
PROPOXUR
EXPLANATION
Propoxur was previously evaluated by the WHO Expert Group in
1973. This evaluation resulted in the allocation of an ADI of 0-0.02
mg/kg bw (WHO/FAO, 1974). Since the 1973 evaluation new data on
virtually all toxicological aspects have become available. In the
present monograph addendum these data are summarized and the safety
of propoxur is re-evaluated.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution and excretion
Female ICR mice, fasted for 12 hours, were given a single dose
of 1 mg/kg bw 14C-propoxur (ring-labelled) directly into the
stomach. The amount of 14C absorbed from the intestines (expressed
as % of the applied amount) was 25, 48, 54, 66 and 74% after 1, 5,
15, 30 and 60 minutes respectively. At 5 minutes after dose
administration 14C was present in blood, liver and carcass. Only
trace amounts of 14C activity were found in captured CO2. In
urine, 16, 28 and 50% of the applied amount was found after 15, 30
and 60 minutes respectively (Ahdaya et al. 1981).
The distribution of radioactivity was followed in a whole body
autoradiographic study in rats for the 72 hours after administering
a single dose of about 5 mg/kg bw 14C-propoxur (ring labelled).
During the first 8 hours, the highest concentrations were found in
kidneys, gastrointestinal tract contents, urinary bladder contents,
lymph fluid and in the nasal and pharyngal mucosa. Somewhat lower
levels were present in blood, lung, salivary gland, parotis and
connective tissues (skin, cartilages, bones, ligaments, testes,
epididymis, seminal vesicle membranes). Next highest in
concentration were the spleen, adrenal gland and infraorbital gland.
Low concentrations were found in muscle tissues, fat, brain, spinal
marrow and thymus. At 24 hours after application, the radioactivity
concentrations had declined markedly in most tissues. Excretion
proceeded slower towards the end of the test period. The
autoradiographs indicated rapid excretion, mostly in urine and, to a
lesser degree, in feces (Weber, 1988).
Male and female Long-Evans rats were given a single oral dose
of 14C-propoxur (position of label not reported). In the period up
to 48 hours after application, 62-91% of the dose was excreted in
urine and 3-33% in feces (Abd-Elraof et al. 1981).
A group of 42 male rats received a daily dose of 30 mg/kg bw
propoxur for 2 weeks followed by 50 mg/kg bw/day for 4 weeks.
Concentrations of propoxur and the metabolite 2-isopropoxyphenol
(M2) were measured in kidneys, liver, blood and brain on days 1, 7,
14, 28 and 42. Propoxur concentrations were highest in kidneys
(1.6-7.0 mg/kg) followed by liver (0.7-1.4 mg/kg), blood (0.27-0.49
mg/l) and brain (0.22-0.29 mg/kg). The concentration distribution
for the metabolite was comparable. Apart from the increases noted
after elevation of the dose level, the only consistent trend in the
propoxur concentrations in tissues was a time-correlated increase in
the concentrations in the kidneys (Krechniak & Foss, 1983a).
The distribution and excretion of the metabolite 2-
isopropoxyphenol were investigated after administration of a single
intravenous dose of 50 mg/kg bw to male Wistar rats. The
elimination kinetics had a two-phase character. During the first
phase, lasting about 20-60 minutes, over 85% of the substance was
eliminated from the organs and tissues. Highest concentrations
occurred in blood and kidneys. Over a 5-day period, 53% of the
administered dose was excreted via urine, 95% of this amount being
recovered during the first 24 hours (Krechniak & Foss 1983b).
Dermal absorption of propoxur was measured in groups of male
Sprague Dawley rats receiving a single dose of 0.65, 6.9, 70 or 690
wµg 14C-propoxur/cm2to a shaven, intact skin area of 15 cm2. The
vehicle was a mixture of ethanol and deionized water. Skin
penetration was calculated from 14C determinations in urine, feces,
blood, carcass and application skin site, carried out at 0.5, 1, 2,
4, 8 and 24 hours after dose application. After 8 hours of exposure
the absorption percentages were 46, 54, 22 and 17% at the dose
levels of 0.65, 6.9, 70 and 690 µg/cm2 respectively (Eigenberg,
1988).
A lactating cow was given a single oral dose of 0.21 mg/kg bw
14C-propoxur (label in ring) in the form of a gelatin capsule. 14C
concentrations in blood, urine, feces and milk were measured up to 3
days after application. Total 14C levels in blood reached a peak
activity at 1 hour after application. In urine a total of 96% of
the dose was excreted, excretion being completed after 32 hours. In
feces, only 0.74% was found and in milk, less than 0.1%. At 7 days
after the first dose, the cow received an identical dose and was
sacrificed 2.5 hours later for determination of 14C in tissues.
Concentrations in kidneys, liver, fat, heart, muscle and brain were
0.355, 0.051, 0.017, 0.016, 0.009 and <0.003 ppm (expressed as
propoxur equivalents), respectively. 2-Isopropoxyphenol was
identied as major metabolite in liver, kidneys and urine (Bell &
Gronberg, 1975).
Biotransformation
Qualitative metabolite pattern
Studies were carried out in mice, rats, hamsters, monkeys and
humans; the results are summarized below.
Ten male Wistar rats were maintained on a diet containing 8000
ppm propoxur for 13 weeks. In urine, collected over a 24-hour
period, a large number of metabolites was identified. The results
show the biotransformation pathway in rats to comprise
depropoxylation, hydrolysis of the ester bond, N-methyl
hydroxylation and demethylation, and ring hydroxylation at ring
positions 3, 4 and 5. The proposed metabolism scheme of propoxur in
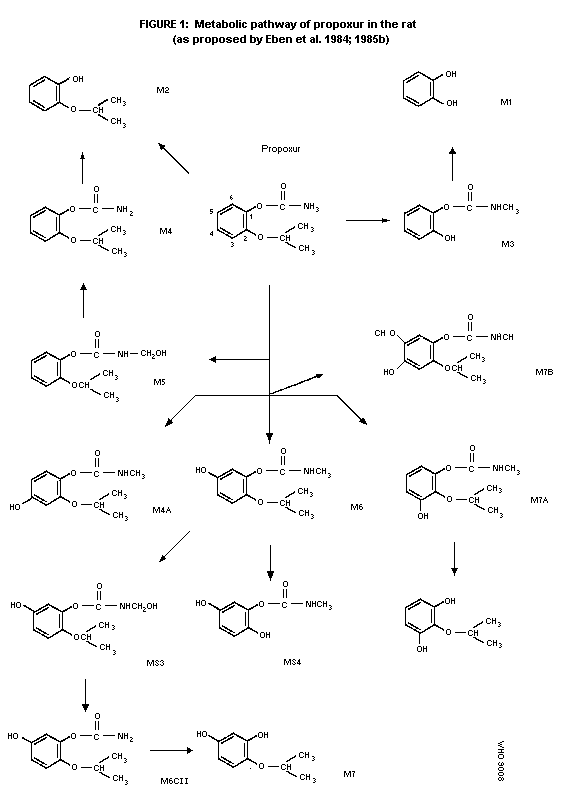
rats is presented in figure 1 (Eben et al. 1984; 1985b).
The same investigators have performed similar studies in NMRI
mice and Golden hamsters. The main metabolite pattern found in
these species was comparable to the one in rats: depropoxylation,
hydrolysis of the ester bond, N-demethylation and ring hydroxylation
at ring positions 3, 4 and 5 were also found in mice and hamsters.
The metabolites MS3, MS4 and M6CII however, identified in rats,
could not be confirmed in mice and hamsters ( Eben et al. 1986a;
1987).
A similar study in Rhesus monkeys (dose level 40 mg/kg bw/day
for 13 weeks) revealed that the biotransformation in primates, like
that in rodents involves depropoxylation, hydrolysis of the ester
bond and N-demethylation. Ring hydroxylation, however, in monkeys
occurs only at the 4- and 5-position of the ring, unlike in rodents
where it occurs at the 3-position also. The following metabolites,
identified in rats, were not found in monkeys: M4A, MS3, M6CII,
MS4, M7A and M8 (Eben et al. 1986b).
Human data are limited. In urine obtained from a person, who in
a suicidal attempt had ingested large amounts of an EC-formulation
of propoxur, a large number of propoxur metabolites was identified.
The metabolites were present as the free compounds or conjugated
with glucuronide or sulfate. The results indicate that, like in
other species, the biotransformation pathways comprise
depropoxylation, hydrolysis of the ester bond and ring
hydroxylation. Like monkeys, and contrary to rodents, in humans the
ring hydroxylation occurs at the 4- and 5- positions only (the 3
position is not hydroxylated) (Eben et al. 1985a).
Quantitative metabolite pattern
Male and female Long-Evans rats received a single dose of 14C-
propoxur. Urine and feces were collected up to 48 hours after
dosing; TLC was done to identify metabolites. Of the radioactivity
present in urine, 34% was unchanged propoxur, 8% was present as M2,
5% as M3 and 52% was not identified. In feces, 37% was unchanged
compound, 40% was present as M2, 9% as M3 and 14% was not identified
(Abd-Elraof et al. 1981).
The following study was done to determine the extent to which
the renal metabolite pattern depends upon diet and dose level. The
diets used were semisynthetic casein basic feed and Altromin
standard feed. Groups of 5 female Wistar rats received 50, 250 or
5000 ppm propoxur in the two diets for 4 weeks followed by a single
oral dose of 1 mg/kg bw 14C-propoxur (ring labelled). More than
 90% of the administered 14C was recovered in urine in all groups.
The urinary conjugated metabolite pattern was comparable for the
different dose levels and the different diets. At higher (5000 ppm)
dose levels, there was a trend for a higher percentage of M5 and M6
and a lower for M2 and M7. The 14C distribution over the
conjugated metabolites is summarized in Table 1.
TABLE 1. DISTRIBUTION OF 14C OVER URINARY METABOLITES IN RATS
METABOLITE % OF 14C PRESENT IN
URINE (RANGE FOR ALL
DOSE LEVELS AND DIETS)
M1 7.0-15.5
M2 17.2-25.2
M3 22.2-30.2
M5 0.6- 2.5
M6 4.5- 7.9
MS3 0.3- 1.2
M7 14.0-16.3
M7A 5.9- 8.3
M8 1.7- 4.1
(Karl, 1986; Karl & Schneider, 1987)
Metabolism of propoxur in the liver was studied further in in
vivo studies, using post-mitochondrial fractions of livers of
rats, mice, hamsters and monkeys. A similar study was done in human
liver preparations. The results show M5 to be the major metabolite
in rat liver, M3 and M6 being formed to a lesser degree. The
percentage of M5 in relation to overall metabolism decreases from
mouse to hamster and monkeys. In the liver preparation from Rhesus
monkeys, M3 and M6 were formed more than in M5. In additional tests
it was observed that in liver cell fractions of rats and mice
further metabolization of M5 does not take place in hamsters it
occurs only to a limited degree. In contrast, the liver cell
fractions of Rhesus monkeys and humans are able to further
metabolize M5 to 4 other metabolites (not identified) (Schmidt,
1987).
Effect on cholinesterase activity
Male and female Wistar rats were given a single oral dose of 0,
1, 5 or 25 mg/kg bw via stomach tube. Cholinesterase activity was
measured in plasma, erythrocytes and brain at intervals varying from
0.5 hours to 14 days after dose application. At the highest dose
level, cholinesterase activity in plasma and erythrocytes was
depressed after 30 and 60 minutes; after 3 hours the depressions
were no longer apparent. Brain cholinesterase activity was depressed
after 3-5 hours in the same group; 3 days after dosing this was no
longer apparent. At 1 and 5 mg/kg bw no effect was seen (Heimann,
1982c).
In male rats given a single oral dose of 2.1, 7.0, 20.9, 50 or
70 mg/kg bw propoxur, cholinesterase activity in whole blood was
decreased at all dose levels (at 2.1 mg/kg bw the decrease was
slight) after 10 minutes; at 24 hours after dose application the
depressions were no longer present. The same investigators tested
for cholinesterase depression in whole blood and brain in male rats
dosed orally with 30 mg/kg bw/day for 14 days followed by 50 mg/kg
bw via the same route for 28 days. A decreased activity in both
blood and brain was noted during the first 14 days; the depression
gradually disappeared during the following 28 days of treatment.
This indicates adaptation (Krechniak & Foss, 1982).
In an inhalation study, male and female rats were exposed to
0.4, 1.2, 9.0, 30.0, 78.0 or 172.0 mg/m3 propoxur for 6 hours.
Directly after cessation of exposure, plasma and erythrocyte
cholinesterase activities were measured and compared to pre-test
values. At 30, 78 and 172 mg/m3, depressions were observed. In
addition, at 172 mg/m3 cholinergic symptoms were seen at 30 minutes
after commencement of exposure (Kimmerle & Eben, 1978a).
Effect on liver enzyme activity
Mice
Male mice received propoxur (purity 98.8%) in the drinking
water at concentrations increasing with each successive week from 50
to 2000 ppm over a total period of 6 weeks. In animals thus treated
for six weeks, the LD50 for propoxur was significantly higher than
in untreated controls, a finding indicating that tolerance to
propoxur acute toxicity had developed. Hexobarbital sleeping time
was significantly reduced, indicating induction of hepatic
microsomal enzymes. Determinations of carboxy esterase activities
in liver, plasma and brain, however, did not show any significant
increase in the propoxur-treated animals (Costa et al. 1981).
Rats
Propoxur was administered orally for 5 days to male and female
rats at dose levels of 0, 15 or 30 mg/kg bw/day. The rats treated
with propoxur did not exhibit induction of the mixed function
oxidases in comparison to control rats. (A positive control group
was treated with sodium phenobarbitone 50 mg/kg bw/day for 5 days
and induction of mixed function oxidases was observed) (Mihail,
1982).
Concomitant to a short-term study in female rats, liver tissue
enzyme activities were measured after 3, 7, 14 and 28 days of
feeding on a diet containing 0 or 5000 ppm propoxur. From day 3,
cytochrome P-450 dependent mono-oxygenases (i.e. 7-ethoxycoumarin
deethylase, ethoxyresorufin deethylase, aldrin epoxidase) were
induced (increased with a factor of 2-3). The activity of the
microsomal epoxide hydroxylase was increased by the same magnitude.
In contrast, the cytosolic glutathione-S-transferase exhibited only
a slight change compared to the control group. Altromin diet was
used in this study. An identical study in which a casein semi-
synthetic diet was used also showed induction of mono-oxygenase
activity by propoxur. The absolute activity levels reached in the
latter study, however, were about 50% lower compared to the values
observed in the concurrent control and treatment groups of the
Altromin diet study (Machemer & Schmidt, 1988).
Toxicological studies
Acute toxicity
The acute toxicity of propoxur to rats and mice is presented in
Table 2.
The observed intoxication symptoms were indicative of
inhibition of cholinesterase: convulsions, muscular tremors,
muscular spasms, dyspnoea, salivation (Flucke, 1980; Heimann,
1982b).
Short-term toxicity
Oral studies
Rats
The toxicity of propoxur technical (purity 98.6%) and propoxur
recrystallized (purity 99.2%) was compared in a 5 day-study. Groups
of Wistar rats (5/sex/group) received oral doses of 0, 15 or 30
mg/kg bw/day of propoxur of both purities via stomach tube for 5
days. Appearance, behaviour and body weight were recorded daily.
After 5 days, all animals were sacrificed and submitted to gross
pathology. The weights of liver and kidneys were determined.
Clinical chemistry was performed on all animals. N-demethylase, O-
demethylase, cytochrome P-450 and triglycerides were measured in
liver homogenates (cholinesterase activity not determined). The
only adverse effects noted were convulsions and apathy, occurring in
all treatment groups in a dose-related manner. No difference in
toxicity between the two purities were found (Heimann, 1983).
TABLE 2. ACUTE TOXICITY OF PROPOXUR IN ANIMALS
LD50 LC50
SPECIES SEX ROUTE (mg/kg bw) (mg/m3) REFERENCES
Mouse M oral 37 - Haley et al. 1974
F idem 39 - Haley et al. 1974
Rat M* oral 39 - Thyssen et al. 1977
M* oral 45 - Flucke, 1984
M oral 196 - Flucke, 1980
F oral 126 - Flucke, 1980
M* oral 94 - Flucke, 1980
F* oral 68 - Flucke, 1980
M oral 167 - Heimann, 1982b
F oral 96 - Heimann, 1982b
M* oral 69 - Heimann, 1982b
F* oral 47 - Heimann, 1982b
M i.p. 16 - Heimann, 1982b
F i.p. 13 - Heimann, 1982b
M&F dermal >5000 - Flucke, 1980
M&F inhal. - >498 Pauluhn, 1988
* Fasted animals
Dogs
Beagle dogs (6/sex/group) were given diets containing 0, 200 or
600 ppm propoxur (purity 99.4%) for 52 weeks. An additional group
received 1800 ppm from week 1 through week 40, 3600 ppm from week 41
through 44 and 5400 ppm from week 45 through 52 (the increases were
established in order to produce overt toxic signs). Appearance,
behaviour and body weight were recorded. On a number of occasions
throughout the study reflex tests, ophthalmoscopy, hematology,
clinical chemistry, urinalysis and cholinesterase activity (in
plasma and erythrocytes) were determined. At sacrifice, organs were
weighed and complete gross pathology and histopathology were carried
out. In livers N-demethylase and cytochrome P-450 were measured.
Cholinergic symptoms were observed at the highest dose level after
elevation of the dose level to 5400 ppm and 1/6 animals died. In
addition, the following parameters were increased in this group:
thrombocyte, leucocyte and reticulocyte counts, incidence of Heinz
bodies, ALAT and SAP, liver weight and thyroid weight; thymus weight
was decreased (also noted: medium thymus atrophy). At the highest
dose level and at 600 ppm also, growth was retarded and plasma
cholesterol and liver N-demethylase were increased. The NOAEL in
this study is 200 ppm (Hoffmann & Gröning, 1984).
Inhalation studies
Groups of Wistar rats (10/sex/group) were exposed to aerosols
containing propoxur (purity 98.9%) in concentrations of 0, 5.7, 18.7
or 31.7 mg/m3 6 hours per day, 5 days per week over a period of 12
weeks. There was no effect on behaviour, growth, hematology,
clincial chemistry, urinalysis, organ weights or histopathology.
The only effect observed was a depression of cholinesterase activity
in plasma, erythrocytes and brain, occuring at 31.7 mg/m3 only
(Kimmerle & Iyatomi, 1976).
Groups of Wistar rats (5/sex/group) were exposed to aerosols
containing propoxur (purity 99.6%) in concentrations of 0, 15.3,
45.3 or 139.6 mg/m3 during 6 hours per day, 5 days per week over a
period of 4 or 8 weeks. The observations included clinical signs,
body weight, cholinesterase activity in plasma, erythrocytes and
brain at sacrifice after 4 and 8 weeks, urinalysis, gross pathology
(all organs) and histopathology (4 tissues/animal) and organ weights
(4 organs/animal). Cholinergic symptoms were observed at 139.6
mg/m3. Cholinesterase activies in brain were depressed in week 4
at 45.3 and 139.6 mg/m3 and in week 8 at 15.3 mg/m3 also. There
were no signs of specific organ damage or an alteration in the
urinary bladder epithelium (Pauluhn & Rühl, 1985).
Long term carcinogenicity studies
Mice
Groups of 50 male and 50 female CF1/W74 mice were fed diets
containing 0, 700, 2000 or 6000 ppm propoxur (purity 99.6%) for 24
months. Satellite groups of 10 male and 10 female mice were fed at
the same dose levels and were used for interim sacrifice after 6
months. Observations included clinical signs, body weight, food
consumption, hematology and clinical chemistry. The weights of 6
organs/animal were recorded. Gross pathology and limited
histopathogy (about 20 tissues/animal) were carried out. Slight
growth retardation was oberved in the 700, 2000 and 6000 ppm males;
ALAT was increased in 6000 ppm-females after 6 months only. The
relative weights of testes and spleen were increased or decreased,
respectively, at all dose levels to a dose-related degree. The
tumour incidence was not increased (Bomhard & Löser, 1981; Reid
Patterson, 1980).
Rats
In a chronic toxicity study summarized in the WHO/FAO monograph
from 1974, with dietary dose levels of 0, 250, 750, 2000 and 6000
ppm (purity 99.8%),the effects were: growth retardation and reduced
food consumption at 200 and 6000 ppm and increased relative liver
weight at 6000 ppm (NOAEL in this study: 250 ppm) (WHO/FAO, 1974).
The histological sections produced in this study were reexamined.
Histopathological appraisal of urinary bladder sections confirmed
the absence of alterations in this organ at all dose levels
(Luckhaus, 1984).
Groups of 20 male and 20 female Wistar rats were fed diets
containing 0, 50, 200 or 800 ppm propoxur (purity 97.25%) for 18
months. The observations included clinical signs, food intake, body
weight, hematology and clinical chemistry. At termination all
animals were sacrificed. Organ weights were determined and
histopathology was carried out. Growth was slightly decreased in
the 800 ppm females. At the end of the study cholinesterase
activities in whole blood and brain were inhibited at 800 ppm. The
NOAEL in this study is 200 ppm (Jurek, 1978).
Groups of 50 male and 50 female Wistar rats were fed diets
containing 0, 200, 1000 or 5000 ppm propoxur (purity 99.4%) for 2
years. Additional groups of 10 rats/sex/group were treated at the
same dose levels and were used for interim sacrifice after 1 year.
The observations included clinical signs, body weight, gross
pathology and histopathology. Growth was retarded at 1000 and 5000
ppm. At 5000 ppm slight neuromuscular changes (i.e. slightly
increased incidences of peripheral neuropathy and muscular atrophy
of the rear extremities) were noted. Also at 5000 ppm, ASAT was
decreased (males and females) and urea was increased (females only).
At the same dose level the relative weights of a number of organs
(heart, lung, liver, kidney, adrenal) were increased. At interim
autopsy the incidence of hyperplasia of the urinary bladder was
increased at 1000 and 5000 ppm (incidences 0/20, 0/20, 6/20 and
19/20 in the 0, 200, 1000 and 5000 ppm groups, respectively). The
findings in the urinary bladder at terminal sacrifice are presented
in Table 3.
TABLE 3. INCIDENCE OF URINARY BLADDER ALTERATIONS
IN MALE AND FEMALE RATS
OCCURRENCE CONTROL 200 ppm 1000 ppm 5000 ppm
Hyperplasia 1/98 1/96 15/99 92/97
Papilloma 0/98 0/96 1/99 53/97
Carcinoma 0/98 0/96 0/99 13/97
The NOAEL in this study is 200 ppm (determined to be equal to
9.6 mg/kg bw/day) (Suberg & Löser, 1984, Glaister, 1984).
For further determination of the dose-response/exposure-time
relationship with regard to the effect on the urinary bladder,
groups of 70 female Wistar rats were fed diets containing 0, 50,
250, 1000, 3000, 5000 or 8000 ppm propoxur (purity 99.6-99.9%) for
periods up to 104 weeks. After 4, 7, 12, 26, 53 and 78 weeks, 5 or
10 rats/group were sacrificed for interim autopsy. The animals were
observed for clinical signs, food intake, water intake and body
weight. Organ weights were determined. Gross pathology and
histopathology (kidney, urinary bladder, ureter, liver) were carried
out. Growth was retarded at 3000 ppm and higher dose levels. The
relative weights of liver and kidneys were increased at 3000, 5000
and 8000 ppm. Hyperplasia of the bladder epithelium was observed at
1000 ppm (from week 53), at 3000 ppm (from week 12), 5000 ppm (from
week 4) and at 8000 ppm (from week 2). At the latter two dose
levels the effect had developed to severe hyperplasia with recent
vascularization and papillary and nodular hyperplasia after 53
weeks. After 104 weeks, dose-related increases were observed on
hyperplasia, papilloma and carincoma of the urinary bladder in
female rats.
The NOAEL in this study is 250 ppm (Hahnemann & Rühl-Fehlert,
1988f)
In a number of additional studies the effect of propoxur on the
urinary bladder was yet further examined. Issues to be elucidated
were strain and species specificity, influence of the diet used and
effect of vitamin C supplementation.
Strain specificity
Possible strain specificity of the effect of propoxur on the
urinary bladder, observed in Wistar rats, was examined through an
oral study in Sprague Dawley rats. Groups of 50 female Sprague
Dawley rats were fed diets containing 0, 3000 or 8000 ppm propoxur
(purity 99.6%-99.9%) for periods up to 52 weeks. Growth was
retarded and the relative weights of liver, lung and kidneys were
increased at both dose levels. Simple hyperplasia of the urinary
bladder was observed at 3000 and 8000 ppm from week 4 on. At 8000
ppm hyperplasia with neovascularization, papillary hyperplasia and
incipient nodular hyperplasia were found in the urinary bladder from
week 27. Thus, this strain of rats is as sensitive as is the Wistar
rat with regard to the formation of urinary bladder hyperplasia by
propoxur (Hahnemann & Rühl-Fehlert, 1988b).
Species specificity
In the two oral short-term toxicity studies in dogs (summarized
in the relevant paragraphs of the present monograph and the 1974
WHO/FAO monograph respectively) and in the long-term study in mice
(summarized in the paragraph on long-term studies of the present
monograph) no effect on the urinary bladder epithelium was observed.
Further evidence of species specificity was obtained in the
following studies.
Mice
Groups of 50 female NMRI mice were fed diets containing 0, 3000
or 8000 ppm propoxur (purity 99.6%-99.9%) for 53 weeks. Growth was
slightly decreased at 8000 ppm. Increased liver weight and fatty
degeneration occurred at 3000 and 8000 ppm. Relative lung weight
was increased at 8000 ppm only. No adverse effect on urinary
bladder epithelium was noted (Hahnemann & Rühl-Fehlert, 1988c).
Hamsters
Groups of 50 female Syrian golden hamsters were fed diets
containing 0, 3000 or 8000 ppm propoxur (purity 99.6-99.9%) for 53
weeks. At both dose levels the incidence of mortality was slightly
increased, impairment of the general state of the animals (not
specified) was noted and growth was retarded. The relative weights
of kidneys and adrenals were increased at 8000 ppm only. No adverse
effect on urinary bladder epithelium was observed (Hahnemann & Rühl-
Fehlert, 1988a).
Rhesus monkeys
A group of 6 Rhesus monkeys (3 per sex) received oral doses of
40 mg/kg bw/day propoxur (purity 99.6%) via oral intubation for 13
weeks. This daily dose was previously determined to be the maximum
tolerable dose. No control group was used. Cholinergic symptoms
were observed following compound administration. No adverse effect
on the urinary bladder epithelium was noted (Hoffmann & Rühl, 1985).
Effect of vitamin C supplementation
Groups of 50 female Wistar rats were fed diets containing 1%
vitamin C and propoxur (purity 99.6-99.9%) at concentrations of 0,
1000, 3000 or 8000 ppm for a period of 49 weeks. Additional groups
received the same propoxur dose levels in unsupplemented diet.
Growth was retarded in all propoxur-treated groups. The relative
weights of liver (8000 ppm only) and kidneys and lungs (all dose
levels) were increased. The propoxur-induced hyperplastic changes
of the urinary bladder epithelium were observed in all treatment
groups, being present to an equal degree after feeding of the
supplemented and the unsupplemented diets. Thus, vitamin C did not
influence the effect of propoxur on the rat urinary bladder
epithelium (Hahnemann & Rühl-Fehlert, 1988e).
Effect of diet
In the rat studies in which propoxur was found to produce the
urinary bladder alterations, the diet used was Altromin B21 standard
diet. To examine if the diet used was a relevant factor for the
occurrence of the urinary bladder changes, two additional studies
were carried out using a semisynthetic diet (Casein diet no. 1/0).
Groups of 50 female Wistar rats were fed Casein semi-synthetic
diet no. 1/0 containing 0 or 8000 ppm propoxur (purity 99.9%) for
4,8 or 14 weeks. Growth retardation and reduced water intake were
observed in the treated animals. In addition, relative liver and
kidney weights were increased. No urinary bladder changes were
found at histopathology (Hahnemann & Rühl-Fehlert, 1988d).
Groups of 50 female Wistar rats were fed Casein diet no. 1/0
containing 0, 3000 or 8000 ppm propoxur (purity 99.6%) for periods
up to 100 weeks. Growth was retarded at 3000 and 8000 ppm.
Relative weights of lung, kidney and liver were increased at 8000
ppm. Histopathology revealed no treatment-related changes in the
urinary bladders of treated animals (Hahnemann & Rühl-Fehlert,
1988g).
In a study using 14C-propoxur, possible differences in
absorption of propoxur from the Altromin diet and the semisynthetic
case in diet were ruled out. Thus, the absence of urinary bladder
effects when the casein diet is used is not caused by lower
absorption of propoxur from this diet (Weber, 1986). Another
relevant result was observed in a study in rats in which the renal
metabolite pattern was determined after a single oral dose of 14C-
propoxur (label in ring) after preceding administration of 0, 250 or
5000 ppm propoxur either in Altromin standard diet or in the
semisynthetic casein diet for 4 weeks. No differences in the
metabolite pattern were found (Karl, 1986; Karl & Schneider, 1987).
Special studies on combination toxicity
The acute oral application of equitoxic doses of propoxur and
azinphosmethyl to male Wistar rats revealed an additive toxic effect
of the combination of the two pesticides (Thyssen, 1977).
The LD50-value in male Wistar rats for an equitoxic mixture of
propoxur (purity 99.3%) and cyfluthrin (93.7%) proved to be less
than the value expected on the basis of simple addition of effects
(Flucke, 1984).
Special studies on embryotoxicity and teratogenicity
Rats
Groups of 24 pregnant female Wistar rats received 0, 3, 9 or 27
mg/kg bw/day propoxur (purity 99.4%) p.o. by gavage from day 6
through 15 of gestation. Appearance, behaviour, body weight and
food consumption were recorded daily. At day 21 of gestation all
animals were sacrificed and the fetuses were delivered by Cesarean
section. The number of implantations, resorptions (early and late)
and corpora lutea were determined. The fetuses were counted and
weighed; gross pathology and histopathology (skeletal and visceral)
were carried out. At 27 mg/kg bw, 3 animals died before the end of
the test. At 9 and 27 mg/kg bw, symptoms (increased grooming,
chewing motions, teeth grinding) were noted in the hours following
dose application during the entire treatment; at 27 mg/kg bw, in
addition, tremors and ventral recumbency were observed. At the same
dose levels, food consumption and growth of dams were decreased in a
dose related fashion. No other effects were seen. The NOAEL for
maternal toxicity in this study is 3 mg/kg bw/day (Becker et al.
1989a).
Rabbits
Groups of 15 Himalayan rabbits received 0, 1, 3 or 10 mg/kg
bw/day propoxur (purity 99.6%) p.o. from day 6 through 18 of
gestation. There were no indications of maternally toxic,
embryotoxic or teratogenic effects. However, the study was limited
with respect to soft tissues examination (Schlüter, 1981).
Groups of 16 pregnant Chinchilla rabbits received 0, 3, 10 or
30 mg/kg bw/day propoxur (purity 99.4%) p.o. by gavage from day 6
through 18 of gestation. Appearance, behaviour, body weight and
food consumption were recorded daily. At day 28 of gestation all
animals were sacrificed and the fetuses were delivered by Cesarean
section. The number of implantations, resorptions (early and late)
and corpora lutea were determined. The fetuses were counted and
weighed; gross pathology and histopathology (skeletal and visceral)
were carried out. At 30 mg/kg bw restless behaviour and dyspnoea
were observed after dose application on the first 3 treatment days;
in the same group 3 animals died before test end. In addition, body
weight loss (day 6-9 of gestation) occurred in this group. Also, at
30 mg/kg bw post-implantation loss was increased (number of pups per
dam decreased consequently). The NOAEL for maternal toxicity and
embryotoxicity in this study is 10 mg/kg bw/day (Becker et al.
1989b)
Special studies on mutagenicity
A large number of mutagenicity tests has been carried out with
propoxur. The results are summarized in Table 2 ( in vitro assays)
and Table 3 ( in vivo assays). In addition, in vitro tests in
prokaryotes have been performed with a number of metabolites of
propoxur. The results of these studies are summarized in Table 6.
Special studies on skin and eye irritation and sensitization
Undiluted propoxur (purity 99.2%) was tested for irritation to
shaven intact and shaven abraded skin areas of 6 New Zealand
rabbits. Exposure was for 24 or 72 hours. No irritation was observed
(Thyssen, 1978).
A dose of 0.5 g propoxur (purity 99.6%) moistened with purified
water, was applied under occlusive conditions to the shaven intact
back skin of 6 male New Zealand White rabbits for 4 hours. No skin
irritation was observed up to 72 hours after application (Yamane,
1986a).
In groups of 3 or 5 New Zealand rabbits an eye irritation study
was carried out with undiluted propoxur (purity 99.2%). Eyes were
rinsed after 5 minutes or 24 hours of exposure. The animals were
observed for 7 days. The only sign of irritation was slight erythema
of the conjunctivae of 2/3 animals that were exposed for 24 hours.
At 24 hours after rinsing this was no longer present (Thyssen,
1978).
Application of 0.1 g propoxur (purity 99.6%) into the eyes of 9
male New Zealand White rabbits caused severe miosis, which
disappeared within 24 hours after application. No irritation
effects were seen up to 96 hours post application (Yamane, 1986b).
Propoxur (purity 98.8%) did not exhibit a sensitizing effect in
the maximization test (Magnusson & Kligman) in guinea pigs (Heimann,
1982a).
Observations in humans
Dermal absorption
0.1 ml 14C-propoxur in acetone was applied to the ventral
forearm of 6 humans (sex not reported) (skin area 2.8-20 cm2;
applied amount 5 wµg propoxur/cm2). The skin sites were not
protected and subjects were asked not to wash the area for 24 hours.
Urine, collected for 5 days after beginning of exposure, was
monitored for 14C. The data were corrected for incomplete urinary
recovery using the 14C found in urine after administration of an
intravenous dose. A skin absorption of 19.6% of the dose was found
(Feldmann & Maibach, 1974).
