
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 41
QUINTOZENE
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Orgnization
Geneva, 1984
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
ISBN 92 4 154181 4
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1984
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR QUINTOZENE
1. SUMMARY AND RECOMMENDATIONS
1.1. Summary
1.1.1. Identity, analytical methods, and
sources of exposure
1.1.2. Environmental concentrations and
exposures
1.1.3. Kinetics and metabolism
1.1.4. Studies on experimental animals
1.1.5. Effects on man
1.1.6. Effects on the environment
1.2. Recommendations
2. IDENTITY, PROPERTIES AND ANALYTICAL METHODS
2.1. Identity
2.2. Properties and analytical methods
2.2.1. Physical and chemical properties
2.2.2. Analytical methods
3. USES, ENVIRONMENTAL LEVELS AND EXPOSURES,
TRANSPORT AND DISTRIBUTION
3.1. Uses
3.2. Levels and exposure
3.3. Transport and distribution
3.3.1. Abiotic degradation and bioaccumulation
4. KINETICS AND METABOLISM
4.1. Absorption
4.1.1. Inhalation
4.1.2. Gastrointestinal tract
4.1.3. Dermal exposure
4.2. Distribution and storage
4.3. Biotransformation
4.4. Elimination
4.4.1. Human studies
4.4.2. Animal studies
5. STUDIES ON EXPERIMENTAL ANIMALS
5.1. Short-term studies
5.1.1. Single dose
5.1.2. Repeated dose
5.2. Reproduction studies
5.3. Mutagenicity
5.4. Carcinogenicity
6. EFFECTS ON MAN
7. EFFECTS ON THE ENVIRONMENT
7.1. Toxicity for aquatic organisms
7.2. Toxicity for terrestrial organisms
7.2.1. Plants
7.2.2. Earthworms
7.2.3. Bees
7.2.4. Birds
7.3. Toxicity for microorganism
7.4. Bioaccumulation and biomagnification
8. PREVIOUS EVALUATIONS OF QUINTOZENE BY INTERNATIONAL BODIES
9. EVALUATION OF HEALTH RISKS FOR MAN AND EFFECTS ON THE ENVIRONMENT
9.1. Evaluation of health risks for man
9.2. Evaluation of overall environmental effects
9.3. Conclusions
REFERENCES
NOTE TO READERS OF THE CRITERIA DOCUMENTS
While every effort has been made to present information in the
criteria documents as accurately as possible without unduly
delaying their publication, mistakes might have occurred and are
likely to occur in the future. In the interest of all users of the
environmental health criteria documents, readers are kindly
requested to communicate any errors found to the Manager of the
International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland, in order that they may be
included in corrigenda, which will appear in subsequent volumes.
In addition, experts in any particular field dealt with in the
criteria documents are kindly requested to make available to the
WHO Secretariat any important published information that may have
inadvertently been omitted and which may change the evaluation of
health risks from exposure to the environmental agent under
examination, so that the information may be considered in the event
of updating and re-evaluation of the conclusions contained in the
criteria documents.
* * *
A detailed data profile and a legal file can be obtained from
the International Register of Potentially Toxic Chemicals, Palais
des Nations, 1211 Geneva 10, Switzerland (Telephone no. 988400 -
985850)
TASK GROUP MEETING ON ENVIRONMENTAL HEALTH CRITERIA FOR
ORGANOCHLORINE PESTICIDES OTHER THAN DDT (ENDOSULFAN,
QUINTOZENE, TECNAZENE, TETRADIFON)
Members
Dr E. Astolfi, Faculty of Medicine of Buenos Aires, Buenos
Aires, Argentina
Dr I. Desi, Department of Environmental Hygienic Toxicology,
National Institute of Hygiene, Budapest, Hungary
(Vice-Chairman)
Dr R. Drew, Department of Clinical Pharmacology, Flinders
University of South Australia, Bedford Park, South
Australia
Dr S.K. Kashyap, National Institute of Occupational Health,
Ahmedabad, India
Dr A.N. Mohammed, University of Calabar, Calabar, Nigeria
Dr O.E. Paynter, Office of Pesticide Programs, US
Environmental Protection Agency, Washington DC, USA
Dr W.O. Phoon, Department of Social Medicine and Public
Health, Faculty of Medicine, University of Singapore,
Outram Hill, Singapore (Chairman)
Dr D. Wassermann, Department of Occupational Health, The
Hebrew University, Hadassah Medical School, Jerusalem,
Israel
Representatives of Other Organizations
Dr H. Kaufmann, International Group of National Associations
of Agrochemical Manufacturers (GIFAP)
Dr V.E.F. Solman, International Union for Conservation of
Nature and Natural Resources (IUCN), Ottawa, Ontario,
Canada
Secretariat
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood
Experimental Station, Abbots Ripton, Huntingdon, United
Kingdom (Temporary Adviser)
Dr M. Gilbert, International Register for Potentially Toxic
Chemicals, United Nations Environment Programme, Geneva,
Switzerland
Dr K.W. Jager, Division of Environmental Health, International
Programme on Chemical Safety, World Health Organization,
Geneva, Switzerland (Secretary)
Dr D.C. Villeneuve, Health Protection Branch, Department of
National Health and Welfare, Tunney's Pasture, Ottawa,
Ontario, Canada (Temporary Adviser) (Rapporteur)
Mr J.D. Wilbourn, Unit of Carcinogen Identification and
Evaluation, International Agency for Research on Cancer,
Lyons, France
ENVIRONMENTAL HEALTH CRITERIA FOR QUINTOZENE
Following the recommendations of the United Nations Conference
on the Human Environment held in Stockholm in 1972, and in response
to a number of World Health Resolutions (WHA23.60, WHA24.47,
WHA25.58, WHA26.68), and the recommendation of the Governing
Council of the United Nations Environment Programme, (UNEP/GC/10,
3 July 1973), a programme on the integrated assessment of the
health effects of environmental pollution was initiated in 1973.
The programme, known as the WHO Environmental Health Criteria
Programme, has been implemented with the support of the Environment
Fund of the United Nations Environment Programme. In 1980, the
Environmental Health Criteria Programme was incorporated into the
International Programme on Chemical Safety (IPCS). The result of
the Environmental Health Criteria Programme is a series of criteria
documents.
A WHO Task Group on Environmental Health Criteria for
Organochlorine Pesticides other than DDT (Endosulfan, Quintozene,
Tecnazene, Tetradifon) was held at the Health Protection Branch,
Department of National Health and Welfare Ottawa from 28 May -
1 June, 1984. The meeting was opened by Dr E. Somers, Director-
General, Environmental Health Directorate, and Dr K.W. Jager
welcomed the participants on behalf of the three co-sponsoring
organizations of the IPCS (UNEP/ILO/WHO). The Task Group reviewed
and revised the draft criteria document and made an evaluation of
the health risks of exposure to quintozene.
The drafts of this document were prepared by Dr D.C. Villeneuve
of Canada and Dr S. Dobson of the United Kingdom.
The efforts of all who helped in the preparation and
finalization of the document are gratefully acknowledged.
* * *
Partial financial support for the publication of this criteria
document was kindly provided by the United States Department of
Health and Human Services, through a contract from the National
Institute of Environmental Health Sciences, Research Triangle Park,
North Carolina, USA - a WHO Collaborating Centre for Environmental
Health Effects.
1. SUMMARY AND RECOMMENDATIONS
1.1. Summary
1.1.1. Identity, analytical methods, and sources of exposure
Technical quintozene (pentachloronitrobenzene) is a white solid
with a musty odour, that, is used in formulation as a soil
fungicide and as a seed dressing. Hexachlorobenzene is a possible
major contaminant in technical quintozene.
Gas chromatography combined with electron capture detection is
used for the analytical determination of quintozene.
Exposure of the general population is mainly via residues in
the food.
1.1.2. Environmental concentrations and exposures
Quintozene persists in soil with a half-life of approximately
4 - 10 months. Part of it is lost from the soil by volatilization.
Biodegradation, mainly to pentachloroaniline is an important route
of conversion. Photodegradation is not important.
1.1.3. Kinetics and metabolism
There are large animal species differences in the absorption of
quintozene from the gastrointestinal tract. There is no information
on absorption via inhalation or via the skin. After ingestion,
faecal elimination of unchanged material is an important route of
excretion. Pentachloroaniline and mercapturic acid conjugates are
the major metabolites found in urine. There is no tendency for
bioaccumulation.
1.1.4. Studies on experimental animals
Quintozene is practically non-toxic according to the scale of
Hodge & Sterner (1956). The oral LD50 for quintozene in the rat
ranges from 1650 to more than 30 000 mg/kg body weight. WHO (1984)
classified quintozene in the category of technical products
unlikely to present an acute hazard in normal use.
In long-term studies, no-observed-adverse-effect levels are
1.25 mg/kg body weight and 0.75 mg/kg body weight for rats and
dogs, respectively. At higher dosages, there is liver hypertrophy
with some histopathological changes. In dogs, dose-related liver
damage including fibrosis was induced.
Purified quintozene was not teratogenic at levels of up to 500
mg/kg body weight in mice. Positive results obtained with
technical quintozene in mice (500 mg/kg body weight) implicate the
involvement of hexachlorobenzene in the teratogenic response.
Quintozene was not teratogenic in rats at levels up to 1563 mg/kg.
Quintozene is generally negative in short-term tests for
genetic activity. In carcinogenicity studies where rats and mice
were fed quintozene at levels up to 1200 mg/kg diet, equivocal or
negative findings have been reported. Hexachlorobenzene, a possible
impurity in technical quintozene, is carcinogenic to mice, rats,
and hamsters.
1.1.5. Effects on man
Quintozene is a weak skin sensitizer, but not an irritant.
Except for 1 case of conjunctivitis in an occupational setting,
instances of accidental overexposure have not been reported.
1.1.6. Effects on the environment
There are indications that quintozene applied at recommended
rates as a soil fungicide could produce a significant adverse
effect on earthworm survival. There is no evidence that quintozene
represents a threat to other organisms tested. Its bioaccumulation
in fish is low.
1.2. Recommendations
1. Further data on absorption resulting from
different routes of exposure to quintozene are
required.
2. Levels of impurities, especially hexachloro-
benzene, in quintozene should be kept to a minimum.
3. Adequate carcinogenicity studies are required on
quintozene.
2. IDENTITY, PROPERTIES AND ANALYTICAL METHODS
2.1. Identity
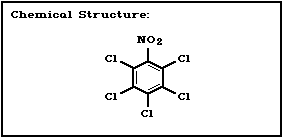 Molecular formula: C6Cl5NO2
CAS chemical name: pentachloronitrobenzene
Common trade names: avicol, botrilex, brassicol, earthcide,
fartox, folosan, fomac 2, fungiclor,
GC 3944-3-4, kobu, kobutol, KP 2,
NCI-C00419, olpisan, PCNB, entagen,
pterraclor, terrafum, tilcarex,
tritisan. A complete list of trade
names is available from IRPTC (1983).
CAS registry number: 82-68-8
Relative molecular mass: 295.36
2.2. Properties and Analytical Methods
2.2.1. Physical and chemical properties
Quintozene is a pale yellow-to-white (depending on the
purity) solid with a musty odour and has a melting point of 142 -
146 °C. It is soluble in carbon disulfide, benzene, chloroform,
ketones, and aromatic and chlorinated hydrocarbons but is
practically insoluble in water (0.44 mg/litre at 20 °C); in ethanol
its solubility is 2% at 25 °C (IARC, 1974). It has a vapour
pressure at 20 °C of 10-8 x 667 kPa (Berkowitz et al., 1976).
Hexaachlorobenzene is often found as a contaminant in quintozene
and levels can range up to 3% (in the past, levels as high as 30%
were found).
It is quite stable in soil but eventually degrades to
pentachloroaniline (PCA).
Quintozene is primarily registered for use as a soil fungicide
for use in agriculture and on field crops, selected vegetables,
horticultural crops, and in greenhouses. It is also used as a
seed-treatment fungicide for crop seeds such as cotton, peanuts,
soybeans, and grain. It has been formulated as wettable powder,
dust, emulsifiable concentrate, granules, and combination products.
It has been sold under a variety of trade names.
The first laboratory synthesis was reported in 1868 (Berkowitz
et al., 1976). It was first introduced in Germany as a soil
fungicide in the 1930s. It has been produced in the USA since 1962
(IARC, 1974).
Quintozene is produced by either the chlorination of
nitrobenzene or the nitration of chlorinated benzenes. In 1972,
production levels in the USA were estimated to be 1.3 million kg,
of which 30 - 40% was exported (Berkowitz et al., 1976).
2.2.2. Analytical methods
Methods of cleanup and analysis for quintozene have been
summarized by Berkowitz et al. (1976). These include a
colorimetric and a gas chromatographic method. The latter is the
most sensitive and can be used in combination with a
microcoulometric (Burke & Holswade, 1964) or electron capture
detector (Kuchar et al., 1969). Cleanup of extracts can be
accomplished by column chromatography using silicic acid (Methratta
et al., 1967) or florisil (US DHEW, 1973).
3. USES, ENVIRONMENTAL LEVELS AND EXPOSURES, TRANSPORT AND
DISTRIBUTION
3.1. Uses
The major uses of quintozene are summarized in Table 1 and some
information is provided on the quantities used.
Table 1. Usage data for quintozene in selected countriesa
-------------------------------------------------------------------
Area Quantity Year Uses
-------------------------------------------------------------------
Colombia 12 305 kg 1982 fungicide recommended for
15 926 kg 1981 treatment of millet, corn,
23 965 kg 1980 and sorghum
Malaysia fungicide
Mexico 313 000 kg 1983 seed treatment
Sweden 10 000 kg 1981 fungicide in various crops;
2000 kg 1981 Home and garden fungicide
on lawns
Tanzania 500 tonne 1981-82 applied to bananas,
cereals, beans, etc.
United Kingdom 22.89 tonne 1975-79 fungicide used on non-
edible crops and turf; as
a dust applied to soil
before sowing or planting
edible crops; used on
onion seed; as a paste
applied to the stems of
cucumber plants
USA 2043 - 2183 1982 fungicide
tonne
-------------------------------------------------------------------
a From: IRPTC, personal communication, 1984.
3.2. Levels and Exposures
General population
No data are available for the concentrations of quintozene in
air or water.
It is persistant in soil, and is often present in crops grown
on treated soil. In a market basket survey in the USA, residues
ranged from 0.001 to 0.003 mg/kg in 6 out of 240 composites
examined. Three of the composites contained only trace amounts.
It was most common in the fats and oils class. Quintozene residues
on lettuce in greenhouses sprayed at levels of 30 g/m2 in the open
air declined from 60 µg/g after 5 days to almost zero after 7 weeks
(Dunsing & Windschild, 1976). In a study by Heikes (1980), it was
found that residues in 11 samples of peanut butter averaged 5.05
µg/kg.
Infants and children
In a market basket survey by the US FDA (Johnson et al., 1979),
quintozene was found in 2 out of 10 samples of food composites for
infants 6-months-old, and one of these had only a trace amount.
The residue values ranged from trace to 0.03 mg/kg. In the food
composites for toddlers 2-years-old, PCNB was found in 4 out of 10
samples, 1 containing a trace amount, and the residues in the other
sample ranging from 0.004 to 0.016 mg/kg. The residues occurred in
the oils and fats class as in the adult survey.
PCNB does not appear to accumulate to any appreciable degree in
cows' milk (Goursaud et al., 1972; Glofke, 1973).
3.3. Transport and Distribution
(a) Air
Contamination of the air is often due to volatilization from
the soil. Quintozene has a relatively high volatility (10-6 x 1775
kPa at 25 °C) and thus the principal mechanism of loss from the
soil is volatilization (Lee, 1975). It has an affinity for the
air-water interface and thus moist air passing over the soil could
account for a percentage of the loss by a "codistillation"
process. In a study done by Caseley, this amount was found to be
62% (Berkowitz et al., 1976). Such loss would be greater
immediately after application and would depend on the adsorbent
properties of the soil (Lee, 1975).
(b) Water
Since quintozene is practically insoluble in water, it may be
assumed that leaching is negligible (Leistra & Smelt, 1974).
However, few data are available on residues in water bodies and
drinking-water. In one study, urban storm run-off was analysed and
quintozene was found in only negligible amounts or not at all.
However, this has not been correlated with use patterns in the area
(Dappen, 1974).
(c) Soil
The fate of quintozene in soil has been more extensively
studied. It persists in Californian soils and has a half-life of
4.7 - 9.7 months (Wang & Broadbent, 1973). Longer half-lives were
associated with soils rich in organic matter. In an analysis of 22
samples collected from potato fields, treated for 11 years,
residues averaged 7.06 mg/kg with a range of 0.06 - 25.25 mg/kg
(Beck & Hansen, 1974). In a study by Rautapaa et al. (1977),
quintozene levels of 0.05 - 27.0 mg/kg were found in the soil of
forest tree nurseries. In one case, it was found that all the
quintozene applied (20 - 40 kg/ha) was left in the soil. No
explanation was given for this phenomenon. The authors found that,
in general, residues in cereal and clover fields were less than
those in forest-tree nurseries. Residues in the soil of those
cereal and clover fields were 0.4%. Appreciable levels of the
associated impurities and metabolites were also found.
3.3.1. Abiotic degradation and bioccumulation
Studies on the photoreduction of quintozene have shown that
ultraviolet irradiation results mainly in reductive dechlorination.
Irradiation of solutions in hexane produces pentachlorobenzene,
1,2,4,5-tetrachlorobenzene, and 2,3,4,6-and 2,3,4,5-tetra-
chloronitrobenzene (Crosby & Hamadmad, 1971). However, this
process is very slow and thus not likely to be important as a
degradation route. Biologically, soil microorganisms convert
quintozene to pentachloroaniline (PCA) and methylthiopenta-
chlorobenzene (MTPCB) (Sijpensteijn et al., 1977).
Under aerobic growth conditions, fungi and actinomycetes have
been shown to convert low concentrations of quintozene to PCA and
MTPCB. In a related study, Ko & Farley (1969) observed that
microorganisms converted quintozene to PCA and that this process
was greatest in submerged soil. Although quintozene is taken up
from the soil by plants, it does not accumulate to any important
degree in animals (section 4.2)
4. KINETICS AND METABOLISM
4.1. Absorption
4.1.1. Inhalation
No information on the uptake of quintozene through inhalation
is available.
4.1.2. Gastrointestinal tract
There are no studies investigating the absorption of quintozene
from the gastrointestinal tract. However, studies relating to the
faecal elimination of the parent material suggest that absorption
by this route may be limited and species-dependent (section 4.4.2).
4.1.3. Dermal exposure
No information on the uptake of quintozene through dermal
exposure is available.
4.2. Distribution and Storage
Human studies
No data are available to indicate the extent of storage and
distribution of quintozene in man.
Animal studies
After feeding quintozene to dogs in the diet at levels of up to
1080 mg/kg, for 2 years, none was found in the kidney, brain,
skeletal muscle, liver, spleen, fat, bile, blood, urine, and faeces
(Borzelleca et al., 1971). Nor was quintozene detected in the
skeletal muscle, liver, kidney, fat, or faeces of rats fed up to
500 mg/kg diet for 33 weeks (Borzelleca et al., 1971). Storage of
quintozene did not occur in fat, skeletal muscle, liver, or kidney
of cows administered an amount equivalent to 10 mg/kg diet for 12
weeks (Borzelleca et al., 1971). Only negligible amounts were
detected in the milk of these cows. Borzelleca et al. (1971) did,
however, find tissue storage of hexachlorobenzene and penta-
chlorobenzene, contaminants of technical quintozene, in rats, dogs,
and cows in degrees paralleling their contents in the quintozene.
After sheep were dosed orally with quintozene (31 - 32 mg/kg body
weight), quintozene was detected in the omental fat (0.5 mg/kg) for
only 1 day (Avrahami & White, 1976). Sixteen weeks of feeding
chickens a diet containing 300 mg quintozene/kg resulted in fat
levels of 0.85 mg/kg and levels in the egg yolk of approximately
0.02 mg/kg (Simon et al., 1979). Quintozene was not detected in
the bile, gall bladder, liver, blood, or muscle of these animals.
In another study where pregnant rats were administered
quintozene at levels of 50 - 200 mg/kg body weight, from day 6 to
15 of gestation, residues were not detected in either maternal
tissues (brain, liver, heart, spleen, kidney, adipose tissue) or in
fetuses removed by Cesarean section (Villeneuve & Khera, 1975). In
mice, 4 daily doses of 500 mg/kg body weight led to the appearance
of the metabolites, pentachloroanisole and pentachlorophenyl
sulfide in the fatty tissue of pregnant mice and fetuses; these
results indicated placental transfer of the metabolites (Courtney
et al., 1976).
Analyses of tissue from 2 Rhesus monkeys sacrificed 24 and 48 h
after being administered 2 mg/kg body weight orally showed that
quintozene was eliminated quickly and had virtually no tendency to
accumulate (Koegel et al., 1979).
In another study, where Rhesus monkeys were fed a diet
containing 14C-quintozene at 2 mg/kg for 550 days, a storage curve
was constructed by subtracting the excreted from the administered
radioactivity. The storage curve reached a steady state plateau of
2 - 3% of the administered dose after 30 - 40 days of treatment
(Muller et al., 1978).
4.3. Biotransformation
The proposed metabolic and excretory pathway for quintozene is
shown in Fig. 1. In animals, the major biotransformation products
that appear in urine are pentachloroaniline (PCA), formed by
reduction of the nitro group, and mercapturic acids formed after
replacement of the nitro group with glutathione and subsequent
further metabolism. The relative contribution of each of these
reactions to the overall biotransformation of quintozene is species
dependent. Rabbits dosed orally with 2 g quintozene excreted 14%
in the urine as N-acetyl- S-pentachlorophenyl-cysteine (PCC)
and 12% as free and conjugated PCA. The balance of the dose was
excreted unchanged in the faeces (Betts et al., 1955) (section
4.4.2). In rhesus monkeys and sheep, PCA was identified as the
major metabolite (Avrahami & White, 1976; Muller et al., 1978),
whereas, in the rat, PCC is the major metabolite (O'Grodnick et
al., 1981).
In addition to the above primary metabolites, a number of minor
metabolites have been identified. The nitro group can be replaced
either with a methylthio group to form pentachlorothioanisole
(PCTA) or with a hydroxyl group to form pentachlorophenol (PCP).
PCTA has been shown to be formed in rats, dogs, chickens, and
monkeys (Borzelleca et al., 1971; Muller et al., 1978; Dunn et al.,
1979) and PCP in rabbits, rats, and monkeys (Betts et al., 1955;
Muller et al., 1978; O'Grodnick et al., 1981). Following
administration of quintozene pentachlorobenzene and tetrachloro-bis
(methyl mercapto)-benzene were found in monkey urine (Muller et
al., 1978) and pentachlorophenyl sulfide in rat urine (O'Grodnick
et al., 1981).
Quintozene is converted by soil microorganisms and on plants to
PCA and PCTA (Kuchar et al., 1969; Berkowitz et al., 1976;
Sijpesteijn et al., 1977).
Molecular formula: C6Cl5NO2
CAS chemical name: pentachloronitrobenzene
Common trade names: avicol, botrilex, brassicol, earthcide,
fartox, folosan, fomac 2, fungiclor,
GC 3944-3-4, kobu, kobutol, KP 2,
NCI-C00419, olpisan, PCNB, entagen,
pterraclor, terrafum, tilcarex,
tritisan. A complete list of trade
names is available from IRPTC (1983).
CAS registry number: 82-68-8
Relative molecular mass: 295.36
2.2. Properties and Analytical Methods
2.2.1. Physical and chemical properties
Quintozene is a pale yellow-to-white (depending on the
purity) solid with a musty odour and has a melting point of 142 -
146 °C. It is soluble in carbon disulfide, benzene, chloroform,
ketones, and aromatic and chlorinated hydrocarbons but is
practically insoluble in water (0.44 mg/litre at 20 °C); in ethanol
its solubility is 2% at 25 °C (IARC, 1974). It has a vapour
pressure at 20 °C of 10-8 x 667 kPa (Berkowitz et al., 1976).
