
FAO/PL:1968/M/9/1
WHO/FOOD ADD./69.35
1968 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
Issued jointly by FAO and WHO
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party of Experts and the WHO Expert
Committee on Pesticide Residues, which met in Geneva, 9-16 December,
1968.
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
WORLD HEALTH ORGANIZATION
Geneva, 1969
CHLOROPROPYLATE
IDENTITY
Chemical names
isopropyl-2-hydroxy-2,2-di(p-chlorophenyl) acetate;
isopropyl 4,4'-dichlorobenzilate (IUPAC)
Synonyms
Acaralata(R), Rospin(R), Gesakar(R), Chlormite(R)
Structural formula
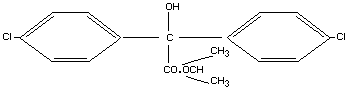 Other information on identity and properties
The technical material is a yellowish crystalline preparation with a
purity of approximately 95 per cent. No data have been submitted on
the composition of the technical product.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
When carbon14 labelled chloropropylate was administered orally to
pairs of male and female rats at about 1.6 mg/kg the radioactivity was
excreted in the urine and faeces. No radio-active carbon dioxide was
expired. The excretion pattern of the radioactivity differed between
the two sexes, the male voiding 75 per cent of the dose in the
faeces, and six per cent in the urine, and the female 49 per cent in
the faeces and 31 per cent in the urine. Most of the radioactivity
was eliminated in the first 48 hours. The total tissue residue of
radioactivity 120 hours after administration was 0.9 per cent and 1.0
per cent in the male and female respectively. The majority of these
residues were in the liver and fat (Cassidy et al., 1968).
Metabolic studies extending over 10 days were performed on pairs of
dogs (one male and one female) treated orally with technical
chloropropylate at 12.8 or 64.1 mg/kg on five consecutive days.
Urinary excretion accounted for 5.2 and and 33.2 per cent of the
total dose in the urine of the males at low and high dose levels, the
corresponding figures for the female being 3.2 and 33.7 per cent. In
the faeces, 6.4 and 3.4 per cent were detected in the male, at low and
high doses respectively, and 16.9 and 7.3 per cent in the female. No
residues were detected in blood, brain, fat, liver, kidney or muscle
at sacrifice. The method of analysis employed would not detect
dichlorobenzilic acid conjugates. (Hazleton Laboratories, Inc., 1964)
The presence of the probable metabolite, dichlorobenzilic acid has
been demonstrated in dog urine, by thin layer chromatography (Mattson
et al., 1965).
Acute toxicity (active ingredient) (oral)
Animal Route Formulation or solvent LD50 mg/kg Reference
body-weight
Mouse Oral 20 per cent suspension >5 000 Stenger, 1962
in gum arabic
Rat Oral 20 per cent suspension >5 000 Stenger, 1963a
on gum arabic
Rat Oral 40 per cent wettable >13 840 Ind. Bio-Test,
powder 1965a
Rat Oral 25 per cent emulsifiable 5 000 Ind. Bio-Test,
solution 1965b
Fowl Oral 25 per cent emulsifiable 2 500 Sanderson, 1963
solution
Short-term studies
Avian wildlife
No deaths resulted when mallard ducks were fed diets containing 0.8
per cent chloropropylate for seven days, although reduced food
consumption and body-weight occurred at this dose level (Woodard
Research Corp, 1966a).
Bobwhite quail were fed for seven days on diets containing 0.8, 0.4
and 0.2 per cent chloropropylate. The LD50 was about 0.4 per cent
and reduced food intake and weight loss were apparent in all groups.
(Woodard Research Corp., 1966b)
Rat
Groups of five male and five female rats dosed orally by intubation
six times weekly for four weeks with 50, 250 or 500 mg/kg/day
chloropropylate suspended in gum arabic failed to show any toxic
effects as judged by changes in survival, body-weight gain and
microscopic examination of tissues after autopsy (Geigy, 1964).
Administration over 12 weeks of 0, 100, 1000, 5000 and 25 000 ppm of
chloropropylate in the diet to groups of 21 male and 21 female rats
resulted in a mortality of 29 per cent at the 5000 ppm level and 70
per cent at the 25 000 ppm level. At the highest level, hair loss
occurred after two weeks, and bloody nasal discharge was frequently
observed. Body-weight gain was doubtfully depressed in males at 1000
ppm and definitely depressed in both sexes at higher dose levels. Food
intake levels were reduced in both sexes at 25 000 ppm.
Histopathological examination of tissues revealed dose related changes
in the liver, and an increasing incidence of testicular atrophy at
doses of 1000 ppm and above. (Stenger, 1963b)
Dog
Administration over three months of 0, 100, 500 or 3000 ppm of
chloropropylate in a dry diet to groups of three male and three female
dogs resulted in slight depression of average bodyweight gain in male
dogs at 3000 ppm. Haematology, biochemical studies, urinalysis, gross
and histopathology were all comparable to controls. Organ to
body-weight ratios were increased in the case of liver and kidney at
3000 ppm in both sexes (Hazleton Laboratories, Inc., 1965).
A one-year interim report of a two-year feeding study using
chloropropylate 40 per cent wettable formulation in groups of three
male and three female dogs at 0, 100, 500 and 3000 ppm active
ingredient indicates some depression of female growth rate in two out
of three female dogs at 500 ppm. At 3000 ppm, body-weight loss or
insufficient gain (five out of six) gastric stress and trauma (five
out of six), elevated alkaline phosphatase (four out of four, day 90),
and mortality (three out of six) occurred. The survivors were reduced
to an intake of 2000 ppm after 104 days and were taken off the test at
the one-hundred-and-sixteenth day. They were again dosed with 2000 ppm
from the one-hundred-and-ninety-eighth day of the study. After one
year, this 2000 ppm group showed no adverse effect, except slightly
high urinary albumin in one male and one female animal (Industrial
Bio-Test Laboratories 1967).
Long-term studies
Rat
Three groups of 30 male and 30 female rats were fed 0, 40, or 125 ppm
active ingredient as the 50 per cent wettable formulation in the diet
for two years. The majority of the parameters, including mortality,
body-weight, food intake, haematology and incidence of neoplasms were
comparable to the controls. However, at 125 ppm the decrease in
prostate weight, apparent already as a trend at 40 ppm, became
significant. Similarly an increased incidence of chronic renal
disease, and doubtful fatty changes in the liver were more commonly
encountered at 125 ppm (Woodard, 1966c).
Special studies
(a) Reproduction
In a three-generation study, three groups of 10 male and 20 female
rats were fed 0, 25 or 50 ppm of chloropropylate in their diet. The
litters from the second matings were used to provide the new
generations. The F1b litter did not receive the test diet until 28
days after weaning, but it is not stated whether there was a similar
delay period in the case of the F2b litter. The F0 animals showed no
toxic effects as measured by changes in body-weight, mortality or
reproductive capacity. Litters from treated groups were comparable to
the controls in numbers of litters per group, litter size, mean birth
weight, mean weanling weights, and survival to weaning. Abnormalities
were within normal limits. Histopathology of the F3b weanling rats
indicated a slightly higher frequency of hepatic cell vacuolization,
and mineralization of renal tubules than that encountered in the
controls but such pathological abnormalities are frequently
encountered in normal rats (Woodard, 1966-67).
(b) Studies on the metabolite
Dichlorbenzilic acid, was fed to groups of 20 male and 20 female rats
for 99 days at dose levels of 0, 20, 100, 500 and 2500 ppm active
ingredient in a 20 per cent powder. Food consumption body-weight
gain, mortality, organ weights and gross and histopathology showed no
significant changes from the controls. A tendency to slight depression
of kidney and testes weight was noted at 2500 ppm (Domenjoz, 1965).
Comments
In one report of the two 12-week rat studies, the body-weight data for
the males show a dose-related decrease throughout the study. It is
improbable that the lower dose levels reduce body-weight
significantly.
In the rat three-generation study, the data indicate that there was a
delay between weaning and starting the animals on the test-diet, in
the case of one litter. The reason for this delay and the extent of it
are not specified. Such a delay period could affect the maturation of
the reproductive organs and, consequently, fertility data in this
study are of little value. There is no evidence of teratogenic
activity, although detailed skeletal or soft tissue examinations have
not been made.
In the interim report of the dog study, the female body-weight gain is
apparently depressed in two out of the three dogs at 500 ppm. No such
depression is apparent following return to dietary level of 2000 ppm
after temporary discontinuation of the 3000 ppm and so this apparent
depression of body-weight gain may be an artifact. It should, however,
be borne in mind: (1) that a tolerance may have developed in the
animals receiving the high dose following this earlier exposure, and
(2) that the rate of growth would be less in one-year old dogs than in
the much younger dogs exposed at the initiation of the study.
Attention is drawn to the presence of chronic renal disease in the rat
two-year study, and to the increased urinary albumen in two dogs at
the top dose level. Furthermore, there is the possibility of
metabolites of unknown toxicity being formed.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effects
Rat: 40 ppm equivalent to 2 mg/kg body-weight per day.
Estimate of temporary acceptable daily intake for man
0-0.01 mg/kg body weight.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Pre-harvest treatments
Chloropropylate is used in several countries as a contact acaracide
for the control of mites on apples, pears, stone fruit, citrus fruit,
soft fruit, grapes, olives, vegetables, tea and cotton.