TABLE 4. RESULTS OF IN VITRO MUTAGENICITY ASSAYS ON PROPOXUR
TEST SYSTEM TEST OBJECT CONCENTRATION PURITY RESULTS REFERENCE
Ames test * S. typhimurium 50 nmol/plate ca. 95% Negative Blevins et al. 1977b
TA98, TA100, TA1535
TA1537, TA1538
Ames test * S. typhimurium 0.1-1000 µg/pl 98.0% Negative Inukai & Iyatomi, 1978
TA98, TA100, TA1535 solvent DMSO (1)
TA1537, TA1538
Ames test * S. typhimurium 10-1500 µg/pl >96% Negative De Lorenzo et al. 1978
TA98, TA100, TA1535 (1)
TA1537, TA1538
Ames test S. typhimurium 0.25-100 µg/ml 97% Negative Jaszczuk et al. 1979
TA98, TA100, TA1535
TA1537, TA1538
Ames test * S. typhimurium 20-12500 µg/pl 98.6% Negative Herbold, 1982
TA98, TA100, TA1535 (1)
TA1537, TA1538
Reversion assay * Saccharomyces 75-10000 µg/ml 99.8% Negative Herbold, 1985e
cerevisiae D7 solvent DMSO (1)
Reverse mutation test E. coli WP2 hcr. 20 µl/disk ? Negative Shirasu et al. 1976
B/r try WP2
Reverse mutation E. coli WP2 hcr. 10-5000 µg/pl 98.05 Negative Shirasu et al. 1979
test * S. typh. (1)
TA98, TA100, TA1535
TA1537, TA1538
TABLE 4 (CONTD)
TEST SYSTEM TEST OBJECT CONCENTRATION PURITY RESULTS REFERENCE
Reverse mutation E. coli WP2 hcr. 500-25000 µg/pl 98.0% Negative Ohta & Moriya, 1983
test * S. typh. (1)
TA98, TA100, TA1535
TA1537, TA1538
HGPRT-test * Chinese hamster 25-125 µg/ml 99.6% Negative Lehn, 1988
ovary (CHO) cells (without S9 mix) (1)
600-1500 µg/ml (with S9 mix)
Mitotic gene Saccharomyces 2 ml of suspension 99.8% Negative Siebert & Lemperle, 1974;
conversion test cerevisiae D4 (containing 1000 ppm Siebert & Eisenbrand, 1974
a.i.) at 5 x 10 cells;
solvent DMSO
Pol Al-test * E. coli pol A+ 62.5-10000 µg/pl 98.5% Negative Herbold, 1983a
E. coli pol A- solvent DMSO (1)
Rec-assay Bacillus subtilis 3-300 µg/disk 98.0% Negative Inukai & Iyatomi, 1978
NIG17, NIG45 solvent DMSO (1)
Rec-assay Bacillus subtilis 20 µg/disk ? Negative Shirasu et al. 1976
H17 Rec+, M45 Rec- solvent DMSO
Rec-assay Bacillus subtilis 20-2000 µg/disk 98.0% Negative Shirasu ET AL. 1976
H17 Rec+, M45 Rec- (1)
Rec-assay Bacillus subtilis 50-10000 µg/disk 98.0% Negative Ohta & Moriya, 1983
H17 Rec+, M45 Rec- (1)
TABLE 4 (CONTD)
TEST SYSTEM TEST OBJECT CONCENTRATION PURITY RESULTS REFERENCE
Sister chromatid Human lymphocytes without S9 mix: 99.6% Negative Herbold, 1985d
exchange assay * 125-500 µg/ml (1)
with S9 mix:
250-1000 µg/ml
solvent DMSO
Single-strand Human fibroblasts 10-5 M approx Negative Blevins et al. 1977a
break assay 95%
Chromosome aberr. Chinese hamster without S9 mix. 97.8% Negative Putman & Morris, 1988
assay ovary (CHO) cells 157-625 µg/ml (1)
with S9 mix:
615 and 1250 µg/ml
solvent DMSO
* Test was carried out both with and without metabolic activation.
(1) Positive control yielded positive results.
TABLE 5. RESULTS OF IN VIVO MUTAGENICITY ASSAYS ON PROPOXUR
TEST SYSTEM TEST OBJECT CONCENTRATION PURITY RESULTS REFERENCE
DNA metabolism Male rat spleen cells 10 mg/kg bw; p.o. ? Negative Klein, 1984
studies (1)
Sister chromatid Chinese hamster bone 75 or 150 mg/kg bw 99.6% Negative Herbold, 1985c
exchange assay marrow cells p.o. (1)
Cytogenic study Chinese hamster 2 x 75 mg/kg bw 99.6% Negative Herbold, 1986
spermatogonia 2 x 150 mg/kg bw (1)
Cytogenic study Chinese hamster bone 75-300 mg/kg bw; 99.6% Negative Herbold, 1988
marrow cells p.o. (1)
Micronucleus test Male and female ICR- 25 mg/kg bw + 25 ? Negative Seiler, 1977
mice bone marrow cells mg/kg bw NaNO2; p.o.
Micronucleus test Male and female NMRI- 2 x 5 mg/kg bw; 99.2% Negative Herbold, 1980b
mice bone marrow cells 2 x 10 mg/kg bw; p.o. (1)
Dominant lethal Male mice 5 x 25 mg/kg bw ? Positive Tyrkiel, 1977
test 5 x 50 mg/kg (#) (1)
Dominant lethal Male mice 10 mg/kg bw; p.o. 99.2% Negative Herbold, 1980a
(#) Herbold (1978), in a critical review, points out the equivocality of the results, thus showing the questionable
validity of the author's conclusion.
(1) Postive control yielded positive results.
TABLE 6. RESULTS OF MUTAGENICITY ASSAYS ON PROPOXUR METABOLITES
TEST SYSTEM TEST OBJECT CONCENTRATION RESULTS REFERENCE
M1
Ames test * S. typhimurium 20 and 12500 µg/plate Negative Herbold, 1983c
TA98, TA100,
TA1535, TA1537
Poly A1- test *1 E. coli pol A+ 625-6075 µg/plate Negative Herbold, 1984b
E. coli pol A1-
M2
Ames test * S. typhimurium 20-12500 µg/plate Negative Herbold, 1983b
TA98, TA100
TA1535, TA1537
Mitotic recombination Saccharomyces 185.9-30000 µg/plate Negative Herbold, 1984e
assay * cerevisiae D7
M3
Ames test * S. typhimurium 312.5-5000 µg/plate Negative Herbold, 1984c
TA98. TA100, TA1535
TA1537. TA1538
DNA metabolism studies Male rat spleen 10 mg/kg bw Negative Klein, 1984
cells (1)
M4
Ames test * S. typhimurium 312.5-5000 µg/plate Negative Herbold, 1984a
TA98, TA100, TA1535
TA1537, TA1538
DNA metabolism studies Male rat spleen cells 10 mg/kg bw Negative Klein, 1984
TABLE 6 (CONTD)
TEST SYSTEM TEST OBJECT CONCENTRATION RESULTS REFERENCE
M5
Ames test * S. typhimurium 8-8748 µg/plate Negative Herbold, 1984d
TA98, TA100, TA1535
TA1537, TA1538
DNA metabolism studies Male rat spleen cells 10 mg/kg bw Negative Klein, 1984
(1)
M7
Not evaluable in Ames test due to breakdown in test medium
M8
Ames test * S. typhimurium evaluated range: Negative Herbold, 1984f
TA98, TA100 8-1800 µg/plate
TA1535, TA1537
Propoxur urine (rat, 8000 ppm in feed)
Ames test S. typhimurium 767 µl/plate Negative Herbold, 1985b
TA98, TA100
TA1535, TA1537
Propoxur urine extract (rat, 8000 in feed)
Ames test S. typhimurium evaluated range (2) Herbold, 1985a
TA98, TA100 14.5-29 µl/plate
TA1535, TA1537
* Test was carried out both with and without metabolic activation.
(1) Suppression of semi-conservative DNA synthesis was seen.
(2) Test outline and result inadequately reported.
Baygon spray (containing 2.0% propoxur and 0.5% dichlorvos) was
sprayed on the upper arm of 4 humans (3 males, 1 female) 6 times (1
second per spraying) at intervals of 10 minutes. The amount of
propoxur applied was 41 mg per person. Dermal absorption was
assessed via measurement of cholinesterase activity in plasma and
erythrocytes and of blood propoxur concentrations up to 6 hours
after treatment began. Urine samples, collected for 24 or 48 hours,
were monitored for the concentration of propoxur metabolite
isopropoxyphenol. No skin penetration of propoxur were found. The
identical procedure was used in a study in which 500 mg Baygon Dust
(1% w/w propoxur) was applied for 2 hours under an occlusive pad to
the abraded skin of the upper arm of 4 male subjects (skin sites
were abraded through application of an adhesive plaster and removal
of it, 30 minutes later). There were no signs indicating skin
penetration by propoxur (Eben & Kimmerle, 1974b).
Cholinesterase inhibition
Four human subjects (3 male and 1 female) were exposed by
inhalation to an aerosol containing 3 mg/m3 propoxur (purity 100%)
for 4 hours. The concentration of propoxur in the blood and
cholinesterase activity in plasma were determined up to 120 minutes
after treatment. Urine was monitored for the propoxur metabolite 2-
isopropoxyphenol up to 72 hours after exposure. In blood propoxur
was not found and cholinesterase activity was not depressed. 2-
Isopropoxyphenol was present in urine; the observed concentration
had decreased to trace level after 24 hours (and the compound was
absent thereafter), indicating excretion within this interval
(Kimmerle & Eben 1978b).
COMMENTS
After oral administration to rats the compound is rapidly
excreted, almost exclusively via the urine; only small quantities
are found in the feces. In urine the compound is excreted unchanged
or as one of a large number of metabolites which are present as free
compounds or as glucuronide or sulfate conjugates. The
biotransformation pathways in all species studied comprise
depropoxylation, hydrolysis of the ester bond and N-demethylation.
Ring hydroxylation also occurs: in rodents at ring positions 3, 4
and 5, and in primates at the 4- and 5- positions only. The
biotransformation pathway in humans is the same as in the Rhesus
monkey.
Propoxur induces drug metabolizing enzymes in the liver of
rats. This effect was greater with Altromin diet than with a semi-
synthetic diet.
The compound showed high acute oral toxicity in the species
examined.
Short-term administration of propoxur to rats (gavage, 5 days)
and dogs (dietary, 52 weeks) revealed cholinergic signs, growth
retardation and inhibition of cholinesterase activity in blood and
brain as the main toxicological effects. In the dog an increase of
microsomal enzyme activity was also observed. The NOAEL in the dog
study was 200 ppm (equivalent to 300 mg/kg bw/day).
In a long-term feeding study in mice there was significant
inhibition of growth at the 6000 ppm level. The NOAEL was 2000 ppm
(equivalent to 300 mg/kg bw/day).
In long-term feeding studies in Wistar rats, growth
retardation, ChE inhibition and urinary bladder alterations were the
main effects observed. Hyperplastic and neoplastic changes, which
were dependent on the diet, were found in the bladder of rats. The
hyperplasia could be reduced by the administration of ammonium
chloride in the predisposing diet, presumably via its effect on
urinary pH. These changes were not seen in mice, hamsters, dogs or
Rhesus monkeys.
The NOAEL in rats for the formation of hyperplasia of the
urinary bladder epithelium was 200 ppm (equal to 10 mg/kg bw/day).
This is also the NOAEL for AChE inhibition.
Increased post-implantation losses were observed in a
teratogenicity study in rabbits at 30 mg/kg bw/day. The NOAEL was
10 mg/kg bw/day. No embryotoxicity was observed at the highest dose
tested in rats (27 mg/kg bw/day). No teratogenic effects were
observed in either species.
After reviewing all available in vitro and in vivo short-
term tests, the Meeting concluded that there was no evidence of
genotoxicity.
Based on a re-evaluation of the human data available from the
1973 JMPR, a single oral dose of 0.2 mg/kg bw could be considered as
a NOAEL for humans since the depression of erythrocyte
cholinesterase did not exceed 20% and the recovery was very rapid.
These data have been reviewed by a WHO Expert Committee (WHO, 1973).
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse 2000 ppm in the diet, equivalent to 300 mg/kg bw/day
(ChE activity not measured)
Rat: 200 ppm in the diet, equal to 10 mg/kg bw/day
Dog: 200 ppm in the diet, equivalent to 5 mg/kg bw/day
Human 0.1 mg/kg bw
Estimate of acceptable daily intake for man
0-0.02 mg /kg bw
Studies which will provide information valuable in the continued
evaluation of the compound
Further observations in humans.
REFERENCES
Abd-Elraof, T.K., Dauterman, W.C. & Mailman, R.B. (1981) In vivo
metabolism and excretion of propoxur and malathion in the rat:
Effect of lead treatment. Toxicol. Appl. Pharmacol., 59: 324-330.
Ahdaya, S.M., Monroe, R.J. & Guthrie, F.E. (1981) Absorption and
distribution of intubated insecticides in fasted mice. Pesticide
Biochem. and Physiol. 16: 38-46.
Becker, H., Mladenovic, P. & Terrier, Ch. ( 1989a) Embryotoxicity
study (including teratogenicity) with BOQ 5812315 (c.n. propoxur) in
the rat. Unpublished Report No. R 4686 (project 207270) dated March
1, 1989 from RCC-Research & Consulting Company AG, Itingen,
Switzerland. Submitted to WHO by Bayer AG, Leverkusen, Federal
Republic of Germany.
Becker, H., Mladenovic, P. & Terrier, Ch. (1989b) Embryotoxicity
study (including teratogenicity) with BOQ 5812315 (c.n. propoxur) in
the rabbit. Unpublished Report No. R 4684 (Project 207292) dated
March 1, 1989 from RCC-Research & Consulting Company AG, Itingen,
Switzerland. Submitted to WHO by Bayer AG, Leverkusen, Federal
Republic of Germany.
Bell, R.L. & Gronberg, R.R. (1975) The metabolic fate of BAYGON in
the lactating dairy cow. Unpublished Report No. 44771 dated July 18,
1975 from from Chemagro Agricultural Division - Mobay Chemical
Corporation. Submitted to WHO by Bayer A.G., Leverkusen, Federal
Republic of Germany.
Blevins, R.D., Lijnski, W. & Regan, J.D. (1977a) Nitrosated
methylcarbamate insecticides: effect on the DNA of human cells.
Mut. Res. 44: 1-7.
Blevins, R.D., Lee, M. & Regan, J.D. (1977b) Mutagenicity screening
of five methyl carbamate insecticides and their nitroso derivatives
using mutants of Salmonella typhimurium LT2, Mutat. Res. 56: 1-
6.
Bomhard, E. & Löser, E. (1981) Bö 58 12 315 Chronic toxicological
study on mice (Feeding study over two years). Unpublished Report No.
9954 dated May 12, 1981 from Bayer A.G. Institute of Toxicology,
Wuppertal-Elberfeld. Submitted to WHO by Bayer A.G., Leverkusen,
Federal Republic of Germany.
Costa, L.G., Hand, H., Schwab, Bw & Murphy, S.D. (1981) Tolerance to
the carbamate insecticide propoxur. Toxicology, 21: 267-278.
De Lorenzo, F., Staiano, N., Silengo, L. & Cortese, R. (1978)
Mutagenicity of diallate, sulfallate, triallate and relationship
between structure and mutagenic effects of carbamates used widely in
agriculture. Cancer Res., 38: 13-15.
Eben, A., Karl, W. & Machemer, L. (1984) Studies on the
biotransformation of propoxur in the rat. Unpublished Report No.
12866 (KWN 15) dated August 17, 1984 from Bayer AG Institut für
Toxikologie, Wuppertal-Elberfeld. Submitted to WHO by Bayer AG,
Leverkusen, Federal Republic of Germany.
Eben, A., Karl, W. & Machemer, L. (1985a) Studies on
biotransformation of propoxur in humans. Unpublished Report No.
13947 (KWN 26) dated October 24, 1985 from Bayer AG Institut für
Toxikologie, Wuppertal-Elberfeld. Submitted to WHO by Bayer AG,
Leverkusen, Federal Republic of Germany.
Eben, A., Karl, W. & Machemer, L. (1985b) Supplementary studies on
biotransformation of propoxur in the rat. Unpublished Report no.
14148 dated December 13, 1985 from Bayer AG Institut für
Toxikologie, Wuppertal-Elberfeld. Submitted to WHO by Bayer AG.
Leverkusen, Federal Republic of Germany.
Eben, A., Karl, W. Machemer, L. (1986a) The biotransformation of
propoxur in golden hamsters. Unpublished Report No. 14690 (KWN 37)
dated June 9, 1986 from Bayer AG Institute of Toxicology, Wuppertal-
Elberfeld. Submitted to WHO by Bayer AG, Leverkusen, Federal
Republic of Germany.
Eben, A., Karl. W. & Machemer, L. (1986b) Propoxur [The active
ingredient of RBaygon] Biotransformation study on monkeys.
Unpublished Report No. 15056 (KWN 40) dated September 11, 1986 from
Bayer AG Fachbereich Toxikologie, Wuppertal-Elberfeld. Submitted to
WHO by Bayer AG, Leverkusen, Federal Republic of Germany.
Eben, A., Karl, W. & Machemer, L. (1987) Investigation on the
biotransformation of propoxur in mice. Unpublished Report No. 15697
(KWN 46) dated April 3, 1987 from Bayer AG Fachbereich Toxikologie
Landwirtschaft ZF-Zentrale Analytik Structure Research, Wuppertal.
Submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Eben, A. & Kimmerle, G. (1974b) 1% Baygon Dust. Resorption study on
skin of test volunteers. Unpublished Report No. 4461 dated February
5, 1974 from Bayer AG-Institut für Toxikologie, Wuppertal-Elberfeld.
Submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany. Test volunteers. Unpublished Report No. 4462 dated February
8, 1974 from Bayer AG Institut für Toxikologie, Wuppertal-Elberfeld.
Submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Eigenberg, D.A. (1988) Dermal absorption of propoxur technical in
rat using 14C-propoxur. Unpublished report No. 1097 (study number
88-721-AT) dated December 28, 1988 from Mobay Corporation, Health,
Environment and Safety, Corporate Toxicology Department, Stilwell,
Kansas, USA. Submitted to WHO by Bayer AG, Leverkusen, Federal
Republic of Germany.
Feldmann, R.J. & Maibach, H.I. (1974) Percutaneous penetration of
some pesticides and herbicides in man. Toxicol. Appl. Pharmacol.
28: 126-132.
Flucke, W. (1980) Boe 5812315 (propoxur). Acute toxicity studies.
Unpublished Report No. 9295 dated July 8, 1980 from Bayer AG
Institut für Toxikologie, Wuppertal-Elberfeld. Submitted to WHO by
Bayer AG, Leverkusen, Federal Republic of Germany.
Flucke, W. (1984) FCR (cyfluthrin) BOQ (propoxur) Study for
combination toxicity. Unpublished Report No. 12544 dated March 14,
1984 from Bayer AG Institute of Toxikology, Wuppertal-Elberfeld.
Submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Glaister, J.R. (1984) BOQ 5812315: 2 year carcinogenicity/chronic
toxicity study in the rat. Histopathology Report. Hazleton
Laboratories Harrogate, June 1984. Data submitted to WHO by Bayer AG
Leverkusen, Federal Republic of Germany.
Hahnemann, S. & Rühl-Fehlert, C. (1988a) BOQ 5812315 (common name:
propoxur). Chronic feeding test on Syrian gold hamsters (species
sensitivity). Unpublished Report No. 16801 dated June 15, 1988 from
Bayer AG Toxicology Division, Wuppertal (study no. T 0018434).
Submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Hahnemann, S. & Rühl-Fehlert, C. (1988b) BOQ 5812315 (common name:
propoxur). Chronic feeding test on Sprague-Dawley rats (strain
sensitivity). Unpublished report from Bayer AG Toxicology Division,
Wuppertal (study no. T 1018435). Submitted to WHO by Bayer AG,
Leverkusen, Federal Republic of Germany.
Hahnemann, S. & Rühl-Fehlert, C. (1988c) BOQ 5812315 (common name:
propoxur). Chronic feeding test on NMRI mice (species sensitivity).
Unpublished report no. 16803 dated June 15, 1988 from Bayer AG
Toxicology Division, Wuppertal (study no. T 9018433). Submitted to
WHO by Bayer AG, Leverkusen, Federal Republic of Germany.
Hahnemann, S. & Rühl-Fehlert, C. (1988d) BOQ 5812315 (common name:
propoxur). Sub-chronic feeding test on female Wistar rats (effect of
feed quality). Unpublished Report No. 16897 dated July 13, 1988 from
Bayer AG Toxicology Division, Wuppertal (study no. T 5019041).
Submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Hahnemann, S. & Rühl-Fehlert, C. (1988e) BOQ 5812315 (common name:
propoxur). Chronic feeding test on female Wistar rats with added 1%
L-(+)ascorbic acid. Unpublished Report No. 16979 dated August 2,
1988 from Bayer AG Toxicology Division, Wuppertal (study no. T
8018432). Submitted to WHO by Bayer AG, Leverkusen, Federal Republic
of Germany.
Hahnemann, S. & Rühl-Fehlert, C. (1988f) BOQ 5812315 (common name:
propoxur). Chronic feeding test on female Wistar rats over 2 years
(dose-effect-time relationship). Unpublished Report No. 16980 dated
August 2, 1988 from Bayer AG Toxicology Division, Wuppertal (study
no. T 6018430). Submitted to WHO by Bayer AG, Leverkusen, Federal
Republic of Germany.
Hahnemann, S. & Rühl-Fehlert, C. (1988g) BOQ 5812315 (common name:
propoxur). Chronic feeding study on female rats (Effect of feed and
drinking water type). Unpublished Report No. 17146 dated September
13, 1988 from Bayer AG Fachbereich Toxikologie, Wuppertal. Submitted
to WHO by Bayer AG, Leverkusen, Federal Republic of Germany.
Haley, T.J., Farmer, J.H., Dooley, K.L., Harmon, J.R. & Peoples, A.
(1974) Determination of the LD01 and extrapolation of the LD001 for
five methylcarbamate pesticides. J. Européen de Toxicologie, 7(3):
152-158.
Heimann, K.G. (1982a) Propoxur (the active ingredient of RBaygon
and UndenR). Study of sensitization effect on guinea pigs.
Unpublished Report No. 11218 dated October 15, 1982 from Bayer AG
Institut für Toxikologie, Wuppertal-Elberfeld. Submitted to WHO by
Bayer AG, Leverkusen, Federal Republic of Germany.
Heimann, K.G. (1982b) Carbamate UN, technical. Study for acute
toxicity on rat. Unpublished Report No. 11329 dated December 15,
1982 from Bayer AG Institute of Toxicology, Wuppertal-Elberfeld.
Submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Heimann, K.G. (1982c). Carbamate UN, Technical product: acute study
of the effect on the activity of the cholinesterases in blood
plasma, erythrocytes and brains of rats compared with carbamate UN
recrystallised product. Unpublished Report No. 11330 dated December
15, 1982 from Bayer AG Institute of Toxicology, Wuppertal-Elberfeld.
Submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Heimann, K.G. (1983) Carbamate UN, technical (c.n. propoxur, Unden
active ingredient; Baygon active ingredient) Subacute study on rats
compared with carbamate UN, recrystallised. Unpublished Report No.
11621 dated March 8, 1983 from Bayer AG Institute of Toxicology
Wuppertal-Elberfeld. Submitted to WHO by Bayer AG, Leverkusen,
Federal Republic of Germany.
Herbold, B. (1980a) BOE 5812315, dominant lethal study on male mouse
to test for mutagenic effects. Unpublished Report No. 8808 dated
January 7, 1980 from Bayer AG, Institute of Toxicology, Wuppertal-
Elberfeld. Submitted to WHO by Bayer AG, Leverkusen, Federal
Republic of Germany.
Herbold, B. (1980b) BOE 5812315, microncucleus test on mouse to
evaluate BOE 5812315 for mutagenic potential. Unpublished Report No.
9274 dated June 27, 1980 from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld. Submitted to WHO by Bayer AG, Leverkusen,
Federal Republic of Germany.
Herbold, B. (1982) Carbamate UN technical, Salmonella/microsome
test to evaluate for point mutation. Unpublished report no. 11301
dated December 6, 1982 from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld. Submitted to WHO by Bayer AG, Leverkusen,
Federal Republic of Germany.
Herbold, B. (1983a) Carbamate UN technical - Pol A1- test on E.
coli for potential DNA damage. Unpublished Report No. 11403 dated
January 6, 1983 from Bayer AG, Institute of Toxicology, Wuppertal-
Elberfeld. Submitted to WHO by Bayer AG, Leverkusen, Federal
Republic of Germany.
Herbold, B. (1983b) Isopropoxyphenol, Salmonella/microsome test to
evaluate for point mutation. Unpublished Report No. 12321 dated
December 20, 1983 from Bayer AG, Institute of Toxicology, Wuppertal-
Elberfeld. Submitted to WHO by Bayer AG, Leverkusen, Federal
Republic of Germany.
Herbold, B. (1983c) Brenzcatechin, Salmonella/microsome test to
evaluate for potential point mutation. Unpublished Report No. 12322
dated December 20, 1983 from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld. Data submitted to WHO by Bayer AG, Leverkusen,
Federal Republic of Germany.
Herbold, B. (1984a) THS 1240, Salmonella/microsome test to
evaluate for potential point mutation. Unpublished Report No. 12483
dated February 24, 1984 from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld. Submitted to WHO by Bayer AG, Leverkusen,
Federal Republic of Germany.
Herbold, B. (1984b) Brenzcatechin, POL-test of E. coli to evaluate
for potential DNA damage. Unpublished Report No. 12497 dated
February 29, 1984 from Bayer AG, Wuppertal-Elberfeld. Submitted to
WHO by Bayer AG, Leverkusen, Federal Republic of Germany.
Herbold, B. (1984c) THS 2490, Salmonella/microsome test to
evaluate for point mutation. Unpublished Report No. 12529 dated
March 6, 1984 from Bayer AG, Institute of Toxicology, Wuppertal-
Elberfeld. Submitted to WHO by Bayer AG, Leverkusen, Federal
Republic of Germany.
Herbold, B. (1984d) THS 1241b, Salmonella/microsome test to
evaluate for potential point mutation. Unpublished Report No. 12795
dated July 9, 1984 from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld. Submitted to WHO by Bayer AG, Leverkusen,
Federal Republic of Germany.
Herbold, B. (1984e). Isopropoxyphenol, test on S.cerevisiae D7 for
the induction of mitotic recombination. Unpublished Report No. 12876
dated August 20, 1984 from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld. Submitted to WHO by Bayer AG, Leverkusen,
Federal Republic of Germany.
Herbold, B. (1984f) THS 2647, Salmonella/microsome test to
evaluate for potential point mutation. Unpublished Report No. 12996
dated October 24, 1984 from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld. Submitted to WHO by Bayer AG, Leverkusen,
Federal Republic of Germany.
Herbold, B. (1985a) Propoxur urine extract compared with control
urine extract, Salmonella microsome test to evaluate for potential
point mutation. Unpublished Report No. 13350 dated March 14, 1985
from Bayer AG, Institute of Toxicology, Wuppertal-Elberfeld.
Submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Herbold, B. (1985b) Propoxur urine, Salmonella/microsome test to
evaluate for potential point mutation. Unpublished Report No. 13395
dated March 27, 1985 from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld. Submitted to WHO by Bayer AG, Leverkusen,
Federal Republic of Germany.
Herbold, B. (1985c) Propoxur (BOQ 5812315), sister chromatid
exchange in the bone marrow of the Chinese Hamster in vivo to
evaluate for harmful effect on DNA. Unpublished Report No. 13501
dated May 22, 1985 from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld. Submitted to WHO by Bayer AG, Leverkusen,
Federal Republic of Germany.
Herbold, B. (1985d) BOQ 5812315, sister chromatid exchange in human
lymphocyte cultures in vitro to test for DNA-modifying effects.
Unpublished Report No. 13871 dated October 9, 1985 from Bayer AG,
Institute of Toxicology, Wuppertal-Elberfeld. Submitted to WHO by
Bayer AG, Leverkusen, Federal Republic of Germany.
Herbold, B. (1985e) BOQ 5812315, test on S.cerevisiae D7 to
evaluate for point mutagenic effect. Unpublished Report No. 13966
dated October 30, 1985 from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld. Submitted to WHO by Bayer AG, Leverkusen,
Federal Republic of Germany.
Herbold, B. (1986) BOQ 5812315 (c.n. propoxur), cytogenetic study of
chromosome damage using spermatogonia of Chinese Hamsters in vivo.
Unpublished Report No. 14984 dated August 20, 1986 from Bayer AG,
Institute of Toxicology, Wuppertal-Elberfeld. Submitted to WHO by
Bayer AG, Leverkusen, Federal Republic of Germany.
Herbold, B. (1988) BOQ 5812315 (c.n. propoxur), cytogenetic study on
bone marrow of Chinese Hamster in vivo to detect chromosomal
damage. Unpublished Report No. 17111 dated September 6, 1988 from
Bayer AG Institute of Toxicology, Wuppertal-Elberfeld. Submitted to
WHO by Bayer AG, Leverkusen, Federal Republic of Germany.
Hoffmann, K. & Gröning, P. (1984) BOQ 5812315 (BOE 5812315, c.n.
propoxur) Chronic toxicity to dogs on oral administration (12-months
feeding study). Unpublished Report No. 12605 dated April 11, 1984
from Bayer AG Institute of Toxicology, Wuppertal-Elberfeld.
Submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Hoffmann, K. & Ruehl, Chr. (1985) Propoxur (BOQ 5812315). Subchronic
study of toxicity to Rhesus monkeys after oral administration by
stomach tube for 13 weeks to check for possible findings in the
urinary bladder. Unpublished Report No. 13779 dated August 27, 1985
from Bayer AG Institute of Toxicology, Wuppertal-Elberfeld.
Submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Inukai, H. & Iyatomi, A. (1978) Propoxur. Mutagenicity test on
bacterial systems. Unpublished Report No. 103 dated February 24,
1978 from Nitokuno Agricultural Chemicals Institute, Toyoda, Japan.
Submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Jaszcuk, E., Syrowatka, T. & Cybulski, J. (1979) Mutagenic activity
of propoxur, carbaryl and their nitroso derivatives: induction of
reversion in Salmonella typhimurium. Roczniki Panstwowzgo Zakladu
Higieny Warszawa, 30(1): 81-88. (English summary only).
Jurek, A. (1978) Chronische Langzeittoxizität des
Propoxurkarbaminats, Roczniki Panstwowzgo Zakladu Higieny Warszawa,
29(3): 327-338.
Karl, W. (1986) Biotransformation of propoxur: quantitative
determination of the metabolic pattern in rats given a single dose
of 14C-propoxur after a subchronic prefeeding period in two diet
groups and three dose groups. Unpublished Report No. KWN 42 dated
October 15, 1986 from Bayer AG Institute of Toxicology, Wuppertal-
Elberfeld. Submitted to WHO by Bayer Leverkusen, Federal Republic of
Germany.
Karl, W. & Schneider, J. (1987) Isolation and spectroscopic
structure elucidation of the renal metabolite conjugates of
propoxur. Unpublished Report No. KWN 44 dated August 26, 1987 from
Bayer AG Institute of Toxicology, Wuppertal-Elberfeld. Submitted to
WHO by Bayer AG, Federal Republic of Germany.
Kimmerle, G. & Eben, A. (1978a) Propoxur - Acute inhalation study on
rats with determination of acetylcholinesterase activity in blood
and elimination of 2-isopropoxyphenol in urine. Unpublished Report
No. 7555 dated May 1978 from Bayer AG Institute für Toxikologie,
Wuppertal-Elberfeld. Submitted to WHO by Bayer AG Leverkusen,
Federal Republic of Germany.
Kimmerle, G. & Eben, A. (1978b) Propoxur - Single exposure of
persons to propoxur with determination of acetylcholinesterase
activity in plasma and erythrocytes, propoxur concentration in blood
and elimination of 2-isopropoxyphenol in urine. Unpublished Report
No. 7554 dated May, 1978 from Bayer AG, Institut für Toxikologie,
Wuppertal-Elberfeld. Submitted to WHO by Bayer AG Leverkusen,
Federal Republic of Germany.
Kimmerle, G. & Iyatomi, A. (1976) Toxicity of propoxur to rats by
subacute inhalation. Jap. J. Ind. Health, 18: 375-382.
Klein, W. (1984) Effect of an active ingredient and three
metabolites on the DNA metabolism. Unpublished Report R3346 dated
March 1984 from Osterreichisches Forschungszentrum Seiberdorf
Biochemistry Department/Toxicology Department. (Research commisioned
by Bayer AG). Submitted to WHO by Bayer AG Leverkusen, Federal
Republic of Germany.
Krechniak, J. & Foss, W. (1982) Cholinesterase activity in rats
treated with propoxur. Bull. Environm. Contam. Toxicol., 29: 599-
604.
Krechniak, J. & Foss, W. (1983a) Behaviour of propoxur after
repeated administration. Bromat. Chem. Toksykol., 16 (3-4): 205-
208. (English translation).
Krechniak, J. & Foss, W. (1983b) Distribution and excretion of
isopropoxyphenol in rats. Bromat. Chem. Toksykol., 6 (3-4): 209-
211 (English translation).
Lehn, H. (1988) BOQ 5812315 (c.n. propoxur), mutagenicity study for
the detection of induced forward mutations in the CHO-HGPRT assay
in vitro. Unpublished Report No. 17090 dated August 31, 1988 from
Bayer AG, Fachbereich Toxikologie, Wuppertal. Data submitted to WHO
by Bayer AG, Leverkusen, Federal Republic of Germany.