Hexaachlorobenzene is often found as a contaminant in quintozene
and levels can range up to 3% (in the past, levels as high as 30%
were found).
It is quite stable in soil but eventually degrades to
pentachloroaniline (PCA).
Quintozene is primarily registered for use as a soil fungicide
for use in agriculture and on field crops, selected vegetables,
horticultural crops, and in greenhouses. It is also used as a
seed-treatment fungicide for crop seeds such as cotton, peanuts,
soybeans, and grain. It has been formulated as wettable powder,
dust, emulsifiable concentrate, granules, and combination products.
It has been sold under a variety of trade names.
The first laboratory synthesis was reported in 1868 (Berkowitz
et al., 1976). It was first introduced in Germany as a soil
fungicide in the 1930s. It has been produced in the USA since 1962
(IARC, 1974).
Quintozene is produced by either the chlorination of
nitrobenzene or the nitration of chlorinated benzenes. In 1972,
production levels in the USA were estimated to be 1.3 million kg,
of which 30 - 40% was exported (Berkowitz et al., 1976).
2.2.2. Analytical methods
Methods of cleanup and analysis for quintozene have been
summarized by Berkowitz et al. (1976). These include a
colorimetric and a gas chromatographic method. The latter is the
most sensitive and can be used in combination with a
microcoulometric (Burke & Holswade, 1964) or electron capture
detector (Kuchar et al., 1969). Cleanup of extracts can be
accomplished by column chromatography using silicic acid (Methratta
et al., 1967) or florisil (US DHEW, 1973).
3. USES, ENVIRONMENTAL LEVELS AND EXPOSURES, TRANSPORT AND
DISTRIBUTION
3.1. Uses
The major uses of quintozene are summarized in Table 1 and some
information is provided on the quantities used.
Table 1. Usage data for quintozene in selected countriesa
-------------------------------------------------------------------
Area Quantity Year Uses
-------------------------------------------------------------------
Colombia 12 305 kg 1982 fungicide recommended for
15 926 kg 1981 treatment of millet, corn,
23 965 kg 1980 and sorghum
Malaysia fungicide
Mexico 313 000 kg 1983 seed treatment
Sweden 10 000 kg 1981 fungicide in various crops;
2000 kg 1981 Home and garden fungicide
on lawns
Tanzania 500 tonne 1981-82 applied to bananas,
cereals, beans, etc.
United Kingdom 22.89 tonne 1975-79 fungicide used on non-
edible crops and turf; as
a dust applied to soil
before sowing or planting
edible crops; used on
onion seed; as a paste
applied to the stems of
cucumber plants
USA 2043 - 2183 1982 fungicide
tonne
-------------------------------------------------------------------
a From: IRPTC, personal communication, 1984.
3.2. Levels and Exposures
General population
No data are available for the concentrations of quintozene in
air or water.
It is persistant in soil, and is often present in crops grown
on treated soil. In a market basket survey in the USA, residues
ranged from 0.001 to 0.003 mg/kg in 6 out of 240 composites
examined. Three of the composites contained only trace amounts.
It was most common in the fats and oils class. Quintozene residues
on lettuce in greenhouses sprayed at levels of 30 g/m2 in the open
air declined from 60 µg/g after 5 days to almost zero after 7 weeks
(Dunsing & Windschild, 1976). In a study by Heikes (1980), it was
found that residues in 11 samples of peanut butter averaged 5.05
µg/kg.
Infants and children
In a market basket survey by the US FDA (Johnson et al., 1979),
quintozene was found in 2 out of 10 samples of food composites for
infants 6-months-old, and one of these had only a trace amount.
The residue values ranged from trace to 0.03 mg/kg. In the food
composites for toddlers 2-years-old, PCNB was found in 4 out of 10
samples, 1 containing a trace amount, and the residues in the other
sample ranging from 0.004 to 0.016 mg/kg. The residues occurred in
the oils and fats class as in the adult survey.
PCNB does not appear to accumulate to any appreciable degree in
cows' milk (Goursaud et al., 1972; Glofke, 1973).
3.3. Transport and Distribution
(a) Air
Contamination of the air is often due to volatilization from
the soil. Quintozene has a relatively high volatility (10-6 x 1775
kPa at 25 °C) and thus the principal mechanism of loss from the
soil is volatilization (Lee, 1975). It has an affinity for the
air-water interface and thus moist air passing over the soil could
account for a percentage of the loss by a "codistillation"
process. In a study done by Caseley, this amount was found to be
62% (Berkowitz et al., 1976). Such loss would be greater
immediately after application and would depend on the adsorbent
properties of the soil (Lee, 1975).
(b) Water
Since quintozene is practically insoluble in water, it may be
assumed that leaching is negligible (Leistra & Smelt, 1974).
However, few data are available on residues in water bodies and
drinking-water. In one study, urban storm run-off was analysed and
quintozene was found in only negligible amounts or not at all.
However, this has not been correlated with use patterns in the area
(Dappen, 1974).
(c) Soil
The fate of quintozene in soil has been more extensively
studied. It persists in Californian soils and has a half-life of
4.7 - 9.7 months (Wang & Broadbent, 1973). Longer half-lives were
associated with soils rich in organic matter. In an analysis of 22
samples collected from potato fields, treated for 11 years,
residues averaged 7.06 mg/kg with a range of 0.06 - 25.25 mg/kg
(Beck & Hansen, 1974). In a study by Rautapaa et al. (1977),
quintozene levels of 0.05 - 27.0 mg/kg were found in the soil of
forest tree nurseries. In one case, it was found that all the
quintozene applied (20 - 40 kg/ha) was left in the soil. No
explanation was given for this phenomenon. The authors found that,
in general, residues in cereal and clover fields were less than
those in forest-tree nurseries. Residues in the soil of those
cereal and clover fields were 0.4%. Appreciable levels of the
associated impurities and metabolites were also found.
3.3.1. Abiotic degradation and bioccumulation
Studies on the photoreduction of quintozene have shown that
ultraviolet irradiation results mainly in reductive dechlorination.
Irradiation of solutions in hexane produces pentachlorobenzene,
1,2,4,5-tetrachlorobenzene, and 2,3,4,6-and 2,3,4,5-tetra-
chloronitrobenzene (Crosby & Hamadmad, 1971). However, this
process is very slow and thus not likely to be important as a
degradation route. Biologically, soil microorganisms convert
quintozene to pentachloroaniline (PCA) and methylthiopenta-
chlorobenzene (MTPCB) (Sijpensteijn et al., 1977).
Under aerobic growth conditions, fungi and actinomycetes have
been shown to convert low concentrations of quintozene to PCA and
MTPCB. In a related study, Ko & Farley (1969) observed that
microorganisms converted quintozene to PCA and that this process
was greatest in submerged soil. Although quintozene is taken up
from the soil by plants, it does not accumulate to any important
degree in animals (section 4.2)
4. KINETICS AND METABOLISM
4.1. Absorption
4.1.1. Inhalation
No information on the uptake of quintozene through inhalation
is available.
4.1.2. Gastrointestinal tract
There are no studies investigating the absorption of quintozene
from the gastrointestinal tract. However, studies relating to the
faecal elimination of the parent material suggest that absorption
by this route may be limited and species-dependent (section 4.4.2).
4.1.3. Dermal exposure
No information on the uptake of quintozene through dermal
exposure is available.
4.2. Distribution and Storage
Human studies
No data are available to indicate the extent of storage and
distribution of quintozene in man.
Animal studies
After feeding quintozene to dogs in the diet at levels of up to
1080 mg/kg, for 2 years, none was found in the kidney, brain,
skeletal muscle, liver, spleen, fat, bile, blood, urine, and faeces
(Borzelleca et al., 1971). Nor was quintozene detected in the
skeletal muscle, liver, kidney, fat, or faeces of rats fed up to
500 mg/kg diet for 33 weeks (Borzelleca et al., 1971). Storage of
quintozene did not occur in fat, skeletal muscle, liver, or kidney
of cows administered an amount equivalent to 10 mg/kg diet for 12
weeks (Borzelleca et al., 1971). Only negligible amounts were
detected in the milk of these cows. Borzelleca et al. (1971) did,
however, find tissue storage of hexachlorobenzene and penta-
chlorobenzene, contaminants of technical quintozene, in rats, dogs,
and cows in degrees paralleling their contents in the quintozene.
After sheep were dosed orally with quintozene (31 - 32 mg/kg body
weight), quintozene was detected in the omental fat (0.5 mg/kg) for
only 1 day (Avrahami & White, 1976). Sixteen weeks of feeding
chickens a diet containing 300 mg quintozene/kg resulted in fat
levels of 0.85 mg/kg and levels in the egg yolk of approximately
0.02 mg/kg (Simon et al., 1979). Quintozene was not detected in
the bile, gall bladder, liver, blood, or muscle of these animals.
In another study where pregnant rats were administered
quintozene at levels of 50 - 200 mg/kg body weight, from day 6 to
15 of gestation, residues were not detected in either maternal
tissues (brain, liver, heart, spleen, kidney, adipose tissue) or in
fetuses removed by Cesarean section (Villeneuve & Khera, 1975). In
mice, 4 daily doses of 500 mg/kg body weight led to the appearance
of the metabolites, pentachloroanisole and pentachlorophenyl
sulfide in the fatty tissue of pregnant mice and fetuses; these
results indicated placental transfer of the metabolites (Courtney
et al., 1976).
Analyses of tissue from 2 Rhesus monkeys sacrificed 24 and 48 h
after being administered 2 mg/kg body weight orally showed that
quintozene was eliminated quickly and had virtually no tendency to
accumulate (Koegel et al., 1979).
In another study, where Rhesus monkeys were fed a diet
containing 14C-quintozene at 2 mg/kg for 550 days, a storage curve
was constructed by subtracting the excreted from the administered
radioactivity. The storage curve reached a steady state plateau of
2 - 3% of the administered dose after 30 - 40 days of treatment
(Muller et al., 1978).
4.3. Biotransformation
The proposed metabolic and excretory pathway for quintozene is
shown in Fig. 1. In animals, the major biotransformation products
that appear in urine are pentachloroaniline (PCA), formed by
reduction of the nitro group, and mercapturic acids formed after
replacement of the nitro group with glutathione and subsequent
further metabolism. The relative contribution of each of these
reactions to the overall biotransformation of quintozene is species
dependent. Rabbits dosed orally with 2 g quintozene excreted 14%
in the urine as N-acetyl- S-pentachlorophenyl-cysteine (PCC)
and 12% as free and conjugated PCA. The balance of the dose was
excreted unchanged in the faeces (Betts et al., 1955) (section
4.4.2). In rhesus monkeys and sheep, PCA was identified as the
major metabolite (Avrahami & White, 1976; Muller et al., 1978),
whereas, in the rat, PCC is the major metabolite (O'Grodnick et
al., 1981).
In addition to the above primary metabolites, a number of minor
metabolites have been identified. The nitro group can be replaced
either with a methylthio group to form pentachlorothioanisole
(PCTA) or with a hydroxyl group to form pentachlorophenol (PCP).
PCTA has been shown to be formed in rats, dogs, chickens, and
monkeys (Borzelleca et al., 1971; Muller et al., 1978; Dunn et al.,
1979) and PCP in rabbits, rats, and monkeys (Betts et al., 1955;
Muller et al., 1978; O'Grodnick et al., 1981). Following
administration of quintozene pentachlorobenzene and tetrachloro-bis
(methyl mercapto)-benzene were found in monkey urine (Muller et
al., 1978) and pentachlorophenyl sulfide in rat urine (O'Grodnick
et al., 1981).
Quintozene is converted by soil microorganisms and on plants to
PCA and PCTA (Kuchar et al., 1969; Berkowitz et al., 1976;
Sijpesteijn et al., 1977).
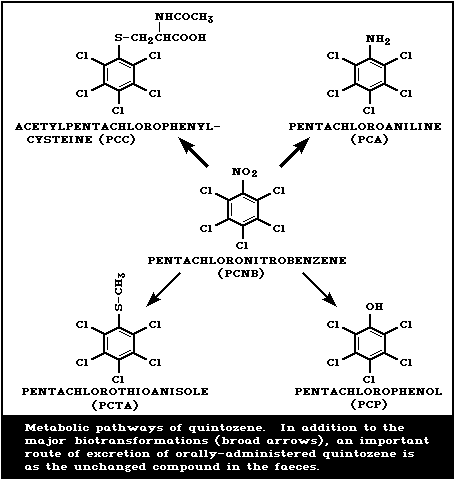 4.4. Elimination
4.4.1. Human studies
No information on the elimination of quintozene in man was
found.
4.4.2. Animal studies
The major routes of elimination of ingested quintozene are via
the faeces as unchanged material or in the urine as metabolites
(Fig. 1)
The amount of quintozene eliminated unchanged in the faeces is
species-dependent. Betts et al. (1955) administered 1,2, or 3 g of
quintozene to rabbits by stomach tube and found an average of 46,
62, and 59% of the dose was eliminated unchanged in the faeces over
72 h. The faecal elimination was also variable (range 27 - 82% of
the dose). In sheep dosed orally with 31 mg/kg body weight,
approximately 80% of the dose was eliminated unchanged via the
faeces (Avrahami & White, 1976). In metabolic studies carried out
on Rhesus monkeys (Koegel et al., 1979), only 7.4% of the
administered oral dose (2 mg/kg body weight) was excreted as
quintozene in the faeces. After feeding rats with a diet
containing 500 mg/kg diet, no detectable quintozene was found in
the faeces, but unmetabolized quintozene was found in the faeces of
dogs fed 1080 mg/kg diet for 2 years (Borzelleca et al., 1971).
Once absorbed, the elimination of quintozene is primarily as
metabolites in the urine (section 4.3). Although quintozene has
been found in bile, the relative importance of this finding for the
elimination or enterohepatic circulation of quintozene is unknown.
Nevertheless, it would appear that elimination by this route is
species-dependent, since quintozene has been identified in the bile
of monkeys (Kogel et al., 1979) and mice (Courtney et al., 1976)
but not in the bile of chickens (Kuchar et al., 1969; WHO 1975) or
dogs (Borzelleca et al., 1971).
When a lactating cow was fed 5 mg/kg diet for 3 days, the
parent compound was not detected in the milk using a method in
which the sensitivity was 0.01 mg/kg (St. John et al., 1965).
Traces of quintozene were found in milk from cows treated orally
with the equivalent of 10 mg/kg diet for 8 weeks (Borzelleca et
al., 1971). It was also found in the milk of untreated cows and
analyses of the feed showed quintozene levels of 0.002 - 0.006
mg/kg.
Rhesus monkeys were fed diets containing quintozene at 2 mg/kg
for 550 days. A storage curve was constructed by subtracting the
excreted from the administered radioactivity. The storage curve
levelled out after 30 - 40 days of treatment, resulting in a
storage plateau of only 2 - 3% of the administered dose (Muller et
al., 1978).
There was little accumulation of quintozene in fat tissue;
slightly elevated concentrations are found in the liver and kidney
as well as the thymus, lymph nodes, and bone marrow.
Residual levels in various tissues are given in Table 2.
Table 2. Tissue residue levels in Rhesus monkeys fed 2 mg/kg PCNB
in the diet for 550 daysa
---------------------------------------------------------------
Tissue PCNB Tissue PCNB
(mg/kg) (mg/kg)
---------------------------------------------------------------
Blood 0.07 adrenal cortex 0.08
Muscle 0.01 thymus 0.20
Brain 0.03 lymph nodes (large intestine) 0.12
Liver 0.19 bone marrow 0.13
Kidney 0.14 omental fat 0.21
---------------------------------------------------------------
a From: Muller et al. (1978).
As part of the same study, a Rhesus monkey dosed orally with
radio-labelled quintozene at 2 mg/kg eliminated 92% of the
radioactivity after 5 days, 91% of which was in the form of
metabolites (Muller et al., 1978).
5. STUDIES ON EXPERIMENTAL ANIMALS
The toxicity and the residue data on quintozene have been
reviewed several times by international bodies such as FAO/WHO
(1970, 1974, 1976, 1978), IARC (1974), and CEC (1981). For their
conclusion, refer to section 8. We refer to these reports, which
contain more detailed information on the toxicity studies and
residue data than the present report. Moreover, several
unpublished studies have been evaluated and reported there.
5.1. Short-Term Studies
5.1.1. Single dose
Data on the acute toxicity of quintozene are summarized in
Table 3.
Table 3. Acute toxicity of quintozene
-------------------------------------------------------------------
Animal Route LD50 (mg/kg body weight) References
-------------------------------------------------------------------
rat (M) oral 1710 (oil solution) FAO/WHO (1970)
rat (F) oral 1650 (oil solution) FAO/WHO (1970)
rat oral > 30 000 (aqueous suspension) FAO/WHO (1970)
rat ip 5000 (aqueous suspension) FAO/WHO (1970)
dog oral no deaths up to 2500 mg/kg Berkowitz et al.
(1976)
rabbit dermal no deaths up to 4000 mg/kg Berkowitz et al.
(1976)
-------------------------------------------------------------------
Rabbits were dosed dermally once, with quintozene as a 30%
solution in dimethyl phthalate at 2 dose levels (10 and 13.3 ml/kg)
and observed for 14 days (Borzelleca et al., 1971). There was no
evidence of toxicity or skin irritation.
Cats
Quintozene, dissolved in corn oil, administered orally to cats
once at a level of 1600 mg/kg, caused a significant elevation in
methaemoglobin levels and an approximately 8-fold increase in the
number of erythrocytes containing Heinz bodies (Schumann &
Borzelleca, 1978). This latter finding, together with the fact
that a greater percentage of erythrocytes failed to stain properly
in the course of time, suggested that a functional impairment of
the erythrocyte had occurred.
5.1.2. Repeated dose
Rat
Five groups of 7 male and 7 female albino rats of weaning age
were fed diets containing technical quintozene at 0, 63.5, 635,
1250, 2500, or 5000 mg/kg for 3 months. Growth and survival were
adversely affected at 5000 mg/kg in both sexes and also in males at
2500 mg/kg. Liver hypertrophy was observed at all levels except in
females fed 63.5 mg/kg. No haematological changes were seen and
histological alterations were limited to fine vacuolization of
liver cell cytoplasm at 5000 mg/kg (Finnegan et al., 1958). An
unspecified number of young rats were fed diets containing 0 or
2000 mg quintozene/kg for 10 weeks (Wit et al., 1957). No gross
effects other than decreased growth rate in the males were noted.
Groups of 10 male and 10 female rats were fed diets containing 0,
1000, 5000, or 10 000 mg quintozene/kg for 90 days. The animals
showed a slight growth depression at 5000 mg/kg and marked growth
depression at 10 000 mg/kg (Hoechst, unpublished data, 1964).
Groups of 10 male and 10 female rats were fed diets containing
technical quintozene at concentrations of 0, 25, 100, 300, 1000, or
2500 mg/kg diet for 2 years. No changes in blood haematology were
found. In females, growth depression was observed at doses of 100
mg/kg diet and above (Finnegan et al., 1958).
Dogs
Groups of 3 mongrel dogs were fed diets containing 25, 200, or
1000 mg quintozene/kg for 1 year. No adverse effects were noted on
body weight or survival. No haematological changes were seen and
histopathological changes were restricted to liver cell enlarge-
ment, which was not dose-dependent (Finnegan et al., 1958). In a
2-year study, groups of 3 male and 3 female dogs were fed diets
containing 0, 500, 1000, or 5000 mg quintozene/kg. Liver changes
occurred in all groups in a dose-related manner. The 5000 mg/kg
level produced severe liver damage including fibrosis, narrowing of
hepatic cell cords, increased size of the periportal areas, and
leukocyte infiltration. At 1000 and 500 mg/kg, the changes were
similar but less pronounced. Reduced haematopoiesis and atrophy of
bone marrow were observed in animals receiving the highest dose
(FAO/WHO, 1970). Purebred beagles (4 per sex per dose) were fed
diets containing quintozene at levels ranging from 5 to 1080 mg/kg
for 2 years. Haematocrit values were depressed at 18 months in
males receiving 30 and 180 mg/kg, but not in animals receiving 1080
mg/kg. No dose-related effects were observed on urine analysis,
blood chemistry, mortality, body weight, food consumption, or
estrous cycle. Organ weight data revealed higher values for livers
in dogs fed 1080 mg quintozene/kg. Histologically, dogs sacrificed
at 2 years after receiving 180 or 1080 mg/kg showed hepatic and
renal effects deemed reversible by the authors (Borzelleca et al.,
1971).
Monkeys
Two male and two female rhesus monkeys were fed 2 mg
quintozene/kg for 70 days. The haematological variables
(haemoglobin, haematocrit, RBC, WBC), and the histopathological
examination of the liver, stomach, small and large intestine,
spleen, kidneys, heart, lung, thymus, cerebrum, cerebellum, pons,
medulla, spinal cord, and bone marrow were carried out in 1 male
and female monkey
after 70 days. The histopathology, clinical chemistry, and serum
cortisol levels remained within normal limits (Muller et al.,
1978).
5.2. Reproduction Studies
Reproduction studies were carried out on rats fed a diet
containing 0, 5, 50, or 500 mg quintozene/kg until the F/3b litters
were weaned. Quintozene had no effect on fertility
(pregnancies/mating), gestation (litters cast/pregnancies),
viability (live at 4 days/live at birth), or lactation (weaned/live
minus discards) indices. No dose-related histopathological
abnormalities were recorded in any of the F3b pups. It was not
teratogenic to rats at dosages up to 1563 mg/kg body weight (Jordan &
Borzelleca, 1973; Khera & Villeneuve, 1975; Courtney et al., 1976).
Levels of 500 mg/kg body weight of technical quintozene (87% pure)
administered from day 7 to 11 of gestation produced renal agenesis
in C57B1/6 mice, but none were produced with purified material
(99%) (Courtney et al., 1976). Hexachlorobenzene, a major
contaminant in the technical material, was implicated in the
teratogenic response. Quintozene did not produce any teratogenic
response in AKR mice when administered at levels up to 500 mg/kg
diet (Berkowitz et al., 1976).
5.3. Mutagenicity
Quintozene was reported to give a positive mutagenic
response in a host cell reactivation deficient strain of
E. coli (Clarke, 1971). It was not mutagenic when studied in an
Ames test system consisting of several bacterial strains using
Aroclor 1254 activation (Mohn, 1971). It was reported to be
negative in a reverse mutation assay using 5 tester strains of
Salmonella typhimurium and E. coli (Moriya et al., 1983). It was
shown to be negative in a dominant lethal test in mice where the
chemical was administered for 7 weeks in the diet (no
concentrations specified) (Van Logten, 1977). It produced no
significant increase in mutation rates in Salmonella typhimurium
and Serratia marcescens; it also gave negative results in spot
tests against the same strains of Salmonella typhimurium and
Serratia marcescens (Buselmaier et al., 1973). Both FAO/WHO
(1978) and CEC (1981) concluded that there were no indications for
mutagenic activity.
5.4. Carcinogenicity
The carcinogenicity of quintozene was evaluated by IARC in
1973 (IARC, 1974). Studies evaluated at that time as well as
additional studies are summarized below.
In a large screening study, 18 male and 18 female (C57BL/6
x C3H/Anf)F1 mice and similar numbers of (C57BL/6 x AKR)F1
mice were given single doses of 464 mg quintozene/kg body
weight (unspecified purity) by stomach tube, when the animals
were 7 days of age, and this same absolute dose was then given
daily until the animals were 28 days of age. This was followed by
a diet containing 1206 mg/kg diet, which was administered up to 78
weeks. Hepatomas were the only tumours found in excess over the
controls; 2/18 male and 4/18 female (C57BL/6 x C3H/Anf)F1 mice
developed hepatomas compared with 8/79 and 0/87 in controls. Of
the (C57BL/6/ x AKR)F1 mice, 10/17 males and 1/17 females developed
hepatomas compared with 5/90 and 1/82 in controls. The incidence
of other tumours was similar in treated and control animals (Innes
et al., 1969).
