the 15 mg/kg bw/day
animals. Significant plasma and erythrocyte cholinesterase
depression were seen in all animals except in females at 7.5 mg/kg
bw/day. A NOAEL could be not be determined in this study (Dubost
et al., 1963).
In another one-month study, groups of 4 beagle dogs/sex/dose
were administered phosalone (100% pure) at dietary concentrations of
0, 12.5, 25 or 37.5 ppm. No deaths were observed nor were there
abnormal clinical signs. There was no adverse effect on body
weight. Plasma cholinesterase activity was depressed by > 20% in
the high-dose group. Erythrocyte and brain cholinesterase activity
were not affected. At terminal autopsy a mauve discoloration was
observed in the bladder of all high- and mid-dose animals and in one
female at the low dose. Since the bladder discoloration was not seen
in any other dog study, the NOAEL was the highest dose of 37.5 ppm,
equal to 0.81 mg/kg bw/day (Noel et al., 1970).
In a 6-month study, groups of beagle dogs (4/sex/dose) received
phosalone at 0, 10 or 25 ppm in the diet. No haematological,
biochemical or histological changes attributable to phosalone were
observed except in erythrocyte and plasma cholinesterase. Brain
cholinesterase activity was not depressed. Depression of plasma
cholinesterase activity of about 40% and 25% was observed in the 25
ppm and 10 ppm groups, respectively. Decreased erythrocyte
cholinesterase activity was seen only at 25 ppm towards the end of
the study. The NOAEL was the highest dose (25 ppm, equivalent to
0.63 mg/kg bw/day) (Fournel et al,. 1966).
In a 107-week study, phosalone (purity unknown) was
administered in the diet to groups of 4 beagle dogs/sex/dose at
concentrations of 0, 100, 200 or 1000 ppm. One female at 1000 ppm
died during the study. Although marked plasma and erythrocyte
cholinesterase depression were observed, the immediate cause of
death seemed to be an infection. Body-weight loss was seen at 100
ppm, and cholinergic symptomatology was seen in two animals at the
highest dose and in one animal at 100 ppm. Abnormal ECGs (increased
T-waves) were seen at 1000 ppm. Biochemical and haematological
investigations demonstrated no changes that appeared to be compound-
related apart from elevations in ALAT in 4 animals at 1000 ppm.
Reductions in plasma cholinesterase > 20% were observed at 100 ppm
and above, and in erythrocyte cholinesterase at 200 ppm and above.
The degree of brain cholinesterase inhibition was variable, with a
mean in the 1000 ppm group of 79% of controls. Inhibition at
termination of the study was considerable in 3/8 animals and almost
complete in 2 animals. The NOAEL was 200 ppm, equivalent to 5 mg/kg
bw/day, based on clinical signs, body-weight loss, elevated ALAT and
brain cholinesterase depression at 1000 ppm (Donoso et al., 1967).
Groups of 6 beagle dogs (6/sex/dose) received 0, 5, 25 or 300
ppm phosalone (94.5% pure) in the diet for 52 weeks. Three deaths
in different groups were observed. During week 43, all animals
receiving 300 ppm vomited and lost weight and 5 showed reduced food
consumption. Some recovery in weight occurred towards the end of
the study. No changes of toxicological importance occurred on
ophthalmological, haematological, biochemical or urinary examination
except for cholinesterase activity. Reduction in plasma
cholinesterase > 20% compared to controls occurred in the 25 and
300 ppm groups (both sexes and combined and at all time points after
dosing commenced). At 5 ppm, significant reductions occurred in
males only at 4, 26, and 52 weeks. These reductions were just under
20%. Red cell cholinesterase was significantly reduced at 300 ppm
at all time points. At 25 ppm, this enzyme was reduced compared to
the control group in males at 26 weeks and in both sexes at 52
weeks. The reduction in this group compared to pre-dosing levels
was < 15%. Brain cholinesterase was significantly reduced in both
sexes at 300 ppm to about 60% of control values. No significant
differences between groups were observed in organ weights or after
macroscopic or histopathological examination of organs. The NOAEL
was 25 ppm, equal to 0.89 mg/kg bw/day (males) and 0.97 mg/kg bw/day
(females), based on depression of brain cholinesterase activity and
clinical effects at 300 ppm (Barker at al., 1992).
Long-term toxicity/carcinogenicity studies
Mice
In a 2-year study in CD-1 mice (60-65/sex/group), phosalone
(95.3% pure) was administered at dietary concentrations of 0, 15, 50
or 150 ppm. Five mice of each sex were sacrificed at 6 weeks and at
study termination at 105 weeks for plasma, erythrocyte and brain
cholinesterase determinations. Plasma and erythrocyte
cholinesterase were reduced by at least 20% in the 150 ppm group at
both 6 and 105 weeks. In some cases, particularly with plasma
cholinesterase, the reduction was much greater. Brain
cholinesterase was not reduced compared to controls. Statistically
significant increases in adrenal weights were found at all dose
levels and in both sexes, with the exception of females at the
lowest dose. These increases were both in relative and absolute
weights and were significant except for relative weight at 15 ppm in
males. Decrease in absolute brain weight was significant at 50 ppm
and 150 ppm in males. Relative increases in testicular weights (50
ppm), kidney weights in females only (15 ppm) and relative and
absolute increases in pituitary weights (males: 15 ppm) were also
significant. No neoplastic or non-neoplastic histopathological
findings were attributable to phosalone. There were adrenal weight
changes, which in the absence of histopathological changes, were
considered to be adaptive, and changes in testicular weights at 50
ppm and kidney weight in one sex at 15 ppm which were likely to be
insignificant in view of a lack of dose-response. The decrease in
absolute brain weight in males was not dose-related. The NOAEL was
thus the highest level of 150 ppm (equal to 23 and 31 mg/kg bw/day
in males and females, respectively), based on the lack of depression
of brain cholinesterase activity at this level (Nelson et al.,
1980).
Rats
In a 2-year study, groups of 30 rats/sex/dose (albino: strain
not stated) received phosalone of unknown purity at dietary
concentrations of 0, 25, 50 or 250 ppm (half of these concentrations
for the first four weeks). Mortality during the course of the study
was similar in all groups. There was some depression in weight gain
in the highest-dose animals compared to controls, especially in
males at 103 weeks. No haematological changes attributable to the
test material were observed. There were marked (up to three-fold)
differences in group mean plasma cholinesterase activities between
sexes, females having much higher activity; this raised some doubt
about the reliability of these assays. Inhibition of erythrocyte
and plasma cholinesterase activity was seen at 250 ppm. Inhibition
of brain cholinesterase activity > 20% was seen at 250 ppm in
males. No histopathological changes related to the test material
were seen. Although a few instances of testicular activity were
seen, there was no indication of any relationship between occurrence
of this finding and administration of the test material. The NOAEL
was 50 ppm, equivalent to 2.5 mg/kg bw/day, based on brain
cholinesterase inhibition at the next highest dose (Woodard Research
Corporation, 1967).
In a second 2-year study, Sprague-Dawley CD rats (50/sex/dose)
were given phosalone (purity 94.5%) at doses of 0, 5, 50 or 1000
ppm, the highest dose being reduced to 500 ppm at week 27. These
concentrations were equal to 0, 0.2, 1.8 or 20 mg/kg bw/day for
males and 0, 0.2, 2.5 or 31 mg/kg bw/day for females, on the basis
of consumption data from week 27. Satellite groups of animals
(15/sex/dose), were killed at 52 weeks. There was no effect on
mortality and clinical effects were mainly confined to the high-dose
group. Depression of plasma and erythrocyte cholinesterase was found
at the high and medium concentrations and depression of brain
cholinesterase activity was found in the highest dose group. There
were some treatment-related changes in the adrenals. A dose-response
across all groups in the prevalence of testicular atrophy was
observed (1/27, 3/34, 6/30 10/42 in survivors to the terminal kill).
Reductions in testis weight were dose-related, but were
statistically significant only at the high and mid-dose levels (5.03
g, 4.66 g, 4.51 g and 4.22 g, for the control, low, medium and high-
dose groups, respectively). The NOAEL was < 5 ppm, equal to 0.2
mg/kg bw/day (Barker & Sortwell, 1993).
Reproduction studies
Rats
In a three-generation reproduction study, groups of albino rats
(10 males and 20 females/group) were administered phosalone (purity
unknown) at dietary concentrations of 0, 25 or 50 ppm. Only half
the nominal dietary concentration was given during the initial three
weeks of the diet, on the grounds that young rats eat about twice as
much daily during this period; this procedure was repeated in each
generation. When the rats had received phosalone for 74 days, 10
females in each group were paired with the 10 males from the same
group for 10 days, after which the other 10 females were mated with
the 10 males. The animals from these litters (F1a) were sacrificed
after weaning, weighed and examined. Microscopic histopathological
examination was not carried out. The F0 rats were then remated as
different pairs to produce the F1b litters. Litters were examined
at weaning and representative male and female pups were placed on
control diet to constitute the next study groups. Within 21 days of
weaning, the F1b generation received 25 ppm or 50 ppm phosalone,
while controls continued to be fed control diet. The F1b rats were
mated in the same way as the F0 generation, F2a pups being
sacrificed and examined as above, while the F1b parents were also
killed and examined. Uteruses from females failing to bear two
litters were removed and examined for implantation sites. Rats were
selected from each F2b litter to give 10 males and 20 females in
each group, the remaining F2b pups were sacrificed and examined as
before without microscopic pathology. The retained F2b pups in the
two test groups received the test diet within 24 days of weaning.
After 51 days on the diet, the males and 10 females were paired for
10 days, the males being paired with the other 10 females after they
had all been on the diet for 71 days. The F3a pups were sacrificed
after weaning and examined as above. Within 10 days, F2b females
were re-mated with different males to produce the F3b litters.
After weaning, the pups were sacrificed and the main organs
examined. Tissues from one male and one female in each F3b litter
were examined histopathologically. The F2b parents were mated for
a third time to produce F3c pups litters, which were treated in the
same way as the F3b pups.
The test groups of rats were similar to the controls throughout
the study. Three F0 females died in the 50 ppm group and one in
the 25 ppm group while none died in the control group. Test
substance-related effects on fetal resorption were not observed and
malformations were observed only in 3 pups from one litter of the 25
ppm group. The three pups exhibited flaccid paralysis of the hind
limbs. Organs from the test and control weanlings from the F3b and
F3c groups did not differ significantly in weight or
histopathology. No adverse effect was determined in the study and
the NOAEL was > 50 ppm, equivalent to 2.5 mg/kg bw/day (Jones et
al., 1967a).
In a two-generation reproduction study, groups of 32 male and
32 female rats (6 week-old Crl: CD(SD)VSAF/Plus BR) were given
phosalone (96.8% pure) at dietary concentrations of 0, 10, 50 or 400
ppm. Phosalone was administered for 10 weeks, after which the rats
were mated for 20 days. The dams were allowed to rear their young
and from these litters, 21 days postpartum, 28 rats/sex/group were
selected as the F1 generation. F1 animals were selected and
treated from weaning until 16 weeks when they were mated. After
weaning of their litters the F1 parents were sacrificed as were the
F2 pups.
At 400 ppm, there was a lower body weight from conception to
day 17 in the F0 generation, and lower mean body-weights of the F1
males and females. There were 2 total litter losses post-partum in
the F0 generation and three in the F1 generation. There was a
reduced litter size in the F0 animals and increased pup mortality
to day 4 in both generations. Retarded pup growth and marked plasma
and erythrocyte cholinesterase depression were noted in both
generations. At 50 ppm, there was marked plasma and erythrocyte
cholinesterase depression in both generations, while at 10 ppm
cholinesterase reduction was < 20%. Brain cholinesterase activity
was not measured. The NOAEL was 50 ppm, equivalent to 2.5 mg/kg
bw/day, based on retarded pup growth and erythrocyte cholinesterase
depression (Bryson et al., 1991).
Special studies on embryotoxicity/teratogenicity
A study was carried out on chick embryos (white Leghorn) at
doses of 0.2, 0.6 or 1.8 mg/egg, using 30 eggs/group. Two further
groups of 30 eggs served as solvent (dimethylsulfoxide) and
untreated controls. At 18 days, the surviving embryos were removed,
weighed and examined. Phosalone had no significant effect on
mortality, weight or structure (Pasquet, 1969).
Groups of 25 female rats (Wistar HAN) were treated with 0, 2,
10 or 20 mg/kg bw/day of phosalone by gavage, from days 6 through 15
of gestation. Controls received distilled water with 4% CMC. The
animals were killed 21 days after coitus and the fetuses were
removed by caesarean section. Dams and fetuses were examined
macroscopically and microscopically. Oral administration of
phosalone at the highest dose produced clinical signs (chewing
movements, piloerection and dyspnea). Additionally, reduced food
consumption, weight loss, reduction in litter size, due to increased
resorptions and a slight increase in mean fetal body weight, were
observed. There was no evidence of fetal abnormality. At 2 and 10
mg/kg bw/day, maternal and fetal parameters were similar to
controls. The NOAEL was 10 mg/kg bw/day for both maternal toxicity
and fetotoxicity (Allen et al., 1989a).
Groups of 25 Fauve de Bourgogne rabbits were orally treated by
capsules with undiluted phosalone (purity unstated) from days 6
through 16 of gestation with doses of 0, 2, 6 or 18 mg/kg bw/day. A
positive control group was treated with thalidomide. The does were
sacrificed at 28 days. The percentage of animals becoming pregnant,
the number of resorptions, the number of fetuses recovered alive
after caesarian section and their weight did not differ
significantly from the untreated controls. The incidence of
malformed fetuses was low in all groups and the malformations were
trivial. By contrast malformations were observed in the thalidomide
group. The NOAEL was 18 mg/kg bw/day, the highest dose tested
(Pasquet, 1969).
Phosalone (93.5% pure) was administered in
carboxymethylcellulose (4%) by gavage to Chinchilla KFM/CHIN rabbits
(16/dose) at doses of 0, 1, 10 or 20 mg/kg bw/day from days 6
through 18 post-coitum. Clinical signs of cholinergic intoxication,
particularly dyspnea and extensor spasms, reduced food consumption,
loss of body weight and increased incidence of resorption of embryos
were observed at the highest dose. Post-implantation loss was
increased at 10 and 20 mg/kg bw/day compared to concurrent controls
but was within the range of historical controls. No adverse effect
was seen on fetuses at any dose, or on maternal parameters at doses
of 1 or 10 mg/kg bw/day. The NOAEL was 10 mg/kg bw/day, based on
both fetal and maternal toxicity (Allen et al., 1989b; Allen,
1989).
Special studies on genotoxicity
Based on the results of genotoxicity assays given in Table 2,
the Meeting concluded that phosalone was not genotoxic.
Special studies on neurotoxicity
Groups of white Leghorn hens (10/group/dose) were given dietary
doses of 0, 50, 163 or 500 ppm phosalone or TOCP at 500 ppm for 45
days. Hens were examined daily during the study. At termination,
brain, three levels of spinal cord and a portion of sciatic nerve
were taken from the controls, the TOCP-treated birds and the highest
dose of phosalone-treated hens. Although some signs of cholinergic
toxicity occurred, no phosalone-treated birds showed any indication
of organophosphate-induced delayed neuropathy. TOCP-treated hens
showed paralysis from 13 days onwards, and the only deaths seen,
were amongst the TOCP-treated fowl. Histopathological appearances
in tissues taken from the control and phosalone-treated animals were
within normal limits. Although there was some demyelination in the
TOCP group of animals, particularly in the sciatic nerve, this was
not significant (Woodard et al., 1966).
