
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 28
ACRYLONITRILE
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Orgnization
Geneva, 1983
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
ISBN 92 4 154088 5
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1983
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR ACRYLONITRILE
1. SUMMARY AND RECOMMENDATIONS FOR FURTHER RESEARCH
1.1. Summary
1.1.1. Properties and analytical methods
1.1.2. Sources of exposure
1.1.3. Industrial and environmental levels of exposure
1.1.4. Monitoring of acrylonitrile uptake
1.1.5. Absorption, distribution, biotransformation,
and elimination
1.1.6. Effects on experimental animals
1.1.7. Effects on man
1.2. Recommendations for further research
2. PROPERTIES AND ANALYTICAL METHODS
2.1. Physical and chemical properties of acrylonitrile
2.1.1. Physical properties
2.1.2. Chemical properties
2.2. Analytical methods
2.2.1. Sampling methods
2.2.2. Analytical methods for determining acrylonitrile
2.2.2.1 Determination of acrylonitrile and its
metabolites in biological materials
3. SOURCES OF INDUSTRIAL AND ENVIRONMENTAL EXPOSURE TO ACRYLONITRILE
3.1. Natural occurrence
3.2. Industrial technology, production data, and projection
3.3. Use patterns
3.4. Disposal of wastes
3.5. Accidental release
3.6. Environmental persistence
4. INDUSTRIAL AND ENVIRONMENTAL SOURCES AND LEVELS OF EXPOSURE
4.1. Exposure of the general population
4.1.1. Air
4.1.2. Water
4.1.3. Food
4.1.4. Other sources of exposure
4.2. Occupational exposure
4.3. Estimate of human exposure from all environmental media
5. CHEMOBIOKINETICS AND METABOLISM
5.1. Absorption
5.1.1. Human studies
5.1.1.1 Uptake through inhalation
5.1.1.2 Dermal absorption
5.1.1.3 Uptake by other routes
5.1.2. Experimental animal studies
5.1.2.1 Uptake through inhalation
5.1.2.2 Dermal absorption
5.1.2.3 Uptake by other routes
5.2. Distribution and toxicokinetics
5.2.1. Human studies
5.2.2. Experimental animal studies
5.3. Biotransformation and elimination
5.3.1. Human studies
5.3.2. Experimental animal studies
5.3.2.1 The oxidative pathways of acrylonitrile
metabolism
5.3.2.2 Mercapturic acids formed in
acrylonitrile biotransformation
5.3.2.3 The glucuronic acid conjugate
of acrylonitrile metabolism
5.3.2.4 Quantitative aspects of acrylonitrile
biotransformation and elimination of
its metabolites
6. BIOLOGICAL MONITORING OF ACRYLONITRILE UPTAKE
7. EFFECTS ON EXPERIMENTAL ANIMALS AND CELL SYSTEMS
7.1. Acute toxicity
7.1.1. Lethal doses and concentrations
7.1.1.1 Lethal doses
7.1.1.2 Lethal concentrations in air
7.1.1.3 Lethal concentrations in water
7.1.2. Clinical observations
7.1.3. Biochemical changes and mechanisms of
acrylonitrile toxicity
7.1.3.1 Effect on cytochrome oxidase
7.1.3.2 Effect on sulfhydryls
7.1.3.3 Interaction with the microsomal oxidation system
as a possible mechanism of toxicity
7.1.3.4 Observations on the possible participation of
membrane lipid peroxidation in the mechanism
of toxicity
7.1.3.5 Studies on antidotes
7.2. Subacute toxicity
7.2.1. Inhalation exposure
7.2.2. Oral administration
7.2.3. Subcutaneous administration and
intraperitoneal administration
7.2.4. Clinical observations in animal studies
7.2.4.1 Body weight, food and water consumption
7.2.4.2 Organ weights and pathology
7.2.4.3 Blood
7.2.4.4 Immune system
7.2.4.5 Nervous system
7.2.4.6 Urine
7.2.4.7 Adrenals
7.2.4.8 Metabolism
7.3. Chronic toxicity
7.3.1. Body weight, food and water intake
7.3.2. Organ weights
7.3.3. Pathology and histology
7.3.4. Haematology and clinical chemistry
7.3.5. Nervous system
7.3.6. Kidney function
7.4. Teratogenicity and embryotoxicity
7.5. Mutagenicity
7.5.1. Bacterial systems
7.5.2. Yeast assays
7.5.3. Drosophila melanogaster
7.5.4. Mammalian cell in vitro assays
7.5.5. Mammalian in vivo assays
7.6. Carcinogenicity
8. EFFECTS ON MAN
8.1. Acrylonitrile
8.1.1. Acute toxicity
8.1.1.1 Inhalation exposure
8.1.1.2 Dermal exposure
8.1.2. Chronic toxicity - occupational exposure
8.1.2.1 Clinical observations
8.1.2.2 Haematology
8.1.2.3 Other organs
8.1.2.4 Nervous system
8.1.2.5 Dermal effects
8.2. Mutagenicity
8.3. Carcinogenicity
8.4. Simultaneous occupational exposure to acrylonitrile and
other chemicals
8.4.1. Acute toxicity
8.4.2. Chronic toxicity
9. EVALUATION OF HEALTH RISKS TO MAN FROM EXPOSURE TO
ACRYLONITRILE
9.1. Sources and levels of exposure
9.2. Acrylonitrile toxicity
REFERENCES
NOTE TO READERS OF THE CRITERIA DOCUMENTS
While every effort has been made to present information in
the criteria documents as accurately as possible without unduly
delaying their publication, mistakes might have occurred and are
likely to occur in the future. In the interest of all users of
the environmental health criteria documents, readers are kindly
requested to communicate any errors found to the Manager of the
International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland, in order that they may be
included in corrigenda, which will appear in subsequent volumes.
In addition, experts in any particular field dealt with in
the criteria documents are kindly requested to make available to
the WHO Secretariat any important published information that may
have inadvertently been omitted and which may change the
evaluation of health risks from exposure to the environmental
agent under examination, so that the information may be
considered in the event of updating and re-evaluation of the
conclusions contained in the criteria documents.
* * *
A detailed data profile and a legal file can be obtained from
the International Register of Potentially Toxic Chemicals, Palais
des Nations, 1211 Geneva 10, Switzerland (Telephone no. 988400 -
985850).
TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR ACRYLONITRILE
Members
Dr I. Gut, Institute of Hygiene & Epidemiology, Prague,
Czechoslovakia
Dr V.V. Ivanov, State Medical Institute, Krasnoyarsk, USSR
Dr J. Kopecky, Institute of Hygiene & Epidemiology, Prague,
Czechoslovakia
Dr W.N. Rom, Rocky Mountain Center for Occupational &
Environmental Health, School of Medicine, University of
Utah, Salt Lake City, Utah, USA
Dr M. Sharratt, BP Group Occupational Health Centre,
Sunbury-on-Thames, England (Chairman)
Dr J. Sokal, Institute of Occupational Medicine, Lodz, Poland
(Rapporteur)
Dr. L. Zisser, Department of Occupational Medicine, Kupat
Holin - District Yehuda, Rehovoth, Israel (Vice-Chairman)
Representatives of other organizations
Dr A. Berlin, Health & Safety Directorate, Commission of the
European Communities, Luxembourg
Dr R.A. Baxter, Monsanto Europe, Brussels (representing the
Association of Plastic Manufacturers in Europe - APME)
Secretariat
Dr M.H. Draper, Medical Officer-Toxicologist, International
Programme on Chemical Safety (Secretary)
Dr K.W. Jager, Consultant, International Programme on Chemical
Safety
ENVIRONMENTAL HEALTH CRITERIA FOR ACRYLONITRILE
Further to the recommendations of the Stockholm United
Nations Conference on the Human Environment in 1972, and in
response to a number of World Health Assembly resolutions
(WHA23.60, WHA24.47, WHA25.58, WHA26.68) and the recommendations
of the Governing Council of the United Nations Environment
Programme, (UNEP/GC/10, July 3 1973), a programme on the
integrated assessment of the health effects of environmental
pollution was initiated in 1973. The programme, known as the WHO
Environmental Health Criteria Programme, has been implemented
with the support of the Environment Fund of the United Nations
Environment Programme. In 1980, the Environmental Health
Criteria Programme was incorporated into the International
Programme on Chemical Safety (IPCS). The result of the
Environmental Health Criteria Programme is a series of criteria
documents.
The Institute of Hygiene and Epidemiology (Director,
Professor Bohumir Rosicky), Prague, was responsible, as a Lead
Institution of the IPCS, for the preparation of the first and
second drafts, which were written and coordinated by Dr I. Gut
and Dr J. Kopecky of that Institute.
The Task Group for the Environmental Health Criteria for
Acrylonitrile met in Prague in the Institute of Hygiene and
Epidemiology from 4-8 July 1983. The meeting was opened by
Professor B. Rosicky, and Dr M.H. Draper welcomed the
participants and representatives of the organizations on behalf
of the three organizations co-sponsoring the IPCS (UNEP/ILO/WHO).
The Task Group reviewed and revised the second draft criteria
document and made an evaluation of the health risks of exposure
to acrylonitrile.
The efforts of all who helped in the preparation and the
finalization of the document are gratefully acknowledged.
* * *
Partial financial support for the publication of this
criteria document was kindly provided by the United States
Department of Health and Human Services through a contract from
the National Institute of Environmental Health Sciences, Research
Triangle Park, North Carolina, USA - a WHO Collaborating Centre
for Environmental Health Effects.
1. SUMMARY AND RECOMMENDATIONS FOR FURTHER RESEARCH
1.1. Summary
1.1.1. Properties and analytical methods
Acrylonitrile (CH2=CH-C-N) is a volatile, colourless,
flammable liquid with a sweet characteristic odour. It is used
in the production of acrylic and modacrylic fibres, resins and
rubbers, and as a chemical intermediate. It has been employed as
a fumigant. Exposure to both the vapour and the liquid can occur
at the workplace, the highest atmospheric concentrations
occurring in acrylic fibre production.
For the control of exposure to acrylonitrile at the
workplace, sampling should preferably be from the breathing zone
of the worker; active and passive sampling techniques are
available.
The most widely used analytical techniques are the gas
chromatographic techniques; these are particularly sensitive if
nitrogen-sensitive and specific sensors are used. High-pressure
liquid chromatographic, infra-red, and colorimetric methods may
be useful, where gas chromatography is not available. Methods
have been developed for the determination of acrylonitrile in
blood, food, water, etc. Determination of acrylonitrile-derived
mercapturic acids in urine may prove to be of value for the
biological monitoring of exposure.
1.1.2. Sources of exposure
Acrylonitrile is emitted from industrial plants in the form
of vapours and in aqueous effluents; exposure of the population
living near plants cannot therefore be excluded. The total
emissions from acrylonitrile plants have been estimated to be
about 2.2% of total production, but these figures have decreased
recently. Polymers contain various concentrations of free
acrylonitrile; when used for packaging in the food industry,
minute amounts of the monomer may pass into the food.
Acrylonitrile may also enter the environment accidentally, during
its storage and transport.
1.1.3. Industrial and environmental levels of exposure
Contamination of water and food is possible but, with the
exception of the contamination of water supplies through
accidental spillage, levels of exposure would be low. The
highest potential for exposure is at the workplace, both through
inhalation of vapour and contamination of the skin by liquid
acrylonitrile.
1.1.4. Monitoring of acrylonitrile uptake
The most significant uptake of acrylonitrile vapour is
through the respiratory tract. Exposure is commonly monitored by
determining the time-weighted average atmospheric concentrations.
Estimation of acrylonitrile-derived mercapturic acids in
urine is a promising method for the biological monitoring of
exposure, but further validating studies are needed.
1.1.5. Absorption, distribution, biotransformation, and
elimination
In animals, acrylonitrile is readily absorbed both through
the skin and by inhalation. Systemic and even fatal effects are
possible via these routes.
The distribution of acrylonitrile within the animal body is
fairly uniform. There are no indications of accumulation in
animal tissues following prolonged exposure.
At least 10 different metabolites of acrylonitrile have been
identified. Mercapturic acids are the major metabolites of
acrylonitrile in vivo. Urinary excretion of acrylonitrile-
derived mercapturic acids is proportional to the internal
concentration of acrylonitrile.
Elimination of acrylonitrile, as such, in expired air is
negligible, but a small percentage is eliminated in the urine.
1.1.6. Effects on experimental animals
Acrylonitrile induces a variety of toxic effects. Effects
due to over-exposure are non-specific and mainly related to the
gastro-intestinal and respiratory tracts, the central nervous
system, and the kidneys. Respiratory distress, lethargy,
convulsions, and coma occur with lethal or near-lethal exposures
(7500 mg/m3, inhalation). Dogs are most sensitive, and rats
least sensitive to acrylonitrile, with mice, guinea-pigs, cats,
and monkeys in an intermediate position. However, the
information available from these studies is too fragmentary to
indicate clear no-observed-adverse-effect levels.
Extensive dermal exposure to the liquid may be lethal. At
lower exposures, irritation of the skin and mucous membranes can
occur.
The most typical biochemical changes caused by acrylonitrile
are inhibition of sulfhydryl-dependent enzymes (lactate
dehydrogenase, LDH (EC 1.1.1.27), sorbitol dehydrogenase, SDH (EC
1.1.1.14), pyruvate oxidase (EC 1.2.3.3)) and a reduction in the
concentrations of glutathione and protein sulfhydryls in the
blood and various organs, resulting in a disturbance of glucose
utilization. The cyanide generated causes inhibition of
cytochrome oxidase (EC 1.9.3.1) but this seems to be of less
significance than the above-mentioned metabolic disturbances, at
low exposure levels.
Exposure to some organic solvents in addition to
acrylonitrile may significantly enhance its toxic effects.
Acrylonitrile can cause embryotoxic and teratogenic effects,
but only at levels near the toxic dose level for the specific
experimental animal.
It is probable that acrylonitrile is not mutagenic itself,
but that its metabolites are responsible for the positive effects
in various test systems. It is mutagenic in in vitro systems
(bacterial tests and cell cultures), but not in in vivo systems,
such as the dominant lethal assay.
On the basis of the results of several animal studies, using
a wide dose-range, there is sufficient evidence to suggest that
acrylonitrile is a carcinogen in the rat.
1.1.7. Effects on man
Symptoms of over-exposure in man are non-specific. They are
related to the gastrointestinal and respiratory tracts, and to
the central nervous system and include headache, insomnia,
nausea, vomiting, diarrhoea, fatigue, mild jaundice, and
irritation and inflammation of the respiratory tract and mucous
membranes. In more severe cases, unconsciousness and convulsions
may occur. Fatalities have been reported following exposure to
acrylonitrile, especially following its use as a fumigant.
Dermal exposure, especially to liquid acrylonitrile, may cause
irritation, erythema, and blisters. Toxic and allergic dermatitis
can occur.
While a correlation between exposure to acrylonitrile and the
incidence of cancer in man has not been demonstrated conclusively
in human epidemiological studies, the findings are not
incompatible with this supposition. Thus, there is no reason to
disregard the evidence that has been provided by animal studies.
It follows that exposure to acrylonitrile should be kept as
low as possible at the workplace and in the general environment,
and that skin contact with liquid acrylonitrile should be
avoided.
1.2. Recommendations for Further Research
The Task Group noted that valuable information from industry,
while available to national and international bodies, had not
been published. This greatly reduces the value of these studies,
as they are unavailab1e for peer review and critical examination
by the scientific community.
The Group recommended the following studies:
(a) Improvement and validation of passive sampling techniques
with special attention to interfering substances;
(b) Validation of the measurement of acrylonitrile and
acrylonitrile-derived mercapturic acids in urine as
methods for biological monitoring for workplace exposure,
with regard to analytical aspects and sampling conditions;
(c) Investigation of the environmental fate of acrylonitrile
including photochemical degradation;
(d) Further investigation of the mechanisms of action and the
nature of acute and chronic toxic effects in conditions
relevant to human exposure;
(e) Studies on the carcinogenicity of acrylonitrile in
relation to animal species other than the rat;
(f) Further investigation of the metabolism and toxicokinetics
of acrylonitrile in different animal species, in order to
obtain information that will assist in the interpretation
of biological monitoring data in man;
(g) Further examination of the immunological aspects of the
action of acrylonitrile in man and animals;
(h) Further studies on the effects of acrylonitrile on
reproduction;
(i) Investigations on reproductive outcome and mutagenicity in
human beings occupationally exposed to acrylonitrile.
Epidemiological data with good indications of past and
present exposure levels should be available, to ensure an
adequate health risk evaluation.
2. PROPERTIES AND ANALYTICAL METHODS
2.1. Physical and Chemical Properties of Acrylonitrile
2.1.1. Physical properties
Acrylonitrile (CH2=CH-C-N) is a volatile, colourless,
flammable liquid with a sweet, characteristic odour. It is
slightly soluble in water and miscible with most organic solvents
(American Cyanamid, 1959). The vapours are explosive, cyanide
gas being produced. The explosive range in air at 25 °C has a
lower limit of 3.05%, and an upper limit of 17.0%, by volume
(Patty, 1963). The olfactory threshold level for acrylonitrile
averages 40.4 mg/m3 (18.6 ppm) and ranges from 0.007 to 109.4
mg/m3 (0.0031 to 50.4 ppm) (Baker, 1963). Important physical
constants and properties of acrylonitrile are summarized in
Table 1.
2.1.2. Chemical properties
Structural formula: H H
\ /
\3 2/
C = C
/ \
/ \
H C - N
1
Synonyms: cyanoethylene, 2-propenenitrile, vinyl
cyanide.
CAS Registry Number: 107-13-1.
The reactions of acrylonitrile involve the double bond (C=C)
and/or the cyano group (CN) (American Cyanamid, 1959). It
polymerizes to polyacrylonitrile, and copolymerizes with, e.g.,
styrene, butadiene, esters of acrylic or methacrylic acid, to
form various resins, nitrile rubber, and acrylic and modacrylic
fibres. Hydration produces acrylamide or acrylic acid and
esterification the corresponding acrylic esters. Reductive
coupling produces adiponitrile. With compounds containing active
hydrogen(s) (AH molecules such as the biologically-important
compounds containing the nucleophilic -CH, -NH, and -SH groups),
cyanoethylation takes place:
A-H + CH2 = CH-CN + A-CH2CH2CN
(American Cyanamid, 1959). This reaction is of particular
importance in relation to its fate in biological systems;
covalent binding of acrylonitrile to the tissue components has
been demonstrated (section 7.1.3.3). Direct oxidation of
acrylonitrile with hydroperoxide compounds affects the cyano
group of acrylonitrile, although in biological systems, it is
probable that oxidation of the double bond to the oxirane,
glycidonitrile (CH2 - CH-CN) occurs (Kopecky et al., 1980a,b).
\ /
O
Table 1. Physical properties of acrylonitrilea
-----------------------------------------------------------------
appearance colourless liquid
boiling point 77.3 °C at 760 mm pressure
density 0.8060 (20 °C), 0.8004 (25 °C)
flash point (tag open cup) 0 °C
(closed cup) -4.4 °C
freezing point -83.55, ± 0.05 °C
ignition temperature 481 °C
relative molecular mass 53.06
octanol/H2O partition coefficient 0.12b
odour faintly pungent
refractive index nD 25 = 1.3888
% solubility in waterc 7.2% (0 °C)
7.35% (20 °C)
7.9% (40 °C)
vapour pressure (mm Hg) 50 (8.7 °C)
100 (23.6 °C)
250 (45.5 °C)
500 (64.7 °C)
760 (77.3 °C)
partial vapour pressure log P = 7.518 - 1644.7
water azeotrope TK
(i.e., 80 mm at 20 °C)
Conversion factor for vapour 1 mg/m3 = 0.4605 ppm
(25 °C; 760 mm Hg) 1 ppm = 2.17 mg/m3
1 mg/litre water = 1 ppm
-----------------------------------------------------------------
a From: American Cyanamid (1959, 1974).
b From: Dorigan et al. (1976); antilog of -0.92.
c Acrylonitrile is miscible with most organic solvents.
There have not been any experimental studies but, as a
reactive olefine, it would be expected that acrylonitrile would
be oxidized in the atmosphere under the influence of ultraviolet
radiation (UVR) or by reactive oxygen species (atomic oxygen, OH
radicals, ozone). The atmospheric half-life of acrylonitrile is
estimated to be 9-10 h (Suta, 1979).
Technical-grade acrylonitrile is more than 99% pure. Except
for water, impurities and stabilizers are present at mg/kg levels
only. Possible contaminants are shown in Table 2. Spontaneous
explosive polymerization of pure acrylonitrile may occur, in the
absence of oxygen, on exposure to visible light or alkali
(DuPont, 1977). A yellow colour may slowly develop on standing,
particularly after excessive exposure to light. Water improves
the stability of acrylonitrile, and the technical-grade product
is stabilized against self-polymerization and colour formation by
the addition of hydroquinone monomethyl ether and water.
2.2. Analytical Methods
In this section, sampling methods, sample storage, and
analytical methods for determining acrylonitrile and its
metabolites are discussed. The only breakdown products
considered are those detected in vivo, as these are the only
ones of importance for assessing levels of exposure to
acrylonitrile.
2.2.1. Sampling methods
Sorption tubes are widely used for sampling acrylonitrile in
air, because samples can be taken over a prolonged period from
the breathing zone of the worker. The solid sorbent gas samplers
have been critically reviewed by Crisp (1980). Of the solid
sorbents, activated charcoal, porous polymers, or silica gel are
most commonly used. Adsorbed acrylonitrile is later desorbed,
generally by a solvent (methanol or carbon disulfide) or
thermally, and determined by gas chromatography. Several devices
have been developed for sampling workplace air. A sorbent
sampling tube fastened to the worker's shoulder and a pump
fastened to the belt may be worn for a whole working shift
without discomfort. Muhtarova (1977) described significant
differences between the results of static sampling and personal
monitoring in determining acrylonitrile exposure in workers.
Personal monitoring gives a better indication. Area
concentrations can be determined by detector tubes, to give an
immediate indication of the level (CIA, 1978; Grote et al.,
1978).
In the widely-used NIOSH method S156 (NIOSH, 1976), a known
volume of air is drawn through a charcoal tube (divided into 2
sections in order to check that the adsorption capacity has not
been swamped), and the charcoal is desorbed by methanol for 30
min. This method was validated by NIOSH over a concentration
range of 17.5-70.0 mg/m3 (8.1-32.3 ppm) at 22 °C and 760 mm Hg
using a 20-litre sample; the coefficient of variation was 0.073.
However, the suspicion that acrylonitrile may be a human
carcinogen (NIOSH, 1978) led to the need to determine lower
concentrations of acrylonitrile in air. With a simple
modification in method S156, using a desorbing solvent of 2% v/v
acetone solution in carbon disulfide, Gagnon & Posner (1979) were
able to achieve a sensitivity of 1.1 mg/m3 (0.5 ppm) based on an
air sample volume of 15 litres. The samples are stable for at
least a week, even in the absence of a stabilizer. A similar
method, developed by the Midwest Research Institute for sampling
air near acrylonitrile plants (Going et al., 1979), involves the
use of charcoal tubes, sampling air at 1 litre/min, desorbing the
sample with carbon disulfide, and analysing by gas
chromatography. However, high humidity and interference from
other substances can reduce collection efficiency on charcoal;
these problems can be overcome by the use of porous polymer
absorbents and thermal desorption techniques (Campbell & Moore,
1979; United Kingdom Health and Safety Executive, 1981).
Table 2. Specifications for acrylonitrile from two producersa
------------------------------------------------------------------
Specifications DuPont Monsanto
------------------------------------------------------------------
acetone, mg/kg max. n.r.b 300
acetonitrile, mg/kg max. 500 500
aldehydes, as acetaldehyde
mg/kg max. 50 50
iron, mg/kg max. 0.1 0.2
hydrocyanic acid, mg/kg max. 10 5
peroxides, as hydrogen
peroxide, mg/kg max. 0.3 1.0
water, % 2.5-4.5 2.5-4.5
inhibitor, MEHQc, mg/kg 35 - 50 35 - 50
acidity, as acetic acid,
mg/kg max. 35 20
pH, 5% aqueous solution 5.5-7.5 n.r.b
non-volatile matter,
mg/kg max. 100 100
refractive index at 25 °C 1.3880 - 1.3892 1.3880 - 1.3892
appearance clear & free clear & free
flowing flowing
------------------------------------------------------------------
a From: DuPont (1977) and Monsanto (1977a).
b n.r. = not reported.
c MEHQ - hydroquinone monomethyl ether (methylhydroquinone).
While many industrial hygiene personal monitoring
measurements have been carried out using these methods, over the
last 3-4 years an increasing number of "passive" samplers (gas
badges) (Silverstein, 1977) have been developed. The advantages
of these devices are that there are no moving parts to break
down, regular flow calibration is unnecessary, and no bulky,
expensive pumps are required.
Benson & Boyce (1981) and Benson et al. (1981) described a
passive dosimeter in which acrylonitrile was adsorbed on a porous
polymer (PorapakRN) contained in a removable element, and
determined by thermal desorption gas chromatography. It can be
used satisfactorily for determining acrylonitrile concentrations
in air under a range of atmospheric conditions, when working to
a control limit of 8.7 mg/m3 (4 ppm) but, at a concentration of
4.4 mg/m3 (2 ppm), a 40% error has been reported. These devices
are now considered to be as reliable as the more conventional
pump and tube methods (Rose & Perkins, 1982).
The head-space sampling method is useful for the
determination of residual acrylonitrile monomer in copolymers and
by-products, since it is more sensitive (detection limit 1.1
mg/m3 (0.5 ppm)) than direct injection (detection limit 21.7
mg/m3 (10 ppm)) (Steichen, 1976). It involves the equilibration
of a solid polymer with air in a closed vessel. Free monomer is
partitioned between the polymer phase and the "head-space" air,
and the monomer concentration in the head-space is then
determined (Steichen, 1976). Oomens (1980) gives a detection
limit for acrylonitrile of 0.02 mg/m3 (0.01 ppm) with the aid of
a similar method, applying the more sensitive and specific PND
detector. The procedure has been used for determining the
acrylonitrile monomer in copolymer solutions (McNeal & Breder,
1981), plastic packaging, and beverages (Gawell, 1979). Gawell's
method is suitable for determining acrylonitrile at
concentrations as low as 0.1 mg/kg, in plastics, and 0.005 mg/kg,
in beverages. The method has also been used for determining
acrylonitrile in food-simulating solvents (US FDA, 1977a) and,
with a detection limit of 0.5 mg/kg, in acrylonitrile-derived
copolymers (Steichen, 1976).
Continuously recording gas chromatographic methods have been
developed for monitoring atmospheric concentrations of
acrylonitrile.
Samples of water containing acrylonitrile can be acidified by
sulfuric acid to a pH < 4 and then kept at 0 °C until analysed
(Going et al., 1979).
2.2.2. Analytical methods for determinating acrylonitrile
Acrylonitrile can be determined using instrumental methods:
gas chromatography, possibly high-pressure liquid chromatography,
infrared spectroscopy, polarography, and chemical titrimetric and
colorimetric methods.
(a) Gas chromatography
This is the most frequently used method for acrylonitrile
determination, particularly in conjunction with the charcoal
sampling method. A number of gas chromatographic procedures have
been developed for different types of samples. Until recently,
almost all involved flame ionization detection, but attention is
now being paid to thermoionic nitrogen-selective detectors
(Shevchik, 1976) in the determination of acrylonitrile (e.g., US
FDA, 1977a; Gawell, 1979; McNeal & Breder, 1981).
Various column packings have been evaluated for the
determination of acrylonitrile by gas chromatography, e.g., in
the air (Parsons & Mitzner, 1975; Russell, 1975) (Table 3).
Porous polymer column packings have the advantage of resolving
acrylonitrile from methanol (frequently used to desorb
acrylonitrile from charcoal) and of being useful for direct
injection of aqueous acrylonitrile samples.
Examples of gas chromatographic methods for determining
acrylonitrile in a variety of products and samples containing
acrylonitrile are given in Table 4, together with the detection
limits.
Borg-Warner Chemicals (1977) developed a continuous-recording
gas chromatograph that reportedly detects acrylonitrile below 1.1
mg/m3 (0.5 ppm). A portable gas chromatograph for the
determination of acrylonitrile in air was developed by Vistron
(personal communication, 1978) and a direct injection gas
chromatograph for acrylonitrile determinations was tested by
Union Carbide Corporation (1977); preliminary results indicate a
detection limit below 2.2 mg/m3 (1 ppm).
(b) High-pressure liquid chromatography
A high-pressure liquid chromatograph method has been
developed for the determination of residual acrylonitrile monomer
in acrylic polymer and fibre (US Consumer Product Safety
Commission, 1978). The acrylic polymer or fibre is heated above
its glass transition temperature and refluxed continuously under
water. The extract is distilled and analysed. No interference
from contaminants has been noted.
(c) Infrared spectroscopy
Direct determination of acrylonitrile in air by IR
spectroscopy, using wavelength 10.49 µm, 20 °C and 760 mm Hg, and
a 250 cm gas cell, has been reported to have a detection limit of
about 0.5 ppm (v/v). The equipment is expensive, requires skill
to use, and is sensitive to physical damage. A portable IR
analyser for "on-the-spot" detection of acrylonitrile in air,
with a detection limit of 0.4 mg/m3 (0.2 ppm), has been
recommended by Jacobs & Syrjala (1978).
Table 3. Gas chromatographic conditions for acrylonitrile determination
--------------------------------------------------------------------------------------------------------
Packing Conditions Comments Reference
--------------------------------------------------------------------------------------------------------
Tenax 80 °C, 15 cc/min N2, -, Used by American Cyanamid
60 x 0.3 cm, Teflon for water analysis
0.4% Carbowax 1500 on 100 °C, 30 cc/min He, -, Head space analysis of Steichen (1976)
Carbopax A 80 x 0.3 cm, stainless steel residual monomer
Porapak Q, 50/80 mesh 155 °C, 50 cc/min N2, -, NIOSH method for acrylo- NIOSH (1976)
120 x 0.6 cm stainless steel nitrile in air
Porapak Q, 50/80 mesh 160 °C, 30 cc/min N2, Poor resolution from Barrett (1974)
3.2 min, 150 x 0.3 cm methanol
stainless steel
Porapak N, 50/80 mesh 170 °C, 40 cc/min N2, Resolved from methanol Barrett (1974)
10.5 min, 270 x 0.3 cm
stainless steel
Chromosorb 101, 50/60 or 110 °C to 200 °C at ASTM approved method for ASTM (1981)
porous styrene divinyl 10 °C/min, 25 ml/min He, nitriles in water
benzene polymer 240 x 0.3 cm stainless
steel
Porapak Q, 50/80 mesh 156 °C, 50 cc/min He, Used with a trapping Bellar & Sigsby
11.8 min, 360 x 0.3 cm column for combustion (1980)
stainless steel effluents
10% SP - 1000, 60/80 150 °C, 45 cm/min Acrylonitrile plus various Marano et al. (1978)
mesh supelcopore organic vapours
--------------------------------------------------------------------------------------------------------
a Column temperature, carrier gas and flow rate, retention time, column parameters.
Table 4. Determination of acrylonitrile in different acrylonitrile-containing
samples and products
-------------------------------------------------------------------------------
Sample source Detection limit Reference
-------------------------------------------------------------------------------
water solution 10 mg/kg Ramstad & Nicholson (1982)
polyacrylonitrile 10 - 100 mg/kg Reichle & Tengler (1968)
vinylidene chloride- 10 mg/kg UK Ministry of Agriculture,
acrylonitrile coated film Fisheries & Food (1982)
food samples 0.01 - 0.02 mg/kg UK Ministry of Agriculture,
Fisheries & Food (1982)
acrylic co-polymers 0.5 mg/kg Steichen (1976)
70 mg/kg McNeal & Breder (1981)
carbonated beverage (simulated) 1 mg/kg McNeal & Breder (1982)
fumigant residue in cereals & 0.1 mg/kg Heuser & Scudmore (1969)
other foods
air of acrylonitrile plants n.s. Cincolella et al. (1981)
acetone extract of styrene- 1 mg/kg US Consumer Product Safety
acrylonitrile resins Commission (1978)
-------------------------------------------------------------------------------
n.s. = not stated
(d) Polarography
A polarographic method for the determination of acrylonitrile
was first reported by Bird & Hale (1952). Berck (1960) used the
method of Daues & Hamner (1957) to determine acrylonitrile
residues in walnuts. Aqueous extracts of styrene-acrylonitrile
copolymer (Petrova et al., 1972), the volatile fractions of
styrene copolymer (Uhde & Koehler, 1967), and industrial waste
water (Ponomarev et al., 1974) have also been analysed using
polarography. A method developed by Rogaczewska (1964) had a
sensitivity of 10 mg/litre and 40 mg/m3 for the determination of
acrylonitrile in solution and in air, respectively.
(e) Colorimetric methods
In one method, the acrylonitrile-containing sample is
hydrolysed by a strong base to ammonia, which is determined by
the Nessler reagent (Rogaczewska, 1965; Aarato & Bittera, 1972).
The detection limit of this method is about 6 mg/m3 (3 ppm) in
air. A modification using hypochlorite and sodium salicylate has
a detection limit of 0.5 mg/m3 (Rogaczewska, 1976).
A modified hydrolytic method using hydrogen peroxide under
acidic conditions has been developed for the determination of
acrylonitrile in air (American Industrial Hygiene Association,
1970; Maddock et al., 1977). The sensitivity is in the range of
20-300 µg/ml of absorbing solution.
Another colorimetric method is based on the formation of
cyanogen bromide under the influence of UVR and the production of
a pink colour by coupling the cyanogen bromide with benzidine in
pyridine solution. Using this method, Kanai & Hashimoto (1965)
determined acrylonitrile in the expired air, blood, and urine of
exposed animals. This method has been further used for the
determination of acrylonitrile in air (Krynska, 1970; Tada, 1971;
Russkih, 1972, 1973) with a detection limit of 0.4 - 0.5 mg/m3,
and in food (Kroeller, 1970) and waste water (Ghersin et al.,
1969) with a detection limit of 2 mg/1itre. When the sample
contains both acrylonitrile and cyanide, the cyanide should be
removed before analysis (Aldridge, 1944; Bruce et al., 1955;
Kanai & Hashimoto, 1965).
(f) Titrimetric methods
A titrimetric method based on the cyanoethylation of a
sulfhydryl compound (lauryl mercaptan), by acrylonitrile, has
been described (Haslam & Newlands, 1955). An excess of the thiol
is added to the acrylonitrile sample and, after the reaction, it
is determined by iodometric or amperometric titration or by
Ellman's reagent. Although this method is specific, it is
neither rapid nor sensitive enough.
A titrimetric method for determining acrylonitrile, developed
by Terent'ev & Obtemperanskaya (1956), consists of the release of
sodium hydroxide by the reaction of acrylonitrile with sodium
sulfite. A paper-strip modification of this method has recently
been reported by Rajendran & Muthu (1981). It is used for the
detection of acrylonitrile in air and fumigated foodstuffs.
(g) Other analytical methods
Other methods are not frequently used. The
spectrophotometric method of Hall & Stevens (1977), in which
formation of a pyridine-acrylonitrile complex is determined at
435.4 nm, suffers from interference from cyanide, which must be
separated out of the solution.
2.2.2.1. Determination of acrylonitrile and its metabolites in
biological materials
(a) Acrylonitrile in urine
Sato et al. (1975) have modified the method of Aldridge
(1944); acrylonitrile in urine is separated by azeotropic
distillation and then determined by gas chromatography. The
detection limit is 5 µg/litre.
More recently, Houthuijs et al. (1982) developed a method
using head-space chromatography, which has a detection limit of 2
µg/litre. Two millilitre aliquots of urine are equilibrated at
90 °C in 25 ml vials for 3-5 h; the vapour phase is injected
automatically in a gas chromatograph, with a 15% carbowax column
and a phosphor-nitrogen detector.
(b) Acrylonitrile metabolites in urine
(i) Acrylonitrile-derived mercapturic acids
A gas-chromatographic method has been developed by Draminski
& Trojanowska (in press) for the determination of 2-cyanoethyl-
mercapturic acid in the urine of workers exposed to acrylonitrile.
The mercapturic acid is extracted from urine and derivatized by
diazomethane to the methyl ester. The precision of this method is
10% for 2-cyanoethyl-mercapturic acid in the concentration range
of 50-350 mg/litre of urine.
A general procedure for determining the total amount of
mercapturic acids (more generally the total amount of thioethers)
in urine has been described by Seutter-Berlage et al. (1977,
1978) and modified by Van Doorn et al. (1979) and Buffoni et al.
(1982). Deproteinized urine is hydrolysed with sodium hydroxide
for 50 min at 100 °C; this converts the mercapturic acids (and
generally all thioethers) to the corresponding thiols. After
cooling and acidification, SH-groups are assayed by the method of
Ellman (1959).
(ii) Thiocyanate levels
The colorimetric method (the formation of a coloured complex
of thiocyanate with ferric ion) was developed in 1943 (Lawton et
al., 1943).
Very recently, Imanari et al. (1982) applied the high-
performance liquid chromatographic technique, using a strong base
ion-exchanger column for such determinations. The method has
been shown to differentiate well between the urinary thiocyanate
levels found in smokers and non-smokers.
3. SOURCES OF INDUSTRIAL AND ENVIRONMENTAL EXPOSURE TO ACRYLONITRILE
3.1. Natural Occurrence
Acrylonitrile does not occur as a natural product.
3.2. Industrial Technology, Production Data and Projection
Current production is based on catalytic ammoxidation of
propylene in the vapour phase (Idol, 1974):
catalyst
2CH2 = CH-CH3 + 2 NH3 + 3 O2 -------> 2 CH2 = CH-CN + 6 H2O
Bismuth phosphomolybdate is the most frequently used catalyst.
The chief by-products are acetonitrile and hydrogen cyanide.
Processes previously used in the production of acrylonitrile
were: (i) the catalytic addition of hydrogen cyanide to
acetylene; (ii) the catalytic dehydration of ethylene to
cyanohydrin; and (iii) the catalytic reaction of propylene with
nitric oxide. These processes are no longer used by the major
manufacturers in the world.
In 1976, the known production of acrylonitrile was about 2.4
million tonnes (IARC, 1979). US manufacturers produced 0.69
million tonnes, Western European manufacturers, 0.92 million
tonnes, and Japanese manufacturers, 0.63 million tonnes.
Production figures for East European countries and the USSR are
not available.
The average annual growth of acrylonitrile production was
about 11% during 1965-1975 (Anonymous, 1977). While further
growth was expected during the early 1980s, because of increased
demands for polyacrylamide in tertiary oil recovery (Pujado et
al., 1977), this did not occur owing to a general recession in
world trade. The West European figure for 1981 is of the order
of 800 000 tonnes (Personal communication), approximately 15%
less than in 1976.
3.3. Use Patterns
The use pattern for acrylonitrile and its products in the USA
in 1976 and Western Europe in 1977 are presented in Table 5
(IARC, 1979).
In a mixture with carbon tetrachloride, acrylonitrile has
also been used as a fumigant for tobacco (Berg, ed., 1977) and
for flour milling and bakery equipment.
Table 5. The use patterns of acrylonitrile and its products in the
USA (1976) and Western Europe (1977)a
-------------------------------------------------------------------------
Product % of % of product production
acrylonitrile
production
-------------------------------------------------------------------------
USA W. Europe
acrylic and 48 68 82 - clothing and home
modacrylic fibres furnishings
18 - export
acrylonitrile- 21 15 88 - pipe fittings, automotive
butadiene-styrene and vehicle components, etc.
acrylonitrile-styrene 12 - automobile instrument
resins panels, household items
etc.
adiponitrile 12 -- mainly hexamethylenediamine
other products 19 17 21 - nitrile elastomers
21 - acrylamide
16 - barrier resins
42 - polyether polymer
polyols, fatty diamines,
etc.
-------------------------------------------------------------------------
a From: IARC (1979).
Pesticides containing acrylonitrile have been withdrawn by
the manufacturers. Acrylonitrile polymers and copolymers are
components of products intended for use in contact with food,
e.g., (i) vinyl resin coatings; (ii) adhesives; (iii)
cellophane; (iv) paper and paperboard components (limited); (v)
polyolefin films; (vi) elastomers - for repeated use; and (vii)
rigid, semi-rigid, and modified acrylic and vinyl plastics. In
the USA, the amount of acrylic component may not exceed that
which is reasonably required to produce the intended effect (US
FDA, 1977b).
The acrylonitrile content of containers fabricated from
acrylonitrile copolymers and the possible migration of
acrylonitrile into foods and beverages have been reviewed (US
FDA, 1977a). The use of copolymers of acrylonitrile for making
beverage bottles was banned in the USA in September, 1977.
The Canada Food and Drugs Act and Regulations (1982) prohibit
the sale of any food in packaging containing acrylonitrile, such
that the compound may pass into the food.
Table 6 shows the levels of residual acrylonitrile in several
polymers, some acrylonitrile derivatives, and products fumigated
with acrylonitrile (US Consumer Product Safety Commission, 1978).
Table 6. Levels of residual acrylonitrile found in various
products
---------------------------------------------------------------
Product Acrylonitrile
content
---------------------------------------------------------------
acrylic and modacrylic fibres 1 mg/kga
acrylonitrile-butadiene-styrene resins 30-50 mg/kga
styrene-acrylonitrile resins 15 mg/kga
nitrile rubber and latex material 0-750 mg/kga
acrylamide 25-50 mg/kga
polyether polymer polyols 100-300 mg/kga
shelled walnuts 0-8.5 mg/kgb
US cigarettes (non-filtered) 1-2 mg/100 cigarettesc
---------------------------------------------------------------
a From: US Consumer Product Safety Commission (1978).
b 38 days after fumigation with a mixture of acrylonitrile
and carbon tetrachloride (Berck, 1960).
c From: Buérin et al. (1974); Wynder & Hoffmann (1967).
The total emissions from acrylonitrile plants in the USA, in
1974, have been estimated to be about 2.2% of the total
production (Table 7) (Patterson et al., 1976). More recent
estimates (Suta, personal communication, 1982), following the
introduction of stricter emission controls, indicate an overall
reduction in emissions and a change in pattern (for 1981, 800
tonnes for acrylonitrile production and 3000 tonnes for end-
product manufacture).
3.4. Disposal of Wastes
Acrylonitrile may also enter the environment during storage,
transport, transfer, and end-use. A detailed study on the entry
of acrylonitrile into the environment was carried out by the
Midwest Research Institute for the EPA (Going et al., 1979).
Air, water, and soil were sampled at, and near to, acrylonitrile
and acrylamide production plants and acrylonitrile-derived resin,
fibre, and elastomer production plants.
During acrylonitrile production, the fo1lowing wastes are
produced: gaseous wastes; liquid wastes (waste water column
bottoms, acetonitrile column bottoms, heavy ends, crude
acetonitrile, hydrogen cyanide); and solid wastes (catalyst fines
and organic polymers). Three types of on-site disposal methods
have been described by Hughes & Horn (1977): (a) flare; (b)
thermal incineration; and (c) deep-well pond and deep-well
injection.
Table 7. Acrylonitrile emissions from plants
in the USA in 1974a
-----------------------------------------------
Source Emission (tonnes)
-----------------------------------------------
acrylonitrile production 6400
end-product manufacture 5900
bulk storage 1800
total emission 14 100
-----------------------------------------------
a From: Patterson et al. (1976).
Much liquid waste from acrylonitrile-manufacturing plants is
discharged directly into deep wells, after pre-treatment using
alkaline hydrolysis, the biodegradable effluent being disposed of
in publicly-owned treatment works. In some cases, organic wastes
are incinerated (Lowenbach et al., 1978).
Deep-well injection is no longer considered a viable method
in the USA; to control the drilling of new wells, an industrial
discharger must re-apply for a permit (US EPA, 1977).
Lowenbach et al. (1978) extensively reviewed the alternative
biological, chemical, and physical methods of treating waste
waters from acrylonitrile manufacture, but a detailed discussion
of these is not within the scope of this report.
3.5. Accidental Release
Acrylonitrile may be released accidentally into the
environment. Its half-life in air is estimated to be 9-10 h
(section 2.1.2). In water, the half-life, as estimated by the
BOD test, is 5-7 days. Although these data would indicate that
small spillages would not present a problem, initial high levels
of acrylonitrile may have severe local effects. No
bioaccumulation or food chain concentration potential has been
noted (US Dept of Transportation, 1974), but it was observed that
the concentration of acylonitrile in the ground water increased
when it rained several months after an accidental spillage
occurred. The persistence of acrylonitrile in the water of wells
located within 30 m of a spill of 91 000 litres of acrylonitrile
from a tank car was followed for about 1 year (Miller & Villaume,
1978). No attempt was made to contain or clean up the spill for
108 days and water from 5 wells showed acrylonitrile
concentrations ranging from 46 up to 3520 mg/litre during this
time. On day 108, contaminated soil was removed, but levels of
acrylonitrile actually increased in some wells. Levels decreased
after about 170 days, when contaminated ground water was pumped
away; a sample of this ground water contained an acrylonitrile
concentration of 144 mg/litre. It is possible that the high
concentration of acrylonitrile produced by the spill was lethal
to bacteria, precluding biological degradation. However, no
quantitative measurements of soil or water organisms were made.
3.6. Environmental Persistence
Acrylonitrile is readily degraded by acclimated anaerobic
microorganisms (Mills & Stack, 1955). Aerobic degradation with
activated sludge is complete in 20 days (Miller & Villaume, 1978;
Freeman et al., 1981). The residual level after aerated
activated sludge treatment was below 0.1 mg/kg. Acrylonitrile has
been shown to inhibit anaerobic organisms (For fish toxicity, see
Table 11).
4. INDUSTRIAL AND ENVIRONMENTAL SOURCES AND LEVELS OF EXPOSURE
4.1. Exposure of the General Population
4.1.1. Air
The possibility of exposure to acrylonitrile-contaminated air
is limited to residents near industrial production and processing
sites. In the vicinity of 2 plants producing acrylonitrile, high
concentrations of the monomer, ranging from 390 to 608 mg/m3
(180-280 ppm) were found near the exhausts of both ships and
storage tanks (Sato et al., 1979). Going et al. (1979) determined
acrylonitrile concentrations in samples of air, soil, water, and
sediments around 11 industrial sites. The concentrations of
acrylonitrile in air varied from < 0.1 - 325 µg/m3 ; the highest
levels were found at an acrylonitrile-, butadiene-styrene resin
plant and an acrylonitrile/acrylamide plant. The occurrence of
acrylonitrile was highly correlated with the wind patterns; the
highest levels were found downwind of the plant or at points
crosswind but close to the plant. The air also contained
xylenes, ethylbenzene, dichlorobenzenes, toluene,
trimethylbenzenes, and styrene.
4.1.2. Water
Acrylonitrile was present in effluent discharged from
chemical and latex manufacturing plants (Shackelford & Keith,
1976), and was detected at 0.1 g/litre in effluent discharged
from an acrylic fibre-manufacturing plant in the USA (Europ-Cost,
1976). Near 11 industrial sites (Going et al., 1979), the
highest acrylonitrile levels in water were 3.5 and 4.3 mg/litre
from an acrylic/modacrylic fibre plant and a nitrile elastomer
plant, respectively. There was no apparent correlation between
air levels and water concentrations. No acrylonitrile was found
in the soil and sediments. Water samples from some plants also
contained propionitrile.
4.1.3. Food
Contamination of food from polymer packaging material
containing free acrylonitrile has been reported. Following a
study on the migration of acrylonitrile from ABS and AS resins,
Tatsuno et al. (1979) concluded that after long-term preservation
of food in ABS and AS resins the concentration of acrylonitrile
in food may rise to 0.05 mg/kg. Further studies on food-
simulating solvents showed that migration of acrylonitrile
occurred from ABS and AS resins into 4% acetic acid, 20% ethanol,
heptane, and olive oil; it was concluded that resins containing
acrylonitrile levels of more than 10 mg/kg should not be used for
packaging foods containing alcohol (Tatsuno et al., 1980).
Nitrile resins made from copolymers of acrylonitrile and other
monomers (e.g., methyl acrylate) are no longer used in the USA to
make beverage bottles (US FDA, 1977a). In a study performed in
Sweden, the amount of acrylonitrile monomer found in nitrile
resin bottles was 2-5 mg/kg. The amount in the beverage was
generally 0.002 - 0.003 mg/kg, but two samples contained as much
as 0.009 mg/kg (Vaz, 1981, personal communication). A government
survey of the acrylonitrile content of food suggested that the
average intake of acrylonitrile in the United Kingdom was likely
to be less than 0.3 µg/person per day (United Kingdom Ministry of
Agriculture, Fisheries & Food, 1982).
An acrylonitrile concentration of 0-19 mg/kg was detected in
dry food fumigated with acrylonitrile at a concentration of 10
g/m3 . The study was carried out using radioactive acrylonitrile
and provided information that acrylonitrile levels in the stored
food decreased by 30-70% over a period of 2 months (Pfeilsticker
et al., 1977).
4.1.4. Other sources of exposure
Free acrylonitrile monomer has been found in commercial
acrylonitrile polymers at levels of less than 1 mg/kg (acrylic
and modacrylic fibres), 15 mg/kg (styrene-acrylonitrile resins),
30-50 mg/kg (ABS resins) and 0-750 mg/kg (nitrile rubbers and
latex materials) (US Consumer Product Safety Commission, 1978).
Another possible source of acrylonitrile environmental
exposure is accidental spillage during transport. The following
estimates have been made of the incidence of the accidental
release of acrylonitrile per year: during transport in barges -
0.0117; in trucks - 0.063; and by rail - 0.17 (Miller & Villaume,
1978). This means, for example, that during transport by rail,
one accident would occur approximately every 6 years.
4.2. Occupational Exposure
Up to 12 000 workers in the USA were thought to have come
into major contact with acrylonitrile during 1976 and possibly
some 125 000 workers were exposed, to some extent (Miller &
Villaume, 1978). It has also been estimated that as many as
400 000 may have had some contact with acrylonitrile in 1976.
The exposures reported in several countries are shown in Table 8.
The introduction of a lower exposure limit in several
countries is likely to have decreased the actual exposure to
acrylonitrile at the workplace.
As acrylonitrile vapour is twice as dense as air, spills
and leaks in enclosed buildings may lead to harmful
accumulations of vapour, especially in low-lying areas
(Baxter, 1979). The same author describes various
possibilities for preventing this, such as the use of double
mechanical seals, enclosed drainage systems, well-ventilated
sampling points, etc. Plant design should aim at complete
containment of acrylonitrile, both as a liquid and a vapour.
Table 8. Concentration of acrylonitrile in the air at work-places
-------------------------------------------------------------------------------------------
Operation Acrylonitrile in Reference
work-place air (mg/m3)
Average level Range
-------------------------------------------------------------------------------------------
Acrylonitrile production 5 - 0.5b - Zotova (1975a)
during loading (open air) 5 0.2 - 60 Cincolella et al. (1981)
near ACN tanks or pumps 45 4 - 125 Cincolella et al. (1981)
- 4.2 - 7.2 Gincheva et al. (1977)
Acrylic fibre production 3 - 20 Orusev et al. (1973)
- <11 Enikeeva et al. (1976)
- <11 Sakurai & Kusumota (1972)
- <45
4.6 - 31a,s <2.2 - >43 Sakurai et al. (1978)
0.2 - 9.1a,t -
polymerization 8 <4 - >20 Czajkowska et al. (1969)
<4 <1 - >10 Lodz Sanit. Inspec. Survey (1981)
25 2 - 103 Cincolella et al. (1981)
spinning 6 1.5 - 20 Czajkowska et al. (1969)
<4 <1 - >10 Lodz Sanit. Inspec. Survey (1981)
9.5 Sakurai et al. (1978)
Thermosetting plastic plant 1.4 Scupakas (1968)
Rubber footwear plant 1 - 11 Volkova & Bagdinov (1969)
Unspecified chemical 0.6 - 6 Babanov et al. (1959)
conversions
Production of acryl- 4a,s,t 0 - 22 Iwasaki et al. (1980)
butadiene-styrene resin
(A.B.S)
polymerization 30 0 - 200 Cincolella et al. (1981)
Production of nitrile rubber
rubber - polymerization 4 1 - 27 Cincolella et al. (1981)
reactor cleaning 36t 5 - 54 Cincolella et al. (1981)
Acrylic dispersions 78 9 - 600 Cincolella et al. (1981)
(Latex production
polymerization)
-------------------------------------------------------------------------------------------
a 2 or more factories evaluated.
b average levels over 5 years.
t time-weighted average concentration.
s spot.
A code of practice has recently been published for the safe
design, construction, and use of plants (CIA, 1978). Safe
handling, engineering, and work practices, controls, compliance
programmes, personal protective equipment, housekeeping, employee
information and training, signs and labels, etc. for work with
acrylonitrile have been described by the OSHA (1981).
Exposure to acrylonitrile may also occur through skin
contact. Acrylonitrile was shown to contaminate the skin of
workers, their clothing and tools, also the equipment, walls,
windows, handrails, handles, etc. in the workplace and was not
easy to remove. A protective paste of household soap, mineral
oil, glycerine, and china clay was said to reduce contamination
of the palms of the hands by 67% (Zotova, 1975a).
Acrylonitrile can penetrate clothing and leather shoes
(American Cyanamid, 1976). Dermal contact with liquid
acrylonitrile may cause local skin damage, severe dermatitis, and
systemic toxicity, and must therefore be prevented by high
standards of industrial hygiene.
4.3. Estimate of Human Exposure from All Environmental Media
The production and use of acrylonitrile at the workplace
provide the greatest potential for exposure. Airborne exposure
to acrylonitrile near industrial sites appears to pose the
highest potential risk for the general population; the potential
for exposure through water and food appears to be low by
comparison.
5. CHEMOBIOKINETICS AND METABOLISM
5.1. Absorption
5.1.1. Human studies
5.1.1.1. Uptake through inhalation
The retention of acrylonitrile in the respiratory tract in 3
volunteers exposed to a concentration of 20 mg/m3 for up to 4 h
was 46 ± 1.6% and did not change throughout the inhalation period
(Rogaczewska & Piotrowski, 1968).
5.1.1.2. Dermal absorption
Rogaczewska & Piotrowski (1968) applied liquid acrylonitrile
to the forearm skin of 4 human volunteers and estimated that the
average absorption rate was 0.6 mg/cm2 per h.
5.1.1.3. Uptake by other routes
No data avai1able.
5.1.2. Experimental animal studies
5.1.2.1. Uptake through inhalation
Young et al. (1977) determined the recovery of 14C
acrylonitrile in rats exposed to 11 or 220 mg/m3 (5 or 100 ppm)
for 6 h in a "nose only" inhalation chamber. In the first 9 days
following the start of inhalation, 82.2% of the radioactivity was
recovered from the urine, after the higher dose, and 68.5%, after
the lower dose, 3-4% occurred in the faeces; and 6% and 2.6%,
respectively, were expired as 14CO2.
5.1.2.2. Dermal absorption
Three rabbits breathing pure air while their skin (315-350
cm2 ) was exposed to an atmosphere containing an acrylonitrile
concentration of 1-4.2 g/m3, survived, whereas 3 other rabbits
breathing pure air with the skin exposed to 44-62 g/m3 died
within 2.5-4 h. Inhalation exposure to 0.58-0.67 g/m3 was fatal
for 3 rabbits within 2-3 h (Rogaczcwska, 1975). The author
interprets these data as suggesting that dermal absorption of
vapour is about 100 times less efficient than its pulmonary
absorption. The immersion of rabbit ear in liquid acrylonitrile
was fatal for the animal within a few hours (Rogaczewska, 1971).
Subcutaneous (sc) or intravenous (iv) administration of
14C-acrylonitrile at 0.5 mmole/kg body weight to rats resulted
in faster and greater elimination of radioactivity in the first 4
h than after oral administration (Gut et al., 1980).
5.1.2.3. Uptake by other routes
Young et al. (1977) calculated that after oral administration
of 0.1 mg or 10 mg of 14C-acrylonitrile per kg body weight, 85-
100% of acrylonitrile was absorbed in rats. The absorption rate
was lower in rats after oral administration than after sc or ip
administration (Nerudova et al., 1980a; Gut et al., 1981). After
ip administration, the blood concentration of acrylonitrile
reached a maximum in several minutes and then decreased rapidly
(Nerudova et al., 1980a; Gut et al., 1981). After ip and oral
administration of 1,2-14C acrylonitrile and acrylo14C-nitrile to
rats, 82-93% of the radioactivity was recovered from the urine
and some 3-7% exhaled unchanged in the breath in 24 h (Sapota,
1982).
5.2. Distribution and Toxicokinetics
5.2.1. Human studies
No data available.
5.2.2. Experimental animal studies
Acrylonitrile concentrations in blood and liver reach higher
levels after iv or ip administration than after oral
administration; concentrations rapidly decrease in blood (t0.5 =
19 min) and liver (t0.5 = 15 min after iv and 21 min after ip
administration) (Nerudova et al., 1980a; Gut et al., 1981). The
apparent t0.5 after oral administration is 61 min in blood and 70
min in liver, but this appears to be due to slow absorption
rather than to slow elimination. The area under the acrylonitrile
concentration/time curve for blood was higher than for liver
after oral, iv, or ip administration (Gut et al., 1981),
indicating rapid transformation of acrylonitrile by the liver.
Extrapolation of acrylonitrile blood levels after ip or iv
administration in rats to zero time indicated that the apparent
volume of distribution was unity, and that concentrations of free
acrylonitrile in the rest of the body were unlikely to be greater
than that in the blood (Nerudova et al., 1980a).
Young et al. (1977) followed the distribution of
radioactivity in rats after a single oral or iv dose of 14 C-
acrylonitrile. Radioactivity was found in the lung, liver,
kidney, stomach, intestines, skeletal muscle, blood, and other
organs and tissues, but high levels of radioactivity occurred in
erythrocytes, skin, and stomach regardless of the dose and route.
The high levels in the stomach wall after iv administration
support the observation of Nerudova et al. (1980a) that, after iv
administration, acrylonitrile is excreted into the stomach lumen.
After single intraperitoneal and oral administration to
rats of 1,2-14C acrylonitrile and acrylo14C-nitrile, most of
the 14C found in the tissues was associated with erythrocytes,
liver, and kidneys, lower levels being found in the lung and
brain. The 14C in the erythrocytes was still largely retained
48 h after administration. Significant differences in the
rates of 14C loss from tissues occurred with 1,2-14C
acrylonitrile and acrylo14C-nitrile given orally (Sapota &
Draminski, 1981; Sapota, 1982).
After oral administration to rats, up to a maximum of 94%
of 14C from 1-14C acrylonitrile in erythrocytes was found to
be covalently bound to cytoplasmic and membrane proteins, whereas
90% of the radioactivity from potassium cyanide in erythrocytes
was found in the haem fraction of haemoglobin (Farooqui & Ahmed,
1982).
After a single ip injection of 2,3-14C acrylonitrile in
male rats, radioactivity was generally highest in the blood,
intermediate in the spleen, liver, and kidney, and lower in other
tissues. The percentage of the dose remaining in the body after
9 days was estimated to be about 5% of the administered dose
(Hashimoto & Kimura, 1977).
A semi-quantitative study using whole-body autoradiography
(Sandberg & Slanina, 1980) confirmed that, after iv
administration to rats, acrylonitrile and/or its metabolites
accumulate in the blood, liver, kidney, stomach mucosa, adrenal
cortex, intestinal contents, and hair follicles of rats. After
oral administration to the monkey (Sandberg & Slanina, 1980),
high radioactivity levels were detected in the liver, kidney,
intestinal mucosa, adrenal cortex, and blood. As total
radioactivity was measured in the studies of Young et al. (1977),
Sandberg & Slanina (1980), and Sapota & Draminski (1981), it was
impossible to differentiate acrylonitrile from its metabolites or
from acrylonitrile bound covalently to proteins (Bolt et al.,
1978; Gut et al., 1981); thus, these studies are difficult to
interpret from the point of view of the chemobiokinetics of free
acrylonitrile.
Peter & Bolt (1981) found that 12 h after ip or iv
administration of 2,3-14C acrylonitrile, about half of the
radioactivity remaining in the tissues was irreversibly bound to
proteins. The rapid elimination of acrylonitrile mercapturic
acid after iv, ip, or sc administration (Gut et al., 1981a)
indicates that most of the acrylonitrile-derived radioactivity in
the distribution studies was associated with cyanoethylglutathione,
or subsequent intermediate metabolites including acrylonitrile
mercapturic acid.
Thus, it is impossible to determine conclusively from the
present data whether the relatively high levels of acrylonitrile-
14C radioactivity in the erythrocytes, kidney, spleen, liver,
adrenals, stomach walls, and skin are due to free acrylonitrile,
its metabolites, or cyanoethylated proteins. However, the
chemobiokinetics of free acrylonitrile in blood and liver (Nerudova
et al., 1981) suggest that its distribution is fairly uniform and
that higher levels of radioactivity in some organs and erythrocytes
are due to reaction products of acrylonitrile with soluble and
protein sulfhydryls.
Information on the subcellular distribution of 1,14C
acrylonitrile in rat can be found in Ahmed et al. (1982). Sato et
al. (1982) studied the distribution and accumulation of 2,3-14C
acrylonitrile in the rat. They observed a longer retention of
acrylonitrile in brain and muscle. The cytosol fractions of brain,
liver, and kidney showed a relatively high specific radioactivity.
The evidence, available at present, on the distribution of
acrylonitrile in the body, and on tissue damage following exposure,
does not indicate increased accumulation in any particular tissue
or organ, except erythrocytes, and there is no indication from
animal studies of tissue accumulation following long-term exposure.
5.3. Biotransformation and Elimination
Levels of acrylonitrile metabolites in blood and their
relationship to atmospheric acrylonitrile concentrations or to the
dose administered are usually considered together, in studies on
the relationship between the dose or concentration of acrylonitrile
and the elimination of metabolites in urine. They will therefore be
considered together in the following section.
5.3.1. Human studies
Acrylonitrile is metabolized partly to thiocyanate. Blood
thiocyanate levels of volunteers exposed to acrylonitrile
concentrations below 45 mg/m3 (22 ppm) for 30 min returned to normal
after 24 h, while elevated levels were still present 12 h after
exposure to 110 mg/m3 (50 ppm) for 30 min (Wilson & McCormick,
1949).
Draminski & Trojanowska (in press) reported that at airborne
acrylonitrile concentrations of between 3 and 10 mg/m3,
concentrations of S-(2-cyanoethyl) mercapturic acid in the urine of
13 workers exposed to acrylonitrile, fell in the range of 50-200
mg/litre.
5.3.2. Experimental animal studies
Acrylonitrile is partly metabolized to cyanide, which is then
transformed by rhodanese (EC 2.8.1.1) to thiocyanate and eliminated
in urine (Dudley & Neal, 1942; Brieger et al., 1952; Ghiringhelli,
1954). However, the fate of the major portion of administered
acrylonitrile was not clear until recently. Recent studies have
shown that the major urinary metabolites in rats, hamsters, guinea-
pigs, rabbits, and dogs are mercapturic acids resulting from the
glutathione-S-transferase(s) (EC 2.5.1.18) -catalysed conjugation
of acrylonitrile or glycidonitrile with glutathione (section
5.3.2.2). At present, at least 10 acrylonitrile metabolites have
been isolated and/or identified in animal urine.
The oxidative pathway leads to the liberation of cyanide via an
epoxide (glycidonitrile) and cyanohydrin (Kopecky et al., 1980a,b).
Cyanohydrin spontaneously decomposes to cyanide and glycolaldehyde
which, together with 2-cyanoethanol, cyanoacetic acid, and acetic
acid, have been found as in vitro metabolites of acrylonitrile
(Duverger-van Bogaert et al., 1981). Only 2-cyanoethanol and
cyanoacetic acid were detected in the urine of rats administered
acrylonitri1e intraperitoneally (Lambotte-Vandepaer et al., 1981a).
The proposed routes of the oxidative pathway are shown
diagrammatically in Fig. 1; some of the biotransformation steps are
speculative.
The existence of a glucuronoconjugate of acrylonitrile was
reported in the urine of rats after oral administration of
acrylonitrile (Hoffman et al., 1976). Two metabolites of
acrylonitrile ( S-[2-cyanoethyl] cysteine and S-[2-cyanoethyl]
mercapturic acid) were identified by Dahm (1977) in rats given
radiolabelled acrylonitrile, but he was unable to identify a
third metabolite, as it was unstable. Young et al. (1977)
found that acrylamide was not a metabolite as had been
suggested by Hashimoto & Kanai (1965). The same authors also
identified carbon dioxide as a metabolite in rats, but they
were unable to detect significant quantities of free
acrylonitrile or cyanide in the urine of exposed rats, though
Hashimoto & Kanai (1965) estimated that 15% of an iv dose of
acrylonitrile was eliminated unchanged in the urine and
expired air of the rabbit.
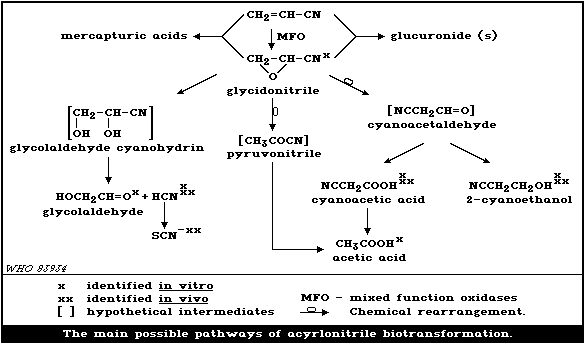 5.3.2.1. The oxidative pathway of acrylonitrile metabolism
The oxidative pathway of acrylonitrile biotransformation
includes a number of consecutive enzyme-catalyzed or spontaneous
reactions. The first step, oxidation of acrylonitrile to
glycidonitrile, is catalyzed by hepatic microsomal mono-oxygenases
(Abreu & Ahmed 1980; Kopecky et al., 1980a,b; Guengerich et al.,
1981; Ahmed & Abreu, 1982). Glycidonitrile is a reactive
intermediate, and a number of its metabolites have been recorded;
in in vitro experiments it is transformed by epoxide hydrolase (EC
3.3.2.3) to glycolaldehyde cyanohydrin, which decomposes
spontaneously to hydrocyanic acid (cyanide) and glycoealdehyde
(Kopecky et al., 1979, 1980a,b; Abreu & Ahmed, 1980; Duverger-van
Bogaert, 1981a). The yield of cyanide in the in vitro experiments
depends on the techniques used (Nerudova et al., 1980b). Besides
forming conjugation products with glutathione (section 5.3.2.2),
glycidonitrile rearranges to cyanoacetaldehyde, which is further
reduced to 2-cyanoethanol or oxidized to cyanoacetic acid. Acetic
acid is also present (Duverger-van Bogaert, 1981).
The results of animal studies have shown that cyanide formed in
vivo is subsequently converted by rhodanese (EC 2.8.1.1) to
thiocyanate and eliminated in urine (e.g., Dudley & Neal, 1942;
Brieger et al., 1952; Ahmed & Patel, 1981). Thiocyanate has been
directly measured in the urine of various animals after
acrylonitrile administration (Lawton et al., 1943; Mallette, 1943;
Czajkowska, 1971; Efremov, 1976b). Rats administered acrylonitrile
at 60 mg/kg body weight, excreted thiocyanate in the urine at a
constant rate of 0.53 mg/h with an excretion half period of 13 h
(Czajkowska, 1971). Sulfhydryl compounds (cysteine, BAL, and
Unithiol) increase the activity of rhodanese in the conversion of
cyanide to thiocyanate in vitro, as well as in vivo (e.g.,
Frankenberg, 1980). A similar increase with acrylonitrile has not
been convincingly demonstrated (Gut et al., 1975), perhaps because
of the inhibiting properties of acrylonitrile on rhodanese.
5.3.2.2. Mercapturic acids formed in acrylonitrile biotransformation
Cyanoethylation of naturally-occurring sulfhydryl compounds
plays an important role in acrylonitrile metabolism. Acrylonitrile
forms stable conjugates with L-cysteine and L-glutathione in vitro
(Hashimoto & Kanai, 1965; Gut et al., 1975) and a portion of
absorbed acrylonitrile is thus prevented from being metabolized to
cyanide. Depressed levels of sulfhydryl compounds have been
reported following acrylonitrile administration (e.g., Wisniewska-
Knypl et al., 1970; Hashimoto & Kanai, 1972; Vainio & Mäkinen,
1977; Dinu & Klein, 1976; Szabo et al., 1977). The spontaneous
conjugation of glutathione with acrylonitrile or glycidonitrile
proceeds very slowly; glycidonitrile forms S-(2-cyano-2-
hydroxyethyl)-L-glutathione and S-(1-cyano-2-hydroxyethyl)-L-
glutathione in the ratio of about 1:1. In the enzyme-catalysed
conjugation this ratio shifts to about 3:1 (Holechek & Kopecky,
1981). These authors demonstrated that no cyanide was released
from the conjugation product of acrylonitrile with GSH, while
cyanide was released from the conjugation product of glycidonitrile
with GSH. This study confirmed the findings of Boyland & Chasseaud
(1967, 1968) concerning the participation of glutathione- S-
alkylene transferase(s) (EC 2.5.2.18) in the cyanoethylation
reaction of glutathione. Since glutathione conjugates are
precursors of mercapturic acids, the occurrence of mercapturic
acids derived from acrylonitrile and glycidonitrile may be expected
in the urine of animals exposed to acrylonitrile.
The major metabolite of acrylonitrile in the rat, rabbit, and
other animals was found to be 2-cyanoethylmercapturic acid (Dahm,
1977; Wright, 1977; Ahmed & Patel, 1979; Kopecky et al., 1979,
1980a,b,c, 1981; Langvardt et al., 1980; Sapota & Draminski, 1981;
Sapota & Chmielnicka, 1981; Van Bladeren et al., 1981; Ghanayem &
Ahmed, 1982). While 2-cyanoethylmer-capturic acid was the sole
mercapturic acid identified in the urine of rats after iv
administration of acrylonitrile, a second mercapturic acid of
unestablished structure was also excreted after oral
administration. Langvardt et al. (1980), using 1-14C- or 2,3-14C-
acrylonitrile, found seven radioactive metabolites in rat urine.
The 3 major metabolites included thiocyanate and 2-
cyanoethylmercapturic acid. The third was tentatively identified
as 4-acetyl-5-cyanotetra-hydro-1,4-2 H-thiazine-3-carboxylic acid.
The 4 remaining metabolites represented at least one third of the
total activity excreted; their chemical structures are not known,
but none contained the -CN group of acrylonitrile. Different
results were reported by van Bladeren et al. (1981). In common
with Kopecky & Langvardt and colleagues, they isolated
2-cyanoethylmercapturic acid from the urine of orally-dosed rats;
however, 2-hydroxyethylmercapturic acid was also excreted. It is
suggested that this second mercapturic acid may be formed via one
of the conjugates of glutathione with glycidonitrile, S-(2-cyano-
2-hydroxyethyl)-L-glutathione. The amount of mercapturic acids
excreted relative to the dose was approximately constant up to a
dose of acrylonitrile of 26.5 mg/kg body weight. At higher doses,
the amount of mercapturic acids excreted remained constant. These
authors and Wright (1977) suggested that this might be a
consequence of the depletion of available glutathion at the higher
dose levels. It seems likely that, at high exposure levels, the
preferred metabolic pathway (conjugation of glutathione with
acrylonitrile or its metabolite) is overloaded, and another unknown
metabolic pathway takes over. After an oral dose to rats of 1-14C
acrylonitrile, 4 metabolites were found in the bile, 2 major
metabolites being GSH conjugates of acrylonitrile (Ghanayem &
Ahmed, 1982).
The report by Dahm (1977) that rats administered acrylonitrile
excreted S-(2-cyanoethyl)-L-cysteine has not been confirmed by any
of the authors who have examined the glutathione conjugation
pathway of acrylonitrile biotransformation. Fig. 2 illustrates
the proposed routes of mercaptide formation from acrylonitrile.
5.3.2.1. The oxidative pathway of acrylonitrile metabolism
The oxidative pathway of acrylonitrile biotransformation
includes a number of consecutive enzyme-catalyzed or spontaneous
reactions. The first step, oxidation of acrylonitrile to
glycidonitrile, is catalyzed by hepatic microsomal mono-oxygenases
(Abreu & Ahmed 1980; Kopecky et al., 1980a,b; Guengerich et al.,
1981; Ahmed & Abreu, 1982). Glycidonitrile is a reactive
intermediate, and a number of its metabolites have been recorded;
in in vitro experiments it is transformed by epoxide hydrolase (EC
3.3.2.3) to glycolaldehyde cyanohydrin, which decomposes
spontaneously to hydrocyanic acid (cyanide) and glycoealdehyde
(Kopecky et al., 1979, 1980a,b; Abreu & Ahmed, 1980; Duverger-van
Bogaert, 1981a). The yield of cyanide in the in vitro experiments
depends on the techniques used (Nerudova et al., 1980b). Besides
forming conjugation products with glutathione (section 5.3.2.2),
glycidonitrile rearranges to cyanoacetaldehyde, which is further
reduced to 2-cyanoethanol or oxidized to cyanoacetic acid. Acetic
acid is also present (Duverger-van Bogaert, 1981).
The results of animal studies have shown that cyanide formed in
vivo is subsequently converted by rhodanese (EC 2.8.1.1) to
thiocyanate and eliminated in urine (e.g., Dudley & Neal, 1942;
Brieger et al., 1952; Ahmed & Patel, 1981). Thiocyanate has been
directly measured in the urine of various animals after
acrylonitrile administration (Lawton et al., 1943; Mallette, 1943;
Czajkowska, 1971; Efremov, 1976b). Rats administered acrylonitrile
at 60 mg/kg body weight, excreted thiocyanate in the urine at a
constant rate of 0.53 mg/h with an excretion half period of 13 h
(Czajkowska, 1971). Sulfhydryl compounds (cysteine, BAL, and
Unithiol) increase the activity of rhodanese in the conversion of
cyanide to thiocyanate in vitro, as well as in vivo (e.g.,
Frankenberg, 1980). A similar increase with acrylonitrile has not
been convincingly demonstrated (Gut et al., 1975), perhaps because
of the inhibiting properties of acrylonitrile on rhodanese.
5.3.2.2. Mercapturic acids formed in acrylonitrile biotransformation
Cyanoethylation of naturally-occurring sulfhydryl compounds
plays an important role in acrylonitrile metabolism. Acrylonitrile
forms stable conjugates with L-cysteine and L-glutathione in vitro
(Hashimoto & Kanai, 1965; Gut et al., 1975) and a portion of
absorbed acrylonitrile is thus prevented from being metabolized to
cyanide. Depressed levels of sulfhydryl compounds have been
reported following acrylonitrile administration (e.g., Wisniewska-
Knypl et al., 1970; Hashimoto & Kanai, 1972; Vainio & Mäkinen,
1977; Dinu & Klein, 1976; Szabo et al., 1977). The spontaneous
conjugation of glutathione with acrylonitrile or glycidonitrile
proceeds very slowly; glycidonitrile forms S-(2-cyano-2-
hydroxyethyl)-L-glutathione and S-(1-cyano-2-hydroxyethyl)-L-
glutathione in the ratio of about 1:1. In the enzyme-catalysed
conjugation this ratio shifts to about 3:1 (Holechek & Kopecky,
1981). These authors demonstrated that no cyanide was released
from the conjugation product of acrylonitrile with GSH, while
cyanide was released from the conjugation product of glycidonitrile
with GSH. This study confirmed the findings of Boyland & Chasseaud
(1967, 1968) concerning the participation of glutathione- S-
alkylene transferase(s) (EC 2.5.2.18) in the cyanoethylation
reaction of glutathione. Since glutathione conjugates are
precursors of mercapturic acids, the occurrence of mercapturic
acids derived from acrylonitrile and glycidonitrile may be expected
in the urine of animals exposed to acrylonitrile.
The major metabolite of acrylonitrile in the rat, rabbit, and
other animals was found to be 2-cyanoethylmercapturic acid (Dahm,
1977; Wright, 1977; Ahmed & Patel, 1979; Kopecky et al., 1979,
1980a,b,c, 1981; Langvardt et al., 1980; Sapota & Draminski, 1981;
Sapota & Chmielnicka, 1981; Van Bladeren et al., 1981; Ghanayem &
Ahmed, 1982). While 2-cyanoethylmer-capturic acid was the sole
mercapturic acid identified in the urine of rats after iv
administration of acrylonitrile, a second mercapturic acid of
unestablished structure was also excreted after oral
administration. Langvardt et al. (1980), using 1-14C- or 2,3-14C-
acrylonitrile, found seven radioactive metabolites in rat urine.
The 3 major metabolites included thiocyanate and 2-
cyanoethylmercapturic acid. The third was tentatively identified
as 4-acetyl-5-cyanotetra-hydro-1,4-2 H-thiazine-3-carboxylic acid.
The 4 remaining metabolites represented at least one third of the
total activity excreted; their chemical structures are not known,
but none contained the -CN group of acrylonitrile. Different
results were reported by van Bladeren et al. (1981). In common
with Kopecky & Langvardt and colleagues, they isolated
2-cyanoethylmercapturic acid from the urine of orally-dosed rats;
however, 2-hydroxyethylmercapturic acid was also excreted. It is
suggested that this second mercapturic acid may be formed via one
of the conjugates of glutathione with glycidonitrile, S-(2-cyano-
2-hydroxyethyl)-L-glutathione. The amount of mercapturic acids
excreted relative to the dose was approximately constant up to a
dose of acrylonitrile of 26.5 mg/kg body weight. At higher doses,
the amount of mercapturic acids excreted remained constant. These
authors and Wright (1977) suggested that this might be a
consequence of the depletion of available glutathion at the higher
dose levels. It seems likely that, at high exposure levels, the
preferred metabolic pathway (conjugation of glutathione with
acrylonitrile or its metabolite) is overloaded, and another unknown
metabolic pathway takes over. After an oral dose to rats of 1-14C
acrylonitrile, 4 metabolites were found in the bile, 2 major
metabolites being GSH conjugates of acrylonitrile (Ghanayem &
Ahmed, 1982).
The report by Dahm (1977) that rats administered acrylonitrile
excreted S-(2-cyanoethyl)-L-cysteine has not been confirmed by any
of the authors who have examined the glutathione conjugation
pathway of acrylonitrile biotransformation. Fig. 2 illustrates
the proposed routes of mercaptide formation from acrylonitrile.
 5.3.2.3. The glucuronic acid conjugates of acrylonitrile metabolism
Rats treated with doses of acrylonitrile ranging from 20 to 40
mg/kg body weight (Hoffman et al., 1976) excreted significantly
more glucuronic acid than untreated controls or rats administered
10 mg acrylonitrile/kg body weight. This suggests that
acrylonitrile-derived glucuronide might be the alternative
substance to conjugate metabolites (van Bladereu et al., 1981).
The results of Lambotte-Vandepaer et al. (1980) support this
theory. The mutagenicity of rat urine after administration of
acrylonitrile at 30 mg/kg body weight was enhanced by treatment
with beta-D-glucuronidase (EC 3.2.1.31) prior to the Ames'
mutagenicity assay. This indicates that a glucuronide was cleaved
to give a free mutagenic agent derived from acrylonitrile. The
dose fits the dose range that evokes a significant increase in
glucuronic acid excretion (Hoffmann et al., 1976) and is of the
same magnitude as that at which van Bladeren et al. (1981)
demonstrated a depletion of glutathione in rat liver.
5.3.2.4. Quantitative aspects of acrylonitrile bio-transformation
and elimination of its metabolites
(a) Effect of acrylonitrile concentration and dose
The relationship between acrylonitrile concentrations in the
air, cyanide and thiocyanate in the blood, and thiocyanate in the
urine was described by Brieger et al. (1952). At acrylonitrile
concentrations between 55 and 220 mg/m3 (25 and 100 ppm), the blood
and urine thiocyanate concentrations were proportional to inhaled
acrylonitrile concentrations in rats. However, the cyanide content
of blood was measurable only at the highest acrylonitrile
concentration. In dogs, cyanide could be detected in blood at an
acrylonitrile concentration of 110 mg/m3 and cyanide concentrations
in blood were proportional to the inhaled acrylonitrile
concentrations in the range of 110-220 mg/m3 (50 - 100 ppm). Data
indicate that a certain acrylonitrile concentration must be exceeded
to provide conditions for the formation of enough cyanide to surpass
the metabolic capacity of rhodanese or the supply of co-factors;
this concentration is lower in the dog than in the rat.
In mice and rats, the dose of acrylonitrile was directly
related to the cyanide levels in blood, liver, kidney, and brain
(Ahmed & Patel, 1981), and, in rats, the ip administration of
acrylonitrile at 20-60 mg/kg body weight or oral administration at
15-60 mg/kg body weight also produced a proportional increase in
thiocyanate excretion in the urine.
However, thiocyanate is always present in urine (9 mg/litre in
rats) (Brieger et al., 1952), and the acrylonitrile exposures
required to exceed this level significantly are high. Thus, urine
thiocyanate levels would not give an accurate estimate of exposure
at the atmospheric acrylonitrile concentrations found in industry,
at present.
The observation of Hoffmann et al. (1976) suggested a possible
alternative conjugating route for metabolites at higher
acrylonitrile exposure levels involving glucuronic acid. Before
this is confirmed, the effects on carbohydrate metabolism and
glucose utilization in rats must be considered, as well as the
possibility that this alternative pathway of glucose metabolism
leading to the formation of glucuronic acid, and thus elevated
glucuronic acid levels in urine, may be stimulated by acrylonitrile.
From the standpoint of a possible exposure test, however, it is
emphasized that high doses of acrylonitrile are required to
increase excretion of glucuronic acid in urine, but such doses
would only occur in cases of accidental overexposure.
(b) Differences between species
The work of Brieger et al. (1952) revealed that, at the same
acrylonitrile exposure concentrations, cyanide blood levels in dogs
were far higher than in rats. This was apparently due to a less
efficient detoxification of cyanide to thiocyanate in dogs since,
when exposed to an acrylonitrile concentration of 217 mg/m3 (100
ppm), the total sum of cyanide and thiocyanate concentrations in
blood was about 260 µmol/litre in dogs and 840 µmol/litre in rats.
Although the normal thiocyanate blood level was about 150
µmol/litre in the rat and only about 55 µmol/litre in the dog, the
elevation caused by acrylonitrile was far higher in rats,
suggesting that rats metabolize acrylonitrile to cyanide at a
substantially higher rate and are able to detoxify it more
efficiently than dogs.
Mice excrete more thiocyanate than rats, at a given dose of
acrylonitrile, even though detoxification of cyanide to thiocyanate
in mice is apparently less efficient than in rats. Co-administration
of acrylonitrile and thiosulfate resulted in a 3-fold increase in
thiocyanate excretion in mice, while in rats the effect was much
smaller (Gut et al. 1975; Silver et al., 1982). Moreover, the
thiosulfate significantly reduced mortality in mice, but the
reduction in rat mortality was only slight, confirming that
enhanced detoxification of cyanide in mice is important.
Ahmed & Patel (1981) also observed that the rate of metabolism
of acrylonitrile was higher in mice than in rats.
(c) Time course of elimination of acrylonitrile metabolites
The excretion in urine of 14C-acrylonitrile-derived mercapturic
acids follows shortly after ip, sc, iv, or oral administration of
14C-acrylonitrile in rats (Gut et al., 1981a) and rapidly
decreases, whereas the excretion of thiocyanate from acrylonitrile
given orally or intra-peritoneally increases after a time lag
culminating between hours 8 and 12 in rats, but sooner in mice and
Chinese hamsters (Gut et al., 1975). The time course of
acrylonitrile-derived mercapturic acid excretion in rats was
closely correlated with free acrylonitrile concentrations in blood
and liver (Nerudova et al., 1980a; Gut et al., 1981a), while that
of thiocyanate was not, whatever the route of administration.
(d) Effect of the route of administration
The excretion of thiocyanate by rats, mice, and Chinese
hamsters after oral, ip, sc, and iv administration of 14C-
acrylonitrile represented 20-40%, 5%, 5%, and 1%, respectively, of
the dose administered. However, urinary excretion of radioactivity
was almost quantitative (Gut et al., 1981a); subtracting the
thiocyanate excretion from total urinary metabolites (radioactivity)
revealed that excretion of acrylonitrile-mercapturic acids (and
other possible acrylonitrile metabolites) is independent of the
route of administration (Kopecky et al., 1980a). When 1-14C-
acrylonitrile was administered orally to rats, 27% of the dose had
been excreted in the bile in 6 h, mainly in the form of 2
glutathione conjugates of acrylonitrile (Ghanayem & Ahmed, 1982).
(e) Metabolic interactions of acrylonitrile with other xenobiotics
Oral administration of an equimolar dose of acrylonitrile (0.5
mmol/kg body weight) to rats did not influence the elimination of
phenol from benzene. However, benzene, toluene, ethylbenzene, or
styrene (0.5 mmol/kg body weight) markedly decreased the rate and
total excretion of thiocyanate from an equal dose of acrylonitrile
given orally; higher doses of the solvents caused greater
inhibition (Gut et al., 1981). On the other hand, subcutaneous
administration of benzene and styrene increased the excretion of an
equal dose of 14C-acrylonitrile (0.5 mmol/kg body weight) during
the first 4 h and decreased it between the 8th and l2th hours
(owing to inhibited thiocyanate formation and excretion). The
total of metabolites excreted was unaffected. The co-administration
of industrial solvents markedly increased the lethality of
acrylonitrile (Gut et al., 1981a). Inhibition of the oxidative
metabolism of acrylonitrile in rats by a cytochrome P-450 inhibitor
(1-phenylimidazole) inhibited completely the excretion of N-
acetyl- S-(2-hydroxyethyl) L-cysteine in favour of the excretion of
N-acetyl- S-(2-cyanoethyl)-L-cysteine (van Bladeren et al., 1981).
The latter compound, the authors considered, resulted from direct
cyanoethylation of glutathione, whereas the former was formed via
the epoxide, glycidonitrile. Overnight fasting and cobaltous
chloride pre-treatment increased the biliary excretion of
metabolites, while phenobarbital did not induce any change, and
dimethyl maleate significantly decreased the excretion (Ghanayem &
Ahmed, 1982).
6. BIOLOGICAL MONITORING OF ACRYLONITRILE UPTAKE
Studies, particularly animal studies, on the absorption,
distribution, biotransformation, and elimination of acrylonitrile
have shown that a small fraction of the acrylonitrile absorbed is
rapidly eliminated in the urine without biotransformation, while
the remainder is biotransformed via several pathways, a number of
metabolites being excreted in urine; some of these metabolites are
unique to acrylonitrile.
The absorption studies have also clearly shown that, in
addition to uptake of acrylonitrile by inhalation, skin penetration
can be an important route of entry, particularly in the presence of
liquid acrylonitrile. Thus, in human studies, unless performed
under controlled conditions, a good correlation cannot necessarily
be expected between a bioindicator of uptake and ambient air
measurements of acrylonitrile, even when carried out with personal
samplers.
Possible indicators of acrylonitrile uptake at present include:
acrylonitrile in urine, acrylonitrile-derived mercapturic acids in
urine, total thioethers in urine, and thiocyanates in urine.
Houthuijs et al. (1982) studied the excretion pattern of
acrylonitrile in the urine of 15 exposed workers over a 7-day
period, with a control group of 41 unexposed workers. They noted
that the concentrations of acrylonitrile in urine peaked at the
end, or shortly after the end, of the working day, decreasing
rapidly until the beginning of the next working day without,
however, falling to the levels in the control group. Correlations
have been found between acrylonitrile concentrations in air and
those in urine. In the control group, a significant increase in
the acrylonitrile excretion in urine was found with the number of
cigarettes smoked. For a mean acrylonitrile concentration in air
of 0.28 mg/m3 (0.13 ppm), the mean acrylonitrile level in urine at
the end of the working day was 38 µg/litre, using the headspace
chromatographic technique. In the control group for non-smokers,
the mean level of acrylonitrile in urine was 2 µg/litre and for
smokers (20-30 cigarettes per day) 9.0 µg/litre.
Sakurai et al. (1978) have also established a relationship
between acrylonitrile concentrations in air and levels in urine for
a group of 102 exposed workers and compared them with 62 controls.
For an air concentration of 0.2 mg/m3 (0.1 ppm) (as measured by
personal samplers), an acrylonitrile level in urine of 3.0 µg/litre
was found, using the Sato et al. (1975) method of analysis
(separation by azeotropic distillation and determination by gas
chromatography). The urine samples were collected at the end of
the working day. At an air concentration of 1.1 mg/m3 (0.5 ppm),
the level of acrylonitrile in urine was 19.7 µg/litre, and at a
concentration in air of 9 mg/m3 (4.2 ppm) the corresponding level
in urine was 359.6 µg/litre. Acrylonitrile could not be detected
in the urine of controls. At the same time, an increase in the
thiocyanate level in urine was noted, particularly at the higher
exposure levels.
According to Houthuijs et al. (1982), the differences found
between the urine levels of acrylonitrile in the two studies are
most likely due to differences in analytical techniques.
The validity of the determination of urine levels of
acrylonitrile using gas chromatography-head space analysis for
monitoring acrylonitrile-exposed workers was established by Benchev
et al. (1982).
A promising method for estimating total acrylonitrile uptake
seems to be the determination of acrylonitrile-derived mercapturic
acids; such acids are specific for acrylonitrile and are absent
from normal urine. They have been shown in experimental animal
studies to be well correlated with the free acrylonitrile
concentration in blood (Nerudova et al., 1980; Gut et al.,
1981a,b); animal data also indicate that the capacity of the enzyme
systems to produce the acrylonitrile-derived mercapturic acids is
unlikely to be exceeded at the exposure levels of interest (van
Bladeren et al., 1981). Draminski & Trojanowska (1983) established
the presence of S-(2-cyanoethyl) mercapturic acid in the urine of
13 workers exposed to acrylonitrile, using a gas chromatographic
technique. The concentrations ranged between 50 and 200 mg/litre
for ambient acrylonitrile levels between 3.3 and 9.8 mg/m3 (1.5 and
4.5 ppm). The "total thioethers" were also determined in the urine
samples by a spectrophotometric method (Kopecky, 1982) and shown to
be strongly correlated with the S-(2-cyanoethyl) mercapturic acid
excretion, indicating that, in the case of exposure to pure
acrylonitrile, the major part of the sum of "thioethers" is
represented by this specific mercapturic acid.
Increased glucuronic acid excretion was reported by Ostrovskaja
et al. (1976) in 45.5% of workers exposed to acrylonitrile
concentrations of 0.7-1.5 mg/m3 (0.3-0.7 ppm).
The recent studies reported above show that biological
monitoring may become a suitable approach for assessing
acrylonitrile uptake, in particular in relation to the working
environment. Both acrylonitrile in urine and acrylonitrile-derived
mercapturic acids in urine seem to be the most suitable
bioindicators of uptake, at present, as they have the advantage of
specificity. More work is needed to resolve the apparent
discrepancies due to analytical techniques and to determine the
half-lives. This should make it possible to establish the most
appropriate sampling time with respect to exposure and help in the
determination of the concentrations of concern.
Interest in the determination of total "thioethers" in urine as
a bioindicator of uptake lies in the greater simplicity of the
analytical techniques used. However, more work is needed,
particularly with regard to interferences and half-lives.
The possibility of estimating acrylonitrile exposure in smokers
was suggested by Della Fiorentina & De Wiest (1979), who observed
that determination of carboxyhaemoglobin in blood makes it possible
to calculate the amount of thiocyanate present in urine that is due
to smoking, and thus to calculate the uptake of acrylonitrile.
However, experience shows that there can be marked variations in
thiocyanate levels in smokers, which greatly exceed those in non-
smokers occupationally-exposed to acrylonitrile (Czajkowska et al.,
1969).
7. EFFECTS ON EXPERIMENTAL ANIMALS AND CELL SYSTEMS
7.1. Acute Toxicity
7.1.1. Lethal doses and concentrations
7.1.1.1. Lethal doses
The range of acute LD50 values for acrylonitrile in different
laboratory mammals is generally between 25 and 186 mg/kg body
weight (Table 9), though a value of 282 mg/kg body weight was
observed when liquid acrylonitrile was applied to the skin of the
tail of male rats (Zotova, 1976). Mice are more sensitive than
rats, guinea-pigs, and rabbits. There seems to be little
consistency in the effects of route or vehicle of administration,
or of sex, on the LD50 level. The LD50 for dogs was not reported,
but they tolerated iv administration of acrylonitrile at 50 mg/kg
body weight and died after 300 mg/kg (Graham, 1965). The LD50
values reported are an order of magnitude higher than the LD50 for
cyanide (one of the metabolites of acrylonitrile), but markedly
lower than those for industrial solvents and monomers of plastics
(the LD50 for benzene and its derivatives being about 2000-3000
mg/kg body weight).
7.1.1.2. Lethal concentrations in the air
The range of acute LC50 s for 4-h inhalation of acrylonitrile is
between 150 and 1250 mg/m3 (Table 10). Dogs appeared to be the
most sensitive of the species tested and the sensitivity decreased
in the following order: mice, rabbits, cats, rats, guinea-pigs, the
latter being apparently the most resistant to inhalation exposure.
The exposure of 315-350 cm2 of the skin of rabbits to an
acrylonitrile concentration of 44-62 g/m3, in an exposure chamber,
such that the animals were breathing pure air, proved fatal after
2.5-4 h. Inhalation exposure to 0.58-0.67 g/m3 was fatal for 3
rabbits within 2-3 h (Rogaczewska, 1975).
In the 3 species of insects tested in a fumigation chamber
for 8 h, the LC50 value was found to be 700-1900 mg/m3 (Bond &
Buckland, 1976). Lindgren et al. (1954) exposed 8 insect
species for 2 or 6 h and found LC50 values of 1000-4500 mg/m3 .
Table 9. Acute LD50 values for acrylonitrile: effect of animal species
strain and route of administration
---------------------------------------------------------------------------------------------
Species/strain/sex Number Route LD50 (mg/kg Vehicle Reference
body weight)
---------------------------------------------------------------------------------------------
mouse/-/male M + F 333 oral 36 water Tullar (1947)
mouse/-/female M + F 333 oral 48 water Tullar (1947)
mouse/-/M + F 169 oral 40 olive oil Tullar (1947)
mouse/H strain/- - oral 25 physiol. Benesh & Cherna
saline (1959)
mouse/-/- - oral 40-46 - American Cyanamid
(1951)
mouse/-/female M + F 325 ip 48 water Tullar (1947)
mouse/-/male M + F 325 ip 40 water Tullar (1947)
mouse/NMRI or "SPF"/- - ip 50 - Zeller et al.
(1969)
mouse/ICR/female - ip 47 - Yoshikawa (1968)
mouse/H strain/- - sc 35("technical physiol. Benesh & Cherna
AN") saline (1959)
mouse/"inbred"/male 60 sc 50 (2 h) physiol. Graham (1965)
saline
25 (24 h)
mouse/BN/male 60 sc 34 - Knobloch et al.
(1971)
rat/Sherman/- groups of oral 93 - Smyth & Carpenter
6-10 (1948)
rat/Wistar/- - oral 101 - Paulet & Vidal
(1975)
rat/Wistar or Stock/- - oral 128 - Zellar et al.
(1969)
rat/Wistar-Stamm/male - oral 82 - von Borchardt
et al. (1970)
rat/Wistar-Stamm/female - oral 86 - von Borchardt
et al. (1970)
rat/-/M + F 80 oral 84 water Tullar (1947)
rat/-/M + F 51 oral 72 olive oil Tullar (1947)
rat/Wistar/- - oral 78 physiol. Benesh & Cherna
saline (1959)
rat/Sprague-Dawley/male 20 oral 186 water Monsanto (1975)
rat/Sprague-Dawley/female 20 oral 186 water Monsanto (1975)
Table 9. (contd.)
---------------------------------------------------------------------------------------------
Species/strain/sex Number Route LD50 (mg/kg Vehicle Reference
body weight)
---------------------------------------------------------------------------------------------
rat/Wistar/male 110 ip 100 - Knobloch et al.
(1971)
rat/Wistar/- - ip 65 poly- Paulet & Vidal
ethylene (1975)
glycol
rat/Wistar/male 110 sc 80 - Knobloch et al.
(1971)
rat/"albino"/male - sc 96 water Magos (1962)
rat/"white"/male - skin of 282 liquid Zotova (1976)
tail acrylonitrile
rat/"white"/male - skin of 148 liquid Zotova (1976)
abdomen acrylonitrile
guinea-pig/-/- - oral 50 - Carpenter et al.
(1949)
guinea-pig/-/- - oral 85 olive oil Tullar (1947)
guinea-pig/-/M & F 30 oral 56 - Jedlicka et al.
(1958)
guinea-pig/-/- - sc 130 - Ghiringhelli (1954)
guinea-pig/-/- 11 iv 72 water Tullar (1947)
guinea-pig/Hartley-/male 12 or more intact 0.46 ml/kg - Roudabush et al.
skin (1965)
abraded 0.86 ml/kg - Roudabush et al.
skin (1965)
guinea-pig/-/- - skin 0.25 ml/kg - Smyth & Carpenter
(1948)
rabbit/-/- - oral 93 - Lefaux (1966)
rabbit/-/- - iv 69 - Paulet & Desnos
(1961)
rabbit/"white"/M & F 12 or more abraded 0.28 ml/kg - Roudabush et al.
skin (1965)
---------------------------------------------------------------------------------------------
Table 10. Acute lethal effect of single inhalation of acrylonitrile: effect of
duration and concentration of acrylonitrile
---------------------------------------------------------------------------------------------------
Species/strain/sex Number Concentration Duration Mortality Reference
(mg/m3) (h) (died/
tested)
---------------------------------------------------------------------------------------------------
white mouse/stock/- 6 600 0.5 0/6 McOmie (1949)
6 1500 0.5 5/6 McOmie (1949)
6 5800 0.5 5/6 McOmie (1949)
6 900 1 1/6 McOmie (1949)
6 900 2 3/6 McOmie (1949)
6 1700 1 6/6 McOmie (1949)
mouse/BN/male 12 300 4 LC50 Knobloch et al. (1971)
rat/Sherman/- 6 1085 4 0/6 Smyth & Carpenter et al. (1971)
6 2170 4 6/6 Smyth & Carpenter et al. (1971)
rat/Sherman/female 6 1085 4 2/6 to Carpenter et al. (1949)
4/6
rat/Wistar/- 20 54 7 0/20 Brieger et al. (1952)
20 109 7 0/20 Brieger et al. (1952)
20 163 7 0/20 Brieger et al. (1952)
20 217 7 4/20 Brieger et al. (1952)
rat/Wistar/male 12 470 4 LC50 Knobloch et al. (1971)
rat/Osborne-Mendel/- 16 2750 1 0/16 Dudley & Neal (1942)
16 3230 1 4/16 Dudley & Neal (1942)
16 5300 1 13/16 Dudley & Neal (1942)
16 660 2 0/16 Dudley & Neal (1942)
16 1290 2 6/16 Dudley & Neal (1942)
16 2730 2 16/16 Dudley & Neal (1942)
16 280 4 0/16 Dudley & Neal (1942)
16 680 4 5/16 Dudley & Neal (1942)
16 1380 4 16/16 Dudley & Neal (1942)
16 290 8 0/16 Dudley & Neal (1942)
16 460 8 1/16 Dudley & Neal (1942)
16 590 8 7/16 Dudley & Neal (1942)
16 690 8 15/16 Dudley & Neal (1942)
---------------------------------------------------------------------------------------------------
Table 10. (contd.)
---------------------------------------------------------------------------------------------------
Species/strain/sex Number Concentration Duration Mortality Reference
(mg/m3) (h) (died/
tested)
---------------------------------------------------------------------------------------------------
rat/Wistar/male 3 650 3 1/3 Appel et al. (1981)
3 1100 2 3/3 Appel et al. (1981)
3 2600 0.5 1/3 Appel et al. (1981)
6 3000 0.5 6/6 Appel et al. (1981)
guinea-pig/-/- 8 580 4 0/8 Dudley & Neal (1942)
8 1250 4 5/8 Dudley & Neal (1942)
8 2520 4 8/8 Dudley & Neal (1942)
guinea-pig/-/- 12 990 4 LC50 Knobloch et al. (1971)
rabbit/"albino"/- 2 290 4 0/2 Dudley & Neal (1942)
2 560 4 2/2 Dudley & Neal (1942)
2 1260 4 2/2 Dudley & Neal (1942)
rabbit/-/- 5 670 - 1100 2-3 5/5 Rogaczewska (1975)
cat/-/- 4 210 4 0/4 Dudley & Neal (1942)
2 600 4 0/2 Dudley & Neal (1942)
2 1300 4 2/2 Dudley & Neal (1942)
dog/-/- 3 63 4 0/3 Dudley & Neal (1942)
2 140 4 1/2 Dudley & Neal (1942)
3 213 4 0/3 Dudley & Neal (1942)
2 240 4 2/2 Dudley & Neal (1942)
dog/-/- 4 108 7 0/4 Brieger et al. (1952)
4 163 7 0/4 Brieger et al. (1952)
6 213 7 6/6 Brieger et al. (1952)
Rhesus monkey/-/- 3 163 7 1/3 Brieger et al. (1952)
---------------------------------------------------------------------------------------------------
7.1.1.3. Lethal concentrations in water
(a) Fish
Acute toxicity, determined by a static bioassay at 25 °C,
revealed TLm (median tolerance limit, i.e., a concentration of
acrylonitrile killing 50% of the test organisms within a specified
time) values ranging from 25.5 to 44.6 mg/litre at 24 h, and from
11.8 to 33.5 mg/litre at 96 h. There were no apparent significant
differences in the sensitivity of various kinds of fish (Table 11).
(b) Invertebrates
For the brown shrimp (Crangon crangon), the LC50 for a 24-h
exposure was 10-33 mg/litre (Portman & Wilson, 1971). Bandt (1953)
exposed several species of arthropods (a shrimp-like crustaceae and
3 types of larvae) to 20-100 mg acrylonitrile/litre water and found
marked species and individual differences: a lethal effect was
observed in some species with 25 mg/litre after 48 h, while other
species were not affected after 3 days. The most resistant species
were unaffected by 100 mg/litre after 24-48 h. The results of
studies by Rajendran & Muthu (1981) showed that acrylonitrile
affects the activity of the phosphorylase and acetylcholinesterase
enzymes in Tribolium castaneum Herbst, and Trogoderma granarium
Everts.
7.1.2. Clinical observations
The inhalation studies of Dudley & Neal (1942), Brieger et al.
(1952), and Rogaczewska (1975), and the results of oral and
parenteral administration (Ghiringhelli, 1954; Benesh & Cherna,
1959; Paulet & Despos, 1961; Graham, 1965; Paulet et al., 1966)
indicate that animals inhaling lethal concentrations of
acrylonitrile, or administered lethal dosages of acrylonitrile
orally or parenterally, showed rather similar effects: excitability
and stimulated breathing, shallow rapid breathing, slow gasping
breathing, apnoea, convulsions, and death. Vomiting occurred in
cats, dogs, and monkeys after inhaling acrylonitrile, and in rats
following parenteral administration. Reddening of the skin of the
ears, nose, and feet (in rhesus monkeys, also of the face and
genital organs) and mucosa was accompanied by lachrymation, nasal
discharge, and salivation, not only after inhalation exposure, but
also following oral and sc administration, while hind-leg
incoordination, paresis or paralysis, were observed in rats after
oral administration, and in rabbits after iv administration.
