
PROPIONYLPROMAZINE
First Draft Prepared by
Dr. William C. Keller
Food and Drug Administration
Rockville, Maryland, USA
and
Dr D.M. Pugh
University College
Dublin, Ireland
1. EXPLANATION
Propionylpromazine (PPZ) is a crystalline slightly yellow
powder with a melting point around 70 °C. It is a neuroleptic
phenothiazine derivative. Other similar drugs with wide veterinary
use include promazine and chlorpromazine. In the dog, PPZ is two to
four times more potent than chlorpromazine, but is in all other
respects clinically indistinguishable. In veterinary medicine the
hydrochloride salt is used as a 1% aqueous injectable solution.
Phenothiazine neuroleptic compounds are most often used as
antiemetics, antipruritics, and as premedication for anęsthesia in
veterinary medicine.
PPZ was in common use in veterinary practice in the 1950s and
1960s. PPZ is of interest to JECFA because of the illicit use at
pharmacological dosage (< 1 mg/kg i.m.) in the immediate
pre-slaughter period. PPZ is used to lessen weight loss, trauma,
disease, aggression and/or the prevalence of pale, soft exudative
(PSE) pork produced from stress-susceptible animals (Zacharias,
1975). This use of PPZ was reported as long ago as 1961 (Kaemmerer,
1961); it has caused concern and stimulated efforts to identify
resulting tissue residues (Haagsma, et al., 1988; Keukens and Aerts,
1989).
PPZ is reportedly used widely in horse racing to alter
performance, although approval for its use in horses was withdrawn
in the US following reports that its use caused irreversible penile
paralysis in stallions.
PPZ has not been evaluated previously by the Expert Committee.
Synonyms: Propiopromazine, Combelene, Tranvet, Tranvex
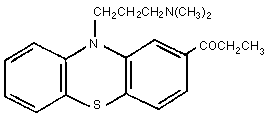 Molecular formula: C20H24N2OS
Molecular weight: 340.55
Chemical name: 1[10[3-(dimethylamino)-propyl]-10H-pheno-
thiazin-2-yl]-1-propanone
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, Distribution, and Excretion
The comparative distribution and depletion of PPZ was studied
in the rat and pig. Female Wistar rats and Landrace pigs were
injected with PPZ. Resulting tissue levels are shown at Tables 1 and
2.
Table 1. Propionylpromazine concentrations in tissues of the rat after i.v.
administration of 4 mg propiopromazine phosphate per kg body weight
Time after injection Propiopromazine concentration (µg/g)1
(h)
Kidney Liver Brain
0.2 16.2 ± 4.8 1.3 ± 0.4 5.6 ± 1.3
0.5 9.9 ± 3.2 1.2 ± 0.2 5.4 ± 0.8
1.0 7.0 ± 3.0 1.1 ± 0.3 2.7 ± 0.4
2.0 3.3 ± 1.5 0.6 ± 0.2 0.6 ± 0.2
1. Mean standard deviation of four individual rats.
Table 2. Propionylpromazine concentrations in pig tissues at different times after
i.m. injection of 0.5 mg of propiopromazine phosphate per kg body
weight (approximately 50 mg per animal)
Time after Sex* Concentrations (ng/g) Quantity (mg)
injection
(h)
Kidney Liver Brain Diaphragm Injection site
s 340 260 210 50 28.8
s 340 300 200 30 10.2
2 21.6 ± 8.0**
b 90 80 210 70 24.0
b 90 70 130 50 23.6
Table 2. cont'd
Time after Sex* Concentrations (ng/g) Quantity (mg)
injection
(h)
Kidney Liver Brain Diaphragm Injection site
s 150 200 190 50 15.0
s 150 240 130 30 21.4
8 19.3 ± 3.2**
b 50 80 190 60 18.5
b 110 170 180 60 22.1
s 50 240 40 < 10 4.6
s 40 370 50 10 7.4
24 5.7 ± 2.6**
b 40 120 50 10 8.3
b 80 190 100 20 2.6
Control s < 2 < 10 < 10 < 10 <0.001***
b < 2 < 10 < 10 < 10 <0.001***
* s, Sows; b, castrated boars
** Mean ± standard deviation
*** Same site as in treated animals
The investigators concluded there was a significant difference
in the ratios of kidney and liver residue concentrations in rats and
pigs, a potential sex-related difference in liver/kidney
distribution in swine, and an apparent distribution to liver at 24
hours in swine. All residues were in sub ppm concentration in pigs
and by 24 hours the liver was the target organ (x = 230 ng/g), a
concentration which differed little from that at two and eight hours
post-dosing. Kidney, brain and diaphragm concentrations had fallen
to about 25% of the 2 hour values by 24 hours. At 24 hours 10% to
20% of the administered dose remained at the injection site,
indicating a depot effect which can confuse interpretation of the
time-concentration data. Nonetheless, the 24-hour muscle
concentrations were at the limit of detection for this method.
(Olling et al., 1981).
Five pigs were dosed with PPZ at 0.5 mg base/kg body weight
intramuscularly and killed at two and four hours afterwards.
Residues in muscle, fat, kidney, liver, blood and urine were assayed
for PPZ and its sulfoxide by GLC. Use of a protease was beneficial
in increasing analytic yields from tissues, emphasizing the
importance of binding to protein as a feature of the distribution
phase of phenothiazine derivatives. The report stressed also the
ease with which light and oxygen can cause molecular change in this
group and, hence, analytical problems. No tissue concentration was
in the ppm range other than injection site. Depot fat was richest in
parent compound (< 367 µg/kg at 4 h), again a feature to be
anticipated with this highly lipophilic drug. Injection site
concentrations were in the trace to 863 µg/kg range five days after
dosing. In no case did tissue PPZ sulfoxide concentrations exceed
those of parent compound. In contrast, when urine was first
subjected to ß-glucuronidase the sulfoxide metabolite almost always
exceeded the concentration of PPZ itself. Detectable concentrations
persisted for up to 48 hours in three pigs (Arneth, 1986).
Other authors have described different analytical methods
capable of detecting PPZ in pig kidney in the open literature:
Keukens & Aerts (1989) used HPLC, Friedrich (1988) HPLC,
Scheutwinkel-Reich et al. (1982) GC-MS, Van Ginkel et al. (1988) LC
plus UV spectrum, Haagsma et al. (1988) 2-dimensional TLC. All the
above studies but one (Haagsma et al.) were methodological
evaluations using spiked tissue and yielded recoveries between 13%
and 95% with detection limits between 1 and 25 ppb.
Medicated pigs were dosed at recommended levels and slaughtered
two, five and eight hours later. Six pigs were used, two were
slaughtered at each interval and kidney, diaphragm, and injection
site were sampled. Only diaphragm tissue sampled after eight hours
yielded no detectable residue. Injection site tissues provided the
highest concentration of PPZ (Haagsma et al. (1988).
Several proprietary reports, all dating from 1977, report a
satisfactory fluorometric method for pig tissue residue studies of
PPZ and its sulfoxide (Putter, 1977), a study of PPZ concentration
in plasma (Putter, et al 1977), and PPZ concentration in various
tissues (Putter and Bauditz, 1977). Each reported concentrations
following a single intramuscular injection of PPZ at 2 mg/kg. In
plasma, trace concentrations were reached by 48 hours (n = 3), but
in tissues had not been reached by 72 hours (n = 6). Liver was the
target organ (88-119 µg/kg at 72 k). Urine was not studied.
2.1.2 Biotransformation
PPZ metabolites suitable for detection of illicit doping of
racehorses were investigated in horse urine. Three metabolites were
identified. The recommended metabolite for detection of doping was
2-(1-hydroxypropyl)promazine sulfoxide (Dewey and Maylin, 1984). In
a later paper (Park et al., 1989) the metabolism and
pharmacokinetics of PPZ in horses were also studied. The parent and
3 metabolites, 2-(1-hydroxy-propyl)promazine,
2-(1-propenyl)promazine, and 7-hydroxyprop-ionylpromazine were
identified in urine. The serum t“ was 5 hour.
2.1.3 Effects on enzymes and other biochemical parameters
The effects of PPZ and other tranquillizers on cerebrospinal
fluid and serum constituents were studied in the anaesthetized dog.
PPZ at 0.3 mg/kg decreased the concentration of urea N and Na+ in
cerebrospinal fluid. PPZ decreased serum urea N, increased serum
Ca++ and Cl-, and had no effect on glucose, creatinine,
potassium, or inorganic phosphates in cerebrospinal fluid or serum.