In another study, 10 Rhode Island red hens were dosed with 350
mg/kg bw phosalone (=LD50) as a 35% formulation, while protected by
atropine and pralidoxime methanesulfonate (P2S). The dose was
repeated after 3 weeks. There were 4 untreated controls and 4
positive controls treated with a neuropathic dose of mipafox. One
phosalone-treated hen died at 4 days, and another at 29 days. No
pathological changes were seen in the spinal cord. The positive
controls became severely ataxic at 13 days and showed serious
effects in the spinal cord. The untreated controls were clinically
and pathologically normal (Heath et al., 1967).
Groups of 20 New Hampshire red hens received TOCP (750 mg/kg bw
p.o., positive control), or 0 or 600 mg/kg bw p.o., phosalone.
Dosed and positive control animals also received atropine 15 min
before dosing. The vehicle control and phosalone group were redosed
at day 22 and sacrificed at day 41. Birds from the first positive
control group were killed on day 22 of the study at which time the
10 birds from the second positive control group were dosed and
sacrificed 19 days later. No significant clinical signs were seen
in the vehicle control fowl. Neuropathological examination was
performed on central and peripheral nervous tissue from all terminal
sacrificed animals and seven decedents in the phosalone group.
Table 2. Results of genotoxicity assays on phosalone
Test Object Concentration phosalone Purity Results Reference
In vitro
Ames test (1) S. typhimurium 1-1000 µg/plate ? Negative Benezet & Cartier, 1980
(strains TA98, 100, 125-1000 µg/plate
1535, 1537)
Ames test (1) S. typhimurium 250-10 000 94% Negative Haworth & Lawlor, 1989
(strains TA98, 100, µg/plate
1535, 1537, 1538)
HPRT test (1) CHO/K1 cells 0.1-50 µg/ml 95% Negative Cordier & Bonneau, 1985
Chromosomal CHO/K1 cells 4, 40, 75 µg/ml 95% Negative Cordier & Fournier, 1989
aberration in
CHO cells (1)
Chromosomal CHO/WBL cells 16.7, 500 µg/ml 94% Negative Murli, 1989
aberration in (also 5 µg/ml without
CHO cells (1) metabolic activation)
UDS in vitro Rat primary 0.005-252 µg/ml 94% weak Cifone, 1989
hepatocyte positive
In vivo
Micronucleus CD-1 mice 10, 20, 40 mg/kg bw/day 99.5% Negative Pasquet & Fournier, 1980
Dominant lethal CD-1 mice 10, 30, 75 mg/kg 95.3% Negative Goldenthal et al.,
test 1978
(1) With and without metabolic activation.
Tissues examined were spinal cord (cervical, thoracic and
lumbosacral), medulla, sciatic and tibial nerves. In the positive
control groups, all except one bird showed clinical signs of delayed
neuropathy, appearing from days 13-24. In both positive controls,
histopathological evidence of delayed neuropathy was found. In the
phosalone group, 4 birds showed signs of cholinergic toxicity after
the first dose and two birds died. On day 22 after the second dose,
six hens showed acute toxicity. Seven hens died on days 23-24.
Histopathological evidence of nerve degeneration was not more
frequent in this group than in the vehicle controls (Morris, 1983).
The Meeting concluded that there is no evidence that phosalone
has potential to cause organophosphate-induced delayed neuropathy.
Antidote studies of phosalone intoxication
Ten groups of 10 mice each were given phosalone orally followed
immediately by i.p. administration of combinations of pralidoxime
methylsulfate (Contrathion) at 0, 25, 50, 100 or 200 mg/kg bw and
atropine at 0, 10, 20 or 40 mg/kg bw. Both antidotes were effective
in preventing death although pralidoxime methylsulfate at 100 and
200 mg/kg bw appeared to be toxic. Some aspects of the results were
difficult to interpret since doses of pralidoxime methylsulfate used
were high compared to the recommended human dose of 5-6 mg/kg bw
(SERB, 1988). Moreover the i.p. LD50 of a similar pralidoxime salt
is 125-250 mg/kg bw in the mouse (Marrs, 1991). Doses of atropine
used in this study were also high (Dubost et al., 1963).
In another study using orally administered phosalone (vehicle
unstated), groups of rats (9-10/group) were given 50 or 100 mg/kg bw
phosalone. Phosalone (100 mg/kg bw) was administered to another
group of 10 rats, followed by pralidoxime methanesulfonate (50 mg/kg
bw s.c.) and atropine (17.5 mg/kg bw s.c.) 15-20 minutes after
clinical signs of anticholinesterase poisoning developed. All
animals in the 100 mg/kg bw group not treated with the antidotes
showed considerably reduced erythrocyte anticholinesterase activity
and died within 24 hours. Five out of 10 rats survived 24 hours in
the 50 mg/kg bw group and their cholinesterase activity was less
depressed. When treated with the antidotal regime, 9/10 rats dosed
with phosalone at 100 mg/kg bw survived and cholinesterase activity
was less depressed than that of animals treated with 50 mg/kg bw
phosalone but untreated with antidotes (Hayward, 1969).
In a study of the antidotal effects of bis-pyridinium oxime
(obidoxime), groups of mice (4/sex) were administered phosalone by
gavage at a dose of 250 mg/kg bw, a dose well above the LD50.
Immediately afterwards, obidoxime and atropine were injected
intraperitoneally at obidoxime doses of 0, 25, 50 or 100 mg/kg bw
and at atropine doses of 0, 10, 20 or 40 mg/kg bw in various
combinations. When no antidote was given, phosalone was invariably
fatal whereas survival was considerably improved with combinations
of antidote except at the highest dose of atropine and obidoxime.
Obidoxime alone was clearly more beneficial than atropine alone.
Synergism was not observed, because of the study design (Pasquet &
Masuret, 1970).
It was concluded from these studies that atropine is an
effective antidote to phosalone. Pralidoxime (either methylsulfate
or methanesulfonate) reactivated phosalone-inhibited rat erythrocyte
cholinesterase and was an effective antidote. Obidoxime was also an
effective antidote but the usual synergism of atropine and
pralidoxime salts or obidoxime was not noted probably due to the
design of the studies.
Special studies on metabolites and intermediates
A number of acute toxicity and genotoxicity studies have been
conducted with phosalone metabolites and intermediates. The results
are summarized in Tables 3-6.
Other studies
3-Hydroxymethyl 6-chlorobenzoxolone was non-irritant to the
intact rabbit skin but very irritant to the scarified skin (Pasquet
& Mazuret, 1973b). 3-Chloromethyl 6-chlorobenzoxolone was non-
irritant to the intact rabbit skin (Pasquet et al., 1983b).
In a 13-week study, groups of 15 male and 15 female rats
(Sprague-Dawley-derived Crl:CD(SD)BR) were given 6-chlorobenzoxolone
at dietary concentrations of 0,5, 15 or 45 mg/kg bw/day for 13
weeks. Groups of 10 male and female controls and high-dose animals
underwent a subsequent 28-day treatment-free period. The only
effect of the treatment was suppression of weight gain in all
treated males and in the high-dose females. A compensatory weight
gain was observed in the animals that underwent the treatment-free
period (Owens, 1985).
A study was performed to determine the potential of Di-syston
(disulfoton) to potentiate the activity of phosalone. Groups of 8
beagle dogs were fed diets containing phosalone at concentrations of
7.5, 15 or 25 ppm for 19 weeks. In each case, Di-syston was added
at a concentration of 1 ppm from week 14 to week 19. There were no
changes in physical appearance, haematology or pathology that could
be ascribed to the treatment. No treatment-related changes in
plasma or erythrocyte cholinesterase were seen (Jones et al.,
1967b).
Table 3. Acute toxicity of phosalone metabolites
Species Strain Sex Route LD50 References
(mg/kg bw)
Phosalone-oxon
Mouse CD-1(COBS) M PO 35 Fournel et al., 1968
(30-41)
Mouse CD-1(COBS) F PO 40 Fournel et al., 1968
(33-49)
Mouse ? Mix PO 32 Rhône-Poulenc, 1968
Rat CD(COBS) M PO 36 Fournel et al., 1968
(29-44)
Rat CD(COBS) F PO 21 Pasquet et al., 1976
(12-28)
Rat CD(COBS) F PO 20 Fournel et al., 1968
(15-29)
Rat CD(COBS) F PC 380 Pasquet & Mazuret, 1973a
(240-610)
3-methylthiomethyl 6-chlorobenzoxazolone
Mouse OF1 M PO 590 Pasquet & Mazuret, 1980
(499-698)
OF1 F PO 837 Pasquet & Mazuret, 1980
(706-991)
3-methylsulfinylmethyl 6-chlorobenzoxazolone
Mouse OF1 M PO 405 Pasquet & Mazuret (1980)
OF1 F PO 315 Pasquet & Mazuret (1980)
(256-386)
Table 3 (contd)
Species Strain Sex Route LD50 References
(mg/kg bw)
3-methylsulfonylmethyl 6-chlorobenzoxazolone
Mouse OF1 M PO > 5000 Pasquet & Mazuret 1980
OF1 F PO > 5000 Pasquet & Mazuret 1980
Table 4. Acute toxicity of intermediates and soil metabolites
Species Strain Sex Route LD50 References
(mg/kg bw)
3-hydroxymethyl 6-chlorobenzoxolone
Mouse CD-1(COBS) Mix p.o. 730 Pasquet & Mazuret, 1973b
(557-956)
Benzoxolone
Mouse CD-1(COBS) Mix p.o. 580 Pasquet & Mazuret, 1973b
3-chloromethyl 6-chlorobenzoxolone
Rat CD Sprague- Mix i.p. 305 Pasquet et al., 1983a
Dawley
Table 5. Genotoxicity of phosalone intermediates
Compound Test object Concentration Purity Result References
intermediate
Unknown intermediate
Ames test (1) Salmonella typhimurium 0.005-0.5 µg/plate ? -ve Weill, 1988
TA98, TA100, TA1535,
TA1537, TA1538
Table 6. Genotoxicity of phosalone soil metabolites
Compound Test object Concentration Purity Result Reference
intermediate
3-amino 7-chloro 2-phenoxazone
Ames test (1,2) Salmonella typhimurium 10-100 µg/plate (-S9) 95% +ve Vanrel et al., 1989
TA98, TA100. TA1535, 25-125 µg/plate (+S9)
TA1537, TA1538
(1) With and without metabolic activation.
(2) Positive with metabolic activation and in TA1537, and TA100 only.
Phosalone (96% pure) was applied to the skin of rabbits (New
Zeeland white) for 21 days at dose levels of 0.4, 2 or 10 mg/kg
bw/day. Azinphosethyl was applied at 2 mg/kg bw/day. Both
chemicals were applied to intact or abraded skin. Inhibition of
brain cholinesterase > 20% was not noted in any phosalone group,
but marked depression was seen with azinphosethyl. Phosalone did
not cause red blood cell or plasma cholinesterase depression (Kynoch
et al., 1979).
In studies to elicit delayed sensitization in guinea-pigs
(Pasquet & Mazuret, 1977; Cunny & Siglin, 1989) no sensitization was
seen. Phosalone was moderately irritant to the eye in New Zeeland
white rabbits (Siglin et al., 1989). Irritancy to the rabbit eye
was also observed by Safonov (1968).
Observations in humans
In a study performed on 14 male volunteers to establish a re-
entry period after applying phosalone in citrus groves, dislodgeable
residues up to 3.6 µg/cm2 (leaf) produced no adverse effect other
than some plasma cholinesterase depression (Knaak et al., 1978).
In 1987, several cases of illness resembling anticholinesterase
poisoning were reported in California. On investigation by the
California Department of Food and Agriculture, it was found that the
illness had occurred in workers harvesting grapes from vineyards
treated with a preparation containing phosalone. A study was
therefore carried out in which grapes were treated with phosalone (3
lb/acre) twice, 24 days apart. Fourteen days after the second
treatment, 30 volunteers harvested the crop for 6 consecutive days
while 22 volunteers harvested a crop from an untreated vineyard.
Assignment to the treated or control group was random. Blood was
collected for plasma and erythrocyte cholinesterase activity in the
afternoon of days 4, 3 and 2 pre-harvest, daily during harvest, and
three days thereafter. Samples were transported cooled in ice and
assayed within six hours by a modified Ellman method. Urinary
diethylphosphates were assayed on early morning urine samples
collected on days 6, 5, 4 and 1 before harvest, daily during
harvest, and days 1 and 2 thereafter. No symptoms or clinical signs
were recorded that could be attributed to anticholinesterase
effects. There was a gradual decline in plasma cholinesterase
levels during the harvest, which recovered after the end of the
harvest. This effect was not seen in controls nor in red blood cell
cholinesterase level in either test or control groups. Ethyl
phosphate metabolites were found in the urine of the test group
(Baugher, 1989). A similar study was carried out on French apple
pickers (Vogel, 1991). No clinical signs, symptoms suggestive of
anticholinesterase toxicity, or significant depression of plasma or
erythrocyte cholinesterase were observed.
In 1966-1979, eleven pesticide incidents involving the
agricultural use of phosalone were reported to the USEPA's Pesticide
Incident Monitoring System (Hodgson & Smith, 1992). The antidote
dietixim (a cholinesterase reactivator) was effective in human
phosalone poisoning (Kundiev & Kagan, 1992).
COMMENTS
After oral administration, phosalone was moderately well
absorbed, 15-25% appearing in the faeces. Phosalone was extensively
metabolized to phosphorothioates, phosphorodithioates and
3-methylthiomethyl 6-chloro-benzoxazolone, the last of which is
subsequently metabolized ultimately to 3-methylsulfonylmethyl 6-
chlorobenzoxazolone.
Pure phosalone is almost certainly not a cholinesterase
inhibitor, but acquires inhibitory activity after conversion to
phosalone oxon in vivo.
The acute oral toxicity varies with species, but is in the
region of 100-200 mg/kg bw in rodents. Phosalone has been
classified as moderately hazardous by WHO.
There were two short-term studies in rats which could be used
to give NOAELs. In a five-week oral gavage study in rats at doses
of 0, 7.5 or 15 mg/kg bw/day, the NOAEL or 7.5 mg/kg bw/day based on
brain cholinesterase inhibition. In an eight-week study in rats
using dietary concentrations of 0, 10, 100, 300, 600 or 1200 ppm,
the NOAEL was 10 ppm (equal to 0.87 mg/kg bw/day), based on brain
cholinesterase inhibition. It is possible that NOAELs could have
been established at 100 or 300 ppm, but the dose rates for those
groups were increased to 2400 and 4800 ppm, respectively, after 5
weeks to establish a maximum tolerated dose.
