
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 114
DIMETHYLFORMAMIDE
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
First draft prepared by Dr. A. Bainova,
Institute of Hygeine and Occupational Health, Sofia, Bulgaria
World Health Orgnization
Geneva, 1991
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
WHO Library Cataloguing in Publication Data
Dimethylformamide.
(Environmental health criteria ; 114)
1.Dimethylformamide - adverse effects 2.Dimethylformamide - toxicity
I.Series
ISBN 92 4 157114 4 (NLM Classification: QV 633)
ISSN 0250-863X
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1991
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR DIMETHYLFORMAMIDE
1. SUMMARY AND EVALUATION, CONCLUSIONS, RECOMMENDATIONS
1.1. Summary and evaluation
1.1.1. General properties
1.1.2. Environmental transport, distribution, and transformation
1.1.3. Environmental levels and human exposure
1.1.4. Kinetics and metabolism
1.1.5. Effects on organisms in the environment
1.1.6. Effects on experimental animals and in vitro test systems
1.1.7. Effects on human beings
1.2. Conclusions
1.3. Recommendations
1.3.1. Safe handling
1.3.2. Further research
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Organoleptic properties
2.4. Analytical methods
2.4.1. Determination of DMF in workplace air
2.4.2. Determination of DMF and metabolites in biological media
2.4.3. Determination of DMF in soil, plants, and food
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
3.2. Man-made sources
3.2.1. Production and uses
3.2.1.1 Production
3.2.1.2 Uses
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Transport and distribution between media
4.1.1. Air
4.1.2. Water
4.1.3. Soil
4.1.4. Bioaccumulation
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Air
5.1.2. Water
5.1.3. Soil
5.2. General population exposure
5.3. Occupational exposure
5.3.1. Concentrations in the workplace air
5.3.2. Dermal exposure
6. KINETICS AND METABOLISM
6.1. Animal studies
6.1.1. Absorption
6.1.2. Distribution
6.1.3. Metabolic transformation
6.1.4. Elimination and excretion
6.1.5. Metabolic interaction between DMF and ethanol
6.2. Human studies
6.2.1. Absorption, distribution, metabolism, excretion
6.2.2. The influence of ethanol on DMF
metabolism in human volunteers
6.2.3. Biological monitoring of workers
6.2.3.1 Determination of NMF in the urine
6.2.3.2 N,N-dimethylformamide determination in the
expired air
6.2.3.3 Appraisal
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single exposures
8.2. Skin and eye irritation, sensitization
8.2.1. Skin irritation
8.2.2. Eye irritation
8.2.3. Sensitization
8.3. Repeated exposure
8.4. Specific organ toxicity
8.4.1. Liver
8.4.2. Gastrointestinal tract
8.4.3. Cardiovascular system
8.4.4. Kidney
8.4.5. Nervous system
8.4.6. Lungs
8.4.7. Haematopoietic system
8.4.8. Adrenals
8.4.9. Gonads
8.5. Developmental toxicity and reproduction
8.5.1. Developmental toxicity
8.5.1.1 Mouse
8.5.1.2 Rat
8.5.1.3 Rabbit
8.5.1.4 Appraisal
8.6. Mutagenicity and related end-points
8.6.1. In vitro studies
8.6.2. In vivo studies
8.6.3. Appraisal
8.7. Carcinogenicity
8.8. Induction of tumour cell differentiation
8.9. Mechanism of toxicity, mode of action
9. EFFECTS ON HUMAN BEINGS
9.1. General population exposure
9.2. Occupational exposure
9.2.1. Accidental poisoning
9.2.2. Long-term exposure
9.2.3. Epidemiological studies on carcinogenicity
9.2.4. Alcohol intolerance
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
RESUME ET EVALUATION, CONCLUSIONS, RECOMMANDATIONS
RESUMEN Y EVALUACION, CONCLUSIONES, RECOMENDACIONES
TASK GROUP MEETING ON ENVIRONMENTAL HEALTH CRITERIA FOR DIMETHYLFORMAMIDE
Members
Dr A. Aitio, International Agency for Research on Cancer, World Health
Organization, Lyon, France (Chairman)
Dr A. Bainova, Institute of Hygiene and Occupational Health, Sofia,
Bulgaria (Co-rapporteur)
Ms J. Favilla, Office of Toxic Substances, US Environmental Protection
Agency, Washington, USA
Dr G.L. Kennedy, Jr, Haskell Laboratory for Toxicology and Industrial
Medicine, EI du Pont de Nemours & Co., Newark, Delaware, USA (Co-
rapporteur)
Professor N.P. Misra, Department of Medicine, Gandhi Medical College,
Bhopal, India
Dr K. Morimoto, Division of Medical Chemistry, National Institute of
Hygienic Sciences, Tokyo, Japan (Vice-Chairman)
Dr C. Sadarangani, Petrochemical Industries Co.KSC., Ahmadi, Kuwait
Dr V. Scailteur, Procter and Gamble GMBH, Frankfurt, Federal
Republic of Germany
Dr Yu Hui Qin, Institute of Environmental Health Monitoring, Chinese
Academy of Preventive Medicine, Beijing, People's Republic of China
Observers
Dr R. Jäckh, European Chemical Industries Ecology and Toxicology
Centre, Brussels, Belgium
Secretariat
Dr R. Hertel, Fraunhofer Institute for Toxicology and Aerosol Research,
Hanover, Federal Republic of Germany
Dr K.W. Jager, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Secretary)
Dr P.G. Jenkins, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
NOTE TO READERS OF THE CRITERIA DOCUMENTS
Every effort has been made to present information in the criteria
documents as accurately as possible without unduly delaying their
publication. In the interest of all users of the environmental health
criteria documents, readers are kindly requested to communicate any
errors that may have occurred to the Manager of the International
Programme on Chemical Safety, World Health Organization, Geneva,
Switzerland, in order that they may be included in corrigenda, which
will appear in subsequent volumes.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Palais des
Nations, 1211 Geneva 10, Switzerland (Telephone no. 7988400 -
7985850).
ENVIRONMENTAL HEALTH CRITERIA FOR DIMETHYLFORMAMIDE
A WHO Task Group on Environmental Health Criteria for
Dimethylformamide, which met in Wolfsburg from 13 to 17 March 1989,
was organized by the Fraunhofer Institute for Toxicology and Aerosol
Research, Hanover, Federal Republic of Germany. The meeting was
sponsored by the Federal Government. Dr K.W. Jager of the IPCS opened
the meeting and welcomed the participants on behalf of the three
cooperating organizations of the IPCS (UNEP/ILO/WHO). The Task Group
reviewed and revised the draft criteria document and made an
evaluation of the risks for human health and the environment from
exposure to dimethylformamide.
The first and second drafts of this document were prepared by Dr
A. BAINOVA of the Institute of Hygiene and Occupational Health, Sofia,
Bulgaria. Dr K.W. JAGER of the Central Unit, International Programme
on Chemical Safety was responsible for the scientific content of the
document and Mrs M.O. HEAD of Oxford for the editing.
The efforts of all who helped in the preparation and finalization
of the document are gratefully acknowledged.
1. SUMMARY AND EVALUATION, CONCLUSIONS, RECOMMENDATIONS
1.1 Summary and evaluation
1.1.1 General properties
N,N-dimethylformamide (dimethylformamide, DMF, CAS 68-12-2)
is an organic solvent produced in large quantities throughout the
world. It is used in the chemical industry as a solvent, an
intermediate, and an additive. DMF is a colourless liquid with an
unpleasant slight odour that, nevertheless, has poor warning
properties. It is usually stable but, when it comes in contact with
strong oxidizers, halogens, alkylaluminium, or halogenated
hydrocarbons (especially in combination with metals), it may cause
fires and explosions. DMF is completely miscible with water and most
organic solvents. It has a relatively low vapour pressure.
Gas chromatographic procedures for determining DMF are available.
1.1.2 Environmental transport, distribution, and transformation
DMF is stable in ambient air, but may undergo microbial and algal
degradation in water. Adapted microorganisms and activated sludge
efficiently biodegrade DMF. As a result of its complete solubility in
water, DMF moves readily through soils and would not be expected to
accumulate in the food chain.
1.1.3 Environmental levels and human exposure
DMF does not occur naturally. There are few data concerning
environmental levels or the exposure of the general population to DMF.
Concentrations in the air in the range of 0.02-0.12 mg/m3 have been
found in residential areas, near industrial sites. DMF has rarely
been detected in the water of heavily industrialized river basins, and
then only at concentrations below 0.01 mg/litre.
Data are not available on the levels of DMF in soil, plants,
wildlife, and food.
Occupational exposure occurs via skin contact with DMF liquid and
vapour, and through the inhalation of vapour. Concentrations of 3-86
mg/m3 air have been detected, with peaks of up to 600 mg/m3, during
the repair or maintenance of machines. In a few unusual situations,
levels of up to 4500 mg/m3 have been reported.
1.1.4 Kinetics and metabolism
Toxic amounts of DMF may be absorbed by inhalation and through the
skin. Absorbed DMF is distributed uniformly. The metabolic
transformation of DMF takes place mainly in the liver, with the aid of
microsomal enzyme systems. In animals and human beings, the main
product of DMF biotransformation is N-hydroxymethyl- N-methylformamide
(DMF-OH). This metabolite is converted during gas chromatographic
analysis to N-methylformamide, which is itself (together with N-
hydroxy methylformamide and formamide) a minor metabolite. Thus,
metabolic studies and biological monitoring, urinary concentrations of
metabolites are measured and expressed as NMF, though DMF-OH is the
major contributor to this concentration. The determination of NMF/DMF-
OH in the urine may be a suitable biological indicator of total DMF
exposure.
In experimental animals, it has been demonstrated that DMF
metabolism is saturated at high exposure levels and, at very high
levels, DMF inhibits its own metabolism.
Metabolic interaction occurs between DMF and ethanol.
1.1.5 Effects on organisms in the environment
The effects of DMF on the environment have not been well studied.
The toxicity for aquatic organisms appears to be low.
1.1.6 Effects on experimental animals and in vitro test systems
The acute toxicity of DMF in a variety of species is low (in rats,
the oral LD50 is approximately 3000 mg/kg, the dermal LD50,
approximately 5000 mg/kg, and the inhalational LC50, approximately
10 000 mg/m3). It is a slight to moderate skin and eye irritant.
One study on guinea-pigs indicated no sensitization potential. DMF
can facilitate the absorption of other chemical substances through the
skin.
Exposure of experimental animals to DMF via all routes of exposure
may cause dose-related liver injury. Regeneration, after exposure has
ceased, has been demonstrated. In some studies, signs of toxicity in
the myocardium and kidneys have also been described.
DMF has not been shown to be toxic to the testes or ovaries of
rats and effects on fertility have not been demonstrated. DMF has
been found to be embryotoxic and a weak teratogen in rats, mice, and
rabbits. The rabbit was found to be the most sensitive species when
exposed via inhalation: teratogenic effects were observed at 1350
mg/m3 (450 ppm) and above, but not at 450 ppm (150 ppm). After dermal
exposure, a very low incidence of embryotoxic and teratogenic effects
was observed in some studies at dose levels of between 100 and 400
mg/kg per day.
DMF was generally found to be inactive, both in vitro and in
vivo, in an extensive set of short-term tests for genetic and related
effects.
No adequate long-term carcinogenicity studies on experimental
animals have been reported.
1.1.7 Effects on human beings
No adverse effects of DMF on the general population have been
clearly demonstrated.
Skin irritation and conjunctivitis have been reported after direct
contact with DMF.
After accidental exposure to high levels of DMF, abdominal pain,
nausea, vomiting, dizziness, and fatigue occur within 48 h. Liver
function may be disturbed, and blood pressure changes, tachycardia,
and ECG abnormalities have been reported. Recovery is usually
complete.
Following long-term repeated exposure, symptoms include headache,
loss of appetite, and fatigue. Biochemical signs of liver dysfunction
may be observed. Liver damage seems to occur only when the DMF
exposure level exceeds 30 mg/m3, in the absence of skin contact. This
airborne level corresponds to approximately 40 mg NMF/DMF-OH/g
creatinine in a post-shift urine sample.
Exposure to DMF, even at concentrations below 30 mg/m3, may cause
alcohol intolerance. Symptoms may include a sudden facial flush,
tightness in the chest, and dizziness, sometimes accompanied by nausea
and dyspnoea. They last from 2 to 4 h and disappear without
treatment.
There is limited evidence that DMF is carcinogenic for human
beings. An increased incidence of testicular tumours was reported in
one study, whereas another study showed an increased incidence of
tumours of the buccal cavity and pharynx, but not of the testes.
In two studies, which provide few details, an increased frequency
of miscarriages was reported in women exposed to DMF, among other
chemicals.
1.2 Conclusions
1. In view of the present uses of DMF, general population
exposure is probably very low.
2. DMF is readily absorbed through the skin as well as via
inhalation. Determination of urinary NMF/DMF-OH is a useful
means of estimating the total amount of DMF absorbed.
3. The risk of liver damage is low, when the level of DMF in
ambient air is kept below 30 mg/m3 and there is no skin
contact. A tentative value for the corresponding urinary
NMF/DMF-OH level in a post-shift sample is 40 mg/g creatinine.
4. DMF is embryotoxic and a weak teratogen in rats, mice, and
rabbits.
5. There is limited evidence of carcinogenicity of DMF for human
beings.
6. Available data indicate low environmental toxicity. It is
unlikely that bioaccumulation takes place.
1.3 Recommendations
1.3.1 Safe handling
1. Airborne concentrations should be maintained below 30 mg/m3
and skin contact should be prevented.
2. Urinary NMF/DMF-OH, as an index of total exposure, should be
monitored and maintained below 40 mg NMF/g creatinine in post-
shift samples. If this level is exceeded, action should be
taken to reduce exposure.
1.3.2 Further research
1. The possible carcinogenic effects of DMF in human beings
should be investigated by means of studies on experimental
animals and human populations.
2. More information is needed on the extrapolation of the
embryotoxicity and teratogenicity of DMF from animal studies
to human beings. Comparison of the kinetics of DMF in human
beings and animals would be valuable.
3. There is a need for more information on the mechanisms of
action and the relative potency of the metabolites of DMF in
both animals and human beings.
4. The relationships should be refined between: (a) urinary
metabolite concentrations and atmospheric exposure levels (in
the absence of skin contact), and (b) total dose via all
routes (as indicated by post-shift urinary NMF levels) and the
absence of hepatotoxicity.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Chemical structure: H3C O
\ //
N -- C
/ \
H3C H
Chemical formula: C3H7NO
Common name: dimethylformamide
Common synonyms: N,N-dimethylformamide,
DMF, DMFA, formdimethylamide
CAS registry number: 68-12-2
Relative molecular mass 73.1
Conversion factors: 1 ppm = 3 mg/m3
(at 20 °C) 1 mg/m3 = 0.33 ppm
2.2 Physical and chemical properties
Some physical properties of DMF (Eberling, 1980) are given in
Table 1. DMF is a colourless, organic solvent, free from suspended
matter. Technical DMF may contain impurities, depending on the
manufacturing and purification processes.
DMF is stable. It is hygroscopic and easily absorbs water from a
humid atmosphere and should therefore be kept under dry nitrogen. High
purity DMF, required for acrylic fibres, is best stored in aluminium
tanks. DMF does not change under light or oxygen and does not
polymerize spontaneously. Temperatures > 350 °C may cause
decomposition to form dimethylamine and carbon dioxide, with pressure
developing in closed containers (Farhi et al., 1968; US NIOSH, 1978).
In a fire involving DMF, or at temperatures > 350 °C, the toxic gases
and vapours consist primarily of dimethylamine and carbon monoxide.
DMF reacts readily with alkylaluminiums. Contact with carbon
tetrachloride and other halogenated hydrocarbons, particularly when in
contact with iron, as well as contact with strong oxidizing agents
(e.g., methylene diisocyanate, halogens, and permanganates) may cause
fires and explosions. In acidic solution (pH 3.8), DMF can be
nitrosated by sodium nitrate yielding small amounts of N-nitroso-
dimethylamine (0.04% at 37 °C and 1% at 90 °C).
Table 1. Physical properties of DMF
----------------------------------------------------------------------------
Property Value
----------------------------------------------------------------------------
Melting point (°C) - 60.5
Boiling point (°C) 153
Flash point (°C) 58 (closed cup)
67 (open cup)
Auto-ignition temperature (°C) 445
Density at 25 °C (specific gravity) (g/ml) 0.9445
Relative vapour density 2.51
Vapour pressure (mmHg/kPa)
at 20 °C 2.65/0.35
at 25 °C 3.7/0.48
at 60 °C 26/3.46
Vapour concentration in saturated air at
25 °C (mg/m3) 14 800
Explosive limits in air at 20 °C
(101 kPa/1 atm./%vol.)
lower limit 2.2 (70g/m3)
upper limit 16 (500 g/m3)
n- Octanol/water partitition coefficient 0.13
Solubility in water Miscible in all proportions
Solubility in organic solvents Miscible with ether, ketones,
aromatic hydrocarbons,
ethanol, but not with
aliphatic hydrocarbons
Dielectric constant at 20 °C 36.7
----------------------------------------------------------------------------
2.3 Organoleptic properties
DMF is a colourless liquid with an unpleasant taste and an
ammonia-like, specific odour that has poor warning properties (US
NIOSH, 1978). The odour threshold for the most sensitive people
ranges from 0.12 to 0.15 mg/m3 (Odoshashvili, 1963; Lazarev & Levina,
1976; Amster et al., 1983; Clay & Spittler, 1983). For some people,
the odour threshold has been reported to be as high as 60 mg/m3
(Leonardous et al., 1965).
2.4 Analytical methods
2.4.1 Determination of DMF in workplace air
Colorimetric methods, based on the development of a red colour
after the addition of hydroxylamine chloride as alkaline solution, are
not specific (Farhi et al., 1968). Lauwerys et al. (1980) described a
simple spectrophotometric method for measuring DMF vapour
concentrations. Gas-liquid chromatography is now the method of choice
(Kimmerle & Eben, 1975a; US NIOSH, 1977; Muravieva & Anvaer, 1979;
Brugnone et al., 1980a; Muravieva, 1983; Stransky, 1986). Detector
tubes, certified by US NIOSH, or other direct-reading devices
calibrated to measure DMF (Krivanek et al., 1978; US NIOSH, 1978) can
be used. High-performance liquid chroma tographic analysis (Lipski,
1982) can also be used. Mass spectrometric analysis for DMF in
expired air has been described by Wilson & Ottley (1981), with a lower
limit of detection of 0.5 mg/m3.
2.4.2 Determination of DMF and metabolites in biological media
Barnes & Henry (1974) developed a method for the gas
chromatographic determination of NMF ( N-methylformamide) (thought to
be the principal metabolite of DMF) in urine at concentrations of
between 5 and 500 µg/litre by either direct injection of the urine or
of urine extracts. Methods for simultaneous gas chromatographic
determination of DMF and NMF in the same blood sample (0.2 ml) and of
DMF, NMF, and formamide in 1 ml 24-h urine have been published by
Kimmerle & Eben (1975a) and Muravieva & Anvaer (1979). Similar
techniques were reported by Krivanek et al. (1978), Sanotsky et al.
(1978), and Lauwerys et al. (1980), involving primarily the
determination of NMF in the urine (Table 2).
2.4.3 Determination of DMF in soil, plants, and food
Analytical methods for the determination of DMF in these media
have not been described.
Table 2. Analytical methods for the determination of DMF, NMF (DMF-OH),
and formamide (NMF-OH) in urine, blood, and other biological tissues
-------------------------------------------------------------------------------------------------------------------
Biological Analytical method Detection limits Reference
tissue DMF NMF (DMF-OH) Formamide
(NMF-OH)
-------------------------------------------------------------------------------------------------------------------
Urine gas chromatography 0.5 mg/litre Barnes & Henry (1974)
gas chromatography 1.5 mg/litre 1 mg/litre 3.5 mg/litre Kimmerle & Eben (1975a)
gas chromatography 0.1 mg/litre Krivanek et al. (1978)
gas chromatography 1.5 mg/litre 3 mg/litre 10 mg/litre Muravieva & Anvaer (1979)
gas chromatography 0.8 mg/litre Mráz et al. (1987)
gas chromatography Lauwerys et al. (1980)
Blood gas chromatography 1 mg/litre 1.5 mg/litre Kimmerle & Eben (1975a)
gas chromatography 0.03 mg/litre 0.3 mg/litre 10 mg/litre Sanotsky et al. (1978)
gas chromatography 1.5 mg/litre 3 mg/litre Muravieva & Anvaer (1979)
gas chromatography 0.4 mmol/litre Lundberg et al. (1983)
Livera gas chromatography 0.2 mmol/kg Lundberg et al. (1983)
Kidney 0.6 mmol/kg
Brain 0.3 mmol/kg
Adrenals 0.9 mmol/kg
-------------------------------------------------------------------------------------------------------------------
a Tissue homogenate.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
DMF does not occur naturally.
3.2 Man-made sources
3.2.1 Production and uses
3.2.1.1 Production
DMF was first synthesized in 1893 from carbon monoxide and
dimethylamine (Kennedy, 1986). It is usually manufactured by a one-
stage reaction of carbon monoxide with dimethylamine:
catalyst
CO + (CH3)2NH ----> (CH3)2
or by a two-stage reaction with methylformate and dimethylamine
(Eberling, 1980):
catalyst
CO + CH3OH ----> HCOOCH3
HCOOCH3 + (CH3)2NH ----> HCON(CH3)2 + CH3OH
DMF can also be manufactured from carbon dioxide, hydrogen, and
dimethylamine, in the presence of halogen-containing transition metal
compounds.
DMF is shipped in tank trucks and tank containers, and is also
marketed in 200-kg steel drums. The materials for DMF handling and
storage are usually (carbon) steels, austenitic steels, and aluminium.
Seals and pipelines should be made of polytetrafluoro-ethylene,
polyethylene, or polypropylene of high relative molecular mass.
Ethylene-propylene rubber can also be used.
The world production capacity of DMF is about 225 x 103
tonnes/year (Eberling, 1980). Production in the USA in 1979 was
15 000 tonnes. In 1980, NIOSH estimated that 69 000 US workers, in
various occupations in 25 major industries, were exposed to DMF.
Data are not available on losses of DMF into the environment and
into the ambient air during its production and use.
DMF can be recovered from the air by scrubbing with water and from
aqueous solution by distillation.
3.2.1.2 Uses
DMF is a universal industrial solvent, because of its water
solubility, organic nature, and high dielectric constant. The main
use (65-75%) of DMF is as solvent for acrylic fibres and
polyurethanes; 15-20% is used in the production of pharmaceutical
products (Eberling, 1980).
DMF is used as:
- a spinning solvent for synthetic textiles, based on
polyacrylonitrile or cellulose triacetate;
- a resin, rubber, and polymer solvent;
- a solvent for dyes and pigments for use with textiles, wood,
leather, films, paper, and plastics;
- a solvent in pesticide formulations;
- a booster solvent in coating, printing, and adhesive
formulations;
- a chemical intermediate, catalyst, and reaction medium in
chemical manufacturing and the pharmaceutical industry;
- a solvent in the production of polyurethane and other synthetic
leathers, or synthetic rubber;
- a selective absorption and extraction solvent for recovery,
purification, absorption, separation, and desulfurization of
non-paraffinic compounds from paraffin hydrocarbons;
- in the manufacture of paint stripper components for the removal
of vinyl films, epoxy coatings, and varnish finishes; in the
production of wire enamels, based on polyamides, polyurethanes,
and other polymers;
- in the pigment and dye industry to improve dyeing properties;
- a crystallization solvent in the pharmaceutical industry;
- a solvent for carbonaceous deposit cleaning applications for
high-voltage capacitors;
- an oil sludge dispersing agent;
- an anti-stall gasoline additive;
- a laboratory solvent and as a solvent for the extraction of
biological material in chemical analysis.
DMF (itself, or as a component in consumer products) is not
generally available to the general population (Farhi et al., 1968;
Bainova, 1980; Lundberg, 1982; Tanaka & Utsunomiya, 1982; Barral-
Chamaillard & Rouzioux, 1983; Kennedy, 1986; US EPA, 1986).
Because of its hepatotoxicity, DMF is not used as a solvent in
pharmaceutical or cosmetic products.
DMF has been approved by the US FDA as a component of adhesives,
for use in the packaging, transport, or storage of food.
DMF is present in some registered pesticides as an inert solvent.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Transport and distribution between media
4.1.1 Air
DMF is stable in air. Concentrations in ambient air are related
to its industrial use. No data have been found on the rates of
reaction of DMF with hydroxyl radicals, ozone, or other atmospheric
pollutants. Darnall et al. (1976) reported DMF to have a half-life of
9.9 days in a polluted atmosphere. In oxidizing smog-chamber studies
(Laity et al., 1973; Farley, 1977; Sickles et al., 1980), no
photochemical oxidation of DMF occurred. The ultraviolet (UV)
absorption spectrum for DMF indicated no absorption > 290 nm
(Grasselli, 1973), showing that no photodegradation should be expected
in the environment. The water solubility of DMF suggests that it
should be easily removed from air by rainfall.
The DMF levels in the air of working environments depend on the
rate of usage, technology, and industrial hygiene practices (Aldyreva
& Gafurov, 1980; Brugnone et al., 1980a; Lauwerys et al., 1980;
Yonemoto & Suzuki, 1980; Koudela & Spazier, 1981; Taccola et al.,
1981; Paoletti & Iannaccone, 1982; Tomasini et al., 1983; Sala et al.,
1984; Kennedy, 1986; US EPA, 1986).
4.1.2 Water
According to Eberling (1980), aqueous solutions of DMF undergo
slight hydrolysis at neutral pH. After 120 h of refluxing, only 0.17%
of a 50% solution was hydrolysed. The hydrolysis of DMF is
accelerated by acids and alkalis. No data about the oxidation or
photodegradation of DMF are available.
DMF is susceptible to biodegradation by activated sludges, though
an acclimation period is usually required. Water from the Vistula
River was reported to biodegrade DMF, as was an unspecified bacterial
culture isolated from soil exposed to petroleum + petroleum products
(Chromek et al., 1983). Dojlido (1979) reported that, in an activated
sludge system, 100% of the 70 mg DMF/litre was degraded in 38 days.
In a river die-away test, under light aeration conditions, 28 mg
DMF/litre were degraded in the water with a lag time of 2 days. The
lag time decreased when acclimatized microorganisms were used in the
test.
Chromek et al. (1983) determined the changes in respiration rate
in algal cultures of Scenedesmus quadricauda, after treatment with
1000 mg DMF/litre. DMF degradation via dimethylamine to ammonia
occurred within 3 days. The rate of DMF degradation to ammonia
depended on the degree of adaptation of the heterotrophic mixed
cultures (activated sludge) and varied between 35 and 70 mg/g per h.
The dimethylamine decomposition rate was about 25 mg/g per h.
Gubser (1969) reported that, in a continuous-flow activated sludge
system, DMF was reduced by 90-100% within 10 days at concentrations of
20 and 50 mg/litre, and within 28 days at a concentration of 81
mg/litre. Chromek et al. (1983) found that the alga Scenedesmus
quadricauda in cultures was able to degrade DMF to dimethylamine and
ammonia in 3 days. The DMF concentration tested was about 1000
mg/litre; this corresponds to values seen in industrial effluents.
After the formation of an adaptive enzymatic system, the DMF
concentration decreased at a constant rate of about 40 mg/g per h.
Adaptation of the culture resulted in an enhanced rate of degradation.
Pseudomonas sp., Pseudomonas sp.II, and Vibrio aeromonas, isolated
from sewage effluents, degraded DMF (US EPA, 1986). Begert (1975)
proposed several series of aerobic bacterial systems, which eliminated
more than 90% of the DMF in the sewage from a chemical textile plant.
The complete water solubility and low n-octanol/water partition
coefficient (Table 1) of DMF suggest that adsorption on sediments in
water is not an important environmental process. DMF is not expected
to evaporate from the aquatic environment to any significant rate
because of its volatility and high water solubility (US EPA, 1986).
4.1.3 Soil
Contamination of soil with DMF may occur through spillage or
leakage during its production, transport, storage, or use. DMF's high
solubility in water and its low n-octanol/water partition coefficient
show that it can seep down into soil and potentially into ground
water. DMF was completely biodegraded by a bacterial culture,
isolated from soil that had been in contact with low levels of
petroleum and petroleum products for several years. This culture was
used for the purification of waste waters containing 250 mg DMF/litre
in an aerated tank; the addition of activated sludge for 18 h resulted
in the biodegradation of 94% of the DMF (Romadina, 1975).
4.1.4 Bioaccumulation
Sasaki (1978) found that DMF did not bioaccumulate in the carp;
the low partition coefficient was considered to be the explanation.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
Air-monitoring for DMF was conducted at distances ranging from 25
to 300 m from an artificial fibre plant in the USSR. Odoshashvili
(1963) found that DMF levels were only below the proposed allowable
limit of 0.03 mg/m3 at 300 m from the plant.
Residents of private homes within a 0.5 mile radius of a chemical
waste recycling site complained of unpleasant odours. DMF was found
to be the major atmopheric contaminant in concentrations of up to 0.12
mg/m3, but it originated primarily from the industrial sites nearby
and not from the soil or the waste site (Clay & Spittler, 1983).
Amster et al. (1983) studied another abandoned chemical waste facility
in the USA, in response to complaints from nearby residents about
odour, with similar results, i.e., air levels of 0.024-0.15 mg DMF/m3
originated from a neighbouring industry.
5.1.2 Water
Very low concentrations of DMF were found in effluent waters from
sewage-treatment plants or municipal sewage-treatment systems (US EPA,
1986). A concentration of 2 µg/litre was measured in a sample taken
from a sewage-treatment plant on the western shore of Lake Michigan.
Ewing et al. (1977) examined 204 water samples from 14 heavily
industrialized river basins in the USA. DMF was found in only one
sample, at a concentration of 2 µg/litre. Samples of 63 effluent and
22 intake waters from various chemical manufacturers were collected in
areas throughout the USA (Perry et al., 1979) and analysed for organic
pollutants. Over 570 compounds were tentatively identified, of which
33 were important pollutants. DMF was detected once at a concentration
< 10 µg/litre.
Chromek et al. (1983) reported that DMF concentrations of
approximately 1000 mg/litre were found in effluents from the
production of synthetic leather.
5.1.3 Soil
No data are available on DMF levels in soil and plants.
5.2 General population exposure
No data are available on exposure of the general population to
DMF.
However, DMF may be a component of coatings, adhesives, engine
degreasing agents, and photographic developers for consumer use.
Exposure through the use of DMF in food processing, food
packaging, and pesticides may occur, but data are not available.
5.3 Occupational exposure
5.3.1 Concentrations in the workplace air
DMF is not highly volatile and is manufactured in closed systems.
Data on DMF concentrations in plants manufacturing DMF are not
available.
Concentrations of DMF in the workplace air in various industrial
applications, are listed in Table 3. In most cases, the mean
concentrations are less than 30 mg/m3, but certain jobs, particularly
those involving mixing operations, result in higher concentrations.
The cleaning of equipment or tanks that have contained DMF can involve
exposure to levels of up to 147 mg/m3. Kang-de & Hui-lan (1981)
reported an unusually high DMF concentration of 4525 mg/m3 during
repairs following an accident. The ranges of concentration reported
vary considerably, but the time of sampling is not generally
specified. The highest values have been found during repair or
maintenance work, in accidents, and where batch sampling (opening the
reactor system) was being conducted.
5.3.2 Dermal exposure
The relative importance of dermal exposure to liquid or vapour DMF
(versus inhalation of vapour) was studied by Aldyreva & Gafurov
(1980), Lauwerys et al. (1980), Bortsevich (1984), and Sala et al.
(1984).
Lauwerys et al. (1980) studied 7 workers from a spinning mill in a
polyacrylic fibre factory. During the first week, the workers wore
gloves and during the second week, a barrier cream was applied twice
each day to the hands and forearms. On the first day of the third
week, the skin was not protected, but the workers were equipped with
self-contained breathing equipment. The average N-methylformamide
(NMF) concentration in the urine at the end of the day, when there was
no dermal protection, was about 3 times higher than that during the
first week. Eight hours after the start of exposure without skin
protection, one worker reported abdominal pains; a second worker had
to stop working 48 h later because of severe gastric pain. Hence,
from the second day, the workers were requested to resume wearing
their impermeable gloves. Urinary NMF concentrations returned to the
values found during the first week. This convinced the workers of the
need to avoid all contact with the DMF solution and to use protective
gloves correctly. The study also showed that gloves were more
effective than silicone or glycerol barrier creams in preventing skin
absorption of DMF.
In a new plant producing artificial leather, Aldyreva et al. (1980)
found DMF in nearly all washings from the operators' hands.
According to Bortsevich (1984), the quantity of DMF absorbed
through the skin might be twice the quantity taken up through
inhalation. The author reported significant DMF concentrations in the
skin washings from the palms of hands, shoulders, back, thighs, and
abdomen. Part of the dermal uptake of DMF may result from its
presence in the air and part from contaminated clothing.
Table 3. DMF concentrations in air in various industrial applications
----------------------------------------------------------------------------------------------------------------------------
Factory product Job description Mean DMF Range of DMF Reference
concentrations concentrations
(mg/m3) (mg/m3)
----------------------------------------------------------------------------------------------------------------------------
Polyacrylic fibres spinning line - maintenance - 1-46.6 Lauwerys et al. (1980)
Artificial leather various (pre-improvement) - 0-60 Aldyreva & Gafurov (1980)
various (post-improvement) 1/3 samples below
detection
production 5.3 1.9-8.3 Brugnone et al. (1980a)
production (highest in mixing) > 30 < 150 Taccola et al. (1981)
production - normal 4.2-66 Paoletti & Iannaccone
opening reactor - < 549 (1982)
maintenance of rollers < 120
production - mixing > 34 Tomasini et al. (1983)
soaking and drying 12.1 (± 40.2) - Bortsevich (1984)
coating and colouring 32.3 (± 98.7) -
mixing resins 22.7 and 85.2 2-117 Sala et al. (1984)
spreading "transfer" system 33.8 8-72
spreading "coagulate" system 14 2-49
tank cleaning 86.3 9-147
machine cleaning 24.1 12-35
Surface-treating handlers 0-15.4 - Yonemoto & Suzuki (1980)
agents
Solvents - often > 30 peak 105-600 Lyle et al. (1979)
Synthetic rubber repairing, accidents, - 9.5-4525 Kang-de & Hui-lan (1981)
sampling with system opened,
extracting < 10 -
Unspecified chemicals unspecified - 50-250 Koudela & Spazier (1981)
----------------------------------------------------------------------------------------------------------------------------
Sala et al. (1984) reported that the total daily excretion of NMF
(DMF-OHa and NMF) in the 24-h urine samples of a worker who usually
cleaned the tanks in a factory where artificial polyurethane leathers
were produced, was 95-725 mg or 35-390 mg NMF/litre. This is higher
than would have been expected in a subject with a mean airborne
exposure of 100 mg DMF/m3. The worker usually operated without using
any personal protection.
Penetration through various glove materials has been studied.
Breakthrough time was > 480 min for butyl rubber, 6-66 min for
neoprene, and 5-22 min for polyvinylchloride and polyvinyl alcohol
(Henry & Schlatter, 1981).
Similarly, Sansone & Tewari (1978) showed that < 0.1% DMF passed
through neoprene gloves, 0.1-1% through natural rubber gloves, 1-10%
through nitrile gloves, and > 10% through poly-vinylchloride gloves,
in half an hour.
---------------------------------------------------------------------------
a DMF-OH = N-hydroxymethyl- N-methylformamide.
6. KINETICS AND METABOLISM
6.1 Animal studies
6.1.1 Absorption
Sanotsky et al. (1978) determined DMF concentrations in the blood
of rats, 24 h after the oral administration of 200-4000 mg DMF/kg body
weight and found mean blood levels ranging from 40 to 1870 mg/litre.
DMF is readily absorbed via inhalation and dermally. Maximal blood
and tissue concentrations were observed in rats up to 3 h after
exposure to 438 and 6015 mg DMF/m3 (Kimmerle & Eben, 1975a) or to 1690
and 6700 mg DMF/m3 (Lundberg et al., 1983). According to Massmann
(1956), at least 0.8 ml of 100% DMF was absorbed through 14 cm2 of
exposed skin of the tails of rats in the course of 8 h, which is
equivalent to an absorption rate of about 57 mg/cm2 per 8 h.
6.1.2 Distribution
Twenty-four hours after an ip dose of 14C-DMF in male rats, about
4% of the radioactivity was recovered in the blood, less than 1% in
the brain, heart, lungs, stomach, intestines, spleen, and kidneys, and
1-3% in the liver, adipose tissue, and muscles (Scailteur & Lauwerys,
1984).
Kimmerle & Eben (1975a) studied DMF and NMF (DMF-OH)a
concentrations in the blood of rats and dogs after single and repeated
respiratory exposure. At the highest airborne concentration (6015
mg/m3), DMF was still detectable in the blood of male rats up to 2
days after the end of a 3-h exposure. At lower concentrations, DMF
levels in the blood decreased rapidly (Table 4). After 3 h exposure
to 63 mg/m3 or 6 h exposure to 87 mg/m3, similar levels of NMF were
found in the blood at the end of the periods of exposure, but no NMF
was detectable 3 h after the end of exposure. Only after a 3-h
exposure to a very high concentration (6015 mg/m3) did NMF levels in
blood continue to increase for the 2 days following exposure (Table 4).
Blood concentrations of DMF in male dogs also decreased rapidly
following a 6-h single exposure. However, NMF could be detected in
the blood at higher concentrations and for a longer period of time
after exposure (Table 5).
---------------------------------------------------------------------------
a DMF = dimethylformamide;
DMF-OH= N-hydroxymethyl- N-methylformamide;
NMF = N-methylformamide;
NMF-OH = N-hydroxymethylformamide;
F = formamide.
Table 4. Concentrations of DMF and NMF in the blood of male rats after
a single inhalation exposure
---------------------------------------------------------------------
Hours after Inhalation exposure to DMF (3 h)
end of 6015 mg/m3 438 mg/m3 63mg/m3
exposure --------------------------------------------------------
DMF NMF DMF NMF DMF NMF
(mg/litre) (mg/litre) (mg/litre)
---------------------------------------------------------------------
0 1190 11.5 25.7 7.3 NDa 2.5
0.5 1166 12.1 21.7 6.9 1.9
1 1329 15.8 20.7 10.2 1.2
2.5 1275 20.9 10.5 11.8 0.5
4.5 1322 25.9 1.8 10.6 ND
21 824 50.3
45 46 84.3
-----------------------------------------------------------------------
a ND = not detectable.
When male rats were exposed to 1050 ± 126 mg/m3, 6 h/day, for 5
days, the levels of DMF and NMF in the blood returned to ND levels
before each consecutive exposure. However, when male dogs were
exposed to 177 ± 36 mg NFM/m3, 6 h/day, for 5 days, NMF accumulated in
the blood (10 mg/litre, 2 h after the first exposure; 30 mg/litre, 3 h
after the fifth exposure). In contrast, in female dogs, exposed to 69
± 12 mg/m3, 6 h/day, for 5 days, the daily NMF concentration in the
blood remained almost constant, returning to a low level of about 1-
1.5 mg/ml, before each new exposure.
Table 5. Concentrations of DMF and NMF in the blood of male dogs
after a single inhalation exposure
------------------------------------------------------------------------
Hours after Inhalation exposure to DMF (6 h)
end of 513 ± 114 mg/m3 60 ± 9 mg/m3
exposure ------------------------------------------------------------
DMF NMF DMF NMF
(mg/litre) (mg/litre) (mg/litre) (mg/litre)
------------------------------------------------------------------------
0 51.6 9.7 7.4 10.5
0.5 54.9 13.7 5.6 11.9
1 47.7 14.9 4.1 12.1
2 39.4 17.4 0.7 13.3
3 38.7 23.6 NDa 13.3
27 3.1
------------------------------------------------------------------------
a ND = not detectable.
Finally, in male and female dogs exposed to 63 ± 9 mg/m3, 6 h/day,
for 5 days a week over 4 weeks, DMF levels went back to ND before each
new exposure. There was no accumulation of NMF. The weekly average
concentrations of NMF were slightly higher in males than in females.
Lundberg et al. (1983) measured DMF and NMF concentrations in
various organs of the rat after a single 4-h inhalation exposure to
1690 or 6700 DMF mg/m3; DMF and NMF were distributed uniformly
throughout the tissues (Tables 6 and 7). Blood levels of NMF (DMF-OH)
for the first 3 h following exposure were lower after exposure to 6700
mg/m3 than after exposure to 1690 mg/m3 (Table 6 and 7). The authors
suggested that high DMF doses inhibit DMF biotransformation. This
interpretation is supported by the results of Kimmerle & Eben (1975a),
who reported that NMF concentrations in the blood (11-21 mg/litre)
during the first 3 h following a 3-h exposure to 6015 mg DMF/m3 were
lower than those following a 6-h exposure to 513 mg/m3.
6.1.3 Metabolic transformation
After iv injection of DMF in cats, Massman (1956) found that only
a small amount of the compound was excreted unchanged in the urine.
He could not detect any hydrolysis of the amide to dimethylamine and
formic acid. Barnes & Ranta (1972) identified a urinary metabolite,
NMF, in the urine of rats treated with sc injections of DMF.
After single or repeated respiratory exposure to DMF, Kimmerle &
Eben (1975a) identified NMF and formamide in the urine of rats and
dogs. The authors proposed a model of successive N-demethylations of
DMF.
In in vitro studies, Barnes & Ranta (1972) measured a low level
of formaldehyde, when rat liver homogenates were incubated with DMF in
the presence of an NADPH-generating system. They concluded that DMF
was N-demethylated in the liver with the help of microsomal enzymes.
This was in agreement with previous in vivo findings.
Later on, however, it was shown that the incubation of various rat
tissues with DMF did not release formaldehyde in vitro. Furthermore,
neither formaldehyde nor any other monocarbon derivative (CO, CH3OH,
CH4, HCOOH) was detected, when DMF was incubated with fortified liver
microsomes. However, a metabolite determined by gas chromatography
(GC) was identified as NMF. This led to speculation that DMF-OH was a
probable metabolite of DMF that was broken down (demethylated) to form
NMF during gas chromatographic analysis (Scailteur et al., 1984).
Brindley et al. (1983) indicated that a stable precursor of
formaldehyde was present in the urine of mice treated with DMF.
Direct evidence that DMF-OH is a metabolite of DMF was only
obtained by investigating urine samples of animals treated with DMF.
DMF-OH was identified in rat urine using HPLC combined with chemical
ionization mass spectrometry (Scailteur et al., 1984) and in mouse
urine high-field H-NMR spectroscopy and radio thin layer
chromatography (Kestell et al., 1986).
Table 6. Concentrations of DMF and NMF in rat tissues after a 4-h exposure to 6700 mg DMF/m3
------------------------------------------------------------------------------------------------------------------------
Hours after Blood Liver Kidney Brain Adrenals
end of (mg/litre) (mmol/kg)
exposure DMF NMF DMF NMF DMF NMF DMF NMF DMF NMF
------------------------------------------------------------------------------------------------------------------------
0 965 < 24 9.8 < 0.3 11.0 0.8 11.4 0.4 8.6 < 1.0
3 1089 < 24 11.7 0.5 12.8 < 0.6 2.7 < 0.3 8.8 < 1.0
6 950 71 10.1 0.7 11.5 1.3 10.1 0.5 9.0 1.2
20 263 295 2.6 1.9 3.1 2.3 1.5 2.1 1.9 1.9
48 < 29 < 24 < 0.2 < 0.3 < 0.6 < 0.6 < 0.3 < 0.3 < 0.9 < 1.0
------------------------------------------------------------------------------------------------------------------------
Table 7. Concentrations of DMF and NMF in rat tissues after a 4-h exposure to 1690 mg DMF/m3
------------------------------------------------------------------------------------------------------------------------
Hours after Blood Liver Kidney Brain Adrenals
end of (mg/litre) (mmol/kg)
exposure DMF NMF DMF NMF DMF NMF DMF NMF DMF NMF
------------------------------------------------------------------------------------------------------------------------
0 373 41 2.8 0.5 3.1 0.9 3.1 0.52 .1 < 1.0
3 205 47 1.8 0.5 2.8 0.9 2.0 0.6 1.6 < 1.0
6 197 47 1.8 0.6 2.0 1.2 1.9 0.7 1.5 1.0
20 < 29 < 24 < 0.5 < 0.3 < 0.6 0.6 < 0.3 < 0.3 < 0.9 < 1.0
------------------------------------------------------------------------------------------------------------------------
Using GC combined with mass spectrometry, Scailteur & Lauwerys
(1984a,b) showed that besides the major metabolite, DMF-OH, a small
amount of NMF could also be identified in the urine of DMF-treated
rats. This was confirmed by Kestell et al. (1986) using H-NMR
spectroscopy. Thus when urine samples are analysed after DMF
administration, using gas chromatography, the combination of DMF-OH +
NMF is determined as NMF and the combination of hydroxymethylformamide
(NMF-OH) + formamide, as formamide (Scailteur et al., 1984). Using
GC/MS, Scailteur & Lauwerys (1984a,b) could not identify NMF in the
urine of DMF-OH-treated rats. The authors therefore suggested that
NMF is not a product of DMF-OH biotransformation, but is directly
formed from DMF.
Hepatectomy markedly reduced the in vivo transformation of DMF
into DMF-OH, confirming that the liver is the main site of metabolic
degradation (Scailteur et al., 1984).
In parallel with the hypothesis of Lundberg et al. (1983) that
high doses of DMF could inhibit its biotransformation, Scailteur et
al. (1984) showed that the urinary excretion of metabolites (DMF-OH +
NMF, NMF-OH + F) was the same, following 2 daily ip injections of 0.5
mg/kg body weight or 2 daily ip injections of 1 ml/kg.
Scailteur & Lauwerys (1984a) studied the mechanism of the in vitro
and in vivo oxidative biotransformation of DMF. Addition of catalase
or superoxide dismutase to liver microsomes, incubated with DMF,
decreased the level of DMF-OH production. in vitro and in vivo, DMF
transformation was also diminished in the presence of radical
scavengers, such as dimethylsulfoxide, tert-butyl alcohol,
hydroquinone, and trichloroacetonitrile. Addition of IRON/EDTAa to
microsomes, incubated with DMF in vitro, stimulated DMF oxidation.
The authors concluded that the metabolic transformation of DMF to DMF-
OH involved hydroxyl radicals.
Metabolites, other than DMF-OH (NMF) and NMF-OH (F), appear to be
formed from DMF. Indeed, about 20% of an ip dose was recovered in the
urine of mice (Brindley et al., 1983) and rats (Scailteur & Lauwerys,
1984a,b), as unidentified chemicals.
Kestell et al. (1986, 1987) identified low levels of methylamine
and dimethylamine in the urine of DMF-treated mice (about 4%).
A metabolic transformation scheme is presented in Fig. 1, based on
the above data.
---------------------------------------------------------------------------
a EDTA = ethylene diamine tetra acetate.
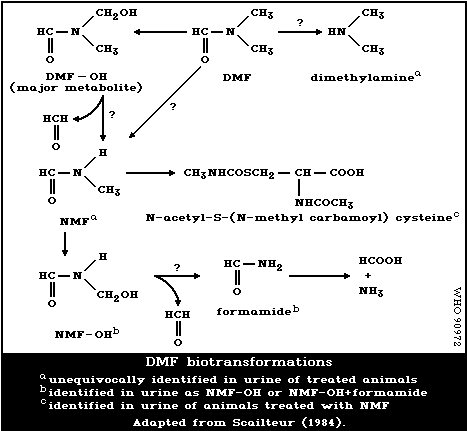 6.1.4 Elimination and excretion
The transformation and excretion of DMF in rodents is rapid. When
14C-labelled DMF in 0.1 ml saline was injected ip at 6.8 mmol/kg body
weight in mice, about 83% of the radioactivity was recovered in the
urine within 24 h following injection. Of this amount, only 5% was
unchanged DMF and 56% was C-hydroxylated or N-demethylated
derivatives. About 18% of the dose was excreted in the form of
unknown chemicals (Brindley et al., 1983).
Similarly, 24 h after ip injection of 400 mg DMF/kg body weight in
0.2 ml saline in mice, about 56% of the dose was excreted in the urine
as DMF-OH and only 5% as unchanged DMF (Kestell et al., 1986).
Within 72 h of an ip administration of 1 ml 14C-DMF/kg to male
or female rats, 70% of the injected radioactivity was recovered in the
urine. Approximately 15% was excreted as unchanged DMF, 50% as DMF-OH
(NMF), and 5% as NMF-OH (F). About 20% was excreted as unidentified
metabolite(s) (Scailteur & Lauwerys, 1984a,b).
After oral exposure to DMF (40-2000 mg/kg), Sanotsky et al. (1978)
determined that about 6% of the dose was excreted in 24 h.
The elimination of DMF, NMF (DMF-OH), and formamide (NMF-OH) was
measured after single or repeated inhalation exposure in rats and dogs
(Kimmerle & Eben, 1975a). Twenty-four hours after a single exposure to
63 mg NMF/m3 for 3 h, or 87 mg/m3 for 6 h, no NMF was found in the
urine of male rats. Under the same conditions, exposure to 513 mg/m3
for 6 h or to 6015 mg/m3 for 3 h led to excretion of 4 mg and 14 mg
NMF (DMF-OH), respectively, during the 24 h following the start of
exposure. Only in the last case was DMF also measured in the urine.
After repeated exposure of male rats to DMF (1050 mg/m3, 6 h/day, for
5 days), urinary levels of NMF (DMF-OH) remained practically constant
for the first 3 days, then slightly decreased from the fourth day of
exposure. Excretion of F (NMF-OH) was much lower than excretion of NMF
(DMF-OH).
While no accumulation of urinary NMF (DMF-OH) was observed in male
rats, male dogs exposed to 177 mg DMF/m3 (6 h/day for 5 days) excreted
increasing concentrations of NMF (DMF-OH) (36 mg/24 h after the first
inhalation; 87 mg/24 h after the 4th inhalation). Urinary excretion
of formamide (NMF-OH) varied between 10 and 20 mg/24 h. Excretion of
unchanged DMF was very low (< 2 mg/24 h). However, in female dogs
exposed to 69 mg/m3 (6 h/day for 5 days), no urinary accumulation of
NMF or F was observed. When male or female rats were exposed for 4
weeks to 63 mg/m3 (6 h/day, 5 days per week), NMF and F concentrations
in the urine remained practically constant during the exposure period.
Male dogs generally excreted slightly higher levels of metabolites
than female dogs (Kimmerle & Eben, 1975a).
In rats treated with repeated, high, ip doses of DMF (4 daily
injections of 1 ml/kg body weight), Scailteur et al. (1984) showed
that females excreted higher amounts of unchanged DMF than males. The
pattern of metabolite (NMF, F) excretion was similar in both sexes
after single or repeated ip administration.
6.1.5 Metabolic interaction between DMF and ethanol
DMF and ethanol appear to interact metabolically.
The alterations in blood metabolites depend on the dose of DMF,
the time interval between DMF and ethanol administration, and the
respective routes of administration.
The various studies performed are summarized in Table 8. Blood
concentrations of DMF and NMF, ethanol, and acetaldehyde were measured
using GC methods.
The influence of DMF on ethanol oxidation might be explained, at
least partially, by its inhibitory effect on the activity of alcohol
dehydrogenase in vitro and in vivo (Sharkawi, 1979) and aldehyde
dehydrogenase in vivo (Elovaara et al., 1983).
Table 8. Metabolic interaction between DMF and ethanol
-----------------------------------------------------------------------------------------------------------------------------------------
Species Ethanol Time of DMF Effects on blood concentrations of: Reference
dose administration dose
(route) (route) DMF and Ethanol and
NMF acetaldehyde
-----------------------------------------------------------------------------------------------------------------------------------------
Rat .2 g/kg immediately before 312 mg/m3 No effects on not measured Eben & Kimmerle (1976)
(oral) DMF exposure 2 h (inhalation) DMF and NMF
Rat 2 g/kg immediately before 261 or 627 mg/m3 DMF increased not measured Eben & Kimmerle (1976)
(oral) DMF exposure 2 h (inhalation) NMF formation
Rat 2 g/kg per day daily immediately about 600 mg/m3 DMF increased ethanol increased Eben & Kimmerle (1976)
for 5 days before DMF 2 h/day 5 days NMF formation
(oral) exposure (inhalation)
Dog 2 g/kg immediately before about 630 mg/m3 DMF increased not measured Eben & Kimmerle (1976)
(oral) DMF exposure 2 h (inhalation) NMF decreased
Dog 2 g/kg immediately after 630 mg/m3 DMF increased not measured Eben & Kimmerle (1976)
(oral) DMF exposure 2 h (inhalation) NMF decreased
Rat 2 g/kg 1 h after last 3000 mg/m3 not measured acetaldehyde Hanasono et al. (1977)
(oral) DMF exposure 4 h/day 3 days increased
(inhalation)
Rat 2 g/kg 1 h after last 6000 mg/m3 not measured ethanol increased Hanasono et al. (1977)
(oral) DMF exposure 4 h/day 3 days acetaldehyde
(inhalation) decreased
Mouse 1 g/kg 2 h after DMF 1.2 ml/kg not measured ethanol increased Sharkawi (1980)
(ip) exposure (ip)
Rat 2 g/kg 3 h after DMF 0.15 g/kg not measured ethanol increased Hanasono et al. (1977)
(oral) exposure (oral) acetaldehyde
decreased
Rat 2 g/kg 18 h after DMF 0.15 g/kg not measured acetaldehydea Hanasono et al. (1977)
(oral) exposure (oral) increased
Table 8. (continued)
-----------------------------------------------------------------------------------------------------------------------------------------
Species Ethanol Time of DMF Effects on blood concentrations of: Reference
dose administration dose
(route) (route) DMF and Ethanol and
NMF acetaldehyde
-----------------------------------------------------------------------------------------------------------------------------------------
Rat 2 g/kg 18 h after DMF 1.5 g/kg not measured ethanol increased Hanasono et al. (1977)
(oral) exposure (oral)
Rat 2 g/kg 24 h after last 3000 mg/m3 not measured acetaldehyde Hanasono et al. (1977)
(oral) DMF exposure 4 h/day 3 days increased
(inhalation)
Rat 2 g/kg 24 h after last 12 000 mg/m3 not measured acetaldehyde Hanasono et al. (1977)
(oral) DMF exposure 4 h/day 3 days increased
(inhalation)
-----------------------------------------------------------------------------------------------------------------------------------------
a Increased acetaldehyde level observed after this dose of DMF was equivalent to that produced by an equimolar dose of disulfiram (antabuse).
6.2 Human studies
6.2.1 Absorption, distribution, metabolism, excretion
In vitro studies on excised human skin (Bortsevich, 1984) showed
a relationship between the amount of DMF absorbed through the dermal
barrier and the DMF concentrations in water, as well as the exposure
time. DMF enhances its own penetration. Some of the results are
given in Table 9. They are of practical value, because such solutions
are used in synthetic fibre production.
After respiratory exposure to DMF, lung retention in workers in an
artificial leather factory was 72% (Brugnone, 1980a,b).
Table 9. Quantities of DMF absorbed in in vitro studies on
excised human skin
--------------------------------------------------------------
Exposure period DMF solutions in water
(h) 100% 60% 30% 15%
% DMF absorbed through the skin (mg/cm2)
--------------------------------------------------------------
0.5 0.046 NDa NDa NDa
1-1.5 7.400 0.035 0.013 0.006
2-2.5 20.550 0.087 0.048 0.009
3-3.5 40.810 0.222 0.097 0.017
4-4.5 51.730 0.300 0.160 0.069
--------------------------------------------------------------
a ND = Not detectable.
The relative importance of skin versus inhalation for DMF
absorption was studied in volunteers by Maxfield et al. (1975),
Kimmerle & Eben (1975a), and Krivanek et al. (1978) (section 6.2.3.1).
As in animals, the major human metabolite of DMF has been reported
to be DMF-OH and not NMF. However, it is measured as NMF when using
gas chromatography including the small amount of NMF excreted in the
urine (Scailteur & Lauwerys, 1987).
When a male volunteer inhaled the DMF vapours that were produced
over liquid DMF in a beaker for 6 h, Mraz & Turecek (1987) identified
the metabolite N-acetyl- S-( N-methylcarbamoyl) cysteine in the urine.
Malonova & Bardodej (1983) reported a possible increase in the
urinary excretion of mercapturates in workers exposed to unknown
concentrations of DMF (approximately twice the excretion in controls
(smokers)).
6.2.2 The influence of ethanol on DMF metabolism in human volunteers
Eben & Kimmerle (1976) exposed 4 subjects via inhalation to DMF
(159 mg/m3) for 2 h with, and without, ingestion of 19 g ethanol (50
ml 38% gin), 10 min before they inhaled the DMF. No changes in DMF
concentrations in blood were found. The comparatively lower NMF
concentrations in the blood of subjects with combined exposure to
ethanol and DMF indicated that the ethanol decreased the
biotransformation of DMF. No significant differences in the blood
levels of ethanol and acetaldehyde were detected in subjects with, or
without, ethanol exposure, which differed from the effects observed in
animal studies. The authors suggested that this was because of the
relatively low concentrations of DMF used in the human studies.
6.2.3 Biological monitoring of workers
N-Hydroxymethyl- N-methylformamide (DMF-OH) has been identified as
the main urinary metabolite of DMF. It is measured, using gas
chromatography, as NMF together with the small proportion of NMF
excreted in the urine. Some results of studies on the correlation
between exposure levels to DMF and NMF excretion in workers and human
volunteers are given in Table 10.
6.2.3.1 Determination of NMF in the urine
NMF (DMF-OH) in the urine is a sensitive biological parameter of
human DMF exposure. NMF levels in the urine are usually greater at
the end of the shift than on the morning after the exposure. Lauwerys
et al. (1980) compared a group of 22 male workers from the spinning
mill in a polyacrylic fibre plant with 28 controls. The workers in the
spinning department wore gloves and long sleeves, but did not have any
respiratory protection. Spot urine samples were collected before, and
after, the work shift for 5 consecutive days, to determine NMF and
creatinine concentrations. NMF was notdetected in the urine of
control workers, who were not exposed to DMF. There was a poor
correlation, on an individual basis, between the integrated DMF
exposure and the NMF concentration in the urine collected at the end
of the shift, or in that collected before resuming work the following
day. However, on a group basis, there was a good correlation between
the intensity of exposure and NMF levels in the urine collected at the
end of the shift.
In a second study in the polyacrylic fibre plant, Lauwerys et al.
(1980) studied the NMF levels in the urine of 7 workers for 3 weeks,
when different types of personal protective devices were used.
Absorption of DMF vapours through the skin was more important than
through inhalation. In the absence of skin contact, a concentration
of 40-50 mg NMF/g creatinine, in post-shift samples, corresponded to
an average concentration of DMF vapour of 13 mg/m3 (45 ppm) during a
6-h exposure period.
Table 10. NMF levels in urine as a test for DMF exposure
------------------------------------------------------------------------------------------------------------------
Subjects DMF concentrations NMF concentrations Time of sampling Reference
in the air in the urine
------------------------------------------------------------------------------------------------------------------
4 volunteers 78 ± 24 mg/m3a 24 mg/24 h Kimmerle & Eben (1975a)
261 ± 75 mg/m3a 97.4 mg/24 h
63 ± 12 mg/m3b 30 mg/24 h
4 volunteers 159 ± 96 mg/m3a 44.8 mg/24 h Eben & Kimmerle (1976)
4 volunteers 32.4 ± 2.1 mg/m3a,c 5 mg/24 h Maxfield et al. (1975)
8 volunteers 26.4 ± 0.9 mg/m3b 2.5 mg/24 h Krivanek et al. (1978)
22 workers 13 mg/m3b 20-40 mg/g post-shift samples Lauwerys et al. (1980)
creatinine
9 workers 15.4 mg/m3b 0.4-19.6 mg/24 h Yonemoto & Suzuki (1980)
85 workers 30-150 mg/m3b,c 0.104-0.224 mg/ml Aldyreva et al. (1980)
23 workers above 30 mg/m3b 20-40 mg/24 h Taccola et al. (1981)
2 volunteers 30 mg/m3b 102.6 µmol/8 h Wicarova & Dadak (1981)
39 workers 217.5 µmol/24 h
30 workers 14-86.3 mg/m3b 12-188.3 mg/g 4 h after the work shift Sala et al. (1984)
creatinine different work areas
------------------------------------------------------------------------------------------------------------------
a Single inhalation exposure to DMF (2, 4, or 6 h/day).
b Repeated inhalation exposure to DMF (6, 7, 7.5 h/day).
c Dermal absorption.
Yonemoto & Suzuki (1980) studied the relationship between the
individual occupational exposure to DMF and the amount of NMF in the
urine of 9 male workers who handled polyurethane surface-treating
agents for synthetic leather. The time-weighted average individual
exposures ranged from 0 to 15.4 mg DMF/m3. The amount of NMF excreted
daily ranged from 0.4 to 19.56 mg/24 h. The excretion rate of NMF
(mg/h) increased from the beginning of exposure and reached a maximum
in the urine samples collected in the evening. The relationship
between the total daily NMF excretion in the urine and the level of
exposure was represented as a linear regression, indicating that the
best biological index of DMF exposure is the determination of NMF in
the 24-h urine (Fig. 2). At an 8-h integrated DMF exposure of 15
mg/m3, the NMF level in the urine of the workers was less than 20
mg/24 h. This value is higher than those obtained for volunteers
(Kimmerle & Eben, 1975b; Krivanek et al., 1978) or for workers
(Lauwerys et al., 1980). Yonemoto & Suzuki (1980) stated that the
difference might be due to dermal absorption of DMF, because the
workers did not use protective gloves or special working overalls.
6.1.4 Elimination and excretion
The transformation and excretion of DMF in rodents is rapid. When
14C-labelled DMF in 0.1 ml saline was injected ip at 6.8 mmol/kg body
weight in mice, about 83% of the radioactivity was recovered in the
urine within 24 h following injection. Of this amount, only 5% was
unchanged DMF and 56% was C-hydroxylated or N-demethylated
derivatives. About 18% of the dose was excreted in the form of
unknown chemicals (Brindley et al., 1983).
Similarly, 24 h after ip injection of 400 mg DMF/kg body weight in
0.2 ml saline in mice, about 56% of the dose was excreted in the urine
as DMF-OH and only 5% as unchanged DMF (Kestell et al., 1986).
Within 72 h of an ip administration of 1 ml 14C-DMF/kg to male
or female rats, 70% of the injected radioactivity was recovered in the
urine. Approximately 15% was excreted as unchanged DMF, 50% as DMF-OH
(NMF), and 5% as NMF-OH (F). About 20% was excreted as unidentified
metabolite(s) (Scailteur & Lauwerys, 1984a,b).
After oral exposure to DMF (40-2000 mg/kg), Sanotsky et al. (1978)
determined that about 6% of the dose was excreted in 24 h.
The elimination of DMF, NMF (DMF-OH), and formamide (NMF-OH) was
measured after single or repeated inhalation exposure in rats and dogs
(Kimmerle & Eben, 1975a). Twenty-four hours after a single exposure to
63 mg NMF/m3 for 3 h, or 87 mg/m3 for 6 h, no NMF was found in the
urine of male rats. Under the same conditions, exposure to 513 mg/m3
for 6 h or to 6015 mg/m3 for 3 h led to excretion of 4 mg and 14 mg
NMF (DMF-OH), respectively, during the 24 h following the start of
exposure. Only in the last case was DMF also measured in the urine.
After repeated exposure of male rats to DMF (1050 mg/m3, 6 h/day, for
5 days), urinary levels of NMF (DMF-OH) remained practically constant
for the first 3 days, then slightly decreased from the fourth day of
exposure. Excretion of F (NMF-OH) was much lower than excretion of NMF
(DMF-OH).
While no accumulation of urinary NMF (DMF-OH) was observed in male
rats, male dogs exposed to 177 mg DMF/m3 (6 h/day for 5 days) excreted
increasing concentrations of NMF (DMF-OH) (36 mg/24 h after the first
inhalation; 87 mg/24 h after the 4th inhalation). Urinary excretion
of formamide (NMF-OH) varied between 10 and 20 mg/24 h. Excretion of
unchanged DMF was very low (< 2 mg/24 h). However, in female dogs
exposed to 69 mg/m3 (6 h/day for 5 days), no urinary accumulation of
NMF or F was observed. When male or female rats were exposed for 4
weeks to 63 mg/m3 (6 h/day, 5 days per week), NMF and F concentrations
in the urine remained practically constant during the exposure period.
Male dogs generally excreted slightly higher levels of metabolites
than female dogs (Kimmerle & Eben, 1975a).
In rats treated with repeated, high, ip doses of DMF (4 daily
injections of 1 ml/kg body weight), Scailteur et al. (1984) showed
that females excreted higher amounts of unchanged DMF than males. The
pattern of metabolite (NMF, F) excretion was similar in both sexes
after single or repeated ip administration.
6.1.5 Metabolic interaction between DMF and ethanol
DMF and ethanol appear to interact metabolically.
The alterations in blood metabolites depend on the dose of DMF,
the time interval between DMF and ethanol administration, and the
respective routes of administration.
The various studies performed are summarized in Table 8. Blood
concentrations of DMF and NMF, ethanol, and acetaldehyde were measured
using GC methods.
The influence of DMF on ethanol oxidation might be explained, at
least partially, by its inhibitory effect on the activity of alcohol
dehydrogenase in vitro and in vivo (Sharkawi, 1979) and aldehyde
dehydrogenase in vivo (Elovaara et al., 1983).
Table 8. Metabolic interaction between DMF and ethanol
-----------------------------------------------------------------------------------------------------------------------------------------
Species Ethanol Time of DMF Effects on blood concentrations of: Reference
dose administration dose
(route) (route) DMF and Ethanol and
NMF acetaldehyde
-----------------------------------------------------------------------------------------------------------------------------------------
Rat .2 g/kg immediately before 312 mg/m3 No effects on not measured Eben & Kimmerle (1976)
(oral) DMF exposure 2 h (inhalation) DMF and NMF
Rat 2 g/kg immediately before 261 or 627 mg/m3 DMF increased not measured Eben & Kimmerle (1976)
(oral) DMF exposure 2 h (inhalation) NMF formation
Rat 2 g/kg per day daily immediately about 600 mg/m3 DMF increased ethanol increased Eben & Kimmerle (1976)
for 5 days before DMF 2 h/day 5 days NMF formation
(oral) exposure (inhalation)
Dog 2 g/kg immediately before about 630 mg/m3 DMF increased not measured Eben & Kimmerle (1976)
(oral) DMF exposure 2 h (inhalation) NMF decreased
Dog 2 g/kg immediately after 630 mg/m3 DMF increased not measured Eben & Kimmerle (1976)
(oral) DMF exposure 2 h (inhalation) NMF decreased
Rat 2 g/kg 1 h after last 3000 mg/m3 not measured acetaldehyde Hanasono et al. (1977)
(oral) DMF exposure 4 h/day 3 days increased
(inhalation)
Rat 2 g/kg 1 h after last 6000 mg/m3 not measured ethanol increased Hanasono et al. (1977)
(oral) DMF exposure 4 h/day 3 days acetaldehyde
(inhalation) decreased
Mouse 1 g/kg 2 h after DMF 1.2 ml/kg not measured ethanol increased Sharkawi (1980)
(ip) exposure (ip)
Rat 2 g/kg 3 h after DMF 0.15 g/kg not measured ethanol increased Hanasono et al. (1977)
(oral) exposure (oral) acetaldehyde
decreased
Rat 2 g/kg 18 h after DMF 0.15 g/kg not measured acetaldehydea Hanasono et al. (1977)
(oral) exposure (oral) increased
Table 8. (continued)
-----------------------------------------------------------------------------------------------------------------------------------------
Species Ethanol Time of DMF Effects on blood concentrations of: Reference
dose administration dose
(route) (route) DMF and Ethanol and
NMF acetaldehyde
-----------------------------------------------------------------------------------------------------------------------------------------
Rat 2 g/kg 18 h after DMF 1.5 g/kg not measured ethanol increased Hanasono et al. (1977)
(oral) exposure (oral)
Rat 2 g/kg 24 h after last 3000 mg/m3 not measured acetaldehyde Hanasono et al. (1977)
(oral) DMF exposure 4 h/day 3 days increased
(inhalation)
Rat 2 g/kg 24 h after last 12 000 mg/m3 not measured acetaldehyde Hanasono et al. (1977)
(oral) DMF exposure 4 h/day 3 days increased
(inhalation)
-----------------------------------------------------------------------------------------------------------------------------------------
a Increased acetaldehyde level observed after this dose of DMF was equivalent to that produced by an equimolar dose of disulfiram (antabuse).
6.2 Human studies
6.2.1 Absorption, distribution, metabolism, excretion
In vitro studies on excised human skin (Bortsevich, 1984) showed
a relationship between the amount of DMF absorbed through the dermal
barrier and the DMF concentrations in water, as well as the exposure
time. DMF enhances its own penetration. Some of the results are
given in Table 9. They are of practical value, because such solutions
are used in synthetic fibre production.
After respiratory exposure to DMF, lung retention in workers in an
artificial leather factory was 72% (Brugnone, 1980a,b).
Table 9. Quantities of DMF absorbed in in vitro studies on
excised human skin
--------------------------------------------------------------
Exposure period DMF solutions in water
(h) 100% 60% 30% 15%
% DMF absorbed through the skin (mg/cm2)
--------------------------------------------------------------
0.5 0.046 NDa NDa NDa
1-1.5 7.400 0.035 0.013 0.006
2-2.5 20.550 0.087 0.048 0.009
3-3.5 40.810 0.222 0.097 0.017
4-4.5 51.730 0.300 0.160 0.069
--------------------------------------------------------------
a ND = Not detectable.
The relative importance of skin versus inhalation for DMF
absorption was studied in volunteers by Maxfield et al. (1975),
Kimmerle & Eben (1975a), and Krivanek et al. (1978) (section 6.2.3.1).
As in animals, the major human metabolite of DMF has been reported
to be DMF-OH and not NMF. However, it is measured as NMF when using
gas chromatography including the small amount of NMF excreted in the
urine (Scailteur & Lauwerys, 1987).
When a male volunteer inhaled the DMF vapours that were produced
over liquid DMF in a beaker for 6 h, Mraz & Turecek (1987) identified
the metabolite N-acetyl- S-( N-methylcarbamoyl) cysteine in the urine.
Malonova & Bardodej (1983) reported a possible increase in the
urinary excretion of mercapturates in workers exposed to unknown
concentrations of DMF (approximately twice the excretion in controls
(smokers)).
6.2.2 The influence of ethanol on DMF metabolism in human volunteers
Eben & Kimmerle (1976) exposed 4 subjects via inhalation to DMF
(159 mg/m3) for 2 h with, and without, ingestion of 19 g ethanol (50
ml 38% gin), 10 min before they inhaled the DMF. No changes in DMF
concentrations in blood were found. The comparatively lower NMF
concentrations in the blood of subjects with combined exposure to
ethanol and DMF indicated that the ethanol decreased the
biotransformation of DMF. No significant differences in the blood
levels of ethanol and acetaldehyde were detected in subjects with, or
without, ethanol exposure, which differed from the effects observed in
animal studies. The authors suggested that this was because of the
relatively low concentrations of DMF used in the human studies.
6.2.3 Biological monitoring of workers
N-Hydroxymethyl- N-methylformamide (DMF-OH) has been identified as
the main urinary metabolite of DMF. It is measured, using gas
chromatography, as NMF together with the small proportion of NMF
excreted in the urine. Some results of studies on the correlation
between exposure levels to DMF and NMF excretion in workers and human
volunteers are given in Table 10.
6.2.3.1 Determination of NMF in the urine
NMF (DMF-OH) in the urine is a sensitive biological parameter of
human DMF exposure. NMF levels in the urine are usually greater at
the end of the shift than on the morning after the exposure. Lauwerys
et al. (1980) compared a group of 22 male workers from the spinning
mill in a polyacrylic fibre plant with 28 controls. The workers in the
spinning department wore gloves and long sleeves, but did not have any
respiratory protection. Spot urine samples were collected before, and
after, the work shift for 5 consecutive days, to determine NMF and
creatinine concentrations. NMF was notdetected in the urine of
control workers, who were not exposed to DMF. There was a poor
correlation, on an individual basis, between the integrated DMF
exposure and the NMF concentration in the urine collected at the end
of the shift, or in that collected before resuming work the following
day. However, on a group basis, there was a good correlation between
the intensity of exposure and NMF levels in the urine collected at the
end of the shift.
In a second study in the polyacrylic fibre plant, Lauwerys et al.
(1980) studied the NMF levels in the urine of 7 workers for 3 weeks,
when different types of personal protective devices were used.
Absorption of DMF vapours through the skin was more important than
through inhalation. In the absence of skin contact, a concentration
of 40-50 mg NMF/g creatinine, in post-shift samples, corresponded to
an average concentration of DMF vapour of 13 mg/m3 (45 ppm) during a
6-h exposure period.
Table 10. NMF levels in urine as a test for DMF exposure
------------------------------------------------------------------------------------------------------------------
Subjects DMF concentrations NMF concentrations Time of sampling Reference
in the air in the urine
------------------------------------------------------------------------------------------------------------------
4 volunteers 78 ± 24 mg/m3a 24 mg/24 h Kimmerle & Eben (1975a)
261 ± 75 mg/m3a 97.4 mg/24 h
63 ± 12 mg/m3b 30 mg/24 h
4 volunteers 159 ± 96 mg/m3a 44.8 mg/24 h Eben & Kimmerle (1976)
4 volunteers 32.4 ± 2.1 mg/m3a,c 5 mg/24 h Maxfield et al. (1975)
8 volunteers 26.4 ± 0.9 mg/m3b 2.5 mg/24 h Krivanek et al. (1978)
22 workers 13 mg/m3b 20-40 mg/g post-shift samples Lauwerys et al. (1980)
creatinine
9 workers 15.4 mg/m3b 0.4-19.6 mg/24 h Yonemoto & Suzuki (1980)
85 workers 30-150 mg/m3b,c 0.104-0.224 mg/ml Aldyreva et al. (1980)
23 workers above 30 mg/m3b 20-40 mg/24 h Taccola et al. (1981)
2 volunteers 30 mg/m3b 102.6 µmol/8 h Wicarova & Dadak (1981)
39 workers 217.5 µmol/24 h
30 workers 14-86.3 mg/m3b 12-188.3 mg/g 4 h after the work shift Sala et al. (1984)
creatinine different work areas
------------------------------------------------------------------------------------------------------------------
a Single inhalation exposure to DMF (2, 4, or 6 h/day).
b Repeated inhalation exposure to DMF (6, 7, 7.5 h/day).
c Dermal absorption.
Yonemoto & Suzuki (1980) studied the relationship between the
individual occupational exposure to DMF and the amount of NMF in the
urine of 9 male workers who handled polyurethane surface-treating
agents for synthetic leather. The time-weighted average individual
exposures ranged from 0 to 15.4 mg DMF/m3. The amount of NMF excreted
daily ranged from 0.4 to 19.56 mg/24 h. The excretion rate of NMF
(mg/h) increased from the beginning of exposure and reached a maximum
in the urine samples collected in the evening. The relationship
between the total daily NMF excretion in the urine and the level of
exposure was represented as a linear regression, indicating that the
best biological index of DMF exposure is the determination of NMF in
the 24-h urine (Fig. 2). At an 8-h integrated DMF exposure of 15
mg/m3, the NMF level in the urine of the workers was less than 20
mg/24 h. This value is higher than those obtained for volunteers
(Kimmerle & Eben, 1975b; Krivanek et al., 1978) or for workers
(Lauwerys et al., 1980). Yonemoto & Suzuki (1980) stated that the
difference might be due to dermal absorption of DMF, because the
workers did not use protective gloves or special working overalls.
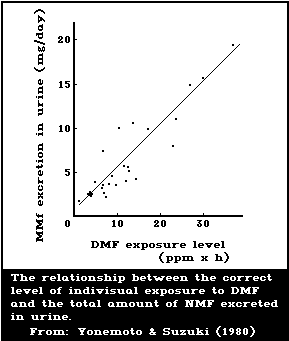 Wicarova & Dadak (1981) studied the relationship between the
amount of NMF in the shift urine (8 h) or the all-day urine (24 h) of
workers and DMF concentrations in the air (0-100 mg/m3) in an
artificial leather plant . The relationship was linear for the shift
urine samples. For the 24-h urine samples, the relationship was
linear only in the range of 0-80 mg DMF/m3 (see also Table 10).
When Dixon et al. (1983) studied the urinary NMF excretion in a
group of 32-37 workers who were exposed to similar air levels of DMF
for either 8 h per shift (5 days/week) or 12 h per shift (4
days/week), they found higher concentrations of NMF in the urine when
the workers were working 8-h shifts. A possible explanation was that
a 13% reduction in urine volume was seen in workers on 8-h shifts
during the summer months compared with higher urine outputs seen in
the same workers on 12-h shifts during the winter months.
Sala et al. (1984) found a correlation between urinary NMF levels,
4 h after workplace exposure, and the workers' exposure levels to DMF
in 5 job categories relating to artificial leather production. They
reported airborne DMF concentrations of 4.5-14 mg/m3 for spreading
"coagulate" system workers, with a mean NMF in urine of 16 mg/g
creatinine, 9.4 mg DMF/m3 for finishing workers, with a mean NMF
urinary value of 12 mg/g creatinine (low exposures), and 86.3 mg
DMF/m3 in tank cleaning workers with a corresponding urinary value of
188.3 mg NMF/g creatinine (highest exposure).
