
METHIDATHION
First draft prepared by M. Caris,
Bureau of Chemical Safety
Health and Welfare Canada, Ottawa, Canada
EXPLANATION
Methidathion is a broad-spectrum organophosphate insecticide,
whose mode of action is by inhibition of acetylcholinesterase.
Methidathion has been previously evaluated by the Joint Meeting in
1972 (Annex I, reference 18) when a Temporary ADI of 0.005 mg/kg bw
was allocated and in 1975 (Annex I, reference 24), when an ADI of
0.005 mg/kg bw was allocated.
The purpose of the present evaluation was to review additional
toxicity data which had been generated in an effort to compliment
and update the existing data base on methidathion. The first
comprehensive review of methidathion (Annex I, reference 19)
indicated that the technical material contained a minimum of 95%
pure active ingredient. The purity of the technical material
reported in the presently reviewed studies ranged from 92.6-99.95%.
In order to facilitate a comprehensive review of the toxicology
data on methidathion, relevant summaries from previously published
monographs and monograph addenda (Annex I, references 19 and 25)
have been included herein.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution, and excretion
Mice
Male and female CFI mice were treated dermally with 14C-
radiolabelled methidathion in the carbonyl carbon of the thiadiazole
ring in acetone solution or formulated in petroleum hydrocarbon with
emulsifier (the ratio of active ingredient to petroleum hydrocarbon
to emulsifier was 6:10:1). The actual dermal dose was stated to be
12 mg/kg bw. The test solutions for both sexes were well absorbed
through the skin as measured over a 72-h period, with residual
radioactivity on the skin in the acetone group of 0.47-0.67% and in
the formulated product of 0.35-1.27% of the dose. The highest
concentrations of radioactivity were recovered in the expired CO2
(50.85-64.1%) and urine (14.48-23.47%). Radioactivity found in
tissues (0.3-0.7%) and blood (0.03-0.21%) was minimal. Total
recovery represented 83-94% of the administered dose. The half-lives
for the testing solutions on the skin were calculated for the
acetone group to be 9.1 and 10.5 h for males and females, and for
the formulated group to be 10.4 and 10.9 h for males and females,
respectively. Blood and tissue levels appeared to plateau
approximately 4 h post-dosing, with tissue levels rarely exceeding 4
ppm (Simoneaux & Marco, 1984).
Rats
Methidathion, 14C-radiolabelled in the carbonyl carbon of the
thiadiazole ring was administered by gavage to groups of 5 male and
5 female Charles River CD rats as a single oral nominal dose of 0.25
or 2.5 mg/kg bw. A third test group was pretreated with unlabelled
methidathion for 14 days at the low dose of 0.25 mg/kg bw/day
followed by a single oral 14C-radiolabelled dose of methidathion
at 0.25 mg/kg bw. Two rats/sex served as vehicle (3% cornstarch
suspension with 0.5% polysorbate-80) controls. The total recovered
radioactivity accounted for 75.1-92.9% of the administered dose. The
data generated from the present study indicate that the principal
route of elimination was via the urine, with 30.3-37.1% of the
radioactivity excreted at the low dose and from 41.8-57% excreted at
the high dose. Residual tissue levels determined 7 days post-dosing,
generally accounted for less than 1% of the dose with highest
concentrations of radioactivity found in the liver, carcass and
bone. Faeces contained only 2.2-2.6% of the administered
radioactivity. Radioactivity in respired CO2 was not measured. The
half-lives of elimination in the urine of 14C-labelled
methidathion ranged from 7.4 to 9.7 h. There were no obvious
differences in the distribution patterns when consideration was
given to dose levels administered, pretreatment or sex (Szolics &
Simoneaux, 1987a).
Groups of 2 SD rats/sex were treated orally by gavage with a
single dose of 14C-methidathion radiolabelled on the carbonyl
group at a dose of 0.295 or 2.949 mg/kg bw (as suspensions in 3%
cornstarch suspension containing 0.5% polysorbate-80). Overall
radioactive recovery accounted for 99.6-102.5% of the administered
dose. The primary route of elimination was via the urine with 54.2-
56.9% of the dose excreted after 96 h. The second highest source of
radioactivity was recovered from expired CO2, representing 32.2-
34.4% of the radioactivity at the low dose and from 41.2-43.5% of
the administered dose at the higher dose level. Radiolabelled CO2
was detected within 4 h post-dosing at both dose levels ranging from
3.3-7.5% of the dose, suggesting early fragmentation of the
thiadiazole ring. Radioactivity in the faeces accounted for 2.9-4.5%
and from 8.2-11.6% of the dose for the low- and high dose level,
respectively. There were no major differences in elimination
patterns with respect to sex (Szolics & Simoneaux, 1987b).
Methidathion, 14C-labelled in the methoxy group was
administered as a single oral dose to 2 SD rats/sex at a dose of
2.597 mg/kg bw (in a 3% cornstarch suspension containing 0.5%
polysorbate-80). Total radioactive recovery represented 93% of the
administered dose. The major route of elimination was via the urine,
which in males comprised 39.8% and in females, 48.3% of the
administered radiolabel. The level of radioactivity in expired
volatiles was 33-39.3% of the dose, with recovery as early as 4 h
post-dosing. The radioactivity in the faeces (7.6% and 8.3% for M
and F, respectively) and carcass (6.3% and 3.9% for M and F,
respectively) contributed minimally to the total recovery (Szolics &
Simoneaux, 1987c).
Methidathion, 14C-radiolabelled in the ring carbon adjacent
to the methoxy group was given to SD rats (2/sex/group) by gavage at
a single oral dose of 0.25 or 2.52 mg/kg bw (in a 3% cornstarch
suspension containing 0.5% polysorbate-80). The principal route of
elimination was via the urine, which represented 52.4-69% of the
recovered radioactivity. Radioactivity recovered from the faeces
ranged from 5.5-7.6% of the dose and from 3.6-6.7% in the carcass.
The majority of the excreted urinary and faecal radiolabel was
recovered within 24 h of dosing. Expired CO2 accounted for
approximately 10% of the administered dose, with maximum recovery at
24 h and detection as early as 4 h post-dosing. The distribution of
radioactivity was not significantly affected by dose or sex. Overall
recovery of radioactive label was 77.6-91.5% of the administered
dose (Szolics & Simoneaux, 1987d).
Male and female SD rats were treated dermally with methidathion
14C-labelled in the carbonyl carbon of the thiadiazole ring in
acetone solution or formulated in petroleum hydrocarbon with
emulsifier (the ratio of active ingredient to petroleum hydrocarbon
to emulsifier was 6:10:1). The dermal dose was stated to be
equivalent to 12 mg/kg bw. Methidathion was well absorbed through
the skin as measured over a 72-h period. Higher percentages of
residual radioactivity were found in the skin of both sexes treated
with the formulated product (4.5-9.8%) when compared to the acetone
group (0.3-1.9%), suggesting slower absorption of the formulated
material over the 72-h period. There were no other differences with
respect to sex or testing solution. The highest concentrations of
radioactivity, in order of magnitude, were recovered in the expired
CO2, urine and carcass. Total recovery represented 86-95% of the
administered dose. The half-lives for the testing solutions on the
skin were calculated for the acetone group to be 16.9 and 15.9 h for
males and females, and for the formulated group to be 17.2 and 17.4
h for males and females, respectively. Plasma and tissue levels
generally attained plateau levels 48-h post-dosing, with tissue
levels rarely exceeding 2 ppm (Marco & Simoneaux, 1982).
Hens
Methidathion, 14C-radiolabelled at the second carbon adjacent
to the methoxy group was administered to a white leghorn chicken in
gelatin capsules equivalent to 45.3 ppm for a period of 16 days.
Total radiolabel recovered was 92.7%, of which 89.96% was found in
the excreta. Expired CO2 comprised 1.7% of the dose, whereas the
remainder of the radioactivity was divided among the egg yolk
(0.21%), egg white (0.21%), tissues (0.49%) and blood (0.06%).
Radioactive levels in the egg were observed to plateau between days
9 and 14 of treatment. Total residual radioactivity accounted for
0.49% of the administered dose. The highest residue levels in the
individual tissues were found in the liver (3.85 ppm) and kidney
(2.02 ppm) (Szolics & Simoneaux, 1985).
Goats
A single lactating goat was treated daily with a capsule of
methidathion, 14C-labelled in the ring carbonyl group at a level
equivalent to 5 ppm in the diet for a period of 10 consecutive days.
Total radioactive recovery from all sources was 91.5% of the
administered dose. The principal route of elimination was by expired
CO2, representing 65.8% of the dose. Urinary and faecal radiolabel
accounted for 20.4% and 3.4% of the dose, respectively. The
remaining radioactivity was dispersed among the various fractions,
rarely exceeding 1% of the total admin-istered dose. Residual levels
in body tissues were less than 0.35% of the dose and ranged from
0.02 to 0.15 ppm. Highest levels were found in the liver. Recovery
of radioactivity from milk reached plateau levels at 0.11 ppm after
5 days of treatment (Staley et al. 1987).
A single lactating goat was treated daily with a capsule of
methidathion, 14C-labelled in the ring carbon adjacent to the
methoxy group at a level equivalent to 5 ppm in the diet for a
period of 10 consecutive days. The majority of the radioactive label
was eliminated via the urine, accounting for 18.1% of the
administered dose. Expiration by CO2 and elimination via the
faeces represented 9.6% and 6.4% of the dose, respectively. Recovery
of the remaining radioactivity was distributed in the blood (2.9%),
liver (1.2%) and generally less than 1% of the dose was found in
each of the samples from milk, perirenal and omental fat, kidney,
leg muscle and tenderloin. Total recovery of radioactivity was only
40.1% of the administered dose. It has been postulated that this may
be due to a neutral volatile e.g., methane, which could not be
trapped in the standard volatile or CO2 trap (Staley & Simoneaux,
1987).
Biotransformation
Rats
The proposed metabolic pathway in animals is depicted in Figure
1 (Szolics & Simoneaux, 1987h).
Partitioning of urine collected from rats treated orally with
14C-carbonyl labelled methidathion at 0.295 or 2.949 mg/kg bw
(Szolics & Simoneaux, 1987b) indicated that the urine consisted of
mainly organic soluble metabolites, which accounted for 79% in male
and 66% in female of the total urinary radioactivity. The major
metabolite was the sulfide derivative accounting for 44-45% of the
radioactivity. The other principal metabolites were the sulfone
(14.2 and 8% for males and females, respectively) and the sulfoxide
(11.1 and 3.3% for males and females, respectively). The RH compound
represented only 2.4% of the urinary radioactivity whereas the
parent, methidathion, was present as only 0.7%. The oxygen analog
was not identified. The remainder of the total urinary radioactivity
(21-34%) was aqueous soluble metabolites. Two of the three
chromatographed peaks, displayed elution patterns similar to the
cysteine conjugate (2.1%) and desmonomethyl derivative (15.3%) of
methidathion (Szolics & Simoneaux, 1987e).
The urine of rats treated with 14C-labelled methidathion in
the methoxy group at 2.597 mg/kg bw (Szolics & Simoneaux, 1987c)
comprised both organic (67-72%) and aqueous (28-33%) soluble
metabolites. The major organic metabolites were the sulfoxide
(40.5%) and sulfone (23%). The remainder were identified as the RH
compound (1%), unchanged parent (0.8% - female only), the sulfide
(0.3% - male only) and oxygen analog (0.2% - male only). The aqueous
soluble metabolites were characterized as the cysteine conjugate
(2.1%) and desmonomethyl derivative (10.5%) of methidathion (Szolics
& Simoneaux, 1987f).
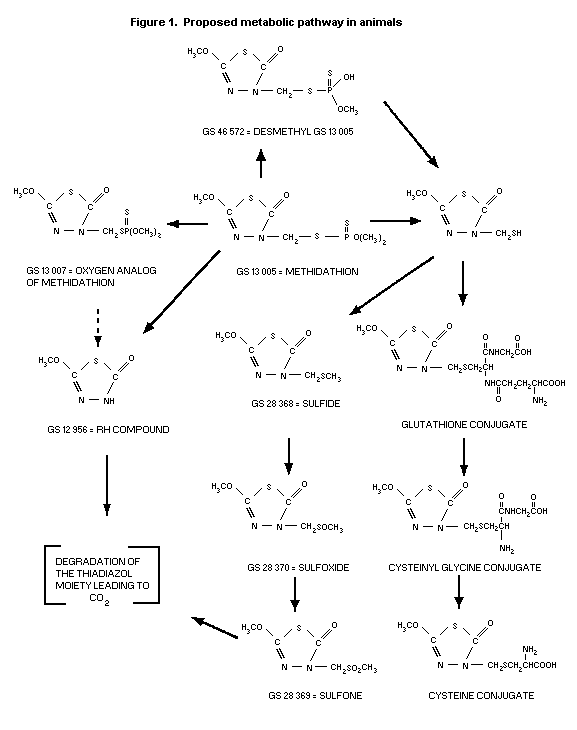 Metabolites from the urine of rats administered single oral
doses of 14C-labelled methidathion in the ring carbon adjacent to
the methoxy group at 0.25 or 2.52 mg/kg bw (Szolics & Simoneaux,
1987d) were identified. Organic soluble metabolites represented 61-
63%, whereas aqueous soluble metabolites accounted for 37-39% of the
urinary radioactivity. The major organic metabolite was the sulfide
(35.4%). Similar amounts of the sulfoxide (8.6%) and the sulfone
(8.2%) were detected. The RH compound represented 2.1% of the
radioactivity, and the oxygen analog represented 0.6%. No unchanged
parent was identified. With respect to the aqueous soluble
metabolites, the desmonomethyl derivative of methidathion accounted
for 20% (Szolics & Simoneaux, 1987g).
Goats
The urine of a single lactating goat treated daily by capsule
equivalent to a dietary intake level of 5 ppm for 10 days (Staley
et al. 1987) was selected for characterization of metabolites.
Partitioning data revealed that 84.5% of the urinary radioactivity
were aqueous soluble and 5.5% were organic soluble metabolites. The
principal aqueous soluble metabolites were the desmonomethyl
derivative (59.7%) and cysteine conjugate (10.4%) of methidathion.
Chromatography of the organic soluble components reportedly showed
that the metabolites in the goat were qualitatively the same as
those found in rat urine. The proposed predominant metabolic pathway
of methidathion in the goat was 0-demethylation (Szolics &
Simoneaux, 1987h).
Toxicological studies
Acute toxicity studies
Acute toxicity studies conducted in both the male and female
Tif.RAIf rat (Table 1), reveal that technical methidathion, upon
oral administration is highly toxic with LD50 values of 26 to 43.8
mg/kg bw. The acute dermal studies indicate that methidathion is
slightly to moderately toxic. Clinical signs of toxicity, upon oral
and dermal dosing were generally manifest as curved or ventral body
position, dacryorrhea/chromodacryorrhea, diarrhoea, dyspnea,
exophthalmus, ruffled fur, sedation, tonic/clonic muscle spasms and
trismus. The symptoms were reversible in surviving animals.
The acute oral toxicity of methidathion has been investigated
in several animal species (Table 2). The results indicate that
methidathion is moderately to highly toxic in all species tested
with LD50 values ranging from 17 to 200 mg/kg bw.
Table 1. Acute toxicity of technical methidathion
Species Sex Route Vehicle LD50 Purity Reference
(strain) (mg/kg bw)
Rat M&F oral CMC, 2% 43.8 ? Bathe (1973a)
(Tif. RAIf)
Rat M&F oral PEG, 400 26 96.9% Bathe & Sachsse
(Tif. RAIf) (1980a)
Rat M&F oral PEG, 400 26 92.7% Bathe & Sachsse
(Tif. RAIf) (1980b)
Rat M&F dermal CMC, 2% 1546 ? Bathe, (1973b)
(Tif. RAIf)
Rat M&F dermal CMC, 2% 1663 ? Bathe & Sachsse
(Tif. RAIf) (1975)
Rat M&F dermal CMC, 2% + 297 92.6% Sarasin (1980)
(Tif. RAIf) Tween 80, 0.1%
Short-term toxicity studies
Rats
Five groups of ten male rats received by gavage 0, 0.25, 0.83,
2.5 or 8.3 mg methidiathion/kg bw/day five days a week for four
weeks. Signs of cholinesterase inhibition occurred during the first
week at the 8.3 mg/kg bw/day level, but not later in this or in
other groups. Dose-related cholinesterase inhibition occurred in RBC
and plasma, the no-effect level being 0.25 mg/kg bw/day. Plasma
cholinesterase had returned to normal three days after treatment was
stopped, but RBC cholinesterase had not reached normal figures after
21 days (Noakes & Watson, 1964b).
Five groups of five male and five female rats received by
gavage 0. 2.5, 5, 10 or 20 mg methidathion/kg bw/day six days a week
for four weeks. In the 10 and 20 mg/kg bw/day groups, four and nine
animals died, respectively. Body-weight gain was depressed in all
groups, but no relation to dosage was apparent. There was a slight
increase in fat deposition in the liver at 5 mg/kg bw/day and at the
highest level this was more marked (Stenger & Roulet, 1963).
Groups of 24 male and 24 female rats were fed for 22 weeks on
diets containing 0, 1, 4, 16 or 64 ppm methidathion. In a similar
study in the same laboratory, groups of 24 male and 24 female rats
were fed for 26 weeks on diets supplying 0, 128 or 256 ppm
methidathion. The rate of body-weight gain was reduced at 64 ppm and
above in females but not in males. Histopathological examination of
liver, spleen and kidneys showed a dose-related increase in fat
deposition in the liver at doses above 64 ppm in both sexes. No
abnormalities in haematological indices or in results of urinalyses
were found (Stenger & Roulet, 1965).
Table 2. Acute oral toxicity of methidathion1
Species Sex LD50 Reference
(mg/kg bw)
Mouse F 17 Noakes & Sanderson, 1964
Hamster F 30 Noakes & Sanderson, 1964
Rat M&F 20-81 Slenger, 1964a,b, 1966a,b;
Aeppli, 1969a,b; 1970a,b;
Noakes & Sanderson, 1964
Rat M 26-65 Slenger, 1964a
Noakes & Sanderson, 1964
Guinea-pig F 25 Slenger, 1964a
Noakes & Sanderson, 1964
Rabbit M 80 Sachsse, 1971
Dog M&F 200 Noakes & Sanderson, 1964
Chicken F 80 Annex I, 19
1 Formulations calculated as a.i.
Groups of 20 male and 20 female rats were fed for six months on
diets containing 0, 0.5, 2, 10, 50 or 250 ppm methidathion. At the
250 ppm level weight gain was slightly depressed and clinical signs
of cholinesterase inhibition were seen, particularly in females.
Plasma cholinesterase was inhibited in the 250 ppm group and
erythrocyte cholinesterase in groups receiving 10 ppm and above.
Experimental groups were similar to controls with regard to
survival, food intake, weights and microscopic appearance of liver,
kidneys, spleen and testes and the macroscopic appearance of other
organs (Noakes & Watson, 1964a).
Rabbits
A dermal study was undertaken with groups of 5 New Zeeland
rabbits/sex administered methidathion (purity unknown) topically for
6-h daily non-occlusive exposure periods at doses of 0 (vehicle
control, PEG 300), 1, 5 or 20 mg/kg bw/day for a period of 22
consecutive days. There were no significant effects of treatment on
survival, food consumption, haematology, blood chemistry,
cholinesterase activity, organ weights, gross morphologic or
histopathological alterations. Based on minimal effects noted as
hypoactivity in a single male at 20 mg/kg bw, occasional incidences
of soft faeces or diarrhoea in treated males and a slight trend to
decreased body-weight gain in the high dose 20 mg/kg bw/day treated
males, a conservative NOAEL may be set at 5 mg/kg bw/day (Folinusz
et al. 1986).
A second dermal study was conducted with groups of New Zeeland
HRP:SPF rabbits (4-6 per sex) topically administered methidathion
(95% purity) daily for a 6-h occlusive exposure period at doses of 0
(vehicle control, PEG 400), 1, 10, 40 or 80 mg/kg bw for a period of
21 days. Treatment with methidathion resulted in mortality in males
at all dose levels (0/5, 2/5, 2/6, 3/5 and 3/5) and in females at 40
mg/kg bw/day and higher (0/5, 0/5, 0/4, 2/5 and 4/5). Clinical signs
of toxicity in males treated at dose levels of 1 mg/kg bw/day and
higher and in females treated at dose levels of 40 and 80 mg/kg
bw/day were manifest as anorexia, ataxia, hunched posture, languid
appearance and laboured respiration. Tremors were observed at dose
levels of 10 mg/kg bw/day and higher, whereas convulsions were
reported in a single high-dose (80 mg/kg bw/day) treated female.
Cholinesterase assessments revealed significant depression in the
mean plasma (38-86%), RBC (40-80%) and brain (37-88%) cholinesterase
values for both sexes at dose levels of 10 mg/kg bw/day and higher.
Treatment-related microscopic alterations were evidenced in the
liver and gall bladder. Principal findings in the liver were denoted
as hepatocytic clearing and congestion at dose levels as low as 1
mg/kg bw/day. Capsular/subcapsular necrosis with acute inflammation
was also noted in several of the treated animals. Lesions of the
gall bladder were present in both sexes at dose levels of 10 mg/kg
bw/day and higher and were attributed to bile reflux due to
hyperperistalsis. Subacute inflammation of the myocardium and
degeneration of the medial aorta occasionally with mineralization
were observed in several of the rabbits dying during the study
period. There were no specific effects of treatment on body-weight,
food consumption, ophthalmoscopy, haematological and blood
biochemical parameters or organ weights. The NOAEL was determined to
be 1 mg/kg bw/day by the author (Osheroff, 1987).
The results of the present study when interpreted
independently, have not unequivocally demonstrated 1 mg/kg bw/day to
be a NOAEL. The author has provided valid argument that evaluation
of primary toxicity was complicated by the additive effects of
stress especially during clinical observations. The occlusive rubber
binding used may not only have enhanced the absorption of the test
material but in combination with the neck collar, may have increased
the stress factor, thus augmenting the toxicity of the test
material. In view of the intrinsic variables in the present study
design, it is considered appropriate to critically assess the
results of the present study in conjunction with those generated
from the previously conducted rabbit dermal study, wherein dermal
administration of methidathion under non-occlusive means failed to
produce effects in rabbits at dose levels as high as 5 mg/kg bw/day
(Folinusz et al. 1986).
Dogs
Four groups of three male and three female beagle dogs received
diets containing 0, 4, 16 or 65 ppm methidathion for two years. The
animals were starved of diet one day each week and received a double
ration on the next day. Administration of methidathion was
discontinued from week 16 to 19. Erythrocyte cholinesterase was
inhibited in the 64 ppm group, but brain cholinesterase was
unaffected by treatment. SGPT was markedly elevated in the 64 and 16
ppm groups and slightly raised in males of the 4 ppm group. During
weeks 16 to 19 these levels fell, but only the 4 ppm group returned
to normal. SGOT levels were not the same as controls at all
treatment levels, but serum alkaline phosphatase was elevated and
sulfobromophthalein retention increased in the 16 and 64 ppm groups.
The livers of dogs receiving 16 and 64 ppm were pigmented on
macroscopic examination. Microscopically, pigmentation could be seen
in macrophages and hepatic cells (principally centrilobular) in the
16 and 64 ppm groups, the intensity of deposit being dose-related.
The Perl's reaction showed that the pigment did not contain
appreciable quantities of iron. The kidneys of the 64 ppm group also
showed pigmentation. It was questionable whether the livers of the 4
ppm group contained excess pigment. Control and test groups were
indistinguishable regarding behaviour, results of clinical tests
including neurological examination, haematological findings, organ
weights and macroscopic and microscopic appearance of organs other
than those mentioned. An additional two dogs received 64 ppm
methidathion in the diet for four weeks. The SGOT was elevated at
two and four weeks and at autopsy the livers were dark in colour.
Moderate diffuse pigmentation was seen microscopically in the liver
of one animal.
The plasma enzyme and liver histology changes at 30 days of
treatment at 64 ppm were not increased after treatment for two years
at this dose. Furthermore, the serum enzyme changes at 16-19 weeks
of treatment at 64 ppm were reversible and returned to normal three
weeks after cessation of treatment. The NOAEL was 4 ppm (Johnston,
1967).
The histological slides of the liver tissue from the original
study were re-evaluated and submitted to the 1975 Joint Meeting
Annex 1, reference 25). Intrahepatic cholestasis was observed in
dogs fed 16 and 64 ppm methidathion. Neither degenerative nor
inflammatory changes were associated with this lesion. Pigmentation
occurred as bile-plugs in biliary ductules, or as amorphous deposits
in Kupffer cells or as lipofuscin in both hepatic and Kupffer cells.
Haemolysis was not detected. A minimal degree lipofuscin
pigmentation was also observed at 4 ppm and in the control group.
Mild irregular fatty changes of the hepatocytes with occasional
periportal histiolymphocytic infiltration of the liver were observed
in both treated and control animals (Hess, 1975).
Methidathion (97% purity) was administered to groups of 4
beagle dogs per sex in the daily feed at dietary levels of 0, 0.5,
4, 45 or 140 ppm equal to 0, 0.02, 0.16, 1.96 or 5.67 mg/kg bw/day,
respectively, for a period of 90 days. An additional group of 4 dogs
per sex was treated orally by gelatin capsule at a dose level of
0.14 mg/kg bw/day, equal to a daily dietary intake level of 4 ppm,
for a similar treatment duration. A NOAEL of 4 ppm was assessed
based upon the evidence of cholestasis in all male and female dogs
treated at 45 and 140 ppm. Cholestasis was similarly described in a
single male dog treated by capsule at 0.14 mg/kg bw/day. Other
consequences of treatment evident in both sexes at dietary levels of
45 ppm and higher were discoloration of the liver and markedly
enhanced enzyme activity, expressed as increased levels of ALP,
SGOT, SGPT, GGT and sorbitol dehydrogenase. RBC cholinesterase
activity was significantly (75-88%) inhibited in dogs of both sexes
treated at 140 ppm. Brain cholinesterase activity was inhibited
(26.8%) in the 140 ppm treated females when compared to the
controls. There were no effects of methidathion treatment on serum
cholinesterase levels. Clinical signs of tremor and reduced activity
post-dosing, observed during the latter part of the study, were
exhibited in a single male dog at the high dose level. Reduced
activity was also noted in a single male treated at 45 ppm. Other
effects of treatment were significantly decreased mean food intake
values in the high dose treated males when compared to the control
group. There were no treatment-related effects observed with respect
to survival, body-weights, ophthalmoscopic examination,
haematological parameters, urinalysis or organ weights (Chang &
Wyand, 1990).
Six groups of 4 beagle dogs/sex were treated with methidathion
(96% purity) at dietary levels of 0, 0.5, 2, 4, 40 or 140 ppm, equal
to 0, 0.02, 0.07, 0.15, 1.34 or 4.51 mg/kg bw/day, respectively, for
a period of 12 months. A NOAEL of 4 ppm was indicated based on
treatment-related hepatic effects observed grossly as general
discoloration of the liver and characterized histomorphologically as
cholestasis and chronic inflammation of the liver in both sexes at
dietary levels of 40 ppm and higher. Associated changes in blood
biochemical parameters were denoted by elevated ALP, SGOT, SGPT,
sorbitol dehydrogenase and bilirubin levels. Other significant
clinical changes recorded only in the females were increased GGT and
decreased total protein and albumin values. RBC choline-sterase
activity was markedly inhibited (76-87%) in both male and females
dogs treated with methidathion at 140 ppm. Serum cholinesterase
activity was not adversely affected by treatment at any dietary
level. Brain cholinesterase activity was significantly depressed
(16-27%) in both sexes treated at 140 ppm when compared to the
controls. Another effect of treatment was related to decreased food
consumption in male dogs treated with methidation at a dietary level
of 140 ppm. There were no effects of treatment on survival, clinical
signs, body-weights, ophthalmoscopy, haematology, urinalysis, faecal
examination or organ weights (Chang and Walberg, 1991).
Monkeys
Three groups of Rhesus monkeys (approximately equal number of
each sex) were administered 0, 0.25 or 1.0 mg methidathion/kg bw/day
by stomach tube, 6 days a week for 23 months. Two of each group were
autopsied after 12 months. Plasma and erythrocyte cholinesterase
activity were inhibited in the 1 mg/kg bw/day group, but not at the
lower level. Brain cholinesterase was unaltered by treatment.
Growth, results of haemological tests, results of chemical analyses
of serum (including SGPT and ALP) and macro- and microscopic
examination of tissues were similar in control and test groups
(Fabran et al., 1971).
Long-term toxicity/carcinogenicity studies
Mice
A 23-month study was conducted with groups of 50 Charles River
CD-1 mice per sex/group fed diets containing methidathion (purity
not stated) at levels of 0, 3, 10, 50 or 100 ppm equal to 0.43,
1.42, 6.99 or 13.70 mg/kg bw/day, respectively. An additional 120
mice/sex/group were assigned to the chronic phase of this bioassay.
Interim sacrifices of 20 mice/sex/group were scheduled after 3, 6,
12 and 13 months. Animals sacrificed at 13 months were maintained on
control diet for one month as recovery animals following 12 months
of continuous treatment. The remaining animals (40/sex/group,
maximum) were sacrificed after 18 months of treatment. Treatment of
mice with methidathion resulted in slightly decreased survival of
the 100 ppm treated males when compared to the controls. The only
clinical sign remarked upon was discoloration of the urine in the 50
and 100 ppm treated males. Potentially reversible increases in liver
enzyme activity were reported in males at 50 ppm and higher and in
females at 100 ppm. Significantly decreased but potentially
reversible RBC cholinesterase activity values (26-46%) were
registered in males at 100 ppm and in females at 50 ppm and higher.
Brain cholinesterase activity was markedly inhibited (15-49%) in
both sexes at 100 ppm. There were no inhibitory effects of treatment
on plasma cholinesterase activity. There were no effects of
methidathion treatment noted with respect to body-weights, food and
water consumption or ophthalmoscopy. Treatment-related target organ
alterations were manifest in the gall bladder and liver at dietary
levels of 50 ppm and higher in males and in females at 100 ppm.
Microscopic changes were described in the gall bladder as
cholecystitis and hyperplasia and, hepatic findings were denoted as
bile duct hyperplasia, bile stasis, cholangiofibrosis, chronic
hepatitis and hypertrophy. Increased extramedullary haematopoiesis
of the spleen, associated with increased spleen weights were
observed in the 100 ppm treated males. Treatment of male mice with
methidathion resulted in a significantly increased incidence of
hepatocellular tumours (adenomas, carcinomas and adenomas/carcinomas
combined).
0 3 10 50 100 ppm
Adenoma 1/46 9/45 7/47 8/43 21/45
Carcinoma 8/46 6/45 4/47 13/43 17/45
Combined 9/46 15/45 11/47 21/43 38/45
Historical control data (Quest et al. 1990) generated from 14
studies conducted at the same test facility with the same strain of
mouse, revealed that the incidences of hepatocellular tumours
reported in the present study exceeded the historical control range
at dietary levels of 50 ppm and higher. Latency, expressed in terms
of time to appearance of first tumour, was not apparently decreased
in the treated male groups when compared to the concurrent controls.
The incidence of hepatocellular tumours was not increased in female
mice treated with methidathion The NOAEL in this study was 10 ppm,
equal to 1.4 mg/kg bw/day (Goldenthal, 1986).
Rats
In order to investigate the long-term toxic effects and
possible carcinogenic action of methidathion, four groups of 25 male
and 25 female rats each were fed for three weeks on diets containing
0, 2, 8 or 32 ppm and for a further 101 weeks on diets containing 0,
4, 16 or 64 ppm methidathion. The rate of gain in body weight was
reduced from week 8 in male animals of the 64 ppm group. After the
first year the rates of gain became erratic in all groups, making
interpretation of the findings difficult. Female rats on test diets
grew at a rate comparable to controls. Erythrocyte cholinesterase
was inhibited in the 16 and 64 ppm groups, while plasma
cholinesterase showed minimal inhibition at 100 weeks in the 64 ppm
group only. Brain cholinesterase was inhibited in the 64 ppm group,
with marginal and no reduction in the 16 and 4 ppm groups,
respectively. Decreased relative adrenal weights were found in
females of the 16 ppm and 64 ppm groups, and decreased ovary weights
in the 64 ppm group. The relative kidney weights of males was
increased in the 16 and 64 ppm groups. A greater frequency of
hepatic degenerative changes was noted in rats fed methidathion in
the diet; the high incidence of pulmonary infections in the rats
renders this finding of doubtful toxicological significance. The
food intake, results of haematological investigations and chemical
analysis of serum (including SPGT) and the survival rate were
similar in the test groups and in the control group. The incidence
of tumours was variable between groups but was low and not dose-
related, and no unusual tumours were found. The NOAEL in this study
was 4 ppm methidathion in the diet (Johnston, 1967).