Ten stock albino mice of each sex were painted twice weekly
with 0.2 ml of a 0.3% solution of quintozene in acetone for 12
weeks. This was followed by twice-weekly paintings with a 0.5%
solution of croton-oil in acetone for 20 weeks followed by
observation for 40 weeks. In a control group, acetone alone was
given followed by treatment with croton oil. The total number of
skin tumours at the end of croton-oil treatment was 12 in 9
surviving controls and 50 in 13 survivors in the quintozene group.
One tumour in the quintozene group had progressed to a squamous-
cell carcinoma. An infiltrating squamous-cell carcinoma was also
observed in 1 control mouse killed 31 weeks from the start of the
croton-oil treatment (Searle, 1966).
Two unpublished studies on the carcinogenicity of quintozene
(containing 2.7% hexachlorobenzene) were reviewed by the FAO/WHO
Joint Meeting on Pesticide Residues in 1975. Groups of 100 male
and 100 female Swiss mice and 50 male and 50 female Wistar rats
were administered levels of 0, 100, 400, or 1200 mg/kg diet. In
mice, a non-dose-related increase in the incidence of liver
hyperplastic nodules was observed in males and an increased
incidence of subcutaneous fibrosarcomas was observed in females at
the highest dose level. No increased tumour incidence was reported
in rats (FAO/WHO, 1976). No further details of this study are
available.
Groups of 50 male and 50 females Osborne-Mendel rats and
B6C3F1 mice were given technical grade quintozene (purity 97%
with 12 impurities) in their diet for 78 weeks. In rats, the
average dietary concentrations were 10 064 and 5417 mg/kg of
diet for males and 14 635 and 7875 for females; in mice,
average dietary concentrations were 5213 and 2606 for males
and 8187 and 4093 for females. Observation continued for 33 -
35 additional weeks in rats and for 14 - 15 additional weeks
in mice. Adequate numbers of animals survived long enough to
permit the detection of late developing tumours. No
statistically-significant increase in the incidence of
neoplasms was seen in either species. It was concluded that
quintozene was not carcinogenic under the conditions of this
bioassay (NCI, 1978).
Hexachlorobenzene, a potential impurity in quintozene, is
carcinogenic in mice, rats, and hamsters producing tumours of
the liver (IARC, 1979; Smith & Cabral, 1980).
6. EFFECTS ON MAN
In patch tests, a quarter-inch square of cotton cloth was
moistened with water, dipped in a 75% quintozene wettable powder
(Olin formulation), and then placed on the volar surface of the
right forearms of 50 human volunteers. The patches were then
covered with a 1-inch square of aluminum foil held in place by a 2-
inch square of adhesive tape. After 48 h, the patches were
removed. No evidence of irritation was seen in any of the
subjects. Two weeks later, the same test was repeated on the left
arm of the same subjects. After 48 h, 46 of the 50 subjects showed
no signs or irritation. In 3 of the 4 reported reactions, a 1-inch
square area showed erythema, oedema, and small vesicle formation
with marked itching; the 4th subject had only erythema and itching.
Of the 46 subjects who were negative when the second patch was
removed, 9 developed a delayed reaction. Time of onset varied from
approximately 8 h to several days. In 2 of these persons, the
reaction included erythema, oedema, small vesicle formation, and
itching. The skin reaction reached a peak during the first few
days of symptoms and subsided with time, with some scaling of the
skin (Finnegan et al., 1958). One instance of keratoconjunctivitis
has been reported in the literature (Fujita et al., 1976) and
resulted from the application of the pesticide. Remission of the
condition took a month.
7. EFFECTS ON THE ENVIRONMENT
7.1. Toxicity for Aquatic Organims
Quintozene is of low toxicity for aquatic organisms. The only
data on the toxicity of quintozene for aquatic organisms is from
Nishiuchi & Yoshida (1972) who quote a 48-h LC50 value of 10 000
µg/litre for carp and a 3-h LC50 value for Daphnia of 40 000
µg/litre.
7.2. Toxicity for Terrestrial Organisms
7.2.1. Plants
Vishunavat & Shukla (1981) examined the effects of quintozene
on seed germination, plant stand, and yield of lentils. There were
no significant effects. Brown et al. (1982) did not find any
effects on the germination of orchids when 99% pure quintozene was
applied at concentrations of 25 and 50 mg/litre. At 100 mg/litre,
Cattleya elongata did not germinate, but germination of Laelia
was unaffected and Vanda tricolor showed improved germination.
Quintozene eliminated growth of excised shoot tips in orchids of
the Cymbidium family.
7.2.2. Earthworms
Roark & Dale (1979) reared earthworms Eisenia foetida in
soil pre-mixed with quintozene at a dose of 0.679 g/4719 cm3
of soil, corresponding to 226.8 g over 929 cm2 of turf. The
dose was calculated as the total of 3 applications of the
recommended dosage for turf. The worms did not reproduce in
treated soil. Survival of worms was not significantly reduced
within the first 10 days, but fell to zero within 29 days of
treatment with quintozene.
7.2.3. Bees
No information is available on toxicity of quintozene for bees.
However, since the main uses of quintozene are on soil or as a seed
dressing, it is unlikely that bees would be exposed.
7.2.4. Birds
Dunn et al. (1979a) fed white leghorns with concentrations
of 0, 10, 50, 100, or 1000 mg quintozene/kg diet and examined
egg production and hatchability. None of these concentrations
caused obvious toxic effects, death, or histopathological changes
in either control or treated groups. Egg production during the
25th to the 35th week was not significantly affected. However, at
a higher dose level of 1000 mg/kg diet, onset of egg production was
delayed for 1 month, and the number of chicks hatched from fertile
eggs significantly decreased from 91% in the controls to 69% in
treated birds. Shell strength of the eggs was not significantly
altered (Dunn et al., 1979a). In another study (Dunn et al.,
1979b), the authors reported that bioaccumulation of PCNB or its
metabolites only occurred in trace concentrations; body weight
gains were significantly lower in hens fed 1000 mg quintozene/kg
diet.
7.3. Toxicity for Microorganisms
Tu (1980) reported that quintozene at doses up to 5000 mg/litre
did not induce any effects on 3 strains of the bacterium Rhizobium
japonicum in culture. Quintozene also did not show any effects
on 25 strains of Rhizobium bacteria, isolated from root nodules of
red clover, at doses up to 1000 mg/litre in the culture medium
(Heinonen-Tanski et al., 1982). Smiley & Craven (1979) applied
quintozene 9 times annually for 3 years, at weekly intervals during
July and August, to a turf of Kentucky blue grass. There were no
significant effects on the populations of bacteria, actinomycetes,
or fungi. The effects of quintozene on carbon dioxide evolution
and on the enzyme activities in organisms in soil were observed by
Mitterer et al. (1981). The recommended soil dosage of the
fungicide caused an increase in carbon dioxide evolution from soil
cultures. After a second and third application, this initial
increase was followed by a decrease in carbon dioxide release to
below the value for untreated control soil. Quintozene showed a
severe and continuous inhibition of xylanase activity, in marked
contrast to other fungicides tested.
7.4. Bioaccumulation and Biomagnification
Ogiso & Tanabe (1982) measured residues in different tissues of
crop plants. High concentrations of quintozene in plant tissues
relative to soil levels were only found in the outer layers of
roots and tubers directly in contact with soil. There is little
evidence of systemic uptake.
Kanazawa (1981) reported a bioconcentration factor for
quintozene by top mouth gudgeon Pseudorasbora parva of 238. The
flow-through system maintained water concentrations of between 5
and 20 µg quintozene/litre.
8. PREVIOUS EVALUATIONS OF QUINTOZENE BY INTERNATIONAL BODIES
The Joint Meeting on Pesticide Residues (JMPR) reviewed
residues and toxicity data on quintozene in 1969, 1973, 1975, and
1977 (FAO/WHO, 1970, 1974, 1976, 1978). The conclusion in 1977 was
that 25 mg/kg diet, equivalent to 1.25 mg/kg body weight was a no-
observed-effect-level in the rat and 30 mg/kg diet, equivalent to
0.75 mg/kg body weight in the dog. On the basis of this, the
estimate of an acceptable daily intake (ADI) for man was 0 - 0.007
mg/kg body weight.
IARC (1974) did not come to a conclusion on the carcinogenicity
of quintozene because of lack of data at the time. FAO/WHO (1978)
concluded that there were no indications that administration of
quintozene resulted in carcinogenic activity.
CEC (1981) concluded that there was a need to set limits on the
impurities present in technical quintozene.
WHO, in its "Guidelines to the Use of the WHO Recommended
Classification of Pesticides by Hazard" (WHO, 1984), classified
quintozene in the category of technical products unlikely to
present an acute hazard in normal use.
Regulatory standards established by national bodies in 12
different countries (Argentina, Brazil, Czechoslovakia, the Federal
Republic of Germany, India, Japan, Kenya, Mexico, Sweden, the
United Kingdom, the USA, and the USSR) and the EEC can be found in
the IRPTC (International Register of Potentially Toxic Chemicals)
legal file (IRPTC, 1983).
9. EVALUATION OF HEALTH RISKS FOR MAN AND EFFECTS ON THE
ENVIRONMENT
9.1. Evaluation of Health Risks for Man
Quintozene toxicity
Quintozene is practically non-toxic according to the scale of
Hodge & Sterner (1956). The oral LD50 in rats was 1650 to more
than 30 000 mg/kg body weight. WHO (1984) classified quintozene in
the category of technical products unlikely to present an acute
hazard in normal use.
No-observed-adverse-effect levels in long-term studies on the
rat and the dog were 1.25 and 0.75 mg/kg body weight (25 and 30
mg/kg diet), respectively. In long-term studies with rats and at
higher dosages (63 mg/kg diet), quintozene can give rise to liver
hypertrophy with some histopathological changes and in dogs to more
severe liver damage with fibrosis (5000 mg/kg diet). In short-term
studies on female rats, quintozene caused induction of mixed-
function oxidases.
Quintozene is both metabolised and excreted unchanged and
does not accumulate in tissues.
Quintozene is not considered to be teratogenic.
Quintozene is generally negative in short-term tests for
genetic activity. In carcinogenicity studies on rats and
mice, equivocal or negative findings have been reported.
Hexachlorobenzene, a possible impurity in technical quintozene
is carcinogenic for mice, rats, and hamsters.
Except for 1 case of conjunctivitis in an occupational setting,
no other cases of poisoning or adverse effects have been reported
in man.
Exposure to quintozene
The general population can be exposed via residues in food,
especially in oils and fats. Information on exposure from other
sources is lacking. No cases of accidental or occupational
overexposure have been reported.
Hazard assessment
With the exception of some data on residues in food, no human
exposure data are available for quintozene. It is therefore
difficult to evaluate the hazard for man of present exposure to
this substance. Nevertheless, in view of its low toxicity in
short-term and long-term animal studies, the data available on
quintozene would indicate a low degree of concern in relation to
human health effects.
9.2. Evaluation of Overall Environmental Effects
The only significant adverse effect reported for quintozene is
on earthworms. According to laboratory tests, quintozene applied
at recommended doses as a soil fungicide appears to have long-term
toxic effects on the earthworm. Unfortunately, no observations of
the effects on earthworms of quintozene alone, during field use,
are available.
There is no evidence that quintozene represents a threat to
non-target organisms. It has a very low acute toxicity for fish
and Daphnia.
Its bioaccumulation by fish is low, and no effects have been
reported on terrestrial plants, birds, or microorganisms.
9.3. Conclusions
1. The general population does not appear to be at
risk from residues of quintozene in food.
2. Exposure of the general population via air and
drinking-water could not be evaluated because of lack
of data.
3. Occupational exposure has not been reported to
cause any adverse effects.
4. There is limited information on the effects of
quintozene in the general environment. It has been
shown to be toxic to earthworms, in laboratory
tests. Data on other organisms suggest that
quintozene is not a problem in the general
environment.
5. Quintozene does not biomagnify.
6. The major toxicological concern with quintozene
is the presence of hexachlorobenzene as an impurity.
REFERENCES
ANONYMOUS (1976) Fungicides. In: Analytical methods for
pesticides and plant growth regulators. VII. Government
regulations, pheromone analysis, additional pesticides, New
York, Academic Press, pp. 251-331.
ANONYMOUS (1977a) Rebuttable presumption against
registration and continued registration of pesticide products
containing PCNB. Fed. Regist., 42: 56072-56100.
ANONYMOUS (1977b) Surface water quality in Canada - an
overview. Fish. Environ. Can., 1-45.
AVRAHAMI, M. & WHITE, D.A. (1976) Rapid elimination of PCNB
and metabolite from sheep fat. N.Z. J. exp. Agric., 4: 299-302.
BECK, J. & HANSEN, K.E. (1974) The degradation of
quintozene, pentachlorobenzene, hexachlorobenzene and
petachloroaniline in soil. Science, 5: 41-48.
BERG, G.L., ed. (1978) Farm chemicals handbook, Willoughby,
Ohio, Meister Publishing Company, p. D133.
BERKOWITZ. J., STEVENS, J., ARNOLD, D., GOYER, M., SENECHAL,
D., HARRISON, J., LUDWIG, R., & NEWMEYER, J. (1976)
Substitute chemical program: initial scientific review of
PCNB, Washington DC, US Environmental Protection Agency,
Office of Pesticide Programs, Criteria and Evaluation Division
(EPA 540/1-75-016).
BETTS, J.J., JAMES, S.P., & THORPE, W.V. (1955) The
metabolism of pentachloronitrobenzene and 2,3,4,6-tetra-
chloronitrobenzene and the formation of mercapturic acids in
the rabbit. Biochem. J., 61: 611-617.
BORZELLECA, J.F., LARSON, P.S., CRAWFORD, E.M., HENNIGAR,
G.R., KUCHAR, E.J., & KLEIN, H.H. (1971) Toxicologic and
metabolic studies on pentachloronitrobenzene. Toxicol. appl.
Pharmacol., 18: 522-534.
BROWN, D.M., GROOM, C.L., CVITANIK, M., BROWN, M., COOPER,
J.L., & ARDITTI, J. (1982) Effects of fungicides and
bactericides on orchid seed germination and shoot tip cultures
in vitro. Plant Cell, Tissue and Organ Cult., 1: 165-180.
BURKE, G. & HOLSWADE, W. (1964) Gas chromatogrphy with
microcoulometric detection for pesticide residue analysis.
J. Assoc. Off. Anal. Chem., 47: 845-859.
BUSELMAIER, W., ROHRBORN, G., & PROPPING, P. (1973)
Comparative investigations on the mutagenicity of pesticides
in mammalian test systems. Mutat. Res., 21: 25-26.
CLARKE, C.H. (1971) The mutagenic specificities of
pentachloronitrobenzene and captan to environmental mutagens.
Mutat. Res., 11: 247-248.
CEC (1981) Criteria (dose/effect relationships) for
organochlorine pesticides, Oxford, Pergamon Press.
COURTNEY, K.D., COPELAND, M.F., & ROBBINS, A. (1976) The
effects of PCNB, hexachlorobenzene and related compounds on
fetal development. Toxicol. appl. Pharmacol., 35: 239-256.
CROSBY, D.G. & HAMADMAD, N. (1971) Photo-reduction of
pentachlorobenzenes. J. agric. food Chem., 19: 1171-1174.
DAPPEN, G. (1974) Pesticide analysis from urban storm
runoff, Washington DC, US Department of Commerce, National
Technical Information Service (PB Report, Issue No.
238593/8GA, 44).
DUNN, J.S., BUSH, P.B., BOOTH, N.H., FARRELL, R.L., THOMASON,
D.M., & GOETSCH, D.D. (1979a) Effect of pentachloronitro-
benzene upon egg production, hatchability, and residue
accumulation in the tissue of white leghorn hens. Toxicol.
appl. Pharmacol., 48: 425-433.
DUNN, J.S., BUSH, P.B., BOOTH, N.H., FARRELL, R.L., THOMASON,
D.M., & GOETSCH, D.D. (1979b) Tissue residues from feeding
pentachloronitrobenzene (quintozene) to white leghorn
chickens. Am. J. vet. Res., 40: 1227-1230.
DUNSING, M. & WINDSCHILD, J. (1976) [Residues of quintozene,
hexachlorobenzene, and pentachloroaniline in lettuce and
soil.] Nachr. Pflanzenschutzd., 30: 106-108 (in German).
FAO/WHO (1970) Quintozene. In: 1969 Evaluations of some
pesticide residues in foods, Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1974) Quintozene. In: 1973 Evaluations of some
pesticide residues in foods, Rome, Food and Agriculture
Organization of the United Nations, pp. 379-396.
FAO/WHO (1976) Quintozene. In: 1975 Evaluations of some
pesticide residues in foods, Rome, Food and Agriculture
Organization of the United Nations, pp. 357-363.
FAO/WHO (1978) Quintozene. In: 1977 Evaluations of some
pesticide residues in foods, Rome, Food and Agriculture
Organization of the United Nations, pp. 439-440.
FINNEGAN, J.K., LARSON, P.S., SMITH, R.B. Jr, HAAG, H.B., &
HENNIGAR, G.R. (1958) Acute and chronic toxicity studies on
pentachloronitrobenzene. Arch. int. Pharmacodyn., 114: 38-52.
FUJITA, K., SUZUKI, H., & OCHIAI, F. (1976) [Kerato-
conjunctivitis due to pesticides of relatively low acute
toxicity.] Clin. Opthalmol., 30: 419-423 (in Japanese).
GLOFKE, E. (1973) [Pesticide in milk and milk products.]
Milchwirt. Ber. Bundesanst. Wolfpassing Rotholz., 34: 37-40
(in German).
GOURSAUD, J., LUQUET, F.M., BOUDIER, J.F., & CASALIS, J.
(1972) Sur la pollution du lait par les residues
d'hexachlorobenzene (HCB). Ind. Aliment. Agric., 89: 31-35.
HEIKES, D.L. (1980) Residues of pentachloronitrobenzene and
related compounds in peanut butter. Bull. environ. Contam.
Toxicol., 24: 338-343.
HEINONEN-TANSKI, H., OROS, G., & KECSKES, M. (1982) The
effect of soil pesticides on the growth of red clover
Rhizobia. Acta agric. Scand., 32: 283-288.
HODGE, H.C. & STERNER, J.H. (1956) Combined tabulation of
toxicity classes. In: Spector, W.S., ed. Handbook of
toxicology, Philadelphia, W.B. Saunders Company, Vol. 1.
IARC (1974) Some organochlorine pesticides, Lyons,
International Agency for Research on Cancer, pp. 211-218
(Monographs on the Evaluation of the Carcinogenic Risk of
Chemicals to Man, No. 5).
IARC (1979) Hexachlorobenzene, Lyons, International Agency
for Research on Cancer, Vol. 20, pp. 155-179 (Monographs on
the Evaluation of the Carcinogenic Risk of Chemicals to Man).
INNES, J.R.M., ULLAND, B.M., VALERIO, M.G., PETRUCELLI, L.,
FISHBEIN, L., MART, O.R., PALLOTTA, A.J., BATTES, R.R., FALK,
R., GART, J.J., KLEIN, N., MITCHELL, I., & PETERS, J. (1969)
Bioassay of assay of pesticides and industrial chemicals for
tumorigenicity in mice: A preliminary note. J. Natl Cancer
Inst., 42: 1011-1114.
IRPTC (1983) IRPTC legal file 1983, Geneva, International
Register of Potentially Toxic Chemicals, United Nations
Environment Programme, Vol. 1 & 2.
JOHNSON, R.D., MANSKE, D.D., NEW, D.H., & PODEREBARAC, D.S.
(1969) Pesticides and other chemical residues in infant and
toddler total diet samples: 1 August 1974-July 1975, Pest.
Monit. J., 13: 87-98.
JORDAN, R.L. & BORZELLECA, J.F. (1973) Teratogenic studies
with pentachlorinitrobenzene in rats. Toxicol. appl.
Pharmacol., 25: 454.
KANAZAWA, J. (1981) Measurement of the bioconcentration
factors of pesticides by fresh water fish and their
correlation with physico-chemical properties or acute
toxicities. Pestic. Sci., 12: 417-424.
KHERA, K.S. & VILLENEUVE, D.C. (1975) Teratogenicity studies
on halogenated benzenes (pentachloro-, pentachlorinitro-, and
hexabromo-) in rats. Toxicology, 5: 117-122.
KO, W.H. & FARLEY, J.D. (1969) Conversion of
pentachloronitrobenzene to pentachloroaniline in soil and the
effect of these compounds on soil microorganisms.
Phytopathology, 59: 64-67.
KOEGEL, W., MULLER, W.F., COULSTON, F., & KORTE, F. (1979)
Fate and effects of pentachloronitrobenzene in Rhesus
monkeys. J. agric. food Chem., 27: 1181-1185.
KUCHAR, E.J., GEENTY, F.O., GRIFFITH, W.P., & THOMAS, R.J.
(1969) Analytical studies of metabolism of terrachlor in
beagle dogs, rats and plants. J. agric. food Chem., 17:
1237-1240.
LEE, R.E. Jr, ed. (1975) Air pollution from pesticides:
Sources, occurrence and dispersion. In: Air pollution from
pesticides and agricultural processes, Florida, CRC Press.
LEISTRA, M. & SMELT, J.H. (1974) Concentrations of
quintozene at different depths in bulb-growing soils. Bull.
environ. Contam. Toxicol., 11: 241-243.
METHRATTA, T.P., MONTAGNA, R.W., & GRIFFITH, W.D. (1967)
Determination of teraclor in crops and soil by electron-
capture gas chromatography. J. agric. food Chem., 15: 648-650.
MITTERER, M., BAYER, H., & SCHINNER, F. (1981) [The
influence of fungicides on microbiol acitivity in soil.]
Z. Pflanzenernaehr. Bodenk. D., 144: 463-471 (in German).
MOHN, G. (1971) Microorganisms as test systems of
mutagenicity. Arch. Toxicol., 28: 93-104.
MORIYA, M., OHTA, T., WATANABE, K., MIYAZAWA, T., KATO, K., &
SHIRASU, Y. (1983) Further mutagenicity studies on
pesticides in bacterial reversion assay systems. Mutat. Res.,
116: 185-216.
MULLER, W.F., SCHEUNERT, I., ROZMAN, K., KOGEL, W., FREITAG,
D., RICHTER, E., COULSTON, F., & KORTE, F. (1978)
Comparative metabolism of hexachlorobenzene in plants, rats
and Rhesus monkeys. Ecotoxicol. environ. Saf., 2: 437-445.
MUSTY, P.R. & NICKLESS, G. (1974) The extraction and
recovery of chlorinated insecticides and polychlorinated
biphenyls from water using porous polyurethane foams.
J. Chromatog., 100: 83-93.
NCI (1978) Bioassay of pentachloronitrobenzene for possible
carcinogenicity, Bethesda, Maryland, National Cancer Institute
(Technical Report Series No. 61).
NISHIUCHI, Y. & YOSHIDA, K. (1972) Noyaku Kensasho Hokoku,
p. 122 (in Japanese) (in English in Kanazawa (1981)).
OGISO, M. & TANABE, H. (1982) Residue of quintozene, its
metabolites, and hexachlorobenzene in the soil and crops.
J. Pestic. Sci. (Nihon Noyaku Gakkaishi), 7: 391-396.
O'GRODNICK, J.S., ADAMOVICS, J.A., BLAKE, SH., & WEDIG, J.
(1981) The metabolic fate of 14C-labelled pentachloronitro-
benzene in Osborne-Mendell rats. Chemosphere, 10: 67-72.
RAUTAPAA, J., PYYSALO, H., & BLOMQVIST, H. (1977) Quintozene
in some soils and plants in Finland. Ann. Agric. Fenn., 16:
277-282.
ROARK, J.H. & DALE, J.L. (1979) The effect of turf
fungicides on earthworms. Proc. Arkansas Acad. Sci., 33: 71-74.
SCHUMANN, A.M. & BORZELLECA, J.F. (1978) An assessment of
the methaemoglobin and Heinz-body-inducing capacity of
pentachloronitrobenzene in the cat. Toxicol. appl. Pharmacol.,
44: 523-529.
SEARLE, C.E. (1966) Tumor initiatory activity of
chloromono-nitrobenzenes and other compounds. Cancer Res.,
26: 12-17.