(Hassan et al., 1985a). The effects of PPZ and other tranquillizers
on cerebrospinal fluid constituents were further studied in the
anęsthetized dog at 0.6 mg/kg. PPZ lowered potassium levels
significantly but had no other effect. (Hassan et al., 1985b)
Serum and pituitary LH and FSH were reduced when 1.5 mg/kg PPZ
was given to mature male rats i.p. daily for 2 weeks (Ibrahim et
al., 1987).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
Species Sex Route LD50 (mg/kg) Reference
Mouse M&F i.v. 38(35.7-40.5) Silvestrini &
Quadri (1970)
2.2.2 Short-term studies
No information available.
2.2.3 Long-term/carcinogenicity studies
No information available.
2.2.4 Reproduction studies
No information available
2.2.7 Special studies on genotoxicity
Test system Test object Concentration Results Reference
Ames test S. typhimurium 1 mg, 5 mg Negative Preiss et al.
TA 1535, 100, (1983)
98, 1537, 1538
2.3 Observations in humans
There are no reports available from clinical studies of PPZ in
humans. The structure of PPZ, shown in Figure 1, is almost identical
to that of its structural isomer, propiomazine (Fig 2) (Booth,
1988). The effects of propiomazine have been reported in humans for
whom it is used as a tranquillizer and sleep-inducing agent. Its use
is particularly common in Scandinavia, where it has been used for
decades.
Molecular formula: C20H24N2OS
Molecular weight: 340.55
Chemical name: 1[10[3-(dimethylamino)-propyl]-10H-pheno-
thiazin-2-yl]-1-propanone
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, Distribution, and Excretion
The comparative distribution and depletion of PPZ was studied
in the rat and pig. Female Wistar rats and Landrace pigs were
injected with PPZ. Resulting tissue levels are shown at Tables 1 and
2.
Table 1. Propionylpromazine concentrations in tissues of the rat after i.v.
administration of 4 mg propiopromazine phosphate per kg body weight
Time after injection Propiopromazine concentration (µg/g)1
(h)
Kidney Liver Brain
0.2 16.2 ± 4.8 1.3 ± 0.4 5.6 ± 1.3
0.5 9.9 ± 3.2 1.2 ± 0.2 5.4 ± 0.8
1.0 7.0 ± 3.0 1.1 ± 0.3 2.7 ± 0.4
2.0 3.3 ± 1.5 0.6 ± 0.2 0.6 ± 0.2
1. Mean standard deviation of four individual rats.
Table 2. Propionylpromazine concentrations in pig tissues at different times after
i.m. injection of 0.5 mg of propiopromazine phosphate per kg body
weight (approximately 50 mg per animal)
Time after Sex* Concentrations (ng/g) Quantity (mg)
injection
(h)
Kidney Liver Brain Diaphragm Injection site
s 340 260 210 50 28.8
s 340 300 200 30 10.2
2 21.6 ± 8.0**
b 90 80 210 70 24.0
b 90 70 130 50 23.6
Table 2. cont'd
Time after Sex* Concentrations (ng/g) Quantity (mg)
injection
(h)
Kidney Liver Brain Diaphragm Injection site
s 150 200 190 50 15.0
s 150 240 130 30 21.4
8 19.3 ± 3.2**
b 50 80 190 60 18.5
b 110 170 180 60 22.1
s 50 240 40 < 10 4.6
s 40 370 50 10 7.4
24 5.7 ± 2.6**
b 40 120 50 10 8.3
b 80 190 100 20 2.6
Control s < 2 < 10 < 10 < 10 <0.001***
b < 2 < 10 < 10 < 10 <0.001***
* s, Sows; b, castrated boars
** Mean ± standard deviation
*** Same site as in treated animals
The investigators concluded there was a significant difference
in the ratios of kidney and liver residue concentrations in rats and
pigs, a potential sex-related difference in liver/kidney
distribution in swine, and an apparent distribution to liver at 24
hours in swine. All residues were in sub ppm concentration in pigs
and by 24 hours the liver was the target organ (x = 230 ng/g), a
concentration which differed little from that at two and eight hours
post-dosing. Kidney, brain and diaphragm concentrations had fallen
to about 25% of the 2 hour values by 24 hours. At 24 hours 10% to
20% of the administered dose remained at the injection site,
indicating a depot effect which can confuse interpretation of the
time-concentration data. Nonetheless, the 24-hour muscle
concentrations were at the limit of detection for this method.
(Olling et al., 1981).
Five pigs were dosed with PPZ at 0.5 mg base/kg body weight
intramuscularly and killed at two and four hours afterwards.
Residues in muscle, fat, kidney, liver, blood and urine were assayed
for PPZ and its sulfoxide by GLC. Use of a protease was beneficial
in increasing analytic yields from tissues, emphasizing the
importance of binding to protein as a feature of the distribution
phase of phenothiazine derivatives. The report stressed also the
ease with which light and oxygen can cause molecular change in this
group and, hence, analytical problems. No tissue concentration was
in the ppm range other than injection site. Depot fat was richest in
parent compound (< 367 µg/kg at 4 h), again a feature to be
anticipated with this highly lipophilic drug. Injection site
concentrations were in the trace to 863 µg/kg range five days after
dosing. In no case did tissue PPZ sulfoxide concentrations exceed
those of parent compound. In contrast, when urine was first
subjected to ß-glucuronidase the sulfoxide metabolite almost always
exceeded the concentration of PPZ itself. Detectable concentrations
persisted for up to 48 hours in three pigs (Arneth, 1986).
Other authors have described different analytical methods
capable of detecting PPZ in pig kidney in the open literature:
Keukens & Aerts (1989) used HPLC, Friedrich (1988) HPLC,
Scheutwinkel-Reich et al. (1982) GC-MS, Van Ginkel et al. (1988) LC
plus UV spectrum, Haagsma et al. (1988) 2-dimensional TLC. All the
above studies but one (Haagsma et al.) were methodological
evaluations using spiked tissue and yielded recoveries between 13%
and 95% with detection limits between 1 and 25 ppb.
Medicated pigs were dosed at recommended levels and slaughtered
two, five and eight hours later. Six pigs were used, two were
slaughtered at each interval and kidney, diaphragm, and injection
site were sampled. Only diaphragm tissue sampled after eight hours
yielded no detectable residue. Injection site tissues provided the
highest concentration of PPZ (Haagsma et al. (1988).
Several proprietary reports, all dating from 1977, report a
satisfactory fluorometric method for pig tissue residue studies of
PPZ and its sulfoxide (Putter, 1977), a study of PPZ concentration
in plasma (Putter, et al 1977), and PPZ concentration in various
tissues (Putter and Bauditz, 1977). Each reported concentrations
following a single intramuscular injection of PPZ at 2 mg/kg. In
plasma, trace concentrations were reached by 48 hours (n = 3), but
in tissues had not been reached by 72 hours (n = 6). Liver was the
target organ (88-119 µg/kg at 72 k). Urine was not studied.
2.1.2 Biotransformation
PPZ metabolites suitable for detection of illicit doping of
racehorses were investigated in horse urine. Three metabolites were
identified. The recommended metabolite for detection of doping was
2-(1-hydroxypropyl)promazine sulfoxide (Dewey and Maylin, 1984). In
a later paper (Park et al., 1989) the metabolism and
pharmacokinetics of PPZ in horses were also studied. The parent and
3 metabolites, 2-(1-hydroxy-propyl)promazine,
2-(1-propenyl)promazine, and 7-hydroxyprop-ionylpromazine were
identified in urine. The serum t“ was 5 hour.
2.1.3 Effects on enzymes and other biochemical parameters
The effects of PPZ and other tranquillizers on cerebrospinal
fluid and serum constituents were studied in the anaesthetized dog.
PPZ at 0.3 mg/kg decreased the concentration of urea N and Na+ in
cerebrospinal fluid. PPZ decreased serum urea N, increased serum
Ca++ and Cl-, and had no effect on glucose, creatinine,
potassium, or inorganic phosphates in cerebrospinal fluid or serum.