Luckhaus, G. (1984) Propoxur, two-year oral toxicity study in rats,
addendum to HRC Report No. 2809/69/235, histological follow-up
investigation of the urinary bladder. Unpublished Report No. 13012
dated November 2, 1984 from Bayer AG, Institute of Toxicology,
Wuppertal. Data submitted to WHO by Bayer AG, Leverkusen, Federal
Republic of Germany.
Machemer, L. & Schmidt, U. (1988) Propoxur. Status of the studies
and assessment regarding oncogenic potential to the urinary bladder.
Unpublished report dated September 30, 1988 from Bayer AG
Fachbereich Toxikologie, Institut für Toxikologie Landwirtschaft,
Wuppertal-Elberfeld. Data submitted to WHO by Bayer AG, Leverkusen,
Federal Republic of Germany.
Mihail, F. (1982) BOQ 5812315. Test for induction of the microsomal
liver enzymes. Unpublished Report No. 10976 dated June 29, 1982 from
Bayer AG, Institute of Toxicology, Wuppertal-Elberfeld. Data
submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Ohta, T. & Moriya, M. (1983) Propoxur, microbial mutagenicity study.
Unpublished Report from Institute of Environmental Toxicology
(Japan) dated February 28, 1983. Data submitted to WHO by Bayer AG,
Leverkusen, Federal Republic of Germany.
Pauluhn, J. (1988) BOQ 5812315 (common name: propoxur) study of the
inhalation toxicity in accordance with OECD Guideline No. 403.
Unpublished Report No. 16966 dated July, 1988 from Bayer AG
Fachbereich Toxikolgie, Wuppertal. Submitted to WHO by Bayer AG,
Leverkusen, Federal Republic of Germany.
Pauluhn, J. & Rühl, C. (1985) BOQ 5812315, study for subacute
inhalation toxicity to the rat. Unpublished Report No. 13297 dated
February, 20, 1985 from Bayer AG, Institute of Toxicology Wuppertal-
Elberfeld. Data submitted to WHO by Bayer AG, Leverkusen, Federal
Republic of Germany.
Putman, D.L. & Morris, M.J. (1988) Chromosome aberrations in Chinese
Hamster Ovary (CHO) cells, Baygon technical. Unpublished Report No.
1094 dated December 22, 1988 from Microbiological Associates Inc.
Maryland, USA. Submitted to WHO by Bayer AG, Leverkusen, Federal
Republic of Germany.
Reid Patterson, D. (1980) Histopathology report on Boe 5812315 mouse
study. Unpublished Report No. 2317-262/13 dated May, 1980 from
Hazleton Laboratories Europe, Harrogate, England to Bayer AG
Institut für Toxikologie, Wuppertal. Submitted to WHO by Bayer AG,
Leverkusen, Federal Republic of Germany.
Schlüter, G. (1981) BOQ 5812315. Evaluation for embryotoxic and
teratogenic effects after oral administration to the rabbit.
Unpublished Report No. 10183 dated September 9, 1981 from Bayer AG,
Institute of Toxikologie, Wuppertal-Elberfeld. Data submitted to WHO
by Bayer AG, Leverkusen, Federal Republic of Germany.
Schmidt, U. (1987) Investigations of interspecies differences in
primary metabolism with liver-cell fractions from rat, mouse,
hamster, monkey and man. Unpublished Report No. 16237 dated November
19, 1987 from Bayer AG, Fachbereich Toxikologie, Wuppertal.
Submitted to WHO by etc.
Seiler, J.P. (1977) Nitrosation in vitro and in vivo by sodium
nitrite, and mutagenicity of nitrogenous pesticides. Mutat. Res.
48: 225-236.
Shirasu, Y., Moriya, M., Kato, K., Furuhashi, A. & Kada, T. (1976)
Mutagenicity screening of pesticides in the microbial system.
Mutat. Res. 40: 19-30.
Shirasu, Y., Moriya, M. & Sugiyama, F. (1979) Propoxur, mutagenicity
test on bacterial systems. Unpublished Report from Institute of
Environmental Toxikologie (Japan) dated August 28, 1979. Data
submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Siebert, D. & Eisenbrand, G. (1974) Induction of mitotic gene
conversion in Saccharomyces cerevisiae. Mutat. Res. 22: 121-126.
Siebert, D. & Lemperle, E. (1974) Genetic effects of herbicides:
induction of mitotic gene conversion in Saccharomyces cerevisiae.
Mutat. Res. 22: 111-120.
Suberg, H. & Löser, E. (1984) BOQ 5812315, chronic toxicological
study with rats (feeding study over 106 weeks). Unpublished Report
No. 12870 dated August 20, 1984 from Bayer AG, Institute of
Toxicology, Wuppertal. Data submitted to WHO by Bayer AG,
Leverkusen, Federal Republic of Germany.
Thyssen, J. (1977) Study for combination toxicity of azinphos-methyl
and propoxur. Unpublished Report No. 7174 dated December 14, 1977
from Bayer AG, Institute of Toxicology, Wuppertal-Elberfeld. Data
submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Thyssen, J. (1978) Propoxur-Untersuchungen an der Haut und am Auge
von Kaninchen. Unpublished report dated September 19, 1978 from
Bayer AG, Institut für Toxikologie, Wuppertal-Elberfeld. Submitted
to WHO by Bayer AG, Leverkusen, Federal Republic of Germany.
Tyrkiel, E. (1977) Mutagenne addzialywannie, o-izopropoksyfenoyl-N-
methylo-karaminianu (propoksura) na komorki pleiowe myszy. Rocz.
Panstw. Zakl. Hig. 28 (6): 601-613.
Weber, H. (1986) Comparison of the absorption of a tracer dose of
(Phenyl-U-14C) propoxur from a basic casein diet and a standard
Altromin 1324 diet by nonradioactively pretreated Wistar rats.
Unpublished Report No. 2504 dated February 4, 1986 from Bayer AG,
Institute of Metabolism Research , Wuppertal. Data submitted to WHO
by Bayer AG, Leverkusen, Federal Republic of Germany.
Weber, H. (1988) [Phenyl-UL-14C] Propoxur: whole-study
autoradiographic distribution of the radioactivity in the rat.
Unpublished Report No.2988 dated April 21, 1988 from Bayer AG,
Institute for Metabolism Research, Leverkusen. Data submitted to WHO
by Bayer AG, Leverkusen, Federal Republic of Germany.
WHO/FAO (1974) 1973 Evaluations of some pesticide residues in food,
WHO Pesticides Residues Series, No. 3. World Health Organization,
Geneva.
Yamane, S. (1986a) Primary skin irritation study of propoxur in
rabbits. Unpublished Report No. D-0714 (DT-9) dated June 9, 1986
from Hita Research Laboratories, Japan. Data submitted to WHO by
Bayer AG, Leverkusen, Federal Republic of Germany.
Yamane, S. (1986b) Primary eye irritation study of propoxur in
rabbits. Unpublished Report No. D-0736 (DT-10) dated September 12,
1986 from Hita Research Laboratories, Japan. Data submitted to WHO
by Bayer AG, Leverkusen, Federal Republic of Germany.
90% of the administered 14C was recovered in urine in all groups.
The urinary conjugated metabolite pattern was comparable for the
different dose levels and the different diets. At higher (5000 ppm)
dose levels, there was a trend for a higher percentage of M5 and M6
and a lower for M2 and M7. The 14C distribution over the
conjugated metabolites is summarized in Table 1.
TABLE 1. DISTRIBUTION OF 14C OVER URINARY METABOLITES IN RATS
METABOLITE % OF 14C PRESENT IN
URINE (RANGE FOR ALL
DOSE LEVELS AND DIETS)
M1 7.0-15.5
M2 17.2-25.2
M3 22.2-30.2
M5 0.6- 2.5
M6 4.5- 7.9
MS3 0.3- 1.2
M7 14.0-16.3
M7A 5.9- 8.3
M8 1.7- 4.1
(Karl, 1986; Karl & Schneider, 1987)
Metabolism of propoxur in the liver was studied further in in
vivo studies, using post-mitochondrial fractions of livers of
rats, mice, hamsters and monkeys. A similar study was done in human
liver preparations. The results show M5 to be the major metabolite
in rat liver, M3 and M6 being formed to a lesser degree. The
percentage of M5 in relation to overall metabolism decreases from
mouse to hamster and monkeys. In the liver preparation from Rhesus
monkeys, M3 and M6 were formed more than in M5. In additional tests
it was observed that in liver cell fractions of rats and mice
further metabolization of M5 does not take place in hamsters it
occurs only to a limited degree. In contrast, the liver cell
fractions of Rhesus monkeys and humans are able to further
metabolize M5 to 4 other metabolites (not identified) (Schmidt,
1987).
Effect on cholinesterase activity
Male and female Wistar rats were given a single oral dose of 0,
1, 5 or 25 mg/kg bw via stomach tube. Cholinesterase activity was
measured in plasma, erythrocytes and brain at intervals varying from
0.5 hours to 14 days after dose application. At the highest dose
level, cholinesterase activity in plasma and erythrocytes was
depressed after 30 and 60 minutes; after 3 hours the depressions
were no longer apparent. Brain cholinesterase activity was depressed
after 3-5 hours in the same group; 3 days after dosing this was no
longer apparent. At 1 and 5 mg/kg bw no effect was seen (Heimann,
1982c).
In male rats given a single oral dose of 2.1, 7.0, 20.9, 50 or
70 mg/kg bw propoxur, cholinesterase activity in whole blood was
decreased at all dose levels (at 2.1 mg/kg bw the decrease was
slight) after 10 minutes; at 24 hours after dose application the
depressions were no longer present. The same investigators tested
for cholinesterase depression in whole blood and brain in male rats
dosed orally with 30 mg/kg bw/day for 14 days followed by 50 mg/kg
bw via the same route for 28 days. A decreased activity in both
blood and brain was noted during the first 14 days; the depression
gradually disappeared during the following 28 days of treatment.
This indicates adaptation (Krechniak & Foss, 1982).
In an inhalation study, male and female rats were exposed to
0.4, 1.2, 9.0, 30.0, 78.0 or 172.0 mg/m3 propoxur for 6 hours.
Directly after cessation of exposure, plasma and erythrocyte
cholinesterase activities were measured and compared to pre-test
values. At 30, 78 and 172 mg/m3, depressions were observed. In
addition, at 172 mg/m3 cholinergic symptoms were seen at 30 minutes
after commencement of exposure (Kimmerle & Eben, 1978a).
Effect on liver enzyme activity
Mice
Male mice received propoxur (purity 98.8%) in the drinking
water at concentrations increasing with each successive week from 50
to 2000 ppm over a total period of 6 weeks. In animals thus treated
for six weeks, the LD50 for propoxur was significantly higher than
in untreated controls, a finding indicating that tolerance to
propoxur acute toxicity had developed. Hexobarbital sleeping time
was significantly reduced, indicating induction of hepatic
microsomal enzymes. Determinations of carboxy esterase activities
in liver, plasma and brain, however, did not show any significant
increase in the propoxur-treated animals (Costa et al. 1981).
Rats
Propoxur was administered orally for 5 days to male and female
rats at dose levels of 0, 15 or 30 mg/kg bw/day. The rats treated
with propoxur did not exhibit induction of the mixed function
oxidases in comparison to control rats. (A positive control group
was treated with sodium phenobarbitone 50 mg/kg bw/day for 5 days
and induction of mixed function oxidases was observed) (Mihail,
1982).
Concomitant to a short-term study in female rats, liver tissue
enzyme activities were measured after 3, 7, 14 and 28 days of
feeding on a diet containing 0 or 5000 ppm propoxur. From day 3,
cytochrome P-450 dependent mono-oxygenases (i.e. 7-ethoxycoumarin
deethylase, ethoxyresorufin deethylase, aldrin epoxidase) were
induced (increased with a factor of 2-3). The activity of the
microsomal epoxide hydroxylase was increased by the same magnitude.
In contrast, the cytosolic glutathione-S-transferase exhibited only
a slight change compared to the control group. Altromin diet was
used in this study. An identical study in which a casein semi-
synthetic diet was used also showed induction of mono-oxygenase
activity by propoxur. The absolute activity levels reached in the
latter study, however, were about 50% lower compared to the values
observed in the concurrent control and treatment groups of the
Altromin diet study (Machemer & Schmidt, 1988).
Toxicological studies
Acute toxicity
The acute toxicity of propoxur to rats and mice is presented in
Table 2.
The observed intoxication symptoms were indicative of
inhibition of cholinesterase: convulsions, muscular tremors,
muscular spasms, dyspnoea, salivation (Flucke, 1980; Heimann,
1982b).
Short-term toxicity
Oral studies
Rats
The toxicity of propoxur technical (purity 98.6%) and propoxur
recrystallized (purity 99.2%) was compared in a 5 day-study. Groups
of Wistar rats (5/sex/group) received oral doses of 0, 15 or 30
mg/kg bw/day of propoxur of both purities via stomach tube for 5
days. Appearance, behaviour and body weight were recorded daily.
After 5 days, all animals were sacrificed and submitted to gross
pathology. The weights of liver and kidneys were determined.
Clinical chemistry was performed on all animals. N-demethylase, O-
demethylase, cytochrome P-450 and triglycerides were measured in
liver homogenates (cholinesterase activity not determined). The
only adverse effects noted were convulsions and apathy, occurring in
all treatment groups in a dose-related manner. No difference in
toxicity between the two purities were found (Heimann, 1983).
TABLE 2. ACUTE TOXICITY OF PROPOXUR IN ANIMALS
LD50 LC50
SPECIES SEX ROUTE (mg/kg bw) (mg/m3) REFERENCES
Mouse M oral 37 - Haley et al. 1974
F idem 39 - Haley et al. 1974
Rat M* oral 39 - Thyssen et al. 1977
M* oral 45 - Flucke, 1984
M oral 196 - Flucke, 1980
F oral 126 - Flucke, 1980
M* oral 94 - Flucke, 1980
F* oral 68 - Flucke, 1980
M oral 167 - Heimann, 1982b
F oral 96 - Heimann, 1982b
M* oral 69 - Heimann, 1982b
F* oral 47 - Heimann, 1982b
M i.p. 16 - Heimann, 1982b
F i.p. 13 - Heimann, 1982b
M&F dermal >5000 - Flucke, 1980
M&F inhal. - >498 Pauluhn, 1988
* Fasted animals
Dogs
Beagle dogs (6/sex/group) were given diets containing 0, 200 or
600 ppm propoxur (purity 99.4%) for 52 weeks. An additional group
received 1800 ppm from week 1 through week 40, 3600 ppm from week 41
through 44 and 5400 ppm from week 45 through 52 (the increases were
established in order to produce overt toxic signs). Appearance,
behaviour and body weight were recorded. On a number of occasions
throughout the study reflex tests, ophthalmoscopy, hematology,
clinical chemistry, urinalysis and cholinesterase activity (in
plasma and erythrocytes) were determined. At sacrifice, organs were
weighed and complete gross pathology and histopathology were carried
out. In livers N-demethylase and cytochrome P-450 were measured.
Cholinergic symptoms were observed at the highest dose level after
elevation of the dose level to 5400 ppm and 1/6 animals died. In
addition, the following parameters were increased in this group:
thrombocyte, leucocyte and reticulocyte counts, incidence of Heinz
bodies, ALAT and SAP, liver weight and thyroid weight; thymus weight
was decreased (also noted: medium thymus atrophy). At the highest
dose level and at 600 ppm also, growth was retarded and plasma
cholesterol and liver N-demethylase were increased. The NOAEL in
this study is 200 ppm (Hoffmann & Gröning, 1984).
Inhalation studies
Groups of Wistar rats (10/sex/group) were exposed to aerosols
containing propoxur (purity 98.9%) in concentrations of 0, 5.7, 18.7
or 31.7 mg/m3 6 hours per day, 5 days per week over a period of 12
weeks. There was no effect on behaviour, growth, hematology,
clincial chemistry, urinalysis, organ weights or histopathology.
The only effect observed was a depression of cholinesterase activity
in plasma, erythrocytes and brain, occuring at 31.7 mg/m3 only
(Kimmerle & Iyatomi, 1976).
Groups of Wistar rats (5/sex/group) were exposed to aerosols
containing propoxur (purity 99.6%) in concentrations of 0, 15.3,
45.3 or 139.6 mg/m3 during 6 hours per day, 5 days per week over a
period of 4 or 8 weeks. The observations included clinical signs,
body weight, cholinesterase activity in plasma, erythrocytes and
brain at sacrifice after 4 and 8 weeks, urinalysis, gross pathology
(all organs) and histopathology (4 tissues/animal) and organ weights
(4 organs/animal). Cholinergic symptoms were observed at 139.6
mg/m3. Cholinesterase activies in brain were depressed in week 4
at 45.3 and 139.6 mg/m3 and in week 8 at 15.3 mg/m3 also. There
were no signs of specific organ damage or an alteration in the
urinary bladder epithelium (Pauluhn & Rühl, 1985).
Long term carcinogenicity studies
Mice
Groups of 50 male and 50 female CF1/W74 mice were fed diets
containing 0, 700, 2000 or 6000 ppm propoxur (purity 99.6%) for 24
months. Satellite groups of 10 male and 10 female mice were fed at
the same dose levels and were used for interim sacrifice after 6
months. Observations included clinical signs, body weight, food
consumption, hematology and clinical chemistry. The weights of 6
organs/animal were recorded. Gross pathology and limited
histopathogy (about 20 tissues/animal) were carried out. Slight
growth retardation was oberved in the 700, 2000 and 6000 ppm males;
ALAT was increased in 6000 ppm-females after 6 months only. The
relative weights of testes and spleen were increased or decreased,
respectively, at all dose levels to a dose-related degree. The
tumour incidence was not increased (Bomhard & Löser, 1981; Reid
Patterson, 1980).
Rats
In a chronic toxicity study summarized in the WHO/FAO monograph
from 1974, with dietary dose levels of 0, 250, 750, 2000 and 6000
ppm (purity 99.8%),the effects were: growth retardation and reduced
food consumption at 200 and 6000 ppm and increased relative liver
weight at 6000 ppm (NOAEL in this study: 250 ppm) (WHO/FAO, 1974).
The histological sections produced in this study were reexamined.
Histopathological appraisal of urinary bladder sections confirmed
the absence of alterations in this organ at all dose levels
(Luckhaus, 1984).
Groups of 20 male and 20 female Wistar rats were fed diets
containing 0, 50, 200 or 800 ppm propoxur (purity 97.25%) for 18
months. The observations included clinical signs, food intake, body
weight, hematology and clinical chemistry. At termination all
animals were sacrificed. Organ weights were determined and
histopathology was carried out. Growth was slightly decreased in
the 800 ppm females. At the end of the study cholinesterase
activities in whole blood and brain were inhibited at 800 ppm. The
NOAEL in this study is 200 ppm (Jurek, 1978).
Groups of 50 male and 50 female Wistar rats were fed diets
containing 0, 200, 1000 or 5000 ppm propoxur (purity 99.4%) for 2
years. Additional groups of 10 rats/sex/group were treated at the
same dose levels and were used for interim sacrifice after 1 year.
The observations included clinical signs, body weight, gross
pathology and histopathology. Growth was retarded at 1000 and 5000
ppm. At 5000 ppm slight neuromuscular changes (i.e. slightly
increased incidences of peripheral neuropathy and muscular atrophy
of the rear extremities) were noted. Also at 5000 ppm, ASAT was
decreased (males and females) and urea was increased (females only).
At the same dose level the relative weights of a number of organs
(heart, lung, liver, kidney, adrenal) were increased. At interim
autopsy the incidence of hyperplasia of the urinary bladder was
increased at 1000 and 5000 ppm (incidences 0/20, 0/20, 6/20 and
19/20 in the 0, 200, 1000 and 5000 ppm groups, respectively). The
findings in the urinary bladder at terminal sacrifice are presented
in Table 3.
TABLE 3. INCIDENCE OF URINARY BLADDER ALTERATIONS
IN MALE AND FEMALE RATS
OCCURRENCE CONTROL 200 ppm 1000 ppm 5000 ppm
Hyperplasia 1/98 1/96 15/99 92/97
Papilloma 0/98 0/96 1/99 53/97
Carcinoma 0/98 0/96 0/99 13/97
The NOAEL in this study is 200 ppm (determined to be equal to
9.6 mg/kg bw/day) (Suberg & Löser, 1984, Glaister, 1984).
For further determination of the dose-response/exposure-time
relationship with regard to the effect on the urinary bladder,
groups of 70 female Wistar rats were fed diets containing 0, 50,
250, 1000, 3000, 5000 or 8000 ppm propoxur (purity 99.6-99.9%) for
periods up to 104 weeks. After 4, 7, 12, 26, 53 and 78 weeks, 5 or
10 rats/group were sacrificed for interim autopsy. The animals were
observed for clinical signs, food intake, water intake and body
weight. Organ weights were determined. Gross pathology and
histopathology (kidney, urinary bladder, ureter, liver) were carried
out. Growth was retarded at 3000 ppm and higher dose levels. The
relative weights of liver and kidneys were increased at 3000, 5000
and 8000 ppm. Hyperplasia of the bladder epithelium was observed at
1000 ppm (from week 53), at 3000 ppm (from week 12), 5000 ppm (from
week 4) and at 8000 ppm (from week 2). At the latter two dose
levels the effect had developed to severe hyperplasia with recent
vascularization and papillary and nodular hyperplasia after 53
weeks. After 104 weeks, dose-related increases were observed on
hyperplasia, papilloma and carincoma of the urinary bladder in
female rats.
The NOAEL in this study is 250 ppm (Hahnemann & Rühl-Fehlert,
1988f)
In a number of additional studies the effect of propoxur on the
urinary bladder was yet further examined. Issues to be elucidated
were strain and species specificity, influence of the diet used and
effect of vitamin C supplementation.
Strain specificity
Possible strain specificity of the effect of propoxur on the
urinary bladder, observed in Wistar rats, was examined through an
oral study in Sprague Dawley rats. Groups of 50 female Sprague
Dawley rats were fed diets containing 0, 3000 or 8000 ppm propoxur
(purity 99.6%-99.9%) for periods up to 52 weeks. Growth was
retarded and the relative weights of liver, lung and kidneys were
increased at both dose levels. Simple hyperplasia of the urinary
bladder was observed at 3000 and 8000 ppm from week 4 on. At 8000
ppm hyperplasia with neovascularization, papillary hyperplasia and
incipient nodular hyperplasia were found in the urinary bladder from
week 27. Thus, this strain of rats is as sensitive as is the Wistar
rat with regard to the formation of urinary bladder hyperplasia by
propoxur (Hahnemann & Rühl-Fehlert, 1988b).
Species specificity
In the two oral short-term toxicity studies in dogs (summarized
in the relevant paragraphs of the present monograph and the 1974
WHO/FAO monograph respectively) and in the long-term study in mice
(summarized in the paragraph on long-term studies of the present
monograph) no effect on the urinary bladder epithelium was observed.
Further evidence of species specificity was obtained in the
following studies.
Mice
Groups of 50 female NMRI mice were fed diets containing 0, 3000
or 8000 ppm propoxur (purity 99.6%-99.9%) for 53 weeks. Growth was
slightly decreased at 8000 ppm. Increased liver weight and fatty
degeneration occurred at 3000 and 8000 ppm. Relative lung weight
was increased at 8000 ppm only. No adverse effect on urinary
bladder epithelium was noted (Hahnemann & Rühl-Fehlert, 1988c).
Hamsters
Groups of 50 female Syrian golden hamsters were fed diets
containing 0, 3000 or 8000 ppm propoxur (purity 99.6-99.9%) for 53
weeks. At both dose levels the incidence of mortality was slightly
increased, impairment of the general state of the animals (not
specified) was noted and growth was retarded. The relative weights
of kidneys and adrenals were increased at 8000 ppm only. No adverse
effect on urinary bladder epithelium was observed (Hahnemann & Rühl-
Fehlert, 1988a).
Rhesus monkeys
A group of 6 Rhesus monkeys (3 per sex) received oral doses of
40 mg/kg bw/day propoxur (purity 99.6%) via oral intubation for 13
weeks. This daily dose was previously determined to be the maximum
tolerable dose. No control group was used. Cholinergic symptoms
were observed following compound administration. No adverse effect
on the urinary bladder epithelium was noted (Hoffmann & Rühl, 1985).
Effect of vitamin C supplementation
Groups of 50 female Wistar rats were fed diets containing 1%
vitamin C and propoxur (purity 99.6-99.9%) at concentrations of 0,
1000, 3000 or 8000 ppm for a period of 49 weeks. Additional groups
received the same propoxur dose levels in unsupplemented diet.
Growth was retarded in all propoxur-treated groups. The relative
weights of liver (8000 ppm only) and kidneys and lungs (all dose
levels) were increased. The propoxur-induced hyperplastic changes
of the urinary bladder epithelium were observed in all treatment
groups, being present to an equal degree after feeding of the
supplemented and the unsupplemented diets. Thus, vitamin C did not
influence the effect of propoxur on the rat urinary bladder
epithelium (Hahnemann & Rühl-Fehlert, 1988e).
Effect of diet
In the rat studies in which propoxur was found to produce the
urinary bladder alterations, the diet used was Altromin B21 standard
diet. To examine if the diet used was a relevant factor for the
occurrence of the urinary bladder changes, two additional studies
were carried out using a semisynthetic diet (Casein diet no. 1/0).
Groups of 50 female Wistar rats were fed Casein semi-synthetic
diet no. 1/0 containing 0 or 8000 ppm propoxur (purity 99.9%) for
4,8 or 14 weeks. Growth retardation and reduced water intake were
observed in the treated animals. In addition, relative liver and
kidney weights were increased. No urinary bladder changes were
found at histopathology (Hahnemann & Rühl-Fehlert, 1988d).
Groups of 50 female Wistar rats were fed Casein diet no. 1/0
containing 0, 3000 or 8000 ppm propoxur (purity 99.6%) for periods
up to 100 weeks. Growth was retarded at 3000 and 8000 ppm.
Relative weights of lung, kidney and liver were increased at 8000
ppm. Histopathology revealed no treatment-related changes in the
urinary bladders of treated animals (Hahnemann & Rühl-Fehlert,
1988g).
In a study using 14C-propoxur, possible differences in
absorption of propoxur from the Altromin diet and the semisynthetic
case in diet were ruled out. Thus, the absence of urinary bladder
effects when the casein diet is used is not caused by lower
absorption of propoxur from this diet (Weber, 1986). Another
relevant result was observed in a study in rats in which the renal
metabolite pattern was determined after a single oral dose of 14C-
propoxur (label in ring) after preceding administration of 0, 250 or
5000 ppm propoxur either in Altromin standard diet or in the
semisynthetic casein diet for 4 weeks. No differences in the
metabolite pattern were found (Karl, 1986; Karl & Schneider, 1987).
Special studies on combination toxicity
The acute oral application of equitoxic doses of propoxur and
azinphosmethyl to male Wistar rats revealed an additive toxic effect
of the combination of the two pesticides (Thyssen, 1977).
The LD50-value in male Wistar rats for an equitoxic mixture of
propoxur (purity 99.3%) and cyfluthrin (93.7%) proved to be less
than the value expected on the basis of simple addition of effects
(Flucke, 1984).
Special studies on embryotoxicity and teratogenicity
Rats
Groups of 24 pregnant female Wistar rats received 0, 3, 9 or 27
mg/kg bw/day propoxur (purity 99.4%) p.o. by gavage from day 6
through 15 of gestation. Appearance, behaviour, body weight and
food consumption were recorded daily. At day 21 of gestation all
animals were sacrificed and the fetuses were delivered by Cesarean
section. The number of implantations, resorptions (early and late)
and corpora lutea were determined. The fetuses were counted and
weighed; gross pathology and histopathology (skeletal and visceral)
were carried out. At 27 mg/kg bw, 3 animals died before the end of
the test. At 9 and 27 mg/kg bw, symptoms (increased grooming,
chewing motions, teeth grinding) were noted in the hours following
dose application during the entire treatment; at 27 mg/kg bw, in
addition, tremors and ventral recumbency were observed. At the same
dose levels, food consumption and growth of dams were decreased in a
dose related fashion. No other effects were seen. The NOAEL for
maternal toxicity in this study is 3 mg/kg bw/day (Becker et al.
1989a).
Rabbits
Groups of 15 Himalayan rabbits received 0, 1, 3 or 10 mg/kg
bw/day propoxur (purity 99.6%) p.o. from day 6 through 18 of
gestation. There were no indications of maternally toxic,
embryotoxic or teratogenic effects. However, the study was limited
with respect to soft tissues examination (Schlüter, 1981).
Groups of 16 pregnant Chinchilla rabbits received 0, 3, 10 or
30 mg/kg bw/day propoxur (purity 99.4%) p.o. by gavage from day 6
through 18 of gestation. Appearance, behaviour, body weight and
food consumption were recorded daily. At day 28 of gestation all
animals were sacrificed and the fetuses were delivered by Cesarean
section. The number of implantations, resorptions (early and late)
and corpora lutea were determined. The fetuses were counted and
weighed; gross pathology and histopathology (skeletal and visceral)
were carried out. At 30 mg/kg bw restless behaviour and dyspnoea
were observed after dose application on the first 3 treatment days;
in the same group 3 animals died before test end. In addition, body
weight loss (day 6-9 of gestation) occurred in this group. Also, at
30 mg/kg bw post-implantation loss was increased (number of pups per
dam decreased consequently). The NOAEL for maternal toxicity and
embryotoxicity in this study is 10 mg/kg bw/day (Becker et al.
1989b)
Special studies on mutagenicity
A large number of mutagenicity tests has been carried out with
propoxur. The results are summarized in Table 2 ( in vitro assays)
and Table 3 ( in vivo assays). In addition, in vitro tests in
prokaryotes have been performed with a number of metabolites of
propoxur. The results of these studies are summarized in Table 6.
Special studies on skin and eye irritation and sensitization
Undiluted propoxur (purity 99.2%) was tested for irritation to
shaven intact and shaven abraded skin areas of 6 New Zealand
rabbits. Exposure was for 24 or 72 hours. No irritation was observed
(Thyssen, 1978).
A dose of 0.5 g propoxur (purity 99.6%) moistened with purified
water, was applied under occlusive conditions to the shaven intact
back skin of 6 male New Zealand White rabbits for 4 hours. No skin
irritation was observed up to 72 hours after application (Yamane,
1986a).
In groups of 3 or 5 New Zealand rabbits an eye irritation study
was carried out with undiluted propoxur (purity 99.2%). Eyes were
rinsed after 5 minutes or 24 hours of exposure. The animals were
observed for 7 days. The only sign of irritation was slight erythema
of the conjunctivae of 2/3 animals that were exposed for 24 hours.
At 24 hours after rinsing this was no longer present (Thyssen,
1978).
Application of 0.1 g propoxur (purity 99.6%) into the eyes of 9
male New Zealand White rabbits caused severe miosis, which
disappeared within 24 hours after application. No irritation
effects were seen up to 96 hours post application (Yamane, 1986b).
Propoxur (purity 98.8%) did not exhibit a sensitizing effect in
the maximization test (Magnusson & Kligman) in guinea pigs (Heimann,
1982a).
Observations in humans
Dermal absorption
0.1 ml 14C-propoxur in acetone was applied to the ventral
forearm of 6 humans (sex not reported) (skin area 2.8-20 cm2;
applied amount 5 wµg propoxur/cm2). The skin sites were not
protected and subjects were asked not to wash the area for 24 hours.
Urine, collected for 5 days after beginning of exposure, was
monitored for 14C. The data were corrected for incomplete urinary
recovery using the 14C found in urine after administration of an
intravenous dose. A skin absorption of 19.6% of the dose was found
(Feldmann & Maibach, 1974).
TABLE 4. RESULTS OF IN VITRO MUTAGENICITY ASSAYS ON PROPOXUR
TEST SYSTEM TEST OBJECT CONCENTRATION PURITY RESULTS REFERENCE
Ames test * S. typhimurium 50 nmol/plate ca. 95% Negative Blevins et al. 1977b
TA98, TA100, TA1535
TA1537, TA1538
Ames test * S. typhimurium 0.1-1000 µg/pl 98.0% Negative Inukai & Iyatomi, 1978
TA98, TA100, TA1535 solvent DMSO (1)
TA1537, TA1538
Ames test * S. typhimurium 10-1500 µg/pl >96% Negative De Lorenzo et al. 1978
TA98, TA100, TA1535 (1)
TA1537, TA1538
Ames test S. typhimurium 0.25-100 µg/ml 97% Negative Jaszczuk et al. 1979
TA98, TA100, TA1535
TA1537, TA1538
Ames test * S. typhimurium 20-12500 µg/pl 98.6% Negative Herbold, 1982
TA98, TA100, TA1535 (1)
TA1537, TA1538
Reversion assay * Saccharomyces 75-10000 µg/ml 99.8% Negative Herbold, 1985e
cerevisiae D7 solvent DMSO (1)
Reverse mutation test E. coli WP2 hcr. 20 µl/disk ? Negative Shirasu et al. 1976
B/r try WP2
Reverse mutation E. coli WP2 hcr. 10-5000 µg/pl 98.05 Negative Shirasu et al. 1979
test * S. typh. (1)
TA98, TA100, TA1535
TA1537, TA1538
TABLE 4 (CONTD)
TEST SYSTEM TEST OBJECT CONCENTRATION PURITY RESULTS REFERENCE
Reverse mutation E. coli WP2 hcr. 500-25000 µg/pl 98.0% Negative Ohta & Moriya, 1983
test * S. typh. (1)
TA98, TA100, TA1535
TA1537, TA1538
HGPRT-test * Chinese hamster 25-125 µg/ml 99.6% Negative Lehn, 1988
ovary (CHO) cells (without S9 mix) (1)
600-1500 µg/ml (with S9 mix)
Mitotic gene Saccharomyces 2 ml of suspension 99.8% Negative Siebert & Lemperle, 1974;
conversion test cerevisiae D4 (containing 1000 ppm Siebert & Eisenbrand, 1974
a.i.) at 5 x 10 cells;
solvent DMSO
Pol Al-test * E. coli pol A+ 62.5-10000 µg/pl 98.5% Negative Herbold, 1983a
E. coli pol A- solvent DMSO (1)
Rec-assay Bacillus subtilis 3-300 µg/disk 98.0% Negative Inukai & Iyatomi, 1978
NIG17, NIG45 solvent DMSO (1)
Rec-assay Bacillus subtilis 20 µg/disk ? Negative Shirasu et al. 1976
H17 Rec+, M45 Rec- solvent DMSO
Rec-assay Bacillus subtilis 20-2000 µg/disk 98.0% Negative Shirasu ET AL. 1976
H17 Rec+, M45 Rec- (1)
Rec-assay Bacillus subtilis 50-10000 µg/disk 98.0% Negative Ohta & Moriya, 1983
H17 Rec+, M45 Rec- (1)
TABLE 4 (CONTD)
TEST SYSTEM TEST OBJECT CONCENTRATION PURITY RESULTS REFERENCE
Sister chromatid Human lymphocytes without S9 mix: 99.6% Negative Herbold, 1985d
exchange assay * 125-500 µg/ml (1)
with S9 mix:
250-1000 µg/ml
solvent DMSO
Single-strand Human fibroblasts 10-5 M approx Negative Blevins et al. 1977a
break assay 95%
Chromosome aberr. Chinese hamster without S9 mix. 97.8% Negative Putman & Morris, 1988
assay ovary (CHO) cells 157-625 µg/ml (1)
with S9 mix:
615 and 1250 µg/ml
solvent DMSO
* Test was carried out both with and without metabolic activation.