Groups of 65 Crl:COBS CD(SD) BR rats/sex were treated with
methidathion (97.3% purity) at dietary levels of 0, 4, 40 or 100 ppm
equal to 0.16, 1.72 or 4.91 mg/kg bw/day, respectively for a period
of 104 weeks. For interim sacrifice purposes, an additional 15
rats/sex/group were assigned on study. At 52 weeks, 10
rats/sex/group were sacrificed with the remaining 5 rats/sex/ group
sacrificed at week 93 of study. Treatment with methidathion resulted
in clinical signs expressed in the 40 and 100 ppm groups as
alopecia, chromorhinorrhea, hyperactivity, skin lesions, tremors,
hypersensitivity to the touch and fasciculation. Mean body-weights
and weight gains were decreased in both sexes at 100 ppm throughout
the course of treatment. Body-weight gains were decreased at a
dietary level of 40 ppm during the initial few weeks of treatment,
with comparable weight gain thereafter. Mean food intake was
significantly increased in both sexes treated at dietary levels of
40 ppm and higher, whereas water consumption was decreased at 100
ppm and occasionally at 40 ppm. Treatment-related haematological
findings were exhibited at a dietary level of 100 ppm as inverted
neutrophil:lymphocyte ratios, reduced RBC parameters and increased
platelet counts. Variations in blood biochemical parameters were
observed at 40 ppm and higher as decreased total bilirubin,
decreased total protein as well as changes in electrolyte levels
(decreased potassium and calcium, and increased inorganic phosphorus
and chloride). Significant inhibition of RBC (14-38%), serum (22-
66%) and brain (42-74%) cholinesterase activity were determined in
both sexes of rats treated at dietary levels of 40 ppm and higher.
Notable changes in urinalyses were revealed as decreased volume and
increased specific gravity at 40 ppm and higher which correlated
well with the reduced fluid intake reported in these groups. Gross
necropsy findings showed an increased incidence of skin lesions in
the 40 and 100 ppm levels which were associated microscopically with
ulceration and/or chronic purulent inflammation. Other pathological
alterations were related to an increased incidence in focal
accumulations of foamy macrophages in the alveoli of the 100 ppm
treated males and females. There were no effects of treatment on
survival or ophthalmoscopy. The NOAEL for in-life parameters was
determined to be 4 ppm, equal to 0.16 mg/kg bw/day. There was no
evidence of methidathion-induced carcinogenic potential (Yau et
al., 1986).
Reproduction studies
Rats
In a three-generation study, three groups of 10 male and 20
female rats were fed on a diet containing 0, 2 or 16 ppm
methidathion for three weeks, and thereafter on a diet containing 0,
4 or 32 ppm methidathion. Litters from the second mating were used
to provide the new generations. The F1b litters did not receive
test diets until 26 days and the F2b until 22 days after weaning.
F0, Flb and F2b generations received diets for 27-28 weeks
during which they produced two litters. The number of young
surviving at weaning was reduced in all generations of litters from
animals fed 32 ppm methidathion and the mean liver weight of F3b
weanlings of this group was slightly increased. Body weights,
reproductive capacity and mortality of parents and the number of
litters, litter size, mean birth and weaning weights of test groups
were comparable to controls. The number of stillbirths and incidence
of congenital abnormalities were unaltered by treatment. No
histological damage was found in the organs of the F3b animals
examined. The NOAEL in this study was 4 ppm methidathion (Lobdell &
Johnston, 1966).
A two-generation (one litter per generation) reproduction study
was conducted with groups of 15 male and 30 female Charles River SD
CR1:CD BR rats fed methidathion (purity not specified) at dietary
levels of 0, 5, 25 or 50 ppm equal to 0, 0.43, 2.08 or 4.23 mg/kg
bw/day, respectively. With respect to reproductive performance,
statistically (p < 0.05) reduced mating indices were recorded for
the 25 and 50 ppm groups in the F1 generation. Although the mating
indices were reduced in these groups (69% and 66%, respectively)
relative to the concurrent F1 control group (88%), they were
nevertheless not significantly different from those calculated for
the F0 generation control (73%) or treated groups (60-65%). The
fertility and gestation indices were comparable among groups.
Treatment-related effects on maternal animals fed dietary levels of
25 ppm and higher were revealed by clinical signs of toxicity
manifest during lactation as slight and/or intermittent tremors.
Mean body-weights of F1 50 ppm-treated males were decreased prior
to and during the 12-week premating period. Mean body-weights gains
were not, however, significantly different from controls. Body-
weights of females recorded during lactation of both generations
were decreased at 50 ppm when compared to the concurrent control.
Decreased ovary weights recorded in females at 25 and 50 ppm were
not correlated with histopathological changes. Effects of treatment
on progeny were expressed as decreased survival in the 50 ppm group
of the F1 generation, decreased body-weights and clinical signs in
both generations at 25 and 50 ppm. The clinical signs were
suggestive of poor maternal care and were described as
weakness/lethargy, coolness to the touch and starving appearance. On
the basis of the reported findings, a NOAEL of 5 ppm, equal to 0.43
mg/kg bw/day, was indicated (Salamon, 1987).
Special studies on teratogenicity
Rats
A teratology study was conducted with groups of 25 mated female
Crl: COBS CD(SD)BR rats administered methidathion (93.2-96% purity)
at 0.25, 1.0 and 2.5 mg/kg bw/day or the control (vehicle, 3%
aqueous cornstarch with 0.5% Tween 80) orally by gavage from days 6
to 15 of gestation, inclusive. Confirmation of mating was determined
by presence of sperm in the vaginal washing, and this day was
designated day 0 of gestation. A NOAEL for maternal toxicity was
indicated at 1.0 mg/kg bw/day based on mortality, decreased body-
weights, food intake and clinical signs at 2.5 mg/kg bw/day.
Clinical signs of toxicity in the high dose (2.5 mg/kg bw/day) group
were suggestive of organophosphorus intoxication including,
lethargy, tremors, lacrimation, salivation, raspy respiration,
exophthalmia, chromodacryorrhea. A NOAEL for embryofetal toxicity
was set at the highest dose level tested of 2.5 mg/kg bw/day. There
were no significant differences noted with respect to the mean
number of implantations, live fetuses or mean number of resorptions
on a litter basis. There was similarly no significant variability in
the post-implantation loss, fetal sex ratio or in the mean fetal
litter weight in the treated groups when compared to the control.
Treatment with methidathion failed to uncover any evidence of
teratogenic potential (Mainiero et al. 1987).
Rabbits
Methidathion (purity not specified) was administered orally by
gavage at 0 (vehicle control, 3% cornstarch with 0.5% Tween 80), 2,
6 or 12 mg/kg bw/day to groups of 19 artificially inseminated New
Zeeland white rabbits on days 7 through 19 of gestation. The day of
artificial insemination was designated as day 0 of gestation.
Treatment-related clinical signs of toxicity at the high dose (12
mg/kg bw/day) treated animals were manifest as ataxia, tremors,
salivation and miosis. In the absence of similar effects in the
control or other treated groups, the NOAEL for maternal toxicity was
demonstrated to be 6 mg/kg bw/day. There was no evidence of
embryofetal developmental toxicity or potential for teratogenicity
at any of the dose levels investigated, including the highest dose
of 12 mg/kg bw/day (Hummel et al. 1987).
Special studies on genotoxicity
A battery of mutagenicity assays were conducted with technical
methidathion, the results of which are presented in Table 3. The
tests performed to evaluate potential for gene mutation in bacteria,
DNA damage as well as chromosome aberration were negative. A
dominant lethal study in mice was also negative. Positive responses
were observed in the in vitro assays with Saccharomyces
cerevisiae and in the Chinese hamster test for sister chromatid
exchange. In vivo tests conducted to examine the similar
endpoints, did not however elicit any evidence of mutagenic
potential in mammalian cells.
Special studies on delayed neurotoxicity
Four adult hens received four subcutaneous injections of 50 mg
methidathion/kg body-weight (the maximum tolerated dose) at weekly
intervals and they were observed for a further four weeks. The signs
of acute poisoning lasted two to three days each time, but birds
remained in good condition and no paralysis developed.
Neuropathological examinations were not performed (Noakes, 1964).
Five groups of ten adult hens were fed diets containing 0, 16,
52 or 160 ppm methidathion or 316 ppm tri-orthocresylphosphate for
45-47 days. No abnormal neurological signs were found in birds fed
methidathion, but those on tri-orthocresylphosphate showed leg
weakness, lack of balance and ataxia during the final week of
treatment. Unequivocal evidence of demyelination of neural tissue
was not found in methidathion or tri-orthocresylphosphate-treated
animals (Johnston, 1965).
The delayed neurotoxic potential of methidathion (purity not
specified) was investigated in groups of 10-30 female white leghorn
hens. The animals were administered the test material twice, 21 days
apart, at 0 (vehicle, CMC 2%), 43.75, 87.5, 175 or 350 mg/kg bw.
Animals receiving methidathion at 350 mg/kg bw were pretreated with
an intramuscular injection of atropine sulfate, 10 mg/kg bw, one
hour prior to treatment. Groups of 3 male and 3 female hens received
a single oral dose of the positive control, TOCP at 215, 464, 600,
1000 or 2150 mg/kg bw and were then observed for 21 days post-
dosing. A preliminary acute oral study indicated that the LD50 of
methidathion in the hen was 175 mg/kg bw. Clinical signs of
intoxication were expressed as ataxia, slight tremor, curved or
ventral body position and sedation, with subsequent recovery in
surviving animals, 8-10 h post-dosing. Histopathological evaluation
of the spinal cord and peripheral nerves did not reveal any
treatment-related lesions of the nervous system, whereas TOCP
toxicity was evidenced as central-peripheral, distally accentuated
neuropathy. Treatment with methidathion did not produce any signs of
delayed neurotoxicity (Ullmann et al. 1977).
Table 3. Genotoxicity of technical methidathion
Test Test system Concentration Purity Results References
(vehicle)
Reverse mutation S. typhimurium 25, 75, 225, 675, 2025 98.4% negative Arni & Muller (1980a)
(in vivo) TA98, 100, 1535, 1537 µg/0.1 mL (DMSO) 1., 2.
S. typhimurium 25, 75, 225, 675, 2025 93.4% negative Arni & Muller (1980b)
TA98, 100, 1535, 1537 µg/0.1 mL (DMSO) 1., 2.
S. typhimurium 0, 0.1, 0.5, 1, 5, 10, 50 99.95% negative Satou et al. (1979)
TA98, 100, 1535, 1537 mg/mL (DMSO) 1., 2.
E. coli
B/r WP2 Try- Hcr-
S. typhimurium 10, 50, 100, 500, 1000, ? negative Simmon et al. (1977)
TA98, 100, 1535, 1537, 5000 µg/plate (DMSO) 1., 2.
1538
Rec assay (in vitro) B. subtilis 5, 25, 100 mg/mL (DMSO) 99.95% negative Satou et al. (1979)
H17, M45
Reverse mutation/ S. typhimurium 10, 20, 40 mg/kg bw ? negative Simmon et al. (1977)
Host-mediated TA1535, 1538/Male Swiss (single oral doses)
(in vivo) Webster mouse
(inocculated ip)
5, 10, 20 mg/kg bw negative
(5 oral doses) (DMSO)
S. typhimurium 0, 5, 10, 20 mg/kg bw 93.4% negative Arni & Muller (1980c)
TA98, 100, 1537/ (3 oral doses: 2 h, 1 h
male mouse and prior to innoculation)
(inocculated iv) (CMC, 0.5%)
Table 3 (cont'd)
Test Test system Concentration Purity Results References
(vehicle)
Gene conversion/ S. cerevisiae MP-1 675, 1250, 2500, 5000, 93.4 conversion: Arni & Muller (1981)
forward mutation 10000 µg/mL (DMSO) slight (+ ve)
(in vivo) mutation:
(+ ve)
Forward mutation Mouse lymphoma cells - 0, 15 mg/kg bw (3 oral 93.4% negative Strasser & Muller (1980)
host-mediated (in L5 178Y/mouse doses post-innoculation)
vivo) (DBA/Bom) (CMC)
DNA repair Mouse (male CD-1) 5 x 10-7 to 1% (DMSO) ? negative Tong (1982a)
(in vivo) hepatocytes
Rat (male F344) 5 x 10-9 to 1% (DMSO) ? negative Tong (1982b)
hepatocytes
Rat hepatocytes 0.128, 0.64, 3.2, 16 97.2% negative Puri & Muller (1982a)
µg/mL (DMSO)
Rat hepatocytes 1.85, 5.56, 16.67, 50, 100, 96% negative Hertner & Arni (1990)
200 µg/mL (DMSO)
Human fibroblasts 1.024, 5.12, 25.6, 128 ? negative Puri & Muller (1982b)
µg/mL (DMSO)
SCE (in vivo) Chinese hamster 0, 10, 20, 40, 80 µg/mL 99.1% slight (+ ve) Chem et al. (1981)
cell line V79 (DMSO) at 40, 80
ug/mL
SCE (in vivo) Chinese hamster 0, 17, 34, 68 mg/kg bw 93.4% negative Hool & Muller (1980)
(bone marrow) (CMC)
Table 3 (cont'd)
Test Test system Concentration Purity Results References
(vehicle)
Chromosome Chinese hamster ovary 43, 75, 87.5, 175, 350 96% negative Strasser & Arni (1990)
aberration CCL 61 µg/mL (DMSO)
(in vivo) 96.9% negative Hool et al. (1980)
Nucleus anomaly Chinese hamster 0, 17, 34, 68 mg/kg bw
(in vivo) (bone marrow) (2 oral doses) (CMC) 98.4% negative Fritz (1976)
Dominant lethal Mice, NMRI 0, 15, 45 mg/kg bw (CMC)
(in vivo)
1. = in the presence of metabolic activation
2. = in the absence of metabolic activation
Special studies on irritation and sensitization
The eye irritation potential of technical methidathion was
investigated in 3 English Silver strain rabbits/sex. Administration
of 0.1 grams of methidathion into the conjunctival sac of the left
eye (right eye served as control) produced irritating conjunctival
reaction in the unwashed eye of a single male rabbit (Sachsse,
1973a).
Technical methidathion (as a 50% polyethylene glycol
suspension) was applied under occlusive conditions to the shaved
backs of male and female English Silver strain rabbits for 24 h.
Skin reactions were observed as very slight erythema in one male and
moderate to severe erythema in association with slight oedema in one
female rabbit (Sachsse, 1973b).
The shaved backs of male Hartley albino guinea-pigs were
repeatedly treated with technical methidathion as a 10% solution in
diethyl ether. There was no evidence of skin sensitization potential
(Cannelongo, 1984).
Special studies on potentiation
The potentiating effects of methidathion (purity not specified)
with profenofos (Sachsse & Bathe, 1977b) and methacrifos (Sachsse &
Bathe, 1978) were investigated in male and female Tif:RAIf rats by
comparing the theoretical LD50 values, based on an assumption of
strictly additive toxicity, with that of the experimentally derived
LD50 values for the equitoxic mixtures. There was no potentiating
effect of methidathion with profenofos, whereas a slight enhancement
of acute toxicity was observed with an equitoxic mixture of
methidathion and methacrifos.
Special studies on promoting activity
Male F344 DuCrj strain rats received a single ip injection of
the initiator, N-nitrosodiethylamine (DEN) at 200 mg/kg bw. Two
weeks thereafter, the rats were treated with methidathion (92.7%
purity) at dietary levels of 0, 10, 30, 100 or 300 ppm (equal to 0,
1.0, 2.3, 7.6 or 22.9 mg/kg bw, respectively) or the positive
control, 3'-methyl-4-dimethyl amino azobenzene (3'-Me-DAB) at 600
ppm (equal to 33.9 mg/kg bw) for a period of 6 weeks. Three weeks
following treatment with DEN, partial hepatectomy was performed on
all animals. Histochemical evaluation of 4 liver sections from each
animal after 8 weeks on study, revealed a significantly increased
number of GGT positive foci per unit area in rats treated with
methidathion at 100 and 300 ppm and in the 600 ppm 3'-Me-DAB
positive control group. No increases in the number of GGT positive
foci were observed in additional groups of rats treated with
methidathion at 100 and 300 ppm alone, without previous initiation
with DEN. The number of foci in rats treated with methidathion at 30
ppm and lower were not different from the controls (Arai, 1981).
A second short-term study of the promoting activity in the rat
liver was conducted with groups of male Fischer 344 DuCrj strain
rats administered methidathion (98.6% purity) at dietary levels of
0, 35, 46, 59, 77 or 100 ppm (equal to 0, 2.7, 3.5, 4.8, 6.0 or 7.6
mg/kg bw/day, respectively) for a period of 6 weeks. Two weeks prior
to feeding with methidathion, the rats were pretreated with a single
ip injection of the initiator DEN at 200 mg/kg bw. Partial (two-
thirds) hepatectomy was performed three weeks after initiation and
the animals were sacrificed after 8 weeks on study. Histochemical
analysis unveiled a significant dose-related increase in the number
of GGT positive foci in rats treated with methidathion at dietary
levels of 59 ppm and higher. The number of GGT positive foci at
dietary levels of 46 ppm and lower were comparable to the controls.
No increases in the number of GGT positive foci were recorded in an
additional group of rats treated with methidathion at 100 ppm alone,
without previous initiation with DEN (Daiyu-Kai Institute of Medical
Science, 1983).
Special studies on therapeutic activity of antidotes
The therapeutic potential of antidotes was tested by
administering methidathion (purity not specified) orally by gavage
to Tif.RAIf rats at the LD80 of 46 mg/kg bw. The therapeutic
agents, atropine sulfate alone, toxogonin alone, PAM alone and
toxogonin combined with atropine sulfate were then administered
intramuscularly after the first appearance of clinical signs of
poisoning. A further aspect of therapeutics was investigated, by
administering 5 repeated daily oral doses of methidathion at the
LD50 (34 mg/kg bw) to rats and then, upon observation of clinical
signs, commencing with the respective combination of antidotes. All
therapeutic agents were, according to the treatment regimen,
effective against the oral LD80 of methidathion. A positive
protective effect against repeated LD50 doses of methidathion were
observed with atropine and toxogonin. PAM and toxogonin in
combination with atropine had minimal protective effect against
repeated challenges to methidathion (Sachsse & Bathe, 1977a).
Observations in humans
One male subject took 4 mg/day (equal to 0.04 mg/kg bw/day) for
17 days, and 8 mg/day (equal to 0.08 mg/kg bw/day) of methidathion
for 27 days. No effect was found on RBC and plasma cholinesterase,
the thrombocyte count and stability or on the clinical condition of
the subject (Payot, 1965).
Two groups of eight men received 0.04 or 0.11 mg
methidathion/kg bw/day orally in capsules for 6 weeks. Four men
received placebo capsules. The treatment with methidathion was
without effect on plasma and RBC cholinesterase, SGPT and SGOT and
results of urinalyses or on EEG pattern or clinical condition of the
subject (Coulston, 1970).
A case of massive poisoning was reported to have occurred where
a 25-year old man weighing 60 kg ingested a number of mouthfuls of
supracide 40 (methidathion, 40% active ingredient). The man was
discovered approximately 2 hours after the event, in an unconscious,
semi-comatose condition. Upon admission to hospital the patient was
treated intravenously with atropine and toxogonin. The
cholinesterase activity in the serum was found to be zero a few
hours after admission. The patient was discharged after 15 days of
hospitalization. The laboratory findings were normal with exception
of cholinesterase activity which remained low. Follow-up examination
at 2, 5 and 10 months revealed no abnormalities with no evidence of
delayed neurotoxicity (Teitelman et al. 1975).
A case of attempted suicide occurred in a 50-year old man
weighing 67 kg who had ingested approximately 6.2 g or 40 ml of
Supracid 20 (methidathion, 15.5% active ingredient). About 1.5 h
after the incident, the subject was admitted to hospital suffering
from mental confusion, muscle fasciculations, bradycardia, miosis,
sweating, salivation and lacrimation. The patient underwent gastric
lavage followed by treatment with atropine sulphate and pralidoxime.
Six hours after ingestion of methidathion, the incidence of muscle
fasciculations increased followed by bronchorrhea and coma. Serum
and RBC cholinesterase values, as measured over eight days following
poisoning were generally less than 50% of normal values.
Cholinesterase values assessed after 45 days were normal.
Lymphocytic NTE activity was normal as determined on days 5, 10, 17
and 45 after poisoning. The patient was discharged after 21 days of
hospitalization. A follow-up examination after 7 months failed to
establish any evidence of delayed polyneuropathy or other
neurological abnormality (Zoppellari et al. 1990).
COMMENTS
Methidathion was extensively absorbed when administered orally
to rats. The routes of elimination were via the urine and expired
CO2. There were no significant differences in elimination patterns
with regard to dose levels administered, pre-treatment or sex.
In the rat, the predominant urinary metabolites were the
sulfide, sulfoxide, sulfone and desmonomethyl derivative. Negligible
quantities of the parent and oxygen analog were detected in the
urine. The predominant metabolic pathway of methidathion in the goat
was via O-demethylation with the desmonomethyl derivative as the
principal urinary metabolite. Cysteine conjugates were identified in
each species.
Methidathion has a high acute oral toxicity. The World Health
Organization has classified methidathion as highly hazardous (WHO,
1992).
Methidathion was administered to dogs for 90 days at dietary
concentrations of 0, 0.5, 4, 45 or 140 ppm, or 0.14 mg/kg bw/day
(equal to 4 ppm) by capsule, and for a period of 12 months at 0,
0.5, 2, 4, 40, or 140 ppm in the diet. In both studies the dietary
NOAEL was determined to be 4 ppm, equal to 0.16 mg/kg bw/day, based
on liver effects, most notably cholestasis and increased liver
enzymatic activity in serum at dietary levels of 40 ppm and above.
Cholestasis was observed in a single male dog treated by capsule at
0.14 mg/kg bw/day. Erythrocyte and brain cholinesterase activities
were affected only at the highest level of 140 ppm.
Long-term dietary treatment of mice with methidathion for 23
months at 0, 3, 10, 50 or 100 ppm revealed an increased incidence of
hepatocellular tumours in males at 50 ppm and above, resulting in a
NOAEL of 10 ppm, equal to 1.4 mg/kg bw/day. Erythrocyte
cholinesterase was inhibited at 50 ppm (equal to 7 mg/kg bw/day) and
above, whereas brain cholinesterase activity was affected at 100 ppm
(equal to 13.7 mg/kg bw/day).
A 104-week long-term toxicity/carcinogenicity study in rats fed
methidathion at 0, 4, 40 or 100 ppm indicated a NOAEL of 4 ppm,
equal to 0.16 mg/kg bw/day, based on inhibition of erythrocyte and
brain cholinesterase activity at 40 ppm and above. Methidathion was
not carcinogenic in rats.
In a two-generation reproduction study in rats at dietary
concentrations of 0, 5, 25 or 50 ppm, the NOAEL was 5 ppm, equal to
0.43 mg/kg bw/day. At 25 ppm, reduced mating indices in the F1
generation and decreased progeny body weights were observed.
There were no teratogenic effects observed when methidathion
was administered by gavage to rats at doses of 0, 0.25, 1.0, or 2.5
mg/kg bw/day or to rabbits at doses of 0, 2, 6 or 12 mg/kg bw/day.
Maternal effects were demonstrated at the highest doses in both the
rat and rabbit as clinical signs of toxicity. In the rat, increased
mortality as well as decreased body-weights and food consumption
were also observed. The NOAELs in the rat and rabbit were determined
to be 1 and 6 mg/kg bw/day, respectively.
Treatment of hens with methidathion did not produce any
clinical or pathological evidence of delayed neurotoxicity.
In two reported cases of poisoning with methidathion, each of
the male subjects exhibited classic clinical and biochemical signs
of organophosphorus intoxication. Both subjects recovered, with no
evidence of delayed neurotoxicity uncovered upon follow-up
examination. In one of the cases jaundice was reported during the
recovery period.
Two human volunteer studies failed to reveal any inhibition of
erythrocyte or serum cholinesterase activity at doses up to 0.11
mg/kg bw/day.
After reviewing the available genotoxicity data, the Meeting
concluded that methidathion was not genotoxic.
In the dog, the effects on the liver appeared to have occurred
at levels lower than the levels causing inhibition of cholinesterase
activity, giving a NOAEL of 0.1 mg/kg bw/day. Whether or not there
was a relationship to the potential induction of liver effects in
man could not be ascertained.
The Meeting concluded, after consideration of the
hepatocellular tumours found in male mice in the long-term toxicity
study, together with the lack of genotoxicity, that methidathion did
not present a carcinogenic hazard for humans.
The previous ADI, based on a NOAEL in man of 0.11 mg/kg bw/day,
was revised. The revised ADI is based on the NOAEL in the dog and a
100-fold safety factor.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 10 ppm, equal to 1.4 mg/kg bw/day (23-month study)
Rat 4 ppm, equal to 0.16 mg/kg bw/day (104-week study)
5 ppm, equal to 0.43 mg/kg bw/day (reproduction
study)
Dog: 0.1 mg/kg bw/day (90-day, one-year, and two-year
studies)
Human: 0.11 mg/kg bw/day.
Estimate of acceptable daily intake for humans
0 - 0.001 mg/kg bw
Studies which will provide information valuable in the continued
evaluation of the compound
Further observations in humans
REFERENCES
Aeppli, L. (1969a) Akute Toxizität, Ratte per os, A 2039 E (0 40
WP). Unveröffentlicht. Geigy, J.R. AG.
Aeppli, L. (1969b) Akute Toxizität, Ratte per os, A 3628 E (0 40
WP). Unveröffentlicht. Geigy, J.R. AG.
Aeppli, L. (1970a) Akute Toxizität, Ratte per os, A 3628
blaugefärbt. Unveröffentlicht. Geigy, J.R. AG.
Aeppli, L. (1970b) Akute Toxizität, Ratte per os, A 3389 C (= 40
ES). Unveröffentlicht. Geigy, J.R. AG.
Arai, M. (1981). In vivo short term study on the promoting
activity of methidathion (CG-831) (translation). Unpublished report
dated October 31, 1981 from Daiyu-Kai Ikagaku Kenkyu-Sho, Japan.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Arni, P. & Muller, D. (1980a). Salmonella/mammalian-microsome
mutagenicity test with GS 13005 (Test for mutagenic properties in
bacteria). Project No. 79/1556. Unpublished report dated April 17,
1980 from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Arni, P. & Muller, D. (1980b). Salmonella/mammalian-microsome
mutagenicity test with GS 13005 (Test for mutagenic properties in
bacteria). Project No. 801488. Unpublished report dated October 29,
1980 from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Arni, P. & Muller, D., 1980c. Intrasanguine host-mediated assay with
S. typhimurium with GS 13005 (Test for the demonstration of point
mutations in bacteria in vivo). Project No. 801494. Unpublished
report dated October 31, 1980 from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Arni, P. & Muller, D. (1981). Mutagenicity test on Saccharomyces
cerevisiae MP-1 in vitro with GS 13005 (Test for mutagenic
properties in yeast cells). Project No. 802030. Unpublished report
dated November 5, 1981 from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Bathe, R. (1973a). Acute oral LD50 of technical GS 13005 in the
rat. Unpublished report dated May 25, 1973 from Ciba-Geigy Ltd.,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Bathe, R. (1973b). Acute dermal LD50 of technical GS 13005 in the
rat. Project No. Siss 2929. Unpublished report dated October 3, 1973
from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Bathe, R. & Sachsse, K. (1975). Acute dermal LD50 in the rat of
technical GS 13005. Project No. Siss 4650. Unpublished report dated
September 15, 1975 from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Bathe, R. & Sachsse, K. (1980a). Acute oral LD50 in the rat of
technical GS 13005. Project No. 791668. Unpublished report dated
January 9, 1980 from Ciba-Geigy Ltd., Basle, Switzerland. Submitted
to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Bathe, R. & Sachsse, K. (1980b). Acute oral LD50 in the rat of
technical GS 13005. Project No. 791667. Unpublished report dated
January 10, 1980 from Ciba-Geigy Ltd., Basle, Switzerland. Submitted
to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Cannelongo, B. (1984). Guinea pig skin sensitization. Supracide
technical FL 830958. Project No. 3152-83. Unpublished report dated
January 9, 1984 from Stillmeadow, Inc., Houston, USA. Submitted to
WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Chang J. and Walberg, J. (1991). One-year dietary study in Beagle
dogs. Project No. F00028. Unpublished report dated June 24, 1991
from Ciba-Geigy Corporation, Framington, USA. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Chang, J. & Wyand, S. (1990). 90-day oral toxicity study in Beagle
dogs. Project No. F-00023. Unpublished report dated March 21, 1990
from Ciba-Geigy Corporation, Farmington, USA. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Chen, H., Hsueh, J., Sirianni, S., & Huang, C. (1981). Induction of
sister-chromatid exchanges and cell cycle delay in cultured
mammalian cells treated with eight organophosphorus pesticides.
Mutation Research, 88: 307-316.
Coulston, F. (1970). Effects on man of small daily doses of GS13005.
Unpublished report of Albany Medical College. Submitted to WHO in
1972 and cited in Annex I, 19.
Daiyu-Kai Institute of Medical Science (1983). In vivo short-term
study on dose-dependence and threshold level of promoting activity
of GS13005 in the rat liver. Unpublished report received June 1983
from Daiyu-Kai Institute of Medical Science, Japan. Submitted to WHO
by Ciba-Geigy Ltd., Basle, Switzerland.
Fabran, J.E., Golberg, L. and Coulston, F. (1971). Two-year safety
evaluation study of GS13005 on Rhesus monkey. Unpublished report of
Albany Medical College. Submitted to WHO in 1972, and cited in Annex
I, 19.
Folinusz, P., Huber, K., Schiavo, D., Hazelette, J., & Green, J.
(1986). Methidathion: 21-day dermal toxicity study in rabbits.
Project No. 86019. Unpublished report dated August 28, 1986 from
Ciba-Geigy Corporation, Summit, USA. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Fritz, H. (1976). Dominant lethal study on GS 13005 technical, mouse
(test for cytotoxic or mutagenic effects on male germinal cells).
Project No. 327633. Unpublished report dated August 3, 1976,
supplemented May 26, 1987, from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Goldenthal, E. (1986). Two-year dietary oncogenicity study in mice.
Project No. 382-087. Unpublished report dated March 7, 1986 from
International Research and Development Corporation, Mattawan, USA.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Hertner, T. & Arni, P. (1990). Autoradiographic DNA repair test on
rat hepatocytes. Project No. 891344. Unpublished report dated
February 6, 1990 from Ciba-Geigy Ltd., Basle, Switzerland. Submitted
to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Hess, R. (1975) Nature of the liver changes observed in a two-year
feeding study in dogs. Unpublished report from the
Toxicology/Pathology Dept., Ciba-Geigy. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Hool, G., Langauer, M., & Muller, D. (1980). Nucleus anomaly test in
somatic interphase nuclei of Chinese hamster. Project No. 800437.
Unpublished report dated July 2, 1980, supplemented May 26, 1987,
from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Hool, G. & Muller, D. (1980). Sister chromatid exchange study GS
13005 Chinese hamster (test for mutagenic effects of bone marrow
cells). Project No. 801489. Unpublished report dated November 4,
1980, supplemented May 26, 1987, from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Hummel, H., Youreneff, M., Giknis, M., Arthur, A. & Yau, E., 1987.
Methidathion: a teratology (segment II) study in rabbits. Project
No. 86131. Unpublished report dated January 13, 1987 from Ciba-Geigy
Corporation, Summit, USA. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Johnston, C.D. (1965) GS 13 005. Demyelination study in the chicken.
Report from Woodard Research Corp. (unpublished).
Johnston, C.D. (1967). GS 13 005. safety evaluation by two-year
feeding studies in rats and dogs. Final report from Woodard Research
Corp. (unpublished).
Lobdell, B.J. & Johnston, C.D. (1966). GS 13 005. Three-generation
reproduction study in the rat. Report from Woodard Research Corp.
(unpublished).
Mainiero, J., Levy, E., Infurna, R., & Yau, E. (1987). Methidathion
technical: a teratology (segment II) study in rats. Project No.:
86172. Unpublished report dated January 15, 1987 from Ciba-Geigy
Corporation, Summit, USA. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Marco, G. & Simoneaux, B. (1982). Dermal absorption of thiadiazole-
14C-methidathion by rats. Project No. ABR-81058. Unpublished
report dated April 22, 1982 from Ciba-Geigy Corporation, Greensboro,
USA. Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Noakes, D.N. & Sanderson, D.M. (1964) The toxicology of GS 13 005
and GS12968. Species variation in acute oral tosicity. Report from
Chesterford Park Research Station (unpublished).
Noakes, D.N. (1964) The toxicology of GS 13 005 and GS 12968. Tests
for delayed neurotoxic effects in the hen. Report from Chesterford
Park Research Station (unpublished).
Noakes, D.N. & Watson, W.A. (1964a) The toxicology of GS 13 005 and
GS 12968. Dietary toxicity to the rat: 6-month study. Report from
Chesterford Park Research Station (unpublished).
Noakes, D.N. & Watson, W.A. (1964b) The toxicology of GS 12 968 (NC
2962) and GS 13 005 (NC 2964). Cumulative oral toxicity to the rat.
Report from Chesterford Park Research Station (unpublished).
Osheroff, M. (1987). 21-day dermal toxicity study in rabbits with
methidathion technical. Project No. 483-254. Unpublished report
dated January 15, 1987 from Hazleton Laboratories America, Inc.,
Vienna, USA. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Payot, H.P., GS13005. Subchionische toxizitat am menschen (Einzel
und Selbstversuch). Unveröffentlichter Berict der Geigy J.R., Ag.