Table 11. Median tolerance limit values (TLm)a for various fish exposed to
acrylonitrile
-------------------------------------------------------------------------------------
Species Water type TLm (mg/litre) Reference
24 h 48 h 96 h
-------------------------------------------------------------------------------------
Fathead minnow hard 32.7 16.7 14.3 Henderson et al. (1961)
(Pimphales promelas) soft 34.3 21.5 18.1 Henderson et al. (1961)
Minnow (Phoxinus - 38.2 17.6 - Marcoci & Ionescu (1974)
phoxinus)
Bluegill (Lepomis soft 25.5 14.3 11.8 Henderson et al. (1961)
macrochirus)
Guppy (Lebistes soft 44.6 33.5 33.5 Henderson et al. (1961)
reticulatus)
Goldfish (Carassius - - - 40 Paulet & Vidal (1975)
sp.)
Carp (Cyprinus - 37.4 24.0 - Marcoci & Ionescu (1974)
carpio)
Rainbow trout hard - 70 - Jackson & Brown (1970)
(Salmo gairdneri)
Pin perch sea 24.5 - - Daugherty & Garett (1951)
(marine fish) (30/l tank)
(Lagodon rhomboides)
Rainbow trout tap, - 5b - Sloof (1979)
(Salmo gairdneri) dechlorinated,
3.6 mg/litre
hard 15 - - Sloof (1979)
Zebra fish same (LC50)
-------------------------------------------------------------------------------------
a TLm median tolerance limit, a concentration of acrylonitrile killing
50% of the test organisms within a specified time.
b Minimal concentration changing respiratory frequency.
(a) Effects on the skin
Direct application of liquid acrylonitrile to the shaved skin
of rabbits induced slight local vasodilation immediately, without
any systemic effect (1-2 ml covering 100-200 cm2) or with an
increased respiratory rate (3 ml over 300 cm2) (McOmie, 1949).
Tuller (1947) observed erythema in only one of 3 areas of abraded
skin, following application of 1 ml of acrylonitrile on a gauze pad
covered by rubber sheeting. However, Zeller et al. (1969) found
that a 15-min application of acrylonitrile on a cotton pad to
shaved skin resulted in skin oedema, and a 20-h application, in
slight necrosis. Guinea-pigs appear to be more sensitive than
rabbits; the application of a 2% solution of acrylonitrile in
acetone for 24 h, under occlusion, did not induce any effects, but
8% or higher concentrations induced dose-dependent erythema
followed by desquamation and mild necrosis (Gut et al., unpublished
data). Erythema of the nose, face, ears, legs, and genital organs
may follow inhalation and oral administration of acrylonitrile.
(b) Effects on the eye
McOmie (1949) instilled one drop of acrylonitrile into the eye
of a rabbit. After 1 h, there was mild conjunctivitis without
corneal clouding or pupillary damage and no effects were observed
after 24 h. Oedema and slight necrosis of the conjunctiva after 8
days were observed in rabbits by Zeller et al. (1969).
(c) Effects on respiration
It was stressed by Paulet et al. (1966) that, after a lethal
intravenous dose of acrylonitrile (120 mg/kg body weight), the
respiratory rate in rabbits did not increase as is characteristic
in cyanide poisoning. However, respiratory disturbance was
observed in: guinea-pigs given a sc lethal dose of acrylonitrile
(130 mg/kg body weight) (Ghiringhelli, 1954), anaesthetized dogs
given 100 mg/kg body weight intravenously (Graham, 1965), mice
given an oral lethal dose (Benesh & Cherna, 1959), and in guinea-
pigs given 100 mg/kg body weight orally (Jedlicka et al., 1958).
Pulmonary oedema was also seen.
An increased respiratory rate followed the application of
liquid acrylonitrile (3 ml/kg body weight) to the skin of rabbits
(McOmie, 1949), and "respiratory distress" was reported in rhesus
monkeys exposed to 163 mg/m3 for 7 h (Brieger et al., 1952). When
rats, rabbits, cats, dogs, and monkeys were exposed to lethal
concentrations of acrylonitrile, Dudley & Neal (1942) observed an
initial stimulation of respiration followed by shallow rapid
breathing, slow gasping breathing, convulsions, coma, and death.
These respiratory effects were absent in guinea-pigs, but
irritation of the pulmonary membranes and some delayed deaths from
lung oedema occurred.
(d) Effects on circulation
Acrylonitrile administered iv at 13, 27, 55, or 110 mg/kg body
weight had little effect on the respiratory and blood pressure
responses of anaesthetized rabbits to adrenalin, noradrenalin, or
acetylcholine, and Gravczyk & Zwierzchovski (1973) believed that
the circulation was not the primary target organ in acrylonitrile
poisoning. However, lethal doses (50 or 100 mg/kg body weight) in
guinea-pigs caused dilation of the right ventricle, congestion of
the coronary blood vessels, hepatic and splenic hyperaemia, and
inflammation of the intestinal mucosa (Jedlicka et al., 1958). In
Sprague-Dawley rats administered a lethal dose of acrylonitrile,
there were haemorrhagic areas in the lung and liver and acute
gastrointestinal inflammation (Monsanto, 1975). Whether the
reddening of nose, ears, legs, face, and genital organs in rats and
other species, after inhalation and oral administration of
acrylonitrile, is due to a direct effect on small vessels or is an
inflammatory response is not known.
(e) Effects on adrenals
The effect of lethal doses of acrylonitrile on the adrenals
became evident in the reports of Szabo & Selye (1971, 1972) and
Szabo et al. (1976). After iv administration of high doses (150 or
200 mg/kg body weight), haemorrhage was observed in both adrenals
of most animals, and there was adrenal haemorrhage in some rats
following oral administration of 10, 15, or 20 mg/kg body weight.
Various types of histological damage were observed in the adrenal
cortex and medulla, some of them within 30 min of acrylonitrile
administration.
A possible mechanism involving the peroxidative action of
acrylonitrile in acrylonitrile-induced adrenal injury has been
suggested recently by Silver & Szabo (1982). Szabo et al. (1980)
investigated the pathogenesis of experimental adrenal haemorrhagic
necrosis using various morphological, biochemical, and
pharmacological methods. Their results suggest that the presence
of a functional adrenocortex is necessary for the development of
cortical damage.
(f) Blood chemistry
Intraperitoneal administration of acrylonitrile to male rats at
33 mg/kg body weight per day for 3 days decreased serum
corticosterone to 30%, prolactin to 40%, but increased follicle-
stimulating hormone (FSH) to 200% of control levels and did not
affect luteinizing hormone (LH) (Nilsen et al., 1980). In adult
male Wistar rats, a single ip administration of acrylonitrile of 10
mg/kg body weight did not have any effect on serum glutamic
oxaloacetic transferase (SGOT) and serum glutamic pyruvate
transaminase (SGPT) activity, but increased lactate dehydrogenase
(EC 1.1.1.27) (LDH) to 200% and sorbitol dehydrogenase (SDH) (EC
1.1.1.14) to 300% compared with the controls (Noel et al., 1978).
The same dose in male rats inhibited butyrylcholineesterase (EC
3.1.1.8), did not have any effect on alkaline phosphatase (EC
3.1.3.1), and increased fructose monophosphate aldolase activity,
suggesting that there had been an adverse effect on the liver
(Ivanov et al., 1979). Administraton of L-cysteine, alpha
tocopherol, or ionol prevented these effects. A single oral dose
of acrylonitrile (l/2 LD50, 41 mg/kg body weight) in rats resulted
in changes in the elution patterns of serum gel chromatography and
paper-electrophoresis of globulins (Franzen & Wagner, 1978). Serum
SDH was significantly elevated (approximately 4-fold) in rats, 24 h
after administration of acrylonitrile at 150 mg/kg body weight. A
60% increase in serum SDH was found in rats administered
acrylonitrile at 500 mg/litre in drinking-water for 21 days (Silver
et al., 1982).
(g) Effects on other organs
Focal superficial necrosis of the liver associated with
haemorrhagic gastritis was found in rats necropsied 24 h after
administration of acrylonitrile at 150 mg/kg body weight in the
drinking-water (Silver et al., 1982).
Acrylonitrile shows an inhibitory effect on K-stimulated
respiration of guinea-pig brain cortex slices at 1 mM, but little
effect on the liver at the same concentration. A stronger
anaesthetic action of acrylonitrile was detected in vitro on the
sciatic nerve of Rana nigra maculata, compared with some other
anaesthetic agents (Hashimoto & Kanai, 1965). The recovery phase of
nerve excitation was also affected by acrylonitrile (Ando &
Hashimoto, 1967).
7.1.3. Biochemical changes and mechanisms of acrylonitrile
toxicity
7.1.3.1. Effect on cytochrome oxidase
Evidence has been presented that cytochrome oxidase (EC
1.9.3.1) activity may be significantly inhibited in acrylonitrile
poisoning. This was suspected after Dudley & Neal (1942) and
Brieger et al. (1952) had reported significant concentrations of
cyanide in dogs and monkeys exposed to acrylonitrile vapours.
Tarkowski (1968) observed inhibited cytochrome oxidase activity in
the brain, kidneys, and liver of rats after ip injection of
acrylonitrile at 100 mg/kg body weight. In vitro, a 50%
inhibition of the enzyme was observed in homogenates of brain,
kidneys, and liver with an acrylonitrile concentration of 10-3M.
Such concentrations have been observed in vivo, shortly after
lethal doses of acrylonitrile (Tarkowski, 1968; Nerudova et al.,
1980; Gut et al., 1981b), but acrylonitrile has a short half-life
in blood and liver after ip or iv administration (15-20 min).
There are correspondingly increased blood and liver concentrations
of cyanide in rats after acrylonitrile administration (up to 180 µM,
Gut et al., 1981b), together with an even greater inhibition of
cytochrome oxidase, and a much higher sensitivity of cytochrome
oxidase activity to cyanide (50% inhibition in vitro at 10-8M
(Tarkowski, 1966).
A significantly decreased ratio of oxidized to reduced
nicotinamide-adenine dinucleotides was observed by Sokal et al.
(1972, 1977) in the brain of rats after sc administration of
acrylonitrile at 100-120 mg/kg body weight, indicating inhibition
of NADH oxidation in the mitochondria, possibly also at the level
of cytochrome oxidase. These changes seem to be of biological
importance, because their magnitude was similar to that observed at
the death of animals subjected to experimental hypoxia. Thus, the
cyanide-mediated inhibition of cytochrome oxidase would seem to be
of importance in the later stages of intoxication and death. This
"cyanide effect" is apparently more pronounced at higher
acrylonitrile doses (Willhite & Smith, 1981) and appears to be more
significant in mice and dogs (Brieger et al., 1952; Benesh &
Cherna, 1959; Gut et al., 1981b) than in rats. This is in
agreement with the greater efficacy in mice than in rats of
thiosulfate (an antidote to cyanide) in acrylonitrile poisoning,
and with the higher cyanide concentration in the blood of dogs than
in that of rats (101 µM versus 10 µM), after breathing the same
concentration of acrylonitrile (217 mg/m3 for 7 h) (Brieger et al.,
1952). The protective effect of another cyanide antidote, nitrite
(Dudley & Neal, 1942; Ghiringhelli, 1954; Benesh & Cherna, 1959),
in acrylonitrile poisoning also points to the participation of
cyanide in lethal acrylonitrile poisoning.
7.1.3.2. Effect on sulfhydryls
There is considerable evidence to demonstrate that
acrylonitrile significantly depresses the concentrations of soluble
glutathione and protein sulfhydryls in the blood, liver, brain, and
kidney. Acrylonitrile also inhibits some SH-dependent enzymes that
participate in carbohydrate metabolism. Wisniewska-Knypl (1970,
1978), Hashimoto & Kanai (1972), and Vainio & Mäkinen (1977)
observed that the inhibition of sulfhydryls was dose-dependent in
the range of 10-100 mg/kg body weight in vivo, and in the
concentraton range 0.01-10 nM in vitro (Wisniewska-Knypl, 1978).
A significant decrease in brain sulfhydryls was reported after a
single dermal application of acrylonitrile of as little as 2.82
mg/kg body weight (Zotova, 1976). These effects were observed
after sc, ip, or iv administration to rats, rabbits, hamsters,
guinea-pigs, and mice. There were some decreases in the activity
of serum or tissue - SH-dependent enzymes including oxoglutarate
dehydrogenase (EC 1.2.4.2). However, the activity of succinate
dehydrogenase (EC 1.3.99.1) was not reduced (Wisniewska-Knypl,
1978), and there were corresponding increases in the liver, blood,
and brain concentrations of glucose, pyruvate, and lactate
(Hashimoto & Ando, 1966; Dinu & Klein, 1976).
It was shown by Zitting et al. (1981) that short-term exposure
to acrylonitrile decreased the liver glutathione content within 4 h
of poisoning, but that the glutathione contents returned to normal
in brain, liver, and kidney, within 24 h. At the same time, the
activity of cerebral succinate dehydrogenase and of ethoxycoumarin
demethylase in liver and kidney decreased. Increased glucose,
pyruvate, and lactate concentrations in blood, liver, and brain
were also found, immediately after the fifth exposure, in rats
exposed through inhalation to an acrylonitrile concentration of 300
mg/m3, 8 h daily, for 5 days. In protein, sulfhydryl-dependent
enzyme inhibition was absent and the glutathione level was
significantly reduced in the liver but not in the brain (Gut et
al., 1982). The effects of acrylonitrile on sulfhydryls were
significantly reduced by co-administering L-cysteine and other
sulfhydryls and there were corresponding decreases in lethal
effects (Hashimoto & Kanai, 1965; Bondarev et al., 1976; McLaughlin
et al., 1976; Appel et al., 1981). The results of these studies
demonstrate the protective role of SH-groups in acrylonitrile
poisoning.
The role of hypoxia in the acute thiol-depressive effect of
acrylonitrile in male rats was investigated by Jaeger (1978) and
Jaeger & Cote (1982). Hypoxia was found to enhance non-protein SH
loss in the liver, when there was exposure to acrylonitrile (Jaeger
& Cote, 1982).
Evidence has been presented (Holechek & Kopecky, 1981) that
inhibition of tissue sulfhydryls may be due not only to the
acrylonitrile itself, but also to its reactive metabolite,
glycidonitrile.
7.1.3.3. Interaction with the microsomal oxidation system as a
possible mechanism of toxicity
Acrylonitrile added to mouse, rat, and human liver microsomes
caused characteristic spectral complexes with cytochrome P-450
(Ivanov et al., 1979; Appel et al., 1981).
It was shown in vitro that glycidonitrile, which is
generated in rat-liver microsomes by mixed-function oxidases (EC
1.14.14.1), covalently binds to microsomal membrane and albumin
(Ivanov et al., 1982). The biological significance of this
phenomenon may be inferred from experiments with inhibitors and
inducers of the mixed-function oxidases (Ivanov, 1981). In rats
pre-treated with phenobarbital, an increased amount of
glycidonitrile was covalently bound to macromolecules and
substantially higher activity of fructose-bisphosphate aldolase (EC
4.1.2.13) was observed in the blood, indicating liver damage. On
the other hand, SKF-525A, the inhibitor of cytochrome P-450,
reduced both effects in vivo and in vitro . Activation of
acrylonitrile by cytochrome P-450 may therefore result in a
cytotoxic effect.
A previous injection of SKF-525A or of cobalt(II) chloride
(CoCl2), another inhibitor of cytochrome P-450, resulted in
significant protection against the gastrointestinal bleeding in
rats caused by acrylonitrile (Ghanayem & Ahmed, 1982).
Some data show that acrylonitrile and its epoxide,
glycidonitrile, bind covalently, in vitro, to DNA and RNA
(Guengenrich et al., 1981; Peter et al., 1983a). However, the
quantitative extent of truly irreversible binding is much less than
that observed in experiments with other vinyl monomers (Peter et
al., 1983).
A lower content of cytochrome P-450 in the liver and reduced
oxidative microsomal metabolism of xenobiotics were observed in
rats after an ip injection of acrylonitrile at 10 or 33 mg/kg body
weight, for 3 days (Noel et al., 1978; Nilsen et al., 1980), after
inhalation exposure to 300 mg/m3 of acrylonitrile, 8 h day, for 5
days (Gut et al., in press), and in Chinese hamsters after an ip
injection of 30 mg/kg (Zitting et al., 1981). Inhibition of
microsomal oxidation of xenobiotics by acrylonitrile was observed
in vitro (Ivanov et al., 1979).
Pre-treatment of rats with inducers of microsomal oxidases such
as phenobarbital, 3-methylcholantrene, or Arochlor 1254, nullified
the effect of acrylonitrile on the total cytochrome P-450 content.
The activity of other microsomal enzymes, glucose-6-phosphatase (EC
3.1.3.9), and NADPH cytochrome-c-reductase (EC 1.6.2.4), was
unaffected by acrylonitrile (Duverger-van Bogaert et al., 1978;
Noel et al., 1978).
Ghanayem & Ahmed (1982) showed that Arochlor 1254 drastically
increased acrylonitrile-induced gastric bleeding in rats.
Phenobarbital significantly increased acrylonitrile-induced
hepatocyte damage in rats (Ivanov, 1981).
Acrylonitrile binds with liver microsomal and S-9 fractions, in
vitro, by direct alkylation. The microsomal activation of
acrylonitrile into reactive intermediates was also detected
(Duverger-van Bogaert et al., 1982a). The irreversible binding of
acrylonitrile to liver microsomal proteins was inhibited by thiols
and even more by dithiocarb (Peter & Bolt, 1981).
7.1.3.4. Observations on the possible participation of membrane
lipid peroxidation in the mechanism of toxicity
The ip administration of acrylonitrile at 10 mg/kg body weight
induced lipid peroxidation in rat liver (Dinu, 1975a; Ivanov et
al., 1979) and erythrocyte membranes (Ivanov et al., 1982),
indicating possible damage of cellular membranes. NADPH-dependent
lipid peroxidation in rat liver microsomes was only slightly
stimulated (Ivanov et al., 1978; Duverger-van Bogaert et al., 1981)
whereas substantial stimulation was found in the post-mitochondrial
fraction of rat liver, lung, and brain, as well as in brain-marrow
homogenate (Ivanov et al., 1978; Ivanov, 1979; Al'shansky et al.,
1980). There was a correlation between an increased amount of
malondialdehyde and a decreased content of -SH groups in the post-
mitochondrial supernatant of rat liver (Ivanov, 1981) and brain
(Al'shansky et al., 1980). Conjugated diene concentrations in rat
liver microsomes were significantly elevated after iv
administration of acrylonitrile to rats (150 mg/kg body weight),
but no change was seen in the adrenal glands (Silver & Szabo,
1982).
Pre-treatment of rats with antioxidants, in doses equivalent to
those of the acrylonitrile administered, afforded protection
against the pro-oxidant effect of acrylonitrile and elevation of
blood fructose-1-phosphate aldolase, decreased the activity of
butyrylcholine esterase (EC 3.1.1.8) (Ivanov et al., 1979), and
reduced the GABA content and activity of glutamate decarboxylase
(EC 4.1.1.15) in the brain (Al'shansky et al., 1980).
7.1.3.5. Studies on antidotes
Appel et al. (1981a) found that the cyanide antidotes, 4-
dimethylaminophenol plus thiosulfate, protected rats against the
lethal effects of orally-administered acrylonitrile. A comparative
evaluation was made by McLaughlin et al. (1975) of the efficacy of
thiols (cysteine hydrochloride) and cyanide antidotes. The authors
showed that thiols were more effective in protecting rats against
acrylonitrile poisoning. Bondarev et al. (1976) demonstrated the
protective role of some sulfur-containing compounds in the
acrylonitrile poisoning of rats. The protective effects of some
antioxidants, such as vitamin E and ionol, have also been
demonstrated (Ivanov et al., 1979).
The possible toxic mechanisms and theoretical protective
mechanisms are summarized in Fig. 3, which indicates the complex
nature of the interference of acrylonitrile with cellular
mechanisms, as far as can be derived from current knowledge.
5.3.2.3. The glucuronic acid conjugates of acrylonitrile metabolism
Rats treated with doses of acrylonitrile ranging from 20 to 40
mg/kg body weight (Hoffman et al., 1976) excreted significantly
more glucuronic acid than untreated controls or rats administered
10 mg acrylonitrile/kg body weight. This suggests that
acrylonitrile-derived glucuronide might be the alternative
substance to conjugate metabolites (van Bladereu et al., 1981).
The results of Lambotte-Vandepaer et al. (1980) support this
theory. The mutagenicity of rat urine after administration of
acrylonitrile at 30 mg/kg body weight was enhanced by treatment
with beta-D-glucuronidase (EC 3.2.1.31) prior to the Ames'
mutagenicity assay. This indicates that a glucuronide was cleaved
to give a free mutagenic agent derived from acrylonitrile. The
dose fits the dose range that evokes a significant increase in
glucuronic acid excretion (Hoffmann et al., 1976) and is of the
same magnitude as that at which van Bladeren et al. (1981)
demonstrated a depletion of glutathione in rat liver.
5.3.2.4. Quantitative aspects of acrylonitrile bio-transformation
and elimination of its metabolites
(a) Effect of acrylonitrile concentration and dose
The relationship between acrylonitrile concentrations in the
air, cyanide and thiocyanate in the blood, and thiocyanate in the
urine was described by Brieger et al. (1952). At acrylonitrile
concentrations between 55 and 220 mg/m3 (25 and 100 ppm), the blood
and urine thiocyanate concentrations were proportional to inhaled
acrylonitrile concentrations in rats. However, the cyanide content
of blood was measurable only at the highest acrylonitrile
concentration. In dogs, cyanide could be detected in blood at an
acrylonitrile concentration of 110 mg/m3 and cyanide concentrations
in blood were proportional to the inhaled acrylonitrile
concentrations in the range of 110-220 mg/m3 (50 - 100 ppm). Data
indicate that a certain acrylonitrile concentration must be exceeded
to provide conditions for the formation of enough cyanide to surpass
the metabolic capacity of rhodanese or the supply of co-factors;
this concentration is lower in the dog than in the rat.
In mice and rats, the dose of acrylonitrile was directly
related to the cyanide levels in blood, liver, kidney, and brain
(Ahmed & Patel, 1981), and, in rats, the ip administration of
acrylonitrile at 20-60 mg/kg body weight or oral administration at
15-60 mg/kg body weight also produced a proportional increase in
thiocyanate excretion in the urine.
However, thiocyanate is always present in urine (9 mg/litre in
rats) (Brieger et al., 1952), and the acrylonitrile exposures
required to exceed this level significantly are high. Thus, urine
thiocyanate levels would not give an accurate estimate of exposure
at the atmospheric acrylonitrile concentrations found in industry,
at present.
The observation of Hoffmann et al. (1976) suggested a possible
alternative conjugating route for metabolites at higher
acrylonitrile exposure levels involving glucuronic acid. Before
this is confirmed, the effects on carbohydrate metabolism and
glucose utilization in rats must be considered, as well as the
possibility that this alternative pathway of glucose metabolism
leading to the formation of glucuronic acid, and thus elevated
glucuronic acid levels in urine, may be stimulated by acrylonitrile.
From the standpoint of a possible exposure test, however, it is
emphasized that high doses of acrylonitrile are required to
increase excretion of glucuronic acid in urine, but such doses
would only occur in cases of accidental overexposure.
(b) Differences between species
The work of Brieger et al. (1952) revealed that, at the same
acrylonitrile exposure concentrations, cyanide blood levels in dogs
were far higher than in rats. This was apparently due to a less
efficient detoxification of cyanide to thiocyanate in dogs since,
when exposed to an acrylonitrile concentration of 217 mg/m3 (100
ppm), the total sum of cyanide and thiocyanate concentrations in
blood was about 260 µmol/litre in dogs and 840 µmol/litre in rats.
Although the normal thiocyanate blood level was about 150
µmol/litre in the rat and only about 55 µmol/litre in the dog, the
elevation caused by acrylonitrile was far higher in rats,
suggesting that rats metabolize acrylonitrile to cyanide at a
substantially higher rate and are able to detoxify it more
efficiently than dogs.
Mice excrete more thiocyanate than rats, at a given dose of
acrylonitrile, even though detoxification of cyanide to thiocyanate
in mice is apparently less efficient than in rats. Co-administration
of acrylonitrile and thiosulfate resulted in a 3-fold increase in
thiocyanate excretion in mice, while in rats the effect was much
smaller (Gut et al. 1975; Silver et al., 1982). Moreover, the
thiosulfate significantly reduced mortality in mice, but the
reduction in rat mortality was only slight, confirming that
enhanced detoxification of cyanide in mice is important.
Ahmed & Patel (1981) also observed that the rate of metabolism
of acrylonitrile was higher in mice than in rats.
(c) Time course of elimination of acrylonitrile metabolites
The excretion in urine of 14C-acrylonitrile-derived mercapturic
acids follows shortly after ip, sc, iv, or oral administration of
14C-acrylonitrile in rats (Gut et al., 1981a) and rapidly
decreases, whereas the excretion of thiocyanate from acrylonitrile
given orally or intra-peritoneally increases after a time lag
culminating between hours 8 and 12 in rats, but sooner in mice and
Chinese hamsters (Gut et al., 1975). The time course of
acrylonitrile-derived mercapturic acid excretion in rats was
closely correlated with free acrylonitrile concentrations in blood
and liver (Nerudova et al., 1980a; Gut et al., 1981a), while that
of thiocyanate was not, whatever the route of administration.
(d) Effect of the route of administration
The excretion of thiocyanate by rats, mice, and Chinese
hamsters after oral, ip, sc, and iv administration of 14C-
acrylonitrile represented 20-40%, 5%, 5%, and 1%, respectively, of
the dose administered. However, urinary excretion of radioactivity
was almost quantitative (Gut et al., 1981a); subtracting the
thiocyanate excretion from total urinary metabolites (radioactivity)
revealed that excretion of acrylonitrile-mercapturic acids (and
other possible acrylonitrile metabolites) is independent of the
route of administration (Kopecky et al., 1980a). When 1-14C-
acrylonitrile was administered orally to rats, 27% of the dose had
been excreted in the bile in 6 h, mainly in the form of 2
glutathione conjugates of acrylonitrile (Ghanayem & Ahmed, 1982).
(e) Metabolic interactions of acrylonitrile with other xenobiotics
Oral administration of an equimolar dose of acrylonitrile (0.5
mmol/kg body weight) to rats did not influence the elimination of
phenol from benzene. However, benzene, toluene, ethylbenzene, or
styrene (0.5 mmol/kg body weight) markedly decreased the rate and
total excretion of thiocyanate from an equal dose of acrylonitrile
given orally; higher doses of the solvents caused greater
inhibition (Gut et al., 1981). On the other hand, subcutaneous
administration of benzene and styrene increased the excretion of an
equal dose of 14C-acrylonitrile (0.5 mmol/kg body weight) during
the first 4 h and decreased it between the 8th and l2th hours
(owing to inhibited thiocyanate formation and excretion). The
total of metabolites excreted was unaffected. The co-administration
of industrial solvents markedly increased the lethality of
acrylonitrile (Gut et al., 1981a). Inhibition of the oxidative
metabolism of acrylonitrile in rats by a cytochrome P-450 inhibitor
(1-phenylimidazole) inhibited completely the excretion of N-
acetyl- S-(2-hydroxyethyl) L-cysteine in favour of the excretion of
N-acetyl- S-(2-cyanoethyl)-L-cysteine (van Bladeren et al., 1981).
The latter compound, the authors considered, resulted from direct
cyanoethylation of glutathione, whereas the former was formed via
the epoxide, glycidonitrile. Overnight fasting and cobaltous
chloride pre-treatment increased the biliary excretion of
metabolites, while phenobarbital did not induce any change, and
dimethyl maleate significantly decreased the excretion (Ghanayem &
Ahmed, 1982).
6. BIOLOGICAL MONITORING OF ACRYLONITRILE UPTAKE
Studies, particularly animal studies, on the absorption,
distribution, biotransformation, and elimination of acrylonitrile
have shown that a small fraction of the acrylonitrile absorbed is
rapidly eliminated in the urine without biotransformation, while
the remainder is biotransformed via several pathways, a number of
metabolites being excreted in urine; some of these metabolites are
unique to acrylonitrile.
The absorption studies have also clearly shown that, in
addition to uptake of acrylonitrile by inhalation, skin penetration
can be an important route of entry, particularly in the presence of
liquid acrylonitrile. Thus, in human studies, unless performed
under controlled conditions, a good correlation cannot necessarily
be expected between a bioindicator of uptake and ambient air
measurements of acrylonitrile, even when carried out with personal
samplers.
Possible indicators of acrylonitrile uptake at present include:
acrylonitrile in urine, acrylonitrile-derived mercapturic acids in
urine, total thioethers in urine, and thiocyanates in urine.
Houthuijs et al. (1982) studied the excretion pattern of
acrylonitrile in the urine of 15 exposed workers over a 7-day
period, with a control group of 41 unexposed workers. They noted
that the concentrations of acrylonitrile in urine peaked at the
end, or shortly after the end, of the working day, decreasing
rapidly until the beginning of the next working day without,
however, falling to the levels in the control group. Correlations
have been found between acrylonitrile concentrations in air and
those in urine. In the control group, a significant increase in
the acrylonitrile excretion in urine was found with the number of
cigarettes smoked. For a mean acrylonitrile concentration in air
of 0.28 mg/m3 (0.13 ppm), the mean acrylonitrile level in urine at
the end of the working day was 38 µg/litre, using the headspace
chromatographic technique. In the control group for non-smokers,
the mean level of acrylonitrile in urine was 2 µg/litre and for
smokers (20-30 cigarettes per day) 9.0 µg/litre.
Sakurai et al. (1978) have also established a relationship
between acrylonitrile concentrations in air and levels in urine for
a group of 102 exposed workers and compared them with 62 controls.
For an air concentration of 0.2 mg/m3 (0.1 ppm) (as measured by
personal samplers), an acrylonitrile level in urine of 3.0 µg/litre
was found, using the Sato et al. (1975) method of analysis
(separation by azeotropic distillation and determination by gas
chromatography). The urine samples were collected at the end of
the working day. At an air concentration of 1.1 mg/m3 (0.5 ppm),
the level of acrylonitrile in urine was 19.7 µg/litre, and at a
concentration in air of 9 mg/m3 (4.2 ppm) the corresponding level
in urine was 359.6 µg/litre. Acrylonitrile could not be detected
in the urine of controls. At the same time, an increase in the
thiocyanate level in urine was noted, particularly at the higher
exposure levels.
According to Houthuijs et al. (1982), the differences found
between the urine levels of acrylonitrile in the two studies are
most likely due to differences in analytical techniques.
The validity of the determination of urine levels of
acrylonitrile using gas chromatography-head space analysis for
monitoring acrylonitrile-exposed workers was established by Benchev
et al. (1982).
A promising method for estimating total acrylonitrile uptake
seems to be the determination of acrylonitrile-derived mercapturic
acids; such acids are specific for acrylonitrile and are absent
from normal urine. They have been shown in experimental animal
studies to be well correlated with the free acrylonitrile
concentration in blood (Nerudova et al., 1980; Gut et al.,
1981a,b); animal data also indicate that the capacity of the enzyme
systems to produce the acrylonitrile-derived mercapturic acids is
unlikely to be exceeded at the exposure levels of interest (van
Bladeren et al., 1981). Draminski & Trojanowska (1983) established
the presence of S-(2-cyanoethyl) mercapturic acid in the urine of
13 workers exposed to acrylonitrile, using a gas chromatographic
technique. The concentrations ranged between 50 and 200 mg/litre
for ambient acrylonitrile levels between 3.3 and 9.8 mg/m3 (1.5 and
4.5 ppm). The "total thioethers" were also determined in the urine
samples by a spectrophotometric method (Kopecky, 1982) and shown to
be strongly correlated with the S-(2-cyanoethyl) mercapturic acid
excretion, indicating that, in the case of exposure to pure
acrylonitrile, the major part of the sum of "thioethers" is
represented by this specific mercapturic acid.
Increased glucuronic acid excretion was reported by Ostrovskaja
et al. (1976) in 45.5% of workers exposed to acrylonitrile
concentrations of 0.7-1.5 mg/m3 (0.3-0.7 ppm).
The recent studies reported above show that biological
monitoring may become a suitable approach for assessing
acrylonitrile uptake, in particular in relation to the working
environment. Both acrylonitrile in urine and acrylonitrile-derived
mercapturic acids in urine seem to be the most suitable
bioindicators of uptake, at present, as they have the advantage of
specificity. More work is needed to resolve the apparent
discrepancies due to analytical techniques and to determine the
half-lives. This should make it possible to establish the most
appropriate sampling time with respect to exposure and help in the
determination of the concentrations of concern.
Interest in the determination of total "thioethers" in urine as
a bioindicator of uptake lies in the greater simplicity of the
analytical techniques used. However, more work is needed,
particularly with regard to interferences and half-lives.
The possibility of estimating acrylonitrile exposure in smokers
was suggested by Della Fiorentina & De Wiest (1979), who observed
that determination of carboxyhaemoglobin in blood makes it possible
to calculate the amount of thiocyanate present in urine that is due
to smoking, and thus to calculate the uptake of acrylonitrile.
However, experience shows that there can be marked variations in
thiocyanate levels in smokers, which greatly exceed those in non-
smokers occupationally-exposed to acrylonitrile (Czajkowska et al.,
1969).
7. EFFECTS ON EXPERIMENTAL ANIMALS AND CELL SYSTEMS
7.1. Acute Toxicity
7.1.1. Lethal doses and concentrations
7.1.1.1. Lethal doses
The range of acute LD50 values for acrylonitrile in different
laboratory mammals is generally between 25 and 186 mg/kg body
weight (Table 9), though a value of 282 mg/kg body weight was
observed when liquid acrylonitrile was applied to the skin of the
tail of male rats (Zotova, 1976). Mice are more sensitive than
rats, guinea-pigs, and rabbits. There seems to be little
consistency in the effects of route or vehicle of administration,
or of sex, on the LD50 level. The LD50 for dogs was not reported,
but they tolerated iv administration of acrylonitrile at 50 mg/kg
body weight and died after 300 mg/kg (Graham, 1965). The LD50
values reported are an order of magnitude higher than the LD50 for
cyanide (one of the metabolites of acrylonitrile), but markedly
lower than those for industrial solvents and monomers of plastics
(the LD50 for benzene and its derivatives being about 2000-3000
mg/kg body weight).
7.1.1.2. Lethal concentrations in the air
The range of acute LC50 s for 4-h inhalation of acrylonitrile is
between 150 and 1250 mg/m3 (Table 10). Dogs appeared to be the
most sensitive of the species tested and the sensitivity decreased
in the following order: mice, rabbits, cats, rats, guinea-pigs, the
latter being apparently the most resistant to inhalation exposure.
The exposure of 315-350 cm2 of the skin of rabbits to an
acrylonitrile concentration of 44-62 g/m3, in an exposure chamber,
such that the animals were breathing pure air, proved fatal after
2.5-4 h. Inhalation exposure to 0.58-0.67 g/m3 was fatal for 3
rabbits within 2-3 h (Rogaczewska, 1975).
In the 3 species of insects tested in a fumigation chamber
for 8 h, the LC50 value was found to be 700-1900 mg/m3 (Bond &
Buckland, 1976). Lindgren et al. (1954) exposed 8 insect
species for 2 or 6 h and found LC50 values of 1000-4500 mg/m3 .
Table 9. Acute LD50 values for acrylonitrile: effect of animal species
strain and route of administration
---------------------------------------------------------------------------------------------
Species/strain/sex Number Route LD50 (mg/kg Vehicle Reference
body weight)
---------------------------------------------------------------------------------------------
mouse/-/male M + F 333 oral 36 water Tullar (1947)
mouse/-/female M + F 333 oral 48 water Tullar (1947)
mouse/-/M + F 169 oral 40 olive oil Tullar (1947)
mouse/H strain/- - oral 25 physiol. Benesh & Cherna
saline (1959)
mouse/-/- - oral 40-46 - American Cyanamid
(1951)
mouse/-/female M + F 325 ip 48 water Tullar (1947)
mouse/-/male M + F 325 ip 40 water Tullar (1947)
mouse/NMRI or "SPF"/- - ip 50 - Zeller et al.
(1969)
mouse/ICR/female - ip 47 - Yoshikawa (1968)
mouse/H strain/- - sc 35("technical physiol. Benesh & Cherna
AN") saline (1959)
mouse/"inbred"/male 60 sc 50 (2 h) physiol. Graham (1965)
saline
25 (24 h)
mouse/BN/male 60 sc 34 - Knobloch et al.
(1971)
rat/Sherman/- groups of oral 93 - Smyth & Carpenter
6-10 (1948)
rat/Wistar/- - oral 101 - Paulet & Vidal
(1975)
rat/Wistar or Stock/- - oral 128 - Zellar et al.
(1969)
rat/Wistar-Stamm/male - oral 82 - von Borchardt
et al. (1970)
rat/Wistar-Stamm/female - oral 86 - von Borchardt
et al. (1970)
rat/-/M + F 80 oral 84 water Tullar (1947)
rat/-/M + F 51 oral 72 olive oil Tullar (1947)
rat/Wistar/- - oral 78 physiol. Benesh & Cherna
saline (1959)
rat/Sprague-Dawley/male 20 oral 186 water Monsanto (1975)
rat/Sprague-Dawley/female 20 oral 186 water Monsanto (1975)
Table 9. (contd.)
---------------------------------------------------------------------------------------------
Species/strain/sex Number Route LD50 (mg/kg Vehicle Reference
body weight)
---------------------------------------------------------------------------------------------
rat/Wistar/male 110 ip 100 - Knobloch et al.
(1971)
rat/Wistar/- - ip 65 poly- Paulet & Vidal
ethylene (1975)
glycol
rat/Wistar/male 110 sc 80 - Knobloch et al.
(1971)
rat/"albino"/male - sc 96 water Magos (1962)
rat/"white"/male - skin of 282 liquid Zotova (1976)
tail acrylonitrile
rat/"white"/male - skin of 148 liquid Zotova (1976)
abdomen acrylonitrile
guinea-pig/-/- - oral 50 - Carpenter et al.
(1949)
guinea-pig/-/- - oral 85 olive oil Tullar (1947)
guinea-pig/-/M & F 30 oral 56 - Jedlicka et al.
(1958)
guinea-pig/-/- - sc 130 - Ghiringhelli (1954)
guinea-pig/-/- 11 iv 72 water Tullar (1947)
guinea-pig/Hartley-/male 12 or more intact 0.46 ml/kg - Roudabush et al.
skin (1965)
abraded 0.86 ml/kg - Roudabush et al.
skin (1965)
guinea-pig/-/- - skin 0.25 ml/kg - Smyth & Carpenter
(1948)
rabbit/-/- - oral 93 - Lefaux (1966)
rabbit/-/- - iv 69 - Paulet & Desnos
(1961)
rabbit/"white"/M & F 12 or more abraded 0.28 ml/kg - Roudabush et al.
skin (1965)
---------------------------------------------------------------------------------------------
Table 10. Acute lethal effect of single inhalation of acrylonitrile: effect of
duration and concentration of acrylonitrile
---------------------------------------------------------------------------------------------------
Species/strain/sex Number Concentration Duration Mortality Reference
(mg/m3) (h) (died/
tested)
---------------------------------------------------------------------------------------------------
white mouse/stock/- 6 600 0.5 0/6 McOmie (1949)
6 1500 0.5 5/6 McOmie (1949)
6 5800 0.5 5/6 McOmie (1949)
6 900 1 1/6 McOmie (1949)
6 900 2 3/6 McOmie (1949)
6 1700 1 6/6 McOmie (1949)
mouse/BN/male 12 300 4 LC50 Knobloch et al. (1971)
rat/Sherman/- 6 1085 4 0/6 Smyth & Carpenter et al. (1971)
6 2170 4 6/6 Smyth & Carpenter et al. (1971)
rat/Sherman/female 6 1085 4 2/6 to Carpenter et al. (1949)
4/6
rat/Wistar/- 20 54 7 0/20 Brieger et al. (1952)
20 109 7 0/20 Brieger et al. (1952)
20 163 7 0/20 Brieger et al. (1952)
20 217 7 4/20 Brieger et al. (1952)
rat/Wistar/male 12 470 4 LC50 Knobloch et al. (1971)
rat/Osborne-Mendel/- 16 2750 1 0/16 Dudley & Neal (1942)
16 3230 1 4/16 Dudley & Neal (1942)
16 5300 1 13/16 Dudley & Neal (1942)
16 660 2 0/16 Dudley & Neal (1942)
16 1290 2 6/16 Dudley & Neal (1942)
16 2730 2 16/16 Dudley & Neal (1942)
16 280 4 0/16 Dudley & Neal (1942)
16 680 4 5/16 Dudley & Neal (1942)
16 1380 4 16/16 Dudley & Neal (1942)
16 290 8 0/16 Dudley & Neal (1942)
16 460 8 1/16 Dudley & Neal (1942)
16 590 8 7/16 Dudley & Neal (1942)
16 690 8 15/16 Dudley & Neal (1942)
---------------------------------------------------------------------------------------------------
Table 10. (contd.)
---------------------------------------------------------------------------------------------------
Species/strain/sex Number Concentration Duration Mortality Reference
(mg/m3) (h) (died/
tested)
---------------------------------------------------------------------------------------------------
rat/Wistar/male 3 650 3 1/3 Appel et al. (1981)
3 1100 2 3/3 Appel et al. (1981)
3 2600 0.5 1/3 Appel et al. (1981)
6 3000 0.5 6/6 Appel et al. (1981)
guinea-pig/-/- 8 580 4 0/8 Dudley & Neal (1942)
8 1250 4 5/8 Dudley & Neal (1942)
8 2520 4 8/8 Dudley & Neal (1942)
guinea-pig/-/- 12 990 4 LC50 Knobloch et al. (1971)
rabbit/"albino"/- 2 290 4 0/2 Dudley & Neal (1942)
2 560 4 2/2 Dudley & Neal (1942)
2 1260 4 2/2 Dudley & Neal (1942)
rabbit/-/- 5 670 - 1100 2-3 5/5 Rogaczewska (1975)
cat/-/- 4 210 4 0/4 Dudley & Neal (1942)
2 600 4 0/2 Dudley & Neal (1942)
2 1300 4 2/2 Dudley & Neal (1942)
dog/-/- 3 63 4 0/3 Dudley & Neal (1942)
2 140 4 1/2 Dudley & Neal (1942)
3 213 4 0/3 Dudley & Neal (1942)
2 240 4 2/2 Dudley & Neal (1942)
dog/-/- 4 108 7 0/4 Brieger et al. (1952)
4 163 7 0/4 Brieger et al. (1952)
6 213 7 6/6 Brieger et al. (1952)
Rhesus monkey/-/- 3 163 7 1/3 Brieger et al. (1952)
---------------------------------------------------------------------------------------------------
7.1.1.3. Lethal concentrations in water
(a) Fish
Acute toxicity, determined by a static bioassay at 25 °C,
revealed TLm (median tolerance limit, i.e., a concentration of
acrylonitrile killing 50% of the test organisms within a specified
time) values ranging from 25.5 to 44.6 mg/litre at 24 h, and from
11.8 to 33.5 mg/litre at 96 h. There were no apparent significant
differences in the sensitivity of various kinds of fish (Table 11).
(b) Invertebrates
For the brown shrimp (Crangon crangon), the LC50 for a 24-h
exposure was 10-33 mg/litre (Portman & Wilson, 1971). Bandt (1953)
exposed several species of arthropods (a shrimp-like crustaceae and
3 types of larvae) to 20-100 mg acrylonitrile/litre water and found
marked species and individual differences: a lethal effect was
observed in some species with 25 mg/litre after 48 h, while other
species were not affected after 3 days. The most resistant species
were unaffected by 100 mg/litre after 24-48 h. The results of
studies by Rajendran & Muthu (1981) showed that acrylonitrile
affects the activity of the phosphorylase and acetylcholinesterase
enzymes in Tribolium castaneum Herbst, and Trogoderma granarium
Everts.
7.1.2. Clinical observations
The inhalation studies of Dudley & Neal (1942), Brieger et al.
(1952), and Rogaczewska (1975), and the results of oral and
parenteral administration (Ghiringhelli, 1954; Benesh & Cherna,
1959; Paulet & Despos, 1961; Graham, 1965; Paulet et al., 1966)
indicate that animals inhaling lethal concentrations of
acrylonitrile, or administered lethal dosages of acrylonitrile
orally or parenterally, showed rather similar effects: excitability
and stimulated breathing, shallow rapid breathing, slow gasping
breathing, apnoea, convulsions, and death. Vomiting occurred in
cats, dogs, and monkeys after inhaling acrylonitrile, and in rats
following parenteral administration. Reddening of the skin of the
ears, nose, and feet (in rhesus monkeys, also of the face and
genital organs) and mucosa was accompanied by lachrymation, nasal
discharge, and salivation, not only after inhalation exposure, but
also following oral and sc administration, while hind-leg
incoordination, paresis or paralysis, were observed in rats after
oral administration, and in rabbits after iv administration.
Table 11. Median tolerance limit values (TLm)a for various fish exposed to
acrylonitrile
-------------------------------------------------------------------------------------
Species Water type TLm (mg/litre) Reference
24 h 48 h 96 h
-------------------------------------------------------------------------------------
Fathead minnow hard 32.7 16.7 14.3 Henderson et al. (1961)
(Pimphales promelas) soft 34.3 21.5 18.1 Henderson et al. (1961)
Minnow (Phoxinus - 38.2 17.6 - Marcoci & Ionescu (1974)
phoxinus)
Bluegill (Lepomis soft 25.5 14.3 11.8 Henderson et al. (1961)
macrochirus)
Guppy (Lebistes soft 44.6 33.5 33.5 Henderson et al. (1961)
reticulatus)
Goldfish (Carassius - - - 40 Paulet & Vidal (1975)
sp.)
Carp (Cyprinus - 37.4 24.0 - Marcoci & Ionescu (1974)
carpio)
Rainbow trout hard - 70 - Jackson & Brown (1970)
(Salmo gairdneri)
Pin perch sea 24.5 - - Daugherty & Garett (1951)
(marine fish) (30/l tank)
(Lagodon rhomboides)
Rainbow trout tap, - 5b - Sloof (1979)
(Salmo gairdneri) dechlorinated,
3.6 mg/litre
hard 15 - - Sloof (1979)
Zebra fish same (LC50)
-------------------------------------------------------------------------------------
a TLm median tolerance limit, a concentration of acrylonitrile killing
50% of the test organisms within a specified time.
b Minimal concentration changing respiratory frequency.
(a) Effects on the skin
Direct application of liquid acrylonitrile to the shaved skin
of rabbits induced slight local vasodilation immediately, without
any systemic effect (1-2 ml covering 100-200 cm2) or with an
increased respiratory rate (3 ml over 300 cm2) (McOmie, 1949).
Tuller (1947) observed erythema in only one of 3 areas of abraded
skin, following application of 1 ml of acrylonitrile on a gauze pad
covered by rubber sheeting. However, Zeller et al. (1969) found
that a 15-min application of acrylonitrile on a cotton pad to
shaved skin resulted in skin oedema, and a 20-h application, in
slight necrosis. Guinea-pigs appear to be more sensitive than
rabbits; the application of a 2% solution of acrylonitrile in
acetone for 24 h, under occlusion, did not induce any effects, but
8% or higher concentrations induced dose-dependent erythema
followed by desquamation and mild necrosis (Gut et al., unpublished
data). Erythema of the nose, face, ears, legs, and genital organs
may follow inhalation and oral administration of acrylonitrile.
(b) Effects on the eye
McOmie (1949) instilled one drop of acrylonitrile into the eye
of a rabbit. After 1 h, there was mild conjunctivitis without
corneal clouding or pupillary damage and no effects were observed
after 24 h. Oedema and slight necrosis of the conjunctiva after 8
days were observed in rabbits by Zeller et al. (1969).
(c) Effects on respiration
It was stressed by Paulet et al. (1966) that, after a lethal
intravenous dose of acrylonitrile (120 mg/kg body weight), the
respiratory rate in rabbits did not increase as is characteristic
in cyanide poisoning. However, respiratory disturbance was
observed in: guinea-pigs given a sc lethal dose of acrylonitrile
(130 mg/kg body weight) (Ghiringhelli, 1954), anaesthetized dogs
given 100 mg/kg body weight intravenously (Graham, 1965), mice
given an oral lethal dose (Benesh & Cherna, 1959), and in guinea-
pigs given 100 mg/kg body weight orally (Jedlicka et al., 1958).
Pulmonary oedema was also seen.
An increased respiratory rate followed the application of
liquid acrylonitrile (3 ml/kg body weight) to the skin of rabbits
(McOmie, 1949), and "respiratory distress" was reported in rhesus
monkeys exposed to 163 mg/m3 for 7 h (Brieger et al., 1952). When
rats, rabbits, cats, dogs, and monkeys were exposed to lethal
concentrations of acrylonitrile, Dudley & Neal (1942) observed an
initial stimulation of respiration followed by shallow rapid
breathing, slow gasping breathing, convulsions, coma, and death.
These respiratory effects were absent in guinea-pigs, but
irritation of the pulmonary membranes and some delayed deaths from
lung oedema occurred.
(d) Effects on circulation
Acrylonitrile administered iv at 13, 27, 55, or 110 mg/kg body
weight had little effect on the respiratory and blood pressure
responses of anaesthetized rabbits to adrenalin, noradrenalin, or
acetylcholine, and Gravczyk & Zwierzchovski (1973) believed that
the circulation was not the primary target organ in acrylonitrile
poisoning. However, lethal doses (50 or 100 mg/kg body weight) in
guinea-pigs caused dilation of the right ventricle, congestion of
the coronary blood vessels, hepatic and splenic hyperaemia, and
inflammation of the intestinal mucosa (Jedlicka et al., 1958). In
Sprague-Dawley rats administered a lethal dose of acrylonitrile,
there were haemorrhagic areas in the lung and liver and acute
gastrointestinal inflammation (Monsanto, 1975). Whether the
reddening of nose, ears, legs, face, and genital organs in rats and
other species, after inhalation and oral administration of
acrylonitrile, is due to a direct effect on small vessels or is an
inflammatory response is not known.
(e) Effects on adrenals
The effect of lethal doses of acrylonitrile on the adrenals
became evident in the reports of Szabo & Selye (1971, 1972) and
Szabo et al. (1976). After iv administration of high doses (150 or
200 mg/kg body weight), haemorrhage was observed in both adrenals
of most animals, and there was adrenal haemorrhage in some rats
following oral administration of 10, 15, or 20 mg/kg body weight.
Various types of histological damage were observed in the adrenal
cortex and medulla, some of them within 30 min of acrylonitrile
administration.
A possible mechanism involving the peroxidative action of
acrylonitrile in acrylonitrile-induced adrenal injury has been
suggested recently by Silver & Szabo (1982). Szabo et al. (1980)
investigated the pathogenesis of experimental adrenal haemorrhagic
necrosis using various morphological, biochemical, and
pharmacological methods. Their results suggest that the presence
of a functional adrenocortex is necessary for the development of
cortical damage.
(f) Blood chemistry
Intraperitoneal administration of acrylonitrile to male rats at
33 mg/kg body weight per day for 3 days decreased serum
corticosterone to 30%, prolactin to 40%, but increased follicle-
stimulating hormone (FSH) to 200% of control levels and did not
affect luteinizing hormone (LH) (Nilsen et al., 1980). In adult
male Wistar rats, a single ip administration of acrylonitrile of 10
mg/kg body weight did not have any effect on serum glutamic
oxaloacetic transferase (SGOT) and serum glutamic pyruvate
transaminase (SGPT) activity, but increased lactate dehydrogenase
(EC 1.1.1.27) (LDH) to 200% and sorbitol dehydrogenase (SDH) (EC
1.1.1.14) to 300% compared with the controls (Noel et al., 1978).
The same dose in male rats inhibited butyrylcholineesterase (EC
3.1.1.8), did not have any effect on alkaline phosphatase (EC
3.1.3.1), and increased fructose monophosphate aldolase activity,
suggesting that there had been an adverse effect on the liver
(Ivanov et al., 1979). Administraton of L-cysteine, alpha
tocopherol, or ionol prevented these effects. A single oral dose
of acrylonitrile (l/2 LD50, 41 mg/kg body weight) in rats resulted
in changes in the elution patterns of serum gel chromatography and
paper-electrophoresis of globulins (Franzen & Wagner, 1978). Serum
SDH was significantly elevated (approximately 4-fold) in rats, 24 h
after administration of acrylonitrile at 150 mg/kg body weight. A
60% increase in serum SDH was found in rats administered
acrylonitrile at 500 mg/litre in drinking-water for 21 days (Silver
et al., 1982).
(g) Effects on other organs
Focal superficial necrosis of the liver associated with
haemorrhagic gastritis was found in rats necropsied 24 h after
administration of acrylonitrile at 150 mg/kg body weight in the
drinking-water (Silver et al., 1982).
Acrylonitrile shows an inhibitory effect on K-stimulated
respiration of guinea-pig brain cortex slices at 1 mM, but little
effect on the liver at the same concentration. A stronger
anaesthetic action of acrylonitrile was detected in vitro on the
sciatic nerve of Rana nigra maculata, compared with some other
anaesthetic agents (Hashimoto & Kanai, 1965). The recovery phase of
nerve excitation was also affected by acrylonitrile (Ando &
Hashimoto, 1967).
7.1.3. Biochemical changes and mechanisms of acrylonitrile
toxicity
7.1.3.1. Effect on cytochrome oxidase
Evidence has been presented that cytochrome oxidase (EC
1.9.3.1) activity may be significantly inhibited in acrylonitrile
poisoning. This was suspected after Dudley & Neal (1942) and
Brieger et al. (1952) had reported significant concentrations of
cyanide in dogs and monkeys exposed to acrylonitrile vapours.
Tarkowski (1968) observed inhibited cytochrome oxidase activity in
the brain, kidneys, and liver of rats after ip injection of
acrylonitrile at 100 mg/kg body weight. In vitro, a 50%
inhibition of the enzyme was observed in homogenates of brain,
kidneys, and liver with an acrylonitrile concentration of 10-3M.
Such concentrations have been observed in vivo, shortly after
lethal doses of acrylonitrile (Tarkowski, 1968; Nerudova et al.,
1980; Gut et al., 1981b), but acrylonitrile has a short half-life
in blood and liver after ip or iv administration (15-20 min).
There are correspondingly increased blood and liver concentrations
of cyanide in rats after acrylonitrile administration (up to 180 µM,
Gut et al., 1981b), together with an even greater inhibition of
cytochrome oxidase, and a much higher sensitivity of cytochrome
oxidase activity to cyanide (50% inhibition in vitro at 10-8M
(Tarkowski, 1966).
A significantly decreased ratio of oxidized to reduced
nicotinamide-adenine dinucleotides was observed by Sokal et al.
(1972, 1977) in the brain of rats after sc administration of
acrylonitrile at 100-120 mg/kg body weight, indicating inhibition
of NADH oxidation in the mitochondria, possibly also at the level
of cytochrome oxidase. These changes seem to be of biological
importance, because their magnitude was similar to that observed at
the death of animals subjected to experimental hypoxia. Thus, the
cyanide-mediated inhibition of cytochrome oxidase would seem to be
of importance in the later stages of intoxication and death. This
"cyanide effect" is apparently more pronounced at higher
acrylonitrile doses (Willhite & Smith, 1981) and appears to be more
significant in mice and dogs (Brieger et al., 1952; Benesh &
Cherna, 1959; Gut et al., 1981b) than in rats. This is in
agreement with the greater efficacy in mice than in rats of
thiosulfate (an antidote to cyanide) in acrylonitrile poisoning,
and with the higher cyanide concentration in the blood of dogs than
in that of rats (101 µM versus 10 µM), after breathing the same
concentration of acrylonitrile (217 mg/m3 for 7 h) (Brieger et al.,
1952). The protective effect of another cyanide antidote, nitrite
(Dudley & Neal, 1942; Ghiringhelli, 1954; Benesh & Cherna, 1959),
in acrylonitrile poisoning also points to the participation of
cyanide in lethal acrylonitrile poisoning.
7.1.3.2. Effect on sulfhydryls
There is considerable evidence to demonstrate that
acrylonitrile significantly depresses the concentrations of soluble
glutathione and protein sulfhydryls in the blood, liver, brain, and
kidney. Acrylonitrile also inhibits some SH-dependent enzymes that
participate in carbohydrate metabolism. Wisniewska-Knypl (1970,
1978), Hashimoto & Kanai (1972), and Vainio & Mäkinen (1977)
observed that the inhibition of sulfhydryls was dose-dependent in
the range of 10-100 mg/kg body weight in vivo, and in the
concentraton range 0.01-10 nM in vitro (Wisniewska-Knypl, 1978).
A significant decrease in brain sulfhydryls was reported after a
single dermal application of acrylonitrile of as little as 2.82
mg/kg body weight (Zotova, 1976). These effects were observed
after sc, ip, or iv administration to rats, rabbits, hamsters,
guinea-pigs, and mice. There were some decreases in the activity
of serum or tissue - SH-dependent enzymes including oxoglutarate
dehydrogenase (EC 1.2.4.2). However, the activity of succinate
dehydrogenase (EC 1.3.99.1) was not reduced (Wisniewska-Knypl,
1978), and there were corresponding increases in the liver, blood,
and brain concentrations of glucose, pyruvate, and lactate
(Hashimoto & Ando, 1966; Dinu & Klein, 1976).
It was shown by Zitting et al. (1981) that short-term exposure
to acrylonitrile decreased the liver glutathione content within 4 h
of poisoning, but that the glutathione contents returned to normal
in brain, liver, and kidney, within 24 h. At the same time, the
activity of cerebral succinate dehydrogenase and of ethoxycoumarin
demethylase in liver and kidney decreased. Increased glucose,
pyruvate, and lactate concentrations in blood, liver, and brain
were also found, immediately after the fifth exposure, in rats
exposed through inhalation to an acrylonitrile concentration of 300
mg/m3, 8 h daily, for 5 days. In protein, sulfhydryl-dependent
enzyme inhibition was absent and the glutathione level was
significantly reduced in the liver but not in the brain (Gut et
al., 1982). The effects of acrylonitrile on sulfhydryls were
significantly reduced by co-administering L-cysteine and other
sulfhydryls and there were corresponding decreases in lethal
effects (Hashimoto & Kanai, 1965; Bondarev et al., 1976; McLaughlin
et al., 1976; Appel et al., 1981). The results of these studies
demonstrate the protective role of SH-groups in acrylonitrile
poisoning.
The role of hypoxia in the acute thiol-depressive effect of
acrylonitrile in male rats was investigated by Jaeger (1978) and
Jaeger & Cote (1982). Hypoxia was found to enhance non-protein SH
loss in the liver, when there was exposure to acrylonitrile (Jaeger
& Cote, 1982).
Evidence has been presented (Holechek & Kopecky, 1981) that
inhibition of tissue sulfhydryls may be due not only to the
acrylonitrile itself, but also to its reactive metabolite,
glycidonitrile.
7.1.3.3. Interaction with the microsomal oxidation system as a
possible mechanism of toxicity
Acrylonitrile added to mouse, rat, and human liver microsomes
caused characteristic spectral complexes with cytochrome P-450
(Ivanov et al., 1979; Appel et al., 1981).
It was shown in vitro that glycidonitrile, which is
generated in rat-liver microsomes by mixed-function oxidases (EC
1.14.14.1), covalently binds to microsomal membrane and albumin
(Ivanov et al., 1982). The biological significance of this
phenomenon may be inferred from experiments with inhibitors and
inducers of the mixed-function oxidases (Ivanov, 1981). In rats
pre-treated with phenobarbital, an increased amount of
glycidonitrile was covalently bound to macromolecules and
substantially higher activity of fructose-bisphosphate aldolase (EC
4.1.2.13) was observed in the blood, indicating liver damage. On
the other hand, SKF-525A, the inhibitor of cytochrome P-450,
reduced both effects in vivo and in vitro . Activation of
acrylonitrile by cytochrome P-450 may therefore result in a
cytotoxic effect.
A previous injection of SKF-525A or of cobalt(II) chloride
(CoCl2), another inhibitor of cytochrome P-450, resulted in
significant protection against the gastrointestinal bleeding in
rats caused by acrylonitrile (Ghanayem & Ahmed, 1982).
Some data show that acrylonitrile and its epoxide,
glycidonitrile, bind covalently, in vitro, to DNA and RNA
(Guengenrich et al., 1981; Peter et al., 1983a). However, the
quantitative extent of truly irreversible binding is much less than
that observed in experiments with other vinyl monomers (Peter et
al., 1983).
A lower content of cytochrome P-450 in the liver and reduced
oxidative microsomal metabolism of xenobiotics were observed in
rats after an ip injection of acrylonitrile at 10 or 33 mg/kg body
weight, for 3 days (Noel et al., 1978; Nilsen et al., 1980), after
inhalation exposure to 300 mg/m3 of acrylonitrile, 8 h day, for 5
days (Gut et al., in press), and in Chinese hamsters after an ip
injection of 30 mg/kg (Zitting et al., 1981). Inhibition of
microsomal oxidation of xenobiotics by acrylonitrile was observed
in vitro (Ivanov et al., 1979).
Pre-treatment of rats with inducers of microsomal oxidases such
as phenobarbital, 3-methylcholantrene, or Arochlor 1254, nullified
the effect of acrylonitrile on the total cytochrome P-450 content.
The activity of other microsomal enzymes, glucose-6-phosphatase (EC
3.1.3.9), and NADPH cytochrome-c-reductase (EC 1.6.2.4), was
unaffected by acrylonitrile (Duverger-van Bogaert et al., 1978;
Noel et al., 1978).
Ghanayem & Ahmed (1982) showed that Arochlor 1254 drastically
increased acrylonitrile-induced gastric bleeding in rats.
Phenobarbital significantly increased acrylonitrile-induced
hepatocyte damage in rats (Ivanov, 1981).
Acrylonitrile binds with liver microsomal and S-9 fractions, in
vitro, by direct alkylation. The microsomal activation of
acrylonitrile into reactive intermediates was also detected
(Duverger-van Bogaert et al., 1982a). The irreversible binding of
acrylonitrile to liver microsomal proteins was inhibited by thiols
and even more by dithiocarb (Peter & Bolt, 1981).
7.1.3.4. Observations on the possible participation of membrane
lipid peroxidation in the mechanism of toxicity
The ip administration of acrylonitrile at 10 mg/kg body weight
induced lipid peroxidation in rat liver (Dinu, 1975a; Ivanov et
al., 1979) and erythrocyte membranes (Ivanov et al., 1982),
indicating possible damage of cellular membranes. NADPH-dependent
lipid peroxidation in rat liver microsomes was only slightly
stimulated (Ivanov et al., 1978; Duverger-van Bogaert et al., 1981)
whereas substantial stimulation was found in the post-mitochondrial
fraction of rat liver, lung, and brain, as well as in brain-marrow
homogenate (Ivanov et al., 1978; Ivanov, 1979; Al'shansky et al.,
1980). There was a correlation between an increased amount of
malondialdehyde and a decreased content of -SH groups in the post-
mitochondrial supernatant of rat liver (Ivanov, 1981) and brain
(Al'shansky et al., 1980). Conjugated diene concentrations in rat
liver microsomes were significantly elevated after iv
administration of acrylonitrile to rats (150 mg/kg body weight),
but no change was seen in the adrenal glands (Silver & Szabo,
1982).
Pre-treatment of rats with antioxidants, in doses equivalent to
those of the acrylonitrile administered, afforded protection
against the pro-oxidant effect of acrylonitrile and elevation of
blood fructose-1-phosphate aldolase, decreased the activity of
butyrylcholine esterase (EC 3.1.1.8) (Ivanov et al., 1979), and
reduced the GABA content and activity of glutamate decarboxylase
(EC 4.1.1.15) in the brain (Al'shansky et al., 1980).
7.1.3.5. Studies on antidotes
Appel et al. (1981a) found that the cyanide antidotes, 4-
dimethylaminophenol plus thiosulfate, protected rats against the
lethal effects of orally-administered acrylonitrile. A comparative
evaluation was made by McLaughlin et al. (1975) of the efficacy of
thiols (cysteine hydrochloride) and cyanide antidotes. The authors
showed that thiols were more effective in protecting rats against
acrylonitrile poisoning. Bondarev et al. (1976) demonstrated the
protective role of some sulfur-containing compounds in the
acrylonitrile poisoning of rats. The protective effects of some
antioxidants, such as vitamin E and ionol, have also been
demonstrated (Ivanov et al., 1979).
The possible toxic mechanisms and theoretical protective
mechanisms are summarized in Fig. 3, which indicates the complex
nature of the interference of acrylonitrile with cellular
mechanisms, as far as can be derived from current knowledge.
 7.2. Subacute Toxicity
7.2.1. Inhalation exposure
Rats, guinea-pigs, rabbits, monkeys, and cats were exposed to
acrylonitrile at 330 mg/m3 air for 4 h per day, 5 days a week, for
8 weeks. All adult rats survived 8 weeks, but 5 out of 8 young
rats died by the 6th week, 3 out of 16 guinea-pigs and 1 out of 4
rabbits died during the 5th week, and 1 out of 2 monkeys died after
6 weeks of exposure. At 220 mg/m3 for 4 h/day, 5 days a week, for
8 weeks, all rats, rabbits, and guinea-pigs survived for 8 weeks,
but 1 out of 4 cats died during the third week. After the first 4-
h exposure to 120 mg/m3, 1 out of 2 dogs died, but the other
survived 4 weeks' exposure. Four rhesus monkeys survived 4 weeks'
exposure to 120 mg/m3 for 4 h/day (Dudley et al., 1942).
CD-1 mice, Charles River rats, and beagle dogs were exposed to
acrylonitrile 57 times for 6 h a day, 5 days a week, over a 90-day
period. Some dogs were killed by exposure to 117 mg/m3 (54 ppm)
but not 58 mg/m3 (24 ppm). Mice and rats were unaffected by these
concentrations, but a concentration of 234 mg/m3 (108 ppm) was
lethal for half the rats and mice. As with acute exposures, dogs
were more sensitive than rats and mice, but mice did not appear to
be more sensitive than rats. Atmospheric concentrations of
acrylonitrile of 58, 117, and 117 mg/m3 did not induce any lethal
effects in dogs, rats, and mice, respectively (Brewer, 1976).
7.2.2. Oral administration
Over a period of 7 weeks, 6 rats were administered orally 15
doses of acrylonitrile at 30 mg/kg body weight, then 7 doses at 50
mg/kg, followed by 13 doses at 75 mg/kg, without lethal effects
being induced (Barnes, 1970). No deaths occurred when Sprague-
Dawley rats were offered 85 mg or less of acrylonitrile per litre
of drinking-water for 90 days (Humiston & Frauson, 1975). Given
the slow absorption of acrylonitrile from the gastrointestinal
tract, blood levels could have been low, and it can be calculated
that the daily dose could have been about 8.5 mg/kg body weight.
The studies are compatible with the view that acrylonitrile is
unlikely to have a significant cumulative effect.
7.2.3. Subcutaneous administration and intraperitoneal
administration
Daily sc doses of 40 mg/kg body weight over 4 weeks, or daily
ip injections of 20 mg acrylonitrile/kg body weight over 6 weeks,
were not fatal for rats (Krysiak & Knobloch, 1971).
7.2.4. Clinical observations in animal studies
Rats exposed to acrylonitrile concentrations of 220 mg/m3 for 4
h daily, 5 days a week (Dudley et al., 1942) over a period of 8
weeks, showed slight lethargy but gained weight, as did guinea-
pigs. Rabbits failed to gain weight and were listless, while cats
became listless, vomited, and lost weight. One cat developed a
transitory weakness of the hind legs after the third exposure and
died after the eleventh exposure; the 3 remaining cats survived 8
weeks with few untoward effects. Exposure to an atmospheric
concentration of 330 mg/m3 resulted in weight loss in rats, their
coats became rough, and their general physical condition poor
(Dudley et al., 1942). Young rats and guinea-pigs showed impaired
growth and marked irritation of the eyes and nose during the first
week of exposure. Marked eye and nose irritation was also seen in
rabbits and cats and the latter developed transitory weakness of
the hind legs. Monkeys appeared sleepy and weak and frequently
salivated and vomited. Thus, the 220 mg/m3 exposure level markedly
affected cats, while rabbits and guinea-pigs were little affected;
a concentration of 330 mg/m3 induced various effects including
death. Brewer (1976) exposed CD-1 mice, Charles River rats, and
beagle dogs to acrylonitrile concentrations of 0, 58, 117, 234
mg/m3 (0, 24, 54, or 108 ppm) for 6 h daily, 5 days a week, for 13
weeks. Signs observed included ataxia, ptosis, emaciation,
rhinitis, and diuresis. As is common with acrylonitrile over-
exposure, convulsions usually preceded death (e.g., Benesh &
Cherna, 1959; Paulet et al., 1966).
7.2.4.1. Body weight, food and water consumption
Loss of body weight or failure to gain body weight was seen in
rats exposed to acrylonitrile at 330 mg/m3 and in cats and rabbits
exposed to 220 mg/m3 for 4 h a day, 5 days a week, for 13 weeks.
Loss of appetite was seen in rhesus monkeys exposed to 330 mg/m3,
while 120 mg/m3 did not elicit any toxic effects (Dudley et al.,
1942). No adverse effects were seen in 6 rats administered
successively 15 doses of 30 mg/kg body weight, 7 doses of 50 mg/kg,
and 13 doses of 75 mg/kg over a period of 7 weeks (Barnes, 1970).
Adult Sprague-Dawley rats received between 0 and 42 mg
acrylonitrile/kg body weight in drinking-water for 90 days. The
body weight was depressed in males receiving 42 mg/kg, while
females were affected by 22 mg/kg, but only after the 57th day.
The mean weekly food consumption of males was lower for 7 weeks on
38 mg/kg and for 2 weeks on l7 mg/kg. Food consumption decreased
in females receiving 42 or 22 mg/kg for 6 weeks and 1 week,
respectively (Humiston & Frauson, 1975).
7.2.4.2. Organ weights and pathology
The weights of the liver, kidney, spleen, pituitary gland,
lungs, gonads, thyroid gland, adrenals, heart, and brain of rats,
mice, and dogs exposed to an acrylonitrile concentration of 234
mg/m3 for 6 h a day, 5 days a week, for 13 weeks, were within
normal limits (Brewer, 1976). In rats receiving acrylonitrile in
drinking-water for 90 days, no changes in absolute or relative
organ weights were seen in males receiving 4 mg/kg body weight or
females receiving 5 mg/kg, daily (Humiston & Frauson, 1975). The
relative liver weight was significantly increased in males and
females receiving 17 mg/kg (males) or 22 mg/kg (females) or more.
The weights of the heart and liver were increased significantly in
adult Wistar rats administered 50 mg acrylonitrile/kg body weight,
intraperitoneally, daily, for 3 weeks (Knobloch et al., 1971);
their weight loss caused an increase in the relative weights not
only of the heart and liver, but also of the kidney and spleen.
Dudley et al. (1942) examined the livers of rats, guinea-pigs,
rabbits, cats, dogs, and rhesus monkeys exposed to acrylonitrile at
220 or 230 mg/m3, and observed histological changes only in cats.
Liver parenchymal degeneration was reported in adult Wistar rats
after daily ip administration of 50 mg/kg body weight for 3 weeks
(Knobloch et al., 1971). In the above-mentioned study, Dudley et
al. (1942) also reported signs of renal damage, such as hyaline
casts in the straight collecting tubules of all species, and
limited subacute interstitial nephritis; this was especially seen
in guinea-pigs and rabbits. Parenchymal degeneration of the
kidneys was reported by Knobloch et al. (1971). In the study by
Dudley et al. (1942), lungs were affected by subacute
bronchopneumonia, congestion, and oedema of the alveolar walls,
extravasation of erythrocytes and serum into the alveoli, focal
collection of lymphyocytes and polymorphonuclear leukocytes, in
most guinea-pigs, rabbits, the monkey, and 1 out of 3 of the rats.
The authors also reported slight haemosiderosis in the spleen of
rats, but negligible siderosis in cats, guinea-pigs, and rabbits.
Exposure of rats to acrylonitrile (22 mg/m3, 10 ppm) for 7 weeks
and (100 mg/m3, 50 ppm) for another 6 weeks caused enlargement of
the liver, kidney, heart, and spleen, but co-administration of
vitamins B1, B2, and cystine had a protective effect against
enlargement of the heart. Alcohol dehydrogenase activity in the
liver decreased after exposure, but the above-mentioned drugs
alleviated this decrease, to some extent (Takagi et al., 1968).
7.2.4.3. Blood
A normal haematological picture was reported in rats and dogs
repeatedly exposed to acrylonitrile vapours at up to 240 mg/m3
(Brewer, 1976), and in rats and rabbits (except for a raised
eosinophil count) repeatedly exposed to up to 330 mg/m3 (Dudley et
al., 1942).
Minami et al. (1973) exposed male rabbits to 54 mg/m3 for 1 day
per week (8 h) for 8 weeks; haematocrit and haemoglobin were
unaffected, but pO2 and pH were raised and pCO2 lowered by the
treatment.
In rats receiving acrylonitrile in the drinking-water for 90
days, the only significant haematological change was a decrease in
the red-cell count, on the 83rd day, in females receiving 42 mg/kg
body weight per day (Humiston & Frauson, 1975). The blood urea and
alkaline phosphatase (EC 3.1.3.1) levels in the males receiving 38
mg/kg body weight per day were raised, but SGPT activity was
normal. While rats administered ip 50 mg acrylonitrile/kg body
weight, daily, for 3 weeks (Knobloch et al., 1971) developed
leukocytosis and increased serum asparagine-oxo-aminotransferase
(EC 2.6.1.14) activity, mice exposed for 70 days to 225 mg/m3 (100
ppm) or 340 mg/m3 (150 ppm) 6 h daily, did not develop any
haematological abnormalities (Hashimoto, 1962). Rats exposed to
9.7 mg/m3, 4 h daily, 5 days a week for 2 months, did not show any
effects on the erythrocyte count or on the haemoglobin
concentration (Vissarionova et al., 1979).
As a whole, the studies failed to demonstrate any consistent
effect of acrylonitrile on red or white blood cell production or
viability. Leukocytosis following repeated intraperitoneal
administration of an irritant material is to be expected.
7.2.4.4. Immune system
Wistar rats were exposed to an acrylonitrile concentration of
10 mg/m3 for 6 h daily, 5 days a week, for 16 weeks. Acrylonitrile
depressed both T helper and T suppressor functions, and a
diminished degree of B lymphocyte transformation was observed.
Alpha tocopherol (im 0.21 mmol/kg body weight on alternate days for
16 weeks) protected against this effect (Krivova et al., 1982).
7.2.4.5. Nervous system
Some findings in the experimental animal studies on
acrylonitrile were indicative of an effect on the nervous system.
Rats, mice, and dogs exposed to up to 240 mg/m3, for 6 h daily, 5
days per week, for 13 weeks, showed ataxia and convulsions, prior
to death (Brewer, 1976). Transitory hind-leg weakness was seen in
cats exposed for 8 weeks (4 h per day, 5 days per week) to 330
mg/m3 (Dudley et al., 1942). Krysiak & Knobloch (1971) found that
rats receiving acrylonitrile intraperitoneally at 20 mg/kg body
weight daily for 6 weeks, or sc at 40 mg/kg daily for 4 weeks,
showed a significant lengthening of the time to perform correctly
in a conditioned food reflex test, and a significant decrease in
the number of correct reactions, compared with pre-treatment
observations or controls. Performance improved when the treatment
was discontinued. Daily ip administration to rats of acrylonitrile
at 50 mg/kg body weight for 3 weeks caused a vacuolization of
neuronal cells of the cortex and brain stem (Knobloch et al.,
1971).
7.2.4.6. Urine
No significant changes in urine composition were observed in
the experimental studies of Humiston & Frauson (1975) (sections
7.2.4.1 - 7.2.4.3).
7.2.4.7. Adrenals
The adrenals of rats exposed for 21-60 days to acrylonitrile
in drinking-water (0.05% and 0.2%) showed an atrophic zona
fasciculata and an enlarged zona glomerulosa. The animals on the
higher dose had a reduced plasma corticosteroid level and an
increased plasma Na+Concentration. The K+ level was unchanged
(Szabo et al., 1976). Serum corticosterone in rats was decreased
by 3 ip doses of acrylonitrile (33 mg/kg body weight) on successive
days (Nilsen et al., 1980).
7.2.4.8. Metabolism
After repeated exposure of rabbits to acrylonitrile, the in
vitro metabolism in the liver of acrylonitrile into cyanide and
thiocyanate decreased with time, while the excretion of unchanged
acrylonitrile in the urine increased (Sato, 1978).
7.3. Chronic Toxicity
Observations have been made on animals administered
acrylonitrile in drinking-water, food, through inhalation, and by
dermal application.
Tuller (1947) administered acrylonitrile at 500 mg/litre in the
drinking-water or acrylonitrile-fumigated food (dose not precisely
specified) to rats. After 2 years, the mortality was higher in
rats drinking acrylonitrile solution (50% deaths) than in paired
controls (25%), another control group (15%), and in rats on
acrylonitrile-fumigated food (5%). However, when acrylonitrile was
administered at 0.5, 5, and 90 mg/litre in the drinking-water to
male and female CFW rats for 2 years, the mortality rate was
unaffected (Svirbely & Floyd, 1961). Groups of 4 male and 4 female
beagle dogs were administered acrylonitrile at concentrations of
100, 200, or 300 mg/litre in the drinking-water, for 6 months.
Average intakes of acrylonitrile were the following for males
(females): 10(8) mg/kg body weight at 100 mg/litre; 16(17) mg/kg at
200 mg/litre; and 17(18) mg/kg at 300 mg/litre. Five dogs died, or
were killed because debilitated, in each of the 2 higher dosage
groups (Quast et al., 1975).
In the dogs receiving acrylonitrile at 100-300 mg/litre in the
drinking-water, early signs of toxicity included roughening of the
coat and, later, retching and vomiting. Terminal signs of lethargy,
weakness, emaciation, and respiratory distress were noted (Quast et
al., 1975).
7.3.1. Body weight, food and water intake
Body-weight gain was reduced during 4 of the 11 weeks at the
higher dose level (240 mg/m3) in a test in which male and female
Wistar rats and albino rabbits were exposed to acrylonitrile vapour
at concentrations of 0, 50, and 240 mg/m3, for 3 h/day, 6 days a
week, for 6 months (Knobloch et al., 1972).
Growth retardation was observed in male rats drinking 500 mg
acrylonitrile per litre water, for 2 years (Tuller, 1947). Svirbely
& Floyd (1961) administered acrylonitrile at 0.5, 5, or 50 mg/litre
in the drinking-water of rats and found a slight decrease in water
consumption at the highest concentration in both sexes.
Statistically significant reductions in the body weight of rats
receiving drinking-water containing acrylonitrile concentrations of
35, 100, or 300 mg/litre were associated with decreased water
consumption and decreased food consumption at 300 mg/litre in males
and 100 mg/litre in females (Ouast et al., 1977). Decreased food
and water consumption and body weight was reported with beagle dogs
drinking acrylonitrile at 200 or 300 mg/litre water for 6 months
(Quast et al., 1975). Marked weight decreases were seen in dogs
that eventually died or had to be killed.
7.3.2. Organ weights
In rabbits exposed to atmospheric acrylonitrile concentrations
of 250 mg/m3, for 3 h/day, 6 days a week for 6 months, a
significant increase in the heart weight was noted, and some
fluctuations in blood pressure were described (Knobloch et al.,
1972).
Ferin et al. (1961) exposed rats to drinking-water containing
an acrylonitrile concentration of 20 or 1000 mg/litre for 6 months.
At the higher dose, increased relative weights of the liver,
spleen, and kidneys were noted. The relative weights of the heart,
liver, and brain in males, and of the liver and kidneys in females,
were increased in rats receiving 300 mg/litre in drinking-water.
The males drinking 100 mg/litre showed a significantly increased
brain weight, and the females receiving 100 mg/litre had
significantly lower relative heart weights (Quast et al., 1977).
The weights of brain, heart, liver, and kidneys of beagle dogs
drinking an acrylonitrile concentration of 100 mg/litre in
drinking-water (Quast et al., 1977) were normal but, at 200
mg/litre, the 2 remaining males had a lower absolute brain weight
and higher relative kidney weight than controls. The 2 remaining
females receiving 300 mg/litre also had significantly lower relative
brain weights compared with controls.
7.3.3. Pathology and histology
Inflammation of the pulmonary system accompanied by an
inflammatory exudate into the bronchial lumen occurred in rats
exposed to acrylonitrile at 240 mg/m3 for 3 h a day, 6 days a
week, for 6 months (Knobloch et al., 1972). Various pathological
changes occurred in male and female rats maintained on water
containing acrylonitrile at 35, 100, or 300 mg/litre for 12 months
(Quast et al., 1977). Males and females on the 2 higher doses
developed paleness and thickening of the mucosa, erosions, ulcers,
and sometimes papilloma formations in the non-glandular portion of
the stomach. Three females receiving 300 and 100 mg/litre and one
male at 300 mg/litre had ear canal tumours. Microscopic findings
of tissue with tumorous growth revealed an increased frequency of
gastric cell papillomas, Zymbal (sebaceous) gland tumours of the
ear canal, and microtumours of the nervous system, in rats
receiving a concentration of acrylonitrile of 100 or 300 mg/litre.
These tumours do not occur spontaneously at such a high frequency
in the strain of rat used. The nervous system lesions were
consistent with the diagnosis of astrocytoma.
Minimal lesions were seen in the liver of rats drinking
acrylonitrile at 100 or 300 mg/litre and chronic renal disease
occurred in females drinking 300 mg/litre. The squamous epithelium
of the stomach was hyperplastic in rats drinking 100 or 300
mg/litre.
Histopathological changes in dogs receiving acrylonitrile
concentrations of 200 and 300 mg/litre in water (Quast et al.,
1975) were similar to those in untreated controls. The pneumonia
present may have resulted from irritation of the mucosa of the
tongue and oesophagus, which produced abnormal swallowing,
resulting in aspiration of some food.
7.3.4. Haematology and clinical chemistry
In rats exposed for 3 h/day, 6 days a week, for 6 months to
acrylonitrile in air concentrations of either 50 or 240 mg/m3, the
eosinophil count had significantly increased after 4 months. Total
serum protein was unchanged, while albumin and alpha-globulin had
increased and gamma-globulin decreased in both test groups
(Knobloch et al., 1971). Leukocytosis was observed in rats
drinking acrylonitrile in water at 1000 mg/litre (Ferin et al.,
1961). Periodic examinations of rats drinking 0.5, 5, or 50
mg/litre in water (Svirbely & Floyd, 1961), or 0 - 300 mg/litre in
water (Quast et al., 1977), showed normal haematological findings.
A significant elevation in alkaline phosphatase activity was found
in female rats exposed to 300 mg/litre. Half-way through the
study, beagle dogs drinking acrylonitrile concentrations of 0, 100,
200, or 300 mg/litre in water showed a significant decrease in
haematocrit, erythrocyte count, and haemoglobin concentration at
300 mg/litre and decreased erythrocyte count at 200 mg/litre, in
males. Females on 300 mg/litre also showed a significant decrease
in the erythrocyte count. However, all haematological findings
were within normal ranges towards the end of the study, except in
males at 300 mg/litre, which had a lower erythrocyte count. Blood
urea nitrogen, serum alkaline phosphatase activity, SGPT, and SGOT
were measured periodically; the findings in males were always
within normal limits, but females receiving 300 and 200 mg/litre
showed some increase in SGOT and SPGT activity. Total and
individual serum proteins were unaffected at the end of the study.
Zotova (1976) exposed rats by applying acrylonitrile solution to
the skin of the tail at doses of 2.82, 0.56, or 0.11 mg/kg body
weight per day and observed a decreased haemoglobin concentration
at the highest dosage level after 2 months. Blood catalase (EC
1.11.1.6) activity increased, and blood peroxidase (EC 1.11.1.7)
activity decreased, initially, but later became normal; there was
no change in sulfhydryl levels.
A decrease in blood -SH groups was reported in rats exposed to
acrylonitrile at 10 mg/m3 for 5 days/week, 4 h a day, for 4 months
(Efremov, 1976d).
7.3.5. Nervous system
Changes in central nervous function, as elicited by a
conditioned avoidance test, were found in rats drinking water
containing acrylonitrile at 20 mg/litre, for 6 months (Ferin et
al., 1961). Rats exposed through inhalation to acrylonitrile
concentrations of 50 and 240 mg/m3 for 3 h a day, 6 days a week,
for 6 months, showed significant defects in performance in a "Y"
maze (Krysiak, 1971). Rats exposed to 10 mg/m3, 5 days/week, 4 h a
day, for 4 months, showed a 59% decrease in the activity of brain
catalase, a 59% decrease in brain peroxidase, and a 37% decrease in
-SH groups (Efremov, 1976d). Histopathological changes in the
nervous system, consistent with a diagnosis of astrocytoma, were
observed in rats exposed to acrylonitrile at 35, 100, or 300
mg/litre in drinking-water (Quast et al., 1977).
7.3.6. Kidney function
Knobloch et al. (1972) exposed rats to 50 or 240 mg/m3, for
3 h per day, 6 days per week, for 6 months; kidney dysfunction was
indicated by increased urinary output at both concentrations and an
increase in urinary protein and areas of degenerated proximal
convoluted tubules at the higher concentration.
There were no abnormalities in the urine chemistry of rats
drinking water containing acrylonitrile at 0, 35, 100, or 300
mg/litre (Quast et al., 1977), or of dogs (Quast et al., 1975),
drinking water containing 100, 200, or 300 mg/litre. When
acrylonitrile was applied to the skin of the tail of rats, daily,
at doses of 2.82, 0.56, or 0.11 mg/kg body weight for 4.5 months,
the excretion of urinary chlorides increased on day 10 of exposure
at the 2 higher doses and decreased at the lowest dose (Zotova,
1976).
7.4. Teratogenicity and Embryotoxicity
The teratogenic potential of ingested or inhaled
acrylonitrile was investigated by Murray et al. (1978).
Groups of pregnant SD rats were given acrylonitrile at 0, 10,
25, or 65 mg/kg body weight per day, by gavage, from day 6 to
day 15 of gestation. Groups of 30 pregnant SD rats were
exposed 6 h per day to 0, 87, or 174 mg/m3 (0, 40, or 80 ppm)
acrylonitrile by inhalation, during the same period of
pregnancy. A dose of 65 mg/kg body weight per day caused
marked maternal toxicity, significant embryotoxicity, and an
increased incidence of fetal malformations. Findings of the
two studies suggesting a teratogenic effect were noted at 25
mg/kg per day and at 174 mg/m3 (80 ppm). At 10 mg/kg body
weight per day and 87 mg/m3 (40 ppm), no embryotoxicity or
teratogenicity was found. There was no apparent correlation
between the degree of toxicity seen in the individual dams and
the occurrance of malformations in their offspring.
Embryotoxic effects in pregnant mice of 3 strains were
described after intraperitoneal administration of unspecified doses
of acrylonitrile (Scheufler, 1976). A single ip injection of
acrylonitrile of 32 mg/kg body weight, given on the 5th or 7th day
of pregnancy, induced an embryotoxic effect in mice from an inbred
strain of AB Jena-Hall, but not in DBA and C57 C1 mice (Scheufler,
1980).
Kankaanpää et al. (1979) studied the embryotoxic effects of
acrylonitrile using chick eggs, but did not find any clear evidence
of its teratogenicity.
The exposure of Sprague-Dawley rats to an acrylonitrile
concentration in drinking-water of 500 mg/litre led to decreased
fertility and decreased viability of the young, and the females
developed a progressive muscular weakness in the hind legs about
16-19 weeks after the weaning of the second litter (Svirbely &
Floyd, 1961).
Willhite (1981a,b) observed skeletal malformations in hamster
fetuses after the administration of acrylonitrile at 80 mg/kg body
weight to pregnant hamsters. The histological study of both early
embryos and term fetuses revealed mesodermal changes, including a
reduction in the number of cells, shrinkage of the cell cytoplasm,
and enlarged extracellular spaces. In addition, a reduction in
mitotic figures and focal necrosis were noted. The affected
embryos were smaller and their development was delayed compared
with untreated controls. Teratogenic effects were only observed
when there was simultaneous maternal toxicity.
7.5. Mutagenicity
7.5.1. Bacterial systems
(a) Ames test with Salmonella typhimurium strains
Tests have been carried out on several strains, with and
without metabolic activation, using several methods of treatment
with acrylonitrile. Negative results were obtained, with and
without activation, in 5 tested strains in 2 studies (Litton
Bionetics, 1975; Stanford Research Institute, 1976). A weak, but
reproducible, positive effect was observed with metabolic
activation using between 0.5 and 1.5 mg acrylonitrile per plate in
strain TA 1535 (Haskell Laboratory, 1975; De Meester et al., 1978,
1979). Three methods of exposure were examined by Milwy & Wolff
(1977) in 3 strains of S. typhimurium. A low level of mutagenic
activity was noted in strain TA 1535, when plates were sprayed with
acrylonitrile or when it was mixed with the medium, with
activation.
Exposure to acrylonitrile vapour of the strains TA 1535 and TA
100 also demonstrated acrylonitrile mutagenicity (Duverger-Van
Bogaert et al., 1981; Ivanov, 1981). Zhurkov et al. (1983) tested
the mutagenicity of acrylonitrile in strains TA 1535 and TA 1538,
with and without microsomal activation, and found a dose-dependent
effect in strain TA 1535.
Urine collected from rats and mice treated with acrylonitrile
was mutagenic in S. typhimurium strain TA 1530, in the absence of
metabolic activation. Pre-treatment of the animals with
phenobarbital abolished the direct mutagenicity of urine from rats
and reduced that from mice. The addition of beta-glucuronidase (EC
3.2.1.31) to the incubation mixtures enhanced the mutagenic
activity of urine from acrylonitrile-treated animals (Lambotte-
Vandepaer et al., 1980, 1981a). Duverger-van Bogaert et al. (1982b)
suggested that glutathione might play a role in the formation of a
mutagenic metabolite of acrylonitrile. The mutagenic activity of
acrylonitrile vapours towards S. typhimurium strains was strictly
dependent on the presence of an activation system, confirming the
report of Milwy & Wolff (1977). Lambotte-Vandepaer et al. (1980)
indicated that animal urine might retain its mutagenic activity for
as long as a week after collection. The acrylonitrile-derived
epoxide, glycidonitrile, synthesized by Kopecky & Smejkal
(unpublished data, 1979), was shown to be the principal substance
that exerted mutagenic activity in the absence of metabolic
activation, whereas acrylonitrile itself required metabolic
activation in the S. typhimurium Ames test (Cherna et al., 1981).
(b) Mutagenicity in Escherichia coli
One of 3 strains of E. coli (WP2) revealed mutagenic activity
of acrylonitrile; activation did not have any effect (Venitt et
al., 1977). The mutagenic activity of acrylonitrile was confirmed
in other experiments using the simplified fluctuation test of Green
et al. (1976). The results suggested that acrylonitrile caused
non-excisable mis-repair of DNA associated with the generation of
DNA strand breaks (Venitt et al., 1977). The method of Slater et
al. (1971) did not reveal any effect with or without an activation
system at 10 µg acrylonitrile per plate (Litton Bionetics, 1976).
The variability of the results, even when the same kinds of
assays are used, could be because of differences in purity of the
acrylonitrile, in method, or in bacterial sensitivity. However, the
mutagenicity of acrylonitrile in bacterial systems seems to have
been established.
7.5.2. Yeast assays
Possible mutagenic activity of acrylonitrile was found with
Saccharomyces cerevisiae, but metabolic activation was without
effect (Litton Bionetics, 1975).
7.5.3. Drosophila melanogaster
A negative result was obtained when 0.1% acrylonitrile was
administered by intra-abdominal injection into D. melanogaster in
order to examine its ability to induce a recessive lethal effect in
the X chromosomes (Benesh & Shram, 1969).
7.5.4. Mammalian cell in vitro assays
The L5178Y kinase mouse lymphoma cell assay (Litton Bionetics,
1976) failed to show mutagenic activity of acrylonitrile using the
procedure of Clive & Spector (1975). Chinese hamster ovary cells
showed an increase in sister chromatid exchange (SCE) after
exposure to acrylonitrile, when co-cultured with rat hepatocytes
(Ved Brat & Williams, 1982). No effect was found without the
latter.
Acrylonitrile induced a slight increase in the SCE of cultured
human lymphocytes in the presence of S-9 mix and increased
unscheduled DNA synthesis with a very high concentration (0.5 M)
(Perocco et al., 1982). Application of acrylonitrile to primary
Syrian golden hamster embryo cells in culture produced foci of
morphologically-transformed cells. Pre-treatment with simian
adenovirus (SA7) caused an 8 to 9-fold increase in the frequency of
virus-transformed foci. When 3H-thymidine-labelled cells were
treated with acrylonitrile and their DNA subjected to alkaline
sucrose gradients, a shift in the sedimentation pattern occurred,
which was reminiscent of that observed with carcinogen treatment.
These observations added support to recent studies indicating that
acrylonitrile may be carcinogenic (Parent & Castro, 1979).
7.5.5. Mammalian in vivo assays
The inhalation exposure of 16 Sprague-Dawley male rats to
acrylonitrile levels up to 1085 mg/m3 (500 ppm) for 90 days did not
reveal chromatid or chromosomal aberrations or bone-marrow
abnormalities (Johnson et al., 1978). The results of Rabello-Gray
& Ahmed (1980) and the recent results of Leonard et al. (1981) also
showed that acrylonitrile fails to induce chromosomal aberrations
in somatic and germ cells.
Similar negative results were reported by Zhurkov et al. (1983)
following inhalation exposure of mice to acrylonitrile concentrations
of both 100 mg/m3 and 20 mg/m3 . They also reported negative
results in a dominant lethal assay in mice.
From preliminary results concerning DNA-alkylation by
acrylonitrile and vinyl chloride monomer (Peter et al., 1983), it
appears that DNA-alkylation occurs to a much lesser extent with
acrylonitrile than with vinyl chloride monomer. This is consistent
with the absence of mutagenic effects in vivo.
7.6. Carcinogenicity
Although full data were not available to the Group, there was
strong evidence from the data considered that acrylonitrile is a
carcinogen in rats.
Maltoni et al. (1977, 1982) investigated the carcinogenicity of
acrylonitrile administered to Sprague-Dawley rats by inhalation at
87, 44, 22, and 11 mg/m3 (40, 20, 10, and 5 ppm), 4 h daily, 5
times a week, or by stomach tube as a solution in olive oil, at a
dose of 5 mg/kg body weight, once a day, 3 times a week. In each
case, the rats were treated for 52 weeks and then kept without
further treatment until death. An increased incidence of some
tumours was noted in the acrylonitrile-treated animals, e.g.,
mammary tumours, forestomach papillomas and acanthomas, and
encephalic tumours (gliomas).
Two-year studies on Sprague-Dawley rats, following inhalation
exposure to acrylonitrile or ingestion in drinking-water, have been
performed at the Dow Chemical Company laboratories (Quast et al.,
1980a,b). In the inhalation studies, rats were exposed to 0, 44,
or 174 mg/m3 (0, 20, or 80 ppm) for 6 h/day, 5 days a week, for 24
months. Treatment-related tumours were found in the central nervous
system, Zymbal gland, tongue, stomach, small intestine, mammary
gland, and nasal turbinates. An apparent decrease in tumours of
the pituitary gland, the adrenals, the thyroid, the pancreas, and
testes was observed in the exposed rats.
In the ingestion study, rats were maintained on water
containing acrylonitrile levels of 0, 35, 100, and 300 mg/litre,
equivalent to mean dosage levels of 0, 4, 9, or 22 mg/kg body
weight per day. Evidence of oncogenicity was found in rats at all
dose levels of acrylonitrile. An increased tumour incidence was
observed in the treated rats affecting, particularly, the centra1
nervous system and also the Zymbal gland, tongue, stomach, small
intestine, and mammary gland. A decreased incidence of tumours was
observed at some sites: pituitary, thyroid, adrenals, pancreas, and
uterus.
In studies performed by Hogen & Rinehart (1980), acrylonitrile
was administered to Sprague-Dawley rats in the drinking-water at 1
or 100 mg/litre for 19-22 months, or by gavage at 0.1 or 10 mg/kg
body weight per day in water for about 20 months. A second group
of Fisher 344 rats received acrylonitrile in the drinking-water at
1, 3, 10, 30, or 100 mg/litre for 23-26 months. A statistically
significant increase in tumours was reported in the group receiving
acrylonitrile at 10 mg/kg body weight by gavage and in the groups
receiving 10, 30, or 100 mg acrylonitrile/litre drinking-water.
So far, no information is available on the carcinogenicity of
acrylonitrile for animal species other than rats.
After reviewing these data, IARC (IARC, 1982) and COC (UK
Ministry of Agriculture, Fisheries and Food, 1982) concluded that
acrylonitrile was a carcinogen in experimental animals.
8. EFFECTS ON MAN
Acrylonitrile has long been known to be a toxic substance that
induces systemic as well as local injury in both animals and man.
It has frequently been used in combination with other chemicals;
they may modify its toxicity, as was the case when it was used as a
fumigant.
8.1. Acrylonitrile
8.1.1. Acute Toxicity
8.1.1.1. Inhalation exposure
A 22-year-old chemist, who was exposed to acrylonitrile vapours
when a distillation apparatus leaked, developed headache, vertigo,
vomiting, tremors, uncoordinated movements, and convulsions
(Sartorelli, 1966). Vomiting and nausea persisted for 24 h. One
day after exposure, slight liver enlargement and congestion of the
oral pharynx, but no disorders of the CNS, were noted. After 4
days, no kidney, liver, cardiac, or respiratory abnormalities were
detected. Workers exposed to "mild" concentrations of acrylonitrile
in synthetic rubber manufacture developed nausea, vomiting,
weakness, nasal irritation, and an "oppressive feeling" in the
upper respiratory tract (Wilson, 1944). Headache, fatigue, and
diarrhoea were observed in some cases, and mild jaundice lasting
for several days and accompanied by liver tenderness and low-grade
anaemia in a few others. Jaundice lasted for 4 weeks in 1 case;
this individual complained of lassitude and fatigue after one year.
Zeller et al. (1969) observed that in 16 cases of acute inhalation
of acrylonitrile fumes by workers, nausea, vomiting, headache, and
vertigo appeared within 5-15 min; none of the workers needed
hospitalization. The authors described 50 cases of skin contact
with irritation, erythema, and blistering appearing within 5 min to
24 h, but with no systemic consequences. Workmen exposed to
concentrations varying from 35 to 220 mg/m3 (16-100 ppm) for 20-45
min during cleaning operations in polymerizers frequently
complained of a dull headache, fullness in the chest, irritation of
the eyes, nose, and throat, and feelings of apprehension and
nervous irritability. Some workmen had "intolerable itching" of
the skin, but no accompanying dermatitis.
8.1.1.2. Dermal exposure
A male laboratory worker who spilled "small quantities" of
liquid acrylonitrile on his hands, developed diffuse erythema on
both hands and wrists after 24 h, and blisters on the fingertips by
the third day. The hands were slightly swollen, erythematous,
itchy, and painful. The fingers remained dry and scaly on the 10th
day (Dudley & Neal, 1942). Wilson et al. (1948) observed that
direct skin contact led to irritation and erythema followed by scab
formation; healing was slow. Development of allergic dermatitis is
possible; a 27-year-old individual developed a rash on his finger
following the use for 6 weeks of a finger splint made from an
acrylonitrile/methyl methacrylate copolymer. Patch testing gave
positive reactions to the copolymer and 0.1% acrylonitrile (Balda,
1975). In another case report, skin lesions were first observed at
the site of contact with liquid acrylonitrile, which then spread
rapidly to other neighbouring regions. Several days after contact,
the lesions spread rapidly to other parts of the body that had not
been exposed, and these extensions were assumed to be an allergic
reaction (Hashimoto & Kobayashi, 1961).
In addition to local dermal toxicity, dermal absorption of
acrylonitrile may lead to systemic poisoning. Grunske (1949)
described a fatal case in which a 3-year-old girl had entered a
room that had recently been sprayed with an acrylonitrile-
containing insecticide (Ventox). Exposure was mainly through
inhalation, but skin exposure was possible, too. Another fatal
case was reported by Lorz (1950) in which a 10-year-old girl had
been treated on the scalp for lice with an insecticide that was
identified as containing acrylonitrile (Ventox). She had impetigo
and widespread scratches on the skin of the scalp. This could have
increased the absorption of acrylonitrile.
Two workers who spilled liquid acrylonitrile on their legs,
immediately washed their legs and dried their shoes, but put them
on again. Blisters developed at the sites of contact, 6-8 h after
the spill. Therapy lasted 21 and 38 days, respectively. The skin
of 2 workers who were cleaning apparatus (temperature 50 °C), came
into contact with 5% acrylonitrile solution; other possible
substances in the mixture were not specified. Serious skin burns
developed. Therapy lasted 35 and 72 days, respectively (Babanov,
1957). Zeller et al. (1969) reported 50 cases of skin damage
resulting from occupational contact with acrylonitrile. A burning
sensation developed within 5 min to 24 h followed by a reddening of
the area, which often blistered after 1 day.
8.1.2. Chronic toxicity - occupational exposure
Chronic effects can potentially occur after prolonged exposure
to acrylonitrile, both in the vapour and liquid forms.
8.1.2.1. Clinical observations
Complaints of poor health, headache, decreased work capacity,
poor sleep, irritability, chest pains, poor appetite, and skin
irritation (during the first months of employment only) came from
workers employed in the manufacture of acrylonitrile (Zotova,
1975a).
In a study by Sakurai & Kusumoto (1972), workers employed in
acrylonitrile manufacture also complained of headache, weakness,
fatigue, nausea, vomiting, nosebleeds, and insomnia; the symptoms
correlated well with the length, but not with the level of exposure
or with the age of the workers. A total of 4439 examinations were
made over about 10 years prior to 1970, in 576 workers who formed
2 cohorts, one exposed to concentrations of acrylonitrile below 11
mg/m3 (5 ppm), the other below 45 mg/m3 (20 ppm). However, the
authors later stated that these exposure levels were not reliably
reported (Sakurai et al., 1978).
Babanov et al. (1959) reported that workers exposed to
acrylonitrile concentrations at 0.6-6 mg/m3 for approximately 3
years suffered from headache, insomnia, pains in the heart region,
general weakness, decreased working capacity, and increased
irritability. The vocal cords were inflamed, and non-specific
changes in the vestibular apparatus and pale mucous membranes and
skin were seen. Blood pressure was said to be reduced.