(1) Positive control yielded positive results.
TABLE 5. RESULTS OF IN VIVO MUTAGENICITY ASSAYS ON PROPOXUR
TEST SYSTEM TEST OBJECT CONCENTRATION PURITY RESULTS REFERENCE
DNA metabolism Male rat spleen cells 10 mg/kg bw; p.o. ? Negative Klein, 1984
studies (1)
Sister chromatid Chinese hamster bone 75 or 150 mg/kg bw 99.6% Negative Herbold, 1985c
exchange assay marrow cells p.o. (1)
Cytogenic study Chinese hamster 2 x 75 mg/kg bw 99.6% Negative Herbold, 1986
spermatogonia 2 x 150 mg/kg bw (1)
Cytogenic study Chinese hamster bone 75-300 mg/kg bw; 99.6% Negative Herbold, 1988
marrow cells p.o. (1)
Micronucleus test Male and female ICR- 25 mg/kg bw + 25 ? Negative Seiler, 1977
mice bone marrow cells mg/kg bw NaNO2; p.o.
Micronucleus test Male and female NMRI- 2 x 5 mg/kg bw; 99.2% Negative Herbold, 1980b
mice bone marrow cells 2 x 10 mg/kg bw; p.o. (1)
Dominant lethal Male mice 5 x 25 mg/kg bw ? Positive Tyrkiel, 1977
test 5 x 50 mg/kg (#) (1)
Dominant lethal Male mice 10 mg/kg bw; p.o. 99.2% Negative Herbold, 1980a
(#) Herbold (1978), in a critical review, points out the equivocality of the results, thus showing the questionable
validity of the author's conclusion.
(1) Postive control yielded positive results.
TABLE 6. RESULTS OF MUTAGENICITY ASSAYS ON PROPOXUR METABOLITES
TEST SYSTEM TEST OBJECT CONCENTRATION RESULTS REFERENCE
M1
Ames test * S. typhimurium 20 and 12500 µg/plate Negative Herbold, 1983c
TA98, TA100,
TA1535, TA1537
Poly A1- test *1 E. coli pol A+ 625-6075 µg/plate Negative Herbold, 1984b
E. coli pol A1-
M2
Ames test * S. typhimurium 20-12500 µg/plate Negative Herbold, 1983b
TA98, TA100
TA1535, TA1537
Mitotic recombination Saccharomyces 185.9-30000 µg/plate Negative Herbold, 1984e
assay * cerevisiae D7
M3
Ames test * S. typhimurium 312.5-5000 µg/plate Negative Herbold, 1984c
TA98. TA100, TA1535
TA1537. TA1538
DNA metabolism studies Male rat spleen 10 mg/kg bw Negative Klein, 1984
cells (1)
M4
Ames test * S. typhimurium 312.5-5000 µg/plate Negative Herbold, 1984a
TA98, TA100, TA1535
TA1537, TA1538
DNA metabolism studies Male rat spleen cells 10 mg/kg bw Negative Klein, 1984
TABLE 6 (CONTD)
TEST SYSTEM TEST OBJECT CONCENTRATION RESULTS REFERENCE
M5
Ames test * S. typhimurium 8-8748 µg/plate Negative Herbold, 1984d
TA98, TA100, TA1535
TA1537, TA1538
DNA metabolism studies Male rat spleen cells 10 mg/kg bw Negative Klein, 1984
(1)
M7
Not evaluable in Ames test due to breakdown in test medium
M8
Ames test * S. typhimurium evaluated range: Negative Herbold, 1984f
TA98, TA100 8-1800 µg/plate
TA1535, TA1537
Propoxur urine (rat, 8000 ppm in feed)
Ames test S. typhimurium 767 µl/plate Negative Herbold, 1985b
TA98, TA100
TA1535, TA1537
Propoxur urine extract (rat, 8000 in feed)
Ames test S. typhimurium evaluated range (2) Herbold, 1985a
TA98, TA100 14.5-29 µl/plate
TA1535, TA1537
* Test was carried out both with and without metabolic activation.
(1) Suppression of semi-conservative DNA synthesis was seen.
(2) Test outline and result inadequately reported.
Baygon spray (containing 2.0% propoxur and 0.5% dichlorvos) was
sprayed on the upper arm of 4 humans (3 males, 1 female) 6 times (1
second per spraying) at intervals of 10 minutes. The amount of
propoxur applied was 41 mg per person. Dermal absorption was
assessed via measurement of cholinesterase activity in plasma and
erythrocytes and of blood propoxur concentrations up to 6 hours
after treatment began. Urine samples, collected for 24 or 48 hours,
were monitored for the concentration of propoxur metabolite
isopropoxyphenol. No skin penetration of propoxur were found. The
identical procedure was used in a study in which 500 mg Baygon Dust
(1% w/w propoxur) was applied for 2 hours under an occlusive pad to
the abraded skin of the upper arm of 4 male subjects (skin sites
were abraded through application of an adhesive plaster and removal
of it, 30 minutes later). There were no signs indicating skin
penetration by propoxur (Eben & Kimmerle, 1974b).
Cholinesterase inhibition
Four human subjects (3 male and 1 female) were exposed by
inhalation to an aerosol containing 3 mg/m3 propoxur (purity 100%)
for 4 hours. The concentration of propoxur in the blood and
cholinesterase activity in plasma were determined up to 120 minutes
after treatment. Urine was monitored for the propoxur metabolite 2-
isopropoxyphenol up to 72 hours after exposure. In blood propoxur
was not found and cholinesterase activity was not depressed. 2-
Isopropoxyphenol was present in urine; the observed concentration
had decreased to trace level after 24 hours (and the compound was
absent thereafter), indicating excretion within this interval
(Kimmerle & Eben 1978b).
COMMENTS
After oral administration to rats the compound is rapidly
excreted, almost exclusively via the urine; only small quantities
are found in the feces. In urine the compound is excreted unchanged
or as one of a large number of metabolites which are present as free
compounds or as glucuronide or sulfate conjugates. The
biotransformation pathways in all species studied comprise
depropoxylation, hydrolysis of the ester bond and N-demethylation.
Ring hydroxylation also occurs: in rodents at ring positions 3, 4
and 5, and in primates at the 4- and 5- positions only. The
biotransformation pathway in humans is the same as in the Rhesus
monkey.
Propoxur induces drug metabolizing enzymes in the liver of
rats. This effect was greater with Altromin diet than with a semi-
synthetic diet.
The compound showed high acute oral toxicity in the species
examined.
Short-term administration of propoxur to rats (gavage, 5 days)
and dogs (dietary, 52 weeks) revealed cholinergic signs, growth
retardation and inhibition of cholinesterase activity in blood and
brain as the main toxicological effects. In the dog an increase of
microsomal enzyme activity was also observed. The NOAEL in the dog
study was 200 ppm (equivalent to 300 mg/kg bw/day).
In a long-term feeding study in mice there was significant
inhibition of growth at the 6000 ppm level. The NOAEL was 2000 ppm
(equivalent to 300 mg/kg bw/day).
In long-term feeding studies in Wistar rats, growth
retardation, ChE inhibition and urinary bladder alterations were the
main effects observed. Hyperplastic and neoplastic changes, which
were dependent on the diet, were found in the bladder of rats. The
hyperplasia could be reduced by the administration of ammonium
chloride in the predisposing diet, presumably via its effect on
urinary pH. These changes were not seen in mice, hamsters, dogs or
Rhesus monkeys.
The NOAEL in rats for the formation of hyperplasia of the
urinary bladder epithelium was 200 ppm (equal to 10 mg/kg bw/day).
This is also the NOAEL for AChE inhibition.
Increased post-implantation losses were observed in a
teratogenicity study in rabbits at 30 mg/kg bw/day. The NOAEL was
10 mg/kg bw/day. No embryotoxicity was observed at the highest dose
tested in rats (27 mg/kg bw/day). No teratogenic effects were
observed in either species.
After reviewing all available in vitro and in vivo short-
term tests, the Meeting concluded that there was no evidence of
genotoxicity.
Based on a re-evaluation of the human data available from the
1973 JMPR, a single oral dose of 0.2 mg/kg bw could be considered as
a NOAEL for humans since the depression of erythrocyte
cholinesterase did not exceed 20% and the recovery was very rapid.
These data have been reviewed by a WHO Expert Committee (WHO, 1973).
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse 2000 ppm in the diet, equivalent to 300 mg/kg bw/day
(ChE activity not measured)
Rat: 200 ppm in the diet, equal to 10 mg/kg bw/day
Dog: 200 ppm in the diet, equivalent to 5 mg/kg bw/day
Human 0.1 mg/kg bw
Estimate of acceptable daily intake for man
0-0.02 mg /kg bw
Studies which will provide information valuable in the continued
evaluation of the compound
Further observations in humans.
REFERENCES
Abd-Elraof, T.K., Dauterman, W.C. & Mailman, R.B. (1981) In vivo
metabolism and excretion of propoxur and malathion in the rat:
Effect of lead treatment. Toxicol. Appl. Pharmacol., 59: 324-330.
Ahdaya, S.M., Monroe, R.J. & Guthrie, F.E. (1981) Absorption and
distribution of intubated insecticides in fasted mice. Pesticide
Biochem. and Physiol. 16: 38-46.
Becker, H., Mladenovic, P. & Terrier, Ch. ( 1989a) Embryotoxicity
study (including teratogenicity) with BOQ 5812315 (c.n. propoxur) in
the rat. Unpublished Report No. R 4686 (project 207270) dated March
1, 1989 from RCC-Research & Consulting Company AG, Itingen,
Switzerland. Submitted to WHO by Bayer AG, Leverkusen, Federal
Republic of Germany.
Becker, H., Mladenovic, P. & Terrier, Ch. (1989b) Embryotoxicity
study (including teratogenicity) with BOQ 5812315 (c.n. propoxur) in
the rabbit. Unpublished Report No. R 4684 (Project 207292) dated
March 1, 1989 from RCC-Research & Consulting Company AG, Itingen,
Switzerland. Submitted to WHO by Bayer AG, Leverkusen, Federal
Republic of Germany.
Bell, R.L. & Gronberg, R.R. (1975) The metabolic fate of BAYGON in
the lactating dairy cow. Unpublished Report No. 44771 dated July 18,
1975 from from Chemagro Agricultural Division - Mobay Chemical
Corporation. Submitted to WHO by Bayer A.G., Leverkusen, Federal
Republic of Germany.
Blevins, R.D., Lijnski, W. & Regan, J.D. (1977a) Nitrosated
methylcarbamate insecticides: effect on the DNA of human cells.
Mut. Res. 44: 1-7.
Blevins, R.D., Lee, M. & Regan, J.D. (1977b) Mutagenicity screening
of five methyl carbamate insecticides and their nitroso derivatives
using mutants of Salmonella typhimurium LT2, Mutat. Res. 56: 1-
6.
Bomhard, E. & Löser, E. (1981) Bö 58 12 315 Chronic toxicological
study on mice (Feeding study over two years). Unpublished Report No.
9954 dated May 12, 1981 from Bayer A.G. Institute of Toxicology,
Wuppertal-Elberfeld. Submitted to WHO by Bayer A.G., Leverkusen,
Federal Republic of Germany.
Costa, L.G., Hand, H., Schwab, Bw & Murphy, S.D. (1981) Tolerance to
the carbamate insecticide propoxur. Toxicology, 21: 267-278.
De Lorenzo, F., Staiano, N., Silengo, L. & Cortese, R. (1978)
Mutagenicity of diallate, sulfallate, triallate and relationship
between structure and mutagenic effects of carbamates used widely in
agriculture. Cancer Res., 38: 13-15.
Eben, A., Karl, W. & Machemer, L. (1984) Studies on the
biotransformation of propoxur in the rat. Unpublished Report No.
12866 (KWN 15) dated August 17, 1984 from Bayer AG Institut für
Toxikologie, Wuppertal-Elberfeld. Submitted to WHO by Bayer AG,
Leverkusen, Federal Republic of Germany.
Eben, A., Karl, W. & Machemer, L. (1985a) Studies on
biotransformation of propoxur in humans. Unpublished Report No.
13947 (KWN 26) dated October 24, 1985 from Bayer AG Institut für
Toxikologie, Wuppertal-Elberfeld. Submitted to WHO by Bayer AG,
Leverkusen, Federal Republic of Germany.
Eben, A., Karl, W. & Machemer, L. (1985b) Supplementary studies on
biotransformation of propoxur in the rat. Unpublished Report no.
14148 dated December 13, 1985 from Bayer AG Institut für
Toxikologie, Wuppertal-Elberfeld. Submitted to WHO by Bayer AG.
Leverkusen, Federal Republic of Germany.
Eben, A., Karl, W. Machemer, L. (1986a) The biotransformation of
propoxur in golden hamsters. Unpublished Report No. 14690 (KWN 37)
dated June 9, 1986 from Bayer AG Institute of Toxicology, Wuppertal-
Elberfeld. Submitted to WHO by Bayer AG, Leverkusen, Federal
Republic of Germany.
Eben, A., Karl. W. & Machemer, L. (1986b) Propoxur [The active
ingredient of RBaygon] Biotransformation study on monkeys.
Unpublished Report No. 15056 (KWN 40) dated September 11, 1986 from
Bayer AG Fachbereich Toxikologie, Wuppertal-Elberfeld. Submitted to
WHO by Bayer AG, Leverkusen, Federal Republic of Germany.
Eben, A., Karl, W. & Machemer, L. (1987) Investigation on the
biotransformation of propoxur in mice. Unpublished Report No. 15697
(KWN 46) dated April 3, 1987 from Bayer AG Fachbereich Toxikologie
Landwirtschaft ZF-Zentrale Analytik Structure Research, Wuppertal.
Submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Eben, A. & Kimmerle, G. (1974b) 1% Baygon Dust. Resorption study on
skin of test volunteers. Unpublished Report No. 4461 dated February
5, 1974 from Bayer AG-Institut für Toxikologie, Wuppertal-Elberfeld.
Submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany. Test volunteers. Unpublished Report No. 4462 dated February
8, 1974 from Bayer AG Institut für Toxikologie, Wuppertal-Elberfeld.
Submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Eigenberg, D.A. (1988) Dermal absorption of propoxur technical in
rat using 14C-propoxur. Unpublished report No. 1097 (study number
88-721-AT) dated December 28, 1988 from Mobay Corporation, Health,
Environment and Safety, Corporate Toxicology Department, Stilwell,
Kansas, USA. Submitted to WHO by Bayer AG, Leverkusen, Federal
Republic of Germany.
Feldmann, R.J. & Maibach, H.I. (1974) Percutaneous penetration of
some pesticides and herbicides in man. Toxicol. Appl. Pharmacol.
28: 126-132.
Flucke, W. (1980) Boe 5812315 (propoxur). Acute toxicity studies.
Unpublished Report No. 9295 dated July 8, 1980 from Bayer AG
Institut für Toxikologie, Wuppertal-Elberfeld. Submitted to WHO by
Bayer AG, Leverkusen, Federal Republic of Germany.
Flucke, W. (1984) FCR (cyfluthrin) BOQ (propoxur) Study for
combination toxicity. Unpublished Report No. 12544 dated March 14,
1984 from Bayer AG Institute of Toxikology, Wuppertal-Elberfeld.
Submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Glaister, J.R. (1984) BOQ 5812315: 2 year carcinogenicity/chronic
toxicity study in the rat. Histopathology Report. Hazleton
Laboratories Harrogate, June 1984. Data submitted to WHO by Bayer AG
Leverkusen, Federal Republic of Germany.
Hahnemann, S. & Rühl-Fehlert, C. (1988a) BOQ 5812315 (common name:
propoxur). Chronic feeding test on Syrian gold hamsters (species
sensitivity). Unpublished Report No. 16801 dated June 15, 1988 from
Bayer AG Toxicology Division, Wuppertal (study no. T 0018434).
Submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Hahnemann, S. & Rühl-Fehlert, C. (1988b) BOQ 5812315 (common name:
propoxur). Chronic feeding test on Sprague-Dawley rats (strain
sensitivity). Unpublished report from Bayer AG Toxicology Division,
Wuppertal (study no. T 1018435). Submitted to WHO by Bayer AG,
Leverkusen, Federal Republic of Germany.
Hahnemann, S. & Rühl-Fehlert, C. (1988c) BOQ 5812315 (common name:
propoxur). Chronic feeding test on NMRI mice (species sensitivity).
Unpublished report no. 16803 dated June 15, 1988 from Bayer AG
Toxicology Division, Wuppertal (study no. T 9018433). Submitted to
WHO by Bayer AG, Leverkusen, Federal Republic of Germany.
Hahnemann, S. & Rühl-Fehlert, C. (1988d) BOQ 5812315 (common name:
propoxur). Sub-chronic feeding test on female Wistar rats (effect of
feed quality). Unpublished Report No. 16897 dated July 13, 1988 from
Bayer AG Toxicology Division, Wuppertal (study no. T 5019041).
Submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Hahnemann, S. & Rühl-Fehlert, C. (1988e) BOQ 5812315 (common name:
propoxur). Chronic feeding test on female Wistar rats with added 1%
L-(+)ascorbic acid. Unpublished Report No. 16979 dated August 2,
1988 from Bayer AG Toxicology Division, Wuppertal (study no. T
8018432). Submitted to WHO by Bayer AG, Leverkusen, Federal Republic
of Germany.
Hahnemann, S. & Rühl-Fehlert, C. (1988f) BOQ 5812315 (common name:
propoxur). Chronic feeding test on female Wistar rats over 2 years
(dose-effect-time relationship). Unpublished Report No. 16980 dated
August 2, 1988 from Bayer AG Toxicology Division, Wuppertal (study
no. T 6018430). Submitted to WHO by Bayer AG, Leverkusen, Federal
Republic of Germany.
Hahnemann, S. & Rühl-Fehlert, C. (1988g) BOQ 5812315 (common name:
propoxur). Chronic feeding study on female rats (Effect of feed and
drinking water type). Unpublished Report No. 17146 dated September
13, 1988 from Bayer AG Fachbereich Toxikologie, Wuppertal. Submitted
to WHO by Bayer AG, Leverkusen, Federal Republic of Germany.
Haley, T.J., Farmer, J.H., Dooley, K.L., Harmon, J.R. & Peoples, A.
(1974) Determination of the LD01 and extrapolation of the LD001 for
five methylcarbamate pesticides. J. Européen de Toxicologie, 7(3):
152-158.
Heimann, K.G. (1982a) Propoxur (the active ingredient of RBaygon
and UndenR). Study of sensitization effect on guinea pigs.
Unpublished Report No. 11218 dated October 15, 1982 from Bayer AG
Institut für Toxikologie, Wuppertal-Elberfeld. Submitted to WHO by
Bayer AG, Leverkusen, Federal Republic of Germany.
Heimann, K.G. (1982b) Carbamate UN, technical. Study for acute
toxicity on rat. Unpublished Report No. 11329 dated December 15,
1982 from Bayer AG Institute of Toxicology, Wuppertal-Elberfeld.
Submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Heimann, K.G. (1982c). Carbamate UN, Technical product: acute study
of the effect on the activity of the cholinesterases in blood
plasma, erythrocytes and brains of rats compared with carbamate UN
recrystallised product. Unpublished Report No. 11330 dated December
15, 1982 from Bayer AG Institute of Toxicology, Wuppertal-Elberfeld.
Submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Heimann, K.G. (1983) Carbamate UN, technical (c.n. propoxur, Unden
active ingredient; Baygon active ingredient) Subacute study on rats
compared with carbamate UN, recrystallised. Unpublished Report No.
11621 dated March 8, 1983 from Bayer AG Institute of Toxicology
Wuppertal-Elberfeld. Submitted to WHO by Bayer AG, Leverkusen,
Federal Republic of Germany.
Herbold, B. (1980a) BOE 5812315, dominant lethal study on male mouse
to test for mutagenic effects. Unpublished Report No. 8808 dated
January 7, 1980 from Bayer AG, Institute of Toxicology, Wuppertal-
Elberfeld. Submitted to WHO by Bayer AG, Leverkusen, Federal
Republic of Germany.
Herbold, B. (1980b) BOE 5812315, microncucleus test on mouse to
evaluate BOE 5812315 for mutagenic potential. Unpublished Report No.
9274 dated June 27, 1980 from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld. Submitted to WHO by Bayer AG, Leverkusen,
Federal Republic of Germany.
Herbold, B. (1982) Carbamate UN technical, Salmonella/microsome
test to evaluate for point mutation. Unpublished report no. 11301
dated December 6, 1982 from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld. Submitted to WHO by Bayer AG, Leverkusen,
Federal Republic of Germany.
Herbold, B. (1983a) Carbamate UN technical - Pol A1- test on E.
coli for potential DNA damage. Unpublished Report No. 11403 dated
January 6, 1983 from Bayer AG, Institute of Toxicology, Wuppertal-
Elberfeld. Submitted to WHO by Bayer AG, Leverkusen, Federal
Republic of Germany.
Herbold, B. (1983b) Isopropoxyphenol, Salmonella/microsome test to
evaluate for point mutation. Unpublished Report No. 12321 dated
December 20, 1983 from Bayer AG, Institute of Toxicology, Wuppertal-
Elberfeld. Submitted to WHO by Bayer AG, Leverkusen, Federal
Republic of Germany.
Herbold, B. (1983c) Brenzcatechin, Salmonella/microsome test to
evaluate for potential point mutation. Unpublished Report No. 12322
dated December 20, 1983 from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld. Data submitted to WHO by Bayer AG, Leverkusen,
Federal Republic of Germany.
Herbold, B. (1984a) THS 1240, Salmonella/microsome test to
evaluate for potential point mutation. Unpublished Report No. 12483
dated February 24, 1984 from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld. Submitted to WHO by Bayer AG, Leverkusen,
Federal Republic of Germany.
Herbold, B. (1984b) Brenzcatechin, POL-test of E. coli to evaluate
for potential DNA damage. Unpublished Report No. 12497 dated
February 29, 1984 from Bayer AG, Wuppertal-Elberfeld. Submitted to
WHO by Bayer AG, Leverkusen, Federal Republic of Germany.
Herbold, B. (1984c) THS 2490, Salmonella/microsome test to
evaluate for point mutation. Unpublished Report No. 12529 dated
March 6, 1984 from Bayer AG, Institute of Toxicology, Wuppertal-
Elberfeld. Submitted to WHO by Bayer AG, Leverkusen, Federal
Republic of Germany.
Herbold, B. (1984d) THS 1241b, Salmonella/microsome test to
evaluate for potential point mutation. Unpublished Report No. 12795
dated July 9, 1984 from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld. Submitted to WHO by Bayer AG, Leverkusen,
Federal Republic of Germany.
Herbold, B. (1984e). Isopropoxyphenol, test on S.cerevisiae D7 for
the induction of mitotic recombination. Unpublished Report No. 12876
dated August 20, 1984 from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld. Submitted to WHO by Bayer AG, Leverkusen,
Federal Republic of Germany.
Herbold, B. (1984f) THS 2647, Salmonella/microsome test to
evaluate for potential point mutation. Unpublished Report No. 12996
dated October 24, 1984 from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld. Submitted to WHO by Bayer AG, Leverkusen,
Federal Republic of Germany.
Herbold, B. (1985a) Propoxur urine extract compared with control
urine extract, Salmonella microsome test to evaluate for potential
point mutation. Unpublished Report No. 13350 dated March 14, 1985
from Bayer AG, Institute of Toxicology, Wuppertal-Elberfeld.
Submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Herbold, B. (1985b) Propoxur urine, Salmonella/microsome test to
evaluate for potential point mutation. Unpublished Report No. 13395
dated March 27, 1985 from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld. Submitted to WHO by Bayer AG, Leverkusen,
Federal Republic of Germany.
Herbold, B. (1985c) Propoxur (BOQ 5812315), sister chromatid
exchange in the bone marrow of the Chinese Hamster in vivo to
evaluate for harmful effect on DNA. Unpublished Report No. 13501
dated May 22, 1985 from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld. Submitted to WHO by Bayer AG, Leverkusen,
Federal Republic of Germany.
Herbold, B. (1985d) BOQ 5812315, sister chromatid exchange in human
lymphocyte cultures in vitro to test for DNA-modifying effects.
Unpublished Report No. 13871 dated October 9, 1985 from Bayer AG,
Institute of Toxicology, Wuppertal-Elberfeld. Submitted to WHO by
Bayer AG, Leverkusen, Federal Republic of Germany.
Herbold, B. (1985e) BOQ 5812315, test on S.cerevisiae D7 to
evaluate for point mutagenic effect. Unpublished Report No. 13966
dated October 30, 1985 from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld. Submitted to WHO by Bayer AG, Leverkusen,
Federal Republic of Germany.
Herbold, B. (1986) BOQ 5812315 (c.n. propoxur), cytogenetic study of
chromosome damage using spermatogonia of Chinese Hamsters in vivo.
Unpublished Report No. 14984 dated August 20, 1986 from Bayer AG,
Institute of Toxicology, Wuppertal-Elberfeld. Submitted to WHO by
Bayer AG, Leverkusen, Federal Republic of Germany.
Herbold, B. (1988) BOQ 5812315 (c.n. propoxur), cytogenetic study on
bone marrow of Chinese Hamster in vivo to detect chromosomal
damage. Unpublished Report No. 17111 dated September 6, 1988 from
Bayer AG Institute of Toxicology, Wuppertal-Elberfeld. Submitted to
WHO by Bayer AG, Leverkusen, Federal Republic of Germany.
Hoffmann, K. & Gröning, P. (1984) BOQ 5812315 (BOE 5812315, c.n.
propoxur) Chronic toxicity to dogs on oral administration (12-months
feeding study). Unpublished Report No. 12605 dated April 11, 1984
from Bayer AG Institute of Toxicology, Wuppertal-Elberfeld.
Submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Hoffmann, K. & Ruehl, Chr. (1985) Propoxur (BOQ 5812315). Subchronic
study of toxicity to Rhesus monkeys after oral administration by
stomach tube for 13 weeks to check for possible findings in the
urinary bladder. Unpublished Report No. 13779 dated August 27, 1985
from Bayer AG Institute of Toxicology, Wuppertal-Elberfeld.
Submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Inukai, H. & Iyatomi, A. (1978) Propoxur. Mutagenicity test on
bacterial systems. Unpublished Report No. 103 dated February 24,
1978 from Nitokuno Agricultural Chemicals Institute, Toyoda, Japan.
Submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Jaszcuk, E., Syrowatka, T. & Cybulski, J. (1979) Mutagenic activity
of propoxur, carbaryl and their nitroso derivatives: induction of
reversion in Salmonella typhimurium. Roczniki Panstwowzgo Zakladu
Higieny Warszawa, 30(1): 81-88. (English summary only).
Jurek, A. (1978) Chronische Langzeittoxizität des
Propoxurkarbaminats, Roczniki Panstwowzgo Zakladu Higieny Warszawa,
29(3): 327-338.
Karl, W. (1986) Biotransformation of propoxur: quantitative
determination of the metabolic pattern in rats given a single dose
of 14C-propoxur after a subchronic prefeeding period in two diet
groups and three dose groups. Unpublished Report No. KWN 42 dated
October 15, 1986 from Bayer AG Institute of Toxicology, Wuppertal-
Elberfeld. Submitted to WHO by Bayer Leverkusen, Federal Republic of
Germany.
Karl, W. & Schneider, J. (1987) Isolation and spectroscopic
structure elucidation of the renal metabolite conjugates of
propoxur. Unpublished Report No. KWN 44 dated August 26, 1987 from
Bayer AG Institute of Toxicology, Wuppertal-Elberfeld. Submitted to
WHO by Bayer AG, Federal Republic of Germany.
Kimmerle, G. & Eben, A. (1978a) Propoxur - Acute inhalation study on
rats with determination of acetylcholinesterase activity in blood
and elimination of 2-isopropoxyphenol in urine. Unpublished Report
No. 7555 dated May 1978 from Bayer AG Institute für Toxikologie,
Wuppertal-Elberfeld. Submitted to WHO by Bayer AG Leverkusen,
Federal Republic of Germany.
Kimmerle, G. & Eben, A. (1978b) Propoxur - Single exposure of
persons to propoxur with determination of acetylcholinesterase
activity in plasma and erythrocytes, propoxur concentration in blood
and elimination of 2-isopropoxyphenol in urine. Unpublished Report
No. 7554 dated May, 1978 from Bayer AG, Institut für Toxikologie,
Wuppertal-Elberfeld. Submitted to WHO by Bayer AG Leverkusen,
Federal Republic of Germany.
Kimmerle, G. & Iyatomi, A. (1976) Toxicity of propoxur to rats by
subacute inhalation. Jap. J. Ind. Health, 18: 375-382.
Klein, W. (1984) Effect of an active ingredient and three
metabolites on the DNA metabolism. Unpublished Report R3346 dated
March 1984 from Osterreichisches Forschungszentrum Seiberdorf
Biochemistry Department/Toxicology Department. (Research commisioned
by Bayer AG). Submitted to WHO by Bayer AG Leverkusen, Federal
Republic of Germany.
Krechniak, J. & Foss, W. (1982) Cholinesterase activity in rats
treated with propoxur. Bull. Environm. Contam. Toxicol., 29: 599-
604.
Krechniak, J. & Foss, W. (1983a) Behaviour of propoxur after
repeated administration. Bromat. Chem. Toksykol., 16 (3-4): 205-
208. (English translation).
Krechniak, J. & Foss, W. (1983b) Distribution and excretion of
isopropoxyphenol in rats. Bromat. Chem. Toksykol., 6 (3-4): 209-
211 (English translation).
Lehn, H. (1988) BOQ 5812315 (c.n. propoxur), mutagenicity study for
the detection of induced forward mutations in the CHO-HGPRT assay
in vitro. Unpublished Report No. 17090 dated August 31, 1988 from
Bayer AG, Fachbereich Toxikologie, Wuppertal. Data submitted to WHO
by Bayer AG, Leverkusen, Federal Republic of Germany.
Luckhaus, G. (1984) Propoxur, two-year oral toxicity study in rats,
addendum to HRC Report No. 2809/69/235, histological follow-up
investigation of the urinary bladder. Unpublished Report No. 13012
dated November 2, 1984 from Bayer AG, Institute of Toxicology,
Wuppertal. Data submitted to WHO by Bayer AG, Leverkusen, Federal
Republic of Germany.
Machemer, L. & Schmidt, U. (1988) Propoxur. Status of the studies
and assessment regarding oncogenic potential to the urinary bladder.
Unpublished report dated September 30, 1988 from Bayer AG
Fachbereich Toxikologie, Institut für Toxikologie Landwirtschaft,
Wuppertal-Elberfeld. Data submitted to WHO by Bayer AG, Leverkusen,
Federal Republic of Germany.
Mihail, F. (1982) BOQ 5812315. Test for induction of the microsomal
liver enzymes. Unpublished Report No. 10976 dated June 29, 1982 from
Bayer AG, Institute of Toxicology, Wuppertal-Elberfeld. Data
submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Ohta, T. & Moriya, M. (1983) Propoxur, microbial mutagenicity study.
Unpublished Report from Institute of Environmental Toxicology
(Japan) dated February 28, 1983. Data submitted to WHO by Bayer AG,
Leverkusen, Federal Republic of Germany.
Pauluhn, J. (1988) BOQ 5812315 (common name: propoxur) study of the
inhalation toxicity in accordance with OECD Guideline No. 403.
Unpublished Report No. 16966 dated July, 1988 from Bayer AG
Fachbereich Toxikolgie, Wuppertal. Submitted to WHO by Bayer AG,
Leverkusen, Federal Republic of Germany.
Pauluhn, J. & Rühl, C. (1985) BOQ 5812315, study for subacute
inhalation toxicity to the rat. Unpublished Report No. 13297 dated
February, 20, 1985 from Bayer AG, Institute of Toxicology Wuppertal-
Elberfeld. Data submitted to WHO by Bayer AG, Leverkusen, Federal
Republic of Germany.
Putman, D.L. & Morris, M.J. (1988) Chromosome aberrations in Chinese
Hamster Ovary (CHO) cells, Baygon technical. Unpublished Report No.
1094 dated December 22, 1988 from Microbiological Associates Inc.
Maryland, USA. Submitted to WHO by Bayer AG, Leverkusen, Federal
Republic of Germany.