Submitted to WHO in 1972 and cited in Annex I, 19.
Puri E. & Muller D. (1982a). Autoradiographic DNA repair test on rat
hepatocytes GS 13005 ( in vitro test for DNA-damaging properties).
Project No. 820585. Unpublished report dated October 19, 1982 from
Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Puri E. & Muller D. (1982b). Autoradiographic DNA repair test on
human fibroblasts GS 13005 ( in vitro test for DNA-damaging
properties). Project No. 820586. Unpublished report dated October
18, 1982 from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO
by Ciba-Geigy Ltd., Basle, Switzerland.
Quest, J., Copley, M., Hamernik, K., Rinde, E., Fisher, B., Engler,
R., Burnam, W. & Fenner-Crisp, P. (1990). Evaluation of the
carcinogenic potential of pesticides, 2. Methidathion. Regulatory
Toxicology and Pharmacology, 12: 117-126.
Sachsse, K. (1971) Acute oral LD50 of technical GS 13 005 in the
dog. Report from Ciba-Geigy Ltd (unpublished).
Sachsse, K. (1973a). Irritation of technical GS 13005 in the rabbit
eye. Project No. Siss 2929. Unpublished report dated June 6, 1973
from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Sachsse, K. (1973b). Skin irritation in the rabbit after single
application of technical GS 13005. Project No. Siss 2929.
Unpublished report dated June 6, 1973 from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Sachsse, K. & Bathe, R. (1977a). The therapeutic activity of
atropine sulfate, pralidoxim (PAM) and toxogonin with regard to GS
13005 in the rat. Unpublished report dated December 5, 1977 from
Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Sachsse, K. & Bathe, R. (1977b). Potentiation study: CGA 15324
versus 2 insecticides, GS 13005 (methidathion) and G 24480
(diazinon) in the rat. Unpublished report dated February 15, 1977
from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Sachsse, K. & Bathe, R., 1978. CGA 20168 versus 6 insecticides, C
177 (DDVP), C 570 (phosphamidon), GS 13005 (methidathion), G 24480
(diazinon), CGA 15324 and malathion. Project No. 404478-Siss 6526.
Unpublished report dated June 23, 1978 from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Salamon, C. (1987). Two-generation reproduction study in albino rats
with methidathion technical. Project No. 450-2125. Unpublished
report dated January 15, 1987 from American Biogenic Corporation,
Decatur, USA. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Sarasin G. (1980). Acute dermal LD50 in the rat of GS 13005
technical Rohschmelze (=crude melt) (Ex Monthey). Project No.
801726. Unpublished report dated December 17, 1980 from Ciba-Geigy
Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Satou, S., Kimura, Y., Yamamoto, K. & Ichihara, A. (1979). In vitro
microbial assays for mutagenicity testing of DMTP (methidathion).
Project No. NRI-79-2884. Unpublished report dated August 31, 1979
from Nomura Research Institute, Japan. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Simmon, V., Poole, D. Nevell, G., & Skinner, W. (1977). In vitro
and in vivo microbiological assays of six Ciba-Geigy chemicals.
Project No. LSC-5686. Unpublished report dated March 1977 from
Stanford Research Institute, Menlo Park, USA. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Simoneaux, B. & Marco, G. (1984). Dermal absorption of thiadiazole-
14C-methidathion by mice. Project No. ABR-84015. Unpublished
report dated April 12, 1984 from Ciba-Geigy Corporation, Greensboro,
USA. Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Staley, J., Murphy, T., & Simoneaux, B. (1987). Metabolism of 14C
(Carbonyl) labelled methidathion in a goat. Project No. ABR-86112.
Unpublished report dated February 26, 1987 from Ciba-Geigy
Corporation, Greensboro, USA. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Stenger, E.G. & Roulet, F. (1963) Subchronische Toxizität, Ratte per
os, GS 13005 rein. Unveröffentlichter Bericht der Geigy, J.R. AG.
Stenger, E.G. (1964a) Akute Toxizität, Ratte per os, GS 13005 40 WP.
Unveröffentlichter Bericht der Geigy, J.R. AG.
Stenger, E.G. (1964b) Akute Atoxizität, Ratte per os, GS 13005 40
ES. Unveröffentlichter Bericht der Geigy, J.R. AG.
Stenger, E.G. & Roulet, F. (1965) Chronische Toxizität, Ratte,
Fütterungsversuche 4-22- Wochen, GS 13 005. Unveröffenlichter
Bericht der Geigy, J.R. AG.
Stenger, E.G. (1966a) Akute Toxizität, Ratte per os, GS 13 005 40
WP. Unveröffentlichter Bericht der Geigy, J.R. AG.
Stenger, E.G. (1966b) Akute Toxizität, Ratte per os, A 2041 C (= 40
ES). Unveröffentlichter Bericht der Geigy, J.R. AG.
Staley, J. & Simoneaux, B. (1987). Metabolism of methidathion
labelled with 14C on the ring carbon adjacent to the methoxy group
in a goat. Project No. ABR-86118. Unpublished report dated March 2,
1987 from Ciba-Geigy Corporation, Greensboro, USA. Submitted to WHO
by Ciba-Geigy Ltd., Basle, Switzerland.
Strasser, F. & Arni, P. (1990). Chromosome studies on Chinese
hamster ovary cell line CCL 61 in vitro. Project No. 891202.
Unpublished report dated January 30, 1990 from Ciba-Geigy Ltd.,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Strasser, F. & Muller, D. (1980). Point mutation assay with mouse
lymphoma cells, host mediated assay with GS 13005 (test for
mutagenic properties in mammalian cells). Project No. 801495.
Unpublished report dated October 21, 1980 from Ciba-Geigy Ltd.,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Szolics, I. & Simoneaux, B. (1985). Partitioning characteristics and
14C distribution in a chicken dosed with 2 -14C-methidathion for
16 days. Project No. ABR-85039. Unpublished report dated July 22,
1985 from Ciba-Geigy Corporation, Greensboro, USA. Submitted to WHO
by Ciba-Geigy Ltd., Basle, Switzerland.
Szolics, I. & Simoneaux, B. (1987a). Disposition of methidathion in
the rat. Project No.: ABR-86122. Unpublished report dated March 6,
1987 from Ciba-Geigy Corporation, Greensboro, USA. Submitted to WHO
by Ciba-Geigy Ltd., Basle, Switzerland.
Szolics, I. & Simoneaux, B. (1987b). The disposition of
radioactivity in rats dosed with carbonyl 14C-methidathion.
Project No. ABR-86084. Unpublished report dated February 26, 1987
from Ciba-Geigy Corporation, Greensboro, USA. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Szolics, I. & Simoneaux, B. (1987c). The disposition of
radioactivity in rats dosed with methoxy labelled 14C-
methidathion. Project No. ABR-86115. Unpublished report dated March
2, 1987 from Ciba-Geigy Corporation, Greensboro, USA. Submitted to
WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Szolics, I. & Simoneaux, B. (1987d). The disposition of
radioactivity in rats dosed with 14C-methidathion labelled in the
ring carbon adjacent to the methoxy group. Project No. ABR-86095.
Unpublished report dated February 26, 1987 from Ciba-Geigy
Corporation, Greensboro, USA. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Szolics, I. & Simoneaux, B. (1987e). Characterization of carbonyl
14C-labelled methidathion metabolites in rat urine. Project No.
ABR-86107. Unpublished report dated March 2, 1987 from Ciba-Geigy
Corporation, Greensboro, USA. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Szolics, I. & Simoneaux, B. (1987f). Characterization of methoxy
14C-labelled methidathion metabolites in rat urine. Project No.
ABR-86116. Unpublished report dated February 26, 1987 from Ciba-
Geigy Corporation, Greensboro, USA. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Szolics, I. & Simoneaux, B. (1987g). Characterization of 14C-
methidathion metabolites in rat urine - label 14C adjacent to the
methoxy group. Project No. ABR-86114. Unpublished report dated March
2, 1987 from Ciba-Geigy Corporation, Greensboro, USA. Submitted to
WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Szolics, I. & Simoneaux, B. (1987h). Characterization of 14C-
methidathion metabolites in goat urine. Project No. ABR-86113.
Unpublished report dated March 3, 1987 from Ciba-Geigy Corporation,
Greensboro, USA. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Teitelman, U., Adler, M., Levy, I., & Dikstein, S. (1975). Treatment
of massive poisoning by the organophosphate methidathion. Clinical
Toxicology, 8(3), 277-282.
Tong C. (1982a) The hepatocyte primary culture/DNA repair assay on
compound GS 13005 - 008266 using mouse hepatocytes in culture.
Project No. M 111881. Unpublished report dated February 10, 1982
from Naylor Dana Institute, Valhalla, USA. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Tong C. (1982b) The hepatocyte primary culture/DNA repair assay on
compound GS 13005 - 008266 using rat hepatocytes in culture. Project
No. M 111881. Unpublished report dated February 10, 1982 from Naylor
Dana Institute, Valhalla, USA. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Ullmann, L., Krinke, G., Sachsse, K. & Hess, R. (1977). Acute oral
toxicity and neurotoxicity study of technical GS 13005 in the
domestic fowl ( Gallus domesticus). Project No. Siss 5927.
Unpublished report dated November 8, 1977 from Ciba-Geigy Ltd.,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Yau, E., McMartin, D., Zaidi, I., & Green, J. (1986). Methidathion:
2-year oral oncogenicity and toxicity study in albino rats. Project
No. 86061. Unpublished report dated May 23, 1986 from Ciba-Geigy
Corporation, Summit, USA. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Zoppellari, R., Targa, L., Tonini, P., & Zatelli, R., 1990. Acute
poisoning with methidathion: A case. Human & Experimental
Toxicology, 9(6), 415-419.
Metabolites from the urine of rats administered single oral
doses of 14C-labelled methidathion in the ring carbon adjacent to
the methoxy group at 0.25 or 2.52 mg/kg bw (Szolics & Simoneaux,
1987d) were identified. Organic soluble metabolites represented 61-
63%, whereas aqueous soluble metabolites accounted for 37-39% of the
urinary radioactivity. The major organic metabolite was the sulfide
(35.4%). Similar amounts of the sulfoxide (8.6%) and the sulfone
(8.2%) were detected. The RH compound represented 2.1% of the
radioactivity, and the oxygen analog represented 0.6%. No unchanged
parent was identified. With respect to the aqueous soluble
metabolites, the desmonomethyl derivative of methidathion accounted
for 20% (Szolics & Simoneaux, 1987g).
Goats
The urine of a single lactating goat treated daily by capsule
equivalent to a dietary intake level of 5 ppm for 10 days (Staley
et al. 1987) was selected for characterization of metabolites.
Partitioning data revealed that 84.5% of the urinary radioactivity
were aqueous soluble and 5.5% were organic soluble metabolites. The
principal aqueous soluble metabolites were the desmonomethyl
derivative (59.7%) and cysteine conjugate (10.4%) of methidathion.
Chromatography of the organic soluble components reportedly showed
that the metabolites in the goat were qualitatively the same as
those found in rat urine. The proposed predominant metabolic pathway
of methidathion in the goat was 0-demethylation (Szolics &
Simoneaux, 1987h).
Toxicological studies
Acute toxicity studies
Acute toxicity studies conducted in both the male and female
Tif.RAIf rat (Table 1), reveal that technical methidathion, upon
oral administration is highly toxic with LD50 values of 26 to 43.8
mg/kg bw. The acute dermal studies indicate that methidathion is
slightly to moderately toxic. Clinical signs of toxicity, upon oral
and dermal dosing were generally manifest as curved or ventral body
position, dacryorrhea/chromodacryorrhea, diarrhoea, dyspnea,
exophthalmus, ruffled fur, sedation, tonic/clonic muscle spasms and
trismus. The symptoms were reversible in surviving animals.
The acute oral toxicity of methidathion has been investigated
in several animal species (Table 2). The results indicate that
methidathion is moderately to highly toxic in all species tested
with LD50 values ranging from 17 to 200 mg/kg bw.
Table 1. Acute toxicity of technical methidathion
Species Sex Route Vehicle LD50 Purity Reference
(strain) (mg/kg bw)
Rat M&F oral CMC, 2% 43.8 ? Bathe (1973a)
(Tif. RAIf)
Rat M&F oral PEG, 400 26 96.9% Bathe & Sachsse
(Tif. RAIf) (1980a)
Rat M&F oral PEG, 400 26 92.7% Bathe & Sachsse
(Tif. RAIf) (1980b)
Rat M&F dermal CMC, 2% 1546 ? Bathe, (1973b)
(Tif. RAIf)
Rat M&F dermal CMC, 2% 1663 ? Bathe & Sachsse
(Tif. RAIf) (1975)
Rat M&F dermal CMC, 2% + 297 92.6% Sarasin (1980)
(Tif. RAIf) Tween 80, 0.1%
Short-term toxicity studies
Rats
Five groups of ten male rats received by gavage 0, 0.25, 0.83,
2.5 or 8.3 mg methidiathion/kg bw/day five days a week for four
weeks. Signs of cholinesterase inhibition occurred during the first
week at the 8.3 mg/kg bw/day level, but not later in this or in
other groups. Dose-related cholinesterase inhibition occurred in RBC
and plasma, the no-effect level being 0.25 mg/kg bw/day. Plasma
cholinesterase had returned to normal three days after treatment was
stopped, but RBC cholinesterase had not reached normal figures after
21 days (Noakes & Watson, 1964b).
Five groups of five male and five female rats received by
gavage 0. 2.5, 5, 10 or 20 mg methidathion/kg bw/day six days a week
for four weeks. In the 10 and 20 mg/kg bw/day groups, four and nine
animals died, respectively. Body-weight gain was depressed in all
groups, but no relation to dosage was apparent. There was a slight
increase in fat deposition in the liver at 5 mg/kg bw/day and at the
highest level this was more marked (Stenger & Roulet, 1963).
Groups of 24 male and 24 female rats were fed for 22 weeks on
diets containing 0, 1, 4, 16 or 64 ppm methidathion. In a similar
study in the same laboratory, groups of 24 male and 24 female rats
were fed for 26 weeks on diets supplying 0, 128 or 256 ppm
methidathion. The rate of body-weight gain was reduced at 64 ppm and
above in females but not in males. Histopathological examination of
liver, spleen and kidneys showed a dose-related increase in fat
deposition in the liver at doses above 64 ppm in both sexes. No
abnormalities in haematological indices or in results of urinalyses
were found (Stenger & Roulet, 1965).
Table 2. Acute oral toxicity of methidathion1
Species Sex LD50 Reference
(mg/kg bw)
Mouse F 17 Noakes & Sanderson, 1964
Hamster F 30 Noakes & Sanderson, 1964
Rat M&F 20-81 Slenger, 1964a,b, 1966a,b;
Aeppli, 1969a,b; 1970a,b;
Noakes & Sanderson, 1964
Rat M 26-65 Slenger, 1964a
Noakes & Sanderson, 1964
Guinea-pig F 25 Slenger, 1964a
Noakes & Sanderson, 1964
Rabbit M 80 Sachsse, 1971
Dog M&F 200 Noakes & Sanderson, 1964
Chicken F 80 Annex I, 19
1 Formulations calculated as a.i.
Groups of 20 male and 20 female rats were fed for six months on
diets containing 0, 0.5, 2, 10, 50 or 250 ppm methidathion. At the
250 ppm level weight gain was slightly depressed and clinical signs
of cholinesterase inhibition were seen, particularly in females.
Plasma cholinesterase was inhibited in the 250 ppm group and
erythrocyte cholinesterase in groups receiving 10 ppm and above.
Experimental groups were similar to controls with regard to
survival, food intake, weights and microscopic appearance of liver,
kidneys, spleen and testes and the macroscopic appearance of other
organs (Noakes & Watson, 1964a).
Rabbits
A dermal study was undertaken with groups of 5 New Zeeland
rabbits/sex administered methidathion (purity unknown) topically for
6-h daily non-occlusive exposure periods at doses of 0 (vehicle
control, PEG 300), 1, 5 or 20 mg/kg bw/day for a period of 22
consecutive days. There were no significant effects of treatment on
survival, food consumption, haematology, blood chemistry,
cholinesterase activity, organ weights, gross morphologic or
histopathological alterations. Based on minimal effects noted as
hypoactivity in a single male at 20 mg/kg bw, occasional incidences
of soft faeces or diarrhoea in treated males and a slight trend to
decreased body-weight gain in the high dose 20 mg/kg bw/day treated
males, a conservative NOAEL may be set at 5 mg/kg bw/day (Folinusz
et al. 1986).
A second dermal study was conducted with groups of New Zeeland
HRP:SPF rabbits (4-6 per sex) topically administered methidathion
(95% purity) daily for a 6-h occlusive exposure period at doses of 0
(vehicle control, PEG 400), 1, 10, 40 or 80 mg/kg bw for a period of
21 days. Treatment with methidathion resulted in mortality in males
at all dose levels (0/5, 2/5, 2/6, 3/5 and 3/5) and in females at 40
mg/kg bw/day and higher (0/5, 0/5, 0/4, 2/5 and 4/5). Clinical signs
of toxicity in males treated at dose levels of 1 mg/kg bw/day and
higher and in females treated at dose levels of 40 and 80 mg/kg
bw/day were manifest as anorexia, ataxia, hunched posture, languid
appearance and laboured respiration. Tremors were observed at dose
levels of 10 mg/kg bw/day and higher, whereas convulsions were
reported in a single high-dose (80 mg/kg bw/day) treated female.
Cholinesterase assessments revealed significant depression in the
mean plasma (38-86%), RBC (40-80%) and brain (37-88%) cholinesterase
values for both sexes at dose levels of 10 mg/kg bw/day and higher.
Treatment-related microscopic alterations were evidenced in the
liver and gall bladder. Principal findings in the liver were denoted
as hepatocytic clearing and congestion at dose levels as low as 1
mg/kg bw/day. Capsular/subcapsular necrosis with acute inflammation
was also noted in several of the treated animals. Lesions of the
gall bladder were present in both sexes at dose levels of 10 mg/kg
bw/day and higher and were attributed to bile reflux due to
hyperperistalsis. Subacute inflammation of the myocardium and
degeneration of the medial aorta occasionally with mineralization
were observed in several of the rabbits dying during the study
period. There were no specific effects of treatment on body-weight,
food consumption, ophthalmoscopy, haematological and blood
biochemical parameters or organ weights. The NOAEL was determined to
be 1 mg/kg bw/day by the author (Osheroff, 1987).
The results of the present study when interpreted
independently, have not unequivocally demonstrated 1 mg/kg bw/day to
be a NOAEL. The author has provided valid argument that evaluation
of primary toxicity was complicated by the additive effects of
stress especially during clinical observations. The occlusive rubber
binding used may not only have enhanced the absorption of the test
material but in combination with the neck collar, may have increased
the stress factor, thus augmenting the toxicity of the test
material. In view of the intrinsic variables in the present study
design, it is considered appropriate to critically assess the
results of the present study in conjunction with those generated
from the previously conducted rabbit dermal study, wherein dermal
administration of methidathion under non-occlusive means failed to
produce effects in rabbits at dose levels as high as 5 mg/kg bw/day
(Folinusz et al. 1986).
Dogs
Four groups of three male and three female beagle dogs received
diets containing 0, 4, 16 or 65 ppm methidathion for two years. The
animals were starved of diet one day each week and received a double
ration on the next day. Administration of methidathion was
discontinued from week 16 to 19. Erythrocyte cholinesterase was
inhibited in the 64 ppm group, but brain cholinesterase was
unaffected by treatment. SGPT was markedly elevated in the 64 and 16
ppm groups and slightly raised in males of the 4 ppm group. During
weeks 16 to 19 these levels fell, but only the 4 ppm group returned
to normal. SGOT levels were not the same as controls at all
treatment levels, but serum alkaline phosphatase was elevated and
sulfobromophthalein retention increased in the 16 and 64 ppm groups.
The livers of dogs receiving 16 and 64 ppm were pigmented on
macroscopic examination. Microscopically, pigmentation could be seen
in macrophages and hepatic cells (principally centrilobular) in the
16 and 64 ppm groups, the intensity of deposit being dose-related.
The Perl's reaction showed that the pigment did not contain
appreciable quantities of iron. The kidneys of the 64 ppm group also
showed pigmentation. It was questionable whether the livers of the 4
ppm group contained excess pigment. Control and test groups were
indistinguishable regarding behaviour, results of clinical tests
including neurological examination, haematological findings, organ
weights and macroscopic and microscopic appearance of organs other
than those mentioned. An additional two dogs received 64 ppm
methidathion in the diet for four weeks. The SGOT was elevated at
two and four weeks and at autopsy the livers were dark in colour.
Moderate diffuse pigmentation was seen microscopically in the liver
of one animal.
The plasma enzyme and liver histology changes at 30 days of
treatment at 64 ppm were not increased after treatment for two years
at this dose. Furthermore, the serum enzyme changes at 16-19 weeks
of treatment at 64 ppm were reversible and returned to normal three
weeks after cessation of treatment. The NOAEL was 4 ppm (Johnston,
1967).
The histological slides of the liver tissue from the original
study were re-evaluated and submitted to the 1975 Joint Meeting
Annex 1, reference 25). Intrahepatic cholestasis was observed in
dogs fed 16 and 64 ppm methidathion. Neither degenerative nor
inflammatory changes were associated with this lesion. Pigmentation
occurred as bile-plugs in biliary ductules, or as amorphous deposits
in Kupffer cells or as lipofuscin in both hepatic and Kupffer cells.
Haemolysis was not detected. A minimal degree lipofuscin
pigmentation was also observed at 4 ppm and in the control group.
Mild irregular fatty changes of the hepatocytes with occasional
periportal histiolymphocytic infiltration of the liver were observed
in both treated and control animals (Hess, 1975).
Methidathion (97% purity) was administered to groups of 4
beagle dogs per sex in the daily feed at dietary levels of 0, 0.5,
4, 45 or 140 ppm equal to 0, 0.02, 0.16, 1.96 or 5.67 mg/kg bw/day,
respectively, for a period of 90 days. An additional group of 4 dogs
per sex was treated orally by gelatin capsule at a dose level of
0.14 mg/kg bw/day, equal to a daily dietary intake level of 4 ppm,
for a similar treatment duration. A NOAEL of 4 ppm was assessed
based upon the evidence of cholestasis in all male and female dogs
treated at 45 and 140 ppm. Cholestasis was similarly described in a
single male dog treated by capsule at 0.14 mg/kg bw/day. Other
consequences of treatment evident in both sexes at dietary levels of
45 ppm and higher were discoloration of the liver and markedly
enhanced enzyme activity, expressed as increased levels of ALP,
SGOT, SGPT, GGT and sorbitol dehydrogenase. RBC cholinesterase
activity was significantly (75-88%) inhibited in dogs of both sexes
treated at 140 ppm. Brain cholinesterase activity was inhibited
(26.8%) in the 140 ppm treated females when compared to the
controls. There were no effects of methidathion treatment on serum
cholinesterase levels. Clinical signs of tremor and reduced activity
post-dosing, observed during the latter part of the study, were
exhibited in a single male dog at the high dose level. Reduced
activity was also noted in a single male treated at 45 ppm. Other
effects of treatment were significantly decreased mean food intake
values in the high dose treated males when compared to the control
group. There were no treatment-related effects observed with respect
to survival, body-weights, ophthalmoscopic examination,
haematological parameters, urinalysis or organ weights (Chang &
Wyand, 1990).
Six groups of 4 beagle dogs/sex were treated with methidathion
(96% purity) at dietary levels of 0, 0.5, 2, 4, 40 or 140 ppm, equal
to 0, 0.02, 0.07, 0.15, 1.34 or 4.51 mg/kg bw/day, respectively, for
a period of 12 months. A NOAEL of 4 ppm was indicated based on
treatment-related hepatic effects observed grossly as general
discoloration of the liver and characterized histomorphologically as
cholestasis and chronic inflammation of the liver in both sexes at
dietary levels of 40 ppm and higher. Associated changes in blood
biochemical parameters were denoted by elevated ALP, SGOT, SGPT,
sorbitol dehydrogenase and bilirubin levels. Other significant
clinical changes recorded only in the females were increased GGT and
decreased total protein and albumin values. RBC choline-sterase
activity was markedly inhibited (76-87%) in both male and females
dogs treated with methidathion at 140 ppm. Serum cholinesterase
activity was not adversely affected by treatment at any dietary
level. Brain cholinesterase activity was significantly depressed
(16-27%) in both sexes treated at 140 ppm when compared to the
controls. Another effect of treatment was related to decreased food
consumption in male dogs treated with methidation at a dietary level
of 140 ppm. There were no effects of treatment on survival, clinical
signs, body-weights, ophthalmoscopy, haematology, urinalysis, faecal
examination or organ weights (Chang and Walberg, 1991).
Monkeys
Three groups of Rhesus monkeys (approximately equal number of
each sex) were administered 0, 0.25 or 1.0 mg methidathion/kg bw/day
by stomach tube, 6 days a week for 23 months. Two of each group were
autopsied after 12 months. Plasma and erythrocyte cholinesterase
activity were inhibited in the 1 mg/kg bw/day group, but not at the
lower level. Brain cholinesterase was unaltered by treatment.
Growth, results of haemological tests, results of chemical analyses
of serum (including SGPT and ALP) and macro- and microscopic
examination of tissues were similar in control and test groups
(Fabran et al., 1971).
Long-term toxicity/carcinogenicity studies
Mice
A 23-month study was conducted with groups of 50 Charles River
CD-1 mice per sex/group fed diets containing methidathion (purity
not stated) at levels of 0, 3, 10, 50 or 100 ppm equal to 0.43,
1.42, 6.99 or 13.70 mg/kg bw/day, respectively. An additional 120
mice/sex/group were assigned to the chronic phase of this bioassay.
Interim sacrifices of 20 mice/sex/group were scheduled after 3, 6,
12 and 13 months. Animals sacrificed at 13 months were maintained on
control diet for one month as recovery animals following 12 months
of continuous treatment. The remaining animals (40/sex/group,
maximum) were sacrificed after 18 months of treatment. Treatment of
mice with methidathion resulted in slightly decreased survival of
the 100 ppm treated males when compared to the controls. The only
clinical sign remarked upon was discoloration of the urine in the 50
and 100 ppm treated males. Potentially reversible increases in liver
enzyme activity were reported in males at 50 ppm and higher and in
females at 100 ppm. Significantly decreased but potentially
reversible RBC cholinesterase activity values (26-46%) were
registered in males at 100 ppm and in females at 50 ppm and higher.
Brain cholinesterase activity was markedly inhibited (15-49%) in
both sexes at 100 ppm. There were no inhibitory effects of treatment
on plasma cholinesterase activity. There were no effects of
methidathion treatment noted with respect to body-weights, food and
water consumption or ophthalmoscopy. Treatment-related target organ
alterations were manifest in the gall bladder and liver at dietary
levels of 50 ppm and higher in males and in females at 100 ppm.
Microscopic changes were described in the gall bladder as
cholecystitis and hyperplasia and, hepatic findings were denoted as
bile duct hyperplasia, bile stasis, cholangiofibrosis, chronic
hepatitis and hypertrophy. Increased extramedullary haematopoiesis
of the spleen, associated with increased spleen weights were
observed in the 100 ppm treated males. Treatment of male mice with
methidathion resulted in a significantly increased incidence of
hepatocellular tumours (adenomas, carcinomas and adenomas/carcinomas
combined).
0 3 10 50 100 ppm
Adenoma 1/46 9/45 7/47 8/43 21/45
Carcinoma 8/46 6/45 4/47 13/43 17/45
Combined 9/46 15/45 11/47 21/43 38/45
Historical control data (Quest et al. 1990) generated from 14
studies conducted at the same test facility with the same strain of
mouse, revealed that the incidences of hepatocellular tumours
reported in the present study exceeded the historical control range
at dietary levels of 50 ppm and higher. Latency, expressed in terms
of time to appearance of first tumour, was not apparently decreased
in the treated male groups when compared to the concurrent controls.
The incidence of hepatocellular tumours was not increased in female
mice treated with methidathion The NOAEL in this study was 10 ppm,
equal to 1.4 mg/kg bw/day (Goldenthal, 1986).
Rats
In order to investigate the long-term toxic effects and
possible carcinogenic action of methidathion, four groups of 25 male
and 25 female rats each were fed for three weeks on diets containing
0, 2, 8 or 32 ppm and for a further 101 weeks on diets containing 0,
4, 16 or 64 ppm methidathion. The rate of gain in body weight was
reduced from week 8 in male animals of the 64 ppm group. After the
first year the rates of gain became erratic in all groups, making
interpretation of the findings difficult. Female rats on test diets
grew at a rate comparable to controls. Erythrocyte cholinesterase
was inhibited in the 16 and 64 ppm groups, while plasma
cholinesterase showed minimal inhibition at 100 weeks in the 64 ppm
group only. Brain cholinesterase was inhibited in the 64 ppm group,
with marginal and no reduction in the 16 and 4 ppm groups,
respectively. Decreased relative adrenal weights were found in
females of the 16 ppm and 64 ppm groups, and decreased ovary weights
in the 64 ppm group. The relative kidney weights of males was
increased in the 16 and 64 ppm groups. A greater frequency of
hepatic degenerative changes was noted in rats fed methidathion in
the diet; the high incidence of pulmonary infections in the rats
renders this finding of doubtful toxicological significance. The
food intake, results of haematological investigations and chemical
analysis of serum (including SPGT) and the survival rate were
similar in the test groups and in the control group. The incidence
of tumours was variable between groups but was low and not dose-
related, and no unusual tumours were found. The NOAEL in this study
was 4 ppm methidathion in the diet (Johnston, 1967).
Groups of 65 Crl:COBS CD(SD) BR rats/sex were treated with
methidathion (97.3% purity) at dietary levels of 0, 4, 40 or 100 ppm
equal to 0.16, 1.72 or 4.91 mg/kg bw/day, respectively for a period
of 104 weeks. For interim sacrifice purposes, an additional 15
rats/sex/group were assigned on study. At 52 weeks, 10
rats/sex/group were sacrificed with the remaining 5 rats/sex/ group
sacrificed at week 93 of study. Treatment with methidathion resulted
in clinical signs expressed in the 40 and 100 ppm groups as
alopecia, chromorhinorrhea, hyperactivity, skin lesions, tremors,
hypersensitivity to the touch and fasciculation. Mean body-weights
and weight gains were decreased in both sexes at 100 ppm throughout
the course of treatment. Body-weight gains were decreased at a
dietary level of 40 ppm during the initial few weeks of treatment,
with comparable weight gain thereafter. Mean food intake was
significantly increased in both sexes treated at dietary levels of
40 ppm and higher, whereas water consumption was decreased at 100
ppm and occasionally at 40 ppm. Treatment-related haematological
findings were exhibited at a dietary level of 100 ppm as inverted
neutrophil:lymphocyte ratios, reduced RBC parameters and increased
platelet counts. Variations in blood biochemical parameters were
observed at 40 ppm and higher as decreased total bilirubin,
decreased total protein as well as changes in electrolyte levels
(decreased potassium and calcium, and increased inorganic phosphorus
and chloride). Significant inhibition of RBC (14-38%), serum (22-
66%) and brain (42-74%) cholinesterase activity were determined in
both sexes of rats treated at dietary levels of 40 ppm and higher.
Notable changes in urinalyses were revealed as decreased volume and
increased specific gravity at 40 ppm and higher which correlated
well with the reduced fluid intake reported in these groups. Gross
necropsy findings showed an increased incidence of skin lesions in
the 40 and 100 ppm levels which were associated microscopically with
ulceration and/or chronic purulent inflammation. Other pathological
alterations were related to an increased incidence in focal
accumulations of foamy macrophages in the alveoli of the 100 ppm
treated males and females. There were no effects of treatment on
survival or ophthalmoscopy. The NOAEL for in-life parameters was
determined to be 4 ppm, equal to 0.16 mg/kg bw/day. There was no
evidence of methidathion-induced carcinogenic potential (Yau et
al., 1986).
Reproduction studies
Rats
In a three-generation study, three groups of 10 male and 20
female rats were fed on a diet containing 0, 2 or 16 ppm
methidathion for three weeks, and thereafter on a diet containing 0,
4 or 32 ppm methidathion. Litters from the second mating were used
to provide the new generations. The F1b litters did not receive
test diets until 26 days and the F2b until 22 days after weaning.
F0, Flb and F2b generations received diets for 27-28 weeks
during which they produced two litters. The number of young
surviving at weaning was reduced in all generations of litters from
animals fed 32 ppm methidathion and the mean liver weight of F3b
weanlings of this group was slightly increased. Body weights,
reproductive capacity and mortality of parents and the number of
litters, litter size, mean birth and weaning weights of test groups
were comparable to controls. The number of stillbirths and incidence
of congenital abnormalities were unaltered by treatment. No
histological damage was found in the organs of the F3b animals
examined. The NOAEL in this study was 4 ppm methidathion (Lobdell &
Johnston, 1966).