The recommended rates of application range from 20 to 60 g/100 l, and
the amount of spray liquid applied is generally 500-1000 l/ha for
low-grown crops (vegetables, etc.) and 2000-2500 l/ha for high-grown
crops (e.g. fruit orchards). (US Dept. of Agric., 1967)
Post-harvest treatments
No post-harvest treatment is recommended.
Other uses
No other uses are recommended.
Residues resulting from supervised trials
Residue data are available from supervised trials on several food
crops, grown under various conditions, using various rates of
application and pro-harvest intervals (Geigy, 1963 and 1968) These
data refer to apples and pears, stone fruit (apricots, peaches, plums,
prunes), citrus fruit (oranges, lemons), small fruit (strawberries,
blueberries), vegetables (tomatoes, muskmelons, cucumbers). In most
cases normal dosage rates were applied in accordance with label
recommendations; in a few experiments higher dosages were included.
The residues were analysed with a GLC method using microcoulometric
detection, sensitive to 0.05 ppm. Results of supervised trials and
available information on application mainly refer to work in the
United States of America. From other countries only few relevant data
are available. The following table presents a summary of typical
residue data:
Rate of Post-treatment Residue
application Number of interval whole fruit
(g/100 l) treatments (days) basis (ppm)
Apples 25 1 1 1.2
25 1 21 0.9
50 1 1 2.0
50 1 21 1.3
1.12 kg* 1 1 3.2
1.12 kg* 1 21 1.5
2.24 kg* 1 1 4.6
2.24 kg* 1 21 3.6
Pears 0.28 kg* 1 1 0.8
0.28 kg* 1 7 0.1
1.12 kg* 1 7 0.6
Grapefruit 30 1 1 2.1**
30 1 21 1.2**
90 1 1 12.5**
90 1 21 6.1**
Oranges 30 1 1 0.6**
30 1 21 0.7**
90 1 1 1.0**
90 1 21 0.9**
Tomatoes 0.56 kg* 1 3 0.1
0.56 kg* 1 14 0.05 max.
2.24 kg* 1 3 0.12
2.24 kg* 1 14 0.09
(continued)
Rate of Post-treatment Residue
application Number of interval whole fruit
(g/100 l) treatments (days) basis (ppm)
Cantaloupes 2.24 kg* 1 1 0.22
2.24 kg* 1 14 0.07
* Rate application/ha.
** Residue in peel : pulp after seven days was <0.1.
Fate of residues
General comments
Chloropropylate can be considered as a persistent compound. More
information is needed on the nature of terminal residues in plants,
animals and their products.
In soils
No information available.
In plants
Experiments have been performed to examine if and to what extent
breakdown products derived from chloropropylate could be found, with
particular reference to the possible occurrence of
4,4'-dichlorobenzilic acid.
Apple trees were sprayed with 2 lb/100 gal; samples were taken three,
14 and 21 days after the application. The sensitivity of the
analytical method was 0.1 ppm. In recovery studies it was shown that
4,4'-dichlorobenzilic acid could be detected without interference by
chloropropylate. It appeared from this study that chloropropylate is
found mainly on the outer surface of the treated apples. No unchanged
chloropropylate could be detected in the pulp. None of the treated
apples contained detectable amounts of 4,4'-dichlorobenzilic acid
(Murphy et al., 1966).
In animals
No information available other than that referring to special
toxicological and biochemical work.
In storage and processing
No data are available on the fate of residues during storage and
processing. Since the residue remains mainly on the skin and migrates
only to a very small extent or not at all into the pulp, it may be
expected that washing and peeling of treated fruit will remove most of
the residue. No data are available on the effects of cooking.
Evidence of residues in food, in commerce or at consumption
Food moving in commerce
No information available.
Food at the time of consumption
No information available.
Methods of residue analysis
See chlorobenzilate.
National tolerances
Country Crop Tolerance (ppm)
United States of America Apples, pears 5
(United States of America Federal Register)
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
Appraisal
Chloropropylate is a persistent and specific acaracide with biological
properties related to those of chlorobenzilate. The compound, although
registered for use in a relatively large number of countries, does not
seem to be extensively applied. No information was available on the
extent of use of this compound. Furthermore, the total composition of
the technical material has not been furnished. The limited residue
data which have been made available are mainly based upon experiments
in the United States of America and Switzerland. Residue data from
other countries are desired. No data have been furnished on the level
of residues in food moving in commerce, and on the rate of
disappearance during storage and processing.
In certain cases, e.g. grapes and blueberries, recommendations for
tolerances cannot be made until further data are available on the
possible carry-over of the residue in wine, and the necessity for the
utilization of the compound on blueberries. No information is
available on the nature of the terminal residues in plants and animal
products.
A referee method of analysis has not been established. However,
gas-liquid chromatography appears suitable for this purpose, but must
be further developed by collaborative studies. Comparative evaluation
of the different detectors used in gas chromatographic methods, and
evaluation of the different methods of extraction are needed.
Recommendations
Temporary tolerances
The following temporary tolerances (to be in effect until 1972) are to
apply to raw agricultural products moving in commerce unless otherwise
indicated. In the case of fruits and vegetables the tolerances should
be applied as soon as practicable after harvest and in any event prior
to actual retail to the public. In the case of commodities entering
international trade, the tolerances should be applied by the importing
country at the point of entry or as soon as practicable thereafter.
The figures refer to the parent compound only.
Apples, pears, citrus (on whole-fruit basis) -3.0 ppm
Tomatoes, cantaloupes -1.0 ppm
Further work or information
Required before 30 June 1972:
1. Information on the composition of the technical product.
2. Information on the nature of terminal residues in plants, animals,
and their products.
3. Data on the extent of use in various countries.
4. Data from countries other than the United States of America and
Switzerland on the required rates and frequencies of application,
pre-harvest intervals, and the resultant residues.
5. Data on residue levels in raw agricultural products moving in
commerce.
6. Data on the disappearance of residues during storage and
processing.
7. Data on the possible carry-over of residues into wine as a result
of the treatment of grapes.
8. Comparative evaluation of the different detectors used in
gas-liquid chromatographic methods and of different methods of
extraction for regulatory purposes.
9. Identification of metabolites other than dichlorobenzilic acid and
investigation of their toxicology.
10. Adequate reproduction studies in the rat or other species.
11. Further investigation of kidney function and excretion.
Desirable
Collaborative studies to establish a referee method.
REFERENCES
Cassidy, J. E., Min, B. and Murphy, R. T. (1968) Metabolic fate of
C14 chloropropylate, in white rats. A balance study. Geigy Chemical
Corp. Unpublished report
Domenjoz, R. (1965) Dichlorbenzilsäure Toxizität bei chronischer
Verabreichung. Institute of Pharmacology, Rheinische
Friedrich-Wilhelm's University, Bonn, Unpublished report
Geigy S.A., J.R. (1963) Chlorpropylat im Vergleich zu Chlorbenzilat.
Unpublished report
Geigy. (1964) Toxicology of chloropropylate (G24163). Geigy Chemical
Corp., unpublished report
Geigy S.A., J.R. (1968) Chloropropylate working paper. Unpublished
report
Hazleton Laboratories Inc. (1964) Method development for the analysis
of chloropropylate and chlorobenzilate in biological fluids and tissue
specimens. Metabolic distribution and excretion of chloropropylate and
chlorobenzilate in dogs, Unpublished report
Hazleton Laboratories Inc. (1965) Three-month dietary administration.
Dogs. Final report. Chloropropylate 50W, Unpublished report
Industrial Bio-Test Laboratories. (1965a) Acute toxicity studies on
chloropropylate 40W, Unpublished report
Industrial Bio-Test Laboratories. (1965b) Acute toxicity studies on
chloropropylate 25E, Unpublished report
Industrial Bio-Test Laboratories. (1967) Two-year chronic oral
toxicity of chloropropylate 40W. Beagle dogs. Twelve-month status
report, Unpublished report
Mattson, A. M., Beaudoin, R. L. and Schneller, J. (1965) Comparison of
urinary metabolites in dogs after administration of chlorobenzilate or
chloropropylate. Geigy Chemical Corp., Unpublished report
Murphy, R., Kahrs, R. and Mattson, A. M. (1966) Dissipation of
residues of chlorobenzilate and chloropropylate on apples. Analytical
Department of Geigy Research Laboratories, Division of Geigy Chem.
Corp., Ardsley, New York, Unpublished report
Sanderson, D. M. (1953) The avian toxicity of chloropropylate.
Chesterford Park Research Station. Fisons Pest Control Ltd.,
Unpublished report
Stenger. (1962) Chlorpropylat (G24163). Akute Toxizität - Maus per os.
Geigy Chemical Corp., Unpublished report
Stenger. (1963) G24163 (Chlorpropylat). Akute Toxizität - Ratte per
os. Geigy Chemical Corp., Unpublished report
Stenger. (1963b) G24103 (Chlorpropylat). Subchronische toxizität
- Ratte. Geigy Chemical Corp., Unpublished report
United States of America Federal Register 27, F.R. 12092, pav. 120-218
United States of America Department of Agriculture. (1967) Summary of
registered pesticide chemical uses, 30 September
Woodard. (1966a) Chloropropylate. Subacute toxicity in mallard ducks,
Unpublished report, Woodard Research Corp.