6.2.3.2 N,N-Dimethylformamide determination in the expired air
Airborne DMF concentrations change considerably during the work
shift and from one workplace to another. Brugnone et al. (1980a)
measured the DMF concentrations in the alveolar air every hour during
the 8-h shift of 8 workers employed in an artificial leather plant.
The alveolar DMF concentration in 6 workers was correlated with the
DMF concentration in the air of the respective workplaces.
In a second study, Brugnone et al. (1984) studied 8 exposed
workers by determining the DMF concentrations in the environmental
air, alveolar air, blood, and urine. Air samples were collected at
hourly intervals during an 8-h work shift, blood samples, at 2-h
intervals, and urine samples, at 4-h intervals. No DMF was found in
the blood or urine. A good correlation between the alveolar and
environmental DMF concentrations was found in 6 out of the 8 workers,
and at all subsequent hours, except for the fourth hour.
In practice, the alveolar air test is more difficult to perform
and use for routine examination than measurement of NMF levels in
urine samples, and is not recommended for biological monitoring.
6.2.3.3 Appraisal
The level of NMF in a post-shift urine sample is the most
appropriate biological parameter for total DMF exposure (inhalation
plus dermal) during the shift.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
The effects of DMF on organisms in the environment have been
reviewed by Kennedy (1986) and by US EPA (1986).
The LC50s of DMF for various aquatic species, given in Table 11,
indicate a low toxicity for the species tested.
DMF is commonly used to facilitate the solution of lipophilic
compounds in water during aquatic toxicity tests.
Cardwell et al. (1978) studied the long-term toxicity of DMF for
fathead minnow (Pimephales promelas), brown trout (Salvelinus
fontinalis), and bluegill (Lepomis macrochirus), and found threshold
limits of between 43 and 98 mg DMF/litre for the brook trout and
between 5 and 10 mg/litre for the fathead minnow. LeBlanc &
Surprenant (1983) showed that a level of 0.1 ml DMF/litre was
acceptable for long-term aquatic toxicity tests. In a study by
Tonogai et al. (1982), the 24-h and 48-h static median tolerance
limits for the Himedaka (Oryzias latipes) were > 1000 mg DMF/litre.
A no-observed-effect level (NOEL) of 7700 mg/litre was reported
for the rainbow trout by Shubat et al. (1982).
Solutions of DMF of 25 g/litre (2.5%) were shown to be lethal
within 0.5 h for eggs of sea urchins (Lythechinus variegatus, Arbacia
punctulata, Lythechinus pictus), the surf clam (Spisule solidissima),
and the annelid (Pectinaria) (Rebhun & Sawada, 1969).
Hughes & Vilkas (1983) determined that the highest concentration
that had no significant effect on the green alga Selenastrum
capricornatum, was 1 ml/litre and the no-effect level was 0.5
ml/litre.
Concentrations ranging from 0.085-0.340% DMF had an inhibitory
effect on cultures of Streptomyces aureofaciens (Welward & Halama,
1978).
Table 11. Medial lethal (LC50) concentrations (mg/litre) for aquatic
organisms exposed to dimethylformamide (DMF)
----------------------------------------------------------------------------------------------------
Species LC50 Reference
---------------------------------------
24-h 48-h 96-h
----------------------------------------------------------------------------------------------------
Guppy 1300 Dojlido (1979)
(Paecilia reticulata)
Rainbow trout 9800 Poirier et al. (1986)
(Salmo gairdneri) 9860 Shubat et al. (1982)
Fathead minnow 10 600 Poirier et al. (1986)
(Pimephales promelas)
Bluegill 7100 Poirier et al. (1986)
(Lepomis macrochirus)
Midge (Paratanytarsus 36 200 Poirier et al. (1986)
parthenogeneticus)
Daphnid (Daphnia magna) 14 500 Poirier et al. (1986)
12 300 LeBlanc & Surprenant
(approx.) (1983)
Larvae (Aedes aegypti) 68 000 Kramer et al. (1983)
(approx.)
----------------------------------------------------------------------------------------------------
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposures
Data on the acute toxicity of DMF in different laboratory animals,
when administered by different routes, have been reviewed by Kennedy
(1986). The acute toxicity in a number of species, following oral,
dermal, inhalation (Table 12), or parenteral (Table 13) administration
of DMF is relatively low, with lethal doses generally in the g/kg
range for the oral, dermal, and parenteral routes and in the g/m3 for
inhalation exposures. Animals given large single doses of DMF or
exposed to high air levels showed general depression, anaesthesia,
loss of appetite, loss of body weight, tremors, laboured breathing,
convulsions, haemorrage of the nose and mouth, liver injury, and coma
immediately preceding death.
In mice and rats, exposed to DMF via inhalation, signs of mucous
membrane irritation were seen (Lobanova, 1958; Lundberg et al., 1986),
and lung damage was detected histologically (Clayton et al., 1963).
Where tissue pathology was included in the study, the prominent
organ showing damage was the liver (Massmann, 1956; Sanotsky et al.,
1978; Mathew et al., 1980; Lundberg et al., 1981). No obvious species
differences were observed with regard to acute lethality, but young
rats appeared more sensitive to DMF-induced lethality than older rats
(Kimura et al., 1971).
8.2 Skin and eye irritation, sensitization
DMF was reported to be irritating to the eyes, mucous membranes,
and the skin (Hamilton & Hardy, 1974; Aldyreva & Gafurov, 1980).
8.2.1 Skin irritation
Rat tails dipped in DMF at 40 °C for 8 h became mummified in a few
days (Massmann, 1956).
A single application of 500 mg DMF/kg resulted in transient
irritation within 2-3 h in mice, but no irritation in rats (Wiles &
Narcisse, 1971). DMF was slightly irritating for mice at doses of
2500 and 5000 mg/kg. No skin irritation was detected in rabbits with
applications of 100, 200, or 500 mg DMF/kg. Single applications of
DMF on the skin of rats and guinea-pigs did not cause irritation
(Kiss, 1979; Bainova, 1985). Repeated 28-day treatments with 960 or
1920 mg/kg did not induce marked local dermal effects in rats (Bainova
et al., 1985).
Table 12. LD50 and LC50 values of DMF after oral, dermal, or
inhalation exposure in various animal species
----------------------------------------------------------------------------------------------------
Species Oral LD50 Dermal LD50 Inhalation LC50 Reference
(mg/kg) (mg/kg) (mg/m3)
----------------------------------------------------------------------------------------------------
Rat 3000 Thiersch (1962)
5000 9432 US NIOSH (1977)
3920 Massmann (1956)
11 140 12 000 Schottek (1970, 1972)
4000 Sanotsky et al. (1978)
> 11 520 Bainova & Antov (1980)
15 000 Clayton et al. (1963)
4320 Lazarev & Levina (1976)
11 000a Stula & Krauss (1977)
> 13 440 Lundberg et al. (1986)
3200 Qin & Gue (1976)
14 000 Cai & Huang (1979)
7170 Bartsch et al. (1976)
Mouse 3950 Lazarev & Levina (1976)
5550 Lazarev & Levina (1976)
> 5000 Wiles & Narcisse (1971)
6420 Bartsch et al. (1976)
3700 6000-9400 Lobanova (1958)
5400, 6200 Qin & Gue (1976)
18 300 Cai & Huang (1979)
Rabbit > 5000 > 500 Wiles & Narcisse (1971)
1500a Stula & Krauss (1977)
Mongolian gerbil 3929 Llewellyn et al. (1974)
----------------------------------------------------------------------------------------------------
a Pregnant females.
Table 13. LD50s (mg/kg body weight) of DMF after parenteral administration
in various animal species
----------------------------------------------------------------------------------------------------------
Species Intraperitoneal Intravenous Intramuscular Subcutaneous Reference
----------------------------------------------------------------------------------------------------------
Rat 1480 4030 Massmann (1956)
2500 Thiersch (1962)
4440 2830 Bartsch et al. (1976)
4600 Pham Huu Chanh et al. (1971)
5470 Shottek (1970, 1972)
Mouse 300 Massmann (1956)
3500 3800 4500 US NIOSH (1977)
650 Barral-Chamaillard
& Rouzioux (1983)
1454 Burgun et al. (1975)
2000 Antoine et al. (1983)
3150 2800 Wiles & Narcisse (1971)
5200 Pham Huu Chanh et al. (1971)
5850 3490 Bartsch et al. (1976)
Rabbit 945 1800 Massmann (1956)
1000 Wiles & Narcisse (1971)
5000 US NIOSH (1977)
Guinea-pig 1300 Wahlberg & Boman (1979)
1030 US NIOSH (1977)
4000 Ungar et al. (1976)
Dog 470 Barral-Chamaillard
& Rouzioux (1983)
Cat 500 Massmann (1956)
----------------------------------------------------------------------------------------------------------
After repeated application of DMF to the skin of guinea-pigs for
21 days (Bainova, 1985), the mean irritative dose was 31% DMF (range
17-56%).
Dermal irritation was not seen in rabbits treated dermally with 2
g DMF/kg for 6 h, daily 15 times during a 4-week period (Kennedy,
1986).
8.2.2 Eye irritation
A 25% (25 g/litre) solution of DMF in water, injected into the
conjunctival sac of the rabbit, did not produce any effects; 50% was
slightly irritating, and 75-100% produced more severe irritation
(Massmann, 1956). Single dose DMF instillation (0.1 ml) produced
moderate corneal damage and conjunctival redness that was most
pronounced at 2-3 days. By day 14, a mild degree of conjunctival
redness, moderate corneal damage with an area of severe injury, slight
surface distortion, and subsurface vascularization were observed
(Kennedy & Sherman, 1986). In another study, the same authors
reported that, after a single DMF instillation, the eye inflammation
subsided and disappeared by the 8th day.
8.2.3 Sensitization
DMF was tested, using a maximization technique, on guinea-pigs to
determine skin sensitization; it did not induce any response (Bainova,
1985).
8.3 Repeated exposure
The effects of repeated oral, dermal, or inhalation exposure to
DMF in various animal species have been reviewed by Kennedy (1986) and
these data, together with other new information are summarized in
Table 14. In all species tested, except the dog, liver damage was
produced, the degree of damage generally being proportional to the
dose administered. In the two reported studies on the dog (Clayton et
al., 1963; Kimmerle & Eben, 1975a), the inhalation exposure conditions
appeared to be too low (60 mg/m3) to produce damage, though 1 out of
the 4 dogs tested by Clayton did have altered liver function tests.
Higher levels were not tested. Some evidence of recovery from the
hepatotoxic effects of DMF was found in rats (Kennedy & Sherman,
1986).
Higher, intermittent doses of DMF appeared to produce more
pronounced effects in male rats than continuous dosing (Bainova et
al., 1981a; Bainova, 1985). Tanaka (1971) found more pronounced liver
damage in rats following one rather than three weeks of exposure and
considered that the high regenerative capacity of liver tissue was
responsible for the observation.
Other tissues and organs that are affected, particularly by high
doses of DMF, will be discussed in section 8.4.
8.4 Specific organ toxicity
8.4.1 Liver
The potency of DMF as a hepatotoxic agent has been reviewed by
Kennedy (1986) and by Scailteur & Lauwerys (1987). The effects of DMF
on the liver were studied after single or repeated inhalation, dermal,
or oral treatment of rats, mice, and rabbits (Massmann, 1956; Clayton
et al., 1963; Shottek, 1970; Tanaka, 1971; Kimmerle & Eben, 1975a;
Medyankin, 1975; Sanotsky et al., 1978; Germanova et al., 1979; Mathew
et al., 1980; Bainova et al., 1981a; Lundberg et al., 1981; Lundberg,
1982; Brondeau et al., 1983; Bainova, 1985; Kennedy & Sherman, 1986;
Scailteur & Lauwerys, 1987). Single oral administrations of 2250-5000
mg DMF/kg in rats (Kennedy & Sherman, 1986) caused clay-coloured
liver, congestion, and centrilobular necrosis of hepatocytes. Lower
doses resulted in deviations in liver function, such as decreased
excretion of cholic acid in the bile, bromosulfthalein retention,
increased serum activities of GOT, GPT, LAP, OCT, AlcP, ChE, LDH, and
gamma-GT, and significant enhancement of cholesterol, triglyceride,
and bilirubin contents in the serum and liver homogenates. In rats,
following both intraperitoneal (ip) and inhalation exposure, there
were no increases in SDH levels at 420 and 840 mg/m3 but a lower level
(210 mg/m3) raised the serum activity of SDH (Lundberg et al., 1986).
Pathomorphological investigation demonstrated lipid degeneration and
cloudy swelling of hepatocytes in the central zones of the lobules
followed by signs of regeneration.
DMF at 0.6 ml/kg, administered intraperitoneally, caused mild
changes in rat liver lobules. Marked centrilobular necrosis and
central phlebitis were found in the rats treated with single ip doses
of 0.9 and 1.2 ml DMF/kg (Mathew et al., 1980). A single ip dose of
0.5 ml DMF/kg to hamsters caused centrilobular necrosis accompanied by
haemosiderosis (Ungar et al., 1976). Morphological changes were
reported in the liver by Clayton et al. (1963), Shottek (1970), Tanaka
(1971), and Santa Cruz & Corpino (1978) after repeated DMF exposure of
young animals, with periodic peaks (Table 14).
Table 14. Effects of repeated oral, dermal, or inhalation exposure to DMF in various animal species
________________________________________________________________________________________________________
Species Route of Dose Duration Effects Reference
exposure
________________________________________________________________________________________________________
Mongolian oral 10 000 mg/kg 30 days no changes in body weight, liver, Llewellyn
gerbil drinking-water or kidney et al. (1974)
10 000 mg/kg 200 days mortality in 25% of animals;
drinking-water liver necrosis
17 000 mg/kg 80 days mortality with liver necrosis;
drinking-water LD50 cumulative 90 206 mg/kg
body weight
34 000 mg/kg 6 days mortality with liver necrosis;
drinking-water LD50 cumulative 3846 mg/kg body
weight
Mouse oral 620 or 1240 30 days anorexia, loss of body weight Qin & Gue
mg/kg diet (1976)
160, 540, 119 days dose-related increase in relative Becci et al.
1850 mg/kg diet and absolute liver weights; no (1983)
other histological or biochemical
changes; NOEL, 246-326 mg/kg
diet per day
Rat oral 320 or 640 30 days anorexia, loss of body weight Qin & Gue
mg/kg diet (1976)
50, 500, 5000 100 days body weight decrease; liver Qin & Gue
mg/litre damage at 5000 mg/litre; (1976)
drinking-water increase in liver to body weight
ratio at 500 and 5000 mg/litre;
structural liver changes and
regeneration at 5000 mg/litre;
NOEL, 50 mg/litre
Table 14 (continued)
________________________________________________________________________________________________________
Species Route of Dose Duration Effects Reference
exposure
________________________________________________________________________________________________________
Rat oral 102, 497, 1000 14 days no behavioural changes at 102 or Savolainen
mg/litre 49 days 497 mg/litre for 49 days; dose- (1981)
drinking-water related deviations in cerebral
and glial cell enzyme activities
215, 750, 2500 104 days dose-related increase in relative Becci et al.
mg/kg diet and absolute liver weights, (1983)
considered to be physiological
adaptation; NOEL, 210-235 mg/kg
diet per day
200, 1000, 5000 90 days slight anaemia and leukocytosis, Kennedy &
mg/kg diet hypercholesterolaemia at 1000 and Sherman (1986)
(equivalent to 5000 mg/kg diet; NOEL, 200 mg/kg
12, 60, 300 mg/ diet
kg/body weight
per day)
0.1, 0.5, 1.0 14 days dose-related increase in liver/ Elovaara
g/litre in 49 days body weight ratios; in liver et al.
drinking-water and kidneys, increased values of (1983)
reduced glutathione, microsomal
UDP glucuronosyl transferase,
and ethoxycoumarin O-demethylase
activities; no changes in liver
microsomal cytochrome P-450 or
ADPH-cytochrome reductase
activity
Rat dermal 470 mg/kg per 30 days continuous dosing caused Schottek
day for 29 days hepatoxicity and did not protect (1970)
and 11 140 mg/kg against lethality; pretreatment
on the 30th day did not enhance toxic reactions
after application of the LD50
in 30-day pretreated rats
Table 14 (continued).
________________________________________________________________________________________________________
Species Route of Dose Duration Effects Reference
exposure
________________________________________________________________________________________________________
215, 430, 960, 30 days dose-related changes in GOT, Bainova &
or 4800 mg/kg GPT, AlcP, ChE, gamma-GT, lipid Antov (1980)
per day fractions in serum and liver
homogenates; NOEL, 215 mg/kg
Rat dermal 215, 320, 960, 30 days dose-related changes (at doses Bainova (1985)
or 4800 mg/kg > 320 mg/kg) in enzyme
activities per day in liver,
myocardium, and kidney
homogenates; NOEL, 215 mg/kg
Rat dermal 960 mg/kg daily 28 days functional, biochemical, and Bainova et al.
or 1920 mg/kg pathomorphological changes in (1981a)
applied liver; and lipid metabolism Bainova (1985)
intermittentlya on the 4th, 8th, 14th, and 28th
day of the tests; changes more
pronounced after intermittent
exposure
4-h dipping of 60 days concentration-related changes Medyankin
tails in 60, 65, in liver and nervous system; (1975)
70, or 80% DMF NOEL, 60% DMF in water
in water
4-h dipping of 120 days no changes at 30% DMF contact and Medyankin
tails in 5 mg DMF/m3 inhalation; adverse (1975)
30 or 60% DMF effects at other concentrations
and inhalation of
5 or 10 mg DMF/m3,
6 h daily
Table 14 (continued).
________________________________________________________________________________________________________
Species Route of Dose Duration Effects Reference
exposure
________________________________________________________________________________________________________
Rabbit dermal 50, 100% water 7 days died at 5-8 day of application Huang et al.
solution, 3 at 100% DMF; liver biochemical (1981)
times/day, 2 ml/ and histological changes
application
2000 mg/kg per 9 days anorexia, cyanosis, and mortality Kennedy &
day with liver necrosis Sherman (1986)
Guinea dermal 50, 75, 100% 7 days died 2-4 days after application of Huang et al.
-pig solution, 75 or 100% and 4-9 days after 50%; (1981)
3 times/day, loss of body weight; liver damage
2 ml/application
Rat inhalation 1800 mg/m3 for 6 days concentration-related mortality; Schottek
6 h daily cumulation of hepatoxic effect (1970)
750 and 1500 6 days
mg/m3, 6 h daily
30 mg/m3 for 8 days no changes in the function of Sanotsky &
6 h daily the thyroid or adrenal glands Ulanova (1975)
aerosol for 3 or liver and kidney necrosis, lung Santa Cruz &
0.5 h daily 30 days changes, arterial changes in Corpino (1978);
(concentration myocardium Santa Cruz &
unknown) Maccioni (1978)
22 ± 1.6 mg/m3 18 weeks liver changes, no other responses Cai & Huang
for 6 h daily, (1979)
6 days a week
Table 14 (continued).
________________________________________________________________________________________________________
Species Route of Dose Duration Effects Reference
exposure
________________________________________________________________________________________________________
Rat inhalation 130 mg/m3 for 27 days functional changes in kidneys Germanova et al.
4 h daily and liver; arterial blood pressure (1979)
more pronounced after additional
300 mg/m3 in 27 days single administration of 500 mg
5 peaks of DMF/kg on the 1st, 8th, and
15 min at 27th days of the studies, and
40-min intervals after intermittent exposure
7569 mg/m3 5 days weakness, weight loss, Kennedy &
for 6 h daily dehydration, liver necrosis Sherman (1986)
Young rat inhalation 600 mg/m3 for 28 days increased serum GOT and GPT; Tanaka (1971)
(3-12 8 h daily morphological liver changes,
weeks old) mainly in 3-week-old rats;
no histological abnormalities
in other organs
Young rat inhalation 600 mg/m3 for 28 days liver changes at the 1st, 2nd, Tanaka (1971)
(3 weeks 8 h daily and 3rd, and 4th week of test more
old) 600 mg/m3 for intense in the group exposed
1 h daily for 8 h daily; no cumulation of
hepatoxic effect
Table 14 (continued).
________________________________________________________________________________________________________
Species Route of Dose Duration Effects Reference
exposure
________________________________________________________________________________________________________
Rat, inhalation 450, 900, 1800, 60 days increased serum GOT, GPT, AlcP, Craig et al.
mouse 3600 mg/m3 cholesterol, anaemia and (1984)
for 6 h daily histological liver changes at
900 mg/m3 or more; liver weight
increase at 450 mg/m3; NOEL
below 450 mg/m3 in both species
Rat, cat inhalation 300, 690, 1350 120 days anorexia, weight loss, liver Massmann
mg/m3, 8 h daily degeneration, and necrosis; (1956)
changes in brain, myocardium,
and kidneys; no abnormalities
in blood tests or ECG
Rabbit inhalation 22 ± 1.6 mg/m3 18 weeks no changes in ECG or liver Cai & Huang
for 6 h daily, parameters (1979)
6 days per week
317 ± 37.8 mg/m3 14 weeks body weight changes; liver damage Cai & Huang
for 6 h daily, functionally and structurally; by (1979)
6 days a week congestion and haemorrhage
120 mg/m3 for 50 days microscopic and electron- Arena et al.
8 h daily microscopic changes in the (1982)
myocardium
Dog inhalation 60 mg/m3 for 107 days reversible changes in blood Clayton et al.
6 h daily pressure, ECG, and in liver (1963)
functions
63 mg/m3 for
6 h daily 28 days normal GOT, GPT, bilirubin, urea, Kimmerle & Eben
and creatinine in plasma; (1975a)
NOEL, 63 mg DMF/m3
___________________________________________________________________________________________________________
a Two alternative intermittent regimes were used: (i) 1920 mg/kg per day for 2 days, followed by no
treatment for 2 days; (ii) 1920 mg/kg every second day.
Diets supplemented with DMF at levels of 215, 750, or 2500 mg/kg
for 104 days for rats and 160, 540, or 1850 mg/kg for 119 days for
mice, resulted in significant dose-related increases in relative liver
weights in all experimental animals. No deviations were reported in
the serum activities of GOT, GPT, AlcP, other than an increase in GPT
activity in mice fed a diet containing 1850 mg DMF/kg. Histo-
pathological evaluation did not reveal any hepatotoxicity (Becci et
al., 1983). The oral administration of a 10% water solution of DMF
(400 mg/kg body weight) for 14 days (Leshik & Feoktistova, 1984) to
guinea-pigs significantly decreased the ascorbic acid content and the
concentration of cytochrome P-450 in the liver. The daily intake of
0.1, 0.5, or 1.0 g DMF/litre in the drinking-water for 2 or 7 weeks
(Elovaara et al., 1983) increased the liver/body weight ratios, the
microsomal UDP-glucuronosyl-transferase and 7-ethoxycoumarin- O-
demethylase activity, and reduced the glutathione concentration in
liver homogenates.
No liver injury was seen following inhalation exposure of dogs to
63 mg DMF/m3 (Kimmerle & Eben, 1975a). However, in another study, 1
out of 4 dogs exposed to 60 mg/m3 showed increased enzyme values
(Clayton et al., 1963)). Liver injury was also not seen: after
inhalation exposure of rats at levels of < 450 mg/m3 (Craig et al.,
1984), after dermal exposure of rats at a level of 240 mg/kg (Bainova,
1985), and at dietary levels of 215 mg/kg body weight per day for rats
and 160 mg/kg for mice (Becci et al., 1983).
8.4.2 Gastrointestinal tract
Toxic gastroenteritis with pathomorphological deviations was
described in the experimental animals treated at high doses or
concentrations in studies reported in Table 14 (Massmann, 1956;
Clayton et al., 1963; Shottek, 1970).
8.4.3 Cardiovascular system
Microscopic examination did not reveal any myocardial lesions in
rats and mice after ingestion of dietary levels of 215, 750, or 2500
mg DMF/kg for 104 days, or 160, 540, or 1850 mg DMF/kg for 119 days,
respectively (Becci et al., 1983).
Clayton et al. (1963) and Germanova et al. (1979) reported
decreases in blood pressure in dogs (not cats) following exposure to
DMF. The changes described were not great and, in the absence of
confirmatory data in other test models (and in man), are of
questionable significance. Large iv doses (500 mg/kg) did not produce
any changes in the contractile force of myocardial tissue in dogs
(Pham Huu Chanh et al., 1973).
Santa Cruz & Maccioni (1978) described histological changes in the
myocardium of the rat and Clayton et al. (1963) described subtle blood
pressure changes in the dog. The findings in the rat followed high
exposures; the blood pressure changes in the dog were minimal and hard
to differentiate from those in control animals.
8.4.4 Kidney
Swelling of the kidney tubules occurred after a single oral
administration of 2250-5000 mg DMF/kg in rats (Kennedy & Sherman,
1986). Short-term feeding studies in rats and mice (Table 14) did not
reveal any histopathological lesions in the kidneys (Becci et al.,
1983; Kennedy & Sherman, 1986).
A number of pathomorphological studies revealed vacuolar
degeneration, mainly in the renal tubules (Massmann, 1956; Clayton et
al., 1963; Santa Cruz & Corpino, 1978; Lundberg et al., 1983). Costa
et al. (1978) observed histological, histochemical, and electron-
microscopic renal lesions in groups of rats, exposed to DMF aerosols
(dose not stated) for 1 h/day for 15 days or for 0.5 h/day for 30
days. Degeneration took place in the proximal part of tubules and in
the visceral epithelium of the glomerulus with marked mitochondrial
changes.
Repeated inhalation exposure to 130 or 300 mg DMF/m3 increased the
kidney/body weight ratio, and decreased diuresis, and the total
protein, sodium chloride, and potassium contents in the urine of rats
(Germanova et al., 1979).
Elovaara et al. (1983) reported enhanced activities of 7-ethoxy-
coumarin- O-demethylase, and UDP-glucuronosyltransferase, and a
decrease in cytosolic formaldehyde dehydrogenase activity in rats
orally exposed to DMF. Bainova & Antov (1980) and Bainova et al.
(1981b) reported that the 30-day dermal application of 960 or 4800 mg
DMF/kg resulted in dose-related increased activities of SucDH, G-6-
PDH, and LDH in rat kidney homogenates.
8.4.5 Nervous system
Functional changes in the nervous system were observed after
administration of high doses or exposure to high concentrations of DMF
(Massmann, 1956; Clayton et al., 1963) and after prolonged treatment
with moderate doses of DMF (Medyankin, 1975; Sanotsky et al., 1978;
Germanova et al., 1979; Bainova, 1985) (Table 14). Doses within the
range of lethal levels resulted in anaesthesia, depression, or coma.
Moderate doses caused inhibition of motor activity.
No effects on the behaviour of rats were noted after they drank
DMF in the drinking-water for 2 and 7 weeks at doses ranging from 1.4
to 13.7 mmol/litre with a cumulative dose of 3200 mg/kg in the rats
(Savolainen, 1981). The same dose enhanced the activities of acid
proteinase and 2,3-cyclicnucleotide-3'-phosphohydrolase in the glial
cells.
Massmann (1956) and Clayton et al. (1963) observed
pathomorphological changes in the brains of experimental animals after
treatment with high doses of DMF (Table 14).
8.4.6 Lungs
Lung congestion and oedema were found in rats after single oral
application of 2250-5000 mg DMF/kg (Kennedy & Sherman, 1986).
Bronchopneumonic changes were observed in experimental animals
after inhalation of high DMF concentrations (Massmann, 1956; Clayton
et al., 1963; Shottek, 1970; Santa Cruz & Corpino, 1978) (Table 14).
According to the authors, the changes were related to injury of the
small arterial vessels and, to some extent, to local irritation caused
by DMF.
8.4.7 Haematopoietic system
High levels of DMF might cause some anaemia, but no other changes
in the erythrocytes of experimental animals have been reported
(Massmann, 1956; Clayton et al., 1963; Sanotsky et al., 1978;
Germanova et al., 1979; Bainova & Antov, 1980) (Table 14). Depressed
bone marrow activity was reported in rats after a single oral
administration of 2250-5000 mg DMF/kg (Kennedy & Sherman, 1986). Pham
Huu Chanh et al. (1971) found leukocytosis in rats after repeated ip
injections of DMF.
Pathomorphological changes were observed in the spleen after
exposure to high doses of DMF. They were accompanied by an increase
in the spleen/body weight ratio (Massmann, 1956; Clayton et al., 1963;
Bainova & Antov, 1980; Bainova, 1985). Medyankin (1975) found
inhibited phagocytosis activity of leukocytes and decreased glycogen
content in the neutrophiles of rats as a result of combined dermal and
inhalation exposure to DMF.
8.4.8 Adrenals
Clayton et al. (1963) observed histological changes in the adrenal
glands after inhalation of DMF. Decreased ascorbic acid content in
the adrenals of rats was reported by Germanova et al. (1979), during
intermittent and continuous inhalation exposure to DMF (Table 14).
8.4.9 Gonads
Male rats were exposed to 584-616 mg DMF/m3 or 49-51 mg/m3 for 4 h
daily for 2, 4, or 8 days; female rats were exposed to 2.3 or 10.7 mg
DMF/m3, for 4 h daily, for 30 days (Sheveleva et al., 1979).
Examination of the sperm and the histological study of the testes and
ovaries did not show any pathological signs.
Lewis et al. (1979) exposed male rats at 90 or 900 mg DMF/m3, for
6 h daily, over 5 days. Gross and histological examination of the
testes did not reveal any pathological changes.
The histological examination of male rats, treated orally in
short-term studies (Becci et al., 1983; Kennedy & Sherman, 1986) with
a variety of doses (Table 14), did not result in changes in the
testes. No lesions were noted in the male rats after a 30-day dermal
application of DMF (Bainova, 1985). Craig et al. (1984) did not find
testicular or ovarian lesions in rats and mice after short-term
inhalation exposure to DMF at concentrations of up to 3600 mg/m3
(Table 14).
Examination of the gonads in a large number of the acute and
repeated-dose toxicity studies discussed earlier did not reveal them
to be a target for DMF toxicity.
8.5 Developmental toxicity and reproduction
DMF was investigated for developmental toxicity in mice, rats, and
rabbits using the oral, dermal, and inhalation routes and parenteral
injection. According to present-day requirements, most of the older
studies were not adequately designed or described. A survey is given
in Tables 15, 16, 17.
No 3-generation reproduction studies were available.
8.5.1 Developmental toxicity
8.5.1.1 Mouse
Administration by gavage of 580 or 193 µl DMF/kg per day, from day
6 to 15 after conception, to 26 mice per dose group led to a dose-
dependent decrease in fetal weights and an increase in the number of
retardations and variations. At 580 µl/kg per day, 17 out of 241
fetuses were malformed (cleft palate, exencephaly, hydro-cephalus
internus, aplasia of presphenoid). No maternal effects were recorded;
the number of live fetuses remained unchanged. At 193 µl/kg, 4 out of
245 fetuses showed malformations. In untreated control groups for
each dose group, 2 out of 229 and 1 out of 310 fetuses, respectively,
had a cleft palate (Hellwig et al., in press).
After an ip injection of 600 or 1100 mg DMF/kg per day, from day 1
to 14 after conception, embryotoxic and teratogenic effects were
registered. The malformation rates were 18 and 75%, respectively, and
the effects consisted of absence or retardation of posterior skull
ossification, open eye-lids, cerebral oedema, sternal haematomas, and
spina bifida-like defects in the thoracic region. Embryotoxic effects
recorded were late resorptions. Doses of 170 mg/kg per day from day 1
to 14 after conception, and 250 mg/kg per day from day 6-14, and
single doses of 2100 mg/kg each on days 3, 7, 9, or 11 after
conception, did not produce any effects (Scheufler & Freye, 1975).
In another ip injection study on mice, 6 animals per dose group
were treated with 0.4 or 1.0 mg DMF/kg per day from day 11 to 15 after
conception. Maternal body weight gain was reduced at 1.0 mg/kg per
day, 2 out of 6 animals had abortions, 7 out of 36 fetuses showed
exencephaly and 1 had a cleft palate. No effects were observed at 0.4
mg/kg (Hellwig et al., in press).
The studies indicate that DMF may be teratogenic for mice.
Table 15. DMF administration to pregnant mice
----------------------------------------------------------------------------------------------------
Route Dosea Maternal Embryotoxicity Malformations Reference
toxicity
----------------------------------------------------------------------------------------------------
Gavage control- - - Hellwig et al.
193 µl/kg - fetal weights 4 of 245 living (in press)
(gavage; day decreased fetuses showed
6-15 pc) malformations
580 µl/kg NRb fetal weights 17 of 241 living
(gavage; day decreased fetuses showed
6-15 pc) malformations
Intra- control - - - Scheufler &
peritoneal 170 mg/kg NRb - - Freye (1975)
injection (day 1-14 pc)
250 mg/kg NRb - -
(day 6-14 pc)
600 mg/kg NRb late malformation
1100 mg/kg resorptions rates 18 and 75%
(day 1-14 pc) respectively
Intra- 0.4 mg/kg - - - Hellwig et al.
peritoneal (day 11-15 pc) (in press)
injection 1.0 mg/kg + 2/6 abortions 8/36
(day 11-15 pc) malformations
----------------------------------------------------------------------------------------------------
a pc = Post conception.
b NR = Not reported.
Table 16. DMF administration to pregnant rats
---------------------------------------------------------------------------------------------------------
Route Dosea Maternal Embryotoxicity Malformations Reference
toxicity
---------------------------------------------------------------------------------------------------------
Gavage control - - - Hellwig et al.
(day 6-15 pc)
533 µl/kg - some embryo-
lethality in the
early phase, +
reduced fetal
weights
+ retardations
+ variations
1600 µl/kg weight 63% embyro- 12% of the 85
stationary lethality in living fetuses
between the median were malformed
day 6-15 phase
Dermal day 6-10 and Hellwig et al.
0 µl/kg - - -
100 µl/kg - - 2.46%
500 µl/kg - - 3.05%
1000 µl/kg - slight reduced 5.46%
fetal length (increase in rib
and vertebral
abnormalities)
Dermal control - - - Stula &
600 mg/kg reduced slight Krauss (1977)
(days 9, 10 + weight embryolethality
11, 11 + 12, gain
12 + 13 pc)
1200 mg/kg reduced slight
(day 10 + 11 pc) weight embryolethality -
gain
Table 16. (contd.)
---------------------------------------------------------------------------------------------------------
Route Dosea Maternal Embryotoxicity Malformations Reference
toxicity
---------------------------------------------------------------------------------------------------------
Dermal 1200 mg/kg reduced slight
(contd.) (day 12 + 13 pc) weight embryolethality -
gain
2400 mg/kg stationary embryolethality -
(day 10 + 11 pc) weight
400 or 200 reduced high embryo- -
mg/kg (applied weight lethality
6 times/day gain
days 11 + 12
+ 13 pc)
Inhalation control - - - Hellwig et al.
(day 0-1, 4-8, weight weights
11-15, 18-19 pc) gain resorptions
861 mg/m3 (287 ppm) reduced reduced fetal -
(day 0-3, 6-10, weight weights
13-18 pc) gain retardations
resorptions
variations
660 mg/m3 (220 ppm) - reduced -
(day 4-8 pc) weight and
length
1560 mg/m3 (520 ppm) reduced embyro- -
(day 4-8 pc) weight lethality;
gain reduced weights
Table 16. (contd.)
---------------------------------------------------------------------------------------------------------
Route Dosea Maternal Embryotoxicity Malformations Reference
toxicity
---------------------------------------------------------------------------------------------------------
Inhalation control - - - Kimmerele &
54 mg/m3 (18 ppm) - - - Machemer
516 mg/m3 (172 ppm) - reduced fetal - (1975)
(6-15 day pc) weights
Inhalation control - - - Keller & Lewis
903 mg/m3 (301 ppm) reduced ossification
(day 6-15 pc) weight variations
gain slightly increased
from 60 to 75% -
Inhalation control - - - Shottek
1200 mg/m3 (400 ppm) total resorp- - (1964)
(4 h/day, 10-20 day pc) NR tions in some
animals, no
retardations
Inhalation 0.05 mg/litre ca.48 mg/m3 weight - Sheveleva &
(ca. 16 ppm) NR decrease Osina (1973)
(day 0-20 pc)
0.8 mg/litre ca.600 mg/m3 embryolethality; -
(ca. 200 ppm) NR weight decrease
Intravenous control - - - Parkie & Webb
injection 0.5 g/kg on - - (1983)
days 10, 11, NR
or 12 pc)
---------------------------------------------------------------------------------------------------------
a pc = Post conception.
b NR = Not reported.
Table 17. DMF administration to pregnant rabbits
---------------------------------------------------------------------------------------------------------
Route Dosea Maternal Embryotoxicity Malformations Reference
toxicity
---------------------------------------------------------------------------------------------------------
Gavage control - - - Merkle &
46.4 µl/kg - - 1 hydrocephalus Zeller (1980)
(day 6-18 pc)
68.1 µl/kg - decrease in number 3 hydrocephalus
(day 6-18 pc) of implantations
and % living
implantations
200 µl/kg decrease in decrease in +
(day 8-16 pc) food intake, fetal weight, 16 fetuses in
weight gain, 3 abortions, 7 litters were
and placental placental weight malformed:
weight decrease
Dermal control - - - Stula &
200 mg/kg some - Krauss (1977)
(day 8-16 pc) embryolethality
Dermal control 100 mg/kg - - - Hellwig et al.
occlusive litter skeletal
(day 6-28 pc) anomalies; 3.3%
agenesia of gall
bladder
Dermal 200 mg/kg per day - - -
400 mg/kg per day decrease in 23.5% fetuses
body weight per litter sternal
on day 16 and anomalies; 2.45%
18 hernia umbilicalis,
6.08% agenesia
of gall bladder
Table 17 (contd.)
---------------------------------------------------------------------------------------------------------
Route Dosea Maternal Embryotoxicity Malformations Reference
toxicity
---------------------------------------------------------------------------------------------------------
Inhalation Control - - - Hellwig et al.
50 ppm)
(day 7-19 pc)
450 mg/m3 decrease in increase in 1 hernia
(150 ppm) body weight sternal umbilicalis
gain variations (50 fetuses)
1350 mg/m3 decrease in decrease in incidence of hernia
(450 ppm) body weight weights, umbilicalis
gain increase in increased;
variations skeletal and soft
(pseudoanky- tissue anomalies
losis)
---------------------------------------------------------------------------------------------------------
a pc = Post conception.
8.5.1.2 Rat
In a gavage study on groups of 26 rats administered 1600 µl DMF/kg
per day from day 6 to 15 after conception, maternal toxicity was
observed in the form of decreased body weight. Sixty-three percent of
the implantations were resorbed and 12% of the surviving 85 (36.64%)
fetuses were malformed (9 cases of diffuse anasarca, 2 cases of tail
aplasia, 1 micrognathia, furthermore several fetuses had anomalies of
the ribs, sternum, and vertebral column). A dose of 533 µl DMF/kg per
day caused some early fetal deaths, in the absence of clinical signs
of maternal toxicity, reduced fetal weight, and also some
malformations, as well as an increase in variations and skeletal
retardations. The malformations consisted of: 2 cases of tail
aplasia, 2 cases of cleft palate, 1 atresia ani, 1 anasarca, 1 open
eye, and several fetuses with split and aplastic vertebrae. At 176
µl/kg, decreased placental weights and some decreases in fetal length
and increases in fetal weight were seen. All other parameters were
within the range of biological variability (Hellwig et al., in press).
The dermal studies on rats did not fulfil today's criteria for a
valid study.
In one series of studies (Hellwig et al., in press), 0, 100, 500,
or 1000 µl DMF/kg per day (undiluted material) were administered in an
uncovered dermal system from day 6 to 10 and then from day 13 to 15
after conception (26 animals per dose group; 10 in the control group).
Under these conditions, 1000 µl/kg per day caused a slightly retarded
weight gain among the dams and significant dermal irritation. The
fetuses were slightly smaller. Malformations consisted of split
thoracic vertebrae and anomalies of the ribs. The rate of the
malformations in live fetuses was 0% per litter in the controls and
2.46%, 3.05%, and 5.46% with increasing dose level. This may indicate
a weak dose-related teratogenic effect.
In another study (Stula & Krauss, 1977), rats received dermal
doses of up to 2400 mg (undiluted DMF)/kg per day, at least every 2
days, from day 9 to 13 after conception, under non-occlusive
conditions. There was clear evidence of embryolethality at 2400 mg/kg
per day on gestation days 10 and 11 (26%, i.e., 7 rats) and at 1200
and 2400 mg/kg per day (in 6 portions of 200 and 400 mg) on gestation
days 11, 12, and 13 with incidences of 43 and 30%, respectively.
Maternal weight gain and average fetal weights were also suppressed.
Fetal abnormalities were not observed, with the exception of a few
subcutaneous haematomas, which occurred at a rate also seen in
historical controls at this laboratory.
Several inhalation studies were carried out on rats. In one
study, 23 animals per dose group were exposed to 54 or 516 mg DMF/m3
(18 or 172 ppm) over 6 h per day from day 6 to 15 after conception.
There were 22 animals in the control group. The higher exposure level
caused a decrease in fetal weights in the absence of signs of maternal
toxicity. No effects were seen on the numbers of implantations,
resorption rates, placental weights, number of fetuses weighing less
than 3 g (runts), variations in skeletal development or malformations;
the lower exposure level (54 mg/m3) did not cause any adverse effects
(Kimmerle & Machemer, 1975).
In another rat study, exposure to 903 mg/m3 (301 ppm) for 6 h per
day, from day 6 to 15 after conception (19 animals per group) led to a
reduction in maternal weight gain and to a slightly increased
incidence of skeletal (ossification) variations of from 60 to 75%.
Exposure to 96 mg/m3 (32 ppm) did not produce any effect (Keller &
Lewis, 1981).
Following 10 exposures to 1200 mg/m3 (400 ppm) for 4 h per day,
from day 10 to 20 after conception, dead implants (54% total
resorptions compared with 15% in the controls) occurred. The numbers
of animals in the treated and control groups were not reported
(Shottek, 1964).
Fetal weight decreases and fetal deaths were reported in another
study on rats after exposure to 600 mg/m3 (200 ppm) from day 0
throughout the gestation period. Exposure to 48 mg/m3 (16 ppm) was
said to have caused reduced fetal weights. However, the description
of the study was inadequate (Sheveleva & Osina, 1973).
A series of inhalation studies on rats is described (Hellwig et
al., in press) in which the exposure periods did not fully cover the
critical period of the gestation phase (e.g., "window dosing" or non-
exposure during weekends, see Table 17). In one set of these
experiments, exposure to concentrations of 660 and 1560 mg/m3 (220 and
520 ppm), for 6 h per day (days 0-3, 6-10, 11-18 after conception; 18
animals per group) caused decreased fetal weights, retardations, and
an increase in embryolethality. Another exposure regimen, i.e., 861
mg/m3 (287 ppm), for 6 h per day, was administered on days 1, 4-8,
11-15, and 18-19 after conception to a group of 30 rats. Twenty
animals were subjected to caesarian section on day 20 after
conception, the offsprings of the other 10 animals were raised until
day 21 after birth. Thirty rats served as untreated controls. There
was retarded maternal weight gain from the beginning of the treatment;
fetal weights were decreased, and the numbers of variations and
retardations were increased. No malformations were found.
No effects were detected after single iv injections of 0.5 g/kg
body weight between days 10, 11, or 12 after conception (Parkie &
Webb, 1983). Furthermore, earlier investigations on rats after single
ip or sc injections did not give any indication of teratogenicity;
however, such studies are of limited value for a toxicity assessment.
The above studies indicate that DMF is embryotoxic in the rat.
After dermal and oral administration, teratogenicity may also occur.
8.5.1.3 Rabbit
DMF caused maternal toxicity and embryotoxicity, including
teratogenicity, in rabbits after administration by gavage at 200 µl/kg
per day from day 6-18 after conception. All 11 animals in the dose
group became pregnant and showed reduced food intake and weight gain.
Placental weights were significantly lower and 3 abortions occurred.
The fetuses showed weight reduction. The main findings recorded on
fetal examination were hernia umbilicalis (7 cases), hydrocephalus
internus (6 cases), eventratio simplex (3 cases), exophthalmus (2
cases), cleft palate (1 case), and malposition of limbs (1 case). The
number of implantations was not adversely influenced. At 68.1 µl/kg
per day, no maternal effects were observed among 16 pregnant animals
(18 inseminated); decreased numbers of total implantations and of live
fetuses occurred, also an increase in skeletal variations and
retardations per litter; 3 cases of hydrocephalus internus were
present. At 46.4 µl/kg per day (10/12 does were pregnant) 1 case of
hydrocephalus internus occurred; all other parameters were in the
range of biological variability. No malformations occurred in the
untreated control group (22/24 animals) (Merkle & Zeller, 1980).
In a dermal study, 5 rabbits (4 animals in the control group)
received 200 mg DMF/kg per day, dermally, from day 8 to day 16 after
conception. The test material was applied in undiluted form on the
intact skin, apparently under non-occlusive conditions. Embryo
mortality was 6% compared with 3% in the controls. The average fetal
weight was 32.9 g in the litters of treated animals compared with 28.4
g among controls. Fetal abnormalities were not detected (Stula &
Krauss, 1977).
In another dermal study (Hellwig et al., in press), dose levels of
undiluted DMF of 0, 100, 200, or 400 mg/kg per day were applied for 6
h/day, under semi-occlusive conditions (15 animals per dose group). A
5-6% decrease in maternal body weights occurred in the highest dose
group, towards the end of the treatment period (days 16-18 after
conception). At this dose level, an increase in skeletal (sternal)
malformations was found in 15 fetuses in 7 litters investigated
(23.5%), and also 5 cases of missing gall bladder (in 2 litters). No
malformations occurred in animals in the 200 mg/kg per day group or in
the untreated control group. At 100 mg/kg per day, one fetus had a
sternal anomaly, 2 fetuses had gall bladder agenesis, and one of the
latter a hypertrophic-dilatative cardiac-aortic malformation.
In a recent inhalation study, exposure levels were 0, 150, 450,
and 1350 mg/m3 (0, 50, 150, and 450 ppm) over 6 h per day from day 7
to day 19 after conception (fifteen animals per dose group). Animals
in the highest exposure group showed a slight retardation in body
weight gain as a sign of maternal toxicity. The fetal weights were
significantly lower in this group, and there was a significant
increase in malformations, mostly hernia umbilicalis (7 out of 86
fetuses, in 4 out of 15 litters) and some soft-tissue malformations,
such as missing gall bladder, without statistical significance. In
addition, anomalies of the sternum, an increase in split vertebrae,
and a number of variations were also recorded. At 450 mg/m3, maternal
body weights were slightly retarded during the exposure period and the
corrected body weight gain was marginally, but significantly,
decreased. One case of hernia umbilicalis among 75 fetuses and an
increase in sternal variations were observed. At 150 mg/m3, neither
fetuses nor does showed any indications of response to treatment. In
summary, signs of embryotoxicity and teratogenicity were seen at the
highest concentration (1350 mg/m3) and to a lesser degree at 450
mg/m3. The maternal toxicity seen at 1350 mg/m3 and 450 mg/m3 is in
accordance with the maternal toxicity observed at 900 mg/m3 in a
previous range-finding study (Hellwig et al., in press).
These studies indicate that DMF may be teratogenic for rabbits.
8.5.1.4 Appraisal
The overall conclusion from all studies is that DMF may lead to
embryotoxic and teratogenic effects in rats, mice, and rabbits. An
increase in malformations in the absence of maternal toxicity is
clearly visible after gavage and ip administration, with a smaller
incidence after dermal administration. In general, the rabbit
appeared to be more sensitive to the teratogenic effects of DMF than
the rat. After inhalation exposure, fetal toxicity and teratogenic
effects appear to be confined to conditions of maternal toxicity.
8.6 Mutagenicity and related end-points
DMF has been tested extensively in mutagenicity and genotoxicity
assays. DMF was one of the 42 chemicals selected for study in the
International Collaborative Program for the Evaluation of Short-Term
Tests for Carcinogenicity (Serres & Ashby, 1981).
The genetic toxicity studies on DMF have been reviewed by Purchase
et al. (1978), Kennedy (1986), US EPA (1986), and IARC (1989).
8.6.1 In vitro studies
In different in vitro assays, DMF did not induce mutations or
genotoxic effects (Table 18). Negative results were obtained with
both in Salmonella typhimurium and Escherichia coli. DMF did not
induce unscheduled DNA synthesis, sister chromatid exchanges,
chromosomal aberrations, or gene mutation in mammalian cells, or
mitotic gene conversion or crossing over in yeasts (Serres & Ashby,
1981).
Table 18. Short-term genotoxicity tests on DMF ( in vitro )
------------------------------------------------------------------------------------------------------------
Method Concentration Condition; comment Results Reference
------------------------------------------------------------------------------------------------------------
Ames test 0.65 x 10-5-1.3 x 10-3 TA 98, 100, 1535, - Antoine et al.
mol/litre 1537, 1538, (1983)
with and without
liver microsome
B. subtilis maximum dose 20 mg/disk - S9, + S9 (rat, yellow - Serres &
Spore rec-assay tail, clam) Ashby (1981)
E. coli differential highest concentration WP2, WP67, CM871 - Serres &
killing test 30 µl/plate Ashby (1981)
E. coli rec-assay highest concentration 1 g/ml 2921, 9239, 8471, 5519, - Serres &
7623, 7689 Ashby (1981)
DNA polymerase deficient 100 µl/ml + S9 and - S9 - Serres &
assay Ashby (1981)
E. coli W3110 -
P3478
Yeast mutation S. pombe - Serres &
Ashby (1981)
S. cerevisiae (XV185-14C) - Serres &
Ashby (1981)
Mitotic recombination S. cerevisiae (JD1) + Serres &
Ashby (1981)
Mitotic crossing-over 10-1000 µg/ml S. cerevisiae (T1, T2) - Serres &
assay Ashby (1981)
Mitotic aneuploidy lowest effective S. cerevisiae (D6) - S9 + Serres &
concentration 100 µg/ml Ashby (1981)
Mitotic gene conversion 5 µl/ml S. cerevisiae (D7) + S9 - Serres &
Ashby (1981)
Mitotic gene conversion 500 µg/ml S. cerevisiae (JD1) - Serres &
Ashby (1981)
Repair test using yeast minimum effective S. cerevisiae (wild & rad) + Serres &
strains (cell growth concentration Ashby (1981)
inhibition) (MEC) 300 µg/ml
Nuclear enlargement 0.01-100 µg/ml human fibroblasts - Serres &
8-200 µg/ml HeLa cells + Ashby (1981)
Table 18. (contd.)
------------------------------------------------------------------------------------------------------------
Method Concentration Condition; comment Results Reference
------------------------------------------------------------------------------------------------------------
UDS test 1.1-90 µg/ml (-S9) human fibroblasts - Serres &
2-30 µg/ml (+S9) (WI - 38) -, + S9 Ashby (1981)
0.032-100 µg/ml human fibroblasts - Serres &
(from skin biopsies) Ashby (1981)
0.1-100 µg/ml HeLa cells - Serres &
Ashby (1981)
Sister chromatid exchange 0.00625-0.1% CHO cells (-, + S9) - Serres &
(SCE) Ashby (1981)
0.1-100 µg/ml CHO cells - Serres &
Ashby (1981)
RL Chromosome assay 75-300 µg/ml Rat liver epithelial type - Serres &
cell line (RL1) Ashby (1981)
Mouse lymphoma 46.9-3000 µg/ml Mouse lymphoma cells - Serres &
mutagenesis assay (L5178Y) Ashby (1981)
-, + S9 rat liver
Human fibroblast 0.2-0.5 mg/ml human lung fibroblast - Serres &
Diphtheria toxin (HSC172) Ashby (1981)
Resistance test
Cell transformation test 500 µg/ml Baby hamster kidney cells + Serres &
(BHK21C13/HRC1) Ashby (1981)
BHK-21 cell - Serres &
Ashby (1981)
Integration enhancement 0.005-0.5 C3H2K cell - Serres &
test (MLV Test) Ashby (1981)
Cytogenetic analysis 1.1 x 10-2-1.1 human peripheral - Antoine et al.
mol/litre lymphocytes (1983)
Cytogenetic analysis 10-20% human peripheral + Koudela &
lymphocytes Spazier (1979)
------------------------------------------------------------------------------------------------------------
Comment: Positive results were obtained with very high concentrations, such as 100-500 µg/ml and 10-20%.
In one study, DMF did not induce any increase in chromosomal
aberrations or sister chromatid exchange in human peripheral blood
lymphocytes in vitro (Antoine et al., 1983). However, in another
study, chromosomal aberrations were reported in human peripheral
lymphocyte cultures treated with DMF (Koudela & Spazier, 1979). The
authors performed cytogenetic analyses of human peripheral lymphocytes
treated with DMF (dilution from 10-7 to 10-2 mol/litre. Compared
with the positive control, thio-TEPA, the clastogenic activity of DMF
was 3- to 4-fold lower. Chromosome aberrations were concentration-
related at DMF levels of 10-20%.
Cytogenetic analysis of peripheral lymphocytes in 40 workers
exposed to 35-180 mg DMF/m3 was performed, first at 4-month
intervals, and later at 6-month intervals. Increased frequencies of
non-specified chromosomal aberrations of 3.82 and 2.74%, respectively,
were found (Koudela & Spazier, 1981). In further sampling periods,
after technological adjustments to decrease the DMF exposure to about
30 mg/m3, the authors established lower frequencies of cell
aberrations in most of the workers, i.e., 1.59, 1.58, and 1.49%, in
the various periods under study. The aberrant cells in the control
group were 1.61-1.10% (Koudela & Spazier, 1979).
8.6.2 In vivo studies
In in vivo studies, DMF was negative in dominant lethal
mutagenic assays, tests for chromosome aberrations and sperm
abnormalities, and micronucleus tests, (Table 19).
8.6.3 Appraisal
The results obtained in the in vitro and in vivo test systems
showed that DMF did not induce damage in genetic material.
8.7 Carcinogenicity
The carcinogenic activity of DMF has not been examined in 2-year
studies on test-animals (Purchase et al., 1978; Barral-Chamaillard &
Rouzioux, 1983; Kennedy, 1986; US EPA, 1986). However, there are some
data concerning DMF, applied as a solvent, and for shorter periods of
time.
In a study by Druckrey et al. (1967) two groups of 15 and 5 BD
rats were treated, with 75 and 150 mg DMF/litre in the drinking-water
for 500 and 250 days, respectively (total doses 38 000 mg/litre in
drinking-water). The animals were observed for up to a maximum of 750
days, with an average survival of 532 days. Similarly, two groups of
12 rats each were given weekly, subcutaneous injections of 200 and 400
mg DMF/kg (total doses 8000 and 20 000 mg/kg, respectively) and
observed for 732 and 766 days, respectively. No tumorigenic effects
were reported in this small group of rats.
Table 19. Short-term genotoxicity tests of DMF ( in vivo )
--------------------------------------------------------------------------------------------------------------
Method Animal Dose route Results Reference
--------------------------------------------------------------------------------------------------------------
Dominant lethal male rat 90, 900 mg/m3 - Lewis et al.
mutagenic bioassay inhalation 6 h daily (1979)
for 5 consecutive days
Chromosome male and 2.3-600 mg/m3 - Sheveleva
aberrations female rat inhalation et al. (1979)
Micronucleus test BALB/C mouse 0.2-2000 mg/kg ip - Antoine
et al. (1983)
Micronucleus test B6C3F1 mouse 80% of LD50 ip - Serres & Ashby (1981)
Micronucleus test ICR mouse 0.425-1.7 mg/kg ip - Serres & Ashby (1981)
Micronucleus test CD-1 mouse 0.4-1.6 mg/kg ip - Serres & Ashby (1981)
Sperm morphology (CBA x BALB/c)F1 0.1-1.5 mg/kg ip - Serres & Ashby (1981)
assay male mouse
--------------------------------------------------------------------------------------------------------------
Kommineni (1973) reported that 9 out of 18 male, and 11 out of 19
female rats, developed tumours in different organs following ip
administrations of 100 mg DMF per rat, once a week, for 10 weeks. The
incidence of tumours in male control rats was 4 out of 14 and, in
females, 5 out of 14, also in different organs. No specific organ or
tumour type predominated in either the test or control group. Three
testicular tumours were seen, a bilateral interstitial cell tumour in
the controls, and an interstitial cell tumour and an embryonal cell
carcinoma in the test group.
8.8 Induction of tumour cell differentiation
Borenfreund et al. (1975) reported a decrease in the malignancy
of the Friend erythroleukaemic cells and a marked increase in their
differentiation along the erythroid pathway after their treatment with
0.5 and 1% DMF solutions.
DMF induction of cell differentiation and a marked reduction of
tumorigenicity was established by Dexter (1977) in transplantable
murine rhabdomyosarcoma cells. In another study, Dexter & Hager
(1980) used 4 carcinoma cell lines derived from two specimens of
adenosarcoma of the human sigmoid colon and showed changes in the
carcinoma cells towards less malignant cell types. Hager et al.
(1980) demonstrated that a cultured human colon carcinoma cell
responded to DMF by more differentiated development, again suggesting
an antitumour effect.
Chakrabarty et al. (1984) studied the effects of DMF on AKR-2B and
AKR-MCA cells in vitro and found that complete loss of anchorage
independent growth occurred and the reduced expression of membrane
antigens was restored.
The DMF induction of tumour cell differentiation has been studied
by Kimball & Hixon (1983) in relation to the deviation of the nuclear
protein. Cordeiro & Savarese (1984) studied it in relation to the
effects on cysteine/glutathione metabolism, and Levine et al. (1985)
in relationship to the changes in receptor occupation and growth
factor responsiveness. Chen et al. (1986) studied the induction of
tumour cells by DMF in relation to the rate of nucleoside transport in
the cells.
A review of the studies on the effects of the induction of
alkylformamides on terminal differentiation of tumour cells (Langdon &
Hickman, 1987) shows that DMF should not be used for such purposes.
The anti-neoplastic activity of DMF, determined in vitro and in vivo,
does not appear to be sufficient for therapeutic use.
8.9 Mechanism of toxicity, mode of action
Several hypotheses on the possible mechanism of DMF hepatotoxicity
have been tested. No experimental support for lipid peroxidation,
lysosome labilization, or glutathione depletion has been reported.
The critical biological effects leading to DMF hepatotoxicity have not
been identified and still need to be elucidated (Scailteur & Lauwerys,
1987).
9. EFFECTS ON HUMAN BEINGS
9.1 General population exposure
No effects of DMF on the general population have been reported.
9.2 Occupational exposure
Reports of occupational poisonings with DMF have been reviewed by
Kennedy (1986), US EPA (1986), and Scailteur & Lauwerys (1987).
9.2.1 Accidental poisoning
Several cases of acute accidental occupational poisoning with DMF
have been reported (Tolot et al., 1968; Potter, 1973; Chary, 1974;
Chivers, 1978; Aldyreva & Gafurov, 1980; Kang-de & Hui-lan, 1981;
Shlygina & Nemolshev, 1981; Paoletti et al., 1982a,b). They were
caused by the malfunctioning of the equipment, splashing of the
organic solvent over the body, or working in plants without taking
protective measures. Over-exposure has occurred via the skin and/or
inhalation. Usually, the symptoms appeared from several hours up to
several days after the accident. The major symptoms were epigastric
or abdominal pain, which was irradiating and progressive, accompanied
by dizziness, nausea, anorexia, vomiting, fatigue, alcohol
intolerance, and skin irritation. Clinical laboratory tests showed
liver function disturbance. Radioisotope diagnostic tests and liver
biopsy revealed morphological changes in the liver. No clinical
manifestations of renal dysfunction were reported. The patients
recovered with symptomatic therapy in hospital for 2-3 weeks. Liver
function tests returned to normal. Some of the patients, who were
followed for several months or several years after the acute
poisoning, had normal function tests.
9.2.2 Long-term exposure
After occupational exposure to DMF (intensity and length of
exposure unspecified, no control groups), eye irritation, headache,
anorexia, gastrointestinal disturbances, and sometimes hepatomegaly
with biochemical signs of liver damage were reported. Some
haematological changes were also observed (Tolot et al., 1958; Weiss,
1971; Dilorenzo & Grazioli, 1972). In studies in which exposure was
quantified, subjective complaints of headache, fatigue, and
gastrointestinal and cardiovascular changes were reported.
Disturbances of liver function could be measured by changes in plasma
bilirubin levels, and increases in the serum activity of liver enzymes
(transaminases, alkaline phosphatase, glutamyl-transpep-tidase).
Alcohol intolerance occurred. Haematological changes and ECG
deviations were also observed (Table 20).
In a questionnaire study, for which little detail is available,
Schottek (1972) reported 14% miscarriages in a group of women exposed
to about 100 mg DMF/m3 compared with 10% in the control group. No
statistical analysis was performed. Aldyreva & Gafurov (1980)
reported perturbations in menstruation in 26 out of 70 women who had
been exposed to 30-150 mg DMF/m3 for about a year. No data are
available on controls. On the basis of company statistics, general
morbidity associated with gynaecological changes appeared to be
increased among DMF-exposed women.
Farquharson et al. (1983) reported miscarriages in 3 out of 9
women, who had been exposed to DMF as well as a number of other
chemicals.
Because of its effect on the stratum corneum, DMF interferes with
the barrier function of the skin; this was demonstrated in human
volunteers by increased water loss following DMF exposure (Baker,
1968).
9.2.3 Epidemiological studies on carcinogenicity
Ducatman et al. (1986) reported three cases of testicular germ-
cell tumours in 1981-83 among 153 white men who repaired the exterior
surfaces and electrical components of F4 Phantom jet aircraft, in the
USA. This finding led to surveys of two other repair shops at
different geographical locations; in one of the shops, the same type
of aircraft was repaired, while in the other, different types of
aircraft were repaired. Four out of 680 white male workers in the
same type of repair shop had a history of testicular germ-cell cancers
(0.95 expected) occurring in 1970-83. No case of testicular germ-cell
cancer was found among the 446 white men employed at the facility
where different types of aircraft were repaired. Of the 7 cases of
testicular germ cell tumours, 5 were seminomas and 2 were embryonal-
cell carcinomas. All 7 men had long histories of working in aircraft
repair. The time from first exposure to diagnosis ranged from 4 to 19
years. There were many common exposures to solvents in the three
facilities, the only exposure identified as unique to the F4 Phantom
jet aircraft repair facilities, where the cases occurred, being to a
solvent mixture containing 80% dimethyl-formamide (20% unspecified).
No quantitative exposure data exist. Three of the cases had certainly
been exposed to this mixture and 3 cases, probably exposed. The cases
were found through foremen and from filed death certificates, and the
authors suggested that under-reporting was possible. No other cases
of cancer were investigated.
Levin et al. (1987) described 3 cases of embryonal-cell carcinoma
of the testes in workers at one leather tannery in the USA, all of
whom had worked as swabbers on the spray lines in leather finishing.
The latency period was from 8 to 14 years. According to the authors,
all the tanneries surveyed used dimethylformamide, as well as a wide
range of dyes, solvents, and other chemicals. No quantitative
exposure data are available. The number of workers from which these 3
cases arose was not given, and other cancers were not looked for.
Table 20. Studies on workers with long-term exposures
---------------------------------------------------------------------------------------------------------------------
Number Number Length DMF exposure Urinary Hepato- Alcohol Other effects Reference
of of non- of (mg/m3) NMF toxicity intoler-
exposed exposed exposure ance
subjects controls (years)
---------------------------------------------------------------------------------------------------------------------
22 28 5 1-47 (usually 20-63 - + NR Lauwerys
< 30; gloves mg/g et al. (1980)
worn) creatinine
11 - 3 3-15 0.4-20 - +(6)a NR Yonemoto &
mg/24 h Suzuki (1980)
28 29 3-5 30-60 NR - NR complaints of eye and Hinkova et al.
respiratory tract (1980)
irritation; no haemato-
logical changes
115 67 1-1.5 30-150 with NR + NR complaints of gastro- Aldyreva &
higher peaks + (a few out intestinal tract or Gafurov (1980)
skin exposure of 29) cardiovascular and
ovarian distur-
bances (29)
177 - 3-5 10-30 NR - NR complaints of Aldyreva &
cardiovascular Gafurov (1980)
disturbances (45)
81 96 3.5 < 10 NR +(10) NR complaints of gastro- Kang-de &
accidental intestinal tract and Hui-Lan (1981)
peak levels up cardiovascular
to 4525 + skin disturbances, ECG
exposure changes
23 - 2 > 30 peaks 10-40 NR NR No ECG changes com- Taccola et al.
up to 150 mg/day pared with pre-exposure (1981)
27 237 2 2-80 peaks NR +(2) +(8) complaints of gastro- Paoletti &
up to 549 intestinal tract Iannaccone
disturbances (15); (1982)
headache (6)
---------------------------------------------------------------------------------------------------------------------
Table 20. (contd.)
---------------------------------------------------------------------------------------------------------------------
Number Number Length DMF exposure Urinary Hepato- Alcohol Other effects Reference
of of non- of (mg/m3) NMF toxicity intoler-
exposed exposed exposure ance
subjects controls (years)
---------------------------------------------------------------------------------------------------------------------
13 - < 4 14-60 NR +(2) +(8) complaints of gastro- Tomasini
(mean: 29) intestinal tract et al (1983)
disturbances (8); eye
irritation (11); kidney
function test and
haematology, normal
26 54 > 5 2-5 NR - NR NR Catenacci
(mean: 3) et al.(1984)
28 54 > 5 12-25 NR - NR NR Catenacci
(mean: 18) et al. (1984)
100 100 5 8-58 NR + +(39) complaints of headache, Cirla et al.
(mean: 22) (8 versus eye and throat (1984)
2 controls) irritation, gastro-
intestinal tract and
cardiovascular
disturbance
24 29 5 10-60 NR NR NR irritative dermatitis Bainova (1985)
15 28 6-10 20-30 NR NR NR increased coagulation Imbriani
(median 27) time et al. (1986)
---------------------------------------------------------------------------------------------------------------------
a Incidence is indicated when available.
NR = not reported
To evaluate the significance of this cluster, an analysis of the
New York State Cancer Registry was conducted. Occupations were
determined from cancer registries and from death certificates for all
residents in Fulton County who were diagnosed as having testicular
cancer from 1974-88. From preliminary results, it is estimated that
workers who are employed in the leather tanning industry are 5-6 times
more likely to develop testicular cancer than those who are not
leather workers. However, the testicular cancer rates in this county
were lower than expected within this period, and an adjacent county
showed the same number of cases of testicular cancer, none of the
affected individuals having ever worked in the leather industry
(Walrath et al., 1988).
O'Berg et al. (1985) and Chen et al. (1988a) studied the cancer
incidence among 2530 actively employed workers with potential exposure
to dimethylformamide between 1956 and 1984, 1329 employees with
exposure to dimethylformamide and acrylonitrile at an acrylic fibre
manufacturing plant in South Carolina, USA, and 1130 controls from the
same plant. Cancer incidence rates for the company (1956-84) and USA
national rates (1973-77) were used to calculate the expected number of
cases. For all workers exposed to DMF (alone or with acrylonitrile),
the standardized incidence ratio (SIR), based on company rates for all
cancers combined, was 110 [95% confidence interval (CI), 88-136]a (88
cases); the SIR on the basis of national rates was 92. The SIR for
cancer of the buccal cavity and pharynx was 344 [CI, 172-615]a (11
cases), on the basis of company rates, and 167, on the basis of US
rates. More cancer cases than expected from company rates (34 cases:
SIR 134 [CI, 98-195]a) were found among employees exposed to
dimethylformamide alone, due mainly to 8 carcinomas of the buccal
cavity and pharynx versus 1.0 expected (SIR, 800 [CI, 345-1580]a).
All of these cases either smoked or chewed tobacco, but no information
was available on the smoking, tobacco chewing, or drinking habits of
the cohort. An additional case occurred among employees exposed to
dimethyl-formamide alone (SIR, 167); 4 of these tumours were cancers
of the lip. No such excess was found among the workers exposed to
both dimethylformamide and acrylonitrile (2 observed; SIR, 125, on the
basis of company rates). The authors reported that there was no
association with intensity or duration of exposure: "low" and
"moderate" exposure SIR 420 (5 cases); "high" exposure SIR 300 (6
cases). "Low" exposure conditions included: no direct contact with
liquids containing any dimethylformamide, even wearing protective
equipment; and workplace air levels consistently below 30 mg DMF/m3
(10 ppm) (no odour of DMF evident). "Moderate" exposure conditions
included: intermittent contact with liquids containing > 5%
dimethylformamide; workplace air levels sometimes higher than 30 mg
DMF/m3 (10 ppm) (more than once per week); DMF-laden materials
handled, but air levels of DMF maintained at above levels. "High"
exposure conditions included: frequent contact with liquids containing
> 5% dimethylformamide; workplace air levels often > 30 mg DMF/m3
(> 10 ppm); use of breathing protection often required for periods of
15 min-1 h; DMF vapour levels frequently > 30 mg/m3 (> 10 ppm)
(when handling pure dimethylformamide or dimethylformamide-containing
---------------------------------------------------------------------------
a As calculated by IARC (1989).
materials). One case of testicular cancer was found among the 3859
workers exposed to DMF (alone or with acrylonitrile) with 1.7 expected
on the basis of company rates, and no cases of liver cancer. The
company rates may be more relevant for comparison, as there were only
actively employed persons among the exposed and because the USA rates
are based on a limited time period, 1973-77.
Chen et al. (1988b) analysed mortality from 1950-82 among both
active and pensioned employees in the same cohort. Expected numbers
(adjusted for age and time period) were based on company rates. For
all workers exposed to DMF (alone or with acrylonitrile), the
standardized mortality ratio (SMR) for lung cancer was 124 (33 cases
[95% CI, 85-174]a). An increased risk of lung cancer was found in the
cohort exposed only to DMF (19 cases; SMR 141 [CI, 84-219]a), but not
in the cohort exposed to DMF and acrylonitrile. There were 3 deaths
from cancer of the buccal cavity and pharynx (SIR 188) in all persons
exposed to DMF (alone or with acrylo-nitrile). No other excess cancer
risk was reported. No information is given in this report on loss to
follow-up, death certificates, or whether these deaths were included
in the incidence study reported above.
Walrath et al. (1988) reported a case-control study on cancer
among 8724 Du Pont employees with potential exposure to DMF in 4 other
USA plants. Summary analyses for all plants combined did not show any
statistically significant association between ever having been exposed
to DMF and subsequent development of cancers of the buccal cavity and
pharynx, liver, malignant melanoma, prostate, and testes. When odds
ratios are examined according to plant site, prostate cancer at one
site was significantly elevated, on the basis of 3 cases exposed out
of 4, but no statistically significant association was observed among
employees similarly exposed to DMF in the other 3 plants. The recency
of exposure to DMF, the low exposures received, and the absence of
similar excesses at other plants argue against a causal association
between DMF exposure and prostate cancer at the one site. Assessment
of highest DMF exposure rank, duration of exposure, and latent period,
does not show any patterns suggesting an association between DMF and
cancers of the buccal cavity and pharynx, liver, malignant melanoma,
prostate, or testes.
9.2.4 Alcohol intolerance
Reviews on the synergistic action of ethanol with organic solvents
have been published by Haguenoer et al. (1982), Hills & Venable
(1982), and Stockley (1983).
Episodes of alcohol intolerance among workers exposed to DMF have
been repeatedly described at all levels of exposure (see sections
9.2.1 and 9.2.2, and Table 20).
Symptoms include flushing of the face, dizziness, nausea,
tightness of the chest, sometimes dyspnoea, and cardiac palpitations.
The reactions were reported within 24 h of DMF exposure and very
shortly after alcohol ingestion. These episodes lasted for up to 2 h
---------------------------------------------------------------------------
a As calculated by IARC (1989).
(Chivers, 1978; Lyle et al., 1979; Yonemoto & Suzuki, 1980; Paoletti
et al., 1982a; Cirla et al., 1984).
According to Loos (1979), abnormal liver function tests were
already discovered among workers who drank only 50-70 g alcohol per
day, but were also exposed to 45-66 mg DMF/m3, while the threshold
consumption for functional liver changes was 80-100 g alcohol per day
in control individuals. It should be noted that the test group was
also exposed to other solvents, mainly tetrahydrofuran, toluene, and
xylene.
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The International Agency for Research on Cancer (IARC) evaluated
the carcinogenicity of dimethylformamide in 1988 (IARC, 1989), and
concluded that:
- there is limited evidence for the carcinogenicity of
dimethylformamide in humans;
- there is inadequate evidence for the carcinogenicity of
dimethylformamide in experimental animals.
Overall evaluation: Dimethylformamide is possibly carcinogenic
to humans (Group 2B).
REFERENCES
ALDYREVA, M.V. & GAFUROV, S.A. (1980) [ Labour protection in the production
of artificial leather.] Moscow, Medizina, 158 pp (in Russian).
ALDYREVA, M.V., BORTSEVICH, S.V., PALAGUSHINA, A.I., SIDOROVA, N.V., &
TARASOVA, L.A. (1980) [Effect of dimethylformamide on workers' health in
the production of polyurethane synthetic leather.] Gig. Tr. prof. Zabol.,
6: 24-28 (in Russian).
AMSTER, M.B., HIJAZI, N., & CHAN, R. (1983) Real time monitoring of low
level air contaminants from hazardous waste sites. In: Proceedings of the
National Conference on Management of Uncontrolled Hazardous Waste Sites.
Washington, DC, pp. 98-99.
ANTOINE, J.L., ARANY, J., LEONARD, A., HENROTTE, J., JENAR-DUBUISSON, G., &
DECAT, G. (1983) Lack of mutagenic activity of dimethylformamide.
Toxicology, 26: 207- 212.
ARENA, N., SANTA CRUZ, G., ALIA, E.F., BALDUS, M., CORGIOLU, T., & ALIA,
E.E. (1982) [Structural and ultrastructural changes in the myocardium of
rabbits exposed to dimethylformamide vapours.] Boll. Soc. Ital. Biol.
Sper., 58: 1496-1501 (in Italian).
BAINOVA, A. (1980) [Contemporary views on the hygiene standardization of
dimethyl- formamide.] Letopisi HEI, 37: 62-68 (in Bulgarian).
BAINOVA, A. (1985) [ Toxicological problems due to the action of chemical
substances on the skin (dermatotoxicology).] Sofia, Referat, pp. 1-60 (D.
Sci. Thesis) (in Bulgarian).
BAINOVA, A. & ANTOV, G. (1980) Dermal toxicity of dimethylformamide in
rats. In: Abstracts of the 5th International Symposium on Occupational
Health in the Production of Artificial Fibres, Belgirate, Italy, 16-20
September, 1980, Modena, Permanent Commission and International Association
on Occupational Health, pp. 73-74.
BAINOVA, A., SPASSOVSKI, M., HINKOVA, L., & TASHEVA, M. (1981a) [Effect
of intermittent and continuous action of dimethylformamide on the
adaptation process.] Probl. hig., 6: 27-35 (in Bulgarian).
BAINOVA, A., ANTOV, G.P., & IVANOVICH, E.H. (1981b) Combined action of
dimethylformamide and noise on kidneys of white rats. CR. Acad. bulg. Sci.,
34: 1609-1611.
BAKER, H. (1968) The effects of dimethylsulfoxide, dimethylformamide, and
dimethyl- acetamide on the cutaneous barrier to water in human skin. J.
invest. Dermatol., 50: 283- 288.
BARNES, J.R. & HENRY, N.W. (1974) The determination of N-methylformamide
and N-methylacetamide in urine. Am. Ind. Hyg. Assoc. J., 35: 84-87.
BARNES, J.R. & RANTA, K.E. (1972) The metabolism of dimethylformamide
and dimethylacetamide. Toxicol. appl. Pharmacol., 23: 271-276.
BARRAL-CHAMAILLARD, J. & ROUZIOUX, M. (1983) Dimethylformamide. Arch.
Mal. prof., 44: 203-208.
BARTSCH, W., SPONER, G., DIETMAN, K., & FUCHS, G. (1976) Acute toxicity
of various solvents in the mouse and rat. Arzneimittelforschung, 26: 1581-
1583.
BECCI, P.J., VOSS, K.A., JOHNSON, W.D., GALLO, M.A., & BABISH, J.G. (1983)
Subchronic feeding study of N,N-dimethylformamide in rats and mice. J. Am.
Coll. Toxicol., 2(6): 371-378.
BEGERT, A. (1975) [Purification of chemical textile plant sewage.]
Oesterr. Abwasser- Rundsch., 20: 98-102 (in German).
BORENFREUND, E., STEINGLASS, M., KORNGOLD, G., & BENDICH, A. (1975)
Effect of dimethylsulfoxide and dimethylformamide on the growth and
morphology of tumour cells. Ann. N.Y. Acad. Sci., 243: 164-171.
BORTSEVICH, S.V. (1984) [The hygienic importance of dimethylformamide
absorption through the skin.] Gig. Tr. prof. Zabol., 11: 55-57 (in
Russian).
BRINDLEY, C., GESCHER, A., & ROSS, D. (1983) Studies of the metabolism of
dimethylformamide in mice. Chem.-biol. Interact., 45: 387-392.
BRONDEAU, M.T., BONNET, P., GUENIER, J.P., & DE CEAURRIZ, J. (1983)
Short-term inhalation test for evaluating industrial hepatotoxicants in
rats. Toxicol. Lett., 19: 139-146.
BRUGNONE, F., PERBELLINI, L., & GAFFURI, E. (1980a) N,N-Dimethylformamide
concentration in environmental and alveolar air in a artificial leather
factory. Br. J. ind. Med., 37: 185-188.
BRUGNONE, F., PERBELLINI, L., GAFFURI, E., & ASASTOLI, P. (1980b)
Biomonitoring of industrial solvent exposures in workers' alveolar air.