(Hassan et al., 1985a). The effects of PPZ and other tranquillizers
on cerebrospinal fluid constituents were further studied in the
anęsthetized dog at 0.6 mg/kg. PPZ lowered potassium levels
significantly but had no other effect. (Hassan et al., 1985b)
Serum and pituitary LH and FSH were reduced when 1.5 mg/kg PPZ
was given to mature male rats i.p. daily for 2 weeks (Ibrahim et
al., 1987).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
Species Sex Route LD50 (mg/kg) Reference
Mouse M&F i.v. 38(35.7-40.5) Silvestrini &
Quadri (1970)
2.2.2 Short-term studies
No information available.
2.2.3 Long-term/carcinogenicity studies
No information available.
2.2.4 Reproduction studies
No information available
2.2.7 Special studies on genotoxicity
Test system Test object Concentration Results Reference
Ames test S. typhimurium 1 mg, 5 mg Negative Preiss et al.
TA 1535, 100, (1983)
98, 1537, 1538
2.3 Observations in humans
There are no reports available from clinical studies of PPZ in
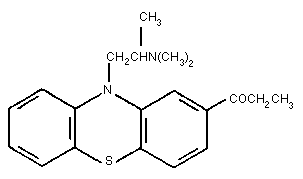
humans. The structure of PPZ, shown in Figure 1, is almost identical
to that of its structural isomer, propiomazine (Fig 2) (Booth,
1988). The effects of propiomazine have been reported in humans for
whom it is used as a tranquillizer and sleep-inducing agent. Its use
is particularly common in Scandinavia, where it has been used for
decades.
 Two recent clinical reports on the effects of propiomazine in
humans are summarized below:
The effects of propiomazine on sleep were studied in 10 healthy
adult volunteers. The subjects received 25 mg propiomazine orally on
5 consecutive nights. A decrease in sleep latency and suppression of
REM sleep, and an increase in sleep quality were reported (Almqvist
et al., 1987).
In another study 25 mg of propiomazine was given orally to 40
elderly subjects to study effect on sleep. Propiomazine was very
effective in 20 normal subjects, and also increased the duration of
sleep in psychogeriatric subjects (Viukari and Miettinen, 1984).
3. COMMENTS
There are no useful reports available from studies conducted to
determine the no effect level for PPZ in animals.
The manufacturer was unable to supply the Committee with data
on the pharmacology and toxicology of propionylpromazine and the
published literature has yielded relatively little information.
However, the drug is of known efficacy in clinical use. Both pigs
and horses are able to metabolize propionylpromazine, at least in
part, but neither case was a full distribution, metabolism,
elimination, or balance study available. A single value of 863 µg/kg
has been recorded at the injection site in a pig 5 days after the
intramuscular injection of 0.5 mg/kg of propionylpromazine. The drug
binds extensively to tissue, and to proteins, and also accumulates
in fatty tissues. In rats and pigs, the drug is able to enter the
brain and, in dogs, has been shown to cause minor changes in
cerebrospinal fluid and serum. The NOEL for these changes was
0.6 mg/kg given intravenously.
In rats, a reduction in the concentrations of pituitary
gonadotrophins has been demonstrated in brain and serum following
the daily intraperitoneal injection of propionylpromazine at
1.5 mg/kg for 2 weeks.
The intravenous LD50 of propionylpromazine in mice was
38 mg/kg.
In a 4-week study of groups of 5 female and 5 male rats exposed
to propionylpromazine in the diet at 0, 60, 360, and 2160 mg/kg
b.w./day, a decreased rate of weight gain was seen each week in both
sexes at the highest dose and in females at 360 mg/kg b.w./day,
which was associated with reduced feed intake. A reduction in
ovarian weight in all treated females and a dose-dependent reduction
in thyroid weight in males prevented the establishment of a NOEL in
this study. Slight periportal fatty infiltration, which sometimes
involved mid-zone and centrilobular hepatocytes, was seen in all
treated animals and was greatest in the high-dose group.
No short- or long-term studies, no systematic studies of the
effects of the drug on reproduction, or of its teratogenicity,
mutagenicity, carcinogenicity, or immunotoxicity, and no reports on
its use in humans were available to the Committee. However, several
studies have reported the presence of propionylpromazine in pig
kidneys collected from abattoirs so that human exposure must
therefore be presumed.
4. EVALUATION
The absence of information in several major areas of
pharmacological and toxicological importance made it impossible for
the Committee to establish an ADI for propionylpromazine. This is
regrettable because the Committee was aware that propionylpromazine
is used in circumstances in which the consumer will be exposed to
residues that may be capable of exerting a pharmacological effect.
5. REFERENCES
ALMQVIST, M., LILJENBERG, B., HETTA, J., RIMON, R., HAMBERT, G., &
ROOS, B. (1987) Effects of propiomazine on the EEG sleep of normal
subjects. Pharm and Toxicol., 61: 278-81.
ARNETH, W. (1986) Untersuchungen zur Verteilung von Combelen und
Combelen-Sulfoxid-Ruchstanden im Schwein. Fleischwirtschaft,
66(5): 922-925.
BOOTH, N.H. (1988) Psychotropic agents. In: Veterinary Pharmacology
and Therapeutics. ed Booth and McDonald Iowa State University
Press, Ames Iowa, 363-81.
DEWEY, E.A. & MAYLIN, G.A. (1984) Analysis of propionylpromazine and
its metabolites in horse urine. Cornell Veterinarian, 74: 38-49.
FRIEDRICH, A. (1988) Screening-Test zum qualiftativen Nachweis der
gebrauchlichsten Transportprotektoren (Sedativa, Betablocker) mit
Hilfe der Hochdruch flussigkeitschromatographie. Tierarztliche
Umschau., 43: 493-501.
Van GINKEL, L.A., SCHWILLENS, P.L.W.J. & OLLING, M. (1989) Liquid
chromatographic method with on-line UV spectrum identification and
off-line thin layer chromatographic confirmation for the defection
of tranquillisers and carazolol in pig kidneys. Analytica Chemica
Acta, 225: 137-146.
HAAGSMA, N, BATHELT, E.R., ENGELSMA, J.W. (1988) Thin-layer
chromatographic screening method for the tranquillizers azaperone,
propiopromazine and carazolol in pig tissues. J. Chromatography,
436: 73-9.
HASSAN, A.B., EL-KHALIK, A.A., ATTA, A.H., HASHIM, M. & ZAGHLOL,
H.A. (1985a) Effect of some tranquilizers on cerebrospinal fluid and
serum constituents in dogs, Vet Med J., 33: 2, 199-209.
HASSAN, A.B., EL-KHALIK, A.A., & ZAGHLOL, H.A. (1985b) Effect of
some tranquilizers on cerebrospinal fluid (CSF) in dogs. Egypt J.
Vet Sci., 21(2): 279-86.
IBRAHIM, S.S., EL-SADET, S.E. & ABOUL-ELA, A. (1987) Effects of some
tranquilizers on the levels of gonadotropins and thyroid activity in
mature male rats. Vet Med J., 35(1): 129-37.
KAEMMERER, K. (1961) Erfahrungen bei transportversuchen unter
Combelen-Schutz. Veterinarmedizinische Nachrichten, No. 2: 51-64.
KEUKENS, H.J. & AERTS, M.M. (1989) Determination of residues of
carazolol and a number of tranquillisers in swine kidney by high
performance liquid chromatography with ultraviolet and fluorescence
detection. Journal of Chromatography, 464: 149-161.
OLLING, M., STEPHANY, R.W. & RAUWS, A.G. (1981) The determination of
propiopromazine in animal tissue. Journal of Veterinary
Pharmacology and Therapeutics, 4: 291-294.
PARK, J., SHIN, Y.O. & CHOO, H.P. (1989) Metabolism and
pharmacokinetic studies of propionylpromazine in horses. Journal of
Chromatography, 489: 313-321.
PREISS A., SCHEUTWINKLE-REICH, M. & STAN, H.J. (1983) Mutagenic
nature of tranquilizers used in animal fattening as revealed in the
Salmonella/microsome test. Fleischwirtschaft (abs), 63(2):
243-4.
PUTTER, J. (1977) Determination of Combelen and its sulphoxide in
biological material. Unpublished Report (PHARMA REPORT No. 6733)
from Bayer AG. Submitted to WHO by Bayer AG, Leverkusen, Germany.
PUTTER, J., BAUDITZ, R., & DORN, H. (1977) Unpublished Report
(PHARMA REPORT No. 6790) from Bayer AG. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
PUTTER, J. & BAUDITZ, R. Combelen: Residue investigations in pigs.