Reid Patterson, D. (1980) Histopathology report on Boe 5812315 mouse
study. Unpublished Report No. 2317-262/13 dated May, 1980 from
Hazleton Laboratories Europe, Harrogate, England to Bayer AG
Institut für Toxikologie, Wuppertal. Submitted to WHO by Bayer AG,
Leverkusen, Federal Republic of Germany.
Schlüter, G. (1981) BOQ 5812315. Evaluation for embryotoxic and
teratogenic effects after oral administration to the rabbit.
Unpublished Report No. 10183 dated September 9, 1981 from Bayer AG,
Institute of Toxikologie, Wuppertal-Elberfeld. Data submitted to WHO
by Bayer AG, Leverkusen, Federal Republic of Germany.
Schmidt, U. (1987) Investigations of interspecies differences in
primary metabolism with liver-cell fractions from rat, mouse,
hamster, monkey and man. Unpublished Report No. 16237 dated November
19, 1987 from Bayer AG, Fachbereich Toxikologie, Wuppertal.
Submitted to WHO by etc.
Seiler, J.P. (1977) Nitrosation in vitro and in vivo by sodium
nitrite, and mutagenicity of nitrogenous pesticides. Mutat. Res.
48: 225-236.
Shirasu, Y., Moriya, M., Kato, K., Furuhashi, A. & Kada, T. (1976)
Mutagenicity screening of pesticides in the microbial system.
Mutat. Res. 40: 19-30.
Shirasu, Y., Moriya, M. & Sugiyama, F. (1979) Propoxur, mutagenicity
test on bacterial systems. Unpublished Report from Institute of
Environmental Toxikologie (Japan) dated August 28, 1979. Data
submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Siebert, D. & Eisenbrand, G. (1974) Induction of mitotic gene
conversion in Saccharomyces cerevisiae. Mutat. Res. 22: 121-126.
Siebert, D. & Lemperle, E. (1974) Genetic effects of herbicides:
induction of mitotic gene conversion in Saccharomyces cerevisiae.
Mutat. Res. 22: 111-120.
Suberg, H. & Löser, E. (1984) BOQ 5812315, chronic toxicological
study with rats (feeding study over 106 weeks). Unpublished Report
No. 12870 dated August 20, 1984 from Bayer AG, Institute of
Toxicology, Wuppertal. Data submitted to WHO by Bayer AG,
Leverkusen, Federal Republic of Germany.
Thyssen, J. (1977) Study for combination toxicity of azinphos-methyl
and propoxur. Unpublished Report No. 7174 dated December 14, 1977
from Bayer AG, Institute of Toxicology, Wuppertal-Elberfeld. Data
submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Thyssen, J. (1978) Propoxur-Untersuchungen an der Haut und am Auge
von Kaninchen. Unpublished report dated September 19, 1978 from
Bayer AG, Institut für Toxikologie, Wuppertal-Elberfeld. Submitted
to WHO by Bayer AG, Leverkusen, Federal Republic of Germany.
Tyrkiel, E. (1977) Mutagenne addzialywannie, o-izopropoksyfenoyl-N-
methylo-karaminianu (propoksura) na komorki pleiowe myszy. Rocz.
Panstw. Zakl. Hig. 28 (6): 601-613.
Weber, H. (1986) Comparison of the absorption of a tracer dose of
(Phenyl-U-14C) propoxur from a basic casein diet and a standard
Altromin 1324 diet by nonradioactively pretreated Wistar rats.
Unpublished Report No. 2504 dated February 4, 1986 from Bayer AG,
Institute of Metabolism Research , Wuppertal. Data submitted to WHO
by Bayer AG, Leverkusen, Federal Republic of Germany.
Weber, H. (1988) [Phenyl-UL-14C] Propoxur: whole-study
autoradiographic distribution of the radioactivity in the rat.
Unpublished Report No.2988 dated April 21, 1988 from Bayer AG,
Institute for Metabolism Research, Leverkusen. Data submitted to WHO
by Bayer AG, Leverkusen, Federal Republic of Germany.
WHO/FAO (1974) 1973 Evaluations of some pesticide residues in food,
WHO Pesticides Residues Series, No. 3. World Health Organization,
Geneva.
Yamane, S. (1986a) Primary skin irritation study of propoxur in
rabbits. Unpublished Report No. D-0714 (DT-9) dated June 9, 1986
from Hita Research Laboratories, Japan. Data submitted to WHO by
Bayer AG, Leverkusen, Federal Republic of Germany.
Yamane, S. (1986b) Primary eye irritation study of propoxur in
rabbits. Unpublished Report No. D-0736 (DT-10) dated September 12,
1986 from Hita Research Laboratories, Japan. Data submitted to WHO
by Bayer AG, Leverkusen, Federal Republic of Germany.
 90% of the administered 14C was recovered in urine in all groups.
The urinary conjugated metabolite pattern was comparable for the
different dose levels and the different diets. At higher (5000 ppm)
dose levels, there was a trend for a higher percentage of M5 and M6
and a lower for M2 and M7. The 14C distribution over the
conjugated metabolites is summarized in Table 1.
TABLE 1. DISTRIBUTION OF 14C OVER URINARY METABOLITES IN RATS
METABOLITE % OF 14C PRESENT IN
URINE (RANGE FOR ALL
DOSE LEVELS AND DIETS)
M1 7.0-15.5
M2 17.2-25.2
M3 22.2-30.2
M5 0.6- 2.5
M6 4.5- 7.9
MS3 0.3- 1.2
M7 14.0-16.3
M7A 5.9- 8.3
M8 1.7- 4.1
(Karl, 1986; Karl & Schneider, 1987)
Metabolism of propoxur in the liver was studied further in in
vivo studies, using post-mitochondrial fractions of livers of
rats, mice, hamsters and monkeys. A similar study was done in human
liver preparations. The results show M5 to be the major metabolite
in rat liver, M3 and M6 being formed to a lesser degree. The
percentage of M5 in relation to overall metabolism decreases from
mouse to hamster and monkeys. In the liver preparation from Rhesus
monkeys, M3 and M6 were formed more than in M5. In additional tests
it was observed that in liver cell fractions of rats and mice
further metabolization of M5 does not take place in hamsters it
occurs only to a limited degree. In contrast, the liver cell
fractions of Rhesus monkeys and humans are able to further
metabolize M5 to 4 other metabolites (not identified) (Schmidt,
1987).
Effect on cholinesterase activity
Male and female Wistar rats were given a single oral dose of 0,
1, 5 or 25 mg/kg bw via stomach tube. Cholinesterase activity was
measured in plasma, erythrocytes and brain at intervals varying from
0.5 hours to 14 days after dose application. At the highest dose
level, cholinesterase activity in plasma and erythrocytes was
depressed after 30 and 60 minutes; after 3 hours the depressions
were no longer apparent. Brain cholinesterase activity was depressed
after 3-5 hours in the same group; 3 days after dosing this was no
longer apparent. At 1 and 5 mg/kg bw no effect was seen (Heimann,
1982c).
In male rats given a single oral dose of 2.1, 7.0, 20.9, 50 or
70 mg/kg bw propoxur, cholinesterase activity in whole blood was
decreased at all dose levels (at 2.1 mg/kg bw the decrease was
slight) after 10 minutes; at 24 hours after dose application the
depressions were no longer present. The same investigators tested
for cholinesterase depression in whole blood and brain in male rats
dosed orally with 30 mg/kg bw/day for 14 days followed by 50 mg/kg
bw via the same route for 28 days. A decreased activity in both
blood and brain was noted during the first 14 days; the depression
gradually disappeared during the following 28 days of treatment.
This indicates adaptation (Krechniak & Foss, 1982).
In an inhalation study, male and female rats were exposed to
0.4, 1.2, 9.0, 30.0, 78.0 or 172.0 mg/m3 propoxur for 6 hours.
Directly after cessation of exposure, plasma and erythrocyte
cholinesterase activities were measured and compared to pre-test
values. At 30, 78 and 172 mg/m3, depressions were observed. In
addition, at 172 mg/m3 cholinergic symptoms were seen at 30 minutes
after commencement of exposure (Kimmerle & Eben, 1978a).
Effect on liver enzyme activity
Mice
Male mice received propoxur (purity 98.8%) in the drinking
water at concentrations increasing with each successive week from 50
to 2000 ppm over a total period of 6 weeks. In animals thus treated
for six weeks, the LD50 for propoxur was significantly higher than
in untreated controls, a finding indicating that tolerance to
propoxur acute toxicity had developed. Hexobarbital sleeping time
was significantly reduced, indicating induction of hepatic
microsomal enzymes. Determinations of carboxy esterase activities
in liver, plasma and brain, however, did not show any significant
increase in the propoxur-treated animals (Costa et al. 1981).
Rats
Propoxur was administered orally for 5 days to male and female
rats at dose levels of 0, 15 or 30 mg/kg bw/day. The rats treated
with propoxur did not exhibit induction of the mixed function
oxidases in comparison to control rats. (A positive control group
was treated with sodium phenobarbitone 50 mg/kg bw/day for 5 days
and induction of mixed function oxidases was observed) (Mihail,
1982).
Concomitant to a short-term study in female rats, liver tissue
enzyme activities were measured after 3, 7, 14 and 28 days of
feeding on a diet containing 0 or 5000 ppm propoxur. From day 3,
cytochrome P-450 dependent mono-oxygenases (i.e. 7-ethoxycoumarin
deethylase, ethoxyresorufin deethylase, aldrin epoxidase) were
induced (increased with a factor of 2-3). The activity of the
microsomal epoxide hydroxylase was increased by the same magnitude.
In contrast, the cytosolic glutathione-S-transferase exhibited only
a slight change compared to the control group. Altromin diet was
used in this study. An identical study in which a casein semi-
synthetic diet was used also showed induction of mono-oxygenase
activity by propoxur. The absolute activity levels reached in the
latter study, however, were about 50% lower compared to the values
observed in the concurrent control and treatment groups of the
Altromin diet study (Machemer & Schmidt, 1988).
Toxicological studies
Acute toxicity
The acute toxicity of propoxur to rats and mice is presented in
Table 2.
The observed intoxication symptoms were indicative of
inhibition of cholinesterase: convulsions, muscular tremors,
muscular spasms, dyspnoea, salivation (Flucke, 1980; Heimann,
1982b).
Short-term toxicity
Oral studies
Rats
The toxicity of propoxur technical (purity 98.6%) and propoxur
recrystallized (purity 99.2%) was compared in a 5 day-study. Groups
of Wistar rats (5/sex/group) received oral doses of 0, 15 or 30
mg/kg bw/day of propoxur of both purities via stomach tube for 5
days. Appearance, behaviour and body weight were recorded daily.
After 5 days, all animals were sacrificed and submitted to gross
pathology. The weights of liver and kidneys were determined.
Clinical chemistry was performed on all animals. N-demethylase, O-
demethylase, cytochrome P-450 and triglycerides were measured in
liver homogenates (cholinesterase activity not determined). The
only adverse effects noted were convulsions and apathy, occurring in
all treatment groups in a dose-related manner. No difference in
toxicity between the two purities were found (Heimann, 1983).
TABLE 2. ACUTE TOXICITY OF PROPOXUR IN ANIMALS
LD50 LC50
SPECIES SEX ROUTE (mg/kg bw) (mg/m3) REFERENCES
Mouse M oral 37 - Haley et al. 1974
F idem 39 - Haley et al. 1974
Rat M* oral 39 - Thyssen et al. 1977
M* oral 45 - Flucke, 1984
M oral 196 - Flucke, 1980
F oral 126 - Flucke, 1980
M* oral 94 - Flucke, 1980
F* oral 68 - Flucke, 1980
M oral 167 - Heimann, 1982b
F oral 96 - Heimann, 1982b
M* oral 69 - Heimann, 1982b
F* oral 47 - Heimann, 1982b
M i.p. 16 - Heimann, 1982b
F i.p. 13 - Heimann, 1982b
M&F dermal >5000 - Flucke, 1980
M&F inhal. - >498 Pauluhn, 1988
* Fasted animals
Dogs
Beagle dogs (6/sex/group) were given diets containing 0, 200 or
600 ppm propoxur (purity 99.4%) for 52 weeks. An additional group
received 1800 ppm from week 1 through week 40, 3600 ppm from week 41
through 44 and 5400 ppm from week 45 through 52 (the increases were
established in order to produce overt toxic signs). Appearance,
behaviour and body weight were recorded. On a number of occasions
throughout the study reflex tests, ophthalmoscopy, hematology,
clinical chemistry, urinalysis and cholinesterase activity (in
plasma and erythrocytes) were determined. At sacrifice, organs were
weighed and complete gross pathology and histopathology were carried
out. In livers N-demethylase and cytochrome P-450 were measured.
Cholinergic symptoms were observed at the highest dose level after
elevation of the dose level to 5400 ppm and 1/6 animals died. In
addition, the following parameters were increased in this group:
thrombocyte, leucocyte and reticulocyte counts, incidence of Heinz
bodies, ALAT and SAP, liver weight and thyroid weight; thymus weight
was decreased (also noted: medium thymus atrophy). At the highest
dose level and at 600 ppm also, growth was retarded and plasma
cholesterol and liver N-demethylase were increased. The NOAEL in
this study is 200 ppm (Hoffmann & Gröning, 1984).
Inhalation studies
Groups of Wistar rats (10/sex/group) were exposed to aerosols
containing propoxur (purity 98.9%) in concentrations of 0, 5.7, 18.7
or 31.7 mg/m3 6 hours per day, 5 days per week over a period of 12
weeks. There was no effect on behaviour, growth, hematology,
clincial chemistry, urinalysis, organ weights or histopathology.
The only effect observed was a depression of cholinesterase activity
in plasma, erythrocytes and brain, occuring at 31.7 mg/m3 only
(Kimmerle & Iyatomi, 1976).
Groups of Wistar rats (5/sex/group) were exposed to aerosols
containing propoxur (purity 99.6%) in concentrations of 0, 15.3,
45.3 or 139.6 mg/m3 during 6 hours per day, 5 days per week over a
period of 4 or 8 weeks. The observations included clinical signs,
body weight, cholinesterase activity in plasma, erythrocytes and
brain at sacrifice after 4 and 8 weeks, urinalysis, gross pathology
(all organs) and histopathology (4 tissues/animal) and organ weights
(4 organs/animal). Cholinergic symptoms were observed at 139.6
mg/m3. Cholinesterase activies in brain were depressed in week 4
at 45.3 and 139.6 mg/m3 and in week 8 at 15.3 mg/m3 also. There
were no signs of specific organ damage or an alteration in the
urinary bladder epithelium (Pauluhn & Rühl, 1985).
Long term carcinogenicity studies
Mice
Groups of 50 male and 50 female CF1/W74 mice were fed diets
containing 0, 700, 2000 or 6000 ppm propoxur (purity 99.6%) for 24
months. Satellite groups of 10 male and 10 female mice were fed at
the same dose levels and were used for interim sacrifice after 6
months. Observations included clinical signs, body weight, food
consumption, hematology and clinical chemistry. The weights of 6
organs/animal were recorded. Gross pathology and limited
histopathogy (about 20 tissues/animal) were carried out. Slight
growth retardation was oberved in the 700, 2000 and 6000 ppm males;
ALAT was increased in 6000 ppm-females after 6 months only. The
relative weights of testes and spleen were increased or decreased,
respectively, at all dose levels to a dose-related degree. The
tumour incidence was not increased (Bomhard & Löser, 1981; Reid
Patterson, 1980).
Rats
In a chronic toxicity study summarized in the WHO/FAO monograph
from 1974, with dietary dose levels of 0, 250, 750, 2000 and 6000
ppm (purity 99.8%),the effects were: growth retardation and reduced
food consumption at 200 and 6000 ppm and increased relative liver
weight at 6000 ppm (NOAEL in this study: 250 ppm) (WHO/FAO, 1974).
The histological sections produced in this study were reexamined.
Histopathological appraisal of urinary bladder sections confirmed
the absence of alterations in this organ at all dose levels
(Luckhaus, 1984).
Groups of 20 male and 20 female Wistar rats were fed diets
containing 0, 50, 200 or 800 ppm propoxur (purity 97.25%) for 18
months. The observations included clinical signs, food intake, body
weight, hematology and clinical chemistry. At termination all
animals were sacrificed. Organ weights were determined and
histopathology was carried out. Growth was slightly decreased in
the 800 ppm females. At the end of the study cholinesterase
activities in whole blood and brain were inhibited at 800 ppm. The
NOAEL in this study is 200 ppm (Jurek, 1978).
Groups of 50 male and 50 female Wistar rats were fed diets
containing 0, 200, 1000 or 5000 ppm propoxur (purity 99.4%) for 2
years. Additional groups of 10 rats/sex/group were treated at the
same dose levels and were used for interim sacrifice after 1 year.
The observations included clinical signs, body weight, gross
pathology and histopathology. Growth was retarded at 1000 and 5000
ppm. At 5000 ppm slight neuromuscular changes (i.e. slightly
increased incidences of peripheral neuropathy and muscular atrophy
of the rear extremities) were noted. Also at 5000 ppm, ASAT was
decreased (males and females) and urea was increased (females only).
At the same dose level the relative weights of a number of organs
(heart, lung, liver, kidney, adrenal) were increased. At interim
autopsy the incidence of hyperplasia of the urinary bladder was
increased at 1000 and 5000 ppm (incidences 0/20, 0/20, 6/20 and
19/20 in the 0, 200, 1000 and 5000 ppm groups, respectively). The
findings in the urinary bladder at terminal sacrifice are presented
in Table 3.
TABLE 3. INCIDENCE OF URINARY BLADDER ALTERATIONS
IN MALE AND FEMALE RATS
OCCURRENCE CONTROL 200 ppm 1000 ppm 5000 ppm
Hyperplasia 1/98 1/96 15/99 92/97
Papilloma 0/98 0/96 1/99 53/97
Carcinoma 0/98 0/96 0/99 13/97
The NOAEL in this study is 200 ppm (determined to be equal to
9.6 mg/kg bw/day) (Suberg & Löser, 1984, Glaister, 1984).
For further determination of the dose-response/exposure-time
relationship with regard to the effect on the urinary bladder,
groups of 70 female Wistar rats were fed diets containing 0, 50,
250, 1000, 3000, 5000 or 8000 ppm propoxur (purity 99.6-99.9%) for
periods up to 104 weeks. After 4, 7, 12, 26, 53 and 78 weeks, 5 or
10 rats/group were sacrificed for interim autopsy. The animals were
observed for clinical signs, food intake, water intake and body
weight. Organ weights were determined. Gross pathology and
histopathology (kidney, urinary bladder, ureter, liver) were carried
out. Growth was retarded at 3000 ppm and higher dose levels. The
relative weights of liver and kidneys were increased at 3000, 5000
and 8000 ppm. Hyperplasia of the bladder epithelium was observed at
1000 ppm (from week 53), at 3000 ppm (from week 12), 5000 ppm (from
week 4) and at 8000 ppm (from week 2). At the latter two dose
levels the effect had developed to severe hyperplasia with recent
vascularization and papillary and nodular hyperplasia after 53
weeks. After 104 weeks, dose-related increases were observed on
hyperplasia, papilloma and carincoma of the urinary bladder in
female rats.
The NOAEL in this study is 250 ppm (Hahnemann & Rühl-Fehlert,
1988f)
In a number of additional studies the effect of propoxur on the
urinary bladder was yet further examined. Issues to be elucidated
were strain and species specificity, influence of the diet used and
effect of vitamin C supplementation.
Strain specificity
Possible strain specificity of the effect of propoxur on the
urinary bladder, observed in Wistar rats, was examined through an
oral study in Sprague Dawley rats. Groups of 50 female Sprague
Dawley rats were fed diets containing 0, 3000 or 8000 ppm propoxur
(purity 99.6%-99.9%) for periods up to 52 weeks. Growth was
retarded and the relative weights of liver, lung and kidneys were
increased at both dose levels. Simple hyperplasia of the urinary
bladder was observed at 3000 and 8000 ppm from week 4 on. At 8000
ppm hyperplasia with neovascularization, papillary hyperplasia and
incipient nodular hyperplasia were found in the urinary bladder from
week 27. Thus, this strain of rats is as sensitive as is the Wistar
rat with regard to the formation of urinary bladder hyperplasia by
propoxur (Hahnemann & Rühl-Fehlert, 1988b).
Species specificity
In the two oral short-term toxicity studies in dogs (summarized
in the relevant paragraphs of the present monograph and the 1974
WHO/FAO monograph respectively) and in the long-term study in mice
(summarized in the paragraph on long-term studies of the present
monograph) no effect on the urinary bladder epithelium was observed.
Further evidence of species specificity was obtained in the
following studies.
Mice
Groups of 50 female NMRI mice were fed diets containing 0, 3000
or 8000 ppm propoxur (purity 99.6%-99.9%) for 53 weeks. Growth was
slightly decreased at 8000 ppm. Increased liver weight and fatty
degeneration occurred at 3000 and 8000 ppm. Relative lung weight
was increased at 8000 ppm only. No adverse effect on urinary
bladder epithelium was noted (Hahnemann & Rühl-Fehlert, 1988c).
Hamsters
Groups of 50 female Syrian golden hamsters were fed diets
containing 0, 3000 or 8000 ppm propoxur (purity 99.6-99.9%) for 53
weeks. At both dose levels the incidence of mortality was slightly
increased, impairment of the general state of the animals (not
specified) was noted and growth was retarded. The relative weights
of kidneys and adrenals were increased at 8000 ppm only. No adverse
effect on urinary bladder epithelium was observed (Hahnemann & Rühl-
Fehlert, 1988a).
Rhesus monkeys
A group of 6 Rhesus monkeys (3 per sex) received oral doses of
40 mg/kg bw/day propoxur (purity 99.6%) via oral intubation for 13
weeks. This daily dose was previously determined to be the maximum
tolerable dose. No control group was used. Cholinergic symptoms
were observed following compound administration. No adverse effect
on the urinary bladder epithelium was noted (Hoffmann & Rühl, 1985).
Effect of vitamin C supplementation
Groups of 50 female Wistar rats were fed diets containing 1%
vitamin C and propoxur (purity 99.6-99.9%) at concentrations of 0,
1000, 3000 or 8000 ppm for a period of 49 weeks. Additional groups
received the same propoxur dose levels in unsupplemented diet.
Growth was retarded in all propoxur-treated groups. The relative
weights of liver (8000 ppm only) and kidneys and lungs (all dose
levels) were increased. The propoxur-induced hyperplastic changes
of the urinary bladder epithelium were observed in all treatment
groups, being present to an equal degree after feeding of the
supplemented and the unsupplemented diets. Thus, vitamin C did not
influence the effect of propoxur on the rat urinary bladder
epithelium (Hahnemann & Rühl-Fehlert, 1988e).
Effect of diet
In the rat studies in which propoxur was found to produce the
urinary bladder alterations, the diet used was Altromin B21 standard
diet. To examine if the diet used was a relevant factor for the
occurrence of the urinary bladder changes, two additional studies
were carried out using a semisynthetic diet (Casein diet no. 1/0).
Groups of 50 female Wistar rats were fed Casein semi-synthetic
diet no. 1/0 containing 0 or 8000 ppm propoxur (purity 99.9%) for
4,8 or 14 weeks. Growth retardation and reduced water intake were
observed in the treated animals. In addition, relative liver and
kidney weights were increased. No urinary bladder changes were
found at histopathology (Hahnemann & Rühl-Fehlert, 1988d).
Groups of 50 female Wistar rats were fed Casein diet no. 1/0
containing 0, 3000 or 8000 ppm propoxur (purity 99.6%) for periods
up to 100 weeks. Growth was retarded at 3000 and 8000 ppm.
Relative weights of lung, kidney and liver were increased at 8000
ppm. Histopathology revealed no treatment-related changes in the
urinary bladders of treated animals (Hahnemann & Rühl-Fehlert,
1988g).
In a study using 14C-propoxur, possible differences in
absorption of propoxur from the Altromin diet and the semisynthetic
case in diet were ruled out. Thus, the absence of urinary bladder
effects when the casein diet is used is not caused by lower
absorption of propoxur from this diet (Weber, 1986). Another
relevant result was observed in a study in rats in which the renal
metabolite pattern was determined after a single oral dose of 14C-
propoxur (label in ring) after preceding administration of 0, 250 or
5000 ppm propoxur either in Altromin standard diet or in the
semisynthetic casein diet for 4 weeks. No differences in the
metabolite pattern were found (Karl, 1986; Karl & Schneider, 1987).
Special studies on combination toxicity
The acute oral application of equitoxic doses of propoxur and
azinphosmethyl to male Wistar rats revealed an additive toxic effect
of the combination of the two pesticides (Thyssen, 1977).
The LD50-value in male Wistar rats for an equitoxic mixture of
propoxur (purity 99.3%) and cyfluthrin (93.7%) proved to be less
than the value expected on the basis of simple addition of effects
(Flucke, 1984).
Special studies on embryotoxicity and teratogenicity
Rats
Groups of 24 pregnant female Wistar rats received 0, 3, 9 or 27
mg/kg bw/day propoxur (purity 99.4%) p.o. by gavage from day 6
through 15 of gestation. Appearance, behaviour, body weight and
food consumption were recorded daily. At day 21 of gestation all
animals were sacrificed and the fetuses were delivered by Cesarean
section. The number of implantations, resorptions (early and late)
and corpora lutea were determined. The fetuses were counted and
weighed; gross pathology and histopathology (skeletal and visceral)
were carried out. At 27 mg/kg bw, 3 animals died before the end of
the test. At 9 and 27 mg/kg bw, symptoms (increased grooming,
chewing motions, teeth grinding) were noted in the hours following
dose application during the entire treatment; at 27 mg/kg bw, in
addition, tremors and ventral recumbency were observed. At the same
dose levels, food consumption and growth of dams were decreased in a
dose related fashion. No other effects were seen. The NOAEL for
maternal toxicity in this study is 3 mg/kg bw/day (Becker et al.
1989a).
Rabbits
Groups of 15 Himalayan rabbits received 0, 1, 3 or 10 mg/kg
bw/day propoxur (purity 99.6%) p.o. from day 6 through 18 of
gestation. There were no indications of maternally toxic,
embryotoxic or teratogenic effects. However, the study was limited
with respect to soft tissues examination (Schlüter, 1981).
Groups of 16 pregnant Chinchilla rabbits received 0, 3, 10 or
30 mg/kg bw/day propoxur (purity 99.4%) p.o. by gavage from day 6
through 18 of gestation. Appearance, behaviour, body weight and
food consumption were recorded daily. At day 28 of gestation all
animals were sacrificed and the fetuses were delivered by Cesarean
section. The number of implantations, resorptions (early and late)
and corpora lutea were determined. The fetuses were counted and
weighed; gross pathology and histopathology (skeletal and visceral)
were carried out. At 30 mg/kg bw restless behaviour and dyspnoea
were observed after dose application on the first 3 treatment days;
in the same group 3 animals died before test end. In addition, body
weight loss (day 6-9 of gestation) occurred in this group. Also, at
30 mg/kg bw post-implantation loss was increased (number of pups per
dam decreased consequently). The NOAEL for maternal toxicity and
embryotoxicity in this study is 10 mg/kg bw/day (Becker et al.
1989b)
Special studies on mutagenicity
A large number of mutagenicity tests has been carried out with
propoxur. The results are summarized in Table 2 ( in vitro assays)
and Table 3 ( in vivo assays). In addition, in vitro tests in
prokaryotes have been performed with a number of metabolites of
propoxur. The results of these studies are summarized in Table 6.
Special studies on skin and eye irritation and sensitization
Undiluted propoxur (purity 99.2%) was tested for irritation to
shaven intact and shaven abraded skin areas of 6 New Zealand
rabbits. Exposure was for 24 or 72 hours. No irritation was observed
(Thyssen, 1978).
A dose of 0.5 g propoxur (purity 99.6%) moistened with purified
water, was applied under occlusive conditions to the shaven intact
back skin of 6 male New Zealand White rabbits for 4 hours. No skin
irritation was observed up to 72 hours after application (Yamane,
1986a).
In groups of 3 or 5 New Zealand rabbits an eye irritation study
was carried out with undiluted propoxur (purity 99.2%). Eyes were
rinsed after 5 minutes or 24 hours of exposure. The animals were
observed for 7 days. The only sign of irritation was slight erythema
of the conjunctivae of 2/3 animals that were exposed for 24 hours.
At 24 hours after rinsing this was no longer present (Thyssen,
1978).
Application of 0.1 g propoxur (purity 99.6%) into the eyes of 9
male New Zealand White rabbits caused severe miosis, which
disappeared within 24 hours after application. No irritation
effects were seen up to 96 hours post application (Yamane, 1986b).
Propoxur (purity 98.8%) did not exhibit a sensitizing effect in
the maximization test (Magnusson & Kligman) in guinea pigs (Heimann,
1982a).
Observations in humans
Dermal absorption
0.1 ml 14C-propoxur in acetone was applied to the ventral
forearm of 6 humans (sex not reported) (skin area 2.8-20 cm2;
applied amount 5 wµg propoxur/cm2). The skin sites were not
protected and subjects were asked not to wash the area for 24 hours.
Urine, collected for 5 days after beginning of exposure, was
monitored for 14C. The data were corrected for incomplete urinary
recovery using the 14C found in urine after administration of an
intravenous dose. A skin absorption of 19.6% of the dose was found
(Feldmann & Maibach, 1974).
TABLE 4. RESULTS OF IN VITRO MUTAGENICITY ASSAYS ON PROPOXUR
TEST SYSTEM TEST OBJECT CONCENTRATION PURITY RESULTS REFERENCE
Ames test * S. typhimurium 50 nmol/plate ca. 95% Negative Blevins et al. 1977b
TA98, TA100, TA1535
TA1537, TA1538
Ames test * S. typhimurium 0.1-1000 µg/pl 98.0% Negative Inukai & Iyatomi, 1978
TA98, TA100, TA1535 solvent DMSO (1)
TA1537, TA1538
Ames test * S. typhimurium 10-1500 µg/pl >96% Negative De Lorenzo et al. 1978
TA98, TA100, TA1535 (1)
TA1537, TA1538
Ames test S. typhimurium 0.25-100 µg/ml 97% Negative Jaszczuk et al. 1979
TA98, TA100, TA1535
TA1537, TA1538
Ames test * S. typhimurium 20-12500 µg/pl 98.6% Negative Herbold, 1982
TA98, TA100, TA1535 (1)
TA1537, TA1538
Reversion assay * Saccharomyces 75-10000 µg/ml 99.8% Negative Herbold, 1985e
cerevisiae D7 solvent DMSO (1)
Reverse mutation test E. coli WP2 hcr. 20 µl/disk ? Negative Shirasu et al. 1976
B/r try WP2
Reverse mutation E. coli WP2 hcr. 10-5000 µg/pl 98.05 Negative Shirasu et al. 1979
test * S. typh. (1)
TA98, TA100, TA1535
TA1537, TA1538
TABLE 4 (CONTD)
TEST SYSTEM TEST OBJECT CONCENTRATION PURITY RESULTS REFERENCE
Reverse mutation E. coli WP2 hcr. 500-25000 µg/pl 98.0% Negative Ohta & Moriya, 1983
test * S. typh. (1)
TA98, TA100, TA1535
TA1537, TA1538
HGPRT-test * Chinese hamster 25-125 µg/ml 99.6% Negative Lehn, 1988
ovary (CHO) cells (without S9 mix) (1)
600-1500 µg/ml (with S9 mix)
Mitotic gene Saccharomyces 2 ml of suspension 99.8% Negative Siebert & Lemperle, 1974;
conversion test cerevisiae D4 (containing 1000 ppm Siebert & Eisenbrand, 1974
a.i.) at 5 x 10 cells;
solvent DMSO
Pol Al-test * E. coli pol A+ 62.5-10000 µg/pl 98.5% Negative Herbold, 1983a
E. coli pol A- solvent DMSO (1)
Rec-assay Bacillus subtilis 3-300 µg/disk 98.0% Negative Inukai & Iyatomi, 1978
NIG17, NIG45 solvent DMSO (1)
Rec-assay Bacillus subtilis 20 µg/disk ? Negative Shirasu et al. 1976
H17 Rec+, M45 Rec- solvent DMSO
Rec-assay Bacillus subtilis 20-2000 µg/disk 98.0% Negative Shirasu ET AL. 1976
H17 Rec+, M45 Rec- (1)
Rec-assay Bacillus subtilis 50-10000 µg/disk 98.0% Negative Ohta & Moriya, 1983
H17 Rec+, M45 Rec- (1)
TABLE 4 (CONTD)
TEST SYSTEM TEST OBJECT CONCENTRATION PURITY RESULTS REFERENCE
Sister chromatid Human lymphocytes without S9 mix: 99.6% Negative Herbold, 1985d
exchange assay * 125-500 µg/ml (1)
with S9 mix:
250-1000 µg/ml
solvent DMSO
Single-strand Human fibroblasts 10-5 M approx Negative Blevins et al. 1977a
break assay 95%
Chromosome aberr. Chinese hamster without S9 mix. 97.8% Negative Putman & Morris, 1988
assay ovary (CHO) cells 157-625 µg/ml (1)
with S9 mix:
615 and 1250 µg/ml
solvent DMSO
* Test was carried out both with and without metabolic activation.
(1) Positive control yielded positive results.
TABLE 5. RESULTS OF IN VIVO MUTAGENICITY ASSAYS ON PROPOXUR
TEST SYSTEM TEST OBJECT CONCENTRATION PURITY RESULTS REFERENCE
DNA metabolism Male rat spleen cells 10 mg/kg bw; p.o. ? Negative Klein, 1984
studies (1)
Sister chromatid Chinese hamster bone 75 or 150 mg/kg bw 99.6% Negative Herbold, 1985c
exchange assay marrow cells p.o. (1)
Cytogenic study Chinese hamster 2 x 75 mg/kg bw 99.6% Negative Herbold, 1986
spermatogonia 2 x 150 mg/kg bw (1)
Cytogenic study Chinese hamster bone 75-300 mg/kg bw; 99.6% Negative Herbold, 1988
marrow cells p.o. (1)
Micronucleus test Male and female ICR- 25 mg/kg bw + 25 ? Negative Seiler, 1977
mice bone marrow cells mg/kg bw NaNO2; p.o.