Woodard. (1966b) Chloropropylate. Safety evaluation on fish and wild
life (Bobwhite quail, rainbow trout, bluegill sunfish and goldfish),
Unpublished report, Woodard Research Corp.
Woodard. (1966c) Chloropropylate. Safety evaluation by dietary feeding
to rats for 104 weeks, Unpublished report, Woodard Research Corp.
Woodard. (1966-67) Chloropropylate. Three generation reproduction
study in the rat. Histological report addenda, Unpublished report,
Woodard Research Corp.
Other information on identity and properties
The technical material is a yellowish crystalline preparation with a
purity of approximately 95 per cent. No data have been submitted on
the composition of the technical product.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
When carbon14 labelled chloropropylate was administered orally to
pairs of male and female rats at about 1.6 mg/kg the radioactivity was
excreted in the urine and faeces. No radio-active carbon dioxide was
expired. The excretion pattern of the radioactivity differed between
the two sexes, the male voiding 75 per cent of the dose in the
faeces, and six per cent in the urine, and the female 49 per cent in
the faeces and 31 per cent in the urine. Most of the radioactivity
was eliminated in the first 48 hours. The total tissue residue of
radioactivity 120 hours after administration was 0.9 per cent and 1.0
per cent in the male and female respectively. The majority of these
residues were in the liver and fat (Cassidy et al., 1968).
Metabolic studies extending over 10 days were performed on pairs of
dogs (one male and one female) treated orally with technical
chloropropylate at 12.8 or 64.1 mg/kg on five consecutive days.
Urinary excretion accounted for 5.2 and and 33.2 per cent of the
total dose in the urine of the males at low and high dose levels, the
corresponding figures for the female being 3.2 and 33.7 per cent. In
the faeces, 6.4 and 3.4 per cent were detected in the male, at low and
high doses respectively, and 16.9 and 7.3 per cent in the female. No
residues were detected in blood, brain, fat, liver, kidney or muscle
at sacrifice. The method of analysis employed would not detect
dichlorobenzilic acid conjugates. (Hazleton Laboratories, Inc., 1964)
The presence of the probable metabolite, dichlorobenzilic acid has
been demonstrated in dog urine, by thin layer chromatography (Mattson
et al., 1965).
Acute toxicity (active ingredient) (oral)
Animal Route Formulation or solvent LD50 mg/kg Reference
body-weight
Mouse Oral 20 per cent suspension >5 000 Stenger, 1962
in gum arabic
Rat Oral 20 per cent suspension >5 000 Stenger, 1963a
on gum arabic
Rat Oral 40 per cent wettable >13 840 Ind. Bio-Test,
powder 1965a
Rat Oral 25 per cent emulsifiable 5 000 Ind. Bio-Test,
solution 1965b
Fowl Oral 25 per cent emulsifiable 2 500 Sanderson, 1963
solution
Short-term studies
Avian wildlife
No deaths resulted when mallard ducks were fed diets containing 0.8
per cent chloropropylate for seven days, although reduced food
consumption and body-weight occurred at this dose level (Woodard
Research Corp, 1966a).
Bobwhite quail were fed for seven days on diets containing 0.8, 0.4
and 0.2 per cent chloropropylate. The LD50 was about 0.4 per cent
and reduced food intake and weight loss were apparent in all groups.
(Woodard Research Corp., 1966b)
Rat
Groups of five male and five female rats dosed orally by intubation
six times weekly for four weeks with 50, 250 or 500 mg/kg/day
chloropropylate suspended in gum arabic failed to show any toxic
effects as judged by changes in survival, body-weight gain and
microscopic examination of tissues after autopsy (Geigy, 1964).
Administration over 12 weeks of 0, 100, 1000, 5000 and 25 000 ppm of
chloropropylate in the diet to groups of 21 male and 21 female rats
resulted in a mortality of 29 per cent at the 5000 ppm level and 70
per cent at the 25 000 ppm level. At the highest level, hair loss
occurred after two weeks, and bloody nasal discharge was frequently
observed. Body-weight gain was doubtfully depressed in males at 1000
ppm and definitely depressed in both sexes at higher dose levels. Food
intake levels were reduced in both sexes at 25 000 ppm.
Histopathological examination of tissues revealed dose related changes
in the liver, and an increasing incidence of testicular atrophy at
doses of 1000 ppm and above. (Stenger, 1963b)
Dog
Administration over three months of 0, 100, 500 or 3000 ppm of
chloropropylate in a dry diet to groups of three male and three female
dogs resulted in slight depression of average bodyweight gain in male
dogs at 3000 ppm. Haematology, biochemical studies, urinalysis, gross
and histopathology were all comparable to controls. Organ to
body-weight ratios were increased in the case of liver and kidney at
3000 ppm in both sexes (Hazleton Laboratories, Inc., 1965).
A one-year interim report of a two-year feeding study using
chloropropylate 40 per cent wettable formulation in groups of three
male and three female dogs at 0, 100, 500 and 3000 ppm active
ingredient indicates some depression of female growth rate in two out
of three female dogs at 500 ppm. At 3000 ppm, body-weight loss or
insufficient gain (five out of six) gastric stress and trauma (five
out of six), elevated alkaline phosphatase (four out of four, day 90),
and mortality (three out of six) occurred. The survivors were reduced
to an intake of 2000 ppm after 104 days and were taken off the test at
the one-hundred-and-sixteenth day. They were again dosed with 2000 ppm
from the one-hundred-and-ninety-eighth day of the study. After one
year, this 2000 ppm group showed no adverse effect, except slightly
high urinary albumin in one male and one female animal (Industrial
Bio-Test Laboratories 1967).
Long-term studies
Rat
Three groups of 30 male and 30 female rats were fed 0, 40, or 125 ppm
active ingredient as the 50 per cent wettable formulation in the diet
for two years. The majority of the parameters, including mortality,
body-weight, food intake, haematology and incidence of neoplasms were
comparable to the controls. However, at 125 ppm the decrease in
prostate weight, apparent already as a trend at 40 ppm, became
significant. Similarly an increased incidence of chronic renal
disease, and doubtful fatty changes in the liver were more commonly
encountered at 125 ppm (Woodard, 1966c).
Special studies
(a) Reproduction
In a three-generation study, three groups of 10 male and 20 female
rats were fed 0, 25 or 50 ppm of chloropropylate in their diet. The
litters from the second matings were used to provide the new
generations. The F1b litter did not receive the test diet until 28
days after weaning, but it is not stated whether there was a similar
delay period in the case of the F2b litter. The F0 animals showed no
toxic effects as measured by changes in body-weight, mortality or
reproductive capacity. Litters from treated groups were comparable to
the controls in numbers of litters per group, litter size, mean birth
weight, mean weanling weights, and survival to weaning. Abnormalities
were within normal limits. Histopathology of the F3b weanling rats
indicated a slightly higher frequency of hepatic cell vacuolization,
and mineralization of renal tubules than that encountered in the
controls but such pathological abnormalities are frequently
encountered in normal rats (Woodard, 1966-67).
(b) Studies on the metabolite
Dichlorbenzilic acid, was fed to groups of 20 male and 20 female rats
for 99 days at dose levels of 0, 20, 100, 500 and 2500 ppm active
ingredient in a 20 per cent powder. Food consumption body-weight
gain, mortality, organ weights and gross and histopathology showed no
significant changes from the controls. A tendency to slight depression
of kidney and testes weight was noted at 2500 ppm (Domenjoz, 1965).
Comments
In one report of the two 12-week rat studies, the body-weight data for
the males show a dose-related decrease throughout the study. It is
improbable that the lower dose levels reduce body-weight
significantly.
In the rat three-generation study, the data indicate that there was a
delay between weaning and starting the animals on the test-diet, in
the case of one litter. The reason for this delay and the extent of it
are not specified. Such a delay period could affect the maturation of
the reproductive organs and, consequently, fertility data in this
study are of little value. There is no evidence of teratogenic
activity, although detailed skeletal or soft tissue examinations have
not been made.
In the interim report of the dog study, the female body-weight gain is
apparently depressed in two out of the three dogs at 500 ppm. No such
depression is apparent following return to dietary level of 2000 ppm
after temporary discontinuation of the 3000 ppm and so this apparent
depression of body-weight gain may be an artifact. It should, however,
be borne in mind: (1) that a tolerance may have developed in the
animals receiving the high dose following this earlier exposure, and
(2) that the rate of growth would be less in one-year old dogs than in
the much younger dogs exposed at the initiation of the study.
Attention is drawn to the presence of chronic renal disease in the rat
two-year study, and to the increased urinary albumen in two dogs at
the top dose level. Furthermore, there is the possibility of
metabolites of unknown toxicity being formed.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effects
Rat: 40 ppm equivalent to 2 mg/kg body-weight per day.
Estimate of temporary acceptable daily intake for man
0-0.01 mg/kg body weight.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Pre-harvest treatments
Chloropropylate is used in several countries as a contact acaracide
for the control of mites on apples, pears, stone fruit, citrus fruit,
soft fruit, grapes, olives, vegetables, tea and cotton.