Int. Arch. occup. environ. Health, 47: 245-261.
BRUGNONE, F., PERBELLINI, L., GAFFURI, E., & TURRI, P.V. (1984)
Monitoring of industrial exposure to dimethylformamide by analysis of
alveolar air. Med. Lav., 6: 139- 141.
BURGUN, J., MARTZ, R., FORNEY, R.B., & KIPLINGER, G.F. (1975) The acute
toxicity of dimethylformamide and its combined effects with ethanol in the
mouse. Toxicol. appl. Pharmacol., 33: 149-150.
CAI, S.X. & HUANG, M.Y. (1979) Investigation on occupational hazard in a
butadiene monomer workshop of a cis-butadiene rubber plant. J. Hyg. Res.,
8(1): 22-49 (in Chinese).
CARDWELL, R.D., FOREMAN, D.G., PAYNE, T.R., & WILBUR, D.J. (1978) Acute
and chronic toxicity of four chemicals to fish. Duluth, Minnesota, US
Environmental Protection Agency (Contract 68-01-0711).
CATENACCI, G., GRAMPELLA, D., TERZI, R., SALA, H., & POLLINI, G., (1984)
Hepatic function in subjects exposed to environmental concentrations of DMF
lower than the actually proposed TLV. Med. Lav., 6: 157-158.
CHAKRABARTY, S., MCRAE, L.J., LEVINE, A.E., & BRATTAIX, M.G. (1984)
Restoration of normal growth control and membrane antigen composition in
malignant cells by N,N-dimethylformamide. Cancer Res., 44: 2181-2185.
CHARY, S. (1974) Dimethylformamide. A case of acute pancreatitis. Lancet,
2(7876): 356.
CHEN, J.L., FAYERWEATHER, W.E., & PELL, S. (1988a) Cancer incidence of
workers exposed to dimethylformamide and/or acrylonitrile. J. occup. Med.,
30: 813-818.
CHEN, J.L., FAYERWEATHER, W.E., & PELL, S. (1988b) Mortality study of
workers exposed to dimethylformamide and/or acrylonitrile. J. occup. Med.,
30: 819-821.
CHEN, S.-F., CLEAVELAND, J.S., HOLLMAN, A.B., WIEMANN, M.C., PARKS, R.E., &
STOECKLER, J.D. (1986) Changes in nucleoside transport of HL-60 human
promyelocytic cells during N,N-dimethylformamide induced differentiation.
Cancer Res., 46: 3449-3455.
CHIVERS, C.P. (1978) Disulfiram effect from inhalation of
dimethylformamide. Lancet, 1(8059): 331.
CHROMEK, J., KUPEK, J., MLADEK, M., & MARVAN, P. (1983) A study of
respiration of the alga Sceadesmus quadricauda in batch conditions under
influence of N,N-dimethylformamide and dimethylamine. Arch. Hydrobiol.,
64(Suppl.): 441-460.
CIRLA, A.M., PISATI, G., INVERNIZZI, E., & TORRICELLI, P. (1984)
Epidemiological study on workers exposed to low dimethylformamide
concentrations. G. Ital. Med. Lav., 6: 149-156.
CLAY, P.F. & SPITTLER, T.M. (1983) Determination of airborne volatile
nitrogen compounds using four independent techniques. In: Proceedings of
the National Conference on Management of Uncontrolled Hazardous Waste
Sites, Washington, DC, pp. 100-104.
CLAYTON, J.W., BARNES, J.R., HOOD, D.B., & SCHEPERS, G.W. (1963) The
inhalation toxicity of dimethylformamide (DMF). Am. Ind. Hyg. Assoc. J.,
24: 144-154.
CORDEIRO, R.F. & SAVARESE, T.M. (1984) Reversal by L-cysteine of the
growth inhibitory and glutathione-depleting effects of N-methylformamide
and N,N-dimethylformamide. Biochem. biophys. Res. Commun., 122: 798-803.
COSTA, V., FRONGIA, N., & SANTA CRUZ, G. (1978) [Histological and
ultrastructural changes of the rat renal glomerulus caused by
dimethylformamide inhalation.] Boll. Soc. Ital. Biol. Sper., 54: 1723-1728
(in Italian).
CRAIG, D.K., WEIR, R.J., WAGNER, W., & GROTH, D. (1984) Subchronic
inhalation toxicity of dimethylformamide in rats and mice. Drug chem.
Toxicol., 7: 551-571.
DARNALL, K.R., LLOYD, A.C., WINER, A.M., & PITTS, J.N. (1976) Reactivity
scale for atmospheric hydrocarbons based on reaction with hydroxyl radical.
Environ. Sci. Technol., 7: 692-696.
DEXTER, D.L. (1977) N,N-Dimethylformamide-induced morphological
differentiation and reduction of tumorigenicity in cultured mouse
rhabdomyosarcoma cells. Cancer Res., 37: 3136-3140.
DEXTER, D.L. & HAGER, J.C. (1980) Maturation induction of tumour cells
using a human colon carcinoma model. Cancer, 45: 1178-1184.
DILORENZO, F. & GRAZIOLI, C. (1972) [Hematological, hematochemical, and
gastric findings in workers exposed to breathing vapours of
dimethylformamide.] Lav. Um., 24: 96-106 (in Italian).
DIXON, S.W., GRAEPEL, G.J., & LOONEY, W.C. (1983) Seasonal effects on
concentrations of monomethylformamide in urine samples. Am. Ind. Hyg.
Assoc. J., 44: 273-275.
DOJLIDO, J.R. (1979) Investigations of biodegradability and toxicity of
organic compounds. Washington, DC, US Environmental Protection Agency
(Unpublished report No. EPA-600/2-79-163).
DRUCKREY, H., PREUSSMANN, R., IVANKOVIC, S., & SCHMAHL, D. (1967)
[Organotropic carcinogenic effects of 65 different N-nitroso-compounds on
B.D. rats.] Z. Krebsforsch., 69: 103-201 (in German).
DUCATMAN, A.M., CONWILL, D.E., & CRAWL, J. (1986) Germ cell tumours of
the testicle among aircraft repairmen. J. Urol., 136: 834-836.
EBEN, A. & KIMMERLE, G. (1976) Metabolism studies in N,N-
dimethylformamide. III. Studies on the influence of ethanol in persons and
laboratory animals. Int. Arch. occup. environ. Health, 36: 243-265.
EBERLING, C.L. (1980) Formic acid and derivatives (DMF). In: Kirk-
Othmer encyclopedia of chemical technology, 3rd ed., New York, Chichester,
Brisbane, Toronto, John Wiley & Sons, Vol. 11, pp. 263-268.
ELOVAARA, E., MARSELOS, M., & VAINO, H. (1983) N,N-Dimethylformamide-
induced effects on hepatic and renal xenobiotic enzymes with emphasis on
aldehyde metabolism in the rat. Acta. pharmacol. toxicol., 53: 159-165.
EWING, B.B., CHIAN, E.S.K., COOK, J.C., EVANS, C.A., HOPKE, P.A., &
PERKINS, E.G. (1977) Monitoring to detect previously unrecognized
pollutants in surface water. Appendix: Organic analysis data, Washington,
DC, US Environmental Protection Agency, (Unpublished report No. EPA-560/6-
77-015).
FARHI, M., MOREL, M., & CAVIGNEAUX, A. (1968) Dimethylformamide.
NCON(CH3)2. Cah. Notes doc., 50: 91-93.
FARLEY, F.F. (1977) Photochemical reactivity classification of
hydrocarbons and other organic compounds. In: International Conference on
Photochemical Oxidant Pollution and Its Containment, Research Triangle
Park, North Carolina, US Environmental Protection Agency. (Unpublished
report No. EPA-600/3-77-0018).
FARQUHARSON, R.O., HALL, M.A., & FULLERTON, W.T. (1983) Poor obstetric
outcome in three quality control laboratory workers. Lancet, 30: 983-984.
GERMANOVA, A.L., HALEPO, A.I., AVILOVA, G.G., ANVAER, L., HOROCHULOVA,
N.V., MALTSEVA, N.M., & MIGUKINA, N.V. (1979) [Adaptation after
continuous and intermittent exposure to dimethylformamide.] In: [ The
toxicology of new industrial chemicals.] Moscow, Medizina, Vol. 15, pp. 69-
76 (in Russian).
GRASSELLI, J.G. (1973) Atlas of spectral data and physical constants for
organic compounds, Cleveland, Ohio, USA, The Chemical Rubber Co.
GUBSER, H. (1969) Purification of chemical waste waters. Gas Wasser
Abwasser, 49: 175-181.
HAGER, J.C., GOLD, D.V., BARBOSA, J.A., FLIGIEL, L., MILLER, F., & DEXTER,
D.L. (1980) N,N-Dimethylformamide-induced modulation of organ and tumour
associated markers in cultured human colon carcinoma cells. J. Natl Cancer
Inst., 64: 439-445.
HAGUENOER, J.M., BOURRINET, P., & FRIMAT, P. (1982) Interrelations
between alcoholism and exposure to industrial poisons. Arch. Mal. prof.,
43: 461-473.
HAMILTON, A. & HARDY, H.L. (1974) Industrial toxicology, 3rd ed., Acton,
Massachusetts, Publishing Science Group Inc., 349 pp.
HANASONO, G.K., FULLER, R.W., BRODDLE, W.D., & GIBSON, W.R. (1977)
Studies on the effects of N,N-dimethylformamide on ethanol disposition and
on monoamine oxidase activity in rats. Toxicol. appl. Pharmacol., 39: 461-
472.
HELLWIG, J., MERKLE, J., KLIMISCH, H.J., & JÄCKH, R. (in press)
Investigations on the prenatal toxicity of N,N-dimethylformamide (DMF) in
mice, rats and rabbits. Food chem. Toxicol..
HENRY, N.W. & SCHLATTER, C.N. (1981) The development of a standard method
for evaluating chemical protective clothing to permeation by hazardous
liquids. Am. Ind. Hyg. Assoc. J., 42: 202-207.
HILLS, B.W. & VENABLE (1982) The interaction of ethyl alcohol and
industrial chemicals. Am. J. ind. Med., 3: 321-333.
HINKOVA, L., GINCHEVA, N., STAMOVA, N., HRISTEVA, V., CHOLAKOV, B., &
SASSOVSKY, M. (1980) [Influence of dimethylformamide on workers' health.]
Probl. hyg., Sofia, 5: 75-81 (in Bulgarian).
HUANG, M.Y., LUO, Y.Z., GENG, T.B., MENG, D.S., LIU, J., HUANG, M.F., &
WANG, Y.S. (1981) [Studies on the dermal toxicity of dimethylformamide.]
J. Hyg. Res., 10(4): 21-26 (in Chinese).
HUGHES, J.S. & VILKAS A.G. (1983) Toxicity of N,N-dimethylformamide used
as a solvent in toxicity tests with the green alga Selenastrum capricornum.
Bull. environ. Contam. Toxicol., 31: 98-104.
IARC (1989) Dimethylformamide. In: Some organic solvents, resin monomers
and related compounds, pigments and occupational exposures in paint
manufacture and painting, Lyon, International Agency for Research on
Cancer, pp. 171-197 (IARC Monograph on the Evaluation of Carcinogenic Risks
to Humans, Vol. 47).
IMBRIANI, M., CHITTORI, S., PRESTINONI, A., LONGONI, P., CASCONE, G., &
GAMBA, G. (1986) Effects of dimethylformamide (DMF) on coagulation and
platelet activity. Arch. environ. Health, 41: 90-93.
KANG-DE, C. & HUI-LAN, Z. (1981) Observation on the effects of
dimethylformamide on human health. In: Abstracts of the 9th International
Congress on Occupational Health in the Chemical Industry, Aswan, Egypt, 15-
17 September 1981, Aswan, Egypt, Permanent Commission and International
Association on Occupational Health, pp. 22-23.
KELLER, C.A. & LEWIS, S.C. (1981) Inhalation teratology study of N,N-
dimethyl- formamide (DMF). Teratology, 23: 45A.
KENNEDY, G.L., Jr (1986) Biological effects of acetamide, formamide, and
their monomethyl and dimethyl derivatives. CRC crit. Rev. Toxicol., 17(2),
129-182.
KENNEDY, G.L. & SHERMAN, H. (1986) Acute and subchronic toxicity of
dimethylformamide and dimethylacetamide following various routes of
administration. Drug chem. Toxicol., 9: 147-170.
KESTELL, P., GILL, M.H., THREADGILL, M.D., GESCHER, A., HOWARTH, O.W., &
CURZON, E.H. (1986) Identification by proton NMR of N-(hydroxymethyl)- N-
methylformamide as the major urinary metabolite of N,N-dimethylformamide
in mice. Life Sci., 38: 719- 724.
KESTELL, P., THREADGILL, M.D., GESCHER, A., GLEDHILL, A.P., SHAW, A.J., &
FARMER, R.B. (1987) An investigation of the relationship between the
hepatotoxicity and the metabolism of N-alkylformamides. J. Pharmacol. exp.
Ther., 270: 265-270.
KIMBALL, P.M. & HIXON, S. (1983) Nuclear protein changes following N,N-
dimethylformamide (DMF) induced maturation. J. cell. Biochem., 22: 245-249.
KIMMERLE, G. & EBEN, A. (1975a) Metabolism studies of N,N-
dimethylformamide. I. Studies in rats and dogs. Int. Arch. Arbeitsmed.,
34: 109-126.
KIMMERLE, G. & EBEN, A. (1975b) Metabolism studies of N,N-
dimethylformamide. II. Studies in persons. Int. Arch. Arbeitsmed., 34:
127-136.
KIMMERLE, G. & MACHEMER, L. (1975) Studies with N,N-dimethylformamide for
embryotoxic and teratogenic effects on rats after dynamic inhalation. Int.
Arch. Arbeitsmed., 34: 167-175.
KIMURA, E.T., EBERT, D.M., & DODGE, P.W. (1971) Acute toxicity and limits
of solvent residue for sixteen organic solvents. Toxicol. appl.
Pharmacol., 19(4): 699-704.
KISS, G. (1979) [Study of the irritative action of dimethylformamide.]
Börg. Venerol., 55: 203 (in Hungarian).
KOMMINENI, C. (1973) Pathological studies of aflatoxin fractions and
dimethylformamide in MRC rats, Omaha, University of Nebraska (Dissertation,
December 1972).
KOUDELA, K. & SPAZIER, K. (1979) [Effects of dimethylformamide on human
peripheral lymphocytes.] Cesk. Hyg., 24: 432-436 (in Czech).
KOUDELA, K. & SPAZIER, K. (1981) [Increased concentration of
dimethylformamide vapours in the atmosphere.] Prac. Lek., 33: 121-123 (in
Czech).
KRAMER, V.C., SCHNELL, D.J., & NICKERSON, K.W. (1983) Relative toxicity
of organic solvents to Aedes aegypti larvae. J. invertebr. Pathol., 42:
285-287.
KRIVANEK, N.D., MCLAUGHLIN, M., & FAYERWEATHER, W.E. (1978)
Monomethylformamide levels in human urine after repetitive exposure to
dimethylformamide vapour. J. occup. Med., 20: 179-182.
LAITY, J.L., BURSTEIN, I.G., & APPEL, B.R. (1973) Photochemical smog and
the atmospheric reactions of solvents. In: Solvents theory and practice,
pp. 95-112, Washington, DC, American Chemical Society (Advances in
Chemistry Series, Vol. 124).
LANGDON, S.P. & HICKMAN, J.A. (1987) Alkylformamides as inducers of
tumour cell differentiation - a mini-review. Toxicology, 43: 239-249.
LAUWERYS, R. (1986) Dimethylformamide. In: Alessio, L., Berlin, A., Boni,
M., & Roi, R., ed. Biological indicators for the assessment of human
exposure to industrial chemicals. Brussels, Luxembourg, Commission of the
European Communities Joint Research Center, pp. 19-27.
LAUWERYS, R.R., KIVITS, A., LHOIR, M., RIGOLET, P., HOUBAT, D., BUCHET,
J.P., & ROELS, H.A. (1980) Biological surveillance of workers exposed to
dimethylformamide and the influence of skin protection on its
percutaneous absorption. Int. Arch. occup. environ. Health, 45: 189-203.
LAZAREV, N.V. & LEVINA, E.N. (1976) [Dimethylformamide.] In: [Harmful
substances in industry.] Leningrad, Himia, Vol. 2, pp. 36-38 (in Russian).
LEBLANC, G.A. & SURPRENANT, D.C. (1983) The acute and chronic toxicity of
acetone, dimethylformamide, and triethylene glycol to Daphnia magna
(Strauss). Arch. environ. Contam. Toxicol., 12: 305-310.
LESHIK, J.A.D. & FEOKTISTOVA, A.J. (1984) [Ascorbic acid supply and
cytochrome P-450 level in guinea-pig liver during dimethylformamide
poisoning.] J. Vopr. Pitan., 5: 65-67 (in Russian).
LEVIN, S.M., BAKER, D.B., LANDRIGAN, P.J., MONAGHAN, S.V., FRUMIN, E.,
BRAITHWAITE, M., & TOWNE, W. (1987) Testicular cancer in leather tanners
exposed to dimethylformamide. Lancet, II(8568): 1153.
LEVINE, A.E., MCRAE, L.J., & BRATTAIN, M.G. (1985) Changes in receptor
occupancy and growth factor responsiveness induced by treatment of a
transformed mouse embryo cell line with N,N-dimethylformamide. Cancer Res.,
45: 6401-6405.
LEWIS, S.C., RINEHART, W.E., SCHROEDER, R.E., & THAKARA, J.W. (1979)
Dominant lethal mutagenic bioassay of dimethylformamide (DMF). Environ.
Mutagen., 1: 166.
LIPSKI, K. (1982) Liquid chromatographic determinations of
dimethylformamide, methylene bisphenyl isocyanate, and methylene bisphenyl
amine in air samples. Ann. occup. Hyg., 25: 1-4.
LLEWELLYN, G.C., HASTINGS, W.C., & KIMBROUGH, T.D. (1974) The effects of
dimethylformamide on female Mongolian gerbils Meriones unguiculatus. Bull.
environ. Contam. Toxicol., 11: 467-473.
LOBANOVA, K.P. (1958) [Toxicity of dimethylformamide.] Gig. i Sanit.,
23: 31- 37 (in Russian).
LOOS, H. (1979) Hazards, health supervision, and potentiation of alcohol
by mixtures of organic solvents, especially dimethylformamide (DMF).
Arbeitsmed. Sozialmed. Präventivmed., 14(5): 127-129.
LUNDBERG, I., LUNDBERG, S., & KRONEVY, T. (1981) Some observations on
dimethylformamide hepatotoxicity. Toxicology, 22: 1-7.
LUNDBERG, I., PEHRSSON, A., LUNDBERG, S., KRONEVY, T., & LIDUMS, V. (1983)
Delayed dimethyformamide biotransformation after high exposures in rats.
Toxicol. Lett., 17: 29- 34.
LUNDBERG, I., EKDAHL, M., KRONEVI, T., LIDUMS, V., & LUNDBERG, S. (1986)
Relative hepatotoxicity of some industrial solvents after intraperitoneal
injection or inhalation exposure in rats. Environ. Res., 40: 411-420.
LUNDBERG, S. (1982) [ Nordic group of experts for documentation of
threshold limit values - 38. Dimethylformamide.] Solna, Sweden, National
Institute of Occupational Health, pp. 32 (Report 1982:28) (in Swedish).
LYLE, W.H., SPENCE, T.W.M., MCKINNELEY, W.M., & DUCKERS, K. (1979)
Dimethylformamide and alcohol intolerance. Br. J. ind. Med., 36: 63-66.
MALONOVA, H. & BARDODEJ, Z. (1983) Urinary excretion of mercapturates as
a biological indicator of exposure to electrophilic agents. J. Hyg. epidem.
Microbiol. Immunol., 27(3): 319-328.
MASSMANN, W. (1956) Toxicological investigations on dimethylformamide.
Br. J. ind. Med., 13: 51-54.
MATHEW, T., KARUNANITHY, R., YEE, M.H., & NATARAJAN, P.N. (1980)
Hepatotoxicity of dimethylformamide and dimethylsulfoxide at and above the
levels used in some aflatoxin studies. Lab. Invest., 42: 257-262.
MAXFIELD, M.E., BARNES, J.R., AZAR, A., & TROCHIMOWIEZ, H.T. (1975)
Urinary excretion of metabolite following experimental human exposure to
dimethylformamide or to dimethylacetamide. J. occup. Med., 17: 506-511.
MEDYANKIN, A.V. (1975) [Complex action of dimethylformamide under
conditions of a long-term experiment.] Gig. i Sanit., 9: 39-42 (in
Russian).
MERKLE, J. VON. & ZELLER, H. (1980) [Studies on acetamides and formamides
for embryotoxic and teratogenic activities in the rabbit.]
Arzneimittelforschung, 30: 1557-1562 (in German).
MRAZ, J. & TURECEK, F. (1987) Identification of N-acetyl- S-
( N-methylcarbamoyl) cysteine, a human metabolite of N',N'-dimethyl-
formamide and N-methylformamide. J. Chromatogr., 414: 399-404.
MRAZ, J., MRAZ, M., SEDIVEC, V., & FLEK, J. (1987) [Gas chromatographic
determination of N-methylformamide in urine.] Pra. Lek., 39: 352-355
(in Czech).
MURAVIEVA, S.I. (1983) [Improvement of the methods for monitoring the
content of harmful substances in the air of worksite.] Gig. Tr. prof.
Zabol., 6: 39-41 (in Russian).
MURAVIEVA, S.I. & ANVAER, L.P. (1979) [Determination of dimethylformamide
and its metabolites in biological liquids by gas chromatographic method.]
Gig. Tr. prof. Zabol., 6: 58-59 (in Russian).
O'BERG, M.T., CHEN, J.L., & BURKE, C.A. (1985) Epidemiologic study of
workers exposed to acrylonitrile, an update. J. occup. Med., 27: 835-840.
ODOSHASHVILI, D.G. (1963) [Hygienic evaluation of atmospheric air
pollution with dimethylformamide.] In: [Literature on air pollution and
related occupational diseases.] Vol. 9, pp. 169-177 (in Russian).
PAOLETTI, A. & IANNACCONE, A. (1982) [Risk from dimethylformamide
intoxication in a plant for synthetic leather.] Ann. Ist. Super. Sanit.,
18: 567-570 (in Italian).
PAOLETTI, A., FABRI, G., & MASCI, O. (1982a) [Antabuse effect from
solvents: comparison between dimethylformamide and trichloroethylene.]
Ann. Ist. Super. Sanit., 14: 1099-1100 (in Italian).
PAOLETTI, A., FABRI, G., & MARINI BETTOLO, P. (1982b) [An isolated case
of "acute abdomen". Intoxication from dimethylformamide.] Minerva Med., 73:
3407-3410 (in Italian).
PARKHIE, M. & WEBB, M. (1983) Embryotoxicity and teratogenicity of
thalidomide in rats. Teratology, 27: 327.
PERRY, D.L., CHUANG, C.C., JUNGCLAUS, G.A., & WARNER, J.S. (1979)
Identification of organic compounds in industrial effluent discharges,
Athens, Georgia, US Environmental Protection Agency, Office of Research and
Development (Unpublished report No. EPA-600/4-79-016, NTIS PB-294794).
PHAM HUU CHANH, NGUYEN DAT XUONG, & AZUM-GELADE, M.-C. (1971) Etude
toxicologique de la formamide et de ses dérivés N-méthylés et N-éthylés.
Thérapie, 26:409-424.
PHAM HUU CHANH, AZUM-GELADE, M.-C., NGUYEN VAN BAC, & NGUYEN DAT XUONG
(1973) Cardiovascular activity of N,N-dimethylformamide. Toxicology, 1:
135- 142.
POIRIER, S.H., KNUTH, M.L., ANDERSON-BUCHOU, C.D., BROOKE, L.T., LIMA,
A.R., & SHUBAT, P.J. (1986) Comparative toxicity of methanol and N,N-
dimethylformamide to freshwater fish and invertebrates. Bull. environ.
Contam. Toxicol., 37: 615-621.
POTTER, H.P. (1973) Dimethylformamide induced abdominal pain and liver
injury. Arch. environ. Health, 27: 340-341.
PURCHASE, I.F.H., LONGSTAFF, E., ASHBY, J., STYLES, J.A., ANDERSON, D.,
LEFEVRE, P.A., & WESTWOOD, F.R. (1978) An evaluation of six short-term
tests for detecting organic chemical cancerogens. Br. J. Cancer, 37: 873-
903.
QIN, Y.H. & GUE, R.R. (1976) [Studies on the maximum allowalble
concentration of dimethylformamide in surface water.] J. Hyg. Res., 5(2):
161-167 (in Chinese).
REBHUN, L.J. & SAWADA, N. (1969) Augmentation and dispersion of the in
vivo mitotic apparatus of living marine eggs. Protoplasma, 68: 1-22.
ROMADINA, E.S. (1975) [Direct action of microorganisms - one way of
increasing the effectiveness of biological purification of waste waters.]
In: Telitchenko, M., ed. [Biological self-purification in water quality
management: Proceedings of the All-Union Symposium on Sanitary
Hydrobiology.] Moscow, Nauka, pp. 110-112 (in Russian).
SALA, C., BERNABEO, F., COLOMBO, G., INVERNIZZI, E., & MENEGHEL, G. (1984)
Dimethylformamide risk. An evaluation in the production of artificial
organic leather. Med. Lav. 6: 143-148.
SANOTSKY, I.V. & ULANOVA, I.P. (1975) [ Criteria for harmfulness in
hygiene and toxicology for evaluation of hazards from chemical compounds.]
Moscow, Medizina, 372 pp (in Russian).
SANOTSKY, I.V., MURAVIEVA, S.I., ZAEVA, G.N., ANVAER, L., & SEMILETKINA,
N.N. (1978) [Metabolism of dimethylformamide depending on the intensity
of its action.] Gig. Tr. prof. Zabol., 11: 24-27 (in Russian).
SANSONE, E.B. & TEWARI, Y.B. (1978) The permeability of laboratory gloves
to selected solvents. Am. Ind. Hyg. Assoc. J., 39(2): 169-174.
SANTA CRUZ, G. & CORPINO, P. (1978) [Preliminary morphological studies on
acute experimental inhalation exposure in the rat.] Boll. Soc. Ital. Biol.
Sper., 54: 1710- 1717 (in Italian).
SANTA CRUZ, G. & MACCIONI, A. (1978) [Experimental study on
dimethylformamide toxicity. Changes of the myocardium after prolonged
inhalation treatment in the rat.] Boll. Soc. Ital. Biol. Sper., 54: 1717-
1722 (in Italian).
SASAKI, S. (1978) The scientific aspects of the chemical substance
control law in Japan. In: Hutzinger, O., Lelyveld, L.H., van, & Zoeteman,
B.C.J., ed., Aquatic pollutants: Transformation and biological effects,
Oxford, New York, Pergamon Press.
SAVOLAINEN, H. (1981) Dose-dependant effects of peroral dimethylformamide
administration on rat brain. Acta neuropathol., 53: 249-252.
SCAILTEUR, V. (1984) Contribution à l'étude de la relation toxicité-
metabolisme de la dimethylformamide chez le rat. Université Catholique de
Louvain, 255 pp. (Thèse).
SCAILTEUR, V. & LAUWERYS, R. (1984a) In vivo and in vitro oxidative
biotransformation of dimethylformamide in rat. Chem.-biol. Interact., 50:
327-337.
SCAILTEUR, V. & LAUWERYS, R. (1984b) In vivo metabolism of
dimethylformamide and relationship to toxicity in the male rat. Arch.
Toxicol., 56: 87-91.
SCAILTEUR, V. & LAUWERYS, R.R. (1987) Dimethylformamide (DMF)
hepatotoxicity. Toxicology, 43: 231-238.
SCAILTEUR, V., HOFFMANN, E., BUCHET, J.P., & LAUWERYS, R. (1984) Study on
in vivo and in vitro metabolism of dimethylformamide in male and female
rats. Toxicology, 29: 222-234.
SCHEUFLER, H., VON, & FREYE, H.-A. (1975) [Embryotoxic and teratogenic
effects of dimethylformamide.] Dtsch. Gesundheitswes., 30: 455-459 (in
German).
SCHOTTEK, W. (1964) [Problems with the standardization of embryotoxic
substances.] Z. ärztl. Fortbild., 64: 1158-1162 (in German).
SCHOTTEK, W. (1970) [Experimental dimethylformamide toxicity studies on
experimental animals after repeated treatment.] Acta. biol. med. Germ., 25:
359-361 (in German).
SCHOTTEK, W. (1972) [Towards the problem of hygiene standardization of
chemicals having embryotoxic action.] In: Sanotsky, I.V., ed. [Hygine
standardization in study of remote effects of industrial substances.]
Moscow, Medizina, pp. 119-123 (in Russian).
SERRES, F.J., DE & ASHBY, J., ed. (1981) Evaluation of short-term tests
for carcinogens, Amsterdam, Oxford, New York, Elsevier Science Publishers,
827 pp (Progress in Mutation Research, Vol. 1).
SHARKAWI, M. (1980) Inhibition of alcoholdehydrogenase by dimethyl-
formamide and dimethylsulfoxide. Toxicol. Lett., 4: 493-497.
SHEVELEVA, G.A. & OSINA, S.A. (1973) [Experimental investigation of the
embryotropic action of dimethylformamide.] In: [ The toxicology of new
industrial chemicals.] Moscow, Medizina, Vol. 13, pp. 75-82 (in Russian).
SHEVELEVA, G.A., STREKALOVA, E.E., & CHIRKOVA, E.M. (1979) [Study of the
embryotropic, mutagenic, and gonadotropic effects of dimethylformamide
after inhibition exposure.] In: [ The toxicology of new industrial
chemicals.] Moscow, Medizina, Vol. 15, pp. 21-25 (in Russian).
SHLYGINA, O.E. & NEMOLCHEV, V.M. (1981) [The use of radio-isotope methods
in studies of acute poisoning with dimethylformamide.] In: [ Proceedings of
the Moscow Scientific Research Institute of First Aid.] Moscow, Medizina,
Vol. 45: 130-132 (in Russian).
SHUBAT, P.J., POIRIER, S.H., KNUTH, M.L., & BROOKE, L.T. (1982) Acute
toxicity of tetrachloroethylene and tetrachloroethylene with
dimethylformamide to rainbow trout, Salmo gairdneri. Bull. environ. Contam.
Toxicol., 28: 7-10.
SICKLES, J.E., WRIGHT, R.S., SUTCLIFFE, C.R., BLAKARD, A.L., & DAYTON, D.P.
(1980) Smog chamber studies of the reactivity of volatile organic
compounds, In: Proceedings of the 73rd Annual Meeting of the Air Pollution
Control Association, (Paper 80-50.1).
STOCKLEY, I.H. (1983) Drugs, foods, and environmental chemical agents
which can initiate Antabuse-like reactions with alcohol. Pharmacol.
Interact., 4: 12-16.
STRANSKY, V. (1986) The determination of N,N-dimethylformamide in working
atmosphere by the method of gas chromatography after sampling on activated
charcoal. Prac. Lek., 38: 15-19.
STULA, E.F. & KRAUSS, W.C. (1977) Embryotoxicity in rats and rabbits from
cutaneous application of amide type solvents and substituted ureas.
Toxicol. appl. Pharmacol., 41: 35-55.
TACCOLA, A., CATENACCI, G., & BARUFFINI, A. (1981) Cardiotoxicity of
dimethylformamide (DMF). Electrocardiographic findings and continuous
electro- cardiographic monitoring. G. Ital. Med. Lav., 3: 149-151.
TANAKA, K.I. (1971) Toxicity of dimethylformamide (DMF) to the young
female rat. Int. Arch. Arbeitsmed., 28: 98-105.
TANAKA, K.I. & UTSUNOMIYA, T. (1982) [The toxicity of N,N-
dimethylformamide (DMF).] Jpn. J. ind. Health, 24: 3-12 (in Japanese).
THIERSCH, J.E. (1962) Effects of acetamides and formamides on the rat
litter in utero. J. Reprod. Fertil., 4: 220.
TOLOT, F., DROIN, M., & GENEVOIS, J. (1958) Intoxication par la
diméthylformamide. Arch. Mal. prof., 19: 602-606.
TOLOT, F., ARCADIO, F., LENGLET, J.-P., & ROCHE, L. (1968) Intoxication
par la diméthylformamide. Arch. Mal. prof., 29: 714-717.
TOMASINI, M., TODARO, A., PIAZZONI, M., & PERUZZO, G.F. (1983) [Pathology
due to dimethylformamide. Observation of 14 cases.] Med. Lav., 74: 217-220
(in Italian).
TONOGAI, Y., OGAWA, S., ITO, Y., & IWAIDA, M. (1982) Actual survey on TLM
(median tolerance limit) values of environmental pollutants, especially on
amines, nitriles, aromatic nitrogen compounds and artificial dyes. J.
toxicol. Sci., 7: 193-203.
UNGAR, H., SULLMAN, S.F., & ZUCKERMAN, A.J. (1976) Acute and protracted
changes in the liver of Syrian hamsters induced by a single dose of
aflatoxin B1. Observations on pathological effects of the solvent
dimethylformamide. Br. J. exp. Pathol., 57: 157- 164.
US EPA (1986) Health and environmental effects profile for N,N-
dimethylformamide. Cincinnati, Ohio, US Environmental Protection Agency,
Health and Environmental Assessment, Environment Criteria and Assessment
Office, 106 pp (Unpublished data).
US NIOSH (1978) Occupational health guideline for dimethylformamide,
Cincinnati, Ohio, US National Institute for Occupational Safety and Health,
5 pp.
US NIOSH (1977) Manual of analytical methods, Cincinnati, Ohio, US
National Institute for Occupational Safety and Health, Vol. 3 (No. S-255).
WAHLBERG, J.E. & BOMAN, A. (1979) Comparative percutaneous toxicity of
ten industrial solvents in the guinea-pig. Scand. J. Work Environ. Health,
5: 345-351.
WALRATH, J., FAYERWEATHER, M.P.H., & GILBY, C.I.H. (1988) A case-control
study of cancer among Du Pont employees with potential for exposure to
dimethylformamide, Wilmington, Delaware, E.I. Du Pont de Nemours Co.
(Unpublished report).
WEISS, G. (1971) [Industrial dimethylformamide intoxication and the
question of its recognition as an occupational disease.] Zbl. Arbeitsmed.,
11.: 345-346 (in German).
WELWARD, L. & HALAMA, D. (1978) Influence of anti-microbial agents on
contamination and chlorotetracycline production. Folia microbiol., 23: 12-
17.
WICAROVA, O. & DADAK, O. (1981) [Urinary N-methylformamide levels in
persons exposed to dimethylformamide vapour.] Prac. Lek., 33: 42-46 (in
Czech).
WILES, J.S. & NARCISSE, J.K., Jr (1971) The acute toxicity of
dimethylformamides in several animal species. Am. Ind. Hyg. Assoc. J., 32:
539-545.
WILSON, H.K. & OTTLEY, T.W. (1981) The use of a transportable mass
spectrometer for the direct measurement of industrial solvents in breath.
Biomed. mass. Spectrom., 8: 606-610.
YONEMOTO, J. & SUZUKI, S. (1980) Relation of exposure to
dimethylformamide vapour and the metabolite, methylformamide, in urine of
workers. Int. Arch. occup. environ. Health, 46: 159-165.
RESUME ET EVALUATION, CONCLUSIONS, RECOMMANDATIONS
1. Résumé et évaluation
1.1 Propriétés générales
Le N,N-diméthylformamide (diméthylformamide, DMF, CSA 68-12-1)
est un solvant organique qui est produit en grandes quantités dans
l'ensemble du monde. On l'utilise dans l'industrie chimique comme
solvant, comme produit intermédiaire et comme additif. Le DMF est un
liquide incolore d'odeur légère mais désagréable qui, néanmoins, est
insuffisante pour attirer l'attention. Il est généralement stable
mais il peut entraîner des incendies et des explosions par contact
avec des oxydants forts, des halogènes, des dérivés alkylaluminiques
ou des hydrocarbures halogénés (en particulier combinés à des métaux).
Le DMF est entièrement miscible à l'eau et à la plupart des solvants
organiques. Sa tension de vapeur est relativement faible.
Du point de vue analytique, on a recours à la chromatographie
en phase gazeuse.
1.2 Transport, distribution, et transformation dans l'environnement
Le DMF est stable dans l'air ambiant mais il peut subir une
décomposition microbienne et algaire dans l'eau. Les microorganismes
adaptés et les boues activées assurent une biodégradation efficace du
DMF. Etant soluble dans l'eau en toutes proportions, le DMF est très
mobile dans les sols et il ne devrait pas s'accumuler dans la chaîne
alimentaire.
1.3 Concentration dans l'environnement et exposition humaine
Le DMF n'existe pas à l'état naturel. On ne possède que peu de
données sur sa concentration dans l'environnement ou sur l'exposition
de la population générale. Dans l'air de zones résidentielles situées
à proximité d'installations industrielles, on a trouvé des
concentrations allant de 0,02 à 0,12 mg/m3. Il est rare qu'on décèle
la présence de DMF dans l'eau des bassins fluviaux très industrialisés
et encore, les concentrations ne dépassent pas 0,01 mg/litre.
On ne possède pas de données sur la concentration du DMF dans le
sol, les végétaux, la faune sauvage et les produits alimentaires.
L'exposition professionnelle se produit par contact cutané avec le
liquide ou la vapeur ou par inhalation de la vapeur. On a décelé des
concentrations de 3 à 86 mg/m3 dans l'air avec des maxima allant
jusqu'à 600 mg/m3, au cours de travaux de réparation ou d'entretien de
machines. Dans quelques cas exceptionnels, des concentrations allant
jusqu'à 4500 mg/m3 ont été signalées.
1.4 Cinétique et métabolisme
Des quantités toxiques de DMF peuvent être absorbées par
inhalation ou pénétration percutanée. Une fois absorbé, le DMF se
distribue de façon uniforme dans l'organisme. Sa métabolisation a
lieu principalement dans le foie sous l'action des enzymes
microsomiales. Chez l'animal et l'homme, le principal produit de la
biotransformation du DMF est le N-hydroxyméthyl- N-méthyl-formamide
(DMF-OH). Au cours de l'analyse par chromatographie en phase gazeuse,
ce métabolite est transformé en N-méthyl-formamide (NMF) qui est lui-
même (ainsi que le N-hydroxyméthyl et le formamide) un métabolite
mineur du DMF. Par conséquent, lorsqu'on procède à des études
métaboliques et que l'on effectue la surveillance biologique du DMF,
les concentrations urinaires de métabolites sont mesurées et exprimées
en NMF, même si le DMF-OH en est le constituant essentiel. Le dosage
du NMF/DMF-OH dans les urines peut donner une bonne indication
biologique de l'exposition totale au DMF.
Chez l'animal d'expérience, on a montré que le mécanisme de
métabolisation du DMF se sature lorsque l'exposition est intense, le
DMF jouant le rôle de rétroinhibiteur de son propre métabolisme à
concentration très élevée.
Il y a interaction métabolique entre le DMF et l'éthanol.
1.5 Effets sur les êtres vivant dans leur milieu naturel
On n'a pas très bien étudié des effets du DMF sur l'environnement.
Il semble que sa toxicité pour les organismes aquatiques soit faible.
1.6 Effets sur les animaux d'expérience et les systèmes d'épreuve
in vitro
Le DMF présente pour diverses espèces une faible toxicité aiguë
(chez le rat la DL50 par voie orale est d'environ 3000 mg/kg, la DL50
dermique d'environ 5000 mg/kg et la CL50 par inhalation à peu près
égale à 10 000 mg/m3). Il est légèrement à modérément irritant pour
la peau et les yeux. D'après une étude sur des cobayes, il ne semble
pas doté de pouvoir sensibilisateur. Le DMF peut faciliter
l'absorption percutanée d'autres substances chimiques.
Chez l'animal d'expérience, l'exposition au DMF, quelle que soit
la voie de pénétration, peut provoquer des lésions hépatiques qui
dépendent de la dose. Lorsque l'exposition cesse, on a pu constater
qu'il y avait régénération des tissus. Certaines études on également
permis d'observer des signes de toxicité au niveau du myocarde et des
reins.
On n'a pas constaté de toxicité pour les testicules ou les
ovaires, ni observé d'effets sur la fécondité chez le rat. Chez le
rat, la souris et le lapin, le DMF s'est révélé embryotoxique et
faiblement tératogène. C'est le lapin qui est l'espèce la plus
sensible à l'exposition respiratoire: des effets tératogènes ont été
observés à partir de 1350 mg/m3 (450 ppm), aucun effet n'étant
constaté à 450 mg/m3 (150ppm). Après exposition de la peau, certaines
études ont mis en évidence de très rares effets embryotoxiques et
tératogènes à des doses journalières comprises entre 100 et 400 mg/kg.
De nombreuses épreuves à court terme à la recherche d'effets
génétiques et anomalies de ce genre, on a montré que le DMF était en
général inactif tant in vitro qu' in vivo.
Aucune étude convenable de cancérogénicité à long terme sur
animaux de laboratoire n'est décrite dans la littérature.
1.7 Effets sur l'homme
Aucun effet indésirable sur la population dans son ensemble n'a
été nettement mis en évidence.
On a fait état d'irritation cutanée et de conjonctivites après
contact direct avec du DMF.
Après exposition accidentelle à de fortes concentrations de DMF,
on note dans les 48h. des douleurs abdominales, des nausées, des
étourdissements et de la fatigue. La fonction hépatique peut être
perturbée et on a signalé des modifications de la tension artérielle,
une tachycardie et des anomalies du tracé électrocardiographique. En
général la récupération est totale.
Après des expositions réitérées sur une longue période, on observe
des symptômes tels que céphalées, perte d'appétit et fatigue. Des
signes biochimiques d'insuffisance hépatique peuvent également
s'observer. Des lésions hépatiques n'apparaissent, semble-t-il, qu'à
partir d'une exposition de l'ordre de 30 mg/m3, en l'absence de
contact cutané. Cette concentration atmosphérique correspond à
environ 40 mg de NMF/DMF-OH par gramme de créatinine dans un
échantillon d'urine prélevé à la fin du poste de travail.
Même à des concentrations inférieures à 30 mg/m3, l'exposition
au DMF peut causer une intolérance à l'alcool dont les symptômes
peuvent consister en rougeur soudaine de la face, sensation de
constriction thoracique et étourdissements, quelques fois accompagnés
de nausées et de dyspnée. Ces symptômes durent de 2 à 4 heures et
disparaissent spontanément.
On possède des preuves limitées d'une activité cancérogène du DMF
pour l'homme. C'est ainsi qu'une étude a fait état d'un accroissement
de l'incidence des tumeurs du testicule après exposition à du DMF,
tandis qu'une autre étude à révélé une incidence tumorale accrue au
niveau de la cavité buccale et du pharynx, mais pas au niveau des
testicules.
Deux études peu détaillées font état d'un accroissement de la
fréquence des avortements chez des femmes exposées à du DMF, entre
autres substances chimiques.
2. Conclusions
1. Compte tenu de ses usages actuels, la population dans son
ensemble, n'est probablement que très peu exposée au DMF.
2. Le DMF est facilement résorbé au niveau de la peau et des voies
respiratoires. Le dosage dans les urines du NMF/DMF-OH
constitue un moyen utile pour évaluer la quantité totale de DMF
absorbé.
3. Le risque de lésions hépatiques est faible si la concentration
du DMF dans l'air ambiant est maintenue en dessous de 30 mg/m3
et qu'il n'y a pas de contact cutané. La valeur correspondante
de la teneur des urines en NMF/DMF-OH à la fin du poste de
travail a été fixée provisoirement à 40 mg/g de créatinine.
4. Le DMF est embryotoxique et faiblement tératogène pour le rat,
la souris et le lapin.
5. Il existe des preuves limitées d'une cancérogénicité du DMF
pour l'homme.
6. D'après les données dont on dispose, le DMF est peu toxique
pour l'environnement. Il est peu probable qu'il donne lieu à
une bioaccumulation.
3. Recommandations
3.1 Précautions à prendre pour la manipulation
1. Maintenir la concentration atmosphérique au-dessous de 30 mg/m3
et éviter le contact avec la peau.
2. Surveiller la concentration urinaire de NMF/DMF-OH qui indique
l'exposition totale et la maintenir en-dessous de 40 mg de
NMF/g de créatinine dans les échantillons prélevés à la fin du
poste de travail. Si la concentration dépasse cette valeur,
prendre les mesures nécessaires pour réduire l'exposition.
3.2 Recherches à effectuer
1. Etudier les effets cancérogènes possibles du DMF chez l'homme,
par des études sur des populations humaines et des animaux
d'expérience.
2. Il faudrait disposer de données plus complètes sur la
possibilité d'extrapoler à l'homme les résultats des études
d'embryotoxicité et de tératogénicité effectuées sur l'animal.
Il serait bon d'étudier la cinétique comparée du DMF chez
l'homme et l'animal.
3. Davantage de données sont nécessaires sur le mode d'action et
l'activité relative des métabolites du DMF chez l'homme et
l'animal.
4. Il faudrait affiner les relations entre a) les concentrations
en métabolites urinaires et les taux d'exposition atmosphérique
(en l'absence de contact cutané), et b) la dose totale absorbée
par toutes les voies possibles (indiquée par la concentration
en NMF urinaire à la fin du poste de travail) et l'absence
d'hépatotoxicité.
RESUMEN Y EVALUACION, CONCLUSIONES, RECOMENDACIONES
1. Resumen y evaluación
1.1 Propiedades generales
La N,N-dimetilformamida (dimetilformamida, DMF, CAS 68-12-2) es
un disolvente orgánico que se produce en grandes cantidades en todo el
mundo. Se utiliza en la industria química como disolvente, compuesto
intermedio y aditivo. La DMF es un líquido incoloro con un ligero
olor desagradable que, no obstante, tiene escasas propiedades de
alerta. Es generalmente estable, pero cuando entra en contacto con
oxidantes fuertes, halógenos, alquilaluminio o hidrocarburos
halogenados (especialmente en combinación con metales), puede
prenderse y provocar explosiones. La DMF es totalmente miscible con
el agua y la mayoría de los disolventes orgánicos. Su presión de
vapor es relativamente baja.
Existen procedimientos de cromatografía de gases para la
determinación de la DMF.
1.2 Transporte, distribución y transformación en el medio ambiente
Aunque la DMF es estable en el aire, puede ser objeto en el agua
de degradación por microbios y algas. Los microorganismos adaptados y
los fangos activados biodegradan la DMF de modo eficiente. A
consecuencia de su solubilidad total en el agua, la DMF se desplaza
fácilmente en el suelo y no es de esperar que se acumule en la cadena
alimentaria.
1.3 Niveles medioambientales y exposición humana
La DMF no aparece en la naturaleza. Se dispone de pocos datos
relativos a los niveles medioambientales o a la exposición de la
población general a la DMF. En zonas residenciales cercanas a centros
industriales se han medido concentraciones atmosféricas de 0,02-0,12
mg/m3. Se ha detectado muy raras veces en las aguas de cuencas
fluviales muy industrializadas, y en esos casos sólo en
concentraciones inferiores a 0,01 mg/litro.
No se dispone de datos relativos a los niveles de DMF en el suelo,
los vegetales, los animales silvestres ni los alimentos.
La exposición profesional se produce por contacto cutáneo con la
DMF en forma líquida y de vapor, y por la inhalación de vapores. Se
han detectado concentraciones de 3-86 mg/m3 de aire, con valores
máximos de hasta 600 mg/m3, durante las operaciones de reparación o de
mantenimiento de máquinas. En condiciones muy especiales, se han
registrado concentraciones de hasta 4500 mg/m3.
1.4 Cinética y metabolismo
Pueden absorberse cantidades tóxicas de DMF por inhalación y a
través de la piel. La DMF absorbida se distribuye uniformemente. La
transformación metabólica de la DMF tiene lugar principalmente en el
hígado, con el concurso de sistemas de enzimas microsómicas. En los
animales y el ser humano, el producto principal de la
biotransformación de la DMF es la N-hidroximetil- N-metilformamida
(DMF-OH). Este metabolito principal se convierte durante el análisis
con cromatografía de gases en N-metilformamida, que es a su vez (junto
con la N-hidroximetilformamida y la formamida) uno de los metabolitos
secundarios. Así pues, en los estudios metabólicos y para el
monitoreo biológico, las concentraciones de metabolitos en la orina se
miden y expresan en forma de NMF, aunque la DMF-OH sea el
contribuyente principal a esa concentración. El análisis de la
NMF/DMF-OH en la orina puede ser un indicador biológico adecuado de la
exposición total a la DMF.
En los animales de experimentación, se ha demostrado que el
metabolismo de la DMF se satura a niveles de exposición elevados y
que, a niveles muy elevados, la DMF inhibe su propio metabolismo.
Se produce interacción metabólica entre la DMF y el etanol.
1.5 Efectos en los organismos del medio ambiente
No se han estudiado bien los efectos de la DMF en el medio
ambiente. La toxicidad para los organismos acuáticos parece baja.
1.6 Efectos en los animales de experimentación y en sistemas de
ensayo in vitro
La toxicidad aguda de la DMF en diversas especies es baja (en
ratas, la DL50 es de unos 3000 mg/kg, la DL50 cutánea es de
aproximadamente 5000 mg/kg, y la LC50 por inhalación es de unos 10 000
mg/m3. Su capacidad de irritación de la piel y los ojos es entre
ligera y moderada. En un estudio realizado con cobayas no hubo
indicación alguna de potencial de sensibilización. La DMF puede
facilitar la absorción de otras sustancias químicas a través de la
piel.
La exposición de animales de experimentación a la DMF por todas
las vías de exposición puede provocar lesiones hepáticas dependientes
de la dosis. Se ha demostrado que se produce regeneración al cesar la
exposición. En algunos estudios se han descrito asimismo síntomas de
toxicidad en el miocardio y el riñón.
No se ha demostrado que la DMF sea tóxica para el testículo ni
para el ovario de la rata, ni se han observado efectos en la
fecundidad. Se ha descubierto que la DMF es embriotóxica y
ligeramente teratogénica en la rata, el ratón y el conejo. El conejo
parece ser la especie más sensible a la exposición por inhalación: se
observaron efectos teratogénicos a partir de 1350 mg/m3 (450 ppm),
pero no a 450 mg/m3 (150 ppm). Tras la exposición cutánea, en algunos
estudios se observó una incidencia muy baja de efectos embriotóxicos y
teratogénicos con dosis que variaron entre 100 y 400 mg/kg al día.
En una amplia serie de ensayos a corto plazo en busca de efectos
genéticos y otros afines se encontró que, en general, la DMF es
inactiva, tanto in vitro como in vivo.
No se han comunicado estudios suficientes sobre carcinogenicidad
a largo plazo en animales de experimentación.
1.7 Efectos en el ser humano
No se ha demostrado claramente la existencia de efectos adversos
de la DMF en la población general.
Se han comunicado casos de irritación cutánea y conjuntivitis tras
el contacto directo con DMF.
Al cabo de 48 horas de la exposición accidental a niveles elevados
de DMF, aparecen dolores abdominales, náuseas, vómitos, mareos y
fatiga. La función hepática puede alterarse y se han notificado casos
de cambios en la tensión arterial, taquicardia y anomalías
electroencefalográficas. Por lo general, la recuperación es completa.
Después de una exposición repetida y a largo plazo, aparecen
síntomas como dolor de cabeza, pérdida de apetito y fatiga. Pueden
observarse signos de disfunción hepática. Las lesiones hepáticas
parecen producirse sólo cuando el nivel de exposición a la DMF pasa de
30 mg/m3, en ausencia de contacto cutáneo. Ese nivel en el aire
corresponde a aproximadamente 40 mg de NMF/DMF-OH/creatinina en una
muestra de orina tomada después del turno de trabajo.
La exposición a la DMF, incluso en concentraciones inferiores a 30
mg/m3, puede provocar intolerancia al alcohol. Entre los síntomas
pueden presentarse un acaloramiento facial repentino, opresión en el
pecho y mareos, a veces acompañados de náuseas y disnea. Duran entre
2 y 4 h y desaparecen espontáneamente.
Existen pruebas limitadas de que la DMF es carcinogénica para el
ser humano. En un estudio se comunicó una incidencia mayor de tumores
testiculares, mientras que en otro se observó una incidencia mayor de
tumores de la cavidad oral y la faringe, pero no del testículo.
En dos estudios, que comunican pocos pormenores, se observó una
frecuencia mayor de abortos espontáneos en mujeres expuestas a la DMF,
entre otras sustancias químicas.
2. Conclusiones
1. Dados los usos actuales de la DMF, la exposición de la
población general es probablemente muy baja.
2. La DMF se absorbe fácilmente a través de la piel además de por
inhalación. La determinación de la NMF/DMF-OH en la orina es
un medio muy útil de estimar la cantidad total de DMF
absorbida.
3. El riesgo de lesión hepática es reducido si el nivel de DMF en
el aire se mantiene por debajo de 30 mg/m3 y no hay contacto
cutáneo. Un valor provisional para el nivel correspondiente de
NMF/DMF-OH en la orina en una muestra tomada después del turno
de trabajo es 40 mg/g de creatinina.
4. La DMF es embriotóxica y ligeramente teratogénica en la rata,
el ratón y el conejo.
5. Existen pruebas limitadas de la carcinogenicidad de la DMF para
el ser humano.
6. Los datos disponibles indican que tiene una baja toxicidad
ambiental. Es poco probable que se produzca bioacumulación.
3. Recomendaciones
3.1 Manipulación sin riesgos
1. Las concentraciones en el aire deben mantenerse por debajo de
30 mg/m3 y debe evitarse el contacto con la piel.
2. La NMF/DMF-OH en la orina, como índice de la exposición total,
debe vigilarse y mantenerse por debajo de 40 mg de NMF/g de
creatinina en muestras tomadas después del turno de trabajo.
Si se sobrepasa ese nivel, deben adoptarse medidas para
disminuir la exposición.
3.2 Nuevas investigaciones
1. Los posibles efectos carcinogénicos del DMF en el ser humano
deben investigarse mediante estudios en animales de
experimentación y poblaciones humanas.
2. Se necesita más información para extrapolar de los estudios en
animales al ser humano los datos sobre embriotoxicidad y
teratogenicidad de la DMF. La comparación de la cinética de la
DMF en el ser humano y en los animales sería muy útil.
3. Se necesita más información sobre los mecanismos de acción y la
potencia relativa de los metabolitos de la DMF en animales y en
el ser humano.
4. Deben afinarse las relaciones entre a) las concentraciones de
metabolitos en la orina y los niveles de exposición en la
atmósfera (en ausencia de contacto cutáneo), y b) la dosis
total recibida por todas las vías (indicada por los niveles de
NMF en la orina después del trabajo) y la ausencia de
hepatotoxicidad.
Wicarova & Dadak (1981) studied the relationship between the
amount of NMF in the shift urine (8 h) or the all-day urine (24 h) of
workers and DMF concentrations in the air (0-100 mg/m3) in an
artificial leather plant . The relationship was linear for the shift
urine samples. For the 24-h urine samples, the relationship was
linear only in the range of 0-80 mg DMF/m3 (see also Table 10).
When Dixon et al. (1983) studied the urinary NMF excretion in a
group of 32-37 workers who were exposed to similar air levels of DMF
for either 8 h per shift (5 days/week) or 12 h per shift (4
days/week), they found higher concentrations of NMF in the urine when
the workers were working 8-h shifts. A possible explanation was that
a 13% reduction in urine volume was seen in workers on 8-h shifts
during the summer months compared with higher urine outputs seen in
the same workers on 12-h shifts during the winter months.
Sala et al. (1984) found a correlation between urinary NMF levels,
4 h after workplace exposure, and the workers' exposure levels to DMF
in 5 job categories relating to artificial leather production. They
reported airborne DMF concentrations of 4.5-14 mg/m3 for spreading
"coagulate" system workers, with a mean NMF in urine of 16 mg/g
creatinine, 9.4 mg DMF/m3 for finishing workers, with a mean NMF
urinary value of 12 mg/g creatinine (low exposures), and 86.3 mg
DMF/m3 in tank cleaning workers with a corresponding urinary value of
188.3 mg NMF/g creatinine (highest exposure).
6.2.3.2 N,N-Dimethylformamide determination in the expired air
Airborne DMF concentrations change considerably during the work
shift and from one workplace to another. Brugnone et al. (1980a)
measured the DMF concentrations in the alveolar air every hour during
the 8-h shift of 8 workers employed in an artificial leather plant.
The alveolar DMF concentration in 6 workers was correlated with the
DMF concentration in the air of the respective workplaces.
In a second study, Brugnone et al. (1984) studied 8 exposed
workers by determining the DMF concentrations in the environmental
air, alveolar air, blood, and urine. Air samples were collected at
hourly intervals during an 8-h work shift, blood samples, at 2-h
intervals, and urine samples, at 4-h intervals. No DMF was found in
the blood or urine. A good correlation between the alveolar and
environmental DMF concentrations was found in 6 out of the 8 workers,
and at all subsequent hours, except for the fourth hour.
In practice, the alveolar air test is more difficult to perform
and use for routine examination than measurement of NMF levels in
urine samples, and is not recommended for biological monitoring.
6.2.3.3 Appraisal
The level of NMF in a post-shift urine sample is the most
appropriate biological parameter for total DMF exposure (inhalation
plus dermal) during the shift.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
The effects of DMF on organisms in the environment have been
reviewed by Kennedy (1986) and by US EPA (1986).
The LC50s of DMF for various aquatic species, given in Table 11,
indicate a low toxicity for the species tested.
DMF is commonly used to facilitate the solution of lipophilic
compounds in water during aquatic toxicity tests.
Cardwell et al. (1978) studied the long-term toxicity of DMF for
fathead minnow (Pimephales promelas), brown trout (Salvelinus
fontinalis), and bluegill (Lepomis macrochirus), and found threshold
limits of between 43 and 98 mg DMF/litre for the brook trout and
between 5 and 10 mg/litre for the fathead minnow. LeBlanc &
Surprenant (1983) showed that a level of 0.1 ml DMF/litre was
acceptable for long-term aquatic toxicity tests. In a study by
Tonogai et al. (1982), the 24-h and 48-h static median tolerance
limits for the Himedaka (Oryzias latipes) were > 1000 mg DMF/litre.
A no-observed-effect level (NOEL) of 7700 mg/litre was reported
for the rainbow trout by Shubat et al. (1982).
Solutions of DMF of 25 g/litre (2.5%) were shown to be lethal
within 0.5 h for eggs of sea urchins (Lythechinus variegatus, Arbacia
punctulata, Lythechinus pictus), the surf clam (Spisule solidissima),
and the annelid (Pectinaria) (Rebhun & Sawada, 1969).
Hughes & Vilkas (1983) determined that the highest concentration
that had no significant effect on the green alga Selenastrum
capricornatum, was 1 ml/litre and the no-effect level was 0.5
ml/litre.
Concentrations ranging from 0.085-0.340% DMF had an inhibitory
effect on cultures of Streptomyces aureofaciens (Welward & Halama,
1978).
Table 11. Medial lethal (LC50) concentrations (mg/litre) for aquatic
organisms exposed to dimethylformamide (DMF)
----------------------------------------------------------------------------------------------------
Species LC50 Reference
---------------------------------------
24-h 48-h 96-h
----------------------------------------------------------------------------------------------------
Guppy 1300 Dojlido (1979)
(Paecilia reticulata)
Rainbow trout 9800 Poirier et al. (1986)
(Salmo gairdneri) 9860 Shubat et al. (1982)
Fathead minnow 10 600 Poirier et al. (1986)
(Pimephales promelas)
Bluegill 7100 Poirier et al. (1986)
(Lepomis macrochirus)
Midge (Paratanytarsus 36 200 Poirier et al. (1986)
parthenogeneticus)
Daphnid (Daphnia magna) 14 500 Poirier et al. (1986)
12 300 LeBlanc & Surprenant
(approx.) (1983)
Larvae (Aedes aegypti) 68 000 Kramer et al. (1983)
(approx.)
----------------------------------------------------------------------------------------------------
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposures
Data on the acute toxicity of DMF in different laboratory animals,
when administered by different routes, have been reviewed by Kennedy
(1986). The acute toxicity in a number of species, following oral,
dermal, inhalation (Table 12), or parenteral (Table 13) administration
of DMF is relatively low, with lethal doses generally in the g/kg
range for the oral, dermal, and parenteral routes and in the g/m3 for
inhalation exposures. Animals given large single doses of DMF or
exposed to high air levels showed general depression, anaesthesia,
loss of appetite, loss of body weight, tremors, laboured breathing,
convulsions, haemorrage of the nose and mouth, liver injury, and coma
immediately preceding death.
In mice and rats, exposed to DMF via inhalation, signs of mucous
membrane irritation were seen (Lobanova, 1958; Lundberg et al., 1986),
and lung damage was detected histologically (Clayton et al., 1963).
Where tissue pathology was included in the study, the prominent
organ showing damage was the liver (Massmann, 1956; Sanotsky et al.,
1978; Mathew et al., 1980; Lundberg et al., 1981). No obvious species
differences were observed with regard to acute lethality, but young
rats appeared more sensitive to DMF-induced lethality than older rats
(Kimura et al., 1971).
8.2 Skin and eye irritation, sensitization
DMF was reported to be irritating to the eyes, mucous membranes,
and the skin (Hamilton & Hardy, 1974; Aldyreva & Gafurov, 1980).
8.2.1 Skin irritation
Rat tails dipped in DMF at 40 °C for 8 h became mummified in a few
days (Massmann, 1956).
A single application of 500 mg DMF/kg resulted in transient
irritation within 2-3 h in mice, but no irritation in rats (Wiles &
Narcisse, 1971). DMF was slightly irritating for mice at doses of
2500 and 5000 mg/kg. No skin irritation was detected in rabbits with
applications of 100, 200, or 500 mg DMF/kg. Single applications of
DMF on the skin of rats and guinea-pigs did not cause irritation
(Kiss, 1979; Bainova, 1985). Repeated 28-day treatments with 960 or
1920 mg/kg did not induce marked local dermal effects in rats (Bainova
et al., 1985).
Table 12. LD50 and LC50 values of DMF after oral, dermal, or
inhalation exposure in various animal species
----------------------------------------------------------------------------------------------------
Species Oral LD50 Dermal LD50 Inhalation LC50 Reference
(mg/kg) (mg/kg) (mg/m3)
----------------------------------------------------------------------------------------------------
Rat 3000 Thiersch (1962)
5000 9432 US NIOSH (1977)
3920 Massmann (1956)
11 140 12 000 Schottek (1970, 1972)
4000 Sanotsky et al. (1978)
> 11 520 Bainova & Antov (1980)
15 000 Clayton et al. (1963)
4320 Lazarev & Levina (1976)
11 000a Stula & Krauss (1977)
> 13 440 Lundberg et al. (1986)
3200 Qin & Gue (1976)
14 000 Cai & Huang (1979)
7170 Bartsch et al. (1976)
Mouse 3950 Lazarev & Levina (1976)
5550 Lazarev & Levina (1976)
> 5000 Wiles & Narcisse (1971)
6420 Bartsch et al. (1976)
3700 6000-9400 Lobanova (1958)
5400, 6200 Qin & Gue (1976)
18 300 Cai & Huang (1979)
Rabbit > 5000 > 500 Wiles & Narcisse (1971)
1500a Stula & Krauss (1977)
Mongolian gerbil 3929 Llewellyn et al. (1974)
----------------------------------------------------------------------------------------------------
a Pregnant females.
Table 13. LD50s (mg/kg body weight) of DMF after parenteral administration
in various animal species
----------------------------------------------------------------------------------------------------------
Species Intraperitoneal Intravenous Intramuscular Subcutaneous Reference
----------------------------------------------------------------------------------------------------------
Rat 1480 4030 Massmann (1956)
2500 Thiersch (1962)
4440 2830 Bartsch et al. (1976)
4600 Pham Huu Chanh et al. (1971)
5470 Shottek (1970, 1972)
Mouse 300 Massmann (1956)
3500 3800 4500 US NIOSH (1977)
650 Barral-Chamaillard
& Rouzioux (1983)
1454 Burgun et al. (1975)
2000 Antoine et al. (1983)
3150 2800 Wiles & Narcisse (1971)
5200 Pham Huu Chanh et al. (1971)
5850 3490 Bartsch et al. (1976)
Rabbit 945 1800 Massmann (1956)
1000 Wiles & Narcisse (1971)
5000 US NIOSH (1977)
Guinea-pig 1300 Wahlberg & Boman (1979)
1030 US NIOSH (1977)
4000 Ungar et al. (1976)
Dog 470 Barral-Chamaillard
& Rouzioux (1983)
Cat 500 Massmann (1956)
----------------------------------------------------------------------------------------------------------
After repeated application of DMF to the skin of guinea-pigs for
21 days (Bainova, 1985), the mean irritative dose was 31% DMF (range
17-56%).
Dermal irritation was not seen in rabbits treated dermally with 2
g DMF/kg for 6 h, daily 15 times during a 4-week period (Kennedy,
1986).
8.2.2 Eye irritation
A 25% (25 g/litre) solution of DMF in water, injected into the
conjunctival sac of the rabbit, did not produce any effects; 50% was
slightly irritating, and 75-100% produced more severe irritation
(Massmann, 1956). Single dose DMF instillation (0.1 ml) produced
moderate corneal damage and conjunctival redness that was most
pronounced at 2-3 days. By day 14, a mild degree of conjunctival
redness, moderate corneal damage with an area of severe injury, slight
surface distortion, and subsurface vascularization were observed
(Kennedy & Sherman, 1986). In another study, the same authors
reported that, after a single DMF instillation, the eye inflammation
subsided and disappeared by the 8th day.
8.2.3 Sensitization
DMF was tested, using a maximization technique, on guinea-pigs to
determine skin sensitization; it did not induce any response (Bainova,
1985).
8.3 Repeated exposure
The effects of repeated oral, dermal, or inhalation exposure to
DMF in various animal species have been reviewed by Kennedy (1986) and
these data, together with other new information are summarized in
Table 14. In all species tested, except the dog, liver damage was
produced, the degree of damage generally being proportional to the
dose administered. In the two reported studies on the dog (Clayton et
al., 1963; Kimmerle & Eben, 1975a), the inhalation exposure conditions
appeared to be too low (60 mg/m3) to produce damage, though 1 out of
the 4 dogs tested by Clayton did have altered liver function tests.
Higher levels were not tested. Some evidence of recovery from the
hepatotoxic effects of DMF was found in rats (Kennedy & Sherman,
1986).
Higher, intermittent doses of DMF appeared to produce more
pronounced effects in male rats than continuous dosing (Bainova et
al., 1981a; Bainova, 1985). Tanaka (1971) found more pronounced liver
damage in rats following one rather than three weeks of exposure and
considered that the high regenerative capacity of liver tissue was
responsible for the observation.
Other tissues and organs that are affected, particularly by high
doses of DMF, will be discussed in section 8.4.
8.4 Specific organ toxicity
8.4.1 Liver
The potency of DMF as a hepatotoxic agent has been reviewed by
Kennedy (1986) and by Scailteur & Lauwerys (1987). The effects of DMF
on the liver were studied after single or repeated inhalation, dermal,
or oral treatment of rats, mice, and rabbits (Massmann, 1956; Clayton
et al., 1963; Shottek, 1970; Tanaka, 1971; Kimmerle & Eben, 1975a;
Medyankin, 1975; Sanotsky et al., 1978; Germanova et al., 1979; Mathew
et al., 1980; Bainova et al., 1981a; Lundberg et al., 1981; Lundberg,
1982; Brondeau et al., 1983; Bainova, 1985; Kennedy & Sherman, 1986;
Scailteur & Lauwerys, 1987). Single oral administrations of 2250-5000
mg DMF/kg in rats (Kennedy & Sherman, 1986) caused clay-coloured
liver, congestion, and centrilobular necrosis of hepatocytes. Lower
doses resulted in deviations in liver function, such as decreased
excretion of cholic acid in the bile, bromosulfthalein retention,
increased serum activities of GOT, GPT, LAP, OCT, AlcP, ChE, LDH, and
gamma-GT, and significant enhancement of cholesterol, triglyceride,
and bilirubin contents in the serum and liver homogenates. In rats,
following both intraperitoneal (ip) and inhalation exposure, there
were no increases in SDH levels at 420 and 840 mg/m3 but a lower level
(210 mg/m3) raised the serum activity of SDH (Lundberg et al., 1986).
Pathomorphological investigation demonstrated lipid degeneration and
cloudy swelling of hepatocytes in the central zones of the lobules
followed by signs of regeneration.
DMF at 0.6 ml/kg, administered intraperitoneally, caused mild
changes in rat liver lobules. Marked centrilobular necrosis and
central phlebitis were found in the rats treated with single ip doses
of 0.9 and 1.2 ml DMF/kg (Mathew et al., 1980). A single ip dose of
0.5 ml DMF/kg to hamsters caused centrilobular necrosis accompanied by
haemosiderosis (Ungar et al., 1976). Morphological changes were
reported in the liver by Clayton et al. (1963), Shottek (1970), Tanaka
(1971), and Santa Cruz & Corpino (1978) after repeated DMF exposure of
young animals, with periodic peaks (Table 14).
Table 14. Effects of repeated oral, dermal, or inhalation exposure to DMF in various animal species
________________________________________________________________________________________________________
Species Route of Dose Duration Effects Reference
exposure
________________________________________________________________________________________________________
Mongolian oral 10 000 mg/kg 30 days no changes in body weight, liver, Llewellyn
gerbil drinking-water or kidney et al. (1974)
10 000 mg/kg 200 days mortality in 25% of animals;
drinking-water liver necrosis
17 000 mg/kg 80 days mortality with liver necrosis;
drinking-water LD50 cumulative 90 206 mg/kg
body weight
34 000 mg/kg 6 days mortality with liver necrosis;
drinking-water LD50 cumulative 3846 mg/kg body
weight
Mouse oral 620 or 1240 30 days anorexia, loss of body weight Qin & Gue
mg/kg diet (1976)
160, 540, 119 days dose-related increase in relative Becci et al.
1850 mg/kg diet and absolute liver weights; no (1983)
other histological or biochemical
changes; NOEL, 246-326 mg/kg
diet per day
Rat oral 320 or 640 30 days anorexia, loss of body weight Qin & Gue
mg/kg diet (1976)
50, 500, 5000 100 days body weight decrease; liver Qin & Gue
mg/litre damage at 5000 mg/litre; (1976)
drinking-water increase in liver to body weight
ratio at 500 and 5000 mg/litre;
structural liver changes and
regeneration at 5000 mg/litre;
NOEL, 50 mg/litre
Table 14 (continued)
________________________________________________________________________________________________________
Species Route of Dose Duration Effects Reference
exposure
________________________________________________________________________________________________________
Rat oral 102, 497, 1000 14 days no behavioural changes at 102 or Savolainen
mg/litre 49 days 497 mg/litre for 49 days; dose- (1981)
drinking-water related deviations in cerebral
and glial cell enzyme activities
215, 750, 2500 104 days dose-related increase in relative Becci et al.
mg/kg diet and absolute liver weights, (1983)
considered to be physiological
adaptation; NOEL, 210-235 mg/kg
diet per day
200, 1000, 5000 90 days slight anaemia and leukocytosis, Kennedy &
mg/kg diet hypercholesterolaemia at 1000 and Sherman (1986)
(equivalent to 5000 mg/kg diet; NOEL, 200 mg/kg
12, 60, 300 mg/ diet
kg/body weight
per day)
0.1, 0.5, 1.0 14 days dose-related increase in liver/ Elovaara
g/litre in 49 days body weight ratios; in liver et al.
drinking-water and kidneys, increased values of (1983)
reduced glutathione, microsomal
UDP glucuronosyl transferase,
and ethoxycoumarin O-demethylase
activities; no changes in liver
microsomal cytochrome P-450 or
ADPH-cytochrome reductase
activity
Rat dermal 470 mg/kg per 30 days continuous dosing caused Schottek
day for 29 days hepatoxicity and did not protect (1970)
and 11 140 mg/kg against lethality; pretreatment
on the 30th day did not enhance toxic reactions
after application of the LD50
in 30-day pretreated rats
Table 14 (continued).
________________________________________________________________________________________________________
Species Route of Dose Duration Effects Reference
exposure
________________________________________________________________________________________________________
215, 430, 960, 30 days dose-related changes in GOT, Bainova &
or 4800 mg/kg GPT, AlcP, ChE, gamma-GT, lipid Antov (1980)
per day fractions in serum and liver
homogenates; NOEL, 215 mg/kg
Rat dermal 215, 320, 960, 30 days dose-related changes (at doses Bainova (1985)
or 4800 mg/kg > 320 mg/kg) in enzyme
activities per day in liver,
myocardium, and kidney
homogenates; NOEL, 215 mg/kg
Rat dermal 960 mg/kg daily 28 days functional, biochemical, and Bainova et al.
or 1920 mg/kg pathomorphological changes in (1981a)
applied liver; and lipid metabolism Bainova (1985)
intermittentlya on the 4th, 8th, 14th, and 28th
day of the tests; changes more
pronounced after intermittent
exposure
4-h dipping of 60 days concentration-related changes Medyankin
tails in 60, 65, in liver and nervous system; (1975)
70, or 80% DMF NOEL, 60% DMF in water
in water
4-h dipping of 120 days no changes at 30% DMF contact and Medyankin
tails in 5 mg DMF/m3 inhalation; adverse (1975)
30 or 60% DMF effects at other concentrations
and inhalation of
5 or 10 mg DMF/m3,
6 h daily
Table 14 (continued).
________________________________________________________________________________________________________
Species Route of Dose Duration Effects Reference
exposure
________________________________________________________________________________________________________
Rabbit dermal 50, 100% water 7 days died at 5-8 day of application Huang et al.
solution, 3 at 100% DMF; liver biochemical (1981)
times/day, 2 ml/ and histological changes
application
2000 mg/kg per 9 days anorexia, cyanosis, and mortality Kennedy &
day with liver necrosis Sherman (1986)
Guinea dermal 50, 75, 100% 7 days died 2-4 days after application of Huang et al.
-pig solution, 75 or 100% and 4-9 days after 50%; (1981)
3 times/day, loss of body weight; liver damage
2 ml/application
Rat inhalation 1800 mg/m3 for 6 days concentration-related mortality; Schottek
6 h daily cumulation of hepatoxic effect (1970)
750 and 1500 6 days
mg/m3, 6 h daily
30 mg/m3 for 8 days no changes in the function of Sanotsky &
6 h daily the thyroid or adrenal glands Ulanova (1975)
aerosol for 3 or liver and kidney necrosis, lung Santa Cruz &
0.5 h daily 30 days changes, arterial changes in Corpino (1978);
(concentration myocardium Santa Cruz &
unknown) Maccioni (1978)
22 ± 1.6 mg/m3 18 weeks liver changes, no other responses Cai & Huang
for 6 h daily, (1979)
6 days a week
Table 14 (continued).
________________________________________________________________________________________________________
Species Route of Dose Duration Effects Reference
exposure
________________________________________________________________________________________________________
Rat inhalation 130 mg/m3 for 27 days functional changes in kidneys Germanova et al.
4 h daily and liver; arterial blood pressure (1979)
more pronounced after additional
300 mg/m3 in 27 days single administration of 500 mg
5 peaks of DMF/kg on the 1st, 8th, and
15 min at 27th days of the studies, and
40-min intervals after intermittent exposure
7569 mg/m3 5 days weakness, weight loss, Kennedy &
for 6 h daily dehydration, liver necrosis Sherman (1986)
Young rat inhalation 600 mg/m3 for 28 days increased serum GOT and GPT; Tanaka (1971)
(3-12 8 h daily morphological liver changes,
weeks old) mainly in 3-week-old rats;
no histological abnormalities
in other organs
Young rat inhalation 600 mg/m3 for 28 days liver changes at the 1st, 2nd, Tanaka (1971)
(3 weeks 8 h daily and 3rd, and 4th week of test more
old) 600 mg/m3 for intense in the group exposed
1 h daily for 8 h daily; no cumulation of
hepatoxic effect
Table 14 (continued).
________________________________________________________________________________________________________
Species Route of Dose Duration Effects Reference
exposure
________________________________________________________________________________________________________
Rat, inhalation 450, 900, 1800, 60 days increased serum GOT, GPT, AlcP, Craig et al.
mouse 3600 mg/m3 cholesterol, anaemia and (1984)
for 6 h daily histological liver changes at
900 mg/m3 or more; liver weight
increase at 450 mg/m3; NOEL
below 450 mg/m3 in both species
Rat, cat inhalation 300, 690, 1350 120 days anorexia, weight loss, liver Massmann
mg/m3, 8 h daily degeneration, and necrosis; (1956)
changes in brain, myocardium,
and kidneys; no abnormalities
in blood tests or ECG
Rabbit inhalation 22 ± 1.6 mg/m3 18 weeks no changes in ECG or liver Cai & Huang
for 6 h daily, parameters (1979)
6 days per week
317 ± 37.8 mg/m3 14 weeks body weight changes; liver damage Cai & Huang
for 6 h daily, functionally and structurally; by (1979)
6 days a week congestion and haemorrhage
120 mg/m3 for 50 days microscopic and electron- Arena et al.
8 h daily microscopic changes in the (1982)
myocardium
Dog inhalation 60 mg/m3 for 107 days reversible changes in blood Clayton et al.
6 h daily pressure, ECG, and in liver (1963)
functions
63 mg/m3 for
6 h daily 28 days normal GOT, GPT, bilirubin, urea, Kimmerle & Eben
and creatinine in plasma; (1975a)
NOEL, 63 mg DMF/m3
___________________________________________________________________________________________________________
a Two alternative intermittent regimes were used: (i) 1920 mg/kg per day for 2 days, followed by no
treatment for 2 days; (ii) 1920 mg/kg every second day.