Five studies were carried out in dogs. In a one-month oral
dosing study in dogs, a NOAEL could not be determined as plasma and
erythrocyte cholinesterase depression were seen at the lowest dose
(7.5 mg/kg bw/day). In another one-month study using dietary
concentrations of 0, 12.5, 25 or 37.5 ppm, the NOAEL was the highest
level, which was equal to 0.81 mg/kg bw/day; although plasma
cholinesterase depression was seen in the study, neither erythrocyte
nor brain cholinesterase activity was depressed. In a 6-month study
in dogs using dietary concentrations of 0, 10 or 25 ppm, although
plasma and erythrocyte cholinesterase were depressed, the brain
enzyme was not, so the NOAEL was the highest dose (equivalent to
0.63 mg/kg bw/day). In a two-year study in beagle dogs, males and
females were fed phosalone in the diet at concentrations of 0, 100,
200 or 1000 ppm. The NOAEL was 200 ppm, equivalent to 5 mg/kg
bw/day, based on brain cholinesterase depression, body-weight loss
and elevated alanine aminotransferase at the highest dose. In a
more recent one-year study in dogs using dietary concentrations of
0, 5, 25 or 300 ppm phosalone, the NOAEL was 25 ppm (equal to 0.89
mg/kg bw/day), based on brain cholinesterase depression at 300 ppm.
The overall NOAEL for dogs was considered to be 200 ppm, in view of
the spacing of the doses in the most recent study.
In a lifetime carcinogenicity study in mice, phosalone was
given at dietary concentrations of 0, 15, 50 or 150 ppm. The NOAEL
was 150 ppm, equal to 23 mg/kg bw/day, based on the lack of
depression of brain cholinesterase activity, although plasma and red
blood cell cholinesterase depression were seen at this level. There
was no evidence of carcinogenicity.
In a two-year study in rats using concentrations of 0, 25, 50
or 250 ppm phosalone in the diet, the NOAEL was 50 ppm, equivalent
to 2.5 mg/kg bw/day, based on brain cholinesterase depression at the
highest dose. In a second 2-year study in rats, dietary
concentrations of 0, 5, 50 or 1000 ppm were used, the highest dose
being reduced to 500 ppm later in the study. There was a
statistically significant increase in the prevalence of testicular
atrophy and reduction in testicular weight in both the high and mid-
dose groups and a dose response across all groups for both effects.
The Meeting concluded that the NOAEL was < 5 ppm, equal to 0.2
mg/kg bw/day.
In a teratogenicity study in rats at doses of 0, 2, 10 or 20
mg/kg bw/day, the NOAEL was 10 mg/kg bw/day for both maternal
toxicity and fetotoxicity. In a study in rabbits using doses of 0,
2, 6 or 18 mg/kg bw/day, the NOAEL was the highest dose. In another
study in rabbits, using doses of 0, 1, 10 or 20 mg/kg bw/day, the
NOAEL was 10 mg/kg bw/day based on maternal toxicity. Phosalone was
not teratogenic in either the rat or rabbit.
Two multigeneration reproduction studies in rats were reviewed.
In the first study, using dietary concentrations of phosalone of 25
or 50 ppm, no adverse effects were observed. The NOAEL was > 50
ppm, equivalent to 2.5 mg/kg bw/day. In the second study, in which
phosalone was administered at dietary concentrations of 0, 10, 50 or
400 ppm, the NOAEL was 50 ppm (equivalent to 2.5 mg/kg bw/day) based
on retarded pup growth and plasma and erythrocyte cholinesterase
depression.
Phosalone has been adequately tested in a series of in vitro
and in vivo genotoxicity assays. The Meeting concluded that
phosalone was not genotoxic.
There is no evidence that phosalone has potential to cause
organophosphate-induced delayed neuropathy.
Pralidoxime salts and obidoxime are both effective in
experimental phosalone poisoning.
No human study was available from which a NOAEL could be
derived.
An ADI of 0-0.001 mg/kg bw was established based on the lowest
dose (0.2 mg/kg bw/day) in the recent two-year study in rats. A
200-fold safety factor was used because of concerns that the trend
for the occurrence of testicular atrophy and reduction in testis
weight existed across all groups.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 150 ppm, equal to 23 mg/kg bw/day (two-year study)
Rat: < 5 ppm, equal to 0.2 mg/kg bw/day
(two-year study)
50 ppm, equivalent to 2.5 mg/kg bw/day
(multigeneration reproduction study)
Rabbit: 10 mg/kg bw/day (teratology study)
Dog: 200 ppm, equivalent to 5 mg/kg bw/day
(several studies)
Estimate of acceptable daily intake for humans
0-0.001 mg/kg bw
Studies which will provide information valuable in the continued
evaluation of the compound
Explanation of the testicular atrophy seen in the recent study
in rats.
Observations in humans.
REFERENCES
Allen, P.A. (1989) Unpublished study. Additional evaluation of the
incidence of resorptions in the studies performed by RCC on the
effects of phosalone technical on embryonic and fetal development in
the rabbit. Project 083002, 204423, 082991. RCC Research and
Consulting Company AG, PO Box, CH 4452 Itingen, Switzerland.
Supplied to WHO by Rhône-Poulenc.
Allen, P.A., Frei, D., Mladenovic, P. & Terrier C. (1989a)
Unpublished study. Embryotoxicity study (including teratogenicity)
with phosalone technical in the rat. Project 082980. RCC Research
and Consulting Company AG, PO Box, CH 4452 Itingen, Switzerland.
Supplied to WHO by Rhône-Poulenc.
Allen, P.A., Mladenovic, P. & Terrier, C. (1989b) Unpublished study.
Embryotoxicity study (including teratogenicity) with phosalone
technical in the rabbit. Project 083002. RCC Research and Consulting
Company AG, PO Box, CH 4452 Itingen, Switzerland. Supplied to WHO by
Rhône-Poulenc.
Barker, M.H., Smith, T.G., Buist, D.P., Crook, D., Morrow, J. &
Gopinath, C. (1992) Unpublished study. Phosalone dietary toxicity
study in beagle dogs. HRC report No. RNP 336/91907. Huntingdon
Research Centre Ltd, Wooley, Huntingdon, PE18 6ES, England. Supplied
to WHO by Rhône-Poulenc.
Barker, M.H. & Sortwell, R.J. (1993) Unpublished study. Phosalone
(11974 RP). Potential tumorigenic and toxic effects in prolonged
dietary administration to rats Volume 1. Huntingdon Research Centre
Ltd, PO Box 2, Huntingdon, Cambridgeshire PE18 6ES. Supplied to WHO
by Rhône-Poulenc. England
Baugher (1989). Unpublished study. Exposure of harvesters to Zolone
(R) EC brand insecticide applied to grapes in California, 1988.
Project no 29088. Orius Associates, Frederick, Maryland, USA.
Supplied to WHO by Rhône-Poulenc.
Benezet, F. & Cartier, J.R. (1980) Unpublished study. Phosalone (11
974 RP) mutagenicity study on Salmonella typhimurium. Report
RP/RD/CNG No. 19 234 E of 19th January 1978. Centre Nicolas Grillet,
94400 Vitry-sur-Seine, France. Supplied to WHO by Rhône-Poulenc.
Bryson, A.M., Parker, C.A., Offer, J.M. & Farmer, H. (1991)
Unpublished study. A study of the effect of phosalone on
reproductive function of two generations in the rat. Report No. RNP
326/91277. Huntingdon Research Centre Ltd, PO Box 2, Huntingdon,
Cambridgeshire, PE18 6ES England. Supplied to WHO by Rhône-Poulenc.
Cifone, M.A. (1989) Unpublished study. Mutagenicity test on
phosalone technical in the rat primary hepatocyte unscheduled DNA
synthesis test. HLA study No. 10754-0-447, Hazleton Laboratories
America Inc 5518 Nicholson Lane, Suite 400, Kensington Maryland
20895, USA. Supplied to WHO by Rhône-Poulenc.
Cordier, A. & Bonneau, D. (1985) Unpublished study. Phosalone (11
974 RP) CHO/HPRT test. Report. ST/CRV/Tox. No. 22 363-E of 10th May
1985. Rhône-Poulenc Santé, Centre de Recherches de Vitry, 94403
Vitry-sur-Seine Cedex, France. Supplied to WHO by Rhône-Poulenc.
Cordier, A. & Fournier, E. (1985) Unpublished study. Phosalone (11
974 RP) chromosome aberration test in Chinese hamster ovary cells
(CHO). Report ST/CRV/Tox. No. 435-E of 28th August 1985. Rhône-
Poulenc Santé, Centre de Recherches de Vitry, 94403 Vitry-sur-Seine
Cedex, France. Supplied to WHO by Rhône-Poulenc.
Craine, E.M. (1974a) Unpublished study. Disposition of phosalone-
14C in a lactating cow. Research report No. EMC 74:17 of the 29th
April 1974. Hess and Clark Research Department, Ashland, Ohio, USA.
Supplied to WHO by Rhône-Poulenc.
Craine, E.M (1974b) Unpublished study. Disposition of phosalone-14C
in a lactating cow. Research report No. EMC 74:84 of the 25th
November 1974. Hess and Clark Research Department, Ashland, Ohio,
USA. Supplied to WHO by Rhône-Poulenc.
Craine, E.M. (1974c) Unpublished study. The disposition of
phosalone-14C applied to the skin of a pig. Research report No. EMC
74:30 of the 14th May 1974. Hess and Clark Research Department,
Ashland, Ohio, USA. Supplied to WHO by Rhône-Poulenc.
Craine, E.M. (1975) Unpublished study. The metabolism of phosalone-
14C in dairy cows. Research report No. EMC 75:51 of the 2nd July
1975. Hess and Clark Research Department, Ashland, Ohio, USA.
Supplied to WHO by Rhône-Poulenc.
Cunny, H. & Siglin, J.C. (19789) Unpublished study. Delayed contact
hypersensitivity study in guinea-pigs with phosalone technical.
Study No. 3147.37. Springborn Life Sciences Inc. Spencerville, Ohio
45887, USA. Supplied to WHO by Rhône-Poulenc.
Desmoras, M.J. (1979). Unpublished study. Studies on the metabolism
of phosalone (11974 R.P.), in the mouse, the rat and the rabbit.
Note RP/RD/CNG No 20 234 of the 20 August 1979. Supplied to WHO by
Rhône-Poulenc.
Desmoras, M.J. & Fournel, J. (1968) Unpublished study. Studies on
the degradation of phosalone (11974 R.P.), in mammals. Note RP -
DSPh of the 1st March 1968. Rhône-Poulenc, Laboratoires de
Recherches, 22 Avenue Montaigne, 75 Paris 8, France. Supplied to WHO
by Rhône-Poulenc.
Donoso, J., Woodard, M.W. & Woodard, G. (1967) Unpublished study.
Phosalone safety evaluation by repeated oral administration to dogs
for 107 weeks. Report dated 7th September 1967. Woodard Research
Corporation, Herndon, Virginia 22070 USA. Supplied to WHO by Rhône-
Poulenc.
Dubost, P. Fournel, J., Ganter, P., Julou, L., Koenig, F. & Myon, J.
(1963). Unpublished study. Acute toxicity, local tolerance,
anticholinesterase activity and chronic toxicity in rats and dogs.
Note DSPh 9, 203 of the 29th March 1963. Centre Nicolas Grillet,
94400 Vitry-sur-Seine, France. Supplied to WHO by Rhône-Poulenc.
Fournel, J., Ganter, P., Julou, L., Populaire, P., Myon, J., Pascal,
S. & Pasquet, J. (1966) Unpublished study. Phosalone (11 974 RP)
Sub-acute (6-month) toxicity study in the dog. Note RP-DSPh 11 330 E
of the 14th June 1966. Société des Usines Chimiques Rhône-Poulenc,
Direction Scientifiques, Laboratoires de Recherche, Centre Nicolas
Grillet, 94400 Vitry-sur-Seine, France. Supplied to WHO by Rhône-
Poulenc.
Fournel, J., Julou, L. & Pasquet, J. (1968) 12244 R.P. Unpublished
study. Acute toxicity in mice rats and in vivo anti-cholinesterase
activity in rats. Note RP - DSPh 12663 (=12665E) of the 1st March
1968. Société des Usines Chimiques Rhône-Poulenc, Direction
Scientifiques, Laboratoires de Recherche, 22 Avenue Montaigne, 75
Paris 8,France. Supplied to WHO by Rhône-Poulenc.
Goldenthal, E.I., Jessup, D.C. & Rodwell, D.E. (1978) Unpublished
study. Dominant lethal study in mice. Study carried out for Rhône-
Poulenc dated the 18th December 1978. International Research and
Development Corporation, Mattawan, Michigan, 49071, USA.
Haworth, L. & Lawlor, T.E. (1989) Unpublished study. Mutagenicity
test on phosalone technical in the Ames Salmonella/microsome
reverse mutation assay. HLA study No. 10754-0-401, Hazleton
Laboratories America Inc., 5518 Nicholson Lane, Suite 400,
Kensington Maryland 20895, USA. Supplied to WHO by Rhône-Poulenc.
Heath, S.A.B., Rivett, K.F. & Woolf, N. (1967) Unpublished study.
Phosalone test for neurotoxicity. Study No. 230857, May and Baker
Ltd, Dagenham, Essex, England. Supplied to WHO by Rhône-Poulenc.
Hayward, A. (1969). Unpublished study. Insecticides: a study of the
effects of "Cyanox" (S-4084) and "phosalone" on rat erythrocyte
cholinesterase. Report No. PRG/584 (=274359). May and Baker Ltd,
Ongar, Essex, England. Supplied to WHO by Rhône-Poulenc.
Hodgson, H.J. & Smith, A.D. (1992). Commercial and residential
poisoning with anticholinesterases. In: Ballantyne B and Marrs TC
(ed) Clinical and experimental toxicology of organophosphates and
carbamates, pp. 352-363. Butterworth-Heinemann, Oxford, England.
Hopkins, R., Lewis, C.J. & Lewsley, R. (1991). Unpublished study.
(14C)-phosalone: a study of the absorption, distribution,
metabolism and excretion in the rat. Report No. 5759-68/93R.
Hazleton UK, Harrogate, England. Supplied to WHO by Rhône-Poulenc.
Jones, E.M., Post, K.F., Scott, W.J., Woodyard, M.W., Woodyard, G. &
Cronin, M.T.I. (1967a) Unpublished study. Phosalone three generation
reproduction study in the rat. Report dated September 11th 1967.
Woodyard Research Corporation and Chipman Chemical Company. Supplied
to WHO by Rhône-Poulenc.
Jones, M.E., Woodard, M.W., Imming, R.J., Woodard, G. & Cronin,
M.T.I. (1967b) Unpublished study. Phosalone followed by a
determination of potentiation activity with the compound Di-syston.
Report dated August 10th, 1967. Woodyard Research Corporation and
Chipman Chemical Company. Supplied to WHO by Rhône-Poulenc.
Kehoe, D.F. (1990) Unpublished study. 8-week dietary toxicity study
with phosalone in rats. HLA study No. 6224-149, Hazleton
Laboratories America Inc 3301 Kinsman Boulevard, Madison, Wisconsin
53704, USA. Supplied to WHO by Rhône-Poulenc.