Micronucleus test Male and female NMRI- 2 x 5 mg/kg bw; 99.2% Negative Herbold, 1980b
mice bone marrow cells 2 x 10 mg/kg bw; p.o. (1)
Dominant lethal Male mice 5 x 25 mg/kg bw ? Positive Tyrkiel, 1977
test 5 x 50 mg/kg (#) (1)
Dominant lethal Male mice 10 mg/kg bw; p.o. 99.2% Negative Herbold, 1980a
(#) Herbold (1978), in a critical review, points out the equivocality of the results, thus showing the questionable
validity of the author's conclusion.
(1) Postive control yielded positive results.
TABLE 6. RESULTS OF MUTAGENICITY ASSAYS ON PROPOXUR METABOLITES
TEST SYSTEM TEST OBJECT CONCENTRATION RESULTS REFERENCE
M1
Ames test * S. typhimurium 20 and 12500 µg/plate Negative Herbold, 1983c
TA98, TA100,
TA1535, TA1537
Poly A1- test *1 E. coli pol A+ 625-6075 µg/plate Negative Herbold, 1984b
E. coli pol A1-
M2
Ames test * S. typhimurium 20-12500 µg/plate Negative Herbold, 1983b
TA98, TA100
TA1535, TA1537
Mitotic recombination Saccharomyces 185.9-30000 µg/plate Negative Herbold, 1984e
assay * cerevisiae D7
M3
Ames test * S. typhimurium 312.5-5000 µg/plate Negative Herbold, 1984c
TA98. TA100, TA1535
TA1537. TA1538
DNA metabolism studies Male rat spleen 10 mg/kg bw Negative Klein, 1984
cells (1)
M4
Ames test * S. typhimurium 312.5-5000 µg/plate Negative Herbold, 1984a
TA98, TA100, TA1535
TA1537, TA1538
DNA metabolism studies Male rat spleen cells 10 mg/kg bw Negative Klein, 1984
TABLE 6 (CONTD)
TEST SYSTEM TEST OBJECT CONCENTRATION RESULTS REFERENCE
M5
Ames test * S. typhimurium 8-8748 µg/plate Negative Herbold, 1984d
TA98, TA100, TA1535
TA1537, TA1538
DNA metabolism studies Male rat spleen cells 10 mg/kg bw Negative Klein, 1984
(1)
M7
Not evaluable in Ames test due to breakdown in test medium
M8
Ames test * S. typhimurium evaluated range: Negative Herbold, 1984f
TA98, TA100 8-1800 µg/plate
TA1535, TA1537
Propoxur urine (rat, 8000 ppm in feed)
Ames test S. typhimurium 767 µl/plate Negative Herbold, 1985b
TA98, TA100
TA1535, TA1537
Propoxur urine extract (rat, 8000 in feed)
Ames test S. typhimurium evaluated range (2) Herbold, 1985a
TA98, TA100 14.5-29 µl/plate
TA1535, TA1537
* Test was carried out both with and without metabolic activation.
(1) Suppression of semi-conservative DNA synthesis was seen.
(2) Test outline and result inadequately reported.
Baygon spray (containing 2.0% propoxur and 0.5% dichlorvos) was
sprayed on the upper arm of 4 humans (3 males, 1 female) 6 times (1
second per spraying) at intervals of 10 minutes. The amount of
propoxur applied was 41 mg per person. Dermal absorption was
assessed via measurement of cholinesterase activity in plasma and
erythrocytes and of blood propoxur concentrations up to 6 hours
after treatment began. Urine samples, collected for 24 or 48 hours,
were monitored for the concentration of propoxur metabolite
isopropoxyphenol. No skin penetration of propoxur were found. The
identical procedure was used in a study in which 500 mg Baygon Dust
(1% w/w propoxur) was applied for 2 hours under an occlusive pad to
the abraded skin of the upper arm of 4 male subjects (skin sites
were abraded through application of an adhesive plaster and removal
of it, 30 minutes later). There were no signs indicating skin
penetration by propoxur (Eben & Kimmerle, 1974b).
Cholinesterase inhibition
Four human subjects (3 male and 1 female) were exposed by
inhalation to an aerosol containing 3 mg/m3 propoxur (purity 100%)
for 4 hours. The concentration of propoxur in the blood and
cholinesterase activity in plasma were determined up to 120 minutes
after treatment. Urine was monitored for the propoxur metabolite 2-
isopropoxyphenol up to 72 hours after exposure. In blood propoxur
was not found and cholinesterase activity was not depressed. 2-
Isopropoxyphenol was present in urine; the observed concentration
had decreased to trace level after 24 hours (and the compound was
absent thereafter), indicating excretion within this interval
(Kimmerle & Eben 1978b).
COMMENTS
After oral administration to rats the compound is rapidly
excreted, almost exclusively via the urine; only small quantities
are found in the feces. In urine the compound is excreted unchanged
or as one of a large number of metabolites which are present as free
compounds or as glucuronide or sulfate conjugates. The
biotransformation pathways in all species studied comprise
depropoxylation, hydrolysis of the ester bond and N-demethylation.
Ring hydroxylation also occurs: in rodents at ring positions 3, 4
and 5, and in primates at the 4- and 5- positions only. The
biotransformation pathway in humans is the same as in the Rhesus
monkey.
Propoxur induces drug metabolizing enzymes in the liver of
rats. This effect was greater with Altromin diet than with a semi-
synthetic diet.
The compound showed high acute oral toxicity in the species
examined.
Short-term administration of propoxur to rats (gavage, 5 days)
and dogs (dietary, 52 weeks) revealed cholinergic signs, growth
retardation and inhibition of cholinesterase activity in blood and
brain as the main toxicological effects. In the dog an increase of
microsomal enzyme activity was also observed. The NOAEL in the dog
study was 200 ppm (equivalent to 300 mg/kg bw/day).
In a long-term feeding study in mice there was significant
inhibition of growth at the 6000 ppm level. The NOAEL was 2000 ppm
(equivalent to 300 mg/kg bw/day).
In long-term feeding studies in Wistar rats, growth
retardation, ChE inhibition and urinary bladder alterations were the
main effects observed. Hyperplastic and neoplastic changes, which
were dependent on the diet, were found in the bladder of rats. The
hyperplasia could be reduced by the administration of ammonium
chloride in the predisposing diet, presumably via its effect on
urinary pH. These changes were not seen in mice, hamsters, dogs or
Rhesus monkeys.
The NOAEL in rats for the formation of hyperplasia of the
urinary bladder epithelium was 200 ppm (equal to 10 mg/kg bw/day).
This is also the NOAEL for AChE inhibition.
Increased post-implantation losses were observed in a
teratogenicity study in rabbits at 30 mg/kg bw/day. The NOAEL was
10 mg/kg bw/day. No embryotoxicity was observed at the highest dose
tested in rats (27 mg/kg bw/day). No teratogenic effects were
observed in either species.
After reviewing all available in vitro and in vivo short-
term tests, the Meeting concluded that there was no evidence of
genotoxicity.
Based on a re-evaluation of the human data available from the
1973 JMPR, a single oral dose of 0.2 mg/kg bw could be considered as
a NOAEL for humans since the depression of erythrocyte
cholinesterase did not exceed 20% and the recovery was very rapid.
These data have been reviewed by a WHO Expert Committee (WHO, 1973).
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse 2000 ppm in the diet, equivalent to 300 mg/kg bw/day
(ChE activity not measured)
Rat: 200 ppm in the diet, equal to 10 mg/kg bw/day
Dog: 200 ppm in the diet, equivalent to 5 mg/kg bw/day
Human 0.1 mg/kg bw
Estimate of acceptable daily intake for man
0-0.02 mg /kg bw
Studies which will provide information valuable in the continued
evaluation of the compound
Further observations in humans.
REFERENCES
Abd-Elraof, T.K., Dauterman, W.C. & Mailman, R.B. (1981) In vivo
metabolism and excretion of propoxur and malathion in the rat:
Effect of lead treatment. Toxicol. Appl. Pharmacol., 59: 324-330.
Ahdaya, S.M., Monroe, R.J. & Guthrie, F.E. (1981) Absorption and
distribution of intubated insecticides in fasted mice. Pesticide
Biochem. and Physiol. 16: 38-46.
Becker, H., Mladenovic, P. & Terrier, Ch. ( 1989a) Embryotoxicity
study (including teratogenicity) with BOQ 5812315 (c.n. propoxur) in
the rat. Unpublished Report No. R 4686 (project 207270) dated March
1, 1989 from RCC-Research & Consulting Company AG, Itingen,
Switzerland. Submitted to WHO by Bayer AG, Leverkusen, Federal
Republic of Germany.
Becker, H., Mladenovic, P. & Terrier, Ch. (1989b) Embryotoxicity
study (including teratogenicity) with BOQ 5812315 (c.n. propoxur) in
the rabbit. Unpublished Report No. R 4684 (Project 207292) dated
March 1, 1989 from RCC-Research & Consulting Company AG, Itingen,
Switzerland. Submitted to WHO by Bayer AG, Leverkusen, Federal
Republic of Germany.
Bell, R.L. & Gronberg, R.R. (1975) The metabolic fate of BAYGON in
the lactating dairy cow. Unpublished Report No. 44771 dated July 18,
1975 from from Chemagro Agricultural Division - Mobay Chemical
Corporation. Submitted to WHO by Bayer A.G., Leverkusen, Federal
Republic of Germany.
Blevins, R.D., Lijnski, W. & Regan, J.D. (1977a) Nitrosated
methylcarbamate insecticides: effect on the DNA of human cells.
Mut. Res. 44: 1-7.
Blevins, R.D., Lee, M. & Regan, J.D. (1977b) Mutagenicity screening
of five methyl carbamate insecticides and their nitroso derivatives
using mutants of Salmonella typhimurium LT2, Mutat. Res. 56: 1-
6.
Bomhard, E. & Löser, E. (1981) Bö 58 12 315 Chronic toxicological
study on mice (Feeding study over two years). Unpublished Report No.
9954 dated May 12, 1981 from Bayer A.G. Institute of Toxicology,
Wuppertal-Elberfeld. Submitted to WHO by Bayer A.G., Leverkusen,
Federal Republic of Germany.
Costa, L.G., Hand, H., Schwab, Bw & Murphy, S.D. (1981) Tolerance to
the carbamate insecticide propoxur. Toxicology, 21: 267-278.
De Lorenzo, F., Staiano, N., Silengo, L. & Cortese, R. (1978)
Mutagenicity of diallate, sulfallate, triallate and relationship
between structure and mutagenic effects of carbamates used widely in
agriculture. Cancer Res., 38: 13-15.
Eben, A., Karl, W. & Machemer, L. (1984) Studies on the
biotransformation of propoxur in the rat. Unpublished Report No.
12866 (KWN 15) dated August 17, 1984 from Bayer AG Institut für
Toxikologie, Wuppertal-Elberfeld. Submitted to WHO by Bayer AG,
Leverkusen, Federal Republic of Germany.
Eben, A., Karl, W. & Machemer, L. (1985a) Studies on
biotransformation of propoxur in humans. Unpublished Report No.
13947 (KWN 26) dated October 24, 1985 from Bayer AG Institut für
Toxikologie, Wuppertal-Elberfeld. Submitted to WHO by Bayer AG,
Leverkusen, Federal Republic of Germany.
Eben, A., Karl, W. & Machemer, L. (1985b) Supplementary studies on
biotransformation of propoxur in the rat. Unpublished Report no.
14148 dated December 13, 1985 from Bayer AG Institut für
Toxikologie, Wuppertal-Elberfeld. Submitted to WHO by Bayer AG.
Leverkusen, Federal Republic of Germany.
Eben, A., Karl, W. Machemer, L. (1986a) The biotransformation of
propoxur in golden hamsters. Unpublished Report No. 14690 (KWN 37)
dated June 9, 1986 from Bayer AG Institute of Toxicology, Wuppertal-
Elberfeld. Submitted to WHO by Bayer AG, Leverkusen, Federal
Republic of Germany.
Eben, A., Karl. W. & Machemer, L. (1986b) Propoxur [The active
ingredient of RBaygon] Biotransformation study on monkeys.
Unpublished Report No. 15056 (KWN 40) dated September 11, 1986 from
Bayer AG Fachbereich Toxikologie, Wuppertal-Elberfeld. Submitted to
WHO by Bayer AG, Leverkusen, Federal Republic of Germany.
Eben, A., Karl, W. & Machemer, L. (1987) Investigation on the
biotransformation of propoxur in mice. Unpublished Report No. 15697
(KWN 46) dated April 3, 1987 from Bayer AG Fachbereich Toxikologie
Landwirtschaft ZF-Zentrale Analytik Structure Research, Wuppertal.
Submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Eben, A. & Kimmerle, G. (1974b) 1% Baygon Dust. Resorption study on
skin of test volunteers. Unpublished Report No. 4461 dated February
5, 1974 from Bayer AG-Institut für Toxikologie, Wuppertal-Elberfeld.
Submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany. Test volunteers. Unpublished Report No. 4462 dated February
8, 1974 from Bayer AG Institut für Toxikologie, Wuppertal-Elberfeld.
Submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Eigenberg, D.A. (1988) Dermal absorption of propoxur technical in
rat using 14C-propoxur. Unpublished report No. 1097 (study number
88-721-AT) dated December 28, 1988 from Mobay Corporation, Health,
Environment and Safety, Corporate Toxicology Department, Stilwell,
Kansas, USA. Submitted to WHO by Bayer AG, Leverkusen, Federal
Republic of Germany.
Feldmann, R.J. & Maibach, H.I. (1974) Percutaneous penetration of
some pesticides and herbicides in man. Toxicol. Appl. Pharmacol.
28: 126-132.
Flucke, W. (1980) Boe 5812315 (propoxur). Acute toxicity studies.
Unpublished Report No. 9295 dated July 8, 1980 from Bayer AG
Institut für Toxikologie, Wuppertal-Elberfeld. Submitted to WHO by
Bayer AG, Leverkusen, Federal Republic of Germany.
Flucke, W. (1984) FCR (cyfluthrin) BOQ (propoxur) Study for
combination toxicity. Unpublished Report No. 12544 dated March 14,
1984 from Bayer AG Institute of Toxikology, Wuppertal-Elberfeld.
Submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Glaister, J.R. (1984) BOQ 5812315: 2 year carcinogenicity/chronic
toxicity study in the rat. Histopathology Report. Hazleton
Laboratories Harrogate, June 1984. Data submitted to WHO by Bayer AG
Leverkusen, Federal Republic of Germany.
Hahnemann, S. & Rühl-Fehlert, C. (1988a) BOQ 5812315 (common name:
propoxur). Chronic feeding test on Syrian gold hamsters (species
sensitivity). Unpublished Report No. 16801 dated June 15, 1988 from
Bayer AG Toxicology Division, Wuppertal (study no. T 0018434).
Submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Hahnemann, S. & Rühl-Fehlert, C. (1988b) BOQ 5812315 (common name:
propoxur). Chronic feeding test on Sprague-Dawley rats (strain
sensitivity). Unpublished report from Bayer AG Toxicology Division,
Wuppertal (study no. T 1018435). Submitted to WHO by Bayer AG,
Leverkusen, Federal Republic of Germany.
Hahnemann, S. & Rühl-Fehlert, C. (1988c) BOQ 5812315 (common name:
propoxur). Chronic feeding test on NMRI mice (species sensitivity).
Unpublished report no. 16803 dated June 15, 1988 from Bayer AG
Toxicology Division, Wuppertal (study no. T 9018433). Submitted to
WHO by Bayer AG, Leverkusen, Federal Republic of Germany.
Hahnemann, S. & Rühl-Fehlert, C. (1988d) BOQ 5812315 (common name:
propoxur). Sub-chronic feeding test on female Wistar rats (effect of
feed quality). Unpublished Report No. 16897 dated July 13, 1988 from
Bayer AG Toxicology Division, Wuppertal (study no. T 5019041).
Submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Hahnemann, S. & Rühl-Fehlert, C. (1988e) BOQ 5812315 (common name:
propoxur). Chronic feeding test on female Wistar rats with added 1%
L-(+)ascorbic acid. Unpublished Report No. 16979 dated August 2,
1988 from Bayer AG Toxicology Division, Wuppertal (study no. T
8018432). Submitted to WHO by Bayer AG, Leverkusen, Federal Republic
of Germany.
Hahnemann, S. & Rühl-Fehlert, C. (1988f) BOQ 5812315 (common name:
propoxur). Chronic feeding test on female Wistar rats over 2 years
(dose-effect-time relationship). Unpublished Report No. 16980 dated
August 2, 1988 from Bayer AG Toxicology Division, Wuppertal (study
no. T 6018430). Submitted to WHO by Bayer AG, Leverkusen, Federal
Republic of Germany.
Hahnemann, S. & Rühl-Fehlert, C. (1988g) BOQ 5812315 (common name:
propoxur). Chronic feeding study on female rats (Effect of feed and
drinking water type). Unpublished Report No. 17146 dated September
13, 1988 from Bayer AG Fachbereich Toxikologie, Wuppertal. Submitted
to WHO by Bayer AG, Leverkusen, Federal Republic of Germany.
Haley, T.J., Farmer, J.H., Dooley, K.L., Harmon, J.R. & Peoples, A.
(1974) Determination of the LD01 and extrapolation of the LD001 for
five methylcarbamate pesticides. J. Européen de Toxicologie, 7(3):
152-158.
Heimann, K.G. (1982a) Propoxur (the active ingredient of RBaygon
and UndenR). Study of sensitization effect on guinea pigs.
Unpublished Report No. 11218 dated October 15, 1982 from Bayer AG
Institut für Toxikologie, Wuppertal-Elberfeld. Submitted to WHO by
Bayer AG, Leverkusen, Federal Republic of Germany.
Heimann, K.G. (1982b) Carbamate UN, technical. Study for acute
toxicity on rat. Unpublished Report No. 11329 dated December 15,
1982 from Bayer AG Institute of Toxicology, Wuppertal-Elberfeld.
Submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Heimann, K.G. (1982c). Carbamate UN, Technical product: acute study
of the effect on the activity of the cholinesterases in blood
plasma, erythrocytes and brains of rats compared with carbamate UN
recrystallised product. Unpublished Report No. 11330 dated December
15, 1982 from Bayer AG Institute of Toxicology, Wuppertal-Elberfeld.
Submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Heimann, K.G. (1983) Carbamate UN, technical (c.n. propoxur, Unden
active ingredient; Baygon active ingredient) Subacute study on rats
compared with carbamate UN, recrystallised. Unpublished Report No.
11621 dated March 8, 1983 from Bayer AG Institute of Toxicology
Wuppertal-Elberfeld. Submitted to WHO by Bayer AG, Leverkusen,
Federal Republic of Germany.
Herbold, B. (1980a) BOE 5812315, dominant lethal study on male mouse
to test for mutagenic effects. Unpublished Report No. 8808 dated
January 7, 1980 from Bayer AG, Institute of Toxicology, Wuppertal-
Elberfeld. Submitted to WHO by Bayer AG, Leverkusen, Federal
Republic of Germany.
Herbold, B. (1980b) BOE 5812315, microncucleus test on mouse to
evaluate BOE 5812315 for mutagenic potential. Unpublished Report No.
9274 dated June 27, 1980 from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld. Submitted to WHO by Bayer AG, Leverkusen,
Federal Republic of Germany.
Herbold, B. (1982) Carbamate UN technical, Salmonella/microsome
test to evaluate for point mutation. Unpublished report no. 11301
dated December 6, 1982 from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld. Submitted to WHO by Bayer AG, Leverkusen,
Federal Republic of Germany.
Herbold, B. (1983a) Carbamate UN technical - Pol A1- test on E.
coli for potential DNA damage. Unpublished Report No. 11403 dated
January 6, 1983 from Bayer AG, Institute of Toxicology, Wuppertal-
Elberfeld. Submitted to WHO by Bayer AG, Leverkusen, Federal
Republic of Germany.
Herbold, B. (1983b) Isopropoxyphenol, Salmonella/microsome test to
evaluate for point mutation. Unpublished Report No. 12321 dated
December 20, 1983 from Bayer AG, Institute of Toxicology, Wuppertal-
Elberfeld. Submitted to WHO by Bayer AG, Leverkusen, Federal
Republic of Germany.
Herbold, B. (1983c) Brenzcatechin, Salmonella/microsome test to
evaluate for potential point mutation. Unpublished Report No. 12322
dated December 20, 1983 from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld. Data submitted to WHO by Bayer AG, Leverkusen,
Federal Republic of Germany.
Herbold, B. (1984a) THS 1240, Salmonella/microsome test to
evaluate for potential point mutation. Unpublished Report No. 12483
dated February 24, 1984 from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld. Submitted to WHO by Bayer AG, Leverkusen,
Federal Republic of Germany.
Herbold, B. (1984b) Brenzcatechin, POL-test of E. coli to evaluate
for potential DNA damage. Unpublished Report No. 12497 dated
February 29, 1984 from Bayer AG, Wuppertal-Elberfeld. Submitted to
WHO by Bayer AG, Leverkusen, Federal Republic of Germany.
Herbold, B. (1984c) THS 2490, Salmonella/microsome test to
evaluate for point mutation. Unpublished Report No. 12529 dated
March 6, 1984 from Bayer AG, Institute of Toxicology, Wuppertal-
Elberfeld. Submitted to WHO by Bayer AG, Leverkusen, Federal
Republic of Germany.
Herbold, B. (1984d) THS 1241b, Salmonella/microsome test to
evaluate for potential point mutation. Unpublished Report No. 12795
dated July 9, 1984 from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld. Submitted to WHO by Bayer AG, Leverkusen,
Federal Republic of Germany.
Herbold, B. (1984e). Isopropoxyphenol, test on S.cerevisiae D7 for
the induction of mitotic recombination. Unpublished Report No. 12876
dated August 20, 1984 from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld. Submitted to WHO by Bayer AG, Leverkusen,
Federal Republic of Germany.
Herbold, B. (1984f) THS 2647, Salmonella/microsome test to
evaluate for potential point mutation. Unpublished Report No. 12996
dated October 24, 1984 from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld. Submitted to WHO by Bayer AG, Leverkusen,
Federal Republic of Germany.
Herbold, B. (1985a) Propoxur urine extract compared with control
urine extract, Salmonella microsome test to evaluate for potential
point mutation. Unpublished Report No. 13350 dated March 14, 1985
from Bayer AG, Institute of Toxicology, Wuppertal-Elberfeld.
Submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Herbold, B. (1985b) Propoxur urine, Salmonella/microsome test to
evaluate for potential point mutation. Unpublished Report No. 13395
dated March 27, 1985 from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld. Submitted to WHO by Bayer AG, Leverkusen,
Federal Republic of Germany.
Herbold, B. (1985c) Propoxur (BOQ 5812315), sister chromatid
exchange in the bone marrow of the Chinese Hamster in vivo to
evaluate for harmful effect on DNA. Unpublished Report No. 13501
dated May 22, 1985 from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld. Submitted to WHO by Bayer AG, Leverkusen,
Federal Republic of Germany.
Herbold, B. (1985d) BOQ 5812315, sister chromatid exchange in human
lymphocyte cultures in vitro to test for DNA-modifying effects.
Unpublished Report No. 13871 dated October 9, 1985 from Bayer AG,
Institute of Toxicology, Wuppertal-Elberfeld. Submitted to WHO by
Bayer AG, Leverkusen, Federal Republic of Germany.
Herbold, B. (1985e) BOQ 5812315, test on S.cerevisiae D7 to
evaluate for point mutagenic effect. Unpublished Report No. 13966
dated October 30, 1985 from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld. Submitted to WHO by Bayer AG, Leverkusen,
Federal Republic of Germany.
Herbold, B. (1986) BOQ 5812315 (c.n. propoxur), cytogenetic study of
chromosome damage using spermatogonia of Chinese Hamsters in vivo.
Unpublished Report No. 14984 dated August 20, 1986 from Bayer AG,
Institute of Toxicology, Wuppertal-Elberfeld. Submitted to WHO by
Bayer AG, Leverkusen, Federal Republic of Germany.
Herbold, B. (1988) BOQ 5812315 (c.n. propoxur), cytogenetic study on
bone marrow of Chinese Hamster in vivo to detect chromosomal
damage. Unpublished Report No. 17111 dated September 6, 1988 from
Bayer AG Institute of Toxicology, Wuppertal-Elberfeld. Submitted to
WHO by Bayer AG, Leverkusen, Federal Republic of Germany.
Hoffmann, K. & Gröning, P. (1984) BOQ 5812315 (BOE 5812315, c.n.
propoxur) Chronic toxicity to dogs on oral administration (12-months
feeding study). Unpublished Report No. 12605 dated April 11, 1984
from Bayer AG Institute of Toxicology, Wuppertal-Elberfeld.
Submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Hoffmann, K. & Ruehl, Chr. (1985) Propoxur (BOQ 5812315). Subchronic
study of toxicity to Rhesus monkeys after oral administration by
stomach tube for 13 weeks to check for possible findings in the
urinary bladder. Unpublished Report No. 13779 dated August 27, 1985
from Bayer AG Institute of Toxicology, Wuppertal-Elberfeld.
Submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Inukai, H. & Iyatomi, A. (1978) Propoxur. Mutagenicity test on
bacterial systems. Unpublished Report No. 103 dated February 24,
1978 from Nitokuno Agricultural Chemicals Institute, Toyoda, Japan.
Submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Jaszcuk, E., Syrowatka, T. & Cybulski, J. (1979) Mutagenic activity
of propoxur, carbaryl and their nitroso derivatives: induction of
reversion in Salmonella typhimurium. Roczniki Panstwowzgo Zakladu
Higieny Warszawa, 30(1): 81-88. (English summary only).
Jurek, A. (1978) Chronische Langzeittoxizität des
Propoxurkarbaminats, Roczniki Panstwowzgo Zakladu Higieny Warszawa,
29(3): 327-338.
Karl, W. (1986) Biotransformation of propoxur: quantitative
determination of the metabolic pattern in rats given a single dose
of 14C-propoxur after a subchronic prefeeding period in two diet
groups and three dose groups. Unpublished Report No. KWN 42 dated
October 15, 1986 from Bayer AG Institute of Toxicology, Wuppertal-
Elberfeld. Submitted to WHO by Bayer Leverkusen, Federal Republic of
Germany.
Karl, W. & Schneider, J. (1987) Isolation and spectroscopic
structure elucidation of the renal metabolite conjugates of
propoxur. Unpublished Report No. KWN 44 dated August 26, 1987 from
Bayer AG Institute of Toxicology, Wuppertal-Elberfeld. Submitted to
WHO by Bayer AG, Federal Republic of Germany.
Kimmerle, G. & Eben, A. (1978a) Propoxur - Acute inhalation study on
rats with determination of acetylcholinesterase activity in blood
and elimination of 2-isopropoxyphenol in urine. Unpublished Report
No. 7555 dated May 1978 from Bayer AG Institute für Toxikologie,
Wuppertal-Elberfeld. Submitted to WHO by Bayer AG Leverkusen,
Federal Republic of Germany.
Kimmerle, G. & Eben, A. (1978b) Propoxur - Single exposure of
persons to propoxur with determination of acetylcholinesterase
activity in plasma and erythrocytes, propoxur concentration in blood
and elimination of 2-isopropoxyphenol in urine. Unpublished Report
No. 7554 dated May, 1978 from Bayer AG, Institut für Toxikologie,
Wuppertal-Elberfeld. Submitted to WHO by Bayer AG Leverkusen,
Federal Republic of Germany.
Kimmerle, G. & Iyatomi, A. (1976) Toxicity of propoxur to rats by
subacute inhalation. Jap. J. Ind. Health, 18: 375-382.
Klein, W. (1984) Effect of an active ingredient and three
metabolites on the DNA metabolism. Unpublished Report R3346 dated
March 1984 from Osterreichisches Forschungszentrum Seiberdorf
Biochemistry Department/Toxicology Department. (Research commisioned
by Bayer AG). Submitted to WHO by Bayer AG Leverkusen, Federal
Republic of Germany.
Krechniak, J. & Foss, W. (1982) Cholinesterase activity in rats
treated with propoxur. Bull. Environm. Contam. Toxicol., 29: 599-
604.
Krechniak, J. & Foss, W. (1983a) Behaviour of propoxur after
repeated administration. Bromat. Chem. Toksykol., 16 (3-4): 205-
208. (English translation).
Krechniak, J. & Foss, W. (1983b) Distribution and excretion of
isopropoxyphenol in rats. Bromat. Chem. Toksykol., 6 (3-4): 209-
211 (English translation).
Lehn, H. (1988) BOQ 5812315 (c.n. propoxur), mutagenicity study for
the detection of induced forward mutations in the CHO-HGPRT assay
in vitro. Unpublished Report No. 17090 dated August 31, 1988 from
Bayer AG, Fachbereich Toxikologie, Wuppertal. Data submitted to WHO
by Bayer AG, Leverkusen, Federal Republic of Germany.
Luckhaus, G. (1984) Propoxur, two-year oral toxicity study in rats,
addendum to HRC Report No. 2809/69/235, histological follow-up
investigation of the urinary bladder. Unpublished Report No. 13012
dated November 2, 1984 from Bayer AG, Institute of Toxicology,
Wuppertal. Data submitted to WHO by Bayer AG, Leverkusen, Federal
Republic of Germany.
Machemer, L. & Schmidt, U. (1988) Propoxur. Status of the studies
and assessment regarding oncogenic potential to the urinary bladder.
Unpublished report dated September 30, 1988 from Bayer AG
Fachbereich Toxikologie, Institut für Toxikologie Landwirtschaft,
Wuppertal-Elberfeld. Data submitted to WHO by Bayer AG, Leverkusen,
Federal Republic of Germany.
Mihail, F. (1982) BOQ 5812315. Test for induction of the microsomal
liver enzymes. Unpublished Report No. 10976 dated June 29, 1982 from
Bayer AG, Institute of Toxicology, Wuppertal-Elberfeld. Data
submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Ohta, T. & Moriya, M. (1983) Propoxur, microbial mutagenicity study.
Unpublished Report from Institute of Environmental Toxicology
(Japan) dated February 28, 1983. Data submitted to WHO by Bayer AG,
Leverkusen, Federal Republic of Germany.
Pauluhn, J. (1988) BOQ 5812315 (common name: propoxur) study of the
inhalation toxicity in accordance with OECD Guideline No. 403.
Unpublished Report No. 16966 dated July, 1988 from Bayer AG
Fachbereich Toxikolgie, Wuppertal. Submitted to WHO by Bayer AG,
Leverkusen, Federal Republic of Germany.
Pauluhn, J. & Rühl, C. (1985) BOQ 5812315, study for subacute
inhalation toxicity to the rat. Unpublished Report No. 13297 dated
February, 20, 1985 from Bayer AG, Institute of Toxicology Wuppertal-
Elberfeld. Data submitted to WHO by Bayer AG, Leverkusen, Federal
Republic of Germany.
Putman, D.L. & Morris, M.J. (1988) Chromosome aberrations in Chinese
Hamster Ovary (CHO) cells, Baygon technical. Unpublished Report No.
1094 dated December 22, 1988 from Microbiological Associates Inc.
Maryland, USA. Submitted to WHO by Bayer AG, Leverkusen, Federal
Republic of Germany.
Reid Patterson, D. (1980) Histopathology report on Boe 5812315 mouse
study. Unpublished Report No. 2317-262/13 dated May, 1980 from
Hazleton Laboratories Europe, Harrogate, England to Bayer AG
Institut für Toxikologie, Wuppertal. Submitted to WHO by Bayer AG,
Leverkusen, Federal Republic of Germany.
Schlüter, G. (1981) BOQ 5812315. Evaluation for embryotoxic and
teratogenic effects after oral administration to the rabbit.
Unpublished Report No. 10183 dated September 9, 1981 from Bayer AG,
Institute of Toxikologie, Wuppertal-Elberfeld. Data submitted to WHO
by Bayer AG, Leverkusen, Federal Republic of Germany.
Schmidt, U. (1987) Investigations of interspecies differences in
primary metabolism with liver-cell fractions from rat, mouse,
hamster, monkey and man. Unpublished Report No. 16237 dated November
19, 1987 from Bayer AG, Fachbereich Toxikologie, Wuppertal.
Submitted to WHO by etc.
Seiler, J.P. (1977) Nitrosation in vitro and in vivo by sodium
nitrite, and mutagenicity of nitrogenous pesticides. Mutat. Res.
48: 225-236.
Shirasu, Y., Moriya, M., Kato, K., Furuhashi, A. & Kada, T. (1976)
Mutagenicity screening of pesticides in the microbial system.
Mutat. Res. 40: 19-30.
Shirasu, Y., Moriya, M. & Sugiyama, F. (1979) Propoxur, mutagenicity
test on bacterial systems. Unpublished Report from Institute of
Environmental Toxikologie (Japan) dated August 28, 1979. Data
submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Siebert, D. & Eisenbrand, G. (1974) Induction of mitotic gene
conversion in Saccharomyces cerevisiae. Mutat. Res. 22: 121-126.
Siebert, D. & Lemperle, E. (1974) Genetic effects of herbicides:
induction of mitotic gene conversion in Saccharomyces cerevisiae.
Mutat. Res. 22: 111-120.
Suberg, H. & Löser, E. (1984) BOQ 5812315, chronic toxicological
study with rats (feeding study over 106 weeks). Unpublished Report
No. 12870 dated August 20, 1984 from Bayer AG, Institute of
Toxicology, Wuppertal. Data submitted to WHO by Bayer AG,
Leverkusen, Federal Republic of Germany.