Diets supplemented with DMF at levels of 215, 750, or 2500 mg/kg
for 104 days for rats and 160, 540, or 1850 mg/kg for 119 days for
mice, resulted in significant dose-related increases in relative liver
weights in all experimental animals. No deviations were reported in
the serum activities of GOT, GPT, AlcP, other than an increase in GPT
activity in mice fed a diet containing 1850 mg DMF/kg. Histo-
pathological evaluation did not reveal any hepatotoxicity (Becci et
al., 1983). The oral administration of a 10% water solution of DMF
(400 mg/kg body weight) for 14 days (Leshik & Feoktistova, 1984) to
guinea-pigs significantly decreased the ascorbic acid content and the
concentration of cytochrome P-450 in the liver. The daily intake of
0.1, 0.5, or 1.0 g DMF/litre in the drinking-water for 2 or 7 weeks
(Elovaara et al., 1983) increased the liver/body weight ratios, the
microsomal UDP-glucuronosyl-transferase and 7-ethoxycoumarin- O-
demethylase activity, and reduced the glutathione concentration in
liver homogenates.
No liver injury was seen following inhalation exposure of dogs to
63 mg DMF/m3 (Kimmerle & Eben, 1975a). However, in another study, 1
out of 4 dogs exposed to 60 mg/m3 showed increased enzyme values
(Clayton et al., 1963)). Liver injury was also not seen: after
inhalation exposure of rats at levels of < 450 mg/m3 (Craig et al.,
1984), after dermal exposure of rats at a level of 240 mg/kg (Bainova,
1985), and at dietary levels of 215 mg/kg body weight per day for rats
and 160 mg/kg for mice (Becci et al., 1983).
8.4.2 Gastrointestinal tract
Toxic gastroenteritis with pathomorphological deviations was
described in the experimental animals treated at high doses or
concentrations in studies reported in Table 14 (Massmann, 1956;
Clayton et al., 1963; Shottek, 1970).
8.4.3 Cardiovascular system
Microscopic examination did not reveal any myocardial lesions in
rats and mice after ingestion of dietary levels of 215, 750, or 2500
mg DMF/kg for 104 days, or 160, 540, or 1850 mg DMF/kg for 119 days,
respectively (Becci et al., 1983).
Clayton et al. (1963) and Germanova et al. (1979) reported
decreases in blood pressure in dogs (not cats) following exposure to
DMF. The changes described were not great and, in the absence of
confirmatory data in other test models (and in man), are of
questionable significance. Large iv doses (500 mg/kg) did not produce
any changes in the contractile force of myocardial tissue in dogs
(Pham Huu Chanh et al., 1973).
Santa Cruz & Maccioni (1978) described histological changes in the
myocardium of the rat and Clayton et al. (1963) described subtle blood
pressure changes in the dog. The findings in the rat followed high
exposures; the blood pressure changes in the dog were minimal and hard
to differentiate from those in control animals.
8.4.4 Kidney
Swelling of the kidney tubules occurred after a single oral
administration of 2250-5000 mg DMF/kg in rats (Kennedy & Sherman,
1986). Short-term feeding studies in rats and mice (Table 14) did not
reveal any histopathological lesions in the kidneys (Becci et al.,
1983; Kennedy & Sherman, 1986).
A number of pathomorphological studies revealed vacuolar
degeneration, mainly in the renal tubules (Massmann, 1956; Clayton et
al., 1963; Santa Cruz & Corpino, 1978; Lundberg et al., 1983). Costa
et al. (1978) observed histological, histochemical, and electron-
microscopic renal lesions in groups of rats, exposed to DMF aerosols
(dose not stated) for 1 h/day for 15 days or for 0.5 h/day for 30
days. Degeneration took place in the proximal part of tubules and in
the visceral epithelium of the glomerulus with marked mitochondrial
changes.
Repeated inhalation exposure to 130 or 300 mg DMF/m3 increased the
kidney/body weight ratio, and decreased diuresis, and the total
protein, sodium chloride, and potassium contents in the urine of rats
(Germanova et al., 1979).
Elovaara et al. (1983) reported enhanced activities of 7-ethoxy-
coumarin- O-demethylase, and UDP-glucuronosyltransferase, and a
decrease in cytosolic formaldehyde dehydrogenase activity in rats
orally exposed to DMF. Bainova & Antov (1980) and Bainova et al.
(1981b) reported that the 30-day dermal application of 960 or 4800 mg
DMF/kg resulted in dose-related increased activities of SucDH, G-6-
PDH, and LDH in rat kidney homogenates.
8.4.5 Nervous system
Functional changes in the nervous system were observed after
administration of high doses or exposure to high concentrations of DMF
(Massmann, 1956; Clayton et al., 1963) and after prolonged treatment
with moderate doses of DMF (Medyankin, 1975; Sanotsky et al., 1978;
Germanova et al., 1979; Bainova, 1985) (Table 14). Doses within the
range of lethal levels resulted in anaesthesia, depression, or coma.
Moderate doses caused inhibition of motor activity.
No effects on the behaviour of rats were noted after they drank
DMF in the drinking-water for 2 and 7 weeks at doses ranging from 1.4
to 13.7 mmol/litre with a cumulative dose of 3200 mg/kg in the rats
(Savolainen, 1981). The same dose enhanced the activities of acid
proteinase and 2,3-cyclicnucleotide-3'-phosphohydrolase in the glial
cells.
Massmann (1956) and Clayton et al. (1963) observed
pathomorphological changes in the brains of experimental animals after
treatment with high doses of DMF (Table 14).
8.4.6 Lungs
Lung congestion and oedema were found in rats after single oral
application of 2250-5000 mg DMF/kg (Kennedy & Sherman, 1986).
Bronchopneumonic changes were observed in experimental animals
after inhalation of high DMF concentrations (Massmann, 1956; Clayton
et al., 1963; Shottek, 1970; Santa Cruz & Corpino, 1978) (Table 14).
According to the authors, the changes were related to injury of the
small arterial vessels and, to some extent, to local irritation caused
by DMF.
8.4.7 Haematopoietic system
High levels of DMF might cause some anaemia, but no other changes
in the erythrocytes of experimental animals have been reported
(Massmann, 1956; Clayton et al., 1963; Sanotsky et al., 1978;
Germanova et al., 1979; Bainova & Antov, 1980) (Table 14). Depressed
bone marrow activity was reported in rats after a single oral
administration of 2250-5000 mg DMF/kg (Kennedy & Sherman, 1986). Pham
Huu Chanh et al. (1971) found leukocytosis in rats after repeated ip
injections of DMF.
Pathomorphological changes were observed in the spleen after
exposure to high doses of DMF. They were accompanied by an increase
in the spleen/body weight ratio (Massmann, 1956; Clayton et al., 1963;
Bainova & Antov, 1980; Bainova, 1985). Medyankin (1975) found
inhibited phagocytosis activity of leukocytes and decreased glycogen
content in the neutrophiles of rats as a result of combined dermal and
inhalation exposure to DMF.
8.4.8 Adrenals
Clayton et al. (1963) observed histological changes in the adrenal
glands after inhalation of DMF. Decreased ascorbic acid content in
the adrenals of rats was reported by Germanova et al. (1979), during
intermittent and continuous inhalation exposure to DMF (Table 14).
8.4.9 Gonads
Male rats were exposed to 584-616 mg DMF/m3 or 49-51 mg/m3 for 4 h
daily for 2, 4, or 8 days; female rats were exposed to 2.3 or 10.7 mg
DMF/m3, for 4 h daily, for 30 days (Sheveleva et al., 1979).
Examination of the sperm and the histological study of the testes and
ovaries did not show any pathological signs.
Lewis et al. (1979) exposed male rats at 90 or 900 mg DMF/m3, for
6 h daily, over 5 days. Gross and histological examination of the
testes did not reveal any pathological changes.
The histological examination of male rats, treated orally in
short-term studies (Becci et al., 1983; Kennedy & Sherman, 1986) with
a variety of doses (Table 14), did not result in changes in the
testes. No lesions were noted in the male rats after a 30-day dermal
application of DMF (Bainova, 1985). Craig et al. (1984) did not find
testicular or ovarian lesions in rats and mice after short-term
inhalation exposure to DMF at concentrations of up to 3600 mg/m3
(Table 14).
Examination of the gonads in a large number of the acute and
repeated-dose toxicity studies discussed earlier did not reveal them
to be a target for DMF toxicity.
8.5 Developmental toxicity and reproduction
DMF was investigated for developmental toxicity in mice, rats, and
rabbits using the oral, dermal, and inhalation routes and parenteral
injection. According to present-day requirements, most of the older
studies were not adequately designed or described. A survey is given
in Tables 15, 16, 17.
No 3-generation reproduction studies were available.
8.5.1 Developmental toxicity
8.5.1.1 Mouse
Administration by gavage of 580 or 193 µl DMF/kg per day, from day
6 to 15 after conception, to 26 mice per dose group led to a dose-
dependent decrease in fetal weights and an increase in the number of
retardations and variations. At 580 µl/kg per day, 17 out of 241
fetuses were malformed (cleft palate, exencephaly, hydro-cephalus
internus, aplasia of presphenoid). No maternal effects were recorded;
the number of live fetuses remained unchanged. At 193 µl/kg, 4 out of
245 fetuses showed malformations. In untreated control groups for
each dose group, 2 out of 229 and 1 out of 310 fetuses, respectively,
had a cleft palate (Hellwig et al., in press).
After an ip injection of 600 or 1100 mg DMF/kg per day, from day 1
to 14 after conception, embryotoxic and teratogenic effects were
registered. The malformation rates were 18 and 75%, respectively, and
the effects consisted of absence or retardation of posterior skull
ossification, open eye-lids, cerebral oedema, sternal haematomas, and
spina bifida-like defects in the thoracic region. Embryotoxic effects
recorded were late resorptions. Doses of 170 mg/kg per day from day 1
to 14 after conception, and 250 mg/kg per day from day 6-14, and
single doses of 2100 mg/kg each on days 3, 7, 9, or 11 after
conception, did not produce any effects (Scheufler & Freye, 1975).
In another ip injection study on mice, 6 animals per dose group
were treated with 0.4 or 1.0 mg DMF/kg per day from day 11 to 15 after
conception. Maternal body weight gain was reduced at 1.0 mg/kg per
day, 2 out of 6 animals had abortions, 7 out of 36 fetuses showed
exencephaly and 1 had a cleft palate. No effects were observed at 0.4
mg/kg (Hellwig et al., in press).
The studies indicate that DMF may be teratogenic for mice.
Table 15. DMF administration to pregnant mice
----------------------------------------------------------------------------------------------------
Route Dosea Maternal Embryotoxicity Malformations Reference
toxicity
----------------------------------------------------------------------------------------------------
Gavage control- - - Hellwig et al.
193 µl/kg - fetal weights 4 of 245 living (in press)
(gavage; day decreased fetuses showed
6-15 pc) malformations
580 µl/kg NRb fetal weights 17 of 241 living
(gavage; day decreased fetuses showed
6-15 pc) malformations
Intra- control - - - Scheufler &
peritoneal 170 mg/kg NRb - - Freye (1975)
injection (day 1-14 pc)
250 mg/kg NRb - -
(day 6-14 pc)
600 mg/kg NRb late malformation
1100 mg/kg resorptions rates 18 and 75%
(day 1-14 pc) respectively
Intra- 0.4 mg/kg - - - Hellwig et al.
peritoneal (day 11-15 pc) (in press)
injection 1.0 mg/kg + 2/6 abortions 8/36
(day 11-15 pc) malformations
----------------------------------------------------------------------------------------------------
a pc = Post conception.
b NR = Not reported.
Table 16. DMF administration to pregnant rats
---------------------------------------------------------------------------------------------------------
Route Dosea Maternal Embryotoxicity Malformations Reference
toxicity
---------------------------------------------------------------------------------------------------------
Gavage control - - - Hellwig et al.
(day 6-15 pc)
533 µl/kg - some embryo-
lethality in the
early phase, +
reduced fetal
weights
+ retardations
+ variations
1600 µl/kg weight 63% embyro- 12% of the 85
stationary lethality in living fetuses
between the median were malformed
day 6-15 phase
Dermal day 6-10 and Hellwig et al.
0 µl/kg - - -
100 µl/kg - - 2.46%
500 µl/kg - - 3.05%
1000 µl/kg - slight reduced 5.46%
fetal length (increase in rib
and vertebral
abnormalities)
Dermal control - - - Stula &
600 mg/kg reduced slight Krauss (1977)
(days 9, 10 + weight embryolethality
11, 11 + 12, gain
12 + 13 pc)
1200 mg/kg reduced slight
(day 10 + 11 pc) weight embryolethality -
gain
Table 16. (contd.)
---------------------------------------------------------------------------------------------------------
Route Dosea Maternal Embryotoxicity Malformations Reference
toxicity
---------------------------------------------------------------------------------------------------------
Dermal 1200 mg/kg reduced slight
(contd.) (day 12 + 13 pc) weight embryolethality -
gain
2400 mg/kg stationary embryolethality -
(day 10 + 11 pc) weight
400 or 200 reduced high embryo- -
mg/kg (applied weight lethality
6 times/day gain
days 11 + 12
+ 13 pc)
Inhalation control - - - Hellwig et al.
(day 0-1, 4-8, weight weights
11-15, 18-19 pc) gain resorptions
861 mg/m3 (287 ppm) reduced reduced fetal -
(day 0-3, 6-10, weight weights
13-18 pc) gain retardations
resorptions
variations
660 mg/m3 (220 ppm) - reduced -
(day 4-8 pc) weight and
length
1560 mg/m3 (520 ppm) reduced embyro- -
(day 4-8 pc) weight lethality;
gain reduced weights
Table 16. (contd.)
---------------------------------------------------------------------------------------------------------
Route Dosea Maternal Embryotoxicity Malformations Reference
toxicity
---------------------------------------------------------------------------------------------------------
Inhalation control - - - Kimmerele &
54 mg/m3 (18 ppm) - - - Machemer
516 mg/m3 (172 ppm) - reduced fetal - (1975)
(6-15 day pc) weights
Inhalation control - - - Keller & Lewis
903 mg/m3 (301 ppm) reduced ossification
(day 6-15 pc) weight variations
gain slightly increased
from 60 to 75% -
Inhalation control - - - Shottek
1200 mg/m3 (400 ppm) total resorp- - (1964)
(4 h/day, 10-20 day pc) NR tions in some
animals, no
retardations
Inhalation 0.05 mg/litre ca.48 mg/m3 weight - Sheveleva &
(ca. 16 ppm) NR decrease Osina (1973)
(day 0-20 pc)
0.8 mg/litre ca.600 mg/m3 embryolethality; -
(ca. 200 ppm) NR weight decrease
Intravenous control - - - Parkie & Webb
injection 0.5 g/kg on - - (1983)
days 10, 11, NR
or 12 pc)
---------------------------------------------------------------------------------------------------------
a pc = Post conception.
b NR = Not reported.
Table 17. DMF administration to pregnant rabbits
---------------------------------------------------------------------------------------------------------
Route Dosea Maternal Embryotoxicity Malformations Reference
toxicity
---------------------------------------------------------------------------------------------------------
Gavage control - - - Merkle &
46.4 µl/kg - - 1 hydrocephalus Zeller (1980)
(day 6-18 pc)
68.1 µl/kg - decrease in number 3 hydrocephalus
(day 6-18 pc) of implantations
and % living
implantations
200 µl/kg decrease in decrease in +
(day 8-16 pc) food intake, fetal weight, 16 fetuses in
weight gain, 3 abortions, 7 litters were
and placental placental weight malformed:
weight decrease
Dermal control - - - Stula &
200 mg/kg some - Krauss (1977)
(day 8-16 pc) embryolethality
Dermal control 100 mg/kg - - - Hellwig et al.
occlusive litter skeletal
(day 6-28 pc) anomalies; 3.3%
agenesia of gall
bladder
Dermal 200 mg/kg per day - - -
400 mg/kg per day decrease in 23.5% fetuses
body weight per litter sternal
on day 16 and anomalies; 2.45%
18 hernia umbilicalis,
6.08% agenesia
of gall bladder
Table 17 (contd.)
---------------------------------------------------------------------------------------------------------
Route Dosea Maternal Embryotoxicity Malformations Reference
toxicity
---------------------------------------------------------------------------------------------------------
Inhalation Control - - - Hellwig et al.
50 ppm)
(day 7-19 pc)
450 mg/m3 decrease in increase in 1 hernia
(150 ppm) body weight sternal umbilicalis
gain variations (50 fetuses)
1350 mg/m3 decrease in decrease in incidence of hernia
(450 ppm) body weight weights, umbilicalis
gain increase in increased;
variations skeletal and soft
(pseudoanky- tissue anomalies
losis)
---------------------------------------------------------------------------------------------------------
a pc = Post conception.
8.5.1.2 Rat
In a gavage study on groups of 26 rats administered 1600 µl DMF/kg
per day from day 6 to 15 after conception, maternal toxicity was
observed in the form of decreased body weight. Sixty-three percent of
the implantations were resorbed and 12% of the surviving 85 (36.64%)
fetuses were malformed (9 cases of diffuse anasarca, 2 cases of tail
aplasia, 1 micrognathia, furthermore several fetuses had anomalies of
the ribs, sternum, and vertebral column). A dose of 533 µl DMF/kg per
day caused some early fetal deaths, in the absence of clinical signs
of maternal toxicity, reduced fetal weight, and also some
malformations, as well as an increase in variations and skeletal
retardations. The malformations consisted of: 2 cases of tail
aplasia, 2 cases of cleft palate, 1 atresia ani, 1 anasarca, 1 open
eye, and several fetuses with split and aplastic vertebrae. At 176
µl/kg, decreased placental weights and some decreases in fetal length
and increases in fetal weight were seen. All other parameters were
within the range of biological variability (Hellwig et al., in press).
The dermal studies on rats did not fulfil today's criteria for a
valid study.
In one series of studies (Hellwig et al., in press), 0, 100, 500,
or 1000 µl DMF/kg per day (undiluted material) were administered in an
uncovered dermal system from day 6 to 10 and then from day 13 to 15
after conception (26 animals per dose group; 10 in the control group).
Under these conditions, 1000 µl/kg per day caused a slightly retarded
weight gain among the dams and significant dermal irritation. The
fetuses were slightly smaller. Malformations consisted of split
thoracic vertebrae and anomalies of the ribs. The rate of the
malformations in live fetuses was 0% per litter in the controls and
2.46%, 3.05%, and 5.46% with increasing dose level. This may indicate
a weak dose-related teratogenic effect.
In another study (Stula & Krauss, 1977), rats received dermal
doses of up to 2400 mg (undiluted DMF)/kg per day, at least every 2
days, from day 9 to 13 after conception, under non-occlusive
conditions. There was clear evidence of embryolethality at 2400 mg/kg
per day on gestation days 10 and 11 (26%, i.e., 7 rats) and at 1200
and 2400 mg/kg per day (in 6 portions of 200 and 400 mg) on gestation
days 11, 12, and 13 with incidences of 43 and 30%, respectively.
Maternal weight gain and average fetal weights were also suppressed.
Fetal abnormalities were not observed, with the exception of a few
subcutaneous haematomas, which occurred at a rate also seen in
historical controls at this laboratory.
Several inhalation studies were carried out on rats. In one
study, 23 animals per dose group were exposed to 54 or 516 mg DMF/m3
(18 or 172 ppm) over 6 h per day from day 6 to 15 after conception.
There were 22 animals in the control group. The higher exposure level
caused a decrease in fetal weights in the absence of signs of maternal
toxicity. No effects were seen on the numbers of implantations,
resorption rates, placental weights, number of fetuses weighing less
than 3 g (runts), variations in skeletal development or malformations;
the lower exposure level (54 mg/m3) did not cause any adverse effects
(Kimmerle & Machemer, 1975).
In another rat study, exposure to 903 mg/m3 (301 ppm) for 6 h per
day, from day 6 to 15 after conception (19 animals per group) led to a
reduction in maternal weight gain and to a slightly increased
incidence of skeletal (ossification) variations of from 60 to 75%.
Exposure to 96 mg/m3 (32 ppm) did not produce any effect (Keller &
Lewis, 1981).
Following 10 exposures to 1200 mg/m3 (400 ppm) for 4 h per day,
from day 10 to 20 after conception, dead implants (54% total
resorptions compared with 15% in the controls) occurred. The numbers
of animals in the treated and control groups were not reported
(Shottek, 1964).
Fetal weight decreases and fetal deaths were reported in another
study on rats after exposure to 600 mg/m3 (200 ppm) from day 0
throughout the gestation period. Exposure to 48 mg/m3 (16 ppm) was
said to have caused reduced fetal weights. However, the description
of the study was inadequate (Sheveleva & Osina, 1973).
A series of inhalation studies on rats is described (Hellwig et
al., in press) in which the exposure periods did not fully cover the
critical period of the gestation phase (e.g., "window dosing" or non-
exposure during weekends, see Table 17). In one set of these
experiments, exposure to concentrations of 660 and 1560 mg/m3 (220 and
520 ppm), for 6 h per day (days 0-3, 6-10, 11-18 after conception; 18
animals per group) caused decreased fetal weights, retardations, and
an increase in embryolethality. Another exposure regimen, i.e., 861
mg/m3 (287 ppm), for 6 h per day, was administered on days 1, 4-8,
11-15, and 18-19 after conception to a group of 30 rats. Twenty
animals were subjected to caesarian section on day 20 after
conception, the offsprings of the other 10 animals were raised until
day 21 after birth. Thirty rats served as untreated controls. There
was retarded maternal weight gain from the beginning of the treatment;
fetal weights were decreased, and the numbers of variations and
retardations were increased. No malformations were found.
No effects were detected after single iv injections of 0.5 g/kg
body weight between days 10, 11, or 12 after conception (Parkie &
Webb, 1983). Furthermore, earlier investigations on rats after single
ip or sc injections did not give any indication of teratogenicity;
however, such studies are of limited value for a toxicity assessment.
The above studies indicate that DMF is embryotoxic in the rat.
After dermal and oral administration, teratogenicity may also occur.
8.5.1.3 Rabbit
DMF caused maternal toxicity and embryotoxicity, including
teratogenicity, in rabbits after administration by gavage at 200 µl/kg
per day from day 6-18 after conception. All 11 animals in the dose
group became pregnant and showed reduced food intake and weight gain.
Placental weights were significantly lower and 3 abortions occurred.
The fetuses showed weight reduction. The main findings recorded on
fetal examination were hernia umbilicalis (7 cases), hydrocephalus
internus (6 cases), eventratio simplex (3 cases), exophthalmus (2
cases), cleft palate (1 case), and malposition of limbs (1 case). The
number of implantations was not adversely influenced. At 68.1 µl/kg
per day, no maternal effects were observed among 16 pregnant animals
(18 inseminated); decreased numbers of total implantations and of live
fetuses occurred, also an increase in skeletal variations and
retardations per litter; 3 cases of hydrocephalus internus were
present. At 46.4 µl/kg per day (10/12 does were pregnant) 1 case of
hydrocephalus internus occurred; all other parameters were in the
range of biological variability. No malformations occurred in the
untreated control group (22/24 animals) (Merkle & Zeller, 1980).
In a dermal study, 5 rabbits (4 animals in the control group)
received 200 mg DMF/kg per day, dermally, from day 8 to day 16 after
conception. The test material was applied in undiluted form on the
intact skin, apparently under non-occlusive conditions. Embryo
mortality was 6% compared with 3% in the controls. The average fetal
weight was 32.9 g in the litters of treated animals compared with 28.4
g among controls. Fetal abnormalities were not detected (Stula &
Krauss, 1977).
In another dermal study (Hellwig et al., in press), dose levels of
undiluted DMF of 0, 100, 200, or 400 mg/kg per day were applied for 6
h/day, under semi-occlusive conditions (15 animals per dose group). A
5-6% decrease in maternal body weights occurred in the highest dose
group, towards the end of the treatment period (days 16-18 after
conception). At this dose level, an increase in skeletal (sternal)
malformations was found in 15 fetuses in 7 litters investigated
(23.5%), and also 5 cases of missing gall bladder (in 2 litters). No
malformations occurred in animals in the 200 mg/kg per day group or in
the untreated control group. At 100 mg/kg per day, one fetus had a
sternal anomaly, 2 fetuses had gall bladder agenesis, and one of the
latter a hypertrophic-dilatative cardiac-aortic malformation.
In a recent inhalation study, exposure levels were 0, 150, 450,
and 1350 mg/m3 (0, 50, 150, and 450 ppm) over 6 h per day from day 7
to day 19 after conception (fifteen animals per dose group). Animals
in the highest exposure group showed a slight retardation in body
weight gain as a sign of maternal toxicity. The fetal weights were
significantly lower in this group, and there was a significant
increase in malformations, mostly hernia umbilicalis (7 out of 86
fetuses, in 4 out of 15 litters) and some soft-tissue malformations,
such as missing gall bladder, without statistical significance. In
addition, anomalies of the sternum, an increase in split vertebrae,
and a number of variations were also recorded. At 450 mg/m3, maternal
body weights were slightly retarded during the exposure period and the
corrected body weight gain was marginally, but significantly,
decreased. One case of hernia umbilicalis among 75 fetuses and an
increase in sternal variations were observed. At 150 mg/m3, neither
fetuses nor does showed any indications of response to treatment. In
summary, signs of embryotoxicity and teratogenicity were seen at the
highest concentration (1350 mg/m3) and to a lesser degree at 450
mg/m3. The maternal toxicity seen at 1350 mg/m3 and 450 mg/m3 is in
accordance with the maternal toxicity observed at 900 mg/m3 in a
previous range-finding study (Hellwig et al., in press).
These studies indicate that DMF may be teratogenic for rabbits.
8.5.1.4 Appraisal
The overall conclusion from all studies is that DMF may lead to
embryotoxic and teratogenic effects in rats, mice, and rabbits. An
increase in malformations in the absence of maternal toxicity is
clearly visible after gavage and ip administration, with a smaller
incidence after dermal administration. In general, the rabbit
appeared to be more sensitive to the teratogenic effects of DMF than
the rat. After inhalation exposure, fetal toxicity and teratogenic
effects appear to be confined to conditions of maternal toxicity.
8.6 Mutagenicity and related end-points
DMF has been tested extensively in mutagenicity and genotoxicity
assays. DMF was one of the 42 chemicals selected for study in the
International Collaborative Program for the Evaluation of Short-Term
Tests for Carcinogenicity (Serres & Ashby, 1981).
The genetic toxicity studies on DMF have been reviewed by Purchase
et al. (1978), Kennedy (1986), US EPA (1986), and IARC (1989).
8.6.1 In vitro studies
In different in vitro assays, DMF did not induce mutations or
genotoxic effects (Table 18). Negative results were obtained with
both in Salmonella typhimurium and Escherichia coli. DMF did not
induce unscheduled DNA synthesis, sister chromatid exchanges,
chromosomal aberrations, or gene mutation in mammalian cells, or
mitotic gene conversion or crossing over in yeasts (Serres & Ashby,
1981).
Table 18. Short-term genotoxicity tests on DMF ( in vitro )
------------------------------------------------------------------------------------------------------------
Method Concentration Condition; comment Results Reference
------------------------------------------------------------------------------------------------------------
Ames test 0.65 x 10-5-1.3 x 10-3 TA 98, 100, 1535, - Antoine et al.
mol/litre 1537, 1538, (1983)
with and without
liver microsome
B. subtilis maximum dose 20 mg/disk - S9, + S9 (rat, yellow - Serres &
Spore rec-assay tail, clam) Ashby (1981)
E. coli differential highest concentration WP2, WP67, CM871 - Serres &
killing test 30 µl/plate Ashby (1981)
E. coli rec-assay highest concentration 1 g/ml 2921, 9239, 8471, 5519, - Serres &
7623, 7689 Ashby (1981)
DNA polymerase deficient 100 µl/ml + S9 and - S9 - Serres &
assay Ashby (1981)
E. coli W3110 -
P3478
Yeast mutation S. pombe - Serres &
Ashby (1981)
S. cerevisiae (XV185-14C) - Serres &
Ashby (1981)
Mitotic recombination S. cerevisiae (JD1) + Serres &
Ashby (1981)
Mitotic crossing-over 10-1000 µg/ml S. cerevisiae (T1, T2) - Serres &
assay Ashby (1981)
Mitotic aneuploidy lowest effective S. cerevisiae (D6) - S9 + Serres &
concentration 100 µg/ml Ashby (1981)
Mitotic gene conversion 5 µl/ml S. cerevisiae (D7) + S9 - Serres &
Ashby (1981)
Mitotic gene conversion 500 µg/ml S. cerevisiae (JD1) - Serres &
Ashby (1981)
Repair test using yeast minimum effective S. cerevisiae (wild & rad) + Serres &
strains (cell growth concentration Ashby (1981)
inhibition) (MEC) 300 µg/ml
Nuclear enlargement 0.01-100 µg/ml human fibroblasts - Serres &
8-200 µg/ml HeLa cells + Ashby (1981)
Table 18. (contd.)
------------------------------------------------------------------------------------------------------------
Method Concentration Condition; comment Results Reference
------------------------------------------------------------------------------------------------------------
UDS test 1.1-90 µg/ml (-S9) human fibroblasts - Serres &
2-30 µg/ml (+S9) (WI - 38) -, + S9 Ashby (1981)
0.032-100 µg/ml human fibroblasts - Serres &
(from skin biopsies) Ashby (1981)
0.1-100 µg/ml HeLa cells - Serres &
Ashby (1981)
Sister chromatid exchange 0.00625-0.1% CHO cells (-, + S9) - Serres &
(SCE) Ashby (1981)
0.1-100 µg/ml CHO cells - Serres &
Ashby (1981)
RL Chromosome assay 75-300 µg/ml Rat liver epithelial type - Serres &
cell line (RL1) Ashby (1981)
Mouse lymphoma 46.9-3000 µg/ml Mouse lymphoma cells - Serres &
mutagenesis assay (L5178Y) Ashby (1981)
-, + S9 rat liver
Human fibroblast 0.2-0.5 mg/ml human lung fibroblast - Serres &
Diphtheria toxin (HSC172) Ashby (1981)
Resistance test
Cell transformation test 500 µg/ml Baby hamster kidney cells + Serres &
(BHK21C13/HRC1) Ashby (1981)
BHK-21 cell - Serres &
Ashby (1981)
Integration enhancement 0.005-0.5 C3H2K cell - Serres &
test (MLV Test) Ashby (1981)
Cytogenetic analysis 1.1 x 10-2-1.1 human peripheral - Antoine et al.
mol/litre lymphocytes (1983)
Cytogenetic analysis 10-20% human peripheral + Koudela &
lymphocytes Spazier (1979)
------------------------------------------------------------------------------------------------------------
Comment: Positive results were obtained with very high concentrations, such as 100-500 µg/ml and 10-20%.
In one study, DMF did not induce any increase in chromosomal
aberrations or sister chromatid exchange in human peripheral blood
lymphocytes in vitro (Antoine et al., 1983). However, in another
study, chromosomal aberrations were reported in human peripheral
lymphocyte cultures treated with DMF (Koudela & Spazier, 1979). The
authors performed cytogenetic analyses of human peripheral lymphocytes
treated with DMF (dilution from 10-7 to 10-2 mol/litre. Compared
with the positive control, thio-TEPA, the clastogenic activity of DMF
was 3- to 4-fold lower. Chromosome aberrations were concentration-
related at DMF levels of 10-20%.
Cytogenetic analysis of peripheral lymphocytes in 40 workers
exposed to 35-180 mg DMF/m3 was performed, first at 4-month
intervals, and later at 6-month intervals. Increased frequencies of
non-specified chromosomal aberrations of 3.82 and 2.74%, respectively,
were found (Koudela & Spazier, 1981). In further sampling periods,
after technological adjustments to decrease the DMF exposure to about
30 mg/m3, the authors established lower frequencies of cell
aberrations in most of the workers, i.e., 1.59, 1.58, and 1.49%, in
the various periods under study. The aberrant cells in the control
group were 1.61-1.10% (Koudela & Spazier, 1979).
8.6.2 In vivo studies
In in vivo studies, DMF was negative in dominant lethal
mutagenic assays, tests for chromosome aberrations and sperm
abnormalities, and micronucleus tests, (Table 19).
8.6.3 Appraisal
The results obtained in the in vitro and in vivo test systems
showed that DMF did not induce damage in genetic material.
8.7 Carcinogenicity
The carcinogenic activity of DMF has not been examined in 2-year
studies on test-animals (Purchase et al., 1978; Barral-Chamaillard &
Rouzioux, 1983; Kennedy, 1986; US EPA, 1986). However, there are some
data concerning DMF, applied as a solvent, and for shorter periods of
time.
In a study by Druckrey et al. (1967) two groups of 15 and 5 BD
rats were treated, with 75 and 150 mg DMF/litre in the drinking-water
for 500 and 250 days, respectively (total doses 38 000 mg/litre in
drinking-water). The animals were observed for up to a maximum of 750
days, with an average survival of 532 days. Similarly, two groups of
12 rats each were given weekly, subcutaneous injections of 200 and 400
mg DMF/kg (total doses 8000 and 20 000 mg/kg, respectively) and
observed for 732 and 766 days, respectively. No tumorigenic effects
were reported in this small group of rats.
Table 19. Short-term genotoxicity tests of DMF ( in vivo )
--------------------------------------------------------------------------------------------------------------
Method Animal Dose route Results Reference
--------------------------------------------------------------------------------------------------------------
Dominant lethal male rat 90, 900 mg/m3 - Lewis et al.
mutagenic bioassay inhalation 6 h daily (1979)
for 5 consecutive days
Chromosome male and 2.3-600 mg/m3 - Sheveleva
aberrations female rat inhalation et al. (1979)
Micronucleus test BALB/C mouse 0.2-2000 mg/kg ip - Antoine
et al. (1983)
Micronucleus test B6C3F1 mouse 80% of LD50 ip - Serres & Ashby (1981)
Micronucleus test ICR mouse 0.425-1.7 mg/kg ip - Serres & Ashby (1981)
Micronucleus test CD-1 mouse 0.4-1.6 mg/kg ip - Serres & Ashby (1981)
Sperm morphology (CBA x BALB/c)F1 0.1-1.5 mg/kg ip - Serres & Ashby (1981)
assay male mouse
--------------------------------------------------------------------------------------------------------------
Kommineni (1973) reported that 9 out of 18 male, and 11 out of 19
female rats, developed tumours in different organs following ip
administrations of 100 mg DMF per rat, once a week, for 10 weeks. The
incidence of tumours in male control rats was 4 out of 14 and, in
females, 5 out of 14, also in different organs. No specific organ or
tumour type predominated in either the test or control group. Three
testicular tumours were seen, a bilateral interstitial cell tumour in
the controls, and an interstitial cell tumour and an embryonal cell
carcinoma in the test group.
8.8 Induction of tumour cell differentiation
Borenfreund et al. (1975) reported a decrease in the malignancy
of the Friend erythroleukaemic cells and a marked increase in their
differentiation along the erythroid pathway after their treatment with
0.5 and 1% DMF solutions.
DMF induction of cell differentiation and a marked reduction of
tumorigenicity was established by Dexter (1977) in transplantable
murine rhabdomyosarcoma cells. In another study, Dexter & Hager
(1980) used 4 carcinoma cell lines derived from two specimens of
adenosarcoma of the human sigmoid colon and showed changes in the
carcinoma cells towards less malignant cell types. Hager et al.
(1980) demonstrated that a cultured human colon carcinoma cell
responded to DMF by more differentiated development, again suggesting
an antitumour effect.
Chakrabarty et al. (1984) studied the effects of DMF on AKR-2B and
AKR-MCA cells in vitro and found that complete loss of anchorage
independent growth occurred and the reduced expression of membrane
antigens was restored.
The DMF induction of tumour cell differentiation has been studied
by Kimball & Hixon (1983) in relation to the deviation of the nuclear
protein. Cordeiro & Savarese (1984) studied it in relation to the
effects on cysteine/glutathione metabolism, and Levine et al. (1985)
in relationship to the changes in receptor occupation and growth
factor responsiveness. Chen et al. (1986) studied the induction of
tumour cells by DMF in relation to the rate of nucleoside transport in
the cells.
A review of the studies on the effects of the induction of
alkylformamides on terminal differentiation of tumour cells (Langdon &
Hickman, 1987) shows that DMF should not be used for such purposes.
The anti-neoplastic activity of DMF, determined in vitro and in vivo,
does not appear to be sufficient for therapeutic use.
8.9 Mechanism of toxicity, mode of action
Several hypotheses on the possible mechanism of DMF hepatotoxicity
have been tested. No experimental support for lipid peroxidation,
lysosome labilization, or glutathione depletion has been reported.
The critical biological effects leading to DMF hepatotoxicity have not
been identified and still need to be elucidated (Scailteur & Lauwerys,
1987).
9. EFFECTS ON HUMAN BEINGS
9.1 General population exposure
No effects of DMF on the general population have been reported.
9.2 Occupational exposure
Reports of occupational poisonings with DMF have been reviewed by
Kennedy (1986), US EPA (1986), and Scailteur & Lauwerys (1987).
9.2.1 Accidental poisoning
Several cases of acute accidental occupational poisoning with DMF
have been reported (Tolot et al., 1968; Potter, 1973; Chary, 1974;
Chivers, 1978; Aldyreva & Gafurov, 1980; Kang-de & Hui-lan, 1981;
Shlygina & Nemolshev, 1981; Paoletti et al., 1982a,b). They were
caused by the malfunctioning of the equipment, splashing of the
organic solvent over the body, or working in plants without taking
protective measures. Over-exposure has occurred via the skin and/or
inhalation. Usually, the symptoms appeared from several hours up to
several days after the accident. The major symptoms were epigastric
or abdominal pain, which was irradiating and progressive, accompanied
by dizziness, nausea, anorexia, vomiting, fatigue, alcohol
intolerance, and skin irritation. Clinical laboratory tests showed
liver function disturbance. Radioisotope diagnostic tests and liver
biopsy revealed morphological changes in the liver. No clinical
manifestations of renal dysfunction were reported. The patients
recovered with symptomatic therapy in hospital for 2-3 weeks. Liver
function tests returned to normal. Some of the patients, who were
followed for several months or several years after the acute
poisoning, had normal function tests.
9.2.2 Long-term exposure
After occupational exposure to DMF (intensity and length of
exposure unspecified, no control groups), eye irritation, headache,
anorexia, gastrointestinal disturbances, and sometimes hepatomegaly
with biochemical signs of liver damage were reported. Some
haematological changes were also observed (Tolot et al., 1958; Weiss,
1971; Dilorenzo & Grazioli, 1972). In studies in which exposure was
quantified, subjective complaints of headache, fatigue, and
gastrointestinal and cardiovascular changes were reported.
Disturbances of liver function could be measured by changes in plasma
bilirubin levels, and increases in the serum activity of liver enzymes
(transaminases, alkaline phosphatase, glutamyl-transpep-tidase).
Alcohol intolerance occurred. Haematological changes and ECG
deviations were also observed (Table 20).
In a questionnaire study, for which little detail is available,
Schottek (1972) reported 14% miscarriages in a group of women exposed
to about 100 mg DMF/m3 compared with 10% in the control group. No
statistical analysis was performed. Aldyreva & Gafurov (1980)
reported perturbations in menstruation in 26 out of 70 women who had
been exposed to 30-150 mg DMF/m3 for about a year. No data are
available on controls. On the basis of company statistics, general
morbidity associated with gynaecological changes appeared to be
increased among DMF-exposed women.
Farquharson et al. (1983) reported miscarriages in 3 out of 9
women, who had been exposed to DMF as well as a number of other
chemicals.
Because of its effect on the stratum corneum, DMF interferes with
the barrier function of the skin; this was demonstrated in human
volunteers by increased water loss following DMF exposure (Baker,
1968).
9.2.3 Epidemiological studies on carcinogenicity
Ducatman et al. (1986) reported three cases of testicular germ-
cell tumours in 1981-83 among 153 white men who repaired the exterior
surfaces and electrical components of F4 Phantom jet aircraft, in the
USA. This finding led to surveys of two other repair shops at
different geographical locations; in one of the shops, the same type
of aircraft was repaired, while in the other, different types of
aircraft were repaired. Four out of 680 white male workers in the
same type of repair shop had a history of testicular germ-cell cancers
(0.95 expected) occurring in 1970-83. No case of testicular germ-cell
cancer was found among the 446 white men employed at the facility
where different types of aircraft were repaired. Of the 7 cases of
testicular germ cell tumours, 5 were seminomas and 2 were embryonal-
cell carcinomas. All 7 men had long histories of working in aircraft
repair. The time from first exposure to diagnosis ranged from 4 to 19
years. There were many common exposures to solvents in the three
facilities, the only exposure identified as unique to the F4 Phantom
jet aircraft repair facilities, where the cases occurred, being to a
solvent mixture containing 80% dimethyl-formamide (20% unspecified).
No quantitative exposure data exist. Three of the cases had certainly
been exposed to this mixture and 3 cases, probably exposed. The cases
were found through foremen and from filed death certificates, and the
authors suggested that under-reporting was possible. No other cases
of cancer were investigated.
Levin et al. (1987) described 3 cases of embryonal-cell carcinoma
of the testes in workers at one leather tannery in the USA, all of
whom had worked as swabbers on the spray lines in leather finishing.
The latency period was from 8 to 14 years. According to the authors,
all the tanneries surveyed used dimethylformamide, as well as a wide
range of dyes, solvents, and other chemicals. No quantitative
exposure data are available. The number of workers from which these 3
cases arose was not given, and other cancers were not looked for.
Table 20. Studies on workers with long-term exposures
---------------------------------------------------------------------------------------------------------------------
Number Number Length DMF exposure Urinary Hepato- Alcohol Other effects Reference
of of non- of (mg/m3) NMF toxicity intoler-
exposed exposed exposure ance
subjects controls (years)
---------------------------------------------------------------------------------------------------------------------
22 28 5 1-47 (usually 20-63 - + NR Lauwerys
< 30; gloves mg/g et al. (1980)
worn) creatinine
11 - 3 3-15 0.4-20 - +(6)a NR Yonemoto &
mg/24 h Suzuki (1980)
28 29 3-5 30-60 NR - NR complaints of eye and Hinkova et al.
respiratory tract (1980)
irritation; no haemato-
logical changes
115 67 1-1.5 30-150 with NR + NR complaints of gastro- Aldyreva &
higher peaks + (a few out intestinal tract or Gafurov (1980)
skin exposure of 29) cardiovascular and
ovarian distur-
bances (29)
177 - 3-5 10-30 NR - NR complaints of Aldyreva &
cardiovascular Gafurov (1980)
disturbances (45)
81 96 3.5 < 10 NR +(10) NR complaints of gastro- Kang-de &
accidental intestinal tract and Hui-Lan (1981)
peak levels up cardiovascular
to 4525 + skin disturbances, ECG
exposure changes
23 - 2 > 30 peaks 10-40 NR NR No ECG changes com- Taccola et al.
up to 150 mg/day pared with pre-exposure (1981)
27 237 2 2-80 peaks NR +(2) +(8) complaints of gastro- Paoletti &
up to 549 intestinal tract Iannaccone
disturbances (15); (1982)
headache (6)
---------------------------------------------------------------------------------------------------------------------
Table 20. (contd.)
---------------------------------------------------------------------------------------------------------------------
Number Number Length DMF exposure Urinary Hepato- Alcohol Other effects Reference
of of non- of (mg/m3) NMF toxicity intoler-
exposed exposed exposure ance
subjects controls (years)
---------------------------------------------------------------------------------------------------------------------
13 - < 4 14-60 NR +(2) +(8) complaints of gastro- Tomasini
(mean: 29) intestinal tract et al (1983)
disturbances (8); eye
irritation (11); kidney
function test and
haematology, normal
26 54 > 5 2-5 NR - NR NR Catenacci
(mean: 3) et al.(1984)
28 54 > 5 12-25 NR - NR NR Catenacci
(mean: 18) et al. (1984)
100 100 5 8-58 NR + +(39) complaints of headache, Cirla et al.
(mean: 22) (8 versus eye and throat (1984)
2 controls) irritation, gastro-
intestinal tract and
cardiovascular
disturbance
24 29 5 10-60 NR NR NR irritative dermatitis Bainova (1985)
15 28 6-10 20-30 NR NR NR increased coagulation Imbriani
(median 27) time et al. (1986)
---------------------------------------------------------------------------------------------------------------------
a Incidence is indicated when available.
NR = not reported
To evaluate the significance of this cluster, an analysis of the
New York State Cancer Registry was conducted. Occupations were
determined from cancer registries and from death certificates for all
residents in Fulton County who were diagnosed as having testicular
cancer from 1974-88. From preliminary results, it is estimated that
workers who are employed in the leather tanning industry are 5-6 times
more likely to develop testicular cancer than those who are not
leather workers. However, the testicular cancer rates in this county
were lower than expected within this period, and an adjacent county
showed the same number of cases of testicular cancer, none of the
affected individuals having ever worked in the leather industry
(Walrath et al., 1988).
O'Berg et al. (1985) and Chen et al. (1988a) studied the cancer
incidence among 2530 actively employed workers with potential exposure
to dimethylformamide between 1956 and 1984, 1329 employees with
exposure to dimethylformamide and acrylonitrile at an acrylic fibre
manufacturing plant in South Carolina, USA, and 1130 controls from the
same plant. Cancer incidence rates for the company (1956-84) and USA
national rates (1973-77) were used to calculate the expected number of
cases. For all workers exposed to DMF (alone or with acrylonitrile),
the standardized incidence ratio (SIR), based on company rates for all
cancers combined, was 110 [95% confidence interval (CI), 88-136]a (88
cases); the SIR on the basis of national rates was 92. The SIR for
cancer of the buccal cavity and pharynx was 344 [CI, 172-615]a (11
cases), on the basis of company rates, and 167, on the basis of US
rates. More cancer cases than expected from company rates (34 cases:
SIR 134 [CI, 98-195]a) were found among employees exposed to
dimethylformamide alone, due mainly to 8 carcinomas of the buccal
cavity and pharynx versus 1.0 expected (SIR, 800 [CI, 345-1580]a).
All of these cases either smoked or chewed tobacco, but no information
was available on the smoking, tobacco chewing, or drinking habits of
the cohort. An additional case occurred among employees exposed to
dimethyl-formamide alone (SIR, 167); 4 of these tumours were cancers
of the lip. No such excess was found among the workers exposed to
both dimethylformamide and acrylonitrile (2 observed; SIR, 125, on the
basis of company rates). The authors reported that there was no
association with intensity or duration of exposure: "low" and
"moderate" exposure SIR 420 (5 cases); "high" exposure SIR 300 (6
cases). "Low" exposure conditions included: no direct contact with
liquids containing any dimethylformamide, even wearing protective
equipment; and workplace air levels consistently below 30 mg DMF/m3
(10 ppm) (no odour of DMF evident). "Moderate" exposure conditions
included: intermittent contact with liquids containing > 5%
dimethylformamide; workplace air levels sometimes higher than 30 mg
DMF/m3 (10 ppm) (more than once per week); DMF-laden materials
handled, but air levels of DMF maintained at above levels. "High"
exposure conditions included: frequent contact with liquids containing
> 5% dimethylformamide; workplace air levels often > 30 mg DMF/m3
(> 10 ppm); use of breathing protection often required for periods of
15 min-1 h; DMF vapour levels frequently > 30 mg/m3 (> 10 ppm)
(when handling pure dimethylformamide or dimethylformamide-containing
---------------------------------------------------------------------------
a As calculated by IARC (1989).
materials). One case of testicular cancer was found among the 3859
workers exposed to DMF (alone or with acrylonitrile) with 1.7 expected
on the basis of company rates, and no cases of liver cancer. The
company rates may be more relevant for comparison, as there were only
actively employed persons among the exposed and because the USA rates
are based on a limited time period, 1973-77.
Chen et al. (1988b) analysed mortality from 1950-82 among both
active and pensioned employees in the same cohort. Expected numbers
(adjusted for age and time period) were based on company rates. For
all workers exposed to DMF (alone or with acrylonitrile), the
standardized mortality ratio (SMR) for lung cancer was 124 (33 cases
[95% CI, 85-174]a). An increased risk of lung cancer was found in the
cohort exposed only to DMF (19 cases; SMR 141 [CI, 84-219]a), but not
in the cohort exposed to DMF and acrylonitrile. There were 3 deaths
from cancer of the buccal cavity and pharynx (SIR 188) in all persons
exposed to DMF (alone or with acrylo-nitrile). No other excess cancer
risk was reported. No information is given in this report on loss to
follow-up, death certificates, or whether these deaths were included
in the incidence study reported above.
Walrath et al. (1988) reported a case-control study on cancer
among 8724 Du Pont employees with potential exposure to DMF in 4 other
USA plants. Summary analyses for all plants combined did not show any
statistically significant association between ever having been exposed
to DMF and subsequent development of cancers of the buccal cavity and
pharynx, liver, malignant melanoma, prostate, and testes. When odds
ratios are examined according to plant site, prostate cancer at one
site was significantly elevated, on the basis of 3 cases exposed out
of 4, but no statistically significant association was observed among
employees similarly exposed to DMF in the other 3 plants. The recency
of exposure to DMF, the low exposures received, and the absence of
similar excesses at other plants argue against a causal association
between DMF exposure and prostate cancer at the one site. Assessment
of highest DMF exposure rank, duration of exposure, and latent period,
does not show any patterns suggesting an association between DMF and
cancers of the buccal cavity and pharynx, liver, malignant melanoma,
prostate, or testes.
9.2.4 Alcohol intolerance
Reviews on the synergistic action of ethanol with organic solvents
have been published by Haguenoer et al. (1982), Hills & Venable
(1982), and Stockley (1983).
Episodes of alcohol intolerance among workers exposed to DMF have
been repeatedly described at all levels of exposure (see sections
9.2.1 and 9.2.2, and Table 20).
Symptoms include flushing of the face, dizziness, nausea,
tightness of the chest, sometimes dyspnoea, and cardiac palpitations.
The reactions were reported within 24 h of DMF exposure and very
shortly after alcohol ingestion. These episodes lasted for up to 2 h
---------------------------------------------------------------------------
a As calculated by IARC (1989).
(Chivers, 1978; Lyle et al., 1979; Yonemoto & Suzuki, 1980; Paoletti
et al., 1982a; Cirla et al., 1984).
According to Loos (1979), abnormal liver function tests were
already discovered among workers who drank only 50-70 g alcohol per
day, but were also exposed to 45-66 mg DMF/m3, while the threshold
consumption for functional liver changes was 80-100 g alcohol per day
in control individuals. It should be noted that the test group was
also exposed to other solvents, mainly tetrahydrofuran, toluene, and
xylene.
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The International Agency for Research on Cancer (IARC) evaluated
the carcinogenicity of dimethylformamide in 1988 (IARC, 1989), and
concluded that:
- there is limited evidence for the carcinogenicity of
dimethylformamide in humans;
- there is inadequate evidence for the carcinogenicity of
dimethylformamide in experimental animals.
Overall evaluation: Dimethylformamide is possibly carcinogenic
to humans (Group 2B).
REFERENCES
ALDYREVA, M.V. & GAFUROV, S.A. (1980) [ Labour protection in the production
of artificial leather.] Moscow, Medizina, 158 pp (in Russian).
ALDYREVA, M.V., BORTSEVICH, S.V., PALAGUSHINA, A.I., SIDOROVA, N.V., &
TARASOVA, L.A. (1980) [Effect of dimethylformamide on workers' health in
the production of polyurethane synthetic leather.] Gig. Tr. prof. Zabol.,
6: 24-28 (in Russian).
AMSTER, M.B., HIJAZI, N., & CHAN, R. (1983) Real time monitoring of low
level air contaminants from hazardous waste sites. In: Proceedings of the
National Conference on Management of Uncontrolled Hazardous Waste Sites.
Washington, DC, pp. 98-99.
ANTOINE, J.L., ARANY, J., LEONARD, A., HENROTTE, J., JENAR-DUBUISSON, G., &
DECAT, G. (1983) Lack of mutagenic activity of dimethylformamide.
Toxicology, 26: 207- 212.
ARENA, N., SANTA CRUZ, G., ALIA, E.F., BALDUS, M., CORGIOLU, T., & ALIA,
E.E. (1982) [Structural and ultrastructural changes in the myocardium of
rabbits exposed to dimethylformamide vapours.] Boll. Soc. Ital. Biol.
Sper., 58: 1496-1501 (in Italian).
BAINOVA, A. (1980) [Contemporary views on the hygiene standardization of
dimethyl- formamide.] Letopisi HEI, 37: 62-68 (in Bulgarian).
BAINOVA, A. (1985) [ Toxicological problems due to the action of chemical
substances on the skin (dermatotoxicology).] Sofia, Referat, pp. 1-60 (D.
Sci. Thesis) (in Bulgarian).
BAINOVA, A. & ANTOV, G. (1980) Dermal toxicity of dimethylformamide in
rats. In: Abstracts of the 5th International Symposium on Occupational
Health in the Production of Artificial Fibres, Belgirate, Italy, 16-20
September, 1980, Modena, Permanent Commission and International Association
on Occupational Health, pp. 73-74.
BAINOVA, A., SPASSOVSKI, M., HINKOVA, L., & TASHEVA, M. (1981a) [Effect
of intermittent and continuous action of dimethylformamide on the
adaptation process.] Probl. hig., 6: 27-35 (in Bulgarian).
BAINOVA, A., ANTOV, G.P., & IVANOVICH, E.H. (1981b) Combined action of
dimethylformamide and noise on kidneys of white rats. CR. Acad. bulg. Sci.,
34: 1609-1611.
BAKER, H. (1968) The effects of dimethylsulfoxide, dimethylformamide, and
dimethyl- acetamide on the cutaneous barrier to water in human skin. J.
invest. Dermatol., 50: 283- 288.
BARNES, J.R. & HENRY, N.W. (1974) The determination of N-methylformamide
and N-methylacetamide in urine. Am. Ind. Hyg. Assoc. J., 35: 84-87.
BARNES, J.R. & RANTA, K.E. (1972) The metabolism of dimethylformamide
and dimethylacetamide. Toxicol. appl. Pharmacol., 23: 271-276.
BARRAL-CHAMAILLARD, J. & ROUZIOUX, M. (1983) Dimethylformamide. Arch.
Mal. prof., 44: 203-208.
BARTSCH, W., SPONER, G., DIETMAN, K., & FUCHS, G. (1976) Acute toxicity
of various solvents in the mouse and rat. Arzneimittelforschung, 26: 1581-
1583.
BECCI, P.J., VOSS, K.A., JOHNSON, W.D., GALLO, M.A., & BABISH, J.G. (1983)
Subchronic feeding study of N,N-dimethylformamide in rats and mice. J. Am.
Coll. Toxicol., 2(6): 371-378.
BEGERT, A. (1975) [Purification of chemical textile plant sewage.]
Oesterr. Abwasser- Rundsch., 20: 98-102 (in German).
BORENFREUND, E., STEINGLASS, M., KORNGOLD, G., & BENDICH, A. (1975)
Effect of dimethylsulfoxide and dimethylformamide on the growth and
morphology of tumour cells. Ann. N.Y. Acad. Sci., 243: 164-171.
BORTSEVICH, S.V. (1984) [The hygienic importance of dimethylformamide
absorption through the skin.] Gig. Tr. prof. Zabol., 11: 55-57 (in
Russian).
BRINDLEY, C., GESCHER, A., & ROSS, D. (1983) Studies of the metabolism of
dimethylformamide in mice. Chem.-biol. Interact., 45: 387-392.
BRONDEAU, M.T., BONNET, P., GUENIER, J.P., & DE CEAURRIZ, J. (1983)
Short-term inhalation test for evaluating industrial hepatotoxicants in
rats. Toxicol. Lett., 19: 139-146.
BRUGNONE, F., PERBELLINI, L., & GAFFURI, E. (1980a) N,N-Dimethylformamide
concentration in environmental and alveolar air in a artificial leather
factory. Br. J. ind. Med., 37: 185-188.
BRUGNONE, F., PERBELLINI, L., GAFFURI, E., & ASASTOLI, P. (1980b)
Biomonitoring of industrial solvent exposures in workers' alveolar air.
Int. Arch. occup. environ. Health, 47: 245-261.
BRUGNONE, F., PERBELLINI, L., GAFFURI, E., & TURRI, P.V. (1984)
Monitoring of industrial exposure to dimethylformamide by analysis of
alveolar air. Med. Lav., 6: 139- 141.
BURGUN, J., MARTZ, R., FORNEY, R.B., & KIPLINGER, G.F. (1975) The acute
toxicity of dimethylformamide and its combined effects with ethanol in the
mouse. Toxicol. appl. Pharmacol., 33: 149-150.
CAI, S.X. & HUANG, M.Y. (1979) Investigation on occupational hazard in a
butadiene monomer workshop of a cis-butadiene rubber plant. J. Hyg. Res.,
8(1): 22-49 (in Chinese).
CARDWELL, R.D., FOREMAN, D.G., PAYNE, T.R., & WILBUR, D.J. (1978) Acute
and chronic toxicity of four chemicals to fish. Duluth, Minnesota, US
Environmental Protection Agency (Contract 68-01-0711).
CATENACCI, G., GRAMPELLA, D., TERZI, R., SALA, H., & POLLINI, G., (1984)
Hepatic function in subjects exposed to environmental concentrations of DMF
lower than the actually proposed TLV. Med. Lav., 6: 157-158.
CHAKRABARTY, S., MCRAE, L.J., LEVINE, A.E., & BRATTAIX, M.G. (1984)
Restoration of normal growth control and membrane antigen composition in
malignant cells by N,N-dimethylformamide. Cancer Res., 44: 2181-2185.
CHARY, S. (1974) Dimethylformamide. A case of acute pancreatitis. Lancet,
2(7876): 356.
CHEN, J.L., FAYERWEATHER, W.E., & PELL, S. (1988a) Cancer incidence of
workers exposed to dimethylformamide and/or acrylonitrile. J. occup. Med.,
30: 813-818.
CHEN, J.L., FAYERWEATHER, W.E., & PELL, S. (1988b) Mortality study of
workers exposed to dimethylformamide and/or acrylonitrile. J. occup. Med.,
30: 819-821.
CHEN, S.-F., CLEAVELAND, J.S., HOLLMAN, A.B., WIEMANN, M.C., PARKS, R.E., &
STOECKLER, J.D. (1986) Changes in nucleoside transport of HL-60 human
promyelocytic cells during N,N-dimethylformamide induced differentiation.
Cancer Res., 46: 3449-3455.
CHIVERS, C.P. (1978) Disulfiram effect from inhalation of
dimethylformamide. Lancet, 1(8059): 331.
CHROMEK, J., KUPEK, J., MLADEK, M., & MARVAN, P. (1983) A study of
respiration of the alga Sceadesmus quadricauda in batch conditions under
influence of N,N-dimethylformamide and dimethylamine. Arch. Hydrobiol.,
64(Suppl.): 441-460.
CIRLA, A.M., PISATI, G., INVERNIZZI, E., & TORRICELLI, P. (1984)
Epidemiological study on workers exposed to low dimethylformamide
concentrations. G. Ital. Med. Lav., 6: 149-156.
CLAY, P.F. & SPITTLER, T.M. (1983) Determination of airborne volatile
nitrogen compounds using four independent techniques. In: Proceedings of
the National Conference on Management of Uncontrolled Hazardous Waste
Sites, Washington, DC, pp. 100-104.
CLAYTON, J.W., BARNES, J.R., HOOD, D.B., & SCHEPERS, G.W. (1963) The
inhalation toxicity of dimethylformamide (DMF). Am. Ind. Hyg. Assoc. J.,
24: 144-154.
CORDEIRO, R.F. & SAVARESE, T.M. (1984) Reversal by L-cysteine of the
growth inhibitory and glutathione-depleting effects of N-methylformamide
and N,N-dimethylformamide. Biochem. biophys. Res. Commun., 122: 798-803.
COSTA, V., FRONGIA, N., & SANTA CRUZ, G. (1978) [Histological and
ultrastructural changes of the rat renal glomerulus caused by
dimethylformamide inhalation.] Boll. Soc. Ital. Biol. Sper., 54: 1723-1728
(in Italian).
CRAIG, D.K., WEIR, R.J., WAGNER, W., & GROTH, D. (1984) Subchronic
inhalation toxicity of dimethylformamide in rats and mice. Drug chem.
Toxicol., 7: 551-571.
DARNALL, K.R., LLOYD, A.C., WINER, A.M., & PITTS, J.N. (1976) Reactivity
scale for atmospheric hydrocarbons based on reaction with hydroxyl radical.
Environ. Sci. Technol., 7: 692-696.
DEXTER, D.L. (1977) N,N-Dimethylformamide-induced morphological
differentiation and reduction of tumorigenicity in cultured mouse
rhabdomyosarcoma cells. Cancer Res., 37: 3136-3140.
DEXTER, D.L. & HAGER, J.C. (1980) Maturation induction of tumour cells
using a human colon carcinoma model. Cancer, 45: 1178-1184.
DILORENZO, F. & GRAZIOLI, C. (1972) [Hematological, hematochemical, and
gastric findings in workers exposed to breathing vapours of
dimethylformamide.] Lav. Um., 24: 96-106 (in Italian).
DIXON, S.W., GRAEPEL, G.J., & LOONEY, W.C. (1983) Seasonal effects on
concentrations of monomethylformamide in urine samples. Am. Ind. Hyg.
Assoc. J., 44: 273-275.
DOJLIDO, J.R. (1979) Investigations of biodegradability and toxicity of
organic compounds. Washington, DC, US Environmental Protection Agency
(Unpublished report No. EPA-600/2-79-163).
DRUCKREY, H., PREUSSMANN, R., IVANKOVIC, S., & SCHMAHL, D. (1967)
[Organotropic carcinogenic effects of 65 different N-nitroso-compounds on
B.D. rats.] Z. Krebsforsch., 69: 103-201 (in German).
DUCATMAN, A.M., CONWILL, D.E., & CRAWL, J. (1986) Germ cell tumours of
the testicle among aircraft repairmen. J. Urol., 136: 834-836.
EBEN, A. & KIMMERLE, G. (1976) Metabolism studies in N,N-
dimethylformamide. III. Studies on the influence of ethanol in persons and
laboratory animals. Int. Arch. occup. environ. Health, 36: 243-265.
EBERLING, C.L. (1980) Formic acid and derivatives (DMF). In: Kirk-
Othmer encyclopedia of chemical technology, 3rd ed., New York, Chichester,
Brisbane, Toronto, John Wiley & Sons, Vol. 11, pp. 263-268.
ELOVAARA, E., MARSELOS, M., & VAINO, H. (1983) N,N-Dimethylformamide-
induced effects on hepatic and renal xenobiotic enzymes with emphasis on
aldehyde metabolism in the rat. Acta. pharmacol. toxicol., 53: 159-165.
EWING, B.B., CHIAN, E.S.K., COOK, J.C., EVANS, C.A., HOPKE, P.A., &
PERKINS, E.G. (1977) Monitoring to detect previously unrecognized
pollutants in surface water. Appendix: Organic analysis data, Washington,
DC, US Environmental Protection Agency, (Unpublished report No. EPA-560/6-
77-015).
FARHI, M., MOREL, M., & CAVIGNEAUX, A. (1968) Dimethylformamide.
NCON(CH3)2. Cah. Notes doc., 50: 91-93.
FARLEY, F.F. (1977) Photochemical reactivity classification of
hydrocarbons and other organic compounds. In: International Conference on
Photochemical Oxidant Pollution and Its Containment, Research Triangle
Park, North Carolina, US Environmental Protection Agency. (Unpublished
report No. EPA-600/3-77-0018).
FARQUHARSON, R.O., HALL, M.A., & FULLERTON, W.T. (1983) Poor obstetric
outcome in three quality control laboratory workers. Lancet, 30: 983-984.
GERMANOVA, A.L., HALEPO, A.I., AVILOVA, G.G., ANVAER, L., HOROCHULOVA,
N.V., MALTSEVA, N.M., & MIGUKINA, N.V. (1979) [Adaptation after
continuous and intermittent exposure to dimethylformamide.] In: [ The
toxicology of new industrial chemicals.] Moscow, Medizina, Vol. 15, pp. 69-
76 (in Russian).
GRASSELLI, J.G. (1973) Atlas of spectral data and physical constants for
organic compounds, Cleveland, Ohio, USA, The Chemical Rubber Co.
GUBSER, H. (1969) Purification of chemical waste waters. Gas Wasser
Abwasser, 49: 175-181.
HAGER, J.C., GOLD, D.V., BARBOSA, J.A., FLIGIEL, L., MILLER, F., & DEXTER,
D.L. (1980) N,N-Dimethylformamide-induced modulation of organ and tumour
associated markers in cultured human colon carcinoma cells. J. Natl Cancer
Inst., 64: 439-445.
HAGUENOER, J.M., BOURRINET, P., & FRIMAT, P. (1982) Interrelations
between alcoholism and exposure to industrial poisons. Arch. Mal. prof.,
43: 461-473.
HAMILTON, A. & HARDY, H.L. (1974) Industrial toxicology, 3rd ed., Acton,
Massachusetts, Publishing Science Group Inc., 349 pp.
HANASONO, G.K., FULLER, R.W., BRODDLE, W.D., & GIBSON, W.R. (1977)
Studies on the effects of N,N-dimethylformamide on ethanol disposition and
on monoamine oxidase activity in rats. Toxicol. appl. Pharmacol., 39: 461-
472.
HELLWIG, J., MERKLE, J., KLIMISCH, H.J., & JÄCKH, R. (in press)
Investigations on the prenatal toxicity of N,N-dimethylformamide (DMF) in
mice, rats and rabbits. Food chem. Toxicol..
HENRY, N.W. & SCHLATTER, C.N. (1981) The development of a standard method
for evaluating chemical protective clothing to permeation by hazardous
liquids. Am. Ind. Hyg. Assoc. J., 42: 202-207.
HILLS, B.W. & VENABLE (1982) The interaction of ethyl alcohol and
industrial chemicals. Am. J. ind. Med., 3: 321-333.
HINKOVA, L., GINCHEVA, N., STAMOVA, N., HRISTEVA, V., CHOLAKOV, B., &
SASSOVSKY, M. (1980) [Influence of dimethylformamide on workers' health.]
Probl. hyg., Sofia, 5: 75-81 (in Bulgarian).
HUANG, M.Y., LUO, Y.Z., GENG, T.B., MENG, D.S., LIU, J., HUANG, M.F., &
WANG, Y.S. (1981) [Studies on the dermal toxicity of dimethylformamide.]
J. Hyg. Res., 10(4): 21-26 (in Chinese).
HUGHES, J.S. & VILKAS A.G. (1983) Toxicity of N,N-dimethylformamide used
as a solvent in toxicity tests with the green alga Selenastrum capricornum.
Bull. environ. Contam. Toxicol., 31: 98-104.
IARC (1989) Dimethylformamide. In: Some organic solvents, resin monomers
and related compounds, pigments and occupational exposures in paint
manufacture and painting, Lyon, International Agency for Research on
Cancer, pp. 171-197 (IARC Monograph on the Evaluation of Carcinogenic Risks
to Humans, Vol. 47).
IMBRIANI, M., CHITTORI, S., PRESTINONI, A., LONGONI, P., CASCONE, G., &
GAMBA, G. (1986) Effects of dimethylformamide (DMF) on coagulation and
platelet activity. Arch. environ. Health, 41: 90-93.
KANG-DE, C. & HUI-LAN, Z. (1981) Observation on the effects of
dimethylformamide on human health. In: Abstracts of the 9th International
Congress on Occupational Health in the Chemical Industry, Aswan, Egypt, 15-
17 September 1981, Aswan, Egypt, Permanent Commission and International
Association on Occupational Health, pp. 22-23.
KELLER, C.A. & LEWIS, S.C. (1981) Inhalation teratology study of N,N-
dimethyl- formamide (DMF). Teratology, 23: 45A.
KENNEDY, G.L., Jr (1986) Biological effects of acetamide, formamide, and
their monomethyl and dimethyl derivatives. CRC crit. Rev. Toxicol., 17(2),
129-182.
KENNEDY, G.L. & SHERMAN, H. (1986) Acute and subchronic toxicity of
dimethylformamide and dimethylacetamide following various routes of
administration. Drug chem. Toxicol., 9: 147-170.
KESTELL, P., GILL, M.H., THREADGILL, M.D., GESCHER, A., HOWARTH, O.W., &
CURZON, E.H. (1986) Identification by proton NMR of N-(hydroxymethyl)- N-
methylformamide as the major urinary metabolite of N,N-dimethylformamide
in mice. Life Sci., 38: 719- 724.
KESTELL, P., THREADGILL, M.D., GESCHER, A., GLEDHILL, A.P., SHAW, A.J., &
FARMER, R.B. (1987) An investigation of the relationship between the
hepatotoxicity and the metabolism of N-alkylformamides. J. Pharmacol. exp.
Ther., 270: 265-270.
KIMBALL, P.M. & HIXON, S. (1983) Nuclear protein changes following N,N-
dimethylformamide (DMF) induced maturation. J. cell. Biochem., 22: 245-249.
KIMMERLE, G. & EBEN, A. (1975a) Metabolism studies of N,N-
dimethylformamide. I. Studies in rats and dogs. Int. Arch. Arbeitsmed.,
34: 109-126.
KIMMERLE, G. & EBEN, A. (1975b) Metabolism studies of N,N-
dimethylformamide. II. Studies in persons. Int. Arch. Arbeitsmed., 34:
127-136.
KIMMERLE, G. & MACHEMER, L. (1975) Studies with N,N-dimethylformamide for
embryotoxic and teratogenic effects on rats after dynamic inhalation. Int.
Arch. Arbeitsmed., 34: 167-175.
KIMURA, E.T., EBERT, D.M., & DODGE, P.W. (1971) Acute toxicity and limits
of solvent residue for sixteen organic solvents. Toxicol. appl.
Pharmacol., 19(4): 699-704.
KISS, G. (1979) [Study of the irritative action of dimethylformamide.]
Börg. Venerol., 55: 203 (in Hungarian).
KOMMINENI, C. (1973) Pathological studies of aflatoxin fractions and
dimethylformamide in MRC rats, Omaha, University of Nebraska (Dissertation,
December 1972).
KOUDELA, K. & SPAZIER, K. (1979) [Effects of dimethylformamide on human
peripheral lymphocytes.] Cesk. Hyg., 24: 432-436 (in Czech).
KOUDELA, K. & SPAZIER, K. (1981) [Increased concentration of
dimethylformamide vapours in the atmosphere.] Prac. Lek., 33: 121-123 (in
Czech).
KRAMER, V.C., SCHNELL, D.J., & NICKERSON, K.W. (1983) Relative toxicity
of organic solvents to Aedes aegypti larvae. J. invertebr. Pathol., 42:
285-287.
KRIVANEK, N.D., MCLAUGHLIN, M., & FAYERWEATHER, W.E. (1978)
Monomethylformamide levels in human urine after repetitive exposure to
dimethylformamide vapour. J. occup. Med., 20: 179-182.
LAITY, J.L., BURSTEIN, I.G., & APPEL, B.R. (1973) Photochemical smog and
the atmospheric reactions of solvents. In: Solvents theory and practice,
pp. 95-112, Washington, DC, American Chemical Society (Advances in
Chemistry Series, Vol. 124).
LANGDON, S.P. & HICKMAN, J.A. (1987) Alkylformamides as inducers of
tumour cell differentiation - a mini-review. Toxicology, 43: 239-249.
LAUWERYS, R. (1986) Dimethylformamide. In: Alessio, L., Berlin, A., Boni,
M., & Roi, R., ed. Biological indicators for the assessment of human
exposure to industrial chemicals. Brussels, Luxembourg, Commission of the
European Communities Joint Research Center, pp. 19-27.
LAUWERYS, R.R., KIVITS, A., LHOIR, M., RIGOLET, P., HOUBAT, D., BUCHET,
J.P., & ROELS, H.A. (1980) Biological surveillance of workers exposed to
dimethylformamide and the influence of skin protection on its
percutaneous absorption. Int. Arch. occup. environ. Health, 45: 189-203.
LAZAREV, N.V. & LEVINA, E.N. (1976) [Dimethylformamide.] In: [Harmful
substances in industry.] Leningrad, Himia, Vol. 2, pp. 36-38 (in Russian).
LEBLANC, G.A. & SURPRENANT, D.C. (1983) The acute and chronic toxicity of
acetone, dimethylformamide, and triethylene glycol to Daphnia magna
(Strauss). Arch. environ. Contam. Toxicol., 12: 305-310.
LESHIK, J.A.D. & FEOKTISTOVA, A.J. (1984) [Ascorbic acid supply and
cytochrome P-450 level in guinea-pig liver during dimethylformamide
poisoning.] J. Vopr. Pitan., 5: 65-67 (in Russian).
LEVIN, S.M., BAKER, D.B., LANDRIGAN, P.J., MONAGHAN, S.V., FRUMIN, E.,
BRAITHWAITE, M., & TOWNE, W. (1987) Testicular cancer in leather tanners
exposed to dimethylformamide. Lancet, II(8568): 1153.
LEVINE, A.E., MCRAE, L.J., & BRATTAIN, M.G. (1985) Changes in receptor
occupancy and growth factor responsiveness induced by treatment of a
transformed mouse embryo cell line with N,N-dimethylformamide. Cancer Res.,
45: 6401-6405.
LEWIS, S.C., RINEHART, W.E., SCHROEDER, R.E., & THAKARA, J.W. (1979)
Dominant lethal mutagenic bioassay of dimethylformamide (DMF). Environ.
Mutagen., 1: 166.
LIPSKI, K. (1982) Liquid chromatographic determinations of
dimethylformamide, methylene bisphenyl isocyanate, and methylene bisphenyl
amine in air samples. Ann. occup. Hyg., 25: 1-4.
LLEWELLYN, G.C., HASTINGS, W.C., & KIMBROUGH, T.D. (1974) The effects of
dimethylformamide on female Mongolian gerbils Meriones unguiculatus. Bull.
environ. Contam. Toxicol., 11: 467-473.
LOBANOVA, K.P. (1958) [Toxicity of dimethylformamide.] Gig. i Sanit.,
23: 31- 37 (in Russian).
LOOS, H. (1979) Hazards, health supervision, and potentiation of alcohol
by mixtures of organic solvents, especially dimethylformamide (DMF).
Arbeitsmed. Sozialmed. Präventivmed., 14(5): 127-129.
LUNDBERG, I., LUNDBERG, S., & KRONEVY, T. (1981) Some observations on
dimethylformamide hepatotoxicity. Toxicology, 22: 1-7.
LUNDBERG, I., PEHRSSON, A., LUNDBERG, S., KRONEVY, T., & LIDUMS, V. (1983)
Delayed dimethyformamide biotransformation after high exposures in rats.
Toxicol. Lett., 17: 29- 34.
LUNDBERG, I., EKDAHL, M., KRONEVI, T., LIDUMS, V., & LUNDBERG, S. (1986)
Relative hepatotoxicity of some industrial solvents after intraperitoneal
injection or inhalation exposure in rats. Environ. Res., 40: 411-420.
LUNDBERG, S. (1982) [ Nordic group of experts for documentation of
threshold limit values - 38. Dimethylformamide.] Solna, Sweden, National
Institute of Occupational Health, pp. 32 (Report 1982:28) (in Swedish).
LYLE, W.H., SPENCE, T.W.M., MCKINNELEY, W.M., & DUCKERS, K. (1979)
Dimethylformamide and alcohol intolerance. Br. J. ind. Med., 36: 63-66.
MALONOVA, H. & BARDODEJ, Z. (1983) Urinary excretion of mercapturates as
a biological indicator of exposure to electrophilic agents. J. Hyg. epidem.
Microbiol. Immunol., 27(3): 319-328.
MASSMANN, W. (1956) Toxicological investigations on dimethylformamide.
Br. J. ind. Med., 13: 51-54.
MATHEW, T., KARUNANITHY, R., YEE, M.H., & NATARAJAN, P.N. (1980)
Hepatotoxicity of dimethylformamide and dimethylsulfoxide at and above the
levels used in some aflatoxin studies. Lab. Invest., 42: 257-262.
MAXFIELD, M.E., BARNES, J.R., AZAR, A., & TROCHIMOWIEZ, H.T. (1975)
Urinary excretion of metabolite following experimental human exposure to
dimethylformamide or to dimethylacetamide. J. occup. Med., 17: 506-511.
MEDYANKIN, A.V. (1975) [Complex action of dimethylformamide under
conditions of a long-term experiment.] Gig. i Sanit., 9: 39-42 (in
Russian).
MERKLE, J. VON. & ZELLER, H. (1980) [Studies on acetamides and formamides
for embryotoxic and teratogenic activities in the rabbit.]
Arzneimittelforschung, 30: 1557-1562 (in German).
MRAZ, J. & TURECEK, F. (1987) Identification of N-acetyl- S-
( N-methylcarbamoyl) cysteine, a human metabolite of N',N'-dimethyl-
formamide and N-methylformamide. J. Chromatogr., 414: 399-404.
MRAZ, J., MRAZ, M., SEDIVEC, V., & FLEK, J. (1987) [Gas chromatographic
determination of N-methylformamide in urine.] Pra. Lek., 39: 352-355
(in Czech).
MURAVIEVA, S.I. (1983) [Improvement of the methods for monitoring the
content of harmful substances in the air of worksite.] Gig. Tr. prof.
Zabol., 6: 39-41 (in Russian).
MURAVIEVA, S.I. & ANVAER, L.P. (1979) [Determination of dimethylformamide
and its metabolites in biological liquids by gas chromatographic method.]
Gig. Tr. prof. Zabol., 6: 58-59 (in Russian).