Unpublished Report (PHARMA REPORT No. 6840) from Bayer AG. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
RAUWS, A.G. (1983) Tranquillisers in the transport of pigs destined
for slaughter: a residue problem? Tijdschrift Diergeneeskunde,
108: 659-664.
SCHEUTWINKEL-REICH, M., GROHMANN, H.G., JORDAN, S., PREISS, A.M. &
STAN, H.J. (1982) GC and GC-MS analysis of tranquillisers in meat.
Fresenius Zeitung für Analytische Chemie, 311: 398-399.
SILVESTRINI B., & QUADRI, E. (1970) Investigations on the
specificity of the so-called analgesic activity of non-narcotic
drugs. European J. of Pharmacology, 12: 231-235.
VIUKARI, M. & MIETTINEN, P. (1984) Diazepam, promethazine and
propiomazine as hypnotics in elderly inpatients.
Neuropsychobiology, 12: 134-7.
ZACHARIAS, H. (1975) Sedation von Schlachtschweinen vor der Transort
und die sich daraus ergebendedn veterinarmedizinischen, rechtlichen
wie fleishhygienischen Fragen. Tierartzliche Umschau, 30: 598-600.
Two recent clinical reports on the effects of propiomazine in
humans are summarized below:
The effects of propiomazine on sleep were studied in 10 healthy
adult volunteers. The subjects received 25 mg propiomazine orally on
5 consecutive nights. A decrease in sleep latency and suppression of
REM sleep, and an increase in sleep quality were reported (Almqvist
et al., 1987).
In another study 25 mg of propiomazine was given orally to 40
elderly subjects to study effect on sleep. Propiomazine was very
effective in 20 normal subjects, and also increased the duration of
sleep in psychogeriatric subjects (Viukari and Miettinen, 1984).
3. COMMENTS
There are no useful reports available from studies conducted to
determine the no effect level for PPZ in animals.
The manufacturer was unable to supply the Committee with data
on the pharmacology and toxicology of propionylpromazine and the
published literature has yielded relatively little information.
However, the drug is of known efficacy in clinical use. Both pigs
and horses are able to metabolize propionylpromazine, at least in
part, but neither case was a full distribution, metabolism,
elimination, or balance study available. A single value of 863 µg/kg
has been recorded at the injection site in a pig 5 days after the
intramuscular injection of 0.5 mg/kg of propionylpromazine. The drug
binds extensively to tissue, and to proteins, and also accumulates
in fatty tissues. In rats and pigs, the drug is able to enter the
brain and, in dogs, has been shown to cause minor changes in
cerebrospinal fluid and serum. The NOEL for these changes was
0.6 mg/kg given intravenously.
In rats, a reduction in the concentrations of pituitary
gonadotrophins has been demonstrated in brain and serum following
the daily intraperitoneal injection of propionylpromazine at
1.5 mg/kg for 2 weeks.
The intravenous LD50 of propionylpromazine in mice was
38 mg/kg.
In a 4-week study of groups of 5 female and 5 male rats exposed
to propionylpromazine in the diet at 0, 60, 360, and 2160 mg/kg
b.w./day, a decreased rate of weight gain was seen each week in both
sexes at the highest dose and in females at 360 mg/kg b.w./day,
which was associated with reduced feed intake. A reduction in
ovarian weight in all treated females and a dose-dependent reduction
in thyroid weight in males prevented the establishment of a NOEL in
this study. Slight periportal fatty infiltration, which sometimes
involved mid-zone and centrilobular hepatocytes, was seen in all
treated animals and was greatest in the high-dose group.
No short- or long-term studies, no systematic studies of the
effects of the drug on reproduction, or of its teratogenicity,
mutagenicity, carcinogenicity, or immunotoxicity, and no reports on
its use in humans were available to the Committee. However, several
studies have reported the presence of propionylpromazine in pig
kidneys collected from abattoirs so that human exposure must
therefore be presumed.
4. EVALUATION
The absence of information in several major areas of
pharmacological and toxicological importance made it impossible for
the Committee to establish an ADI for propionylpromazine. This is
regrettable because the Committee was aware that propionylpromazine
is used in circumstances in which the consumer will be exposed to
residues that may be capable of exerting a pharmacological effect.
5. REFERENCES
ALMQVIST, M., LILJENBERG, B., HETTA, J., RIMON, R., HAMBERT, G., &
ROOS, B. (1987) Effects of propiomazine on the EEG sleep of normal
subjects. Pharm and Toxicol., 61: 278-81.
ARNETH, W. (1986) Untersuchungen zur Verteilung von Combelen und
Combelen-Sulfoxid-Ruchstanden im Schwein. Fleischwirtschaft,
66(5): 922-925.
BOOTH, N.H. (1988) Psychotropic agents. In: Veterinary Pharmacology
and Therapeutics. ed Booth and McDonald Iowa State University
Press, Ames Iowa, 363-81.
DEWEY, E.A. & MAYLIN, G.A. (1984) Analysis of propionylpromazine and
its metabolites in horse urine. Cornell Veterinarian, 74: 38-49.
FRIEDRICH, A. (1988) Screening-Test zum qualiftativen Nachweis der
gebrauchlichsten Transportprotektoren (Sedativa, Betablocker) mit
Hilfe der Hochdruch flussigkeitschromatographie. Tierarztliche
Umschau., 43: 493-501.
Van GINKEL, L.A., SCHWILLENS, P.L.W.J. & OLLING, M. (1989) Liquid
chromatographic method with on-line UV spectrum identification and
off-line thin layer chromatographic confirmation for the defection
of tranquillisers and carazolol in pig kidneys. Analytica Chemica
Acta, 225: 137-146.
HAAGSMA, N, BATHELT, E.R., ENGELSMA, J.W. (1988) Thin-layer
chromatographic screening method for the tranquillizers azaperone,
propiopromazine and carazolol in pig tissues. J. Chromatography,
436: 73-9.
HASSAN, A.B., EL-KHALIK, A.A., ATTA, A.H., HASHIM, M. & ZAGHLOL,
H.A. (1985a) Effect of some tranquilizers on cerebrospinal fluid and
serum constituents in dogs, Vet Med J., 33: 2, 199-209.
HASSAN, A.B., EL-KHALIK, A.A., & ZAGHLOL, H.A. (1985b) Effect of
some tranquilizers on cerebrospinal fluid (CSF) in dogs. Egypt J.
Vet Sci., 21(2): 279-86.
IBRAHIM, S.S., EL-SADET, S.E. & ABOUL-ELA, A. (1987) Effects of some
tranquilizers on the levels of gonadotropins and thyroid activity in
mature male rats. Vet Med J., 35(1): 129-37.
KAEMMERER, K. (1961) Erfahrungen bei transportversuchen unter
Combelen-Schutz. Veterinarmedizinische Nachrichten, No. 2: 51-64.
KEUKENS, H.J. & AERTS, M.M. (1989) Determination of residues of
carazolol and a number of tranquillisers in swine kidney by high
performance liquid chromatography with ultraviolet and fluorescence
detection. Journal of Chromatography, 464: 149-161.
OLLING, M., STEPHANY, R.W. & RAUWS, A.G. (1981) The determination of
propiopromazine in animal tissue. Journal of Veterinary
Pharmacology and Therapeutics, 4: 291-294.
PARK, J., SHIN, Y.O. & CHOO, H.P. (1989) Metabolism and
pharmacokinetic studies of propionylpromazine in horses. Journal of
Chromatography, 489: 313-321.
PREISS A., SCHEUTWINKLE-REICH, M. & STAN, H.J. (1983) Mutagenic
nature of tranquilizers used in animal fattening as revealed in the
Salmonella/microsome test. Fleischwirtschaft (abs), 63(2):
243-4.
PUTTER, J. (1977) Determination of Combelen and its sulphoxide in
biological material. Unpublished Report (PHARMA REPORT No. 6733)
from Bayer AG. Submitted to WHO by Bayer AG, Leverkusen, Germany.
PUTTER, J., BAUDITZ, R., & DORN, H. (1977) Unpublished Report
(PHARMA REPORT No. 6790) from Bayer AG. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
PUTTER, J. & BAUDITZ, R. Combelen: Residue investigations in pigs.
Unpublished Report (PHARMA REPORT No. 6840) from Bayer AG. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
RAUWS, A.G. (1983) Tranquillisers in the transport of pigs destined
for slaughter: a residue problem? Tijdschrift Diergeneeskunde,
108: 659-664.