Thyssen, J. (1977) Study for combination toxicity of azinphos-methyl
and propoxur. Unpublished Report No. 7174 dated December 14, 1977
from Bayer AG, Institute of Toxicology, Wuppertal-Elberfeld. Data
submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Thyssen, J. (1978) Propoxur-Untersuchungen an der Haut und am Auge
von Kaninchen. Unpublished report dated September 19, 1978 from
Bayer AG, Institut für Toxikologie, Wuppertal-Elberfeld. Submitted
to WHO by Bayer AG, Leverkusen, Federal Republic of Germany.
Tyrkiel, E. (1977) Mutagenne addzialywannie, o-izopropoksyfenoyl-N-
methylo-karaminianu (propoksura) na komorki pleiowe myszy. Rocz.
Panstw. Zakl. Hig. 28 (6): 601-613.
Weber, H. (1986) Comparison of the absorption of a tracer dose of
(Phenyl-U-14C) propoxur from a basic casein diet and a standard
Altromin 1324 diet by nonradioactively pretreated Wistar rats.
Unpublished Report No. 2504 dated February 4, 1986 from Bayer AG,
Institute of Metabolism Research , Wuppertal. Data submitted to WHO
by Bayer AG, Leverkusen, Federal Republic of Germany.
Weber, H. (1988) [Phenyl-UL-14C] Propoxur: whole-study
autoradiographic distribution of the radioactivity in the rat.
Unpublished Report No.2988 dated April 21, 1988 from Bayer AG,
Institute for Metabolism Research, Leverkusen. Data submitted to WHO
by Bayer AG, Leverkusen, Federal Republic of Germany.
WHO/FAO (1974) 1973 Evaluations of some pesticide residues in food,
WHO Pesticides Residues Series, No. 3. World Health Organization,
Geneva.
Yamane, S. (1986a) Primary skin irritation study of propoxur in
rabbits. Unpublished Report No. D-0714 (DT-9) dated June 9, 1986
from Hita Research Laboratories, Japan. Data submitted to WHO by
Bayer AG, Leverkusen, Federal Republic of Germany.
Yamane, S. (1986b) Primary eye irritation study of propoxur in
rabbits. Unpublished Report No. D-0736 (DT-10) dated September 12,
1986 from Hita Research Laboratories, Japan. Data submitted to WHO
by Bayer AG, Leverkusen, Federal Republic of Germany.
90% of the administered 14C was recovered in urine in all groups.
The urinary conjugated metabolite pattern was comparable for the
different dose levels and the different diets. At higher (5000 ppm)
dose levels, there was a trend for a higher percentage of M5 and M6
and a lower for M2 and M7. The 14C distribution over the
conjugated metabolites is summarized in Table 1.
TABLE 1. DISTRIBUTION OF 14C OVER URINARY METABOLITES IN RATS
METABOLITE % OF 14C PRESENT IN
URINE (RANGE FOR ALL
DOSE LEVELS AND DIETS)
M1 7.0-15.5
M2 17.2-25.2
M3 22.2-30.2
M5 0.6- 2.5
M6 4.5- 7.9
MS3 0.3- 1.2
M7 14.0-16.3
M7A 5.9- 8.3
M8 1.7- 4.1
(Karl, 1986; Karl & Schneider, 1987)
Metabolism of propoxur in the liver was studied further in in
vivo studies, using post-mitochondrial fractions of livers of
rats, mice, hamsters and monkeys. A similar study was done in human
liver preparations. The results show M5 to be the major metabolite
in rat liver, M3 and M6 being formed to a lesser degree. The
percentage of M5 in relation to overall metabolism decreases from
mouse to hamster and monkeys. In the liver preparation from Rhesus
monkeys, M3 and M6 were formed more than in M5. In additional tests
it was observed that in liver cell fractions of rats and mice
further metabolization of M5 does not take place in hamsters it
occurs only to a limited degree. In contrast, the liver cell
fractions of Rhesus monkeys and humans are able to further
metabolize M5 to 4 other metabolites (not identified) (Schmidt,
1987).
Effect on cholinesterase activity
Male and female Wistar rats were given a single oral dose of 0,
1, 5 or 25 mg/kg bw via stomach tube. Cholinesterase activity was
measured in plasma, erythrocytes and brain at intervals varying from
0.5 hours to 14 days after dose application. At the highest dose
level, cholinesterase activity in plasma and erythrocytes was
depressed after 30 and 60 minutes; after 3 hours the depressions
were no longer apparent. Brain cholinesterase activity was depressed
after 3-5 hours in the same group; 3 days after dosing this was no
longer apparent. At 1 and 5 mg/kg bw no effect was seen (Heimann,
1982c).
In male rats given a single oral dose of 2.1, 7.0, 20.9, 50 or
70 mg/kg bw propoxur, cholinesterase activity in whole blood was
decreased at all dose levels (at 2.1 mg/kg bw the decrease was
slight) after 10 minutes; at 24 hours after dose application the
depressions were no longer present. The same investigators tested
for cholinesterase depression in whole blood and brain in male rats
dosed orally with 30 mg/kg bw/day for 14 days followed by 50 mg/kg
bw via the same route for 28 days. A decreased activity in both
blood and brain was noted during the first 14 days; the depression
gradually disappeared during the following 28 days of treatment.
This indicates adaptation (Krechniak & Foss, 1982).
In an inhalation study, male and female rats were exposed to
0.4, 1.2, 9.0, 30.0, 78.0 or 172.0 mg/m3 propoxur for 6 hours.
Directly after cessation of exposure, plasma and erythrocyte
cholinesterase activities were measured and compared to pre-test
values. At 30, 78 and 172 mg/m3, depressions were observed. In
addition, at 172 mg/m3 cholinergic symptoms were seen at 30 minutes
after commencement of exposure (Kimmerle & Eben, 1978a).
Effect on liver enzyme activity
Mice
Male mice received propoxur (purity 98.8%) in the drinking
water at concentrations increasing with each successive week from 50
to 2000 ppm over a total period of 6 weeks. In animals thus treated
for six weeks, the LD50 for propoxur was significantly higher than
in untreated controls, a finding indicating that tolerance to
propoxur acute toxicity had developed. Hexobarbital sleeping time
was significantly reduced, indicating induction of hepatic
microsomal enzymes. Determinations of carboxy esterase activities
in liver, plasma and brain, however, did not show any significant
increase in the propoxur-treated animals (Costa et al. 1981).
Rats
Propoxur was administered orally for 5 days to male and female
rats at dose levels of 0, 15 or 30 mg/kg bw/day. The rats treated
with propoxur did not exhibit induction of the mixed function
oxidases in comparison to control rats. (A positive control group
was treated with sodium phenobarbitone 50 mg/kg bw/day for 5 days
and induction of mixed function oxidases was observed) (Mihail,
1982).
Concomitant to a short-term study in female rats, liver tissue
enzyme activities were measured after 3, 7, 14 and 28 days of
feeding on a diet containing 0 or 5000 ppm propoxur. From day 3,
cytochrome P-450 dependent mono-oxygenases (i.e. 7-ethoxycoumarin
deethylase, ethoxyresorufin deethylase, aldrin epoxidase) were
induced (increased with a factor of 2-3). The activity of the
microsomal epoxide hydroxylase was increased by the same magnitude.
In contrast, the cytosolic glutathione-S-transferase exhibited only
a slight change compared to the control group. Altromin diet was
used in this study. An identical study in which a casein semi-
synthetic diet was used also showed induction of mono-oxygenase
activity by propoxur. The absolute activity levels reached in the
latter study, however, were about 50% lower compared to the values
observed in the concurrent control and treatment groups of the
Altromin diet study (Machemer & Schmidt, 1988).
Toxicological studies
Acute toxicity
The acute toxicity of propoxur to rats and mice is presented in
Table 2.
The observed intoxication symptoms were indicative of
inhibition of cholinesterase: convulsions, muscular tremors,
muscular spasms, dyspnoea, salivation (Flucke, 1980; Heimann,
1982b).
Short-term toxicity
Oral studies
Rats
The toxicity of propoxur technical (purity 98.6%) and propoxur
recrystallized (purity 99.2%) was compared in a 5 day-study. Groups
of Wistar rats (5/sex/group) received oral doses of 0, 15 or 30
mg/kg bw/day of propoxur of both purities via stomach tube for 5
days. Appearance, behaviour and body weight were recorded daily.
After 5 days, all animals were sacrificed and submitted to gross
pathology. The weights of liver and kidneys were determined.
Clinical chemistry was performed on all animals. N-demethylase, O-
demethylase, cytochrome P-450 and triglycerides were measured in
liver homogenates (cholinesterase activity not determined). The
only adverse effects noted were convulsions and apathy, occurring in
all treatment groups in a dose-related manner. No difference in
toxicity between the two purities were found (Heimann, 1983).
TABLE 2. ACUTE TOXICITY OF PROPOXUR IN ANIMALS
LD50 LC50
SPECIES SEX ROUTE (mg/kg bw) (mg/m3) REFERENCES
Mouse M oral 37 - Haley et al. 1974
F idem 39 - Haley et al. 1974
Rat M* oral 39 - Thyssen et al. 1977
M* oral 45 - Flucke, 1984
M oral 196 - Flucke, 1980
F oral 126 - Flucke, 1980
M* oral 94 - Flucke, 1980
F* oral 68 - Flucke, 1980
M oral 167 - Heimann, 1982b
F oral 96 - Heimann, 1982b
M* oral 69 - Heimann, 1982b
F* oral 47 - Heimann, 1982b
M i.p. 16 - Heimann, 1982b
F i.p. 13 - Heimann, 1982b
M&F dermal >5000 - Flucke, 1980
M&F inhal. - >498 Pauluhn, 1988
* Fasted animals
Dogs
Beagle dogs (6/sex/group) were given diets containing 0, 200 or
600 ppm propoxur (purity 99.4%) for 52 weeks. An additional group
received 1800 ppm from week 1 through week 40, 3600 ppm from week 41
through 44 and 5400 ppm from week 45 through 52 (the increases were
established in order to produce overt toxic signs). Appearance,
behaviour and body weight were recorded. On a number of occasions
throughout the study reflex tests, ophthalmoscopy, hematology,
clinical chemistry, urinalysis and cholinesterase activity (in
plasma and erythrocytes) were determined. At sacrifice, organs were
weighed and complete gross pathology and histopathology were carried
out. In livers N-demethylase and cytochrome P-450 were measured.
Cholinergic symptoms were observed at the highest dose level after
elevation of the dose level to 5400 ppm and 1/6 animals died. In
addition, the following parameters were increased in this group:
thrombocyte, leucocyte and reticulocyte counts, incidence of Heinz
bodies, ALAT and SAP, liver weight and thyroid weight; thymus weight
was decreased (also noted: medium thymus atrophy). At the highest
dose level and at 600 ppm also, growth was retarded and plasma
cholesterol and liver N-demethylase were increased. The NOAEL in
this study is 200 ppm (Hoffmann & Gröning, 1984).
Inhalation studies
Groups of Wistar rats (10/sex/group) were exposed to aerosols
containing propoxur (purity 98.9%) in concentrations of 0, 5.7, 18.7
or 31.7 mg/m3 6 hours per day, 5 days per week over a period of 12
weeks. There was no effect on behaviour, growth, hematology,
clincial chemistry, urinalysis, organ weights or histopathology.
The only effect observed was a depression of cholinesterase activity
in plasma, erythrocytes and brain, occuring at 31.7 mg/m3 only
(Kimmerle & Iyatomi, 1976).
Groups of Wistar rats (5/sex/group) were exposed to aerosols
containing propoxur (purity 99.6%) in concentrations of 0, 15.3,
45.3 or 139.6 mg/m3 during 6 hours per day, 5 days per week over a
period of 4 or 8 weeks. The observations included clinical signs,
body weight, cholinesterase activity in plasma, erythrocytes and
brain at sacrifice after 4 and 8 weeks, urinalysis, gross pathology
(all organs) and histopathology (4 tissues/animal) and organ weights
(4 organs/animal). Cholinergic symptoms were observed at 139.6
mg/m3. Cholinesterase activies in brain were depressed in week 4
at 45.3 and 139.6 mg/m3 and in week 8 at 15.3 mg/m3 also. There
were no signs of specific organ damage or an alteration in the
urinary bladder epithelium (Pauluhn & Rühl, 1985).
Long term carcinogenicity studies
Mice
Groups of 50 male and 50 female CF1/W74 mice were fed diets
containing 0, 700, 2000 or 6000 ppm propoxur (purity 99.6%) for 24
months. Satellite groups of 10 male and 10 female mice were fed at
the same dose levels and were used for interim sacrifice after 6
months. Observations included clinical signs, body weight, food
consumption, hematology and clinical chemistry. The weights of 6
organs/animal were recorded. Gross pathology and limited
histopathogy (about 20 tissues/animal) were carried out. Slight
growth retardation was oberved in the 700, 2000 and 6000 ppm males;
ALAT was increased in 6000 ppm-females after 6 months only. The
relative weights of testes and spleen were increased or decreased,
respectively, at all dose levels to a dose-related degree. The
tumour incidence was not increased (Bomhard & Löser, 1981; Reid
Patterson, 1980).
Rats
In a chronic toxicity study summarized in the WHO/FAO monograph
from 1974, with dietary dose levels of 0, 250, 750, 2000 and 6000
ppm (purity 99.8%),the effects were: growth retardation and reduced
food consumption at 200 and 6000 ppm and increased relative liver
weight at 6000 ppm (NOAEL in this study: 250 ppm) (WHO/FAO, 1974).
The histological sections produced in this study were reexamined.
Histopathological appraisal of urinary bladder sections confirmed
the absence of alterations in this organ at all dose levels
(Luckhaus, 1984).
Groups of 20 male and 20 female Wistar rats were fed diets
containing 0, 50, 200 or 800 ppm propoxur (purity 97.25%) for 18
months. The observations included clinical signs, food intake, body
weight, hematology and clinical chemistry. At termination all
animals were sacrificed. Organ weights were determined and
histopathology was carried out. Growth was slightly decreased in
the 800 ppm females. At the end of the study cholinesterase
activities in whole blood and brain were inhibited at 800 ppm. The
NOAEL in this study is 200 ppm (Jurek, 1978).
Groups of 50 male and 50 female Wistar rats were fed diets
containing 0, 200, 1000 or 5000 ppm propoxur (purity 99.4%) for 2
years. Additional groups of 10 rats/sex/group were treated at the
same dose levels and were used for interim sacrifice after 1 year.
The observations included clinical signs, body weight, gross
pathology and histopathology. Growth was retarded at 1000 and 5000
ppm. At 5000 ppm slight neuromuscular changes (i.e. slightly
increased incidences of peripheral neuropathy and muscular atrophy
of the rear extremities) were noted. Also at 5000 ppm, ASAT was
decreased (males and females) and urea was increased (females only).
At the same dose level the relative weights of a number of organs
(heart, lung, liver, kidney, adrenal) were increased. At interim
autopsy the incidence of hyperplasia of the urinary bladder was
increased at 1000 and 5000 ppm (incidences 0/20, 0/20, 6/20 and
19/20 in the 0, 200, 1000 and 5000 ppm groups, respectively). The
findings in the urinary bladder at terminal sacrifice are presented
in Table 3.
TABLE 3. INCIDENCE OF URINARY BLADDER ALTERATIONS
IN MALE AND FEMALE RATS
OCCURRENCE CONTROL 200 ppm 1000 ppm 5000 ppm
Hyperplasia 1/98 1/96 15/99 92/97
Papilloma 0/98 0/96 1/99 53/97
Carcinoma 0/98 0/96 0/99 13/97
The NOAEL in this study is 200 ppm (determined to be equal to
9.6 mg/kg bw/day) (Suberg & Löser, 1984, Glaister, 1984).
For further determination of the dose-response/exposure-time
relationship with regard to the effect on the urinary bladder,
groups of 70 female Wistar rats were fed diets containing 0, 50,
250, 1000, 3000, 5000 or 8000 ppm propoxur (purity 99.6-99.9%) for
periods up to 104 weeks. After 4, 7, 12, 26, 53 and 78 weeks, 5 or
10 rats/group were sacrificed for interim autopsy. The animals were
observed for clinical signs, food intake, water intake and body
weight. Organ weights were determined. Gross pathology and
histopathology (kidney, urinary bladder, ureter, liver) were carried
out. Growth was retarded at 3000 ppm and higher dose levels. The
relative weights of liver and kidneys were increased at 3000, 5000
and 8000 ppm. Hyperplasia of the bladder epithelium was observed at
1000 ppm (from week 53), at 3000 ppm (from week 12), 5000 ppm (from
week 4) and at 8000 ppm (from week 2). At the latter two dose
levels the effect had developed to severe hyperplasia with recent
vascularization and papillary and nodular hyperplasia after 53
weeks. After 104 weeks, dose-related increases were observed on
hyperplasia, papilloma and carincoma of the urinary bladder in
female rats.
The NOAEL in this study is 250 ppm (Hahnemann & Rühl-Fehlert,
1988f)
In a number of additional studies the effect of propoxur on the
urinary bladder was yet further examined. Issues to be elucidated
were strain and species specificity, influence of the diet used and
effect of vitamin C supplementation.
Strain specificity
Possible strain specificity of the effect of propoxur on the
urinary bladder, observed in Wistar rats, was examined through an
oral study in Sprague Dawley rats. Groups of 50 female Sprague
Dawley rats were fed diets containing 0, 3000 or 8000 ppm propoxur
(purity 99.6%-99.9%) for periods up to 52 weeks. Growth was
retarded and the relative weights of liver, lung and kidneys were
increased at both dose levels. Simple hyperplasia of the urinary
bladder was observed at 3000 and 8000 ppm from week 4 on. At 8000
ppm hyperplasia with neovascularization, papillary hyperplasia and
incipient nodular hyperplasia were found in the urinary bladder from
week 27. Thus, this strain of rats is as sensitive as is the Wistar
rat with regard to the formation of urinary bladder hyperplasia by
propoxur (Hahnemann & Rühl-Fehlert, 1988b).
Species specificity
In the two oral short-term toxicity studies in dogs (summarized
in the relevant paragraphs of the present monograph and the 1974
WHO/FAO monograph respectively) and in the long-term study in mice
(summarized in the paragraph on long-term studies of the present
monograph) no effect on the urinary bladder epithelium was observed.
Further evidence of species specificity was obtained in the
following studies.
Mice
Groups of 50 female NMRI mice were fed diets containing 0, 3000
or 8000 ppm propoxur (purity 99.6%-99.9%) for 53 weeks. Growth was
slightly decreased at 8000 ppm. Increased liver weight and fatty
degeneration occurred at 3000 and 8000 ppm. Relative lung weight
was increased at 8000 ppm only. No adverse effect on urinary
bladder epithelium was noted (Hahnemann & Rühl-Fehlert, 1988c).
Hamsters
Groups of 50 female Syrian golden hamsters were fed diets
containing 0, 3000 or 8000 ppm propoxur (purity 99.6-99.9%) for 53
weeks. At both dose levels the incidence of mortality was slightly
increased, impairment of the general state of the animals (not
specified) was noted and growth was retarded. The relative weights
of kidneys and adrenals were increased at 8000 ppm only. No adverse
effect on urinary bladder epithelium was observed (Hahnemann & Rühl-
Fehlert, 1988a).
Rhesus monkeys
A group of 6 Rhesus monkeys (3 per sex) received oral doses of
40 mg/kg bw/day propoxur (purity 99.6%) via oral intubation for 13
weeks. This daily dose was previously determined to be the maximum
tolerable dose. No control group was used. Cholinergic symptoms
were observed following compound administration. No adverse effect
on the urinary bladder epithelium was noted (Hoffmann & Rühl, 1985).
Effect of vitamin C supplementation
Groups of 50 female Wistar rats were fed diets containing 1%
vitamin C and propoxur (purity 99.6-99.9%) at concentrations of 0,
1000, 3000 or 8000 ppm for a period of 49 weeks. Additional groups
received the same propoxur dose levels in unsupplemented diet.
Growth was retarded in all propoxur-treated groups. The relative
weights of liver (8000 ppm only) and kidneys and lungs (all dose
levels) were increased. The propoxur-induced hyperplastic changes
of the urinary bladder epithelium were observed in all treatment
groups, being present to an equal degree after feeding of the
supplemented and the unsupplemented diets. Thus, vitamin C did not
influence the effect of propoxur on the rat urinary bladder
epithelium (Hahnemann & Rühl-Fehlert, 1988e).
Effect of diet
In the rat studies in which propoxur was found to produce the
urinary bladder alterations, the diet used was Altromin B21 standard
diet. To examine if the diet used was a relevant factor for the
occurrence of the urinary bladder changes, two additional studies
were carried out using a semisynthetic diet (Casein diet no. 1/0).
Groups of 50 female Wistar rats were fed Casein semi-synthetic
diet no. 1/0 containing 0 or 8000 ppm propoxur (purity 99.9%) for
4,8 or 14 weeks. Growth retardation and reduced water intake were
observed in the treated animals. In addition, relative liver and
kidney weights were increased. No urinary bladder changes were
found at histopathology (Hahnemann & Rühl-Fehlert, 1988d).
Groups of 50 female Wistar rats were fed Casein diet no. 1/0
containing 0, 3000 or 8000 ppm propoxur (purity 99.6%) for periods
up to 100 weeks. Growth was retarded at 3000 and 8000 ppm.
Relative weights of lung, kidney and liver were increased at 8000
ppm. Histopathology revealed no treatment-related changes in the
urinary bladders of treated animals (Hahnemann & Rühl-Fehlert,
1988g).
In a study using 14C-propoxur, possible differences in
absorption of propoxur from the Altromin diet and the semisynthetic
case in diet were ruled out. Thus, the absence of urinary bladder
effects when the casein diet is used is not caused by lower
absorption of propoxur from this diet (Weber, 1986). Another
relevant result was observed in a study in rats in which the renal
metabolite pattern was determined after a single oral dose of 14C-
propoxur (label in ring) after preceding administration of 0, 250 or
5000 ppm propoxur either in Altromin standard diet or in the
semisynthetic casein diet for 4 weeks. No differences in the
metabolite pattern were found (Karl, 1986; Karl & Schneider, 1987).
Special studies on combination toxicity
The acute oral application of equitoxic doses of propoxur and
azinphosmethyl to male Wistar rats revealed an additive toxic effect
of the combination of the two pesticides (Thyssen, 1977).
The LD50-value in male Wistar rats for an equitoxic mixture of
propoxur (purity 99.3%) and cyfluthrin (93.7%) proved to be less
than the value expected on the basis of simple addition of effects
(Flucke, 1984).
Special studies on embryotoxicity and teratogenicity
Rats
Groups of 24 pregnant female Wistar rats received 0, 3, 9 or 27
mg/kg bw/day propoxur (purity 99.4%) p.o. by gavage from day 6
through 15 of gestation. Appearance, behaviour, body weight and
food consumption were recorded daily. At day 21 of gestation all
animals were sacrificed and the fetuses were delivered by Cesarean
section. The number of implantations, resorptions (early and late)
and corpora lutea were determined. The fetuses were counted and
weighed; gross pathology and histopathology (skeletal and visceral)
were carried out. At 27 mg/kg bw, 3 animals died before the end of
the test. At 9 and 27 mg/kg bw, symptoms (increased grooming,
chewing motions, teeth grinding) were noted in the hours following
dose application during the entire treatment; at 27 mg/kg bw, in
addition, tremors and ventral recumbency were observed. At the same
dose levels, food consumption and growth of dams were decreased in a
dose related fashion. No other effects were seen. The NOAEL for
maternal toxicity in this study is 3 mg/kg bw/day (Becker et al.
1989a).
Rabbits
Groups of 15 Himalayan rabbits received 0, 1, 3 or 10 mg/kg
bw/day propoxur (purity 99.6%) p.o. from day 6 through 18 of
gestation. There were no indications of maternally toxic,
embryotoxic or teratogenic effects. However, the study was limited
with respect to soft tissues examination (Schlüter, 1981).
Groups of 16 pregnant Chinchilla rabbits received 0, 3, 10 or
30 mg/kg bw/day propoxur (purity 99.4%) p.o. by gavage from day 6
through 18 of gestation. Appearance, behaviour, body weight and
food consumption were recorded daily. At day 28 of gestation all
animals were sacrificed and the fetuses were delivered by Cesarean
section. The number of implantations, resorptions (early and late)
and corpora lutea were determined. The fetuses were counted and
weighed; gross pathology and histopathology (skeletal and visceral)
were carried out. At 30 mg/kg bw restless behaviour and dyspnoea
were observed after dose application on the first 3 treatment days;
in the same group 3 animals died before test end. In addition, body
weight loss (day 6-9 of gestation) occurred in this group. Also, at
30 mg/kg bw post-implantation loss was increased (number of pups per
dam decreased consequently). The NOAEL for maternal toxicity and
embryotoxicity in this study is 10 mg/kg bw/day (Becker et al.
1989b)
Special studies on mutagenicity
A large number of mutagenicity tests has been carried out with
propoxur. The results are summarized in Table 2 ( in vitro assays)
and Table 3 ( in vivo assays). In addition, in vitro tests in
prokaryotes have been performed with a number of metabolites of
propoxur. The results of these studies are summarized in Table 6.
Special studies on skin and eye irritation and sensitization
Undiluted propoxur (purity 99.2%) was tested for irritation to
shaven intact and shaven abraded skin areas of 6 New Zealand
rabbits. Exposure was for 24 or 72 hours. No irritation was observed
(Thyssen, 1978).
A dose of 0.5 g propoxur (purity 99.6%) moistened with purified
water, was applied under occlusive conditions to the shaven intact
back skin of 6 male New Zealand White rabbits for 4 hours. No skin
irritation was observed up to 72 hours after application (Yamane,
1986a).
In groups of 3 or 5 New Zealand rabbits an eye irritation study
was carried out with undiluted propoxur (purity 99.2%). Eyes were
rinsed after 5 minutes or 24 hours of exposure. The animals were
observed for 7 days. The only sign of irritation was slight erythema
of the conjunctivae of 2/3 animals that were exposed for 24 hours.
At 24 hours after rinsing this was no longer present (Thyssen,
1978).
Application of 0.1 g propoxur (purity 99.6%) into the eyes of 9
male New Zealand White rabbits caused severe miosis, which
disappeared within 24 hours after application. No irritation
effects were seen up to 96 hours post application (Yamane, 1986b).
Propoxur (purity 98.8%) did not exhibit a sensitizing effect in
the maximization test (Magnusson & Kligman) in guinea pigs (Heimann,
1982a).
Observations in humans
Dermal absorption
0.1 ml 14C-propoxur in acetone was applied to the ventral
forearm of 6 humans (sex not reported) (skin area 2.8-20 cm2;
applied amount 5 wµg propoxur/cm2). The skin sites were not
protected and subjects were asked not to wash the area for 24 hours.
Urine, collected for 5 days after beginning of exposure, was
monitored for 14C. The data were corrected for incomplete urinary
recovery using the 14C found in urine after administration of an
intravenous dose. A skin absorption of 19.6% of the dose was found
(Feldmann & Maibach, 1974).
TABLE 4. RESULTS OF IN VITRO MUTAGENICITY ASSAYS ON PROPOXUR
TEST SYSTEM TEST OBJECT CONCENTRATION PURITY RESULTS REFERENCE
Ames test * S. typhimurium 50 nmol/plate ca. 95% Negative Blevins et al. 1977b
TA98, TA100, TA1535
TA1537, TA1538
Ames test * S. typhimurium 0.1-1000 µg/pl 98.0% Negative Inukai & Iyatomi, 1978
TA98, TA100, TA1535 solvent DMSO (1)
TA1537, TA1538
Ames test * S. typhimurium 10-1500 µg/pl >96% Negative De Lorenzo et al. 1978
TA98, TA100, TA1535 (1)
TA1537, TA1538
Ames test S. typhimurium 0.25-100 µg/ml 97% Negative Jaszczuk et al. 1979
TA98, TA100, TA1535
TA1537, TA1538
Ames test * S. typhimurium 20-12500 µg/pl 98.6% Negative Herbold, 1982
TA98, TA100, TA1535 (1)
TA1537, TA1538
Reversion assay * Saccharomyces 75-10000 µg/ml 99.8% Negative Herbold, 1985e
cerevisiae D7 solvent DMSO (1)
Reverse mutation test E. coli WP2 hcr. 20 µl/disk ? Negative Shirasu et al. 1976
B/r try WP2
Reverse mutation E. coli WP2 hcr. 10-5000 µg/pl 98.05 Negative Shirasu et al. 1979
test * S. typh. (1)
TA98, TA100, TA1535
TA1537, TA1538
TABLE 4 (CONTD)
TEST SYSTEM TEST OBJECT CONCENTRATION PURITY RESULTS REFERENCE
Reverse mutation E. coli WP2 hcr. 500-25000 µg/pl 98.0% Negative Ohta & Moriya, 1983
test * S. typh. (1)
TA98, TA100, TA1535
TA1537, TA1538
HGPRT-test * Chinese hamster 25-125 µg/ml 99.6% Negative Lehn, 1988
ovary (CHO) cells (without S9 mix) (1)
600-1500 µg/ml (with S9 mix)
Mitotic gene Saccharomyces 2 ml of suspension 99.8% Negative Siebert & Lemperle, 1974;
conversion test cerevisiae D4 (containing 1000 ppm Siebert & Eisenbrand, 1974
a.i.) at 5 x 10 cells;
solvent DMSO
Pol Al-test * E. coli pol A+ 62.5-10000 µg/pl 98.5% Negative Herbold, 1983a
E. coli pol A- solvent DMSO (1)
Rec-assay Bacillus subtilis 3-300 µg/disk 98.0% Negative Inukai & Iyatomi, 1978
NIG17, NIG45 solvent DMSO (1)
Rec-assay Bacillus subtilis 20 µg/disk ? Negative Shirasu et al. 1976
H17 Rec+, M45 Rec- solvent DMSO
Rec-assay Bacillus subtilis 20-2000 µg/disk 98.0% Negative Shirasu ET AL. 1976
H17 Rec+, M45 Rec- (1)
Rec-assay Bacillus subtilis 50-10000 µg/disk 98.0% Negative Ohta & Moriya, 1983
H17 Rec+, M45 Rec- (1)
TABLE 4 (CONTD)
TEST SYSTEM TEST OBJECT CONCENTRATION PURITY RESULTS REFERENCE
Sister chromatid Human lymphocytes without S9 mix: 99.6% Negative Herbold, 1985d
exchange assay * 125-500 µg/ml (1)
with S9 mix:
250-1000 µg/ml
solvent DMSO
Single-strand Human fibroblasts 10-5 M approx Negative Blevins et al. 1977a
break assay 95%
Chromosome aberr. Chinese hamster without S9 mix. 97.8% Negative Putman & Morris, 1988
assay ovary (CHO) cells 157-625 µg/ml (1)
with S9 mix:
615 and 1250 µg/ml
solvent DMSO
* Test was carried out both with and without metabolic activation.
(1) Positive control yielded positive results.
TABLE 5. RESULTS OF IN VIVO MUTAGENICITY ASSAYS ON PROPOXUR
TEST SYSTEM TEST OBJECT CONCENTRATION PURITY RESULTS REFERENCE
DNA metabolism Male rat spleen cells 10 mg/kg bw; p.o. ? Negative Klein, 1984
studies (1)
Sister chromatid Chinese hamster bone 75 or 150 mg/kg bw 99.6% Negative Herbold, 1985c
exchange assay marrow cells p.o. (1)
Cytogenic study Chinese hamster 2 x 75 mg/kg bw 99.6% Negative Herbold, 1986
spermatogonia 2 x 150 mg/kg bw (1)
Cytogenic study Chinese hamster bone 75-300 mg/kg bw; 99.6% Negative Herbold, 1988
marrow cells p.o. (1)
Micronucleus test Male and female ICR- 25 mg/kg bw + 25 ? Negative Seiler, 1977
mice bone marrow cells mg/kg bw NaNO2; p.o.
Micronucleus test Male and female NMRI- 2 x 5 mg/kg bw; 99.2% Negative Herbold, 1980b
mice bone marrow cells 2 x 10 mg/kg bw; p.o. (1)
Dominant lethal Male mice 5 x 25 mg/kg bw ? Positive Tyrkiel, 1977
test 5 x 50 mg/kg (#) (1)
Dominant lethal Male mice 10 mg/kg bw; p.o. 99.2% Negative Herbold, 1980a
(#) Herbold (1978), in a critical review, points out the equivocality of the results, thus showing the questionable
validity of the author's conclusion.
(1) Postive control yielded positive results.
TABLE 6. RESULTS OF MUTAGENICITY ASSAYS ON PROPOXUR METABOLITES
TEST SYSTEM TEST OBJECT CONCENTRATION RESULTS REFERENCE
M1
Ames test * S. typhimurium 20 and 12500 µg/plate Negative Herbold, 1983c
TA98, TA100,
TA1535, TA1537
Poly A1- test *1 E. coli pol A+ 625-6075 µg/plate Negative Herbold, 1984b
E. coli pol A1-
M2
Ames test * S. typhimurium 20-12500 µg/plate Negative Herbold, 1983b
TA98, TA100
TA1535, TA1537
Mitotic recombination Saccharomyces 185.9-30000 µg/plate Negative Herbold, 1984e
assay * cerevisiae D7
M3
Ames test * S. typhimurium 312.5-5000 µg/plate Negative Herbold, 1984c
TA98. TA100, TA1535
TA1537. TA1538
DNA metabolism studies Male rat spleen 10 mg/kg bw Negative Klein, 1984
cells (1)
M4
Ames test * S. typhimurium 312.5-5000 µg/plate Negative Herbold, 1984a
TA98, TA100, TA1535
TA1537, TA1538
DNA metabolism studies Male rat spleen cells 10 mg/kg bw Negative Klein, 1984
TABLE 6 (CONTD)
TEST SYSTEM TEST OBJECT CONCENTRATION RESULTS REFERENCE
M5
Ames test * S. typhimurium 8-8748 µg/plate Negative Herbold, 1984d
TA98, TA100, TA1535
TA1537, TA1538
DNA metabolism studies Male rat spleen cells 10 mg/kg bw Negative Klein, 1984
(1)
M7
Not evaluable in Ames test due to breakdown in test medium
M8
Ames test * S. typhimurium evaluated range: Negative Herbold, 1984f
TA98, TA100 8-1800 µg/plate
TA1535, TA1537
Propoxur urine (rat, 8000 ppm in feed)
Ames test S. typhimurium 767 µl/plate Negative Herbold, 1985b
TA98, TA100
TA1535, TA1537
Propoxur urine extract (rat, 8000 in feed)
Ames test S. typhimurium evaluated range (2) Herbold, 1985a
TA98, TA100 14.5-29 µl/plate
TA1535, TA1537
* Test was carried out both with and without metabolic activation.