Changes in the health status and laboratory tests were not
observed in a group of 23 men who had been working for 3-5 years in
an acrylonitrile plant, where, during the warm season, exposure
levels reached 4.2-7.2 mg/m3 (Gincheva et al., 1977). Stamova et
al. (1976) studied workers' health in the related polyacrylic fibre
plant in which acrylonitrile exposure levels ranged around 10
mg/m3, but could fluctuate upwards to 25 mg/m3 . Workers were also
exposed to other chemical substances. An increase was found in
both skin diseases and various "neurasthenic" complaints and
diseases. Dorodnova (1976) did not find any differences in the
gynaecological health status of 410 women working in a polyacrylic
fibre plant in Saratov compared with that of 436 unexposed women.
8.1.2.2. Haematology
Compared with the findings in blood donors, some male and
female employees exposed to acrylonitrile at 2.5-5 mg/m3 showed
a reduced haemoglobin level, erythrocyte count, leukocyte count,
and percentage of neutrophiles, with an increased percentage of
lymphocytes and plasma iron. Inhibition of maturation of
normoblasts in bone marrow was also reported (Shustov, 1968).
Similar results were reported by Zotova (1975b). Lower
erythrocyte, haemoglobin, and total white counts were found in
laboratory workers exposed to acrylonitrile, and in apparatus
operators and machinists. Higher than normal total glutathione
levels were found in male operators and maintenance men and reduced
glutathione levels in male apparatus operators. Oxidized
glutathione was elevated and total sulfhydryl groups decreased in
workers employed in all these occupations.
Lower erythrocyte counts and a relative lymphocytosis were also
observed by Babanov et al. (1959) in the study mentioned above.
8.1.2.3. Other organs
(a) Liver
Sakurai & Kusumoto (1972) (section 8.1.2.1) reported some
abnormal results in liver function tests; however, in a further
study of 102 workers from some of the factories, Sakurai et al.
(1978) did not find any significant liver function test
abnormalities related to acrylonitrile exposure, when exposure
levels had decreased from 11-44 mg/m3 (5-20 ppm) to 9 mg/m3 (4.2
ppm). Increased serum cholinesterase activity, hyperbilirubinaemia,
decreased coloidal stability, and hypergammaglobulinaemia were
described in workers exposed to acrylonitrile concentrations of up
to 5 mg/m3 and to acrylonitrile-polymer dust of up to 1.5 mg/m3
(Enikeeva et al., 1976). These effects have not been reported
elsewhere.
(b) Eye
Blepharoconjunctivitis was reported by Delivanova et al. (1978)
in all of 302 workers examined over a 2-year period; 42 had severe
alterations caused by conjunctivitis, and all disorders were
connected with exposure to acrylonitrile.
(c) Gastro-intestinal effects
Symptoms of gastritis and colitis were observed in workers
exposed to acrylonitrile concentrations of up to 5 mg/m3 (Enikeeva
et al., 1976).
(d) Immune system
Acrylonitrile has been found to have an immunodepressive
effect. The functional activity of T-lymphocytes was found to have
decreased in workers exposed to acrylonitrile (Ivanov, private
communication, 1983).
8.1.2.4. Nervous system
Nausea, vomiting, headache, and vertigo (Wilson, 1944; Wilson
et al., 1948; Zeller et al., 1969; Sakurai & Kusumoto, 1972;
Zotova, 1975) indicate a possible effect of acrylonitrile on the
nervous system. Ageeva (1970) reported a significant decrease in
an "epinephrine-like substance", and an increase in acetylcholine.
Depression, lability of autonomic functions (lowered arterial
pressure, labile pulse, diffuse dermographia, increased sweating,
change in orthostatic reflex) were also observed in workers
involved in acrylonitrile production.
8.1.2.5. Dermal effects
Spassovski (1976) reported irritant and allergic dermatitis in
acrylonitrile workers; dermatitis was also observed by Antonev &
Rogailin (1970) and Stamova et al. (1976).
8.2. Mutagenicity
Thiess & Fleig (1978) examined workers who had been exposed to
acrylonitrile for 15.3 years and workers who had not been exposed.
No differences were found in the incidence of chromosomal
aberrations, including or excluding gaps, in the 100 metaphases
examined for each person.
8.3. Carcinogenicity
In a retrospective cohort epidemiological study of 1345 male
workers with potential exposure to acrylonitrile from 1950-66,
followed until 31 December, 1976, 25 cases of cancer were found
with 20.5 expected, based on company rates (O'Berg, 1980). Of
these, 8 were respiratory cancer cases, with 4.4 expected. Twenty-
three cases occurred among workers first exposed during the start-
up period (1950-52) when exposures were higher; only 12.9 were
expected (P = 0.01).
The standardized incidence ratio (SIR) was 179 for cancer among
the operators and mechanics who had at least 6 months' exposure and
began their assignments during start-up. A "dose response" was
shown with those with longer duration of employment, workers with
estimated higher exposures having higher risk. Latency was also
demonstrated, with 17 of the 24 cases occurring 20 years after the
onset of exposure among those with at least 6 months' employment,
including 6 of the 8 lung cancer cases. It should be pointed out
that, using the National Cancer Institute's expected incidence
rates for 1969-71, the expected rate would be 25.5 rather than 20.5
from the company rates. In a concomitant cancer mortality study,
20 cancer deaths were found with 17.4 expected, using company rates
(not significant); the expected rates exceeded the company rates
using national, state, or regional cancer rates. The author felt
that it might be premature to evaluate mortality statistics,
because of insufficient latency (many cancer cases had been
recently diagnosed and were still living). Smoking habits were not
considered, though the author stated that 7 out of the 8 lung
cancer cases were known to have smoked.
A follow-up study on the mortality rate among 327 employees of
a chemical rubber plant in the USA revealed that the number of
deaths from lung cancer was significantly higher than expected (9
versus 5.9 for US white males and 4.7 for other rubber workers from
the same city). The greatest excess was seen among men who had
worked for 5-14 years and who had started working there at least 15
years before death (Delzell & Monson, 1982). This study was
confounded by the multiple exposure of workers in the nitrile
rubber manufacturing plant.
Kiesselbach et al. (1979) examined the mortality rate, the
cancer rate, and the type of cancer against the period of exposure
to acrylonitrile in 884 workers. The results revealed that the
general mortality of the exposed group was markedly lower than that
of the normal population (58 versus an expected 104), possibly
because of the "healthy worker" effect. The mortality rate for
malignant tumours, cardiovascular, brain, respiratory, and gastro-
intestinal diseases, suicide, and other causes was the same as in
the normal population. No relationship was found between length of
exposure and mortality from tumours.
An excess of deaths from lung cancer was reported in
acrylonitrile workers by Thiess et al. (1980). In addition, 2
cases of Hodgkin's disease contributed to a slight excess of cancer
of the lymphatic tissue. However, exposure to other substances,
some of them known carcinogens, made interpretation of the results
difficult.
A cohort study on men potentially exposed to acrylonitrile
during the start-up of a plant indicated that there was no excess
mortality from lung cancer. There were no deaths from lung cancer
in maintenance workers, who possibly had the highest exposures.
There was an excess of kidney cancers (based on 2 cases only) and
of circulatory disease other than rheumatic and atherosclerotic
(based on 5 cases), accompanied by a deficit of atherosclerotic
heart disease.
Because the cohort was small with only 4 cancer deaths
observed, it could not give an indication of excess cancer risk or
association with duration of exposure to acrylonitrile. An
additional retrospective cohort mortality study, in two
acrylonitrile plants in the USA, on 352 males exposed for 6 months
or more prior to 1968 and followed up until December 1977, did not
show any excess mortality including cancer mortality. There were
15 deaths from all causes, 18.11 being expected, and 3 deaths from
cancer (2.8 expected) (Zack, unpublished data, 1980).a
In a study by Nakamura (1981), 9525 workers employed in the
production of acrylonitrile, acrylonitrile rubbers, and ABS were
studied. Deaths due to cancers in general and to lung and colon
cancers in particular, were not increased, while 7 deaths due to
liver, gall bladder, or cystic duct cancer were found against the 5
that might have been expected.
The mortality of 1111 men who worked on the polymerization of
acrylonitrile and the spinning of acrylic fibre in the United
Kingdom from 1950 to 1968 was surveyed up to the end of 1978.
Seventy-nine deaths were identified in 6 factories. The total
number of deaths among men exposed to acrylonitrile for at least
one year was slightly lower than expected (68 versus 72.4) and a
relative excess of deaths from all cancers was found, arising
mainly from cancers of the lung, stomach, colon and brain,
pancreas, testis, and bladder (21 versus 13 expected). The authors
considered particularly relevant, the excess of lung cancer in
those aged 15-44 years. Nevertheless, the authors considered that
their results were inconclusive and urged continued surveillance
and analysis of the exposed population in the United Kingdom
(Werner & Carter, 1981).
The epidemiological studies provide some indications that
acrylonitrile exposure is associated with cancer, particularly of
the lung. However, the studies reported, while neither conclusive
nor contradictory, are limited by insufficient latency. Other
difficulties, such as cohort identification and selection, and
combined exposures have made interpretation difficult. Further
epidemiological data are therefore of great importance, and
consideration of smoking and single exposures to acrylonitrile is
desirable.
8.4. Simultaneous Exposure to Acrylonitrile and
Other Chemicals
8.4.1. Acute toxicity
Numerous non-fatal and fatal cases of poisoning by
acrylonitrile-containing mixtures have been described (Davis et
al., 1973). In home fumigation, an acrylonitrile mixture with
carbon tetrachloride or methylene chloride is placed in shallow
open pans and the vapours dispersed by fans for 24-72 h, the
operator deciding when the house is safe for occupancy (Davis et
al., 1973). A man working in the polymerization of acrylonitrile,
polybutadiene, and styrene for 2 years complained of numbness of
the fingers and toes, severe fatigue in the lower extremities, and
----------------------------------------------------------------------
a Zack, J.A. (1980) The mortality experience of Monsanto
workers exposed to acrylonitrile (Monsanto Internal
Report).
general malaise. Decreased patellar and achilles tendon reflexes,
and hypoaesthesia in the peripheral parts of the fingers and toes
were observed. Free acrylonitrile was detected in the urine. The
exposure level of acrylonitrile was estimated to exceed 108.5 mg/m3
(50 ppm) (Seki, 1967).
Lachrymation, burning in the throat, coughing, sneezing,
nausea, vomiting, dizziness, visual disturbance, headache, coma,
seizures, and dermatitis have been described in non-fatal cases
(Davis et al., 1973). Fatalities have occurred following exposure
to vapours (Grunske, 1949; Davis et al., 1973) and liquid (Lorz,
1950). Cyanide was detected in the blood in some cases. Symptoms
and signs prior to death varied from case to case; sore throat,
weakness, dizziness, vomiting, eye irritation, respiratory
disorders, pallor, tachycardia, tremors, unconsciousness, and
epidermal necrolysis have been described. Other pathological
conditions occurred, but many could have been the result of pre-
existing disease or of exposure to the other component(s) of the
mixture. Findings in children suggest that they may be more
sensitive to acrylonitrile exposure than adults (Grunske, 1949).
8.4.2. Chronic toxicity
Abnormalities in subjects exposed simultaneously to
acrylonitrile and several other chemicals have been described in
several studies. As the concentrations of the other chemicals were
frequently higher than those of acrylonitrile, it is difficult to
decide whether the abnormalities were caused by acrylonitrile, the
other chemical(s), or a combination of the two.
An abnormally high proportion of workers exposed to
acrylonitrile levels of 3-20 mg/m3, 33 ppm NH3, up to 1 mg
H2SO4/m3, 0.41-0.67 mg NaOH/m3, and 2-10 mg acetic acid/m3 were
described as suffering from a variety of symptoms ascribed to
disorders of the autonomic nervous system ("neurasthenic syndrome")
(e.g., irritability, headache, poor appetite, fatigue). Intolerance
to alcohol has also been observed (Orusev & Popovski, 1973; Orusev
et al., 1973).
Apprentices exposed to acrylonitrile (0.8-1.8 mg/m3), methyl
methacrylate (16-17 mg/m3), and sodium thiosulfate were examined
before exposure, after 1-2 weeks, and after a further unspecified
time. "Neurasthenic" symptoms were rare before, but frequent after
exposure, and the incidence of immunological reactivity against the
chemicals increased, as did the concentration of ceruloplasmin
(Mavrina & Il'ina, 1974). Dermal tests for allergy were also made
by Hromov (1974) in workers who had been in contact with
acrylonitrile, methyl acrylate, and sodium thiocyanate. Intradermal
samples showed positive haemoagglutination reactions in 86.5% of
workers exposed to acrylonitrile, 76.1% exposed to methyl acrylate,
and 53.6% exposed to sodium thiocyanate. Dermatitis, eczema, and
urticaria occurred.
Mavrina & Hromov (1974) and Shustov & Mavrina (1975) reported
abnormalities of the liver, nervous, cardiovascular, and
gastrointestinal systems in workers occupationally exposed to
acrylonitrile, methyl acrylate, and sodium thiocyanate during fibre
production. In particular, symptoms associated with the activity
of the autonomic nervous system were noted among the 340 workers
examined. Dryness, desquamation, fissures, and diffuse erythema of
the skin were also apparent. Women exposed to acrylonitrile and
methyl acrylate (Chobot, 1979) were said to suffer from
disturbances in menstrual function twice as frequently as a control
group. A low incidence of irritant and allergic dermatitis and
vitiligo was noted in workers exposed to acrylonitrile, methyl
acrylate, and dimethylformamide in fibre production (Bainova,
1975). In 11 out of 28 workers, delayed skin sensitization and
allergic dermatitis were observed with dimethylformamide.
Ostrovskaja et al. (1976) observed workers exposed to
acrylonitrile, acetonitrile, and hydrocyanic acid during training
and after 1.5 years and 3 years. In 190 men and women, aged 20 -
30 years, many signs and symptoms were noted including modified
reflexes, changes in blood pressure, ECG, and EEG. However, it is
difficult to attribute the findings of these authors solely to
acrylonitrile exposure.
9. EVALUATION OF HEALTH RISKS TO MAN FROM EXPOSURE TO ACRYLONITRILE
9.1. Sources and Levels of Exposure
Acrylonitrile is a colourless, volatile, chemically reactive
liquid; it does not occur as a natural product. The monomer is
used world-wide, on a large scale, in the manufacture of polymers,
fibres, and rubbers and as a chemical intermediate. Acrylonitrile-
containing polymers have been used in the manufacture of products
that come into contact with food; the amounts of acrylonitrile that
migrate into foods can be reduced to negligible quantities by the
use of good manufacturing practices in the production of the
polymers.
The major sources of contamination of the general environment
are acrylonitrile-producing and -polymerizing plants. The
occurrence of acrylonitrile in air, water, and soil near industrial
plants has been described. There is evidence that acrylonitrile
has persisted in soil for long periods following accidental
spillage; subsequent contamination of ground water has been
demonstrated.
The highest exposures occur in the workplace. Experience shows
that containment of such exposures can more readily be achieved in
production plants than in those in which acrylonitrile is used to
make other products. In a number of countries, exposure limits or
recommended limit values for the workplace have been arrived at;
values recently set have tended to be lower than in the past (Table
12).
Accidental exposure to acrylonitrile liquid and vapour may
occur during the various stages of production, transport, and use.
9.2. Acrylonitrile Toxicity
Inhaled acrylonitrile vapour is readily absorbed. Acute
systemic effects following absorption of vapour have been
described. Symptoms were non-specific and referable to the
gastrointestinal and respiratory tracts, the liver, and the central
nervous system. No acute adverse effects have been reported
following daily exposure (8-h) to up to 45 mg/m3.a At higher
concentrations rising to 220 mg/m3, 20-40 min exposure resulted
in complaints of headache, irritation of the upper respiratory
tract and the eyes, nervous irritability, and itching of the skin.
Fatalities have been reported following the use of fumigant
mixtures containing acrylonitrile together with carbon
tetrachloride and methylene chloride. Exact exposure conditions
are not known, but animal data suggest that inhalation exposure to
acrylonitrile at 500-2000 mg/m3 for 1/2-3 h could be fatal.
Simultaneous exposure to some organic solvents may enhance the
toxicity of acrylonitrile.
---------------------------------------------------------------------------
a This value was for many years the occupational exposure limit
in many countries where acrylonitrile was manufactured.
Table 12. Occupational exposure limits for selected countries
---------------------------------------------------------------
Country TWA mg/m3 STEL mg/m3 Reference
---------------------------------------------------------------
France 9 34*** INRS (1983)
Germany, Federal -* (S) DFG (1982)
Republic of
Hungary 0.5 0.5 Hungary (1979)
Japan 45 - Japan Association
of Industrial
Health (1972)
Poland Poland (1982)
Sweden 4** (S) 13 Sweden (1981)
United Kingdom
USA 4.5** (S) ACGIH (1982)
USSR 0.5 (S) USSR (1982)
---------------------------------------------------------------
* Proved animal carcinogen, strongly suspected of also being
carcinogenic for human beings. No safe concentration can be
listed.
TWA = Time-weighted average.
STEL = Short-term exposure limit.
** Listed as Industrial Substance Suspect of Carcinogenic
Potential for Man.
*** Alarm level.
S = Skin uptake can contribute to overall exposure.
Liquid acrylonitrile is also absorbed through the skin,
reportedly giving rise to non-specific symptoms similar to those
that follow acrylonitrile vapour inhalation. Local injury can
occur a few hours after exposure to liquid acrylonitrile. One
fatality has been reported. It is also an irritant to the eye.
Skin absorption of vapour does not appear to contribute
significantly to overall acrylonitrile uptake in the workplace.
There are no indications that acrylonitrile accumulates in the
body following prolonged exposure to levels found in the workplace.
Skin sensitization has been reported in a few cases; however,
no evidence is available to suggest the occurrence of pulmonary
allergic reactions.
Acrylonitrile has been shown to induce embryotoxic and
teratogenic effects at high dosage levels in experimental animals.
Although acrylonitrile is metabolized partly to cyanide, it has
been demonstrated that the acute toxic actions of acrylonitrile are
not solely due to cyanide, as was once believed.
Acrylonitrile has been shown to be mutagenic in some in vitro
systems in the presence of metabolic activation systems. So far,
mutagenic activity has not been demonstrated in in vivo assay
systems.
Complaints of ill health in workers exposed for a number of
years to acrylonitrile concentrations of less than 45 mg/m3 have
been reported in several studies. The complaints were variable in
nature and no consistent correlation with the extent of exposure
appears to have been established. The studies do not provide
evidence of a specific disease arising from long-term, low-level
exposure.
Several long-term studies in which acrylonitrile was
administered to rats orally and by inhalation demonstrated the
induction of malignant tumours. Data available in summary form
suggest that the incidence was dose-related. Eight epidemiological
studies have been carried out on workers exposed to acrylonitrile.
These studies have not demonstrated conclusively that there is a
correlation between exposure to acrylonitrile and the incidence of
cancer in man. Nevertheless, the findings are not incompatible with
the supposition that acrylonitrile is a potential human carcinogen
and thus give no cause for disregarding the evidence that has been
provided by animal studies.
It is not possible to establish a level below which no adverse
effects occur on the basis of the experimental and epidemiological
data presented in this document. However, it is evident that
exposure to acrylonitrile should be kept as low as possible in both
the workplace and the general environment, and that skin contact
with the liquid should be avoided.
REFERENCES
AARATO, S.E. & BITTERA, E. (1972) [Determination of
acrylonitrile in the air in the presence of dimethylformamide
and ammonia.] Munkavedelem, 18 : 50-51 (in Hungarian, Chem.
Abstr. 79 : 57280b, 1973).
ABREU, M.E. & AHMED, A.E. (1980) Metabolism of acrylonitrile
to cyanide: in vitro studies. Drug Metab. Disposition, 8 (6):
376-379.
ACGIH (1982) Threshold limit values for chemical substances
and physical agents in the work environment with intended
changes for 1982. Cincinnati, USA, American Conference of
Governmental Industrial Hygienists.
AGEEVA, S.T. (1970) [The condition of the metabolism of
mediating substances in workers producing acrylonitrile.] Tr.
Sarat. Med. Inst., 78 : 10-13 (in Russian).
AHMED, A.E. & ABREU, M.E. (1982) Microsomal metabolism of
acrylonitrile in liver and brain. Adv. exp. Med. Biol., 136
(Part B): 1229-1238.
AHMED, A.E. & PATEL, K. (1979) Studies on the metabolism of
aliphatic nitriles. Toxicol. appl. Pharmacol., 48 : A 91
(abstract).
AHMED, A.E. & PATEL, K. (1981) Acrylonitrile - in vivo
metabolism in rats and mice. Drug. Metab. Disposition, 9 (3):
219-222.
AHMED, A.E., FAROOQUI, M.Y.H., UPRETI, R.K., & EL-SHABRAWY,
O. (1982) Distribution and covalent interactions of (1-14C)
acrylonitrile in the rat. Toxicology, 23 : 159-175.
ALDRIDGE, W.N. (1944) A new method for the estimation of
micro quantities of cyanide and thiocyanate. Analyst, 69 :
262-265.
AL'SHANSKY, A.M., IVANOV, V.V., & PROMYSLOV, M.SH. (1980)
[Effect of acrylonitrile on content of Q-aminobutyric acid
glutamate decarboxylase activity and on lipid peroxidation in
rat brain tissue.] Vop. Med. Chimii, 26 (4): 507-509 (in
Russian).
AMERICAN CYANAMID COMPANY (1951) The chemistry of
acrylonitrile. In: Nitrogen chemicals digest, New York,
American Cyanamid Company, Vol. 5.
AMERICAN CYANAMID COMPANY (1959) The chemistry of
acrylonitrile, 2nd ed., New York, American Cyanamid Company,
272 pp.
AMERICAN CYANAMID COMPANY (1974) Handling storage - analyses
of acrylonitrile. Wayne, New Jersey, American Cyanamid
Company, 42 pp.
AMERICAN CYANAMID COMPANY (1976) Material safety data sheet;
Acrylonitrile, Wayne, New Jersey, American Cyanamid Company.
AMERICAN INDUSTRIAL HYGIENE ASSOCIATION (1970)
Acrylonitrile. Am. Ind. Hyg. Assoc. J.,: 529-531.
ANDO, K. & HASHIMOTO, K. (1967) [Effects of acrylonitrile
and acrylamide on peripheral nerve.] Rep. Osaka Prefectural
Inst. pub. Health, 4 : 23-27 (in Japanese).
ANONYMOUS (1977) Acrylo growth won't be bottled up. Chem.
Week, 9 March : 30-31.
ANTON'EV, A.A. & ROGAILIN, V.I. (1970) [Professional
dermatoses caused by acrylonitrile and their prophylaxis.] Tr.
Perm. Gos. Med. Inst., 99 : 103-106 (in Russian).
APPEL, K.E., PETER, H., & BOLT, H.M. (1981a) Effect of
potential antidotes on the acute toxicity of acrylonitrile.
Int. Arch. occup. environ. Health, 49 : 157-163.
APPEL, K.E., PETER, H., BOLT, M., & BOLT, H.M. (1981b)
Interaction of acrylonitrile with hepatic microsomes of rats
and man. Toxicol. Lett., 7 (4-5): 335-340.
ASTM (1981) Annual book of ASTM standards. Method D3371-79:
Testing for nitriles in aqueous solution by gas-liquid
chromatography, Philadelphia, American Society for Testing and
Materials.
BABANOV, G.P. (1957) [Local manifestation of the action of
acrylonitrile on the skin and mucosa.] Vrac. Delo, 5 : 511-514
(in Russian).
BABANOV, G.P., KLJUCHIKOV, V.N., KARAJEVA, N.I., & LILEEVA,
Z.V. (1959) [Clinical symptoms of chronic poisoning by
acrylonitrile.] Vrach. Delo, 8 : 833-836 (in Russian).
BAINOVA, A. (1975) [Assessment of the skin lesions in the
production of Bulana polyacrylonitrile fibers.] Dermatol.
Venerol., 14 (2): 92-97 (in Bulgarian).
BAKER, R.A. (1963) Threshold odors of organic chemicals. J.
Am. Water Works Assoc., 55 : 913-916.
BALDA, B.R. (1975) [Acrylonitrile as a contact allergen.]
Hautarzt, 26 : 599-601 (in German).
BANDT, H.J. (1953) [Acrylonitrile as an effluent.] Z.
Fisch., 2 : 457-461 (in German).
BARNES, J.M. (1970) Effects on rats of compounds related to
acrylamide. Br. J. ind. Med., 27 (2): 147-149.
BARRETT, W.J. (1974) Sampling and analysis of four organic
compounds using solid sorbents, Washington, US Department of
Health, Education and Welfare (Southern Research Institute
Report SORI-EAS-74-301).
BAXTER, R.A. (1979) Evaluation and control of industrial
exposure to acrylonitrile. Ann. occup. Hyg., 22 : 429-435.
BELILES, R.P., PAULIN, H.J., MAKRIS, N.G., & WEIR, R.J.
(1980) Three-generation reproduction study of rats receiving
acrylonitrile in drinking water. Final report. Litton
Bionetics Inc.
BELLAR, T.A. & SIGSBY, J.E., Jr (1970) Direct gas
chromatographic analysis of low molecular weight substituted
organic compounds in emissions. Environ. Sci. Technol., 4 :
150-156.
BENCHEV, I., RIZOV, N., & KOLARSKA, A. (1982) [Acrylonitrile
determination in biological fluids by gas chromatography -
"head space" analysis.] Hyg. Zdrav., 1 : 87-89 (in Bulgarian).
BENESH, V. & CHERNA, V. (1959) [Acrylonitrile. Acute
toxicity and mechanism of action.] J. Hyg. Epidemiol.
Microbiol. Immunol., 3 (1): 106-116 (in German and in Russian).
BENESH, V. & SHRAM, R. (1969) Mutagenic activity of some
pesticides in Drosophila melanogaster. Ind. Med. Surg.,
38 (12): 442-444.
BENSON, G.B. & BOYCE, G.E. (1981) A thermally desorbable
passive dosimeter for personal monitoring for acrylonitrile.
Ann. occup. Hyg., 24 (1): 55-76.
BENSON, G.B., BOYCE, G.E., & HAILE, D.M. (1981) Industrial
hygiene measurements for organic pollutants (acrylonitrile)
using passive dosimeters and automated thermal desorption.
Ann. occup. Hyg., 24 (4): 367-373.
BERCK, B. (1960) Retention of acrylonitrile and carbon
tetrachloride by shelled walnuts fumigated with acrylon. J.
Agric. food Chem., 8 :128-131.
BERG, G.L., ed. (1977) 1977 Farm Chemicals Handbook, 63rd
issue, Willoughby, Ohio, Meister Publishing Co., pp. D5, D50.
BIRD, W.L. & HALE, C.H. (1952) Polarographic determination
of acrylonitrile. Anal. Chem., 24 : 586-587.
BOLLINI, M., SEVES, A., & FOCHER, B. (1974) [Determination
of free monomers in water emulsions of synthetic polymers or
copolymers.] Ind. Carta, 12 : 234-240 (in Italian, Chem.
Abstr., 81 : 121672b).
BOLT, H.M., FILSER, J.G., & HINDERER, R.K. (1978) Rat liver
microsomal uptake and irreversible protein binding of
1,2-14C-vinyl bromide. Toxicol. appl. Pharmacol., 44 : 481-489.
BOND, E.J. & BUCKLAND, C.T. (1976) Control of insects with
fumigants at low temperatures: toxicity of mixtures of methyl
bromide and acrylonitrile to three species of insects. J.
econ. Entomol., 69 : 725-727.
BONDAREV, G.I., STASENKOVA, K.P., & VISSARIONOVA, V.Y.
(1976) [Protective effect of sulfur-containing compounds in
poisoning with acrylic acid nitrile.] Vopr. Pitan., (4) : 55-58
(in Russian).
BORCHARDT, VON K., FRANSEN, E., & HARTMAN, B. (1970) [Acute
oral intoxication with acrylonitrile in animals.] Z. Ges.
Hyg., 16 : 316-319 (in German).
BORG WARNER CHEMICALS (1977) In response to SHA's request
for information on occupational exposure to acrylonitrile.
Fed. Reg., 42 : 33043 (Letter to OSHA Docket Officer, US
Department of Labor).
BOYLAND, E. & CHASSEAUD, L.F. (1967) Enzyme-catalysed
conjugations of glutathione with carbonyl compounds. Biochem.
J., 104 : 95-102.
BOYLAND, E. & CHASSEAUD, L.F. (1968) Enzymes catalysing
conjugations of glutathione with A,B,-unsaturated carbonyl
compounds. Biochem. J., 109 : 651-661.
BREWER, W.E. (1976) 90-day subacute vapor inhalation
toxicity study with acrylonitrile in beagle dogs, albino rats
and albino mice (Industrial Biotest Report No. 74-42, prepared
for the Monsanto Company).
BRIEGER, H., RIEDERS, F., & HODES, A.B. (1952)
Acrylonitrile: spectrophotometric determination, acute
toxicity, and mechanism of action. Ind. Hyg. occup. Med., 6 :
128-140.
BRUCE, R.B., HOWARD, J.W., & HANZAL, R.F. (1955)
Determination of cyanide, thiocyanate and A-hydroxynitriles in
plasma or serum. Anal. Chem., 27 : 1346-1347.
BUFFONI, F., COPPI, C., & SANTONI, C. (1982) Determination
of thioethers in urine. Clin. Chem., 28 : 248-250.
CAMPBELL, D.N. & MOORE, R.H. (1979) The quantitative
determination of acrylonitrile, acrolein, acetonitrile and
acetone in workplace air. Am. Ind. Hyg. Assoc. J., 40 : 904-909.
CANADA FOOD & DRUGS ACT & REGULATIONS (1982) Division 23 -
Section B.23.008. Canada, Ministry of Supply & Services.
CARPENTER, C.P., SMYTH, H.F., & POZZANI, U.C. (1949) The
assay of acute vapour toxicity and the grading and
interpretation of results on 96 chemical compounds. J. ind.
Hyg. Toxicol., 31 : 343-346.
CHANDRA, H., GUPTA, B.H., BHARGAVA, S.K., CLERK, S.H., &
MAHENDRA, P.N. (1980) Chronic cyanide exposure - a
biochemical and industrial hygiene study. J. anal. Toxicol.,
4 : 161-165.
CHERNA, M., KOCHISOVA, J., KODYTKOVA, I., KOPECKY, J., &
SHRAM, R.J. (1981) Mutagenic activity of oxiranecarbonitrile
(glycidonitrile). In: Gut, I., Cikrt, M. & Plaa, G.L., ed.
Industrial & environmental xenobiotics, Berlin, Springer-
Verlag, pp. 251-255.
CHOBOT, A.M. (1979) [Menstrual function in workers of the
polyacrylonitrile fibre industry.] Zdrav. Beloruss., 2 : 24-27
(in Russian).
CIA (1978) Codes of practice for chemicals with major
hazards - acrylonitrile, Chemical Industries Association.
CINCOLELLA, A., VOIRIN, D., HECHT, G., GERBER, J.-M., DUCOS,
P., & LIMASSET, J.-C. (1981) Monitoring of atmospheric
concentrations of acrylonitrile in eleven French factories. G.
Ital. Med. lav., 3 : 165-167.
CLIVE, D. & SPECTOR, F.S. (1975) Laboratory procedure for
assessing specific locus mutagens at the TK locus in cultured
L5178Y mouse lymphoma cells. Mutat. Res., 31 : 17-29.
COLLINS, T.F.V. (1977) Written testimony in the matter of
acrylonitrile copolymers used to fabricate beverage containers
(Docket No. 76N-0070, exhibit G-82).
CRISP, S. (1980) Solid sorbent gas samplers. Ann. occup.
Hyg., 23 : 47-76.
CUPITT, L.T. (1980) Fate of toxic and hazardous materials in
the air environment. (EPA report No. EPA-600/3-80-084).
CZAJKOWSKA, T. (1971) [Excretion of certain metabolites upon
single administration of acrylonitrile.] Med. Pr., 22 (4):
381-385 (in Polish).
CZAJKOWSKA, T., KNOBLOCH, K., KOWALSKI, Z., KRYSIAK, B.,
PIOTROWSKI, J., ROGACZEWSKA, T., STARZYNSKI, Z., STASIK, M.,
SZENDZIKOWSKI, S., & ZWIERZCHOWSKI, Z. (1969) [Evaluation of
the toxicity and exposure of chemical industry workers to
acrylonitrile.] Med. Pr., (Supplement): 11-16 (in Polish).
DAHM, D.J. (1977) Identification of metabolites of
acrylonitrile. Testimony before the US FDA (Industrial Biotest
Report No. 74-42, Appendix 4: A.1-A.19, prepared for the
Monsanto Company).
DAUGHERTY, F.M. & GARRETT, J.T. (1951) Toxicity levels of
hydrocyanic acid and some industrial by-products. Texas J.
Sci., No. 3 : 391-396.
DAUES, G.W. & HAMNER, W.F. (1957) Determination of small
amounts of acrylonitrile in aqueous industrial streams. Anal.
Chem., 29 : 1035-1037.
DAVIS, J.H., DAVIES, J.E., AMERICO RAFONNELLI, R.S., & REICH,
G. (1973) Investigation of fatal acrylonitrile
intoxications. Pesticides and the environment, a continuing
controversy. Inter. Med. Book Corp., 2 : 547-556.
DELIVANOVA, S., POPOVSKI, P., & ORUSEV, T. (1978)
[Blepharoconjunctivitis in workers of the polyacrylonitrile
synthetic fibre industry.] God. Zb. Med. Fak. Skoje, 24 :
279-282 (in Macedonian).
DELLA FIORENTINA, H. & DE WIEST, F. (1979) Recherche d'un
indice biologique d'exposition chronique des travailleurs aux
dérivés cyanés. Arch. Mal. prof., 40 : 700-703.
DELZELL, E. & MONSON, R.R. (1982) Mortality among rubber
workers: VI. Men with potential exposure to acrylonitrile. J.
occup. Med., 24 (10): 767-769.
DE MEESTER, C., PONCELET, F., ROBERFROID, M., & MERCIER, M.
(1978b) Mutagenicity of acrylonitrile. Toxicology, 11 : 19-27.
DE MEESTER, C., DUVERGER-VAN BOGAERT, M., LAMBOTTE-VAN DEPAER,
M., ROBERFROID, M., PONCELET, F., & MERCIER, M. (1979) Liver
extract mediated mutagenicity of acrylonitrile. Toxicology,
13 (1): 7-15.
DFG (1982) Maximum concentrations at the workplace and
biological tolerance values for working materials 1982,
Weinheim, Verlag Chemie Gmbh, Commission for Investigation of
Health Hazards of Chemical Compounds in the Work Area,
Deutsche Forschungsgemeinschaft (Report No. 18).
DINU, V. (1975a) Activity of glutathione peroxidase and
catalase and the concentration of lipid peroxides in acute
intoxication with acrylonitrile. Rev. Roum. Biochim., 12 (1):
11-14.
DINU, V. (1975b) Intracellular thiol concentration in
acrylonitrile intoxication. Rev. Roum. Biochim., 12 (3):
155-158.
DINU, V. & KLEIN, R. (1976) Catalase activity and
glutathione and lactic acid concentrations in rats acutely
poisoned by acrylonitrile. J. Pharmacol., 7 (2): 223-226.
DJURIC, D., RAICEVIC, P., & KONSTANTINOVIC, I. (1962)
Excretion of thiocyanates in urine of smokers. Arch. environ.
Health, 5 : 12-15.
DORIGAN, J., FULLER, B., & DUFFY, R. (1976) Scoring of
organic air pollutants chemistry, production and toxicity of
selected synthetic organic chemicals (chemicals A-C), (MITRE
Tech. Rep. MTR-7248).
DORODNOVA, N.S. (1976) [Gynaecological morbidity and
specific functions of the female organism under the conditions
of chemical production in "Nitron".] Gig. Zabol., 8 : 45-46 (in
Russian).
DRAMANSKI, W. & TROJANOWSKA, B. (in press) Chromatographic
determination of S-cyanoethylmercapturic acid - a metabolite
of acrylonitrile in urine. Arch. Toxicol.
DUDLEY, H.C. & NEAL, P.A. (1942) Toxicology of acrylonitrile
(vinyl cyanide). I. Study of the acute toxicity. J. ind. Hyg.
Toxicol., 24 (2): 27-36.
DUDLEY, H.C., SWEENEY, T.R., & MILLER, J.W. (1942)
Toxicology of acrylonitrile (vinyl cyanide). II. Studies of
effects of daily inhalation. J. ind. Hyg. Toxicol., 24 :
255-258.
DUPONT (1977) In response to OSHA's request for information
on occupational exposure to acrylonitrile, letter to the OSHA
Docket Officer, US Department of Labour, Fed. Reg., 42 : 33043.
DUVERGER-VAN BOGAERT, M., LAMBOTTE-VANDEPAER, M., NOEL, G.,
ROBERFROID, M., & MERCIER, M. (1978) Biochemical effects of
ACN on the rat liver, as influenced by various pre-treatments
of the animals. Biochem. Biophys. Res. Comm., 83 : 1117-1124.
DUVERGER-VAN BOGAERT, M., LAMBOTTE-VANDEPAER, M., DE MEESTER,
C., ROLLMANN, B., PONCELET, F., & MERCIER, M. (1981) Effect
of several factors on the liver extract mediated mutagenicity
of acrylonitrile and identification of four new in vitro
metabolites. Toxicol. Lett., 7 : 311-319.
DUVERGER-VAN BOGAERT, M., LAMBOTTE-VANDEPAER, M., MERCIER, M.,
& PONCELET, F. (1982a) In vitro covalent binding of
acrylonitrile to rat liver proteins. Toxicol. Lett., 13 :
203-209.
DUVERGER-VAN BOGAERT, M., LAMBOTTE-VANDEPAER, M., DE MEESTER,
C., MERCIER, M., & PONCELET, F. (1982b) Role of glutathione
in liver-mediated mutagenicity of acrylonitrile. Toxicol.
Lett., 11 : 305-311.
EFREMOV, A.M. (1976a) [Study of the free radicals of the
blood, brain, liver and spleen of white rats subjected to
chronic acrylonitrile poisoning.] Zdrav. Beloruss., 6 : 86 (in
Russian).
EFREMOV, A.M. (1976b) [Study of the biotransformation of
acrylonitriles in animals.] Zdrav. Beloruss., 7 : 85-86 (in
Russian).
EFREMOV, A.M. (1976c) [State of protective adaptive
reactions in white rats poisoned with acrylonitrile.] Gig. Tr.
Ohr. Zdor. Rab. Neft. Neftehim. Prom-sti., 9 : 120-122 (in
Russian).
EFREMOV, A.M. (1976d) [Activity of catalase, peroxidase and
sulfhydryl groups of the brain, liver, and blood in white rats
poisoned with acrylonitrile.] Gig. Tr. Ohr. Zdor. Rab. Neft.
Neftehim. Prom-sti., 9 : 123-124 (in Russian).
ELLMAN, G.L. (1959) Tissue sulfhydryl groups. Arch.
Biochem. Biophys., 82 : 70-77.
ENIKEEVA, N.A., OSTROVSKAJA, R.S., SYSO, L.V., PODREZ, Z.G.,
BRAGINSKAJA, L.L., GVOZDEV, N.P., NESTEROVA, N.E.,
MUSSERSKAJA, A.N., SUZDALTREVA, N.A., & EFREMOV, A.M. (1976)
[Industrial hygiene and health status of workers involved in
the manufacture of the synthetic fibre Nitron.] Gig. Tr. Ohr.
Zdor. Rab. Nef. Neftehim. Prom-sti., No. 9 : 22-25 (in Russian).
EUROP-COST (1976) A comprehensive list of polluting
substances which have been identified in various fresh waters,
effluent discharges, aquatic animals and plants, and bottom
sediments, 2nd ed. Luxembourg, Commission of the European
Communities, p. 19 (EUCO/MDU/73/76, XII/476/76).
FAROOQUI, M.Y.G. & AHMED, A.E. (1982) Molecular interaction
of acrylonitrile and potassium cyanide with rat blood. Chem.
Biol. Interact., 38 : 145-149.
FERIN, J., URBANKOVA, J., & VLCHKOVA, A. (1961) [Hygienic
and toxicological problems of acrylonitrile and
dimethylformamide.] Prac. Lék., 13 : 466-468 (in Czech).
FRANKENBERG, L. (1980) Enzyme therapy in cyanide poisoning:
effect of rhodanese and sulfur compounds. Arch. Toxicol., 45 :
315-323.
FRANZEN, E. von & WAGNER, G. (1978) [Gelchromatographic and
electrophoretic serum protein pattern in rats after acute
acrylonitrile intoxication.] Z. Gesamte Hyg., 24 : 534-537 (in
German).
FREEMAN, R.A., SCHROY, J.M., KLIEVE, J.R., & ARCHER, S.A.
(1981) Experimental studies on the rate of air stripping of
hazardous chemicals from waste treatment systems. In: APCA
Meeting, Montreal, Canada, June 1980.
GAGNON, Y.T. & POSNER, J.C. (1979) Recovery of acrylonitrile
from charcoal tubes at low levels. Am. Ind. Hyg. Assoc. J.,
40 : 923-925.
GAWELL, G. B-M. (1979) Determination of acrylonitrile
monomer in plastic packaging and beverages by headspace gas
chromatography. Analyst, 104 : 106-110.
GHANAYEM, B.I. & AHMED, A.E. (1982) In vivo
biotransformation and biliary excretion of 1-14C acrylonitrile
in rats. Arch. Toxicol., 50 : 175-185.
GHERSIN, Z., STITZL, A., & MANEA, R. (1969) [Comparative
study of titrimetric and colorimetric methods for determining
acrylonitrile in waste water.] Rev. Chim. (Bucharest), 20 :
689-694 (in Rumanian, Chem. Abstr., 72 : 124853m).
GHIRINGHELLI, L. (1954) [Acrylonitrile: toxicity and
mechanism of action.] Med. lav., 45 (5): 305-312 (in Italian).
GINCHEVA, N., STAMOVA, N., HINKOVA, L., KYURKTCHIEV, S.,
SPASOVSKI, M., BAINOVA, A., MUHTAROVA, M., & HRISTEVA, V.
(1977) Study of the health status of workers from the
acrylonitrile department. In: Proceedings of the International
Symposium on Occupational Health in the Production of
Artificial Fibres - Abstracts, Finland, 6-10 June 1977, p. 41.
GOING, J.E., KUYKENDAHL, P., LONG, S., ONSTOT, J., & THOMAS,
K. (1979) Environmental monitoring near industrial sites:
acrylonitrile. 285 pp (EPA/560/6-79/003).
GRAHAM, J.P.D. (1965) Hydroxycobalamin as an antidote to
acrylonitrile. Toxicol. appl. Pharmacol., 7 : 367-372.
GRAVCZYK, J. & ZWIERZCHOVSKI, Z. (1973) [Effect of acute
experimental poisoning with acrylonitrile on the pressor
responses to adrenaline, noradrenaline and acetylcholine.]
Med. Pr., 24 (2): 151-158 (in Polish).
GREEN, M.L.H., MURIEL, W.J., & BRIDGES, B.A. (1976) Use of
simplified fluctuation test to detect low levels of mutagens.
Mutat. Res., 38 : 33-42.
GROTE, A.A., KIM, W.S., & KUPEL, R.E. (1978) Establishing a
protocol from laboratory studies to be used in field sampling
operations. Am. Ind. Hyg. Assoc. J., 39 : 880-884.
GRUNSKE, F. (1949) [Ventox and ventox-poisoning.] Dtsch.
med. Wochenschr., 74 : 1081 (in German).
GUENGERICH, F.P., GEIGER, L.E., HOGY, L.L., & WRIGHT, P.L.
(1981) In vitro metabolism of acrylonitrile to
2-cyanoethylene oxide, reaction with glutathione, and
irreversible binding to proteins and nucleic acids. Cancer
Res., 41 : 4925-4933.
GUERIN, M.R., OLERICH, G., & HORTON, A.D. (1974) Routine gas
chromatographic component profiling of cigarette smoke for the
identification of biologically significant constituents. J.
Chromatogr. Sci., 12 : 385-391.
GUT, I., NERUDOVA, J., KOPECKY, J., & HOLECHEK, V. (1975)
Acrylonitrile biotransformation in rats, mice, and chinese
hamsters as influenced by the route of administration and by
phenobarbital, SKF-525A, cysteine, dimercaprol, or
thisulphate. Arch. Toxicol., 33 : 151-161.
GUT, I., KOPECKY, J., & FILIP, J. (1981a) Acrylonitrile-14C
metabolism in rats: effect of the route of administration on
the elimination of thiocyanate and other radioactive
metabolites in urine and faeces. J. Hyg. Epidemiol. Microbiol.
Immunol., 25 (1): 12-16.
GUT, I., KOPECKY, J., & NERUDOVA, J. (1981b) Relationship
between acrylonitrile biotransformation, pharmacokinetics, and
acute toxicity. A short review. G. Ital. Med. lav., 3 : 131-136.
GUT, I., PELECH, L., VEJLUPKOVA, J., MATOUSEK, O., & KLENER,
P. (1982) [Effects of induction and inhibition of benzene
metabolism in hematopoiesis in male Wistar rats.] Cesk. Hyg.,
27 (1): 1-13 (in Czech).
GUT, I., NERUDOVA, J., FRANTIK, E., KOPECKY, J., HOLECHEK, V.,
STILBOROVA, A., MIRHEJOVSKA, E., & HOLUSHA, R. (in press)
Effect of acrylonitrile inhalation: Intermediary metabolism
and the excretion of thioethers and thiocyanate in rats. J.
Hyg. Epidemiol. Microbiol. Immunol, 28 : (in press).
HALL, M.E. & STEVENS, J.W., Jr (1977) Spectrophotometric
determination of acrylonitrile. Anal. Chem., 49 (14): 2277-2280.
HASHIMOTO, K. (1962) Studies on the intoxication of
acrylonitrile report IV. Effect of daily inhalation of
acrylonitrile on body weight, bodily activity and hemogram of
mice. Q. J. Labor. Res., 10 : 11-16.
HASHIMOTO, K. & ANDO, K. (1966) Effect of acrylonitrile on
the central and peripheral nervous system. In: Symposium on
Higher Nervous Functions & Occupational Health, pp. 87-89.
HASHIMOTO, K. & KANAI, R. (1965) Studies on the toxicology
of acrylonitrile, metabolism, mode of action and therapy. Ind.
Health, 3 (1-2): 30-45.
HASHIMOTO, K. & KANAI, R. (1972) Effect of acrylonitrile on
sulfhydryls and pyruvate metabolism in tissues. Biochem.
Pharmacol., 21 : 635-640.
HASHIMOTO, K. & KOBAYASI, T. (1961) A case of acute
dermatitis caused by contact with acrylonitrile. Q. J. Labor.
Res., 9 (1-4): 21-24.
HASKELL LABORATORY (1975) In vitro microbial mutagenicity
studies of acrylonitrile. (Report No. 351-357, E.I. Dupont de
Nemours & Co., Haskell Laboratory for Toxicology and
Industrial Medicine).
HASLAM, J. & NEWLANDS, G. (1955) Determination of
acrylonitrile in air. Analyst, 180 : 50-53.
HEINO, J., VILHUNEN, R., & HERNBERG, S. (1968) Urinary
thiocyanate concentrations in smokers and non-smokers.
Work-Environ.-Health, 5 : 12-15.
HENDERSON, C., PICKERING, G.H., & LEMKE, A.E. (1961) The
effect of some organic cyanides (nitriles) on fish. Proc. 15th
Ind. Waste Conf. En. Bull., 45 (2): 120-130.
HEUSER, S.G. & SCUDMORE, K.A. (1969) Determination of
fumigant residues in cereals and other foodstuffs: a
multi-detection scheme for gas chromatography of solvent
extracts. J. Sci. Food Agric., 20 : 566-572.
HOFFMANN, P., KLEINE, D., & FRANZEN, E. (1976) [On the proof
of acrylonitrile exposure: Detoxication of acrylonitrile by
coupling with D-glucuronic acid.] Z. Ges. Hyg., 22 (5): 310-312
(in German).
HOGAN, G.K. & RINEHART, W.E. (1980) A 24-month oral
toxicity/carcinogenicity study of acrylonitrile administered
by inhalation to Spartan rats. Bio/dynamics Inc. (Final report
Vols I & II, Project No. 77-1746).
HOLECHEK, V. & KOPECKY, J. (1981) Conjugation of glutathione
with acrylonitrile and glycidonitrile. In: Gut, I., Cikrt, M.
& Plaa, G.L., ed. Industrial and environmental xenobiotics.
Berlin, Springer Verlag, pp. 239-245.
HOUTHUIJS, D., REMIJN, B., WILLEMS, H., BOLEIJ, J., &
BIERSTEKER, K. (1982) Biological monitoring of acrylonitrile
exposure. Am. J. ind. Med., 3 (3): 313-320.
HROMOV, V.E. (1974) [Detection of circulating and fixed
antibodies in the diagnosis of allergies of chemical
etiology.] Vrach. Delo, 12 : 115-116 (in Russian).
HUGHES, T.W. & HORN, D.A. (1977) Source assessment:
acrylonitrile manufacture (air emission) (NITS Report. No.
PB-271 969).
HUMISTON, C.G. & FRAUSON, L.O. (1975) 90-day oral toxicity
study incorporating acrylonitrile in the drinking water of
rats. Midland, MI, Dow Chemical Toxicology Research
Laboratory, 99 pp.
HUNGARY (1979) Workplace requirements for air purity, Magyar
Nöpköztarsasagi Orszagos Szabvany, MSZ 21461-78 Az MSZ
21461-73 helyett G 23, 1979, 1 January.
IARC (1979) Acrylonitrile, acrylic and modacrylic fibres,
and acrylonitrile- butadiene-styrene and styrene-acrylonitrile
copolymers, pp. 73-113 (IARC Monographs on the Evaluation of
the Carcinogenic Risk of Chemicals to Humans, Vol. 19).
IARC (1982) IARC Monographs on the Evaluation of
Carcinogenic Risk of Chemicals to Humans, 1-29 (Suppl. 4) pp.
25-27.
IDOL, J.D., Jr (1974) Acrylonitrile polymers in prospect and
retrospect. In: Proceedings of the Applied Polymer Symposium
25: Acrylonitrile in Macromolecules, New York, John Wiley, pp.
1-18.
IMANARI, T., TANABE, S., TOIDA, T., SHINZO, T., & TOSHIHIKO,
T. (1982) Simultaneous determination of cyanide and
thiocyanate by high performance liquid chromatography. Chem.
Pharm. Bull. Japan, 30 (10): 3800-3802.
INRS (1983) Valeurs limités pour les concentrations des
substances dangereuses dans l'air des locaux de travail. Cah.
Notes Documen., No. 110, (ND 1410-110-83).
IVANOV, V.V. (1980) [Oxidative transformation of
acrylonitrile as a basis of its toxicological action.]
Farmakol. Toksikol., 43 (3): 383-384 (in Russian).
IVANOV, V.V. (1981) [Evaluation of the significance of
acrylonitrile metabolism in the mechanism of its toxicity.]
Gig. Tr. prof. Zabol., 9 : 48-50 (in Russian).
IVANOV, V.V. & AL'SHANSKY, A.M. (1982) GABA-ergic components
and lipid peroxidation during acute exogenous acrylonitrile
poisoning. Bull. exper. Biol. Med., 94 (7): 40-43.
IVANOV, V.V., GRIOHCHENKO, D.A., & KLIMATSKAJA, L.G. (1979)
[Blood serum enzymes in evaluating the nature of liver damage
and the effectiveness of chemotherapy following acrylonitrile
poisoning.] Vopr. Med. Him., 25 (6): 715-718 (in Russian).
IVANOV, V.V., KURNETSOVA, G.P., & ARCHAKOV, A.I. (1978)
[Stimulation of lipid peroxidation by acrylonitrile in rat
liver tissue.] Vopr. Med. Him., 24 (6): 816-818 (in Russian).
IVANOV, V.V., ZHISNOV, G.F., BACHMANOVA, G.I., MAZUROV, A.V.,
& ARCHALOV, A.I. (1979) [Interaction of acrylonitrile with
the microsomal oxidation system in rat liver tissue.] Vopr.
Med. Him., 25 (4): 468-471 (in Russian).
IVANOV, V.V., ZHIRNOV, G.F., & ARCHAKOV, A.J. (1982)
[Activation of acrylonitrile in the microsomal system of
oxidation.] Vopr. Med. Him., 28 (2): 95-98.
IWASAKI, K., KANNO, S., SAWATARI, K., SAWADA, S., SUGIMOTO,
M., SODA, R., TAKANO, T., NAKAMURA, K., MATSUMURA, Y., MYOJO,
T., SAKURAI, H., & TAKEMOTO, K. (1980) [Determination of
acrylonitrile in the working environments of a few kinds of
plants handling acrylonitrile.] Annual Report of the National
Institute of Industrial Health for 1979, p. 38 (in Japanese).
JACOBS, H.W. & SYRJALA, R.J. (1978) The use of infrared
analyzers for monitoring acrylonitrile. Am. Ind. Hyg. Assoc.
J., 39 : 161-165.
JACKSON, S. & BROWN, V.M. (1970) Effect of toxic wastes on
treatment processes and watercourses. Water Pollut. Control,
69 (3): 292-313.
JAEGER, R.J. (1978) Effect of hypoxia on the toxicity of
1,1-dichloroethylene or ACN. Toxicol. appl. Pharmacol., 45 :
257.
JAEGER, R.J. & COTE, I.L. (1982) Effect of hypoxia on the
acute toxicity of acrylonitrile. Res. Comm. Chem. Pathol.
Pharmacol., 36 (2): 345-348.
JAMES, S.P., WARING, R.H., & WHITE, D.A. (1967) Separation
of mercapturic acids and related compounds by gas
chromatography and a method for the determination of hippuric
acid. J. Chromatogr., 26 : 255-258.
JAPAN ASSOCIATION OF INDUSTRIAL HEALTH (1972) Recommendations
on permissible concentrations.
JEDLICKA, V., PASEK, A., & GOLA, J. (1958) Pesticides in
foods. III. Acrylonitrile as a food insecticide. J. Hyg. Epid.
Microbiol. Immunol., 1 : 116-125.
KANAI, R. & HASHIMOTO, K. (1965) Determination of
acrylonitrile, cyanide and thiocyanate in biological
materials. Ind. Health, 3 (47): 47-52.
KANKAANPÄÄ, E.E., HEMMINKI, K., & VAINIO, H. (1979)
Embryotoxicity of acrolein, acrylonitrile and acryl acid in
developing chick embryos. Toxicol. Lett., 4 : 93-96.
KIESSELBACH, N., KORALLUS, U., LANGA, H.J., NIESS, A., &
ZWINGERS, T. (1979) Acrylonitrile - Epidemiological Study,
Bayer 1977. Zentr. Arbeitsmed. Profyl., 29 (10): 257-259.
KILKIPARI, I. & SAVOLAINEN, H. (1982) Increased urinary
excretion of thioether in new rubber workers. Br. J. ind.
Med., 39 : 401-403.
KLESHCHEVA, M.S., USACHEVA, V.T., & POZHAROVA, V.N. (1971)
[Determination of residual monomers and non-polymerising
impurities in polystyrene plastics by means of gas
chromatography.] Plast. Mass., 7 : 57-58 (in Russian).
KNEZEVICH, A.L. & HOGAN, G.K. (1980) A 24-month oral
toxicity/carcinogenicity study of acrylonitrile administered
in the drinking water to Fischer 344 rats. Bio/Dynamics Inc.
(Final Report Vols I and IV, Project No. 77-1744, Bio-Dynamics
Inc.).
KNOBLOCH, K., SZENDZIKOWSKI, S., CZAJKOWSKA, T., & KRYSIAK,
B. (1971) [Experimental studies on acute and subacute
toxicity of acrylonitrile.] Med. Pr., 22 (3): 257-269 (in
Polish).
KNOBLOCH, K., SZENDZIKOWSKI, S., & CZAJKOWSKA, T. (1972)
[Chronic toxicity of acrylonitrile.] Med. Pr., 23 (3): 243-257
(in Polish).
KOLK, J.J. (1979) Inventory of employees exposed to
acrylonitrile in the Netherlands. Acrylonitrile Consultation
Group (Report of the Medical Sub-Group of the Vereniging van
de Nederlandse Chemische Industrie [VNCI]).
KOPECKY, J. (1982) Development and methodology for
assessment of urinary excretion of S-cyanoethylmercapturic
acid in man. (Final Report to the WHO Regional Office for
Europe, ICP/RCE 903).
KOPECKY, J., ZACHARDOVA, D., GUT, I., & FILIP, J. (1979)
[Metabolism of acrylonitrile in rats in vivo. ] Prac. Lék., 31 :
203-207 (in Czech).
KOPECKY, J., GUT, I., NERUDOVA, J., ZACHARDOVA, D., &
HOLECHEK, V. (1980a) Two routes of acrylonitrile metabolism.
J. Hyg. Epid. Microbiol. Immunol., 24 : 356-362.
KOPECKY, J., GUT, I., NERUDOVA, J., ZACHARDOVA, D., HOLECHEK,
V., & FILIP, J. (1980b) Acrylonitrile metabolism in the rat.
Arch. Toxicol., Suppl. 4 : 322-324.
KOPECKY, J., ZACHARDOVA, D., & GUT, I. (1980c) New findings
on acrylonitrile metabolism. Czech. Med., 3 : 295-301.
KOPECKY, J., GUT, I., NERUDOVA, J., ZACHARDOVA, D., &
HOLECHEK, V. (1981) Metabolic studies on acrylonitrile. In:
Gut, I., Cikrt, M. & Plaa, G.L., ed. Industrial and
environmental xenobiotics, Berlin, Springer-Verlag, pp.
221-231.
KORZHOVA, I.T., KLESHCHEVA, M.S., PORODINA, M.N., KORHOVA,
R.D., BALANDINA, V.A., & HOHLOV, V.A. (1974)
[Gas-chromatographic determination of toxic concentrations of
styrene and acrylonitrile vapours during the synthesis of
polystyrene plastics.] Plast. Mass., 5 : 67-69 (in Russian,
Chem. Abstr., 81 : 110759j).
KRIVOVA, V.N., USTINOVIC, I.P., & ERENBURG, B.E. (1982)
[Functional activity of the immune T-system due to
acrylonitrile poisoning.] In: Metabolicheskie aspecti
deistvija na organism industrial'nih himicheskih soedinenii,
Krasnojarsk, pp. 53-55 (in Russian).
KROELLER, E. (1970) [Residual acrylonitrile determination in
food.] Dtsch Lebensm. Rundsch., 66 : 11-33 (in German).
KRYNSKA, A. (1970) [Method of determination of acrylonitrile
in the air.] Pr. Central. Inst. Ochrony Pr., 20 : 303-310 (in
Polish, Chem. Abstr., 74 : 90798w).
KRYSIAK, B & KNOBLOCH, K. (1971) [Effect of acrylonitrile on
the central nervous system.] Med. Pr., 22 (6): 601-610 (in
Polish).
LAMBOTTE-VANDEPAER, M., DUVERGER-VAN BOGAERT, M., DE MEESTER,
C., PONCELET, F., & MERCIER, M. (1980) Mutagenicity of urine
from rats and mice treated with acrylonitrile. Toxicology, 16 :
67-71.
LAMBOTTE-VANDEPAER, M., DUVERGER-VAN BOGAERT, M., DE MEESTER,
C., ROLLMANN, B., PONCELET, F., & MERCIER, M. (1981a)
Identification of two urinary metabolites of rats treated with
acrylonitrile; influence of several inhibitors on the
mutagenicity of those urines. Toxicol. Lett., 7 : 321-328.
LAMBOTTE-VANDEPAER, M., DUVERGER-VAN BOGAERT, M., GARNY, V.,
PONCELET, F., & MERCIER, M. (1981b) Mutagenicity of
metabolites of acrylonitrile detected in urine. Mutat. Res.,
80 : 269 (abstract).
LANGVARDT, P.W., PUTZIG, C.L., BRAUN, W.H., & YOUNG, J.D.
(1980) Identification of the major urinary metabolites of
acrylonitrile in the rat. J. Toxicol. environ. Health, 6 :
273-282.
LAWTON, A.H., SWEENEY, T.A., & DUDLEY, H.C. (1943)
Toxicology of acrylonitrile (vinyl cyanide): III.
Determination of thiocyanate in blood and urine. J. ind. Hyg.
Toxicol., 25 : 13-19.
LEFAUX, R. (1966) [Chemistry and toxicology of plastics.]
Mainz, Krausskopf Verlag (in German).
LEONARD, A., GARNY, V., PONCELET, F., & MERCIER, M. (1981)
Mutagenicity of acrylonitrile in mouse. Toxicol. Lett., 7 :
329-334.
LINDGREN, D.L., VINCENT, L.E., & KROHNE, H.E. (1954)
Relative effectiveness of ten fumigants to adults of eight
species of stored product insects. J. econ. Entomol., 47 (5):
923-926.
LITTON BIONETICS (1975) Mutagenic evaluation of compounds
MCA 730 (LBI Project No. 2547, prepared for the Manufacturing
Chemists Association).
LITTON BIONETICS (1976) Mutagenicity evaluation of MCA 730
(Final report, LBI Project No. 2548, prepared for the
Manufacturing Chemists Association).
LODZ SANITARY INSPECTION SURVEY (1981) [Annual report on the
concentration of acrylonitrile in the air at workplaces in the
acrylic fibre plant "Chemitex-Anilana" at Lodz.] Lodz, Poland,
Sanitary-Epidemiological Station (in Polish).
LORZ, H. (1950) [On the percutaneous poisoning by
acrylonitrile (Ventox).] Dtsch med. Wochenschr., 75 (33/34):
1087-1088 (in German).
LOWENBACH, W., SCHLESINGER, S., & KING, J. (1978) Toxic
pollutant identification: acrylonitrile manufacture, 160 pp
(EPA-IMPQE series 02178-03).
MADDOCK, J.T., BYER, W.L., MALSEED, R.T., KERESZTESY, J.,
TAYLOR, T.J., & KATZ, A.B. (1977) Acrylonitrile (Monograph
prepared for US Consumer Product Safety Commission, Washington
DC).
MAGOS, L. (1962) A study of acrylonitrile poisoning in
relation to methemoglobin-CN complex formation. Br. J. ind.
Med., 19 : 283-286.
MALLETTE, F.S. (1943) Industrial hygiene in synthetic rubber
manufacture. Ind. Med., 12 : 495-499.
MALTONI, C., CILIBERTI, A., & DI MAIO, V. (1977)
Carcinogenicity bioassays on rats of acrylonitrile
administered by inhalation and by ingestion. Med. lav., 68 :
401-411.
MALTONI, C., CILIBERTI, A., & CARRETTI, D. (1982)
Experimental contributions in identifying brain potential
carcinogens in the petrochemical industry. Ann. N.Y. Acad.
Sci., 381 : 216-249.
MARANO, R.R., LEVINE, S.P., & HARVEY, T.M. (1978) Trace
determination of subnanogram amounts of acrylonitrile in
complex matrices by gas chromatography with a
nitrogen-selective detector. Anal. Chem., 50 (13): 1948-1951.
MARCOCI, S. & IONESCU, M. (1974) [The toxic action of
acrylonitrile on fish.] Strd. Prof. Calitatii Apelor, 16 : 7-22
(in Rumanian).
MARKELOV, M.A. & SEMENENKO, E.I. (1976) [Determination of
microconcentrations of substances migrating from polymer
materials to water.] Plast. Mass., 1 : 57-59 (in Russian, Chem.
Abstr., 84 : 155747g).
MAVRINA, E.A. & HROMOV, W.E. (1974) [Functional changes in
the body of workers engaged in production of nitron fibre.]
Vrach. Delo, 6 : 129-131 (in Russian).
MAVRINA, E.A. & IL'INA, V.A. (1974) [Change in the
immunological reactivity of the bodies of students of a
technical-trade school during training in a nitron production
plant.] Gig. i Sanit., 5 : 109-110 (in Russian).
MCLAUGHLIN, M., KRIVANEK, N.D., & TROCHIMOWICH, H.J. (1976)
Evaluation of antidotes for acrylonitrile poisoning (Abstr.).
Toxicol. appl. Pharmacol., 37 : 133-134.
MCNEAL, T. & BREDER, C.V. (1981) Head-space sampling and
gas-solid chromatographic determination of residual
acrylonitrile in acrylonitrile copolymer solutions. J. Assoc.
Off. Anal. Chem., 64 (2): 270-275.
MCNEAL, T. & BREDER, C.V. (1982) Manual headspace gas-solid
chromatographic determination of sub-parts-per-trillion levels
of acrylonitrile in 3% acetic acid. J. Assoc. Off. Anal.
Chem., 65 (1): 184-187.
MCOMIE, W.A. (1949) Comparative toxicity of
methacrylonitrile and acrylonitrile. J. ind. Hyg. Toxicol.,
31 : 113-116.
MILLER, L.M. & VILLAUME, J.E. (1978) Investigation of
selected potential environmental contaminants: acrylonitrile.
Washington DC, US EPA, 234 pp (Prepared for the Office of
Toxic Substances).
MILLS, E.J., Jr & STACK, V.T., Jr (1955) Acclimation of
microorganisms for the oxidation of pure organic chemicals.
In: Proceedings of the 9th Industrial Waste Conference Purdue
University, pp. 449-464.
MILWY, P. (1978) Letter to the editor. Reply to S. Venitt.
Mutat. Res., 57 : 110-112.
MILWY, P. & WOLFF, M. (1977) Mutagenic studies with
acrylonitrile. Mutat. Res., 48 : 271-278.
MINAMI, M., SATO, M., SUSUKI, A., YAMAMURA, K., & ISHIZU, S.
(1973) [Acrylonitrile poisoning. I. Result of exposure to
acrylonitrile vapour.] Tokyo Joshi Ika Daigaku Zasshi, 43 (10):
849-855 (in Japanese).
MLETZKO, H.G. von, & HENKEL, W. (1978) Animal experiments
with exposure to noise and acrylonitrile from the
chronobiological aspect. Z. Gesamte Hyg., 24 : 515-518.
MONSANTO (1975) Joint toxic action between acrylonitrile and
potassium cyanide. St Louis, MO, Monsanto Co. Medical
Department.
MONSANTO (1977a) Acrylonitrile sales specification, St
Louis, MO, Monsanto Chemicals Intermed. Co. (Monsanto data
sheet).
MONSANTO (1977b) In response to OSHA's request for
information on acrylonitrile (42 FR 34327) (Letter to the OSHA
Docket Officer, US Department of Labor).
MONSON, R.R. & FINE, L.J. (1978) Cancer mortality and
morbidity among rubber workers. J. Natl Cancer Inst., 61 (4):
1047.
MUHTAROVA, M. (1977) [Personal sample for evaluation of the
professional risk due to poisonings.] Hyg. Zdrav., 5 : 447-450
(in Bulgarian).
MURRAY, F.J., SCHWETZ, B.A., NITSCHE, K.D., JOHN, J.A.,
NORRIS, J.M., & GEHRING, P.J. (1978) Teratogenicity of
acrylonitrile given to rats by gavage or by inhalation. Food
cosmet. Toxicol., 16 (6): 547-551.
MURRAY, F.J., NITSCHKE, K.D., JOHN, J.A., SMITH, F.A., QUAST,
J.F., BLOGG, C.D., & SCHWETZ, B.A. (1976) Teratologic
evaluation of acrylonitrile monomer given to rats by gavage.
Midland MI, USA, Dow Toxicological Research Laboratory
(prepared for the Manufac. Chem. Assoc.).
NAKAMURA, K. (1981) [Mortality study of acrylonitrile
workers.] In: Annual Report of the National Institute of
Industrial Health for 1980, p. 31 (in Japanese).
NERUDOVA, J., HOLECHEK, V., GUT, I., & KOPECKY, J. (1980a)
[Relation between kinetics of acrylonitrile after different
routes of administration and its conversion to thiocyanate.]
Prakt. Lék., 32 : 15-18 (in Czech).
NERUDOVA, J., KOPECKY, J., GUT, I., & HOLECHEK, V. (1980b)
[Metabolism of acrylonitrile in the rats in vitro.] Prakt.
Lék., 32 : 98-103 (in Czech).
NERUDOVA, J., GUT, I., & KOPECKY, J. (1981) Cyanide effect
in acute acrylonitrile poisoning in mice. In: Gut, I., Cikrt,
M., & Plaa, G.L., ed. Industrial and environmental
xenobiotics, Berlin, Springer Verlag, pp. 245-250.
NILSEN, O.G., TOFTGARD, R., & ENEROTH, P. (1980) Effects of
acrylonitrile on rat liver cytochrome P-450, benz( a)pyrene
metabolism, and serum hormone levels. Toxicol. Lett., 6 :
399-404.
NIOSH (1976) NIOSH analytical methods for set K, Washington,
DC, NIOSH (NTIS Report No. PB-254 227).
NIOSH (1978) A recommended standard for occupational
exposure to acrylonitrile, Cincinnati, OH, Department of
Health, Education and Welfare (NIOSH), (Publ. No. 78-116).
NOEL, G., LAMBOTTE-VANDEPAER, M., DUVERGER-VAN BOGAERT, M.,
ROBERFROID, M., & MERCIER, M. (1978) Hepatotoxic effects of
acrylonitrile in rats. Arch. int. Physiol. Biochem., 86 (4):
951-952.
O'BERG, M.T. (1980) Epidemiologic study of workers exposed
to acrylonitrile. J. occup. Med., 22 (4): 245-252.
OOMENS, A.C. (1980) Experience with a dual detector
headspace gas chromatograph for acrylonitrile analysis. In:
Kolb, B., ed. Applied headspace gaschromatography, Heydon, pp.
111-116.
ORUSEV, T. & POPOVSKI, P. (1973) [Clinical symptoms in
workers occupationally exposed to acrylonitrile.] God. Zb.
Med. Fak. Skopje, 19 : 187-192 (in Macedonian).
ORUSEV, T., BAUER, S., & POPOVSKI, P. (1973) [Occupational
exposure to acrylonitrile in a plant for production of acrylic
synthetic fibres.] God. Zb. Med. Fak. Skopje, 19 : 445-449 (in
Macedonian).
OSHA (1978a) Emergency temporary and permanent standards for
occupational exposure to acrylonitrile (vinyl cyanide). Fed.
Reg., 43 : 2600, 4562.
OSHA (1978b) Permanent standard for exposure to acrylo-
nitrile. Fed. Reg., 43 : 45809-45817.
OSHA (1981) General Industry - Safety & Health Standards, 29
CFR 1910. US Department of Labor, pp. 830-850, 220 pp.
OSTROVSKAJA, R.S., PODREZ, Z.G., BRAGINSKAJA, L.L., BOKLOG,
E.P., EFREMOV, A.M., & VOLKOVA, G.A. (1976) [Health status
of workers currently engaged in the production of
acrylonitrile.] Gig. Tr. Prof. Zabol., 6 : 8-12 (in Russian).
PANOVA, R.V., BELOVA, R.A., & LAVRUSHINA, T.S. (1969)
[Chromatographic determination of residual monomers in
latexes.] Prom. Sin. Kauch. Nauch.-Tekh. Sb., 1 : 21-23 (in
Russian, Chem. Abstr., 74 : 43238d).
PARENT, R.A. & CASTRO, B.C. (1979) Effect of acrylonitrile
on primary Syrian golden hamster embryo cells in culture:
Transformation and DNA fragmentation. J. Natl Cancer Inst.,
62 (4): 1025-1029.
PARSONS, J.S. & MITZNER, S. (1975) Gas chromatographic
method for concentration and analysis of traces of industrial
organic pollutants in environmental air and stacks. Environ.
Sci. Technol., 9 : 1053-1058.
PATTERSON, R.M., BORNSTEIN, M.I., & GARSHICK, E. (1976)
Assessment of acrylonitrile as a potential air pollution
problem, Vol. VI (US Environmental Protection Agency, Contract
No. 68-02-1337, Task 8, Springfield, VA, National Technical
Information Service, No. PB-258 358).
PATTY, F.A., ed. (1963) Acrylonitrile, CH2=CHCN (vinyl
cyanide propenenitrile). In: Industrial hygiene and
toxicology, 2nd Revised ed., Vol. 2, pp. 2009-2012.
PAULET, G. & DESNOS, J. (1961) L'acrylonitrile,
toxicité-méchanisme d'action-thérapeutique. Arch. int.
Pharmacodyn., 131 (1-2): 54-83.
PAULET, G. & VIDAL, M. (1975) De la toxicité de quelques
esters acryliques et méthacryliques de l'acrylamide et des
polyacryliques. Arch. Mal. prof., 36 (1-2): 58-60.
PAULET, G., DESNOS, J., & BATTIG, J. (1966) De la toxicité
de l'acrylonitrile. Arch. Mal. prof., 27 (12): 849-856.
PEROCCO, P., PANE, G., BOLOGNESI, S., & ZANNOTTI, M. (1982)
Increase of sister chromatid exchange and unscheduled
synthesis of deoxyribonucleic acid by acrylonitrile in human
lymphocytes in vitro. Scand. J. Work Environ. Health, 8 :
290-293.
PETER, H. & BOLT, H.M. (1981) Irreversible protein binding
of acrylonitrile. Xenobiotica, 11 : 51-56.
PETER, H., APPEL, K.E., BERG, R., & BOLT, H.M. (1983)
Irreversible binding of acrylonitrile to nucleic acids.
Xenobiotica, 13 (1): 19-25.
PETROVA, L.I., NOSKOVA, M.P., BALANDINA, V.A., & GURICHEVA,
Z.G. (1972) [Polarographic determination of acrylonitrile in
aqueous extract of styrene- acrylonitrile copolymers.] Gig. i
Sanit., 37 : 62-65 (in Russian, Chem. Abstr., 76: 127898y).
PFEILSTICKER, K., INKMANN, K., & BOCHISCH, M. (1977)
Residues of (1-14C) ACN in fumigated dry food. Z.
Lebensmittel-Untersuch. Forsch., 164 (4): 274-276.
POLAND (1982) Decree of the Minister of Labour, Wages, and
Social Affairs, 22 December, 1982 (Low Gaz. No. 43, 29
December, 1982, Entry 287).
PONOMAREV, Y.P., ANUFRIEVA, T.L., & SHKORBATOVA, T.L. (1974)
[Polarographic determination of acrylonitrile in waste waters
using extraction.] Vodosnabz., Kanaliz. Gidroteh. Sooruz., 17:
67-70 (in Russian, Chem. Abstr., 82 : 34803x).
PORTMANN, J.K. & WILSON, K.W. (1971) The toxicity of 140
substances to the brown shrimp and other marine animals. 12 pp
(Ministry Agriculture Fisheries & Food Laboratories, England;
Shellfish Inf. Leafl. No. 22).
PUJADO, P.R., VORS, B.V., & KRUEDING, A.P. (1977) Newest
acrylonitrile process. Hydrocarbon Process, (May, 1977):
169-172.
QUAST, J.F., HUMISTON, C.G., SCHWETZ, B.A., FRAUSON, L.A.,
WADE, C.E., & NORRIS, J.M. (1975) A six-month oral toxicity
study incorporating acrylonitrile in the drinking water of
pure-bred beagle dogs. Midland MI, USA, Dow Toxicology
Research Laboratory, (prepared for the Manuf. Chem. Assoc.).
QUAST, J.F., ENRIQUEZ, R.M., WADE, C.E., HUMISTON, C.C., &
SCHWETZ, B.A. (1977) Toxicity and drinking water containing
acrylonitrile in rats: results after 12 months. Midland MI,
USA, Dow Toxicology Research Laboratory (prepared for the
Manuf. Chem. Assoc.).
QUAST, J.F., SCHUETZ, D.J., BALMER, M.F., GUSHOW, T.S., PARK,
C.N., & McKENNA, M.J. (1980a) A two-year toxicity and
oncogenicity study with acrylonitrile following inhalation
exposure of rats. Final Report. Midland MI, USA, Dow
Toxicology Research Laboratory.
QUAST, J.F., WADE, C.E., HUMISTON, C.G., CARREON, R.M.,
HERMANN, E.A., PARK, C.N., & SCHWETZ, B.A. (1980b) A
two-year toxicity and oncogenicity study with acrylonitrile
incorporated in the drinking water of rats. Final Report.
Midland MI, USA, Dow Toxicology Research Laboratory.
RABELLO-GAY, M.N. & AHMED, E. (1980) Acrylonitrile: in vivo
cytogenetic studies in mice and rats. Mut. Res., 79 : 249-255.
RAJENDRAN, S. & MUTHU, M. (1981) Detection of acrylonitrile
and ethylene oxide in air and fumigated foodstuffs. Bull.
environ. Contam. Toxicol., 27 : 426-431.
RAJENDRAN, S. & MUTHU, M. (1981) Effect of acrylonitrile on
trehalase, phosphorylase and acetylcholinesterase activities
in Tribolium castenlum Herbst and Trogoderma granarium Everts.
Experientia (Basel), 37 (8): 886.
RAMSTAD, T. & NICHOLSON, L.W. (1982) Determination of
sub-parts-per-billion levels of acrylonitrile in aqueous
solutions. Anal. Chem., 54 : 1191-1196.
REICHLE, A. & TENGLER, H. (1968) [Gas-chromatographic
procedures for determining residual monomers in the polymer.
Determination of acrylonitrile in polyacrylonitrile.]
Plastverarbeiter, 19 : 921-924 (in German, Chem. Abstr., 70 :
97206c).
ROGACZEWSKA, T. (1964) [Polarographic determination of
acrylonitrile in air.] Chem. Anal., 9 : 417-423 (in Polish).
ROGACZEWSKA, T. (1965) [Colorimetric method of acrylonitrile
determination in air.] Med. Pr., 16 (6): 465-475 (in Polish).
ROGACZEWSKA, T. (1971) The problems of obtaining the
evaluation of deleterious effects of acrylonitrile vapours in
industrial conditions. Thesis, Lodz, Poland, Institute of
Industrial Medicine.
ROGACZEWSKA, T. (1975) [Percutaneous absorption of
acrylonitrile vapours in animals.] Med. Pr., 26 (6): 459-465
(in Polish).
ROGACZEWSKA, T. (1976) [Determination of acrylonitrile in
air.] Med. Pr., 27 (2): 115-126 (in Polish).
ROGACZEWSKA, T. & PIOTROWSKI, J. (1968) [Routes of
acrylonitrile absorption in man.] Med. Pr., 19 (4): 349-353 (in
Polish).
ROSE, V.E. & PERKINS, J.L. (1982) Passive dosimetry - state
of the art review. Am. Ind. Hyg. Assoc. J., 43 : 605-621.
ROUDABUSH, R.L., TERHAAR, C.J., FASSETT, D.W., & DZIUBA, S.P.
(1965) Comparative acute effects of some chemicals on the
skin of rabbits and guinea-pigs. Toxicol. appl. Pharmacol., 7 :
559-565.
RUSSELL, J.W. (1975) Analysis of air pollutants using
sampling tubes and gas chromatography. Environ. Sci. Technol.,
9 : 1175-1178.
RUSSKIH, A.A. (1972) [Determination of acetone cyanohydrin
and acrylonitrile in air.] In: Bezhaev, M.S., ed. Proceedings
of the 4th Conference of People Employed in Universities and
Industrial Laboratories of South-east USSR on Physico-chemical
Methods of Analytical Control of Industrial Products, USSR,
Dagestan State University, Vol. 3, p. 100 (in Russian, Chem.
Abstr., 81 : 16301g).
RUSSKIH, A.A. (1973) [Photometric determination of
acrylonitrile in air.] Zavod. Lab., 39 (1): 5-6 (in Russian).
SAKURAI, H. & KUSUMOTO, M. (1972) [Epidemiologic study of
health impairment among acrylonitrile workers.] J. Sci. Labour
(Tokyo), 48 (5): 273-282 (in Japanese).
SAKURAI, H., ONODERA, M., UTSUNOMIYA, T., MINAKUCHI, H., IWAI,
H., & MATYUMURA, H. (1978) Health effects of acrylonitrile
in acrylic fibre factories. Br. J. ind. Med., 35 (3): 219-225.
SANDBERG, E.C. & SLANINA, P. (1980) Distribution of (1-14C)
acrylonitrile in rat and monkey. Toxicol. Lett., 6 : 187-191.
SAPOTA, A. (1982) The disposition of 14C-acrylonitrile in
rats. Xenobiotica, 12 (4): 259-264.
SAPOTA, A. & CHMIELNICKA, J. (1981) [Toxicity and metabolism
of acrylonitrile.] Med. Pr., 32 (1): 25-33 (in Polish).
SAPOTA, A. & DRAMINSKI, W. (1981) The fate of
14C-acrylonitrile in rats. In: Gut, I., Cikrt, M., & Plaa,
G.L., ed. Industrial and environmental xenobiotics, Berlin,
Springer Verlag, pp. 231-237.
SARTORELLI, E. (1966) [Acute acrylonitrile intoxication.]
Med. lav., 57 (3): 184-187 (in Italian).
SATO, M. (1978) [Studies on the toxic effect of
acrylonitrile - its metabolism, absorption and excretion.]
Jpn. J. Hyg., 33 (3): 497-505 (in Japanese).
SATO, M., ISHIZU, S., & MOMOTANI, H. (1975) [Determination
of acrylonitrile, cyanide and thiocyanate in blood and urine.]
Sangyo Igaku, 17 : 99-105 (in Japanese, Chem. Abstr., 100292z).
SATO, M., HIRASAWA, F., OGATA, M., TAKIZAWA, Y., KOJIMA, H., &
YOSHIDA, T. (1982) Distribution and accumulation of
(2,3-14C) acrylonitrile in rat after single injection.
Ecotoxicol. environ. Safety, 6 (5): 489-494.
SATO, S., KOSHIO, H., MIMURA, M., NAKAMURA, S., & ICHIMURA,
M. (1979) Investigation in counter-measure to the sources of
unspecified harmful chemicals in 1978. pp. 1-19 (in Japanese,
Report of the study sponsored by the Environmental Agency).
SCHEUFLER, H. von (1976) Experimental testing of chemical
agents for embryotoxicity, teratogenicity and mutagenicity -
ontogenic reactions of the laboratory mouse to these
injections and their evaluation - a critical analysis method.
Biol. Rundsch., 14 (4): 227-229.
SCHEUFLER, H. von (1980) [On the embryotoxic effectiveness
of acrylonitrile in the laboratory mouse.] Z. Gesamte Hyg.,
26 : 564-565 (in German).
SCUPAKAS, D. (1968) [Industrial hygiene conditions during
the reworking of plastics and problems improving them.] In:
Yavnaist, E.Y., ed. Proceedings of the first conference on
actual problems of industrial hygiene and occupational
pathology, 1967, Riga, Medical Institute, pp. 128-132 (in
Russian, Chem. Abstr., 72 : 47094k).
SEKI, S. (1967) [On acrylonitrile poisoning in a synthetic
resin plant.] Jpn. J. Hyg., 22 (1): 257 (in Japanese).
SEUTTER-BERLAGE, F., VAN DORP, H.L., KOSSE, H.G.J., &
HENDERSON, P.T. (1977) Urinary mercapturic acid excretion as
a biological parameter of exposure to alkylating agents. Int.
Arch. occup. environ. Health, 39 : 45-51.
SEUTTER-BERLAGE, F., DELBRESSINE, L.P.C., SMEETS, F.L.M., &
KETELAARS, H.C.J. (1978) Identification of three
sulfur-containing urinary metabolites of styrene in the rat.
Xenobiotica, 8 : 413-418.
SHACKELFORD, W.M. & KEITH, L.H. (1976) Frequency of organic
compounds identified in water, Athens GA, USA, US
Environmental Protection Agency, p. 55 (EPA-600/4-76-062).
SHEVCHIK, J. (1976) Detectors in gas chromatography,
Amsterdam, Elsevier, 192 pp.
SHUSTOV, V.J. (1968) [Clinical manifestation and
hematological changes in workers employed in the production of
acrylonitrile at the Saratov Chemical Combine.] Gig. Tr. prof.
Zabol., 3 : 27-29 (in Russian).
SHUSTOV, V.Y. & MAVRINA, E.A. (1975) [Clinical picture of
chronic poisoning in the production of nitrone.] Gig. Tr.
prof. Zabol., 3 : 27-29 (in Russian).
SILVER, E.H. & SZABO, S. (1982) Possible role of lipid
peroxidation in the action of acrylonitrile on the adrenals,
liver and gastrointestinal tract. Res. Comm. Chem. Pathol.
Pharmacol., 36 (1): 33-43.
SILVER, E.H., KUTTAB, S.H., HASAN, T., & HASSAN, M. (1982a)
Structural considerations in the metabolism of nitriles to
cyanide in vivo. Drug Metab. Disposition, 10 : 495-498.
SILVER, E.H., McCOMB, D.J., KOVACS, K., & SZABO, S. (1982b)
Limited hepatotoxic potential of acrylonitrile in rats.
Toxicol. appl. Pharmacol., 64 : 131-139.
SILVERSTEIN, L.G. (1977) Calidation of abcor GASBADGE for
acrylonitrile and improved desorption efficiency. Am. Ind.
Hyg. Assoc. J., 38 (8): 412-413.
SKVOSTSOV, Ju.I. (1980) [Variation in the amino acid
spectrum of blood serum in workers employed in ACN production
and nutritive ways of its correction. Vopr. Pitan., No. 2 :
31-33 (in Russian).
SLATER, E.E., ANDERSON, M.D., & ROSENKRANZ, S. (1971) Rapid
detection of mutagens and carcinogens. Cancer Res., 31 (7):
970-973.
SLOOF, W. (1979) Detection limits of a biological monitoring
system based on fish respiration. Bull environ. Contam.
Toxicol., 23 : 517-523.
SMYTH, H.F. & CARPENTER, C.P. (1948) Further experience with
the range-finding test in the industrial toxicology
laboratory. J. ind. Hyg. Toxicol., 30 : 63-68.
SOKAL, J.A. & KLYSZEJKO-STEFANOWICZ, L. (1972)
[Nicotinamide-adenine dinucleotides in acute poisoning with
some toxic agents.] Lodz Tow. Nauk., No. 112: 64-101 (in
Polish, Chem. Abstr., 79 : 62271d).
SOKAL, J.A., KNOBLOCH, K., MAJKA, J., SAPOTA, A., &
SZENDZIKOWSKI, S. (1977) [Toxicity of acrylate compounds
used in industry.] Prakt. Lek., 29 (4): 157-161 (in Czech).
SPASSOVSKI, M. (1976) Health hazards in the production and
processing of some fibres, resins, and plastics in Bulgaria.
Environ. Health Perspect., 17 : 199-202.
STAMOVA, N., GINCHEVA, N., SPASOVSKI, M., BAINOVA, A.,
IVANOVA, S., KURKCHIEV, S., HINKOVA, L., KHRISTEVA, V.,
MUKHTAROVA, M., KARADZHOVA, N., TSANEVA, L., & FIKOVA, E.
(1976) [Labour hygiene during the production of synthetic
fibres "Bulana".] Hyg. Zdrav., 2 : 134-140 (in Russian).
STANFORD RESEARCH INSTITUTE (1976) Interim Report: In vitro
microbiological mutagenicity studies of Dow Chemical Company
Compounds (SRI Project No. LSC-4378, prepared for Dow Chemical
Company).
STEICHEN, R.J. (1976) Modified solution approach for the gas
chromatographic determination of residual monomers by
head-space analysis. Anal. Chem., 48 : 1398-1402.
SUTA, B.E. (1979) Assessment of human exposures to
atmospheric acrylonitrile (Prepared by the Stanford Research
Institute - Contract 68-02-2835-EPA).
SVIRBELY, J.L. & FLOYD, E.P. (1961) Toxicologic studies of
acrylonitrile and B,B'-oxydipropionitrile. III. Chronic
studies. Meeting paper AINA-ACSIH, Detroit MI, USA.
SWEDEN (1981) Ordinance issued by the National Swedish Board
of Occupational Safety and Health Concerning Hygiene Limit
Values (in Swedish).
SZABO, S. & SELYE, H. (1971) Adrenal apoplexy and necrosis
produced by acrylonitrile. Endocrinol., 57 (3): 405-408.
SZABO, S. & SELYE, H. (1972) Effects of phenobarbital and
steroids on the adrenal apoplexy produced by acrylonitrile in
rats. Endocrinol. Exp., 6 (3): 141-146.
SZABO, S., REYNOLDS, E.S., KOMANICKY, P., MOSLEN, M.T., &
MELBY, J.C. (1976) Effect of chronic acrylonitrile ingestion
on rat adrenal. Toxicol. appl. Pharmacol., 37 (1): 133.
SZABO, S., BAILEY, K.A., BOOR, P.J., & JAEGER, R.J. (1977)
Acrylonitrile and tissue glutathion - differential effect of
acute and chronic interactions. Biochem. Biophys. Res.
Commun., 79 (1): 32-37.
SZABO, S., HUTTNER, I., KOVACS, K., HORVATH, E., SZABO, D., &
HORNER, H.C. (1980) Pathogenesis of experimental adrenal
hemorrhagic necrosis ("apoplexy"). Ultrastructural,
biochemical, neuropharmacologic and blood coagulation studies
with acrylonitrile in the rat. Lab. Invest., 42 (5): 533-545.
TADA, O. (1971) [Methods for determinations of toxic
substances in air-organic compounds.] J. Sci. Lab., (Part II),
47 : 789-796.
TAKAGI, N., TAKAGI, K., MATSUDA, T., & KOIZUMI, S. (1968)
[Effect of administration of vitimins B1 or B2 plus L-cysteine
to rats exposed to acrylonitrile gas over a long period.] J.
Sci. Lab., 44 (8): 454-464 (in Japanese).
TARKOWSKI, S. (1966) [Effect of cobalt on the inhibition by
potassium cyanide of cytochrome oxidase activity.] Med. Pr.,
17 (2): 116-119 (in Polish).
TARKOWSKI, S. (1968) [Effect of acrylonitrile on certain
properties of cytochrome oxidase.] Med. Pr., 19 (6): 525-531
(in Polish).
TATSUNO, T., INOUE, T., & TANIMURA, A. (1979) [Hygienic
chemical studies on plastics (II). Residual acrylonitrile in
ABS resin or AS resin and its migration into water.] Report of
the National Institute of Hygiene, 97 : 93-97 (in Japanese).
TATSUNO, T., INOUE, T., & TANIMURA, A. (1980) [Hygienic
chemical studies on plastics (V). Migration of acrylonitrile
from acrylonitrile-butadiene-styrene copolymer and
acrylonitrile-styrene copolymer into various food simulating
solvents in long period.] Report of the National Institute of
Hygiene, 98 : 110-115 (in Japanese).
TERENT'EV, A.P. & OBTEMPERANSKAJA, S.I. (1956) [Quantitative
determination of acrylonitrile with sodium sulfite.] Z. Anal.
Him., 11 : 638-639 (in Russian).
THIESS, A.M. & FLEIG, I. (1978) Analysis of chromosomes of
workers exposed to acrylonitrile. Arch. Toxicol., 41 (2):
149-152.
THIESS, A.M., FRENTZEL-BEYME, R., LINK, R., & WILD, H.
(1980) [Mortality study in chemical workers in different
production plants with exposure also to acrylonitrile.]
Zentralbl. Arbeitsmed. Arbeitsschutz Prophyl. Ergonomie,
30 (7): 259-267 (in German).
TULLAR, P.E. (1947) Final report on the pharmacology and
toxicology of acrylonitrile and acrylon. Washington,
Palusowski Memorial Research Laboratory, George Washington
University.
UHDE, W.J. & KOEHLER, U. (1967) [Testing of plastic
articles. Determination of monomeric compounds in the volatile
fraction of styrene polymers.] Z. Lebensm. Unters.-Forsch.,
135 : 135-140 (in German).
UNION CARBIDE CORPORATION (1977) In response to OSHA's
request for information on occupational exposure to
acrylonitrile. Fed. Reg., 42 : 33042 (Unpublished letter to the
OSHA Docket Officer, US Department of Labor).
UNITED KINGDOM HEALTH & SAFETY EXECUTIVE (1981a)
Acrylonitrile in air. Laboratory method using charcoal
adsorption tubes and gas chromatography. Methods for the
Determination of Hazardous Substances, 1, March 1981.
UNITED KINGDOM HEALTH & SAFETY EXECUTIVE (1981b)
Acrylonitrile in air. Laboratory method using porous polymer
adsorption tubes, and thermal desorption with gas
chromatographic analysis. Methods for the Determination of
Hazardous Substances, 2, March 1981.
UNITED KINGDOM MINISTRY OF AGRICULTURE, FISHERIES & FOOD
(1982) Survey of acrylonitrile and methacrylonitrile in food
contact materials and in food, London, HMSO (Food Surveillance
Paper No. 6).
US CONSUMER PRODUCT SAFETY COMMISSION (1978) Assessment of
acrylonitrile contained in consumer products. Washington, DC
(Final report, contract No. CPSC-C-77-0009. Task No. 1014K,
H.I.A. Economic Analysis).
US DEPARTMENT OF TRANSPORTATION (1974) Acrylonitrile. In:
CHRIS hazardous chemical data, Department of Transportation,
Coast Guard, C-G-446-2.
US EPA (1977) Optional procedures for classification of
pesticide uses by regulation. Fed. Reg., 42 : 44170, 44178.
US FDA (1977a) Acrylonitrile copolymer used to fabricate
beverage containers, final decision. Fed. Reg., 42 :
48528-48544.
US FDA (1977b) Substances for use only as components of
adhesives. US Code Fed. Regul., Title 21, parts 175.105,
175.300, 175.320, 176.170, 176.180, 177.1010, 177.1020,
177.1040, 177.1200, 177.2600, 178.3790, 180.22, pp. 438, 445,
452, 455, 465, 471-472, 482-483, 486, 488, 496, 501-504, 555,
596, 609-611.
USSR (1982) System of standards of work safety. The air of
the working zone. General sanitary-hygienic requirements (GOST
12.1.005-76). MAC of harmful substances in the air of working
zone (Suppl. to Tab. 4, SN 245-71, No 7-11). MAC of harmful
substances in the air of working zone (part 12-19), confirmed
in 1977-1982 years.
VAINIO, M. & MAKINEN, M. (1977) Styrene and acrylonitrile
induced depression of hepatic nonprotein sulfhydryl content in
various rodent species. Res. Commun. chem. Pathol. Pharmacol.,
17 (1): 115-124.
VAN BLADEREN, P.J., DELBRESSINE, L.P.C., HOOGETERP, J.J.,
BEAUMONT, A.H.G.M., BREIMER, D.D., SEUTTER-BERLAGE, F., & VAN
DER GEN, A. (1981) Formation of mercapturic acids from
acrylonitrile, crotononitrile, and cinnamonitrile by direct
conjugation and via an intermediate oxidation process. Drug
Metab. Disposition, 9 : 246-249.
VAN DOORN, R., BOS, R.P., LEIJDEKKERS, C.-M., WAGENAAS-ZEGERS,
M.A.P., THEUWS, J.L.G., & HENDERSON, P.T. (1979) Thioether
concentration and mutagenicity of urine from cigarette
smokers. Int. Arch. occup. environ. Health, 43 : 159-166.
VED BRAT, S. & WILLIAMS, G.M. (1982) Hepatocyte-mediated
production of sister chromatid exchanges in co-cultured cells
by acrylonitrile: evidence for extra cellular transport of a
stable reactive metabolite. Cancer Lett., 17 (2): 213-216.
VENITT, S., BUSHELL, C.T., & OSBORNE, M. (1977) Mutagenicity
of acrylonitrile (cyanoethylene) in Escherichia coli. Mutat.
Res., 45 (2): 283-288.
VERSCHUEREN, K. (1977) Handbook of environmental data on
organic chemicals, Van Nostrand Reinhold, pp. 78-81.
VISSARIONOVA, V.U., BONDAREV, G.I., STASENKOVA, K.P., &
ZEMLJANSKAJA, T.A. (1979) [Study of the effect of rations
with different protein content on rats following acrylonitrile
poisoning.] Vopr. Pitan., (1) : 36-40 (in Russian).
VOLKOVA, Z.A. & BAGDINOV, Z.M. (1969) [Industrial hygiene
problems in vulcanization processes of rubber production.]
Gig. i Sanit., 34 : 33-40 (in Russian).
WERNER, J.B. & CARTER, J.T. (1981) Mortality of United
Kingdom acrylonitrile polymerisation workers. Br. J. ind.
Med., 38 : 247-253.
WILLHITE, C.C. (1981a) Inhalation toxicology of acute
exposure to aliphatic nitriles. Clin. Toxicol., 18 (8):
991-1003.
WILLHITE, C.C. (1981b) Teratogenic effects of aliphatic
nitriles. Teratology, 23 : 317-323.
WILLHITE, C.C. & SMITH, R.P. (1981) The role of cyanide
liberation in the acute toxicity of aliphatic nitriles.
Toxicol. appl. Pharmacol., 59 : 589-602.
WILSON, R.H. (1944) Health hazards encountered in the
manufacture of synthetic rubber. J. Am. Med. Assoc., 124 :
701-703.
WILSON, R.H. & McCORMICK, W.E. (1949) Acrylonitrile: its
physiology and toxicology. Ind. Med., 18 : 223-245.
WILSON, R.H., HOUGH, G.V., & McCORMICK, W.E. (1948) Medical
problems encountered in the manufacture of American-made
rubber. Ind. Med., 17 : 199-207.
WISNIEWSKA-KNYPL, J.M., KNOBLOCH, J.M., JABLONSKA, J., & RUTA,
U. (1970) [Decrease of tissue respiration activity of
oxoglutarate dehydrogenase and level of sulfhydryl groups in
acute acrylonitrile intoxication in rats.] Med. Pr., 21 (6):
543-549 (in Polish).
WISNIEWSKA-KNYPL, J.M., TARKOWSKI, S., KLIMZAK, J., &
DRAMINSKI, W. (1978) [ Metabolism of vinyl chloride in the
liver of rats.] (in Polish) (Final report on the research
project of the Institute of Occupational Medicine in Lodz,
Poland).
WRIGHT, P.L. (1977) Studies on the metabolism of
acrylonitrile. Testimony before the FDA. In: Monsanto Co.
(1976b) Appendix A, pp. A-20 - A-28.
WYNDER, E.L. & HOFFMANN, D. (1967) Tobacco and tobacco
smoke. Studies in experimental carcinogenesis. New York,
Academic Press, p. 450.
YOSHIKAWA, H. (1968) [Toxicity of nitrile compounds. II.
Aliphatic nitriles.] Igak. Scibut., 77 (1): 1-4 (in Japanese).
YOUNG, J.D., SLAURER, R.W., & KARBOWSKI, R.J. (1977) The
pharmacokinetic and metabolism profile of 14 C-acrylonitrile
given to rats by three routes. Midland MI, USA, Toxicol. Res.
Lab. Dow Chem., (prepared for the Manufacturing Chem. Assoc.).
ZAYTSEVA, N.A., TOLSTOBROVA, S.A., & IL'IN, D.T. (1972)
[Chromatographic determination of unreacted monomers in
emulsions of acrylic copolymers.] Soviet Chem. Ind., 48 :
298-299 (in Russian).
ZELLER, H., HOFMANN, H.T., THIESS, A.M., & HEY, W. (1969)
[Toxicity of nitriles. Results of animal experiments and
industrial experiences during 15 years.] Zent. Arbeitsmed.
Arbeitsschutz, 19 (8): 226-238 (in German).
ZHURKOV, V.S., SHRAM, R.L. & DUGAN, A.M. (1983) [Analysis of
mutagenic activity of acrylonitrile.] Gig. i. Sanit., 1 : 71-72
(in Russian).
ZITTING, A., TENHUNEN, R., & SAVOLAINEN, H. (1981) Effects
of intraperitoneally injected acrylonitrile on liver, kidney
and brain. Acta pharmacol. toxicol., 49 : 412-415.
ZOTOVA, L.V. (1975a) [Working conditions in the production
of acrylonitrile and their effect on workers.] Gig. Tr. prof.
Zabol., No. 8 : 8-11 (in Russian).
ZOTOVA, L.V. (1976) [Toxic effect of acrylonitrile on
experimental animals following its absorption through the
skin.] Gig. i Sanit., 41 (10): 103-105 (in Russian).
7.2. Subacute Toxicity
7.2.1. Inhalation exposure
Rats, guinea-pigs, rabbits, monkeys, and cats were exposed to
acrylonitrile at 330 mg/m3 air for 4 h per day, 5 days a week, for
8 weeks. All adult rats survived 8 weeks, but 5 out of 8 young
rats died by the 6th week, 3 out of 16 guinea-pigs and 1 out of 4
rabbits died during the 5th week, and 1 out of 2 monkeys died after
6 weeks of exposure. At 220 mg/m3 for 4 h/day, 5 days a week, for
8 weeks, all rats, rabbits, and guinea-pigs survived for 8 weeks,
but 1 out of 4 cats died during the third week. After the first 4-
h exposure to 120 mg/m3, 1 out of 2 dogs died, but the other
survived 4 weeks' exposure. Four rhesus monkeys survived 4 weeks'
exposure to 120 mg/m3 for 4 h/day (Dudley et al., 1942).
CD-1 mice, Charles River rats, and beagle dogs were exposed to
acrylonitrile 57 times for 6 h a day, 5 days a week, over a 90-day
period. Some dogs were killed by exposure to 117 mg/m3 (54 ppm)
but not 58 mg/m3 (24 ppm). Mice and rats were unaffected by these
concentrations, but a concentration of 234 mg/m3 (108 ppm) was
lethal for half the rats and mice. As with acute exposures, dogs
were more sensitive than rats and mice, but mice did not appear to
be more sensitive than rats. Atmospheric concentrations of
acrylonitrile of 58, 117, and 117 mg/m3 did not induce any lethal
effects in dogs, rats, and mice, respectively (Brewer, 1976).
7.2.2. Oral administration
Over a period of 7 weeks, 6 rats were administered orally 15
doses of acrylonitrile at 30 mg/kg body weight, then 7 doses at 50
mg/kg, followed by 13 doses at 75 mg/kg, without lethal effects
being induced (Barnes, 1970). No deaths occurred when Sprague-
Dawley rats were offered 85 mg or less of acrylonitrile per litre
of drinking-water for 90 days (Humiston & Frauson, 1975). Given
the slow absorption of acrylonitrile from the gastrointestinal
tract, blood levels could have been low, and it can be calculated
that the daily dose could have been about 8.5 mg/kg body weight.
The studies are compatible with the view that acrylonitrile is
unlikely to have a significant cumulative effect.
7.2.3. Subcutaneous administration and intraperitoneal
administration
Daily sc doses of 40 mg/kg body weight over 4 weeks, or daily
ip injections of 20 mg acrylonitrile/kg body weight over 6 weeks,
were not fatal for rats (Krysiak & Knobloch, 1971).