SIJPESTEIJN, A.K., DEKHUIJZEN, H.M., & VONK, J.W. (1977)
Biological conversions of fungicides in plants and micro-
organisms. In: Siegel, M.R. & Sisler, H.D., ed. Antifungal
compounds, Vol. 2: Interactions in biological and ecological
systems, New York, Marcel Dekker Inc., Vol. 2.
SIMON, G.S., KUCHAR, E.J., KLEIN, H.H., & BORZELLECA, J.F.
(1979) Distribution and clearance of pentachloronitrobenzene
in chickens. Toxicol. appl. Pharmacol., 50: 401-406.
SMILEY, R.W. & CRAVEN, M.M. (1979) Microflora of turfgrass
treated with fungicides. Soil Biol. Biochem., 11: 349-353.
SMITH, A.G. & CABRAL, J.R.P. (1980) Liver cell tumours in
rats fed hexachlorobenzene. Cancer Lett., 11: 169-172.
ST. JOHN, L.E. Jr, AMMERING, J.W., WAGNER, D.G., WARNER, R.G.,
& LISK, D.J. (1965) Fate of 4,6-dinitro-2-isobutylphenol,
2-chloro-4,6-bis(ethylamino)-S-triazine, and PCNB in the dairy
cow. J. dairy Sci., 48: 502-503.
TU, C.M. (1980) Effects of fungicides on growth of Rhizobium
japonicum in vitro. Bull. environ. Contam. Toxicol., 25:
364-368.
US DHEW (1973) Pesticide analytical manual, Maryland, US
Department of Health, Education, and Welfare, Food and Drug
Administration.
VAN LOGTEN, M. (1977) Quintozene. (Working Paper for 1977
JMPR) (FAO/WHO FOS/RES/77.46A).
VILLENEUVE, D.C. & KHERA, K.S. (1975) Placental transfer of
halogenated benzenes (Pentachloro-, Pentachloronitro-, and
Hexabromo-) in rats. Environ. Physiol. Biochem., 5: 328-331.
VISHUNAVAT, K. & SHUKLA, P. (1981) Effects of seed treatment
of lentil upon germination, plant stand, and yield.
Pesticides, 15: 15-16.
WANG, C.H. & BROADBENT, F.E. (1973) Effect of soil
treatments on losses of two chloronitrobenzene fungicides.
J. environ. Qual., 2: 511-515.
WHO (1975) Pesticide residues in food, Geneva, World Health
Organization, Vol. 97, pp. 7-37 (Technical Report Series No.
574 FAO Agricultural Studies).
WHO (1984) The WHO recommended classification of pesticides
by hazard, Geneva, World Health Organization (Unpublished
Report VBC/84.2).
WIT, S.L., VAN ESCH, G.J., & VAN GENDEREN, H. (1957)
Toxicity of some chloronitrobenzene compounds (trichlorodi-
nitrobenzene, trichloronitrobenzene, tetrachloronitrobenzene,
and pentachloronitrobenzene) to laboratory rats and residues
found in crops treated with these fungicides. In: Proceedings
of the 4th International Congress of Crop Protection, Hamburg.
4.4. Elimination
4.4.1. Human studies
No information on the elimination of quintozene in man was
found.
4.4.2. Animal studies
The major routes of elimination of ingested quintozene are via
the faeces as unchanged material or in the urine as metabolites
(Fig. 1)
The amount of quintozene eliminated unchanged in the faeces is
species-dependent. Betts et al. (1955) administered 1,2, or 3 g of
quintozene to rabbits by stomach tube and found an average of 46,
62, and 59% of the dose was eliminated unchanged in the faeces over
72 h. The faecal elimination was also variable (range 27 - 82% of
the dose). In sheep dosed orally with 31 mg/kg body weight,
approximately 80% of the dose was eliminated unchanged via the
faeces (Avrahami & White, 1976). In metabolic studies carried out
on Rhesus monkeys (Koegel et al., 1979), only 7.4% of the
administered oral dose (2 mg/kg body weight) was excreted as
quintozene in the faeces. After feeding rats with a diet
containing 500 mg/kg diet, no detectable quintozene was found in
the faeces, but unmetabolized quintozene was found in the faeces of
dogs fed 1080 mg/kg diet for 2 years (Borzelleca et al., 1971).
Once absorbed, the elimination of quintozene is primarily as
metabolites in the urine (section 4.3). Although quintozene has
been found in bile, the relative importance of this finding for the
elimination or enterohepatic circulation of quintozene is unknown.
Nevertheless, it would appear that elimination by this route is
species-dependent, since quintozene has been identified in the bile
of monkeys (Kogel et al., 1979) and mice (Courtney et al., 1976)
but not in the bile of chickens (Kuchar et al., 1969; WHO 1975) or
dogs (Borzelleca et al., 1971).
When a lactating cow was fed 5 mg/kg diet for 3 days, the
parent compound was not detected in the milk using a method in
which the sensitivity was 0.01 mg/kg (St. John et al., 1965).
Traces of quintozene were found in milk from cows treated orally
with the equivalent of 10 mg/kg diet for 8 weeks (Borzelleca et
al., 1971). It was also found in the milk of untreated cows and
analyses of the feed showed quintozene levels of 0.002 - 0.006
mg/kg.
Rhesus monkeys were fed diets containing quintozene at 2 mg/kg
for 550 days. A storage curve was constructed by subtracting the
excreted from the administered radioactivity. The storage curve
levelled out after 30 - 40 days of treatment, resulting in a
storage plateau of only 2 - 3% of the administered dose (Muller et
al., 1978).
There was little accumulation of quintozene in fat tissue;
slightly elevated concentrations are found in the liver and kidney
as well as the thymus, lymph nodes, and bone marrow.
Residual levels in various tissues are given in Table 2.
Table 2. Tissue residue levels in Rhesus monkeys fed 2 mg/kg PCNB
in the diet for 550 daysa
---------------------------------------------------------------
Tissue PCNB Tissue PCNB
(mg/kg) (mg/kg)
---------------------------------------------------------------
Blood 0.07 adrenal cortex 0.08
Muscle 0.01 thymus 0.20
Brain 0.03 lymph nodes (large intestine) 0.12
Liver 0.19 bone marrow 0.13
Kidney 0.14 omental fat 0.21
---------------------------------------------------------------
a From: Muller et al. (1978).
As part of the same study, a Rhesus monkey dosed orally with
radio-labelled quintozene at 2 mg/kg eliminated 92% of the
radioactivity after 5 days, 91% of which was in the form of
metabolites (Muller et al., 1978).
5. STUDIES ON EXPERIMENTAL ANIMALS
The toxicity and the residue data on quintozene have been
reviewed several times by international bodies such as FAO/WHO
(1970, 1974, 1976, 1978), IARC (1974), and CEC (1981). For their
conclusion, refer to section 8. We refer to these reports, which
contain more detailed information on the toxicity studies and
residue data than the present report. Moreover, several
unpublished studies have been evaluated and reported there.
5.1. Short-Term Studies
5.1.1. Single dose
Data on the acute toxicity of quintozene are summarized in
Table 3.
Table 3. Acute toxicity of quintozene
-------------------------------------------------------------------
Animal Route LD50 (mg/kg body weight) References
-------------------------------------------------------------------
rat (M) oral 1710 (oil solution) FAO/WHO (1970)
rat (F) oral 1650 (oil solution) FAO/WHO (1970)
rat oral > 30 000 (aqueous suspension) FAO/WHO (1970)
rat ip 5000 (aqueous suspension) FAO/WHO (1970)
dog oral no deaths up to 2500 mg/kg Berkowitz et al.
(1976)
rabbit dermal no deaths up to 4000 mg/kg Berkowitz et al.
(1976)
-------------------------------------------------------------------
Rabbits were dosed dermally once, with quintozene as a 30%
solution in dimethyl phthalate at 2 dose levels (10 and 13.3 ml/kg)
and observed for 14 days (Borzelleca et al., 1971). There was no
evidence of toxicity or skin irritation.
Cats
Quintozene, dissolved in corn oil, administered orally to cats
once at a level of 1600 mg/kg, caused a significant elevation in
methaemoglobin levels and an approximately 8-fold increase in the
number of erythrocytes containing Heinz bodies (Schumann &
Borzelleca, 1978). This latter finding, together with the fact
that a greater percentage of erythrocytes failed to stain properly
in the course of time, suggested that a functional impairment of
the erythrocyte had occurred.
5.1.2. Repeated dose
Rat
Five groups of 7 male and 7 female albino rats of weaning age
were fed diets containing technical quintozene at 0, 63.5, 635,
1250, 2500, or 5000 mg/kg for 3 months. Growth and survival were
adversely affected at 5000 mg/kg in both sexes and also in males at
2500 mg/kg. Liver hypertrophy was observed at all levels except in
females fed 63.5 mg/kg. No haematological changes were seen and
histological alterations were limited to fine vacuolization of
liver cell cytoplasm at 5000 mg/kg (Finnegan et al., 1958). An
unspecified number of young rats were fed diets containing 0 or
2000 mg quintozene/kg for 10 weeks (Wit et al., 1957). No gross
effects other than decreased growth rate in the males were noted.
Groups of 10 male and 10 female rats were fed diets containing 0,
1000, 5000, or 10 000 mg quintozene/kg for 90 days. The animals
showed a slight growth depression at 5000 mg/kg and marked growth
depression at 10 000 mg/kg (Hoechst, unpublished data, 1964).
Groups of 10 male and 10 female rats were fed diets containing
technical quintozene at concentrations of 0, 25, 100, 300, 1000, or
2500 mg/kg diet for 2 years. No changes in blood haematology were
found. In females, growth depression was observed at doses of 100
mg/kg diet and above (Finnegan et al., 1958).
Dogs
Groups of 3 mongrel dogs were fed diets containing 25, 200, or
1000 mg quintozene/kg for 1 year. No adverse effects were noted on
body weight or survival. No haematological changes were seen and
histopathological changes were restricted to liver cell enlarge-
ment, which was not dose-dependent (Finnegan et al., 1958). In a
2-year study, groups of 3 male and 3 female dogs were fed diets
containing 0, 500, 1000, or 5000 mg quintozene/kg. Liver changes
occurred in all groups in a dose-related manner. The 5000 mg/kg
level produced severe liver damage including fibrosis, narrowing of
hepatic cell cords, increased size of the periportal areas, and
leukocyte infiltration. At 1000 and 500 mg/kg, the changes were
similar but less pronounced. Reduced haematopoiesis and atrophy of
bone marrow were observed in animals receiving the highest dose
(FAO/WHO, 1970). Purebred beagles (4 per sex per dose) were fed
diets containing quintozene at levels ranging from 5 to 1080 mg/kg
for 2 years. Haematocrit values were depressed at 18 months in
males receiving 30 and 180 mg/kg, but not in animals receiving 1080
mg/kg. No dose-related effects were observed on urine analysis,
blood chemistry, mortality, body weight, food consumption, or
estrous cycle. Organ weight data revealed higher values for livers
in dogs fed 1080 mg quintozene/kg. Histologically, dogs sacrificed
at 2 years after receiving 180 or 1080 mg/kg showed hepatic and
renal effects deemed reversible by the authors (Borzelleca et al.,
1971).
Monkeys
Two male and two female rhesus monkeys were fed 2 mg
quintozene/kg for 70 days. The haematological variables
(haemoglobin, haematocrit, RBC, WBC), and the histopathological
examination of the liver, stomach, small and large intestine,
spleen, kidneys, heart, lung, thymus, cerebrum, cerebellum, pons,
medulla, spinal cord, and bone marrow were carried out in 1 male
and female monkey
after 70 days. The histopathology, clinical chemistry, and serum
cortisol levels remained within normal limits (Muller et al.,
1978).
5.2. Reproduction Studies
Reproduction studies were carried out on rats fed a diet
containing 0, 5, 50, or 500 mg quintozene/kg until the F/3b litters
were weaned. Quintozene had no effect on fertility
(pregnancies/mating), gestation (litters cast/pregnancies),
viability (live at 4 days/live at birth), or lactation (weaned/live
minus discards) indices. No dose-related histopathological
abnormalities were recorded in any of the F3b pups. It was not
teratogenic to rats at dosages up to 1563 mg/kg body weight (Jordan &
Borzelleca, 1973; Khera & Villeneuve, 1975; Courtney et al., 1976).
Levels of 500 mg/kg body weight of technical quintozene (87% pure)
administered from day 7 to 11 of gestation produced renal agenesis
in C57B1/6 mice, but none were produced with purified material
(99%) (Courtney et al., 1976). Hexachlorobenzene, a major
contaminant in the technical material, was implicated in the
teratogenic response. Quintozene did not produce any teratogenic
response in AKR mice when administered at levels up to 500 mg/kg
diet (Berkowitz et al., 1976).
5.3. Mutagenicity
Quintozene was reported to give a positive mutagenic
response in a host cell reactivation deficient strain of
E. coli (Clarke, 1971). It was not mutagenic when studied in an
Ames test system consisting of several bacterial strains using
Aroclor 1254 activation (Mohn, 1971). It was reported to be
negative in a reverse mutation assay using 5 tester strains of
Salmonella typhimurium and E. coli (Moriya et al., 1983). It was
shown to be negative in a dominant lethal test in mice where the
chemical was administered for 7 weeks in the diet (no
concentrations specified) (Van Logten, 1977). It produced no
significant increase in mutation rates in Salmonella typhimurium
and Serratia marcescens; it also gave negative results in spot
tests against the same strains of Salmonella typhimurium and
Serratia marcescens (Buselmaier et al., 1973). Both FAO/WHO
(1978) and CEC (1981) concluded that there were no indications for
mutagenic activity.
5.4. Carcinogenicity
The carcinogenicity of quintozene was evaluated by IARC in
1973 (IARC, 1974). Studies evaluated at that time as well as
additional studies are summarized below.
In a large screening study, 18 male and 18 female (C57BL/6
x C3H/Anf)F1 mice and similar numbers of (C57BL/6 x AKR)F1
mice were given single doses of 464 mg quintozene/kg body
weight (unspecified purity) by stomach tube, when the animals
were 7 days of age, and this same absolute dose was then given
daily until the animals were 28 days of age. This was followed by
a diet containing 1206 mg/kg diet, which was administered up to 78
weeks. Hepatomas were the only tumours found in excess over the
controls; 2/18 male and 4/18 female (C57BL/6 x C3H/Anf)F1 mice
developed hepatomas compared with 8/79 and 0/87 in controls. Of
the (C57BL/6/ x AKR)F1 mice, 10/17 males and 1/17 females developed
hepatomas compared with 5/90 and 1/82 in controls. The incidence
of other tumours was similar in treated and control animals (Innes
et al., 1969).
Ten stock albino mice of each sex were painted twice weekly
with 0.2 ml of a 0.3% solution of quintozene in acetone for 12
weeks. This was followed by twice-weekly paintings with a 0.5%
solution of croton-oil in acetone for 20 weeks followed by
observation for 40 weeks. In a control group, acetone alone was
given followed by treatment with croton oil. The total number of
skin tumours at the end of croton-oil treatment was 12 in 9
surviving controls and 50 in 13 survivors in the quintozene group.
One tumour in the quintozene group had progressed to a squamous-
cell carcinoma. An infiltrating squamous-cell carcinoma was also
observed in 1 control mouse killed 31 weeks from the start of the
croton-oil treatment (Searle, 1966).
Two unpublished studies on the carcinogenicity of quintozene
(containing 2.7% hexachlorobenzene) were reviewed by the FAO/WHO
Joint Meeting on Pesticide Residues in 1975. Groups of 100 male
and 100 female Swiss mice and 50 male and 50 female Wistar rats
were administered levels of 0, 100, 400, or 1200 mg/kg diet. In
mice, a non-dose-related increase in the incidence of liver
hyperplastic nodules was observed in males and an increased
incidence of subcutaneous fibrosarcomas was observed in females at
the highest dose level. No increased tumour incidence was reported
in rats (FAO/WHO, 1976). No further details of this study are
available.
Groups of 50 male and 50 females Osborne-Mendel rats and
B6C3F1 mice were given technical grade quintozene (purity 97%
with 12 impurities) in their diet for 78 weeks. In rats, the
average dietary concentrations were 10 064 and 5417 mg/kg of
diet for males and 14 635 and 7875 for females; in mice,
average dietary concentrations were 5213 and 2606 for males
and 8187 and 4093 for females. Observation continued for 33 -
35 additional weeks in rats and for 14 - 15 additional weeks
in mice. Adequate numbers of animals survived long enough to
permit the detection of late developing tumours. No
statistically-significant increase in the incidence of
neoplasms was seen in either species. It was concluded that
quintozene was not carcinogenic under the conditions of this
bioassay (NCI, 1978).
Hexachlorobenzene, a potential impurity in quintozene, is
carcinogenic in mice, rats, and hamsters producing tumours of
the liver (IARC, 1979; Smith & Cabral, 1980).
6. EFFECTS ON MAN
In patch tests, a quarter-inch square of cotton cloth was
moistened with water, dipped in a 75% quintozene wettable powder
(Olin formulation), and then placed on the volar surface of the
right forearms of 50 human volunteers. The patches were then
covered with a 1-inch square of aluminum foil held in place by a 2-
inch square of adhesive tape. After 48 h, the patches were
removed. No evidence of irritation was seen in any of the
subjects. Two weeks later, the same test was repeated on the left
arm of the same subjects. After 48 h, 46 of the 50 subjects showed
no signs or irritation. In 3 of the 4 reported reactions, a 1-inch
square area showed erythema, oedema, and small vesicle formation
with marked itching; the 4th subject had only erythema and itching.
Of the 46 subjects who were negative when the second patch was
removed, 9 developed a delayed reaction. Time of onset varied from
approximately 8 h to several days. In 2 of these persons, the
reaction included erythema, oedema, small vesicle formation, and
itching. The skin reaction reached a peak during the first few
days of symptoms and subsided with time, with some scaling of the
skin (Finnegan et al., 1958). One instance of keratoconjunctivitis
has been reported in the literature (Fujita et al., 1976) and
resulted from the application of the pesticide. Remission of the
condition took a month.
7. EFFECTS ON THE ENVIRONMENT
7.1. Toxicity for Aquatic Organims
Quintozene is of low toxicity for aquatic organisms. The only
data on the toxicity of quintozene for aquatic organisms is from
Nishiuchi & Yoshida (1972) who quote a 48-h LC50 value of 10 000
µg/litre for carp and a 3-h LC50 value for Daphnia of 40 000
µg/litre.
7.2. Toxicity for Terrestrial Organisms
7.2.1. Plants
Vishunavat & Shukla (1981) examined the effects of quintozene
on seed germination, plant stand, and yield of lentils. There were
no significant effects. Brown et al. (1982) did not find any
effects on the germination of orchids when 99% pure quintozene was
applied at concentrations of 25 and 50 mg/litre. At 100 mg/litre,
Cattleya elongata did not germinate, but germination of Laelia
was unaffected and Vanda tricolor showed improved germination.
Quintozene eliminated growth of excised shoot tips in orchids of
the Cymbidium family.
7.2.2. Earthworms
Roark & Dale (1979) reared earthworms Eisenia foetida in
soil pre-mixed with quintozene at a dose of 0.679 g/4719 cm3
of soil, corresponding to 226.8 g over 929 cm2 of turf. The
dose was calculated as the total of 3 applications of the
recommended dosage for turf. The worms did not reproduce in
treated soil. Survival of worms was not significantly reduced
within the first 10 days, but fell to zero within 29 days of
treatment with quintozene.
7.2.3. Bees
No information is available on toxicity of quintozene for bees.
However, since the main uses of quintozene are on soil or as a seed
dressing, it is unlikely that bees would be exposed.
7.2.4. Birds
Dunn et al. (1979a) fed white leghorns with concentrations
of 0, 10, 50, 100, or 1000 mg quintozene/kg diet and examined
egg production and hatchability. None of these concentrations
caused obvious toxic effects, death, or histopathological changes
in either control or treated groups. Egg production during the
25th to the 35th week was not significantly affected. However, at
a higher dose level of 1000 mg/kg diet, onset of egg production was
delayed for 1 month, and the number of chicks hatched from fertile
eggs significantly decreased from 91% in the controls to 69% in
treated birds. Shell strength of the eggs was not significantly
altered (Dunn et al., 1979a). In another study (Dunn et al.,
1979b), the authors reported that bioaccumulation of PCNB or its
metabolites only occurred in trace concentrations; body weight
gains were significantly lower in hens fed 1000 mg quintozene/kg
diet.
7.3. Toxicity for Microorganisms
Tu (1980) reported that quintozene at doses up to 5000 mg/litre
did not induce any effects on 3 strains of the bacterium Rhizobium
japonicum in culture. Quintozene also did not show any effects
on 25 strains of Rhizobium bacteria, isolated from root nodules of
red clover, at doses up to 1000 mg/litre in the culture medium
(Heinonen-Tanski et al., 1982). Smiley & Craven (1979) applied
quintozene 9 times annually for 3 years, at weekly intervals during
July and August, to a turf of Kentucky blue grass. There were no
significant effects on the populations of bacteria, actinomycetes,
or fungi. The effects of quintozene on carbon dioxide evolution
and on the enzyme activities in organisms in soil were observed by
Mitterer et al. (1981). The recommended soil dosage of the
fungicide caused an increase in carbon dioxide evolution from soil
cultures. After a second and third application, this initial
increase was followed by a decrease in carbon dioxide release to
below the value for untreated control soil. Quintozene showed a
severe and continuous inhibition of xylanase activity, in marked
contrast to other fungicides tested.
7.4. Bioaccumulation and Biomagnification
Ogiso & Tanabe (1982) measured residues in different tissues of
crop plants. High concentrations of quintozene in plant tissues
relative to soil levels were only found in the outer layers of
roots and tubers directly in contact with soil. There is little
evidence of systemic uptake.
Kanazawa (1981) reported a bioconcentration factor for
quintozene by top mouth gudgeon Pseudorasbora parva of 238. The
flow-through system maintained water concentrations of between 5
and 20 µg quintozene/litre.
8. PREVIOUS EVALUATIONS OF QUINTOZENE BY INTERNATIONAL BODIES
The Joint Meeting on Pesticide Residues (JMPR) reviewed
residues and toxicity data on quintozene in 1969, 1973, 1975, and
1977 (FAO/WHO, 1970, 1974, 1976, 1978). The conclusion in 1977 was
that 25 mg/kg diet, equivalent to 1.25 mg/kg body weight was a no-
observed-effect-level in the rat and 30 mg/kg diet, equivalent to
0.75 mg/kg body weight in the dog. On the basis of this, the
estimate of an acceptable daily intake (ADI) for man was 0 - 0.007
mg/kg body weight.
IARC (1974) did not come to a conclusion on the carcinogenicity
of quintozene because of lack of data at the time. FAO/WHO (1978)
concluded that there were no indications that administration of
quintozene resulted in carcinogenic activity.
CEC (1981) concluded that there was a need to set limits on the
impurities present in technical quintozene.
WHO, in its "Guidelines to the Use of the WHO Recommended
Classification of Pesticides by Hazard" (WHO, 1984), classified
quintozene in the category of technical products unlikely to
present an acute hazard in normal use.
Regulatory standards established by national bodies in 12
different countries (Argentina, Brazil, Czechoslovakia, the Federal
Republic of Germany, India, Japan, Kenya, Mexico, Sweden, the
United Kingdom, the USA, and the USSR) and the EEC can be found in
the IRPTC (International Register of Potentially Toxic Chemicals)
legal file (IRPTC, 1983).
9. EVALUATION OF HEALTH RISKS FOR MAN AND EFFECTS ON THE
ENVIRONMENT
9.1. Evaluation of Health Risks for Man
Quintozene toxicity
Quintozene is practically non-toxic according to the scale of
Hodge & Sterner (1956). The oral LD50 in rats was 1650 to more
than 30 000 mg/kg body weight. WHO (1984) classified quintozene in
the category of technical products unlikely to present an acute
hazard in normal use.