A two-generation (one litter per generation) reproduction study
was conducted with groups of 15 male and 30 female Charles River SD
CR1:CD BR rats fed methidathion (purity not specified) at dietary
levels of 0, 5, 25 or 50 ppm equal to 0, 0.43, 2.08 or 4.23 mg/kg
bw/day, respectively. With respect to reproductive performance,
statistically (p < 0.05) reduced mating indices were recorded for
the 25 and 50 ppm groups in the F1 generation. Although the mating
indices were reduced in these groups (69% and 66%, respectively)
relative to the concurrent F1 control group (88%), they were
nevertheless not significantly different from those calculated for
the F0 generation control (73%) or treated groups (60-65%). The
fertility and gestation indices were comparable among groups.
Treatment-related effects on maternal animals fed dietary levels of
25 ppm and higher were revealed by clinical signs of toxicity
manifest during lactation as slight and/or intermittent tremors.
Mean body-weights of F1 50 ppm-treated males were decreased prior
to and during the 12-week premating period. Mean body-weights gains
were not, however, significantly different from controls. Body-
weights of females recorded during lactation of both generations
were decreased at 50 ppm when compared to the concurrent control.
Decreased ovary weights recorded in females at 25 and 50 ppm were
not correlated with histopathological changes. Effects of treatment
on progeny were expressed as decreased survival in the 50 ppm group
of the F1 generation, decreased body-weights and clinical signs in
both generations at 25 and 50 ppm. The clinical signs were
suggestive of poor maternal care and were described as
weakness/lethargy, coolness to the touch and starving appearance. On
the basis of the reported findings, a NOAEL of 5 ppm, equal to 0.43
mg/kg bw/day, was indicated (Salamon, 1987).
Special studies on teratogenicity
Rats
A teratology study was conducted with groups of 25 mated female
Crl: COBS CD(SD)BR rats administered methidathion (93.2-96% purity)
at 0.25, 1.0 and 2.5 mg/kg bw/day or the control (vehicle, 3%
aqueous cornstarch with 0.5% Tween 80) orally by gavage from days 6
to 15 of gestation, inclusive. Confirmation of mating was determined
by presence of sperm in the vaginal washing, and this day was
designated day 0 of gestation. A NOAEL for maternal toxicity was
indicated at 1.0 mg/kg bw/day based on mortality, decreased body-
weights, food intake and clinical signs at 2.5 mg/kg bw/day.
Clinical signs of toxicity in the high dose (2.5 mg/kg bw/day) group
were suggestive of organophosphorus intoxication including,
lethargy, tremors, lacrimation, salivation, raspy respiration,
exophthalmia, chromodacryorrhea. A NOAEL for embryofetal toxicity
was set at the highest dose level tested of 2.5 mg/kg bw/day. There
were no significant differences noted with respect to the mean
number of implantations, live fetuses or mean number of resorptions
on a litter basis. There was similarly no significant variability in
the post-implantation loss, fetal sex ratio or in the mean fetal
litter weight in the treated groups when compared to the control.
Treatment with methidathion failed to uncover any evidence of
teratogenic potential (Mainiero et al. 1987).
Rabbits
Methidathion (purity not specified) was administered orally by
gavage at 0 (vehicle control, 3% cornstarch with 0.5% Tween 80), 2,
6 or 12 mg/kg bw/day to groups of 19 artificially inseminated New
Zeeland white rabbits on days 7 through 19 of gestation. The day of
artificial insemination was designated as day 0 of gestation.
Treatment-related clinical signs of toxicity at the high dose (12
mg/kg bw/day) treated animals were manifest as ataxia, tremors,
salivation and miosis. In the absence of similar effects in the
control or other treated groups, the NOAEL for maternal toxicity was
demonstrated to be 6 mg/kg bw/day. There was no evidence of
embryofetal developmental toxicity or potential for teratogenicity
at any of the dose levels investigated, including the highest dose
of 12 mg/kg bw/day (Hummel et al. 1987).
Special studies on genotoxicity
A battery of mutagenicity assays were conducted with technical
methidathion, the results of which are presented in Table 3. The
tests performed to evaluate potential for gene mutation in bacteria,
DNA damage as well as chromosome aberration were negative. A
dominant lethal study in mice was also negative. Positive responses
were observed in the in vitro assays with Saccharomyces
cerevisiae and in the Chinese hamster test for sister chromatid
exchange. In vivo tests conducted to examine the similar
endpoints, did not however elicit any evidence of mutagenic
potential in mammalian cells.
Special studies on delayed neurotoxicity
Four adult hens received four subcutaneous injections of 50 mg
methidathion/kg body-weight (the maximum tolerated dose) at weekly
intervals and they were observed for a further four weeks. The signs
of acute poisoning lasted two to three days each time, but birds
remained in good condition and no paralysis developed.
Neuropathological examinations were not performed (Noakes, 1964).
Five groups of ten adult hens were fed diets containing 0, 16,
52 or 160 ppm methidathion or 316 ppm tri-orthocresylphosphate for
45-47 days. No abnormal neurological signs were found in birds fed
methidathion, but those on tri-orthocresylphosphate showed leg
weakness, lack of balance and ataxia during the final week of
treatment. Unequivocal evidence of demyelination of neural tissue
was not found in methidathion or tri-orthocresylphosphate-treated
animals (Johnston, 1965).
The delayed neurotoxic potential of methidathion (purity not
specified) was investigated in groups of 10-30 female white leghorn
hens. The animals were administered the test material twice, 21 days
apart, at 0 (vehicle, CMC 2%), 43.75, 87.5, 175 or 350 mg/kg bw.
Animals receiving methidathion at 350 mg/kg bw were pretreated with
an intramuscular injection of atropine sulfate, 10 mg/kg bw, one
hour prior to treatment. Groups of 3 male and 3 female hens received
a single oral dose of the positive control, TOCP at 215, 464, 600,
1000 or 2150 mg/kg bw and were then observed for 21 days post-
dosing. A preliminary acute oral study indicated that the LD50 of
methidathion in the hen was 175 mg/kg bw. Clinical signs of
intoxication were expressed as ataxia, slight tremor, curved or
ventral body position and sedation, with subsequent recovery in
surviving animals, 8-10 h post-dosing. Histopathological evaluation
of the spinal cord and peripheral nerves did not reveal any
treatment-related lesions of the nervous system, whereas TOCP
toxicity was evidenced as central-peripheral, distally accentuated
neuropathy. Treatment with methidathion did not produce any signs of
delayed neurotoxicity (Ullmann et al. 1977).
Table 3. Genotoxicity of technical methidathion
Test Test system Concentration Purity Results References
(vehicle)
Reverse mutation S. typhimurium 25, 75, 225, 675, 2025 98.4% negative Arni & Muller (1980a)
(in vivo) TA98, 100, 1535, 1537 µg/0.1 mL (DMSO) 1., 2.
S. typhimurium 25, 75, 225, 675, 2025 93.4% negative Arni & Muller (1980b)
TA98, 100, 1535, 1537 µg/0.1 mL (DMSO) 1., 2.
S. typhimurium 0, 0.1, 0.5, 1, 5, 10, 50 99.95% negative Satou et al. (1979)
TA98, 100, 1535, 1537 mg/mL (DMSO) 1., 2.
E. coli
B/r WP2 Try- Hcr-
S. typhimurium 10, 50, 100, 500, 1000, ? negative Simmon et al. (1977)
TA98, 100, 1535, 1537, 5000 µg/plate (DMSO) 1., 2.
1538
Rec assay (in vitro) B. subtilis 5, 25, 100 mg/mL (DMSO) 99.95% negative Satou et al. (1979)
H17, M45
Reverse mutation/ S. typhimurium 10, 20, 40 mg/kg bw ? negative Simmon et al. (1977)
Host-mediated TA1535, 1538/Male Swiss (single oral doses)
(in vivo) Webster mouse
(inocculated ip)
5, 10, 20 mg/kg bw negative
(5 oral doses) (DMSO)
S. typhimurium 0, 5, 10, 20 mg/kg bw 93.4% negative Arni & Muller (1980c)
TA98, 100, 1537/ (3 oral doses: 2 h, 1 h
male mouse and prior to innoculation)
(inocculated iv) (CMC, 0.5%)
Table 3 (cont'd)
Test Test system Concentration Purity Results References
(vehicle)
Gene conversion/ S. cerevisiae MP-1 675, 1250, 2500, 5000, 93.4 conversion: Arni & Muller (1981)
forward mutation 10000 µg/mL (DMSO) slight (+ ve)
(in vivo) mutation:
(+ ve)
Forward mutation Mouse lymphoma cells - 0, 15 mg/kg bw (3 oral 93.4% negative Strasser & Muller (1980)
host-mediated (in L5 178Y/mouse doses post-innoculation)
vivo) (DBA/Bom) (CMC)
DNA repair Mouse (male CD-1) 5 x 10-7 to 1% (DMSO) ? negative Tong (1982a)
(in vivo) hepatocytes
Rat (male F344) 5 x 10-9 to 1% (DMSO) ? negative Tong (1982b)
hepatocytes
Rat hepatocytes 0.128, 0.64, 3.2, 16 97.2% negative Puri & Muller (1982a)
µg/mL (DMSO)
Rat hepatocytes 1.85, 5.56, 16.67, 50, 100, 96% negative Hertner & Arni (1990)
200 µg/mL (DMSO)
Human fibroblasts 1.024, 5.12, 25.6, 128 ? negative Puri & Muller (1982b)
µg/mL (DMSO)
SCE (in vivo) Chinese hamster 0, 10, 20, 40, 80 µg/mL 99.1% slight (+ ve) Chem et al. (1981)
cell line V79 (DMSO) at 40, 80
ug/mL
SCE (in vivo) Chinese hamster 0, 17, 34, 68 mg/kg bw 93.4% negative Hool & Muller (1980)
(bone marrow) (CMC)
Table 3 (cont'd)
Test Test system Concentration Purity Results References
(vehicle)
Chromosome Chinese hamster ovary 43, 75, 87.5, 175, 350 96% negative Strasser & Arni (1990)
aberration CCL 61 µg/mL (DMSO)
(in vivo) 96.9% negative Hool et al. (1980)
Nucleus anomaly Chinese hamster 0, 17, 34, 68 mg/kg bw
(in vivo) (bone marrow) (2 oral doses) (CMC) 98.4% negative Fritz (1976)
Dominant lethal Mice, NMRI 0, 15, 45 mg/kg bw (CMC)
(in vivo)
1. = in the presence of metabolic activation
2. = in the absence of metabolic activation
Special studies on irritation and sensitization
The eye irritation potential of technical methidathion was
investigated in 3 English Silver strain rabbits/sex. Administration
of 0.1 grams of methidathion into the conjunctival sac of the left
eye (right eye served as control) produced irritating conjunctival
reaction in the unwashed eye of a single male rabbit (Sachsse,
1973a).
Technical methidathion (as a 50% polyethylene glycol
suspension) was applied under occlusive conditions to the shaved
backs of male and female English Silver strain rabbits for 24 h.
Skin reactions were observed as very slight erythema in one male and
moderate to severe erythema in association with slight oedema in one
female rabbit (Sachsse, 1973b).
The shaved backs of male Hartley albino guinea-pigs were
repeatedly treated with technical methidathion as a 10% solution in
diethyl ether. There was no evidence of skin sensitization potential
(Cannelongo, 1984).
Special studies on potentiation
The potentiating effects of methidathion (purity not specified)
with profenofos (Sachsse & Bathe, 1977b) and methacrifos (Sachsse &
Bathe, 1978) were investigated in male and female Tif:RAIf rats by
comparing the theoretical LD50 values, based on an assumption of
strictly additive toxicity, with that of the experimentally derived
LD50 values for the equitoxic mixtures. There was no potentiating
effect of methidathion with profenofos, whereas a slight enhancement
of acute toxicity was observed with an equitoxic mixture of
methidathion and methacrifos.
Special studies on promoting activity
Male F344 DuCrj strain rats received a single ip injection of
the initiator, N-nitrosodiethylamine (DEN) at 200 mg/kg bw. Two
weeks thereafter, the rats were treated with methidathion (92.7%
purity) at dietary levels of 0, 10, 30, 100 or 300 ppm (equal to 0,
1.0, 2.3, 7.6 or 22.9 mg/kg bw, respectively) or the positive
control, 3'-methyl-4-dimethyl amino azobenzene (3'-Me-DAB) at 600
ppm (equal to 33.9 mg/kg bw) for a period of 6 weeks. Three weeks
following treatment with DEN, partial hepatectomy was performed on
all animals. Histochemical evaluation of 4 liver sections from each
animal after 8 weeks on study, revealed a significantly increased
number of GGT positive foci per unit area in rats treated with
methidathion at 100 and 300 ppm and in the 600 ppm 3'-Me-DAB
positive control group. No increases in the number of GGT positive
foci were observed in additional groups of rats treated with
methidathion at 100 and 300 ppm alone, without previous initiation
with DEN. The number of foci in rats treated with methidathion at 30
ppm and lower were not different from the controls (Arai, 1981).
A second short-term study of the promoting activity in the rat
liver was conducted with groups of male Fischer 344 DuCrj strain
rats administered methidathion (98.6% purity) at dietary levels of
0, 35, 46, 59, 77 or 100 ppm (equal to 0, 2.7, 3.5, 4.8, 6.0 or 7.6
mg/kg bw/day, respectively) for a period of 6 weeks. Two weeks prior
to feeding with methidathion, the rats were pretreated with a single
ip injection of the initiator DEN at 200 mg/kg bw. Partial (two-
thirds) hepatectomy was performed three weeks after initiation and
the animals were sacrificed after 8 weeks on study. Histochemical
analysis unveiled a significant dose-related increase in the number
of GGT positive foci in rats treated with methidathion at dietary
levels of 59 ppm and higher. The number of GGT positive foci at
dietary levels of 46 ppm and lower were comparable to the controls.
No increases in the number of GGT positive foci were recorded in an
additional group of rats treated with methidathion at 100 ppm alone,
without previous initiation with DEN (Daiyu-Kai Institute of Medical
Science, 1983).
Special studies on therapeutic activity of antidotes
The therapeutic potential of antidotes was tested by
administering methidathion (purity not specified) orally by gavage
to Tif.RAIf rats at the LD80 of 46 mg/kg bw. The therapeutic
agents, atropine sulfate alone, toxogonin alone, PAM alone and
toxogonin combined with atropine sulfate were then administered
intramuscularly after the first appearance of clinical signs of
poisoning. A further aspect of therapeutics was investigated, by
administering 5 repeated daily oral doses of methidathion at the
LD50 (34 mg/kg bw) to rats and then, upon observation of clinical
signs, commencing with the respective combination of antidotes. All
therapeutic agents were, according to the treatment regimen,
effective against the oral LD80 of methidathion. A positive
protective effect against repeated LD50 doses of methidathion were
observed with atropine and toxogonin. PAM and toxogonin in
combination with atropine had minimal protective effect against
repeated challenges to methidathion (Sachsse & Bathe, 1977a).
Observations in humans
One male subject took 4 mg/day (equal to 0.04 mg/kg bw/day) for
17 days, and 8 mg/day (equal to 0.08 mg/kg bw/day) of methidathion
for 27 days. No effect was found on RBC and plasma cholinesterase,
the thrombocyte count and stability or on the clinical condition of
the subject (Payot, 1965).
Two groups of eight men received 0.04 or 0.11 mg
methidathion/kg bw/day orally in capsules for 6 weeks. Four men
received placebo capsules. The treatment with methidathion was
without effect on plasma and RBC cholinesterase, SGPT and SGOT and
results of urinalyses or on EEG pattern or clinical condition of the
subject (Coulston, 1970).
A case of massive poisoning was reported to have occurred where
a 25-year old man weighing 60 kg ingested a number of mouthfuls of
supracide 40 (methidathion, 40% active ingredient). The man was
discovered approximately 2 hours after the event, in an unconscious,
semi-comatose condition. Upon admission to hospital the patient was
treated intravenously with atropine and toxogonin. The
cholinesterase activity in the serum was found to be zero a few
hours after admission. The patient was discharged after 15 days of
hospitalization. The laboratory findings were normal with exception
of cholinesterase activity which remained low. Follow-up examination
at 2, 5 and 10 months revealed no abnormalities with no evidence of
delayed neurotoxicity (Teitelman et al. 1975).
A case of attempted suicide occurred in a 50-year old man
weighing 67 kg who had ingested approximately 6.2 g or 40 ml of
Supracid 20 (methidathion, 15.5% active ingredient). About 1.5 h
after the incident, the subject was admitted to hospital suffering
from mental confusion, muscle fasciculations, bradycardia, miosis,
sweating, salivation and lacrimation. The patient underwent gastric
lavage followed by treatment with atropine sulphate and pralidoxime.
Six hours after ingestion of methidathion, the incidence of muscle
fasciculations increased followed by bronchorrhea and coma. Serum
and RBC cholinesterase values, as measured over eight days following
poisoning were generally less than 50% of normal values.
Cholinesterase values assessed after 45 days were normal.
Lymphocytic NTE activity was normal as determined on days 5, 10, 17
and 45 after poisoning. The patient was discharged after 21 days of
hospitalization. A follow-up examination after 7 months failed to
establish any evidence of delayed polyneuropathy or other
neurological abnormality (Zoppellari et al. 1990).
COMMENTS
Methidathion was extensively absorbed when administered orally
to rats. The routes of elimination were via the urine and expired
CO2. There were no significant differences in elimination patterns
with regard to dose levels administered, pre-treatment or sex.
In the rat, the predominant urinary metabolites were the
sulfide, sulfoxide, sulfone and desmonomethyl derivative. Negligible
quantities of the parent and oxygen analog were detected in the
urine. The predominant metabolic pathway of methidathion in the goat
was via O-demethylation with the desmonomethyl derivative as the
principal urinary metabolite. Cysteine conjugates were identified in
each species.
Methidathion has a high acute oral toxicity. The World Health
Organization has classified methidathion as highly hazardous (WHO,
1992).
Methidathion was administered to dogs for 90 days at dietary
concentrations of 0, 0.5, 4, 45 or 140 ppm, or 0.14 mg/kg bw/day
(equal to 4 ppm) by capsule, and for a period of 12 months at 0,
0.5, 2, 4, 40, or 140 ppm in the diet. In both studies the dietary
NOAEL was determined to be 4 ppm, equal to 0.16 mg/kg bw/day, based
on liver effects, most notably cholestasis and increased liver
enzymatic activity in serum at dietary levels of 40 ppm and above.
Cholestasis was observed in a single male dog treated by capsule at
0.14 mg/kg bw/day. Erythrocyte and brain cholinesterase activities
were affected only at the highest level of 140 ppm.
Long-term dietary treatment of mice with methidathion for 23
months at 0, 3, 10, 50 or 100 ppm revealed an increased incidence of
hepatocellular tumours in males at 50 ppm and above, resulting in a
NOAEL of 10 ppm, equal to 1.4 mg/kg bw/day. Erythrocyte
cholinesterase was inhibited at 50 ppm (equal to 7 mg/kg bw/day) and
above, whereas brain cholinesterase activity was affected at 100 ppm
(equal to 13.7 mg/kg bw/day).
A 104-week long-term toxicity/carcinogenicity study in rats fed
methidathion at 0, 4, 40 or 100 ppm indicated a NOAEL of 4 ppm,
equal to 0.16 mg/kg bw/day, based on inhibition of erythrocyte and
brain cholinesterase activity at 40 ppm and above. Methidathion was
not carcinogenic in rats.
In a two-generation reproduction study in rats at dietary
concentrations of 0, 5, 25 or 50 ppm, the NOAEL was 5 ppm, equal to
0.43 mg/kg bw/day. At 25 ppm, reduced mating indices in the F1
generation and decreased progeny body weights were observed.
There were no teratogenic effects observed when methidathion
was administered by gavage to rats at doses of 0, 0.25, 1.0, or 2.5
mg/kg bw/day or to rabbits at doses of 0, 2, 6 or 12 mg/kg bw/day.
Maternal effects were demonstrated at the highest doses in both the
rat and rabbit as clinical signs of toxicity. In the rat, increased
mortality as well as decreased body-weights and food consumption
were also observed. The NOAELs in the rat and rabbit were determined
to be 1 and 6 mg/kg bw/day, respectively.
Treatment of hens with methidathion did not produce any
clinical or pathological evidence of delayed neurotoxicity.
In two reported cases of poisoning with methidathion, each of
the male subjects exhibited classic clinical and biochemical signs
of organophosphorus intoxication. Both subjects recovered, with no
evidence of delayed neurotoxicity uncovered upon follow-up
examination. In one of the cases jaundice was reported during the
recovery period.
Two human volunteer studies failed to reveal any inhibition of
erythrocyte or serum cholinesterase activity at doses up to 0.11
mg/kg bw/day.
After reviewing the available genotoxicity data, the Meeting
concluded that methidathion was not genotoxic.
In the dog, the effects on the liver appeared to have occurred
at levels lower than the levels causing inhibition of cholinesterase
activity, giving a NOAEL of 0.1 mg/kg bw/day. Whether or not there
was a relationship to the potential induction of liver effects in
man could not be ascertained.
The Meeting concluded, after consideration of the
hepatocellular tumours found in male mice in the long-term toxicity
study, together with the lack of genotoxicity, that methidathion did
not present a carcinogenic hazard for humans.
The previous ADI, based on a NOAEL in man of 0.11 mg/kg bw/day,
was revised. The revised ADI is based on the NOAEL in the dog and a
100-fold safety factor.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 10 ppm, equal to 1.4 mg/kg bw/day (23-month study)
Rat 4 ppm, equal to 0.16 mg/kg bw/day (104-week study)
5 ppm, equal to 0.43 mg/kg bw/day (reproduction
study)
Dog: 0.1 mg/kg bw/day (90-day, one-year, and two-year
studies)
Human: 0.11 mg/kg bw/day.
Estimate of acceptable daily intake for humans
0 - 0.001 mg/kg bw
Studies which will provide information valuable in the continued
evaluation of the compound
Further observations in humans
REFERENCES
Aeppli, L. (1969a) Akute Toxizität, Ratte per os, A 2039 E (0 40
WP). Unveröffentlicht. Geigy, J.R. AG.
Aeppli, L. (1969b) Akute Toxizität, Ratte per os, A 3628 E (0 40
WP). Unveröffentlicht. Geigy, J.R. AG.
Aeppli, L. (1970a) Akute Toxizität, Ratte per os, A 3628
blaugefärbt. Unveröffentlicht. Geigy, J.R. AG.
Aeppli, L. (1970b) Akute Toxizität, Ratte per os, A 3389 C (= 40
ES). Unveröffentlicht. Geigy, J.R. AG.
Arai, M. (1981). In vivo short term study on the promoting
activity of methidathion (CG-831) (translation). Unpublished report
dated October 31, 1981 from Daiyu-Kai Ikagaku Kenkyu-Sho, Japan.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Arni, P. & Muller, D. (1980a). Salmonella/mammalian-microsome
mutagenicity test with GS 13005 (Test for mutagenic properties in
bacteria). Project No. 79/1556. Unpublished report dated April 17,
1980 from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Arni, P. & Muller, D. (1980b). Salmonella/mammalian-microsome
mutagenicity test with GS 13005 (Test for mutagenic properties in
bacteria). Project No. 801488. Unpublished report dated October 29,
1980 from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Arni, P. & Muller, D., 1980c. Intrasanguine host-mediated assay with
S. typhimurium with GS 13005 (Test for the demonstration of point
mutations in bacteria in vivo). Project No. 801494. Unpublished
report dated October 31, 1980 from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Arni, P. & Muller, D. (1981). Mutagenicity test on Saccharomyces
cerevisiae MP-1 in vitro with GS 13005 (Test for mutagenic
properties in yeast cells). Project No. 802030. Unpublished report
dated November 5, 1981 from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Bathe, R. (1973a). Acute oral LD50 of technical GS 13005 in the
rat. Unpublished report dated May 25, 1973 from Ciba-Geigy Ltd.,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Bathe, R. (1973b). Acute dermal LD50 of technical GS 13005 in the
rat. Project No. Siss 2929. Unpublished report dated October 3, 1973
from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Bathe, R. & Sachsse, K. (1975). Acute dermal LD50 in the rat of
technical GS 13005. Project No. Siss 4650. Unpublished report dated
September 15, 1975 from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Bathe, R. & Sachsse, K. (1980a). Acute oral LD50 in the rat of
technical GS 13005. Project No. 791668. Unpublished report dated
January 9, 1980 from Ciba-Geigy Ltd., Basle, Switzerland. Submitted
to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Bathe, R. & Sachsse, K. (1980b). Acute oral LD50 in the rat of
technical GS 13005. Project No. 791667. Unpublished report dated
January 10, 1980 from Ciba-Geigy Ltd., Basle, Switzerland. Submitted
to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Cannelongo, B. (1984). Guinea pig skin sensitization. Supracide
technical FL 830958. Project No. 3152-83. Unpublished report dated
January 9, 1984 from Stillmeadow, Inc., Houston, USA. Submitted to
WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Chang J. and Walberg, J. (1991). One-year dietary study in Beagle
dogs. Project No. F00028. Unpublished report dated June 24, 1991
from Ciba-Geigy Corporation, Framington, USA. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Chang, J. & Wyand, S. (1990). 90-day oral toxicity study in Beagle
dogs. Project No. F-00023. Unpublished report dated March 21, 1990
from Ciba-Geigy Corporation, Farmington, USA. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Chen, H., Hsueh, J., Sirianni, S., & Huang, C. (1981). Induction of
sister-chromatid exchanges and cell cycle delay in cultured
mammalian cells treated with eight organophosphorus pesticides.
Mutation Research, 88: 307-316.
Coulston, F. (1970). Effects on man of small daily doses of GS13005.
Unpublished report of Albany Medical College. Submitted to WHO in
1972 and cited in Annex I, 19.
Daiyu-Kai Institute of Medical Science (1983). In vivo short-term
study on dose-dependence and threshold level of promoting activity
of GS13005 in the rat liver. Unpublished report received June 1983
from Daiyu-Kai Institute of Medical Science, Japan. Submitted to WHO
by Ciba-Geigy Ltd., Basle, Switzerland.
Fabran, J.E., Golberg, L. and Coulston, F. (1971). Two-year safety
evaluation study of GS13005 on Rhesus monkey. Unpublished report of
Albany Medical College. Submitted to WHO in 1972, and cited in Annex
I, 19.
Folinusz, P., Huber, K., Schiavo, D., Hazelette, J., & Green, J.
(1986). Methidathion: 21-day dermal toxicity study in rabbits.
Project No. 86019. Unpublished report dated August 28, 1986 from
Ciba-Geigy Corporation, Summit, USA. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Fritz, H. (1976). Dominant lethal study on GS 13005 technical, mouse
(test for cytotoxic or mutagenic effects on male germinal cells).
Project No. 327633. Unpublished report dated August 3, 1976,
supplemented May 26, 1987, from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Goldenthal, E. (1986). Two-year dietary oncogenicity study in mice.
Project No. 382-087. Unpublished report dated March 7, 1986 from
International Research and Development Corporation, Mattawan, USA.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Hertner, T. & Arni, P. (1990). Autoradiographic DNA repair test on
rat hepatocytes. Project No. 891344. Unpublished report dated
February 6, 1990 from Ciba-Geigy Ltd., Basle, Switzerland. Submitted
to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Hess, R. (1975) Nature of the liver changes observed in a two-year
feeding study in dogs. Unpublished report from the
Toxicology/Pathology Dept., Ciba-Geigy. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Hool, G., Langauer, M., & Muller, D. (1980). Nucleus anomaly test in
somatic interphase nuclei of Chinese hamster. Project No. 800437.
Unpublished report dated July 2, 1980, supplemented May 26, 1987,
from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Hool, G. & Muller, D. (1980). Sister chromatid exchange study GS
13005 Chinese hamster (test for mutagenic effects of bone marrow
cells). Project No. 801489. Unpublished report dated November 4,
1980, supplemented May 26, 1987, from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Hummel, H., Youreneff, M., Giknis, M., Arthur, A. & Yau, E., 1987.
Methidathion: a teratology (segment II) study in rabbits. Project
No. 86131. Unpublished report dated January 13, 1987 from Ciba-Geigy
Corporation, Summit, USA. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Johnston, C.D. (1965) GS 13 005. Demyelination study in the chicken.
Report from Woodard Research Corp. (unpublished).
Johnston, C.D. (1967). GS 13 005. safety evaluation by two-year
feeding studies in rats and dogs. Final report from Woodard Research
Corp. (unpublished).
Lobdell, B.J. & Johnston, C.D. (1966). GS 13 005. Three-generation
reproduction study in the rat. Report from Woodard Research Corp.
(unpublished).
Mainiero, J., Levy, E., Infurna, R., & Yau, E. (1987). Methidathion
technical: a teratology (segment II) study in rats. Project No.:
86172. Unpublished report dated January 15, 1987 from Ciba-Geigy
Corporation, Summit, USA. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Marco, G. & Simoneaux, B. (1982). Dermal absorption of thiadiazole-
14C-methidathion by rats. Project No. ABR-81058. Unpublished
report dated April 22, 1982 from Ciba-Geigy Corporation, Greensboro,
USA. Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Noakes, D.N. & Sanderson, D.M. (1964) The toxicology of GS 13 005
and GS12968. Species variation in acute oral tosicity. Report from
Chesterford Park Research Station (unpublished).
Noakes, D.N. (1964) The toxicology of GS 13 005 and GS 12968. Tests
for delayed neurotoxic effects in the hen. Report from Chesterford
Park Research Station (unpublished).
Noakes, D.N. & Watson, W.A. (1964a) The toxicology of GS 13 005 and
GS 12968. Dietary toxicity to the rat: 6-month study. Report from
Chesterford Park Research Station (unpublished).
Noakes, D.N. & Watson, W.A. (1964b) The toxicology of GS 12 968 (NC
2962) and GS 13 005 (NC 2964). Cumulative oral toxicity to the rat.
Report from Chesterford Park Research Station (unpublished).
Osheroff, M. (1987). 21-day dermal toxicity study in rabbits with
methidathion technical. Project No. 483-254. Unpublished report
dated January 15, 1987 from Hazleton Laboratories America, Inc.,
Vienna, USA. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Payot, H.P., GS13005. Subchionische toxizitat am menschen (Einzel
und Selbstversuch). Unveröffentlichter Berict der Geigy J.R., Ag.
Submitted to WHO in 1972 and cited in Annex I, 19.
Puri E. & Muller D. (1982a). Autoradiographic DNA repair test on rat
hepatocytes GS 13005 ( in vitro test for DNA-damaging properties).
Project No. 820585. Unpublished report dated October 19, 1982 from
Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Puri E. & Muller D. (1982b). Autoradiographic DNA repair test on
human fibroblasts GS 13005 ( in vitro test for DNA-damaging
properties). Project No. 820586. Unpublished report dated October
18, 1982 from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO
by Ciba-Geigy Ltd., Basle, Switzerland.
Quest, J., Copley, M., Hamernik, K., Rinde, E., Fisher, B., Engler,
R., Burnam, W. & Fenner-Crisp, P. (1990). Evaluation of the
carcinogenic potential of pesticides, 2. Methidathion. Regulatory
Toxicology and Pharmacology, 12: 117-126.
Sachsse, K. (1971) Acute oral LD50 of technical GS 13 005 in the
dog. Report from Ciba-Geigy Ltd (unpublished).
Sachsse, K. (1973a). Irritation of technical GS 13005 in the rabbit
eye. Project No. Siss 2929. Unpublished report dated June 6, 1973
from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Sachsse, K. (1973b). Skin irritation in the rabbit after single
application of technical GS 13005. Project No. Siss 2929.
Unpublished report dated June 6, 1973 from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Sachsse, K. & Bathe, R. (1977a). The therapeutic activity of
atropine sulfate, pralidoxim (PAM) and toxogonin with regard to GS
13005 in the rat. Unpublished report dated December 5, 1977 from
Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Sachsse, K. & Bathe, R. (1977b). Potentiation study: CGA 15324
versus 2 insecticides, GS 13005 (methidathion) and G 24480
(diazinon) in the rat. Unpublished report dated February 15, 1977
from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Sachsse, K. & Bathe, R., 1978. CGA 20168 versus 6 insecticides, C
177 (DDVP), C 570 (phosphamidon), GS 13005 (methidathion), G 24480
(diazinon), CGA 15324 and malathion. Project No. 404478-Siss 6526.
Unpublished report dated June 23, 1978 from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Salamon, C. (1987). Two-generation reproduction study in albino rats
with methidathion technical. Project No. 450-2125. Unpublished
report dated January 15, 1987 from American Biogenic Corporation,
Decatur, USA. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Sarasin G. (1980). Acute dermal LD50 in the rat of GS 13005
technical Rohschmelze (=crude melt) (Ex Monthey). Project No.
801726. Unpublished report dated December 17, 1980 from Ciba-Geigy
Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Satou, S., Kimura, Y., Yamamoto, K. & Ichihara, A. (1979). In vitro
microbial assays for mutagenicity testing of DMTP (methidathion).
Project No. NRI-79-2884. Unpublished report dated August 31, 1979
from Nomura Research Institute, Japan. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Simmon, V., Poole, D. Nevell, G., & Skinner, W. (1977). In vitro
and in vivo microbiological assays of six Ciba-Geigy chemicals.