SCHEUTWINKEL-REICH, M., GROHMANN, H.G., JORDAN, S., PREISS, A.M. &
STAN, H.J. (1982) GC and GC-MS analysis of tranquillisers in meat.
Fresenius Zeitung für Analytische Chemie, 311: 398-399.
SILVESTRINI B., & QUADRI, E. (1970) Investigations on the
specificity of the so-called analgesic activity of non-narcotic
drugs. European J. of Pharmacology, 12: 231-235.
VIUKARI, M. & MIETTINEN, P. (1984) Diazepam, promethazine and
propiomazine as hypnotics in elderly inpatients.
Neuropsychobiology, 12: 134-7.
ZACHARIAS, H. (1975) Sedation von Schlachtschweinen vor der Transort
und die sich daraus ergebendedn veterinarmedizinischen, rechtlichen
wie fleishhygienischen Fragen. Tierartzliche Umschau, 30: 598-600.
 Molecular formula: C20H24N2OS
Molecular weight: 340.55
Chemical name: 1[10[3-(dimethylamino)-propyl]-10H-pheno-
thiazin-2-yl]-1-propanone
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, Distribution, and Excretion
The comparative distribution and depletion of PPZ was studied
in the rat and pig. Female Wistar rats and Landrace pigs were
injected with PPZ. Resulting tissue levels are shown at Tables 1 and
2.
Table 1. Propionylpromazine concentrations in tissues of the rat after i.v.
administration of 4 mg propiopromazine phosphate per kg body weight
Time after injection Propiopromazine concentration (µg/g)1
(h)
Kidney Liver Brain
0.2 16.2 ± 4.8 1.3 ± 0.4 5.6 ± 1.3
0.5 9.9 ± 3.2 1.2 ± 0.2 5.4 ± 0.8
1.0 7.0 ± 3.0 1.1 ± 0.3 2.7 ± 0.4
2.0 3.3 ± 1.5 0.6 ± 0.2 0.6 ± 0.2
1. Mean standard deviation of four individual rats.
Table 2. Propionylpromazine concentrations in pig tissues at different times after
i.m. injection of 0.5 mg of propiopromazine phosphate per kg body
weight (approximately 50 mg per animal)
Time after Sex* Concentrations (ng/g) Quantity (mg)
injection
(h)
Kidney Liver Brain Diaphragm Injection site
s 340 260 210 50 28.8
s 340 300 200 30 10.2
2 21.6 ± 8.0**
b 90 80 210 70 24.0
b 90 70 130 50 23.6
Table 2. cont'd
Time after Sex* Concentrations (ng/g) Quantity (mg)
injection
(h)
Kidney Liver Brain Diaphragm Injection site
s 150 200 190 50 15.0
s 150 240 130 30 21.4
8 19.3 ± 3.2**
b 50 80 190 60 18.5
b 110 170 180 60 22.1
s 50 240 40 < 10 4.6
s 40 370 50 10 7.4
24 5.7 ± 2.6**
b 40 120 50 10 8.3
b 80 190 100 20 2.6
Control s < 2 < 10 < 10 < 10 <0.001***
b < 2 < 10 < 10 < 10 <0.001***
* s, Sows; b, castrated boars
** Mean ± standard deviation
*** Same site as in treated animals
The investigators concluded there was a significant difference
in the ratios of kidney and liver residue concentrations in rats and
pigs, a potential sex-related difference in liver/kidney
distribution in swine, and an apparent distribution to liver at 24
hours in swine. All residues were in sub ppm concentration in pigs
and by 24 hours the liver was the target organ (x = 230 ng/g), a
concentration which differed little from that at two and eight hours
post-dosing. Kidney, brain and diaphragm concentrations had fallen
to about 25% of the 2 hour values by 24 hours. At 24 hours 10% to
20% of the administered dose remained at the injection site,
indicating a depot effect which can confuse interpretation of the
time-concentration data. Nonetheless, the 24-hour muscle
concentrations were at the limit of detection for this method.
(Olling et al., 1981).
Five pigs were dosed with PPZ at 0.5 mg base/kg body weight
intramuscularly and killed at two and four hours afterwards.
Residues in muscle, fat, kidney, liver, blood and urine were assayed
for PPZ and its sulfoxide by GLC. Use of a protease was beneficial
in increasing analytic yields from tissues, emphasizing the
importance of binding to protein as a feature of the distribution
phase of phenothiazine derivatives. The report stressed also the
ease with which light and oxygen can cause molecular change in this
group and, hence, analytical problems. No tissue concentration was
in the ppm range other than injection site. Depot fat was richest in
parent compound (< 367 µg/kg at 4 h), again a feature to be
anticipated with this highly lipophilic drug. Injection site
concentrations were in the trace to 863 µg/kg range five days after
dosing. In no case did tissue PPZ sulfoxide concentrations exceed
those of parent compound. In contrast, when urine was first
subjected to ß-glucuronidase the sulfoxide metabolite almost always
exceeded the concentration of PPZ itself. Detectable concentrations
persisted for up to 48 hours in three pigs (Arneth, 1986).
Other authors have described different analytical methods
capable of detecting PPZ in pig kidney in the open literature:
Keukens & Aerts (1989) used HPLC, Friedrich (1988) HPLC,
Scheutwinkel-Reich et al. (1982) GC-MS, Van Ginkel et al. (1988) LC
plus UV spectrum, Haagsma et al. (1988) 2-dimensional TLC. All the
above studies but one (Haagsma et al.) were methodological
evaluations using spiked tissue and yielded recoveries between 13%
and 95% with detection limits between 1 and 25 ppb.
Medicated pigs were dosed at recommended levels and slaughtered
two, five and eight hours later. Six pigs were used, two were
slaughtered at each interval and kidney, diaphragm, and injection
site were sampled. Only diaphragm tissue sampled after eight hours
yielded no detectable residue. Injection site tissues provided the
highest concentration of PPZ (Haagsma et al. (1988).
Several proprietary reports, all dating from 1977, report a
satisfactory fluorometric method for pig tissue residue studies of
PPZ and its sulfoxide (Putter, 1977), a study of PPZ concentration
in plasma (Putter, et al 1977), and PPZ concentration in various
tissues (Putter and Bauditz, 1977). Each reported concentrations
following a single intramuscular injection of PPZ at 2 mg/kg. In
plasma, trace concentrations were reached by 48 hours (n = 3), but
in tissues had not been reached by 72 hours (n = 6). Liver was the
target organ (88-119 µg/kg at 72 k). Urine was not studied.
2.1.2 Biotransformation
PPZ metabolites suitable for detection of illicit doping of
racehorses were investigated in horse urine. Three metabolites were
identified. The recommended metabolite for detection of doping was
2-(1-hydroxypropyl)promazine sulfoxide (Dewey and Maylin, 1984). In
a later paper (Park et al., 1989) the metabolism and
pharmacokinetics of PPZ in horses were also studied. The parent and
3 metabolites, 2-(1-hydroxy-propyl)promazine,
2-(1-propenyl)promazine, and 7-hydroxyprop-ionylpromazine were
identified in urine. The serum t“ was 5 hour.
2.1.3 Effects on enzymes and other biochemical parameters
The effects of PPZ and other tranquillizers on cerebrospinal
fluid and serum constituents were studied in the anaesthetized dog.
PPZ at 0.3 mg/kg decreased the concentration of urea N and Na+ in
cerebrospinal fluid. PPZ decreased serum urea N, increased serum
Ca++ and Cl-, and had no effect on glucose, creatinine,
potassium, or inorganic phosphates in cerebrospinal fluid or serum.
(Hassan et al., 1985a). The effects of PPZ and other tranquillizers
on cerebrospinal fluid constituents were further studied in the
anęsthetized dog at 0.6 mg/kg. PPZ lowered potassium levels
significantly but had no other effect. (Hassan et al., 1985b)
Serum and pituitary LH and FSH were reduced when 1.5 mg/kg PPZ
was given to mature male rats i.p. daily for 2 weeks (Ibrahim et
al., 1987).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
Species Sex Route LD50 (mg/kg) Reference
Mouse M&F i.v. 38(35.7-40.5) Silvestrini &
Quadri (1970)
2.2.2 Short-term studies
No information available.
2.2.3 Long-term/carcinogenicity studies
No information available.