Knaak, J.B., Maddy, K.T., Gallo, M.A., Lillie, D.T., Craine, E.M &
Serat, W.F. (1978) Worker re-entry study involving phosalone
application to citrus groves. Toxicol Appl. Pharmacol., 46 363-
374.
Kundiev, Y.I. & Kagan, Y.S. (1992) Anticholinesterses used in the
USSR: poisoning, treatment and preventative measures. In: Ballantyne
B and Marrs TC (ed) Clinical and experimental toxicology of
organophosphates and carbamates.pp 494-501. Butterworth-Heinemann,
Oxford, England.
Kynoch, S.R., Lloyd, G.K., Mallard, J.R., Street, A.E., Gibson,
W.A., Wadsworth, P.F. & Prentice, D.E. (1979) Unpublished study. The
effect of repeated applications of phosalone 11974 RP to the skin of
rabbits for 21 days. Report No. RNP 125/79335. Huntingdon Research
Centre Ltd, PO Box 2, Huntingdon, Cambridgeshire, PE18 6ES, England.
Supplied to WHO by Rhône-Poulenc.
Marrs, T.C. (1991). Toxicology of oximes used in the treatment of
organophosphate poisoning. Adverse drug reactions and Toxicology
Reviews, 10: 61-72.
Mazuret, A. (1971) Unpublished study. Phosalone,methyl-azinphos and
parathion acute toxicity in the rat. Report No. RP -DSPh No. 15544-
E. Rhône-Poulenc Santé, Centre de Recherches de Vitry, 94403 Vitry-
sur-Seine Cedex, France. Supplied to WHO by Rhône-Poulenc.
Morris, J.M. (1983) Unpublished study. Acute delayed neurotoxicity
study in hens with phosalone. GSRI project No. 411-B51-40, Gulf
South Research Institute, PO Box 1177, New Iberia, Louisiana 70560,
USA. Supplied to WHO by Rhône-Poulenc.
Murli, H. (1989) Unpublished study. Mutagenicity test on phosalone
technical in an in vitro cytogenetic assay measuring chromosomal
aberration frequencies in Chinese hamster ovary (CHO) cells. HLA
study No. 10754-0-437, Hazleton Laboratories America Inc., 5518
Nicholson Lane, Suite 400, Kensington, Maryland 20895, USA. Supplied
to WHO by Rhône-Poulenc.
Nelson, L.W., Geil, R.G. & Spicer, E.J. (1980) Unpublished study.
Phosalone lifetime oncogenicity study in mice. International
Research and Development Corporation, Mattawan, Michigan 49071, USA.
Report 347-009, submitted June 23rd, 1980. Supplied to WHO by Rhône-
Poulenc.
Noel, P.R., Rivett, K.F., Osborne, B.E. & Street, A.E. (1970)
Unpublished study. Phosalone dietary intake in beagle dogs for four
weeks. Report No. 3619/70/431. Huntingdon Research Centre Ltd, PO
Box 2, Huntingdon, Cambridgeshire, PE18 6ES, England. Supplied to
WHO by Rhône-Poulenc.
Owen, P.E. (1985) Unpublished study.6-chlorobenzoxolone: 13 week
oral (dietary administration) study in the rat with a 28-day
treatment-free period. Report No. 4229-198/14. Hazleton UK, Otley
Road, Harrogate, England. Supplied to WHO by Rhône-Poulenc.
Pasquet, J. (1969) Unpublished study. Study of the teratogenic
activity of phosalone (11974 RP) on the chick embryo and on the
rabbit. Note RP - DSPh 13447E of the 7th February 1969 (translation
no 2707). Centre Nicolas Grillet, 94400 Vitry-sur-Seine, France.
Supplied to WHO by Rhône-Poulenc.
Pasquet, J. (1971) Unpublished study. Phosalone (11 974 R.P.)
toxicité aigüe chez la ratte par voie percutanée. Note RP - DSPh
15481 of the 28th June 1971. Centre Nicolas Grillet, 94400 Vitry-
sur-Seine, France. Supplied to WHO by Rhône-Poulenc.
Pasquet, J. & Fournier, E. (1980) Unpublished study. Phosalone (11
974 RP) micronucleus test in the mouse. Report.RP/RD/CNG No 557-E of
8th April 1980. Rhône-Poulenc Santé, Centre de Recherches de Vitry,
94403 Vitry-sur-Seine Cedex, France. Supplied to WHO by Rhône-
Poulenc.
Pasquet, J. & Mazuret, A. (1970) Unpublished study. Obidoxime
chloride (Toxogonin R Merck = 24,590 R.P. chloride) acute
intraperitoneal toxicity in the mouse and antidotal effect on the
acute oral toxicity in the mouse of phosalone (11974 RP) RP - DSPh
No. 14514-E. Rhône-Poulenc Santé, Centre de Recherches de Vitry,
94403 Vitry-sur-Seine Cedex, France. Supplied to WHO by Rhône-
Poulenc.
Pasquet, J., Mazuret, A., Vaissere, J. & Gaildrat (1983a)
Unpublished study. Chloromethyl-3 chloro-6 benzoxolone (16891 R.P.)
- acute toxicity in the rat by the intraperitoneal route. Report
ST.CRV/Tox No. 21971. Rhône-Poulenc Santé, Centre de Recherches de
Vitry, 94407 Vitry-sur-Seine Cedex, France. Supplied to WHO by
Rhône-Poulenc.
Pasquet, J., Mazuret, A. & Vaissere, J. (1983b) Unpublished study.
Chlorométhyl-3 chloro-6 benzoxolone (16891 R.P.) - eau de lavage de
la solution chlorométhyléique - irritation cutanée primaire chez le
lapin. Note No ST.CRV/Tox. No. 21972. Rhône-Poulenc Santé, Centre de
Recherches de Vitry, 94407 Vitry-sur-Seine Cedex, France. Supplied
to WHO by Rhône-Poulenc.
Pasquet, J. & Mazuret, A. (1973a). Unpublished study. Phosalone
oxygen analog (12244 R.P.) acute percutaneous toxicity in the rat.
SUCRP - DSPh No.16979. Rhône-Poulenc,Laboratoires de Recherches, 22
Avenue Montaigne, 75 Paris 8, France. Supplied to WHO by Rhône-
Poulenc.
Pasquet, J. & Mazuret, A. (1973b). Unpublished study. Hydroxymethyl-
3 chloro-6 benzoxolone (16 236 RP) toxicité et tolérance locale. RP)
acute percutaneous toxicity in the rat. Note SUCRP - DSPh No.
17144.Rhône-Poulenc Santé, Centre de Recherches de Vitry, 94403
Vitry-sur-Seine Cedex, France. Supplied to WHO by Rhône-Poulenc.
Supplied to WHO by Rhône-Poulenc.
Pasquet, J. & Mazuret, A. (1977). Unpublished study. Phosalone (11
974 RP) guinea-pig skin sensitization study. Report RP/RD/CNG No.
19117 of 27th April 1977. Centre Nicolas Grillet, 94400 Vitry-sur-
Seine, France. Supplied to WHO by Rhône-Poulenc.
Pasquet, J. & Mazuret, A. (1980). Unpublished study. Metabolites of
phosalone (11 974 RP) : compounds 19889 RP, 19889 RP and 19914 RP.
Acute oral toxicity in the mouse. Report C.R. Vitry/CNG. No. 20 736-
E of 12th November 1980. Centre Nicolas Grillet, 94400 Vitry-sur-
Seine, France. Supplied to WHO by Rhône-Poulenc.
Pasquet, J., Mazuret, A., Fournel, M. & Koenig, M. (1976)
Unpublished study. Acute oral and percutaneous toxicity of phosalone
(R.P.11974) in the rat, in comparison with azinphosmethyl and
parathion. Note RP/RD/CNG No. 18 550 EP/LR of 27th February 1976.
Centre Nicolas Grillet, 94400 Vitry-sur-Seine, France. Supplied to
WHO by Rhône-Poulenc.
Reddy, S.J., Reddy, B.V. & Ramamurthy, R. (1992). Impact of chronic
phosalone toxicity on Bohr factor and oxygen equilibrium curves of
rat. Biochem Inter. 26: 171-179.
Rhône-Poulenc (1968) Unpublished study. 12244 R.P. oxygen analog of
phosalone acute oral toxicity and anticholinesterasic activity in
vitro. Note RP - DSPh 12664 of the 20th February 1968. Société des
Usines Chimiques Rhône-Poulenc, Direction Scientifiques,
Laboratoires de Recherche, 22 Avenue Montaigne, 75 Paris 8, France.
Supplied to WHO by Rhône-Poulenc.
Rhône-Poulenc Inc. (1979) Unpublished study. Phosalone dairy cow
feeding study. ADC project #475A, B of the 6th July 1968. Rhône-
Poulenc Inc., Agricultural Division, PO Box 125, Black Horse Lane,
Monmouth Junction, New Jersey 08852, USA. Supplied to WHO by Rhône-
Poulenc.
Rhône-Poulenc Inc. (1980a) Unpublished study. Residue determination
of phosalone and its oxygen analog in the milk and tissues of dairy
cattle by electron capture gas chromatography. ADC project #475D of
the 22nd September 1968. Rhône-Poulenc Inc., Agricultural division,
PO Box 125, Black Horse Lane, Monmouth Junction New Jersey 08852,
USA. Supplied to WHO by Rhône-Poulenc.
Rhône-Poulenc Inc. (1980b) Unpublished study. Residue determination
of metabolites containing the chlorobenzoxazole and
chloroaminoiphenol moieties in the milk and tissues from dairy
cattle fed phosalone. ADC project #475D1 of the 22nd September 1968.
Rhône-Poulenc Inc., Agricultural division, PO Box 125, Black Horse
Lane, Monmouth Junction, New Jersey 08852, USA. Supplied to WHO by
Rhône-Poulenc.
Rhône-Poulenc Inc. (1980c) Unpublished study. Validation of
analytical methods of phosalone and its metabolites in milk and
animal tissues. ADC project #496 of the 22nd September 1968. Rhône-
Poulenc Inc., Agricultural Division, PO Box 125, Black Horse Lane,
Monmouth Junction, New Jersey 08852, USA. Supplied to WHO by Rhône-
Poulenc.
Safonov, N.M. (1968) (Toxicological characteristics of a new
organophosphorus insecticide: phosalone preliminary results).
Gigiena truda professional, nye zabolevanija, 12, 43 - Translation
supplied to WHO by Rhône-Poulenc.
SERB (1988) Contrathion, pralidoxime, pralidoxima, Data Sheet.
Laboratoires SERB, 53 Rue de l'Isle-Adam, 75020, Paris, France.
Siglin, J.C., Jenkins, P.K., Rush, R.E., Liao, J.T. & Rodwell, D.E.
(1979) Unpublished study. Primary eye irritation study of phosalone
technical in rabbits with washout (EPA). Study No. 3147.36.
Springborn Life Sciences, Inc., Spencerville, Ohio 45887, USA.
Supplied to WHO by Rhône-Poulenc.
Vanrel, B., Thenaisie, S., Gascoin, M.N., Vanrell, B., Glomot, R. &
Le Bigot, J.F. (1989) Unpublished study. Metabolite of phosalone in
soil reverse mutation assay in vitro Ames test. Study No. 5137
MMO. Rhône-Poulenc Agrochimie, Lyon, France. Supplied to WHO by
Rhône-Poulenc.
Vogel, W.(1991). Unpublished study. Exposure of harvesters to
residues of Zolone(R) flo (Phosalone) insecticide applied to apples
in France in 1989. RCC project No 241683. RCC Umweltchemie AG, PO
Itingen CH 4452 Switzerland. Supplied to WHO by Rhône-Poulenc.
Woodard, M.W., Cockrell, K.O., Woodyard, G. & Cronin, M.T.I. (1966)
Unpublished study. Phosalone demyelination study in chickens. Report
submitted April 7th, 1966 to the Chipman Chemical Company. Woodard
Research Corporation. Supplied to WHO by Rhône-Poulenc.
Woodard Research Corporation (1967) Unpublished study. Phosalone
safety evaluation by repeated oral administration to rats for 103-
104 weeks. Report submitted April 7th, 1966 to the Chipman Chemical
Company. Supplied to WHO by Rhône-Poulenc.
Weill, N. (1988) Unpublished study. Ames test on Salmonella
typhimurium on 50221 RP. Report No. 803380. Hazleton France.
Supplied to WHO by Rhône-Poulenc.
WHO (1986) Environmental Health Criteria 63. Organophosphorus
insecticides: a general introduction. World Health Organization,
Geneva, p 63.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
technical grade phosalone is probably due to small amounts of oxon
present (up to 0.5% of the present technical material). The reason
that phosalone oxon did not inhibit rat brain cholinesterase at
single doses up to 9 mg/kg bw, whereas phosalone did at 27 mg/kg bw
(surprising in view of the much higher acute toxicity of the former)
is obscure (Fournel et al., 1968). Inhibition of brain
cholinesterase by phosalone was also observed by Pasquet et al.
(1976) with an I50 of 31 mg/kg bw p.o. Phosalone was reported to
shift to the right the oxygen dissociation curve in rats (Reddy et
al., 1992).
Toxicological studies
Acute toxicity studies
A summary of acute toxicity data for phosalone is shown in
Table 1. Phosalone has been classified as moderately hazardous by
WHO (WHO, 1992).
Short-term toxicity studies
Rats
A dose of 50 mg/kg bw/day administered by gavage (vehicle
unstated) to white rats for 14 days was lethal (Safonov, 1968).
Phosalone was administered as an oil solution to male rats
(10/dose) (strain unstated) at doses of 7.5 or 15 mg/kg bw/day for 5
weeks. The only death seen in the study was a control rat and no
abnormal clinical signs were observed. Slight reductions in body-
weight gain noted at both dose levels were not statistically
significant. No major differences between test and control groups
were seen in haematology or clinical chemistry except in
cholinesterase activity. Depression of blood cholinesterase
activity (> 20%) was observed at both doses. Brain cholinesterase
activity was reduced at the high dose only. No statistically
significant differences in organ weights or pathological findings
were seen between test groups and controls. The NOAEL was 7.5 mg/kg
bw/day, based on brain cholinesterase inhibition (Dubost et al.,
1963).
In an 8-week study, groups of 10 Crl:CD(SD) rats/sex/dose
received 10, 100, 300, 600 or 1200 ppm phosalone (94.1% pure) in the
diet. After 5 weeks, the doses administered to the 100 and 300 ppm
groups were increased to 2400 and 4800 ppm, respectively, in order
to establish the maximum tolerated dose. At 300/4800 ppm, one
female died and all remainder animals were sacrificed in extremis.