Project No. LSC-5686. Unpublished report dated March 1977 from
Stanford Research Institute, Menlo Park, USA. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Simoneaux, B. & Marco, G. (1984). Dermal absorption of thiadiazole-
14C-methidathion by mice. Project No. ABR-84015. Unpublished
report dated April 12, 1984 from Ciba-Geigy Corporation, Greensboro,
USA. Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Staley, J., Murphy, T., & Simoneaux, B. (1987). Metabolism of 14C
(Carbonyl) labelled methidathion in a goat. Project No. ABR-86112.
Unpublished report dated February 26, 1987 from Ciba-Geigy
Corporation, Greensboro, USA. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Stenger, E.G. & Roulet, F. (1963) Subchronische Toxizität, Ratte per
os, GS 13005 rein. Unveröffentlichter Bericht der Geigy, J.R. AG.
Stenger, E.G. (1964a) Akute Toxizität, Ratte per os, GS 13005 40 WP.
Unveröffentlichter Bericht der Geigy, J.R. AG.
Stenger, E.G. (1964b) Akute Atoxizität, Ratte per os, GS 13005 40
ES. Unveröffentlichter Bericht der Geigy, J.R. AG.
Stenger, E.G. & Roulet, F. (1965) Chronische Toxizität, Ratte,
Fütterungsversuche 4-22- Wochen, GS 13 005. Unveröffenlichter
Bericht der Geigy, J.R. AG.
Stenger, E.G. (1966a) Akute Toxizität, Ratte per os, GS 13 005 40
WP. Unveröffentlichter Bericht der Geigy, J.R. AG.
Stenger, E.G. (1966b) Akute Toxizität, Ratte per os, A 2041 C (= 40
ES). Unveröffentlichter Bericht der Geigy, J.R. AG.
Staley, J. & Simoneaux, B. (1987). Metabolism of methidathion
labelled with 14C on the ring carbon adjacent to the methoxy group
in a goat. Project No. ABR-86118. Unpublished report dated March 2,
1987 from Ciba-Geigy Corporation, Greensboro, USA. Submitted to WHO
by Ciba-Geigy Ltd., Basle, Switzerland.
Strasser, F. & Arni, P. (1990). Chromosome studies on Chinese
hamster ovary cell line CCL 61 in vitro. Project No. 891202.
Unpublished report dated January 30, 1990 from Ciba-Geigy Ltd.,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Strasser, F. & Muller, D. (1980). Point mutation assay with mouse
lymphoma cells, host mediated assay with GS 13005 (test for
mutagenic properties in mammalian cells). Project No. 801495.
Unpublished report dated October 21, 1980 from Ciba-Geigy Ltd.,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Szolics, I. & Simoneaux, B. (1985). Partitioning characteristics and
14C distribution in a chicken dosed with 2 -14C-methidathion for
16 days. Project No. ABR-85039. Unpublished report dated July 22,
1985 from Ciba-Geigy Corporation, Greensboro, USA. Submitted to WHO
by Ciba-Geigy Ltd., Basle, Switzerland.
Szolics, I. & Simoneaux, B. (1987a). Disposition of methidathion in
the rat. Project No.: ABR-86122. Unpublished report dated March 6,
1987 from Ciba-Geigy Corporation, Greensboro, USA. Submitted to WHO
by Ciba-Geigy Ltd., Basle, Switzerland.
Szolics, I. & Simoneaux, B. (1987b). The disposition of
radioactivity in rats dosed with carbonyl 14C-methidathion.
Project No. ABR-86084. Unpublished report dated February 26, 1987
from Ciba-Geigy Corporation, Greensboro, USA. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Szolics, I. & Simoneaux, B. (1987c). The disposition of
radioactivity in rats dosed with methoxy labelled 14C-
methidathion. Project No. ABR-86115. Unpublished report dated March
2, 1987 from Ciba-Geigy Corporation, Greensboro, USA. Submitted to
WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Szolics, I. & Simoneaux, B. (1987d). The disposition of
radioactivity in rats dosed with 14C-methidathion labelled in the
ring carbon adjacent to the methoxy group. Project No. ABR-86095.
Unpublished report dated February 26, 1987 from Ciba-Geigy
Corporation, Greensboro, USA. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Szolics, I. & Simoneaux, B. (1987e). Characterization of carbonyl
14C-labelled methidathion metabolites in rat urine. Project No.
ABR-86107. Unpublished report dated March 2, 1987 from Ciba-Geigy
Corporation, Greensboro, USA. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Szolics, I. & Simoneaux, B. (1987f). Characterization of methoxy
14C-labelled methidathion metabolites in rat urine. Project No.
ABR-86116. Unpublished report dated February 26, 1987 from Ciba-
Geigy Corporation, Greensboro, USA. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Szolics, I. & Simoneaux, B. (1987g). Characterization of 14C-
methidathion metabolites in rat urine - label 14C adjacent to the
methoxy group. Project No. ABR-86114. Unpublished report dated March
2, 1987 from Ciba-Geigy Corporation, Greensboro, USA. Submitted to
WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Szolics, I. & Simoneaux, B. (1987h). Characterization of 14C-
methidathion metabolites in goat urine. Project No. ABR-86113.
Unpublished report dated March 3, 1987 from Ciba-Geigy Corporation,
Greensboro, USA. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Teitelman, U., Adler, M., Levy, I., & Dikstein, S. (1975). Treatment
of massive poisoning by the organophosphate methidathion. Clinical
Toxicology, 8(3), 277-282.
Tong C. (1982a) The hepatocyte primary culture/DNA repair assay on
compound GS 13005 - 008266 using mouse hepatocytes in culture.
Project No. M 111881. Unpublished report dated February 10, 1982
from Naylor Dana Institute, Valhalla, USA. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Tong C. (1982b) The hepatocyte primary culture/DNA repair assay on
compound GS 13005 - 008266 using rat hepatocytes in culture. Project
No. M 111881. Unpublished report dated February 10, 1982 from Naylor
Dana Institute, Valhalla, USA. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Ullmann, L., Krinke, G., Sachsse, K. & Hess, R. (1977). Acute oral
toxicity and neurotoxicity study of technical GS 13005 in the
domestic fowl ( Gallus domesticus). Project No. Siss 5927.
Unpublished report dated November 8, 1977 from Ciba-Geigy Ltd.,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Yau, E., McMartin, D., Zaidi, I., & Green, J. (1986). Methidathion:
2-year oral oncogenicity and toxicity study in albino rats. Project
No. 86061. Unpublished report dated May 23, 1986 from Ciba-Geigy
Corporation, Summit, USA. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Zoppellari, R., Targa, L., Tonini, P., & Zatelli, R., 1990. Acute
poisoning with methidathion: A case. Human & Experimental
Toxicology, 9(6), 415-419.
 Metabolites from the urine of rats administered single oral
doses of 14C-labelled methidathion in the ring carbon adjacent to
the methoxy group at 0.25 or 2.52 mg/kg bw (Szolics & Simoneaux,
1987d) were identified. Organic soluble metabolites represented 61-
63%, whereas aqueous soluble metabolites accounted for 37-39% of the
urinary radioactivity. The major organic metabolite was the sulfide
(35.4%). Similar amounts of the sulfoxide (8.6%) and the sulfone
(8.2%) were detected. The RH compound represented 2.1% of the
radioactivity, and the oxygen analog represented 0.6%. No unchanged
parent was identified. With respect to the aqueous soluble
metabolites, the desmonomethyl derivative of methidathion accounted
for 20% (Szolics & Simoneaux, 1987g).
Goats
The urine of a single lactating goat treated daily by capsule
equivalent to a dietary intake level of 5 ppm for 10 days (Staley
et al. 1987) was selected for characterization of metabolites.
Partitioning data revealed that 84.5% of the urinary radioactivity
were aqueous soluble and 5.5% were organic soluble metabolites. The
principal aqueous soluble metabolites were the desmonomethyl
derivative (59.7%) and cysteine conjugate (10.4%) of methidathion.
Chromatography of the organic soluble components reportedly showed
that the metabolites in the goat were qualitatively the same as
those found in rat urine. The proposed predominant metabolic pathway
of methidathion in the goat was 0-demethylation (Szolics &
Simoneaux, 1987h).
Toxicological studies
Acute toxicity studies
Acute toxicity studies conducted in both the male and female
Tif.RAIf rat (Table 1), reveal that technical methidathion, upon
oral administration is highly toxic with LD50 values of 26 to 43.8
mg/kg bw. The acute dermal studies indicate that methidathion is
slightly to moderately toxic. Clinical signs of toxicity, upon oral
and dermal dosing were generally manifest as curved or ventral body
position, dacryorrhea/chromodacryorrhea, diarrhoea, dyspnea,
exophthalmus, ruffled fur, sedation, tonic/clonic muscle spasms and
trismus. The symptoms were reversible in surviving animals.
The acute oral toxicity of methidathion has been investigated
in several animal species (Table 2). The results indicate that
methidathion is moderately to highly toxic in all species tested
with LD50 values ranging from 17 to 200 mg/kg bw.
Table 1. Acute toxicity of technical methidathion
Species Sex Route Vehicle LD50 Purity Reference
(strain) (mg/kg bw)
Rat M&F oral CMC, 2% 43.8 ? Bathe (1973a)
(Tif. RAIf)
Rat M&F oral PEG, 400 26 96.9% Bathe & Sachsse
(Tif. RAIf) (1980a)
Rat M&F oral PEG, 400 26 92.7% Bathe & Sachsse
(Tif. RAIf) (1980b)
Rat M&F dermal CMC, 2% 1546 ? Bathe, (1973b)
(Tif. RAIf)
Rat M&F dermal CMC, 2% 1663 ? Bathe & Sachsse
(Tif. RAIf) (1975)
Rat M&F dermal CMC, 2% + 297 92.6% Sarasin (1980)
(Tif. RAIf) Tween 80, 0.1%
Short-term toxicity studies
Rats
Five groups of ten male rats received by gavage 0, 0.25, 0.83,
2.5 or 8.3 mg methidiathion/kg bw/day five days a week for four
weeks. Signs of cholinesterase inhibition occurred during the first
week at the 8.3 mg/kg bw/day level, but not later in this or in
other groups. Dose-related cholinesterase inhibition occurred in RBC
and plasma, the no-effect level being 0.25 mg/kg bw/day. Plasma
cholinesterase had returned to normal three days after treatment was
stopped, but RBC cholinesterase had not reached normal figures after
21 days (Noakes & Watson, 1964b).
Five groups of five male and five female rats received by
gavage 0. 2.5, 5, 10 or 20 mg methidathion/kg bw/day six days a week
for four weeks. In the 10 and 20 mg/kg bw/day groups, four and nine
animals died, respectively. Body-weight gain was depressed in all
groups, but no relation to dosage was apparent. There was a slight
increase in fat deposition in the liver at 5 mg/kg bw/day and at the
highest level this was more marked (Stenger & Roulet, 1963).
Groups of 24 male and 24 female rats were fed for 22 weeks on
diets containing 0, 1, 4, 16 or 64 ppm methidathion. In a similar
study in the same laboratory, groups of 24 male and 24 female rats
were fed for 26 weeks on diets supplying 0, 128 or 256 ppm
methidathion. The rate of body-weight gain was reduced at 64 ppm and
above in females but not in males. Histopathological examination of
liver, spleen and kidneys showed a dose-related increase in fat
deposition in the liver at doses above 64 ppm in both sexes. No
abnormalities in haematological indices or in results of urinalyses
were found (Stenger & Roulet, 1965).
Table 2. Acute oral toxicity of methidathion1
Species Sex LD50 Reference
(mg/kg bw)
Mouse F 17 Noakes & Sanderson, 1964
Hamster F 30 Noakes & Sanderson, 1964
Rat M&F 20-81 Slenger, 1964a,b, 1966a,b;
Aeppli, 1969a,b; 1970a,b;
Noakes & Sanderson, 1964
Rat M 26-65 Slenger, 1964a
Noakes & Sanderson, 1964
Guinea-pig F 25 Slenger, 1964a
Noakes & Sanderson, 1964
Rabbit M 80 Sachsse, 1971
Dog M&F 200 Noakes & Sanderson, 1964
Chicken F 80 Annex I, 19
1 Formulations calculated as a.i.
Groups of 20 male and 20 female rats were fed for six months on
diets containing 0, 0.5, 2, 10, 50 or 250 ppm methidathion. At the
250 ppm level weight gain was slightly depressed and clinical signs
of cholinesterase inhibition were seen, particularly in females.
Plasma cholinesterase was inhibited in the 250 ppm group and
erythrocyte cholinesterase in groups receiving 10 ppm and above.
Experimental groups were similar to controls with regard to
survival, food intake, weights and microscopic appearance of liver,
kidneys, spleen and testes and the macroscopic appearance of other
organs (Noakes & Watson, 1964a).
Rabbits
A dermal study was undertaken with groups of 5 New Zeeland
rabbits/sex administered methidathion (purity unknown) topically for
6-h daily non-occlusive exposure periods at doses of 0 (vehicle
control, PEG 300), 1, 5 or 20 mg/kg bw/day for a period of 22
consecutive days. There were no significant effects of treatment on
survival, food consumption, haematology, blood chemistry,
cholinesterase activity, organ weights, gross morphologic or
histopathological alterations. Based on minimal effects noted as
hypoactivity in a single male at 20 mg/kg bw, occasional incidences
of soft faeces or diarrhoea in treated males and a slight trend to
decreased body-weight gain in the high dose 20 mg/kg bw/day treated
males, a conservative NOAEL may be set at 5 mg/kg bw/day (Folinusz
et al. 1986).
A second dermal study was conducted with groups of New Zeeland
HRP:SPF rabbits (4-6 per sex) topically administered methidathion
(95% purity) daily for a 6-h occlusive exposure period at doses of 0
(vehicle control, PEG 400), 1, 10, 40 or 80 mg/kg bw for a period of
21 days. Treatment with methidathion resulted in mortality in males
at all dose levels (0/5, 2/5, 2/6, 3/5 and 3/5) and in females at 40
mg/kg bw/day and higher (0/5, 0/5, 0/4, 2/5 and 4/5). Clinical signs
of toxicity in males treated at dose levels of 1 mg/kg bw/day and
higher and in females treated at dose levels of 40 and 80 mg/kg
bw/day were manifest as anorexia, ataxia, hunched posture, languid
appearance and laboured respiration. Tremors were observed at dose
levels of 10 mg/kg bw/day and higher, whereas convulsions were
reported in a single high-dose (80 mg/kg bw/day) treated female.
Cholinesterase assessments revealed significant depression in the
mean plasma (38-86%), RBC (40-80%) and brain (37-88%) cholinesterase
values for both sexes at dose levels of 10 mg/kg bw/day and higher.
Treatment-related microscopic alterations were evidenced in the
liver and gall bladder. Principal findings in the liver were denoted
as hepatocytic clearing and congestion at dose levels as low as 1
mg/kg bw/day. Capsular/subcapsular necrosis with acute inflammation
was also noted in several of the treated animals. Lesions of the
gall bladder were present in both sexes at dose levels of 10 mg/kg
bw/day and higher and were attributed to bile reflux due to
hyperperistalsis. Subacute inflammation of the myocardium and
degeneration of the medial aorta occasionally with mineralization
were observed in several of the rabbits dying during the study
period. There were no specific effects of treatment on body-weight,
food consumption, ophthalmoscopy, haematological and blood
biochemical parameters or organ weights. The NOAEL was determined to
be 1 mg/kg bw/day by the author (Osheroff, 1987).
The results of the present study when interpreted
independently, have not unequivocally demonstrated 1 mg/kg bw/day to
be a NOAEL. The author has provided valid argument that evaluation
of primary toxicity was complicated by the additive effects of
stress especially during clinical observations. The occlusive rubber
binding used may not only have enhanced the absorption of the test
material but in combination with the neck collar, may have increased
the stress factor, thus augmenting the toxicity of the test
material. In view of the intrinsic variables in the present study
design, it is considered appropriate to critically assess the
results of the present study in conjunction with those generated
from the previously conducted rabbit dermal study, wherein dermal
administration of methidathion under non-occlusive means failed to
produce effects in rabbits at dose levels as high as 5 mg/kg bw/day
(Folinusz et al. 1986).
Dogs
Four groups of three male and three female beagle dogs received
diets containing 0, 4, 16 or 65 ppm methidathion for two years. The
animals were starved of diet one day each week and received a double
ration on the next day. Administration of methidathion was
discontinued from week 16 to 19. Erythrocyte cholinesterase was
inhibited in the 64 ppm group, but brain cholinesterase was
unaffected by treatment. SGPT was markedly elevated in the 64 and 16
ppm groups and slightly raised in males of the 4 ppm group. During
weeks 16 to 19 these levels fell, but only the 4 ppm group returned
to normal. SGOT levels were not the same as controls at all
treatment levels, but serum alkaline phosphatase was elevated and
sulfobromophthalein retention increased in the 16 and 64 ppm groups.
The livers of dogs receiving 16 and 64 ppm were pigmented on
macroscopic examination. Microscopically, pigmentation could be seen
in macrophages and hepatic cells (principally centrilobular) in the
16 and 64 ppm groups, the intensity of deposit being dose-related.
The Perl's reaction showed that the pigment did not contain
appreciable quantities of iron. The kidneys of the 64 ppm group also
showed pigmentation. It was questionable whether the livers of the 4
ppm group contained excess pigment. Control and test groups were
indistinguishable regarding behaviour, results of clinical tests
including neurological examination, haematological findings, organ
weights and macroscopic and microscopic appearance of organs other
than those mentioned. An additional two dogs received 64 ppm
methidathion in the diet for four weeks. The SGOT was elevated at
two and four weeks and at autopsy the livers were dark in colour.
Moderate diffuse pigmentation was seen microscopically in the liver
of one animal.
The plasma enzyme and liver histology changes at 30 days of
treatment at 64 ppm were not increased after treatment for two years
at this dose. Furthermore, the serum enzyme changes at 16-19 weeks
of treatment at 64 ppm were reversible and returned to normal three
weeks after cessation of treatment. The NOAEL was 4 ppm (Johnston,
1967).
The histological slides of the liver tissue from the original
study were re-evaluated and submitted to the 1975 Joint Meeting
Annex 1, reference 25). Intrahepatic cholestasis was observed in
dogs fed 16 and 64 ppm methidathion. Neither degenerative nor
inflammatory changes were associated with this lesion. Pigmentation
occurred as bile-plugs in biliary ductules, or as amorphous deposits
in Kupffer cells or as lipofuscin in both hepatic and Kupffer cells.
Haemolysis was not detected. A minimal degree lipofuscin
pigmentation was also observed at 4 ppm and in the control group.
Mild irregular fatty changes of the hepatocytes with occasional
periportal histiolymphocytic infiltration of the liver were observed
in both treated and control animals (Hess, 1975).
Methidathion (97% purity) was administered to groups of 4
beagle dogs per sex in the daily feed at dietary levels of 0, 0.5,
4, 45 or 140 ppm equal to 0, 0.02, 0.16, 1.96 or 5.67 mg/kg bw/day,
respectively, for a period of 90 days. An additional group of 4 dogs
per sex was treated orally by gelatin capsule at a dose level of
0.14 mg/kg bw/day, equal to a daily dietary intake level of 4 ppm,
for a similar treatment duration. A NOAEL of 4 ppm was assessed
based upon the evidence of cholestasis in all male and female dogs
treated at 45 and 140 ppm. Cholestasis was similarly described in a
single male dog treated by capsule at 0.14 mg/kg bw/day. Other
consequences of treatment evident in both sexes at dietary levels of
45 ppm and higher were discoloration of the liver and markedly
enhanced enzyme activity, expressed as increased levels of ALP,
SGOT, SGPT, GGT and sorbitol dehydrogenase. RBC cholinesterase
activity was significantly (75-88%) inhibited in dogs of both sexes
treated at 140 ppm. Brain cholinesterase activity was inhibited
(26.8%) in the 140 ppm treated females when compared to the
controls. There were no effects of methidathion treatment on serum
cholinesterase levels. Clinical signs of tremor and reduced activity
post-dosing, observed during the latter part of the study, were
exhibited in a single male dog at the high dose level. Reduced
activity was also noted in a single male treated at 45 ppm. Other
effects of treatment were significantly decreased mean food intake
values in the high dose treated males when compared to the control
group. There were no treatment-related effects observed with respect
to survival, body-weights, ophthalmoscopic examination,
haematological parameters, urinalysis or organ weights (Chang &
Wyand, 1990).
Six groups of 4 beagle dogs/sex were treated with methidathion
(96% purity) at dietary levels of 0, 0.5, 2, 4, 40 or 140 ppm, equal
to 0, 0.02, 0.07, 0.15, 1.34 or 4.51 mg/kg bw/day, respectively, for
a period of 12 months. A NOAEL of 4 ppm was indicated based on
treatment-related hepatic effects observed grossly as general
discoloration of the liver and characterized histomorphologically as
cholestasis and chronic inflammation of the liver in both sexes at
dietary levels of 40 ppm and higher. Associated changes in blood
biochemical parameters were denoted by elevated ALP, SGOT, SGPT,
sorbitol dehydrogenase and bilirubin levels. Other significant
clinical changes recorded only in the females were increased GGT and
decreased total protein and albumin values. RBC choline-sterase
activity was markedly inhibited (76-87%) in both male and females
dogs treated with methidathion at 140 ppm. Serum cholinesterase
activity was not adversely affected by treatment at any dietary
level. Brain cholinesterase activity was significantly depressed
(16-27%) in both sexes treated at 140 ppm when compared to the
controls. Another effect of treatment was related to decreased food
consumption in male dogs treated with methidation at a dietary level
of 140 ppm. There were no effects of treatment on survival, clinical
signs, body-weights, ophthalmoscopy, haematology, urinalysis, faecal
examination or organ weights (Chang and Walberg, 1991).
Monkeys
Three groups of Rhesus monkeys (approximately equal number of
each sex) were administered 0, 0.25 or 1.0 mg methidathion/kg bw/day
by stomach tube, 6 days a week for 23 months. Two of each group were
autopsied after 12 months. Plasma and erythrocyte cholinesterase
activity were inhibited in the 1 mg/kg bw/day group, but not at the
lower level. Brain cholinesterase was unaltered by treatment.
Growth, results of haemological tests, results of chemical analyses
of serum (including SGPT and ALP) and macro- and microscopic
examination of tissues were similar in control and test groups
(Fabran et al., 1971).
Long-term toxicity/carcinogenicity studies
Mice
A 23-month study was conducted with groups of 50 Charles River
CD-1 mice per sex/group fed diets containing methidathion (purity
not stated) at levels of 0, 3, 10, 50 or 100 ppm equal to 0.43,
1.42, 6.99 or 13.70 mg/kg bw/day, respectively. An additional 120
mice/sex/group were assigned to the chronic phase of this bioassay.
Interim sacrifices of 20 mice/sex/group were scheduled after 3, 6,
12 and 13 months. Animals sacrificed at 13 months were maintained on
control diet for one month as recovery animals following 12 months
of continuous treatment. The remaining animals (40/sex/group,
maximum) were sacrificed after 18 months of treatment. Treatment of
mice with methidathion resulted in slightly decreased survival of
the 100 ppm treated males when compared to the controls. The only
clinical sign remarked upon was discoloration of the urine in the 50
and 100 ppm treated males. Potentially reversible increases in liver
enzyme activity were reported in males at 50 ppm and higher and in
females at 100 ppm. Significantly decreased but potentially
reversible RBC cholinesterase activity values (26-46%) were
registered in males at 100 ppm and in females at 50 ppm and higher.
Brain cholinesterase activity was markedly inhibited (15-49%) in
both sexes at 100 ppm. There were no inhibitory effects of treatment
on plasma cholinesterase activity. There were no effects of
methidathion treatment noted with respect to body-weights, food and
water consumption or ophthalmoscopy. Treatment-related target organ
alterations were manifest in the gall bladder and liver at dietary
levels of 50 ppm and higher in males and in females at 100 ppm.
Microscopic changes were described in the gall bladder as
cholecystitis and hyperplasia and, hepatic findings were denoted as
bile duct hyperplasia, bile stasis, cholangiofibrosis, chronic
hepatitis and hypertrophy. Increased extramedullary haematopoiesis
of the spleen, associated with increased spleen weights were
observed in the 100 ppm treated males. Treatment of male mice with
methidathion resulted in a significantly increased incidence of
hepatocellular tumours (adenomas, carcinomas and adenomas/carcinomas
combined).
0 3 10 50 100 ppm
Adenoma 1/46 9/45 7/47 8/43 21/45
Carcinoma 8/46 6/45 4/47 13/43 17/45
Combined 9/46 15/45 11/47 21/43 38/45
Historical control data (Quest et al. 1990) generated from 14
studies conducted at the same test facility with the same strain of
mouse, revealed that the incidences of hepatocellular tumours
reported in the present study exceeded the historical control range
at dietary levels of 50 ppm and higher. Latency, expressed in terms
of time to appearance of first tumour, was not apparently decreased
in the treated male groups when compared to the concurrent controls.
The incidence of hepatocellular tumours was not increased in female
mice treated with methidathion The NOAEL in this study was 10 ppm,
equal to 1.4 mg/kg bw/day (Goldenthal, 1986).
Rats
In order to investigate the long-term toxic effects and
possible carcinogenic action of methidathion, four groups of 25 male
and 25 female rats each were fed for three weeks on diets containing
0, 2, 8 or 32 ppm and for a further 101 weeks on diets containing 0,
4, 16 or 64 ppm methidathion. The rate of gain in body weight was
reduced from week 8 in male animals of the 64 ppm group. After the
first year the rates of gain became erratic in all groups, making
interpretation of the findings difficult. Female rats on test diets
grew at a rate comparable to controls. Erythrocyte cholinesterase
was inhibited in the 16 and 64 ppm groups, while plasma
cholinesterase showed minimal inhibition at 100 weeks in the 64 ppm
group only. Brain cholinesterase was inhibited in the 64 ppm group,
with marginal and no reduction in the 16 and 4 ppm groups,
respectively. Decreased relative adrenal weights were found in
females of the 16 ppm and 64 ppm groups, and decreased ovary weights
in the 64 ppm group. The relative kidney weights of males was
increased in the 16 and 64 ppm groups. A greater frequency of
hepatic degenerative changes was noted in rats fed methidathion in
the diet; the high incidence of pulmonary infections in the rats
renders this finding of doubtful toxicological significance. The
food intake, results of haematological investigations and chemical
analysis of serum (including SPGT) and the survival rate were
similar in the test groups and in the control group. The incidence
of tumours was variable between groups but was low and not dose-
related, and no unusual tumours were found. The NOAEL in this study
was 4 ppm methidathion in the diet (Johnston, 1967).
Groups of 65 Crl:COBS CD(SD) BR rats/sex were treated with
methidathion (97.3% purity) at dietary levels of 0, 4, 40 or 100 ppm
equal to 0.16, 1.72 or 4.91 mg/kg bw/day, respectively for a period
of 104 weeks. For interim sacrifice purposes, an additional 15
rats/sex/group were assigned on study. At 52 weeks, 10
rats/sex/group were sacrificed with the remaining 5 rats/sex/ group
sacrificed at week 93 of study. Treatment with methidathion resulted
in clinical signs expressed in the 40 and 100 ppm groups as
alopecia, chromorhinorrhea, hyperactivity, skin lesions, tremors,
hypersensitivity to the touch and fasciculation. Mean body-weights
and weight gains were decreased in both sexes at 100 ppm throughout
the course of treatment. Body-weight gains were decreased at a
dietary level of 40 ppm during the initial few weeks of treatment,
with comparable weight gain thereafter. Mean food intake was
significantly increased in both sexes treated at dietary levels of
40 ppm and higher, whereas water consumption was decreased at 100
ppm and occasionally at 40 ppm. Treatment-related haematological
findings were exhibited at a dietary level of 100 ppm as inverted
neutrophil:lymphocyte ratios, reduced RBC parameters and increased
platelet counts. Variations in blood biochemical parameters were
observed at 40 ppm and higher as decreased total bilirubin,
decreased total protein as well as changes in electrolyte levels
(decreased potassium and calcium, and increased inorganic phosphorus
and chloride). Significant inhibition of RBC (14-38%), serum (22-
66%) and brain (42-74%) cholinesterase activity were determined in
both sexes of rats treated at dietary levels of 40 ppm and higher.
Notable changes in urinalyses were revealed as decreased volume and
increased specific gravity at 40 ppm and higher which correlated
well with the reduced fluid intake reported in these groups. Gross
necropsy findings showed an increased incidence of skin lesions in
the 40 and 100 ppm levels which were associated microscopically with
ulceration and/or chronic purulent inflammation. Other pathological
alterations were related to an increased incidence in focal
accumulations of foamy macrophages in the alveoli of the 100 ppm
treated males and females. There were no effects of treatment on
survival or ophthalmoscopy. The NOAEL for in-life parameters was
determined to be 4 ppm, equal to 0.16 mg/kg bw/day. There was no
evidence of methidathion-induced carcinogenic potential (Yau et
al., 1986).
Reproduction studies
Rats
In a three-generation study, three groups of 10 male and 20
female rats were fed on a diet containing 0, 2 or 16 ppm
methidathion for three weeks, and thereafter on a diet containing 0,
4 or 32 ppm methidathion. Litters from the second mating were used
to provide the new generations. The F1b litters did not receive
test diets until 26 days and the F2b until 22 days after weaning.
F0, Flb and F2b generations received diets for 27-28 weeks
during which they produced two litters. The number of young
surviving at weaning was reduced in all generations of litters from
animals fed 32 ppm methidathion and the mean liver weight of F3b
weanlings of this group was slightly increased. Body weights,
reproductive capacity and mortality of parents and the number of
litters, litter size, mean birth and weaning weights of test groups
were comparable to controls. The number of stillbirths and incidence
of congenital abnormalities were unaltered by treatment. No
histological damage was found in the organs of the F3b animals
examined. The NOAEL in this study was 4 ppm methidathion (Lobdell &
Johnston, 1966).
A two-generation (one litter per generation) reproduction study
was conducted with groups of 15 male and 30 female Charles River SD
CR1:CD BR rats fed methidathion (purity not specified) at dietary
levels of 0, 5, 25 or 50 ppm equal to 0, 0.43, 2.08 or 4.23 mg/kg
bw/day, respectively. With respect to reproductive performance,
statistically (p < 0.05) reduced mating indices were recorded for
the 25 and 50 ppm groups in the F1 generation. Although the mating
indices were reduced in these groups (69% and 66%, respectively)
relative to the concurrent F1 control group (88%), they were
nevertheless not significantly different from those calculated for
the F0 generation control (73%) or treated groups (60-65%). The
fertility and gestation indices were comparable among groups.
Treatment-related effects on maternal animals fed dietary levels of
25 ppm and higher were revealed by clinical signs of toxicity
manifest during lactation as slight and/or intermittent tremors.
Mean body-weights of F1 50 ppm-treated males were decreased prior
to and during the 12-week premating period. Mean body-weights gains
were not, however, significantly different from controls. Body-
weights of females recorded during lactation of both generations
were decreased at 50 ppm when compared to the concurrent control.
Decreased ovary weights recorded in females at 25 and 50 ppm were
not correlated with histopathological changes. Effects of treatment
on progeny were expressed as decreased survival in the 50 ppm group
of the F1 generation, decreased body-weights and clinical signs in
both generations at 25 and 50 ppm. The clinical signs were
suggestive of poor maternal care and were described as
weakness/lethargy, coolness to the touch and starving appearance. On
the basis of the reported findings, a NOAEL of 5 ppm, equal to 0.43
mg/kg bw/day, was indicated (Salamon, 1987).
Special studies on teratogenicity
Rats
A teratology study was conducted with groups of 25 mated female
Crl: COBS CD(SD)BR rats administered methidathion (93.2-96% purity)
at 0.25, 1.0 and 2.5 mg/kg bw/day or the control (vehicle, 3%
aqueous cornstarch with 0.5% Tween 80) orally by gavage from days 6
to 15 of gestation, inclusive. Confirmation of mating was determined
by presence of sperm in the vaginal washing, and this day was
designated day 0 of gestation. A NOAEL for maternal toxicity was
indicated at 1.0 mg/kg bw/day based on mortality, decreased body-
weights, food intake and clinical signs at 2.5 mg/kg bw/day.
Clinical signs of toxicity in the high dose (2.5 mg/kg bw/day) group
were suggestive of organophosphorus intoxication including,
lethargy, tremors, lacrimation, salivation, raspy respiration,
exophthalmia, chromodacryorrhea. A NOAEL for embryofetal toxicity
was set at the highest dose level tested of 2.5 mg/kg bw/day. There
were no significant differences noted with respect to the mean
number of implantations, live fetuses or mean number of resorptions
on a litter basis. There was similarly no significant variability in
the post-implantation loss, fetal sex ratio or in the mean fetal
litter weight in the treated groups when compared to the control.
Treatment with methidathion failed to uncover any evidence of
teratogenic potential (Mainiero et al. 1987).
Rabbits
Methidathion (purity not specified) was administered orally by
gavage at 0 (vehicle control, 3% cornstarch with 0.5% Tween 80), 2,
6 or 12 mg/kg bw/day to groups of 19 artificially inseminated New
Zeeland white rabbits on days 7 through 19 of gestation. The day of
artificial insemination was designated as day 0 of gestation.