2.2.4 Reproduction studies
No information available
2.2.7 Special studies on genotoxicity
Test system Test object Concentration Results Reference
Ames test S. typhimurium 1 mg, 5 mg Negative Preiss et al.
TA 1535, 100, (1983)
98, 1537, 1538
2.3 Observations in humans
There are no reports available from clinical studies of PPZ in
humans. The structure of PPZ, shown in Figure 1, is almost identical
to that of its structural isomer, propiomazine (Fig 2) (Booth,
1988). The effects of propiomazine have been reported in humans for
whom it is used as a tranquillizer and sleep-inducing agent. Its use
is particularly common in Scandinavia, where it has been used for
decades.
Molecular formula: C20H24N2OS
Molecular weight: 340.55
Chemical name: 1[10[3-(dimethylamino)-propyl]-10H-pheno-
thiazin-2-yl]-1-propanone
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, Distribution, and Excretion
The comparative distribution and depletion of PPZ was studied
in the rat and pig. Female Wistar rats and Landrace pigs were
injected with PPZ. Resulting tissue levels are shown at Tables 1 and
2.
Table 1. Propionylpromazine concentrations in tissues of the rat after i.v.
administration of 4 mg propiopromazine phosphate per kg body weight
Time after injection Propiopromazine concentration (µg/g)1
(h)
Kidney Liver Brain
0.2 16.2 ± 4.8 1.3 ± 0.4 5.6 ± 1.3
0.5 9.9 ± 3.2 1.2 ± 0.2 5.4 ± 0.8
1.0 7.0 ± 3.0 1.1 ± 0.3 2.7 ± 0.4
2.0 3.3 ± 1.5 0.6 ± 0.2 0.6 ± 0.2
1. Mean standard deviation of four individual rats.
Table 2. Propionylpromazine concentrations in pig tissues at different times after
i.m. injection of 0.5 mg of propiopromazine phosphate per kg body
weight (approximately 50 mg per animal)
Time after Sex* Concentrations (ng/g) Quantity (mg)
injection
(h)
Kidney Liver Brain Diaphragm Injection site
s 340 260 210 50 28.8
s 340 300 200 30 10.2
2 21.6 ± 8.0**
b 90 80 210 70 24.0
b 90 70 130 50 23.6
Table 2. cont'd
Time after Sex* Concentrations (ng/g) Quantity (mg)
injection
(h)
Kidney Liver Brain Diaphragm Injection site
s 150 200 190 50 15.0
s 150 240 130 30 21.4
8 19.3 ± 3.2**
b 50 80 190 60 18.5
b 110 170 180 60 22.1
s 50 240 40 < 10 4.6
s 40 370 50 10 7.4
24 5.7 ± 2.6**
b 40 120 50 10 8.3
b 80 190 100 20 2.6
Control s < 2 < 10 < 10 < 10 <0.001***
b < 2 < 10 < 10 < 10 <0.001***
* s, Sows; b, castrated boars
** Mean ± standard deviation
*** Same site as in treated animals
The investigators concluded there was a significant difference
in the ratios of kidney and liver residue concentrations in rats and
pigs, a potential sex-related difference in liver/kidney
distribution in swine, and an apparent distribution to liver at 24
hours in swine. All residues were in sub ppm concentration in pigs
and by 24 hours the liver was the target organ (x = 230 ng/g), a
concentration which differed little from that at two and eight hours
post-dosing. Kidney, brain and diaphragm concentrations had fallen
to about 25% of the 2 hour values by 24 hours. At 24 hours 10% to
20% of the administered dose remained at the injection site,
indicating a depot effect which can confuse interpretation of the
time-concentration data. Nonetheless, the 24-hour muscle
concentrations were at the limit of detection for this method.
(Olling et al., 1981).
Five pigs were dosed with PPZ at 0.5 mg base/kg body weight
intramuscularly and killed at two and four hours afterwards.
Residues in muscle, fat, kidney, liver, blood and urine were assayed
for PPZ and its sulfoxide by GLC. Use of a protease was beneficial
in increasing analytic yields from tissues, emphasizing the
importance of binding to protein as a feature of the distribution
phase of phenothiazine derivatives. The report stressed also the
ease with which light and oxygen can cause molecular change in this
group and, hence, analytical problems. No tissue concentration was
in the ppm range other than injection site. Depot fat was richest in
parent compound (< 367 µg/kg at 4 h), again a feature to be
anticipated with this highly lipophilic drug. Injection site
concentrations were in the trace to 863 µg/kg range five days after
dosing. In no case did tissue PPZ sulfoxide concentrations exceed
those of parent compound. In contrast, when urine was first
subjected to ß-glucuronidase the sulfoxide metabolite almost always
exceeded the concentration of PPZ itself. Detectable concentrations
persisted for up to 48 hours in three pigs (Arneth, 1986).
Other authors have described different analytical methods
capable of detecting PPZ in pig kidney in the open literature:
Keukens & Aerts (1989) used HPLC, Friedrich (1988) HPLC,
Scheutwinkel-Reich et al. (1982) GC-MS, Van Ginkel et al. (1988) LC
plus UV spectrum, Haagsma et al. (1988) 2-dimensional TLC. All the
above studies but one (Haagsma et al.) were methodological
evaluations using spiked tissue and yielded recoveries between 13%
and 95% with detection limits between 1 and 25 ppb.
Medicated pigs were dosed at recommended levels and slaughtered
two, five and eight hours later. Six pigs were used, two were
slaughtered at each interval and kidney, diaphragm, and injection
site were sampled. Only diaphragm tissue sampled after eight hours
yielded no detectable residue. Injection site tissues provided the
highest concentration of PPZ (Haagsma et al. (1988).
Several proprietary reports, all dating from 1977, report a
satisfactory fluorometric method for pig tissue residue studies of
PPZ and its sulfoxide (Putter, 1977), a study of PPZ concentration
in plasma (Putter, et al 1977), and PPZ concentration in various
tissues (Putter and Bauditz, 1977). Each reported concentrations
following a single intramuscular injection of PPZ at 2 mg/kg. In
plasma, trace concentrations were reached by 48 hours (n = 3), but
in tissues had not been reached by 72 hours (n = 6). Liver was the
target organ (88-119 µg/kg at 72 k). Urine was not studied.
2.1.2 Biotransformation
PPZ metabolites suitable for detection of illicit doping of
racehorses were investigated in horse urine. Three metabolites were
identified. The recommended metabolite for detection of doping was
2-(1-hydroxypropyl)promazine sulfoxide (Dewey and Maylin, 1984). In
a later paper (Park et al., 1989) the metabolism and
pharmacokinetics of PPZ in horses were also studied. The parent and
3 metabolites, 2-(1-hydroxy-propyl)promazine,
2-(1-propenyl)promazine, and 7-hydroxyprop-ionylpromazine were
identified in urine. The serum t“ was 5 hour.
2.1.3 Effects on enzymes and other biochemical parameters
The effects of PPZ and other tranquillizers on cerebrospinal
fluid and serum constituents were studied in the anaesthetized dog.
PPZ at 0.3 mg/kg decreased the concentration of urea N and Na+ in
cerebrospinal fluid. PPZ decreased serum urea N, increased serum
Ca++ and Cl-, and had no effect on glucose, creatinine,
potassium, or inorganic phosphates in cerebrospinal fluid or serum.
(Hassan et al., 1985a). The effects of PPZ and other tranquillizers
on cerebrospinal fluid constituents were further studied in the
anęsthetized dog at 0.6 mg/kg. PPZ lowered potassium levels
significantly but had no other effect. (Hassan et al., 1985b)
Serum and pituitary LH and FSH were reduced when 1.5 mg/kg PPZ
was given to mature male rats i.p. daily for 2 weeks (Ibrahim et
al., 1987).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
Species Sex Route LD50 (mg/kg) Reference
Mouse M&F i.v. 38(35.7-40.5) Silvestrini &
Quadri (1970)
2.2.2 Short-term studies
No information available.
2.2.3 Long-term/carcinogenicity studies
No information available.
2.2.4 Reproduction studies
No information available
2.2.7 Special studies on genotoxicity
Test system Test object Concentration Results Reference
Ames test S. typhimurium 1 mg, 5 mg Negative Preiss et al.