7.2.4. Clinical observations in animal studies
Rats exposed to acrylonitrile concentrations of 220 mg/m3 for 4
h daily, 5 days a week (Dudley et al., 1942) over a period of 8
weeks, showed slight lethargy but gained weight, as did guinea-
pigs. Rabbits failed to gain weight and were listless, while cats
became listless, vomited, and lost weight. One cat developed a
transitory weakness of the hind legs after the third exposure and
died after the eleventh exposure; the 3 remaining cats survived 8
weeks with few untoward effects. Exposure to an atmospheric
concentration of 330 mg/m3 resulted in weight loss in rats, their
coats became rough, and their general physical condition poor
(Dudley et al., 1942). Young rats and guinea-pigs showed impaired
growth and marked irritation of the eyes and nose during the first
week of exposure. Marked eye and nose irritation was also seen in
rabbits and cats and the latter developed transitory weakness of
the hind legs. Monkeys appeared sleepy and weak and frequently
salivated and vomited. Thus, the 220 mg/m3 exposure level markedly
affected cats, while rabbits and guinea-pigs were little affected;
a concentration of 330 mg/m3 induced various effects including
death. Brewer (1976) exposed CD-1 mice, Charles River rats, and
beagle dogs to acrylonitrile concentrations of 0, 58, 117, 234
mg/m3 (0, 24, 54, or 108 ppm) for 6 h daily, 5 days a week, for 13
weeks. Signs observed included ataxia, ptosis, emaciation,
rhinitis, and diuresis. As is common with acrylonitrile over-
exposure, convulsions usually preceded death (e.g., Benesh &
Cherna, 1959; Paulet et al., 1966).
7.2.4.1. Body weight, food and water consumption
Loss of body weight or failure to gain body weight was seen in
rats exposed to acrylonitrile at 330 mg/m3 and in cats and rabbits
exposed to 220 mg/m3 for 4 h a day, 5 days a week, for 13 weeks.
Loss of appetite was seen in rhesus monkeys exposed to 330 mg/m3,
while 120 mg/m3 did not elicit any toxic effects (Dudley et al.,
1942). No adverse effects were seen in 6 rats administered
successively 15 doses of 30 mg/kg body weight, 7 doses of 50 mg/kg,
and 13 doses of 75 mg/kg over a period of 7 weeks (Barnes, 1970).
Adult Sprague-Dawley rats received between 0 and 42 mg
acrylonitrile/kg body weight in drinking-water for 90 days. The
body weight was depressed in males receiving 42 mg/kg, while
females were affected by 22 mg/kg, but only after the 57th day.
The mean weekly food consumption of males was lower for 7 weeks on
38 mg/kg and for 2 weeks on l7 mg/kg. Food consumption decreased
in females receiving 42 or 22 mg/kg for 6 weeks and 1 week,
respectively (Humiston & Frauson, 1975).
7.2.4.2. Organ weights and pathology
The weights of the liver, kidney, spleen, pituitary gland,
lungs, gonads, thyroid gland, adrenals, heart, and brain of rats,
mice, and dogs exposed to an acrylonitrile concentration of 234
mg/m3 for 6 h a day, 5 days a week, for 13 weeks, were within
normal limits (Brewer, 1976). In rats receiving acrylonitrile in
drinking-water for 90 days, no changes in absolute or relative
organ weights were seen in males receiving 4 mg/kg body weight or
females receiving 5 mg/kg, daily (Humiston & Frauson, 1975). The
relative liver weight was significantly increased in males and
females receiving 17 mg/kg (males) or 22 mg/kg (females) or more.
The weights of the heart and liver were increased significantly in
adult Wistar rats administered 50 mg acrylonitrile/kg body weight,
intraperitoneally, daily, for 3 weeks (Knobloch et al., 1971);
their weight loss caused an increase in the relative weights not
only of the heart and liver, but also of the kidney and spleen.
Dudley et al. (1942) examined the livers of rats, guinea-pigs,
rabbits, cats, dogs, and rhesus monkeys exposed to acrylonitrile at
220 or 230 mg/m3, and observed histological changes only in cats.
Liver parenchymal degeneration was reported in adult Wistar rats
after daily ip administration of 50 mg/kg body weight for 3 weeks
(Knobloch et al., 1971). In the above-mentioned study, Dudley et
al. (1942) also reported signs of renal damage, such as hyaline
casts in the straight collecting tubules of all species, and
limited subacute interstitial nephritis; this was especially seen
in guinea-pigs and rabbits. Parenchymal degeneration of the
kidneys was reported by Knobloch et al. (1971). In the study by
Dudley et al. (1942), lungs were affected by subacute
bronchopneumonia, congestion, and oedema of the alveolar walls,
extravasation of erythrocytes and serum into the alveoli, focal
collection of lymphyocytes and polymorphonuclear leukocytes, in
most guinea-pigs, rabbits, the monkey, and 1 out of 3 of the rats.
The authors also reported slight haemosiderosis in the spleen of
rats, but negligible siderosis in cats, guinea-pigs, and rabbits.
Exposure of rats to acrylonitrile (22 mg/m3, 10 ppm) for 7 weeks
and (100 mg/m3, 50 ppm) for another 6 weeks caused enlargement of
the liver, kidney, heart, and spleen, but co-administration of
vitamins B1, B2, and cystine had a protective effect against
enlargement of the heart. Alcohol dehydrogenase activity in the
liver decreased after exposure, but the above-mentioned drugs
alleviated this decrease, to some extent (Takagi et al., 1968).
7.2.4.3. Blood
A normal haematological picture was reported in rats and dogs
repeatedly exposed to acrylonitrile vapours at up to 240 mg/m3
(Brewer, 1976), and in rats and rabbits (except for a raised
eosinophil count) repeatedly exposed to up to 330 mg/m3 (Dudley et
al., 1942).
Minami et al. (1973) exposed male rabbits to 54 mg/m3 for 1 day
per week (8 h) for 8 weeks; haematocrit and haemoglobin were
unaffected, but pO2 and pH were raised and pCO2 lowered by the
treatment.
In rats receiving acrylonitrile in the drinking-water for 90
days, the only significant haematological change was a decrease in
the red-cell count, on the 83rd day, in females receiving 42 mg/kg
body weight per day (Humiston & Frauson, 1975). The blood urea and
alkaline phosphatase (EC 3.1.3.1) levels in the males receiving 38
mg/kg body weight per day were raised, but SGPT activity was
normal. While rats administered ip 50 mg acrylonitrile/kg body
weight, daily, for 3 weeks (Knobloch et al., 1971) developed
leukocytosis and increased serum asparagine-oxo-aminotransferase
(EC 2.6.1.14) activity, mice exposed for 70 days to 225 mg/m3 (100
ppm) or 340 mg/m3 (150 ppm) 6 h daily, did not develop any
haematological abnormalities (Hashimoto, 1962). Rats exposed to
9.7 mg/m3, 4 h daily, 5 days a week for 2 months, did not show any
effects on the erythrocyte count or on the haemoglobin
concentration (Vissarionova et al., 1979).
As a whole, the studies failed to demonstrate any consistent
effect of acrylonitrile on red or white blood cell production or
viability. Leukocytosis following repeated intraperitoneal
administration of an irritant material is to be expected.
7.2.4.4. Immune system
Wistar rats were exposed to an acrylonitrile concentration of
10 mg/m3 for 6 h daily, 5 days a week, for 16 weeks. Acrylonitrile
depressed both T helper and T suppressor functions, and a
diminished degree of B lymphocyte transformation was observed.
Alpha tocopherol (im 0.21 mmol/kg body weight on alternate days for
16 weeks) protected against this effect (Krivova et al., 1982).
7.2.4.5. Nervous system
Some findings in the experimental animal studies on
acrylonitrile were indicative of an effect on the nervous system.
Rats, mice, and dogs exposed to up to 240 mg/m3, for 6 h daily, 5
days per week, for 13 weeks, showed ataxia and convulsions, prior
to death (Brewer, 1976). Transitory hind-leg weakness was seen in
cats exposed for 8 weeks (4 h per day, 5 days per week) to 330
mg/m3 (Dudley et al., 1942). Krysiak & Knobloch (1971) found that
rats receiving acrylonitrile intraperitoneally at 20 mg/kg body
weight daily for 6 weeks, or sc at 40 mg/kg daily for 4 weeks,
showed a significant lengthening of the time to perform correctly
in a conditioned food reflex test, and a significant decrease in
the number of correct reactions, compared with pre-treatment
observations or controls. Performance improved when the treatment
was discontinued. Daily ip administration to rats of acrylonitrile
at 50 mg/kg body weight for 3 weeks caused a vacuolization of
neuronal cells of the cortex and brain stem (Knobloch et al.,
1971).
7.2.4.6. Urine
No significant changes in urine composition were observed in
the experimental studies of Humiston & Frauson (1975) (sections
7.2.4.1 - 7.2.4.3).
7.2.4.7. Adrenals
The adrenals of rats exposed for 21-60 days to acrylonitrile
in drinking-water (0.05% and 0.2%) showed an atrophic zona
fasciculata and an enlarged zona glomerulosa. The animals on the
higher dose had a reduced plasma corticosteroid level and an
increased plasma Na+Concentration. The K+ level was unchanged
(Szabo et al., 1976). Serum corticosterone in rats was decreased
by 3 ip doses of acrylonitrile (33 mg/kg body weight) on successive
days (Nilsen et al., 1980).
7.2.4.8. Metabolism
After repeated exposure of rabbits to acrylonitrile, the in
vitro metabolism in the liver of acrylonitrile into cyanide and
thiocyanate decreased with time, while the excretion of unchanged
acrylonitrile in the urine increased (Sato, 1978).
7.3. Chronic Toxicity
Observations have been made on animals administered
acrylonitrile in drinking-water, food, through inhalation, and by
dermal application.
Tuller (1947) administered acrylonitrile at 500 mg/litre in the
drinking-water or acrylonitrile-fumigated food (dose not precisely
specified) to rats. After 2 years, the mortality was higher in
rats drinking acrylonitrile solution (50% deaths) than in paired
controls (25%), another control group (15%), and in rats on
acrylonitrile-fumigated food (5%). However, when acrylonitrile was
administered at 0.5, 5, and 90 mg/litre in the drinking-water to
male and female CFW rats for 2 years, the mortality rate was
unaffected (Svirbely & Floyd, 1961). Groups of 4 male and 4 female
beagle dogs were administered acrylonitrile at concentrations of
100, 200, or 300 mg/litre in the drinking-water, for 6 months.
Average intakes of acrylonitrile were the following for males
(females): 10(8) mg/kg body weight at 100 mg/litre; 16(17) mg/kg at
200 mg/litre; and 17(18) mg/kg at 300 mg/litre. Five dogs died, or
were killed because debilitated, in each of the 2 higher dosage
groups (Quast et al., 1975).
In the dogs receiving acrylonitrile at 100-300 mg/litre in the
drinking-water, early signs of toxicity included roughening of the
coat and, later, retching and vomiting. Terminal signs of lethargy,
weakness, emaciation, and respiratory distress were noted (Quast et
al., 1975).
7.3.1. Body weight, food and water intake
Body-weight gain was reduced during 4 of the 11 weeks at the
higher dose level (240 mg/m3) in a test in which male and female
Wistar rats and albino rabbits were exposed to acrylonitrile vapour
at concentrations of 0, 50, and 240 mg/m3, for 3 h/day, 6 days a
week, for 6 months (Knobloch et al., 1972).
Growth retardation was observed in male rats drinking 500 mg
acrylonitrile per litre water, for 2 years (Tuller, 1947). Svirbely
& Floyd (1961) administered acrylonitrile at 0.5, 5, or 50 mg/litre
in the drinking-water of rats and found a slight decrease in water
consumption at the highest concentration in both sexes.
Statistically significant reductions in the body weight of rats
receiving drinking-water containing acrylonitrile concentrations of
35, 100, or 300 mg/litre were associated with decreased water
consumption and decreased food consumption at 300 mg/litre in males
and 100 mg/litre in females (Ouast et al., 1977). Decreased food
and water consumption and body weight was reported with beagle dogs
drinking acrylonitrile at 200 or 300 mg/litre water for 6 months
(Quast et al., 1975). Marked weight decreases were seen in dogs
that eventually died or had to be killed.
7.3.2. Organ weights
In rabbits exposed to atmospheric acrylonitrile concentrations
of 250 mg/m3, for 3 h/day, 6 days a week for 6 months, a
significant increase in the heart weight was noted, and some
fluctuations in blood pressure were described (Knobloch et al.,
1972).
Ferin et al. (1961) exposed rats to drinking-water containing
an acrylonitrile concentration of 20 or 1000 mg/litre for 6 months.
At the higher dose, increased relative weights of the liver,
spleen, and kidneys were noted. The relative weights of the heart,
liver, and brain in males, and of the liver and kidneys in females,
were increased in rats receiving 300 mg/litre in drinking-water.
The males drinking 100 mg/litre showed a significantly increased
brain weight, and the females receiving 100 mg/litre had
significantly lower relative heart weights (Quast et al., 1977).
The weights of brain, heart, liver, and kidneys of beagle dogs
drinking an acrylonitrile concentration of 100 mg/litre in
drinking-water (Quast et al., 1977) were normal but, at 200
mg/litre, the 2 remaining males had a lower absolute brain weight
and higher relative kidney weight than controls. The 2 remaining
females receiving 300 mg/litre also had significantly lower relative
brain weights compared with controls.
7.3.3. Pathology and histology
Inflammation of the pulmonary system accompanied by an
inflammatory exudate into the bronchial lumen occurred in rats
exposed to acrylonitrile at 240 mg/m3 for 3 h a day, 6 days a
week, for 6 months (Knobloch et al., 1972). Various pathological
changes occurred in male and female rats maintained on water
containing acrylonitrile at 35, 100, or 300 mg/litre for 12 months
(Quast et al., 1977). Males and females on the 2 higher doses
developed paleness and thickening of the mucosa, erosions, ulcers,
and sometimes papilloma formations in the non-glandular portion of
the stomach. Three females receiving 300 and 100 mg/litre and one
male at 300 mg/litre had ear canal tumours. Microscopic findings
of tissue with tumorous growth revealed an increased frequency of
gastric cell papillomas, Zymbal (sebaceous) gland tumours of the
ear canal, and microtumours of the nervous system, in rats
receiving a concentration of acrylonitrile of 100 or 300 mg/litre.
These tumours do not occur spontaneously at such a high frequency
in the strain of rat used. The nervous system lesions were
consistent with the diagnosis of astrocytoma.
Minimal lesions were seen in the liver of rats drinking
acrylonitrile at 100 or 300 mg/litre and chronic renal disease
occurred in females drinking 300 mg/litre. The squamous epithelium
of the stomach was hyperplastic in rats drinking 100 or 300
mg/litre.
Histopathological changes in dogs receiving acrylonitrile
concentrations of 200 and 300 mg/litre in water (Quast et al.,
1975) were similar to those in untreated controls. The pneumonia
present may have resulted from irritation of the mucosa of the
tongue and oesophagus, which produced abnormal swallowing,
resulting in aspiration of some food.
7.3.4. Haematology and clinical chemistry
In rats exposed for 3 h/day, 6 days a week, for 6 months to
acrylonitrile in air concentrations of either 50 or 240 mg/m3, the
eosinophil count had significantly increased after 4 months. Total
serum protein was unchanged, while albumin and alpha-globulin had
increased and gamma-globulin decreased in both test groups
(Knobloch et al., 1971). Leukocytosis was observed in rats
drinking acrylonitrile in water at 1000 mg/litre (Ferin et al.,
1961). Periodic examinations of rats drinking 0.5, 5, or 50
mg/litre in water (Svirbely & Floyd, 1961), or 0 - 300 mg/litre in
water (Quast et al., 1977), showed normal haematological findings.
A significant elevation in alkaline phosphatase activity was found
in female rats exposed to 300 mg/litre. Half-way through the
study, beagle dogs drinking acrylonitrile concentrations of 0, 100,
200, or 300 mg/litre in water showed a significant decrease in
haematocrit, erythrocyte count, and haemoglobin concentration at
300 mg/litre and decreased erythrocyte count at 200 mg/litre, in
males. Females on 300 mg/litre also showed a significant decrease
in the erythrocyte count. However, all haematological findings
were within normal ranges towards the end of the study, except in
males at 300 mg/litre, which had a lower erythrocyte count. Blood
urea nitrogen, serum alkaline phosphatase activity, SGPT, and SGOT
were measured periodically; the findings in males were always
within normal limits, but females receiving 300 and 200 mg/litre
showed some increase in SGOT and SPGT activity. Total and
individual serum proteins were unaffected at the end of the study.
Zotova (1976) exposed rats by applying acrylonitrile solution to
the skin of the tail at doses of 2.82, 0.56, or 0.11 mg/kg body
weight per day and observed a decreased haemoglobin concentration
at the highest dosage level after 2 months. Blood catalase (EC
1.11.1.6) activity increased, and blood peroxidase (EC 1.11.1.7)
activity decreased, initially, but later became normal; there was
no change in sulfhydryl levels.
A decrease in blood -SH groups was reported in rats exposed to
acrylonitrile at 10 mg/m3 for 5 days/week, 4 h a day, for 4 months
(Efremov, 1976d).
7.3.5. Nervous system
Changes in central nervous function, as elicited by a
conditioned avoidance test, were found in rats drinking water
containing acrylonitrile at 20 mg/litre, for 6 months (Ferin et
al., 1961). Rats exposed through inhalation to acrylonitrile
concentrations of 50 and 240 mg/m3 for 3 h a day, 6 days a week,
for 6 months, showed significant defects in performance in a "Y"
maze (Krysiak, 1971). Rats exposed to 10 mg/m3, 5 days/week, 4 h a
day, for 4 months, showed a 59% decrease in the activity of brain
catalase, a 59% decrease in brain peroxidase, and a 37% decrease in
-SH groups (Efremov, 1976d). Histopathological changes in the
nervous system, consistent with a diagnosis of astrocytoma, were
observed in rats exposed to acrylonitrile at 35, 100, or 300
mg/litre in drinking-water (Quast et al., 1977).
7.3.6. Kidney function
Knobloch et al. (1972) exposed rats to 50 or 240 mg/m3, for
3 h per day, 6 days per week, for 6 months; kidney dysfunction was
indicated by increased urinary output at both concentrations and an
increase in urinary protein and areas of degenerated proximal
convoluted tubules at the higher concentration.
There were no abnormalities in the urine chemistry of rats
drinking water containing acrylonitrile at 0, 35, 100, or 300
mg/litre (Quast et al., 1977), or of dogs (Quast et al., 1975),
drinking water containing 100, 200, or 300 mg/litre. When
acrylonitrile was applied to the skin of the tail of rats, daily,
at doses of 2.82, 0.56, or 0.11 mg/kg body weight for 4.5 months,
the excretion of urinary chlorides increased on day 10 of exposure
at the 2 higher doses and decreased at the lowest dose (Zotova,
1976).
7.4. Teratogenicity and Embryotoxicity
The teratogenic potential of ingested or inhaled
acrylonitrile was investigated by Murray et al. (1978).
Groups of pregnant SD rats were given acrylonitrile at 0, 10,
25, or 65 mg/kg body weight per day, by gavage, from day 6 to
day 15 of gestation. Groups of 30 pregnant SD rats were
exposed 6 h per day to 0, 87, or 174 mg/m3 (0, 40, or 80 ppm)
acrylonitrile by inhalation, during the same period of
pregnancy. A dose of 65 mg/kg body weight per day caused
marked maternal toxicity, significant embryotoxicity, and an
increased incidence of fetal malformations. Findings of the
two studies suggesting a teratogenic effect were noted at 25
mg/kg per day and at 174 mg/m3 (80 ppm). At 10 mg/kg body
weight per day and 87 mg/m3 (40 ppm), no embryotoxicity or
teratogenicity was found. There was no apparent correlation
between the degree of toxicity seen in the individual dams and
the occurrance of malformations in their offspring.
Embryotoxic effects in pregnant mice of 3 strains were
described after intraperitoneal administration of unspecified doses
of acrylonitrile (Scheufler, 1976). A single ip injection of
acrylonitrile of 32 mg/kg body weight, given on the 5th or 7th day
of pregnancy, induced an embryotoxic effect in mice from an inbred
strain of AB Jena-Hall, but not in DBA and C57 C1 mice (Scheufler,
1980).
Kankaanpää et al. (1979) studied the embryotoxic effects of
acrylonitrile using chick eggs, but did not find any clear evidence
of its teratogenicity.
The exposure of Sprague-Dawley rats to an acrylonitrile
concentration in drinking-water of 500 mg/litre led to decreased
fertility and decreased viability of the young, and the females
developed a progressive muscular weakness in the hind legs about
16-19 weeks after the weaning of the second litter (Svirbely &
Floyd, 1961).
Willhite (1981a,b) observed skeletal malformations in hamster
fetuses after the administration of acrylonitrile at 80 mg/kg body
weight to pregnant hamsters. The histological study of both early
embryos and term fetuses revealed mesodermal changes, including a
reduction in the number of cells, shrinkage of the cell cytoplasm,
and enlarged extracellular spaces. In addition, a reduction in
mitotic figures and focal necrosis were noted. The affected
embryos were smaller and their development was delayed compared
with untreated controls. Teratogenic effects were only observed
when there was simultaneous maternal toxicity.
7.5. Mutagenicity
7.5.1. Bacterial systems
(a) Ames test with Salmonella typhimurium strains
Tests have been carried out on several strains, with and
without metabolic activation, using several methods of treatment
with acrylonitrile. Negative results were obtained, with and
without activation, in 5 tested strains in 2 studies (Litton
Bionetics, 1975; Stanford Research Institute, 1976). A weak, but
reproducible, positive effect was observed with metabolic
activation using between 0.5 and 1.5 mg acrylonitrile per plate in
strain TA 1535 (Haskell Laboratory, 1975; De Meester et al., 1978,
1979). Three methods of exposure were examined by Milwy & Wolff
(1977) in 3 strains of S. typhimurium. A low level of mutagenic
activity was noted in strain TA 1535, when plates were sprayed with
acrylonitrile or when it was mixed with the medium, with
activation.
Exposure to acrylonitrile vapour of the strains TA 1535 and TA
100 also demonstrated acrylonitrile mutagenicity (Duverger-Van
Bogaert et al., 1981; Ivanov, 1981). Zhurkov et al. (1983) tested
the mutagenicity of acrylonitrile in strains TA 1535 and TA 1538,
with and without microsomal activation, and found a dose-dependent
effect in strain TA 1535.
Urine collected from rats and mice treated with acrylonitrile
was mutagenic in S. typhimurium strain TA 1530, in the absence of
metabolic activation. Pre-treatment of the animals with
phenobarbital abolished the direct mutagenicity of urine from rats
and reduced that from mice. The addition of beta-glucuronidase (EC
3.2.1.31) to the incubation mixtures enhanced the mutagenic
activity of urine from acrylonitrile-treated animals (Lambotte-
Vandepaer et al., 1980, 1981a). Duverger-van Bogaert et al. (1982b)
suggested that glutathione might play a role in the formation of a
mutagenic metabolite of acrylonitrile. The mutagenic activity of
acrylonitrile vapours towards S. typhimurium strains was strictly
dependent on the presence of an activation system, confirming the
report of Milwy & Wolff (1977). Lambotte-Vandepaer et al. (1980)
indicated that animal urine might retain its mutagenic activity for
as long as a week after collection. The acrylonitrile-derived
epoxide, glycidonitrile, synthesized by Kopecky & Smejkal
(unpublished data, 1979), was shown to be the principal substance
that exerted mutagenic activity in the absence of metabolic
activation, whereas acrylonitrile itself required metabolic
activation in the S. typhimurium Ames test (Cherna et al., 1981).
(b) Mutagenicity in Escherichia coli
One of 3 strains of E. coli (WP2) revealed mutagenic activity
of acrylonitrile; activation did not have any effect (Venitt et
al., 1977). The mutagenic activity of acrylonitrile was confirmed
in other experiments using the simplified fluctuation test of Green
et al. (1976). The results suggested that acrylonitrile caused
non-excisable mis-repair of DNA associated with the generation of
DNA strand breaks (Venitt et al., 1977). The method of Slater et
al. (1971) did not reveal any effect with or without an activation
system at 10 µg acrylonitrile per plate (Litton Bionetics, 1976).
The variability of the results, even when the same kinds of
assays are used, could be because of differences in purity of the
acrylonitrile, in method, or in bacterial sensitivity. However, the
mutagenicity of acrylonitrile in bacterial systems seems to have
been established.
7.5.2. Yeast assays
Possible mutagenic activity of acrylonitrile was found with
Saccharomyces cerevisiae, but metabolic activation was without
effect (Litton Bionetics, 1975).
7.5.3. Drosophila melanogaster
A negative result was obtained when 0.1% acrylonitrile was
administered by intra-abdominal injection into D. melanogaster in
order to examine its ability to induce a recessive lethal effect in
the X chromosomes (Benesh & Shram, 1969).
7.5.4. Mammalian cell in vitro assays
The L5178Y kinase mouse lymphoma cell assay (Litton Bionetics,
1976) failed to show mutagenic activity of acrylonitrile using the
procedure of Clive & Spector (1975). Chinese hamster ovary cells
showed an increase in sister chromatid exchange (SCE) after
exposure to acrylonitrile, when co-cultured with rat hepatocytes
(Ved Brat & Williams, 1982). No effect was found without the
latter.
Acrylonitrile induced a slight increase in the SCE of cultured
human lymphocytes in the presence of S-9 mix and increased
unscheduled DNA synthesis with a very high concentration (0.5 M)
(Perocco et al., 1982). Application of acrylonitrile to primary
Syrian golden hamster embryo cells in culture produced foci of
morphologically-transformed cells. Pre-treatment with simian
adenovirus (SA7) caused an 8 to 9-fold increase in the frequency of
virus-transformed foci. When 3H-thymidine-labelled cells were
treated with acrylonitrile and their DNA subjected to alkaline
sucrose gradients, a shift in the sedimentation pattern occurred,
which was reminiscent of that observed with carcinogen treatment.
These observations added support to recent studies indicating that
acrylonitrile may be carcinogenic (Parent & Castro, 1979).
7.5.5. Mammalian in vivo assays
The inhalation exposure of 16 Sprague-Dawley male rats to
acrylonitrile levels up to 1085 mg/m3 (500 ppm) for 90 days did not
reveal chromatid or chromosomal aberrations or bone-marrow
abnormalities (Johnson et al., 1978). The results of Rabello-Gray
& Ahmed (1980) and the recent results of Leonard et al. (1981) also
showed that acrylonitrile fails to induce chromosomal aberrations
in somatic and germ cells.
Similar negative results were reported by Zhurkov et al. (1983)
following inhalation exposure of mice to acrylonitrile concentrations
of both 100 mg/m3 and 20 mg/m3 . They also reported negative
results in a dominant lethal assay in mice.
From preliminary results concerning DNA-alkylation by
acrylonitrile and vinyl chloride monomer (Peter et al., 1983), it
appears that DNA-alkylation occurs to a much lesser extent with
acrylonitrile than with vinyl chloride monomer. This is consistent
with the absence of mutagenic effects in vivo.
7.6. Carcinogenicity
Although full data were not available to the Group, there was
strong evidence from the data considered that acrylonitrile is a
carcinogen in rats.
Maltoni et al. (1977, 1982) investigated the carcinogenicity of
acrylonitrile administered to Sprague-Dawley rats by inhalation at
87, 44, 22, and 11 mg/m3 (40, 20, 10, and 5 ppm), 4 h daily, 5
times a week, or by stomach tube as a solution in olive oil, at a
dose of 5 mg/kg body weight, once a day, 3 times a week. In each
case, the rats were treated for 52 weeks and then kept without
further treatment until death. An increased incidence of some
tumours was noted in the acrylonitrile-treated animals, e.g.,
mammary tumours, forestomach papillomas and acanthomas, and
encephalic tumours (gliomas).
Two-year studies on Sprague-Dawley rats, following inhalation
exposure to acrylonitrile or ingestion in drinking-water, have been
performed at the Dow Chemical Company laboratories (Quast et al.,
1980a,b). In the inhalation studies, rats were exposed to 0, 44,
or 174 mg/m3 (0, 20, or 80 ppm) for 6 h/day, 5 days a week, for 24
months. Treatment-related tumours were found in the central nervous
system, Zymbal gland, tongue, stomach, small intestine, mammary
gland, and nasal turbinates. An apparent decrease in tumours of
the pituitary gland, the adrenals, the thyroid, the pancreas, and
testes was observed in the exposed rats.
In the ingestion study, rats were maintained on water
containing acrylonitrile levels of 0, 35, 100, and 300 mg/litre,
equivalent to mean dosage levels of 0, 4, 9, or 22 mg/kg body
weight per day. Evidence of oncogenicity was found in rats at all
dose levels of acrylonitrile. An increased tumour incidence was
observed in the treated rats affecting, particularly, the centra1
nervous system and also the Zymbal gland, tongue, stomach, small
intestine, and mammary gland. A decreased incidence of tumours was
observed at some sites: pituitary, thyroid, adrenals, pancreas, and
uterus.
In studies performed by Hogen & Rinehart (1980), acrylonitrile
was administered to Sprague-Dawley rats in the drinking-water at 1
or 100 mg/litre for 19-22 months, or by gavage at 0.1 or 10 mg/kg
body weight per day in water for about 20 months. A second group
of Fisher 344 rats received acrylonitrile in the drinking-water at
1, 3, 10, 30, or 100 mg/litre for 23-26 months. A statistically
significant increase in tumours was reported in the group receiving
acrylonitrile at 10 mg/kg body weight by gavage and in the groups
receiving 10, 30, or 100 mg acrylonitrile/litre drinking-water.
So far, no information is available on the carcinogenicity of
acrylonitrile for animal species other than rats.
After reviewing these data, IARC (IARC, 1982) and COC (UK
Ministry of Agriculture, Fisheries and Food, 1982) concluded that
acrylonitrile was a carcinogen in experimental animals.
8. EFFECTS ON MAN
Acrylonitrile has long been known to be a toxic substance that
induces systemic as well as local injury in both animals and man.
It has frequently been used in combination with other chemicals;
they may modify its toxicity, as was the case when it was used as a
fumigant.
8.1. Acrylonitrile
8.1.1. Acute Toxicity
8.1.1.1. Inhalation exposure
A 22-year-old chemist, who was exposed to acrylonitrile vapours
when a distillation apparatus leaked, developed headache, vertigo,
vomiting, tremors, uncoordinated movements, and convulsions
(Sartorelli, 1966). Vomiting and nausea persisted for 24 h. One
day after exposure, slight liver enlargement and congestion of the
oral pharynx, but no disorders of the CNS, were noted. After 4
days, no kidney, liver, cardiac, or respiratory abnormalities were
detected. Workers exposed to "mild" concentrations of acrylonitrile
in synthetic rubber manufacture developed nausea, vomiting,
weakness, nasal irritation, and an "oppressive feeling" in the
upper respiratory tract (Wilson, 1944). Headache, fatigue, and
diarrhoea were observed in some cases, and mild jaundice lasting
for several days and accompanied by liver tenderness and low-grade
anaemia in a few others. Jaundice lasted for 4 weeks in 1 case;
this individual complained of lassitude and fatigue after one year.
Zeller et al. (1969) observed that in 16 cases of acute inhalation
of acrylonitrile fumes by workers, nausea, vomiting, headache, and
vertigo appeared within 5-15 min; none of the workers needed
hospitalization. The authors described 50 cases of skin contact
with irritation, erythema, and blistering appearing within 5 min to
24 h, but with no systemic consequences. Workmen exposed to
concentrations varying from 35 to 220 mg/m3 (16-100 ppm) for 20-45
min during cleaning operations in polymerizers frequently
complained of a dull headache, fullness in the chest, irritation of
the eyes, nose, and throat, and feelings of apprehension and
nervous irritability. Some workmen had "intolerable itching" of
the skin, but no accompanying dermatitis.
8.1.1.2. Dermal exposure
A male laboratory worker who spilled "small quantities" of
liquid acrylonitrile on his hands, developed diffuse erythema on
both hands and wrists after 24 h, and blisters on the fingertips by
the third day. The hands were slightly swollen, erythematous,
itchy, and painful. The fingers remained dry and scaly on the 10th
day (Dudley & Neal, 1942). Wilson et al. (1948) observed that
direct skin contact led to irritation and erythema followed by scab
formation; healing was slow. Development of allergic dermatitis is
possible; a 27-year-old individual developed a rash on his finger
following the use for 6 weeks of a finger splint made from an
acrylonitrile/methyl methacrylate copolymer. Patch testing gave
positive reactions to the copolymer and 0.1% acrylonitrile (Balda,
1975). In another case report, skin lesions were first observed at
the site of contact with liquid acrylonitrile, which then spread
rapidly to other neighbouring regions. Several days after contact,
the lesions spread rapidly to other parts of the body that had not
been exposed, and these extensions were assumed to be an allergic
reaction (Hashimoto & Kobayashi, 1961).
In addition to local dermal toxicity, dermal absorption of
acrylonitrile may lead to systemic poisoning. Grunske (1949)
described a fatal case in which a 3-year-old girl had entered a
room that had recently been sprayed with an acrylonitrile-
containing insecticide (Ventox). Exposure was mainly through
inhalation, but skin exposure was possible, too. Another fatal
case was reported by Lorz (1950) in which a 10-year-old girl had
been treated on the scalp for lice with an insecticide that was
identified as containing acrylonitrile (Ventox). She had impetigo
and widespread scratches on the skin of the scalp. This could have
increased the absorption of acrylonitrile.
Two workers who spilled liquid acrylonitrile on their legs,
immediately washed their legs and dried their shoes, but put them
on again. Blisters developed at the sites of contact, 6-8 h after
the spill. Therapy lasted 21 and 38 days, respectively. The skin
of 2 workers who were cleaning apparatus (temperature 50 °C), came
into contact with 5% acrylonitrile solution; other possible
substances in the mixture were not specified. Serious skin burns
developed. Therapy lasted 35 and 72 days, respectively (Babanov,
1957). Zeller et al. (1969) reported 50 cases of skin damage
resulting from occupational contact with acrylonitrile. A burning
sensation developed within 5 min to 24 h followed by a reddening of
the area, which often blistered after 1 day.
8.1.2. Chronic toxicity - occupational exposure
Chronic effects can potentially occur after prolonged exposure
to acrylonitrile, both in the vapour and liquid forms.
8.1.2.1. Clinical observations
Complaints of poor health, headache, decreased work capacity,
poor sleep, irritability, chest pains, poor appetite, and skin
irritation (during the first months of employment only) came from
workers employed in the manufacture of acrylonitrile (Zotova,
1975a).
In a study by Sakurai & Kusumoto (1972), workers employed in
acrylonitrile manufacture also complained of headache, weakness,
fatigue, nausea, vomiting, nosebleeds, and insomnia; the symptoms
correlated well with the length, but not with the level of exposure
or with the age of the workers. A total of 4439 examinations were
made over about 10 years prior to 1970, in 576 workers who formed
2 cohorts, one exposed to concentrations of acrylonitrile below 11
mg/m3 (5 ppm), the other below 45 mg/m3 (20 ppm). However, the
authors later stated that these exposure levels were not reliably
reported (Sakurai et al., 1978).
Babanov et al. (1959) reported that workers exposed to
acrylonitrile concentrations at 0.6-6 mg/m3 for approximately 3
years suffered from headache, insomnia, pains in the heart region,
general weakness, decreased working capacity, and increased
irritability. The vocal cords were inflamed, and non-specific
changes in the vestibular apparatus and pale mucous membranes and
skin were seen. Blood pressure was said to be reduced.
Changes in the health status and laboratory tests were not
observed in a group of 23 men who had been working for 3-5 years in
an acrylonitrile plant, where, during the warm season, exposure
levels reached 4.2-7.2 mg/m3 (Gincheva et al., 1977). Stamova et
al. (1976) studied workers' health in the related polyacrylic fibre
plant in which acrylonitrile exposure levels ranged around 10
mg/m3, but could fluctuate upwards to 25 mg/m3 . Workers were also
exposed to other chemical substances. An increase was found in
both skin diseases and various "neurasthenic" complaints and
diseases. Dorodnova (1976) did not find any differences in the
gynaecological health status of 410 women working in a polyacrylic
fibre plant in Saratov compared with that of 436 unexposed women.
8.1.2.2. Haematology
Compared with the findings in blood donors, some male and
female employees exposed to acrylonitrile at 2.5-5 mg/m3 showed
a reduced haemoglobin level, erythrocyte count, leukocyte count,
and percentage of neutrophiles, with an increased percentage of
lymphocytes and plasma iron. Inhibition of maturation of
normoblasts in bone marrow was also reported (Shustov, 1968).
Similar results were reported by Zotova (1975b). Lower
erythrocyte, haemoglobin, and total white counts were found in
laboratory workers exposed to acrylonitrile, and in apparatus
operators and machinists. Higher than normal total glutathione
levels were found in male operators and maintenance men and reduced
glutathione levels in male apparatus operators. Oxidized
glutathione was elevated and total sulfhydryl groups decreased in
workers employed in all these occupations.
Lower erythrocyte counts and a relative lymphocytosis were also
observed by Babanov et al. (1959) in the study mentioned above.
8.1.2.3. Other organs
(a) Liver
Sakurai & Kusumoto (1972) (section 8.1.2.1) reported some
abnormal results in liver function tests; however, in a further
study of 102 workers from some of the factories, Sakurai et al.
(1978) did not find any significant liver function test
abnormalities related to acrylonitrile exposure, when exposure
levels had decreased from 11-44 mg/m3 (5-20 ppm) to 9 mg/m3 (4.2
ppm). Increased serum cholinesterase activity, hyperbilirubinaemia,
decreased coloidal stability, and hypergammaglobulinaemia were
described in workers exposed to acrylonitrile concentrations of up
to 5 mg/m3 and to acrylonitrile-polymer dust of up to 1.5 mg/m3
(Enikeeva et al., 1976). These effects have not been reported
elsewhere.
(b) Eye
Blepharoconjunctivitis was reported by Delivanova et al. (1978)
in all of 302 workers examined over a 2-year period; 42 had severe
alterations caused by conjunctivitis, and all disorders were
connected with exposure to acrylonitrile.
(c) Gastro-intestinal effects
Symptoms of gastritis and colitis were observed in workers
exposed to acrylonitrile concentrations of up to 5 mg/m3 (Enikeeva
et al., 1976).
(d) Immune system
Acrylonitrile has been found to have an immunodepressive
effect. The functional activity of T-lymphocytes was found to have
decreased in workers exposed to acrylonitrile (Ivanov, private
communication, 1983).
8.1.2.4. Nervous system
Nausea, vomiting, headache, and vertigo (Wilson, 1944; Wilson
et al., 1948; Zeller et al., 1969; Sakurai & Kusumoto, 1972;
Zotova, 1975) indicate a possible effect of acrylonitrile on the
nervous system. Ageeva (1970) reported a significant decrease in
an "epinephrine-like substance", and an increase in acetylcholine.
Depression, lability of autonomic functions (lowered arterial
pressure, labile pulse, diffuse dermographia, increased sweating,
change in orthostatic reflex) were also observed in workers
involved in acrylonitrile production.
8.1.2.5. Dermal effects
Spassovski (1976) reported irritant and allergic dermatitis in
acrylonitrile workers; dermatitis was also observed by Antonev &
Rogailin (1970) and Stamova et al. (1976).
8.2. Mutagenicity
Thiess & Fleig (1978) examined workers who had been exposed to
acrylonitrile for 15.3 years and workers who had not been exposed.
No differences were found in the incidence of chromosomal
aberrations, including or excluding gaps, in the 100 metaphases
examined for each person.
8.3. Carcinogenicity
In a retrospective cohort epidemiological study of 1345 male
workers with potential exposure to acrylonitrile from 1950-66,
followed until 31 December, 1976, 25 cases of cancer were found
with 20.5 expected, based on company rates (O'Berg, 1980). Of
these, 8 were respiratory cancer cases, with 4.4 expected. Twenty-
three cases occurred among workers first exposed during the start-
up period (1950-52) when exposures were higher; only 12.9 were
expected (P = 0.01).
The standardized incidence ratio (SIR) was 179 for cancer among
the operators and mechanics who had at least 6 months' exposure and
began their assignments during start-up. A "dose response" was
shown with those with longer duration of employment, workers with
estimated higher exposures having higher risk. Latency was also
demonstrated, with 17 of the 24 cases occurring 20 years after the
onset of exposure among those with at least 6 months' employment,
including 6 of the 8 lung cancer cases. It should be pointed out
that, using the National Cancer Institute's expected incidence
rates for 1969-71, the expected rate would be 25.5 rather than 20.5
from the company rates. In a concomitant cancer mortality study,
20 cancer deaths were found with 17.4 expected, using company rates
(not significant); the expected rates exceeded the company rates
using national, state, or regional cancer rates. The author felt
that it might be premature to evaluate mortality statistics,
because of insufficient latency (many cancer cases had been
recently diagnosed and were still living). Smoking habits were not
considered, though the author stated that 7 out of the 8 lung
cancer cases were known to have smoked.
A follow-up study on the mortality rate among 327 employees of
a chemical rubber plant in the USA revealed that the number of
deaths from lung cancer was significantly higher than expected (9
versus 5.9 for US white males and 4.7 for other rubber workers from
the same city). The greatest excess was seen among men who had
worked for 5-14 years and who had started working there at least 15
years before death (Delzell & Monson, 1982). This study was
confounded by the multiple exposure of workers in the nitrile
rubber manufacturing plant.
Kiesselbach et al. (1979) examined the mortality rate, the
cancer rate, and the type of cancer against the period of exposure
to acrylonitrile in 884 workers. The results revealed that the
general mortality of the exposed group was markedly lower than that
of the normal population (58 versus an expected 104), possibly
because of the "healthy worker" effect. The mortality rate for
malignant tumours, cardiovascular, brain, respiratory, and gastro-
intestinal diseases, suicide, and other causes was the same as in
the normal population. No relationship was found between length of
exposure and mortality from tumours.
An excess of deaths from lung cancer was reported in
acrylonitrile workers by Thiess et al. (1980). In addition, 2
cases of Hodgkin's disease contributed to a slight excess of cancer
of the lymphatic tissue. However, exposure to other substances,
some of them known carcinogens, made interpretation of the results
difficult.
A cohort study on men potentially exposed to acrylonitrile
during the start-up of a plant indicated that there was no excess
mortality from lung cancer. There were no deaths from lung cancer
in maintenance workers, who possibly had the highest exposures.
There was an excess of kidney cancers (based on 2 cases only) and
of circulatory disease other than rheumatic and atherosclerotic
(based on 5 cases), accompanied by a deficit of atherosclerotic
heart disease.
Because the cohort was small with only 4 cancer deaths
observed, it could not give an indication of excess cancer risk or
association with duration of exposure to acrylonitrile. An
additional retrospective cohort mortality study, in two
acrylonitrile plants in the USA, on 352 males exposed for 6 months
or more prior to 1968 and followed up until December 1977, did not
show any excess mortality including cancer mortality. There were
15 deaths from all causes, 18.11 being expected, and 3 deaths from
cancer (2.8 expected) (Zack, unpublished data, 1980).a
In a study by Nakamura (1981), 9525 workers employed in the
production of acrylonitrile, acrylonitrile rubbers, and ABS were
studied. Deaths due to cancers in general and to lung and colon
cancers in particular, were not increased, while 7 deaths due to
liver, gall bladder, or cystic duct cancer were found against the 5
that might have been expected.
The mortality of 1111 men who worked on the polymerization of
acrylonitrile and the spinning of acrylic fibre in the United
Kingdom from 1950 to 1968 was surveyed up to the end of 1978.
Seventy-nine deaths were identified in 6 factories. The total
number of deaths among men exposed to acrylonitrile for at least
one year was slightly lower than expected (68 versus 72.4) and a
relative excess of deaths from all cancers was found, arising
mainly from cancers of the lung, stomach, colon and brain,
pancreas, testis, and bladder (21 versus 13 expected). The authors
considered particularly relevant, the excess of lung cancer in
those aged 15-44 years. Nevertheless, the authors considered that
their results were inconclusive and urged continued surveillance
and analysis of the exposed population in the United Kingdom
(Werner & Carter, 1981).
The epidemiological studies provide some indications that
acrylonitrile exposure is associated with cancer, particularly of
the lung. However, the studies reported, while neither conclusive
nor contradictory, are limited by insufficient latency. Other
difficulties, such as cohort identification and selection, and
combined exposures have made interpretation difficult. Further
epidemiological data are therefore of great importance, and
consideration of smoking and single exposures to acrylonitrile is
desirable.
8.4. Simultaneous Exposure to Acrylonitrile and
Other Chemicals
8.4.1. Acute toxicity
Numerous non-fatal and fatal cases of poisoning by
acrylonitrile-containing mixtures have been described (Davis et
al., 1973). In home fumigation, an acrylonitrile mixture with
carbon tetrachloride or methylene chloride is placed in shallow
open pans and the vapours dispersed by fans for 24-72 h, the
operator deciding when the house is safe for occupancy (Davis et
al., 1973). A man working in the polymerization of acrylonitrile,
polybutadiene, and styrene for 2 years complained of numbness of
the fingers and toes, severe fatigue in the lower extremities, and
----------------------------------------------------------------------
a Zack, J.A. (1980) The mortality experience of Monsanto
workers exposed to acrylonitrile (Monsanto Internal
Report).
general malaise. Decreased patellar and achilles tendon reflexes,
and hypoaesthesia in the peripheral parts of the fingers and toes
were observed. Free acrylonitrile was detected in the urine. The
exposure level of acrylonitrile was estimated to exceed 108.5 mg/m3
(50 ppm) (Seki, 1967).
Lachrymation, burning in the throat, coughing, sneezing,
nausea, vomiting, dizziness, visual disturbance, headache, coma,
seizures, and dermatitis have been described in non-fatal cases
(Davis et al., 1973). Fatalities have occurred following exposure
to vapours (Grunske, 1949; Davis et al., 1973) and liquid (Lorz,
1950). Cyanide was detected in the blood in some cases. Symptoms
and signs prior to death varied from case to case; sore throat,
weakness, dizziness, vomiting, eye irritation, respiratory
disorders, pallor, tachycardia, tremors, unconsciousness, and
epidermal necrolysis have been described. Other pathological
conditions occurred, but many could have been the result of pre-
existing disease or of exposure to the other component(s) of the
mixture. Findings in children suggest that they may be more
sensitive to acrylonitrile exposure than adults (Grunske, 1949).
8.4.2. Chronic toxicity
Abnormalities in subjects exposed simultaneously to
acrylonitrile and several other chemicals have been described in
several studies. As the concentrations of the other chemicals were
frequently higher than those of acrylonitrile, it is difficult to
decide whether the abnormalities were caused by acrylonitrile, the
other chemical(s), or a combination of the two.
An abnormally high proportion of workers exposed to
acrylonitrile levels of 3-20 mg/m3, 33 ppm NH3, up to 1 mg
H2SO4/m3, 0.41-0.67 mg NaOH/m3, and 2-10 mg acetic acid/m3 were
described as suffering from a variety of symptoms ascribed to
disorders of the autonomic nervous system ("neurasthenic syndrome")
(e.g., irritability, headache, poor appetite, fatigue). Intolerance
to alcohol has also been observed (Orusev & Popovski, 1973; Orusev
et al., 1973).
Apprentices exposed to acrylonitrile (0.8-1.8 mg/m3), methyl
methacrylate (16-17 mg/m3), and sodium thiosulfate were examined
before exposure, after 1-2 weeks, and after a further unspecified
time. "Neurasthenic" symptoms were rare before, but frequent after
exposure, and the incidence of immunological reactivity against the
chemicals increased, as did the concentration of ceruloplasmin
(Mavrina & Il'ina, 1974). Dermal tests for allergy were also made
by Hromov (1974) in workers who had been in contact with
acrylonitrile, methyl acrylate, and sodium thiocyanate. Intradermal
samples showed positive haemoagglutination reactions in 86.5% of
workers exposed to acrylonitrile, 76.1% exposed to methyl acrylate,
and 53.6% exposed to sodium thiocyanate. Dermatitis, eczema, and
urticaria occurred.
Mavrina & Hromov (1974) and Shustov & Mavrina (1975) reported
abnormalities of the liver, nervous, cardiovascular, and
gastrointestinal systems in workers occupationally exposed to
acrylonitrile, methyl acrylate, and sodium thiocyanate during fibre
production. In particular, symptoms associated with the activity
of the autonomic nervous system were noted among the 340 workers
examined. Dryness, desquamation, fissures, and diffuse erythema of
the skin were also apparent. Women exposed to acrylonitrile and
methyl acrylate (Chobot, 1979) were said to suffer from
disturbances in menstrual function twice as frequently as a control
group. A low incidence of irritant and allergic dermatitis and
vitiligo was noted in workers exposed to acrylonitrile, methyl
acrylate, and dimethylformamide in fibre production (Bainova,
1975). In 11 out of 28 workers, delayed skin sensitization and
allergic dermatitis were observed with dimethylformamide.
Ostrovskaja et al. (1976) observed workers exposed to
acrylonitrile, acetonitrile, and hydrocyanic acid during training
and after 1.5 years and 3 years. In 190 men and women, aged 20 -
30 years, many signs and symptoms were noted including modified
reflexes, changes in blood pressure, ECG, and EEG. However, it is
difficult to attribute the findings of these authors solely to
acrylonitrile exposure.
9. EVALUATION OF HEALTH RISKS TO MAN FROM EXPOSURE TO ACRYLONITRILE
9.1. Sources and Levels of Exposure
Acrylonitrile is a colourless, volatile, chemically reactive
liquid; it does not occur as a natural product. The monomer is
used world-wide, on a large scale, in the manufacture of polymers,
fibres, and rubbers and as a chemical intermediate. Acrylonitrile-
containing polymers have been used in the manufacture of products
that come into contact with food; the amounts of acrylonitrile that
migrate into foods can be reduced to negligible quantities by the
use of good manufacturing practices in the production of the
polymers.
The major sources of contamination of the general environment
are acrylonitrile-producing and -polymerizing plants. The
occurrence of acrylonitrile in air, water, and soil near industrial
plants has been described. There is evidence that acrylonitrile
has persisted in soil for long periods following accidental
spillage; subsequent contamination of ground water has been
demonstrated.
The highest exposures occur in the workplace. Experience shows
that containment of such exposures can more readily be achieved in
production plants than in those in which acrylonitrile is used to
make other products. In a number of countries, exposure limits or
recommended limit values for the workplace have been arrived at;
values recently set have tended to be lower than in the past (Table
12).
Accidental exposure to acrylonitrile liquid and vapour may
occur during the various stages of production, transport, and use.
9.2. Acrylonitrile Toxicity
Inhaled acrylonitrile vapour is readily absorbed. Acute
systemic effects following absorption of vapour have been
described. Symptoms were non-specific and referable to the
gastrointestinal and respiratory tracts, the liver, and the central
nervous system. No acute adverse effects have been reported
following daily exposure (8-h) to up to 45 mg/m3.a At higher
concentrations rising to 220 mg/m3, 20-40 min exposure resulted
in complaints of headache, irritation of the upper respiratory
tract and the eyes, nervous irritability, and itching of the skin.
Fatalities have been reported following the use of fumigant
mixtures containing acrylonitrile together with carbon
tetrachloride and methylene chloride. Exact exposure conditions
are not known, but animal data suggest that inhalation exposure to
acrylonitrile at 500-2000 mg/m3 for 1/2-3 h could be fatal.
Simultaneous exposure to some organic solvents may enhance the
toxicity of acrylonitrile.
---------------------------------------------------------------------------
a This value was for many years the occupational exposure limit
in many countries where acrylonitrile was manufactured.
Table 12. Occupational exposure limits for selected countries
---------------------------------------------------------------
Country TWA mg/m3 STEL mg/m3 Reference
---------------------------------------------------------------
France 9 34*** INRS (1983)
Germany, Federal -* (S) DFG (1982)
Republic of
Hungary 0.5 0.5 Hungary (1979)
Japan 45 - Japan Association
of Industrial
Health (1972)
Poland Poland (1982)
Sweden 4** (S) 13 Sweden (1981)
United Kingdom
USA 4.5** (S) ACGIH (1982)
USSR 0.5 (S) USSR (1982)
---------------------------------------------------------------
* Proved animal carcinogen, strongly suspected of also being
carcinogenic for human beings. No safe concentration can be
listed.
TWA = Time-weighted average.
STEL = Short-term exposure limit.
** Listed as Industrial Substance Suspect of Carcinogenic
Potential for Man.
*** Alarm level.
S = Skin uptake can contribute to overall exposure.
Liquid acrylonitrile is also absorbed through the skin,
reportedly giving rise to non-specific symptoms similar to those
that follow acrylonitrile vapour inhalation. Local injury can
occur a few hours after exposure to liquid acrylonitrile. One
fatality has been reported. It is also an irritant to the eye.
Skin absorption of vapour does not appear to contribute
significantly to overall acrylonitrile uptake in the workplace.
There are no indications that acrylonitrile accumulates in the
body following prolonged exposure to levels found in the workplace.
Skin sensitization has been reported in a few cases; however,
no evidence is available to suggest the occurrence of pulmonary
allergic reactions.
Acrylonitrile has been shown to induce embryotoxic and
teratogenic effects at high dosage levels in experimental animals.
Although acrylonitrile is metabolized partly to cyanide, it has
been demonstrated that the acute toxic actions of acrylonitrile are
not solely due to cyanide, as was once believed.
Acrylonitrile has been shown to be mutagenic in some in vitro
systems in the presence of metabolic activation systems. So far,
mutagenic activity has not been demonstrated in in vivo assay
systems.
Complaints of ill health in workers exposed for a number of
years to acrylonitrile concentrations of less than 45 mg/m3 have
been reported in several studies. The complaints were variable in
nature and no consistent correlation with the extent of exposure
appears to have been established. The studies do not provide
evidence of a specific disease arising from long-term, low-level
exposure.
Several long-term studies in which acrylonitrile was
administered to rats orally and by inhalation demonstrated the
induction of malignant tumours. Data available in summary form
suggest that the incidence was dose-related. Eight epidemiological
studies have been carried out on workers exposed to acrylonitrile.
These studies have not demonstrated conclusively that there is a
correlation between exposure to acrylonitrile and the incidence of
cancer in man. Nevertheless, the findings are not incompatible with
the supposition that acrylonitrile is a potential human carcinogen
and thus give no cause for disregarding the evidence that has been
provided by animal studies.
It is not possible to establish a level below which no adverse
effects occur on the basis of the experimental and epidemiological
data presented in this document. However, it is evident that
exposure to acrylonitrile should be kept as low as possible in both
the workplace and the general environment, and that skin contact
with the liquid should be avoided.
REFERENCES
AARATO, S.E. & BITTERA, E. (1972) [Determination of
acrylonitrile in the air in the presence of dimethylformamide
and ammonia.] Munkavedelem, 18 : 50-51 (in Hungarian, Chem.
Abstr. 79 : 57280b, 1973).
ABREU, M.E. & AHMED, A.E. (1980) Metabolism of acrylonitrile
to cyanide: in vitro studies. Drug Metab. Disposition, 8 (6):
376-379.
ACGIH (1982) Threshold limit values for chemical substances
and physical agents in the work environment with intended
changes for 1982. Cincinnati, USA, American Conference of
Governmental Industrial Hygienists.
AGEEVA, S.T. (1970) [The condition of the metabolism of
mediating substances in workers producing acrylonitrile.] Tr.
Sarat. Med. Inst., 78 : 10-13 (in Russian).
AHMED, A.E. & ABREU, M.E. (1982) Microsomal metabolism of
acrylonitrile in liver and brain. Adv. exp. Med. Biol., 136
(Part B): 1229-1238.
AHMED, A.E. & PATEL, K. (1979) Studies on the metabolism of
aliphatic nitriles. Toxicol. appl. Pharmacol., 48 : A 91
(abstract).
AHMED, A.E. & PATEL, K. (1981) Acrylonitrile - in vivo
metabolism in rats and mice. Drug. Metab. Disposition, 9 (3):
219-222.
AHMED, A.E., FAROOQUI, M.Y.H., UPRETI, R.K., & EL-SHABRAWY,
O. (1982) Distribution and covalent interactions of (1-14C)
acrylonitrile in the rat. Toxicology, 23 : 159-175.
ALDRIDGE, W.N. (1944) A new method for the estimation of
micro quantities of cyanide and thiocyanate. Analyst, 69 :
262-265.
AL'SHANSKY, A.M., IVANOV, V.V., & PROMYSLOV, M.SH. (1980)
[Effect of acrylonitrile on content of Q-aminobutyric acid
glutamate decarboxylase activity and on lipid peroxidation in
rat brain tissue.] Vop. Med. Chimii, 26 (4): 507-509 (in
Russian).
AMERICAN CYANAMID COMPANY (1951) The chemistry of
acrylonitrile. In: Nitrogen chemicals digest, New York,
American Cyanamid Company, Vol. 5.
AMERICAN CYANAMID COMPANY (1959) The chemistry of
acrylonitrile, 2nd ed., New York, American Cyanamid Company,
272 pp.
AMERICAN CYANAMID COMPANY (1974) Handling storage - analyses
of acrylonitrile. Wayne, New Jersey, American Cyanamid
Company, 42 pp.
AMERICAN CYANAMID COMPANY (1976) Material safety data sheet;
Acrylonitrile, Wayne, New Jersey, American Cyanamid Company.
AMERICAN INDUSTRIAL HYGIENE ASSOCIATION (1970)
Acrylonitrile. Am. Ind. Hyg. Assoc. J.,: 529-531.
ANDO, K. & HASHIMOTO, K. (1967) [Effects of acrylonitrile
and acrylamide on peripheral nerve.] Rep. Osaka Prefectural
Inst. pub. Health, 4 : 23-27 (in Japanese).
ANONYMOUS (1977) Acrylo growth won't be bottled up. Chem.
Week, 9 March : 30-31.
ANTON'EV, A.A. & ROGAILIN, V.I. (1970) [Professional
dermatoses caused by acrylonitrile and their prophylaxis.] Tr.
Perm. Gos. Med. Inst., 99 : 103-106 (in Russian).
APPEL, K.E., PETER, H., & BOLT, H.M. (1981a) Effect of
potential antidotes on the acute toxicity of acrylonitrile.
Int. Arch. occup. environ. Health, 49 : 157-163.
APPEL, K.E., PETER, H., BOLT, M., & BOLT, H.M. (1981b)
Interaction of acrylonitrile with hepatic microsomes of rats
and man. Toxicol. Lett., 7 (4-5): 335-340.
ASTM (1981) Annual book of ASTM standards. Method D3371-79:
Testing for nitriles in aqueous solution by gas-liquid
chromatography, Philadelphia, American Society for Testing and
Materials.
BABANOV, G.P. (1957) [Local manifestation of the action of
acrylonitrile on the skin and mucosa.] Vrac. Delo, 5 : 511-514
(in Russian).
BABANOV, G.P., KLJUCHIKOV, V.N., KARAJEVA, N.I., & LILEEVA,
Z.V. (1959) [Clinical symptoms of chronic poisoning by
acrylonitrile.] Vrach. Delo, 8 : 833-836 (in Russian).
BAINOVA, A. (1975) [Assessment of the skin lesions in the
production of Bulana polyacrylonitrile fibers.] Dermatol.
Venerol., 14 (2): 92-97 (in Bulgarian).
BAKER, R.A. (1963) Threshold odors of organic chemicals. J.
Am. Water Works Assoc., 55 : 913-916.
BALDA, B.R. (1975) [Acrylonitrile as a contact allergen.]
Hautarzt, 26 : 599-601 (in German).
BANDT, H.J. (1953) [Acrylonitrile as an effluent.] Z.
Fisch., 2 : 457-461 (in German).
BARNES, J.M. (1970) Effects on rats of compounds related to
acrylamide. Br. J. ind. Med., 27 (2): 147-149.
BARRETT, W.J. (1974) Sampling and analysis of four organic
compounds using solid sorbents, Washington, US Department of
Health, Education and Welfare (Southern Research Institute
Report SORI-EAS-74-301).
BAXTER, R.A. (1979) Evaluation and control of industrial
exposure to acrylonitrile. Ann. occup. Hyg., 22 : 429-435.
BELILES, R.P., PAULIN, H.J., MAKRIS, N.G., & WEIR, R.J.
(1980) Three-generation reproduction study of rats receiving
acrylonitrile in drinking water. Final report. Litton
Bionetics Inc.
BELLAR, T.A. & SIGSBY, J.E., Jr (1970) Direct gas
chromatographic analysis of low molecular weight substituted
organic compounds in emissions. Environ. Sci. Technol., 4 :
150-156.
BENCHEV, I., RIZOV, N., & KOLARSKA, A. (1982) [Acrylonitrile
determination in biological fluids by gas chromatography -
"head space" analysis.] Hyg. Zdrav., 1 : 87-89 (in Bulgarian).
BENESH, V. & CHERNA, V. (1959) [Acrylonitrile. Acute
toxicity and mechanism of action.] J. Hyg. Epidemiol.
Microbiol. Immunol., 3 (1): 106-116 (in German and in Russian).
BENESH, V. & SHRAM, R. (1969) Mutagenic activity of some
pesticides in Drosophila melanogaster. Ind. Med. Surg.,
38 (12): 442-444.
BENSON, G.B. & BOYCE, G.E. (1981) A thermally desorbable
passive dosimeter for personal monitoring for acrylonitrile.
Ann. occup. Hyg., 24 (1): 55-76.
BENSON, G.B., BOYCE, G.E., & HAILE, D.M. (1981) Industrial
hygiene measurements for organic pollutants (acrylonitrile)
using passive dosimeters and automated thermal desorption.
Ann. occup. Hyg., 24 (4): 367-373.
BERCK, B. (1960) Retention of acrylonitrile and carbon
tetrachloride by shelled walnuts fumigated with acrylon. J.
Agric. food Chem., 8 :128-131.
BERG, G.L., ed. (1977) 1977 Farm Chemicals Handbook, 63rd
issue, Willoughby, Ohio, Meister Publishing Co., pp. D5, D50.
BIRD, W.L. & HALE, C.H. (1952) Polarographic determination
of acrylonitrile. Anal. Chem., 24 : 586-587.
BOLLINI, M., SEVES, A., & FOCHER, B. (1974) [Determination
of free monomers in water emulsions of synthetic polymers or
copolymers.] Ind. Carta, 12 : 234-240 (in Italian, Chem.
Abstr., 81 : 121672b).
BOLT, H.M., FILSER, J.G., & HINDERER, R.K. (1978) Rat liver
microsomal uptake and irreversible protein binding of
1,2-14C-vinyl bromide. Toxicol. appl. Pharmacol., 44 : 481-489.
BOND, E.J. & BUCKLAND, C.T. (1976) Control of insects with
fumigants at low temperatures: toxicity of mixtures of methyl
bromide and acrylonitrile to three species of insects. J.
econ. Entomol., 69 : 725-727.
BONDAREV, G.I., STASENKOVA, K.P., & VISSARIONOVA, V.Y.
(1976) [Protective effect of sulfur-containing compounds in
poisoning with acrylic acid nitrile.] Vopr. Pitan., (4) : 55-58
(in Russian).
BORCHARDT, VON K., FRANSEN, E., & HARTMAN, B. (1970) [Acute
oral intoxication with acrylonitrile in animals.] Z. Ges.
Hyg., 16 : 316-319 (in German).
BORG WARNER CHEMICALS (1977) In response to SHA's request
for information on occupational exposure to acrylonitrile.
Fed. Reg., 42 : 33043 (Letter to OSHA Docket Officer, US
Department of Labor).
BOYLAND, E. & CHASSEAUD, L.F. (1967) Enzyme-catalysed
conjugations of glutathione with carbonyl compounds. Biochem.
J., 104 : 95-102.
BOYLAND, E. & CHASSEAUD, L.F. (1968) Enzymes catalysing
conjugations of glutathione with A,B,-unsaturated carbonyl
compounds. Biochem. J., 109 : 651-661.
BREWER, W.E. (1976) 90-day subacute vapor inhalation
toxicity study with acrylonitrile in beagle dogs, albino rats
and albino mice (Industrial Biotest Report No. 74-42, prepared
for the Monsanto Company).
BRIEGER, H., RIEDERS, F., & HODES, A.B. (1952)
Acrylonitrile: spectrophotometric determination, acute
toxicity, and mechanism of action. Ind. Hyg. occup. Med., 6 :
128-140.
BRUCE, R.B., HOWARD, J.W., & HANZAL, R.F. (1955)
Determination of cyanide, thiocyanate and A-hydroxynitriles in
plasma or serum. Anal. Chem., 27 : 1346-1347.
BUFFONI, F., COPPI, C., & SANTONI, C. (1982) Determination
of thioethers in urine. Clin. Chem., 28 : 248-250.
CAMPBELL, D.N. & MOORE, R.H. (1979) The quantitative
determination of acrylonitrile, acrolein, acetonitrile and
acetone in workplace air. Am. Ind. Hyg. Assoc. J., 40 : 904-909.
CANADA FOOD & DRUGS ACT & REGULATIONS (1982) Division 23 -
Section B.23.008. Canada, Ministry of Supply & Services.
CARPENTER, C.P., SMYTH, H.F., & POZZANI, U.C. (1949) The
assay of acute vapour toxicity and the grading and
interpretation of results on 96 chemical compounds. J. ind.
Hyg. Toxicol., 31 : 343-346.
CHANDRA, H., GUPTA, B.H., BHARGAVA, S.K., CLERK, S.H., &
MAHENDRA, P.N. (1980) Chronic cyanide exposure - a
biochemical and industrial hygiene study. J. anal. Toxicol.,
4 : 161-165.
CHERNA, M., KOCHISOVA, J., KODYTKOVA, I., KOPECKY, J., &
SHRAM, R.J. (1981) Mutagenic activity of oxiranecarbonitrile
(glycidonitrile). In: Gut, I., Cikrt, M. & Plaa, G.L., ed.
Industrial & environmental xenobiotics, Berlin, Springer-
Verlag, pp. 251-255.
CHOBOT, A.M. (1979) [Menstrual function in workers of the
polyacrylonitrile fibre industry.] Zdrav. Beloruss., 2 : 24-27
(in Russian).
CIA (1978) Codes of practice for chemicals with major
hazards - acrylonitrile, Chemical Industries Association.
CINCOLELLA, A., VOIRIN, D., HECHT, G., GERBER, J.-M., DUCOS,
P., & LIMASSET, J.-C. (1981) Monitoring of atmospheric
concentrations of acrylonitrile in eleven French factories. G.
Ital. Med. lav., 3 : 165-167.
CLIVE, D. & SPECTOR, F.S. (1975) Laboratory procedure for
assessing specific locus mutagens at the TK locus in cultured
L5178Y mouse lymphoma cells. Mutat. Res., 31 : 17-29.
COLLINS, T.F.V. (1977) Written testimony in the matter of
acrylonitrile copolymers used to fabricate beverage containers
(Docket No. 76N-0070, exhibit G-82).
CRISP, S. (1980) Solid sorbent gas samplers. Ann. occup.
Hyg., 23 : 47-76.
CUPITT, L.T. (1980) Fate of toxic and hazardous materials in
the air environment. (EPA report No. EPA-600/3-80-084).
CZAJKOWSKA, T. (1971) [Excretion of certain metabolites upon
single administration of acrylonitrile.] Med. Pr., 22 (4):
381-385 (in Polish).
CZAJKOWSKA, T., KNOBLOCH, K., KOWALSKI, Z., KRYSIAK, B.,
PIOTROWSKI, J., ROGACZEWSKA, T., STARZYNSKI, Z., STASIK, M.,
SZENDZIKOWSKI, S., & ZWIERZCHOWSKI, Z. (1969) [Evaluation of
the toxicity and exposure of chemical industry workers to
acrylonitrile.] Med. Pr., (Supplement): 11-16 (in Polish).
DAHM, D.J. (1977) Identification of metabolites of
acrylonitrile. Testimony before the US FDA (Industrial Biotest
Report No. 74-42, Appendix 4: A.1-A.19, prepared for the
Monsanto Company).
DAUGHERTY, F.M. & GARRETT, J.T. (1951) Toxicity levels of
hydrocyanic acid and some industrial by-products. Texas J.
Sci., No. 3 : 391-396.
DAUES, G.W. & HAMNER, W.F. (1957) Determination of small
amounts of acrylonitrile in aqueous industrial streams. Anal.
Chem., 29 : 1035-1037.
DAVIS, J.H., DAVIES, J.E., AMERICO RAFONNELLI, R.S., & REICH,
G. (1973) Investigation of fatal acrylonitrile
intoxications. Pesticides and the environment, a continuing
controversy. Inter. Med. Book Corp., 2 : 547-556.
DELIVANOVA, S., POPOVSKI, P., & ORUSEV, T. (1978)
[Blepharoconjunctivitis in workers of the polyacrylonitrile
synthetic fibre industry.] God. Zb. Med. Fak. Skoje, 24 :
279-282 (in Macedonian).
DELLA FIORENTINA, H. & DE WIEST, F. (1979) Recherche d'un
indice biologique d'exposition chronique des travailleurs aux
dérivés cyanés. Arch. Mal. prof., 40 : 700-703.
DELZELL, E. & MONSON, R.R. (1982) Mortality among rubber
workers: VI. Men with potential exposure to acrylonitrile. J.
occup. Med., 24 (10): 767-769.
DE MEESTER, C., PONCELET, F., ROBERFROID, M., & MERCIER, M.
(1978b) Mutagenicity of acrylonitrile. Toxicology, 11 : 19-27.
DE MEESTER, C., DUVERGER-VAN BOGAERT, M., LAMBOTTE-VAN DEPAER,
M., ROBERFROID, M., PONCELET, F., & MERCIER, M. (1979) Liver
extract mediated mutagenicity of acrylonitrile. Toxicology,
13 (1): 7-15.
DFG (1982) Maximum concentrations at the workplace and
biological tolerance values for working materials 1982,
Weinheim, Verlag Chemie Gmbh, Commission for Investigation of
Health Hazards of Chemical Compounds in the Work Area,
Deutsche Forschungsgemeinschaft (Report No. 18).
DINU, V. (1975a) Activity of glutathione peroxidase and
catalase and the concentration of lipid peroxides in acute
intoxication with acrylonitrile. Rev. Roum. Biochim., 12 (1):
11-14.
DINU, V. (1975b) Intracellular thiol concentration in
acrylonitrile intoxication. Rev. Roum. Biochim., 12 (3):
155-158.
DINU, V. & KLEIN, R. (1976) Catalase activity and
glutathione and lactic acid concentrations in rats acutely
poisoned by acrylonitrile. J. Pharmacol., 7 (2): 223-226.
DJURIC, D., RAICEVIC, P., & KONSTANTINOVIC, I. (1962)
Excretion of thiocyanates in urine of smokers. Arch. environ.
Health, 5 : 12-15.
DORIGAN, J., FULLER, B., & DUFFY, R. (1976) Scoring of
organic air pollutants chemistry, production and toxicity of
selected synthetic organic chemicals (chemicals A-C), (MITRE
Tech. Rep. MTR-7248).
DORODNOVA, N.S. (1976) [Gynaecological morbidity and
specific functions of the female organism under the conditions
of chemical production in "Nitron".] Gig. Zabol., 8 : 45-46 (in
Russian).
DRAMANSKI, W. & TROJANOWSKA, B. (in press) Chromatographic
determination of S-cyanoethylmercapturic acid - a metabolite
of acrylonitrile in urine. Arch. Toxicol.
DUDLEY, H.C. & NEAL, P.A. (1942) Toxicology of acrylonitrile
(vinyl cyanide). I. Study of the acute toxicity. J. ind. Hyg.
Toxicol., 24 (2): 27-36.
DUDLEY, H.C., SWEENEY, T.R., & MILLER, J.W. (1942)
Toxicology of acrylonitrile (vinyl cyanide). II. Studies of
effects of daily inhalation. J. ind. Hyg. Toxicol., 24 :
255-258.
DUPONT (1977) In response to OSHA's request for information
on occupational exposure to acrylonitrile, letter to the OSHA
Docket Officer, US Department of Labour, Fed. Reg., 42 : 33043.
DUVERGER-VAN BOGAERT, M., LAMBOTTE-VANDEPAER, M., NOEL, G.,
ROBERFROID, M., & MERCIER, M. (1978) Biochemical effects of
ACN on the rat liver, as influenced by various pre-treatments
of the animals. Biochem. Biophys. Res. Comm., 83 : 1117-1124.
DUVERGER-VAN BOGAERT, M., LAMBOTTE-VANDEPAER, M., DE MEESTER,
C., ROLLMANN, B., PONCELET, F., & MERCIER, M. (1981) Effect
of several factors on the liver extract mediated mutagenicity
of acrylonitrile and identification of four new in vitro
metabolites. Toxicol. Lett., 7 : 311-319.
DUVERGER-VAN BOGAERT, M., LAMBOTTE-VANDEPAER, M., MERCIER, M.,
& PONCELET, F. (1982a) In vitro covalent binding of
acrylonitrile to rat liver proteins. Toxicol. Lett., 13 :
203-209.
DUVERGER-VAN BOGAERT, M., LAMBOTTE-VANDEPAER, M., DE MEESTER,
C., MERCIER, M., & PONCELET, F. (1982b) Role of glutathione
in liver-mediated mutagenicity of acrylonitrile. Toxicol.
Lett., 11 : 305-311.
EFREMOV, A.M. (1976a) [Study of the free radicals of the
blood, brain, liver and spleen of white rats subjected to
chronic acrylonitrile poisoning.] Zdrav. Beloruss., 6 : 86 (in
Russian).
EFREMOV, A.M. (1976b) [Study of the biotransformation of
acrylonitriles in animals.] Zdrav. Beloruss., 7 : 85-86 (in
Russian).
EFREMOV, A.M. (1976c) [State of protective adaptive
reactions in white rats poisoned with acrylonitrile.] Gig. Tr.
Ohr. Zdor. Rab. Neft. Neftehim. Prom-sti., 9 : 120-122 (in
Russian).
EFREMOV, A.M. (1976d) [Activity of catalase, peroxidase and
sulfhydryl groups of the brain, liver, and blood in white rats
poisoned with acrylonitrile.] Gig. Tr. Ohr. Zdor. Rab. Neft.
Neftehim. Prom-sti., 9 : 123-124 (in Russian).
ELLMAN, G.L. (1959) Tissue sulfhydryl groups. Arch.
Biochem. Biophys., 82 : 70-77.
ENIKEEVA, N.A., OSTROVSKAJA, R.S., SYSO, L.V., PODREZ, Z.G.,
BRAGINSKAJA, L.L., GVOZDEV, N.P., NESTEROVA, N.E.,
MUSSERSKAJA, A.N., SUZDALTREVA, N.A., & EFREMOV, A.M. (1976)
[Industrial hygiene and health status of workers involved in
the manufacture of the synthetic fibre Nitron.] Gig. Tr. Ohr.
Zdor. Rab. Nef. Neftehim. Prom-sti., No. 9 : 22-25 (in Russian).
EUROP-COST (1976) A comprehensive list of polluting
substances which have been identified in various fresh waters,
effluent discharges, aquatic animals and plants, and bottom
sediments, 2nd ed. Luxembourg, Commission of the European
Communities, p. 19 (EUCO/MDU/73/76, XII/476/76).
FAROOQUI, M.Y.G. & AHMED, A.E. (1982) Molecular interaction
of acrylonitrile and potassium cyanide with rat blood. Chem.
Biol. Interact., 38 : 145-149.
FERIN, J., URBANKOVA, J., & VLCHKOVA, A. (1961) [Hygienic
and toxicological problems of acrylonitrile and
dimethylformamide.] Prac. Lék., 13 : 466-468 (in Czech).
FRANKENBERG, L. (1980) Enzyme therapy in cyanide poisoning:
effect of rhodanese and sulfur compounds. Arch. Toxicol., 45 :
315-323.
FRANZEN, E. von & WAGNER, G. (1978) [Gelchromatographic and
electrophoretic serum protein pattern in rats after acute
acrylonitrile intoxication.] Z. Gesamte Hyg., 24 : 534-537 (in
German).
FREEMAN, R.A., SCHROY, J.M., KLIEVE, J.R., & ARCHER, S.A.
(1981) Experimental studies on the rate of air stripping of
hazardous chemicals from waste treatment systems. In: APCA
Meeting, Montreal, Canada, June 1980.
GAGNON, Y.T. & POSNER, J.C. (1979) Recovery of acrylonitrile
from charcoal tubes at low levels. Am. Ind. Hyg. Assoc. J.,
40 : 923-925.
GAWELL, G. B-M. (1979) Determination of acrylonitrile
monomer in plastic packaging and beverages by headspace gas
chromatography. Analyst, 104 : 106-110.
GHANAYEM, B.I. & AHMED, A.E. (1982) In vivo
biotransformation and biliary excretion of 1-14C acrylonitrile
in rats. Arch. Toxicol., 50 : 175-185.
GHERSIN, Z., STITZL, A., & MANEA, R. (1969) [Comparative
study of titrimetric and colorimetric methods for determining
acrylonitrile in waste water.] Rev. Chim. (Bucharest), 20 :
689-694 (in Rumanian, Chem. Abstr., 72 : 124853m).
GHIRINGHELLI, L. (1954) [Acrylonitrile: toxicity and
mechanism of action.] Med. lav., 45 (5): 305-312 (in Italian).
GINCHEVA, N., STAMOVA, N., HINKOVA, L., KYURKTCHIEV, S.,
SPASOVSKI, M., BAINOVA, A., MUHTAROVA, M., & HRISTEVA, V.
(1977) Study of the health status of workers from the
acrylonitrile department. In: Proceedings of the International
Symposium on Occupational Health in the Production of
Artificial Fibres - Abstracts, Finland, 6-10 June 1977, p. 41.
GOING, J.E., KUYKENDAHL, P., LONG, S., ONSTOT, J., & THOMAS,
K. (1979) Environmental monitoring near industrial sites:
acrylonitrile. 285 pp (EPA/560/6-79/003).
GRAHAM, J.P.D. (1965) Hydroxycobalamin as an antidote to
acrylonitrile. Toxicol. appl. Pharmacol., 7 : 367-372.
GRAVCZYK, J. & ZWIERZCHOVSKI, Z. (1973) [Effect of acute
experimental poisoning with acrylonitrile on the pressor
responses to adrenaline, noradrenaline and acetylcholine.]
Med. Pr., 24 (2): 151-158 (in Polish).
GREEN, M.L.H., MURIEL, W.J., & BRIDGES, B.A. (1976) Use of
simplified fluctuation test to detect low levels of mutagens.
Mutat. Res., 38 : 33-42.
GROTE, A.A., KIM, W.S., & KUPEL, R.E. (1978) Establishing a
protocol from laboratory studies to be used in field sampling
operations. Am. Ind. Hyg. Assoc. J., 39 : 880-884.
GRUNSKE, F. (1949) [Ventox and ventox-poisoning.] Dtsch.
med. Wochenschr., 74 : 1081 (in German).
GUENGERICH, F.P., GEIGER, L.E., HOGY, L.L., & WRIGHT, P.L.
(1981) In vitro metabolism of acrylonitrile to
2-cyanoethylene oxide, reaction with glutathione, and
irreversible binding to proteins and nucleic acids. Cancer
Res., 41 : 4925-4933.
GUERIN, M.R., OLERICH, G., & HORTON, A.D. (1974) Routine gas
chromatographic component profiling of cigarette smoke for the
identification of biologically significant constituents. J.
Chromatogr. Sci., 12 : 385-391.
GUT, I., NERUDOVA, J., KOPECKY, J., & HOLECHEK, V. (1975)
Acrylonitrile biotransformation in rats, mice, and chinese
hamsters as influenced by the route of administration and by
phenobarbital, SKF-525A, cysteine, dimercaprol, or
thisulphate. Arch. Toxicol., 33 : 151-161.
GUT, I., KOPECKY, J., & FILIP, J. (1981a) Acrylonitrile-14C
metabolism in rats: effect of the route of administration on
the elimination of thiocyanate and other radioactive
metabolites in urine and faeces. J. Hyg. Epidemiol. Microbiol.
Immunol., 25 (1): 12-16.
GUT, I., KOPECKY, J., & NERUDOVA, J. (1981b) Relationship
between acrylonitrile biotransformation, pharmacokinetics, and
acute toxicity. A short review. G. Ital. Med. lav., 3 : 131-136.
GUT, I., PELECH, L., VEJLUPKOVA, J., MATOUSEK, O., & KLENER,
P. (1982) [Effects of induction and inhibition of benzene
metabolism in hematopoiesis in male Wistar rats.] Cesk. Hyg.,
27 (1): 1-13 (in Czech).
GUT, I., NERUDOVA, J., FRANTIK, E., KOPECKY, J., HOLECHEK, V.,
STILBOROVA, A., MIRHEJOVSKA, E., & HOLUSHA, R. (in press)
Effect of acrylonitrile inhalation: Intermediary metabolism
and the excretion of thioethers and thiocyanate in rats. J.
Hyg. Epidemiol. Microbiol. Immunol, 28 : (in press).
HALL, M.E. & STEVENS, J.W., Jr (1977) Spectrophotometric
determination of acrylonitrile. Anal. Chem., 49 (14): 2277-2280.
HASHIMOTO, K. (1962) Studies on the intoxication of
acrylonitrile report IV. Effect of daily inhalation of
acrylonitrile on body weight, bodily activity and hemogram of
mice. Q. J. Labor. Res., 10 : 11-16.
HASHIMOTO, K. & ANDO, K. (1966) Effect of acrylonitrile on
the central and peripheral nervous system. In: Symposium on
Higher Nervous Functions & Occupational Health, pp. 87-89.
HASHIMOTO, K. & KANAI, R. (1965) Studies on the toxicology
of acrylonitrile, metabolism, mode of action and therapy. Ind.
Health, 3 (1-2): 30-45.
HASHIMOTO, K. & KANAI, R. (1972) Effect of acrylonitrile on
sulfhydryls and pyruvate metabolism in tissues. Biochem.
Pharmacol., 21 : 635-640.
HASHIMOTO, K. & KOBAYASI, T. (1961) A case of acute
dermatitis caused by contact with acrylonitrile. Q. J. Labor.
Res., 9 (1-4): 21-24.
HASKELL LABORATORY (1975) In vitro microbial mutagenicity
studies of acrylonitrile. (Report No. 351-357, E.I. Dupont de
Nemours & Co., Haskell Laboratory for Toxicology and
Industrial Medicine).
HASLAM, J. & NEWLANDS, G. (1955) Determination of
acrylonitrile in air. Analyst, 180 : 50-53.
HEINO, J., VILHUNEN, R., & HERNBERG, S. (1968) Urinary
thiocyanate concentrations in smokers and non-smokers.
Work-Environ.-Health, 5 : 12-15.
HENDERSON, C., PICKERING, G.H., & LEMKE, A.E. (1961) The
effect of some organic cyanides (nitriles) on fish. Proc. 15th
Ind. Waste Conf. En. Bull., 45 (2): 120-130.
HEUSER, S.G. & SCUDMORE, K.A. (1969) Determination of
fumigant residues in cereals and other foodstuffs: a
multi-detection scheme for gas chromatography of solvent
extracts. J. Sci. Food Agric., 20 : 566-572.
HOFFMANN, P., KLEINE, D., & FRANZEN, E. (1976) [On the proof
of acrylonitrile exposure: Detoxication of acrylonitrile by
coupling with D-glucuronic acid.] Z. Ges. Hyg., 22 (5): 310-312
(in German).
HOGAN, G.K. & RINEHART, W.E. (1980) A 24-month oral
toxicity/carcinogenicity study of acrylonitrile administered
by inhalation to Spartan rats. Bio/dynamics Inc. (Final report
Vols I & II, Project No. 77-1746).
HOLECHEK, V. & KOPECKY, J. (1981) Conjugation of glutathione
with acrylonitrile and glycidonitrile. In: Gut, I., Cikrt, M.
& Plaa, G.L., ed. Industrial and environmental xenobiotics.
Berlin, Springer Verlag, pp. 239-245.
HOUTHUIJS, D., REMIJN, B., WILLEMS, H., BOLEIJ, J., &
BIERSTEKER, K. (1982) Biological monitoring of acrylonitrile
exposure. Am. J. ind. Med., 3 (3): 313-320.
HROMOV, V.E. (1974) [Detection of circulating and fixed
antibodies in the diagnosis of allergies of chemical
etiology.] Vrach. Delo, 12 : 115-116 (in Russian).
HUGHES, T.W. & HORN, D.A. (1977) Source assessment:
acrylonitrile manufacture (air emission) (NITS Report. No.
PB-271 969).
HUMISTON, C.G. & FRAUSON, L.O. (1975) 90-day oral toxicity
study incorporating acrylonitrile in the drinking water of
rats. Midland, MI, Dow Chemical Toxicology Research
Laboratory, 99 pp.
HUNGARY (1979) Workplace requirements for air purity, Magyar
Nöpköztarsasagi Orszagos Szabvany, MSZ 21461-78 Az MSZ
21461-73 helyett G 23, 1979, 1 January.
IARC (1979) Acrylonitrile, acrylic and modacrylic fibres,
and acrylonitrile- butadiene-styrene and styrene-acrylonitrile
copolymers, pp. 73-113 (IARC Monographs on the Evaluation of
the Carcinogenic Risk of Chemicals to Humans, Vol. 19).
IARC (1982) IARC Monographs on the Evaluation of
Carcinogenic Risk of Chemicals to Humans, 1-29 (Suppl. 4) pp.
25-27.
IDOL, J.D., Jr (1974) Acrylonitrile polymers in prospect and
retrospect. In: Proceedings of the Applied Polymer Symposium
25: Acrylonitrile in Macromolecules, New York, John Wiley, pp.
1-18.
IMANARI, T., TANABE, S., TOIDA, T., SHINZO, T., & TOSHIHIKO,
T. (1982) Simultaneous determination of cyanide and
thiocyanate by high performance liquid chromatography. Chem.
Pharm. Bull. Japan, 30 (10): 3800-3802.
INRS (1983) Valeurs limités pour les concentrations des
substances dangereuses dans l'air des locaux de travail. Cah.
Notes Documen., No. 110, (ND 1410-110-83).
IVANOV, V.V. (1980) [Oxidative transformation of
acrylonitrile as a basis of its toxicological action.]
Farmakol. Toksikol., 43 (3): 383-384 (in Russian).
IVANOV, V.V. (1981) [Evaluation of the significance of
acrylonitrile metabolism in the mechanism of its toxicity.]
Gig. Tr. prof. Zabol., 9 : 48-50 (in Russian).
IVANOV, V.V. & AL'SHANSKY, A.M. (1982) GABA-ergic components
and lipid peroxidation during acute exogenous acrylonitrile
poisoning. Bull. exper. Biol. Med., 94 (7): 40-43.
IVANOV, V.V., GRIOHCHENKO, D.A., & KLIMATSKAJA, L.G. (1979)
[Blood serum enzymes in evaluating the nature of liver damage
and the effectiveness of chemotherapy following acrylonitrile
poisoning.] Vopr. Med. Him., 25 (6): 715-718 (in Russian).
IVANOV, V.V., KURNETSOVA, G.P., & ARCHAKOV, A.I. (1978)
[Stimulation of lipid peroxidation by acrylonitrile in rat
liver tissue.] Vopr. Med. Him., 24 (6): 816-818 (in Russian).
IVANOV, V.V., ZHISNOV, G.F., BACHMANOVA, G.I., MAZUROV, A.V.,
& ARCHALOV, A.I. (1979) [Interaction of acrylonitrile with
the microsomal oxidation system in rat liver tissue.] Vopr.
Med. Him., 25 (4): 468-471 (in Russian).
IVANOV, V.V., ZHIRNOV, G.F., & ARCHAKOV, A.J. (1982)
[Activation of acrylonitrile in the microsomal system of
oxidation.] Vopr. Med. Him., 28 (2): 95-98.
IWASAKI, K., KANNO, S., SAWATARI, K., SAWADA, S., SUGIMOTO,
M., SODA, R., TAKANO, T., NAKAMURA, K., MATSUMURA, Y., MYOJO,
T., SAKURAI, H., & TAKEMOTO, K. (1980) [Determination of
acrylonitrile in the working environments of a few kinds of
plants handling acrylonitrile.] Annual Report of the National
Institute of Industrial Health for 1979, p. 38 (in Japanese).
JACOBS, H.W. & SYRJALA, R.J. (1978) The use of infrared
analyzers for monitoring acrylonitrile. Am. Ind. Hyg. Assoc.
J., 39 : 161-165.
JACKSON, S. & BROWN, V.M. (1970) Effect of toxic wastes on
treatment processes and watercourses. Water Pollut. Control,
69 (3): 292-313.
JAEGER, R.J. (1978) Effect of hypoxia on the toxicity of
1,1-dichloroethylene or ACN. Toxicol. appl. Pharmacol., 45 :
257.
JAEGER, R.J. & COTE, I.L. (1982) Effect of hypoxia on the
acute toxicity of acrylonitrile. Res. Comm. Chem. Pathol.
Pharmacol., 36 (2): 345-348.
JAMES, S.P., WARING, R.H., & WHITE, D.A. (1967) Separation
of mercapturic acids and related compounds by gas
chromatography and a method for the determination of hippuric
acid. J. Chromatogr., 26 : 255-258.
JAPAN ASSOCIATION OF INDUSTRIAL HEALTH (1972) Recommendations
on permissible concentrations.
JEDLICKA, V., PASEK, A., & GOLA, J. (1958) Pesticides in
foods. III. Acrylonitrile as a food insecticide. J. Hyg. Epid.
Microbiol. Immunol., 1 : 116-125.
KANAI, R. & HASHIMOTO, K. (1965) Determination of
acrylonitrile, cyanide and thiocyanate in biological
materials. Ind. Health, 3 (47): 47-52.
KANKAANPÄÄ, E.E., HEMMINKI, K., & VAINIO, H. (1979)
Embryotoxicity of acrolein, acrylonitrile and acryl acid in
developing chick embryos. Toxicol. Lett., 4 : 93-96.
KIESSELBACH, N., KORALLUS, U., LANGA, H.J., NIESS, A., &
ZWINGERS, T. (1979) Acrylonitrile - Epidemiological Study,
Bayer 1977. Zentr. Arbeitsmed. Profyl., 29 (10): 257-259.
KILKIPARI, I. & SAVOLAINEN, H. (1982) Increased urinary
excretion of thioether in new rubber workers. Br. J. ind.
Med., 39 : 401-403.
KLESHCHEVA, M.S., USACHEVA, V.T., & POZHAROVA, V.N. (1971)
[Determination of residual monomers and non-polymerising
impurities in polystyrene plastics by means of gas
chromatography.] Plast. Mass., 7 : 57-58 (in Russian).
KNEZEVICH, A.L. & HOGAN, G.K. (1980) A 24-month oral
toxicity/carcinogenicity study of acrylonitrile administered
in the drinking water to Fischer 344 rats. Bio/Dynamics Inc.
(Final Report Vols I and IV, Project No. 77-1744, Bio-Dynamics
Inc.).
KNOBLOCH, K., SZENDZIKOWSKI, S., CZAJKOWSKA, T., & KRYSIAK,
B. (1971) [Experimental studies on acute and subacute
toxicity of acrylonitrile.] Med. Pr., 22 (3): 257-269 (in
Polish).
KNOBLOCH, K., SZENDZIKOWSKI, S., & CZAJKOWSKA, T. (1972)
[Chronic toxicity of acrylonitrile.] Med. Pr., 23 (3): 243-257
(in Polish).
KOLK, J.J. (1979) Inventory of employees exposed to
acrylonitrile in the Netherlands. Acrylonitrile Consultation
Group (Report of the Medical Sub-Group of the Vereniging van
de Nederlandse Chemische Industrie [VNCI]).
KOPECKY, J. (1982) Development and methodology for
assessment of urinary excretion of S-cyanoethylmercapturic
acid in man. (Final Report to the WHO Regional Office for
Europe, ICP/RCE 903).
KOPECKY, J., ZACHARDOVA, D., GUT, I., & FILIP, J. (1979)
[Metabolism of acrylonitrile in rats in vivo. ] Prac. Lék., 31 :
203-207 (in Czech).
KOPECKY, J., GUT, I., NERUDOVA, J., ZACHARDOVA, D., &
HOLECHEK, V. (1980a) Two routes of acrylonitrile metabolism.
J. Hyg. Epid. Microbiol. Immunol., 24 : 356-362.
KOPECKY, J., GUT, I., NERUDOVA, J., ZACHARDOVA, D., HOLECHEK,
V., & FILIP, J. (1980b) Acrylonitrile metabolism in the rat.
Arch. Toxicol., Suppl. 4 : 322-324.
KOPECKY, J., ZACHARDOVA, D., & GUT, I. (1980c) New findings
on acrylonitrile metabolism. Czech. Med., 3 : 295-301.
KOPECKY, J., GUT, I., NERUDOVA, J., ZACHARDOVA, D., &
HOLECHEK, V. (1981) Metabolic studies on acrylonitrile. In:
Gut, I., Cikrt, M. & Plaa, G.L., ed. Industrial and
environmental xenobiotics, Berlin, Springer-Verlag, pp.
221-231.
KORZHOVA, I.T., KLESHCHEVA, M.S., PORODINA, M.N., KORHOVA,
R.D., BALANDINA, V.A., & HOHLOV, V.A. (1974)
[Gas-chromatographic determination of toxic concentrations of
styrene and acrylonitrile vapours during the synthesis of
polystyrene plastics.] Plast. Mass., 5 : 67-69 (in Russian,
Chem. Abstr., 81 : 110759j).
KRIVOVA, V.N., USTINOVIC, I.P., & ERENBURG, B.E. (1982)
[Functional activity of the immune T-system due to
acrylonitrile poisoning.] In: Metabolicheskie aspecti
deistvija na organism industrial'nih himicheskih soedinenii,
Krasnojarsk, pp. 53-55 (in Russian).
KROELLER, E. (1970) [Residual acrylonitrile determination in
food.] Dtsch Lebensm. Rundsch., 66 : 11-33 (in German).
KRYNSKA, A. (1970) [Method of determination of acrylonitrile
in the air.] Pr. Central. Inst. Ochrony Pr., 20 : 303-310 (in
Polish, Chem. Abstr., 74 : 90798w).
KRYSIAK, B & KNOBLOCH, K. (1971) [Effect of acrylonitrile on
the central nervous system.] Med. Pr., 22 (6): 601-610 (in
Polish).
LAMBOTTE-VANDEPAER, M., DUVERGER-VAN BOGAERT, M., DE MEESTER,
C., PONCELET, F., & MERCIER, M. (1980) Mutagenicity of urine
from rats and mice treated with acrylonitrile. Toxicology, 16 :
67-71.
LAMBOTTE-VANDEPAER, M., DUVERGER-VAN BOGAERT, M., DE MEESTER,
C., ROLLMANN, B., PONCELET, F., & MERCIER, M. (1981a)
Identification of two urinary metabolites of rats treated with
acrylonitrile; influence of several inhibitors on the
mutagenicity of those urines. Toxicol. Lett., 7 : 321-328.
LAMBOTTE-VANDEPAER, M., DUVERGER-VAN BOGAERT, M., GARNY, V.,
PONCELET, F., & MERCIER, M. (1981b) Mutagenicity of
metabolites of acrylonitrile detected in urine. Mutat. Res.,
80 : 269 (abstract).
LANGVARDT, P.W., PUTZIG, C.L., BRAUN, W.H., & YOUNG, J.D.
(1980) Identification of the major urinary metabolites of
acrylonitrile in the rat. J. Toxicol. environ. Health, 6 :
273-282.
LAWTON, A.H., SWEENEY, T.A., & DUDLEY, H.C. (1943)
Toxicology of acrylonitrile (vinyl cyanide): III.
Determination of thiocyanate in blood and urine. J. ind. Hyg.
Toxicol., 25 : 13-19.
LEFAUX, R. (1966) [Chemistry and toxicology of plastics.]
Mainz, Krausskopf Verlag (in German).
LEONARD, A., GARNY, V., PONCELET, F., & MERCIER, M. (1981)
Mutagenicity of acrylonitrile in mouse. Toxicol. Lett., 7 :
329-334.
LINDGREN, D.L., VINCENT, L.E., & KROHNE, H.E. (1954)
Relative effectiveness of ten fumigants to adults of eight
species of stored product insects. J. econ. Entomol., 47 (5):
923-926.
LITTON BIONETICS (1975) Mutagenic evaluation of compounds
MCA 730 (LBI Project No. 2547, prepared for the Manufacturing
Chemists Association).
LITTON BIONETICS (1976) Mutagenicity evaluation of MCA 730
(Final report, LBI Project No. 2548, prepared for the
Manufacturing Chemists Association).
LODZ SANITARY INSPECTION SURVEY (1981) [Annual report on the
concentration of acrylonitrile in the air at workplaces in the
acrylic fibre plant "Chemitex-Anilana" at Lodz.] Lodz, Poland,
Sanitary-Epidemiological Station (in Polish).
LORZ, H. (1950) [On the percutaneous poisoning by
acrylonitrile (Ventox).] Dtsch med. Wochenschr., 75 (33/34):
1087-1088 (in German).
LOWENBACH, W., SCHLESINGER, S., & KING, J. (1978) Toxic
pollutant identification: acrylonitrile manufacture, 160 pp
(EPA-IMPQE series 02178-03).
MADDOCK, J.T., BYER, W.L., MALSEED, R.T., KERESZTESY, J.,
TAYLOR, T.J., & KATZ, A.B. (1977) Acrylonitrile (Monograph
prepared for US Consumer Product Safety Commission, Washington
DC).
MAGOS, L. (1962) A study of acrylonitrile poisoning in
relation to methemoglobin-CN complex formation. Br. J. ind.
Med., 19 : 283-286.
MALLETTE, F.S. (1943) Industrial hygiene in synthetic rubber
manufacture. Ind. Med., 12 : 495-499.
MALTONI, C., CILIBERTI, A., & DI MAIO, V. (1977)
Carcinogenicity bioassays on rats of acrylonitrile
administered by inhalation and by ingestion. Med. lav., 68 :
401-411.
MALTONI, C., CILIBERTI, A., & CARRETTI, D. (1982)
Experimental contributions in identifying brain potential
carcinogens in the petrochemical industry. Ann. N.Y. Acad.
Sci., 381 : 216-249.
MARANO, R.R., LEVINE, S.P., & HARVEY, T.M. (1978) Trace
determination of subnanogram amounts of acrylonitrile in
complex matrices by gas chromatography with a
nitrogen-selective detector. Anal. Chem., 50 (13): 1948-1951.
MARCOCI, S. & IONESCU, M. (1974) [The toxic action of
acrylonitrile on fish.] Strd. Prof. Calitatii Apelor, 16 : 7-22
(in Rumanian).
MARKELOV, M.A. & SEMENENKO, E.I. (1976) [Determination of
microconcentrations of substances migrating from polymer
materials to water.] Plast. Mass., 1 : 57-59 (in Russian, Chem.
Abstr., 84 : 155747g).
MAVRINA, E.A. & HROMOV, W.E. (1974) [Functional changes in
the body of workers engaged in production of nitron fibre.]
Vrach. Delo, 6 : 129-131 (in Russian).
MAVRINA, E.A. & IL'INA, V.A. (1974) [Change in the
immunological reactivity of the bodies of students of a
technical-trade school during training in a nitron production
plant.] Gig. i Sanit., 5 : 109-110 (in Russian).
MCLAUGHLIN, M., KRIVANEK, N.D., & TROCHIMOWICH, H.J. (1976)
Evaluation of antidotes for acrylonitrile poisoning (Abstr.).
Toxicol. appl. Pharmacol., 37 : 133-134.
MCNEAL, T. & BREDER, C.V. (1981) Head-space sampling and
gas-solid chromatographic determination of residual
acrylonitrile in acrylonitrile copolymer solutions. J. Assoc.
Off. Anal. Chem., 64 (2): 270-275.
MCNEAL, T. & BREDER, C.V. (1982) Manual headspace gas-solid
chromatographic determination of sub-parts-per-trillion levels
of acrylonitrile in 3% acetic acid. J. Assoc. Off. Anal.
Chem., 65 (1): 184-187.
MCOMIE, W.A. (1949) Comparative toxicity of
methacrylonitrile and acrylonitrile. J. ind. Hyg. Toxicol.,
31 : 113-116.
MILLER, L.M. & VILLAUME, J.E. (1978) Investigation of
selected potential environmental contaminants: acrylonitrile.
Washington DC, US EPA, 234 pp (Prepared for the Office of
Toxic Substances).
MILLS, E.J., Jr & STACK, V.T., Jr (1955) Acclimation of
microorganisms for the oxidation of pure organic chemicals.
In: Proceedings of the 9th Industrial Waste Conference Purdue
University, pp. 449-464.
MILWY, P. (1978) Letter to the editor. Reply to S. Venitt.
Mutat. Res., 57 : 110-112.
MILWY, P. & WOLFF, M. (1977) Mutagenic studies with
acrylonitrile. Mutat. Res., 48 : 271-278.
MINAMI, M., SATO, M., SUSUKI, A., YAMAMURA, K., & ISHIZU, S.
(1973) [Acrylonitrile poisoning. I. Result of exposure to
acrylonitrile vapour.] Tokyo Joshi Ika Daigaku Zasshi, 43 (10):
849-855 (in Japanese).
MLETZKO, H.G. von, & HENKEL, W. (1978) Animal experiments
with exposure to noise and acrylonitrile from the
chronobiological aspect. Z. Gesamte Hyg., 24 : 515-518.
MONSANTO (1975) Joint toxic action between acrylonitrile and
potassium cyanide. St Louis, MO, Monsanto Co. Medical
Department.
MONSANTO (1977a) Acrylonitrile sales specification, St
Louis, MO, Monsanto Chemicals Intermed. Co. (Monsanto data
sheet).
MONSANTO (1977b) In response to OSHA's request for
information on acrylonitrile (42 FR 34327) (Letter to the OSHA
Docket Officer, US Department of Labor).
MONSON, R.R. & FINE, L.J. (1978) Cancer mortality and
morbidity among rubber workers. J. Natl Cancer Inst., 61 (4):
1047.
MUHTAROVA, M. (1977) [Personal sample for evaluation of the
professional risk due to poisonings.] Hyg. Zdrav., 5 : 447-450
(in Bulgarian).
MURRAY, F.J., SCHWETZ, B.A., NITSCHE, K.D., JOHN, J.A.,
NORRIS, J.M., & GEHRING, P.J. (1978) Teratogenicity of
acrylonitrile given to rats by gavage or by inhalation. Food
cosmet. Toxicol., 16 (6): 547-551.
MURRAY, F.J., NITSCHKE, K.D., JOHN, J.A., SMITH, F.A., QUAST,
J.F., BLOGG, C.D., & SCHWETZ, B.A. (1976) Teratologic
evaluation of acrylonitrile monomer given to rats by gavage.
Midland MI, USA, Dow Toxicological Research Laboratory
(prepared for the Manufac. Chem. Assoc.).