At 100/2400 ppm all females had to be sacrificed. Males at both
these doses survived but showed reduced body weight and food
Table 1. Acute toxicity of phosalone
Species Strain Sex Route LD50 (mg/kg bw) References
Mouse CD-1(COBS) M p.o. 118 (97-144) Fournel et al. (1968)
Mouse CD-1(COBS) F p.o. 93 (74-116) Fournel et al. (1968)
Mouse ? ? p.o. 180* 435** Dubost et al. (1963)
Mouse ? ? p.o. 205 Dubost et al. (1963)
Mouse ? ? p.o. 180 Safonov (1968)
Rat ? M p.o. 120 Dubost et al. (1963)
Rat CD(COBS) M p.o. 125 (102-152) Fournel et al. (1968)
Rat ? F p.o. 170 Dubost et al. (1963)
Rat ? F p.o. 135 Dubost et al. (1963)
Rat CD(COBS) F p.o. 90 (68-119) Fournel et al. (1968)
Rat ? ? p.o. 50 Hayward (1969)
Rat ? ? p.o. 135 Safonov (1968)
Rat CD(COBS) M p.o. 130 (104-163) Pasquet et al. (1976)
Rat CD(COBS) F p.o. 110 (87-139) Pasquet et al. (1976)
Rat ? F p.c. 390 Dubost et al. (1963)
Rat CD(COBS) F p.c. 700 (480-1015) Pasquet (1971)
Rat CD(COBS) F p.c. 1530 Mazuret (1971)
Rat ? F p.c. > 2560 Pasquet & Mazuret (1973a)
Rat CD(COBS) F p.c. 1530 Pasquet et al. (1976)
Rabbit ? ? p.c. > 1000 Dubost et al. (1963)
Guinea-pig ? ? p.o. 150 Dubost et al. (1963)
Guinea-pig ? ? p.o. 82 Dubost et al. (1963)
Hens Rhode Island red F s.c. 350 Heath et al (1967)
* Aqueous Suspension
** Oil solution
consumption. Body weight and cumulative body-weight gain were
significantly lower in females receiving 1200 ppm. No changes were
seen in the eyes. Significantly lower plasma and erythrocyte
cholinesterase were seen at 100 ppm in both sexes. Brain
cholinesterase activity was decreased at 600 ppm to about 80% and
50% of controls in males and females, respectively. Other changes
in clinical chemistry parameters were seen at higher doses,
including lower blood glucose, total protein and albumen and higher
cholesterol and blood urea. No treatment-related changes were seen
in organ weights or in organs on macroscopic or microscopic
examination. The NOAEL was 10 ppm, equal to 0.87 mg/kg bw/day in
males and 0.93 mg/kg bw/day in females, based on brain
cholinesterase inhibition (Kehoe, 1990).
Rabbits
A 3-week percutaneous study was undertaken in rabbits using
phosalone at doses of 0.4, 2.0 or 10 mg/kg bw/day
acetone/ethanol/arachid oil. There were no deaths. A 20%
depression in brain cholinesterase occurred in the highest dose
females. Some irritancy was observed (Kynoch et al., 1979).
Dogs
Phosalone was administered orally to dogs in oil solution for 1
month at dose levels of 7.5, 15 or 30 mg/kg bw/day. One animal of
each sex was used at each dose without controls. Neither clinical
signs nor significant weight changes were noted. Albumen/globulin
ratios in both animals treated with phosalone at 7.5 mg/kg bw/day
were depressed; however, this was not seen in the 15 mg/kg bw/day
animals. Significant plasma and erythrocyte cholinesterase
depression were seen in all animals except in females at 7.5 mg/kg
bw/day. A NOAEL could be not be determined in this study (Dubost
et al., 1963).
In another one-month study, groups of 4 beagle dogs/sex/dose
were administered phosalone (100% pure) at dietary concentrations of
0, 12.5, 25 or 37.5 ppm. No deaths were observed nor were there
abnormal clinical signs. There was no adverse effect on body
weight. Plasma cholinesterase activity was depressed by > 20% in
the high-dose group. Erythrocyte and brain cholinesterase activity
were not affected. At terminal autopsy a mauve discoloration was
observed in the bladder of all high- and mid-dose animals and in one
female at the low dose. Since the bladder discoloration was not seen
in any other dog study, the NOAEL was the highest dose of 37.5 ppm,
equal to 0.81 mg/kg bw/day (Noel et al., 1970).
In a 6-month study, groups of beagle dogs (4/sex/dose) received
phosalone at 0, 10 or 25 ppm in the diet. No haematological,
biochemical or histological changes attributable to phosalone were
observed except in erythrocyte and plasma cholinesterase. Brain
cholinesterase activity was not depressed. Depression of plasma
cholinesterase activity of about 40% and 25% was observed in the 25
ppm and 10 ppm groups, respectively. Decreased erythrocyte
cholinesterase activity was seen only at 25 ppm towards the end of
the study. The NOAEL was the highest dose (25 ppm, equivalent to
0.63 mg/kg bw/day) (Fournel et al,. 1966).
In a 107-week study, phosalone (purity unknown) was
administered in the diet to groups of 4 beagle dogs/sex/dose at
concentrations of 0, 100, 200 or 1000 ppm. One female at 1000 ppm
died during the study. Although marked plasma and erythrocyte
cholinesterase depression were observed, the immediate cause of
death seemed to be an infection. Body-weight loss was seen at 100
ppm, and cholinergic symptomatology was seen in two animals at the
highest dose and in one animal at 100 ppm. Abnormal ECGs (increased
T-waves) were seen at 1000 ppm. Biochemical and haematological
investigations demonstrated no changes that appeared to be compound-
related apart from elevations in ALAT in 4 animals at 1000 ppm.
Reductions in plasma cholinesterase > 20% were observed at 100 ppm
and above, and in erythrocyte cholinesterase at 200 ppm and above.
The degree of brain cholinesterase inhibition was variable, with a
mean in the 1000 ppm group of 79% of controls. Inhibition at
termination of the study was considerable in 3/8 animals and almost
complete in 2 animals. The NOAEL was 200 ppm, equivalent to 5 mg/kg
bw/day, based on clinical signs, body-weight loss, elevated ALAT and
brain cholinesterase depression at 1000 ppm (Donoso et al., 1967).
Groups of 6 beagle dogs (6/sex/dose) received 0, 5, 25 or 300
ppm phosalone (94.5% pure) in the diet for 52 weeks. Three deaths
in different groups were observed. During week 43, all animals
receiving 300 ppm vomited and lost weight and 5 showed reduced food
consumption. Some recovery in weight occurred towards the end of
the study. No changes of toxicological importance occurred on
ophthalmological, haematological, biochemical or urinary examination
except for cholinesterase activity. Reduction in plasma
cholinesterase > 20% compared to controls occurred in the 25 and
300 ppm groups (both sexes and combined and at all time points after
dosing commenced). At 5 ppm, significant reductions occurred in
males only at 4, 26, and 52 weeks. These reductions were just under
20%. Red cell cholinesterase was significantly reduced at 300 ppm
at all time points. At 25 ppm, this enzyme was reduced compared to
the control group in males at 26 weeks and in both sexes at 52
weeks. The reduction in this group compared to pre-dosing levels
was < 15%. Brain cholinesterase was significantly reduced in both
sexes at 300 ppm to about 60% of control values. No significant
differences between groups were observed in organ weights or after
macroscopic or histopathological examination of organs. The NOAEL
was 25 ppm, equal to 0.89 mg/kg bw/day (males) and 0.97 mg/kg bw/day
(females), based on depression of brain cholinesterase activity and
clinical effects at 300 ppm (Barker at al., 1992).
Long-term toxicity/carcinogenicity studies
Mice
In a 2-year study in CD-1 mice (60-65/sex/group), phosalone
(95.3% pure) was administered at dietary concentrations of 0, 15, 50
or 150 ppm. Five mice of each sex were sacrificed at 6 weeks and at
study termination at 105 weeks for plasma, erythrocyte and brain
cholinesterase determinations. Plasma and erythrocyte
cholinesterase were reduced by at least 20% in the 150 ppm group at
both 6 and 105 weeks. In some cases, particularly with plasma
cholinesterase, the reduction was much greater. Brain
cholinesterase was not reduced compared to controls. Statistically
significant increases in adrenal weights were found at all dose
levels and in both sexes, with the exception of females at the
lowest dose. These increases were both in relative and absolute
weights and were significant except for relative weight at 15 ppm in
males. Decrease in absolute brain weight was significant at 50 ppm
and 150 ppm in males. Relative increases in testicular weights (50
ppm), kidney weights in females only (15 ppm) and relative and
absolute increases in pituitary weights (males: 15 ppm) were also
significant. No neoplastic or non-neoplastic histopathological
findings were attributable to phosalone. There were adrenal weight
changes, which in the absence of histopathological changes, were
considered to be adaptive, and changes in testicular weights at 50
ppm and kidney weight in one sex at 15 ppm which were likely to be
insignificant in view of a lack of dose-response. The decrease in
absolute brain weight in males was not dose-related. The NOAEL was
thus the highest level of 150 ppm (equal to 23 and 31 mg/kg bw/day
in males and females, respectively), based on the lack of depression
of brain cholinesterase activity at this level (Nelson et al.,
1980).
Rats
In a 2-year study, groups of 30 rats/sex/dose (albino: strain
not stated) received phosalone of unknown purity at dietary
concentrations of 0, 25, 50 or 250 ppm (half of these concentrations
for the first four weeks). Mortality during the course of the study
was similar in all groups. There was some depression in weight gain
in the highest-dose animals compared to controls, especially in
males at 103 weeks. No haematological changes attributable to the
test material were observed. There were marked (up to three-fold)
differences in group mean plasma cholinesterase activities between
sexes, females having much higher activity; this raised some doubt
about the reliability of these assays. Inhibition of erythrocyte
and plasma cholinesterase activity was seen at 250 ppm. Inhibition
of brain cholinesterase activity > 20% was seen at 250 ppm in
males. No histopathological changes related to the test material
were seen. Although a few instances of testicular activity were
seen, there was no indication of any relationship between occurrence
of this finding and administration of the test material. The NOAEL
was 50 ppm, equivalent to 2.5 mg/kg bw/day, based on brain
cholinesterase inhibition at the next highest dose (Woodard Research
Corporation, 1967).
In a second 2-year study, Sprague-Dawley CD rats (50/sex/dose)
were given phosalone (purity 94.5%) at doses of 0, 5, 50 or 1000
ppm, the highest dose being reduced to 500 ppm at week 27. These
concentrations were equal to 0, 0.2, 1.8 or 20 mg/kg bw/day for
males and 0, 0.2, 2.5 or 31 mg/kg bw/day for females, on the basis
of consumption data from week 27. Satellite groups of animals
(15/sex/dose), were killed at 52 weeks. There was no effect on
mortality and clinical effects were mainly confined to the high-dose
group. Depression of plasma and erythrocyte cholinesterase was found
at the high and medium concentrations and depression of brain
cholinesterase activity was found in the highest dose group. There
were some treatment-related changes in the adrenals. A dose-response
across all groups in the prevalence of testicular atrophy was
observed (1/27, 3/34, 6/30 10/42 in survivors to the terminal kill).
Reductions in testis weight were dose-related, but were
statistically significant only at the high and mid-dose levels (5.03
g, 4.66 g, 4.51 g and 4.22 g, for the control, low, medium and high-
dose groups, respectively). The NOAEL was < 5 ppm, equal to 0.2
mg/kg bw/day (Barker & Sortwell, 1993).
Reproduction studies
Rats
In a three-generation reproduction study, groups of albino rats
(10 males and 20 females/group) were administered phosalone (purity
unknown) at dietary concentrations of 0, 25 or 50 ppm. Only half
the nominal dietary concentration was given during the initial three
weeks of the diet, on the grounds that young rats eat about twice as
much daily during this period; this procedure was repeated in each
generation. When the rats had received phosalone for 74 days, 10
females in each group were paired with the 10 males from the same
group for 10 days, after which the other 10 females were mated with
the 10 males. The animals from these litters (F1a) were sacrificed
after weaning, weighed and examined. Microscopic histopathological
examination was not carried out. The F0 rats were then remated as
different pairs to produce the F1b litters. Litters were examined
at weaning and representative male and female pups were placed on
control diet to constitute the next study groups. Within 21 days of
weaning, the F1b generation received 25 ppm or 50 ppm phosalone,
while controls continued to be fed control diet. The F1b rats were
mated in the same way as the F0 generation, F2a pups being
sacrificed and examined as above, while the F1b parents were also
killed and examined. Uteruses from females failing to bear two
litters were removed and examined for implantation sites. Rats were
selected from each F2b litter to give 10 males and 20 females in
each group, the remaining F2b pups were sacrificed and examined as
before without microscopic pathology. The retained F2b pups in the
two test groups received the test diet within 24 days of weaning.
After 51 days on the diet, the males and 10 females were paired for
10 days, the males being paired with the other 10 females after they
had all been on the diet for 71 days. The F3a pups were sacrificed
after weaning and examined as above. Within 10 days, F2b females
were re-mated with different males to produce the F3b litters.
After weaning, the pups were sacrificed and the main organs
examined. Tissues from one male and one female in each F3b litter
were examined histopathologically. The F2b parents were mated for
a third time to produce F3c pups litters, which were treated in the
same way as the F3b pups.
The test groups of rats were similar to the controls throughout
the study. Three F0 females died in the 50 ppm group and one in
the 25 ppm group while none died in the control group. Test
substance-related effects on fetal resorption were not observed and
malformations were observed only in 3 pups from one litter of the 25
ppm group. The three pups exhibited flaccid paralysis of the hind
limbs. Organs from the test and control weanlings from the F3b and
F3c groups did not differ significantly in weight or
histopathology. No adverse effect was determined in the study and
the NOAEL was > 50 ppm, equivalent to 2.5 mg/kg bw/day (Jones et
al., 1967a).
In a two-generation reproduction study, groups of 32 male and
32 female rats (6 week-old Crl: CD(SD)VSAF/Plus BR) were given
phosalone (96.8% pure) at dietary concentrations of 0, 10, 50 or 400
ppm. Phosalone was administered for 10 weeks, after which the rats
were mated for 20 days. The dams were allowed to rear their young
and from these litters, 21 days postpartum, 28 rats/sex/group were
selected as the F1 generation. F1 animals were selected and
treated from weaning until 16 weeks when they were mated. After
weaning of their litters the F1 parents were sacrificed as were the
F2 pups.
At 400 ppm, there was a lower body weight from conception to
day 17 in the F0 generation, and lower mean body-weights of the F1
males and females. There were 2 total litter losses post-partum in
the F0 generation and three in the F1 generation. There was a
reduced litter size in the F0 animals and increased pup mortality
to day 4 in both generations. Retarded pup growth and marked plasma
and erythrocyte cholinesterase depression were noted in both
generations. At 50 ppm, there was marked plasma and erythrocyte
cholinesterase depression in both generations, while at 10 ppm
cholinesterase reduction was < 20%. Brain cholinesterase activity
was not measured. The NOAEL was 50 ppm, equivalent to 2.5 mg/kg
bw/day, based on retarded pup growth and erythrocyte cholinesterase
depression (Bryson et al., 1991).
Special studies on embryotoxicity/teratogenicity
A study was carried out on chick embryos (white Leghorn) at
doses of 0.2, 0.6 or 1.8 mg/egg, using 30 eggs/group. Two further
groups of 30 eggs served as solvent (dimethylsulfoxide) and
untreated controls. At 18 days, the surviving embryos were removed,
weighed and examined. Phosalone had no significant effect on
mortality, weight or structure (Pasquet, 1969).