The recommended rates of application range from 20 to 60 g/100 l, and
the amount of spray liquid applied is generally 500-1000 l/ha for
low-grown crops (vegetables, etc.) and 2000-2500 l/ha for high-grown
crops (e.g. fruit orchards). (US Dept. of Agric., 1967)
Post-harvest treatments
No post-harvest treatment is recommended.
Other uses
No other uses are recommended.
Residues resulting from supervised trials
Residue data are available from supervised trials on several food
crops, grown under various conditions, using various rates of
application and pro-harvest intervals (Geigy, 1963 and 1968) These
data refer to apples and pears, stone fruit (apricots, peaches, plums,
prunes), citrus fruit (oranges, lemons), small fruit (strawberries,
blueberries), vegetables (tomatoes, muskmelons, cucumbers). In most
cases normal dosage rates were applied in accordance with label
recommendations; in a few experiments higher dosages were included.
The residues were analysed with a GLC method using microcoulometric
detection, sensitive to 0.05 ppm. Results of supervised trials and
available information on application mainly refer to work in the
United States of America. From other countries only few relevant data
are available. The following table presents a summary of typical
residue data:
Rate of Post-treatment Residue
application Number of interval whole fruit
(g/100 l) treatments (days) basis (ppm)
Apples 25 1 1 1.2
25 1 21 0.9
50 1 1 2.0
50 1 21 1.3
1.12 kg* 1 1 3.2
1.12 kg* 1 21 1.5
2.24 kg* 1 1 4.6
2.24 kg* 1 21 3.6
Pears 0.28 kg* 1 1 0.8
0.28 kg* 1 7 0.1
1.12 kg* 1 7 0.6
Grapefruit 30 1 1 2.1**
30 1 21 1.2**
90 1 1 12.5**
90 1 21 6.1**
Oranges 30 1 1 0.6**
30 1 21 0.7**
90 1 1 1.0**
90 1 21 0.9**
Tomatoes 0.56 kg* 1 3 0.1
0.56 kg* 1 14 0.05 max.
2.24 kg* 1 3 0.12
2.24 kg* 1 14 0.09
(continued)
Rate of Post-treatment Residue
application Number of interval whole fruit
(g/100 l) treatments (days) basis (ppm)
Cantaloupes 2.24 kg* 1 1 0.22
2.24 kg* 1 14 0.07
* Rate application/ha.
** Residue in peel : pulp after seven days was <0.1.
Fate of residues
General comments
Chloropropylate can be considered as a persistent compound. More
information is needed on the nature of terminal residues in plants,
animals and their products.
In soils
No information available.
In plants
Experiments have been performed to examine if and to what extent
breakdown products derived from chloropropylate could be found, with
particular reference to the possible occurrence of
4,4'-dichlorobenzilic acid.
Apple trees were sprayed with 2 lb/100 gal; samples were taken three,
14 and 21 days after the application. The sensitivity of the
analytical method was 0.1 ppm. In recovery studies it was shown that
4,4'-dichlorobenzilic acid could be detected without interference by
chloropropylate. It appeared from this study that chloropropylate is
found mainly on the outer surface of the treated apples. No unchanged
chloropropylate could be detected in the pulp. None of the treated
apples contained detectable amounts of 4,4'-dichlorobenzilic acid
(Murphy et al., 1966).
In animals
No information available other than that referring to special
toxicological and biochemical work.
In storage and processing
No data are available on the fate of residues during storage and
processing. Since the residue remains mainly on the skin and migrates
only to a very small extent or not at all into the pulp, it may be
expected that washing and peeling of treated fruit will remove most of
the residue. No data are available on the effects of cooking.
Evidence of residues in food, in commerce or at consumption
Food moving in commerce
No information available.
Food at the time of consumption
No information available.
Methods of residue analysis
See chlorobenzilate.
National tolerances
Country Crop Tolerance (ppm)
United States of America Apples, pears 5
(United States of America Federal Register)
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
Appraisal
Chloropropylate is a persistent and specific acaracide with biological
properties related to those of chlorobenzilate. The compound, although
registered for use in a relatively large number of countries, does not
seem to be extensively applied. No information was available on the
extent of use of this compound. Furthermore, the total composition of
the technical material has not been furnished. The limited residue
data which have been made available are mainly based upon experiments
in the United States of America and Switzerland. Residue data from
other countries are desired. No data have been furnished on the level
of residues in food moving in commerce, and on the rate of
disappearance during storage and processing.
In certain cases, e.g. grapes and blueberries, recommendations for
tolerances cannot be made until further data are available on the
possible carry-over of the residue in wine, and the necessity for the
utilization of the compound on blueberries. No information is
available on the nature of the terminal residues in plants and animal
products.
A referee method of analysis has not been established. However,
gas-liquid chromatography appears suitable for this purpose, but must
be further developed by collaborative studies. Comparative evaluation
of the different detectors used in gas chromatographic methods, and
evaluation of the different methods of extraction are needed.
Recommendations
Temporary tolerances
The following temporary tolerances (to be in effect until 1972) are to
apply to raw agricultural products moving in commerce unless otherwise
indicated. In the case of fruits and vegetables the tolerances should
be applied as soon as practicable after harvest and in any event prior
to actual retail to the public. In the case of commodities entering
international trade, the tolerances should be applied by the importing
country at the point of entry or as soon as practicable thereafter.
The figures refer to the parent compound only.
Apples, pears, citrus (on whole-fruit basis) -3.0 ppm
Tomatoes, cantaloupes -1.0 ppm
Further work or information
Required before 30 June 1972:
1. Information on the composition of the technical product.
2. Information on the nature of terminal residues in plants, animals,
and their products.
3. Data on the extent of use in various countries.
4. Data from countries other than the United States of America and
Switzerland on the required rates and frequencies of application,
pre-harvest intervals, and the resultant residues.
5. Data on residue levels in raw agricultural products moving in
commerce.
6. Data on the disappearance of residues during storage and
processing.
7. Data on the possible carry-over of residues into wine as a result
of the treatment of grapes.
8. Comparative evaluation of the different detectors used in
gas-liquid chromatographic methods and of different methods of
extraction for regulatory purposes.
9. Identification of metabolites other than dichlorobenzilic acid and
investigation of their toxicology.
10. Adequate reproduction studies in the rat or other species.
11. Further investigation of kidney function and excretion.
Desirable
Collaborative studies to establish a referee method.
REFERENCES
Cassidy, J. E., Min, B. and Murphy, R. T. (1968) Metabolic fate of
C14 chloropropylate, in white rats. A balance study. Geigy Chemical
Corp. Unpublished report
Domenjoz, R. (1965) Dichlorbenzilsäure Toxizität bei chronischer
Verabreichung. Institute of Pharmacology, Rheinische
Friedrich-Wilhelm's University, Bonn, Unpublished report
Geigy S.A., J.R. (1963) Chlorpropylat im Vergleich zu Chlorbenzilat.
Unpublished report
Geigy. (1964) Toxicology of chloropropylate (G24163). Geigy Chemical
Corp., unpublished report
Geigy S.A., J.R. (1968) Chloropropylate working paper. Unpublished
report
Hazleton Laboratories Inc. (1964) Method development for the analysis
of chloropropylate and chlorobenzilate in biological fluids and tissue
specimens. Metabolic distribution and excretion of chloropropylate and
chlorobenzilate in dogs, Unpublished report
Hazleton Laboratories Inc. (1965) Three-month dietary administration.
Dogs. Final report. Chloropropylate 50W, Unpublished report
Industrial Bio-Test Laboratories. (1965a) Acute toxicity studies on
chloropropylate 40W, Unpublished report
Industrial Bio-Test Laboratories. (1965b) Acute toxicity studies on
chloropropylate 25E, Unpublished report
Industrial Bio-Test Laboratories. (1967) Two-year chronic oral
toxicity of chloropropylate 40W. Beagle dogs. Twelve-month status
report, Unpublished report
Mattson, A. M., Beaudoin, R. L. and Schneller, J. (1965) Comparison of
urinary metabolites in dogs after administration of chlorobenzilate or
chloropropylate. Geigy Chemical Corp., Unpublished report
Murphy, R., Kahrs, R. and Mattson, A. M. (1966) Dissipation of
residues of chlorobenzilate and chloropropylate on apples. Analytical
Department of Geigy Research Laboratories, Division of Geigy Chem.
Corp., Ardsley, New York, Unpublished report
Sanderson, D. M. (1953) The avian toxicity of chloropropylate.
Chesterford Park Research Station. Fisons Pest Control Ltd.,
Unpublished report
Stenger. (1962) Chlorpropylat (G24163). Akute Toxizität - Maus per os.
Geigy Chemical Corp., Unpublished report
Stenger. (1963) G24163 (Chlorpropylat). Akute Toxizität - Ratte per
os. Geigy Chemical Corp., Unpublished report
Stenger. (1963b) G24103 (Chlorpropylat). Subchronische toxizität
- Ratte. Geigy Chemical Corp., Unpublished report
United States of America Federal Register 27, F.R. 12092, pav. 120-218
United States of America Department of Agriculture. (1967) Summary of
registered pesticide chemical uses, 30 September
Woodard. (1966a) Chloropropylate. Subacute toxicity in mallard ducks,
Unpublished report, Woodard Research Corp.
Woodard. (1966b) Chloropropylate. Safety evaluation on fish and wild
life (Bobwhite quail, rainbow trout, bluegill sunfish and goldfish),
Unpublished report, Woodard Research Corp.