NAKAMURA, K. (1981) [Mortality study of acrylonitrile
workers.] In: Annual Report of the National Institute of
Industrial Health for 1980, p. 31 (in Japanese).
NERUDOVA, J., HOLECHEK, V., GUT, I., & KOPECKY, J. (1980a)
[Relation between kinetics of acrylonitrile after different
routes of administration and its conversion to thiocyanate.]
Prakt. Lék., 32 : 15-18 (in Czech).
NERUDOVA, J., KOPECKY, J., GUT, I., & HOLECHEK, V. (1980b)
[Metabolism of acrylonitrile in the rats in vitro.] Prakt.
Lék., 32 : 98-103 (in Czech).
NERUDOVA, J., GUT, I., & KOPECKY, J. (1981) Cyanide effect
in acute acrylonitrile poisoning in mice. In: Gut, I., Cikrt,
M., & Plaa, G.L., ed. Industrial and environmental
xenobiotics, Berlin, Springer Verlag, pp. 245-250.
NILSEN, O.G., TOFTGARD, R., & ENEROTH, P. (1980) Effects of
acrylonitrile on rat liver cytochrome P-450, benz( a)pyrene
metabolism, and serum hormone levels. Toxicol. Lett., 6 :
399-404.
NIOSH (1976) NIOSH analytical methods for set K, Washington,
DC, NIOSH (NTIS Report No. PB-254 227).
NIOSH (1978) A recommended standard for occupational
exposure to acrylonitrile, Cincinnati, OH, Department of
Health, Education and Welfare (NIOSH), (Publ. No. 78-116).
NOEL, G., LAMBOTTE-VANDEPAER, M., DUVERGER-VAN BOGAERT, M.,
ROBERFROID, M., & MERCIER, M. (1978) Hepatotoxic effects of
acrylonitrile in rats. Arch. int. Physiol. Biochem., 86 (4):
951-952.
O'BERG, M.T. (1980) Epidemiologic study of workers exposed
to acrylonitrile. J. occup. Med., 22 (4): 245-252.
OOMENS, A.C. (1980) Experience with a dual detector
headspace gas chromatograph for acrylonitrile analysis. In:
Kolb, B., ed. Applied headspace gaschromatography, Heydon, pp.
111-116.
ORUSEV, T. & POPOVSKI, P. (1973) [Clinical symptoms in
workers occupationally exposed to acrylonitrile.] God. Zb.
Med. Fak. Skopje, 19 : 187-192 (in Macedonian).
ORUSEV, T., BAUER, S., & POPOVSKI, P. (1973) [Occupational
exposure to acrylonitrile in a plant for production of acrylic
synthetic fibres.] God. Zb. Med. Fak. Skopje, 19 : 445-449 (in
Macedonian).
OSHA (1978a) Emergency temporary and permanent standards for
occupational exposure to acrylonitrile (vinyl cyanide). Fed.
Reg., 43 : 2600, 4562.
OSHA (1978b) Permanent standard for exposure to acrylo-
nitrile. Fed. Reg., 43 : 45809-45817.
OSHA (1981) General Industry - Safety & Health Standards, 29
CFR 1910. US Department of Labor, pp. 830-850, 220 pp.
OSTROVSKAJA, R.S., PODREZ, Z.G., BRAGINSKAJA, L.L., BOKLOG,
E.P., EFREMOV, A.M., & VOLKOVA, G.A. (1976) [Health status
of workers currently engaged in the production of
acrylonitrile.] Gig. Tr. Prof. Zabol., 6 : 8-12 (in Russian).
PANOVA, R.V., BELOVA, R.A., & LAVRUSHINA, T.S. (1969)
[Chromatographic determination of residual monomers in
latexes.] Prom. Sin. Kauch. Nauch.-Tekh. Sb., 1 : 21-23 (in
Russian, Chem. Abstr., 74 : 43238d).
PARENT, R.A. & CASTRO, B.C. (1979) Effect of acrylonitrile
on primary Syrian golden hamster embryo cells in culture:
Transformation and DNA fragmentation. J. Natl Cancer Inst.,
62 (4): 1025-1029.
PARSONS, J.S. & MITZNER, S. (1975) Gas chromatographic
method for concentration and analysis of traces of industrial
organic pollutants in environmental air and stacks. Environ.
Sci. Technol., 9 : 1053-1058.
PATTERSON, R.M., BORNSTEIN, M.I., & GARSHICK, E. (1976)
Assessment of acrylonitrile as a potential air pollution
problem, Vol. VI (US Environmental Protection Agency, Contract
No. 68-02-1337, Task 8, Springfield, VA, National Technical
Information Service, No. PB-258 358).
PATTY, F.A., ed. (1963) Acrylonitrile, CH2=CHCN (vinyl
cyanide propenenitrile). In: Industrial hygiene and
toxicology, 2nd Revised ed., Vol. 2, pp. 2009-2012.
PAULET, G. & DESNOS, J. (1961) L'acrylonitrile,
toxicité-méchanisme d'action-thérapeutique. Arch. int.
Pharmacodyn., 131 (1-2): 54-83.
PAULET, G. & VIDAL, M. (1975) De la toxicité de quelques
esters acryliques et méthacryliques de l'acrylamide et des
polyacryliques. Arch. Mal. prof., 36 (1-2): 58-60.
PAULET, G., DESNOS, J., & BATTIG, J. (1966) De la toxicité
de l'acrylonitrile. Arch. Mal. prof., 27 (12): 849-856.
PEROCCO, P., PANE, G., BOLOGNESI, S., & ZANNOTTI, M. (1982)
Increase of sister chromatid exchange and unscheduled
synthesis of deoxyribonucleic acid by acrylonitrile in human
lymphocytes in vitro. Scand. J. Work Environ. Health, 8 :
290-293.
PETER, H. & BOLT, H.M. (1981) Irreversible protein binding
of acrylonitrile. Xenobiotica, 11 : 51-56.
PETER, H., APPEL, K.E., BERG, R., & BOLT, H.M. (1983)
Irreversible binding of acrylonitrile to nucleic acids.
Xenobiotica, 13 (1): 19-25.
PETROVA, L.I., NOSKOVA, M.P., BALANDINA, V.A., & GURICHEVA,
Z.G. (1972) [Polarographic determination of acrylonitrile in
aqueous extract of styrene- acrylonitrile copolymers.] Gig. i
Sanit., 37 : 62-65 (in Russian, Chem. Abstr., 76: 127898y).
PFEILSTICKER, K., INKMANN, K., & BOCHISCH, M. (1977)
Residues of (1-14C) ACN in fumigated dry food. Z.
Lebensmittel-Untersuch. Forsch., 164 (4): 274-276.
POLAND (1982) Decree of the Minister of Labour, Wages, and
Social Affairs, 22 December, 1982 (Low Gaz. No. 43, 29
December, 1982, Entry 287).
PONOMAREV, Y.P., ANUFRIEVA, T.L., & SHKORBATOVA, T.L. (1974)
[Polarographic determination of acrylonitrile in waste waters
using extraction.] Vodosnabz., Kanaliz. Gidroteh. Sooruz., 17:
67-70 (in Russian, Chem. Abstr., 82 : 34803x).
PORTMANN, J.K. & WILSON, K.W. (1971) The toxicity of 140
substances to the brown shrimp and other marine animals. 12 pp
(Ministry Agriculture Fisheries & Food Laboratories, England;
Shellfish Inf. Leafl. No. 22).
PUJADO, P.R., VORS, B.V., & KRUEDING, A.P. (1977) Newest
acrylonitrile process. Hydrocarbon Process, (May, 1977):
169-172.
QUAST, J.F., HUMISTON, C.G., SCHWETZ, B.A., FRAUSON, L.A.,
WADE, C.E., & NORRIS, J.M. (1975) A six-month oral toxicity
study incorporating acrylonitrile in the drinking water of
pure-bred beagle dogs. Midland MI, USA, Dow Toxicology
Research Laboratory, (prepared for the Manuf. Chem. Assoc.).
QUAST, J.F., ENRIQUEZ, R.M., WADE, C.E., HUMISTON, C.C., &
SCHWETZ, B.A. (1977) Toxicity and drinking water containing
acrylonitrile in rats: results after 12 months. Midland MI,
USA, Dow Toxicology Research Laboratory (prepared for the
Manuf. Chem. Assoc.).
QUAST, J.F., SCHUETZ, D.J., BALMER, M.F., GUSHOW, T.S., PARK,
C.N., & McKENNA, M.J. (1980a) A two-year toxicity and
oncogenicity study with acrylonitrile following inhalation
exposure of rats. Final Report. Midland MI, USA, Dow
Toxicology Research Laboratory.
QUAST, J.F., WADE, C.E., HUMISTON, C.G., CARREON, R.M.,
HERMANN, E.A., PARK, C.N., & SCHWETZ, B.A. (1980b) A
two-year toxicity and oncogenicity study with acrylonitrile
incorporated in the drinking water of rats. Final Report.
Midland MI, USA, Dow Toxicology Research Laboratory.
RABELLO-GAY, M.N. & AHMED, E. (1980) Acrylonitrile: in vivo
cytogenetic studies in mice and rats. Mut. Res., 79 : 249-255.
RAJENDRAN, S. & MUTHU, M. (1981) Detection of acrylonitrile
and ethylene oxide in air and fumigated foodstuffs. Bull.
environ. Contam. Toxicol., 27 : 426-431.
RAJENDRAN, S. & MUTHU, M. (1981) Effect of acrylonitrile on
trehalase, phosphorylase and acetylcholinesterase activities
in Tribolium castenlum Herbst and Trogoderma granarium Everts.
Experientia (Basel), 37 (8): 886.
RAMSTAD, T. & NICHOLSON, L.W. (1982) Determination of
sub-parts-per-billion levels of acrylonitrile in aqueous
solutions. Anal. Chem., 54 : 1191-1196.
REICHLE, A. & TENGLER, H. (1968) [Gas-chromatographic
procedures for determining residual monomers in the polymer.
Determination of acrylonitrile in polyacrylonitrile.]
Plastverarbeiter, 19 : 921-924 (in German, Chem. Abstr., 70 :
97206c).
ROGACZEWSKA, T. (1964) [Polarographic determination of
acrylonitrile in air.] Chem. Anal., 9 : 417-423 (in Polish).
ROGACZEWSKA, T. (1965) [Colorimetric method of acrylonitrile
determination in air.] Med. Pr., 16 (6): 465-475 (in Polish).
ROGACZEWSKA, T. (1971) The problems of obtaining the
evaluation of deleterious effects of acrylonitrile vapours in
industrial conditions. Thesis, Lodz, Poland, Institute of
Industrial Medicine.
ROGACZEWSKA, T. (1975) [Percutaneous absorption of
acrylonitrile vapours in animals.] Med. Pr., 26 (6): 459-465
(in Polish).
ROGACZEWSKA, T. (1976) [Determination of acrylonitrile in
air.] Med. Pr., 27 (2): 115-126 (in Polish).
ROGACZEWSKA, T. & PIOTROWSKI, J. (1968) [Routes of
acrylonitrile absorption in man.] Med. Pr., 19 (4): 349-353 (in
Polish).
ROSE, V.E. & PERKINS, J.L. (1982) Passive dosimetry - state
of the art review. Am. Ind. Hyg. Assoc. J., 43 : 605-621.
ROUDABUSH, R.L., TERHAAR, C.J., FASSETT, D.W., & DZIUBA, S.P.
(1965) Comparative acute effects of some chemicals on the
skin of rabbits and guinea-pigs. Toxicol. appl. Pharmacol., 7 :
559-565.
RUSSELL, J.W. (1975) Analysis of air pollutants using
sampling tubes and gas chromatography. Environ. Sci. Technol.,
9 : 1175-1178.
RUSSKIH, A.A. (1972) [Determination of acetone cyanohydrin
and acrylonitrile in air.] In: Bezhaev, M.S., ed. Proceedings
of the 4th Conference of People Employed in Universities and
Industrial Laboratories of South-east USSR on Physico-chemical
Methods of Analytical Control of Industrial Products, USSR,
Dagestan State University, Vol. 3, p. 100 (in Russian, Chem.
Abstr., 81 : 16301g).
RUSSKIH, A.A. (1973) [Photometric determination of
acrylonitrile in air.] Zavod. Lab., 39 (1): 5-6 (in Russian).
SAKURAI, H. & KUSUMOTO, M. (1972) [Epidemiologic study of
health impairment among acrylonitrile workers.] J. Sci. Labour
(Tokyo), 48 (5): 273-282 (in Japanese).
SAKURAI, H., ONODERA, M., UTSUNOMIYA, T., MINAKUCHI, H., IWAI,
H., & MATYUMURA, H. (1978) Health effects of acrylonitrile
in acrylic fibre factories. Br. J. ind. Med., 35 (3): 219-225.
SANDBERG, E.C. & SLANINA, P. (1980) Distribution of (1-14C)
acrylonitrile in rat and monkey. Toxicol. Lett., 6 : 187-191.
SAPOTA, A. (1982) The disposition of 14C-acrylonitrile in
rats. Xenobiotica, 12 (4): 259-264.
SAPOTA, A. & CHMIELNICKA, J. (1981) [Toxicity and metabolism
of acrylonitrile.] Med. Pr., 32 (1): 25-33 (in Polish).
SAPOTA, A. & DRAMINSKI, W. (1981) The fate of
14C-acrylonitrile in rats. In: Gut, I., Cikrt, M., & Plaa,
G.L., ed. Industrial and environmental xenobiotics, Berlin,
Springer Verlag, pp. 231-237.
SARTORELLI, E. (1966) [Acute acrylonitrile intoxication.]
Med. lav., 57 (3): 184-187 (in Italian).
SATO, M. (1978) [Studies on the toxic effect of
acrylonitrile - its metabolism, absorption and excretion.]
Jpn. J. Hyg., 33 (3): 497-505 (in Japanese).
SATO, M., ISHIZU, S., & MOMOTANI, H. (1975) [Determination
of acrylonitrile, cyanide and thiocyanate in blood and urine.]
Sangyo Igaku, 17 : 99-105 (in Japanese, Chem. Abstr., 100292z).
SATO, M., HIRASAWA, F., OGATA, M., TAKIZAWA, Y., KOJIMA, H., &
YOSHIDA, T. (1982) Distribution and accumulation of
(2,3-14C) acrylonitrile in rat after single injection.
Ecotoxicol. environ. Safety, 6 (5): 489-494.
SATO, S., KOSHIO, H., MIMURA, M., NAKAMURA, S., & ICHIMURA,
M. (1979) Investigation in counter-measure to the sources of
unspecified harmful chemicals in 1978. pp. 1-19 (in Japanese,
Report of the study sponsored by the Environmental Agency).
SCHEUFLER, H. von (1976) Experimental testing of chemical
agents for embryotoxicity, teratogenicity and mutagenicity -
ontogenic reactions of the laboratory mouse to these
injections and their evaluation - a critical analysis method.
Biol. Rundsch., 14 (4): 227-229.
SCHEUFLER, H. von (1980) [On the embryotoxic effectiveness
of acrylonitrile in the laboratory mouse.] Z. Gesamte Hyg.,
26 : 564-565 (in German).
SCUPAKAS, D. (1968) [Industrial hygiene conditions during
the reworking of plastics and problems improving them.] In:
Yavnaist, E.Y., ed. Proceedings of the first conference on
actual problems of industrial hygiene and occupational
pathology, 1967, Riga, Medical Institute, pp. 128-132 (in
Russian, Chem. Abstr., 72 : 47094k).
SEKI, S. (1967) [On acrylonitrile poisoning in a synthetic
resin plant.] Jpn. J. Hyg., 22 (1): 257 (in Japanese).
SEUTTER-BERLAGE, F., VAN DORP, H.L., KOSSE, H.G.J., &
HENDERSON, P.T. (1977) Urinary mercapturic acid excretion as
a biological parameter of exposure to alkylating agents. Int.
Arch. occup. environ. Health, 39 : 45-51.
SEUTTER-BERLAGE, F., DELBRESSINE, L.P.C., SMEETS, F.L.M., &
KETELAARS, H.C.J. (1978) Identification of three
sulfur-containing urinary metabolites of styrene in the rat.
Xenobiotica, 8 : 413-418.
SHACKELFORD, W.M. & KEITH, L.H. (1976) Frequency of organic
compounds identified in water, Athens GA, USA, US
Environmental Protection Agency, p. 55 (EPA-600/4-76-062).
SHEVCHIK, J. (1976) Detectors in gas chromatography,
Amsterdam, Elsevier, 192 pp.
SHUSTOV, V.J. (1968) [Clinical manifestation and
hematological changes in workers employed in the production of
acrylonitrile at the Saratov Chemical Combine.] Gig. Tr. prof.
Zabol., 3 : 27-29 (in Russian).
SHUSTOV, V.Y. & MAVRINA, E.A. (1975) [Clinical picture of
chronic poisoning in the production of nitrone.] Gig. Tr.
prof. Zabol., 3 : 27-29 (in Russian).
SILVER, E.H. & SZABO, S. (1982) Possible role of lipid
peroxidation in the action of acrylonitrile on the adrenals,
liver and gastrointestinal tract. Res. Comm. Chem. Pathol.
Pharmacol., 36 (1): 33-43.
SILVER, E.H., KUTTAB, S.H., HASAN, T., & HASSAN, M. (1982a)
Structural considerations in the metabolism of nitriles to
cyanide in vivo. Drug Metab. Disposition, 10 : 495-498.
SILVER, E.H., McCOMB, D.J., KOVACS, K., & SZABO, S. (1982b)
Limited hepatotoxic potential of acrylonitrile in rats.
Toxicol. appl. Pharmacol., 64 : 131-139.
SILVERSTEIN, L.G. (1977) Calidation of abcor GASBADGE for
acrylonitrile and improved desorption efficiency. Am. Ind.
Hyg. Assoc. J., 38 (8): 412-413.
SKVOSTSOV, Ju.I. (1980) [Variation in the amino acid
spectrum of blood serum in workers employed in ACN production
and nutritive ways of its correction. Vopr. Pitan., No. 2 :
31-33 (in Russian).
SLATER, E.E., ANDERSON, M.D., & ROSENKRANZ, S. (1971) Rapid
detection of mutagens and carcinogens. Cancer Res., 31 (7):
970-973.
SLOOF, W. (1979) Detection limits of a biological monitoring
system based on fish respiration. Bull environ. Contam.
Toxicol., 23 : 517-523.
SMYTH, H.F. & CARPENTER, C.P. (1948) Further experience with
the range-finding test in the industrial toxicology
laboratory. J. ind. Hyg. Toxicol., 30 : 63-68.
SOKAL, J.A. & KLYSZEJKO-STEFANOWICZ, L. (1972)
[Nicotinamide-adenine dinucleotides in acute poisoning with
some toxic agents.] Lodz Tow. Nauk., No. 112: 64-101 (in
Polish, Chem. Abstr., 79 : 62271d).
SOKAL, J.A., KNOBLOCH, K., MAJKA, J., SAPOTA, A., &
SZENDZIKOWSKI, S. (1977) [Toxicity of acrylate compounds
used in industry.] Prakt. Lek., 29 (4): 157-161 (in Czech).
SPASSOVSKI, M. (1976) Health hazards in the production and
processing of some fibres, resins, and plastics in Bulgaria.
Environ. Health Perspect., 17 : 199-202.
STAMOVA, N., GINCHEVA, N., SPASOVSKI, M., BAINOVA, A.,
IVANOVA, S., KURKCHIEV, S., HINKOVA, L., KHRISTEVA, V.,
MUKHTAROVA, M., KARADZHOVA, N., TSANEVA, L., & FIKOVA, E.
(1976) [Labour hygiene during the production of synthetic
fibres "Bulana".] Hyg. Zdrav., 2 : 134-140 (in Russian).
STANFORD RESEARCH INSTITUTE (1976) Interim Report: In vitro
microbiological mutagenicity studies of Dow Chemical Company
Compounds (SRI Project No. LSC-4378, prepared for Dow Chemical
Company).
STEICHEN, R.J. (1976) Modified solution approach for the gas
chromatographic determination of residual monomers by
head-space analysis. Anal. Chem., 48 : 1398-1402.
SUTA, B.E. (1979) Assessment of human exposures to
atmospheric acrylonitrile (Prepared by the Stanford Research
Institute - Contract 68-02-2835-EPA).
SVIRBELY, J.L. & FLOYD, E.P. (1961) Toxicologic studies of
acrylonitrile and B,B'-oxydipropionitrile. III. Chronic
studies. Meeting paper AINA-ACSIH, Detroit MI, USA.
SWEDEN (1981) Ordinance issued by the National Swedish Board
of Occupational Safety and Health Concerning Hygiene Limit
Values (in Swedish).
SZABO, S. & SELYE, H. (1971) Adrenal apoplexy and necrosis
produced by acrylonitrile. Endocrinol., 57 (3): 405-408.
SZABO, S. & SELYE, H. (1972) Effects of phenobarbital and
steroids on the adrenal apoplexy produced by acrylonitrile in
rats. Endocrinol. Exp., 6 (3): 141-146.
SZABO, S., REYNOLDS, E.S., KOMANICKY, P., MOSLEN, M.T., &
MELBY, J.C. (1976) Effect of chronic acrylonitrile ingestion
on rat adrenal. Toxicol. appl. Pharmacol., 37 (1): 133.
SZABO, S., BAILEY, K.A., BOOR, P.J., & JAEGER, R.J. (1977)
Acrylonitrile and tissue glutathion - differential effect of
acute and chronic interactions. Biochem. Biophys. Res.
Commun., 79 (1): 32-37.
SZABO, S., HUTTNER, I., KOVACS, K., HORVATH, E., SZABO, D., &
HORNER, H.C. (1980) Pathogenesis of experimental adrenal
hemorrhagic necrosis ("apoplexy"). Ultrastructural,
biochemical, neuropharmacologic and blood coagulation studies
with acrylonitrile in the rat. Lab. Invest., 42 (5): 533-545.
TADA, O. (1971) [Methods for determinations of toxic
substances in air-organic compounds.] J. Sci. Lab., (Part II),
47 : 789-796.
TAKAGI, N., TAKAGI, K., MATSUDA, T., & KOIZUMI, S. (1968)
[Effect of administration of vitimins B1 or B2 plus L-cysteine
to rats exposed to acrylonitrile gas over a long period.] J.
Sci. Lab., 44 (8): 454-464 (in Japanese).
TARKOWSKI, S. (1966) [Effect of cobalt on the inhibition by
potassium cyanide of cytochrome oxidase activity.] Med. Pr.,
17 (2): 116-119 (in Polish).
TARKOWSKI, S. (1968) [Effect of acrylonitrile on certain
properties of cytochrome oxidase.] Med. Pr., 19 (6): 525-531
(in Polish).
TATSUNO, T., INOUE, T., & TANIMURA, A. (1979) [Hygienic
chemical studies on plastics (II). Residual acrylonitrile in
ABS resin or AS resin and its migration into water.] Report of
the National Institute of Hygiene, 97 : 93-97 (in Japanese).
TATSUNO, T., INOUE, T., & TANIMURA, A. (1980) [Hygienic
chemical studies on plastics (V). Migration of acrylonitrile
from acrylonitrile-butadiene-styrene copolymer and
acrylonitrile-styrene copolymer into various food simulating
solvents in long period.] Report of the National Institute of
Hygiene, 98 : 110-115 (in Japanese).
TERENT'EV, A.P. & OBTEMPERANSKAJA, S.I. (1956) [Quantitative
determination of acrylonitrile with sodium sulfite.] Z. Anal.
Him., 11 : 638-639 (in Russian).
THIESS, A.M. & FLEIG, I. (1978) Analysis of chromosomes of
workers exposed to acrylonitrile. Arch. Toxicol., 41 (2):
149-152.
THIESS, A.M., FRENTZEL-BEYME, R., LINK, R., & WILD, H.
(1980) [Mortality study in chemical workers in different
production plants with exposure also to acrylonitrile.]
Zentralbl. Arbeitsmed. Arbeitsschutz Prophyl. Ergonomie,
30 (7): 259-267 (in German).
TULLAR, P.E. (1947) Final report on the pharmacology and
toxicology of acrylonitrile and acrylon. Washington,
Palusowski Memorial Research Laboratory, George Washington
University.
UHDE, W.J. & KOEHLER, U. (1967) [Testing of plastic
articles. Determination of monomeric compounds in the volatile
fraction of styrene polymers.] Z. Lebensm. Unters.-Forsch.,
135 : 135-140 (in German).
UNION CARBIDE CORPORATION (1977) In response to OSHA's
request for information on occupational exposure to
acrylonitrile. Fed. Reg., 42 : 33042 (Unpublished letter to the
OSHA Docket Officer, US Department of Labor).
UNITED KINGDOM HEALTH & SAFETY EXECUTIVE (1981a)
Acrylonitrile in air. Laboratory method using charcoal
adsorption tubes and gas chromatography. Methods for the
Determination of Hazardous Substances, 1, March 1981.
UNITED KINGDOM HEALTH & SAFETY EXECUTIVE (1981b)
Acrylonitrile in air. Laboratory method using porous polymer
adsorption tubes, and thermal desorption with gas
chromatographic analysis. Methods for the Determination of
Hazardous Substances, 2, March 1981.
UNITED KINGDOM MINISTRY OF AGRICULTURE, FISHERIES & FOOD
(1982) Survey of acrylonitrile and methacrylonitrile in food
contact materials and in food, London, HMSO (Food Surveillance
Paper No. 6).
US CONSUMER PRODUCT SAFETY COMMISSION (1978) Assessment of
acrylonitrile contained in consumer products. Washington, DC
(Final report, contract No. CPSC-C-77-0009. Task No. 1014K,
H.I.A. Economic Analysis).
US DEPARTMENT OF TRANSPORTATION (1974) Acrylonitrile. In:
CHRIS hazardous chemical data, Department of Transportation,
Coast Guard, C-G-446-2.
US EPA (1977) Optional procedures for classification of
pesticide uses by regulation. Fed. Reg., 42 : 44170, 44178.
US FDA (1977a) Acrylonitrile copolymer used to fabricate
beverage containers, final decision. Fed. Reg., 42 :
48528-48544.
US FDA (1977b) Substances for use only as components of
adhesives. US Code Fed. Regul., Title 21, parts 175.105,
175.300, 175.320, 176.170, 176.180, 177.1010, 177.1020,
177.1040, 177.1200, 177.2600, 178.3790, 180.22, pp. 438, 445,
452, 455, 465, 471-472, 482-483, 486, 488, 496, 501-504, 555,
596, 609-611.
USSR (1982) System of standards of work safety. The air of
the working zone. General sanitary-hygienic requirements (GOST
12.1.005-76). MAC of harmful substances in the air of working
zone (Suppl. to Tab. 4, SN 245-71, No 7-11). MAC of harmful
substances in the air of working zone (part 12-19), confirmed
in 1977-1982 years.
VAINIO, M. & MAKINEN, M. (1977) Styrene and acrylonitrile
induced depression of hepatic nonprotein sulfhydryl content in
various rodent species. Res. Commun. chem. Pathol. Pharmacol.,
17 (1): 115-124.
VAN BLADEREN, P.J., DELBRESSINE, L.P.C., HOOGETERP, J.J.,
BEAUMONT, A.H.G.M., BREIMER, D.D., SEUTTER-BERLAGE, F., & VAN
DER GEN, A. (1981) Formation of mercapturic acids from
acrylonitrile, crotononitrile, and cinnamonitrile by direct
conjugation and via an intermediate oxidation process. Drug
Metab. Disposition, 9 : 246-249.
VAN DOORN, R., BOS, R.P., LEIJDEKKERS, C.-M., WAGENAAS-ZEGERS,
M.A.P., THEUWS, J.L.G., & HENDERSON, P.T. (1979) Thioether
concentration and mutagenicity of urine from cigarette
smokers. Int. Arch. occup. environ. Health, 43 : 159-166.
VED BRAT, S. & WILLIAMS, G.M. (1982) Hepatocyte-mediated
production of sister chromatid exchanges in co-cultured cells
by acrylonitrile: evidence for extra cellular transport of a
stable reactive metabolite. Cancer Lett., 17 (2): 213-216.
VENITT, S., BUSHELL, C.T., & OSBORNE, M. (1977) Mutagenicity
of acrylonitrile (cyanoethylene) in Escherichia coli. Mutat.
Res., 45 (2): 283-288.
VERSCHUEREN, K. (1977) Handbook of environmental data on
organic chemicals, Van Nostrand Reinhold, pp. 78-81.
VISSARIONOVA, V.U., BONDAREV, G.I., STASENKOVA, K.P., &
ZEMLJANSKAJA, T.A. (1979) [Study of the effect of rations
with different protein content on rats following acrylonitrile
poisoning.] Vopr. Pitan., (1) : 36-40 (in Russian).
VOLKOVA, Z.A. & BAGDINOV, Z.M. (1969) [Industrial hygiene
problems in vulcanization processes of rubber production.]
Gig. i Sanit., 34 : 33-40 (in Russian).
WERNER, J.B. & CARTER, J.T. (1981) Mortality of United
Kingdom acrylonitrile polymerisation workers. Br. J. ind.
Med., 38 : 247-253.
WILLHITE, C.C. (1981a) Inhalation toxicology of acute
exposure to aliphatic nitriles. Clin. Toxicol., 18 (8):
991-1003.
WILLHITE, C.C. (1981b) Teratogenic effects of aliphatic
nitriles. Teratology, 23 : 317-323.
WILLHITE, C.C. & SMITH, R.P. (1981) The role of cyanide
liberation in the acute toxicity of aliphatic nitriles.
Toxicol. appl. Pharmacol., 59 : 589-602.
WILSON, R.H. (1944) Health hazards encountered in the
manufacture of synthetic rubber. J. Am. Med. Assoc., 124 :
701-703.
WILSON, R.H. & McCORMICK, W.E. (1949) Acrylonitrile: its
physiology and toxicology. Ind. Med., 18 : 223-245.
WILSON, R.H., HOUGH, G.V., & McCORMICK, W.E. (1948) Medical
problems encountered in the manufacture of American-made
rubber. Ind. Med., 17 : 199-207.
WISNIEWSKA-KNYPL, J.M., KNOBLOCH, J.M., JABLONSKA, J., & RUTA,
U. (1970) [Decrease of tissue respiration activity of
oxoglutarate dehydrogenase and level of sulfhydryl groups in
acute acrylonitrile intoxication in rats.] Med. Pr., 21 (6):
543-549 (in Polish).
WISNIEWSKA-KNYPL, J.M., TARKOWSKI, S., KLIMZAK, J., &
DRAMINSKI, W. (1978) [ Metabolism of vinyl chloride in the
liver of rats.] (in Polish) (Final report on the research
project of the Institute of Occupational Medicine in Lodz,
Poland).
WRIGHT, P.L. (1977) Studies on the metabolism of
acrylonitrile. Testimony before the FDA. In: Monsanto Co.
(1976b) Appendix A, pp. A-20 - A-28.
WYNDER, E.L. & HOFFMANN, D. (1967) Tobacco and tobacco
smoke. Studies in experimental carcinogenesis. New York,
Academic Press, p. 450.
YOSHIKAWA, H. (1968) [Toxicity of nitrile compounds. II.
Aliphatic nitriles.] Igak. Scibut., 77 (1): 1-4 (in Japanese).
YOUNG, J.D., SLAURER, R.W., & KARBOWSKI, R.J. (1977) The
pharmacokinetic and metabolism profile of 14 C-acrylonitrile
given to rats by three routes. Midland MI, USA, Toxicol. Res.
Lab. Dow Chem., (prepared for the Manufacturing Chem. Assoc.).
ZAYTSEVA, N.A., TOLSTOBROVA, S.A., & IL'IN, D.T. (1972)
[Chromatographic determination of unreacted monomers in
emulsions of acrylic copolymers.] Soviet Chem. Ind., 48 :
298-299 (in Russian).
ZELLER, H., HOFMANN, H.T., THIESS, A.M., & HEY, W. (1969)
[Toxicity of nitriles. Results of animal experiments and
industrial experiences during 15 years.] Zent. Arbeitsmed.
Arbeitsschutz, 19 (8): 226-238 (in German).
ZHURKOV, V.S., SHRAM, R.L. & DUGAN, A.M. (1983) [Analysis of
mutagenic activity of acrylonitrile.] Gig. i. Sanit., 1 : 71-72
(in Russian).
ZITTING, A., TENHUNEN, R., & SAVOLAINEN, H. (1981) Effects
of intraperitoneally injected acrylonitrile on liver, kidney
and brain. Acta pharmacol. toxicol., 49 : 412-415.
ZOTOVA, L.V. (1975a) [Working conditions in the production
of acrylonitrile and their effect on workers.] Gig. Tr. prof.
Zabol., No. 8 : 8-11 (in Russian).
ZOTOVA, L.V. (1976) [Toxic effect of acrylonitrile on
experimental animals following its absorption through the
skin.] Gig. i Sanit., 41 (10): 103-105 (in Russian).
 5.3.2.1. The oxidative pathway of acrylonitrile metabolism
The oxidative pathway of acrylonitrile biotransformation
includes a number of consecutive enzyme-catalyzed or spontaneous
reactions. The first step, oxidation of acrylonitrile to
glycidonitrile, is catalyzed by hepatic microsomal mono-oxygenases
(Abreu & Ahmed 1980; Kopecky et al., 1980a,b; Guengerich et al.,
1981; Ahmed & Abreu, 1982). Glycidonitrile is a reactive
intermediate, and a number of its metabolites have been recorded;
in in vitro experiments it is transformed by epoxide hydrolase (EC
3.3.2.3) to glycolaldehyde cyanohydrin, which decomposes
spontaneously to hydrocyanic acid (cyanide) and glycoealdehyde
(Kopecky et al., 1979, 1980a,b; Abreu & Ahmed, 1980; Duverger-van
Bogaert, 1981a). The yield of cyanide in the in vitro experiments
depends on the techniques used (Nerudova et al., 1980b). Besides
forming conjugation products with glutathione (section 5.3.2.2),
glycidonitrile rearranges to cyanoacetaldehyde, which is further
reduced to 2-cyanoethanol or oxidized to cyanoacetic acid. Acetic
acid is also present (Duverger-van Bogaert, 1981).
The results of animal studies have shown that cyanide formed in
vivo is subsequently converted by rhodanese (EC 2.8.1.1) to
thiocyanate and eliminated in urine (e.g., Dudley & Neal, 1942;
Brieger et al., 1952; Ahmed & Patel, 1981). Thiocyanate has been
directly measured in the urine of various animals after
acrylonitrile administration (Lawton et al., 1943; Mallette, 1943;
Czajkowska, 1971; Efremov, 1976b). Rats administered acrylonitrile
at 60 mg/kg body weight, excreted thiocyanate in the urine at a
constant rate of 0.53 mg/h with an excretion half period of 13 h
(Czajkowska, 1971). Sulfhydryl compounds (cysteine, BAL, and
Unithiol) increase the activity of rhodanese in the conversion of
cyanide to thiocyanate in vitro, as well as in vivo (e.g.,
Frankenberg, 1980). A similar increase with acrylonitrile has not
been convincingly demonstrated (Gut et al., 1975), perhaps because
of the inhibiting properties of acrylonitrile on rhodanese.
5.3.2.2. Mercapturic acids formed in acrylonitrile biotransformation
Cyanoethylation of naturally-occurring sulfhydryl compounds
plays an important role in acrylonitrile metabolism. Acrylonitrile
forms stable conjugates with L-cysteine and L-glutathione in vitro
(Hashimoto & Kanai, 1965; Gut et al., 1975) and a portion of
absorbed acrylonitrile is thus prevented from being metabolized to
cyanide. Depressed levels of sulfhydryl compounds have been
reported following acrylonitrile administration (e.g., Wisniewska-
Knypl et al., 1970; Hashimoto & Kanai, 1972; Vainio & Mäkinen,
1977; Dinu & Klein, 1976; Szabo et al., 1977). The spontaneous
conjugation of glutathione with acrylonitrile or glycidonitrile
proceeds very slowly; glycidonitrile forms S-(2-cyano-2-
hydroxyethyl)-L-glutathione and S-(1-cyano-2-hydroxyethyl)-L-
glutathione in the ratio of about 1:1. In the enzyme-catalysed
conjugation this ratio shifts to about 3:1 (Holechek & Kopecky,
1981). These authors demonstrated that no cyanide was released
from the conjugation product of acrylonitrile with GSH, while
cyanide was released from the conjugation product of glycidonitrile
with GSH. This study confirmed the findings of Boyland & Chasseaud
(1967, 1968) concerning the participation of glutathione- S-
alkylene transferase(s) (EC 2.5.2.18) in the cyanoethylation
reaction of glutathione. Since glutathione conjugates are
precursors of mercapturic acids, the occurrence of mercapturic
acids derived from acrylonitrile and glycidonitrile may be expected
in the urine of animals exposed to acrylonitrile.
The major metabolite of acrylonitrile in the rat, rabbit, and
other animals was found to be 2-cyanoethylmercapturic acid (Dahm,
1977; Wright, 1977; Ahmed & Patel, 1979; Kopecky et al., 1979,
1980a,b,c, 1981; Langvardt et al., 1980; Sapota & Draminski, 1981;
Sapota & Chmielnicka, 1981; Van Bladeren et al., 1981; Ghanayem &
Ahmed, 1982). While 2-cyanoethylmer-capturic acid was the sole
mercapturic acid identified in the urine of rats after iv
administration of acrylonitrile, a second mercapturic acid of
unestablished structure was also excreted after oral
administration. Langvardt et al. (1980), using 1-14C- or 2,3-14C-
acrylonitrile, found seven radioactive metabolites in rat urine.
The 3 major metabolites included thiocyanate and 2-
cyanoethylmercapturic acid. The third was tentatively identified
as 4-acetyl-5-cyanotetra-hydro-1,4-2 H-thiazine-3-carboxylic acid.
The 4 remaining metabolites represented at least one third of the
total activity excreted; their chemical structures are not known,
but none contained the -CN group of acrylonitrile. Different
results were reported by van Bladeren et al. (1981). In common
with Kopecky & Langvardt and colleagues, they isolated
2-cyanoethylmercapturic acid from the urine of orally-dosed rats;
however, 2-hydroxyethylmercapturic acid was also excreted. It is
suggested that this second mercapturic acid may be formed via one
of the conjugates of glutathione with glycidonitrile, S-(2-cyano-
2-hydroxyethyl)-L-glutathione. The amount of mercapturic acids
excreted relative to the dose was approximately constant up to a
dose of acrylonitrile of 26.5 mg/kg body weight. At higher doses,
the amount of mercapturic acids excreted remained constant. These
authors and Wright (1977) suggested that this might be a
consequence of the depletion of available glutathion at the higher
dose levels. It seems likely that, at high exposure levels, the
preferred metabolic pathway (conjugation of glutathione with
acrylonitrile or its metabolite) is overloaded, and another unknown
metabolic pathway takes over. After an oral dose to rats of 1-14C
acrylonitrile, 4 metabolites were found in the bile, 2 major
metabolites being GSH conjugates of acrylonitrile (Ghanayem &
Ahmed, 1982).
The report by Dahm (1977) that rats administered acrylonitrile
excreted S-(2-cyanoethyl)-L-cysteine has not been confirmed by any
of the authors who have examined the glutathione conjugation
pathway of acrylonitrile biotransformation. Fig. 2 illustrates
the proposed routes of mercaptide formation from acrylonitrile.
5.3.2.1. The oxidative pathway of acrylonitrile metabolism
The oxidative pathway of acrylonitrile biotransformation
includes a number of consecutive enzyme-catalyzed or spontaneous
reactions. The first step, oxidation of acrylonitrile to
glycidonitrile, is catalyzed by hepatic microsomal mono-oxygenases
(Abreu & Ahmed 1980; Kopecky et al., 1980a,b; Guengerich et al.,
1981; Ahmed & Abreu, 1982). Glycidonitrile is a reactive
intermediate, and a number of its metabolites have been recorded;
in in vitro experiments it is transformed by epoxide hydrolase (EC
3.3.2.3) to glycolaldehyde cyanohydrin, which decomposes
spontaneously to hydrocyanic acid (cyanide) and glycoealdehyde
(Kopecky et al., 1979, 1980a,b; Abreu & Ahmed, 1980; Duverger-van
Bogaert, 1981a). The yield of cyanide in the in vitro experiments
depends on the techniques used (Nerudova et al., 1980b). Besides
forming conjugation products with glutathione (section 5.3.2.2),
glycidonitrile rearranges to cyanoacetaldehyde, which is further
reduced to 2-cyanoethanol or oxidized to cyanoacetic acid. Acetic
acid is also present (Duverger-van Bogaert, 1981).
The results of animal studies have shown that cyanide formed in
vivo is subsequently converted by rhodanese (EC 2.8.1.1) to
thiocyanate and eliminated in urine (e.g., Dudley & Neal, 1942;
Brieger et al., 1952; Ahmed & Patel, 1981). Thiocyanate has been
directly measured in the urine of various animals after
acrylonitrile administration (Lawton et al., 1943; Mallette, 1943;
Czajkowska, 1971; Efremov, 1976b). Rats administered acrylonitrile
at 60 mg/kg body weight, excreted thiocyanate in the urine at a
constant rate of 0.53 mg/h with an excretion half period of 13 h
(Czajkowska, 1971). Sulfhydryl compounds (cysteine, BAL, and
Unithiol) increase the activity of rhodanese in the conversion of
cyanide to thiocyanate in vitro, as well as in vivo (e.g.,
Frankenberg, 1980). A similar increase with acrylonitrile has not
been convincingly demonstrated (Gut et al., 1975), perhaps because
of the inhibiting properties of acrylonitrile on rhodanese.
5.3.2.2. Mercapturic acids formed in acrylonitrile biotransformation
Cyanoethylation of naturally-occurring sulfhydryl compounds
plays an important role in acrylonitrile metabolism. Acrylonitrile
forms stable conjugates with L-cysteine and L-glutathione in vitro
(Hashimoto & Kanai, 1965; Gut et al., 1975) and a portion of
absorbed acrylonitrile is thus prevented from being metabolized to
cyanide. Depressed levels of sulfhydryl compounds have been
reported following acrylonitrile administration (e.g., Wisniewska-
Knypl et al., 1970; Hashimoto & Kanai, 1972; Vainio & Mäkinen,
1977; Dinu & Klein, 1976; Szabo et al., 1977). The spontaneous
conjugation of glutathione with acrylonitrile or glycidonitrile
proceeds very slowly; glycidonitrile forms S-(2-cyano-2-
hydroxyethyl)-L-glutathione and S-(1-cyano-2-hydroxyethyl)-L-
glutathione in the ratio of about 1:1. In the enzyme-catalysed
conjugation this ratio shifts to about 3:1 (Holechek & Kopecky,
1981). These authors demonstrated that no cyanide was released
from the conjugation product of acrylonitrile with GSH, while
cyanide was released from the conjugation product of glycidonitrile
with GSH. This study confirmed the findings of Boyland & Chasseaud
(1967, 1968) concerning the participation of glutathione- S-
alkylene transferase(s) (EC 2.5.2.18) in the cyanoethylation
reaction of glutathione. Since glutathione conjugates are
precursors of mercapturic acids, the occurrence of mercapturic
acids derived from acrylonitrile and glycidonitrile may be expected
in the urine of animals exposed to acrylonitrile.
The major metabolite of acrylonitrile in the rat, rabbit, and
other animals was found to be 2-cyanoethylmercapturic acid (Dahm,
1977; Wright, 1977; Ahmed & Patel, 1979; Kopecky et al., 1979,
1980a,b,c, 1981; Langvardt et al., 1980; Sapota & Draminski, 1981;
Sapota & Chmielnicka, 1981; Van Bladeren et al., 1981; Ghanayem &
Ahmed, 1982). While 2-cyanoethylmer-capturic acid was the sole
mercapturic acid identified in the urine of rats after iv
administration of acrylonitrile, a second mercapturic acid of
unestablished structure was also excreted after oral
administration. Langvardt et al. (1980), using 1-14C- or 2,3-14C-
acrylonitrile, found seven radioactive metabolites in rat urine.
The 3 major metabolites included thiocyanate and 2-
cyanoethylmercapturic acid. The third was tentatively identified
as 4-acetyl-5-cyanotetra-hydro-1,4-2 H-thiazine-3-carboxylic acid.
The 4 remaining metabolites represented at least one third of the
total activity excreted; their chemical structures are not known,
but none contained the -CN group of acrylonitrile. Different
results were reported by van Bladeren et al. (1981). In common
with Kopecky & Langvardt and colleagues, they isolated
2-cyanoethylmercapturic acid from the urine of orally-dosed rats;
however, 2-hydroxyethylmercapturic acid was also excreted. It is
suggested that this second mercapturic acid may be formed via one
of the conjugates of glutathione with glycidonitrile, S-(2-cyano-
2-hydroxyethyl)-L-glutathione. The amount of mercapturic acids
excreted relative to the dose was approximately constant up to a
dose of acrylonitrile of 26.5 mg/kg body weight. At higher doses,
the amount of mercapturic acids excreted remained constant. These
authors and Wright (1977) suggested that this might be a
consequence of the depletion of available glutathion at the higher
dose levels. It seems likely that, at high exposure levels, the
preferred metabolic pathway (conjugation of glutathione with
acrylonitrile or its metabolite) is overloaded, and another unknown
metabolic pathway takes over. After an oral dose to rats of 1-14C
acrylonitrile, 4 metabolites were found in the bile, 2 major
metabolites being GSH conjugates of acrylonitrile (Ghanayem &
Ahmed, 1982).
The report by Dahm (1977) that rats administered acrylonitrile
excreted S-(2-cyanoethyl)-L-cysteine has not been confirmed by any
of the authors who have examined the glutathione conjugation
pathway of acrylonitrile biotransformation. Fig. 2 illustrates
the proposed routes of mercaptide formation from acrylonitrile.
 5.3.2.3. The glucuronic acid conjugates of acrylonitrile metabolism
Rats treated with doses of acrylonitrile ranging from 20 to 40
mg/kg body weight (Hoffman et al., 1976) excreted significantly
more glucuronic acid than untreated controls or rats administered
10 mg acrylonitrile/kg body weight. This suggests that
acrylonitrile-derived glucuronide might be the alternative
substance to conjugate metabolites (van Bladereu et al., 1981).
The results of Lambotte-Vandepaer et al. (1980) support this
theory. The mutagenicity of rat urine after administration of
acrylonitrile at 30 mg/kg body weight was enhanced by treatment
with beta-D-glucuronidase (EC 3.2.1.31) prior to the Ames'
mutagenicity assay. This indicates that a glucuronide was cleaved
to give a free mutagenic agent derived from acrylonitrile. The
dose fits the dose range that evokes a significant increase in
glucuronic acid excretion (Hoffmann et al., 1976) and is of the
same magnitude as that at which van Bladeren et al. (1981)
demonstrated a depletion of glutathione in rat liver.
5.3.2.4. Quantitative aspects of acrylonitrile bio-transformation
and elimination of its metabolites
(a) Effect of acrylonitrile concentration and dose
The relationship between acrylonitrile concentrations in the
air, cyanide and thiocyanate in the blood, and thiocyanate in the
urine was described by Brieger et al. (1952). At acrylonitrile
concentrations between 55 and 220 mg/m3 (25 and 100 ppm), the blood
and urine thiocyanate concentrations were proportional to inhaled
acrylonitrile concentrations in rats. However, the cyanide content
of blood was measurable only at the highest acrylonitrile
concentration. In dogs, cyanide could be detected in blood at an
acrylonitrile concentration of 110 mg/m3 and cyanide concentrations
in blood were proportional to the inhaled acrylonitrile
concentrations in the range of 110-220 mg/m3 (50 - 100 ppm). Data
indicate that a certain acrylonitrile concentration must be exceeded
to provide conditions for the formation of enough cyanide to surpass
the metabolic capacity of rhodanese or the supply of co-factors;
this concentration is lower in the dog than in the rat.
In mice and rats, the dose of acrylonitrile was directly
related to the cyanide levels in blood, liver, kidney, and brain
(Ahmed & Patel, 1981), and, in rats, the ip administration of
acrylonitrile at 20-60 mg/kg body weight or oral administration at
15-60 mg/kg body weight also produced a proportional increase in
thiocyanate excretion in the urine.
However, thiocyanate is always present in urine (9 mg/litre in
rats) (Brieger et al., 1952), and the acrylonitrile exposures
required to exceed this level significantly are high. Thus, urine
thiocyanate levels would not give an accurate estimate of exposure
at the atmospheric acrylonitrile concentrations found in industry,
at present.
The observation of Hoffmann et al. (1976) suggested a possible
alternative conjugating route for metabolites at higher
acrylonitrile exposure levels involving glucuronic acid. Before
this is confirmed, the effects on carbohydrate metabolism and
glucose utilization in rats must be considered, as well as the
possibility that this alternative pathway of glucose metabolism
leading to the formation of glucuronic acid, and thus elevated
glucuronic acid levels in urine, may be stimulated by acrylonitrile.
From the standpoint of a possible exposure test, however, it is
emphasized that high doses of acrylonitrile are required to
increase excretion of glucuronic acid in urine, but such doses
would only occur in cases of accidental overexposure.
(b) Differences between species
The work of Brieger et al. (1952) revealed that, at the same
acrylonitrile exposure concentrations, cyanide blood levels in dogs
were far higher than in rats. This was apparently due to a less
efficient detoxification of cyanide to thiocyanate in dogs since,
when exposed to an acrylonitrile concentration of 217 mg/m3 (100
ppm), the total sum of cyanide and thiocyanate concentrations in
blood was about 260 µmol/litre in dogs and 840 µmol/litre in rats.
Although the normal thiocyanate blood level was about 150
µmol/litre in the rat and only about 55 µmol/litre in the dog, the
elevation caused by acrylonitrile was far higher in rats,
suggesting that rats metabolize acrylonitrile to cyanide at a
substantially higher rate and are able to detoxify it more
efficiently than dogs.
Mice excrete more thiocyanate than rats, at a given dose of
acrylonitrile, even though detoxification of cyanide to thiocyanate
in mice is apparently less efficient than in rats. Co-administration
of acrylonitrile and thiosulfate resulted in a 3-fold increase in
thiocyanate excretion in mice, while in rats the effect was much
smaller (Gut et al. 1975; Silver et al., 1982). Moreover, the
thiosulfate significantly reduced mortality in mice, but the
reduction in rat mortality was only slight, confirming that
enhanced detoxification of cyanide in mice is important.
Ahmed & Patel (1981) also observed that the rate of metabolism
of acrylonitrile was higher in mice than in rats.
(c) Time course of elimination of acrylonitrile metabolites
The excretion in urine of 14C-acrylonitrile-derived mercapturic
acids follows shortly after ip, sc, iv, or oral administration of
14C-acrylonitrile in rats (Gut et al., 1981a) and rapidly
decreases, whereas the excretion of thiocyanate from acrylonitrile
given orally or intra-peritoneally increases after a time lag
culminating between hours 8 and 12 in rats, but sooner in mice and
Chinese hamsters (Gut et al., 1975). The time course of
acrylonitrile-derived mercapturic acid excretion in rats was
closely correlated with free acrylonitrile concentrations in blood
and liver (Nerudova et al., 1980a; Gut et al., 1981a), while that
of thiocyanate was not, whatever the route of administration.
(d) Effect of the route of administration
The excretion of thiocyanate by rats, mice, and Chinese
hamsters after oral, ip, sc, and iv administration of 14C-
acrylonitrile represented 20-40%, 5%, 5%, and 1%, respectively, of
the dose administered. However, urinary excretion of radioactivity
was almost quantitative (Gut et al., 1981a); subtracting the
thiocyanate excretion from total urinary metabolites (radioactivity)
revealed that excretion of acrylonitrile-mercapturic acids (and
other possible acrylonitrile metabolites) is independent of the
route of administration (Kopecky et al., 1980a). When 1-14C-
acrylonitrile was administered orally to rats, 27% of the dose had
been excreted in the bile in 6 h, mainly in the form of 2
glutathione conjugates of acrylonitrile (Ghanayem & Ahmed, 1982).
(e) Metabolic interactions of acrylonitrile with other xenobiotics
Oral administration of an equimolar dose of acrylonitrile (0.5
mmol/kg body weight) to rats did not influence the elimination of
phenol from benzene. However, benzene, toluene, ethylbenzene, or
styrene (0.5 mmol/kg body weight) markedly decreased the rate and
total excretion of thiocyanate from an equal dose of acrylonitrile
given orally; higher doses of the solvents caused greater
inhibition (Gut et al., 1981). On the other hand, subcutaneous
administration of benzene and styrene increased the excretion of an
equal dose of 14C-acrylonitrile (0.5 mmol/kg body weight) during
the first 4 h and decreased it between the 8th and l2th hours
(owing to inhibited thiocyanate formation and excretion). The
total of metabolites excreted was unaffected. The co-administration
of industrial solvents markedly increased the lethality of
acrylonitrile (Gut et al., 1981a). Inhibition of the oxidative
metabolism of acrylonitrile in rats by a cytochrome P-450 inhibitor
(1-phenylimidazole) inhibited completely the excretion of N-
acetyl- S-(2-hydroxyethyl) L-cysteine in favour of the excretion of
N-acetyl- S-(2-cyanoethyl)-L-cysteine (van Bladeren et al., 1981).
The latter compound, the authors considered, resulted from direct
cyanoethylation of glutathione, whereas the former was formed via
the epoxide, glycidonitrile. Overnight fasting and cobaltous
chloride pre-treatment increased the biliary excretion of
metabolites, while phenobarbital did not induce any change, and
dimethyl maleate significantly decreased the excretion (Ghanayem &
Ahmed, 1982).
6. BIOLOGICAL MONITORING OF ACRYLONITRILE UPTAKE
Studies, particularly animal studies, on the absorption,
distribution, biotransformation, and elimination of acrylonitrile
have shown that a small fraction of the acrylonitrile absorbed is
rapidly eliminated in the urine without biotransformation, while
the remainder is biotransformed via several pathways, a number of
metabolites being excreted in urine; some of these metabolites are
unique to acrylonitrile.
The absorption studies have also clearly shown that, in
addition to uptake of acrylonitrile by inhalation, skin penetration
can be an important route of entry, particularly in the presence of
liquid acrylonitrile. Thus, in human studies, unless performed
under controlled conditions, a good correlation cannot necessarily
be expected between a bioindicator of uptake and ambient air
measurements of acrylonitrile, even when carried out with personal
samplers.
Possible indicators of acrylonitrile uptake at present include:
acrylonitrile in urine, acrylonitrile-derived mercapturic acids in
urine, total thioethers in urine, and thiocyanates in urine.
Houthuijs et al. (1982) studied the excretion pattern of
acrylonitrile in the urine of 15 exposed workers over a 7-day
period, with a control group of 41 unexposed workers. They noted
that the concentrations of acrylonitrile in urine peaked at the
end, or shortly after the end, of the working day, decreasing
rapidly until the beginning of the next working day without,
however, falling to the levels in the control group. Correlations
have been found between acrylonitrile concentrations in air and
those in urine. In the control group, a significant increase in
the acrylonitrile excretion in urine was found with the number of
cigarettes smoked. For a mean acrylonitrile concentration in air
of 0.28 mg/m3 (0.13 ppm), the mean acrylonitrile level in urine at
the end of the working day was 38 µg/litre, using the headspace
chromatographic technique. In the control group for non-smokers,
the mean level of acrylonitrile in urine was 2 µg/litre and for
smokers (20-30 cigarettes per day) 9.0 µg/litre.
Sakurai et al. (1978) have also established a relationship
between acrylonitrile concentrations in air and levels in urine for
a group of 102 exposed workers and compared them with 62 controls.
For an air concentration of 0.2 mg/m3 (0.1 ppm) (as measured by
personal samplers), an acrylonitrile level in urine of 3.0 µg/litre
was found, using the Sato et al. (1975) method of analysis
(separation by azeotropic distillation and determination by gas
chromatography). The urine samples were collected at the end of
the working day. At an air concentration of 1.1 mg/m3 (0.5 ppm),
the level of acrylonitrile in urine was 19.7 µg/litre, and at a
concentration in air of 9 mg/m3 (4.2 ppm) the corresponding level
in urine was 359.6 µg/litre. Acrylonitrile could not be detected
in the urine of controls. At the same time, an increase in the
thiocyanate level in urine was noted, particularly at the higher
exposure levels.
According to Houthuijs et al. (1982), the differences found
between the urine levels of acrylonitrile in the two studies are
most likely due to differences in analytical techniques.
The validity of the determination of urine levels of
acrylonitrile using gas chromatography-head space analysis for
monitoring acrylonitrile-exposed workers was established by Benchev
et al. (1982).
A promising method for estimating total acrylonitrile uptake
seems to be the determination of acrylonitrile-derived mercapturic
acids; such acids are specific for acrylonitrile and are absent
from normal urine. They have been shown in experimental animal
studies to be well correlated with the free acrylonitrile
concentration in blood (Nerudova et al., 1980; Gut et al.,
1981a,b); animal data also indicate that the capacity of the enzyme
systems to produce the acrylonitrile-derived mercapturic acids is
unlikely to be exceeded at the exposure levels of interest (van
Bladeren et al., 1981). Draminski & Trojanowska (1983) established
the presence of S-(2-cyanoethyl) mercapturic acid in the urine of
13 workers exposed to acrylonitrile, using a gas chromatographic
technique. The concentrations ranged between 50 and 200 mg/litre
for ambient acrylonitrile levels between 3.3 and 9.8 mg/m3 (1.5 and
4.5 ppm). The "total thioethers" were also determined in the urine
samples by a spectrophotometric method (Kopecky, 1982) and shown to
be strongly correlated with the S-(2-cyanoethyl) mercapturic acid
excretion, indicating that, in the case of exposure to pure
acrylonitrile, the major part of the sum of "thioethers" is
represented by this specific mercapturic acid.
Increased glucuronic acid excretion was reported by Ostrovskaja
et al. (1976) in 45.5% of workers exposed to acrylonitrile
concentrations of 0.7-1.5 mg/m3 (0.3-0.7 ppm).
The recent studies reported above show that biological
monitoring may become a suitable approach for assessing
acrylonitrile uptake, in particular in relation to the working
environment. Both acrylonitrile in urine and acrylonitrile-derived
mercapturic acids in urine seem to be the most suitable
bioindicators of uptake, at present, as they have the advantage of
specificity. More work is needed to resolve the apparent
discrepancies due to analytical techniques and to determine the
half-lives. This should make it possible to establish the most
appropriate sampling time with respect to exposure and help in the
determination of the concentrations of concern.
Interest in the determination of total "thioethers" in urine as
a bioindicator of uptake lies in the greater simplicity of the
analytical techniques used. However, more work is needed,
particularly with regard to interferences and half-lives.
The possibility of estimating acrylonitrile exposure in smokers
was suggested by Della Fiorentina & De Wiest (1979), who observed
that determination of carboxyhaemoglobin in blood makes it possible
to calculate the amount of thiocyanate present in urine that is due
to smoking, and thus to calculate the uptake of acrylonitrile.
However, experience shows that there can be marked variations in
thiocyanate levels in smokers, which greatly exceed those in non-
smokers occupationally-exposed to acrylonitrile (Czajkowska et al.,
1969).
7. EFFECTS ON EXPERIMENTAL ANIMALS AND CELL SYSTEMS
7.1. Acute Toxicity
7.1.1. Lethal doses and concentrations
7.1.1.1. Lethal doses
The range of acute LD50 values for acrylonitrile in different
laboratory mammals is generally between 25 and 186 mg/kg body
weight (Table 9), though a value of 282 mg/kg body weight was
observed when liquid acrylonitrile was applied to the skin of the
tail of male rats (Zotova, 1976). Mice are more sensitive than
rats, guinea-pigs, and rabbits. There seems to be little
consistency in the effects of route or vehicle of administration,
or of sex, on the LD50 level. The LD50 for dogs was not reported,
but they tolerated iv administration of acrylonitrile at 50 mg/kg
body weight and died after 300 mg/kg (Graham, 1965). The LD50
values reported are an order of magnitude higher than the LD50 for
cyanide (one of the metabolites of acrylonitrile), but markedly
lower than those for industrial solvents and monomers of plastics
(the LD50 for benzene and its derivatives being about 2000-3000
mg/kg body weight).
7.1.1.2. Lethal concentrations in the air
The range of acute LC50 s for 4-h inhalation of acrylonitrile is
between 150 and 1250 mg/m3 (Table 10). Dogs appeared to be the
most sensitive of the species tested and the sensitivity decreased
in the following order: mice, rabbits, cats, rats, guinea-pigs, the
latter being apparently the most resistant to inhalation exposure.
The exposure of 315-350 cm2 of the skin of rabbits to an
acrylonitrile concentration of 44-62 g/m3, in an exposure chamber,
such that the animals were breathing pure air, proved fatal after
2.5-4 h. Inhalation exposure to 0.58-0.67 g/m3 was fatal for 3
rabbits within 2-3 h (Rogaczewska, 1975).
In the 3 species of insects tested in a fumigation chamber
for 8 h, the LC50 value was found to be 700-1900 mg/m3 (Bond &
Buckland, 1976). Lindgren et al. (1954) exposed 8 insect
species for 2 or 6 h and found LC50 values of 1000-4500 mg/m3 .
Table 9. Acute LD50 values for acrylonitrile: effect of animal species
strain and route of administration
---------------------------------------------------------------------------------------------
Species/strain/sex Number Route LD50 (mg/kg Vehicle Reference
body weight)
---------------------------------------------------------------------------------------------
mouse/-/male M + F 333 oral 36 water Tullar (1947)
mouse/-/female M + F 333 oral 48 water Tullar (1947)
mouse/-/M + F 169 oral 40 olive oil Tullar (1947)
mouse/H strain/- - oral 25 physiol. Benesh & Cherna
saline (1959)
mouse/-/- - oral 40-46 - American Cyanamid
(1951)
mouse/-/female M + F 325 ip 48 water Tullar (1947)
mouse/-/male M + F 325 ip 40 water Tullar (1947)
mouse/NMRI or "SPF"/- - ip 50 - Zeller et al.
(1969)
mouse/ICR/female - ip 47 - Yoshikawa (1968)
mouse/H strain/- - sc 35("technical physiol. Benesh & Cherna
AN") saline (1959)
mouse/"inbred"/male 60 sc 50 (2 h) physiol. Graham (1965)
saline
25 (24 h)
mouse/BN/male 60 sc 34 - Knobloch et al.
(1971)
rat/Sherman/- groups of oral 93 - Smyth & Carpenter
6-10 (1948)
rat/Wistar/- - oral 101 - Paulet & Vidal
(1975)
rat/Wistar or Stock/- - oral 128 - Zellar et al.
(1969)
rat/Wistar-Stamm/male - oral 82 - von Borchardt
et al. (1970)
rat/Wistar-Stamm/female - oral 86 - von Borchardt
et al. (1970)
rat/-/M + F 80 oral 84 water Tullar (1947)
rat/-/M + F 51 oral 72 olive oil Tullar (1947)
rat/Wistar/- - oral 78 physiol. Benesh & Cherna
saline (1959)
rat/Sprague-Dawley/male 20 oral 186 water Monsanto (1975)
rat/Sprague-Dawley/female 20 oral 186 water Monsanto (1975)
Table 9. (contd.)
---------------------------------------------------------------------------------------------
Species/strain/sex Number Route LD50 (mg/kg Vehicle Reference
body weight)
---------------------------------------------------------------------------------------------
rat/Wistar/male 110 ip 100 - Knobloch et al.
(1971)
rat/Wistar/- - ip 65 poly- Paulet & Vidal
ethylene (1975)
glycol
rat/Wistar/male 110 sc 80 - Knobloch et al.
(1971)
rat/"albino"/male - sc 96 water Magos (1962)
rat/"white"/male - skin of 282 liquid Zotova (1976)
tail acrylonitrile
rat/"white"/male - skin of 148 liquid Zotova (1976)
abdomen acrylonitrile
guinea-pig/-/- - oral 50 - Carpenter et al.
(1949)
guinea-pig/-/- - oral 85 olive oil Tullar (1947)
guinea-pig/-/M & F 30 oral 56 - Jedlicka et al.
(1958)
guinea-pig/-/- - sc 130 - Ghiringhelli (1954)
guinea-pig/-/- 11 iv 72 water Tullar (1947)
guinea-pig/Hartley-/male 12 or more intact 0.46 ml/kg - Roudabush et al.
skin (1965)
abraded 0.86 ml/kg - Roudabush et al.
skin (1965)
guinea-pig/-/- - skin 0.25 ml/kg - Smyth & Carpenter
(1948)
rabbit/-/- - oral 93 - Lefaux (1966)
rabbit/-/- - iv 69 - Paulet & Desnos
(1961)
rabbit/"white"/M & F 12 or more abraded 0.28 ml/kg - Roudabush et al.
skin (1965)
---------------------------------------------------------------------------------------------
Table 10. Acute lethal effect of single inhalation of acrylonitrile: effect of
duration and concentration of acrylonitrile
---------------------------------------------------------------------------------------------------
Species/strain/sex Number Concentration Duration Mortality Reference
(mg/m3) (h) (died/
tested)
---------------------------------------------------------------------------------------------------
white mouse/stock/- 6 600 0.5 0/6 McOmie (1949)
6 1500 0.5 5/6 McOmie (1949)
6 5800 0.5 5/6 McOmie (1949)
6 900 1 1/6 McOmie (1949)
6 900 2 3/6 McOmie (1949)
6 1700 1 6/6 McOmie (1949)
mouse/BN/male 12 300 4 LC50 Knobloch et al. (1971)
rat/Sherman/- 6 1085 4 0/6 Smyth & Carpenter et al. (1971)
6 2170 4 6/6 Smyth & Carpenter et al. (1971)
rat/Sherman/female 6 1085 4 2/6 to Carpenter et al. (1949)
4/6
rat/Wistar/- 20 54 7 0/20 Brieger et al. (1952)
20 109 7 0/20 Brieger et al. (1952)
20 163 7 0/20 Brieger et al. (1952)
20 217 7 4/20 Brieger et al. (1952)
rat/Wistar/male 12 470 4 LC50 Knobloch et al. (1971)
rat/Osborne-Mendel/- 16 2750 1 0/16 Dudley & Neal (1942)
16 3230 1 4/16 Dudley & Neal (1942)
16 5300 1 13/16 Dudley & Neal (1942)
16 660 2 0/16 Dudley & Neal (1942)
16 1290 2 6/16 Dudley & Neal (1942)
16 2730 2 16/16 Dudley & Neal (1942)
16 280 4 0/16 Dudley & Neal (1942)
16 680 4 5/16 Dudley & Neal (1942)
16 1380 4 16/16 Dudley & Neal (1942)
16 290 8 0/16 Dudley & Neal (1942)
16 460 8 1/16 Dudley & Neal (1942)
16 590 8 7/16 Dudley & Neal (1942)
16 690 8 15/16 Dudley & Neal (1942)
---------------------------------------------------------------------------------------------------
Table 10. (contd.)
---------------------------------------------------------------------------------------------------
Species/strain/sex Number Concentration Duration Mortality Reference
(mg/m3) (h) (died/
tested)
---------------------------------------------------------------------------------------------------
rat/Wistar/male 3 650 3 1/3 Appel et al. (1981)
3 1100 2 3/3 Appel et al. (1981)
3 2600 0.5 1/3 Appel et al. (1981)
6 3000 0.5 6/6 Appel et al. (1981)
guinea-pig/-/- 8 580 4 0/8 Dudley & Neal (1942)
8 1250 4 5/8 Dudley & Neal (1942)
8 2520 4 8/8 Dudley & Neal (1942)
guinea-pig/-/- 12 990 4 LC50 Knobloch et al. (1971)
rabbit/"albino"/- 2 290 4 0/2 Dudley & Neal (1942)
2 560 4 2/2 Dudley & Neal (1942)
2 1260 4 2/2 Dudley & Neal (1942)
rabbit/-/- 5 670 - 1100 2-3 5/5 Rogaczewska (1975)
cat/-/- 4 210 4 0/4 Dudley & Neal (1942)
2 600 4 0/2 Dudley & Neal (1942)
2 1300 4 2/2 Dudley & Neal (1942)
dog/-/- 3 63 4 0/3 Dudley & Neal (1942)
2 140 4 1/2 Dudley & Neal (1942)
3 213 4 0/3 Dudley & Neal (1942)
2 240 4 2/2 Dudley & Neal (1942)
dog/-/- 4 108 7 0/4 Brieger et al. (1952)
4 163 7 0/4 Brieger et al. (1952)
6 213 7 6/6 Brieger et al. (1952)
Rhesus monkey/-/- 3 163 7 1/3 Brieger et al. (1952)
---------------------------------------------------------------------------------------------------
7.1.1.3. Lethal concentrations in water
(a) Fish
Acute toxicity, determined by a static bioassay at 25 °C,
revealed TLm (median tolerance limit, i.e., a concentration of
acrylonitrile killing 50% of the test organisms within a specified
time) values ranging from 25.5 to 44.6 mg/litre at 24 h, and from
11.8 to 33.5 mg/litre at 96 h. There were no apparent significant
differences in the sensitivity of various kinds of fish (Table 11).
(b) Invertebrates
For the brown shrimp (Crangon crangon), the LC50 for a 24-h
exposure was 10-33 mg/litre (Portman & Wilson, 1971). Bandt (1953)
exposed several species of arthropods (a shrimp-like crustaceae and
3 types of larvae) to 20-100 mg acrylonitrile/litre water and found
marked species and individual differences: a lethal effect was
observed in some species with 25 mg/litre after 48 h, while other
species were not affected after 3 days. The most resistant species
were unaffected by 100 mg/litre after 24-48 h. The results of
studies by Rajendran & Muthu (1981) showed that acrylonitrile
affects the activity of the phosphorylase and acetylcholinesterase
enzymes in Tribolium castaneum Herbst, and Trogoderma granarium
Everts.
7.1.2. Clinical observations
The inhalation studies of Dudley & Neal (1942), Brieger et al.
(1952), and Rogaczewska (1975), and the results of oral and
parenteral administration (Ghiringhelli, 1954; Benesh & Cherna,
1959; Paulet & Despos, 1961; Graham, 1965; Paulet et al., 1966)
indicate that animals inhaling lethal concentrations of
acrylonitrile, or administered lethal dosages of acrylonitrile
orally or parenterally, showed rather similar effects: excitability
and stimulated breathing, shallow rapid breathing, slow gasping
breathing, apnoea, convulsions, and death. Vomiting occurred in
cats, dogs, and monkeys after inhaling acrylonitrile, and in rats
following parenteral administration. Reddening of the skin of the
ears, nose, and feet (in rhesus monkeys, also of the face and
genital organs) and mucosa was accompanied by lachrymation, nasal
discharge, and salivation, not only after inhalation exposure, but
also following oral and sc administration, while hind-leg
incoordination, paresis or paralysis, were observed in rats after
oral administration, and in rabbits after iv administration.
Table 11. Median tolerance limit values (TLm)a for various fish exposed to
acrylonitrile
-------------------------------------------------------------------------------------
Species Water type TLm (mg/litre) Reference
24 h 48 h 96 h
-------------------------------------------------------------------------------------
Fathead minnow hard 32.7 16.7 14.3 Henderson et al. (1961)
(Pimphales promelas) soft 34.3 21.5 18.1 Henderson et al. (1961)
Minnow (Phoxinus - 38.2 17.6 - Marcoci & Ionescu (1974)
phoxinus)
Bluegill (Lepomis soft 25.5 14.3 11.8 Henderson et al. (1961)
macrochirus)
Guppy (Lebistes soft 44.6 33.5 33.5 Henderson et al. (1961)
reticulatus)
Goldfish (Carassius - - - 40 Paulet & Vidal (1975)
sp.)
Carp (Cyprinus - 37.4 24.0 - Marcoci & Ionescu (1974)
carpio)
Rainbow trout hard - 70 - Jackson & Brown (1970)
(Salmo gairdneri)
Pin perch sea 24.5 - - Daugherty & Garett (1951)
(marine fish) (30/l tank)
(Lagodon rhomboides)
Rainbow trout tap, - 5b - Sloof (1979)
(Salmo gairdneri) dechlorinated,
3.6 mg/litre
hard 15 - - Sloof (1979)
Zebra fish same (LC50)
-------------------------------------------------------------------------------------
a TLm median tolerance limit, a concentration of acrylonitrile killing
50% of the test organisms within a specified time.
b Minimal concentration changing respiratory frequency.
(a) Effects on the skin
Direct application of liquid acrylonitrile to the shaved skin
of rabbits induced slight local vasodilation immediately, without
any systemic effect (1-2 ml covering 100-200 cm2) or with an
increased respiratory rate (3 ml over 300 cm2) (McOmie, 1949).
Tuller (1947) observed erythema in only one of 3 areas of abraded
skin, following application of 1 ml of acrylonitrile on a gauze pad
covered by rubber sheeting. However, Zeller et al. (1969) found
that a 15-min application of acrylonitrile on a cotton pad to
shaved skin resulted in skin oedema, and a 20-h application, in
slight necrosis. Guinea-pigs appear to be more sensitive than
rabbits; the application of a 2% solution of acrylonitrile in
acetone for 24 h, under occlusion, did not induce any effects, but
8% or higher concentrations induced dose-dependent erythema
followed by desquamation and mild necrosis (Gut et al., unpublished
data). Erythema of the nose, face, ears, legs, and genital organs
may follow inhalation and oral administration of acrylonitrile.
(b) Effects on the eye
McOmie (1949) instilled one drop of acrylonitrile into the eye
of a rabbit. After 1 h, there was mild conjunctivitis without
corneal clouding or pupillary damage and no effects were observed
after 24 h. Oedema and slight necrosis of the conjunctiva after 8
days were observed in rabbits by Zeller et al. (1969).
(c) Effects on respiration
It was stressed by Paulet et al. (1966) that, after a lethal
intravenous dose of acrylonitrile (120 mg/kg body weight), the
respiratory rate in rabbits did not increase as is characteristic
in cyanide poisoning. However, respiratory disturbance was
observed in: guinea-pigs given a sc lethal dose of acrylonitrile
(130 mg/kg body weight) (Ghiringhelli, 1954), anaesthetized dogs
given 100 mg/kg body weight intravenously (Graham, 1965), mice
given an oral lethal dose (Benesh & Cherna, 1959), and in guinea-
pigs given 100 mg/kg body weight orally (Jedlicka et al., 1958).
Pulmonary oedema was also seen.
An increased respiratory rate followed the application of
liquid acrylonitrile (3 ml/kg body weight) to the skin of rabbits
(McOmie, 1949), and "respiratory distress" was reported in rhesus
monkeys exposed to 163 mg/m3 for 7 h (Brieger et al., 1952). When
rats, rabbits, cats, dogs, and monkeys were exposed to lethal
concentrations of acrylonitrile, Dudley & Neal (1942) observed an
initial stimulation of respiration followed by shallow rapid
breathing, slow gasping breathing, convulsions, coma, and death.
These respiratory effects were absent in guinea-pigs, but
irritation of the pulmonary membranes and some delayed deaths from
lung oedema occurred.
(d) Effects on circulation
Acrylonitrile administered iv at 13, 27, 55, or 110 mg/kg body
weight had little effect on the respiratory and blood pressure
responses of anaesthetized rabbits to adrenalin, noradrenalin, or
acetylcholine, and Gravczyk & Zwierzchovski (1973) believed that
the circulation was not the primary target organ in acrylonitrile
poisoning. However, lethal doses (50 or 100 mg/kg body weight) in
guinea-pigs caused dilation of the right ventricle, congestion of
the coronary blood vessels, hepatic and splenic hyperaemia, and
inflammation of the intestinal mucosa (Jedlicka et al., 1958). In
Sprague-Dawley rats administered a lethal dose of acrylonitrile,
there were haemorrhagic areas in the lung and liver and acute
gastrointestinal inflammation (Monsanto, 1975). Whether the
reddening of nose, ears, legs, face, and genital organs in rats and
other species, after inhalation and oral administration of
acrylonitrile, is due to a direct effect on small vessels or is an
inflammatory response is not known.
(e) Effects on adrenals
The effect of lethal doses of acrylonitrile on the adrenals
became evident in the reports of Szabo & Selye (1971, 1972) and
Szabo et al. (1976). After iv administration of high doses (150 or
200 mg/kg body weight), haemorrhage was observed in both adrenals
of most animals, and there was adrenal haemorrhage in some rats
following oral administration of 10, 15, or 20 mg/kg body weight.
Various types of histological damage were observed in the adrenal
cortex and medulla, some of them within 30 min of acrylonitrile
administration.
A possible mechanism involving the peroxidative action of
acrylonitrile in acrylonitrile-induced adrenal injury has been
suggested recently by Silver & Szabo (1982). Szabo et al. (1980)
investigated the pathogenesis of experimental adrenal haemorrhagic
necrosis using various morphological, biochemical, and
pharmacological methods. Their results suggest that the presence
of a functional adrenocortex is necessary for the development of
cortical damage.
(f) Blood chemistry
Intraperitoneal administration of acrylonitrile to male rats at
33 mg/kg body weight per day for 3 days decreased serum
corticosterone to 30%, prolactin to 40%, but increased follicle-
stimulating hormone (FSH) to 200% of control levels and did not
affect luteinizing hormone (LH) (Nilsen et al., 1980). In adult
male Wistar rats, a single ip administration of acrylonitrile of 10
mg/kg body weight did not have any effect on serum glutamic
oxaloacetic transferase (SGOT) and serum glutamic pyruvate
transaminase (SGPT) activity, but increased lactate dehydrogenase
(EC 1.1.1.27) (LDH) to 200% and sorbitol dehydrogenase (SDH) (EC
1.1.1.14) to 300% compared with the controls (Noel et al., 1978).
The same dose in male rats inhibited butyrylcholineesterase (EC
3.1.1.8), did not have any effect on alkaline phosphatase (EC
3.1.3.1), and increased fructose monophosphate aldolase activity,
suggesting that there had been an adverse effect on the liver
(Ivanov et al., 1979). Administraton of L-cysteine, alpha
tocopherol, or ionol prevented these effects. A single oral dose
of acrylonitrile (l/2 LD50, 41 mg/kg body weight) in rats resulted
in changes in the elution patterns of serum gel chromatography and
paper-electrophoresis of globulins (Franzen & Wagner, 1978). Serum
SDH was significantly elevated (approximately 4-fold) in rats, 24 h
after administration of acrylonitrile at 150 mg/kg body weight. A
60% increase in serum SDH was found in rats administered
acrylonitrile at 500 mg/litre in drinking-water for 21 days (Silver
et al., 1982).
(g) Effects on other organs
Focal superficial necrosis of the liver associated with
haemorrhagic gastritis was found in rats necropsied 24 h after
administration of acrylonitrile at 150 mg/kg body weight in the
drinking-water (Silver et al., 1982).
Acrylonitrile shows an inhibitory effect on K-stimulated
respiration of guinea-pig brain cortex slices at 1 mM, but little
effect on the liver at the same concentration. A stronger
anaesthetic action of acrylonitrile was detected in vitro on the
sciatic nerve of Rana nigra maculata, compared with some other
anaesthetic agents (Hashimoto & Kanai, 1965). The recovery phase of
nerve excitation was also affected by acrylonitrile (Ando &
Hashimoto, 1967).
7.1.3. Biochemical changes and mechanisms of acrylonitrile
toxicity
7.1.3.1. Effect on cytochrome oxidase
Evidence has been presented that cytochrome oxidase (EC
1.9.3.1) activity may be significantly inhibited in acrylonitrile
poisoning. This was suspected after Dudley & Neal (1942) and
Brieger et al. (1952) had reported significant concentrations of
cyanide in dogs and monkeys exposed to acrylonitrile vapours.
Tarkowski (1968) observed inhibited cytochrome oxidase activity in
the brain, kidneys, and liver of rats after ip injection of
acrylonitrile at 100 mg/kg body weight. In vitro, a 50%
inhibition of the enzyme was observed in homogenates of brain,
kidneys, and liver with an acrylonitrile concentration of 10-3M.
Such concentrations have been observed in vivo, shortly after
lethal doses of acrylonitrile (Tarkowski, 1968; Nerudova et al.,
1980; Gut et al., 1981b), but acrylonitrile has a short half-life
in blood and liver after ip or iv administration (15-20 min).
There are correspondingly increased blood and liver concentrations
of cyanide in rats after acrylonitrile administration (up to 180 µM,
Gut et al., 1981b), together with an even greater inhibition of
cytochrome oxidase, and a much higher sensitivity of cytochrome
oxidase activity to cyanide (50% inhibition in vitro at 10-8M
(Tarkowski, 1966).
A significantly decreased ratio of oxidized to reduced
nicotinamide-adenine dinucleotides was observed by Sokal et al.
(1972, 1977) in the brain of rats after sc administration of
acrylonitrile at 100-120 mg/kg body weight, indicating inhibition
of NADH oxidation in the mitochondria, possibly also at the level
of cytochrome oxidase. These changes seem to be of biological
importance, because their magnitude was similar to that observed at
the death of animals subjected to experimental hypoxia. Thus, the
cyanide-mediated inhibition of cytochrome oxidase would seem to be
of importance in the later stages of intoxication and death. This
"cyanide effect" is apparently more pronounced at higher
acrylonitrile doses (Willhite & Smith, 1981) and appears to be more
significant in mice and dogs (Brieger et al., 1952; Benesh &
Cherna, 1959; Gut et al., 1981b) than in rats. This is in
agreement with the greater efficacy in mice than in rats of
thiosulfate (an antidote to cyanide) in acrylonitrile poisoning,
and with the higher cyanide concentration in the blood of dogs than
in that of rats (101 µM versus 10 µM), after breathing the same
concentration of acrylonitrile (217 mg/m3 for 7 h) (Brieger et al.,
1952). The protective effect of another cyanide antidote, nitrite
(Dudley & Neal, 1942; Ghiringhelli, 1954; Benesh & Cherna, 1959),
in acrylonitrile poisoning also points to the participation of
cyanide in lethal acrylonitrile poisoning.
7.1.3.2. Effect on sulfhydryls
There is considerable evidence to demonstrate that
acrylonitrile significantly depresses the concentrations of soluble
glutathione and protein sulfhydryls in the blood, liver, brain, and
kidney. Acrylonitrile also inhibits some SH-dependent enzymes that
participate in carbohydrate metabolism. Wisniewska-Knypl (1970,
1978), Hashimoto & Kanai (1972), and Vainio & Mäkinen (1977)
observed that the inhibition of sulfhydryls was dose-dependent in
the range of 10-100 mg/kg body weight in vivo, and in the
concentraton range 0.01-10 nM in vitro (Wisniewska-Knypl, 1978).
A significant decrease in brain sulfhydryls was reported after a
single dermal application of acrylonitrile of as little as 2.82
mg/kg body weight (Zotova, 1976). These effects were observed
after sc, ip, or iv administration to rats, rabbits, hamsters,
guinea-pigs, and mice. There were some decreases in the activity
of serum or tissue - SH-dependent enzymes including oxoglutarate
dehydrogenase (EC 1.2.4.2). However, the activity of succinate
dehydrogenase (EC 1.3.99.1) was not reduced (Wisniewska-Knypl,
1978), and there were corresponding increases in the liver, blood,
and brain concentrations of glucose, pyruvate, and lactate
(Hashimoto & Ando, 1966; Dinu & Klein, 1976).
It was shown by Zitting et al. (1981) that short-term exposure
to acrylonitrile decreased the liver glutathione content within 4 h
of poisoning, but that the glutathione contents returned to normal
in brain, liver, and kidney, within 24 h. At the same time, the
activity of cerebral succinate dehydrogenase and of ethoxycoumarin
demethylase in liver and kidney decreased. Increased glucose,
pyruvate, and lactate concentrations in blood, liver, and brain
were also found, immediately after the fifth exposure, in rats
exposed through inhalation to an acrylonitrile concentration of 300
mg/m3, 8 h daily, for 5 days. In protein, sulfhydryl-dependent
enzyme inhibition was absent and the glutathione level was
significantly reduced in the liver but not in the brain (Gut et
al., 1982). The effects of acrylonitrile on sulfhydryls were
significantly reduced by co-administering L-cysteine and other
sulfhydryls and there were corresponding decreases in lethal
effects (Hashimoto & Kanai, 1965; Bondarev et al., 1976; McLaughlin
et al., 1976; Appel et al., 1981). The results of these studies
demonstrate the protective role of SH-groups in acrylonitrile
poisoning.
The role of hypoxia in the acute thiol-depressive effect of
acrylonitrile in male rats was investigated by Jaeger (1978) and
Jaeger & Cote (1982). Hypoxia was found to enhance non-protein SH
loss in the liver, when there was exposure to acrylonitrile (Jaeger
& Cote, 1982).
Evidence has been presented (Holechek & Kopecky, 1981) that
inhibition of tissue sulfhydryls may be due not only to the
acrylonitrile itself, but also to its reactive metabolite,
glycidonitrile.
7.1.3.3. Interaction with the microsomal oxidation system as a
possible mechanism of toxicity
Acrylonitrile added to mouse, rat, and human liver microsomes
caused characteristic spectral complexes with cytochrome P-450
(Ivanov et al., 1979; Appel et al., 1981).
It was shown in vitro that glycidonitrile, which is
generated in rat-liver microsomes by mixed-function oxidases (EC
1.14.14.1), covalently binds to microsomal membrane and albumin
(Ivanov et al., 1982). The biological significance of this
phenomenon may be inferred from experiments with inhibitors and
inducers of the mixed-function oxidases (Ivanov, 1981). In rats
pre-treated with phenobarbital, an increased amount of
glycidonitrile was covalently bound to macromolecules and
substantially higher activity of fructose-bisphosphate aldolase (EC
4.1.2.13) was observed in the blood, indicating liver damage. On
the other hand, SKF-525A, the inhibitor of cytochrome P-450,
reduced both effects in vivo and in vitro . Activation of
acrylonitrile by cytochrome P-450 may therefore result in a
cytotoxic effect.
A previous injection of SKF-525A or of cobalt(II) chloride
(CoCl2), another inhibitor of cytochrome P-450, resulted in
significant protection against the gastrointestinal bleeding in
rats caused by acrylonitrile (Ghanayem & Ahmed, 1982).
Some data show that acrylonitrile and its epoxide,
glycidonitrile, bind covalently, in vitro, to DNA and RNA
(Guengenrich et al., 1981; Peter et al., 1983a). However, the
quantitative extent of truly irreversible binding is much less than
that observed in experiments with other vinyl monomers (Peter et
al., 1983).
A lower content of cytochrome P-450 in the liver and reduced
oxidative microsomal metabolism of xenobiotics were observed in
rats after an ip injection of acrylonitrile at 10 or 33 mg/kg body
weight, for 3 days (Noel et al., 1978; Nilsen et al., 1980), after
inhalation exposure to 300 mg/m3 of acrylonitrile, 8 h day, for 5
days (Gut et al., in press), and in Chinese hamsters after an ip
injection of 30 mg/kg (Zitting et al., 1981). Inhibition of
microsomal oxidation of xenobiotics by acrylonitrile was observed
in vitro (Ivanov et al., 1979).
Pre-treatment of rats with inducers of microsomal oxidases such
as phenobarbital, 3-methylcholantrene, or Arochlor 1254, nullified
the effect of acrylonitrile on the total cytochrome P-450 content.
The activity of other microsomal enzymes, glucose-6-phosphatase (EC
3.1.3.9), and NADPH cytochrome-c-reductase (EC 1.6.2.4), was
unaffected by acrylonitrile (Duverger-van Bogaert et al., 1978;
Noel et al., 1978).
Ghanayem & Ahmed (1982) showed that Arochlor 1254 drastically
increased acrylonitrile-induced gastric bleeding in rats.
Phenobarbital significantly increased acrylonitrile-induced
hepatocyte damage in rats (Ivanov, 1981).
Acrylonitrile binds with liver microsomal and S-9 fractions, in
vitro, by direct alkylation. The microsomal activation of
acrylonitrile into reactive intermediates was also detected
(Duverger-van Bogaert et al., 1982a). The irreversible binding of
acrylonitrile to liver microsomal proteins was inhibited by thiols
and even more by dithiocarb (Peter & Bolt, 1981).
7.1.3.4. Observations on the possible participation of membrane
lipid peroxidation in the mechanism of toxicity
The ip administration of acrylonitrile at 10 mg/kg body weight
induced lipid peroxidation in rat liver (Dinu, 1975a; Ivanov et
al., 1979) and erythrocyte membranes (Ivanov et al., 1982),
indicating possible damage of cellular membranes. NADPH-dependent
lipid peroxidation in rat liver microsomes was only slightly
stimulated (Ivanov et al., 1978; Duverger-van Bogaert et al., 1981)
whereas substantial stimulation was found in the post-mitochondrial
fraction of rat liver, lung, and brain, as well as in brain-marrow
homogenate (Ivanov et al., 1978; Ivanov, 1979; Al'shansky et al.,
1980). There was a correlation between an increased amount of
malondialdehyde and a decreased content of -SH groups in the post-
mitochondrial supernatant of rat liver (Ivanov, 1981) and brain
(Al'shansky et al., 1980). Conjugated diene concentrations in rat
liver microsomes were significantly elevated after iv
administration of acrylonitrile to rats (150 mg/kg body weight),
but no change was seen in the adrenal glands (Silver & Szabo,
1982).
Pre-treatment of rats with antioxidants, in doses equivalent to
those of the acrylonitrile administered, afforded protection
against the pro-oxidant effect of acrylonitrile and elevation of
blood fructose-1-phosphate aldolase, decreased the activity of
butyrylcholine esterase (EC 3.1.1.8) (Ivanov et al., 1979), and
reduced the GABA content and activity of glutamate decarboxylase
(EC 4.1.1.15) in the brain (Al'shansky et al., 1980).
7.1.3.5. Studies on antidotes
Appel et al. (1981a) found that the cyanide antidotes, 4-
dimethylaminophenol plus thiosulfate, protected rats against the
lethal effects of orally-administered acrylonitrile. A comparative
evaluation was made by McLaughlin et al. (1975) of the efficacy of
thiols (cysteine hydrochloride) and cyanide antidotes. The authors
showed that thiols were more effective in protecting rats against
acrylonitrile poisoning. Bondarev et al. (1976) demonstrated the
protective role of some sulfur-containing compounds in the
acrylonitrile poisoning of rats. The protective effects of some
antioxidants, such as vitamin E and ionol, have also been
demonstrated (Ivanov et al., 1979).
The possible toxic mechanisms and theoretical protective
mechanisms are summarized in Fig. 3, which indicates the complex
nature of the interference of acrylonitrile with cellular
mechanisms, as far as can be derived from current knowledge.
5.3.2.3. The glucuronic acid conjugates of acrylonitrile metabolism
Rats treated with doses of acrylonitrile ranging from 20 to 40
mg/kg body weight (Hoffman et al., 1976) excreted significantly
more glucuronic acid than untreated controls or rats administered
10 mg acrylonitrile/kg body weight. This suggests that
acrylonitrile-derived glucuronide might be the alternative
substance to conjugate metabolites (van Bladereu et al., 1981).
The results of Lambotte-Vandepaer et al. (1980) support this
theory. The mutagenicity of rat urine after administration of
acrylonitrile at 30 mg/kg body weight was enhanced by treatment
with beta-D-glucuronidase (EC 3.2.1.31) prior to the Ames'
mutagenicity assay. This indicates that a glucuronide was cleaved
to give a free mutagenic agent derived from acrylonitrile. The
dose fits the dose range that evokes a significant increase in
glucuronic acid excretion (Hoffmann et al., 1976) and is of the
same magnitude as that at which van Bladeren et al. (1981)
demonstrated a depletion of glutathione in rat liver.
5.3.2.4. Quantitative aspects of acrylonitrile bio-transformation
and elimination of its metabolites
(a) Effect of acrylonitrile concentration and dose
The relationship between acrylonitrile concentrations in the
air, cyanide and thiocyanate in the blood, and thiocyanate in the
urine was described by Brieger et al. (1952). At acrylonitrile
concentrations between 55 and 220 mg/m3 (25 and 100 ppm), the blood
and urine thiocyanate concentrations were proportional to inhaled
acrylonitrile concentrations in rats. However, the cyanide content
of blood was measurable only at the highest acrylonitrile
concentration. In dogs, cyanide could be detected in blood at an
acrylonitrile concentration of 110 mg/m3 and cyanide concentrations
in blood were proportional to the inhaled acrylonitrile
concentrations in the range of 110-220 mg/m3 (50 - 100 ppm). Data
indicate that a certain acrylonitrile concentration must be exceeded
to provide conditions for the formation of enough cyanide to surpass
the metabolic capacity of rhodanese or the supply of co-factors;
this concentration is lower in the dog than in the rat.
In mice and rats, the dose of acrylonitrile was directly
related to the cyanide levels in blood, liver, kidney, and brain
(Ahmed & Patel, 1981), and, in rats, the ip administration of
acrylonitrile at 20-60 mg/kg body weight or oral administration at
15-60 mg/kg body weight also produced a proportional increase in
thiocyanate excretion in the urine.
However, thiocyanate is always present in urine (9 mg/litre in
rats) (Brieger et al., 1952), and the acrylonitrile exposures
required to exceed this level significantly are high. Thus, urine
thiocyanate levels would not give an accurate estimate of exposure
at the atmospheric acrylonitrile concentrations found in industry,
at present.
The observation of Hoffmann et al. (1976) suggested a possible
alternative conjugating route for metabolites at higher
acrylonitrile exposure levels involving glucuronic acid. Before
this is confirmed, the effects on carbohydrate metabolism and
glucose utilization in rats must be considered, as well as the
possibility that this alternative pathway of glucose metabolism
leading to the formation of glucuronic acid, and thus elevated
glucuronic acid levels in urine, may be stimulated by acrylonitrile.
From the standpoint of a possible exposure test, however, it is
emphasized that high doses of acrylonitrile are required to
increase excretion of glucuronic acid in urine, but such doses
would only occur in cases of accidental overexposure.
(b) Differences between species
The work of Brieger et al. (1952) revealed that, at the same
acrylonitrile exposure concentrations, cyanide blood levels in dogs
were far higher than in rats. This was apparently due to a less
efficient detoxification of cyanide to thiocyanate in dogs since,
when exposed to an acrylonitrile concentration of 217 mg/m3 (100
ppm), the total sum of cyanide and thiocyanate concentrations in
blood was about 260 µmol/litre in dogs and 840 µmol/litre in rats.
Although the normal thiocyanate blood level was about 150
µmol/litre in the rat and only about 55 µmol/litre in the dog, the
elevation caused by acrylonitrile was far higher in rats,
suggesting that rats metabolize acrylonitrile to cyanide at a
substantially higher rate and are able to detoxify it more
efficiently than dogs.
Mice excrete more thiocyanate than rats, at a given dose of
acrylonitrile, even though detoxification of cyanide to thiocyanate
in mice is apparently less efficient than in rats. Co-administration
of acrylonitrile and thiosulfate resulted in a 3-fold increase in
thiocyanate excretion in mice, while in rats the effect was much
smaller (Gut et al. 1975; Silver et al., 1982). Moreover, the
thiosulfate significantly reduced mortality in mice, but the
reduction in rat mortality was only slight, confirming that
enhanced detoxification of cyanide in mice is important.
Ahmed & Patel (1981) also observed that the rate of metabolism
of acrylonitrile was higher in mice than in rats.
(c) Time course of elimination of acrylonitrile metabolites
The excretion in urine of 14C-acrylonitrile-derived mercapturic
acids follows shortly after ip, sc, iv, or oral administration of
14C-acrylonitrile in rats (Gut et al., 1981a) and rapidly
decreases, whereas the excretion of thiocyanate from acrylonitrile
given orally or intra-peritoneally increases after a time lag
culminating between hours 8 and 12 in rats, but sooner in mice and
Chinese hamsters (Gut et al., 1975). The time course of
acrylonitrile-derived mercapturic acid excretion in rats was
closely correlated with free acrylonitrile concentrations in blood
and liver (Nerudova et al., 1980a; Gut et al., 1981a), while that
of thiocyanate was not, whatever the route of administration.
(d) Effect of the route of administration
The excretion of thiocyanate by rats, mice, and Chinese
hamsters after oral, ip, sc, and iv administration of 14C-
acrylonitrile represented 20-40%, 5%, 5%, and 1%, respectively, of
the dose administered. However, urinary excretion of radioactivity
was almost quantitative (Gut et al., 1981a); subtracting the
thiocyanate excretion from total urinary metabolites (radioactivity)
revealed that excretion of acrylonitrile-mercapturic acids (and
other possible acrylonitrile metabolites) is independent of the
route of administration (Kopecky et al., 1980a). When 1-14C-
acrylonitrile was administered orally to rats, 27% of the dose had
been excreted in the bile in 6 h, mainly in the form of 2
glutathione conjugates of acrylonitrile (Ghanayem & Ahmed, 1982).
(e) Metabolic interactions of acrylonitrile with other xenobiotics
Oral administration of an equimolar dose of acrylonitrile (0.5
mmol/kg body weight) to rats did not influence the elimination of
phenol from benzene. However, benzene, toluene, ethylbenzene, or
styrene (0.5 mmol/kg body weight) markedly decreased the rate and
total excretion of thiocyanate from an equal dose of acrylonitrile
given orally; higher doses of the solvents caused greater
inhibition (Gut et al., 1981). On the other hand, subcutaneous
administration of benzene and styrene increased the excretion of an
equal dose of 14C-acrylonitrile (0.5 mmol/kg body weight) during
the first 4 h and decreased it between the 8th and l2th hours
(owing to inhibited thiocyanate formation and excretion). The
total of metabolites excreted was unaffected. The co-administration
of industrial solvents markedly increased the lethality of
acrylonitrile (Gut et al., 1981a). Inhibition of the oxidative
metabolism of acrylonitrile in rats by a cytochrome P-450 inhibitor
(1-phenylimidazole) inhibited completely the excretion of N-
acetyl- S-(2-hydroxyethyl) L-cysteine in favour of the excretion of
N-acetyl- S-(2-cyanoethyl)-L-cysteine (van Bladeren et al., 1981).
The latter compound, the authors considered, resulted from direct
cyanoethylation of glutathione, whereas the former was formed via
the epoxide, glycidonitrile. Overnight fasting and cobaltous
chloride pre-treatment increased the biliary excretion of
metabolites, while phenobarbital did not induce any change, and
dimethyl maleate significantly decreased the excretion (Ghanayem &
Ahmed, 1982).
6. BIOLOGICAL MONITORING OF ACRYLONITRILE UPTAKE
Studies, particularly animal studies, on the absorption,
distribution, biotransformation, and elimination of acrylonitrile
have shown that a small fraction of the acrylonitrile absorbed is
rapidly eliminated in the urine without biotransformation, while
the remainder is biotransformed via several pathways, a number of
metabolites being excreted in urine; some of these metabolites are
unique to acrylonitrile.
The absorption studies have also clearly shown that, in
addition to uptake of acrylonitrile by inhalation, skin penetration
can be an important route of entry, particularly in the presence of
liquid acrylonitrile. Thus, in human studies, unless performed
under controlled conditions, a good correlation cannot necessarily
be expected between a bioindicator of uptake and ambient air
measurements of acrylonitrile, even when carried out with personal
samplers.
Possible indicators of acrylonitrile uptake at present include:
acrylonitrile in urine, acrylonitrile-derived mercapturic acids in
urine, total thioethers in urine, and thiocyanates in urine.
Houthuijs et al. (1982) studied the excretion pattern of
acrylonitrile in the urine of 15 exposed workers over a 7-day
period, with a control group of 41 unexposed workers. They noted
that the concentrations of acrylonitrile in urine peaked at the
end, or shortly after the end, of the working day, decreasing
rapidly until the beginning of the next working day without,
however, falling to the levels in the control group. Correlations
have been found between acrylonitrile concentrations in air and
those in urine. In the control group, a significant increase in
the acrylonitrile excretion in urine was found with the number of
cigarettes smoked. For a mean acrylonitrile concentration in air
of 0.28 mg/m3 (0.13 ppm), the mean acrylonitrile level in urine at
the end of the working day was 38 µg/litre, using the headspace
chromatographic technique. In the control group for non-smokers,
the mean level of acrylonitrile in urine was 2 µg/litre and for
smokers (20-30 cigarettes per day) 9.0 µg/litre.
Sakurai et al. (1978) have also established a relationship
between acrylonitrile concentrations in air and levels in urine for
a group of 102 exposed workers and compared them with 62 controls.
For an air concentration of 0.2 mg/m3 (0.1 ppm) (as measured by
personal samplers), an acrylonitrile level in urine of 3.0 µg/litre
was found, using the Sato et al. (1975) method of analysis
(separation by azeotropic distillation and determination by gas
chromatography). The urine samples were collected at the end of
the working day. At an air concentration of 1.1 mg/m3 (0.5 ppm),
the level of acrylonitrile in urine was 19.7 µg/litre, and at a
concentration in air of 9 mg/m3 (4.2 ppm) the corresponding level
in urine was 359.6 µg/litre. Acrylonitrile could not be detected
in the urine of controls. At the same time, an increase in the
thiocyanate level in urine was noted, particularly at the higher
exposure levels.
According to Houthuijs et al. (1982), the differences found
between the urine levels of acrylonitrile in the two studies are
most likely due to differences in analytical techniques.
The validity of the determination of urine levels of
acrylonitrile using gas chromatography-head space analysis for
monitoring acrylonitrile-exposed workers was established by Benchev
et al. (1982).
A promising method for estimating total acrylonitrile uptake
seems to be the determination of acrylonitrile-derived mercapturic
acids; such acids are specific for acrylonitrile and are absent
from normal urine. They have been shown in experimental animal
studies to be well correlated with the free acrylonitrile
concentration in blood (Nerudova et al., 1980; Gut et al.,
1981a,b); animal data also indicate that the capacity of the enzyme
systems to produce the acrylonitrile-derived mercapturic acids is
unlikely to be exceeded at the exposure levels of interest (van
Bladeren et al., 1981). Draminski & Trojanowska (1983) established
the presence of S-(2-cyanoethyl) mercapturic acid in the urine of
13 workers exposed to acrylonitrile, using a gas chromatographic
technique. The concentrations ranged between 50 and 200 mg/litre
for ambient acrylonitrile levels between 3.3 and 9.8 mg/m3 (1.5 and
4.5 ppm). The "total thioethers" were also determined in the urine
samples by a spectrophotometric method (Kopecky, 1982) and shown to
be strongly correlated with the S-(2-cyanoethyl) mercapturic acid
excretion, indicating that, in the case of exposure to pure
acrylonitrile, the major part of the sum of "thioethers" is
represented by this specific mercapturic acid.
Increased glucuronic acid excretion was reported by Ostrovskaja
et al. (1976) in 45.5% of workers exposed to acrylonitrile
concentrations of 0.7-1.5 mg/m3 (0.3-0.7 ppm).
The recent studies reported above show that biological
monitoring may become a suitable approach for assessing
acrylonitrile uptake, in particular in relation to the working
environment. Both acrylonitrile in urine and acrylonitrile-derived
mercapturic acids in urine seem to be the most suitable
bioindicators of uptake, at present, as they have the advantage of
specificity. More work is needed to resolve the apparent
discrepancies due to analytical techniques and to determine the
half-lives. This should make it possible to establish the most
appropriate sampling time with respect to exposure and help in the
determination of the concentrations of concern.