No-observed-adverse-effect levels in long-term studies on the
rat and the dog were 1.25 and 0.75 mg/kg body weight (25 and 30
mg/kg diet), respectively. In long-term studies with rats and at
higher dosages (63 mg/kg diet), quintozene can give rise to liver
hypertrophy with some histopathological changes and in dogs to more
severe liver damage with fibrosis (5000 mg/kg diet). In short-term
studies on female rats, quintozene caused induction of mixed-
function oxidases.
Quintozene is both metabolised and excreted unchanged and
does not accumulate in tissues.
Quintozene is not considered to be teratogenic.
Quintozene is generally negative in short-term tests for
genetic activity. In carcinogenicity studies on rats and
mice, equivocal or negative findings have been reported.
Hexachlorobenzene, a possible impurity in technical quintozene
is carcinogenic for mice, rats, and hamsters.
Except for 1 case of conjunctivitis in an occupational setting,
no other cases of poisoning or adverse effects have been reported
in man.
Exposure to quintozene
The general population can be exposed via residues in food,
especially in oils and fats. Information on exposure from other
sources is lacking. No cases of accidental or occupational
overexposure have been reported.
Hazard assessment
With the exception of some data on residues in food, no human
exposure data are available for quintozene. It is therefore
difficult to evaluate the hazard for man of present exposure to
this substance. Nevertheless, in view of its low toxicity in
short-term and long-term animal studies, the data available on
quintozene would indicate a low degree of concern in relation to
human health effects.
9.2. Evaluation of Overall Environmental Effects
The only significant adverse effect reported for quintozene is
on earthworms. According to laboratory tests, quintozene applied
at recommended doses as a soil fungicide appears to have long-term
toxic effects on the earthworm. Unfortunately, no observations of
the effects on earthworms of quintozene alone, during field use,
are available.
There is no evidence that quintozene represents a threat to
non-target organisms. It has a very low acute toxicity for fish
and Daphnia.
Its bioaccumulation by fish is low, and no effects have been
reported on terrestrial plants, birds, or microorganisms.
9.3. Conclusions
1. The general population does not appear to be at
risk from residues of quintozene in food.
2. Exposure of the general population via air and
drinking-water could not be evaluated because of lack
of data.
3. Occupational exposure has not been reported to
cause any adverse effects.
4. There is limited information on the effects of
quintozene in the general environment. It has been
shown to be toxic to earthworms, in laboratory
tests. Data on other organisms suggest that
quintozene is not a problem in the general
environment.
5. Quintozene does not biomagnify.
6. The major toxicological concern with quintozene
is the presence of hexachlorobenzene as an impurity.
REFERENCES
ANONYMOUS (1976) Fungicides. In: Analytical methods for
pesticides and plant growth regulators. VII. Government
regulations, pheromone analysis, additional pesticides, New
York, Academic Press, pp. 251-331.
ANONYMOUS (1977a) Rebuttable presumption against
registration and continued registration of pesticide products
containing PCNB. Fed. Regist., 42: 56072-56100.
ANONYMOUS (1977b) Surface water quality in Canada - an
overview. Fish. Environ. Can., 1-45.
AVRAHAMI, M. & WHITE, D.A. (1976) Rapid elimination of PCNB
and metabolite from sheep fat. N.Z. J. exp. Agric., 4: 299-302.
BECK, J. & HANSEN, K.E. (1974) The degradation of
quintozene, pentachlorobenzene, hexachlorobenzene and
petachloroaniline in soil. Science, 5: 41-48.
BERG, G.L., ed. (1978) Farm chemicals handbook, Willoughby,
Ohio, Meister Publishing Company, p. D133.
BERKOWITZ. J., STEVENS, J., ARNOLD, D., GOYER, M., SENECHAL,
D., HARRISON, J., LUDWIG, R., & NEWMEYER, J. (1976)
Substitute chemical program: initial scientific review of
PCNB, Washington DC, US Environmental Protection Agency,
Office of Pesticide Programs, Criteria and Evaluation Division
(EPA 540/1-75-016).
BETTS, J.J., JAMES, S.P., & THORPE, W.V. (1955) The
metabolism of pentachloronitrobenzene and 2,3,4,6-tetra-
chloronitrobenzene and the formation of mercapturic acids in
the rabbit. Biochem. J., 61: 611-617.
BORZELLECA, J.F., LARSON, P.S., CRAWFORD, E.M., HENNIGAR,
G.R., KUCHAR, E.J., & KLEIN, H.H. (1971) Toxicologic and
metabolic studies on pentachloronitrobenzene. Toxicol. appl.
Pharmacol., 18: 522-534.
BROWN, D.M., GROOM, C.L., CVITANIK, M., BROWN, M., COOPER,
J.L., & ARDITTI, J. (1982) Effects of fungicides and
bactericides on orchid seed germination and shoot tip cultures
in vitro. Plant Cell, Tissue and Organ Cult., 1: 165-180.
BURKE, G. & HOLSWADE, W. (1964) Gas chromatogrphy with
microcoulometric detection for pesticide residue analysis.
J. Assoc. Off. Anal. Chem., 47: 845-859.
BUSELMAIER, W., ROHRBORN, G., & PROPPING, P. (1973)
Comparative investigations on the mutagenicity of pesticides
in mammalian test systems. Mutat. Res., 21: 25-26.
CLARKE, C.H. (1971) The mutagenic specificities of
pentachloronitrobenzene and captan to environmental mutagens.
Mutat. Res., 11: 247-248.
CEC (1981) Criteria (dose/effect relationships) for
organochlorine pesticides, Oxford, Pergamon Press.
COURTNEY, K.D., COPELAND, M.F., & ROBBINS, A. (1976) The
effects of PCNB, hexachlorobenzene and related compounds on
fetal development. Toxicol. appl. Pharmacol., 35: 239-256.
CROSBY, D.G. & HAMADMAD, N. (1971) Photo-reduction of
pentachlorobenzenes. J. agric. food Chem., 19: 1171-1174.
DAPPEN, G. (1974) Pesticide analysis from urban storm
runoff, Washington DC, US Department of Commerce, National
Technical Information Service (PB Report, Issue No.
238593/8GA, 44).
DUNN, J.S., BUSH, P.B., BOOTH, N.H., FARRELL, R.L., THOMASON,
D.M., & GOETSCH, D.D. (1979a) Effect of pentachloronitro-
benzene upon egg production, hatchability, and residue
accumulation in the tissue of white leghorn hens. Toxicol.
appl. Pharmacol., 48: 425-433.
DUNN, J.S., BUSH, P.B., BOOTH, N.H., FARRELL, R.L., THOMASON,
D.M., & GOETSCH, D.D. (1979b) Tissue residues from feeding
pentachloronitrobenzene (quintozene) to white leghorn
chickens. Am. J. vet. Res., 40: 1227-1230.
DUNSING, M. & WINDSCHILD, J. (1976) [Residues of quintozene,
hexachlorobenzene, and pentachloroaniline in lettuce and
soil.] Nachr. Pflanzenschutzd., 30: 106-108 (in German).
FAO/WHO (1970) Quintozene. In: 1969 Evaluations of some
pesticide residues in foods, Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1974) Quintozene. In: 1973 Evaluations of some
pesticide residues in foods, Rome, Food and Agriculture
Organization of the United Nations, pp. 379-396.
FAO/WHO (1976) Quintozene. In: 1975 Evaluations of some
pesticide residues in foods, Rome, Food and Agriculture
Organization of the United Nations, pp. 357-363.
FAO/WHO (1978) Quintozene. In: 1977 Evaluations of some
pesticide residues in foods, Rome, Food and Agriculture
Organization of the United Nations, pp. 439-440.
FINNEGAN, J.K., LARSON, P.S., SMITH, R.B. Jr, HAAG, H.B., &
HENNIGAR, G.R. (1958) Acute and chronic toxicity studies on
pentachloronitrobenzene. Arch. int. Pharmacodyn., 114: 38-52.
FUJITA, K., SUZUKI, H., & OCHIAI, F. (1976) [Kerato-
conjunctivitis due to pesticides of relatively low acute
toxicity.] Clin. Opthalmol., 30: 419-423 (in Japanese).
GLOFKE, E. (1973) [Pesticide in milk and milk products.]
Milchwirt. Ber. Bundesanst. Wolfpassing Rotholz., 34: 37-40
(in German).
GOURSAUD, J., LUQUET, F.M., BOUDIER, J.F., & CASALIS, J.
(1972) Sur la pollution du lait par les residues
d'hexachlorobenzene (HCB). Ind. Aliment. Agric., 89: 31-35.
HEIKES, D.L. (1980) Residues of pentachloronitrobenzene and
related compounds in peanut butter. Bull. environ. Contam.
Toxicol., 24: 338-343.
HEINONEN-TANSKI, H., OROS, G., & KECSKES, M. (1982) The
effect of soil pesticides on the growth of red clover
Rhizobia. Acta agric. Scand., 32: 283-288.
HODGE, H.C. & STERNER, J.H. (1956) Combined tabulation of
toxicity classes. In: Spector, W.S., ed. Handbook of
toxicology, Philadelphia, W.B. Saunders Company, Vol. 1.
IARC (1974) Some organochlorine pesticides, Lyons,
International Agency for Research on Cancer, pp. 211-218
(Monographs on the Evaluation of the Carcinogenic Risk of
Chemicals to Man, No. 5).
IARC (1979) Hexachlorobenzene, Lyons, International Agency
for Research on Cancer, Vol. 20, pp. 155-179 (Monographs on
the Evaluation of the Carcinogenic Risk of Chemicals to Man).
INNES, J.R.M., ULLAND, B.M., VALERIO, M.G., PETRUCELLI, L.,
FISHBEIN, L., MART, O.R., PALLOTTA, A.J., BATTES, R.R., FALK,
R., GART, J.J., KLEIN, N., MITCHELL, I., & PETERS, J. (1969)
Bioassay of assay of pesticides and industrial chemicals for
tumorigenicity in mice: A preliminary note. J. Natl Cancer
Inst., 42: 1011-1114.
IRPTC (1983) IRPTC legal file 1983, Geneva, International
Register of Potentially Toxic Chemicals, United Nations
Environment Programme, Vol. 1 & 2.
JOHNSON, R.D., MANSKE, D.D., NEW, D.H., & PODEREBARAC, D.S.
(1969) Pesticides and other chemical residues in infant and
toddler total diet samples: 1 August 1974-July 1975, Pest.
Monit. J., 13: 87-98.
JORDAN, R.L. & BORZELLECA, J.F. (1973) Teratogenic studies
with pentachlorinitrobenzene in rats. Toxicol. appl.
Pharmacol., 25: 454.
KANAZAWA, J. (1981) Measurement of the bioconcentration
factors of pesticides by fresh water fish and their
correlation with physico-chemical properties or acute
toxicities. Pestic. Sci., 12: 417-424.
KHERA, K.S. & VILLENEUVE, D.C. (1975) Teratogenicity studies
on halogenated benzenes (pentachloro-, pentachlorinitro-, and
hexabromo-) in rats. Toxicology, 5: 117-122.
KO, W.H. & FARLEY, J.D. (1969) Conversion of
pentachloronitrobenzene to pentachloroaniline in soil and the
effect of these compounds on soil microorganisms.
Phytopathology, 59: 64-67.
KOEGEL, W., MULLER, W.F., COULSTON, F., & KORTE, F. (1979)
Fate and effects of pentachloronitrobenzene in Rhesus
monkeys. J. agric. food Chem., 27: 1181-1185.
KUCHAR, E.J., GEENTY, F.O., GRIFFITH, W.P., & THOMAS, R.J.
(1969) Analytical studies of metabolism of terrachlor in
beagle dogs, rats and plants. J. agric. food Chem., 17:
1237-1240.
LEE, R.E. Jr, ed. (1975) Air pollution from pesticides:
Sources, occurrence and dispersion. In: Air pollution from
pesticides and agricultural processes, Florida, CRC Press.
LEISTRA, M. & SMELT, J.H. (1974) Concentrations of
quintozene at different depths in bulb-growing soils. Bull.
environ. Contam. Toxicol., 11: 241-243.
METHRATTA, T.P., MONTAGNA, R.W., & GRIFFITH, W.D. (1967)
Determination of teraclor in crops and soil by electron-
capture gas chromatography. J. agric. food Chem., 15: 648-650.
MITTERER, M., BAYER, H., & SCHINNER, F. (1981) [The
influence of fungicides on microbiol acitivity in soil.]
Z. Pflanzenernaehr. Bodenk. D., 144: 463-471 (in German).
MOHN, G. (1971) Microorganisms as test systems of
mutagenicity. Arch. Toxicol., 28: 93-104.
MORIYA, M., OHTA, T., WATANABE, K., MIYAZAWA, T., KATO, K., &
SHIRASU, Y. (1983) Further mutagenicity studies on
pesticides in bacterial reversion assay systems. Mutat. Res.,
116: 185-216.
MULLER, W.F., SCHEUNERT, I., ROZMAN, K., KOGEL, W., FREITAG,
D., RICHTER, E., COULSTON, F., & KORTE, F. (1978)
Comparative metabolism of hexachlorobenzene in plants, rats
and Rhesus monkeys. Ecotoxicol. environ. Saf., 2: 437-445.
MUSTY, P.R. & NICKLESS, G. (1974) The extraction and
recovery of chlorinated insecticides and polychlorinated
biphenyls from water using porous polyurethane foams.
J. Chromatog., 100: 83-93.
NCI (1978) Bioassay of pentachloronitrobenzene for possible
carcinogenicity, Bethesda, Maryland, National Cancer Institute
(Technical Report Series No. 61).
NISHIUCHI, Y. & YOSHIDA, K. (1972) Noyaku Kensasho Hokoku,
p. 122 (in Japanese) (in English in Kanazawa (1981)).
OGISO, M. & TANABE, H. (1982) Residue of quintozene, its
metabolites, and hexachlorobenzene in the soil and crops.
J. Pestic. Sci. (Nihon Noyaku Gakkaishi), 7: 391-396.
O'GRODNICK, J.S., ADAMOVICS, J.A., BLAKE, SH., & WEDIG, J.
(1981) The metabolic fate of 14C-labelled pentachloronitro-
benzene in Osborne-Mendell rats. Chemosphere, 10: 67-72.
RAUTAPAA, J., PYYSALO, H., & BLOMQVIST, H. (1977) Quintozene
in some soils and plants in Finland. Ann. Agric. Fenn., 16:
277-282.
ROARK, J.H. & DALE, J.L. (1979) The effect of turf
fungicides on earthworms. Proc. Arkansas Acad. Sci., 33: 71-74.
SCHUMANN, A.M. & BORZELLECA, J.F. (1978) An assessment of
the methaemoglobin and Heinz-body-inducing capacity of
pentachloronitrobenzene in the cat. Toxicol. appl. Pharmacol.,
44: 523-529.
SEARLE, C.E. (1966) Tumor initiatory activity of
chloromono-nitrobenzenes and other compounds. Cancer Res.,
26: 12-17.
SIJPESTEIJN, A.K., DEKHUIJZEN, H.M., & VONK, J.W. (1977)
Biological conversions of fungicides in plants and micro-
organisms. In: Siegel, M.R. & Sisler, H.D., ed. Antifungal
compounds, Vol. 2: Interactions in biological and ecological
systems, New York, Marcel Dekker Inc., Vol. 2.
SIMON, G.S., KUCHAR, E.J., KLEIN, H.H., & BORZELLECA, J.F.
(1979) Distribution and clearance of pentachloronitrobenzene
in chickens. Toxicol. appl. Pharmacol., 50: 401-406.
SMILEY, R.W. & CRAVEN, M.M. (1979) Microflora of turfgrass
treated with fungicides. Soil Biol. Biochem., 11: 349-353.
SMITH, A.G. & CABRAL, J.R.P. (1980) Liver cell tumours in
rats fed hexachlorobenzene. Cancer Lett., 11: 169-172.
ST. JOHN, L.E. Jr, AMMERING, J.W., WAGNER, D.G., WARNER, R.G.,
& LISK, D.J. (1965) Fate of 4,6-dinitro-2-isobutylphenol,
2-chloro-4,6-bis(ethylamino)-S-triazine, and PCNB in the dairy
cow. J. dairy Sci., 48: 502-503.
TU, C.M. (1980) Effects of fungicides on growth of Rhizobium
japonicum in vitro. Bull. environ. Contam. Toxicol., 25:
364-368.
US DHEW (1973) Pesticide analytical manual, Maryland, US
Department of Health, Education, and Welfare, Food and Drug
Administration.
VAN LOGTEN, M. (1977) Quintozene. (Working Paper for 1977
JMPR) (FAO/WHO FOS/RES/77.46A).
VILLENEUVE, D.C. & KHERA, K.S. (1975) Placental transfer of
halogenated benzenes (Pentachloro-, Pentachloronitro-, and
Hexabromo-) in rats. Environ. Physiol. Biochem., 5: 328-331.
VISHUNAVAT, K. & SHUKLA, P. (1981) Effects of seed treatment
of lentil upon germination, plant stand, and yield.
Pesticides, 15: 15-16.
WANG, C.H. & BROADBENT, F.E. (1973) Effect of soil
treatments on losses of two chloronitrobenzene fungicides.
J. environ. Qual., 2: 511-515.
WHO (1975) Pesticide residues in food, Geneva, World Health
Organization, Vol. 97, pp. 7-37 (Technical Report Series No.
574 FAO Agricultural Studies).
WHO (1984) The WHO recommended classification of pesticides
by hazard, Geneva, World Health Organization (Unpublished
Report VBC/84.2).
WIT, S.L., VAN ESCH, G.J., & VAN GENDEREN, H. (1957)
Toxicity of some chloronitrobenzene compounds (trichlorodi-
nitrobenzene, trichloronitrobenzene, tetrachloronitrobenzene,
and pentachloronitrobenzene) to laboratory rats and residues
found in crops treated with these fungicides. In: Proceedings
of the 4th International Congress of Crop Protection, Hamburg.
 Molecular formula: C6Cl5NO2
CAS chemical name: pentachloronitrobenzene
Common trade names: avicol, botrilex, brassicol, earthcide,
fartox, folosan, fomac 2, fungiclor,
GC 3944-3-4, kobu, kobutol, KP 2,
NCI-C00419, olpisan, PCNB, entagen,
pterraclor, terrafum, tilcarex,
tritisan. A complete list of trade
names is available from IRPTC (1983).
CAS registry number:
Molecular formula: C6Cl5NO2
CAS chemical name: pentachloronitrobenzene
Common trade names: avicol, botrilex, brassicol, earthcide,
fartox, folosan, fomac 2, fungiclor,
GC 3944-3-4, kobu, kobutol, KP 2,
NCI-C00419, olpisan, PCNB, entagen,
pterraclor, terrafum, tilcarex,
tritisan. A complete list of trade
names is available from IRPTC (1983).
CAS registry number:  4.4. Elimination
4.4.1. Human studies
No information on the elimination of quintozene in man was
found.
4.4.2. Animal studies
The major routes of elimination of ingested quintozene are via
the faeces as unchanged material or in the urine as metabolites
(Fig. 1)
The amount of quintozene eliminated unchanged in the faeces is
species-dependent. Betts et al. (1955) administered 1,2, or 3 g of
quintozene to rabbits by stomach tube and found an average of 46,
62, and 59% of the dose was eliminated unchanged in the faeces over
72 h. The faecal elimination was also variable (range 27 - 82% of
the dose). In sheep dosed orally with 31 mg/kg body weight,
approximately 80% of the dose was eliminated unchanged via the
faeces (Avrahami & White, 1976). In metabolic studies carried out
on Rhesus monkeys (Koegel et al., 1979), only 7.4% of the
administered oral dose (2 mg/kg body weight) was excreted as
quintozene in the faeces. After feeding rats with a diet
containing 500 mg/kg diet, no detectable quintozene was found in
the faeces, but unmetabolized quintozene was found in the faeces of
dogs fed 1080 mg/kg diet for 2 years (Borzelleca et al., 1971).
Once absorbed, the elimination of quintozene is primarily as
metabolites in the urine (section 4.3). Although quintozene has
been found in bile, the relative importance of this finding for the
elimination or enterohepatic circulation of quintozene is unknown.
Nevertheless, it would appear that elimination by this route is
species-dependent, since quintozene has been identified in the bile
of monkeys (Kogel et al., 1979) and mice (Courtney et al., 1976)
but not in the bile of chickens (Kuchar et al., 1969; WHO 1975) or
dogs (Borzelleca et al., 1971).
When a lactating cow was fed 5 mg/kg diet for 3 days, the
parent compound was not detected in the milk using a method in
which the sensitivity was 0.01 mg/kg (St. John et al., 1965).
Traces of quintozene were found in milk from cows treated orally
with the equivalent of 10 mg/kg diet for 8 weeks (Borzelleca et
al., 1971). It was also found in the milk of untreated cows and
analyses of the feed showed quintozene levels of 0.002 - 0.006
mg/kg.
Rhesus monkeys were fed diets containing quintozene at 2 mg/kg
for 550 days. A storage curve was constructed by subtracting the
excreted from the administered radioactivity. The storage curve
levelled out after 30 - 40 days of treatment, resulting in a
storage plateau of only 2 - 3% of the administered dose (Muller et
al., 1978).
There was little accumulation of quintozene in fat tissue;
slightly elevated concentrations are found in the liver and kidney
as well as the thymus, lymph nodes, and bone marrow.
Residual levels in various tissues are given in Table 2.
Table 2. Tissue residue levels in Rhesus monkeys fed 2 mg/kg PCNB
in the diet for 550 daysa
---------------------------------------------------------------
Tissue PCNB Tissue PCNB
(mg/kg) (mg/kg)
---------------------------------------------------------------
Blood 0.07 adrenal cortex 0.08
Muscle 0.01 thymus 0.20
Brain 0.03 lymph nodes (large intestine) 0.12
Liver 0.19 bone marrow 0.13
Kidney 0.14 omental fat 0.21
---------------------------------------------------------------
a From: Muller et al. (1978).
As part of the same study, a Rhesus monkey dosed orally with
radio-labelled quintozene at 2 mg/kg eliminated 92% of the
radioactivity after 5 days, 91% of which was in the form of
metabolites (Muller et al., 1978).
5. STUDIES ON EXPERIMENTAL ANIMALS
The toxicity and the residue data on quintozene have been
reviewed several times by international bodies such as FAO/WHO
(1970, 1974, 1976, 1978), IARC (1974), and CEC (1981). For their
conclusion, refer to section 8. We refer to these reports, which
contain more detailed information on the toxicity studies and
residue data than the present report. Moreover, several
unpublished studies have been evaluated and reported there.
5.1. Short-Term Studies
5.1.1. Single dose
Data on the acute toxicity of quintozene are summarized in
Table 3.
Table 3. Acute toxicity of quintozene
-------------------------------------------------------------------
Animal Route LD50 (mg/kg body weight) References
-------------------------------------------------------------------
rat (M) oral 1710 (oil solution) FAO/WHO (1970)
rat (F) oral 1650 (oil solution) FAO/WHO (1970)
rat oral > 30 000 (aqueous suspension) FAO/WHO (1970)
rat ip 5000 (aqueous suspension) FAO/WHO (1970)
dog oral no deaths up to 2500 mg/kg Berkowitz et al.
(1976)
rabbit dermal no deaths up to 4000 mg/kg Berkowitz et al.
(1976)
-------------------------------------------------------------------
Rabbits were dosed dermally once, with quintozene as a 30%
solution in dimethyl phthalate at 2 dose levels (10 and 13.3 ml/kg)
and observed for 14 days (Borzelleca et al., 1971). There was no
evidence of toxicity or skin irritation.
Cats
Quintozene, dissolved in corn oil, administered orally to cats
once at a level of 1600 mg/kg, caused a significant elevation in
methaemoglobin levels and an approximately 8-fold increase in the
number of erythrocytes containing Heinz bodies (Schumann &
Borzelleca, 1978). This latter finding, together with the fact
that a greater percentage of erythrocytes failed to stain properly
in the course of time, suggested that a functional impairment of
the erythrocyte had occurred.