Woodard. (1966c) Chloropropylate. Safety evaluation by dietary feeding
to rats for 104 weeks, Unpublished report, Woodard Research Corp.
Woodard. (1966-67) Chloropropylate. Three generation reproduction
study in the rat. Histological report addenda, Unpublished report,
Woodard Research Corp.
 Other information on identity and properties
The technical material is a yellowish crystalline preparation with a
purity of approximately 95 per cent. No data have been submitted on
the composition of the technical product.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
When carbon14 labelled chloropropylate was administered orally to
pairs of male and female rats at about 1.6 mg/kg the radioactivity was
excreted in the urine and faeces. No radio-active carbon dioxide was
expired. The excretion pattern of the radioactivity differed between
the two sexes, the male voiding 75 per cent of the dose in the
faeces, and six per cent in the urine, and the female 49 per cent in
the faeces and 31 per cent in the urine. Most of the radioactivity
was eliminated in the first 48 hours. The total tissue residue of
radioactivity 120 hours after administration was 0.9 per cent and 1.0
per cent in the male and female respectively. The majority of these
residues were in the liver and fat (Cassidy et al., 1968).
Metabolic studies extending over 10 days were performed on pairs of
dogs (one male and one female) treated orally with technical
chloropropylate at 12.8 or 64.1 mg/kg on five consecutive days.
Urinary excretion accounted for 5.2 and and 33.2 per cent of the
total dose in the urine of the males at low and high dose levels, the
corresponding figures for the female being 3.2 and 33.7 per cent. In
the faeces, 6.4 and 3.4 per cent were detected in the male, at low and
high doses respectively, and 16.9 and 7.3 per cent in the female. No
residues were detected in blood, brain, fat, liver, kidney or muscle
at sacrifice. The method of analysis employed would not detect
dichlorobenzilic acid conjugates. (Hazleton Laboratories, Inc., 1964)
The presence of the probable metabolite, dichlorobenzilic acid has
been demonstrated in dog urine, by thin layer chromatography (Mattson
et al., 1965).
Acute toxicity (active ingredient) (oral)
Animal Route Formulation or solvent LD50 mg/kg Reference
body-weight
Mouse Oral 20 per cent suspension >5 000 Stenger, 1962
in gum arabic
Rat Oral 20 per cent suspension >5 000 Stenger, 1963a
on gum arabic
Rat Oral 40 per cent wettable >13 840 Ind. Bio-Test,
powder 1965a
Rat Oral 25 per cent emulsifiable 5 000 Ind. Bio-Test,
solution 1965b
Fowl Oral 25 per cent emulsifiable 2 500 Sanderson, 1963
solution
Short-term studies
Avian wildlife
No deaths resulted when mallard ducks were fed diets containing 0.8
per cent chloropropylate for seven days, although reduced food
consumption and body-weight occurred at this dose level (Woodard
Research Corp, 1966a).
Bobwhite quail were fed for seven days on diets containing 0.8, 0.4
and 0.2 per cent chloropropylate. The LD50 was about 0.4 per cent
and reduced food intake and weight loss were apparent in all groups.
(Woodard Research Corp., 1966b)
Rat
Groups of five male and five female rats dosed orally by intubation
six times weekly for four weeks with 50, 250 or 500 mg/kg/day
chloropropylate suspended in gum arabic failed to show any toxic
effects as judged by changes in survival, body-weight gain and
microscopic examination of tissues after autopsy (Geigy, 1964).
Administration over 12 weeks of 0, 100, 1000, 5000 and 25 000 ppm of
chloropropylate in the diet to groups of 21 male and 21 female rats
resulted in a mortality of 29 per cent at the 5000 ppm level and 70
per cent at the 25 000 ppm level. At the highest level, hair loss
occurred after two weeks, and bloody nasal discharge was frequently
observed. Body-weight gain was doubtfully depressed in males at 1000
ppm and definitely depressed in both sexes at higher dose levels. Food
intake levels were reduced in both sexes at 25 000 ppm.
Histopathological examination of tissues revealed dose related changes
in the liver, and an increasing incidence of testicular atrophy at
doses of 1000 ppm and above. (Stenger, 1963b)
Dog
Administration over three months of 0, 100, 500 or 3000 ppm of
chloropropylate in a dry diet to groups of three male and three female
dogs resulted in slight depression of average bodyweight gain in male
dogs at 3000 ppm. Haematology, biochemical studies, urinalysis, gross
and histopathology were all comparable to controls. Organ to
body-weight ratios were increased in the case of liver and kidney at
3000 ppm in both sexes (Hazleton Laboratories, Inc., 1965).
A one-year interim report of a two-year feeding study using
chloropropylate 40 per cent wettable formulation in groups of three
male and three female dogs at 0, 100, 500 and 3000 ppm active
ingredient indicates some depression of female growth rate in two out
of three female dogs at 500 ppm. At 3000 ppm, body-weight loss or
insufficient gain (five out of six) gastric stress and trauma (five
out of six), elevated alkaline phosphatase (four out of four, day 90),
and mortality (three out of six) occurred. The survivors were reduced
to an intake of 2000 ppm after 104 days and were taken off the test at
the one-hundred-and-sixteenth day. They were again dosed with 2000 ppm
from the one-hundred-and-ninety-eighth day of the study. After one
year, this 2000 ppm group showed no adverse effect, except slightly
high urinary albumin in one male and one female animal (Industrial
Bio-Test Laboratories 1967).
Long-term studies
Rat
Three groups of 30 male and 30 female rats were fed 0, 40, or 125 ppm
active ingredient as the 50 per cent wettable formulation in the diet
for two years. The majority of the parameters, including mortality,
body-weight, food intake, haematology and incidence of neoplasms were
comparable to the controls. However, at 125 ppm the decrease in
prostate weight, apparent already as a trend at 40 ppm, became
significant. Similarly an increased incidence of chronic renal
disease, and doubtful fatty changes in the liver were more commonly
encountered at 125 ppm (Woodard, 1966c).
Special studies
(a) Reproduction
In a three-generation study, three groups of 10 male and 20 female
rats were fed 0, 25 or 50 ppm of chloropropylate in their diet. The
litters from the second matings were used to provide the new
generations. The F1b litter did not receive the test diet until 28
days after weaning, but it is not stated whether there was a similar
delay period in the case of the F2b litter. The F0 animals showed no
toxic effects as measured by changes in body-weight, mortality or
reproductive capacity. Litters from treated groups were comparable to
the controls in numbers of litters per group, litter size, mean birth
weight, mean weanling weights, and survival to weaning. Abnormalities
were within normal limits. Histopathology of the F3b weanling rats
indicated a slightly higher frequency of hepatic cell vacuolization,
and mineralization of renal tubules than that encountered in the
controls but such pathological abnormalities are frequently
encountered in normal rats (Woodard, 1966-67).
(b) Studies on the metabolite
Dichlorbenzilic acid, was fed to groups of 20 male and 20 female rats
for 99 days at dose levels of 0, 20, 100, 500 and 2500 ppm active
ingredient in a 20 per cent powder. Food consumption body-weight
gain, mortality, organ weights and gross and histopathology showed no
significant changes from the controls. A tendency to slight depression
of kidney and testes weight was noted at 2500 ppm (Domenjoz, 1965).
Comments
In one report of the two 12-week rat studies, the body-weight data for
the males show a dose-related decrease throughout the study. It is
improbable that the lower dose levels reduce body-weight
significantly.
In the rat three-generation study, the data indicate that there was a
delay between weaning and starting the animals on the test-diet, in
the case of one litter. The reason for this delay and the extent of it
are not specified. Such a delay period could affect the maturation of
the reproductive organs and, consequently, fertility data in this
study are of little value. There is no evidence of teratogenic
activity, although detailed skeletal or soft tissue examinations have
not been made.
In the interim report of the dog study, the female body-weight gain is
apparently depressed in two out of the three dogs at 500 ppm. No such
depression is apparent following return to dietary level of 2000 ppm
after temporary discontinuation of the 3000 ppm and so this apparent
depression of body-weight gain may be an artifact. It should, however,
be borne in mind: (1) that a tolerance may have developed in the
animals receiving the high dose following this earlier exposure, and
(2) that the rate of growth would be less in one-year old dogs than in
the much younger dogs exposed at the initiation of the study.
Attention is drawn to the presence of chronic renal disease in the rat
two-year study, and to the increased urinary albumen in two dogs at
the top dose level. Furthermore, there is the possibility of
metabolites of unknown toxicity being formed.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effects
Rat: 40 ppm equivalent to 2 mg/kg body-weight per day.
Estimate of temporary acceptable daily intake for man
0-0.01 mg/kg body weight.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Pre-harvest treatments
Chloropropylate is used in several countries as a contact acaracide
for the control of mites on apples, pears, stone fruit, citrus fruit,
soft fruit, grapes, olives, vegetables, tea and cotton.
The recommended rates of application range from 20 to 60 g/100 l, and
the amount of spray liquid applied is generally 500-1000 l/ha for
low-grown crops (vegetables, etc.) and 2000-2500 l/ha for high-grown
crops (e.g. fruit orchards). (US Dept. of Agric., 1967)
Post-harvest treatments
No post-harvest treatment is recommended.
Other uses
No other uses are recommended.