Interest in the determination of total "thioethers" in urine as
a bioindicator of uptake lies in the greater simplicity of the
analytical techniques used. However, more work is needed,
particularly with regard to interferences and half-lives.
The possibility of estimating acrylonitrile exposure in smokers
was suggested by Della Fiorentina & De Wiest (1979), who observed
that determination of carboxyhaemoglobin in blood makes it possible
to calculate the amount of thiocyanate present in urine that is due
to smoking, and thus to calculate the uptake of acrylonitrile.
However, experience shows that there can be marked variations in
thiocyanate levels in smokers, which greatly exceed those in non-
smokers occupationally-exposed to acrylonitrile (Czajkowska et al.,
1969).
7. EFFECTS ON EXPERIMENTAL ANIMALS AND CELL SYSTEMS
7.1. Acute Toxicity
7.1.1. Lethal doses and concentrations
7.1.1.1. Lethal doses
The range of acute LD50 values for acrylonitrile in different
laboratory mammals is generally between 25 and 186 mg/kg body
weight (Table 9), though a value of 282 mg/kg body weight was
observed when liquid acrylonitrile was applied to the skin of the
tail of male rats (Zotova, 1976). Mice are more sensitive than
rats, guinea-pigs, and rabbits. There seems to be little
consistency in the effects of route or vehicle of administration,
or of sex, on the LD50 level. The LD50 for dogs was not reported,
but they tolerated iv administration of acrylonitrile at 50 mg/kg
body weight and died after 300 mg/kg (Graham, 1965). The LD50
values reported are an order of magnitude higher than the LD50 for
cyanide (one of the metabolites of acrylonitrile), but markedly
lower than those for industrial solvents and monomers of plastics
(the LD50 for benzene and its derivatives being about 2000-3000
mg/kg body weight).
7.1.1.2. Lethal concentrations in the air
The range of acute LC50 s for 4-h inhalation of acrylonitrile is
between 150 and 1250 mg/m3 (Table 10). Dogs appeared to be the
most sensitive of the species tested and the sensitivity decreased
in the following order: mice, rabbits, cats, rats, guinea-pigs, the
latter being apparently the most resistant to inhalation exposure.
The exposure of 315-350 cm2 of the skin of rabbits to an
acrylonitrile concentration of 44-62 g/m3, in an exposure chamber,
such that the animals were breathing pure air, proved fatal after
2.5-4 h. Inhalation exposure to 0.58-0.67 g/m3 was fatal for 3
rabbits within 2-3 h (Rogaczewska, 1975).
In the 3 species of insects tested in a fumigation chamber
for 8 h, the LC50 value was found to be 700-1900 mg/m3 (Bond &
Buckland, 1976). Lindgren et al. (1954) exposed 8 insect
species for 2 or 6 h and found LC50 values of 1000-4500 mg/m3 .
Table 9. Acute LD50 values for acrylonitrile: effect of animal species
strain and route of administration
---------------------------------------------------------------------------------------------
Species/strain/sex Number Route LD50 (mg/kg Vehicle Reference
body weight)
---------------------------------------------------------------------------------------------
mouse/-/male M + F 333 oral 36 water Tullar (1947)
mouse/-/female M + F 333 oral 48 water Tullar (1947)
mouse/-/M + F 169 oral 40 olive oil Tullar (1947)
mouse/H strain/- - oral 25 physiol. Benesh & Cherna
saline (1959)
mouse/-/- - oral 40-46 - American Cyanamid
(1951)
mouse/-/female M + F 325 ip 48 water Tullar (1947)
mouse/-/male M + F 325 ip 40 water Tullar (1947)
mouse/NMRI or "SPF"/- - ip 50 - Zeller et al.
(1969)
mouse/ICR/female - ip 47 - Yoshikawa (1968)
mouse/H strain/- - sc 35("technical physiol. Benesh & Cherna
AN") saline (1959)
mouse/"inbred"/male 60 sc 50 (2 h) physiol. Graham (1965)
saline
25 (24 h)
mouse/BN/male 60 sc 34 - Knobloch et al.
(1971)
rat/Sherman/- groups of oral 93 - Smyth & Carpenter
6-10 (1948)
rat/Wistar/- - oral 101 - Paulet & Vidal
(1975)
rat/Wistar or Stock/- - oral 128 - Zellar et al.
(1969)
rat/Wistar-Stamm/male - oral 82 - von Borchardt
et al. (1970)
rat/Wistar-Stamm/female - oral 86 - von Borchardt
et al. (1970)
rat/-/M + F 80 oral 84 water Tullar (1947)
rat/-/M + F 51 oral 72 olive oil Tullar (1947)
rat/Wistar/- - oral 78 physiol. Benesh & Cherna
saline (1959)
rat/Sprague-Dawley/male 20 oral 186 water Monsanto (1975)
rat/Sprague-Dawley/female 20 oral 186 water Monsanto (1975)
Table 9. (contd.)
---------------------------------------------------------------------------------------------
Species/strain/sex Number Route LD50 (mg/kg Vehicle Reference
body weight)
---------------------------------------------------------------------------------------------
rat/Wistar/male 110 ip 100 - Knobloch et al.
(1971)
rat/Wistar/- - ip 65 poly- Paulet & Vidal
ethylene (1975)
glycol
rat/Wistar/male 110 sc 80 - Knobloch et al.
(1971)
rat/"albino"/male - sc 96 water Magos (1962)
rat/"white"/male - skin of 282 liquid Zotova (1976)
tail acrylonitrile
rat/"white"/male - skin of 148 liquid Zotova (1976)
abdomen acrylonitrile
guinea-pig/-/- - oral 50 - Carpenter et al.
(1949)
guinea-pig/-/- - oral 85 olive oil Tullar (1947)
guinea-pig/-/M & F 30 oral 56 - Jedlicka et al.
(1958)
guinea-pig/-/- - sc 130 - Ghiringhelli (1954)
guinea-pig/-/- 11 iv 72 water Tullar (1947)
guinea-pig/Hartley-/male 12 or more intact 0.46 ml/kg - Roudabush et al.
skin (1965)
abraded 0.86 ml/kg - Roudabush et al.
skin (1965)
guinea-pig/-/- - skin 0.25 ml/kg - Smyth & Carpenter
(1948)
rabbit/-/- - oral 93 - Lefaux (1966)
rabbit/-/- - iv 69 - Paulet & Desnos
(1961)
rabbit/"white"/M & F 12 or more abraded 0.28 ml/kg - Roudabush et al.
skin (1965)
---------------------------------------------------------------------------------------------
Table 10. Acute lethal effect of single inhalation of acrylonitrile: effect of
duration and concentration of acrylonitrile
---------------------------------------------------------------------------------------------------
Species/strain/sex Number Concentration Duration Mortality Reference
(mg/m3) (h) (died/
tested)
---------------------------------------------------------------------------------------------------
white mouse/stock/- 6 600 0.5 0/6 McOmie (1949)
6 1500 0.5 5/6 McOmie (1949)
6 5800 0.5 5/6 McOmie (1949)
6 900 1 1/6 McOmie (1949)
6 900 2 3/6 McOmie (1949)
6 1700 1 6/6 McOmie (1949)
mouse/BN/male 12 300 4 LC50 Knobloch et al. (1971)
rat/Sherman/- 6 1085 4 0/6 Smyth & Carpenter et al. (1971)
6 2170 4 6/6 Smyth & Carpenter et al. (1971)
rat/Sherman/female 6 1085 4 2/6 to Carpenter et al. (1949)
4/6
rat/Wistar/- 20 54 7 0/20 Brieger et al. (1952)
20 109 7 0/20 Brieger et al. (1952)
20 163 7 0/20 Brieger et al. (1952)
20 217 7 4/20 Brieger et al. (1952)
rat/Wistar/male 12 470 4 LC50 Knobloch et al. (1971)
rat/Osborne-Mendel/- 16 2750 1 0/16 Dudley & Neal (1942)
16 3230 1 4/16 Dudley & Neal (1942)
16 5300 1 13/16 Dudley & Neal (1942)
16 660 2 0/16 Dudley & Neal (1942)
16 1290 2 6/16 Dudley & Neal (1942)
16 2730 2 16/16 Dudley & Neal (1942)
16 280 4 0/16 Dudley & Neal (1942)
16 680 4 5/16 Dudley & Neal (1942)
16 1380 4 16/16 Dudley & Neal (1942)
16 290 8 0/16 Dudley & Neal (1942)
16 460 8 1/16 Dudley & Neal (1942)
16 590 8 7/16 Dudley & Neal (1942)
16 690 8 15/16 Dudley & Neal (1942)
---------------------------------------------------------------------------------------------------
Table 10. (contd.)
---------------------------------------------------------------------------------------------------
Species/strain/sex Number Concentration Duration Mortality Reference
(mg/m3) (h) (died/
tested)
---------------------------------------------------------------------------------------------------
rat/Wistar/male 3 650 3 1/3 Appel et al. (1981)
3 1100 2 3/3 Appel et al. (1981)
3 2600 0.5 1/3 Appel et al. (1981)
6 3000 0.5 6/6 Appel et al. (1981)
guinea-pig/-/- 8 580 4 0/8 Dudley & Neal (1942)
8 1250 4 5/8 Dudley & Neal (1942)
8 2520 4 8/8 Dudley & Neal (1942)
guinea-pig/-/- 12 990 4 LC50 Knobloch et al. (1971)
rabbit/"albino"/- 2 290 4 0/2 Dudley & Neal (1942)
2 560 4 2/2 Dudley & Neal (1942)
2 1260 4 2/2 Dudley & Neal (1942)
rabbit/-/- 5 670 - 1100 2-3 5/5 Rogaczewska (1975)
cat/-/- 4 210 4 0/4 Dudley & Neal (1942)
2 600 4 0/2 Dudley & Neal (1942)
2 1300 4 2/2 Dudley & Neal (1942)
dog/-/- 3 63 4 0/3 Dudley & Neal (1942)
2 140 4 1/2 Dudley & Neal (1942)
3 213 4 0/3 Dudley & Neal (1942)
2 240 4 2/2 Dudley & Neal (1942)
dog/-/- 4 108 7 0/4 Brieger et al. (1952)
4 163 7 0/4 Brieger et al. (1952)
6 213 7 6/6 Brieger et al. (1952)
Rhesus monkey/-/- 3 163 7 1/3 Brieger et al. (1952)
---------------------------------------------------------------------------------------------------
7.1.1.3. Lethal concentrations in water
(a) Fish
Acute toxicity, determined by a static bioassay at 25 °C,
revealed TLm (median tolerance limit, i.e., a concentration of
acrylonitrile killing 50% of the test organisms within a specified
time) values ranging from 25.5 to 44.6 mg/litre at 24 h, and from
11.8 to 33.5 mg/litre at 96 h. There were no apparent significant
differences in the sensitivity of various kinds of fish (Table 11).
(b) Invertebrates
For the brown shrimp (Crangon crangon), the LC50 for a 24-h
exposure was 10-33 mg/litre (Portman & Wilson, 1971). Bandt (1953)
exposed several species of arthropods (a shrimp-like crustaceae and
3 types of larvae) to 20-100 mg acrylonitrile/litre water and found
marked species and individual differences: a lethal effect was
observed in some species with 25 mg/litre after 48 h, while other
species were not affected after 3 days. The most resistant species
were unaffected by 100 mg/litre after 24-48 h. The results of
studies by Rajendran & Muthu (1981) showed that acrylonitrile
affects the activity of the phosphorylase and acetylcholinesterase
enzymes in Tribolium castaneum Herbst, and Trogoderma granarium
Everts.
7.1.2. Clinical observations
The inhalation studies of Dudley & Neal (1942), Brieger et al.
(1952), and Rogaczewska (1975), and the results of oral and
parenteral administration (Ghiringhelli, 1954; Benesh & Cherna,
1959; Paulet & Despos, 1961; Graham, 1965; Paulet et al., 1966)
indicate that animals inhaling lethal concentrations of
acrylonitrile, or administered lethal dosages of acrylonitrile
orally or parenterally, showed rather similar effects: excitability
and stimulated breathing, shallow rapid breathing, slow gasping
breathing, apnoea, convulsions, and death. Vomiting occurred in
cats, dogs, and monkeys after inhaling acrylonitrile, and in rats
following parenteral administration. Reddening of the skin of the
ears, nose, and feet (in rhesus monkeys, also of the face and
genital organs) and mucosa was accompanied by lachrymation, nasal
discharge, and salivation, not only after inhalation exposure, but
also following oral and sc administration, while hind-leg
incoordination, paresis or paralysis, were observed in rats after
oral administration, and in rabbits after iv administration.
Table 11. Median tolerance limit values (TLm)a for various fish exposed to
acrylonitrile
-------------------------------------------------------------------------------------
Species Water type TLm (mg/litre) Reference
24 h 48 h 96 h
-------------------------------------------------------------------------------------
Fathead minnow hard 32.7 16.7 14.3 Henderson et al. (1961)
(Pimphales promelas) soft 34.3 21.5 18.1 Henderson et al. (1961)
Minnow (Phoxinus - 38.2 17.6 - Marcoci & Ionescu (1974)
phoxinus)
Bluegill (Lepomis soft 25.5 14.3 11.8 Henderson et al. (1961)
macrochirus)
Guppy (Lebistes soft 44.6 33.5 33.5 Henderson et al. (1961)
reticulatus)
Goldfish (Carassius - - - 40 Paulet & Vidal (1975)
sp.)
Carp (Cyprinus - 37.4 24.0 - Marcoci & Ionescu (1974)
carpio)
Rainbow trout hard - 70 - Jackson & Brown (1970)
(Salmo gairdneri)
Pin perch sea 24.5 - - Daugherty & Garett (1951)
(marine fish) (30/l tank)
(Lagodon rhomboides)
Rainbow trout tap, - 5b - Sloof (1979)
(Salmo gairdneri) dechlorinated,
3.6 mg/litre
hard 15 - - Sloof (1979)
Zebra fish same (LC50)
-------------------------------------------------------------------------------------
a TLm median tolerance limit, a concentration of acrylonitrile killing
50% of the test organisms within a specified time.
b Minimal concentration changing respiratory frequency.
(a) Effects on the skin
Direct application of liquid acrylonitrile to the shaved skin
of rabbits induced slight local vasodilation immediately, without
any systemic effect (1-2 ml covering 100-200 cm2) or with an
increased respiratory rate (3 ml over 300 cm2) (McOmie, 1949).
Tuller (1947) observed erythema in only one of 3 areas of abraded
skin, following application of 1 ml of acrylonitrile on a gauze pad
covered by rubber sheeting. However, Zeller et al. (1969) found
that a 15-min application of acrylonitrile on a cotton pad to
shaved skin resulted in skin oedema, and a 20-h application, in
slight necrosis. Guinea-pigs appear to be more sensitive than
rabbits; the application of a 2% solution of acrylonitrile in
acetone for 24 h, under occlusion, did not induce any effects, but
8% or higher concentrations induced dose-dependent erythema
followed by desquamation and mild necrosis (Gut et al., unpublished
data). Erythema of the nose, face, ears, legs, and genital organs
may follow inhalation and oral administration of acrylonitrile.
(b) Effects on the eye
McOmie (1949) instilled one drop of acrylonitrile into the eye
of a rabbit. After 1 h, there was mild conjunctivitis without
corneal clouding or pupillary damage and no effects were observed
after 24 h. Oedema and slight necrosis of the conjunctiva after 8
days were observed in rabbits by Zeller et al. (1969).
(c) Effects on respiration
It was stressed by Paulet et al. (1966) that, after a lethal
intravenous dose of acrylonitrile (120 mg/kg body weight), the
respiratory rate in rabbits did not increase as is characteristic
in cyanide poisoning. However, respiratory disturbance was
observed in: guinea-pigs given a sc lethal dose of acrylonitrile
(130 mg/kg body weight) (Ghiringhelli, 1954), anaesthetized dogs
given 100 mg/kg body weight intravenously (Graham, 1965), mice
given an oral lethal dose (Benesh & Cherna, 1959), and in guinea-
pigs given 100 mg/kg body weight orally (Jedlicka et al., 1958).
Pulmonary oedema was also seen.
An increased respiratory rate followed the application of
liquid acrylonitrile (3 ml/kg body weight) to the skin of rabbits
(McOmie, 1949), and "respiratory distress" was reported in rhesus
monkeys exposed to 163 mg/m3 for 7 h (Brieger et al., 1952). When
rats, rabbits, cats, dogs, and monkeys were exposed to lethal
concentrations of acrylonitrile, Dudley & Neal (1942) observed an
initial stimulation of respiration followed by shallow rapid
breathing, slow gasping breathing, convulsions, coma, and death.
These respiratory effects were absent in guinea-pigs, but
irritation of the pulmonary membranes and some delayed deaths from
lung oedema occurred.
(d) Effects on circulation
Acrylonitrile administered iv at 13, 27, 55, or 110 mg/kg body
weight had little effect on the respiratory and blood pressure
responses of anaesthetized rabbits to adrenalin, noradrenalin, or
acetylcholine, and Gravczyk & Zwierzchovski (1973) believed that
the circulation was not the primary target organ in acrylonitrile
poisoning. However, lethal doses (50 or 100 mg/kg body weight) in
guinea-pigs caused dilation of the right ventricle, congestion of
the coronary blood vessels, hepatic and splenic hyperaemia, and
inflammation of the intestinal mucosa (Jedlicka et al., 1958). In
Sprague-Dawley rats administered a lethal dose of acrylonitrile,
there were haemorrhagic areas in the lung and liver and acute
gastrointestinal inflammation (Monsanto, 1975). Whether the
reddening of nose, ears, legs, face, and genital organs in rats and
other species, after inhalation and oral administration of
acrylonitrile, is due to a direct effect on small vessels or is an
inflammatory response is not known.
(e) Effects on adrenals
The effect of lethal doses of acrylonitrile on the adrenals
became evident in the reports of Szabo & Selye (1971, 1972) and
Szabo et al. (1976). After iv administration of high doses (150 or
200 mg/kg body weight), haemorrhage was observed in both adrenals
of most animals, and there was adrenal haemorrhage in some rats
following oral administration of 10, 15, or 20 mg/kg body weight.
Various types of histological damage were observed in the adrenal
cortex and medulla, some of them within 30 min of acrylonitrile
administration.
A possible mechanism involving the peroxidative action of
acrylonitrile in acrylonitrile-induced adrenal injury has been
suggested recently by Silver & Szabo (1982). Szabo et al. (1980)
investigated the pathogenesis of experimental adrenal haemorrhagic
necrosis using various morphological, biochemical, and
pharmacological methods. Their results suggest that the presence
of a functional adrenocortex is necessary for the development of
cortical damage.
(f) Blood chemistry
Intraperitoneal administration of acrylonitrile to male rats at
33 mg/kg body weight per day for 3 days decreased serum
corticosterone to 30%, prolactin to 40%, but increased follicle-
stimulating hormone (FSH) to 200% of control levels and did not
affect luteinizing hormone (LH) (Nilsen et al., 1980). In adult
male Wistar rats, a single ip administration of acrylonitrile of 10
mg/kg body weight did not have any effect on serum glutamic
oxaloacetic transferase (SGOT) and serum glutamic pyruvate
transaminase (SGPT) activity, but increased lactate dehydrogenase
(EC 1.1.1.27) (LDH) to 200% and sorbitol dehydrogenase (SDH) (EC
1.1.1.14) to 300% compared with the controls (Noel et al., 1978).
The same dose in male rats inhibited butyrylcholineesterase (EC
3.1.1.8), did not have any effect on alkaline phosphatase (EC
3.1.3.1), and increased fructose monophosphate aldolase activity,
suggesting that there had been an adverse effect on the liver
(Ivanov et al., 1979). Administraton of L-cysteine, alpha
tocopherol, or ionol prevented these effects. A single oral dose
of acrylonitrile (l/2 LD50, 41 mg/kg body weight) in rats resulted
in changes in the elution patterns of serum gel chromatography and
paper-electrophoresis of globulins (Franzen & Wagner, 1978). Serum
SDH was significantly elevated (approximately 4-fold) in rats, 24 h
after administration of acrylonitrile at 150 mg/kg body weight. A
60% increase in serum SDH was found in rats administered
acrylonitrile at 500 mg/litre in drinking-water for 21 days (Silver
et al., 1982).
(g) Effects on other organs
Focal superficial necrosis of the liver associated with
haemorrhagic gastritis was found in rats necropsied 24 h after
administration of acrylonitrile at 150 mg/kg body weight in the
drinking-water (Silver et al., 1982).
Acrylonitrile shows an inhibitory effect on K-stimulated
respiration of guinea-pig brain cortex slices at 1 mM, but little
effect on the liver at the same concentration. A stronger
anaesthetic action of acrylonitrile was detected in vitro on the
sciatic nerve of Rana nigra maculata, compared with some other
anaesthetic agents (Hashimoto & Kanai, 1965). The recovery phase of
nerve excitation was also affected by acrylonitrile (Ando &
Hashimoto, 1967).
7.1.3. Biochemical changes and mechanisms of acrylonitrile
toxicity
7.1.3.1. Effect on cytochrome oxidase
Evidence has been presented that cytochrome oxidase (EC
1.9.3.1) activity may be significantly inhibited in acrylonitrile
poisoning. This was suspected after Dudley & Neal (1942) and
Brieger et al. (1952) had reported significant concentrations of
cyanide in dogs and monkeys exposed to acrylonitrile vapours.
Tarkowski (1968) observed inhibited cytochrome oxidase activity in
the brain, kidneys, and liver of rats after ip injection of
acrylonitrile at 100 mg/kg body weight. In vitro, a 50%
inhibition of the enzyme was observed in homogenates of brain,
kidneys, and liver with an acrylonitrile concentration of 10-3M.
Such concentrations have been observed in vivo, shortly after
lethal doses of acrylonitrile (Tarkowski, 1968; Nerudova et al.,
1980; Gut et al., 1981b), but acrylonitrile has a short half-life
in blood and liver after ip or iv administration (15-20 min).
There are correspondingly increased blood and liver concentrations
of cyanide in rats after acrylonitrile administration (up to 180 µM,
Gut et al., 1981b), together with an even greater inhibition of
cytochrome oxidase, and a much higher sensitivity of cytochrome
oxidase activity to cyanide (50% inhibition in vitro at 10-8M
(Tarkowski, 1966).
A significantly decreased ratio of oxidized to reduced
nicotinamide-adenine dinucleotides was observed by Sokal et al.
(1972, 1977) in the brain of rats after sc administration of
acrylonitrile at 100-120 mg/kg body weight, indicating inhibition
of NADH oxidation in the mitochondria, possibly also at the level
of cytochrome oxidase. These changes seem to be of biological
importance, because their magnitude was similar to that observed at
the death of animals subjected to experimental hypoxia. Thus, the
cyanide-mediated inhibition of cytochrome oxidase would seem to be
of importance in the later stages of intoxication and death. This
"cyanide effect" is apparently more pronounced at higher
acrylonitrile doses (Willhite & Smith, 1981) and appears to be more
significant in mice and dogs (Brieger et al., 1952; Benesh &
Cherna, 1959; Gut et al., 1981b) than in rats. This is in
agreement with the greater efficacy in mice than in rats of
thiosulfate (an antidote to cyanide) in acrylonitrile poisoning,
and with the higher cyanide concentration in the blood of dogs than
in that of rats (101 µM versus 10 µM), after breathing the same
concentration of acrylonitrile (217 mg/m3 for 7 h) (Brieger et al.,
1952). The protective effect of another cyanide antidote, nitrite
(Dudley & Neal, 1942; Ghiringhelli, 1954; Benesh & Cherna, 1959),
in acrylonitrile poisoning also points to the participation of
cyanide in lethal acrylonitrile poisoning.
7.1.3.2. Effect on sulfhydryls
There is considerable evidence to demonstrate that
acrylonitrile significantly depresses the concentrations of soluble
glutathione and protein sulfhydryls in the blood, liver, brain, and
kidney. Acrylonitrile also inhibits some SH-dependent enzymes that
participate in carbohydrate metabolism. Wisniewska-Knypl (1970,
1978), Hashimoto & Kanai (1972), and Vainio & Mäkinen (1977)
observed that the inhibition of sulfhydryls was dose-dependent in
the range of 10-100 mg/kg body weight in vivo, and in the
concentraton range 0.01-10 nM in vitro (Wisniewska-Knypl, 1978).
A significant decrease in brain sulfhydryls was reported after a
single dermal application of acrylonitrile of as little as 2.82
mg/kg body weight (Zotova, 1976). These effects were observed
after sc, ip, or iv administration to rats, rabbits, hamsters,
guinea-pigs, and mice. There were some decreases in the activity
of serum or tissue - SH-dependent enzymes including oxoglutarate
dehydrogenase (EC 1.2.4.2). However, the activity of succinate
dehydrogenase (EC 1.3.99.1) was not reduced (Wisniewska-Knypl,
1978), and there were corresponding increases in the liver, blood,
and brain concentrations of glucose, pyruvate, and lactate
(Hashimoto & Ando, 1966; Dinu & Klein, 1976).
It was shown by Zitting et al. (1981) that short-term exposure
to acrylonitrile decreased the liver glutathione content within 4 h
of poisoning, but that the glutathione contents returned to normal
in brain, liver, and kidney, within 24 h. At the same time, the
activity of cerebral succinate dehydrogenase and of ethoxycoumarin
demethylase in liver and kidney decreased. Increased glucose,
pyruvate, and lactate concentrations in blood, liver, and brain
were also found, immediately after the fifth exposure, in rats
exposed through inhalation to an acrylonitrile concentration of 300
mg/m3, 8 h daily, for 5 days. In protein, sulfhydryl-dependent
enzyme inhibition was absent and the glutathione level was
significantly reduced in the liver but not in the brain (Gut et
al., 1982). The effects of acrylonitrile on sulfhydryls were
significantly reduced by co-administering L-cysteine and other
sulfhydryls and there were corresponding decreases in lethal
effects (Hashimoto & Kanai, 1965; Bondarev et al., 1976; McLaughlin
et al., 1976; Appel et al., 1981). The results of these studies
demonstrate the protective role of SH-groups in acrylonitrile
poisoning.
The role of hypoxia in the acute thiol-depressive effect of
acrylonitrile in male rats was investigated by Jaeger (1978) and
Jaeger & Cote (1982). Hypoxia was found to enhance non-protein SH
loss in the liver, when there was exposure to acrylonitrile (Jaeger
& Cote, 1982).
Evidence has been presented (Holechek & Kopecky, 1981) that
inhibition of tissue sulfhydryls may be due not only to the
acrylonitrile itself, but also to its reactive metabolite,
glycidonitrile.
7.1.3.3. Interaction with the microsomal oxidation system as a
possible mechanism of toxicity
Acrylonitrile added to mouse, rat, and human liver microsomes
caused characteristic spectral complexes with cytochrome P-450
(Ivanov et al., 1979; Appel et al., 1981).
It was shown in vitro that glycidonitrile, which is
generated in rat-liver microsomes by mixed-function oxidases (EC
1.14.14.1), covalently binds to microsomal membrane and albumin
(Ivanov et al., 1982). The biological significance of this
phenomenon may be inferred from experiments with inhibitors and
inducers of the mixed-function oxidases (Ivanov, 1981). In rats
pre-treated with phenobarbital, an increased amount of
glycidonitrile was covalently bound to macromolecules and
substantially higher activity of fructose-bisphosphate aldolase (EC
4.1.2.13) was observed in the blood, indicating liver damage. On
the other hand, SKF-525A, the inhibitor of cytochrome P-450,
reduced both effects in vivo and in vitro . Activation of
acrylonitrile by cytochrome P-450 may therefore result in a
cytotoxic effect.
A previous injection of SKF-525A or of cobalt(II) chloride
(CoCl2), another inhibitor of cytochrome P-450, resulted in
significant protection against the gastrointestinal bleeding in
rats caused by acrylonitrile (Ghanayem & Ahmed, 1982).
Some data show that acrylonitrile and its epoxide,
glycidonitrile, bind covalently, in vitro, to DNA and RNA
(Guengenrich et al., 1981; Peter et al., 1983a). However, the
quantitative extent of truly irreversible binding is much less than
that observed in experiments with other vinyl monomers (Peter et
al., 1983).
A lower content of cytochrome P-450 in the liver and reduced
oxidative microsomal metabolism of xenobiotics were observed in
rats after an ip injection of acrylonitrile at 10 or 33 mg/kg body
weight, for 3 days (Noel et al., 1978; Nilsen et al., 1980), after
inhalation exposure to 300 mg/m3 of acrylonitrile, 8 h day, for 5
days (Gut et al., in press), and in Chinese hamsters after an ip
injection of 30 mg/kg (Zitting et al., 1981). Inhibition of
microsomal oxidation of xenobiotics by acrylonitrile was observed
in vitro (Ivanov et al., 1979).
Pre-treatment of rats with inducers of microsomal oxidases such
as phenobarbital, 3-methylcholantrene, or Arochlor 1254, nullified
the effect of acrylonitrile on the total cytochrome P-450 content.
The activity of other microsomal enzymes, glucose-6-phosphatase (EC
3.1.3.9), and NADPH cytochrome-c-reductase (EC 1.6.2.4), was
unaffected by acrylonitrile (Duverger-van Bogaert et al., 1978;
Noel et al., 1978).
Ghanayem & Ahmed (1982) showed that Arochlor 1254 drastically
increased acrylonitrile-induced gastric bleeding in rats.
Phenobarbital significantly increased acrylonitrile-induced
hepatocyte damage in rats (Ivanov, 1981).
Acrylonitrile binds with liver microsomal and S-9 fractions, in
vitro, by direct alkylation. The microsomal activation of
acrylonitrile into reactive intermediates was also detected
(Duverger-van Bogaert et al., 1982a). The irreversible binding of
acrylonitrile to liver microsomal proteins was inhibited by thiols
and even more by dithiocarb (Peter & Bolt, 1981).
7.1.3.4. Observations on the possible participation of membrane
lipid peroxidation in the mechanism of toxicity
The ip administration of acrylonitrile at 10 mg/kg body weight
induced lipid peroxidation in rat liver (Dinu, 1975a; Ivanov et
al., 1979) and erythrocyte membranes (Ivanov et al., 1982),
indicating possible damage of cellular membranes. NADPH-dependent
lipid peroxidation in rat liver microsomes was only slightly
stimulated (Ivanov et al., 1978; Duverger-van Bogaert et al., 1981)
whereas substantial stimulation was found in the post-mitochondrial
fraction of rat liver, lung, and brain, as well as in brain-marrow
homogenate (Ivanov et al., 1978; Ivanov, 1979; Al'shansky et al.,
1980). There was a correlation between an increased amount of
malondialdehyde and a decreased content of -SH groups in the post-
mitochondrial supernatant of rat liver (Ivanov, 1981) and brain
(Al'shansky et al., 1980). Conjugated diene concentrations in rat
liver microsomes were significantly elevated after iv
administration of acrylonitrile to rats (150 mg/kg body weight),
but no change was seen in the adrenal glands (Silver & Szabo,
1982).
Pre-treatment of rats with antioxidants, in doses equivalent to
those of the acrylonitrile administered, afforded protection
against the pro-oxidant effect of acrylonitrile and elevation of
blood fructose-1-phosphate aldolase, decreased the activity of
butyrylcholine esterase (EC 3.1.1.8) (Ivanov et al., 1979), and
reduced the GABA content and activity of glutamate decarboxylase
(EC 4.1.1.15) in the brain (Al'shansky et al., 1980).
7.1.3.5. Studies on antidotes
Appel et al. (1981a) found that the cyanide antidotes, 4-
dimethylaminophenol plus thiosulfate, protected rats against the
lethal effects of orally-administered acrylonitrile. A comparative
evaluation was made by McLaughlin et al. (1975) of the efficacy of
thiols (cysteine hydrochloride) and cyanide antidotes. The authors
showed that thiols were more effective in protecting rats against
acrylonitrile poisoning. Bondarev et al. (1976) demonstrated the
protective role of some sulfur-containing compounds in the
acrylonitrile poisoning of rats. The protective effects of some
antioxidants, such as vitamin E and ionol, have also been
demonstrated (Ivanov et al., 1979).
The possible toxic mechanisms and theoretical protective
mechanisms are summarized in Fig. 3, which indicates the complex
nature of the interference of acrylonitrile with cellular
mechanisms, as far as can be derived from current knowledge.
 7.2. Subacute Toxicity
7.2.1. Inhalation exposure
Rats, guinea-pigs, rabbits, monkeys, and cats were exposed to
acrylonitrile at 330 mg/m3 air for 4 h per day, 5 days a week, for
8 weeks. All adult rats survived 8 weeks, but 5 out of 8 young
rats died by the 6th week, 3 out of 16 guinea-pigs and 1 out of 4
rabbits died during the 5th week, and 1 out of 2 monkeys died after
6 weeks of exposure. At 220 mg/m3 for 4 h/day, 5 days a week, for
8 weeks, all rats, rabbits, and guinea-pigs survived for 8 weeks,
but 1 out of 4 cats died during the third week. After the first 4-
h exposure to 120 mg/m3, 1 out of 2 dogs died, but the other
survived 4 weeks' exposure. Four rhesus monkeys survived 4 weeks'
exposure to 120 mg/m3 for 4 h/day (Dudley et al., 1942).
CD-1 mice, Charles River rats, and beagle dogs were exposed to
acrylonitrile 57 times for 6 h a day, 5 days a week, over a 90-day
period. Some dogs were killed by exposure to 117 mg/m3 (54 ppm)
but not 58 mg/m3 (24 ppm). Mice and rats were unaffected by these
concentrations, but a concentration of 234 mg/m3 (108 ppm) was
lethal for half the rats and mice. As with acute exposures, dogs
were more sensitive than rats and mice, but mice did not appear to
be more sensitive than rats. Atmospheric concentrations of
acrylonitrile of 58, 117, and 117 mg/m3 did not induce any lethal
effects in dogs, rats, and mice, respectively (Brewer, 1976).
7.2.2. Oral administration
Over a period of 7 weeks, 6 rats were administered orally 15
doses of acrylonitrile at 30 mg/kg body weight, then 7 doses at 50
mg/kg, followed by 13 doses at 75 mg/kg, without lethal effects
being induced (Barnes, 1970). No deaths occurred when Sprague-
Dawley rats were offered 85 mg or less of acrylonitrile per litre
of drinking-water for 90 days (Humiston & Frauson, 1975). Given
the slow absorption of acrylonitrile from the gastrointestinal
tract, blood levels could have been low, and it can be calculated
that the daily dose could have been about 8.5 mg/kg body weight.
The studies are compatible with the view that acrylonitrile is
unlikely to have a significant cumulative effect.
7.2.3. Subcutaneous administration and intraperitoneal
administration
Daily sc doses of 40 mg/kg body weight over 4 weeks, or daily
ip injections of 20 mg acrylonitrile/kg body weight over 6 weeks,
were not fatal for rats (Krysiak & Knobloch, 1971).
7.2.4. Clinical observations in animal studies
Rats exposed to acrylonitrile concentrations of 220 mg/m3 for 4
h daily, 5 days a week (Dudley et al., 1942) over a period of 8
weeks, showed slight lethargy but gained weight, as did guinea-
pigs. Rabbits failed to gain weight and were listless, while cats
became listless, vomited, and lost weight. One cat developed a
transitory weakness of the hind legs after the third exposure and
died after the eleventh exposure; the 3 remaining cats survived 8
weeks with few untoward effects. Exposure to an atmospheric
concentration of 330 mg/m3 resulted in weight loss in rats, their
coats became rough, and their general physical condition poor
(Dudley et al., 1942). Young rats and guinea-pigs showed impaired
growth and marked irritation of the eyes and nose during the first
week of exposure. Marked eye and nose irritation was also seen in
rabbits and cats and the latter developed transitory weakness of
the hind legs. Monkeys appeared sleepy and weak and frequently
salivated and vomited. Thus, the 220 mg/m3 exposure level markedly
affected cats, while rabbits and guinea-pigs were little affected;
a concentration of 330 mg/m3 induced various effects including
death. Brewer (1976) exposed CD-1 mice, Charles River rats, and
beagle dogs to acrylonitrile concentrations of 0, 58, 117, 234
mg/m3 (0, 24, 54, or 108 ppm) for 6 h daily, 5 days a week, for 13
weeks. Signs observed included ataxia, ptosis, emaciation,
rhinitis, and diuresis. As is common with acrylonitrile over-
exposure, convulsions usually preceded death (e.g., Benesh &
Cherna, 1959; Paulet et al., 1966).
7.2.4.1. Body weight, food and water consumption
Loss of body weight or failure to gain body weight was seen in
rats exposed to acrylonitrile at 330 mg/m3 and in cats and rabbits
exposed to 220 mg/m3 for 4 h a day, 5 days a week, for 13 weeks.
Loss of appetite was seen in rhesus monkeys exposed to 330 mg/m3,
while 120 mg/m3 did not elicit any toxic effects (Dudley et al.,
1942). No adverse effects were seen in 6 rats administered
successively 15 doses of 30 mg/kg body weight, 7 doses of 50 mg/kg,
and 13 doses of 75 mg/kg over a period of 7 weeks (Barnes, 1970).
Adult Sprague-Dawley rats received between 0 and 42 mg
acrylonitrile/kg body weight in drinking-water for 90 days. The
body weight was depressed in males receiving 42 mg/kg, while
females were affected by 22 mg/kg, but only after the 57th day.
The mean weekly food consumption of males was lower for 7 weeks on
38 mg/kg and for 2 weeks on l7 mg/kg. Food consumption decreased
in females receiving 42 or 22 mg/kg for 6 weeks and 1 week,
respectively (Humiston & Frauson, 1975).
7.2.4.2. Organ weights and pathology
The weights of the liver, kidney, spleen, pituitary gland,
lungs, gonads, thyroid gland, adrenals, heart, and brain of rats,
mice, and dogs exposed to an acrylonitrile concentration of 234
mg/m3 for 6 h a day, 5 days a week, for 13 weeks, were within
normal limits (Brewer, 1976). In rats receiving acrylonitrile in
drinking-water for 90 days, no changes in absolute or relative
organ weights were seen in males receiving 4 mg/kg body weight or
females receiving 5 mg/kg, daily (Humiston & Frauson, 1975). The
relative liver weight was significantly increased in males and
females receiving 17 mg/kg (males) or 22 mg/kg (females) or more.
The weights of the heart and liver were increased significantly in
adult Wistar rats administered 50 mg acrylonitrile/kg body weight,
intraperitoneally, daily, for 3 weeks (Knobloch et al., 1971);
their weight loss caused an increase in the relative weights not
only of the heart and liver, but also of the kidney and spleen.
Dudley et al. (1942) examined the livers of rats, guinea-pigs,
rabbits, cats, dogs, and rhesus monkeys exposed to acrylonitrile at
220 or 230 mg/m3, and observed histological changes only in cats.
Liver parenchymal degeneration was reported in adult Wistar rats
after daily ip administration of 50 mg/kg body weight for 3 weeks
(Knobloch et al., 1971). In the above-mentioned study, Dudley et
al. (1942) also reported signs of renal damage, such as hyaline
casts in the straight collecting tubules of all species, and
limited subacute interstitial nephritis; this was especially seen
in guinea-pigs and rabbits. Parenchymal degeneration of the
kidneys was reported by Knobloch et al. (1971). In the study by
Dudley et al. (1942), lungs were affected by subacute
bronchopneumonia, congestion, and oedema of the alveolar walls,
extravasation of erythrocytes and serum into the alveoli, focal
collection of lymphyocytes and polymorphonuclear leukocytes, in
most guinea-pigs, rabbits, the monkey, and 1 out of 3 of the rats.
The authors also reported slight haemosiderosis in the spleen of
rats, but negligible siderosis in cats, guinea-pigs, and rabbits.
Exposure of rats to acrylonitrile (22 mg/m3, 10 ppm) for 7 weeks
and (100 mg/m3, 50 ppm) for another 6 weeks caused enlargement of
the liver, kidney, heart, and spleen, but co-administration of
vitamins B1, B2, and cystine had a protective effect against
enlargement of the heart. Alcohol dehydrogenase activity in the
liver decreased after exposure, but the above-mentioned drugs
alleviated this decrease, to some extent (Takagi et al., 1968).
7.2.4.3. Blood
A normal haematological picture was reported in rats and dogs
repeatedly exposed to acrylonitrile vapours at up to 240 mg/m3
(Brewer, 1976), and in rats and rabbits (except for a raised
eosinophil count) repeatedly exposed to up to 330 mg/m3 (Dudley et
al., 1942).
Minami et al. (1973) exposed male rabbits to 54 mg/m3 for 1 day
per week (8 h) for 8 weeks; haematocrit and haemoglobin were
unaffected, but pO2 and pH were raised and pCO2 lowered by the
treatment.
In rats receiving acrylonitrile in the drinking-water for 90
days, the only significant haematological change was a decrease in
the red-cell count, on the 83rd day, in females receiving 42 mg/kg
body weight per day (Humiston & Frauson, 1975). The blood urea and
alkaline phosphatase (EC 3.1.3.1) levels in the males receiving 38
mg/kg body weight per day were raised, but SGPT activity was
normal. While rats administered ip 50 mg acrylonitrile/kg body
weight, daily, for 3 weeks (Knobloch et al., 1971) developed
leukocytosis and increased serum asparagine-oxo-aminotransferase
(EC 2.6.1.14) activity, mice exposed for 70 days to 225 mg/m3 (100
ppm) or 340 mg/m3 (150 ppm) 6 h daily, did not develop any
haematological abnormalities (Hashimoto, 1962). Rats exposed to
9.7 mg/m3, 4 h daily, 5 days a week for 2 months, did not show any
effects on the erythrocyte count or on the haemoglobin
concentration (Vissarionova et al., 1979).
As a whole, the studies failed to demonstrate any consistent
effect of acrylonitrile on red or white blood cell production or
viability. Leukocytosis following repeated intraperitoneal
administration of an irritant material is to be expected.
7.2.4.4. Immune system
Wistar rats were exposed to an acrylonitrile concentration of
10 mg/m3 for 6 h daily, 5 days a week, for 16 weeks. Acrylonitrile
depressed both T helper and T suppressor functions, and a
diminished degree of B lymphocyte transformation was observed.
Alpha tocopherol (im 0.21 mmol/kg body weight on alternate days for
16 weeks) protected against this effect (Krivova et al., 1982).
7.2.4.5. Nervous system
Some findings in the experimental animal studies on
acrylonitrile were indicative of an effect on the nervous system.
Rats, mice, and dogs exposed to up to 240 mg/m3, for 6 h daily, 5
days per week, for 13 weeks, showed ataxia and convulsions, prior
to death (Brewer, 1976). Transitory hind-leg weakness was seen in
cats exposed for 8 weeks (4 h per day, 5 days per week) to 330
mg/m3 (Dudley et al., 1942). Krysiak & Knobloch (1971) found that
rats receiving acrylonitrile intraperitoneally at 20 mg/kg body
weight daily for 6 weeks, or sc at 40 mg/kg daily for 4 weeks,
showed a significant lengthening of the time to perform correctly
in a conditioned food reflex test, and a significant decrease in
the number of correct reactions, compared with pre-treatment
observations or controls. Performance improved when the treatment
was discontinued. Daily ip administration to rats of acrylonitrile
at 50 mg/kg body weight for 3 weeks caused a vacuolization of
neuronal cells of the cortex and brain stem (Knobloch et al.,
1971).
7.2.4.6. Urine
No significant changes in urine composition were observed in
the experimental studies of Humiston & Frauson (1975) (sections
7.2.4.1 - 7.2.4.3).
7.2.4.7. Adrenals
The adrenals of rats exposed for 21-60 days to acrylonitrile
in drinking-water (0.05% and 0.2%) showed an atrophic zona
fasciculata and an enlarged zona glomerulosa. The animals on the
higher dose had a reduced plasma corticosteroid level and an
increased plasma Na+Concentration. The K+ level was unchanged
(Szabo et al., 1976). Serum corticosterone in rats was decreased
by 3 ip doses of acrylonitrile (33 mg/kg body weight) on successive
days (Nilsen et al., 1980).
7.2.4.8. Metabolism
After repeated exposure of rabbits to acrylonitrile, the in
vitro metabolism in the liver of acrylonitrile into cyanide and
thiocyanate decreased with time, while the excretion of unchanged
acrylonitrile in the urine increased (Sato, 1978).
7.3. Chronic Toxicity
Observations have been made on animals administered
acrylonitrile in drinking-water, food, through inhalation, and by
dermal application.
Tuller (1947) administered acrylonitrile at 500 mg/litre in the
drinking-water or acrylonitrile-fumigated food (dose not precisely
specified) to rats. After 2 years, the mortality was higher in
rats drinking acrylonitrile solution (50% deaths) than in paired
controls (25%), another control group (15%), and in rats on
acrylonitrile-fumigated food (5%). However, when acrylonitrile was
administered at 0.5, 5, and 90 mg/litre in the drinking-water to
male and female CFW rats for 2 years, the mortality rate was
unaffected (Svirbely & Floyd, 1961). Groups of 4 male and 4 female
beagle dogs were administered acrylonitrile at concentrations of
100, 200, or 300 mg/litre in the drinking-water, for 6 months.
Average intakes of acrylonitrile were the following for males
(females): 10(8) mg/kg body weight at 100 mg/litre; 16(17) mg/kg at
200 mg/litre; and 17(18) mg/kg at 300 mg/litre. Five dogs died, or
were killed because debilitated, in each of the 2 higher dosage
groups (Quast et al., 1975).
In the dogs receiving acrylonitrile at 100-300 mg/litre in the
drinking-water, early signs of toxicity included roughening of the
coat and, later, retching and vomiting. Terminal signs of lethargy,
weakness, emaciation, and respiratory distress were noted (Quast et
al., 1975).
7.3.1. Body weight, food and water intake
Body-weight gain was reduced during 4 of the 11 weeks at the
higher dose level (240 mg/m3) in a test in which male and female
Wistar rats and albino rabbits were exposed to acrylonitrile vapour
at concentrations of 0, 50, and 240 mg/m3, for 3 h/day, 6 days a
week, for 6 months (Knobloch et al., 1972).
Growth retardation was observed in male rats drinking 500 mg
acrylonitrile per litre water, for 2 years (Tuller, 1947). Svirbely
& Floyd (1961) administered acrylonitrile at 0.5, 5, or 50 mg/litre
in the drinking-water of rats and found a slight decrease in water
consumption at the highest concentration in both sexes.
Statistically significant reductions in the body weight of rats
receiving drinking-water containing acrylonitrile concentrations of
35, 100, or 300 mg/litre were associated with decreased water
consumption and decreased food consumption at 300 mg/litre in males
and 100 mg/litre in females (Ouast et al., 1977). Decreased food
and water consumption and body weight was reported with beagle dogs
drinking acrylonitrile at 200 or 300 mg/litre water for 6 months
(Quast et al., 1975). Marked weight decreases were seen in dogs
that eventually died or had to be killed.
7.3.2. Organ weights
In rabbits exposed to atmospheric acrylonitrile concentrations
of 250 mg/m3, for 3 h/day, 6 days a week for 6 months, a
significant increase in the heart weight was noted, and some
fluctuations in blood pressure were described (Knobloch et al.,
1972).
Ferin et al. (1961) exposed rats to drinking-water containing
an acrylonitrile concentration of 20 or 1000 mg/litre for 6 months.
At the higher dose, increased relative weights of the liver,
spleen, and kidneys were noted. The relative weights of the heart,
liver, and brain in males, and of the liver and kidneys in females,
were increased in rats receiving 300 mg/litre in drinking-water.
The males drinking 100 mg/litre showed a significantly increased
brain weight, and the females receiving 100 mg/litre had
significantly lower relative heart weights (Quast et al., 1977).
The weights of brain, heart, liver, and kidneys of beagle dogs
drinking an acrylonitrile concentration of 100 mg/litre in
drinking-water (Quast et al., 1977) were normal but, at 200
mg/litre, the 2 remaining males had a lower absolute brain weight
and higher relative kidney weight than controls. The 2 remaining
females receiving 300 mg/litre also had significantly lower relative
brain weights compared with controls.
7.3.3. Pathology and histology
Inflammation of the pulmonary system accompanied by an
inflammatory exudate into the bronchial lumen occurred in rats
exposed to acrylonitrile at 240 mg/m3 for 3 h a day, 6 days a
week, for 6 months (Knobloch et al., 1972). Various pathological
changes occurred in male and female rats maintained on water
containing acrylonitrile at 35, 100, or 300 mg/litre for 12 months
(Quast et al., 1977). Males and females on the 2 higher doses
developed paleness and thickening of the mucosa, erosions, ulcers,
and sometimes papilloma formations in the non-glandular portion of
the stomach. Three females receiving 300 and 100 mg/litre and one
male at 300 mg/litre had ear canal tumours. Microscopic findings
of tissue with tumorous growth revealed an increased frequency of
gastric cell papillomas, Zymbal (sebaceous) gland tumours of the
ear canal, and microtumours of the nervous system, in rats
receiving a concentration of acrylonitrile of 100 or 300 mg/litre.
These tumours do not occur spontaneously at such a high frequency
in the strain of rat used. The nervous system lesions were
consistent with the diagnosis of astrocytoma.
Minimal lesions were seen in the liver of rats drinking
acrylonitrile at 100 or 300 mg/litre and chronic renal disease
occurred in females drinking 300 mg/litre. The squamous epithelium
of the stomach was hyperplastic in rats drinking 100 or 300
mg/litre.
Histopathological changes in dogs receiving acrylonitrile
concentrations of 200 and 300 mg/litre in water (Quast et al.,
1975) were similar to those in untreated controls. The pneumonia
present may have resulted from irritation of the mucosa of the
tongue and oesophagus, which produced abnormal swallowing,
resulting in aspiration of some food.
7.3.4. Haematology and clinical chemistry
In rats exposed for 3 h/day, 6 days a week, for 6 months to
acrylonitrile in air concentrations of either 50 or 240 mg/m3, the
eosinophil count had significantly increased after 4 months. Total
serum protein was unchanged, while albumin and alpha-globulin had
increased and gamma-globulin decreased in both test groups
(Knobloch et al., 1971). Leukocytosis was observed in rats
drinking acrylonitrile in water at 1000 mg/litre (Ferin et al.,
1961). Periodic examinations of rats drinking 0.5, 5, or 50
mg/litre in water (Svirbely & Floyd, 1961), or 0 - 300 mg/litre in
water (Quast et al., 1977), showed normal haematological findings.
A significant elevation in alkaline phosphatase activity was found
in female rats exposed to 300 mg/litre. Half-way through the
study, beagle dogs drinking acrylonitrile concentrations of 0, 100,
200, or 300 mg/litre in water showed a significant decrease in
haematocrit, erythrocyte count, and haemoglobin concentration at
300 mg/litre and decreased erythrocyte count at 200 mg/litre, in
males. Females on 300 mg/litre also showed a significant decrease
in the erythrocyte count. However, all haematological findings
were within normal ranges towards the end of the study, except in
males at 300 mg/litre, which had a lower erythrocyte count. Blood
urea nitrogen, serum alkaline phosphatase activity, SGPT, and SGOT
were measured periodically; the findings in males were always
within normal limits, but females receiving 300 and 200 mg/litre
showed some increase in SGOT and SPGT activity. Total and
individual serum proteins were unaffected at the end of the study.
Zotova (1976) exposed rats by applying acrylonitrile solution to
the skin of the tail at doses of 2.82, 0.56, or 0.11 mg/kg body
weight per day and observed a decreased haemoglobin concentration
at the highest dosage level after 2 months. Blood catalase (EC
1.11.1.6) activity increased, and blood peroxidase (EC 1.11.1.7)
activity decreased, initially, but later became normal; there was
no change in sulfhydryl levels.
A decrease in blood -SH groups was reported in rats exposed to
acrylonitrile at 10 mg/m3 for 5 days/week, 4 h a day, for 4 months
(Efremov, 1976d).
7.3.5. Nervous system
Changes in central nervous function, as elicited by a
conditioned avoidance test, were found in rats drinking water
containing acrylonitrile at 20 mg/litre, for 6 months (Ferin et
al., 1961). Rats exposed through inhalation to acrylonitrile
concentrations of 50 and 240 mg/m3 for 3 h a day, 6 days a week,
for 6 months, showed significant defects in performance in a "Y"
maze (Krysiak, 1971). Rats exposed to 10 mg/m3, 5 days/week, 4 h a
day, for 4 months, showed a 59% decrease in the activity of brain
catalase, a 59% decrease in brain peroxidase, and a 37% decrease in
-SH groups (Efremov, 1976d). Histopathological changes in the
nervous system, consistent with a diagnosis of astrocytoma, were
observed in rats exposed to acrylonitrile at 35, 100, or 300
mg/litre in drinking-water (Quast et al., 1977).
7.3.6. Kidney function
Knobloch et al. (1972) exposed rats to 50 or 240 mg/m3, for
3 h per day, 6 days per week, for 6 months; kidney dysfunction was
indicated by increased urinary output at both concentrations and an
increase in urinary protein and areas of degenerated proximal
convoluted tubules at the higher concentration.
There were no abnormalities in the urine chemistry of rats
drinking water containing acrylonitrile at 0, 35, 100, or 300
mg/litre (Quast et al., 1977), or of dogs (Quast et al., 1975),
drinking water containing 100, 200, or 300 mg/litre. When
acrylonitrile was applied to the skin of the tail of rats, daily,
at doses of 2.82, 0.56, or 0.11 mg/kg body weight for 4.5 months,
the excretion of urinary chlorides increased on day 10 of exposure
at the 2 higher doses and decreased at the lowest dose (Zotova,
1976).
7.4. Teratogenicity and Embryotoxicity
The teratogenic potential of ingested or inhaled
acrylonitrile was investigated by Murray et al. (1978).
Groups of pregnant SD rats were given acrylonitrile at 0, 10,
25, or 65 mg/kg body weight per day, by gavage, from day 6 to
day 15 of gestation. Groups of 30 pregnant SD rats were
exposed 6 h per day to 0, 87, or 174 mg/m3 (0, 40, or 80 ppm)
acrylonitrile by inhalation, during the same period of
pregnancy. A dose of 65 mg/kg body weight per day caused
marked maternal toxicity, significant embryotoxicity, and an
increased incidence of fetal malformations. Findings of the
two studies suggesting a teratogenic effect were noted at 25
mg/kg per day and at 174 mg/m3 (80 ppm). At 10 mg/kg body
weight per day and 87 mg/m3 (40 ppm), no embryotoxicity or
teratogenicity was found. There was no apparent correlation
between the degree of toxicity seen in the individual dams and
the occurrance of malformations in their offspring.
Embryotoxic effects in pregnant mice of 3 strains were
described after intraperitoneal administration of unspecified doses
of acrylonitrile (Scheufler, 1976). A single ip injection of
acrylonitrile of 32 mg/kg body weight, given on the 5th or 7th day
of pregnancy, induced an embryotoxic effect in mice from an inbred
strain of AB Jena-Hall, but not in DBA and C57 C1 mice (Scheufler,
1980).
Kankaanpää et al. (1979) studied the embryotoxic effects of
acrylonitrile using chick eggs, but did not find any clear evidence
of its teratogenicity.
The exposure of Sprague-Dawley rats to an acrylonitrile
concentration in drinking-water of 500 mg/litre led to decreased
fertility and decreased viability of the young, and the females
developed a progressive muscular weakness in the hind legs about
16-19 weeks after the weaning of the second litter (Svirbely &
Floyd, 1961).
Willhite (1981a,b) observed skeletal malformations in hamster
fetuses after the administration of acrylonitrile at 80 mg/kg body
weight to pregnant hamsters. The histological study of both early
embryos and term fetuses revealed mesodermal changes, including a
reduction in the number of cells, shrinkage of the cell cytoplasm,
and enlarged extracellular spaces. In addition, a reduction in
mitotic figures and focal necrosis were noted. The affected
embryos were smaller and their development was delayed compared
with untreated controls. Teratogenic effects were only observed
when there was simultaneous maternal toxicity.
7.5. Mutagenicity
7.5.1. Bacterial systems
(a) Ames test with Salmonella typhimurium strains
Tests have been carried out on several strains, with and
without metabolic activation, using several methods of treatment
with acrylonitrile. Negative results were obtained, with and
without activation, in 5 tested strains in 2 studies (Litton
Bionetics, 1975; Stanford Research Institute, 1976). A weak, but
reproducible, positive effect was observed with metabolic
activation using between 0.5 and 1.5 mg acrylonitrile per plate in
strain TA 1535 (Haskell Laboratory, 1975; De Meester et al., 1978,
1979). Three methods of exposure were examined by Milwy & Wolff
(1977) in 3 strains of S. typhimurium. A low level of mutagenic
activity was noted in strain TA 1535, when plates were sprayed with
acrylonitrile or when it was mixed with the medium, with
activation.
Exposure to acrylonitrile vapour of the strains TA 1535 and TA
100 also demonstrated acrylonitrile mutagenicity (Duverger-Van
Bogaert et al., 1981; Ivanov, 1981). Zhurkov et al. (1983) tested
the mutagenicity of acrylonitrile in strains TA 1535 and TA 1538,
with and without microsomal activation, and found a dose-dependent
effect in strain TA 1535.
Urine collected from rats and mice treated with acrylonitrile
was mutagenic in S. typhimurium strain TA 1530, in the absence of
metabolic activation. Pre-treatment of the animals with
phenobarbital abolished the direct mutagenicity of urine from rats
and reduced that from mice. The addition of beta-glucuronidase (EC
3.2.1.31) to the incubation mixtures enhanced the mutagenic
activity of urine from acrylonitrile-treated animals (Lambotte-
Vandepaer et al., 1980, 1981a). Duverger-van Bogaert et al. (1982b)
suggested that glutathione might play a role in the formation of a
mutagenic metabolite of acrylonitrile. The mutagenic activity of
acrylonitrile vapours towards S. typhimurium strains was strictly
dependent on the presence of an activation system, confirming the
report of Milwy & Wolff (1977). Lambotte-Vandepaer et al. (1980)
indicated that animal urine might retain its mutagenic activity for
as long as a week after collection. The acrylonitrile-derived
epoxide, glycidonitrile, synthesized by Kopecky & Smejkal
(unpublished data, 1979), was shown to be the principal substance
that exerted mutagenic activity in the absence of metabolic
activation, whereas acrylonitrile itself required metabolic
activation in the S. typhimurium Ames test (Cherna et al., 1981).
(b) Mutagenicity in Escherichia coli
One of 3 strains of E. coli (WP2) revealed mutagenic activity
of acrylonitrile; activation did not have any effect (Venitt et
al., 1977). The mutagenic activity of acrylonitrile was confirmed
in other experiments using the simplified fluctuation test of Green
et al. (1976). The results suggested that acrylonitrile caused
non-excisable mis-repair of DNA associated with the generation of
DNA strand breaks (Venitt et al., 1977). The method of Slater et
al. (1971) did not reveal any effect with or without an activation
system at 10 µg acrylonitrile per plate (Litton Bionetics, 1976).
The variability of the results, even when the same kinds of
assays are used, could be because of differences in purity of the
acrylonitrile, in method, or in bacterial sensitivity. However, the
mutagenicity of acrylonitrile in bacterial systems seems to have
been established.
7.5.2. Yeast assays
Possible mutagenic activity of acrylonitrile was found with
Saccharomyces cerevisiae, but metabolic activation was without
effect (Litton Bionetics, 1975).
7.5.3. Drosophila melanogaster
A negative result was obtained when 0.1% acrylonitrile was
administered by intra-abdominal injection into D. melanogaster in
order to examine its ability to induce a recessive lethal effect in
the X chromosomes (Benesh & Shram, 1969).
7.5.4. Mammalian cell in vitro assays
The L5178Y kinase mouse lymphoma cell assay (Litton Bionetics,
1976) failed to show mutagenic activity of acrylonitrile using the
procedure of Clive & Spector (1975). Chinese hamster ovary cells
showed an increase in sister chromatid exchange (SCE) after
exposure to acrylonitrile, when co-cultured with rat hepatocytes
(Ved Brat & Williams, 1982). No effect was found without the
latter.
Acrylonitrile induced a slight increase in the SCE of cultured
human lymphocytes in the presence of S-9 mix and increased
unscheduled DNA synthesis with a very high concentration (0.5 M)
(Perocco et al., 1982). Application of acrylonitrile to primary
Syrian golden hamster embryo cells in culture produced foci of
morphologically-transformed cells. Pre-treatment with simian
adenovirus (SA7) caused an 8 to 9-fold increase in the frequency of
virus-transformed foci. When 3H-thymidine-labelled cells were
treated with acrylonitrile and their DNA subjected to alkaline
sucrose gradients, a shift in the sedimentation pattern occurred,
which was reminiscent of that observed with carcinogen treatment.
These observations added support to recent studies indicating that
acrylonitrile may be carcinogenic (Parent & Castro, 1979).
7.5.5. Mammalian in vivo assays
The inhalation exposure of 16 Sprague-Dawley male rats to
acrylonitrile levels up to 1085 mg/m3 (500 ppm) for 90 days did not
reveal chromatid or chromosomal aberrations or bone-marrow
abnormalities (Johnson et al., 1978). The results of Rabello-Gray
& Ahmed (1980) and the recent results of Leonard et al. (1981) also
showed that acrylonitrile fails to induce chromosomal aberrations
in somatic and germ cells.
Similar negative results were reported by Zhurkov et al. (1983)
following inhalation exposure of mice to acrylonitrile concentrations
of both 100 mg/m3 and 20 mg/m3 . They also reported negative
results in a dominant lethal assay in mice.
From preliminary results concerning DNA-alkylation by
acrylonitrile and vinyl chloride monomer (Peter et al., 1983), it
appears that DNA-alkylation occurs to a much lesser extent with
acrylonitrile than with vinyl chloride monomer. This is consistent
with the absence of mutagenic effects in vivo.
7.6. Carcinogenicity
Although full data were not available to the Group, there was
strong evidence from the data considered that acrylonitrile is a
carcinogen in rats.
Maltoni et al. (1977, 1982) investigated the carcinogenicity of
acrylonitrile administered to Sprague-Dawley rats by inhalation at
87, 44, 22, and 11 mg/m3 (40, 20, 10, and 5 ppm), 4 h daily, 5
times a week, or by stomach tube as a solution in olive oil, at a
dose of 5 mg/kg body weight, once a day, 3 times a week. In each
case, the rats were treated for 52 weeks and then kept without
further treatment until death. An increased incidence of some
tumours was noted in the acrylonitrile-treated animals, e.g.,
mammary tumours, forestomach papillomas and acanthomas, and
encephalic tumours (gliomas).
Two-year studies on Sprague-Dawley rats, following inhalation
exposure to acrylonitrile or ingestion in drinking-water, have been
performed at the Dow Chemical Company laboratories (Quast et al.,
1980a,b). In the inhalation studies, rats were exposed to 0, 44,
or 174 mg/m3 (0, 20, or 80 ppm) for 6 h/day, 5 days a week, for 24
months. Treatment-related tumours were found in the central nervous
system, Zymbal gland, tongue, stomach, small intestine, mammary
gland, and nasal turbinates. An apparent decrease in tumours of
the pituitary gland, the adrenals, the thyroid, the pancreas, and
testes was observed in the exposed rats.
In the ingestion study, rats were maintained on water
containing acrylonitrile levels of 0, 35, 100, and 300 mg/litre,
equivalent to mean dosage levels of 0, 4, 9, or 22 mg/kg body
weight per day. Evidence of oncogenicity was found in rats at all
dose levels of acrylonitrile. An increased tumour incidence was
observed in the treated rats affecting, particularly, the centra1
nervous system and also the Zymbal gland, tongue, stomach, small
intestine, and mammary gland. A decreased incidence of tumours was
observed at some sites: pituitary, thyroid, adrenals, pancreas, and
uterus.
In studies performed by Hogen & Rinehart (1980), acrylonitrile
was administered to Sprague-Dawley rats in the drinking-water at 1
or 100 mg/litre for 19-22 months, or by gavage at 0.1 or 10 mg/kg
body weight per day in water for about 20 months. A second group
of Fisher 344 rats received acrylonitrile in the drinking-water at
1, 3, 10, 30, or 100 mg/litre for 23-26 months. A statistically
significant increase in tumours was reported in the group receiving
acrylonitrile at 10 mg/kg body weight by gavage and in the groups
receiving 10, 30, or 100 mg acrylonitrile/litre drinking-water.
So far, no information is available on the carcinogenicity of
acrylonitrile for animal species other than rats.
After reviewing these data, IARC (IARC, 1982) and COC (UK
Ministry of Agriculture, Fisheries and Food, 1982) concluded that
acrylonitrile was a carcinogen in experimental animals.
8. EFFECTS ON MAN
Acrylonitrile has long been known to be a toxic substance that
induces systemic as well as local injury in both animals and man.
It has frequently been used in combination with other chemicals;
they may modify its toxicity, as was the case when it was used as a
fumigant.
8.1. Acrylonitrile
8.1.1. Acute Toxicity
8.1.1.1. Inhalation exposure
A 22-year-old chemist, who was exposed to acrylonitrile vapours
when a distillation apparatus leaked, developed headache, vertigo,
vomiting, tremors, uncoordinated movements, and convulsions
(Sartorelli, 1966). Vomiting and nausea persisted for 24 h. One
day after exposure, slight liver enlargement and congestion of the
oral pharynx, but no disorders of the CNS, were noted. After 4
days, no kidney, liver, cardiac, or respiratory abnormalities were
detected. Workers exposed to "mild" concentrations of acrylonitrile
in synthetic rubber manufacture developed nausea, vomiting,
weakness, nasal irritation, and an "oppressive feeling" in the
upper respiratory tract (Wilson, 1944). Headache, fatigue, and
diarrhoea were observed in some cases, and mild jaundice lasting
for several days and accompanied by liver tenderness and low-grade
anaemia in a few others. Jaundice lasted for 4 weeks in 1 case;
this individual complained of lassitude and fatigue after one year.
Zeller et al. (1969) observed that in 16 cases of acute inhalation
of acrylonitrile fumes by workers, nausea, vomiting, headache, and
vertigo appeared within 5-15 min; none of the workers needed
hospitalization. The authors described 50 cases of skin contact
with irritation, erythema, and blistering appearing within 5 min to
24 h, but with no systemic consequences. Workmen exposed to
concentrations varying from 35 to 220 mg/m3 (16-100 ppm) for 20-45
min during cleaning operations in polymerizers frequently
complained of a dull headache, fullness in the chest, irritation of
the eyes, nose, and throat, and feelings of apprehension and
nervous irritability. Some workmen had "intolerable itching" of
the skin, but no accompanying dermatitis.
8.1.1.2. Dermal exposure
A male laboratory worker who spilled "small quantities" of
liquid acrylonitrile on his hands, developed diffuse erythema on
both hands and wrists after 24 h, and blisters on the fingertips by
the third day. The hands were slightly swollen, erythematous,
itchy, and painful. The fingers remained dry and scaly on the 10th
day (Dudley & Neal, 1942). Wilson et al. (1948) observed that
direct skin contact led to irritation and erythema followed by scab
formation; healing was slow. Development of allergic dermatitis is
possible; a 27-year-old individual developed a rash on his finger
following the use for 6 weeks of a finger splint made from an
acrylonitrile/methyl methacrylate copolymer. Patch testing gave
positive reactions to the copolymer and 0.1% acrylonitrile (Balda,
1975). In another case report, skin lesions were first observed at
the site of contact with liquid acrylonitrile, which then spread
rapidly to other neighbouring regions. Several days after contact,
the lesions spread rapidly to other parts of the body that had not
been exposed, and these extensions were assumed to be an allergic
reaction (Hashimoto & Kobayashi, 1961).
In addition to local dermal toxicity, dermal absorption of
acrylonitrile may lead to systemic poisoning. Grunske (1949)
described a fatal case in which a 3-year-old girl had entered a
room that had recently been sprayed with an acrylonitrile-
containing insecticide (Ventox). Exposure was mainly through
inhalation, but skin exposure was possible, too. Another fatal
case was reported by Lorz (1950) in which a 10-year-old girl had
been treated on the scalp for lice with an insecticide that was
identified as containing acrylonitrile (Ventox). She had impetigo
and widespread scratches on the skin of the scalp. This could have
increased the absorption of acrylonitrile.
Two workers who spilled liquid acrylonitrile on their legs,
immediately washed their legs and dried their shoes, but put them
on again. Blisters developed at the sites of contact, 6-8 h after
the spill. Therapy lasted 21 and 38 days, respectively. The skin
of 2 workers who were cleaning apparatus (temperature 50 °C), came
into contact with 5% acrylonitrile solution; other possible
substances in the mixture were not specified. Serious skin burns
developed. Therapy lasted 35 and 72 days, respectively (Babanov,
1957). Zeller et al. (1969) reported 50 cases of skin damage
resulting from occupational contact with acrylonitrile. A burning
sensation developed within 5 min to 24 h followed by a reddening of
the area, which often blistered after 1 day.
8.1.2. Chronic toxicity - occupational exposure
Chronic effects can potentially occur after prolonged exposure
to acrylonitrile, both in the vapour and liquid forms.
8.1.2.1. Clinical observations
Complaints of poor health, headache, decreased work capacity,
poor sleep, irritability, chest pains, poor appetite, and skin
irritation (during the first months of employment only) came from
workers employed in the manufacture of acrylonitrile (Zotova,
1975a).
In a study by Sakurai & Kusumoto (1972), workers employed in
acrylonitrile manufacture also complained of headache, weakness,
fatigue, nausea, vomiting, nosebleeds, and insomnia; the symptoms
correlated well with the length, but not with the level of exposure
or with the age of the workers. A total of 4439 examinations were
made over about 10 years prior to 1970, in 576 workers who formed
2 cohorts, one exposed to concentrations of acrylonitrile below 11
mg/m3 (5 ppm), the other below 45 mg/m3 (20 ppm). However, the
authors later stated that these exposure levels were not reliably
reported (Sakurai et al., 1978).
Babanov et al. (1959) reported that workers exposed to
acrylonitrile concentrations at 0.6-6 mg/m3 for approximately 3
years suffered from headache, insomnia, pains in the heart region,
general weakness, decreased working capacity, and increased
irritability. The vocal cords were inflamed, and non-specific
changes in the vestibular apparatus and pale mucous membranes and
skin were seen. Blood pressure was said to be reduced.
Changes in the health status and laboratory tests were not
observed in a group of 23 men who had been working for 3-5 years in
an acrylonitrile plant, where, during the warm season, exposure
levels reached 4.2-7.2 mg/m3 (Gincheva et al., 1977). Stamova et
al. (1976) studied workers' health in the related polyacrylic fibre
plant in which acrylonitrile exposure levels ranged around 10
mg/m3, but could fluctuate upwards to 25 mg/m3 . Workers were also
exposed to other chemical substances. An increase was found in
both skin diseases and various "neurasthenic" complaints and
diseases. Dorodnova (1976) did not find any differences in the
gynaecological health status of 410 women working in a polyacrylic
fibre plant in Saratov compared with that of 436 unexposed women.
8.1.2.2. Haematology
Compared with the findings in blood donors, some male and
female employees exposed to acrylonitrile at 2.5-5 mg/m3 showed
a reduced haemoglobin level, erythrocyte count, leukocyte count,
and percentage of neutrophiles, with an increased percentage of
lymphocytes and plasma iron. Inhibition of maturation of
normoblasts in bone marrow was also reported (Shustov, 1968).
Similar results were reported by Zotova (1975b). Lower
erythrocyte, haemoglobin, and total white counts were found in
laboratory workers exposed to acrylonitrile, and in apparatus
operators and machinists. Higher than normal total glutathione
levels were found in male operators and maintenance men and reduced
glutathione levels in male apparatus operators. Oxidized
glutathione was elevated and total sulfhydryl groups decreased in
workers employed in all these occupations.
Lower erythrocyte counts and a relative lymphocytosis were also
observed by Babanov et al. (1959) in the study mentioned above.
8.1.2.3. Other organs
(a) Liver
Sakurai & Kusumoto (1972) (section 8.1.2.1) reported some
abnormal results in liver function tests; however, in a further
study of 102 workers from some of the factories, Sakurai et al.
(1978) did not find any significant liver function test
abnormalities related to acrylonitrile exposure, when exposure
levels had decreased from 11-44 mg/m3 (5-20 ppm) to 9 mg/m3 (4.2
ppm). Increased serum cholinesterase activity, hyperbilirubinaemia,
decreased coloidal stability, and hypergammaglobulinaemia were
described in workers exposed to acrylonitrile concentrations of up
to 5 mg/m3 and to acrylonitrile-polymer dust of up to 1.5 mg/m3
(Enikeeva et al., 1976). These effects have not been reported
elsewhere.
(b) Eye
Blepharoconjunctivitis was reported by Delivanova et al. (1978)
in all of 302 workers examined over a 2-year period; 42 had severe
alterations caused by conjunctivitis, and all disorders were
connected with exposure to acrylonitrile.
(c) Gastro-intestinal effects
Symptoms of gastritis and colitis were observed in workers
exposed to acrylonitrile concentrations of up to 5 mg/m3 (Enikeeva
et al., 1976).
(d) Immune system
Acrylonitrile has been found to have an immunodepressive
effect. The functional activity of T-lymphocytes was found to have
decreased in workers exposed to acrylonitrile (Ivanov, private
communication, 1983).
8.1.2.4. Nervous system
Nausea, vomiting, headache, and vertigo (Wilson, 1944; Wilson
et al., 1948; Zeller et al., 1969; Sakurai & Kusumoto, 1972;
Zotova, 1975) indicate a possible effect of acrylonitrile on the
nervous system. Ageeva (1970) reported a significant decrease in
an "epinephrine-like substance", and an increase in acetylcholine.
Depression, lability of autonomic functions (lowered arterial
pressure, labile pulse, diffuse dermographia, increased sweating,
change in orthostatic reflex) were also observed in workers
involved in acrylonitrile production.
8.1.2.5. Dermal effects
Spassovski (1976) reported irritant and allergic dermatitis in
acrylonitrile workers; dermatitis was also observed by Antonev &
Rogailin (1970) and Stamova et al. (1976).
8.2. Mutagenicity
Thiess & Fleig (1978) examined workers who had been exposed to
acrylonitrile for 15.3 years and workers who had not been exposed.
No differences were found in the incidence of chromosomal
aberrations, including or excluding gaps, in the 100 metaphases
examined for each person.
8.3. Carcinogenicity
In a retrospective cohort epidemiological study of 1345 male
workers with potential exposure to acrylonitrile from 1950-66,
followed until 31 December, 1976, 25 cases of cancer were found
with 20.5 expected, based on company rates (O'Berg, 1980). Of
these, 8 were respiratory cancer cases, with 4.4 expected. Twenty-
three cases occurred among workers first exposed during the start-
up period (1950-52) when exposures were higher; only 12.9 were
expected (P = 0.01).
The standardized incidence ratio (SIR) was 179 for cancer among
the operators and mechanics who had at least 6 months' exposure and
began their assignments during start-up. A "dose response" was
shown with those with longer duration of employment, workers with
estimated higher exposures having higher risk. Latency was also
demonstrated, with 17 of the 24 cases occurring 20 years after the
onset of exposure among those with at least 6 months' employment,
including 6 of the 8 lung cancer cases. It should be pointed out
that, using the National Cancer Institute's expected incidence
rates for 1969-71, the expected rate would be 25.5 rather than 20.5
from the company rates. In a concomitant cancer mortality study,
20 cancer deaths were found with 17.4 expected, using company rates
(not significant); the expected rates exceeded the company rates
using national, state, or regional cancer rates. The author felt
that it might be premature to evaluate mortality statistics,
because of insufficient latency (many cancer cases had been
recently diagnosed and were still living). Smoking habits were not
considered, though the author stated that 7 out of the 8 lung
cancer cases were known to have smoked.
A follow-up study on the mortality rate among 327 employees of
a chemical rubber plant in the USA revealed that the number of
deaths from lung cancer was significantly higher than expected (9
versus 5.9 for US white males and 4.7 for other rubber workers from
the same city). The greatest excess was seen among men who had
worked for 5-14 years and who had started working there at least 15
years before death (Delzell & Monson, 1982). This study was
confounded by the multiple exposure of workers in the nitrile
rubber manufacturing plant.
Kiesselbach et al. (1979) examined the mortality rate, the
cancer rate, and the type of cancer against the period of exposure
to acrylonitrile in 884 workers. The results revealed that the
general mortality of the exposed group was markedly lower than that
of the normal population (58 versus an expected 104), possibly
because of the "healthy worker" effect. The mortality rate for
malignant tumours, cardiovascular, brain, respiratory, and gastro-
intestinal diseases, suicide, and other causes was the same as in
the normal population. No relationship was found between length of
exposure and mortality from tumours.
An excess of deaths from lung cancer was reported in
acrylonitrile workers by Thiess et al. (1980). In addition, 2
cases of Hodgkin's disease contributed to a slight excess of cancer
of the lymphatic tissue. However, exposure to other substances,
some of them known carcinogens, made interpretation of the results
difficult.
A cohort study on men potentially exposed to acrylonitrile
during the start-up of a plant indicated that there was no excess
mortality from lung cancer. There were no deaths from lung cancer
in maintenance workers, who possibly had the highest exposures.
There was an excess of kidney cancers (based on 2 cases only) and
of circulatory disease other than rheumatic and atherosclerotic
(based on 5 cases), accompanied by a deficit of atherosclerotic
heart disease.
Because the cohort was small with only 4 cancer deaths
observed, it could not give an indication of excess cancer risk or
association with duration of exposure to acrylonitrile. An
additional retrospective cohort mortality study, in two
acrylonitrile plants in the USA, on 352 males exposed for 6 months
or more prior to 1968 and followed up until December 1977, did not
show any excess mortality including cancer mortality. There were
15 deaths from all causes, 18.11 being expected, and 3 deaths from
cancer (2.8 expected) (Zack, unpublished data, 1980).a
In a study by Nakamura (1981), 9525 workers employed in the
production of acrylonitrile, acrylonitrile rubbers, and ABS were
studied. Deaths due to cancers in general and to lung and colon
cancers in particular, were not increased, while 7 deaths due to
liver, gall bladder, or cystic duct cancer were found against the 5
that might have been expected.
The mortality of 1111 men who worked on the polymerization of
acrylonitrile and the spinning of acrylic fibre in the United
Kingdom from 1950 to 1968 was surveyed up to the end of 1978.
Seventy-nine deaths were identified in 6 factories. The total
number of deaths among men exposed to acrylonitrile for at least
one year was slightly lower than expected (68 versus 72.4) and a
relative excess of deaths from all cancers was found, arising
mainly from cancers of the lung, stomach, colon and brain,
pancreas, testis, and bladder (21 versus 13 expected). The authors
considered particularly relevant, the excess of lung cancer in
those aged 15-44 years. Nevertheless, the authors considered that
their results were inconclusive and urged continued surveillance
and analysis of the exposed population in the United Kingdom
(Werner & Carter, 1981).
The epidemiological studies provide some indications that
acrylonitrile exposure is associated with cancer, particularly of
the lung. However, the studies reported, while neither conclusive
nor contradictory, are limited by insufficient latency. Other
difficulties, such as cohort identification and selection, and
combined exposures have made interpretation difficult. Further
epidemiological data are therefore of great importance, and
consideration of smoking and single exposures to acrylonitrile is
desirable.
8.4. Simultaneous Exposure to Acrylonitrile and
Other Chemicals
8.4.1. Acute toxicity
Numerous non-fatal and fatal cases of poisoning by
acrylonitrile-containing mixtures have been described (Davis et
al., 1973). In home fumigation, an acrylonitrile mixture with
carbon tetrachloride or methylene chloride is placed in shallow
open pans and the vapours dispersed by fans for 24-72 h, the
operator deciding when the house is safe for occupancy (Davis et
al., 1973). A man working in the polymerization of acrylonitrile,
polybutadiene, and styrene for 2 years complained of numbness of
the fingers and toes, severe fatigue in the lower extremities, and
----------------------------------------------------------------------
a Zack, J.A. (1980) The mortality experience of Monsanto
workers exposed to acrylonitrile (Monsanto Internal
Report).
general malaise. Decreased patellar and achilles tendon reflexes,
and hypoaesthesia in the peripheral parts of the fingers and toes
were observed. Free acrylonitrile was detected in the urine. The
exposure level of acrylonitrile was estimated to exceed 108.5 mg/m3
(50 ppm) (Seki, 1967).
Lachrymation, burning in the throat, coughing, sneezing,
nausea, vomiting, dizziness, visual disturbance, headache, coma,
seizures, and dermatitis have been described in non-fatal cases
(Davis et al., 1973). Fatalities have occurred following exposure
to vapours (Grunske, 1949; Davis et al., 1973) and liquid (Lorz,
1950). Cyanide was detected in the blood in some cases. Symptoms
and signs prior to death varied from case to case; sore throat,
weakness, dizziness, vomiting, eye irritation, respiratory
disorders, pallor, tachycardia, tremors, unconsciousness, and
epidermal necrolysis have been described. Other pathological
conditions occurred, but many could have been the result of pre-
existing disease or of exposure to the other component(s) of the
mixture. Findings in children suggest that they may be more
sensitive to acrylonitrile exposure than adults (Grunske, 1949).
8.4.2. Chronic toxicity
Abnormalities in subjects exposed simultaneously to
acrylonitrile and several other chemicals have been described in
several studies. As the concentrations of the other chemicals were
frequently higher than those of acrylonitrile, it is difficult to
decide whether the abnormalities were caused by acrylonitrile, the
other chemical(s), or a combination of the two.
An abnormally high proportion of workers exposed to
acrylonitrile levels of 3-20 mg/m3, 33 ppm NH3, up to 1 mg
H2SO4/m3, 0.41-0.67 mg NaOH/m3, and 2-10 mg acetic acid/m3 were
described as suffering from a variety of symptoms ascribed to
disorders of the autonomic nervous system ("neurasthenic syndrome")
(e.g., irritability, headache, poor appetite, fatigue). Intolerance
to alcohol has also been observed (Orusev & Popovski, 1973; Orusev
et al., 1973).
Apprentices exposed to acrylonitrile (0.8-1.8 mg/m3), methyl
methacrylate (16-17 mg/m3), and sodium thiosulfate were examined
before exposure, after 1-2 weeks, and after a further unspecified
time. "Neurasthenic" symptoms were rare before, but frequent after
exposure, and the incidence of immunological reactivity against the
chemicals increased, as did the concentration of ceruloplasmin
(Mavrina & Il'ina, 1974). Dermal tests for allergy were also made
by Hromov (1974) in workers who had been in contact with
acrylonitrile, methyl acrylate, and sodium thiocyanate. Intradermal
samples showed positive haemoagglutination reactions in 86.5% of
workers exposed to acrylonitrile, 76.1% exposed to methyl acrylate,
and 53.6% exposed to sodium thiocyanate. Dermatitis, eczema, and
urticaria occurred.
Mavrina & Hromov (1974) and Shustov & Mavrina (1975) reported
abnormalities of the liver, nervous, cardiovascular, and
gastrointestinal systems in workers occupationally exposed to
acrylonitrile, methyl acrylate, and sodium thiocyanate during fibre
production. In particular, symptoms associated with the activity
of the autonomic nervous system were noted among the 340 workers
examined. Dryness, desquamation, fissures, and diffuse erythema of
the skin were also apparent. Women exposed to acrylonitrile and
methyl acrylate (Chobot, 1979) were said to suffer from
disturbances in menstrual function twice as frequently as a control
group. A low incidence of irritant and allergic dermatitis and
vitiligo was noted in workers exposed to acrylonitrile, methyl
acrylate, and dimethylformamide in fibre production (Bainova,
1975). In 11 out of 28 workers, delayed skin sensitization and
allergic dermatitis were observed with dimethylformamide.
Ostrovskaja et al. (1976) observed workers exposed to
acrylonitrile, acetonitrile, and hydrocyanic acid during training
and after 1.5 years and 3 years. In 190 men and women, aged 20 -
30 years, many signs and symptoms were noted including modified
reflexes, changes in blood pressure, ECG, and EEG. However, it is
difficult to attribute the findings of these authors solely to
acrylonitrile exposure.
9. EVALUATION OF HEALTH RISKS TO MAN FROM EXPOSURE TO ACRYLONITRILE
9.1. Sources and Levels of Exposure
Acrylonitrile is a colourless, volatile, chemically reactive
liquid; it does not occur as a natural product. The monomer is
used world-wide, on a large scale, in the manufacture of polymers,
fibres, and rubbers and as a chemical intermediate. Acrylonitrile-
containing polymers have been used in the manufacture of products
that come into contact with food; the amounts of acrylonitrile that
migrate into foods can be reduced to negligible quantities by the
use of good manufacturing practices in the production of the
polymers.
The major sources of contamination of the general environment
are acrylonitrile-producing and -polymerizing plants. The
occurrence of acrylonitrile in air, water, and soil near industrial
plants has been described. There is evidence that acrylonitrile
has persisted in soil for long periods following accidental
spillage; subsequent contamination of ground water has been
demonstrated.
The highest exposures occur in the workplace. Experience shows
that containment of such exposures can more readily be achieved in
production plants than in those in which acrylonitrile is used to
make other products. In a number of countries, exposure limits or
recommended limit values for the workplace have been arrived at;
values recently set have tended to be lower than in the past (Table
12).
Accidental exposure to acrylonitrile liquid and vapour may
occur during the various stages of production, transport, and use.
9.2. Acrylonitrile Toxicity
Inhaled acrylonitrile vapour is readily absorbed. Acute
systemic effects following absorption of vapour have been
described. Symptoms were non-specific and referable to the
gastrointestinal and respiratory tracts, the liver, and the central
nervous system. No acute adverse effects have been reported
following daily exposure (8-h) to up to 45 mg/m3.a At higher
concentrations rising to 220 mg/m3, 20-40 min exposure resulted
in complaints of headache, irritation of the upper respiratory
tract and the eyes, nervous irritability, and itching of the skin.
Fatalities have been reported following the use of fumigant
mixtures containing acrylonitrile together with carbon
tetrachloride and methylene chloride. Exact exposure conditions
are not known, but animal data suggest that inhalation exposure to
acrylonitrile at 500-2000 mg/m3 for 1/2-3 h could be fatal.
Simultaneous exposure to some organic solvents may enhance the
toxicity of acrylonitrile.
---------------------------------------------------------------------------
a This value was for many years the occupational exposure limit
in many countries where acrylonitrile was manufactured.
Table 12. Occupational exposure limits for selected countries
---------------------------------------------------------------
Country TWA mg/m3 STEL mg/m3 Reference
---------------------------------------------------------------
France 9 34*** INRS (1983)
Germany, Federal -* (S) DFG (1982)
Republic of
Hungary 0.5 0.5 Hungary (1979)
Japan 45 - Japan Association
of Industrial
Health (1972)
Poland Poland (1982)
Sweden 4** (S) 13 Sweden (1981)
United Kingdom
USA 4.5** (S) ACGIH (1982)
USSR 0.5 (S) USSR (1982)
---------------------------------------------------------------
* Proved animal carcinogen, strongly suspected of also being
carcinogenic for human beings. No safe concentration can be
listed.
TWA = Time-weighted average.
STEL = Short-term exposure limit.
** Listed as Industrial Substance Suspect of Carcinogenic
Potential for Man.
*** Alarm level.
S = Skin uptake can contribute to overall exposure.
Liquid acrylonitrile is also absorbed through the skin,
reportedly giving rise to non-specific symptoms similar to those
that follow acrylonitrile vapour inhalation. Local injury can
occur a few hours after exposure to liquid acrylonitrile. One
fatality has been reported. It is also an irritant to the eye.
Skin absorption of vapour does not appear to contribute
significantly to overall acrylonitrile uptake in the workplace.
There are no indications that acrylonitrile accumulates in the
body following prolonged exposure to levels found in the workplace.
Skin sensitization has been reported in a few cases; however,
no evidence is available to suggest the occurrence of pulmonary
allergic reactions.
Acrylonitrile has been shown to induce embryotoxic and
teratogenic effects at high dosage levels in experimental animals.
Although acrylonitrile is metabolized partly to cyanide, it has
been demonstrated that the acute toxic actions of acrylonitrile are
not solely due to cyanide, as was once believed.
Acrylonitrile has been shown to be mutagenic in some in vitro
systems in the presence of metabolic activation systems. So far,
mutagenic activity has not been demonstrated in in vivo assay
systems.
Complaints of ill health in workers exposed for a number of
years to acrylonitrile concentrations of less than 45 mg/m3 have
been reported in several studies. The complaints were variable in
nature and no consistent correlation with the extent of exposure
appears to have been established. The studies do not provide
evidence of a specific disease arising from long-term, low-level
exposure.
Several long-term studies in which acrylonitrile was
administered to rats orally and by inhalation demonstrated the
induction of malignant tumours. Data available in summary form
suggest that the incidence was dose-related. Eight epidemiological
studies have been carried out on workers exposed to acrylonitrile.
These studies have not demonstrated conclusively that there is a
correlation between exposure to acrylonitrile and the incidence of
cancer in man. Nevertheless, the findings are not incompatible with
the supposition that acrylonitrile is a potential human carcinogen
and thus give no cause for disregarding the evidence that has been
provided by animal studies.
It is not possible to establish a level below which no adverse
effects occur on the basis of the experimental and epidemiological
data presented in this document. However, it is evident that
exposure to acrylonitrile should be kept as low as possible in both
the workplace and the general environment, and that skin contact
with the liquid should be avoided.
REFERENCES
AARATO, S.E. & BITTERA, E. (1972) [Determination of
acrylonitrile in the air in the presence of dimethylformamide
and ammonia.] Munkavedelem, 18 : 50-51 (in Hungarian, Chem.
Abstr. 79 : 57280b, 1973).
ABREU, M.E. & AHMED, A.E. (1980) Metabolism of acrylonitrile
to cyanide: in vitro studies. Drug Metab. Disposition, 8 (6):
376-379.
ACGIH (1982) Threshold limit values for chemical substances
and physical agents in the work environment with intended
changes for 1982. Cincinnati, USA, American Conference of
Governmental Industrial Hygienists.
AGEEVA, S.T. (1970) [The condition of the metabolism of
mediating substances in workers producing acrylonitrile.] Tr.
Sarat. Med. Inst., 78 : 10-13 (in Russian).
AHMED, A.E. & ABREU, M.E. (1982) Microsomal metabolism of
acrylonitrile in liver and brain. Adv. exp. Med. Biol., 136
(Part B): 1229-1238.
AHMED, A.E. & PATEL, K. (1979) Studies on the metabolism of
aliphatic nitriles. Toxicol. appl. Pharmacol., 48 : A 91
(abstract).
AHMED, A.E. & PATEL, K. (1981) Acrylonitrile - in vivo
metabolism in rats and mice. Drug. Metab. Disposition, 9 (3):
219-222.
AHMED, A.E., FAROOQUI, M.Y.H., UPRETI, R.K., & EL-SHABRAWY,
O. (1982) Distribution and covalent interactions of (1-14C)
acrylonitrile in the rat. Toxicology, 23 : 159-175.
ALDRIDGE, W.N. (1944) A new method for the estimation of
micro quantities of cyanide and thiocyanate. Analyst, 69 :
262-265.
AL'SHANSKY, A.M., IVANOV, V.V., & PROMYSLOV, M.SH. (1980)
[Effect of acrylonitrile on content of Q-aminobutyric acid
glutamate decarboxylase activity and on lipid peroxidation in
rat brain tissue.] Vop. Med. Chimii, 26 (4): 507-509 (in
Russian).
AMERICAN CYANAMID COMPANY (1951) The chemistry of
acrylonitrile. In: Nitrogen chemicals digest, New York,
American Cyanamid Company, Vol. 5.
AMERICAN CYANAMID COMPANY (1959) The chemistry of
acrylonitrile, 2nd ed., New York, American Cyanamid Company,
272 pp.
AMERICAN CYANAMID COMPANY (1974) Handling storage - analyses
of acrylonitrile. Wayne, New Jersey, American Cyanamid
Company, 42 pp.
AMERICAN CYANAMID COMPANY (1976) Material safety data sheet;
Acrylonitrile, Wayne, New Jersey, American Cyanamid Company.
AMERICAN INDUSTRIAL HYGIENE ASSOCIATION (1970)
Acrylonitrile. Am. Ind. Hyg. Assoc. J.,: 529-531.
ANDO, K. & HASHIMOTO, K. (1967) [Effects of acrylonitrile
and acrylamide on peripheral nerve.] Rep. Osaka Prefectural
Inst. pub. Health, 4 : 23-27 (in Japanese).
ANONYMOUS (1977) Acrylo growth won't be bottled up. Chem.
Week, 9 March : 30-31.
ANTON'EV, A.A. & ROGAILIN, V.I. (1970) [Professional
dermatoses caused by acrylonitrile and their prophylaxis.] Tr.
Perm. Gos. Med. Inst., 99 : 103-106 (in Russian).
APPEL, K.E., PETER, H., & BOLT, H.M. (1981a) Effect of
potential antidotes on the acute toxicity of acrylonitrile.
Int. Arch. occup. environ. Health, 49 : 157-163.
APPEL, K.E., PETER, H., BOLT, M., & BOLT, H.M. (1981b)
Interaction of acrylonitrile with hepatic microsomes of rats
and man. Toxicol. Lett., 7 (4-5): 335-340.
ASTM (1981) Annual book of ASTM standards. Method D3371-79:
Testing for nitriles in aqueous solution by gas-liquid
chromatography, Philadelphia, American Society for Testing and
Materials.
BABANOV, G.P. (1957) [Local manifestation of the action of
acrylonitrile on the skin and mucosa.] Vrac. Delo, 5 : 511-514
(in Russian).
BABANOV, G.P., KLJUCHIKOV, V.N., KARAJEVA, N.I., & LILEEVA,
Z.V. (1959) [Clinical symptoms of chronic poisoning by
acrylonitrile.] Vrach. Delo, 8 : 833-836 (in Russian).
BAINOVA, A. (1975) [Assessment of the skin lesions in the
production of Bulana polyacrylonitrile fibers.] Dermatol.
Venerol., 14 (2): 92-97 (in Bulgarian).
BAKER, R.A. (1963) Threshold odors of organic chemicals. J.
Am. Water Works Assoc., 55 : 913-916.
BALDA, B.R. (1975) [Acrylonitrile as a contact allergen.]
Hautarzt, 26 : 599-601 (in German).
BANDT, H.J. (1953) [Acrylonitrile as an effluent.] Z.
Fisch., 2 : 457-461 (in German).
BARNES, J.M. (1970) Effects on rats of compounds related to
acrylamide. Br. J. ind. Med., 27 (2): 147-149.
BARRETT, W.J. (1974) Sampling and analysis of four organic
compounds using solid sorbents, Washington, US Department of
Health, Education and Welfare (Southern Research Institute
Report SORI-EAS-74-301).
BAXTER, R.A. (1979) Evaluation and control of industrial
exposure to acrylonitrile. Ann. occup. Hyg., 22 : 429-435.
BELILES, R.P., PAULIN, H.J., MAKRIS, N.G., & WEIR, R.J.
(1980) Three-generation reproduction study of rats receiving
acrylonitrile in drinking water. Final report. Litton
Bionetics Inc.
BELLAR, T.A. & SIGSBY, J.E., Jr (1970) Direct gas
chromatographic analysis of low molecular weight substituted
organic compounds in emissions. Environ. Sci. Technol., 4 :
150-156.
BENCHEV, I., RIZOV, N., & KOLARSKA, A. (1982) [Acrylonitrile
determination in biological fluids by gas chromatography -
"head space" analysis.] Hyg. Zdrav., 1 : 87-89 (in Bulgarian).
BENESH, V. & CHERNA, V. (1959) [Acrylonitrile. Acute
toxicity and mechanism of action.] J. Hyg. Epidemiol.
Microbiol. Immunol., 3 (1): 106-116 (in German and in Russian).
BENESH, V. & SHRAM, R. (1969) Mutagenic activity of some
pesticides in Drosophila melanogaster. Ind. Med. Surg.,
38 (12): 442-444.
BENSON, G.B. & BOYCE, G.E. (1981) A thermally desorbable
passive dosimeter for personal monitoring for acrylonitrile.
Ann. occup. Hyg., 24 (1): 55-76.
BENSON, G.B., BOYCE, G.E., & HAILE, D.M. (1981) Industrial
hygiene measurements for organic pollutants (acrylonitrile)
using passive dosimeters and automated thermal desorption.
Ann. occup. Hyg., 24 (4): 367-373.
BERCK, B. (1960) Retention of acrylonitrile and carbon
tetrachloride by shelled walnuts fumigated with acrylon. J.
Agric. food Chem., 8 :128-131.
BERG, G.L., ed. (1977) 1977 Farm Chemicals Handbook, 63rd
issue, Willoughby, Ohio, Meister Publishing Co., pp. D5, D50.
BIRD, W.L. & HALE, C.H. (1952) Polarographic determination
of acrylonitrile. Anal. Chem., 24 : 586-587.
BOLLINI, M., SEVES, A., & FOCHER, B. (1974) [Determination
of free monomers in water emulsions of synthetic polymers or
copolymers.] Ind. Carta, 12 : 234-240 (in Italian, Chem.
Abstr., 81 : 121672b).
BOLT, H.M., FILSER, J.G., & HINDERER, R.K. (1978) Rat liver
microsomal uptake and irreversible protein binding of
1,2-14C-vinyl bromide. Toxicol. appl. Pharmacol., 44 : 481-489.
BOND, E.J. & BUCKLAND, C.T. (1976) Control of insects with
fumigants at low temperatures: toxicity of mixtures of methyl
bromide and acrylonitrile to three species of insects. J.
econ. Entomol., 69 : 725-727.
BONDAREV, G.I., STASENKOVA, K.P., & VISSARIONOVA, V.Y.
(1976) [Protective effect of sulfur-containing compounds in
poisoning with acrylic acid nitrile.] Vopr. Pitan., (4) : 55-58
(in Russian).
BORCHARDT, VON K., FRANSEN, E., & HARTMAN, B. (1970) [Acute
oral intoxication with acrylonitrile in animals.] Z. Ges.
Hyg., 16 : 316-319 (in German).
BORG WARNER CHEMICALS (1977) In response to SHA's request
for information on occupational exposure to acrylonitrile.
Fed. Reg., 42 : 33043 (Letter to OSHA Docket Officer, US
Department of Labor).
BOYLAND, E. & CHASSEAUD, L.F. (1967) Enzyme-catalysed
conjugations of glutathione with carbonyl compounds. Biochem.
J., 104 : 95-102.
BOYLAND, E. & CHASSEAUD, L.F. (1968) Enzymes catalysing
conjugations of glutathione with A,B,-unsaturated carbonyl
compounds. Biochem. J., 109 : 651-661.
BREWER, W.E. (1976) 90-day subacute vapor inhalation
toxicity study with acrylonitrile in beagle dogs, albino rats
and albino mice (Industrial Biotest Report No. 74-42, prepared
for the Monsanto Company).
BRIEGER, H., RIEDERS, F., & HODES, A.B. (1952)
Acrylonitrile: spectrophotometric determination, acute
toxicity, and mechanism of action. Ind. Hyg. occup. Med., 6 :
128-140.
BRUCE, R.B., HOWARD, J.W., & HANZAL, R.F. (1955)
Determination of cyanide, thiocyanate and A-hydroxynitriles in
plasma or serum. Anal. Chem., 27 : 1346-1347.
BUFFONI, F., COPPI, C., & SANTONI, C. (1982) Determination
of thioethers in urine. Clin. Chem., 28 : 248-250.
CAMPBELL, D.N. & MOORE, R.H. (1979) The quantitative
determination of acrylonitrile, acrolein, acetonitrile and
acetone in workplace air. Am. Ind. Hyg. Assoc. J., 40 : 904-909.
CANADA FOOD & DRUGS ACT & REGULATIONS (1982) Division 23 -
Section B.23.008. Canada, Ministry of Supply & Services.
CARPENTER, C.P., SMYTH, H.F., & POZZANI, U.C. (1949) The
assay of acute vapour toxicity and the grading and
interpretation of results on 96 chemical compounds. J. ind.
Hyg. Toxicol., 31 : 343-346.
CHANDRA, H., GUPTA, B.H., BHARGAVA, S.K., CLERK, S.H., &
MAHENDRA, P.N. (1980) Chronic cyanide exposure - a
biochemical and industrial hygiene study. J. anal. Toxicol.,
4 : 161-165.
CHERNA, M., KOCHISOVA, J., KODYTKOVA, I., KOPECKY, J., &
SHRAM, R.J. (1981) Mutagenic activity of oxiranecarbonitrile
(glycidonitrile). In: Gut, I., Cikrt, M. & Plaa, G.L., ed.
Industrial & environmental xenobiotics, Berlin, Springer-
Verlag, pp. 251-255.
CHOBOT, A.M. (1979) [Menstrual function in workers of the
polyacrylonitrile fibre industry.] Zdrav. Beloruss., 2 : 24-27
(in Russian).
CIA (1978) Codes of practice for chemicals with major
hazards - acrylonitrile, Chemical Industries Association.
CINCOLELLA, A., VOIRIN, D., HECHT, G., GERBER, J.-M., DUCOS,
P., & LIMASSET, J.-C. (1981) Monitoring of atmospheric
concentrations of acrylonitrile in eleven French factories. G.
Ital. Med. lav., 3 : 165-167.
CLIVE, D. & SPECTOR, F.S. (1975) Laboratory procedure for
assessing specific locus mutagens at the TK locus in cultured
L5178Y mouse lymphoma cells. Mutat. Res., 31 : 17-29.
COLLINS, T.F.V. (1977) Written testimony in the matter of
acrylonitrile copolymers used to fabricate beverage containers
(Docket No. 76N-0070, exhibit G-82).
CRISP, S. (1980) Solid sorbent gas samplers. Ann. occup.
Hyg., 23 : 47-76.
CUPITT, L.T. (1980) Fate of toxic and hazardous materials in
the air environment. (EPA report No. EPA-600/3-80-084).
CZAJKOWSKA, T. (1971) [Excretion of certain metabolites upon
single administration of acrylonitrile.] Med. Pr., 22 (4):
381-385 (in Polish).
CZAJKOWSKA, T., KNOBLOCH, K., KOWALSKI, Z., KRYSIAK, B.,
PIOTROWSKI, J., ROGACZEWSKA, T., STARZYNSKI, Z., STASIK, M.,
SZENDZIKOWSKI, S., & ZWIERZCHOWSKI, Z. (1969) [Evaluation of
the toxicity and exposure of chemical industry workers to
acrylonitrile.] Med. Pr., (Supplement): 11-16 (in Polish).
DAHM, D.J. (1977) Identification of metabolites of
acrylonitrile. Testimony before the US FDA (Industrial Biotest
Report No. 74-42, Appendix 4: A.1-A.19, prepared for the
Monsanto Company).