Groups of 25 female rats (Wistar HAN) were treated with 0, 2,
10 or 20 mg/kg bw/day of phosalone by gavage, from days 6 through 15
of gestation. Controls received distilled water with 4% CMC. The
animals were killed 21 days after coitus and the fetuses were
removed by caesarean section. Dams and fetuses were examined
macroscopically and microscopically. Oral administration of
phosalone at the highest dose produced clinical signs (chewing
movements, piloerection and dyspnea). Additionally, reduced food
consumption, weight loss, reduction in litter size, due to increased
resorptions and a slight increase in mean fetal body weight, were
observed. There was no evidence of fetal abnormality. At 2 and 10
mg/kg bw/day, maternal and fetal parameters were similar to
controls. The NOAEL was 10 mg/kg bw/day for both maternal toxicity
and fetotoxicity (Allen et al., 1989a).
Groups of 25 Fauve de Bourgogne rabbits were orally treated by
capsules with undiluted phosalone (purity unstated) from days 6
through 16 of gestation with doses of 0, 2, 6 or 18 mg/kg bw/day. A
positive control group was treated with thalidomide. The does were
sacrificed at 28 days. The percentage of animals becoming pregnant,
the number of resorptions, the number of fetuses recovered alive
after caesarian section and their weight did not differ
significantly from the untreated controls. The incidence of
malformed fetuses was low in all groups and the malformations were
trivial. By contrast malformations were observed in the thalidomide
group. The NOAEL was 18 mg/kg bw/day, the highest dose tested
(Pasquet, 1969).
Phosalone (93.5% pure) was administered in
carboxymethylcellulose (4%) by gavage to Chinchilla KFM/CHIN rabbits
(16/dose) at doses of 0, 1, 10 or 20 mg/kg bw/day from days 6
through 18 post-coitum. Clinical signs of cholinergic intoxication,
particularly dyspnea and extensor spasms, reduced food consumption,
loss of body weight and increased incidence of resorption of embryos
were observed at the highest dose. Post-implantation loss was
increased at 10 and 20 mg/kg bw/day compared to concurrent controls
but was within the range of historical controls. No adverse effect
was seen on fetuses at any dose, or on maternal parameters at doses
of 1 or 10 mg/kg bw/day. The NOAEL was 10 mg/kg bw/day, based on
both fetal and maternal toxicity (Allen et al., 1989b; Allen,
1989).
Special studies on genotoxicity
Based on the results of genotoxicity assays given in Table 2,
the Meeting concluded that phosalone was not genotoxic.
Special studies on neurotoxicity
Groups of white Leghorn hens (10/group/dose) were given dietary
doses of 0, 50, 163 or 500 ppm phosalone or TOCP at 500 ppm for 45
days. Hens were examined daily during the study. At termination,
brain, three levels of spinal cord and a portion of sciatic nerve
were taken from the controls, the TOCP-treated birds and the highest
dose of phosalone-treated hens. Although some signs of cholinergic
toxicity occurred, no phosalone-treated birds showed any indication
of organophosphate-induced delayed neuropathy. TOCP-treated hens
showed paralysis from 13 days onwards, and the only deaths seen,
were amongst the TOCP-treated fowl. Histopathological appearances
in tissues taken from the control and phosalone-treated animals were
within normal limits. Although there was some demyelination in the
TOCP group of animals, particularly in the sciatic nerve, this was
not significant (Woodard et al., 1966).
In another study, 10 Rhode Island red hens were dosed with 350
mg/kg bw phosalone (=LD50) as a 35% formulation, while protected by
atropine and pralidoxime methanesulfonate (P2S). The dose was
repeated after 3 weeks. There were 4 untreated controls and 4
positive controls treated with a neuropathic dose of mipafox. One
phosalone-treated hen died at 4 days, and another at 29 days. No
pathological changes were seen in the spinal cord. The positive
controls became severely ataxic at 13 days and showed serious
effects in the spinal cord. The untreated controls were clinically
and pathologically normal (Heath et al., 1967).
Groups of 20 New Hampshire red hens received TOCP (750 mg/kg bw
p.o., positive control), or 0 or 600 mg/kg bw p.o., phosalone.
Dosed and positive control animals also received atropine 15 min
before dosing. The vehicle control and phosalone group were redosed
at day 22 and sacrificed at day 41. Birds from the first positive
control group were killed on day 22 of the study at which time the
10 birds from the second positive control group were dosed and
sacrificed 19 days later. No significant clinical signs were seen
in the vehicle control fowl. Neuropathological examination was
performed on central and peripheral nervous tissue from all terminal
sacrificed animals and seven decedents in the phosalone group.
Table 2. Results of genotoxicity assays on phosalone
Test Object Concentration phosalone Purity Results Reference
In vitro
Ames test (1) S. typhimurium 1-1000 µg/plate ? Negative Benezet & Cartier, 1980
(strains TA98, 100, 125-1000 µg/plate
1535, 1537)
Ames test (1) S. typhimurium 250-10 000 94% Negative Haworth & Lawlor, 1989
(strains TA98, 100, µg/plate
1535, 1537, 1538)
HPRT test (1) CHO/K1 cells 0.1-50 µg/ml 95% Negative Cordier & Bonneau, 1985
Chromosomal CHO/K1 cells 4, 40, 75 µg/ml 95% Negative Cordier & Fournier, 1989
aberration in
CHO cells (1)
Chromosomal CHO/WBL cells 16.7, 500 µg/ml 94% Negative Murli, 1989
aberration in (also 5 µg/ml without
CHO cells (1) metabolic activation)
UDS in vitro Rat primary 0.005-252 µg/ml 94% weak Cifone, 1989
hepatocyte positive
In vivo
Micronucleus CD-1 mice 10, 20, 40 mg/kg bw/day 99.5% Negative Pasquet & Fournier, 1980
Dominant lethal CD-1 mice 10, 30, 75 mg/kg 95.3% Negative Goldenthal et al.,
test 1978
(1) With and without metabolic activation.
Tissues examined were spinal cord (cervical, thoracic and
lumbosacral), medulla, sciatic and tibial nerves. In the positive
control groups, all except one bird showed clinical signs of delayed
neuropathy, appearing from days 13-24. In both positive controls,
histopathological evidence of delayed neuropathy was found. In the
phosalone group, 4 birds showed signs of cholinergic toxicity after
the first dose and two birds died. On day 22 after the second dose,
six hens showed acute toxicity. Seven hens died on days 23-24.
Histopathological evidence of nerve degeneration was not more
frequent in this group than in the vehicle controls (Morris, 1983).
The Meeting concluded that there is no evidence that phosalone
has potential to cause organophosphate-induced delayed neuropathy.
Antidote studies of phosalone intoxication
Ten groups of 10 mice each were given phosalone orally followed
immediately by i.p. administration of combinations of pralidoxime
methylsulfate (Contrathion) at 0, 25, 50, 100 or 200 mg/kg bw and
atropine at 0, 10, 20 or 40 mg/kg bw. Both antidotes were effective
in preventing death although pralidoxime methylsulfate at 100 and
200 mg/kg bw appeared to be toxic. Some aspects of the results were
difficult to interpret since doses of pralidoxime methylsulfate used
were high compared to the recommended human dose of 5-6 mg/kg bw
(SERB, 1988). Moreover the i.p. LD50 of a similar pralidoxime salt
is 125-250 mg/kg bw in the mouse (Marrs, 1991). Doses of atropine
used in this study were also high (Dubost et al., 1963).
In another study using orally administered phosalone (vehicle
unstated), groups of rats (9-10/group) were given 50 or 100 mg/kg bw
phosalone. Phosalone (100 mg/kg bw) was administered to another
group of 10 rats, followed by pralidoxime methanesulfonate (50 mg/kg
bw s.c.) and atropine (17.5 mg/kg bw s.c.) 15-20 minutes after
clinical signs of anticholinesterase poisoning developed. All
animals in the 100 mg/kg bw group not treated with the antidotes
showed considerably reduced erythrocyte anticholinesterase activity
and died within 24 hours. Five out of 10 rats survived 24 hours in
the 50 mg/kg bw group and their cholinesterase activity was less
depressed. When treated with the antidotal regime, 9/10 rats dosed
with phosalone at 100 mg/kg bw survived and cholinesterase activity
was less depressed than that of animals treated with 50 mg/kg bw
phosalone but untreated with antidotes (Hayward, 1969).
In a study of the antidotal effects of bis-pyridinium oxime
(obidoxime), groups of mice (4/sex) were administered phosalone by
gavage at a dose of 250 mg/kg bw, a dose well above the LD50.
Immediately afterwards, obidoxime and atropine were injected
intraperitoneally at obidoxime doses of 0, 25, 50 or 100 mg/kg bw
and at atropine doses of 0, 10, 20 or 40 mg/kg bw in various
combinations. When no antidote was given, phosalone was invariably
fatal whereas survival was considerably improved with combinations
of antidote except at the highest dose of atropine and obidoxime.
Obidoxime alone was clearly more beneficial than atropine alone.
Synergism was not observed, because of the study design (Pasquet &
Masuret, 1970).
It was concluded from these studies that atropine is an
effective antidote to phosalone. Pralidoxime (either methylsulfate
or methanesulfonate) reactivated phosalone-inhibited rat erythrocyte
cholinesterase and was an effective antidote. Obidoxime was also an
effective antidote but the usual synergism of atropine and
pralidoxime salts or obidoxime was not noted probably due to the
design of the studies.
Special studies on metabolites and intermediates
A number of acute toxicity and genotoxicity studies have been
conducted with phosalone metabolites and intermediates. The results
are summarized in Tables 3-6.
Other studies
3-Hydroxymethyl 6-chlorobenzoxolone was non-irritant to the
intact rabbit skin but very irritant to the scarified skin (Pasquet
& Mazuret, 1973b). 3-Chloromethyl 6-chlorobenzoxolone was non-
irritant to the intact rabbit skin (Pasquet et al., 1983b).
In a 13-week study, groups of 15 male and 15 female rats
(Sprague-Dawley-derived Crl:CD(SD)BR) were given 6-chlorobenzoxolone
at dietary concentrations of 0,5, 15 or 45 mg/kg bw/day for 13
weeks. Groups of 10 male and female controls and high-dose animals
underwent a subsequent 28-day treatment-free period. The only
effect of the treatment was suppression of weight gain in all
treated males and in the high-dose females. A compensatory weight
gain was observed in the animals that underwent the treatment-free
period (Owens, 1985).
A study was performed to determine the potential of Di-syston
(disulfoton) to potentiate the activity of phosalone. Groups of 8
beagle dogs were fed diets containing phosalone at concentrations of
7.5, 15 or 25 ppm for 19 weeks. In each case, Di-syston was added
at a concentration of 1 ppm from week 14 to week 19. There were no
changes in physical appearance, haematology or pathology that could
be ascribed to the treatment. No treatment-related changes in
plasma or erythrocyte cholinesterase were seen (Jones et al.,
1967b).
Table 3. Acute toxicity of phosalone metabolites
Species Strain Sex Route LD50 References
(mg/kg bw)
Phosalone-oxon
Mouse CD-1(COBS) M PO 35 Fournel et al., 1968
(30-41)
Mouse CD-1(COBS) F PO 40 Fournel et al., 1968
(33-49)
Mouse ? Mix PO 32 Rhône-Poulenc, 1968
Rat CD(COBS) M PO 36 Fournel et al., 1968
(29-44)
Rat CD(COBS) F PO 21 Pasquet et al., 1976
(12-28)
Rat CD(COBS) F PO 20 Fournel et al., 1968
(15-29)
Rat CD(COBS) F PC 380 Pasquet & Mazuret, 1973a
(240-610)
3-methylthiomethyl 6-chlorobenzoxazolone
Mouse OF1 M PO 590 Pasquet & Mazuret, 1980
(499-698)
OF1 F PO 837 Pasquet & Mazuret, 1980
(706-991)
3-methylsulfinylmethyl 6-chlorobenzoxazolone
Mouse OF1 M PO 405 Pasquet & Mazuret (1980)
OF1 F PO 315 Pasquet & Mazuret (1980)
(256-386)
Table 3 (contd)
Species Strain Sex Route LD50 References
(mg/kg bw)
3-methylsulfonylmethyl 6-chlorobenzoxazolone
Mouse OF1 M PO > 5000 Pasquet & Mazuret 1980
OF1 F PO > 5000 Pasquet & Mazuret 1980
Table 4. Acute toxicity of intermediates and soil metabolites
Species Strain Sex Route LD50 References
(mg/kg bw)
3-hydroxymethyl 6-chlorobenzoxolone
Mouse CD-1(COBS) Mix p.o. 730 Pasquet & Mazuret, 1973b
(557-956)
Benzoxolone
Mouse CD-1(COBS) Mix p.o. 580 Pasquet & Mazuret, 1973b
3-chloromethyl 6-chlorobenzoxolone
Rat CD Sprague- Mix i.p. 305 Pasquet et al., 1983a
Dawley
Table 5. Genotoxicity of phosalone intermediates
Compound Test object Concentration Purity Result References
intermediate
Unknown intermediate
Ames test (1) Salmonella typhimurium 0.005-0.5 µg/plate ? -ve Weill, 1988
TA98, TA100, TA1535,
TA1537, TA1538
Table 6. Genotoxicity of phosalone soil metabolites
Compound Test object Concentration Purity Result Reference
intermediate
3-amino 7-chloro 2-phenoxazone
Ames test (1,2) Salmonella typhimurium 10-100 µg/plate (-S9) 95% +ve Vanrel et al., 1989
TA98, TA100. TA1535, 25-125 µg/plate (+S9)
TA1537, TA1538
(1) With and without metabolic activation.
(2) Positive with metabolic activation and in TA1537, and TA100 only.
Phosalone (96% pure) was applied to the skin of rabbits (New
Zeeland white) for 21 days at dose levels of 0.4, 2 or 10 mg/kg
bw/day. Azinphosethyl was applied at 2 mg/kg bw/day. Both
chemicals were applied to intact or abraded skin. Inhibition of
brain cholinesterase > 20% was not noted in any phosalone group,
but marked depression was seen with azinphosethyl. Phosalone did
not cause red blood cell or plasma cholinesterase depression (Kynoch
et al., 1979).
In studies to elicit delayed sensitization in guinea-pigs
(Pasquet & Mazuret, 1977; Cunny & Siglin, 1989) no sensitization was
seen. Phosalone was moderately irritant to the eye in New Zeeland
white rabbits (Siglin et al., 1989). Irritancy to the rabbit eye
was also observed by Safonov (1968).
Observations in humans
In a study performed on 14 male volunteers to establish a re-
entry period after applying phosalone in citrus groves, dislodgeable
residues up to 3.6 µg/cm2 (leaf) produced no adverse effect other
than some plasma cholinesterase depression (Knaak et al., 1978).
In 1987, several cases of illness resembling anticholinesterase
poisoning were reported in California. On investigation by the
California Department of Food and Agriculture, it was found that the
illness had occurred in workers harvesting grapes from vineyards
treated with a preparation containing phosalone. A study was
therefore carried out in which grapes were treated with phosalone (3
lb/acre) twice, 24 days apart. Fourteen days after the second
treatment, 30 volunteers harvested the crop for 6 consecutive days
while 22 volunteers harvested a crop from an untreated vineyard.