Residues resulting from supervised trials
Residue data are available from supervised trials on several food
crops, grown under various conditions, using various rates of
application and pro-harvest intervals (Geigy, 1963 and 1968) These
data refer to apples and pears, stone fruit (apricots, peaches, plums,
prunes), citrus fruit (oranges, lemons), small fruit (strawberries,
blueberries), vegetables (tomatoes, muskmelons, cucumbers). In most
cases normal dosage rates were applied in accordance with label
recommendations; in a few experiments higher dosages were included.
The residues were analysed with a GLC method using microcoulometric
detection, sensitive to 0.05 ppm. Results of supervised trials and
available information on application mainly refer to work in the
United States of America. From other countries only few relevant data
are available. The following table presents a summary of typical
residue data:
Rate of Post-treatment Residue
application Number of interval whole fruit
(g/100 l) treatments (days) basis (ppm)
Apples 25 1 1 1.2
25 1 21 0.9
50 1 1 2.0
50 1 21 1.3
1.12 kg* 1 1 3.2
1.12 kg* 1 21 1.5
2.24 kg* 1 1 4.6
2.24 kg* 1 21 3.6
Pears 0.28 kg* 1 1 0.8
0.28 kg* 1 7 0.1
1.12 kg* 1 7 0.6
Grapefruit 30 1 1 2.1**
30 1 21 1.2**
90 1 1 12.5**
90 1 21 6.1**
Oranges 30 1 1 0.6**
30 1 21 0.7**
90 1 1 1.0**
90 1 21 0.9**
Tomatoes 0.56 kg* 1 3 0.1
0.56 kg* 1 14 0.05 max.
2.24 kg* 1 3 0.12
2.24 kg* 1 14 0.09
(continued)
Rate of Post-treatment Residue
application Number of interval whole fruit
(g/100 l) treatments (days) basis (ppm)
Cantaloupes 2.24 kg* 1 1 0.22
2.24 kg* 1 14 0.07
* Rate application/ha.
** Residue in peel : pulp after seven days was <0.1.
Fate of residues
General comments
Chloropropylate can be considered as a persistent compound. More
information is needed on the nature of terminal residues in plants,
animals and their products.
In soils
No information available.
In plants
Experiments have been performed to examine if and to what extent
breakdown products derived from chloropropylate could be found, with
particular reference to the possible occurrence of
4,4'-dichlorobenzilic acid.
Apple trees were sprayed with 2 lb/100 gal; samples were taken three,
14 and 21 days after the application. The sensitivity of the
analytical method was 0.1 ppm. In recovery studies it was shown that
4,4'-dichlorobenzilic acid could be detected without interference by
chloropropylate. It appeared from this study that chloropropylate is
found mainly on the outer surface of the treated apples. No unchanged
chloropropylate could be detected in the pulp. None of the treated
apples contained detectable amounts of 4,4'-dichlorobenzilic acid
(Murphy et al., 1966).
In animals
No information available other than that referring to special
toxicological and biochemical work.
In storage and processing
No data are available on the fate of residues during storage and
processing. Since the residue remains mainly on the skin and migrates
only to a very small extent or not at all into the pulp, it may be
expected that washing and peeling of treated fruit will remove most of
the residue. No data are available on the effects of cooking.
Evidence of residues in food, in commerce or at consumption
Food moving in commerce
No information available.
Food at the time of consumption
No information available.
Methods of residue analysis
See chlorobenzilate.
National tolerances
Country Crop Tolerance (ppm)
United States of America Apples, pears 5
(United States of America Federal Register)
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
Appraisal
Chloropropylate is a persistent and specific acaracide with biological
properties related to those of chlorobenzilate. The compound, although
registered for use in a relatively large number of countries, does not
seem to be extensively applied. No information was available on the
extent of use of this compound. Furthermore, the total composition of
the technical material has not been furnished. The limited residue
data which have been made available are mainly based upon experiments
in the United States of America and Switzerland. Residue data from
other countries are desired. No data have been furnished on the level
of residues in food moving in commerce, and on the rate of
disappearance during storage and processing.
In certain cases, e.g. grapes and blueberries, recommendations for
tolerances cannot be made until further data are available on the
possible carry-over of the residue in wine, and the necessity for the
utilization of the compound on blueberries. No information is
available on the nature of the terminal residues in plants and animal
products.
A referee method of analysis has not been established. However,
gas-liquid chromatography appears suitable for this purpose, but must
be further developed by collaborative studies. Comparative evaluation
of the different detectors used in gas chromatographic methods, and
evaluation of the different methods of extraction are needed.
Recommendations
Temporary tolerances
The following temporary tolerances (to be in effect until 1972) are to
apply to raw agricultural products moving in commerce unless otherwise
indicated. In the case of fruits and vegetables the tolerances should
be applied as soon as practicable after harvest and in any event prior
to actual retail to the public. In the case of commodities entering
international trade, the tolerances should be applied by the importing
country at the point of entry or as soon as practicable thereafter.
The figures refer to the parent compound only.
Apples, pears, citrus (on whole-fruit basis) -3.0 ppm
Tomatoes, cantaloupes -1.0 ppm
Further work or information
Required before 30 June 1972:
1. Information on the composition of the technical product.
2. Information on the nature of terminal residues in plants, animals,
and their products.
3. Data on the extent of use in various countries.
4. Data from countries other than the United States of America and
Switzerland on the required rates and frequencies of application,
pre-harvest intervals, and the resultant residues.
5. Data on residue levels in raw agricultural products moving in
commerce.
6. Data on the disappearance of residues during storage and
processing.
7. Data on the possible carry-over of residues into wine as a result
of the treatment of grapes.
8. Comparative evaluation of the different detectors used in
gas-liquid chromatographic methods and of different methods of
extraction for regulatory purposes.
9. Identification of metabolites other than dichlorobenzilic acid and
investigation of their toxicology.
10. Adequate reproduction studies in the rat or other species.
11. Further investigation of kidney function and excretion.
Desirable
Collaborative studies to establish a referee method.
REFERENCES
Cassidy, J. E., Min, B. and Murphy, R. T. (1968) Metabolic fate of
C14 chloropropylate, in white rats. A balance study. Geigy Chemical
Corp. Unpublished report
Domenjoz, R. (1965) Dichlorbenzilsäure Toxizität bei chronischer
Verabreichung. Institute of Pharmacology, Rheinische
Friedrich-Wilhelm's University, Bonn, Unpublished report
Geigy S.A., J.R. (1963) Chlorpropylat im Vergleich zu Chlorbenzilat.
Unpublished report
Geigy. (1964) Toxicology of chloropropylate (G24163). Geigy Chemical
Corp., unpublished report
Geigy S.A., J.R. (1968) Chloropropylate working paper. Unpublished
report
Hazleton Laboratories Inc. (1964) Method development for the analysis
of chloropropylate and chlorobenzilate in biological fluids and tissue
specimens. Metabolic distribution and excretion of chloropropylate and
chlorobenzilate in dogs, Unpublished report
Hazleton Laboratories Inc. (1965) Three-month dietary administration.
Dogs. Final report. Chloropropylate 50W, Unpublished report
Industrial Bio-Test Laboratories. (1965a) Acute toxicity studies on
chloropropylate 40W, Unpublished report
Industrial Bio-Test Laboratories. (1965b) Acute toxicity studies on
chloropropylate 25E, Unpublished report
Industrial Bio-Test Laboratories. (1967) Two-year chronic oral
toxicity of chloropropylate 40W. Beagle dogs. Twelve-month status
report, Unpublished report
Mattson, A. M., Beaudoin, R. L. and Schneller, J. (1965) Comparison of
urinary metabolites in dogs after administration of chlorobenzilate or
chloropropylate. Geigy Chemical Corp., Unpublished report
Murphy, R., Kahrs, R. and Mattson, A. M. (1966) Dissipation of
residues of chlorobenzilate and chloropropylate on apples. Analytical
Department of Geigy Research Laboratories, Division of Geigy Chem.
Corp., Ardsley, New York, Unpublished report
Sanderson, D. M. (1953) The avian toxicity of chloropropylate.
Chesterford Park Research Station. Fisons Pest Control Ltd.,
Unpublished report
Stenger. (1962) Chlorpropylat (G24163). Akute Toxizität - Maus per os.
Geigy Chemical Corp., Unpublished report
Stenger. (1963) G24163 (Chlorpropylat). Akute Toxizität - Ratte per
os. Geigy Chemical Corp., Unpublished report
Stenger. (1963b) G24103 (Chlorpropylat). Subchronische toxizität
- Ratte. Geigy Chemical Corp., Unpublished report
United States of America Federal Register 27, F.R. 12092, pav. 120-218
United States of America Department of Agriculture. (1967) Summary of
registered pesticide chemical uses, 30 September
Woodard. (1966a) Chloropropylate. Subacute toxicity in mallard ducks,
Unpublished report, Woodard Research Corp.
Woodard. (1966b) Chloropropylate. Safety evaluation on fish and wild
life (Bobwhite quail, rainbow trout, bluegill sunfish and goldfish),
Unpublished report, Woodard Research Corp.
Woodard. (1966c) Chloropropylate. Safety evaluation by dietary feeding
to rats for 104 weeks, Unpublished report, Woodard Research Corp.