Treatment-related clinical signs of toxicity at the high dose (12
mg/kg bw/day) treated animals were manifest as ataxia, tremors,
salivation and miosis. In the absence of similar effects in the
control or other treated groups, the NOAEL for maternal toxicity was
demonstrated to be 6 mg/kg bw/day. There was no evidence of
embryofetal developmental toxicity or potential for teratogenicity
at any of the dose levels investigated, including the highest dose
of 12 mg/kg bw/day (Hummel et al. 1987).
Special studies on genotoxicity
A battery of mutagenicity assays were conducted with technical
methidathion, the results of which are presented in Table 3. The
tests performed to evaluate potential for gene mutation in bacteria,
DNA damage as well as chromosome aberration were negative. A
dominant lethal study in mice was also negative. Positive responses
were observed in the in vitro assays with Saccharomyces
cerevisiae and in the Chinese hamster test for sister chromatid
exchange. In vivo tests conducted to examine the similar
endpoints, did not however elicit any evidence of mutagenic
potential in mammalian cells.
Special studies on delayed neurotoxicity
Four adult hens received four subcutaneous injections of 50 mg
methidathion/kg body-weight (the maximum tolerated dose) at weekly
intervals and they were observed for a further four weeks. The signs
of acute poisoning lasted two to three days each time, but birds
remained in good condition and no paralysis developed.
Neuropathological examinations were not performed (Noakes, 1964).
Five groups of ten adult hens were fed diets containing 0, 16,
52 or 160 ppm methidathion or 316 ppm tri-orthocresylphosphate for
45-47 days. No abnormal neurological signs were found in birds fed
methidathion, but those on tri-orthocresylphosphate showed leg
weakness, lack of balance and ataxia during the final week of
treatment. Unequivocal evidence of demyelination of neural tissue
was not found in methidathion or tri-orthocresylphosphate-treated
animals (Johnston, 1965).
The delayed neurotoxic potential of methidathion (purity not
specified) was investigated in groups of 10-30 female white leghorn
hens. The animals were administered the test material twice, 21 days
apart, at 0 (vehicle, CMC 2%), 43.75, 87.5, 175 or 350 mg/kg bw.
Animals receiving methidathion at 350 mg/kg bw were pretreated with
an intramuscular injection of atropine sulfate, 10 mg/kg bw, one
hour prior to treatment. Groups of 3 male and 3 female hens received
a single oral dose of the positive control, TOCP at 215, 464, 600,
1000 or 2150 mg/kg bw and were then observed for 21 days post-
dosing. A preliminary acute oral study indicated that the LD50 of
methidathion in the hen was 175 mg/kg bw. Clinical signs of
intoxication were expressed as ataxia, slight tremor, curved or
ventral body position and sedation, with subsequent recovery in
surviving animals, 8-10 h post-dosing. Histopathological evaluation
of the spinal cord and peripheral nerves did not reveal any
treatment-related lesions of the nervous system, whereas TOCP
toxicity was evidenced as central-peripheral, distally accentuated
neuropathy. Treatment with methidathion did not produce any signs of
delayed neurotoxicity (Ullmann et al. 1977).
Table 3. Genotoxicity of technical methidathion
Test Test system Concentration Purity Results References
(vehicle)
Reverse mutation S. typhimurium 25, 75, 225, 675, 2025 98.4% negative Arni & Muller (1980a)
(in vivo) TA98, 100, 1535, 1537 µg/0.1 mL (DMSO) 1., 2.
S. typhimurium 25, 75, 225, 675, 2025 93.4% negative Arni & Muller (1980b)
TA98, 100, 1535, 1537 µg/0.1 mL (DMSO) 1., 2.
S. typhimurium 0, 0.1, 0.5, 1, 5, 10, 50 99.95% negative Satou et al. (1979)
TA98, 100, 1535, 1537 mg/mL (DMSO) 1., 2.
E. coli
B/r WP2 Try- Hcr-
S. typhimurium 10, 50, 100, 500, 1000, ? negative Simmon et al. (1977)
TA98, 100, 1535, 1537, 5000 µg/plate (DMSO) 1., 2.
1538
Rec assay (in vitro) B. subtilis 5, 25, 100 mg/mL (DMSO) 99.95% negative Satou et al. (1979)
H17, M45
Reverse mutation/ S. typhimurium 10, 20, 40 mg/kg bw ? negative Simmon et al. (1977)
Host-mediated TA1535, 1538/Male Swiss (single oral doses)
(in vivo) Webster mouse
(inocculated ip)
5, 10, 20 mg/kg bw negative
(5 oral doses) (DMSO)
S. typhimurium 0, 5, 10, 20 mg/kg bw 93.4% negative Arni & Muller (1980c)
TA98, 100, 1537/ (3 oral doses: 2 h, 1 h
male mouse and prior to innoculation)
(inocculated iv) (CMC, 0.5%)
Table 3 (cont'd)
Test Test system Concentration Purity Results References
(vehicle)
Gene conversion/ S. cerevisiae MP-1 675, 1250, 2500, 5000, 93.4 conversion: Arni & Muller (1981)
forward mutation 10000 µg/mL (DMSO) slight (+ ve)
(in vivo) mutation:
(+ ve)
Forward mutation Mouse lymphoma cells - 0, 15 mg/kg bw (3 oral 93.4% negative Strasser & Muller (1980)
host-mediated (in L5 178Y/mouse doses post-innoculation)
vivo) (DBA/Bom) (CMC)
DNA repair Mouse (male CD-1) 5 x 10-7 to 1% (DMSO) ? negative Tong (1982a)
(in vivo) hepatocytes
Rat (male F344) 5 x 10-9 to 1% (DMSO) ? negative Tong (1982b)
hepatocytes
Rat hepatocytes 0.128, 0.64, 3.2, 16 97.2% negative Puri & Muller (1982a)
µg/mL (DMSO)
Rat hepatocytes 1.85, 5.56, 16.67, 50, 100, 96% negative Hertner & Arni (1990)
200 µg/mL (DMSO)
Human fibroblasts 1.024, 5.12, 25.6, 128 ? negative Puri & Muller (1982b)
µg/mL (DMSO)
SCE (in vivo) Chinese hamster 0, 10, 20, 40, 80 µg/mL 99.1% slight (+ ve) Chem et al. (1981)
cell line V79 (DMSO) at 40, 80
ug/mL
SCE (in vivo) Chinese hamster 0, 17, 34, 68 mg/kg bw 93.4% negative Hool & Muller (1980)
(bone marrow) (CMC)
Table 3 (cont'd)
Test Test system Concentration Purity Results References
(vehicle)
Chromosome Chinese hamster ovary 43, 75, 87.5, 175, 350 96% negative Strasser & Arni (1990)
aberration CCL 61 µg/mL (DMSO)
(in vivo) 96.9% negative Hool et al. (1980)
Nucleus anomaly Chinese hamster 0, 17, 34, 68 mg/kg bw
(in vivo) (bone marrow) (2 oral doses) (CMC) 98.4% negative Fritz (1976)
Dominant lethal Mice, NMRI 0, 15, 45 mg/kg bw (CMC)
(in vivo)
1. = in the presence of metabolic activation
2. = in the absence of metabolic activation
Special studies on irritation and sensitization
The eye irritation potential of technical methidathion was
investigated in 3 English Silver strain rabbits/sex. Administration
of 0.1 grams of methidathion into the conjunctival sac of the left
eye (right eye served as control) produced irritating conjunctival
reaction in the unwashed eye of a single male rabbit (Sachsse,
1973a).
Technical methidathion (as a 50% polyethylene glycol
suspension) was applied under occlusive conditions to the shaved
backs of male and female English Silver strain rabbits for 24 h.
Skin reactions were observed as very slight erythema in one male and
moderate to severe erythema in association with slight oedema in one
female rabbit (Sachsse, 1973b).
The shaved backs of male Hartley albino guinea-pigs were
repeatedly treated with technical methidathion as a 10% solution in
diethyl ether. There was no evidence of skin sensitization potential
(Cannelongo, 1984).
Special studies on potentiation
The potentiating effects of methidathion (purity not specified)
with profenofos (Sachsse & Bathe, 1977b) and methacrifos (Sachsse &
Bathe, 1978) were investigated in male and female Tif:RAIf rats by
comparing the theoretical LD50 values, based on an assumption of
strictly additive toxicity, with that of the experimentally derived
LD50 values for the equitoxic mixtures. There was no potentiating
effect of methidathion with profenofos, whereas a slight enhancement
of acute toxicity was observed with an equitoxic mixture of
methidathion and methacrifos.
Special studies on promoting activity
Male F344 DuCrj strain rats received a single ip injection of
the initiator, N-nitrosodiethylamine (DEN) at 200 mg/kg bw. Two
weeks thereafter, the rats were treated with methidathion (92.7%
purity) at dietary levels of 0, 10, 30, 100 or 300 ppm (equal to 0,
1.0, 2.3, 7.6 or 22.9 mg/kg bw, respectively) or the positive
control, 3'-methyl-4-dimethyl amino azobenzene (3'-Me-DAB) at 600
ppm (equal to 33.9 mg/kg bw) for a period of 6 weeks. Three weeks
following treatment with DEN, partial hepatectomy was performed on
all animals. Histochemical evaluation of 4 liver sections from each
animal after 8 weeks on study, revealed a significantly increased
number of GGT positive foci per unit area in rats treated with
methidathion at 100 and 300 ppm and in the 600 ppm 3'-Me-DAB
positive control group. No increases in the number of GGT positive
foci were observed in additional groups of rats treated with
methidathion at 100 and 300 ppm alone, without previous initiation
with DEN. The number of foci in rats treated with methidathion at 30
ppm and lower were not different from the controls (Arai, 1981).
A second short-term study of the promoting activity in the rat
liver was conducted with groups of male Fischer 344 DuCrj strain
rats administered methidathion (98.6% purity) at dietary levels of
0, 35, 46, 59, 77 or 100 ppm (equal to 0, 2.7, 3.5, 4.8, 6.0 or 7.6
mg/kg bw/day, respectively) for a period of 6 weeks. Two weeks prior
to feeding with methidathion, the rats were pretreated with a single
ip injection of the initiator DEN at 200 mg/kg bw. Partial (two-
thirds) hepatectomy was performed three weeks after initiation and
the animals were sacrificed after 8 weeks on study. Histochemical
analysis unveiled a significant dose-related increase in the number
of GGT positive foci in rats treated with methidathion at dietary
levels of 59 ppm and higher. The number of GGT positive foci at
dietary levels of 46 ppm and lower were comparable to the controls.
No increases in the number of GGT positive foci were recorded in an
additional group of rats treated with methidathion at 100 ppm alone,
without previous initiation with DEN (Daiyu-Kai Institute of Medical
Science, 1983).
Special studies on therapeutic activity of antidotes
The therapeutic potential of antidotes was tested by
administering methidathion (purity not specified) orally by gavage
to Tif.RAIf rats at the LD80 of 46 mg/kg bw. The therapeutic
agents, atropine sulfate alone, toxogonin alone, PAM alone and
toxogonin combined with atropine sulfate were then administered
intramuscularly after the first appearance of clinical signs of
poisoning. A further aspect of therapeutics was investigated, by
administering 5 repeated daily oral doses of methidathion at the
LD50 (34 mg/kg bw) to rats and then, upon observation of clinical
signs, commencing with the respective combination of antidotes. All
therapeutic agents were, according to the treatment regimen,
effective against the oral LD80 of methidathion. A positive
protective effect against repeated LD50 doses of methidathion were
observed with atropine and toxogonin. PAM and toxogonin in
combination with atropine had minimal protective effect against
repeated challenges to methidathion (Sachsse & Bathe, 1977a).
Observations in humans
One male subject took 4 mg/day (equal to 0.04 mg/kg bw/day) for
17 days, and 8 mg/day (equal to 0.08 mg/kg bw/day) of methidathion
for 27 days. No effect was found on RBC and plasma cholinesterase,
the thrombocyte count and stability or on the clinical condition of
the subject (Payot, 1965).
Two groups of eight men received 0.04 or 0.11 mg
methidathion/kg bw/day orally in capsules for 6 weeks. Four men
received placebo capsules. The treatment with methidathion was
without effect on plasma and RBC cholinesterase, SGPT and SGOT and
results of urinalyses or on EEG pattern or clinical condition of the
subject (Coulston, 1970).
A case of massive poisoning was reported to have occurred where
a 25-year old man weighing 60 kg ingested a number of mouthfuls of
supracide 40 (methidathion, 40% active ingredient). The man was
discovered approximately 2 hours after the event, in an unconscious,
semi-comatose condition. Upon admission to hospital the patient was
treated intravenously with atropine and toxogonin. The
cholinesterase activity in the serum was found to be zero a few
hours after admission. The patient was discharged after 15 days of
hospitalization. The laboratory findings were normal with exception
of cholinesterase activity which remained low. Follow-up examination
at 2, 5 and 10 months revealed no abnormalities with no evidence of
delayed neurotoxicity (Teitelman et al. 1975).
A case of attempted suicide occurred in a 50-year old man
weighing 67 kg who had ingested approximately 6.2 g or 40 ml of
Supracid 20 (methidathion, 15.5% active ingredient). About 1.5 h
after the incident, the subject was admitted to hospital suffering
from mental confusion, muscle fasciculations, bradycardia, miosis,
sweating, salivation and lacrimation. The patient underwent gastric
lavage followed by treatment with atropine sulphate and pralidoxime.
Six hours after ingestion of methidathion, the incidence of muscle
fasciculations increased followed by bronchorrhea and coma. Serum
and RBC cholinesterase values, as measured over eight days following
poisoning were generally less than 50% of normal values.
Cholinesterase values assessed after 45 days were normal.
Lymphocytic NTE activity was normal as determined on days 5, 10, 17
and 45 after poisoning. The patient was discharged after 21 days of
hospitalization. A follow-up examination after 7 months failed to
establish any evidence of delayed polyneuropathy or other
neurological abnormality (Zoppellari et al. 1990).
COMMENTS
Methidathion was extensively absorbed when administered orally
to rats. The routes of elimination were via the urine and expired
CO2. There were no significant differences in elimination patterns
with regard to dose levels administered, pre-treatment or sex.
In the rat, the predominant urinary metabolites were the
sulfide, sulfoxide, sulfone and desmonomethyl derivative. Negligible
quantities of the parent and oxygen analog were detected in the
urine. The predominant metabolic pathway of methidathion in the goat
was via O-demethylation with the desmonomethyl derivative as the
principal urinary metabolite. Cysteine conjugates were identified in
each species.
Methidathion has a high acute oral toxicity. The World Health
Organization has classified methidathion as highly hazardous (WHO,
1992).
Methidathion was administered to dogs for 90 days at dietary
concentrations of 0, 0.5, 4, 45 or 140 ppm, or 0.14 mg/kg bw/day
(equal to 4 ppm) by capsule, and for a period of 12 months at 0,
0.5, 2, 4, 40, or 140 ppm in the diet. In both studies the dietary
NOAEL was determined to be 4 ppm, equal to 0.16 mg/kg bw/day, based
on liver effects, most notably cholestasis and increased liver
enzymatic activity in serum at dietary levels of 40 ppm and above.
Cholestasis was observed in a single male dog treated by capsule at
0.14 mg/kg bw/day. Erythrocyte and brain cholinesterase activities
were affected only at the highest level of 140 ppm.
Long-term dietary treatment of mice with methidathion for 23
months at 0, 3, 10, 50 or 100 ppm revealed an increased incidence of
hepatocellular tumours in males at 50 ppm and above, resulting in a
NOAEL of 10 ppm, equal to 1.4 mg/kg bw/day. Erythrocyte
cholinesterase was inhibited at 50 ppm (equal to 7 mg/kg bw/day) and
above, whereas brain cholinesterase activity was affected at 100 ppm
(equal to 13.7 mg/kg bw/day).
A 104-week long-term toxicity/carcinogenicity study in rats fed
methidathion at 0, 4, 40 or 100 ppm indicated a NOAEL of 4 ppm,
equal to 0.16 mg/kg bw/day, based on inhibition of erythrocyte and
brain cholinesterase activity at 40 ppm and above. Methidathion was
not carcinogenic in rats.
In a two-generation reproduction study in rats at dietary
concentrations of 0, 5, 25 or 50 ppm, the NOAEL was 5 ppm, equal to
0.43 mg/kg bw/day. At 25 ppm, reduced mating indices in the F1
generation and decreased progeny body weights were observed.
There were no teratogenic effects observed when methidathion
was administered by gavage to rats at doses of 0, 0.25, 1.0, or 2.5
mg/kg bw/day or to rabbits at doses of 0, 2, 6 or 12 mg/kg bw/day.
Maternal effects were demonstrated at the highest doses in both the
rat and rabbit as clinical signs of toxicity. In the rat, increased
mortality as well as decreased body-weights and food consumption
were also observed. The NOAELs in the rat and rabbit were determined
to be 1 and 6 mg/kg bw/day, respectively.
Treatment of hens with methidathion did not produce any
clinical or pathological evidence of delayed neurotoxicity.
In two reported cases of poisoning with methidathion, each of
the male subjects exhibited classic clinical and biochemical signs
of organophosphorus intoxication. Both subjects recovered, with no
evidence of delayed neurotoxicity uncovered upon follow-up
examination. In one of the cases jaundice was reported during the
recovery period.
Two human volunteer studies failed to reveal any inhibition of
erythrocyte or serum cholinesterase activity at doses up to 0.11
mg/kg bw/day.
After reviewing the available genotoxicity data, the Meeting
concluded that methidathion was not genotoxic.
In the dog, the effects on the liver appeared to have occurred
at levels lower than the levels causing inhibition of cholinesterase
activity, giving a NOAEL of 0.1 mg/kg bw/day. Whether or not there
was a relationship to the potential induction of liver effects in
man could not be ascertained.
The Meeting concluded, after consideration of the
hepatocellular tumours found in male mice in the long-term toxicity
study, together with the lack of genotoxicity, that methidathion did
not present a carcinogenic hazard for humans.
The previous ADI, based on a NOAEL in man of 0.11 mg/kg bw/day,
was revised. The revised ADI is based on the NOAEL in the dog and a
100-fold safety factor.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 10 ppm, equal to 1.4 mg/kg bw/day (23-month study)
Rat 4 ppm, equal to 0.16 mg/kg bw/day (104-week study)
5 ppm, equal to 0.43 mg/kg bw/day (reproduction
study)
Dog: 0.1 mg/kg bw/day (90-day, one-year, and two-year
studies)
Human: 0.11 mg/kg bw/day.
Estimate of acceptable daily intake for humans
0 - 0.001 mg/kg bw
Studies which will provide information valuable in the continued
evaluation of the compound
Further observations in humans
REFERENCES
Aeppli, L. (1969a) Akute Toxizität, Ratte per os, A 2039 E (0 40
WP). Unveröffentlicht. Geigy, J.R. AG.
Aeppli, L. (1969b) Akute Toxizität, Ratte per os, A 3628 E (0 40
WP). Unveröffentlicht. Geigy, J.R. AG.
Aeppli, L. (1970a) Akute Toxizität, Ratte per os, A 3628
blaugefärbt. Unveröffentlicht. Geigy, J.R. AG.
Aeppli, L. (1970b) Akute Toxizität, Ratte per os, A 3389 C (= 40
ES). Unveröffentlicht. Geigy, J.R. AG.
Arai, M. (1981). In vivo short term study on the promoting
activity of methidathion (CG-831) (translation). Unpublished report
dated October 31, 1981 from Daiyu-Kai Ikagaku Kenkyu-Sho, Japan.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Arni, P. & Muller, D. (1980a). Salmonella/mammalian-microsome
mutagenicity test with GS 13005 (Test for mutagenic properties in
bacteria). Project No. 79/1556. Unpublished report dated April 17,
1980 from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Arni, P. & Muller, D. (1980b). Salmonella/mammalian-microsome
mutagenicity test with GS 13005 (Test for mutagenic properties in
bacteria). Project No. 801488. Unpublished report dated October 29,
1980 from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Arni, P. & Muller, D., 1980c. Intrasanguine host-mediated assay with
S. typhimurium with GS 13005 (Test for the demonstration of point
mutations in bacteria in vivo). Project No. 801494. Unpublished
report dated October 31, 1980 from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Arni, P. & Muller, D. (1981). Mutagenicity test on Saccharomyces
cerevisiae MP-1 in vitro with GS 13005 (Test for mutagenic
properties in yeast cells). Project No. 802030. Unpublished report
dated November 5, 1981 from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Bathe, R. (1973a). Acute oral LD50 of technical GS 13005 in the
rat. Unpublished report dated May 25, 1973 from Ciba-Geigy Ltd.,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Bathe, R. (1973b). Acute dermal LD50 of technical GS 13005 in the
rat. Project No. Siss 2929. Unpublished report dated October 3, 1973
from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Bathe, R. & Sachsse, K. (1975). Acute dermal LD50 in the rat of
technical GS 13005. Project No. Siss 4650. Unpublished report dated
September 15, 1975 from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Bathe, R. & Sachsse, K. (1980a). Acute oral LD50 in the rat of
technical GS 13005. Project No. 791668. Unpublished report dated
January 9, 1980 from Ciba-Geigy Ltd., Basle, Switzerland. Submitted
to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Bathe, R. & Sachsse, K. (1980b). Acute oral LD50 in the rat of
technical GS 13005. Project No. 791667. Unpublished report dated
January 10, 1980 from Ciba-Geigy Ltd., Basle, Switzerland. Submitted
to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Cannelongo, B. (1984). Guinea pig skin sensitization. Supracide
technical FL 830958. Project No. 3152-83. Unpublished report dated
January 9, 1984 from Stillmeadow, Inc., Houston, USA. Submitted to
WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Chang J. and Walberg, J. (1991). One-year dietary study in Beagle
dogs. Project No. F00028. Unpublished report dated June 24, 1991
from Ciba-Geigy Corporation, Framington, USA. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Chang, J. & Wyand, S. (1990). 90-day oral toxicity study in Beagle
dogs. Project No. F-00023. Unpublished report dated March 21, 1990
from Ciba-Geigy Corporation, Farmington, USA. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Chen, H., Hsueh, J., Sirianni, S., & Huang, C. (1981). Induction of
sister-chromatid exchanges and cell cycle delay in cultured
mammalian cells treated with eight organophosphorus pesticides.
Mutation Research, 88: 307-316.
Coulston, F. (1970). Effects on man of small daily doses of GS13005.
Unpublished report of Albany Medical College. Submitted to WHO in
1972 and cited in Annex I, 19.
Daiyu-Kai Institute of Medical Science (1983). In vivo short-term
study on dose-dependence and threshold level of promoting activity
of GS13005 in the rat liver. Unpublished report received June 1983
from Daiyu-Kai Institute of Medical Science, Japan. Submitted to WHO
by Ciba-Geigy Ltd., Basle, Switzerland.
Fabran, J.E., Golberg, L. and Coulston, F. (1971). Two-year safety
evaluation study of GS13005 on Rhesus monkey. Unpublished report of
Albany Medical College. Submitted to WHO in 1972, and cited in Annex
I, 19.
Folinusz, P., Huber, K., Schiavo, D., Hazelette, J., & Green, J.
(1986). Methidathion: 21-day dermal toxicity study in rabbits.
Project No. 86019. Unpublished report dated August 28, 1986 from
Ciba-Geigy Corporation, Summit, USA. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Fritz, H. (1976). Dominant lethal study on GS 13005 technical, mouse
(test for cytotoxic or mutagenic effects on male germinal cells).
Project No. 327633. Unpublished report dated August 3, 1976,
supplemented May 26, 1987, from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Goldenthal, E. (1986). Two-year dietary oncogenicity study in mice.
Project No. 382-087. Unpublished report dated March 7, 1986 from
International Research and Development Corporation, Mattawan, USA.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Hertner, T. & Arni, P. (1990). Autoradiographic DNA repair test on
rat hepatocytes. Project No. 891344. Unpublished report dated
February 6, 1990 from Ciba-Geigy Ltd., Basle, Switzerland. Submitted
to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Hess, R. (1975) Nature of the liver changes observed in a two-year
feeding study in dogs. Unpublished report from the
Toxicology/Pathology Dept., Ciba-Geigy. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Hool, G., Langauer, M., & Muller, D. (1980). Nucleus anomaly test in
somatic interphase nuclei of Chinese hamster. Project No. 800437.
Unpublished report dated July 2, 1980, supplemented May 26, 1987,
from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Hool, G. & Muller, D. (1980). Sister chromatid exchange study GS
13005 Chinese hamster (test for mutagenic effects of bone marrow
cells). Project No. 801489. Unpublished report dated November 4,
1980, supplemented May 26, 1987, from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Hummel, H., Youreneff, M., Giknis, M., Arthur, A. & Yau, E., 1987.
Methidathion: a teratology (segment II) study in rabbits. Project
No. 86131. Unpublished report dated January 13, 1987 from Ciba-Geigy
Corporation, Summit, USA. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Johnston, C.D. (1965) GS 13 005. Demyelination study in the chicken.
Report from Woodard Research Corp. (unpublished).
Johnston, C.D. (1967). GS 13 005. safety evaluation by two-year
feeding studies in rats and dogs. Final report from Woodard Research
Corp. (unpublished).
Lobdell, B.J. & Johnston, C.D. (1966). GS 13 005. Three-generation
reproduction study in the rat. Report from Woodard Research Corp.
(unpublished).
Mainiero, J., Levy, E., Infurna, R., & Yau, E. (1987). Methidathion
technical: a teratology (segment II) study in rats. Project No.:
86172. Unpublished report dated January 15, 1987 from Ciba-Geigy
Corporation, Summit, USA. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Marco, G. & Simoneaux, B. (1982). Dermal absorption of thiadiazole-
14C-methidathion by rats. Project No. ABR-81058. Unpublished
report dated April 22, 1982 from Ciba-Geigy Corporation, Greensboro,
USA. Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Noakes, D.N. & Sanderson, D.M. (1964) The toxicology of GS 13 005
and GS12968. Species variation in acute oral tosicity. Report from
Chesterford Park Research Station (unpublished).
Noakes, D.N. (1964) The toxicology of GS 13 005 and GS 12968. Tests
for delayed neurotoxic effects in the hen. Report from Chesterford
Park Research Station (unpublished).
Noakes, D.N. & Watson, W.A. (1964a) The toxicology of GS 13 005 and
GS 12968. Dietary toxicity to the rat: 6-month study. Report from
Chesterford Park Research Station (unpublished).
Noakes, D.N. & Watson, W.A. (1964b) The toxicology of GS 12 968 (NC
2962) and GS 13 005 (NC 2964). Cumulative oral toxicity to the rat.
Report from Chesterford Park Research Station (unpublished).
Osheroff, M. (1987). 21-day dermal toxicity study in rabbits with
methidathion technical. Project No. 483-254. Unpublished report
dated January 15, 1987 from Hazleton Laboratories America, Inc.,
Vienna, USA. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Payot, H.P., GS13005. Subchionische toxizitat am menschen (Einzel
und Selbstversuch). Unveröffentlichter Berict der Geigy J.R., Ag.
Submitted to WHO in 1972 and cited in Annex I, 19.
Puri E. & Muller D. (1982a). Autoradiographic DNA repair test on rat
hepatocytes GS 13005 ( in vitro test for DNA-damaging properties).
Project No. 820585. Unpublished report dated October 19, 1982 from
Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Puri E. & Muller D. (1982b). Autoradiographic DNA repair test on
human fibroblasts GS 13005 ( in vitro test for DNA-damaging
properties). Project No. 820586. Unpublished report dated October
18, 1982 from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO
by Ciba-Geigy Ltd., Basle, Switzerland.
Quest, J., Copley, M., Hamernik, K., Rinde, E., Fisher, B., Engler,
R., Burnam, W. & Fenner-Crisp, P. (1990). Evaluation of the
carcinogenic potential of pesticides, 2. Methidathion. Regulatory
Toxicology and Pharmacology, 12: 117-126.
Sachsse, K. (1971) Acute oral LD50 of technical GS 13 005 in the
dog. Report from Ciba-Geigy Ltd (unpublished).
Sachsse, K. (1973a). Irritation of technical GS 13005 in the rabbit
eye. Project No. Siss 2929. Unpublished report dated June 6, 1973
from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Sachsse, K. (1973b). Skin irritation in the rabbit after single
application of technical GS 13005. Project No. Siss 2929.
Unpublished report dated June 6, 1973 from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Sachsse, K. & Bathe, R. (1977a). The therapeutic activity of
atropine sulfate, pralidoxim (PAM) and toxogonin with regard to GS
13005 in the rat. Unpublished report dated December 5, 1977 from
Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Sachsse, K. & Bathe, R. (1977b). Potentiation study: CGA 15324
versus 2 insecticides, GS 13005 (methidathion) and G 24480
(diazinon) in the rat. Unpublished report dated February 15, 1977
from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Sachsse, K. & Bathe, R., 1978. CGA 20168 versus 6 insecticides, C
177 (DDVP), C 570 (phosphamidon), GS 13005 (methidathion), G 24480
(diazinon), CGA 15324 and malathion. Project No. 404478-Siss 6526.
Unpublished report dated June 23, 1978 from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Salamon, C. (1987). Two-generation reproduction study in albino rats
with methidathion technical. Project No. 450-2125. Unpublished
report dated January 15, 1987 from American Biogenic Corporation,
Decatur, USA. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Sarasin G. (1980). Acute dermal LD50 in the rat of GS 13005
technical Rohschmelze (=crude melt) (Ex Monthey). Project No.
801726. Unpublished report dated December 17, 1980 from Ciba-Geigy
Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Satou, S., Kimura, Y., Yamamoto, K. & Ichihara, A. (1979). In vitro
microbial assays for mutagenicity testing of DMTP (methidathion).
Project No. NRI-79-2884. Unpublished report dated August 31, 1979
from Nomura Research Institute, Japan. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Simmon, V., Poole, D. Nevell, G., & Skinner, W. (1977). In vitro
and in vivo microbiological assays of six Ciba-Geigy chemicals.
Project No. LSC-5686. Unpublished report dated March 1977 from
Stanford Research Institute, Menlo Park, USA. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Simoneaux, B. & Marco, G. (1984). Dermal absorption of thiadiazole-
14C-methidathion by mice. Project No. ABR-84015. Unpublished
report dated April 12, 1984 from Ciba-Geigy Corporation, Greensboro,
USA. Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Staley, J., Murphy, T., & Simoneaux, B. (1987). Metabolism of 14C
(Carbonyl) labelled methidathion in a goat. Project No. ABR-86112.
Unpublished report dated February 26, 1987 from Ciba-Geigy
Corporation, Greensboro, USA. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Stenger, E.G. & Roulet, F. (1963) Subchronische Toxizität, Ratte per
os, GS 13005 rein. Unveröffentlichter Bericht der Geigy, J.R. AG.
Stenger, E.G. (1964a) Akute Toxizität, Ratte per os, GS 13005 40 WP.
Unveröffentlichter Bericht der Geigy, J.R. AG.
Stenger, E.G. (1964b) Akute Atoxizität, Ratte per os, GS 13005 40
ES. Unveröffentlichter Bericht der Geigy, J.R. AG.
Stenger, E.G. & Roulet, F. (1965) Chronische Toxizität, Ratte,
Fütterungsversuche 4-22- Wochen, GS 13 005. Unveröffenlichter
Bericht der Geigy, J.R. AG.
Stenger, E.G. (1966a) Akute Toxizität, Ratte per os, GS 13 005 40
WP. Unveröffentlichter Bericht der Geigy, J.R. AG.
Stenger, E.G. (1966b) Akute Toxizität, Ratte per os, A 2041 C (= 40
ES). Unveröffentlichter Bericht der Geigy, J.R. AG.
Staley, J. & Simoneaux, B. (1987). Metabolism of methidathion
labelled with 14C on the ring carbon adjacent to the methoxy group
in a goat. Project No. ABR-86118. Unpublished report dated March 2,
1987 from Ciba-Geigy Corporation, Greensboro, USA. Submitted to WHO
by Ciba-Geigy Ltd., Basle, Switzerland.
Strasser, F. & Arni, P. (1990). Chromosome studies on Chinese
hamster ovary cell line CCL 61 in vitro. Project No. 891202.
Unpublished report dated January 30, 1990 from Ciba-Geigy Ltd.,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Strasser, F. & Muller, D. (1980). Point mutation assay with mouse
lymphoma cells, host mediated assay with GS 13005 (test for
mutagenic properties in mammalian cells). Project No. 801495.
Unpublished report dated October 21, 1980 from Ciba-Geigy Ltd.,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Szolics, I. & Simoneaux, B. (1985). Partitioning characteristics and
14C distribution in a chicken dosed with 2 -14C-methidathion for
16 days. Project No. ABR-85039. Unpublished report dated July 22,
1985 from Ciba-Geigy Corporation, Greensboro, USA. Submitted to WHO
by Ciba-Geigy Ltd., Basle, Switzerland.
Szolics, I. & Simoneaux, B. (1987a). Disposition of methidathion in
the rat. Project No.: ABR-86122. Unpublished report dated March 6,
1987 from Ciba-Geigy Corporation, Greensboro, USA. Submitted to WHO
by Ciba-Geigy Ltd., Basle, Switzerland.
Szolics, I. & Simoneaux, B. (1987b). The disposition of
radioactivity in rats dosed with carbonyl 14C-methidathion.