O'BERG, M.T., CHEN, J.L., & BURKE, C.A. (1985) Epidemiologic study of
workers exposed to acrylonitrile, an update. J. occup. Med., 27: 835-840.
ODOSHASHVILI, D.G. (1963) [Hygienic evaluation of atmospheric air
pollution with dimethylformamide.] In: [Literature on air pollution and
related occupational diseases.] Vol. 9, pp. 169-177 (in Russian).
PAOLETTI, A. & IANNACCONE, A. (1982) [Risk from dimethylformamide
intoxication in a plant for synthetic leather.] Ann. Ist. Super. Sanit.,
18: 567-570 (in Italian).
PAOLETTI, A., FABRI, G., & MASCI, O. (1982a) [Antabuse effect from
solvents: comparison between dimethylformamide and trichloroethylene.]
Ann. Ist. Super. Sanit., 14: 1099-1100 (in Italian).
PAOLETTI, A., FABRI, G., & MARINI BETTOLO, P. (1982b) [An isolated case
of "acute abdomen". Intoxication from dimethylformamide.] Minerva Med., 73:
3407-3410 (in Italian).
PARKHIE, M. & WEBB, M. (1983) Embryotoxicity and teratogenicity of
thalidomide in rats. Teratology, 27: 327.
PERRY, D.L., CHUANG, C.C., JUNGCLAUS, G.A., & WARNER, J.S. (1979)
Identification of organic compounds in industrial effluent discharges,
Athens, Georgia, US Environmental Protection Agency, Office of Research and
Development (Unpublished report No. EPA-600/4-79-016, NTIS PB-294794).
PHAM HUU CHANH, NGUYEN DAT XUONG, & AZUM-GELADE, M.-C. (1971) Etude
toxicologique de la formamide et de ses dérivés N-méthylés et N-éthylés.
Thérapie, 26:409-424.
PHAM HUU CHANH, AZUM-GELADE, M.-C., NGUYEN VAN BAC, & NGUYEN DAT XUONG
(1973) Cardiovascular activity of N,N-dimethylformamide. Toxicology, 1:
135- 142.
POIRIER, S.H., KNUTH, M.L., ANDERSON-BUCHOU, C.D., BROOKE, L.T., LIMA,
A.R., & SHUBAT, P.J. (1986) Comparative toxicity of methanol and N,N-
dimethylformamide to freshwater fish and invertebrates. Bull. environ.
Contam. Toxicol., 37: 615-621.
POTTER, H.P. (1973) Dimethylformamide induced abdominal pain and liver
injury. Arch. environ. Health, 27: 340-341.
PURCHASE, I.F.H., LONGSTAFF, E., ASHBY, J., STYLES, J.A., ANDERSON, D.,
LEFEVRE, P.A., & WESTWOOD, F.R. (1978) An evaluation of six short-term
tests for detecting organic chemical cancerogens. Br. J. Cancer, 37: 873-
903.
QIN, Y.H. & GUE, R.R. (1976) [Studies on the maximum allowalble
concentration of dimethylformamide in surface water.] J. Hyg. Res., 5(2):
161-167 (in Chinese).
REBHUN, L.J. & SAWADA, N. (1969) Augmentation and dispersion of the in
vivo mitotic apparatus of living marine eggs. Protoplasma, 68: 1-22.
ROMADINA, E.S. (1975) [Direct action of microorganisms - one way of
increasing the effectiveness of biological purification of waste waters.]
In: Telitchenko, M., ed. [Biological self-purification in water quality
management: Proceedings of the All-Union Symposium on Sanitary
Hydrobiology.] Moscow, Nauka, pp. 110-112 (in Russian).
SALA, C., BERNABEO, F., COLOMBO, G., INVERNIZZI, E., & MENEGHEL, G. (1984)
Dimethylformamide risk. An evaluation in the production of artificial
organic leather. Med. Lav. 6: 143-148.
SANOTSKY, I.V. & ULANOVA, I.P. (1975) [ Criteria for harmfulness in
hygiene and toxicology for evaluation of hazards from chemical compounds.]
Moscow, Medizina, 372 pp (in Russian).
SANOTSKY, I.V., MURAVIEVA, S.I., ZAEVA, G.N., ANVAER, L., & SEMILETKINA,
N.N. (1978) [Metabolism of dimethylformamide depending on the intensity
of its action.] Gig. Tr. prof. Zabol., 11: 24-27 (in Russian).
SANSONE, E.B. & TEWARI, Y.B. (1978) The permeability of laboratory gloves
to selected solvents. Am. Ind. Hyg. Assoc. J., 39(2): 169-174.
SANTA CRUZ, G. & CORPINO, P. (1978) [Preliminary morphological studies on
acute experimental inhalation exposure in the rat.] Boll. Soc. Ital. Biol.
Sper., 54: 1710- 1717 (in Italian).
SANTA CRUZ, G. & MACCIONI, A. (1978) [Experimental study on
dimethylformamide toxicity. Changes of the myocardium after prolonged
inhalation treatment in the rat.] Boll. Soc. Ital. Biol. Sper., 54: 1717-
1722 (in Italian).
SASAKI, S. (1978) The scientific aspects of the chemical substance
control law in Japan. In: Hutzinger, O., Lelyveld, L.H., van, & Zoeteman,
B.C.J., ed., Aquatic pollutants: Transformation and biological effects,
Oxford, New York, Pergamon Press.
SAVOLAINEN, H. (1981) Dose-dependant effects of peroral dimethylformamide
administration on rat brain. Acta neuropathol., 53: 249-252.
SCAILTEUR, V. (1984) Contribution à l'étude de la relation toxicité-
metabolisme de la dimethylformamide chez le rat. Université Catholique de
Louvain, 255 pp. (Thèse).
SCAILTEUR, V. & LAUWERYS, R. (1984a) In vivo and in vitro oxidative
biotransformation of dimethylformamide in rat. Chem.-biol. Interact., 50:
327-337.
SCAILTEUR, V. & LAUWERYS, R. (1984b) In vivo metabolism of
dimethylformamide and relationship to toxicity in the male rat. Arch.
Toxicol., 56: 87-91.
SCAILTEUR, V. & LAUWERYS, R.R. (1987) Dimethylformamide (DMF)
hepatotoxicity. Toxicology, 43: 231-238.
SCAILTEUR, V., HOFFMANN, E., BUCHET, J.P., & LAUWERYS, R. (1984) Study on
in vivo and in vitro metabolism of dimethylformamide in male and female
rats. Toxicology, 29: 222-234.
SCHEUFLER, H., VON, & FREYE, H.-A. (1975) [Embryotoxic and teratogenic
effects of dimethylformamide.] Dtsch. Gesundheitswes., 30: 455-459 (in
German).
SCHOTTEK, W. (1964) [Problems with the standardization of embryotoxic
substances.] Z. ärztl. Fortbild., 64: 1158-1162 (in German).
SCHOTTEK, W. (1970) [Experimental dimethylformamide toxicity studies on
experimental animals after repeated treatment.] Acta. biol. med. Germ., 25:
359-361 (in German).
SCHOTTEK, W. (1972) [Towards the problem of hygiene standardization of
chemicals having embryotoxic action.] In: Sanotsky, I.V., ed. [Hygine
standardization in study of remote effects of industrial substances.]
Moscow, Medizina, pp. 119-123 (in Russian).
SERRES, F.J., DE & ASHBY, J., ed. (1981) Evaluation of short-term tests
for carcinogens, Amsterdam, Oxford, New York, Elsevier Science Publishers,
827 pp (Progress in Mutation Research, Vol. 1).
SHARKAWI, M. (1980) Inhibition of alcoholdehydrogenase by dimethyl-
formamide and dimethylsulfoxide. Toxicol. Lett., 4: 493-497.
SHEVELEVA, G.A. & OSINA, S.A. (1973) [Experimental investigation of the
embryotropic action of dimethylformamide.] In: [ The toxicology of new
industrial chemicals.] Moscow, Medizina, Vol. 13, pp. 75-82 (in Russian).
SHEVELEVA, G.A., STREKALOVA, E.E., & CHIRKOVA, E.M. (1979) [Study of the
embryotropic, mutagenic, and gonadotropic effects of dimethylformamide
after inhibition exposure.] In: [ The toxicology of new industrial
chemicals.] Moscow, Medizina, Vol. 15, pp. 21-25 (in Russian).
SHLYGINA, O.E. & NEMOLCHEV, V.M. (1981) [The use of radio-isotope methods
in studies of acute poisoning with dimethylformamide.] In: [ Proceedings of
the Moscow Scientific Research Institute of First Aid.] Moscow, Medizina,
Vol. 45: 130-132 (in Russian).
SHUBAT, P.J., POIRIER, S.H., KNUTH, M.L., & BROOKE, L.T. (1982) Acute
toxicity of tetrachloroethylene and tetrachloroethylene with
dimethylformamide to rainbow trout, Salmo gairdneri. Bull. environ. Contam.
Toxicol., 28: 7-10.
SICKLES, J.E., WRIGHT, R.S., SUTCLIFFE, C.R., BLAKARD, A.L., & DAYTON, D.P.
(1980) Smog chamber studies of the reactivity of volatile organic
compounds, In: Proceedings of the 73rd Annual Meeting of the Air Pollution
Control Association, (Paper 80-50.1).
STOCKLEY, I.H. (1983) Drugs, foods, and environmental chemical agents
which can initiate Antabuse-like reactions with alcohol. Pharmacol.
Interact., 4: 12-16.
STRANSKY, V. (1986) The determination of N,N-dimethylformamide in working
atmosphere by the method of gas chromatography after sampling on activated
charcoal. Prac. Lek., 38: 15-19.
STULA, E.F. & KRAUSS, W.C. (1977) Embryotoxicity in rats and rabbits from
cutaneous application of amide type solvents and substituted ureas.
Toxicol. appl. Pharmacol., 41: 35-55.
TACCOLA, A., CATENACCI, G., & BARUFFINI, A. (1981) Cardiotoxicity of
dimethylformamide (DMF). Electrocardiographic findings and continuous
electro- cardiographic monitoring. G. Ital. Med. Lav., 3: 149-151.
TANAKA, K.I. (1971) Toxicity of dimethylformamide (DMF) to the young
female rat. Int. Arch. Arbeitsmed., 28: 98-105.
TANAKA, K.I. & UTSUNOMIYA, T. (1982) [The toxicity of N,N-
dimethylformamide (DMF).] Jpn. J. ind. Health, 24: 3-12 (in Japanese).
THIERSCH, J.E. (1962) Effects of acetamides and formamides on the rat
litter in utero. J. Reprod. Fertil., 4: 220.
TOLOT, F., DROIN, M., & GENEVOIS, J. (1958) Intoxication par la
diméthylformamide. Arch. Mal. prof., 19: 602-606.
TOLOT, F., ARCADIO, F., LENGLET, J.-P., & ROCHE, L. (1968) Intoxication
par la diméthylformamide. Arch. Mal. prof., 29: 714-717.
TOMASINI, M., TODARO, A., PIAZZONI, M., & PERUZZO, G.F. (1983) [Pathology
due to dimethylformamide. Observation of 14 cases.] Med. Lav., 74: 217-220
(in Italian).
TONOGAI, Y., OGAWA, S., ITO, Y., & IWAIDA, M. (1982) Actual survey on TLM
(median tolerance limit) values of environmental pollutants, especially on
amines, nitriles, aromatic nitrogen compounds and artificial dyes. J.
toxicol. Sci., 7: 193-203.
UNGAR, H., SULLMAN, S.F., & ZUCKERMAN, A.J. (1976) Acute and protracted
changes in the liver of Syrian hamsters induced by a single dose of
aflatoxin B1. Observations on pathological effects of the solvent
dimethylformamide. Br. J. exp. Pathol., 57: 157- 164.
US EPA (1986) Health and environmental effects profile for N,N-
dimethylformamide. Cincinnati, Ohio, US Environmental Protection Agency,
Health and Environmental Assessment, Environment Criteria and Assessment
Office, 106 pp (Unpublished data).
US NIOSH (1978) Occupational health guideline for dimethylformamide,
Cincinnati, Ohio, US National Institute for Occupational Safety and Health,
5 pp.
US NIOSH (1977) Manual of analytical methods, Cincinnati, Ohio, US
National Institute for Occupational Safety and Health, Vol. 3 (No. S-255).
WAHLBERG, J.E. & BOMAN, A. (1979) Comparative percutaneous toxicity of
ten industrial solvents in the guinea-pig. Scand. J. Work Environ. Health,
5: 345-351.
WALRATH, J., FAYERWEATHER, M.P.H., & GILBY, C.I.H. (1988) A case-control
study of cancer among Du Pont employees with potential for exposure to
dimethylformamide, Wilmington, Delaware, E.I. Du Pont de Nemours Co.
(Unpublished report).
WEISS, G. (1971) [Industrial dimethylformamide intoxication and the
question of its recognition as an occupational disease.] Zbl. Arbeitsmed.,
11.: 345-346 (in German).
WELWARD, L. & HALAMA, D. (1978) Influence of anti-microbial agents on
contamination and chlorotetracycline production. Folia microbiol., 23: 12-
17.
WICAROVA, O. & DADAK, O. (1981) [Urinary N-methylformamide levels in
persons exposed to dimethylformamide vapour.] Prac. Lek., 33: 42-46 (in
Czech).
WILES, J.S. & NARCISSE, J.K., Jr (1971) The acute toxicity of
dimethylformamides in several animal species. Am. Ind. Hyg. Assoc. J., 32:
539-545.
WILSON, H.K. & OTTLEY, T.W. (1981) The use of a transportable mass
spectrometer for the direct measurement of industrial solvents in breath.
Biomed. mass. Spectrom., 8: 606-610.
YONEMOTO, J. & SUZUKI, S. (1980) Relation of exposure to
dimethylformamide vapour and the metabolite, methylformamide, in urine of
workers. Int. Arch. occup. environ. Health, 46: 159-165.
RESUME ET EVALUATION, CONCLUSIONS, RECOMMANDATIONS
1. Résumé et évaluation
1.1 Propriétés générales
Le N,N-diméthylformamide (diméthylformamide, DMF, CSA 68-12-1)
est un solvant organique qui est produit en grandes quantités dans
l'ensemble du monde. On l'utilise dans l'industrie chimique comme
solvant, comme produit intermédiaire et comme additif. Le DMF est un
liquide incolore d'odeur légère mais désagréable qui, néanmoins, est
insuffisante pour attirer l'attention. Il est généralement stable
mais il peut entraîner des incendies et des explosions par contact
avec des oxydants forts, des halogènes, des dérivés alkylaluminiques
ou des hydrocarbures halogénés (en particulier combinés à des métaux).
Le DMF est entièrement miscible à l'eau et à la plupart des solvants
organiques. Sa tension de vapeur est relativement faible.
Du point de vue analytique, on a recours à la chromatographie
en phase gazeuse.
1.2 Transport, distribution, et transformation dans l'environnement
Le DMF est stable dans l'air ambiant mais il peut subir une
décomposition microbienne et algaire dans l'eau. Les microorganismes
adaptés et les boues activées assurent une biodégradation efficace du
DMF. Etant soluble dans l'eau en toutes proportions, le DMF est très
mobile dans les sols et il ne devrait pas s'accumuler dans la chaîne
alimentaire.
1.3 Concentration dans l'environnement et exposition humaine
Le DMF n'existe pas à l'état naturel. On ne possède que peu de
données sur sa concentration dans l'environnement ou sur l'exposition
de la population générale. Dans l'air de zones résidentielles situées
à proximité d'installations industrielles, on a trouvé des
concentrations allant de 0,02 à 0,12 mg/m3. Il est rare qu'on décèle
la présence de DMF dans l'eau des bassins fluviaux très industrialisés
et encore, les concentrations ne dépassent pas 0,01 mg/litre.
On ne possède pas de données sur la concentration du DMF dans le
sol, les végétaux, la faune sauvage et les produits alimentaires.
L'exposition professionnelle se produit par contact cutané avec le
liquide ou la vapeur ou par inhalation de la vapeur. On a décelé des
concentrations de 3 à 86 mg/m3 dans l'air avec des maxima allant
jusqu'à 600 mg/m3, au cours de travaux de réparation ou d'entretien de
machines. Dans quelques cas exceptionnels, des concentrations allant
jusqu'à 4500 mg/m3 ont été signalées.
1.4 Cinétique et métabolisme
Des quantités toxiques de DMF peuvent être absorbées par
inhalation ou pénétration percutanée. Une fois absorbé, le DMF se
distribue de façon uniforme dans l'organisme. Sa métabolisation a
lieu principalement dans le foie sous l'action des enzymes
microsomiales. Chez l'animal et l'homme, le principal produit de la
biotransformation du DMF est le N-hydroxyméthyl- N-méthyl-formamide
(DMF-OH). Au cours de l'analyse par chromatographie en phase gazeuse,
ce métabolite est transformé en N-méthyl-formamide (NMF) qui est lui-
même (ainsi que le N-hydroxyméthyl et le formamide) un métabolite
mineur du DMF. Par conséquent, lorsqu'on procède à des études
métaboliques et que l'on effectue la surveillance biologique du DMF,
les concentrations urinaires de métabolites sont mesurées et exprimées
en NMF, même si le DMF-OH en est le constituant essentiel. Le dosage
du NMF/DMF-OH dans les urines peut donner une bonne indication
biologique de l'exposition totale au DMF.
Chez l'animal d'expérience, on a montré que le mécanisme de
métabolisation du DMF se sature lorsque l'exposition est intense, le
DMF jouant le rôle de rétroinhibiteur de son propre métabolisme à
concentration très élevée.
Il y a interaction métabolique entre le DMF et l'éthanol.
1.5 Effets sur les êtres vivant dans leur milieu naturel
On n'a pas très bien étudié des effets du DMF sur l'environnement.
Il semble que sa toxicité pour les organismes aquatiques soit faible.
1.6 Effets sur les animaux d'expérience et les systèmes d'épreuve
in vitro
Le DMF présente pour diverses espèces une faible toxicité aiguë
(chez le rat la DL50 par voie orale est d'environ 3000 mg/kg, la DL50
dermique d'environ 5000 mg/kg et la CL50 par inhalation à peu près
égale à 10 000 mg/m3). Il est légèrement à modérément irritant pour
la peau et les yeux. D'après une étude sur des cobayes, il ne semble
pas doté de pouvoir sensibilisateur. Le DMF peut faciliter
l'absorption percutanée d'autres substances chimiques.
Chez l'animal d'expérience, l'exposition au DMF, quelle que soit
la voie de pénétration, peut provoquer des lésions hépatiques qui
dépendent de la dose. Lorsque l'exposition cesse, on a pu constater
qu'il y avait régénération des tissus. Certaines études on également
permis d'observer des signes de toxicité au niveau du myocarde et des
reins.
On n'a pas constaté de toxicité pour les testicules ou les
ovaires, ni observé d'effets sur la fécondité chez le rat. Chez le
rat, la souris et le lapin, le DMF s'est révélé embryotoxique et
faiblement tératogène. C'est le lapin qui est l'espèce la plus
sensible à l'exposition respiratoire: des effets tératogènes ont été
observés à partir de 1350 mg/m3 (450 ppm), aucun effet n'étant
constaté à 450 mg/m3 (150ppm). Après exposition de la peau, certaines
études ont mis en évidence de très rares effets embryotoxiques et
tératogènes à des doses journalières comprises entre 100 et 400 mg/kg.
De nombreuses épreuves à court terme à la recherche d'effets
génétiques et anomalies de ce genre, on a montré que le DMF était en
général inactif tant in vitro qu' in vivo.
Aucune étude convenable de cancérogénicité à long terme sur
animaux de laboratoire n'est décrite dans la littérature.
1.7 Effets sur l'homme
Aucun effet indésirable sur la population dans son ensemble n'a
été nettement mis en évidence.
On a fait état d'irritation cutanée et de conjonctivites après
contact direct avec du DMF.
Après exposition accidentelle à de fortes concentrations de DMF,
on note dans les 48h. des douleurs abdominales, des nausées, des
étourdissements et de la fatigue. La fonction hépatique peut être
perturbée et on a signalé des modifications de la tension artérielle,
une tachycardie et des anomalies du tracé électrocardiographique. En
général la récupération est totale.
Après des expositions réitérées sur une longue période, on observe
des symptômes tels que céphalées, perte d'appétit et fatigue. Des
signes biochimiques d'insuffisance hépatique peuvent également
s'observer. Des lésions hépatiques n'apparaissent, semble-t-il, qu'à
partir d'une exposition de l'ordre de 30 mg/m3, en l'absence de
contact cutané. Cette concentration atmosphérique correspond à
environ 40 mg de NMF/DMF-OH par gramme de créatinine dans un
échantillon d'urine prélevé à la fin du poste de travail.
Même à des concentrations inférieures à 30 mg/m3, l'exposition
au DMF peut causer une intolérance à l'alcool dont les symptômes
peuvent consister en rougeur soudaine de la face, sensation de
constriction thoracique et étourdissements, quelques fois accompagnés
de nausées et de dyspnée. Ces symptômes durent de 2 à 4 heures et
disparaissent spontanément.
On possède des preuves limitées d'une activité cancérogène du DMF
pour l'homme. C'est ainsi qu'une étude a fait état d'un accroissement
de l'incidence des tumeurs du testicule après exposition à du DMF,
tandis qu'une autre étude à révélé une incidence tumorale accrue au
niveau de la cavité buccale et du pharynx, mais pas au niveau des
testicules.
Deux études peu détaillées font état d'un accroissement de la
fréquence des avortements chez des femmes exposées à du DMF, entre
autres substances chimiques.
2. Conclusions
1. Compte tenu de ses usages actuels, la population dans son
ensemble, n'est probablement que très peu exposée au DMF.
2. Le DMF est facilement résorbé au niveau de la peau et des voies
respiratoires. Le dosage dans les urines du NMF/DMF-OH
constitue un moyen utile pour évaluer la quantité totale de DMF
absorbé.
3. Le risque de lésions hépatiques est faible si la concentration
du DMF dans l'air ambiant est maintenue en dessous de 30 mg/m3
et qu'il n'y a pas de contact cutané. La valeur correspondante
de la teneur des urines en NMF/DMF-OH à la fin du poste de
travail a été fixée provisoirement à 40 mg/g de créatinine.
4. Le DMF est embryotoxique et faiblement tératogène pour le rat,
la souris et le lapin.
5. Il existe des preuves limitées d'une cancérogénicité du DMF
pour l'homme.
6. D'après les données dont on dispose, le DMF est peu toxique
pour l'environnement. Il est peu probable qu'il donne lieu à
une bioaccumulation.
3. Recommandations
3.1 Précautions à prendre pour la manipulation
1. Maintenir la concentration atmosphérique au-dessous de 30 mg/m3
et éviter le contact avec la peau.
2. Surveiller la concentration urinaire de NMF/DMF-OH qui indique
l'exposition totale et la maintenir en-dessous de 40 mg de
NMF/g de créatinine dans les échantillons prélevés à la fin du
poste de travail. Si la concentration dépasse cette valeur,
prendre les mesures nécessaires pour réduire l'exposition.
3.2 Recherches à effectuer
1. Etudier les effets cancérogènes possibles du DMF chez l'homme,
par des études sur des populations humaines et des animaux
d'expérience.
2. Il faudrait disposer de données plus complètes sur la
possibilité d'extrapoler à l'homme les résultats des études
d'embryotoxicité et de tératogénicité effectuées sur l'animal.
Il serait bon d'étudier la cinétique comparée du DMF chez
l'homme et l'animal.
3. Davantage de données sont nécessaires sur le mode d'action et
l'activité relative des métabolites du DMF chez l'homme et
l'animal.
4. Il faudrait affiner les relations entre a) les concentrations
en métabolites urinaires et les taux d'exposition atmosphérique
(en l'absence de contact cutané), et b) la dose totale absorbée
par toutes les voies possibles (indiquée par la concentration
en NMF urinaire à la fin du poste de travail) et l'absence
d'hépatotoxicité.
RESUMEN Y EVALUACION, CONCLUSIONES, RECOMENDACIONES
1. Resumen y evaluación
1.1 Propiedades generales
La N,N-dimetilformamida (dimetilformamida, DMF, CAS 68-12-2) es
un disolvente orgánico que se produce en grandes cantidades en todo el
mundo. Se utiliza en la industria química como disolvente, compuesto
intermedio y aditivo. La DMF es un líquido incoloro con un ligero
olor desagradable que, no obstante, tiene escasas propiedades de
alerta. Es generalmente estable, pero cuando entra en contacto con
oxidantes fuertes, halógenos, alquilaluminio o hidrocarburos
halogenados (especialmente en combinación con metales), puede
prenderse y provocar explosiones. La DMF es totalmente miscible con
el agua y la mayoría de los disolventes orgánicos. Su presión de
vapor es relativamente baja.
Existen procedimientos de cromatografía de gases para la
determinación de la DMF.
1.2 Transporte, distribución y transformación en el medio ambiente
Aunque la DMF es estable en el aire, puede ser objeto en el agua
de degradación por microbios y algas. Los microorganismos adaptados y
los fangos activados biodegradan la DMF de modo eficiente. A
consecuencia de su solubilidad total en el agua, la DMF se desplaza
fácilmente en el suelo y no es de esperar que se acumule en la cadena
alimentaria.
1.3 Niveles medioambientales y exposición humana
La DMF no aparece en la naturaleza. Se dispone de pocos datos
relativos a los niveles medioambientales o a la exposición de la
población general a la DMF. En zonas residenciales cercanas a centros
industriales se han medido concentraciones atmosféricas de 0,02-0,12
mg/m3. Se ha detectado muy raras veces en las aguas de cuencas
fluviales muy industrializadas, y en esos casos sólo en
concentraciones inferiores a 0,01 mg/litro.
No se dispone de datos relativos a los niveles de DMF en el suelo,
los vegetales, los animales silvestres ni los alimentos.
La exposición profesional se produce por contacto cutáneo con la
DMF en forma líquida y de vapor, y por la inhalación de vapores. Se
han detectado concentraciones de 3-86 mg/m3 de aire, con valores
máximos de hasta 600 mg/m3, durante las operaciones de reparación o de
mantenimiento de máquinas. En condiciones muy especiales, se han
registrado concentraciones de hasta 4500 mg/m3.
1.4 Cinética y metabolismo
Pueden absorberse cantidades tóxicas de DMF por inhalación y a
través de la piel. La DMF absorbida se distribuye uniformemente. La
transformación metabólica de la DMF tiene lugar principalmente en el
hígado, con el concurso de sistemas de enzimas microsómicas. En los
animales y el ser humano, el producto principal de la
biotransformación de la DMF es la N-hidroximetil- N-metilformamida
(DMF-OH). Este metabolito principal se convierte durante el análisis
con cromatografía de gases en N-metilformamida, que es a su vez (junto
con la N-hidroximetilformamida y la formamida) uno de los metabolitos
secundarios. Así pues, en los estudios metabólicos y para el
monitoreo biológico, las concentraciones de metabolitos en la orina se
miden y expresan en forma de NMF, aunque la DMF-OH sea el
contribuyente principal a esa concentración. El análisis de la
NMF/DMF-OH en la orina puede ser un indicador biológico adecuado de la
exposición total a la DMF.
En los animales de experimentación, se ha demostrado que el
metabolismo de la DMF se satura a niveles de exposición elevados y
que, a niveles muy elevados, la DMF inhibe su propio metabolismo.
Se produce interacción metabólica entre la DMF y el etanol.
1.5 Efectos en los organismos del medio ambiente
No se han estudiado bien los efectos de la DMF en el medio
ambiente. La toxicidad para los organismos acuáticos parece baja.
1.6 Efectos en los animales de experimentación y en sistemas de
ensayo in vitro
La toxicidad aguda de la DMF en diversas especies es baja (en
ratas, la DL50 es de unos 3000 mg/kg, la DL50 cutánea es de
aproximadamente 5000 mg/kg, y la LC50 por inhalación es de unos 10 000
mg/m3. Su capacidad de irritación de la piel y los ojos es entre
ligera y moderada. En un estudio realizado con cobayas no hubo
indicación alguna de potencial de sensibilización. La DMF puede
facilitar la absorción de otras sustancias químicas a través de la
piel.
La exposición de animales de experimentación a la DMF por todas
las vías de exposición puede provocar lesiones hepáticas dependientes
de la dosis. Se ha demostrado que se produce regeneración al cesar la
exposición. En algunos estudios se han descrito asimismo síntomas de
toxicidad en el miocardio y el riñón.
No se ha demostrado que la DMF sea tóxica para el testículo ni
para el ovario de la rata, ni se han observado efectos en la
fecundidad. Se ha descubierto que la DMF es embriotóxica y
ligeramente teratogénica en la rata, el ratón y el conejo. El conejo
parece ser la especie más sensible a la exposición por inhalación: se
observaron efectos teratogénicos a partir de 1350 mg/m3 (450 ppm),
pero no a 450 mg/m3 (150 ppm). Tras la exposición cutánea, en algunos
estudios se observó una incidencia muy baja de efectos embriotóxicos y
teratogénicos con dosis que variaron entre 100 y 400 mg/kg al día.
En una amplia serie de ensayos a corto plazo en busca de efectos
genéticos y otros afines se encontró que, en general, la DMF es
inactiva, tanto in vitro como in vivo.
No se han comunicado estudios suficientes sobre carcinogenicidad
a largo plazo en animales de experimentación.
1.7 Efectos en el ser humano
No se ha demostrado claramente la existencia de efectos adversos
de la DMF en la población general.
Se han comunicado casos de irritación cutánea y conjuntivitis tras
el contacto directo con DMF.
Al cabo de 48 horas de la exposición accidental a niveles elevados
de DMF, aparecen dolores abdominales, náuseas, vómitos, mareos y
fatiga. La función hepática puede alterarse y se han notificado casos
de cambios en la tensión arterial, taquicardia y anomalías
electroencefalográficas. Por lo general, la recuperación es completa.
Después de una exposición repetida y a largo plazo, aparecen
síntomas como dolor de cabeza, pérdida de apetito y fatiga. Pueden
observarse signos de disfunción hepática. Las lesiones hepáticas
parecen producirse sólo cuando el nivel de exposición a la DMF pasa de
30 mg/m3, en ausencia de contacto cutáneo. Ese nivel en el aire
corresponde a aproximadamente 40 mg de NMF/DMF-OH/creatinina en una
muestra de orina tomada después del turno de trabajo.
La exposición a la DMF, incluso en concentraciones inferiores a 30
mg/m3, puede provocar intolerancia al alcohol. Entre los síntomas
pueden presentarse un acaloramiento facial repentino, opresión en el
pecho y mareos, a veces acompañados de náuseas y disnea. Duran entre
2 y 4 h y desaparecen espontáneamente.
Existen pruebas limitadas de que la DMF es carcinogénica para el
ser humano. En un estudio se comunicó una incidencia mayor de tumores
testiculares, mientras que en otro se observó una incidencia mayor de
tumores de la cavidad oral y la faringe, pero no del testículo.
En dos estudios, que comunican pocos pormenores, se observó una
frecuencia mayor de abortos espontáneos en mujeres expuestas a la DMF,
entre otras sustancias químicas.
2. Conclusiones
1. Dados los usos actuales de la DMF, la exposición de la
población general es probablemente muy baja.
2. La DMF se absorbe fácilmente a través de la piel además de por
inhalación. La determinación de la NMF/DMF-OH en la orina es
un medio muy útil de estimar la cantidad total de DMF
absorbida.
3. El riesgo de lesión hepática es reducido si el nivel de DMF en
el aire se mantiene por debajo de 30 mg/m3 y no hay contacto
cutáneo. Un valor provisional para el nivel correspondiente de
NMF/DMF-OH en la orina en una muestra tomada después del turno
de trabajo es 40 mg/g de creatinina.
4. La DMF es embriotóxica y ligeramente teratogénica en la rata,
el ratón y el conejo.
5. Existen pruebas limitadas de la carcinogenicidad de la DMF para
el ser humano.
6. Los datos disponibles indican que tiene una baja toxicidad
ambiental. Es poco probable que se produzca bioacumulación.
3. Recomendaciones
3.1 Manipulación sin riesgos
1. Las concentraciones en el aire deben mantenerse por debajo de
30 mg/m3 y debe evitarse el contacto con la piel.
2. La NMF/DMF-OH en la orina, como índice de la exposición total,
debe vigilarse y mantenerse por debajo de 40 mg de NMF/g de
creatinina en muestras tomadas después del turno de trabajo.
Si se sobrepasa ese nivel, deben adoptarse medidas para
disminuir la exposición.
3.2 Nuevas investigaciones
1. Los posibles efectos carcinogénicos del DMF en el ser humano
deben investigarse mediante estudios en animales de
experimentación y poblaciones humanas.
2. Se necesita más información para extrapolar de los estudios en
animales al ser humano los datos sobre embriotoxicidad y
teratogenicidad de la DMF. La comparación de la cinética de la
DMF en el ser humano y en los animales sería muy útil.
3. Se necesita más información sobre los mecanismos de acción y la
potencia relativa de los metabolitos de la DMF en animales y en
el ser humano.
4. Deben afinarse las relaciones entre a) las concentraciones de
metabolitos en la orina y los niveles de exposición en la
atmósfera (en ausencia de contacto cutáneo), y b) la dosis
total recibida por todas las vías (indicada por los niveles de
NMF en la orina después del trabajo) y la ausencia de
hepatotoxicidad.
 6.1.4 Elimination and excretion
The transformation and excretion of DMF in rodents is rapid. When
14C-labelled DMF in 0.1 ml saline was injected ip at 6.8 mmol/kg body
weight in mice, about 83% of the radioactivity was recovered in the
urine within 24 h following injection. Of this amount, only 5% was
unchanged DMF and 56% was C-hydroxylated or N-demethylated
derivatives. About 18% of the dose was excreted in the form of
unknown chemicals (Brindley et al., 1983).
Similarly, 24 h after ip injection of 400 mg DMF/kg body weight in
0.2 ml saline in mice, about 56% of the dose was excreted in the urine
as DMF-OH and only 5% as unchanged DMF (Kestell et al., 1986).
Within 72 h of an ip administration of 1 ml 14C-DMF/kg to male
or female rats, 70% of the injected radioactivity was recovered in the
urine. Approximately 15% was excreted as unchanged DMF, 50% as DMF-OH
(NMF), and 5% as NMF-OH (F). About 20% was excreted as unidentified
metabolite(s) (Scailteur & Lauwerys, 1984a,b).
After oral exposure to DMF (40-2000 mg/kg), Sanotsky et al. (1978)
determined that about 6% of the dose was excreted in 24 h.
The elimination of DMF, NMF (DMF-OH), and formamide (NMF-OH) was
measured after single or repeated inhalation exposure in rats and dogs
(Kimmerle & Eben, 1975a). Twenty-four hours after a single exposure to
63 mg NMF/m3 for 3 h, or 87 mg/m3 for 6 h, no NMF was found in the
urine of male rats. Under the same conditions, exposure to 513 mg/m3
for 6 h or to 6015 mg/m3 for 3 h led to excretion of 4 mg and 14 mg
NMF (DMF-OH), respectively, during the 24 h following the start of
exposure. Only in the last case was DMF also measured in the urine.
After repeated exposure of male rats to DMF (1050 mg/m3, 6 h/day, for
5 days), urinary levels of NMF (DMF-OH) remained practically constant
for the first 3 days, then slightly decreased from the fourth day of
exposure. Excretion of F (NMF-OH) was much lower than excretion of NMF
(DMF-OH).
While no accumulation of urinary NMF (DMF-OH) was observed in male
rats, male dogs exposed to 177 mg DMF/m3 (6 h/day for 5 days) excreted
increasing concentrations of NMF (DMF-OH) (36 mg/24 h after the first
inhalation; 87 mg/24 h after the 4th inhalation). Urinary excretion
of formamide (NMF-OH) varied between 10 and 20 mg/24 h. Excretion of
unchanged DMF was very low (< 2 mg/24 h). However, in female dogs
exposed to 69 mg/m3 (6 h/day for 5 days), no urinary accumulation of
NMF or F was observed. When male or female rats were exposed for 4
weeks to 63 mg/m3 (6 h/day, 5 days per week), NMF and F concentrations
in the urine remained practically constant during the exposure period.
Male dogs generally excreted slightly higher levels of metabolites
than female dogs (Kimmerle & Eben, 1975a).
In rats treated with repeated, high, ip doses of DMF (4 daily
injections of 1 ml/kg body weight), Scailteur et al. (1984) showed
that females excreted higher amounts of unchanged DMF than males. The
pattern of metabolite (NMF, F) excretion was similar in both sexes
after single or repeated ip administration.
6.1.5 Metabolic interaction between DMF and ethanol
DMF and ethanol appear to interact metabolically.
The alterations in blood metabolites depend on the dose of DMF,
the time interval between DMF and ethanol administration, and the
respective routes of administration.
The various studies performed are summarized in Table 8. Blood
concentrations of DMF and NMF, ethanol, and acetaldehyde were measured
using GC methods.
The influence of DMF on ethanol oxidation might be explained, at
least partially, by its inhibitory effect on the activity of alcohol
dehydrogenase in vitro and in vivo (Sharkawi, 1979) and aldehyde
dehydrogenase in vivo (Elovaara et al., 1983).
Table 8. Metabolic interaction between DMF and ethanol
-----------------------------------------------------------------------------------------------------------------------------------------
Species Ethanol Time of DMF Effects on blood concentrations of: Reference
dose administration dose
(route) (route) DMF and Ethanol and
NMF acetaldehyde
-----------------------------------------------------------------------------------------------------------------------------------------
Rat .2 g/kg immediately before 312 mg/m3 No effects on not measured Eben & Kimmerle (1976)
(oral) DMF exposure 2 h (inhalation) DMF and NMF
Rat 2 g/kg immediately before 261 or 627 mg/m3 DMF increased not measured Eben & Kimmerle (1976)
(oral) DMF exposure 2 h (inhalation) NMF formation
Rat 2 g/kg per day daily immediately about 600 mg/m3 DMF increased ethanol increased Eben & Kimmerle (1976)
for 5 days before DMF 2 h/day 5 days NMF formation
(oral) exposure (inhalation)
Dog 2 g/kg immediately before about 630 mg/m3 DMF increased not measured Eben & Kimmerle (1976)
(oral) DMF exposure 2 h (inhalation) NMF decreased
Dog 2 g/kg immediately after 630 mg/m3 DMF increased not measured Eben & Kimmerle (1976)
(oral) DMF exposure 2 h (inhalation) NMF decreased
Rat 2 g/kg 1 h after last 3000 mg/m3 not measured acetaldehyde Hanasono et al. (1977)
(oral) DMF exposure 4 h/day 3 days increased
(inhalation)
Rat 2 g/kg 1 h after last 6000 mg/m3 not measured ethanol increased Hanasono et al. (1977)
(oral) DMF exposure 4 h/day 3 days acetaldehyde
(inhalation) decreased
Mouse 1 g/kg 2 h after DMF 1.2 ml/kg not measured ethanol increased Sharkawi (1980)
(ip) exposure (ip)
Rat 2 g/kg 3 h after DMF 0.15 g/kg not measured ethanol increased Hanasono et al. (1977)
(oral) exposure (oral) acetaldehyde
decreased
Rat 2 g/kg 18 h after DMF 0.15 g/kg not measured acetaldehydea Hanasono et al. (1977)
(oral) exposure (oral) increased
Table 8. (continued)
-----------------------------------------------------------------------------------------------------------------------------------------
Species Ethanol Time of DMF Effects on blood concentrations of: Reference
dose administration dose
(route) (route) DMF and Ethanol and
NMF acetaldehyde
-----------------------------------------------------------------------------------------------------------------------------------------
Rat 2 g/kg 18 h after DMF 1.5 g/kg not measured ethanol increased Hanasono et al. (1977)
(oral) exposure (oral)
Rat 2 g/kg 24 h after last 3000 mg/m3 not measured acetaldehyde Hanasono et al. (1977)
(oral) DMF exposure 4 h/day 3 days increased
(inhalation)
Rat 2 g/kg 24 h after last 12 000 mg/m3 not measured acetaldehyde Hanasono et al. (1977)
(oral) DMF exposure 4 h/day 3 days increased
(inhalation)
-----------------------------------------------------------------------------------------------------------------------------------------
a Increased acetaldehyde level observed after this dose of DMF was equivalent to that produced by an equimolar dose of disulfiram (antabuse).
6.2 Human studies
6.2.1 Absorption, distribution, metabolism, excretion
In vitro studies on excised human skin (Bortsevich, 1984) showed
a relationship between the amount of DMF absorbed through the dermal
barrier and the DMF concentrations in water, as well as the exposure
time. DMF enhances its own penetration. Some of the results are
given in Table 9. They are of practical value, because such solutions
are used in synthetic fibre production.
After respiratory exposure to DMF, lung retention in workers in an
artificial leather factory was 72% (Brugnone, 1980a,b).
Table 9. Quantities of DMF absorbed in in vitro studies on
excised human skin
--------------------------------------------------------------
Exposure period DMF solutions in water
(h) 100% 60% 30% 15%
% DMF absorbed through the skin (mg/cm2)
--------------------------------------------------------------
0.5 0.046 NDa NDa NDa
1-1.5 7.400 0.035 0.013 0.006
2-2.5 20.550 0.087 0.048 0.009
3-3.5 40.810 0.222 0.097 0.017
4-4.5 51.730 0.300 0.160 0.069
--------------------------------------------------------------
a ND = Not detectable.
The relative importance of skin versus inhalation for DMF
absorption was studied in volunteers by Maxfield et al. (1975),
Kimmerle & Eben (1975a), and Krivanek et al. (1978) (section 6.2.3.1).
As in animals, the major human metabolite of DMF has been reported
to be DMF-OH and not NMF. However, it is measured as NMF when using
gas chromatography including the small amount of NMF excreted in the
urine (Scailteur & Lauwerys, 1987).
When a male volunteer inhaled the DMF vapours that were produced
over liquid DMF in a beaker for 6 h, Mraz & Turecek (1987) identified
the metabolite N-acetyl- S-( N-methylcarbamoyl) cysteine in the urine.
Malonova & Bardodej (1983) reported a possible increase in the
urinary excretion of mercapturates in workers exposed to unknown
concentrations of DMF (approximately twice the excretion in controls
(smokers)).
6.2.2 The influence of ethanol on DMF metabolism in human volunteers
Eben & Kimmerle (1976) exposed 4 subjects via inhalation to DMF
(159 mg/m3) for 2 h with, and without, ingestion of 19 g ethanol (50
ml 38% gin), 10 min before they inhaled the DMF. No changes in DMF
concentrations in blood were found. The comparatively lower NMF
concentrations in the blood of subjects with combined exposure to
ethanol and DMF indicated that the ethanol decreased the
biotransformation of DMF. No significant differences in the blood
levels of ethanol and acetaldehyde were detected in subjects with, or
without, ethanol exposure, which differed from the effects observed in
animal studies. The authors suggested that this was because of the
relatively low concentrations of DMF used in the human studies.
6.2.3 Biological monitoring of workers
N-Hydroxymethyl- N-methylformamide (DMF-OH) has been identified as
the main urinary metabolite of DMF. It is measured, using gas
chromatography, as NMF together with the small proportion of NMF
excreted in the urine. Some results of studies on the correlation
between exposure levels to DMF and NMF excretion in workers and human
volunteers are given in Table 10.
6.2.3.1 Determination of NMF in the urine
NMF (DMF-OH) in the urine is a sensitive biological parameter of
human DMF exposure. NMF levels in the urine are usually greater at
the end of the shift than on the morning after the exposure. Lauwerys
et al. (1980) compared a group of 22 male workers from the spinning
mill in a polyacrylic fibre plant with 28 controls. The workers in the
spinning department wore gloves and long sleeves, but did not have any
respiratory protection. Spot urine samples were collected before, and
after, the work shift for 5 consecutive days, to determine NMF and
creatinine concentrations. NMF was notdetected in the urine of
control workers, who were not exposed to DMF. There was a poor
correlation, on an individual basis, between the integrated DMF
exposure and the NMF concentration in the urine collected at the end
of the shift, or in that collected before resuming work the following
day. However, on a group basis, there was a good correlation between
the intensity of exposure and NMF levels in the urine collected at the
end of the shift.
In a second study in the polyacrylic fibre plant, Lauwerys et al.
(1980) studied the NMF levels in the urine of 7 workers for 3 weeks,
when different types of personal protective devices were used.
Absorption of DMF vapours through the skin was more important than
through inhalation. In the absence of skin contact, a concentration
of 40-50 mg NMF/g creatinine, in post-shift samples, corresponded to
an average concentration of DMF vapour of 13 mg/m3 (45 ppm) during a
6-h exposure period.
Table 10. NMF levels in urine as a test for DMF exposure
------------------------------------------------------------------------------------------------------------------
Subjects DMF concentrations NMF concentrations Time of sampling Reference
in the air in the urine
------------------------------------------------------------------------------------------------------------------
4 volunteers 78 ± 24 mg/m3a 24 mg/24 h Kimmerle & Eben (1975a)
261 ± 75 mg/m3a 97.4 mg/24 h
63 ± 12 mg/m3b 30 mg/24 h
4 volunteers 159 ± 96 mg/m3a 44.8 mg/24 h Eben & Kimmerle (1976)
4 volunteers 32.4 ± 2.1 mg/m3a,c 5 mg/24 h Maxfield et al. (1975)
8 volunteers 26.4 ± 0.9 mg/m3b 2.5 mg/24 h Krivanek et al. (1978)
22 workers 13 mg/m3b 20-40 mg/g post-shift samples Lauwerys et al. (1980)
creatinine
9 workers 15.4 mg/m3b 0.4-19.6 mg/24 h Yonemoto & Suzuki (1980)
85 workers 30-150 mg/m3b,c 0.104-0.224 mg/ml Aldyreva et al. (1980)
23 workers above 30 mg/m3b 20-40 mg/24 h Taccola et al. (1981)
2 volunteers 30 mg/m3b 102.6 µmol/8 h Wicarova & Dadak (1981)
39 workers 217.5 µmol/24 h
30 workers 14-86.3 mg/m3b 12-188.3 mg/g 4 h after the work shift Sala et al. (1984)
creatinine different work areas
------------------------------------------------------------------------------------------------------------------
a Single inhalation exposure to DMF (2, 4, or 6 h/day).
b Repeated inhalation exposure to DMF (6, 7, 7.5 h/day).
c Dermal absorption.
Yonemoto & Suzuki (1980) studied the relationship between the
individual occupational exposure to DMF and the amount of NMF in the
urine of 9 male workers who handled polyurethane surface-treating
agents for synthetic leather. The time-weighted average individual
exposures ranged from 0 to 15.4 mg DMF/m3. The amount of NMF excreted
daily ranged from 0.4 to 19.56 mg/24 h. The excretion rate of NMF
(mg/h) increased from the beginning of exposure and reached a maximum
in the urine samples collected in the evening. The relationship
between the total daily NMF excretion in the urine and the level of
exposure was represented as a linear regression, indicating that the
best biological index of DMF exposure is the determination of NMF in
the 24-h urine (Fig. 2). At an 8-h integrated DMF exposure of 15
mg/m3, the NMF level in the urine of the workers was less than 20
mg/24 h. This value is higher than those obtained for volunteers
(Kimmerle & Eben, 1975b; Krivanek et al., 1978) or for workers
(Lauwerys et al., 1980). Yonemoto & Suzuki (1980) stated that the
difference might be due to dermal absorption of DMF, because the
workers did not use protective gloves or special working overalls.
6.1.4 Elimination and excretion
The transformation and excretion of DMF in rodents is rapid. When
14C-labelled DMF in 0.1 ml saline was injected ip at 6.8 mmol/kg body
weight in mice, about 83% of the radioactivity was recovered in the
urine within 24 h following injection. Of this amount, only 5% was
unchanged DMF and 56% was C-hydroxylated or N-demethylated
derivatives. About 18% of the dose was excreted in the form of
unknown chemicals (Brindley et al., 1983).
Similarly, 24 h after ip injection of 400 mg DMF/kg body weight in
0.2 ml saline in mice, about 56% of the dose was excreted in the urine
as DMF-OH and only 5% as unchanged DMF (Kestell et al., 1986).
Within 72 h of an ip administration of 1 ml 14C-DMF/kg to male
or female rats, 70% of the injected radioactivity was recovered in the
urine. Approximately 15% was excreted as unchanged DMF, 50% as DMF-OH
(NMF), and 5% as NMF-OH (F). About 20% was excreted as unidentified
metabolite(s) (Scailteur & Lauwerys, 1984a,b).
After oral exposure to DMF (40-2000 mg/kg), Sanotsky et al. (1978)
determined that about 6% of the dose was excreted in 24 h.
The elimination of DMF, NMF (DMF-OH), and formamide (NMF-OH) was
measured after single or repeated inhalation exposure in rats and dogs
(Kimmerle & Eben, 1975a). Twenty-four hours after a single exposure to
63 mg NMF/m3 for 3 h, or 87 mg/m3 for 6 h, no NMF was found in the
urine of male rats. Under the same conditions, exposure to 513 mg/m3
for 6 h or to 6015 mg/m3 for 3 h led to excretion of 4 mg and 14 mg
NMF (DMF-OH), respectively, during the 24 h following the start of
exposure. Only in the last case was DMF also measured in the urine.
After repeated exposure of male rats to DMF (1050 mg/m3, 6 h/day, for
5 days), urinary levels of NMF (DMF-OH) remained practically constant
for the first 3 days, then slightly decreased from the fourth day of
exposure. Excretion of F (NMF-OH) was much lower than excretion of NMF
(DMF-OH).
While no accumulation of urinary NMF (DMF-OH) was observed in male
rats, male dogs exposed to 177 mg DMF/m3 (6 h/day for 5 days) excreted
increasing concentrations of NMF (DMF-OH) (36 mg/24 h after the first
inhalation; 87 mg/24 h after the 4th inhalation). Urinary excretion
of formamide (NMF-OH) varied between 10 and 20 mg/24 h. Excretion of
unchanged DMF was very low (< 2 mg/24 h). However, in female dogs
exposed to 69 mg/m3 (6 h/day for 5 days), no urinary accumulation of
NMF or F was observed. When male or female rats were exposed for 4
weeks to 63 mg/m3 (6 h/day, 5 days per week), NMF and F concentrations
in the urine remained practically constant during the exposure period.
Male dogs generally excreted slightly higher levels of metabolites
than female dogs (Kimmerle & Eben, 1975a).
In rats treated with repeated, high, ip doses of DMF (4 daily
injections of 1 ml/kg body weight), Scailteur et al. (1984) showed
that females excreted higher amounts of unchanged DMF than males. The
pattern of metabolite (NMF, F) excretion was similar in both sexes
after single or repeated ip administration.
6.1.5 Metabolic interaction between DMF and ethanol
DMF and ethanol appear to interact metabolically.
The alterations in blood metabolites depend on the dose of DMF,
the time interval between DMF and ethanol administration, and the
respective routes of administration.
The various studies performed are summarized in Table 8. Blood
concentrations of DMF and NMF, ethanol, and acetaldehyde were measured
using GC methods.
The influence of DMF on ethanol oxidation might be explained, at
least partially, by its inhibitory effect on the activity of alcohol
dehydrogenase in vitro and in vivo (Sharkawi, 1979) and aldehyde
dehydrogenase in vivo (Elovaara et al., 1983).
Table 8. Metabolic interaction between DMF and ethanol
-----------------------------------------------------------------------------------------------------------------------------------------
Species Ethanol Time of DMF Effects on blood concentrations of: Reference
dose administration dose
(route) (route) DMF and Ethanol and
NMF acetaldehyde
-----------------------------------------------------------------------------------------------------------------------------------------
Rat .2 g/kg immediately before 312 mg/m3 No effects on not measured Eben & Kimmerle (1976)
(oral) DMF exposure 2 h (inhalation) DMF and NMF
Rat 2 g/kg immediately before 261 or 627 mg/m3 DMF increased not measured Eben & Kimmerle (1976)
(oral) DMF exposure 2 h (inhalation) NMF formation
Rat 2 g/kg per day daily immediately about 600 mg/m3 DMF increased ethanol increased Eben & Kimmerle (1976)
for 5 days before DMF 2 h/day 5 days NMF formation
(oral) exposure (inhalation)
Dog 2 g/kg immediately before about 630 mg/m3 DMF increased not measured Eben & Kimmerle (1976)
(oral) DMF exposure 2 h (inhalation) NMF decreased
Dog 2 g/kg immediately after 630 mg/m3 DMF increased not measured Eben & Kimmerle (1976)
(oral) DMF exposure 2 h (inhalation) NMF decreased
Rat 2 g/kg 1 h after last 3000 mg/m3 not measured acetaldehyde Hanasono et al. (1977)
(oral) DMF exposure 4 h/day 3 days increased
(inhalation)
Rat 2 g/kg 1 h after last 6000 mg/m3 not measured ethanol increased Hanasono et al. (1977)
(oral) DMF exposure 4 h/day 3 days acetaldehyde
(inhalation) decreased
Mouse 1 g/kg 2 h after DMF 1.2 ml/kg not measured ethanol increased Sharkawi (1980)
(ip) exposure (ip)
Rat 2 g/kg 3 h after DMF 0.15 g/kg not measured ethanol increased Hanasono et al. (1977)
(oral) exposure (oral) acetaldehyde
decreased
Rat 2 g/kg 18 h after DMF 0.15 g/kg not measured acetaldehydea Hanasono et al. (1977)
(oral) exposure (oral) increased
Table 8. (continued)
-----------------------------------------------------------------------------------------------------------------------------------------
Species Ethanol Time of DMF Effects on blood concentrations of: Reference
dose administration dose
(route) (route) DMF and Ethanol and
NMF acetaldehyde
-----------------------------------------------------------------------------------------------------------------------------------------
Rat 2 g/kg 18 h after DMF 1.5 g/kg not measured ethanol increased Hanasono et al. (1977)
(oral) exposure (oral)
Rat 2 g/kg 24 h after last 3000 mg/m3 not measured acetaldehyde Hanasono et al. (1977)
(oral) DMF exposure 4 h/day 3 days increased
(inhalation)
Rat 2 g/kg 24 h after last 12 000 mg/m3 not measured acetaldehyde Hanasono et al. (1977)
(oral) DMF exposure 4 h/day 3 days increased
(inhalation)
-----------------------------------------------------------------------------------------------------------------------------------------
a Increased acetaldehyde level observed after this dose of DMF was equivalent to that produced by an equimolar dose of disulfiram (antabuse).
6.2 Human studies
6.2.1 Absorption, distribution, metabolism, excretion
In vitro studies on excised human skin (Bortsevich, 1984) showed
a relationship between the amount of DMF absorbed through the dermal
barrier and the DMF concentrations in water, as well as the exposure
time. DMF enhances its own penetration. Some of the results are
given in Table 9. They are of practical value, because such solutions
are used in synthetic fibre production.
After respiratory exposure to DMF, lung retention in workers in an
artificial leather factory was 72% (Brugnone, 1980a,b).
Table 9. Quantities of DMF absorbed in in vitro studies on
excised human skin
--------------------------------------------------------------
Exposure period DMF solutions in water
(h) 100% 60% 30% 15%
% DMF absorbed through the skin (mg/cm2)
--------------------------------------------------------------
0.5 0.046 NDa NDa NDa
1-1.5 7.400 0.035 0.013 0.006
2-2.5 20.550 0.087 0.048 0.009
3-3.5 40.810 0.222 0.097 0.017
4-4.5 51.730 0.300 0.160 0.069
--------------------------------------------------------------
a ND = Not detectable.
The relative importance of skin versus inhalation for DMF
absorption was studied in volunteers by Maxfield et al. (1975),
Kimmerle & Eben (1975a), and Krivanek et al. (1978) (section 6.2.3.1).
As in animals, the major human metabolite of DMF has been reported
to be DMF-OH and not NMF. However, it is measured as NMF when using
gas chromatography including the small amount of NMF excreted in the
urine (Scailteur & Lauwerys, 1987).
When a male volunteer inhaled the DMF vapours that were produced
over liquid DMF in a beaker for 6 h, Mraz & Turecek (1987) identified
the metabolite N-acetyl- S-( N-methylcarbamoyl) cysteine in the urine.
Malonova & Bardodej (1983) reported a possible increase in the
urinary excretion of mercapturates in workers exposed to unknown
concentrations of DMF (approximately twice the excretion in controls
(smokers)).
6.2.2 The influence of ethanol on DMF metabolism in human volunteers
Eben & Kimmerle (1976) exposed 4 subjects via inhalation to DMF
(159 mg/m3) for 2 h with, and without, ingestion of 19 g ethanol (50
ml 38% gin), 10 min before they inhaled the DMF. No changes in DMF
concentrations in blood were found. The comparatively lower NMF
concentrations in the blood of subjects with combined exposure to
ethanol and DMF indicated that the ethanol decreased the
biotransformation of DMF. No significant differences in the blood
levels of ethanol and acetaldehyde were detected in subjects with, or
without, ethanol exposure, which differed from the effects observed in
animal studies. The authors suggested that this was because of the
relatively low concentrations of DMF used in the human studies.
6.2.3 Biological monitoring of workers
N-Hydroxymethyl- N-methylformamide (DMF-OH) has been identified as
the main urinary metabolite of DMF. It is measured, using gas
chromatography, as NMF together with the small proportion of NMF
excreted in the urine. Some results of studies on the correlation
between exposure levels to DMF and NMF excretion in workers and human
volunteers are given in Table 10.
6.2.3.1 Determination of NMF in the urine
NMF (DMF-OH) in the urine is a sensitive biological parameter of
human DMF exposure. NMF levels in the urine are usually greater at
the end of the shift than on the morning after the exposure. Lauwerys
et al. (1980) compared a group of 22 male workers from the spinning
mill in a polyacrylic fibre plant with 28 controls. The workers in the
spinning department wore gloves and long sleeves, but did not have any
respiratory protection. Spot urine samples were collected before, and
after, the work shift for 5 consecutive days, to determine NMF and
creatinine concentrations. NMF was notdetected in the urine of
control workers, who were not exposed to DMF. There was a poor
correlation, on an individual basis, between the integrated DMF
exposure and the NMF concentration in the urine collected at the end
of the shift, or in that collected before resuming work the following
day. However, on a group basis, there was a good correlation between
the intensity of exposure and NMF levels in the urine collected at the
end of the shift.
In a second study in the polyacrylic fibre plant, Lauwerys et al.
(1980) studied the NMF levels in the urine of 7 workers for 3 weeks,
when different types of personal protective devices were used.
Absorption of DMF vapours through the skin was more important than
through inhalation. In the absence of skin contact, a concentration
of 40-50 mg NMF/g creatinine, in post-shift samples, corresponded to
an average concentration of DMF vapour of 13 mg/m3 (45 ppm) during a
6-h exposure period.
Table 10. NMF levels in urine as a test for DMF exposure
------------------------------------------------------------------------------------------------------------------
Subjects DMF concentrations NMF concentrations Time of sampling Reference
in the air in the urine
------------------------------------------------------------------------------------------------------------------
4 volunteers 78 ± 24 mg/m3a 24 mg/24 h Kimmerle & Eben (1975a)
261 ± 75 mg/m3a 97.4 mg/24 h
63 ± 12 mg/m3b 30 mg/24 h
4 volunteers 159 ± 96 mg/m3a 44.8 mg/24 h Eben & Kimmerle (1976)
4 volunteers 32.4 ± 2.1 mg/m3a,c 5 mg/24 h Maxfield et al. (1975)
8 volunteers 26.4 ± 0.9 mg/m3b 2.5 mg/24 h Krivanek et al. (1978)
22 workers 13 mg/m3b 20-40 mg/g post-shift samples Lauwerys et al. (1980)
creatinine
9 workers 15.4 mg/m3b 0.4-19.6 mg/24 h Yonemoto & Suzuki (1980)
85 workers 30-150 mg/m3b,c 0.104-0.224 mg/ml Aldyreva et al. (1980)
23 workers above 30 mg/m3b 20-40 mg/24 h Taccola et al. (1981)
2 volunteers 30 mg/m3b 102.6 µmol/8 h Wicarova & Dadak (1981)
39 workers 217.5 µmol/24 h
30 workers 14-86.3 mg/m3b 12-188.3 mg/g 4 h after the work shift Sala et al. (1984)
creatinine different work areas
------------------------------------------------------------------------------------------------------------------
a Single inhalation exposure to DMF (2, 4, or 6 h/day).
b Repeated inhalation exposure to DMF (6, 7, 7.5 h/day).
c Dermal absorption.
Yonemoto & Suzuki (1980) studied the relationship between the
individual occupational exposure to DMF and the amount of NMF in the
urine of 9 male workers who handled polyurethane surface-treating
agents for synthetic leather. The time-weighted average individual
exposures ranged from 0 to 15.4 mg DMF/m3. The amount of NMF excreted
daily ranged from 0.4 to 19.56 mg/24 h. The excretion rate of NMF
(mg/h) increased from the beginning of exposure and reached a maximum
in the urine samples collected in the evening. The relationship
between the total daily NMF excretion in the urine and the level of
exposure was represented as a linear regression, indicating that the
best biological index of DMF exposure is the determination of NMF in
the 24-h urine (Fig. 2). At an 8-h integrated DMF exposure of 15
mg/m3, the NMF level in the urine of the workers was less than 20
mg/24 h. This value is higher than those obtained for volunteers
(Kimmerle & Eben, 1975b; Krivanek et al., 1978) or for workers
(Lauwerys et al., 1980). Yonemoto & Suzuki (1980) stated that the
difference might be due to dermal absorption of DMF, because the
workers did not use protective gloves or special working overalls.
 Wicarova & Dadak (1981) studied the relationship between the
amount of NMF in the shift urine (8 h) or the all-day urine (24 h) of
workers and DMF concentrations in the air (0-100 mg/m3) in an
artificial leather plant . The relationship was linear for the shift
urine samples. For the 24-h urine samples, the relationship was
linear only in the range of 0-80 mg DMF/m3 (see also Table 10).
When Dixon et al. (1983) studied the urinary NMF excretion in a
group of 32-37 workers who were exposed to similar air levels of DMF
for either 8 h per shift (5 days/week) or 12 h per shift (4
days/week), they found higher concentrations of NMF in the urine when
the workers were working 8-h shifts. A possible explanation was that
a 13% reduction in urine volume was seen in workers on 8-h shifts
during the summer months compared with higher urine outputs seen in
the same workers on 12-h shifts during the winter months.
Sala et al. (1984) found a correlation between urinary NMF levels,
4 h after workplace exposure, and the workers' exposure levels to DMF
in 5 job categories relating to artificial leather production. They
reported airborne DMF concentrations of 4.5-14 mg/m3 for spreading
"coagulate" system workers, with a mean NMF in urine of 16 mg/g
creatinine, 9.4 mg DMF/m3 for finishing workers, with a mean NMF
urinary value of 12 mg/g creatinine (low exposures), and 86.3 mg
DMF/m3 in tank cleaning workers with a corresponding urinary value of
188.3 mg NMF/g creatinine (highest exposure).
6.2.3.2 N,N-Dimethylformamide determination in the expired air
Airborne DMF concentrations change considerably during the work
shift and from one workplace to another. Brugnone et al. (1980a)
measured the DMF concentrations in the alveolar air every hour during
the 8-h shift of 8 workers employed in an artificial leather plant.
The alveolar DMF concentration in 6 workers was correlated with the
DMF concentration in the air of the respective workplaces.
In a second study, Brugnone et al. (1984) studied 8 exposed
workers by determining the DMF concentrations in the environmental
air, alveolar air, blood, and urine. Air samples were collected at
hourly intervals during an 8-h work shift, blood samples, at 2-h
intervals, and urine samples, at 4-h intervals. No DMF was found in
the blood or urine. A good correlation between the alveolar and
environmental DMF concentrations was found in 6 out of the 8 workers,
and at all subsequent hours, except for the fourth hour.
In practice, the alveolar air test is more difficult to perform
and use for routine examination than measurement of NMF levels in
urine samples, and is not recommended for biological monitoring.
6.2.3.3 Appraisal
The level of NMF in a post-shift urine sample is the most
appropriate biological parameter for total DMF exposure (inhalation
plus dermal) during the shift.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
The effects of DMF on organisms in the environment have been
reviewed by Kennedy (1986) and by US EPA (1986).
The LC50s of DMF for various aquatic species, given in Table 11,
indicate a low toxicity for the species tested.
DMF is commonly used to facilitate the solution of lipophilic
compounds in water during aquatic toxicity tests.
Cardwell et al. (1978) studied the long-term toxicity of DMF for
fathead minnow (Pimephales promelas), brown trout (Salvelinus
fontinalis), and bluegill (Lepomis macrochirus), and found threshold
limits of between 43 and 98 mg DMF/litre for the brook trout and
between 5 and 10 mg/litre for the fathead minnow. LeBlanc &
Surprenant (1983) showed that a level of 0.1 ml DMF/litre was
acceptable for long-term aquatic toxicity tests. In a study by
Tonogai et al. (1982), the 24-h and 48-h static median tolerance
limits for the Himedaka (Oryzias latipes) were > 1000 mg DMF/litre.
A no-observed-effect level (NOEL) of 7700 mg/litre was reported
for the rainbow trout by Shubat et al. (1982).
Solutions of DMF of 25 g/litre (2.5%) were shown to be lethal
within 0.5 h for eggs of sea urchins (Lythechinus variegatus, Arbacia
punctulata, Lythechinus pictus), the surf clam (Spisule solidissima),
and the annelid (Pectinaria) (Rebhun & Sawada, 1969).
Hughes & Vilkas (1983) determined that the highest concentration
that had no significant effect on the green alga Selenastrum
capricornatum, was 1 ml/litre and the no-effect level was 0.5
ml/litre.
Concentrations ranging from 0.085-0.340% DMF had an inhibitory
effect on cultures of Streptomyces aureofaciens (Welward & Halama,
1978).
Table 11. Medial lethal (LC50) concentrations (mg/litre) for aquatic
organisms exposed to dimethylformamide (DMF)
----------------------------------------------------------------------------------------------------
Species LC50 Reference
---------------------------------------
24-h 48-h 96-h
----------------------------------------------------------------------------------------------------
Guppy 1300 Dojlido (1979)
(Paecilia reticulata)
Rainbow trout 9800 Poirier et al. (1986)
(Salmo gairdneri) 9860 Shubat et al. (1982)
Fathead minnow 10 600 Poirier et al. (1986)
(Pimephales promelas)
Bluegill 7100 Poirier et al. (1986)
(Lepomis macrochirus)
Midge (Paratanytarsus 36 200 Poirier et al. (1986)
parthenogeneticus)
Daphnid (Daphnia magna) 14 500 Poirier et al. (1986)
12 300 LeBlanc & Surprenant
(approx.) (1983)
Larvae (Aedes aegypti) 68 000 Kramer et al. (1983)
(approx.)
----------------------------------------------------------------------------------------------------
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposures
Data on the acute toxicity of DMF in different laboratory animals,
when administered by different routes, have been reviewed by Kennedy
(1986). The acute toxicity in a number of species, following oral,
dermal, inhalation (Table 12), or parenteral (Table 13) administration
of DMF is relatively low, with lethal doses generally in the g/kg
range for the oral, dermal, and parenteral routes and in the g/m3 for
inhalation exposures. Animals given large single doses of DMF or
exposed to high air levels showed general depression, anaesthesia,
loss of appetite, loss of body weight, tremors, laboured breathing,
convulsions, haemorrage of the nose and mouth, liver injury, and coma
immediately preceding death.
In mice and rats, exposed to DMF via inhalation, signs of mucous
membrane irritation were seen (Lobanova, 1958; Lundberg et al., 1986),
and lung damage was detected histologically (Clayton et al., 1963).
Where tissue pathology was included in the study, the prominent
organ showing damage was the liver (Massmann, 1956; Sanotsky et al.,
1978; Mathew et al., 1980; Lundberg et al., 1981). No obvious species
differences were observed with regard to acute lethality, but young
rats appeared more sensitive to DMF-induced lethality than older rats
(Kimura et al., 1971).
8.2 Skin and eye irritation, sensitization
DMF was reported to be irritating to the eyes, mucous membranes,
and the skin (Hamilton & Hardy, 1974; Aldyreva & Gafurov, 1980).
8.2.1 Skin irritation
Rat tails dipped in DMF at 40 °C for 8 h became mummified in a few
days (Massmann, 1956).
A single application of 500 mg DMF/kg resulted in transient
irritation within 2-3 h in mice, but no irritation in rats (Wiles &
Narcisse, 1971). DMF was slightly irritating for mice at doses of
2500 and 5000 mg/kg. No skin irritation was detected in rabbits with
applications of 100, 200, or 500 mg DMF/kg. Single applications of
DMF on the skin of rats and guinea-pigs did not cause irritation
(Kiss, 1979; Bainova, 1985). Repeated 28-day treatments with 960 or
1920 mg/kg did not induce marked local dermal effects in rats (Bainova
et al., 1985).
Table 12. LD50 and LC50 values of DMF after oral, dermal, or
inhalation exposure in various animal species
----------------------------------------------------------------------------------------------------
Species Oral LD50 Dermal LD50 Inhalation LC50 Reference
(mg/kg) (mg/kg) (mg/m3)
----------------------------------------------------------------------------------------------------
Rat 3000 Thiersch (1962)
5000 9432 US NIOSH (1977)
3920 Massmann (1956)
11 140 12 000 Schottek (1970, 1972)
4000 Sanotsky et al. (1978)
> 11 520 Bainova & Antov (1980)
15 000 Clayton et al. (1963)
4320 Lazarev & Levina (1976)
11 000a Stula & Krauss (1977)
> 13 440 Lundberg et al. (1986)
3200 Qin & Gue (1976)
14 000 Cai & Huang (1979)
7170 Bartsch et al. (1976)
Mouse 3950 Lazarev & Levina (1976)
5550 Lazarev & Levina (1976)
> 5000 Wiles & Narcisse (1971)
6420 Bartsch et al. (1976)
3700 6000-9400 Lobanova (1958)
5400, 6200 Qin & Gue (1976)
18 300 Cai & Huang (1979)
Rabbit > 5000 > 500 Wiles & Narcisse (1971)
1500a Stula & Krauss (1977)
Mongolian gerbil 3929 Llewellyn et al. (1974)
----------------------------------------------------------------------------------------------------
a Pregnant females.
Table 13. LD50s (mg/kg body weight) of DMF after parenteral administration
in various animal species
----------------------------------------------------------------------------------------------------------
Species Intraperitoneal Intravenous Intramuscular Subcutaneous Reference
----------------------------------------------------------------------------------------------------------
Rat 1480 4030 Massmann (1956)
2500 Thiersch (1962)
4440 2830 Bartsch et al. (1976)
4600 Pham Huu Chanh et al. (1971)
5470 Shottek (1970, 1972)
Mouse 300 Massmann (1956)
3500 3800 4500 US NIOSH (1977)
650 Barral-Chamaillard
& Rouzioux (1983)
1454 Burgun et al. (1975)
2000 Antoine et al. (1983)
3150 2800 Wiles & Narcisse (1971)
5200 Pham Huu Chanh et al. (1971)
5850 3490 Bartsch et al. (1976)
Rabbit 945 1800 Massmann (1956)
1000 Wiles & Narcisse (1971)
5000 US NIOSH (1977)
Guinea-pig 1300 Wahlberg & Boman (1979)
1030 US NIOSH (1977)
4000 Ungar et al. (1976)
Dog 470 Barral-Chamaillard
& Rouzioux (1983)
Cat 500 Massmann (1956)
----------------------------------------------------------------------------------------------------------
After repeated application of DMF to the skin of guinea-pigs for
21 days (Bainova, 1985), the mean irritative dose was 31% DMF (range
17-56%).
Dermal irritation was not seen in rabbits treated dermally with 2
g DMF/kg for 6 h, daily 15 times during a 4-week period (Kennedy,
1986).
8.2.2 Eye irritation
A 25% (25 g/litre) solution of DMF in water, injected into the
conjunctival sac of the rabbit, did not produce any effects; 50% was
slightly irritating, and 75-100% produced more severe irritation
(Massmann, 1956). Single dose DMF instillation (0.1 ml) produced
moderate corneal damage and conjunctival redness that was most
pronounced at 2-3 days. By day 14, a mild degree of conjunctival
redness, moderate corneal damage with an area of severe injury, slight
surface distortion, and subsurface vascularization were observed
(Kennedy & Sherman, 1986). In another study, the same authors
reported that, after a single DMF instillation, the eye inflammation
subsided and disappeared by the 8th day.
8.2.3 Sensitization
DMF was tested, using a maximization technique, on guinea-pigs to
determine skin sensitization; it did not induce any response (Bainova,
1985).
8.3 Repeated exposure
The effects of repeated oral, dermal, or inhalation exposure to
DMF in various animal species have been reviewed by Kennedy (1986) and
these data, together with other new information are summarized in
Table 14. In all species tested, except the dog, liver damage was
produced, the degree of damage generally being proportional to the
dose administered. In the two reported studies on the dog (Clayton et
al., 1963; Kimmerle & Eben, 1975a), the inhalation exposure conditions
appeared to be too low (60 mg/m3) to produce damage, though 1 out of
the 4 dogs tested by Clayton did have altered liver function tests.
Higher levels were not tested. Some evidence of recovery from the
hepatotoxic effects of DMF was found in rats (Kennedy & Sherman,
1986).
Higher, intermittent doses of DMF appeared to produce more
pronounced effects in male rats than continuous dosing (Bainova et
al., 1981a; Bainova, 1985). Tanaka (1971) found more pronounced liver
damage in rats following one rather than three weeks of exposure and
considered that the high regenerative capacity of liver tissue was
responsible for the observation.
Other tissues and organs that are affected, particularly by high
doses of DMF, will be discussed in section 8.4.
8.4 Specific organ toxicity
8.4.1 Liver
The potency of DMF as a hepatotoxic agent has been reviewed by
Kennedy (1986) and by Scailteur & Lauwerys (1987). The effects of DMF
on the liver were studied after single or repeated inhalation, dermal,
or oral treatment of rats, mice, and rabbits (Massmann, 1956; Clayton
et al., 1963; Shottek, 1970; Tanaka, 1971; Kimmerle & Eben, 1975a;
Medyankin, 1975; Sanotsky et al., 1978; Germanova et al., 1979; Mathew
et al., 1980; Bainova et al., 1981a; Lundberg et al., 1981; Lundberg,
1982; Brondeau et al., 1983; Bainova, 1985; Kennedy & Sherman, 1986;
Scailteur & Lauwerys, 1987). Single oral administrations of 2250-5000
mg DMF/kg in rats (Kennedy & Sherman, 1986) caused clay-coloured
liver, congestion, and centrilobular necrosis of hepatocytes. Lower
doses resulted in deviations in liver function, such as decreased
excretion of cholic acid in the bile, bromosulfthalein retention,
increased serum activities of GOT, GPT, LAP, OCT, AlcP, ChE, LDH, and
gamma-GT, and significant enhancement of cholesterol, triglyceride,
and bilirubin contents in the serum and liver homogenates. In rats,
following both intraperitoneal (ip) and inhalation exposure, there
were no increases in SDH levels at 420 and 840 mg/m3 but a lower level
(210 mg/m3) raised the serum activity of SDH (Lundberg et al., 1986).
Pathomorphological investigation demonstrated lipid degeneration and
cloudy swelling of hepatocytes in the central zones of the lobules
followed by signs of regeneration.
DMF at 0.6 ml/kg, administered intraperitoneally, caused mild
changes in rat liver lobules. Marked centrilobular necrosis and
central phlebitis were found in the rats treated with single ip doses
of 0.9 and 1.2 ml DMF/kg (Mathew et al., 1980). A single ip dose of
0.5 ml DMF/kg to hamsters caused centrilobular necrosis accompanied by
haemosiderosis (Ungar et al., 1976). Morphological changes were
reported in the liver by Clayton et al. (1963), Shottek (1970), Tanaka
(1971), and Santa Cruz & Corpino (1978) after repeated DMF exposure of
young animals, with periodic peaks (Table 14).
Table 14. Effects of repeated oral, dermal, or inhalation exposure to DMF in various animal species
________________________________________________________________________________________________________
Species Route of Dose Duration Effects Reference
exposure
________________________________________________________________________________________________________
Mongolian oral 10 000 mg/kg 30 days no changes in body weight, liver, Llewellyn
gerbil drinking-water or kidney et al. (1974)
10 000 mg/kg 200 days mortality in 25% of animals;
drinking-water liver necrosis
17 000 mg/kg 80 days mortality with liver necrosis;
drinking-water LD50 cumulative 90 206 mg/kg
body weight
34 000 mg/kg 6 days mortality with liver necrosis;
drinking-water LD50 cumulative 3846 mg/kg body
weight
Mouse oral 620 or 1240 30 days anorexia, loss of body weight Qin & Gue
mg/kg diet (1976)
160, 540, 119 days dose-related increase in relative Becci et al.