Woodard. (1966-67) Chloropropylate. Three generation reproduction
study in the rat. Histological report addenda, Unpublished report,
Woodard Research Corp.
Other information on identity and properties
The technical material is a yellowish crystalline preparation with a
purity of approximately 95 per cent. No data have been submitted on
the composition of the technical product.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
When carbon14 labelled chloropropylate was administered orally to
pairs of male and female rats at about 1.6 mg/kg the radioactivity was
excreted in the urine and faeces. No radio-active carbon dioxide was
expired. The excretion pattern of the radioactivity differed between
the two sexes, the male voiding 75 per cent of the dose in the
faeces, and six per cent in the urine, and the female 49 per cent in
the faeces and 31 per cent in the urine. Most of the radioactivity
was eliminated in the first 48 hours. The total tissue residue of
radioactivity 120 hours after administration was 0.9 per cent and 1.0
per cent in the male and female respectively. The majority of these
residues were in the liver and fat (Cassidy et al., 1968).
Metabolic studies extending over 10 days were performed on pairs of
dogs (one male and one female) treated orally with technical
chloropropylate at 12.8 or 64.1 mg/kg on five consecutive days.
Urinary excretion accounted for 5.2 and and 33.2 per cent of the
total dose in the urine of the males at low and high dose levels, the
corresponding figures for the female being 3.2 and 33.7 per cent. In
the faeces, 6.4 and 3.4 per cent were detected in the male, at low and
high doses respectively, and 16.9 and 7.3 per cent in the female. No
residues were detected in blood, brain, fat, liver, kidney or muscle
at sacrifice. The method of analysis employed would not detect
dichlorobenzilic acid conjugates. (Hazleton Laboratories, Inc., 1964)
The presence of the probable metabolite, dichlorobenzilic acid has
been demonstrated in dog urine, by thin layer chromatography (Mattson
et al., 1965).
Acute toxicity (active ingredient) (oral)
Animal Route Formulation or solvent LD50 mg/kg Reference
body-weight
Mouse Oral 20 per cent suspension >5 000 Stenger, 1962
in gum arabic
Rat Oral 20 per cent suspension >5 000 Stenger, 1963a
on gum arabic
Rat Oral 40 per cent wettable >13 840 Ind. Bio-Test,
powder 1965a
Rat Oral 25 per cent emulsifiable 5 000 Ind. Bio-Test,
solution 1965b
Fowl Oral 25 per cent emulsifiable 2 500 Sanderson, 1963
solution
Short-term studies
Avian wildlife
No deaths resulted when mallard ducks were fed diets containing 0.8
per cent chloropropylate for seven days, although reduced food
consumption and body-weight occurred at this dose level (Woodard
Research Corp, 1966a).
Bobwhite quail were fed for seven days on diets containing 0.8, 0.4
and 0.2 per cent chloropropylate. The LD50 was about 0.4 per cent
and reduced food intake and weight loss were apparent in all groups.
(Woodard Research Corp., 1966b)
Rat
Groups of five male and five female rats dosed orally by intubation
six times weekly for four weeks with 50, 250 or 500 mg/kg/day
chloropropylate suspended in gum arabic failed to show any toxic
effects as judged by changes in survival, body-weight gain and
microscopic examination of tissues after autopsy (Geigy, 1964).
Administration over 12 weeks of 0, 100, 1000, 5000 and 25 000 ppm of
chloropropylate in the diet to groups of 21 male and 21 female rats
resulted in a mortality of 29 per cent at the 5000 ppm level and 70
per cent at the 25 000 ppm level. At the highest level, hair loss
occurred after two weeks, and bloody nasal discharge was frequently
observed. Body-weight gain was doubtfully depressed in males at 1000
ppm and definitely depressed in both sexes at higher dose levels. Food
intake levels were reduced in both sexes at 25 000 ppm.
Histopathological examination of tissues revealed dose related changes
in the liver, and an increasing incidence of testicular atrophy at
doses of 1000 ppm and above. (Stenger, 1963b)
Dog
Administration over three months of 0, 100, 500 or 3000 ppm of
chloropropylate in a dry diet to groups of three male and three female
dogs resulted in slight depression of average bodyweight gain in male
dogs at 3000 ppm. Haematology, biochemical studies, urinalysis, gross
and histopathology were all comparable to controls. Organ to
body-weight ratios were increased in the case of liver and kidney at
3000 ppm in both sexes (Hazleton Laboratories, Inc., 1965).
A one-year interim report of a two-year feeding study using
chloropropylate 40 per cent wettable formulation in groups of three
male and three female dogs at 0, 100, 500 and 3000 ppm active
ingredient indicates some depression of female growth rate in two out
of three female dogs at 500 ppm. At 3000 ppm, body-weight loss or
insufficient gain (five out of six) gastric stress and trauma (five
out of six), elevated alkaline phosphatase (four out of four, day 90),
and mortality (three out of six) occurred. The survivors were reduced
to an intake of 2000 ppm after 104 days and were taken off the test at
the one-hundred-and-sixteenth day. They were again dosed with 2000 ppm
from the one-hundred-and-ninety-eighth day of the study. After one
year, this 2000 ppm group showed no adverse effect, except slightly
high urinary albumin in one male and one female animal (Industrial
Bio-Test Laboratories 1967).
Long-term studies
Rat
Three groups of 30 male and 30 female rats were fed 0, 40, or 125 ppm
active ingredient as the 50 per cent wettable formulation in the diet
for two years. The majority of the parameters, including mortality,
body-weight, food intake, haematology and incidence of neoplasms were
comparable to the controls. However, at 125 ppm the decrease in
prostate weight, apparent already as a trend at 40 ppm, became
significant. Similarly an increased incidence of chronic renal
disease, and doubtful fatty changes in the liver were more commonly
encountered at 125 ppm (Woodard, 1966c).
Special studies
(a) Reproduction
In a three-generation study, three groups of 10 male and 20 female
rats were fed 0, 25 or 50 ppm of chloropropylate in their diet. The
litters from the second matings were used to provide the new
generations. The F1b litter did not receive the test diet until 28
days after weaning, but it is not stated whether there was a similar
delay period in the case of the F2b litter. The F0 animals showed no
toxic effects as measured by changes in body-weight, mortality or
reproductive capacity. Litters from treated groups were comparable to
the controls in numbers of litters per group, litter size, mean birth
weight, mean weanling weights, and survival to weaning. Abnormalities
were within normal limits. Histopathology of the F3b weanling rats
indicated a slightly higher frequency of hepatic cell vacuolization,
and mineralization of renal tubules than that encountered in the
controls but such pathological abnormalities are frequently
encountered in normal rats (Woodard, 1966-67).
(b) Studies on the metabolite
Dichlorbenzilic acid, was fed to groups of 20 male and 20 female rats
for 99 days at dose levels of 0, 20, 100, 500 and 2500 ppm active
ingredient in a 20 per cent powder. Food consumption body-weight
gain, mortality, organ weights and gross and histopathology showed no
significant changes from the controls. A tendency to slight depression
of kidney and testes weight was noted at 2500 ppm (Domenjoz, 1965).
Comments
In one report of the two 12-week rat studies, the body-weight data for
the males show a dose-related decrease throughout the study. It is
improbable that the lower dose levels reduce body-weight
significantly.
In the rat three-generation study, the data indicate that there was a
delay between weaning and starting the animals on the test-diet, in
the case of one litter. The reason for this delay and the extent of it
are not specified. Such a delay period could affect the maturation of
the reproductive organs and, consequently, fertility data in this
study are of little value. There is no evidence of teratogenic
activity, although detailed skeletal or soft tissue examinations have
not been made.
In the interim report of the dog study, the female body-weight gain is
apparently depressed in two out of the three dogs at 500 ppm. No such
depression is apparent following return to dietary level of 2000 ppm
after temporary discontinuation of the 3000 ppm and so this apparent
depression of body-weight gain may be an artifact. It should, however,
be borne in mind: (1) that a tolerance may have developed in the
animals receiving the high dose following this earlier exposure, and
(2) that the rate of growth would be less in one-year old dogs than in
the much younger dogs exposed at the initiation of the study.
Attention is drawn to the presence of chronic renal disease in the rat
two-year study, and to the increased urinary albumen in two dogs at
the top dose level. Furthermore, there is the possibility of
metabolites of unknown toxicity being formed.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effects
Rat: 40 ppm equivalent to 2 mg/kg body-weight per day.
Estimate of temporary acceptable daily intake for man
0-0.01 mg/kg body weight.
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Pre-harvest treatments
Chloropropylate is used in several countries as a contact acaracide
for the control of mites on apples, pears, stone fruit, citrus fruit,
soft fruit, grapes, olives, vegetables, tea and cotton.
The recommended rates of application range from 20 to 60 g/100 l, and
the amount of spray liquid applied is generally 500-1000 l/ha for
low-grown crops (vegetables, etc.) and 2000-2500 l/ha for high-grown
crops (e.g. fruit orchards). (US Dept. of Agric., 1967)
Post-harvest treatments
No post-harvest treatment is recommended.
Other uses
No other uses are recommended.