(1) Suppression of semi-conservative DNA synthesis was seen.
(2) Test outline and result inadequately reported.
Baygon spray (containing 2.0% propoxur and 0.5% dichlorvos) was
sprayed on the upper arm of 4 humans (3 males, 1 female) 6 times (1
second per spraying) at intervals of 10 minutes. The amount of
propoxur applied was 41 mg per person. Dermal absorption was
assessed via measurement of cholinesterase activity in plasma and
erythrocytes and of blood propoxur concentrations up to 6 hours
after treatment began. Urine samples, collected for 24 or 48 hours,
were monitored for the concentration of propoxur metabolite
isopropoxyphenol. No skin penetration of propoxur were found. The
identical procedure was used in a study in which 500 mg Baygon Dust
(1% w/w propoxur) was applied for 2 hours under an occlusive pad to
the abraded skin of the upper arm of 4 male subjects (skin sites
were abraded through application of an adhesive plaster and removal
of it, 30 minutes later). There were no signs indicating skin
penetration by propoxur (Eben & Kimmerle, 1974b).
Cholinesterase inhibition
Four human subjects (3 male and 1 female) were exposed by
inhalation to an aerosol containing 3 mg/m3 propoxur (purity 100%)
for 4 hours. The concentration of propoxur in the blood and
cholinesterase activity in plasma were determined up to 120 minutes
after treatment. Urine was monitored for the propoxur metabolite 2-
isopropoxyphenol up to 72 hours after exposure. In blood propoxur
was not found and cholinesterase activity was not depressed. 2-
Isopropoxyphenol was present in urine; the observed concentration
had decreased to trace level after 24 hours (and the compound was
absent thereafter), indicating excretion within this interval
(Kimmerle & Eben 1978b).
COMMENTS
After oral administration to rats the compound is rapidly
excreted, almost exclusively via the urine; only small quantities
are found in the feces. In urine the compound is excreted unchanged
or as one of a large number of metabolites which are present as free
compounds or as glucuronide or sulfate conjugates. The
biotransformation pathways in all species studied comprise
depropoxylation, hydrolysis of the ester bond and N-demethylation.
Ring hydroxylation also occurs: in rodents at ring positions 3, 4
and 5, and in primates at the 4- and 5- positions only. The
biotransformation pathway in humans is the same as in the Rhesus
monkey.
Propoxur induces drug metabolizing enzymes in the liver of
rats. This effect was greater with Altromin diet than with a semi-
synthetic diet.
The compound showed high acute oral toxicity in the species
examined.
Short-term administration of propoxur to rats (gavage, 5 days)
and dogs (dietary, 52 weeks) revealed cholinergic signs, growth
retardation and inhibition of cholinesterase activity in blood and
brain as the main toxicological effects. In the dog an increase of
microsomal enzyme activity was also observed. The NOAEL in the dog
study was 200 ppm (equivalent to 300 mg/kg bw/day).
In a long-term feeding study in mice there was significant
inhibition of growth at the 6000 ppm level. The NOAEL was 2000 ppm
(equivalent to 300 mg/kg bw/day).
In long-term feeding studies in Wistar rats, growth
retardation, ChE inhibition and urinary bladder alterations were the
main effects observed. Hyperplastic and neoplastic changes, which
were dependent on the diet, were found in the bladder of rats. The
hyperplasia could be reduced by the administration of ammonium
chloride in the predisposing diet, presumably via its effect on
urinary pH. These changes were not seen in mice, hamsters, dogs or
Rhesus monkeys.
The NOAEL in rats for the formation of hyperplasia of the
urinary bladder epithelium was 200 ppm (equal to 10 mg/kg bw/day).
This is also the NOAEL for AChE inhibition.
Increased post-implantation losses were observed in a
teratogenicity study in rabbits at 30 mg/kg bw/day. The NOAEL was
10 mg/kg bw/day. No embryotoxicity was observed at the highest dose
tested in rats (27 mg/kg bw/day). No teratogenic effects were
observed in either species.
After reviewing all available in vitro and in vivo short-
term tests, the Meeting concluded that there was no evidence of
genotoxicity.
Based on a re-evaluation of the human data available from the
1973 JMPR, a single oral dose of 0.2 mg/kg bw could be considered as
a NOAEL for humans since the depression of erythrocyte
cholinesterase did not exceed 20% and the recovery was very rapid.
These data have been reviewed by a WHO Expert Committee (WHO, 1973).
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse 2000 ppm in the diet, equivalent to 300 mg/kg bw/day
(ChE activity not measured)
Rat: 200 ppm in the diet, equal to 10 mg/kg bw/day
Dog: 200 ppm in the diet, equivalent to 5 mg/kg bw/day
Human 0.1 mg/kg bw
Estimate of acceptable daily intake for man
0-0.02 mg /kg bw
Studies which will provide information valuable in the continued
evaluation of the compound
Further observations in humans.
REFERENCES
Abd-Elraof, T.K., Dauterman, W.C. & Mailman, R.B. (1981) In vivo
metabolism and excretion of propoxur and malathion in the rat:
Effect of lead treatment. Toxicol. Appl. Pharmacol., 59: 324-330.
Ahdaya, S.M., Monroe, R.J. & Guthrie, F.E. (1981) Absorption and
distribution of intubated insecticides in fasted mice. Pesticide
Biochem. and Physiol. 16: 38-46.
Becker, H., Mladenovic, P. & Terrier, Ch. ( 1989a) Embryotoxicity
study (including teratogenicity) with BOQ 5812315 (c.n. propoxur) in
the rat. Unpublished Report No. R 4686 (project 207270) dated March
1, 1989 from RCC-Research & Consulting Company AG, Itingen,
Switzerland. Submitted to WHO by Bayer AG, Leverkusen, Federal
Republic of Germany.
Becker, H., Mladenovic, P. & Terrier, Ch. (1989b) Embryotoxicity
study (including teratogenicity) with BOQ 5812315 (c.n. propoxur) in
the rabbit. Unpublished Report No. R 4684 (Project 207292) dated
March 1, 1989 from RCC-Research & Consulting Company AG, Itingen,
Switzerland. Submitted to WHO by Bayer AG, Leverkusen, Federal
Republic of Germany.
Bell, R.L. & Gronberg, R.R. (1975) The metabolic fate of BAYGON in
the lactating dairy cow. Unpublished Report No. 44771 dated July 18,
1975 from from Chemagro Agricultural Division - Mobay Chemical
Corporation. Submitted to WHO by Bayer A.G., Leverkusen, Federal
Republic of Germany.
Blevins, R.D., Lijnski, W. & Regan, J.D. (1977a) Nitrosated
methylcarbamate insecticides: effect on the DNA of human cells.
Mut. Res. 44: 1-7.
Blevins, R.D., Lee, M. & Regan, J.D. (1977b) Mutagenicity screening
of five methyl carbamate insecticides and their nitroso derivatives
using mutants of Salmonella typhimurium LT2, Mutat. Res. 56: 1-
6.
Bomhard, E. & Löser, E. (1981) Bö 58 12 315 Chronic toxicological
study on mice (Feeding study over two years). Unpublished Report No.
9954 dated May 12, 1981 from Bayer A.G. Institute of Toxicology,
Wuppertal-Elberfeld. Submitted to WHO by Bayer A.G., Leverkusen,
Federal Republic of Germany.
Costa, L.G., Hand, H., Schwab, Bw & Murphy, S.D. (1981) Tolerance to
the carbamate insecticide propoxur. Toxicology, 21: 267-278.
De Lorenzo, F., Staiano, N., Silengo, L. & Cortese, R. (1978)
Mutagenicity of diallate, sulfallate, triallate and relationship
between structure and mutagenic effects of carbamates used widely in
agriculture. Cancer Res., 38: 13-15.
Eben, A., Karl, W. & Machemer, L. (1984) Studies on the
biotransformation of propoxur in the rat. Unpublished Report No.
12866 (KWN 15) dated August 17, 1984 from Bayer AG Institut für
Toxikologie, Wuppertal-Elberfeld. Submitted to WHO by Bayer AG,
Leverkusen, Federal Republic of Germany.
Eben, A., Karl, W. & Machemer, L. (1985a) Studies on
biotransformation of propoxur in humans. Unpublished Report No.
13947 (KWN 26) dated October 24, 1985 from Bayer AG Institut für
Toxikologie, Wuppertal-Elberfeld. Submitted to WHO by Bayer AG,
Leverkusen, Federal Republic of Germany.
Eben, A., Karl, W. & Machemer, L. (1985b) Supplementary studies on
biotransformation of propoxur in the rat. Unpublished Report no.
14148 dated December 13, 1985 from Bayer AG Institut für
Toxikologie, Wuppertal-Elberfeld. Submitted to WHO by Bayer AG.
Leverkusen, Federal Republic of Germany.
Eben, A., Karl, W. Machemer, L. (1986a) The biotransformation of
propoxur in golden hamsters. Unpublished Report No. 14690 (KWN 37)
dated June 9, 1986 from Bayer AG Institute of Toxicology, Wuppertal-
Elberfeld. Submitted to WHO by Bayer AG, Leverkusen, Federal
Republic of Germany.
Eben, A., Karl. W. & Machemer, L. (1986b) Propoxur [The active
ingredient of RBaygon] Biotransformation study on monkeys.
Unpublished Report No. 15056 (KWN 40) dated September 11, 1986 from
Bayer AG Fachbereich Toxikologie, Wuppertal-Elberfeld. Submitted to
WHO by Bayer AG, Leverkusen, Federal Republic of Germany.
Eben, A., Karl, W. & Machemer, L. (1987) Investigation on the
biotransformation of propoxur in mice. Unpublished Report No. 15697
(KWN 46) dated April 3, 1987 from Bayer AG Fachbereich Toxikologie
Landwirtschaft ZF-Zentrale Analytik Structure Research, Wuppertal.
Submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Eben, A. & Kimmerle, G. (1974b) 1% Baygon Dust. Resorption study on
skin of test volunteers. Unpublished Report No. 4461 dated February
5, 1974 from Bayer AG-Institut für Toxikologie, Wuppertal-Elberfeld.
Submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany. Test volunteers. Unpublished Report No. 4462 dated February
8, 1974 from Bayer AG Institut für Toxikologie, Wuppertal-Elberfeld.
Submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Eigenberg, D.A. (1988) Dermal absorption of propoxur technical in
rat using 14C-propoxur. Unpublished report No. 1097 (study number
88-721-AT) dated December 28, 1988 from Mobay Corporation, Health,
Environment and Safety, Corporate Toxicology Department, Stilwell,
Kansas, USA. Submitted to WHO by Bayer AG, Leverkusen, Federal
Republic of Germany.
Feldmann, R.J. & Maibach, H.I. (1974) Percutaneous penetration of
some pesticides and herbicides in man. Toxicol. Appl. Pharmacol.
28: 126-132.
Flucke, W. (1980) Boe 5812315 (propoxur). Acute toxicity studies.
Unpublished Report No. 9295 dated July 8, 1980 from Bayer AG
Institut für Toxikologie, Wuppertal-Elberfeld. Submitted to WHO by
Bayer AG, Leverkusen, Federal Republic of Germany.
Flucke, W. (1984) FCR (cyfluthrin) BOQ (propoxur) Study for
combination toxicity. Unpublished Report No. 12544 dated March 14,
1984 from Bayer AG Institute of Toxikology, Wuppertal-Elberfeld.
Submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Glaister, J.R. (1984) BOQ 5812315: 2 year carcinogenicity/chronic
toxicity study in the rat. Histopathology Report. Hazleton
Laboratories Harrogate, June 1984. Data submitted to WHO by Bayer AG
Leverkusen, Federal Republic of Germany.
Hahnemann, S. & Rühl-Fehlert, C. (1988a) BOQ 5812315 (common name:
propoxur). Chronic feeding test on Syrian gold hamsters (species
sensitivity). Unpublished Report No. 16801 dated June 15, 1988 from
Bayer AG Toxicology Division, Wuppertal (study no. T 0018434).
Submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Hahnemann, S. & Rühl-Fehlert, C. (1988b) BOQ 5812315 (common name:
propoxur). Chronic feeding test on Sprague-Dawley rats (strain
sensitivity). Unpublished report from Bayer AG Toxicology Division,
Wuppertal (study no. T 1018435). Submitted to WHO by Bayer AG,
Leverkusen, Federal Republic of Germany.
Hahnemann, S. & Rühl-Fehlert, C. (1988c) BOQ 5812315 (common name:
propoxur). Chronic feeding test on NMRI mice (species sensitivity).
Unpublished report no. 16803 dated June 15, 1988 from Bayer AG
Toxicology Division, Wuppertal (study no. T 9018433). Submitted to
WHO by Bayer AG, Leverkusen, Federal Republic of Germany.
Hahnemann, S. & Rühl-Fehlert, C. (1988d) BOQ 5812315 (common name:
propoxur). Sub-chronic feeding test on female Wistar rats (effect of
feed quality). Unpublished Report No. 16897 dated July 13, 1988 from
Bayer AG Toxicology Division, Wuppertal (study no. T 5019041).
Submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Hahnemann, S. & Rühl-Fehlert, C. (1988e) BOQ 5812315 (common name:
propoxur). Chronic feeding test on female Wistar rats with added 1%
L-(+)ascorbic acid. Unpublished Report No. 16979 dated August 2,
1988 from Bayer AG Toxicology Division, Wuppertal (study no. T
8018432). Submitted to WHO by Bayer AG, Leverkusen, Federal Republic
of Germany.
Hahnemann, S. & Rühl-Fehlert, C. (1988f) BOQ 5812315 (common name:
propoxur). Chronic feeding test on female Wistar rats over 2 years
(dose-effect-time relationship). Unpublished Report No. 16980 dated
August 2, 1988 from Bayer AG Toxicology Division, Wuppertal (study
no. T 6018430). Submitted to WHO by Bayer AG, Leverkusen, Federal
Republic of Germany.
Hahnemann, S. & Rühl-Fehlert, C. (1988g) BOQ 5812315 (common name:
propoxur). Chronic feeding study on female rats (Effect of feed and
drinking water type). Unpublished Report No. 17146 dated September
13, 1988 from Bayer AG Fachbereich Toxikologie, Wuppertal. Submitted
to WHO by Bayer AG, Leverkusen, Federal Republic of Germany.
Haley, T.J., Farmer, J.H., Dooley, K.L., Harmon, J.R. & Peoples, A.
(1974) Determination of the LD01 and extrapolation of the LD001 for
five methylcarbamate pesticides. J. Européen de Toxicologie, 7(3):
152-158.
Heimann, K.G. (1982a) Propoxur (the active ingredient of RBaygon
and UndenR). Study of sensitization effect on guinea pigs.
Unpublished Report No. 11218 dated October 15, 1982 from Bayer AG
Institut für Toxikologie, Wuppertal-Elberfeld. Submitted to WHO by
Bayer AG, Leverkusen, Federal Republic of Germany.
Heimann, K.G. (1982b) Carbamate UN, technical. Study for acute
toxicity on rat. Unpublished Report No. 11329 dated December 15,
1982 from Bayer AG Institute of Toxicology, Wuppertal-Elberfeld.
Submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Heimann, K.G. (1982c). Carbamate UN, Technical product: acute study
of the effect on the activity of the cholinesterases in blood
plasma, erythrocytes and brains of rats compared with carbamate UN
recrystallised product. Unpublished Report No. 11330 dated December
15, 1982 from Bayer AG Institute of Toxicology, Wuppertal-Elberfeld.
Submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Heimann, K.G. (1983) Carbamate UN, technical (c.n. propoxur, Unden
active ingredient; Baygon active ingredient) Subacute study on rats
compared with carbamate UN, recrystallised. Unpublished Report No.
11621 dated March 8, 1983 from Bayer AG Institute of Toxicology
Wuppertal-Elberfeld. Submitted to WHO by Bayer AG, Leverkusen,
Federal Republic of Germany.
Herbold, B. (1980a) BOE 5812315, dominant lethal study on male mouse
to test for mutagenic effects. Unpublished Report No. 8808 dated
January 7, 1980 from Bayer AG, Institute of Toxicology, Wuppertal-
Elberfeld. Submitted to WHO by Bayer AG, Leverkusen, Federal
Republic of Germany.
Herbold, B. (1980b) BOE 5812315, microncucleus test on mouse to
evaluate BOE 5812315 for mutagenic potential. Unpublished Report No.
9274 dated June 27, 1980 from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld. Submitted to WHO by Bayer AG, Leverkusen,
Federal Republic of Germany.
Herbold, B. (1982) Carbamate UN technical, Salmonella/microsome
test to evaluate for point mutation. Unpublished report no. 11301
dated December 6, 1982 from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld. Submitted to WHO by Bayer AG, Leverkusen,
Federal Republic of Germany.
Herbold, B. (1983a) Carbamate UN technical - Pol A1- test on E.
coli for potential DNA damage. Unpublished Report No. 11403 dated
January 6, 1983 from Bayer AG, Institute of Toxicology, Wuppertal-
Elberfeld. Submitted to WHO by Bayer AG, Leverkusen, Federal
Republic of Germany.
Herbold, B. (1983b) Isopropoxyphenol, Salmonella/microsome test to
evaluate for point mutation. Unpublished Report No. 12321 dated
December 20, 1983 from Bayer AG, Institute of Toxicology, Wuppertal-
Elberfeld. Submitted to WHO by Bayer AG, Leverkusen, Federal
Republic of Germany.
Herbold, B. (1983c) Brenzcatechin, Salmonella/microsome test to
evaluate for potential point mutation. Unpublished Report No. 12322
dated December 20, 1983 from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld. Data submitted to WHO by Bayer AG, Leverkusen,
Federal Republic of Germany.
Herbold, B. (1984a) THS 1240, Salmonella/microsome test to
evaluate for potential point mutation. Unpublished Report No. 12483
dated February 24, 1984 from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld. Submitted to WHO by Bayer AG, Leverkusen,
Federal Republic of Germany.
Herbold, B. (1984b) Brenzcatechin, POL-test of E. coli to evaluate
for potential DNA damage. Unpublished Report No. 12497 dated
February 29, 1984 from Bayer AG, Wuppertal-Elberfeld. Submitted to
WHO by Bayer AG, Leverkusen, Federal Republic of Germany.
Herbold, B. (1984c) THS 2490, Salmonella/microsome test to
evaluate for point mutation. Unpublished Report No. 12529 dated
March 6, 1984 from Bayer AG, Institute of Toxicology, Wuppertal-
Elberfeld. Submitted to WHO by Bayer AG, Leverkusen, Federal
Republic of Germany.
Herbold, B. (1984d) THS 1241b, Salmonella/microsome test to
evaluate for potential point mutation. Unpublished Report No. 12795
dated July 9, 1984 from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld. Submitted to WHO by Bayer AG, Leverkusen,
Federal Republic of Germany.
Herbold, B. (1984e). Isopropoxyphenol, test on S.cerevisiae D7 for
the induction of mitotic recombination. Unpublished Report No. 12876
dated August 20, 1984 from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld. Submitted to WHO by Bayer AG, Leverkusen,
Federal Republic of Germany.
Herbold, B. (1984f) THS 2647, Salmonella/microsome test to
evaluate for potential point mutation. Unpublished Report No. 12996
dated October 24, 1984 from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld. Submitted to WHO by Bayer AG, Leverkusen,
Federal Republic of Germany.
Herbold, B. (1985a) Propoxur urine extract compared with control
urine extract, Salmonella microsome test to evaluate for potential
point mutation. Unpublished Report No. 13350 dated March 14, 1985
from Bayer AG, Institute of Toxicology, Wuppertal-Elberfeld.
Submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Herbold, B. (1985b) Propoxur urine, Salmonella/microsome test to
evaluate for potential point mutation. Unpublished Report No. 13395
dated March 27, 1985 from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld. Submitted to WHO by Bayer AG, Leverkusen,
Federal Republic of Germany.
Herbold, B. (1985c) Propoxur (BOQ 5812315), sister chromatid
exchange in the bone marrow of the Chinese Hamster in vivo to
evaluate for harmful effect on DNA. Unpublished Report No. 13501
dated May 22, 1985 from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld. Submitted to WHO by Bayer AG, Leverkusen,
Federal Republic of Germany.
Herbold, B. (1985d) BOQ 5812315, sister chromatid exchange in human
lymphocyte cultures in vitro to test for DNA-modifying effects.
Unpublished Report No. 13871 dated October 9, 1985 from Bayer AG,
Institute of Toxicology, Wuppertal-Elberfeld. Submitted to WHO by
Bayer AG, Leverkusen, Federal Republic of Germany.
Herbold, B. (1985e) BOQ 5812315, test on S.cerevisiae D7 to
evaluate for point mutagenic effect. Unpublished Report No. 13966
dated October 30, 1985 from Bayer AG, Institute of Toxicology,
Wuppertal-Elberfeld. Submitted to WHO by Bayer AG, Leverkusen,
Federal Republic of Germany.
Herbold, B. (1986) BOQ 5812315 (c.n. propoxur), cytogenetic study of
chromosome damage using spermatogonia of Chinese Hamsters in vivo.
Unpublished Report No. 14984 dated August 20, 1986 from Bayer AG,
Institute of Toxicology, Wuppertal-Elberfeld. Submitted to WHO by
Bayer AG, Leverkusen, Federal Republic of Germany.
Herbold, B. (1988) BOQ 5812315 (c.n. propoxur), cytogenetic study on
bone marrow of Chinese Hamster in vivo to detect chromosomal
damage. Unpublished Report No. 17111 dated September 6, 1988 from
Bayer AG Institute of Toxicology, Wuppertal-Elberfeld. Submitted to
WHO by Bayer AG, Leverkusen, Federal Republic of Germany.
Hoffmann, K. & Gröning, P. (1984) BOQ 5812315 (BOE 5812315, c.n.
propoxur) Chronic toxicity to dogs on oral administration (12-months
feeding study). Unpublished Report No. 12605 dated April 11, 1984
from Bayer AG Institute of Toxicology, Wuppertal-Elberfeld.
Submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Hoffmann, K. & Ruehl, Chr. (1985) Propoxur (BOQ 5812315). Subchronic
study of toxicity to Rhesus monkeys after oral administration by
stomach tube for 13 weeks to check for possible findings in the
urinary bladder. Unpublished Report No. 13779 dated August 27, 1985
from Bayer AG Institute of Toxicology, Wuppertal-Elberfeld.
Submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Inukai, H. & Iyatomi, A. (1978) Propoxur. Mutagenicity test on
bacterial systems. Unpublished Report No. 103 dated February 24,
1978 from Nitokuno Agricultural Chemicals Institute, Toyoda, Japan.
Submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Jaszcuk, E., Syrowatka, T. & Cybulski, J. (1979) Mutagenic activity
of propoxur, carbaryl and their nitroso derivatives: induction of
reversion in Salmonella typhimurium. Roczniki Panstwowzgo Zakladu
Higieny Warszawa, 30(1): 81-88. (English summary only).
Jurek, A. (1978) Chronische Langzeittoxizität des
Propoxurkarbaminats, Roczniki Panstwowzgo Zakladu Higieny Warszawa,
29(3): 327-338.
Karl, W. (1986) Biotransformation of propoxur: quantitative
determination of the metabolic pattern in rats given a single dose
of 14C-propoxur after a subchronic prefeeding period in two diet
groups and three dose groups. Unpublished Report No. KWN 42 dated
October 15, 1986 from Bayer AG Institute of Toxicology, Wuppertal-
Elberfeld. Submitted to WHO by Bayer Leverkusen, Federal Republic of
Germany.
Karl, W. & Schneider, J. (1987) Isolation and spectroscopic
structure elucidation of the renal metabolite conjugates of
propoxur. Unpublished Report No. KWN 44 dated August 26, 1987 from
Bayer AG Institute of Toxicology, Wuppertal-Elberfeld. Submitted to
WHO by Bayer AG, Federal Republic of Germany.
Kimmerle, G. & Eben, A. (1978a) Propoxur - Acute inhalation study on
rats with determination of acetylcholinesterase activity in blood
and elimination of 2-isopropoxyphenol in urine. Unpublished Report
No. 7555 dated May 1978 from Bayer AG Institute für Toxikologie,
Wuppertal-Elberfeld. Submitted to WHO by Bayer AG Leverkusen,
Federal Republic of Germany.
Kimmerle, G. & Eben, A. (1978b) Propoxur - Single exposure of
persons to propoxur with determination of acetylcholinesterase
activity in plasma and erythrocytes, propoxur concentration in blood
and elimination of 2-isopropoxyphenol in urine. Unpublished Report
No. 7554 dated May, 1978 from Bayer AG, Institut für Toxikologie,
Wuppertal-Elberfeld. Submitted to WHO by Bayer AG Leverkusen,
Federal Republic of Germany.
Kimmerle, G. & Iyatomi, A. (1976) Toxicity of propoxur to rats by
subacute inhalation. Jap. J. Ind. Health, 18: 375-382.
Klein, W. (1984) Effect of an active ingredient and three
metabolites on the DNA metabolism. Unpublished Report R3346 dated
March 1984 from Osterreichisches Forschungszentrum Seiberdorf
Biochemistry Department/Toxicology Department. (Research commisioned
by Bayer AG). Submitted to WHO by Bayer AG Leverkusen, Federal
Republic of Germany.
Krechniak, J. & Foss, W. (1982) Cholinesterase activity in rats
treated with propoxur. Bull. Environm. Contam. Toxicol., 29: 599-
604.
Krechniak, J. & Foss, W. (1983a) Behaviour of propoxur after
repeated administration. Bromat. Chem. Toksykol., 16 (3-4): 205-
208. (English translation).
Krechniak, J. & Foss, W. (1983b) Distribution and excretion of
isopropoxyphenol in rats. Bromat. Chem. Toksykol., 6 (3-4): 209-
211 (English translation).
Lehn, H. (1988) BOQ 5812315 (c.n. propoxur), mutagenicity study for
the detection of induced forward mutations in the CHO-HGPRT assay
in vitro. Unpublished Report No. 17090 dated August 31, 1988 from
Bayer AG, Fachbereich Toxikologie, Wuppertal. Data submitted to WHO
by Bayer AG, Leverkusen, Federal Republic of Germany.
Luckhaus, G. (1984) Propoxur, two-year oral toxicity study in rats,
addendum to HRC Report No. 2809/69/235, histological follow-up
investigation of the urinary bladder. Unpublished Report No. 13012
dated November 2, 1984 from Bayer AG, Institute of Toxicology,
Wuppertal. Data submitted to WHO by Bayer AG, Leverkusen, Federal
Republic of Germany.
Machemer, L. & Schmidt, U. (1988) Propoxur. Status of the studies
and assessment regarding oncogenic potential to the urinary bladder.
Unpublished report dated September 30, 1988 from Bayer AG
Fachbereich Toxikologie, Institut für Toxikologie Landwirtschaft,
Wuppertal-Elberfeld. Data submitted to WHO by Bayer AG, Leverkusen,
Federal Republic of Germany.
Mihail, F. (1982) BOQ 5812315. Test for induction of the microsomal
liver enzymes. Unpublished Report No. 10976 dated June 29, 1982 from
Bayer AG, Institute of Toxicology, Wuppertal-Elberfeld. Data
submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Ohta, T. & Moriya, M. (1983) Propoxur, microbial mutagenicity study.
Unpublished Report from Institute of Environmental Toxicology
(Japan) dated February 28, 1983. Data submitted to WHO by Bayer AG,
Leverkusen, Federal Republic of Germany.
Pauluhn, J. (1988) BOQ 5812315 (common name: propoxur) study of the
inhalation toxicity in accordance with OECD Guideline No. 403.
Unpublished Report No. 16966 dated July, 1988 from Bayer AG
Fachbereich Toxikolgie, Wuppertal. Submitted to WHO by Bayer AG,
Leverkusen, Federal Republic of Germany.
Pauluhn, J. & Rühl, C. (1985) BOQ 5812315, study for subacute
inhalation toxicity to the rat. Unpublished Report No. 13297 dated
February, 20, 1985 from Bayer AG, Institute of Toxicology Wuppertal-
Elberfeld. Data submitted to WHO by Bayer AG, Leverkusen, Federal
Republic of Germany.
Putman, D.L. & Morris, M.J. (1988) Chromosome aberrations in Chinese
Hamster Ovary (CHO) cells, Baygon technical. Unpublished Report No.
1094 dated December 22, 1988 from Microbiological Associates Inc.
Maryland, USA. Submitted to WHO by Bayer AG, Leverkusen, Federal
Republic of Germany.
Reid Patterson, D. (1980) Histopathology report on Boe 5812315 mouse
study. Unpublished Report No. 2317-262/13 dated May, 1980 from
Hazleton Laboratories Europe, Harrogate, England to Bayer AG
Institut für Toxikologie, Wuppertal. Submitted to WHO by Bayer AG,
Leverkusen, Federal Republic of Germany.
Schlüter, G. (1981) BOQ 5812315. Evaluation for embryotoxic and
teratogenic effects after oral administration to the rabbit.
Unpublished Report No. 10183 dated September 9, 1981 from Bayer AG,
Institute of Toxikologie, Wuppertal-Elberfeld. Data submitted to WHO
by Bayer AG, Leverkusen, Federal Republic of Germany.
Schmidt, U. (1987) Investigations of interspecies differences in
primary metabolism with liver-cell fractions from rat, mouse,
hamster, monkey and man. Unpublished Report No. 16237 dated November
19, 1987 from Bayer AG, Fachbereich Toxikologie, Wuppertal.
Submitted to WHO by etc.
Seiler, J.P. (1977) Nitrosation in vitro and in vivo by sodium
nitrite, and mutagenicity of nitrogenous pesticides. Mutat. Res.
48: 225-236.
Shirasu, Y., Moriya, M., Kato, K., Furuhashi, A. & Kada, T. (1976)
Mutagenicity screening of pesticides in the microbial system.
Mutat. Res. 40: 19-30.
Shirasu, Y., Moriya, M. & Sugiyama, F. (1979) Propoxur, mutagenicity
test on bacterial systems. Unpublished Report from Institute of
Environmental Toxikologie (Japan) dated August 28, 1979. Data
submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Siebert, D. & Eisenbrand, G. (1974) Induction of mitotic gene
conversion in Saccharomyces cerevisiae. Mutat. Res. 22: 121-126.
Siebert, D. & Lemperle, E. (1974) Genetic effects of herbicides:
induction of mitotic gene conversion in Saccharomyces cerevisiae.
Mutat. Res. 22: 111-120.
Suberg, H. & Löser, E. (1984) BOQ 5812315, chronic toxicological
study with rats (feeding study over 106 weeks). Unpublished Report
No. 12870 dated August 20, 1984 from Bayer AG, Institute of
Toxicology, Wuppertal. Data submitted to WHO by Bayer AG,
Leverkusen, Federal Republic of Germany.
Thyssen, J. (1977) Study for combination toxicity of azinphos-methyl
and propoxur. Unpublished Report No. 7174 dated December 14, 1977
from Bayer AG, Institute of Toxicology, Wuppertal-Elberfeld. Data
submitted to WHO by Bayer AG, Leverkusen, Federal Republic of
Germany.
Thyssen, J. (1978) Propoxur-Untersuchungen an der Haut und am Auge
von Kaninchen. Unpublished report dated September 19, 1978 from
Bayer AG, Institut für Toxikologie, Wuppertal-Elberfeld. Submitted
to WHO by Bayer AG, Leverkusen, Federal Republic of Germany.
Tyrkiel, E. (1977) Mutagenne addzialywannie, o-izopropoksyfenoyl-N-
methylo-karaminianu (propoksura) na komorki pleiowe myszy. Rocz.
Panstw. Zakl. Hig. 28 (6): 601-613.
Weber, H. (1986) Comparison of the absorption of a tracer dose of
(Phenyl-U-14C) propoxur from a basic casein diet and a standard
Altromin 1324 diet by nonradioactively pretreated Wistar rats.
Unpublished Report No. 2504 dated February 4, 1986 from Bayer AG,
Institute of Metabolism Research , Wuppertal. Data submitted to WHO
by Bayer AG, Leverkusen, Federal Republic of Germany.
Weber, H. (1988) [Phenyl-UL-14C] Propoxur: whole-study
autoradiographic distribution of the radioactivity in the rat.
Unpublished Report No.2988 dated April 21, 1988 from Bayer AG,
Institute for Metabolism Research, Leverkusen. Data submitted to WHO
by Bayer AG, Leverkusen, Federal Republic of Germany.
WHO/FAO (1974) 1973 Evaluations of some pesticide residues in food,
WHO Pesticides Residues Series, No. 3. World Health Organization,
Geneva.
Yamane, S. (1986a) Primary skin irritation study of propoxur in
rabbits. Unpublished Report No. D-0714 (DT-9) dated June 9, 1986
from Hita Research Laboratories, Japan. Data submitted to WHO by
Bayer AG, Leverkusen, Federal Republic of Germany.
Yamane, S. (1986b) Primary eye irritation study of propoxur in
rabbits. Unpublished Report No. D-0736 (DT-10) dated September 12,
1986 from Hita Research Laboratories, Japan. Data submitted to WHO
by Bayer AG, Leverkusen, Federal Republic of Germany.