1850 mg/kg diet and absolute liver weights; no (1983)
other histological or biochemical
changes; NOEL, 246-326 mg/kg
diet per day
Rat oral 320 or 640 30 days anorexia, loss of body weight Qin & Gue
mg/kg diet (1976)
50, 500, 5000 100 days body weight decrease; liver Qin & Gue
mg/litre damage at 5000 mg/litre; (1976)
drinking-water increase in liver to body weight
ratio at 500 and 5000 mg/litre;
structural liver changes and
regeneration at 5000 mg/litre;
NOEL, 50 mg/litre
Table 14 (continued)
________________________________________________________________________________________________________
Species Route of Dose Duration Effects Reference
exposure
________________________________________________________________________________________________________
Rat oral 102, 497, 1000 14 days no behavioural changes at 102 or Savolainen
mg/litre 49 days 497 mg/litre for 49 days; dose- (1981)
drinking-water related deviations in cerebral
and glial cell enzyme activities
215, 750, 2500 104 days dose-related increase in relative Becci et al.
mg/kg diet and absolute liver weights, (1983)
considered to be physiological
adaptation; NOEL, 210-235 mg/kg
diet per day
200, 1000, 5000 90 days slight anaemia and leukocytosis, Kennedy &
mg/kg diet hypercholesterolaemia at 1000 and Sherman (1986)
(equivalent to 5000 mg/kg diet; NOEL, 200 mg/kg
12, 60, 300 mg/ diet
kg/body weight
per day)
0.1, 0.5, 1.0 14 days dose-related increase in liver/ Elovaara
g/litre in 49 days body weight ratios; in liver et al.
drinking-water and kidneys, increased values of (1983)
reduced glutathione, microsomal
UDP glucuronosyl transferase,
and ethoxycoumarin O-demethylase
activities; no changes in liver
microsomal cytochrome P-450 or
ADPH-cytochrome reductase
activity
Rat dermal 470 mg/kg per 30 days continuous dosing caused Schottek
day for 29 days hepatoxicity and did not protect (1970)
and 11 140 mg/kg against lethality; pretreatment
on the 30th day did not enhance toxic reactions
after application of the LD50
in 30-day pretreated rats
Table 14 (continued).
________________________________________________________________________________________________________
Species Route of Dose Duration Effects Reference
exposure
________________________________________________________________________________________________________
215, 430, 960, 30 days dose-related changes in GOT, Bainova &
or 4800 mg/kg GPT, AlcP, ChE, gamma-GT, lipid Antov (1980)
per day fractions in serum and liver
homogenates; NOEL, 215 mg/kg
Rat dermal 215, 320, 960, 30 days dose-related changes (at doses Bainova (1985)
or 4800 mg/kg > 320 mg/kg) in enzyme
activities per day in liver,
myocardium, and kidney
homogenates; NOEL, 215 mg/kg
Rat dermal 960 mg/kg daily 28 days functional, biochemical, and Bainova et al.
or 1920 mg/kg pathomorphological changes in (1981a)
applied liver; and lipid metabolism Bainova (1985)
intermittentlya on the 4th, 8th, 14th, and 28th
day of the tests; changes more
pronounced after intermittent
exposure
4-h dipping of 60 days concentration-related changes Medyankin
tails in 60, 65, in liver and nervous system; (1975)
70, or 80% DMF NOEL, 60% DMF in water
in water
4-h dipping of 120 days no changes at 30% DMF contact and Medyankin
tails in 5 mg DMF/m3 inhalation; adverse (1975)
30 or 60% DMF effects at other concentrations
and inhalation of
5 or 10 mg DMF/m3,
6 h daily
Table 14 (continued).
________________________________________________________________________________________________________
Species Route of Dose Duration Effects Reference
exposure
________________________________________________________________________________________________________
Rabbit dermal 50, 100% water 7 days died at 5-8 day of application Huang et al.
solution, 3 at 100% DMF; liver biochemical (1981)
times/day, 2 ml/ and histological changes
application
2000 mg/kg per 9 days anorexia, cyanosis, and mortality Kennedy &
day with liver necrosis Sherman (1986)
Guinea dermal 50, 75, 100% 7 days died 2-4 days after application of Huang et al.
-pig solution, 75 or 100% and 4-9 days after 50%; (1981)
3 times/day, loss of body weight; liver damage
2 ml/application
Rat inhalation 1800 mg/m3 for 6 days concentration-related mortality; Schottek
6 h daily cumulation of hepatoxic effect (1970)
750 and 1500 6 days
mg/m3, 6 h daily
30 mg/m3 for 8 days no changes in the function of Sanotsky &
6 h daily the thyroid or adrenal glands Ulanova (1975)
aerosol for 3 or liver and kidney necrosis, lung Santa Cruz &
0.5 h daily 30 days changes, arterial changes in Corpino (1978);
(concentration myocardium Santa Cruz &
unknown) Maccioni (1978)
22 ± 1.6 mg/m3 18 weeks liver changes, no other responses Cai & Huang
for 6 h daily, (1979)
6 days a week
Table 14 (continued).
________________________________________________________________________________________________________
Species Route of Dose Duration Effects Reference
exposure
________________________________________________________________________________________________________
Rat inhalation 130 mg/m3 for 27 days functional changes in kidneys Germanova et al.
4 h daily and liver; arterial blood pressure (1979)
more pronounced after additional
300 mg/m3 in 27 days single administration of 500 mg
5 peaks of DMF/kg on the 1st, 8th, and
15 min at 27th days of the studies, and
40-min intervals after intermittent exposure
7569 mg/m3 5 days weakness, weight loss, Kennedy &
for 6 h daily dehydration, liver necrosis Sherman (1986)
Young rat inhalation 600 mg/m3 for 28 days increased serum GOT and GPT; Tanaka (1971)
(3-12 8 h daily morphological liver changes,
weeks old) mainly in 3-week-old rats;
no histological abnormalities
in other organs
Young rat inhalation 600 mg/m3 for 28 days liver changes at the 1st, 2nd, Tanaka (1971)
(3 weeks 8 h daily and 3rd, and 4th week of test more
old) 600 mg/m3 for intense in the group exposed
1 h daily for 8 h daily; no cumulation of
hepatoxic effect
Table 14 (continued).
________________________________________________________________________________________________________
Species Route of Dose Duration Effects Reference
exposure
________________________________________________________________________________________________________
Rat, inhalation 450, 900, 1800, 60 days increased serum GOT, GPT, AlcP, Craig et al.
mouse 3600 mg/m3 cholesterol, anaemia and (1984)
for 6 h daily histological liver changes at
900 mg/m3 or more; liver weight
increase at 450 mg/m3; NOEL
below 450 mg/m3 in both species
Rat, cat inhalation 300, 690, 1350 120 days anorexia, weight loss, liver Massmann
mg/m3, 8 h daily degeneration, and necrosis; (1956)
changes in brain, myocardium,
and kidneys; no abnormalities
in blood tests or ECG
Rabbit inhalation 22 ± 1.6 mg/m3 18 weeks no changes in ECG or liver Cai & Huang
for 6 h daily, parameters (1979)
6 days per week
317 ± 37.8 mg/m3 14 weeks body weight changes; liver damage Cai & Huang
for 6 h daily, functionally and structurally; by (1979)
6 days a week congestion and haemorrhage
120 mg/m3 for 50 days microscopic and electron- Arena et al.
8 h daily microscopic changes in the (1982)
myocardium
Dog inhalation 60 mg/m3 for 107 days reversible changes in blood Clayton et al.
6 h daily pressure, ECG, and in liver (1963)
functions
63 mg/m3 for
6 h daily 28 days normal GOT, GPT, bilirubin, urea, Kimmerle & Eben
and creatinine in plasma; (1975a)
NOEL, 63 mg DMF/m3
___________________________________________________________________________________________________________
a Two alternative intermittent regimes were used: (i) 1920 mg/kg per day for 2 days, followed by no
treatment for 2 days; (ii) 1920 mg/kg every second day.
Diets supplemented with DMF at levels of 215, 750, or 2500 mg/kg
for 104 days for rats and 160, 540, or 1850 mg/kg for 119 days for
mice, resulted in significant dose-related increases in relative liver
weights in all experimental animals. No deviations were reported in
the serum activities of GOT, GPT, AlcP, other than an increase in GPT
activity in mice fed a diet containing 1850 mg DMF/kg. Histo-
pathological evaluation did not reveal any hepatotoxicity (Becci et
al., 1983). The oral administration of a 10% water solution of DMF
(400 mg/kg body weight) for 14 days (Leshik & Feoktistova, 1984) to
guinea-pigs significantly decreased the ascorbic acid content and the
concentration of cytochrome P-450 in the liver. The daily intake of
0.1, 0.5, or 1.0 g DMF/litre in the drinking-water for 2 or 7 weeks
(Elovaara et al., 1983) increased the liver/body weight ratios, the
microsomal UDP-glucuronosyl-transferase and 7-ethoxycoumarin- O-
demethylase activity, and reduced the glutathione concentration in
liver homogenates.
No liver injury was seen following inhalation exposure of dogs to
63 mg DMF/m3 (Kimmerle & Eben, 1975a). However, in another study, 1
out of 4 dogs exposed to 60 mg/m3 showed increased enzyme values
(Clayton et al., 1963)). Liver injury was also not seen: after
inhalation exposure of rats at levels of < 450 mg/m3 (Craig et al.,
1984), after dermal exposure of rats at a level of 240 mg/kg (Bainova,
1985), and at dietary levels of 215 mg/kg body weight per day for rats
and 160 mg/kg for mice (Becci et al., 1983).
8.4.2 Gastrointestinal tract
Toxic gastroenteritis with pathomorphological deviations was
described in the experimental animals treated at high doses or
concentrations in studies reported in Table 14 (Massmann, 1956;
Clayton et al., 1963; Shottek, 1970).
8.4.3 Cardiovascular system
Microscopic examination did not reveal any myocardial lesions in
rats and mice after ingestion of dietary levels of 215, 750, or 2500
mg DMF/kg for 104 days, or 160, 540, or 1850 mg DMF/kg for 119 days,
respectively (Becci et al., 1983).
Clayton et al. (1963) and Germanova et al. (1979) reported
decreases in blood pressure in dogs (not cats) following exposure to
DMF. The changes described were not great and, in the absence of
confirmatory data in other test models (and in man), are of
questionable significance. Large iv doses (500 mg/kg) did not produce
any changes in the contractile force of myocardial tissue in dogs
(Pham Huu Chanh et al., 1973).
Santa Cruz & Maccioni (1978) described histological changes in the
myocardium of the rat and Clayton et al. (1963) described subtle blood
pressure changes in the dog. The findings in the rat followed high
exposures; the blood pressure changes in the dog were minimal and hard
to differentiate from those in control animals.
8.4.4 Kidney
Swelling of the kidney tubules occurred after a single oral
administration of 2250-5000 mg DMF/kg in rats (Kennedy & Sherman,
1986). Short-term feeding studies in rats and mice (Table 14) did not
reveal any histopathological lesions in the kidneys (Becci et al.,
1983; Kennedy & Sherman, 1986).
A number of pathomorphological studies revealed vacuolar
degeneration, mainly in the renal tubules (Massmann, 1956; Clayton et
al., 1963; Santa Cruz & Corpino, 1978; Lundberg et al., 1983). Costa
et al. (1978) observed histological, histochemical, and electron-
microscopic renal lesions in groups of rats, exposed to DMF aerosols
(dose not stated) for 1 h/day for 15 days or for 0.5 h/day for 30
days. Degeneration took place in the proximal part of tubules and in
the visceral epithelium of the glomerulus with marked mitochondrial
changes.
Repeated inhalation exposure to 130 or 300 mg DMF/m3 increased the
kidney/body weight ratio, and decreased diuresis, and the total
protein, sodium chloride, and potassium contents in the urine of rats
(Germanova et al., 1979).
Elovaara et al. (1983) reported enhanced activities of 7-ethoxy-
coumarin- O-demethylase, and UDP-glucuronosyltransferase, and a
decrease in cytosolic formaldehyde dehydrogenase activity in rats
orally exposed to DMF. Bainova & Antov (1980) and Bainova et al.
(1981b) reported that the 30-day dermal application of 960 or 4800 mg
DMF/kg resulted in dose-related increased activities of SucDH, G-6-
PDH, and LDH in rat kidney homogenates.
8.4.5 Nervous system
Functional changes in the nervous system were observed after
administration of high doses or exposure to high concentrations of DMF
(Massmann, 1956; Clayton et al., 1963) and after prolonged treatment
with moderate doses of DMF (Medyankin, 1975; Sanotsky et al., 1978;
Germanova et al., 1979; Bainova, 1985) (Table 14). Doses within the
range of lethal levels resulted in anaesthesia, depression, or coma.
Moderate doses caused inhibition of motor activity.
No effects on the behaviour of rats were noted after they drank
DMF in the drinking-water for 2 and 7 weeks at doses ranging from 1.4
to 13.7 mmol/litre with a cumulative dose of 3200 mg/kg in the rats
(Savolainen, 1981). The same dose enhanced the activities of acid
proteinase and 2,3-cyclicnucleotide-3'-phosphohydrolase in the glial
cells.
Massmann (1956) and Clayton et al. (1963) observed
pathomorphological changes in the brains of experimental animals after
treatment with high doses of DMF (Table 14).
8.4.6 Lungs
Lung congestion and oedema were found in rats after single oral
application of 2250-5000 mg DMF/kg (Kennedy & Sherman, 1986).
Bronchopneumonic changes were observed in experimental animals
after inhalation of high DMF concentrations (Massmann, 1956; Clayton
et al., 1963; Shottek, 1970; Santa Cruz & Corpino, 1978) (Table 14).
According to the authors, the changes were related to injury of the
small arterial vessels and, to some extent, to local irritation caused
by DMF.
8.4.7 Haematopoietic system
High levels of DMF might cause some anaemia, but no other changes
in the erythrocytes of experimental animals have been reported
(Massmann, 1956; Clayton et al., 1963; Sanotsky et al., 1978;
Germanova et al., 1979; Bainova & Antov, 1980) (Table 14). Depressed
bone marrow activity was reported in rats after a single oral
administration of 2250-5000 mg DMF/kg (Kennedy & Sherman, 1986). Pham
Huu Chanh et al. (1971) found leukocytosis in rats after repeated ip
injections of DMF.
Pathomorphological changes were observed in the spleen after
exposure to high doses of DMF. They were accompanied by an increase
in the spleen/body weight ratio (Massmann, 1956; Clayton et al., 1963;
Bainova & Antov, 1980; Bainova, 1985). Medyankin (1975) found
inhibited phagocytosis activity of leukocytes and decreased glycogen
content in the neutrophiles of rats as a result of combined dermal and
inhalation exposure to DMF.
8.4.8 Adrenals
Clayton et al. (1963) observed histological changes in the adrenal
glands after inhalation of DMF. Decreased ascorbic acid content in
the adrenals of rats was reported by Germanova et al. (1979), during
intermittent and continuous inhalation exposure to DMF (Table 14).
8.4.9 Gonads
Male rats were exposed to 584-616 mg DMF/m3 or 49-51 mg/m3 for 4 h
daily for 2, 4, or 8 days; female rats were exposed to 2.3 or 10.7 mg
DMF/m3, for 4 h daily, for 30 days (Sheveleva et al., 1979).
Examination of the sperm and the histological study of the testes and
ovaries did not show any pathological signs.
Lewis et al. (1979) exposed male rats at 90 or 900 mg DMF/m3, for
6 h daily, over 5 days. Gross and histological examination of the
testes did not reveal any pathological changes.
The histological examination of male rats, treated orally in
short-term studies (Becci et al., 1983; Kennedy & Sherman, 1986) with
a variety of doses (Table 14), did not result in changes in the
testes. No lesions were noted in the male rats after a 30-day dermal
application of DMF (Bainova, 1985). Craig et al. (1984) did not find
testicular or ovarian lesions in rats and mice after short-term
inhalation exposure to DMF at concentrations of up to 3600 mg/m3
(Table 14).
Examination of the gonads in a large number of the acute and
repeated-dose toxicity studies discussed earlier did not reveal them
to be a target for DMF toxicity.
8.5 Developmental toxicity and reproduction
DMF was investigated for developmental toxicity in mice, rats, and
rabbits using the oral, dermal, and inhalation routes and parenteral
injection. According to present-day requirements, most of the older
studies were not adequately designed or described. A survey is given
in Tables 15, 16, 17.
No 3-generation reproduction studies were available.
8.5.1 Developmental toxicity
8.5.1.1 Mouse
Administration by gavage of 580 or 193 µl DMF/kg per day, from day
6 to 15 after conception, to 26 mice per dose group led to a dose-
dependent decrease in fetal weights and an increase in the number of
retardations and variations. At 580 µl/kg per day, 17 out of 241
fetuses were malformed (cleft palate, exencephaly, hydro-cephalus
internus, aplasia of presphenoid). No maternal effects were recorded;
the number of live fetuses remained unchanged. At 193 µl/kg, 4 out of
245 fetuses showed malformations. In untreated control groups for
each dose group, 2 out of 229 and 1 out of 310 fetuses, respectively,
had a cleft palate (Hellwig et al., in press).
After an ip injection of 600 or 1100 mg DMF/kg per day, from day 1
to 14 after conception, embryotoxic and teratogenic effects were
registered. The malformation rates were 18 and 75%, respectively, and
the effects consisted of absence or retardation of posterior skull
ossification, open eye-lids, cerebral oedema, sternal haematomas, and
spina bifida-like defects in the thoracic region. Embryotoxic effects
recorded were late resorptions. Doses of 170 mg/kg per day from day 1
to 14 after conception, and 250 mg/kg per day from day 6-14, and
single doses of 2100 mg/kg each on days 3, 7, 9, or 11 after
conception, did not produce any effects (Scheufler & Freye, 1975).
In another ip injection study on mice, 6 animals per dose group
were treated with 0.4 or 1.0 mg DMF/kg per day from day 11 to 15 after
conception. Maternal body weight gain was reduced at 1.0 mg/kg per
day, 2 out of 6 animals had abortions, 7 out of 36 fetuses showed
exencephaly and 1 had a cleft palate. No effects were observed at 0.4
mg/kg (Hellwig et al., in press).
The studies indicate that DMF may be teratogenic for mice.
Table 15. DMF administration to pregnant mice
----------------------------------------------------------------------------------------------------
Route Dosea Maternal Embryotoxicity Malformations Reference
toxicity
----------------------------------------------------------------------------------------------------
Gavage control- - - Hellwig et al.
193 µl/kg - fetal weights 4 of 245 living (in press)
(gavage; day decreased fetuses showed
6-15 pc) malformations
580 µl/kg NRb fetal weights 17 of 241 living
(gavage; day decreased fetuses showed
6-15 pc) malformations
Intra- control - - - Scheufler &
peritoneal 170 mg/kg NRb - - Freye (1975)
injection (day 1-14 pc)
250 mg/kg NRb - -
(day 6-14 pc)
600 mg/kg NRb late malformation
1100 mg/kg resorptions rates 18 and 75%
(day 1-14 pc) respectively
Intra- 0.4 mg/kg - - - Hellwig et al.
peritoneal (day 11-15 pc) (in press)
injection 1.0 mg/kg + 2/6 abortions 8/36
(day 11-15 pc) malformations
----------------------------------------------------------------------------------------------------
a pc = Post conception.
b NR = Not reported.
Table 16. DMF administration to pregnant rats
---------------------------------------------------------------------------------------------------------
Route Dosea Maternal Embryotoxicity Malformations Reference
toxicity
---------------------------------------------------------------------------------------------------------
Gavage control - - - Hellwig et al.
(day 6-15 pc)
533 µl/kg - some embryo-
lethality in the
early phase, +
reduced fetal
weights
+ retardations
+ variations
1600 µl/kg weight 63% embyro- 12% of the 85
stationary lethality in living fetuses
between the median were malformed
day 6-15 phase
Dermal day 6-10 and Hellwig et al.
0 µl/kg - - -
100 µl/kg - - 2.46%
500 µl/kg - - 3.05%
1000 µl/kg - slight reduced 5.46%
fetal length (increase in rib
and vertebral
abnormalities)
Dermal control - - - Stula &
600 mg/kg reduced slight Krauss (1977)
(days 9, 10 + weight embryolethality
11, 11 + 12, gain
12 + 13 pc)
1200 mg/kg reduced slight
(day 10 + 11 pc) weight embryolethality -
gain
Table 16. (contd.)
---------------------------------------------------------------------------------------------------------
Route Dosea Maternal Embryotoxicity Malformations Reference
toxicity
---------------------------------------------------------------------------------------------------------
Dermal 1200 mg/kg reduced slight
(contd.) (day 12 + 13 pc) weight embryolethality -
gain
2400 mg/kg stationary embryolethality -
(day 10 + 11 pc) weight
400 or 200 reduced high embryo- -
mg/kg (applied weight lethality
6 times/day gain
days 11 + 12
+ 13 pc)
Inhalation control - - - Hellwig et al.
(day 0-1, 4-8, weight weights
11-15, 18-19 pc) gain resorptions
861 mg/m3 (287 ppm) reduced reduced fetal -
(day 0-3, 6-10, weight weights
13-18 pc) gain retardations
resorptions
variations
660 mg/m3 (220 ppm) - reduced -
(day 4-8 pc) weight and
length
1560 mg/m3 (520 ppm) reduced embyro- -
(day 4-8 pc) weight lethality;
gain reduced weights
Table 16. (contd.)
---------------------------------------------------------------------------------------------------------
Route Dosea Maternal Embryotoxicity Malformations Reference
toxicity
---------------------------------------------------------------------------------------------------------
Inhalation control - - - Kimmerele &
54 mg/m3 (18 ppm) - - - Machemer
516 mg/m3 (172 ppm) - reduced fetal - (1975)
(6-15 day pc) weights
Inhalation control - - - Keller & Lewis
903 mg/m3 (301 ppm) reduced ossification
(day 6-15 pc) weight variations
gain slightly increased
from 60 to 75% -
Inhalation control - - - Shottek
1200 mg/m3 (400 ppm) total resorp- - (1964)
(4 h/day, 10-20 day pc) NR tions in some
animals, no
retardations
Inhalation 0.05 mg/litre ca.48 mg/m3 weight - Sheveleva &
(ca. 16 ppm) NR decrease Osina (1973)
(day 0-20 pc)
0.8 mg/litre ca.600 mg/m3 embryolethality; -
(ca. 200 ppm) NR weight decrease
Intravenous control - - - Parkie & Webb
injection 0.5 g/kg on - - (1983)
days 10, 11, NR
or 12 pc)
---------------------------------------------------------------------------------------------------------
a pc = Post conception.
b NR = Not reported.
Table 17. DMF administration to pregnant rabbits
---------------------------------------------------------------------------------------------------------
Route Dosea Maternal Embryotoxicity Malformations Reference
toxicity
---------------------------------------------------------------------------------------------------------
Gavage control - - - Merkle &
46.4 µl/kg - - 1 hydrocephalus Zeller (1980)
(day 6-18 pc)
68.1 µl/kg - decrease in number 3 hydrocephalus
(day 6-18 pc) of implantations
and % living
implantations
200 µl/kg decrease in decrease in +
(day 8-16 pc) food intake, fetal weight, 16 fetuses in
weight gain, 3 abortions, 7 litters were
and placental placental weight malformed:
weight decrease
Dermal control - - - Stula &
200 mg/kg some - Krauss (1977)
(day 8-16 pc) embryolethality
Dermal control 100 mg/kg - - - Hellwig et al.
occlusive litter skeletal
(day 6-28 pc) anomalies; 3.3%
agenesia of gall
bladder
Dermal 200 mg/kg per day - - -
400 mg/kg per day decrease in 23.5% fetuses
body weight per litter sternal
on day 16 and anomalies; 2.45%
18 hernia umbilicalis,
6.08% agenesia
of gall bladder
Table 17 (contd.)
---------------------------------------------------------------------------------------------------------
Route Dosea Maternal Embryotoxicity Malformations Reference
toxicity
---------------------------------------------------------------------------------------------------------
Inhalation Control - - - Hellwig et al.
50 ppm)
(day 7-19 pc)
450 mg/m3 decrease in increase in 1 hernia
(150 ppm) body weight sternal umbilicalis
gain variations (50 fetuses)
1350 mg/m3 decrease in decrease in incidence of hernia
(450 ppm) body weight weights, umbilicalis
gain increase in increased;
variations skeletal and soft
(pseudoanky- tissue anomalies
losis)
---------------------------------------------------------------------------------------------------------
a pc = Post conception.
8.5.1.2 Rat
In a gavage study on groups of 26 rats administered 1600 µl DMF/kg
per day from day 6 to 15 after conception, maternal toxicity was
observed in the form of decreased body weight. Sixty-three percent of
the implantations were resorbed and 12% of the surviving 85 (36.64%)
fetuses were malformed (9 cases of diffuse anasarca, 2 cases of tail
aplasia, 1 micrognathia, furthermore several fetuses had anomalies of
the ribs, sternum, and vertebral column). A dose of 533 µl DMF/kg per
day caused some early fetal deaths, in the absence of clinical signs
of maternal toxicity, reduced fetal weight, and also some
malformations, as well as an increase in variations and skeletal
retardations. The malformations consisted of: 2 cases of tail
aplasia, 2 cases of cleft palate, 1 atresia ani, 1 anasarca, 1 open
eye, and several fetuses with split and aplastic vertebrae. At 176
µl/kg, decreased placental weights and some decreases in fetal length
and increases in fetal weight were seen. All other parameters were
within the range of biological variability (Hellwig et al., in press).
The dermal studies on rats did not fulfil today's criteria for a
valid study.
In one series of studies (Hellwig et al., in press), 0, 100, 500,
or 1000 µl DMF/kg per day (undiluted material) were administered in an
uncovered dermal system from day 6 to 10 and then from day 13 to 15
after conception (26 animals per dose group; 10 in the control group).
Under these conditions, 1000 µl/kg per day caused a slightly retarded
weight gain among the dams and significant dermal irritation. The
fetuses were slightly smaller. Malformations consisted of split
thoracic vertebrae and anomalies of the ribs. The rate of the
malformations in live fetuses was 0% per litter in the controls and
2.46%, 3.05%, and 5.46% with increasing dose level. This may indicate
a weak dose-related teratogenic effect.
In another study (Stula & Krauss, 1977), rats received dermal
doses of up to 2400 mg (undiluted DMF)/kg per day, at least every 2
days, from day 9 to 13 after conception, under non-occlusive
conditions. There was clear evidence of embryolethality at 2400 mg/kg
per day on gestation days 10 and 11 (26%, i.e., 7 rats) and at 1200
and 2400 mg/kg per day (in 6 portions of 200 and 400 mg) on gestation
days 11, 12, and 13 with incidences of 43 and 30%, respectively.
Maternal weight gain and average fetal weights were also suppressed.
Fetal abnormalities were not observed, with the exception of a few
subcutaneous haematomas, which occurred at a rate also seen in
historical controls at this laboratory.
Several inhalation studies were carried out on rats. In one
study, 23 animals per dose group were exposed to 54 or 516 mg DMF/m3
(18 or 172 ppm) over 6 h per day from day 6 to 15 after conception.
There were 22 animals in the control group. The higher exposure level
caused a decrease in fetal weights in the absence of signs of maternal
toxicity. No effects were seen on the numbers of implantations,
resorption rates, placental weights, number of fetuses weighing less
than 3 g (runts), variations in skeletal development or malformations;
the lower exposure level (54 mg/m3) did not cause any adverse effects
(Kimmerle & Machemer, 1975).
In another rat study, exposure to 903 mg/m3 (301 ppm) for 6 h per
day, from day 6 to 15 after conception (19 animals per group) led to a
reduction in maternal weight gain and to a slightly increased
incidence of skeletal (ossification) variations of from 60 to 75%.
Exposure to 96 mg/m3 (32 ppm) did not produce any effect (Keller &
Lewis, 1981).
Following 10 exposures to 1200 mg/m3 (400 ppm) for 4 h per day,
from day 10 to 20 after conception, dead implants (54% total
resorptions compared with 15% in the controls) occurred. The numbers
of animals in the treated and control groups were not reported
(Shottek, 1964).
Fetal weight decreases and fetal deaths were reported in another
study on rats after exposure to 600 mg/m3 (200 ppm) from day 0
throughout the gestation period. Exposure to 48 mg/m3 (16 ppm) was
said to have caused reduced fetal weights. However, the description
of the study was inadequate (Sheveleva & Osina, 1973).
A series of inhalation studies on rats is described (Hellwig et
al., in press) in which the exposure periods did not fully cover the
critical period of the gestation phase (e.g., "window dosing" or non-
exposure during weekends, see Table 17). In one set of these
experiments, exposure to concentrations of 660 and 1560 mg/m3 (220 and
520 ppm), for 6 h per day (days 0-3, 6-10, 11-18 after conception; 18
animals per group) caused decreased fetal weights, retardations, and
an increase in embryolethality. Another exposure regimen, i.e., 861
mg/m3 (287 ppm), for 6 h per day, was administered on days 1, 4-8,
11-15, and 18-19 after conception to a group of 30 rats. Twenty
animals were subjected to caesarian section on day 20 after
conception, the offsprings of the other 10 animals were raised until
day 21 after birth. Thirty rats served as untreated controls. There
was retarded maternal weight gain from the beginning of the treatment;
fetal weights were decreased, and the numbers of variations and
retardations were increased. No malformations were found.
No effects were detected after single iv injections of 0.5 g/kg
body weight between days 10, 11, or 12 after conception (Parkie &
Webb, 1983). Furthermore, earlier investigations on rats after single
ip or sc injections did not give any indication of teratogenicity;
however, such studies are of limited value for a toxicity assessment.
The above studies indicate that DMF is embryotoxic in the rat.
After dermal and oral administration, teratogenicity may also occur.
8.5.1.3 Rabbit
DMF caused maternal toxicity and embryotoxicity, including
teratogenicity, in rabbits after administration by gavage at 200 µl/kg
per day from day 6-18 after conception. All 11 animals in the dose
group became pregnant and showed reduced food intake and weight gain.
Placental weights were significantly lower and 3 abortions occurred.
The fetuses showed weight reduction. The main findings recorded on
fetal examination were hernia umbilicalis (7 cases), hydrocephalus
internus (6 cases), eventratio simplex (3 cases), exophthalmus (2
cases), cleft palate (1 case), and malposition of limbs (1 case). The
number of implantations was not adversely influenced. At 68.1 µl/kg
per day, no maternal effects were observed among 16 pregnant animals
(18 inseminated); decreased numbers of total implantations and of live
fetuses occurred, also an increase in skeletal variations and
retardations per litter; 3 cases of hydrocephalus internus were
present. At 46.4 µl/kg per day (10/12 does were pregnant) 1 case of
hydrocephalus internus occurred; all other parameters were in the
range of biological variability. No malformations occurred in the
untreated control group (22/24 animals) (Merkle & Zeller, 1980).
In a dermal study, 5 rabbits (4 animals in the control group)
received 200 mg DMF/kg per day, dermally, from day 8 to day 16 after
conception. The test material was applied in undiluted form on the
intact skin, apparently under non-occlusive conditions. Embryo
mortality was 6% compared with 3% in the controls. The average fetal
weight was 32.9 g in the litters of treated animals compared with 28.4
g among controls. Fetal abnormalities were not detected (Stula &
Krauss, 1977).
In another dermal study (Hellwig et al., in press), dose levels of
undiluted DMF of 0, 100, 200, or 400 mg/kg per day were applied for 6
h/day, under semi-occlusive conditions (15 animals per dose group). A
5-6% decrease in maternal body weights occurred in the highest dose
group, towards the end of the treatment period (days 16-18 after
conception). At this dose level, an increase in skeletal (sternal)
malformations was found in 15 fetuses in 7 litters investigated
(23.5%), and also 5 cases of missing gall bladder (in 2 litters). No
malformations occurred in animals in the 200 mg/kg per day group or in
the untreated control group. At 100 mg/kg per day, one fetus had a
sternal anomaly, 2 fetuses had gall bladder agenesis, and one of the
latter a hypertrophic-dilatative cardiac-aortic malformation.
In a recent inhalation study, exposure levels were 0, 150, 450,
and 1350 mg/m3 (0, 50, 150, and 450 ppm) over 6 h per day from day 7
to day 19 after conception (fifteen animals per dose group). Animals
in the highest exposure group showed a slight retardation in body
weight gain as a sign of maternal toxicity. The fetal weights were
significantly lower in this group, and there was a significant
increase in malformations, mostly hernia umbilicalis (7 out of 86
fetuses, in 4 out of 15 litters) and some soft-tissue malformations,
such as missing gall bladder, without statistical significance. In
addition, anomalies of the sternum, an increase in split vertebrae,
and a number of variations were also recorded. At 450 mg/m3, maternal
body weights were slightly retarded during the exposure period and the
corrected body weight gain was marginally, but significantly,
decreased. One case of hernia umbilicalis among 75 fetuses and an
increase in sternal variations were observed. At 150 mg/m3, neither
fetuses nor does showed any indications of response to treatment. In
summary, signs of embryotoxicity and teratogenicity were seen at the
highest concentration (1350 mg/m3) and to a lesser degree at 450
mg/m3. The maternal toxicity seen at 1350 mg/m3 and 450 mg/m3 is in
accordance with the maternal toxicity observed at 900 mg/m3 in a
previous range-finding study (Hellwig et al., in press).
These studies indicate that DMF may be teratogenic for rabbits.
8.5.1.4 Appraisal
The overall conclusion from all studies is that DMF may lead to
embryotoxic and teratogenic effects in rats, mice, and rabbits. An
increase in malformations in the absence of maternal toxicity is
clearly visible after gavage and ip administration, with a smaller
incidence after dermal administration. In general, the rabbit
appeared to be more sensitive to the teratogenic effects of DMF than
the rat. After inhalation exposure, fetal toxicity and teratogenic
effects appear to be confined to conditions of maternal toxicity.
8.6 Mutagenicity and related end-points
DMF has been tested extensively in mutagenicity and genotoxicity
assays. DMF was one of the 42 chemicals selected for study in the
International Collaborative Program for the Evaluation of Short-Term
Tests for Carcinogenicity (Serres & Ashby, 1981).
The genetic toxicity studies on DMF have been reviewed by Purchase
et al. (1978), Kennedy (1986), US EPA (1986), and IARC (1989).
8.6.1 In vitro studies
In different in vitro assays, DMF did not induce mutations or
genotoxic effects (Table 18). Negative results were obtained with
both in Salmonella typhimurium and Escherichia coli. DMF did not
induce unscheduled DNA synthesis, sister chromatid exchanges,
chromosomal aberrations, or gene mutation in mammalian cells, or
mitotic gene conversion or crossing over in yeasts (Serres & Ashby,
1981).
Table 18. Short-term genotoxicity tests on DMF ( in vitro )
------------------------------------------------------------------------------------------------------------
Method Concentration Condition; comment Results Reference
------------------------------------------------------------------------------------------------------------
Ames test 0.65 x 10-5-1.3 x 10-3 TA 98, 100, 1535, - Antoine et al.
mol/litre 1537, 1538, (1983)
with and without
liver microsome
B. subtilis maximum dose 20 mg/disk - S9, + S9 (rat, yellow - Serres &
Spore rec-assay tail, clam) Ashby (1981)
E. coli differential highest concentration WP2, WP67, CM871 - Serres &
killing test 30 µl/plate Ashby (1981)
E. coli rec-assay highest concentration 1 g/ml 2921, 9239, 8471, 5519, - Serres &
7623, 7689 Ashby (1981)
DNA polymerase deficient 100 µl/ml + S9 and - S9 - Serres &
assay Ashby (1981)
E. coli W3110 -
P3478
Yeast mutation S. pombe - Serres &
Ashby (1981)
S. cerevisiae (XV185-14C) - Serres &
Ashby (1981)
Mitotic recombination S. cerevisiae (JD1) + Serres &
Ashby (1981)
Mitotic crossing-over 10-1000 µg/ml S. cerevisiae (T1, T2) - Serres &
assay Ashby (1981)
Mitotic aneuploidy lowest effective S. cerevisiae (D6) - S9 + Serres &
concentration 100 µg/ml Ashby (1981)
Mitotic gene conversion 5 µl/ml S. cerevisiae (D7) + S9 - Serres &
Ashby (1981)
Mitotic gene conversion 500 µg/ml S. cerevisiae (JD1) - Serres &
Ashby (1981)
Repair test using yeast minimum effective S. cerevisiae (wild & rad) + Serres &
strains (cell growth concentration Ashby (1981)
inhibition) (MEC) 300 µg/ml
Nuclear enlargement 0.01-100 µg/ml human fibroblasts - Serres &
8-200 µg/ml HeLa cells + Ashby (1981)
Table 18. (contd.)
------------------------------------------------------------------------------------------------------------
Method Concentration Condition; comment Results Reference
------------------------------------------------------------------------------------------------------------
UDS test 1.1-90 µg/ml (-S9) human fibroblasts - Serres &
2-30 µg/ml (+S9) (WI - 38) -, + S9 Ashby (1981)
0.032-100 µg/ml human fibroblasts - Serres &
(from skin biopsies) Ashby (1981)
0.1-100 µg/ml HeLa cells - Serres &
Ashby (1981)
Sister chromatid exchange 0.00625-0.1% CHO cells (-, + S9) - Serres &
(SCE) Ashby (1981)
0.1-100 µg/ml CHO cells - Serres &
Ashby (1981)
RL Chromosome assay 75-300 µg/ml Rat liver epithelial type - Serres &
cell line (RL1) Ashby (1981)
Mouse lymphoma 46.9-3000 µg/ml Mouse lymphoma cells - Serres &
mutagenesis assay (L5178Y) Ashby (1981)
-, + S9 rat liver
Human fibroblast 0.2-0.5 mg/ml human lung fibroblast - Serres &
Diphtheria toxin (HSC172) Ashby (1981)
Resistance test
Cell transformation test 500 µg/ml Baby hamster kidney cells + Serres &
(BHK21C13/HRC1) Ashby (1981)
BHK-21 cell - Serres &
Ashby (1981)
Integration enhancement 0.005-0.5 C3H2K cell - Serres &
test (MLV Test) Ashby (1981)
Cytogenetic analysis 1.1 x 10-2-1.1 human peripheral - Antoine et al.
mol/litre lymphocytes (1983)
Cytogenetic analysis 10-20% human peripheral + Koudela &
lymphocytes Spazier (1979)
------------------------------------------------------------------------------------------------------------
Comment: Positive results were obtained with very high concentrations, such as 100-500 µg/ml and 10-20%.
In one study, DMF did not induce any increase in chromosomal
aberrations or sister chromatid exchange in human peripheral blood
lymphocytes in vitro (Antoine et al., 1983). However, in another
study, chromosomal aberrations were reported in human peripheral
lymphocyte cultures treated with DMF (Koudela & Spazier, 1979). The
authors performed cytogenetic analyses of human peripheral lymphocytes
treated with DMF (dilution from 10-7 to 10-2 mol/litre. Compared
with the positive control, thio-TEPA, the clastogenic activity of DMF
was 3- to 4-fold lower. Chromosome aberrations were concentration-
related at DMF levels of 10-20%.
Cytogenetic analysis of peripheral lymphocytes in 40 workers
exposed to 35-180 mg DMF/m3 was performed, first at 4-month
intervals, and later at 6-month intervals. Increased frequencies of
non-specified chromosomal aberrations of 3.82 and 2.74%, respectively,
were found (Koudela & Spazier, 1981). In further sampling periods,
after technological adjustments to decrease the DMF exposure to about
30 mg/m3, the authors established lower frequencies of cell
aberrations in most of the workers, i.e., 1.59, 1.58, and 1.49%, in
the various periods under study. The aberrant cells in the control
group were 1.61-1.10% (Koudela & Spazier, 1979).
8.6.2 In vivo studies
In in vivo studies, DMF was negative in dominant lethal
mutagenic assays, tests for chromosome aberrations and sperm
abnormalities, and micronucleus tests, (Table 19).
8.6.3 Appraisal
The results obtained in the in vitro and in vivo test systems
showed that DMF did not induce damage in genetic material.
8.7 Carcinogenicity
The carcinogenic activity of DMF has not been examined in 2-year
studies on test-animals (Purchase et al., 1978; Barral-Chamaillard &
Rouzioux, 1983; Kennedy, 1986; US EPA, 1986). However, there are some
data concerning DMF, applied as a solvent, and for shorter periods of
time.
In a study by Druckrey et al. (1967) two groups of 15 and 5 BD
rats were treated, with 75 and 150 mg DMF/litre in the drinking-water
for 500 and 250 days, respectively (total doses 38 000 mg/litre in
drinking-water). The animals were observed for up to a maximum of 750
days, with an average survival of 532 days. Similarly, two groups of
12 rats each were given weekly, subcutaneous injections of 200 and 400
mg DMF/kg (total doses 8000 and 20 000 mg/kg, respectively) and
observed for 732 and 766 days, respectively. No tumorigenic effects
were reported in this small group of rats.
Table 19. Short-term genotoxicity tests of DMF ( in vivo )
--------------------------------------------------------------------------------------------------------------
Method Animal Dose route Results Reference
--------------------------------------------------------------------------------------------------------------
Dominant lethal male rat 90, 900 mg/m3 - Lewis et al.
mutagenic bioassay inhalation 6 h daily (1979)
for 5 consecutive days
Chromosome male and 2.3-600 mg/m3 - Sheveleva
aberrations female rat inhalation et al. (1979)
Micronucleus test BALB/C mouse 0.2-2000 mg/kg ip - Antoine
et al. (1983)
Micronucleus test B6C3F1 mouse 80% of LD50 ip - Serres & Ashby (1981)
Micronucleus test ICR mouse 0.425-1.7 mg/kg ip - Serres & Ashby (1981)
Micronucleus test CD-1 mouse 0.4-1.6 mg/kg ip - Serres & Ashby (1981)
Sperm morphology (CBA x BALB/c)F1 0.1-1.5 mg/kg ip - Serres & Ashby (1981)
assay male mouse
--------------------------------------------------------------------------------------------------------------
Kommineni (1973) reported that 9 out of 18 male, and 11 out of 19
female rats, developed tumours in different organs following ip
administrations of 100 mg DMF per rat, once a week, for 10 weeks. The
incidence of tumours in male control rats was 4 out of 14 and, in
females, 5 out of 14, also in different organs. No specific organ or
tumour type predominated in either the test or control group. Three
testicular tumours were seen, a bilateral interstitial cell tumour in
the controls, and an interstitial cell tumour and an embryonal cell
carcinoma in the test group.
8.8 Induction of tumour cell differentiation
Borenfreund et al. (1975) reported a decrease in the malignancy
of the Friend erythroleukaemic cells and a marked increase in their
differentiation along the erythroid pathway after their treatment with
0.5 and 1% DMF solutions.
DMF induction of cell differentiation and a marked reduction of
tumorigenicity was established by Dexter (1977) in transplantable
murine rhabdomyosarcoma cells. In another study, Dexter & Hager
(1980) used 4 carcinoma cell lines derived from two specimens of
adenosarcoma of the human sigmoid colon and showed changes in the
carcinoma cells towards less malignant cell types. Hager et al.
(1980) demonstrated that a cultured human colon carcinoma cell
responded to DMF by more differentiated development, again suggesting
an antitumour effect.
Chakrabarty et al. (1984) studied the effects of DMF on AKR-2B and
AKR-MCA cells in vitro and found that complete loss of anchorage
independent growth occurred and the reduced expression of membrane
antigens was restored.
The DMF induction of tumour cell differentiation has been studied
by Kimball & Hixon (1983) in relation to the deviation of the nuclear
protein. Cordeiro & Savarese (1984) studied it in relation to the
effects on cysteine/glutathione metabolism, and Levine et al. (1985)
in relationship to the changes in receptor occupation and growth
factor responsiveness. Chen et al. (1986) studied the induction of
tumour cells by DMF in relation to the rate of nucleoside transport in
the cells.
A review of the studies on the effects of the induction of
alkylformamides on terminal differentiation of tumour cells (Langdon &
Hickman, 1987) shows that DMF should not be used for such purposes.
The anti-neoplastic activity of DMF, determined in vitro and in vivo,
does not appear to be sufficient for therapeutic use.
8.9 Mechanism of toxicity, mode of action
Several hypotheses on the possible mechanism of DMF hepatotoxicity
have been tested. No experimental support for lipid peroxidation,
lysosome labilization, or glutathione depletion has been reported.
The critical biological effects leading to DMF hepatotoxicity have not
been identified and still need to be elucidated (Scailteur & Lauwerys,
1987).
9. EFFECTS ON HUMAN BEINGS
9.1 General population exposure
No effects of DMF on the general population have been reported.
9.2 Occupational exposure
Reports of occupational poisonings with DMF have been reviewed by
Kennedy (1986), US EPA (1986), and Scailteur & Lauwerys (1987).
9.2.1 Accidental poisoning
Several cases of acute accidental occupational poisoning with DMF
have been reported (Tolot et al., 1968; Potter, 1973; Chary, 1974;
Chivers, 1978; Aldyreva & Gafurov, 1980; Kang-de & Hui-lan, 1981;
Shlygina & Nemolshev, 1981; Paoletti et al., 1982a,b). They were
caused by the malfunctioning of the equipment, splashing of the
organic solvent over the body, or working in plants without taking
protective measures. Over-exposure has occurred via the skin and/or
inhalation. Usually, the symptoms appeared from several hours up to
several days after the accident. The major symptoms were epigastric
or abdominal pain, which was irradiating and progressive, accompanied
by dizziness, nausea, anorexia, vomiting, fatigue, alcohol
intolerance, and skin irritation. Clinical laboratory tests showed
liver function disturbance. Radioisotope diagnostic tests and liver
biopsy revealed morphological changes in the liver. No clinical
manifestations of renal dysfunction were reported. The patients
recovered with symptomatic therapy in hospital for 2-3 weeks. Liver
function tests returned to normal. Some of the patients, who were
followed for several months or several years after the acute
poisoning, had normal function tests.
9.2.2 Long-term exposure
After occupational exposure to DMF (intensity and length of
exposure unspecified, no control groups), eye irritation, headache,
anorexia, gastrointestinal disturbances, and sometimes hepatomegaly
with biochemical signs of liver damage were reported. Some
haematological changes were also observed (Tolot et al., 1958; Weiss,
1971; Dilorenzo & Grazioli, 1972). In studies in which exposure was
quantified, subjective complaints of headache, fatigue, and
gastrointestinal and cardiovascular changes were reported.
Disturbances of liver function could be measured by changes in plasma
bilirubin levels, and increases in the serum activity of liver enzymes
(transaminases, alkaline phosphatase, glutamyl-transpep-tidase).
Alcohol intolerance occurred. Haematological changes and ECG
deviations were also observed (Table 20).
In a questionnaire study, for which little detail is available,
Schottek (1972) reported 14% miscarriages in a group of women exposed
to about 100 mg DMF/m3 compared with 10% in the control group. No
statistical analysis was performed. Aldyreva & Gafurov (1980)
reported perturbations in menstruation in 26 out of 70 women who had
been exposed to 30-150 mg DMF/m3 for about a year. No data are
available on controls. On the basis of company statistics, general
morbidity associated with gynaecological changes appeared to be
increased among DMF-exposed women.
Farquharson et al. (1983) reported miscarriages in 3 out of 9
women, who had been exposed to DMF as well as a number of other
chemicals.
Because of its effect on the stratum corneum, DMF interferes with
the barrier function of the skin; this was demonstrated in human
volunteers by increased water loss following DMF exposure (Baker,
1968).
9.2.3 Epidemiological studies on carcinogenicity
Ducatman et al. (1986) reported three cases of testicular germ-
cell tumours in 1981-83 among 153 white men who repaired the exterior
surfaces and electrical components of F4 Phantom jet aircraft, in the
USA. This finding led to surveys of two other repair shops at
different geographical locations; in one of the shops, the same type
of aircraft was repaired, while in the other, different types of
aircraft were repaired. Four out of 680 white male workers in the
same type of repair shop had a history of testicular germ-cell cancers
(0.95 expected) occurring in 1970-83. No case of testicular germ-cell
cancer was found among the 446 white men employed at the facility
where different types of aircraft were repaired. Of the 7 cases of
testicular germ cell tumours, 5 were seminomas and 2 were embryonal-
cell carcinomas. All 7 men had long histories of working in aircraft
repair. The time from first exposure to diagnosis ranged from 4 to 19
years. There were many common exposures to solvents in the three
facilities, the only exposure identified as unique to the F4 Phantom
jet aircraft repair facilities, where the cases occurred, being to a
solvent mixture containing 80% dimethyl-formamide (20% unspecified).
No quantitative exposure data exist. Three of the cases had certainly
been exposed to this mixture and 3 cases, probably exposed. The cases
were found through foremen and from filed death certificates, and the
authors suggested that under-reporting was possible. No other cases
of cancer were investigated.
Levin et al. (1987) described 3 cases of embryonal-cell carcinoma
of the testes in workers at one leather tannery in the USA, all of
whom had worked as swabbers on the spray lines in leather finishing.
The latency period was from 8 to 14 years. According to the authors,
all the tanneries surveyed used dimethylformamide, as well as a wide
range of dyes, solvents, and other chemicals. No quantitative
exposure data are available. The number of workers from which these 3
cases arose was not given, and other cancers were not looked for.
Table 20. Studies on workers with long-term exposures
---------------------------------------------------------------------------------------------------------------------
Number Number Length DMF exposure Urinary Hepato- Alcohol Other effects Reference
of of non- of (mg/m3) NMF toxicity intoler-
exposed exposed exposure ance
subjects controls (years)
---------------------------------------------------------------------------------------------------------------------
22 28 5 1-47 (usually 20-63 - + NR Lauwerys
< 30; gloves mg/g et al. (1980)
worn) creatinine
11 - 3 3-15 0.4-20 - +(6)a NR Yonemoto &
mg/24 h Suzuki (1980)
28 29 3-5 30-60 NR - NR complaints of eye and Hinkova et al.
respiratory tract (1980)
irritation; no haemato-
logical changes
115 67 1-1.5 30-150 with NR + NR complaints of gastro- Aldyreva &
higher peaks + (a few out intestinal tract or Gafurov (1980)
skin exposure of 29) cardiovascular and
ovarian distur-
bances (29)
177 - 3-5 10-30 NR - NR complaints of Aldyreva &
cardiovascular Gafurov (1980)
disturbances (45)
81 96 3.5 < 10 NR +(10) NR complaints of gastro- Kang-de &
accidental intestinal tract and Hui-Lan (1981)
peak levels up cardiovascular
to 4525 + skin disturbances, ECG
exposure changes
23 - 2 > 30 peaks 10-40 NR NR No ECG changes com- Taccola et al.
up to 150 mg/day pared with pre-exposure (1981)
27 237 2 2-80 peaks NR +(2) +(8) complaints of gastro- Paoletti &
up to 549 intestinal tract Iannaccone
disturbances (15); (1982)
headache (6)
---------------------------------------------------------------------------------------------------------------------
Table 20. (contd.)
---------------------------------------------------------------------------------------------------------------------
Number Number Length DMF exposure Urinary Hepato- Alcohol Other effects Reference
of of non- of (mg/m3) NMF toxicity intoler-
exposed exposed exposure ance
subjects controls (years)
---------------------------------------------------------------------------------------------------------------------
13 - < 4 14-60 NR +(2) +(8) complaints of gastro- Tomasini
(mean: 29) intestinal tract et al (1983)
disturbances (8); eye
irritation (11); kidney
function test and
haematology, normal
26 54 > 5 2-5 NR - NR NR Catenacci
(mean: 3) et al.(1984)
28 54 > 5 12-25 NR - NR NR Catenacci
(mean: 18) et al. (1984)
100 100 5 8-58 NR + +(39) complaints of headache, Cirla et al.
(mean: 22) (8 versus eye and throat (1984)
2 controls) irritation, gastro-
intestinal tract and
cardiovascular
disturbance
24 29 5 10-60 NR NR NR irritative dermatitis Bainova (1985)
15 28 6-10 20-30 NR NR NR increased coagulation Imbriani
(median 27) time et al. (1986)
---------------------------------------------------------------------------------------------------------------------
a Incidence is indicated when available.
NR = not reported
To evaluate the significance of this cluster, an analysis of the
New York State Cancer Registry was conducted. Occupations were
determined from cancer registries and from death certificates for all
residents in Fulton County who were diagnosed as having testicular
cancer from 1974-88. From preliminary results, it is estimated that
workers who are employed in the leather tanning industry are 5-6 times
more likely to develop testicular cancer than those who are not
leather workers. However, the testicular cancer rates in this county
were lower than expected within this period, and an adjacent county
showed the same number of cases of testicular cancer, none of the
affected individuals having ever worked in the leather industry
(Walrath et al., 1988).
O'Berg et al. (1985) and Chen et al. (1988a) studied the cancer
incidence among 2530 actively employed workers with potential exposure
to dimethylformamide between 1956 and 1984, 1329 employees with
exposure to dimethylformamide and acrylonitrile at an acrylic fibre
manufacturing plant in South Carolina, USA, and 1130 controls from the
same plant. Cancer incidence rates for the company (1956-84) and USA
national rates (1973-77) were used to calculate the expected number of
cases. For all workers exposed to DMF (alone or with acrylonitrile),
the standardized incidence ratio (SIR), based on company rates for all
cancers combined, was 110 [95% confidence interval (CI), 88-136]a (88
cases); the SIR on the basis of national rates was 92. The SIR for
cancer of the buccal cavity and pharynx was 344 [CI, 172-615]a (11
cases), on the basis of company rates, and 167, on the basis of US
rates. More cancer cases than expected from company rates (34 cases:
SIR 134 [CI, 98-195]a) were found among employees exposed to
dimethylformamide alone, due mainly to 8 carcinomas of the buccal
cavity and pharynx versus 1.0 expected (SIR, 800 [CI, 345-1580]a).
All of these cases either smoked or chewed tobacco, but no information
was available on the smoking, tobacco chewing, or drinking habits of
the cohort. An additional case occurred among employees exposed to
dimethyl-formamide alone (SIR, 167); 4 of these tumours were cancers
of the lip. No such excess was found among the workers exposed to
both dimethylformamide and acrylonitrile (2 observed; SIR, 125, on the
basis of company rates). The authors reported that there was no
association with intensity or duration of exposure: "low" and
"moderate" exposure SIR 420 (5 cases); "high" exposure SIR 300 (6
cases). "Low" exposure conditions included: no direct contact with
liquids containing any dimethylformamide, even wearing protective
equipment; and workplace air levels consistently below 30 mg DMF/m3
(10 ppm) (no odour of DMF evident). "Moderate" exposure conditions
included: intermittent contact with liquids containing > 5%
dimethylformamide; workplace air levels sometimes higher than 30 mg
DMF/m3 (10 ppm) (more than once per week); DMF-laden materials
handled, but air levels of DMF maintained at above levels. "High"
exposure conditions included: frequent contact with liquids containing
> 5% dimethylformamide; workplace air levels often > 30 mg DMF/m3
(> 10 ppm); use of breathing protection often required for periods of
15 min-1 h; DMF vapour levels frequently > 30 mg/m3 (> 10 ppm)
(when handling pure dimethylformamide or dimethylformamide-containing
---------------------------------------------------------------------------
a As calculated by IARC (1989).
materials). One case of testicular cancer was found among the 3859
workers exposed to DMF (alone or with acrylonitrile) with 1.7 expected
on the basis of company rates, and no cases of liver cancer. The
company rates may be more relevant for comparison, as there were only
actively employed persons among the exposed and because the USA rates
are based on a limited time period, 1973-77.
Chen et al. (1988b) analysed mortality from 1950-82 among both
active and pensioned employees in the same cohort. Expected numbers
(adjusted for age and time period) were based on company rates. For
all workers exposed to DMF (alone or with acrylonitrile), the
standardized mortality ratio (SMR) for lung cancer was 124 (33 cases
[95% CI, 85-174]a). An increased risk of lung cancer was found in the
cohort exposed only to DMF (19 cases; SMR 141 [CI, 84-219]a), but not
in the cohort exposed to DMF and acrylonitrile. There were 3 deaths
from cancer of the buccal cavity and pharynx (SIR 188) in all persons
exposed to DMF (alone or with acrylo-nitrile). No other excess cancer
risk was reported. No information is given in this report on loss to
follow-up, death certificates, or whether these deaths were included
in the incidence study reported above.
Walrath et al. (1988) reported a case-control study on cancer
among 8724 Du Pont employees with potential exposure to DMF in 4 other
USA plants. Summary analyses for all plants combined did not show any
statistically significant association between ever having been exposed
to DMF and subsequent development of cancers of the buccal cavity and
pharynx, liver, malignant melanoma, prostate, and testes. When odds
ratios are examined according to plant site, prostate cancer at one
site was significantly elevated, on the basis of 3 cases exposed out
of 4, but no statistically significant association was observed among
employees similarly exposed to DMF in the other 3 plants. The recency
of exposure to DMF, the low exposures received, and the absence of
similar excesses at other plants argue against a causal association
between DMF exposure and prostate cancer at the one site. Assessment
of highest DMF exposure rank, duration of exposure, and latent period,
does not show any patterns suggesting an association between DMF and
cancers of the buccal cavity and pharynx, liver, malignant melanoma,
prostate, or testes.
9.2.4 Alcohol intolerance
Reviews on the synergistic action of ethanol with organic solvents
have been published by Haguenoer et al. (1982), Hills & Venable
(1982), and Stockley (1983).
Episodes of alcohol intolerance among workers exposed to DMF have
been repeatedly described at all levels of exposure (see sections
9.2.1 and 9.2.2, and Table 20).
Symptoms include flushing of the face, dizziness, nausea,
tightness of the chest, sometimes dyspnoea, and cardiac palpitations.
The reactions were reported within 24 h of DMF exposure and very
shortly after alcohol ingestion. These episodes lasted for up to 2 h
---------------------------------------------------------------------------
a As calculated by IARC (1989).
(Chivers, 1978; Lyle et al., 1979; Yonemoto & Suzuki, 1980; Paoletti
et al., 1982a; Cirla et al., 1984).
According to Loos (1979), abnormal liver function tests were
already discovered among workers who drank only 50-70 g alcohol per
day, but were also exposed to 45-66 mg DMF/m3, while the threshold
consumption for functional liver changes was 80-100 g alcohol per day
in control individuals. It should be noted that the test group was
also exposed to other solvents, mainly tetrahydrofuran, toluene, and
xylene.
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The International Agency for Research on Cancer (IARC) evaluated
the carcinogenicity of dimethylformamide in 1988 (IARC, 1989), and
concluded that:
- there is limited evidence for the carcinogenicity of
dimethylformamide in humans;
- there is inadequate evidence for the carcinogenicity of
dimethylformamide in experimental animals.
Overall evaluation: Dimethylformamide is possibly carcinogenic
to humans (Group 2B).
REFERENCES
ALDYREVA, M.V. & GAFUROV, S.A. (1980) [ Labour protection in the production
of artificial leather.] Moscow, Medizina, 158 pp (in Russian).
ALDYREVA, M.V., BORTSEVICH, S.V., PALAGUSHINA, A.I., SIDOROVA, N.V., &
TARASOVA, L.A. (1980) [Effect of dimethylformamide on workers' health in
the production of polyurethane synthetic leather.] Gig. Tr. prof. Zabol.,
6: 24-28 (in Russian).
AMSTER, M.B., HIJAZI, N., & CHAN, R. (1983) Real time monitoring of low
level air contaminants from hazardous waste sites. In: Proceedings of the
National Conference on Management of Uncontrolled Hazardous Waste Sites.
Washington, DC, pp. 98-99.
ANTOINE, J.L., ARANY, J., LEONARD, A., HENROTTE, J., JENAR-DUBUISSON, G., &
DECAT, G. (1983) Lack of mutagenic activity of dimethylformamide.
Toxicology, 26: 207- 212.
ARENA, N., SANTA CRUZ, G., ALIA, E.F., BALDUS, M., CORGIOLU, T., & ALIA,
E.E. (1982) [Structural and ultrastructural changes in the myocardium of
rabbits exposed to dimethylformamide vapours.] Boll. Soc. Ital. Biol.
Sper., 58: 1496-1501 (in Italian).
BAINOVA, A. (1980) [Contemporary views on the hygiene standardization of
dimethyl- formamide.] Letopisi HEI, 37: 62-68 (in Bulgarian).
BAINOVA, A. (1985) [ Toxicological problems due to the action of chemical
substances on the skin (dermatotoxicology).] Sofia, Referat, pp. 1-60 (D.
Sci. Thesis) (in Bulgarian).
BAINOVA, A. & ANTOV, G. (1980) Dermal toxicity of dimethylformamide in
rats. In: Abstracts of the 5th International Symposium on Occupational
Health in the Production of Artificial Fibres, Belgirate, Italy, 16-20
September, 1980, Modena, Permanent Commission and International Association
on Occupational Health, pp. 73-74.
BAINOVA, A., SPASSOVSKI, M., HINKOVA, L., & TASHEVA, M. (1981a) [Effect
of intermittent and continuous action of dimethylformamide on the
adaptation process.] Probl. hig., 6: 27-35 (in Bulgarian).
BAINOVA, A., ANTOV, G.P., & IVANOVICH, E.H. (1981b) Combined action of
dimethylformamide and noise on kidneys of white rats. CR. Acad. bulg. Sci.,
34: 1609-1611.
BAKER, H. (1968) The effects of dimethylsulfoxide, dimethylformamide, and
dimethyl- acetamide on the cutaneous barrier to water in human skin. J.
invest. Dermatol., 50: 283- 288.
BARNES, J.R. & HENRY, N.W. (1974) The determination of N-methylformamide
and N-methylacetamide in urine. Am. Ind. Hyg. Assoc. J., 35: 84-87.
BARNES, J.R. & RANTA, K.E. (1972) The metabolism of dimethylformamide
and dimethylacetamide. Toxicol. appl. Pharmacol., 23: 271-276.
BARRAL-CHAMAILLARD, J. & ROUZIOUX, M. (1983) Dimethylformamide. Arch.
Mal. prof., 44: 203-208.
BARTSCH, W., SPONER, G., DIETMAN, K., & FUCHS, G. (1976) Acute toxicity
of various solvents in the mouse and rat. Arzneimittelforschung, 26: 1581-
1583.
BECCI, P.J., VOSS, K.A., JOHNSON, W.D., GALLO, M.A., & BABISH, J.G. (1983)
Subchronic feeding study of N,N-dimethylformamide in rats and mice. J. Am.
Coll. Toxicol., 2(6): 371-378.
BEGERT, A. (1975) [Purification of chemical textile plant sewage.]
Oesterr. Abwasser- Rundsch., 20: 98-102 (in German).
BORENFREUND, E., STEINGLASS, M., KORNGOLD, G., & BENDICH, A. (1975)
Effect of dimethylsulfoxide and dimethylformamide on the growth and
morphology of tumour cells. Ann. N.Y. Acad. Sci., 243: 164-171.
BORTSEVICH, S.V. (1984) [The hygienic importance of dimethylformamide
absorption through the skin.] Gig. Tr. prof. Zabol., 11: 55-57 (in
Russian).
BRINDLEY, C., GESCHER, A., & ROSS, D. (1983) Studies of the metabolism of
dimethylformamide in mice. Chem.-biol. Interact., 45: 387-392.
BRONDEAU, M.T., BONNET, P., GUENIER, J.P., & DE CEAURRIZ, J. (1983)
Short-term inhalation test for evaluating industrial hepatotoxicants in
rats. Toxicol. Lett., 19: 139-146.
BRUGNONE, F., PERBELLINI, L., & GAFFURI, E. (1980a) N,N-Dimethylformamide
concentration in environmental and alveolar air in a artificial leather
factory. Br. J. ind. Med., 37: 185-188.
BRUGNONE, F., PERBELLINI, L., GAFFURI, E., & ASASTOLI, P. (1980b)
Biomonitoring of industrial solvent exposures in workers' alveolar air.
Int. Arch. occup. environ. Health, 47: 245-261.
BRUGNONE, F., PERBELLINI, L., GAFFURI, E., & TURRI, P.V. (1984)
Monitoring of industrial exposure to dimethylformamide by analysis of
alveolar air. Med. Lav., 6: 139- 141.
BURGUN, J., MARTZ, R., FORNEY, R.B., & KIPLINGER, G.F. (1975) The acute
toxicity of dimethylformamide and its combined effects with ethanol in the
mouse. Toxicol. appl. Pharmacol., 33: 149-150.
CAI, S.X. & HUANG, M.Y. (1979) Investigation on occupational hazard in a
butadiene monomer workshop of a cis-butadiene rubber plant. J. Hyg. Res.,
8(1): 22-49 (in Chinese).
CARDWELL, R.D., FOREMAN, D.G., PAYNE, T.R., & WILBUR, D.J. (1978) Acute
and chronic toxicity of four chemicals to fish. Duluth, Minnesota, US
Environmental Protection Agency (Contract 68-01-0711).
CATENACCI, G., GRAMPELLA, D., TERZI, R., SALA, H., & POLLINI, G., (1984)
Hepatic function in subjects exposed to environmental concentrations of DMF
lower than the actually proposed TLV. Med. Lav., 6: 157-158.
CHAKRABARTY, S., MCRAE, L.J., LEVINE, A.E., & BRATTAIX, M.G. (1984)
Restoration of normal growth control and membrane antigen composition in
malignant cells by N,N-dimethylformamide. Cancer Res., 44: 2181-2185.
CHARY, S. (1974) Dimethylformamide. A case of acute pancreatitis. Lancet,
2(7876): 356.
CHEN, J.L., FAYERWEATHER, W.E., & PELL, S. (1988a) Cancer incidence of
workers exposed to dimethylformamide and/or acrylonitrile. J. occup. Med.,
30: 813-818.
CHEN, J.L., FAYERWEATHER, W.E., & PELL, S. (1988b) Mortality study of
workers exposed to dimethylformamide and/or acrylonitrile. J. occup. Med.,
30: 819-821.
CHEN, S.-F., CLEAVELAND, J.S., HOLLMAN, A.B., WIEMANN, M.C., PARKS, R.E., &
STOECKLER, J.D. (1986) Changes in nucleoside transport of HL-60 human
promyelocytic cells during N,N-dimethylformamide induced differentiation.
Cancer Res., 46: 3449-3455.
CHIVERS, C.P. (1978) Disulfiram effect from inhalation of
dimethylformamide. Lancet, 1(8059): 331.
CHROMEK, J., KUPEK, J., MLADEK, M., & MARVAN, P. (1983) A study of
respiration of the alga Sceadesmus quadricauda in batch conditions under
influence of N,N-dimethylformamide and dimethylamine. Arch. Hydrobiol.,
64(Suppl.): 441-460.
CIRLA, A.M., PISATI, G., INVERNIZZI, E., & TORRICELLI, P. (1984)
Epidemiological study on workers exposed to low dimethylformamide
concentrations. G. Ital. Med. Lav., 6: 149-156.
CLAY, P.F. & SPITTLER, T.M. (1983) Determination of airborne volatile
nitrogen compounds using four independent techniques. In: Proceedings of
the National Conference on Management of Uncontrolled Hazardous Waste
Sites, Washington, DC, pp. 100-104.
CLAYTON, J.W., BARNES, J.R., HOOD, D.B., & SCHEPERS, G.W. (1963) The
inhalation toxicity of dimethylformamide (DMF). Am. Ind. Hyg. Assoc. J.,
24: 144-154.
CORDEIRO, R.F. & SAVARESE, T.M. (1984) Reversal by L-cysteine of the
growth inhibitory and glutathione-depleting effects of N-methylformamide
and N,N-dimethylformamide. Biochem. biophys. Res. Commun., 122: 798-803.
COSTA, V., FRONGIA, N., & SANTA CRUZ, G. (1978) [Histological and
ultrastructural changes of the rat renal glomerulus caused by
dimethylformamide inhalation.] Boll. Soc. Ital. Biol. Sper., 54: 1723-1728
(in Italian).
CRAIG, D.K., WEIR, R.J., WAGNER, W., & GROTH, D. (1984) Subchronic
inhalation toxicity of dimethylformamide in rats and mice. Drug chem.
Toxicol., 7: 551-571.
DARNALL, K.R., LLOYD, A.C., WINER, A.M., & PITTS, J.N. (1976) Reactivity
scale for atmospheric hydrocarbons based on reaction with hydroxyl radical.
Environ. Sci. Technol., 7: 692-696.
DEXTER, D.L. (1977) N,N-Dimethylformamide-induced morphological
differentiation and reduction of tumorigenicity in cultured mouse
rhabdomyosarcoma cells. Cancer Res., 37: 3136-3140.
DEXTER, D.L. & HAGER, J.C. (1980) Maturation induction of tumour cells
using a human colon carcinoma model. Cancer, 45: 1178-1184.
DILORENZO, F. & GRAZIOLI, C. (1972) [Hematological, hematochemical, and
gastric findings in workers exposed to breathing vapours of
dimethylformamide.] Lav. Um., 24: 96-106 (in Italian).
DIXON, S.W., GRAEPEL, G.J., & LOONEY, W.C. (1983) Seasonal effects on
concentrations of monomethylformamide in urine samples. Am. Ind. Hyg.
Assoc. J., 44: 273-275.
DOJLIDO, J.R. (1979) Investigations of biodegradability and toxicity of
organic compounds. Washington, DC, US Environmental Protection Agency
(Unpublished report No. EPA-600/2-79-163).
DRUCKREY, H., PREUSSMANN, R., IVANKOVIC, S., & SCHMAHL, D. (1967)
[Organotropic carcinogenic effects of 65 different N-nitroso-compounds on
B.D. rats.] Z. Krebsforsch., 69: 103-201 (in German).
DUCATMAN, A.M., CONWILL, D.E., & CRAWL, J. (1986) Germ cell tumours of
the testicle among aircraft repairmen. J. Urol., 136: 834-836.
EBEN, A. & KIMMERLE, G. (1976) Metabolism studies in N,N-
dimethylformamide. III. Studies on the influence of ethanol in persons and
laboratory animals. Int. Arch. occup. environ. Health, 36: 243-265.
EBERLING, C.L. (1980) Formic acid and derivatives (DMF). In: Kirk-
Othmer encyclopedia of chemical technology, 3rd ed., New York, Chichester,
Brisbane, Toronto, John Wiley & Sons, Vol. 11, pp. 263-268.
ELOVAARA, E., MARSELOS, M., & VAINO, H. (1983) N,N-Dimethylformamide-
induced effects on hepatic and renal xenobiotic enzymes with emphasis on
aldehyde metabolism in the rat. Acta. pharmacol. toxicol., 53: 159-165.
EWING, B.B., CHIAN, E.S.K., COOK, J.C., EVANS, C.A., HOPKE, P.A., &
PERKINS, E.G. (1977) Monitoring to detect previously unrecognized
pollutants in surface water. Appendix: Organic analysis data, Washington,
DC, US Environmental Protection Agency, (Unpublished report No. EPA-560/6-
77-015).
FARHI, M., MOREL, M., & CAVIGNEAUX, A. (1968) Dimethylformamide.
NCON(CH3)2. Cah. Notes doc., 50: 91-93.
FARLEY, F.F. (1977) Photochemical reactivity classification of
hydrocarbons and other organic compounds. In: International Conference on
Photochemical Oxidant Pollution and Its Containment, Research Triangle
Park, North Carolina, US Environmental Protection Agency. (Unpublished
report No. EPA-600/3-77-0018).
FARQUHARSON, R.O., HALL, M.A., & FULLERTON, W.T. (1983) Poor obstetric
outcome in three quality control laboratory workers. Lancet, 30: 983-984.
GERMANOVA, A.L., HALEPO, A.I., AVILOVA, G.G., ANVAER, L., HOROCHULOVA,
N.V., MALTSEVA, N.M., & MIGUKINA, N.V. (1979) [Adaptation after
continuous and intermittent exposure to dimethylformamide.] In: [ The
toxicology of new industrial chemicals.] Moscow, Medizina, Vol. 15, pp. 69-
76 (in Russian).
GRASSELLI, J.G. (1973) Atlas of spectral data and physical constants for
organic compounds, Cleveland, Ohio, USA, The Chemical Rubber Co.
GUBSER, H. (1969) Purification of chemical waste waters. Gas Wasser
Abwasser, 49: 175-181.
HAGER, J.C., GOLD, D.V., BARBOSA, J.A., FLIGIEL, L., MILLER, F., & DEXTER,
D.L. (1980) N,N-Dimethylformamide-induced modulation of organ and tumour
associated markers in cultured human colon carcinoma cells. J. Natl Cancer
Inst., 64: 439-445.
HAGUENOER, J.M., BOURRINET, P., & FRIMAT, P. (1982) Interrelations
between alcoholism and exposure to industrial poisons. Arch. Mal. prof.,
43: 461-473.
HAMILTON, A. & HARDY, H.L. (1974) Industrial toxicology, 3rd ed., Acton,
Massachusetts, Publishing Science Group Inc., 349 pp.
HANASONO, G.K., FULLER, R.W., BRODDLE, W.D., & GIBSON, W.R. (1977)
Studies on the effects of N,N-dimethylformamide on ethanol disposition and
on monoamine oxidase activity in rats. Toxicol. appl. Pharmacol., 39: 461-
472.
HELLWIG, J., MERKLE, J., KLIMISCH, H.J., & JÄCKH, R. (in press)
Investigations on the prenatal toxicity of N,N-dimethylformamide (DMF) in
mice, rats and rabbits. Food chem. Toxicol..
HENRY, N.W. & SCHLATTER, C.N. (1981) The development of a standard method
for evaluating chemical protective clothing to permeation by hazardous
liquids. Am. Ind. Hyg. Assoc. J., 42: 202-207.
HILLS, B.W. & VENABLE (1982) The interaction of ethyl alcohol and
industrial chemicals. Am. J. ind. Med., 3: 321-333.
HINKOVA, L., GINCHEVA, N., STAMOVA, N., HRISTEVA, V., CHOLAKOV, B., &
SASSOVSKY, M. (1980) [Influence of dimethylformamide on workers' health.]
Probl. hyg., Sofia, 5: 75-81 (in Bulgarian).
HUANG, M.Y., LUO, Y.Z., GENG, T.B., MENG, D.S., LIU, J., HUANG, M.F., &
WANG, Y.S. (1981) [Studies on the dermal toxicity of dimethylformamide.]
J. Hyg. Res., 10(4): 21-26 (in Chinese).
HUGHES, J.S. & VILKAS A.G. (1983) Toxicity of N,N-dimethylformamide used
as a solvent in toxicity tests with the green alga Selenastrum capricornum.
Bull. environ. Contam. Toxicol., 31: 98-104.
IARC (1989) Dimethylformamide. In: Some organic solvents, resin monomers
and related compounds, pigments and occupational exposures in paint
manufacture and painting, Lyon, International Agency for Research on
Cancer, pp. 171-197 (IARC Monograph on the Evaluation of Carcinogenic Risks
to Humans, Vol. 47).
IMBRIANI, M., CHITTORI, S., PRESTINONI, A., LONGONI, P., CASCONE, G., &
GAMBA, G. (1986) Effects of dimethylformamide (DMF) on coagulation and
platelet activity. Arch. environ. Health, 41: 90-93.
KANG-DE, C. & HUI-LAN, Z. (1981) Observation on the effects of
dimethylformamide on human health. In: Abstracts of the 9th International
Congress on Occupational Health in the Chemical Industry, Aswan, Egypt, 15-
17 September 1981, Aswan, Egypt, Permanent Commission and International
Association on Occupational Health, pp. 22-23.
KELLER, C.A. & LEWIS, S.C. (1981) Inhalation teratology study of N,N-
dimethyl- formamide (DMF). Teratology, 23: 45A.
KENNEDY, G.L., Jr (1986) Biological effects of acetamide, formamide, and
their monomethyl and dimethyl derivatives. CRC crit. Rev. Toxicol., 17(2),
129-182.
KENNEDY, G.L. & SHERMAN, H. (1986) Acute and subchronic toxicity of
dimethylformamide and dimethylacetamide following various routes of
administration. Drug chem. Toxicol., 9: 147-170.
KESTELL, P., GILL, M.H., THREADGILL, M.D., GESCHER, A., HOWARTH, O.W., &
CURZON, E.H. (1986) Identification by proton NMR of N-(hydroxymethyl)- N-
methylformamide as the major urinary metabolite of N,N-dimethylformamide
in mice. Life Sci., 38: 719- 724.
KESTELL, P., THREADGILL, M.D., GESCHER, A., GLEDHILL, A.P., SHAW, A.J., &
FARMER, R.B. (1987) An investigation of the relationship between the
hepatotoxicity and the metabolism of N-alkylformamides. J. Pharmacol. exp.
Ther., 270: 265-270.
KIMBALL, P.M. & HIXON, S. (1983) Nuclear protein changes following N,N-
dimethylformamide (DMF) induced maturation. J. cell. Biochem., 22: 245-249.
KIMMERLE, G. & EBEN, A. (1975a) Metabolism studies of N,N-
dimethylformamide. I. Studies in rats and dogs. Int. Arch. Arbeitsmed.,
34: 109-126.
KIMMERLE, G. & EBEN, A. (1975b) Metabolism studies of N,N-
dimethylformamide. II. Studies in persons. Int. Arch. Arbeitsmed., 34:
127-136.
KIMMERLE, G. & MACHEMER, L. (1975) Studies with N,N-dimethylformamide for
embryotoxic and teratogenic effects on rats after dynamic inhalation. Int.
Arch. Arbeitsmed., 34: 167-175.
KIMURA, E.T., EBERT, D.M., & DODGE, P.W. (1971) Acute toxicity and limits
of solvent residue for sixteen organic solvents. Toxicol. appl.
Pharmacol., 19(4): 699-704.
KISS, G. (1979) [Study of the irritative action of dimethylformamide.]
Börg. Venerol., 55: 203 (in Hungarian).
KOMMINENI, C. (1973) Pathological studies of aflatoxin fractions and
dimethylformamide in MRC rats, Omaha, University of Nebraska (Dissertation,
December 1972).
KOUDELA, K. & SPAZIER, K. (1979) [Effects of dimethylformamide on human
peripheral lymphocytes.] Cesk. Hyg., 24: 432-436 (in Czech).
KOUDELA, K. & SPAZIER, K. (1981) [Increased concentration of
dimethylformamide vapours in the atmosphere.] Prac. Lek., 33: 121-123 (in
Czech).
KRAMER, V.C., SCHNELL, D.J., & NICKERSON, K.W. (1983) Relative toxicity
of organic solvents to Aedes aegypti larvae. J. invertebr. Pathol., 42:
285-287.