DAUGHERTY, F.M. & GARRETT, J.T. (1951) Toxicity levels of
hydrocyanic acid and some industrial by-products. Texas J.
Sci., No. 3 : 391-396.
DAUES, G.W. & HAMNER, W.F. (1957) Determination of small
amounts of acrylonitrile in aqueous industrial streams. Anal.
Chem., 29 : 1035-1037.
DAVIS, J.H., DAVIES, J.E., AMERICO RAFONNELLI, R.S., & REICH,
G. (1973) Investigation of fatal acrylonitrile
intoxications. Pesticides and the environment, a continuing
controversy. Inter. Med. Book Corp., 2 : 547-556.
DELIVANOVA, S., POPOVSKI, P., & ORUSEV, T. (1978)
[Blepharoconjunctivitis in workers of the polyacrylonitrile
synthetic fibre industry.] God. Zb. Med. Fak. Skoje, 24 :
279-282 (in Macedonian).
DELLA FIORENTINA, H. & DE WIEST, F. (1979) Recherche d'un
indice biologique d'exposition chronique des travailleurs aux
dérivés cyanés. Arch. Mal. prof., 40 : 700-703.
DELZELL, E. & MONSON, R.R. (1982) Mortality among rubber
workers: VI. Men with potential exposure to acrylonitrile. J.
occup. Med., 24 (10): 767-769.
DE MEESTER, C., PONCELET, F., ROBERFROID, M., & MERCIER, M.
(1978b) Mutagenicity of acrylonitrile. Toxicology, 11 : 19-27.
DE MEESTER, C., DUVERGER-VAN BOGAERT, M., LAMBOTTE-VAN DEPAER,
M., ROBERFROID, M., PONCELET, F., & MERCIER, M. (1979) Liver
extract mediated mutagenicity of acrylonitrile. Toxicology,
13 (1): 7-15.
DFG (1982) Maximum concentrations at the workplace and
biological tolerance values for working materials 1982,
Weinheim, Verlag Chemie Gmbh, Commission for Investigation of
Health Hazards of Chemical Compounds in the Work Area,
Deutsche Forschungsgemeinschaft (Report No. 18).
DINU, V. (1975a) Activity of glutathione peroxidase and
catalase and the concentration of lipid peroxides in acute
intoxication with acrylonitrile. Rev. Roum. Biochim., 12 (1):
11-14.
DINU, V. (1975b) Intracellular thiol concentration in
acrylonitrile intoxication. Rev. Roum. Biochim., 12 (3):
155-158.
DINU, V. & KLEIN, R. (1976) Catalase activity and
glutathione and lactic acid concentrations in rats acutely
poisoned by acrylonitrile. J. Pharmacol., 7 (2): 223-226.
DJURIC, D., RAICEVIC, P., & KONSTANTINOVIC, I. (1962)
Excretion of thiocyanates in urine of smokers. Arch. environ.
Health, 5 : 12-15.
DORIGAN, J., FULLER, B., & DUFFY, R. (1976) Scoring of
organic air pollutants chemistry, production and toxicity of
selected synthetic organic chemicals (chemicals A-C), (MITRE
Tech. Rep. MTR-7248).
DORODNOVA, N.S. (1976) [Gynaecological morbidity and
specific functions of the female organism under the conditions
of chemical production in "Nitron".] Gig. Zabol., 8 : 45-46 (in
Russian).
DRAMANSKI, W. & TROJANOWSKA, B. (in press) Chromatographic
determination of S-cyanoethylmercapturic acid - a metabolite
of acrylonitrile in urine. Arch. Toxicol.
DUDLEY, H.C. & NEAL, P.A. (1942) Toxicology of acrylonitrile
(vinyl cyanide). I. Study of the acute toxicity. J. ind. Hyg.
Toxicol., 24 (2): 27-36.
DUDLEY, H.C., SWEENEY, T.R., & MILLER, J.W. (1942)
Toxicology of acrylonitrile (vinyl cyanide). II. Studies of
effects of daily inhalation. J. ind. Hyg. Toxicol., 24 :
255-258.
DUPONT (1977) In response to OSHA's request for information
on occupational exposure to acrylonitrile, letter to the OSHA
Docket Officer, US Department of Labour, Fed. Reg., 42 : 33043.
DUVERGER-VAN BOGAERT, M., LAMBOTTE-VANDEPAER, M., NOEL, G.,
ROBERFROID, M., & MERCIER, M. (1978) Biochemical effects of
ACN on the rat liver, as influenced by various pre-treatments
of the animals. Biochem. Biophys. Res. Comm., 83 : 1117-1124.
DUVERGER-VAN BOGAERT, M., LAMBOTTE-VANDEPAER, M., DE MEESTER,
C., ROLLMANN, B., PONCELET, F., & MERCIER, M. (1981) Effect
of several factors on the liver extract mediated mutagenicity
of acrylonitrile and identification of four new in vitro
metabolites. Toxicol. Lett., 7 : 311-319.
DUVERGER-VAN BOGAERT, M., LAMBOTTE-VANDEPAER, M., MERCIER, M.,
& PONCELET, F. (1982a) In vitro covalent binding of
acrylonitrile to rat liver proteins. Toxicol. Lett., 13 :
203-209.
DUVERGER-VAN BOGAERT, M., LAMBOTTE-VANDEPAER, M., DE MEESTER,
C., MERCIER, M., & PONCELET, F. (1982b) Role of glutathione
in liver-mediated mutagenicity of acrylonitrile. Toxicol.
Lett., 11 : 305-311.
EFREMOV, A.M. (1976a) [Study of the free radicals of the
blood, brain, liver and spleen of white rats subjected to
chronic acrylonitrile poisoning.] Zdrav. Beloruss., 6 : 86 (in
Russian).
EFREMOV, A.M. (1976b) [Study of the biotransformation of
acrylonitriles in animals.] Zdrav. Beloruss., 7 : 85-86 (in
Russian).
EFREMOV, A.M. (1976c) [State of protective adaptive
reactions in white rats poisoned with acrylonitrile.] Gig. Tr.
Ohr. Zdor. Rab. Neft. Neftehim. Prom-sti., 9 : 120-122 (in
Russian).
EFREMOV, A.M. (1976d) [Activity of catalase, peroxidase and
sulfhydryl groups of the brain, liver, and blood in white rats
poisoned with acrylonitrile.] Gig. Tr. Ohr. Zdor. Rab. Neft.
Neftehim. Prom-sti., 9 : 123-124 (in Russian).
ELLMAN, G.L. (1959) Tissue sulfhydryl groups. Arch.
Biochem. Biophys., 82 : 70-77.
ENIKEEVA, N.A., OSTROVSKAJA, R.S., SYSO, L.V., PODREZ, Z.G.,
BRAGINSKAJA, L.L., GVOZDEV, N.P., NESTEROVA, N.E.,
MUSSERSKAJA, A.N., SUZDALTREVA, N.A., & EFREMOV, A.M. (1976)
[Industrial hygiene and health status of workers involved in
the manufacture of the synthetic fibre Nitron.] Gig. Tr. Ohr.
Zdor. Rab. Nef. Neftehim. Prom-sti., No. 9 : 22-25 (in Russian).
EUROP-COST (1976) A comprehensive list of polluting
substances which have been identified in various fresh waters,
effluent discharges, aquatic animals and plants, and bottom
sediments, 2nd ed. Luxembourg, Commission of the European
Communities, p. 19 (EUCO/MDU/73/76, XII/476/76).
FAROOQUI, M.Y.G. & AHMED, A.E. (1982) Molecular interaction
of acrylonitrile and potassium cyanide with rat blood. Chem.
Biol. Interact., 38 : 145-149.
FERIN, J., URBANKOVA, J., & VLCHKOVA, A. (1961) [Hygienic
and toxicological problems of acrylonitrile and
dimethylformamide.] Prac. Lék., 13 : 466-468 (in Czech).
FRANKENBERG, L. (1980) Enzyme therapy in cyanide poisoning:
effect of rhodanese and sulfur compounds. Arch. Toxicol., 45 :
315-323.
FRANZEN, E. von & WAGNER, G. (1978) [Gelchromatographic and
electrophoretic serum protein pattern in rats after acute
acrylonitrile intoxication.] Z. Gesamte Hyg., 24 : 534-537 (in
German).
FREEMAN, R.A., SCHROY, J.M., KLIEVE, J.R., & ARCHER, S.A.
(1981) Experimental studies on the rate of air stripping of
hazardous chemicals from waste treatment systems. In: APCA
Meeting, Montreal, Canada, June 1980.
GAGNON, Y.T. & POSNER, J.C. (1979) Recovery of acrylonitrile
from charcoal tubes at low levels. Am. Ind. Hyg. Assoc. J.,
40 : 923-925.
GAWELL, G. B-M. (1979) Determination of acrylonitrile
monomer in plastic packaging and beverages by headspace gas
chromatography. Analyst, 104 : 106-110.
GHANAYEM, B.I. & AHMED, A.E. (1982) In vivo
biotransformation and biliary excretion of 1-14C acrylonitrile
in rats. Arch. Toxicol., 50 : 175-185.
GHERSIN, Z., STITZL, A., & MANEA, R. (1969) [Comparative
study of titrimetric and colorimetric methods for determining
acrylonitrile in waste water.] Rev. Chim. (Bucharest), 20 :
689-694 (in Rumanian, Chem. Abstr., 72 : 124853m).
GHIRINGHELLI, L. (1954) [Acrylonitrile: toxicity and
mechanism of action.] Med. lav., 45 (5): 305-312 (in Italian).
GINCHEVA, N., STAMOVA, N., HINKOVA, L., KYURKTCHIEV, S.,
SPASOVSKI, M., BAINOVA, A., MUHTAROVA, M., & HRISTEVA, V.
(1977) Study of the health status of workers from the
acrylonitrile department. In: Proceedings of the International
Symposium on Occupational Health in the Production of
Artificial Fibres - Abstracts, Finland, 6-10 June 1977, p. 41.
GOING, J.E., KUYKENDAHL, P., LONG, S., ONSTOT, J., & THOMAS,
K. (1979) Environmental monitoring near industrial sites:
acrylonitrile. 285 pp (EPA/560/6-79/003).
GRAHAM, J.P.D. (1965) Hydroxycobalamin as an antidote to
acrylonitrile. Toxicol. appl. Pharmacol., 7 : 367-372.
GRAVCZYK, J. & ZWIERZCHOVSKI, Z. (1973) [Effect of acute
experimental poisoning with acrylonitrile on the pressor
responses to adrenaline, noradrenaline and acetylcholine.]
Med. Pr., 24 (2): 151-158 (in Polish).
GREEN, M.L.H., MURIEL, W.J., & BRIDGES, B.A. (1976) Use of
simplified fluctuation test to detect low levels of mutagens.
Mutat. Res., 38 : 33-42.
GROTE, A.A., KIM, W.S., & KUPEL, R.E. (1978) Establishing a
protocol from laboratory studies to be used in field sampling
operations. Am. Ind. Hyg. Assoc. J., 39 : 880-884.
GRUNSKE, F. (1949) [Ventox and ventox-poisoning.] Dtsch.
med. Wochenschr., 74 : 1081 (in German).
GUENGERICH, F.P., GEIGER, L.E., HOGY, L.L., & WRIGHT, P.L.
(1981) In vitro metabolism of acrylonitrile to
2-cyanoethylene oxide, reaction with glutathione, and
irreversible binding to proteins and nucleic acids. Cancer
Res., 41 : 4925-4933.
GUERIN, M.R., OLERICH, G., & HORTON, A.D. (1974) Routine gas
chromatographic component profiling of cigarette smoke for the
identification of biologically significant constituents. J.
Chromatogr. Sci., 12 : 385-391.
GUT, I., NERUDOVA, J., KOPECKY, J., & HOLECHEK, V. (1975)
Acrylonitrile biotransformation in rats, mice, and chinese
hamsters as influenced by the route of administration and by
phenobarbital, SKF-525A, cysteine, dimercaprol, or
thisulphate. Arch. Toxicol., 33 : 151-161.
GUT, I., KOPECKY, J., & FILIP, J. (1981a) Acrylonitrile-14C
metabolism in rats: effect of the route of administration on
the elimination of thiocyanate and other radioactive
metabolites in urine and faeces. J. Hyg. Epidemiol. Microbiol.
Immunol., 25 (1): 12-16.
GUT, I., KOPECKY, J., & NERUDOVA, J. (1981b) Relationship
between acrylonitrile biotransformation, pharmacokinetics, and
acute toxicity. A short review. G. Ital. Med. lav., 3 : 131-136.
GUT, I., PELECH, L., VEJLUPKOVA, J., MATOUSEK, O., & KLENER,
P. (1982) [Effects of induction and inhibition of benzene
metabolism in hematopoiesis in male Wistar rats.] Cesk. Hyg.,
27 (1): 1-13 (in Czech).
GUT, I., NERUDOVA, J., FRANTIK, E., KOPECKY, J., HOLECHEK, V.,
STILBOROVA, A., MIRHEJOVSKA, E., & HOLUSHA, R. (in press)
Effect of acrylonitrile inhalation: Intermediary metabolism
and the excretion of thioethers and thiocyanate in rats. J.
Hyg. Epidemiol. Microbiol. Immunol, 28 : (in press).
HALL, M.E. & STEVENS, J.W., Jr (1977) Spectrophotometric
determination of acrylonitrile. Anal. Chem., 49 (14): 2277-2280.
HASHIMOTO, K. (1962) Studies on the intoxication of
acrylonitrile report IV. Effect of daily inhalation of
acrylonitrile on body weight, bodily activity and hemogram of
mice. Q. J. Labor. Res., 10 : 11-16.
HASHIMOTO, K. & ANDO, K. (1966) Effect of acrylonitrile on
the central and peripheral nervous system. In: Symposium on
Higher Nervous Functions & Occupational Health, pp. 87-89.
HASHIMOTO, K. & KANAI, R. (1965) Studies on the toxicology
of acrylonitrile, metabolism, mode of action and therapy. Ind.
Health, 3 (1-2): 30-45.
HASHIMOTO, K. & KANAI, R. (1972) Effect of acrylonitrile on
sulfhydryls and pyruvate metabolism in tissues. Biochem.
Pharmacol., 21 : 635-640.
HASHIMOTO, K. & KOBAYASI, T. (1961) A case of acute
dermatitis caused by contact with acrylonitrile. Q. J. Labor.
Res., 9 (1-4): 21-24.
HASKELL LABORATORY (1975) In vitro microbial mutagenicity
studies of acrylonitrile. (Report No. 351-357, E.I. Dupont de
Nemours & Co., Haskell Laboratory for Toxicology and
Industrial Medicine).
HASLAM, J. & NEWLANDS, G. (1955) Determination of
acrylonitrile in air. Analyst, 180 : 50-53.
HEINO, J., VILHUNEN, R., & HERNBERG, S. (1968) Urinary
thiocyanate concentrations in smokers and non-smokers.
Work-Environ.-Health, 5 : 12-15.
HENDERSON, C., PICKERING, G.H., & LEMKE, A.E. (1961) The
effect of some organic cyanides (nitriles) on fish. Proc. 15th
Ind. Waste Conf. En. Bull., 45 (2): 120-130.
HEUSER, S.G. & SCUDMORE, K.A. (1969) Determination of
fumigant residues in cereals and other foodstuffs: a
multi-detection scheme for gas chromatography of solvent
extracts. J. Sci. Food Agric., 20 : 566-572.
HOFFMANN, P., KLEINE, D., & FRANZEN, E. (1976) [On the proof
of acrylonitrile exposure: Detoxication of acrylonitrile by
coupling with D-glucuronic acid.] Z. Ges. Hyg., 22 (5): 310-312
(in German).
HOGAN, G.K. & RINEHART, W.E. (1980) A 24-month oral
toxicity/carcinogenicity study of acrylonitrile administered
by inhalation to Spartan rats. Bio/dynamics Inc. (Final report
Vols I & II, Project No. 77-1746).
HOLECHEK, V. & KOPECKY, J. (1981) Conjugation of glutathione
with acrylonitrile and glycidonitrile. In: Gut, I., Cikrt, M.
& Plaa, G.L., ed. Industrial and environmental xenobiotics.
Berlin, Springer Verlag, pp. 239-245.
HOUTHUIJS, D., REMIJN, B., WILLEMS, H., BOLEIJ, J., &
BIERSTEKER, K. (1982) Biological monitoring of acrylonitrile
exposure. Am. J. ind. Med., 3 (3): 313-320.
HROMOV, V.E. (1974) [Detection of circulating and fixed
antibodies in the diagnosis of allergies of chemical
etiology.] Vrach. Delo, 12 : 115-116 (in Russian).
HUGHES, T.W. & HORN, D.A. (1977) Source assessment:
acrylonitrile manufacture (air emission) (NITS Report. No.
PB-271 969).
HUMISTON, C.G. & FRAUSON, L.O. (1975) 90-day oral toxicity
study incorporating acrylonitrile in the drinking water of
rats. Midland, MI, Dow Chemical Toxicology Research
Laboratory, 99 pp.
HUNGARY (1979) Workplace requirements for air purity, Magyar
Nöpköztarsasagi Orszagos Szabvany, MSZ 21461-78 Az MSZ
21461-73 helyett G 23, 1979, 1 January.
IARC (1979) Acrylonitrile, acrylic and modacrylic fibres,
and acrylonitrile- butadiene-styrene and styrene-acrylonitrile
copolymers, pp. 73-113 (IARC Monographs on the Evaluation of
the Carcinogenic Risk of Chemicals to Humans, Vol. 19).
IARC (1982) IARC Monographs on the Evaluation of
Carcinogenic Risk of Chemicals to Humans, 1-29 (Suppl. 4) pp.
25-27.
IDOL, J.D., Jr (1974) Acrylonitrile polymers in prospect and
retrospect. In: Proceedings of the Applied Polymer Symposium
25: Acrylonitrile in Macromolecules, New York, John Wiley, pp.
1-18.
IMANARI, T., TANABE, S., TOIDA, T., SHINZO, T., & TOSHIHIKO,
T. (1982) Simultaneous determination of cyanide and
thiocyanate by high performance liquid chromatography. Chem.
Pharm. Bull. Japan, 30 (10): 3800-3802.
INRS (1983) Valeurs limités pour les concentrations des
substances dangereuses dans l'air des locaux de travail. Cah.
Notes Documen., No. 110, (ND 1410-110-83).
IVANOV, V.V. (1980) [Oxidative transformation of
acrylonitrile as a basis of its toxicological action.]
Farmakol. Toksikol., 43 (3): 383-384 (in Russian).
IVANOV, V.V. (1981) [Evaluation of the significance of
acrylonitrile metabolism in the mechanism of its toxicity.]
Gig. Tr. prof. Zabol., 9 : 48-50 (in Russian).
IVANOV, V.V. & AL'SHANSKY, A.M. (1982) GABA-ergic components
and lipid peroxidation during acute exogenous acrylonitrile
poisoning. Bull. exper. Biol. Med., 94 (7): 40-43.
IVANOV, V.V., GRIOHCHENKO, D.A., & KLIMATSKAJA, L.G. (1979)
[Blood serum enzymes in evaluating the nature of liver damage
and the effectiveness of chemotherapy following acrylonitrile
poisoning.] Vopr. Med. Him., 25 (6): 715-718 (in Russian).
IVANOV, V.V., KURNETSOVA, G.P., & ARCHAKOV, A.I. (1978)
[Stimulation of lipid peroxidation by acrylonitrile in rat
liver tissue.] Vopr. Med. Him., 24 (6): 816-818 (in Russian).
IVANOV, V.V., ZHISNOV, G.F., BACHMANOVA, G.I., MAZUROV, A.V.,
& ARCHALOV, A.I. (1979) [Interaction of acrylonitrile with
the microsomal oxidation system in rat liver tissue.] Vopr.
Med. Him., 25 (4): 468-471 (in Russian).
IVANOV, V.V., ZHIRNOV, G.F., & ARCHAKOV, A.J. (1982)
[Activation of acrylonitrile in the microsomal system of
oxidation.] Vopr. Med. Him., 28 (2): 95-98.
IWASAKI, K., KANNO, S., SAWATARI, K., SAWADA, S., SUGIMOTO,
M., SODA, R., TAKANO, T., NAKAMURA, K., MATSUMURA, Y., MYOJO,
T., SAKURAI, H., & TAKEMOTO, K. (1980) [Determination of
acrylonitrile in the working environments of a few kinds of
plants handling acrylonitrile.] Annual Report of the National
Institute of Industrial Health for 1979, p. 38 (in Japanese).
JACOBS, H.W. & SYRJALA, R.J. (1978) The use of infrared
analyzers for monitoring acrylonitrile. Am. Ind. Hyg. Assoc.
J., 39 : 161-165.
JACKSON, S. & BROWN, V.M. (1970) Effect of toxic wastes on
treatment processes and watercourses. Water Pollut. Control,
69 (3): 292-313.
JAEGER, R.J. (1978) Effect of hypoxia on the toxicity of
1,1-dichloroethylene or ACN. Toxicol. appl. Pharmacol., 45 :
257.
JAEGER, R.J. & COTE, I.L. (1982) Effect of hypoxia on the
acute toxicity of acrylonitrile. Res. Comm. Chem. Pathol.
Pharmacol., 36 (2): 345-348.
JAMES, S.P., WARING, R.H., & WHITE, D.A. (1967) Separation
of mercapturic acids and related compounds by gas
chromatography and a method for the determination of hippuric
acid. J. Chromatogr., 26 : 255-258.
JAPAN ASSOCIATION OF INDUSTRIAL HEALTH (1972) Recommendations
on permissible concentrations.
JEDLICKA, V., PASEK, A., & GOLA, J. (1958) Pesticides in
foods. III. Acrylonitrile as a food insecticide. J. Hyg. Epid.
Microbiol. Immunol., 1 : 116-125.
KANAI, R. & HASHIMOTO, K. (1965) Determination of
acrylonitrile, cyanide and thiocyanate in biological
materials. Ind. Health, 3 (47): 47-52.
KANKAANPÄÄ, E.E., HEMMINKI, K., & VAINIO, H. (1979)
Embryotoxicity of acrolein, acrylonitrile and acryl acid in
developing chick embryos. Toxicol. Lett., 4 : 93-96.
KIESSELBACH, N., KORALLUS, U., LANGA, H.J., NIESS, A., &
ZWINGERS, T. (1979) Acrylonitrile - Epidemiological Study,
Bayer 1977. Zentr. Arbeitsmed. Profyl., 29 (10): 257-259.
KILKIPARI, I. & SAVOLAINEN, H. (1982) Increased urinary
excretion of thioether in new rubber workers. Br. J. ind.
Med., 39 : 401-403.
KLESHCHEVA, M.S., USACHEVA, V.T., & POZHAROVA, V.N. (1971)
[Determination of residual monomers and non-polymerising
impurities in polystyrene plastics by means of gas
chromatography.] Plast. Mass., 7 : 57-58 (in Russian).
KNEZEVICH, A.L. & HOGAN, G.K. (1980) A 24-month oral
toxicity/carcinogenicity study of acrylonitrile administered
in the drinking water to Fischer 344 rats. Bio/Dynamics Inc.
(Final Report Vols I and IV, Project No. 77-1744, Bio-Dynamics
Inc.).
KNOBLOCH, K., SZENDZIKOWSKI, S., CZAJKOWSKA, T., & KRYSIAK,
B. (1971) [Experimental studies on acute and subacute
toxicity of acrylonitrile.] Med. Pr., 22 (3): 257-269 (in
Polish).
KNOBLOCH, K., SZENDZIKOWSKI, S., & CZAJKOWSKA, T. (1972)
[Chronic toxicity of acrylonitrile.] Med. Pr., 23 (3): 243-257
(in Polish).
KOLK, J.J. (1979) Inventory of employees exposed to
acrylonitrile in the Netherlands. Acrylonitrile Consultation
Group (Report of the Medical Sub-Group of the Vereniging van
de Nederlandse Chemische Industrie [VNCI]).
KOPECKY, J. (1982) Development and methodology for
assessment of urinary excretion of S-cyanoethylmercapturic
acid in man. (Final Report to the WHO Regional Office for
Europe, ICP/RCE 903).
KOPECKY, J., ZACHARDOVA, D., GUT, I., & FILIP, J. (1979)
[Metabolism of acrylonitrile in rats in vivo. ] Prac. Lék., 31 :
203-207 (in Czech).
KOPECKY, J., GUT, I., NERUDOVA, J., ZACHARDOVA, D., &
HOLECHEK, V. (1980a) Two routes of acrylonitrile metabolism.
J. Hyg. Epid. Microbiol. Immunol., 24 : 356-362.
KOPECKY, J., GUT, I., NERUDOVA, J., ZACHARDOVA, D., HOLECHEK,
V., & FILIP, J. (1980b) Acrylonitrile metabolism in the rat.
Arch. Toxicol., Suppl. 4 : 322-324.
KOPECKY, J., ZACHARDOVA, D., & GUT, I. (1980c) New findings
on acrylonitrile metabolism. Czech. Med., 3 : 295-301.
KOPECKY, J., GUT, I., NERUDOVA, J., ZACHARDOVA, D., &
HOLECHEK, V. (1981) Metabolic studies on acrylonitrile. In:
Gut, I., Cikrt, M. & Plaa, G.L., ed. Industrial and
environmental xenobiotics, Berlin, Springer-Verlag, pp.
221-231.
KORZHOVA, I.T., KLESHCHEVA, M.S., PORODINA, M.N., KORHOVA,
R.D., BALANDINA, V.A., & HOHLOV, V.A. (1974)
[Gas-chromatographic determination of toxic concentrations of
styrene and acrylonitrile vapours during the synthesis of
polystyrene plastics.] Plast. Mass., 5 : 67-69 (in Russian,
Chem. Abstr., 81 : 110759j).
KRIVOVA, V.N., USTINOVIC, I.P., & ERENBURG, B.E. (1982)
[Functional activity of the immune T-system due to
acrylonitrile poisoning.] In: Metabolicheskie aspecti
deistvija na organism industrial'nih himicheskih soedinenii,
Krasnojarsk, pp. 53-55 (in Russian).
KROELLER, E. (1970) [Residual acrylonitrile determination in
food.] Dtsch Lebensm. Rundsch., 66 : 11-33 (in German).
KRYNSKA, A. (1970) [Method of determination of acrylonitrile
in the air.] Pr. Central. Inst. Ochrony Pr., 20 : 303-310 (in
Polish, Chem. Abstr., 74 : 90798w).
KRYSIAK, B & KNOBLOCH, K. (1971) [Effect of acrylonitrile on
the central nervous system.] Med. Pr., 22 (6): 601-610 (in
Polish).
LAMBOTTE-VANDEPAER, M., DUVERGER-VAN BOGAERT, M., DE MEESTER,
C., PONCELET, F., & MERCIER, M. (1980) Mutagenicity of urine
from rats and mice treated with acrylonitrile. Toxicology, 16 :
67-71.
LAMBOTTE-VANDEPAER, M., DUVERGER-VAN BOGAERT, M., DE MEESTER,
C., ROLLMANN, B., PONCELET, F., & MERCIER, M. (1981a)
Identification of two urinary metabolites of rats treated with
acrylonitrile; influence of several inhibitors on the
mutagenicity of those urines. Toxicol. Lett., 7 : 321-328.
LAMBOTTE-VANDEPAER, M., DUVERGER-VAN BOGAERT, M., GARNY, V.,
PONCELET, F., & MERCIER, M. (1981b) Mutagenicity of
metabolites of acrylonitrile detected in urine. Mutat. Res.,
80 : 269 (abstract).
LANGVARDT, P.W., PUTZIG, C.L., BRAUN, W.H., & YOUNG, J.D.
(1980) Identification of the major urinary metabolites of
acrylonitrile in the rat. J. Toxicol. environ. Health, 6 :
273-282.
LAWTON, A.H., SWEENEY, T.A., & DUDLEY, H.C. (1943)
Toxicology of acrylonitrile (vinyl cyanide): III.
Determination of thiocyanate in blood and urine. J. ind. Hyg.
Toxicol., 25 : 13-19.
LEFAUX, R. (1966) [Chemistry and toxicology of plastics.]
Mainz, Krausskopf Verlag (in German).
LEONARD, A., GARNY, V., PONCELET, F., & MERCIER, M. (1981)
Mutagenicity of acrylonitrile in mouse. Toxicol. Lett., 7 :
329-334.
LINDGREN, D.L., VINCENT, L.E., & KROHNE, H.E. (1954)
Relative effectiveness of ten fumigants to adults of eight
species of stored product insects. J. econ. Entomol., 47 (5):
923-926.
LITTON BIONETICS (1975) Mutagenic evaluation of compounds
MCA 730 (LBI Project No. 2547, prepared for the Manufacturing
Chemists Association).
LITTON BIONETICS (1976) Mutagenicity evaluation of MCA 730
(Final report, LBI Project No. 2548, prepared for the
Manufacturing Chemists Association).
LODZ SANITARY INSPECTION SURVEY (1981) [Annual report on the
concentration of acrylonitrile in the air at workplaces in the
acrylic fibre plant "Chemitex-Anilana" at Lodz.] Lodz, Poland,
Sanitary-Epidemiological Station (in Polish).
LORZ, H. (1950) [On the percutaneous poisoning by
acrylonitrile (Ventox).] Dtsch med. Wochenschr., 75 (33/34):
1087-1088 (in German).
LOWENBACH, W., SCHLESINGER, S., & KING, J. (1978) Toxic
pollutant identification: acrylonitrile manufacture, 160 pp
(EPA-IMPQE series 02178-03).
MADDOCK, J.T., BYER, W.L., MALSEED, R.T., KERESZTESY, J.,
TAYLOR, T.J., & KATZ, A.B. (1977) Acrylonitrile (Monograph
prepared for US Consumer Product Safety Commission, Washington
DC).
MAGOS, L. (1962) A study of acrylonitrile poisoning in
relation to methemoglobin-CN complex formation. Br. J. ind.
Med., 19 : 283-286.
MALLETTE, F.S. (1943) Industrial hygiene in synthetic rubber
manufacture. Ind. Med., 12 : 495-499.
MALTONI, C., CILIBERTI, A., & DI MAIO, V. (1977)
Carcinogenicity bioassays on rats of acrylonitrile
administered by inhalation and by ingestion. Med. lav., 68 :
401-411.
MALTONI, C., CILIBERTI, A., & CARRETTI, D. (1982)
Experimental contributions in identifying brain potential
carcinogens in the petrochemical industry. Ann. N.Y. Acad.
Sci., 381 : 216-249.
MARANO, R.R., LEVINE, S.P., & HARVEY, T.M. (1978) Trace
determination of subnanogram amounts of acrylonitrile in
complex matrices by gas chromatography with a
nitrogen-selective detector. Anal. Chem., 50 (13): 1948-1951.
MARCOCI, S. & IONESCU, M. (1974) [The toxic action of
acrylonitrile on fish.] Strd. Prof. Calitatii Apelor, 16 : 7-22
(in Rumanian).
MARKELOV, M.A. & SEMENENKO, E.I. (1976) [Determination of
microconcentrations of substances migrating from polymer
materials to water.] Plast. Mass., 1 : 57-59 (in Russian, Chem.
Abstr., 84 : 155747g).
MAVRINA, E.A. & HROMOV, W.E. (1974) [Functional changes in
the body of workers engaged in production of nitron fibre.]
Vrach. Delo, 6 : 129-131 (in Russian).
MAVRINA, E.A. & IL'INA, V.A. (1974) [Change in the
immunological reactivity of the bodies of students of a
technical-trade school during training in a nitron production
plant.] Gig. i Sanit., 5 : 109-110 (in Russian).
MCLAUGHLIN, M., KRIVANEK, N.D., & TROCHIMOWICH, H.J. (1976)
Evaluation of antidotes for acrylonitrile poisoning (Abstr.).
Toxicol. appl. Pharmacol., 37 : 133-134.
MCNEAL, T. & BREDER, C.V. (1981) Head-space sampling and
gas-solid chromatographic determination of residual
acrylonitrile in acrylonitrile copolymer solutions. J. Assoc.
Off. Anal. Chem., 64 (2): 270-275.
MCNEAL, T. & BREDER, C.V. (1982) Manual headspace gas-solid
chromatographic determination of sub-parts-per-trillion levels
of acrylonitrile in 3% acetic acid. J. Assoc. Off. Anal.
Chem., 65 (1): 184-187.
MCOMIE, W.A. (1949) Comparative toxicity of
methacrylonitrile and acrylonitrile. J. ind. Hyg. Toxicol.,
31 : 113-116.
MILLER, L.M. & VILLAUME, J.E. (1978) Investigation of
selected potential environmental contaminants: acrylonitrile.
Washington DC, US EPA, 234 pp (Prepared for the Office of
Toxic Substances).
MILLS, E.J., Jr & STACK, V.T., Jr (1955) Acclimation of
microorganisms for the oxidation of pure organic chemicals.
In: Proceedings of the 9th Industrial Waste Conference Purdue
University, pp. 449-464.
MILWY, P. (1978) Letter to the editor. Reply to S. Venitt.
Mutat. Res., 57 : 110-112.
MILWY, P. & WOLFF, M. (1977) Mutagenic studies with
acrylonitrile. Mutat. Res., 48 : 271-278.
MINAMI, M., SATO, M., SUSUKI, A., YAMAMURA, K., & ISHIZU, S.
(1973) [Acrylonitrile poisoning. I. Result of exposure to
acrylonitrile vapour.] Tokyo Joshi Ika Daigaku Zasshi, 43 (10):
849-855 (in Japanese).
MLETZKO, H.G. von, & HENKEL, W. (1978) Animal experiments
with exposure to noise and acrylonitrile from the
chronobiological aspect. Z. Gesamte Hyg., 24 : 515-518.
MONSANTO (1975) Joint toxic action between acrylonitrile and
potassium cyanide. St Louis, MO, Monsanto Co. Medical
Department.
MONSANTO (1977a) Acrylonitrile sales specification, St
Louis, MO, Monsanto Chemicals Intermed. Co. (Monsanto data
sheet).
MONSANTO (1977b) In response to OSHA's request for
information on acrylonitrile (42 FR 34327) (Letter to the OSHA
Docket Officer, US Department of Labor).
MONSON, R.R. & FINE, L.J. (1978) Cancer mortality and
morbidity among rubber workers. J. Natl Cancer Inst., 61 (4):
1047.
MUHTAROVA, M. (1977) [Personal sample for evaluation of the
professional risk due to poisonings.] Hyg. Zdrav., 5 : 447-450
(in Bulgarian).
MURRAY, F.J., SCHWETZ, B.A., NITSCHE, K.D., JOHN, J.A.,
NORRIS, J.M., & GEHRING, P.J. (1978) Teratogenicity of
acrylonitrile given to rats by gavage or by inhalation. Food
cosmet. Toxicol., 16 (6): 547-551.
MURRAY, F.J., NITSCHKE, K.D., JOHN, J.A., SMITH, F.A., QUAST,
J.F., BLOGG, C.D., & SCHWETZ, B.A. (1976) Teratologic
evaluation of acrylonitrile monomer given to rats by gavage.
Midland MI, USA, Dow Toxicological Research Laboratory
(prepared for the Manufac. Chem. Assoc.).
NAKAMURA, K. (1981) [Mortality study of acrylonitrile
workers.] In: Annual Report of the National Institute of
Industrial Health for 1980, p. 31 (in Japanese).
NERUDOVA, J., HOLECHEK, V., GUT, I., & KOPECKY, J. (1980a)
[Relation between kinetics of acrylonitrile after different
routes of administration and its conversion to thiocyanate.]
Prakt. Lék., 32 : 15-18 (in Czech).
NERUDOVA, J., KOPECKY, J., GUT, I., & HOLECHEK, V. (1980b)
[Metabolism of acrylonitrile in the rats in vitro.] Prakt.
Lék., 32 : 98-103 (in Czech).
NERUDOVA, J., GUT, I., & KOPECKY, J. (1981) Cyanide effect
in acute acrylonitrile poisoning in mice. In: Gut, I., Cikrt,
M., & Plaa, G.L., ed. Industrial and environmental
xenobiotics, Berlin, Springer Verlag, pp. 245-250.
NILSEN, O.G., TOFTGARD, R., & ENEROTH, P. (1980) Effects of
acrylonitrile on rat liver cytochrome P-450, benz( a)pyrene
metabolism, and serum hormone levels. Toxicol. Lett., 6 :
399-404.
NIOSH (1976) NIOSH analytical methods for set K, Washington,
DC, NIOSH (NTIS Report No. PB-254 227).
NIOSH (1978) A recommended standard for occupational
exposure to acrylonitrile, Cincinnati, OH, Department of
Health, Education and Welfare (NIOSH), (Publ. No. 78-116).
NOEL, G., LAMBOTTE-VANDEPAER, M., DUVERGER-VAN BOGAERT, M.,
ROBERFROID, M., & MERCIER, M. (1978) Hepatotoxic effects of
acrylonitrile in rats. Arch. int. Physiol. Biochem., 86 (4):
951-952.
O'BERG, M.T. (1980) Epidemiologic study of workers exposed
to acrylonitrile. J. occup. Med., 22 (4): 245-252.
OOMENS, A.C. (1980) Experience with a dual detector
headspace gas chromatograph for acrylonitrile analysis. In:
Kolb, B., ed. Applied headspace gaschromatography, Heydon, pp.
111-116.
ORUSEV, T. & POPOVSKI, P. (1973) [Clinical symptoms in
workers occupationally exposed to acrylonitrile.] God. Zb.
Med. Fak. Skopje, 19 : 187-192 (in Macedonian).
ORUSEV, T., BAUER, S., & POPOVSKI, P. (1973) [Occupational
exposure to acrylonitrile in a plant for production of acrylic
synthetic fibres.] God. Zb. Med. Fak. Skopje, 19 : 445-449 (in
Macedonian).
OSHA (1978a) Emergency temporary and permanent standards for
occupational exposure to acrylonitrile (vinyl cyanide). Fed.
Reg., 43 : 2600, 4562.
OSHA (1978b) Permanent standard for exposure to acrylo-
nitrile. Fed. Reg., 43 : 45809-45817.
OSHA (1981) General Industry - Safety & Health Standards, 29
CFR 1910. US Department of Labor, pp. 830-850, 220 pp.
OSTROVSKAJA, R.S., PODREZ, Z.G., BRAGINSKAJA, L.L., BOKLOG,
E.P., EFREMOV, A.M., & VOLKOVA, G.A. (1976) [Health status
of workers currently engaged in the production of
acrylonitrile.] Gig. Tr. Prof. Zabol., 6 : 8-12 (in Russian).
PANOVA, R.V., BELOVA, R.A., & LAVRUSHINA, T.S. (1969)
[Chromatographic determination of residual monomers in
latexes.] Prom. Sin. Kauch. Nauch.-Tekh. Sb., 1 : 21-23 (in
Russian, Chem. Abstr., 74 : 43238d).
PARENT, R.A. & CASTRO, B.C. (1979) Effect of acrylonitrile
on primary Syrian golden hamster embryo cells in culture:
Transformation and DNA fragmentation. J. Natl Cancer Inst.,
62 (4): 1025-1029.
PARSONS, J.S. & MITZNER, S. (1975) Gas chromatographic
method for concentration and analysis of traces of industrial
organic pollutants in environmental air and stacks. Environ.
Sci. Technol., 9 : 1053-1058.
PATTERSON, R.M., BORNSTEIN, M.I., & GARSHICK, E. (1976)
Assessment of acrylonitrile as a potential air pollution
problem, Vol. VI (US Environmental Protection Agency, Contract
No. 68-02-1337, Task 8, Springfield, VA, National Technical
Information Service, No. PB-258 358).
PATTY, F.A., ed. (1963) Acrylonitrile, CH2=CHCN (vinyl
cyanide propenenitrile). In: Industrial hygiene and
toxicology, 2nd Revised ed., Vol. 2, pp. 2009-2012.
PAULET, G. & DESNOS, J. (1961) L'acrylonitrile,
toxicité-méchanisme d'action-thérapeutique. Arch. int.
Pharmacodyn., 131 (1-2): 54-83.
PAULET, G. & VIDAL, M. (1975) De la toxicité de quelques
esters acryliques et méthacryliques de l'acrylamide et des
polyacryliques. Arch. Mal. prof., 36 (1-2): 58-60.
PAULET, G., DESNOS, J., & BATTIG, J. (1966) De la toxicité
de l'acrylonitrile. Arch. Mal. prof., 27 (12): 849-856.
PEROCCO, P., PANE, G., BOLOGNESI, S., & ZANNOTTI, M. (1982)
Increase of sister chromatid exchange and unscheduled
synthesis of deoxyribonucleic acid by acrylonitrile in human
lymphocytes in vitro. Scand. J. Work Environ. Health, 8 :
290-293.
PETER, H. & BOLT, H.M. (1981) Irreversible protein binding
of acrylonitrile. Xenobiotica, 11 : 51-56.
PETER, H., APPEL, K.E., BERG, R., & BOLT, H.M. (1983)
Irreversible binding of acrylonitrile to nucleic acids.
Xenobiotica, 13 (1): 19-25.
PETROVA, L.I., NOSKOVA, M.P., BALANDINA, V.A., & GURICHEVA,
Z.G. (1972) [Polarographic determination of acrylonitrile in
aqueous extract of styrene- acrylonitrile copolymers.] Gig. i
Sanit., 37 : 62-65 (in Russian, Chem. Abstr., 76: 127898y).
PFEILSTICKER, K., INKMANN, K., & BOCHISCH, M. (1977)
Residues of (1-14C) ACN in fumigated dry food. Z.
Lebensmittel-Untersuch. Forsch., 164 (4): 274-276.
POLAND (1982) Decree of the Minister of Labour, Wages, and
Social Affairs, 22 December, 1982 (Low Gaz. No. 43, 29
December, 1982, Entry 287).
PONOMAREV, Y.P., ANUFRIEVA, T.L., & SHKORBATOVA, T.L. (1974)
[Polarographic determination of acrylonitrile in waste waters
using extraction.] Vodosnabz., Kanaliz. Gidroteh. Sooruz., 17:
67-70 (in Russian, Chem. Abstr., 82 : 34803x).
PORTMANN, J.K. & WILSON, K.W. (1971) The toxicity of 140
substances to the brown shrimp and other marine animals. 12 pp
(Ministry Agriculture Fisheries & Food Laboratories, England;
Shellfish Inf. Leafl. No. 22).
PUJADO, P.R., VORS, B.V., & KRUEDING, A.P. (1977) Newest
acrylonitrile process. Hydrocarbon Process, (May, 1977):
169-172.
QUAST, J.F., HUMISTON, C.G., SCHWETZ, B.A., FRAUSON, L.A.,
WADE, C.E., & NORRIS, J.M. (1975) A six-month oral toxicity
study incorporating acrylonitrile in the drinking water of
pure-bred beagle dogs. Midland MI, USA, Dow Toxicology
Research Laboratory, (prepared for the Manuf. Chem. Assoc.).
QUAST, J.F., ENRIQUEZ, R.M., WADE, C.E., HUMISTON, C.C., &
SCHWETZ, B.A. (1977) Toxicity and drinking water containing
acrylonitrile in rats: results after 12 months. Midland MI,
USA, Dow Toxicology Research Laboratory (prepared for the
Manuf. Chem. Assoc.).
QUAST, J.F., SCHUETZ, D.J., BALMER, M.F., GUSHOW, T.S., PARK,
C.N., & McKENNA, M.J. (1980a) A two-year toxicity and
oncogenicity study with acrylonitrile following inhalation
exposure of rats. Final Report. Midland MI, USA, Dow
Toxicology Research Laboratory.
QUAST, J.F., WADE, C.E., HUMISTON, C.G., CARREON, R.M.,
HERMANN, E.A., PARK, C.N., & SCHWETZ, B.A. (1980b) A
two-year toxicity and oncogenicity study with acrylonitrile
incorporated in the drinking water of rats. Final Report.
Midland MI, USA, Dow Toxicology Research Laboratory.
RABELLO-GAY, M.N. & AHMED, E. (1980) Acrylonitrile: in vivo
cytogenetic studies in mice and rats. Mut. Res., 79 : 249-255.
RAJENDRAN, S. & MUTHU, M. (1981) Detection of acrylonitrile
and ethylene oxide in air and fumigated foodstuffs. Bull.
environ. Contam. Toxicol., 27 : 426-431.
RAJENDRAN, S. & MUTHU, M. (1981) Effect of acrylonitrile on
trehalase, phosphorylase and acetylcholinesterase activities
in Tribolium castenlum Herbst and Trogoderma granarium Everts.
Experientia (Basel), 37 (8): 886.
RAMSTAD, T. & NICHOLSON, L.W. (1982) Determination of
sub-parts-per-billion levels of acrylonitrile in aqueous
solutions. Anal. Chem., 54 : 1191-1196.
REICHLE, A. & TENGLER, H. (1968) [Gas-chromatographic
procedures for determining residual monomers in the polymer.
Determination of acrylonitrile in polyacrylonitrile.]
Plastverarbeiter, 19 : 921-924 (in German, Chem. Abstr., 70 :
97206c).
ROGACZEWSKA, T. (1964) [Polarographic determination of
acrylonitrile in air.] Chem. Anal., 9 : 417-423 (in Polish).
ROGACZEWSKA, T. (1965) [Colorimetric method of acrylonitrile
determination in air.] Med. Pr., 16 (6): 465-475 (in Polish).
ROGACZEWSKA, T. (1971) The problems of obtaining the
evaluation of deleterious effects of acrylonitrile vapours in
industrial conditions. Thesis, Lodz, Poland, Institute of
Industrial Medicine.
ROGACZEWSKA, T. (1975) [Percutaneous absorption of
acrylonitrile vapours in animals.] Med. Pr., 26 (6): 459-465
(in Polish).
ROGACZEWSKA, T. (1976) [Determination of acrylonitrile in
air.] Med. Pr., 27 (2): 115-126 (in Polish).
ROGACZEWSKA, T. & PIOTROWSKI, J. (1968) [Routes of
acrylonitrile absorption in man.] Med. Pr., 19 (4): 349-353 (in
Polish).
ROSE, V.E. & PERKINS, J.L. (1982) Passive dosimetry - state
of the art review. Am. Ind. Hyg. Assoc. J., 43 : 605-621.
ROUDABUSH, R.L., TERHAAR, C.J., FASSETT, D.W., & DZIUBA, S.P.
(1965) Comparative acute effects of some chemicals on the
skin of rabbits and guinea-pigs. Toxicol. appl. Pharmacol., 7 :
559-565.
RUSSELL, J.W. (1975) Analysis of air pollutants using
sampling tubes and gas chromatography. Environ. Sci. Technol.,
9 : 1175-1178.
RUSSKIH, A.A. (1972) [Determination of acetone cyanohydrin
and acrylonitrile in air.] In: Bezhaev, M.S., ed. Proceedings
of the 4th Conference of People Employed in Universities and
Industrial Laboratories of South-east USSR on Physico-chemical
Methods of Analytical Control of Industrial Products, USSR,
Dagestan State University, Vol. 3, p. 100 (in Russian, Chem.
Abstr., 81 : 16301g).
RUSSKIH, A.A. (1973) [Photometric determination of
acrylonitrile in air.] Zavod. Lab., 39 (1): 5-6 (in Russian).
SAKURAI, H. & KUSUMOTO, M. (1972) [Epidemiologic study of
health impairment among acrylonitrile workers.] J. Sci. Labour
(Tokyo), 48 (5): 273-282 (in Japanese).
SAKURAI, H., ONODERA, M., UTSUNOMIYA, T., MINAKUCHI, H., IWAI,
H., & MATYUMURA, H. (1978) Health effects of acrylonitrile
in acrylic fibre factories. Br. J. ind. Med., 35 (3): 219-225.
SANDBERG, E.C. & SLANINA, P. (1980) Distribution of (1-14C)
acrylonitrile in rat and monkey. Toxicol. Lett., 6 : 187-191.
SAPOTA, A. (1982) The disposition of 14C-acrylonitrile in
rats. Xenobiotica, 12 (4): 259-264.
SAPOTA, A. & CHMIELNICKA, J. (1981) [Toxicity and metabolism
of acrylonitrile.] Med. Pr., 32 (1): 25-33 (in Polish).
SAPOTA, A. & DRAMINSKI, W. (1981) The fate of
14C-acrylonitrile in rats. In: Gut, I., Cikrt, M., & Plaa,
G.L., ed. Industrial and environmental xenobiotics, Berlin,
Springer Verlag, pp. 231-237.
SARTORELLI, E. (1966) [Acute acrylonitrile intoxication.]
Med. lav., 57 (3): 184-187 (in Italian).
SATO, M. (1978) [Studies on the toxic effect of
acrylonitrile - its metabolism, absorption and excretion.]
Jpn. J. Hyg., 33 (3): 497-505 (in Japanese).
SATO, M., ISHIZU, S., & MOMOTANI, H. (1975) [Determination
of acrylonitrile, cyanide and thiocyanate in blood and urine.]
Sangyo Igaku, 17 : 99-105 (in Japanese, Chem. Abstr., 100292z).
SATO, M., HIRASAWA, F., OGATA, M., TAKIZAWA, Y., KOJIMA, H., &
YOSHIDA, T. (1982) Distribution and accumulation of
(2,3-14C) acrylonitrile in rat after single injection.
Ecotoxicol. environ. Safety, 6 (5): 489-494.
SATO, S., KOSHIO, H., MIMURA, M., NAKAMURA, S., & ICHIMURA,
M. (1979) Investigation in counter-measure to the sources of
unspecified harmful chemicals in 1978. pp. 1-19 (in Japanese,
Report of the study sponsored by the Environmental Agency).
SCHEUFLER, H. von (1976) Experimental testing of chemical
agents for embryotoxicity, teratogenicity and mutagenicity -
ontogenic reactions of the laboratory mouse to these
injections and their evaluation - a critical analysis method.
Biol. Rundsch., 14 (4): 227-229.
SCHEUFLER, H. von (1980) [On the embryotoxic effectiveness
of acrylonitrile in the laboratory mouse.] Z. Gesamte Hyg.,
26 : 564-565 (in German).
SCUPAKAS, D. (1968) [Industrial hygiene conditions during
the reworking of plastics and problems improving them.] In:
Yavnaist, E.Y., ed. Proceedings of the first conference on
actual problems of industrial hygiene and occupational
pathology, 1967, Riga, Medical Institute, pp. 128-132 (in
Russian, Chem. Abstr., 72 : 47094k).
SEKI, S. (1967) [On acrylonitrile poisoning in a synthetic
resin plant.] Jpn. J. Hyg., 22 (1): 257 (in Japanese).
SEUTTER-BERLAGE, F., VAN DORP, H.L., KOSSE, H.G.J., &
HENDERSON, P.T. (1977) Urinary mercapturic acid excretion as
a biological parameter of exposure to alkylating agents. Int.
Arch. occup. environ. Health, 39 : 45-51.
SEUTTER-BERLAGE, F., DELBRESSINE, L.P.C., SMEETS, F.L.M., &
KETELAARS, H.C.J. (1978) Identification of three
sulfur-containing urinary metabolites of styrene in the rat.
Xenobiotica, 8 : 413-418.
SHACKELFORD, W.M. & KEITH, L.H. (1976) Frequency of organic
compounds identified in water, Athens GA, USA, US
Environmental Protection Agency, p. 55 (EPA-600/4-76-062).
SHEVCHIK, J. (1976) Detectors in gas chromatography,
Amsterdam, Elsevier, 192 pp.
SHUSTOV, V.J. (1968) [Clinical manifestation and
hematological changes in workers employed in the production of
acrylonitrile at the Saratov Chemical Combine.] Gig. Tr. prof.
Zabol., 3 : 27-29 (in Russian).
SHUSTOV, V.Y. & MAVRINA, E.A. (1975) [Clinical picture of
chronic poisoning in the production of nitrone.] Gig. Tr.
prof. Zabol., 3 : 27-29 (in Russian).
SILVER, E.H. & SZABO, S. (1982) Possible role of lipid
peroxidation in the action of acrylonitrile on the adrenals,
liver and gastrointestinal tract. Res. Comm. Chem. Pathol.
Pharmacol., 36 (1): 33-43.
SILVER, E.H., KUTTAB, S.H., HASAN, T., & HASSAN, M. (1982a)
Structural considerations in the metabolism of nitriles to
cyanide in vivo. Drug Metab. Disposition, 10 : 495-498.
SILVER, E.H., McCOMB, D.J., KOVACS, K., & SZABO, S. (1982b)
Limited hepatotoxic potential of acrylonitrile in rats.
Toxicol. appl. Pharmacol., 64 : 131-139.
SILVERSTEIN, L.G. (1977) Calidation of abcor GASBADGE for
acrylonitrile and improved desorption efficiency. Am. Ind.
Hyg. Assoc. J., 38 (8): 412-413.
SKVOSTSOV, Ju.I. (1980) [Variation in the amino acid
spectrum of blood serum in workers employed in ACN production
and nutritive ways of its correction. Vopr. Pitan., No. 2 :
31-33 (in Russian).
SLATER, E.E., ANDERSON, M.D., & ROSENKRANZ, S. (1971) Rapid
detection of mutagens and carcinogens. Cancer Res., 31 (7):
970-973.
SLOOF, W. (1979) Detection limits of a biological monitoring
system based on fish respiration. Bull environ. Contam.
Toxicol., 23 : 517-523.
SMYTH, H.F. & CARPENTER, C.P. (1948) Further experience with
the range-finding test in the industrial toxicology
laboratory. J. ind. Hyg. Toxicol., 30 : 63-68.
SOKAL, J.A. & KLYSZEJKO-STEFANOWICZ, L. (1972)
[Nicotinamide-adenine dinucleotides in acute poisoning with
some toxic agents.] Lodz Tow. Nauk., No. 112: 64-101 (in
Polish, Chem. Abstr., 79 : 62271d).
SOKAL, J.A., KNOBLOCH, K., MAJKA, J., SAPOTA, A., &
SZENDZIKOWSKI, S. (1977) [Toxicity of acrylate compounds
used in industry.] Prakt. Lek., 29 (4): 157-161 (in Czech).
SPASSOVSKI, M. (1976) Health hazards in the production and
processing of some fibres, resins, and plastics in Bulgaria.
Environ. Health Perspect., 17 : 199-202.
STAMOVA, N., GINCHEVA, N., SPASOVSKI, M., BAINOVA, A.,
IVANOVA, S., KURKCHIEV, S., HINKOVA, L., KHRISTEVA, V.,
MUKHTAROVA, M., KARADZHOVA, N., TSANEVA, L., & FIKOVA, E.
(1976) [Labour hygiene during the production of synthetic
fibres "Bulana".] Hyg. Zdrav., 2 : 134-140 (in Russian).
STANFORD RESEARCH INSTITUTE (1976) Interim Report: In vitro
microbiological mutagenicity studies of Dow Chemical Company
Compounds (SRI Project No. LSC-4378, prepared for Dow Chemical
Company).
STEICHEN, R.J. (1976) Modified solution approach for the gas
chromatographic determination of residual monomers by
head-space analysis. Anal. Chem., 48 : 1398-1402.
SUTA, B.E. (1979) Assessment of human exposures to
atmospheric acrylonitrile (Prepared by the Stanford Research
Institute - Contract 68-02-2835-EPA).
SVIRBELY, J.L. & FLOYD, E.P. (1961) Toxicologic studies of
acrylonitrile and B,B'-oxydipropionitrile. III. Chronic
studies. Meeting paper AINA-ACSIH, Detroit MI, USA.
SWEDEN (1981) Ordinance issued by the National Swedish Board
of Occupational Safety and Health Concerning Hygiene Limit
Values (in Swedish).
SZABO, S. & SELYE, H. (1971) Adrenal apoplexy and necrosis
produced by acrylonitrile. Endocrinol., 57 (3): 405-408.
SZABO, S. & SELYE, H. (1972) Effects of phenobarbital and
steroids on the adrenal apoplexy produced by acrylonitrile in
rats. Endocrinol. Exp., 6 (3): 141-146.
SZABO, S., REYNOLDS, E.S., KOMANICKY, P., MOSLEN, M.T., &
MELBY, J.C. (1976) Effect of chronic acrylonitrile ingestion
on rat adrenal. Toxicol. appl. Pharmacol., 37 (1): 133.
SZABO, S., BAILEY, K.A., BOOR, P.J., & JAEGER, R.J. (1977)
Acrylonitrile and tissue glutathion - differential effect of
acute and chronic interactions. Biochem. Biophys. Res.
Commun., 79 (1): 32-37.
SZABO, S., HUTTNER, I., KOVACS, K., HORVATH, E., SZABO, D., &
HORNER, H.C. (1980) Pathogenesis of experimental adrenal
hemorrhagic necrosis ("apoplexy"). Ultrastructural,
biochemical, neuropharmacologic and blood coagulation studies
with acrylonitrile in the rat. Lab. Invest., 42 (5): 533-545.
TADA, O. (1971) [Methods for determinations of toxic
substances in air-organic compounds.] J. Sci. Lab., (Part II),
47 : 789-796.
TAKAGI, N., TAKAGI, K., MATSUDA, T., & KOIZUMI, S. (1968)
[Effect of administration of vitimins B1 or B2 plus L-cysteine
to rats exposed to acrylonitrile gas over a long period.] J.
Sci. Lab., 44 (8): 454-464 (in Japanese).
TARKOWSKI, S. (1966) [Effect of cobalt on the inhibition by
potassium cyanide of cytochrome oxidase activity.] Med. Pr.,
17 (2): 116-119 (in Polish).
TARKOWSKI, S. (1968) [Effect of acrylonitrile on certain
properties of cytochrome oxidase.] Med. Pr., 19 (6): 525-531
(in Polish).
TATSUNO, T., INOUE, T., & TANIMURA, A. (1979) [Hygienic
chemical studies on plastics (II). Residual acrylonitrile in
ABS resin or AS resin and its migration into water.] Report of
the National Institute of Hygiene, 97 : 93-97 (in Japanese).
TATSUNO, T., INOUE, T., & TANIMURA, A. (1980) [Hygienic
chemical studies on plastics (V). Migration of acrylonitrile
from acrylonitrile-butadiene-styrene copolymer and
acrylonitrile-styrene copolymer into various food simulating
solvents in long period.] Report of the National Institute of
Hygiene, 98 : 110-115 (in Japanese).
TERENT'EV, A.P. & OBTEMPERANSKAJA, S.I. (1956) [Quantitative
determination of acrylonitrile with sodium sulfite.] Z. Anal.
Him., 11 : 638-639 (in Russian).
THIESS, A.M. & FLEIG, I. (1978) Analysis of chromosomes of
workers exposed to acrylonitrile. Arch. Toxicol., 41 (2):
149-152.
THIESS, A.M., FRENTZEL-BEYME, R., LINK, R., & WILD, H.
(1980) [Mortality study in chemical workers in different
production plants with exposure also to acrylonitrile.]
Zentralbl. Arbeitsmed. Arbeitsschutz Prophyl. Ergonomie,
30 (7): 259-267 (in German).
TULLAR, P.E. (1947) Final report on the pharmacology and
toxicology of acrylonitrile and acrylon. Washington,
Palusowski Memorial Research Laboratory, George Washington
University.
UHDE, W.J. & KOEHLER, U. (1967) [Testing of plastic
articles. Determination of monomeric compounds in the volatile
fraction of styrene polymers.] Z. Lebensm. Unters.-Forsch.,
135 : 135-140 (in German).
UNION CARBIDE CORPORATION (1977) In response to OSHA's
request for information on occupational exposure to
acrylonitrile. Fed. Reg., 42 : 33042 (Unpublished letter to the
OSHA Docket Officer, US Department of Labor).
UNITED KINGDOM HEALTH & SAFETY EXECUTIVE (1981a)
Acrylonitrile in air. Laboratory method using charcoal
adsorption tubes and gas chromatography. Methods for the
Determination of Hazardous Substances, 1, March 1981.
UNITED KINGDOM HEALTH & SAFETY EXECUTIVE (1981b)
Acrylonitrile in air. Laboratory method using porous polymer
adsorption tubes, and thermal desorption with gas
chromatographic analysis. Methods for the Determination of
Hazardous Substances, 2, March 1981.
UNITED KINGDOM MINISTRY OF AGRICULTURE, FISHERIES & FOOD
(1982) Survey of acrylonitrile and methacrylonitrile in food
contact materials and in food, London, HMSO (Food Surveillance
Paper No. 6).
US CONSUMER PRODUCT SAFETY COMMISSION (1978) Assessment of
acrylonitrile contained in consumer products. Washington, DC
(Final report, contract No. CPSC-C-77-0009. Task No. 1014K,
H.I.A. Economic Analysis).
US DEPARTMENT OF TRANSPORTATION (1974) Acrylonitrile. In:
CHRIS hazardous chemical data, Department of Transportation,
Coast Guard, C-G-446-2.
US EPA (1977) Optional procedures for classification of
pesticide uses by regulation. Fed. Reg., 42 : 44170, 44178.
US FDA (1977a) Acrylonitrile copolymer used to fabricate
beverage containers, final decision. Fed. Reg., 42 :
48528-48544.
US FDA (1977b) Substances for use only as components of
adhesives. US Code Fed. Regul., Title 21, parts 175.105,
175.300, 175.320, 176.170, 176.180, 177.1010, 177.1020,
177.1040, 177.1200, 177.2600, 178.3790, 180.22, pp. 438, 445,
452, 455, 465, 471-472, 482-483, 486, 488, 496, 501-504, 555,
596, 609-611.
USSR (1982) System of standards of work safety. The air of
the working zone. General sanitary-hygienic requirements (GOST
12.1.005-76). MAC of harmful substances in the air of working
zone (Suppl. to Tab. 4, SN 245-71, No 7-11). MAC of harmful
substances in the air of working zone (part 12-19), confirmed
in 1977-1982 years.
VAINIO, M. & MAKINEN, M. (1977) Styrene and acrylonitrile
induced depression of hepatic nonprotein sulfhydryl content in
various rodent species. Res. Commun. chem. Pathol. Pharmacol.,
17 (1): 115-124.
VAN BLADEREN, P.J., DELBRESSINE, L.P.C., HOOGETERP, J.J.,
BEAUMONT, A.H.G.M., BREIMER, D.D., SEUTTER-BERLAGE, F., & VAN
DER GEN, A. (1981) Formation of mercapturic acids from
acrylonitrile, crotononitrile, and cinnamonitrile by direct
conjugation and via an intermediate oxidation process. Drug
Metab. Disposition, 9 : 246-249.
VAN DOORN, R., BOS, R.P., LEIJDEKKERS, C.-M., WAGENAAS-ZEGERS,
M.A.P., THEUWS, J.L.G., & HENDERSON, P.T. (1979) Thioether
concentration and mutagenicity of urine from cigarette
smokers. Int. Arch. occup. environ. Health, 43 : 159-166.
VED BRAT, S. & WILLIAMS, G.M. (1982) Hepatocyte-mediated
production of sister chromatid exchanges in co-cultured cells
by acrylonitrile: evidence for extra cellular transport of a
stable reactive metabolite. Cancer Lett., 17 (2): 213-216.
VENITT, S., BUSHELL, C.T., & OSBORNE, M. (1977) Mutagenicity
of acrylonitrile (cyanoethylene) in Escherichia coli. Mutat.
Res., 45 (2): 283-288.
VERSCHUEREN, K. (1977) Handbook of environmental data on
organic chemicals, Van Nostrand Reinhold, pp. 78-81.
VISSARIONOVA, V.U., BONDAREV, G.I., STASENKOVA, K.P., &
ZEMLJANSKAJA, T.A. (1979) [Study of the effect of rations
with different protein content on rats following acrylonitrile
poisoning.] Vopr. Pitan., (1) : 36-40 (in Russian).
VOLKOVA, Z.A. & BAGDINOV, Z.M. (1969) [Industrial hygiene
problems in vulcanization processes of rubber production.]
Gig. i Sanit., 34 : 33-40 (in Russian).
WERNER, J.B. & CARTER, J.T. (1981) Mortality of United
Kingdom acrylonitrile polymerisation workers. Br. J. ind.
Med., 38 : 247-253.
WILLHITE, C.C. (1981a) Inhalation toxicology of acute
exposure to aliphatic nitriles. Clin. Toxicol., 18 (8):
991-1003.
WILLHITE, C.C. (1981b) Teratogenic effects of aliphatic
nitriles. Teratology, 23 : 317-323.
WILLHITE, C.C. & SMITH, R.P. (1981) The role of cyanide
liberation in the acute toxicity of aliphatic nitriles.
Toxicol. appl. Pharmacol., 59 : 589-602.
WILSON, R.H. (1944) Health hazards encountered in the
manufacture of synthetic rubber. J. Am. Med. Assoc., 124 :
701-703.
WILSON, R.H. & McCORMICK, W.E. (1949) Acrylonitrile: its
physiology and toxicology. Ind. Med., 18 : 223-245.
WILSON, R.H., HOUGH, G.V., & McCORMICK, W.E. (1948) Medical
problems encountered in the manufacture of American-made
rubber. Ind. Med., 17 : 199-207.
WISNIEWSKA-KNYPL, J.M., KNOBLOCH, J.M., JABLONSKA, J., & RUTA,
U. (1970) [Decrease of tissue respiration activity of
oxoglutarate dehydrogenase and level of sulfhydryl groups in
acute acrylonitrile intoxication in rats.] Med. Pr., 21 (6):
543-549 (in Polish).
WISNIEWSKA-KNYPL, J.M., TARKOWSKI, S., KLIMZAK, J., &
DRAMINSKI, W. (1978) [ Metabolism of vinyl chloride in the
liver of rats.] (in Polish) (Final report on the research
project of the Institute of Occupational Medicine in Lodz,
Poland).
WRIGHT, P.L. (1977) Studies on the metabolism of
acrylonitrile. Testimony before the FDA. In: Monsanto Co.
(1976b) Appendix A, pp. A-20 - A-28.
WYNDER, E.L. & HOFFMANN, D. (1967) Tobacco and tobacco
smoke. Studies in experimental carcinogenesis. New York,
Academic Press, p. 450.
YOSHIKAWA, H. (1968) [Toxicity of nitrile compounds. II.
Aliphatic nitriles.] Igak. Scibut., 77 (1): 1-4 (in Japanese).
YOUNG, J.D., SLAURER, R.W., & KARBOWSKI, R.J. (1977) The
pharmacokinetic and metabolism profile of 14 C-acrylonitrile
given to rats by three routes. Midland MI, USA, Toxicol. Res.
Lab. Dow Chem., (prepared for the Manufacturing Chem. Assoc.).
ZAYTSEVA, N.A., TOLSTOBROVA, S.A., & IL'IN, D.T. (1972)
[Chromatographic determination of unreacted monomers in
emulsions of acrylic copolymers.] Soviet Chem. Ind., 48 :
298-299 (in Russian).
ZELLER, H., HOFMANN, H.T., THIESS, A.M., & HEY, W. (1969)
[Toxicity of nitriles. Results of animal experiments and
industrial experiences during 15 years.] Zent. Arbeitsmed.
Arbeitsschutz, 19 (8): 226-238 (in German).
ZHURKOV, V.S., SHRAM, R.L. & DUGAN, A.M. (1983) [Analysis of
mutagenic activity of acrylonitrile.] Gig. i. Sanit., 1 : 71-72
(in Russian).
ZITTING, A., TENHUNEN, R., & SAVOLAINEN, H. (1981) Effects
of intraperitoneally injected acrylonitrile on liver, kidney
and brain. Acta pharmacol. toxicol., 49 : 412-415.
ZOTOVA, L.V. (1975a) [Working conditions in the production
of acrylonitrile and their effect on workers.] Gig. Tr. prof.
Zabol., No. 8 : 8-11 (in Russian).
ZOTOVA, L.V. (1976) [Toxic effect of acrylonitrile on
experimental animals following its absorption through the
skin.] Gig. i Sanit., 41 (10): 103-105 (in Russian).
7.2. Subacute Toxicity
7.2.1. Inhalation exposure
Rats, guinea-pigs, rabbits, monkeys, and cats were exposed to
acrylonitrile at 330 mg/m3 air for 4 h per day, 5 days a week, for
8 weeks. All adult rats survived 8 weeks, but 5 out of 8 young
rats died by the 6th week, 3 out of 16 guinea-pigs and 1 out of 4
rabbits died during the 5th week, and 1 out of 2 monkeys died after
6 weeks of exposure. At 220 mg/m3 for 4 h/day, 5 days a week, for
8 weeks, all rats, rabbits, and guinea-pigs survived for 8 weeks,
but 1 out of 4 cats died during the third week. After the first 4-
h exposure to 120 mg/m3, 1 out of 2 dogs died, but the other
survived 4 weeks' exposure. Four rhesus monkeys survived 4 weeks'
exposure to 120 mg/m3 for 4 h/day (Dudley et al., 1942).
CD-1 mice, Charles River rats, and beagle dogs were exposed to
acrylonitrile 57 times for 6 h a day, 5 days a week, over a 90-day
period. Some dogs were killed by exposure to 117 mg/m3 (54 ppm)
but not 58 mg/m3 (24 ppm). Mice and rats were unaffected by these
concentrations, but a concentration of 234 mg/m3 (108 ppm) was
lethal for half the rats and mice. As with acute exposures, dogs
were more sensitive than rats and mice, but mice did not appear to
be more sensitive than rats. Atmospheric concentrations of
acrylonitrile of 58, 117, and 117 mg/m3 did not induce any lethal
effects in dogs, rats, and mice, respectively (Brewer, 1976).
7.2.2. Oral administration
Over a period of 7 weeks, 6 rats were administered orally 15
doses of acrylonitrile at 30 mg/kg body weight, then 7 doses at 50
mg/kg, followed by 13 doses at 75 mg/kg, without lethal effects
being induced (Barnes, 1970). No deaths occurred when Sprague-
Dawley rats were offered 85 mg or less of acrylonitrile per litre
of drinking-water for 90 days (Humiston & Frauson, 1975). Given
the slow absorption of acrylonitrile from the gastrointestinal
tract, blood levels could have been low, and it can be calculated
that the daily dose could have been about 8.5 mg/kg body weight.
The studies are compatible with the view that acrylonitrile is
unlikely to have a significant cumulative effect.
7.2.3. Subcutaneous administration and intraperitoneal
administration
Daily sc doses of 40 mg/kg body weight over 4 weeks, or daily
ip injections of 20 mg acrylonitrile/kg body weight over 6 weeks,
were not fatal for rats (Krysiak & Knobloch, 1971).
7.2.4. Clinical observations in animal studies
Rats exposed to acrylonitrile concentrations of 220 mg/m3 for 4
h daily, 5 days a week (Dudley et al., 1942) over a period of 8
weeks, showed slight lethargy but gained weight, as did guinea-
pigs. Rabbits failed to gain weight and were listless, while cats
became listless, vomited, and lost weight. One cat developed a
transitory weakness of the hind legs after the third exposure and
died after the eleventh exposure; the 3 remaining cats survived 8
weeks with few untoward effects. Exposure to an atmospheric
concentration of 330 mg/m3 resulted in weight loss in rats, their
coats became rough, and their general physical condition poor
(Dudley et al., 1942). Young rats and guinea-pigs showed impaired
growth and marked irritation of the eyes and nose during the first
week of exposure. Marked eye and nose irritation was also seen in
rabbits and cats and the latter developed transitory weakness of
the hind legs. Monkeys appeared sleepy and weak and frequently
salivated and vomited. Thus, the 220 mg/m3 exposure level markedly
affected cats, while rabbits and guinea-pigs were little affected;
a concentration of 330 mg/m3 induced various effects including
death. Brewer (1976) exposed CD-1 mice, Charles River rats, and
beagle dogs to acrylonitrile concentrations of 0, 58, 117, 234
mg/m3 (0, 24, 54, or 108 ppm) for 6 h daily, 5 days a week, for 13
weeks. Signs observed included ataxia, ptosis, emaciation,
rhinitis, and diuresis. As is common with acrylonitrile over-
exposure, convulsions usually preceded death (e.g., Benesh &
Cherna, 1959; Paulet et al., 1966).
7.2.4.1. Body weight, food and water consumption
Loss of body weight or failure to gain body weight was seen in
rats exposed to acrylonitrile at 330 mg/m3 and in cats and rabbits
exposed to 220 mg/m3 for 4 h a day, 5 days a week, for 13 weeks.
Loss of appetite was seen in rhesus monkeys exposed to 330 mg/m3,
while 120 mg/m3 did not elicit any toxic effects (Dudley et al.,
1942). No adverse effects were seen in 6 rats administered
successively 15 doses of 30 mg/kg body weight, 7 doses of 50 mg/kg,
and 13 doses of 75 mg/kg over a period of 7 weeks (Barnes, 1970).
Adult Sprague-Dawley rats received between 0 and 42 mg
acrylonitrile/kg body weight in drinking-water for 90 days. The
body weight was depressed in males receiving 42 mg/kg, while
females were affected by 22 mg/kg, but only after the 57th day.
The mean weekly food consumption of males was lower for 7 weeks on
38 mg/kg and for 2 weeks on l7 mg/kg. Food consumption decreased
in females receiving 42 or 22 mg/kg for 6 weeks and 1 week,
respectively (Humiston & Frauson, 1975).
7.2.4.2. Organ weights and pathology
The weights of the liver, kidney, spleen, pituitary gland,
lungs, gonads, thyroid gland, adrenals, heart, and brain of rats,
mice, and dogs exposed to an acrylonitrile concentration of 234
mg/m3 for 6 h a day, 5 days a week, for 13 weeks, were within
normal limits (Brewer, 1976). In rats receiving acrylonitrile in
drinking-water for 90 days, no changes in absolute or relative
organ weights were seen in males receiving 4 mg/kg body weight or
females receiving 5 mg/kg, daily (Humiston & Frauson, 1975). The
relative liver weight was significantly increased in males and
females receiving 17 mg/kg (males) or 22 mg/kg (females) or more.
The weights of the heart and liver were increased significantly in
adult Wistar rats administered 50 mg acrylonitrile/kg body weight,
intraperitoneally, daily, for 3 weeks (Knobloch et al., 1971);
their weight loss caused an increase in the relative weights not
only of the heart and liver, but also of the kidney and spleen.
Dudley et al. (1942) examined the livers of rats, guinea-pigs,
rabbits, cats, dogs, and rhesus monkeys exposed to acrylonitrile at
220 or 230 mg/m3, and observed histological changes only in cats.
Liver parenchymal degeneration was reported in adult Wistar rats
after daily ip administration of 50 mg/kg body weight for 3 weeks
(Knobloch et al., 1971). In the above-mentioned study, Dudley et
al. (1942) also reported signs of renal damage, such as hyaline
casts in the straight collecting tubules of all species, and
limited subacute interstitial nephritis; this was especially seen
in guinea-pigs and rabbits. Parenchymal degeneration of the
kidneys was reported by Knobloch et al. (1971). In the study by
Dudley et al. (1942), lungs were affected by subacute
bronchopneumonia, congestion, and oedema of the alveolar walls,
extravasation of erythrocytes and serum into the alveoli, focal
collection of lymphyocytes and polymorphonuclear leukocytes, in
most guinea-pigs, rabbits, the monkey, and 1 out of 3 of the rats.
The authors also reported slight haemosiderosis in the spleen of
rats, but negligible siderosis in cats, guinea-pigs, and rabbits.
Exposure of rats to acrylonitrile (22 mg/m3, 10 ppm) for 7 weeks
and (100 mg/m3, 50 ppm) for another 6 weeks caused enlargement of
the liver, kidney, heart, and spleen, but co-administration of
vitamins B1, B2, and cystine had a protective effect against
enlargement of the heart. Alcohol dehydrogenase activity in the
liver decreased after exposure, but the above-mentioned drugs
alleviated this decrease, to some extent (Takagi et al., 1968).
7.2.4.3. Blood
A normal haematological picture was reported in rats and dogs
repeatedly exposed to acrylonitrile vapours at up to 240 mg/m3
(Brewer, 1976), and in rats and rabbits (except for a raised
eosinophil count) repeatedly exposed to up to 330 mg/m3 (Dudley et
al., 1942).
Minami et al. (1973) exposed male rabbits to 54 mg/m3 for 1 day
per week (8 h) for 8 weeks; haematocrit and haemoglobin were
unaffected, but pO2 and pH were raised and pCO2 lowered by the
treatment.
In rats receiving acrylonitrile in the drinking-water for 90
days, the only significant haematological change was a decrease in
the red-cell count, on the 83rd day, in females receiving 42 mg/kg
body weight per day (Humiston & Frauson, 1975). The blood urea and
alkaline phosphatase (EC 3.1.3.1) levels in the males receiving 38
mg/kg body weight per day were raised, but SGPT activity was
normal. While rats administered ip 50 mg acrylonitrile/kg body
weight, daily, for 3 weeks (Knobloch et al., 1971) developed
leukocytosis and increased serum asparagine-oxo-aminotransferase
(EC 2.6.1.14) activity, mice exposed for 70 days to 225 mg/m3 (100
ppm) or 340 mg/m3 (150 ppm) 6 h daily, did not develop any
haematological abnormalities (Hashimoto, 1962). Rats exposed to
9.7 mg/m3, 4 h daily, 5 days a week for 2 months, did not show any
effects on the erythrocyte count or on the haemoglobin
concentration (Vissarionova et al., 1979).
As a whole, the studies failed to demonstrate any consistent
effect of acrylonitrile on red or white blood cell production or
viability. Leukocytosis following repeated intraperitoneal
administration of an irritant material is to be expected.
7.2.4.4. Immune system
Wistar rats were exposed to an acrylonitrile concentration of
10 mg/m3 for 6 h daily, 5 days a week, for 16 weeks. Acrylonitrile
depressed both T helper and T suppressor functions, and a
diminished degree of B lymphocyte transformation was observed.
Alpha tocopherol (im 0.21 mmol/kg body weight on alternate days for
16 weeks) protected against this effect (Krivova et al., 1982).
7.2.4.5. Nervous system
Some findings in the experimental animal studies on
acrylonitrile were indicative of an effect on the nervous system.
Rats, mice, and dogs exposed to up to 240 mg/m3, for 6 h daily, 5
days per week, for 13 weeks, showed ataxia and convulsions, prior
to death (Brewer, 1976). Transitory hind-leg weakness was seen in
cats exposed for 8 weeks (4 h per day, 5 days per week) to 330
mg/m3 (Dudley et al., 1942). Krysiak & Knobloch (1971) found that
rats receiving acrylonitrile intraperitoneally at 20 mg/kg body
weight daily for 6 weeks, or sc at 40 mg/kg daily for 4 weeks,
showed a significant lengthening of the time to perform correctly
in a conditioned food reflex test, and a significant decrease in
the number of correct reactions, compared with pre-treatment
observations or controls. Performance improved when the treatment
was discontinued. Daily ip administration to rats of acrylonitrile
at 50 mg/kg body weight for 3 weeks caused a vacuolization of
neuronal cells of the cortex and brain stem (Knobloch et al.,
1971).
7.2.4.6. Urine
No significant changes in urine composition were observed in
the experimental studies of Humiston & Frauson (1975) (sections
7.2.4.1 - 7.2.4.3).
7.2.4.7. Adrenals
The adrenals of rats exposed for 21-60 days to acrylonitrile
in drinking-water (0.05% and 0.2%) showed an atrophic zona
fasciculata and an enlarged zona glomerulosa. The animals on the
higher dose had a reduced plasma corticosteroid level and an
increased plasma Na+Concentration. The K+ level was unchanged
(Szabo et al., 1976). Serum corticosterone in rats was decreased
by 3 ip doses of acrylonitrile (33 mg/kg body weight) on successive
days (Nilsen et al., 1980).
7.2.4.8. Metabolism
After repeated exposure of rabbits to acrylonitrile, the in
vitro metabolism in the liver of acrylonitrile into cyanide and
thiocyanate decreased with time, while the excretion of unchanged
acrylonitrile in the urine increased (Sato, 1978).
7.3. Chronic Toxicity
Observations have been made on animals administered
acrylonitrile in drinking-water, food, through inhalation, and by
dermal application.
Tuller (1947) administered acrylonitrile at 500 mg/litre in the
drinking-water or acrylonitrile-fumigated food (dose not precisely
specified) to rats. After 2 years, the mortality was higher in
rats drinking acrylonitrile solution (50% deaths) than in paired
controls (25%), another control group (15%), and in rats on
acrylonitrile-fumigated food (5%). However, when acrylonitrile was
administered at 0.5, 5, and 90 mg/litre in the drinking-water to
male and female CFW rats for 2 years, the mortality rate was
unaffected (Svirbely & Floyd, 1961). Groups of 4 male and 4 female
beagle dogs were administered acrylonitrile at concentrations of
100, 200, or 300 mg/litre in the drinking-water, for 6 months.
Average intakes of acrylonitrile were the following for males
(females): 10(8) mg/kg body weight at 100 mg/litre; 16(17) mg/kg at
200 mg/litre; and 17(18) mg/kg at 300 mg/litre. Five dogs died, or
were killed because debilitated, in each of the 2 higher dosage
groups (Quast et al., 1975).
In the dogs receiving acrylonitrile at 100-300 mg/litre in the
drinking-water, early signs of toxicity included roughening of the
coat and, later, retching and vomiting. Terminal signs of lethargy,
weakness, emaciation, and respiratory distress were noted (Quast et
al., 1975).
7.3.1. Body weight, food and water intake
Body-weight gain was reduced during 4 of the 11 weeks at the
higher dose level (240 mg/m3) in a test in which male and female
Wistar rats and albino rabbits were exposed to acrylonitrile vapour
at concentrations of 0, 50, and 240 mg/m3, for 3 h/day, 6 days a
week, for 6 months (Knobloch et al., 1972).
Growth retardation was observed in male rats drinking 500 mg
acrylonitrile per litre water, for 2 years (Tuller, 1947). Svirbely
& Floyd (1961) administered acrylonitrile at 0.5, 5, or 50 mg/litre
in the drinking-water of rats and found a slight decrease in water
consumption at the highest concentration in both sexes.
Statistically significant reductions in the body weight of rats
receiving drinking-water containing acrylonitrile concentrations of
35, 100, or 300 mg/litre were associated with decreased water
consumption and decreased food consumption at 300 mg/litre in males
and 100 mg/litre in females (Ouast et al., 1977). Decreased food
and water consumption and body weight was reported with beagle dogs
drinking acrylonitrile at 200 or 300 mg/litre water for 6 months
(Quast et al., 1975). Marked weight decreases were seen in dogs
that eventually died or had to be killed.
7.3.2. Organ weights
In rabbits exposed to atmospheric acrylonitrile concentrations
of 250 mg/m3, for 3 h/day, 6 days a week for 6 months, a
significant increase in the heart weight was noted, and some
fluctuations in blood pressure were described (Knobloch et al.,
1972).
Ferin et al. (1961) exposed rats to drinking-water containing
an acrylonitrile concentration of 20 or 1000 mg/litre for 6 months.
At the higher dose, increased relative weights of the liver,
spleen, and kidneys were noted. The relative weights of the heart,
liver, and brain in males, and of the liver and kidneys in females,
were increased in rats receiving 300 mg/litre in drinking-water.
The males drinking 100 mg/litre showed a significantly increased
brain weight, and the females receiving 100 mg/litre had
significantly lower relative heart weights (Quast et al., 1977).
The weights of brain, heart, liver, and kidneys of beagle dogs
drinking an acrylonitrile concentration of 100 mg/litre in
drinking-water (Quast et al., 1977) were normal but, at 200
mg/litre, the 2 remaining males had a lower absolute brain weight
and higher relative kidney weight than controls. The 2 remaining
females receiving 300 mg/litre also had significantly lower relative
brain weights compared with controls.
7.3.3. Pathology and histology
Inflammation of the pulmonary system accompanied by an
inflammatory exudate into the bronchial lumen occurred in rats
exposed to acrylonitrile at 240 mg/m3 for 3 h a day, 6 days a
week, for 6 months (Knobloch et al., 1972). Various pathological
changes occurred in male and female rats maintained on water
containing acrylonitrile at 35, 100, or 300 mg/litre for 12 months
(Quast et al., 1977). Males and females on the 2 higher doses
developed paleness and thickening of the mucosa, erosions, ulcers,
and sometimes papilloma formations in the non-glandular portion of
the stomach. Three females receiving 300 and 100 mg/litre and one
male at 300 mg/litre had ear canal tumours. Microscopic findings
of tissue with tumorous growth revealed an increased frequency of
gastric cell papillomas, Zymbal (sebaceous) gland tumours of the
ear canal, and microtumours of the nervous system, in rats
receiving a concentration of acrylonitrile of 100 or 300 mg/litre.
These tumours do not occur spontaneously at such a high frequency
in the strain of rat used. The nervous system lesions were
consistent with the diagnosis of astrocytoma.
Minimal lesions were seen in the liver of rats drinking
acrylonitrile at 100 or 300 mg/litre and chronic renal disease
occurred in females drinking 300 mg/litre. The squamous epithelium
of the stomach was hyperplastic in rats drinking 100 or 300
mg/litre.
Histopathological changes in dogs receiving acrylonitrile
concentrations of 200 and 300 mg/litre in water (Quast et al.,
1975) were similar to those in untreated controls. The pneumonia
present may have resulted from irritation of the mucosa of the
tongue and oesophagus, which produced abnormal swallowing,
resulting in aspiration of some food.
7.3.4. Haematology and clinical chemistry
In rats exposed for 3 h/day, 6 days a week, for 6 months to
acrylonitrile in air concentrations of either 50 or 240 mg/m3, the
eosinophil count had significantly increased after 4 months. Total
serum protein was unchanged, while albumin and alpha-globulin had
increased and gamma-globulin decreased in both test groups
(Knobloch et al., 1971). Leukocytosis was observed in rats
drinking acrylonitrile in water at 1000 mg/litre (Ferin et al.,
1961). Periodic examinations of rats drinking 0.5, 5, or 50
mg/litre in water (Svirbely & Floyd, 1961), or 0 - 300 mg/litre in
water (Quast et al., 1977), showed normal haematological findings.
A significant elevation in alkaline phosphatase activity was found
in female rats exposed to 300 mg/litre. Half-way through the
study, beagle dogs drinking acrylonitrile concentrations of 0, 100,
200, or 300 mg/litre in water showed a significant decrease in
haematocrit, erythrocyte count, and haemoglobin concentration at
300 mg/litre and decreased erythrocyte count at 200 mg/litre, in
males. Females on 300 mg/litre also showed a significant decrease
in the erythrocyte count. However, all haematological findings
were within normal ranges towards the end of the study, except in
males at 300 mg/litre, which had a lower erythrocyte count. Blood
urea nitrogen, serum alkaline phosphatase activity, SGPT, and SGOT
were measured periodically; the findings in males were always
within normal limits, but females receiving 300 and 200 mg/litre
showed some increase in SGOT and SPGT activity. Total and
individual serum proteins were unaffected at the end of the study.
Zotova (1976) exposed rats by applying acrylonitrile solution to
the skin of the tail at doses of 2.82, 0.56, or 0.11 mg/kg body
weight per day and observed a decreased haemoglobin concentration
at the highest dosage level after 2 months. Blood catalase (EC
1.11.1.6) activity increased, and blood peroxidase (EC 1.11.1.7)
activity decreased, initially, but later became normal; there was
no change in sulfhydryl levels.
A decrease in blood -SH groups was reported in rats exposed to
acrylonitrile at 10 mg/m3 for 5 days/week, 4 h a day, for 4 months
(Efremov, 1976d).
7.3.5. Nervous system
Changes in central nervous function, as elicited by a
conditioned avoidance test, were found in rats drinking water
containing acrylonitrile at 20 mg/litre, for 6 months (Ferin et
al., 1961). Rats exposed through inhalation to acrylonitrile
concentrations of 50 and 240 mg/m3 for 3 h a day, 6 days a week,
for 6 months, showed significant defects in performance in a "Y"
maze (Krysiak, 1971). Rats exposed to 10 mg/m3, 5 days/week, 4 h a
day, for 4 months, showed a 59% decrease in the activity of brain
catalase, a 59% decrease in brain peroxidase, and a 37% decrease in
-SH groups (Efremov, 1976d). Histopathological changes in the
nervous system, consistent with a diagnosis of astrocytoma, were
observed in rats exposed to acrylonitrile at 35, 100, or 300
mg/litre in drinking-water (Quast et al., 1977).
7.3.6. Kidney function
Knobloch et al. (1972) exposed rats to 50 or 240 mg/m3, for
3 h per day, 6 days per week, for 6 months; kidney dysfunction was
indicated by increased urinary output at both concentrations and an
increase in urinary protein and areas of degenerated proximal
convoluted tubules at the higher concentration.
There were no abnormalities in the urine chemistry of rats
drinking water containing acrylonitrile at 0, 35, 100, or 300
mg/litre (Quast et al., 1977), or of dogs (Quast et al., 1975),
drinking water containing 100, 200, or 300 mg/litre. When
acrylonitrile was applied to the skin of the tail of rats, daily,
at doses of 2.82, 0.56, or 0.11 mg/kg body weight for 4.5 months,
the excretion of urinary chlorides increased on day 10 of exposure
at the 2 higher doses and decreased at the lowest dose (Zotova,
1976).
7.4. Teratogenicity and Embryotoxicity
The teratogenic potential of ingested or inhaled
acrylonitrile was investigated by Murray et al. (1978).
Groups of pregnant SD rats were given acrylonitrile at 0, 10,
25, or 65 mg/kg body weight per day, by gavage, from day 6 to
day 15 of gestation. Groups of 30 pregnant SD rats were
exposed 6 h per day to 0, 87, or 174 mg/m3 (0, 40, or 80 ppm)
acrylonitrile by inhalation, during the same period of
pregnancy. A dose of 65 mg/kg body weight per day caused
marked maternal toxicity, significant embryotoxicity, and an
increased incidence of fetal malformations. Findings of the
two studies suggesting a teratogenic effect were noted at 25
mg/kg per day and at 174 mg/m3 (80 ppm). At 10 mg/kg body
weight per day and 87 mg/m3 (40 ppm), no embryotoxicity or
teratogenicity was found. There was no apparent correlation
between the degree of toxicity seen in the individual dams and
the occurrance of malformations in their offspring.
Embryotoxic effects in pregnant mice of 3 strains were
described after intraperitoneal administration of unspecified doses
of acrylonitrile (Scheufler, 1976). A single ip injection of
acrylonitrile of 32 mg/kg body weight, given on the 5th or 7th day
of pregnancy, induced an embryotoxic effect in mice from an inbred
strain of AB Jena-Hall, but not in DBA and C57 C1 mice (Scheufler,
1980).
Kankaanpää et al. (1979) studied the embryotoxic effects of
acrylonitrile using chick eggs, but did not find any clear evidence
of its teratogenicity.
The exposure of Sprague-Dawley rats to an acrylonitrile
concentration in drinking-water of 500 mg/litre led to decreased
fertility and decreased viability of the young, and the females
developed a progressive muscular weakness in the hind legs about
16-19 weeks after the weaning of the second litter (Svirbely &
Floyd, 1961).
Willhite (1981a,b) observed skeletal malformations in hamster
fetuses after the administration of acrylonitrile at 80 mg/kg body
weight to pregnant hamsters. The histological study of both early
embryos and term fetuses revealed mesodermal changes, including a
reduction in the number of cells, shrinkage of the cell cytoplasm,
and enlarged extracellular spaces. In addition, a reduction in
mitotic figures and focal necrosis were noted. The affected
embryos were smaller and their development was delayed compared
with untreated controls. Teratogenic effects were only observed
when there was simultaneous maternal toxicity.
7.5. Mutagenicity
7.5.1. Bacterial systems
(a) Ames test with Salmonella typhimurium strains
Tests have been carried out on several strains, with and
without metabolic activation, using several methods of treatment
with acrylonitrile. Negative results were obtained, with and
without activation, in 5 tested strains in 2 studies (Litton
Bionetics, 1975; Stanford Research Institute, 1976). A weak, but
reproducible, positive effect was observed with metabolic
activation using between 0.5 and 1.5 mg acrylonitrile per plate in
strain TA 1535 (Haskell Laboratory, 1975; De Meester et al., 1978,
1979). Three methods of exposure were examined by Milwy & Wolff
(1977) in 3 strains of S. typhimurium. A low level of mutagenic
activity was noted in strain TA 1535, when plates were sprayed with
acrylonitrile or when it was mixed with the medium, with
activation.
Exposure to acrylonitrile vapour of the strains TA 1535 and TA
100 also demonstrated acrylonitrile mutagenicity (Duverger-Van
Bogaert et al., 1981; Ivanov, 1981). Zhurkov et al. (1983) tested
the mutagenicity of acrylonitrile in strains TA 1535 and TA 1538,
with and without microsomal activation, and found a dose-dependent
effect in strain TA 1535.
Urine collected from rats and mice treated with acrylonitrile
was mutagenic in S. typhimurium strain TA 1530, in the absence of
metabolic activation. Pre-treatment of the animals with
phenobarbital abolished the direct mutagenicity of urine from rats
and reduced that from mice. The addition of beta-glucuronidase (EC
3.2.1.31) to the incubation mixtures enhanced the mutagenic
activity of urine from acrylonitrile-treated animals (Lambotte-
Vandepaer et al., 1980, 1981a). Duverger-van Bogaert et al. (1982b)
suggested that glutathione might play a role in the formation of a
mutagenic metabolite of acrylonitrile. The mutagenic activity of
acrylonitrile vapours towards S. typhimurium strains was strictly
dependent on the presence of an activation system, confirming the
report of Milwy & Wolff (1977). Lambotte-Vandepaer et al. (1980)
indicated that animal urine might retain its mutagenic activity for
as long as a week after collection. The acrylonitrile-derived
epoxide, glycidonitrile, synthesized by Kopecky & Smejkal
(unpublished data, 1979), was shown to be the principal substance
that exerted mutagenic activity in the absence of metabolic
activation, whereas acrylonitrile itself required metabolic
activation in the S. typhimurium Ames test (Cherna et al., 1981).
(b) Mutagenicity in Escherichia coli
One of 3 strains of E. coli (WP2) revealed mutagenic activity
of acrylonitrile; activation did not have any effect (Venitt et
al., 1977). The mutagenic activity of acrylonitrile was confirmed
in other experiments using the simplified fluctuation test of Green
et al. (1976). The results suggested that acrylonitrile caused
non-excisable mis-repair of DNA associated with the generation of
DNA strand breaks (Venitt et al., 1977). The method of Slater et
al. (1971) did not reveal any effect with or without an activation
system at 10 µg acrylonitrile per plate (Litton Bionetics, 1976).
The variability of the results, even when the same kinds of
assays are used, could be because of differences in purity of the
acrylonitrile, in method, or in bacterial sensitivity. However, the
mutagenicity of acrylonitrile in bacterial systems seems to have
been established.
7.5.2. Yeast assays
Possible mutagenic activity of acrylonitrile was found with
Saccharomyces cerevisiae, but metabolic activation was without
effect (Litton Bionetics, 1975).
7.5.3. Drosophila melanogaster
A negative result was obtained when 0.1% acrylonitrile was
administered by intra-abdominal injection into D. melanogaster in
order to examine its ability to induce a recessive lethal effect in
the X chromosomes (Benesh & Shram, 1969).
7.5.4. Mammalian cell in vitro assays
The L5178Y kinase mouse lymphoma cell assay (Litton Bionetics,
1976) failed to show mutagenic activity of acrylonitrile using the
procedure of Clive & Spector (1975). Chinese hamster ovary cells
showed an increase in sister chromatid exchange (SCE) after
exposure to acrylonitrile, when co-cultured with rat hepatocytes
(Ved Brat & Williams, 1982). No effect was found without the
latter.
Acrylonitrile induced a slight increase in the SCE of cultured
human lymphocytes in the presence of S-9 mix and increased
unscheduled DNA synthesis with a very high concentration (0.5 M)
(Perocco et al., 1982). Application of acrylonitrile to primary
Syrian golden hamster embryo cells in culture produced foci of
morphologically-transformed cells. Pre-treatment with simian
adenovirus (SA7) caused an 8 to 9-fold increase in the frequency of
virus-transformed foci. When 3H-thymidine-labelled cells were
treated with acrylonitrile and their DNA subjected to alkaline
sucrose gradients, a shift in the sedimentation pattern occurred,
which was reminiscent of that observed with carcinogen treatment.
These observations added support to recent studies indicating that
acrylonitrile may be carcinogenic (Parent & Castro, 1979).
7.5.5. Mammalian in vivo assays
The inhalation exposure of 16 Sprague-Dawley male rats to
acrylonitrile levels up to 1085 mg/m3 (500 ppm) for 90 days did not
reveal chromatid or chromosomal aberrations or bone-marrow
abnormalities (Johnson et al., 1978). The results of Rabello-Gray
& Ahmed (1980) and the recent results of Leonard et al. (1981) also
showed that acrylonitrile fails to induce chromosomal aberrations
in somatic and germ cells.
Similar negative results were reported by Zhurkov et al. (1983)
following inhalation exposure of mice to acrylonitrile concentrations
of both 100 mg/m3 and 20 mg/m3 . They also reported negative
results in a dominant lethal assay in mice.
From preliminary results concerning DNA-alkylation by
acrylonitrile and vinyl chloride monomer (Peter et al., 1983), it
appears that DNA-alkylation occurs to a much lesser extent with
acrylonitrile than with vinyl chloride monomer. This is consistent
with the absence of mutagenic effects in vivo.
7.6. Carcinogenicity
Although full data were not available to the Group, there was
strong evidence from the data considered that acrylonitrile is a
carcinogen in rats.
Maltoni et al. (1977, 1982) investigated the carcinogenicity of
acrylonitrile administered to Sprague-Dawley rats by inhalation at
87, 44, 22, and 11 mg/m3 (40, 20, 10, and 5 ppm), 4 h daily, 5
times a week, or by stomach tube as a solution in olive oil, at a
dose of 5 mg/kg body weight, once a day, 3 times a week. In each
case, the rats were treated for 52 weeks and then kept without
further treatment until death. An increased incidence of some
tumours was noted in the acrylonitrile-treated animals, e.g.,
mammary tumours, forestomach papillomas and acanthomas, and
encephalic tumours (gliomas).
Two-year studies on Sprague-Dawley rats, following inhalation
exposure to acrylonitrile or ingestion in drinking-water, have been
performed at the Dow Chemical Company laboratories (Quast et al.,
1980a,b). In the inhalation studies, rats were exposed to 0, 44,
or 174 mg/m3 (0, 20, or 80 ppm) for 6 h/day, 5 days a week, for 24
months. Treatment-related tumours were found in the central nervous
system, Zymbal gland, tongue, stomach, small intestine, mammary
gland, and nasal turbinates. An apparent decrease in tumours of
the pituitary gland, the adrenals, the thyroid, the pancreas, and
testes was observed in the exposed rats.
In the ingestion study, rats were maintained on water
containing acrylonitrile levels of 0, 35, 100, and 300 mg/litre,
equivalent to mean dosage levels of 0, 4, 9, or 22 mg/kg body
weight per day. Evidence of oncogenicity was found in rats at all
dose levels of acrylonitrile. An increased tumour incidence was
observed in the treated rats affecting, particularly, the centra1
nervous system and also the Zymbal gland, tongue, stomach, small
intestine, and mammary gland. A decreased incidence of tumours was
observed at some sites: pituitary, thyroid, adrenals, pancreas, and
uterus.
In studies performed by Hogen & Rinehart (1980), acrylonitrile
was administered to Sprague-Dawley rats in the drinking-water at 1
or 100 mg/litre for 19-22 months, or by gavage at 0.1 or 10 mg/kg
body weight per day in water for about 20 months. A second group
of Fisher 344 rats received acrylonitrile in the drinking-water at
1, 3, 10, 30, or 100 mg/litre for 23-26 months. A statistically
significant increase in tumours was reported in the group receiving
acrylonitrile at 10 mg/kg body weight by gavage and in the groups
receiving 10, 30, or 100 mg acrylonitrile/litre drinking-water.
So far, no information is available on the carcinogenicity of
acrylonitrile for animal species other than rats.
After reviewing these data, IARC (IARC, 1982) and COC (UK
Ministry of Agriculture, Fisheries and Food, 1982) concluded that
acrylonitrile was a carcinogen in experimental animals.
8. EFFECTS ON MAN
Acrylonitrile has long been known to be a toxic substance that
induces systemic as well as local injury in both animals and man.
It has frequently been used in combination with other chemicals;
they may modify its toxicity, as was the case when it was used as a
fumigant.
8.1. Acrylonitrile
8.1.1. Acute Toxicity
8.1.1.1. Inhalation exposure
A 22-year-old chemist, who was exposed to acrylonitrile vapours
when a distillation apparatus leaked, developed headache, vertigo,
vomiting, tremors, uncoordinated movements, and convulsions
(Sartorelli, 1966). Vomiting and nausea persisted for 24 h. One
day after exposure, slight liver enlargement and congestion of the
oral pharynx, but no disorders of the CNS, were noted. After 4
days, no kidney, liver, cardiac, or respiratory abnormalities were
detected. Workers exposed to "mild" concentrations of acrylonitrile
in synthetic rubber manufacture developed nausea, vomiting,
weakness, nasal irritation, and an "oppressive feeling" in the
upper respiratory tract (Wilson, 1944). Headache, fatigue, and
diarrhoea were observed in some cases, and mild jaundice lasting
for several days and accompanied by liver tenderness and low-grade
anaemia in a few others. Jaundice lasted for 4 weeks in 1 case;
this individual complained of lassitude and fatigue after one year.
Zeller et al. (1969) observed that in 16 cases of acute inhalation
of acrylonitrile fumes by workers, nausea, vomiting, headache, and
vertigo appeared within 5-15 min; none of the workers needed
hospitalization. The authors described 50 cases of skin contact
with irritation, erythema, and blistering appearing within 5 min to
24 h, but with no systemic consequences. Workmen exposed to
concentrations varying from 35 to 220 mg/m3 (16-100 ppm) for 20-45
min during cleaning operations in polymerizers frequently
complained of a dull headache, fullness in the chest, irritation of
the eyes, nose, and throat, and feelings of apprehension and
nervous irritability. Some workmen had "intolerable itching" of
the skin, but no accompanying dermatitis.
8.1.1.2. Dermal exposure
A male laboratory worker who spilled "small quantities" of
liquid acrylonitrile on his hands, developed diffuse erythema on
both hands and wrists after 24 h, and blisters on the fingertips by
the third day. The hands were slightly swollen, erythematous,
itchy, and painful. The fingers remained dry and scaly on the 10th
day (Dudley & Neal, 1942). Wilson et al. (1948) observed that
direct skin contact led to irritation and erythema followed by scab
formation; healing was slow. Development of allergic dermatitis is
possible; a 27-year-old individual developed a rash on his finger
following the use for 6 weeks of a finger splint made from an
acrylonitrile/methyl methacrylate copolymer. Patch testing gave
positive reactions to the copolymer and 0.1% acrylonitrile (Balda,
1975). In another case report, skin lesions were first observed at
the site of contact with liquid acrylonitrile, which then spread
rapidly to other neighbouring regions. Several days after contact,
the lesions spread rapidly to other parts of the body that had not
been exposed, and these extensions were assumed to be an allergic
reaction (Hashimoto & Kobayashi, 1961).