5.1.2. Repeated dose
Rat
Five groups of 7 male and 7 female albino rats of weaning age
were fed diets containing technical quintozene at 0, 63.5, 635,
1250, 2500, or 5000 mg/kg for 3 months. Growth and survival were
adversely affected at 5000 mg/kg in both sexes and also in males at
2500 mg/kg. Liver hypertrophy was observed at all levels except in
females fed 63.5 mg/kg. No haematological changes were seen and
histological alterations were limited to fine vacuolization of
liver cell cytoplasm at 5000 mg/kg (Finnegan et al., 1958). An
unspecified number of young rats were fed diets containing 0 or
2000 mg quintozene/kg for 10 weeks (Wit et al., 1957). No gross
effects other than decreased growth rate in the males were noted.
Groups of 10 male and 10 female rats were fed diets containing 0,
1000, 5000, or 10 000 mg quintozene/kg for 90 days. The animals
showed a slight growth depression at 5000 mg/kg and marked growth
depression at 10 000 mg/kg (Hoechst, unpublished data, 1964).
Groups of 10 male and 10 female rats were fed diets containing
technical quintozene at concentrations of 0, 25, 100, 300, 1000, or
2500 mg/kg diet for 2 years. No changes in blood haematology were
found. In females, growth depression was observed at doses of 100
mg/kg diet and above (Finnegan et al., 1958).
Dogs
Groups of 3 mongrel dogs were fed diets containing 25, 200, or
1000 mg quintozene/kg for 1 year. No adverse effects were noted on
body weight or survival. No haematological changes were seen and
histopathological changes were restricted to liver cell enlarge-
ment, which was not dose-dependent (Finnegan et al., 1958). In a
2-year study, groups of 3 male and 3 female dogs were fed diets
containing 0, 500, 1000, or 5000 mg quintozene/kg. Liver changes
occurred in all groups in a dose-related manner. The 5000 mg/kg
level produced severe liver damage including fibrosis, narrowing of
hepatic cell cords, increased size of the periportal areas, and
leukocyte infiltration. At 1000 and 500 mg/kg, the changes were
similar but less pronounced. Reduced haematopoiesis and atrophy of
bone marrow were observed in animals receiving the highest dose
(FAO/WHO, 1970). Purebred beagles (4 per sex per dose) were fed
diets containing quintozene at levels ranging from 5 to 1080 mg/kg
for 2 years. Haematocrit values were depressed at 18 months in
males receiving 30 and 180 mg/kg, but not in animals receiving 1080
mg/kg. No dose-related effects were observed on urine analysis,
blood chemistry, mortality, body weight, food consumption, or
estrous cycle. Organ weight data revealed higher values for livers
in dogs fed 1080 mg quintozene/kg. Histologically, dogs sacrificed
at 2 years after receiving 180 or 1080 mg/kg showed hepatic and
renal effects deemed reversible by the authors (Borzelleca et al.,
1971).
Monkeys
Two male and two female rhesus monkeys were fed 2 mg
quintozene/kg for 70 days. The haematological variables
(haemoglobin, haematocrit, RBC, WBC), and the histopathological
examination of the liver, stomach, small and large intestine,
spleen, kidneys, heart, lung, thymus, cerebrum, cerebellum, pons,
medulla, spinal cord, and bone marrow were carried out in 1 male
and female monkey
after 70 days. The histopathology, clinical chemistry, and serum
cortisol levels remained within normal limits (Muller et al.,
1978).
5.2. Reproduction Studies
Reproduction studies were carried out on rats fed a diet
containing 0, 5, 50, or 500 mg quintozene/kg until the F/3b litters
were weaned. Quintozene had no effect on fertility
(pregnancies/mating), gestation (litters cast/pregnancies),
viability (live at 4 days/live at birth), or lactation (weaned/live
minus discards) indices. No dose-related histopathological
abnormalities were recorded in any of the F3b pups. It was not
teratogenic to rats at dosages up to 1563 mg/kg body weight (Jordan &
Borzelleca, 1973; Khera & Villeneuve, 1975; Courtney et al., 1976).
Levels of 500 mg/kg body weight of technical quintozene (87% pure)
administered from day 7 to 11 of gestation produced renal agenesis
in C57B1/6 mice, but none were produced with purified material
(99%) (Courtney et al., 1976). Hexachlorobenzene, a major
contaminant in the technical material, was implicated in the
teratogenic response. Quintozene did not produce any teratogenic
response in AKR mice when administered at levels up to 500 mg/kg
diet (Berkowitz et al., 1976).
5.3. Mutagenicity
Quintozene was reported to give a positive mutagenic
response in a host cell reactivation deficient strain of
E. coli (Clarke, 1971). It was not mutagenic when studied in an
Ames test system consisting of several bacterial strains using
Aroclor 1254 activation (Mohn, 1971). It was reported to be
negative in a reverse mutation assay using 5 tester strains of
Salmonella typhimurium and E. coli (Moriya et al., 1983). It was
shown to be negative in a dominant lethal test in mice where the
chemical was administered for 7 weeks in the diet (no
concentrations specified) (Van Logten, 1977). It produced no
significant increase in mutation rates in Salmonella typhimurium
and Serratia marcescens; it also gave negative results in spot
tests against the same strains of Salmonella typhimurium and
Serratia marcescens (Buselmaier et al., 1973). Both FAO/WHO
(1978) and CEC (1981) concluded that there were no indications for
mutagenic activity.
5.4. Carcinogenicity
The carcinogenicity of quintozene was evaluated by IARC in
1973 (IARC, 1974). Studies evaluated at that time as well as
additional studies are summarized below.
In a large screening study, 18 male and 18 female (C57BL/6
x C3H/Anf)F1 mice and similar numbers of (C57BL/6 x AKR)F1
mice were given single doses of 464 mg quintozene/kg body
weight (unspecified purity) by stomach tube, when the animals
were 7 days of age, and this same absolute dose was then given
daily until the animals were 28 days of age. This was followed by
a diet containing 1206 mg/kg diet, which was administered up to 78
weeks. Hepatomas were the only tumours found in excess over the
controls; 2/18 male and 4/18 female (C57BL/6 x C3H/Anf)F1 mice
developed hepatomas compared with 8/79 and 0/87 in controls. Of
the (C57BL/6/ x AKR)F1 mice, 10/17 males and 1/17 females developed
hepatomas compared with 5/90 and 1/82 in controls. The incidence
of other tumours was similar in treated and control animals (Innes
et al., 1969).
Ten stock albino mice of each sex were painted twice weekly
with 0.2 ml of a 0.3% solution of quintozene in acetone for 12
weeks. This was followed by twice-weekly paintings with a 0.5%
solution of croton-oil in acetone for 20 weeks followed by
observation for 40 weeks. In a control group, acetone alone was
given followed by treatment with croton oil. The total number of
skin tumours at the end of croton-oil treatment was 12 in 9
surviving controls and 50 in 13 survivors in the quintozene group.
One tumour in the quintozene group had progressed to a squamous-
cell carcinoma. An infiltrating squamous-cell carcinoma was also
observed in 1 control mouse killed 31 weeks from the start of the
croton-oil treatment (Searle, 1966).
Two unpublished studies on the carcinogenicity of quintozene
(containing 2.7% hexachlorobenzene) were reviewed by the FAO/WHO
Joint Meeting on Pesticide Residues in 1975. Groups of 100 male
and 100 female Swiss mice and 50 male and 50 female Wistar rats
were administered levels of 0, 100, 400, or 1200 mg/kg diet. In
mice, a non-dose-related increase in the incidence of liver
hyperplastic nodules was observed in males and an increased
incidence of subcutaneous fibrosarcomas was observed in females at
the highest dose level. No increased tumour incidence was reported
in rats (FAO/WHO, 1976). No further details of this study are
available.
Groups of 50 male and 50 females Osborne-Mendel rats and
B6C3F1 mice were given technical grade quintozene (purity 97%
with 12 impurities) in their diet for 78 weeks. In rats, the
average dietary concentrations were 10 064 and 5417 mg/kg of
diet for males and 14 635 and 7875 for females; in mice,
average dietary concentrations were 5213 and 2606 for males
and 8187 and 4093 for females. Observation continued for 33 -
35 additional weeks in rats and for 14 - 15 additional weeks
in mice. Adequate numbers of animals survived long enough to
permit the detection of late developing tumours. No
statistically-significant increase in the incidence of
neoplasms was seen in either species. It was concluded that
quintozene was not carcinogenic under the conditions of this
bioassay (NCI, 1978).
Hexachlorobenzene, a potential impurity in quintozene, is
carcinogenic in mice, rats, and hamsters producing tumours of
the liver (IARC, 1979; Smith & Cabral, 1980).
6. EFFECTS ON MAN
In patch tests, a quarter-inch square of cotton cloth was
moistened with water, dipped in a 75% quintozene wettable powder
(Olin formulation), and then placed on the volar surface of the
right forearms of 50 human volunteers. The patches were then
covered with a 1-inch square of aluminum foil held in place by a 2-
inch square of adhesive tape. After 48 h, the patches were
removed. No evidence of irritation was seen in any of the
subjects. Two weeks later, the same test was repeated on the left
arm of the same subjects. After 48 h, 46 of the 50 subjects showed
no signs or irritation. In 3 of the 4 reported reactions, a 1-inch
square area showed erythema, oedema, and small vesicle formation
with marked itching; the 4th subject had only erythema and itching.
Of the 46 subjects who were negative when the second patch was
removed, 9 developed a delayed reaction. Time of onset varied from
approximately 8 h to several days. In 2 of these persons, the
reaction included erythema, oedema, small vesicle formation, and
itching. The skin reaction reached a peak during the first few
days of symptoms and subsided with time, with some scaling of the
skin (Finnegan et al., 1958). One instance of keratoconjunctivitis
has been reported in the literature (Fujita et al., 1976) and
resulted from the application of the pesticide. Remission of the
condition took a month.
7. EFFECTS ON THE ENVIRONMENT
7.1. Toxicity for Aquatic Organims
Quintozene is of low toxicity for aquatic organisms. The only
data on the toxicity of quintozene for aquatic organisms is from
Nishiuchi & Yoshida (1972) who quote a 48-h LC50 value of 10 000
µg/litre for carp and a 3-h LC50 value for Daphnia of 40 000
µg/litre.
7.2. Toxicity for Terrestrial Organisms
7.2.1. Plants
Vishunavat & Shukla (1981) examined the effects of quintozene
on seed germination, plant stand, and yield of lentils. There were
no significant effects. Brown et al. (1982) did not find any
effects on the germination of orchids when 99% pure quintozene was
applied at concentrations of 25 and 50 mg/litre. At 100 mg/litre,
Cattleya elongata did not germinate, but germination of Laelia
was unaffected and Vanda tricolor showed improved germination.
Quintozene eliminated growth of excised shoot tips in orchids of
the Cymbidium family.
7.2.2. Earthworms
Roark & Dale (1979) reared earthworms Eisenia foetida in
soil pre-mixed with quintozene at a dose of 0.679 g/4719 cm3
of soil, corresponding to 226.8 g over 929 cm2 of turf. The
dose was calculated as the total of 3 applications of the
recommended dosage for turf. The worms did not reproduce in
treated soil. Survival of worms was not significantly reduced
within the first 10 days, but fell to zero within 29 days of
treatment with quintozene.
7.2.3. Bees
No information is available on toxicity of quintozene for bees.
However, since the main uses of quintozene are on soil or as a seed
dressing, it is unlikely that bees would be exposed.
7.2.4. Birds
Dunn et al. (1979a) fed white leghorns with concentrations
of 0, 10, 50, 100, or 1000 mg quintozene/kg diet and examined
egg production and hatchability. None of these concentrations
caused obvious toxic effects, death, or histopathological changes
in either control or treated groups. Egg production during the
25th to the 35th week was not significantly affected. However, at
a higher dose level of 1000 mg/kg diet, onset of egg production was
delayed for 1 month, and the number of chicks hatched from fertile
eggs significantly decreased from 91% in the controls to 69% in
treated birds. Shell strength of the eggs was not significantly
altered (Dunn et al., 1979a). In another study (Dunn et al.,
1979b), the authors reported that bioaccumulation of PCNB or its
metabolites only occurred in trace concentrations; body weight
gains were significantly lower in hens fed 1000 mg quintozene/kg
diet.
7.3. Toxicity for Microorganisms
Tu (1980) reported that quintozene at doses up to 5000 mg/litre
did not induce any effects on 3 strains of the bacterium Rhizobium
japonicum in culture. Quintozene also did not show any effects
on 25 strains of Rhizobium bacteria, isolated from root nodules of
red clover, at doses up to 1000 mg/litre in the culture medium
(Heinonen-Tanski et al., 1982). Smiley & Craven (1979) applied
quintozene 9 times annually for 3 years, at weekly intervals during
July and August, to a turf of Kentucky blue grass. There were no
significant effects on the populations of bacteria, actinomycetes,
or fungi. The effects of quintozene on carbon dioxide evolution
and on the enzyme activities in organisms in soil were observed by
Mitterer et al. (1981). The recommended soil dosage of the
fungicide caused an increase in carbon dioxide evolution from soil
cultures. After a second and third application, this initial
increase was followed by a decrease in carbon dioxide release to
below the value for untreated control soil. Quintozene showed a
severe and continuous inhibition of xylanase activity, in marked
contrast to other fungicides tested.
7.4. Bioaccumulation and Biomagnification
Ogiso & Tanabe (1982) measured residues in different tissues of
crop plants. High concentrations of quintozene in plant tissues
relative to soil levels were only found in the outer layers of
roots and tubers directly in contact with soil. There is little
evidence of systemic uptake.
Kanazawa (1981) reported a bioconcentration factor for
quintozene by top mouth gudgeon Pseudorasbora parva of 238. The
flow-through system maintained water concentrations of between 5
and 20 µg quintozene/litre.
8. PREVIOUS EVALUATIONS OF QUINTOZENE BY INTERNATIONAL BODIES
The Joint Meeting on Pesticide Residues (JMPR) reviewed
residues and toxicity data on quintozene in 1969, 1973, 1975, and
1977 (FAO/WHO, 1970, 1974, 1976, 1978). The conclusion in 1977 was
that 25 mg/kg diet, equivalent to 1.25 mg/kg body weight was a no-
observed-effect-level in the rat and 30 mg/kg diet, equivalent to
0.75 mg/kg body weight in the dog. On the basis of this, the
estimate of an acceptable daily intake (ADI) for man was 0 - 0.007
mg/kg body weight.
IARC (1974) did not come to a conclusion on the carcinogenicity
of quintozene because of lack of data at the time. FAO/WHO (1978)
concluded that there were no indications that administration of
quintozene resulted in carcinogenic activity.
CEC (1981) concluded that there was a need to set limits on the
impurities present in technical quintozene.
WHO, in its "Guidelines to the Use of the WHO Recommended
Classification of Pesticides by Hazard" (WHO, 1984), classified
quintozene in the category of technical products unlikely to
present an acute hazard in normal use.
Regulatory standards established by national bodies in 12
different countries (Argentina, Brazil, Czechoslovakia, the Federal
Republic of Germany, India, Japan, Kenya, Mexico, Sweden, the
United Kingdom, the USA, and the USSR) and the EEC can be found in
the IRPTC (International Register of Potentially Toxic Chemicals)
legal file (IRPTC, 1983).
9. EVALUATION OF HEALTH RISKS FOR MAN AND EFFECTS ON THE
ENVIRONMENT
9.1. Evaluation of Health Risks for Man
Quintozene toxicity
Quintozene is practically non-toxic according to the scale of
Hodge & Sterner (1956). The oral LD50 in rats was 1650 to more
than 30 000 mg/kg body weight. WHO (1984) classified quintozene in
the category of technical products unlikely to present an acute
hazard in normal use.
No-observed-adverse-effect levels in long-term studies on the
rat and the dog were 1.25 and 0.75 mg/kg body weight (25 and 30
mg/kg diet), respectively. In long-term studies with rats and at
higher dosages (63 mg/kg diet), quintozene can give rise to liver
hypertrophy with some histopathological changes and in dogs to more
severe liver damage with fibrosis (5000 mg/kg diet). In short-term
studies on female rats, quintozene caused induction of mixed-
function oxidases.
Quintozene is both metabolised and excreted unchanged and
does not accumulate in tissues.
Quintozene is not considered to be teratogenic.
Quintozene is generally negative in short-term tests for
genetic activity. In carcinogenicity studies on rats and
mice, equivocal or negative findings have been reported.
Hexachlorobenzene, a possible impurity in technical quintozene
is carcinogenic for mice, rats, and hamsters.
Except for 1 case of conjunctivitis in an occupational setting,
no other cases of poisoning or adverse effects have been reported
in man.
Exposure to quintozene
The general population can be exposed via residues in food,
especially in oils and fats. Information on exposure from other
sources is lacking. No cases of accidental or occupational
overexposure have been reported.
Hazard assessment
With the exception of some data on residues in food, no human
exposure data are available for quintozene. It is therefore
difficult to evaluate the hazard for man of present exposure to
this substance. Nevertheless, in view of its low toxicity in
short-term and long-term animal studies, the data available on
quintozene would indicate a low degree of concern in relation to
human health effects.
9.2. Evaluation of Overall Environmental Effects
The only significant adverse effect reported for quintozene is
on earthworms. According to laboratory tests, quintozene applied
at recommended doses as a soil fungicide appears to have long-term
toxic effects on the earthworm. Unfortunately, no observations of
the effects on earthworms of quintozene alone, during field use,
are available.
There is no evidence that quintozene represents a threat to
non-target organisms. It has a very low acute toxicity for fish
and Daphnia.
Its bioaccumulation by fish is low, and no effects have been
reported on terrestrial plants, birds, or microorganisms.
9.3. Conclusions
1. The general population does not appear to be at
risk from residues of quintozene in food.
2. Exposure of the general population via air and
drinking-water could not be evaluated because of lack
of data.
3. Occupational exposure has not been reported to
cause any adverse effects.
4. There is limited information on the effects of
quintozene in the general environment. It has been
shown to be toxic to earthworms, in laboratory
tests. Data on other organisms suggest that
quintozene is not a problem in the general
environment.
5. Quintozene does not biomagnify.
6. The major toxicological concern with quintozene
is the presence of hexachlorobenzene as an impurity.
REFERENCES
ANONYMOUS (1976) Fungicides. In: Analytical methods for
pesticides and plant growth regulators. VII. Government
regulations, pheromone analysis, additional pesticides, New
York, Academic Press, pp. 251-331.
ANONYMOUS (1977a) Rebuttable presumption against
registration and continued registration of pesticide products
containing PCNB. Fed. Regist., 42: 56072-56100.
ANONYMOUS (1977b) Surface water quality in Canada - an
overview. Fish. Environ. Can., 1-45.
AVRAHAMI, M. & WHITE, D.A. (1976) Rapid elimination of PCNB
and metabolite from sheep fat. N.Z. J. exp. Agric., 4: 299-302.
BECK, J. & HANSEN, K.E. (1974) The degradation of
quintozene, pentachlorobenzene, hexachlorobenzene and
petachloroaniline in soil. Science, 5: 41-48.
BERG, G.L., ed. (1978) Farm chemicals handbook, Willoughby,
Ohio, Meister Publishing Company, p. D133.
BERKOWITZ. J., STEVENS, J., ARNOLD, D., GOYER, M., SENECHAL,
D., HARRISON, J., LUDWIG, R., & NEWMEYER, J. (1976)
Substitute chemical program: initial scientific review of
PCNB, Washington DC, US Environmental Protection Agency,
Office of Pesticide Programs, Criteria and Evaluation Division
(EPA 540/1-75-016).
BETTS, J.J., JAMES, S.P., & THORPE, W.V. (1955) The
metabolism of pentachloronitrobenzene and 2,3,4,6-tetra-
chloronitrobenzene and the formation of mercapturic acids in
the rabbit. Biochem. J., 61: 611-617.
BORZELLECA, J.F., LARSON, P.S., CRAWFORD, E.M., HENNIGAR,
G.R., KUCHAR, E.J., & KLEIN, H.H. (1971) Toxicologic and
metabolic studies on pentachloronitrobenzene. Toxicol. appl.
Pharmacol., 18: 522-534.
BROWN, D.M., GROOM, C.L., CVITANIK, M., BROWN, M., COOPER,
J.L., & ARDITTI, J. (1982) Effects of fungicides and
bactericides on orchid seed germination and shoot tip cultures
in vitro. Plant Cell, Tissue and Organ Cult., 1: 165-180.
BURKE, G. & HOLSWADE, W. (1964) Gas chromatogrphy with
microcoulometric detection for pesticide residue analysis.
J. Assoc. Off. Anal. Chem., 47: 845-859.
BUSELMAIER, W., ROHRBORN, G., & PROPPING, P. (1973)
Comparative investigations on the mutagenicity of pesticides
in mammalian test systems. Mutat. Res., 21: 25-26.
CLARKE, C.H. (1971) The mutagenic specificities of
pentachloronitrobenzene and captan to environmental mutagens.
Mutat. Res., 11: 247-248.
CEC (1981) Criteria (dose/effect relationships) for
organochlorine pesticides, Oxford, Pergamon Press.
COURTNEY, K.D., COPELAND, M.F., & ROBBINS, A. (1976) The
effects of PCNB, hexachlorobenzene and related compounds on
fetal development. Toxicol. appl. Pharmacol., 35: 239-256.
CROSBY, D.G. & HAMADMAD, N. (1971) Photo-reduction of
pentachlorobenzenes. J. agric. food Chem., 19: 1171-1174.
DAPPEN, G. (1974) Pesticide analysis from urban storm
runoff, Washington DC, US Department of Commerce, National
Technical Information Service (PB Report, Issue No.
238593/8GA, 44).
DUNN, J.S., BUSH, P.B., BOOTH, N.H., FARRELL, R.L., THOMASON,
D.M., & GOETSCH, D.D. (1979a) Effect of pentachloronitro-
benzene upon egg production, hatchability, and residue
accumulation in the tissue of white leghorn hens. Toxicol.
appl. Pharmacol., 48: 425-433.
DUNN, J.S., BUSH, P.B., BOOTH, N.H., FARRELL, R.L., THOMASON,
D.M., & GOETSCH, D.D. (1979b) Tissue residues from feeding
pentachloronitrobenzene (quintozene) to white leghorn
chickens. Am. J. vet. Res., 40: 1227-1230.
DUNSING, M. & WINDSCHILD, J. (1976) [Residues of quintozene,
hexachlorobenzene, and pentachloroaniline in lettuce and
soil.] Nachr. Pflanzenschutzd., 30: 106-108 (in German).
FAO/WHO (1970) Quintozene. In: 1969 Evaluations of some
pesticide residues in foods, Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1974) Quintozene. In: 1973 Evaluations of some
pesticide residues in foods, Rome, Food and Agriculture
Organization of the United Nations, pp. 379-396.
FAO/WHO (1976) Quintozene. In: 1975 Evaluations of some
pesticide residues in foods, Rome, Food and Agriculture
Organization of the United Nations, pp. 357-363.
FAO/WHO (1978) Quintozene. In: 1977 Evaluations of some
pesticide residues in foods, Rome, Food and Agriculture
Organization of the United Nations, pp. 439-440.
FINNEGAN, J.K., LARSON, P.S., SMITH, R.B. Jr, HAAG, H.B., &
HENNIGAR, G.R. (1958) Acute and chronic toxicity studies on
pentachloronitrobenzene. Arch. int. Pharmacodyn., 114: 38-52.
FUJITA, K., SUZUKI, H., & OCHIAI, F. (1976) [Kerato-
conjunctivitis due to pesticides of relatively low acute
toxicity.] Clin. Opthalmol., 30: 419-423 (in Japanese).
GLOFKE, E. (1973) [Pesticide in milk and milk products.]
Milchwirt. Ber. Bundesanst. Wolfpassing Rotholz., 34: 37-40
(in German).
GOURSAUD, J., LUQUET, F.M., BOUDIER, J.F., & CASALIS, J.
(1972) Sur la pollution du lait par les residues
d'hexachlorobenzene (HCB). Ind. Aliment. Agric., 89: 31-35.
HEIKES, D.L. (1980) Residues of pentachloronitrobenzene and
related compounds in peanut butter. Bull. environ. Contam.