KRIVANEK, N.D., MCLAUGHLIN, M., & FAYERWEATHER, W.E. (1978)
Monomethylformamide levels in human urine after repetitive exposure to
dimethylformamide vapour. J. occup. Med., 20: 179-182.
LAITY, J.L., BURSTEIN, I.G., & APPEL, B.R. (1973) Photochemical smog and
the atmospheric reactions of solvents. In: Solvents theory and practice,
pp. 95-112, Washington, DC, American Chemical Society (Advances in
Chemistry Series, Vol. 124).
LANGDON, S.P. & HICKMAN, J.A. (1987) Alkylformamides as inducers of
tumour cell differentiation - a mini-review. Toxicology, 43: 239-249.
LAUWERYS, R. (1986) Dimethylformamide. In: Alessio, L., Berlin, A., Boni,
M., & Roi, R., ed. Biological indicators for the assessment of human
exposure to industrial chemicals. Brussels, Luxembourg, Commission of the
European Communities Joint Research Center, pp. 19-27.
LAUWERYS, R.R., KIVITS, A., LHOIR, M., RIGOLET, P., HOUBAT, D., BUCHET,
J.P., & ROELS, H.A. (1980) Biological surveillance of workers exposed to
dimethylformamide and the influence of skin protection on its
percutaneous absorption. Int. Arch. occup. environ. Health, 45: 189-203.
LAZAREV, N.V. & LEVINA, E.N. (1976) [Dimethylformamide.] In: [Harmful
substances in industry.] Leningrad, Himia, Vol. 2, pp. 36-38 (in Russian).
LEBLANC, G.A. & SURPRENANT, D.C. (1983) The acute and chronic toxicity of
acetone, dimethylformamide, and triethylene glycol to Daphnia magna
(Strauss). Arch. environ. Contam. Toxicol., 12: 305-310.
LESHIK, J.A.D. & FEOKTISTOVA, A.J. (1984) [Ascorbic acid supply and
cytochrome P-450 level in guinea-pig liver during dimethylformamide
poisoning.] J. Vopr. Pitan., 5: 65-67 (in Russian).
LEVIN, S.M., BAKER, D.B., LANDRIGAN, P.J., MONAGHAN, S.V., FRUMIN, E.,
BRAITHWAITE, M., & TOWNE, W. (1987) Testicular cancer in leather tanners
exposed to dimethylformamide. Lancet, II(8568): 1153.
LEVINE, A.E., MCRAE, L.J., & BRATTAIN, M.G. (1985) Changes in receptor
occupancy and growth factor responsiveness induced by treatment of a
transformed mouse embryo cell line with N,N-dimethylformamide. Cancer Res.,
45: 6401-6405.
LEWIS, S.C., RINEHART, W.E., SCHROEDER, R.E., & THAKARA, J.W. (1979)
Dominant lethal mutagenic bioassay of dimethylformamide (DMF). Environ.
Mutagen., 1: 166.
LIPSKI, K. (1982) Liquid chromatographic determinations of
dimethylformamide, methylene bisphenyl isocyanate, and methylene bisphenyl
amine in air samples. Ann. occup. Hyg., 25: 1-4.
LLEWELLYN, G.C., HASTINGS, W.C., & KIMBROUGH, T.D. (1974) The effects of
dimethylformamide on female Mongolian gerbils Meriones unguiculatus. Bull.
environ. Contam. Toxicol., 11: 467-473.
LOBANOVA, K.P. (1958) [Toxicity of dimethylformamide.] Gig. i Sanit.,
23: 31- 37 (in Russian).
LOOS, H. (1979) Hazards, health supervision, and potentiation of alcohol
by mixtures of organic solvents, especially dimethylformamide (DMF).
Arbeitsmed. Sozialmed. Präventivmed., 14(5): 127-129.
LUNDBERG, I., LUNDBERG, S., & KRONEVY, T. (1981) Some observations on
dimethylformamide hepatotoxicity. Toxicology, 22: 1-7.
LUNDBERG, I., PEHRSSON, A., LUNDBERG, S., KRONEVY, T., & LIDUMS, V. (1983)
Delayed dimethyformamide biotransformation after high exposures in rats.
Toxicol. Lett., 17: 29- 34.
LUNDBERG, I., EKDAHL, M., KRONEVI, T., LIDUMS, V., & LUNDBERG, S. (1986)
Relative hepatotoxicity of some industrial solvents after intraperitoneal
injection or inhalation exposure in rats. Environ. Res., 40: 411-420.
LUNDBERG, S. (1982) [ Nordic group of experts for documentation of
threshold limit values - 38. Dimethylformamide.] Solna, Sweden, National
Institute of Occupational Health, pp. 32 (Report 1982:28) (in Swedish).
LYLE, W.H., SPENCE, T.W.M., MCKINNELEY, W.M., & DUCKERS, K. (1979)
Dimethylformamide and alcohol intolerance. Br. J. ind. Med., 36: 63-66.
MALONOVA, H. & BARDODEJ, Z. (1983) Urinary excretion of mercapturates as
a biological indicator of exposure to electrophilic agents. J. Hyg. epidem.
Microbiol. Immunol., 27(3): 319-328.
MASSMANN, W. (1956) Toxicological investigations on dimethylformamide.
Br. J. ind. Med., 13: 51-54.
MATHEW, T., KARUNANITHY, R., YEE, M.H., & NATARAJAN, P.N. (1980)
Hepatotoxicity of dimethylformamide and dimethylsulfoxide at and above the
levels used in some aflatoxin studies. Lab. Invest., 42: 257-262.
MAXFIELD, M.E., BARNES, J.R., AZAR, A., & TROCHIMOWIEZ, H.T. (1975)
Urinary excretion of metabolite following experimental human exposure to
dimethylformamide or to dimethylacetamide. J. occup. Med., 17: 506-511.
MEDYANKIN, A.V. (1975) [Complex action of dimethylformamide under
conditions of a long-term experiment.] Gig. i Sanit., 9: 39-42 (in
Russian).
MERKLE, J. VON. & ZELLER, H. (1980) [Studies on acetamides and formamides
for embryotoxic and teratogenic activities in the rabbit.]
Arzneimittelforschung, 30: 1557-1562 (in German).
MRAZ, J. & TURECEK, F. (1987) Identification of N-acetyl- S-
( N-methylcarbamoyl) cysteine, a human metabolite of N',N'-dimethyl-
formamide and N-methylformamide. J. Chromatogr., 414: 399-404.
MRAZ, J., MRAZ, M., SEDIVEC, V., & FLEK, J. (1987) [Gas chromatographic
determination of N-methylformamide in urine.] Pra. Lek., 39: 352-355
(in Czech).
MURAVIEVA, S.I. (1983) [Improvement of the methods for monitoring the
content of harmful substances in the air of worksite.] Gig. Tr. prof.
Zabol., 6: 39-41 (in Russian).
MURAVIEVA, S.I. & ANVAER, L.P. (1979) [Determination of dimethylformamide
and its metabolites in biological liquids by gas chromatographic method.]
Gig. Tr. prof. Zabol., 6: 58-59 (in Russian).
O'BERG, M.T., CHEN, J.L., & BURKE, C.A. (1985) Epidemiologic study of
workers exposed to acrylonitrile, an update. J. occup. Med., 27: 835-840.
ODOSHASHVILI, D.G. (1963) [Hygienic evaluation of atmospheric air
pollution with dimethylformamide.] In: [Literature on air pollution and
related occupational diseases.] Vol. 9, pp. 169-177 (in Russian).
PAOLETTI, A. & IANNACCONE, A. (1982) [Risk from dimethylformamide
intoxication in a plant for synthetic leather.] Ann. Ist. Super. Sanit.,
18: 567-570 (in Italian).
PAOLETTI, A., FABRI, G., & MASCI, O. (1982a) [Antabuse effect from
solvents: comparison between dimethylformamide and trichloroethylene.]
Ann. Ist. Super. Sanit., 14: 1099-1100 (in Italian).
PAOLETTI, A., FABRI, G., & MARINI BETTOLO, P. (1982b) [An isolated case
of "acute abdomen". Intoxication from dimethylformamide.] Minerva Med., 73:
3407-3410 (in Italian).
PARKHIE, M. & WEBB, M. (1983) Embryotoxicity and teratogenicity of
thalidomide in rats. Teratology, 27: 327.
PERRY, D.L., CHUANG, C.C., JUNGCLAUS, G.A., & WARNER, J.S. (1979)
Identification of organic compounds in industrial effluent discharges,
Athens, Georgia, US Environmental Protection Agency, Office of Research and
Development (Unpublished report No. EPA-600/4-79-016, NTIS PB-294794).
PHAM HUU CHANH, NGUYEN DAT XUONG, & AZUM-GELADE, M.-C. (1971) Etude
toxicologique de la formamide et de ses dérivés N-méthylés et N-éthylés.
Thérapie, 26:409-424.
PHAM HUU CHANH, AZUM-GELADE, M.-C., NGUYEN VAN BAC, & NGUYEN DAT XUONG
(1973) Cardiovascular activity of N,N-dimethylformamide. Toxicology, 1:
135- 142.
POIRIER, S.H., KNUTH, M.L., ANDERSON-BUCHOU, C.D., BROOKE, L.T., LIMA,
A.R., & SHUBAT, P.J. (1986) Comparative toxicity of methanol and N,N-
dimethylformamide to freshwater fish and invertebrates. Bull. environ.
Contam. Toxicol., 37: 615-621.
POTTER, H.P. (1973) Dimethylformamide induced abdominal pain and liver
injury. Arch. environ. Health, 27: 340-341.
PURCHASE, I.F.H., LONGSTAFF, E., ASHBY, J., STYLES, J.A., ANDERSON, D.,
LEFEVRE, P.A., & WESTWOOD, F.R. (1978) An evaluation of six short-term
tests for detecting organic chemical cancerogens. Br. J. Cancer, 37: 873-
903.
QIN, Y.H. & GUE, R.R. (1976) [Studies on the maximum allowalble
concentration of dimethylformamide in surface water.] J. Hyg. Res., 5(2):
161-167 (in Chinese).
REBHUN, L.J. & SAWADA, N. (1969) Augmentation and dispersion of the in
vivo mitotic apparatus of living marine eggs. Protoplasma, 68: 1-22.
ROMADINA, E.S. (1975) [Direct action of microorganisms - one way of
increasing the effectiveness of biological purification of waste waters.]
In: Telitchenko, M., ed. [Biological self-purification in water quality
management: Proceedings of the All-Union Symposium on Sanitary
Hydrobiology.] Moscow, Nauka, pp. 110-112 (in Russian).
SALA, C., BERNABEO, F., COLOMBO, G., INVERNIZZI, E., & MENEGHEL, G. (1984)
Dimethylformamide risk. An evaluation in the production of artificial
organic leather. Med. Lav. 6: 143-148.
SANOTSKY, I.V. & ULANOVA, I.P. (1975) [ Criteria for harmfulness in
hygiene and toxicology for evaluation of hazards from chemical compounds.]
Moscow, Medizina, 372 pp (in Russian).
SANOTSKY, I.V., MURAVIEVA, S.I., ZAEVA, G.N., ANVAER, L., & SEMILETKINA,
N.N. (1978) [Metabolism of dimethylformamide depending on the intensity
of its action.] Gig. Tr. prof. Zabol., 11: 24-27 (in Russian).
SANSONE, E.B. & TEWARI, Y.B. (1978) The permeability of laboratory gloves
to selected solvents. Am. Ind. Hyg. Assoc. J., 39(2): 169-174.
SANTA CRUZ, G. & CORPINO, P. (1978) [Preliminary morphological studies on
acute experimental inhalation exposure in the rat.] Boll. Soc. Ital. Biol.
Sper., 54: 1710- 1717 (in Italian).
SANTA CRUZ, G. & MACCIONI, A. (1978) [Experimental study on
dimethylformamide toxicity. Changes of the myocardium after prolonged
inhalation treatment in the rat.] Boll. Soc. Ital. Biol. Sper., 54: 1717-
1722 (in Italian).
SASAKI, S. (1978) The scientific aspects of the chemical substance
control law in Japan. In: Hutzinger, O., Lelyveld, L.H., van, & Zoeteman,
B.C.J., ed., Aquatic pollutants: Transformation and biological effects,
Oxford, New York, Pergamon Press.
SAVOLAINEN, H. (1981) Dose-dependant effects of peroral dimethylformamide
administration on rat brain. Acta neuropathol., 53: 249-252.
SCAILTEUR, V. (1984) Contribution à l'étude de la relation toxicité-
metabolisme de la dimethylformamide chez le rat. Université Catholique de
Louvain, 255 pp. (Thèse).
SCAILTEUR, V. & LAUWERYS, R. (1984a) In vivo and in vitro oxidative
biotransformation of dimethylformamide in rat. Chem.-biol. Interact., 50:
327-337.
SCAILTEUR, V. & LAUWERYS, R. (1984b) In vivo metabolism of
dimethylformamide and relationship to toxicity in the male rat. Arch.
Toxicol., 56: 87-91.
SCAILTEUR, V. & LAUWERYS, R.R. (1987) Dimethylformamide (DMF)
hepatotoxicity. Toxicology, 43: 231-238.
SCAILTEUR, V., HOFFMANN, E., BUCHET, J.P., & LAUWERYS, R. (1984) Study on
in vivo and in vitro metabolism of dimethylformamide in male and female
rats. Toxicology, 29: 222-234.
SCHEUFLER, H., VON, & FREYE, H.-A. (1975) [Embryotoxic and teratogenic
effects of dimethylformamide.] Dtsch. Gesundheitswes., 30: 455-459 (in
German).
SCHOTTEK, W. (1964) [Problems with the standardization of embryotoxic
substances.] Z. ärztl. Fortbild., 64: 1158-1162 (in German).
SCHOTTEK, W. (1970) [Experimental dimethylformamide toxicity studies on
experimental animals after repeated treatment.] Acta. biol. med. Germ., 25:
359-361 (in German).
SCHOTTEK, W. (1972) [Towards the problem of hygiene standardization of
chemicals having embryotoxic action.] In: Sanotsky, I.V., ed. [Hygine
standardization in study of remote effects of industrial substances.]
Moscow, Medizina, pp. 119-123 (in Russian).
SERRES, F.J., DE & ASHBY, J., ed. (1981) Evaluation of short-term tests
for carcinogens, Amsterdam, Oxford, New York, Elsevier Science Publishers,
827 pp (Progress in Mutation Research, Vol. 1).
SHARKAWI, M. (1980) Inhibition of alcoholdehydrogenase by dimethyl-
formamide and dimethylsulfoxide. Toxicol. Lett., 4: 493-497.
SHEVELEVA, G.A. & OSINA, S.A. (1973) [Experimental investigation of the
embryotropic action of dimethylformamide.] In: [ The toxicology of new
industrial chemicals.] Moscow, Medizina, Vol. 13, pp. 75-82 (in Russian).
SHEVELEVA, G.A., STREKALOVA, E.E., & CHIRKOVA, E.M. (1979) [Study of the
embryotropic, mutagenic, and gonadotropic effects of dimethylformamide
after inhibition exposure.] In: [ The toxicology of new industrial
chemicals.] Moscow, Medizina, Vol. 15, pp. 21-25 (in Russian).
SHLYGINA, O.E. & NEMOLCHEV, V.M. (1981) [The use of radio-isotope methods
in studies of acute poisoning with dimethylformamide.] In: [ Proceedings of
the Moscow Scientific Research Institute of First Aid.] Moscow, Medizina,
Vol. 45: 130-132 (in Russian).
SHUBAT, P.J., POIRIER, S.H., KNUTH, M.L., & BROOKE, L.T. (1982) Acute
toxicity of tetrachloroethylene and tetrachloroethylene with
dimethylformamide to rainbow trout, Salmo gairdneri. Bull. environ. Contam.
Toxicol., 28: 7-10.
SICKLES, J.E., WRIGHT, R.S., SUTCLIFFE, C.R., BLAKARD, A.L., & DAYTON, D.P.
(1980) Smog chamber studies of the reactivity of volatile organic
compounds, In: Proceedings of the 73rd Annual Meeting of the Air Pollution
Control Association, (Paper 80-50.1).
STOCKLEY, I.H. (1983) Drugs, foods, and environmental chemical agents
which can initiate Antabuse-like reactions with alcohol. Pharmacol.
Interact., 4: 12-16.
STRANSKY, V. (1986) The determination of N,N-dimethylformamide in working
atmosphere by the method of gas chromatography after sampling on activated
charcoal. Prac. Lek., 38: 15-19.
STULA, E.F. & KRAUSS, W.C. (1977) Embryotoxicity in rats and rabbits from
cutaneous application of amide type solvents and substituted ureas.
Toxicol. appl. Pharmacol., 41: 35-55.
TACCOLA, A., CATENACCI, G., & BARUFFINI, A. (1981) Cardiotoxicity of
dimethylformamide (DMF). Electrocardiographic findings and continuous
electro- cardiographic monitoring. G. Ital. Med. Lav., 3: 149-151.
TANAKA, K.I. (1971) Toxicity of dimethylformamide (DMF) to the young
female rat. Int. Arch. Arbeitsmed., 28: 98-105.
TANAKA, K.I. & UTSUNOMIYA, T. (1982) [The toxicity of N,N-
dimethylformamide (DMF).] Jpn. J. ind. Health, 24: 3-12 (in Japanese).
THIERSCH, J.E. (1962) Effects of acetamides and formamides on the rat
litter in utero. J. Reprod. Fertil., 4: 220.
TOLOT, F., DROIN, M., & GENEVOIS, J. (1958) Intoxication par la
diméthylformamide. Arch. Mal. prof., 19: 602-606.
TOLOT, F., ARCADIO, F., LENGLET, J.-P., & ROCHE, L. (1968) Intoxication
par la diméthylformamide. Arch. Mal. prof., 29: 714-717.
TOMASINI, M., TODARO, A., PIAZZONI, M., & PERUZZO, G.F. (1983) [Pathology
due to dimethylformamide. Observation of 14 cases.] Med. Lav., 74: 217-220
(in Italian).
TONOGAI, Y., OGAWA, S., ITO, Y., & IWAIDA, M. (1982) Actual survey on TLM
(median tolerance limit) values of environmental pollutants, especially on
amines, nitriles, aromatic nitrogen compounds and artificial dyes. J.
toxicol. Sci., 7: 193-203.
UNGAR, H., SULLMAN, S.F., & ZUCKERMAN, A.J. (1976) Acute and protracted
changes in the liver of Syrian hamsters induced by a single dose of
aflatoxin B1. Observations on pathological effects of the solvent
dimethylformamide. Br. J. exp. Pathol., 57: 157- 164.
US EPA (1986) Health and environmental effects profile for N,N-
dimethylformamide. Cincinnati, Ohio, US Environmental Protection Agency,
Health and Environmental Assessment, Environment Criteria and Assessment
Office, 106 pp (Unpublished data).
US NIOSH (1978) Occupational health guideline for dimethylformamide,
Cincinnati, Ohio, US National Institute for Occupational Safety and Health,
5 pp.
US NIOSH (1977) Manual of analytical methods, Cincinnati, Ohio, US
National Institute for Occupational Safety and Health, Vol. 3 (No. S-255).
WAHLBERG, J.E. & BOMAN, A. (1979) Comparative percutaneous toxicity of
ten industrial solvents in the guinea-pig. Scand. J. Work Environ. Health,
5: 345-351.
WALRATH, J., FAYERWEATHER, M.P.H., & GILBY, C.I.H. (1988) A case-control
study of cancer among Du Pont employees with potential for exposure to
dimethylformamide, Wilmington, Delaware, E.I. Du Pont de Nemours Co.
(Unpublished report).
WEISS, G. (1971) [Industrial dimethylformamide intoxication and the
question of its recognition as an occupational disease.] Zbl. Arbeitsmed.,
11.: 345-346 (in German).
WELWARD, L. & HALAMA, D. (1978) Influence of anti-microbial agents on
contamination and chlorotetracycline production. Folia microbiol., 23: 12-
17.
WICAROVA, O. & DADAK, O. (1981) [Urinary N-methylformamide levels in
persons exposed to dimethylformamide vapour.] Prac. Lek., 33: 42-46 (in
Czech).
WILES, J.S. & NARCISSE, J.K., Jr (1971) The acute toxicity of
dimethylformamides in several animal species. Am. Ind. Hyg. Assoc. J., 32:
539-545.
WILSON, H.K. & OTTLEY, T.W. (1981) The use of a transportable mass
spectrometer for the direct measurement of industrial solvents in breath.
Biomed. mass. Spectrom., 8: 606-610.
YONEMOTO, J. & SUZUKI, S. (1980) Relation of exposure to
dimethylformamide vapour and the metabolite, methylformamide, in urine of
workers. Int. Arch. occup. environ. Health, 46: 159-165.
RESUME ET EVALUATION, CONCLUSIONS, RECOMMANDATIONS
1. Résumé et évaluation
1.1 Propriétés générales
Le N,N-diméthylformamide (diméthylformamide, DMF, CSA 68-12-1)
est un solvant organique qui est produit en grandes quantités dans
l'ensemble du monde. On l'utilise dans l'industrie chimique comme
solvant, comme produit intermédiaire et comme additif. Le DMF est un
liquide incolore d'odeur légère mais désagréable qui, néanmoins, est
insuffisante pour attirer l'attention. Il est généralement stable
mais il peut entraîner des incendies et des explosions par contact
avec des oxydants forts, des halogènes, des dérivés alkylaluminiques
ou des hydrocarbures halogénés (en particulier combinés à des métaux).
Le DMF est entièrement miscible à l'eau et à la plupart des solvants
organiques. Sa tension de vapeur est relativement faible.
Du point de vue analytique, on a recours à la chromatographie
en phase gazeuse.
1.2 Transport, distribution, et transformation dans l'environnement
Le DMF est stable dans l'air ambiant mais il peut subir une
décomposition microbienne et algaire dans l'eau. Les microorganismes
adaptés et les boues activées assurent une biodégradation efficace du
DMF. Etant soluble dans l'eau en toutes proportions, le DMF est très
mobile dans les sols et il ne devrait pas s'accumuler dans la chaîne
alimentaire.
1.3 Concentration dans l'environnement et exposition humaine
Le DMF n'existe pas à l'état naturel. On ne possède que peu de
données sur sa concentration dans l'environnement ou sur l'exposition
de la population générale. Dans l'air de zones résidentielles situées
à proximité d'installations industrielles, on a trouvé des
concentrations allant de 0,02 à 0,12 mg/m3. Il est rare qu'on décèle
la présence de DMF dans l'eau des bassins fluviaux très industrialisés
et encore, les concentrations ne dépassent pas 0,01 mg/litre.
On ne possède pas de données sur la concentration du DMF dans le
sol, les végétaux, la faune sauvage et les produits alimentaires.
L'exposition professionnelle se produit par contact cutané avec le
liquide ou la vapeur ou par inhalation de la vapeur. On a décelé des
concentrations de 3 à 86 mg/m3 dans l'air avec des maxima allant
jusqu'à 600 mg/m3, au cours de travaux de réparation ou d'entretien de
machines. Dans quelques cas exceptionnels, des concentrations allant
jusqu'à 4500 mg/m3 ont été signalées.
1.4 Cinétique et métabolisme
Des quantités toxiques de DMF peuvent être absorbées par
inhalation ou pénétration percutanée. Une fois absorbé, le DMF se
distribue de façon uniforme dans l'organisme. Sa métabolisation a
lieu principalement dans le foie sous l'action des enzymes
microsomiales. Chez l'animal et l'homme, le principal produit de la
biotransformation du DMF est le N-hydroxyméthyl- N-méthyl-formamide
(DMF-OH). Au cours de l'analyse par chromatographie en phase gazeuse,
ce métabolite est transformé en N-méthyl-formamide (NMF) qui est lui-
même (ainsi que le N-hydroxyméthyl et le formamide) un métabolite
mineur du DMF. Par conséquent, lorsqu'on procède à des études
métaboliques et que l'on effectue la surveillance biologique du DMF,
les concentrations urinaires de métabolites sont mesurées et exprimées
en NMF, même si le DMF-OH en est le constituant essentiel. Le dosage
du NMF/DMF-OH dans les urines peut donner une bonne indication
biologique de l'exposition totale au DMF.
Chez l'animal d'expérience, on a montré que le mécanisme de
métabolisation du DMF se sature lorsque l'exposition est intense, le
DMF jouant le rôle de rétroinhibiteur de son propre métabolisme à
concentration très élevée.
Il y a interaction métabolique entre le DMF et l'éthanol.
1.5 Effets sur les êtres vivant dans leur milieu naturel
On n'a pas très bien étudié des effets du DMF sur l'environnement.
Il semble que sa toxicité pour les organismes aquatiques soit faible.
1.6 Effets sur les animaux d'expérience et les systèmes d'épreuve
in vitro
Le DMF présente pour diverses espèces une faible toxicité aiguë
(chez le rat la DL50 par voie orale est d'environ 3000 mg/kg, la DL50
dermique d'environ 5000 mg/kg et la CL50 par inhalation à peu près
égale à 10 000 mg/m3). Il est légèrement à modérément irritant pour
la peau et les yeux. D'après une étude sur des cobayes, il ne semble
pas doté de pouvoir sensibilisateur. Le DMF peut faciliter
l'absorption percutanée d'autres substances chimiques.
Chez l'animal d'expérience, l'exposition au DMF, quelle que soit
la voie de pénétration, peut provoquer des lésions hépatiques qui
dépendent de la dose. Lorsque l'exposition cesse, on a pu constater
qu'il y avait régénération des tissus. Certaines études on également
permis d'observer des signes de toxicité au niveau du myocarde et des
reins.
On n'a pas constaté de toxicité pour les testicules ou les
ovaires, ni observé d'effets sur la fécondité chez le rat. Chez le
rat, la souris et le lapin, le DMF s'est révélé embryotoxique et
faiblement tératogène. C'est le lapin qui est l'espèce la plus
sensible à l'exposition respiratoire: des effets tératogènes ont été
observés à partir de 1350 mg/m3 (450 ppm), aucun effet n'étant
constaté à 450 mg/m3 (150ppm). Après exposition de la peau, certaines
études ont mis en évidence de très rares effets embryotoxiques et
tératogènes à des doses journalières comprises entre 100 et 400 mg/kg.
De nombreuses épreuves à court terme à la recherche d'effets
génétiques et anomalies de ce genre, on a montré que le DMF était en
général inactif tant in vitro qu' in vivo.
Aucune étude convenable de cancérogénicité à long terme sur
animaux de laboratoire n'est décrite dans la littérature.
1.7 Effets sur l'homme
Aucun effet indésirable sur la population dans son ensemble n'a
été nettement mis en évidence.
On a fait état d'irritation cutanée et de conjonctivites après
contact direct avec du DMF.
Après exposition accidentelle à de fortes concentrations de DMF,
on note dans les 48h. des douleurs abdominales, des nausées, des
étourdissements et de la fatigue. La fonction hépatique peut être
perturbée et on a signalé des modifications de la tension artérielle,
une tachycardie et des anomalies du tracé électrocardiographique. En
général la récupération est totale.
Après des expositions réitérées sur une longue période, on observe
des symptômes tels que céphalées, perte d'appétit et fatigue. Des
signes biochimiques d'insuffisance hépatique peuvent également
s'observer. Des lésions hépatiques n'apparaissent, semble-t-il, qu'à
partir d'une exposition de l'ordre de 30 mg/m3, en l'absence de
contact cutané. Cette concentration atmosphérique correspond à
environ 40 mg de NMF/DMF-OH par gramme de créatinine dans un
échantillon d'urine prélevé à la fin du poste de travail.
Même à des concentrations inférieures à 30 mg/m3, l'exposition
au DMF peut causer une intolérance à l'alcool dont les symptômes
peuvent consister en rougeur soudaine de la face, sensation de
constriction thoracique et étourdissements, quelques fois accompagnés
de nausées et de dyspnée. Ces symptômes durent de 2 à 4 heures et
disparaissent spontanément.
On possède des preuves limitées d'une activité cancérogène du DMF
pour l'homme. C'est ainsi qu'une étude a fait état d'un accroissement
de l'incidence des tumeurs du testicule après exposition à du DMF,
tandis qu'une autre étude à révélé une incidence tumorale accrue au
niveau de la cavité buccale et du pharynx, mais pas au niveau des
testicules.
Deux études peu détaillées font état d'un accroissement de la
fréquence des avortements chez des femmes exposées à du DMF, entre
autres substances chimiques.
2. Conclusions
1. Compte tenu de ses usages actuels, la population dans son
ensemble, n'est probablement que très peu exposée au DMF.
2. Le DMF est facilement résorbé au niveau de la peau et des voies
respiratoires. Le dosage dans les urines du NMF/DMF-OH
constitue un moyen utile pour évaluer la quantité totale de DMF
absorbé.
3. Le risque de lésions hépatiques est faible si la concentration
du DMF dans l'air ambiant est maintenue en dessous de 30 mg/m3
et qu'il n'y a pas de contact cutané. La valeur correspondante
de la teneur des urines en NMF/DMF-OH à la fin du poste de
travail a été fixée provisoirement à 40 mg/g de créatinine.
4. Le DMF est embryotoxique et faiblement tératogène pour le rat,
la souris et le lapin.
5. Il existe des preuves limitées d'une cancérogénicité du DMF
pour l'homme.
6. D'après les données dont on dispose, le DMF est peu toxique
pour l'environnement. Il est peu probable qu'il donne lieu à
une bioaccumulation.
3. Recommandations
3.1 Précautions à prendre pour la manipulation
1. Maintenir la concentration atmosphérique au-dessous de 30 mg/m3
et éviter le contact avec la peau.
2. Surveiller la concentration urinaire de NMF/DMF-OH qui indique
l'exposition totale et la maintenir en-dessous de 40 mg de
NMF/g de créatinine dans les échantillons prélevés à la fin du
poste de travail. Si la concentration dépasse cette valeur,
prendre les mesures nécessaires pour réduire l'exposition.
3.2 Recherches à effectuer
1. Etudier les effets cancérogènes possibles du DMF chez l'homme,
par des études sur des populations humaines et des animaux
d'expérience.
2. Il faudrait disposer de données plus complètes sur la
possibilité d'extrapoler à l'homme les résultats des études
d'embryotoxicité et de tératogénicité effectuées sur l'animal.
Il serait bon d'étudier la cinétique comparée du DMF chez
l'homme et l'animal.
3. Davantage de données sont nécessaires sur le mode d'action et
l'activité relative des métabolites du DMF chez l'homme et
l'animal.
4. Il faudrait affiner les relations entre a) les concentrations
en métabolites urinaires et les taux d'exposition atmosphérique
(en l'absence de contact cutané), et b) la dose totale absorbée
par toutes les voies possibles (indiquée par la concentration
en NMF urinaire à la fin du poste de travail) et l'absence
d'hépatotoxicité.
RESUMEN Y EVALUACION, CONCLUSIONES, RECOMENDACIONES
1. Resumen y evaluación
1.1 Propiedades generales
La N,N-dimetilformamida (dimetilformamida, DMF, CAS
Wicarova & Dadak (1981) studied the relationship between the
amount of NMF in the shift urine (8 h) or the all-day urine (24 h) of
workers and DMF concentrations in the air (0-100 mg/m3) in an
artificial leather plant . The relationship was linear for the shift
urine samples. For the 24-h urine samples, the relationship was
linear only in the range of 0-80 mg DMF/m3 (see also Table 10).
When Dixon et al. (1983) studied the urinary NMF excretion in a
group of 32-37 workers who were exposed to similar air levels of DMF
for either 8 h per shift (5 days/week) or 12 h per shift (4
days/week), they found higher concentrations of NMF in the urine when
the workers were working 8-h shifts. A possible explanation was that
a 13% reduction in urine volume was seen in workers on 8-h shifts
during the summer months compared with higher urine outputs seen in
the same workers on 12-h shifts during the winter months.
Sala et al. (1984) found a correlation between urinary NMF levels,
4 h after workplace exposure, and the workers' exposure levels to DMF
in 5 job categories relating to artificial leather production. They
reported airborne DMF concentrations of 4.5-14 mg/m3 for spreading
"coagulate" system workers, with a mean NMF in urine of 16 mg/g
creatinine, 9.4 mg DMF/m3 for finishing workers, with a mean NMF
urinary value of 12 mg/g creatinine (low exposures), and 86.3 mg
DMF/m3 in tank cleaning workers with a corresponding urinary value of
188.3 mg NMF/g creatinine (highest exposure).
6.2.3.2 N,N-Dimethylformamide determination in the expired air
Airborne DMF concentrations change considerably during the work
shift and from one workplace to another. Brugnone et al. (1980a)
measured the DMF concentrations in the alveolar air every hour during
the 8-h shift of 8 workers employed in an artificial leather plant.
The alveolar DMF concentration in 6 workers was correlated with the
DMF concentration in the air of the respective workplaces.
In a second study, Brugnone et al. (1984) studied 8 exposed
workers by determining the DMF concentrations in the environmental
air, alveolar air, blood, and urine. Air samples were collected at
hourly intervals during an 8-h work shift, blood samples, at 2-h
intervals, and urine samples, at 4-h intervals. No DMF was found in
the blood or urine. A good correlation between the alveolar and
environmental DMF concentrations was found in 6 out of the 8 workers,
and at all subsequent hours, except for the fourth hour.
In practice, the alveolar air test is more difficult to perform
and use for routine examination than measurement of NMF levels in
urine samples, and is not recommended for biological monitoring.
6.2.3.3 Appraisal
The level of NMF in a post-shift urine sample is the most
appropriate biological parameter for total DMF exposure (inhalation
plus dermal) during the shift.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
The effects of DMF on organisms in the environment have been
reviewed by Kennedy (1986) and by US EPA (1986).
The LC50s of DMF for various aquatic species, given in Table 11,
indicate a low toxicity for the species tested.
DMF is commonly used to facilitate the solution of lipophilic
compounds in water during aquatic toxicity tests.
Cardwell et al. (1978) studied the long-term toxicity of DMF for
fathead minnow (Pimephales promelas), brown trout (Salvelinus
fontinalis), and bluegill (Lepomis macrochirus), and found threshold
limits of between 43 and 98 mg DMF/litre for the brook trout and
between 5 and 10 mg/litre for the fathead minnow. LeBlanc &
Surprenant (1983) showed that a level of 0.1 ml DMF/litre was
acceptable for long-term aquatic toxicity tests. In a study by
Tonogai et al. (1982), the 24-h and 48-h static median tolerance
limits for the Himedaka (Oryzias latipes) were > 1000 mg DMF/litre.
A no-observed-effect level (NOEL) of 7700 mg/litre was reported
for the rainbow trout by Shubat et al. (1982).
Solutions of DMF of 25 g/litre (2.5%) were shown to be lethal
within 0.5 h for eggs of sea urchins (Lythechinus variegatus, Arbacia
punctulata, Lythechinus pictus), the surf clam (Spisule solidissima),
and the annelid (Pectinaria) (Rebhun & Sawada, 1969).
Hughes & Vilkas (1983) determined that the highest concentration
that had no significant effect on the green alga Selenastrum
capricornatum, was 1 ml/litre and the no-effect level was 0.5
ml/litre.
Concentrations ranging from 0.085-0.340% DMF had an inhibitory
effect on cultures of Streptomyces aureofaciens (Welward & Halama,
1978).
Table 11. Medial lethal (LC50) concentrations (mg/litre) for aquatic
organisms exposed to dimethylformamide (DMF)
----------------------------------------------------------------------------------------------------
Species LC50 Reference
---------------------------------------
24-h 48-h 96-h
----------------------------------------------------------------------------------------------------
Guppy 1300 Dojlido (1979)
(Paecilia reticulata)
Rainbow trout 9800 Poirier et al. (1986)
(Salmo gairdneri) 9860 Shubat et al. (1982)
Fathead minnow 10 600 Poirier et al. (1986)
(Pimephales promelas)
Bluegill 7100 Poirier et al. (1986)
(Lepomis macrochirus)
Midge (Paratanytarsus 36 200 Poirier et al. (1986)
parthenogeneticus)
Daphnid (Daphnia magna) 14 500 Poirier et al. (1986)
12 300 LeBlanc & Surprenant
(approx.) (1983)
Larvae (Aedes aegypti) 68 000 Kramer et al. (1983)
(approx.)
----------------------------------------------------------------------------------------------------
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposures
Data on the acute toxicity of DMF in different laboratory animals,
when administered by different routes, have been reviewed by Kennedy
(1986). The acute toxicity in a number of species, following oral,
dermal, inhalation (Table 12), or parenteral (Table 13) administration
of DMF is relatively low, with lethal doses generally in the g/kg
range for the oral, dermal, and parenteral routes and in the g/m3 for
inhalation exposures. Animals given large single doses of DMF or
exposed to high air levels showed general depression, anaesthesia,
loss of appetite, loss of body weight, tremors, laboured breathing,
convulsions, haemorrage of the nose and mouth, liver injury, and coma
immediately preceding death.
In mice and rats, exposed to DMF via inhalation, signs of mucous
membrane irritation were seen (Lobanova, 1958; Lundberg et al., 1986),
and lung damage was detected histologically (Clayton et al., 1963).
Where tissue pathology was included in the study, the prominent
organ showing damage was the liver (Massmann, 1956; Sanotsky et al.,
1978; Mathew et al., 1980; Lundberg et al., 1981). No obvious species
differences were observed with regard to acute lethality, but young
rats appeared more sensitive to DMF-induced lethality than older rats
(Kimura et al., 1971).
8.2 Skin and eye irritation, sensitization
DMF was reported to be irritating to the eyes, mucous membranes,
and the skin (Hamilton & Hardy, 1974; Aldyreva & Gafurov, 1980).
8.2.1 Skin irritation
Rat tails dipped in DMF at 40 °C for 8 h became mummified in a few
days (Massmann, 1956).
A single application of 500 mg DMF/kg resulted in transient
irritation within 2-3 h in mice, but no irritation in rats (Wiles &
Narcisse, 1971). DMF was slightly irritating for mice at doses of
2500 and 5000 mg/kg. No skin irritation was detected in rabbits with
applications of 100, 200, or 500 mg DMF/kg. Single applications of
DMF on the skin of rats and guinea-pigs did not cause irritation
(Kiss, 1979; Bainova, 1985). Repeated 28-day treatments with 960 or
1920 mg/kg did not induce marked local dermal effects in rats (Bainova
et al., 1985).
Table 12. LD50 and LC50 values of DMF after oral, dermal, or
inhalation exposure in various animal species
----------------------------------------------------------------------------------------------------
Species Oral LD50 Dermal LD50 Inhalation LC50 Reference
(mg/kg) (mg/kg) (mg/m3)
----------------------------------------------------------------------------------------------------
Rat 3000 Thiersch (1962)
5000 9432 US NIOSH (1977)
3920 Massmann (1956)
11 140 12 000 Schottek (1970, 1972)
4000 Sanotsky et al. (1978)
> 11 520 Bainova & Antov (1980)
15 000 Clayton et al. (1963)
4320 Lazarev & Levina (1976)
11 000a Stula & Krauss (1977)
> 13 440 Lundberg et al. (1986)
3200 Qin & Gue (1976)
14 000 Cai & Huang (1979)
7170 Bartsch et al. (1976)
Mouse 3950 Lazarev & Levina (1976)
5550 Lazarev & Levina (1976)
> 5000 Wiles & Narcisse (1971)
6420 Bartsch et al. (1976)
3700 6000-9400 Lobanova (1958)
5400, 6200 Qin & Gue (1976)
18 300 Cai & Huang (1979)
Rabbit > 5000 > 500 Wiles & Narcisse (1971)
1500a Stula & Krauss (1977)
Mongolian gerbil 3929 Llewellyn et al. (1974)
----------------------------------------------------------------------------------------------------
a Pregnant females.
Table 13. LD50s (mg/kg body weight) of DMF after parenteral administration
in various animal species
----------------------------------------------------------------------------------------------------------
Species Intraperitoneal Intravenous Intramuscular Subcutaneous Reference
----------------------------------------------------------------------------------------------------------
Rat 1480 4030 Massmann (1956)
2500 Thiersch (1962)
4440 2830 Bartsch et al. (1976)
4600 Pham Huu Chanh et al. (1971)
5470 Shottek (1970, 1972)
Mouse 300 Massmann (1956)
3500 3800 4500 US NIOSH (1977)
650 Barral-Chamaillard
& Rouzioux (1983)
1454 Burgun et al. (1975)
2000 Antoine et al. (1983)
3150 2800 Wiles & Narcisse (1971)
5200 Pham Huu Chanh et al. (1971)
5850 3490 Bartsch et al. (1976)
Rabbit 945 1800 Massmann (1956)
1000 Wiles & Narcisse (1971)
5000 US NIOSH (1977)
Guinea-pig 1300 Wahlberg & Boman (1979)
1030 US NIOSH (1977)
4000 Ungar et al. (1976)
Dog 470 Barral-Chamaillard
& Rouzioux (1983)
Cat 500 Massmann (1956)
----------------------------------------------------------------------------------------------------------
After repeated application of DMF to the skin of guinea-pigs for
21 days (Bainova, 1985), the mean irritative dose was 31% DMF (range
17-56%).
Dermal irritation was not seen in rabbits treated dermally with 2
g DMF/kg for 6 h, daily 15 times during a 4-week period (Kennedy,
1986).
8.2.2 Eye irritation
A 25% (25 g/litre) solution of DMF in water, injected into the
conjunctival sac of the rabbit, did not produce any effects; 50% was
slightly irritating, and 75-100% produced more severe irritation
(Massmann, 1956). Single dose DMF instillation (0.1 ml) produced
moderate corneal damage and conjunctival redness that was most
pronounced at 2-3 days. By day 14, a mild degree of conjunctival
redness, moderate corneal damage with an area of severe injury, slight
surface distortion, and subsurface vascularization were observed
(Kennedy & Sherman, 1986). In another study, the same authors
reported that, after a single DMF instillation, the eye inflammation
subsided and disappeared by the 8th day.
8.2.3 Sensitization
DMF was tested, using a maximization technique, on guinea-pigs to
determine skin sensitization; it did not induce any response (Bainova,
1985).
8.3 Repeated exposure
The effects of repeated oral, dermal, or inhalation exposure to
DMF in various animal species have been reviewed by Kennedy (1986) and
these data, together with other new information are summarized in
Table 14. In all species tested, except the dog, liver damage was
produced, the degree of damage generally being proportional to the
dose administered. In the two reported studies on the dog (Clayton et
al., 1963; Kimmerle & Eben, 1975a), the inhalation exposure conditions
appeared to be too low (60 mg/m3) to produce damage, though 1 out of
the 4 dogs tested by Clayton did have altered liver function tests.
Higher levels were not tested. Some evidence of recovery from the
hepatotoxic effects of DMF was found in rats (Kennedy & Sherman,
1986).
Higher, intermittent doses of DMF appeared to produce more
pronounced effects in male rats than continuous dosing (Bainova et
al., 1981a; Bainova, 1985). Tanaka (1971) found more pronounced liver
damage in rats following one rather than three weeks of exposure and
considered that the high regenerative capacity of liver tissue was
responsible for the observation.
Other tissues and organs that are affected, particularly by high
doses of DMF, will be discussed in section 8.4.
8.4 Specific organ toxicity
8.4.1 Liver
The potency of DMF as a hepatotoxic agent has been reviewed by
Kennedy (1986) and by Scailteur & Lauwerys (1987). The effects of DMF
on the liver were studied after single or repeated inhalation, dermal,
or oral treatment of rats, mice, and rabbits (Massmann, 1956; Clayton
et al., 1963; Shottek, 1970; Tanaka, 1971; Kimmerle & Eben, 1975a;
Medyankin, 1975; Sanotsky et al., 1978; Germanova et al., 1979; Mathew
et al., 1980; Bainova et al., 1981a; Lundberg et al., 1981; Lundberg,
1982; Brondeau et al., 1983; Bainova, 1985; Kennedy & Sherman, 1986;
Scailteur & Lauwerys, 1987). Single oral administrations of 2250-5000
mg DMF/kg in rats (Kennedy & Sherman, 1986) caused clay-coloured
liver, congestion, and centrilobular necrosis of hepatocytes. Lower
doses resulted in deviations in liver function, such as decreased
excretion of cholic acid in the bile, bromosulfthalein retention,
increased serum activities of GOT, GPT, LAP, OCT, AlcP, ChE, LDH, and
gamma-GT, and significant enhancement of cholesterol, triglyceride,
and bilirubin contents in the serum and liver homogenates. In rats,
following both intraperitoneal (ip) and inhalation exposure, there
were no increases in SDH levels at 420 and 840 mg/m3 but a lower level
(210 mg/m3) raised the serum activity of SDH (Lundberg et al., 1986).
Pathomorphological investigation demonstrated lipid degeneration and
cloudy swelling of hepatocytes in the central zones of the lobules
followed by signs of regeneration.
DMF at 0.6 ml/kg, administered intraperitoneally, caused mild
changes in rat liver lobules. Marked centrilobular necrosis and
central phlebitis were found in the rats treated with single ip doses
of 0.9 and 1.2 ml DMF/kg (Mathew et al., 1980). A single ip dose of
0.5 ml DMF/kg to hamsters caused centrilobular necrosis accompanied by
haemosiderosis (Ungar et al., 1976). Morphological changes were
reported in the liver by Clayton et al. (1963), Shottek (1970), Tanaka
(1971), and Santa Cruz & Corpino (1978) after repeated DMF exposure of
young animals, with periodic peaks (Table 14).
Table 14. Effects of repeated oral, dermal, or inhalation exposure to DMF in various animal species
________________________________________________________________________________________________________
Species Route of Dose Duration Effects Reference
exposure
________________________________________________________________________________________________________
Mongolian oral 10 000 mg/kg 30 days no changes in body weight, liver, Llewellyn
gerbil drinking-water or kidney et al. (1974)
10 000 mg/kg 200 days mortality in 25% of animals;
drinking-water liver necrosis
17 000 mg/kg 80 days mortality with liver necrosis;
drinking-water LD50 cumulative 90 206 mg/kg
body weight
34 000 mg/kg 6 days mortality with liver necrosis;
drinking-water LD50 cumulative 3846 mg/kg body
weight
Mouse oral 620 or 1240 30 days anorexia, loss of body weight Qin & Gue
mg/kg diet (1976)
160, 540, 119 days dose-related increase in relative Becci et al.
1850 mg/kg diet and absolute liver weights; no (1983)
other histological or biochemical
changes; NOEL, 246-326 mg/kg
diet per day
Rat oral 320 or 640 30 days anorexia, loss of body weight Qin & Gue
mg/kg diet (1976)
50, 500, 5000 100 days body weight decrease; liver Qin & Gue
mg/litre damage at 5000 mg/litre; (1976)
drinking-water increase in liver to body weight
ratio at 500 and 5000 mg/litre;
structural liver changes and
regeneration at 5000 mg/litre;
NOEL, 50 mg/litre
Table 14 (continued)
________________________________________________________________________________________________________
Species Route of Dose Duration Effects Reference
exposure
________________________________________________________________________________________________________
Rat oral 102, 497, 1000 14 days no behavioural changes at 102 or Savolainen
mg/litre 49 days 497 mg/litre for 49 days; dose- (1981)
drinking-water related deviations in cerebral
and glial cell enzyme activities
215, 750, 2500 104 days dose-related increase in relative Becci et al.
mg/kg diet and absolute liver weights, (1983)
considered to be physiological
adaptation; NOEL, 210-235 mg/kg
diet per day
200, 1000, 5000 90 days slight anaemia and leukocytosis, Kennedy &
mg/kg diet hypercholesterolaemia at 1000 and Sherman (1986)
(equivalent to 5000 mg/kg diet; NOEL, 200 mg/kg
12, 60, 300 mg/ diet
kg/body weight
per day)
0.1, 0.5, 1.0 14 days dose-related increase in liver/ Elovaara
g/litre in 49 days body weight ratios; in liver et al.
drinking-water and kidneys, increased values of (1983)
reduced glutathione, microsomal
UDP glucuronosyl transferase,
and ethoxycoumarin O-demethylase
activities; no changes in liver
microsomal cytochrome P-450 or
ADPH-cytochrome reductase
activity
Rat dermal 470 mg/kg per 30 days continuous dosing caused Schottek
day for 29 days hepatoxicity and did not protect (1970)
and 11 140 mg/kg against lethality; pretreatment
on the 30th day did not enhance toxic reactions
after application of the LD50
in 30-day pretreated rats
Table 14 (continued).
________________________________________________________________________________________________________
Species Route of Dose Duration Effects Reference
exposure
________________________________________________________________________________________________________
215, 430, 960, 30 days dose-related changes in GOT, Bainova &
or 4800 mg/kg GPT, AlcP, ChE, gamma-GT, lipid Antov (1980)
per day fractions in serum and liver
homogenates; NOEL, 215 mg/kg
Rat dermal 215, 320, 960, 30 days dose-related changes (at doses Bainova (1985)
or 4800 mg/kg > 320 mg/kg) in enzyme
activities per day in liver,
myocardium, and kidney
homogenates; NOEL, 215 mg/kg
Rat dermal 960 mg/kg daily 28 days functional, biochemical, and Bainova et al.
or 1920 mg/kg pathomorphological changes in (1981a)
applied liver; and lipid metabolism Bainova (1985)
intermittentlya on the 4th, 8th, 14th, and 28th
day of the tests; changes more
pronounced after intermittent
exposure
4-h dipping of 60 days concentration-related changes Medyankin
tails in 60, 65, in liver and nervous system; (1975)
70, or 80% DMF NOEL, 60% DMF in water
in water
4-h dipping of 120 days no changes at 30% DMF contact and Medyankin
tails in 5 mg DMF/m3 inhalation; adverse (1975)
30 or 60% DMF effects at other concentrations
and inhalation of
5 or 10 mg DMF/m3,
6 h daily
Table 14 (continued).
________________________________________________________________________________________________________
Species Route of Dose Duration Effects Reference
exposure
________________________________________________________________________________________________________
Rabbit dermal 50, 100% water 7 days died at 5-8 day of application Huang et al.
solution, 3 at 100% DMF; liver biochemical (1981)
times/day, 2 ml/ and histological changes
application
2000 mg/kg per 9 days anorexia, cyanosis, and mortality Kennedy &
day with liver necrosis Sherman (1986)
Guinea dermal 50, 75, 100% 7 days died 2-4 days after application of Huang et al.
-pig solution, 75 or 100% and 4-9 days after 50%; (1981)
3 times/day, loss of body weight; liver damage
2 ml/application
Rat inhalation 1800 mg/m3 for 6 days concentration-related mortality; Schottek
6 h daily cumulation of hepatoxic effect (1970)
750 and 1500 6 days
mg/m3, 6 h daily
30 mg/m3 for 8 days no changes in the function of Sanotsky &
6 h daily the thyroid or adrenal glands Ulanova (1975)
aerosol for 3 or liver and kidney necrosis, lung Santa Cruz &
0.5 h daily 30 days changes, arterial changes in Corpino (1978);
(concentration myocardium Santa Cruz &
unknown) Maccioni (1978)
22 ± 1.6 mg/m3 18 weeks liver changes, no other responses Cai & Huang
for 6 h daily, (1979)
6 days a week
Table 14 (continued).
________________________________________________________________________________________________________
Species Route of Dose Duration Effects Reference
exposure
________________________________________________________________________________________________________
Rat inhalation 130 mg/m3 for 27 days functional changes in kidneys Germanova et al.
4 h daily and liver; arterial blood pressure (1979)
more pronounced after additional
300 mg/m3 in 27 days single administration of 500 mg
5 peaks of DMF/kg on the 1st, 8th, and
15 min at 27th days of the studies, and
40-min intervals after intermittent exposure
7569 mg/m3 5 days weakness, weight loss, Kennedy &
for 6 h daily dehydration, liver necrosis Sherman (1986)
Young rat inhalation 600 mg/m3 for 28 days increased serum GOT and GPT; Tanaka (1971)
(3-12 8 h daily morphological liver changes,
weeks old) mainly in 3-week-old rats;
no histological abnormalities
in other organs
Young rat inhalation 600 mg/m3 for 28 days liver changes at the 1st, 2nd, Tanaka (1971)
(3 weeks 8 h daily and 3rd, and 4th week of test more
old) 600 mg/m3 for intense in the group exposed
1 h daily for 8 h daily; no cumulation of
hepatoxic effect
Table 14 (continued).
________________________________________________________________________________________________________
Species Route of Dose Duration Effects Reference
exposure
________________________________________________________________________________________________________
Rat, inhalation 450, 900, 1800, 60 days increased serum GOT, GPT, AlcP, Craig et al.
mouse 3600 mg/m3 cholesterol, anaemia and (1984)
for 6 h daily histological liver changes at
900 mg/m3 or more; liver weight
increase at 450 mg/m3; NOEL
below 450 mg/m3 in both species
Rat, cat inhalation 300, 690, 1350 120 days anorexia, weight loss, liver Massmann
mg/m3, 8 h daily degeneration, and necrosis; (1956)
changes in brain, myocardium,
and kidneys; no abnormalities
in blood tests or ECG
Rabbit inhalation 22 ± 1.6 mg/m3 18 weeks no changes in ECG or liver Cai & Huang
for 6 h daily, parameters (1979)
6 days per week
317 ± 37.8 mg/m3 14 weeks body weight changes; liver damage Cai & Huang
for 6 h daily, functionally and structurally; by (1979)
6 days a week congestion and haemorrhage
120 mg/m3 for 50 days microscopic and electron- Arena et al.
8 h daily microscopic changes in the (1982)
myocardium
Dog inhalation 60 mg/m3 for 107 days reversible changes in blood Clayton et al.
6 h daily pressure, ECG, and in liver (1963)
functions
63 mg/m3 for
6 h daily 28 days normal GOT, GPT, bilirubin, urea, Kimmerle & Eben
and creatinine in plasma; (1975a)
NOEL, 63 mg DMF/m3
___________________________________________________________________________________________________________
a Two alternative intermittent regimes were used: (i) 1920 mg/kg per day for 2 days, followed by no
treatment for 2 days; (ii) 1920 mg/kg every second day.
Diets supplemented with DMF at levels of 215, 750, or 2500 mg/kg
for 104 days for rats and 160, 540, or 1850 mg/kg for 119 days for
mice, resulted in significant dose-related increases in relative liver
weights in all experimental animals. No deviations were reported in
the serum activities of GOT, GPT, AlcP, other than an increase in GPT
activity in mice fed a diet containing 1850 mg DMF/kg. Histo-
pathological evaluation did not reveal any hepatotoxicity (Becci et
al., 1983). The oral administration of a 10% water solution of DMF
(400 mg/kg body weight) for 14 days (Leshik & Feoktistova, 1984) to
guinea-pigs significantly decreased the ascorbic acid content and the
concentration of cytochrome P-450 in the liver. The daily intake of
0.1, 0.5, or 1.0 g DMF/litre in the drinking-water for 2 or 7 weeks
(Elovaara et al., 1983) increased the liver/body weight ratios, the
microsomal UDP-glucuronosyl-transferase and 7-ethoxycoumarin- O-
demethylase activity, and reduced the glutathione concentration in
liver homogenates.
No liver injury was seen following inhalation exposure of dogs to
63 mg DMF/m3 (Kimmerle & Eben, 1975a). However, in another study, 1
out of 4 dogs exposed to 60 mg/m3 showed increased enzyme values
(Clayton et al., 1963)). Liver injury was also not seen: after
inhalation exposure of rats at levels of < 450 mg/m3 (Craig et al.,
1984), after dermal exposure of rats at a level of 240 mg/kg (Bainova,
1985), and at dietary levels of 215 mg/kg body weight per day for rats
and 160 mg/kg for mice (Becci et al., 1983).
8.4.2 Gastrointestinal tract
Toxic gastroenteritis with pathomorphological deviations was
described in the experimental animals treated at high doses or
concentrations in studies reported in Table 14 (Massmann, 1956;
Clayton et al., 1963; Shottek, 1970).
8.4.3 Cardiovascular system
Microscopic examination did not reveal any myocardial lesions in
rats and mice after ingestion of dietary levels of 215, 750, or 2500
mg DMF/kg for 104 days, or 160, 540, or 1850 mg DMF/kg for 119 days,
respectively (Becci et al., 1983).
Clayton et al. (1963) and Germanova et al. (1979) reported
decreases in blood pressure in dogs (not cats) following exposure to
DMF. The changes described were not great and, in the absence of
confirmatory data in other test models (and in man), are of
questionable significance. Large iv doses (500 mg/kg) did not produce
any changes in the contractile force of myocardial tissue in dogs
(Pham Huu Chanh et al., 1973).
Santa Cruz & Maccioni (1978) described histological changes in the
myocardium of the rat and Clayton et al. (1963) described subtle blood
pressure changes in the dog. The findings in the rat followed high
exposures; the blood pressure changes in the dog were minimal and hard
to differentiate from those in control animals.
8.4.4 Kidney
Swelling of the kidney tubules occurred after a single oral
administration of 2250-5000 mg DMF/kg in rats (Kennedy & Sherman,
1986). Short-term feeding studies in rats and mice (Table 14) did not
reveal any histopathological lesions in the kidneys (Becci et al.,
1983; Kennedy & Sherman, 1986).
A number of pathomorphological studies revealed vacuolar
degeneration, mainly in the renal tubules (Massmann, 1956; Clayton et
al., 1963; Santa Cruz & Corpino, 1978; Lundberg et al., 1983). Costa
et al. (1978) observed histological, histochemical, and electron-
microscopic renal lesions in groups of rats, exposed to DMF aerosols
(dose not stated) for 1 h/day for 15 days or for 0.5 h/day for 30
days. Degeneration took place in the proximal part of tubules and in
the visceral epithelium of the glomerulus with marked mitochondrial
changes.
Repeated inhalation exposure to 130 or 300 mg DMF/m3 increased the
kidney/body weight ratio, and decreased diuresis, and the total
protein, sodium chloride, and potassium contents in the urine of rats
(Germanova et al., 1979).
Elovaara et al. (1983) reported enhanced activities of 7-ethoxy-
coumarin- O-demethylase, and UDP-glucuronosyltransferase, and a
decrease in cytosolic formaldehyde dehydrogenase activity in rats
orally exposed to DMF. Bainova & Antov (1980) and Bainova et al.
(1981b) reported that the 30-day dermal application of 960 or 4800 mg
DMF/kg resulted in dose-related increased activities of SucDH, G-6-
PDH, and LDH in rat kidney homogenates.
8.4.5 Nervous system
Functional changes in the nervous system were observed after
administration of high doses or exposure to high concentrations of DMF
(Massmann, 1956; Clayton et al., 1963) and after prolonged treatment
with moderate doses of DMF (Medyankin, 1975; Sanotsky et al., 1978;
Germanova et al., 1979; Bainova, 1985) (Table 14). Doses within the
range of lethal levels resulted in anaesthesia, depression, or coma.
Moderate doses caused inhibition of motor activity.
No effects on the behaviour of rats were noted after they drank
DMF in the drinking-water for 2 and 7 weeks at doses ranging from 1.4
to 13.7 mmol/litre with a cumulative dose of 3200 mg/kg in the rats
(Savolainen, 1981). The same dose enhanced the activities of acid
proteinase and 2,3-cyclicnucleotide-3'-phosphohydrolase in the glial
cells.
Massmann (1956) and Clayton et al. (1963) observed
pathomorphological changes in the brains of experimental animals after
treatment with high doses of DMF (Table 14).
8.4.6 Lungs
Lung congestion and oedema were found in rats after single oral
application of 2250-5000 mg DMF/kg (Kennedy & Sherman, 1986).
Bronchopneumonic changes were observed in experimental animals
after inhalation of high DMF concentrations (Massmann, 1956; Clayton
et al., 1963; Shottek, 1970; Santa Cruz & Corpino, 1978) (Table 14).
According to the authors, the changes were related to injury of the
small arterial vessels and, to some extent, to local irritation caused
by DMF.
8.4.7 Haematopoietic system
High levels of DMF might cause some anaemia, but no other changes
in the erythrocytes of experimental animals have been reported
(Massmann, 1956; Clayton et al., 1963; Sanotsky et al., 1978;
Germanova et al., 1979; Bainova & Antov, 1980) (Table 14). Depressed
bone marrow activity was reported in rats after a single oral
administration of 2250-5000 mg DMF/kg (Kennedy & Sherman, 1986). Pham
Huu Chanh et al. (1971) found leukocytosis in rats after repeated ip
injections of DMF.
Pathomorphological changes were observed in the spleen after
exposure to high doses of DMF. They were accompanied by an increase
in the spleen/body weight ratio (Massmann, 1956; Clayton et al., 1963;
Bainova & Antov, 1980; Bainova, 1985). Medyankin (1975) found
inhibited phagocytosis activity of leukocytes and decreased glycogen
content in the neutrophiles of rats as a result of combined dermal and
inhalation exposure to DMF.
8.4.8 Adrenals
Clayton et al. (1963) observed histological changes in the adrenal
glands after inhalation of DMF. Decreased ascorbic acid content in
the adrenals of rats was reported by Germanova et al. (1979), during
intermittent and continuous inhalation exposure to DMF (Table 14).
8.4.9 Gonads
Male rats were exposed to 584-616 mg DMF/m3 or 49-51 mg/m3 for 4 h
daily for 2, 4, or 8 days; female rats were exposed to 2.3 or 10.7 mg
DMF/m3, for 4 h daily, for 30 days (Sheveleva et al., 1979).
Examination of the sperm and the histological study of the testes and
ovaries did not show any pathological signs.
Lewis et al. (1979) exposed male rats at 90 or 900 mg DMF/m3, for
6 h daily, over 5 days. Gross and histological examination of the
testes did not reveal any pathological changes.
The histological examination of male rats, treated orally in
short-term studies (Becci et al., 1983; Kennedy & Sherman, 1986) with
a variety of doses (Table 14), did not result in changes in the
testes. No lesions were noted in the male rats after a 30-day dermal
application of DMF (Bainova, 1985). Craig et al. (1984) did not find
testicular or ovarian lesions in rats and mice after short-term
inhalation exposure to DMF at concentrations of up to 3600 mg/m3
(Table 14).
Examination of the gonads in a large number of the acute and
repeated-dose toxicity studies discussed earlier did not reveal them
to be a target for DMF toxicity.
8.5 Developmental toxicity and reproduction
DMF was investigated for developmental toxicity in mice, rats, and
rabbits using the oral, dermal, and inhalation routes and parenteral
injection. According to present-day requirements, most of the older
studies were not adequately designed or described. A survey is given
in Tables 15, 16, 17.
No 3-generation reproduction studies were available.
8.5.1 Developmental toxicity
8.5.1.1 Mouse
Administration by gavage of 580 or 193 µl DMF/kg per day, from day
6 to 15 after conception, to 26 mice per dose group led to a dose-
dependent decrease in fetal weights and an increase in the number of
retardations and variations. At 580 µl/kg per day, 17 out of 241
fetuses were malformed (cleft palate, exencephaly, hydro-cephalus
internus, aplasia of presphenoid). No maternal effects were recorded;
the number of live fetuses remained unchanged. At 193 µl/kg, 4 out of
245 fetuses showed malformations. In untreated control groups for
each dose group, 2 out of 229 and 1 out of 310 fetuses, respectively,
had a cleft palate (Hellwig et al., in press).
After an ip injection of 600 or 1100 mg DMF/kg per day, from day 1
to 14 after conception, embryotoxic and teratogenic effects were
registered. The malformation rates were 18 and 75%, respectively, and
the effects consisted of absence or retardation of posterior skull
ossification, open eye-lids, cerebral oedema, sternal haematomas, and
spina bifida-like defects in the thoracic region. Embryotoxic effects
recorded were late resorptions. Doses of 170 mg/kg per day from day 1
to 14 after conception, and 250 mg/kg per day from day 6-14, and
single doses of 2100 mg/kg each on days 3, 7, 9, or 11 after
conception, did not produce any effects (Scheufler & Freye, 1975).
In another ip injection study on mice, 6 animals per dose group
were treated with 0.4 or 1.0 mg DMF/kg per day from day 11 to 15 after
conception. Maternal body weight gain was reduced at 1.0 mg/kg per
day, 2 out of 6 animals had abortions, 7 out of 36 fetuses showed
exencephaly and 1 had a cleft palate. No effects were observed at 0.4
mg/kg (Hellwig et al., in press).
The studies indicate that DMF may be teratogenic for mice.
Table 15. DMF administration to pregnant mice
----------------------------------------------------------------------------------------------------
Route Dosea Maternal Embryotoxicity Malformations Reference
toxicity
----------------------------------------------------------------------------------------------------
Gavage control- - - Hellwig et al.
193 µl/kg - fetal weights 4 of 245 living (in press)
(gavage; day decreased fetuses showed
6-15 pc) malformations
580 µl/kg NRb fetal weights 17 of 241 living
(gavage; day decreased fetuses showed
6-15 pc) malformations
Intra- control - - - Scheufler &
peritoneal 170 mg/kg NRb - - Freye (1975)
injection (day 1-14 pc)
250 mg/kg NRb - -
(day 6-14 pc)
600 mg/kg NRb late malformation
1100 mg/kg resorptions rates 18 and 75%
(day 1-14 pc) respectively
Intra- 0.4 mg/kg - - - Hellwig et al.
peritoneal (day 11-15 pc) (in press)
injection 1.0 mg/kg + 2/6 abortions 8/36
(day 11-15 pc) malformations
----------------------------------------------------------------------------------------------------
a pc = Post conception.
b NR = Not reported.
Table 16. DMF administration to pregnant rats
---------------------------------------------------------------------------------------------------------
Route Dosea Maternal Embryotoxicity Malformations Reference
toxicity
---------------------------------------------------------------------------------------------------------
Gavage control - - - Hellwig et al.
(day 6-15 pc)
533 µl/kg - some embryo-
lethality in the
early phase, +
reduced fetal
weights
+ retardations
+ variations
1600 µl/kg weight 63% embyro- 12% of the 85
stationary lethality in living fetuses
between the median were malformed
day 6-15 phase
Dermal day 6-10 and Hellwig et al.
0 µl/kg - - -
100 µl/kg - - 2.46%
500 µl/kg - - 3.05%
1000 µl/kg - slight reduced 5.46%
fetal length (increase in rib
and vertebral
abnormalities)
Dermal control - - - Stula &
600 mg/kg reduced slight Krauss (1977)
(days 9, 10 + weight embryolethality
11, 11 + 12, gain
12 + 13 pc)
1200 mg/kg reduced slight
(day 10 + 11 pc) weight embryolethality -
gain
Table 16. (contd.)
---------------------------------------------------------------------------------------------------------
Route Dosea Maternal Embryotoxicity Malformations Reference
toxicity
---------------------------------------------------------------------------------------------------------
Dermal 1200 mg/kg reduced slight
(contd.) (day 12 + 13 pc) weight embryolethality -
gain
2400 mg/kg stationary embryolethality -
(day 10 + 11 pc) weight
400 or 200 reduced high embryo- -
mg/kg (applied weight lethality
6 times/day gain
days 11 + 12
+ 13 pc)
Inhalation control - - - Hellwig et al.
(day 0-1, 4-8, weight weights
11-15, 18-19 pc) gain resorptions
861 mg/m3 (287 ppm) reduced reduced fetal -
(day 0-3, 6-10, weight weights
13-18 pc) gain retardations
resorptions
variations
660 mg/m3 (220 ppm) - reduced -
(day 4-8 pc) weight and
length
1560 mg/m3 (520 ppm) reduced embyro- -
(day 4-8 pc) weight lethality;
gain reduced weights
Table 16. (contd.)
---------------------------------------------------------------------------------------------------------
Route Dosea Maternal Embryotoxicity Malformations Reference
toxicity
---------------------------------------------------------------------------------------------------------
Inhalation control - - - Kimmerele &
54 mg/m3 (18 ppm) - - - Machemer
516 mg/m3 (172 ppm) - reduced fetal - (1975)
(6-15 day pc) weights
Inhalation control - - - Keller & Lewis
903 mg/m3 (301 ppm) reduced ossification
(day 6-15 pc) weight variations
gain slightly increased
from 60 to 75% -
Inhalation control - - - Shottek
1200 mg/m3 (400 ppm) total resorp- - (1964)
(4 h/day, 10-20 day pc) NR tions in some
animals, no
retardations
Inhalation 0.05 mg/litre ca.48 mg/m3 weight - Sheveleva &
(ca. 16 ppm) NR decrease Osina (1973)
(day 0-20 pc)
0.8 mg/litre ca.600 mg/m3 embryolethality; -
(ca. 200 ppm) NR weight decrease
Intravenous control - - - Parkie & Webb
injection 0.5 g/kg on - - (1983)
days 10, 11, NR
or 12 pc)
---------------------------------------------------------------------------------------------------------
a pc = Post conception.
b NR = Not reported.
Table 17. DMF administration to pregnant rabbits
---------------------------------------------------------------------------------------------------------
Route Dosea Maternal Embryotoxicity Malformations Reference
toxicity
---------------------------------------------------------------------------------------------------------
Gavage control - - - Merkle &
46.4 µl/kg - - 1 hydrocephalus Zeller (1980)
(day 6-18 pc)
68.1 µl/kg - decrease in number 3 hydrocephalus
(day 6-18 pc) of implantations
and % living
implantations
200 µl/kg decrease in decrease in +
(day 8-16 pc) food intake, fetal weight, 16 fetuses in
weight gain, 3 abortions, 7 litters were
and placental placental weight malformed:
weight decrease
Dermal control - - - Stula &
200 mg/kg some - Krauss (1977)
(day 8-16 pc) embryolethality
Dermal control 100 mg/kg - - - Hellwig et al.
occlusive litter skeletal
(day 6-28 pc) anomalies; 3.3%
agenesia of gall
bladder
Dermal 200 mg/kg per day - - -
400 mg/kg per day decrease in 23.5% fetuses
body weight per litter sternal
on day 16 and anomalies; 2.45%
18 hernia umbilicalis,
6.08% agenesia
of gall bladder
Table 17 (contd.)
---------------------------------------------------------------------------------------------------------
Route Dosea Maternal Embryotoxicity Malformations Reference
toxicity
---------------------------------------------------------------------------------------------------------
Inhalation Control - - - Hellwig et al.
50 ppm)
(day 7-19 pc)
450 mg/m3 decrease in increase in 1 hernia
(150 ppm) body weight sternal umbilicalis
gain variations (50 fetuses)
1350 mg/m3 decrease in decrease in incidence of hernia
(450 ppm) body weight weights, umbilicalis
gain increase in increased;
variations skeletal and soft
(pseudoanky- tissue anomalies
losis)
---------------------------------------------------------------------------------------------------------
a pc = Post conception.
8.5.1.2 Rat
In a gavage study on groups of 26 rats administered 1600 µl DMF/kg
per day from day 6 to 15 after conception, maternal toxicity was
observed in the form of decreased body weight. Sixty-three percent of
the implantations were resorbed and 12% of the surviving 85 (36.64%)
fetuses were malformed (9 cases of diffuse anasarca, 2 cases of tail
aplasia, 1 micrognathia, furthermore several fetuses had anomalies of
the ribs, sternum, and vertebral column). A dose of 533 µl DMF/kg per
day caused some early fetal deaths, in the absence of clinical signs
of maternal toxicity, reduced fetal weight, and also some
malformations, as well as an increase in variations and skeletal
retardations. The malformations consisted of: 2 cases of tail
aplasia, 2 cases of cleft palate, 1 atresia ani, 1 anasarca, 1 open
eye, and several fetuses with split and aplastic vertebrae. At 176
µl/kg, decreased placental weights and some decreases in fetal length
and increases in fetal weight were seen. All other parameters were
within the range of biological variability (Hellwig et al., in press).
The dermal studies on rats did not fulfil today's criteria for a
valid study.
In one series of studies (Hellwig et al., in press), 0, 100, 500,
or 1000 µl DMF/kg per day (undiluted material) were administered in an
uncovered dermal system from day 6 to 10 and then from day 13 to 15
after conception (26 animals per dose group; 10 in the control group).
Under these conditions, 1000 µl/kg per day caused a slightly retarded
weight gain among the dams and significant dermal irritation. The
fetuses were slightly smaller. Malformations consisted of split
thoracic vertebrae and anomalies of the ribs. The rate of the
malformations in live fetuses was 0% per litter in the controls and
2.46%, 3.05%, and 5.46% with increasing dose level. This may indicate
a weak dose-related teratogenic effect.
In another study (Stula & Krauss, 1977), rats received dermal
doses of up to 2400 mg (undiluted DMF)/kg per day, at least every 2
days, from day 9 to 13 after conception, under non-occlusive
conditions. There was clear evidence of embryolethality at 2400 mg/kg
per day on gestation days 10 and 11 (26%, i.e., 7 rats) and at 1200
and 2400 mg/kg per day (in 6 portions of 200 and 400 mg) on gestation
days 11, 12, and 13 with incidences of 43 and 30%, respectively.
Maternal weight gain and average fetal weights were also suppressed.
Fetal abnormalities were not observed, with the exception of a few
subcutaneous haematomas, which occurred at a rate also seen in
historical controls at this laboratory.
Several inhalation studies were carried out on rats. In one
study, 23 animals per dose group were exposed to 54 or 516 mg DMF/m3
(18 or 172 ppm) over 6 h per day from day 6 to 15 after conception.
There were 22 animals in the control group. The higher exposure level
caused a decrease in fetal weights in the absence of signs of maternal
toxicity. No effects were seen on the numbers of implantations,
resorption rates, placental weights, number of fetuses weighing less
than 3 g (runts), variations in skeletal development or malformations;
the lower exposure level (54 mg/m3) did not cause any adverse effects
(Kimmerle & Machemer, 1975).
In another rat study, exposure to 903 mg/m3 (301 ppm) for 6 h per
day, from day 6 to 15 after conception (19 animals per group) led to a
reduction in maternal weight gain and to a slightly increased
incidence of skeletal (ossification) variations of from 60 to 75%.
Exposure to 96 mg/m3 (32 ppm) did not produce any effect (Keller &
Lewis, 1981).
Following 10 exposures to 1200 mg/m3 (400 ppm) for 4 h per day,
from day 10 to 20 after conception, dead implants (54% total
resorptions compared with 15% in the controls) occurred. The numbers
of animals in the treated and control groups were not reported
(Shottek, 1964).
Fetal weight decreases and fetal deaths were reported in another
study on rats after exposure to 600 mg/m3 (200 ppm) from day 0
throughout the gestation period. Exposure to 48 mg/m3 (16 ppm) was
said to have caused reduced fetal weights. However, the description
of the study was inadequate (Sheveleva & Osina, 1973).
A series of inhalation studies on rats is described (Hellwig et
al., in press) in which the exposure periods did not fully cover the
critical period of the gestation phase (e.g., "window dosing" or non-
exposure during weekends, see Table 17). In one set of these
experiments, exposure to concentrations of 660 and 1560 mg/m3 (220 and
520 ppm), for 6 h per day (days 0-3, 6-10, 11-18 after conception; 18
animals per group) caused decreased fetal weights, retardations, and
an increase in embryolethality. Another exposure regimen, i.e., 861
mg/m3 (287 ppm), for 6 h per day, was administered on days 1, 4-8,
11-15, and 18-19 after conception to a group of 30 rats. Twenty
animals were subjected to caesarian section on day 20 after
conception, the offsprings of the other 10 animals were raised until
day 21 after birth. Thirty rats served as untreated controls. There
was retarded maternal weight gain from the beginning of the treatment;
fetal weights were decreased, and the numbers of variations and
retardations were increased. No malformations were found.
No effects were detected after single iv injections of 0.5 g/kg
body weight between days 10, 11, or 12 after conception (Parkie &
Webb, 1983). Furthermore, earlier investigations on rats after single
ip or sc injections did not give any indication of teratogenicity;
however, such studies are of limited value for a toxicity assessment.
The above studies indicate that DMF is embryotoxic in the rat.
After dermal and oral administration, teratogenicity may also occur.
8.5.1.3 Rabbit
DMF caused maternal toxicity and embryotoxicity, including
teratogenicity, in rabbits after administration by gavage at 200 µl/kg
per day from day 6-18 after conception. All 11 animals in the dose
group became pregnant and showed reduced food intake and weight gain.
Placental weights were significantly lower and 3 abortions occurred.
The fetuses showed weight reduction. The main findings recorded on
fetal examination were hernia umbilicalis (7 cases), hydrocephalus
internus (6 cases), eventratio simplex (3 cases), exophthalmus (2
cases), cleft palate (1 case), and malposition of limbs (1 case). The
number of implantations was not adversely influenced. At 68.1 µl/kg
per day, no maternal effects were observed among 16 pregnant animals
(18 inseminated); decreased numbers of total implantations and of live
fetuses occurred, also an increase in skeletal variations and
retardations per litter; 3 cases of hydrocephalus internus were
present. At 46.4 µl/kg per day (10/12 does were pregnant) 1 case of
hydrocephalus internus occurred; all other parameters were in the
range of biological variability. No malformations occurred in the
untreated control group (22/24 animals) (Merkle & Zeller, 1980).
In a dermal study, 5 rabbits (4 animals in the control group)
received 200 mg DMF/kg per day, dermally, from day 8 to day 16 after
conception. The test material was applied in undiluted form on the
intact skin, apparently under non-occlusive conditions. Embryo
mortality was 6% compared with 3% in the controls. The average fetal
weight was 32.9 g in the litters of treated animals compared with 28.4
g among controls. Fetal abnormalities were not detected (Stula &
Krauss, 1977).
In another dermal study (Hellwig et al., in press), dose levels of
undiluted DMF of 0, 100, 200, or 400 mg/kg per day were applied for 6
h/day, under semi-occlusive conditions (15 animals per dose group). A
5-6% decrease in maternal body weights occurred in the highest dose
group, towards the end of the treatment period (days 16-18 after
conception). At this dose level, an increase in skeletal (sternal)
malformations was found in 15 fetuses in 7 litters investigated
(23.5%), and also 5 cases of missing gall bladder (in 2 litters). No
malformations occurred in animals in the 200 mg/kg per day group or in
the untreated control group. At 100 mg/kg per day, one fetus had a
sternal anomaly, 2 fetuses had gall bladder agenesis, and one of the
latter a hypertrophic-dilatative cardiac-aortic malformation.