Assignment to the treated or control group was random. Blood was
collected for plasma and erythrocyte cholinesterase activity in the
afternoon of days 4, 3 and 2 pre-harvest, daily during harvest, and
three days thereafter. Samples were transported cooled in ice and
assayed within six hours by a modified Ellman method. Urinary
diethylphosphates were assayed on early morning urine samples
collected on days 6, 5, 4 and 1 before harvest, daily during
harvest, and days 1 and 2 thereafter. No symptoms or clinical signs
were recorded that could be attributed to anticholinesterase
effects. There was a gradual decline in plasma cholinesterase
levels during the harvest, which recovered after the end of the
harvest. This effect was not seen in controls nor in red blood cell
cholinesterase level in either test or control groups. Ethyl
phosphate metabolites were found in the urine of the test group
(Baugher, 1989). A similar study was carried out on French apple
pickers (Vogel, 1991). No clinical signs, symptoms suggestive of
anticholinesterase toxicity, or significant depression of plasma or
erythrocyte cholinesterase were observed.
In 1966-1979, eleven pesticide incidents involving the
agricultural use of phosalone were reported to the USEPA's Pesticide
Incident Monitoring System (Hodgson & Smith, 1992). The antidote
dietixim (a cholinesterase reactivator) was effective in human
phosalone poisoning (Kundiev & Kagan, 1992).
COMMENTS
After oral administration, phosalone was moderately well
absorbed, 15-25% appearing in the faeces. Phosalone was extensively
metabolized to phosphorothioates, phosphorodithioates and
3-methylthiomethyl 6-chloro-benzoxazolone, the last of which is
subsequently metabolized ultimately to 3-methylsulfonylmethyl 6-
chlorobenzoxazolone.
Pure phosalone is almost certainly not a cholinesterase
inhibitor, but acquires inhibitory activity after conversion to
phosalone oxon in vivo.
The acute oral toxicity varies with species, but is in the
region of 100-200 mg/kg bw in rodents. Phosalone has been
classified as moderately hazardous by WHO.
There were two short-term studies in rats which could be used
to give NOAELs. In a five-week oral gavage study in rats at doses
of 0, 7.5 or 15 mg/kg bw/day, the NOAEL or 7.5 mg/kg bw/day based on
brain cholinesterase inhibition. In an eight-week study in rats
using dietary concentrations of 0, 10, 100, 300, 600 or 1200 ppm,
the NOAEL was 10 ppm (equal to 0.87 mg/kg bw/day), based on brain
cholinesterase inhibition. It is possible that NOAELs could have
been established at 100 or 300 ppm, but the dose rates for those
groups were increased to 2400 and 4800 ppm, respectively, after 5
weeks to establish a maximum tolerated dose.
Five studies were carried out in dogs. In a one-month oral
dosing study in dogs, a NOAEL could not be determined as plasma and
erythrocyte cholinesterase depression were seen at the lowest dose
(7.5 mg/kg bw/day). In another one-month study using dietary
concentrations of 0, 12.5, 25 or 37.5 ppm, the NOAEL was the highest
level, which was equal to 0.81 mg/kg bw/day; although plasma
cholinesterase depression was seen in the study, neither erythrocyte
nor brain cholinesterase activity was depressed. In a 6-month study
in dogs using dietary concentrations of 0, 10 or 25 ppm, although
plasma and erythrocyte cholinesterase were depressed, the brain
enzyme was not, so the NOAEL was the highest dose (equivalent to
0.63 mg/kg bw/day). In a two-year study in beagle dogs, males and
females were fed phosalone in the diet at concentrations of 0, 100,
200 or 1000 ppm. The NOAEL was 200 ppm, equivalent to 5 mg/kg
bw/day, based on brain cholinesterase depression, body-weight loss
and elevated alanine aminotransferase at the highest dose. In a
more recent one-year study in dogs using dietary concentrations of
0, 5, 25 or 300 ppm phosalone, the NOAEL was 25 ppm (equal to 0.89
mg/kg bw/day), based on brain cholinesterase depression at 300 ppm.
The overall NOAEL for dogs was considered to be 200 ppm, in view of
the spacing of the doses in the most recent study.
In a lifetime carcinogenicity study in mice, phosalone was
given at dietary concentrations of 0, 15, 50 or 150 ppm. The NOAEL
was 150 ppm, equal to 23 mg/kg bw/day, based on the lack of
depression of brain cholinesterase activity, although plasma and red
blood cell cholinesterase depression were seen at this level. There
was no evidence of carcinogenicity.
In a two-year study in rats using concentrations of 0, 25, 50
or 250 ppm phosalone in the diet, the NOAEL was 50 ppm, equivalent
to 2.5 mg/kg bw/day, based on brain cholinesterase depression at the
highest dose. In a second 2-year study in rats, dietary
concentrations of 0, 5, 50 or 1000 ppm were used, the highest dose
being reduced to 500 ppm later in the study. There was a
statistically significant increase in the prevalence of testicular
atrophy and reduction in testicular weight in both the high and mid-
dose groups and a dose response across all groups for both effects.
The Meeting concluded that the NOAEL was < 5 ppm, equal to 0.2
mg/kg bw/day.
In a teratogenicity study in rats at doses of 0, 2, 10 or 20
mg/kg bw/day, the NOAEL was 10 mg/kg bw/day for both maternal
toxicity and fetotoxicity. In a study in rabbits using doses of 0,
2, 6 or 18 mg/kg bw/day, the NOAEL was the highest dose. In another
study in rabbits, using doses of 0, 1, 10 or 20 mg/kg bw/day, the
NOAEL was 10 mg/kg bw/day based on maternal toxicity. Phosalone was
not teratogenic in either the rat or rabbit.
Two multigeneration reproduction studies in rats were reviewed.
In the first study, using dietary concentrations of phosalone of 25
or 50 ppm, no adverse effects were observed. The NOAEL was > 50
ppm, equivalent to 2.5 mg/kg bw/day. In the second study, in which
phosalone was administered at dietary concentrations of 0, 10, 50 or
400 ppm, the NOAEL was 50 ppm (equivalent to 2.5 mg/kg bw/day) based
on retarded pup growth and plasma and erythrocyte cholinesterase
depression.
Phosalone has been adequately tested in a series of in vitro
and in vivo genotoxicity assays. The Meeting concluded that
phosalone was not genotoxic.
There is no evidence that phosalone has potential to cause
organophosphate-induced delayed neuropathy.
Pralidoxime salts and obidoxime are both effective in
experimental phosalone poisoning.
No human study was available from which a NOAEL could be
derived.
An ADI of 0-0.001 mg/kg bw was established based on the lowest
dose (0.2 mg/kg bw/day) in the recent two-year study in rats. A
200-fold safety factor was used because of concerns that the trend
for the occurrence of testicular atrophy and reduction in testis
weight existed across all groups.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 150 ppm, equal to 23 mg/kg bw/day (two-year study)
Rat: < 5 ppm, equal to 0.2 mg/kg bw/day
(two-year study)
50 ppm, equivalent to 2.5 mg/kg bw/day
(multigeneration reproduction study)
Rabbit: 10 mg/kg bw/day (teratology study)
Dog: 200 ppm, equivalent to 5 mg/kg bw/day
(several studies)
Estimate of acceptable daily intake for humans
0-0.001 mg/kg bw
Studies which will provide information valuable in the continued
evaluation of the compound
Explanation of the testicular atrophy seen in the recent study
in rats.
Observations in humans.
REFERENCES
Allen, P.A. (1989) Unpublished study. Additional evaluation of the
incidence of resorptions in the studies performed by RCC on the
effects of phosalone technical on embryonic and fetal development in
the rabbit. Project 083002, 204423, 082991. RCC Research and
Consulting Company AG, PO Box, CH 4452 Itingen, Switzerland.
Supplied to WHO by Rhône-Poulenc.
Allen, P.A., Frei, D., Mladenovic, P. & Terrier C. (1989a)
Unpublished study. Embryotoxicity study (including teratogenicity)
with phosalone technical in the rat. Project 082980. RCC Research
and Consulting Company AG, PO Box, CH 4452 Itingen, Switzerland.
Supplied to WHO by Rhône-Poulenc.
Allen, P.A., Mladenovic, P. & Terrier, C. (1989b) Unpublished study.
Embryotoxicity study (including teratogenicity) with phosalone
technical in the rabbit. Project 083002. RCC Research and Consulting
Company AG, PO Box, CH 4452 Itingen, Switzerland. Supplied to WHO by
Rhône-Poulenc.
Barker, M.H., Smith, T.G., Buist, D.P., Crook, D., Morrow, J. &
Gopinath, C. (1992) Unpublished study. Phosalone dietary toxicity
study in beagle dogs. HRC report No. RNP 336/91907. Huntingdon
Research Centre Ltd, Wooley, Huntingdon, PE18 6ES, England. Supplied
to WHO by Rhône-Poulenc.
Barker, M.H. & Sortwell, R.J. (1993) Unpublished study. Phosalone
(11974 RP). Potential tumorigenic and toxic effects in prolonged
dietary administration to rats Volume 1. Huntingdon Research Centre
Ltd, PO Box 2, Huntingdon, Cambridgeshire PE18 6ES. Supplied to WHO
by Rhône-Poulenc. England
Baugher (1989). Unpublished study. Exposure of harvesters to Zolone
(R) EC brand insecticide applied to grapes in California, 1988.
Project no 29088. Orius Associates, Frederick, Maryland, USA.
Supplied to WHO by Rhône-Poulenc.
Benezet, F. & Cartier, J.R. (1980) Unpublished study. Phosalone (11
974 RP) mutagenicity study on Salmonella typhimurium. Report
RP/RD/CNG No. 19 234 E of 19th January 1978. Centre Nicolas Grillet,
94400 Vitry-sur-Seine, France. Supplied to WHO by Rhône-Poulenc.
Bryson, A.M., Parker, C.A., Offer, J.M. & Farmer, H. (1991)
Unpublished study. A study of the effect of phosalone on
reproductive function of two generations in the rat. Report No. RNP
326/91277. Huntingdon Research Centre Ltd, PO Box 2, Huntingdon,
Cambridgeshire, PE18 6ES England. Supplied to WHO by Rhône-Poulenc.
Cifone, M.A. (1989) Unpublished study. Mutagenicity test on
phosalone technical in the rat primary hepatocyte unscheduled DNA
synthesis test. HLA study No. 10754-0-447, Hazleton Laboratories
America Inc 5518 Nicholson Lane, Suite 400, Kensington Maryland
20895, USA. Supplied to WHO by Rhône-Poulenc.
Cordier, A. & Bonneau, D. (1985) Unpublished study. Phosalone (11
974 RP) CHO/HPRT test. Report. ST/CRV/Tox. No. 22 363-E of 10th May
1985. Rhône-Poulenc Santé, Centre de Recherches de Vitry, 94403
Vitry-sur-Seine Cedex, France. Supplied to WHO by Rhône-Poulenc.
Cordier, A. & Fournier, E. (1985) Unpublished study. Phosalone (11
974 RP) chromosome aberration test in Chinese hamster ovary cells
(CHO). Report ST/CRV/Tox. No. 435-E of 28th August 1985. Rhône-
Poulenc Santé, Centre de Recherches de Vitry, 94403 Vitry-sur-Seine
Cedex, France. Supplied to WHO by Rhône-Poulenc.
Craine, E.M. (1974a) Unpublished study. Disposition of phosalone-
14C in a lactating cow. Research report No. EMC 74:17 of the 29th
April 1974. Hess and Clark Research Department, Ashland, Ohio, USA.
Supplied to WHO by Rhône-Poulenc.
Craine, E.M (1974b) Unpublished study. Disposition of phosalone-14C
in a lactating cow. Research report No. EMC 74:84 of the 25th
November 1974. Hess and Clark Research Department, Ashland, Ohio,
USA. Supplied to WHO by Rhône-Poulenc.
Craine, E.M. (1974c) Unpublished study. The disposition of
phosalone-14C applied to the skin of a pig. Research report No. EMC
74:30 of the 14th May 1974. Hess and Clark Research Department,
Ashland, Ohio, USA. Supplied to WHO by Rhône-Poulenc.
Craine, E.M. (1975) Unpublished study. The metabolism of phosalone-
14C in dairy cows. Research report No. EMC 75:51 of the 2nd July
1975. Hess and Clark Research Department, Ashland, Ohio, USA.
Supplied to WHO by Rhône-Poulenc.
Cunny, H. & Siglin, J.C. (19789) Unpublished study. Delayed contact
hypersensitivity study in guinea-pigs with phosalone technical.
Study No. 3147.37. Springborn Life Sciences Inc. Spencerville, Ohio
45887, USA. Supplied to WHO by Rhône-Poulenc.
Desmoras, M.J. (1979). Unpublished study. Studies on the metabolism
of phosalone (11974 R.P.), in the mouse, the rat and the rabbit.
Note RP/RD/CNG No 20 234 of the 20 August 1979. Supplied to WHO by
Rhône-Poulenc.
Desmoras, M.J. & Fournel, J. (1968) Unpublished study. Studies on
the degradation of phosalone (11974 R.P.), in mammals. Note RP -
DSPh of the 1st March 1968. Rhône-Poulenc, Laboratoires de
Recherches, 22 Avenue Montaigne, 75 Paris 8, France. Supplied to WHO
by Rhône-Poulenc.
Donoso, J., Woodard, M.W. & Woodard, G. (1967) Unpublished study.
Phosalone safety evaluation by repeated oral administration to dogs
for 107 weeks. Report dated 7th September 1967. Woodard Research
Corporation, Herndon, Virginia 22070 USA. Supplied to WHO by Rhône-
Poulenc.
Dubost, P. Fournel, J., Ganter, P., Julou, L., Koenig, F. & Myon, J.
(1963). Unpublished study. Acute toxicity, local tolerance,
anticholinesterase activity and chronic toxicity in rats and dogs.
Note DSPh 9, 203 of the 29th March 1963. Centre Nicolas Grillet,
94400 Vitry-sur-Seine, France. Supplied to WHO by Rhône-Poulenc.
Fournel, J., Ganter, P., Julou, L., Populaire, P., Myon, J., Pascal,
S. & Pasquet, J. (1966) Unpublished study. Phosalone (11 974 RP)
Sub-acute (6-month) toxicity study in the dog. Note RP-DSPh 11 330 E
of the 14th June 1966. Société des Usines Chimiques Rhône-Poulenc,
Direction Scientifiques, Laboratoires de Recherche, Centre Nicolas
Grillet, 94400 Vitry-sur-Seine, France. Supplied to WHO by Rhône-
Poulenc.
Fournel, J., Julou, L. & Pasquet, J. (1968) 12244 R.P. Unpublished
study. Acute toxicity in mice rats and in vivo anti-cholinesterase
activity in rats. Note RP - DSPh 12663 (=12665E) of the 1st March
1968. Société des Usines Chimiques Rhône-Poulenc, Direction
Scientifiques, Laboratoires de Recherche, 22 Avenue Montaigne, 75
Paris 8,France. Supplied to WHO by Rhône-Poulenc.
Goldenthal, E.I., Jessup, D.C. & Rodwell, D.E. (1978) Unpublished
study. Dominant lethal study in mice. Study carried out for Rhône-
Poulenc dated the 18th December 1978. International Research and
Development Corporation, Mattawan, Michigan, 49071, USA.