Toxicol., 24: 338-343.
HEINONEN-TANSKI, H., OROS, G., & KECSKES, M. (1982) The
effect of soil pesticides on the growth of red clover
Rhizobia. Acta agric. Scand., 32: 283-288.
HODGE, H.C. & STERNER, J.H. (1956) Combined tabulation of
toxicity classes. In: Spector, W.S., ed. Handbook of
toxicology, Philadelphia, W.B. Saunders Company, Vol. 1.
IARC (1974) Some organochlorine pesticides, Lyons,
International Agency for Research on Cancer, pp. 211-218
(Monographs on the Evaluation of the Carcinogenic Risk of
Chemicals to Man, No. 5).
IARC (1979) Hexachlorobenzene, Lyons, International Agency
for Research on Cancer, Vol. 20, pp. 155-179 (Monographs on
the Evaluation of the Carcinogenic Risk of Chemicals to Man).
INNES, J.R.M., ULLAND, B.M., VALERIO, M.G., PETRUCELLI, L.,
FISHBEIN, L., MART, O.R., PALLOTTA, A.J., BATTES, R.R., FALK,
R., GART, J.J., KLEIN, N., MITCHELL, I., & PETERS, J. (1969)
Bioassay of assay of pesticides and industrial chemicals for
tumorigenicity in mice: A preliminary note. J. Natl Cancer
Inst., 42: 1011-1114.
IRPTC (1983) IRPTC legal file 1983, Geneva, International
Register of Potentially Toxic Chemicals, United Nations
Environment Programme, Vol. 1 & 2.
JOHNSON, R.D., MANSKE, D.D., NEW, D.H., & PODEREBARAC, D.S.
(1969) Pesticides and other chemical residues in infant and
toddler total diet samples: 1 August 1974-July 1975, Pest.
Monit. J., 13: 87-98.
JORDAN, R.L. & BORZELLECA, J.F. (1973) Teratogenic studies
with pentachlorinitrobenzene in rats. Toxicol. appl.
Pharmacol., 25: 454.
KANAZAWA, J. (1981) Measurement of the bioconcentration
factors of pesticides by fresh water fish and their
correlation with physico-chemical properties or acute
toxicities. Pestic. Sci., 12: 417-424.
KHERA, K.S. & VILLENEUVE, D.C. (1975) Teratogenicity studies
on halogenated benzenes (pentachloro-, pentachlorinitro-, and
hexabromo-) in rats. Toxicology, 5: 117-122.
KO, W.H. & FARLEY, J.D. (1969) Conversion of
pentachloronitrobenzene to pentachloroaniline in soil and the
effect of these compounds on soil microorganisms.
Phytopathology, 59: 64-67.
KOEGEL, W., MULLER, W.F., COULSTON, F., & KORTE, F. (1979)
Fate and effects of pentachloronitrobenzene in Rhesus
monkeys. J. agric. food Chem., 27: 1181-1185.
KUCHAR, E.J., GEENTY, F.O., GRIFFITH, W.P., & THOMAS, R.J.
(1969) Analytical studies of metabolism of terrachlor in
beagle dogs, rats and plants. J. agric. food Chem., 17:
1237-1240.
LEE, R.E. Jr, ed. (1975) Air pollution from pesticides:
Sources, occurrence and dispersion. In: Air pollution from
pesticides and agricultural processes, Florida, CRC Press.
LEISTRA, M. & SMELT, J.H. (1974) Concentrations of
quintozene at different depths in bulb-growing soils. Bull.
environ. Contam. Toxicol., 11: 241-243.
METHRATTA, T.P., MONTAGNA, R.W., & GRIFFITH, W.D. (1967)
Determination of teraclor in crops and soil by electron-
capture gas chromatography. J. agric. food Chem., 15: 648-650.
MITTERER, M., BAYER, H., & SCHINNER, F. (1981) [The
influence of fungicides on microbiol acitivity in soil.]
Z. Pflanzenernaehr. Bodenk. D., 144: 463-471 (in German).
MOHN, G. (1971) Microorganisms as test systems of
mutagenicity. Arch. Toxicol., 28: 93-104.
MORIYA, M., OHTA, T., WATANABE, K., MIYAZAWA, T., KATO, K., &
SHIRASU, Y. (1983) Further mutagenicity studies on
pesticides in bacterial reversion assay systems. Mutat. Res.,
116: 185-216.
MULLER, W.F., SCHEUNERT, I., ROZMAN, K., KOGEL, W., FREITAG,
D., RICHTER, E., COULSTON, F., & KORTE, F. (1978)
Comparative metabolism of hexachlorobenzene in plants, rats
and Rhesus monkeys. Ecotoxicol. environ. Saf., 2: 437-445.
MUSTY, P.R. & NICKLESS, G. (1974) The extraction and
recovery of chlorinated insecticides and polychlorinated
biphenyls from water using porous polyurethane foams.
J. Chromatog., 100: 83-93.
NCI (1978) Bioassay of pentachloronitrobenzene for possible
carcinogenicity, Bethesda, Maryland, National Cancer Institute
(Technical Report Series No. 61).
NISHIUCHI, Y. & YOSHIDA, K. (1972) Noyaku Kensasho Hokoku,
p. 122 (in Japanese) (in English in Kanazawa (1981)).
OGISO, M. & TANABE, H. (1982) Residue of quintozene, its
metabolites, and hexachlorobenzene in the soil and crops.
J. Pestic. Sci. (Nihon Noyaku Gakkaishi), 7: 391-396.
O'GRODNICK, J.S., ADAMOVICS, J.A., BLAKE, SH., & WEDIG, J.
(1981) The metabolic fate of 14C-labelled pentachloronitro-
benzene in Osborne-Mendell rats. Chemosphere, 10: 67-72.
RAUTAPAA, J., PYYSALO, H., & BLOMQVIST, H. (1977) Quintozene
in some soils and plants in Finland. Ann. Agric. Fenn., 16:
277-282.
ROARK, J.H. & DALE, J.L. (1979) The effect of turf
fungicides on earthworms. Proc. Arkansas Acad. Sci., 33: 71-74.
SCHUMANN, A.M. & BORZELLECA, J.F. (1978) An assessment of
the methaemoglobin and Heinz-body-inducing capacity of
pentachloronitrobenzene in the cat. Toxicol. appl. Pharmacol.,
44: 523-529.
SEARLE, C.E. (1966) Tumor initiatory activity of
chloromono-nitrobenzenes and other compounds. Cancer Res.,
26: 12-17.
SIJPESTEIJN, A.K., DEKHUIJZEN, H.M., & VONK, J.W. (1977)
Biological conversions of fungicides in plants and micro-
organisms. In: Siegel, M.R. & Sisler, H.D., ed. Antifungal
compounds, Vol. 2: Interactions in biological and ecological
systems, New York, Marcel Dekker Inc., Vol. 2.
SIMON, G.S., KUCHAR, E.J., KLEIN, H.H., & BORZELLECA, J.F.
(1979) Distribution and clearance of pentachloronitrobenzene
in chickens. Toxicol. appl. Pharmacol., 50: 401-406.
SMILEY, R.W. & CRAVEN, M.M. (1979) Microflora of turfgrass
treated with fungicides. Soil Biol. Biochem., 11: 349-353.
SMITH, A.G. & CABRAL, J.R.P. (1980) Liver cell tumours in
rats fed hexachlorobenzene. Cancer Lett., 11: 169-172.
ST. JOHN, L.E. Jr, AMMERING, J.W., WAGNER, D.G., WARNER, R.G.,
& LISK, D.J. (1965) Fate of 4,6-dinitro-2-isobutylphenol,
2-chloro-4,6-bis(ethylamino)-S-triazine, and PCNB in the dairy
cow. J. dairy Sci., 48: 502-503.
TU, C.M. (1980) Effects of fungicides on growth of Rhizobium
japonicum in vitro. Bull. environ. Contam. Toxicol., 25:
364-368.
US DHEW (1973) Pesticide analytical manual, Maryland, US
Department of Health, Education, and Welfare, Food and Drug
Administration.
VAN LOGTEN, M. (1977) Quintozene. (Working Paper for 1977
JMPR) (FAO/WHO FOS/RES/77.46A).
VILLENEUVE, D.C. & KHERA, K.S. (1975) Placental transfer of
halogenated benzenes (Pentachloro-, Pentachloronitro-, and
Hexabromo-) in rats. Environ. Physiol. Biochem., 5: 328-331.
VISHUNAVAT, K. & SHUKLA, P. (1981) Effects of seed treatment
of lentil upon germination, plant stand, and yield.
Pesticides, 15: 15-16.
WANG, C.H. & BROADBENT, F.E. (1973) Effect of soil
treatments on losses of two chloronitrobenzene fungicides.
J. environ. Qual., 2: 511-515.
WHO (1975) Pesticide residues in food, Geneva, World Health
Organization, Vol. 97, pp. 7-37 (Technical Report Series No.
574 FAO Agricultural Studies).
WHO (1984) The WHO recommended classification of pesticides
by hazard, Geneva, World Health Organization (Unpublished
Report VBC/84.2).
WIT, S.L., VAN ESCH, G.J., & VAN GENDEREN, H. (1957)
Toxicity of some chloronitrobenzene compounds (trichlorodi-
nitrobenzene, trichloronitrobenzene, tetrachloronitrobenzene,
and pentachloronitrobenzene) to laboratory rats and residues
found in crops treated with these fungicides. In: Proceedings
of the 4th International Congress of Crop Protection, Hamburg.
4.4. Elimination
4.4.1. Human studies
No information on the elimination of quintozene in man was
found.
4.4.2. Animal studies
The major routes of elimination of ingested quintozene are via
the faeces as unchanged material or in the urine as metabolites
(Fig. 1)
The amount of quintozene eliminated unchanged in the faeces is
species-dependent. Betts et al. (1955) administered 1,2, or 3 g of
quintozene to rabbits by stomach tube and found an average of 46,
62, and 59% of the dose was eliminated unchanged in the faeces over
72 h. The faecal elimination was also variable (range 27 - 82% of
the dose). In sheep dosed orally with 31 mg/kg body weight,
approximately 80% of the dose was eliminated unchanged via the
faeces (Avrahami & White, 1976). In metabolic studies carried out
on Rhesus monkeys (Koegel et al., 1979), only 7.4% of the
administered oral dose (2 mg/kg body weight) was excreted as
quintozene in the faeces. After feeding rats with a diet
containing 500 mg/kg diet, no detectable quintozene was found in
the faeces, but unmetabolized quintozene was found in the faeces of
dogs fed 1080 mg/kg diet for 2 years (Borzelleca et al., 1971).
Once absorbed, the elimination of quintozene is primarily as
metabolites in the urine (section 4.3). Although quintozene has
been found in bile, the relative importance of this finding for the
elimination or enterohepatic circulation of quintozene is unknown.
Nevertheless, it would appear that elimination by this route is
species-dependent, since quintozene has been identified in the bile
of monkeys (Kogel et al., 1979) and mice (Courtney et al., 1976)
but not in the bile of chickens (Kuchar et al., 1969; WHO 1975) or
dogs (Borzelleca et al., 1971).
When a lactating cow was fed 5 mg/kg diet for 3 days, the
parent compound was not detected in the milk using a method in
which the sensitivity was 0.01 mg/kg (St. John et al., 1965).
Traces of quintozene were found in milk from cows treated orally
with the equivalent of 10 mg/kg diet for 8 weeks (Borzelleca et
al., 1971). It was also found in the milk of untreated cows and
analyses of the feed showed quintozene levels of 0.002 - 0.006
mg/kg.
Rhesus monkeys were fed diets containing quintozene at 2 mg/kg
for 550 days. A storage curve was constructed by subtracting the
excreted from the administered radioactivity. The storage curve
levelled out after 30 - 40 days of treatment, resulting in a
storage plateau of only 2 - 3% of the administered dose (Muller et
al., 1978).
There was little accumulation of quintozene in fat tissue;
slightly elevated concentrations are found in the liver and kidney
as well as the thymus, lymph nodes, and bone marrow.
Residual levels in various tissues are given in Table 2.
Table 2. Tissue residue levels in Rhesus monkeys fed 2 mg/kg PCNB
in the diet for 550 daysa
---------------------------------------------------------------
Tissue PCNB Tissue PCNB
(mg/kg) (mg/kg)
---------------------------------------------------------------
Blood 0.07 adrenal cortex 0.08
Muscle 0.01 thymus 0.20
Brain 0.03 lymph nodes (large intestine) 0.12
Liver 0.19 bone marrow 0.13
Kidney 0.14 omental fat 0.21
---------------------------------------------------------------
a From: Muller et al. (1978).
As part of the same study, a Rhesus monkey dosed orally with
radio-labelled quintozene at 2 mg/kg eliminated 92% of the
radioactivity after 5 days, 91% of which was in the form of
metabolites (Muller et al., 1978).
5. STUDIES ON EXPERIMENTAL ANIMALS
The toxicity and the residue data on quintozene have been
reviewed several times by international bodies such as FAO/WHO
(1970, 1974, 1976, 1978), IARC (1974), and CEC (1981). For their
conclusion, refer to section 8. We refer to these reports, which
contain more detailed information on the toxicity studies and
residue data than the present report. Moreover, several
unpublished studies have been evaluated and reported there.
5.1. Short-Term Studies
5.1.1. Single dose
Data on the acute toxicity of quintozene are summarized in
Table 3.
Table 3. Acute toxicity of quintozene
-------------------------------------------------------------------
Animal Route LD50 (mg/kg body weight) References
-------------------------------------------------------------------
rat (M) oral 1710 (oil solution) FAO/WHO (1970)
rat (F) oral 1650 (oil solution) FAO/WHO (1970)
rat oral > 30 000 (aqueous suspension) FAO/WHO (1970)
rat ip 5000 (aqueous suspension) FAO/WHO (1970)
dog oral no deaths up to 2500 mg/kg Berkowitz et al.
(1976)
rabbit dermal no deaths up to 4000 mg/kg Berkowitz et al.
(1976)
-------------------------------------------------------------------
Rabbits were dosed dermally once, with quintozene as a 30%
solution in dimethyl phthalate at 2 dose levels (10 and 13.3 ml/kg)
and observed for 14 days (Borzelleca et al., 1971). There was no
evidence of toxicity or skin irritation.
Cats
Quintozene, dissolved in corn oil, administered orally to cats
once at a level of 1600 mg/kg, caused a significant elevation in
methaemoglobin levels and an approximately 8-fold increase in the
number of erythrocytes containing Heinz bodies (Schumann &
Borzelleca, 1978). This latter finding, together with the fact
that a greater percentage of erythrocytes failed to stain properly
in the course of time, suggested that a functional impairment of
the erythrocyte had occurred.
5.1.2. Repeated dose
Rat
Five groups of 7 male and 7 female albino rats of weaning age
were fed diets containing technical quintozene at 0, 63.5, 635,
1250, 2500, or 5000 mg/kg for 3 months. Growth and survival were
adversely affected at 5000 mg/kg in both sexes and also in males at
2500 mg/kg. Liver hypertrophy was observed at all levels except in
females fed 63.5 mg/kg. No haematological changes were seen and
histological alterations were limited to fine vacuolization of
liver cell cytoplasm at 5000 mg/kg (Finnegan et al., 1958). An
unspecified number of young rats were fed diets containing 0 or
2000 mg quintozene/kg for 10 weeks (Wit et al., 1957). No gross
effects other than decreased growth rate in the males were noted.
Groups of 10 male and 10 female rats were fed diets containing 0,
1000, 5000, or 10 000 mg quintozene/kg for 90 days. The animals
showed a slight growth depression at 5000 mg/kg and marked growth
depression at 10 000 mg/kg (Hoechst, unpublished data, 1964).
Groups of 10 male and 10 female rats were fed diets containing
technical quintozene at concentrations of 0, 25, 100, 300, 1000, or
2500 mg/kg diet for 2 years. No changes in blood haematology were
found. In females, growth depression was observed at doses of 100
mg/kg diet and above (Finnegan et al., 1958).
Dogs
Groups of 3 mongrel dogs were fed diets containing 25, 200, or
1000 mg quintozene/kg for 1 year. No adverse effects were noted on
body weight or survival. No haematological changes were seen and
histopathological changes were restricted to liver cell enlarge-
ment, which was not dose-dependent (Finnegan et al., 1958). In a
2-year study, groups of 3 male and 3 female dogs were fed diets
containing 0, 500, 1000, or 5000 mg quintozene/kg. Liver changes
occurred in all groups in a dose-related manner. The 5000 mg/kg
level produced severe liver damage including fibrosis, narrowing of
hepatic cell cords, increased size of the periportal areas, and
leukocyte infiltration. At 1000 and 500 mg/kg, the changes were
similar but less pronounced. Reduced haematopoiesis and atrophy of
bone marrow were observed in animals receiving the highest dose
(FAO/WHO, 1970). Purebred beagles (4 per sex per dose) were fed
diets containing quintozene at levels ranging from 5 to 1080 mg/kg
for 2 years. Haematocrit values were depressed at 18 months in
males receiving 30 and 180 mg/kg, but not in animals receiving 1080
mg/kg. No dose-related effects were observed on urine analysis,
blood chemistry, mortality, body weight, food consumption, or
estrous cycle. Organ weight data revealed higher values for livers
in dogs fed 1080 mg quintozene/kg. Histologically, dogs sacrificed
at 2 years after receiving 180 or 1080 mg/kg showed hepatic and
renal effects deemed reversible by the authors (Borzelleca et al.,
1971).
Monkeys
Two male and two female rhesus monkeys were fed 2 mg
quintozene/kg for 70 days. The haematological variables
(haemoglobin, haematocrit, RBC, WBC), and the histopathological
examination of the liver, stomach, small and large intestine,
spleen, kidneys, heart, lung, thymus, cerebrum, cerebellum, pons,
medulla, spinal cord, and bone marrow were carried out in 1 male
and female monkey
after 70 days. The histopathology, clinical chemistry, and serum
cortisol levels remained within normal limits (Muller et al.,
1978).
5.2. Reproduction Studies
Reproduction studies were carried out on rats fed a diet
containing 0, 5, 50, or 500 mg quintozene/kg until the F/3b litters
were weaned. Quintozene had no effect on fertility
(pregnancies/mating), gestation (litters cast/pregnancies),
viability (live at 4 days/live at birth), or lactation (weaned/live
minus discards) indices. No dose-related histopathological
abnormalities were recorded in any of the F3b pups. It was not
teratogenic to rats at dosages up to 1563 mg/kg body weight (Jordan &
Borzelleca, 1973; Khera & Villeneuve, 1975; Courtney et al., 1976).
Levels of 500 mg/kg body weight of technical quintozene (87% pure)
administered from day 7 to 11 of gestation produced renal agenesis
in C57B1/6 mice, but none were produced with purified material
(99%) (Courtney et al., 1976). Hexachlorobenzene, a major
contaminant in the technical material, was implicated in the
teratogenic response. Quintozene did not produce any teratogenic
response in AKR mice when administered at levels up to 500 mg/kg
diet (Berkowitz et al., 1976).
5.3. Mutagenicity
Quintozene was reported to give a positive mutagenic
response in a host cell reactivation deficient strain of
E. coli (Clarke, 1971). It was not mutagenic when studied in an
Ames test system consisting of several bacterial strains using
Aroclor 1254 activation (Mohn, 1971). It was reported to be
negative in a reverse mutation assay using 5 tester strains of
Salmonella typhimurium and E. coli (Moriya et al., 1983). It was
shown to be negative in a dominant lethal test in mice where the
chemical was administered for 7 weeks in the diet (no
concentrations specified) (Van Logten, 1977). It produced no
significant increase in mutation rates in Salmonella typhimurium
and Serratia marcescens; it also gave negative results in spot
tests against the same strains of Salmonella typhimurium and
Serratia marcescens (Buselmaier et al., 1973). Both FAO/WHO
(1978) and CEC (1981) concluded that there were no indications for
mutagenic activity.
5.4. Carcinogenicity
The carcinogenicity of quintozene was evaluated by IARC in
1973 (IARC, 1974). Studies evaluated at that time as well as
additional studies are summarized below.
In a large screening study, 18 male and 18 female (C57BL/6
x C3H/Anf)F1 mice and similar numbers of (C57BL/6 x AKR)F1
mice were given single doses of 464 mg quintozene/kg body
weight (unspecified purity) by stomach tube, when the animals
were 7 days of age, and this same absolute dose was then given
daily until the animals were 28 days of age. This was followed by
a diet containing 1206 mg/kg diet, which was administered up to 78
weeks. Hepatomas were the only tumours found in excess over the
controls; 2/18 male and 4/18 female (C57BL/6 x C3H/Anf)F1 mice
developed hepatomas compared with 8/79 and 0/87 in controls. Of
the (C57BL/6/ x AKR)F1 mice, 10/17 males and 1/17 females developed
hepatomas compared with 5/90 and 1/82 in controls. The incidence
of other tumours was similar in treated and control animals (Innes
et al., 1969).
Ten stock albino mice of each sex were painted twice weekly
with 0.2 ml of a 0.3% solution of quintozene in acetone for 12
weeks. This was followed by twice-weekly paintings with a 0.5%
solution of croton-oil in acetone for 20 weeks followed by
observation for 40 weeks. In a control group, acetone alone was
given followed by treatment with croton oil. The total number of
skin tumours at the end of croton-oil treatment was 12 in 9
surviving controls and 50 in 13 survivors in the quintozene group.
One tumour in the quintozene group had progressed to a squamous-
cell carcinoma. An infiltrating squamous-cell carcinoma was also
observed in 1 control mouse killed 31 weeks from the start of the
croton-oil treatment (Searle, 1966).
Two unpublished studies on the carcinogenicity of quintozene
(containing 2.7% hexachlorobenzene) were reviewed by the FAO/WHO
Joint Meeting on Pesticide Residues in 1975. Groups of 100 male
and 100 female Swiss mice and 50 male and 50 female Wistar rats
were administered levels of 0, 100, 400, or 1200 mg/kg diet. In
mice, a non-dose-related increase in the incidence of liver
hyperplastic nodules was observed in males and an increased
incidence of subcutaneous fibrosarcomas was observed in females at
the highest dose level. No increased tumour incidence was reported
in rats (FAO/WHO, 1976). No further details of this study are
available.
Groups of 50 male and 50 females Osborne-Mendel rats and
B6C3F1 mice were given technical grade quintozene (purity 97%
with 12 impurities) in their diet for 78 weeks. In rats, the
average dietary concentrations were 10 064 and 5417 mg/kg of
diet for males and 14 635 and 7875 for females; in mice,
average dietary concentrations were 5213 and 2606 for males
and 8187 and 4093 for females. Observation continued for 33 -
35 additional weeks in rats and for 14 - 15 additional weeks
in mice. Adequate numbers of animals survived long enough to
permit the detection of late developing tumours. No
statistically-significant increase in the incidence of
neoplasms was seen in either species. It was concluded that
quintozene was not carcinogenic under the conditions of this
bioassay (NCI, 1978).
Hexachlorobenzene, a potential impurity in quintozene, is
carcinogenic in mice, rats, and hamsters producing tumours of
the liver (IARC, 1979; Smith & Cabral, 1980).
6. EFFECTS ON MAN
In patch tests, a quarter-inch square of cotton cloth was
moistened with water, dipped in a 75% quintozene wettable powder
(Olin formulation), and then placed on the volar surface of the
right forearms of 50 human volunteers. The patches were then
covered with a 1-inch square of aluminum foil held in place by a 2-
inch square of adhesive tape. After 48 h, the patches were
removed. No evidence of irritation was seen in any of the
subjects. Two weeks later, the same test was repeated on the left
arm of the same subjects. After 48 h, 46 of the 50 subjects showed
no signs or irritation. In 3 of the 4 reported reactions, a 1-inch
square area showed erythema, oedema, and small vesicle formation
with marked itching; the 4th subject had only erythema and itching.