In a recent inhalation study, exposure levels were 0, 150, 450,
and 1350 mg/m3 (0, 50, 150, and 450 ppm) over 6 h per day from day 7
to day 19 after conception (fifteen animals per dose group). Animals
in the highest exposure group showed a slight retardation in body
weight gain as a sign of maternal toxicity. The fetal weights were
significantly lower in this group, and there was a significant
increase in malformations, mostly hernia umbilicalis (7 out of 86
fetuses, in 4 out of 15 litters) and some soft-tissue malformations,
such as missing gall bladder, without statistical significance. In
addition, anomalies of the sternum, an increase in split vertebrae,
and a number of variations were also recorded. At 450 mg/m3, maternal
body weights were slightly retarded during the exposure period and the
corrected body weight gain was marginally, but significantly,
decreased. One case of hernia umbilicalis among 75 fetuses and an
increase in sternal variations were observed. At 150 mg/m3, neither
fetuses nor does showed any indications of response to treatment. In
summary, signs of embryotoxicity and teratogenicity were seen at the
highest concentration (1350 mg/m3) and to a lesser degree at 450
mg/m3. The maternal toxicity seen at 1350 mg/m3 and 450 mg/m3 is in
accordance with the maternal toxicity observed at 900 mg/m3 in a
previous range-finding study (Hellwig et al., in press).
These studies indicate that DMF may be teratogenic for rabbits.
8.5.1.4 Appraisal
The overall conclusion from all studies is that DMF may lead to
embryotoxic and teratogenic effects in rats, mice, and rabbits. An
increase in malformations in the absence of maternal toxicity is
clearly visible after gavage and ip administration, with a smaller
incidence after dermal administration. In general, the rabbit
appeared to be more sensitive to the teratogenic effects of DMF than
the rat. After inhalation exposure, fetal toxicity and teratogenic
effects appear to be confined to conditions of maternal toxicity.
8.6 Mutagenicity and related end-points
DMF has been tested extensively in mutagenicity and genotoxicity
assays. DMF was one of the 42 chemicals selected for study in the
International Collaborative Program for the Evaluation of Short-Term
Tests for Carcinogenicity (Serres & Ashby, 1981).
The genetic toxicity studies on DMF have been reviewed by Purchase
et al. (1978), Kennedy (1986), US EPA (1986), and IARC (1989).
8.6.1 In vitro studies
In different in vitro assays, DMF did not induce mutations or
genotoxic effects (Table 18). Negative results were obtained with
both in Salmonella typhimurium and Escherichia coli. DMF did not
induce unscheduled DNA synthesis, sister chromatid exchanges,
chromosomal aberrations, or gene mutation in mammalian cells, or
mitotic gene conversion or crossing over in yeasts (Serres & Ashby,
1981).
Table 18. Short-term genotoxicity tests on DMF ( in vitro )
------------------------------------------------------------------------------------------------------------
Method Concentration Condition; comment Results Reference
------------------------------------------------------------------------------------------------------------
Ames test 0.65 x 10-5-1.3 x 10-3 TA 98, 100, 1535, - Antoine et al.
mol/litre 1537, 1538, (1983)
with and without
liver microsome
B. subtilis maximum dose 20 mg/disk - S9, + S9 (rat, yellow - Serres &
Spore rec-assay tail, clam) Ashby (1981)
E. coli differential highest concentration WP2, WP67, CM871 - Serres &
killing test 30 µl/plate Ashby (1981)
E. coli rec-assay highest concentration 1 g/ml 2921, 9239, 8471, 5519, - Serres &
7623, 7689 Ashby (1981)
DNA polymerase deficient 100 µl/ml + S9 and - S9 - Serres &
assay Ashby (1981)
E. coli W3110 -
P3478
Yeast mutation S. pombe - Serres &
Ashby (1981)
S. cerevisiae (XV185-14C) - Serres &
Ashby (1981)
Mitotic recombination S. cerevisiae (JD1) + Serres &
Ashby (1981)
Mitotic crossing-over 10-1000 µg/ml S. cerevisiae (T1, T2) - Serres &
assay Ashby (1981)
Mitotic aneuploidy lowest effective S. cerevisiae (D6) - S9 + Serres &
concentration 100 µg/ml Ashby (1981)
Mitotic gene conversion 5 µl/ml S. cerevisiae (D7) + S9 - Serres &
Ashby (1981)
Mitotic gene conversion 500 µg/ml S. cerevisiae (JD1) - Serres &
Ashby (1981)
Repair test using yeast minimum effective S. cerevisiae (wild & rad) + Serres &
strains (cell growth concentration Ashby (1981)
inhibition) (MEC) 300 µg/ml
Nuclear enlargement 0.01-100 µg/ml human fibroblasts - Serres &
8-200 µg/ml HeLa cells + Ashby (1981)
Table 18. (contd.)
------------------------------------------------------------------------------------------------------------
Method Concentration Condition; comment Results Reference
------------------------------------------------------------------------------------------------------------
UDS test 1.1-90 µg/ml (-S9) human fibroblasts - Serres &
2-30 µg/ml (+S9) (WI - 38) -, + S9 Ashby (1981)
0.032-100 µg/ml human fibroblasts - Serres &
(from skin biopsies) Ashby (1981)
0.1-100 µg/ml HeLa cells - Serres &
Ashby (1981)
Sister chromatid exchange 0.00625-0.1% CHO cells (-, + S9) - Serres &
(SCE) Ashby (1981)
0.1-100 µg/ml CHO cells - Serres &
Ashby (1981)
RL Chromosome assay 75-300 µg/ml Rat liver epithelial type - Serres &
cell line (RL1) Ashby (1981)
Mouse lymphoma 46.9-3000 µg/ml Mouse lymphoma cells - Serres &
mutagenesis assay (L5178Y) Ashby (1981)
-, + S9 rat liver
Human fibroblast 0.2-0.5 mg/ml human lung fibroblast - Serres &
Diphtheria toxin (HSC172) Ashby (1981)
Resistance test
Cell transformation test 500 µg/ml Baby hamster kidney cells + Serres &
(BHK21C13/HRC1) Ashby (1981)
BHK-21 cell - Serres &
Ashby (1981)
Integration enhancement 0.005-0.5 C3H2K cell - Serres &
test (MLV Test) Ashby (1981)
Cytogenetic analysis 1.1 x 10-2-1.1 human peripheral - Antoine et al.
mol/litre lymphocytes (1983)
Cytogenetic analysis 10-20% human peripheral + Koudela &
lymphocytes Spazier (1979)
------------------------------------------------------------------------------------------------------------
Comment: Positive results were obtained with very high concentrations, such as 100-500 µg/ml and 10-20%.
In one study, DMF did not induce any increase in chromosomal
aberrations or sister chromatid exchange in human peripheral blood
lymphocytes in vitro (Antoine et al., 1983). However, in another
study, chromosomal aberrations were reported in human peripheral
lymphocyte cultures treated with DMF (Koudela & Spazier, 1979). The
authors performed cytogenetic analyses of human peripheral lymphocytes
treated with DMF (dilution from 10-7 to 10-2 mol/litre. Compared
with the positive control, thio-TEPA, the clastogenic activity of DMF
was 3- to 4-fold lower. Chromosome aberrations were concentration-
related at DMF levels of 10-20%.
Cytogenetic analysis of peripheral lymphocytes in 40 workers
exposed to 35-180 mg DMF/m3 was performed, first at 4-month
intervals, and later at 6-month intervals. Increased frequencies of
non-specified chromosomal aberrations of 3.82 and 2.74%, respectively,
were found (Koudela & Spazier, 1981). In further sampling periods,
after technological adjustments to decrease the DMF exposure to about
30 mg/m3, the authors established lower frequencies of cell
aberrations in most of the workers, i.e., 1.59, 1.58, and 1.49%, in
the various periods under study. The aberrant cells in the control
group were 1.61-1.10% (Koudela & Spazier, 1979).
8.6.2 In vivo studies
In in vivo studies, DMF was negative in dominant lethal
mutagenic assays, tests for chromosome aberrations and sperm
abnormalities, and micronucleus tests, (Table 19).
8.6.3 Appraisal
The results obtained in the in vitro and in vivo test systems
showed that DMF did not induce damage in genetic material.
8.7 Carcinogenicity
The carcinogenic activity of DMF has not been examined in 2-year
studies on test-animals (Purchase et al., 1978; Barral-Chamaillard &
Rouzioux, 1983; Kennedy, 1986; US EPA, 1986). However, there are some
data concerning DMF, applied as a solvent, and for shorter periods of
time.
In a study by Druckrey et al. (1967) two groups of 15 and 5 BD
rats were treated, with 75 and 150 mg DMF/litre in the drinking-water
for 500 and 250 days, respectively (total doses 38 000 mg/litre in
drinking-water). The animals were observed for up to a maximum of 750
days, with an average survival of 532 days. Similarly, two groups of
12 rats each were given weekly, subcutaneous injections of 200 and 400
mg DMF/kg (total doses 8000 and 20 000 mg/kg, respectively) and
observed for 732 and 766 days, respectively. No tumorigenic effects
were reported in this small group of rats.
Table 19. Short-term genotoxicity tests of DMF ( in vivo )
--------------------------------------------------------------------------------------------------------------
Method Animal Dose route Results Reference
--------------------------------------------------------------------------------------------------------------
Dominant lethal male rat 90, 900 mg/m3 - Lewis et al.
mutagenic bioassay inhalation 6 h daily (1979)
for 5 consecutive days
Chromosome male and 2.3-600 mg/m3 - Sheveleva
aberrations female rat inhalation et al. (1979)
Micronucleus test BALB/C mouse 0.2-2000 mg/kg ip - Antoine
et al. (1983)
Micronucleus test B6C3F1 mouse 80% of LD50 ip - Serres & Ashby (1981)
Micronucleus test ICR mouse 0.425-1.7 mg/kg ip - Serres & Ashby (1981)
Micronucleus test CD-1 mouse 0.4-1.6 mg/kg ip - Serres & Ashby (1981)
Sperm morphology (CBA x BALB/c)F1 0.1-1.5 mg/kg ip - Serres & Ashby (1981)
assay male mouse
--------------------------------------------------------------------------------------------------------------
Kommineni (1973) reported that 9 out of 18 male, and 11 out of 19
female rats, developed tumours in different organs following ip
administrations of 100 mg DMF per rat, once a week, for 10 weeks. The
incidence of tumours in male control rats was 4 out of 14 and, in
females, 5 out of 14, also in different organs. No specific organ or
tumour type predominated in either the test or control group. Three
testicular tumours were seen, a bilateral interstitial cell tumour in
the controls, and an interstitial cell tumour and an embryonal cell
carcinoma in the test group.
8.8 Induction of tumour cell differentiation
Borenfreund et al. (1975) reported a decrease in the malignancy
of the Friend erythroleukaemic cells and a marked increase in their
differentiation along the erythroid pathway after their treatment with
0.5 and 1% DMF solutions.
DMF induction of cell differentiation and a marked reduction of
tumorigenicity was established by Dexter (1977) in transplantable
murine rhabdomyosarcoma cells. In another study, Dexter & Hager
(1980) used 4 carcinoma cell lines derived from two specimens of
adenosarcoma of the human sigmoid colon and showed changes in the
carcinoma cells towards less malignant cell types. Hager et al.
(1980) demonstrated that a cultured human colon carcinoma cell
responded to DMF by more differentiated development, again suggesting
an antitumour effect.
Chakrabarty et al. (1984) studied the effects of DMF on AKR-2B and
AKR-MCA cells in vitro and found that complete loss of anchorage
independent growth occurred and the reduced expression of membrane
antigens was restored.
The DMF induction of tumour cell differentiation has been studied
by Kimball & Hixon (1983) in relation to the deviation of the nuclear
protein. Cordeiro & Savarese (1984) studied it in relation to the
effects on cysteine/glutathione metabolism, and Levine et al. (1985)
in relationship to the changes in receptor occupation and growth
factor responsiveness. Chen et al. (1986) studied the induction of
tumour cells by DMF in relation to the rate of nucleoside transport in
the cells.
A review of the studies on the effects of the induction of
alkylformamides on terminal differentiation of tumour cells (Langdon &
Hickman, 1987) shows that DMF should not be used for such purposes.
The anti-neoplastic activity of DMF, determined in vitro and in vivo,
does not appear to be sufficient for therapeutic use.
8.9 Mechanism of toxicity, mode of action
Several hypotheses on the possible mechanism of DMF hepatotoxicity
have been tested. No experimental support for lipid peroxidation,
lysosome labilization, or glutathione depletion has been reported.
The critical biological effects leading to DMF hepatotoxicity have not
been identified and still need to be elucidated (Scailteur & Lauwerys,
1987).
9. EFFECTS ON HUMAN BEINGS
9.1 General population exposure
No effects of DMF on the general population have been reported.
9.2 Occupational exposure
Reports of occupational poisonings with DMF have been reviewed by
Kennedy (1986), US EPA (1986), and Scailteur & Lauwerys (1987).
9.2.1 Accidental poisoning
Several cases of acute accidental occupational poisoning with DMF
have been reported (Tolot et al., 1968; Potter, 1973; Chary, 1974;
Chivers, 1978; Aldyreva & Gafurov, 1980; Kang-de & Hui-lan, 1981;
Shlygina & Nemolshev, 1981; Paoletti et al., 1982a,b). They were
caused by the malfunctioning of the equipment, splashing of the
organic solvent over the body, or working in plants without taking
protective measures. Over-exposure has occurred via the skin and/or
inhalation. Usually, the symptoms appeared from several hours up to
several days after the accident. The major symptoms were epigastric
or abdominal pain, which was irradiating and progressive, accompanied
by dizziness, nausea, anorexia, vomiting, fatigue, alcohol
intolerance, and skin irritation. Clinical laboratory tests showed
liver function disturbance. Radioisotope diagnostic tests and liver
biopsy revealed morphological changes in the liver. No clinical
manifestations of renal dysfunction were reported. The patients
recovered with symptomatic therapy in hospital for 2-3 weeks. Liver
function tests returned to normal. Some of the patients, who were
followed for several months or several years after the acute
poisoning, had normal function tests.
9.2.2 Long-term exposure
After occupational exposure to DMF (intensity and length of
exposure unspecified, no control groups), eye irritation, headache,
anorexia, gastrointestinal disturbances, and sometimes hepatomegaly
with biochemical signs of liver damage were reported. Some
haematological changes were also observed (Tolot et al., 1958; Weiss,
1971; Dilorenzo & Grazioli, 1972). In studies in which exposure was
quantified, subjective complaints of headache, fatigue, and
gastrointestinal and cardiovascular changes were reported.
Disturbances of liver function could be measured by changes in plasma
bilirubin levels, and increases in the serum activity of liver enzymes
(transaminases, alkaline phosphatase, glutamyl-transpep-tidase).
Alcohol intolerance occurred. Haematological changes and ECG
deviations were also observed (Table 20).
In a questionnaire study, for which little detail is available,
Schottek (1972) reported 14% miscarriages in a group of women exposed
to about 100 mg DMF/m3 compared with 10% in the control group. No
statistical analysis was performed. Aldyreva & Gafurov (1980)
reported perturbations in menstruation in 26 out of 70 women who had
been exposed to 30-150 mg DMF/m3 for about a year. No data are
available on controls. On the basis of company statistics, general
morbidity associated with gynaecological changes appeared to be
increased among DMF-exposed women.
Farquharson et al. (1983) reported miscarriages in 3 out of 9
women, who had been exposed to DMF as well as a number of other
chemicals.
Because of its effect on the stratum corneum, DMF interferes with
the barrier function of the skin; this was demonstrated in human
volunteers by increased water loss following DMF exposure (Baker,
1968).
9.2.3 Epidemiological studies on carcinogenicity
Ducatman et al. (1986) reported three cases of testicular germ-
cell tumours in 1981-83 among 153 white men who repaired the exterior
surfaces and electrical components of F4 Phantom jet aircraft, in the
USA. This finding led to surveys of two other repair shops at
different geographical locations; in one of the shops, the same type
of aircraft was repaired, while in the other, different types of
aircraft were repaired. Four out of 680 white male workers in the
same type of repair shop had a history of testicular germ-cell cancers
(0.95 expected) occurring in 1970-83. No case of testicular germ-cell
cancer was found among the 446 white men employed at the facility
where different types of aircraft were repaired. Of the 7 cases of
testicular germ cell tumours, 5 were seminomas and 2 were embryonal-
cell carcinomas. All 7 men had long histories of working in aircraft
repair. The time from first exposure to diagnosis ranged from 4 to 19
years. There were many common exposures to solvents in the three
facilities, the only exposure identified as unique to the F4 Phantom
jet aircraft repair facilities, where the cases occurred, being to a
solvent mixture containing 80% dimethyl-formamide (20% unspecified).
No quantitative exposure data exist. Three of the cases had certainly
been exposed to this mixture and 3 cases, probably exposed. The cases
were found through foremen and from filed death certificates, and the
authors suggested that under-reporting was possible. No other cases
of cancer were investigated.
Levin et al. (1987) described 3 cases of embryonal-cell carcinoma
of the testes in workers at one leather tannery in the USA, all of
whom had worked as swabbers on the spray lines in leather finishing.
The latency period was from 8 to 14 years. According to the authors,
all the tanneries surveyed used dimethylformamide, as well as a wide
range of dyes, solvents, and other chemicals. No quantitative
exposure data are available. The number of workers from which these 3
cases arose was not given, and other cancers were not looked for.
Table 20. Studies on workers with long-term exposures
---------------------------------------------------------------------------------------------------------------------
Number Number Length DMF exposure Urinary Hepato- Alcohol Other effects Reference
of of non- of (mg/m3) NMF toxicity intoler-
exposed exposed exposure ance
subjects controls (years)
---------------------------------------------------------------------------------------------------------------------
22 28 5 1-47 (usually 20-63 - + NR Lauwerys
< 30; gloves mg/g et al. (1980)
worn) creatinine
11 - 3 3-15 0.4-20 - +(6)a NR Yonemoto &
mg/24 h Suzuki (1980)
28 29 3-5 30-60 NR - NR complaints of eye and Hinkova et al.
respiratory tract (1980)
irritation; no haemato-
logical changes
115 67 1-1.5 30-150 with NR + NR complaints of gastro- Aldyreva &
higher peaks + (a few out intestinal tract or Gafurov (1980)
skin exposure of 29) cardiovascular and
ovarian distur-
bances (29)
177 - 3-5 10-30 NR - NR complaints of Aldyreva &
cardiovascular Gafurov (1980)
disturbances (45)
81 96 3.5 < 10 NR +(10) NR complaints of gastro- Kang-de &
accidental intestinal tract and Hui-Lan (1981)
peak levels up cardiovascular
to 4525 + skin disturbances, ECG
exposure changes
23 - 2 > 30 peaks 10-40 NR NR No ECG changes com- Taccola et al.
up to 150 mg/day pared with pre-exposure (1981)
27 237 2 2-80 peaks NR +(2) +(8) complaints of gastro- Paoletti &
up to 549 intestinal tract Iannaccone
disturbances (15); (1982)
headache (6)
---------------------------------------------------------------------------------------------------------------------
Table 20. (contd.)
---------------------------------------------------------------------------------------------------------------------
Number Number Length DMF exposure Urinary Hepato- Alcohol Other effects Reference
of of non- of (mg/m3) NMF toxicity intoler-
exposed exposed exposure ance
subjects controls (years)
---------------------------------------------------------------------------------------------------------------------
13 - < 4 14-60 NR +(2) +(8) complaints of gastro- Tomasini
(mean: 29) intestinal tract et al (1983)
disturbances (8); eye
irritation (11); kidney
function test and
haematology, normal
26 54 > 5 2-5 NR - NR NR Catenacci
(mean: 3) et al.(1984)
28 54 > 5 12-25 NR - NR NR Catenacci
(mean: 18) et al. (1984)
100 100 5 8-58 NR + +(39) complaints of headache, Cirla et al.
(mean: 22) (8 versus eye and throat (1984)
2 controls) irritation, gastro-
intestinal tract and
cardiovascular
disturbance
24 29 5 10-60 NR NR NR irritative dermatitis Bainova (1985)
15 28 6-10 20-30 NR NR NR increased coagulation Imbriani
(median 27) time et al. (1986)
---------------------------------------------------------------------------------------------------------------------
a Incidence is indicated when available.
NR = not reported
To evaluate the significance of this cluster, an analysis of the
New York State Cancer Registry was conducted. Occupations were
determined from cancer registries and from death certificates for all
residents in Fulton County who were diagnosed as having testicular
cancer from 1974-88. From preliminary results, it is estimated that
workers who are employed in the leather tanning industry are 5-6 times
more likely to develop testicular cancer than those who are not
leather workers. However, the testicular cancer rates in this county
were lower than expected within this period, and an adjacent county
showed the same number of cases of testicular cancer, none of the
affected individuals having ever worked in the leather industry
(Walrath et al., 1988).
O'Berg et al. (1985) and Chen et al. (1988a) studied the cancer
incidence among 2530 actively employed workers with potential exposure
to dimethylformamide between 1956 and 1984, 1329 employees with
exposure to dimethylformamide and acrylonitrile at an acrylic fibre
manufacturing plant in South Carolina, USA, and 1130 controls from the
same plant. Cancer incidence rates for the company (1956-84) and USA
national rates (1973-77) were used to calculate the expected number of
cases. For all workers exposed to DMF (alone or with acrylonitrile),
the standardized incidence ratio (SIR), based on company rates for all
cancers combined, was 110 [95% confidence interval (CI), 88-136]a (88
cases); the SIR on the basis of national rates was 92. The SIR for
cancer of the buccal cavity and pharynx was 344 [CI, 172-615]a (11
cases), on the basis of company rates, and 167, on the basis of US
rates. More cancer cases than expected from company rates (34 cases:
SIR 134 [CI, 98-195]a) were found among employees exposed to
dimethylformamide alone, due mainly to 8 carcinomas of the buccal
cavity and pharynx versus 1.0 expected (SIR, 800 [CI, 345-1580]a).
All of these cases either smoked or chewed tobacco, but no information
was available on the smoking, tobacco chewing, or drinking habits of
the cohort. An additional case occurred among employees exposed to
dimethyl-formamide alone (SIR, 167); 4 of these tumours were cancers
of the lip. No such excess was found among the workers exposed to
both dimethylformamide and acrylonitrile (2 observed; SIR, 125, on the
basis of company rates). The authors reported that there was no
association with intensity or duration of exposure: "low" and
"moderate" exposure SIR 420 (5 cases); "high" exposure SIR 300 (6
cases). "Low" exposure conditions included: no direct contact with
liquids containing any dimethylformamide, even wearing protective
equipment; and workplace air levels consistently below 30 mg DMF/m3
(10 ppm) (no odour of DMF evident). "Moderate" exposure conditions
included: intermittent contact with liquids containing > 5%
dimethylformamide; workplace air levels sometimes higher than 30 mg
DMF/m3 (10 ppm) (more than once per week); DMF-laden materials
handled, but air levels of DMF maintained at above levels. "High"
exposure conditions included: frequent contact with liquids containing
> 5% dimethylformamide; workplace air levels often > 30 mg DMF/m3
(> 10 ppm); use of breathing protection often required for periods of
15 min-1 h; DMF vapour levels frequently > 30 mg/m3 (> 10 ppm)
(when handling pure dimethylformamide or dimethylformamide-containing
---------------------------------------------------------------------------
a As calculated by IARC (1989).
materials). One case of testicular cancer was found among the 3859
workers exposed to DMF (alone or with acrylonitrile) with 1.7 expected
on the basis of company rates, and no cases of liver cancer. The
company rates may be more relevant for comparison, as there were only
actively employed persons among the exposed and because the USA rates
are based on a limited time period, 1973-77.
Chen et al. (1988b) analysed mortality from 1950-82 among both
active and pensioned employees in the same cohort. Expected numbers
(adjusted for age and time period) were based on company rates. For
all workers exposed to DMF (alone or with acrylonitrile), the
standardized mortality ratio (SMR) for lung cancer was 124 (33 cases
[95% CI, 85-174]a). An increased risk of lung cancer was found in the
cohort exposed only to DMF (19 cases; SMR 141 [CI, 84-219]a), but not
in the cohort exposed to DMF and acrylonitrile. There were 3 deaths
from cancer of the buccal cavity and pharynx (SIR 188) in all persons
exposed to DMF (alone or with acrylo-nitrile). No other excess cancer
risk was reported. No information is given in this report on loss to
follow-up, death certificates, or whether these deaths were included
in the incidence study reported above.
Walrath et al. (1988) reported a case-control study on cancer
among 8724 Du Pont employees with potential exposure to DMF in 4 other
USA plants. Summary analyses for all plants combined did not show any
statistically significant association between ever having been exposed
to DMF and subsequent development of cancers of the buccal cavity and
pharynx, liver, malignant melanoma, prostate, and testes. When odds
ratios are examined according to plant site, prostate cancer at one
site was significantly elevated, on the basis of 3 cases exposed out
of 4, but no statistically significant association was observed among
employees similarly exposed to DMF in the other 3 plants. The recency
of exposure to DMF, the low exposures received, and the absence of
similar excesses at other plants argue against a causal association
between DMF exposure and prostate cancer at the one site. Assessment
of highest DMF exposure rank, duration of exposure, and latent period,
does not show any patterns suggesting an association between DMF and
cancers of the buccal cavity and pharynx, liver, malignant melanoma,
prostate, or testes.
9.2.4 Alcohol intolerance
Reviews on the synergistic action of ethanol with organic solvents
have been published by Haguenoer et al. (1982), Hills & Venable
(1982), and Stockley (1983).
Episodes of alcohol intolerance among workers exposed to DMF have
been repeatedly described at all levels of exposure (see sections
9.2.1 and 9.2.2, and Table 20).
Symptoms include flushing of the face, dizziness, nausea,
tightness of the chest, sometimes dyspnoea, and cardiac palpitations.
The reactions were reported within 24 h of DMF exposure and very
shortly after alcohol ingestion. These episodes lasted for up to 2 h
---------------------------------------------------------------------------
a As calculated by IARC (1989).
(Chivers, 1978; Lyle et al., 1979; Yonemoto & Suzuki, 1980; Paoletti
et al., 1982a; Cirla et al., 1984).
According to Loos (1979), abnormal liver function tests were
already discovered among workers who drank only 50-70 g alcohol per
day, but were also exposed to 45-66 mg DMF/m3, while the threshold
consumption for functional liver changes was 80-100 g alcohol per day
in control individuals. It should be noted that the test group was
also exposed to other solvents, mainly tetrahydrofuran, toluene, and
xylene.
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The International Agency for Research on Cancer (IARC) evaluated
the carcinogenicity of dimethylformamide in 1988 (IARC, 1989), and
concluded that:
- there is limited evidence for the carcinogenicity of
dimethylformamide in humans;
- there is inadequate evidence for the carcinogenicity of
dimethylformamide in experimental animals.
Overall evaluation: Dimethylformamide is possibly carcinogenic
to humans (Group 2B).
REFERENCES
ALDYREVA, M.V. & GAFUROV, S.A. (1980) [ Labour protection in the production
of artificial leather.] Moscow, Medizina, 158 pp (in Russian).
ALDYREVA, M.V., BORTSEVICH, S.V., PALAGUSHINA, A.I., SIDOROVA, N.V., &
TARASOVA, L.A. (1980) [Effect of dimethylformamide on workers' health in
the production of polyurethane synthetic leather.] Gig. Tr. prof. Zabol.,
6: 24-28 (in Russian).
AMSTER, M.B., HIJAZI, N., & CHAN, R. (1983) Real time monitoring of low
level air contaminants from hazardous waste sites. In: Proceedings of the
National Conference on Management of Uncontrolled Hazardous Waste Sites.
Washington, DC, pp. 98-99.
ANTOINE, J.L., ARANY, J., LEONARD, A., HENROTTE, J., JENAR-DUBUISSON, G., &
DECAT, G. (1983) Lack of mutagenic activity of dimethylformamide.
Toxicology, 26: 207- 212.
ARENA, N., SANTA CRUZ, G., ALIA, E.F., BALDUS, M., CORGIOLU, T., & ALIA,
E.E. (1982) [Structural and ultrastructural changes in the myocardium of
rabbits exposed to dimethylformamide vapours.] Boll. Soc. Ital. Biol.
Sper., 58: 1496-1501 (in Italian).
BAINOVA, A. (1980) [Contemporary views on the hygiene standardization of
dimethyl- formamide.] Letopisi HEI, 37: 62-68 (in Bulgarian).
BAINOVA, A. (1985) [ Toxicological problems due to the action of chemical
substances on the skin (dermatotoxicology).] Sofia, Referat, pp. 1-60 (D.
Sci. Thesis) (in Bulgarian).
BAINOVA, A. & ANTOV, G. (1980) Dermal toxicity of dimethylformamide in
rats. In: Abstracts of the 5th International Symposium on Occupational
Health in the Production of Artificial Fibres, Belgirate, Italy, 16-20
September, 1980, Modena, Permanent Commission and International Association
on Occupational Health, pp. 73-74.
BAINOVA, A., SPASSOVSKI, M., HINKOVA, L., & TASHEVA, M. (1981a) [Effect
of intermittent and continuous action of dimethylformamide on the
adaptation process.] Probl. hig., 6: 27-35 (in Bulgarian).
BAINOVA, A., ANTOV, G.P., & IVANOVICH, E.H. (1981b) Combined action of
dimethylformamide and noise on kidneys of white rats. CR. Acad. bulg. Sci.,
34: 1609-1611.
BAKER, H. (1968) The effects of dimethylsulfoxide, dimethylformamide, and
dimethyl- acetamide on the cutaneous barrier to water in human skin. J.
invest. Dermatol., 50: 283- 288.
BARNES, J.R. & HENRY, N.W. (1974) The determination of N-methylformamide
and N-methylacetamide in urine. Am. Ind. Hyg. Assoc. J., 35: 84-87.
BARNES, J.R. & RANTA, K.E. (1972) The metabolism of dimethylformamide
and dimethylacetamide. Toxicol. appl. Pharmacol., 23: 271-276.
BARRAL-CHAMAILLARD, J. & ROUZIOUX, M. (1983) Dimethylformamide. Arch.
Mal. prof., 44: 203-208.
BARTSCH, W., SPONER, G., DIETMAN, K., & FUCHS, G. (1976) Acute toxicity
of various solvents in the mouse and rat. Arzneimittelforschung, 26: 1581-
1583.
BECCI, P.J., VOSS, K.A., JOHNSON, W.D., GALLO, M.A., & BABISH, J.G. (1983)
Subchronic feeding study of N,N-dimethylformamide in rats and mice. J. Am.
Coll. Toxicol., 2(6): 371-378.
BEGERT, A. (1975) [Purification of chemical textile plant sewage.]
Oesterr. Abwasser- Rundsch., 20: 98-102 (in German).
BORENFREUND, E., STEINGLASS, M., KORNGOLD, G., & BENDICH, A. (1975)
Effect of dimethylsulfoxide and dimethylformamide on the growth and
morphology of tumour cells. Ann. N.Y. Acad. Sci., 243: 164-171.
BORTSEVICH, S.V. (1984) [The hygienic importance of dimethylformamide
absorption through the skin.] Gig. Tr. prof. Zabol., 11: 55-57 (in
Russian).
BRINDLEY, C., GESCHER, A., & ROSS, D. (1983) Studies of the metabolism of
dimethylformamide in mice. Chem.-biol. Interact., 45: 387-392.
BRONDEAU, M.T., BONNET, P., GUENIER, J.P., & DE CEAURRIZ, J. (1983)
Short-term inhalation test for evaluating industrial hepatotoxicants in
rats. Toxicol. Lett., 19: 139-146.
BRUGNONE, F., PERBELLINI, L., & GAFFURI, E. (1980a) N,N-Dimethylformamide
concentration in environmental and alveolar air in a artificial leather
factory. Br. J. ind. Med., 37: 185-188.
BRUGNONE, F., PERBELLINI, L., GAFFURI, E., & ASASTOLI, P. (1980b)
Biomonitoring of industrial solvent exposures in workers' alveolar air.
Int. Arch. occup. environ. Health, 47: 245-261.
BRUGNONE, F., PERBELLINI, L., GAFFURI, E., & TURRI, P.V. (1984)
Monitoring of industrial exposure to dimethylformamide by analysis of
alveolar air. Med. Lav., 6: 139- 141.
BURGUN, J., MARTZ, R., FORNEY, R.B., & KIPLINGER, G.F. (1975) The acute
toxicity of dimethylformamide and its combined effects with ethanol in the
mouse. Toxicol. appl. Pharmacol., 33: 149-150.
CAI, S.X. & HUANG, M.Y. (1979) Investigation on occupational hazard in a
butadiene monomer workshop of a cis-butadiene rubber plant. J. Hyg. Res.,
8(1): 22-49 (in Chinese).
CARDWELL, R.D., FOREMAN, D.G., PAYNE, T.R., & WILBUR, D.J. (1978) Acute
and chronic toxicity of four chemicals to fish. Duluth, Minnesota, US
Environmental Protection Agency (Contract 68-01-0711).
CATENACCI, G., GRAMPELLA, D., TERZI, R., SALA, H., & POLLINI, G., (1984)
Hepatic function in subjects exposed to environmental concentrations of DMF
lower than the actually proposed TLV. Med. Lav., 6: 157-158.
CHAKRABARTY, S., MCRAE, L.J., LEVINE, A.E., & BRATTAIX, M.G. (1984)
Restoration of normal growth control and membrane antigen composition in
malignant cells by N,N-dimethylformamide. Cancer Res., 44: 2181-2185.
CHARY, S. (1974) Dimethylformamide. A case of acute pancreatitis. Lancet,
2(7876): 356.
CHEN, J.L., FAYERWEATHER, W.E., & PELL, S. (1988a) Cancer incidence of
workers exposed to dimethylformamide and/or acrylonitrile. J. occup. Med.,
30: 813-818.
CHEN, J.L., FAYERWEATHER, W.E., & PELL, S. (1988b) Mortality study of
workers exposed to dimethylformamide and/or acrylonitrile. J. occup. Med.,
30: 819-821.
CHEN, S.-F., CLEAVELAND, J.S., HOLLMAN, A.B., WIEMANN, M.C., PARKS, R.E., &
STOECKLER, J.D. (1986) Changes in nucleoside transport of HL-60 human
promyelocytic cells during N,N-dimethylformamide induced differentiation.
Cancer Res., 46: 3449-3455.
CHIVERS, C.P. (1978) Disulfiram effect from inhalation of
dimethylformamide. Lancet, 1(8059): 331.
CHROMEK, J., KUPEK, J., MLADEK, M., & MARVAN, P. (1983) A study of
respiration of the alga Sceadesmus quadricauda in batch conditions under
influence of N,N-dimethylformamide and dimethylamine. Arch. Hydrobiol.,
64(Suppl.): 441-460.
CIRLA, A.M., PISATI, G., INVERNIZZI, E., & TORRICELLI, P. (1984)
Epidemiological study on workers exposed to low dimethylformamide
concentrations. G. Ital. Med. Lav., 6: 149-156.
CLAY, P.F. & SPITTLER, T.M. (1983) Determination of airborne volatile
nitrogen compounds using four independent techniques. In: Proceedings of
the National Conference on Management of Uncontrolled Hazardous Waste
Sites, Washington, DC, pp. 100-104.
CLAYTON, J.W., BARNES, J.R., HOOD, D.B., & SCHEPERS, G.W. (1963) The
inhalation toxicity of dimethylformamide (DMF). Am. Ind. Hyg. Assoc. J.,
24: 144-154.
CORDEIRO, R.F. & SAVARESE, T.M. (1984) Reversal by L-cysteine of the
growth inhibitory and glutathione-depleting effects of N-methylformamide
and N,N-dimethylformamide. Biochem. biophys. Res. Commun., 122: 798-803.
COSTA, V., FRONGIA, N., & SANTA CRUZ, G. (1978) [Histological and
ultrastructural changes of the rat renal glomerulus caused by
dimethylformamide inhalation.] Boll. Soc. Ital. Biol. Sper., 54: 1723-1728
(in Italian).
CRAIG, D.K., WEIR, R.J., WAGNER, W., & GROTH, D. (1984) Subchronic
inhalation toxicity of dimethylformamide in rats and mice. Drug chem.
Toxicol., 7: 551-571.
DARNALL, K.R., LLOYD, A.C., WINER, A.M., & PITTS, J.N. (1976) Reactivity
scale for atmospheric hydrocarbons based on reaction with hydroxyl radical.
Environ. Sci. Technol., 7: 692-696.
DEXTER, D.L. (1977) N,N-Dimethylformamide-induced morphological
differentiation and reduction of tumorigenicity in cultured mouse
rhabdomyosarcoma cells. Cancer Res., 37: 3136-3140.
DEXTER, D.L. & HAGER, J.C. (1980) Maturation induction of tumour cells
using a human colon carcinoma model. Cancer, 45: 1178-1184.
DILORENZO, F. & GRAZIOLI, C. (1972) [Hematological, hematochemical, and
gastric findings in workers exposed to breathing vapours of
dimethylformamide.] Lav. Um., 24: 96-106 (in Italian).
DIXON, S.W., GRAEPEL, G.J., & LOONEY, W.C. (1983) Seasonal effects on
concentrations of monomethylformamide in urine samples. Am. Ind. Hyg.
Assoc. J., 44: 273-275.
DOJLIDO, J.R. (1979) Investigations of biodegradability and toxicity of
organic compounds. Washington, DC, US Environmental Protection Agency
(Unpublished report No. EPA-600/2-79-163).
DRUCKREY, H., PREUSSMANN, R., IVANKOVIC, S., & SCHMAHL, D. (1967)
[Organotropic carcinogenic effects of 65 different N-nitroso-compounds on
B.D. rats.] Z. Krebsforsch., 69: 103-201 (in German).
DUCATMAN, A.M., CONWILL, D.E., & CRAWL, J. (1986) Germ cell tumours of
the testicle among aircraft repairmen. J. Urol., 136: 834-836.
EBEN, A. & KIMMERLE, G. (1976) Metabolism studies in N,N-
dimethylformamide. III. Studies on the influence of ethanol in persons and
laboratory animals. Int. Arch. occup. environ. Health, 36: 243-265.
EBERLING, C.L. (1980) Formic acid and derivatives (DMF). In: Kirk-
Othmer encyclopedia of chemical technology, 3rd ed., New York, Chichester,
Brisbane, Toronto, John Wiley & Sons, Vol. 11, pp. 263-268.
ELOVAARA, E., MARSELOS, M., & VAINO, H. (1983) N,N-Dimethylformamide-
induced effects on hepatic and renal xenobiotic enzymes with emphasis on
aldehyde metabolism in the rat. Acta. pharmacol. toxicol., 53: 159-165.
EWING, B.B., CHIAN, E.S.K., COOK, J.C., EVANS, C.A., HOPKE, P.A., &
PERKINS, E.G. (1977) Monitoring to detect previously unrecognized
pollutants in surface water. Appendix: Organic analysis data, Washington,
DC, US Environmental Protection Agency, (Unpublished report No. EPA-560/6-
77-015).
FARHI, M., MOREL, M., & CAVIGNEAUX, A. (1968) Dimethylformamide.
NCON(CH3)2. Cah. Notes doc., 50: 91-93.
FARLEY, F.F. (1977) Photochemical reactivity classification of
hydrocarbons and other organic compounds. In: International Conference on
Photochemical Oxidant Pollution and Its Containment, Research Triangle
Park, North Carolina, US Environmental Protection Agency. (Unpublished
report No. EPA-600/3-77-0018).
FARQUHARSON, R.O., HALL, M.A., & FULLERTON, W.T. (1983) Poor obstetric
outcome in three quality control laboratory workers. Lancet, 30: 983-984.
GERMANOVA, A.L., HALEPO, A.I., AVILOVA, G.G., ANVAER, L., HOROCHULOVA,
N.V., MALTSEVA, N.M., & MIGUKINA, N.V. (1979) [Adaptation after
continuous and intermittent exposure to dimethylformamide.] In: [ The
toxicology of new industrial chemicals.] Moscow, Medizina, Vol. 15, pp. 69-
76 (in Russian).
GRASSELLI, J.G. (1973) Atlas of spectral data and physical constants for
organic compounds, Cleveland, Ohio, USA, The Chemical Rubber Co.
GUBSER, H. (1969) Purification of chemical waste waters. Gas Wasser
Abwasser, 49: 175-181.
HAGER, J.C., GOLD, D.V., BARBOSA, J.A., FLIGIEL, L., MILLER, F., & DEXTER,
D.L. (1980) N,N-Dimethylformamide-induced modulation of organ and tumour
associated markers in cultured human colon carcinoma cells. J. Natl Cancer
Inst., 64: 439-445.
HAGUENOER, J.M., BOURRINET, P., & FRIMAT, P. (1982) Interrelations
between alcoholism and exposure to industrial poisons. Arch. Mal. prof.,
43: 461-473.
HAMILTON, A. & HARDY, H.L. (1974) Industrial toxicology, 3rd ed., Acton,
Massachusetts, Publishing Science Group Inc., 349 pp.
HANASONO, G.K., FULLER, R.W., BRODDLE, W.D., & GIBSON, W.R. (1977)
Studies on the effects of N,N-dimethylformamide on ethanol disposition and
on monoamine oxidase activity in rats. Toxicol. appl. Pharmacol., 39: 461-
472.
HELLWIG, J., MERKLE, J., KLIMISCH, H.J., & JÄCKH, R. (in press)
Investigations on the prenatal toxicity of N,N-dimethylformamide (DMF) in
mice, rats and rabbits. Food chem. Toxicol..
HENRY, N.W. & SCHLATTER, C.N. (1981) The development of a standard method
for evaluating chemical protective clothing to permeation by hazardous
liquids. Am. Ind. Hyg. Assoc. J., 42: 202-207.
HILLS, B.W. & VENABLE (1982) The interaction of ethyl alcohol and
industrial chemicals. Am. J. ind. Med., 3: 321-333.
HINKOVA, L., GINCHEVA, N., STAMOVA, N., HRISTEVA, V., CHOLAKOV, B., &
SASSOVSKY, M. (1980) [Influence of dimethylformamide on workers' health.]
Probl. hyg., Sofia, 5: 75-81 (in Bulgarian).
HUANG, M.Y., LUO, Y.Z., GENG, T.B., MENG, D.S., LIU, J., HUANG, M.F., &
WANG, Y.S. (1981) [Studies on the dermal toxicity of dimethylformamide.]
J. Hyg. Res., 10(4): 21-26 (in Chinese).
HUGHES, J.S. & VILKAS A.G. (1983) Toxicity of N,N-dimethylformamide used
as a solvent in toxicity tests with the green alga Selenastrum capricornum.
Bull. environ. Contam. Toxicol., 31: 98-104.
IARC (1989) Dimethylformamide. In: Some organic solvents, resin monomers
and related compounds, pigments and occupational exposures in paint
manufacture and painting, Lyon, International Agency for Research on
Cancer, pp. 171-197 (IARC Monograph on the Evaluation of Carcinogenic Risks
to Humans, Vol. 47).
IMBRIANI, M., CHITTORI, S., PRESTINONI, A., LONGONI, P., CASCONE, G., &
GAMBA, G. (1986) Effects of dimethylformamide (DMF) on coagulation and
platelet activity. Arch. environ. Health, 41: 90-93.
KANG-DE, C. & HUI-LAN, Z. (1981) Observation on the effects of
dimethylformamide on human health. In: Abstracts of the 9th International
Congress on Occupational Health in the Chemical Industry, Aswan, Egypt, 15-
17 September 1981, Aswan, Egypt, Permanent Commission and International
Association on Occupational Health, pp. 22-23.
KELLER, C.A. & LEWIS, S.C. (1981) Inhalation teratology study of N,N-
dimethyl- formamide (DMF). Teratology, 23: 45A.
KENNEDY, G.L., Jr (1986) Biological effects of acetamide, formamide, and
their monomethyl and dimethyl derivatives. CRC crit. Rev. Toxicol., 17(2),
129-182.
KENNEDY, G.L. & SHERMAN, H. (1986) Acute and subchronic toxicity of
dimethylformamide and dimethylacetamide following various routes of
administration. Drug chem. Toxicol., 9: 147-170.
KESTELL, P., GILL, M.H., THREADGILL, M.D., GESCHER, A., HOWARTH, O.W., &
CURZON, E.H. (1986) Identification by proton NMR of N-(hydroxymethyl)- N-
methylformamide as the major urinary metabolite of N,N-dimethylformamide
in mice. Life Sci., 38: 719- 724.
KESTELL, P., THREADGILL, M.D., GESCHER, A., GLEDHILL, A.P., SHAW, A.J., &
FARMER, R.B. (1987) An investigation of the relationship between the
hepatotoxicity and the metabolism of N-alkylformamides. J. Pharmacol. exp.
Ther., 270: 265-270.
KIMBALL, P.M. & HIXON, S. (1983) Nuclear protein changes following N,N-
dimethylformamide (DMF) induced maturation. J. cell. Biochem., 22: 245-249.
KIMMERLE, G. & EBEN, A. (1975a) Metabolism studies of N,N-
dimethylformamide. I. Studies in rats and dogs. Int. Arch. Arbeitsmed.,
34: 109-126.
KIMMERLE, G. & EBEN, A. (1975b) Metabolism studies of N,N-
dimethylformamide. II. Studies in persons. Int. Arch. Arbeitsmed., 34:
127-136.
KIMMERLE, G. & MACHEMER, L. (1975) Studies with N,N-dimethylformamide for
embryotoxic and teratogenic effects on rats after dynamic inhalation. Int.
Arch. Arbeitsmed., 34: 167-175.
KIMURA, E.T., EBERT, D.M., & DODGE, P.W. (1971) Acute toxicity and limits
of solvent residue for sixteen organic solvents. Toxicol. appl.
Pharmacol., 19(4): 699-704.
KISS, G. (1979) [Study of the irritative action of dimethylformamide.]
Börg. Venerol., 55: 203 (in Hungarian).
KOMMINENI, C. (1973) Pathological studies of aflatoxin fractions and
dimethylformamide in MRC rats, Omaha, University of Nebraska (Dissertation,
December 1972).
KOUDELA, K. & SPAZIER, K. (1979) [Effects of dimethylformamide on human
peripheral lymphocytes.] Cesk. Hyg., 24: 432-436 (in Czech).
KOUDELA, K. & SPAZIER, K. (1981) [Increased concentration of
dimethylformamide vapours in the atmosphere.] Prac. Lek., 33: 121-123 (in
Czech).
KRAMER, V.C., SCHNELL, D.J., & NICKERSON, K.W. (1983) Relative toxicity
of organic solvents to Aedes aegypti larvae. J. invertebr. Pathol., 42:
285-287.
KRIVANEK, N.D., MCLAUGHLIN, M., & FAYERWEATHER, W.E. (1978)
Monomethylformamide levels in human urine after repetitive exposure to
dimethylformamide vapour. J. occup. Med., 20: 179-182.
LAITY, J.L., BURSTEIN, I.G., & APPEL, B.R. (1973) Photochemical smog and
the atmospheric reactions of solvents. In: Solvents theory and practice,
pp. 95-112, Washington, DC, American Chemical Society (Advances in
Chemistry Series, Vol. 124).
LANGDON, S.P. & HICKMAN, J.A. (1987) Alkylformamides as inducers of
tumour cell differentiation - a mini-review. Toxicology, 43: 239-249.
LAUWERYS, R. (1986) Dimethylformamide. In: Alessio, L., Berlin, A., Boni,
M., & Roi, R., ed. Biological indicators for the assessment of human
exposure to industrial chemicals. Brussels, Luxembourg, Commission of the
European Communities Joint Research Center, pp. 19-27.
LAUWERYS, R.R., KIVITS, A., LHOIR, M., RIGOLET, P., HOUBAT, D., BUCHET,
J.P., & ROELS, H.A. (1980) Biological surveillance of workers exposed to
dimethylformamide and the influence of skin protection on its
percutaneous absorption. Int. Arch. occup. environ. Health, 45: 189-203.
LAZAREV, N.V. & LEVINA, E.N. (1976) [Dimethylformamide.] In: [Harmful
substances in industry.] Leningrad, Himia, Vol. 2, pp. 36-38 (in Russian).
LEBLANC, G.A. & SURPRENANT, D.C. (1983) The acute and chronic toxicity of
acetone, dimethylformamide, and triethylene glycol to Daphnia magna
(Strauss). Arch. environ. Contam. Toxicol., 12: 305-310.
LESHIK, J.A.D. & FEOKTISTOVA, A.J. (1984) [Ascorbic acid supply and
cytochrome P-450 level in guinea-pig liver during dimethylformamide
poisoning.] J. Vopr. Pitan., 5: 65-67 (in Russian).
LEVIN, S.M., BAKER, D.B., LANDRIGAN, P.J., MONAGHAN, S.V., FRUMIN, E.,
BRAITHWAITE, M., & TOWNE, W. (1987) Testicular cancer in leather tanners
exposed to dimethylformamide. Lancet, II(8568): 1153.
LEVINE, A.E., MCRAE, L.J., & BRATTAIN, M.G. (1985) Changes in receptor
occupancy and growth factor responsiveness induced by treatment of a
transformed mouse embryo cell line with N,N-dimethylformamide. Cancer Res.,
45: 6401-6405.
LEWIS, S.C., RINEHART, W.E., SCHROEDER, R.E., & THAKARA, J.W. (1979)
Dominant lethal mutagenic bioassay of dimethylformamide (DMF). Environ.
Mutagen., 1: 166.
LIPSKI, K. (1982) Liquid chromatographic determinations of
dimethylformamide, methylene bisphenyl isocyanate, and methylene bisphenyl
amine in air samples. Ann. occup. Hyg., 25: 1-4.
LLEWELLYN, G.C., HASTINGS, W.C., & KIMBROUGH, T.D. (1974) The effects of
dimethylformamide on female Mongolian gerbils Meriones unguiculatus. Bull.
environ. Contam. Toxicol., 11: 467-473.
LOBANOVA, K.P. (1958) [Toxicity of dimethylformamide.] Gig. i Sanit.,
23: 31- 37 (in Russian).
LOOS, H. (1979) Hazards, health supervision, and potentiation of alcohol
by mixtures of organic solvents, especially dimethylformamide (DMF).
Arbeitsmed. Sozialmed. Präventivmed., 14(5): 127-129.
LUNDBERG, I., LUNDBERG, S., & KRONEVY, T. (1981) Some observations on
dimethylformamide hepatotoxicity. Toxicology, 22: 1-7.
LUNDBERG, I., PEHRSSON, A., LUNDBERG, S., KRONEVY, T., & LIDUMS, V. (1983)
Delayed dimethyformamide biotransformation after high exposures in rats.
Toxicol. Lett., 17: 29- 34.
LUNDBERG, I., EKDAHL, M., KRONEVI, T., LIDUMS, V., & LUNDBERG, S. (1986)
Relative hepatotoxicity of some industrial solvents after intraperitoneal
injection or inhalation exposure in rats. Environ. Res., 40: 411-420.
LUNDBERG, S. (1982) [ Nordic group of experts for documentation of
threshold limit values - 38. Dimethylformamide.] Solna, Sweden, National
Institute of Occupational Health, pp. 32 (Report 1982:28) (in Swedish).
LYLE, W.H., SPENCE, T.W.M., MCKINNELEY, W.M., & DUCKERS, K. (1979)
Dimethylformamide and alcohol intolerance. Br. J. ind. Med., 36: 63-66.
MALONOVA, H. & BARDODEJ, Z. (1983) Urinary excretion of mercapturates as
a biological indicator of exposure to electrophilic agents. J. Hyg. epidem.
Microbiol. Immunol., 27(3): 319-328.
MASSMANN, W. (1956) Toxicological investigations on dimethylformamide.
Br. J. ind. Med., 13: 51-54.
MATHEW, T., KARUNANITHY, R., YEE, M.H., & NATARAJAN, P.N. (1980)
Hepatotoxicity of dimethylformamide and dimethylsulfoxide at and above the
levels used in some aflatoxin studies. Lab. Invest., 42: 257-262.
MAXFIELD, M.E., BARNES, J.R., AZAR, A., & TROCHIMOWIEZ, H.T. (1975)
Urinary excretion of metabolite following experimental human exposure to
dimethylformamide or to dimethylacetamide. J. occup. Med., 17: 506-511.
MEDYANKIN, A.V. (1975) [Complex action of dimethylformamide under
conditions of a long-term experiment.] Gig. i Sanit., 9: 39-42 (in
Russian).
MERKLE, J. VON. & ZELLER, H. (1980) [Studies on acetamides and formamides
for embryotoxic and teratogenic activities in the rabbit.]
Arzneimittelforschung, 30: 1557-1562 (in German).
MRAZ, J. & TURECEK, F. (1987) Identification of N-acetyl- S-
( N-methylcarbamoyl) cysteine, a human metabolite of N',N'-dimethyl-
formamide and N-methylformamide. J. Chromatogr., 414: 399-404.
MRAZ, J., MRAZ, M., SEDIVEC, V., & FLEK, J. (1987) [Gas chromatographic
determination of N-methylformamide in urine.] Pra. Lek., 39: 352-355
(in Czech).
MURAVIEVA, S.I. (1983) [Improvement of the methods for monitoring the
content of harmful substances in the air of worksite.] Gig. Tr. prof.
Zabol., 6: 39-41 (in Russian).
MURAVIEVA, S.I. & ANVAER, L.P. (1979) [Determination of dimethylformamide
and its metabolites in biological liquids by gas chromatographic method.]
Gig. Tr. prof. Zabol., 6: 58-59 (in Russian).
O'BERG, M.T., CHEN, J.L., & BURKE, C.A. (1985) Epidemiologic study of
workers exposed to acrylonitrile, an update. J. occup. Med., 27: 835-840.
ODOSHASHVILI, D.G. (1963) [Hygienic evaluation of atmospheric air
pollution with dimethylformamide.] In: [Literature on air pollution and
related occupational diseases.] Vol. 9, pp. 169-177 (in Russian).
PAOLETTI, A. & IANNACCONE, A. (1982) [Risk from dimethylformamide
intoxication in a plant for synthetic leather.] Ann. Ist. Super. Sanit.,
18: 567-570 (in Italian).
PAOLETTI, A., FABRI, G., & MASCI, O. (1982a) [Antabuse effect from
solvents: comparison between dimethylformamide and trichloroethylene.]
Ann. Ist. Super. Sanit., 14: 1099-1100 (in Italian).
PAOLETTI, A., FABRI, G., & MARINI BETTOLO, P. (1982b) [An isolated case
of "acute abdomen". Intoxication from dimethylformamide.] Minerva Med., 73:
3407-3410 (in Italian).
PARKHIE, M. & WEBB, M. (1983) Embryotoxicity and teratogenicity of
thalidomide in rats. Teratology, 27: 327.
PERRY, D.L., CHUANG, C.C., JUNGCLAUS, G.A., & WARNER, J.S. (1979)
Identification of organic compounds in industrial effluent discharges,
Athens, Georgia, US Environmental Protection Agency, Office of Research and
Development (Unpublished report No. EPA-600/4-79-016, NTIS PB-294794).
PHAM HUU CHANH, NGUYEN DAT XUONG, & AZUM-GELADE, M.-C. (1971) Etude
toxicologique de la formamide et de ses dérivés N-méthylés et N-éthylés.
Thérapie, 26:409-424.
PHAM HUU CHANH, AZUM-GELADE, M.-C., NGUYEN VAN BAC, & NGUYEN DAT XUONG
(1973) Cardiovascular activity of N,N-dimethylformamide. Toxicology, 1:
135- 142.
POIRIER, S.H., KNUTH, M.L., ANDERSON-BUCHOU, C.D., BROOKE, L.T., LIMA,
A.R., & SHUBAT, P.J. (1986) Comparative toxicity of methanol and N,N-
dimethylformamide to freshwater fish and invertebrates. Bull. environ.
Contam. Toxicol., 37: 615-621.
POTTER, H.P. (1973) Dimethylformamide induced abdominal pain and liver
injury. Arch. environ. Health, 27: 340-341.
PURCHASE, I.F.H., LONGSTAFF, E., ASHBY, J., STYLES, J.A., ANDERSON, D.,
LEFEVRE, P.A., & WESTWOOD, F.R. (1978) An evaluation of six short-term
tests for detecting organic chemical cancerogens. Br. J. Cancer, 37: 873-
903.
QIN, Y.H. & GUE, R.R. (1976) [Studies on the maximum allowalble
concentration of dimethylformamide in surface water.] J. Hyg. Res., 5(2):
161-167 (in Chinese).
REBHUN, L.J. & SAWADA, N. (1969) Augmentation and dispersion of the in
vivo mitotic apparatus of living marine eggs. Protoplasma, 68: 1-22.
ROMADINA, E.S. (1975) [Direct action of microorganisms - one way of
increasing the effectiveness of biological purification of waste waters.]
In: Telitchenko, M., ed. [Biological self-purification in water quality
management: Proceedings of the All-Union Symposium on Sanitary
Hydrobiology.] Moscow, Nauka, pp. 110-112 (in Russian).
SALA, C., BERNABEO, F., COLOMBO, G., INVERNIZZI, E., & MENEGHEL, G. (1984)
Dimethylformamide risk. An evaluation in the production of artificial
organic leather. Med. Lav. 6: 143-148.
SANOTSKY, I.V. & ULANOVA, I.P. (1975) [ Criteria for harmfulness in
hygiene and toxicology for evaluation of hazards from chemical compounds.]
Moscow, Medizina, 372 pp (in Russian).
SANOTSKY, I.V., MURAVIEVA, S.I., ZAEVA, G.N., ANVAER, L., & SEMILETKINA,
N.N. (1978) [Metabolism of dimethylformamide depending on the intensity
of its action.] Gig. Tr. prof. Zabol., 11: 24-27 (in Russian).
SANSONE, E.B. & TEWARI, Y.B. (1978) The permeability of laboratory gloves
to selected solvents. Am. Ind. Hyg. Assoc. J., 39(2): 169-174.
SANTA CRUZ, G. & CORPINO, P. (1978) [Preliminary morphological studies on
acute experimental inhalation exposure in the rat.] Boll. Soc. Ital. Biol.
Sper., 54: 1710- 1717 (in Italian).
SANTA CRUZ, G. & MACCIONI, A. (1978) [Experimental study on
dimethylformamide toxicity. Changes of the myocardium after prolonged
inhalation treatment in the rat.] Boll. Soc. Ital. Biol. Sper., 54: 1717-
1722 (in Italian).
SASAKI, S. (1978) The scientific aspects of the chemical substance
control law in Japan. In: Hutzinger, O., Lelyveld, L.H., van, & Zoeteman,
B.C.J., ed., Aquatic pollutants: Transformation and biological effects,
Oxford, New York, Pergamon Press.
SAVOLAINEN, H. (1981) Dose-dependant effects of peroral dimethylformamide
administration on rat brain. Acta neuropathol., 53: 249-252.
SCAILTEUR, V. (1984) Contribution à l'étude de la relation toxicité-
metabolisme de la dimethylformamide chez le rat. Université Catholique de
Louvain, 255 pp. (Thèse).
SCAILTEUR, V. & LAUWERYS, R. (1984a) In vivo and in vitro oxidative
biotransformation of dimethylformamide in rat. Chem.-biol. Interact., 50:
327-337.
SCAILTEUR, V. & LAUWERYS, R. (1984b) In vivo metabolism of
dimethylformamide and relationship to toxicity in the male rat. Arch.
Toxicol., 56: 87-91.
SCAILTEUR, V. & LAUWERYS, R.R. (1987) Dimethylformamide (DMF)
hepatotoxicity. Toxicology, 43: 231-238.
SCAILTEUR, V., HOFFMANN, E., BUCHET, J.P., & LAUWERYS, R. (1984) Study on
in vivo and in vitro metabolism of dimethylformamide in male and female
rats. Toxicology, 29: 222-234.
SCHEUFLER, H., VON, & FREYE, H.-A. (1975) [Embryotoxic and teratogenic
effects of dimethylformamide.] Dtsch. Gesundheitswes., 30: 455-459 (in
German).
SCHOTTEK, W. (1964) [Problems with the standardization of embryotoxic
substances.] Z. ärztl. Fortbild., 64: 1158-1162 (in German).
SCHOTTEK, W. (1970) [Experimental dimethylformamide toxicity studies on
experimental animals after repeated treatment.] Acta. biol. med. Germ., 25:
359-361 (in German).
SCHOTTEK, W. (1972) [Towards the problem of hygiene standardization of
chemicals having embryotoxic action.] In: Sanotsky, I.V., ed. [Hygine
standardization in study of remote effects of industrial substances.]
Moscow, Medizina, pp. 119-123 (in Russian).
SERRES, F.J., DE & ASHBY, J., ed. (1981) Evaluation of short-term tests
for carcinogens, Amsterdam, Oxford, New York, Elsevier Science Publishers,
827 pp (Progress in Mutation Research, Vol. 1).
SHARKAWI, M. (1980) Inhibition of alcoholdehydrogenase by dimethyl-
formamide and dimethylsulfoxide. Toxicol. Lett., 4: 493-497.
SHEVELEVA, G.A. & OSINA, S.A. (1973) [Experimental investigation of the
embryotropic action of dimethylformamide.] In: [ The toxicology of new
industrial chemicals.] Moscow, Medizina, Vol. 13, pp. 75-82 (in Russian).
SHEVELEVA, G.A., STREKALOVA, E.E., & CHIRKOVA, E.M. (1979) [Study of the
embryotropic, mutagenic, and gonadotropic effects of dimethylformamide
after inhibition exposure.] In: [ The toxicology of new industrial
chemicals.] Moscow, Medizina, Vol. 15, pp. 21-25 (in Russian).
SHLYGINA, O.E. & NEMOLCHEV, V.M. (1981) [The use of radio-isotope methods
in studies of acute poisoning with dimethylformamide.] In: [ Proceedings of
the Moscow Scientific Research Institute of First Aid.] Moscow, Medizina,
Vol. 45: 130-132 (in Russian).
SHUBAT, P.J., POIRIER, S.H., KNUTH, M.L., & BROOKE, L.T. (1982) Acute
toxicity of tetrachloroethylene and tetrachloroethylene with
dimethylformamide to rainbow trout, Salmo gairdneri. Bull. environ. Contam.
Toxicol., 28: 7-10.
SICKLES, J.E., WRIGHT, R.S., SUTCLIFFE, C.R., BLAKARD, A.L., & DAYTON, D.P.
(1980) Smog chamber studies of the reactivity of volatile organic
compounds, In: Proceedings of the 73rd Annual Meeting of the Air Pollution
Control Association, (Paper 80-50.1).
STOCKLEY, I.H. (1983) Drugs, foods, and environmental chemical agents
which can initiate Antabuse-like reactions with alcohol. Pharmacol.
Interact., 4: 12-16.
STRANSKY, V. (1986) The determination of N,N-dimethylformamide in working
atmosphere by the method of gas chromatography after sampling on activated
charcoal. Prac. Lek., 38: 15-19.
STULA, E.F. & KRAUSS, W.C. (1977) Embryotoxicity in rats and rabbits from
cutaneous application of amide type solvents and substituted ureas.
Toxicol. appl. Pharmacol., 41: 35-55.
TACCOLA, A., CATENACCI, G., & BARUFFINI, A. (1981) Cardiotoxicity of
dimethylformamide (DMF). Electrocardiographic findings and continuous
electro- cardiographic monitoring. G. Ital. Med. Lav., 3: 149-151.
TANAKA, K.I. (1971) Toxicity of dimethylformamide (DMF) to the young
female rat. Int. Arch. Arbeitsmed., 28: 98-105.
TANAKA, K.I. & UTSUNOMIYA, T. (1982) [The toxicity of N,N-
dimethylformamide (DMF).] Jpn. J. ind. Health, 24: 3-12 (in Japanese).
THIERSCH, J.E. (1962) Effects of acetamides and formamides on the rat
litter in utero. J. Reprod. Fertil., 4: 220.
TOLOT, F., DROIN, M., & GENEVOIS, J. (1958) Intoxication par la
diméthylformamide. Arch. Mal. prof., 19: 602-606.
TOLOT, F., ARCADIO, F., LENGLET, J.-P., & ROCHE, L. (1968) Intoxication
par la diméthylformamide. Arch. Mal. prof., 29: 714-717.
TOMASINI, M., TODARO, A., PIAZZONI, M., & PERUZZO, G.F. (1983) [Pathology
due to dimethylformamide. Observation of 14 cases.] Med. Lav., 74: 217-220
(in Italian).
TONOGAI, Y., OGAWA, S., ITO, Y., & IWAIDA, M. (1982) Actual survey on TLM
(median tolerance limit) values of environmental pollutants, especially on
amines, nitriles, aromatic nitrogen compounds and artificial dyes. J.
toxicol. Sci., 7: 193-203.
UNGAR, H., SULLMAN, S.F., & ZUCKERMAN, A.J. (1976) Acute and protracted
changes in the liver of Syrian hamsters induced by a single dose of
aflatoxin B1. Observations on pathological effects of the solvent
dimethylformamide. Br. J. exp. Pathol., 57: 157- 164.
US EPA (1986) Health and environmental effects profile for N,N-
dimethylformamide. Cincinnati, Ohio, US Environmental Protection Agency,
Health and Environmental Assessment, Environment Criteria and Assessment
Office, 106 pp (Unpublished data).
US NIOSH (1978) Occupational health guideline for dimethylformamide,
Cincinnati, Ohio, US National Institute for Occupational Safety and Health,
5 pp.
US NIOSH (1977) Manual of analytical methods, Cincinnati, Ohio, US
National Institute for Occupational Safety and Health, Vol. 3 (No. S-255).
WAHLBERG, J.E. & BOMAN, A. (1979) Comparative percutaneous toxicity of
ten industrial solvents in the guinea-pig. Scand. J. Work Environ. Health,
5: 345-351.
WALRATH, J., FAYERWEATHER, M.P.H., & GILBY, C.I.H. (1988) A case-control
study of cancer among Du Pont employees with potential for exposure to
dimethylformamide, Wilmington, Delaware, E.I. Du Pont de Nemours Co.
(Unpublished report).
WEISS, G. (1971) [Industrial dimethylformamide intoxication and the
question of its recognition as an occupational disease.] Zbl. Arbeitsmed.,
11.: 345-346 (in German).
WELWARD, L. & HALAMA, D. (1978) Influence of anti-microbial agents on
contamination and chlorotetracycline production. Folia microbiol., 23: 12-
17.
WICAROVA, O. & DADAK, O. (1981) [Urinary N-methylformamide levels in
persons exposed to dimethylformamide vapour.] Prac. Lek., 33: 42-46 (in
Czech).
WILES, J.S. & NARCISSE, J.K., Jr (1971) The acute toxicity of
dimethylformamides in several animal species. Am. Ind. Hyg. Assoc. J., 32:
539-545.
WILSON, H.K. & OTTLEY, T.W. (1981) The use of a transportable mass
spectrometer for the direct measurement of industrial solvents in breath.
Biomed. mass. Spectrom., 8: 606-610.
YONEMOTO, J. & SUZUKI, S. (1980) Relation of exposure to
dimethylformamide vapour and the metabolite, methylformamide, in urine of
workers. Int. Arch. occup. environ. Health, 46: 159-165.
RESUME ET EVALUATION, CONCLUSIONS, RECOMMANDATIONS
1. Résumé et évaluation
1.1 Propriétés générales
Le N,N-diméthylformamide (diméthylformamide, DMF, CSA 68-12-1)
est un solvant organique qui est produit en grandes quantités dans
l'ensemble du monde. On l'utilise dans l'industrie chimique comme
solvant, comme produit intermédiaire et comme additif. Le DMF est un
liquide incolore d'odeur légère mais désagréable qui, néanmoins, est
insuffisante pour attirer l'attention. Il est généralement stable
mais il peut entraîner des incendies et des explosions par contact
avec des oxydants forts, des halogènes, des dérivés alkylaluminiques
ou des hydrocarbures halogénés (en particulier combinés à des métaux).
Le DMF est entièrement miscible à l'eau et à la plupart des solvants
organiques. Sa tension de vapeur est relativement faible.
Du point de vue analytique, on a recours à la chromatographie
en phase gazeuse.
1.2 Transport, distribution, et transformation dans l'environnement
Le DMF est stable dans l'air ambiant mais il peut subir une
décomposition microbienne et algaire dans l'eau. Les microorganismes
adaptés et les boues activées assurent une biodégradation efficace du
DMF. Etant soluble dans l'eau en toutes proportions, le DMF est très
mobile dans les sols et il ne devrait pas s'accumuler dans la chaîne
alimentaire.
1.3 Concentration dans l'environnement et exposition humaine
Le DMF n'existe pas à l'état naturel. On ne possède que peu de
données sur sa concentration dans l'environnement ou sur l'exposition
de la population générale. Dans l'air de zones résidentielles situées
à proximité d'installations industrielles, on a trouvé des
concentrations allant de 0,02 à 0,12 mg/m3. Il est rare qu'on décèle
la présence de DMF dans l'eau des bassins fluviaux très industrialisés
et encore, les concentrations ne dépassent pas 0,01 mg/litre.
On ne possède pas de données sur la concentration du DMF dans le
sol, les végétaux, la faune sauvage et les produits alimentaires.
L'exposition professionnelle se produit par contact cutané avec le
liquide ou la vapeur ou par inhalation de la vapeur. On a décelé des
concentrations de 3 à 86 mg/m3 dans l'air avec des maxima allant
jusqu'à 600 mg/m3, au cours de travaux de réparation ou d'entretien de
machines. Dans quelques cas exceptionnels, des concentrations allant
jusqu'à 4500 mg/m3 ont été signalées.
1.4 Cinétique et métabolisme
Des quantités toxiques de DMF peuvent être absorbées par
inhalation ou pénétration percutanée. Une fois absorbé, le DMF se
distribue de façon uniforme dans l'organisme. Sa métabolisation a
lieu principalement dans le foie sous l'action des enzymes
microsomiales. Chez l'animal et l'homme, le principal produit de la
biotransformation du DMF est le N-hydroxyméthyl- N-méthyl-formamide
(DMF-OH). Au cours de l'analyse par chromatographie en phase gazeuse,
ce métabolite est transformé en N-méthyl-formamide (NMF) qui est lui-
même (ainsi que le N-hydroxyméthyl et le formamide) un métabolite
mineur du DMF. Par conséquent, lorsqu'on procède à des études
métaboliques et que l'on effectue la surveillance biologique du DMF,
les concentrations urinaires de métabolites sont mesurées et exprimées
en NMF, même si le DMF-OH en est le constituant essentiel. Le dosage
du NMF/DMF-OH dans les urines peut donner une bonne indication
biologique de l'exposition totale au DMF.
Chez l'animal d'expérience, on a montré que le mécanisme de
métabolisation du DMF se sature lorsque l'exposition est intense, le
DMF jouant le rôle de rétroinhibiteur de son propre métabolisme à
concentration très élevée.
Il y a interaction métabolique entre le DMF et l'éthanol.
1.5 Effets sur les êtres vivant dans leur milieu naturel
On n'a pas très bien étudié des effets du DMF sur l'environnement.
Il semble que sa toxicité pour les organismes aquatiques soit faible.
1.6 Effets sur les animaux d'expérience et les systèmes d'épreuve
in vitro
Le DMF présente pour diverses espèces une faible toxicité aiguë
(chez le rat la DL50 par voie orale est d'environ 3000 mg/kg, la DL50
dermique d'environ 5000 mg/kg et la CL50 par inhalation à peu près
égale à 10 000 mg/m3). Il est légèrement à modérément irritant pour
la peau et les yeux. D'après une étude sur des cobayes, il ne semble
pas doté de pouvoir sensibilisateur. Le DMF peut faciliter
l'absorption percutanée d'autres substances chimiques.
Chez l'animal d'expérience, l'exposition au DMF, quelle que soit
la voie de pénétration, peut provoquer des lésions hépatiques qui
dépendent de la dose. Lorsque l'exposition cesse, on a pu constater
qu'il y avait régénération des tissus. Certaines études on également
permis d'observer des signes de toxicité au niveau du myocarde et des
reins.
On n'a pas constaté de toxicité pour les testicules ou les
ovaires, ni observé d'effets sur la fécondité chez le rat. Chez le
rat, la souris et le lapin, le DMF s'est révélé embryotoxique et
faiblement tératogène. C'est le lapin qui est l'espèce la plus
sensible à l'exposition respiratoire: des effets tératogènes ont été
observés à partir de 1350 mg/m3 (450 ppm), aucun effet n'étant
constaté à 450 mg/m3 (150ppm). Après exposition de la peau, certaines
études ont mis en évidence de très rares effets embryotoxiques et
tératogènes à des doses journalières comprises entre 100 et 400 mg/kg.
De nombreuses épreuves à court terme à la recherche d'effets
génétiques et anomalies de ce genre, on a montré que le DMF était en
général inactif tant in vitro qu' in vivo.
Aucune étude convenable de cancérogénicité à long terme sur
animaux de laboratoire n'est décrite dans la littérature.
1.7 Effets sur l'homme
Aucun effet indésirable sur la population dans son ensemble n'a
été nettement mis en évidence.
On a fait état d'irritation cutanée et de conjonctivites après
contact direct avec du DMF.
Après exposition accidentelle à de fortes concentrations de DMF,
on note dans les 48h. des douleurs abdominales, des nausées, des
étourdissements et de la fatigue. La fonction hépatique peut être
perturbée et on a signalé des modifications de la tension artérielle,
une tachycardie et des anomalies du tracé électrocardiographique. En
général la récupération est totale.
Après des expositions réitérées sur une longue période, on observe
des symptômes tels que céphalées, perte d'appétit et fatigue. Des
signes biochimiques d'insuffisance hépatique peuvent également
s'observer. Des lésions hépatiques n'apparaissent, semble-t-il, qu'à
partir d'une exposition de l'ordre de 30 mg/m3, en l'absence de
contact cutané. Cette concentration atmosphérique correspond à
environ 40 mg de NMF/DMF-OH par gramme de créatinine dans un
échantillon d'urine prélevé à la fin du poste de travail.
Même à des concentrations inférieures à 30 mg/m3, l'exposition
au DMF peut causer une intolérance à l'alcool dont les symptômes
peuvent consister en rougeur soudaine de la face, sensation de
constriction thoracique et étourdissements, quelques fois accompagnés
de nausées et de dyspnée. Ces symptômes durent de 2 à 4 heures et
disparaissent spontanément.
On possède des preuves limitées d'une activité cancérogène du DMF
pour l'homme. C'est ainsi qu'une étude a fait état d'un accroissement
de l'incidence des tumeurs du testicule après exposition à du DMF,
tandis qu'une autre étude à révélé une incidence tumorale accrue au
niveau de la cavité buccale et du pharynx, mais pas au niveau des
testicules.
Deux études peu détaillées font état d'un accroissement de la
fréquence des avortements chez des femmes exposées à du DMF, entre
autres substances chimiques.
2. Conclusions
1. Compte tenu de ses usages actuels, la population dans son
ensemble, n'est probablement que très peu exposée au DMF.
2. Le DMF est facilement résorbé au niveau de la peau et des voies
respiratoires. Le dosage dans les urines du NMF/DMF-OH
constitue un moyen utile pour évaluer la quantité totale de DMF
absorbé.
3. Le risque de lésions hépatiques est faible si la concentration
du DMF dans l'air ambiant est maintenue en dessous de 30 mg/m3
et qu'il n'y a pas de contact cutané. La valeur correspondante
de la teneur des urines en NMF/DMF-OH à la fin du poste de
travail a été fixée provisoirement à 40 mg/g de créatinine.
4. Le DMF est embryotoxique et faiblement tératogène pour le rat,
la souris et le lapin.
5. Il existe des preuves limitées d'une cancérogénicité du DMF
pour l'homme.
6. D'après les données dont on dispose, le DMF est peu toxique
pour l'environnement. Il est peu probable qu'il donne lieu à
une bioaccumulation.
3. Recommandations
3.1 Précautions à prendre pour la manipulation
1. Maintenir la concentration atmosphérique au-dessous de 30 mg/m3
et éviter le contact avec la peau.
2. Surveiller la concentration urinaire de NMF/DMF-OH qui indique
l'exposition totale et la maintenir en-dessous de 40 mg de
NMF/g de créatinine dans les échantillons prélevés à la fin du
poste de travail. Si la concentration dépasse cette valeur,
prendre les mesures nécessaires pour réduire l'exposition.
3.2 Recherches à effectuer
1. Etudier les effets cancérogènes possibles du DMF chez l'homme,
par des études sur des populations humaines et des animaux
d'expérience.
2. Il faudrait disposer de données plus complètes sur la
possibilité d'extrapoler à l'homme les résultats des études
d'embryotoxicité et de tératogénicité effectuées sur l'animal.
Il serait bon d'étudier la cinétique comparée du DMF chez
l'homme et l'animal.
3. Davantage de données sont nécessaires sur le mode d'action et
l'activité relative des métabolites du DMF chez l'homme et
l'animal.
4. Il faudrait affiner les relations entre a) les concentrations
en métabolites urinaires et les taux d'exposition atmosphérique
(en l'absence de contact cutané), et b) la dose totale absorbée
par toutes les voies possibles (indiquée par la concentration
en NMF urinaire à la fin du poste de travail) et l'absence
d'hépatotoxicité.
RESUMEN Y EVALUACION, CONCLUSIONES, RECOMENDACIONES
1. Resumen y evaluación
1.1 Propiedades generales
La N,N-dimetilformamida (dimetilformamida, DMF, CAS 