Project No. ABR-86084. Unpublished report dated February 26, 1987
from Ciba-Geigy Corporation, Greensboro, USA. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Szolics, I. & Simoneaux, B. (1987c). The disposition of
radioactivity in rats dosed with methoxy labelled 14C-
methidathion. Project No. ABR-86115. Unpublished report dated March
2, 1987 from Ciba-Geigy Corporation, Greensboro, USA. Submitted to
WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Szolics, I. & Simoneaux, B. (1987d). The disposition of
radioactivity in rats dosed with 14C-methidathion labelled in the
ring carbon adjacent to the methoxy group. Project No. ABR-86095.
Unpublished report dated February 26, 1987 from Ciba-Geigy
Corporation, Greensboro, USA. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Szolics, I. & Simoneaux, B. (1987e). Characterization of carbonyl
14C-labelled methidathion metabolites in rat urine. Project No.
ABR-86107. Unpublished report dated March 2, 1987 from Ciba-Geigy
Corporation, Greensboro, USA. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Szolics, I. & Simoneaux, B. (1987f). Characterization of methoxy
14C-labelled methidathion metabolites in rat urine. Project No.
ABR-86116. Unpublished report dated February 26, 1987 from Ciba-
Geigy Corporation, Greensboro, USA. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Szolics, I. & Simoneaux, B. (1987g). Characterization of 14C-
methidathion metabolites in rat urine - label 14C adjacent to the
methoxy group. Project No. ABR-86114. Unpublished report dated March
2, 1987 from Ciba-Geigy Corporation, Greensboro, USA. Submitted to
WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Szolics, I. & Simoneaux, B. (1987h). Characterization of 14C-
methidathion metabolites in goat urine. Project No. ABR-86113.
Unpublished report dated March 3, 1987 from Ciba-Geigy Corporation,
Greensboro, USA. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Teitelman, U., Adler, M., Levy, I., & Dikstein, S. (1975). Treatment
of massive poisoning by the organophosphate methidathion. Clinical
Toxicology, 8(3), 277-282.
Tong C. (1982a) The hepatocyte primary culture/DNA repair assay on
compound GS 13005 - 008266 using mouse hepatocytes in culture.
Project No. M 111881. Unpublished report dated February 10, 1982
from Naylor Dana Institute, Valhalla, USA. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Tong C. (1982b) The hepatocyte primary culture/DNA repair assay on
compound GS 13005 - 008266 using rat hepatocytes in culture. Project
No. M 111881. Unpublished report dated February 10, 1982 from Naylor
Dana Institute, Valhalla, USA. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Ullmann, L., Krinke, G., Sachsse, K. & Hess, R. (1977). Acute oral
toxicity and neurotoxicity study of technical GS 13005 in the
domestic fowl ( Gallus domesticus). Project No. Siss 5927.
Unpublished report dated November 8, 1977 from Ciba-Geigy Ltd.,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Yau, E., McMartin, D., Zaidi, I., & Green, J. (1986). Methidathion:
2-year oral oncogenicity and toxicity study in albino rats. Project
No. 86061. Unpublished report dated May 23, 1986 from Ciba-Geigy
Corporation, Summit, USA. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Zoppellari, R., Targa, L., Tonini, P., & Zatelli, R., 1990. Acute
poisoning with methidathion: A case. Human & Experimental
Toxicology, 9(6), 415-419.
Metabolites from the urine of rats administered single oral
doses of 14C-labelled methidathion in the ring carbon adjacent to
the methoxy group at 0.25 or 2.52 mg/kg bw (Szolics & Simoneaux,
1987d) were identified. Organic soluble metabolites represented 61-
63%, whereas aqueous soluble metabolites accounted for 37-39% of the
urinary radioactivity. The major organic metabolite was the sulfide
(35.4%). Similar amounts of the sulfoxide (8.6%) and the sulfone
(8.2%) were detected. The RH compound represented 2.1% of the
radioactivity, and the oxygen analog represented 0.6%. No unchanged
parent was identified. With respect to the aqueous soluble
metabolites, the desmonomethyl derivative of methidathion accounted
for 20% (Szolics & Simoneaux, 1987g).
Goats
The urine of a single lactating goat treated daily by capsule
equivalent to a dietary intake level of 5 ppm for 10 days (Staley
et al. 1987) was selected for characterization of metabolites.
Partitioning data revealed that 84.5% of the urinary radioactivity
were aqueous soluble and 5.5% were organic soluble metabolites. The
principal aqueous soluble metabolites were the desmonomethyl
derivative (59.7%) and cysteine conjugate (10.4%) of methidathion.
Chromatography of the organic soluble components reportedly showed
that the metabolites in the goat were qualitatively the same as
those found in rat urine. The proposed predominant metabolic pathway
of methidathion in the goat was 0-demethylation (Szolics &
Simoneaux, 1987h).
Toxicological studies
Acute toxicity studies
Acute toxicity studies conducted in both the male and female
Tif.RAIf rat (Table 1), reveal that technical methidathion, upon
oral administration is highly toxic with LD50 values of 26 to 43.8
mg/kg bw. The acute dermal studies indicate that methidathion is
slightly to moderately toxic. Clinical signs of toxicity, upon oral
and dermal dosing were generally manifest as curved or ventral body
position, dacryorrhea/chromodacryorrhea, diarrhoea, dyspnea,
exophthalmus, ruffled fur, sedation, tonic/clonic muscle spasms and
trismus. The symptoms were reversible in surviving animals.
The acute oral toxicity of methidathion has been investigated
in several animal species (Table 2). The results indicate that
methidathion is moderately to highly toxic in all species tested
with LD50 values ranging from 17 to 200 mg/kg bw.
Table 1. Acute toxicity of technical methidathion
Species Sex Route Vehicle LD50 Purity Reference
(strain) (mg/kg bw)
Rat M&F oral CMC, 2% 43.8 ? Bathe (1973a)
(Tif. RAIf)
Rat M&F oral PEG, 400 26 96.9% Bathe & Sachsse
(Tif. RAIf) (1980a)
Rat M&F oral PEG, 400 26 92.7% Bathe & Sachsse
(Tif. RAIf) (1980b)
Rat M&F dermal CMC, 2% 1546 ? Bathe, (1973b)
(Tif. RAIf)
Rat M&F dermal CMC, 2% 1663 ? Bathe & Sachsse
(Tif. RAIf) (1975)
Rat M&F dermal CMC, 2% + 297 92.6% Sarasin (1980)
(Tif. RAIf) Tween 80, 0.1%
Short-term toxicity studies
Rats
Five groups of ten male rats received by gavage 0, 0.25, 0.83,
2.5 or 8.3 mg methidiathion/kg bw/day five days a week for four
weeks. Signs of cholinesterase inhibition occurred during the first
week at the 8.3 mg/kg bw/day level, but not later in this or in
other groups. Dose-related cholinesterase inhibition occurred in RBC
and plasma, the no-effect level being 0.25 mg/kg bw/day. Plasma
cholinesterase had returned to normal three days after treatment was
stopped, but RBC cholinesterase had not reached normal figures after
21 days (Noakes & Watson, 1964b).
Five groups of five male and five female rats received by
gavage 0. 2.5, 5, 10 or 20 mg methidathion/kg bw/day six days a week
for four weeks. In the 10 and 20 mg/kg bw/day groups, four and nine
animals died, respectively. Body-weight gain was depressed in all
groups, but no relation to dosage was apparent. There was a slight
increase in fat deposition in the liver at 5 mg/kg bw/day and at the
highest level this was more marked (Stenger & Roulet, 1963).
Groups of 24 male and 24 female rats were fed for 22 weeks on
diets containing 0, 1, 4, 16 or 64 ppm methidathion. In a similar
study in the same laboratory, groups of 24 male and 24 female rats
were fed for 26 weeks on diets supplying 0, 128 or 256 ppm
methidathion. The rate of body-weight gain was reduced at 64 ppm and
above in females but not in males. Histopathological examination of
liver, spleen and kidneys showed a dose-related increase in fat
deposition in the liver at doses above 64 ppm in both sexes. No
abnormalities in haematological indices or in results of urinalyses
were found (Stenger & Roulet, 1965).
Table 2. Acute oral toxicity of methidathion1
Species Sex LD50 Reference
(mg/kg bw)
Mouse F 17 Noakes & Sanderson, 1964
Hamster F 30 Noakes & Sanderson, 1964
Rat M&F 20-81 Slenger, 1964a,b, 1966a,b;
Aeppli, 1969a,b; 1970a,b;
Noakes & Sanderson, 1964
Rat M 26-65 Slenger, 1964a
Noakes & Sanderson, 1964
Guinea-pig F 25 Slenger, 1964a
Noakes & Sanderson, 1964
Rabbit M 80 Sachsse, 1971
Dog M&F 200 Noakes & Sanderson, 1964
Chicken F 80 Annex I, 19
1 Formulations calculated as a.i.
Groups of 20 male and 20 female rats were fed for six months on
diets containing 0, 0.5, 2, 10, 50 or 250 ppm methidathion. At the
250 ppm level weight gain was slightly depressed and clinical signs
of cholinesterase inhibition were seen, particularly in females.
Plasma cholinesterase was inhibited in the 250 ppm group and
erythrocyte cholinesterase in groups receiving 10 ppm and above.
Experimental groups were similar to controls with regard to
survival, food intake, weights and microscopic appearance of liver,
kidneys, spleen and testes and the macroscopic appearance of other
organs (Noakes & Watson, 1964a).
Rabbits
A dermal study was undertaken with groups of 5 New Zeeland
rabbits/sex administered methidathion (purity unknown) topically for
6-h daily non-occlusive exposure periods at doses of 0 (vehicle
control, PEG 300), 1, 5 or 20 mg/kg bw/day for a period of 22
consecutive days. There were no significant effects of treatment on
survival, food consumption, haematology, blood chemistry,
cholinesterase activity, organ weights, gross morphologic or
histopathological alterations. Based on minimal effects noted as
hypoactivity in a single male at 20 mg/kg bw, occasional incidences
of soft faeces or diarrhoea in treated males and a slight trend to
decreased body-weight gain in the high dose 20 mg/kg bw/day treated
males, a conservative NOAEL may be set at 5 mg/kg bw/day (Folinusz
et al. 1986).
A second dermal study was conducted with groups of New Zeeland
HRP:SPF rabbits (4-6 per sex) topically administered methidathion
(95% purity) daily for a 6-h occlusive exposure period at doses of 0
(vehicle control, PEG 400), 1, 10, 40 or 80 mg/kg bw for a period of
21 days. Treatment with methidathion resulted in mortality in males
at all dose levels (0/5, 2/5, 2/6, 3/5 and 3/5) and in females at 40
mg/kg bw/day and higher (0/5, 0/5, 0/4, 2/5 and 4/5). Clinical signs
of toxicity in males treated at dose levels of 1 mg/kg bw/day and
higher and in females treated at dose levels of 40 and 80 mg/kg
bw/day were manifest as anorexia, ataxia, hunched posture, languid
appearance and laboured respiration. Tremors were observed at dose
levels of 10 mg/kg bw/day and higher, whereas convulsions were
reported in a single high-dose (80 mg/kg bw/day) treated female.
Cholinesterase assessments revealed significant depression in the
mean plasma (38-86%), RBC (40-80%) and brain (37-88%) cholinesterase
values for both sexes at dose levels of 10 mg/kg bw/day and higher.
Treatment-related microscopic alterations were evidenced in the
liver and gall bladder. Principal findings in the liver were denoted
as hepatocytic clearing and congestion at dose levels as low as 1
mg/kg bw/day. Capsular/subcapsular necrosis with acute inflammation
was also noted in several of the treated animals. Lesions of the
gall bladder were present in both sexes at dose levels of 10 mg/kg
bw/day and higher and were attributed to bile reflux due to
hyperperistalsis. Subacute inflammation of the myocardium and
degeneration of the medial aorta occasionally with mineralization
were observed in several of the rabbits dying during the study
period. There were no specific effects of treatment on body-weight,
food consumption, ophthalmoscopy, haematological and blood
biochemical parameters or organ weights. The NOAEL was determined to
be 1 mg/kg bw/day by the author (Osheroff, 1987).
The results of the present study when interpreted
independently, have not unequivocally demonstrated 1 mg/kg bw/day to
be a NOAEL. The author has provided valid argument that evaluation
of primary toxicity was complicated by the additive effects of
stress especially during clinical observations. The occlusive rubber
binding used may not only have enhanced the absorption of the test
material but in combination with the neck collar, may have increased
the stress factor, thus augmenting the toxicity of the test
material. In view of the intrinsic variables in the present study
design, it is considered appropriate to critically assess the
results of the present study in conjunction with those generated
from the previously conducted rabbit dermal study, wherein dermal
administration of methidathion under non-occlusive means failed to
produce effects in rabbits at dose levels as high as 5 mg/kg bw/day
(Folinusz et al. 1986).
Dogs
Four groups of three male and three female beagle dogs received
diets containing 0, 4, 16 or 65 ppm methidathion for two years. The
animals were starved of diet one day each week and received a double
ration on the next day. Administration of methidathion was
discontinued from week 16 to 19. Erythrocyte cholinesterase was
inhibited in the 64 ppm group, but brain cholinesterase was
unaffected by treatment. SGPT was markedly elevated in the 64 and 16
ppm groups and slightly raised in males of the 4 ppm group. During
weeks 16 to 19 these levels fell, but only the 4 ppm group returned
to normal. SGOT levels were not the same as controls at all
treatment levels, but serum alkaline phosphatase was elevated and
sulfobromophthalein retention increased in the 16 and 64 ppm groups.
The livers of dogs receiving 16 and 64 ppm were pigmented on
macroscopic examination. Microscopically, pigmentation could be seen
in macrophages and hepatic cells (principally centrilobular) in the
16 and 64 ppm groups, the intensity of deposit being dose-related.
The Perl's reaction showed that the pigment did not contain
appreciable quantities of iron. The kidneys of the 64 ppm group also
showed pigmentation. It was questionable whether the livers of the 4
ppm group contained excess pigment. Control and test groups were
indistinguishable regarding behaviour, results of clinical tests
including neurological examination, haematological findings, organ
weights and macroscopic and microscopic appearance of organs other
than those mentioned. An additional two dogs received 64 ppm
methidathion in the diet for four weeks. The SGOT was elevated at
two and four weeks and at autopsy the livers were dark in colour.
Moderate diffuse pigmentation was seen microscopically in the liver
of one animal.
The plasma enzyme and liver histology changes at 30 days of
treatment at 64 ppm were not increased after treatment for two years
at this dose. Furthermore, the serum enzyme changes at 16-19 weeks
of treatment at 64 ppm were reversible and returned to normal three
weeks after cessation of treatment. The NOAEL was 4 ppm (Johnston,
1967).
The histological slides of the liver tissue from the original
study were re-evaluated and submitted to the 1975 Joint Meeting
Annex 1, reference 25). Intrahepatic cholestasis was observed in
dogs fed 16 and 64 ppm methidathion. Neither degenerative nor
inflammatory changes were associated with this lesion. Pigmentation
occurred as bile-plugs in biliary ductules, or as amorphous deposits
in Kupffer cells or as lipofuscin in both hepatic and Kupffer cells.
Haemolysis was not detected. A minimal degree lipofuscin
pigmentation was also observed at 4 ppm and in the control group.
Mild irregular fatty changes of the hepatocytes with occasional
periportal histiolymphocytic infiltration of the liver were observed
in both treated and control animals (Hess, 1975).
Methidathion (97% purity) was administered to groups of 4
beagle dogs per sex in the daily feed at dietary levels of 0, 0.5,
4, 45 or 140 ppm equal to 0, 0.02, 0.16, 1.96 or 5.67 mg/kg bw/day,
respectively, for a period of 90 days. An additional group of 4 dogs
per sex was treated orally by gelatin capsule at a dose level of
0.14 mg/kg bw/day, equal to a daily dietary intake level of 4 ppm,
for a similar treatment duration. A NOAEL of 4 ppm was assessed
based upon the evidence of cholestasis in all male and female dogs
treated at 45 and 140 ppm. Cholestasis was similarly described in a
single male dog treated by capsule at 0.14 mg/kg bw/day. Other
consequences of treatment evident in both sexes at dietary levels of
45 ppm and higher were discoloration of the liver and markedly
enhanced enzyme activity, expressed as increased levels of ALP,
SGOT, SGPT, GGT and sorbitol dehydrogenase. RBC cholinesterase
activity was significantly (75-88%) inhibited in dogs of both sexes
treated at 140 ppm. Brain cholinesterase activity was inhibited
(26.8%) in the 140 ppm treated females when compared to the
controls. There were no effects of methidathion treatment on serum
cholinesterase levels. Clinical signs of tremor and reduced activity
post-dosing, observed during the latter part of the study, were
exhibited in a single male dog at the high dose level. Reduced
activity was also noted in a single male treated at 45 ppm. Other
effects of treatment were significantly decreased mean food intake
values in the high dose treated males when compared to the control
group. There were no treatment-related effects observed with respect
to survival, body-weights, ophthalmoscopic examination,
haematological parameters, urinalysis or organ weights (Chang &
Wyand, 1990).
Six groups of 4 beagle dogs/sex were treated with methidathion
(96% purity) at dietary levels of 0, 0.5, 2, 4, 40 or 140 ppm, equal
to 0, 0.02, 0.07, 0.15, 1.34 or 4.51 mg/kg bw/day, respectively, for
a period of 12 months. A NOAEL of 4 ppm was indicated based on
treatment-related hepatic effects observed grossly as general
discoloration of the liver and characterized histomorphologically as
cholestasis and chronic inflammation of the liver in both sexes at
dietary levels of 40 ppm and higher. Associated changes in blood
biochemical parameters were denoted by elevated ALP, SGOT, SGPT,
sorbitol dehydrogenase and bilirubin levels. Other significant
clinical changes recorded only in the females were increased GGT and
decreased total protein and albumin values. RBC choline-sterase
activity was markedly inhibited (76-87%) in both male and females
dogs treated with methidathion at 140 ppm. Serum cholinesterase
activity was not adversely affected by treatment at any dietary
level. Brain cholinesterase activity was significantly depressed
(16-27%) in both sexes treated at 140 ppm when compared to the
controls. Another effect of treatment was related to decreased food
consumption in male dogs treated with methidation at a dietary level
of 140 ppm. There were no effects of treatment on survival, clinical
signs, body-weights, ophthalmoscopy, haematology, urinalysis, faecal
examination or organ weights (Chang and Walberg, 1991).
Monkeys
Three groups of Rhesus monkeys (approximately equal number of
each sex) were administered 0, 0.25 or 1.0 mg methidathion/kg bw/day
by stomach tube, 6 days a week for 23 months. Two of each group were
autopsied after 12 months. Plasma and erythrocyte cholinesterase
activity were inhibited in the 1 mg/kg bw/day group, but not at the
lower level. Brain cholinesterase was unaltered by treatment.
Growth, results of haemological tests, results of chemical analyses
of serum (including SGPT and ALP) and macro- and microscopic
examination of tissues were similar in control and test groups
(Fabran et al., 1971).
Long-term toxicity/carcinogenicity studies
Mice
A 23-month study was conducted with groups of 50 Charles River
CD-1 mice per sex/group fed diets containing methidathion (purity
not stated) at levels of 0, 3, 10, 50 or 100 ppm equal to 0.43,
1.42, 6.99 or 13.70 mg/kg bw/day, respectively. An additional 120
mice/sex/group were assigned to the chronic phase of this bioassay.
Interim sacrifices of 20 mice/sex/group were scheduled after 3, 6,
12 and 13 months. Animals sacrificed at 13 months were maintained on
control diet for one month as recovery animals following 12 months
of continuous treatment. The remaining animals (40/sex/group,
maximum) were sacrificed after 18 months of treatment. Treatment of
mice with methidathion resulted in slightly decreased survival of
the 100 ppm treated males when compared to the controls. The only
clinical sign remarked upon was discoloration of the urine in the 50
and 100 ppm treated males. Potentially reversible increases in liver
enzyme activity were reported in males at 50 ppm and higher and in
females at 100 ppm. Significantly decreased but potentially
reversible RBC cholinesterase activity values (26-46%) were
registered in males at 100 ppm and in females at 50 ppm and higher.
Brain cholinesterase activity was markedly inhibited (15-49%) in
both sexes at 100 ppm. There were no inhibitory effects of treatment
on plasma cholinesterase activity. There were no effects of
methidathion treatment noted with respect to body-weights, food and
water consumption or ophthalmoscopy. Treatment-related target organ
alterations were manifest in the gall bladder and liver at dietary
levels of 50 ppm and higher in males and in females at 100 ppm.
Microscopic changes were described in the gall bladder as
cholecystitis and hyperplasia and, hepatic findings were denoted as
bile duct hyperplasia, bile stasis, cholangiofibrosis, chronic
hepatitis and hypertrophy. Increased extramedullary haematopoiesis
of the spleen, associated with increased spleen weights were
observed in the 100 ppm treated males. Treatment of male mice with
methidathion resulted in a significantly increased incidence of
hepatocellular tumours (adenomas, carcinomas and adenomas/carcinomas
combined).
0 3 10 50 100 ppm
Adenoma 1/46 9/45 7/47 8/43 21/45
Carcinoma 8/46 6/45 4/47 13/43 17/45
Combined 9/46 15/45 11/47 21/43 38/45
Historical control data (Quest et al. 1990) generated from 14
studies conducted at the same test facility with the same strain of
mouse, revealed that the incidences of hepatocellular tumours
reported in the present study exceeded the historical control range
at dietary levels of 50 ppm and higher. Latency, expressed in terms
of time to appearance of first tumour, was not apparently decreased
in the treated male groups when compared to the concurrent controls.
The incidence of hepatocellular tumours was not increased in female
mice treated with methidathion The NOAEL in this study was 10 ppm,
equal to 1.4 mg/kg bw/day (Goldenthal, 1986).
Rats
In order to investigate the long-term toxic effects and
possible carcinogenic action of methidathion, four groups of 25 male
and 25 female rats each were fed for three weeks on diets containing
0, 2, 8 or 32 ppm and for a further 101 weeks on diets containing 0,
4, 16 or 64 ppm methidathion. The rate of gain in body weight was
reduced from week 8 in male animals of the 64 ppm group. After the
first year the rates of gain became erratic in all groups, making
interpretation of the findings difficult. Female rats on test diets
grew at a rate comparable to controls. Erythrocyte cholinesterase
was inhibited in the 16 and 64 ppm groups, while plasma
cholinesterase showed minimal inhibition at 100 weeks in the 64 ppm
group only. Brain cholinesterase was inhibited in the 64 ppm group,
with marginal and no reduction in the 16 and 4 ppm groups,
respectively. Decreased relative adrenal weights were found in
females of the 16 ppm and 64 ppm groups, and decreased ovary weights
in the 64 ppm group. The relative kidney weights of males was
increased in the 16 and 64 ppm groups. A greater frequency of
hepatic degenerative changes was noted in rats fed methidathion in
the diet; the high incidence of pulmonary infections in the rats
renders this finding of doubtful toxicological significance. The
food intake, results of haematological investigations and chemical
analysis of serum (including SPGT) and the survival rate were
similar in the test groups and in the control group. The incidence
of tumours was variable between groups but was low and not dose-
related, and no unusual tumours were found. The NOAEL in this study
was 4 ppm methidathion in the diet (Johnston, 1967).
Groups of 65 Crl:COBS CD(SD) BR rats/sex were treated with
methidathion (97.3% purity) at dietary levels of 0, 4, 40 or 100 ppm
equal to 0.16, 1.72 or 4.91 mg/kg bw/day, respectively for a period
of 104 weeks. For interim sacrifice purposes, an additional 15
rats/sex/group were assigned on study. At 52 weeks, 10
rats/sex/group were sacrificed with the remaining 5 rats/sex/ group
sacrificed at week 93 of study. Treatment with methidathion resulted
in clinical signs expressed in the 40 and 100 ppm groups as
alopecia, chromorhinorrhea, hyperactivity, skin lesions, tremors,
hypersensitivity to the touch and fasciculation. Mean body-weights
and weight gains were decreased in both sexes at 100 ppm throughout
the course of treatment. Body-weight gains were decreased at a
dietary level of 40 ppm during the initial few weeks of treatment,
with comparable weight gain thereafter. Mean food intake was
significantly increased in both sexes treated at dietary levels of
40 ppm and higher, whereas water consumption was decreased at 100
ppm and occasionally at 40 ppm. Treatment-related haematological
findings were exhibited at a dietary level of 100 ppm as inverted
neutrophil:lymphocyte ratios, reduced RBC parameters and increased
platelet counts. Variations in blood biochemical parameters were
observed at 40 ppm and higher as decreased total bilirubin,
decreased total protein as well as changes in electrolyte levels
(decreased potassium and calcium, and increased inorganic phosphorus
and chloride). Significant inhibition of RBC (14-38%), serum (22-
66%) and brain (42-74%) cholinesterase activity were determined in
both sexes of rats treated at dietary levels of 40 ppm and higher.
Notable changes in urinalyses were revealed as decreased volume and
increased specific gravity at 40 ppm and higher which correlated
well with the reduced fluid intake reported in these groups. Gross
necropsy findings showed an increased incidence of skin lesions in
the 40 and 100 ppm levels which were associated microscopically with
ulceration and/or chronic purulent inflammation. Other pathological
alterations were related to an increased incidence in focal
accumulations of foamy macrophages in the alveoli of the 100 ppm
treated males and females. There were no effects of treatment on
survival or ophthalmoscopy. The NOAEL for in-life parameters was
determined to be 4 ppm, equal to 0.16 mg/kg bw/day. There was no
evidence of methidathion-induced carcinogenic potential (Yau et
al., 1986).
Reproduction studies
Rats
In a three-generation study, three groups of 10 male and 20
female rats were fed on a diet containing 0, 2 or 16 ppm
methidathion for three weeks, and thereafter on a diet containing 0,
4 or 32 ppm methidathion. Litters from the second mating were used
to provide the new generations. The F1b litters did not receive
test diets until 26 days and the F2b until 22 days after weaning.
F0, Flb and F2b generations received diets for 27-28 weeks
during which they produced two litters. The number of young
surviving at weaning was reduced in all generations of litters from
animals fed 32 ppm methidathion and the mean liver weight of F3b
weanlings of this group was slightly increased. Body weights,
reproductive capacity and mortality of parents and the number of
litters, litter size, mean birth and weaning weights of test groups
were comparable to controls. The number of stillbirths and incidence
of congenital abnormalities were unaltered by treatment. No
histological damage was found in the organs of the F3b animals
examined. The NOAEL in this study was 4 ppm methidathion (Lobdell &
Johnston, 1966).
A two-generation (one litter per generation) reproduction study
was conducted with groups of 15 male and 30 female Charles River SD
CR1:CD BR rats fed methidathion (purity not specified) at dietary
levels of 0, 5, 25 or 50 ppm equal to 0, 0.43, 2.08 or 4.23 mg/kg
bw/day, respectively. With respect to reproductive performance,
statistically (p < 0.05) reduced mating indices were recorded for
the 25 and 50 ppm groups in the F1 generation. Although the mating
indices were reduced in these groups (69% and 66%, respectively)
relative to the concurrent F1 control group (88%), they were
nevertheless not significantly different from those calculated for
the F0 generation control (73%) or treated groups (60-65%). The
fertility and gestation indices were comparable among groups.
Treatment-related effects on maternal animals fed dietary levels of
25 ppm and higher were revealed by clinical signs of toxicity
manifest during lactation as slight and/or intermittent tremors.
Mean body-weights of F1 50 ppm-treated males were decreased prior
to and during the 12-week premating period. Mean body-weights gains
were not, however, significantly different from controls. Body-
weights of females recorded during lactation of both generations
were decreased at 50 ppm when compared to the concurrent control.
Decreased ovary weights recorded in females at 25 and 50 ppm were
not correlated with histopathological changes. Effects of treatment
on progeny were expressed as decreased survival in the 50 ppm group
of the F1 generation, decreased body-weights and clinical signs in
both generations at 25 and 50 ppm. The clinical signs were
suggestive of poor maternal care and were described as
weakness/lethargy, coolness to the touch and starving appearance. On
the basis of the reported findings, a NOAEL of 5 ppm, equal to 0.43
mg/kg bw/day, was indicated (Salamon, 1987).
Special studies on teratogenicity
Rats
A teratology study was conducted with groups of 25 mated female
Crl: COBS CD(SD)BR rats administered methidathion (93.2-96% purity)
at 0.25, 1.0 and 2.5 mg/kg bw/day or the control (vehicle, 3%
aqueous cornstarch with 0.5% Tween 80) orally by gavage from days 6
to 15 of gestation, inclusive. Confirmation of mating was determined
by presence of sperm in the vaginal washing, and this day was
designated day 0 of gestation. A NOAEL for maternal toxicity was
indicated at 1.0 mg/kg bw/day based on mortality, decreased body-
weights, food intake and clinical signs at 2.5 mg/kg bw/day.
Clinical signs of toxicity in the high dose (2.5 mg/kg bw/day) group
were suggestive of organophosphorus intoxication including,
lethargy, tremors, lacrimation, salivation, raspy respiration,
exophthalmia, chromodacryorrhea. A NOAEL for embryofetal toxicity
was set at the highest dose level tested of 2.5 mg/kg bw/day. There
were no significant differences noted with respect to the mean
number of implantations, live fetuses or mean number of resorptions
on a litter basis. There was similarly no significant variability in
the post-implantation loss, fetal sex ratio or in the mean fetal
litter weight in the treated groups when compared to the control.
Treatment with methidathion failed to uncover any evidence of
teratogenic potential (Mainiero et al. 1987).
Rabbits
Methidathion (purity not specified) was administered orally by
gavage at 0 (vehicle control, 3% cornstarch with 0.5% Tween 80), 2,
6 or 12 mg/kg bw/day to groups of 19 artificially inseminated New
Zeeland white rabbits on days 7 through 19 of gestation. The day of
artificial insemination was designated as day 0 of gestation.
Treatment-related clinical signs of toxicity at the high dose (12
mg/kg bw/day) treated animals were manifest as ataxia, tremors,
salivation and miosis. In the absence of similar effects in the
control or other treated groups, the NOAEL for maternal toxicity was
demonstrated to be 6 mg/kg bw/day. There was no evidence of
embryofetal developmental toxicity or potential for teratogenicity
at any of the dose levels investigated, including the highest dose
of 12 mg/kg bw/day (Hummel et al. 1987).
Special studies on genotoxicity
A battery of mutagenicity assays were conducted with technical
methidathion, the results of which are presented in Table 3. The
tests performed to evaluate potential for gene mutation in bacteria,
DNA damage as well as chromosome aberration were negative. A
dominant lethal study in mice was also negative. Positive responses
were observed in the in vitro assays with Saccharomyces
cerevisiae and in the Chinese hamster test for sister chromatid
exchange. In vivo tests conducted to examine the similar
endpoints, did not however elicit any evidence of mutagenic
potential in mammalian cells.
Special studies on delayed neurotoxicity
Four adult hens received four subcutaneous injections of 50 mg
methidathion/kg body-weight (the maximum tolerated dose) at weekly
intervals and they were observed for a further four weeks. The signs
of acute poisoning lasted two to three days each time, but birds
remained in good condition and no paralysis developed.
Neuropathological examinations were not performed (Noakes, 1964).
Five groups of ten adult hens were fed diets containing 0, 16,
52 or 160 ppm methidathion or 316 ppm tri-orthocresylphosphate for
45-47 days. No abnormal neurological signs were found in birds fed
methidathion, but those on tri-orthocresylphosphate showed leg
weakness, lack of balance and ataxia during the final week of
treatment. Unequivocal evidence of demyelination of neural tissue
was not found in methidathion or tri-orthocresylphosphate-treated
animals (Johnston, 1965).
The delayed neurotoxic potential of methidathion (purity not
specified) was investigated in groups of 10-30 female white leghorn
hens. The animals were administered the test material twice, 21 days
apart, at 0 (vehicle, CMC 2%), 43.75, 87.5, 175 or 350 mg/kg bw.
Animals receiving methidathion at 350 mg/kg bw were pretreated with
an intramuscular injection of atropine sulfate, 10 mg/kg bw, one
hour prior to treatment. Groups of 3 male and 3 female hens received
a single oral dose of the positive control, TOCP at 215, 464, 600,
1000 or 2150 mg/kg bw and were then observed for 21 days post-
dosing. A preliminary acute oral study indicated that the LD50 of
methidathion in the hen was 175 mg/kg bw. Clinical signs of
intoxication were expressed as ataxia, slight tremor, curved or
ventral body position and sedation, with subsequent recovery in
surviving animals, 8-10 h post-dosing. Histopathological evaluation
of the spinal cord and peripheral nerves did not reveal any
treatment-related lesions of the nervous system, whereas TOCP
toxicity was evidenced as central-peripheral, distally accentuated
neuropathy. Treatment with methidathion did not produce any signs of
delayed neurotoxicity (Ullmann et al. 1977).
Table 3. Genotoxicity of technical methidathion
Test Test system Concentration Purity Results References
(vehicle)
Reverse mutation S. typhimurium 25, 75, 225, 675, 2025 98.4% negative Arni & Muller (1980a)
(in vivo) TA98, 100, 1535, 1537 µg/0.1 mL (DMSO) 1., 2.