TA 1535, 100, (1983)
98, 1537, 1538
2.3 Observations in humans
There are no reports available from clinical studies of PPZ in
humans. The structure of PPZ, shown in Figure 1, is almost identical
to that of its structural isomer, propiomazine (Fig 2) (Booth,
1988). The effects of propiomazine have been reported in humans for
whom it is used as a tranquillizer and sleep-inducing agent. Its use
is particularly common in Scandinavia, where it has been used for
decades.
 Two recent clinical reports on the effects of propiomazine in
humans are summarized below:
The effects of propiomazine on sleep were studied in 10 healthy
adult volunteers. The subjects received 25 mg propiomazine orally on
5 consecutive nights. A decrease in sleep latency and suppression of
REM sleep, and an increase in sleep quality were reported (Almqvist
et al., 1987).
In another study 25 mg of propiomazine was given orally to 40
elderly subjects to study effect on sleep. Propiomazine was very
effective in 20 normal subjects, and also increased the duration of
sleep in psychogeriatric subjects (Viukari and Miettinen, 1984).
3. COMMENTS
There are no useful reports available from studies conducted to
determine the no effect level for PPZ in animals.
The manufacturer was unable to supply the Committee with data
on the pharmacology and toxicology of propionylpromazine and the
published literature has yielded relatively little information.
However, the drug is of known efficacy in clinical use. Both pigs
and horses are able to metabolize propionylpromazine, at least in
part, but neither case was a full distribution, metabolism,
elimination, or balance study available. A single value of 863 µg/kg
has been recorded at the injection site in a pig 5 days after the
intramuscular injection of 0.5 mg/kg of propionylpromazine. The drug
binds extensively to tissue, and to proteins, and also accumulates
in fatty tissues. In rats and pigs, the drug is able to enter the
brain and, in dogs, has been shown to cause minor changes in
cerebrospinal fluid and serum. The NOEL for these changes was
0.6 mg/kg given intravenously.
In rats, a reduction in the concentrations of pituitary
gonadotrophins has been demonstrated in brain and serum following
the daily intraperitoneal injection of propionylpromazine at
1.5 mg/kg for 2 weeks.
The intravenous LD50 of propionylpromazine in mice was
38 mg/kg.
In a 4-week study of groups of 5 female and 5 male rats exposed
to propionylpromazine in the diet at 0, 60, 360, and 2160 mg/kg
b.w./day, a decreased rate of weight gain was seen each week in both
sexes at the highest dose and in females at 360 mg/kg b.w./day,
which was associated with reduced feed intake. A reduction in
ovarian weight in all treated females and a dose-dependent reduction
in thyroid weight in males prevented the establishment of a NOEL in
this study. Slight periportal fatty infiltration, which sometimes
involved mid-zone and centrilobular hepatocytes, was seen in all
treated animals and was greatest in the high-dose group.
No short- or long-term studies, no systematic studies of the
effects of the drug on reproduction, or of its teratogenicity,
mutagenicity, carcinogenicity, or immunotoxicity, and no reports on
its use in humans were available to the Committee. However, several
studies have reported the presence of propionylpromazine in pig
kidneys collected from abattoirs so that human exposure must
therefore be presumed.
4. EVALUATION
The absence of information in several major areas of
pharmacological and toxicological importance made it impossible for
the Committee to establish an ADI for propionylpromazine. This is
regrettable because the Committee was aware that propionylpromazine
is used in circumstances in which the consumer will be exposed to
residues that may be capable of exerting a pharmacological effect.
5. REFERENCES
ALMQVIST, M., LILJENBERG, B., HETTA, J., RIMON, R., HAMBERT, G., &
ROOS, B. (1987) Effects of propiomazine on the EEG sleep of normal
subjects. Pharm and Toxicol., 61: 278-81.
ARNETH, W. (1986) Untersuchungen zur Verteilung von Combelen und
Combelen-Sulfoxid-Ruchstanden im Schwein. Fleischwirtschaft,
66(5): 922-925.
BOOTH, N.H. (1988) Psychotropic agents. In: Veterinary Pharmacology
and Therapeutics. ed Booth and McDonald Iowa State University
Press, Ames Iowa, 363-81.
DEWEY, E.A. & MAYLIN, G.A. (1984) Analysis of propionylpromazine and
its metabolites in horse urine. Cornell Veterinarian, 74: 38-49.
FRIEDRICH, A. (1988) Screening-Test zum qualiftativen Nachweis der
gebrauchlichsten Transportprotektoren (Sedativa, Betablocker) mit
Hilfe der Hochdruch flussigkeitschromatographie. Tierarztliche
Umschau., 43: 493-501.
Van GINKEL, L.A., SCHWILLENS, P.L.W.J. & OLLING, M. (1989) Liquid
chromatographic method with on-line UV spectrum identification and
off-line thin layer chromatographic confirmation for the defection
of tranquillisers and carazolol in pig kidneys. Analytica Chemica
Acta, 225: 137-146.
HAAGSMA, N, BATHELT, E.R., ENGELSMA, J.W. (1988) Thin-layer
chromatographic screening method for the tranquillizers azaperone,
propiopromazine and carazolol in pig tissues. J. Chromatography,
436: 73-9.
HASSAN, A.B., EL-KHALIK, A.A., ATTA, A.H., HASHIM, M. & ZAGHLOL,
H.A. (1985a) Effect of some tranquilizers on cerebrospinal fluid and
serum constituents in dogs, Vet Med J., 33: 2, 199-209.
HASSAN, A.B., EL-KHALIK, A.A., & ZAGHLOL, H.A. (1985b) Effect of
some tranquilizers on cerebrospinal fluid (CSF) in dogs. Egypt J.
Vet Sci., 21(2): 279-86.
IBRAHIM, S.S., EL-SADET, S.E. & ABOUL-ELA, A. (1987) Effects of some
tranquilizers on the levels of gonadotropins and thyroid activity in
mature male rats. Vet Med J., 35(1): 129-37.
KAEMMERER, K. (1961) Erfahrungen bei transportversuchen unter
Combelen-Schutz. Veterinarmedizinische Nachrichten, No. 2: 51-64.
KEUKENS, H.J. & AERTS, M.M. (1989) Determination of residues of
carazolol and a number of tranquillisers in swine kidney by high
performance liquid chromatography with ultraviolet and fluorescence
detection. Journal of Chromatography, 464: 149-161.
OLLING, M., STEPHANY, R.W. & RAUWS, A.G. (1981) The determination of
propiopromazine in animal tissue. Journal of Veterinary
Pharmacology and Therapeutics, 4: 291-294.
PARK, J., SHIN, Y.O. & CHOO, H.P. (1989) Metabolism and
pharmacokinetic studies of propionylpromazine in horses. Journal of
Chromatography, 489: 313-321.
PREISS A., SCHEUTWINKLE-REICH, M. & STAN, H.J. (1983) Mutagenic
nature of tranquilizers used in animal fattening as revealed in the
Salmonella/microsome test. Fleischwirtschaft (abs), 63(2):
243-4.
PUTTER, J. (1977) Determination of Combelen and its sulphoxide in
biological material. Unpublished Report (PHARMA REPORT No. 6733)
from Bayer AG. Submitted to WHO by Bayer AG, Leverkusen, Germany.
PUTTER, J., BAUDITZ, R., & DORN, H. (1977) Unpublished Report
(PHARMA REPORT No. 6790) from Bayer AG. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
PUTTER, J. & BAUDITZ, R. Combelen: Residue investigations in pigs.
Unpublished Report (PHARMA REPORT No. 6840) from Bayer AG. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
RAUWS, A.G. (1983) Tranquillisers in the transport of pigs destined
for slaughter: a residue problem? Tijdschrift Diergeneeskunde,
108: 659-664.
SCHEUTWINKEL-REICH, M., GROHMANN, H.G., JORDAN, S., PREISS, A.M. &
STAN, H.J. (1982) GC and GC-MS analysis of tranquillisers in meat.
Fresenius Zeitung für Analytische Chemie, 311: 398-399.
SILVESTRINI B., & QUADRI, E. (1970) Investigations on the
specificity of the so-called analgesic activity of non-narcotic
drugs. European J. of Pharmacology, 12: 231-235.
VIUKARI, M. & MIETTINEN, P. (1984) Diazepam, promethazine and
propiomazine as hypnotics in elderly inpatients.
Neuropsychobiology, 12: 134-7.