In addition to local dermal toxicity, dermal absorption of
acrylonitrile may lead to systemic poisoning. Grunske (1949)
described a fatal case in which a 3-year-old girl had entered a
room that had recently been sprayed with an acrylonitrile-
containing insecticide (Ventox). Exposure was mainly through
inhalation, but skin exposure was possible, too. Another fatal
case was reported by Lorz (1950) in which a 10-year-old girl had
been treated on the scalp for lice with an insecticide that was
identified as containing acrylonitrile (Ventox). She had impetigo
and widespread scratches on the skin of the scalp. This could have
increased the absorption of acrylonitrile.
Two workers who spilled liquid acrylonitrile on their legs,
immediately washed their legs and dried their shoes, but put them
on again. Blisters developed at the sites of contact, 6-8 h after
the spill. Therapy lasted 21 and 38 days, respectively. The skin
of 2 workers who were cleaning apparatus (temperature 50 °C), came
into contact with 5% acrylonitrile solution; other possible
substances in the mixture were not specified. Serious skin burns
developed. Therapy lasted 35 and 72 days, respectively (Babanov,
1957). Zeller et al. (1969) reported 50 cases of skin damage
resulting from occupational contact with acrylonitrile. A burning
sensation developed within 5 min to 24 h followed by a reddening of
the area, which often blistered after 1 day.
8.1.2. Chronic toxicity - occupational exposure
Chronic effects can potentially occur after prolonged exposure
to acrylonitrile, both in the vapour and liquid forms.
8.1.2.1. Clinical observations
Complaints of poor health, headache, decreased work capacity,
poor sleep, irritability, chest pains, poor appetite, and skin
irritation (during the first months of employment only) came from
workers employed in the manufacture of acrylonitrile (Zotova,
1975a).
In a study by Sakurai & Kusumoto (1972), workers employed in
acrylonitrile manufacture also complained of headache, weakness,
fatigue, nausea, vomiting, nosebleeds, and insomnia; the symptoms
correlated well with the length, but not with the level of exposure
or with the age of the workers. A total of 4439 examinations were
made over about 10 years prior to 1970, in 576 workers who formed
2 cohorts, one exposed to concentrations of acrylonitrile below 11
mg/m3 (5 ppm), the other below 45 mg/m3 (20 ppm). However, the
authors later stated that these exposure levels were not reliably
reported (Sakurai et al., 1978).
Babanov et al. (1959) reported that workers exposed to
acrylonitrile concentrations at 0.6-6 mg/m3 for approximately 3
years suffered from headache, insomnia, pains in the heart region,
general weakness, decreased working capacity, and increased
irritability. The vocal cords were inflamed, and non-specific
changes in the vestibular apparatus and pale mucous membranes and
skin were seen. Blood pressure was said to be reduced.
Changes in the health status and laboratory tests were not
observed in a group of 23 men who had been working for 3-5 years in
an acrylonitrile plant, where, during the warm season, exposure
levels reached 4.2-7.2 mg/m3 (Gincheva et al., 1977). Stamova et
al. (1976) studied workers' health in the related polyacrylic fibre
plant in which acrylonitrile exposure levels ranged around 10
mg/m3, but could fluctuate upwards to 25 mg/m3 . Workers were also
exposed to other chemical substances. An increase was found in
both skin diseases and various "neurasthenic" complaints and
diseases. Dorodnova (1976) did not find any differences in the
gynaecological health status of 410 women working in a polyacrylic
fibre plant in Saratov compared with that of 436 unexposed women.
8.1.2.2. Haematology
Compared with the findings in blood donors, some male and
female employees exposed to acrylonitrile at 2.5-5 mg/m3 showed
a reduced haemoglobin level, erythrocyte count, leukocyte count,
and percentage of neutrophiles, with an increased percentage of
lymphocytes and plasma iron. Inhibition of maturation of
normoblasts in bone marrow was also reported (Shustov, 1968).
Similar results were reported by Zotova (1975b). Lower
erythrocyte, haemoglobin, and total white counts were found in
laboratory workers exposed to acrylonitrile, and in apparatus
operators and machinists. Higher than normal total glutathione
levels were found in male operators and maintenance men and reduced
glutathione levels in male apparatus operators. Oxidized
glutathione was elevated and total sulfhydryl groups decreased in
workers employed in all these occupations.
Lower erythrocyte counts and a relative lymphocytosis were also
observed by Babanov et al. (1959) in the study mentioned above.
8.1.2.3. Other organs
(a) Liver
Sakurai & Kusumoto (1972) (section 8.1.2.1) reported some
abnormal results in liver function tests; however, in a further
study of 102 workers from some of the factories, Sakurai et al.
(1978) did not find any significant liver function test
abnormalities related to acrylonitrile exposure, when exposure
levels had decreased from 11-44 mg/m3 (5-20 ppm) to 9 mg/m3 (4.2
ppm). Increased serum cholinesterase activity, hyperbilirubinaemia,
decreased coloidal stability, and hypergammaglobulinaemia were
described in workers exposed to acrylonitrile concentrations of up
to 5 mg/m3 and to acrylonitrile-polymer dust of up to 1.5 mg/m3
(Enikeeva et al., 1976). These effects have not been reported
elsewhere.
(b) Eye
Blepharoconjunctivitis was reported by Delivanova et al. (1978)
in all of 302 workers examined over a 2-year period; 42 had severe
alterations caused by conjunctivitis, and all disorders were
connected with exposure to acrylonitrile.
(c) Gastro-intestinal effects
Symptoms of gastritis and colitis were observed in workers
exposed to acrylonitrile concentrations of up to 5 mg/m3 (Enikeeva
et al., 1976).
(d) Immune system
Acrylonitrile has been found to have an immunodepressive
effect. The functional activity of T-lymphocytes was found to have
decreased in workers exposed to acrylonitrile (Ivanov, private
communication, 1983).
8.1.2.4. Nervous system
Nausea, vomiting, headache, and vertigo (Wilson, 1944; Wilson
et al., 1948; Zeller et al., 1969; Sakurai & Kusumoto, 1972;
Zotova, 1975) indicate a possible effect of acrylonitrile on the
nervous system. Ageeva (1970) reported a significant decrease in
an "epinephrine-like substance", and an increase in acetylcholine.
Depression, lability of autonomic functions (lowered arterial
pressure, labile pulse, diffuse dermographia, increased sweating,
change in orthostatic reflex) were also observed in workers
involved in acrylonitrile production.
8.1.2.5. Dermal effects
Spassovski (1976) reported irritant and allergic dermatitis in
acrylonitrile workers; dermatitis was also observed by Antonev &
Rogailin (1970) and Stamova et al. (1976).
8.2. Mutagenicity
Thiess & Fleig (1978) examined workers who had been exposed to
acrylonitrile for 15.3 years and workers who had not been exposed.
No differences were found in the incidence of chromosomal
aberrations, including or excluding gaps, in the 100 metaphases
examined for each person.
8.3. Carcinogenicity
In a retrospective cohort epidemiological study of 1345 male
workers with potential exposure to acrylonitrile from 1950-66,
followed until 31 December, 1976, 25 cases of cancer were found
with 20.5 expected, based on company rates (O'Berg, 1980). Of
these, 8 were respiratory cancer cases, with 4.4 expected. Twenty-
three cases occurred among workers first exposed during the start-
up period (1950-52) when exposures were higher; only 12.9 were
expected (P = 0.01).
The standardized incidence ratio (SIR) was 179 for cancer among
the operators and mechanics who had at least 6 months' exposure and
began their assignments during start-up. A "dose response" was
shown with those with longer duration of employment, workers with
estimated higher exposures having higher risk. Latency was also
demonstrated, with 17 of the 24 cases occurring 20 years after the
onset of exposure among those with at least 6 months' employment,
including 6 of the 8 lung cancer cases. It should be pointed out
that, using the National Cancer Institute's expected incidence
rates for 1969-71, the expected rate would be 25.5 rather than 20.5
from the company rates. In a concomitant cancer mortality study,
20 cancer deaths were found with 17.4 expected, using company rates
(not significant); the expected rates exceeded the company rates
using national, state, or regional cancer rates. The author felt
that it might be premature to evaluate mortality statistics,
because of insufficient latency (many cancer cases had been
recently diagnosed and were still living). Smoking habits were not
considered, though the author stated that 7 out of the 8 lung
cancer cases were known to have smoked.
A follow-up study on the mortality rate among 327 employees of
a chemical rubber plant in the USA revealed that the number of
deaths from lung cancer was significantly higher than expected (9
versus 5.9 for US white males and 4.7 for other rubber workers from
the same city). The greatest excess was seen among men who had
worked for 5-14 years and who had started working there at least 15
years before death (Delzell & Monson, 1982). This study was
confounded by the multiple exposure of workers in the nitrile
rubber manufacturing plant.
Kiesselbach et al. (1979) examined the mortality rate, the
cancer rate, and the type of cancer against the period of exposure
to acrylonitrile in 884 workers. The results revealed that the
general mortality of the exposed group was markedly lower than that
of the normal population (58 versus an expected 104), possibly
because of the "healthy worker" effect. The mortality rate for
malignant tumours, cardiovascular, brain, respiratory, and gastro-
intestinal diseases, suicide, and other causes was the same as in
the normal population. No relationship was found between length of
exposure and mortality from tumours.
An excess of deaths from lung cancer was reported in
acrylonitrile workers by Thiess et al. (1980). In addition, 2
cases of Hodgkin's disease contributed to a slight excess of cancer
of the lymphatic tissue. However, exposure to other substances,
some of them known carcinogens, made interpretation of the results
difficult.
A cohort study on men potentially exposed to acrylonitrile
during the start-up of a plant indicated that there was no excess
mortality from lung cancer. There were no deaths from lung cancer
in maintenance workers, who possibly had the highest exposures.
There was an excess of kidney cancers (based on 2 cases only) and
of circulatory disease other than rheumatic and atherosclerotic
(based on 5 cases), accompanied by a deficit of atherosclerotic
heart disease.
Because the cohort was small with only 4 cancer deaths
observed, it could not give an indication of excess cancer risk or
association with duration of exposure to acrylonitrile. An
additional retrospective cohort mortality study, in two
acrylonitrile plants in the USA, on 352 males exposed for 6 months
or more prior to 1968 and followed up until December 1977, did not
show any excess mortality including cancer mortality. There were
15 deaths from all causes, 18.11 being expected, and 3 deaths from
cancer (2.8 expected) (Zack, unpublished data, 1980).a
In a study by Nakamura (1981), 9525 workers employed in the
production of acrylonitrile, acrylonitrile rubbers, and ABS were
studied. Deaths due to cancers in general and to lung and colon
cancers in particular, were not increased, while 7 deaths due to
liver, gall bladder, or cystic duct cancer were found against the 5
that might have been expected.
The mortality of 1111 men who worked on the polymerization of
acrylonitrile and the spinning of acrylic fibre in the United
Kingdom from 1950 to 1968 was surveyed up to the end of 1978.
Seventy-nine deaths were identified in 6 factories. The total
number of deaths among men exposed to acrylonitrile for at least
one year was slightly lower than expected (68 versus 72.4) and a
relative excess of deaths from all cancers was found, arising
mainly from cancers of the lung, stomach, colon and brain,
pancreas, testis, and bladder (21 versus 13 expected). The authors
considered particularly relevant, the excess of lung cancer in
those aged 15-44 years. Nevertheless, the authors considered that
their results were inconclusive and urged continued surveillance
and analysis of the exposed population in the United Kingdom
(Werner & Carter, 1981).
The epidemiological studies provide some indications that
acrylonitrile exposure is associated with cancer, particularly of
the lung. However, the studies reported, while neither conclusive
nor contradictory, are limited by insufficient latency. Other
difficulties, such as cohort identification and selection, and
combined exposures have made interpretation difficult. Further
epidemiological data are therefore of great importance, and
consideration of smoking and single exposures to acrylonitrile is
desirable.
8.4. Simultaneous Exposure to Acrylonitrile and
Other Chemicals
8.4.1. Acute toxicity
Numerous non-fatal and fatal cases of poisoning by
acrylonitrile-containing mixtures have been described (Davis et
al., 1973). In home fumigation, an acrylonitrile mixture with
carbon tetrachloride or methylene chloride is placed in shallow
open pans and the vapours dispersed by fans for 24-72 h, the
operator deciding when the house is safe for occupancy (Davis et
al., 1973). A man working in the polymerization of acrylonitrile,
polybutadiene, and styrene for 2 years complained of numbness of
the fingers and toes, severe fatigue in the lower extremities, and
----------------------------------------------------------------------
a Zack, J.A. (1980) The mortality experience of Monsanto
workers exposed to acrylonitrile (Monsanto Internal
Report).
general malaise. Decreased patellar and achilles tendon reflexes,
and hypoaesthesia in the peripheral parts of the fingers and toes
were observed. Free acrylonitrile was detected in the urine. The
exposure level of acrylonitrile was estimated to exceed 108.5 mg/m3
(50 ppm) (Seki, 1967).
Lachrymation, burning in the throat, coughing, sneezing,
nausea, vomiting, dizziness, visual disturbance, headache, coma,
seizures, and dermatitis have been described in non-fatal cases
(Davis et al., 1973). Fatalities have occurred following exposure
to vapours (Grunske, 1949; Davis et al., 1973) and liquid (Lorz,
1950). Cyanide was detected in the blood in some cases. Symptoms
and signs prior to death varied from case to case; sore throat,
weakness, dizziness, vomiting, eye irritation, respiratory
disorders, pallor, tachycardia, tremors, unconsciousness, and
epidermal necrolysis have been described. Other pathological
conditions occurred, but many could have been the result of pre-
existing disease or of exposure to the other component(s) of the
mixture. Findings in children suggest that they may be more
sensitive to acrylonitrile exposure than adults (Grunske, 1949).
8.4.2. Chronic toxicity
Abnormalities in subjects exposed simultaneously to
acrylonitrile and several other chemicals have been described in
several studies. As the concentrations of the other chemicals were
frequently higher than those of acrylonitrile, it is difficult to
decide whether the abnormalities were caused by acrylonitrile, the
other chemical(s), or a combination of the two.
An abnormally high proportion of workers exposed to
acrylonitrile levels of 3-20 mg/m3, 33 ppm NH3, up to 1 mg
H2SO4/m3, 0.41-0.67 mg NaOH/m3, and 2-10 mg acetic acid/m3 were
described as suffering from a variety of symptoms ascribed to
disorders of the autonomic nervous system ("neurasthenic syndrome")
(e.g., irritability, headache, poor appetite, fatigue). Intolerance
to alcohol has also been observed (Orusev & Popovski, 1973; Orusev
et al., 1973).
Apprentices exposed to acrylonitrile (0.8-1.8 mg/m3), methyl
methacrylate (16-17 mg/m3), and sodium thiosulfate were examined
before exposure, after 1-2 weeks, and after a further unspecified
time. "Neurasthenic" symptoms were rare before, but frequent after
exposure, and the incidence of immunological reactivity against the
chemicals increased, as did the concentration of ceruloplasmin
(Mavrina & Il'ina, 1974). Dermal tests for allergy were also made
by Hromov (1974) in workers who had been in contact with
acrylonitrile, methyl acrylate, and sodium thiocyanate. Intradermal
samples showed positive haemoagglutination reactions in 86.5% of
workers exposed to acrylonitrile, 76.1% exposed to methyl acrylate,
and 53.6% exposed to sodium thiocyanate. Dermatitis, eczema, and
urticaria occurred.
Mavrina & Hromov (1974) and Shustov & Mavrina (1975) reported
abnormalities of the liver, nervous, cardiovascular, and
gastrointestinal systems in workers occupationally exposed to
acrylonitrile, methyl acrylate, and sodium thiocyanate during fibre
production. In particular, symptoms associated with the activity
of the autonomic nervous system were noted among the 340 workers
examined. Dryness, desquamation, fissures, and diffuse erythema of
the skin were also apparent. Women exposed to acrylonitrile and
methyl acrylate (Chobot, 1979) were said to suffer from
disturbances in menstrual function twice as frequently as a control
group. A low incidence of irritant and allergic dermatitis and
vitiligo was noted in workers exposed to acrylonitrile, methyl
acrylate, and dimethylformamide in fibre production (Bainova,
1975). In 11 out of 28 workers, delayed skin sensitization and
allergic dermatitis were observed with dimethylformamide.
Ostrovskaja et al. (1976) observed workers exposed to
acrylonitrile, acetonitrile, and hydrocyanic acid during training
and after 1.5 years and 3 years. In 190 men and women, aged 20 -
30 years, many signs and symptoms were noted including modified
reflexes, changes in blood pressure, ECG, and EEG. However, it is
difficult to attribute the findings of these authors solely to
acrylonitrile exposure.
9. EVALUATION OF HEALTH RISKS TO MAN FROM EXPOSURE TO ACRYLONITRILE
9.1. Sources and Levels of Exposure
Acrylonitrile is a colourless, volatile, chemically reactive
liquid; it does not occur as a natural product. The monomer is
used world-wide, on a large scale, in the manufacture of polymers,
fibres, and rubbers and as a chemical intermediate. Acrylonitrile-
containing polymers have been used in the manufacture of products
that come into contact with food; the amounts of acrylonitrile that
migrate into foods can be reduced to negligible quantities by the
use of good manufacturing practices in the production of the
polymers.
The major sources of contamination of the general environment
are acrylonitrile-producing and -polymerizing plants. The
occurrence of acrylonitrile in air, water, and soil near industrial
plants has been described. There is evidence that acrylonitrile
has persisted in soil for long periods following accidental
spillage; subsequent contamination of ground water has been
demonstrated.
The highest exposures occur in the workplace. Experience shows
that containment of such exposures can more readily be achieved in
production plants than in those in which acrylonitrile is used to
make other products. In a number of countries, exposure limits or
recommended limit values for the workplace have been arrived at;
values recently set have tended to be lower than in the past (Table
12).
Accidental exposure to acrylonitrile liquid and vapour may
occur during the various stages of production, transport, and use.
9.2. Acrylonitrile Toxicity
Inhaled acrylonitrile vapour is readily absorbed. Acute
systemic effects following absorption of vapour have been
described. Symptoms were non-specific and referable to the
gastrointestinal and respiratory tracts, the liver, and the central
nervous system. No acute adverse effects have been reported
following daily exposure (8-h) to up to 45 mg/m3.a At higher
concentrations rising to 220 mg/m3, 20-40 min exposure resulted
in complaints of headache, irritation of the upper respiratory
tract and the eyes, nervous irritability, and itching of the skin.
Fatalities have been reported following the use of fumigant
mixtures containing acrylonitrile together with carbon
tetrachloride and methylene chloride. Exact exposure conditions
are not known, but animal data suggest that inhalation exposure to
acrylonitrile at 500-2000 mg/m3 for 1/2-3 h could be fatal.
Simultaneous exposure to some organic solvents may enhance the
toxicity of acrylonitrile.
---------------------------------------------------------------------------
a This value was for many years the occupational exposure limit
in many countries where acrylonitrile was manufactured.
Table 12. Occupational exposure limits for selected countries
---------------------------------------------------------------
Country TWA mg/m3 STEL mg/m3 Reference
---------------------------------------------------------------
France 9 34*** INRS (1983)
Germany, Federal -* (S) DFG (1982)
Republic of
Hungary 0.5 0.5 Hungary (1979)
Japan 45 - Japan Association
of Industrial
Health (1972)
Poland Poland (1982)
Sweden 4** (S) 13 Sweden (1981)
United Kingdom
USA 4.5** (S) ACGIH (1982)
USSR 0.5 (S) USSR (1982)
---------------------------------------------------------------
* Proved animal carcinogen, strongly suspected of also being
carcinogenic for human beings. No safe concentration can be
listed.
TWA = Time-weighted average.
STEL = Short-term exposure limit.
** Listed as Industrial Substance Suspect of Carcinogenic
Potential for Man.
*** Alarm level.
S = Skin uptake can contribute to overall exposure.
Liquid acrylonitrile is also absorbed through the skin,
reportedly giving rise to non-specific symptoms similar to those
that follow acrylonitrile vapour inhalation. Local injury can
occur a few hours after exposure to liquid acrylonitrile. One
fatality has been reported. It is also an irritant to the eye.
Skin absorption of vapour does not appear to contribute
significantly to overall acrylonitrile uptake in the workplace.
There are no indications that acrylonitrile accumulates in the
body following prolonged exposure to levels found in the workplace.
Skin sensitization has been reported in a few cases; however,
no evidence is available to suggest the occurrence of pulmonary
allergic reactions.
Acrylonitrile has been shown to induce embryotoxic and
teratogenic effects at high dosage levels in experimental animals.
Although acrylonitrile is metabolized partly to cyanide, it has
been demonstrated that the acute toxic actions of acrylonitrile are
not solely due to cyanide, as was once believed.
Acrylonitrile has been shown to be mutagenic in some in vitro
systems in the presence of metabolic activation systems. So far,
mutagenic activity has not been demonstrated in in vivo assay
systems.
Complaints of ill health in workers exposed for a number of
years to acrylonitrile concentrations of less than 45 mg/m3 have
been reported in several studies. The complaints were variable in
nature and no consistent correlation with the extent of exposure
appears to have been established. The studies do not provide
evidence of a specific disease arising from long-term, low-level
exposure.
Several long-term studies in which acrylonitrile was
administered to rats orally and by inhalation demonstrated the
induction of malignant tumours. Data available in summary form
suggest that the incidence was dose-related. Eight epidemiological
studies have been carried out on workers exposed to acrylonitrile.
These studies have not demonstrated conclusively that there is a
correlation between exposure to acrylonitrile and the incidence of
cancer in man. Nevertheless, the findings are not incompatible with
the supposition that acrylonitrile is a potential human carcinogen
and thus give no cause for disregarding the evidence that has been
provided by animal studies.
It is not possible to establish a level below which no adverse
effects occur on the basis of the experimental and epidemiological
data presented in this document. However, it is evident that
exposure to acrylonitrile should be kept as low as possible in both
the workplace and the general environment, and that skin contact
with the liquid should be avoided.
REFERENCES
AARATO, S.E. & BITTERA, E. (1972) [Determination of
acrylonitrile in the air in the presence of dimethylformamide
and ammonia.] Munkavedelem, 18 : 50-51 (in Hungarian, Chem.
Abstr. 79 : 57280b, 1973).
ABREU, M.E. & AHMED, A.E. (1980) Metabolism of acrylonitrile
to cyanide: in vitro studies. Drug Metab. Disposition, 8 (6):
376-379.
ACGIH (1982) Threshold limit values for chemical substances
and physical agents in the work environment with intended
changes for 1982. Cincinnati, USA, American Conference of
Governmental Industrial Hygienists.
AGEEVA, S.T. (1970) [The condition of the metabolism of
mediating substances in workers producing acrylonitrile.] Tr.
Sarat. Med. Inst., 78 : 10-13 (in Russian).
AHMED, A.E. & ABREU, M.E. (1982) Microsomal metabolism of
acrylonitrile in liver and brain. Adv. exp. Med. Biol., 136
(Part B): 1229-1238.
AHMED, A.E. & PATEL, K. (1979) Studies on the metabolism of
aliphatic nitriles. Toxicol. appl. Pharmacol., 48 : A 91
(abstract).
AHMED, A.E. & PATEL, K. (1981) Acrylonitrile - in vivo
metabolism in rats and mice. Drug. Metab. Disposition, 9 (3):
219-222.
AHMED, A.E., FAROOQUI, M.Y.H., UPRETI, R.K., & EL-SHABRAWY,
O. (1982) Distribution and covalent interactions of (1-14C)
acrylonitrile in the rat. Toxicology, 23 : 159-175.
ALDRIDGE, W.N. (1944) A new method for the estimation of
micro quantities of cyanide and thiocyanate. Analyst, 69 :
262-265.
AL'SHANSKY, A.M., IVANOV, V.V., & PROMYSLOV, M.SH. (1980)
[Effect of acrylonitrile on content of Q-aminobutyric acid
glutamate decarboxylase activity and on lipid peroxidation in
rat brain tissue.] Vop. Med. Chimii, 26 (4): 507-509 (in
Russian).
AMERICAN CYANAMID COMPANY (1951) The chemistry of
acrylonitrile. In: Nitrogen chemicals digest, New York,
American Cyanamid Company, Vol. 5.
AMERICAN CYANAMID COMPANY (1959) The chemistry of
acrylonitrile, 2nd ed., New York, American Cyanamid Company,
272 pp.
AMERICAN CYANAMID COMPANY (1974) Handling storage - analyses
of acrylonitrile. Wayne, New Jersey, American Cyanamid
Company, 42 pp.
AMERICAN CYANAMID COMPANY (1976) Material safety data sheet;
Acrylonitrile, Wayne, New Jersey, American Cyanamid Company.
AMERICAN INDUSTRIAL HYGIENE ASSOCIATION (1970)
Acrylonitrile. Am. Ind. Hyg. Assoc. J.,: 529-531.
ANDO, K. & HASHIMOTO, K. (1967) [Effects of acrylonitrile
and acrylamide on peripheral nerve.] Rep. Osaka Prefectural
Inst. pub. Health, 4 : 23-27 (in Japanese).
ANONYMOUS (1977) Acrylo growth won't be bottled up. Chem.
Week, 9 March : 30-31.
ANTON'EV, A.A. & ROGAILIN, V.I. (1970) [Professional
dermatoses caused by acrylonitrile and their prophylaxis.] Tr.
Perm. Gos. Med. Inst., 99 : 103-106 (in Russian).
APPEL, K.E., PETER, H., & BOLT, H.M. (1981a) Effect of
potential antidotes on the acute toxicity of acrylonitrile.
Int. Arch. occup. environ. Health, 49 : 157-163.
APPEL, K.E., PETER, H., BOLT, M., & BOLT, H.M. (1981b)
Interaction of acrylonitrile with hepatic microsomes of rats
and man. Toxicol. Lett., 7 (4-5): 335-340.
ASTM (1981) Annual book of ASTM standards. Method D3371-79:
Testing for nitriles in aqueous solution by gas-liquid
chromatography, Philadelphia, American Society for Testing and
Materials.
BABANOV, G.P. (1957) [Local manifestation of the action of
acrylonitrile on the skin and mucosa.] Vrac. Delo, 5 : 511-514
(in Russian).
BABANOV, G.P., KLJUCHIKOV, V.N., KARAJEVA, N.I., & LILEEVA,
Z.V. (1959) [Clinical symptoms of chronic poisoning by
acrylonitrile.] Vrach. Delo, 8 : 833-836 (in Russian).
BAINOVA, A. (1975) [Assessment of the skin lesions in the
production of Bulana polyacrylonitrile fibers.] Dermatol.
Venerol., 14 (2): 92-97 (in Bulgarian).
BAKER, R.A. (1963) Threshold odors of organic chemicals. J.
Am. Water Works Assoc., 55 : 913-916.
BALDA, B.R. (1975) [Acrylonitrile as a contact allergen.]
Hautarzt, 26 : 599-601 (in German).
BANDT, H.J. (1953) [Acrylonitrile as an effluent.] Z.
Fisch., 2 : 457-461 (in German).
BARNES, J.M. (1970) Effects on rats of compounds related to
acrylamide. Br. J. ind. Med., 27 (2): 147-149.
BARRETT, W.J. (1974) Sampling and analysis of four organic
compounds using solid sorbents, Washington, US Department of
Health, Education and Welfare (Southern Research Institute
Report SORI-EAS-74-301).
BAXTER, R.A. (1979) Evaluation and control of industrial
exposure to acrylonitrile. Ann. occup. Hyg., 22 : 429-435.
BELILES, R.P., PAULIN, H.J., MAKRIS, N.G., & WEIR, R.J.
(1980) Three-generation reproduction study of rats receiving
acrylonitrile in drinking water. Final report. Litton
Bionetics Inc.
BELLAR, T.A. & SIGSBY, J.E., Jr (1970) Direct gas
chromatographic analysis of low molecular weight substituted
organic compounds in emissions. Environ. Sci. Technol., 4 :
150-156.
BENCHEV, I., RIZOV, N., & KOLARSKA, A. (1982) [Acrylonitrile
determination in biological fluids by gas chromatography -
"head space" analysis.] Hyg. Zdrav., 1 : 87-89 (in Bulgarian).
BENESH, V. & CHERNA, V. (1959) [Acrylonitrile. Acute
toxicity and mechanism of action.] J. Hyg. Epidemiol.
Microbiol. Immunol., 3 (1): 106-116 (in German and in Russian).
BENESH, V. & SHRAM, R. (1969) Mutagenic activity of some
pesticides in Drosophila melanogaster. Ind. Med. Surg.,
38 (12): 442-444.
BENSON, G.B. & BOYCE, G.E. (1981) A thermally desorbable
passive dosimeter for personal monitoring for acrylonitrile.
Ann. occup. Hyg., 24 (1): 55-76.
BENSON, G.B., BOYCE, G.E., & HAILE, D.M. (1981) Industrial
hygiene measurements for organic pollutants (acrylonitrile)
using passive dosimeters and automated thermal desorption.
Ann. occup. Hyg., 24 (4): 367-373.
BERCK, B. (1960) Retention of acrylonitrile and carbon
tetrachloride by shelled walnuts fumigated with acrylon. J.
Agric. food Chem., 8 :128-131.
BERG, G.L., ed. (1977) 1977 Farm Chemicals Handbook, 63rd
issue, Willoughby, Ohio, Meister Publishing Co., pp. D5, D50.
BIRD, W.L. & HALE, C.H. (1952) Polarographic determination
of acrylonitrile. Anal. Chem., 24 : 586-587.
BOLLINI, M., SEVES, A., & FOCHER, B. (1974) [Determination
of free monomers in water emulsions of synthetic polymers or
copolymers.] Ind. Carta, 12 : 234-240 (in Italian, Chem.
Abstr., 81 : 121672b).
BOLT, H.M., FILSER, J.G., & HINDERER, R.K. (1978) Rat liver
microsomal uptake and irreversible protein binding of
1,2-14C-vinyl bromide. Toxicol. appl. Pharmacol., 44 : 481-489.
BOND, E.J. & BUCKLAND, C.T. (1976) Control of insects with
fumigants at low temperatures: toxicity of mixtures of methyl
bromide and acrylonitrile to three species of insects. J.
econ. Entomol., 69 : 725-727.
BONDAREV, G.I., STASENKOVA, K.P., & VISSARIONOVA, V.Y.
(1976) [Protective effect of sulfur-containing compounds in
poisoning with acrylic acid nitrile.] Vopr. Pitan., (4) : 55-58
(in Russian).
BORCHARDT, VON K., FRANSEN, E., & HARTMAN, B. (1970) [Acute
oral intoxication with acrylonitrile in animals.] Z. Ges.
Hyg., 16 : 316-319 (in German).
BORG WARNER CHEMICALS (1977) In response to SHA's request
for information on occupational exposure to acrylonitrile.
Fed. Reg., 42 : 33043 (Letter to OSHA Docket Officer, US
Department of Labor).
BOYLAND, E. & CHASSEAUD, L.F. (1967) Enzyme-catalysed
conjugations of glutathione with carbonyl compounds. Biochem.
J., 104 : 95-102.
BOYLAND, E. & CHASSEAUD, L.F. (1968) Enzymes catalysing
conjugations of glutathione with A,B,-unsaturated carbonyl
compounds. Biochem. J., 109 : 651-661.
BREWER, W.E. (1976) 90-day subacute vapor inhalation
toxicity study with acrylonitrile in beagle dogs, albino rats
and albino mice (Industrial Biotest Report No. 74-42, prepared
for the Monsanto Company).
BRIEGER, H., RIEDERS, F., & HODES, A.B. (1952)
Acrylonitrile: spectrophotometric determination, acute
toxicity, and mechanism of action. Ind. Hyg. occup. Med., 6 :
128-140.
BRUCE, R.B., HOWARD, J.W., & HANZAL, R.F. (1955)
Determination of cyanide, thiocyanate and A-hydroxynitriles in
plasma or serum. Anal. Chem., 27 : 1346-1347.
BUFFONI, F., COPPI, C., & SANTONI, C. (1982) Determination
of thioethers in urine. Clin. Chem., 28 : 248-250.
CAMPBELL, D.N. & MOORE, R.H. (1979) The quantitative
determination of acrylonitrile, acrolein, acetonitrile and
acetone in workplace air. Am. Ind. Hyg. Assoc. J., 40 : 904-909.
CANADA FOOD & DRUGS ACT & REGULATIONS (1982) Division 23 -
Section B.23.008. Canada, Ministry of Supply & Services.
CARPENTER, C.P., SMYTH, H.F., & POZZANI, U.C. (1949) The
assay of acute vapour toxicity and the grading and
interpretation of results on 96 chemical compounds. J. ind.
Hyg. Toxicol., 31 : 343-346.
CHANDRA, H., GUPTA, B.H., BHARGAVA, S.K., CLERK, S.H., &
MAHENDRA, P.N. (1980) Chronic cyanide exposure - a
biochemical and industrial hygiene study. J. anal. Toxicol.,
4 : 161-165.
CHERNA, M., KOCHISOVA, J., KODYTKOVA, I., KOPECKY, J., &
SHRAM, R.J. (1981) Mutagenic activity of oxiranecarbonitrile
(glycidonitrile). In: Gut, I., Cikrt, M. & Plaa, G.L., ed.
Industrial & environmental xenobiotics, Berlin, Springer-
Verlag, pp. 251-255.
CHOBOT, A.M. (1979) [Menstrual function in workers of the
polyacrylonitrile fibre industry.] Zdrav. Beloruss., 2 : 24-27
(in Russian).
CIA (1978) Codes of practice for chemicals with major
hazards - acrylonitrile, Chemical Industries Association.
CINCOLELLA, A., VOIRIN, D., HECHT, G., GERBER, J.-M., DUCOS,
P., & LIMASSET, J.-C. (1981) Monitoring of atmospheric
concentrations of acrylonitrile in eleven French factories. G.
Ital. Med. lav., 3 : 165-167.
CLIVE, D. & SPECTOR, F.S. (1975) Laboratory procedure for
assessing specific locus mutagens at the TK locus in cultured
L5178Y mouse lymphoma cells. Mutat. Res., 31 : 17-29.
COLLINS, T.F.V. (1977) Written testimony in the matter of
acrylonitrile copolymers used to fabricate beverage containers
(Docket No. 76N-0070, exhibit G-82).
CRISP, S. (1980) Solid sorbent gas samplers. Ann. occup.
Hyg., 23 : 47-76.
CUPITT, L.T. (1980) Fate of toxic and hazardous materials in
the air environment. (EPA report No. EPA-600/3-80-084).
CZAJKOWSKA, T. (1971) [Excretion of certain metabolites upon
single administration of acrylonitrile.] Med. Pr., 22 (4):
381-385 (in Polish).
CZAJKOWSKA, T., KNOBLOCH, K., KOWALSKI, Z., KRYSIAK, B.,
PIOTROWSKI, J., ROGACZEWSKA, T., STARZYNSKI, Z., STASIK, M.,
SZENDZIKOWSKI, S., & ZWIERZCHOWSKI, Z. (1969) [Evaluation of
the toxicity and exposure of chemical industry workers to
acrylonitrile.] Med. Pr., (Supplement): 11-16 (in Polish).
DAHM, D.J. (1977) Identification of metabolites of
acrylonitrile. Testimony before the US FDA (Industrial Biotest
Report No. 74-42, Appendix 4: A.1-A.19, prepared for the
Monsanto Company).
DAUGHERTY, F.M. & GARRETT, J.T. (1951) Toxicity levels of
hydrocyanic acid and some industrial by-products. Texas J.
Sci., No. 3 : 391-396.
DAUES, G.W. & HAMNER, W.F. (1957) Determination of small
amounts of acrylonitrile in aqueous industrial streams. Anal.
Chem., 29 : 1035-1037.
DAVIS, J.H., DAVIES, J.E., AMERICO RAFONNELLI, R.S., & REICH,
G. (1973) Investigation of fatal acrylonitrile
intoxications. Pesticides and the environment, a continuing
controversy. Inter. Med. Book Corp., 2 : 547-556.
DELIVANOVA, S., POPOVSKI, P., & ORUSEV, T. (1978)
[Blepharoconjunctivitis in workers of the polyacrylonitrile
synthetic fibre industry.] God. Zb. Med. Fak. Skoje, 24 :
279-282 (in Macedonian).
DELLA FIORENTINA, H. & DE WIEST, F. (1979) Recherche d'un
indice biologique d'exposition chronique des travailleurs aux
dérivés cyanés. Arch. Mal. prof., 40 : 700-703.
DELZELL, E. & MONSON, R.R. (1982) Mortality among rubber
workers: VI. Men with potential exposure to acrylonitrile. J.
occup. Med., 24 (10): 767-769.
DE MEESTER, C., PONCELET, F., ROBERFROID, M., & MERCIER, M.
(1978b) Mutagenicity of acrylonitrile. Toxicology, 11 : 19-27.
DE MEESTER, C., DUVERGER-VAN BOGAERT, M., LAMBOTTE-VAN DEPAER,
M., ROBERFROID, M., PONCELET, F., & MERCIER, M. (1979) Liver
extract mediated mutagenicity of acrylonitrile. Toxicology,
13 (1): 7-15.
DFG (1982) Maximum concentrations at the workplace and
biological tolerance values for working materials 1982,
Weinheim, Verlag Chemie Gmbh, Commission for Investigation of
Health Hazards of Chemical Compounds in the Work Area,
Deutsche Forschungsgemeinschaft (Report No. 18).
DINU, V. (1975a) Activity of glutathione peroxidase and
catalase and the concentration of lipid peroxides in acute
intoxication with acrylonitrile. Rev. Roum. Biochim., 12 (1):
11-14.
DINU, V. (1975b) Intracellular thiol concentration in
acrylonitrile intoxication. Rev. Roum. Biochim., 12 (3):
155-158.
DINU, V. & KLEIN, R. (1976) Catalase activity and
glutathione and lactic acid concentrations in rats acutely
poisoned by acrylonitrile. J. Pharmacol., 7 (2): 223-226.
DJURIC, D., RAICEVIC, P., & KONSTANTINOVIC, I. (1962)
Excretion of thiocyanates in urine of smokers. Arch. environ.
Health, 5 : 12-15.
DORIGAN, J., FULLER, B., & DUFFY, R. (1976) Scoring of
organic air pollutants chemistry, production and toxicity of
selected synthetic organic chemicals (chemicals A-C), (MITRE
Tech. Rep. MTR-7248).
DORODNOVA, N.S. (1976) [Gynaecological morbidity and
specific functions of the female organism under the conditions
of chemical production in "Nitron".] Gig. Zabol., 8 : 45-46 (in
Russian).
DRAMANSKI, W. & TROJANOWSKA, B. (in press) Chromatographic
determination of S-cyanoethylmercapturic acid - a metabolite
of acrylonitrile in urine. Arch. Toxicol.
DUDLEY, H.C. & NEAL, P.A. (1942) Toxicology of acrylonitrile
(vinyl cyanide). I. Study of the acute toxicity. J. ind. Hyg.
Toxicol., 24 (2): 27-36.
DUDLEY, H.C., SWEENEY, T.R., & MILLER, J.W. (1942)
Toxicology of acrylonitrile (vinyl cyanide). II. Studies of
effects of daily inhalation. J. ind. Hyg. Toxicol., 24 :
255-258.
DUPONT (1977) In response to OSHA's request for information
on occupational exposure to acrylonitrile, letter to the OSHA
Docket Officer, US Department of Labour, Fed. Reg., 42 : 33043.
DUVERGER-VAN BOGAERT, M., LAMBOTTE-VANDEPAER, M., NOEL, G.,
ROBERFROID, M., & MERCIER, M. (1978) Biochemical effects of
ACN on the rat liver, as influenced by various pre-treatments
of the animals. Biochem. Biophys. Res. Comm., 83 : 1117-1124.
DUVERGER-VAN BOGAERT, M., LAMBOTTE-VANDEPAER, M., DE MEESTER,
C., ROLLMANN, B., PONCELET, F., & MERCIER, M. (1981) Effect
of several factors on the liver extract mediated mutagenicity
of acrylonitrile and identification of four new in vitro
metabolites. Toxicol. Lett., 7 : 311-319.
DUVERGER-VAN BOGAERT, M., LAMBOTTE-VANDEPAER, M., MERCIER, M.,
& PONCELET, F. (1982a) In vitro covalent binding of
acrylonitrile to rat liver proteins. Toxicol. Lett., 13 :
203-209.
DUVERGER-VAN BOGAERT, M., LAMBOTTE-VANDEPAER, M., DE MEESTER,
C., MERCIER, M., & PONCELET, F. (1982b) Role of glutathione
in liver-mediated mutagenicity of acrylonitrile. Toxicol.
Lett., 11 : 305-311.
EFREMOV, A.M. (1976a) [Study of the free radicals of the
blood, brain, liver and spleen of white rats subjected to
chronic acrylonitrile poisoning.] Zdrav. Beloruss., 6 : 86 (in
Russian).
EFREMOV, A.M. (1976b) [Study of the biotransformation of
acrylonitriles in animals.] Zdrav. Beloruss., 7 : 85-86 (in
Russian).
EFREMOV, A.M. (1976c) [State of protective adaptive
reactions in white rats poisoned with acrylonitrile.] Gig. Tr.
Ohr. Zdor. Rab. Neft. Neftehim. Prom-sti., 9 : 120-122 (in
Russian).
EFREMOV, A.M. (1976d) [Activity of catalase, peroxidase and
sulfhydryl groups of the brain, liver, and blood in white rats
poisoned with acrylonitrile.] Gig. Tr. Ohr. Zdor. Rab. Neft.
Neftehim. Prom-sti., 9 : 123-124 (in Russian).
ELLMAN, G.L. (1959) Tissue sulfhydryl groups. Arch.
Biochem. Biophys., 82 : 70-77.
ENIKEEVA, N.A., OSTROVSKAJA, R.S., SYSO, L.V., PODREZ, Z.G.,
BRAGINSKAJA, L.L., GVOZDEV, N.P., NESTEROVA, N.E.,
MUSSERSKAJA, A.N., SUZDALTREVA, N.A., & EFREMOV, A.M. (1976)
[Industrial hygiene and health status of workers involved in
the manufacture of the synthetic fibre Nitron.] Gig. Tr. Ohr.
Zdor. Rab. Nef. Neftehim. Prom-sti., No. 9 : 22-25 (in Russian).
EUROP-COST (1976) A comprehensive list of polluting
substances which have been identified in various fresh waters,
effluent discharges, aquatic animals and plants, and bottom
sediments, 2nd ed. Luxembourg, Commission of the European
Communities, p. 19 (EUCO/MDU/73/76, XII/476/76).
FAROOQUI, M.Y.G. & AHMED, A.E. (1982) Molecular interaction
of acrylonitrile and potassium cyanide with rat blood. Chem.
Biol. Interact., 38 : 145-149.
FERIN, J., URBANKOVA, J., & VLCHKOVA, A. (1961) [Hygienic
and toxicological problems of acrylonitrile and
dimethylformamide.] Prac. Lék., 13 : 466-468 (in Czech).
FRANKENBERG, L. (1980) Enzyme therapy in cyanide poisoning:
effect of rhodanese and sulfur compounds. Arch. Toxicol., 45 :
315-323.
FRANZEN, E. von & WAGNER, G. (1978) [Gelchromatographic and
electrophoretic serum protein pattern in rats after acute
acrylonitrile intoxication.] Z. Gesamte Hyg., 24 : 534-537 (in
German).
FREEMAN, R.A., SCHROY, J.M., KLIEVE, J.R., & ARCHER, S.A.
(1981) Experimental studies on the rate of air stripping of
hazardous chemicals from waste treatment systems. In: APCA
Meeting, Montreal, Canada, June 1980.
GAGNON, Y.T. & POSNER, J.C. (1979) Recovery of acrylonitrile
from charcoal tubes at low levels. Am. Ind. Hyg. Assoc. J.,
40 : 923-925.
GAWELL, G. B-M. (1979) Determination of acrylonitrile
monomer in plastic packaging and beverages by headspace gas
chromatography. Analyst, 104 : 106-110.
GHANAYEM, B.I. & AHMED, A.E. (1982) In vivo
biotransformation and biliary excretion of 1-14C acrylonitrile
in rats. Arch. Toxicol., 50 : 175-185.
GHERSIN, Z., STITZL, A., & MANEA, R. (1969) [Comparative
study of titrimetric and colorimetric methods for determining
acrylonitrile in waste water.] Rev. Chim. (Bucharest), 20 :
689-694 (in Rumanian, Chem. Abstr., 72 : 124853m).
GHIRINGHELLI, L. (1954) [Acrylonitrile: toxicity and
mechanism of action.] Med. lav., 45 (5): 305-312 (in Italian).
GINCHEVA, N., STAMOVA, N., HINKOVA, L., KYURKTCHIEV, S.,
SPASOVSKI, M., BAINOVA, A., MUHTAROVA, M., & HRISTEVA, V.
(1977) Study of the health status of workers from the
acrylonitrile department. In: Proceedings of the International
Symposium on Occupational Health in the Production of
Artificial Fibres - Abstracts, Finland, 6-10 June 1977, p. 41.
GOING, J.E., KUYKENDAHL, P., LONG, S., ONSTOT, J., & THOMAS,
K. (1979) Environmental monitoring near industrial sites:
acrylonitrile. 285 pp (EPA/560/6-79/003).
GRAHAM, J.P.D. (1965) Hydroxycobalamin as an antidote to
acrylonitrile. Toxicol. appl. Pharmacol., 7 : 367-372.
GRAVCZYK, J. & ZWIERZCHOVSKI, Z. (1973) [Effect of acute
experimental poisoning with acrylonitrile on the pressor
responses to adrenaline, noradrenaline and acetylcholine.]
Med. Pr., 24 (2): 151-158 (in Polish).
GREEN, M.L.H., MURIEL, W.J., & BRIDGES, B.A. (1976) Use of
simplified fluctuation test to detect low levels of mutagens.
Mutat. Res., 38 : 33-42.
GROTE, A.A., KIM, W.S., & KUPEL, R.E. (1978) Establishing a
protocol from laboratory studies to be used in field sampling
operations. Am. Ind. Hyg. Assoc. J., 39 : 880-884.
GRUNSKE, F. (1949) [Ventox and ventox-poisoning.] Dtsch.
med. Wochenschr., 74 : 1081 (in German).
GUENGERICH, F.P., GEIGER, L.E., HOGY, L.L., & WRIGHT, P.L.
(1981) In vitro metabolism of acrylonitrile to
2-cyanoethylene oxide, reaction with glutathione, and
irreversible binding to proteins and nucleic acids. Cancer
Res., 41 : 4925-4933.
GUERIN, M.R., OLERICH, G., & HORTON, A.D. (1974) Routine gas
chromatographic component profiling of cigarette smoke for the
identification of biologically significant constituents. J.
Chromatogr. Sci., 12 : 385-391.
GUT, I., NERUDOVA, J., KOPECKY, J., & HOLECHEK, V. (1975)
Acrylonitrile biotransformation in rats, mice, and chinese
hamsters as influenced by the route of administration and by
phenobarbital, SKF-525A, cysteine, dimercaprol, or
thisulphate. Arch. Toxicol., 33 : 151-161.
GUT, I., KOPECKY, J., & FILIP, J. (1981a) Acrylonitrile-14C
metabolism in rats: effect of the route of administration on
the elimination of thiocyanate and other radioactive
metabolites in urine and faeces. J. Hyg. Epidemiol. Microbiol.
Immunol., 25 (1): 12-16.
GUT, I., KOPECKY, J., & NERUDOVA, J. (1981b) Relationship
between acrylonitrile biotransformation, pharmacokinetics, and
acute toxicity. A short review. G. Ital. Med. lav., 3 : 131-136.
GUT, I., PELECH, L., VEJLUPKOVA, J., MATOUSEK, O., & KLENER,
P. (1982) [Effects of induction and inhibition of benzene
metabolism in hematopoiesis in male Wistar rats.] Cesk. Hyg.,
27 (1): 1-13 (in Czech).
GUT, I., NERUDOVA, J., FRANTIK, E., KOPECKY, J., HOLECHEK, V.,
STILBOROVA, A., MIRHEJOVSKA, E., & HOLUSHA, R. (in press)
Effect of acrylonitrile inhalation: Intermediary metabolism
and the excretion of thioethers and thiocyanate in rats. J.
Hyg. Epidemiol. Microbiol. Immunol, 28 : (in press).
HALL, M.E. & STEVENS, J.W., Jr (1977) Spectrophotometric
determination of acrylonitrile. Anal. Chem., 49 (14): 2277-2280.
HASHIMOTO, K. (1962) Studies on the intoxication of
acrylonitrile report IV. Effect of daily inhalation of
acrylonitrile on body weight, bodily activity and hemogram of
mice. Q. J. Labor. Res., 10 : 11-16.
HASHIMOTO, K. & ANDO, K. (1966) Effect of acrylonitrile on
the central and peripheral nervous system. In: Symposium on
Higher Nervous Functions & Occupational Health, pp. 87-89.
HASHIMOTO, K. & KANAI, R. (1965) Studies on the toxicology
of acrylonitrile, metabolism, mode of action and therapy. Ind.
Health, 3 (1-2): 30-45.
HASHIMOTO, K. & KANAI, R. (1972) Effect of acrylonitrile on
sulfhydryls and pyruvate metabolism in tissues. Biochem.
Pharmacol., 21 : 635-640.
HASHIMOTO, K. & KOBAYASI, T. (1961) A case of acute
dermatitis caused by contact with acrylonitrile. Q. J. Labor.
Res., 9 (1-4): 21-24.
HASKELL LABORATORY (1975) In vitro microbial mutagenicity
studies of acrylonitrile. (Report No. 351-357, E.I. Dupont de
Nemours & Co., Haskell Laboratory for Toxicology and
Industrial Medicine).
HASLAM, J. & NEWLANDS, G. (1955) Determination of
acrylonitrile in air. Analyst, 180 : 50-53.
HEINO, J., VILHUNEN, R., & HERNBERG, S. (1968) Urinary
thiocyanate concentrations in smokers and non-smokers.
Work-Environ.-Health, 5 : 12-15.
HENDERSON, C., PICKERING, G.H., & LEMKE, A.E. (1961) The
effect of some organic cyanides (nitriles) on fish. Proc. 15th
Ind. Waste Conf. En. Bull., 45 (2): 120-130.
HEUSER, S.G. & SCUDMORE, K.A. (1969) Determination of
fumigant residues in cereals and other foodstuffs: a
multi-detection scheme for gas chromatography of solvent
extracts. J. Sci. Food Agric., 20 : 566-572.
HOFFMANN, P., KLEINE, D., & FRANZEN, E. (1976) [On the proof
of acrylonitrile exposure: Detoxication of acrylonitrile by
coupling with D-glucuronic acid.] Z. Ges. Hyg., 22 (5): 310-312
(in German).
HOGAN, G.K. & RINEHART, W.E. (1980) A 24-month oral
toxicity/carcinogenicity study of acrylonitrile administered
by inhalation to Spartan rats. Bio/dynamics Inc. (Final report
Vols I & II, Project No. 77-1746).
HOLECHEK, V. & KOPECKY, J. (1981) Conjugation of glutathione
with acrylonitrile and glycidonitrile. In: Gut, I., Cikrt, M.
& Plaa, G.L., ed. Industrial and environmental xenobiotics.
Berlin, Springer Verlag, pp. 239-245.
HOUTHUIJS, D., REMIJN, B., WILLEMS, H., BOLEIJ, J., &
BIERSTEKER, K. (1982) Biological monitoring of acrylonitrile
exposure. Am. J. ind. Med., 3 (3): 313-320.
HROMOV, V.E. (1974) [Detection of circulating and fixed
antibodies in the diagnosis of allergies of chemical
etiology.] Vrach. Delo, 12 : 115-116 (in Russian).
HUGHES, T.W. & HORN, D.A. (1977) Source assessment:
acrylonitrile manufacture (air emission) (NITS Report. No.
PB-271 969).
HUMISTON, C.G. & FRAUSON, L.O. (1975) 90-day oral toxicity
study incorporating acrylonitrile in the drinking water of
rats. Midland, MI, Dow Chemical Toxicology Research
Laboratory, 99 pp.
HUNGARY (1979) Workplace requirements for air purity, Magyar
Nöpköztarsasagi Orszagos Szabvany, MSZ 21461-78 Az MSZ
21461-73 helyett G 23, 1979, 1 January.
IARC (1979) Acrylonitrile, acrylic and modacrylic fibres,
and acrylonitrile- butadiene-styrene and styrene-acrylonitrile
copolymers, pp. 73-113 (IARC Monographs on the Evaluation of
the Carcinogenic Risk of Chemicals to Humans, Vol. 19).
IARC (1982) IARC Monographs on the Evaluation of
Carcinogenic Risk of Chemicals to Humans, 1-29 (Suppl. 4) pp.
25-27.
IDOL, J.D., Jr (1974) Acrylonitrile polymers in prospect and
retrospect. In: Proceedings of the Applied Polymer Symposium
25: Acrylonitrile in Macromolecules, New York, John Wiley, pp.
1-18.
IMANARI, T., TANABE, S., TOIDA, T., SHINZO, T., & TOSHIHIKO,
T. (1982) Simultaneous determination of cyanide and
thiocyanate by high performance liquid chromatography. Chem.
Pharm. Bull. Japan, 30 (10): 3800-3802.
INRS (1983) Valeurs limités pour les concentrations des
substances dangereuses dans l'air des locaux de travail. Cah.
Notes Documen., No. 110, (ND 1410-110-83).
IVANOV, V.V. (1980) [Oxidative transformation of
acrylonitrile as a basis of its toxicological action.]
Farmakol. Toksikol., 43 (3): 383-384 (in Russian).
IVANOV, V.V. (1981) [Evaluation of the significance of
acrylonitrile metabolism in the mechanism of its toxicity.]
Gig. Tr. prof. Zabol., 9 : 48-50 (in Russian).
IVANOV, V.V. & AL'SHANSKY, A.M. (1982) GABA-ergic components
and lipid peroxidation during acute exogenous acrylonitrile
poisoning. Bull. exper. Biol. Med., 94 (7): 40-43.
IVANOV, V.V., GRIOHCHENKO, D.A., & KLIMATSKAJA, L.G. (1979)
[Blood serum enzymes in evaluating the nature of liver damage
and the effectiveness of chemotherapy following acrylonitrile
poisoning.] Vopr. Med. Him., 25 (6): 715-718 (in Russian).
IVANOV, V.V., KURNETSOVA, G.P., & ARCHAKOV, A.I. (1978)
[Stimulation of lipid peroxidation by acrylonitrile in rat
liver tissue.] Vopr. Med. Him., 24 (6): 816-818 (in Russian).
IVANOV, V.V., ZHISNOV, G.F., BACHMANOVA, G.I., MAZUROV, A.V.,
& ARCHALOV, A.I. (1979) [Interaction of acrylonitrile with
the microsomal oxidation system in rat liver tissue.] Vopr.
Med. Him., 25 (4): 468-471 (in Russian).
IVANOV, V.V., ZHIRNOV, G.F., & ARCHAKOV, A.J. (1982)
[Activation of acrylonitrile in the microsomal system of
oxidation.] Vopr. Med. Him., 28 (2): 95-98.
IWASAKI, K., KANNO, S., SAWATARI, K., SAWADA, S., SUGIMOTO,
M., SODA, R., TAKANO, T., NAKAMURA, K., MATSUMURA, Y., MYOJO,
T., SAKURAI, H., & TAKEMOTO, K. (1980) [Determination of
acrylonitrile in the working environments of a few kinds of
plants handling acrylonitrile.] Annual Report of the National
Institute of Industrial Health for 1979, p. 38 (in Japanese).
JACOBS, H.W. & SYRJALA, R.J. (1978) The use of infrared
analyzers for monitoring acrylonitrile. Am. Ind. Hyg. Assoc.
J., 39 : 161-165.
JACKSON, S. & BROWN, V.M. (1970) Effect of toxic wastes on
treatment processes and watercourses. Water Pollut. Control,
69 (3): 292-313.
JAEGER, R.J. (1978) Effect of hypoxia on the toxicity of
1,1-dichloroethylene or ACN. Toxicol. appl. Pharmacol., 45 :
257.
JAEGER, R.J. & COTE, I.L. (1982) Effect of hypoxia on the
acute toxicity of acrylonitrile. Res. Comm. Chem. Pathol.
Pharmacol., 36 (2): 345-348.
JAMES, S.P., WARING, R.H., & WHITE, D.A. (1967) Separation
of mercapturic acids and related compounds by gas
chromatography and a method for the determination of hippuric
acid. J. Chromatogr., 26 : 255-258.
JAPAN ASSOCIATION OF INDUSTRIAL HEALTH (1972) Recommendations
on permissible concentrations.
JEDLICKA, V., PASEK, A., & GOLA, J. (1958) Pesticides in
foods. III. Acrylonitrile as a food insecticide. J. Hyg. Epid.
Microbiol. Immunol., 1 : 116-125.
KANAI, R. & HASHIMOTO, K. (1965) Determination of
acrylonitrile, cyanide and thiocyanate in biological
materials. Ind. Health, 3 (47): 47-52.
KANKAANPÄÄ, E.E., HEMMINKI, K., & VAINIO, H. (1979)
Embryotoxicity of acrolein, acrylonitrile and acryl acid in
developing chick embryos. Toxicol. Lett., 4 : 93-96.
KIESSELBACH, N., KORALLUS, U., LANGA, H.J., NIESS, A., &
ZWINGERS, T. (1979) Acrylonitrile - Epidemiological Study,
Bayer 1977. Zentr. Arbeitsmed. Profyl., 29 (10): 257-259.
KILKIPARI, I. & SAVOLAINEN, H. (1982) Increased urinary
excretion of thioether in new rubber workers. Br. J. ind.
Med., 39 : 401-403.
KLESHCHEVA, M.S., USACHEVA, V.T., & POZHAROVA, V.N. (1971)
[Determination of residual monomers and non-polymerising
impurities in polystyrene plastics by means of gas
chromatography.] Plast. Mass., 7 : 57-58 (in Russian).
KNEZEVICH, A.L. & HOGAN, G.K. (1980) A 24-month oral
toxicity/carcinogenicity study of acrylonitrile administered
in the drinking water to Fischer 344 rats. Bio/Dynamics Inc.
(Final Report Vols I and IV, Project No. 77-1744, Bio-Dynamics
Inc.).
KNOBLOCH, K., SZENDZIKOWSKI, S., CZAJKOWSKA, T., & KRYSIAK,
B. (1971) [Experimental studies on acute and subacute
toxicity of acrylonitrile.] Med. Pr., 22 (3): 257-269 (in
Polish).
KNOBLOCH, K., SZENDZIKOWSKI, S., & CZAJKOWSKA, T. (1972)
[Chronic toxicity of acrylonitrile.] Med. Pr., 23 (3): 243-257
(in Polish).
KOLK, J.J. (1979) Inventory of employees exposed to
acrylonitrile in the Netherlands. Acrylonitrile Consultation
Group (Report of the Medical Sub-Group of the Vereniging van
de Nederlandse Chemische Industrie [VNCI]).
KOPECKY, J. (1982) Development and methodology for
assessment of urinary excretion of S-cyanoethylmercapturic
acid in man. (Final Report to the WHO Regional Office for
Europe, ICP/RCE 903).
KOPECKY, J., ZACHARDOVA, D., GUT, I., & FILIP, J. (1979)
[Metabolism of acrylonitrile in rats in vivo. ] Prac. Lék., 31 :
203-207 (in Czech).
KOPECKY, J., GUT, I., NERUDOVA, J., ZACHARDOVA, D., &
HOLECHEK, V. (1980a) Two routes of acrylonitrile metabolism.
J. Hyg. Epid. Microbiol. Immunol., 24 : 356-362.
KOPECKY, J., GUT, I., NERUDOVA, J., ZACHARDOVA, D., HOLECHEK,
V., & FILIP, J. (1980b) Acrylonitrile metabolism in the rat.
Arch. Toxicol., Suppl. 4 : 322-324.
KOPECKY, J., ZACHARDOVA, D., & GUT, I. (1980c) New findings
on acrylonitrile metabolism. Czech. Med., 3 : 295-301.
KOPECKY, J., GUT, I., NERUDOVA, J., ZACHARDOVA, D., &
HOLECHEK, V. (1981) Metabolic studies on acrylonitrile. In:
Gut, I., Cikrt, M. & Plaa, G.L., ed. Industrial and
environmental xenobiotics, Berlin, Springer-Verlag, pp.
221-231.
KORZHOVA, I.T., KLESHCHEVA, M.S., PORODINA, M.N., KORHOVA,
R.D., BALANDINA, V.A., & HOHLOV, V.A. (1974)
[Gas-chromatographic determination of toxic concentrations of
styrene and acrylonitrile vapours during the synthesis of
polystyrene plastics.] Plast. Mass., 5 : 67-69 (in Russian,
Chem. Abstr., 81 : 110759j).
KRIVOVA, V.N., USTINOVIC, I.P., & ERENBURG, B.E. (1982)
[Functional activity of the immune T-system due to
acrylonitrile poisoning.] In: Metabolicheskie aspecti
deistvija na organism industrial'nih himicheskih soedinenii,
Krasnojarsk, pp. 53-55 (in Russian).
KROELLER, E. (1970) [Residual acrylonitrile determination in
food.] Dtsch Lebensm. Rundsch., 66 : 11-33 (in German).
KRYNSKA, A. (1970) [Method of determination of acrylonitrile
in the air.] Pr. Central. Inst. Ochrony Pr., 20 : 303-310 (in
Polish, Chem. Abstr., 74 : 90798w).
KRYSIAK, B & KNOBLOCH, K. (1971) [Effect of acrylonitrile on
the central nervous system.] Med. Pr., 22 (6): 601-610 (in
Polish).
LAMBOTTE-VANDEPAER, M., DUVERGER-VAN BOGAERT, M., DE MEESTER,
C., PONCELET, F., & MERCIER, M. (1980) Mutagenicity of urine
from rats and mice treated with acrylonitrile. Toxicology, 16 :
67-71.
LAMBOTTE-VANDEPAER, M., DUVERGER-VAN BOGAERT, M., DE MEESTER,
C., ROLLMANN, B., PONCELET, F., & MERCIER, M. (1981a)
Identification of two urinary metabolites of rats treated with
acrylonitrile; influence of several inhibitors on the
mutagenicity of those urines. Toxicol. Lett., 7 : 321-328.
LAMBOTTE-VANDEPAER, M., DUVERGER-VAN BOGAERT, M., GARNY, V.,
PONCELET, F., & MERCIER, M. (1981b) Mutagenicity of
metabolites of acrylonitrile detected in urine. Mutat. Res.,
80 : 269 (abstract).
LANGVARDT, P.W., PUTZIG, C.L., BRAUN, W.H., & YOUNG, J.D.
(1980) Identification of the major urinary metabolites of
acrylonitrile in the rat. J. Toxicol. environ. Health, 6 :
273-282.
LAWTON, A.H., SWEENEY, T.A., & DUDLEY, H.C. (1943)
Toxicology of acrylonitrile (vinyl cyanide): III.
Determination of thiocyanate in blood and urine. J. ind. Hyg.
Toxicol., 25 : 13-19.
LEFAUX, R. (1966) [Chemistry and toxicology of plastics.]
Mainz, Krausskopf Verlag (in German).
LEONARD, A., GARNY, V., PONCELET, F., & MERCIER, M. (1981)
Mutagenicity of acrylonitrile in mouse. Toxicol. Lett., 7 :
329-334.
LINDGREN, D.L., VINCENT, L.E., & KROHNE, H.E. (1954)
Relative effectiveness of ten fumigants to adults of eight
species of stored product insects. J. econ. Entomol., 47 (5):
923-926.
LITTON BIONETICS (1975) Mutagenic evaluation of compounds
MCA 730 (LBI Project No. 2547, prepared for the Manufacturing
Chemists Association).
LITTON BIONETICS (1976) Mutagenicity evaluation of MCA 730
(Final report, LBI Project No. 2548, prepared for the
Manufacturing Chemists Association).
LODZ SANITARY INSPECTION SURVEY (1981) [Annual report on the
concentration of acrylonitrile in the air at workplaces in the
acrylic fibre plant "Chemitex-Anilana" at Lodz.] Lodz, Poland,
Sanitary-Epidemiological Station (in Polish).
LORZ, H. (1950) [On the percutaneous poisoning by
acrylonitrile (Ventox).] Dtsch med. Wochenschr., 75 (33/34):
1087-1088 (in German).
LOWENBACH, W., SCHLESINGER, S., & KING, J. (1978) Toxic
pollutant identification: acrylonitrile manufacture, 160 pp
(EPA-IMPQE series 02178-03).
MADDOCK, J.T., BYER, W.L., MALSEED, R.T., KERESZTESY, J.,
TAYLOR, T.J., & KATZ, A.B. (1977) Acrylonitrile (Monograph
prepared for US Consumer Product Safety Commission, Washington
DC).
MAGOS, L. (1962) A study of acrylonitrile poisoning in
relation to methemoglobin-CN complex formation. Br. J. ind.
Med., 19 : 283-286.
MALLETTE, F.S. (1943) Industrial hygiene in synthetic rubber
manufacture. Ind. Med., 12 : 495-499.
MALTONI, C., CILIBERTI, A., & DI MAIO, V. (1977)
Carcinogenicity bioassays on rats of acrylonitrile
administered by inhalation and by ingestion. Med. lav., 68 :
401-411.
MALTONI, C., CILIBERTI, A., & CARRETTI, D. (1982)
Experimental contributions in identifying brain potential
carcinogens in the petrochemical industry. Ann. N.Y. Acad.
Sci., 381 : 216-249.
MARANO, R.R., LEVINE, S.P., & HARVEY, T.M. (1978) Trace
determination of subnanogram amounts of acrylonitrile in
complex matrices by gas chromatography with a
nitrogen-selective detector. Anal. Chem., 50 (13): 1948-1951.
MARCOCI, S. & IONESCU, M. (1974) [The toxic action of
acrylonitrile on fish.] Strd. Prof. Calitatii Apelor, 16 : 7-22
(in Rumanian).
MARKELOV, M.A. & SEMENENKO, E.I. (1976) [Determination of
microconcentrations of substances migrating from polymer
materials to water.] Plast. Mass., 1 : 57-59 (in Russian, Chem.
Abstr., 84 : 155747g).
MAVRINA, E.A. & HROMOV, W.E. (1974) [Functional changes in
the body of workers engaged in production of nitron fibre.]
Vrach. Delo, 6 : 129-131 (in Russian).
MAVRINA, E.A. & IL'INA, V.A. (1974) [Change in the
immunological reactivity of the bodies of students of a
technical-trade school during training in a nitron production
plant.] Gig. i Sanit., 5 : 109-110 (in Russian).
MCLAUGHLIN, M., KRIVANEK, N.D., & TROCHIMOWICH, H.J. (1976)
Evaluation of antidotes for acrylonitrile poisoning (Abstr.).
Toxicol. appl. Pharmacol., 37 : 133-134.
MCNEAL, T. & BREDER, C.V. (1981) Head-space sampling and
gas-solid chromatographic determination of residual
acrylonitrile in acrylonitrile copolymer solutions. J. Assoc.
Off. Anal. Chem., 64 (2): 270-275.
MCNEAL, T. & BREDER, C.V. (1982) Manual headspace gas-solid
chromatographic determination of sub-parts-per-trillion levels
of acrylonitrile in 3% acetic acid. J. Assoc. Off. Anal.
Chem., 65 (1): 184-187.
MCOMIE, W.A. (1949) Comparative toxicity of
methacrylonitrile and acrylonitrile. J. ind. Hyg. Toxicol.,
31 : 113-116.
MILLER, L.M. & VILLAUME, J.E. (1978) Investigation of
selected potential environmental contaminants: acrylonitrile.
Washington DC, US EPA, 234 pp (Prepared for the Office of
Toxic Substances).
MILLS, E.J., Jr & STACK, V.T., Jr (1955) Acclimation of
microorganisms for the oxidation of pure organic chemicals.
In: Proceedings of the 9th Industrial Waste Conference Purdue
University, pp. 449-464.
MILWY, P. (1978) Letter to the editor. Reply to S. Venitt.
Mutat. Res., 57 : 110-112.
MILWY, P. & WOLFF, M. (1977) Mutagenic studies with
acrylonitrile. Mutat. Res., 48 : 271-278.
MINAMI, M., SATO, M., SUSUKI, A., YAMAMURA, K., & ISHIZU, S.
(1973) [Acrylonitrile poisoning. I. Result of exposure to
acrylonitrile vapour.] Tokyo Joshi Ika Daigaku Zasshi, 43 (10):
849-855 (in Japanese).
MLETZKO, H.G. von, & HENKEL, W. (1978) Animal experiments
with exposure to noise and acrylonitrile from the
chronobiological aspect. Z. Gesamte Hyg., 24 : 515-518.
MONSANTO (1975) Joint toxic action between acrylonitrile and
potassium cyanide. St Louis, MO, Monsanto Co. Medical
Department.
MONSANTO (1977a) Acrylonitrile sales specification, St
Louis, MO, Monsanto Chemicals Intermed. Co. (Monsanto data
sheet).
MONSANTO (1977b) In response to OSHA's request for
information on acrylonitrile (42 FR 34327) (Letter to the OSHA
Docket Officer, US Department of Labor).
MONSON, R.R. & FINE, L.J. (1978) Cancer mortality and
morbidity among rubber workers. J. Natl Cancer Inst., 61 (4):
1047.
MUHTAROVA, M. (1977) [Personal sample for evaluation of the
professional risk due to poisonings.] Hyg. Zdrav., 5 : 447-450
(in Bulgarian).
MURRAY, F.J., SCHWETZ, B.A., NITSCHE, K.D., JOHN, J.A.,
NORRIS, J.M., & GEHRING, P.J. (1978) Teratogenicity of
acrylonitrile given to rats by gavage or by inhalation. Food
cosmet. Toxicol., 16 (6): 547-551.
MURRAY, F.J., NITSCHKE, K.D., JOHN, J.A., SMITH, F.A., QUAST,
J.F., BLOGG, C.D., & SCHWETZ, B.A. (1976) Teratologic
evaluation of acrylonitrile monomer given to rats by gavage.
Midland MI, USA, Dow Toxicological Research Laboratory
(prepared for the Manufac. Chem. Assoc.).
NAKAMURA, K. (1981) [Mortality study of acrylonitrile
workers.] In: Annual Report of the National Institute of
Industrial Health for 1980, p. 31 (in Japanese).
NERUDOVA, J., HOLECHEK, V., GUT, I., & KOPECKY, J. (1980a)
[Relation between kinetics of acrylonitrile after different
routes of administration and its conversion to thiocyanate.]
Prakt. Lék., 32 : 15-18 (in Czech).
NERUDOVA, J., KOPECKY, J., GUT, I., & HOLECHEK, V. (1980b)
[Metabolism of acrylonitrile in the rats in vitro.] Prakt.
Lék., 32 : 98-103 (in Czech).
NERUDOVA, J., GUT, I., & KOPECKY, J. (1981) Cyanide effect
in acute acrylonitrile poisoning in mice. In: Gut, I., Cikrt,
M., & Plaa, G.L., ed. Industrial and environmental
xenobiotics, Berlin, Springer Verlag, pp. 245-250.
NILSEN, O.G., TOFTGARD, R., & ENEROTH, P. (1980) Effects of
acrylonitrile on rat liver cytochrome P-450, benz( a)pyrene
metabolism, and serum hormone levels. Toxicol. Lett., 6 :
399-404.
NIOSH (1976) NIOSH analytical methods for set K, Washington,
DC, NIOSH (NTIS Report No. PB-254 227).
NIOSH (1978) A recommended standard for occupational
exposure to acrylonitrile, Cincinnati, OH, Department of
Health, Education and Welfare (NIOSH), (Publ. No. 78-116).
NOEL, G., LAMBOTTE-VANDEPAER, M., DUVERGER-VAN BOGAERT, M.,
ROBERFROID, M., & MERCIER, M. (1978) Hepatotoxic effects of
acrylonitrile in rats. Arch. int. Physiol. Biochem., 86 (4):
951-952.
O'BERG, M.T. (1980) Epidemiologic study of workers exposed
to acrylonitrile. J. occup. Med., 22 (4): 245-252.
OOMENS, A.C. (1980) Experience with a dual detector
headspace gas chromatograph for acrylonitrile analysis. In:
Kolb, B., ed. Applied headspace gaschromatography, Heydon, pp.
111-116.
ORUSEV, T. & POPOVSKI, P. (1973) [Clinical symptoms in
workers occupationally exposed to acrylonitrile.] God. Zb.
Med. Fak. Skopje, 19 : 187-192 (in Macedonian).
ORUSEV, T., BAUER, S., & POPOVSKI, P. (1973) [Occupational
exposure to acrylonitrile in a plant for production of acrylic
synthetic fibres.] God. Zb. Med. Fak. Skopje, 19 : 445-449 (in
Macedonian).
OSHA (1978a) Emergency temporary and permanent standards for
occupational exposure to acrylonitrile (vinyl cyanide). Fed.
Reg., 43 : 2600, 4562.
OSHA (1978b) Permanent standard for exposure to acrylo-
nitrile. Fed. Reg., 43 : 45809-45817.
OSHA (1981) General Industry - Safety & Health Standards, 29
CFR 1910. US Department of Labor, pp. 830-850, 220 pp.
OSTROVSKAJA, R.S., PODREZ, Z.G., BRAGINSKAJA, L.L., BOKLOG,
E.P., EFREMOV, A.M., & VOLKOVA, G.A. (1976) [Health status
of workers currently engaged in the production of
acrylonitrile.] Gig. Tr. Prof. Zabol., 6 : 8-12 (in Russian).
PANOVA, R.V., BELOVA, R.A., & LAVRUSHINA, T.S. (1969)
[Chromatographic determination of residual monomers in
latexes.] Prom. Sin. Kauch. Nauch.-Tekh. Sb., 1 : 21-23 (in
Russian, Chem. Abstr., 74 : 43238d).
PARENT, R.A. & CASTRO, B.C. (1979) Effect of acrylonitrile
on primary Syrian golden hamster embryo cells in culture:
Transformation and DNA fragmentation. J. Natl Cancer Inst.,
62 (4): 1025-1029.
PARSONS, J.S. & MITZNER, S. (1975) Gas chromatographic
method for concentration and analysis of traces of industrial
organic pollutants in environmental air and stacks. Environ.
Sci. Technol., 9 : 1053-1058.
PATTERSON, R.M., BORNSTEIN, M.I., & GARSHICK, E. (1976)
Assessment of acrylonitrile as a potential air pollution
problem, Vol. VI (US Environmental Protection Agency, Contract
No. 68-02-1337, Task 8, Springfield, VA, National Technical
Information Service, No. PB-258 358).
PATTY, F.A., ed. (1963) Acrylonitrile, CH2=CHCN (vinyl
cyanide propenenitrile). In: Industrial hygiene and
toxicology, 2nd Revised ed., Vol. 2, pp. 2009-2012.
PAULET, G. & DESNOS, J. (1961) L'acrylonitrile,
toxicité-méchanisme d'action-thérapeutique. Arch. int.
Pharmacodyn., 131 (1-2): 54-83.
PAULET, G. & VIDAL, M. (1975) De la toxicité de quelques
esters acryliques et méthacryliques de l'acrylamide et des
polyacryliques. Arch. Mal. prof., 36 (1-2): 58-60.
PAULET, G., DESNOS, J., & BATTIG, J. (1966) De la toxicité
de l'acrylonitrile. Arch. Mal. prof., 27 (12): 849-856.
PEROCCO, P., PANE, G., BOLOGNESI, S., & ZANNOTTI, M. (1982)
Increase of sister chromatid exchange and unscheduled
synthesis of deoxyribonucleic acid by acrylonitrile in human
lymphocytes in vitro. Scand. J. Work Environ. Health, 8 :
290-293.
PETER, H. & BOLT, H.M. (1981) Irreversible protein binding
of acrylonitrile. Xenobiotica, 11 : 51-56.
PETER, H., APPEL, K.E., BERG, R., & BOLT, H.M. (1983)
Irreversible binding of acrylonitrile to nucleic acids.
Xenobiotica, 13 (1): 19-25.
PETROVA, L.I., NOSKOVA, M.P., BALANDINA, V.A., & GURICHEVA,
Z.G. (1972) [Polarographic determination of acrylonitrile in
aqueous extract of styrene- acrylonitrile copolymers.] Gig. i
Sanit., 37 : 62-65 (in Russian, Chem. Abstr., 76: 127898y).
PFEILSTICKER, K., INKMANN, K., & BOCHISCH, M. (1977)
Residues of (1-14C) ACN in fumigated dry food. Z.
Lebensmittel-Untersuch. Forsch., 164 (4): 274-276.
POLAND (1982) Decree of the Minister of Labour, Wages, and
Social Affairs, 22 December, 1982 (Low Gaz. No. 43, 29
December, 1982, Entry 287).
PONOMAREV, Y.P., ANUFRIEVA, T.L., & SHKORBATOVA, T.L. (1974)
[Polarographic determination of acrylonitrile in waste waters
using extraction.] Vodosnabz., Kanaliz. Gidroteh. Sooruz., 17:
67-70 (in Russian, Chem. Abstr., 82 : 34803x).
PORTMANN, J.K. & WILSON, K.W. (1971) The toxicity of 140
substances to the brown shrimp and other marine animals. 12 pp
(Ministry Agriculture Fisheries & Food Laboratories, England;
Shellfish Inf. Leafl. No. 22).
PUJADO, P.R., VORS, B.V., & KRUEDING, A.P. (1977) Newest
acrylonitrile process. Hydrocarbon Process, (May, 1977):
169-172.
QUAST, J.F., HUMISTON, C.G., SCHWETZ, B.A., FRAUSON, L.A.,
WADE, C.E., & NORRIS, J.M. (1975) A six-month oral toxicity
study incorporating acrylonitrile in the drinking water of
pure-bred beagle dogs. Midland MI, USA, Dow Toxicology
Research Laboratory, (prepared for the Manuf. Chem. Assoc.).
QUAST, J.F., ENRIQUEZ, R.M., WADE, C.E., HUMISTON, C.C., &
SCHWETZ, B.A. (1977) Toxicity and drinking water containing
acrylonitrile in rats: results after 12 months. Midland MI,
USA, Dow Toxicology Research Laboratory (prepared for the
Manuf. Chem. Assoc.).
QUAST, J.F., SCHUETZ, D.J., BALMER, M.F., GUSHOW, T.S., PARK,
C.N., & McKENNA, M.J. (1980a) A two-year toxicity and
oncogenicity study with acrylonitrile following inhalation
exposure of rats. Final Report. Midland MI, USA, Dow
Toxicology Research Laboratory.
QUAST, J.F., WADE, C.E., HUMISTON, C.G., CARREON, R.M.,
HERMANN, E.A., PARK, C.N., & SCHWETZ, B.A. (1980b) A
two-year toxicity and oncogenicity study with acrylonitrile
incorporated in the drinking water of rats. Final Report.
Midland MI, USA, Dow Toxicology Research Laboratory.
RABELLO-GAY, M.N. & AHMED, E. (1980) Acrylonitrile: in vivo
cytogenetic studies in mice and rats. Mut. Res., 79 : 249-255.
RAJENDRAN, S. & MUTHU, M. (1981) Detection of acrylonitrile
and ethylene oxide in air and fumigated foodstuffs. Bull.
environ. Contam. Toxicol., 27 : 426-431.
RAJENDRAN, S. & MUTHU, M. (1981) Effect of acrylonitrile on
trehalase, phosphorylase and acetylcholinesterase activities
in Tribolium castenlum Herbst and Trogoderma granarium Everts.
Experientia (Basel), 37 (8): 886.
RAMSTAD, T. & NICHOLSON, L.W. (1982) Determination of
sub-parts-per-billion levels of acrylonitrile in aqueous
solutions. Anal. Chem., 54 : 1191-1196.
REICHLE, A. & TENGLER, H. (1968) [Gas-chromatographic
procedures for determining residual monomers in the polymer.
Determination of acrylonitrile in polyacrylonitrile.]
Plastverarbeiter, 19 : 921-924 (in German, Chem. Abstr., 70 :
97206c).
ROGACZEWSKA, T. (1964) [Polarographic determination of
acrylonitrile in air.] Chem. Anal., 9 : 417-423 (in Polish).
ROGACZEWSKA, T. (1965) [Colorimetric method of acrylonitrile
determination in air.] Med. Pr., 16 (6): 465-475 (in Polish).
ROGACZEWSKA, T. (1971) The problems of obtaining the
evaluation of deleterious effects of acrylonitrile vapours in
industrial conditions. Thesis, Lodz, Poland, Institute of
Industrial Medicine.
ROGACZEWSKA, T. (1975) [Percutaneous absorption of
acrylonitrile vapours in animals.] Med. Pr., 26 (6): 459-465
(in Polish).
ROGACZEWSKA, T. (1976) [Determination of acrylonitrile in
air.] Med. Pr., 27 (2): 115-126 (in Polish).
ROGACZEWSKA, T. & PIOTROWSKI, J. (1968) [Routes of
acrylonitrile absorption in man.] Med. Pr., 19 (4): 349-353 (in
Polish).
ROSE, V.E. & PERKINS, J.L. (1982) Passive dosimetry - state
of the art review. Am. Ind. Hyg. Assoc. J., 43 : 605-621.
ROUDABUSH, R.L., TERHAAR, C.J., FASSETT, D.W., & DZIUBA, S.P.
(1965) Comparative acute effects of some chemicals on the
skin of rabbits and guinea-pigs. Toxicol. appl. Pharmacol., 7 :
559-565.
RUSSELL, J.W. (1975) Analysis of air pollutants using
sampling tubes and gas chromatography. Environ. Sci. Technol.,
9 : 1175-1178.
RUSSKIH, A.A. (1972) [Determination of acetone cyanohydrin
and acrylonitrile in air.] In: Bezhaev, M.S., ed. Proceedings
of the 4th Conference of People Employed in Universities and
Industrial Laboratories of South-east USSR on Physico-chemical
Methods of Analytical Control of Industrial Products, USSR,
Dagestan State University, Vol. 3, p. 100 (in Russian, Chem.
Abstr., 81 : 16301g).
RUSSKIH, A.A. (1973) [Photometric determination of
acrylonitrile in air.] Zavod. Lab., 39 (1): 5-6 (in Russian).
SAKURAI, H. & KUSUMOTO, M. (1972) [Epidemiologic study of
health impairment among acrylonitrile workers.] J. Sci. Labour
(Tokyo), 48 (5): 273-282 (in Japanese).
SAKURAI, H., ONODERA, M., UTSUNOMIYA, T., MINAKUCHI, H., IWAI,
H., & MATYUMURA, H. (1978) Health effects of acrylonitrile
in acrylic fibre factories. Br. J. ind. Med., 35 (3): 219-225.
SANDBERG, E.C. & SLANINA, P. (1980) Distribution of (1-14C)
acrylonitrile in rat and monkey. Toxicol. Lett., 6 : 187-191.
SAPOTA, A. (1982) The disposition of 14C-acrylonitrile in
rats. Xenobiotica, 12 (4): 259-264.
SAPOTA, A. & CHMIELNICKA, J. (1981) [Toxicity and metabolism
of acrylonitrile.] Med. Pr., 32 (1): 25-33 (in Polish).
SAPOTA, A. & DRAMINSKI, W. (1981) The fate of
14C-acrylonitrile in rats. In: Gut, I., Cikrt, M., & Plaa,
G.L., ed. Industrial and environmental xenobiotics, Berlin,
Springer Verlag, pp. 231-237.
SARTORELLI, E. (1966) [Acute acrylonitrile intoxication.]
Med. lav., 57 (3): 184-187 (in Italian).
SATO, M. (1978) [Studies on the toxic effect of
acrylonitrile - its metabolism, absorption and excretion.]
Jpn. J. Hyg., 33 (3): 497-505 (in Japanese).
SATO, M., ISHIZU, S., & MOMOTANI, H. (1975) [Determination
of acrylonitrile, cyanide and thiocyanate in blood and urine.]
Sangyo Igaku, 17 : 99-105 (in Japanese, Chem. Abstr., 100292z).
SATO, M., HIRASAWA, F., OGATA, M., TAKIZAWA, Y., KOJIMA, H., &
YOSHIDA, T. (1982) Distribution and accumulation of
(2,3-14C) acrylonitrile in rat after single injection.
Ecotoxicol. environ. Safety, 6 (5): 489-494.
SATO, S., KOSHIO, H., MIMURA, M., NAKAMURA, S., & ICHIMURA,
M. (1979) Investigation in counter-measure to the sources of
unspecified harmful chemicals in 1978. pp. 1-19 (in Japanese,
Report of the study sponsored by the Environmental Agency).
SCHEUFLER, H. von (1976) Experimental testing of chemical
agents for embryotoxicity, teratogenicity and mutagenicity -
ontogenic reactions of the laboratory mouse to these
injections and their evaluation - a critical analysis method.
Biol. Rundsch., 14 (4): 227-229.
SCHEUFLER, H. von (1980) [On the embryotoxic effectiveness
of acrylonitrile in the laboratory mouse.] Z. Gesamte Hyg.,
26 : 564-565 (in German).
SCUPAKAS, D. (1968) [Industrial hygiene conditions during
the reworking of plastics and problems improving them.] In:
Yavnaist, E.Y., ed. Proceedings of the first conference on
actual problems of industrial hygiene and occupational
pathology, 1967, Riga, Medical Institute, pp. 128-132 (in
Russian, Chem. Abstr., 72 : 47094k).
SEKI, S. (1967) [On acrylonitrile poisoning in a synthetic
resin plant.] Jpn. J. Hyg., 22 (1): 257 (in Japanese).
SEUTTER-BERLAGE, F., VAN DORP, H.L., KOSSE, H.G.J., &
HENDERSON, P.T. (1977) Urinary mercapturic acid excretion as
a biological parameter of exposure to alkylating agents. Int.
Arch. occup. environ. Health, 39 : 45-51.
SEUTTER-BERLAGE, F., DELBRESSINE, L.P.C., SMEETS, F.L.M., &
KETELAARS, H.C.J. (1978) Identification of three
sulfur-containing urinary metabolites of styrene in the rat.
Xenobiotica, 8 : 413-418.
SHACKELFORD, W.M. & KEITH, L.H. (1976) Frequency of organic
compounds identified in water, Athens GA, USA, US
Environmental Protection Agency, p. 55 (EPA-600/4-76-062).
SHEVCHIK, J. (1976) Detectors in gas chromatography,
Amsterdam, Elsevier, 192 pp.
SHUSTOV, V.J. (1968) [Clinical manifestation and
hematological changes in workers employed in the production of
acrylonitrile at the Saratov Chemical Combine.] Gig. Tr. prof.
Zabol., 3 : 27-29 (in Russian).
SHUSTOV, V.Y. & MAVRINA, E.A. (1975) [Clinical picture of
chronic poisoning in the production of nitrone.] Gig. Tr.
prof. Zabol., 3 : 27-29 (in Russian).
SILVER, E.H. & SZABO, S. (1982) Possible role of lipid
peroxidation in the action of acrylonitrile on the adrenals,
liver and gastrointestinal tract. Res. Comm. Chem. Pathol.
Pharmacol., 36 (1): 33-43.
SILVER, E.H., KUTTAB, S.H., HASAN, T., & HASSAN, M. (1982a)
Structural considerations in the metabolism of nitriles to
cyanide in vivo. Drug Metab. Disposition, 10 : 495-498.
SILVER, E.H., McCOMB, D.J., KOVACS, K., & SZABO, S. (1982b)
Limited hepatotoxic potential of acrylonitrile in rats.
Toxicol. appl. Pharmacol., 64 : 131-139.
SILVERSTEIN, L.G. (1977) Calidation of abcor GASBADGE for
acrylonitrile and improved desorption efficiency. Am. Ind.
Hyg. Assoc. J., 38 (8): 412-413.
SKVOSTSOV, Ju.I. (1980) [Variation in the amino acid
spectrum of blood serum in workers employed in ACN production
and nutritive ways of its correction. Vopr. Pitan., No. 2 :
31-33 (in Russian).
SLATER, E.E., ANDERSON, M.D., & ROSENKRANZ, S. (1971) Rapid
detection of mutagens and carcinogens. Cancer Res., 31 (7):
970-973.
SLOOF, W. (1979) Detection limits of a biological monitoring
system based on fish respiration. Bull environ. Contam.
Toxicol., 23 : 517-523.
SMYTH, H.F. & CARPENTER, C.P. (1948) Further experience with
the range-finding test in the industrial toxicology
laboratory. J. ind. Hyg. Toxicol., 30 : 63-68.
SOKAL, J.A. & KLYSZEJKO-STEFANOWICZ, L. (1972)
[Nicotinamide-adenine dinucleotides in acute poisoning with
some toxic agents.] Lodz Tow. Nauk., No. 112: 64-101 (in
Polish, Chem. Abstr., 79 : 62271d).
SOKAL, J.A., KNOBLOCH, K., MAJKA, J., SAPOTA, A., &
SZENDZIKOWSKI, S. (1977) [Toxicity of acrylate compounds
used in industry.] Prakt. Lek., 29 (4): 157-161 (in Czech).
SPASSOVSKI, M. (1976) Health hazards in the production and
processing of some fibres, resins, and plastics in Bulgaria.
Environ. Health Perspect., 17 : 199-202.
STAMOVA, N., GINCHEVA, N., SPASOVSKI, M., BAINOVA, A.,
IVANOVA, S., KURKCHIEV, S., HINKOVA, L., KHRISTEVA, V.,
MUKHTAROVA, M., KARADZHOVA, N., TSANEVA, L., & FIKOVA, E.
(1976) [Labour hygiene during the production of synthetic
fibres "Bulana".] Hyg. Zdrav., 2 : 134-140 (in Russian).
STANFORD RESEARCH INSTITUTE (1976) Interim Report: In vitro
microbiological mutagenicity studies of Dow Chemical Company
Compounds (SRI Project No. LSC-4378, prepared for Dow Chemical
Company).
STEICHEN, R.J. (1976) Modified solution approach for the gas
chromatographic determination of residual monomers by
head-space analysis. Anal. Chem., 48 : 1398-1402.
SUTA, B.E. (1979) Assessment of human exposures to
atmospheric acrylonitrile (Prepared by the Stanford Research
Institute - Contract 68-02-2835-EPA).
SVIRBELY, J.L. & FLOYD, E.P. (1961) Toxicologic studies of
acrylonitrile and B,B'-oxydipropionitrile. III. Chronic
studies. Meeting paper AINA-ACSIH, Detroit MI, USA.
SWEDEN (1981) Ordinance issued by the National Swedish Board
of Occupational Safety and Health Concerning Hygiene Limit
Values (in Swedish).
SZABO, S. & SELYE, H. (1971) Adrenal apoplexy and necrosis
produced by acrylonitrile. Endocrinol., 57 (3): 405-408.
SZABO, S. & SELYE, H. (1972) Effects of phenobarbital and
steroids on the adrenal apoplexy produced by acrylonitrile in
rats. Endocrinol. Exp., 6 (3): 141-146.
SZABO, S., REYNOLDS, E.S., KOMANICKY, P., MOSLEN, M.T., &
MELBY, J.C. (1976) Effect of chronic acrylonitrile ingestion
on rat adrenal. Toxicol. appl. Pharmacol., 37 (1): 133.
SZABO, S., BAILEY, K.A., BOOR, P.J., & JAEGER, R.J. (1977)
Acrylonitrile and tissue glutathion - differential effect of
acute and chronic interactions. Biochem. Biophys. Res.
Commun., 79 (1): 32-37.
SZABO, S., HUTTNER, I., KOVACS, K., HORVATH, E., SZABO, D., &
HORNER, H.C. (1980) Pathogenesis of experimental adrenal
hemorrhagic necrosis ("apoplexy"). Ultrastructural,
biochemical, neuropharmacologic and blood coagulation studies
with acrylonitrile in the rat. Lab. Invest., 42 (5): 533-545.
TADA, O. (1971) [Methods for determinations of toxic
substances in air-organic compounds.] J. Sci. Lab., (Part II),
47 : 789-796.
TAKAGI, N., TAKAGI, K., MATSUDA, T., & KOIZUMI, S. (1968)
[Effect of administration of vitimins B1 or B2 plus L-cysteine
to rats exposed to acrylonitrile gas over a long period.] J.
Sci. Lab., 44 (8): 454-464 (in Japanese).
TARKOWSKI, S. (1966) [Effect of cobalt on the inhibition by
potassium cyanide of cytochrome oxidase activity.] Med. Pr.,
17 (2): 116-119 (in Polish).
TARKOWSKI, S. (1968) [Effect of acrylonitrile on certain
properties of cytochrome oxidase.] Med. Pr., 19 (6): 525-531
(in Polish).
TATSUNO, T., INOUE, T., & TANIMURA, A. (1979) [Hygienic
chemical studies on plastics (II). Residual acrylonitrile in
ABS resin or AS resin and its migration into water.] Report of
the National Institute of Hygiene, 97 : 93-97 (in Japanese).
TATSUNO, T., INOUE, T., & TANIMURA, A. (1980) [Hygienic
chemical studies on plastics (V). Migration of acrylonitrile
from acrylonitrile-butadiene-styrene copolymer and
acrylonitrile-styrene copolymer into various food simulating
solvents in long period.] Report of the National Institute of
Hygiene, 98 : 110-115 (in Japanese).
TERENT'EV, A.P. & OBTEMPERANSKAJA, S.I. (1956) [Quantitative
determination of acrylonitrile with sodium sulfite.] Z. Anal.
Him., 11 : 638-639 (in Russian).
THIESS, A.M. & FLEIG, I. (1978) Analysis of chromosomes of
workers exposed to acrylonitrile. Arch. Toxicol., 41 (2):
149-152.
THIESS, A.M., FRENTZEL-BEYME, R., LINK, R., & WILD, H.
(1980) [Mortality study in chemical workers in different
production plants with exposure also to acrylonitrile.]
Zentralbl. Arbeitsmed. Arbeitsschutz Prophyl. Ergonomie,
30 (7): 259-267 (in German).
TULLAR, P.E. (1947) Final report on the pharmacology and
toxicology of acrylonitrile and acrylon. Washington,
Palusowski Memorial Research Laboratory, George Washington
University.
UHDE, W.J. & KOEHLER, U. (1967) [Testing of plastic
articles. Determination of monomeric compounds in the volatile
fraction of styrene polymers.] Z. Lebensm. Unters.-Forsch.,
135 : 135-140 (in German).
UNION CARBIDE CORPORATION (1977) In response to OSHA's
request for information on occupational exposure to
acrylonitrile. Fed. Reg., 42 : 33042 (Unpublished letter to the
OSHA Docket Officer, US Department of Labor).
UNITED KINGDOM HEALTH & SAFETY EXECUTIVE (1981a)
Acrylonitrile in air. Laboratory method using charcoal
adsorption tubes and gas chromatography. Methods for the
Determination of Hazardous Substances, 1, March 1981.
UNITED KINGDOM HEALTH & SAFETY EXECUTIVE (1981b)
Acrylonitrile in air. Laboratory method using porous polymer
adsorption tubes, and thermal desorption with gas
chromatographic analysis. Methods for the Determination of
Hazardous Substances, 2, March 1981.
UNITED KINGDOM MINISTRY OF AGRICULTURE, FISHERIES & FOOD
(1982) Survey of acrylonitrile and methacrylonitrile in food
contact materials and in food, London, HMSO (Food Surveillance
Paper No. 6).
US CONSUMER PRODUCT SAFETY COMMISSION (1978) Assessment of
acrylonitrile contained in consumer products. Washington, DC
(Final report, contract No. CPSC-C-77-0009. Task No. 1014K,
H.I.A. Economic Analysis).
US DEPARTMENT OF TRANSPORTATION (1974) Acrylonitrile. In:
CHRIS hazardous chemical data, Department of Transportation,
Coast Guard, C-G-446-2.
US EPA (1977) Optional procedures for classification of
pesticide uses by regulation. Fed. Reg., 42 : 44170, 44178.
US FDA (1977a) Acrylonitrile copolymer used to fabricate
beverage containers, final decision. Fed. Reg., 42 :
48528-48544.
US FDA (1977b) Substances for use only as components of
adhesives. US Code Fed. Regul., Title 21, parts 175.105,
175.300, 175.320, 176.170, 176.180, 177.1010, 177.1020,
177.1040, 177.1200, 177.2600, 178.3790, 180.22, pp. 438, 445,
452, 455, 465, 471-472, 482-483, 486, 488, 496, 501-504, 555,
596, 609-611.
USSR (1982) System of standards of work safety. The air of
the working zone. General sanitary-hygienic requirements (GOST
12.1.005-76). MAC of harmful substances in the air of working
zone (Suppl. to Tab. 4, SN 245-71, No 7-11). MAC of harmful
substances in the air of working zone (part 12-19), confirmed
in 1977-1982 years.
VAINIO, M. & MAKINEN, M. (1977) Styrene and acrylonitrile
induced depression of hepatic nonprotein sulfhydryl content in
various rodent species. Res. Commun. chem. Pathol. Pharmacol.,
17 (1): 115-124.
VAN BLADEREN, P.J., DELBRESSINE, L.P.C., HOOGETERP, J.J.,
BEAUMONT, A.H.G.M., BREIMER, D.D., SEUTTER-BERLAGE, F., & VAN
DER GEN, A. (1981) Formation of mercapturic acids from
acrylonitrile, crotononitrile, and cinnamonitrile by direct
conjugation and via an intermediate oxidation process. Drug
Metab. Disposition, 9 : 246-249.
VAN DOORN, R., BOS, R.P., LEIJDEKKERS, C.-M., WAGENAAS-ZEGERS,
M.A.P., THEUWS, J.L.G., & HENDERSON, P.T. (1979) Thioether
concentration and mutagenicity of urine from cigarette
smokers. Int. Arch. occup. environ. Health, 43 : 159-166.
VED BRAT, S. & WILLIAMS, G.M. (1982) Hepatocyte-mediated
production of sister chromatid exchanges in co-cultured cells
by acrylonitrile: evidence for extra cellular transport of a
stable reactive metabolite. Cancer Lett., 17 (2): 213-216.
VENITT, S., BUSHELL, C.T., & OSBORNE, M. (1977) Mutagenicity
of acrylonitrile (cyanoethylene) in Escherichia coli. Mutat.
Res., 45 (2): 283-288.
VERSCHUEREN, K. (1977) Handbook of environmental data on
organic chemicals, Van Nostrand Reinhold, pp. 78-81.
VISSARIONOVA, V.U., BONDAREV, G.I., STASENKOVA, K.P., &
ZEMLJANSKAJA, T.A. (1979) [Study of the effect of rations
with different protein content on rats following acrylonitrile
poisoning.] Vopr. Pitan., (1) : 36-40 (in Russian).
VOLKOVA, Z.A. & BAGDINOV, Z.M. (1969) [Industrial hygiene
problems in vulcanization processes of rubber production.]
Gig. i Sanit., 34 : 33-40 (in Russian).
WERNER, J.B. & CARTER, J.T. (1981) Mortality of United
Kingdom acrylonitrile polymerisation workers. Br. J. ind.
Med., 38 : 247-253.
WILLHITE, C.C. (1981a) Inhalation toxicology of acute
exposure to aliphatic nitriles. Clin. Toxicol., 18 (8):
991-1003.
WILLHITE, C.C. (1981b) Teratogenic effects of aliphatic
nitriles. Teratology, 23 : 317-323.
WILLHITE, C.C. & SMITH, R.P. (1981) The role of cyanide
liberation in the acute toxicity of aliphatic nitriles.
Toxicol. appl. Pharmacol., 59 : 589-602.
WILSON, R.H. (1944) Health hazards encountered in the
manufacture of synthetic rubber. J. Am. Med. Assoc., 124 :
701-703.
WILSON, R.H. & McCORMICK, W.E. (1949) Acrylonitrile: its
physiology and toxicology. Ind. Med., 18 : 223-245.
WILSON, R.H., HOUGH, G.V., & McCORMICK, W.E. (1948) Medical
problems encountered in the manufacture of American-made
rubber. Ind. Med., 17 : 199-207.
WISNIEWSKA-KNYPL, J.M., KNOBLOCH, J.M., JABLONSKA, J., & RUTA,
U. (1970) [Decrease of tissue respiration activity of
oxoglutarate dehydrogenase and level of sulfhydryl groups in
acute acrylonitrile intoxication in rats.] Med. Pr., 21 (6):
543-549 (in Polish).
WISNIEWSKA-KNYPL, J.M., TARKOWSKI, S., KLIMZAK, J., &
DRAMINSKI, W. (1978) [ Metabolism of vinyl chloride in the
liver of rats.] (in Polish) (Final report on the research
project of the Institute of Occupational Medicine in Lodz,
Poland).
WRIGHT, P.L. (1977) Studies on the metabolism of
acrylonitrile. Testimony before the FDA. In: Monsanto Co.
(1976b) Appendix A, pp. A-20 - A-28.
WYNDER, E.L. & HOFFMANN, D. (1967) Tobacco and tobacco
smoke. Studies in experimental carcinogenesis. New York,
Academic Press, p. 450.
YOSHIKAWA, H. (1968) [Toxicity of nitrile compounds. II.
Aliphatic nitriles.] Igak. Scibut., 77 (1): 1-4 (in Japanese).
YOUNG, J.D., SLAURER, R.W., & KARBOWSKI, R.J. (1977) The
pharmacokinetic and metabolism profile of 14 C-acrylonitrile
given to rats by three routes. Midland MI, USA, Toxicol. Res.
Lab. Dow Chem., (prepared for the Manufacturing Chem. Assoc.).
ZAYTSEVA, N.A., TOLSTOBROVA, S.A., & IL'IN, D.T. (1972)
[Chromatographic determination of unreacted monomers in
emulsions of acrylic copolymers.] Soviet Chem. Ind., 48 :
298-299 (in Russian).
ZELLER, H., HOFMANN, H.T., THIESS, A.M., & HEY, W. (1969)
[Toxicity of nitriles. Results of animal experiments and
industrial experiences during 15 years.] Zent. Arbeitsmed.
Arbeitsschutz, 19 (8): 226-238 (in German).
ZHURKOV, V.S., SHRAM, R.L. & DUGAN, A.M. (1983) [Analysis of
mutagenic activity of acrylonitrile.] Gig. i. Sanit., 1 : 71-72
(in Russian).
ZITTING, A., TENHUNEN, R., & SAVOLAINEN, H. (1981) Effects
of intraperitoneally injected acrylonitrile on liver, kidney
and brain. Acta pharmacol. toxicol., 49 : 412-415.
ZOTOVA, L.V. (1975a) [Working conditions in the production
of acrylonitrile and their effect on workers.] Gig. Tr. prof.
Zabol., No. 8 : 8-11 (in Russian).
ZOTOVA, L.V. (1976) [Toxic effect of acrylonitrile on
experimental animals following its absorption through the
skin.] Gig. i Sanit., 41 (10): 103-105 (in Russian).