S. typhimurium 25, 75, 225, 675, 2025 93.4% negative Arni & Muller (1980b)
TA98, 100, 1535, 1537 µg/0.1 mL (DMSO) 1., 2.
S. typhimurium 0, 0.1, 0.5, 1, 5, 10, 50 99.95% negative Satou et al. (1979)
TA98, 100, 1535, 1537 mg/mL (DMSO) 1., 2.
E. coli
B/r WP2 Try- Hcr-
S. typhimurium 10, 50, 100, 500, 1000, ? negative Simmon et al. (1977)
TA98, 100, 1535, 1537, 5000 µg/plate (DMSO) 1., 2.
1538
Rec assay (in vitro) B. subtilis 5, 25, 100 mg/mL (DMSO) 99.95% negative Satou et al. (1979)
H17, M45
Reverse mutation/ S. typhimurium 10, 20, 40 mg/kg bw ? negative Simmon et al. (1977)
Host-mediated TA1535, 1538/Male Swiss (single oral doses)
(in vivo) Webster mouse
(inocculated ip)
5, 10, 20 mg/kg bw negative
(5 oral doses) (DMSO)
S. typhimurium 0, 5, 10, 20 mg/kg bw 93.4% negative Arni & Muller (1980c)
TA98, 100, 1537/ (3 oral doses: 2 h, 1 h
male mouse and prior to innoculation)
(inocculated iv) (CMC, 0.5%)
Table 3 (cont'd)
Test Test system Concentration Purity Results References
(vehicle)
Gene conversion/ S. cerevisiae MP-1 675, 1250, 2500, 5000, 93.4 conversion: Arni & Muller (1981)
forward mutation 10000 µg/mL (DMSO) slight (+ ve)
(in vivo) mutation:
(+ ve)
Forward mutation Mouse lymphoma cells - 0, 15 mg/kg bw (3 oral 93.4% negative Strasser & Muller (1980)
host-mediated (in L5 178Y/mouse doses post-innoculation)
vivo) (DBA/Bom) (CMC)
DNA repair Mouse (male CD-1) 5 x 10-7 to 1% (DMSO) ? negative Tong (1982a)
(in vivo) hepatocytes
Rat (male F344) 5 x 10-9 to 1% (DMSO) ? negative Tong (1982b)
hepatocytes
Rat hepatocytes 0.128, 0.64, 3.2, 16 97.2% negative Puri & Muller (1982a)
µg/mL (DMSO)
Rat hepatocytes 1.85, 5.56, 16.67, 50, 100, 96% negative Hertner & Arni (1990)
200 µg/mL (DMSO)
Human fibroblasts 1.024, 5.12, 25.6, 128 ? negative Puri & Muller (1982b)
µg/mL (DMSO)
SCE (in vivo) Chinese hamster 0, 10, 20, 40, 80 µg/mL 99.1% slight (+ ve) Chem et al. (1981)
cell line V79 (DMSO) at 40, 80
ug/mL
SCE (in vivo) Chinese hamster 0, 17, 34, 68 mg/kg bw 93.4% negative Hool & Muller (1980)
(bone marrow) (CMC)
Table 3 (cont'd)
Test Test system Concentration Purity Results References
(vehicle)
Chromosome Chinese hamster ovary 43, 75, 87.5, 175, 350 96% negative Strasser & Arni (1990)
aberration CCL 61 µg/mL (DMSO)
(in vivo) 96.9% negative Hool et al. (1980)
Nucleus anomaly Chinese hamster 0, 17, 34, 68 mg/kg bw
(in vivo) (bone marrow) (2 oral doses) (CMC) 98.4% negative Fritz (1976)
Dominant lethal Mice, NMRI 0, 15, 45 mg/kg bw (CMC)
(in vivo)
1. = in the presence of metabolic activation
2. = in the absence of metabolic activation
Special studies on irritation and sensitization
The eye irritation potential of technical methidathion was
investigated in 3 English Silver strain rabbits/sex. Administration
of 0.1 grams of methidathion into the conjunctival sac of the left
eye (right eye served as control) produced irritating conjunctival
reaction in the unwashed eye of a single male rabbit (Sachsse,
1973a).
Technical methidathion (as a 50% polyethylene glycol
suspension) was applied under occlusive conditions to the shaved
backs of male and female English Silver strain rabbits for 24 h.
Skin reactions were observed as very slight erythema in one male and
moderate to severe erythema in association with slight oedema in one
female rabbit (Sachsse, 1973b).
The shaved backs of male Hartley albino guinea-pigs were
repeatedly treated with technical methidathion as a 10% solution in
diethyl ether. There was no evidence of skin sensitization potential
(Cannelongo, 1984).
Special studies on potentiation
The potentiating effects of methidathion (purity not specified)
with profenofos (Sachsse & Bathe, 1977b) and methacrifos (Sachsse &
Bathe, 1978) were investigated in male and female Tif:RAIf rats by
comparing the theoretical LD50 values, based on an assumption of
strictly additive toxicity, with that of the experimentally derived
LD50 values for the equitoxic mixtures. There was no potentiating
effect of methidathion with profenofos, whereas a slight enhancement
of acute toxicity was observed with an equitoxic mixture of
methidathion and methacrifos.
Special studies on promoting activity
Male F344 DuCrj strain rats received a single ip injection of
the initiator, N-nitrosodiethylamine (DEN) at 200 mg/kg bw. Two
weeks thereafter, the rats were treated with methidathion (92.7%
purity) at dietary levels of 0, 10, 30, 100 or 300 ppm (equal to 0,
1.0, 2.3, 7.6 or 22.9 mg/kg bw, respectively) or the positive
control, 3'-methyl-4-dimethyl amino azobenzene (3'-Me-DAB) at 600
ppm (equal to 33.9 mg/kg bw) for a period of 6 weeks. Three weeks
following treatment with DEN, partial hepatectomy was performed on
all animals. Histochemical evaluation of 4 liver sections from each
animal after 8 weeks on study, revealed a significantly increased
number of GGT positive foci per unit area in rats treated with
methidathion at 100 and 300 ppm and in the 600 ppm 3'-Me-DAB
positive control group. No increases in the number of GGT positive
foci were observed in additional groups of rats treated with
methidathion at 100 and 300 ppm alone, without previous initiation
with DEN. The number of foci in rats treated with methidathion at 30
ppm and lower were not different from the controls (Arai, 1981).
A second short-term study of the promoting activity in the rat
liver was conducted with groups of male Fischer 344 DuCrj strain
rats administered methidathion (98.6% purity) at dietary levels of
0, 35, 46, 59, 77 or 100 ppm (equal to 0, 2.7, 3.5, 4.8, 6.0 or 7.6
mg/kg bw/day, respectively) for a period of 6 weeks. Two weeks prior
to feeding with methidathion, the rats were pretreated with a single
ip injection of the initiator DEN at 200 mg/kg bw. Partial (two-
thirds) hepatectomy was performed three weeks after initiation and
the animals were sacrificed after 8 weeks on study. Histochemical
analysis unveiled a significant dose-related increase in the number
of GGT positive foci in rats treated with methidathion at dietary
levels of 59 ppm and higher. The number of GGT positive foci at
dietary levels of 46 ppm and lower were comparable to the controls.
No increases in the number of GGT positive foci were recorded in an
additional group of rats treated with methidathion at 100 ppm alone,
without previous initiation with DEN (Daiyu-Kai Institute of Medical
Science, 1983).
Special studies on therapeutic activity of antidotes
The therapeutic potential of antidotes was tested by
administering methidathion (purity not specified) orally by gavage
to Tif.RAIf rats at the LD80 of 46 mg/kg bw. The therapeutic
agents, atropine sulfate alone, toxogonin alone, PAM alone and
toxogonin combined with atropine sulfate were then administered
intramuscularly after the first appearance of clinical signs of
poisoning. A further aspect of therapeutics was investigated, by
administering 5 repeated daily oral doses of methidathion at the
LD50 (34 mg/kg bw) to rats and then, upon observation of clinical
signs, commencing with the respective combination of antidotes. All
therapeutic agents were, according to the treatment regimen,
effective against the oral LD80 of methidathion. A positive
protective effect against repeated LD50 doses of methidathion were
observed with atropine and toxogonin. PAM and toxogonin in
combination with atropine had minimal protective effect against
repeated challenges to methidathion (Sachsse & Bathe, 1977a).
Observations in humans
One male subject took 4 mg/day (equal to 0.04 mg/kg bw/day) for
17 days, and 8 mg/day (equal to 0.08 mg/kg bw/day) of methidathion
for 27 days. No effect was found on RBC and plasma cholinesterase,
the thrombocyte count and stability or on the clinical condition of
the subject (Payot, 1965).
Two groups of eight men received 0.04 or 0.11 mg
methidathion/kg bw/day orally in capsules for 6 weeks. Four men
received placebo capsules. The treatment with methidathion was
without effect on plasma and RBC cholinesterase, SGPT and SGOT and
results of urinalyses or on EEG pattern or clinical condition of the
subject (Coulston, 1970).
A case of massive poisoning was reported to have occurred where
a 25-year old man weighing 60 kg ingested a number of mouthfuls of
supracide 40 (methidathion, 40% active ingredient). The man was
discovered approximately 2 hours after the event, in an unconscious,
semi-comatose condition. Upon admission to hospital the patient was
treated intravenously with atropine and toxogonin. The
cholinesterase activity in the serum was found to be zero a few
hours after admission. The patient was discharged after 15 days of
hospitalization. The laboratory findings were normal with exception
of cholinesterase activity which remained low. Follow-up examination
at 2, 5 and 10 months revealed no abnormalities with no evidence of
delayed neurotoxicity (Teitelman et al. 1975).
A case of attempted suicide occurred in a 50-year old man
weighing 67 kg who had ingested approximately 6.2 g or 40 ml of
Supracid 20 (methidathion, 15.5% active ingredient). About 1.5 h
after the incident, the subject was admitted to hospital suffering
from mental confusion, muscle fasciculations, bradycardia, miosis,
sweating, salivation and lacrimation. The patient underwent gastric
lavage followed by treatment with atropine sulphate and pralidoxime.
Six hours after ingestion of methidathion, the incidence of muscle
fasciculations increased followed by bronchorrhea and coma. Serum
and RBC cholinesterase values, as measured over eight days following
poisoning were generally less than 50% of normal values.
Cholinesterase values assessed after 45 days were normal.
Lymphocytic NTE activity was normal as determined on days 5, 10, 17
and 45 after poisoning. The patient was discharged after 21 days of
hospitalization. A follow-up examination after 7 months failed to
establish any evidence of delayed polyneuropathy or other
neurological abnormality (Zoppellari et al. 1990).
COMMENTS
Methidathion was extensively absorbed when administered orally
to rats. The routes of elimination were via the urine and expired
CO2. There were no significant differences in elimination patterns
with regard to dose levels administered, pre-treatment or sex.
In the rat, the predominant urinary metabolites were the
sulfide, sulfoxide, sulfone and desmonomethyl derivative. Negligible
quantities of the parent and oxygen analog were detected in the
urine. The predominant metabolic pathway of methidathion in the goat
was via O-demethylation with the desmonomethyl derivative as the
principal urinary metabolite. Cysteine conjugates were identified in
each species.
Methidathion has a high acute oral toxicity. The World Health
Organization has classified methidathion as highly hazardous (WHO,
1992).
Methidathion was administered to dogs for 90 days at dietary
concentrations of 0, 0.5, 4, 45 or 140 ppm, or 0.14 mg/kg bw/day
(equal to 4 ppm) by capsule, and for a period of 12 months at 0,
0.5, 2, 4, 40, or 140 ppm in the diet. In both studies the dietary
NOAEL was determined to be 4 ppm, equal to 0.16 mg/kg bw/day, based
on liver effects, most notably cholestasis and increased liver
enzymatic activity in serum at dietary levels of 40 ppm and above.
Cholestasis was observed in a single male dog treated by capsule at
0.14 mg/kg bw/day. Erythrocyte and brain cholinesterase activities
were affected only at the highest level of 140 ppm.
Long-term dietary treatment of mice with methidathion for 23
months at 0, 3, 10, 50 or 100 ppm revealed an increased incidence of
hepatocellular tumours in males at 50 ppm and above, resulting in a
NOAEL of 10 ppm, equal to 1.4 mg/kg bw/day. Erythrocyte
cholinesterase was inhibited at 50 ppm (equal to 7 mg/kg bw/day) and
above, whereas brain cholinesterase activity was affected at 100 ppm
(equal to 13.7 mg/kg bw/day).
A 104-week long-term toxicity/carcinogenicity study in rats fed
methidathion at 0, 4, 40 or 100 ppm indicated a NOAEL of 4 ppm,
equal to 0.16 mg/kg bw/day, based on inhibition of erythrocyte and
brain cholinesterase activity at 40 ppm and above. Methidathion was
not carcinogenic in rats.
In a two-generation reproduction study in rats at dietary
concentrations of 0, 5, 25 or 50 ppm, the NOAEL was 5 ppm, equal to
0.43 mg/kg bw/day. At 25 ppm, reduced mating indices in the F1
generation and decreased progeny body weights were observed.
There were no teratogenic effects observed when methidathion
was administered by gavage to rats at doses of 0, 0.25, 1.0, or 2.5
mg/kg bw/day or to rabbits at doses of 0, 2, 6 or 12 mg/kg bw/day.
Maternal effects were demonstrated at the highest doses in both the
rat and rabbit as clinical signs of toxicity. In the rat, increased
mortality as well as decreased body-weights and food consumption
were also observed. The NOAELs in the rat and rabbit were determined
to be 1 and 6 mg/kg bw/day, respectively.
Treatment of hens with methidathion did not produce any
clinical or pathological evidence of delayed neurotoxicity.
In two reported cases of poisoning with methidathion, each of
the male subjects exhibited classic clinical and biochemical signs
of organophosphorus intoxication. Both subjects recovered, with no
evidence of delayed neurotoxicity uncovered upon follow-up
examination. In one of the cases jaundice was reported during the
recovery period.
Two human volunteer studies failed to reveal any inhibition of
erythrocyte or serum cholinesterase activity at doses up to 0.11
mg/kg bw/day.
After reviewing the available genotoxicity data, the Meeting
concluded that methidathion was not genotoxic.
In the dog, the effects on the liver appeared to have occurred
at levels lower than the levels causing inhibition of cholinesterase
activity, giving a NOAEL of 0.1 mg/kg bw/day. Whether or not there
was a relationship to the potential induction of liver effects in
man could not be ascertained.
The Meeting concluded, after consideration of the
hepatocellular tumours found in male mice in the long-term toxicity
study, together with the lack of genotoxicity, that methidathion did
not present a carcinogenic hazard for humans.
The previous ADI, based on a NOAEL in man of 0.11 mg/kg bw/day,
was revised. The revised ADI is based on the NOAEL in the dog and a
100-fold safety factor.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 10 ppm, equal to 1.4 mg/kg bw/day (23-month study)
Rat 4 ppm, equal to 0.16 mg/kg bw/day (104-week study)
5 ppm, equal to 0.43 mg/kg bw/day (reproduction
study)
Dog: 0.1 mg/kg bw/day (90-day, one-year, and two-year
studies)
Human: 0.11 mg/kg bw/day.
Estimate of acceptable daily intake for humans
0 - 0.001 mg/kg bw
Studies which will provide information valuable in the continued
evaluation of the compound
Further observations in humans
REFERENCES
Aeppli, L. (1969a) Akute Toxizität, Ratte per os, A 2039 E (0 40
WP). Unveröffentlicht. Geigy, J.R. AG.
Aeppli, L. (1969b) Akute Toxizität, Ratte per os, A 3628 E (0 40
WP). Unveröffentlicht. Geigy, J.R. AG.
Aeppli, L. (1970a) Akute Toxizität, Ratte per os, A 3628
blaugefärbt. Unveröffentlicht. Geigy, J.R. AG.
Aeppli, L. (1970b) Akute Toxizität, Ratte per os, A 3389 C (= 40
ES). Unveröffentlicht. Geigy, J.R. AG.
Arai, M. (1981). In vivo short term study on the promoting
activity of methidathion (CG-831) (translation). Unpublished report
dated October 31, 1981 from Daiyu-Kai Ikagaku Kenkyu-Sho, Japan.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Arni, P. & Muller, D. (1980a). Salmonella/mammalian-microsome
mutagenicity test with GS 13005 (Test for mutagenic properties in
bacteria). Project No. 79/1556. Unpublished report dated April 17,
1980 from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Arni, P. & Muller, D. (1980b). Salmonella/mammalian-microsome
mutagenicity test with GS 13005 (Test for mutagenic properties in
bacteria). Project No. 801488. Unpublished report dated October 29,
1980 from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Arni, P. & Muller, D., 1980c. Intrasanguine host-mediated assay with
S. typhimurium with GS 13005 (Test for the demonstration of point
mutations in bacteria in vivo). Project No. 801494. Unpublished
report dated October 31, 1980 from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Arni, P. & Muller, D. (1981). Mutagenicity test on Saccharomyces
cerevisiae MP-1 in vitro with GS 13005 (Test for mutagenic
properties in yeast cells). Project No. 802030. Unpublished report
dated November 5, 1981 from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Bathe, R. (1973a). Acute oral LD50 of technical GS 13005 in the
rat. Unpublished report dated May 25, 1973 from Ciba-Geigy Ltd.,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Bathe, R. (1973b). Acute dermal LD50 of technical GS 13005 in the
rat. Project No. Siss 2929. Unpublished report dated October 3, 1973
from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Bathe, R. & Sachsse, K. (1975). Acute dermal LD50 in the rat of
technical GS 13005. Project No. Siss 4650. Unpublished report dated
September 15, 1975 from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Bathe, R. & Sachsse, K. (1980a). Acute oral LD50 in the rat of
technical GS 13005. Project No. 791668. Unpublished report dated
January 9, 1980 from Ciba-Geigy Ltd., Basle, Switzerland. Submitted
to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Bathe, R. & Sachsse, K. (1980b). Acute oral LD50 in the rat of
technical GS 13005. Project No. 791667. Unpublished report dated
January 10, 1980 from Ciba-Geigy Ltd., Basle, Switzerland. Submitted
to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Cannelongo, B. (1984). Guinea pig skin sensitization. Supracide
technical FL 830958. Project No. 3152-83. Unpublished report dated
January 9, 1984 from Stillmeadow, Inc., Houston, USA. Submitted to
WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Chang J. and Walberg, J. (1991). One-year dietary study in Beagle
dogs. Project No. F00028. Unpublished report dated June 24, 1991
from Ciba-Geigy Corporation, Framington, USA. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Chang, J. & Wyand, S. (1990). 90-day oral toxicity study in Beagle
dogs. Project No. F-00023. Unpublished report dated March 21, 1990
from Ciba-Geigy Corporation, Farmington, USA. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Chen, H., Hsueh, J., Sirianni, S., & Huang, C. (1981). Induction of
sister-chromatid exchanges and cell cycle delay in cultured
mammalian cells treated with eight organophosphorus pesticides.
Mutation Research, 88: 307-316.
Coulston, F. (1970). Effects on man of small daily doses of GS13005.
Unpublished report of Albany Medical College. Submitted to WHO in
1972 and cited in Annex I, 19.
Daiyu-Kai Institute of Medical Science (1983). In vivo short-term
study on dose-dependence and threshold level of promoting activity
of GS13005 in the rat liver. Unpublished report received June 1983
from Daiyu-Kai Institute of Medical Science, Japan. Submitted to WHO
by Ciba-Geigy Ltd., Basle, Switzerland.
Fabran, J.E., Golberg, L. and Coulston, F. (1971). Two-year safety
evaluation study of GS13005 on Rhesus monkey. Unpublished report of
Albany Medical College. Submitted to WHO in 1972, and cited in Annex
I, 19.
Folinusz, P., Huber, K., Schiavo, D., Hazelette, J., & Green, J.
(1986). Methidathion: 21-day dermal toxicity study in rabbits.
Project No. 86019. Unpublished report dated August 28, 1986 from
Ciba-Geigy Corporation, Summit, USA. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Fritz, H. (1976). Dominant lethal study on GS 13005 technical, mouse
(test for cytotoxic or mutagenic effects on male germinal cells).
Project No. 327633. Unpublished report dated August 3, 1976,
supplemented May 26, 1987, from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Goldenthal, E. (1986). Two-year dietary oncogenicity study in mice.
Project No. 382-087. Unpublished report dated March 7, 1986 from
International Research and Development Corporation, Mattawan, USA.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Hertner, T. & Arni, P. (1990). Autoradiographic DNA repair test on
rat hepatocytes. Project No. 891344. Unpublished report dated
February 6, 1990 from Ciba-Geigy Ltd., Basle, Switzerland. Submitted
to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Hess, R. (1975) Nature of the liver changes observed in a two-year
feeding study in dogs. Unpublished report from the
Toxicology/Pathology Dept., Ciba-Geigy. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Hool, G., Langauer, M., & Muller, D. (1980). Nucleus anomaly test in
somatic interphase nuclei of Chinese hamster. Project No. 800437.
Unpublished report dated July 2, 1980, supplemented May 26, 1987,
from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Hool, G. & Muller, D. (1980). Sister chromatid exchange study GS
13005 Chinese hamster (test for mutagenic effects of bone marrow
cells). Project No. 801489. Unpublished report dated November 4,
1980, supplemented May 26, 1987, from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Hummel, H., Youreneff, M., Giknis, M., Arthur, A. & Yau, E., 1987.
Methidathion: a teratology (segment II) study in rabbits. Project
No. 86131. Unpublished report dated January 13, 1987 from Ciba-Geigy
Corporation, Summit, USA. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Johnston, C.D. (1965) GS 13 005. Demyelination study in the chicken.
Report from Woodard Research Corp. (unpublished).
Johnston, C.D. (1967). GS 13 005. safety evaluation by two-year
feeding studies in rats and dogs. Final report from Woodard Research
Corp. (unpublished).
Lobdell, B.J. & Johnston, C.D. (1966). GS 13 005. Three-generation
reproduction study in the rat. Report from Woodard Research Corp.
(unpublished).
Mainiero, J., Levy, E., Infurna, R., & Yau, E. (1987). Methidathion
technical: a teratology (segment II) study in rats. Project No.:
86172. Unpublished report dated January 15, 1987 from Ciba-Geigy
Corporation, Summit, USA. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Marco, G. & Simoneaux, B. (1982). Dermal absorption of thiadiazole-
14C-methidathion by rats. Project No. ABR-81058. Unpublished
report dated April 22, 1982 from Ciba-Geigy Corporation, Greensboro,
USA. Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Noakes, D.N. & Sanderson, D.M. (1964) The toxicology of GS 13 005
and GS12968. Species variation in acute oral tosicity. Report from
Chesterford Park Research Station (unpublished).
Noakes, D.N. (1964) The toxicology of GS 13 005 and GS 12968. Tests
for delayed neurotoxic effects in the hen. Report from Chesterford
Park Research Station (unpublished).
Noakes, D.N. & Watson, W.A. (1964a) The toxicology of GS 13 005 and
GS 12968. Dietary toxicity to the rat: 6-month study. Report from
Chesterford Park Research Station (unpublished).
Noakes, D.N. & Watson, W.A. (1964b) The toxicology of GS 12 968 (NC
2962) and GS 13 005 (NC 2964). Cumulative oral toxicity to the rat.
Report from Chesterford Park Research Station (unpublished).
Osheroff, M. (1987). 21-day dermal toxicity study in rabbits with
methidathion technical. Project No. 483-254. Unpublished report
dated January 15, 1987 from Hazleton Laboratories America, Inc.,
Vienna, USA. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Payot, H.P., GS13005. Subchionische toxizitat am menschen (Einzel
und Selbstversuch). Unveröffentlichter Berict der Geigy J.R., Ag.
Submitted to WHO in 1972 and cited in Annex I, 19.
Puri E. & Muller D. (1982a). Autoradiographic DNA repair test on rat
hepatocytes GS 13005 ( in vitro test for DNA-damaging properties).
Project No. 820585. Unpublished report dated October 19, 1982 from
Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Puri E. & Muller D. (1982b). Autoradiographic DNA repair test on
human fibroblasts GS 13005 ( in vitro test for DNA-damaging
properties). Project No. 820586. Unpublished report dated October
18, 1982 from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO
by Ciba-Geigy Ltd., Basle, Switzerland.
Quest, J., Copley, M., Hamernik, K., Rinde, E., Fisher, B., Engler,
R., Burnam, W. & Fenner-Crisp, P. (1990). Evaluation of the
carcinogenic potential of pesticides, 2. Methidathion. Regulatory
Toxicology and Pharmacology, 12: 117-126.
Sachsse, K. (1971) Acute oral LD50 of technical GS 13 005 in the
dog. Report from Ciba-Geigy Ltd (unpublished).
Sachsse, K. (1973a). Irritation of technical GS 13005 in the rabbit
eye. Project No. Siss 2929. Unpublished report dated June 6, 1973
from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Sachsse, K. (1973b). Skin irritation in the rabbit after single
application of technical GS 13005. Project No. Siss 2929.
Unpublished report dated June 6, 1973 from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Sachsse, K. & Bathe, R. (1977a). The therapeutic activity of
atropine sulfate, pralidoxim (PAM) and toxogonin with regard to GS
13005 in the rat. Unpublished report dated December 5, 1977 from
Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Sachsse, K. & Bathe, R. (1977b). Potentiation study: CGA 15324
versus 2 insecticides, GS 13005 (methidathion) and G 24480
(diazinon) in the rat. Unpublished report dated February 15, 1977
from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Sachsse, K. & Bathe, R., 1978. CGA 20168 versus 6 insecticides, C
177 (DDVP), C 570 (phosphamidon), GS 13005 (methidathion), G 24480
(diazinon), CGA 15324 and malathion. Project No. 404478-Siss 6526.
Unpublished report dated June 23, 1978 from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Salamon, C. (1987). Two-generation reproduction study in albino rats
with methidathion technical. Project No. 450-2125. Unpublished
report dated January 15, 1987 from American Biogenic Corporation,
Decatur, USA. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Sarasin G. (1980). Acute dermal LD50 in the rat of GS 13005
technical Rohschmelze (=crude melt) (Ex Monthey). Project No.
801726. Unpublished report dated December 17, 1980 from Ciba-Geigy
Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Satou, S., Kimura, Y., Yamamoto, K. & Ichihara, A. (1979). In vitro
microbial assays for mutagenicity testing of DMTP (methidathion).
Project No. NRI-79-2884. Unpublished report dated August 31, 1979
from Nomura Research Institute, Japan. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Simmon, V., Poole, D. Nevell, G., & Skinner, W. (1977). In vitro
and in vivo microbiological assays of six Ciba-Geigy chemicals.
Project No. LSC-5686. Unpublished report dated March 1977 from
Stanford Research Institute, Menlo Park, USA. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Simoneaux, B. & Marco, G. (1984). Dermal absorption of thiadiazole-
14C-methidathion by mice. Project No. ABR-84015. Unpublished
report dated April 12, 1984 from Ciba-Geigy Corporation, Greensboro,
USA. Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Staley, J., Murphy, T., & Simoneaux, B. (1987). Metabolism of 14C
(Carbonyl) labelled methidathion in a goat. Project No. ABR-86112.
Unpublished report dated February 26, 1987 from Ciba-Geigy
Corporation, Greensboro, USA. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Stenger, E.G. & Roulet, F. (1963) Subchronische Toxizität, Ratte per
os, GS 13005 rein. Unveröffentlichter Bericht der Geigy, J.R. AG.
Stenger, E.G. (1964a) Akute Toxizität, Ratte per os, GS 13005 40 WP.
Unveröffentlichter Bericht der Geigy, J.R. AG.
Stenger, E.G. (1964b) Akute Atoxizität, Ratte per os, GS 13005 40
ES. Unveröffentlichter Bericht der Geigy, J.R. AG.
Stenger, E.G. & Roulet, F. (1965) Chronische Toxizität, Ratte,
Fütterungsversuche 4-22- Wochen, GS 13 005. Unveröffenlichter
Bericht der Geigy, J.R. AG.
Stenger, E.G. (1966a) Akute Toxizität, Ratte per os, GS 13 005 40
WP. Unveröffentlichter Bericht der Geigy, J.R. AG.
Stenger, E.G. (1966b) Akute Toxizität, Ratte per os, A 2041 C (= 40
ES). Unveröffentlichter Bericht der Geigy, J.R. AG.
Staley, J. & Simoneaux, B. (1987). Metabolism of methidathion
labelled with 14C on the ring carbon adjacent to the methoxy group
in a goat. Project No. ABR-86118. Unpublished report dated March 2,
1987 from Ciba-Geigy Corporation, Greensboro, USA. Submitted to WHO
by Ciba-Geigy Ltd., Basle, Switzerland.
Strasser, F. & Arni, P. (1990). Chromosome studies on Chinese
hamster ovary cell line CCL 61 in vitro. Project No. 891202.
Unpublished report dated January 30, 1990 from Ciba-Geigy Ltd.,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Strasser, F. & Muller, D. (1980). Point mutation assay with mouse
lymphoma cells, host mediated assay with GS 13005 (test for
mutagenic properties in mammalian cells). Project No. 801495.
Unpublished report dated October 21, 1980 from Ciba-Geigy Ltd.,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Szolics, I. & Simoneaux, B. (1985). Partitioning characteristics and
14C distribution in a chicken dosed with 2 -14C-methidathion for
16 days. Project No. ABR-85039. Unpublished report dated July 22,
1985 from Ciba-Geigy Corporation, Greensboro, USA. Submitted to WHO
by Ciba-Geigy Ltd., Basle, Switzerland.
Szolics, I. & Simoneaux, B. (1987a). Disposition of methidathion in
the rat. Project No.: ABR-86122. Unpublished report dated March 6,
1987 from Ciba-Geigy Corporation, Greensboro, USA. Submitted to WHO
by Ciba-Geigy Ltd., Basle, Switzerland.
Szolics, I. & Simoneaux, B. (1987b). The disposition of
radioactivity in rats dosed with carbonyl 14C-methidathion.
Project No. ABR-86084. Unpublished report dated February 26, 1987
from Ciba-Geigy Corporation, Greensboro, USA. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Szolics, I. & Simoneaux, B. (1987c). The disposition of
radioactivity in rats dosed with methoxy labelled 14C-
methidathion. Project No. ABR-86115. Unpublished report dated March
2, 1987 from Ciba-Geigy Corporation, Greensboro, USA. Submitted to
WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Szolics, I. & Simoneaux, B. (1987d). The disposition of
radioactivity in rats dosed with 14C-methidathion labelled in the
ring carbon adjacent to the methoxy group. Project No. ABR-86095.
Unpublished report dated February 26, 1987 from Ciba-Geigy
Corporation, Greensboro, USA. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Szolics, I. & Simoneaux, B. (1987e). Characterization of carbonyl
14C-labelled methidathion metabolites in rat urine. Project No.
ABR-86107. Unpublished report dated March 2, 1987 from Ciba-Geigy
Corporation, Greensboro, USA. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Szolics, I. & Simoneaux, B. (1987f). Characterization of methoxy
14C-labelled methidathion metabolites in rat urine. Project No.
ABR-86116. Unpublished report dated February 26, 1987 from Ciba-
Geigy Corporation, Greensboro, USA. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Szolics, I. & Simoneaux, B. (1987g). Characterization of 14C-
methidathion metabolites in rat urine - label 14C adjacent to the
methoxy group. Project No. ABR-86114. Unpublished report dated March
2, 1987 from Ciba-Geigy Corporation, Greensboro, USA. Submitted to
WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Szolics, I. & Simoneaux, B. (1987h). Characterization of 14C-
methidathion metabolites in goat urine. Project No. ABR-86113.
Unpublished report dated March 3, 1987 from Ciba-Geigy Corporation,
Greensboro, USA. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Teitelman, U., Adler, M., Levy, I., & Dikstein, S. (1975). Treatment
of massive poisoning by the organophosphate methidathion. Clinical
Toxicology, 8(3), 277-282.
Tong C. (1982a) The hepatocyte primary culture/DNA repair assay on
compound GS 13005 - 008266 using mouse hepatocytes in culture.
Project No. M 111881. Unpublished report dated February 10, 1982
from Naylor Dana Institute, Valhalla, USA. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Tong C. (1982b) The hepatocyte primary culture/DNA repair assay on
compound GS 13005 - 008266 using rat hepatocytes in culture. Project
No. M 111881. Unpublished report dated February 10, 1982 from Naylor
Dana Institute, Valhalla, USA. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Ullmann, L., Krinke, G., Sachsse, K. & Hess, R. (1977). Acute oral
toxicity and neurotoxicity study of technical GS 13005 in the
domestic fowl ( Gallus domesticus). Project No. Siss 5927.
Unpublished report dated November 8, 1977 from Ciba-Geigy Ltd.,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Yau, E., McMartin, D., Zaidi, I., & Green, J. (1986). Methidathion:
2-year oral oncogenicity and toxicity study in albino rats. Project
No. 86061. Unpublished report dated May 23, 1986 from Ciba-Geigy
Corporation, Summit, USA. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Zoppellari, R., Targa, L., Tonini, P., & Zatelli, R., 1990. Acute
poisoning with methidathion: A case. Human & Experimental
Toxicology, 9(6), 415-419.