Of the 46 subjects who were negative when the second patch was
removed, 9 developed a delayed reaction. Time of onset varied from
approximately 8 h to several days. In 2 of these persons, the
reaction included erythema, oedema, small vesicle formation, and
itching. The skin reaction reached a peak during the first few
days of symptoms and subsided with time, with some scaling of the
skin (Finnegan et al., 1958). One instance of keratoconjunctivitis
has been reported in the literature (Fujita et al., 1976) and
resulted from the application of the pesticide. Remission of the
condition took a month.
7. EFFECTS ON THE ENVIRONMENT
7.1. Toxicity for Aquatic Organims
Quintozene is of low toxicity for aquatic organisms. The only
data on the toxicity of quintozene for aquatic organisms is from
Nishiuchi & Yoshida (1972) who quote a 48-h LC50 value of 10 000
µg/litre for carp and a 3-h LC50 value for Daphnia of 40 000
µg/litre.
7.2. Toxicity for Terrestrial Organisms
7.2.1. Plants
Vishunavat & Shukla (1981) examined the effects of quintozene
on seed germination, plant stand, and yield of lentils. There were
no significant effects. Brown et al. (1982) did not find any
effects on the germination of orchids when 99% pure quintozene was
applied at concentrations of 25 and 50 mg/litre. At 100 mg/litre,
Cattleya elongata did not germinate, but germination of Laelia
was unaffected and Vanda tricolor showed improved germination.
Quintozene eliminated growth of excised shoot tips in orchids of
the Cymbidium family.
7.2.2. Earthworms
Roark & Dale (1979) reared earthworms Eisenia foetida in
soil pre-mixed with quintozene at a dose of 0.679 g/4719 cm3
of soil, corresponding to 226.8 g over 929 cm2 of turf. The
dose was calculated as the total of 3 applications of the
recommended dosage for turf. The worms did not reproduce in
treated soil. Survival of worms was not significantly reduced
within the first 10 days, but fell to zero within 29 days of
treatment with quintozene.
7.2.3. Bees
No information is available on toxicity of quintozene for bees.
However, since the main uses of quintozene are on soil or as a seed
dressing, it is unlikely that bees would be exposed.
7.2.4. Birds
Dunn et al. (1979a) fed white leghorns with concentrations
of 0, 10, 50, 100, or 1000 mg quintozene/kg diet and examined
egg production and hatchability. None of these concentrations
caused obvious toxic effects, death, or histopathological changes
in either control or treated groups. Egg production during the
25th to the 35th week was not significantly affected. However, at
a higher dose level of 1000 mg/kg diet, onset of egg production was
delayed for 1 month, and the number of chicks hatched from fertile
eggs significantly decreased from 91% in the controls to 69% in
treated birds. Shell strength of the eggs was not significantly
altered (Dunn et al., 1979a). In another study (Dunn et al.,
1979b), the authors reported that bioaccumulation of PCNB or its
metabolites only occurred in trace concentrations; body weight
gains were significantly lower in hens fed 1000 mg quintozene/kg
diet.
7.3. Toxicity for Microorganisms
Tu (1980) reported that quintozene at doses up to 5000 mg/litre
did not induce any effects on 3 strains of the bacterium Rhizobium
japonicum in culture. Quintozene also did not show any effects
on 25 strains of Rhizobium bacteria, isolated from root nodules of
red clover, at doses up to 1000 mg/litre in the culture medium
(Heinonen-Tanski et al., 1982). Smiley & Craven (1979) applied
quintozene 9 times annually for 3 years, at weekly intervals during
July and August, to a turf of Kentucky blue grass. There were no
significant effects on the populations of bacteria, actinomycetes,
or fungi. The effects of quintozene on carbon dioxide evolution
and on the enzyme activities in organisms in soil were observed by
Mitterer et al. (1981). The recommended soil dosage of the
fungicide caused an increase in carbon dioxide evolution from soil
cultures. After a second and third application, this initial
increase was followed by a decrease in carbon dioxide release to
below the value for untreated control soil. Quintozene showed a
severe and continuous inhibition of xylanase activity, in marked
contrast to other fungicides tested.
7.4. Bioaccumulation and Biomagnification
Ogiso & Tanabe (1982) measured residues in different tissues of
crop plants. High concentrations of quintozene in plant tissues
relative to soil levels were only found in the outer layers of
roots and tubers directly in contact with soil. There is little
evidence of systemic uptake.
Kanazawa (1981) reported a bioconcentration factor for
quintozene by top mouth gudgeon Pseudorasbora parva of 238. The
flow-through system maintained water concentrations of between 5
and 20 µg quintozene/litre.
8. PREVIOUS EVALUATIONS OF QUINTOZENE BY INTERNATIONAL BODIES
The Joint Meeting on Pesticide Residues (JMPR) reviewed
residues and toxicity data on quintozene in 1969, 1973, 1975, and
1977 (FAO/WHO, 1970, 1974, 1976, 1978). The conclusion in 1977 was
that 25 mg/kg diet, equivalent to 1.25 mg/kg body weight was a no-
observed-effect-level in the rat and 30 mg/kg diet, equivalent to
0.75 mg/kg body weight in the dog. On the basis of this, the
estimate of an acceptable daily intake (ADI) for man was 0 - 0.007
mg/kg body weight.
IARC (1974) did not come to a conclusion on the carcinogenicity
of quintozene because of lack of data at the time. FAO/WHO (1978)
concluded that there were no indications that administration of
quintozene resulted in carcinogenic activity.
CEC (1981) concluded that there was a need to set limits on the
impurities present in technical quintozene.
WHO, in its "Guidelines to the Use of the WHO Recommended
Classification of Pesticides by Hazard" (WHO, 1984), classified
quintozene in the category of technical products unlikely to
present an acute hazard in normal use.
Regulatory standards established by national bodies in 12
different countries (Argentina, Brazil, Czechoslovakia, the Federal
Republic of Germany, India, Japan, Kenya, Mexico, Sweden, the
United Kingdom, the USA, and the USSR) and the EEC can be found in
the IRPTC (International Register of Potentially Toxic Chemicals)
legal file (IRPTC, 1983).
9. EVALUATION OF HEALTH RISKS FOR MAN AND EFFECTS ON THE
ENVIRONMENT
9.1. Evaluation of Health Risks for Man
Quintozene toxicity
Quintozene is practically non-toxic according to the scale of
Hodge & Sterner (1956). The oral LD50 in rats was 1650 to more
than 30 000 mg/kg body weight. WHO (1984) classified quintozene in
the category of technical products unlikely to present an acute
hazard in normal use.
No-observed-adverse-effect levels in long-term studies on the
rat and the dog were 1.25 and 0.75 mg/kg body weight (25 and 30
mg/kg diet), respectively. In long-term studies with rats and at
higher dosages (63 mg/kg diet), quintozene can give rise to liver
hypertrophy with some histopathological changes and in dogs to more
severe liver damage with fibrosis (5000 mg/kg diet). In short-term
studies on female rats, quintozene caused induction of mixed-
function oxidases.
Quintozene is both metabolised and excreted unchanged and
does not accumulate in tissues.
Quintozene is not considered to be teratogenic.
Quintozene is generally negative in short-term tests for
genetic activity. In carcinogenicity studies on rats and
mice, equivocal or negative findings have been reported.
Hexachlorobenzene, a possible impurity in technical quintozene
is carcinogenic for mice, rats, and hamsters.
Except for 1 case of conjunctivitis in an occupational setting,
no other cases of poisoning or adverse effects have been reported
in man.
Exposure to quintozene
The general population can be exposed via residues in food,
especially in oils and fats. Information on exposure from other
sources is lacking. No cases of accidental or occupational
overexposure have been reported.
Hazard assessment
With the exception of some data on residues in food, no human
exposure data are available for quintozene. It is therefore
difficult to evaluate the hazard for man of present exposure to
this substance. Nevertheless, in view of its low toxicity in
short-term and long-term animal studies, the data available on
quintozene would indicate a low degree of concern in relation to
human health effects.
9.2. Evaluation of Overall Environmental Effects
The only significant adverse effect reported for quintozene is
on earthworms. According to laboratory tests, quintozene applied
at recommended doses as a soil fungicide appears to have long-term
toxic effects on the earthworm. Unfortunately, no observations of
the effects on earthworms of quintozene alone, during field use,
are available.
There is no evidence that quintozene represents a threat to
non-target organisms. It has a very low acute toxicity for fish
and Daphnia.
Its bioaccumulation by fish is low, and no effects have been
reported on terrestrial plants, birds, or microorganisms.
9.3. Conclusions
1. The general population does not appear to be at
risk from residues of quintozene in food.
2. Exposure of the general population via air and
drinking-water could not be evaluated because of lack
of data.
3. Occupational exposure has not been reported to
cause any adverse effects.
4. There is limited information on the effects of
quintozene in the general environment. It has been
shown to be toxic to earthworms, in laboratory
tests. Data on other organisms suggest that
quintozene is not a problem in the general
environment.
5. Quintozene does not biomagnify.
6. The major toxicological concern with quintozene
is the presence of hexachlorobenzene as an impurity.
REFERENCES
ANONYMOUS (1976) Fungicides. In: Analytical methods for
pesticides and plant growth regulators. VII. Government
regulations, pheromone analysis, additional pesticides, New
York, Academic Press, pp. 251-331.
ANONYMOUS (1977a) Rebuttable presumption against
registration and continued registration of pesticide products
containing PCNB. Fed. Regist., 42: 56072-56100.
ANONYMOUS (1977b) Surface water quality in Canada - an
overview. Fish. Environ. Can., 1-45.
AVRAHAMI, M. & WHITE, D.A. (1976) Rapid elimination of PCNB
and metabolite from sheep fat. N.Z. J. exp. Agric., 4: 299-302.
BECK, J. & HANSEN, K.E. (1974) The degradation of
quintozene, pentachlorobenzene, hexachlorobenzene and
petachloroaniline in soil. Science, 5: 41-48.
BERG, G.L., ed. (1978) Farm chemicals handbook, Willoughby,
Ohio, Meister Publishing Company, p. D133.
BERKOWITZ. J., STEVENS, J., ARNOLD, D., GOYER, M., SENECHAL,
D., HARRISON, J., LUDWIG, R., & NEWMEYER, J. (1976)
Substitute chemical program: initial scientific review of
PCNB, Washington DC, US Environmental Protection Agency,
Office of Pesticide Programs, Criteria and Evaluation Division
(EPA 540/1-75-016).
BETTS, J.J., JAMES, S.P., & THORPE, W.V. (1955) The
metabolism of pentachloronitrobenzene and 2,3,4,6-tetra-
chloronitrobenzene and the formation of mercapturic acids in
the rabbit. Biochem. J., 61: 611-617.
BORZELLECA, J.F., LARSON, P.S., CRAWFORD, E.M., HENNIGAR,
G.R., KUCHAR, E.J., & KLEIN, H.H. (1971) Toxicologic and
metabolic studies on pentachloronitrobenzene. Toxicol. appl.
Pharmacol., 18: 522-534.
BROWN, D.M., GROOM, C.L., CVITANIK, M., BROWN, M., COOPER,
J.L., & ARDITTI, J. (1982) Effects of fungicides and
bactericides on orchid seed germination and shoot tip cultures
in vitro. Plant Cell, Tissue and Organ Cult., 1: 165-180.
BURKE, G. & HOLSWADE, W. (1964) Gas chromatogrphy with
microcoulometric detection for pesticide residue analysis.
J. Assoc. Off. Anal. Chem., 47: 845-859.
BUSELMAIER, W., ROHRBORN, G., & PROPPING, P. (1973)
Comparative investigations on the mutagenicity of pesticides
in mammalian test systems. Mutat. Res., 21: 25-26.
CLARKE, C.H. (1971) The mutagenic specificities of
pentachloronitrobenzene and captan to environmental mutagens.
Mutat. Res., 11: 247-248.
CEC (1981) Criteria (dose/effect relationships) for
organochlorine pesticides, Oxford, Pergamon Press.
COURTNEY, K.D., COPELAND, M.F., & ROBBINS, A. (1976) The
effects of PCNB, hexachlorobenzene and related compounds on
fetal development. Toxicol. appl. Pharmacol., 35: 239-256.
CROSBY, D.G. & HAMADMAD, N. (1971) Photo-reduction of
pentachlorobenzenes. J. agric. food Chem., 19: 1171-1174.
DAPPEN, G. (1974) Pesticide analysis from urban storm
runoff, Washington DC, US Department of Commerce, National
Technical Information Service (PB Report, Issue No.
238593/8GA, 44).
DUNN, J.S., BUSH, P.B., BOOTH, N.H., FARRELL, R.L., THOMASON,
D.M., & GOETSCH, D.D. (1979a) Effect of pentachloronitro-
benzene upon egg production, hatchability, and residue
accumulation in the tissue of white leghorn hens. Toxicol.
appl. Pharmacol., 48: 425-433.
DUNN, J.S., BUSH, P.B., BOOTH, N.H., FARRELL, R.L., THOMASON,
D.M., & GOETSCH, D.D. (1979b) Tissue residues from feeding
pentachloronitrobenzene (quintozene) to white leghorn
chickens. Am. J. vet. Res., 40: 1227-1230.
DUNSING, M. & WINDSCHILD, J. (1976) [Residues of quintozene,
hexachlorobenzene, and pentachloroaniline in lettuce and
soil.] Nachr. Pflanzenschutzd., 30: 106-108 (in German).
FAO/WHO (1970) Quintozene. In: 1969 Evaluations of some
pesticide residues in foods, Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1974) Quintozene. In: 1973 Evaluations of some
pesticide residues in foods, Rome, Food and Agriculture
Organization of the United Nations, pp. 379-396.
FAO/WHO (1976) Quintozene. In: 1975 Evaluations of some
pesticide residues in foods, Rome, Food and Agriculture
Organization of the United Nations, pp. 357-363.
FAO/WHO (1978) Quintozene. In: 1977 Evaluations of some
pesticide residues in foods, Rome, Food and Agriculture
Organization of the United Nations, pp. 439-440.
FINNEGAN, J.K., LARSON, P.S., SMITH, R.B. Jr, HAAG, H.B., &
HENNIGAR, G.R. (1958) Acute and chronic toxicity studies on
pentachloronitrobenzene. Arch. int. Pharmacodyn., 114: 38-52.
FUJITA, K., SUZUKI, H., & OCHIAI, F. (1976) [Kerato-
conjunctivitis due to pesticides of relatively low acute
toxicity.] Clin. Opthalmol., 30: 419-423 (in Japanese).
GLOFKE, E. (1973) [Pesticide in milk and milk products.]
Milchwirt. Ber. Bundesanst. Wolfpassing Rotholz., 34: 37-40
(in German).
GOURSAUD, J., LUQUET, F.M., BOUDIER, J.F., & CASALIS, J.
(1972) Sur la pollution du lait par les residues
d'hexachlorobenzene (HCB). Ind. Aliment. Agric., 89: 31-35.
HEIKES, D.L. (1980) Residues of pentachloronitrobenzene and
related compounds in peanut butter. Bull. environ. Contam.
Toxicol., 24: 338-343.
HEINONEN-TANSKI, H., OROS, G., & KECSKES, M. (1982) The
effect of soil pesticides on the growth of red clover
Rhizobia. Acta agric. Scand., 32: 283-288.
HODGE, H.C. & STERNER, J.H. (1956) Combined tabulation of
toxicity classes. In: Spector, W.S., ed. Handbook of
toxicology, Philadelphia, W.B. Saunders Company, Vol. 1.
IARC (1974) Some organochlorine pesticides, Lyons,
International Agency for Research on Cancer, pp. 211-218
(Monographs on the Evaluation of the Carcinogenic Risk of
Chemicals to Man, No. 5).
IARC (1979) Hexachlorobenzene, Lyons, International Agency
for Research on Cancer, Vol. 20, pp. 155-179 (Monographs on
the Evaluation of the Carcinogenic Risk of Chemicals to Man).
INNES, J.R.M., ULLAND, B.M., VALERIO, M.G., PETRUCELLI, L.,
FISHBEIN, L., MART, O.R., PALLOTTA, A.J., BATTES, R.R., FALK,
R., GART, J.J., KLEIN, N., MITCHELL, I., & PETERS, J. (1969)
Bioassay of assay of pesticides and industrial chemicals for
tumorigenicity in mice: A preliminary note. J. Natl Cancer
Inst., 42: 1011-1114.
IRPTC (1983) IRPTC legal file 1983, Geneva, International
Register of Potentially Toxic Chemicals, United Nations
Environment Programme, Vol. 1 & 2.
JOHNSON, R.D., MANSKE, D.D., NEW, D.H., & PODEREBARAC, D.S.
(1969) Pesticides and other chemical residues in infant and
toddler total diet samples: 1 August 1974-July 1975, Pest.
Monit. J., 13: 87-98.
JORDAN, R.L. & BORZELLECA, J.F. (1973) Teratogenic studies
with pentachlorinitrobenzene in rats. Toxicol. appl.
Pharmacol., 25: 454.
KANAZAWA, J. (1981) Measurement of the bioconcentration
factors of pesticides by fresh water fish and their
correlation with physico-chemical properties or acute
toxicities. Pestic. Sci., 12: 417-424.
KHERA, K.S. & VILLENEUVE, D.C. (1975) Teratogenicity studies
on halogenated benzenes (pentachloro-, pentachlorinitro-, and
hexabromo-) in rats. Toxicology, 5: 117-122.
KO, W.H. & FARLEY, J.D. (1969) Conversion of
pentachloronitrobenzene to pentachloroaniline in soil and the
effect of these compounds on soil microorganisms.
Phytopathology, 59: 64-67.
KOEGEL, W., MULLER, W.F., COULSTON, F., & KORTE, F. (1979)
Fate and effects of pentachloronitrobenzene in Rhesus
monkeys. J. agric. food Chem., 27: 1181-1185.
KUCHAR, E.J., GEENTY, F.O., GRIFFITH, W.P., & THOMAS, R.J.
(1969) Analytical studies of metabolism of terrachlor in
beagle dogs, rats and plants. J. agric. food Chem., 17:
1237-1240.
LEE, R.E. Jr, ed. (1975) Air pollution from pesticides:
Sources, occurrence and dispersion. In: Air pollution from
pesticides and agricultural processes, Florida, CRC Press.
LEISTRA, M. & SMELT, J.H. (1974) Concentrations of
quintozene at different depths in bulb-growing soils. Bull.
environ. Contam. Toxicol., 11: 241-243.
METHRATTA, T.P., MONTAGNA, R.W., & GRIFFITH, W.D. (1967)
Determination of teraclor in crops and soil by electron-
capture gas chromatography. J. agric. food Chem., 15: 648-650.
MITTERER, M., BAYER, H., & SCHINNER, F. (1981) [The
influence of fungicides on microbiol acitivity in soil.]
Z. Pflanzenernaehr. Bodenk. D., 144: 463-471 (in German).
MOHN, G. (1971) Microorganisms as test systems of
mutagenicity. Arch. Toxicol., 28: 93-104.
MORIYA, M., OHTA, T., WATANABE, K., MIYAZAWA, T., KATO, K., &
SHIRASU, Y. (1983) Further mutagenicity studies on
pesticides in bacterial reversion assay systems. Mutat. Res.,
116: 185-216.
MULLER, W.F., SCHEUNERT, I., ROZMAN, K., KOGEL, W., FREITAG,
D., RICHTER, E., COULSTON, F., & KORTE, F. (1978)
Comparative metabolism of hexachlorobenzene in plants, rats
and Rhesus monkeys. Ecotoxicol. environ. Saf., 2: 437-445.
MUSTY, P.R. & NICKLESS, G. (1974) The extraction and
recovery of chlorinated insecticides and polychlorinated
biphenyls from water using porous polyurethane foams.
J. Chromatog., 100: 83-93.
NCI (1978) Bioassay of pentachloronitrobenzene for possible
carcinogenicity, Bethesda, Maryland, National Cancer Institute
(Technical Report Series No. 61).
NISHIUCHI, Y. & YOSHIDA, K. (1972) Noyaku Kensasho Hokoku,
p. 122 (in Japanese) (in English in Kanazawa (1981)).
OGISO, M. & TANABE, H. (1982) Residue of quintozene, its
metabolites, and hexachlorobenzene in the soil and crops.
J. Pestic. Sci. (Nihon Noyaku Gakkaishi), 7: 391-396.
O'GRODNICK, J.S., ADAMOVICS, J.A., BLAKE, SH., & WEDIG, J.
(1981) The metabolic fate of 14C-labelled pentachloronitro-
benzene in Osborne-Mendell rats. Chemosphere, 10: 67-72.
RAUTAPAA, J., PYYSALO, H., & BLOMQVIST, H. (1977) Quintozene
in some soils and plants in Finland. Ann. Agric. Fenn., 16:
277-282.
ROARK, J.H. & DALE, J.L. (1979) The effect of turf
fungicides on earthworms. Proc. Arkansas Acad. Sci., 33: 71-74.
SCHUMANN, A.M. & BORZELLECA, J.F. (1978) An assessment of
the methaemoglobin and Heinz-body-inducing capacity of
pentachloronitrobenzene in the cat. Toxicol. appl. Pharmacol.,
44: 523-529.
SEARLE, C.E. (1966) Tumor initiatory activity of
chloromono-nitrobenzenes and other compounds. Cancer Res.,
26: 12-17.
SIJPESTEIJN, A.K., DEKHUIJZEN, H.M., & VONK, J.W. (1977)
Biological conversions of fungicides in plants and micro-
organisms. In: Siegel, M.R. & Sisler, H.D., ed. Antifungal
compounds, Vol. 2: Interactions in biological and ecological
systems, New York, Marcel Dekker Inc., Vol. 2.
SIMON, G.S., KUCHAR, E.J., KLEIN, H.H., & BORZELLECA, J.F.
(1979) Distribution and clearance of pentachloronitrobenzene
in chickens. Toxicol. appl. Pharmacol., 50: 401-406.
SMILEY, R.W. & CRAVEN, M.M. (1979) Microflora of turfgrass
treated with fungicides. Soil Biol. Biochem., 11: 349-353.
SMITH, A.G. & CABRAL, J.R.P. (1980) Liver cell tumours in
rats fed hexachlorobenzene. Cancer Lett., 11: 169-172.
ST. JOHN, L.E. Jr, AMMERING, J.W., WAGNER, D.G., WARNER, R.G.,
& LISK, D.J. (1965) Fate of 4,6-dinitro-2-isobutylphenol,
2-chloro-4,6-bis(ethylamino)-S-triazine, and PCNB in the dairy
cow. J. dairy Sci., 48: 502-503.
TU, C.M. (1980) Effects of fungicides on growth of Rhizobium
japonicum in vitro. Bull. environ. Contam. Toxicol., 25:
364-368.
US DHEW (1973) Pesticide analytical manual, Maryland, US
Department of Health, Education, and Welfare, Food and Drug
Administration.
VAN LOGTEN, M. (1977) Quintozene. (Working Paper for 1977
JMPR) (FAO/WHO FOS/RES/77.46A).
VILLENEUVE, D.C. & KHERA, K.S. (1975) Placental transfer of
halogenated benzenes (Pentachloro-, Pentachloronitro-, and
Hexabromo-) in rats. Environ. Physiol. Biochem., 5: 328-331.
VISHUNAVAT, K. & SHUKLA, P. (1981) Effects of seed treatment
of lentil upon germination, plant stand, and yield.
Pesticides, 15: 15-16.
WANG, C.H. & BROADBENT, F.E. (1973) Effect of soil
treatments on losses of two chloronitrobenzene fungicides.
J. environ. Qual., 2: 511-515.
WHO (1975) Pesticide residues in food, Geneva, World Health
Organization, Vol. 97, pp. 7-37 (Technical Report Series No.
574 FAO Agricultural Studies).
WHO (1984) The WHO recommended classification of pesticides
by hazard, Geneva, World Health Organization (Unpublished
Report VBC/84.2).
WIT, S.L., VAN ESCH, G.J., & VAN GENDEREN, H. (1957)
Toxicity of some chloronitrobenzene compounds (trichlorodi-
nitrobenzene, trichloronitrobenzene, tetrachloronitrobenzene,
and pentachloronitrobenzene) to laboratory rats and residues
found in crops treated with these fungicides. In: Proceedings
of the 4th International Congress of Crop Protection, Hamburg.