ZACHARIAS, H. (1975) Sedation von Schlachtschweinen vor der Transort
und die sich daraus ergebendedn veterinarmedizinischen, rechtlichen
wie fleishhygienischen Fragen. Tierartzliche Umschau, 30: 598-600.
Two recent clinical reports on the effects of propiomazine in
humans are summarized below:
The effects of propiomazine on sleep were studied in 10 healthy
adult volunteers. The subjects received 25 mg propiomazine orally on
5 consecutive nights. A decrease in sleep latency and suppression of
REM sleep, and an increase in sleep quality were reported (Almqvist
et al., 1987).
In another study 25 mg of propiomazine was given orally to 40
elderly subjects to study effect on sleep. Propiomazine was very
effective in 20 normal subjects, and also increased the duration of
sleep in psychogeriatric subjects (Viukari and Miettinen, 1984).
3. COMMENTS
There are no useful reports available from studies conducted to
determine the no effect level for PPZ in animals.
The manufacturer was unable to supply the Committee with data
on the pharmacology and toxicology of propionylpromazine and the
published literature has yielded relatively little information.
However, the drug is of known efficacy in clinical use. Both pigs
and horses are able to metabolize propionylpromazine, at least in
part, but neither case was a full distribution, metabolism,
elimination, or balance study available. A single value of 863 µg/kg
has been recorded at the injection site in a pig 5 days after the
intramuscular injection of 0.5 mg/kg of propionylpromazine. The drug
binds extensively to tissue, and to proteins, and also accumulates
in fatty tissues. In rats and pigs, the drug is able to enter the
brain and, in dogs, has been shown to cause minor changes in
cerebrospinal fluid and serum. The NOEL for these changes was
0.6 mg/kg given intravenously.
In rats, a reduction in the concentrations of pituitary
gonadotrophins has been demonstrated in brain and serum following
the daily intraperitoneal injection of propionylpromazine at
1.5 mg/kg for 2 weeks.
The intravenous LD50 of propionylpromazine in mice was
38 mg/kg.
In a 4-week study of groups of 5 female and 5 male rats exposed
to propionylpromazine in the diet at 0, 60, 360, and 2160 mg/kg
b.w./day, a decreased rate of weight gain was seen each week in both
sexes at the highest dose and in females at 360 mg/kg b.w./day,
which was associated with reduced feed intake. A reduction in
ovarian weight in all treated females and a dose-dependent reduction
in thyroid weight in males prevented the establishment of a NOEL in
this study. Slight periportal fatty infiltration, which sometimes
involved mid-zone and centrilobular hepatocytes, was seen in all
treated animals and was greatest in the high-dose group.
No short- or long-term studies, no systematic studies of the
effects of the drug on reproduction, or of its teratogenicity,
mutagenicity, carcinogenicity, or immunotoxicity, and no reports on
its use in humans were available to the Committee. However, several
studies have reported the presence of propionylpromazine in pig
kidneys collected from abattoirs so that human exposure must
therefore be presumed.
4. EVALUATION
The absence of information in several major areas of
pharmacological and toxicological importance made it impossible for
the Committee to establish an ADI for propionylpromazine. This is
regrettable because the Committee was aware that propionylpromazine
is used in circumstances in which the consumer will be exposed to
residues that may be capable of exerting a pharmacological effect.
5. REFERENCES
ALMQVIST, M., LILJENBERG, B., HETTA, J., RIMON, R., HAMBERT, G., &
ROOS, B. (1987) Effects of propiomazine on the EEG sleep of normal
subjects. Pharm and Toxicol., 61: 278-81.
ARNETH, W. (1986) Untersuchungen zur Verteilung von Combelen und
Combelen-Sulfoxid-Ruchstanden im Schwein. Fleischwirtschaft,
66(5): 922-925.
BOOTH, N.H. (1988) Psychotropic agents. In: Veterinary Pharmacology
and Therapeutics. ed Booth and McDonald Iowa State University
Press, Ames Iowa, 363-81.
DEWEY, E.A. & MAYLIN, G.A. (1984) Analysis of propionylpromazine and
its metabolites in horse urine. Cornell Veterinarian, 74: 38-49.
FRIEDRICH, A. (1988) Screening-Test zum qualiftativen Nachweis der
gebrauchlichsten Transportprotektoren (Sedativa, Betablocker) mit
Hilfe der Hochdruch flussigkeitschromatographie. Tierarztliche
Umschau., 43: 493-501.
Van GINKEL, L.A., SCHWILLENS, P.L.W.J. & OLLING, M. (1989) Liquid
chromatographic method with on-line UV spectrum identification and
off-line thin layer chromatographic confirmation for the defection
of tranquillisers and carazolol in pig kidneys. Analytica Chemica
Acta, 225: 137-146.
HAAGSMA, N, BATHELT, E.R., ENGELSMA, J.W. (1988) Thin-layer
chromatographic screening method for the tranquillizers azaperone,
propiopromazine and carazolol in pig tissues. J. Chromatography,
436: 73-9.
HASSAN, A.B., EL-KHALIK, A.A., ATTA, A.H., HASHIM, M. & ZAGHLOL,
H.A. (1985a) Effect of some tranquilizers on cerebrospinal fluid and
serum constituents in dogs, Vet Med J., 33: 2, 199-209.
HASSAN, A.B., EL-KHALIK, A.A., & ZAGHLOL, H.A. (1985b) Effect of
some tranquilizers on cerebrospinal fluid (CSF) in dogs. Egypt J.
Vet Sci., 21(2): 279-86.
IBRAHIM, S.S., EL-SADET, S.E. & ABOUL-ELA, A. (1987) Effects of some
tranquilizers on the levels of gonadotropins and thyroid activity in
mature male rats. Vet Med J., 35(1): 129-37.
KAEMMERER, K. (1961) Erfahrungen bei transportversuchen unter
Combelen-Schutz. Veterinarmedizinische Nachrichten, No. 2: 51-64.
KEUKENS, H.J. & AERTS, M.M. (1989) Determination of residues of
carazolol and a number of tranquillisers in swine kidney by high
performance liquid chromatography with ultraviolet and fluorescence
detection. Journal of Chromatography, 464: 149-161.
OLLING, M., STEPHANY, R.W. & RAUWS, A.G. (1981) The determination of
propiopromazine in animal tissue. Journal of Veterinary
Pharmacology and Therapeutics, 4: 291-294.
PARK, J., SHIN, Y.O. & CHOO, H.P. (1989) Metabolism and
pharmacokinetic studies of propionylpromazine in horses. Journal of
Chromatography, 489: 313-321.
PREISS A., SCHEUTWINKLE-REICH, M. & STAN, H.J. (1983) Mutagenic
nature of tranquilizers used in animal fattening as revealed in the
Salmonella/microsome test. Fleischwirtschaft (abs), 63(2):
243-4.
PUTTER, J. (1977) Determination of Combelen and its sulphoxide in
biological material. Unpublished Report (PHARMA REPORT No. 6733)
from Bayer AG. Submitted to WHO by Bayer AG, Leverkusen, Germany.
PUTTER, J., BAUDITZ, R., & DORN, H. (1977) Unpublished Report
(PHARMA REPORT No. 6790) from Bayer AG. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
PUTTER, J. & BAUDITZ, R. Combelen: Residue investigations in pigs.
Unpublished Report (PHARMA REPORT No. 6840) from Bayer AG. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
RAUWS, A.G. (1983) Tranquillisers in the transport of pigs destined
for slaughter: a residue problem? Tijdschrift Diergeneeskunde,
108: 659-664.
SCHEUTWINKEL-REICH, M., GROHMANN, H.G., JORDAN, S., PREISS, A.M. &
STAN, H.J. (1982) GC and GC-MS analysis of tranquillisers in meat.
Fresenius Zeitung für Analytische Chemie, 311: 398-399.
SILVESTRINI B., & QUADRI, E. (1970) Investigations on the
specificity of the so-called analgesic activity of non-narcotic
drugs. European J. of Pharmacology, 12: 231-235.
VIUKARI, M. & MIETTINEN, P. (1984) Diazepam, promethazine and
propiomazine as hypnotics in elderly inpatients.
Neuropsychobiology, 12: 134-7.
ZACHARIAS, H. (1975) Sedation von Schlachtschweinen vor der Transort
und die sich daraus ergebendedn veterinarmedizinischen, rechtlichen
wie fleishhygienischen Fragen. Tierartzliche Umschau, 30: 598-600.