Haworth, L. & Lawlor, T.E. (1989) Unpublished study. Mutagenicity
test on phosalone technical in the Ames Salmonella/microsome
reverse mutation assay. HLA study No. 10754-0-401, Hazleton
Laboratories America Inc., 5518 Nicholson Lane, Suite 400,
Kensington Maryland 20895, USA. Supplied to WHO by Rhône-Poulenc.
Heath, S.A.B., Rivett, K.F. & Woolf, N. (1967) Unpublished study.
Phosalone test for neurotoxicity. Study No. 230857, May and Baker
Ltd, Dagenham, Essex, England. Supplied to WHO by Rhône-Poulenc.
Hayward, A. (1969). Unpublished study. Insecticides: a study of the
effects of "Cyanox" (S-4084) and "phosalone" on rat erythrocyte
cholinesterase. Report No. PRG/584 (=274359). May and Baker Ltd,
Ongar, Essex, England. Supplied to WHO by Rhône-Poulenc.
Hodgson, H.J. & Smith, A.D. (1992). Commercial and residential
poisoning with anticholinesterases. In: Ballantyne B and Marrs TC
(ed) Clinical and experimental toxicology of organophosphates and
carbamates, pp. 352-363. Butterworth-Heinemann, Oxford, England.
Hopkins, R., Lewis, C.J. & Lewsley, R. (1991). Unpublished study.
(14C)-phosalone: a study of the absorption, distribution,
metabolism and excretion in the rat. Report No. 5759-68/93R.
Hazleton UK, Harrogate, England. Supplied to WHO by Rhône-Poulenc.
Jones, E.M., Post, K.F., Scott, W.J., Woodyard, M.W., Woodyard, G. &
Cronin, M.T.I. (1967a) Unpublished study. Phosalone three generation
reproduction study in the rat. Report dated September 11th 1967.
Woodyard Research Corporation and Chipman Chemical Company. Supplied
to WHO by Rhône-Poulenc.
Jones, M.E., Woodard, M.W., Imming, R.J., Woodard, G. & Cronin,
M.T.I. (1967b) Unpublished study. Phosalone followed by a
determination of potentiation activity with the compound Di-syston.
Report dated August 10th, 1967. Woodyard Research Corporation and
Chipman Chemical Company. Supplied to WHO by Rhône-Poulenc.
Kehoe, D.F. (1990) Unpublished study. 8-week dietary toxicity study
with phosalone in rats. HLA study No. 6224-149, Hazleton
Laboratories America Inc 3301 Kinsman Boulevard, Madison, Wisconsin
53704, USA. Supplied to WHO by Rhône-Poulenc.
Knaak, J.B., Maddy, K.T., Gallo, M.A., Lillie, D.T., Craine, E.M &
Serat, W.F. (1978) Worker re-entry study involving phosalone
application to citrus groves. Toxicol Appl. Pharmacol., 46 363-
374.
Kundiev, Y.I. & Kagan, Y.S. (1992) Anticholinesterses used in the
USSR: poisoning, treatment and preventative measures. In: Ballantyne
B and Marrs TC (ed) Clinical and experimental toxicology of
organophosphates and carbamates.pp 494-501. Butterworth-Heinemann,
Oxford, England.
Kynoch, S.R., Lloyd, G.K., Mallard, J.R., Street, A.E., Gibson,
W.A., Wadsworth, P.F. & Prentice, D.E. (1979) Unpublished study. The
effect of repeated applications of phosalone 11974 RP to the skin of
rabbits for 21 days. Report No. RNP 125/79335. Huntingdon Research
Centre Ltd, PO Box 2, Huntingdon, Cambridgeshire, PE18 6ES, England.
Supplied to WHO by Rhône-Poulenc.
Marrs, T.C. (1991). Toxicology of oximes used in the treatment of
organophosphate poisoning. Adverse drug reactions and Toxicology
Reviews, 10: 61-72.
Mazuret, A. (1971) Unpublished study. Phosalone,methyl-azinphos and
parathion acute toxicity in the rat. Report No. RP -DSPh No. 15544-
E. Rhône-Poulenc Santé, Centre de Recherches de Vitry, 94403 Vitry-
sur-Seine Cedex, France. Supplied to WHO by Rhône-Poulenc.
Morris, J.M. (1983) Unpublished study. Acute delayed neurotoxicity
study in hens with phosalone. GSRI project No. 411-B51-40, Gulf
South Research Institute, PO Box 1177, New Iberia, Louisiana 70560,
USA. Supplied to WHO by Rhône-Poulenc.
Murli, H. (1989) Unpublished study. Mutagenicity test on phosalone
technical in an in vitro cytogenetic assay measuring chromosomal
aberration frequencies in Chinese hamster ovary (CHO) cells. HLA
study No. 10754-0-437, Hazleton Laboratories America Inc., 5518
Nicholson Lane, Suite 400, Kensington, Maryland 20895, USA. Supplied
to WHO by Rhône-Poulenc.
Nelson, L.W., Geil, R.G. & Spicer, E.J. (1980) Unpublished study.
Phosalone lifetime oncogenicity study in mice. International
Research and Development Corporation, Mattawan, Michigan 49071, USA.
Report 347-009, submitted June 23rd, 1980. Supplied to WHO by Rhône-
Poulenc.
Noel, P.R., Rivett, K.F., Osborne, B.E. & Street, A.E. (1970)
Unpublished study. Phosalone dietary intake in beagle dogs for four
weeks. Report No. 3619/70/431. Huntingdon Research Centre Ltd, PO
Box 2, Huntingdon, Cambridgeshire, PE18 6ES, England. Supplied to
WHO by Rhône-Poulenc.
Owen, P.E. (1985) Unpublished study.6-chlorobenzoxolone: 13 week
oral (dietary administration) study in the rat with a 28-day
treatment-free period. Report No. 4229-198/14. Hazleton UK, Otley
Road, Harrogate, England. Supplied to WHO by Rhône-Poulenc.
Pasquet, J. (1969) Unpublished study. Study of the teratogenic
activity of phosalone (11974 RP) on the chick embryo and on the
rabbit. Note RP - DSPh 13447E of the 7th February 1969 (translation
no 2707). Centre Nicolas Grillet, 94400 Vitry-sur-Seine, France.
Supplied to WHO by Rhône-Poulenc.
Pasquet, J. (1971) Unpublished study. Phosalone (11 974 R.P.)
toxicité aigüe chez la ratte par voie percutanée. Note RP - DSPh
15481 of the 28th June 1971. Centre Nicolas Grillet, 94400 Vitry-
sur-Seine, France. Supplied to WHO by Rhône-Poulenc.
Pasquet, J. & Fournier, E. (1980) Unpublished study. Phosalone (11
974 RP) micronucleus test in the mouse. Report.RP/RD/CNG No 557-E of
8th April 1980. Rhône-Poulenc Santé, Centre de Recherches de Vitry,
94403 Vitry-sur-Seine Cedex, France. Supplied to WHO by Rhône-
Poulenc.
Pasquet, J. & Mazuret, A. (1970) Unpublished study. Obidoxime
chloride (Toxogonin R Merck = 24,590 R.P. chloride) acute
intraperitoneal toxicity in the mouse and antidotal effect on the
acute oral toxicity in the mouse of phosalone (11974 RP) RP - DSPh
No. 14514-E. Rhône-Poulenc Santé, Centre de Recherches de Vitry,
94403 Vitry-sur-Seine Cedex, France. Supplied to WHO by Rhône-
Poulenc.
Pasquet, J., Mazuret, A., Vaissere, J. & Gaildrat (1983a)
Unpublished study. Chloromethyl-3 chloro-6 benzoxolone (16891 R.P.)
- acute toxicity in the rat by the intraperitoneal route. Report
ST.CRV/Tox No. 21971. Rhône-Poulenc Santé, Centre de Recherches de
Vitry, 94407 Vitry-sur-Seine Cedex, France. Supplied to WHO by
Rhône-Poulenc.
Pasquet, J., Mazuret, A. & Vaissere, J. (1983b) Unpublished study.
Chlorométhyl-3 chloro-6 benzoxolone (16891 R.P.) - eau de lavage de
la solution chlorométhyléique - irritation cutanée primaire chez le
lapin. Note No ST.CRV/Tox. No. 21972. Rhône-Poulenc Santé, Centre de
Recherches de Vitry, 94407 Vitry-sur-Seine Cedex, France. Supplied
to WHO by Rhône-Poulenc.
Pasquet, J. & Mazuret, A. (1973a). Unpublished study. Phosalone
oxygen analog (12244 R.P.) acute percutaneous toxicity in the rat.
SUCRP - DSPh No.16979. Rhône-Poulenc,Laboratoires de Recherches, 22
Avenue Montaigne, 75 Paris 8, France. Supplied to WHO by Rhône-
Poulenc.
Pasquet, J. & Mazuret, A. (1973b). Unpublished study. Hydroxymethyl-
3 chloro-6 benzoxolone (16 236 RP) toxicité et tolérance locale. RP)
acute percutaneous toxicity in the rat. Note SUCRP - DSPh No.
17144.Rhône-Poulenc Santé, Centre de Recherches de Vitry, 94403
Vitry-sur-Seine Cedex, France. Supplied to WHO by Rhône-Poulenc.
Supplied to WHO by Rhône-Poulenc.
Pasquet, J. & Mazuret, A. (1977). Unpublished study. Phosalone (11
974 RP) guinea-pig skin sensitization study. Report RP/RD/CNG No.
19117 of 27th April 1977. Centre Nicolas Grillet, 94400 Vitry-sur-
Seine, France. Supplied to WHO by Rhône-Poulenc.
Pasquet, J. & Mazuret, A. (1980). Unpublished study. Metabolites of
phosalone (11 974 RP) : compounds 19889 RP, 19889 RP and 19914 RP.
Acute oral toxicity in the mouse. Report C.R. Vitry/CNG. No. 20 736-
E of 12th November 1980. Centre Nicolas Grillet, 94400 Vitry-sur-
Seine, France. Supplied to WHO by Rhône-Poulenc.
Pasquet, J., Mazuret, A., Fournel, M. & Koenig, M. (1976)
Unpublished study. Acute oral and percutaneous toxicity of phosalone
(R.P.11974) in the rat, in comparison with azinphosmethyl and
parathion. Note RP/RD/CNG No. 18 550 EP/LR of 27th February 1976.
Centre Nicolas Grillet, 94400 Vitry-sur-Seine, France. Supplied to
WHO by Rhône-Poulenc.
Reddy, S.J., Reddy, B.V. & Ramamurthy, R. (1992). Impact of chronic
phosalone toxicity on Bohr factor and oxygen equilibrium curves of
rat. Biochem Inter. 26: 171-179.
Rhône-Poulenc (1968) Unpublished study. 12244 R.P. oxygen analog of
phosalone acute oral toxicity and anticholinesterasic activity in
vitro. Note RP - DSPh 12664 of the 20th February 1968. Société des
Usines Chimiques Rhône-Poulenc, Direction Scientifiques,
Laboratoires de Recherche, 22 Avenue Montaigne, 75 Paris 8, France.
Supplied to WHO by Rhône-Poulenc.
Rhône-Poulenc Inc. (1979) Unpublished study. Phosalone dairy cow
feeding study. ADC project #475A, B of the 6th July 1968. Rhône-
Poulenc Inc., Agricultural Division, PO Box 125, Black Horse Lane,
Monmouth Junction, New Jersey 08852, USA. Supplied to WHO by Rhône-
Poulenc.
Rhône-Poulenc Inc. (1980a) Unpublished study. Residue determination
of phosalone and its oxygen analog in the milk and tissues of dairy
cattle by electron capture gas chromatography. ADC project #475D of
the 22nd September 1968. Rhône-Poulenc Inc., Agricultural division,
PO Box 125, Black Horse Lane, Monmouth Junction New Jersey 08852,
USA. Supplied to WHO by Rhône-Poulenc.
Rhône-Poulenc Inc. (1980b) Unpublished study. Residue determination
of metabolites containing the chlorobenzoxazole and
chloroaminoiphenol moieties in the milk and tissues from dairy
cattle fed phosalone. ADC project #475D1 of the 22nd September 1968.
Rhône-Poulenc Inc., Agricultural division, PO Box 125, Black Horse
Lane, Monmouth Junction, New Jersey 08852, USA. Supplied to WHO by
Rhône-Poulenc.
Rhône-Poulenc Inc. (1980c) Unpublished study. Validation of
analytical methods of phosalone and its metabolites in milk and
animal tissues. ADC project #496 of the 22nd September 1968. Rhône-
Poulenc Inc., Agricultural Division, PO Box 125, Black Horse Lane,
Monmouth Junction, New Jersey 08852, USA. Supplied to WHO by Rhône-
Poulenc.
Safonov, N.M. (1968) (Toxicological characteristics of a new
organophosphorus insecticide: phosalone preliminary results).
Gigiena truda professional, nye zabolevanija, 12, 43 - Translation
supplied to WHO by Rhône-Poulenc.
SERB (1988) Contrathion, pralidoxime, pralidoxima, Data Sheet.
Laboratoires SERB, 53 Rue de l'Isle-Adam, 75020, Paris, France.
Siglin, J.C., Jenkins, P.K., Rush, R.E., Liao, J.T. & Rodwell, D.E.
(1979) Unpublished study. Primary eye irritation study of phosalone
technical in rabbits with washout (EPA). Study No. 3147.36.
Springborn Life Sciences, Inc., Spencerville, Ohio 45887, USA.
Supplied to WHO by Rhône-Poulenc.
Vanrel, B., Thenaisie, S., Gascoin, M.N., Vanrell, B., Glomot, R. &
Le Bigot, J.F. (1989) Unpublished study. Metabolite of phosalone in
soil reverse mutation assay in vitro Ames test. Study No. 5137
MMO. Rhône-Poulenc Agrochimie, Lyon, France. Supplied to WHO by
Rhône-Poulenc.
Vogel, W.(1991). Unpublished study. Exposure of harvesters to
residues of Zolone(R) flo (Phosalone) insecticide applied to apples
in France in 1989. RCC project No 241683. RCC Umweltchemie AG, PO
Itingen CH 4452 Switzerland. Supplied to WHO by Rhône-Poulenc.
Woodard, M.W., Cockrell, K.O., Woodyard, G. & Cronin, M.T.I. (1966)
Unpublished study. Phosalone demyelination study in chickens. Report
submitted April 7th, 1966 to the Chipman Chemical Company. Woodard
Research Corporation. Supplied to WHO by Rhône-Poulenc.
Woodard Research Corporation (1967) Unpublished study. Phosalone
safety evaluation by repeated oral administration to rats for 103-
104 weeks. Report submitted April 7th, 1966 to the Chipman Chemical
Company. Supplied to WHO by Rhône-Poulenc.
Weill, N. (1988) Unpublished study. Ames test on Salmonella
typhimurium on 50221 RP. Report No. 803380. Hazleton France.
Supplied to WHO by Rhône-Poulenc.
WHO (1986) Environmental Health Criteria 63. Organophosphorus
insecticides: a general introduction. World Health Organization,
Geneva, p 63.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