Residues resulting from supervised trials
Residue data are available from supervised trials on several food
crops, grown under various conditions, using various rates of
application and pro-harvest intervals (Geigy, 1963 and 1968) These
data refer to apples and pears, stone fruit (apricots, peaches, plums,
prunes), citrus fruit (oranges, lemons), small fruit (strawberries,
blueberries), vegetables (tomatoes, muskmelons, cucumbers). In most
cases normal dosage rates were applied in accordance with label
recommendations; in a few experiments higher dosages were included.
The residues were analysed with a GLC method using microcoulometric
detection, sensitive to 0.05 ppm. Results of supervised trials and
available information on application mainly refer to work in the
United States of America. From other countries only few relevant data
are available. The following table presents a summary of typical
residue data:
Rate of Post-treatment Residue
application Number of interval whole fruit
(g/100 l) treatments (days) basis (ppm)
Apples 25 1 1 1.2
25 1 21 0.9
50 1 1 2.0
50 1 21 1.3
1.12 kg* 1 1 3.2
1.12 kg* 1 21 1.5
2.24 kg* 1 1 4.6
2.24 kg* 1 21 3.6
Pears 0.28 kg* 1 1 0.8
0.28 kg* 1 7 0.1
1.12 kg* 1 7 0.6
Grapefruit 30 1 1 2.1**
30 1 21 1.2**
90 1 1 12.5**
90 1 21 6.1**
Oranges 30 1 1 0.6**
30 1 21 0.7**
90 1 1 1.0**
90 1 21 0.9**
Tomatoes 0.56 kg* 1 3 0.1
0.56 kg* 1 14 0.05 max.
2.24 kg* 1 3 0.12
2.24 kg* 1 14 0.09
(continued)
Rate of Post-treatment Residue
application Number of interval whole fruit
(g/100 l) treatments (days) basis (ppm)
Cantaloupes 2.24 kg* 1 1 0.22
2.24 kg* 1 14 0.07
* Rate application/ha.
** Residue in peel : pulp after seven days was <0.1.
Fate of residues
General comments
Chloropropylate can be considered as a persistent compound. More
information is needed on the nature of terminal residues in plants,
animals and their products.
In soils
No information available.
In plants
Experiments have been performed to examine if and to what extent
breakdown products derived from chloropropylate could be found, with
particular reference to the possible occurrence of
4,4'-dichlorobenzilic acid.
Apple trees were sprayed with 2 lb/100 gal; samples were taken three,
14 and 21 days after the application. The sensitivity of the
analytical method was 0.1 ppm. In recovery studies it was shown that
4,4'-dichlorobenzilic acid could be detected without interference by
chloropropylate. It appeared from this study that chloropropylate is
found mainly on the outer surface of the treated apples. No unchanged
chloropropylate could be detected in the pulp. None of the treated
apples contained detectable amounts of 4,4'-dichlorobenzilic acid
(Murphy et al., 1966).
In animals
No information available other than that referring to special
toxicological and biochemical work.
In storage and processing
No data are available on the fate of residues during storage and
processing. Since the residue remains mainly on the skin and migrates
only to a very small extent or not at all into the pulp, it may be
expected that washing and peeling of treated fruit will remove most of
the residue. No data are available on the effects of cooking.
Evidence of residues in food, in commerce or at consumption
Food moving in commerce
No information available.
Food at the time of consumption
No information available.
Methods of residue analysis
See chlorobenzilate.
National tolerances
Country Crop Tolerance (ppm)
United States of America Apples, pears 5
(United States of America Federal Register)
RECOMMENDATIONS FOR TOLERANCES AND PRACTICAL RESIDUE LIMITS
Appraisal
Chloropropylate is a persistent and specific acaracide with biological
properties related to those of chlorobenzilate. The compound, although
registered for use in a relatively large number of countries, does not
seem to be extensively applied. No information was available on the
extent of use of this compound. Furthermore, the total composition of
the technical material has not been furnished. The limited residue
data which have been made available are mainly based upon experiments
in the United States of America and Switzerland. Residue data from
other countries are desired. No data have been furnished on the level
of residues in food moving in commerce, and on the rate of
disappearance during storage and processing.
In certain cases, e.g. grapes and blueberries, recommendations for
tolerances cannot be made until further data are available on the
possible carry-over of the residue in wine, and the necessity for the
utilization of the compound on blueberries. No information is
available on the nature of the terminal residues in plants and animal
products.
A referee method of analysis has not been established. However,
gas-liquid chromatography appears suitable for this purpose, but must
be further developed by collaborative studies. Comparative evaluation
of the different detectors used in gas chromatographic methods, and
evaluation of the different methods of extraction are needed.
Recommendations
Temporary tolerances
The following temporary tolerances (to be in effect until 1972) are to
apply to raw agricultural products moving in commerce unless otherwise
indicated. In the case of fruits and vegetables the tolerances should
be applied as soon as practicable after harvest and in any event prior
to actual retail to the public. In the case of commodities entering
international trade, the tolerances should be applied by the importing
country at the point of entry or as soon as practicable thereafter.
The figures refer to the parent compound only.
Apples, pears, citrus (on whole-fruit basis) -3.0 ppm
Tomatoes, cantaloupes -1.0 ppm
Further work or information
Required before 30 June 1972:
1. Information on the composition of the technical product.
2. Information on the nature of terminal residues in plants, animals,
and their products.
3. Data on the extent of use in various countries.
4. Data from countries other than the United States of America and
Switzerland on the required rates and frequencies of application,
pre-harvest intervals, and the resultant residues.
5. Data on residue levels in raw agricultural products moving in
commerce.
6. Data on the disappearance of residues during storage and
processing.
7. Data on the possible carry-over of residues into wine as a result
of the treatment of grapes.
8. Comparative evaluation of the different detectors used in
gas-liquid chromatographic methods and of different methods of
extraction for regulatory purposes.
9. Identification of metabolites other than dichlorobenzilic acid and
investigation of their toxicology.
10. Adequate reproduction studies in the rat or other species.
11. Further investigation of kidney function and excretion.
Desirable
Collaborative studies to establish a referee method.
REFERENCES
Cassidy, J. E., Min, B. and Murphy, R. T. (1968) Metabolic fate of
C14 chloropropylate, in white rats. A balance study. Geigy Chemical
Corp. Unpublished report
Domenjoz, R. (1965) Dichlorbenzilsäure Toxizität bei chronischer
Verabreichung. Institute of Pharmacology, Rheinische
Friedrich-Wilhelm's University, Bonn, Unpublished report
Geigy S.A., J.R. (1963) Chlorpropylat im Vergleich zu Chlorbenzilat.
Unpublished report
Geigy. (1964) Toxicology of chloropropylate (G24163). Geigy Chemical
Corp., unpublished report
Geigy S.A., J.R. (1968) Chloropropylate working paper. Unpublished
report
Hazleton Laboratories Inc. (1964) Method development for the analysis
of chloropropylate and chlorobenzilate in biological fluids and tissue
specimens. Metabolic distribution and excretion of chloropropylate and
chlorobenzilate in dogs, Unpublished report
Hazleton Laboratories Inc. (1965) Three-month dietary administration.
Dogs. Final report. Chloropropylate 50W, Unpublished report
Industrial Bio-Test Laboratories. (1965a) Acute toxicity studies on
chloropropylate 40W, Unpublished report
Industrial Bio-Test Laboratories. (1965b) Acute toxicity studies on
chloropropylate 25E, Unpublished report
Industrial Bio-Test Laboratories. (1967) Two-year chronic oral
toxicity of chloropropylate 40W. Beagle dogs. Twelve-month status
report, Unpublished report
Mattson, A. M., Beaudoin, R. L. and Schneller, J. (1965) Comparison of
urinary metabolites in dogs after administration of chlorobenzilate or
chloropropylate. Geigy Chemical Corp., Unpublished report
Murphy, R., Kahrs, R. and Mattson, A. M. (1966) Dissipation of
residues of chlorobenzilate and chloropropylate on apples. Analytical
Department of Geigy Research Laboratories, Division of Geigy Chem.
Corp., Ardsley, New York, Unpublished report
Sanderson, D. M. (1953) The avian toxicity of chloropropylate.
Chesterford Park Research Station. Fisons Pest Control Ltd.,
Unpublished report
Stenger. (1962) Chlorpropylat (G24163). Akute Toxizität - Maus per os.
Geigy Chemical Corp., Unpublished report
Stenger. (1963) G24163 (Chlorpropylat). Akute Toxizität - Ratte per
os. Geigy Chemical Corp., Unpublished report
Stenger. (1963b) G24103 (Chlorpropylat). Subchronische toxizität
- Ratte. Geigy Chemical Corp., Unpublished report
United States of America Federal Register 27, F.R. 12092, pav. 120-218
United States of America Department of Agriculture. (1967) Summary of
registered pesticide chemical uses, 30 September
Woodard. (1966a) Chloropropylate. Subacute toxicity in mallard ducks,
Unpublished report, Woodard Research Corp.
Woodard. (1966b) Chloropropylate. Safety evaluation on fish and wild
life (Bobwhite quail, rainbow trout, bluegill sunfish and goldfish),
Unpublished report, Woodard Research Corp.
Woodard. (1966c) Chloropropylate. Safety evaluation by dietary feeding
to rats for 104 weeks, Unpublished report, Woodard Research Corp.
Woodard. (1966-67) Chloropropylate. Three generation reproduction
study in the rat. Histological report addenda, Unpublished report,
Woodard Research Corp.
